User login
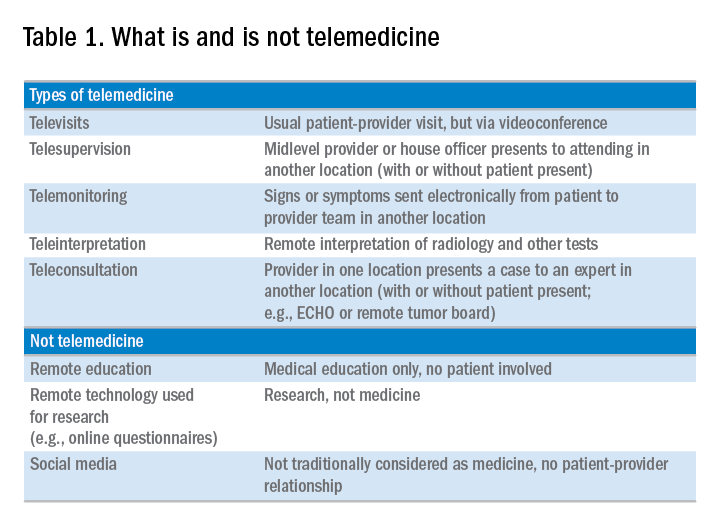
Telemedicine is defined broadly by the World Health Organization as the delivery of health care services at a distance using electronic means for “the diagnosis of treatment, and prevention of disease and injuries, research and evaluation, education of health care providers”1 to improve health. Although no single accepted definition exists, telehealth often is used as the umbrella term to encompass telemedicine (health care delivery) in addition to other activities such as education, research, health surveillance, and public health promotion.2 These various terms often are used interchangeably throughout the literature, leading to confusion.1,3 For the purpose of this review, we will use the term telemedicine to describe any care delivery model whereby patient care is provided at a distance using information technology such as cellphones, computers, or other electronic devices.
In the United States, the use of telemedicine is increasing. According to a 2017 survey of 184 health care executives conducted by the American Telemedicine Association, 88% believed that they would invest in telehealth in the near future, 98% believed that it offered a competitive advantage, with the caveat that 71% believed that lack of coverage and payments were barriers to implementation. Recent studies have shown that telehealth interventions are effective at improving clinical outcomes and decreasing inpatient utilization, with good patient satisfaction in the areas of mental health and chronic disease management. The Veterans Administration has emerged as an early telehealth adopter in chronic disease settings such as mental health, dermatology, hypertension, heart failure, and, as of 2016, has provided care to nearly 700,000 (12%) veterans since its inception.4-6 Despite the increased uptake, significant infrastructure and legal barriers to telemedicine remain and the literature regarding its utility in clinical practice continues to emerge.
Compared with other chronic diseases (e.g., heart failure, diabetes, mental illness) there is a dearth of literature on the use of telemedicine in liver disease. The first portion of this review synthesizes currently published literature of telemedicine/telehealth interventions to improve health care delivery and health outcomes in chronic liver disease including published peer-reviewed articles, abstracts, and ongoing clinical trials. The second portion discusses a framework for the future development of telemedicine and its integration into clinical practice by citing examples currently used throughout the country as well as ways to overcome implementation barriers.
Use of telemedicine in chronic liver disease: A literature review
We performed a systematic review of telemedicine in chronic liver disease. In consultation with a biomedical librarian, we searched for English-language articles for relevant studies with adult participants from July 1984 to May 2017 in PubMed, OVID Medline, American Association for the Study of Liver Disease, EMBASE, Web of Science, ClinicalTrials.gov, Elsevier/Science Direct, and the Cochrane Library (the search strategy is shown in the Supplementary Material at https://doi.org/10.1016/j.cgh.2017.10.004). The references of original publications and of review articles additionally were screened for potentially relevant studies. Abstracts that later resulted in no publications and studies in which telemedicine was used to deliver care, but was neither an exposure nor outcome, were excluded. Social media studies were not considered telemedicine if no patient care was involved. Studies of purely medical education interventions or those that evaluated the accuracy of technology to aid in diagnosis also were excluded.
Supplementary Table 1 (https://doi.org/10.1016/j.cgh.2017.10.004) shows the 20 published articles of telemedicine studies. Among these, there were 9 prospective trials, 3 retrospective studies, 2 case reports, and 6 small case series. One of the studies was randomized prospectively and 10 were uncontrolled.
