User login
Under the Cover of Darkness
When he was about 12, a now 41-year-old man noticed that the skin on his left chest was darkening. For several years afterward, the darkness spread and deepened, and the area became hairy. In young adulthood, he experienced occasional outbreaks of what looked like acne on the lesion; this eventually cleared.
He now finds the hairiness increasingly bothersome, so he shaves the worst parts of it. Upon consulting his primary care provider, he was assured that the lesion is “a birthmark.” Unsatisfied with this answer, the patient took the advice of a friend and decided to consult dermatology.
EXAMINATION
A polygonal, hyperpigmented, hypertrichotic patch covers most of the patient’s left pectoral area. The lateral margin is irregular but well-defined. There is obvious partial regrowth of the shaved hair on the lateral margin, but it stops abruptly at that point.

The breast and surrounding tissue appear normal. No areas of hyperpigmentation or hypertrichosis are seen elsewhere.
What is the diagnosis?
DISCUSSION
First described by William Becker in 1948 (and subsequently named for him), the Becker nevus (BN) received little research attention until a French study of 20,000 young men showed a prevalence of 0.5%. Nearly half of the subjects had first noticed the lesion before the age of 10—a somewhat surprising finding, since abundant evidence implicates androgens in its genesis. (This is supported by the condition’s predominance in males, the increased numbers of androgen receptors and melanocytes in the affected skin, and the prevalence of hypertrichosis.)
The researchers were also surprised to find that only 30% of the reported lesions occurred above the nipple, because the first descriptions of BN gave the impression that the shoulder and chest were most commonly affected. We now know that BN can also be found on arms and legs.
Usually a benign condition, BN can be associated with skeletal or soft-tissue deformities in the affected area (eg, ipsilateral breast hypoplasia). Malignancies—most notably melanoma—have also been reported with BN but are especially uncommon.
The differential includes the café-au-lait macules of neurofibromatosis, Albright disease, and congenital melanocytic nevus. The history of BN (ie, presentation, hypertrichosis, gender of patient, and distribution) usually allow a clinical diagnosis.
Treatment is limited to laser hair removal or laser removal or reduction of pigment.
TAKE-HOME LEARNING POINTS
- Becker nevus (BN) is far more common in males than females.
- BN typically manifests during puberty, which aligns with the suspected androgenic etiology.
- Though the shoulders and chest are the most commonly affected areas, BNs can also appear on the flank, arms, or legs.
- The lesions are rarely associated with serious pathology; hypoplasia of the ipsilateral breast is the most common of these complications.
When he was about 12, a now 41-year-old man noticed that the skin on his left chest was darkening. For several years afterward, the darkness spread and deepened, and the area became hairy. In young adulthood, he experienced occasional outbreaks of what looked like acne on the lesion; this eventually cleared.
He now finds the hairiness increasingly bothersome, so he shaves the worst parts of it. Upon consulting his primary care provider, he was assured that the lesion is “a birthmark.” Unsatisfied with this answer, the patient took the advice of a friend and decided to consult dermatology.
EXAMINATION
A polygonal, hyperpigmented, hypertrichotic patch covers most of the patient’s left pectoral area. The lateral margin is irregular but well-defined. There is obvious partial regrowth of the shaved hair on the lateral margin, but it stops abruptly at that point.

The breast and surrounding tissue appear normal. No areas of hyperpigmentation or hypertrichosis are seen elsewhere.
What is the diagnosis?
DISCUSSION
First described by William Becker in 1948 (and subsequently named for him), the Becker nevus (BN) received little research attention until a French study of 20,000 young men showed a prevalence of 0.5%. Nearly half of the subjects had first noticed the lesion before the age of 10—a somewhat surprising finding, since abundant evidence implicates androgens in its genesis. (This is supported by the condition’s predominance in males, the increased numbers of androgen receptors and melanocytes in the affected skin, and the prevalence of hypertrichosis.)
The researchers were also surprised to find that only 30% of the reported lesions occurred above the nipple, because the first descriptions of BN gave the impression that the shoulder and chest were most commonly affected. We now know that BN can also be found on arms and legs.
Usually a benign condition, BN can be associated with skeletal or soft-tissue deformities in the affected area (eg, ipsilateral breast hypoplasia). Malignancies—most notably melanoma—have also been reported with BN but are especially uncommon.
The differential includes the café-au-lait macules of neurofibromatosis, Albright disease, and congenital melanocytic nevus. The history of BN (ie, presentation, hypertrichosis, gender of patient, and distribution) usually allow a clinical diagnosis.
Treatment is limited to laser hair removal or laser removal or reduction of pigment.
TAKE-HOME LEARNING POINTS
- Becker nevus (BN) is far more common in males than females.
- BN typically manifests during puberty, which aligns with the suspected androgenic etiology.
- Though the shoulders and chest are the most commonly affected areas, BNs can also appear on the flank, arms, or legs.
- The lesions are rarely associated with serious pathology; hypoplasia of the ipsilateral breast is the most common of these complications.
When he was about 12, a now 41-year-old man noticed that the skin on his left chest was darkening. For several years afterward, the darkness spread and deepened, and the area became hairy. In young adulthood, he experienced occasional outbreaks of what looked like acne on the lesion; this eventually cleared.
He now finds the hairiness increasingly bothersome, so he shaves the worst parts of it. Upon consulting his primary care provider, he was assured that the lesion is “a birthmark.” Unsatisfied with this answer, the patient took the advice of a friend and decided to consult dermatology.
EXAMINATION
A polygonal, hyperpigmented, hypertrichotic patch covers most of the patient’s left pectoral area. The lateral margin is irregular but well-defined. There is obvious partial regrowth of the shaved hair on the lateral margin, but it stops abruptly at that point.

The breast and surrounding tissue appear normal. No areas of hyperpigmentation or hypertrichosis are seen elsewhere.
What is the diagnosis?
DISCUSSION
First described by William Becker in 1948 (and subsequently named for him), the Becker nevus (BN) received little research attention until a French study of 20,000 young men showed a prevalence of 0.5%. Nearly half of the subjects had first noticed the lesion before the age of 10—a somewhat surprising finding, since abundant evidence implicates androgens in its genesis. (This is supported by the condition’s predominance in males, the increased numbers of androgen receptors and melanocytes in the affected skin, and the prevalence of hypertrichosis.)
The researchers were also surprised to find that only 30% of the reported lesions occurred above the nipple, because the first descriptions of BN gave the impression that the shoulder and chest were most commonly affected. We now know that BN can also be found on arms and legs.
Usually a benign condition, BN can be associated with skeletal or soft-tissue deformities in the affected area (eg, ipsilateral breast hypoplasia). Malignancies—most notably melanoma—have also been reported with BN but are especially uncommon.
The differential includes the café-au-lait macules of neurofibromatosis, Albright disease, and congenital melanocytic nevus. The history of BN (ie, presentation, hypertrichosis, gender of patient, and distribution) usually allow a clinical diagnosis.
Treatment is limited to laser hair removal or laser removal or reduction of pigment.
TAKE-HOME LEARNING POINTS
- Becker nevus (BN) is far more common in males than females.
- BN typically manifests during puberty, which aligns with the suspected androgenic etiology.
- Though the shoulders and chest are the most commonly affected areas, BNs can also appear on the flank, arms, or legs.
- The lesions are rarely associated with serious pathology; hypoplasia of the ipsilateral breast is the most common of these complications.
New pediatric hypertension guidelines increased hypertension prevalence
New clinical among at-risk youth, according to findings published in Pediatrics.
In a clinical practice guideline (CPG) published in 2017, the American Academy of Pediatrics updated its 2004 guideline to include new reference tables for BP values in addition to new definitions of elevated BP and hypertension, including absolute BP cutoff values for adolescents aged 13 years and older (Pediatrics. 2017 Sep;140[6]:e20173035). This was intended to “emulate the recently updated adult hypertension guidelines and to simplify the process of identifying and classifying hypertension in adolescents,” wrote Michael Khoury, MD, of the Heart Institute at Cincinnati Children’s Hospital Medical Center, and his coauthors.
To evaluate the impact of the new guidelines on the prevalence of hypertension and associations with target organ damage, the investigators used data from a study on obesity and type 2 diabetes in 364 patients aged 10-17 years; 59% were obese, and 30% had type 2 diabetes. Three groups were identified: patients with obesity and type 2 diabetes, patients with obesity but no type 2 diabetes, and healthy (“lean”) controls.
Patients fasted overnight for a minimum of 10 hours, after which body mass index was calculated, blood pressure was taken, and anthropometric, laboratory, echocardiography, and carotid assessments were performed. Average BP measurements were categorized according to the 2004 guideline and to the new CPG.
In carotid ultrasonography assessments, a composite of carotid intima-media thickness was formed from the average of three sites, and a composite carotid intima-media thickness greater than or equal to the 90th percentile of that measured in the lean patients, who were the controls, was considered abnormal. In echocardiography assessments, left ventricular mass (LVM) and LVM index were calculated. Elevated LVM was defined by the pediatric cutoff of LVM index greater than or equal to 38.6 g/m.
For diastolic function, tissue Doppler velocities under the 10th percentile and an average early left ventricular filling/peak early myocardial velocity ratio greater than or equal to the 90th percentile in controls were considered abnormal. Lastly, pulse wave velocity (PWV) was measured to determine arterial stiffness, and a PWV greater than or equal to the 90th percentile for the controls was considered abnormal.
BP classification under the new guideline resulted in an increased prevalence of hypertension at 13% (10% stage 1, 3% stage 2), compared with the 2004 guideline at 8% (6% stage 1, 2% stage 2), with a P value of .007.
Of the 75 patients classified as having elevated BP in the 2004 guideline, 19 (25%) were reclassified as having stage 1 hypertension under the CPG. These 19 patients were older, compared with patients who remained in the elevated blood pressure category (16.5 ± 0.9 vs. 15.5 ± 1.7 years; P = .02). The patients who were reclassified also had higher body mass indexes (38.8 ± 8.2 vs. 33.6 ± 7.4; P = .01) and diastolic blood pressures (76.5 mm Hg ± 8.7 vs. 62.1 ± 12.2 mm Hg; P less than .001), Dr. Khoury and his colleagues reported.
Reclassification to a higher BP category was associated with increased odds of an abnormal target organ damage (TOD) values, and both guidelines produced similar odds, “suggesting that the two guidelines produce similar associations with TOD,” the authors wrote. Reclassification based on the CPG definition accounted for 31% of patients with increased LVM, compared with 20% as defined in the 2004 guideline (P less than .001), and for 33% of patients with abnormal PWV, compared with 23% in the 2004 guideline, suggesting improved sensitivity of hypertension categorization in detecting LVM. A similar effect was seen in other measures of TOD, the authors noted.
The findings suggest that, combined with the increased prevalence of hypertension under the new guidelines, “the CPG may contribute to an increased detection of abnormal LVM and other measures of TOD,” the authors wrote. “This, in turn, may contribute to risk stratification in clinical decision making for youth presenting with BP concerns,” they concluded.
The study was supported by a National Institutes of Health grant. The authors had no relevant disclosures.
SOURCE: Khoury M et al. Pediatrics. 2018 Jul 5. doi: 10.1542/peds.2018-0245.
New clinical among at-risk youth, according to findings published in Pediatrics.
In a clinical practice guideline (CPG) published in 2017, the American Academy of Pediatrics updated its 2004 guideline to include new reference tables for BP values in addition to new definitions of elevated BP and hypertension, including absolute BP cutoff values for adolescents aged 13 years and older (Pediatrics. 2017 Sep;140[6]:e20173035). This was intended to “emulate the recently updated adult hypertension guidelines and to simplify the process of identifying and classifying hypertension in adolescents,” wrote Michael Khoury, MD, of the Heart Institute at Cincinnati Children’s Hospital Medical Center, and his coauthors.
To evaluate the impact of the new guidelines on the prevalence of hypertension and associations with target organ damage, the investigators used data from a study on obesity and type 2 diabetes in 364 patients aged 10-17 years; 59% were obese, and 30% had type 2 diabetes. Three groups were identified: patients with obesity and type 2 diabetes, patients with obesity but no type 2 diabetes, and healthy (“lean”) controls.
Patients fasted overnight for a minimum of 10 hours, after which body mass index was calculated, blood pressure was taken, and anthropometric, laboratory, echocardiography, and carotid assessments were performed. Average BP measurements were categorized according to the 2004 guideline and to the new CPG.
In carotid ultrasonography assessments, a composite of carotid intima-media thickness was formed from the average of three sites, and a composite carotid intima-media thickness greater than or equal to the 90th percentile of that measured in the lean patients, who were the controls, was considered abnormal. In echocardiography assessments, left ventricular mass (LVM) and LVM index were calculated. Elevated LVM was defined by the pediatric cutoff of LVM index greater than or equal to 38.6 g/m.
For diastolic function, tissue Doppler velocities under the 10th percentile and an average early left ventricular filling/peak early myocardial velocity ratio greater than or equal to the 90th percentile in controls were considered abnormal. Lastly, pulse wave velocity (PWV) was measured to determine arterial stiffness, and a PWV greater than or equal to the 90th percentile for the controls was considered abnormal.
BP classification under the new guideline resulted in an increased prevalence of hypertension at 13% (10% stage 1, 3% stage 2), compared with the 2004 guideline at 8% (6% stage 1, 2% stage 2), with a P value of .007.
Of the 75 patients classified as having elevated BP in the 2004 guideline, 19 (25%) were reclassified as having stage 1 hypertension under the CPG. These 19 patients were older, compared with patients who remained in the elevated blood pressure category (16.5 ± 0.9 vs. 15.5 ± 1.7 years; P = .02). The patients who were reclassified also had higher body mass indexes (38.8 ± 8.2 vs. 33.6 ± 7.4; P = .01) and diastolic blood pressures (76.5 mm Hg ± 8.7 vs. 62.1 ± 12.2 mm Hg; P less than .001), Dr. Khoury and his colleagues reported.
Reclassification to a higher BP category was associated with increased odds of an abnormal target organ damage (TOD) values, and both guidelines produced similar odds, “suggesting that the two guidelines produce similar associations with TOD,” the authors wrote. Reclassification based on the CPG definition accounted for 31% of patients with increased LVM, compared with 20% as defined in the 2004 guideline (P less than .001), and for 33% of patients with abnormal PWV, compared with 23% in the 2004 guideline, suggesting improved sensitivity of hypertension categorization in detecting LVM. A similar effect was seen in other measures of TOD, the authors noted.
The findings suggest that, combined with the increased prevalence of hypertension under the new guidelines, “the CPG may contribute to an increased detection of abnormal LVM and other measures of TOD,” the authors wrote. “This, in turn, may contribute to risk stratification in clinical decision making for youth presenting with BP concerns,” they concluded.
The study was supported by a National Institutes of Health grant. The authors had no relevant disclosures.
SOURCE: Khoury M et al. Pediatrics. 2018 Jul 5. doi: 10.1542/peds.2018-0245.
New clinical among at-risk youth, according to findings published in Pediatrics.
In a clinical practice guideline (CPG) published in 2017, the American Academy of Pediatrics updated its 2004 guideline to include new reference tables for BP values in addition to new definitions of elevated BP and hypertension, including absolute BP cutoff values for adolescents aged 13 years and older (Pediatrics. 2017 Sep;140[6]:e20173035). This was intended to “emulate the recently updated adult hypertension guidelines and to simplify the process of identifying and classifying hypertension in adolescents,” wrote Michael Khoury, MD, of the Heart Institute at Cincinnati Children’s Hospital Medical Center, and his coauthors.
To evaluate the impact of the new guidelines on the prevalence of hypertension and associations with target organ damage, the investigators used data from a study on obesity and type 2 diabetes in 364 patients aged 10-17 years; 59% were obese, and 30% had type 2 diabetes. Three groups were identified: patients with obesity and type 2 diabetes, patients with obesity but no type 2 diabetes, and healthy (“lean”) controls.
Patients fasted overnight for a minimum of 10 hours, after which body mass index was calculated, blood pressure was taken, and anthropometric, laboratory, echocardiography, and carotid assessments were performed. Average BP measurements were categorized according to the 2004 guideline and to the new CPG.
In carotid ultrasonography assessments, a composite of carotid intima-media thickness was formed from the average of three sites, and a composite carotid intima-media thickness greater than or equal to the 90th percentile of that measured in the lean patients, who were the controls, was considered abnormal. In echocardiography assessments, left ventricular mass (LVM) and LVM index were calculated. Elevated LVM was defined by the pediatric cutoff of LVM index greater than or equal to 38.6 g/m.
For diastolic function, tissue Doppler velocities under the 10th percentile and an average early left ventricular filling/peak early myocardial velocity ratio greater than or equal to the 90th percentile in controls were considered abnormal. Lastly, pulse wave velocity (PWV) was measured to determine arterial stiffness, and a PWV greater than or equal to the 90th percentile for the controls was considered abnormal.
BP classification under the new guideline resulted in an increased prevalence of hypertension at 13% (10% stage 1, 3% stage 2), compared with the 2004 guideline at 8% (6% stage 1, 2% stage 2), with a P value of .007.
Of the 75 patients classified as having elevated BP in the 2004 guideline, 19 (25%) were reclassified as having stage 1 hypertension under the CPG. These 19 patients were older, compared with patients who remained in the elevated blood pressure category (16.5 ± 0.9 vs. 15.5 ± 1.7 years; P = .02). The patients who were reclassified also had higher body mass indexes (38.8 ± 8.2 vs. 33.6 ± 7.4; P = .01) and diastolic blood pressures (76.5 mm Hg ± 8.7 vs. 62.1 ± 12.2 mm Hg; P less than .001), Dr. Khoury and his colleagues reported.
Reclassification to a higher BP category was associated with increased odds of an abnormal target organ damage (TOD) values, and both guidelines produced similar odds, “suggesting that the two guidelines produce similar associations with TOD,” the authors wrote. Reclassification based on the CPG definition accounted for 31% of patients with increased LVM, compared with 20% as defined in the 2004 guideline (P less than .001), and for 33% of patients with abnormal PWV, compared with 23% in the 2004 guideline, suggesting improved sensitivity of hypertension categorization in detecting LVM. A similar effect was seen in other measures of TOD, the authors noted.
The findings suggest that, combined with the increased prevalence of hypertension under the new guidelines, “the CPG may contribute to an increased detection of abnormal LVM and other measures of TOD,” the authors wrote. “This, in turn, may contribute to risk stratification in clinical decision making for youth presenting with BP concerns,” they concluded.
The study was supported by a National Institutes of Health grant. The authors had no relevant disclosures.
SOURCE: Khoury M et al. Pediatrics. 2018 Jul 5. doi: 10.1542/peds.2018-0245.
FROM PEDIATRICS
Key clinical point: New clinical guidelines for pediatric hypertension resulted in increased prevalence of the condition and improved sensitivity in detecting target organ damage.
Major finding: BP classification under the new guideline resulted in an increased hypertension prevalence of 13% versus 8% with the 2004 guideline (P = .007).
Study details: The impact of the new guidelines was evaluated using data on 364 patients aged 10-18 years in an obesity and type 2 diabetes mellitus trial.
Disclosures: The study was supported by a National Institutes of Health grant. The authors had no relevant disclosures to report.
Source: Khoury M et al. Pediatrics. 2018 Jul 5. doi: 10.1542/peds.2018-0245.
AGA CPU: Extraesophageal symptoms attributed to GERD
When patients lack typical symptoms of gastroesophageal reflux disease (GERD) and have extraesophageal symptoms, ENT, allergy, and pulmonary work-ups are “essential and often should be performed initially,” experts note in an American Gastroenterological Association clinical practice update.
Extraesophageal symptoms often are unrelated to GERD or are multifactorial, wrote Michael F. Vaezi, MD, PhD, of Vanderbilt University Medical Center in Nashville, Tenn., and his associates in Clinical Gastroenterology and Hepatology. Gastroenterologists often are asked to look for reflux as the cause of extraesophageal symptoms before other etiologies have been ruled out.
Proposed extraesophageal manifestations of GERD range from chronic throat clearing and dysphonia to otitis, pulmonary fibrosis, laryngeal cancer, and even lung transplant rejection. Stronger evidence links GERD with symptoms of asthma, cough, and hoarseness, the experts note. “When less stringent criteria are used, the attributions are broader and could include sore throat, sinusitis, ear pain, and pulmonary fibrosis.”
When asked to assess whether GERD is causing extraesophageal symptoms, consider the “constellation” of patient presentation, test results, and treatment response, according to the clinical practice update. No diagnostic tests “unequivocally link any suspected extraesophageal symptom to GERD.” For patients who have both extraesophageal symptoms and typical symptoms of GERD, the authors suggest an evaluator regimen of 6-8 weeks of empiric, aggressive (twice-daily) proton pump inhibitor (PPI) therapy. If aggressive acid suppression therapy appears to improve extraesophageal symptoms, patients should be titrated to the lowest effective treatment dose.If symptoms persist despite an aggressive trial of a PPI, and patients have a body mass index under 25, and a seemingly low probability of GERD, then the experts recommend pH testing “off” therapy and seeking other etiologies for extraesophageal symptoms. If symptoms persist and a patients’ BMI exceeds 25 with a high suspicion of GERD, they recommend evaluations for concomitant asthma or lung disease. If these work-ups are positive, they recommend multichannel intraluminal impedance testing or pH monitoring on treatment.
The clinical practice update strongly discourages surgical treatment of extraesophageal GERD symptoms except in specific populations, such as when patients have objective signs of treatment-refractory GERD and have not responded to comprehensive therapy for other possible causes of extraesophageal symptoms. Recent data suggest that surgery can benefit patients with confirmed structural defects, such as hiatal hernia, which are causing symptomatic, volume-based regurgitation, the experts note. Ideally, these patients should first undergo pH and impedance monitoring to objectively measure the effects of reflux. Additionally, surgical fundoplication “might be beneficial” for patients whose extraesophageal symptoms clearly have responded to PPI therapy but who refuse long-term PPI therapy or who develop unacceptable side effects.
The practice update also extensively discusses the role of testing to evaluate the role of GERD in extraesophageal symptoms. Barium esophagography is insensitive for GERD and is useful only for evaluating dysphagia and the size and type of a hiatal hernia, the experts note. Abnormal laryngoscopy or pharyngoscopic findings are more useful but should not be the “initial driving force” behind a GERD diagnosis and do not necessarily link GERD to extraesophageal symptoms. Likewise, esophagogastroduodenoscopy can identify esophagitis, which signifies GERD but does not establish it as etiologic.
Positive ambulatory pH or impedance monitoring or pharyngeal pH tests also do not definitively link reflux to suspected extraesophageal symptoms, the experts note. They suggest considering “on” therapy monitoring to evaluate treatment efficacy and to time reflux events relative to symptoms in patients with esophagitis, Barrett’s esophagus, or a large hiatal hernia. Conversely, they recommend considering “off” treatment testing to rule out GERD in patients who have no history of confirmed or suspected reflux and who have not responded to PPI therapy.
