User login
Out-of-pocket costs put HIV prevention drug out of reach for many at risk
Since brand-name Truvada was approved for HIV prevention six years ago, its average wholesale price has increased by about 45 percent. Now, the drug – which rakes in billions of dollars in annual global revenue for its manufacturer, Gilead Sciences – carries a list price of close to $2,000 for a 30-day supply.
Most insurers cover the pill, also known as pre-exposure prophylaxis, or PrEP. It has been shown to be more than 90 percent effective in HIV prevention when taken daily.
But patients can get stuck with out-of-pocket costs that make it unaffordable.
“If there is any example of the dysfunction in the American pharmaceutical system, it is this case,” said James Krellenstein, a member of the AIDS advocacy group ACT UP New York. “We have the most effective tool for ending the HIV epidemic, and one reason we’re unable to scale up is because it costs so [much] unnecessarily.”
As policymakers and the health system debate how to control ever-climbing drug prices, experts say this case underscores how patients are left holding the bag.
Private health plans are making patients responsible for a larger share of drug costs. And more are restricting use of the “copay coupons” pharmaceutical companies have used to shield patients from out-of-pocket expenses. Insurers say the drug companies use coupons to steer consumers toward pricier meds. One way health plans are limiting their use is by no longer allowing them to count toward patients’ deductibles.
“This is one more thing that is going to push people off their medications,” said Jim Pickett, a senior director at the AIDS Foundation of Chicago.
Jared Wile, who lives in Chicago, started taking PrEP about three years ago, when he was dating someone with HIV. Wile, who has a $2,750 deductible, used a coupon to obtain the drug. He never paid anything out-of-pocket, he said.
Gilead waives up to $4,800 in out-of-pocket expenses for commercially insured patients.
That changed for Wile this past May, when Wile learned the coupon no longer counted toward his deductible and that he would have to pay the full cost of the prescription – $1,600 per month – until he hit his deductible. Wile said he felt “blindsided” and stopped taking the medication.
Gilead spokesman Ryan McKeel said the company has made extra efforts to help patients overcome financial barriers. He cited assistance programs for uninsured and underinsured people.
“We have designed our assistance programs with the intent that people can benefit from their full value, and we cannot control the actions or decisions of health insurers,” McKeel said in an emailed statement.
The federal Centers for Disease Control and Prevention estimates that more than 1 million people are at high risk of contracting HIV, but Gilead says only about 167,000 people currently take PrEP.
Beyond the money crunch
Price is one of many barriers – alongside patients’ lack of awareness and doctors’ hesitation to prescribe – that threaten to exacerbate the already stark disparities in PrEP use and HIV infection rates.
One major disparity is along geographic lines. The South, for example, accounts for over half of new HIV diagnoses but only about 30 percent of new PrEP users, according to data from AIDSVu, which maps HIV disease and PrEP use. HIV rates and PrEP use also vary by race and ethnicity.
“We are not necessarily seeing that those most at risk are the ones starting PrEP,” said Kristin Keglovitz Baker, chief operating officer of Howard Brown Health, a Chicago health center.
Gilead has recently gone all-in with advertising to reach people at risk, including print campaigns and TV ads that will air through the summer. Since 2012, it has spent $28 million to fund U.S. organizations that seek to raise awareness of HIV, McKeel, the company spokesman, said.
“We recognize that many people who are at high risk for HIV infection still face challenges in accessing Truvada for PrEP, and we are in regular dialogue with public health officials, advocates and physicians to better understand and, where possible, help to address these challenges,” he added.
But price is also an impediment for publicly funded programs, which have limited budgets and are now shelling out more cash for the prevention effort.
“If it was only pennies ... we would be throwing it around,” said Joey Mattingly, an assistant professor at the University of Maryland School of Pharmacy. “Because of how costly it is, we have to control it.”
Some states – California and Florida among them – have launched PrEP assistance programs that can help patients cover the cost of the medication, along with required lab work and medical visits.
Beyond these state-based programs, some public health departments and HIV service organizations are hiring PrEP navigators to help patients navigate the maze of copays and deductibles, and to improve recruitment and retention of new PrEP users.
Washington, D.C.’s health department has doubled down on prevention, and Truvada is key in that effort, said Michael Kharfen, the department’s senior deputy director for HIV/AIDS, Hepatitis, STD and TB Administration.
Insurance usually covers PrEP, and patient assistance programs should fill any financial gaps, he said. But when that isn’t feasible, the department steps in, distributing free Truvada starter packs to at-risk patients.
Kharfen said the city has in the past three years spent almost a million dollars just on Truvada pills, which it purchases at a discounted rate through the federal 340B program, which benefits certain health care providers that treat low-income people. And because of new publicity efforts, he expects the department will need to buy and distribute more pills – posing a conundrum.
Treating more people is net positive, he said. But “how do we sustain this?”
Medicaid programs generally cover PrEP, so they confront a similar situation. Outreach efforts lead to more beneficiaries who take the drug, but that, in turn, could subject the states’ Medicaid budgets to financial hardship.
States are spending millions of dollars on the drug. California’s Medicaid program, for example, spent about $50 million in 2017 and expects the costs to continue climbing. But officials said the expense is offset by long-term savings in preventing new HIV cases.
Massachusetts’ Medicaid program spent about $22 million on Truvada that same year – about $18,000 per beneficiary, according to a spokeswoman for the agency’s Executive Office of Health and Human Services. Those figures don’t account for rebates the state receives from Gilead, which are undisclosed and considered proprietary information.
A complex solution, and no competition
PrEP is only one part of HIV prevention, so help paying for the pill is only one piece of the puzzle.
Patients also need regular HIV testing and medical care, which add to the cost borne both by patients and the health system. Some experts warn that Truvada’s high price point could financially undermine such broad prevention efforts.
Competition could help.
A generic version of the drug, manufactured by Teva Pharmaceuticals, is available abroad and gained approval for use last year from the federal Food and Drug Administration. When it becomes available in the United States, it could bring down prices, though it’s unclear when that will happen. Gilead’s own forecasts reflect that expectation, showing declines in future revenue from Truvada.
“When generics enter, brands lose market share,” said David Howard, a health economist and professor at Emory University, who previously worked in the pharmaceutical industry.
For now, though, Truvada is the only PrEP option available in the U.S., he said. “From a company standpoint ... their best strategy is to make as much money as they can.”
KHN’s coverage of these topics is supported by Laura and John Arnold Foundation and Blue Shield of California Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Since brand-name Truvada was approved for HIV prevention six years ago, its average wholesale price has increased by about 45 percent. Now, the drug – which rakes in billions of dollars in annual global revenue for its manufacturer, Gilead Sciences – carries a list price of close to $2,000 for a 30-day supply.
Most insurers cover the pill, also known as pre-exposure prophylaxis, or PrEP. It has been shown to be more than 90 percent effective in HIV prevention when taken daily.
But patients can get stuck with out-of-pocket costs that make it unaffordable.
“If there is any example of the dysfunction in the American pharmaceutical system, it is this case,” said James Krellenstein, a member of the AIDS advocacy group ACT UP New York. “We have the most effective tool for ending the HIV epidemic, and one reason we’re unable to scale up is because it costs so [much] unnecessarily.”
As policymakers and the health system debate how to control ever-climbing drug prices, experts say this case underscores how patients are left holding the bag.
Private health plans are making patients responsible for a larger share of drug costs. And more are restricting use of the “copay coupons” pharmaceutical companies have used to shield patients from out-of-pocket expenses. Insurers say the drug companies use coupons to steer consumers toward pricier meds. One way health plans are limiting their use is by no longer allowing them to count toward patients’ deductibles.
“This is one more thing that is going to push people off their medications,” said Jim Pickett, a senior director at the AIDS Foundation of Chicago.
Jared Wile, who lives in Chicago, started taking PrEP about three years ago, when he was dating someone with HIV. Wile, who has a $2,750 deductible, used a coupon to obtain the drug. He never paid anything out-of-pocket, he said.
Gilead waives up to $4,800 in out-of-pocket expenses for commercially insured patients.
That changed for Wile this past May, when Wile learned the coupon no longer counted toward his deductible and that he would have to pay the full cost of the prescription – $1,600 per month – until he hit his deductible. Wile said he felt “blindsided” and stopped taking the medication.
Gilead spokesman Ryan McKeel said the company has made extra efforts to help patients overcome financial barriers. He cited assistance programs for uninsured and underinsured people.
“We have designed our assistance programs with the intent that people can benefit from their full value, and we cannot control the actions or decisions of health insurers,” McKeel said in an emailed statement.
The federal Centers for Disease Control and Prevention estimates that more than 1 million people are at high risk of contracting HIV, but Gilead says only about 167,000 people currently take PrEP.
Beyond the money crunch
Price is one of many barriers – alongside patients’ lack of awareness and doctors’ hesitation to prescribe – that threaten to exacerbate the already stark disparities in PrEP use and HIV infection rates.
One major disparity is along geographic lines. The South, for example, accounts for over half of new HIV diagnoses but only about 30 percent of new PrEP users, according to data from AIDSVu, which maps HIV disease and PrEP use. HIV rates and PrEP use also vary by race and ethnicity.
“We are not necessarily seeing that those most at risk are the ones starting PrEP,” said Kristin Keglovitz Baker, chief operating officer of Howard Brown Health, a Chicago health center.
Gilead has recently gone all-in with advertising to reach people at risk, including print campaigns and TV ads that will air through the summer. Since 2012, it has spent $28 million to fund U.S. organizations that seek to raise awareness of HIV, McKeel, the company spokesman, said.
“We recognize that many people who are at high risk for HIV infection still face challenges in accessing Truvada for PrEP, and we are in regular dialogue with public health officials, advocates and physicians to better understand and, where possible, help to address these challenges,” he added.
But price is also an impediment for publicly funded programs, which have limited budgets and are now shelling out more cash for the prevention effort.
“If it was only pennies ... we would be throwing it around,” said Joey Mattingly, an assistant professor at the University of Maryland School of Pharmacy. “Because of how costly it is, we have to control it.”
Some states – California and Florida among them – have launched PrEP assistance programs that can help patients cover the cost of the medication, along with required lab work and medical visits.
Beyond these state-based programs, some public health departments and HIV service organizations are hiring PrEP navigators to help patients navigate the maze of copays and deductibles, and to improve recruitment and retention of new PrEP users.
Washington, D.C.’s health department has doubled down on prevention, and Truvada is key in that effort, said Michael Kharfen, the department’s senior deputy director for HIV/AIDS, Hepatitis, STD and TB Administration.
Insurance usually covers PrEP, and patient assistance programs should fill any financial gaps, he said. But when that isn’t feasible, the department steps in, distributing free Truvada starter packs to at-risk patients.
Kharfen said the city has in the past three years spent almost a million dollars just on Truvada pills, which it purchases at a discounted rate through the federal 340B program, which benefits certain health care providers that treat low-income people. And because of new publicity efforts, he expects the department will need to buy and distribute more pills – posing a conundrum.
Treating more people is net positive, he said. But “how do we sustain this?”
Medicaid programs generally cover PrEP, so they confront a similar situation. Outreach efforts lead to more beneficiaries who take the drug, but that, in turn, could subject the states’ Medicaid budgets to financial hardship.
States are spending millions of dollars on the drug. California’s Medicaid program, for example, spent about $50 million in 2017 and expects the costs to continue climbing. But officials said the expense is offset by long-term savings in preventing new HIV cases.
Massachusetts’ Medicaid program spent about $22 million on Truvada that same year – about $18,000 per beneficiary, according to a spokeswoman for the agency’s Executive Office of Health and Human Services. Those figures don’t account for rebates the state receives from Gilead, which are undisclosed and considered proprietary information.
A complex solution, and no competition
PrEP is only one part of HIV prevention, so help paying for the pill is only one piece of the puzzle.
Patients also need regular HIV testing and medical care, which add to the cost borne both by patients and the health system. Some experts warn that Truvada’s high price point could financially undermine such broad prevention efforts.
Competition could help.
A generic version of the drug, manufactured by Teva Pharmaceuticals, is available abroad and gained approval for use last year from the federal Food and Drug Administration. When it becomes available in the United States, it could bring down prices, though it’s unclear when that will happen. Gilead’s own forecasts reflect that expectation, showing declines in future revenue from Truvada.
“When generics enter, brands lose market share,” said David Howard, a health economist and professor at Emory University, who previously worked in the pharmaceutical industry.
For now, though, Truvada is the only PrEP option available in the U.S., he said. “From a company standpoint ... their best strategy is to make as much money as they can.”
KHN’s coverage of these topics is supported by Laura and John Arnold Foundation and Blue Shield of California Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Since brand-name Truvada was approved for HIV prevention six years ago, its average wholesale price has increased by about 45 percent. Now, the drug – which rakes in billions of dollars in annual global revenue for its manufacturer, Gilead Sciences – carries a list price of close to $2,000 for a 30-day supply.
Most insurers cover the pill, also known as pre-exposure prophylaxis, or PrEP. It has been shown to be more than 90 percent effective in HIV prevention when taken daily.
But patients can get stuck with out-of-pocket costs that make it unaffordable.
“If there is any example of the dysfunction in the American pharmaceutical system, it is this case,” said James Krellenstein, a member of the AIDS advocacy group ACT UP New York. “We have the most effective tool for ending the HIV epidemic, and one reason we’re unable to scale up is because it costs so [much] unnecessarily.”
As policymakers and the health system debate how to control ever-climbing drug prices, experts say this case underscores how patients are left holding the bag.
Private health plans are making patients responsible for a larger share of drug costs. And more are restricting use of the “copay coupons” pharmaceutical companies have used to shield patients from out-of-pocket expenses. Insurers say the drug companies use coupons to steer consumers toward pricier meds. One way health plans are limiting their use is by no longer allowing them to count toward patients’ deductibles.
“This is one more thing that is going to push people off their medications,” said Jim Pickett, a senior director at the AIDS Foundation of Chicago.
Jared Wile, who lives in Chicago, started taking PrEP about three years ago, when he was dating someone with HIV. Wile, who has a $2,750 deductible, used a coupon to obtain the drug. He never paid anything out-of-pocket, he said.
Gilead waives up to $4,800 in out-of-pocket expenses for commercially insured patients.
That changed for Wile this past May, when Wile learned the coupon no longer counted toward his deductible and that he would have to pay the full cost of the prescription – $1,600 per month – until he hit his deductible. Wile said he felt “blindsided” and stopped taking the medication.
Gilead spokesman Ryan McKeel said the company has made extra efforts to help patients overcome financial barriers. He cited assistance programs for uninsured and underinsured people.
“We have designed our assistance programs with the intent that people can benefit from their full value, and we cannot control the actions or decisions of health insurers,” McKeel said in an emailed statement.
The federal Centers for Disease Control and Prevention estimates that more than 1 million people are at high risk of contracting HIV, but Gilead says only about 167,000 people currently take PrEP.
Beyond the money crunch
Price is one of many barriers – alongside patients’ lack of awareness and doctors’ hesitation to prescribe – that threaten to exacerbate the already stark disparities in PrEP use and HIV infection rates.
