User login
First-time, mild diverticulitis: Antibiotics or watchful waiting?
ILLUSTRATIVE CASE
A 58-year-old man presents to your office with a 2-day history of moderate (6/10) left lower quadrant pain, mild fever (none currently), 2 episodes of vomiting, no diarrhea, and no relief with over-the-counter medications. You suspect diverticulitis and obtain an abdominal computed tomography (CT) scan, which shows mild, uncomplicated (Hinchey stage 1a) diverticulitis.
How would you treat him?
Diverticulitis is common; about 200,000 people per year are admitted to the hospital because of diverticulitis in the United States.2,3 Health care providers typically treat diverticular disease with antibiotics and bowel rest.2,3 While severe forms of diverticulitis often require parenteral antibiotics and/or surgery, practitioners are increasingly managing the condition with oral antibiotics.4
One previous randomized control trial (RCT; N=623) found that antibiotic treatment (compared with no antibiotic treatment) for acute uncomplicated diverticulitis did not speed recovery or prevent complications (perforation or abscess formation) or recurrence at 12 months.5 The study’s strengths included limiting enrollment to people with CT-proven diverticulitis, using a good randomization and concealment process, and employing intention-to-treat analysis. The study was limited by a lack of a standardized antibiotic regimen across centers, previous diverticulitis diagnoses in 40% of patients, non-uniform follow-up processes to confirm anatomic resolution, and the lack of assessment to confirm resolution.5
STUDY SUMMARY
RCT finds that watchful waiting is just as effective as antibiotic Tx
This newer study was a single-blind RCT that compared treatment with antibiotics to observation among 528 adult patients in the Netherlands. Patients were enrolled if they had CT-proven, primary, left-sided, uncomplicated acute diverticulitis (Hinchey stage 1a and 1b).1 (The Hinchey classification is based on radiologic findings, with 0 for clinical diverticulitis only, 1a for confined pericolic inflammation or phlegmon, and 1b for pericolic or mesocolic abscess.6) Exclusion criteria included suspicion of colonic cancer by CT or ultrasound (US), previous CT/US-proven diverticulitis, sepsis, pregnancy, or antibiotic use in the previous 4 weeks.1
Observational vs antibiotic treatment. Enrolled patients were randomized to receive IV administration of amoxicillin-clavulanate 1200 mg 4 times daily for at least 48 hours followed by 625 mg PO 3 times daily for 10 total days of antibiotic treatment (n=266) or to be observed (n=262). Computerized randomization, with a random varying block size and stratified by Hinchey classification and center, was performed, and allocation was concealed. The investigators were masked to the allocation until all analyses were completed.1
The primary outcome was the time to functional recovery (resumption of pre-illness work activities) during a 6-month follow-up period. Secondary outcomes included hospital readmission rate; complicated, ongoing, and recurrent diverticulitis; sigmoid resection; other nonsurgical intervention; antibiotic treatment adverse effects; and all-cause mortality.
Continue to: Results
Results. Median recovery time for observational treatment was not inferior to antibiotic treatment (14 days vs 12 days; P=.15; hazard ratio [HR] for functional recovery=0.91; lower limit of 1-sided 95% confidence interval, 0.78). Observation was not inferior to antibiotics for any of the secondary endpoints at 6 and 12 months of follow-up (complicated diverticulitis, 3.8% vs 2.6%, respectively; P=.377), recurrent diverticulitis (3.4% vs 3%; P=.494), readmission (17.6% vs 12%; P=.148), or adverse events (48.5% vs 54.5%; P=.221). Initial hospitalization length of stay was shorter in the observation group (2 vs 3 days; P=.006). The researchers conducted a 24-month telephone follow-up, but no differences from the 12-month follow-up were noted.1
WHAT’S NEW
A study that looks at a true patient-oriented outcome
Previous studies of treatment options for acute uncomplicated diverticulitis looked at short-term outcomes, or at readmission, recurrence, and surgical intervention rate, or requirement for percutaneous drainage.7,8 This study is the first one to look at functional return to work (a true patient-oriented outcome). And it is the only study to look out to 24 months to gauge long-term outcomes with observational treatment.
CAVEATS
Can’t generalize findings to patients with worse forms of diverticulitis
It is worth noting that the findings of this study apply only to the mildest form of CT-proven acute diverticulitis (those patients classified as having Hinchey 1a disease), and is not generalizable to patients with more severe forms. Not enough patients with Hinchey 1b acute diverticulitis were enrolled in the study to reach any conclusions about treatment.
Various guidelines issued outside the United States recommend antibiotics for uncomplicated diverticulitis; however, the American Gastroenterological Association (AGA) indicates that antibiotics should be used selectively.1,9,10 This recommendation was based on an emerging understanding that diverticulitis maybe more inflammatory than infectious in nature. The AGA guideline authors acknowledge that their conclusion was based on low-quality evidence.9
Continuet to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Daniels L, Ünlü Ç, de Korte N, et al, for the Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104:52-61.
2. Wheat CL, Strate LL. Trends in hospitalization for diverticulitis and diverticular bleeding in the United States from 2000 to 2010. Clin Gastroenterol Hepatol. 2016;14:96-103.e1.
3. Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
4. Shabanzadeh DM, Wille-Jørgensen P. Antibiotics for uncomplicated diverticulitis. Cochrane Database Syst Rev. 2012;11:CD009092.
5. Chabok A, Påhlman L, Hjern F, et al. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99:532-539.
6. Klarenbeek BR, de Korte N, van der Peet DL, et al. Review of current classifications for diverticular disease and a translation into clinical practice. Int J Colorectal Dis. 2012;27:207-214.
7. Tandon A, Fretwell VL, Nunes QM, et al. Antibiotics versus no antibiotics in the treatment of acute uncomplicated diverticulitis - a systematic review and meta-analysis. Colorectal Dis. 2018 Jan 11. doi: 10.1111/codi.14013.
8. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57:284-294.
9. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149:1944-1949.
10. Sartelli M, Viale P, Catena F, et al. 2013 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2013;8:3.
ILLUSTRATIVE CASE
A 58-year-old man presents to your office with a 2-day history of moderate (6/10) left lower quadrant pain, mild fever (none currently), 2 episodes of vomiting, no diarrhea, and no relief with over-the-counter medications. You suspect diverticulitis and obtain an abdominal computed tomography (CT) scan, which shows mild, uncomplicated (Hinchey stage 1a) diverticulitis.
How would you treat him?
Diverticulitis is common; about 200,000 people per year are admitted to the hospital because of diverticulitis in the United States.2,3 Health care providers typically treat diverticular disease with antibiotics and bowel rest.2,3 While severe forms of diverticulitis often require parenteral antibiotics and/or surgery, practitioners are increasingly managing the condition with oral antibiotics.4
One previous randomized control trial (RCT; N=623) found that antibiotic treatment (compared with no antibiotic treatment) for acute uncomplicated diverticulitis did not speed recovery or prevent complications (perforation or abscess formation) or recurrence at 12 months.5 The study’s strengths included limiting enrollment to people with CT-proven diverticulitis, using a good randomization and concealment process, and employing intention-to-treat analysis. The study was limited by a lack of a standardized antibiotic regimen across centers, previous diverticulitis diagnoses in 40% of patients, non-uniform follow-up processes to confirm anatomic resolution, and the lack of assessment to confirm resolution.5
STUDY SUMMARY
RCT finds that watchful waiting is just as effective as antibiotic Tx
This newer study was a single-blind RCT that compared treatment with antibiotics to observation among 528 adult patients in the Netherlands. Patients were enrolled if they had CT-proven, primary, left-sided, uncomplicated acute diverticulitis (Hinchey stage 1a and 1b).1 (The Hinchey classification is based on radiologic findings, with 0 for clinical diverticulitis only, 1a for confined pericolic inflammation or phlegmon, and 1b for pericolic or mesocolic abscess.6) Exclusion criteria included suspicion of colonic cancer by CT or ultrasound (US), previous CT/US-proven diverticulitis, sepsis, pregnancy, or antibiotic use in the previous 4 weeks.1
Observational vs antibiotic treatment. Enrolled patients were randomized to receive IV administration of amoxicillin-clavulanate 1200 mg 4 times daily for at least 48 hours followed by 625 mg PO 3 times daily for 10 total days of antibiotic treatment (n=266) or to be observed (n=262). Computerized randomization, with a random varying block size and stratified by Hinchey classification and center, was performed, and allocation was concealed. The investigators were masked to the allocation until all analyses were completed.1
The primary outcome was the time to functional recovery (resumption of pre-illness work activities) during a 6-month follow-up period. Secondary outcomes included hospital readmission rate; complicated, ongoing, and recurrent diverticulitis; sigmoid resection; other nonsurgical intervention; antibiotic treatment adverse effects; and all-cause mortality.
Continue to: Results
Results. Median recovery time for observational treatment was not inferior to antibiotic treatment (14 days vs 12 days; P=.15; hazard ratio [HR] for functional recovery=0.91; lower limit of 1-sided 95% confidence interval, 0.78). Observation was not inferior to antibiotics for any of the secondary endpoints at 6 and 12 months of follow-up (complicated diverticulitis, 3.8% vs 2.6%, respectively; P=.377), recurrent diverticulitis (3.4% vs 3%; P=.494), readmission (17.6% vs 12%; P=.148), or adverse events (48.5% vs 54.5%; P=.221). Initial hospitalization length of stay was shorter in the observation group (2 vs 3 days; P=.006). The researchers conducted a 24-month telephone follow-up, but no differences from the 12-month follow-up were noted.1
WHAT’S NEW
A study that looks at a true patient-oriented outcome
Previous studies of treatment options for acute uncomplicated diverticulitis looked at short-term outcomes, or at readmission, recurrence, and surgical intervention rate, or requirement for percutaneous drainage.7,8 This study is the first one to look at functional return to work (a true patient-oriented outcome). And it is the only study to look out to 24 months to gauge long-term outcomes with observational treatment.
CAVEATS
Can’t generalize findings to patients with worse forms of diverticulitis
It is worth noting that the findings of this study apply only to the mildest form of CT-proven acute diverticulitis (those patients classified as having Hinchey 1a disease), and is not generalizable to patients with more severe forms. Not enough patients with Hinchey 1b acute diverticulitis were enrolled in the study to reach any conclusions about treatment.
Various guidelines issued outside the United States recommend antibiotics for uncomplicated diverticulitis; however, the American Gastroenterological Association (AGA) indicates that antibiotics should be used selectively.1,9,10 This recommendation was based on an emerging understanding that diverticulitis maybe more inflammatory than infectious in nature. The AGA guideline authors acknowledge that their conclusion was based on low-quality evidence.9
Continuet to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 58-year-old man presents to your office with a 2-day history of moderate (6/10) left lower quadrant pain, mild fever (none currently), 2 episodes of vomiting, no diarrhea, and no relief with over-the-counter medications. You suspect diverticulitis and obtain an abdominal computed tomography (CT) scan, which shows mild, uncomplicated (Hinchey stage 1a) diverticulitis.
How would you treat him?
Diverticulitis is common; about 200,000 people per year are admitted to the hospital because of diverticulitis in the United States.2,3 Health care providers typically treat diverticular disease with antibiotics and bowel rest.2,3 While severe forms of diverticulitis often require parenteral antibiotics and/or surgery, practitioners are increasingly managing the condition with oral antibiotics.4
One previous randomized control trial (RCT; N=623) found that antibiotic treatment (compared with no antibiotic treatment) for acute uncomplicated diverticulitis did not speed recovery or prevent complications (perforation or abscess formation) or recurrence at 12 months.5 The study’s strengths included limiting enrollment to people with CT-proven diverticulitis, using a good randomization and concealment process, and employing intention-to-treat analysis. The study was limited by a lack of a standardized antibiotic regimen across centers, previous diverticulitis diagnoses in 40% of patients, non-uniform follow-up processes to confirm anatomic resolution, and the lack of assessment to confirm resolution.5
STUDY SUMMARY
RCT finds that watchful waiting is just as effective as antibiotic Tx
This newer study was a single-blind RCT that compared treatment with antibiotics to observation among 528 adult patients in the Netherlands. Patients were enrolled if they had CT-proven, primary, left-sided, uncomplicated acute diverticulitis (Hinchey stage 1a and 1b).1 (The Hinchey classification is based on radiologic findings, with 0 for clinical diverticulitis only, 1a for confined pericolic inflammation or phlegmon, and 1b for pericolic or mesocolic abscess.6) Exclusion criteria included suspicion of colonic cancer by CT or ultrasound (US), previous CT/US-proven diverticulitis, sepsis, pregnancy, or antibiotic use in the previous 4 weeks.1
Observational vs antibiotic treatment. Enrolled patients were randomized to receive IV administration of amoxicillin-clavulanate 1200 mg 4 times daily for at least 48 hours followed by 625 mg PO 3 times daily for 10 total days of antibiotic treatment (n=266) or to be observed (n=262). Computerized randomization, with a random varying block size and stratified by Hinchey classification and center, was performed, and allocation was concealed. The investigators were masked to the allocation until all analyses were completed.1
The primary outcome was the time to functional recovery (resumption of pre-illness work activities) during a 6-month follow-up period. Secondary outcomes included hospital readmission rate; complicated, ongoing, and recurrent diverticulitis; sigmoid resection; other nonsurgical intervention; antibiotic treatment adverse effects; and all-cause mortality.
Continue to: Results
Results. Median recovery time for observational treatment was not inferior to antibiotic treatment (14 days vs 12 days; P=.15; hazard ratio [HR] for functional recovery=0.91; lower limit of 1-sided 95% confidence interval, 0.78). Observation was not inferior to antibiotics for any of the secondary endpoints at 6 and 12 months of follow-up (complicated diverticulitis, 3.8% vs 2.6%, respectively; P=.377), recurrent diverticulitis (3.4% vs 3%; P=.494), readmission (17.6% vs 12%; P=.148), or adverse events (48.5% vs 54.5%; P=.221). Initial hospitalization length of stay was shorter in the observation group (2 vs 3 days; P=.006). The researchers conducted a 24-month telephone follow-up, but no differences from the 12-month follow-up were noted.1
WHAT’S NEW
A study that looks at a true patient-oriented outcome
Previous studies of treatment options for acute uncomplicated diverticulitis looked at short-term outcomes, or at readmission, recurrence, and surgical intervention rate, or requirement for percutaneous drainage.7,8 This study is the first one to look at functional return to work (a true patient-oriented outcome). And it is the only study to look out to 24 months to gauge long-term outcomes with observational treatment.
CAVEATS
Can’t generalize findings to patients with worse forms of diverticulitis
It is worth noting that the findings of this study apply only to the mildest form of CT-proven acute diverticulitis (those patients classified as having Hinchey 1a disease), and is not generalizable to patients with more severe forms. Not enough patients with Hinchey 1b acute diverticulitis were enrolled in the study to reach any conclusions about treatment.
Various guidelines issued outside the United States recommend antibiotics for uncomplicated diverticulitis; however, the American Gastroenterological Association (AGA) indicates that antibiotics should be used selectively.1,9,10 This recommendation was based on an emerging understanding that diverticulitis maybe more inflammatory than infectious in nature. The AGA guideline authors acknowledge that their conclusion was based on low-quality evidence.9
Continuet to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Daniels L, Ünlü Ç, de Korte N, et al, for the Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104:52-61.
2. Wheat CL, Strate LL. Trends in hospitalization for diverticulitis and diverticular bleeding in the United States from 2000 to 2010. Clin Gastroenterol Hepatol. 2016;14:96-103.e1.
3. Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
4. Shabanzadeh DM, Wille-Jørgensen P. Antibiotics for uncomplicated diverticulitis. Cochrane Database Syst Rev. 2012;11:CD009092.
5. Chabok A, Påhlman L, Hjern F, et al. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99:532-539.
6. Klarenbeek BR, de Korte N, van der Peet DL, et al. Review of current classifications for diverticular disease and a translation into clinical practice. Int J Colorectal Dis. 2012;27:207-214.
7. Tandon A, Fretwell VL, Nunes QM, et al. Antibiotics versus no antibiotics in the treatment of acute uncomplicated diverticulitis - a systematic review and meta-analysis. Colorectal Dis. 2018 Jan 11. doi: 10.1111/codi.14013.
8. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57:284-294.
9. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149:1944-1949.
10. Sartelli M, Viale P, Catena F, et al. 2013 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2013;8:3.
1. Daniels L, Ünlü Ç, de Korte N, et al, for the Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104:52-61.
2. Wheat CL, Strate LL. Trends in hospitalization for diverticulitis and diverticular bleeding in the United States from 2000 to 2010. Clin Gastroenterol Hepatol. 2016;14:96-103.e1.
3. Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
4. Shabanzadeh DM, Wille-Jørgensen P. Antibiotics for uncomplicated diverticulitis. Cochrane Database Syst Rev. 2012;11:CD009092.
5. Chabok A, Påhlman L, Hjern F, et al. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99:532-539.
6. Klarenbeek BR, de Korte N, van der Peet DL, et al. Review of current classifications for diverticular disease and a translation into clinical practice. Int J Colorectal Dis. 2012;27:207-214.
7. Tandon A, Fretwell VL, Nunes QM, et al. Antibiotics versus no antibiotics in the treatment of acute uncomplicated diverticulitis - a systematic review and meta-analysis. Colorectal Dis. 2018 Jan 11. doi: 10.1111/codi.14013.
8. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57:284-294.
9. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149:1944-1949.
10. Sartelli M, Viale P, Catena F, et al. 2013 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2013;8:3.
PRACTICE CHANGER
For mild, computed tomography-proven acute diverticulitis, consider observation only instead of antibiotic therapy.
STRENGTH OF RECOMMENDATION
B: Based on a single randomized controlled trial.
Daniels L, Ünlü Ç, de Korte N, et al, for the Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104:52-61.1
Body-wide, pruritic, papular rash • scalp lesion • excoriation • Dx?
THE CASE
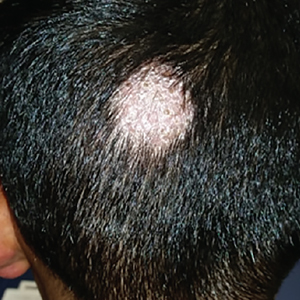
A 7-year-old boy presented with a one-week history of a pruritic rash, which first appeared on his back and continued to spread across his entire body. The patient’s medical history was significant for a scalp lesion (FIGURE 1) that was being treated with oral griseofulvin (started 3 days earlier). He had no history of seasonal allergies, asthma, recent illness, or recent immunizations.
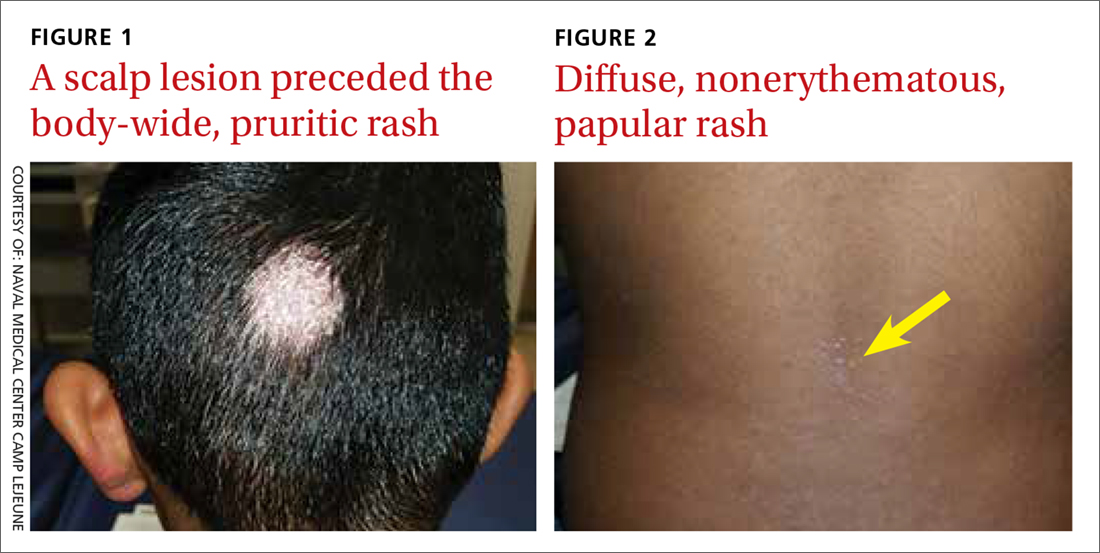
The physical exam was significant for a body-wide, nonerythematous, papular rash (FIGURE 2). There was evidence of excoriation due to itching. No mucosal involvement was appreciated. The remainder of the examination was unremarkable.
QUESTION
Based on the patient’s history and physical exam, which of the following is the most likely diagnosis?
A. Gianotti-Crosti syndrome
B. Atopic dermatitis
C. Dermatophytid reaction
D. Morbilliform drug eruption.
Continue to: THE DIAGNOSIS
THE DIAGNOSIS
The answer is C, dermatophytid reaction.
DISCUSSION
A dermatophytid reaction is a type of id reaction, or autoeczematization. An id reaction is when a localized dermatitis becomes a generalized pruritic eruption.1 In this case, the patient’s dermatitis was the result of a dermatophyte infection (tinea capitis), but an id reaction can also occur in response to noninfectious dermatitides and may be of an atopic, contact, or seborrheic nature.1
Dermatophytid reactions occur in up to 5% of all dermatophyte infections (most commonly tinea pedis) and are proposed to be type IV hypersensitivity reactions to the release of fungal antigens.1 These reactions can occur either before or after the initiation of antifungal treatment. They manifest as symmetric, pruritic, papulovesicular eruptions with fine scaling and commonly affect the face, trunk, extremities, palms, and interdigital spaces.1
What about other possible diagnoses?
Gianotti-Crosti syndrome is an asymptomatic, symmetric, papulovesicular dermatosis that involves the face, limbs, and buttocks of children 2 to 6 years of age.2 The lesions develop in response to a respiratory or gastrointestinal illness.2 They are typically associated with Epstein-Barr virus, hepatitis B, cytomegalovirus, respiratory syncytial virus, and coxsackievirus, but can occur with bacterial infections or following administration of routine immunizations.2
The lesions are self-limited and resolve within 2 months.2 Symptomatic lesions may be treated with oral antihistamines or steroids (topical or systemic).2
Continue to: Atopic dermatitis
Atopic dermatitis is characterized by symmetric involvement of the flexural surfaces of the body with a pruritic, erythematous rash that may have a fine scale.3 It usually manifests prior to 2 years of age, is recurrent, and is commonly associated with allergic rhinitis and asthma.3 Treatment involves trigger avoidance, topical emollients, topical corticosteroids, dilute bleach baths, and topical calcineurin inhibitors.3,4 For patients with significant nocturnal symptoms and sleep loss, oral antihistamines may be helpful.4
Morbilliform drug eruptions are the most common type of dermatologic drug reaction.5 These rashes occur approximately one to 2 weeks after exposure to a causative drug; they consist of pruritic, erythematous papules or macules that start centrally and may spread to the proximal extremities.5 Treatment involves discontinuation of the offending agent. Symptomatic relief may be achieved with oral antihistamines or topical or systemic corticosteroids.5
Treatment of dermatophytid reactions
While the initial impulse in the treatment of a dermatophytid reaction may be to discontinue oral antifungals, these treatments actually help resolve the underlying dermatophyte infection and should be continued. For children with tinea capitis, at least 6 weeks of treatment with an oral antifungal agent is warranted. Medications approved by the US Food and Drug Administration include terbinafine (for patients >4 years of age) and griseofulvin (for patients >2 years of age). Dosages are weight-based. (Fluconazole and itraconazole are not approved for this indication.) Lubricants, topical corticosteroids, and oral antihistamines can be used for acute management of pruritus.1
Our patient was treated successfully with griseofulvin and an oral antihistamine. However, he experienced headaches attributed to griseofulvin and was switched to terbinafine 5 mg/kg/d for 4 weeks. His tinea capitis was resolved at 8 weeks.
CORRESPONDENCE
Richard Temple, MD, CAPT, MC, USN. Department of Family Medicine, Naval Medical Center Camp Lejeune, 100 Brewster Blvd, Camp Lejeune, NC 28547; [email protected].
1. Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:e453-e457.
2. Brandt O, Abeck D, Gianotti R, et al. Gianotti-Crosti syndrome. J Am Acad Dermatol. 2006;54:136-145.
3. Berke R, Singh A, Guralnick M. Atopic dermatitis: an overview. Am Fam Physician. 2012;86:35-42.
4. Eichenfield LF, Boguniewicz M, Simpson EL, et al. Translating atopic dermatitis management guidelines into practice for primary care providers. Pediatrics. 2015;136:554-565.
5. Riedl MA, Casillas AM. Adverse drug reactions: types and treatment options. Am Fam Physician. 2003;68:1781-1790.
THE CASE
A 7-year-old boy presented with a one-week history of a pruritic rash, which first appeared on his back and continued to spread across his entire body. The patient’s medical history was significant for a scalp lesion (FIGURE 1) that was being treated with oral griseofulvin (started 3 days earlier). He had no history of seasonal allergies, asthma, recent illness, or recent immunizations.