Novel tests, such as salivary pepsin and mucosal impedance, have “no clear role in establishing GERD as the cause of extraesophageal symptoms,” the experts emphasize. Clinician scientists also debate the exact pathophysiology of extraesophageal GERD sequelae. While chronic exposure to gastric refluxate clearly can harm proximal structures such as the pharynx, larynx, and bronchial tree, it remains unclear how much acid is necessary to cause injury and whether bile, pepsin, or neurogenic stimulation play a role.
Dr. Vaezi reported having no conflicts of interest. Senior author Frank Zerbib, MD, PhD, reported receiving devices for research purposes from Medtronic and Sandhill Scientific.
SOURCE: Vaezi MF et al. Clin Gastroenterol Hepatol. 2018 Feb 7. doi: 10.1016/j.cgh.2018.02.001.
When patients lack typical symptoms of gastroesophageal reflux disease (GERD) and have extraesophageal symptoms, ENT, allergy, and pulmonary work-ups are “essential and often should be performed initially,” experts note in an American Gastroenterological Association clinical practice update.
Extraesophageal symptoms often are unrelated to GERD or are multifactorial, wrote Michael F. Vaezi, MD, PhD, of Vanderbilt University Medical Center in Nashville, Tenn., and his associates in Clinical Gastroenterology and Hepatology. Gastroenterologists often are asked to look for reflux as the cause of extraesophageal symptoms before other etiologies have been ruled out.
Proposed extraesophageal manifestations of GERD range from chronic throat clearing and dysphonia to otitis, pulmonary fibrosis, laryngeal cancer, and even lung transplant rejection. Stronger evidence links GERD with symptoms of asthma, cough, and hoarseness, the experts note. “When less stringent criteria are used, the attributions are broader and could include sore throat, sinusitis, ear pain, and pulmonary fibrosis.”
When asked to assess whether GERD is causing extraesophageal symptoms, consider the “constellation” of patient presentation, test results, and treatment response, according to the clinical practice update. No diagnostic tests “unequivocally link any suspected extraesophageal symptom to GERD.” For patients who have both extraesophageal symptoms and typical symptoms of GERD, the authors suggest an evaluator regimen of 6-8 weeks of empiric, aggressive (twice-daily) proton pump inhibitor (PPI) therapy. If aggressive acid suppression therapy appears to improve extraesophageal symptoms, patients should be titrated to the lowest effective treatment dose.If symptoms persist despite an aggressive trial of a PPI, and patients have a body mass index under 25, and a seemingly low probability of GERD, then the experts recommend pH testing “off” therapy and seeking other etiologies for extraesophageal symptoms. If symptoms persist and a patients’ BMI exceeds 25 with a high suspicion of GERD, they recommend evaluations for concomitant asthma or lung disease. If these work-ups are positive, they recommend multichannel intraluminal impedance testing or pH monitoring on treatment.
The clinical practice update strongly discourages surgical treatment of extraesophageal GERD symptoms except in specific populations, such as when patients have objective signs of treatment-refractory GERD and have not responded to comprehensive therapy for other possible causes of extraesophageal symptoms. Recent data suggest that surgery can benefit patients with confirmed structural defects, such as hiatal hernia, which are causing symptomatic, volume-based regurgitation, the experts note. Ideally, these patients should first undergo pH and impedance monitoring to objectively measure the effects of reflux. Additionally, surgical fundoplication “might be beneficial” for patients whose extraesophageal symptoms clearly have responded to PPI therapy but who refuse long-term PPI therapy or who develop unacceptable side effects.
The practice update also extensively discusses the role of testing to evaluate the role of GERD in extraesophageal symptoms. Barium esophagography is insensitive for GERD and is useful only for evaluating dysphagia and the size and type of a hiatal hernia, the experts note. Abnormal laryngoscopy or pharyngoscopic findings are more useful but should not be the “initial driving force” behind a GERD diagnosis and do not necessarily link GERD to extraesophageal symptoms. Likewise, esophagogastroduodenoscopy can identify esophagitis, which signifies GERD but does not establish it as etiologic.
Positive ambulatory pH or impedance monitoring or pharyngeal pH tests also do not definitively link reflux to suspected extraesophageal symptoms, the experts note. They suggest considering “on” therapy monitoring to evaluate treatment efficacy and to time reflux events relative to symptoms in patients with esophagitis, Barrett’s esophagus, or a large hiatal hernia. Conversely, they recommend considering “off” treatment testing to rule out GERD in patients who have no history of confirmed or suspected reflux and who have not responded to PPI therapy.
Novel tests, such as salivary pepsin and mucosal impedance, have “no clear role in establishing GERD as the cause of extraesophageal symptoms,” the experts emphasize. Clinician scientists also debate the exact pathophysiology of extraesophageal GERD sequelae. While chronic exposure to gastric refluxate clearly can harm proximal structures such as the pharynx, larynx, and bronchial tree, it remains unclear how much acid is necessary to cause injury and whether bile, pepsin, or neurogenic stimulation play a role.
Dr. Vaezi reported having no conflicts of interest. Senior author Frank Zerbib, MD, PhD, reported receiving devices for research purposes from Medtronic and Sandhill Scientific.
SOURCE: Vaezi MF et al. Clin Gastroenterol Hepatol. 2018 Feb 7. doi: 10.1016/j.cgh.2018.02.001.
When patients lack typical symptoms of gastroesophageal reflux disease (GERD) and have extraesophageal symptoms, ENT, allergy, and pulmonary work-ups are “essential and often should be performed initially,” experts note in an American Gastroenterological Association clinical practice update.
Extraesophageal symptoms often are unrelated to GERD or are multifactorial, wrote Michael F. Vaezi, MD, PhD, of Vanderbilt University Medical Center in Nashville, Tenn., and his associates in Clinical Gastroenterology and Hepatology. Gastroenterologists often are asked to look for reflux as the cause of extraesophageal symptoms before other etiologies have been ruled out.
Proposed extraesophageal manifestations of GERD range from chronic throat clearing and dysphonia to otitis, pulmonary fibrosis, laryngeal cancer, and even lung transplant rejection. Stronger evidence links GERD with symptoms of asthma, cough, and hoarseness, the experts note. “When less stringent criteria are used, the attributions are broader and could include sore throat, sinusitis, ear pain, and pulmonary fibrosis.”
When asked to assess whether GERD is causing extraesophageal symptoms, consider the “constellation” of patient presentation, test results, and treatment response, according to the clinical practice update. No diagnostic tests “unequivocally link any suspected extraesophageal symptom to GERD.” For patients who have both extraesophageal symptoms and typical symptoms of GERD, the authors suggest an evaluator regimen of 6-8 weeks of empiric, aggressive (twice-daily) proton pump inhibitor (PPI) therapy. If aggressive acid suppression therapy appears to improve extraesophageal symptoms, patients should be titrated to the lowest effective treatment dose.If symptoms persist despite an aggressive trial of a PPI, and patients have a body mass index under 25, and a seemingly low probability of GERD, then the experts recommend pH testing “off” therapy and seeking other etiologies for extraesophageal symptoms. If symptoms persist and a patients’ BMI exceeds 25 with a high suspicion of GERD, they recommend evaluations for concomitant asthma or lung disease. If these work-ups are positive, they recommend multichannel intraluminal impedance testing or pH monitoring on treatment.
The clinical practice update strongly discourages surgical treatment of extraesophageal GERD symptoms except in specific populations, such as when patients have objective signs of treatment-refractory GERD and have not responded to comprehensive therapy for other possible causes of extraesophageal symptoms. Recent data suggest that surgery can benefit patients with confirmed structural defects, such as hiatal hernia, which are causing symptomatic, volume-based regurgitation, the experts note. Ideally, these patients should first undergo pH and impedance monitoring to objectively measure the effects of reflux. Additionally, surgical fundoplication “might be beneficial” for patients whose extraesophageal symptoms clearly have responded to PPI therapy but who refuse long-term PPI therapy or who develop unacceptable side effects.
The practice update also extensively discusses the role of testing to evaluate the role of GERD in extraesophageal symptoms. Barium esophagography is insensitive for GERD and is useful only for evaluating dysphagia and the size and type of a hiatal hernia, the experts note. Abnormal laryngoscopy or pharyngoscopic findings are more useful but should not be the “initial driving force” behind a GERD diagnosis and do not necessarily link GERD to extraesophageal symptoms. Likewise, esophagogastroduodenoscopy can identify esophagitis, which signifies GERD but does not establish it as etiologic.
Positive ambulatory pH or impedance monitoring or pharyngeal pH tests also do not definitively link reflux to suspected extraesophageal symptoms, the experts note. They suggest considering “on” therapy monitoring to evaluate treatment efficacy and to time reflux events relative to symptoms in patients with esophagitis, Barrett’s esophagus, or a large hiatal hernia. Conversely, they recommend considering “off” treatment testing to rule out GERD in patients who have no history of confirmed or suspected reflux and who have not responded to PPI therapy.
Novel tests, such as salivary pepsin and mucosal impedance, have “no clear role in establishing GERD as the cause of extraesophageal symptoms,” the experts emphasize. Clinician scientists also debate the exact pathophysiology of extraesophageal GERD sequelae. While chronic exposure to gastric refluxate clearly can harm proximal structures such as the pharynx, larynx, and bronchial tree, it remains unclear how much acid is necessary to cause injury and whether bile, pepsin, or neurogenic stimulation play a role.
Dr. Vaezi reported having no conflicts of interest. Senior author Frank Zerbib, MD, PhD, reported receiving devices for research purposes from Medtronic and Sandhill Scientific.
SOURCE: Vaezi MF et al. Clin Gastroenterol Hepatol. 2018 Feb 7. doi: 10.1016/j.cgh.2018.02.001.
Caring Under a Microscope
I write this editorial at the end of June as summer officially begins. Much of the country—my New Mexico home included—is suffering under an unbearable heat wave in which even those without belief pray for rain. Summer for many is associated with vacations, family trips, and happy hours in the swimming pool among other enjoyable activities that provide a welcome and much deserved break from routine and relief from the grind of work and school. In the words of the George Gershwin tune, “Summertime, and the livin’ is easy.”
In stark contrast to this season, where there is more lightness in being, is the heaviness of the news reports about the Department of Veteran Affairs (VA) that have been featured in the media and the federal press. I suspect I am not alone in having a hard time opening those e-mails; feeling once more the weight of failure on the VA and the employees who have dedicated a good part of their careers to its mission. Even for the VA, June has seen an exceptional string of bad press. I ask as you read this column to think about what the adjective bad means in this context. In the conclusion to this column, I will suggest that the meaning is multivalent.
Among the most distressing stories was the USA Today and Boston Globe headline, “Secret VA nursing home ratings hide poor quality of care from the public.”2 In an all too predictable sequence, this led justifiably to a cascade of demands from the fifth estate, congressional representatives, the administration, veterans and their families, and watchdog organizations for release of the data, investigation of the allegedly deplorable conditions, and rapid fixes to the problems along with the punishment of the guilty.
As an ethicist I am committed to the principles of transparency and accountability that these entities rightly adjure in the wake of any disclosure of a breach of duty to treat each veteran with the best we have—especially the disabled, elderly, and vulnerable. But I have come to believe that the way in which this cycle of scandal and reaction plays out over and over again in VA facilities across the country, what I call “caring under the microscope,” is actually undermining the righteous goals it seeks to achieve.
I encourage you to try this online. Search for the phrase, “VA under microscope” and see what you get. Briefly read the summary, or the entire story if you have the inclination, and then take a few minutes to reflect on the emotional impact of what you read. Under a microscope is an idiom coined to capture the experience of being the object of close inspection and intense scrutiny. As most everyone knows from their own science education, microscopes magnify images that cannot normally be seen with the human eye, allowing us to observe a more detailed and focused image. The microscope surely helped revolutionize medicine and science. But what effect does such amplified and constant observation have on VA employees?3
For the thousands of staff members who do their job every day with all the empathy and skill, integrity, and dedication they can muster, there is demoralization. Researchers in the health professions describe it as “a feeling state of dejection, hopelessness, and a sense of personal ‘incompetence’ that may be tied to a loss of or threat to one’s own goals or values. It has an existential dimension when beliefs and values about oneself are disconfirmed.”4 If you are a nurse assigned to one of VA’s nursing homes, daily striving to ensure patients are clean and comfortable, or a therapist in a continuing living center using all your training to maximize an elder’s mobility and participation in activities, you might well begin to doubt your ability as a professional and question the worth of your work. This is exactly the opposite outcome that the microscopic oversight is intended to attain.
The impact of demoralization on health professionals directly contributes to unprecedented burnout and turnover. Were this not damaging enough, it also has an insidious rippling effect—like contaminated groundwater that poisons where it should be reviving. The humanistic, even spiritual, heart of all the health professions is the relationship between the practitioner and the patient, ideally a relationship of mutual respect and trust. Waves of negative news triggering harsh and unyielding criticism distort even the strongest, purest therapeutic alliances with fear and distrust, just as a microscope not properly focused changes a beautiful image into a blurred muddle.
Worried families of veterans staring at this picture invariably are drawn into the hyper media focus, feeling alarmed and betrayed, even when their loved one may be receiving excellent VA care. In 20 years as a physician and ethicist in VA hospitals, clinics, and community living centers, I know well that bad things happen to good people (both patients and staff). Yet VA patients, families, and staff are seldom offered the wider corrective vision that would note that bad things also happen in other health care institutions and good care is delivered in the VA. Acting Secretary of Veterans Affairs Peter O’Rourke crisply summarized in his response to the nursing home story.5
No veteran or any other human being in a VA or any other nursing home should ever be medicated into a zombie state or left alone in pain like those patients reported in the news story. And if the USA Today story improves the care of a single VA patient, then good has been done at least in the short run. Yet we must also take the long view and consider the moral and psychological outcome of prolonged demoralization on the very staff who must carry out the congressional mandates.
In the same time frame as the nursing home scandal, the VA Office of Inspector General also issued a report on the continued understaffing in the VA.6 This may be the most concerning aftermath of demoralization. One of my best residents had thought about the VA but in the end made a different choice when he completed his training. When I asked him why he told me, “I am afraid to end up in the newspaper.”
Summer will go by far too quickly. Enjoy it while you can so that with renewed strength we may all search for a better way that the light of truth and heat of power can do what they must while also not withering the spirit of caring that animates the people of the VA.
1. Camus A. O’Brien J, trans. The Myth of Sisyphus and Other Essays. New York, New York: Vintage Books, 1955.
2. Slack D, Estes A. Secret VA nursing home ratings hide poor quality of care from the public. USA Today. June 17, 2018. https://www.usatoday.com/story/news/politics/2018/06/17/secret-va-nursing-home-ratings-hide-poor-quality-care/674829002. Accessed June 25, 2018.
3. Gabel S. Demoralization in health professional practice: development, amelioration, and, implications for continuing education.” J Contin Educ Health Prof. 2013;33(2):118-126.
4. Hanlon A. How the microscope redefined the fact. T he Atlantic. February 11, 2016. https://www.theatlantic.com/technology/archive/2016/02/microscope-history-data/462234. Accessed June 27, 2018.
5. O’Rourke P. VA: USA Today’s article is misleading. USA Today. June 20, 2018. https://www.usatoday.com/story/opinion/2018/06/20/va-usa-today-article-misleading-editorials-debates/36223067. Updated June 21, 2018. Accessed June 27, 2018.
6. US Department of Veterans Affairs, Office of the Inspector General. OIG determination of Veterans Health Administration’s occupational staffing shortages. https://www.va.gov/oig/pubs/VAOIG-18-01693-196.pdf. Published June 14, 2018. Accessed June 25, 2018.
I write this editorial at the end of June as summer officially begins. Much of the country—my New Mexico home included—is suffering under an unbearable heat wave in which even those without belief pray for rain. Summer for many is associated with vacations, family trips, and happy hours in the swimming pool among other enjoyable activities that provide a welcome and much deserved break from routine and relief from the grind of work and school. In the words of the George Gershwin tune, “Summertime, and the livin’ is easy.”
In stark contrast to this season, where there is more lightness in being, is the heaviness of the news reports about the Department of Veteran Affairs (VA) that have been featured in the media and the federal press. I suspect I am not alone in having a hard time opening those e-mails; feeling once more the weight of failure on the VA and the employees who have dedicated a good part of their careers to its mission. Even for the VA, June has seen an exceptional string of bad press. I ask as you read this column to think about what the adjective bad means in this context. In the conclusion to this column, I will suggest that the meaning is multivalent.
Among the most distressing stories was the USA Today and Boston Globe headline, “Secret VA nursing home ratings hide poor quality of care from the public.”2 In an all too predictable sequence, this led justifiably to a cascade of demands from the fifth estate, congressional representatives, the administration, veterans and their families, and watchdog organizations for release of the data, investigation of the allegedly deplorable conditions, and rapid fixes to the problems along with the punishment of the guilty.
As an ethicist I am committed to the principles of transparency and accountability that these entities rightly adjure in the wake of any disclosure of a breach of duty to treat each veteran with the best we have—especially the disabled, elderly, and vulnerable. But I have come to believe that the way in which this cycle of scandal and reaction plays out over and over again in VA facilities across the country, what I call “caring under the microscope,” is actually undermining the righteous goals it seeks to achieve.
I encourage you to try this online. Search for the phrase, “VA under microscope” and see what you get. Briefly read the summary, or the entire story if you have the inclination, and then take a few minutes to reflect on the emotional impact of what you read. Under a microscope is an idiom coined to capture the experience of being the object of close inspection and intense scrutiny. As most everyone knows from their own science education, microscopes magnify images that cannot normally be seen with the human eye, allowing us to observe a more detailed and focused image. The microscope surely helped revolutionize medicine and science. But what effect does such amplified and constant observation have on VA employees?3
For the thousands of staff members who do their job every day with all the empathy and skill, integrity, and dedication they can muster, there is demoralization. Researchers in the health professions describe it as “a feeling state of dejection, hopelessness, and a sense of personal ‘incompetence’ that may be tied to a loss of or threat to one’s own goals or values. It has an existential dimension when beliefs and values about oneself are disconfirmed.”4 If you are a nurse assigned to one of VA’s nursing homes, daily striving to ensure patients are clean and comfortable, or a therapist in a continuing living center using all your training to maximize an elder’s mobility and participation in activities, you might well begin to doubt your ability as a professional and question the worth of your work. This is exactly the opposite outcome that the microscopic oversight is intended to attain.
The impact of demoralization on health professionals directly contributes to unprecedented burnout and turnover. Were this not damaging enough, it also has an insidious rippling effect—like contaminated groundwater that poisons where it should be reviving. The humanistic, even spiritual, heart of all the health professions is the relationship between the practitioner and the patient, ideally a relationship of mutual respect and trust. Waves of negative news triggering harsh and unyielding criticism distort even the strongest, purest therapeutic alliances with fear and distrust, just as a microscope not properly focused changes a beautiful image into a blurred muddle.
Worried families of veterans staring at this picture invariably are drawn into the hyper media focus, feeling alarmed and betrayed, even when their loved one may be receiving excellent VA care. In 20 years as a physician and ethicist in VA hospitals, clinics, and community living centers, I know well that bad things happen to good people (both patients and staff). Yet VA patients, families, and staff are seldom offered the wider corrective vision that would note that bad things also happen in other health care institutions and good care is delivered in the VA. Acting Secretary of Veterans Affairs Peter O’Rourke crisply summarized in his response to the nursing home story.5
No veteran or any other human being in a VA or any other nursing home should ever be medicated into a zombie state or left alone in pain like those patients reported in the news story. And if the USA Today story improves the care of a single VA patient, then good has been done at least in the short run. Yet we must also take the long view and consider the moral and psychological outcome of prolonged demoralization on the very staff who must carry out the congressional mandates.
In the same time frame as the nursing home scandal, the VA Office of Inspector General also issued a report on the continued understaffing in the VA.6 This may be the most concerning aftermath of demoralization. One of my best residents had thought about the VA but in the end made a different choice when he completed his training. When I asked him why he told me, “I am afraid to end up in the newspaper.”
Summer will go by far too quickly. Enjoy it while you can so that with renewed strength we may all search for a better way that the light of truth and heat of power can do what they must while also not withering the spirit of caring that animates the people of the VA.
I write this editorial at the end of June as summer officially begins. Much of the country—my New Mexico home included—is suffering under an unbearable heat wave in which even those without belief pray for rain. Summer for many is associated with vacations, family trips, and happy hours in the swimming pool among other enjoyable activities that provide a welcome and much deserved break from routine and relief from the grind of work and school. In the words of the George Gershwin tune, “Summertime, and the livin’ is easy.”
In stark contrast to this season, where there is more lightness in being, is the heaviness of the news reports about the Department of Veteran Affairs (VA) that have been featured in the media and the federal press. I suspect I am not alone in having a hard time opening those e-mails; feeling once more the weight of failure on the VA and the employees who have dedicated a good part of their careers to its mission. Even for the VA, June has seen an exceptional string of bad press. I ask as you read this column to think about what the adjective bad means in this context. In the conclusion to this column, I will suggest that the meaning is multivalent.
Among the most distressing stories was the USA Today and Boston Globe headline, “Secret VA nursing home ratings hide poor quality of care from the public.”2 In an all too predictable sequence, this led justifiably to a cascade of demands from the fifth estate, congressional representatives, the administration, veterans and their families, and watchdog organizations for release of the data, investigation of the allegedly deplorable conditions, and rapid fixes to the problems along with the punishment of the guilty.
As an ethicist I am committed to the principles of transparency and accountability that these entities rightly adjure in the wake of any disclosure of a breach of duty to treat each veteran with the best we have—especially the disabled, elderly, and vulnerable. But I have come to believe that the way in which this cycle of scandal and reaction plays out over and over again in VA facilities across the country, what I call “caring under the microscope,” is actually undermining the righteous goals it seeks to achieve.
I encourage you to try this online. Search for the phrase, “VA under microscope” and see what you get. Briefly read the summary, or the entire story if you have the inclination, and then take a few minutes to reflect on the emotional impact of what you read. Under a microscope is an idiom coined to capture the experience of being the object of close inspection and intense scrutiny. As most everyone knows from their own science education, microscopes magnify images that cannot normally be seen with the human eye, allowing us to observe a more detailed and focused image. The microscope surely helped revolutionize medicine and science. But what effect does such amplified and constant observation have on VA employees?3
For the thousands of staff members who do their job every day with all the empathy and skill, integrity, and dedication they can muster, there is demoralization. Researchers in the health professions describe it as “a feeling state of dejection, hopelessness, and a sense of personal ‘incompetence’ that may be tied to a loss of or threat to one’s own goals or values. It has an existential dimension when beliefs and values about oneself are disconfirmed.”4 If you are a nurse assigned to one of VA’s nursing homes, daily striving to ensure patients are clean and comfortable, or a therapist in a continuing living center using all your training to maximize an elder’s mobility and participation in activities, you might well begin to doubt your ability as a professional and question the worth of your work. This is exactly the opposite outcome that the microscopic oversight is intended to attain.
The impact of demoralization on health professionals directly contributes to unprecedented burnout and turnover. Were this not damaging enough, it also has an insidious rippling effect—like contaminated groundwater that poisons where it should be reviving. The humanistic, even spiritual, heart of all the health professions is the relationship between the practitioner and the patient, ideally a relationship of mutual respect and trust. Waves of negative news triggering harsh and unyielding criticism distort even the strongest, purest therapeutic alliances with fear and distrust, just as a microscope not properly focused changes a beautiful image into a blurred muddle.