One major disparity is along geographic lines. The South, for example, accounts for over half of new HIV diagnoses but only about 30 percent of new PrEP users, according to data from AIDSVu, which maps HIV disease and PrEP use. HIV rates and PrEP use also vary by race and ethnicity.
“We are not necessarily seeing that those most at risk are the ones starting PrEP,” said Kristin Keglovitz Baker, chief operating officer of Howard Brown Health, a Chicago health center.
Gilead has recently gone all-in with advertising to reach people at risk, including print campaigns and TV ads that will air through the summer. Since 2012, it has spent $28 million to fund U.S. organizations that seek to raise awareness of HIV, McKeel, the company spokesman, said.
“We recognize that many people who are at high risk for HIV infection still face challenges in accessing Truvada for PrEP, and we are in regular dialogue with public health officials, advocates and physicians to better understand and, where possible, help to address these challenges,” he added.
But price is also an impediment for publicly funded programs, which have limited budgets and are now shelling out more cash for the prevention effort.
“If it was only pennies ... we would be throwing it around,” said Joey Mattingly, an assistant professor at the University of Maryland School of Pharmacy. “Because of how costly it is, we have to control it.”
Some states – California and Florida among them – have launched PrEP assistance programs that can help patients cover the cost of the medication, along with required lab work and medical visits.
Beyond these state-based programs, some public health departments and HIV service organizations are hiring PrEP navigators to help patients navigate the maze of copays and deductibles, and to improve recruitment and retention of new PrEP users.
Washington, D.C.’s health department has doubled down on prevention, and Truvada is key in that effort, said Michael Kharfen, the department’s senior deputy director for HIV/AIDS, Hepatitis, STD and TB Administration.
Insurance usually covers PrEP, and patient assistance programs should fill any financial gaps, he said. But when that isn’t feasible, the department steps in, distributing free Truvada starter packs to at-risk patients.
Kharfen said the city has in the past three years spent almost a million dollars just on Truvada pills, which it purchases at a discounted rate through the federal 340B program, which benefits certain health care providers that treat low-income people. And because of new publicity efforts, he expects the department will need to buy and distribute more pills – posing a conundrum.
Treating more people is net positive, he said. But “how do we sustain this?”
Medicaid programs generally cover PrEP, so they confront a similar situation. Outreach efforts lead to more beneficiaries who take the drug, but that, in turn, could subject the states’ Medicaid budgets to financial hardship.
States are spending millions of dollars on the drug. California’s Medicaid program, for example, spent about $50 million in 2017 and expects the costs to continue climbing. But officials said the expense is offset by long-term savings in preventing new HIV cases.
Massachusetts’ Medicaid program spent about $22 million on Truvada that same year – about $18,000 per beneficiary, according to a spokeswoman for the agency’s Executive Office of Health and Human Services. Those figures don’t account for rebates the state receives from Gilead, which are undisclosed and considered proprietary information.
A complex solution, and no competition
PrEP is only one part of HIV prevention, so help paying for the pill is only one piece of the puzzle.
Patients also need regular HIV testing and medical care, which add to the cost borne both by patients and the health system. Some experts warn that Truvada’s high price point could financially undermine such broad prevention efforts.
Competition could help.
A generic version of the drug, manufactured by Teva Pharmaceuticals, is available abroad and gained approval for use last year from the federal Food and Drug Administration. When it becomes available in the United States, it could bring down prices, though it’s unclear when that will happen. Gilead’s own forecasts reflect that expectation, showing declines in future revenue from Truvada.
“When generics enter, brands lose market share,” said David Howard, a health economist and professor at Emory University, who previously worked in the pharmaceutical industry.
For now, though, Truvada is the only PrEP option available in the U.S., he said. “From a company standpoint ... their best strategy is to make as much money as they can.”
KHN’s coverage of these topics is supported by Laura and John Arnold Foundation and Blue Shield of California Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Despite U.S. court’s ruling, Medicaid work requirements advance in other states
.
The decision by Judge James Boasberg immediately blocked Kentucky from enacting the provision in Campbell County, which had been set to start Sunday and roll out statewide later this year. Within 36 hours, Kentucky Gov. Matt Bevin, a Republican, eliminated vision and dental benefits to nearly 500,000 Medicaid enrollees, saying the state could no longer afford it.
Meanwhile, Arkansas, New Hampshire and Indiana are moving ahead with the implementation of their versions of a Medicaid work requirement. It is not clear how or if Boasberg’s ruling invalidating the Trump administration’s approval of Kentucky’s plan affects these states.
Arkansas is implementing its requirement this summer while New Hampshire and Indiana plan to phase in their rules beginning In January.
Virginia health officials say they still plan to seek federal permission to enact a work requirement but it isn’t needed in order to expand Medicaid eligibility on Jan.1.
Virginia lawmakers approved Medicaid expansion in June with the condition the state apply for federal permission to include the new mandate.
“We remain focused on the work necessary to ensure that new health coverage for Virginia adults is available beginning on January 1, 2019,” Dr. Jennifer Lee, director of the Virginia Department of Medical Assistance Services, said in a statement. “Developing a waiver is a separate and ongoing process, as described in the final state budget.”
Virginia Medicaid is in discussions with the U.S. Centers for Medicare & Medicaid Services about its waiver, which has not yet been submitted.
Many Republican lawmakers in Virginia voted to expand Medicaid only after it was assured new enrollees would have to work or do volunteer service.
Dr. Scott Garrett, a Virginia House member from Lynchburg, Va., said he was under the impression the bill signed in June by Virginia Gov. Ralph Northam, a Democrat, meant the Medicaid expansion would begin only with a work requirement in place.
“The intent of the General Assembly ... was that you could not do one absent the other,” he said.
Garrett, a Republican, said he long opposed plans to add 400,000 adults to Medicaid because of cost concerns. He said requiring these enrollees to work or do volunteer service would make them healthier and improve their well-being.
Patricia Boozang, senior managing director for Manatt Health, a consulting firm, said she is not surprised states are moving ahead with work requirement plans regardless of the court ruling, which was specific to Kentucky.
She said the decision would cause the Trump administration to review the pending applications more closely so they could withstand a judicial review.
The federal court said Health and Human Services Secretary Alex Azar did not adequately take into account how many people would lose coverage for the work requirement and did not prove such a provision would improve enrollees’ health.
“It’s going to be challenging for them to make the case that health and well-being is going to be improved by the [work requirement] waiver,” she said.
In Arkansas, some Medicaid enrollees face a Thursday deadline to register their status – that they worked, did volunteer service in June or meet one of the state’s many exemptions.
“The ruling does not have an immediate effect on Arkansas’ work requirement,” said spokeswoman Marci Manley.
Advocates for low-income people are weighing whether to file lawsuits to stop the work requirement in other states that have won federal approval.
“We have ... partnerships with state legal advocates in these states and are exploring enforcement and litigation options with them,” said Jane Perkins, legal director of the National Health Law Program, which filed the suit on behalf of Medicaid enrollees in Kentucky to block the work requirements.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
.
The decision by Judge James Boasberg immediately blocked Kentucky from enacting the provision in Campbell County, which had been set to start Sunday and roll out statewide later this year. Within 36 hours, Kentucky Gov. Matt Bevin, a Republican, eliminated vision and dental benefits to nearly 500,000 Medicaid enrollees, saying the state could no longer afford it.
Meanwhile, Arkansas, New Hampshire and Indiana are moving ahead with the implementation of their versions of a Medicaid work requirement. It is not clear how or if Boasberg’s ruling invalidating the Trump administration’s approval of Kentucky’s plan affects these states.
Arkansas is implementing its requirement this summer while New Hampshire and Indiana plan to phase in their rules beginning In January.
Virginia health officials say they still plan to seek federal permission to enact a work requirement but it isn’t needed in order to expand Medicaid eligibility on Jan.1.
Virginia lawmakers approved Medicaid expansion in June with the condition the state apply for federal permission to include the new mandate.
“We remain focused on the work necessary to ensure that new health coverage for Virginia adults is available beginning on January 1, 2019,” Dr. Jennifer Lee, director of the Virginia Department of Medical Assistance Services, said in a statement. “Developing a waiver is a separate and ongoing process, as described in the final state budget.”
Virginia Medicaid is in discussions with the U.S. Centers for Medicare & Medicaid Services about its waiver, which has not yet been submitted.
Many Republican lawmakers in Virginia voted to expand Medicaid only after it was assured new enrollees would have to work or do volunteer service.
Dr. Scott Garrett, a Virginia House member from Lynchburg, Va., said he was under the impression the bill signed in June by Virginia Gov. Ralph Northam, a Democrat, meant the Medicaid expansion would begin only with a work requirement in place.
“The intent of the General Assembly ... was that you could not do one absent the other,” he said.
Garrett, a Republican, said he long opposed plans to add 400,000 adults to Medicaid because of cost concerns. He said requiring these enrollees to work or do volunteer service would make them healthier and improve their well-being.
Patricia Boozang, senior managing director for Manatt Health, a consulting firm, said she is not surprised states are moving ahead with work requirement plans regardless of the court ruling, which was specific to Kentucky.
She said the decision would cause the Trump administration to review the pending applications more closely so they could withstand a judicial review.
The federal court said Health and Human Services Secretary Alex Azar did not adequately take into account how many people would lose coverage for the work requirement and did not prove such a provision would improve enrollees’ health.
“It’s going to be challenging for them to make the case that health and well-being is going to be improved by the [work requirement] waiver,” she said.
In Arkansas, some Medicaid enrollees face a Thursday deadline to register their status – that they worked, did volunteer service in June or meet one of the state’s many exemptions.
“The ruling does not have an immediate effect on Arkansas’ work requirement,” said spokeswoman Marci Manley.
Advocates for low-income people are weighing whether to file lawsuits to stop the work requirement in other states that have won federal approval.
“We have ... partnerships with state legal advocates in these states and are exploring enforcement and litigation options with them,” said Jane Perkins, legal director of the National Health Law Program, which filed the suit on behalf of Medicaid enrollees in Kentucky to block the work requirements.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
.
The decision by Judge James Boasberg immediately blocked Kentucky from enacting the provision in Campbell County, which had been set to start Sunday and roll out statewide later this year. Within 36 hours, Kentucky Gov. Matt Bevin, a Republican, eliminated vision and dental benefits to nearly 500,000 Medicaid enrollees, saying the state could no longer afford it.
Meanwhile, Arkansas, New Hampshire and Indiana are moving ahead with the implementation of their versions of a Medicaid work requirement. It is not clear how or if Boasberg’s ruling invalidating the Trump administration’s approval of Kentucky’s plan affects these states.
Arkansas is implementing its requirement this summer while New Hampshire and Indiana plan to phase in their rules beginning In January.
Virginia health officials say they still plan to seek federal permission to enact a work requirement but it isn’t needed in order to expand Medicaid eligibility on Jan.1.
Virginia lawmakers approved Medicaid expansion in June with the condition the state apply for federal permission to include the new mandate.
“We remain focused on the work necessary to ensure that new health coverage for Virginia adults is available beginning on January 1, 2019,” Dr. Jennifer Lee, director of the Virginia Department of Medical Assistance Services, said in a statement. “Developing a waiver is a separate and ongoing process, as described in the final state budget.”
Virginia Medicaid is in discussions with the U.S. Centers for Medicare & Medicaid Services about its waiver, which has not yet been submitted.
Many Republican lawmakers in Virginia voted to expand Medicaid only after it was assured new enrollees would have to work or do volunteer service.
Dr. Scott Garrett, a Virginia House member from Lynchburg, Va., said he was under the impression the bill signed in June by Virginia Gov. Ralph Northam, a Democrat, meant the Medicaid expansion would begin only with a work requirement in place.
“The intent of the General Assembly ... was that you could not do one absent the other,” he said.
Garrett, a Republican, said he long opposed plans to add 400,000 adults to Medicaid because of cost concerns. He said requiring these enrollees to work or do volunteer service would make them healthier and improve their well-being.
Patricia Boozang, senior managing director for Manatt Health, a consulting firm, said she is not surprised states are moving ahead with work requirement plans regardless of the court ruling, which was specific to Kentucky.
She said the decision would cause the Trump administration to review the pending applications more closely so they could withstand a judicial review.
The federal court said Health and Human Services Secretary Alex Azar did not adequately take into account how many people would lose coverage for the work requirement and did not prove such a provision would improve enrollees’ health.
“It’s going to be challenging for them to make the case that health and well-being is going to be improved by the [work requirement] waiver,” she said.
In Arkansas, some Medicaid enrollees face a Thursday deadline to register their status – that they worked, did volunteer service in June or meet one of the state’s many exemptions.
“The ruling does not have an immediate effect on Arkansas’ work requirement,” said spokeswoman Marci Manley.
Advocates for low-income people are weighing whether to file lawsuits to stop the work requirement in other states that have won federal approval.
“We have ... partnerships with state legal advocates in these states and are exploring enforcement and litigation options with them,” said Jane Perkins, legal director of the National Health Law Program, which filed the suit on behalf of Medicaid enrollees in Kentucky to block the work requirements.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
HPV testing detects cervical precancers earlier than cytology
Women who received only a primary human papillomavirus test were 58% less likely to develop grade 3 or worse cervical intraepithelial neoplasia (CIN3+) by 48 months than women who had the traditional Pap cytology screen.
The primary HPV test also reduced the 2-year risk of CIN2+ neoplasia, compared with Pap smear alone, Gina Suzanne Ogilvie, MD, and her colleagues reported in JAMA.
“These results have demonstrated that primary HPV testing detects cervical neoplasia earlier and more accurately than cytology,” wrote Dr. Ogilvie of the University of British Columbia, Vancouver, and her colleagues.
HPV FOCAL (the Human Papilloma Virus For Cervical Cancers Screening trial) enrolled 19,009 Canadian women aged 25-65 years and randomized them to two cervical cancer screening paradigms: Pap liquid-based cytology (LBC) or primary HPV testing.
The intervention group (9,552) had cervical cancer screening with a high-risk HPV DNA test that detects types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. If either test was positive, they were referred for colposcopy. If both tests were negative, they returned for their final screen with both tests at 48 months.
The control group underwent primary LBC testing, followed by HPV testing for women with atypical squamous cells of unknown significance (ASCUS). If these tests were both positive, they were referred for colposcopy. Women who were positive for ASCUS and HPV negative returned in 12 months and were referred for colposcopy if they had ASCUS or any higher-grade abnormality. At 48 months, they also returned and underwent both screening tests.
The primary outcome was the rate of CIN3+ at 48 months. Secondary endpoints included the 48-month rate of CIN2+, the threshold for colposcopy referral, and the effect of primary HPV testing on colposcopy.
In the first round of screening, HPV testing detected significantly more cases of CIN3+ than did LBC (risk ratio, 1.61). This was an absolute difference of 2.67 more cases per 1,000 screened women.
At 48 months, the rate of CIN3+ was significantly lower in the intervention group than in the control group (2.3 vs. 5.5 per 1,000; RR, 0.42). This represents an absolute difference of 3.2 fewer cases per 1,000, the investigators said.
Overall, however, the two methods detected about the same number of cases by 48 months, the investigators said.
“Cumulative CIN3+ incidence curves show no significantly different disease detection across trial groups in the intervention group. The cumulative incidence was higher earlier in the trial at 18 months and 42 months, compared with the control group. ... By the end of trial follow-up (72 months), incidence was similar across both groups.”
