User login
JAK inhibition linked to B-cell lymphoma
New research indicates that JAK inhibitors may increase the risk of lymphoma in patients with myelofibrosis (MF).
The patients studied had a 15- to 25-fold higher risk of developing B-cell lymphoma if they received treatment with JAK inhibitors.
The researchers speculate that screening MF patients for a pre-existing B-cell clone before starting JAK inhibitor therapy may help prevent lymphoma development.
Heinz Gisslinger, MD, of the Medical University of Vienna in Austria, and his colleagues conducted this research and reported the findings in Blood.
“[W]e started noticing sporadic cases of lymphomas developing in patients being treated for myeloproliferative neoplasms and wanted to know if this phenomenon was connected to treatment,” Dr Gisslinger said.
Therefore, he and his colleagues assessed 626 patients receiving treatment for myeloproliferative neoplasms (MPNs) at the Medical University of Vienna.
The incidence of B-cell lymphoma was 5.8% (4/69) in patients treated with JAK inhibitors and 0.36% (2/557) in patients who did not receive JAK inhibitors. That amounts to a 16-fold increased risk of lymphoma in patients receiving JAK inhibitors.
When the researchers analyzed only patients with primary MF (n=216), the increased risk of B-cell lymphoma was even greater. The incidence of lymphoma was 9.68% (3/31) in patients treated with JAK inhibitors and 0.54% (1/185) in patients who did not receive JAK inhibitors.
That corresponds to a 19-fold increased risk of B-cell lymphoma in primary MF patients treated with JAK inhibitors. When the researchers adjusted for age, there was a 21-fold greater risk. When they adjusted for sex, the risk was 25 times higher.
In a second cohort of 929 MPN patients, the incidence of B-cell lymphoma was 3.51% (2/57) in patients who received JAK inhibitors and 0.23% (2/872) in patients who did not. This corresponds to a 15-fold increased risk of lymphoma in the JAK inhibitor recipients.
Lymphoma cases
In all, there were 6 patients who developed lymphoma after JAK inhibitor treatment. Five developed diffuse large B-cell lymphoma, and 1 had high-grade B-cell lymphoma not otherwise specified.
Four of the patients had primary MF, 1 had post-polycythemia vera MF, and 1 had post-essential thrombocythemia (ET) MF. Five patients had a JAK2V617F mutation, and 1 (the post-ET MF patient) had a CALR mutation.
All 6 patients had received treatment with ruxolitinib. One patient also received fedratinib.
B-cell clone
The researchers studied bone marrow samples from 54 of the 69 patients treated with JAK inhibitors in the first cohort. The team found a pre-existing B-cell clone in 3 of the 4 patients who developed lymphoma. Further investigation suggested this was the clone that later transformed into lymphoma.
The researchers also found an association between JAK inhibition and an increased frequency of aggressive B-cell lymphomas in mouse models.
“By replicating this link between this B-cell clone and aggressive lymphoma, we hope to speed the discovery of an alternative therapy for myelofibrosis,” said study author Veronica Sexl, MD, of the University of Veterinary Medicine in Vienna. “These findings are going to be valuable in clinical care.”
“We determined that patients with this pre-existing B-cell clone in their bone marrow are most at risk for developing aggressive lymphoma,” added study author Ulrich Jäger, MD, of the Medical University of Vienna.
“We also know that up to 16% of people with myelofibrosis have immunoglobulin gene rearrangements like this B-cell clone. Therefore, our findings suggest that all patients with myelofibrosis should be tested for such gene rearrangements before prescribing JAK inhibitors to treat their disease.”
New research indicates that JAK inhibitors may increase the risk of lymphoma in patients with myelofibrosis (MF).
The patients studied had a 15- to 25-fold higher risk of developing B-cell lymphoma if they received treatment with JAK inhibitors.
The researchers speculate that screening MF patients for a pre-existing B-cell clone before starting JAK inhibitor therapy may help prevent lymphoma development.
Heinz Gisslinger, MD, of the Medical University of Vienna in Austria, and his colleagues conducted this research and reported the findings in Blood.
“[W]e started noticing sporadic cases of lymphomas developing in patients being treated for myeloproliferative neoplasms and wanted to know if this phenomenon was connected to treatment,” Dr Gisslinger said.
Therefore, he and his colleagues assessed 626 patients receiving treatment for myeloproliferative neoplasms (MPNs) at the Medical University of Vienna.
The incidence of B-cell lymphoma was 5.8% (4/69) in patients treated with JAK inhibitors and 0.36% (2/557) in patients who did not receive JAK inhibitors. That amounts to a 16-fold increased risk of lymphoma in patients receiving JAK inhibitors.
When the researchers analyzed only patients with primary MF (n=216), the increased risk of B-cell lymphoma was even greater. The incidence of lymphoma was 9.68% (3/31) in patients treated with JAK inhibitors and 0.54% (1/185) in patients who did not receive JAK inhibitors.
That corresponds to a 19-fold increased risk of B-cell lymphoma in primary MF patients treated with JAK inhibitors. When the researchers adjusted for age, there was a 21-fold greater risk. When they adjusted for sex, the risk was 25 times higher.
In a second cohort of 929 MPN patients, the incidence of B-cell lymphoma was 3.51% (2/57) in patients who received JAK inhibitors and 0.23% (2/872) in patients who did not. This corresponds to a 15-fold increased risk of lymphoma in the JAK inhibitor recipients.
Lymphoma cases
In all, there were 6 patients who developed lymphoma after JAK inhibitor treatment. Five developed diffuse large B-cell lymphoma, and 1 had high-grade B-cell lymphoma not otherwise specified.
Four of the patients had primary MF, 1 had post-polycythemia vera MF, and 1 had post-essential thrombocythemia (ET) MF. Five patients had a JAK2V617F mutation, and 1 (the post-ET MF patient) had a CALR mutation.
All 6 patients had received treatment with ruxolitinib. One patient also received fedratinib.
B-cell clone
The researchers studied bone marrow samples from 54 of the 69 patients treated with JAK inhibitors in the first cohort. The team found a pre-existing B-cell clone in 3 of the 4 patients who developed lymphoma. Further investigation suggested this was the clone that later transformed into lymphoma.
The researchers also found an association between JAK inhibition and an increased frequency of aggressive B-cell lymphomas in mouse models.
“By replicating this link between this B-cell clone and aggressive lymphoma, we hope to speed the discovery of an alternative therapy for myelofibrosis,” said study author Veronica Sexl, MD, of the University of Veterinary Medicine in Vienna. “These findings are going to be valuable in clinical care.”
“We determined that patients with this pre-existing B-cell clone in their bone marrow are most at risk for developing aggressive lymphoma,” added study author Ulrich Jäger, MD, of the Medical University of Vienna.
“We also know that up to 16% of people with myelofibrosis have immunoglobulin gene rearrangements like this B-cell clone. Therefore, our findings suggest that all patients with myelofibrosis should be tested for such gene rearrangements before prescribing JAK inhibitors to treat their disease.”
New research indicates that JAK inhibitors may increase the risk of lymphoma in patients with myelofibrosis (MF).
The patients studied had a 15- to 25-fold higher risk of developing B-cell lymphoma if they received treatment with JAK inhibitors.
The researchers speculate that screening MF patients for a pre-existing B-cell clone before starting JAK inhibitor therapy may help prevent lymphoma development.
Heinz Gisslinger, MD, of the Medical University of Vienna in Austria, and his colleagues conducted this research and reported the findings in Blood.
“[W]e started noticing sporadic cases of lymphomas developing in patients being treated for myeloproliferative neoplasms and wanted to know if this phenomenon was connected to treatment,” Dr Gisslinger said.
