User login
Rheumatoid Arthritis vs Osteoarthritis: Comparison of Demographics and Trends of Joint Replacement Data from the Nationwide Inpatient Sample
ABSTRACT
Current literature regarding complications following total joint arthroplasty have primarily focused on patients with osteoarthritis (OA), with less emphasis on the trends and in-hospital outcomes of rheumatoid arthritis (RA) patients undergoing these procedures. The purpose of this study is to analyze the outcomes and trends of RA patients undergoing total knee arthroplasty (TKA) or total hip arthroplasty (THA) compared to OA patients.
Data from the Nationwide Inpatient Sample from 2006 to 2011 was extracted using the International Classification of Diseases, Ninth Revision codes for patients that received a TKA or THA. Outcome measures included cardiovascular complications, cerebrovascular complications, pulmonary complications, wound dehiscence, and infection. Inpatient and hospital demographics including primary diagnosis, age, gender, primary payer, hospital teaching status, Charlson Comorbidity Index score, hospital bed size, location, and median household income were analyzed.
Logistic regression analysis of OA vs RA patients with patient outcomes revealed that osteoarthritic THA candidates had lower risk for cardiovascular complications, pulmonary complications, wound dehiscence, infections, and systemic complications, compared to rheumatoid patients. There was a significantly elevated risk of cerebrovascular complication in osteoarthritic THA compared to RA THA. OA patients undergoing TKA had significantly higher risk for cardiovascular and cerebrovascular complications. There were significant decreases in mechanical wounds, infection, and systemic complications in the OA TKA patients.
RA patients are at higher risk for postoperative infection, wound dehiscence, and systemic complications after TKA and THA compared to OA patients. These findings highlight the importance of preoperative medical clearance and management to optimize RA patients and improve the postoperative outcomes.
Continue to: RA is a chronic systemic inflammatory disease...
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that causes joint deterioration, leading to pain, disability, systemic complications, short lifespan, and decline in quality of life.1-3 The deterioration primarily affects the synovial membranes of joints, causing arthritis and resulting in extra-articular sequelae such as cardiovascular disease,4 pulmonary disease,5 and increased infection rates.3,6 RA is the most prevalent inflammatory arthritis worldwide and affects up to 50 cases per 100,000 in both the US and northern Europe.2,7-9 Although the gold standard of care for these patients is medical management with immunosuppressant drugs such as disease-modifying anti-rheumatic drugs (DMARDs), total joint arthroplasty (TJA) remains an important tool in the management of joint deterioration in such patients.
Total knee arthroplasty (TKA) and total hip arthroplasty (THA) are common procedures utilized to treat disorders that cause joint pain and hindered joint mobility, including osteoarthritis (OA) and RA. Given the aging population, the amount of TKAs and THAs performed in the US has consistently increased each year, with the vast majority of this increase composed of patients with OA.10 As a result, previous studies investigated the trends and outcomes of these procedures in patients with OA, but relatively less is known about the outcomes and trends of patients with RA undergoing the same surgeries.
Given that RA is a fundamentally different condition with its own pathological characteristics, an understanding of how these differences may impact postoperative outcomes in patients with RA is important. This study aims to present a comparative analysis of the trends and postoperative outcomes between patients with RA and OA undergoing TKA and THA (Figure 1, Tables 1 and 2).
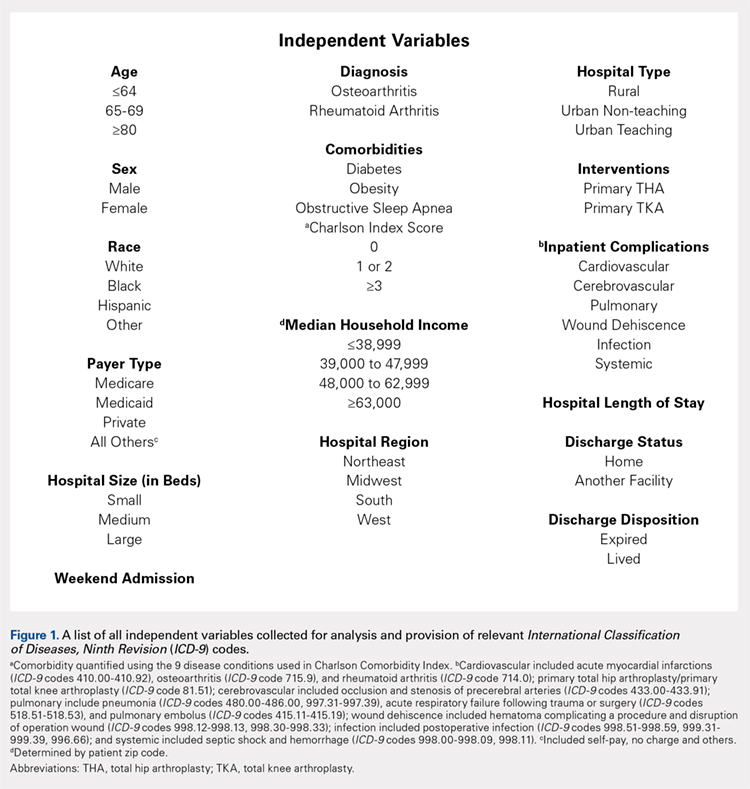
Table 1. Demographics of Total Knee Arthroplasty Patients Based on Primary Diagnosis of Osteoarthritis
| OA | RA | Total | P Value | |||
| No. | Percent | No. | Percent | No. | Percent | (RA vs OA) |
Age group |
|
|
|
|
|
| <.0001 |
<64 years | 295,637 | 42.42 | 11,325 | 48.90 | 306,962 | 42.63 |
|
65 to 79 years | 329,034 | 47.22 | 10,055 | 43.42 | 339,089 | 47.09 |
|
≥80 years | 72,197 | 10.36 | 1780 | 7.69 | 73,977 | 10.27 |
|
Gender |
|
|
|
|
|
| <.0001 |
Male | 259,192 | 37.19 | 4887 | 21.12 | 264,079 | 36.68 |
|
Female | 435,855 | 62.54 | 18,248 | 78.88 | 454,103 | 63.07 |
|
Race |
|
|
|
|
|
| <.0001 |
White | 468,632 | 67.25 | 14,532 | 77.18 | 483,164 | 67.10 |
|
Black | 39,691 | 5.7 | 2119 | 11.25 | 41,810 | 5.81 |
|
Hispanic | 28,573 | 4.1 | 1395 | 7.41 | 29,968 | 4.16 |
|
Other | 21,306 | 3.06 | 783 | 4.16 | 22,089 | 3.07 |
|
Region of hospital |
|
|
|
|
|
| <.0001 |
Northeast | 112,031 | 16.08 | 3417 | 14.75 | 115,448 | 16.03 |
|
Midwest | 192,595 | 27.64 | 5975 | 25.80 | 198,570 | 27.58 |
|
South | 257,855 | 37 | 9422 | 40.68 | 267,277 | 37.12 |
|
West | 134,387 | 19.28 | 4346 | 18.77 | 138,733 | 19.27 |
|
Location/teaching status of hospital |
|
|
|
|
|
| <.0001 |
Rural | 86,321 | 12.39 | 2709 | 11.79 | 89,030 | 12.36 |
|
Urban non-teaching | 333,043 | 47.79 | 10,905 | 47.46 | 343,948 | 47.77 |
|
Urban teaching | 273,326 | 39.22 | 9363 | 40.75 | 282,689 | 39.26 |
|
Hospital location |
|
|
|
|
|
| .0024 |
Rural | 86,321 | 12.39 | 2709 | 11.79 | 89,030 | 12.36 |
|
Urban | 606,369 | 87.01 | 20,268 | 88.21 | 626,637 | 87.03 |
|
Hospital teaching status |
|
|
|
|
|
| <.0001 |
Teaching | 409,465 | 58.76 | 13,275 | 57.78 | 422,740 | 58.71 |
|
Non-teaching | 283,225 | 40.64 | 9702 | 42.22 | 292,927 | 40.68 |
|
Comorbidities |
|
|
|
|
|
|
|
Obstructive sleep apnea | 65,342 | 9.38 | 1946 | 8.40 | 67,288 | 9.35 | <.0001 |
Diabetes | 147,292 | 21.14 | 4289 | 18.52 | 151,581 | 21.05 | <.0001 |
Obesity | 129,277 | 18.55 | 3730 | 16.11 | 133,007 | 18.47 | <.0001 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis.
Table 2. Demographics of Total Hip Arthroplasty Patients Based on Primary Diagnosis of Osteoarthritis or Rheumatoid Arthritis
| OA | RA | Total | P Value | |||
| No. | Percent | No. | Percent | No. | Percent | (RA vs OA) |
Age group |
|
|
|
|
|
| <.0001 |
<64 years | 133,645 | 45.18 | 4679 | 48.02 | 138,324 | 45.27 |
|
65 to 79 years | 123,628 | 41.8 | 3992 | 40.97 | 127,620 | 41.77 |
|
≥80 years | 38,513 | 13.02 | 1073 | 11.01 | 39,586 | 12.96 |
|
Gender |
|
|
|
|
|
| <.0001 |
Male | 129,708 | 43.85 | 2457 | 25.24 | 132,165 | 43.26 |
|
Female | 165,010 | 55.79 | 7278 | 74.76 | 172,288 | 56.39 |
|
Race |
|
|
|
|
|
| <.0001 |
White | 207,005 | 69.98 | 6322 | 80.08 | 213,327 | 69.82 |
|
Black | 15,505 | 5.24 | 771 | 9.77 | 16,276 | 5.33 |
|
Hispanic | 6784 | 2.29 | 522 | 6.61 | 7306 | 2.39 |
|
Other | 7209 | 2.44 | 280 | 3.55 | 7489 | 2.45 |
|
Region of hospital |
|
|
|
|
|
| <.0001 |
Northeast | 58,525 | 19.79 | 1683 | 17.27 | 60,208 | 19.71 |
|
Midwest | 79,040 | 26.72 | 2446 | 25.10 | 81,486 | 26.67 |
|
South | 95,337 | 32.23 | 3716 | 38.14 | 99,053 | 32.42 |
|
West | 62,884 | 21.26 | 1899 | 19.49 | 64,783 | 21.20 |
|
Location/teaching status of hospital |
|
|
|
|
|
| .0065 |
Rural | 30,954 | 10.46 | 993 | 10.26 | 31,947 | 10.46 |
|
Urban non-teaching | 133,061 | 44.99 | 4245 | 43.87 | 137,306 | 44.94 |
|
Urban teaching | 130,150 | 44 | 4439 | 45.87 | 134,589 | 44.05 |
|
Hospital location |
|
|
|
|
|
| .4098 |
Rural | 30,954 | 10.46 | 993 | 10.26 | 31,947 | 10.46 |
|
Urban | 263,211 | 88.99 | 8684 | 89.74 | 271,895 | 88.99 |
|
Hospital teaching status |
|
|
|
|
|
| .0077 |
Teaching | 159,313 | 53.86 | 5108 | 52.78 | 164,421 | 53.82 |
|
Non-teaching | 134,852 | 45.59 | 4569 | 47.22 | 139,421 | 45.63 |
|
Comorbidities |
|
|
|
|
|
|
|
Obstructive sleep apnea | 19,760 | 6.68 | 573 | 5.88 | 20,333 | 6.65 | .0028 |
Diabetes | 41,929 | 14.18 | 1325 | 13.60 | 43,254 | 14.16 | .1077 |
Obesity | 38,808 | 13.12 | 1100 | 11.29 | 39,908 | 13.06 | <.0001 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis
Continue to: Methods...
METHODS
Exemptions were obtained from the Institutional Review Board. Data from the Nationwide Inpatient Sample (NIS) from 2006 to 2011 were extracted using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for patients that received primary TKA or THA, as well as their comorbid conditions. No patients or populations were excluded from the sampling process. A list of all independent variables collected for analysis and provision of relevant ICD-9 codes is included in Figure 1. The NIS is the largest all-payer stratified survey of inpatient care in the US healthcare system. As of 2011, each year provides information on approximately 8 million inpatient stays from about 1000 hospitals in 46 states. All discharges from sampled hospitals are also represented in the database. All patient information is protected, and all methods were conducted in accordance with the highest ethical standards of Human and Animal Rights Research.
STATISTICAL ANALYSIS
SAS 9.2 and PROC FREQ statistics software were used to generate P values (chi square result) and analyze the trends (Cochran-Armitage). Results were weighted utilizing standard discharge weights from the NIS to ensure accurate comparison of data from different time points. P < .05 was considered statistically significant. Multivariable logistic regression analyses were performed to generate odds ratio and 95% confidence limits to assess outcomes across different demographic variables.
RESULTS
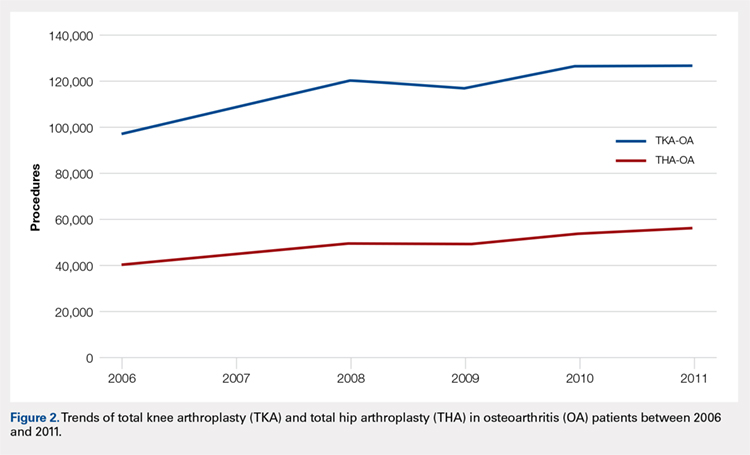
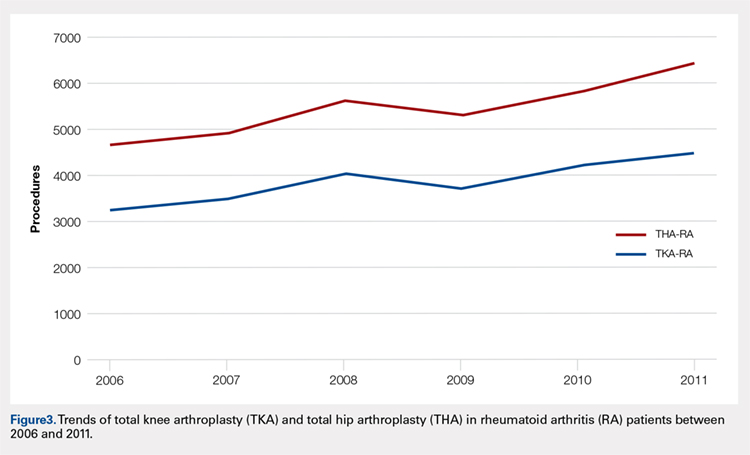
Data on 337,082 and 1,362,241 patients undergoing THA or TKA, respectively, between 2006 and 2011 were analyzed. Patients in both groups were further differentiated by a diagnosis of either OA or RA. OA was the most common diagnosis, constituting 96.8% of all arthritic THA and TKA patients. From 2006 to 2011, a 36% and 34% increase in total number of THAs and TKAs, respectively, were reported. The number of patients with OA undergoing THA and TKA steadily increased from 2006 to 2011 (Figure 2). The number of THA and TKA procedures in patients with RA followed a similar trend but at a comparatively slower rate (Figure 3). The TKA geographical trends mirrored those observed with THA. The majority of operations were performed at urban hospitals (89% THA, 87% TKA; P < .0001). Among patients with RA and OA, the majority of TKAs (47.77%; P < .0001) took place in urban non-teaching hospitals than in urban teaching hospitals (39.26%). This pattern was not the same for THA, with 44.94% being performed at urban teaching hospitals and 44.05% at urban non-teaching institutions (P < .0001). Rural hospitals accounted for a low percentage of operations for both procedures: 10.46% of THA and 12.36% of TKA (P < .0001). Large institutions (based on the number of beds) claimed the majority of cases (59% of THA and TKA).
Logistic regression analysis and odds ratios of patients with OA vs those with RA with patient outcomes adjusted for age, Charlson Comorbidity Index (CCI) score, and gender revealed that patients with OA undergoing THA had lower risk for cardiovascular (0.674; confidence interval (CI) 0.587-0.774) and pulmonary complications (0.416; CI 0.384-0.450), wound dehiscence (0.647; CI 0.561-0.747), infections (0.258; CI 0.221-0.301), and systemic complications (0.625; CI 0.562-0.695) than patients with RA. Patients with OA exhibited statistically significantly higher odds of experiencing cerebrovascular complications after THA than those with RA (1.946; CI 1.673-2.236) (Table 3). In a similar logistic regression analysis of OA vs RA in TKA, which was adjusted for age, CCI score, and gender, patients with OA had significantly higher risk for cardiovascular (1.329; CI 1.069-1.651) and cerebrovascular complications (1.635; CI 1.375-1.943) than patients with RA. Significant decreases in wound dehiscence (0.757; CI 0.639-0.896), infection (0.331; CI 0.286-0.383), and systemic complication (0.641; CI 0.565-0.729) were noted in the patients with OA and TKA (Table 4).
Table 3. Odds Ratio for In-Hospital Complications Following THA for OA Patients vs RA Patients
| Odds Ratio | Confidence Limits |
Cardiovascular complication | .674 | .587-.744 |
Cerebrovascular complication | 1.946 | 1.673-2.236 |
Pulmonary complication | .416 | .384-.450 |
Wound dehiscence | .647 | .561-.747 |
Infection | .258 | .221-.301 |
Systemic complication | .625 | .562-.695 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis; THA, total hip arthroplasty.
Table 4. Odds Ratio for In-Hospital Complications Following TKA for OA Patients vs RA Patients
| Odds Ratio | Confidence Limits |
Cardiovascular complication | 1.329 | 1.069-1.651 |
Cerebrovascular complication | 1.635 | 1.375-1.943 |
Pulmonary complication | 1.03 | .995-1.223 |
Wound dehiscence | .757 | .639-.896 |
Infection | .331 | .286-.383 |
Systemic complication | .641 | .565-.729 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis; TKA, total knee arthroplasty.
Continue to: Discussion...
DISCUSSION
Our results showed a continuous yearly increase from 2006 to 2011 in THA and TKA procedures at a rate of 36% and 34%, respectively; this result was consistent with existing literature.11 Despite a substantial increase in the amount of total THA and TKA procedures, the ratio of patients with RA undergoing these operations has decreased or remained nearly the same. Similar effects were found in Japan and the US when examining patients with RA undergoing TJA procedures between 2001 and 2007 and between 1992 and 2005, respectively.12-14 This observation may be explained by the advances and early initiation of pharmacologic treatment and the widespread use of DMARDs such as methotrexate (MTX), azathioprine, leflunomide, hydroxychloroquine, and biological response modifiers TNF-α and interleukin-1.15 These medications have drastically improved survival rates of patients with RA with impressive capabilities in symptom relief.15 With the increasing use of DMARDs and aggressive treatment early on in the disease process, patients with RA are showing markedly slow progression of joint deterioration, leading to a decreased need for orthopedic intervention compared with the general population.13,15
When analyzing the complication rates for patients undergoing TKA and THA, we observed that patients with RA exhibited a significant increase in the rates of infections, wound dehiscence, and systemic complications prior to discharge from the hospital compared with the OA population. The increased risk of infections was reported in previous studies assessing postoperative complication rates in TJA.16,17 A study utilizing the Norwegian Arthroplasty Registry noted an increased risk of late infection in patients with RA, leading to increased rates of revision TJA in comparison with patients with OA.16 Another study, which was based on the Canadian Institute for Health Information Discharge Abstract Database, showed that patients with RA are at an increased risk of infection only after THA and interestingly not after TKA.17 Although our study did not identify the causes of the increased infection rate, the inherent nature of the disease and the immunomodulatory drugs used to treat it may contribute to this increased infectious risk in patients with RA.6,18 Immunosuppressive DMARDs are some of the widely used medications employed to treat RA and are prime suspects of causing increased infection rates.15 The perioperative use of MTX has not been shown to cause short-term increases in infection for patients undergoing orthopedic intervention, but leflunomide and TNF-α inhibitors have been shown to cause a significant several-fold increase in risk for surgical wound infections.19,20
All patients with RA presented with significant increases for infection, wound dehiscence, and systemic complications, whereas only patients with RA undergoing THA showed increased risk of pulmonary and cardiovascular complications when compared with patients with OA. Surprisingly, in TKA, patients with RA were at a significantly decreased risk of cardiovascular complications. This observation was interesting due to cardiovascular disease being one of RA's most notable extra-articular features.4,21
Patients with RA undergoing TJA also showed significantly lower cerebrovascular complications than patients with OA. The significant reduction in risk for these complications has not been previously reported in the current literature, and it was an unexpected finding as past studies have found an increased risk in cerebrovascular disease in patients with RA. RA is an inflammatory disease exhibiting the upregulation of procoagulation factors,22 so we expected patients with RA to be at an increased risk for cerebrovascular and cardiovascular complications over patients with OA. Although we are unsure why these results were observed, we postulate that pharmaceutical interventions may confer some protection to patients with RA. For example, aspirin is commonly utilized in RA for its protective anti-platelet effect23 and may be a contributing factor to why we found low postoperative complication rates in cerebrovascular disease. However, the reason why aspirin may be protective against cerebrovascular and not cardiovascular complications remains unclear. Moreover, most guidelines suggest that aspirin be stopped prior to surgery.24 Although patients with RA were younger than those with OA, age was accounted for when analyzing the data.
A major strength of the study was the large sample size and the adjustment of potential confounding variables when examining the difference in complications between RA and OA. It is also a national US study that utilizes a validated database. Given that the patient samples in the NIS are reported in a uniform and de-identified manner, the database is considered ideal and has been extensively used for retrospective large observational cohort studies.25 However, the study also had some limitations due to the retrospective and administrative nature of the NIS database. Only data concerning patient complications during their inpatient stay at the hospital were available. Patients who may develop complications following discharge were not included in the data, providing a very small window of time for analysis. Another limitation with the database was its lack of ability to identify the severity of each patient's disease process or the medical treatment they received perioperatively. Finally, no patient-reported outcomes were determined, which would provide information on whether these complications affect the patients’ postoperational satisfaction in regard to their pain and disability.
CONCLUSION
As RA patients continue to utilize joint arthroplasty to repair deteriorated joints, understanding of how the disease process and its medical management may impact patient outcomes is important. This article reports significantly higher postoperational infection rates in RA than in patients with OA, which may be due to the medical management of the disease. Although new medications have been introduced and are being used to treat patients with RA, they have not altered the complication rate following TJA in this patient population. Thus, surgeons and other members of the management team should be familiar with the common medical conditions, co-morbidities, and medical treatments/side effects that are encountered in patients with RA. Future studies should delve into possible differences in long-term outcomes of patients with RA undergoing TKA and THA, as well as whether certain perioperative strategies and therapies (medical or physical) may decrease complications and improve outcomes.