Women who were HPV negative at baseline reaped the biggest benefit. The 48-month HPV incidence rate among them was 1.4 per 1,000, compared with 5.4 per 1,000 in the control group. This 75% risk reduction (RR, 0.25) represents an absolute reduction of 4 cases per 1,000 women.
The intervention group was 61% more likely to have a CIN2+ result by 12 months (RR, 1.61), but 53% less likely to have it at 48 months (RR, 0.47).
“By 48 months, significantly fewer CIN2+ cases were detected overall and across all age groups in the intervention group, compared with the control group,” the team said. At 48 months, the CIN2+ rate was 5 per 1,000 vs. 10.6 per 1,000 – a 53% reduced risk (RR, 0.47) and an absolute reduction of 5.6 cases per 1,000.
Again, the benefit accrued early and mostly in women who were negative by HPV or cytology at baseline. Among these, the CIN2+ risk for the intervention, compared with the control group, was 64% lower (RR, 0.36), and the absolute difference in incidence was 6.38 per 1,000.
This early detection came at a cost, however. Colposcopies were significantly more common in the intervention group in the first screening round (57 vs. 30.8). However, by 48 months, colposcopy rates were lower in the intervention group, compared with the control group (49.2 vs. 70.5). By the end of the study, cumulative colposcopy referral rates were similar (106.2 vs. 101.5).
Dr. Ogilvie and her colleagues suggested that this ultimate similarity in colposcopy rate shows that fears about overdiagnosis with HPV testing are unfounded.
“One of the concerns for adopting HPV-based screening is the lower CIN2+ specificity of HPV testing, compared with cytology, leading to higher screen positive rates and the resulting need for more colposcopies and biopsies. Unnecessary colposcopies potentially cause unintended harm for women and increased costs to health care systems. In this trial, round 1 colposcopy rates in the HPV-tested group were significantly higher than the cytology-tested group. However, by 48 months, the colposcopy rate in the intervention group was reduced while the control group rate increased.
“This increase is partly a result of HPV and cytology co-testing at trial end. Of the 513 control-group women referred for colposcopy at exit, 304 (59%) were cytology negative and HPV positive. In the HPV-tested group, the colposcopy rate decreased in the second round of screening, which more accurately reflects the ongoing impact of HPV-based screening on a colposcopy program. The baseline colposcopy referral rate reflects what happens when HPV-based screening is first implemented, when both prevalent and incident infections will be detected,” the investigators said.
The Canadian Institute of Health Research funded the study. Dr. Ogilvie was a coinvestigator on adjunct studies funded by Hologic and Roche, designed to compare the performance of different HPV assays. Funding for the adjunct studies was not applied to the main HPV FOCAL trial.
SOURCE: Ogilvie GS et al. JAMA. 2018;320:43-52.
Primary HPV testing has been available since 2014 in the United States, but has yet to replace Pap smears, L. Stewart Massad, MD, said in an accompanying editorial (JAMA. 2018;320:35-37).
The reasons are complex and numerous, beginning with the probability that the more sensitive HPV test can run up alarming, but unnecessary, red flags, especially for younger women.
“Adoption of primary HPV screening has been delayed by the suboptimal specificity of this approach, estimated at 85%-95%, especially among populations of young women who often carry HPV infections that regress without oncogenic consequence. These HPV infections represent true-positive HPV assays, but are false-positive cancer screens.”
Lack of patient education is another factor.
“HPV is almost universally acquired by sexually active adults. The virus usually clears in response to immune recognition, although clearance of this intraepithelial virus may take more than a year, and yet HPV may recur and first be detected decades later in the context of long-term monogamy or abstinence.
“Communicating a positive HPV test result requires sensitivity by the clinician and may entail lengthy counseling about the natural history of HPV, the lack of curative therapy, and the low absolute risk of progression to cancer. The clinical implications of an HPV diagnosis for sexual partners and offspring are marginal yet may be quite distressing for an affected woman.”
The HPV vaccine is already affecting cervical cancer rates and will complicate the picture even more. HPV FOCAL completed recruitment in 2012. Since then, rates of HPV 16 and 18, the most cervically carcinogenic serotypes, have fallen in the wake of the 2006 vaccine approval.
This reduction is becoming more apparent as women who were adolescents at vaccination and at the time the study was launched are now aging into screening cohorts. Lower prevalence of cervical precancer has changed the accuracy of screening tests in ways that are only now being appreciated, but that will further favor adoption of primary HPV screening by lowering its false-positive rate.”
The HPV test used in the FOCAL study was also suboptimal, compared with newer versions. The test incorporated all carcinogenic HPV serotypes in a single positive or negative result.
“More recent assays provide HPV genotyping that allows nuanced risk stratification, especially immediate referral to colposcopy for women who screen positive for HPV 16 or HPV 18. Triage for women who test positive for HPV was by cytology, which may not be the optimal triage test because other assays that do not depend on cytotechnologists’ vigilance are becoming available for this purpose. However, these advances should result in fewer false-positive results, further favoring HPV screening over cytology.”
The future of the Pap test remains unclear. Organizations that develop cancer screening guidelines continue to debate the issue.
“A draft recommendation on cervical screening from the U.S. Preventive Services Task Force recommended either cytology testing at 3-year intervals or primary HPV testing at 5-year intervals for women 30-65 years of age, but the final recommendation statement has not yet been released. Fortunately for women, both modalities are so effective for cancer screening that an adequately powered comparative effectiveness trial is likely impossible.”
Dr. Massad is a gynecologic oncology surgeon at Washington University, St. Louis. He has consulted with malpractice attorneys in cases alleging missed cervical cancer but has no financial ties with pharmaceutical companies.
Primary HPV testing has been available since 2014 in the United States, but has yet to replace Pap smears, L. Stewart Massad, MD, said in an accompanying editorial (JAMA. 2018;320:35-37).
The reasons are complex and numerous, beginning with the probability that the more sensitive HPV test can run up alarming, but unnecessary, red flags, especially for younger women.
“Adoption of primary HPV screening has been delayed by the suboptimal specificity of this approach, estimated at 85%-95%, especially among populations of young women who often carry HPV infections that regress without oncogenic consequence. These HPV infections represent true-positive HPV assays, but are false-positive cancer screens.”
Lack of patient education is another factor.
“HPV is almost universally acquired by sexually active adults. The virus usually clears in response to immune recognition, although clearance of this intraepithelial virus may take more than a year, and yet HPV may recur and first be detected decades later in the context of long-term monogamy or abstinence.
“Communicating a positive HPV test result requires sensitivity by the clinician and may entail lengthy counseling about the natural history of HPV, the lack of curative therapy, and the low absolute risk of progression to cancer. The clinical implications of an HPV diagnosis for sexual partners and offspring are marginal yet may be quite distressing for an affected woman.”
The HPV vaccine is already affecting cervical cancer rates and will complicate the picture even more. HPV FOCAL completed recruitment in 2012. Since then, rates of HPV 16 and 18, the most cervically carcinogenic serotypes, have fallen in the wake of the 2006 vaccine approval.
This reduction is becoming more apparent as women who were adolescents at vaccination and at the time the study was launched are now aging into screening cohorts. Lower prevalence of cervical precancer has changed the accuracy of screening tests in ways that are only now being appreciated, but that will further favor adoption of primary HPV screening by lowering its false-positive rate.”
The HPV test used in the FOCAL study was also suboptimal, compared with newer versions. The test incorporated all carcinogenic HPV serotypes in a single positive or negative result.
“More recent assays provide HPV genotyping that allows nuanced risk stratification, especially immediate referral to colposcopy for women who screen positive for HPV 16 or HPV 18. Triage for women who test positive for HPV was by cytology, which may not be the optimal triage test because other assays that do not depend on cytotechnologists’ vigilance are becoming available for this purpose. However, these advances should result in fewer false-positive results, further favoring HPV screening over cytology.”
The future of the Pap test remains unclear. Organizations that develop cancer screening guidelines continue to debate the issue.
“A draft recommendation on cervical screening from the U.S. Preventive Services Task Force recommended either cytology testing at 3-year intervals or primary HPV testing at 5-year intervals for women 30-65 years of age, but the final recommendation statement has not yet been released. Fortunately for women, both modalities are so effective for cancer screening that an adequately powered comparative effectiveness trial is likely impossible.”
Dr. Massad is a gynecologic oncology surgeon at Washington University, St. Louis. He has consulted with malpractice attorneys in cases alleging missed cervical cancer but has no financial ties with pharmaceutical companies.
Primary HPV testing has been available since 2014 in the United States, but has yet to replace Pap smears, L. Stewart Massad, MD, said in an accompanying editorial (JAMA. 2018;320:35-37).
The reasons are complex and numerous, beginning with the probability that the more sensitive HPV test can run up alarming, but unnecessary, red flags, especially for younger women.
“Adoption of primary HPV screening has been delayed by the suboptimal specificity of this approach, estimated at 85%-95%, especially among populations of young women who often carry HPV infections that regress without oncogenic consequence. These HPV infections represent true-positive HPV assays, but are false-positive cancer screens.”
Lack of patient education is another factor.
“HPV is almost universally acquired by sexually active adults. The virus usually clears in response to immune recognition, although clearance of this intraepithelial virus may take more than a year, and yet HPV may recur and first be detected decades later in the context of long-term monogamy or abstinence.
“Communicating a positive HPV test result requires sensitivity by the clinician and may entail lengthy counseling about the natural history of HPV, the lack of curative therapy, and the low absolute risk of progression to cancer. The clinical implications of an HPV diagnosis for sexual partners and offspring are marginal yet may be quite distressing for an affected woman.”
The HPV vaccine is already affecting cervical cancer rates and will complicate the picture even more. HPV FOCAL completed recruitment in 2012. Since then, rates of HPV 16 and 18, the most cervically carcinogenic serotypes, have fallen in the wake of the 2006 vaccine approval.
This reduction is becoming more apparent as women who were adolescents at vaccination and at the time the study was launched are now aging into screening cohorts. Lower prevalence of cervical precancer has changed the accuracy of screening tests in ways that are only now being appreciated, but that will further favor adoption of primary HPV screening by lowering its false-positive rate.”
The HPV test used in the FOCAL study was also suboptimal, compared with newer versions. The test incorporated all carcinogenic HPV serotypes in a single positive or negative result.
“More recent assays provide HPV genotyping that allows nuanced risk stratification, especially immediate referral to colposcopy for women who screen positive for HPV 16 or HPV 18. Triage for women who test positive for HPV was by cytology, which may not be the optimal triage test because other assays that do not depend on cytotechnologists’ vigilance are becoming available for this purpose. However, these advances should result in fewer false-positive results, further favoring HPV screening over cytology.”
The future of the Pap test remains unclear. Organizations that develop cancer screening guidelines continue to debate the issue.
“A draft recommendation on cervical screening from the U.S. Preventive Services Task Force recommended either cytology testing at 3-year intervals or primary HPV testing at 5-year intervals for women 30-65 years of age, but the final recommendation statement has not yet been released. Fortunately for women, both modalities are so effective for cancer screening that an adequately powered comparative effectiveness trial is likely impossible.”
Dr. Massad is a gynecologic oncology surgeon at Washington University, St. Louis. He has consulted with malpractice attorneys in cases alleging missed cervical cancer but has no financial ties with pharmaceutical companies.
Women who received only a primary human papillomavirus test were 58% less likely to develop grade 3 or worse cervical intraepithelial neoplasia (CIN3+) by 48 months than women who had the traditional Pap cytology screen.
The primary HPV test also reduced the 2-year risk of CIN2+ neoplasia, compared with Pap smear alone, Gina Suzanne Ogilvie, MD, and her colleagues reported in JAMA.
“These results have demonstrated that primary HPV testing detects cervical neoplasia earlier and more accurately than cytology,” wrote Dr. Ogilvie of the University of British Columbia, Vancouver, and her colleagues.
HPV FOCAL (the Human Papilloma Virus For Cervical Cancers Screening trial) enrolled 19,009 Canadian women aged 25-65 years and randomized them to two cervical cancer screening paradigms: Pap liquid-based cytology (LBC) or primary HPV testing.
The intervention group (9,552) had cervical cancer screening with a high-risk HPV DNA test that detects types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. If either test was positive, they were referred for colposcopy. If both tests were negative, they returned for their final screen with both tests at 48 months.
The control group underwent primary LBC testing, followed by HPV testing for women with atypical squamous cells of unknown significance (ASCUS). If these tests were both positive, they were referred for colposcopy. Women who were positive for ASCUS and HPV negative returned in 12 months and were referred for colposcopy if they had ASCUS or any higher-grade abnormality. At 48 months, they also returned and underwent both screening tests.
The primary outcome was the rate of CIN3+ at 48 months. Secondary endpoints included the 48-month rate of CIN2+, the threshold for colposcopy referral, and the effect of primary HPV testing on colposcopy.
In the first round of screening, HPV testing detected significantly more cases of CIN3+ than did LBC (risk ratio, 1.61). This was an absolute difference of 2.67 more cases per 1,000 screened women.
At 48 months, the rate of CIN3+ was significantly lower in the intervention group than in the control group (2.3 vs. 5.5 per 1,000; RR, 0.42). This represents an absolute difference of 3.2 fewer cases per 1,000, the investigators said.
Overall, however, the two methods detected about the same number of cases by 48 months, the investigators said.
“Cumulative CIN3+ incidence curves show no significantly different disease detection across trial groups in the intervention group. The cumulative incidence was higher earlier in the trial at 18 months and 42 months, compared with the control group. ... By the end of trial follow-up (72 months), incidence was similar across both groups.”
Women who were HPV negative at baseline reaped the biggest benefit. The 48-month HPV incidence rate among them was 1.4 per 1,000, compared with 5.4 per 1,000 in the control group. This 75% risk reduction (RR, 0.25) represents an absolute reduction of 4 cases per 1,000 women.
The intervention group was 61% more likely to have a CIN2+ result by 12 months (RR, 1.61), but 53% less likely to have it at 48 months (RR, 0.47).
“By 48 months, significantly fewer CIN2+ cases were detected overall and across all age groups in the intervention group, compared with the control group,” the team said. At 48 months, the CIN2+ rate was 5 per 1,000 vs. 10.6 per 1,000 – a 53% reduced risk (RR, 0.47) and an absolute reduction of 5.6 cases per 1,000.
Again, the benefit accrued early and mostly in women who were negative by HPV or cytology at baseline. Among these, the CIN2+ risk for the intervention, compared with the control group, was 64% lower (RR, 0.36), and the absolute difference in incidence was 6.38 per 1,000.
This early detection came at a cost, however. Colposcopies were significantly more common in the intervention group in the first screening round (57 vs. 30.8). However, by 48 months, colposcopy rates were lower in the intervention group, compared with the control group (49.2 vs. 70.5). By the end of the study, cumulative colposcopy referral rates were similar (106.2 vs. 101.5).
Dr. Ogilvie and her colleagues suggested that this ultimate similarity in colposcopy rate shows that fears about overdiagnosis with HPV testing are unfounded.