Telemedicine in hepatitis C treatment
Telemedicine to aid in procedural/surgical management
A few reports have been published in the use of synchronous video and digital technology to aid in periprocedural management in liver disease. A case report highlighted a successful example of gastroenterologist-led teleproctoring using basic video technology to enable a surgeon to perform sclerotherapy for hemostasis in the setting of a variceal bleed.9 Another case report described the transmission of smart phone images from surgical trainees to an attending physician to make a real-time decision regarding a possibly questionable liver procurement, which took place 545 km away from the university hospital.10 A retrospective case series described the feasibility and successful use of high-resolution digital macroscopic photography and electronic transmission between liver transplant centers in the United Kingdom to increase the utilization of split liver transplantation, a setting in which detailed knowledge of vessel anatomy is needed for advanced surgical planning.11 Similarly, an uncontrolled case series from Greece reported on the feasibility and reliability of macroscopic image transmission to aid in the evaluation of liver grafts for transplantation.12
Telemedicine to support evaluation and management of hepatocellular carcinoma
One recent abstract reported on the use of asynchronous store-and-forward telemedicine for screening and management of hepatocellular carcinoma and evaluated process outcomes of specialty care access for newly diagnosed patients.13 A multifaceted approach included live video teleconferencing and centralized radiology review, which was conducted by a multidisciplinary tumor board at an expert hub site, which provided expert opinion and subsequent care (e.g., locoregional therapy, liver transplant evaluation) to spoke sites. As a result of the initiative, the time to specialty evaluation and receipt of hepatocellular carcinoma therapy decreased by 23 and 25 days, respectively.
Remote monitoring interventions
Proposed framework for advancing telemedicine in liver disease: The case for more research and policy changes
Telemedicine can serve two main goals in liver disease: improve access to specialty care, and improve care between visits. For the first goal, the technology is straightforward and limited research is required; the main barriers are regulatory and reimbursement. As an example, one of the authors (M.L.V.) uses telemedicine to perform liver transplant evaluations in Las Vegas, N.V., a state without a liver transplant program. Patients are seen initially by a nurse practitioner who resides in Las Vegas, and those patients needing transplant evaluation are scheduled for a video visit with the attending physician who is physically in California. This works well and patients love it; however, the business model is dependent on the downstream financial incentive of transplantation. In addition, various regulatory requirements must be satisfied such as monthly in-person visits. For the second goal, a number of exciting possibilities exist such as remote monitoring and patient disease management, but more research is needed.
Research
According to the Pew Research Center, 95% of American adults own a cellphone and 77% own a smartphone. These devices passively gather an extraordinary amount of data that could be harnessed to identify early warning signs of complications (remote monitoring). Another potentially fruitful area of research is patient disease management. This includes using technology (e.g., reminder texts) to effect behavior change such as with medication adherence, lifestyle modification, education, or peer mentoring. As an example, the coauthor (M.S.) is leading a study to promote physical activity among liver transplant recipients by using an online web portal developed by researchers at the University of Pennsylvania (Way to Health), which interfaces with patient cell phones and digital accelerometer devices. Participants receive daily feedback through text messages with their step counts, and small financial incentives are provided for adequate levels of physical activity. Technology also can facilitate the development of disease management platforms, which could improve both access and in-between visit monitoring, especially in remote areas. One of the authors (M.L.V.) currently is leading the development of a remote disease management program with funding from the American Association for the Study of Liver Diseases.
Despite the tremendous promise, traditional research methods in telemedicine may be challenging given the rapid and increasing uptake of health technology among patients and health systems. As such, the classic paradigm of randomized controlled trials to evaluate the success of an intervention or change in care delivery often is not feasible. We believe there is a need to recalibrate the definition of what constitutes a high-quality telemedicine study. For example, pragmatic trials and those designed within an implementation science framework that evaluate feasibility, scalability, and cost, in parallel with traditional clinical outcomes, may be better suited and should be accepted more widely.17
Policy
Even when the technology is available and research shows efficacy, the implementation of telemedicine in clinical practice faces regulatory and reimbursement barriers. The first regulatory question is whether a patient–provider relationship is being established (with the exception of limited provider–provider curbside consultation, the answer usually is yes). If so, the practice then is subject to all the usual regulatory concerns. The provider needs to be licensed at the site of origin (where the patient is located) and hold malpractice coverage for that location, and the video and medical record transmission should be compliant with the Health Insurance Portability and Accountability Act. The next challenge is reimbursement. Medicare only pays for video consultation if the patient lives in a designated rural Health Professional Shortage Area (www.cms.gov), and reimbursement by private payers varies. Even this is dependent on ever-changing state laws. Reimbursement for remote patient monitoring is even more limited (the National Telehealth Policy Research Center publishes a useful handbook: http://www.cchpca.org/sites/default/files/resources/50%20State%20FINAL%20April%202016.pdf). Absent a bipartisan Congressional effort to remedy this situation, the best hope for removing reimbursement barriers lies with payment reform. The Medicare Access and CHIP Reauthorization Act of 2015 mandates that the Centers for Medicare and Medicaid Services shift from fee-for-service to alternative payment models in the coming years. In these alternate payment models, providers are responsible for the overall quality and total cost of care for a population of patients. In this scenario, there may be a financial incentive for telemedicine, especially remote monitoring, to keep patients out of the hospital. Until then, under current payment models, reimbursement is limited and the barriers to widespread implementation are high.
Conclusions
Telemedicine has continued to increase in uptake and shows tremendous promise in expanding access to health care, promoting patient disease management, and facilitating in-between health care visit monitoring. Although the future is bright, more research is needed to determine optimal ways to integrate telemedicine — especially remote monitoring — into routine clinical care. We call on our specialty societies to send a clear political advocacy message that policy changes are needed to overcome regulatory and reimbursement challenges.
Acknowledgments
The authors would like to thank Lauren Jones and Mackenzie McDougal for their assistance with the literature review.