The physical exam was significant for a body-wide, nonerythematous, papular rash (FIGURE 2). There was evidence of excoriation due to itching. No mucosal involvement was appreciated. The remainder of the examination was unremarkable.
QUESTION
Based on the patient’s history and physical exam, which of the following is the most likely diagnosis?
A. Gianotti-Crosti syndrome
B. Atopic dermatitis
C. Dermatophytid reaction
D. Morbilliform drug eruption.
Continue to: THE DIAGNOSIS
THE DIAGNOSIS
The answer is C, dermatophytid reaction.
DISCUSSION
A dermatophytid reaction is a type of id reaction, or autoeczematization. An id reaction is when a localized dermatitis becomes a generalized pruritic eruption.1 In this case, the patient’s dermatitis was the result of a dermatophyte infection (tinea capitis), but an id reaction can also occur in response to noninfectious dermatitides and may be of an atopic, contact, or seborrheic nature.1
Dermatophytid reactions occur in up to 5% of all dermatophyte infections (most commonly tinea pedis) and are proposed to be type IV hypersensitivity reactions to the release of fungal antigens.1 These reactions can occur either before or after the initiation of antifungal treatment. They manifest as symmetric, pruritic, papulovesicular eruptions with fine scaling and commonly affect the face, trunk, extremities, palms, and interdigital spaces.1
What about other possible diagnoses?
Gianotti-Crosti syndrome is an asymptomatic, symmetric, papulovesicular dermatosis that involves the face, limbs, and buttocks of children 2 to 6 years of age.2 The lesions develop in response to a respiratory or gastrointestinal illness.2 They are typically associated with Epstein-Barr virus, hepatitis B, cytomegalovirus, respiratory syncytial virus, and coxsackievirus, but can occur with bacterial infections or following administration of routine immunizations.2
The lesions are self-limited and resolve within 2 months.2 Symptomatic lesions may be treated with oral antihistamines or steroids (topical or systemic).2
Continue to: Atopic dermatitis
Atopic dermatitis is characterized by symmetric involvement of the flexural surfaces of the body with a pruritic, erythematous rash that may have a fine scale.3 It usually manifests prior to 2 years of age, is recurrent, and is commonly associated with allergic rhinitis and asthma.3 Treatment involves trigger avoidance, topical emollients, topical corticosteroids, dilute bleach baths, and topical calcineurin inhibitors.3,4 For patients with significant nocturnal symptoms and sleep loss, oral antihistamines may be helpful.4
Morbilliform drug eruptions are the most common type of dermatologic drug reaction.5 These rashes occur approximately one to 2 weeks after exposure to a causative drug; they consist of pruritic, erythematous papules or macules that start centrally and may spread to the proximal extremities.5 Treatment involves discontinuation of the offending agent. Symptomatic relief may be achieved with oral antihistamines or topical or systemic corticosteroids.5
Treatment of dermatophytid reactions
While the initial impulse in the treatment of a dermatophytid reaction may be to discontinue oral antifungals, these treatments actually help resolve the underlying dermatophyte infection and should be continued. For children with tinea capitis, at least 6 weeks of treatment with an oral antifungal agent is warranted. Medications approved by the US Food and Drug Administration include terbinafine (for patients >4 years of age) and griseofulvin (for patients >2 years of age). Dosages are weight-based. (Fluconazole and itraconazole are not approved for this indication.) Lubricants, topical corticosteroids, and oral antihistamines can be used for acute management of pruritus.1
Our patient was treated successfully with griseofulvin and an oral antihistamine. However, he experienced headaches attributed to griseofulvin and was switched to terbinafine 5 mg/kg/d for 4 weeks. His tinea capitis was resolved at 8 weeks.
CORRESPONDENCE
Richard Temple, MD, CAPT, MC, USN. Department of Family Medicine, Naval Medical Center Camp Lejeune, 100 Brewster Blvd, Camp Lejeune, NC 28547; [email protected].
THE CASE
A 7-year-old boy presented with a one-week history of a pruritic rash, which first appeared on his back and continued to spread across his entire body. The patient’s medical history was significant for a scalp lesion (FIGURE 1) that was being treated with oral griseofulvin (started 3 days earlier). He had no history of seasonal allergies, asthma, recent illness, or recent immunizations.

The physical exam was significant for a body-wide, nonerythematous, papular rash (FIGURE 2). There was evidence of excoriation due to itching. No mucosal involvement was appreciated. The remainder of the examination was unremarkable.
QUESTION
Based on the patient’s history and physical exam, which of the following is the most likely diagnosis?
A. Gianotti-Crosti syndrome
B. Atopic dermatitis
C. Dermatophytid reaction
D. Morbilliform drug eruption.
Continue to: THE DIAGNOSIS
THE DIAGNOSIS
The answer is C, dermatophytid reaction.
DISCUSSION
A dermatophytid reaction is a type of id reaction, or autoeczematization. An id reaction is when a localized dermatitis becomes a generalized pruritic eruption.1 In this case, the patient’s dermatitis was the result of a dermatophyte infection (tinea capitis), but an id reaction can also occur in response to noninfectious dermatitides and may be of an atopic, contact, or seborrheic nature.1
Dermatophytid reactions occur in up to 5% of all dermatophyte infections (most commonly tinea pedis) and are proposed to be type IV hypersensitivity reactions to the release of fungal antigens.1 These reactions can occur either before or after the initiation of antifungal treatment. They manifest as symmetric, pruritic, papulovesicular eruptions with fine scaling and commonly affect the face, trunk, extremities, palms, and interdigital spaces.1
What about other possible diagnoses?
Gianotti-Crosti syndrome is an asymptomatic, symmetric, papulovesicular dermatosis that involves the face, limbs, and buttocks of children 2 to 6 years of age.2 The lesions develop in response to a respiratory or gastrointestinal illness.2 They are typically associated with Epstein-Barr virus, hepatitis B, cytomegalovirus, respiratory syncytial virus, and coxsackievirus, but can occur with bacterial infections or following administration of routine immunizations.2
The lesions are self-limited and resolve within 2 months.2 Symptomatic lesions may be treated with oral antihistamines or steroids (topical or systemic).2
Continue to: Atopic dermatitis
Atopic dermatitis is characterized by symmetric involvement of the flexural surfaces of the body with a pruritic, erythematous rash that may have a fine scale.3 It usually manifests prior to 2 years of age, is recurrent, and is commonly associated with allergic rhinitis and asthma.3 Treatment involves trigger avoidance, topical emollients, topical corticosteroids, dilute bleach baths, and topical calcineurin inhibitors.3,4 For patients with significant nocturnal symptoms and sleep loss, oral antihistamines may be helpful.4
Morbilliform drug eruptions are the most common type of dermatologic drug reaction.5 These rashes occur approximately one to 2 weeks after exposure to a causative drug; they consist of pruritic, erythematous papules or macules that start centrally and may spread to the proximal extremities.5 Treatment involves discontinuation of the offending agent. Symptomatic relief may be achieved with oral antihistamines or topical or systemic corticosteroids.5
Treatment of dermatophytid reactions
While the initial impulse in the treatment of a dermatophytid reaction may be to discontinue oral antifungals, these treatments actually help resolve the underlying dermatophyte infection and should be continued. For children with tinea capitis, at least 6 weeks of treatment with an oral antifungal agent is warranted. Medications approved by the US Food and Drug Administration include terbinafine (for patients >4 years of age) and griseofulvin (for patients >2 years of age). Dosages are weight-based. (Fluconazole and itraconazole are not approved for this indication.) Lubricants, topical corticosteroids, and oral antihistamines can be used for acute management of pruritus.1
Our patient was treated successfully with griseofulvin and an oral antihistamine. However, he experienced headaches attributed to griseofulvin and was switched to terbinafine 5 mg/kg/d for 4 weeks. His tinea capitis was resolved at 8 weeks.
CORRESPONDENCE
Richard Temple, MD, CAPT, MC, USN. Department of Family Medicine, Naval Medical Center Camp Lejeune, 100 Brewster Blvd, Camp Lejeune, NC 28547; [email protected].
1. Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:e453-e457.
2. Brandt O, Abeck D, Gianotti R, et al. Gianotti-Crosti syndrome. J Am Acad Dermatol. 2006;54:136-145.
3. Berke R, Singh A, Guralnick M. Atopic dermatitis: an overview. Am Fam Physician. 2012;86:35-42.
4. Eichenfield LF, Boguniewicz M, Simpson EL, et al. Translating atopic dermatitis management guidelines into practice for primary care providers. Pediatrics. 2015;136:554-565.
5. Riedl MA, Casillas AM. Adverse drug reactions: types and treatment options. Am Fam Physician. 2003;68:1781-1790.
1. Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:e453-e457.
2. Brandt O, Abeck D, Gianotti R, et al. Gianotti-Crosti syndrome. J Am Acad Dermatol. 2006;54:136-145.
3. Berke R, Singh A, Guralnick M. Atopic dermatitis: an overview. Am Fam Physician. 2012;86:35-42.
4. Eichenfield LF, Boguniewicz M, Simpson EL, et al. Translating atopic dermatitis management guidelines into practice for primary care providers. Pediatrics. 2015;136:554-565.
5. Riedl MA, Casillas AM. Adverse drug reactions: types and treatment options. Am Fam Physician. 2003;68:1781-1790.
Dietary recommendations for patients with diabetes
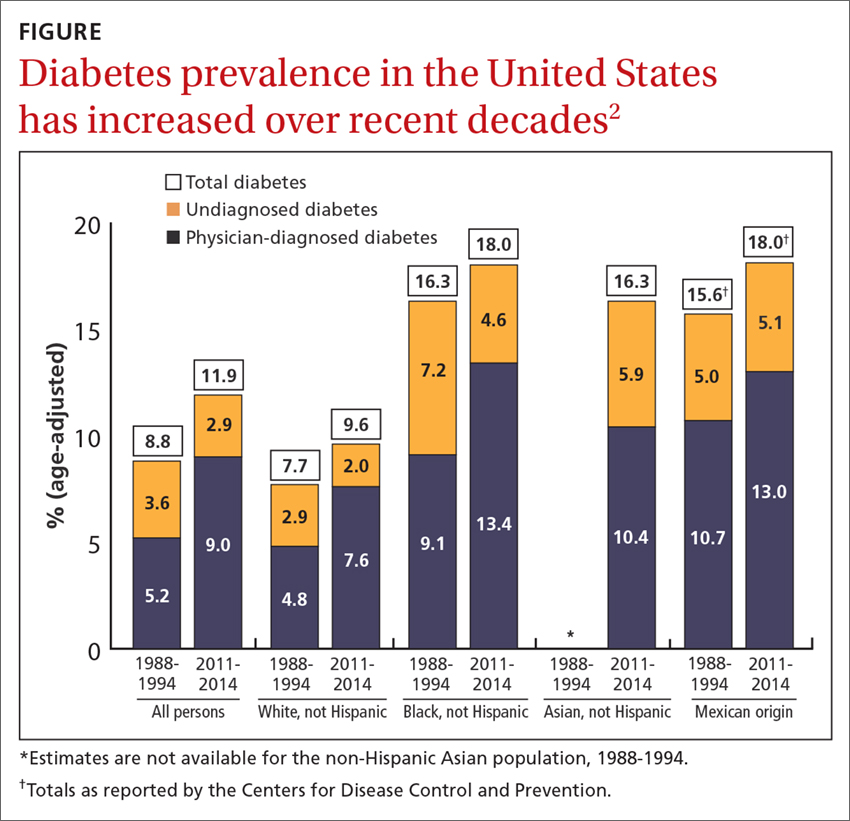
Diabetes affects approximately 9.4% of the US population (more than 30 million people),1 and it is one of the most common conditions treated by family physicians. Additionally, more than 80 million Americans meet the criteria for prediabetes.1 The prevalence of diabetes has increased in adults between the time periods 1988-1994 and 2011-2014, and it varies by race and ethnicity, with the highest prevalence, 18%, among African Americans and Mexican Americans, and the lowest, 9.6%, among non-Hispanic whites (FIGURE).2
Diet is the cornerstone of diabetes treatment
The foundation of a comprehensive management plan for type 2 diabetes mellitus (T2DM) is an appropriate diet. A growing body of evidence shows that a well-structured diet is important in controlling diabetes, delaying or preventing the onset of diabetes, and, in some instances, contributing to its remission. Diabetes UK, the United Kingdom’s equivalent of the American Diabetes Association (ADA), recently updated its clinical guideline for physicians and patients on the role of nutrition in managing and preventing diabetes, and it is consistent with one published by the ADA in 2013.3,4
The Diabetes UK guideline is the result of an evidence-based process that meets the standards recommended by the National Academy of Medicine (previously the Institute of Medicine): a systematic review and formal assessment of the quality of the evidence, and recommendations based on the highest quality evidence available, with the level of evidence stated for each recommendation.5 Assessing the level of evidence and determining the strengths of recommendations were done using the Grades of Recommendation Assessment, Development, and Evaluation (GRADE) system, which uses an approach similar to that of the Strength of Recommendation Taxonomy (SORT).
What, and what not, to focus on. The first set of recommendations states that everyone with, or at risk for, diabetes should receive structured, personalized, and ongoing nutritional advice from a dietician who is coordinated with their clinical care. Nutritional advice should focus on the quality and quantity of food, not on specific nutrients (fat and carbohydrates), since there is no good evidence on what proportion of such nutrients is optimal. And it should be tailored to the culture and eating preferences of the patient.
The type of diet with the strongest evidence base for preventing T2DM is a Mediterranean diet, which is supported by level-4, high-quality evidence. Important aspects of a Mediterranean diet are the regular consumption of nuts, whole grains, fruits, and vegetables; use of olive oil instead of butter; and favoring fish over red meat.6 Other dietary patterns associated with reduced risk but supported only by level-2, low-quality evidence, include Dietary Approaches to Stop Hypertension (DASH), vegetarian, vegan, and Nordic healthy diets. Moderate carbohydrate restriction is supported only by level-1, very low-quality evidence.
The UK guideline, too, recommends preferentially eating whole grains, fruits, and green leafy vegetables, as well as yogurt, cheese, tea, and coffee. And it advises reducing consumption of red processed meats, potatoes (especially French fries), sugar-sweetened beverages, and refined carbohydrates. However, these specific food preferences are supported only by low-level evidence.
Plant stanols and plant sterols are found in a variety of plant foods such as cereals, vegetable oils, seeds, and nuts, and are now being added to some food products. (For more on plant stanols and plant sterols.) They have a chemical structure similar to cholesterol and reduce the intestinal absorption of cholesterol, thereby lowering total serum cholesterol and LDL-cholesterol. Both Diabetes UK and the ADA recommend 2 to 3 grams of stanols/sterols per day.
Continue to: Alcohol intake
Alcohol intake. And what about alcohol intake in those with T2DM? Once again, both guidelines are in concert by stating that alcohol use in those with diabetes should be moderate, defined by the ADA as one or fewer drinks/d for women and 2 or fewer for men.
Weight loss and exercise are important, too. Those who are overweight or obese with T2DM can improve glycemic control with a 5% weight loss achieved by reducing caloric intake and by increasing energy expenditure with 150 minutes of moderate physical activity per week over at least 3 days.3 This recommendation is supported by high-quality evidence.
A 15-kg weight loss is recommended for those attempting diabetes remission (supported by moderate-level evidence).3 One small study in the United Kingdom found that more than half of those with T2DM could achieve remission with weight loss of 10 kg or more; 86% with weight loss of 15 kg or more.7 The Diabetes UK guideline panel rated this as having moderate-level evidence.
The bottom line. Diet and exercise are key interventions for the prevention and treatment of diabetes and can lead to remission if sufficient weight loss is achieved. To achieve and maintain an optimal diet, patients need individualized professional advice and followup. The evidence base for nutritional advice is growing and can be used to improve the quality of these patient-provider interactions.
1. America Diabetes Association. Statistics About Diabetes. http://www.diabetes.org/diabetes-basics/statistics/. Accessed May 13, 2018.
2. CDC. National Center for Health Statistics. Health, United States, 2016. Available at: https://www.cdc.gov/nchs/data/hus/hus16.pdf. Accessed May 21, 2018.
3. Dyson PA, Twenefour D, Breen C, et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2018;35:541-547.
4. Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821-3842.
5. IOM (Institute of Medicine). 2011. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press.
6. Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222.
7. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551.
Diabetes affects approximately 9.4% of the US population (more than 30 million people),1 and it is one of the most common conditions treated by family physicians. Additionally, more than 80 million Americans meet the criteria for prediabetes.1 The prevalence of diabetes has increased in adults between the time periods 1988-1994 and 2011-2014, and it varies by race and ethnicity, with the highest prevalence, 18%, among African Americans and Mexican Americans, and the lowest, 9.6%, among non-Hispanic whites (FIGURE).2

Diet is the cornerstone of diabetes treatment
The foundation of a comprehensive management plan for type 2 diabetes mellitus (T2DM) is an appropriate diet. A growing body of evidence shows that a well-structured diet is important in controlling diabetes, delaying or preventing the onset of diabetes, and, in some instances, contributing to its remission. Diabetes UK, the United Kingdom’s equivalent of the American Diabetes Association (ADA), recently updated its clinical guideline for physicians and patients on the role of nutrition in managing and preventing diabetes, and it is consistent with one published by the ADA in 2013.3,4
The Diabetes UK guideline is the result of an evidence-based process that meets the standards recommended by the National Academy of Medicine (previously the Institute of Medicine): a systematic review and formal assessment of the quality of the evidence, and recommendations based on the highest quality evidence available, with the level of evidence stated for each recommendation.5 Assessing the level of evidence and determining the strengths of recommendations were done using the Grades of Recommendation Assessment, Development, and Evaluation (GRADE) system, which uses an approach similar to that of the Strength of Recommendation Taxonomy (SORT).
What, and what not, to focus on. The first set of recommendations states that everyone with, or at risk for, diabetes should receive structured, personalized, and ongoing nutritional advice from a dietician who is coordinated with their clinical care. Nutritional advice should focus on the quality and quantity of food, not on specific nutrients (fat and carbohydrates), since there is no good evidence on what proportion of such nutrients is optimal. And it should be tailored to the culture and eating preferences of the patient.
The type of diet with the strongest evidence base for preventing T2DM is a Mediterranean diet, which is supported by level-4, high-quality evidence. Important aspects of a Mediterranean diet are the regular consumption of nuts, whole grains, fruits, and vegetables; use of olive oil instead of butter; and favoring fish over red meat.6 Other dietary patterns associated with reduced risk but supported only by level-2, low-quality evidence, include Dietary Approaches to Stop Hypertension (DASH), vegetarian, vegan, and Nordic healthy diets. Moderate carbohydrate restriction is supported only by level-1, very low-quality evidence.
The UK guideline, too, recommends preferentially eating whole grains, fruits, and green leafy vegetables, as well as yogurt, cheese, tea, and coffee. And it advises reducing consumption of red processed meats, potatoes (especially French fries), sugar-sweetened beverages, and refined carbohydrates. However, these specific food preferences are supported only by low-level evidence.
Plant stanols and plant sterols are found in a variety of plant foods such as cereals, vegetable oils, seeds, and nuts, and are now being added to some food products. (For more on plant stanols and plant sterols.) They have a chemical structure similar to cholesterol and reduce the intestinal absorption of cholesterol, thereby lowering total serum cholesterol and LDL-cholesterol. Both Diabetes UK and the ADA recommend 2 to 3 grams of stanols/sterols per day.
Continue to: Alcohol intake
Alcohol intake. And what about alcohol intake in those with T2DM? Once again, both guidelines are in concert by stating that alcohol use in those with diabetes should be moderate, defined by the ADA as one or fewer drinks/d for women and 2 or fewer for men.
Weight loss and exercise are important, too. Those who are overweight or obese with T2DM can improve glycemic control with a 5% weight loss achieved by reducing caloric intake and by increasing energy expenditure with 150 minutes of moderate physical activity per week over at least 3 days.3 This recommendation is supported by high-quality evidence.
A 15-kg weight loss is recommended for those attempting diabetes remission (supported by moderate-level evidence).3 One small study in the United Kingdom found that more than half of those with T2DM could achieve remission with weight loss of 10 kg or more; 86% with weight loss of 15 kg or more.7 The Diabetes UK guideline panel rated this as having moderate-level evidence.
The bottom line. Diet and exercise are key interventions for the prevention and treatment of diabetes and can lead to remission if sufficient weight loss is achieved. To achieve and maintain an optimal diet, patients need individualized professional advice and followup. The evidence base for nutritional advice is growing and can be used to improve the quality of these patient-provider interactions.
Diabetes affects approximately 9.4% of the US population (more than 30 million people),1 and it is one of the most common conditions treated by family physicians. Additionally, more than 80 million Americans meet the criteria for prediabetes.1 The prevalence of diabetes has increased in adults between the time periods 1988-1994 and 2011-2014, and it varies by race and ethnicity, with the highest prevalence, 18%, among African Americans and Mexican Americans, and the lowest, 9.6%, among non-Hispanic whites (FIGURE).2