This paper will be judged for the Resident Writer’s Award.
- Myasoedova E, Davis JM 3rd, Crowson CS, Gabriel SE. Epidemiology of rheumatoid arthritis: rheumatoid arthritis and mortality. Curr Rheumatol Rep. 2010;12(5):379-385. doi:10.1007/s11926-010-0117-y.
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356-361. doi:10.1038/nature01661.
- Gullick NJ, Scott DL. Co-morbidities in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2011;25(4):469-483. doi:10.1016/j.berh.2011.10.009.
- Masuda H, Miyazaki T, Shimada K, et al. Disease duration and severity impacts on long-term cardiovascular events in Japanese patients with rheumatoid arthritis. J Cardiol. 2014;64(5):366-370. doi:10.1016/j.jjcc.2014.02.018.
- Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum.2010;62(6):1583-1591. doi:10.1002/art.27405.
- Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46(9):2287-2293. doi:10.1002/art.10524.
- Rossini M, Rossi E, Bernardi D, et al. Prevalence and incidence of rheumatoid arthritis in Italy. Rheumatol Int. 2014;34(5):659664. doi:10.1007/s00296-014-2974-6.
- Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006;36(3):182-188. doi:10.1016/j.semarthrit.2006.08.006.
- Carbonell J, Cobo T, Balsa A, Descalzo MA, Carmona L. The incidence of rheumatoid arthritis in Spain: results from a nationwide primary care registry. Rheumatology.2008;47(7):1088-1092. doi:10.1093/rheumatology/ken205.
- Skytta ET, Honkanen PB, Eskelinen A, Huhtala H, Remes V. Fewer and older patients with rheumatoid arthritis need total knee replacement. Scand J Rheumatol. 2012;41(5):345-349. doi:10.3109/03009742.2012.681061.
- Singh JA, Vessely MB, Harmsen WS, et al. A population-based study of trends in the use of total hip and total knee arthroplasty, 1969–2008. Mayo Clin Proc. 2010;85(10):898-904. doi:10.4065/mcp.2010.0115.
- Momohara S, Inoue E, Ikari K, et al. Decrease in orthopaedic operations, including total joint replacements, in patients with rheumatoid arthritis between 2001 and 2007: data from Japanese outpatients in a single institute-based large observational cohort (IORRA). Ann Rheum Dis. 2010;69(1):312-313. doi:10.1136/ard.2009.107599.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551. doi:10.5435/00124635-200609000-00004.
- Schrama JC, Espehaug B, Hallan G, et al. Risk of revision for infection in primary total hip and knee arthroplasty in patients with rheumatoid arthritis compared with osteoarthritis: a prospective, population-based study on 108,786 hip and knee joint arthroplasties from the Norwegian Arthroplasty Register. Arthritis Care Res. 2010;62(4):473-479. doi:10.1002/acr.20036.
- Ravi B, Croxford R, Hollands S, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263. doi:10.1002/art.38231.
- Au K, Reed G, Curtis JR, et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(5):785-791. doi:10.1136/ard.2010.128637.
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275-2285. doi:10.1001/jama.295.19.2275.
- Scherrer CB, Mannion AF, Kyburz D, Vogt M, Kramers-de Quervain IA. Infection risk after orthopedic surgery in patients with inflammatory rheumatic diseases treated with immunosuppressive drugs. Arthritis Care Res. 2013;65(12):2032-2040. doi:10.1002/acr.22077.
- Bacani AK, Gabriel SE, Crowson CS, Heit JA, Matteson EL. Noncardiac vascular disease in rheumatoid arthritis: increase in venous thromboembolic events? Arthritis Rheum.2012;64(1):53-61. doi:10.1002/art.33322.
- Wallberg-Jonsson S, Dahlen GH, Nilsson TK, Ranby M, Rantapaa-Dahlqvist S. Tissue plasminogen activator, plasminogen activator inhibitor-1 and von Willebrand factor in rheumatoid arthritis. Clin Rheumatol. 1993;12(3):318324.
- van Heereveld HA, Laan RF, van den Hoogen FH, Malefijt MC, Novakova IR, van de Putte LB. Prevention of symptomatic thrombosis with short term (low molecular weight) heparin in patients with rheumatoid arthritis after hip or knee replacement. Ann Rheum Dis.2001;60(10):974-976. doi:10.1136/ard.60.10.974.
- Mont MA, Jacobs JJ, Boggio LN, et al. Preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg.2011;19(12):768-776.
- Bozic KJ, Bashyal RK, Anthony SG, Chiu V, Shulman B, Rubash HE. Is administratively coded comorbidity and complication data in total joint arthroplasty valid? Clin Orthop Relat Res. 2013;471(1):201-205. doi:10.1007/s11999-012-2352-1.
ABSTRACT
Current literature regarding complications following total joint arthroplasty have primarily focused on patients with osteoarthritis (OA), with less emphasis on the trends and in-hospital outcomes of rheumatoid arthritis (RA) patients undergoing these procedures. The purpose of this study is to analyze the outcomes and trends of RA patients undergoing total knee arthroplasty (TKA) or total hip arthroplasty (THA) compared to OA patients.
Data from the Nationwide Inpatient Sample from 2006 to 2011 was extracted using the International Classification of Diseases, Ninth Revision codes for patients that received a TKA or THA. Outcome measures included cardiovascular complications, cerebrovascular complications, pulmonary complications, wound dehiscence, and infection. Inpatient and hospital demographics including primary diagnosis, age, gender, primary payer, hospital teaching status, Charlson Comorbidity Index score, hospital bed size, location, and median household income were analyzed.
Logistic regression analysis of OA vs RA patients with patient outcomes revealed that osteoarthritic THA candidates had lower risk for cardiovascular complications, pulmonary complications, wound dehiscence, infections, and systemic complications, compared to rheumatoid patients. There was a significantly elevated risk of cerebrovascular complication in osteoarthritic THA compared to RA THA. OA patients undergoing TKA had significantly higher risk for cardiovascular and cerebrovascular complications. There were significant decreases in mechanical wounds, infection, and systemic complications in the OA TKA patients.
RA patients are at higher risk for postoperative infection, wound dehiscence, and systemic complications after TKA and THA compared to OA patients. These findings highlight the importance of preoperative medical clearance and management to optimize RA patients and improve the postoperative outcomes.
Continue to: RA is a chronic systemic inflammatory disease...
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that causes joint deterioration, leading to pain, disability, systemic complications, short lifespan, and decline in quality of life.1-3 The deterioration primarily affects the synovial membranes of joints, causing arthritis and resulting in extra-articular sequelae such as cardiovascular disease,4 pulmonary disease,5 and increased infection rates.3,6 RA is the most prevalent inflammatory arthritis worldwide and affects up to 50 cases per 100,000 in both the US and northern Europe.2,7-9 Although the gold standard of care for these patients is medical management with immunosuppressant drugs such as disease-modifying anti-rheumatic drugs (DMARDs), total joint arthroplasty (TJA) remains an important tool in the management of joint deterioration in such patients.
Total knee arthroplasty (TKA) and total hip arthroplasty (THA) are common procedures utilized to treat disorders that cause joint pain and hindered joint mobility, including osteoarthritis (OA) and RA. Given the aging population, the amount of TKAs and THAs performed in the US has consistently increased each year, with the vast majority of this increase composed of patients with OA.10 As a result, previous studies investigated the trends and outcomes of these procedures in patients with OA, but relatively less is known about the outcomes and trends of patients with RA undergoing the same surgeries.
Given that RA is a fundamentally different condition with its own pathological characteristics, an understanding of how these differences may impact postoperative outcomes in patients with RA is important. This study aims to present a comparative analysis of the trends and postoperative outcomes between patients with RA and OA undergoing TKA and THA (Figure 1, Tables 1 and 2).

Table 1. Demographics of Total Knee Arthroplasty Patients Based on Primary Diagnosis of Osteoarthritis
| OA | RA | Total | P Value | |||
| No. | Percent | No. | Percent | No. | Percent | (RA vs OA) |
Age group |
|
|
|
|
|
| <.0001 |
<64 years | 295,637 | 42.42 | 11,325 | 48.90 | 306,962 | 42.63 |
|
65 to 79 years | 329,034 | 47.22 | 10,055 | 43.42 | 339,089 | 47.09 |
|
≥80 years | 72,197 | 10.36 | 1780 | 7.69 | 73,977 | 10.27 |
|
Gender |
|
|
|
|
|
| <.0001 |
Male | 259,192 | 37.19 | 4887 | 21.12 | 264,079 | 36.68 |
|
Female | 435,855 | 62.54 | 18,248 | 78.88 | 454,103 | 63.07 |
|
Race |
|
|
|
|
|
| <.0001 |
White | 468,632 | 67.25 | 14,532 | 77.18 | 483,164 | 67.10 |
|
Black | 39,691 | 5.7 | 2119 | 11.25 | 41,810 | 5.81 |
|
Hispanic | 28,573 | 4.1 | 1395 | 7.41 | 29,968 | 4.16 |
|
Other | 21,306 | 3.06 | 783 | 4.16 | 22,089 | 3.07 |
|
Region of hospital |
|
|
|
|
|
| <.0001 |
Northeast | 112,031 | 16.08 | 3417 | 14.75 | 115,448 | 16.03 |
|
Midwest | 192,595 | 27.64 | 5975 | 25.80 | 198,570 | 27.58 |
|
South | 257,855 | 37 | 9422 | 40.68 | 267,277 | 37.12 |
|
West | 134,387 | 19.28 | 4346 | 18.77 | 138,733 | 19.27 |
|
Location/teaching status of hospital |
|
|
|
|
|
| <.0001 |
Rural | 86,321 | 12.39 | 2709 | 11.79 | 89,030 | 12.36 |
|
Urban non-teaching | 333,043 | 47.79 | 10,905 | 47.46 | 343,948 | 47.77 |
|
Urban teaching | 273,326 | 39.22 | 9363 | 40.75 | 282,689 | 39.26 |
|
Hospital location |
|
|
|
|
|
| .0024 |
Rural | 86,321 | 12.39 | 2709 | 11.79 | 89,030 | 12.36 |
|
Urban | 606,369 | 87.01 | 20,268 | 88.21 | 626,637 | 87.03 |
|
Hospital teaching status |
|
|
|
|
|
| <.0001 |
Teaching | 409,465 | 58.76 | 13,275 | 57.78 | 422,740 | 58.71 |
|
Non-teaching | 283,225 | 40.64 | 9702 | 42.22 | 292,927 | 40.68 |
|
Comorbidities |
|
|
|
|
|
|
|
Obstructive sleep apnea | 65,342 | 9.38 | 1946 | 8.40 | 67,288 | 9.35 | <.0001 |
Diabetes | 147,292 | 21.14 | 4289 | 18.52 | 151,581 | 21.05 | <.0001 |
Obesity | 129,277 | 18.55 | 3730 | 16.11 | 133,007 | 18.47 | <.0001 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis.
Table 2. Demographics of Total Hip Arthroplasty Patients Based on Primary Diagnosis of Osteoarthritis or Rheumatoid Arthritis
| OA | RA | Total | P Value | |||
| No. | Percent | No. | Percent | No. | Percent | (RA vs OA) |
Age group |
|
|
|
|
|
| <.0001 |
<64 years | 133,645 | 45.18 | 4679 | 48.02 | 138,324 | 45.27 |
|
65 to 79 years | 123,628 | 41.8 | 3992 | 40.97 | 127,620 | 41.77 |
|
≥80 years | 38,513 | 13.02 | 1073 | 11.01 | 39,586 | 12.96 |
|
Gender |
|
|
|
|
|
| <.0001 |
Male | 129,708 | 43.85 | 2457 | 25.24 | 132,165 | 43.26 |
|
Female | 165,010 | 55.79 | 7278 | 74.76 | 172,288 | 56.39 |
|
Race |
|
|
|
|
|
| <.0001 |
White | 207,005 | 69.98 | 6322 | 80.08 | 213,327 | 69.82 |
|
Black | 15,505 | 5.24 | 771 | 9.77 | 16,276 | 5.33 |
|
Hispanic | 6784 | 2.29 | 522 | 6.61 | 7306 | 2.39 |
|
Other | 7209 | 2.44 | 280 | 3.55 | 7489 | 2.45 |
|
Region of hospital |
|
|
|
|
|
| <.0001 |
Northeast | 58,525 | 19.79 | 1683 | 17.27 | 60,208 | 19.71 |
|
Midwest | 79,040 | 26.72 | 2446 | 25.10 | 81,486 | 26.67 |
|
South | 95,337 | 32.23 | 3716 | 38.14 | 99,053 | 32.42 |
|
West | 62,884 | 21.26 | 1899 | 19.49 | 64,783 | 21.20 |
|
Location/teaching status of hospital |
|
|
|
|
|
| .0065 |
Rural | 30,954 | 10.46 | 993 | 10.26 | 31,947 | 10.46 |
|
Urban non-teaching | 133,061 | 44.99 | 4245 | 43.87 | 137,306 | 44.94 |
|
Urban teaching | 130,150 | 44 | 4439 | 45.87 | 134,589 | 44.05 |
|
Hospital location |
|
|
|
|
|
| .4098 |
Rural | 30,954 | 10.46 | 993 | 10.26 | 31,947 | 10.46 |
|
Urban | 263,211 | 88.99 | 8684 | 89.74 | 271,895 | 88.99 |
|
Hospital teaching status |
|
|
|
|
|
| .0077 |
Teaching | 159,313 | 53.86 | 5108 | 52.78 | 164,421 | 53.82 |
|
Non-teaching | 134,852 | 45.59 | 4569 | 47.22 | 139,421 | 45.63 |
|
Comorbidities |
|
|
|
|
|
|
|
Obstructive sleep apnea | 19,760 | 6.68 | 573 | 5.88 | 20,333 | 6.65 | .0028 |
Diabetes | 41,929 | 14.18 | 1325 | 13.60 | 43,254 | 14.16 | .1077 |
Obesity | 38,808 | 13.12 | 1100 | 11.29 | 39,908 | 13.06 | <.0001 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis
Continue to: Methods...
METHODS
Exemptions were obtained from the Institutional Review Board. Data from the Nationwide Inpatient Sample (NIS) from 2006 to 2011 were extracted using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for patients that received primary TKA or THA, as well as their comorbid conditions. No patients or populations were excluded from the sampling process. A list of all independent variables collected for analysis and provision of relevant ICD-9 codes is included in Figure 1. The NIS is the largest all-payer stratified survey of inpatient care in the US healthcare system. As of 2011, each year provides information on approximately 8 million inpatient stays from about 1000 hospitals in 46 states. All discharges from sampled hospitals are also represented in the database. All patient information is protected, and all methods were conducted in accordance with the highest ethical standards of Human and Animal Rights Research.
STATISTICAL ANALYSIS
SAS 9.2 and PROC FREQ statistics software were used to generate P values (chi square result) and analyze the trends (Cochran-Armitage). Results were weighted utilizing standard discharge weights from the NIS to ensure accurate comparison of data from different time points. P < .05 was considered statistically significant. Multivariable logistic regression analyses were performed to generate odds ratio and 95% confidence limits to assess outcomes across different demographic variables.
RESULTS
Data on 337,082 and 1,362,241 patients undergoing THA or TKA, respectively, between 2006 and 2011 were analyzed. Patients in both groups were further differentiated by a diagnosis of either OA or RA. OA was the most common diagnosis, constituting 96.8% of all arthritic THA and TKA patients. From 2006 to 2011, a 36% and 34% increase in total number of THAs and TKAs, respectively, were reported. The number of patients with OA undergoing THA and TKA steadily increased from 2006 to 2011 (Figure 2). The number of THA and TKA procedures in patients with RA followed a similar trend but at a comparatively slower rate (Figure 3). The TKA geographical trends mirrored those observed with THA. The majority of operations were performed at urban hospitals (89% THA, 87% TKA; P < .0001). Among patients with RA and OA, the majority of TKAs (47.77%; P < .0001) took place in urban non-teaching hospitals than in urban teaching hospitals (39.26%). This pattern was not the same for THA, with 44.94% being performed at urban teaching hospitals and 44.05% at urban non-teaching institutions (P < .0001). Rural hospitals accounted for a low percentage of operations for both procedures: 10.46% of THA and 12.36% of TKA (P < .0001). Large institutions (based on the number of beds) claimed the majority of cases (59% of THA and TKA).


Logistic regression analysis and odds ratios of patients with OA vs those with RA with patient outcomes adjusted for age, Charlson Comorbidity Index (CCI) score, and gender revealed that patients with OA undergoing THA had lower risk for cardiovascular (0.674; confidence interval (CI) 0.587-0.774) and pulmonary complications (0.416; CI 0.384-0.450), wound dehiscence (0.647; CI 0.561-0.747), infections (0.258; CI 0.221-0.301), and systemic complications (0.625; CI 0.562-0.695) than patients with RA. Patients with OA exhibited statistically significantly higher odds of experiencing cerebrovascular complications after THA than those with RA (1.946; CI 1.673-2.236) (Table 3). In a similar logistic regression analysis of OA vs RA in TKA, which was adjusted for age, CCI score, and gender, patients with OA had significantly higher risk for cardiovascular (1.329; CI 1.069-1.651) and cerebrovascular complications (1.635; CI 1.375-1.943) than patients with RA. Significant decreases in wound dehiscence (0.757; CI 0.639-0.896), infection (0.331; CI 0.286-0.383), and systemic complication (0.641; CI 0.565-0.729) were noted in the patients with OA and TKA (Table 4).
Table 3. Odds Ratio for In-Hospital Complications Following THA for OA Patients vs RA Patients
| Odds Ratio | Confidence Limits |
Cardiovascular complication | .674 | .587-.744 |
Cerebrovascular complication | 1.946 | 1.673-2.236 |
Pulmonary complication | .416 | .384-.450 |
Wound dehiscence | .647 | .561-.747 |
Infection | .258 | .221-.301 |
Systemic complication | .625 | .562-.695 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis; THA, total hip arthroplasty.
Table 4. Odds Ratio for In-Hospital Complications Following TKA for OA Patients vs RA Patients
| Odds Ratio | Confidence Limits |
Cardiovascular complication | 1.329 | 1.069-1.651 |
Cerebrovascular complication | 1.635 | 1.375-1.943 |
Pulmonary complication | 1.03 | .995-1.223 |
Wound dehiscence | .757 | .639-.896 |
Infection | .331 | .286-.383 |
Systemic complication | .641 | .565-.729 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis; TKA, total knee arthroplasty.
Continue to: Discussion...
DISCUSSION
Our results showed a continuous yearly increase from 2006 to 2011 in THA and TKA procedures at a rate of 36% and 34%, respectively; this result was consistent with existing literature.11 Despite a substantial increase in the amount of total THA and TKA procedures, the ratio of patients with RA undergoing these operations has decreased or remained nearly the same. Similar effects were found in Japan and the US when examining patients with RA undergoing TJA procedures between 2001 and 2007 and between 1992 and 2005, respectively.12-14 This observation may be explained by the advances and early initiation of pharmacologic treatment and the widespread use of DMARDs such as methotrexate (MTX), azathioprine, leflunomide, hydroxychloroquine, and biological response modifiers TNF-α and interleukin-1.15 These medications have drastically improved survival rates of patients with RA with impressive capabilities in symptom relief.15 With the increasing use of DMARDs and aggressive treatment early on in the disease process, patients with RA are showing markedly slow progression of joint deterioration, leading to a decreased need for orthopedic intervention compared with the general population.13,15
When analyzing the complication rates for patients undergoing TKA and THA, we observed that patients with RA exhibited a significant increase in the rates of infections, wound dehiscence, and systemic complications prior to discharge from the hospital compared with the OA population. The increased risk of infections was reported in previous studies assessing postoperative complication rates in TJA.16,17 A study utilizing the Norwegian Arthroplasty Registry noted an increased risk of late infection in patients with RA, leading to increased rates of revision TJA in comparison with patients with OA.16 Another study, which was based on the Canadian Institute for Health Information Discharge Abstract Database, showed that patients with RA are at an increased risk of infection only after THA and interestingly not after TKA.17 Although our study did not identify the causes of the increased infection rate, the inherent nature of the disease and the immunomodulatory drugs used to treat it may contribute to this increased infectious risk in patients with RA.6,18 Immunosuppressive DMARDs are some of the widely used medications employed to treat RA and are prime suspects of causing increased infection rates.15 The perioperative use of MTX has not been shown to cause short-term increases in infection for patients undergoing orthopedic intervention, but leflunomide and TNF-α inhibitors have been shown to cause a significant several-fold increase in risk for surgical wound infections.19,20
All patients with RA presented with significant increases for infection, wound dehiscence, and systemic complications, whereas only patients with RA undergoing THA showed increased risk of pulmonary and cardiovascular complications when compared with patients with OA. Surprisingly, in TKA, patients with RA were at a significantly decreased risk of cardiovascular complications. This observation was interesting due to cardiovascular disease being one of RA's most notable extra-articular features.4,21
Patients with RA undergoing TJA also showed significantly lower cerebrovascular complications than patients with OA. The significant reduction in risk for these complications has not been previously reported in the current literature, and it was an unexpected finding as past studies have found an increased risk in cerebrovascular disease in patients with RA. RA is an inflammatory disease exhibiting the upregulation of procoagulation factors,22 so we expected patients with RA to be at an increased risk for cerebrovascular and cardiovascular complications over patients with OA. Although we are unsure why these results were observed, we postulate that pharmaceutical interventions may confer some protection to patients with RA. For example, aspirin is commonly utilized in RA for its protective anti-platelet effect23 and may be a contributing factor to why we found low postoperative complication rates in cerebrovascular disease. However, the reason why aspirin may be protective against cerebrovascular and not cardiovascular complications remains unclear. Moreover, most guidelines suggest that aspirin be stopped prior to surgery.24 Although patients with RA were younger than those with OA, age was accounted for when analyzing the data.
A major strength of the study was the large sample size and the adjustment of potential confounding variables when examining the difference in complications between RA and OA. It is also a national US study that utilizes a validated database. Given that the patient samples in the NIS are reported in a uniform and de-identified manner, the database is considered ideal and has been extensively used for retrospective large observational cohort studies.25 However, the study also had some limitations due to the retrospective and administrative nature of the NIS database. Only data concerning patient complications during their inpatient stay at the hospital were available. Patients who may develop complications following discharge were not included in the data, providing a very small window of time for analysis. Another limitation with the database was its lack of ability to identify the severity of each patient's disease process or the medical treatment they received perioperatively. Finally, no patient-reported outcomes were determined, which would provide information on whether these complications affect the patients’ postoperational satisfaction in regard to their pain and disability.