Worried families of veterans staring at this picture invariably are drawn into the hyper media focus, feeling alarmed and betrayed, even when their loved one may be receiving excellent VA care. In 20 years as a physician and ethicist in VA hospitals, clinics, and community living centers, I know well that bad things happen to good people (both patients and staff). Yet VA patients, families, and staff are seldom offered the wider corrective vision that would note that bad things also happen in other health care institutions and good care is delivered in the VA. Acting Secretary of Veterans Affairs Peter O’Rourke crisply summarized in his response to the nursing home story.5
No veteran or any other human being in a VA or any other nursing home should ever be medicated into a zombie state or left alone in pain like those patients reported in the news story. And if the USA Today story improves the care of a single VA patient, then good has been done at least in the short run. Yet we must also take the long view and consider the moral and psychological outcome of prolonged demoralization on the very staff who must carry out the congressional mandates.
In the same time frame as the nursing home scandal, the VA Office of Inspector General also issued a report on the continued understaffing in the VA.6 This may be the most concerning aftermath of demoralization. One of my best residents had thought about the VA but in the end made a different choice when he completed his training. When I asked him why he told me, “I am afraid to end up in the newspaper.”
Summer will go by far too quickly. Enjoy it while you can so that with renewed strength we may all search for a better way that the light of truth and heat of power can do what they must while also not withering the spirit of caring that animates the people of the VA.
1. Camus A. O’Brien J, trans. The Myth of Sisyphus and Other Essays. New York, New York: Vintage Books, 1955.
2. Slack D, Estes A. Secret VA nursing home ratings hide poor quality of care from the public. USA Today. June 17, 2018. https://www.usatoday.com/story/news/politics/2018/06/17/secret-va-nursing-home-ratings-hide-poor-quality-care/674829002. Accessed June 25, 2018.
3. Gabel S. Demoralization in health professional practice: development, amelioration, and, implications for continuing education.” J Contin Educ Health Prof. 2013;33(2):118-126.
4. Hanlon A. How the microscope redefined the fact. T he Atlantic. February 11, 2016. https://www.theatlantic.com/technology/archive/2016/02/microscope-history-data/462234. Accessed June 27, 2018.
5. O’Rourke P. VA: USA Today’s article is misleading. USA Today. June 20, 2018. https://www.usatoday.com/story/opinion/2018/06/20/va-usa-today-article-misleading-editorials-debates/36223067. Updated June 21, 2018. Accessed June 27, 2018.
6. US Department of Veterans Affairs, Office of the Inspector General. OIG determination of Veterans Health Administration’s occupational staffing shortages. https://www.va.gov/oig/pubs/VAOIG-18-01693-196.pdf. Published June 14, 2018. Accessed June 25, 2018.
1. Camus A. O’Brien J, trans. The Myth of Sisyphus and Other Essays. New York, New York: Vintage Books, 1955.
2. Slack D, Estes A. Secret VA nursing home ratings hide poor quality of care from the public. USA Today. June 17, 2018. https://www.usatoday.com/story/news/politics/2018/06/17/secret-va-nursing-home-ratings-hide-poor-quality-care/674829002. Accessed June 25, 2018.
3. Gabel S. Demoralization in health professional practice: development, amelioration, and, implications for continuing education.” J Contin Educ Health Prof. 2013;33(2):118-126.
4. Hanlon A. How the microscope redefined the fact. T he Atlantic. February 11, 2016. https://www.theatlantic.com/technology/archive/2016/02/microscope-history-data/462234. Accessed June 27, 2018.
5. O’Rourke P. VA: USA Today’s article is misleading. USA Today. June 20, 2018. https://www.usatoday.com/story/opinion/2018/06/20/va-usa-today-article-misleading-editorials-debates/36223067. Updated June 21, 2018. Accessed June 27, 2018.
6. US Department of Veterans Affairs, Office of the Inspector General. OIG determination of Veterans Health Administration’s occupational staffing shortages. https://www.va.gov/oig/pubs/VAOIG-18-01693-196.pdf. Published June 14, 2018. Accessed June 25, 2018.
Do carbs drive obesity? With evidence inconclusive, debate continues
While the debate continues, David S. Ludwig, MD, PhD, and Cara B. Ebbeling, PhD, argued in a recent clinical review that diet does indeed affect metabolism and body composition.
While evidence from human studies remains limited, animal research findings are consistent with a carbohydrate-insulin model of obesity, according to Dr. Ludwig and Dr. Ebbeling, who are with the New Balance Foundation Obesity Prevention Center at Boston Children’s Hospital and Harvard Medical School.
The carbohydrate-insulin model holds that eating processed, high–glycemic load carbohydrates causes hormonal changes that promote calorie deposition in fat tissue, aggravate hunger, and reduce energy expenditure, they said in JAMA Internal Medicine.
“The conventional way of thinking assumes that the individual has primary control over their calorie balance, and thus, bases conventional treatment on a target of establishing a negative energy balance – so that is 1,000 variations of the ‘eat less, move more’ recommendation,” Dr. Ludwig said in an interview.
The alternative to that established view has proven controversial. The Endocrine Society, in a recent scientific statement, said diet’s effect on obesity risk is largely explainable by calorie intake, rather than some special adverse effect on internal metabolism or energy expenditure.
“Stated differently, ‘a calorie is a calorie,’ ” the authors of the scientific statement said. “Thus, habitual consumption of highly palatable and energy-dense diets predispose to excess weight gain irrespective of macronutrient content.”
Others have sought to refute the carbohydrate-insulin hypothesis in recent reviews, such as an invited commentary in JAMA Internal Medicine by Kevin D. Hall, PhD, of the National Institute of Diabetes and Digestive and Kidney Diseases, and his coauthors.
“Although it is plausible that variables related to insulin signaling could be involved in obesity pathogenesis, the hypothesis that carbohydrate-stimulated insulin secretion is the primary cause of common obesity via direct effects on adipocytes is difficult to reconcile with current evidence,” Dr. Hall and his coauthors wrote in the commentary (JAMA Intern Med. 2018 Jul 2. doi: 10.1001/jamainternmed.2018.2920).
The conventional calorie balance model is a “straw man” that omits neuroendocrine mechanisms known to regulate homeostasis, added Dr. Hall and his coauthors, stating that accurate models of obesity should include physiological processes resisting weight loss and promoting weight gain.
“They might claim that this is a straw man argument, but I would claim that there is a case of the emperor’s new clothing,” Dr. Ludwig countered in the interview. “They argue that body weight is controlled by biology, and that that’s recognized in the conventional view, but how does that view inform treatment in any way? In the absence of any specific testable hypotheses for why the obesity epidemic has emerged so suddenly, conventional recommendations inevitably resort to advice to ‘eat less and move more.’ ”
Dr. Ludwig and Dr. Ebbeling have both conducted research studies examining the carbohydrate-insulin model, or the view that a high-carbohydrate diet results in postprandial hyperinsulinemia and promotes deposition of calories in adipocytes, leading to weight gain through slowing metabolism, increased hunger, or both.
In a study published in the Lancet, Dr. Ludwig and his coinvestigators found that rats fed a high–glycemic index (GI) diet for 18 weeks had more body fat (97.8 grams vs. 57.3 grams; P = .0152) and less lean body mass versus rats fed a low-GI diet. Rats on the high-GI diet also had greater increases over time in blood glucose and plasma insulin after oral glucose. Similarly, mice on a high-GI diet had nearly twice the body fat of mice on low-GI diet, after 9 weeks of feeding (Lancet. 2004 Aug 28. doi: 10.1016/S0140-6736(04)16937-7).
“There’s no way to explain that finding in view of the conventional view that all calories are alike to the body,” Dr. Ludwig said.
“Contrary to prediction of the conventional model, the inherently lower energy density of low-fat diets does not spontaneously produce sustained weight loss. In fact, several recent meta-analyses found that low-fat diets are inferior to all higher-fat [and thus low-glycemic] comparisons. However, these studies characteristically rely on dietary counseling, a method with limitations for testing mechanistic hypotheses owing to varying levels of noncompliance over the long-term,” Dr. Ludwig and Dr. Ebbeling wrote.
Criticisms that claim to refute the carbohydrate-insulin hypothesis are based in part on misinterpretation of recent feeding studies, according to Dr. Ludwig and Dr. Ebbeling. Multiple studies testing whether or not high–glycemic load meals lead to increased fat storage have reported no meaningful differences between low-fat and low-carbohydrate diets. However, these short-term studies, mostly 2 weeks in duration, preclude definitive findings, according to the review.
That’s because the process of adapting to a high-fat diet after having consumed a high-carbohydrate diet takes weeks, which is a well-recognized phenomenon, Dr. Ludwig said.
“If you put sedentary people into military boot camp and tested their biological state after 6 days, you’d probably find that they were fatigued, weak, and had higher inflammation in their muscles, but clearly, you wouldn’t conclude that fitness training is bad for your health,” he said in the interview. “But yet, these are the sort of data that are being used to ‘falsify’ the carbohydrate-insulin model.
“We acknowledge that there aren’t definitive human data,” he continued, “but the conventional model has failed to both explain the obesity epidemic and control it, and the latest public health data suggests that rates are higher today than ever before, despite 50 years of focusing on calorie balance.”
SOURCE: Ludwig DS et al. JAMA Intern Med. 2018 Jul 2. doi:10.1001/jamainternmed.2018.2933.
While the debate continues, David S. Ludwig, MD, PhD, and Cara B. Ebbeling, PhD, argued in a recent clinical review that diet does indeed affect metabolism and body composition.
While evidence from human studies remains limited, animal research findings are consistent with a carbohydrate-insulin model of obesity, according to Dr. Ludwig and Dr. Ebbeling, who are with the New Balance Foundation Obesity Prevention Center at Boston Children’s Hospital and Harvard Medical School.
The carbohydrate-insulin model holds that eating processed, high–glycemic load carbohydrates causes hormonal changes that promote calorie deposition in fat tissue, aggravate hunger, and reduce energy expenditure, they said in JAMA Internal Medicine.
“The conventional way of thinking assumes that the individual has primary control over their calorie balance, and thus, bases conventional treatment on a target of establishing a negative energy balance – so that is 1,000 variations of the ‘eat less, move more’ recommendation,” Dr. Ludwig said in an interview.
The alternative to that established view has proven controversial. The Endocrine Society, in a recent scientific statement, said diet’s effect on obesity risk is largely explainable by calorie intake, rather than some special adverse effect on internal metabolism or energy expenditure.
“Stated differently, ‘a calorie is a calorie,’ ” the authors of the scientific statement said. “Thus, habitual consumption of highly palatable and energy-dense diets predispose to excess weight gain irrespective of macronutrient content.”
Others have sought to refute the carbohydrate-insulin hypothesis in recent reviews, such as an invited commentary in JAMA Internal Medicine by Kevin D. Hall, PhD, of the National Institute of Diabetes and Digestive and Kidney Diseases, and his coauthors.
“Although it is plausible that variables related to insulin signaling could be involved in obesity pathogenesis, the hypothesis that carbohydrate-stimulated insulin secretion is the primary cause of common obesity via direct effects on adipocytes is difficult to reconcile with current evidence,” Dr. Hall and his coauthors wrote in the commentary (JAMA Intern Med. 2018 Jul 2. doi: 10.1001/jamainternmed.2018.2920).
The conventional calorie balance model is a “straw man” that omits neuroendocrine mechanisms known to regulate homeostasis, added Dr. Hall and his coauthors, stating that accurate models of obesity should include physiological processes resisting weight loss and promoting weight gain.
“They might claim that this is a straw man argument, but I would claim that there is a case of the emperor’s new clothing,” Dr. Ludwig countered in the interview. “They argue that body weight is controlled by biology, and that that’s recognized in the conventional view, but how does that view inform treatment in any way? In the absence of any specific testable hypotheses for why the obesity epidemic has emerged so suddenly, conventional recommendations inevitably resort to advice to ‘eat less and move more.’ ”
Dr. Ludwig and Dr. Ebbeling have both conducted research studies examining the carbohydrate-insulin model, or the view that a high-carbohydrate diet results in postprandial hyperinsulinemia and promotes deposition of calories in adipocytes, leading to weight gain through slowing metabolism, increased hunger, or both.
In a study published in the Lancet, Dr. Ludwig and his coinvestigators found that rats fed a high–glycemic index (GI) diet for 18 weeks had more body fat (97.8 grams vs. 57.3 grams; P = .0152) and less lean body mass versus rats fed a low-GI diet. Rats on the high-GI diet also had greater increases over time in blood glucose and plasma insulin after oral glucose. Similarly, mice on a high-GI diet had nearly twice the body fat of mice on low-GI diet, after 9 weeks of feeding (Lancet. 2004 Aug 28. doi: 10.1016/S0140-6736(04)16937-7).
“There’s no way to explain that finding in view of the conventional view that all calories are alike to the body,” Dr. Ludwig said.
“Contrary to prediction of the conventional model, the inherently lower energy density of low-fat diets does not spontaneously produce sustained weight loss. In fact, several recent meta-analyses found that low-fat diets are inferior to all higher-fat [and thus low-glycemic] comparisons. However, these studies characteristically rely on dietary counseling, a method with limitations for testing mechanistic hypotheses owing to varying levels of noncompliance over the long-term,” Dr. Ludwig and Dr. Ebbeling wrote.
Criticisms that claim to refute the carbohydrate-insulin hypothesis are based in part on misinterpretation of recent feeding studies, according to Dr. Ludwig and Dr. Ebbeling. Multiple studies testing whether or not high–glycemic load meals lead to increased fat storage have reported no meaningful differences between low-fat and low-carbohydrate diets. However, these short-term studies, mostly 2 weeks in duration, preclude definitive findings, according to the review.
That’s because the process of adapting to a high-fat diet after having consumed a high-carbohydrate diet takes weeks, which is a well-recognized phenomenon, Dr. Ludwig said.
“If you put sedentary people into military boot camp and tested their biological state after 6 days, you’d probably find that they were fatigued, weak, and had higher inflammation in their muscles, but clearly, you wouldn’t conclude that fitness training is bad for your health,” he said in the interview. “But yet, these are the sort of data that are being used to ‘falsify’ the carbohydrate-insulin model.
“We acknowledge that there aren’t definitive human data,” he continued, “but the conventional model has failed to both explain the obesity epidemic and control it, and the latest public health data suggests that rates are higher today than ever before, despite 50 years of focusing on calorie balance.”
SOURCE: Ludwig DS et al. JAMA Intern Med. 2018 Jul 2. doi:10.1001/jamainternmed.2018.2933.
While the debate continues, David S. Ludwig, MD, PhD, and Cara B. Ebbeling, PhD, argued in a recent clinical review that diet does indeed affect metabolism and body composition.
While evidence from human studies remains limited, animal research findings are consistent with a carbohydrate-insulin model of obesity, according to Dr. Ludwig and Dr. Ebbeling, who are with the New Balance Foundation Obesity Prevention Center at Boston Children’s Hospital and Harvard Medical School.
The carbohydrate-insulin model holds that eating processed, high–glycemic load carbohydrates causes hormonal changes that promote calorie deposition in fat tissue, aggravate hunger, and reduce energy expenditure, they said in JAMA Internal Medicine.
“The conventional way of thinking assumes that the individual has primary control over their calorie balance, and thus, bases conventional treatment on a target of establishing a negative energy balance – so that is 1,000 variations of the ‘eat less, move more’ recommendation,” Dr. Ludwig said in an interview.
The alternative to that established view has proven controversial. The Endocrine Society, in a recent scientific statement, said diet’s effect on obesity risk is largely explainable by calorie intake, rather than some special adverse effect on internal metabolism or energy expenditure.
“Stated differently, ‘a calorie is a calorie,’ ” the authors of the scientific statement said. “Thus, habitual consumption of highly palatable and energy-dense diets predispose to excess weight gain irrespective of macronutrient content.”
Others have sought to refute the carbohydrate-insulin hypothesis in recent reviews, such as an invited commentary in JAMA Internal Medicine by Kevin D. Hall, PhD, of the National Institute of Diabetes and Digestive and Kidney Diseases, and his coauthors.
“Although it is plausible that variables related to insulin signaling could be involved in obesity pathogenesis, the hypothesis that carbohydrate-stimulated insulin secretion is the primary cause of common obesity via direct effects on adipocytes is difficult to reconcile with current evidence,” Dr. Hall and his coauthors wrote in the commentary (JAMA Intern Med. 2018 Jul 2. doi: 10.1001/jamainternmed.2018.2920).
The conventional calorie balance model is a “straw man” that omits neuroendocrine mechanisms known to regulate homeostasis, added Dr. Hall and his coauthors, stating that accurate models of obesity should include physiological processes resisting weight loss and promoting weight gain.
“They might claim that this is a straw man argument, but I would claim that there is a case of the emperor’s new clothing,” Dr. Ludwig countered in the interview. “They argue that body weight is controlled by biology, and that that’s recognized in the conventional view, but how does that view inform treatment in any way? In the absence of any specific testable hypotheses for why the obesity epidemic has emerged so suddenly, conventional recommendations inevitably resort to advice to ‘eat less and move more.’ ”
Dr. Ludwig and Dr. Ebbeling have both conducted research studies examining the carbohydrate-insulin model, or the view that a high-carbohydrate diet results in postprandial hyperinsulinemia and promotes deposition of calories in adipocytes, leading to weight gain through slowing metabolism, increased hunger, or both.
In a study published in the Lancet, Dr. Ludwig and his coinvestigators found that rats fed a high–glycemic index (GI) diet for 18 weeks had more body fat (97.8 grams vs. 57.3 grams; P = .0152) and less lean body mass versus rats fed a low-GI diet. Rats on the high-GI diet also had greater increases over time in blood glucose and plasma insulin after oral glucose. Similarly, mice on a high-GI diet had nearly twice the body fat of mice on low-GI diet, after 9 weeks of feeding (Lancet. 2004 Aug 28. doi: 10.1016/S0140-6736(04)16937-7).
“There’s no way to explain that finding in view of the conventional view that all calories are alike to the body,” Dr. Ludwig said.
“Contrary to prediction of the conventional model, the inherently lower energy density of low-fat diets does not spontaneously produce sustained weight loss. In fact, several recent meta-analyses found that low-fat diets are inferior to all higher-fat [and thus low-glycemic] comparisons. However, these studies characteristically rely on dietary counseling, a method with limitations for testing mechanistic hypotheses owing to varying levels of noncompliance over the long-term,” Dr. Ludwig and Dr. Ebbeling wrote.
Criticisms that claim to refute the carbohydrate-insulin hypothesis are based in part on misinterpretation of recent feeding studies, according to Dr. Ludwig and Dr. Ebbeling. Multiple studies testing whether or not high–glycemic load meals lead to increased fat storage have reported no meaningful differences between low-fat and low-carbohydrate diets. However, these short-term studies, mostly 2 weeks in duration, preclude definitive findings, according to the review.
That’s because the process of adapting to a high-fat diet after having consumed a high-carbohydrate diet takes weeks, which is a well-recognized phenomenon, Dr. Ludwig said.
“If you put sedentary people into military boot camp and tested their biological state after 6 days, you’d probably find that they were fatigued, weak, and had higher inflammation in their muscles, but clearly, you wouldn’t conclude that fitness training is bad for your health,” he said in the interview. “But yet, these are the sort of data that are being used to ‘falsify’ the carbohydrate-insulin model.
“We acknowledge that there aren’t definitive human data,” he continued, “but the conventional model has failed to both explain the obesity epidemic and control it, and the latest public health data suggests that rates are higher today than ever before, despite 50 years of focusing on calorie balance.”
SOURCE: Ludwig DS et al. JAMA Intern Med. 2018 Jul 2. doi:10.1001/jamainternmed.2018.2933.
FROM JAMA INTERNAL MEDICINE
Diet and Dermatology: Google Search Results for Acne, Psoriasis, and Eczema
Researching medical information currently is the third most common use of the Internet in the United States,1 with the majority of adults using the Web as their first source for health information before seeing a physician.2 When assessing health-related information online, resources can be grouped into 4 categories: (1) those attributed to self-proclaimed experts, (2) promotional, (3) social media, and (4) educational.3 Access to such a wide range of sources may give readers the opportunity to share personal anecdotes and opinions, thereby serving as a forum for information that essentially cannot be validated. Although such websites may include useful information and cite current literature, in other instances health-related information may be misleading or fabricated.3
In a study evaluating 291 skin conditions and related Google trends, acne, psoriasis, and eczema were among the most burdensome diseases, with acne yielding the highest number of search results.4 Results of the study indicated a positive correlation between disease burden and online search interest.4 The impact of these online searches and the validity of Google search results are topics worth considering, as more dermatology patients are relying on holistic and nonpharmaceutical approaches to treatment and disease management.5 The purpose of this study was to evaluate content on diet and dermatology available on the Internet for acne, psoriasis, and eczema.
Methods
Google searches were performed in December 2017 using the terms diet and acne, diet and psoriasis, and diet and eczema. The first 10 results for each respective search were reviewed for recommendations about which foods to incorporate in the diet and which to avoid. They also were classified according to the following 4 website categories: (1) those attributed to self-proclaimed experts, (2) promotional, (3) social media, and (4) educational. The recommendations gathered from the 30 websites were then compared to the current literature assessing the impact of diet on these respective conditions by conducting PubMed searches of articles indexed for MEDLINE using the same terms.
Results
The results of this study are outlined in the eTable.