Therefore, he and his colleagues assessed 626 patients receiving treatment for myeloproliferative neoplasms (MPNs) at the Medical University of Vienna.
The incidence of B-cell lymphoma was 5.8% (4/69) in patients treated with JAK inhibitors and 0.36% (2/557) in patients who did not receive JAK inhibitors. That amounts to a 16-fold increased risk of lymphoma in patients receiving JAK inhibitors.
When the researchers analyzed only patients with primary MF (n=216), the increased risk of B-cell lymphoma was even greater. The incidence of lymphoma was 9.68% (3/31) in patients treated with JAK inhibitors and 0.54% (1/185) in patients who did not receive JAK inhibitors.
That corresponds to a 19-fold increased risk of B-cell lymphoma in primary MF patients treated with JAK inhibitors. When the researchers adjusted for age, there was a 21-fold greater risk. When they adjusted for sex, the risk was 25 times higher.
In a second cohort of 929 MPN patients, the incidence of B-cell lymphoma was 3.51% (2/57) in patients who received JAK inhibitors and 0.23% (2/872) in patients who did not. This corresponds to a 15-fold increased risk of lymphoma in the JAK inhibitor recipients.
Lymphoma cases
In all, there were 6 patients who developed lymphoma after JAK inhibitor treatment. Five developed diffuse large B-cell lymphoma, and 1 had high-grade B-cell lymphoma not otherwise specified.
Four of the patients had primary MF, 1 had post-polycythemia vera MF, and 1 had post-essential thrombocythemia (ET) MF. Five patients had a JAK2V617F mutation, and 1 (the post-ET MF patient) had a CALR mutation.
All 6 patients had received treatment with ruxolitinib. One patient also received fedratinib.
B-cell clone
The researchers studied bone marrow samples from 54 of the 69 patients treated with JAK inhibitors in the first cohort. The team found a pre-existing B-cell clone in 3 of the 4 patients who developed lymphoma. Further investigation suggested this was the clone that later transformed into lymphoma.
The researchers also found an association between JAK inhibition and an increased frequency of aggressive B-cell lymphomas in mouse models.
“By replicating this link between this B-cell clone and aggressive lymphoma, we hope to speed the discovery of an alternative therapy for myelofibrosis,” said study author Veronica Sexl, MD, of the University of Veterinary Medicine in Vienna. “These findings are going to be valuable in clinical care.”
“We determined that patients with this pre-existing B-cell clone in their bone marrow are most at risk for developing aggressive lymphoma,” added study author Ulrich Jäger, MD, of the Medical University of Vienna.
“We also know that up to 16% of people with myelofibrosis have immunoglobulin gene rearrangements like this B-cell clone. Therefore, our findings suggest that all patients with myelofibrosis should be tested for such gene rearrangements before prescribing JAK inhibitors to treat their disease.”
Cell therapy receives RMAT designation
The US Food and Drug Administration (FDA) has granted regenerative medicine advanced therapy (RMAT) designation for romyelocel-L, a myeloid progenitor cell therapy that doesn’t require HLA matching.
Romyelocel-L (CLT-008) is being developed as prophylaxis for serious bacterial and fungal infections in patients with de novo acute myeloid leukemia (AML) who develop neutropenia while receiving induction chemotherapy.
The FDA grants RMAT designation to therapies intended to treat serious or life-threatening conditions if there is preliminary clinical evidence that the therapies could address unmet medical needs.
RMAT designation provides similar advantages as breakthrough therapy designation, including early interactions with the FDA to discuss potential ways to accelerate the development of a therapy toward regulatory approval.
The FDA granted romyelocel-L RMAT designation based on a randomized, phase 2 trial of newly diagnosed AML patients who received induction consisting of cytarabine and an anthracycline.
Results from this trial were presented at the 2018 ASCO Annual Meeting (abstract 7043).
The trial enrolled 163 AML patients and randomized them, on the first day of induction, to receive:
- Daily granulocyte colony-stimulating factor (G-CSF) starting on day 14 (n=84)
- Romyelocel-L (7.5 x 106cells/kg) on day 9 plus daily G-CSF starting on day 14 (n=79).
Patients received G-CSF until neutrophil recovery to at least 500/µL.
Baseline characteristics were well balanced between the treatment arms.
There were 120 evaluable patients—59 in the romyelocel-L arm and 61 in the control arm.
The study’s primary endpoint was days in a febrile episode (DFE). The mean DFE from day 9 to 28 was 6.46 days in the romyelocel-L arm and 6.86 days in the control arm (P=0.350). The mean DFE for days 15 to 28 was 2.36 and 3.90, respectively (P=0.020).
The incidence of microbiologically or clinically diagnosed infection from day 9 to 28 was 35.6% in the romyelocel-L arm and 47.5% in the control arm, a decrease of 25% (P=0.089).
From day 15 to 28 the incidence of infection was 6.8% in the romyelocel-L arm and 27.9% in the control arm, a decrease of 76% (P=0.002).
There were no infectious deaths in the romyelocel-L arm but 2 deaths attributed to pneumonia in the control arm.
The mean hospital stay was 25.5 days in the romyelocel-L arm and 28.7 days in the control arm (P=0.002).
The proportion of patients with serious adverse events (AEs) was 14% in the romyelocel-L arm and 18% in the control arm. The proportion of patients with infectious serious AEs was 50% and 77%, respectively.
The most frequent treatment-emergent AEs (in the romyelocel-L and control arms, respectively) were febrile neutropenia (31.4% and 31%), diarrhea (25.7% and 32.4%), hypokalemia (31.4% and 25.4%), hypophosphatemia (21.4% and 23.9%), and pyrexia (22.9% and 22.5%).
There were no cases of graft-versus-host disease.
The US Food and Drug Administration (FDA) has granted regenerative medicine advanced therapy (RMAT) designation for romyelocel-L, a myeloid progenitor cell therapy that doesn’t require HLA matching.
Romyelocel-L (CLT-008) is being developed as prophylaxis for serious bacterial and fungal infections in patients with de novo acute myeloid leukemia (AML) who develop neutropenia while receiving induction chemotherapy.
The FDA grants RMAT designation to therapies intended to treat serious or life-threatening conditions if there is preliminary clinical evidence that the therapies could address unmet medical needs.
RMAT designation provides similar advantages as breakthrough therapy designation, including early interactions with the FDA to discuss potential ways to accelerate the development of a therapy toward regulatory approval.
The FDA granted romyelocel-L RMAT designation based on a randomized, phase 2 trial of newly diagnosed AML patients who received induction consisting of cytarabine and an anthracycline.
Results from this trial were presented at the 2018 ASCO Annual Meeting (abstract 7043).
The trial enrolled 163 AML patients and randomized them, on the first day of induction, to receive:
- Daily granulocyte colony-stimulating factor (G-CSF) starting on day 14 (n=84)
- Romyelocel-L (7.5 x 106cells/kg) on day 9 plus daily G-CSF starting on day 14 (n=79).
Patients received G-CSF until neutrophil recovery to at least 500/µL.
Baseline characteristics were well balanced between the treatment arms.
There were 120 evaluable patients—59 in the romyelocel-L arm and 61 in the control arm.
The study’s primary endpoint was days in a febrile episode (DFE). The mean DFE from day 9 to 28 was 6.46 days in the romyelocel-L arm and 6.86 days in the control arm (P=0.350). The mean DFE for days 15 to 28 was 2.36 and 3.90, respectively (P=0.020).
The incidence of microbiologically or clinically diagnosed infection from day 9 to 28 was 35.6% in the romyelocel-L arm and 47.5% in the control arm, a decrease of 25% (P=0.089).