CONCLUSION
As RA patients continue to utilize joint arthroplasty to repair deteriorated joints, understanding of how the disease process and its medical management may impact patient outcomes is important. This article reports significantly higher postoperational infection rates in RA than in patients with OA, which may be due to the medical management of the disease. Although new medications have been introduced and are being used to treat patients with RA, they have not altered the complication rate following TJA in this patient population. Thus, surgeons and other members of the management team should be familiar with the common medical conditions, co-morbidities, and medical treatments/side effects that are encountered in patients with RA. Future studies should delve into possible differences in long-term outcomes of patients with RA undergoing TKA and THA, as well as whether certain perioperative strategies and therapies (medical or physical) may decrease complications and improve outcomes.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Current literature regarding complications following total joint arthroplasty have primarily focused on patients with osteoarthritis (OA), with less emphasis on the trends and in-hospital outcomes of rheumatoid arthritis (RA) patients undergoing these procedures. The purpose of this study is to analyze the outcomes and trends of RA patients undergoing total knee arthroplasty (TKA) or total hip arthroplasty (THA) compared to OA patients.
Data from the Nationwide Inpatient Sample from 2006 to 2011 was extracted using the International Classification of Diseases, Ninth Revision codes for patients that received a TKA or THA. Outcome measures included cardiovascular complications, cerebrovascular complications, pulmonary complications, wound dehiscence, and infection. Inpatient and hospital demographics including primary diagnosis, age, gender, primary payer, hospital teaching status, Charlson Comorbidity Index score, hospital bed size, location, and median household income were analyzed.
Logistic regression analysis of OA vs RA patients with patient outcomes revealed that osteoarthritic THA candidates had lower risk for cardiovascular complications, pulmonary complications, wound dehiscence, infections, and systemic complications, compared to rheumatoid patients. There was a significantly elevated risk of cerebrovascular complication in osteoarthritic THA compared to RA THA. OA patients undergoing TKA had significantly higher risk for cardiovascular and cerebrovascular complications. There were significant decreases in mechanical wounds, infection, and systemic complications in the OA TKA patients.
RA patients are at higher risk for postoperative infection, wound dehiscence, and systemic complications after TKA and THA compared to OA patients. These findings highlight the importance of preoperative medical clearance and management to optimize RA patients and improve the postoperative outcomes.
Continue to: RA is a chronic systemic inflammatory disease...
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that causes joint deterioration, leading to pain, disability, systemic complications, short lifespan, and decline in quality of life.1-3 The deterioration primarily affects the synovial membranes of joints, causing arthritis and resulting in extra-articular sequelae such as cardiovascular disease,4 pulmonary disease,5 and increased infection rates.3,6 RA is the most prevalent inflammatory arthritis worldwide and affects up to 50 cases per 100,000 in both the US and northern Europe.2,7-9 Although the gold standard of care for these patients is medical management with immunosuppressant drugs such as disease-modifying anti-rheumatic drugs (DMARDs), total joint arthroplasty (TJA) remains an important tool in the management of joint deterioration in such patients.
Total knee arthroplasty (TKA) and total hip arthroplasty (THA) are common procedures utilized to treat disorders that cause joint pain and hindered joint mobility, including osteoarthritis (OA) and RA. Given the aging population, the amount of TKAs and THAs performed in the US has consistently increased each year, with the vast majority of this increase composed of patients with OA.10 As a result, previous studies investigated the trends and outcomes of these procedures in patients with OA, but relatively less is known about the outcomes and trends of patients with RA undergoing the same surgeries.
Given that RA is a fundamentally different condition with its own pathological characteristics, an understanding of how these differences may impact postoperative outcomes in patients with RA is important. This study aims to present a comparative analysis of the trends and postoperative outcomes between patients with RA and OA undergoing TKA and THA (Figure 1, Tables 1 and 2).

Table 1. Demographics of Total Knee Arthroplasty Patients Based on Primary Diagnosis of Osteoarthritis
| OA | RA | Total | P Value | |||
| No. | Percent | No. | Percent | No. | Percent | (RA vs OA) |
Age group |
|
|
|
|
|
| <.0001 |
<64 years | 295,637 | 42.42 | 11,325 | 48.90 | 306,962 | 42.63 |
|
65 to 79 years | 329,034 | 47.22 | 10,055 | 43.42 | 339,089 | 47.09 |
|
≥80 years | 72,197 | 10.36 | 1780 | 7.69 | 73,977 | 10.27 |
|
Gender |
|
|
|
|
|
| <.0001 |
Male | 259,192 | 37.19 | 4887 | 21.12 | 264,079 | 36.68 |
|
Female | 435,855 | 62.54 | 18,248 | 78.88 | 454,103 | 63.07 |
|
Race |
|
|
|
|
|
| <.0001 |
White | 468,632 | 67.25 | 14,532 | 77.18 | 483,164 | 67.10 |
|
Black | 39,691 | 5.7 | 2119 | 11.25 | 41,810 | 5.81 |
|
Hispanic | 28,573 | 4.1 | 1395 | 7.41 | 29,968 | 4.16 |
|
Other | 21,306 | 3.06 | 783 | 4.16 | 22,089 | 3.07 |
|
Region of hospital |
|
|
|
|
|
| <.0001 |
Northeast | 112,031 | 16.08 | 3417 | 14.75 | 115,448 | 16.03 |
|
Midwest | 192,595 | 27.64 | 5975 | 25.80 | 198,570 | 27.58 |
|
South | 257,855 | 37 | 9422 | 40.68 | 267,277 | 37.12 |
|
West | 134,387 | 19.28 | 4346 | 18.77 | 138,733 | 19.27 |
|
Location/teaching status of hospital |
|
|
|
|
|
| <.0001 |
Rural | 86,321 | 12.39 | 2709 | 11.79 | 89,030 | 12.36 |
|
Urban non-teaching | 333,043 | 47.79 | 10,905 | 47.46 | 343,948 | 47.77 |
|
Urban teaching | 273,326 | 39.22 | 9363 | 40.75 | 282,689 | 39.26 |
|
Hospital location |
|
|
|
|
|
| .0024 |
Rural | 86,321 | 12.39 | 2709 | 11.79 | 89,030 | 12.36 |
|
Urban | 606,369 | 87.01 | 20,268 | 88.21 | 626,637 | 87.03 |
|
Hospital teaching status |
|
|
|
|
|
| <.0001 |
Teaching | 409,465 | 58.76 | 13,275 | 57.78 | 422,740 | 58.71 |
|
Non-teaching | 283,225 | 40.64 | 9702 | 42.22 | 292,927 | 40.68 |
|
Comorbidities |
|
|
|
|
|
|
|
Obstructive sleep apnea | 65,342 | 9.38 | 1946 | 8.40 | 67,288 | 9.35 | <.0001 |
Diabetes | 147,292 | 21.14 | 4289 | 18.52 | 151,581 | 21.05 | <.0001 |
Obesity | 129,277 | 18.55 | 3730 | 16.11 | 133,007 | 18.47 | <.0001 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis.
Table 2. Demographics of Total Hip Arthroplasty Patients Based on Primary Diagnosis of Osteoarthritis or Rheumatoid Arthritis
| OA | RA | Total | P Value | |||
| No. | Percent | No. | Percent | No. | Percent | (RA vs OA) |
Age group |
|
|
|
|
|
| <.0001 |
<64 years | 133,645 | 45.18 | 4679 | 48.02 | 138,324 | 45.27 |
|
65 to 79 years | 123,628 | 41.8 | 3992 | 40.97 | 127,620 | 41.77 |
|
≥80 years | 38,513 | 13.02 | 1073 | 11.01 | 39,586 | 12.96 |
|
Gender |
|
|
|
|
|
| <.0001 |
Male | 129,708 | 43.85 | 2457 | 25.24 | 132,165 | 43.26 |
|
Female | 165,010 | 55.79 | 7278 | 74.76 | 172,288 | 56.39 |
|
Race |
|
|
|
|
|
| <.0001 |
White | 207,005 | 69.98 | 6322 | 80.08 | 213,327 | 69.82 |
|
Black | 15,505 | 5.24 | 771 | 9.77 | 16,276 | 5.33 |
|
Hispanic | 6784 | 2.29 | 522 | 6.61 | 7306 | 2.39 |
|
Other | 7209 | 2.44 | 280 | 3.55 | 7489 | 2.45 |
|
Region of hospital |
|
|
|
|
|
| <.0001 |
Northeast | 58,525 | 19.79 | 1683 | 17.27 | 60,208 | 19.71 |
|
Midwest | 79,040 | 26.72 | 2446 | 25.10 | 81,486 | 26.67 |
|
South | 95,337 | 32.23 | 3716 | 38.14 | 99,053 | 32.42 |
|
West | 62,884 | 21.26 | 1899 | 19.49 | 64,783 | 21.20 |
|
Location/teaching status of hospital |
|
|
|
|
|
| .0065 |
Rural | 30,954 | 10.46 | 993 | 10.26 | 31,947 | 10.46 |
|
Urban non-teaching | 133,061 | 44.99 | 4245 | 43.87 | 137,306 | 44.94 |
|
Urban teaching | 130,150 | 44 | 4439 | 45.87 | 134,589 | 44.05 |
|
Hospital location |
|
|
|
|
|
| .4098 |
Rural | 30,954 | 10.46 | 993 | 10.26 | 31,947 | 10.46 |
|
Urban | 263,211 | 88.99 | 8684 | 89.74 | 271,895 | 88.99 |
|
Hospital teaching status |
|
|
|
|
|
| .0077 |
Teaching | 159,313 | 53.86 | 5108 | 52.78 | 164,421 | 53.82 |
|
Non-teaching | 134,852 | 45.59 | 4569 | 47.22 | 139,421 | 45.63 |
|
Comorbidities |
|
|
|
|
|
|
|
Obstructive sleep apnea | 19,760 | 6.68 | 573 | 5.88 | 20,333 | 6.65 | .0028 |
Diabetes | 41,929 | 14.18 | 1325 | 13.60 | 43,254 | 14.16 | .1077 |
Obesity | 38,808 | 13.12 | 1100 | 11.29 | 39,908 | 13.06 | <.0001 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis
Continue to: Methods...
METHODS
Exemptions were obtained from the Institutional Review Board. Data from the Nationwide Inpatient Sample (NIS) from 2006 to 2011 were extracted using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for patients that received primary TKA or THA, as well as their comorbid conditions. No patients or populations were excluded from the sampling process. A list of all independent variables collected for analysis and provision of relevant ICD-9 codes is included in Figure 1. The NIS is the largest all-payer stratified survey of inpatient care in the US healthcare system. As of 2011, each year provides information on approximately 8 million inpatient stays from about 1000 hospitals in 46 states. All discharges from sampled hospitals are also represented in the database. All patient information is protected, and all methods were conducted in accordance with the highest ethical standards of Human and Animal Rights Research.
STATISTICAL ANALYSIS
SAS 9.2 and PROC FREQ statistics software were used to generate P values (chi square result) and analyze the trends (Cochran-Armitage). Results were weighted utilizing standard discharge weights from the NIS to ensure accurate comparison of data from different time points. P < .05 was considered statistically significant. Multivariable logistic regression analyses were performed to generate odds ratio and 95% confidence limits to assess outcomes across different demographic variables.
RESULTS
Data on 337,082 and 1,362,241 patients undergoing THA or TKA, respectively, between 2006 and 2011 were analyzed. Patients in both groups were further differentiated by a diagnosis of either OA or RA. OA was the most common diagnosis, constituting 96.8% of all arthritic THA and TKA patients. From 2006 to 2011, a 36% and 34% increase in total number of THAs and TKAs, respectively, were reported. The number of patients with OA undergoing THA and TKA steadily increased from 2006 to 2011 (Figure 2). The number of THA and TKA procedures in patients with RA followed a similar trend but at a comparatively slower rate (Figure 3). The TKA geographical trends mirrored those observed with THA. The majority of operations were performed at urban hospitals (89% THA, 87% TKA; P < .0001). Among patients with RA and OA, the majority of TKAs (47.77%; P < .0001) took place in urban non-teaching hospitals than in urban teaching hospitals (39.26%). This pattern was not the same for THA, with 44.94% being performed at urban teaching hospitals and 44.05% at urban non-teaching institutions (P < .0001). Rural hospitals accounted for a low percentage of operations for both procedures: 10.46% of THA and 12.36% of TKA (P < .0001). Large institutions (based on the number of beds) claimed the majority of cases (59% of THA and TKA).


Logistic regression analysis and odds ratios of patients with OA vs those with RA with patient outcomes adjusted for age, Charlson Comorbidity Index (CCI) score, and gender revealed that patients with OA undergoing THA had lower risk for cardiovascular (0.674; confidence interval (CI) 0.587-0.774) and pulmonary complications (0.416; CI 0.384-0.450), wound dehiscence (0.647; CI 0.561-0.747), infections (0.258; CI 0.221-0.301), and systemic complications (0.625; CI 0.562-0.695) than patients with RA. Patients with OA exhibited statistically significantly higher odds of experiencing cerebrovascular complications after THA than those with RA (1.946; CI 1.673-2.236) (Table 3). In a similar logistic regression analysis of OA vs RA in TKA, which was adjusted for age, CCI score, and gender, patients with OA had significantly higher risk for cardiovascular (1.329; CI 1.069-1.651) and cerebrovascular complications (1.635; CI 1.375-1.943) than patients with RA. Significant decreases in wound dehiscence (0.757; CI 0.639-0.896), infection (0.331; CI 0.286-0.383), and systemic complication (0.641; CI 0.565-0.729) were noted in the patients with OA and TKA (Table 4).
Table 3. Odds Ratio for In-Hospital Complications Following THA for OA Patients vs RA Patients
| Odds Ratio | Confidence Limits |
Cardiovascular complication | .674 | .587-.744 |
Cerebrovascular complication | 1.946 | 1.673-2.236 |
Pulmonary complication | .416 | .384-.450 |
Wound dehiscence | .647 | .561-.747 |
Infection | .258 | .221-.301 |
Systemic complication | .625 | .562-.695 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis; THA, total hip arthroplasty.
Table 4. Odds Ratio for In-Hospital Complications Following TKA for OA Patients vs RA Patients
| Odds Ratio | Confidence Limits |
Cardiovascular complication | 1.329 | 1.069-1.651 |
Cerebrovascular complication | 1.635 | 1.375-1.943 |
Pulmonary complication | 1.03 | .995-1.223 |
Wound dehiscence | .757 | .639-.896 |
Infection | .331 | .286-.383 |
Systemic complication | .641 | .565-.729 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis; TKA, total knee arthroplasty.
Continue to: Discussion...
DISCUSSION
Our results showed a continuous yearly increase from 2006 to 2011 in THA and TKA procedures at a rate of 36% and 34%, respectively; this result was consistent with existing literature.11 Despite a substantial increase in the amount of total THA and TKA procedures, the ratio of patients with RA undergoing these operations has decreased or remained nearly the same. Similar effects were found in Japan and the US when examining patients with RA undergoing TJA procedures between 2001 and 2007 and between 1992 and 2005, respectively.12-14 This observation may be explained by the advances and early initiation of pharmacologic treatment and the widespread use of DMARDs such as methotrexate (MTX), azathioprine, leflunomide, hydroxychloroquine, and biological response modifiers TNF-α and interleukin-1.15 These medications have drastically improved survival rates of patients with RA with impressive capabilities in symptom relief.15 With the increasing use of DMARDs and aggressive treatment early on in the disease process, patients with RA are showing markedly slow progression of joint deterioration, leading to a decreased need for orthopedic intervention compared with the general population.13,15
When analyzing the complication rates for patients undergoing TKA and THA, we observed that patients with RA exhibited a significant increase in the rates of infections, wound dehiscence, and systemic complications prior to discharge from the hospital compared with the OA population. The increased risk of infections was reported in previous studies assessing postoperative complication rates in TJA.16,17 A study utilizing the Norwegian Arthroplasty Registry noted an increased risk of late infection in patients with RA, leading to increased rates of revision TJA in comparison with patients with OA.16 Another study, which was based on the Canadian Institute for Health Information Discharge Abstract Database, showed that patients with RA are at an increased risk of infection only after THA and interestingly not after TKA.17 Although our study did not identify the causes of the increased infection rate, the inherent nature of the disease and the immunomodulatory drugs used to treat it may contribute to this increased infectious risk in patients with RA.6,18 Immunosuppressive DMARDs are some of the widely used medications employed to treat RA and are prime suspects of causing increased infection rates.15 The perioperative use of MTX has not been shown to cause short-term increases in infection for patients undergoing orthopedic intervention, but leflunomide and TNF-α inhibitors have been shown to cause a significant several-fold increase in risk for surgical wound infections.19,20
All patients with RA presented with significant increases for infection, wound dehiscence, and systemic complications, whereas only patients with RA undergoing THA showed increased risk of pulmonary and cardiovascular complications when compared with patients with OA. Surprisingly, in TKA, patients with RA were at a significantly decreased risk of cardiovascular complications. This observation was interesting due to cardiovascular disease being one of RA's most notable extra-articular features.4,21
Patients with RA undergoing TJA also showed significantly lower cerebrovascular complications than patients with OA. The significant reduction in risk for these complications has not been previously reported in the current literature, and it was an unexpected finding as past studies have found an increased risk in cerebrovascular disease in patients with RA. RA is an inflammatory disease exhibiting the upregulation of procoagulation factors,22 so we expected patients with RA to be at an increased risk for cerebrovascular and cardiovascular complications over patients with OA. Although we are unsure why these results were observed, we postulate that pharmaceutical interventions may confer some protection to patients with RA. For example, aspirin is commonly utilized in RA for its protective anti-platelet effect23 and may be a contributing factor to why we found low postoperative complication rates in cerebrovascular disease. However, the reason why aspirin may be protective against cerebrovascular and not cardiovascular complications remains unclear. Moreover, most guidelines suggest that aspirin be stopped prior to surgery.24 Although patients with RA were younger than those with OA, age was accounted for when analyzing the data.
A major strength of the study was the large sample size and the adjustment of potential confounding variables when examining the difference in complications between RA and OA. It is also a national US study that utilizes a validated database. Given that the patient samples in the NIS are reported in a uniform and de-identified manner, the database is considered ideal and has been extensively used for retrospective large observational cohort studies.25 However, the study also had some limitations due to the retrospective and administrative nature of the NIS database. Only data concerning patient complications during their inpatient stay at the hospital were available. Patients who may develop complications following discharge were not included in the data, providing a very small window of time for analysis. Another limitation with the database was its lack of ability to identify the severity of each patient's disease process or the medical treatment they received perioperatively. Finally, no patient-reported outcomes were determined, which would provide information on whether these complications affect the patients’ postoperational satisfaction in regard to their pain and disability.
CONCLUSION
As RA patients continue to utilize joint arthroplasty to repair deteriorated joints, understanding of how the disease process and its medical management may impact patient outcomes is important. This article reports significantly higher postoperational infection rates in RA than in patients with OA, which may be due to the medical management of the disease. Although new medications have been introduced and are being used to treat patients with RA, they have not altered the complication rate following TJA in this patient population. Thus, surgeons and other members of the management team should be familiar with the common medical conditions, co-morbidities, and medical treatments/side effects that are encountered in patients with RA. Future studies should delve into possible differences in long-term outcomes of patients with RA undergoing TKA and THA, as well as whether certain perioperative strategies and therapies (medical or physical) may decrease complications and improve outcomes.
This paper will be judged for the Resident Writer’s Award.
- Myasoedova E, Davis JM 3rd, Crowson CS, Gabriel SE. Epidemiology of rheumatoid arthritis: rheumatoid arthritis and mortality. Curr Rheumatol Rep. 2010;12(5):379-385. doi:10.1007/s11926-010-0117-y.
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356-361. doi:10.1038/nature01661.
- Gullick NJ, Scott DL. Co-morbidities in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2011;25(4):469-483. doi:10.1016/j.berh.2011.10.009.
- Masuda H, Miyazaki T, Shimada K, et al. Disease duration and severity impacts on long-term cardiovascular events in Japanese patients with rheumatoid arthritis. J Cardiol. 2014;64(5):366-370. doi:10.1016/j.jjcc.2014.02.018.
- Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum.2010;62(6):1583-1591. doi:10.1002/art.27405.
- Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46(9):2287-2293. doi:10.1002/art.10524.
- Rossini M, Rossi E, Bernardi D, et al. Prevalence and incidence of rheumatoid arthritis in Italy. Rheumatol Int. 2014;34(5):659664. doi:10.1007/s00296-014-2974-6.
- Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006;36(3):182-188. doi:10.1016/j.semarthrit.2006.08.006.
- Carbonell J, Cobo T, Balsa A, Descalzo MA, Carmona L. The incidence of rheumatoid arthritis in Spain: results from a nationwide primary care registry. Rheumatology.2008;47(7):1088-1092. doi:10.1093/rheumatology/ken205.
- Skytta ET, Honkanen PB, Eskelinen A, Huhtala H, Remes V. Fewer and older patients with rheumatoid arthritis need total knee replacement. Scand J Rheumatol. 2012;41(5):345-349. doi:10.3109/03009742.2012.681061.
- Singh JA, Vessely MB, Harmsen WS, et al. A population-based study of trends in the use of total hip and total knee arthroplasty, 1969–2008. Mayo Clin Proc. 2010;85(10):898-904. doi:10.4065/mcp.2010.0115.
- Momohara S, Inoue E, Ikari K, et al. Decrease in orthopaedic operations, including total joint replacements, in patients with rheumatoid arthritis between 2001 and 2007: data from Japanese outpatients in a single institute-based large observational cohort (IORRA). Ann Rheum Dis. 2010;69(1):312-313. doi:10.1136/ard.2009.107599.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551. doi:10.5435/00124635-200609000-00004.
- Schrama JC, Espehaug B, Hallan G, et al. Risk of revision for infection in primary total hip and knee arthroplasty in patients with rheumatoid arthritis compared with osteoarthritis: a prospective, population-based study on 108,786 hip and knee joint arthroplasties from the Norwegian Arthroplasty Register. Arthritis Care Res. 2010;62(4):473-479. doi:10.1002/acr.20036.
- Ravi B, Croxford R, Hollands S, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263. doi:10.1002/art.38231.
- Au K, Reed G, Curtis JR, et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(5):785-791. doi:10.1136/ard.2010.128637.
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275-2285. doi:10.1001/jama.295.19.2275.
- Scherrer CB, Mannion AF, Kyburz D, Vogt M, Kramers-de Quervain IA. Infection risk after orthopedic surgery in patients with inflammatory rheumatic diseases treated with immunosuppressive drugs. Arthritis Care Res. 2013;65(12):2032-2040. doi:10.1002/acr.22077.
- Bacani AK, Gabriel SE, Crowson CS, Heit JA, Matteson EL. Noncardiac vascular disease in rheumatoid arthritis: increase in venous thromboembolic events? Arthritis Rheum.2012;64(1):53-61. doi:10.1002/art.33322.