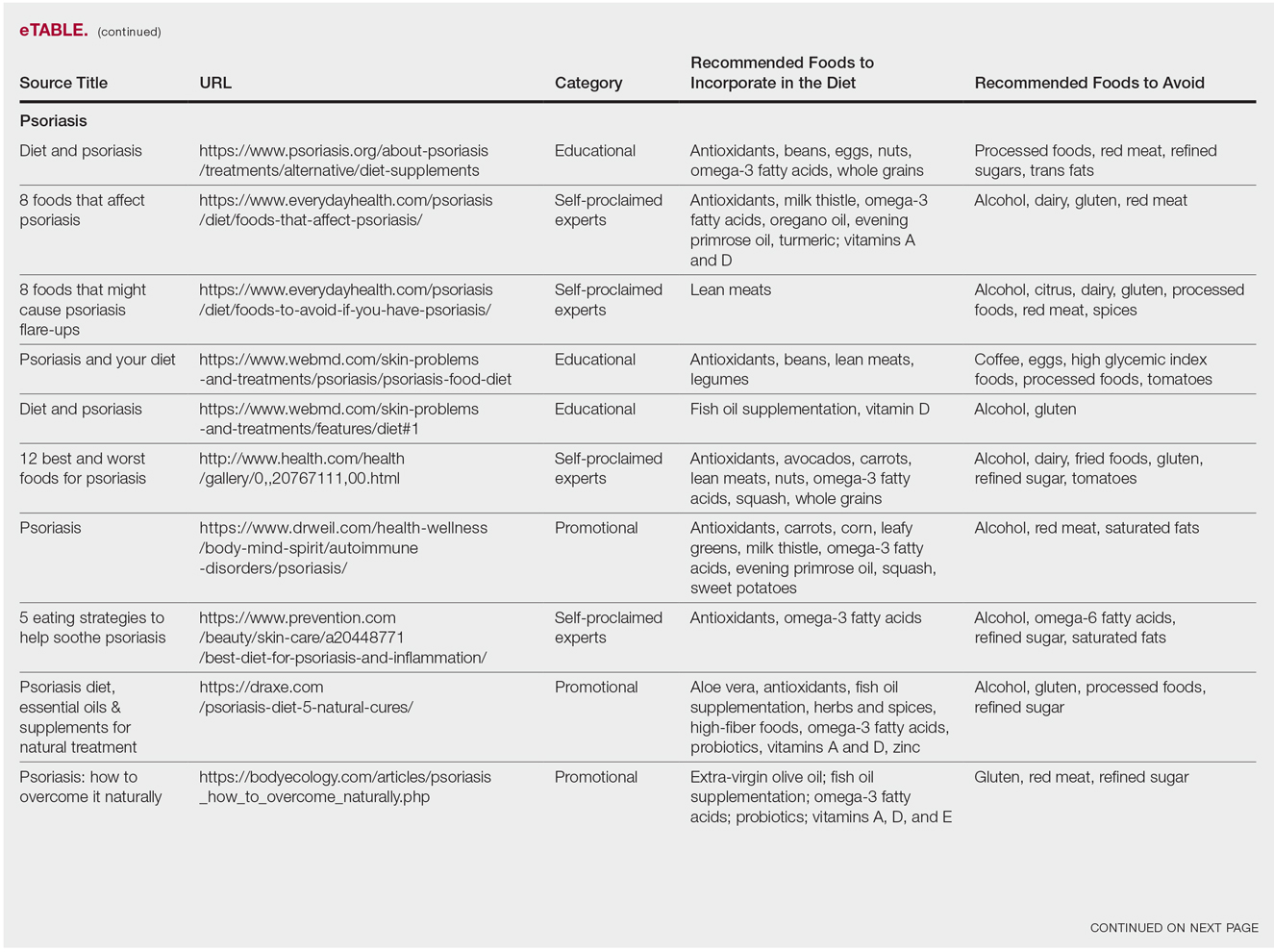
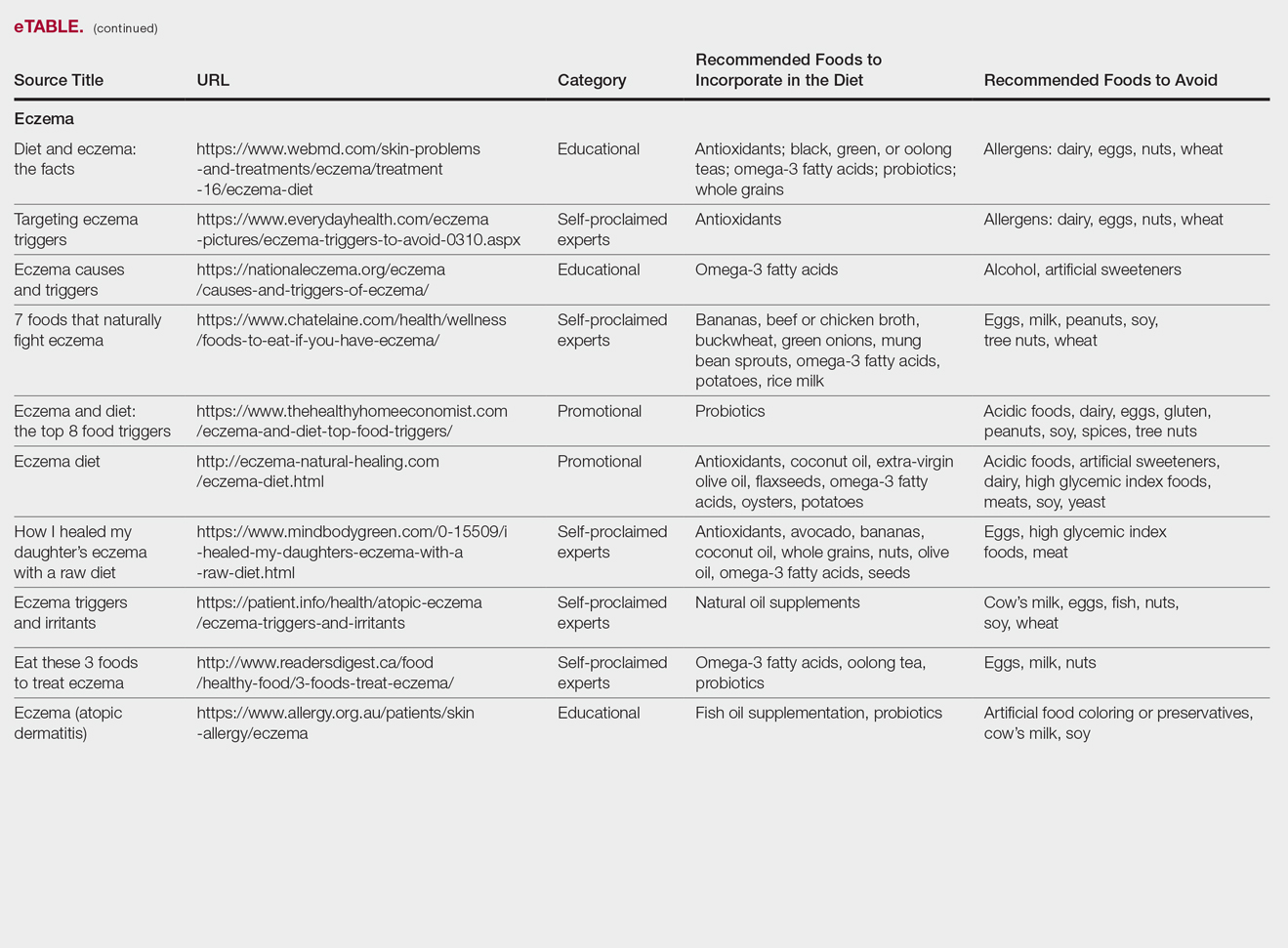
Acne
Our Google search using the term diet and acne produced 17,500,000 results. Of the first 10 search results, 40% (4/10) were websites attributed to self-proclaimed experts, 40% (4/10) were educational resources, and 20% (2/10) were promotional websites. Most of the websites advised acne patients to avoid high glycemic index foods (90% [9/10]) and dairy products (90% [9/10]). When discussing which foods to include in the diet, 70% (7/10) of websites recommended that patients incorporate omega-3 fatty acids and antioxidants in the diet.
Research has shown that a low glycemic index diet can lead to a decrease in patients’ acne lesion counts in some instances.6,7 In a case-controlled study of 2258 patients on a popular weight loss diet that emphasized low glycemic index foods, 87% of participants reported a reduction in acne and 91% reported a decrease in their dosage or number of acne medications.7 Still, the exact correlation between acne development and consumption of glycemic index foods has not been confirmed. However, high glycemic index diets have been linked to hyperinsulinemia, indicating that insulin levels may play a role in acne formation.8 The majority of other currently available studies evaluated the potential link between dairy consumption and acne. A retrospective analysis of 47,355 women spanning 12 weeks showed a positive link between increased dairy consumption, specifically skim milk, and acne formation. Despite the positive trend, limitations such as recall bias made it difficult to draw a conclusion based on these findings.9 However, results of a longitudinal questionnaire-based population study evaluating the impact of dairy consumption on acne in 2489 adolescent patients confirmed a positive correlation.10 Studies conducted in 2009 and 2011 concluded that milk consumption results in elevated insulinlike growth factor 1 levels, which were linked to comedogenesis.8,11
Currently, there are well-described mechanisms to explain the association of dairy consumption and glycemic index with acne. Confirming a correlation between acne development and dairy consumption suggests that a dairy-free diet may benefit acne patients.5 Other trials indicate that low glycemic index diets are beneficial in treating acne.6,7 Therefore, some of the recommendations made in our search results may be of merit; however, there is minimal evidence proving the benefits of the other dietary recommendations made in the websites we evaluated.
Psoriasis
Our Google search using the term diet and psoriasis yielded a total of 9,420,000 results. Of the first 10 search results, 40% (4/10) were websites attributed to self-proclaimed experts, 30% (3/10) were promotional, and 30% (3/10) were educational. Seventy percent (7/10) of websites recommended avoiding alcohol and 60% (6/10) recommended avoiding gluten, with others discouraging consumption of red meat. Most of the websites encouraged patients to consume omega-3 fatty acids and antioxidants, while a few also recommended vitamins A, D, and E, as well as evening primrose oil supplements.
Although current research indicates a positive correlation between excessive alcohol use and psoriasis severity, it is still unclear whether alcohol consumption can be directly linked to the disease.12-14 Likewise, despite belief that increased oxidative stress likely contributes to inflammation in psoriasis, there is little evidence linking antioxidants to improvement in psoriasis symptoms.12 However, the current literature is inconsistent regarding the effects of fish oil supplementation on psoriasis.12 In a randomized double-blind study of 145 patients, there was no significant difference in psoriasis area and severity index scores between a control group and a treatment group receiving fish oil supplementation.15 In another RCT of 45 participants, those given daily very long-chain omega-3 fatty acid supplements saw no difference in psoriasis symptoms.15 Despite debate, literature assessing the impact of gluten-free diets has described improvement in psoriasis lesions in patients with celiac-specific antibodies.16 Although some observational studies described vitamin D supplementation to be beneficial in the treatment of psoriatic lesions, a more recent RCT found no significant difference between control and treatment groups.17-19
Studies also have revealed that certain eating patterns, such as those associated with the Mediterranean diet that is rich in fruits, vegetables, whole grains, and omega-3 fatty acids may be linked to improved endothelial function scores and reduced C-reactive protein and IL-18levels.20,21 In a double-blind RCT of 75 patients with plaque psoriasis, mean (SD) psoriasis area and severity index scores decreased by 11.2 (9.8) in a group treated with omega-3 fatty acids compared to 7.5 (8.8) with omega-6 fatty acids (P=.048).22
Although excessive alcohol use may be linked to psoriasis, there is no conclusive evidence indicating causation, thereby discrediting online claims.12-14 Research has revealed that gluten-free diets in psoriasis patients with celiac disease may improve psoriasis treatment16; however, sufficient evidence is lacking for diets low in gluten and high in polyunsaturated fatty acids or antioxidant supplementation. Of the dietary supplements recommended in the search results we reviewed, fish oil appears to be the most promising, but no recommendations can be made based on the current research.
Eczema
Our Google search using the term diet and eczema yielded 1,160,000 results, with 50% (5/10) of websites attributed to self-proclaimed experts, 30% (3/10) to educational websites, and 20% (2/10) to promotional sites. Of the first 10 results, 80% (8/10) recommended that patients with eczema avoid milk/dairy and 50% (5/10) advised to avoid soy and wheat/gluten. Other websites indicated to avoid eggs, nuts, and artificial sweeteners. Patients were encouraged to incorporate omega-3 fatty acids in their diets, and a few sites recommended bananas, coconut oil, olive oil, and various teas.
In a review of 11 studies with a total of 596 participants, supplementation with vitamins D and E, fish oil, olive oil, and linoleic acid was evaluated for the treatment of eczema.23 Although results indicated modest improvement of eczema severity with supplementation of fish oil, evidence favoring this treatment is limited and unconvincing. Furthermore, some evidence indicates that elimination diets are only appropriate for patients with food allergies.24 In a study evaluating an egg-free and dairy-free diet for eczema patients, only participants with positive egg-specific serum IgE levels saw improvement in disease severity.23 Even though IgE-mediated food allergies have been reported in 40% of children with moderate eczema, the contribution of these allergies to eczema is questionable.25
There is little evidence in the literature to indicate a definitive correlation between the foods mentioned in the search results we evaluated and the development of eczema; however, for patients with food allergies and eczema, elimination diets may decrease disease severity.25,26 There is insufficient evidence to suggest a benefit from evening primrose oil or fish oil supplementation, thereby debunking claims found online.
Comment
Although our Google search results included a wide range of sources and information regarding diet and dermatologic conditions such as acne, psoriasis, and eczema, most of the information we found was either unfounded or misleading. Study limitations in the current literature include small sample size, potential recall bias, lack of appropriate controls, incomplete reported results, and the failure to clearly define skin changes.
When considering the accuracy and type of information regarding skin conditions that is available on the Internet, it is important to note that most of the results we reviewed were webpages attributed to self-proclaimed experts. Although educational websites also were included in the search results, whether or not patients prefer or understand the content of such websites is still unknown; therefore, health organizations should consider revising online patient education materials to allow universal comprehension.27
Furthermore, it is important to consider the impact that widespread Internet access may have on the physician-patient relationship. Having access to health-related information online and being able to potentially self-diagnose could delay or deter patients from seeking professional advice or care.3 A study evaluating the impact of online searches on the physician-patient relationship among 175 patients determined that 36.5% of patients gathered information online prior to their consultation with a physician, while 67.3% chose to complement the information given to them by their physician with online resources.28 Based on these statistics, it is important that physicians be up-to-date with Internet discourse to discredit unfounded recommendations. Ultimately, when it comes to diet and dermatology, patients ought to be skeptical of the information currently available on the Internet, given that most of it is unsubstantiated by medical research.
- Fox S. Online health search 2006. Pew Research Center website. http://www.pewinternet.org/2006/10/29/online-health-search-2006/. Published October 29, 2006. Accessed May 3, 2018.
- Prestin A, Vieux SN, Chou WY. Is online health activity alive and well or flatlining? findings from 10 years of the health information national trends survey. J Health Commun. 2015;20:790-798.
- Zeichner JA, Del Rosso JQ. Acne and the internet. Dermatol Clin. 2016;34:129-132.
- Whitsitt J, Karimkhani C, Boyers LN, et al. Comparing burden of dermatologic disease to search interest on Google trends. Dermatol Online J. 2015;21. pii:13030/qt5xg811qp.
- Shokeen D. Influence of diet in acne vulgaris and atopic dermatitis. Cutis. 2016;98:E28-E29.
- Veith WB, Silverberg NB. The association of acne vulgaris with diet. Cutis. 2011;88:84-91.
- Rouhani P. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(3, suppl 1):P706.
- Melnick BC. Evidence for acne-promoting effect of milk and other insulinotropic dairy products. Nestle Nutr Worksop Ser Pediatr Program. 2011;67:131-145.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. High school dietary diary intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Ulvestad M, Bjertness E, Dalgard F, et al. Acne and dairy products in adolescence: results from a Norwegian longitudinal study [published online July 16, 2016]. J Eur Acad Dermatol Venereol. 2017;31:530-535.
- Melnick BC, Schmitz G. Role of insulin, insulin like growth factor 1, hyperglycemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Murzaku EC, Bronsnick T, Rao BK. Diet in dermatology: part II. melanoma, chronic urticaria, and psoriasis. J Am Acad Dermatol. 2014;71:1053.E1-1053.E16.
- Tobin AM, Higgins EM, Norris S, et al. Prevalence of psoriasis in patients with alcoholic liver disease. Clin Exp Dermatol. 2009;34:698-701.
- Kirby B, Richards HL, Mason DL, et al. Alcohol consumption and psychological distress in patients with psoriasis. Br J Dermatol. 2008;158:138-140.
- Søyland E, Funk J, Rajika G, et al. Effect of dietary supplementation with very long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44-51.
- Morimoto S, Yoshikawa K. Psoriasis and vitamin D3. a review of our experience. Arch Dermatol. 1989;125:231-234.
- Smith EL, Pincus SH, Donovan L, et al. A novel approach for the evaluation and treatment of psoriasis. oral or topical use of 1,25-dihydroxyvitamin D3 can be a safe and effective therapy for psoriasis. J Am Acad Dermatol. 1988;19:516-528.
- Siddiqui MA, Al-Khawajah MM. Vitamin D3 and psoriasis: a randomized double-blind placebo-controlled study. J Dermatol Treat. 1990;1:243-245.
- Wang Y, Gao H, Loyd CM, et al. Chronic skin-specific inflammation promotes vascular inflammation and thrombosis. J Invest Dermatol. 2012;132:2067-2075.
- Barrea L, Nappi F, Di Somma C, et al. Environmental risk factors in psoriasis: the point of view of the nutritionist. Int J Environ Res Public Health. 2016;13. pii:E743. doi:10.3390/ijerph13070743.
- Mayser P, Mrowietz U, Arenberger P, et al. Omega-3 fatty acid-based lipid infusion in patients with chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, multicenter trial. J Am Acad Dermatol. 1998;38:539-547.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012;2:CD005205.
- Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer [published online November 15, 2014]. J Am Acad Dermatol. 2014;71:1039.E1-1039.E12.
- Campbell DE. The role of food allergy in childhood atopic dermatitis. J Paediatr Child Health. 2012;48:1058-1064.
- Werfel T, Erdmann S, Fuchs T, et al. Approach to suspected food allergy in atopic dermatitis. guideline of the Task Force on Food Allergy of the German Society of Allergology and Clinical Immunology (DGAKI) and the Medical Association of German Allergologists (ADA) and the German Society of Pediatric Allergology (GPA). J Dtsch Dermatol Ges. 2009;3:265-271.
- John AM, John ES, Hansberry DR, et al. Assessment of online patient education materials from major dermatologic associations. J Clin Aesthet Dermatol. 2016;9:23-28.
- Orgaz-Molina J, Cotugno M, Girón-Prieto MS, et al. A study of internet searches for medical information in dermatology patients: the patient-physician relationship. Actas Dermosifiliogr. 2015;106:493-499.
Researching medical information currently is the third most common use of the Internet in the United States,1 with the majority of adults using the Web as their first source for health information before seeing a physician.2 When assessing health-related information online, resources can be grouped into 4 categories: (1) those attributed to self-proclaimed experts, (2) promotional, (3) social media, and (4) educational.3 Access to such a wide range of sources may give readers the opportunity to share personal anecdotes and opinions, thereby serving as a forum for information that essentially cannot be validated. Although such websites may include useful information and cite current literature, in other instances health-related information may be misleading or fabricated.3
In a study evaluating 291 skin conditions and related Google trends, acne, psoriasis, and eczema were among the most burdensome diseases, with acne yielding the highest number of search results.4 Results of the study indicated a positive correlation between disease burden and online search interest.4 The impact of these online searches and the validity of Google search results are topics worth considering, as more dermatology patients are relying on holistic and nonpharmaceutical approaches to treatment and disease management.5 The purpose of this study was to evaluate content on diet and dermatology available on the Internet for acne, psoriasis, and eczema.
Methods
Google searches were performed in December 2017 using the terms diet and acne, diet and psoriasis, and diet and eczema. The first 10 results for each respective search were reviewed for recommendations about which foods to incorporate in the diet and which to avoid. They also were classified according to the following 4 website categories: (1) those attributed to self-proclaimed experts, (2) promotional, (3) social media, and (4) educational. The recommendations gathered from the 30 websites were then compared to the current literature assessing the impact of diet on these respective conditions by conducting PubMed searches of articles indexed for MEDLINE using the same terms.
Results
The results of this study are outlined in the eTable.



Acne
Our Google search using the term diet and acne produced 17,500,000 results. Of the first 10 search results, 40% (4/10) were websites attributed to self-proclaimed experts, 40% (4/10) were educational resources, and 20% (2/10) were promotional websites. Most of the websites advised acne patients to avoid high glycemic index foods (90% [9/10]) and dairy products (90% [9/10]). When discussing which foods to include in the diet, 70% (7/10) of websites recommended that patients incorporate omega-3 fatty acids and antioxidants in the diet.
Research has shown that a low glycemic index diet can lead to a decrease in patients’ acne lesion counts in some instances.6,7 In a case-controlled study of 2258 patients on a popular weight loss diet that emphasized low glycemic index foods, 87% of participants reported a reduction in acne and 91% reported a decrease in their dosage or number of acne medications.7 Still, the exact correlation between acne development and consumption of glycemic index foods has not been confirmed. However, high glycemic index diets have been linked to hyperinsulinemia, indicating that insulin levels may play a role in acne formation.8 The majority of other currently available studies evaluated the potential link between dairy consumption and acne. A retrospective analysis of 47,355 women spanning 12 weeks showed a positive link between increased dairy consumption, specifically skim milk, and acne formation. Despite the positive trend, limitations such as recall bias made it difficult to draw a conclusion based on these findings.9 However, results of a longitudinal questionnaire-based population study evaluating the impact of dairy consumption on acne in 2489 adolescent patients confirmed a positive correlation.10 Studies conducted in 2009 and 2011 concluded that milk consumption results in elevated insulinlike growth factor 1 levels, which were linked to comedogenesis.8,11
Currently, there are well-described mechanisms to explain the association of dairy consumption and glycemic index with acne. Confirming a correlation between acne development and dairy consumption suggests that a dairy-free diet may benefit acne patients.5 Other trials indicate that low glycemic index diets are beneficial in treating acne.6,7 Therefore, some of the recommendations made in our search results may be of merit; however, there is minimal evidence proving the benefits of the other dietary recommendations made in the websites we evaluated.
Psoriasis
Our Google search using the term diet and psoriasis yielded a total of 9,420,000 results. Of the first 10 search results, 40% (4/10) were websites attributed to self-proclaimed experts, 30% (3/10) were promotional, and 30% (3/10) were educational. Seventy percent (7/10) of websites recommended avoiding alcohol and 60% (6/10) recommended avoiding gluten, with others discouraging consumption of red meat. Most of the websites encouraged patients to consume omega-3 fatty acids and antioxidants, while a few also recommended vitamins A, D, and E, as well as evening primrose oil supplements.
Although current research indicates a positive correlation between excessive alcohol use and psoriasis severity, it is still unclear whether alcohol consumption can be directly linked to the disease.12-14 Likewise, despite belief that increased oxidative stress likely contributes to inflammation in psoriasis, there is little evidence linking antioxidants to improvement in psoriasis symptoms.12 However, the current literature is inconsistent regarding the effects of fish oil supplementation on psoriasis.12 In a randomized double-blind study of 145 patients, there was no significant difference in psoriasis area and severity index scores between a control group and a treatment group receiving fish oil supplementation.15 In another RCT of 45 participants, those given daily very long-chain omega-3 fatty acid supplements saw no difference in psoriasis symptoms.15 Despite debate, literature assessing the impact of gluten-free diets has described improvement in psoriasis lesions in patients with celiac-specific antibodies.16 Although some observational studies described vitamin D supplementation to be beneficial in the treatment of psoriatic lesions, a more recent RCT found no significant difference between control and treatment groups.17-19
Studies also have revealed that certain eating patterns, such as those associated with the Mediterranean diet that is rich in fruits, vegetables, whole grains, and omega-3 fatty acids may be linked to improved endothelial function scores and reduced C-reactive protein and IL-18levels.20,21 In a double-blind RCT of 75 patients with plaque psoriasis, mean (SD) psoriasis area and severity index scores decreased by 11.2 (9.8) in a group treated with omega-3 fatty acids compared to 7.5 (8.8) with omega-6 fatty acids (P=.048).22
Although excessive alcohol use may be linked to psoriasis, there is no conclusive evidence indicating causation, thereby discrediting online claims.12-14 Research has revealed that gluten-free diets in psoriasis patients with celiac disease may improve psoriasis treatment16; however, sufficient evidence is lacking for diets low in gluten and high in polyunsaturated fatty acids or antioxidant supplementation. Of the dietary supplements recommended in the search results we reviewed, fish oil appears to be the most promising, but no recommendations can be made based on the current research.