Diet is the cornerstone of diabetes treatment
The foundation of a comprehensive management plan for type 2 diabetes mellitus (T2DM) is an appropriate diet. A growing body of evidence shows that a well-structured diet is important in controlling diabetes, delaying or preventing the onset of diabetes, and, in some instances, contributing to its remission. Diabetes UK, the United Kingdom’s equivalent of the American Diabetes Association (ADA), recently updated its clinical guideline for physicians and patients on the role of nutrition in managing and preventing diabetes, and it is consistent with one published by the ADA in 2013.3,4
The Diabetes UK guideline is the result of an evidence-based process that meets the standards recommended by the National Academy of Medicine (previously the Institute of Medicine): a systematic review and formal assessment of the quality of the evidence, and recommendations based on the highest quality evidence available, with the level of evidence stated for each recommendation.5 Assessing the level of evidence and determining the strengths of recommendations were done using the Grades of Recommendation Assessment, Development, and Evaluation (GRADE) system, which uses an approach similar to that of the Strength of Recommendation Taxonomy (SORT).
What, and what not, to focus on. The first set of recommendations states that everyone with, or at risk for, diabetes should receive structured, personalized, and ongoing nutritional advice from a dietician who is coordinated with their clinical care. Nutritional advice should focus on the quality and quantity of food, not on specific nutrients (fat and carbohydrates), since there is no good evidence on what proportion of such nutrients is optimal. And it should be tailored to the culture and eating preferences of the patient.
The type of diet with the strongest evidence base for preventing T2DM is a Mediterranean diet, which is supported by level-4, high-quality evidence. Important aspects of a Mediterranean diet are the regular consumption of nuts, whole grains, fruits, and vegetables; use of olive oil instead of butter; and favoring fish over red meat.6 Other dietary patterns associated with reduced risk but supported only by level-2, low-quality evidence, include Dietary Approaches to Stop Hypertension (DASH), vegetarian, vegan, and Nordic healthy diets. Moderate carbohydrate restriction is supported only by level-1, very low-quality evidence.
The UK guideline, too, recommends preferentially eating whole grains, fruits, and green leafy vegetables, as well as yogurt, cheese, tea, and coffee. And it advises reducing consumption of red processed meats, potatoes (especially French fries), sugar-sweetened beverages, and refined carbohydrates. However, these specific food preferences are supported only by low-level evidence.
Plant stanols and plant sterols are found in a variety of plant foods such as cereals, vegetable oils, seeds, and nuts, and are now being added to some food products. (For more on plant stanols and plant sterols.) They have a chemical structure similar to cholesterol and reduce the intestinal absorption of cholesterol, thereby lowering total serum cholesterol and LDL-cholesterol. Both Diabetes UK and the ADA recommend 2 to 3 grams of stanols/sterols per day.
Continue to: Alcohol intake
Alcohol intake. And what about alcohol intake in those with T2DM? Once again, both guidelines are in concert by stating that alcohol use in those with diabetes should be moderate, defined by the ADA as one or fewer drinks/d for women and 2 or fewer for men.
Weight loss and exercise are important, too. Those who are overweight or obese with T2DM can improve glycemic control with a 5% weight loss achieved by reducing caloric intake and by increasing energy expenditure with 150 minutes of moderate physical activity per week over at least 3 days.3 This recommendation is supported by high-quality evidence.
A 15-kg weight loss is recommended for those attempting diabetes remission (supported by moderate-level evidence).3 One small study in the United Kingdom found that more than half of those with T2DM could achieve remission with weight loss of 10 kg or more; 86% with weight loss of 15 kg or more.7 The Diabetes UK guideline panel rated this as having moderate-level evidence.
The bottom line. Diet and exercise are key interventions for the prevention and treatment of diabetes and can lead to remission if sufficient weight loss is achieved. To achieve and maintain an optimal diet, patients need individualized professional advice and followup. The evidence base for nutritional advice is growing and can be used to improve the quality of these patient-provider interactions.
1. America Diabetes Association. Statistics About Diabetes. http://www.diabetes.org/diabetes-basics/statistics/. Accessed May 13, 2018.
2. CDC. National Center for Health Statistics. Health, United States, 2016. Available at: https://www.cdc.gov/nchs/data/hus/hus16.pdf. Accessed May 21, 2018.
3. Dyson PA, Twenefour D, Breen C, et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2018;35:541-547.
4. Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821-3842.
5. IOM (Institute of Medicine). 2011. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press.
6. Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222.
7. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551.
1. America Diabetes Association. Statistics About Diabetes. http://www.diabetes.org/diabetes-basics/statistics/. Accessed May 13, 2018.
2. CDC. National Center for Health Statistics. Health, United States, 2016. Available at: https://www.cdc.gov/nchs/data/hus/hus16.pdf. Accessed May 21, 2018.
3. Dyson PA, Twenefour D, Breen C, et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2018;35:541-547.
4. Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821-3842.
5. IOM (Institute of Medicine). 2011. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press.
6. Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222.
7. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551.
Gun addiction
I’d like to add several comments to Dr. John Hickner’s editorial, “We need to treat gun violence like an epidemic” (J Fam Pract. 2018;67:198).
First, when I hear gun rights advocates (GRAs) discuss the shooting deaths of innocent fellow Americans, I hear the same type of language that I hear from people with substance, tobacco, or alcohol use disorders. They blame everyone else rather than looking at themselves and what their love of weapons does to those around them. When confronted with ever-increasing numbers of gun-related deaths, they say, “Guns don’t kill people; people kill people.” But when used in the way they were intended to be used, guns—especially semi-automatic weapons—will maim or kill people.
And GRAs will not give up their gun rights—even if it means that their friends, neighbors, or relatives might die. GRAs ignore the unalienable rights described in the Declaration of Independence that guarantee “life, liberty, and the pursuit of happiness”—rights that are potentially annihilated when a person is the target of a loaded gun.
Because GRAs will not give up their weapons voluntarily, the only way to escape the continuously widening web of gun violence is to repeal the Second Amendment and allow local communities to vote either in favor or against gun control, setting whatever limits they deem appropriate on gun possession. Repealing the Second Amendment will not eliminate the killing of innocent Americans, but hopefully it will reduce the number of people who die from gun violence.
W.E. Feeman Jr., MD
Bowling Green, Ohio
I’d like to add several comments to Dr. John Hickner’s editorial, “We need to treat gun violence like an epidemic” (J Fam Pract. 2018;67:198).
First, when I hear gun rights advocates (GRAs) discuss the shooting deaths of innocent fellow Americans, I hear the same type of language that I hear from people with substance, tobacco, or alcohol use disorders. They blame everyone else rather than looking at themselves and what their love of weapons does to those around them. When confronted with ever-increasing numbers of gun-related deaths, they say, “Guns don’t kill people; people kill people.” But when used in the way they were intended to be used, guns—especially semi-automatic weapons—will maim or kill people.
And GRAs will not give up their gun rights—even if it means that their friends, neighbors, or relatives might die. GRAs ignore the unalienable rights described in the Declaration of Independence that guarantee “life, liberty, and the pursuit of happiness”—rights that are potentially annihilated when a person is the target of a loaded gun.
Because GRAs will not give up their weapons voluntarily, the only way to escape the continuously widening web of gun violence is to repeal the Second Amendment and allow local communities to vote either in favor or against gun control, setting whatever limits they deem appropriate on gun possession. Repealing the Second Amendment will not eliminate the killing of innocent Americans, but hopefully it will reduce the number of people who die from gun violence.
W.E. Feeman Jr., MD
Bowling Green, Ohio
I’d like to add several comments to Dr. John Hickner’s editorial, “We need to treat gun violence like an epidemic” (J Fam Pract. 2018;67:198).
First, when I hear gun rights advocates (GRAs) discuss the shooting deaths of innocent fellow Americans, I hear the same type of language that I hear from people with substance, tobacco, or alcohol use disorders. They blame everyone else rather than looking at themselves and what their love of weapons does to those around them. When confronted with ever-increasing numbers of gun-related deaths, they say, “Guns don’t kill people; people kill people.” But when used in the way they were intended to be used, guns—especially semi-automatic weapons—will maim or kill people.
And GRAs will not give up their gun rights—even if it means that their friends, neighbors, or relatives might die. GRAs ignore the unalienable rights described in the Declaration of Independence that guarantee “life, liberty, and the pursuit of happiness”—rights that are potentially annihilated when a person is the target of a loaded gun.
Because GRAs will not give up their weapons voluntarily, the only way to escape the continuously widening web of gun violence is to repeal the Second Amendment and allow local communities to vote either in favor or against gun control, setting whatever limits they deem appropriate on gun possession. Repealing the Second Amendment will not eliminate the killing of innocent Americans, but hopefully it will reduce the number of people who die from gun violence.
W.E. Feeman Jr., MD
Bowling Green, Ohio
The problem with blood pressure guidelines
In this issue of JFP, MacLaughlin and colleagues echo the recommendations of the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guidelines on high blood pressure (BP).1
This guideline, however, is not endorsed by primary care organizations. Both the American College of Physicians (ACP) and the American Academy of Family Physicians (AAFP) released their own evidence-based guideline in 2017.2 (The European Society of Cardiology also declined to endorse the ACC/AHA guideline.3) So how do we make sense of the different recommendations? And how do we decide which guideline is most trustworthy?4
Evidence based vs evidence informed
Both guideline writing groups are highly respected and affiliated with influential organizations. Both claim their guidelines are based on scientific evidence and are crafted with the intention to improve health. The 2 guidelines, however, differ in their fidelity to the evidence-based process and in their willingness to generalize disease-centered interventions to non-diseased populations.
Evidence-based guidelines differ from evidence-informed guidelines. Evidence-based guidelines have an established methodology that includes well-designed specific critical questions, a literature review with clearly defined inclusion and exclusion criteria, an evidence grading system, and a systematic approach to creating recommendations. Evidence-based guidelines are limited in scope and are often controversial because the evidence may not comport with the narrative promulgated by experts. Indeed, the controversy surrounding the 2014 Eighth Joint National Committee (JNC 8) guideline that I co-chaired focused on the one recommendation with the strongest evidence.5,6
Comprehensive guidelines written by experts are by their very nature evidence-informed guidelines. The ACC/AHA guidelines are comprehensive, providing a panoply of recommendations. When such guidelines are written for primary care, the generalizability of specialized disease-centered knowledge is limited,7 and the risk of overdiagnosis and overtreatment rises,8 especially when the primary care community is not invited as equal partners in the guideline development process.
Trustworthy guidelines require management of conflicts of interests. A hidden contributor to guideline panel membership and content is organizational sponsorship. Advocacy organizations and specialty societies have governing boards that have fiduciary responsibilities to their organizations. Such responsibilities may supersede the responsibilities of guideline panel members and influence content. JNC 8’s appointed panel members chose to release the 2014 guideline independently, so as not to cede editorial authority to governing boards of associations with potential conflicts of interest.
As Paul Frame said, “An ounce of prevention is a ton of work.”9
Dr. Frame, a family medicine pioneer who applied evidence-based medicine to preventive practice, encouraged us to ask critical questions that must be supported by scientific evidence before implementing these practices in healthy populations.10 The ACC/AHA guidelines advocate recommendations based on untested assumptions: that improved health results from earlier “diagnosis” and disease labeling of individuals with risks (healthy patients), and that such patients should receive aggressive “prevention” with daily and lifelong medications requiring physician monitoring.11 To support their new diagnostic standards, the authors cite similar relative risk (RR) reductions (an outcome-based measure), while discounting the smaller absolute risk (AR) reductions (a population-based measure) in studies supporting lower BP goals.
Continue to: Let's examine what this means
Let’s examine what this means
In 1967, a study of 143 hypertensive patients showed that treating high BP (average diastolic BP between 115 and 129 mm Hg) dramatically improved important health outcomes.12 The number needed to treat (NNT) after about 1.5 years showed that for every 1.4 people treated, 1 benefited.8 This is strong and effective medicine.
Successive randomized controlled trials of lower BP goals showed consistent RR reductions; however, AR reductions were much lower, reflecting a higher NNT.8 To prove BP-lowering benefits were not a random effect, higher numbers of participants were needed (SPRINT required over 9300 participants).13 The AR reduction in SPRINT was 1.6% (meaning no benefit was seen in 98.4% receiving the intensive intervention). One participant with high cardiovascular disease risk benefited for every 63 subjects given the intensive therapy compared with usual care (BP goal of 120 mm Hg vs 140 mm Hg).13,14 The researchers noted serious harms in 1 of 22 subjects treated. Treating younger patients to lower BP goals labels healthy people with risk factors as “sick” and commits them to lifelong medications. It exposes them to more frequent harms than benefits. For healthy patients who are unlikely to benefit from taking more antihypertensive medication, these harms matter.
Interpreting the benefits of BP Tx when the benefit to individuals appears small
If only there were a biomarker that could tell us who is most likely to benefit from antihypertensive medication treatment, FPs could ensure that the correct patients are treated. The ACP/AAFP guideline points the way. There is a biomarker, and it is called BP. Systolic BP above 150 mm Hg signals urgency to treat with medications.
A call to advocate. We must all advocate for better guideline processes. The status quo in guideline development and its reliance on special interest funding requires ongoing vigilance to advocate on behalf of our patients. High-value medical care is expensive and hard work. When it is applied to the wrong people at the wrong time, we don’t deliver on our promises.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
2. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430-437.
3. Phend C. Europe stands pat on hypertension thresholds. ESC doesn’t follow ACC/AHA diagnotic cutoff, focuses on control rates. Medpage Today. Available at: https://www.medpagetoday.com/cardiology/hypertension/73384?xid=NL_breakingnews_2018-06-09&eun=g1206318d0r&utm_source=Sailthru&utm_medium=email&utm_campaign=BreakingNews_060918&utm_term=Breaking%20News%20Targeted. Accessed June 19, 2018.
4. Institute of Medicine (US). Committee on Standards for Developing Trustworthy Clinical Practice Guidelines; eds, Graham R, Mancher M, Miller Wolman D, et al. Clinical Practice Guidelines We Can Trust. Washington, DC: National Academies Press; 2011.
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
6. Wright JT Jr., Fine LJ, Lackland DT, et al. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499-503.
7. Graham R, James P, Cowan T. Are clinical practice guidelines valid for primary care? J Clin Epidemiol. 2000;53:949-954.
8. Welch HG, Schwartz LM, Woloshin S. Overdiagnosed: Making People Sick in the Pursuit of Health. Boston, Mass: Beacon Press; 2011.
9. Clancy CM, Kamerow DB. Evidence-based medicine meets cost-effectiveness analysis. JAMA. 1996;276:329-330.
10. Frame PS. A critical review of adult health maintenance. Part 1: Prevention of atherosclerotic diseases. J Fam Pract. 1986;22:341-346.
11. Starfield B, Hyde Jervas J, Heath I. Glossary: the concept of prevention: a good idea gone astray? J Epidemiol Community Health. 2008;62:580-583.
12. Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA. 1967;202:1028-1034.
13. Wright JT Jr., Whelton PK, Reboussin DM. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2016;374:2294.
14. Ortiz E, James PA. Let’s not SPRINT to judgment about new blood pressure goals. Ann Intern Med. 2016;164:692-693.
In this issue of JFP, MacLaughlin and colleagues echo the recommendations of the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guidelines on high blood pressure (BP).1
This guideline, however, is not endorsed by primary care organizations. Both the American College of Physicians (ACP) and the American Academy of Family Physicians (AAFP) released their own evidence-based guideline in 2017.2 (The European Society of Cardiology also declined to endorse the ACC/AHA guideline.3) So how do we make sense of the different recommendations? And how do we decide which guideline is most trustworthy?4
Evidence based vs evidence informed
Both guideline writing groups are highly respected and affiliated with influential organizations. Both claim their guidelines are based on scientific evidence and are crafted with the intention to improve health. The 2 guidelines, however, differ in their fidelity to the evidence-based process and in their willingness to generalize disease-centered interventions to non-diseased populations.
Evidence-based guidelines differ from evidence-informed guidelines. Evidence-based guidelines have an established methodology that includes well-designed specific critical questions, a literature review with clearly defined inclusion and exclusion criteria, an evidence grading system, and a systematic approach to creating recommendations. Evidence-based guidelines are limited in scope and are often controversial because the evidence may not comport with the narrative promulgated by experts. Indeed, the controversy surrounding the 2014 Eighth Joint National Committee (JNC 8) guideline that I co-chaired focused on the one recommendation with the strongest evidence.5,6
Comprehensive guidelines written by experts are by their very nature evidence-informed guidelines. The ACC/AHA guidelines are comprehensive, providing a panoply of recommendations. When such guidelines are written for primary care, the generalizability of specialized disease-centered knowledge is limited,7 and the risk of overdiagnosis and overtreatment rises,8 especially when the primary care community is not invited as equal partners in the guideline development process.
Trustworthy guidelines require management of conflicts of interests. A hidden contributor to guideline panel membership and content is organizational sponsorship. Advocacy organizations and specialty societies have governing boards that have fiduciary responsibilities to their organizations. Such responsibilities may supersede the responsibilities of guideline panel members and influence content. JNC 8’s appointed panel members chose to release the 2014 guideline independently, so as not to cede editorial authority to governing boards of associations with potential conflicts of interest.
As Paul Frame said, “An ounce of prevention is a ton of work.”9
Dr. Frame, a family medicine pioneer who applied evidence-based medicine to preventive practice, encouraged us to ask critical questions that must be supported by scientific evidence before implementing these practices in healthy populations.10 The ACC/AHA guidelines advocate recommendations based on untested assumptions: that improved health results from earlier “diagnosis” and disease labeling of individuals with risks (healthy patients), and that such patients should receive aggressive “prevention” with daily and lifelong medications requiring physician monitoring.11 To support their new diagnostic standards, the authors cite similar relative risk (RR) reductions (an outcome-based measure), while discounting the smaller absolute risk (AR) reductions (a population-based measure) in studies supporting lower BP goals.
Continue to: Let's examine what this means
Let’s examine what this means
In 1967, a study of 143 hypertensive patients showed that treating high BP (average diastolic BP between 115 and 129 mm Hg) dramatically improved important health outcomes.12 The number needed to treat (NNT) after about 1.5 years showed that for every 1.4 people treated, 1 benefited.8 This is strong and effective medicine.
Successive randomized controlled trials of lower BP goals showed consistent RR reductions; however, AR reductions were much lower, reflecting a higher NNT.8 To prove BP-lowering benefits were not a random effect, higher numbers of participants were needed (SPRINT required over 9300 participants).13 The AR reduction in SPRINT was 1.6% (meaning no benefit was seen in 98.4% receiving the intensive intervention). One participant with high cardiovascular disease risk benefited for every 63 subjects given the intensive therapy compared with usual care (BP goal of 120 mm Hg vs 140 mm Hg).13,14 The researchers noted serious harms in 1 of 22 subjects treated. Treating younger patients to lower BP goals labels healthy people with risk factors as “sick” and commits them to lifelong medications. It exposes them to more frequent harms than benefits. For healthy patients who are unlikely to benefit from taking more antihypertensive medication, these harms matter.
Interpreting the benefits of BP Tx when the benefit to individuals appears small
If only there were a biomarker that could tell us who is most likely to benefit from antihypertensive medication treatment, FPs could ensure that the correct patients are treated. The ACP/AAFP guideline points the way. There is a biomarker, and it is called BP. Systolic BP above 150 mm Hg signals urgency to treat with medications.
A call to advocate. We must all advocate for better guideline processes. The status quo in guideline development and its reliance on special interest funding requires ongoing vigilance to advocate on behalf of our patients. High-value medical care is expensive and hard work. When it is applied to the wrong people at the wrong time, we don’t deliver on our promises.
In this issue of JFP, MacLaughlin and colleagues echo the recommendations of the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guidelines on high blood pressure (BP).1
This guideline, however, is not endorsed by primary care organizations. Both the American College of Physicians (ACP) and the American Academy of Family Physicians (AAFP) released their own evidence-based guideline in 2017.2 (The European Society of Cardiology also declined to endorse the ACC/AHA guideline.3) So how do we make sense of the different recommendations? And how do we decide which guideline is most trustworthy?4
Evidence based vs evidence informed
Both guideline writing groups are highly respected and affiliated with influential organizations. Both claim their guidelines are based on scientific evidence and are crafted with the intention to improve health. The 2 guidelines, however, differ in their fidelity to the evidence-based process and in their willingness to generalize disease-centered interventions to non-diseased populations.
Evidence-based guidelines differ from evidence-informed guidelines. Evidence-based guidelines have an established methodology that includes well-designed specific critical questions, a literature review with clearly defined inclusion and exclusion criteria, an evidence grading system, and a systematic approach to creating recommendations. Evidence-based guidelines are limited in scope and are often controversial because the evidence may not comport with the narrative promulgated by experts. Indeed, the controversy surrounding the 2014 Eighth Joint National Committee (JNC 8) guideline that I co-chaired focused on the one recommendation with the strongest evidence.5,6
Comprehensive guidelines written by experts are by their very nature evidence-informed guidelines. The ACC/AHA guidelines are comprehensive, providing a panoply of recommendations. When such guidelines are written for primary care, the generalizability of specialized disease-centered knowledge is limited,7 and the risk of overdiagnosis and overtreatment rises,8 especially when the primary care community is not invited as equal partners in the guideline development process.
Trustworthy guidelines require management of conflicts of interests. A hidden contributor to guideline panel membership and content is organizational sponsorship. Advocacy organizations and specialty societies have governing boards that have fiduciary responsibilities to their organizations. Such responsibilities may supersede the responsibilities of guideline panel members and influence content. JNC 8’s appointed panel members chose to release the 2014 guideline independently, so as not to cede editorial authority to governing boards of associations with potential conflicts of interest.
As Paul Frame said, “An ounce of prevention is a ton of work.”9
Dr. Frame, a family medicine pioneer who applied evidence-based medicine to preventive practice, encouraged us to ask critical questions that must be supported by scientific evidence before implementing these practices in healthy populations.10 The ACC/AHA guidelines advocate recommendations based on untested assumptions: that improved health results from earlier “diagnosis” and disease labeling of individuals with risks (healthy patients), and that such patients should receive aggressive “prevention” with daily and lifelong medications requiring physician monitoring.11 To support their new diagnostic standards, the authors cite similar relative risk (RR) reductions (an outcome-based measure), while discounting the smaller absolute risk (AR) reductions (a population-based measure) in studies supporting lower BP goals.
Continue to: Let's examine what this means
Let’s examine what this means
In 1967, a study of 143 hypertensive patients showed that treating high BP (average diastolic BP between 115 and 129 mm Hg) dramatically improved important health outcomes.12 The number needed to treat (NNT) after about 1.5 years showed that for every 1.4 people treated, 1 benefited.8 This is strong and effective medicine.
Successive randomized controlled trials of lower BP goals showed consistent RR reductions; however, AR reductions were much lower, reflecting a higher NNT.8 To prove BP-lowering benefits were not a random effect, higher numbers of participants were needed (SPRINT required over 9300 participants).13 The AR reduction in SPRINT was 1.6% (meaning no benefit was seen in 98.4% receiving the intensive intervention). One participant with high cardiovascular disease risk benefited for every 63 subjects given the intensive therapy compared with usual care (BP goal of 120 mm Hg vs 140 mm Hg).13,14 The researchers noted serious harms in 1 of 22 subjects treated. Treating younger patients to lower BP goals labels healthy people with risk factors as “sick” and commits them to lifelong medications. It exposes them to more frequent harms than benefits. For healthy patients who are unlikely to benefit from taking more antihypertensive medication, these harms matter.
Interpreting the benefits of BP Tx when the benefit to individuals appears small
If only there were a biomarker that could tell us who is most likely to benefit from antihypertensive medication treatment, FPs could ensure that the correct patients are treated. The ACP/AAFP guideline points the way. There is a biomarker, and it is called BP. Systolic BP above 150 mm Hg signals urgency to treat with medications.
A call to advocate. We must all advocate for better guideline processes. The status quo in guideline development and its reliance on special interest funding requires ongoing vigilance to advocate on behalf of our patients. High-value medical care is expensive and hard work. When it is applied to the wrong people at the wrong time, we don’t deliver on our promises.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
2. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430-437.
3. Phend C. Europe stands pat on hypertension thresholds. ESC doesn’t follow ACC/AHA diagnotic cutoff, focuses on control rates. Medpage Today. Available at: https://www.medpagetoday.com/cardiology/hypertension/73384?xid=NL_breakingnews_2018-06-09&eun=g1206318d0r&utm_source=Sailthru&utm_medium=email&utm_campaign=BreakingNews_060918&utm_term=Breaking%20News%20Targeted. Accessed June 19, 2018.
4. Institute of Medicine (US). Committee on Standards for Developing Trustworthy Clinical Practice Guidelines; eds, Graham R, Mancher M, Miller Wolman D, et al. Clinical Practice Guidelines We Can Trust. Washington, DC: National Academies Press; 2011.
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
6. Wright JT Jr., Fine LJ, Lackland DT, et al. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499-503.
7. Graham R, James P, Cowan T. Are clinical practice guidelines valid for primary care? J Clin Epidemiol. 2000;53:949-954.
8. Welch HG, Schwartz LM, Woloshin S. Overdiagnosed: Making People Sick in the Pursuit of Health. Boston, Mass: Beacon Press; 2011.
9. Clancy CM, Kamerow DB. Evidence-based medicine meets cost-effectiveness analysis. JAMA. 1996;276:329-330.
10. Frame PS. A critical review of adult health maintenance. Part 1: Prevention of atherosclerotic diseases. J Fam Pract. 1986;22:341-346.
11. Starfield B, Hyde Jervas J, Heath I. Glossary: the concept of prevention: a good idea gone astray? J Epidemiol Community Health. 2008;62:580-583.
12. Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA. 1967;202:1028-1034.
13. Wright JT Jr., Whelton PK, Reboussin DM. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2016;374:2294.
14. Ortiz E, James PA. Let’s not SPRINT to judgment about new blood pressure goals. Ann Intern Med. 2016;164:692-693.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
2. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430-437.
3. Phend C. Europe stands pat on hypertension thresholds. ESC doesn’t follow ACC/AHA diagnotic cutoff, focuses on control rates. Medpage Today. Available at: https://www.medpagetoday.com/cardiology/hypertension/73384?xid=NL_breakingnews_2018-06-09&eun=g1206318d0r&utm_source=Sailthru&utm_medium=email&utm_campaign=BreakingNews_060918&utm_term=Breaking%20News%20Targeted. Accessed June 19, 2018.
4. Institute of Medicine (US). Committee on Standards for Developing Trustworthy Clinical Practice Guidelines; eds, Graham R, Mancher M, Miller Wolman D, et al. Clinical Practice Guidelines We Can Trust. Washington, DC: National Academies Press; 2011.
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
6. Wright JT Jr., Fine LJ, Lackland DT, et al. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499-503.
7. Graham R, James P, Cowan T. Are clinical practice guidelines valid for primary care? J Clin Epidemiol. 2000;53:949-954.
8. Welch HG, Schwartz LM, Woloshin S. Overdiagnosed: Making People Sick in the Pursuit of Health. Boston, Mass: Beacon Press; 2011.
9. Clancy CM, Kamerow DB. Evidence-based medicine meets cost-effectiveness analysis. JAMA. 1996;276:329-330.
10. Frame PS. A critical review of adult health maintenance. Part 1: Prevention of atherosclerotic diseases. J Fam Pract. 1986;22:341-346.
11. Starfield B, Hyde Jervas J, Heath I. Glossary: the concept of prevention: a good idea gone astray? J Epidemiol Community Health. 2008;62:580-583.
12. Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA. 1967;202:1028-1034.
13. Wright JT Jr., Whelton PK, Reboussin DM. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2016;374:2294.
14. Ortiz E, James PA. Let’s not SPRINT to judgment about new blood pressure goals. Ann Intern Med. 2016;164:692-693.
Diabetes in the elderly: Matching meds to needs
As members of the baby boomer generation (adults ≥65 years) age, the number of people at risk for diabetes increases. Already nearly one-quarter of people over age 65 have type 2 diabetes (T2DM).1 With a proliferation of new medications to treat diabetes, deciding which ones to use in older patients is becoming complex.
In this article we review the important issues to consider when prescribing and monitoring diabetes medications in older adults. To provide optimal patient-centered care, it’s necessary to assess comorbid conditions as well as the costs, risks, and benefits of each medication. Determining appropriate goals of therapy and selecting agents that minimize the risk of hypoglycemia will help ensure safe and effective management of older patients with diabetes.
What makes elderly patients unique
The pathophysiology of T2DM in the elderly is unique in that it involves not just insulin resistance but also age-related loss of beta-cell function, leading to reduced insulin secretion and altered effectiveness of pharmacotherapy.2 The addition of second and third medications may be needed for those with longstanding T2DM, although these agents often reduce the A1C level to a lesser extent than when used as monotherapy in patients whose beta-cell function is still intact. In addition to physiologic changes, older adults with diabetes have varied general health statuses and care support systems. The goal for glycemic management should be personalized based on an individual’s comorbidities and physical and cognitive functional status (TABLE 13,4).2
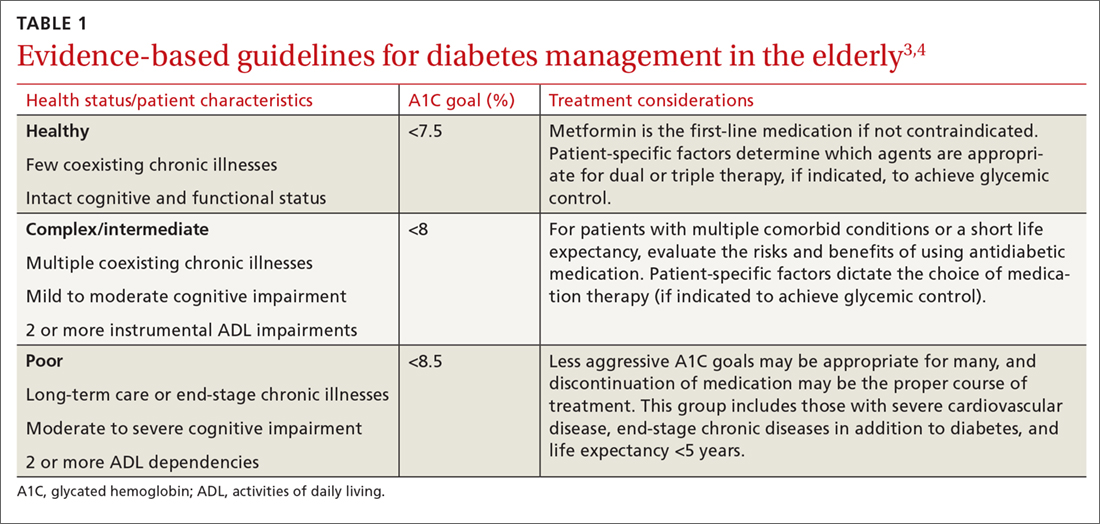
Higher A1C goals can be acceptable for elderly patients with comorbid conditions such as cognitive dysfunction, dementia, or cardiovascular or renal disease. Evaluate cognition when determining appropriate pharmacotherapy. Assess a patient’s awareness of hypoglycemia and ability to adhere to the regimen prescribed. Visual impairment, decreased dexterity, baseline weight, nutritional and functional status, as well as social support, finances, and formulary restrictions should all be considered when determining the most appropriate regimen for a patient. Also take into account patient and family goals of care.2 TABLE 22-4 summarizes key risks and benefits of the medications we discuss next.