- Wallberg-Jonsson S, Dahlen GH, Nilsson TK, Ranby M, Rantapaa-Dahlqvist S. Tissue plasminogen activator, plasminogen activator inhibitor-1 and von Willebrand factor in rheumatoid arthritis. Clin Rheumatol. 1993;12(3):318324.
- van Heereveld HA, Laan RF, van den Hoogen FH, Malefijt MC, Novakova IR, van de Putte LB. Prevention of symptomatic thrombosis with short term (low molecular weight) heparin in patients with rheumatoid arthritis after hip or knee replacement. Ann Rheum Dis.2001;60(10):974-976. doi:10.1136/ard.60.10.974.
- Mont MA, Jacobs JJ, Boggio LN, et al. Preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg.2011;19(12):768-776.
- Bozic KJ, Bashyal RK, Anthony SG, Chiu V, Shulman B, Rubash HE. Is administratively coded comorbidity and complication data in total joint arthroplasty valid? Clin Orthop Relat Res. 2013;471(1):201-205. doi:10.1007/s11999-012-2352-1.
- Myasoedova E, Davis JM 3rd, Crowson CS, Gabriel SE. Epidemiology of rheumatoid arthritis: rheumatoid arthritis and mortality. Curr Rheumatol Rep. 2010;12(5):379-385. doi:10.1007/s11926-010-0117-y.
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356-361. doi:10.1038/nature01661.
- Gullick NJ, Scott DL. Co-morbidities in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2011;25(4):469-483. doi:10.1016/j.berh.2011.10.009.
- Masuda H, Miyazaki T, Shimada K, et al. Disease duration and severity impacts on long-term cardiovascular events in Japanese patients with rheumatoid arthritis. J Cardiol. 2014;64(5):366-370. doi:10.1016/j.jjcc.2014.02.018.
- Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum.2010;62(6):1583-1591. doi:10.1002/art.27405.
- Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46(9):2287-2293. doi:10.1002/art.10524.
- Rossini M, Rossi E, Bernardi D, et al. Prevalence and incidence of rheumatoid arthritis in Italy. Rheumatol Int. 2014;34(5):659664. doi:10.1007/s00296-014-2974-6.
- Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006;36(3):182-188. doi:10.1016/j.semarthrit.2006.08.006.
- Carbonell J, Cobo T, Balsa A, Descalzo MA, Carmona L. The incidence of rheumatoid arthritis in Spain: results from a nationwide primary care registry. Rheumatology.2008;47(7):1088-1092. doi:10.1093/rheumatology/ken205.
- Skytta ET, Honkanen PB, Eskelinen A, Huhtala H, Remes V. Fewer and older patients with rheumatoid arthritis need total knee replacement. Scand J Rheumatol. 2012;41(5):345-349. doi:10.3109/03009742.2012.681061.
- Singh JA, Vessely MB, Harmsen WS, et al. A population-based study of trends in the use of total hip and total knee arthroplasty, 1969–2008. Mayo Clin Proc. 2010;85(10):898-904. doi:10.4065/mcp.2010.0115.
- Momohara S, Inoue E, Ikari K, et al. Decrease in orthopaedic operations, including total joint replacements, in patients with rheumatoid arthritis between 2001 and 2007: data from Japanese outpatients in a single institute-based large observational cohort (IORRA). Ann Rheum Dis. 2010;69(1):312-313. doi:10.1136/ard.2009.107599.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551. doi:10.5435/00124635-200609000-00004.
- Schrama JC, Espehaug B, Hallan G, et al. Risk of revision for infection in primary total hip and knee arthroplasty in patients with rheumatoid arthritis compared with osteoarthritis: a prospective, population-based study on 108,786 hip and knee joint arthroplasties from the Norwegian Arthroplasty Register. Arthritis Care Res. 2010;62(4):473-479. doi:10.1002/acr.20036.
- Ravi B, Croxford R, Hollands S, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263. doi:10.1002/art.38231.
- Au K, Reed G, Curtis JR, et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(5):785-791. doi:10.1136/ard.2010.128637.
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275-2285. doi:10.1001/jama.295.19.2275.
- Scherrer CB, Mannion AF, Kyburz D, Vogt M, Kramers-de Quervain IA. Infection risk after orthopedic surgery in patients with inflammatory rheumatic diseases treated with immunosuppressive drugs. Arthritis Care Res. 2013;65(12):2032-2040. doi:10.1002/acr.22077.
- Bacani AK, Gabriel SE, Crowson CS, Heit JA, Matteson EL. Noncardiac vascular disease in rheumatoid arthritis: increase in venous thromboembolic events? Arthritis Rheum.2012;64(1):53-61. doi:10.1002/art.33322.
- Wallberg-Jonsson S, Dahlen GH, Nilsson TK, Ranby M, Rantapaa-Dahlqvist S. Tissue plasminogen activator, plasminogen activator inhibitor-1 and von Willebrand factor in rheumatoid arthritis. Clin Rheumatol. 1993;12(3):318324.
- van Heereveld HA, Laan RF, van den Hoogen FH, Malefijt MC, Novakova IR, van de Putte LB. Prevention of symptomatic thrombosis with short term (low molecular weight) heparin in patients with rheumatoid arthritis after hip or knee replacement. Ann Rheum Dis.2001;60(10):974-976. doi:10.1136/ard.60.10.974.
- Mont MA, Jacobs JJ, Boggio LN, et al. Preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg.2011;19(12):768-776.
- Bozic KJ, Bashyal RK, Anthony SG, Chiu V, Shulman B, Rubash HE. Is administratively coded comorbidity and complication data in total joint arthroplasty valid? Clin Orthop Relat Res. 2013;471(1):201-205. doi:10.1007/s11999-012-2352-1.
TAKE-HOME POINTS
- Patients undergoing THA for OA, when compared to those with RA undergoing THA, had lower risk for postoperative cardiovascular, pulmonary, wound dehiscence, infections, and systemic complications.
- Patients with OA undergoing THA had statistically significant higher risk of cerebrovascular complication compared to RA patients undergoing the same procedure.
- In TKA, OA patients had significantly higher risk for cardiovascular and cerebrovascular complications, and a significant lower risk for mechanical wounds, infection, and systemic complications.
- RA patients are at higher risk for postoperative infection, wound dehiscence, and systemic complications after TKA and THA compared to OA patients.
- These findings highlight the importance of preoperative medical clearance and management to optimize RA patients and improve the postoperative outcomes.
New look at ATLAS suggests rivaroxaban may still have role in ACS
In a new analysis comparing only clinically similar outcomes in patients with acute coronary syndrome, the addition of rivaroxaban to standard antiplatelet therapy resulted in 115 fewer fatal or irreversible ischemic events per 10,000 patient-years than placebo, at the expense of only 10 additional fatal or seriously harmful events.
This new interpretation of the ATLAS ACS 2-TIMI 51 trial (Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome–Thrombolysis in Myocardial Infarction-51) suggests that the factor Xa inhibitor may still carve out a place for itself in ACS therapy, despite Food and Drug Administration rejections for this indication.
Not only did the survival benefit of rivaroxaban appear early in postevent treatment, it continued to protect patients over time, C. Michael Gibson, MD, and colleagues reported in the Journal of the American College of Cardiology.
“Time-to-event analysis demonstrated that the risk of fatal or irreversible harm remained low and constant over time, whereas reduction in fatal or irreversible ischemic events expanded,” wrote Dr. Gibson, professor of medicine at Beth Israel Deaconess Medical Center, Boston, and his coinvestigators. “By 720 days, a net of 142 fatal or irreversible events would have been prevented by 2.5-mg oral doses twice per day of rivaroxaban. Additional time-to-event sensitivity analyses demonstrated similar results, even when TIMI major bleeding was included as a fatal or irreversible event.”
In conducting the new analysis, Dr. Gibson and his team argued that the original interpretation of the results of ATLAS ACS 2-TIMI 51 lumped both fatal and nonfatal events together in composite endpoints, resulting in an inaccurate real-life picture of rivaroxaban’s therapeutic potential. “All types of events [were] weighted equally; for example, reversible nonintracranial hemorrhage, nonfatal bleeds that can be managed with supportive care, are weighted equally with death and disabling stroke. Second, stroke can be either hemorrhagic or ischemic, and the relative contributions of hemorrhagic or ischemic stroke may not be appropriately assigned to risk-versus-benefit categories in many analyses.”
The net result was that, while rivaroxaban did reduce the risk of the composite endpoint (cardiovascular death, MI, or stroke), the 1.7% absolute difference in cardiovascular mortality was almost completely offset by a 1.3% increase in major bleeding. However, most of those bleeds were reversible and nonfatal, associated with a drop in hemoglobin and/or blood transfusion. The drug did not increase the risk of fatal bleeding.
Giving equal statistical weight to clinically equal events provides a clearer focus, the investigators said.
“In this form of analysis, only fatal or irreversible events were included so that benefit and seriously harmful events of similar clinical impact were compared,” they wrote. “This is particularly important when the endpoints and analyses do not include measurements of subjective clinical impact such as utility measurements or preference weights. This approach also uses risk differences rather than relative measurements such as hazard ratios, so the number of events prevented and caused are clearly distinguished.”
ATLAS comprised more than 15,000 patients with ST-segment elevation MI, non-STEMI, or unstable angina. They were randomized to either rivaroxaban 2.5 mg orally twice per day, 5 mg orally twice per day, or to placebo, in addition to standard of care, which included low-dose aspirin. Patients were stratified by the optional use of clopidogrel/ticlopidine.
Dr. Gibson and his team reanalyzed the data by comparing outcomes they judged as having a similar clinical impact: fatal and irreversible cardiovascular death, MI, and ischemic stroke. They also assessed all bleeding, TIMI life-threatening bleeding, and TIMI major bleeding.
In this analysis, the 2.5-mg dose was associated with 115 fewer fatal or irreversible ischemic deaths per 10,000 patient-years of exposure than placebo (548 vs. 663 nonbleeding cardiovascular deaths, MIs, or ischemic strokes).
However, the same dose was also associated with 10 more excessive, fatal, or irreversibly serious harmful events, compared with placebo per 10,000 patient years (33 fatal bleeds or intracranial hemorrhage vs. 23 for placebo).
“Considered together, there would be 105 fatal or irreversible events prevented per 10,000 patient-years of exposure to 2.5 mg of rivaroxaban taken orally twice a day, compared with placebo. An alternate interpretation of the data is that there would be 11 [10 of 115] fatal or irreversible ischemic events prevented for each fatal or irreversible harmful event caused,” Dr. Gibson and his colleagues wrote.
The benefit held when the outcomes were individually reckoned as well. If periprocedural MIs were excluded, rivaroxaban would still prevent 115 fatal or irreversible ischemic events. If only nonbleeding cardiovascular death or ischemic strokes were included, then 90 fatal or irreversible events would be prevented. And if only nonbleeding cardiovascular death was included, then 95 events would be prevented per 10,000 patient-years of exposure in the group taking rivaroxaban 2.5 mg twice daily.
“In all cases, the fatal or irreversible events prevented are 9-11 times the fatal or irreversible seriously harmful events caused,” the investigators said.
ATLAS ACS 2-TIMI 51 was supported by Johnson & Johnson and Bayer Healthcare. Dr. Gibson has received institutional funding, grants, and honoraria from those companies and from Portola Pharmaceuticals.
SOURCE: Gibson CM et al. J Am Coll Cardiol. 2018;72:129-36.
Balancing the risks and benefits of anticoagulation therapy after an acute coronary event leaves physicians on the horns of a dilemma. How do we choose the most effective and the least harmful antiplatelet and/or antithrombotic strategy?
To support decision making, a careful and thoughtful interpretation of the existing evidence is essential, with an explicit focus on the risk-versus-benefit assessment. Even the most well-designed trial can contain ambiguities, the study investigators noted, and ATLAS was one of these.
The reanalysis of ATLAS by Gibson et al. is an attempt to cut through some of these ambiguities. By comparing only serious or fatal outcomes, the investigators aimed to bring clinically meaningful insight into the picture. Such a way of reporting provides readers with an extra piece of information to assist in deciding whether a treatment should be used.
The analysis isn’t perfect. It doesn’t include less-serious bleeding events, which still may contribute to a poor prognosis. And the analysis didn’t take into about ischemia-driven revascularizations.
Although commonly successful, repeat revascularizations are not free from complications, which may include occurrence of large infarctions, stroke, and serious bleeding.
Nevertheless, the study enhances our understanding of how to best employ low-dose rivaroxaban therapy in addition to antiplatelet agents.
Although we are getting closer to therapy optimization, the final word regarding the use of low-dose rivaroxaban and other agents for secondary prevention of cardiovascular diseases has not yet been said. This is primarily because of substantial variation in the magnitude of the risks and benefits across a population. Comprehensive, individualized profiling of the patients with respect to their ischemic and bleeding risks is crucial to further improve acute coronary syndrome–related outcomes.
Eugenia Nikolsy, MD, PhD, and Freek Verheugt, MD, made these comments in an accompanying editorial (J Am Coll Cardiol. 2018;72:137-40). Dr. Nikolsy is director of clinical research in invasive cardiology at Rambam Academic Hospital, Haifa, Israel. Dr. Verheugt is a professor of cardiology at the Heart-Lung Centre at University Medical Centre, Nijmegen, the Netherlands.
Balancing the risks and benefits of anticoagulation therapy after an acute coronary event leaves physicians on the horns of a dilemma. How do we choose the most effective and the least harmful antiplatelet and/or antithrombotic strategy?
To support decision making, a careful and thoughtful interpretation of the existing evidence is essential, with an explicit focus on the risk-versus-benefit assessment. Even the most well-designed trial can contain ambiguities, the study investigators noted, and ATLAS was one of these.
The reanalysis of ATLAS by Gibson et al. is an attempt to cut through some of these ambiguities. By comparing only serious or fatal outcomes, the investigators aimed to bring clinically meaningful insight into the picture. Such a way of reporting provides readers with an extra piece of information to assist in deciding whether a treatment should be used.
The analysis isn’t perfect. It doesn’t include less-serious bleeding events, which still may contribute to a poor prognosis. And the analysis didn’t take into about ischemia-driven revascularizations.
Although commonly successful, repeat revascularizations are not free from complications, which may include occurrence of large infarctions, stroke, and serious bleeding.
Nevertheless, the study enhances our understanding of how to best employ low-dose rivaroxaban therapy in addition to antiplatelet agents.
Although we are getting closer to therapy optimization, the final word regarding the use of low-dose rivaroxaban and other agents for secondary prevention of cardiovascular diseases has not yet been said. This is primarily because of substantial variation in the magnitude of the risks and benefits across a population. Comprehensive, individualized profiling of the patients with respect to their ischemic and bleeding risks is crucial to further improve acute coronary syndrome–related outcomes.
Eugenia Nikolsy, MD, PhD, and Freek Verheugt, MD, made these comments in an accompanying editorial (J Am Coll Cardiol. 2018;72:137-40). Dr. Nikolsy is director of clinical research in invasive cardiology at Rambam Academic Hospital, Haifa, Israel. Dr. Verheugt is a professor of cardiology at the Heart-Lung Centre at University Medical Centre, Nijmegen, the Netherlands.
Balancing the risks and benefits of anticoagulation therapy after an acute coronary event leaves physicians on the horns of a dilemma. How do we choose the most effective and the least harmful antiplatelet and/or antithrombotic strategy?
To support decision making, a careful and thoughtful interpretation of the existing evidence is essential, with an explicit focus on the risk-versus-benefit assessment. Even the most well-designed trial can contain ambiguities, the study investigators noted, and ATLAS was one of these.
The reanalysis of ATLAS by Gibson et al. is an attempt to cut through some of these ambiguities. By comparing only serious or fatal outcomes, the investigators aimed to bring clinically meaningful insight into the picture. Such a way of reporting provides readers with an extra piece of information to assist in deciding whether a treatment should be used.
The analysis isn’t perfect. It doesn’t include less-serious bleeding events, which still may contribute to a poor prognosis. And the analysis didn’t take into about ischemia-driven revascularizations.
Although commonly successful, repeat revascularizations are not free from complications, which may include occurrence of large infarctions, stroke, and serious bleeding.
Nevertheless, the study enhances our understanding of how to best employ low-dose rivaroxaban therapy in addition to antiplatelet agents.
Although we are getting closer to therapy optimization, the final word regarding the use of low-dose rivaroxaban and other agents for secondary prevention of cardiovascular diseases has not yet been said. This is primarily because of substantial variation in the magnitude of the risks and benefits across a population. Comprehensive, individualized profiling of the patients with respect to their ischemic and bleeding risks is crucial to further improve acute coronary syndrome–related outcomes.
Eugenia Nikolsy, MD, PhD, and Freek Verheugt, MD, made these comments in an accompanying editorial (J Am Coll Cardiol. 2018;72:137-40). Dr. Nikolsy is director of clinical research in invasive cardiology at Rambam Academic Hospital, Haifa, Israel. Dr. Verheugt is a professor of cardiology at the Heart-Lung Centre at University Medical Centre, Nijmegen, the Netherlands.
In a new analysis comparing only clinically similar outcomes in patients with acute coronary syndrome, the addition of rivaroxaban to standard antiplatelet therapy resulted in 115 fewer fatal or irreversible ischemic events per 10,000 patient-years than placebo, at the expense of only 10 additional fatal or seriously harmful events.
This new interpretation of the ATLAS ACS 2-TIMI 51 trial (Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome–Thrombolysis in Myocardial Infarction-51) suggests that the factor Xa inhibitor may still carve out a place for itself in ACS therapy, despite Food and Drug Administration rejections for this indication.
Not only did the survival benefit of rivaroxaban appear early in postevent treatment, it continued to protect patients over time, C. Michael Gibson, MD, and colleagues reported in the Journal of the American College of Cardiology.
“Time-to-event analysis demonstrated that the risk of fatal or irreversible harm remained low and constant over time, whereas reduction in fatal or irreversible ischemic events expanded,” wrote Dr. Gibson, professor of medicine at Beth Israel Deaconess Medical Center, Boston, and his coinvestigators. “By 720 days, a net of 142 fatal or irreversible events would have been prevented by 2.5-mg oral doses twice per day of rivaroxaban. Additional time-to-event sensitivity analyses demonstrated similar results, even when TIMI major bleeding was included as a fatal or irreversible event.”
In conducting the new analysis, Dr. Gibson and his team argued that the original interpretation of the results of ATLAS ACS 2-TIMI 51 lumped both fatal and nonfatal events together in composite endpoints, resulting in an inaccurate real-life picture of rivaroxaban’s therapeutic potential. “All types of events [were] weighted equally; for example, reversible nonintracranial hemorrhage, nonfatal bleeds that can be managed with supportive care, are weighted equally with death and disabling stroke. Second, stroke can be either hemorrhagic or ischemic, and the relative contributions of hemorrhagic or ischemic stroke may not be appropriately assigned to risk-versus-benefit categories in many analyses.”
The net result was that, while rivaroxaban did reduce the risk of the composite endpoint (cardiovascular death, MI, or stroke), the 1.7% absolute difference in cardiovascular mortality was almost completely offset by a 1.3% increase in major bleeding. However, most of those bleeds were reversible and nonfatal, associated with a drop in hemoglobin and/or blood transfusion. The drug did not increase the risk of fatal bleeding.
Giving equal statistical weight to clinically equal events provides a clearer focus, the investigators said.
“In this form of analysis, only fatal or irreversible events were included so that benefit and seriously harmful events of similar clinical impact were compared,” they wrote. “This is particularly important when the endpoints and analyses do not include measurements of subjective clinical impact such as utility measurements or preference weights. This approach also uses risk differences rather than relative measurements such as hazard ratios, so the number of events prevented and caused are clearly distinguished.”
ATLAS comprised more than 15,000 patients with ST-segment elevation MI, non-STEMI, or unstable angina. They were randomized to either rivaroxaban 2.5 mg orally twice per day, 5 mg orally twice per day, or to placebo, in addition to standard of care, which included low-dose aspirin. Patients were stratified by the optional use of clopidogrel/ticlopidine.
Dr. Gibson and his team reanalyzed the data by comparing outcomes they judged as having a similar clinical impact: fatal and irreversible cardiovascular death, MI, and ischemic stroke. They also assessed all bleeding, TIMI life-threatening bleeding, and TIMI major bleeding.
In this analysis, the 2.5-mg dose was associated with 115 fewer fatal or irreversible ischemic deaths per 10,000 patient-years of exposure than placebo (548 vs. 663 nonbleeding cardiovascular deaths, MIs, or ischemic strokes).
However, the same dose was also associated with 10 more excessive, fatal, or irreversibly serious harmful events, compared with placebo per 10,000 patient years (33 fatal bleeds or intracranial hemorrhage vs. 23 for placebo).
“Considered together, there would be 105 fatal or irreversible events prevented per 10,000 patient-years of exposure to 2.5 mg of rivaroxaban taken orally twice a day, compared with placebo. An alternate interpretation of the data is that there would be 11 [10 of 115] fatal or irreversible ischemic events prevented for each fatal or irreversible harmful event caused,” Dr. Gibson and his colleagues wrote.
The benefit held when the outcomes were individually reckoned as well. If periprocedural MIs were excluded, rivaroxaban would still prevent 115 fatal or irreversible ischemic events. If only nonbleeding cardiovascular death or ischemic strokes were included, then 90 fatal or irreversible events would be prevented. And if only nonbleeding cardiovascular death was included, then 95 events would be prevented per 10,000 patient-years of exposure in the group taking rivaroxaban 2.5 mg twice daily.
“In all cases, the fatal or irreversible events prevented are 9-11 times the fatal or irreversible seriously harmful events caused,” the investigators said.
ATLAS ACS 2-TIMI 51 was supported by Johnson & Johnson and Bayer Healthcare. Dr. Gibson has received institutional funding, grants, and honoraria from those companies and from Portola Pharmaceuticals.
SOURCE: Gibson CM et al. J Am Coll Cardiol. 2018;72:129-36.
In a new analysis comparing only clinically similar outcomes in patients with acute coronary syndrome, the addition of rivaroxaban to standard antiplatelet therapy resulted in 115 fewer fatal or irreversible ischemic events per 10,000 patient-years than placebo, at the expense of only 10 additional fatal or seriously harmful events.
This new interpretation of the ATLAS ACS 2-TIMI 51 trial (Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome–Thrombolysis in Myocardial Infarction-51) suggests that the factor Xa inhibitor may still carve out a place for itself in ACS therapy, despite Food and Drug Administration rejections for this indication.
Not only did the survival benefit of rivaroxaban appear early in postevent treatment, it continued to protect patients over time, C. Michael Gibson, MD, and colleagues reported in the Journal of the American College of Cardiology.