From day 15 to 28 the incidence of infection was 6.8% in the romyelocel-L arm and 27.9% in the control arm, a decrease of 76% (P=0.002).
There were no infectious deaths in the romyelocel-L arm but 2 deaths attributed to pneumonia in the control arm.
The mean hospital stay was 25.5 days in the romyelocel-L arm and 28.7 days in the control arm (P=0.002).
The proportion of patients with serious adverse events (AEs) was 14% in the romyelocel-L arm and 18% in the control arm. The proportion of patients with infectious serious AEs was 50% and 77%, respectively.
The most frequent treatment-emergent AEs (in the romyelocel-L and control arms, respectively) were febrile neutropenia (31.4% and 31%), diarrhea (25.7% and 32.4%), hypokalemia (31.4% and 25.4%), hypophosphatemia (21.4% and 23.9%), and pyrexia (22.9% and 22.5%).
There were no cases of graft-versus-host disease.
The US Food and Drug Administration (FDA) has granted regenerative medicine advanced therapy (RMAT) designation for romyelocel-L, a myeloid progenitor cell therapy that doesn’t require HLA matching.
Romyelocel-L (CLT-008) is being developed as prophylaxis for serious bacterial and fungal infections in patients with de novo acute myeloid leukemia (AML) who develop neutropenia while receiving induction chemotherapy.
The FDA grants RMAT designation to therapies intended to treat serious or life-threatening conditions if there is preliminary clinical evidence that the therapies could address unmet medical needs.
RMAT designation provides similar advantages as breakthrough therapy designation, including early interactions with the FDA to discuss potential ways to accelerate the development of a therapy toward regulatory approval.
The FDA granted romyelocel-L RMAT designation based on a randomized, phase 2 trial of newly diagnosed AML patients who received induction consisting of cytarabine and an anthracycline.
Results from this trial were presented at the 2018 ASCO Annual Meeting (abstract 7043).
The trial enrolled 163 AML patients and randomized them, on the first day of induction, to receive:
- Daily granulocyte colony-stimulating factor (G-CSF) starting on day 14 (n=84)
- Romyelocel-L (7.5 x 106cells/kg) on day 9 plus daily G-CSF starting on day 14 (n=79).
Patients received G-CSF until neutrophil recovery to at least 500/µL.
Baseline characteristics were well balanced between the treatment arms.
There were 120 evaluable patients—59 in the romyelocel-L arm and 61 in the control arm.
The study’s primary endpoint was days in a febrile episode (DFE). The mean DFE from day 9 to 28 was 6.46 days in the romyelocel-L arm and 6.86 days in the control arm (P=0.350). The mean DFE for days 15 to 28 was 2.36 and 3.90, respectively (P=0.020).
The incidence of microbiologically or clinically diagnosed infection from day 9 to 28 was 35.6% in the romyelocel-L arm and 47.5% in the control arm, a decrease of 25% (P=0.089).
From day 15 to 28 the incidence of infection was 6.8% in the romyelocel-L arm and 27.9% in the control arm, a decrease of 76% (P=0.002).
There were no infectious deaths in the romyelocel-L arm but 2 deaths attributed to pneumonia in the control arm.
The mean hospital stay was 25.5 days in the romyelocel-L arm and 28.7 days in the control arm (P=0.002).
The proportion of patients with serious adverse events (AEs) was 14% in the romyelocel-L arm and 18% in the control arm. The proportion of patients with infectious serious AEs was 50% and 77%, respectively.
The most frequent treatment-emergent AEs (in the romyelocel-L and control arms, respectively) were febrile neutropenia (31.4% and 31%), diarrhea (25.7% and 32.4%), hypokalemia (31.4% and 25.4%), hypophosphatemia (21.4% and 23.9%), and pyrexia (22.9% and 22.5%).
There were no cases of graft-versus-host disease.
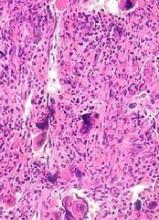
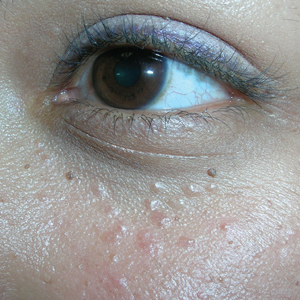
Bumps under eyes
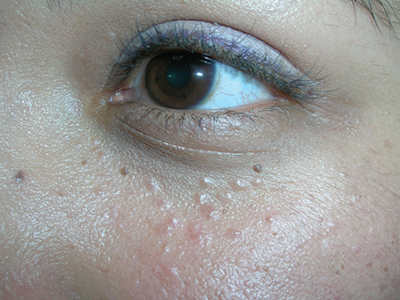
The FP diagnosed syringomas in this patient.
He explained that the bumps are benign tumors that occur frequently on the lower eyelids and upper cheeks. They are completely unrelated to the birth control pill and can develop in men, and run in families, too. While syringomas appear to occur more often in women than men, there are no known causative agents. These are benign growths of the eccrine sweat glands.
Treatment options include cryosurgery, electrosurgery, or chemical destruction with trichloroacetic acid. All of these approaches need to be performed carefully, as the syringomas are so close to the eye. Also, these treatments are only modestly effective; new syringomas can form. And there are no preventive treatments.
In this case, the patient had light brown skin, so there was a risk of causing permanent hypopigmentation with any of these destructive methods. The patient was reassured about the benign nature of the condition; she decided not to seek therapy.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Epidermal nevus and nevus sebaceous. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:958-962.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP diagnosed syringomas in this patient.
He explained that the bumps are benign tumors that occur frequently on the lower eyelids and upper cheeks. They are completely unrelated to the birth control pill and can develop in men, and run in families, too. While syringomas appear to occur more often in women than men, there are no known causative agents. These are benign growths of the eccrine sweat glands.
Treatment options include cryosurgery, electrosurgery, or chemical destruction with trichloroacetic acid. All of these approaches need to be performed carefully, as the syringomas are so close to the eye. Also, these treatments are only modestly effective; new syringomas can form. And there are no preventive treatments.
In this case, the patient had light brown skin, so there was a risk of causing permanent hypopigmentation with any of these destructive methods. The patient was reassured about the benign nature of the condition; she decided not to seek therapy.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Epidermal nevus and nevus sebaceous. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:958-962.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP diagnosed syringomas in this patient.
He explained that the bumps are benign tumors that occur frequently on the lower eyelids and upper cheeks. They are completely unrelated to the birth control pill and can develop in men, and run in families, too. While syringomas appear to occur more often in women than men, there are no known causative agents. These are benign growths of the eccrine sweat glands.
Treatment options include cryosurgery, electrosurgery, or chemical destruction with trichloroacetic acid. All of these approaches need to be performed carefully, as the syringomas are so close to the eye. Also, these treatments are only modestly effective; new syringomas can form. And there are no preventive treatments.
In this case, the patient had light brown skin, so there was a risk of causing permanent hypopigmentation with any of these destructive methods. The patient was reassured about the benign nature of the condition; she decided not to seek therapy.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Epidermal nevus and nevus sebaceous. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:958-962.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Brand Who? Brand You!
During the early days of the American Academy of Nurse Practitioners (incorporated in 1985), I spotted a full-page ad by the Campaign Consultants of America addressed to professional fundraisers. What caught my eye was a photograph of a mother with the tagline, “There’s only one person who understands you better than we do, and she still doesn’t understand what you do for a living.” I pulled the page from the magazine and made a note to consider using it to promote the NP profession. What we needed at the time, despite being an established profession, was to public
Historically, branding has been a task undertaken by a company’s marketing department or an advertising agency to identify elements that differentiate their product from the competition’s. Designing a logo, creating a jingle (Oscar-Mayer, anyone?), or recording a sound bite are the means to emphasize the difference. It paints the mental picture people have of a company, a product, or a provider. These cues remind the consumer about the product. So, how does this apply to the NP (and PA) profession?