Metformin
Metformin is recommended as first-line therapy for those with T2DM for a number of reasons, including its potential to reduce cardiovascular events and mortality.3,5 It also significantly reduces A1C levels by 1% to 1.5%,6 while imparting a low risk of hypoglycemia. Metformin is cost effective and well tolerated, making it an excellent choice for use in older patients.
The most common adverse effects are abdominal discomfort, diarrhea, and weight loss. The use of extended-release preparations, as well as slow titration of dosing, can improve gastrointestinal (GI) tolerance. Weight loss may be an attractive side effect in patients who are overweight or obese, but weight loss and diarrhea are concerning effects in frail older adults who may have poor nutritional reserves.6
Monitor renal function frequently in older patients receiving metformin.3 Renal failure is a risk factor for adverse events such as lactic acidosis, and metformin is therefore contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m2.4 With this in mind, metformin should not be started in patients with an eGFR below 45 mL/min/1.73 m2. And for patients already taking metformin, reduce the total daily dose if the eGFR falls to between 30 and 45 mL/min/1.73 m2.4
Metformin can cause a reduction in vitamin B12 levels after long-term use in up to 30% of patients, likely due to decreased absorption from the ileum.7 Monitor vitamin B12 serum concentrations periodically with long-term therapy, particularly in patients with peripheral neuropathy or anemia, as these conditions may be exacerbated by vitamin B12 deficiency.3,4
Continue to: Sulfonylureas
Sulfonylureas
Sulfonylureas increase the secretion of insulin from pancreatic beta cells, significantly lower blood glucose, and reduce A1C levels by 1% to 2%.6 Because hypoglycemia is a serious risk with sulfonylureas, they should be used conservatively in the elderly.2 Avoid using sulfonylurea formulations with long half-lives or active metabolites, which can cause severe and prolonged hypoglycemia.8,9
Glyburide is broken down into active metabolites that accumulate in patients who have renal insufficiency; it should be avoided in older adults due to the risk of life-threatening hypoglycemic events.10 Glipizide has no active metabolites and has the lowest risk of hypoglycemia in the setting of decreased renal function, making it the preferred sulfonylurea for use in the elderly.3,10
Thiazolidinediones
Thiazolidinediones (TZDs) reduce insulin resistance and decrease hepatic glucose production without increasing the risk of hypoglycemia. These agents effectively lower A1C levels by 1% to 1.5%.11 Despite their efficacy, TZDs have limited benefit because of adverse effects. Serious complications include fluid retention that can exacerbate or lead to worsening heart failure, weight gain, macular edema, and hepatic failure.
Specifically, with pioglitazone, there is also a slightly increased risk of bladder cancer.2 In one study involving more than 30,000 patients taking pioglitazone, an increase in bladder cancer was noted among those using the medication for more than 2 years.12 Still, the hazard ratio was only 1.2, with 90 cases diagnosed over the course of the study. A prudent strategy would be to avoid its use in those with high risk of developing bladder cancer. TZDs are contraindicated in patients with New York Heart Association class III or IV heart failure.8
Increased fracture risk has been identified in both men and women and is a concerning adverse effect in the elderly.8 Fracture risk with TZDs has been approximately twice that of placebo, noted in a study of older women where the fracture rate was 5.1% vs 2.5%, respectively.11 TZDs can be of value in lowering A1C levels without the risk of hypoglycemia. But, due to their adverse effect profile, use TZDs cautiously in older adults at risk for heart failure, falls, or fractures.3
Continue to: DPP-4 inhibitors
DPP-4 inhibitors
Dipeptidyl peptidase-4 (DPP-4) inhibitors work by suppressing the enzyme that degrades 2 incretin hormones, glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP). The resulting enhancement of incretin activity increases glucose-dependent insulin secretion, decreases glucagon secretion, and promotes satiety.6 These agents have modest efficacy with the potential to lower A1C by 0.5% to 0.9%.8,13 Studies show that DPP-4 inhibitors are well tolerated with a minimal risk of hypoglycemia in the elderly.13 These agents are ideal for combination therapy or for monotherapy in older patients who are not good candidates for metformin or a sulfonylurea.
The safety profile, neutral effect on weight, and once-daily dosing make these agents advantageous for use in frail and debilitated elderly patients, as well as in patients with cognitive dysfunction, decreased dexterity, inconsistent meal patterns, or adherence issues. Dose adjustment is required in renal impairment, with the exception of linagliptin. High cost or formulary restrictions may impact use of these agents.
The DPP-4 inhibitors were well tolerated in short-term studies, but long-term safety has yet to be established.6 Reported post-marketing adverse effects include acute renal failure, allergic reactions, and acute pancreatitis.6,14 These agents should be avoided in any patient with a history of pancreatitis.14 In addition, trials investigating the cardiovascular safety and efficacy of DPP-4 inhibitors point to an increased risk of heart failure with the use of saxagliptin and alogliptin, regardless of age.15,16 The potential for adverse effects warrants increased patient monitoring when using these agents in older patients.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are injectable agents that potentiate the actions of the naturally occurring incretin GLP-1, which increases glucose-dependent insulin secretion, inhibits glucagon release, reduces hepatic glucose production, and delays gastric emptying. These agents have a pronounced effect on satiety and promote weight loss. The most common adverse effects are nausea, vomiting, and diarrhea, which occur most commonly during treatment initiation and titration. Studies in elderly patients confirm A1C reductions of 1% to 1.5% and a low risk of hypoglycemia when used alone.17,18
GLP-1 RAs can be used as monotherapy in older patients at risk for hypoglycemia or in those with hypoglycemic unawareness. They can also be used in combination therapy with other agents, including insulin, though concomitant use with insulin or insulin secretagogues increases the risk of hypoglycemia.3 Weight loss and GI adverse effects may limit the use of these agents in frail or undernourished elderly patients.6
Continue to: Since these agents are injected...
Since these agents are injected, they require intact visual, motor, and cognitive skills and thus may not be appropriate in older patients with cognitive or visual impairment or decreased dexterity. In addition, the high cost of these agents may limit their use.
Select a GLP-1 RA based on the frequency of administration, type of glucose control required (fasting or post-prandial), and the patient’s ability to use the administration device. Dose adjustment is required in renal impairment, except with dulaglutide and liraglutide. Use with caution in patients with a history of pancreatitis, and stop GLP-1 RAs if pancreatitis is suspected during treatment.4 Avoid GLP-1 RAs in patients with a personal or family history of thyroid-related cancers, as these agents have been associated with medullary thyroid tumors in animals.4
A new indication. Recent evidence suggests the GLP-1 RAs may offer additional cardiovascular benefit in patients with diabetes.18,19 In August 2017, liraglutide gained an additional FDA indication to reduce the risk of major adverse cardiovascular events in adults with T2DM and established cardiovascular disease.
This new indication was based on the Novo Nordisk- and National Institutes of Health-sponsored LEADER trial, in which liraglutide reduced the risk of cardiovascular death, nonfatal heart attack, or nonfatal stroke by 13% vs placebo (P=.01) with an absolute risk reduction (ARR) of 1.9%.19 Liraglutide demonstrated a 22% reduction in cardiovascular death and a 15% reduction in all-cause death (ARR 1.3%, 1.4% respectively).19 The new cardiovascular indication may impact the choice of add-on therapy to metformin in patients with preexisting cardiovascular conditions.
Continue to: Sodium glucose cotransporter-2 inhibitors
Sodium glucose cotransporter-2 inhibitors
SGLT-2 inhibitors prevent the reabsorption of renal-filtered glucose, resulting in decreased blood glucose levels and increased urinary excretion of glucose without stimulating insulin secretion, and therefore without increasing the risk of hypoglycemia. Additional effects include decreased blood pressure and weight loss.20 Dose adjustment is required in renal impairment.
SGLT-2 inhibitors can be used as monotherapy or in combination with other agents, including insulin, and the relatively low risk of hypoglycemia and moderate A1C lowering potential of 0.5% to 1% provide an oral option for select older patients.20 Common adverse events include hypotension, hyperkalemia, increased low-density lipoprotein (LDL) levels, acute kidney injury, genital mycotic infections, and hypoglycemia when used in combination with insulin or insulin secretagogues.20
Additional warnings have been issued by the FDA for the risk of urinary tract infection with sepsis, as well as diabetic ketoacidosis associated with SGLT-2 inhibitor use.21 The FDA has reported bone fracture risk and decreased bone mineral density with canagliflozin.21 Avoid using SGLT-2 inhibitors in patients with osteopenia or osteoporosis, as the risks outweigh the benefits. Drug-specific warnings may further impact individual use of an agent, with canagliflozin most recently having been associated with increased risk of leg and foot amputations.21
Given the adverse effect profile of SGLT-2 inhibitors, assess their risks and benefits in older patients on a case-by-case basis. Before initiating therapy, evaluate each patient’s volume status. A higher incidence of adverse effects related to intravascular volume depletion has been reported in those 65 or older, with a more prominent increase seen in patients 75 or older.22 However, the risk of hypoglycemia does not seem to increase with age.22
Although many adverse effects have been reported with SGLT-2 inhibitors, empagliflozin was associated with significantly lower rates of all-cause and cardiovascular death and lower risk of hospitalization for heart failure in the only SGLT-2 inhibitor cardiovascular outcomes trial reported to date.23 If this cardiovascular benefit is replicated in additional trials of the other SGLT-2 inhibitors, use of this drug class may increase.
Continue to: Insulin
Insulin
Many patients will ultimately require insulin due to the progressive loss of beta-cell function that occurs in advanced diabetes. Starting insulin therapy early on in the disease may actually restore beta-cell function and reduce glucotoxicity.24 In elderly patients with uncontrolled diabetes, early treatment with basal insulin results in better glycemic control and less hypoglycemia than continuing to titrate oral agents.25
Despite these benefits, however, insulin use often is not optimized in the elderly due to concerns about hypoglycemia and difficulty of administration. Safe use of insulin requires careful selection of an appropriate insulin regimen, since insulin use has been identified as an independent predictor of severe hypoglycemia in the elderly.8,26 Before initiating insulin therapy, evaluate whether an older patient is cognitively and physically able to safely use insulin.
Multiple daily injections may be challenging for some older adults. Limit such insulin regimens to use in high-functioning patients. Although all types of insulin can cause hypoglycemia, regimens that mimic insulin’s normal physiologic pattern introduce less hypoglycemic risk. Using basal insulin that mimics the body’s sustained insulin level throughout the day is associated with a lower frequency of hypoglycemia in older people with diabetes than conventional insulin regimens. Long-acting insulins such as glargine, detemir, and degludec offer a lower risk of hypoglycemia, particularly nocturnal hypoglycemia which may contribute to falls.2,27
Neutral protamine Hagedorn insulin and regular insulin are not recommended for use in the elderly, as they do not mimic the body’s natural basal-bolus insulin production and thus put patients at higher risk of hypoglycemia.4 If insulin intensification is needed after optimizing basal insulin, consider adding mealtime insulin with a bolus of rapid-acting insulin (insulin aspart, insulin lispro, or insulin glulisine). It is important to note that the kidneys are responsible for 30% to 80% of insulin clearance from the body.28 Because insulin action is prolonged in renal insufficiency, prevent hypoglycemia by decreasing basal and bolus doses when the eGFR is below 50 mL/min/1.73m2.28
Dosing errors. Whenever possible, use insulin preparations that minimize dosing errors. Insulin pen formulations, if financially feasible, allow more accurate dosing and are more acceptable to older patients compared with syringes and vials.29 Pen formulations are particularly preferable for older patients with impaired vision or dexterity.29 In addition, when patients must mix insulins, errors are more likely to occur. The use of premixed insulin vials has been shown to increase dosing accuracy when used by the elderly.30
Continue to: Combining antidiabetes agents
Combining antidiabetes agents
However, for older patients already taking metformin who are not at their A1C goal, consider adding a second agent, if not contraindicated. Potential agents include a GLP-1 RA, SGLT-2 inhibitor, DDP-4 inhibitor, or short-acting sulfonylurea (glipizide). Alternatively, basal insulin may be added. However, avoid combining a sulfonylurea with insulin, which greatly increases the risk of hypoglycemia.32 Consider adding a GLP-1 RA or basal insulin if the patient is not at his/her target A1C on oral therapy with multiple agents.3
CORRESPONDENCE
Barbara Keber, MD, Glen Cove Hospital, 101 St. Andrews Lane, Glen Cove, NY; [email protected].
1. CDC. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA, U.S. Department of Health and Human Services, 2017.
2. Lee PG, Halter JB. The pathophysiology of hyperglycemia in older adults: clinical considerations. Diabetes Care. 2017;40:444-452.
3. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(Suppl 1):S1–S138.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2017 Executive Summary. Endocr Pract. 2017;23:207–238.
5. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
6. Kezerle L, Shalev L, Barski L. Treating the elderly diabetic patient: special considerations. Diabetes Metab Syndr Obes. 2014;7:391-400.
7. Singh J, Tushar B. Metformin use and vitamin B12 deficiency in patients with type-2 diabetes mellitus. MVP J Med Sci. 2016:3:67-70.
8. Fravel MA, McDanel DL, Ross MB, et al. Special considerations for treatment of type 2 diabetes mellitus in the elderly. Am J Health Syst Pharm. 2011;68:500-509.
9. Hanlon JT, Semla TP, Schmader KE. Alternative medications for medications in the use of high-risk medications in the elderly and potentially harmful drug-disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63:e8–e18.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Schernthaner G, Curie CJ, Schernthaner GH. Do we still need pioglitazone for the treatment of type 2 diabetes? A risk-benefit critique in 2013. Diabetes Care. 2013;36(Suppl 2):S155-S161.
12. Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922.
13. Avogaro A, Dardano A, de Kreutzenberg SV, et al. Dipeptidyl peptidase-4 inhibitors can minimize the hypoglycaemic burden and enhance safety in elderly people with diabetes. Diabetes Obes Metab. 2015;17:107-115.
14. DeVries JH, RosenstocK J. DPP-4 inhibitor-related pancreatitis: rare but real! Diabetes Care. 2017;40:161-163.
15. Leiter LA, Teoh H, Braunwald E, et al. Efficacy and safety of saxagliptin in older participants in the SAVOR-TIMI 53 trial. Diabetes Care. 2015;38:1145-1153.
16. , , , et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. 2011;162:620-626.
17. Raccah D, Miossec P, Esposito V, et al. Efficacy and safety of lixisenatide in elderly (≥65 years old) and very elderly (≥75 years old) patients with type 2 diabetes: an analysis from the GetGoal phase III programme. Diabetes Metab Res Rev. 2015;31:204-211.
18. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
19. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee, LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
20. Lusk KA, Barnes NE. Role of sodium-glucose cotransporter 2 (SGLT2) inhibitors. US Pharm. 2016;41:26-29.
21. U.S. Food and Drug Administration. Sodium-glucose cotransporter-2 (SGLT2) inhibitors. Available at: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm446852.htm. Accessed May 18, 2018.
22. Miller EM. Overview of the efficacy and safety of SGLT-2 inhibitors in type 2 diabetes mellitus. J Fam Pract. 2017;66(2 Suppl):S5-S12.
23. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
24. Owens DR. Clinical evidence for the earlier initiation of insulin therapy in type 2 diabetes. Diabetes Technol Ther. 2013;15:776-785.
25. Papa G, Fedele V, Chiavetta A, et al. Therapeutic options for elderly diabetic subjects: open label, randomized clinical trial of insulin glargine added to oral antidiabetic drugs versus increased dosage of oral antidiabetic drugs. Acta Diabetol. 2008;45:53-59.
26. Fu H, Xie W, Curtis B, et al. Identifying factors associated with hypoglycemia-related hospitalizations among elderly patients with T2DM in the US: a novel approach using influential variable analysis. Curr Med Res Opin. 2014;30:1787-1793.
27. Sorli C, Warren M, Oyer D, et al. Elderly patients with diabetes experience a lower rate of nocturnal hypoglycaemia with insulin degludec than with insulin glargine: a meta-analysis of phase IIIa trials. Drugs Aging. 2013;30:1009-1018.
28. Sampanis CH. Management of hyperglycemia in patients with diabetes mellitus and chronic renal failure. Hippokratia. 2008;12:22-27.
29. Corsi A, Torre E, Coronel GA, et al. Pre-filled insulin pen in newly insulin-treated diabetic patients over 60 years old. Diab Nutr Metab. 1997;10:78-81.
30. Coscelli C, Calabrese G, Fedele D, et al. Use of premixed insulin among the elderly. Reduction of errors in patient preparation of mixtures. Diabetes Care. 1992;15:1628-1630.
31. American Geriatrics Society. Ten things clinicians and patients should question. Available at: http://www.choosingwisely.org/societies/american-geriatrics-society/. Accessed May 18, 2018.
32. Mogensen UM, Andersson C, Fosbøl EL, et al. Sulfonylurea in combination with insulin is associated with increased mortality compared with a combination of insulin and metformin in a retrospective Danish nationwide study. Diabetologia. 2015;58:50-58.
As members of the baby boomer generation (adults ≥65 years) age, the number of people at risk for diabetes increases. Already nearly one-quarter of people over age 65 have type 2 diabetes (T2DM).1 With a proliferation of new medications to treat diabetes, deciding which ones to use in older patients is becoming complex.
In this article we review the important issues to consider when prescribing and monitoring diabetes medications in older adults. To provide optimal patient-centered care, it’s necessary to assess comorbid conditions as well as the costs, risks, and benefits of each medication. Determining appropriate goals of therapy and selecting agents that minimize the risk of hypoglycemia will help ensure safe and effective management of older patients with diabetes.
What makes elderly patients unique
The pathophysiology of T2DM in the elderly is unique in that it involves not just insulin resistance but also age-related loss of beta-cell function, leading to reduced insulin secretion and altered effectiveness of pharmacotherapy.2 The addition of second and third medications may be needed for those with longstanding T2DM, although these agents often reduce the A1C level to a lesser extent than when used as monotherapy in patients whose beta-cell function is still intact. In addition to physiologic changes, older adults with diabetes have varied general health statuses and care support systems. The goal for glycemic management should be personalized based on an individual’s comorbidities and physical and cognitive functional status (TABLE 13,4).2