“One of the concerns for adopting HPV-based screening is the lower CIN2+ specificity of HPV testing, compared with cytology, leading to higher screen positive rates and the resulting need for more colposcopies and biopsies. Unnecessary colposcopies potentially cause unintended harm for women and increased costs to health care systems. In this trial, round 1 colposcopy rates in the HPV-tested group were significantly higher than the cytology-tested group. However, by 48 months, the colposcopy rate in the intervention group was reduced while the control group rate increased.
“This increase is partly a result of HPV and cytology co-testing at trial end. Of the 513 control-group women referred for colposcopy at exit, 304 (59%) were cytology negative and HPV positive. In the HPV-tested group, the colposcopy rate decreased in the second round of screening, which more accurately reflects the ongoing impact of HPV-based screening on a colposcopy program. The baseline colposcopy referral rate reflects what happens when HPV-based screening is first implemented, when both prevalent and incident infections will be detected,” the investigators said.
The Canadian Institute of Health Research funded the study. Dr. Ogilvie was a coinvestigator on adjunct studies funded by Hologic and Roche, designed to compare the performance of different HPV assays. Funding for the adjunct studies was not applied to the main HPV FOCAL trial.
SOURCE: Ogilvie GS et al. JAMA. 2018;320:43-52.
Women who received only a primary human papillomavirus test were 58% less likely to develop grade 3 or worse cervical intraepithelial neoplasia (CIN3+) by 48 months than women who had the traditional Pap cytology screen.
The primary HPV test also reduced the 2-year risk of CIN2+ neoplasia, compared with Pap smear alone, Gina Suzanne Ogilvie, MD, and her colleagues reported in JAMA.
“These results have demonstrated that primary HPV testing detects cervical neoplasia earlier and more accurately than cytology,” wrote Dr. Ogilvie of the University of British Columbia, Vancouver, and her colleagues.
HPV FOCAL (the Human Papilloma Virus For Cervical Cancers Screening trial) enrolled 19,009 Canadian women aged 25-65 years and randomized them to two cervical cancer screening paradigms: Pap liquid-based cytology (LBC) or primary HPV testing.
The intervention group (9,552) had cervical cancer screening with a high-risk HPV DNA test that detects types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. If either test was positive, they were referred for colposcopy. If both tests were negative, they returned for their final screen with both tests at 48 months.
The control group underwent primary LBC testing, followed by HPV testing for women with atypical squamous cells of unknown significance (ASCUS). If these tests were both positive, they were referred for colposcopy. Women who were positive for ASCUS and HPV negative returned in 12 months and were referred for colposcopy if they had ASCUS or any higher-grade abnormality. At 48 months, they also returned and underwent both screening tests.
The primary outcome was the rate of CIN3+ at 48 months. Secondary endpoints included the 48-month rate of CIN2+, the threshold for colposcopy referral, and the effect of primary HPV testing on colposcopy.
In the first round of screening, HPV testing detected significantly more cases of CIN3+ than did LBC (risk ratio, 1.61). This was an absolute difference of 2.67 more cases per 1,000 screened women.
At 48 months, the rate of CIN3+ was significantly lower in the intervention group than in the control group (2.3 vs. 5.5 per 1,000; RR, 0.42). This represents an absolute difference of 3.2 fewer cases per 1,000, the investigators said.
Overall, however, the two methods detected about the same number of cases by 48 months, the investigators said.
“Cumulative CIN3+ incidence curves show no significantly different disease detection across trial groups in the intervention group. The cumulative incidence was higher earlier in the trial at 18 months and 42 months, compared with the control group. ... By the end of trial follow-up (72 months), incidence was similar across both groups.”
Women who were HPV negative at baseline reaped the biggest benefit. The 48-month HPV incidence rate among them was 1.4 per 1,000, compared with 5.4 per 1,000 in the control group. This 75% risk reduction (RR, 0.25) represents an absolute reduction of 4 cases per 1,000 women.
The intervention group was 61% more likely to have a CIN2+ result by 12 months (RR, 1.61), but 53% less likely to have it at 48 months (RR, 0.47).
“By 48 months, significantly fewer CIN2+ cases were detected overall and across all age groups in the intervention group, compared with the control group,” the team said. At 48 months, the CIN2+ rate was 5 per 1,000 vs. 10.6 per 1,000 – a 53% reduced risk (RR, 0.47) and an absolute reduction of 5.6 cases per 1,000.
Again, the benefit accrued early and mostly in women who were negative by HPV or cytology at baseline. Among these, the CIN2+ risk for the intervention, compared with the control group, was 64% lower (RR, 0.36), and the absolute difference in incidence was 6.38 per 1,000.
This early detection came at a cost, however. Colposcopies were significantly more common in the intervention group in the first screening round (57 vs. 30.8). However, by 48 months, colposcopy rates were lower in the intervention group, compared with the control group (49.2 vs. 70.5). By the end of the study, cumulative colposcopy referral rates were similar (106.2 vs. 101.5).
Dr. Ogilvie and her colleagues suggested that this ultimate similarity in colposcopy rate shows that fears about overdiagnosis with HPV testing are unfounded.
“One of the concerns for adopting HPV-based screening is the lower CIN2+ specificity of HPV testing, compared with cytology, leading to higher screen positive rates and the resulting need for more colposcopies and biopsies. Unnecessary colposcopies potentially cause unintended harm for women and increased costs to health care systems. In this trial, round 1 colposcopy rates in the HPV-tested group were significantly higher than the cytology-tested group. However, by 48 months, the colposcopy rate in the intervention group was reduced while the control group rate increased.
“This increase is partly a result of HPV and cytology co-testing at trial end. Of the 513 control-group women referred for colposcopy at exit, 304 (59%) were cytology negative and HPV positive. In the HPV-tested group, the colposcopy rate decreased in the second round of screening, which more accurately reflects the ongoing impact of HPV-based screening on a colposcopy program. The baseline colposcopy referral rate reflects what happens when HPV-based screening is first implemented, when both prevalent and incident infections will be detected,” the investigators said.
The Canadian Institute of Health Research funded the study. Dr. Ogilvie was a coinvestigator on adjunct studies funded by Hologic and Roche, designed to compare the performance of different HPV assays. Funding for the adjunct studies was not applied to the main HPV FOCAL trial.
SOURCE: Ogilvie GS et al. JAMA. 2018;320:43-52.
FROM JAMA
Key clinical point: Compared with a Pap smear, HPV testing detected CIN3+ more often and earlier.
Major finding: Women who had HPV testing were 58% less likely to be CIN3+ 48 months later.
Study details: The prospective randomized trial comprised 19,009 women.
Disclosures: The Canadian Institute of Health Research funded the study, Dr. Ogilvie was a coinvestigator on adjunct studies funded by Hologic and Roche, designed to compare the performance of different HPV assays. Funding for the adjunct studies was not applied to the main HPV FOCAL trial.
Source: Ogilvie GS et al. JAMA. 2018;320:43-52.
Can an Algorithm Optimize Caffeine Dosing Strategies?
Adjusting the timing of caffeine intake may improve psychomotor vigilance without increasing total caffeine consumption.
BALTIMORE—A new algorithm may be key to optimizing alertness with caffeine, a study suggests. Caffeine is the most widely used stimulant to counter the effects of sleep loss on neurobehavioral performance, but no tools exist to guide the timing and amount of caffeine consumption to optimize its benefits, said Jaques Reifman, PhD, and colleagues. “By using our algorithm, which determines when and how much caffeine a subject should consume, we can improve alertness by up to 64% while consuming the same total amount of caffeine” as in previous studies of caffeine use during chronic sleep restriction, said Dr. Reifman. “Alternatively, a subject can reduce caffeine consumption by up to 65% and still achieve equivalent improvements in alertness.”
The study was presented at the 32nd Annual Meeting of the Associated Professional Sleep Societies and published online ahead of print in the Journal of Sleep Research. Dr. Reifman is a senior research scientist and Director of the Department of Defense Biotechnology High Performance Computing Software Applications Institute and the Telemedicine and Advanced Technology Research Center at the US Army Medical Research and Materiel Command in Fort Detrick, Maryland.
The researchers used a validated mathematical model that predicts the effects of sleep loss and caffeine on psychomotor vigilance task (PVT) performance and combined it with a computationally efficient optimization algorithm to determine when and how much caffeine should be consumed to safely maximize alertness during sleep loss. They used the algorithm to determine ideal dosing strategies for four previously published experimental studies of chronic sleep restriction. The researchers computed dosing strategies that enhanced the predicted PVT performance using the same total amount of caffeine as in the original studies, as well as strategies that achieved an equivalent level of performance, but used less caffeine overall.
The algorithm tailored the timing and amount of caffeine to the sleep–wake schedule of each study condition to maximize caffeine’s benefits, such as by postponing the first dose on certain days, prescribing more caffeine during longer periods of wakefulness, and allocating doses near the end of the day.
The algorithm is designed to predict caffeine’s effects on simple tasks, so dosing strategies for other tasks may differ, the researchers noted. In addition, the algorithm does not account for the effects of sleep debt on caffeine’s benefits or individual differences in caffeine sensitivity.
Suggested Reading
Vital-Lopez FG, Ramakrishnan S, Doty TJ, et al. Caffeine dosing strategies to optimize alertness during sleep loss. J Sleep Res. 2018 May 28 [Epub ahead of print].
Adjusting the timing of caffeine intake may improve psychomotor vigilance without increasing total caffeine consumption.
Adjusting the timing of caffeine intake may improve psychomotor vigilance without increasing total caffeine consumption.
BALTIMORE—A new algorithm may be key to optimizing alertness with caffeine, a study suggests. Caffeine is the most widely used stimulant to counter the effects of sleep loss on neurobehavioral performance, but no tools exist to guide the timing and amount of caffeine consumption to optimize its benefits, said Jaques Reifman, PhD, and colleagues. “By using our algorithm, which determines when and how much caffeine a subject should consume, we can improve alertness by up to 64% while consuming the same total amount of caffeine” as in previous studies of caffeine use during chronic sleep restriction, said Dr. Reifman. “Alternatively, a subject can reduce caffeine consumption by up to 65% and still achieve equivalent improvements in alertness.”
The study was presented at the 32nd Annual Meeting of the Associated Professional Sleep Societies and published online ahead of print in the Journal of Sleep Research. Dr. Reifman is a senior research scientist and Director of the Department of Defense Biotechnology High Performance Computing Software Applications Institute and the Telemedicine and Advanced Technology Research Center at the US Army Medical Research and Materiel Command in Fort Detrick, Maryland.
The researchers used a validated mathematical model that predicts the effects of sleep loss and caffeine on psychomotor vigilance task (PVT) performance and combined it with a computationally efficient optimization algorithm to determine when and how much caffeine should be consumed to safely maximize alertness during sleep loss. They used the algorithm to determine ideal dosing strategies for four previously published experimental studies of chronic sleep restriction. The researchers computed dosing strategies that enhanced the predicted PVT performance using the same total amount of caffeine as in the original studies, as well as strategies that achieved an equivalent level of performance, but used less caffeine overall.
The algorithm tailored the timing and amount of caffeine to the sleep–wake schedule of each study condition to maximize caffeine’s benefits, such as by postponing the first dose on certain days, prescribing more caffeine during longer periods of wakefulness, and allocating doses near the end of the day.
The algorithm is designed to predict caffeine’s effects on simple tasks, so dosing strategies for other tasks may differ, the researchers noted. In addition, the algorithm does not account for the effects of sleep debt on caffeine’s benefits or individual differences in caffeine sensitivity.
Suggested Reading
Vital-Lopez FG, Ramakrishnan S, Doty TJ, et al. Caffeine dosing strategies to optimize alertness during sleep loss. J Sleep Res. 2018 May 28 [Epub ahead of print].
BALTIMORE—A new algorithm may be key to optimizing alertness with caffeine, a study suggests. Caffeine is the most widely used stimulant to counter the effects of sleep loss on neurobehavioral performance, but no tools exist to guide the timing and amount of caffeine consumption to optimize its benefits, said Jaques Reifman, PhD, and colleagues. “By using our algorithm, which determines when and how much caffeine a subject should consume, we can improve alertness by up to 64% while consuming the same total amount of caffeine” as in previous studies of caffeine use during chronic sleep restriction, said Dr. Reifman. “Alternatively, a subject can reduce caffeine consumption by up to 65% and still achieve equivalent improvements in alertness.”
The study was presented at the 32nd Annual Meeting of the Associated Professional Sleep Societies and published online ahead of print in the Journal of Sleep Research. Dr. Reifman is a senior research scientist and Director of the Department of Defense Biotechnology High Performance Computing Software Applications Institute and the Telemedicine and Advanced Technology Research Center at the US Army Medical Research and Materiel Command in Fort Detrick, Maryland.
The researchers used a validated mathematical model that predicts the effects of sleep loss and caffeine on psychomotor vigilance task (PVT) performance and combined it with a computationally efficient optimization algorithm to determine when and how much caffeine should be consumed to safely maximize alertness during sleep loss. They used the algorithm to determine ideal dosing strategies for four previously published experimental studies of chronic sleep restriction. The researchers computed dosing strategies that enhanced the predicted PVT performance using the same total amount of caffeine as in the original studies, as well as strategies that achieved an equivalent level of performance, but used less caffeine overall.
The algorithm tailored the timing and amount of caffeine to the sleep–wake schedule of each study condition to maximize caffeine’s benefits, such as by postponing the first dose on certain days, prescribing more caffeine during longer periods of wakefulness, and allocating doses near the end of the day.
The algorithm is designed to predict caffeine’s effects on simple tasks, so dosing strategies for other tasks may differ, the researchers noted. In addition, the algorithm does not account for the effects of sleep debt on caffeine’s benefits or individual differences in caffeine sensitivity.
Suggested Reading
Vital-Lopez FG, Ramakrishnan S, Doty TJ, et al. Caffeine dosing strategies to optimize alertness during sleep loss. J Sleep Res. 2018 May 28 [Epub ahead of print].