Eczema
Our Google search using the term diet and eczema yielded 1,160,000 results, with 50% (5/10) of websites attributed to self-proclaimed experts, 30% (3/10) to educational websites, and 20% (2/10) to promotional sites. Of the first 10 results, 80% (8/10) recommended that patients with eczema avoid milk/dairy and 50% (5/10) advised to avoid soy and wheat/gluten. Other websites indicated to avoid eggs, nuts, and artificial sweeteners. Patients were encouraged to incorporate omega-3 fatty acids in their diets, and a few sites recommended bananas, coconut oil, olive oil, and various teas.
In a review of 11 studies with a total of 596 participants, supplementation with vitamins D and E, fish oil, olive oil, and linoleic acid was evaluated for the treatment of eczema.23 Although results indicated modest improvement of eczema severity with supplementation of fish oil, evidence favoring this treatment is limited and unconvincing. Furthermore, some evidence indicates that elimination diets are only appropriate for patients with food allergies.24 In a study evaluating an egg-free and dairy-free diet for eczema patients, only participants with positive egg-specific serum IgE levels saw improvement in disease severity.23 Even though IgE-mediated food allergies have been reported in 40% of children with moderate eczema, the contribution of these allergies to eczema is questionable.25
There is little evidence in the literature to indicate a definitive correlation between the foods mentioned in the search results we evaluated and the development of eczema; however, for patients with food allergies and eczema, elimination diets may decrease disease severity.25,26 There is insufficient evidence to suggest a benefit from evening primrose oil or fish oil supplementation, thereby debunking claims found online.
Comment
Although our Google search results included a wide range of sources and information regarding diet and dermatologic conditions such as acne, psoriasis, and eczema, most of the information we found was either unfounded or misleading. Study limitations in the current literature include small sample size, potential recall bias, lack of appropriate controls, incomplete reported results, and the failure to clearly define skin changes.
When considering the accuracy and type of information regarding skin conditions that is available on the Internet, it is important to note that most of the results we reviewed were webpages attributed to self-proclaimed experts. Although educational websites also were included in the search results, whether or not patients prefer or understand the content of such websites is still unknown; therefore, health organizations should consider revising online patient education materials to allow universal comprehension.27
Furthermore, it is important to consider the impact that widespread Internet access may have on the physician-patient relationship. Having access to health-related information online and being able to potentially self-diagnose could delay or deter patients from seeking professional advice or care.3 A study evaluating the impact of online searches on the physician-patient relationship among 175 patients determined that 36.5% of patients gathered information online prior to their consultation with a physician, while 67.3% chose to complement the information given to them by their physician with online resources.28 Based on these statistics, it is important that physicians be up-to-date with Internet discourse to discredit unfounded recommendations. Ultimately, when it comes to diet and dermatology, patients ought to be skeptical of the information currently available on the Internet, given that most of it is unsubstantiated by medical research.
Researching medical information currently is the third most common use of the Internet in the United States,1 with the majority of adults using the Web as their first source for health information before seeing a physician.2 When assessing health-related information online, resources can be grouped into 4 categories: (1) those attributed to self-proclaimed experts, (2) promotional, (3) social media, and (4) educational.3 Access to such a wide range of sources may give readers the opportunity to share personal anecdotes and opinions, thereby serving as a forum for information that essentially cannot be validated. Although such websites may include useful information and cite current literature, in other instances health-related information may be misleading or fabricated.3
In a study evaluating 291 skin conditions and related Google trends, acne, psoriasis, and eczema were among the most burdensome diseases, with acne yielding the highest number of search results.4 Results of the study indicated a positive correlation between disease burden and online search interest.4 The impact of these online searches and the validity of Google search results are topics worth considering, as more dermatology patients are relying on holistic and nonpharmaceutical approaches to treatment and disease management.5 The purpose of this study was to evaluate content on diet and dermatology available on the Internet for acne, psoriasis, and eczema.
Methods
Google searches were performed in December 2017 using the terms diet and acne, diet and psoriasis, and diet and eczema. The first 10 results for each respective search were reviewed for recommendations about which foods to incorporate in the diet and which to avoid. They also were classified according to the following 4 website categories: (1) those attributed to self-proclaimed experts, (2) promotional, (3) social media, and (4) educational. The recommendations gathered from the 30 websites were then compared to the current literature assessing the impact of diet on these respective conditions by conducting PubMed searches of articles indexed for MEDLINE using the same terms.
Results
The results of this study are outlined in the eTable.



Acne
Our Google search using the term diet and acne produced 17,500,000 results. Of the first 10 search results, 40% (4/10) were websites attributed to self-proclaimed experts, 40% (4/10) were educational resources, and 20% (2/10) were promotional websites. Most of the websites advised acne patients to avoid high glycemic index foods (90% [9/10]) and dairy products (90% [9/10]). When discussing which foods to include in the diet, 70% (7/10) of websites recommended that patients incorporate omega-3 fatty acids and antioxidants in the diet.
Research has shown that a low glycemic index diet can lead to a decrease in patients’ acne lesion counts in some instances.6,7 In a case-controlled study of 2258 patients on a popular weight loss diet that emphasized low glycemic index foods, 87% of participants reported a reduction in acne and 91% reported a decrease in their dosage or number of acne medications.7 Still, the exact correlation between acne development and consumption of glycemic index foods has not been confirmed. However, high glycemic index diets have been linked to hyperinsulinemia, indicating that insulin levels may play a role in acne formation.8 The majority of other currently available studies evaluated the potential link between dairy consumption and acne. A retrospective analysis of 47,355 women spanning 12 weeks showed a positive link between increased dairy consumption, specifically skim milk, and acne formation. Despite the positive trend, limitations such as recall bias made it difficult to draw a conclusion based on these findings.9 However, results of a longitudinal questionnaire-based population study evaluating the impact of dairy consumption on acne in 2489 adolescent patients confirmed a positive correlation.10 Studies conducted in 2009 and 2011 concluded that milk consumption results in elevated insulinlike growth factor 1 levels, which were linked to comedogenesis.8,11
Currently, there are well-described mechanisms to explain the association of dairy consumption and glycemic index with acne. Confirming a correlation between acne development and dairy consumption suggests that a dairy-free diet may benefit acne patients.5 Other trials indicate that low glycemic index diets are beneficial in treating acne.6,7 Therefore, some of the recommendations made in our search results may be of merit; however, there is minimal evidence proving the benefits of the other dietary recommendations made in the websites we evaluated.
Psoriasis
Our Google search using the term diet and psoriasis yielded a total of 9,420,000 results. Of the first 10 search results, 40% (4/10) were websites attributed to self-proclaimed experts, 30% (3/10) were promotional, and 30% (3/10) were educational. Seventy percent (7/10) of websites recommended avoiding alcohol and 60% (6/10) recommended avoiding gluten, with others discouraging consumption of red meat. Most of the websites encouraged patients to consume omega-3 fatty acids and antioxidants, while a few also recommended vitamins A, D, and E, as well as evening primrose oil supplements.
Although current research indicates a positive correlation between excessive alcohol use and psoriasis severity, it is still unclear whether alcohol consumption can be directly linked to the disease.12-14 Likewise, despite belief that increased oxidative stress likely contributes to inflammation in psoriasis, there is little evidence linking antioxidants to improvement in psoriasis symptoms.12 However, the current literature is inconsistent regarding the effects of fish oil supplementation on psoriasis.12 In a randomized double-blind study of 145 patients, there was no significant difference in psoriasis area and severity index scores between a control group and a treatment group receiving fish oil supplementation.15 In another RCT of 45 participants, those given daily very long-chain omega-3 fatty acid supplements saw no difference in psoriasis symptoms.15 Despite debate, literature assessing the impact of gluten-free diets has described improvement in psoriasis lesions in patients with celiac-specific antibodies.16 Although some observational studies described vitamin D supplementation to be beneficial in the treatment of psoriatic lesions, a more recent RCT found no significant difference between control and treatment groups.17-19
Studies also have revealed that certain eating patterns, such as those associated with the Mediterranean diet that is rich in fruits, vegetables, whole grains, and omega-3 fatty acids may be linked to improved endothelial function scores and reduced C-reactive protein and IL-18levels.20,21 In a double-blind RCT of 75 patients with plaque psoriasis, mean (SD) psoriasis area and severity index scores decreased by 11.2 (9.8) in a group treated with omega-3 fatty acids compared to 7.5 (8.8) with omega-6 fatty acids (P=.048).22
Although excessive alcohol use may be linked to psoriasis, there is no conclusive evidence indicating causation, thereby discrediting online claims.12-14 Research has revealed that gluten-free diets in psoriasis patients with celiac disease may improve psoriasis treatment16; however, sufficient evidence is lacking for diets low in gluten and high in polyunsaturated fatty acids or antioxidant supplementation. Of the dietary supplements recommended in the search results we reviewed, fish oil appears to be the most promising, but no recommendations can be made based on the current research.
Eczema
Our Google search using the term diet and eczema yielded 1,160,000 results, with 50% (5/10) of websites attributed to self-proclaimed experts, 30% (3/10) to educational websites, and 20% (2/10) to promotional sites. Of the first 10 results, 80% (8/10) recommended that patients with eczema avoid milk/dairy and 50% (5/10) advised to avoid soy and wheat/gluten. Other websites indicated to avoid eggs, nuts, and artificial sweeteners. Patients were encouraged to incorporate omega-3 fatty acids in their diets, and a few sites recommended bananas, coconut oil, olive oil, and various teas.
In a review of 11 studies with a total of 596 participants, supplementation with vitamins D and E, fish oil, olive oil, and linoleic acid was evaluated for the treatment of eczema.23 Although results indicated modest improvement of eczema severity with supplementation of fish oil, evidence favoring this treatment is limited and unconvincing. Furthermore, some evidence indicates that elimination diets are only appropriate for patients with food allergies.24 In a study evaluating an egg-free and dairy-free diet for eczema patients, only participants with positive egg-specific serum IgE levels saw improvement in disease severity.23 Even though IgE-mediated food allergies have been reported in 40% of children with moderate eczema, the contribution of these allergies to eczema is questionable.25
There is little evidence in the literature to indicate a definitive correlation between the foods mentioned in the search results we evaluated and the development of eczema; however, for patients with food allergies and eczema, elimination diets may decrease disease severity.25,26 There is insufficient evidence to suggest a benefit from evening primrose oil or fish oil supplementation, thereby debunking claims found online.
Comment
Although our Google search results included a wide range of sources and information regarding diet and dermatologic conditions such as acne, psoriasis, and eczema, most of the information we found was either unfounded or misleading. Study limitations in the current literature include small sample size, potential recall bias, lack of appropriate controls, incomplete reported results, and the failure to clearly define skin changes.
When considering the accuracy and type of information regarding skin conditions that is available on the Internet, it is important to note that most of the results we reviewed were webpages attributed to self-proclaimed experts. Although educational websites also were included in the search results, whether or not patients prefer or understand the content of such websites is still unknown; therefore, health organizations should consider revising online patient education materials to allow universal comprehension.27
Furthermore, it is important to consider the impact that widespread Internet access may have on the physician-patient relationship. Having access to health-related information online and being able to potentially self-diagnose could delay or deter patients from seeking professional advice or care.3 A study evaluating the impact of online searches on the physician-patient relationship among 175 patients determined that 36.5% of patients gathered information online prior to their consultation with a physician, while 67.3% chose to complement the information given to them by their physician with online resources.28 Based on these statistics, it is important that physicians be up-to-date with Internet discourse to discredit unfounded recommendations. Ultimately, when it comes to diet and dermatology, patients ought to be skeptical of the information currently available on the Internet, given that most of it is unsubstantiated by medical research.
- Fox S. Online health search 2006. Pew Research Center website. http://www.pewinternet.org/2006/10/29/online-health-search-2006/. Published October 29, 2006. Accessed May 3, 2018.
- Prestin A, Vieux SN, Chou WY. Is online health activity alive and well or flatlining? findings from 10 years of the health information national trends survey. J Health Commun. 2015;20:790-798.
- Zeichner JA, Del Rosso JQ. Acne and the internet. Dermatol Clin. 2016;34:129-132.
- Whitsitt J, Karimkhani C, Boyers LN, et al. Comparing burden of dermatologic disease to search interest on Google trends. Dermatol Online J. 2015;21. pii:13030/qt5xg811qp.
- Shokeen D. Influence of diet in acne vulgaris and atopic dermatitis. Cutis. 2016;98:E28-E29.
- Veith WB, Silverberg NB. The association of acne vulgaris with diet. Cutis. 2011;88:84-91.
- Rouhani P. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(3, suppl 1):P706.
- Melnick BC. Evidence for acne-promoting effect of milk and other insulinotropic dairy products. Nestle Nutr Worksop Ser Pediatr Program. 2011;67:131-145.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. High school dietary diary intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Ulvestad M, Bjertness E, Dalgard F, et al. Acne and dairy products in adolescence: results from a Norwegian longitudinal study [published online July 16, 2016]. J Eur Acad Dermatol Venereol. 2017;31:530-535.
- Melnick BC, Schmitz G. Role of insulin, insulin like growth factor 1, hyperglycemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Murzaku EC, Bronsnick T, Rao BK. Diet in dermatology: part II. melanoma, chronic urticaria, and psoriasis. J Am Acad Dermatol. 2014;71:1053.E1-1053.E16.
- Tobin AM, Higgins EM, Norris S, et al. Prevalence of psoriasis in patients with alcoholic liver disease. Clin Exp Dermatol. 2009;34:698-701.
- Kirby B, Richards HL, Mason DL, et al. Alcohol consumption and psychological distress in patients with psoriasis. Br J Dermatol. 2008;158:138-140.
- Søyland E, Funk J, Rajika G, et al. Effect of dietary supplementation with very long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44-51.
- Morimoto S, Yoshikawa K. Psoriasis and vitamin D3. a review of our experience. Arch Dermatol. 1989;125:231-234.
- Smith EL, Pincus SH, Donovan L, et al. A novel approach for the evaluation and treatment of psoriasis. oral or topical use of 1,25-dihydroxyvitamin D3 can be a safe and effective therapy for psoriasis. J Am Acad Dermatol. 1988;19:516-528.
- Siddiqui MA, Al-Khawajah MM. Vitamin D3 and psoriasis: a randomized double-blind placebo-controlled study. J Dermatol Treat. 1990;1:243-245.
- Wang Y, Gao H, Loyd CM, et al. Chronic skin-specific inflammation promotes vascular inflammation and thrombosis. J Invest Dermatol. 2012;132:2067-2075.
- Barrea L, Nappi F, Di Somma C, et al. Environmental risk factors in psoriasis: the point of view of the nutritionist. Int J Environ Res Public Health. 2016;13. pii:E743. doi:10.3390/ijerph13070743.
- Mayser P, Mrowietz U, Arenberger P, et al. Omega-3 fatty acid-based lipid infusion in patients with chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, multicenter trial. J Am Acad Dermatol. 1998;38:539-547.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012;2:CD005205.
- Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer [published online November 15, 2014]. J Am Acad Dermatol. 2014;71:1039.E1-1039.E12.
- Campbell DE. The role of food allergy in childhood atopic dermatitis. J Paediatr Child Health. 2012;48:1058-1064.
- Werfel T, Erdmann S, Fuchs T, et al. Approach to suspected food allergy in atopic dermatitis. guideline of the Task Force on Food Allergy of the German Society of Allergology and Clinical Immunology (DGAKI) and the Medical Association of German Allergologists (ADA) and the German Society of Pediatric Allergology (GPA). J Dtsch Dermatol Ges. 2009;3:265-271.
- John AM, John ES, Hansberry DR, et al. Assessment of online patient education materials from major dermatologic associations. J Clin Aesthet Dermatol. 2016;9:23-28.
- Orgaz-Molina J, Cotugno M, Girón-Prieto MS, et al. A study of internet searches for medical information in dermatology patients: the patient-physician relationship. Actas Dermosifiliogr. 2015;106:493-499.
- Fox S. Online health search 2006. Pew Research Center website. http://www.pewinternet.org/2006/10/29/online-health-search-2006/. Published October 29, 2006. Accessed May 3, 2018.
- Prestin A, Vieux SN, Chou WY. Is online health activity alive and well or flatlining? findings from 10 years of the health information national trends survey. J Health Commun. 2015;20:790-798.
- Zeichner JA, Del Rosso JQ. Acne and the internet. Dermatol Clin. 2016;34:129-132.
- Whitsitt J, Karimkhani C, Boyers LN, et al. Comparing burden of dermatologic disease to search interest on Google trends. Dermatol Online J. 2015;21. pii:13030/qt5xg811qp.
- Shokeen D. Influence of diet in acne vulgaris and atopic dermatitis. Cutis. 2016;98:E28-E29.
- Veith WB, Silverberg NB. The association of acne vulgaris with diet. Cutis. 2011;88:84-91.
- Rouhani P. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(3, suppl 1):P706.
- Melnick BC. Evidence for acne-promoting effect of milk and other insulinotropic dairy products. Nestle Nutr Worksop Ser Pediatr Program. 2011;67:131-145.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. High school dietary diary intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Ulvestad M, Bjertness E, Dalgard F, et al. Acne and dairy products in adolescence: results from a Norwegian longitudinal study [published online July 16, 2016]. J Eur Acad Dermatol Venereol. 2017;31:530-535.
- Melnick BC, Schmitz G. Role of insulin, insulin like growth factor 1, hyperglycemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Murzaku EC, Bronsnick T, Rao BK. Diet in dermatology: part II. melanoma, chronic urticaria, and psoriasis. J Am Acad Dermatol. 2014;71:1053.E1-1053.E16.
- Tobin AM, Higgins EM, Norris S, et al. Prevalence of psoriasis in patients with alcoholic liver disease. Clin Exp Dermatol. 2009;34:698-701.
- Kirby B, Richards HL, Mason DL, et al. Alcohol consumption and psychological distress in patients with psoriasis. Br J Dermatol. 2008;158:138-140.
- Søyland E, Funk J, Rajika G, et al. Effect of dietary supplementation with very long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44-51.
- Morimoto S, Yoshikawa K. Psoriasis and vitamin D3. a review of our experience. Arch Dermatol. 1989;125:231-234.
- Smith EL, Pincus SH, Donovan L, et al. A novel approach for the evaluation and treatment of psoriasis. oral or topical use of 1,25-dihydroxyvitamin D3 can be a safe and effective therapy for psoriasis. J Am Acad Dermatol. 1988;19:516-528.
- Siddiqui MA, Al-Khawajah MM. Vitamin D3 and psoriasis: a randomized double-blind placebo-controlled study. J Dermatol Treat. 1990;1:243-245.
- Wang Y, Gao H, Loyd CM, et al. Chronic skin-specific inflammation promotes vascular inflammation and thrombosis. J Invest Dermatol. 2012;132:2067-2075.
- Barrea L, Nappi F, Di Somma C, et al. Environmental risk factors in psoriasis: the point of view of the nutritionist. Int J Environ Res Public Health. 2016;13. pii:E743. doi:10.3390/ijerph13070743.
- Mayser P, Mrowietz U, Arenberger P, et al. Omega-3 fatty acid-based lipid infusion in patients with chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, multicenter trial. J Am Acad Dermatol. 1998;38:539-547.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012;2:CD005205.
- Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer [published online November 15, 2014]. J Am Acad Dermatol. 2014;71:1039.E1-1039.E12.
- Campbell DE. The role of food allergy in childhood atopic dermatitis. J Paediatr Child Health. 2012;48:1058-1064.
- Werfel T, Erdmann S, Fuchs T, et al. Approach to suspected food allergy in atopic dermatitis. guideline of the Task Force on Food Allergy of the German Society of Allergology and Clinical Immunology (DGAKI) and the Medical Association of German Allergologists (ADA) and the German Society of Pediatric Allergology (GPA). J Dtsch Dermatol Ges. 2009;3:265-271.
- John AM, John ES, Hansberry DR, et al. Assessment of online patient education materials from major dermatologic associations. J Clin Aesthet Dermatol. 2016;9:23-28.
- Orgaz-Molina J, Cotugno M, Girón-Prieto MS, et al. A study of internet searches for medical information in dermatology patients: the patient-physician relationship. Actas Dermosifiliogr. 2015;106:493-499.
Practice Points
- It is important physicians be well-informed regarding Internet discourse to discredit unfounded recommendations.
- It is likely that patients seeking medical advice regarding their dermatologic condition and treatment will have done prior research on the Internet.
- Oftentimes, the information on educational health websites can be confusing to patients.
- Because of widespread Internet access to health-related information, patients may opt to self-diagnose and therefore delay seeking professional care.
Low platelets linked to pregnancy complications
A study that characterized the occurrence and frequency of thrombocytopenia throughout the course of pregnancy found a significant decline in platelet counts during the course of pregnancy, and significant differences between pregnant and nonpregnant women. However, the study – published in the New England Journal of Medicine – also found that women with pregnancy-related complications were more likely to have platelet counts less than 150,000/mm3, even in the absence of known causes of thrombocytopenia.
Jessica Reese, PhD, and her coinvestigators at the University of Oklahoma, Oklahoma City, used data from pregnant women who delivered at a single site from 2011 to 2014. In all, 4,568 women from the study group had uncomplicated pregnancies, and 2,586 had pregnancy-related complications. To be included in the complicated pregnancy group, women needed a diagnosis of hypertension, diabetes, eclampsia or preeclampsia, or abnormal placentation. Another 197 women had preexisting disorders known to be associated with thrombocytopenia.
For the women with uncomplicated pregnancies, Dr. Reese and her colleagues compared platelet counts with those of nonpregnant women who participated in the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2012, using a stratified analysis that accounted for age and racial or ethnic background and excluding NHANES participants with cancer, diabetes, or hypertension.
To look at platelet levels across types of pregnancies and in comparison with nonpregnant women, the investigators established three cutpoints, grouping women into those who had a platelet count of at least 150,000/mm3, those with platelet counts less than 100,000/mm3 but at least 80,000/mm3, and those with platelet counts less than 80,000/mm3.
Only 1% of women with uncomplicated pregnancies had platelet counts less than 100,000/mm3 during pregnancy or at delivery, and just 5 women (0.1%) had unexplained platelet counts below 80,000/mm3. Seven more women with platelet counts less than 80,000/mm3 had an identified cause for their thrombocytopenia.
Overall, mean platelet counts were lower for the women with uncomplicated pregnancies during the first trimester than for nonpregnant women (251,000 vs. 273,000/mm3). These values fell throughout pregnancy to a mean of 217,000/mm3 by the time of delivery at a mean gestation of 39.0 weeks (P less than .001 for all time points). However, mean platelet counts rebounded by the time a postpartum value was obtained at a mean 7.1 weeks after delivery, to 264,000/mm3, a value that wasn’t significantly different from the nonpregnant cohort’s platelet counts.