Higher A1C goals can be acceptable for elderly patients with comorbid conditions such as cognitive dysfunction, dementia, or cardiovascular or renal disease. Evaluate cognition when determining appropriate pharmacotherapy. Assess a patient’s awareness of hypoglycemia and ability to adhere to the regimen prescribed. Visual impairment, decreased dexterity, baseline weight, nutritional and functional status, as well as social support, finances, and formulary restrictions should all be considered when determining the most appropriate regimen for a patient. Also take into account patient and family goals of care.2 TABLE 22-4 summarizes key risks and benefits of the medications we discuss next.

Metformin
Metformin is recommended as first-line therapy for those with T2DM for a number of reasons, including its potential to reduce cardiovascular events and mortality.3,5 It also significantly reduces A1C levels by 1% to 1.5%,6 while imparting a low risk of hypoglycemia. Metformin is cost effective and well tolerated, making it an excellent choice for use in older patients.
The most common adverse effects are abdominal discomfort, diarrhea, and weight loss. The use of extended-release preparations, as well as slow titration of dosing, can improve gastrointestinal (GI) tolerance. Weight loss may be an attractive side effect in patients who are overweight or obese, but weight loss and diarrhea are concerning effects in frail older adults who may have poor nutritional reserves.6
Monitor renal function frequently in older patients receiving metformin.3 Renal failure is a risk factor for adverse events such as lactic acidosis, and metformin is therefore contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m2.4 With this in mind, metformin should not be started in patients with an eGFR below 45 mL/min/1.73 m2. And for patients already taking metformin, reduce the total daily dose if the eGFR falls to between 30 and 45 mL/min/1.73 m2.4
Metformin can cause a reduction in vitamin B12 levels after long-term use in up to 30% of patients, likely due to decreased absorption from the ileum.7 Monitor vitamin B12 serum concentrations periodically with long-term therapy, particularly in patients with peripheral neuropathy or anemia, as these conditions may be exacerbated by vitamin B12 deficiency.3,4
Continue to: Sulfonylureas
Sulfonylureas
Sulfonylureas increase the secretion of insulin from pancreatic beta cells, significantly lower blood glucose, and reduce A1C levels by 1% to 2%.6 Because hypoglycemia is a serious risk with sulfonylureas, they should be used conservatively in the elderly.2 Avoid using sulfonylurea formulations with long half-lives or active metabolites, which can cause severe and prolonged hypoglycemia.8,9
Glyburide is broken down into active metabolites that accumulate in patients who have renal insufficiency; it should be avoided in older adults due to the risk of life-threatening hypoglycemic events.10 Glipizide has no active metabolites and has the lowest risk of hypoglycemia in the setting of decreased renal function, making it the preferred sulfonylurea for use in the elderly.3,10
Thiazolidinediones
Thiazolidinediones (TZDs) reduce insulin resistance and decrease hepatic glucose production without increasing the risk of hypoglycemia. These agents effectively lower A1C levels by 1% to 1.5%.11 Despite their efficacy, TZDs have limited benefit because of adverse effects. Serious complications include fluid retention that can exacerbate or lead to worsening heart failure, weight gain, macular edema, and hepatic failure.
Specifically, with pioglitazone, there is also a slightly increased risk of bladder cancer.2 In one study involving more than 30,000 patients taking pioglitazone, an increase in bladder cancer was noted among those using the medication for more than 2 years.12 Still, the hazard ratio was only 1.2, with 90 cases diagnosed over the course of the study. A prudent strategy would be to avoid its use in those with high risk of developing bladder cancer. TZDs are contraindicated in patients with New York Heart Association class III or IV heart failure.8
Increased fracture risk has been identified in both men and women and is a concerning adverse effect in the elderly.8 Fracture risk with TZDs has been approximately twice that of placebo, noted in a study of older women where the fracture rate was 5.1% vs 2.5%, respectively.11 TZDs can be of value in lowering A1C levels without the risk of hypoglycemia. But, due to their adverse effect profile, use TZDs cautiously in older adults at risk for heart failure, falls, or fractures.3
Continue to: DPP-4 inhibitors
DPP-4 inhibitors
Dipeptidyl peptidase-4 (DPP-4) inhibitors work by suppressing the enzyme that degrades 2 incretin hormones, glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP). The resulting enhancement of incretin activity increases glucose-dependent insulin secretion, decreases glucagon secretion, and promotes satiety.6 These agents have modest efficacy with the potential to lower A1C by 0.5% to 0.9%.8,13 Studies show that DPP-4 inhibitors are well tolerated with a minimal risk of hypoglycemia in the elderly.13 These agents are ideal for combination therapy or for monotherapy in older patients who are not good candidates for metformin or a sulfonylurea.
The safety profile, neutral effect on weight, and once-daily dosing make these agents advantageous for use in frail and debilitated elderly patients, as well as in patients with cognitive dysfunction, decreased dexterity, inconsistent meal patterns, or adherence issues. Dose adjustment is required in renal impairment, with the exception of linagliptin. High cost or formulary restrictions may impact use of these agents.
The DPP-4 inhibitors were well tolerated in short-term studies, but long-term safety has yet to be established.6 Reported post-marketing adverse effects include acute renal failure, allergic reactions, and acute pancreatitis.6,14 These agents should be avoided in any patient with a history of pancreatitis.14 In addition, trials investigating the cardiovascular safety and efficacy of DPP-4 inhibitors point to an increased risk of heart failure with the use of saxagliptin and alogliptin, regardless of age.15,16 The potential for adverse effects warrants increased patient monitoring when using these agents in older patients.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are injectable agents that potentiate the actions of the naturally occurring incretin GLP-1, which increases glucose-dependent insulin secretion, inhibits glucagon release, reduces hepatic glucose production, and delays gastric emptying. These agents have a pronounced effect on satiety and promote weight loss. The most common adverse effects are nausea, vomiting, and diarrhea, which occur most commonly during treatment initiation and titration. Studies in elderly patients confirm A1C reductions of 1% to 1.5% and a low risk of hypoglycemia when used alone.17,18
GLP-1 RAs can be used as monotherapy in older patients at risk for hypoglycemia or in those with hypoglycemic unawareness. They can also be used in combination therapy with other agents, including insulin, though concomitant use with insulin or insulin secretagogues increases the risk of hypoglycemia.3 Weight loss and GI adverse effects may limit the use of these agents in frail or undernourished elderly patients.6
Continue to: Since these agents are injected...
Since these agents are injected, they require intact visual, motor, and cognitive skills and thus may not be appropriate in older patients with cognitive or visual impairment or decreased dexterity. In addition, the high cost of these agents may limit their use.
Select a GLP-1 RA based on the frequency of administration, type of glucose control required (fasting or post-prandial), and the patient’s ability to use the administration device. Dose adjustment is required in renal impairment, except with dulaglutide and liraglutide. Use with caution in patients with a history of pancreatitis, and stop GLP-1 RAs if pancreatitis is suspected during treatment.4 Avoid GLP-1 RAs in patients with a personal or family history of thyroid-related cancers, as these agents have been associated with medullary thyroid tumors in animals.4
A new indication. Recent evidence suggests the GLP-1 RAs may offer additional cardiovascular benefit in patients with diabetes.18,19 In August 2017, liraglutide gained an additional FDA indication to reduce the risk of major adverse cardiovascular events in adults with T2DM and established cardiovascular disease.
This new indication was based on the Novo Nordisk- and National Institutes of Health-sponsored LEADER trial, in which liraglutide reduced the risk of cardiovascular death, nonfatal heart attack, or nonfatal stroke by 13% vs placebo (P=.01) with an absolute risk reduction (ARR) of 1.9%.19 Liraglutide demonstrated a 22% reduction in cardiovascular death and a 15% reduction in all-cause death (ARR 1.3%, 1.4% respectively).19 The new cardiovascular indication may impact the choice of add-on therapy to metformin in patients with preexisting cardiovascular conditions.
Continue to: Sodium glucose cotransporter-2 inhibitors
Sodium glucose cotransporter-2 inhibitors
SGLT-2 inhibitors prevent the reabsorption of renal-filtered glucose, resulting in decreased blood glucose levels and increased urinary excretion of glucose without stimulating insulin secretion, and therefore without increasing the risk of hypoglycemia. Additional effects include decreased blood pressure and weight loss.20 Dose adjustment is required in renal impairment.
SGLT-2 inhibitors can be used as monotherapy or in combination with other agents, including insulin, and the relatively low risk of hypoglycemia and moderate A1C lowering potential of 0.5% to 1% provide an oral option for select older patients.20 Common adverse events include hypotension, hyperkalemia, increased low-density lipoprotein (LDL) levels, acute kidney injury, genital mycotic infections, and hypoglycemia when used in combination with insulin or insulin secretagogues.20
Additional warnings have been issued by the FDA for the risk of urinary tract infection with sepsis, as well as diabetic ketoacidosis associated with SGLT-2 inhibitor use.21 The FDA has reported bone fracture risk and decreased bone mineral density with canagliflozin.21 Avoid using SGLT-2 inhibitors in patients with osteopenia or osteoporosis, as the risks outweigh the benefits. Drug-specific warnings may further impact individual use of an agent, with canagliflozin most recently having been associated with increased risk of leg and foot amputations.21
Given the adverse effect profile of SGLT-2 inhibitors, assess their risks and benefits in older patients on a case-by-case basis. Before initiating therapy, evaluate each patient’s volume status. A higher incidence of adverse effects related to intravascular volume depletion has been reported in those 65 or older, with a more prominent increase seen in patients 75 or older.22 However, the risk of hypoglycemia does not seem to increase with age.22
Although many adverse effects have been reported with SGLT-2 inhibitors, empagliflozin was associated with significantly lower rates of all-cause and cardiovascular death and lower risk of hospitalization for heart failure in the only SGLT-2 inhibitor cardiovascular outcomes trial reported to date.23 If this cardiovascular benefit is replicated in additional trials of the other SGLT-2 inhibitors, use of this drug class may increase.
Continue to: Insulin
Insulin
Many patients will ultimately require insulin due to the progressive loss of beta-cell function that occurs in advanced diabetes. Starting insulin therapy early on in the disease may actually restore beta-cell function and reduce glucotoxicity.24 In elderly patients with uncontrolled diabetes, early treatment with basal insulin results in better glycemic control and less hypoglycemia than continuing to titrate oral agents.25
Despite these benefits, however, insulin use often is not optimized in the elderly due to concerns about hypoglycemia and difficulty of administration. Safe use of insulin requires careful selection of an appropriate insulin regimen, since insulin use has been identified as an independent predictor of severe hypoglycemia in the elderly.8,26 Before initiating insulin therapy, evaluate whether an older patient is cognitively and physically able to safely use insulin.
Multiple daily injections may be challenging for some older adults. Limit such insulin regimens to use in high-functioning patients. Although all types of insulin can cause hypoglycemia, regimens that mimic insulin’s normal physiologic pattern introduce less hypoglycemic risk. Using basal insulin that mimics the body’s sustained insulin level throughout the day is associated with a lower frequency of hypoglycemia in older people with diabetes than conventional insulin regimens. Long-acting insulins such as glargine, detemir, and degludec offer a lower risk of hypoglycemia, particularly nocturnal hypoglycemia which may contribute to falls.2,27
Neutral protamine Hagedorn insulin and regular insulin are not recommended for use in the elderly, as they do not mimic the body’s natural basal-bolus insulin production and thus put patients at higher risk of hypoglycemia.4 If insulin intensification is needed after optimizing basal insulin, consider adding mealtime insulin with a bolus of rapid-acting insulin (insulin aspart, insulin lispro, or insulin glulisine). It is important to note that the kidneys are responsible for 30% to 80% of insulin clearance from the body.28 Because insulin action is prolonged in renal insufficiency, prevent hypoglycemia by decreasing basal and bolus doses when the eGFR is below 50 mL/min/1.73m2.28
Dosing errors. Whenever possible, use insulin preparations that minimize dosing errors. Insulin pen formulations, if financially feasible, allow more accurate dosing and are more acceptable to older patients compared with syringes and vials.29 Pen formulations are particularly preferable for older patients with impaired vision or dexterity.29 In addition, when patients must mix insulins, errors are more likely to occur. The use of premixed insulin vials has been shown to increase dosing accuracy when used by the elderly.30
Continue to: Combining antidiabetes agents
Combining antidiabetes agents
However, for older patients already taking metformin who are not at their A1C goal, consider adding a second agent, if not contraindicated. Potential agents include a GLP-1 RA, SGLT-2 inhibitor, DDP-4 inhibitor, or short-acting sulfonylurea (glipizide). Alternatively, basal insulin may be added. However, avoid combining a sulfonylurea with insulin, which greatly increases the risk of hypoglycemia.32 Consider adding a GLP-1 RA or basal insulin if the patient is not at his/her target A1C on oral therapy with multiple agents.3
CORRESPONDENCE
Barbara Keber, MD, Glen Cove Hospital, 101 St. Andrews Lane, Glen Cove, NY; [email protected].
As members of the baby boomer generation (adults ≥65 years) age, the number of people at risk for diabetes increases. Already nearly one-quarter of people over age 65 have type 2 diabetes (T2DM).1 With a proliferation of new medications to treat diabetes, deciding which ones to use in older patients is becoming complex.
In this article we review the important issues to consider when prescribing and monitoring diabetes medications in older adults. To provide optimal patient-centered care, it’s necessary to assess comorbid conditions as well as the costs, risks, and benefits of each medication. Determining appropriate goals of therapy and selecting agents that minimize the risk of hypoglycemia will help ensure safe and effective management of older patients with diabetes.
What makes elderly patients unique
The pathophysiology of T2DM in the elderly is unique in that it involves not just insulin resistance but also age-related loss of beta-cell function, leading to reduced insulin secretion and altered effectiveness of pharmacotherapy.2 The addition of second and third medications may be needed for those with longstanding T2DM, although these agents often reduce the A1C level to a lesser extent than when used as monotherapy in patients whose beta-cell function is still intact. In addition to physiologic changes, older adults with diabetes have varied general health statuses and care support systems. The goal for glycemic management should be personalized based on an individual’s comorbidities and physical and cognitive functional status (TABLE 13,4).2

Higher A1C goals can be acceptable for elderly patients with comorbid conditions such as cognitive dysfunction, dementia, or cardiovascular or renal disease. Evaluate cognition when determining appropriate pharmacotherapy. Assess a patient’s awareness of hypoglycemia and ability to adhere to the regimen prescribed. Visual impairment, decreased dexterity, baseline weight, nutritional and functional status, as well as social support, finances, and formulary restrictions should all be considered when determining the most appropriate regimen for a patient. Also take into account patient and family goals of care.2 TABLE 22-4 summarizes key risks and benefits of the medications we discuss next.