“Time-to-event analysis demonstrated that the risk of fatal or irreversible harm remained low and constant over time, whereas reduction in fatal or irreversible ischemic events expanded,” wrote Dr. Gibson, professor of medicine at Beth Israel Deaconess Medical Center, Boston, and his coinvestigators. “By 720 days, a net of 142 fatal or irreversible events would have been prevented by 2.5-mg oral doses twice per day of rivaroxaban. Additional time-to-event sensitivity analyses demonstrated similar results, even when TIMI major bleeding was included as a fatal or irreversible event.”
In conducting the new analysis, Dr. Gibson and his team argued that the original interpretation of the results of ATLAS ACS 2-TIMI 51 lumped both fatal and nonfatal events together in composite endpoints, resulting in an inaccurate real-life picture of rivaroxaban’s therapeutic potential. “All types of events [were] weighted equally; for example, reversible nonintracranial hemorrhage, nonfatal bleeds that can be managed with supportive care, are weighted equally with death and disabling stroke. Second, stroke can be either hemorrhagic or ischemic, and the relative contributions of hemorrhagic or ischemic stroke may not be appropriately assigned to risk-versus-benefit categories in many analyses.”
The net result was that, while rivaroxaban did reduce the risk of the composite endpoint (cardiovascular death, MI, or stroke), the 1.7% absolute difference in cardiovascular mortality was almost completely offset by a 1.3% increase in major bleeding. However, most of those bleeds were reversible and nonfatal, associated with a drop in hemoglobin and/or blood transfusion. The drug did not increase the risk of fatal bleeding.
Giving equal statistical weight to clinically equal events provides a clearer focus, the investigators said.
“In this form of analysis, only fatal or irreversible events were included so that benefit and seriously harmful events of similar clinical impact were compared,” they wrote. “This is particularly important when the endpoints and analyses do not include measurements of subjective clinical impact such as utility measurements or preference weights. This approach also uses risk differences rather than relative measurements such as hazard ratios, so the number of events prevented and caused are clearly distinguished.”
ATLAS comprised more than 15,000 patients with ST-segment elevation MI, non-STEMI, or unstable angina. They were randomized to either rivaroxaban 2.5 mg orally twice per day, 5 mg orally twice per day, or to placebo, in addition to standard of care, which included low-dose aspirin. Patients were stratified by the optional use of clopidogrel/ticlopidine.
Dr. Gibson and his team reanalyzed the data by comparing outcomes they judged as having a similar clinical impact: fatal and irreversible cardiovascular death, MI, and ischemic stroke. They also assessed all bleeding, TIMI life-threatening bleeding, and TIMI major bleeding.
In this analysis, the 2.5-mg dose was associated with 115 fewer fatal or irreversible ischemic deaths per 10,000 patient-years of exposure than placebo (548 vs. 663 nonbleeding cardiovascular deaths, MIs, or ischemic strokes).
However, the same dose was also associated with 10 more excessive, fatal, or irreversibly serious harmful events, compared with placebo per 10,000 patient years (33 fatal bleeds or intracranial hemorrhage vs. 23 for placebo).
“Considered together, there would be 105 fatal or irreversible events prevented per 10,000 patient-years of exposure to 2.5 mg of rivaroxaban taken orally twice a day, compared with placebo. An alternate interpretation of the data is that there would be 11 [10 of 115] fatal or irreversible ischemic events prevented for each fatal or irreversible harmful event caused,” Dr. Gibson and his colleagues wrote.
The benefit held when the outcomes were individually reckoned as well. If periprocedural MIs were excluded, rivaroxaban would still prevent 115 fatal or irreversible ischemic events. If only nonbleeding cardiovascular death or ischemic strokes were included, then 90 fatal or irreversible events would be prevented. And if only nonbleeding cardiovascular death was included, then 95 events would be prevented per 10,000 patient-years of exposure in the group taking rivaroxaban 2.5 mg twice daily.
“In all cases, the fatal or irreversible events prevented are 9-11 times the fatal or irreversible seriously harmful events caused,” the investigators said.
ATLAS ACS 2-TIMI 51 was supported by Johnson & Johnson and Bayer Healthcare. Dr. Gibson has received institutional funding, grants, and honoraria from those companies and from Portola Pharmaceuticals.
SOURCE: Gibson CM et al. J Am Coll Cardiol. 2018;72:129-36.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Myeloproliferative neoplasms increase risk for arterial and venous thrombosis
Clinical question: What are the risks for arterial and venous thrombosis in patients with myeloproliferative neoplasms (MPNs)?
Background: Myeloproliferative neoplasms include polycythemia vera, essential thrombocythemia, and primary myelofibrosis. Prior studies have investigated the incidence of arterial and venous thrombosis in patients with myeloproliferative neoplasms, but the actual magnitude of thrombosis risk relative to the general population is unknown.
Study design: Retrospective matched-cohort study.
Setting: Sweden, using the Swedish Inpatient and Cancer Registers.
Synopsis: Using data from 1987 to 2009, 9,429 patients with MPNs were compared with 35,820 control participants to determine hazard ratios for arterial thrombosis, venous thrombosis, and any thrombosis. The highest hazard ratios were seen within 3 months of MPN diagnosis, with hazard ratios of 4.0 (95% confidence interval, 3.6-4.4) for any thrombosis, 3.0 (95% CI, 2.7-3.4) for arterial thrombosis, and 9.7 (95% CI, 7.8-12.0) for venous thrombosis. Risk decreased but remained significantly elevated through follow-up out to 20 years after diagnosis. This decrease was thought to be caused by effective thromboprophylactic and cytoreductive treatment of the MPN.
This study demonstrates significantly elevated risk for thrombosis in patients with MPNs, highest shortly after diagnosis. It suggests the importance of timely diagnosis and treatment of MPNs to decrease early thrombosis risk.
Bottom line: Patients with MPNs have increased rates of arterial and venous thrombosis, with the highest rates within 3 months of diagnosis.
Citation: Hultcrantz M et al. Risk for arterial and venous thrombosis in patients with myeloproliferative neoplasms. Ann Intern Med. 2018 Mar 6;168(5):317-25.
Dr. Komsoukaniants is a hospitalist at UC San Diego Health and an assistant clinical professor at the University of California, San Diego.
Clinical question: What are the risks for arterial and venous thrombosis in patients with myeloproliferative neoplasms (MPNs)?
Background: Myeloproliferative neoplasms include polycythemia vera, essential thrombocythemia, and primary myelofibrosis. Prior studies have investigated the incidence of arterial and venous thrombosis in patients with myeloproliferative neoplasms, but the actual magnitude of thrombosis risk relative to the general population is unknown.
Study design: Retrospective matched-cohort study.
Setting: Sweden, using the Swedish Inpatient and Cancer Registers.
Synopsis: Using data from 1987 to 2009, 9,429 patients with MPNs were compared with 35,820 control participants to determine hazard ratios for arterial thrombosis, venous thrombosis, and any thrombosis. The highest hazard ratios were seen within 3 months of MPN diagnosis, with hazard ratios of 4.0 (95% confidence interval, 3.6-4.4) for any thrombosis, 3.0 (95% CI, 2.7-3.4) for arterial thrombosis, and 9.7 (95% CI, 7.8-12.0) for venous thrombosis. Risk decreased but remained significantly elevated through follow-up out to 20 years after diagnosis. This decrease was thought to be caused by effective thromboprophylactic and cytoreductive treatment of the MPN.
This study demonstrates significantly elevated risk for thrombosis in patients with MPNs, highest shortly after diagnosis. It suggests the importance of timely diagnosis and treatment of MPNs to decrease early thrombosis risk.
Bottom line: Patients with MPNs have increased rates of arterial and venous thrombosis, with the highest rates within 3 months of diagnosis.
Citation: Hultcrantz M et al. Risk for arterial and venous thrombosis in patients with myeloproliferative neoplasms. Ann Intern Med. 2018 Mar 6;168(5):317-25.
Dr. Komsoukaniants is a hospitalist at UC San Diego Health and an assistant clinical professor at the University of California, San Diego.
Clinical question: What are the risks for arterial and venous thrombosis in patients with myeloproliferative neoplasms (MPNs)?
Background: Myeloproliferative neoplasms include polycythemia vera, essential thrombocythemia, and primary myelofibrosis. Prior studies have investigated the incidence of arterial and venous thrombosis in patients with myeloproliferative neoplasms, but the actual magnitude of thrombosis risk relative to the general population is unknown.
Study design: Retrospective matched-cohort study.
Setting: Sweden, using the Swedish Inpatient and Cancer Registers.
Synopsis: Using data from 1987 to 2009, 9,429 patients with MPNs were compared with 35,820 control participants to determine hazard ratios for arterial thrombosis, venous thrombosis, and any thrombosis. The highest hazard ratios were seen within 3 months of MPN diagnosis, with hazard ratios of 4.0 (95% confidence interval, 3.6-4.4) for any thrombosis, 3.0 (95% CI, 2.7-3.4) for arterial thrombosis, and 9.7 (95% CI, 7.8-12.0) for venous thrombosis. Risk decreased but remained significantly elevated through follow-up out to 20 years after diagnosis. This decrease was thought to be caused by effective thromboprophylactic and cytoreductive treatment of the MPN.
This study demonstrates significantly elevated risk for thrombosis in patients with MPNs, highest shortly after diagnosis. It suggests the importance of timely diagnosis and treatment of MPNs to decrease early thrombosis risk.
Bottom line: Patients with MPNs have increased rates of arterial and venous thrombosis, with the highest rates within 3 months of diagnosis.
Citation: Hultcrantz M et al. Risk for arterial and venous thrombosis in patients with myeloproliferative neoplasms. Ann Intern Med. 2018 Mar 6;168(5):317-25.
Dr. Komsoukaniants is a hospitalist at UC San Diego Health and an assistant clinical professor at the University of California, San Diego.
Fentanyl fears drive many opioid users, interviews suggest
SAN DIEGO – Many opioid users on the street are embracing homegrown “risk reduction” techniques to make sure they avoid the danger of fentanyl-laced heroin, while others seek out batches that have caused fatal overdoses because they figure these supplies must be extra-strong and desirable. “Anytime I hear that somebody OD’d off something,” said one user, “... I was like, ‘Oh, that stuff must be good. Where can I get it?’ ”
So say opioid users who are opening up to researchers about the deadly new American drug landscape.
There’s one common thread, said Daniel Ciccarone, MD, MPH, who presented findings from his team’s interviews at the annual meeting of the College on Problems of Drug Dependence. Attitudes about fentanyl are shifting, but not enough to turn the drug – which often is available instead of heroin – into a major crowd-pleaser.
“When we first went into the field, there was a strong negative opinion about fentanyl. It was not wanted; it was imposed,” said Dr. Ciccarone of the University of California at San Francisco. “While there is some shift now, in which some do want or like the fentanyl, it is not strong enough to have shifted the culture. i.e., .”
But fentanyl still is rampant, often making its way to users who do not want and might be actively trying to avoid it. According to the National Institute on Drug Abuse, or NIDA, fentanyl and fentanyl analogs were linked to more than 20,000 of the more than 64,000 fatal U.S. drug overdoses in 2016.
“The risks are greater with fentanyl and not just because it is more potent than heroin,” Dr. Ciccarone said. In addition, “the form (fentanyl vs. fentanyl analogs) and purity shifts on a daily basis, and it’s mixed with heroin in varying amounts. It is these vicissitudes in fentanyl/heroin types and purities that make the street blends dangerous for overdose.”
Dr. Ciccarone and his colleagues interviewed opioid users in Baltimore; Charleston, W. Va.; Lawrence and Lowell, Mass.; and Chicago. Information about some of the interviews was published previously (Intl J Drug Policy. 2017 Aug;46;146-55).
In another study whose results were released at the CPDD conference, researchers from Dartmouth College, Hanover, N.H., interviewed 76 opioid users (91% were white, average age was 34, half were female, and almost all had a history of substance abuse treatment).
Among the other findings of the studies:
- Some opioid users are shocked by the adulteration of heroin by fentanyl, and even dealers are surprised: “When we cut the dope, we don’t use fentanyl. The problem was that we were buying the dope already dirty with that, and we didn’t know it,” said a 42-year-old man from Lawrence, Mass.
- In the New Hampshire interviews, 84% of participants said fentanyl is the leading cause of overdose in the state. “Due to the fentanyl and the heroin, that’s how everyone that I know ended up passing away recently,” said one user.
- Also in New Hampshire, 84% of users interviewed said they’d used fentanyl before, accidentally in some cases. Also, study coauthor Andrea L. Meier said in an interview, “67% of users reported having a prescription for opioids in their lifetime due to some kind of illness or injury. Some were short-term prescriptions (injuries, C-sections, tooth extractions); others were prescribed long-term due to chronic pain or illness.”
Some say pursue fentanyl
In the New Hampshire interviews, 25% of the 84% who’d used fentanyl said they actively seek it out, with one expressing a preference for the “real dope” (heroin laced with fentanyl) versus the “maintenance dope” of heroin alone. Ms. Meier explained what that means: “We’ve heard that once you use fentanyl or fentanyl-laced heroin, heroin doesn’t adequately manage your withdrawal symptoms or give enough of a high. So they will use heroin to get by, but they really want fentanyl or fentanyl-laced heroin.”
She added: “We’ve learned that users are seeking fentanyl due to its potency (a stronger, better high with quicker onset than heroin), cost (cheaper than heroin, so more “bang for your buck”), and once they use fentanyl or fentanyl-laced heroin, heroin doesn’t have the same effect/high.”
As for the risk of overdose, “people want the best high they can get at the cheapest cost,” she said.” They presume it will be potent enough to produce an exceptional high but [they won’t] die from an overdose due to being sufficiently tolerant to handle it. Also, some just felt hopeless and weren’t afraid of an OD.”
Comments from users about fentanyl
- “The high is wonderful. It’s splendidly wonderful. It’s magnified heroin feeling by a great number,” said a 45-year-old man from Baltimore.
- “You spend the same amount of money or even less for it, and you’re getting 3 times as high as you did on heroin,” said one user in New Hampshire. “Who wouldn’t want that?”
Risk-reduction techniques embraced
- In Baltimore, a 39-year-old woman said: “I have a lot of associates that are letting me know, ‘Don’t go to that place because they selling fentanyl.’ ”
- Some users test their heroin. “Like when I get stuff I don’t know what it is, I do a little bit before I do something that I feel,” a Lawrence, Mass., female user in her 20s said. “Like, I want to kind of scale out how much I want to do. Because I don’t want to die. But these people are just doing a gram shot and just ... my friend just died 2 days ago.”
Dr. Ciccarone said some of the biggest proponents of harm-reduction among users were African Americans aged 60 and older who had used for many decades.
“They fear fentanyl and are using old-school harm reduction strategies for staying safer: “tooting” (snorting) and tester shots (small injections to test the quality),” he said. “These may seem like minor strategies, but if enough of the population applies them to their daily drug use, many deaths would be averted. In this crisis, we need all the wisdom we can get, so we should listen to these elders.”
Dr. Ciccarone’s research was funded by the National Institutes of Health and NIDA. Dr. Ciccarone reported no relevant disclosures. The Dartmouth College study was funded by NIDA, and those authors reported no relevant disclosures.
SAN DIEGO – Many opioid users on the street are embracing homegrown “risk reduction” techniques to make sure they avoid the danger of fentanyl-laced heroin, while others seek out batches that have caused fatal overdoses because they figure these supplies must be extra-strong and desirable. “Anytime I hear that somebody OD’d off something,” said one user, “... I was like, ‘Oh, that stuff must be good. Where can I get it?’ ”
So say opioid users who are opening up to researchers about the deadly new American drug landscape.
There’s one common thread, said Daniel Ciccarone, MD, MPH, who presented findings from his team’s interviews at the annual meeting of the College on Problems of Drug Dependence. Attitudes about fentanyl are shifting, but not enough to turn the drug – which often is available instead of heroin – into a major crowd-pleaser.
“When we first went into the field, there was a strong negative opinion about fentanyl. It was not wanted; it was imposed,” said Dr. Ciccarone of the University of California at San Francisco. “While there is some shift now, in which some do want or like the fentanyl, it is not strong enough to have shifted the culture. i.e., .”
But fentanyl still is rampant, often making its way to users who do not want and might be actively trying to avoid it. According to the National Institute on Drug Abuse, or NIDA, fentanyl and fentanyl analogs were linked to more than 20,000 of the more than 64,000 fatal U.S. drug overdoses in 2016.
“The risks are greater with fentanyl and not just because it is more potent than heroin,” Dr. Ciccarone said. In addition, “the form (fentanyl vs. fentanyl analogs) and purity shifts on a daily basis, and it’s mixed with heroin in varying amounts. It is these vicissitudes in fentanyl/heroin types and purities that make the street blends dangerous for overdose.”
Dr. Ciccarone and his colleagues interviewed opioid users in Baltimore; Charleston, W. Va.; Lawrence and Lowell, Mass.; and Chicago. Information about some of the interviews was published previously (Intl J Drug Policy. 2017 Aug;46;146-55).
In another study whose results were released at the CPDD conference, researchers from Dartmouth College, Hanover, N.H., interviewed 76 opioid users (91% were white, average age was 34, half were female, and almost all had a history of substance abuse treatment).
Among the other findings of the studies:
- Some opioid users are shocked by the adulteration of heroin by fentanyl, and even dealers are surprised: “When we cut the dope, we don’t use fentanyl. The problem was that we were buying the dope already dirty with that, and we didn’t know it,” said a 42-year-old man from Lawrence, Mass.
- In the New Hampshire interviews, 84% of participants said fentanyl is the leading cause of overdose in the state. “Due to the fentanyl and the heroin, that’s how everyone that I know ended up passing away recently,” said one user.
- Also in New Hampshire, 84% of users interviewed said they’d used fentanyl before, accidentally in some cases. Also, study coauthor Andrea L. Meier said in an interview, “67% of users reported having a prescription for opioids in their lifetime due to some kind of illness or injury. Some were short-term prescriptions (injuries, C-sections, tooth extractions); others were prescribed long-term due to chronic pain or illness.”
Some say pursue fentanyl
In the New Hampshire interviews, 25% of the 84% who’d used fentanyl said they actively seek it out, with one expressing a preference for the “real dope” (heroin laced with fentanyl) versus the “maintenance dope” of heroin alone. Ms. Meier explained what that means: “We’ve heard that once you use fentanyl or fentanyl-laced heroin, heroin doesn’t adequately manage your withdrawal symptoms or give enough of a high. So they will use heroin to get by, but they really want fentanyl or fentanyl-laced heroin.”
She added: “We’ve learned that users are seeking fentanyl due to its potency (a stronger, better high with quicker onset than heroin), cost (cheaper than heroin, so more “bang for your buck”), and once they use fentanyl or fentanyl-laced heroin, heroin doesn’t have the same effect/high.”
As for the risk of overdose, “people want the best high they can get at the cheapest cost,” she said.” They presume it will be potent enough to produce an exceptional high but [they won’t] die from an overdose due to being sufficiently tolerant to handle it. Also, some just felt hopeless and weren’t afraid of an OD.”
Comments from users about fentanyl
- “The high is wonderful. It’s splendidly wonderful. It’s magnified heroin feeling by a great number,” said a 45-year-old man from Baltimore.
- “You spend the same amount of money or even less for it, and you’re getting 3 times as high as you did on heroin,” said one user in New Hampshire. “Who wouldn’t want that?”
Risk-reduction techniques embraced
- In Baltimore, a 39-year-old woman said: “I have a lot of associates that are letting me know, ‘Don’t go to that place because they selling fentanyl.’ ”
- Some users test their heroin. “Like when I get stuff I don’t know what it is, I do a little bit before I do something that I feel,” a Lawrence, Mass., female user in her 20s said. “Like, I want to kind of scale out how much I want to do. Because I don’t want to die. But these people are just doing a gram shot and just ... my friend just died 2 days ago.”
Dr. Ciccarone said some of the biggest proponents of harm-reduction among users were African Americans aged 60 and older who had used for many decades.
“They fear fentanyl and are using old-school harm reduction strategies for staying safer: “tooting” (snorting) and tester shots (small injections to test the quality),” he said. “These may seem like minor strategies, but if enough of the population applies them to their daily drug use, many deaths would be averted. In this crisis, we need all the wisdom we can get, so we should listen to these elders.”
Dr. Ciccarone’s research was funded by the National Institutes of Health and NIDA. Dr. Ciccarone reported no relevant disclosures. The Dartmouth College study was funded by NIDA, and those authors reported no relevant disclosures.
SAN DIEGO – Many opioid users on the street are embracing homegrown “risk reduction” techniques to make sure they avoid the danger of fentanyl-laced heroin, while others seek out batches that have caused fatal overdoses because they figure these supplies must be extra-strong and desirable. “Anytime I hear that somebody OD’d off something,” said one user, “... I was like, ‘Oh, that stuff must be good. Where can I get it?’ ”
So say opioid users who are opening up to researchers about the deadly new American drug landscape.
There’s one common thread, said Daniel Ciccarone, MD, MPH, who presented findings from his team’s interviews at the annual meeting of the College on Problems of Drug Dependence. Attitudes about fentanyl are shifting, but not enough to turn the drug – which often is available instead of heroin – into a major crowd-pleaser.
“When we first went into the field, there was a strong negative opinion about fentanyl. It was not wanted; it was imposed,” said Dr. Ciccarone of the University of California at San Francisco. “While there is some shift now, in which some do want or like the fentanyl, it is not strong enough to have shifted the culture. i.e., .”
But fentanyl still is rampant, often making its way to users who do not want and might be actively trying to avoid it. According to the National Institute on Drug Abuse, or NIDA, fentanyl and fentanyl analogs were linked to more than 20,000 of the more than 64,000 fatal U.S. drug overdoses in 2016.
“The risks are greater with fentanyl and not just because it is more potent than heroin,” Dr. Ciccarone said. In addition, “the form (fentanyl vs. fentanyl analogs) and purity shifts on a daily basis, and it’s mixed with heroin in varying amounts. It is these vicissitudes in fentanyl/heroin types and purities that make the street blends dangerous for overdose.”
Dr. Ciccarone and his colleagues interviewed opioid users in Baltimore; Charleston, W. Va.; Lawrence and Lowell, Mass.; and Chicago. Information about some of the interviews was published previously (Intl J Drug Policy. 2017 Aug;46;146-55).
In another study whose results were released at the CPDD conference, researchers from Dartmouth College, Hanover, N.H., interviewed 76 opioid users (91% were white, average age was 34, half were female, and almost all had a history of substance abuse treatment).
Among the other findings of the studies:
- Some opioid users are shocked by the adulteration of heroin by fentanyl, and even dealers are surprised: “When we cut the dope, we don’t use fentanyl. The problem was that we were buying the dope already dirty with that, and we didn’t know it,” said a 42-year-old man from Lawrence, Mass.