Supplementary materials and methods
The telemedicine interventions PubMed literature search strategy was as follows: ((“liver diseases”[MeSH Terms] OR (“liver”[All Fields] AND “diseases”[All Fields]) OR “liver diseases”[All Fields] OR (“liver”[All Fields] AND “disease”[All Fields]) OR “liver disease”[All Fields] OR liver dysfunction OR liver dysfunctions)) OR “liver transplantation”[MeSH Terms] OR “liver transplantation” [All Fields] AND (((“telemedicine”[MeSH Terms] OR “telemedicine”[All Fields] OR mobile health OR mhealth OR telehealth OR mhealth)) OR (videoconferencing OR videoconference)).
References
1. Kirsh S., Su G.L., Sales A., et al. Access to outpatient specialty care: solutions from an integrated health care system. Am J Med Qual. 2015;30:88-90.
2. Wilson L.S., Maeder A.J. recent directions in telemedicine: review of trends in research and practice. Healthc Inform Res. 2015;21:213-22.
3. Cross R.K., Kane, S. Integration of telemedicine into clinical gastroenterology and hepatology practice. Clin Gastroenterol Hepatol. 2017;15:175-81.
4. Darkins A., Ryan P., Kobb R., et al. Care coordination/home telehealth: the systematic implementation of health informatics, home telehealth, and disease management to support the care of veteran patients with chronic conditions. Telemed J E Health. 2008;14:1118-26.
5. Tuerk P.W., Fortney J., Bosworth H.B., et al. Toward the development of national telehealth services: the role of Veterans Health Administration and future directions for research. Telemed J E Health. 2010;16:115-7.
6. VA Press Release. Available: https://www.va.gov/opa/pressrel/includes/viewPDF.cfm?id=2789. Accessed: July 27, 2017.
7. Arora S., Kalishman S., Thornton K., et al. Expanding access to hepatitis C virus treatment-extension for Community Healthcare Outcomes (ECHO) project: disruptive innovation in specialty care. Hepatology. 2010;52:1124-33.
8. Mitruka K., Thornton K., Cusick S., et al. Expanding primary care capacity to treat hepatitis C virus infection through an evidence-based care model–Arizona and Utah, 2012-2014. MMWR Morb Mortal Wkly Rep. 2014;63:393-8.
9. Ahmed A., Slosberg E., Prasad P., et al. The successful use of telemedicine in acute variceal hemorrhage. J Clin Gastroenterol. 1999;29:212-3.
10. Croome K.P., Shum J., Al-Basheer M.A., et al. The benefit of smart phone usage in liver organ procurement. J Telemed Telecare. 2011;17:158-60.
11. Bhati C.S., Wigmore S.J., Reddy S., et al. Web-based image transmission: a novel approach to aid communication in split liver transplantation. Clin Transplant. 2010;24:98-103.
12. Mammas C.S., Geropoulos S., Saatsakis G., et al. Telepathology as a method to optimize quality in organ transplantation: a feasibility and reliability study of the virtual benching of liver graft. Stud Health Technol Inform. 2013;190:276-8.
13. Egert E.M., et al. A regional multidisciplinary liver tumor board improves access to hepatocellular carcinoma treatment for patients geographically distant from tertiary medical center. Hepatology. 2015;62:469A
14. Thomson M., Volk M., Kim H.M., et al. An automated telephone monitoring system to identify patients with cirrhosis at risk of re-hospitalization. Dig Dis Sci. 2015;60:3563-9.
15. Ertel A.E., Kaiser T.E., Abbott D.E., et al. Use of video-based education and tele-health home monitoring after liver transplantation: results of a novel pilot study. Surgery. 2016;160:869-76.
16. Thygesen G.B., Andersen H., Damsgaard B.S. et al. The effect of nurse performed telemedical video consultations for patients suffering from alcohol-related liver cirrhosis. J Hepatol. 2017;66:S349
17. Proctor E., Silmere H., Raghavan R. et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65-76.
Dr. Serper is in the division of gastroenterology, University of Pennsylvania Perelman School of Medicine, Philadelphia, and the department of medicine, Corporal Michael J. Crescenz VA Medical Center, Philadelphia; Dr. Volk is in the division of gastroenterology and Transplantation Institute, Loma Linda University, Loma Linda, Calif. The authors disclose no conflicts.
Telemedicine is defined broadly by the World Health Organization as the delivery of health care services at a distance using electronic means for “the diagnosis of treatment, and prevention of disease and injuries, research and evaluation, education of health care providers”1 to improve health. Although no single accepted definition exists, telehealth often is used as the umbrella term to encompass telemedicine (health care delivery) in addition to other activities such as education, research, health surveillance, and public health promotion.2 These various terms often are used interchangeably throughout the literature, leading to confusion.1,3 For the purpose of this review, we will use the term telemedicine to describe any care delivery model whereby patient care is provided at a distance using information technology such as cellphones, computers, or other electronic devices.