Metformin
Metformin is recommended as first-line therapy for those with T2DM for a number of reasons, including its potential to reduce cardiovascular events and mortality.3,5 It also significantly reduces A1C levels by 1% to 1.5%,6 while imparting a low risk of hypoglycemia. Metformin is cost effective and well tolerated, making it an excellent choice for use in older patients.
The most common adverse effects are abdominal discomfort, diarrhea, and weight loss. The use of extended-release preparations, as well as slow titration of dosing, can improve gastrointestinal (GI) tolerance. Weight loss may be an attractive side effect in patients who are overweight or obese, but weight loss and diarrhea are concerning effects in frail older adults who may have poor nutritional reserves.6
Monitor renal function frequently in older patients receiving metformin.3 Renal failure is a risk factor for adverse events such as lactic acidosis, and metformin is therefore contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m2.4 With this in mind, metformin should not be started in patients with an eGFR below 45 mL/min/1.73 m2. And for patients already taking metformin, reduce the total daily dose if the eGFR falls to between 30 and 45 mL/min/1.73 m2.4
Metformin can cause a reduction in vitamin B12 levels after long-term use in up to 30% of patients, likely due to decreased absorption from the ileum.7 Monitor vitamin B12 serum concentrations periodically with long-term therapy, particularly in patients with peripheral neuropathy or anemia, as these conditions may be exacerbated by vitamin B12 deficiency.3,4
Continue to: Sulfonylureas
Sulfonylureas
Sulfonylureas increase the secretion of insulin from pancreatic beta cells, significantly lower blood glucose, and reduce A1C levels by 1% to 2%.6 Because hypoglycemia is a serious risk with sulfonylureas, they should be used conservatively in the elderly.2 Avoid using sulfonylurea formulations with long half-lives or active metabolites, which can cause severe and prolonged hypoglycemia.8,9
Glyburide is broken down into active metabolites that accumulate in patients who have renal insufficiency; it should be avoided in older adults due to the risk of life-threatening hypoglycemic events.10 Glipizide has no active metabolites and has the lowest risk of hypoglycemia in the setting of decreased renal function, making it the preferred sulfonylurea for use in the elderly.3,10
Thiazolidinediones
Thiazolidinediones (TZDs) reduce insulin resistance and decrease hepatic glucose production without increasing the risk of hypoglycemia. These agents effectively lower A1C levels by 1% to 1.5%.11 Despite their efficacy, TZDs have limited benefit because of adverse effects. Serious complications include fluid retention that can exacerbate or lead to worsening heart failure, weight gain, macular edema, and hepatic failure.
Specifically, with pioglitazone, there is also a slightly increased risk of bladder cancer.2 In one study involving more than 30,000 patients taking pioglitazone, an increase in bladder cancer was noted among those using the medication for more than 2 years.12 Still, the hazard ratio was only 1.2, with 90 cases diagnosed over the course of the study. A prudent strategy would be to avoid its use in those with high risk of developing bladder cancer. TZDs are contraindicated in patients with New York Heart Association class III or IV heart failure.8
Increased fracture risk has been identified in both men and women and is a concerning adverse effect in the elderly.8 Fracture risk with TZDs has been approximately twice that of placebo, noted in a study of older women where the fracture rate was 5.1% vs 2.5%, respectively.11 TZDs can be of value in lowering A1C levels without the risk of hypoglycemia. But, due to their adverse effect profile, use TZDs cautiously in older adults at risk for heart failure, falls, or fractures.3
Continue to: DPP-4 inhibitors
DPP-4 inhibitors
Dipeptidyl peptidase-4 (DPP-4) inhibitors work by suppressing the enzyme that degrades 2 incretin hormones, glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP). The resulting enhancement of incretin activity increases glucose-dependent insulin secretion, decreases glucagon secretion, and promotes satiety.6 These agents have modest efficacy with the potential to lower A1C by 0.5% to 0.9%.8,13 Studies show that DPP-4 inhibitors are well tolerated with a minimal risk of hypoglycemia in the elderly.13 These agents are ideal for combination therapy or for monotherapy in older patients who are not good candidates for metformin or a sulfonylurea.
The safety profile, neutral effect on weight, and once-daily dosing make these agents advantageous for use in frail and debilitated elderly patients, as well as in patients with cognitive dysfunction, decreased dexterity, inconsistent meal patterns, or adherence issues. Dose adjustment is required in renal impairment, with the exception of linagliptin. High cost or formulary restrictions may impact use of these agents.
The DPP-4 inhibitors were well tolerated in short-term studies, but long-term safety has yet to be established.6 Reported post-marketing adverse effects include acute renal failure, allergic reactions, and acute pancreatitis.6,14 These agents should be avoided in any patient with a history of pancreatitis.14 In addition, trials investigating the cardiovascular safety and efficacy of DPP-4 inhibitors point to an increased risk of heart failure with the use of saxagliptin and alogliptin, regardless of age.15,16 The potential for adverse effects warrants increased patient monitoring when using these agents in older patients.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are injectable agents that potentiate the actions of the naturally occurring incretin GLP-1, which increases glucose-dependent insulin secretion, inhibits glucagon release, reduces hepatic glucose production, and delays gastric emptying. These agents have a pronounced effect on satiety and promote weight loss. The most common adverse effects are nausea, vomiting, and diarrhea, which occur most commonly during treatment initiation and titration. Studies in elderly patients confirm A1C reductions of 1% to 1.5% and a low risk of hypoglycemia when used alone.17,18
GLP-1 RAs can be used as monotherapy in older patients at risk for hypoglycemia or in those with hypoglycemic unawareness. They can also be used in combination therapy with other agents, including insulin, though concomitant use with insulin or insulin secretagogues increases the risk of hypoglycemia.3 Weight loss and GI adverse effects may limit the use of these agents in frail or undernourished elderly patients.6
Continue to: Since these agents are injected...
Since these agents are injected, they require intact visual, motor, and cognitive skills and thus may not be appropriate in older patients with cognitive or visual impairment or decreased dexterity. In addition, the high cost of these agents may limit their use.
Select a GLP-1 RA based on the frequency of administration, type of glucose control required (fasting or post-prandial), and the patient’s ability to use the administration device. Dose adjustment is required in renal impairment, except with dulaglutide and liraglutide. Use with caution in patients with a history of pancreatitis, and stop GLP-1 RAs if pancreatitis is suspected during treatment.4 Avoid GLP-1 RAs in patients with a personal or family history of thyroid-related cancers, as these agents have been associated with medullary thyroid tumors in animals.4
A new indication. Recent evidence suggests the GLP-1 RAs may offer additional cardiovascular benefit in patients with diabetes.18,19 In August 2017, liraglutide gained an additional FDA indication to reduce the risk of major adverse cardiovascular events in adults with T2DM and established cardiovascular disease.
This new indication was based on the Novo Nordisk- and National Institutes of Health-sponsored LEADER trial, in which liraglutide reduced the risk of cardiovascular death, nonfatal heart attack, or nonfatal stroke by 13% vs placebo (P=.01) with an absolute risk reduction (ARR) of 1.9%.19 Liraglutide demonstrated a 22% reduction in cardiovascular death and a 15% reduction in all-cause death (ARR 1.3%, 1.4% respectively).19 The new cardiovascular indication may impact the choice of add-on therapy to metformin in patients with preexisting cardiovascular conditions.
Continue to: Sodium glucose cotransporter-2 inhibitors
Sodium glucose cotransporter-2 inhibitors
SGLT-2 inhibitors prevent the reabsorption of renal-filtered glucose, resulting in decreased blood glucose levels and increased urinary excretion of glucose without stimulating insulin secretion, and therefore without increasing the risk of hypoglycemia. Additional effects include decreased blood pressure and weight loss.20 Dose adjustment is required in renal impairment.
SGLT-2 inhibitors can be used as monotherapy or in combination with other agents, including insulin, and the relatively low risk of hypoglycemia and moderate A1C lowering potential of 0.5% to 1% provide an oral option for select older patients.20 Common adverse events include hypotension, hyperkalemia, increased low-density lipoprotein (LDL) levels, acute kidney injury, genital mycotic infections, and hypoglycemia when used in combination with insulin or insulin secretagogues.20
Additional warnings have been issued by the FDA for the risk of urinary tract infection with sepsis, as well as diabetic ketoacidosis associated with SGLT-2 inhibitor use.21 The FDA has reported bone fracture risk and decreased bone mineral density with canagliflozin.21 Avoid using SGLT-2 inhibitors in patients with osteopenia or osteoporosis, as the risks outweigh the benefits. Drug-specific warnings may further impact individual use of an agent, with canagliflozin most recently having been associated with increased risk of leg and foot amputations.21
Given the adverse effect profile of SGLT-2 inhibitors, assess their risks and benefits in older patients on a case-by-case basis. Before initiating therapy, evaluate each patient’s volume status. A higher incidence of adverse effects related to intravascular volume depletion has been reported in those 65 or older, with a more prominent increase seen in patients 75 or older.22 However, the risk of hypoglycemia does not seem to increase with age.22
Although many adverse effects have been reported with SGLT-2 inhibitors, empagliflozin was associated with significantly lower rates of all-cause and cardiovascular death and lower risk of hospitalization for heart failure in the only SGLT-2 inhibitor cardiovascular outcomes trial reported to date.23 If this cardiovascular benefit is replicated in additional trials of the other SGLT-2 inhibitors, use of this drug class may increase.
Continue to: Insulin
Insulin
Many patients will ultimately require insulin due to the progressive loss of beta-cell function that occurs in advanced diabetes. Starting insulin therapy early on in the disease may actually restore beta-cell function and reduce glucotoxicity.24 In elderly patients with uncontrolled diabetes, early treatment with basal insulin results in better glycemic control and less hypoglycemia than continuing to titrate oral agents.25
Despite these benefits, however, insulin use often is not optimized in the elderly due to concerns about hypoglycemia and difficulty of administration. Safe use of insulin requires careful selection of an appropriate insulin regimen, since insulin use has been identified as an independent predictor of severe hypoglycemia in the elderly.8,26 Before initiating insulin therapy, evaluate whether an older patient is cognitively and physically able to safely use insulin.
Multiple daily injections may be challenging for some older adults. Limit such insulin regimens to use in high-functioning patients. Although all types of insulin can cause hypoglycemia, regimens that mimic insulin’s normal physiologic pattern introduce less hypoglycemic risk. Using basal insulin that mimics the body’s sustained insulin level throughout the day is associated with a lower frequency of hypoglycemia in older people with diabetes than conventional insulin regimens. Long-acting insulins such as glargine, detemir, and degludec offer a lower risk of hypoglycemia, particularly nocturnal hypoglycemia which may contribute to falls.2,27
Neutral protamine Hagedorn insulin and regular insulin are not recommended for use in the elderly, as they do not mimic the body’s natural basal-bolus insulin production and thus put patients at higher risk of hypoglycemia.4 If insulin intensification is needed after optimizing basal insulin, consider adding mealtime insulin with a bolus of rapid-acting insulin (insulin aspart, insulin lispro, or insulin glulisine). It is important to note that the kidneys are responsible for 30% to 80% of insulin clearance from the body.28 Because insulin action is prolonged in renal insufficiency, prevent hypoglycemia by decreasing basal and bolus doses when the eGFR is below 50 mL/min/1.73m2.28
Dosing errors. Whenever possible, use insulin preparations that minimize dosing errors. Insulin pen formulations, if financially feasible, allow more accurate dosing and are more acceptable to older patients compared with syringes and vials.29 Pen formulations are particularly preferable for older patients with impaired vision or dexterity.29 In addition, when patients must mix insulins, errors are more likely to occur. The use of premixed insulin vials has been shown to increase dosing accuracy when used by the elderly.30
Continue to: Combining antidiabetes agents
Combining antidiabetes agents
However, for older patients already taking metformin who are not at their A1C goal, consider adding a second agent, if not contraindicated. Potential agents include a GLP-1 RA, SGLT-2 inhibitor, DDP-4 inhibitor, or short-acting sulfonylurea (glipizide). Alternatively, basal insulin may be added. However, avoid combining a sulfonylurea with insulin, which greatly increases the risk of hypoglycemia.32 Consider adding a GLP-1 RA or basal insulin if the patient is not at his/her target A1C on oral therapy with multiple agents.3
CORRESPONDENCE
Barbara Keber, MD, Glen Cove Hospital, 101 St. Andrews Lane, Glen Cove, NY; [email protected].
1. CDC. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA, U.S. Department of Health and Human Services, 2017.
2. Lee PG, Halter JB. The pathophysiology of hyperglycemia in older adults: clinical considerations. Diabetes Care. 2017;40:444-452.
3. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(Suppl 1):S1–S138.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2017 Executive Summary. Endocr Pract. 2017;23:207–238.
5. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
6. Kezerle L, Shalev L, Barski L. Treating the elderly diabetic patient: special considerations. Diabetes Metab Syndr Obes. 2014;7:391-400.
7. Singh J, Tushar B. Metformin use and vitamin B12 deficiency in patients with type-2 diabetes mellitus. MVP J Med Sci. 2016:3:67-70.
8. Fravel MA, McDanel DL, Ross MB, et al. Special considerations for treatment of type 2 diabetes mellitus in the elderly. Am J Health Syst Pharm. 2011;68:500-509.
9. Hanlon JT, Semla TP, Schmader KE. Alternative medications for medications in the use of high-risk medications in the elderly and potentially harmful drug-disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63:e8–e18.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Schernthaner G, Curie CJ, Schernthaner GH. Do we still need pioglitazone for the treatment of type 2 diabetes? A risk-benefit critique in 2013. Diabetes Care. 2013;36(Suppl 2):S155-S161.
12. Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922.
13. Avogaro A, Dardano A, de Kreutzenberg SV, et al. Dipeptidyl peptidase-4 inhibitors can minimize the hypoglycaemic burden and enhance safety in elderly people with diabetes. Diabetes Obes Metab. 2015;17:107-115.
14. DeVries JH, RosenstocK J. DPP-4 inhibitor-related pancreatitis: rare but real! Diabetes Care. 2017;40:161-163.
15. Leiter LA, Teoh H, Braunwald E, et al. Efficacy and safety of saxagliptin in older participants in the SAVOR-TIMI 53 trial. Diabetes Care. 2015;38:1145-1153.
16. , , , et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. 2011;162:620-626.
17. Raccah D, Miossec P, Esposito V, et al. Efficacy and safety of lixisenatide in elderly (≥65 years old) and very elderly (≥75 years old) patients with type 2 diabetes: an analysis from the GetGoal phase III programme. Diabetes Metab Res Rev. 2015;31:204-211.
18. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
19. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee, LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
20. Lusk KA, Barnes NE. Role of sodium-glucose cotransporter 2 (SGLT2) inhibitors. US Pharm. 2016;41:26-29.
21. U.S. Food and Drug Administration. Sodium-glucose cotransporter-2 (SGLT2) inhibitors. Available at: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm446852.htm. Accessed May 18, 2018.
22. Miller EM. Overview of the efficacy and safety of SGLT-2 inhibitors in type 2 diabetes mellitus. J Fam Pract. 2017;66(2 Suppl):S5-S12.
23. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
24. Owens DR. Clinical evidence for the earlier initiation of insulin therapy in type 2 diabetes. Diabetes Technol Ther. 2013;15:776-785.
25. Papa G, Fedele V, Chiavetta A, et al. Therapeutic options for elderly diabetic subjects: open label, randomized clinical trial of insulin glargine added to oral antidiabetic drugs versus increased dosage of oral antidiabetic drugs. Acta Diabetol. 2008;45:53-59.
26. Fu H, Xie W, Curtis B, et al. Identifying factors associated with hypoglycemia-related hospitalizations among elderly patients with T2DM in the US: a novel approach using influential variable analysis. Curr Med Res Opin. 2014;30:1787-1793.
27. Sorli C, Warren M, Oyer D, et al. Elderly patients with diabetes experience a lower rate of nocturnal hypoglycaemia with insulin degludec than with insulin glargine: a meta-analysis of phase IIIa trials. Drugs Aging. 2013;30:1009-1018.
28. Sampanis CH. Management of hyperglycemia in patients with diabetes mellitus and chronic renal failure. Hippokratia. 2008;12:22-27.
29. Corsi A, Torre E, Coronel GA, et al. Pre-filled insulin pen in newly insulin-treated diabetic patients over 60 years old. Diab Nutr Metab. 1997;10:78-81.
30. Coscelli C, Calabrese G, Fedele D, et al. Use of premixed insulin among the elderly. Reduction of errors in patient preparation of mixtures. Diabetes Care. 1992;15:1628-1630.
31. American Geriatrics Society. Ten things clinicians and patients should question. Available at: http://www.choosingwisely.org/societies/american-geriatrics-society/. Accessed May 18, 2018.
32. Mogensen UM, Andersson C, Fosbøl EL, et al. Sulfonylurea in combination with insulin is associated with increased mortality compared with a combination of insulin and metformin in a retrospective Danish nationwide study. Diabetologia. 2015;58:50-58.
1. CDC. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA, U.S. Department of Health and Human Services, 2017.
2. Lee PG, Halter JB. The pathophysiology of hyperglycemia in older adults: clinical considerations. Diabetes Care. 2017;40:444-452.
3. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(Suppl 1):S1–S138.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2017 Executive Summary. Endocr Pract. 2017;23:207–238.
5. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
6. Kezerle L, Shalev L, Barski L. Treating the elderly diabetic patient: special considerations. Diabetes Metab Syndr Obes. 2014;7:391-400.
7. Singh J, Tushar B. Metformin use and vitamin B12 deficiency in patients with type-2 diabetes mellitus. MVP J Med Sci. 2016:3:67-70.
8. Fravel MA, McDanel DL, Ross MB, et al. Special considerations for treatment of type 2 diabetes mellitus in the elderly. Am J Health Syst Pharm. 2011;68:500-509.
9. Hanlon JT, Semla TP, Schmader KE. Alternative medications for medications in the use of high-risk medications in the elderly and potentially harmful drug-disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63:e8–e18.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Schernthaner G, Curie CJ, Schernthaner GH. Do we still need pioglitazone for the treatment of type 2 diabetes? A risk-benefit critique in 2013. Diabetes Care. 2013;36(Suppl 2):S155-S161.
12. Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922.
13. Avogaro A, Dardano A, de Kreutzenberg SV, et al. Dipeptidyl peptidase-4 inhibitors can minimize the hypoglycaemic burden and enhance safety in elderly people with diabetes. Diabetes Obes Metab. 2015;17:107-115.
14. DeVries JH, RosenstocK J. DPP-4 inhibitor-related pancreatitis: rare but real! Diabetes Care. 2017;40:161-163.
15. Leiter LA, Teoh H, Braunwald E, et al. Efficacy and safety of saxagliptin in older participants in the SAVOR-TIMI 53 trial. Diabetes Care. 2015;38:1145-1153.
16. , , , et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. 2011;162:620-626.
17. Raccah D, Miossec P, Esposito V, et al. Efficacy and safety of lixisenatide in elderly (≥65 years old) and very elderly (≥75 years old) patients with type 2 diabetes: an analysis from the GetGoal phase III programme. Diabetes Metab Res Rev. 2015;31:204-211.
18. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
19. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee, LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
20. Lusk KA, Barnes NE. Role of sodium-glucose cotransporter 2 (SGLT2) inhibitors. US Pharm. 2016;41:26-29.
21. U.S. Food and Drug Administration. Sodium-glucose cotransporter-2 (SGLT2) inhibitors. Available at: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm446852.htm. Accessed May 18, 2018.
22. Miller EM. Overview of the efficacy and safety of SGLT-2 inhibitors in type 2 diabetes mellitus. J Fam Pract. 2017;66(2 Suppl):S5-S12.
23. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
24. Owens DR. Clinical evidence for the earlier initiation of insulin therapy in type 2 diabetes. Diabetes Technol Ther. 2013;15:776-785.
25. Papa G, Fedele V, Chiavetta A, et al. Therapeutic options for elderly diabetic subjects: open label, randomized clinical trial of insulin glargine added to oral antidiabetic drugs versus increased dosage of oral antidiabetic drugs. Acta Diabetol. 2008;45:53-59.
26. Fu H, Xie W, Curtis B, et al. Identifying factors associated with hypoglycemia-related hospitalizations among elderly patients with T2DM in the US: a novel approach using influential variable analysis. Curr Med Res Opin. 2014;30:1787-1793.
27. Sorli C, Warren M, Oyer D, et al. Elderly patients with diabetes experience a lower rate of nocturnal hypoglycaemia with insulin degludec than with insulin glargine: a meta-analysis of phase IIIa trials. Drugs Aging. 2013;30:1009-1018.
28. Sampanis CH. Management of hyperglycemia in patients with diabetes mellitus and chronic renal failure. Hippokratia. 2008;12:22-27.
29. Corsi A, Torre E, Coronel GA, et al. Pre-filled insulin pen in newly insulin-treated diabetic patients over 60 years old. Diab Nutr Metab. 1997;10:78-81.
30. Coscelli C, Calabrese G, Fedele D, et al. Use of premixed insulin among the elderly. Reduction of errors in patient preparation of mixtures. Diabetes Care. 1992;15:1628-1630.
31. American Geriatrics Society. Ten things clinicians and patients should question. Available at: http://www.choosingwisely.org/societies/american-geriatrics-society/. Accessed May 18, 2018.
32. Mogensen UM, Andersson C, Fosbøl EL, et al. Sulfonylurea in combination with insulin is associated with increased mortality compared with a combination of insulin and metformin in a retrospective Danish nationwide study. Diabetologia. 2015;58:50-58.
From The Journal of Family Practice | 2018;67(7):408-410,412-415.
PRACTICE RECOMMENDATIONS
› Allow higher A1C goals for elderly patients who have such comorbid conditions as cognitive dysfunction, dementia, or cardiovascular or renal disease. B
› Look to metformin first in most instances if there are no contraindications. Monitor renal function frequently and vitamin B12 levels periodically. B
› Consider glucagon-like peptide-1 receptor agonists for patients who also have established cardiovascular disease, or consider starting basal insulin instead of using multiple oral agents. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Blood pressure targets: How low should you go (and for whom)?
For more than a century, clinicians have pondered the significance of elevated blood pressure (BP) and its contribution to cardiovascular disease (CVD). While it is widely understood that high BP increases CVD events, and that treatment lowers that risk, the most appropriate BP goal continues to be a subject of debate.
This article briefly summarizes the evidence to support lower BP goals for patients with hypertension who are commonly seen in family practice, including those needing primary prevention, as well as those with, or at high risk for, atherosclerotic cardiovascular disease (ASCVD), patients with diabetes, and those with chronic kidney disease (CKD). Detailed information regarding specific lifestyle and medication treatment recommendations and thresholds for drug therapy is beyond the scope of this review.
A brief history: ACC/AHA guidelines vs JNC 7 and 8
The most recent comprehensive, evidence-based guideline on the prevention, detection, evaluation, and management of high BP in adults was released in late 2017 by the American College of Cardiology (ACC) and the American Heart Association (AHA).1 It was the first comprehensive BP guideline since the Seventh Report of the Joint National Committee (JNC 7) in 2003.2 The new guideline includes several changes, notably in how BP is classified, the threshold for initiation of antihypertensive drug therapy, and target BP.