- In the New Hampshire interviews, 84% of participants said fentanyl is the leading cause of overdose in the state. “Due to the fentanyl and the heroin, that’s how everyone that I know ended up passing away recently,” said one user.
- Also in New Hampshire, 84% of users interviewed said they’d used fentanyl before, accidentally in some cases. Also, study coauthor Andrea L. Meier said in an interview, “67% of users reported having a prescription for opioids in their lifetime due to some kind of illness or injury. Some were short-term prescriptions (injuries, C-sections, tooth extractions); others were prescribed long-term due to chronic pain or illness.”
Some say pursue fentanyl
In the New Hampshire interviews, 25% of the 84% who’d used fentanyl said they actively seek it out, with one expressing a preference for the “real dope” (heroin laced with fentanyl) versus the “maintenance dope” of heroin alone. Ms. Meier explained what that means: “We’ve heard that once you use fentanyl or fentanyl-laced heroin, heroin doesn’t adequately manage your withdrawal symptoms or give enough of a high. So they will use heroin to get by, but they really want fentanyl or fentanyl-laced heroin.”
She added: “We’ve learned that users are seeking fentanyl due to its potency (a stronger, better high with quicker onset than heroin), cost (cheaper than heroin, so more “bang for your buck”), and once they use fentanyl or fentanyl-laced heroin, heroin doesn’t have the same effect/high.”
As for the risk of overdose, “people want the best high they can get at the cheapest cost,” she said.” They presume it will be potent enough to produce an exceptional high but [they won’t] die from an overdose due to being sufficiently tolerant to handle it. Also, some just felt hopeless and weren’t afraid of an OD.”
Comments from users about fentanyl
- “The high is wonderful. It’s splendidly wonderful. It’s magnified heroin feeling by a great number,” said a 45-year-old man from Baltimore.
- “You spend the same amount of money or even less for it, and you’re getting 3 times as high as you did on heroin,” said one user in New Hampshire. “Who wouldn’t want that?”
Risk-reduction techniques embraced
- In Baltimore, a 39-year-old woman said: “I have a lot of associates that are letting me know, ‘Don’t go to that place because they selling fentanyl.’ ”
- Some users test their heroin. “Like when I get stuff I don’t know what it is, I do a little bit before I do something that I feel,” a Lawrence, Mass., female user in her 20s said. “Like, I want to kind of scale out how much I want to do. Because I don’t want to die. But these people are just doing a gram shot and just ... my friend just died 2 days ago.”
Dr. Ciccarone said some of the biggest proponents of harm-reduction among users were African Americans aged 60 and older who had used for many decades.
“They fear fentanyl and are using old-school harm reduction strategies for staying safer: “tooting” (snorting) and tester shots (small injections to test the quality),” he said. “These may seem like minor strategies, but if enough of the population applies them to their daily drug use, many deaths would be averted. In this crisis, we need all the wisdom we can get, so we should listen to these elders.”
Dr. Ciccarone’s research was funded by the National Institutes of Health and NIDA. Dr. Ciccarone reported no relevant disclosures. The Dartmouth College study was funded by NIDA, and those authors reported no relevant disclosures.
REPORTING FROM CPDD 2018
Daratumumab plus carfilzomib/dexamethasone effective in lenalidomide-refractory myeloma
CHICAGO – Daratumumab in combination with carfilzomib and dexamethasone (D-Kd) was a safe and effective regimen for patients with relapsed multiple myeloma, even in those with disease refractory to lenalidomide, in an open label, phase 1b study.
The regimen was well tolerated, with low rates of neutropenia both overall and in the lenalidomide-refractory subset of patients, according to this subgroup analysis of MMY1001.
The D-Kd regimen produced deep and durable responses, with an “encouraging” median progression-free survival of approximately 14 months for lenalidomide-refractory patients, according to investigator Ajai Chari, MD, of the Tisch Cancer Institute at the Icahn School of Medicine at Mount Sinai, New York.
Patients with lenalidomide-refractory multiple myeloma have often been excluded from recent phase 3 studies in the relapsed/refractory setting, Dr. Chari noted in a presentation of the data at the 2018 annual meeting of the American Society of Clinical Oncology. “With the increasing adoption of lenalidomide maintenance, based on overall survival benefit, clearly there’s a need for more data on lenalidomide-refractory, relapsed refractory myeloma.”
The analysis by Dr. Chari and his colleagues was based on 85 previously treated, carfilzomib-naive patients, of whom 51 were lenalidomide refractory, in the MMY1001 study.
Patients received carfilzomib on days 1, 8, and 15 of 28-day cycles and dexamethasone 40 mg once weekly. They received daratumumab weekly for the first 2 cycles, every 2 weeks for the next 4 cycles, and every 4 weeks thereafter. Ten patients received a standard single first dose of daratumumab, while 75 received a split first dose.
Some grade 3/4 hematologic toxicities were observed, and the rate of grade 3/4 neutropenia was 21% overall. The most common nonhematologic toxicities reaching grade 3/4 included asthenia and hypertension at 12% and 14%, respectively. A similar safety profile was seen in the lenalidomide-refractory subset, according to Dr. Chari.
Grade 3 cardiac treatment-emergent adverse events were seen in seven patients, and resolved in five of them. One patient had a grade 4 event that resolved. Cardiac adverse events improved in grade upon interruption of carfilzomib, Dr. Chari said.
With a median follow-up of 12 months, the response rate was 84% overall, which was comparable to the 79% rate seen in the lenalidomide-refractory patients and 90% rate seen in the patients who were exposed to lenalidomide but not refractory, according to Dr. Chari.
Median progression-free survival had not been reached for the overall patient cohort but was 14.1 months in the lenalidomide-refractory cohort, Dr. Chari said. The 12-month rates of progression-free survival were 71% overall, 62% for lenalidomide-refractory patients, and 87% for lenalidomide-exposed patients.
Median overall survival was not reached overall, not reached in lenalidomide-exposed patients, and was 21.1 months in the lenalidomide-refractory group, he added.
Infusion-related reactions occurred in 5 out of 10 patients who received a standard single first infusion of daratumumab. In patients who received a split first infusion, reactions were seen in 27 (36%) on day 1 and in 3 (4%) on day 2. “Importantly, I think this study highlights the ability to do split dosing, particularly in community practices, and to improve patient convenience,” Dr. Chari said.
Dr. Chari reported disclosures related to Janssen Oncology, the maker of daratumumab, and Amgen, the maker of carfilzomib, as well as Acetylon Pharmaceuticals, Adaptive Biotechnologies, Array Biopharma, Bayer, Biotest, Bristol-Myers Squibb, Celgene, Millennium, Novartis, Onyx, Pharmacyclics, Seattle Genetics, and Takeda Pharmaceutical.
SOURCE: Chari A et al. ASCO 2018, Abstract 8002.
CHICAGO – Daratumumab in combination with carfilzomib and dexamethasone (D-Kd) was a safe and effective regimen for patients with relapsed multiple myeloma, even in those with disease refractory to lenalidomide, in an open label, phase 1b study.
The regimen was well tolerated, with low rates of neutropenia both overall and in the lenalidomide-refractory subset of patients, according to this subgroup analysis of MMY1001.
The D-Kd regimen produced deep and durable responses, with an “encouraging” median progression-free survival of approximately 14 months for lenalidomide-refractory patients, according to investigator Ajai Chari, MD, of the Tisch Cancer Institute at the Icahn School of Medicine at Mount Sinai, New York.
Patients with lenalidomide-refractory multiple myeloma have often been excluded from recent phase 3 studies in the relapsed/refractory setting, Dr. Chari noted in a presentation of the data at the 2018 annual meeting of the American Society of Clinical Oncology. “With the increasing adoption of lenalidomide maintenance, based on overall survival benefit, clearly there’s a need for more data on lenalidomide-refractory, relapsed refractory myeloma.”
The analysis by Dr. Chari and his colleagues was based on 85 previously treated, carfilzomib-naive patients, of whom 51 were lenalidomide refractory, in the MMY1001 study.
Patients received carfilzomib on days 1, 8, and 15 of 28-day cycles and dexamethasone 40 mg once weekly. They received daratumumab weekly for the first 2 cycles, every 2 weeks for the next 4 cycles, and every 4 weeks thereafter. Ten patients received a standard single first dose of daratumumab, while 75 received a split first dose.
Some grade 3/4 hematologic toxicities were observed, and the rate of grade 3/4 neutropenia was 21% overall. The most common nonhematologic toxicities reaching grade 3/4 included asthenia and hypertension at 12% and 14%, respectively. A similar safety profile was seen in the lenalidomide-refractory subset, according to Dr. Chari.
Grade 3 cardiac treatment-emergent adverse events were seen in seven patients, and resolved in five of them. One patient had a grade 4 event that resolved. Cardiac adverse events improved in grade upon interruption of carfilzomib, Dr. Chari said.
With a median follow-up of 12 months, the response rate was 84% overall, which was comparable to the 79% rate seen in the lenalidomide-refractory patients and 90% rate seen in the patients who were exposed to lenalidomide but not refractory, according to Dr. Chari.
Median progression-free survival had not been reached for the overall patient cohort but was 14.1 months in the lenalidomide-refractory cohort, Dr. Chari said. The 12-month rates of progression-free survival were 71% overall, 62% for lenalidomide-refractory patients, and 87% for lenalidomide-exposed patients.
Median overall survival was not reached overall, not reached in lenalidomide-exposed patients, and was 21.1 months in the lenalidomide-refractory group, he added.
Infusion-related reactions occurred in 5 out of 10 patients who received a standard single first infusion of daratumumab. In patients who received a split first infusion, reactions were seen in 27 (36%) on day 1 and in 3 (4%) on day 2. “Importantly, I think this study highlights the ability to do split dosing, particularly in community practices, and to improve patient convenience,” Dr. Chari said.
Dr. Chari reported disclosures related to Janssen Oncology, the maker of daratumumab, and Amgen, the maker of carfilzomib, as well as Acetylon Pharmaceuticals, Adaptive Biotechnologies, Array Biopharma, Bayer, Biotest, Bristol-Myers Squibb, Celgene, Millennium, Novartis, Onyx, Pharmacyclics, Seattle Genetics, and Takeda Pharmaceutical.
SOURCE: Chari A et al. ASCO 2018, Abstract 8002.
CHICAGO – Daratumumab in combination with carfilzomib and dexamethasone (D-Kd) was a safe and effective regimen for patients with relapsed multiple myeloma, even in those with disease refractory to lenalidomide, in an open label, phase 1b study.
The regimen was well tolerated, with low rates of neutropenia both overall and in the lenalidomide-refractory subset of patients, according to this subgroup analysis of MMY1001.
The D-Kd regimen produced deep and durable responses, with an “encouraging” median progression-free survival of approximately 14 months for lenalidomide-refractory patients, according to investigator Ajai Chari, MD, of the Tisch Cancer Institute at the Icahn School of Medicine at Mount Sinai, New York.
Patients with lenalidomide-refractory multiple myeloma have often been excluded from recent phase 3 studies in the relapsed/refractory setting, Dr. Chari noted in a presentation of the data at the 2018 annual meeting of the American Society of Clinical Oncology. “With the increasing adoption of lenalidomide maintenance, based on overall survival benefit, clearly there’s a need for more data on lenalidomide-refractory, relapsed refractory myeloma.”
The analysis by Dr. Chari and his colleagues was based on 85 previously treated, carfilzomib-naive patients, of whom 51 were lenalidomide refractory, in the MMY1001 study.
Patients received carfilzomib on days 1, 8, and 15 of 28-day cycles and dexamethasone 40 mg once weekly. They received daratumumab weekly for the first 2 cycles, every 2 weeks for the next 4 cycles, and every 4 weeks thereafter. Ten patients received a standard single first dose of daratumumab, while 75 received a split first dose.
Some grade 3/4 hematologic toxicities were observed, and the rate of grade 3/4 neutropenia was 21% overall. The most common nonhematologic toxicities reaching grade 3/4 included asthenia and hypertension at 12% and 14%, respectively. A similar safety profile was seen in the lenalidomide-refractory subset, according to Dr. Chari.
Grade 3 cardiac treatment-emergent adverse events were seen in seven patients, and resolved in five of them. One patient had a grade 4 event that resolved. Cardiac adverse events improved in grade upon interruption of carfilzomib, Dr. Chari said.
With a median follow-up of 12 months, the response rate was 84% overall, which was comparable to the 79% rate seen in the lenalidomide-refractory patients and 90% rate seen in the patients who were exposed to lenalidomide but not refractory, according to Dr. Chari.
Median progression-free survival had not been reached for the overall patient cohort but was 14.1 months in the lenalidomide-refractory cohort, Dr. Chari said. The 12-month rates of progression-free survival were 71% overall, 62% for lenalidomide-refractory patients, and 87% for lenalidomide-exposed patients.
Median overall survival was not reached overall, not reached in lenalidomide-exposed patients, and was 21.1 months in the lenalidomide-refractory group, he added.
Infusion-related reactions occurred in 5 out of 10 patients who received a standard single first infusion of daratumumab. In patients who received a split first infusion, reactions were seen in 27 (36%) on day 1 and in 3 (4%) on day 2. “Importantly, I think this study highlights the ability to do split dosing, particularly in community practices, and to improve patient convenience,” Dr. Chari said.
Dr. Chari reported disclosures related to Janssen Oncology, the maker of daratumumab, and Amgen, the maker of carfilzomib, as well as Acetylon Pharmaceuticals, Adaptive Biotechnologies, Array Biopharma, Bayer, Biotest, Bristol-Myers Squibb, Celgene, Millennium, Novartis, Onyx, Pharmacyclics, Seattle Genetics, and Takeda Pharmaceutical.
SOURCE: Chari A et al. ASCO 2018, Abstract 8002.
REPORTING FROM ASCO 2018
Key clinical point: Daratumumab, carfilzomib, and dexamethasone (D-Kd) was safe and effective in patients with relapsed multiple myeloma, regardless of prior lenalidomide exposure.
Major finding: The 12-month rate of progression-free survival was 71% overall, 62% for lenalidomide-refractory patients, and 87% for lenalidomide-exposed patients.
Study details: Subgroup analysis of 85 patients in MMY1001, an open label, phase 1b study.
Disclosures: Dr. Chari reported disclosures related to Janssen Oncology, the maker of daratumumab, and Amgen, the maker of carfilzomib, as well as Acetylon Pharmaceuticals, Adaptive Biotechnologies, Array Biopharma, Bayer, Biotest, Bristol-Myers Squibb, Celgene, Millennium, Novartis, Onyx, Pharmacyclics, Seattle Genetics, and Takeda Pharmaceutical.
Source: Chari A et al. ASCO 2018, Abstract 8002.
Transgender men need counseling on contraceptive and reproductive choices
Researchers have highlighted the need for contraception counseling for transgender men, after a study found around half of transgender men had not been asked by their health care providers about their fertility desires.
Writing in Contraception, researchers reported the results of an anonymous, online survey of 197 female-to-male transgender men, 86% of whom were taking masculinizing hormones.
Overall, 17% of respondents had experienced a pregnancy, with 60 pregnancies reported in total. While participants who had never taken hormones had a nearly 200% higher incidence of pregnancy, compared with those who had taken testosterone, one pregnancy occurred while the subject was taking testosterone, and five of seven reported abortions were in participants who had used testosterone prior to conception.
A total of 30 participants (16.4%) believed testosterone was a contraceptive method, and 10 said that a health care provider had advised them to use testosterone for contraception. However, nearly half of all participants did report using condoms for contraception, making it the most common method. IUDs were the second most common method of contraception currently used.
Nearly one-third of participants said they had used some type of contraceptive pill at some point, 17 said they had tried more than one type of pill, and 36 had used combination pills. However, of those who had used combination pills, nearly half stopped using them because of side effects or because of concern about extra feminine hormones.
The authors noted that most study participants expressed a desire to become a parent. Around one-quarter wanted to bear a child while the majority said they would consider adoption.
One-quarter of respondents had fears about not achieving a desired pregnancy, and for some, those fears began after initiating hormone treatments. Just over half the respondents said their health care provider had not asked about their fertility desires.
“Transgender men have unintended pregnancy as well as future fertility desires, and yet, there is a paucity of reproductive health care best practices research for this unique population,” wrote Alexis Light, MD, MPH, of MedStar Washington Hospital Center, and her coauthors. “This survey confirms earlier studies: Transgender men do become pregnant, both intentionally and unintentionally, and some transgender men engage in behaviors that can lead to unintended pregnancy.”
The study authors called for doctors to be more equipped and prepared to counsel transgender men on contraception and discuss other reproductive health concerns.
The study was supported by MedStar Washington Hospital Center. No conflicts of interest were declared.
SOURCE: Light A et al. Contraception. 2018 Jun 23. doi: 10.1016/j.contraception.2018.06.006.
Researchers have highlighted the need for contraception counseling for transgender men, after a study found around half of transgender men had not been asked by their health care providers about their fertility desires.
Writing in Contraception, researchers reported the results of an anonymous, online survey of 197 female-to-male transgender men, 86% of whom were taking masculinizing hormones.
Overall, 17% of respondents had experienced a pregnancy, with 60 pregnancies reported in total. While participants who had never taken hormones had a nearly 200% higher incidence of pregnancy, compared with those who had taken testosterone, one pregnancy occurred while the subject was taking testosterone, and five of seven reported abortions were in participants who had used testosterone prior to conception.
A total of 30 participants (16.4%) believed testosterone was a contraceptive method, and 10 said that a health care provider had advised them to use testosterone for contraception. However, nearly half of all participants did report using condoms for contraception, making it the most common method. IUDs were the second most common method of contraception currently used.
Nearly one-third of participants said they had used some type of contraceptive pill at some point, 17 said they had tried more than one type of pill, and 36 had used combination pills. However, of those who had used combination pills, nearly half stopped using them because of side effects or because of concern about extra feminine hormones.
The authors noted that most study participants expressed a desire to become a parent. Around one-quarter wanted to bear a child while the majority said they would consider adoption.
One-quarter of respondents had fears about not achieving a desired pregnancy, and for some, those fears began after initiating hormone treatments. Just over half the respondents said their health care provider had not asked about their fertility desires.
“Transgender men have unintended pregnancy as well as future fertility desires, and yet, there is a paucity of reproductive health care best practices research for this unique population,” wrote Alexis Light, MD, MPH, of MedStar Washington Hospital Center, and her coauthors. “This survey confirms earlier studies: Transgender men do become pregnant, both intentionally and unintentionally, and some transgender men engage in behaviors that can lead to unintended pregnancy.”
The study authors called for doctors to be more equipped and prepared to counsel transgender men on contraception and discuss other reproductive health concerns.
The study was supported by MedStar Washington Hospital Center. No conflicts of interest were declared.
SOURCE: Light A et al. Contraception. 2018 Jun 23. doi: 10.1016/j.contraception.2018.06.006.
Researchers have highlighted the need for contraception counseling for transgender men, after a study found around half of transgender men had not been asked by their health care providers about their fertility desires.
Writing in Contraception, researchers reported the results of an anonymous, online survey of 197 female-to-male transgender men, 86% of whom were taking masculinizing hormones.
Overall, 17% of respondents had experienced a pregnancy, with 60 pregnancies reported in total. While participants who had never taken hormones had a nearly 200% higher incidence of pregnancy, compared with those who had taken testosterone, one pregnancy occurred while the subject was taking testosterone, and five of seven reported abortions were in participants who had used testosterone prior to conception.
A total of 30 participants (16.4%) believed testosterone was a contraceptive method, and 10 said that a health care provider had advised them to use testosterone for contraception. However, nearly half of all participants did report using condoms for contraception, making it the most common method. IUDs were the second most common method of contraception currently used.
Nearly one-third of participants said they had used some type of contraceptive pill at some point, 17 said they had tried more than one type of pill, and 36 had used combination pills. However, of those who had used combination pills, nearly half stopped using them because of side effects or because of concern about extra feminine hormones.
The authors noted that most study participants expressed a desire to become a parent. Around one-quarter wanted to bear a child while the majority said they would consider adoption.
One-quarter of respondents had fears about not achieving a desired pregnancy, and for some, those fears began after initiating hormone treatments. Just over half the respondents said their health care provider had not asked about their fertility desires.
“Transgender men have unintended pregnancy as well as future fertility desires, and yet, there is a paucity of reproductive health care best practices research for this unique population,” wrote Alexis Light, MD, MPH, of MedStar Washington Hospital Center, and her coauthors. “This survey confirms earlier studies: Transgender men do become pregnant, both intentionally and unintentionally, and some transgender men engage in behaviors that can lead to unintended pregnancy.”
The study authors called for doctors to be more equipped and prepared to counsel transgender men on contraception and discuss other reproductive health concerns.
The study was supported by MedStar Washington Hospital Center. No conflicts of interest were declared.
SOURCE: Light A et al. Contraception. 2018 Jun 23. doi: 10.1016/j.contraception.2018.06.006.
FROM CONTRACEPTION
Key clinical point: Transgender men need advice on reproductive and contraceptive choices.
Major finding: Half of transgender men were not asked about their fertility desires.
Study details: An online survey of 197 female-to-male transgender men.
Disclosures: The study was supported by MedStar Washington Hospital Center. No conflicts of interest were declared.
Source: Light A et al. Contraception. 2018 Jun 23. doi: 10.1016/j.contraception.2018.06.006.
TAVR-related stroke risk unrelated to anatomy
PARIS – The most appropriate stroke prevention strategy in patients undergoing transcatheter aortic valve replacement is routine use of a cerebroembolic protection device for all, because no identifiable high-risk anatomic subsets exist, Hasan Jilaihawi, MD, said at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“We looked at the anatomy in great detail. I’d hoped to find a strata that was truly high risk, but there is no clear strata that is truly higher risk. So stroke remains an unpredictable event in TAVR, and in the ideal world we would use cerebroembolic protection in everyone,” said Dr. Jilaihawi, codirector of transcatheter valve therapy at New York University.
“I put it to you that, as in carotid stenting, where we routinely use cerebroembolic protection, perhaps we need to consider the same in TAVR,” the cardiologist added.
The SENTINEL trial randomized 363 patients undergoing TAVR 2:1 to the use of the Sentinel intraluminal filter device or no neuroprotection during the procedure. The use of the cerebroembolic protection device was associated with a statistically significant 63% reduction in the incidence of neurologist-adjudicated stroke within 72 hours, from 8.2% to 3.0% (J Am Coll Cardiol. 2017 Jan 31;69[4]:367-77). The device was cleared for marketing by the Food and Drug Administration in 2017 and approved by European authorities several years earlier.
A wealth of evidence shows that the average stroke rate associated with contemporary TAVR is 4.4%, although this figure is probably on the low side because most of the data come from nonrandomized registries, which typically underreport neurologic outcomes. The stroke rate is independent of operator experience and volume, surgical risk score, and institutional TAVR volume. Moreover, in the SENTINEL trial, embolic debris was captured in 99% of patients fitted with the cerebroembolic protection device.