In the United States, the use of telemedicine is increasing. According to a 2017 survey of 184 health care executives conducted by the American Telemedicine Association, 88% believed that they would invest in telehealth in the near future, 98% believed that it offered a competitive advantage, with the caveat that 71% believed that lack of coverage and payments were barriers to implementation. Recent studies have shown that telehealth interventions are effective at improving clinical outcomes and decreasing inpatient utilization, with good patient satisfaction in the areas of mental health and chronic disease management. The Veterans Administration has emerged as an early telehealth adopter in chronic disease settings such as mental health, dermatology, hypertension, heart failure, and, as of 2016, has provided care to nearly 700,000 (12%) veterans since its inception.4-6 Despite the increased uptake, significant infrastructure and legal barriers to telemedicine remain and the literature regarding its utility in clinical practice continues to emerge.
Compared with other chronic diseases (e.g., heart failure, diabetes, mental illness) there is a dearth of literature on the use of telemedicine in liver disease. The first portion of this review synthesizes currently published literature of telemedicine/telehealth interventions to improve health care delivery and health outcomes in chronic liver disease including published peer-reviewed articles, abstracts, and ongoing clinical trials. The second portion discusses a framework for the future development of telemedicine and its integration into clinical practice by citing examples currently used throughout the country as well as ways to overcome implementation barriers.
Use of telemedicine in chronic liver disease: A literature review
We performed a systematic review of telemedicine in chronic liver disease. In consultation with a biomedical librarian, we searched for English-language articles for relevant studies with adult participants from July 1984 to May 2017 in PubMed, OVID Medline, American Association for the Study of Liver Disease, EMBASE, Web of Science, ClinicalTrials.gov, Elsevier/Science Direct, and the Cochrane Library (the search strategy is shown in the Supplementary Material at https://doi.org/10.1016/j.cgh.2017.10.004). The references of original publications and of review articles additionally were screened for potentially relevant studies. Abstracts that later resulted in no publications and studies in which telemedicine was used to deliver care, but was neither an exposure nor outcome, were excluded. Social media studies were not considered telemedicine if no patient care was involved. Studies of purely medical education interventions or those that evaluated the accuracy of technology to aid in diagnosis also were excluded.
Supplementary Table 1 (https://doi.org/10.1016/j.cgh.2017.10.004) shows the 20 published articles of telemedicine studies. Among these, there were 9 prospective trials, 3 retrospective studies, 2 case reports, and 6 small case series. One of the studies was randomized prospectively and 10 were uncontrolled.
Telemedicine in hepatitis C treatment
Telemedicine to aid in procedural/surgical management
A few reports have been published in the use of synchronous video and digital technology to aid in periprocedural management in liver disease. A case report highlighted a successful example of gastroenterologist-led teleproctoring using basic video technology to enable a surgeon to perform sclerotherapy for hemostasis in the setting of a variceal bleed.9 Another case report described the transmission of smart phone images from surgical trainees to an attending physician to make a real-time decision regarding a possibly questionable liver procurement, which took place 545 km away from the university hospital.10 A retrospective case series described the feasibility and successful use of high-resolution digital macroscopic photography and electronic transmission between liver transplant centers in the United Kingdom to increase the utilization of split liver transplantation, a setting in which detailed knowledge of vessel anatomy is needed for advanced surgical planning.11 Similarly, an uncontrolled case series from Greece reported on the feasibility and reliability of macroscopic image transmission to aid in the evaluation of liver grafts for transplantation.12
Telemedicine to support evaluation and management of hepatocellular carcinoma
One recent abstract reported on the use of asynchronous store-and-forward telemedicine for screening and management of hepatocellular carcinoma and evaluated process outcomes of specialty care access for newly diagnosed patients.13 A multifaceted approach included live video teleconferencing and centralized radiology review, which was conducted by a multidisciplinary tumor board at an expert hub site, which provided expert opinion and subsequent care (e.g., locoregional therapy, liver transplant evaluation) to spoke sites. As a result of the initiative, the time to specialty evaluation and receipt of hepatocellular carcinoma therapy decreased by 23 and 25 days, respectively.
Remote monitoring interventions
Proposed framework for advancing telemedicine in liver disease: The case for more research and policy changes
Telemedicine can serve two main goals in liver disease: improve access to specialty care, and improve care between visits. For the first goal, the technology is straightforward and limited research is required; the main barriers are regulatory and reimbursement. As an example, one of the authors (M.L.V.) uses telemedicine to perform liver transplant evaluations in Las Vegas, N.V., a state without a liver transplant program. Patients are seen initially by a nurse practitioner who resides in Las Vegas, and those patients needing transplant evaluation are scheduled for a video visit with the attending physician who is physically in California. This works well and patients love it; however, the business model is dependent on the downstream financial incentive of transplantation. In addition, various regulatory requirements must be satisfied such as monthly in-person visits. For the second goal, a number of exciting possibilities exist such as remote monitoring and patient disease management, but more research is needed.