While widely viewed as positive, the changes in classification, thresholds, and targets for BP therapy have generated controversy and disagreement. Common reasons cited include concern about the data supporting lower thresholds for treatment, the applicability of trial findings to broad patient populations, and the risk of harm with lower BP goals.3 The American Academy of Family Physicians (AAFP) declined to endorse the ACC/AHA guidelines and continues to support the 2014 report by the panel members appointed to the Eighth Joint National Committee (JNC 8) by the National Heart Lung and Blood Institute (NHLBI).4 A primary reason cited for the lack of support for the 2017 guideline is that the majority of recommendations made in the ACC/AHA guideline were not “based on a systematic evidence review.”4 However, there are significant differences in purpose, structure, and scope between the ACC/AHA and JNC 8.
In 2013, the NHLBI announced that it would cease involvement in creating guidelines and transferred responsibility for development to professional organizations.5 Of the 5 guidelines that were in the process of creation (cholesterol, lifestyle intervention, obesity, risk assessment, and high BP), all but the high BP guideline were transferred to the ACC/AHA for completion. The panel members appointed to the JNC 8 elected to publish their recommendations independently and focused only on 3 “critical questions” related to hypertension therapy (eg, therapy initiation, BP goals, and choice of initial agent).6
[polldaddy:10041785]
The JNC 8 report generated significant controversy with the recommendation to relax the BP goal for patients ≥60 years of age to <150/90 mm Hg. Members of the JNC 8 panel who disagreed with this goal published a "minority view" citing concerns about the negative impact the goal would have on CVD and public health, and the "insufficient and inconsistent" evidence supporting relaxed goals.7 The dissenting group cited additional drawbacks of the recommendation, noting that it was highly focused, included data only from randomized controlled trials (RCTs; no meta-analyses or observational data), and did not address or provide guidance on numerous other issues of importance in the care of hypertension.
While the 2017 ACC/AHA guideline also includes formal systematic evidence reviews on major critical questions (ie, optimal BP targets, preferred antihypertensives, the role of home and ambulatory BP monitoring),8 it was designed to be comprehensive and useful for clinicians, providing 106 graded recommendations on commonly encountered questions. It would have been unrealistic to do a formal systematic evidence review and meta-analysis on all clinically relevant questions seen in practice. However, available systematic reviews, meta-analyses, and observational data were scrutinized and used to support the recommendations wherever possible.
Continue to: Say "goodbye" to prehypertension; say "hello" to elevated BP
Say “goodbye” to prehypertension; say “hello” to elevated BP
The 2017 ACC/AHA guideline changed the BP classification for adults (TABLE 11,2). While “normal” remained respectively.1 Removal of the “prehypertension” category and use of the term “elevated” instead was meant to better convey the importance of lifestyle interventions to forestall the development of hypertension.
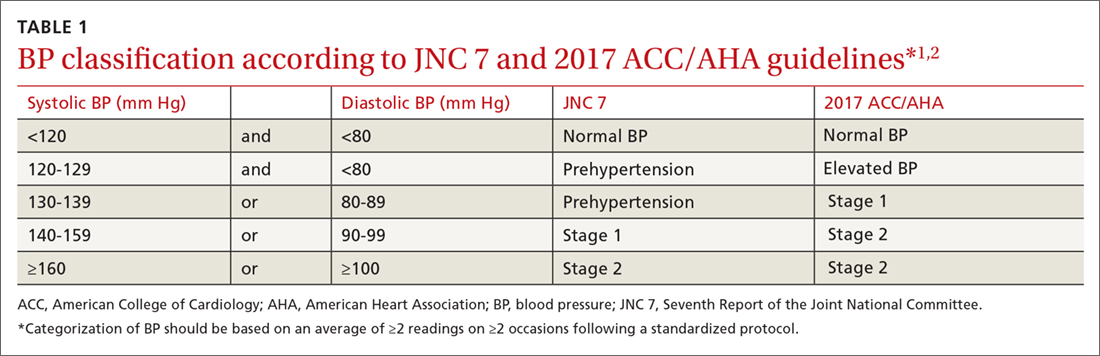
Don’t underestimate the power of BP measurement technique
The importance of appropriate BP measurement technique to confirm the diagnosis of hypertension and assist with medication titration was also emphasized.1 BP measurement technique in usual clinical practice is frequently suboptimal, most commonly resulting in falsely elevated readings.9,10 The guideline recommends the use of out-of-office measurements to confirm elevated clinic readings, screen for white-coat and masked hypertension, and assist in medication adjustment decisions. It is critically important that appropriate BP measurement technique is used, which in many cases, will avoid inappropriate treatment. (See “Getting the hypertension Dx right: Patient positioning matters,” JFP. 2018;67:199-207.)
A look at the evidence supporting lower BP goals
The 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for adults with hypertension commonly seen in clinical practice, including those with CVD or an elevated ASCVD risk (10-year risk ≥10% using the Pooled Cohort Equations11), those with hypertension and low ASCVD risk (10-year risk <10%), and those with hypertension who have concomitant diabetes or CKD.1 The guideline also recommends an SBP goal <130 mm Hg for independently-living, ambulatory older adults (≥65 years) with hypertension.1 TABLE 21,2,6 compares the BP goals in the new 2017 ACC/AHA guidelines to previous recommendations.
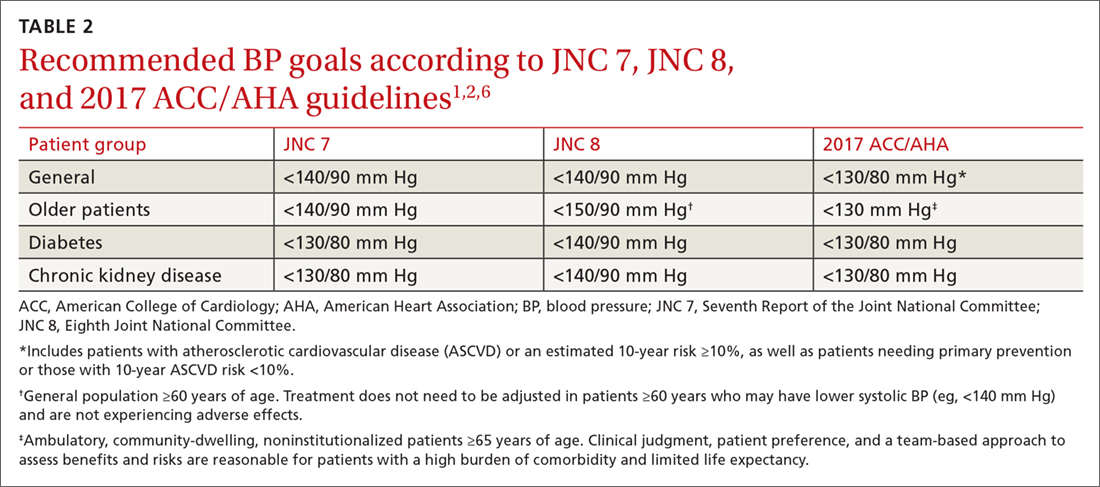
SPRINT. Significant new literature has been generated since the publication of JNC 8 that supports these lower BP goals, particularly in patients with CVD or who are at high ASCVD risk.8,12-15 For example, the Systolic Blood Pressure Intervention Trial (SPRINT) was the largest RCT to assess whether lower BP goals decrease the risk of adverse CVD outcomes.16 In SPRINT, 9361 patients with an SBP ≥130 mm Hg and an increased risk of CVD, but without diabetes or a history of stroke, were randomized to intensive BP treatment (SBP goal <120 mm Hg) or standard treatment (SBP goal <140 mm Hg). After a median follow-up of 3.26 years, the study was stopped early due to a decreased risk in the primary composite outcome of myocardial infarction (MI), other acute coronary syndromes (ACS), stroke, heart failure, or death from CV causes (number needed to treat [NNT] to prevent one event=61).
Intensive treatment was also associated with a lower risk of all-cause mortality (NNT=90), heart failure (NNT=123), death from CV causes (NNT=172), and the primary outcome or death (NNT=52
Continue to: Meta-analyses that have been conducted since SPRINT...
Meta-analyses that have been conducted since SPRINT, and that have incorporated SPRINT data, also support lower BP goals. In the systematic review performed for the 2017 ACC/AHA guideline, an SBP <130 mm Hg compared to a higher BP target was associated with a reduced risk of major CV events, stroke, MI, and heart failure, although not all-cause mortality.8 These findings were largely consistent with other recent meta-analyses.12-15 For example, Bundy et al15 reported significant CV benefit with more vs less intensive BP lowering, whether or not the data from SPRINT were included, with the greatest reduction in risk seen in the groups with highest baseline BP.
It is important to consider a patient’s baseline level of risk when evaluating the absolute benefit of lower BP targets on CV outcomes. For patients with higher CV risk, the absolute benefit of treatment is greater.12-14 These findings support the 2017 ACC/AHA guideline, which recommends initiating drug therapy, in addition to lifestyle modification, in adults with hypertension and high ASCVD risk when the average BP is >130/80 mm Hg, with a goal of <130/80 mm Hg. TABLE 312-15,17-22 summarizes recent systematic reviews and meta-analyses conducted since the publication of JNC 8 that assess the association between intensity of BP lowering and adverse CV and related outcomes.
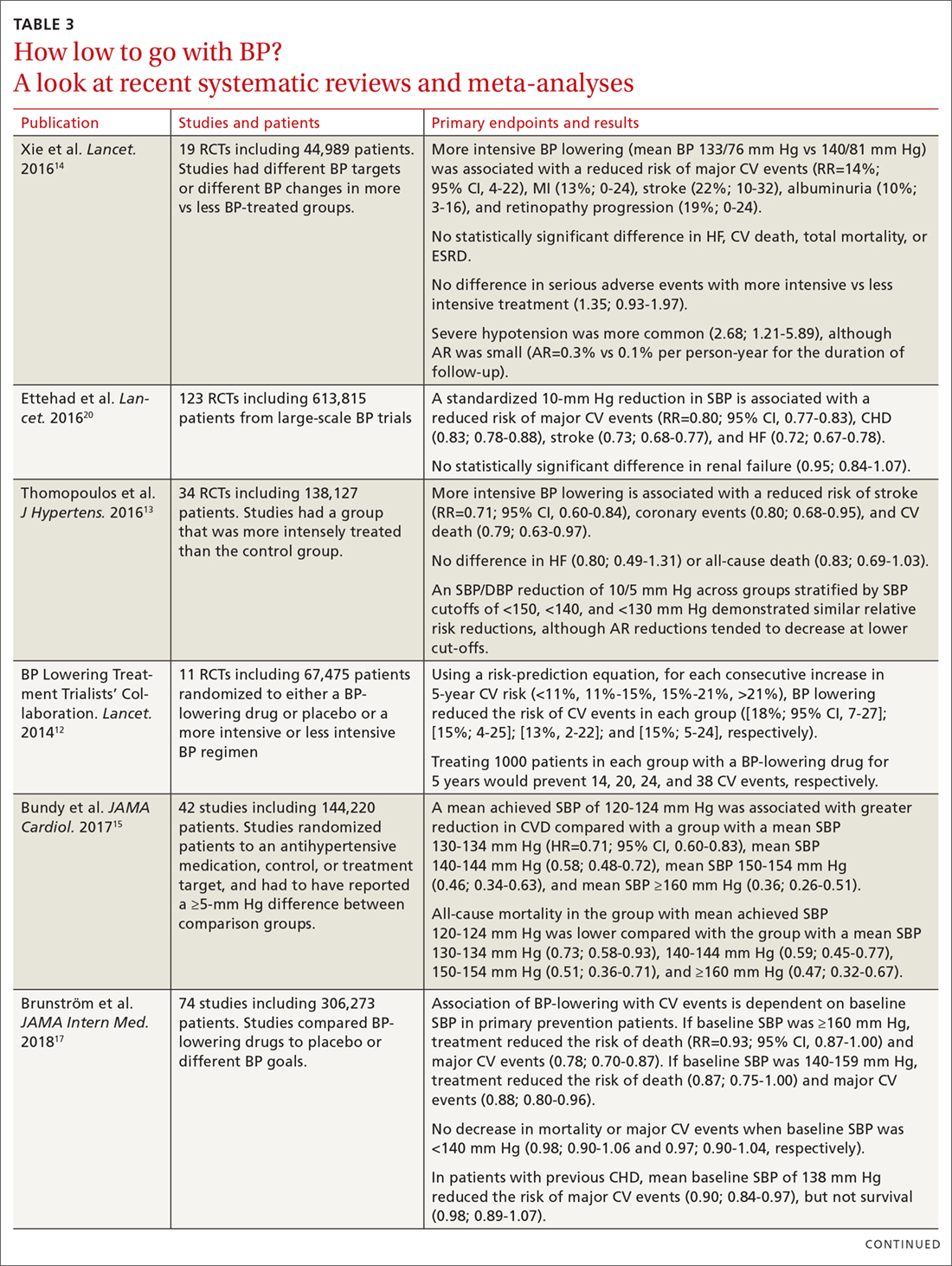
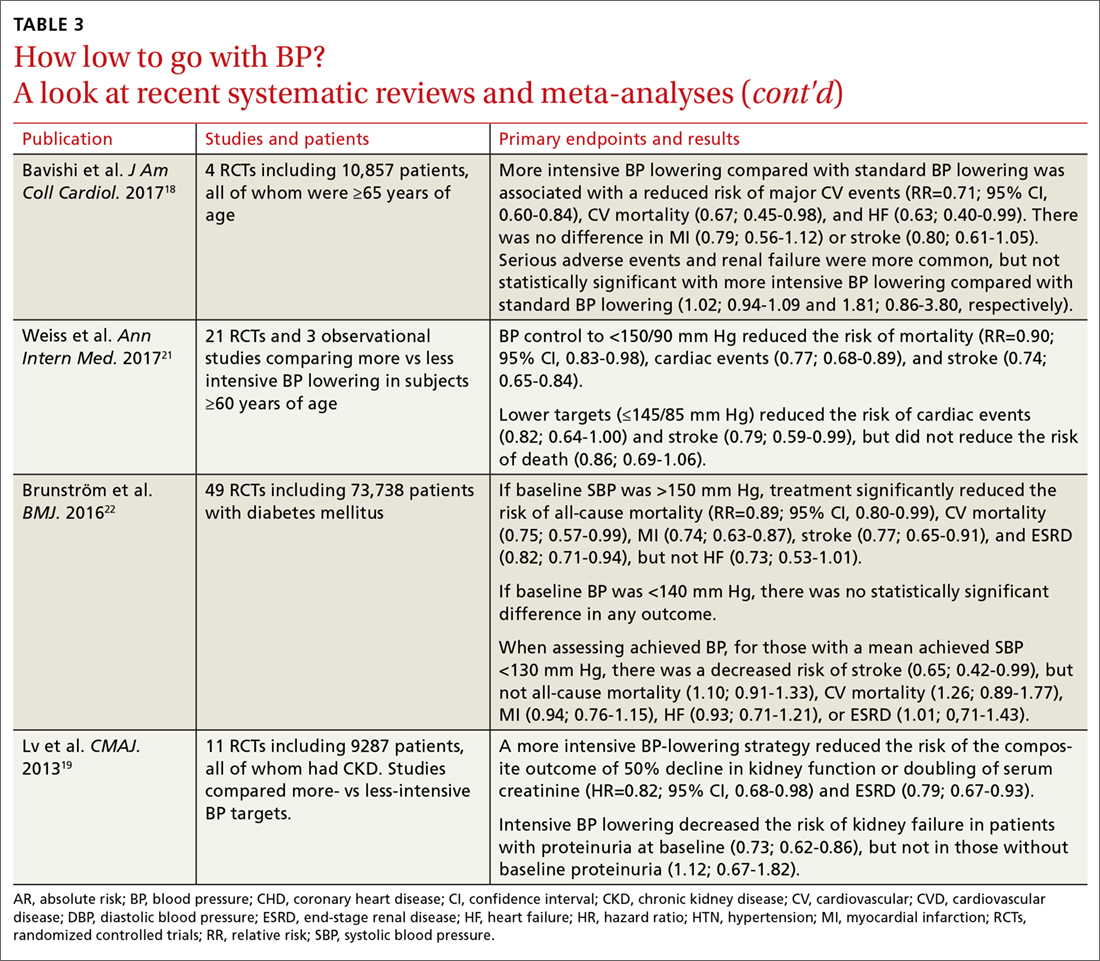
Treating patients with low CV risk
The evidence supporting a lower BP goal in patients with low CV risk is less than for patients at elevated risk. There are no large RCTs for this group that have assessed whether an intensive BP lowering strategy decreases CV outcomes more than a standard BP strategy (eg, <140/90 mm Hg). It is likely that absolute benefit is much smaller than for patients with, or at high risk for, ASCVD.
However, epidemiologic observational studies have indicated a significant log-linear increase in CV mortality starting at an SBP of 115 mm Hg.23 A 20-mm Hg increase in SBP above 115 mm Hg is associated with an approximate doubling of stroke and ischemic heart disease mortality risk.23 Decades worth of exposure to “elevated” BP levels would likely result in significant vascular damage, and attenuation of this process would likely be beneficial.24,25 An RCT specifically designed to test this hypothesis, however, would not be pragmatic considering the substantial number of patient-years that would be required.
Due to insufficient data documenting the value of antihypertensive drug therapy for primary prevention in adults with “elevated” BP and stage 1 hypertension at low risk for CVD, the 2017 ACC/AHA guideline recommends that drug therapy be initiated for all adults only when their BP average is ≥140/90 mm Hg.1 In contrast, for patients needing secondary prevention and for those with elevated CVD risk, the guideline recommends medication in addition to lifestyle modifications once the average BP is ≥130/80 mm Hg. The recommendation to withhold drug therapy until the BP is ≥140/90 mm Hg in patients needing primary prevention is supported by a new meta-analysis of 74 trials with 306,273 participants that aimed to assess the association between BP-lowering treatment and death and CVD at various BP levels.17 In this analysis, pharmacologic treatment was associated with a reduced risk of all-cause mortality, major CVD events, and coronary heart disease if the SBP was ≥140 mm Hg.
Continue to: Treating older patients
Treating older patients
Significant controversy has existed regarding the optimal BP goal in older patients, particularly once the JNC 8 recommended relaxing the SBP goal to <150 mm Hg for pateints ≥60 years of age.6,7 This recommendation was consistent with the guideline from the American College of Physicians (ACP)/AAFP,26 which also recommended a lower SBP of <140 mm Hg in patients with a history of stroke or transient ischemic attack and those at high CV risk.26
Evidence is available, however, supporting more intensive BP goals in older independently-living ambulatory adults. A pre-planned subgroup analysis was conducted in 2636 SPRINT participants ≥75 years of age.27 Similar to the overall experience in SPRINT, lower SBP goals were associated with significant reductions in CV events, including the composite CVD primary outcome (NNT=27), heart failure (NNT=63), nonfatal heart failure (NNT=66), and all-cause mortality (NNT=41). In addition, the relative benefits were approximately equal whether the patients were the most fit, non-fit, or frail, with the absolute benefit being greatest in those who were frail (recognizing that the SPRINT participants were independently-living ambulatory adults). While the absolute rate of serious adverse events was higher in the more intensive BP goal group, there was no statistically significant difference in the incidence of hypotension, orthostatic hypotension, syncope, electrolyte abnormalities, or acute kidney injury or renal failure.
Use of lower BP goals than recommended by JNC 8 was also supported by another recent meta-analysis that compared the outcomes of intensive BP lowering (SBP <140 mm Hg) to a standard BP-lowering strategy (SBP <150 mm Hg).18 Using a random-effects model, more intensive BP lowering was associated with a significant reduction in major adverse CV events (29%), CV mortality (33%), and heart failure (37%), with no increase in serious adverse events or renal failure. Findings with the fixed-effects model used to confirm results were largely consistent, with the exception of a possible increase in renal failure.
Although the evidence supporting lower BP goals in older, ambulatory, noninstitutionalized patients is sound, it is important to consider a patient’s overall disease burden. For older adults with multiple comorbidities and limited life expectancy, as well as those who are nonambulatory or institutionalized, decisions on the intensity of BP lowering should be made using a team-based approach, weighing the risks and benefits.1
Continue to: Treating patients with diabetes
Treating patients with diabetes
The most appropriate BP goal for patients with diabetes has been the subject of much debate, with different goals recommended in different guidelines (TABLE 21,2,6). The most recent American Diabetes Association guideline recommends a BP goal <140/90 mm Hg for most patients, with lower targets (<130/80 mm Hg) for patients at high CV risk if it is achievable without undue treatment burden,28 whereas the 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for all adults with diabetes.1
The ACCORD trial. There is limited evidence to suggest which BP goal is most appropriate for patients with diabetes. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial is the only RCT specifically designed to assess the impact of intensive vs standard BP goals in patients with diabetes.29 In ACCORD, 4733 patients with type 2 diabetes were randomized to either an intensive BP-lowering group (SBP <120 mm Hg) or a standard BP-lowering group (SBP <140 mm Hg). After a mean follow-up of 4.7 years, there was no difference in the primary composite endpoint of nonfatal MI, nonfatal stroke, or death from CV causes. However, the risk of stroke was reduced (NNT=89). Interpretation of ACCORD is limited due to its factorial design and because the trial was significantly underpowered.
Systematic reviews and meta-analyses. Literature supporting lower BP goals in patients with diabetes primarily comes from systematic reviews and meta-analyses.30 In the evidence-based review performed for the 2017 ACC/AHA guidelines, more intensive treatment was associated with a decrease in fatal or nonfatal stroke.8 The results from the ACCORD trial and SPRINT are consistent,31 and a sub-study of SPRINT patients with pre-diabetes showed preservation of CV benefit.32 Also, a meta-analysis of subgroups of trial participants with diabetes showed that more intensive BP lowering in patients is associated with a decrease in major CV events.14
Treating patients with chronic kidney disease
As with diabetes and older patients, recommended goals for patients with CKD have varied (TABLE 21,2,6). The Kidney Disease Improving Global Outcomes (KDIGO) 2012 guideline recommended the same target BP as JNC 7 and the 2017 ACC/AHA guideline: ≤130/80 mm Hg in patients with CKD and urine albumin excretion ≥30 mg/24 hours (or equivalent).1,2,33 KDIGO recommended a more relaxed target (≤140/90 mm Hg), however, for patients with CKD and urine albumin excretion <30 mg/24 hours.1,33
Scant data exist from RCTs designed to assess the CV effects of intensive BP targets in patients with CKD. In SPRINT, where 28% of patients had stage 3 or 4 CKD, benefits of more intensive therapy were similar to those observed in the overall cohort.16,34 While some RCTs have assessed the effect of more intensive BP lowering on progression of CKD, they were not specifically designed or powered to address CV outcomes.35,36
Continue to: In recent meta-analyses assessing the effects...
In recent meta-analyses assessing the effects of intensive BP lowering on renal and CV events in patients with CKD, a lower BP strategy was not associated with a decrease in CV events.8,14,19 However, more intensive therapy was associated with a 17% reduced risk of composite kidney failure events and an 18% reduction in end-stage kidney disease.19 The risk of kidney failure with lower BP goals was 27% lower in patients with baseline proteinuria, but was not significant in patients who did not have proteinuria.19
Evidence supports lower BP goals, but guidelines should guide
The lower BP goals advised in the 2017 ACC/AHA guideline are supported by substantial new high-quality evidence that was not available at the time of the JNC 8 report.1 The strongest evidence for lower goals is found in patients with, or at high risk for, CVD, but other patients commonly seen by primary care providers, including those at lower CVD risk, older patients, and those with diabetes or CKD are also likely to benefit.1
Despite the debates, it is important to remember that guidelines are intended to “guide.” As stated in the guideline, “Guidelines are intended to define practices meeting the needs of patients in most, but not all, circumstances and should not replace clinical judgment.”1 They should be easy to understand and apply, and a consistent, evidence-based BP goal of <130/80 mm Hg for most patients facilitates implementation.
Although more of the US population is categorized as hypertensive under the new guideline (46% now vs 32% before), only 1.9% more require drug therapy, as the vast majority of the newly classified hypertensives are primary prevention patients for whom only lifestyle modification is recommended.37 However, to attain these goals, greater emphasis will be needed on utilizing team-based care, health information technology including electronic medical records and telehealth, performance measures, quality improvement strategies, and financial incentives.1
Finally, as emphasized in the guidelines, BP monitoring technique matters. Clinicians should not accept flawed BP measurement techniques any more than they would accept flawed results from studies performed incorrectly.
CORRESPONDENCE
Eric J. MacLaughlin, PharmD, BCPS, FASHP, FCCP, Texas Tech University Health Sciences Center,1300 S. Coulter Dr., Amarillo, TX 79106; [email protected].
ACKNOWLEDGEMENTS
The authors thank Paul K. Whelton, MB, MD, MSc, FAHA, and Robert M. Carey, MD, FAHA, for their review of this manuscript.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
2. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560-2572.
3. Wilt TJ, Kansagara D, Qaseem A; Clinical Guidelines Committee of the American College of Physicians. Hypertension limbo: balancing benefits, harms, and patient preferences before we lower the bar on blood pressure. Ann Intern Med. 2018;168:369-370.
4. American Academy of Family Physicians. AAFP decides to not endorse AHA/ACC hypertension guideline. Available at: https://www.aafp.org/news/health-of-the-public/20171212notendorseaha-accgdlne.html. Accessed January 9, 2018.
5. Gibbons GH, Shurin SB, Mensah GA, et al. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation. 2013;128:1713-1715.
6. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
7. Wright JT Jr., Fine LJ, Lackland DT, et al. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499-503.
8. Reboussin DM, Allen NB, Griswold ME, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e116-e135.
9. Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation. 2016;134:904-905.
10. Burgess SE, MacLaughlin EJ, Smith PA, et al. Blood pressure rising: differences between current clinical and recommended measurement techniques. J Am Soc Hypertens. 2011;5:484-488.
11. American College of Cardiology. ASCVD Risk Estimator Plus. Available at: http://tools.acc.org/ascvd-risk-estimator-plus/#!/calculate/estimate/. Accessed January 9, 2018.
12. The Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591-598.
13. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels - updated overview and meta-analyses of randomized trials. J Hypertens. 2016;34:613-622.
14. Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435-443.
15. Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2:775-781.
16. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
17. Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:28-36.
18. Bavishi C, Bangalore S, Messerli FH. Outcomes of intensive blood pressure lowering in older hypertensive patients. J Am Coll Cardiol. 2017;69:486-493.
19. Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949-957.
20. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957-967.
21. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med. 2017;166:419-429.
22. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
23. Lewington S, Clarke R, Qizilbash N, et al; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913.
24. Guo X, Zhang X, Guo L, et al. Association between pre-hypertension and cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Curr Hypertens Rep. 2013;15:703-716.
25. Huang Y, Cai X, Li Y, et al. Prehypertension and the risk of stroke: a meta-analysis. Neurology. 2014;82:1153-1161.
26. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430-437.
27. Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673-2682.
28. American Diabetes Association. 9. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S86-S104.
29. The ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
30. Reboldi G, Gentile G, Angeli F, et al. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J Hypertens. 2011;29:1253-1269.
31. Perkovic V, Rodgers A. Redefining blood-pressure targets—SPRINT starts the marathon. N Engl J Med. 2015;373:2175-2178.
32. Bress AP, King JB, Kreider KE, et al. Effect of intensive versus standard blood pressure treatment according to baseline prediabetes status: a post hoc analysis of a randomized trial. Diabetes Care. 2017 Aug 9.
33. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:337-414.
34. Cheung AK, Rahman M, Reboussin DM, et al. Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017;28:2812-2823.
35. Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939-946.
36. Wright JT Jr., Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421-2431.
37. Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 American College of Cardiology/American Heart Association High Blood Pressure Guideline. J Am Coll Cardiol. 2018;71:109-188.
For more than a century, clinicians have pondered the significance of elevated blood pressure (BP) and its contribution to cardiovascular disease (CVD). While it is widely understood that high BP increases CVD events, and that treatment lowers that risk, the most appropriate BP goal continues to be a subject of debate.
This article briefly summarizes the evidence to support lower BP goals for patients with hypertension who are commonly seen in family practice, including those needing primary prevention, as well as those with, or at high risk for, atherosclerotic cardiovascular disease (ASCVD), patients with diabetes, and those with chronic kidney disease (CKD). Detailed information regarding specific lifestyle and medication treatment recommendations and thresholds for drug therapy is beyond the scope of this review.
A brief history: ACC/AHA guidelines vs JNC 7 and 8
The most recent comprehensive, evidence-based guideline on the prevention, detection, evaluation, and management of high BP in adults was released in late 2017 by the American College of Cardiology (ACC) and the American Heart Association (AHA).1 It was the first comprehensive BP guideline since the Seventh Report of the Joint National Committee (JNC 7) in 2003.2 The new guideline includes several changes, notably in how BP is classified, the threshold for initiation of antihypertensive drug therapy, and target BP.
While widely viewed as positive, the changes in classification, thresholds, and targets for BP therapy have generated controversy and disagreement. Common reasons cited include concern about the data supporting lower thresholds for treatment, the applicability of trial findings to broad patient populations, and the risk of harm with lower BP goals.3 The American Academy of Family Physicians (AAFP) declined to endorse the ACC/AHA guidelines and continues to support the 2014 report by the panel members appointed to the Eighth Joint National Committee (JNC 8) by the National Heart Lung and Blood Institute (NHLBI).4 A primary reason cited for the lack of support for the 2017 guideline is that the majority of recommendations made in the ACC/AHA guideline were not “based on a systematic evidence review.”4 However, there are significant differences in purpose, structure, and scope between the ACC/AHA and JNC 8.
In 2013, the NHLBI announced that it would cease involvement in creating guidelines and transferred responsibility for development to professional organizations.5 Of the 5 guidelines that were in the process of creation (cholesterol, lifestyle intervention, obesity, risk assessment, and high BP), all but the high BP guideline were transferred to the ACC/AHA for completion. The panel members appointed to the JNC 8 elected to publish their recommendations independently and focused only on 3 “critical questions” related to hypertension therapy (eg, therapy initiation, BP goals, and choice of initial agent).6
[polldaddy:10041785]
The JNC 8 report generated significant controversy with the recommendation to relax the BP goal for patients ≥60 years of age to <150/90 mm Hg. Members of the JNC 8 panel who disagreed with this goal published a "minority view" citing concerns about the negative impact the goal would have on CVD and public health, and the "insufficient and inconsistent" evidence supporting relaxed goals.7 The dissenting group cited additional drawbacks of the recommendation, noting that it was highly focused, included data only from randomized controlled trials (RCTs; no meta-analyses or observational data), and did not address or provide guidance on numerous other issues of importance in the care of hypertension.
While the 2017 ACC/AHA guideline also includes formal systematic evidence reviews on major critical questions (ie, optimal BP targets, preferred antihypertensives, the role of home and ambulatory BP monitoring),8 it was designed to be comprehensive and useful for clinicians, providing 106 graded recommendations on commonly encountered questions. It would have been unrealistic to do a formal systematic evidence review and meta-analysis on all clinically relevant questions seen in practice. However, available systematic reviews, meta-analyses, and observational data were scrutinized and used to support the recommendations wherever possible.
Continue to: Say "goodbye" to prehypertension; say "hello" to elevated BP
Say “goodbye” to prehypertension; say “hello” to elevated BP
The 2017 ACC/AHA guideline changed the BP classification for adults (TABLE 11,2). While “normal” remained respectively.1 Removal of the “prehypertension” category and use of the term “elevated” instead was meant to better convey the importance of lifestyle interventions to forestall the development of hypertension.