When the investigators looked at mean platelet counts by trimester, they saw no difference between those with uncomplicated and complicated pregnancies until the third trimester. Then, “mean platelet counts decreased at a greater rate among women with pregnancy-related complications,” wrote Dr. Reese and her colleagues; 11.9% of women with complicated pregnancies had platelet counts below 150,000/mm3, while this level was seen in 9.9% of women without complications of pregnancy (P = .01).
At delivery, 2.3% (n = 59) of women with complicated pregnancies had platelet counts below 100,000/mm3, and 31 of these women had counts below 80,000/mm3, representing a significantly higher rate of thrombocytopenia at delivery than seen in the uncomplicated group (P less than .001).
In discussion, Dr. Reese and her coauthors examined the possible mechanisms for decreased levels of circulating platelets during pregnancy. Volume dilution from increased plasma volume is one well-accepted reason. Others include accumulation of platelets within the spleen, which increases in size by about 50% during pregnancy; similarly, the placenta’s circulation is similar to that of the spleen, so platelets may also accumulate there, the authors said. Further support for the placental mechanism comes from the lower average platelet counts for women with twin pregnancies.
The study’s relatively broad definition of pregnancy-related complications may have had the effect of lessening the difference in mean platelet counts between the complicated and uncomplicated pregnancy groups, the investigators acknowledged. Still, their study population had rates of these complications similar to those of the United States population, they said. “Therefore, our data may accurately reflect the platelet counts in women with these pregnancy-related complications,” they noted.
“Severe thrombocytopenia is rare, even in women with pregnancy-related complications,” concluded Dr. Reese and her colleagues. “Our data suggest that, for women with an uncomplicated pregnancy who have a platelet count of less than 100,000/mm3, a cause of thrombocytopenia other than the pregnancy itself should be considered.”
SOURCE: Reese J et al. N Engl J Med. 2018;379:32-43.
A study that characterized the occurrence and frequency of thrombocytopenia throughout the course of pregnancy found a significant decline in platelet counts during the course of pregnancy, and significant differences between pregnant and nonpregnant women. However, the study – published in the New England Journal of Medicine – also found that women with pregnancy-related complications were more likely to have platelet counts less than 150,000/mm3, even in the absence of known causes of thrombocytopenia.
Jessica Reese, PhD, and her coinvestigators at the University of Oklahoma, Oklahoma City, used data from pregnant women who delivered at a single site from 2011 to 2014. In all, 4,568 women from the study group had uncomplicated pregnancies, and 2,586 had pregnancy-related complications. To be included in the complicated pregnancy group, women needed a diagnosis of hypertension, diabetes, eclampsia or preeclampsia, or abnormal placentation. Another 197 women had preexisting disorders known to be associated with thrombocytopenia.
For the women with uncomplicated pregnancies, Dr. Reese and her colleagues compared platelet counts with those of nonpregnant women who participated in the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2012, using a stratified analysis that accounted for age and racial or ethnic background and excluding NHANES participants with cancer, diabetes, or hypertension.
To look at platelet levels across types of pregnancies and in comparison with nonpregnant women, the investigators established three cutpoints, grouping women into those who had a platelet count of at least 150,000/mm3, those with platelet counts less than 100,000/mm3 but at least 80,000/mm3, and those with platelet counts less than 80,000/mm3.
Only 1% of women with uncomplicated pregnancies had platelet counts less than 100,000/mm3 during pregnancy or at delivery, and just 5 women (0.1%) had unexplained platelet counts below 80,000/mm3. Seven more women with platelet counts less than 80,000/mm3 had an identified cause for their thrombocytopenia.
Overall, mean platelet counts were lower for the women with uncomplicated pregnancies during the first trimester than for nonpregnant women (251,000 vs. 273,000/mm3). These values fell throughout pregnancy to a mean of 217,000/mm3 by the time of delivery at a mean gestation of 39.0 weeks (P less than .001 for all time points). However, mean platelet counts rebounded by the time a postpartum value was obtained at a mean 7.1 weeks after delivery, to 264,000/mm3, a value that wasn’t significantly different from the nonpregnant cohort’s platelet counts.
When the investigators looked at mean platelet counts by trimester, they saw no difference between those with uncomplicated and complicated pregnancies until the third trimester. Then, “mean platelet counts decreased at a greater rate among women with pregnancy-related complications,” wrote Dr. Reese and her colleagues; 11.9% of women with complicated pregnancies had platelet counts below 150,000/mm3, while this level was seen in 9.9% of women without complications of pregnancy (P = .01).
At delivery, 2.3% (n = 59) of women with complicated pregnancies had platelet counts below 100,000/mm3, and 31 of these women had counts below 80,000/mm3, representing a significantly higher rate of thrombocytopenia at delivery than seen in the uncomplicated group (P less than .001).
In discussion, Dr. Reese and her coauthors examined the possible mechanisms for decreased levels of circulating platelets during pregnancy. Volume dilution from increased plasma volume is one well-accepted reason. Others include accumulation of platelets within the spleen, which increases in size by about 50% during pregnancy; similarly, the placenta’s circulation is similar to that of the spleen, so platelets may also accumulate there, the authors said. Further support for the placental mechanism comes from the lower average platelet counts for women with twin pregnancies.
The study’s relatively broad definition of pregnancy-related complications may have had the effect of lessening the difference in mean platelet counts between the complicated and uncomplicated pregnancy groups, the investigators acknowledged. Still, their study population had rates of these complications similar to those of the United States population, they said. “Therefore, our data may accurately reflect the platelet counts in women with these pregnancy-related complications,” they noted.
“Severe thrombocytopenia is rare, even in women with pregnancy-related complications,” concluded Dr. Reese and her colleagues. “Our data suggest that, for women with an uncomplicated pregnancy who have a platelet count of less than 100,000/mm3, a cause of thrombocytopenia other than the pregnancy itself should be considered.”
SOURCE: Reese J et al. N Engl J Med. 2018;379:32-43.
A study that characterized the occurrence and frequency of thrombocytopenia throughout the course of pregnancy found a significant decline in platelet counts during the course of pregnancy, and significant differences between pregnant and nonpregnant women. However, the study – published in the New England Journal of Medicine – also found that women with pregnancy-related complications were more likely to have platelet counts less than 150,000/mm3, even in the absence of known causes of thrombocytopenia.
Jessica Reese, PhD, and her coinvestigators at the University of Oklahoma, Oklahoma City, used data from pregnant women who delivered at a single site from 2011 to 2014. In all, 4,568 women from the study group had uncomplicated pregnancies, and 2,586 had pregnancy-related complications. To be included in the complicated pregnancy group, women needed a diagnosis of hypertension, diabetes, eclampsia or preeclampsia, or abnormal placentation. Another 197 women had preexisting disorders known to be associated with thrombocytopenia.
For the women with uncomplicated pregnancies, Dr. Reese and her colleagues compared platelet counts with those of nonpregnant women who participated in the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2012, using a stratified analysis that accounted for age and racial or ethnic background and excluding NHANES participants with cancer, diabetes, or hypertension.
To look at platelet levels across types of pregnancies and in comparison with nonpregnant women, the investigators established three cutpoints, grouping women into those who had a platelet count of at least 150,000/mm3, those with platelet counts less than 100,000/mm3 but at least 80,000/mm3, and those with platelet counts less than 80,000/mm3.
Only 1% of women with uncomplicated pregnancies had platelet counts less than 100,000/mm3 during pregnancy or at delivery, and just 5 women (0.1%) had unexplained platelet counts below 80,000/mm3. Seven more women with platelet counts less than 80,000/mm3 had an identified cause for their thrombocytopenia.
Overall, mean platelet counts were lower for the women with uncomplicated pregnancies during the first trimester than for nonpregnant women (251,000 vs. 273,000/mm3). These values fell throughout pregnancy to a mean of 217,000/mm3 by the time of delivery at a mean gestation of 39.0 weeks (P less than .001 for all time points). However, mean platelet counts rebounded by the time a postpartum value was obtained at a mean 7.1 weeks after delivery, to 264,000/mm3, a value that wasn’t significantly different from the nonpregnant cohort’s platelet counts.
When the investigators looked at mean platelet counts by trimester, they saw no difference between those with uncomplicated and complicated pregnancies until the third trimester. Then, “mean platelet counts decreased at a greater rate among women with pregnancy-related complications,” wrote Dr. Reese and her colleagues; 11.9% of women with complicated pregnancies had platelet counts below 150,000/mm3, while this level was seen in 9.9% of women without complications of pregnancy (P = .01).
At delivery, 2.3% (n = 59) of women with complicated pregnancies had platelet counts below 100,000/mm3, and 31 of these women had counts below 80,000/mm3, representing a significantly higher rate of thrombocytopenia at delivery than seen in the uncomplicated group (P less than .001).
In discussion, Dr. Reese and her coauthors examined the possible mechanisms for decreased levels of circulating platelets during pregnancy. Volume dilution from increased plasma volume is one well-accepted reason. Others include accumulation of platelets within the spleen, which increases in size by about 50% during pregnancy; similarly, the placenta’s circulation is similar to that of the spleen, so platelets may also accumulate there, the authors said. Further support for the placental mechanism comes from the lower average platelet counts for women with twin pregnancies.
The study’s relatively broad definition of pregnancy-related complications may have had the effect of lessening the difference in mean platelet counts between the complicated and uncomplicated pregnancy groups, the investigators acknowledged. Still, their study population had rates of these complications similar to those of the United States population, they said. “Therefore, our data may accurately reflect the platelet counts in women with these pregnancy-related complications,” they noted.
“Severe thrombocytopenia is rare, even in women with pregnancy-related complications,” concluded Dr. Reese and her colleagues. “Our data suggest that, for women with an uncomplicated pregnancy who have a platelet count of less than 100,000/mm3, a cause of thrombocytopenia other than the pregnancy itself should be considered.”
SOURCE: Reese J et al. N Engl J Med. 2018;379:32-43.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Platelet counts less than 150,000/mm3 were more common in complicated pregnancies.
Major finding: Platelet counts were below 150,000/mm3 in 11.9% of complicated versus 9.9% of uncomplicated pregnancies at the time of delivery (P = .01).
Study details: Review of records of 7,351 pregnant women delivering at a single site, compared with NHANES data for 8,885 nonpregnant women.
Disclosures: The National Institutes of Health funded the study. The authors reported having no conflicts of interest.
Source: Reese J et al. N Engl J Med. 2018;379:32-43.
Social networks may influence youth who transition to injection drug use
SAN DIEGO – Youth and young adults who have transitioned from prescription drug misuse to injection drug use tend to reside in dense social networks, results from a novel study suggest.
“A lot of what we know about the transition from prescription opioids to drug use is from populations that have already transitioned to injection drug use, and we’re asking them retrospectively to tell us about their use,” lead study author Alia Al-Tayyib, PhD, said in an interview at the annual meeting of the College on Problems of Drug Dependence.
In an effort to examine the transition from prescription opioid misuse to injection drug use from a social network perspective, Dr. Al-Tayyib and her associates recruited Denver area residents aged 15-24 years to participate in the Social Networks of Abused Prescription Pills in Youth study between October 2015 and April 2017. Participants were recruited via direct outreach and respondent-driven sampling, which is a peer-referral sampling methodology. Individuals were eligible to participate if they were currently misusing prescription opioids or were currently using heroin after a period of prescription opioid misuse.
Study participants completed interviewer-administered behavioral and social network surveys. People from whom data were collected were referred to as “egos,” and they provided information on people in their social networks called “alters.” As a social network prompt, for example, study participants were asked to “think about people you have contact with who have been involved in your life in a significant way during the past month.”
Participants also were asked about places of aggregation with the prompt, “Where does [this person] hang out?” The egos, alters, and locations are all considered “nodes” in the social network. To examine implications on transition, the researchers examined k-plexes, or subgroups of connected nodes.
Dr. Al-Tayyib, an associate research scientist at Denver Public Health, presented results from 80 ego participants and 489 alters. The mean age of ego participants was 21.4 years, 73% were male, 68% were non-Hispanic white, and 60% reported being homeless in the past 12 months. More than three-quarters of ego participants (80%) reported injection drug use, 14% reported misusing prescription opioids, and 6% reported using heroin without injecting. Of the 489 alters, 45.2% reportedly injected, 5.9% used heroin, and 8.6% misused prescription opioids with at least one of the ego participants.
The researchers observed that study participants who transitioned to injection drug use resided in denser social network regions. “It was really hard to capture people who had not already transitioned to injection drug use, partly because that’s not a behavior that’s easily identifiable,” Dr. Al-Tayyib said. “This study is a one look in time, so it’s hard to know which came first: the chicken or the egg. If I’m injecting drugs, do I start hanging out with other people who inject drugs, or is it because I started hanging out with people who inject drugs, and then I started injecting? From a prevention standpoint, it’s engaging youth in positive networks to prevent the transition.”
The study was supported by funding from the National Institute on Drug Abuse. Dr. Al-Tayyib reported having no disclosures.
SAN DIEGO – Youth and young adults who have transitioned from prescription drug misuse to injection drug use tend to reside in dense social networks, results from a novel study suggest.
“A lot of what we know about the transition from prescription opioids to drug use is from populations that have already transitioned to injection drug use, and we’re asking them retrospectively to tell us about their use,” lead study author Alia Al-Tayyib, PhD, said in an interview at the annual meeting of the College on Problems of Drug Dependence.
In an effort to examine the transition from prescription opioid misuse to injection drug use from a social network perspective, Dr. Al-Tayyib and her associates recruited Denver area residents aged 15-24 years to participate in the Social Networks of Abused Prescription Pills in Youth study between October 2015 and April 2017. Participants were recruited via direct outreach and respondent-driven sampling, which is a peer-referral sampling methodology. Individuals were eligible to participate if they were currently misusing prescription opioids or were currently using heroin after a period of prescription opioid misuse.
Study participants completed interviewer-administered behavioral and social network surveys. People from whom data were collected were referred to as “egos,” and they provided information on people in their social networks called “alters.” As a social network prompt, for example, study participants were asked to “think about people you have contact with who have been involved in your life in a significant way during the past month.”
Participants also were asked about places of aggregation with the prompt, “Where does [this person] hang out?” The egos, alters, and locations are all considered “nodes” in the social network. To examine implications on transition, the researchers examined k-plexes, or subgroups of connected nodes.
Dr. Al-Tayyib, an associate research scientist at Denver Public Health, presented results from 80 ego participants and 489 alters. The mean age of ego participants was 21.4 years, 73% were male, 68% were non-Hispanic white, and 60% reported being homeless in the past 12 months. More than three-quarters of ego participants (80%) reported injection drug use, 14% reported misusing prescription opioids, and 6% reported using heroin without injecting. Of the 489 alters, 45.2% reportedly injected, 5.9% used heroin, and 8.6% misused prescription opioids with at least one of the ego participants.
The researchers observed that study participants who transitioned to injection drug use resided in denser social network regions. “It was really hard to capture people who had not already transitioned to injection drug use, partly because that’s not a behavior that’s easily identifiable,” Dr. Al-Tayyib said. “This study is a one look in time, so it’s hard to know which came first: the chicken or the egg. If I’m injecting drugs, do I start hanging out with other people who inject drugs, or is it because I started hanging out with people who inject drugs, and then I started injecting? From a prevention standpoint, it’s engaging youth in positive networks to prevent the transition.”
The study was supported by funding from the National Institute on Drug Abuse. Dr. Al-Tayyib reported having no disclosures.
SAN DIEGO – Youth and young adults who have transitioned from prescription drug misuse to injection drug use tend to reside in dense social networks, results from a novel study suggest.
“A lot of what we know about the transition from prescription opioids to drug use is from populations that have already transitioned to injection drug use, and we’re asking them retrospectively to tell us about their use,” lead study author Alia Al-Tayyib, PhD, said in an interview at the annual meeting of the College on Problems of Drug Dependence.
In an effort to examine the transition from prescription opioid misuse to injection drug use from a social network perspective, Dr. Al-Tayyib and her associates recruited Denver area residents aged 15-24 years to participate in the Social Networks of Abused Prescription Pills in Youth study between October 2015 and April 2017. Participants were recruited via direct outreach and respondent-driven sampling, which is a peer-referral sampling methodology. Individuals were eligible to participate if they were currently misusing prescription opioids or were currently using heroin after a period of prescription opioid misuse.
Study participants completed interviewer-administered behavioral and social network surveys. People from whom data were collected were referred to as “egos,” and they provided information on people in their social networks called “alters.” As a social network prompt, for example, study participants were asked to “think about people you have contact with who have been involved in your life in a significant way during the past month.”
Participants also were asked about places of aggregation with the prompt, “Where does [this person] hang out?” The egos, alters, and locations are all considered “nodes” in the social network. To examine implications on transition, the researchers examined k-plexes, or subgroups of connected nodes.
Dr. Al-Tayyib, an associate research scientist at Denver Public Health, presented results from 80 ego participants and 489 alters. The mean age of ego participants was 21.4 years, 73% were male, 68% were non-Hispanic white, and 60% reported being homeless in the past 12 months. More than three-quarters of ego participants (80%) reported injection drug use, 14% reported misusing prescription opioids, and 6% reported using heroin without injecting. Of the 489 alters, 45.2% reportedly injected, 5.9% used heroin, and 8.6% misused prescription opioids with at least one of the ego participants.
The researchers observed that study participants who transitioned to injection drug use resided in denser social network regions. “It was really hard to capture people who had not already transitioned to injection drug use, partly because that’s not a behavior that’s easily identifiable,” Dr. Al-Tayyib said. “This study is a one look in time, so it’s hard to know which came first: the chicken or the egg. If I’m injecting drugs, do I start hanging out with other people who inject drugs, or is it because I started hanging out with people who inject drugs, and then I started injecting? From a prevention standpoint, it’s engaging youth in positive networks to prevent the transition.”
The study was supported by funding from the National Institute on Drug Abuse. Dr. Al-Tayyib reported having no disclosures.
AT CPDD 2018
Key clinical point: Prevention efforts are needed to target youth and young adults who misuse prescription drugs before they have transitioned to injection drug use.
Major finding:. Study participants who transitioned to injection drug use resided in denser social network regions.
Study details: Responses from 80 Denver area residents aged 15-24 years who participated in the Social Networks of Abused Prescription Pills in Youth study.
Disclosures: The study was supported by funding from the National Institute on Drug Abuse. Dr. Al-Tayyib reported having no disclosures.
Vertebral Artery Dissection in Active-Duty Soldier Due to Mixed Martial Arts Choke Hold
Knowledge of the potential dangers of mixed martial arts is valuable for Department of Defense (DoD) health care providers as the military continues to implement combatives training into regular military instruction. This case study presents an active-duty service member who developed a spontaneous vertebral artery dissection (sVAD) during mixed martial arts training, which led to a cerebellar stroke.
To the authors’ knowledge this is the first documented case of a sVAD with associated stroke related to a mixed martial arts choke hold. Understanding the diagnosis, management, and prognosis of this condition will remain important as hand-to-hand combat instruction continues to be a part of regular military training.
Case Presentation
A 39-year-old active-duty male without significant past medical history presented to the emergency department (ED) at the San Antonio Military Medical Center in Texas for evaluation of severe vertigo with associated nausea and vomiting. He had participated in a Jiu-Jitsu match the evening prior to his presentation and reported that he was placed in a choke hold within the last 12 seconds of the match. He denied losing consciousness during this hold.
Once released, he attempted to stand and developed sudden onset vertigo with severe nausea, leading to multiple bouts of emesis. He additionally developed a throbbing, left-sided headache radiating down the left side of his neck. While the vertigo resolved within an hour, he continued to experience bouts of nausea and emesis, prompting him to present to the ED for further evaluation. The patient’s past medical history was remarkable only for multiple prior concussions, and his only medication was occasional ibuprofen. He denied the usage of recreational drugs.
Upon presentation to the ED, the patient’s vital signs were 139/93 mm Hg blood pressure, 73 beats per minute heart rate, 16 breaths per minute respiration, 100% oxygen saturation on room air, and 97.7° F temperature.
The patient demonstrated normal balance and exhibited no nystagmus or limb/truncal ataxia as evaluated with finger-to-nose/heel-to-shin testing and gait exam. Complete blood count, comprehensive metabolic panel, and coagulation panel all demonstrated no abnormalities.
The patient was admitted to the hospital for symptom control and further monitoring. His headache and nausea were managed with medications, and he began antiplatelet therapy with aspirin 325 mg daily. Given the size of his cerebellar infarction, it was decided that he would be monitored in the hospital for 72 hours for the development of significant cerebellar edema. He remained stable throughout his hospitalization and had only a mild headache at the time of discharge.
The patient was last seen 3 months postinjury with no subjective complaints and a completely normal neurologic exam. The treatment plan for the patient is to continue aspirin for 6 months postinjury at which time a repeat CT will be performed to ensure resolution. He has been counseled to avoid heavy lifting and any activity with potential for sudden movement/force of the neck (grappling/wrestling, chiropractic manipulation, roller coasters, or sit-ups) until the repeat CT has been completed.
Discussion
Spontaneous vertebral artery and carotid artery dissections are collectively referred to as sCADs. Spontaneous cervical artery dissections are a rare condition with a higher incidence of internal carotid dissections than are VADs (1.72 vs 0.97 per 100,000 people).1 In contrast to the general stroke population, patients with sCADs are typically younger (mean age 45.3 years); and more than half of the patients are male.1,2
Spontaneous cervical artery dissections are typically characterized by subintimal tears of the vertebral artery leading to the accumulation of an intramural hematoma and creation of a “false lumen” in the arterial wall.3 A sVAD is more often found in the pars transversaria (V2; 35%) or atlas loop (V3; 34%) segments of the vertebral artery than in the prevertebral (V1; 20%) or intracranial (V4; 11%) segments.3-5 The etiology of these injuries is thought to be minor trauma to the neck in the context of a likely underlying connective tissue disease, though no direct association with a particular disease has been shown.
Biopsy evaluation of the superficial temporal arteries of patients with sCADs have revealed pathologic changes of the media and adventitial layers, including vacuolar degeneration and capillary neoangiogenesis, which are not found in the arteries of control patients.5 Although definitive association with a known connective tissue disease is rare, angiographic evidence of fibromuscular dysplasia, a nonspecific marker of connective tissue disease, is noted in as many as 15% to 20% of patients.6 Consequently, routine connective tissue disease screening is not recommended in these patients. One study found that about 40% of sCAD patients can recall minor cervical trauma in the preceding month in comparison to only 10% of other patients with stroke, leading to the moniker of “bottoms-up” or “beauty-parlor strokes” for these injuries. The most common mechanisms of minor neck trauma causing sCADs include tennis and golf swings, yoga, and roller-coaster rides.7,8
Usually symptomatic at presentation, the most frequently encountered sCAD symptoms are head or neck pain (80%), brain ischemia (56%), and Horner syndrome (25%).1 A study of 161 consecutive patients with internal carotid (n = 135) or vertebral artery (n = 26) dissections revealed that headache was reported by 69% of those with sVADs, and when present, was the initial manifestation in 33%. Headaches typically were ipsilateral to the dissection, located posteriorly in 83% of patients, and lasted an average duration of 72 hours. Neck pain, which was noted in 46% of sVAD patients, was predominantly posterior and ipsilateral in location as well.9 Ischemic symptoms of sVAD may include posterior circulation symptoms, such as vertigo, ataxia, diplopia, and leg weakness as well as lateral medullary (Wallenberg) syndrome characterized by dizziness, postural instability, limb hypotonia/ataxia, blurred vision, and nystagmus.