Research
According to the Pew Research Center, 95% of American adults own a cellphone and 77% own a smartphone. These devices passively gather an extraordinary amount of data that could be harnessed to identify early warning signs of complications (remote monitoring). Another potentially fruitful area of research is patient disease management. This includes using technology (e.g., reminder texts) to effect behavior change such as with medication adherence, lifestyle modification, education, or peer mentoring. As an example, the coauthor (M.S.) is leading a study to promote physical activity among liver transplant recipients by using an online web portal developed by researchers at the University of Pennsylvania (Way to Health), which interfaces with patient cell phones and digital accelerometer devices. Participants receive daily feedback through text messages with their step counts, and small financial incentives are provided for adequate levels of physical activity. Technology also can facilitate the development of disease management platforms, which could improve both access and in-between visit monitoring, especially in remote areas. One of the authors (M.L.V.) currently is leading the development of a remote disease management program with funding from the American Association for the Study of Liver Diseases.
Despite the tremendous promise, traditional research methods in telemedicine may be challenging given the rapid and increasing uptake of health technology among patients and health systems. As such, the classic paradigm of randomized controlled trials to evaluate the success of an intervention or change in care delivery often is not feasible. We believe there is a need to recalibrate the definition of what constitutes a high-quality telemedicine study. For example, pragmatic trials and those designed within an implementation science framework that evaluate feasibility, scalability, and cost, in parallel with traditional clinical outcomes, may be better suited and should be accepted more widely.17
Policy
Even when the technology is available and research shows efficacy, the implementation of telemedicine in clinical practice faces regulatory and reimbursement barriers. The first regulatory question is whether a patient–provider relationship is being established (with the exception of limited provider–provider curbside consultation, the answer usually is yes). If so, the practice then is subject to all the usual regulatory concerns. The provider needs to be licensed at the site of origin (where the patient is located) and hold malpractice coverage for that location, and the video and medical record transmission should be compliant with the Health Insurance Portability and Accountability Act. The next challenge is reimbursement. Medicare only pays for video consultation if the patient lives in a designated rural Health Professional Shortage Area (www.cms.gov), and reimbursement by private payers varies. Even this is dependent on ever-changing state laws. Reimbursement for remote patient monitoring is even more limited (the National Telehealth Policy Research Center publishes a useful handbook: http://www.cchpca.org/sites/default/files/resources/50%20State%20FINAL%20April%202016.pdf). Absent a bipartisan Congressional effort to remedy this situation, the best hope for removing reimbursement barriers lies with payment reform. The Medicare Access and CHIP Reauthorization Act of 2015 mandates that the Centers for Medicare and Medicaid Services shift from fee-for-service to alternative payment models in the coming years. In these alternate payment models, providers are responsible for the overall quality and total cost of care for a population of patients. In this scenario, there may be a financial incentive for telemedicine, especially remote monitoring, to keep patients out of the hospital. Until then, under current payment models, reimbursement is limited and the barriers to widespread implementation are high.
Conclusions
Telemedicine has continued to increase in uptake and shows tremendous promise in expanding access to health care, promoting patient disease management, and facilitating in-between health care visit monitoring. Although the future is bright, more research is needed to determine optimal ways to integrate telemedicine — especially remote monitoring — into routine clinical care. We call on our specialty societies to send a clear political advocacy message that policy changes are needed to overcome regulatory and reimbursement challenges.
Acknowledgments
The authors would like to thank Lauren Jones and Mackenzie McDougal for their assistance with the literature review.
Supplementary materials and methods
The telemedicine interventions PubMed literature search strategy was as follows: ((“liver diseases”[MeSH Terms] OR (“liver”[All Fields] AND “diseases”[All Fields]) OR “liver diseases”[All Fields] OR (“liver”[All Fields] AND “disease”[All Fields]) OR “liver disease”[All Fields] OR liver dysfunction OR liver dysfunctions)) OR “liver transplantation”[MeSH Terms] OR “liver transplantation” [All Fields] AND (((“telemedicine”[MeSH Terms] OR “telemedicine”[All Fields] OR mobile health OR mhealth OR telehealth OR mhealth)) OR (videoconferencing OR videoconference)).
References
1. Kirsh S., Su G.L., Sales A., et al. Access to outpatient specialty care: solutions from an integrated health care system. Am J Med Qual. 2015;30:88-90.
2. Wilson L.S., Maeder A.J. recent directions in telemedicine: review of trends in research and practice. Healthc Inform Res. 2015;21:213-22.
3. Cross R.K., Kane, S. Integration of telemedicine into clinical gastroenterology and hepatology practice. Clin Gastroenterol Hepatol. 2017;15:175-81.
4. Darkins A., Ryan P., Kobb R., et al. Care coordination/home telehealth: the systematic implementation of health informatics, home telehealth, and disease management to support the care of veteran patients with chronic conditions. Telemed J E Health. 2008;14:1118-26.
5. Tuerk P.W., Fortney J., Bosworth H.B., et al. Toward the development of national telehealth services: the role of Veterans Health Administration and future directions for research. Telemed J E Health. 2010;16:115-7.
6. VA Press Release. Available: https://www.va.gov/opa/pressrel/includes/viewPDF.cfm?id=2789. Accessed: July 27, 2017.
7. Arora S., Kalishman S., Thornton K., et al. Expanding access to hepatitis C virus treatment-extension for Community Healthcare Outcomes (ECHO) project: disruptive innovation in specialty care. Hepatology. 2010;52:1124-33.
8. Mitruka K., Thornton K., Cusick S., et al. Expanding primary care capacity to treat hepatitis C virus infection through an evidence-based care model–Arizona and Utah, 2012-2014. MMWR Morb Mortal Wkly Rep. 2014;63:393-8.