“A huge variety of material was captured, including thrombus, valve tissue, calcified material, and – alarmingly – foreign material in 35% of cases,” Dr. Jilaihawi noted.
Nonetheless, the issue of routine versus selective use of cardioembolic protection remains controversial at a time when interventionalists are trying to make TAVR a simpler, briefer procedure, even though the approved Sentinel device is successfully deployed in a median of only 4 minutes. This was the impetus for Dr. Jilaihawi to examine baseline anatomy as a potential predictor of stroke.
He looked at four key anatomic features: aortic arch type, aortic root angulation, aortic arch calcium, and aortic valve calcification. The bottom line: The benefit of cerebroembolic protection with the Sentinel device was consistent across all anatomic subgroups. For example, in patients with an aortic root angulation angle of less than 50 degrees, the incidence of stroke within 3 days post TAVR was 3.2% with and 5.9% without cerebroembolic protection, while in those with an angle of 50 degrees or more the stroke rate was 2.6% with and 9.1% without the Sentinel device. With a total of only 16 strokes by day 3 in the study, those stroke rates in the absence of cerebroembolic protection aren’t significantly different.
There was, however, one unexpected and counterintuitive finding: The greatest stroke reduction with cerebroembolic protection was seen in patients with the least aortic valve calcium. This prompted session cochair Alain Cribier, MD, professor of medicine at the University of Rouen, France, to observe that perhaps valve repositioning is an important factor in TAVR-related strokes. After all, he noted, valve repositioning occurs more often when a patient’s valves are softer and less calcified.
“This is a very important point,” Dr. Jilaihawi responded. “I think there is an interplay between procedural aspects and the anatomy which is not completely captured in this study because we don’t know whose valve was repositioned multiple times.”
He added that the finding that TAVR-related stroke is more common in patients with less calcified aortic valves is consistent with the earlier experience in carotid stenting.
“If you look 10 years ago in the field of carotid stenting, there were a lot of analyses done which concluded that the highest-risk lesions are the least calcified lesions, even though it’s counterintuitive,” he said.
Discussant Saibal Kar, MD, director of interventional cardiac research at Cedars-Sinai Medical Center in Los Angeles, said the take-home point from the SENTINEL analysis is clear: “Cerebroembolic protection is like a seat belt: You should wear it. All patients should wear it.”
The SENTINEL trial was sponsored by Claret Medical. Dr. Jilaihawi reported receiving research grants from Abbott and Medtronic and serving as a consultant to Edwards Lifesciences and Venus Medtech.
SOURCE: Jilaihawi H. EuroPCR 2018.
PARIS – The most appropriate stroke prevention strategy in patients undergoing transcatheter aortic valve replacement is routine use of a cerebroembolic protection device for all, because no identifiable high-risk anatomic subsets exist, Hasan Jilaihawi, MD, said at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“We looked at the anatomy in great detail. I’d hoped to find a strata that was truly high risk, but there is no clear strata that is truly higher risk. So stroke remains an unpredictable event in TAVR, and in the ideal world we would use cerebroembolic protection in everyone,” said Dr. Jilaihawi, codirector of transcatheter valve therapy at New York University.
“I put it to you that, as in carotid stenting, where we routinely use cerebroembolic protection, perhaps we need to consider the same in TAVR,” the cardiologist added.
The SENTINEL trial randomized 363 patients undergoing TAVR 2:1 to the use of the Sentinel intraluminal filter device or no neuroprotection during the procedure. The use of the cerebroembolic protection device was associated with a statistically significant 63% reduction in the incidence of neurologist-adjudicated stroke within 72 hours, from 8.2% to 3.0% (J Am Coll Cardiol. 2017 Jan 31;69[4]:367-77). The device was cleared for marketing by the Food and Drug Administration in 2017 and approved by European authorities several years earlier.
A wealth of evidence shows that the average stroke rate associated with contemporary TAVR is 4.4%, although this figure is probably on the low side because most of the data come from nonrandomized registries, which typically underreport neurologic outcomes. The stroke rate is independent of operator experience and volume, surgical risk score, and institutional TAVR volume. Moreover, in the SENTINEL trial, embolic debris was captured in 99% of patients fitted with the cerebroembolic protection device.
“A huge variety of material was captured, including thrombus, valve tissue, calcified material, and – alarmingly – foreign material in 35% of cases,” Dr. Jilaihawi noted.
Nonetheless, the issue of routine versus selective use of cardioembolic protection remains controversial at a time when interventionalists are trying to make TAVR a simpler, briefer procedure, even though the approved Sentinel device is successfully deployed in a median of only 4 minutes. This was the impetus for Dr. Jilaihawi to examine baseline anatomy as a potential predictor of stroke.
He looked at four key anatomic features: aortic arch type, aortic root angulation, aortic arch calcium, and aortic valve calcification. The bottom line: The benefit of cerebroembolic protection with the Sentinel device was consistent across all anatomic subgroups. For example, in patients with an aortic root angulation angle of less than 50 degrees, the incidence of stroke within 3 days post TAVR was 3.2% with and 5.9% without cerebroembolic protection, while in those with an angle of 50 degrees or more the stroke rate was 2.6% with and 9.1% without the Sentinel device. With a total of only 16 strokes by day 3 in the study, those stroke rates in the absence of cerebroembolic protection aren’t significantly different.
There was, however, one unexpected and counterintuitive finding: The greatest stroke reduction with cerebroembolic protection was seen in patients with the least aortic valve calcium. This prompted session cochair Alain Cribier, MD, professor of medicine at the University of Rouen, France, to observe that perhaps valve repositioning is an important factor in TAVR-related strokes. After all, he noted, valve repositioning occurs more often when a patient’s valves are softer and less calcified.
“This is a very important point,” Dr. Jilaihawi responded. “I think there is an interplay between procedural aspects and the anatomy which is not completely captured in this study because we don’t know whose valve was repositioned multiple times.”
He added that the finding that TAVR-related stroke is more common in patients with less calcified aortic valves is consistent with the earlier experience in carotid stenting.
“If you look 10 years ago in the field of carotid stenting, there were a lot of analyses done which concluded that the highest-risk lesions are the least calcified lesions, even though it’s counterintuitive,” he said.
Discussant Saibal Kar, MD, director of interventional cardiac research at Cedars-Sinai Medical Center in Los Angeles, said the take-home point from the SENTINEL analysis is clear: “Cerebroembolic protection is like a seat belt: You should wear it. All patients should wear it.”
The SENTINEL trial was sponsored by Claret Medical. Dr. Jilaihawi reported receiving research grants from Abbott and Medtronic and serving as a consultant to Edwards Lifesciences and Venus Medtech.
SOURCE: Jilaihawi H. EuroPCR 2018.
PARIS – The most appropriate stroke prevention strategy in patients undergoing transcatheter aortic valve replacement is routine use of a cerebroembolic protection device for all, because no identifiable high-risk anatomic subsets exist, Hasan Jilaihawi, MD, said at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“We looked at the anatomy in great detail. I’d hoped to find a strata that was truly high risk, but there is no clear strata that is truly higher risk. So stroke remains an unpredictable event in TAVR, and in the ideal world we would use cerebroembolic protection in everyone,” said Dr. Jilaihawi, codirector of transcatheter valve therapy at New York University.
“I put it to you that, as in carotid stenting, where we routinely use cerebroembolic protection, perhaps we need to consider the same in TAVR,” the cardiologist added.
The SENTINEL trial randomized 363 patients undergoing TAVR 2:1 to the use of the Sentinel intraluminal filter device or no neuroprotection during the procedure. The use of the cerebroembolic protection device was associated with a statistically significant 63% reduction in the incidence of neurologist-adjudicated stroke within 72 hours, from 8.2% to 3.0% (J Am Coll Cardiol. 2017 Jan 31;69[4]:367-77). The device was cleared for marketing by the Food and Drug Administration in 2017 and approved by European authorities several years earlier.
A wealth of evidence shows that the average stroke rate associated with contemporary TAVR is 4.4%, although this figure is probably on the low side because most of the data come from nonrandomized registries, which typically underreport neurologic outcomes. The stroke rate is independent of operator experience and volume, surgical risk score, and institutional TAVR volume. Moreover, in the SENTINEL trial, embolic debris was captured in 99% of patients fitted with the cerebroembolic protection device.
“A huge variety of material was captured, including thrombus, valve tissue, calcified material, and – alarmingly – foreign material in 35% of cases,” Dr. Jilaihawi noted.
Nonetheless, the issue of routine versus selective use of cardioembolic protection remains controversial at a time when interventionalists are trying to make TAVR a simpler, briefer procedure, even though the approved Sentinel device is successfully deployed in a median of only 4 minutes. This was the impetus for Dr. Jilaihawi to examine baseline anatomy as a potential predictor of stroke.
He looked at four key anatomic features: aortic arch type, aortic root angulation, aortic arch calcium, and aortic valve calcification. The bottom line: The benefit of cerebroembolic protection with the Sentinel device was consistent across all anatomic subgroups. For example, in patients with an aortic root angulation angle of less than 50 degrees, the incidence of stroke within 3 days post TAVR was 3.2% with and 5.9% without cerebroembolic protection, while in those with an angle of 50 degrees or more the stroke rate was 2.6% with and 9.1% without the Sentinel device. With a total of only 16 strokes by day 3 in the study, those stroke rates in the absence of cerebroembolic protection aren’t significantly different.
There was, however, one unexpected and counterintuitive finding: The greatest stroke reduction with cerebroembolic protection was seen in patients with the least aortic valve calcium. This prompted session cochair Alain Cribier, MD, professor of medicine at the University of Rouen, France, to observe that perhaps valve repositioning is an important factor in TAVR-related strokes. After all, he noted, valve repositioning occurs more often when a patient’s valves are softer and less calcified.
“This is a very important point,” Dr. Jilaihawi responded. “I think there is an interplay between procedural aspects and the anatomy which is not completely captured in this study because we don’t know whose valve was repositioned multiple times.”
He added that the finding that TAVR-related stroke is more common in patients with less calcified aortic valves is consistent with the earlier experience in carotid stenting.
“If you look 10 years ago in the field of carotid stenting, there were a lot of analyses done which concluded that the highest-risk lesions are the least calcified lesions, even though it’s counterintuitive,” he said.
Discussant Saibal Kar, MD, director of interventional cardiac research at Cedars-Sinai Medical Center in Los Angeles, said the take-home point from the SENTINEL analysis is clear: “Cerebroembolic protection is like a seat belt: You should wear it. All patients should wear it.”
The SENTINEL trial was sponsored by Claret Medical. Dr. Jilaihawi reported receiving research grants from Abbott and Medtronic and serving as a consultant to Edwards Lifesciences and Venus Medtech.
SOURCE: Jilaihawi H. EuroPCR 2018.
REPORTING FROM EUROPCR 2018
Key clinical point: .
Major finding: Neither aortic arch type, root angulation, nor calcium burden identifies a subgroup of TAVR patients at higher stroke risk.
Study details: The SENTINEL trial randomized 363 TAVR patients 2:1 to the use of a cerebroembolic protection device or no neuroprotection during the procedure.
Disclosures: The SENTINEL trial was sponsored by Claret Medical. The presenter reported having no financial conflicts of interest.
Source: Jilaihawi H. EuroPCR 2018.
Disparities found in access to medication treatment for OUDs
The number of Medicaid enrollees receiving medication treatment with methadone and buprenorphine rose from 2002 to 2009 because of the availability of buprenorphine. A cause for concern, however, is that medication treatment increased at a much higher rate in counties with lower poverty rates – and lower concentrations of black and Hispanic residents.
“Concerted efforts are needed to ensure that [medication treatment] benefits are equitably distributed across society and reach disadvantaged individuals who may be at higher risk of experiencing opioid use disorders,” wrote Bradley D. Stein, MD, PhD, and his colleagues. The report was published in Substance Abuse.
Dr. Stein, of Rand Corporation, and his colleagues set out to assess the changes in medication treatment use over time and how medication treatment was being used at the county level – in addition to the associations between poverty, race/ethnicity, and urbanicity. The research team analyzed Medicaid claims from 2002 to 2009 from 14 states, representing 53% of the U.S. population and 47% of 2009 Medicaid enrollees. The states selected in the analysis, chosen to represent regional and population diversity, were California, Connecticut, Florida, Georgia, Illinois, Louisiana, Massachusetts, Maryland, New York, Pennsylvania, Rhode Island, Texas, Vermont, and Wisconsin. The researchers looked at medication treatment use among 18- to 64-year-old Medicaid enrollees, excluding people who were eligible for both Medicare and Medicaid.
The variables for who received medication treatment and data on county characteristics were well defined. Individuals who had received either methadone or buprenorphine were identified as receiving medication treatment. Some patients (3% or less) used both methadone or buprenorphine but were categorized as methadone users in the analysis to better elucidate the role of buprenorphine in medication treatment. Counties were classified as low poverty if the percentage of the county population was below the median (less than 13.5%) of the counties in the 14 states in the analysis and the federal poverty line.
The racial/ethnic makeup of a county was determined to be low percentage of black people if the percentage of the black population was below the median (less than 5.6%) in all counties. Similarly, a county was considered low percentage of Hispanic residents if the proportion of the Hispanic population was below the median of less than 4.2%, reported Dr. Stein, who also is affiliated with the University of Pittsburgh.
The analysis showed that from 2002 to 2009, the proportion of Medicaid users receiving methadone increased by 20% (42,235 to 50,587), accounting for a fraction of the 62% increase in Medicaid enrollment (42,263 to 68,278). The real driver in increased medication treatment rates was the adoption of buprenorphine, which soared from 75 in 2002 to 19,691 in 2009. In 2009, 29% of Medicaid enrollees received medication treatment with buprenorphine. The growth of medication treatment varied by the characteristics of a county’s population. In 2002, urban counties had substantially higher rates of primarily methadone therapy than did rural counties (P less than.001). But no significant differences were found across the county based the concentration of black residents or poverty. Communities that did not have low concentrations of Hispanic residents experienced higher rates of medication treatment, regardless of poverty (P less than .01 for low poverty and not low poverty)
Those trends changed by 2009. Compared with individuals living in all other types of counties, those living in counties with a lower proportion of black residents and a low poverty rate were much more likely to receive medication treatment. A similar pattern was seen among populations with a lower proportion of Hispanic residents and a low poverty rate, compared with communities with high numbers of Hispanics and not low poverty rate.
Dr. Stein and his colleagues cited several limitations. First, because the study analyzed Medicaid enrollees, it is not known how the findings might translate to uninsured or commercially insured patients. Another limitation is that the study data analyzed patients until 2009, making it difficult to generalize the findings to the population today. Finally, the researchers used a population-based approach.
Nevertheless, they said, the study advances understanding of the impact of buprenorphine on medication treatment among patients who receive Medicaid.
“At a time of intensive policymaker and regulatory efforts to increase [medication treatment] availability, of suffering from these disorders,” Dr. Stein and his colleagues wrote.
The study was supported by a grant from the National Institute on Drug Abuse. The authors disclosed no relevant conflicts of interest.
SOURCE: Stein BD et al. Subst Abuse. 2018 Jun 22. doi: 10.1080/08897077.2018.1449166.
The number of Medicaid enrollees receiving medication treatment with methadone and buprenorphine rose from 2002 to 2009 because of the availability of buprenorphine. A cause for concern, however, is that medication treatment increased at a much higher rate in counties with lower poverty rates – and lower concentrations of black and Hispanic residents.
“Concerted efforts are needed to ensure that [medication treatment] benefits are equitably distributed across society and reach disadvantaged individuals who may be at higher risk of experiencing opioid use disorders,” wrote Bradley D. Stein, MD, PhD, and his colleagues. The report was published in Substance Abuse.
Dr. Stein, of Rand Corporation, and his colleagues set out to assess the changes in medication treatment use over time and how medication treatment was being used at the county level – in addition to the associations between poverty, race/ethnicity, and urbanicity. The research team analyzed Medicaid claims from 2002 to 2009 from 14 states, representing 53% of the U.S. population and 47% of 2009 Medicaid enrollees. The states selected in the analysis, chosen to represent regional and population diversity, were California, Connecticut, Florida, Georgia, Illinois, Louisiana, Massachusetts, Maryland, New York, Pennsylvania, Rhode Island, Texas, Vermont, and Wisconsin. The researchers looked at medication treatment use among 18- to 64-year-old Medicaid enrollees, excluding people who were eligible for both Medicare and Medicaid.
The variables for who received medication treatment and data on county characteristics were well defined. Individuals who had received either methadone or buprenorphine were identified as receiving medication treatment. Some patients (3% or less) used both methadone or buprenorphine but were categorized as methadone users in the analysis to better elucidate the role of buprenorphine in medication treatment. Counties were classified as low poverty if the percentage of the county population was below the median (less than 13.5%) of the counties in the 14 states in the analysis and the federal poverty line.
The racial/ethnic makeup of a county was determined to be low percentage of black people if the percentage of the black population was below the median (less than 5.6%) in all counties. Similarly, a county was considered low percentage of Hispanic residents if the proportion of the Hispanic population was below the median of less than 4.2%, reported Dr. Stein, who also is affiliated with the University of Pittsburgh.
The analysis showed that from 2002 to 2009, the proportion of Medicaid users receiving methadone increased by 20% (42,235 to 50,587), accounting for a fraction of the 62% increase in Medicaid enrollment (42,263 to 68,278). The real driver in increased medication treatment rates was the adoption of buprenorphine, which soared from 75 in 2002 to 19,691 in 2009. In 2009, 29% of Medicaid enrollees received medication treatment with buprenorphine. The growth of medication treatment varied by the characteristics of a county’s population. In 2002, urban counties had substantially higher rates of primarily methadone therapy than did rural counties (P less than.001). But no significant differences were found across the county based the concentration of black residents or poverty. Communities that did not have low concentrations of Hispanic residents experienced higher rates of medication treatment, regardless of poverty (P less than .01 for low poverty and not low poverty)
Those trends changed by 2009. Compared with individuals living in all other types of counties, those living in counties with a lower proportion of black residents and a low poverty rate were much more likely to receive medication treatment. A similar pattern was seen among populations with a lower proportion of Hispanic residents and a low poverty rate, compared with communities with high numbers of Hispanics and not low poverty rate.
Dr. Stein and his colleagues cited several limitations. First, because the study analyzed Medicaid enrollees, it is not known how the findings might translate to uninsured or commercially insured patients. Another limitation is that the study data analyzed patients until 2009, making it difficult to generalize the findings to the population today. Finally, the researchers used a population-based approach.
Nevertheless, they said, the study advances understanding of the impact of buprenorphine on medication treatment among patients who receive Medicaid.
“At a time of intensive policymaker and regulatory efforts to increase [medication treatment] availability, of suffering from these disorders,” Dr. Stein and his colleagues wrote.
The study was supported by a grant from the National Institute on Drug Abuse. The authors disclosed no relevant conflicts of interest.
SOURCE: Stein BD et al. Subst Abuse. 2018 Jun 22. doi: 10.1080/08897077.2018.1449166.
The number of Medicaid enrollees receiving medication treatment with methadone and buprenorphine rose from 2002 to 2009 because of the availability of buprenorphine. A cause for concern, however, is that medication treatment increased at a much higher rate in counties with lower poverty rates – and lower concentrations of black and Hispanic residents.
“Concerted efforts are needed to ensure that [medication treatment] benefits are equitably distributed across society and reach disadvantaged individuals who may be at higher risk of experiencing opioid use disorders,” wrote Bradley D. Stein, MD, PhD, and his colleagues. The report was published in Substance Abuse.
Dr. Stein, of Rand Corporation, and his colleagues set out to assess the changes in medication treatment use over time and how medication treatment was being used at the county level – in addition to the associations between poverty, race/ethnicity, and urbanicity. The research team analyzed Medicaid claims from 2002 to 2009 from 14 states, representing 53% of the U.S. population and 47% of 2009 Medicaid enrollees. The states selected in the analysis, chosen to represent regional and population diversity, were California, Connecticut, Florida, Georgia, Illinois, Louisiana, Massachusetts, Maryland, New York, Pennsylvania, Rhode Island, Texas, Vermont, and Wisconsin. The researchers looked at medication treatment use among 18- to 64-year-old Medicaid enrollees, excluding people who were eligible for both Medicare and Medicaid.
The variables for who received medication treatment and data on county characteristics were well defined. Individuals who had received either methadone or buprenorphine were identified as receiving medication treatment. Some patients (3% or less) used both methadone or buprenorphine but were categorized as methadone users in the analysis to better elucidate the role of buprenorphine in medication treatment. Counties were classified as low poverty if the percentage of the county population was below the median (less than 13.5%) of the counties in the 14 states in the analysis and the federal poverty line.
The racial/ethnic makeup of a county was determined to be low percentage of black people if the percentage of the black population was below the median (less than 5.6%) in all counties. Similarly, a county was considered low percentage of Hispanic residents if the proportion of the Hispanic population was below the median of less than 4.2%, reported Dr. Stein, who also is affiliated with the University of Pittsburgh.
The analysis showed that from 2002 to 2009, the proportion of Medicaid users receiving methadone increased by 20% (42,235 to 50,587), accounting for a fraction of the 62% increase in Medicaid enrollment (42,263 to 68,278). The real driver in increased medication treatment rates was the adoption of buprenorphine, which soared from 75 in 2002 to 19,691 in 2009. In 2009, 29% of Medicaid enrollees received medication treatment with buprenorphine. The growth of medication treatment varied by the characteristics of a county’s population. In 2002, urban counties had substantially higher rates of primarily methadone therapy than did rural counties (P less than.001). But no significant differences were found across the county based the concentration of black residents or poverty. Communities that did not have low concentrations of Hispanic residents experienced higher rates of medication treatment, regardless of poverty (P less than .01 for low poverty and not low poverty)
Those trends changed by 2009. Compared with individuals living in all other types of counties, those living in counties with a lower proportion of black residents and a low poverty rate were much more likely to receive medication treatment. A similar pattern was seen among populations with a lower proportion of Hispanic residents and a low poverty rate, compared with communities with high numbers of Hispanics and not low poverty rate.
Dr. Stein and his colleagues cited several limitations. First, because the study analyzed Medicaid enrollees, it is not known how the findings might translate to uninsured or commercially insured patients. Another limitation is that the study data analyzed patients until 2009, making it difficult to generalize the findings to the population today. Finally, the researchers used a population-based approach.
Nevertheless, they said, the study advances understanding of the impact of buprenorphine on medication treatment among patients who receive Medicaid.
“At a time of intensive policymaker and regulatory efforts to increase [medication treatment] availability, of suffering from these disorders,” Dr. Stein and his colleagues wrote.
The study was supported by a grant from the National Institute on Drug Abuse. The authors disclosed no relevant conflicts of interest.