‘Reverse transitions’ from injecting to noninjecting drug use studied
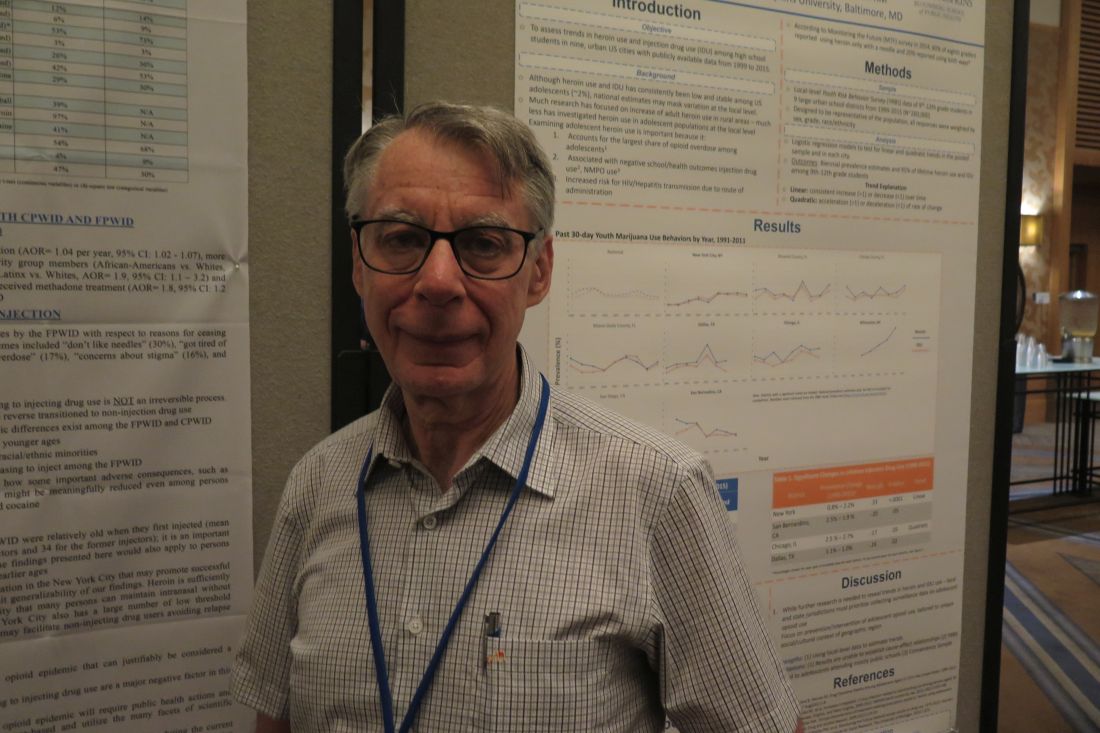
SAN DIEGO – The transition from noninjecting to injecting drug use can be a reversible process, results from a long-term study suggest.
“There’s a common stereotype in popular culture and in academic research that once people start injecting drugs, that will be their dominant route of administration for the rest of their lives,” lead study author Don C. Des Jarlais, PhD, said in an interview at the annual meeting of the College on Problems of Drug Dependence. “We found that people who started injecting injected for several years, but then went back to noninjecting drug use. That so-called ‘reverse transition’ leads to a very big difference in infection with hepatitis C virus as well as reduced risk of overdose and reduced risk of other bacterial infections. Even though these people continued to use drugs, they were doing it in a way that was much safer.”
In an effort to examine the prevalence and characteristics of reverse transitions, Dr. Des Jarlais and his associates recruited injecting and noninjecting drug users aged 18 years and older from the Mount Sinai Beth Israel detoxification and methadone maintenance programs in New York City from 2000 to 2017. The researchers obtained informed consent and conducted a structured interview that included questions on why former injectors had ceased injecting drugs, along with testing for HIV and HCV.
People who were currently injecting were defined as those whose first injection was in 2000 or later and who had injected heroin or cocaine during the 6 months prior to treatment entry. People who formerly injected drugs were defined as those whose first injection was in 2000 or later but who had used heroin or cocaine without injecting drugs during the 6 months prior to treatment entry.
Dr. Des Jarlais, professor of psychiatry at the Icahn School of Medicine at Mount Sinai, New York, reported results from 937 current and 104 former injection drug users. Compared with current injection drug users, former users were older (a mean age of 41 vs. 35, respectively), more likely to be African American (28% vs. 12%), more likely to have received previous methadone treatment (68% vs. 54%), and less likely to be HCV positive (30% vs. 47%; P less than 0.05 for all associations).
The researchers found that 11% of former injection drug users had reverse transitioned to noninjection drug use. Among former injection drug users, the most common reasons for ceasing to inject were “don’t like needles” (30%), “got tired of injecting” (29%), “afraid of overdose” (17%), “concerns about stigma” (16%), and other health concerns (14%).
Dr. Des Jarlais said. “Injecting is messy and it requires private space; it’s not a pleasant way of using drugs compared to just sniffing it. If we’re going to control hepatitis C, we need to find ways of encouraging people that if they’re going to continue to use drugs, they should try to stop injecting.”
The next step in this research area is to develop a more complete understanding of reverse transitions. “These are people doing it on their own, but we don’t have any programs to help them,” he said. “We have needle exchange programs for people to inject safely, but we don’t have any programs where people go from injecting to noninjecting.”
The study received funding support from the National Institute on Drug Abuse. Dr. Des Jarlais reported having no financial disclosures.
SAN DIEGO – The transition from noninjecting to injecting drug use can be a reversible process, results from a long-term study suggest.
“There’s a common stereotype in popular culture and in academic research that once people start injecting drugs, that will be their dominant route of administration for the rest of their lives,” lead study author Don C. Des Jarlais, PhD, said in an interview at the annual meeting of the College on Problems of Drug Dependence. “We found that people who started injecting injected for several years, but then went back to noninjecting drug use. That so-called ‘reverse transition’ leads to a very big difference in infection with hepatitis C virus as well as reduced risk of overdose and reduced risk of other bacterial infections. Even though these people continued to use drugs, they were doing it in a way that was much safer.”
In an effort to examine the prevalence and characteristics of reverse transitions, Dr. Des Jarlais and his associates recruited injecting and noninjecting drug users aged 18 years and older from the Mount Sinai Beth Israel detoxification and methadone maintenance programs in New York City from 2000 to 2017. The researchers obtained informed consent and conducted a structured interview that included questions on why former injectors had ceased injecting drugs, along with testing for HIV and HCV.
People who were currently injecting were defined as those whose first injection was in 2000 or later and who had injected heroin or cocaine during the 6 months prior to treatment entry. People who formerly injected drugs were defined as those whose first injection was in 2000 or later but who had used heroin or cocaine without injecting drugs during the 6 months prior to treatment entry.
Dr. Des Jarlais, professor of psychiatry at the Icahn School of Medicine at Mount Sinai, New York, reported results from 937 current and 104 former injection drug users. Compared with current injection drug users, former users were older (a mean age of 41 vs. 35, respectively), more likely to be African American (28% vs. 12%), more likely to have received previous methadone treatment (68% vs. 54%), and less likely to be HCV positive (30% vs. 47%; P less than 0.05 for all associations).
The researchers found that 11% of former injection drug users had reverse transitioned to noninjection drug use. Among former injection drug users, the most common reasons for ceasing to inject were “don’t like needles” (30%), “got tired of injecting” (29%), “afraid of overdose” (17%), “concerns about stigma” (16%), and other health concerns (14%).
Dr. Des Jarlais said. “Injecting is messy and it requires private space; it’s not a pleasant way of using drugs compared to just sniffing it. If we’re going to control hepatitis C, we need to find ways of encouraging people that if they’re going to continue to use drugs, they should try to stop injecting.”
The next step in this research area is to develop a more complete understanding of reverse transitions. “These are people doing it on their own, but we don’t have any programs to help them,” he said. “We have needle exchange programs for people to inject safely, but we don’t have any programs where people go from injecting to noninjecting.”
The study received funding support from the National Institute on Drug Abuse. Dr. Des Jarlais reported having no financial disclosures.
SAN DIEGO – The transition from noninjecting to injecting drug use can be a reversible process, results from a long-term study suggest.
“There’s a common stereotype in popular culture and in academic research that once people start injecting drugs, that will be their dominant route of administration for the rest of their lives,” lead study author Don C. Des Jarlais, PhD, said in an interview at the annual meeting of the College on Problems of Drug Dependence. “We found that people who started injecting injected for several years, but then went back to noninjecting drug use. That so-called ‘reverse transition’ leads to a very big difference in infection with hepatitis C virus as well as reduced risk of overdose and reduced risk of other bacterial infections. Even though these people continued to use drugs, they were doing it in a way that was much safer.”
In an effort to examine the prevalence and characteristics of reverse transitions, Dr. Des Jarlais and his associates recruited injecting and noninjecting drug users aged 18 years and older from the Mount Sinai Beth Israel detoxification and methadone maintenance programs in New York City from 2000 to 2017. The researchers obtained informed consent and conducted a structured interview that included questions on why former injectors had ceased injecting drugs, along with testing for HIV and HCV.
People who were currently injecting were defined as those whose first injection was in 2000 or later and who had injected heroin or cocaine during the 6 months prior to treatment entry. People who formerly injected drugs were defined as those whose first injection was in 2000 or later but who had used heroin or cocaine without injecting drugs during the 6 months prior to treatment entry.
Dr. Des Jarlais, professor of psychiatry at the Icahn School of Medicine at Mount Sinai, New York, reported results from 937 current and 104 former injection drug users. Compared with current injection drug users, former users were older (a mean age of 41 vs. 35, respectively), more likely to be African American (28% vs. 12%), more likely to have received previous methadone treatment (68% vs. 54%), and less likely to be HCV positive (30% vs. 47%; P less than 0.05 for all associations).
The researchers found that 11% of former injection drug users had reverse transitioned to noninjection drug use. Among former injection drug users, the most common reasons for ceasing to inject were “don’t like needles” (30%), “got tired of injecting” (29%), “afraid of overdose” (17%), “concerns about stigma” (16%), and other health concerns (14%).
Dr. Des Jarlais said. “Injecting is messy and it requires private space; it’s not a pleasant way of using drugs compared to just sniffing it. If we’re going to control hepatitis C, we need to find ways of encouraging people that if they’re going to continue to use drugs, they should try to stop injecting.”
The next step in this research area is to develop a more complete understanding of reverse transitions. “These are people doing it on their own, but we don’t have any programs to help them,” he said. “We have needle exchange programs for people to inject safely, but we don’t have any programs where people go from injecting to noninjecting.”
The study received funding support from the National Institute on Drug Abuse. Dr. Des Jarlais reported having no financial disclosures.
AT CPDD 2018
Key clinical point: To control hepatitis C, patients who continue to use drugs should be encouraged to stop injecting.
Major finding: Among former injection drug users, 11% had reverse transitioned to noninjection drug use.
Study details: A study of 937 current and 104 former injection drug users between 2000 and 2017.
Disclosures: The study received funding support from the National Institute on Drug Abuse. Dr. Des Jarlais reported having no financial disclosures.
Explore Ultrasound Corner
The use of ultrasound is often overlooked when it could very well aid in the diagnosis of a critical illness in a shorter amount of time, while eliminating potential risks that come with many of the usually administered tests.
In 2013, Seth Koenig, MD, FCCP, of Hofstra School of Medicine in New Hyde Park, New York, noticed the need to educate providers about the use of ultrasound in the ICU. Dr. Koenig approached Richard Irwin, MD, Master FCCP, and Editor in Chief of the journal CHEST, with an idea for a new section in the journal. So began “Ultrasound Corner,” an online, video-based series in the journal that provides readers with real cases where ultrasound has played a large role in diagnostic patient care.
Each month, the journal receives two to four submissions from chest medicine clinicians who want to share their critical care ultrasound patient stories. One to two stories are selected and published monthly with real video images that are explained in the manuscript and in a narration done by Dr. Koenig.
“This creates a section where clinicians worldwide can share their experiences so that others may incorporate different methods of diagnosis into their practice,” said Dr. Koenig. “This method of learning challenges the readers to interpret images and integrate the results into a patient management plan.”
Dr. Koenig recommends that clinicians who have experienced benefit using ultrasound in critical care situations submit their cases so that viewers can learn from each other. Share the knowledge you’ve gained from your patient cases. Visit https://mc.manuscriptcentral.com/chest, log in to your account, and click “Start a New Submission” under the “Author” section.
More importantly, Dr. Koenig encourages the journal readership to explore Ultrasound Corner (https://journal.chestnet.org/ultrasound) every month in CHEST to learn of different courses of diagnosis and treatment being used to strengthen patient diagnostic and management plans in new, evolving ways.
The use of ultrasound is often overlooked when it could very well aid in the diagnosis of a critical illness in a shorter amount of time, while eliminating potential risks that come with many of the usually administered tests.
In 2013, Seth Koenig, MD, FCCP, of Hofstra School of Medicine in New Hyde Park, New York, noticed the need to educate providers about the use of ultrasound in the ICU. Dr. Koenig approached Richard Irwin, MD, Master FCCP, and Editor in Chief of the journal CHEST, with an idea for a new section in the journal. So began “Ultrasound Corner,” an online, video-based series in the journal that provides readers with real cases where ultrasound has played a large role in diagnostic patient care.
Each month, the journal receives two to four submissions from chest medicine clinicians who want to share their critical care ultrasound patient stories. One to two stories are selected and published monthly with real video images that are explained in the manuscript and in a narration done by Dr. Koenig.
“This creates a section where clinicians worldwide can share their experiences so that others may incorporate different methods of diagnosis into their practice,” said Dr. Koenig. “This method of learning challenges the readers to interpret images and integrate the results into a patient management plan.”
Dr. Koenig recommends that clinicians who have experienced benefit using ultrasound in critical care situations submit their cases so that viewers can learn from each other. Share the knowledge you’ve gained from your patient cases. Visit https://mc.manuscriptcentral.com/chest, log in to your account, and click “Start a New Submission” under the “Author” section.
More importantly, Dr. Koenig encourages the journal readership to explore Ultrasound Corner (https://journal.chestnet.org/ultrasound) every month in CHEST to learn of different courses of diagnosis and treatment being used to strengthen patient diagnostic and management plans in new, evolving ways.
The use of ultrasound is often overlooked when it could very well aid in the diagnosis of a critical illness in a shorter amount of time, while eliminating potential risks that come with many of the usually administered tests.
In 2013, Seth Koenig, MD, FCCP, of Hofstra School of Medicine in New Hyde Park, New York, noticed the need to educate providers about the use of ultrasound in the ICU. Dr. Koenig approached Richard Irwin, MD, Master FCCP, and Editor in Chief of the journal CHEST, with an idea for a new section in the journal. So began “Ultrasound Corner,” an online, video-based series in the journal that provides readers with real cases where ultrasound has played a large role in diagnostic patient care.
Each month, the journal receives two to four submissions from chest medicine clinicians who want to share their critical care ultrasound patient stories. One to two stories are selected and published monthly with real video images that are explained in the manuscript and in a narration done by Dr. Koenig.
“This creates a section where clinicians worldwide can share their experiences so that others may incorporate different methods of diagnosis into their practice,” said Dr. Koenig. “This method of learning challenges the readers to interpret images and integrate the results into a patient management plan.”
Dr. Koenig recommends that clinicians who have experienced benefit using ultrasound in critical care situations submit their cases so that viewers can learn from each other. Share the knowledge you’ve gained from your patient cases. Visit https://mc.manuscriptcentral.com/chest, log in to your account, and click “Start a New Submission” under the “Author” section.
More importantly, Dr. Koenig encourages the journal readership to explore Ultrasound Corner (https://journal.chestnet.org/ultrasound) every month in CHEST to learn of different courses of diagnosis and treatment being used to strengthen patient diagnostic and management plans in new, evolving ways.
Zolpidem does not boost cannabis abstinence during treatment
SAN DIEGO – Disturbed sleep is one of the symptoms of withdrawal from marijuana, and it can wreak havoc on the lives of people with cannabis use disorder who are trying to quit. Now, a new study provides more evidence about the problem and suggests that the sleep aid zolpidem is not a solution on its own.
Zolpidem seemed to help subjects sleep better, but the effect lasted only as long as they took the drug. And .