In a study of 169 patients with sCAD, brain ischemia occurred in 77% (131 patients) including 67% (n = 114) with ischemic stroke and 10% (n = 17) with transient ischemic attack. Head and/or neck pain was noted in 88% of those with brain ischemia.4 Etiologies for infarction included thromboembolic (85%), hemodynamic (12%), and mixed (3%).10 Isolated local symptoms are rare with one study of 245 patients with sCAD revealing only 20 (8%) presenting with pain only. Of those with pain only, 6 presented with headache, 2 with neck pain, and 12 with both.11
Diagnosis of sVAD requires a high index of suspicion and is confirmed by diagnostic testing. Previously, invasive angiography was the diagnostic gold standard, but with the improvement in quality of CT and MR angiography, these noninvasive modalities have become the tests of choice. There have been no studies to date revealing a definitive benefit of one modality over the other. A meta-analysis of 25 articles that compared the use of CT and MR angiography for the diagnosis of carotid and VAD revealed similar sensitivity and specificity.12 In contrast, a study involving 10 patients with confirmed sVAD who had both CT and MR angiographies during evaluation showed more total findings consistent with dissection on CT than with MR angiography when graded by 2 neuroradiologists. Additionally, the neuroradiologists subjectively rated CT angiography as preferential to MR in showing the imaging findings of dissection in 8 of 10 cases of vertebral dissection.13
Treatment for sCAD remains heavily debated. The use of IV thrombolysis within the standard time window for acute ischemic stroke is advocated for these patients. A meta-analysis of patients with sCAD vs matched patients with stroke from other causes treated with IV thrombolysis showed no difference in mortality at 3 months (9.0% vs 8.8%) or symptomatic intracranial hemorrhage (3.3% vs 3.0%). Additionally, similar percentages of patients had excellent (30.9% vs 37.4%) and favorable (58.2% vs 52.2%) 3-month functional statuses as expressed by the Modified Rankin Score (mRS).14,15
Debate remains regarding subacute therapy for sCAD with either antiplatelet or anticoagulant therapy. A randomized study of 250 patients with cervical artery dissection (118 carotid, 132 vertebral) in which 126 patients were assigned to antiplatelet therapy and 124 patients were assigned to anticoagulant therapy showed an overall low rate of recurrent stroke (2%). There was no significant difference in efficacy between the therapy groups with stroke or death occurring in 3 antiplatelet patients and 1 anticoagulated patient. Adverse effects were very low in both groups with no deaths and only 1 major bleed in the anticoagulation group. Of note, stroke rates were lower in this study than prior observational studies.16
A nonrandomized study of 88 patients with extracranial sCAD showed overall low rates of recurrent ischemic stroke at 3 months with 1/59 (1.7%) in the antiplatelet group and 1/28 (3.6%) in the anticoagulation group (P 17 Given this low overall rate of recurrent stroke in prior studies, a guideline recommendation for antiplatelet or anticoagulant therapy cannot be made at this time.
The overall prognosis for this condition is fair. Functional status and recurrence risk are favorable, with one study finding a mRS score of 1 Additionally, a historic cohort study of 432 patients with first event of sCAD revealed that after a mean follow-up of 31 months, only 4 (0.9%) patients had a recurrent ischemic stroke either due to incomplete recanalization of the artery (n = 2) or recurrent sCAD (n = 2), and only 4 (0.9%) total recurrences of sCAD were report (2 without associated ischemic strokes).18 Further, a prospective study of 61 patients with confirmed sVAD revealed complete recanalization of 45.9% at 3 months, 62.3% at 6 months, and 63.9% at 12 months, suggesting that recanalization occurs mostly during the initial 6 months. There was no identified association between outcome and complete recanalization with favorable outcomes observed in 55 (90.2%) of patients and no further ischemic symptoms during follow-up.19
Neck maneuvers have been cited as a more common cause of sCAD in several previous studies. One retrospective study found chiropractic neck manipulation to be the etiology in 12 of 141 patients with CT- or MR- confirmed sCAD.20 As noted previously, to the authors’ knowledge this is the first reported case of a sVAD occurring after a mixed martial arts choke hold. While sports-related strokes are rare, one evaluation of 70 published cases found that 80% were due to sCAD. Commonly associated sports in this study included football, yoga, wrestling, tennis, golf, and swimming.21 Grappling-related neck manipulation has been noted as an etiology in a few case reports.
Hyperextension of the neck was deemed to be the etiology in boys aged 11 years and 17 years who developed a sCAD while participating in Judo and backyard wrestling, respectively.22,23 In the martial arts realm, there is a case report of a 26-year-old male who developed a sVAD after rapid head turning during a solo Kung Fu maneuver as well as a report of a 41-year-old male experiencing a right VAD complicated by a posterior infarction several days after straining his neck during a mixed martial arts competition.24,25 The patient denied any choke hold or direct blow to the neck.
The present case is different in that it is the first reported case of a sVAD occurring after a submission maneuver. Prior grappling-related sVADs were associated with hyperextension or rapid acceleration/deceleration forces on the neck. Isometric force to the neck is a rarely described mechanism for development of this injury. Although there are isolated and infrequent forensic case reports of carotid dissection with strangulation injuries, the authors believe this is the first documented case of a sVAD attributed to a combatives submission.
In the context of the military health system, it is important to be aware of this potential complication of combatives as instruction in close-quarters combat continues to be an important part of military training.
1. Lee VH, Brown RD Jr, Mandrekar J, Mokri B. Incidence and outcome of cervical artery dissection: a population-based study. Neurology. 2006;67(10):1809-1812.
2. Arnold M, Kappeler L, Georgiadis D, et al. Gender differences in spontaneous cervical artery dissection. Neurology. 2006;67(6):1050-1052.
3. Schvienk W. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
4. Arnold M, Bousser MG, Fahrni G, et al. Vertebral artery dissection: presenting findings and predictors of outcome. Stroke. 2006;37(10):2499-2503.
5. Völker W, Dittrich R, Grewe S, et al. The outer arterial wall layers are primarily affected in spontaneous cervical artery dissection. Neurology. 2011;76(17):1463-1471.
6. Debette S, Markus HS. The genetics of cervical artery dissection: a systematic review. Stroke. 2009;40(6):459-466.
7. DeBehnke D, Brady W. Vertebral artery dissection due to minor neck trauma. J Emerg Med. 1994;12(1):27-31.
8. Engelter ST, Grond-Ginsbach C, Metso TM, et al; Cervical Artery Dissection and Ischemic Stroke Patients Study Group. Cervical artery dissection: trauma and other potential mechanical trigger events. Neurology. 2013;80(21):1950-1957.
9. Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522.
10. Morel A, Naggara O, Touzé E, et al. Mechanism of ischemic infarct in spontaneous cervical artery dissection. Stroke. 2012;43(5):1354-1361.
11. Arnold M, Cumurciuc R, Stapf C, Favrole P, Berthet K, Bousser MG. Pain as the only symptom of cervical artery dissection. J Neurol Neurosurg Psychiatry. 2006;77(9):1021-1024.
12. Provenzale J, Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol. 2009;1939(4):1167-1174.
13. Vertinsky AT, Schwartz NE, Fishbein NJ, Rosenberg J, Albers GW, Zaharchuk G. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol. 2008;29(9):1753-1760.
14. Zinkstok SM, Vergouwen MD, Engelter ST, et al. Safety and functional outcome of thrombolysis in dissection-related ischemic stroke: a meta-analysis of individual patient data. Stroke. 2011;42(9):2515-2520.
15. Engelter S, Rutgers M, Hatz F, et al. Intravenous thrombolysis in stroke attributable to cervical artery dissection. Stroke. 2009;40(12):3772-3776.
16. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomized trial. Lancet Neurol. 2015;14(4):361-367.
17. Kennedy F, Lanfranconi S, Hicks C, et al; CADISS Investigators. Antiplatelets vs. anticoagulation for dissection: CADISS nonrandomized arm and meta-analysis. Neurology. 2012;79(7):686-689.
18. Touze E, Gauvrit JY, Moulin T, Meder JF, Bracard S, Mas JL; Multicenter Survey on Natural History of Cervical Artery Dissection. Risk of stroke and recurrent dissection after a cervical artery dissection: a multicenter study. Neurology. 2003;61(10):1347-1351.
19. Arauz A, Marquez J, Artigas C, Balderrama J, Orrego H. Recanalization of vertebral artery dissection. Stroke. 2010;41(4):717-721.
20. Kennell KA, Daghfal MM, Patel SG, et DeSanto JR, Waterman GS, Bertino RE. Cervical artery dissection related to chiropractic manipulation: one institution’s experience. J Fam Pract. 2017;66(9):556-562.
21. McCrory P. Vertebral artery dissection causing stroke in sport. J Clin Neurosci. 2000;7(4):298-300.
22. Lannuzel A, Moulin T, Amsallem D, Galmiche J, Rumbach L. Vertebral artery dissection following a judo session: a case report. Neuropediatrics. 1994;25(2):106-108.
23. Gupta V, Dhawan N, Bahl J. Minor trauma causing stroke in a young athlete. Case Rep Neurol Med. 2015;2015: 182875.
24. Pacei F, Valvasorri L, Bet L. Vertebral artery dissection during Kung-Fu training. Neurol Sci. 2014;35(2):331-332.
25. Slowey M, Maw G, Furyk J. Case report on vertebral artery dissection in mixed martial arts. Emerg Med Australas. 2012;24(2):203-206.
Knowledge of the potential dangers of mixed martial arts is valuable for Department of Defense (DoD) health care providers as the military continues to implement combatives training into regular military instruction. This case study presents an active-duty service member who developed a spontaneous vertebral artery dissection (sVAD) during mixed martial arts training, which led to a cerebellar stroke.
To the authors’ knowledge this is the first documented case of a sVAD with associated stroke related to a mixed martial arts choke hold. Understanding the diagnosis, management, and prognosis of this condition will remain important as hand-to-hand combat instruction continues to be a part of regular military training.
Case Presentation
A 39-year-old active-duty male without significant past medical history presented to the emergency department (ED) at the San Antonio Military Medical Center in Texas for evaluation of severe vertigo with associated nausea and vomiting. He had participated in a Jiu-Jitsu match the evening prior to his presentation and reported that he was placed in a choke hold within the last 12 seconds of the match. He denied losing consciousness during this hold.
Once released, he attempted to stand and developed sudden onset vertigo with severe nausea, leading to multiple bouts of emesis. He additionally developed a throbbing, left-sided headache radiating down the left side of his neck. While the vertigo resolved within an hour, he continued to experience bouts of nausea and emesis, prompting him to present to the ED for further evaluation. The patient’s past medical history was remarkable only for multiple prior concussions, and his only medication was occasional ibuprofen. He denied the usage of recreational drugs.
Upon presentation to the ED, the patient’s vital signs were 139/93 mm Hg blood pressure, 73 beats per minute heart rate, 16 breaths per minute respiration, 100% oxygen saturation on room air, and 97.7° F temperature.
The patient demonstrated normal balance and exhibited no nystagmus or limb/truncal ataxia as evaluated with finger-to-nose/heel-to-shin testing and gait exam. Complete blood count, comprehensive metabolic panel, and coagulation panel all demonstrated no abnormalities.
The patient was admitted to the hospital for symptom control and further monitoring. His headache and nausea were managed with medications, and he began antiplatelet therapy with aspirin 325 mg daily. Given the size of his cerebellar infarction, it was decided that he would be monitored in the hospital for 72 hours for the development of significant cerebellar edema. He remained stable throughout his hospitalization and had only a mild headache at the time of discharge.
The patient was last seen 3 months postinjury with no subjective complaints and a completely normal neurologic exam. The treatment plan for the patient is to continue aspirin for 6 months postinjury at which time a repeat CT will be performed to ensure resolution. He has been counseled to avoid heavy lifting and any activity with potential for sudden movement/force of the neck (grappling/wrestling, chiropractic manipulation, roller coasters, or sit-ups) until the repeat CT has been completed.
Discussion
Spontaneous vertebral artery and carotid artery dissections are collectively referred to as sCADs. Spontaneous cervical artery dissections are a rare condition with a higher incidence of internal carotid dissections than are VADs (1.72 vs 0.97 per 100,000 people).1 In contrast to the general stroke population, patients with sCADs are typically younger (mean age 45.3 years); and more than half of the patients are male.1,2
Spontaneous cervical artery dissections are typically characterized by subintimal tears of the vertebral artery leading to the accumulation of an intramural hematoma and creation of a “false lumen” in the arterial wall.3 A sVAD is more often found in the pars transversaria (V2; 35%) or atlas loop (V3; 34%) segments of the vertebral artery than in the prevertebral (V1; 20%) or intracranial (V4; 11%) segments.3-5 The etiology of these injuries is thought to be minor trauma to the neck in the context of a likely underlying connective tissue disease, though no direct association with a particular disease has been shown.
Biopsy evaluation of the superficial temporal arteries of patients with sCADs have revealed pathologic changes of the media and adventitial layers, including vacuolar degeneration and capillary neoangiogenesis, which are not found in the arteries of control patients.5 Although definitive association with a known connective tissue disease is rare, angiographic evidence of fibromuscular dysplasia, a nonspecific marker of connective tissue disease, is noted in as many as 15% to 20% of patients.6 Consequently, routine connective tissue disease screening is not recommended in these patients. One study found that about 40% of sCAD patients can recall minor cervical trauma in the preceding month in comparison to only 10% of other patients with stroke, leading to the moniker of “bottoms-up” or “beauty-parlor strokes” for these injuries. The most common mechanisms of minor neck trauma causing sCADs include tennis and golf swings, yoga, and roller-coaster rides.7,8
Usually symptomatic at presentation, the most frequently encountered sCAD symptoms are head or neck pain (80%), brain ischemia (56%), and Horner syndrome (25%).1 A study of 161 consecutive patients with internal carotid (n = 135) or vertebral artery (n = 26) dissections revealed that headache was reported by 69% of those with sVADs, and when present, was the initial manifestation in 33%. Headaches typically were ipsilateral to the dissection, located posteriorly in 83% of patients, and lasted an average duration of 72 hours. Neck pain, which was noted in 46% of sVAD patients, was predominantly posterior and ipsilateral in location as well.9 Ischemic symptoms of sVAD may include posterior circulation symptoms, such as vertigo, ataxia, diplopia, and leg weakness as well as lateral medullary (Wallenberg) syndrome characterized by dizziness, postural instability, limb hypotonia/ataxia, blurred vision, and nystagmus.
In a study of 169 patients with sCAD, brain ischemia occurred in 77% (131 patients) including 67% (n = 114) with ischemic stroke and 10% (n = 17) with transient ischemic attack. Head and/or neck pain was noted in 88% of those with brain ischemia.4 Etiologies for infarction included thromboembolic (85%), hemodynamic (12%), and mixed (3%).10 Isolated local symptoms are rare with one study of 245 patients with sCAD revealing only 20 (8%) presenting with pain only. Of those with pain only, 6 presented with headache, 2 with neck pain, and 12 with both.11
Diagnosis of sVAD requires a high index of suspicion and is confirmed by diagnostic testing. Previously, invasive angiography was the diagnostic gold standard, but with the improvement in quality of CT and MR angiography, these noninvasive modalities have become the tests of choice. There have been no studies to date revealing a definitive benefit of one modality over the other. A meta-analysis of 25 articles that compared the use of CT and MR angiography for the diagnosis of carotid and VAD revealed similar sensitivity and specificity.12 In contrast, a study involving 10 patients with confirmed sVAD who had both CT and MR angiographies during evaluation showed more total findings consistent with dissection on CT than with MR angiography when graded by 2 neuroradiologists. Additionally, the neuroradiologists subjectively rated CT angiography as preferential to MR in showing the imaging findings of dissection in 8 of 10 cases of vertebral dissection.13
Treatment for sCAD remains heavily debated. The use of IV thrombolysis within the standard time window for acute ischemic stroke is advocated for these patients. A meta-analysis of patients with sCAD vs matched patients with stroke from other causes treated with IV thrombolysis showed no difference in mortality at 3 months (9.0% vs 8.8%) or symptomatic intracranial hemorrhage (3.3% vs 3.0%). Additionally, similar percentages of patients had excellent (30.9% vs 37.4%) and favorable (58.2% vs 52.2%) 3-month functional statuses as expressed by the Modified Rankin Score (mRS).14,15
Debate remains regarding subacute therapy for sCAD with either antiplatelet or anticoagulant therapy. A randomized study of 250 patients with cervical artery dissection (118 carotid, 132 vertebral) in which 126 patients were assigned to antiplatelet therapy and 124 patients were assigned to anticoagulant therapy showed an overall low rate of recurrent stroke (2%). There was no significant difference in efficacy between the therapy groups with stroke or death occurring in 3 antiplatelet patients and 1 anticoagulated patient. Adverse effects were very low in both groups with no deaths and only 1 major bleed in the anticoagulation group. Of note, stroke rates were lower in this study than prior observational studies.16
A nonrandomized study of 88 patients with extracranial sCAD showed overall low rates of recurrent ischemic stroke at 3 months with 1/59 (1.7%) in the antiplatelet group and 1/28 (3.6%) in the anticoagulation group (P 17 Given this low overall rate of recurrent stroke in prior studies, a guideline recommendation for antiplatelet or anticoagulant therapy cannot be made at this time.
The overall prognosis for this condition is fair. Functional status and recurrence risk are favorable, with one study finding a mRS score of 1 Additionally, a historic cohort study of 432 patients with first event of sCAD revealed that after a mean follow-up of 31 months, only 4 (0.9%) patients had a recurrent ischemic stroke either due to incomplete recanalization of the artery (n = 2) or recurrent sCAD (n = 2), and only 4 (0.9%) total recurrences of sCAD were report (2 without associated ischemic strokes).18 Further, a prospective study of 61 patients with confirmed sVAD revealed complete recanalization of 45.9% at 3 months, 62.3% at 6 months, and 63.9% at 12 months, suggesting that recanalization occurs mostly during the initial 6 months. There was no identified association between outcome and complete recanalization with favorable outcomes observed in 55 (90.2%) of patients and no further ischemic symptoms during follow-up.19
Neck maneuvers have been cited as a more common cause of sCAD in several previous studies. One retrospective study found chiropractic neck manipulation to be the etiology in 12 of 141 patients with CT- or MR- confirmed sCAD.20 As noted previously, to the authors’ knowledge this is the first reported case of a sVAD occurring after a mixed martial arts choke hold. While sports-related strokes are rare, one evaluation of 70 published cases found that 80% were due to sCAD. Commonly associated sports in this study included football, yoga, wrestling, tennis, golf, and swimming.21 Grappling-related neck manipulation has been noted as an etiology in a few case reports.
Hyperextension of the neck was deemed to be the etiology in boys aged 11 years and 17 years who developed a sCAD while participating in Judo and backyard wrestling, respectively.22,23 In the martial arts realm, there is a case report of a 26-year-old male who developed a sVAD after rapid head turning during a solo Kung Fu maneuver as well as a report of a 41-year-old male experiencing a right VAD complicated by a posterior infarction several days after straining his neck during a mixed martial arts competition.24,25 The patient denied any choke hold or direct blow to the neck.
The present case is different in that it is the first reported case of a sVAD occurring after a submission maneuver. Prior grappling-related sVADs were associated with hyperextension or rapid acceleration/deceleration forces on the neck. Isometric force to the neck is a rarely described mechanism for development of this injury. Although there are isolated and infrequent forensic case reports of carotid dissection with strangulation injuries, the authors believe this is the first documented case of a sVAD attributed to a combatives submission.
In the context of the military health system, it is important to be aware of this potential complication of combatives as instruction in close-quarters combat continues to be an important part of military training.
Knowledge of the potential dangers of mixed martial arts is valuable for Department of Defense (DoD) health care providers as the military continues to implement combatives training into regular military instruction. This case study presents an active-duty service member who developed a spontaneous vertebral artery dissection (sVAD) during mixed martial arts training, which led to a cerebellar stroke.
To the authors’ knowledge this is the first documented case of a sVAD with associated stroke related to a mixed martial arts choke hold. Understanding the diagnosis, management, and prognosis of this condition will remain important as hand-to-hand combat instruction continues to be a part of regular military training.
Case Presentation
A 39-year-old active-duty male without significant past medical history presented to the emergency department (ED) at the San Antonio Military Medical Center in Texas for evaluation of severe vertigo with associated nausea and vomiting. He had participated in a Jiu-Jitsu match the evening prior to his presentation and reported that he was placed in a choke hold within the last 12 seconds of the match. He denied losing consciousness during this hold.
Once released, he attempted to stand and developed sudden onset vertigo with severe nausea, leading to multiple bouts of emesis. He additionally developed a throbbing, left-sided headache radiating down the left side of his neck. While the vertigo resolved within an hour, he continued to experience bouts of nausea and emesis, prompting him to present to the ED for further evaluation. The patient’s past medical history was remarkable only for multiple prior concussions, and his only medication was occasional ibuprofen. He denied the usage of recreational drugs.
Upon presentation to the ED, the patient’s vital signs were 139/93 mm Hg blood pressure, 73 beats per minute heart rate, 16 breaths per minute respiration, 100% oxygen saturation on room air, and 97.7° F temperature.
The patient demonstrated normal balance and exhibited no nystagmus or limb/truncal ataxia as evaluated with finger-to-nose/heel-to-shin testing and gait exam. Complete blood count, comprehensive metabolic panel, and coagulation panel all demonstrated no abnormalities.
The patient was admitted to the hospital for symptom control and further monitoring. His headache and nausea were managed with medications, and he began antiplatelet therapy with aspirin 325 mg daily. Given the size of his cerebellar infarction, it was decided that he would be monitored in the hospital for 72 hours for the development of significant cerebellar edema. He remained stable throughout his hospitalization and had only a mild headache at the time of discharge.
The patient was last seen 3 months postinjury with no subjective complaints and a completely normal neurologic exam. The treatment plan for the patient is to continue aspirin for 6 months postinjury at which time a repeat CT will be performed to ensure resolution. He has been counseled to avoid heavy lifting and any activity with potential for sudden movement/force of the neck (grappling/wrestling, chiropractic manipulation, roller coasters, or sit-ups) until the repeat CT has been completed.
Discussion
Spontaneous vertebral artery and carotid artery dissections are collectively referred to as sCADs. Spontaneous cervical artery dissections are a rare condition with a higher incidence of internal carotid dissections than are VADs (1.72 vs 0.97 per 100,000 people).1 In contrast to the general stroke population, patients with sCADs are typically younger (mean age 45.3 years); and more than half of the patients are male.1,2
Spontaneous cervical artery dissections are typically characterized by subintimal tears of the vertebral artery leading to the accumulation of an intramural hematoma and creation of a “false lumen” in the arterial wall.3 A sVAD is more often found in the pars transversaria (V2; 35%) or atlas loop (V3; 34%) segments of the vertebral artery than in the prevertebral (V1; 20%) or intracranial (V4; 11%) segments.3-5 The etiology of these injuries is thought to be minor trauma to the neck in the context of a likely underlying connective tissue disease, though no direct association with a particular disease has been shown.