The importance of establishing a “brand”—of distinguishing ourselves as competent clinicians with a specific skillset to offer the primary care community—cannot be overstated. Personal branding is a key component of fostering patient loyalty, building your reputation, and increasing referrals to your practice. Understanding the needs and desires of patients, their families, and the community is crucial. Our personal brand emphasizes our assets and expertise. While it can be difficult to look at yourself objectively (especially your assets), it is necessary in today’s competitive world of health care.
NPs constitute the fastest-growing segment of the primary care workforce in the US. More than 50 years of transforming health care as we know it has made us indispensable as health care providers. The literature has long supported the position that NPs provide care that is effective, patient-centered, and evidenced-based. Who we are, what we do, and how well we do it has been documented in myriad reports, surveys, and publications. Yet in many ways, we continue to struggle with an in-between identity. Despite our increasing responsibility in the clinical realm, some are still confused as to who we are.
We are known as nurses first, yet much of the health care we now provide was traditionally in the “physician-only” domain. And because of that history, our ability to function to the fullest extent of our education has been hobbled. These practice restrictions are counterproductive at a time when our nation is facing serious public health challenges.
Over the years, barriers to practice have slowly been whittled away, but full appreciation and recognition of our professional excellence and our contribution to improve the nation’s health is lacking. The fact that much of the research on health status and health ranking fails to include NPs and PAs is testimony that we remain somewhat invisible. And that, my friends, is exactly why it is time to revisit that aforementioned advertisement—not because our mothers don’t know what we do, but because, to some degree, we have eased off the belief that there are still obstacles to full access to NPs as primary care providers. And that is the origin of the need to establish your own brand.
Creating and maintaining your personal brand necessitates that you be multi-functional. You must be a role model, a mentor, and a voice that is respected and reliable. Your brand should advertise what you are known for and what motivates people to seek you, specifically, for their health care needs. Be relentlessly focused on what you do that adds value. As NPs, we have a unique blend of nursing and medicine that allows us to provide the patient-centered care that is central to meeting the existing and future primary care needs of our nation. From our roots in nursing, we offer patients high-quality care and a provider to partner with them in developing their plan of care.
Continue to: A foundational component of building your brand is...
A foundational component of building your brand is positioning yourself as a credible expert and leader. We each have a unique collection of experiences in preventive and primary care. Share that experience by getting involved in your community: participate in health fairs, interact with local news media, or volunteer to serve on your local health board. Emphasize the quality, flexibility, and continuity of care that you can provide. Share any survey findings that demonstrate your ability to anticipate, meet, and even exceed patients’ needs. Demonstrate your ability to deliver quality, accessible health care in a diverse society with increasingly complex medical needs.
As the nation continues to face a shortage of primary care providers and services—a gap that NPs and PAs are equipped to fill—it’s time for us to promote ourselves and advertise all that we can do. This isn’t just for our own sakes, but for our patients’ as well. Give some serious thought (and even more serious effort) to imagining and developing yourself as a brand. Define your brand’s attributes and the qualities or characteristics that make you distinctive from your competitors (or even your colleagues). You are the CEO of brand YOU!
If you have examples of how you promote your personal brand, please share them with me at [email protected].
During the early days of the American Academy of Nurse Practitioners (incorporated in 1985), I spotted a full-page ad by the Campaign Consultants of America addressed to professional fundraisers. What caught my eye was a photograph of a mother with the tagline, “There’s only one person who understands you better than we do, and she still doesn’t understand what you do for a living.” I pulled the page from the magazine and made a note to consider using it to promote the NP profession. What we needed at the time, despite being an established profession, was to public
Historically, branding has been a task undertaken by a company’s marketing department or an advertising agency to identify elements that differentiate their product from the competition’s. Designing a logo, creating a jingle (Oscar-Mayer, anyone?), or recording a sound bite are the means to emphasize the difference. It paints the mental picture people have of a company, a product, or a provider. These cues remind the consumer about the product. So, how does this apply to the NP (and PA) profession?
The importance of establishing a “brand”—of distinguishing ourselves as competent clinicians with a specific skillset to offer the primary care community—cannot be overstated. Personal branding is a key component of fostering patient loyalty, building your reputation, and increasing referrals to your practice. Understanding the needs and desires of patients, their families, and the community is crucial. Our personal brand emphasizes our assets and expertise. While it can be difficult to look at yourself objectively (especially your assets), it is necessary in today’s competitive world of health care.
NPs constitute the fastest-growing segment of the primary care workforce in the US. More than 50 years of transforming health care as we know it has made us indispensable as health care providers. The literature has long supported the position that NPs provide care that is effective, patient-centered, and evidenced-based. Who we are, what we do, and how well we do it has been documented in myriad reports, surveys, and publications. Yet in many ways, we continue to struggle with an in-between identity. Despite our increasing responsibility in the clinical realm, some are still confused as to who we are.
We are known as nurses first, yet much of the health care we now provide was traditionally in the “physician-only” domain. And because of that history, our ability to function to the fullest extent of our education has been hobbled. These practice restrictions are counterproductive at a time when our nation is facing serious public health challenges.
Over the years, barriers to practice have slowly been whittled away, but full appreciation and recognition of our professional excellence and our contribution to improve the nation’s health is lacking. The fact that much of the research on health status and health ranking fails to include NPs and PAs is testimony that we remain somewhat invisible. And that, my friends, is exactly why it is time to revisit that aforementioned advertisement—not because our mothers don’t know what we do, but because, to some degree, we have eased off the belief that there are still obstacles to full access to NPs as primary care providers. And that is the origin of the need to establish your own brand.
Creating and maintaining your personal brand necessitates that you be multi-functional. You must be a role model, a mentor, and a voice that is respected and reliable. Your brand should advertise what you are known for and what motivates people to seek you, specifically, for their health care needs. Be relentlessly focused on what you do that adds value. As NPs, we have a unique blend of nursing and medicine that allows us to provide the patient-centered care that is central to meeting the existing and future primary care needs of our nation. From our roots in nursing, we offer patients high-quality care and a provider to partner with them in developing their plan of care.
Continue to: A foundational component of building your brand is...
A foundational component of building your brand is positioning yourself as a credible expert and leader. We each have a unique collection of experiences in preventive and primary care. Share that experience by getting involved in your community: participate in health fairs, interact with local news media, or volunteer to serve on your local health board. Emphasize the quality, flexibility, and continuity of care that you can provide. Share any survey findings that demonstrate your ability to anticipate, meet, and even exceed patients’ needs. Demonstrate your ability to deliver quality, accessible health care in a diverse society with increasingly complex medical needs.
As the nation continues to face a shortage of primary care providers and services—a gap that NPs and PAs are equipped to fill—it’s time for us to promote ourselves and advertise all that we can do. This isn’t just for our own sakes, but for our patients’ as well. Give some serious thought (and even more serious effort) to imagining and developing yourself as a brand. Define your brand’s attributes and the qualities or characteristics that make you distinctive from your competitors (or even your colleagues). You are the CEO of brand YOU!
If you have examples of how you promote your personal brand, please share them with me at [email protected].