9. Ahmed A., Slosberg E., Prasad P., et al. The successful use of telemedicine in acute variceal hemorrhage. J Clin Gastroenterol. 1999;29:212-3.
10. Croome K.P., Shum J., Al-Basheer M.A., et al. The benefit of smart phone usage in liver organ procurement. J Telemed Telecare. 2011;17:158-60.
11. Bhati C.S., Wigmore S.J., Reddy S., et al. Web-based image transmission: a novel approach to aid communication in split liver transplantation. Clin Transplant. 2010;24:98-103.
12. Mammas C.S., Geropoulos S., Saatsakis G., et al. Telepathology as a method to optimize quality in organ transplantation: a feasibility and reliability study of the virtual benching of liver graft. Stud Health Technol Inform. 2013;190:276-8.
13. Egert E.M., et al. A regional multidisciplinary liver tumor board improves access to hepatocellular carcinoma treatment for patients geographically distant from tertiary medical center. Hepatology. 2015;62:469A
14. Thomson M., Volk M., Kim H.M., et al. An automated telephone monitoring system to identify patients with cirrhosis at risk of re-hospitalization. Dig Dis Sci. 2015;60:3563-9.
15. Ertel A.E., Kaiser T.E., Abbott D.E., et al. Use of video-based education and tele-health home monitoring after liver transplantation: results of a novel pilot study. Surgery. 2016;160:869-76.
16. Thygesen G.B., Andersen H., Damsgaard B.S. et al. The effect of nurse performed telemedical video consultations for patients suffering from alcohol-related liver cirrhosis. J Hepatol. 2017;66:S349
17. Proctor E., Silmere H., Raghavan R. et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65-76.
Dr. Serper is in the division of gastroenterology, University of Pennsylvania Perelman School of Medicine, Philadelphia, and the department of medicine, Corporal Michael J. Crescenz VA Medical Center, Philadelphia; Dr. Volk is in the division of gastroenterology and Transplantation Institute, Loma Linda University, Loma Linda, Calif. The authors disclose no conflicts.
Telemedicine is defined broadly by the World Health Organization as the delivery of health care services at a distance using electronic means for “the diagnosis of treatment, and prevention of disease and injuries, research and evaluation, education of health care providers”1 to improve health. Although no single accepted definition exists, telehealth often is used as the umbrella term to encompass telemedicine (health care delivery) in addition to other activities such as education, research, health surveillance, and public health promotion.2 These various terms often are used interchangeably throughout the literature, leading to confusion.1,3 For the purpose of this review, we will use the term telemedicine to describe any care delivery model whereby patient care is provided at a distance using information technology such as cellphones, computers, or other electronic devices.

In the United States, the use of telemedicine is increasing. According to a 2017 survey of 184 health care executives conducted by the American Telemedicine Association, 88% believed that they would invest in telehealth in the near future, 98% believed that it offered a competitive advantage, with the caveat that 71% believed that lack of coverage and payments were barriers to implementation. Recent studies have shown that telehealth interventions are effective at improving clinical outcomes and decreasing inpatient utilization, with good patient satisfaction in the areas of mental health and chronic disease management. The Veterans Administration has emerged as an early telehealth adopter in chronic disease settings such as mental health, dermatology, hypertension, heart failure, and, as of 2016, has provided care to nearly 700,000 (12%) veterans since its inception.4-6 Despite the increased uptake, significant infrastructure and legal barriers to telemedicine remain and the literature regarding its utility in clinical practice continues to emerge.
Compared with other chronic diseases (e.g., heart failure, diabetes, mental illness) there is a dearth of literature on the use of telemedicine in liver disease. The first portion of this review synthesizes currently published literature of telemedicine/telehealth interventions to improve health care delivery and health outcomes in chronic liver disease including published peer-reviewed articles, abstracts, and ongoing clinical trials. The second portion discusses a framework for the future development of telemedicine and its integration into clinical practice by citing examples currently used throughout the country as well as ways to overcome implementation barriers.
Use of telemedicine in chronic liver disease: A literature review
We performed a systematic review of telemedicine in chronic liver disease. In consultation with a biomedical librarian, we searched for English-language articles for relevant studies with adult participants from July 1984 to May 2017 in PubMed, OVID Medline, American Association for the Study of Liver Disease, EMBASE, Web of Science, ClinicalTrials.gov, Elsevier/Science Direct, and the Cochrane Library (the search strategy is shown in the Supplementary Material at https://doi.org/10.1016/j.cgh.2017.10.004). The references of original publications and of review articles additionally were screened for potentially relevant studies. Abstracts that later resulted in no publications and studies in which telemedicine was used to deliver care, but was neither an exposure nor outcome, were excluded. Social media studies were not considered telemedicine if no patient care was involved. Studies of purely medical education interventions or those that evaluated the accuracy of technology to aid in diagnosis also were excluded.
Supplementary Table 1 (https://doi.org/10.1016/j.cgh.2017.10.004) shows the 20 published articles of telemedicine studies. Among these, there were 9 prospective trials, 3 retrospective studies, 2 case reports, and 6 small case series. One of the studies was randomized prospectively and 10 were uncontrolled.