“Zolpidem or other hypnotics may have some efficacy as a pharmacotherapy to relieve short-term sleep disruption in the treatment of cannabis use disorder. But they should only be used in cases where abstinence-induced insomnia is a significant barrier to cessation and only in combination with other evidence-based interventions to address key aspects of cannabis use disorder,” study coauthor Dustin C. Lee, PhD, of Johns Hopkins University, Baltimore, said in an interview. He presented the study findings at the annual meeting of the College on Problems of Drug Dependence.
Previous research, including studies by some of the authors of the new report, suggests that people trying to withdraw from cannabis often suffer from sleep disturbances.
In a 2010 study, for instance, researchers interviewed 469 adult cannabis users in the Baltimore area who had tried to quit outside of treatment programs. Nearly half reported trouble falling asleep, while 20%-36% reported sleep-related symptoms, such as sleeping more or less than usual, having unusual dreams, and waking during the night (Drug Alcohol Depend. 2010 Sep 1;111[1-2]:120-7).
Another factor could be at play: Cannabis users may sleep better while they use the drug, then lose the benefit when they try to withdraw.
“Studies have demonstrated that acute use of cannabis reduces sleep latency and increases stage 3 sleep, informally referred to as ‘deep’ or ‘slow-wave’ sleep, which means individuals fall asleep faster and remain in deep sleep longer after using cannabis,” Dr. Lee said.
Whatever the explanation, the sleep problems during withdrawal are not helpful to users who are trying to quit. “Research indicates that cannabis cessation results in sleep problems that are a barrier to quitting,” Dr. Lee said.
For the new study, Dr. Lee and his colleagues randomly assigned 127 adults – all in a 12-week clinical trial of cannabis use disorder treatment – to receive extended-release zolpidem or placebo.
Participants underwent urine screens to test for drug use and ambulatory polysomnography to measure sleep.
A preliminary analysis found that those who received zolpidem had higher rates of cannabis abstinence during the 12 weeks and at end of treatment. But analysis showed that the differences were not statistically significant, Dr. Lee said, “which suggests that sleep is not the predominant factor driving successful cessation attempts.”
Researchers also found that sleep efficiency and sleep onset latency deteriorated to a clinically significant degree in week 1 of the study for those who took the placebo (mean sleep efficiency fell from 82% to 74%, while sleep onset latency increased from 28 to 82 minutes).
The zolpidem group did not see any significant change in sleep efficiency or sleep onset latency. However, those participants showed signs of rebound insomnia after they stopped taking zolpidem.
As for the risk of abuse of zolpidem, Dr. Lee said: “We did not see any indication of abuse among the individuals who participated in our study. We monitored this via quantitative urine toxicology testing and remote medication adherence monitoring.”
Moving forward, he said, zolpidem “may have some efficacy as a short-term therapy for sleep disruption in a subset of cannabis users. More research is needed to further evaluate the efficacy of zolpidem or other hypnotic medications in individuals who differ in withdrawal severity or demographics.”
Dr. Lee said evaluating behavioral sleep treatments would be a logical next-step in light of his team’s data.
The National Institute on Drug Abuse funded the study. Three of the authors reported no relevant disclosures, and one reported consulting/advisory links to several drug companies and several state-licensed medical cannabis cultivation or dispensary businesses.
SAN DIEGO – Disturbed sleep is one of the symptoms of withdrawal from marijuana, and it can wreak havoc on the lives of people with cannabis use disorder who are trying to quit. Now, a new study provides more evidence about the problem and suggests that the sleep aid zolpidem is not a solution on its own.
Zolpidem seemed to help subjects sleep better, but the effect lasted only as long as they took the drug. And .
“Zolpidem or other hypnotics may have some efficacy as a pharmacotherapy to relieve short-term sleep disruption in the treatment of cannabis use disorder. But they should only be used in cases where abstinence-induced insomnia is a significant barrier to cessation and only in combination with other evidence-based interventions to address key aspects of cannabis use disorder,” study coauthor Dustin C. Lee, PhD, of Johns Hopkins University, Baltimore, said in an interview. He presented the study findings at the annual meeting of the College on Problems of Drug Dependence.
Previous research, including studies by some of the authors of the new report, suggests that people trying to withdraw from cannabis often suffer from sleep disturbances.
In a 2010 study, for instance, researchers interviewed 469 adult cannabis users in the Baltimore area who had tried to quit outside of treatment programs. Nearly half reported trouble falling asleep, while 20%-36% reported sleep-related symptoms, such as sleeping more or less than usual, having unusual dreams, and waking during the night (Drug Alcohol Depend. 2010 Sep 1;111[1-2]:120-7).
Another factor could be at play: Cannabis users may sleep better while they use the drug, then lose the benefit when they try to withdraw.
“Studies have demonstrated that acute use of cannabis reduces sleep latency and increases stage 3 sleep, informally referred to as ‘deep’ or ‘slow-wave’ sleep, which means individuals fall asleep faster and remain in deep sleep longer after using cannabis,” Dr. Lee said.
Whatever the explanation, the sleep problems during withdrawal are not helpful to users who are trying to quit. “Research indicates that cannabis cessation results in sleep problems that are a barrier to quitting,” Dr. Lee said.
For the new study, Dr. Lee and his colleagues randomly assigned 127 adults – all in a 12-week clinical trial of cannabis use disorder treatment – to receive extended-release zolpidem or placebo.
Participants underwent urine screens to test for drug use and ambulatory polysomnography to measure sleep.
A preliminary analysis found that those who received zolpidem had higher rates of cannabis abstinence during the 12 weeks and at end of treatment. But analysis showed that the differences were not statistically significant, Dr. Lee said, “which suggests that sleep is not the predominant factor driving successful cessation attempts.”
Researchers also found that sleep efficiency and sleep onset latency deteriorated to a clinically significant degree in week 1 of the study for those who took the placebo (mean sleep efficiency fell from 82% to 74%, while sleep onset latency increased from 28 to 82 minutes).
The zolpidem group did not see any significant change in sleep efficiency or sleep onset latency. However, those participants showed signs of rebound insomnia after they stopped taking zolpidem.
As for the risk of abuse of zolpidem, Dr. Lee said: “We did not see any indication of abuse among the individuals who participated in our study. We monitored this via quantitative urine toxicology testing and remote medication adherence monitoring.”
Moving forward, he said, zolpidem “may have some efficacy as a short-term therapy for sleep disruption in a subset of cannabis users. More research is needed to further evaluate the efficacy of zolpidem or other hypnotic medications in individuals who differ in withdrawal severity or demographics.”
Dr. Lee said evaluating behavioral sleep treatments would be a logical next-step in light of his team’s data.
The National Institute on Drug Abuse funded the study. Three of the authors reported no relevant disclosures, and one reported consulting/advisory links to several drug companies and several state-licensed medical cannabis cultivation or dispensary businesses.
SAN DIEGO – Disturbed sleep is one of the symptoms of withdrawal from marijuana, and it can wreak havoc on the lives of people with cannabis use disorder who are trying to quit. Now, a new study provides more evidence about the problem and suggests that the sleep aid zolpidem is not a solution on its own.
Zolpidem seemed to help subjects sleep better, but the effect lasted only as long as they took the drug. And .
“Zolpidem or other hypnotics may have some efficacy as a pharmacotherapy to relieve short-term sleep disruption in the treatment of cannabis use disorder. But they should only be used in cases where abstinence-induced insomnia is a significant barrier to cessation and only in combination with other evidence-based interventions to address key aspects of cannabis use disorder,” study coauthor Dustin C. Lee, PhD, of Johns Hopkins University, Baltimore, said in an interview. He presented the study findings at the annual meeting of the College on Problems of Drug Dependence.
Previous research, including studies by some of the authors of the new report, suggests that people trying to withdraw from cannabis often suffer from sleep disturbances.
In a 2010 study, for instance, researchers interviewed 469 adult cannabis users in the Baltimore area who had tried to quit outside of treatment programs. Nearly half reported trouble falling asleep, while 20%-36% reported sleep-related symptoms, such as sleeping more or less than usual, having unusual dreams, and waking during the night (Drug Alcohol Depend. 2010 Sep 1;111[1-2]:120-7).
Another factor could be at play: Cannabis users may sleep better while they use the drug, then lose the benefit when they try to withdraw.
“Studies have demonstrated that acute use of cannabis reduces sleep latency and increases stage 3 sleep, informally referred to as ‘deep’ or ‘slow-wave’ sleep, which means individuals fall asleep faster and remain in deep sleep longer after using cannabis,” Dr. Lee said.
Whatever the explanation, the sleep problems during withdrawal are not helpful to users who are trying to quit. “Research indicates that cannabis cessation results in sleep problems that are a barrier to quitting,” Dr. Lee said.
For the new study, Dr. Lee and his colleagues randomly assigned 127 adults – all in a 12-week clinical trial of cannabis use disorder treatment – to receive extended-release zolpidem or placebo.
Participants underwent urine screens to test for drug use and ambulatory polysomnography to measure sleep.
A preliminary analysis found that those who received zolpidem had higher rates of cannabis abstinence during the 12 weeks and at end of treatment. But analysis showed that the differences were not statistically significant, Dr. Lee said, “which suggests that sleep is not the predominant factor driving successful cessation attempts.”
Researchers also found that sleep efficiency and sleep onset latency deteriorated to a clinically significant degree in week 1 of the study for those who took the placebo (mean sleep efficiency fell from 82% to 74%, while sleep onset latency increased from 28 to 82 minutes).
The zolpidem group did not see any significant change in sleep efficiency or sleep onset latency. However, those participants showed signs of rebound insomnia after they stopped taking zolpidem.
As for the risk of abuse of zolpidem, Dr. Lee said: “We did not see any indication of abuse among the individuals who participated in our study. We monitored this via quantitative urine toxicology testing and remote medication adherence monitoring.”
Moving forward, he said, zolpidem “may have some efficacy as a short-term therapy for sleep disruption in a subset of cannabis users. More research is needed to further evaluate the efficacy of zolpidem or other hypnotic medications in individuals who differ in withdrawal severity or demographics.”
Dr. Lee said evaluating behavioral sleep treatments would be a logical next-step in light of his team’s data.
The National Institute on Drug Abuse funded the study. Three of the authors reported no relevant disclosures, and one reported consulting/advisory links to several drug companies and several state-licensed medical cannabis cultivation or dispensary businesses.
REPORTING FROM CPDD 2018
Key clinical point: Zolpidem does not boost rates of abstinence during treatment for cannabis use disorder.
Major finding: Researchers did not find a statistically significant difference in cannabis abstinence rates between subjects who took zolpidem during treatment and those who did not.
Study details: Randomized, placebo-controlled trial of 127 adults with cannabis use disorder, all in a 12-week clinical trial of a treatment program, who received extended-release zolpidem or placebo.
Disclosures: Three of the authors reported no relevant disclosures, and one reported consulting/advisory links to several drug companies and several state-licensed medical cannabis cultivation or dispensary businesses.
Fetal exposure to folic acid may reduce youth psychosis risk
Gestational exposure to grain products fortified with folic acid resulted in delayed thinning of the cerebral cortex, and thus may protect against later psychosis, according to results published in JAMA Psychiatry.
In a retrospective, observational cohort study of 292 patients aged 8-18 years, increases in cortical thickness were associated with folic acid exposure in the bilateral frontal and temporal regions of the brain (9.9%-11.6%; corrected P less than .001 to P = .03). Delayed, age-associated thinning in temporal and parietal regions was also observed (beta = –11.1 to –13.9; corrected P = .002), reported Hamdi Eryilmaz, PhD, of the department of psychiatry at Massachusetts General Hospital, Boston, and his coauthors.
The study authors first observed MRI scans in the Massachusetts General Hospital (MGH) cohort, which included the 292 patients aged 8-18 years, and then conducted subsequent analyses on two additional cohorts – the Philadelphia Neurodevelopmental Cohort (PNC) and the National Institutes of Health MRI Study of Normal Brain Development (NIH) cohort – to test the reliability and specificity of cortical development associations and the relevance of MRI changes to psychopathological characteristics, the authors wrote.
Using the U.S. implementation of folic acid fortification in grain foods in 1996 and 1997 to define exposure status, the investigators identified MRI scans of 97 prerollout (not exposed), 96 rollout (partly exposed), and 99 postrollout (fully exposed) unique individuals in the MGH group between January 2005 and March 2015, for patients born between January 1993 and December 2001. They also collected information on demographics; reason for MRI scan; and prior use of psychotropic medications, folic acid, or multivitamins.
The PNC cohort consisted of 861 patients, also aged 8-18 years, from community health settings in Philadelphia who had an MRI assessment and a clinical assessment of psychiatric symptoms. The NIH comparison cohort included 217 patients recruited from six health sites across the United States and born before the fortification rollout.
The MGH analysis contrasted mean cortical thickness and linear and quadratic models of age-associated change in cortical thickness within the fully exposed and nonexposed groups. PNC and NIH analyses evaluated quadratic associations of age with cortical thickness.
In the MGH cohort, increases in cortical thickness were associated with folic acid exposure in the bilateral frontal and temporal regions of the brain (9.9%-11.6%; corrected P less than .001 to P = .03), and delayed, age-associated thinning in the temporal and parietal regions was observed (corrected P = .002). Thickness was higher in the fully exposed group, compared with those in the nonexposed group. The effect was intermediate in the partly exposed group, Dr. Eryilmaz and his coauthors wrote.
In the PNC cohort, delayed, age-related thinning was observed in four clusters overlapping with the MGH analysis: left frontal, right inferior temporal, left inferior parietal, and right inferior parietal. In addition, onset of cortical thinning was found to be between 13.0 and 14.3 years of age in least-square regression analysis. In the comparison NIH cohort, though, only the left frontal cortex demonstrated significant quadratic thinning; the break point occurred at a significantly younger age, compared with those in the PNC group (P less than .001).
In the PNC group, 248 of the 861 patients included in the MRI analysis were typically developing, 199 had a psychosis diagnosis, 105 had attenuated psychotic symptoms, and 309 had various other psychiatric conditions. Best-fit local thinning slopes were calculated for each participant for each region with postfortification thinning, and in three of four regions, less-negative local slopes were associated with significantly reduced adjusted odds of psychosis spectrum diagnosis (odds ratio, 0.37-0.59; P less than .001 to P = .02), the authors reported.
The findings confirm that “fetal exposure to population-wide folic acid fortification was associated with subsequent alterations in cortical development among school-aged youths,” Dr. Eryilmaz and his coauthors wrote. “These cortical changes were associated with reduced risk of psychosis.”
The results also suggest that the protective effects of folic acid in gestation “may extend beyond prevention of neural tube defects and span neurodevelopment during childhood and adolescence,” they concluded.
The study was funded by MQ: Transforming Mental Health, with support from grants from several additional sources, including the National Institutes of Health.
SOURCE: Eryilmaz H et al. JAMA Psychiatry. 2018 Jul 3. doi: 10.1001/jamapsychiatry.2018.1381.
The results of this study complement previous studies in the Netherlands “addressing similar questions using plasma levels of folate in maternal blood during pregnancy,” Tomáš Paus, MD, PhD, wrote in an editorial (JAMA Psychiatry. 2018 Jul 3. doi: 10.1001/jamapsychiatry.2018.1255) published with the study. However, he added, “it is unfortunate that the authors did not report values of cortical surface area in the different groups of individuals studied.”