During the early days of the American Academy of Nurse Practitioners (incorporated in 1985), I spotted a full-page ad by the Campaign Consultants of America addressed to professional fundraisers. What caught my eye was a photograph of a mother with the tagline, “There’s only one person who understands you better than we do, and she still doesn’t understand what you do for a living.” I pulled the page from the magazine and made a note to consider using it to promote the NP profession. What we needed at the time, despite being an established profession, was to public
Historically, branding has been a task undertaken by a company’s marketing department or an advertising agency to identify elements that differentiate their product from the competition’s. Designing a logo, creating a jingle (Oscar-Mayer, anyone?), or recording a sound bite are the means to emphasize the difference. It paints the mental picture people have of a company, a product, or a provider. These cues remind the consumer about the product. So, how does this apply to the NP (and PA) profession?
The importance of establishing a “brand”—of distinguishing ourselves as competent clinicians with a specific skillset to offer the primary care community—cannot be overstated. Personal branding is a key component of fostering patient loyalty, building your reputation, and increasing referrals to your practice. Understanding the needs and desires of patients, their families, and the community is crucial. Our personal brand emphasizes our assets and expertise. While it can be difficult to look at yourself objectively (especially your assets), it is necessary in today’s competitive world of health care.
NPs constitute the fastest-growing segment of the primary care workforce in the US. More than 50 years of transforming health care as we know it has made us indispensable as health care providers. The literature has long supported the position that NPs provide care that is effective, patient-centered, and evidenced-based. Who we are, what we do, and how well we do it has been documented in myriad reports, surveys, and publications. Yet in many ways, we continue to struggle with an in-between identity. Despite our increasing responsibility in the clinical realm, some are still confused as to who we are.
We are known as nurses first, yet much of the health care we now provide was traditionally in the “physician-only” domain. And because of that history, our ability to function to the fullest extent of our education has been hobbled. These practice restrictions are counterproductive at a time when our nation is facing serious public health challenges.
Over the years, barriers to practice have slowly been whittled away, but full appreciation and recognition of our professional excellence and our contribution to improve the nation’s health is lacking. The fact that much of the research on health status and health ranking fails to include NPs and PAs is testimony that we remain somewhat invisible. And that, my friends, is exactly why it is time to revisit that aforementioned advertisement—not because our mothers don’t know what we do, but because, to some degree, we have eased off the belief that there are still obstacles to full access to NPs as primary care providers. And that is the origin of the need to establish your own brand.
Creating and maintaining your personal brand necessitates that you be multi-functional. You must be a role model, a mentor, and a voice that is respected and reliable. Your brand should advertise what you are known for and what motivates people to seek you, specifically, for their health care needs. Be relentlessly focused on what you do that adds value. As NPs, we have a unique blend of nursing and medicine that allows us to provide the patient-centered care that is central to meeting the existing and future primary care needs of our nation. From our roots in nursing, we offer patients high-quality care and a provider to partner with them in developing their plan of care.
Continue to: A foundational component of building your brand is...
A foundational component of building your brand is positioning yourself as a credible expert and leader. We each have a unique collection of experiences in preventive and primary care. Share that experience by getting involved in your community: participate in health fairs, interact with local news media, or volunteer to serve on your local health board. Emphasize the quality, flexibility, and continuity of care that you can provide. Share any survey findings that demonstrate your ability to anticipate, meet, and even exceed patients’ needs. Demonstrate your ability to deliver quality, accessible health care in a diverse society with increasingly complex medical needs.
As the nation continues to face a shortage of primary care providers and services—a gap that NPs and PAs are equipped to fill—it’s time for us to promote ourselves and advertise all that we can do. This isn’t just for our own sakes, but for our patients’ as well. Give some serious thought (and even more serious effort) to imagining and developing yourself as a brand. Define your brand’s attributes and the qualities or characteristics that make you distinctive from your competitors (or even your colleagues). You are the CEO of brand YOU!
If you have examples of how you promote your personal brand, please share them with me at [email protected].
SHORT TAKES
Preoperative physiotherapy reduces postoperative pulmonary complications in patients undergoing elective abdominal surgery
A randomized, controlled trial showed that, in patients having elective abdominal surgery, a 30-minute preoperative physiotherapy session – that focused on breathing exercise training and education – reduced postoperative pulmonary complications, including hospital-acquired pneumonia, by half (hazard ratio, 0.48; number needed to treat, seven).
Severity of thrombocytopenia does not predict bleeding risk in patients with cirrhosis
A subgroup analysis of a prospective cohort study showed that, although platelet counts worsened with progression of liver disease, platelet counts were similar in patients with and without a bleeding event.
Citation: Basili S et al. Platelet count does not predict bleeding in cirrhotic patients: Results from the PRO-LIVER study. Am J Gastroenterol. 2018 Mar;113(3):368-75.
Patent foramen ovale closures are superior to medical therapy in patients with medium to large PFOs
A meta-analysis of four randomized, controlled trials found that patients with cryptogenic stroke who underwent PFO closure had decreased rates of stroke and transient ischemic attack, compared with medical therapy alone, but had increased incidence of atrial fibrillation or atrial flutter.
Citation: De Rosa S et al. Percutaneous closure versus medical treatment in stroke patients with patent foramen ovale: A systematic review and meta-analysis. Ann Intern Med. 2018 Mar 6;168(5):343-50.
Short-run atrial tachyarrhythmias increase the risk of stroke
A retrospective, observational study showed that patients with short-run atrial tachyarrhythmias (more than three consecutive supraventricular ectopic beats lasting less than 5 seconds) have an increased risk of stroke (11.4% vs. 8.3%; P
Citation: Yamada S et al. Risk of stroke in patients with short-run atrial tachyarrhythmia. Stroke. 2017 Dec;48(12):3232-8.
Even one cigarette is too many
A meta-analysis of 55 publications containing 141 cohort studies demonstrated that even those that smoked one cigarette per day were at increased risk of developing coronary artery disease and stroke.
Citation: Hackshaw A et al. Low cigarette consumption and risk of coronary heart disease and stroke: Meta-analysis of 141 cohort studies in 55 study reports. BMJ. 2018 Jan 24;360:j5855.
Preoperative physiotherapy reduces postoperative pulmonary complications in patients undergoing elective abdominal surgery
A randomized, controlled trial showed that, in patients having elective abdominal surgery, a 30-minute preoperative physiotherapy session – that focused on breathing exercise training and education – reduced postoperative pulmonary complications, including hospital-acquired pneumonia, by half (hazard ratio, 0.48; number needed to treat, seven).
Severity of thrombocytopenia does not predict bleeding risk in patients with cirrhosis
A subgroup analysis of a prospective cohort study showed that, although platelet counts worsened with progression of liver disease, platelet counts were similar in patients with and without a bleeding event.
Citation: Basili S et al. Platelet count does not predict bleeding in cirrhotic patients: Results from the PRO-LIVER study. Am J Gastroenterol. 2018 Mar;113(3):368-75.
Patent foramen ovale closures are superior to medical therapy in patients with medium to large PFOs
A meta-analysis of four randomized, controlled trials found that patients with cryptogenic stroke who underwent PFO closure had decreased rates of stroke and transient ischemic attack, compared with medical therapy alone, but had increased incidence of atrial fibrillation or atrial flutter.
Citation: De Rosa S et al. Percutaneous closure versus medical treatment in stroke patients with patent foramen ovale: A systematic review and meta-analysis. Ann Intern Med. 2018 Mar 6;168(5):343-50.
Short-run atrial tachyarrhythmias increase the risk of stroke
A retrospective, observational study showed that patients with short-run atrial tachyarrhythmias (more than three consecutive supraventricular ectopic beats lasting less than 5 seconds) have an increased risk of stroke (11.4% vs. 8.3%; P
Citation: Yamada S et al. Risk of stroke in patients with short-run atrial tachyarrhythmia. Stroke. 2017 Dec;48(12):3232-8.