Telemedicine in hepatitis C treatment
Telemedicine to aid in procedural/surgical management
A few reports have been published in the use of synchronous video and digital technology to aid in periprocedural management in liver disease. A case report highlighted a successful example of gastroenterologist-led teleproctoring using basic video technology to enable a surgeon to perform sclerotherapy for hemostasis in the setting of a variceal bleed.9 Another case report described the transmission of smart phone images from surgical trainees to an attending physician to make a real-time decision regarding a possibly questionable liver procurement, which took place 545 km away from the university hospital.10 A retrospective case series described the feasibility and successful use of high-resolution digital macroscopic photography and electronic transmission between liver transplant centers in the United Kingdom to increase the utilization of split liver transplantation, a setting in which detailed knowledge of vessel anatomy is needed for advanced surgical planning.11 Similarly, an uncontrolled case series from Greece reported on the feasibility and reliability of macroscopic image transmission to aid in the evaluation of liver grafts for transplantation.12
Telemedicine to support evaluation and management of hepatocellular carcinoma
One recent abstract reported on the use of asynchronous store-and-forward telemedicine for screening and management of hepatocellular carcinoma and evaluated process outcomes of specialty care access for newly diagnosed patients.13 A multifaceted approach included live video teleconferencing and centralized radiology review, which was conducted by a multidisciplinary tumor board at an expert hub site, which provided expert opinion and subsequent care (e.g., locoregional therapy, liver transplant evaluation) to spoke sites. As a result of the initiative, the time to specialty evaluation and receipt of hepatocellular carcinoma therapy decreased by 23 and 25 days, respectively.
Remote monitoring interventions
Proposed framework for advancing telemedicine in liver disease: The case for more research and policy changes
Telemedicine can serve two main goals in liver disease: improve access to specialty care, and improve care between visits. For the first goal, the technology is straightforward and limited research is required; the main barriers are regulatory and reimbursement. As an example, one of the authors (M.L.V.) uses telemedicine to perform liver transplant evaluations in Las Vegas, N.V., a state without a liver transplant program. Patients are seen initially by a nurse practitioner who resides in Las Vegas, and those patients needing transplant evaluation are scheduled for a video visit with the attending physician who is physically in California. This works well and patients love it; however, the business model is dependent on the downstream financial incentive of transplantation. In addition, various regulatory requirements must be satisfied such as monthly in-person visits. For the second goal, a number of exciting possibilities exist such as remote monitoring and patient disease management, but more research is needed.
Research
According to the Pew Research Center, 95% of American adults own a cellphone and 77% own a smartphone. These devices passively gather an extraordinary amount of data that could be harnessed to identify early warning signs of complications (remote monitoring). Another potentially fruitful area of research is patient disease management. This includes using technology (e.g., reminder texts) to effect behavior change such as with medication adherence, lifestyle modification, education, or peer mentoring. As an example, the coauthor (M.S.) is leading a study to promote physical activity among liver transplant recipients by using an online web portal developed by researchers at the University of Pennsylvania (Way to Health), which interfaces with patient cell phones and digital accelerometer devices. Participants receive daily feedback through text messages with their step counts, and small financial incentives are provided for adequate levels of physical activity. Technology also can facilitate the development of disease management platforms, which could improve both access and in-between visit monitoring, especially in remote areas. One of the authors (M.L.V.) currently is leading the development of a remote disease management program with funding from the American Association for the Study of Liver Diseases.
Despite the tremendous promise, traditional research methods in telemedicine may be challenging given the rapid and increasing uptake of health technology among patients and health systems. As such, the classic paradigm of randomized controlled trials to evaluate the success of an intervention or change in care delivery often is not feasible. We believe there is a need to recalibrate the definition of what constitutes a high-quality telemedicine study. For example, pragmatic trials and those designed within an implementation science framework that evaluate feasibility, scalability, and cost, in parallel with traditional clinical outcomes, may be better suited and should be accepted more widely.17
Policy
Even when the technology is available and research shows efficacy, the implementation of telemedicine in clinical practice faces regulatory and reimbursement barriers. The first regulatory question is whether a patient–provider relationship is being established (with the exception of limited provider–provider curbside consultation, the answer usually is yes). If so, the practice then is subject to all the usual regulatory concerns. The provider needs to be licensed at the site of origin (where the patient is located) and hold malpractice coverage for that location, and the video and medical record transmission should be compliant with the Health Insurance Portability and Accountability Act. The next challenge is reimbursement. Medicare only pays for video consultation if the patient lives in a designated rural Health Professional Shortage Area (www.cms.gov), and reimbursement by private payers varies. Even this is dependent on ever-changing state laws. Reimbursement for remote patient monitoring is even more limited (the National Telehealth Policy Research Center publishes a useful handbook: http://www.cchpca.org/sites/default/files/resources/50%20State%20FINAL%20April%202016.pdf). Absent a bipartisan Congressional effort to remedy this situation, the best hope for removing reimbursement barriers lies with payment reform. The Medicare Access and CHIP Reauthorization Act of 2015 mandates that the Centers for Medicare and Medicaid Services shift from fee-for-service to alternative payment models in the coming years. In these alternate payment models, providers are responsible for the overall quality and total cost of care for a population of patients. In this scenario, there may be a financial incentive for telemedicine, especially remote monitoring, to keep patients out of the hospital. Until then, under current payment models, reimbursement is limited and the barriers to widespread implementation are high.