Biopsy evaluation of the superficial temporal arteries of patients with sCADs have revealed pathologic changes of the media and adventitial layers, including vacuolar degeneration and capillary neoangiogenesis, which are not found in the arteries of control patients.5 Although definitive association with a known connective tissue disease is rare, angiographic evidence of fibromuscular dysplasia, a nonspecific marker of connective tissue disease, is noted in as many as 15% to 20% of patients.6 Consequently, routine connective tissue disease screening is not recommended in these patients. One study found that about 40% of sCAD patients can recall minor cervical trauma in the preceding month in comparison to only 10% of other patients with stroke, leading to the moniker of “bottoms-up” or “beauty-parlor strokes” for these injuries. The most common mechanisms of minor neck trauma causing sCADs include tennis and golf swings, yoga, and roller-coaster rides.7,8
Usually symptomatic at presentation, the most frequently encountered sCAD symptoms are head or neck pain (80%), brain ischemia (56%), and Horner syndrome (25%).1 A study of 161 consecutive patients with internal carotid (n = 135) or vertebral artery (n = 26) dissections revealed that headache was reported by 69% of those with sVADs, and when present, was the initial manifestation in 33%. Headaches typically were ipsilateral to the dissection, located posteriorly in 83% of patients, and lasted an average duration of 72 hours. Neck pain, which was noted in 46% of sVAD patients, was predominantly posterior and ipsilateral in location as well.9 Ischemic symptoms of sVAD may include posterior circulation symptoms, such as vertigo, ataxia, diplopia, and leg weakness as well as lateral medullary (Wallenberg) syndrome characterized by dizziness, postural instability, limb hypotonia/ataxia, blurred vision, and nystagmus.
In a study of 169 patients with sCAD, brain ischemia occurred in 77% (131 patients) including 67% (n = 114) with ischemic stroke and 10% (n = 17) with transient ischemic attack. Head and/or neck pain was noted in 88% of those with brain ischemia.4 Etiologies for infarction included thromboembolic (85%), hemodynamic (12%), and mixed (3%).10 Isolated local symptoms are rare with one study of 245 patients with sCAD revealing only 20 (8%) presenting with pain only. Of those with pain only, 6 presented with headache, 2 with neck pain, and 12 with both.11
Diagnosis of sVAD requires a high index of suspicion and is confirmed by diagnostic testing. Previously, invasive angiography was the diagnostic gold standard, but with the improvement in quality of CT and MR angiography, these noninvasive modalities have become the tests of choice. There have been no studies to date revealing a definitive benefit of one modality over the other. A meta-analysis of 25 articles that compared the use of CT and MR angiography for the diagnosis of carotid and VAD revealed similar sensitivity and specificity.12 In contrast, a study involving 10 patients with confirmed sVAD who had both CT and MR angiographies during evaluation showed more total findings consistent with dissection on CT than with MR angiography when graded by 2 neuroradiologists. Additionally, the neuroradiologists subjectively rated CT angiography as preferential to MR in showing the imaging findings of dissection in 8 of 10 cases of vertebral dissection.13
Treatment for sCAD remains heavily debated. The use of IV thrombolysis within the standard time window for acute ischemic stroke is advocated for these patients. A meta-analysis of patients with sCAD vs matched patients with stroke from other causes treated with IV thrombolysis showed no difference in mortality at 3 months (9.0% vs 8.8%) or symptomatic intracranial hemorrhage (3.3% vs 3.0%). Additionally, similar percentages of patients had excellent (30.9% vs 37.4%) and favorable (58.2% vs 52.2%) 3-month functional statuses as expressed by the Modified Rankin Score (mRS).14,15
Debate remains regarding subacute therapy for sCAD with either antiplatelet or anticoagulant therapy. A randomized study of 250 patients with cervical artery dissection (118 carotid, 132 vertebral) in which 126 patients were assigned to antiplatelet therapy and 124 patients were assigned to anticoagulant therapy showed an overall low rate of recurrent stroke (2%). There was no significant difference in efficacy between the therapy groups with stroke or death occurring in 3 antiplatelet patients and 1 anticoagulated patient. Adverse effects were very low in both groups with no deaths and only 1 major bleed in the anticoagulation group. Of note, stroke rates were lower in this study than prior observational studies.16
A nonrandomized study of 88 patients with extracranial sCAD showed overall low rates of recurrent ischemic stroke at 3 months with 1/59 (1.7%) in the antiplatelet group and 1/28 (3.6%) in the anticoagulation group (P 17 Given this low overall rate of recurrent stroke in prior studies, a guideline recommendation for antiplatelet or anticoagulant therapy cannot be made at this time.
The overall prognosis for this condition is fair. Functional status and recurrence risk are favorable, with one study finding a mRS score of 1 Additionally, a historic cohort study of 432 patients with first event of sCAD revealed that after a mean follow-up of 31 months, only 4 (0.9%) patients had a recurrent ischemic stroke either due to incomplete recanalization of the artery (n = 2) or recurrent sCAD (n = 2), and only 4 (0.9%) total recurrences of sCAD were report (2 without associated ischemic strokes).18 Further, a prospective study of 61 patients with confirmed sVAD revealed complete recanalization of 45.9% at 3 months, 62.3% at 6 months, and 63.9% at 12 months, suggesting that recanalization occurs mostly during the initial 6 months. There was no identified association between outcome and complete recanalization with favorable outcomes observed in 55 (90.2%) of patients and no further ischemic symptoms during follow-up.19
Neck maneuvers have been cited as a more common cause of sCAD in several previous studies. One retrospective study found chiropractic neck manipulation to be the etiology in 12 of 141 patients with CT- or MR- confirmed sCAD.20 As noted previously, to the authors’ knowledge this is the first reported case of a sVAD occurring after a mixed martial arts choke hold. While sports-related strokes are rare, one evaluation of 70 published cases found that 80% were due to sCAD. Commonly associated sports in this study included football, yoga, wrestling, tennis, golf, and swimming.21 Grappling-related neck manipulation has been noted as an etiology in a few case reports.
Hyperextension of the neck was deemed to be the etiology in boys aged 11 years and 17 years who developed a sCAD while participating in Judo and backyard wrestling, respectively.22,23 In the martial arts realm, there is a case report of a 26-year-old male who developed a sVAD after rapid head turning during a solo Kung Fu maneuver as well as a report of a 41-year-old male experiencing a right VAD complicated by a posterior infarction several days after straining his neck during a mixed martial arts competition.24,25 The patient denied any choke hold or direct blow to the neck.
The present case is different in that it is the first reported case of a sVAD occurring after a submission maneuver. Prior grappling-related sVADs were associated with hyperextension or rapid acceleration/deceleration forces on the neck. Isometric force to the neck is a rarely described mechanism for development of this injury. Although there are isolated and infrequent forensic case reports of carotid dissection with strangulation injuries, the authors believe this is the first documented case of a sVAD attributed to a combatives submission.
In the context of the military health system, it is important to be aware of this potential complication of combatives as instruction in close-quarters combat continues to be an important part of military training.
1. Lee VH, Brown RD Jr, Mandrekar J, Mokri B. Incidence and outcome of cervical artery dissection: a population-based study. Neurology. 2006;67(10):1809-1812.
2. Arnold M, Kappeler L, Georgiadis D, et al. Gender differences in spontaneous cervical artery dissection. Neurology. 2006;67(6):1050-1052.
3. Schvienk W. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
4. Arnold M, Bousser MG, Fahrni G, et al. Vertebral artery dissection: presenting findings and predictors of outcome. Stroke. 2006;37(10):2499-2503.
5. Völker W, Dittrich R, Grewe S, et al. The outer arterial wall layers are primarily affected in spontaneous cervical artery dissection. Neurology. 2011;76(17):1463-1471.
6. Debette S, Markus HS. The genetics of cervical artery dissection: a systematic review. Stroke. 2009;40(6):459-466.
7. DeBehnke D, Brady W. Vertebral artery dissection due to minor neck trauma. J Emerg Med. 1994;12(1):27-31.
8. Engelter ST, Grond-Ginsbach C, Metso TM, et al; Cervical Artery Dissection and Ischemic Stroke Patients Study Group. Cervical artery dissection: trauma and other potential mechanical trigger events. Neurology. 2013;80(21):1950-1957.
9. Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522.
10. Morel A, Naggara O, Touzé E, et al. Mechanism of ischemic infarct in spontaneous cervical artery dissection. Stroke. 2012;43(5):1354-1361.
11. Arnold M, Cumurciuc R, Stapf C, Favrole P, Berthet K, Bousser MG. Pain as the only symptom of cervical artery dissection. J Neurol Neurosurg Psychiatry. 2006;77(9):1021-1024.
12. Provenzale J, Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol. 2009;1939(4):1167-1174.
13. Vertinsky AT, Schwartz NE, Fishbein NJ, Rosenberg J, Albers GW, Zaharchuk G. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol. 2008;29(9):1753-1760.
14. Zinkstok SM, Vergouwen MD, Engelter ST, et al. Safety and functional outcome of thrombolysis in dissection-related ischemic stroke: a meta-analysis of individual patient data. Stroke. 2011;42(9):2515-2520.
15. Engelter S, Rutgers M, Hatz F, et al. Intravenous thrombolysis in stroke attributable to cervical artery dissection. Stroke. 2009;40(12):3772-3776.
16. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomized trial. Lancet Neurol. 2015;14(4):361-367.
17. Kennedy F, Lanfranconi S, Hicks C, et al; CADISS Investigators. Antiplatelets vs. anticoagulation for dissection: CADISS nonrandomized arm and meta-analysis. Neurology. 2012;79(7):686-689.
18. Touze E, Gauvrit JY, Moulin T, Meder JF, Bracard S, Mas JL; Multicenter Survey on Natural History of Cervical Artery Dissection. Risk of stroke and recurrent dissection after a cervical artery dissection: a multicenter study. Neurology. 2003;61(10):1347-1351.
19. Arauz A, Marquez J, Artigas C, Balderrama J, Orrego H. Recanalization of vertebral artery dissection. Stroke. 2010;41(4):717-721.
20. Kennell KA, Daghfal MM, Patel SG, et DeSanto JR, Waterman GS, Bertino RE. Cervical artery dissection related to chiropractic manipulation: one institution’s experience. J Fam Pract. 2017;66(9):556-562.
21. McCrory P. Vertebral artery dissection causing stroke in sport. J Clin Neurosci. 2000;7(4):298-300.
22. Lannuzel A, Moulin T, Amsallem D, Galmiche J, Rumbach L. Vertebral artery dissection following a judo session: a case report. Neuropediatrics. 1994;25(2):106-108.
23. Gupta V, Dhawan N, Bahl J. Minor trauma causing stroke in a young athlete. Case Rep Neurol Med. 2015;2015: 182875.
24. Pacei F, Valvasorri L, Bet L. Vertebral artery dissection during Kung-Fu training. Neurol Sci. 2014;35(2):331-332.
25. Slowey M, Maw G, Furyk J. Case report on vertebral artery dissection in mixed martial arts. Emerg Med Australas. 2012;24(2):203-206.
1. Lee VH, Brown RD Jr, Mandrekar J, Mokri B. Incidence and outcome of cervical artery dissection: a population-based study. Neurology. 2006;67(10):1809-1812.
2. Arnold M, Kappeler L, Georgiadis D, et al. Gender differences in spontaneous cervical artery dissection. Neurology. 2006;67(6):1050-1052.
3. Schvienk W. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
4. Arnold M, Bousser MG, Fahrni G, et al. Vertebral artery dissection: presenting findings and predictors of outcome. Stroke. 2006;37(10):2499-2503.
5. Völker W, Dittrich R, Grewe S, et al. The outer arterial wall layers are primarily affected in spontaneous cervical artery dissection. Neurology. 2011;76(17):1463-1471.
6. Debette S, Markus HS. The genetics of cervical artery dissection: a systematic review. Stroke. 2009;40(6):459-466.
7. DeBehnke D, Brady W. Vertebral artery dissection due to minor neck trauma. J Emerg Med. 1994;12(1):27-31.
8. Engelter ST, Grond-Ginsbach C, Metso TM, et al; Cervical Artery Dissection and Ischemic Stroke Patients Study Group. Cervical artery dissection: trauma and other potential mechanical trigger events. Neurology. 2013;80(21):1950-1957.
9. Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522.
10. Morel A, Naggara O, Touzé E, et al. Mechanism of ischemic infarct in spontaneous cervical artery dissection. Stroke. 2012;43(5):1354-1361.
11. Arnold M, Cumurciuc R, Stapf C, Favrole P, Berthet K, Bousser MG. Pain as the only symptom of cervical artery dissection. J Neurol Neurosurg Psychiatry. 2006;77(9):1021-1024.
12. Provenzale J, Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol. 2009;1939(4):1167-1174.
13. Vertinsky AT, Schwartz NE, Fishbein NJ, Rosenberg J, Albers GW, Zaharchuk G. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol. 2008;29(9):1753-1760.
14. Zinkstok SM, Vergouwen MD, Engelter ST, et al. Safety and functional outcome of thrombolysis in dissection-related ischemic stroke: a meta-analysis of individual patient data. Stroke. 2011;42(9):2515-2520.
15. Engelter S, Rutgers M, Hatz F, et al. Intravenous thrombolysis in stroke attributable to cervical artery dissection. Stroke. 2009;40(12):3772-3776.
16. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomized trial. Lancet Neurol. 2015;14(4):361-367.
17. Kennedy F, Lanfranconi S, Hicks C, et al; CADISS Investigators. Antiplatelets vs. anticoagulation for dissection: CADISS nonrandomized arm and meta-analysis. Neurology. 2012;79(7):686-689.
18. Touze E, Gauvrit JY, Moulin T, Meder JF, Bracard S, Mas JL; Multicenter Survey on Natural History of Cervical Artery Dissection. Risk of stroke and recurrent dissection after a cervical artery dissection: a multicenter study. Neurology. 2003;61(10):1347-1351.
19. Arauz A, Marquez J, Artigas C, Balderrama J, Orrego H. Recanalization of vertebral artery dissection. Stroke. 2010;41(4):717-721.
20. Kennell KA, Daghfal MM, Patel SG, et DeSanto JR, Waterman GS, Bertino RE. Cervical artery dissection related to chiropractic manipulation: one institution’s experience. J Fam Pract. 2017;66(9):556-562.
21. McCrory P. Vertebral artery dissection causing stroke in sport. J Clin Neurosci. 2000;7(4):298-300.
22. Lannuzel A, Moulin T, Amsallem D, Galmiche J, Rumbach L. Vertebral artery dissection following a judo session: a case report. Neuropediatrics. 1994;25(2):106-108.
23. Gupta V, Dhawan N, Bahl J. Minor trauma causing stroke in a young athlete. Case Rep Neurol Med. 2015;2015: 182875.
24. Pacei F, Valvasorri L, Bet L. Vertebral artery dissection during Kung-Fu training. Neurol Sci. 2014;35(2):331-332.
25. Slowey M, Maw G, Furyk J. Case report on vertebral artery dissection in mixed martial arts. Emerg Med Australas. 2012;24(2):203-206.
Lower risk of bleeding with apixaban
Real-world data suggest that apixaban poses a lower risk of bleeding than warfarin, but patients who receive lower doses of apixaban have an increased risk of all-cause mortality.
Compared to warfarin, apixaban was associated with a decreased risk of major bleeding, intracranial bleeding, and gastrointestinal bleeding in certain patients.
Dabigatran and rivaroxaban were associated with a decreased risk of intracranial bleeding for certain patients, compared to warfarin.
However, patients who received rivaroxaban or lower doses of apixaban had an increased risk of all-cause mortality, compared to patients who received warfarin.
Yana Vinogradova, PhD, of University of Nottingham in the UK, and her colleagues reported these results in The BMJ.
The researchers set out to investigate the risks and benefits associated with apixaban, dabigatran, and rivaroxaban compared with warfarin in patients with and without atrial fibrillation (AF).
Using data from 2 large UK primary care databases, the researchers identified 196,061 patients who started or restarted anticoagulants (after more than a 12-month gap) between 2011 and 2016.
There were 132,231 patients taking warfarin, 7744 on dabigatran, 37,863 on rivaroxaban, and 18,223 on apixaban.
Slightly more than half of patients (53%, n=103,270) were diagnosed with AF, and 47% (n=92,791) were prescribed anticoagulants for other conditions.
When compared to warfarin in patients with AF, apixaban was associated with a decreased risk of:
- Major bleeding—adjusted hazard ratio [aHR]=0.66
- Intracranial bleeding—aHR=0.40.
In patients without AF, apixaban was associated with a decreased risk of:
- Major bleeding—aHR=0.60
- Any gastrointestinal bleeding—aHR=0.55
- Upper gastrointestinal bleeding—aHR=0.55.
In patients with AF, dabigatran was associated with a decreased risk of intracranial bleeding—aHR=0.45—compared to warfarin.
In patients without AF, rivaroxaban was associated with a decreased risk of intracranial bleeding—aHR=0.54—compared to warfarin.
Compared to patients taking warfarin, there was an increased risk of all-cause mortality for patients:
- With AF taking rivaroxaban—aHR=1.19
- Without AF taking rivaroxaban— aHR=1.51
- With AF taking lower doses of apixaban—aHR=1.27
- Without AF taking lower doses of apixaban—aHR=1.34.
The researchers said the increased risk of all-cause mortality in these patients may reflect the fact that patients taking warfarin are monitored more closely, or it may be related to underlying conditions.
The team did point out that this is an observational study, so no firm conclusions can be drawn about cause and effect. They also outlined some limitations, such as possible misclassification due to patients not taking their medication.
Nevertheless, the researchers concluded that “the risk of major bleeding is lower in apixaban users, regardless of the reason for prescribing, appearing to show apixaban to be the safest drug.”
Real-world data suggest that apixaban poses a lower risk of bleeding than warfarin, but patients who receive lower doses of apixaban have an increased risk of all-cause mortality.
Compared to warfarin, apixaban was associated with a decreased risk of major bleeding, intracranial bleeding, and gastrointestinal bleeding in certain patients.
Dabigatran and rivaroxaban were associated with a decreased risk of intracranial bleeding for certain patients, compared to warfarin.
However, patients who received rivaroxaban or lower doses of apixaban had an increased risk of all-cause mortality, compared to patients who received warfarin.
Yana Vinogradova, PhD, of University of Nottingham in the UK, and her colleagues reported these results in The BMJ.
The researchers set out to investigate the risks and benefits associated with apixaban, dabigatran, and rivaroxaban compared with warfarin in patients with and without atrial fibrillation (AF).
Using data from 2 large UK primary care databases, the researchers identified 196,061 patients who started or restarted anticoagulants (after more than a 12-month gap) between 2011 and 2016.
There were 132,231 patients taking warfarin, 7744 on dabigatran, 37,863 on rivaroxaban, and 18,223 on apixaban.
Slightly more than half of patients (53%, n=103,270) were diagnosed with AF, and 47% (n=92,791) were prescribed anticoagulants for other conditions.
When compared to warfarin in patients with AF, apixaban was associated with a decreased risk of:
- Major bleeding—adjusted hazard ratio [aHR]=0.66
- Intracranial bleeding—aHR=0.40.
In patients without AF, apixaban was associated with a decreased risk of:
- Major bleeding—aHR=0.60
- Any gastrointestinal bleeding—aHR=0.55
- Upper gastrointestinal bleeding—aHR=0.55.
In patients with AF, dabigatran was associated with a decreased risk of intracranial bleeding—aHR=0.45—compared to warfarin.
In patients without AF, rivaroxaban was associated with a decreased risk of intracranial bleeding—aHR=0.54—compared to warfarin.
Compared to patients taking warfarin, there was an increased risk of all-cause mortality for patients:
- With AF taking rivaroxaban—aHR=1.19
- Without AF taking rivaroxaban— aHR=1.51
- With AF taking lower doses of apixaban—aHR=1.27
- Without AF taking lower doses of apixaban—aHR=1.34.
The researchers said the increased risk of all-cause mortality in these patients may reflect the fact that patients taking warfarin are monitored more closely, or it may be related to underlying conditions.
The team did point out that this is an observational study, so no firm conclusions can be drawn about cause and effect. They also outlined some limitations, such as possible misclassification due to patients not taking their medication.
Nevertheless, the researchers concluded that “the risk of major bleeding is lower in apixaban users, regardless of the reason for prescribing, appearing to show apixaban to be the safest drug.”
Real-world data suggest that apixaban poses a lower risk of bleeding than warfarin, but patients who receive lower doses of apixaban have an increased risk of all-cause mortality.
Compared to warfarin, apixaban was associated with a decreased risk of major bleeding, intracranial bleeding, and gastrointestinal bleeding in certain patients.
Dabigatran and rivaroxaban were associated with a decreased risk of intracranial bleeding for certain patients, compared to warfarin.
However, patients who received rivaroxaban or lower doses of apixaban had an increased risk of all-cause mortality, compared to patients who received warfarin.
Yana Vinogradova, PhD, of University of Nottingham in the UK, and her colleagues reported these results in The BMJ.
The researchers set out to investigate the risks and benefits associated with apixaban, dabigatran, and rivaroxaban compared with warfarin in patients with and without atrial fibrillation (AF).
Using data from 2 large UK primary care databases, the researchers identified 196,061 patients who started or restarted anticoagulants (after more than a 12-month gap) between 2011 and 2016.
There were 132,231 patients taking warfarin, 7744 on dabigatran, 37,863 on rivaroxaban, and 18,223 on apixaban.
Slightly more than half of patients (53%, n=103,270) were diagnosed with AF, and 47% (n=92,791) were prescribed anticoagulants for other conditions.
When compared to warfarin in patients with AF, apixaban was associated with a decreased risk of:
- Major bleeding—adjusted hazard ratio [aHR]=0.66
- Intracranial bleeding—aHR=0.40.
In patients without AF, apixaban was associated with a decreased risk of:
- Major bleeding—aHR=0.60
- Any gastrointestinal bleeding—aHR=0.55
- Upper gastrointestinal bleeding—aHR=0.55.
In patients with AF, dabigatran was associated with a decreased risk of intracranial bleeding—aHR=0.45—compared to warfarin.
In patients without AF, rivaroxaban was associated with a decreased risk of intracranial bleeding—aHR=0.54—compared to warfarin.
Compared to patients taking warfarin, there was an increased risk of all-cause mortality for patients:
- With AF taking rivaroxaban—aHR=1.19
- Without AF taking rivaroxaban— aHR=1.51
- With AF taking lower doses of apixaban—aHR=1.27
- Without AF taking lower doses of apixaban—aHR=1.34.
The researchers said the increased risk of all-cause mortality in these patients may reflect the fact that patients taking warfarin are monitored more closely, or it may be related to underlying conditions.
The team did point out that this is an observational study, so no firm conclusions can be drawn about cause and effect. They also outlined some limitations, such as possible misclassification due to patients not taking their medication.
Nevertheless, the researchers concluded that “the risk of major bleeding is lower in apixaban users, regardless of the reason for prescribing, appearing to show apixaban to be the safest drug.”