Even one cigarette is too many
A meta-analysis of 55 publications containing 141 cohort studies demonstrated that even those that smoked one cigarette per day were at increased risk of developing coronary artery disease and stroke.
Citation: Hackshaw A et al. Low cigarette consumption and risk of coronary heart disease and stroke: Meta-analysis of 141 cohort studies in 55 study reports. BMJ. 2018 Jan 24;360:j5855.
Preoperative physiotherapy reduces postoperative pulmonary complications in patients undergoing elective abdominal surgery
A randomized, controlled trial showed that, in patients having elective abdominal surgery, a 30-minute preoperative physiotherapy session – that focused on breathing exercise training and education – reduced postoperative pulmonary complications, including hospital-acquired pneumonia, by half (hazard ratio, 0.48; number needed to treat, seven).
Severity of thrombocytopenia does not predict bleeding risk in patients with cirrhosis
A subgroup analysis of a prospective cohort study showed that, although platelet counts worsened with progression of liver disease, platelet counts were similar in patients with and without a bleeding event.
Citation: Basili S et al. Platelet count does not predict bleeding in cirrhotic patients: Results from the PRO-LIVER study. Am J Gastroenterol. 2018 Mar;113(3):368-75.
Patent foramen ovale closures are superior to medical therapy in patients with medium to large PFOs
A meta-analysis of four randomized, controlled trials found that patients with cryptogenic stroke who underwent PFO closure had decreased rates of stroke and transient ischemic attack, compared with medical therapy alone, but had increased incidence of atrial fibrillation or atrial flutter.
Citation: De Rosa S et al. Percutaneous closure versus medical treatment in stroke patients with patent foramen ovale: A systematic review and meta-analysis. Ann Intern Med. 2018 Mar 6;168(5):343-50.
Short-run atrial tachyarrhythmias increase the risk of stroke
A retrospective, observational study showed that patients with short-run atrial tachyarrhythmias (more than three consecutive supraventricular ectopic beats lasting less than 5 seconds) have an increased risk of stroke (11.4% vs. 8.3%; P
Citation: Yamada S et al. Risk of stroke in patients with short-run atrial tachyarrhythmia. Stroke. 2017 Dec;48(12):3232-8.
Even one cigarette is too many
A meta-analysis of 55 publications containing 141 cohort studies demonstrated that even those that smoked one cigarette per day were at increased risk of developing coronary artery disease and stroke.
Citation: Hackshaw A et al. Low cigarette consumption and risk of coronary heart disease and stroke: Meta-analysis of 141 cohort studies in 55 study reports. BMJ. 2018 Jan 24;360:j5855.
Oral tecovirimat for smallpox shows efficacy in animals, safety in humans
Tecovirimat, an oral antiviral agent, is being advanced as a treatment for smallpox based on recently reported studies showing efficacy data in animals and safety and pharmacokinetic data in humans.
“The aggregation of the results from these multiple studies involving animals and humans supports tecovirimat as potential smallpox antiviral drug,” Douglas W. Grosenbach, PhD, of SIGA Technologies and his coauthors reported in the New England Journal of Medicine.
Tecovirimat inhibits p37, a protein that is present in all orthopoxviruses, to prevent “formation and egress of enveloped virions, which are essential for virulence,” Dr. Grosenbach and his coauthors wrote in the report.
Smallpox, caused by the variola virus, was eradicated in 1980. However, the disease remains concerning because of the potential for intentional release of variola virus in an act of bioterrorism or biowarfare, according to the authors.
“A single case of smallpox anywhere in the world would be a global health emergency,” Dr. Grosenbach and his colleagues wrote.
Available vaccines are not used because of the risk of side effects and would not be used except in the case of known or suspected variola virus exposure, they added.
Since it would be unethical to intentionally expose humans to variola virus, tecovirimat is being developed in line with the Food and Drug Administration Animal Efficacy Rule, which gives the agency the authority to approve drugs based on animal data for diseases that have a low or nonexistent rate of natural occurrence.
The first treatment developed under the Animal Efficacy Rule, was raxibacumab, a biologic approved by the FDA in December 2012 for treatment of inhalation anthrax. Subsequently, several other agents for prophylaxis or treatment of anthrax and botulism have been developed based on the rule and approved.
Tecovirimat has demonstrated protective efficacy in pilot studies conducted in rabbits infected with rabbitpox virus and nonhuman primates infected with monkeypox virus, Dr. Grosenbach and his colleagues explained in the current report.
In nonhuman primate studies, doses of 3-10 mg/kilogram provided nearly full protection from death with a survival rate of approximately 95% versus 5% for placebo, along with reduced lesion counts and viral loads, according to investigators.
In a tecovirimat clinical trial, 452 volunteers were randomized to receive the antiviral agent twice daily at 600 mg or matching placebo for 14 days. Adverse events occurred in 37.3% of tecovirimat-treated and 33.3% of placebo-treated participants, with events of grade 3 or higher occurring in 1.1% of patients in both groups.
There was one death on the tecovirimat arm, which was caused by a pulmonary embolism in a participant who had a history of recurrent deep-vein thrombosis but was not receiving anticoagulant treatment, investigators said.
Dr. Grosenbach and colleagues also presented pharmacokinetic profiles and exposures for 48 volunteers in a fed state in their report in the New England Journal of Medicine, along with pharmacokinetic data from the animal studies.
The FDA has set a target date of Aug. 8, 2018, for final action on a new drug application submitted for oral tecovirimat for treatment of smallpox, SIGA Technologies said in a May 2018 news release.
Dr. Grosenbach and his coauthors reported a disclosure related to Biomedical Advanced Research and Development Authority (BARDA), grants from NIH during the conduct of the study, and personal fees from SIGA Technologies outside the submitted work.
SOURCE: Grosenbach DW et al. N Engl J Med. 2018 Jul 5;379(1):44-53.
Tecovirimat, an oral antiviral agent, is being advanced as a treatment for smallpox based on recently reported studies showing efficacy data in animals and safety and pharmacokinetic data in humans.
“The aggregation of the results from these multiple studies involving animals and humans supports tecovirimat as potential smallpox antiviral drug,” Douglas W. Grosenbach, PhD, of SIGA Technologies and his coauthors reported in the New England Journal of Medicine.
Tecovirimat inhibits p37, a protein that is present in all orthopoxviruses, to prevent “formation and egress of enveloped virions, which are essential for virulence,” Dr. Grosenbach and his coauthors wrote in the report.
Smallpox, caused by the variola virus, was eradicated in 1980. However, the disease remains concerning because of the potential for intentional release of variola virus in an act of bioterrorism or biowarfare, according to the authors.
“A single case of smallpox anywhere in the world would be a global health emergency,” Dr. Grosenbach and his colleagues wrote.
Available vaccines are not used because of the risk of side effects and would not be used except in the case of known or suspected variola virus exposure, they added.
Since it would be unethical to intentionally expose humans to variola virus, tecovirimat is being developed in line with the Food and Drug Administration Animal Efficacy Rule, which gives the agency the authority to approve drugs based on animal data for diseases that have a low or nonexistent rate of natural occurrence.
The first treatment developed under the Animal Efficacy Rule, was raxibacumab, a biologic approved by the FDA in December 2012 for treatment of inhalation anthrax. Subsequently, several other agents for prophylaxis or treatment of anthrax and botulism have been developed based on the rule and approved.
Tecovirimat has demonstrated protective efficacy in pilot studies conducted in rabbits infected with rabbitpox virus and nonhuman primates infected with monkeypox virus, Dr. Grosenbach and his colleagues explained in the current report.
In nonhuman primate studies, doses of 3-10 mg/kilogram provided nearly full protection from death with a survival rate of approximately 95% versus 5% for placebo, along with reduced lesion counts and viral loads, according to investigators.