Conclusions
Telemedicine has continued to increase in uptake and shows tremendous promise in expanding access to health care, promoting patient disease management, and facilitating in-between health care visit monitoring. Although the future is bright, more research is needed to determine optimal ways to integrate telemedicine — especially remote monitoring — into routine clinical care. We call on our specialty societies to send a clear political advocacy message that policy changes are needed to overcome regulatory and reimbursement challenges.
Acknowledgments
The authors would like to thank Lauren Jones and Mackenzie McDougal for their assistance with the literature review.
Supplementary materials and methods
The telemedicine interventions PubMed literature search strategy was as follows: ((“liver diseases”[MeSH Terms] OR (“liver”[All Fields] AND “diseases”[All Fields]) OR “liver diseases”[All Fields] OR (“liver”[All Fields] AND “disease”[All Fields]) OR “liver disease”[All Fields] OR liver dysfunction OR liver dysfunctions)) OR “liver transplantation”[MeSH Terms] OR “liver transplantation” [All Fields] AND (((“telemedicine”[MeSH Terms] OR “telemedicine”[All Fields] OR mobile health OR mhealth OR telehealth OR mhealth)) OR (videoconferencing OR videoconference)).
References
1. Kirsh S., Su G.L., Sales A., et al. Access to outpatient specialty care: solutions from an integrated health care system. Am J Med Qual. 2015;30:88-90.
2. Wilson L.S., Maeder A.J. recent directions in telemedicine: review of trends in research and practice. Healthc Inform Res. 2015;21:213-22.
3. Cross R.K., Kane, S. Integration of telemedicine into clinical gastroenterology and hepatology practice. Clin Gastroenterol Hepatol. 2017;15:175-81.
4. Darkins A., Ryan P., Kobb R., et al. Care coordination/home telehealth: the systematic implementation of health informatics, home telehealth, and disease management to support the care of veteran patients with chronic conditions. Telemed J E Health. 2008;14:1118-26.
5. Tuerk P.W., Fortney J., Bosworth H.B., et al. Toward the development of national telehealth services: the role of Veterans Health Administration and future directions for research. Telemed J E Health. 2010;16:115-7.
6. VA Press Release. Available: https://www.va.gov/opa/pressrel/includes/viewPDF.cfm?id=2789. Accessed: July 27, 2017.
7. Arora S., Kalishman S., Thornton K., et al. Expanding access to hepatitis C virus treatment-extension for Community Healthcare Outcomes (ECHO) project: disruptive innovation in specialty care. Hepatology. 2010;52:1124-33.
8. Mitruka K., Thornton K., Cusick S., et al. Expanding primary care capacity to treat hepatitis C virus infection through an evidence-based care model–Arizona and Utah, 2012-2014. MMWR Morb Mortal Wkly Rep. 2014;63:393-8.
9. Ahmed A., Slosberg E., Prasad P., et al. The successful use of telemedicine in acute variceal hemorrhage. J Clin Gastroenterol. 1999;29:212-3.
10. Croome K.P., Shum J., Al-Basheer M.A., et al. The benefit of smart phone usage in liver organ procurement. J Telemed Telecare. 2011;17:158-60.
11. Bhati C.S., Wigmore S.J., Reddy S., et al. Web-based image transmission: a novel approach to aid communication in split liver transplantation. Clin Transplant. 2010;24:98-103.
12. Mammas C.S., Geropoulos S., Saatsakis G., et al. Telepathology as a method to optimize quality in organ transplantation: a feasibility and reliability study of the virtual benching of liver graft. Stud Health Technol Inform. 2013;190:276-8.
13. Egert E.M., et al. A regional multidisciplinary liver tumor board improves access to hepatocellular carcinoma treatment for patients geographically distant from tertiary medical center. Hepatology. 2015;62:469A
14. Thomson M., Volk M., Kim H.M., et al. An automated telephone monitoring system to identify patients with cirrhosis at risk of re-hospitalization. Dig Dis Sci. 2015;60:3563-9.
15. Ertel A.E., Kaiser T.E., Abbott D.E., et al. Use of video-based education and tele-health home monitoring after liver transplantation: results of a novel pilot study. Surgery. 2016;160:869-76.
16. Thygesen G.B., Andersen H., Damsgaard B.S. et al. The effect of nurse performed telemedical video consultations for patients suffering from alcohol-related liver cirrhosis. J Hepatol. 2017;66:S349
17. Proctor E., Silmere H., Raghavan R. et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65-76.
Dr. Serper is in the division of gastroenterology, University of Pennsylvania Perelman School of Medicine, Philadelphia, and the department of medicine, Corporal Michael J. Crescenz VA Medical Center, Philadelphia; Dr. Volk is in the division of gastroenterology and Transplantation Institute, Loma Linda University, Loma Linda, Calif. The authors disclose no conflicts.