Previous studies have shown that the children of mothers with high folic acid levels during pregnancy showed greater head growth and larger brain volumes at 6-8 years of age, Dr. Paus wrote, adding that future research should explore the possibility that exposure to folic acid induces effects such as DNA methylation, that may persist over time.
“This folate-methylation hypothesis needs to be tested empirically in large data sets, ideally in conjunction with relevant brain phenotypes, to replicate and expand the initial findings reported” in this study, he concluded.
Dr. Paus is affiliated with the department of psychology at the Rotman Research Institute, Toronto. He reported no conflicts of interest.
The results of this study complement previous studies in the Netherlands “addressing similar questions using plasma levels of folate in maternal blood during pregnancy,” Tomáš Paus, MD, PhD, wrote in an editorial (JAMA Psychiatry. 2018 Jul 3. doi: 10.1001/jamapsychiatry.2018.1255) published with the study. However, he added, “it is unfortunate that the authors did not report values of cortical surface area in the different groups of individuals studied.”
Previous studies have shown that the children of mothers with high folic acid levels during pregnancy showed greater head growth and larger brain volumes at 6-8 years of age, Dr. Paus wrote, adding that future research should explore the possibility that exposure to folic acid induces effects such as DNA methylation, that may persist over time.
“This folate-methylation hypothesis needs to be tested empirically in large data sets, ideally in conjunction with relevant brain phenotypes, to replicate and expand the initial findings reported” in this study, he concluded.
Dr. Paus is affiliated with the department of psychology at the Rotman Research Institute, Toronto. He reported no conflicts of interest.
The results of this study complement previous studies in the Netherlands “addressing similar questions using plasma levels of folate in maternal blood during pregnancy,” Tomáš Paus, MD, PhD, wrote in an editorial (JAMA Psychiatry. 2018 Jul 3. doi: 10.1001/jamapsychiatry.2018.1255) published with the study. However, he added, “it is unfortunate that the authors did not report values of cortical surface area in the different groups of individuals studied.”
Previous studies have shown that the children of mothers with high folic acid levels during pregnancy showed greater head growth and larger brain volumes at 6-8 years of age, Dr. Paus wrote, adding that future research should explore the possibility that exposure to folic acid induces effects such as DNA methylation, that may persist over time.
“This folate-methylation hypothesis needs to be tested empirically in large data sets, ideally in conjunction with relevant brain phenotypes, to replicate and expand the initial findings reported” in this study, he concluded.
Dr. Paus is affiliated with the department of psychology at the Rotman Research Institute, Toronto. He reported no conflicts of interest.
Gestational exposure to grain products fortified with folic acid resulted in delayed thinning of the cerebral cortex, and thus may protect against later psychosis, according to results published in JAMA Psychiatry.
In a retrospective, observational cohort study of 292 patients aged 8-18 years, increases in cortical thickness were associated with folic acid exposure in the bilateral frontal and temporal regions of the brain (9.9%-11.6%; corrected P less than .001 to P = .03). Delayed, age-associated thinning in temporal and parietal regions was also observed (beta = –11.1 to –13.9; corrected P = .002), reported Hamdi Eryilmaz, PhD, of the department of psychiatry at Massachusetts General Hospital, Boston, and his coauthors.
The study authors first observed MRI scans in the Massachusetts General Hospital (MGH) cohort, which included the 292 patients aged 8-18 years, and then conducted subsequent analyses on two additional cohorts – the Philadelphia Neurodevelopmental Cohort (PNC) and the National Institutes of Health MRI Study of Normal Brain Development (NIH) cohort – to test the reliability and specificity of cortical development associations and the relevance of MRI changes to psychopathological characteristics, the authors wrote.
Using the U.S. implementation of folic acid fortification in grain foods in 1996 and 1997 to define exposure status, the investigators identified MRI scans of 97 prerollout (not exposed), 96 rollout (partly exposed), and 99 postrollout (fully exposed) unique individuals in the MGH group between January 2005 and March 2015, for patients born between January 1993 and December 2001. They also collected information on demographics; reason for MRI scan; and prior use of psychotropic medications, folic acid, or multivitamins.
The PNC cohort consisted of 861 patients, also aged 8-18 years, from community health settings in Philadelphia who had an MRI assessment and a clinical assessment of psychiatric symptoms. The NIH comparison cohort included 217 patients recruited from six health sites across the United States and born before the fortification rollout.
The MGH analysis contrasted mean cortical thickness and linear and quadratic models of age-associated change in cortical thickness within the fully exposed and nonexposed groups. PNC and NIH analyses evaluated quadratic associations of age with cortical thickness.
In the MGH cohort, increases in cortical thickness were associated with folic acid exposure in the bilateral frontal and temporal regions of the brain (9.9%-11.6%; corrected P less than .001 to P = .03), and delayed, age-associated thinning in the temporal and parietal regions was observed (corrected P = .002). Thickness was higher in the fully exposed group, compared with those in the nonexposed group. The effect was intermediate in the partly exposed group, Dr. Eryilmaz and his coauthors wrote.
In the PNC cohort, delayed, age-related thinning was observed in four clusters overlapping with the MGH analysis: left frontal, right inferior temporal, left inferior parietal, and right inferior parietal. In addition, onset of cortical thinning was found to be between 13.0 and 14.3 years of age in least-square regression analysis. In the comparison NIH cohort, though, only the left frontal cortex demonstrated significant quadratic thinning; the break point occurred at a significantly younger age, compared with those in the PNC group (P less than .001).
In the PNC group, 248 of the 861 patients included in the MRI analysis were typically developing, 199 had a psychosis diagnosis, 105 had attenuated psychotic symptoms, and 309 had various other psychiatric conditions. Best-fit local thinning slopes were calculated for each participant for each region with postfortification thinning, and in three of four regions, less-negative local slopes were associated with significantly reduced adjusted odds of psychosis spectrum diagnosis (odds ratio, 0.37-0.59; P less than .001 to P = .02), the authors reported.
The findings confirm that “fetal exposure to population-wide folic acid fortification was associated with subsequent alterations in cortical development among school-aged youths,” Dr. Eryilmaz and his coauthors wrote. “These cortical changes were associated with reduced risk of psychosis.”
The results also suggest that the protective effects of folic acid in gestation “may extend beyond prevention of neural tube defects and span neurodevelopment during childhood and adolescence,” they concluded.
The study was funded by MQ: Transforming Mental Health, with support from grants from several additional sources, including the National Institutes of Health.
SOURCE: Eryilmaz H et al. JAMA Psychiatry. 2018 Jul 3. doi: 10.1001/jamapsychiatry.2018.1381.
Gestational exposure to grain products fortified with folic acid resulted in delayed thinning of the cerebral cortex, and thus may protect against later psychosis, according to results published in JAMA Psychiatry.
In a retrospective, observational cohort study of 292 patients aged 8-18 years, increases in cortical thickness were associated with folic acid exposure in the bilateral frontal and temporal regions of the brain (9.9%-11.6%; corrected P less than .001 to P = .03). Delayed, age-associated thinning in temporal and parietal regions was also observed (beta = –11.1 to –13.9; corrected P = .002), reported Hamdi Eryilmaz, PhD, of the department of psychiatry at Massachusetts General Hospital, Boston, and his coauthors.
The study authors first observed MRI scans in the Massachusetts General Hospital (MGH) cohort, which included the 292 patients aged 8-18 years, and then conducted subsequent analyses on two additional cohorts – the Philadelphia Neurodevelopmental Cohort (PNC) and the National Institutes of Health MRI Study of Normal Brain Development (NIH) cohort – to test the reliability and specificity of cortical development associations and the relevance of MRI changes to psychopathological characteristics, the authors wrote.
Using the U.S. implementation of folic acid fortification in grain foods in 1996 and 1997 to define exposure status, the investigators identified MRI scans of 97 prerollout (not exposed), 96 rollout (partly exposed), and 99 postrollout (fully exposed) unique individuals in the MGH group between January 2005 and March 2015, for patients born between January 1993 and December 2001. They also collected information on demographics; reason for MRI scan; and prior use of psychotropic medications, folic acid, or multivitamins.
The PNC cohort consisted of 861 patients, also aged 8-18 years, from community health settings in Philadelphia who had an MRI assessment and a clinical assessment of psychiatric symptoms. The NIH comparison cohort included 217 patients recruited from six health sites across the United States and born before the fortification rollout.
The MGH analysis contrasted mean cortical thickness and linear and quadratic models of age-associated change in cortical thickness within the fully exposed and nonexposed groups. PNC and NIH analyses evaluated quadratic associations of age with cortical thickness.
In the MGH cohort, increases in cortical thickness were associated with folic acid exposure in the bilateral frontal and temporal regions of the brain (9.9%-11.6%; corrected P less than .001 to P = .03), and delayed, age-associated thinning in the temporal and parietal regions was observed (corrected P = .002). Thickness was higher in the fully exposed group, compared with those in the nonexposed group. The effect was intermediate in the partly exposed group, Dr. Eryilmaz and his coauthors wrote.
In the PNC cohort, delayed, age-related thinning was observed in four clusters overlapping with the MGH analysis: left frontal, right inferior temporal, left inferior parietal, and right inferior parietal. In addition, onset of cortical thinning was found to be between 13.0 and 14.3 years of age in least-square regression analysis. In the comparison NIH cohort, though, only the left frontal cortex demonstrated significant quadratic thinning; the break point occurred at a significantly younger age, compared with those in the PNC group (P less than .001).
In the PNC group, 248 of the 861 patients included in the MRI analysis were typically developing, 199 had a psychosis diagnosis, 105 had attenuated psychotic symptoms, and 309 had various other psychiatric conditions. Best-fit local thinning slopes were calculated for each participant for each region with postfortification thinning, and in three of four regions, less-negative local slopes were associated with significantly reduced adjusted odds of psychosis spectrum diagnosis (odds ratio, 0.37-0.59; P less than .001 to P = .02), the authors reported.
The findings confirm that “fetal exposure to population-wide folic acid fortification was associated with subsequent alterations in cortical development among school-aged youths,” Dr. Eryilmaz and his coauthors wrote. “These cortical changes were associated with reduced risk of psychosis.”
The results also suggest that the protective effects of folic acid in gestation “may extend beyond prevention of neural tube defects and span neurodevelopment during childhood and adolescence,” they concluded.
The study was funded by MQ: Transforming Mental Health, with support from grants from several additional sources, including the National Institutes of Health.
SOURCE: Eryilmaz H et al. JAMA Psychiatry. 2018 Jul 3. doi: 10.1001/jamapsychiatry.2018.1381.
FROM JAMA PSYCHIATRY
Key clinical point: Gestational exposure to grain products fortified with folic acid resulted in delayed thinning of the cerebral cortex, and thus may protect against later psychosis.
Major finding: Increases in cortical thickness were associated with folic acid exposure in the bilateral frontal and temporal regions of the brain (9.9%-11.6%; corrected P less than .001 to P = .03).
Study details: A retrospective, observational cohort study of 292 patients aged 8-18 years.
Disclosures: The study was funded by MQ: Transforming Mental Health, with support from grants from several additional sources, including the National Institutes of Health.
Source: Eryilmaz H et al. JAMA Psychiatry. 2018 Jul 3. doi: 10.1001/jamapsychiatry.2018.1381.
Patient survey results highlight disease burden in atopic dermatitis
More than half of the patients with moderate to severe atopic dermatitis (AD) had inadequately controlled disease, which was associated with a higher patient-reported disease burden compared with those who had adequately controlled disease, in a cross-sectional study of adults with AD.
Disease control aside, patient-reported burden was generally higher in those with moderate to severe AD versus patients with mild AD, according to Eric L. Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, and his coauthors.
“These results highlight beyond using measures of disease activity,” the researchers wrote. The study, published in JAMA Dermatology, was conducted before the introduction of dupilumab (Dupixent), the first biologic approved by the Food and Drug Administration for treatment of moderate to severe AD, the authors noted. (The study was supported by the manufacturers of dupilumab.)
The patients were in the Adults With Atopic Dermatitis Reporting on Their Experience (AD-AWARE) study, a cross-sectional analysis of burden of illness in adults with AD in clinical practices at six U.S. academic medical centers. The 1,519 patients completed a self-administered, Internet-based questionnaire during 2013-2014. Among these patients, 830 (54.6%) had moderate to severe AD.
A total of 185 patients with moderate to severe disease received systemic immunomodulators or phototherapy, and of those, more than half (103, or 55.7%) reported inadequate disease control, according to the survey results.
Regardless of disease control, the patients with moderate to severe AD had a greater burden of disease compared with patients with mild AD, according to the investigators. Those burdens included more severe pain and itching, sleep effects, anxiety and depression, and impairment of health-related quality of life, they reported.
Those with moderate to severe disease had a mean of 5.7 days per week with itchy skin, and 22.8% reported itch lasting for more than half a day, compared with a mean of 2.7 days and 2.9%, respectively, for those with mild disease, all significant differences.
Those with moderate to severe disease also reported more trouble sleeping, along with more frequent sleep disturbances, longer time transitioning into sleep, and more use of nonprescription sleep medications than those with mild disease.
Among those with moderate to severe disease, those who were inadequately controlled had a higher level of itch intensity and more frequent itching (a mean of 6.3 days per week), compared with those who were controlled (a mean of 5.7 days per week).
In a previous study looking at patient burden in a phase 2b clinical trial of dupilumab, Dr. Simpson and his coinvestigators found that adults with moderate to severe AD reported a “multidimensional burden” of disease that included disease activity, patient-reported symptoms, quality-of-life impact, and comorbidities (J Am Acad Dermatol. 2016 Mar;74[3]:491-8).
The current analysis based on the AD-AWARE study was supported by dupilumab manufacturers Regeneron Pharmaceuticals and Sanofi. Dr. Simpson reported disclosures related to Amgen, Anacor, Asubio, Celgene, Chugai, Galderma, Genentech, Medicis, Merck, and Regeneron; five of the 15 authors were employees of Sanofi or Regeneron. Other authors reported disclosures related to these and other companies.
SOURCE: Simpson EL et al. JAMA Dermatol. 2018 Jul 3. doi: 10.1001/jamadermatol.2018.1572.
More than half of the patients with moderate to severe atopic dermatitis (AD) had inadequately controlled disease, which was associated with a higher patient-reported disease burden compared with those who had adequately controlled disease, in a cross-sectional study of adults with AD.
Disease control aside, patient-reported burden was generally higher in those with moderate to severe AD versus patients with mild AD, according to Eric L. Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, and his coauthors.
“These results highlight beyond using measures of disease activity,” the researchers wrote. The study, published in JAMA Dermatology, was conducted before the introduction of dupilumab (Dupixent), the first biologic approved by the Food and Drug Administration for treatment of moderate to severe AD, the authors noted. (The study was supported by the manufacturers of dupilumab.)
The patients were in the Adults With Atopic Dermatitis Reporting on Their Experience (AD-AWARE) study, a cross-sectional analysis of burden of illness in adults with AD in clinical practices at six U.S. academic medical centers. The 1,519 patients completed a self-administered, Internet-based questionnaire during 2013-2014. Among these patients, 830 (54.6%) had moderate to severe AD.