SOURCE: Stein BD et al. Subst Abuse. 2018 Jun 22. doi: 10.1080/08897077.2018.1449166.
FROM SUBSTANCE ABUSE
Key clinical point: Medication treatment access for opioid use disorders varies greatly among Medicaid enrollees.
Major finding: Residents of counties with a lower proportion of black residents and a low poverty rate are much more likely to receive medication treatment.
Study details: An analysis of Medicaid claims from 2002 to 2009 from 14 states representing 53% of the U.S. population and 47% of 2009 Medicaid enrollees.
Disclosures: This study was supported by a grant from the National Institute on Drug Abuse. The authors disclosed no relevant conflicts of interest.
Source: Stein BD et al. Subst Abuse. 2018 Jun 22. doi: 10.1080/08897077.2018.1449166.
FDA reverses warning on LABA-containing asthma medications
The combination a by 17%, without increasing the risk of asthma-related intubation or death.
An independent analysis of four large, drug company–sponsored trials supports the Food and Drug Administration’s recent decision to remove the black box warning on LABA/inhaled glucocorticoid products, wrote William W. Busse, MD, and his colleagues. The report was published in the New England Journal of Medicine.
“Our analysis confirmed a lower relative risk of asthma exacerbations of 17% with combination therapy than with an inhaled glucocorticoid alone. This finding corresponds to the lower relative rates of asthma exacerbations that were reported in the sponsored individual trials: by 21% in the GlaxoSmithKline trial [hazard ratio 0.79], by 16% in the AstraZeneca trial [HR. 0.84], and by 11% in the Merck trial [HR 0.89],” wrote Dr. Busse of the University of Wisconsin, Madison, and his coauthors.
The FDA based its December 2017 reversal on an initial review of the studies, which were reviewed by an independent committee and are now public. Dr. Busse led the expert analysis of the studies, which the FDA required after it put the black box warning on the combination products.
In 2010, the FDA advised that LABAs shouldn’t be used as first-line therapy for asthma and required a black box warning on all LABA-containing products. Despite an FDA-conducted meta-analysis that found no increase in serious asthma-related incidents, the agency said there wasn’t enough subgroup evidence to support the safety of LABAs when combined with an inhaled glucocorticoid.
“FDA stated that the small numbers of patients who were enrolled in these studies prevented a definitive conclusion regarding mitigation of serious asthma-related events with the addition of inhaled glucocorticoids,” the investigators stated.
The agency required the four companies marketing a LABA for asthma to conduct prospective randomized trials comparing the safety of LABA/inhaled glucocorticoid to inhaled glucocorticoid alone. The trials by AstraZeneca, GlaxoSmithKline, Merck, and Novartis were identical. Three had complete, 26-week data; Novartis submitted partial data, as it withdrew its product from the American market in 2015. The committee reviewed all of the studies, which comprised a total of 36,010 teens and adults (aged 12-91 years). The primary endpoint was a composite of asthma-related intubation or death; secondary endpoints were a composite of hospitalization, intubation, or death, and individual assessments of each of those events.
Among the four studies, there were three asthma-related intubations: two in the inhaled-glucocorticoid group and one in the combination-therapy group. There were also two asthma-related deaths, both in the combination group.
Serious asthma-related events occurred in 108 of the inhaled glucocorticoid group (0.60%) and in 119 of the combination-therapy group (0.66%), a nonsignificant difference.
However, the combination therapy did confer a significant 17% reduction in asthma exacerbations. Exacerbations occurred in 11.7% of the inhaled glucocorticoid group and in 9.8% of the combination therapy group (relative risk 0.83; P less than 0.001). All four trials showed a similarly decreased risk of exacerbation.
The committee looked at several subgroups, dividing the cohort by age, race/ethnicity/ obesity, and smoking history. The advantage associated with combination therapy remained significant in all these analyses.
“… Our data provide support for the treatment guidelines of both the Global Initiative for Asthma and the Expert Panel Report 3 of the National Asthma Education and Prevention Program, which recommend the use of a low-dose glucocorticoid (step 3) and a medium-dose glucocorticoid (step 4), plus a LABA, with the caution that LABAs should not be used as monotherapy in asthma; the convenience and safety of a combination inhaler is a likely plus,” the committee wrote. “Finally, our combined analysis provides strong evidence to support the recent FDA decision to remove the boxed safety warning for combination therapy with a LABA plus an inhaled glucocorticoid for asthma treatment.”
Dr. Busse disclosed financial relationships with a number of pharmaceutical companies, including Novartis, but noted that none of them were relevant to this work.
SOURCE: Busse WW et al. N Engl J Med. 2018;78:2497-505.
It takes a lot for the FDA to remove a boxed warning on a product, but the data generated by these four manufacturer-led trials of long-acting beta agonists and inhaled corticosteroid combination therapy warrant the regulatory reversal, Sally Seymour, MD, and her colleagues wrote in an accompanying editorial.
“Each completed trial met the prespecified objective and demonstrated noninferiority of combination products to inhaled corticosteroids alone with respect to the composite endpoint of asthma-related death, intubation, or hospitalization.
The majority of events were asthma-related hospitalizations; there were five intubations and deaths overall,” Dr. Seymour and her colleagues wrote.
All of the studies met their primary safety objective, and faced with this – and the consistency of the results among the studies – the path was clear.
“In addition, the observed reduction in asthma exacerbations that required systemic corticosteroids demonstrates a benefit associated with combination products. On the basis of this strong and consistent evidence, we opted to remove the boxed warning right away, without convening an FDA advisory committee meeting.”
There will always be areas of uncertainty, however.
“Admittedly, the results from these trials cannot answer all questions regarding the safety of LABAs. Some uncertainties remain, and we cannot conclude that there is no increase in risk associated with combination products containing an inhaled corticosteroid and a LABA as compared with inhaled corticosteroids alone. Although the trials found that combination therapy reduces the rate of exacerbations that require the administration of systemic corticosteroids, none of them showed a decrease in asthma-related hospitalizations. People with life-threatening asthma were excluded because of safety and ethical concerns, so we don’t know whether the results can be generalized to these patients.”
Nevertheless, the evidence in favor of combination therapy was clear and compelling enough to convince a national regulatory agency to change a stance on safety.
Dr. Seymour is the acting director of the FDA’s Division of Pulmonary, Allergy, & Rheumatology Products.
It takes a lot for the FDA to remove a boxed warning on a product, but the data generated by these four manufacturer-led trials of long-acting beta agonists and inhaled corticosteroid combination therapy warrant the regulatory reversal, Sally Seymour, MD, and her colleagues wrote in an accompanying editorial.
“Each completed trial met the prespecified objective and demonstrated noninferiority of combination products to inhaled corticosteroids alone with respect to the composite endpoint of asthma-related death, intubation, or hospitalization.
The majority of events were asthma-related hospitalizations; there were five intubations and deaths overall,” Dr. Seymour and her colleagues wrote.
All of the studies met their primary safety objective, and faced with this – and the consistency of the results among the studies – the path was clear.
“In addition, the observed reduction in asthma exacerbations that required systemic corticosteroids demonstrates a benefit associated with combination products. On the basis of this strong and consistent evidence, we opted to remove the boxed warning right away, without convening an FDA advisory committee meeting.”
There will always be areas of uncertainty, however.
“Admittedly, the results from these trials cannot answer all questions regarding the safety of LABAs. Some uncertainties remain, and we cannot conclude that there is no increase in risk associated with combination products containing an inhaled corticosteroid and a LABA as compared with inhaled corticosteroids alone. Although the trials found that combination therapy reduces the rate of exacerbations that require the administration of systemic corticosteroids, none of them showed a decrease in asthma-related hospitalizations. People with life-threatening asthma were excluded because of safety and ethical concerns, so we don’t know whether the results can be generalized to these patients.”
Nevertheless, the evidence in favor of combination therapy was clear and compelling enough to convince a national regulatory agency to change a stance on safety.
Dr. Seymour is the acting director of the FDA’s Division of Pulmonary, Allergy, & Rheumatology Products.
It takes a lot for the FDA to remove a boxed warning on a product, but the data generated by these four manufacturer-led trials of long-acting beta agonists and inhaled corticosteroid combination therapy warrant the regulatory reversal, Sally Seymour, MD, and her colleagues wrote in an accompanying editorial.
“Each completed trial met the prespecified objective and demonstrated noninferiority of combination products to inhaled corticosteroids alone with respect to the composite endpoint of asthma-related death, intubation, or hospitalization.
The majority of events were asthma-related hospitalizations; there were five intubations and deaths overall,” Dr. Seymour and her colleagues wrote.
All of the studies met their primary safety objective, and faced with this – and the consistency of the results among the studies – the path was clear.
“In addition, the observed reduction in asthma exacerbations that required systemic corticosteroids demonstrates a benefit associated with combination products. On the basis of this strong and consistent evidence, we opted to remove the boxed warning right away, without convening an FDA advisory committee meeting.”
There will always be areas of uncertainty, however.
“Admittedly, the results from these trials cannot answer all questions regarding the safety of LABAs. Some uncertainties remain, and we cannot conclude that there is no increase in risk associated with combination products containing an inhaled corticosteroid and a LABA as compared with inhaled corticosteroids alone. Although the trials found that combination therapy reduces the rate of exacerbations that require the administration of systemic corticosteroids, none of them showed a decrease in asthma-related hospitalizations. People with life-threatening asthma were excluded because of safety and ethical concerns, so we don’t know whether the results can be generalized to these patients.”
Nevertheless, the evidence in favor of combination therapy was clear and compelling enough to convince a national regulatory agency to change a stance on safety.
Dr. Seymour is the acting director of the FDA’s Division of Pulmonary, Allergy, & Rheumatology Products.
The combination a by 17%, without increasing the risk of asthma-related intubation or death.
An independent analysis of four large, drug company–sponsored trials supports the Food and Drug Administration’s recent decision to remove the black box warning on LABA/inhaled glucocorticoid products, wrote William W. Busse, MD, and his colleagues. The report was published in the New England Journal of Medicine.
“Our analysis confirmed a lower relative risk of asthma exacerbations of 17% with combination therapy than with an inhaled glucocorticoid alone. This finding corresponds to the lower relative rates of asthma exacerbations that were reported in the sponsored individual trials: by 21% in the GlaxoSmithKline trial [hazard ratio 0.79], by 16% in the AstraZeneca trial [HR. 0.84], and by 11% in the Merck trial [HR 0.89],” wrote Dr. Busse of the University of Wisconsin, Madison, and his coauthors.
The FDA based its December 2017 reversal on an initial review of the studies, which were reviewed by an independent committee and are now public. Dr. Busse led the expert analysis of the studies, which the FDA required after it put the black box warning on the combination products.
In 2010, the FDA advised that LABAs shouldn’t be used as first-line therapy for asthma and required a black box warning on all LABA-containing products. Despite an FDA-conducted meta-analysis that found no increase in serious asthma-related incidents, the agency said there wasn’t enough subgroup evidence to support the safety of LABAs when combined with an inhaled glucocorticoid.
“FDA stated that the small numbers of patients who were enrolled in these studies prevented a definitive conclusion regarding mitigation of serious asthma-related events with the addition of inhaled glucocorticoids,” the investigators stated.
The agency required the four companies marketing a LABA for asthma to conduct prospective randomized trials comparing the safety of LABA/inhaled glucocorticoid to inhaled glucocorticoid alone. The trials by AstraZeneca, GlaxoSmithKline, Merck, and Novartis were identical. Three had complete, 26-week data; Novartis submitted partial data, as it withdrew its product from the American market in 2015. The committee reviewed all of the studies, which comprised a total of 36,010 teens and adults (aged 12-91 years). The primary endpoint was a composite of asthma-related intubation or death; secondary endpoints were a composite of hospitalization, intubation, or death, and individual assessments of each of those events.
Among the four studies, there were three asthma-related intubations: two in the inhaled-glucocorticoid group and one in the combination-therapy group. There were also two asthma-related deaths, both in the combination group.
Serious asthma-related events occurred in 108 of the inhaled glucocorticoid group (0.60%) and in 119 of the combination-therapy group (0.66%), a nonsignificant difference.
However, the combination therapy did confer a significant 17% reduction in asthma exacerbations. Exacerbations occurred in 11.7% of the inhaled glucocorticoid group and in 9.8% of the combination therapy group (relative risk 0.83; P less than 0.001). All four trials showed a similarly decreased risk of exacerbation.
The committee looked at several subgroups, dividing the cohort by age, race/ethnicity/ obesity, and smoking history. The advantage associated with combination therapy remained significant in all these analyses.
“… Our data provide support for the treatment guidelines of both the Global Initiative for Asthma and the Expert Panel Report 3 of the National Asthma Education and Prevention Program, which recommend the use of a low-dose glucocorticoid (step 3) and a medium-dose glucocorticoid (step 4), plus a LABA, with the caution that LABAs should not be used as monotherapy in asthma; the convenience and safety of a combination inhaler is a likely plus,” the committee wrote. “Finally, our combined analysis provides strong evidence to support the recent FDA decision to remove the boxed safety warning for combination therapy with a LABA plus an inhaled glucocorticoid for asthma treatment.”
Dr. Busse disclosed financial relationships with a number of pharmaceutical companies, including Novartis, but noted that none of them were relevant to this work.
SOURCE: Busse WW et al. N Engl J Med. 2018;78:2497-505.
The combination a by 17%, without increasing the risk of asthma-related intubation or death.
An independent analysis of four large, drug company–sponsored trials supports the Food and Drug Administration’s recent decision to remove the black box warning on LABA/inhaled glucocorticoid products, wrote William W. Busse, MD, and his colleagues. The report was published in the New England Journal of Medicine.
“Our analysis confirmed a lower relative risk of asthma exacerbations of 17% with combination therapy than with an inhaled glucocorticoid alone. This finding corresponds to the lower relative rates of asthma exacerbations that were reported in the sponsored individual trials: by 21% in the GlaxoSmithKline trial [hazard ratio 0.79], by 16% in the AstraZeneca trial [HR. 0.84], and by 11% in the Merck trial [HR 0.89],” wrote Dr. Busse of the University of Wisconsin, Madison, and his coauthors.
The FDA based its December 2017 reversal on an initial review of the studies, which were reviewed by an independent committee and are now public. Dr. Busse led the expert analysis of the studies, which the FDA required after it put the black box warning on the combination products.
In 2010, the FDA advised that LABAs shouldn’t be used as first-line therapy for asthma and required a black box warning on all LABA-containing products. Despite an FDA-conducted meta-analysis that found no increase in serious asthma-related incidents, the agency said there wasn’t enough subgroup evidence to support the safety of LABAs when combined with an inhaled glucocorticoid.
“FDA stated that the small numbers of patients who were enrolled in these studies prevented a definitive conclusion regarding mitigation of serious asthma-related events with the addition of inhaled glucocorticoids,” the investigators stated.
The agency required the four companies marketing a LABA for asthma to conduct prospective randomized trials comparing the safety of LABA/inhaled glucocorticoid to inhaled glucocorticoid alone. The trials by AstraZeneca, GlaxoSmithKline, Merck, and Novartis were identical. Three had complete, 26-week data; Novartis submitted partial data, as it withdrew its product from the American market in 2015. The committee reviewed all of the studies, which comprised a total of 36,010 teens and adults (aged 12-91 years). The primary endpoint was a composite of asthma-related intubation or death; secondary endpoints were a composite of hospitalization, intubation, or death, and individual assessments of each of those events.
Among the four studies, there were three asthma-related intubations: two in the inhaled-glucocorticoid group and one in the combination-therapy group. There were also two asthma-related deaths, both in the combination group.
Serious asthma-related events occurred in 108 of the inhaled glucocorticoid group (0.60%) and in 119 of the combination-therapy group (0.66%), a nonsignificant difference.
However, the combination therapy did confer a significant 17% reduction in asthma exacerbations. Exacerbations occurred in 11.7% of the inhaled glucocorticoid group and in 9.8% of the combination therapy group (relative risk 0.83; P less than 0.001). All four trials showed a similarly decreased risk of exacerbation.
The committee looked at several subgroups, dividing the cohort by age, race/ethnicity/ obesity, and smoking history. The advantage associated with combination therapy remained significant in all these analyses.
“… Our data provide support for the treatment guidelines of both the Global Initiative for Asthma and the Expert Panel Report 3 of the National Asthma Education and Prevention Program, which recommend the use of a low-dose glucocorticoid (step 3) and a medium-dose glucocorticoid (step 4), plus a LABA, with the caution that LABAs should not be used as monotherapy in asthma; the convenience and safety of a combination inhaler is a likely plus,” the committee wrote. “Finally, our combined analysis provides strong evidence to support the recent FDA decision to remove the boxed safety warning for combination therapy with a LABA plus an inhaled glucocorticoid for asthma treatment.”
Dr. Busse disclosed financial relationships with a number of pharmaceutical companies, including Novartis, but noted that none of them were relevant to this work.
SOURCE: Busse WW et al. N Engl J Med. 2018;78:2497-505.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Combination LABA/inhaled glucocorticoid products appear safe for patients with asthma.
Major finding: The products reduced the risk of an asthma exacerbation by 17%, without increasing the risk of a serious adverse outcome.
Study details: The four randomized studies comprised more than 13,000 patients.
Disclosures: The studies were sponsored by AstraZeneca, GlaxoSmithKline, Merck, and Novartis. Dr. Busse disclosed a financial relationship with Novartis, but said it was not relevant to this work.
Source: Busse WW. N Engl J Med. 2018;78:2497-505.
Death rates rising for 10- to 19-year-olds
Mortality in this age group, which had dropped nearly 33% from 1999 to 2013, climbed from 29.6 per 100,000 population aged 10-19 years in 2013 to 33.1 per 100,000 in 2016, the last year for which data are available. Meanwhile, deaths from injuries – unintentional injuries, suicides, homicides, and legal intervention – went from 19.8 per 100,000 to 23.3, an increase of almost 18%, from 2013 to 2016, and the noninjury death rate “was relatively stable,” Sally C. Curtin and her associates at the NCHS Division of Vital Statistics said in a National Vital Statistics Report.
The recent surge in injury deaths was more substantial in the older half of the age group. The mortality rate for children aged 10-14 years went from a low of 6.4 per 100,000 in 2012 to 7.1 in 2016, an increase of 11%, while the rate for those aged 15-19 rose 19% as it jumped from 32.8 per 100,000 in 2013 to 39.0 in 2016, the investigators wrote in the report.
The rate of unintentional injury deaths in 10- to 19-year-olds shows the same pattern as all deaths and injury deaths: Decline from 1999 to 2013 and then a rise for the last 3 years. That recent rise also can be seen in the most common form of unintentional injury deaths, motor vehicle traffic accidents, and in poisoning deaths, although that uptick began a year later. Homicide deaths declined by one-third from 2007 to 2014 and then increased, while suicide rates have been rising since 2007, the investigators said. Legal intervention deaths, defined as those caused by law enforcement actions, were not included because of relatively small annual numbers.
“Although progress was made in reducing injury deaths among children and adolescents aged 10-19 years during 1999-2013, the recent upturn shows that persistent as well as emerging challenges remain. … Further reductions will require renewed focus and effort,” Ms. Curtin and her associates wrote.
SOURCE: Curtin SC et al. Natl Vital Stat Rep. 2018 Jun;67(4):1-16.
Mortality in this age group, which had dropped nearly 33% from 1999 to 2013, climbed from 29.6 per 100,000 population aged 10-19 years in 2013 to 33.1 per 100,000 in 2016, the last year for which data are available. Meanwhile, deaths from injuries – unintentional injuries, suicides, homicides, and legal intervention – went from 19.8 per 100,000 to 23.3, an increase of almost 18%, from 2013 to 2016, and the noninjury death rate “was relatively stable,” Sally C. Curtin and her associates at the NCHS Division of Vital Statistics said in a National Vital Statistics Report.
The recent surge in injury deaths was more substantial in the older half of the age group. The mortality rate for children aged 10-14 years went from a low of 6.4 per 100,000 in 2012 to 7.1 in 2016, an increase of 11%, while the rate for those aged 15-19 rose 19% as it jumped from 32.8 per 100,000 in 2013 to 39.0 in 2016, the investigators wrote in the report.
The rate of unintentional injury deaths in 10- to 19-year-olds shows the same pattern as all deaths and injury deaths: Decline from 1999 to 2013 and then a rise for the last 3 years. That recent rise also can be seen in the most common form of unintentional injury deaths, motor vehicle traffic accidents, and in poisoning deaths, although that uptick began a year later. Homicide deaths declined by one-third from 2007 to 2014 and then increased, while suicide rates have been rising since 2007, the investigators said. Legal intervention deaths, defined as those caused by law enforcement actions, were not included because of relatively small annual numbers.
“Although progress was made in reducing injury deaths among children and adolescents aged 10-19 years during 1999-2013, the recent upturn shows that persistent as well as emerging challenges remain. … Further reductions will require renewed focus and effort,” Ms. Curtin and her associates wrote.
SOURCE: Curtin SC et al. Natl Vital Stat Rep. 2018 Jun;67(4):1-16.
Mortality in this age group, which had dropped nearly 33% from 1999 to 2013, climbed from 29.6 per 100,000 population aged 10-19 years in 2013 to 33.1 per 100,000 in 2016, the last year for which data are available. Meanwhile, deaths from injuries – unintentional injuries, suicides, homicides, and legal intervention – went from 19.8 per 100,000 to 23.3, an increase of almost 18%, from 2013 to 2016, and the noninjury death rate “was relatively stable,” Sally C. Curtin and her associates at the NCHS Division of Vital Statistics said in a National Vital Statistics Report.
The recent surge in injury deaths was more substantial in the older half of the age group. The mortality rate for children aged 10-14 years went from a low of 6.4 per 100,000 in 2012 to 7.1 in 2016, an increase of 11%, while the rate for those aged 15-19 rose 19% as it jumped from 32.8 per 100,000 in 2013 to 39.0 in 2016, the investigators wrote in the report.
The rate of unintentional injury deaths in 10- to 19-year-olds shows the same pattern as all deaths and injury deaths: Decline from 1999 to 2013 and then a rise for the last 3 years. That recent rise also can be seen in the most common form of unintentional injury deaths, motor vehicle traffic accidents, and in poisoning deaths, although that uptick began a year later. Homicide deaths declined by one-third from 2007 to 2014 and then increased, while suicide rates have been rising since 2007, the investigators said. Legal intervention deaths, defined as those caused by law enforcement actions, were not included because of relatively small annual numbers.
“Although progress was made in reducing injury deaths among children and adolescents aged 10-19 years during 1999-2013, the recent upturn shows that persistent as well as emerging challenges remain. … Further reductions will require renewed focus and effort,” Ms. Curtin and her associates wrote.
SOURCE: Curtin SC et al. Natl Vital Stat Rep. 2018 Jun;67(4):1-16.
FROM NATIONAL VITAL STATISTICS REPORTS