In a tecovirimat clinical trial, 452 volunteers were randomized to receive the antiviral agent twice daily at 600 mg or matching placebo for 14 days. Adverse events occurred in 37.3% of tecovirimat-treated and 33.3% of placebo-treated participants, with events of grade 3 or higher occurring in 1.1% of patients in both groups.
There was one death on the tecovirimat arm, which was caused by a pulmonary embolism in a participant who had a history of recurrent deep-vein thrombosis but was not receiving anticoagulant treatment, investigators said.
Dr. Grosenbach and colleagues also presented pharmacokinetic profiles and exposures for 48 volunteers in a fed state in their report in the New England Journal of Medicine, along with pharmacokinetic data from the animal studies.
The FDA has set a target date of Aug. 8, 2018, for final action on a new drug application submitted for oral tecovirimat for treatment of smallpox, SIGA Technologies said in a May 2018 news release.
Dr. Grosenbach and his coauthors reported a disclosure related to Biomedical Advanced Research and Development Authority (BARDA), grants from NIH during the conduct of the study, and personal fees from SIGA Technologies outside the submitted work.
SOURCE: Grosenbach DW et al. N Engl J Med. 2018 Jul 5;379(1):44-53.
Tecovirimat, an oral antiviral agent, is being advanced as a treatment for smallpox based on recently reported studies showing efficacy data in animals and safety and pharmacokinetic data in humans.
“The aggregation of the results from these multiple studies involving animals and humans supports tecovirimat as potential smallpox antiviral drug,” Douglas W. Grosenbach, PhD, of SIGA Technologies and his coauthors reported in the New England Journal of Medicine.
Tecovirimat inhibits p37, a protein that is present in all orthopoxviruses, to prevent “formation and egress of enveloped virions, which are essential for virulence,” Dr. Grosenbach and his coauthors wrote in the report.
Smallpox, caused by the variola virus, was eradicated in 1980. However, the disease remains concerning because of the potential for intentional release of variola virus in an act of bioterrorism or biowarfare, according to the authors.
“A single case of smallpox anywhere in the world would be a global health emergency,” Dr. Grosenbach and his colleagues wrote.
Available vaccines are not used because of the risk of side effects and would not be used except in the case of known or suspected variola virus exposure, they added.
Since it would be unethical to intentionally expose humans to variola virus, tecovirimat is being developed in line with the Food and Drug Administration Animal Efficacy Rule, which gives the agency the authority to approve drugs based on animal data for diseases that have a low or nonexistent rate of natural occurrence.
The first treatment developed under the Animal Efficacy Rule, was raxibacumab, a biologic approved by the FDA in December 2012 for treatment of inhalation anthrax. Subsequently, several other agents for prophylaxis or treatment of anthrax and botulism have been developed based on the rule and approved.
Tecovirimat has demonstrated protective efficacy in pilot studies conducted in rabbits infected with rabbitpox virus and nonhuman primates infected with monkeypox virus, Dr. Grosenbach and his colleagues explained in the current report.
In nonhuman primate studies, doses of 3-10 mg/kilogram provided nearly full protection from death with a survival rate of approximately 95% versus 5% for placebo, along with reduced lesion counts and viral loads, according to investigators.
In a tecovirimat clinical trial, 452 volunteers were randomized to receive the antiviral agent twice daily at 600 mg or matching placebo for 14 days. Adverse events occurred in 37.3% of tecovirimat-treated and 33.3% of placebo-treated participants, with events of grade 3 or higher occurring in 1.1% of patients in both groups.
There was one death on the tecovirimat arm, which was caused by a pulmonary embolism in a participant who had a history of recurrent deep-vein thrombosis but was not receiving anticoagulant treatment, investigators said.
Dr. Grosenbach and colleagues also presented pharmacokinetic profiles and exposures for 48 volunteers in a fed state in their report in the New England Journal of Medicine, along with pharmacokinetic data from the animal studies.
The FDA has set a target date of Aug. 8, 2018, for final action on a new drug application submitted for oral tecovirimat for treatment of smallpox, SIGA Technologies said in a May 2018 news release.
Dr. Grosenbach and his coauthors reported a disclosure related to Biomedical Advanced Research and Development Authority (BARDA), grants from NIH during the conduct of the study, and personal fees from SIGA Technologies outside the submitted work.
SOURCE: Grosenbach DW et al. N Engl J Med. 2018 Jul 5;379(1):44-53.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Tecovirimat, an oral antiviral agent, is being advanced as a treatment for smallpox based on animal and human study data.
Major finding: The survival rate was approximately 95% in monkeypox-infected primates receiving doses of 3-10 mg/kilogram. In humans, the serious adverse event rate was 1.1% for both tecovirimat and placebo.
Study details: A report on multiple studies in small animals and nonhuman primates, along with a randomized safety trial involving 452 healthy human volunteers.
Disclosures: Study authors reported a disclosure related to Biomedical Advanced Research and Development Authority (BARDA), grants from NIH during the conduct of the study, and personal fees from SIGA Technologies outside the submitted work.
Source: Grosenbach DW et al. N Engl J Med. 2018 Jul 5;379(1):44-53.
Patient autonomy: The moving target
Youth Suicide Rates Steady Climb
Black children aged 5 to 12 years are roughly twice as likely as white children to commit suicide, according to a study funded by the National Institute of Mental Health. But that trend reverses in adolescence: From ages 13 to 17 years, the suicide rates for white children are double those of black children.
The researchers used the CDC’s web-based Injury Statistics Query and Reporting System, analyzing data from 2001-2015 separately for each age group. The data were limited, the researchers say, and did not include information on contributing factors. They add that their findings highlight the need for a greater understanding of age-related racial disparities in youth suicide.
The disturbing findings are part of an overall rise in suicide nationwide. Suicide is the tenth leading cause of death in the US, according to the latest figures from the CDC. In 2016, nearly 45,000 Americans aged ≥ 10 years died by suicide.
In 2017, the CDC released Preventing Suicide: A Technical Package of Policy, Programs, and Practices, with evidence-based strategies (https://www.cdc.gov/media/releases/2018/p0607-suicide-prevention.html). The strategies include creating protective environments by reducing access to lethal means among at-risk individuals and intervening at “suicide hotspots” by, for example, putting barriers on tall structures. “Like most public health problems,” the guide says, “suicide is preventable.”
Black children aged 5 to 12 years are roughly twice as likely as white children to commit suicide, according to a study funded by the National Institute of Mental Health. But that trend reverses in adolescence: From ages 13 to 17 years, the suicide rates for white children are double those of black children.
The researchers used the CDC’s web-based Injury Statistics Query and Reporting System, analyzing data from 2001-2015 separately for each age group. The data were limited, the researchers say, and did not include information on contributing factors. They add that their findings highlight the need for a greater understanding of age-related racial disparities in youth suicide.
The disturbing findings are part of an overall rise in suicide nationwide. Suicide is the tenth leading cause of death in the US, according to the latest figures from the CDC. In 2016, nearly 45,000 Americans aged ≥ 10 years died by suicide.
In 2017, the CDC released Preventing Suicide: A Technical Package of Policy, Programs, and Practices, with evidence-based strategies (https://www.cdc.gov/media/releases/2018/p0607-suicide-prevention.html). The strategies include creating protective environments by reducing access to lethal means among at-risk individuals and intervening at “suicide hotspots” by, for example, putting barriers on tall structures. “Like most public health problems,” the guide says, “suicide is preventable.”