A total of 185 patients with moderate to severe disease received systemic immunomodulators or phototherapy, and of those, more than half (103, or 55.7%) reported inadequate disease control, according to the survey results.
Regardless of disease control, the patients with moderate to severe AD had a greater burden of disease compared with patients with mild AD, according to the investigators. Those burdens included more severe pain and itching, sleep effects, anxiety and depression, and impairment of health-related quality of life, they reported.
Those with moderate to severe disease had a mean of 5.7 days per week with itchy skin, and 22.8% reported itch lasting for more than half a day, compared with a mean of 2.7 days and 2.9%, respectively, for those with mild disease, all significant differences.
Those with moderate to severe disease also reported more trouble sleeping, along with more frequent sleep disturbances, longer time transitioning into sleep, and more use of nonprescription sleep medications than those with mild disease.
Among those with moderate to severe disease, those who were inadequately controlled had a higher level of itch intensity and more frequent itching (a mean of 6.3 days per week), compared with those who were controlled (a mean of 5.7 days per week).
In a previous study looking at patient burden in a phase 2b clinical trial of dupilumab, Dr. Simpson and his coinvestigators found that adults with moderate to severe AD reported a “multidimensional burden” of disease that included disease activity, patient-reported symptoms, quality-of-life impact, and comorbidities (J Am Acad Dermatol. 2016 Mar;74[3]:491-8).
The current analysis based on the AD-AWARE study was supported by dupilumab manufacturers Regeneron Pharmaceuticals and Sanofi. Dr. Simpson reported disclosures related to Amgen, Anacor, Asubio, Celgene, Chugai, Galderma, Genentech, Medicis, Merck, and Regeneron; five of the 15 authors were employees of Sanofi or Regeneron. Other authors reported disclosures related to these and other companies.
SOURCE: Simpson EL et al. JAMA Dermatol. 2018 Jul 3. doi: 10.1001/jamadermatol.2018.1572.
More than half of the patients with moderate to severe atopic dermatitis (AD) had inadequately controlled disease, which was associated with a higher patient-reported disease burden compared with those who had adequately controlled disease, in a cross-sectional study of adults with AD.
Disease control aside, patient-reported burden was generally higher in those with moderate to severe AD versus patients with mild AD, according to Eric L. Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, and his coauthors.
“These results highlight beyond using measures of disease activity,” the researchers wrote. The study, published in JAMA Dermatology, was conducted before the introduction of dupilumab (Dupixent), the first biologic approved by the Food and Drug Administration for treatment of moderate to severe AD, the authors noted. (The study was supported by the manufacturers of dupilumab.)
The patients were in the Adults With Atopic Dermatitis Reporting on Their Experience (AD-AWARE) study, a cross-sectional analysis of burden of illness in adults with AD in clinical practices at six U.S. academic medical centers. The 1,519 patients completed a self-administered, Internet-based questionnaire during 2013-2014. Among these patients, 830 (54.6%) had moderate to severe AD.
A total of 185 patients with moderate to severe disease received systemic immunomodulators or phototherapy, and of those, more than half (103, or 55.7%) reported inadequate disease control, according to the survey results.
Regardless of disease control, the patients with moderate to severe AD had a greater burden of disease compared with patients with mild AD, according to the investigators. Those burdens included more severe pain and itching, sleep effects, anxiety and depression, and impairment of health-related quality of life, they reported.
Those with moderate to severe disease had a mean of 5.7 days per week with itchy skin, and 22.8% reported itch lasting for more than half a day, compared with a mean of 2.7 days and 2.9%, respectively, for those with mild disease, all significant differences.
Those with moderate to severe disease also reported more trouble sleeping, along with more frequent sleep disturbances, longer time transitioning into sleep, and more use of nonprescription sleep medications than those with mild disease.
Among those with moderate to severe disease, those who were inadequately controlled had a higher level of itch intensity and more frequent itching (a mean of 6.3 days per week), compared with those who were controlled (a mean of 5.7 days per week).
In a previous study looking at patient burden in a phase 2b clinical trial of dupilumab, Dr. Simpson and his coinvestigators found that adults with moderate to severe AD reported a “multidimensional burden” of disease that included disease activity, patient-reported symptoms, quality-of-life impact, and comorbidities (J Am Acad Dermatol. 2016 Mar;74[3]:491-8).
The current analysis based on the AD-AWARE study was supported by dupilumab manufacturers Regeneron Pharmaceuticals and Sanofi. Dr. Simpson reported disclosures related to Amgen, Anacor, Asubio, Celgene, Chugai, Galderma, Genentech, Medicis, Merck, and Regeneron; five of the 15 authors were employees of Sanofi or Regeneron. Other authors reported disclosures related to these and other companies.
SOURCE: Simpson EL et al. JAMA Dermatol. 2018 Jul 3. doi: 10.1001/jamadermatol.2018.1572.
FROM JAMA DERMATOLOGY
Key clinical point: Consider the burden of disease in patients, in addition to severity, when evaluating patients with atopic dermatitis.
Major finding: Patients with moderate to severe AD experienced itchy skin a mean of 5.7 days per week, with 22.8% reporting itch lasting for more than half a day, vs. a mean of 2.7 days and 2.9%, respectively, among those with mild disease (P less than .001 for both measures).
Study details: A cross-sectional study of 1,519 adult patients with AD, who answered a questionnaire related to disease burden.
Disclosures: Dr. Simpson reported disclosures related to Amgen, Anacor, Asubio, Celgene, Chugai, Galderma, Genentech, Medicis, Merck, and Regeneron. Five of the 15 authors were employees of Sanofi or Regeneron. Other authors reported disclosures related to these and/or other companies.
Source: Simpson EL et al. JAMA Dermatol. 2018 Jul 3. doi: 10.1001/jamadermatol.2018.1572.
HPV vaccine: New therapeutic option for SCC?
A patient with an aggressive form of squamous cell carcinoma (SCC) experienced complete resolution of tumors following administration of human papillomavirus (HPV) vaccine, a recent case report shows.
The patient, an immunocompetent woman, aged in her 90s, with multiple inoperable cutaneous basaloid SCCs, was treated with a combination of systemic and intratumoral delivery of 9-valent HPV vaccine, authors of the case report wrote in JAMA Dermatology.
All tumors resolved within 11 months of the first intratumoral injection, according to Anna J. Nichols, MD, PhD, department of dermatology and cutaneous surgery, University of Miami, and her coauthors.
‘These findings suggest that the 9-valent HPV vaccine can provide a therapeutic option for inoperable cutaneous SCCs, in addition to its approved use to prevent anogenital HPV infection,” they wrote.
Previously, Dr. Nichols and her coinvestigators reported a reduction in SCCs and basal cell carcinoma in 2 immunocompetent patients who had received quadrivalent HPV vaccine. Those results suggested development of these keratinocyte carcinomas in immunocompetent patients may be driven in part by HPV, the investigators said at the time.
The patient in the current report was treated with 9-valent HPV vaccine in a university-based outpatient clinic between March 2016 and February 2017.
She initially received two doses of intramuscular vaccine 6 weeks apart; 3 weeks later, she received intratumoral administration of the vaccine diluted with sterile saline, followed by three additional intratumoral doses over the next 8 months.
“The marked regression of numerous SCCs after initiation of the intratumoral injections eliminated the need for additional treatment,” Dr. Nichols and her coauthors said in the report.
A reduction in tumor size and number was seen 2 weeks after the second intratumoral dose of the 9-valent HPV vaccine, and within 11 months of the first intratumoral dose, there was no clinical or histologic evidence of SCC, they said.
The patient was left with small, violet-colored scars, along with a small pink papule, histopathologic analysis of which showed mild cellular atypia of basal keratinocytes with hyperkeratosis, according to published details of the case report.
The patient experienced mild pain in some of the tumors on days of intratumoral treatment, but no other side effects were seen, according to the authors.
The patient remained tumor free at the end of follow-up in May 2018, and there was no clinical evidence of SCC recurrence at the patient’s most recent follow-up visit, 24 months after the first intratumoral HPV dose, the researchers wrote.
It’s not clear what role the systemic vaccine doses played in the therapeutic benefit the patient experienced, authors said, noting that tumors not injected directly may have regressed because of either local vaccine dispersion or systemic, immune-mediated mechanisms.
“The potent therapeutic benefit may reflect a combination of immunologic, antiviral, and antitumor effects of 9-valent HPV vaccine,” they concluded.
Dr. Nichols had no disclosures. Study coauthors Tim Ioannides, MD, and Evangelos V. Badiavas, MD, PhD, have a patent pending for the application of human papillomavirus vaccine described in the study.
SOURCE: Nichols AJ et al. JAMA Dermatol. 2018 Jul 3. doi: 10.1001/jamadermatol.2016.5703.
A patient with an aggressive form of squamous cell carcinoma (SCC) experienced complete resolution of tumors following administration of human papillomavirus (HPV) vaccine, a recent case report shows.
The patient, an immunocompetent woman, aged in her 90s, with multiple inoperable cutaneous basaloid SCCs, was treated with a combination of systemic and intratumoral delivery of 9-valent HPV vaccine, authors of the case report wrote in JAMA Dermatology.
All tumors resolved within 11 months of the first intratumoral injection, according to Anna J. Nichols, MD, PhD, department of dermatology and cutaneous surgery, University of Miami, and her coauthors.
‘These findings suggest that the 9-valent HPV vaccine can provide a therapeutic option for inoperable cutaneous SCCs, in addition to its approved use to prevent anogenital HPV infection,” they wrote.
Previously, Dr. Nichols and her coinvestigators reported a reduction in SCCs and basal cell carcinoma in 2 immunocompetent patients who had received quadrivalent HPV vaccine. Those results suggested development of these keratinocyte carcinomas in immunocompetent patients may be driven in part by HPV, the investigators said at the time.
The patient in the current report was treated with 9-valent HPV vaccine in a university-based outpatient clinic between March 2016 and February 2017.
She initially received two doses of intramuscular vaccine 6 weeks apart; 3 weeks later, she received intratumoral administration of the vaccine diluted with sterile saline, followed by three additional intratumoral doses over the next 8 months.
“The marked regression of numerous SCCs after initiation of the intratumoral injections eliminated the need for additional treatment,” Dr. Nichols and her coauthors said in the report.
A reduction in tumor size and number was seen 2 weeks after the second intratumoral dose of the 9-valent HPV vaccine, and within 11 months of the first intratumoral dose, there was no clinical or histologic evidence of SCC, they said.
The patient was left with small, violet-colored scars, along with a small pink papule, histopathologic analysis of which showed mild cellular atypia of basal keratinocytes with hyperkeratosis, according to published details of the case report.
The patient experienced mild pain in some of the tumors on days of intratumoral treatment, but no other side effects were seen, according to the authors.
The patient remained tumor free at the end of follow-up in May 2018, and there was no clinical evidence of SCC recurrence at the patient’s most recent follow-up visit, 24 months after the first intratumoral HPV dose, the researchers wrote.
It’s not clear what role the systemic vaccine doses played in the therapeutic benefit the patient experienced, authors said, noting that tumors not injected directly may have regressed because of either local vaccine dispersion or systemic, immune-mediated mechanisms.
“The potent therapeutic benefit may reflect a combination of immunologic, antiviral, and antitumor effects of 9-valent HPV vaccine,” they concluded.
Dr. Nichols had no disclosures. Study coauthors Tim Ioannides, MD, and Evangelos V. Badiavas, MD, PhD, have a patent pending for the application of human papillomavirus vaccine described in the study.
SOURCE: Nichols AJ et al. JAMA Dermatol. 2018 Jul 3. doi: 10.1001/jamadermatol.2016.5703.
A patient with an aggressive form of squamous cell carcinoma (SCC) experienced complete resolution of tumors following administration of human papillomavirus (HPV) vaccine, a recent case report shows.
The patient, an immunocompetent woman, aged in her 90s, with multiple inoperable cutaneous basaloid SCCs, was treated with a combination of systemic and intratumoral delivery of 9-valent HPV vaccine, authors of the case report wrote in JAMA Dermatology.
All tumors resolved within 11 months of the first intratumoral injection, according to Anna J. Nichols, MD, PhD, department of dermatology and cutaneous surgery, University of Miami, and her coauthors.
‘These findings suggest that the 9-valent HPV vaccine can provide a therapeutic option for inoperable cutaneous SCCs, in addition to its approved use to prevent anogenital HPV infection,” they wrote.
Previously, Dr. Nichols and her coinvestigators reported a reduction in SCCs and basal cell carcinoma in 2 immunocompetent patients who had received quadrivalent HPV vaccine. Those results suggested development of these keratinocyte carcinomas in immunocompetent patients may be driven in part by HPV, the investigators said at the time.
The patient in the current report was treated with 9-valent HPV vaccine in a university-based outpatient clinic between March 2016 and February 2017.
She initially received two doses of intramuscular vaccine 6 weeks apart; 3 weeks later, she received intratumoral administration of the vaccine diluted with sterile saline, followed by three additional intratumoral doses over the next 8 months.
“The marked regression of numerous SCCs after initiation of the intratumoral injections eliminated the need for additional treatment,” Dr. Nichols and her coauthors said in the report.
A reduction in tumor size and number was seen 2 weeks after the second intratumoral dose of the 9-valent HPV vaccine, and within 11 months of the first intratumoral dose, there was no clinical or histologic evidence of SCC, they said.
The patient was left with small, violet-colored scars, along with a small pink papule, histopathologic analysis of which showed mild cellular atypia of basal keratinocytes with hyperkeratosis, according to published details of the case report.
The patient experienced mild pain in some of the tumors on days of intratumoral treatment, but no other side effects were seen, according to the authors.
The patient remained tumor free at the end of follow-up in May 2018, and there was no clinical evidence of SCC recurrence at the patient’s most recent follow-up visit, 24 months after the first intratumoral HPV dose, the researchers wrote.
It’s not clear what role the systemic vaccine doses played in the therapeutic benefit the patient experienced, authors said, noting that tumors not injected directly may have regressed because of either local vaccine dispersion or systemic, immune-mediated mechanisms.
“The potent therapeutic benefit may reflect a combination of immunologic, antiviral, and antitumor effects of 9-valent HPV vaccine,” they concluded.
Dr. Nichols had no disclosures. Study coauthors Tim Ioannides, MD, and Evangelos V. Badiavas, MD, PhD, have a patent pending for the application of human papillomavirus vaccine described in the study.
SOURCE: Nichols AJ et al. JAMA Dermatol. 2018 Jul 3. doi: 10.1001/jamadermatol.2016.5703.
FROM JAMA DERMATOLOGY
Key clinical point: Human papillomavirus (HPV) vaccine may provide a therapeutic option for inoperable cutaneous squamous cell carcinoma (SCC).
Major finding: One patient experienced complete resolution of tumors after systemic and intratumoral delivery of 9-valent HPV vaccine.
Study details: A case report of an immunocompetent woman in her 90s with multiple inoperable cutaneous basaloid SCCs.
Disclosures: Study coauthors Tim Ioannides, MD, and Evangelos V. Badiavas, MD, PhD, have a patent pending for the application of human papillomavirus vaccine described in the study.
Source: Nichols AJ et al. JAMA Dermatol. 2018 Jul 3. doi: 10.1001/jamadermatol.2016.5703.