Black children aged 5 to 12 years are roughly twice as likely as white children to commit suicide, according to a study funded by the National Institute of Mental Health. But that trend reverses in adolescence: From ages 13 to 17 years, the suicide rates for white children are double those of black children.
The researchers used the CDC’s web-based Injury Statistics Query and Reporting System, analyzing data from 2001-2015 separately for each age group. The data were limited, the researchers say, and did not include information on contributing factors. They add that their findings highlight the need for a greater understanding of age-related racial disparities in youth suicide.
The disturbing findings are part of an overall rise in suicide nationwide. Suicide is the tenth leading cause of death in the US, according to the latest figures from the CDC. In 2016, nearly 45,000 Americans aged ≥ 10 years died by suicide.
In 2017, the CDC released Preventing Suicide: A Technical Package of Policy, Programs, and Practices, with evidence-based strategies (https://www.cdc.gov/media/releases/2018/p0607-suicide-prevention.html). The strategies include creating protective environments by reducing access to lethal means among at-risk individuals and intervening at “suicide hotspots” by, for example, putting barriers on tall structures. “Like most public health problems,” the guide says, “suicide is preventable.”
Federal Health Care Data Trends Vietnam Era Veterans
According to the VA, 8,744,000 veterans served in the Armed Forces in the time between the Gulf of Tonkin incident and the signing of the Paris Peace Accords.1 Of those, 3,403,000 were deployed to Southeast Asia. Those who served during this period often were exposed to unique environmental hazards, such as commonly used pesticides and herbicides, as well as diseases attributed to the tropical environment, such as fungal infections, and there were more than 40,000 reported cases of malaria. Upon returning home, these veterans faced a tough readjustment that often magnified the stress associated with combat.
Click here to continue reading.
According to the VA, 8,744,000 veterans served in the Armed Forces in the time between the Gulf of Tonkin incident and the signing of the Paris Peace Accords.1 Of those, 3,403,000 were deployed to Southeast Asia. Those who served during this period often were exposed to unique environmental hazards, such as commonly used pesticides and herbicides, as well as diseases attributed to the tropical environment, such as fungal infections, and there were more than 40,000 reported cases of malaria. Upon returning home, these veterans faced a tough readjustment that often magnified the stress associated with combat.
Click here to continue reading.
According to the VA, 8,744,000 veterans served in the Armed Forces in the time between the Gulf of Tonkin incident and the signing of the Paris Peace Accords.1 Of those, 3,403,000 were deployed to Southeast Asia. Those who served during this period often were exposed to unique environmental hazards, such as commonly used pesticides and herbicides, as well as diseases attributed to the tropical environment, such as fungal infections, and there were more than 40,000 reported cases of malaria. Upon returning home, these veterans faced a tough readjustment that often magnified the stress associated with combat.
Click here to continue reading.
High users of CT pulmonary angiograms have lower diagnostic yields
Clinical question: What physician characteristics are associated with CT pulmonary angiogram (CTPA) diagnostic yield?
Background: Overuse of CTPAs for pulmonary embolism evaluation exposes patients to unnecessary testing and harmful ionizing radiation. Physician characteristics influence ordering practice. Identifying specific characteristics can provide an intervention for reducing overutilization.
Study design: Retrospective analysis.
Setting: Academic teaching hospital in Montreal, Canada.
Synopsis: Investigators reviewed 1,394 CTPAs ordered by 182 physicians at an academic teaching hospital during 2014-2016, with 199 (14.3%) positive studies and 1,195 (85.7%) negative studies. Physician years of experience, physician sex, and emergency medicine specialty were not associated with diagnostic yield. However, the diagnostic yield decreased with the total number of scans ordered per physician. For every 10 additional scans ordered, the odds of a positive test were reduced (odds ratio, 0.76; 95% confidence interval, 0.73-0.79). For physicians who ordered more than 50 studies, the percentage of positive studies was only 5%.
This study’s results show that overuse of CTPA is associated with decreased diagnostic yield. A limitation of the study was that pretest probabilities for pulmonary embolism could not be calculated because of inadequate charting, which would have determined whether CTPA was the appropriate test (as opposed to D-dimer).
Bottom line: Physicians who order higher numbers of CTPAs have lower diagnostic yields.
Citation: Chong J et al. Association of lower diagnostic yield with high users of CT pulmonary angiogram. JAMA Intern Med. 2018 Mar 1;178(3):412-3.
Dr. Komsoukaniants is a hospitalist at UC San Diego Health and an assistant clinical professor at the University of California, San Diego.
Clinical question: What physician characteristics are associated with CT pulmonary angiogram (CTPA) diagnostic yield?
Background: Overuse of CTPAs for pulmonary embolism evaluation exposes patients to unnecessary testing and harmful ionizing radiation. Physician characteristics influence ordering practice. Identifying specific characteristics can provide an intervention for reducing overutilization.
Study design: Retrospective analysis.
Setting: Academic teaching hospital in Montreal, Canada.
Synopsis: Investigators reviewed 1,394 CTPAs ordered by 182 physicians at an academic teaching hospital during 2014-2016, with 199 (14.3%) positive studies and 1,195 (85.7%) negative studies. Physician years of experience, physician sex, and emergency medicine specialty were not associated with diagnostic yield. However, the diagnostic yield decreased with the total number of scans ordered per physician. For every 10 additional scans ordered, the odds of a positive test were reduced (odds ratio, 0.76; 95% confidence interval, 0.73-0.79). For physicians who ordered more than 50 studies, the percentage of positive studies was only 5%.
This study’s results show that overuse of CTPA is associated with decreased diagnostic yield. A limitation of the study was that pretest probabilities for pulmonary embolism could not be calculated because of inadequate charting, which would have determined whether CTPA was the appropriate test (as opposed to D-dimer).
Bottom line: Physicians who order higher numbers of CTPAs have lower diagnostic yields.
Citation: Chong J et al. Association of lower diagnostic yield with high users of CT pulmonary angiogram. JAMA Intern Med. 2018 Mar 1;178(3):412-3.
Dr. Komsoukaniants is a hospitalist at UC San Diego Health and an assistant clinical professor at the University of California, San Diego.
Clinical question: What physician characteristics are associated with CT pulmonary angiogram (CTPA) diagnostic yield?
Background: Overuse of CTPAs for pulmonary embolism evaluation exposes patients to unnecessary testing and harmful ionizing radiation. Physician characteristics influence ordering practice. Identifying specific characteristics can provide an intervention for reducing overutilization.
Study design: Retrospective analysis.
Setting: Academic teaching hospital in Montreal, Canada.
Synopsis: Investigators reviewed 1,394 CTPAs ordered by 182 physicians at an academic teaching hospital during 2014-2016, with 199 (14.3%) positive studies and 1,195 (85.7%) negative studies. Physician years of experience, physician sex, and emergency medicine specialty were not associated with diagnostic yield. However, the diagnostic yield decreased with the total number of scans ordered per physician. For every 10 additional scans ordered, the odds of a positive test were reduced (odds ratio, 0.76; 95% confidence interval, 0.73-0.79). For physicians who ordered more than 50 studies, the percentage of positive studies was only 5%.
This study’s results show that overuse of CTPA is associated with decreased diagnostic yield. A limitation of the study was that pretest probabilities for pulmonary embolism could not be calculated because of inadequate charting, which would have determined whether CTPA was the appropriate test (as opposed to D-dimer).
Bottom line: Physicians who order higher numbers of CTPAs have lower diagnostic yields.
Citation: Chong J et al. Association of lower diagnostic yield with high users of CT pulmonary angiogram. JAMA Intern Med. 2018 Mar 1;178(3):412-3.
Dr. Komsoukaniants is a hospitalist at UC San Diego Health and an assistant clinical professor at the University of California, San Diego.