Don’t underestimate the power of BP measurement technique
The importance of appropriate BP measurement technique to confirm the diagnosis of hypertension and assist with medication titration was also emphasized.1 BP measurement technique in usual clinical practice is frequently suboptimal, most commonly resulting in falsely elevated readings.9,10 The guideline recommends the use of out-of-office measurements to confirm elevated clinic readings, screen for white-coat and masked hypertension, and assist in medication adjustment decisions. It is critically important that appropriate BP measurement technique is used, which in many cases, will avoid inappropriate treatment. (See “Getting the hypertension Dx right: Patient positioning matters,” JFP. 2018;67:199-207.)
A look at the evidence supporting lower BP goals
The 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for adults with hypertension commonly seen in clinical practice, including those with CVD or an elevated ASCVD risk (10-year risk ≥10% using the Pooled Cohort Equations11), those with hypertension and low ASCVD risk (10-year risk <10%), and those with hypertension who have concomitant diabetes or CKD.1 The guideline also recommends an SBP goal <130 mm Hg for independently-living, ambulatory older adults (≥65 years) with hypertension.1 TABLE 21,2,6 compares the BP goals in the new 2017 ACC/AHA guidelines to previous recommendations.

SPRINT. Significant new literature has been generated since the publication of JNC 8 that supports these lower BP goals, particularly in patients with CVD or who are at high ASCVD risk.8,12-15 For example, the Systolic Blood Pressure Intervention Trial (SPRINT) was the largest RCT to assess whether lower BP goals decrease the risk of adverse CVD outcomes.16 In SPRINT, 9361 patients with an SBP ≥130 mm Hg and an increased risk of CVD, but without diabetes or a history of stroke, were randomized to intensive BP treatment (SBP goal <120 mm Hg) or standard treatment (SBP goal <140 mm Hg). After a median follow-up of 3.26 years, the study was stopped early due to a decreased risk in the primary composite outcome of myocardial infarction (MI), other acute coronary syndromes (ACS), stroke, heart failure, or death from CV causes (number needed to treat [NNT] to prevent one event=61).
Intensive treatment was also associated with a lower risk of all-cause mortality (NNT=90), heart failure (NNT=123), death from CV causes (NNT=172), and the primary outcome or death (NNT=52
Continue to: Meta-analyses that have been conducted since SPRINT...
Meta-analyses that have been conducted since SPRINT, and that have incorporated SPRINT data, also support lower BP goals. In the systematic review performed for the 2017 ACC/AHA guideline, an SBP <130 mm Hg compared to a higher BP target was associated with a reduced risk of major CV events, stroke, MI, and heart failure, although not all-cause mortality.8 These findings were largely consistent with other recent meta-analyses.12-15 For example, Bundy et al15 reported significant CV benefit with more vs less intensive BP lowering, whether or not the data from SPRINT were included, with the greatest reduction in risk seen in the groups with highest baseline BP.
It is important to consider a patient’s baseline level of risk when evaluating the absolute benefit of lower BP targets on CV outcomes. For patients with higher CV risk, the absolute benefit of treatment is greater.12-14 These findings support the 2017 ACC/AHA guideline, which recommends initiating drug therapy, in addition to lifestyle modification, in adults with hypertension and high ASCVD risk when the average BP is >130/80 mm Hg, with a goal of <130/80 mm Hg. TABLE 312-15,17-22 summarizes recent systematic reviews and meta-analyses conducted since the publication of JNC 8 that assess the association between intensity of BP lowering and adverse CV and related outcomes.


Treating patients with low CV risk
The evidence supporting a lower BP goal in patients with low CV risk is less than for patients at elevated risk. There are no large RCTs for this group that have assessed whether an intensive BP lowering strategy decreases CV outcomes more than a standard BP strategy (eg, <140/90 mm Hg). It is likely that absolute benefit is much smaller than for patients with, or at high risk for, ASCVD.
However, epidemiologic observational studies have indicated a significant log-linear increase in CV mortality starting at an SBP of 115 mm Hg.23 A 20-mm Hg increase in SBP above 115 mm Hg is associated with an approximate doubling of stroke and ischemic heart disease mortality risk.23 Decades worth of exposure to “elevated” BP levels would likely result in significant vascular damage, and attenuation of this process would likely be beneficial.24,25 An RCT specifically designed to test this hypothesis, however, would not be pragmatic considering the substantial number of patient-years that would be required.
Due to insufficient data documenting the value of antihypertensive drug therapy for primary prevention in adults with “elevated” BP and stage 1 hypertension at low risk for CVD, the 2017 ACC/AHA guideline recommends that drug therapy be initiated for all adults only when their BP average is ≥140/90 mm Hg.1 In contrast, for patients needing secondary prevention and for those with elevated CVD risk, the guideline recommends medication in addition to lifestyle modifications once the average BP is ≥130/80 mm Hg. The recommendation to withhold drug therapy until the BP is ≥140/90 mm Hg in patients needing primary prevention is supported by a new meta-analysis of 74 trials with 306,273 participants that aimed to assess the association between BP-lowering treatment and death and CVD at various BP levels.17 In this analysis, pharmacologic treatment was associated with a reduced risk of all-cause mortality, major CVD events, and coronary heart disease if the SBP was ≥140 mm Hg.
Continue to: Treating older patients
Treating older patients
Significant controversy has existed regarding the optimal BP goal in older patients, particularly once the JNC 8 recommended relaxing the SBP goal to <150 mm Hg for pateints ≥60 years of age.6,7 This recommendation was consistent with the guideline from the American College of Physicians (ACP)/AAFP,26 which also recommended a lower SBP of <140 mm Hg in patients with a history of stroke or transient ischemic attack and those at high CV risk.26
Evidence is available, however, supporting more intensive BP goals in older independently-living ambulatory adults. A pre-planned subgroup analysis was conducted in 2636 SPRINT participants ≥75 years of age.27 Similar to the overall experience in SPRINT, lower SBP goals were associated with significant reductions in CV events, including the composite CVD primary outcome (NNT=27), heart failure (NNT=63), nonfatal heart failure (NNT=66), and all-cause mortality (NNT=41). In addition, the relative benefits were approximately equal whether the patients were the most fit, non-fit, or frail, with the absolute benefit being greatest in those who were frail (recognizing that the SPRINT participants were independently-living ambulatory adults). While the absolute rate of serious adverse events was higher in the more intensive BP goal group, there was no statistically significant difference in the incidence of hypotension, orthostatic hypotension, syncope, electrolyte abnormalities, or acute kidney injury or renal failure.
Use of lower BP goals than recommended by JNC 8 was also supported by another recent meta-analysis that compared the outcomes of intensive BP lowering (SBP <140 mm Hg) to a standard BP-lowering strategy (SBP <150 mm Hg).18 Using a random-effects model, more intensive BP lowering was associated with a significant reduction in major adverse CV events (29%), CV mortality (33%), and heart failure (37%), with no increase in serious adverse events or renal failure. Findings with the fixed-effects model used to confirm results were largely consistent, with the exception of a possible increase in renal failure.
Although the evidence supporting lower BP goals in older, ambulatory, noninstitutionalized patients is sound, it is important to consider a patient’s overall disease burden. For older adults with multiple comorbidities and limited life expectancy, as well as those who are nonambulatory or institutionalized, decisions on the intensity of BP lowering should be made using a team-based approach, weighing the risks and benefits.1
Continue to: Treating patients with diabetes
Treating patients with diabetes
The most appropriate BP goal for patients with diabetes has been the subject of much debate, with different goals recommended in different guidelines (TABLE 21,2,6). The most recent American Diabetes Association guideline recommends a BP goal <140/90 mm Hg for most patients, with lower targets (<130/80 mm Hg) for patients at high CV risk if it is achievable without undue treatment burden,28 whereas the 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for all adults with diabetes.1
The ACCORD trial. There is limited evidence to suggest which BP goal is most appropriate for patients with diabetes. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial is the only RCT specifically designed to assess the impact of intensive vs standard BP goals in patients with diabetes.29 In ACCORD, 4733 patients with type 2 diabetes were randomized to either an intensive BP-lowering group (SBP <120 mm Hg) or a standard BP-lowering group (SBP <140 mm Hg). After a mean follow-up of 4.7 years, there was no difference in the primary composite endpoint of nonfatal MI, nonfatal stroke, or death from CV causes. However, the risk of stroke was reduced (NNT=89). Interpretation of ACCORD is limited due to its factorial design and because the trial was significantly underpowered.

Systematic reviews and meta-analyses. Literature supporting lower BP goals in patients with diabetes primarily comes from systematic reviews and meta-analyses.30 In the evidence-based review performed for the 2017 ACC/AHA guidelines, more intensive treatment was associated with a decrease in fatal or nonfatal stroke.8 The results from the ACCORD trial and SPRINT are consistent,31 and a sub-study of SPRINT patients with pre-diabetes showed preservation of CV benefit.32 Also, a meta-analysis of subgroups of trial participants with diabetes showed that more intensive BP lowering in patients is associated with a decrease in major CV events.14
Treating patients with chronic kidney disease
As with diabetes and older patients, recommended goals for patients with CKD have varied (TABLE 21,2,6). The Kidney Disease Improving Global Outcomes (KDIGO) 2012 guideline recommended the same target BP as JNC 7 and the 2017 ACC/AHA guideline: ≤130/80 mm Hg in patients with CKD and urine albumin excretion ≥30 mg/24 hours (or equivalent).1,2,33 KDIGO recommended a more relaxed target (≤140/90 mm Hg), however, for patients with CKD and urine albumin excretion <30 mg/24 hours.1,33
Scant data exist from RCTs designed to assess the CV effects of intensive BP targets in patients with CKD. In SPRINT, where 28% of patients had stage 3 or 4 CKD, benefits of more intensive therapy were similar to those observed in the overall cohort.16,34 While some RCTs have assessed the effect of more intensive BP lowering on progression of CKD, they were not specifically designed or powered to address CV outcomes.35,36
Continue to: In recent meta-analyses assessing the effects...
In recent meta-analyses assessing the effects of intensive BP lowering on renal and CV events in patients with CKD, a lower BP strategy was not associated with a decrease in CV events.8,14,19 However, more intensive therapy was associated with a 17% reduced risk of composite kidney failure events and an 18% reduction in end-stage kidney disease.19 The risk of kidney failure with lower BP goals was 27% lower in patients with baseline proteinuria, but was not significant in patients who did not have proteinuria.19
Evidence supports lower BP goals, but guidelines should guide
The lower BP goals advised in the 2017 ACC/AHA guideline are supported by substantial new high-quality evidence that was not available at the time of the JNC 8 report.1 The strongest evidence for lower goals is found in patients with, or at high risk for, CVD, but other patients commonly seen by primary care providers, including those at lower CVD risk, older patients, and those with diabetes or CKD are also likely to benefit.1
Despite the debates, it is important to remember that guidelines are intended to “guide.” As stated in the guideline, “Guidelines are intended to define practices meeting the needs of patients in most, but not all, circumstances and should not replace clinical judgment.”1 They should be easy to understand and apply, and a consistent, evidence-based BP goal of <130/80 mm Hg for most patients facilitates implementation.
Although more of the US population is categorized as hypertensive under the new guideline (46% now vs 32% before), only 1.9% more require drug therapy, as the vast majority of the newly classified hypertensives are primary prevention patients for whom only lifestyle modification is recommended.37 However, to attain these goals, greater emphasis will be needed on utilizing team-based care, health information technology including electronic medical records and telehealth, performance measures, quality improvement strategies, and financial incentives.1
Finally, as emphasized in the guidelines, BP monitoring technique matters. Clinicians should not accept flawed BP measurement techniques any more than they would accept flawed results from studies performed incorrectly.
CORRESPONDENCE
Eric J. MacLaughlin, PharmD, BCPS, FASHP, FCCP, Texas Tech University Health Sciences Center,1300 S. Coulter Dr., Amarillo, TX 79106; [email protected].
ACKNOWLEDGEMENTS
The authors thank Paul K. Whelton, MB, MD, MSc, FAHA, and Robert M. Carey, MD, FAHA, for their review of this manuscript.
For more than a century, clinicians have pondered the significance of elevated blood pressure (BP) and its contribution to cardiovascular disease (CVD). While it is widely understood that high BP increases CVD events, and that treatment lowers that risk, the most appropriate BP goal continues to be a subject of debate.
This article briefly summarizes the evidence to support lower BP goals for patients with hypertension who are commonly seen in family practice, including those needing primary prevention, as well as those with, or at high risk for, atherosclerotic cardiovascular disease (ASCVD), patients with diabetes, and those with chronic kidney disease (CKD). Detailed information regarding specific lifestyle and medication treatment recommendations and thresholds for drug therapy is beyond the scope of this review.
A brief history: ACC/AHA guidelines vs JNC 7 and 8
The most recent comprehensive, evidence-based guideline on the prevention, detection, evaluation, and management of high BP in adults was released in late 2017 by the American College of Cardiology (ACC) and the American Heart Association (AHA).1 It was the first comprehensive BP guideline since the Seventh Report of the Joint National Committee (JNC 7) in 2003.2 The new guideline includes several changes, notably in how BP is classified, the threshold for initiation of antihypertensive drug therapy, and target BP.
While widely viewed as positive, the changes in classification, thresholds, and targets for BP therapy have generated controversy and disagreement. Common reasons cited include concern about the data supporting lower thresholds for treatment, the applicability of trial findings to broad patient populations, and the risk of harm with lower BP goals.3 The American Academy of Family Physicians (AAFP) declined to endorse the ACC/AHA guidelines and continues to support the 2014 report by the panel members appointed to the Eighth Joint National Committee (JNC 8) by the National Heart Lung and Blood Institute (NHLBI).4 A primary reason cited for the lack of support for the 2017 guideline is that the majority of recommendations made in the ACC/AHA guideline were not “based on a systematic evidence review.”4 However, there are significant differences in purpose, structure, and scope between the ACC/AHA and JNC 8.
In 2013, the NHLBI announced that it would cease involvement in creating guidelines and transferred responsibility for development to professional organizations.5 Of the 5 guidelines that were in the process of creation (cholesterol, lifestyle intervention, obesity, risk assessment, and high BP), all but the high BP guideline were transferred to the ACC/AHA for completion. The panel members appointed to the JNC 8 elected to publish their recommendations independently and focused only on 3 “critical questions” related to hypertension therapy (eg, therapy initiation, BP goals, and choice of initial agent).6
[polldaddy:10041785]
The JNC 8 report generated significant controversy with the recommendation to relax the BP goal for patients ≥60 years of age to <150/90 mm Hg. Members of the JNC 8 panel who disagreed with this goal published a "minority view" citing concerns about the negative impact the goal would have on CVD and public health, and the "insufficient and inconsistent" evidence supporting relaxed goals.7 The dissenting group cited additional drawbacks of the recommendation, noting that it was highly focused, included data only from randomized controlled trials (RCTs; no meta-analyses or observational data), and did not address or provide guidance on numerous other issues of importance in the care of hypertension.
While the 2017 ACC/AHA guideline also includes formal systematic evidence reviews on major critical questions (ie, optimal BP targets, preferred antihypertensives, the role of home and ambulatory BP monitoring),8 it was designed to be comprehensive and useful for clinicians, providing 106 graded recommendations on commonly encountered questions. It would have been unrealistic to do a formal systematic evidence review and meta-analysis on all clinically relevant questions seen in practice. However, available systematic reviews, meta-analyses, and observational data were scrutinized and used to support the recommendations wherever possible.
Continue to: Say "goodbye" to prehypertension; say "hello" to elevated BP
Say “goodbye” to prehypertension; say “hello” to elevated BP
The 2017 ACC/AHA guideline changed the BP classification for adults (TABLE 11,2). While “normal” remained respectively.1 Removal of the “prehypertension” category and use of the term “elevated” instead was meant to better convey the importance of lifestyle interventions to forestall the development of hypertension.

Don’t underestimate the power of BP measurement technique
The importance of appropriate BP measurement technique to confirm the diagnosis of hypertension and assist with medication titration was also emphasized.1 BP measurement technique in usual clinical practice is frequently suboptimal, most commonly resulting in falsely elevated readings.9,10 The guideline recommends the use of out-of-office measurements to confirm elevated clinic readings, screen for white-coat and masked hypertension, and assist in medication adjustment decisions. It is critically important that appropriate BP measurement technique is used, which in many cases, will avoid inappropriate treatment. (See “Getting the hypertension Dx right: Patient positioning matters,” JFP. 2018;67:199-207.)
A look at the evidence supporting lower BP goals
The 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for adults with hypertension commonly seen in clinical practice, including those with CVD or an elevated ASCVD risk (10-year risk ≥10% using the Pooled Cohort Equations11), those with hypertension and low ASCVD risk (10-year risk <10%), and those with hypertension who have concomitant diabetes or CKD.1 The guideline also recommends an SBP goal <130 mm Hg for independently-living, ambulatory older adults (≥65 years) with hypertension.1 TABLE 21,2,6 compares the BP goals in the new 2017 ACC/AHA guidelines to previous recommendations.

SPRINT. Significant new literature has been generated since the publication of JNC 8 that supports these lower BP goals, particularly in patients with CVD or who are at high ASCVD risk.8,12-15 For example, the Systolic Blood Pressure Intervention Trial (SPRINT) was the largest RCT to assess whether lower BP goals decrease the risk of adverse CVD outcomes.16 In SPRINT, 9361 patients with an SBP ≥130 mm Hg and an increased risk of CVD, but without diabetes or a history of stroke, were randomized to intensive BP treatment (SBP goal <120 mm Hg) or standard treatment (SBP goal <140 mm Hg). After a median follow-up of 3.26 years, the study was stopped early due to a decreased risk in the primary composite outcome of myocardial infarction (MI), other acute coronary syndromes (ACS), stroke, heart failure, or death from CV causes (number needed to treat [NNT] to prevent one event=61).
Intensive treatment was also associated with a lower risk of all-cause mortality (NNT=90), heart failure (NNT=123), death from CV causes (NNT=172), and the primary outcome or death (NNT=52
Continue to: Meta-analyses that have been conducted since SPRINT...
Meta-analyses that have been conducted since SPRINT, and that have incorporated SPRINT data, also support lower BP goals. In the systematic review performed for the 2017 ACC/AHA guideline, an SBP <130 mm Hg compared to a higher BP target was associated with a reduced risk of major CV events, stroke, MI, and heart failure, although not all-cause mortality.8 These findings were largely consistent with other recent meta-analyses.12-15 For example, Bundy et al15 reported significant CV benefit with more vs less intensive BP lowering, whether or not the data from SPRINT were included, with the greatest reduction in risk seen in the groups with highest baseline BP.
It is important to consider a patient’s baseline level of risk when evaluating the absolute benefit of lower BP targets on CV outcomes. For patients with higher CV risk, the absolute benefit of treatment is greater.12-14 These findings support the 2017 ACC/AHA guideline, which recommends initiating drug therapy, in addition to lifestyle modification, in adults with hypertension and high ASCVD risk when the average BP is >130/80 mm Hg, with a goal of <130/80 mm Hg. TABLE 312-15,17-22 summarizes recent systematic reviews and meta-analyses conducted since the publication of JNC 8 that assess the association between intensity of BP lowering and adverse CV and related outcomes.


Treating patients with low CV risk
The evidence supporting a lower BP goal in patients with low CV risk is less than for patients at elevated risk. There are no large RCTs for this group that have assessed whether an intensive BP lowering strategy decreases CV outcomes more than a standard BP strategy (eg, <140/90 mm Hg). It is likely that absolute benefit is much smaller than for patients with, or at high risk for, ASCVD.
However, epidemiologic observational studies have indicated a significant log-linear increase in CV mortality starting at an SBP of 115 mm Hg.23 A 20-mm Hg increase in SBP above 115 mm Hg is associated with an approximate doubling of stroke and ischemic heart disease mortality risk.23 Decades worth of exposure to “elevated” BP levels would likely result in significant vascular damage, and attenuation of this process would likely be beneficial.24,25 An RCT specifically designed to test this hypothesis, however, would not be pragmatic considering the substantial number of patient-years that would be required.
Due to insufficient data documenting the value of antihypertensive drug therapy for primary prevention in adults with “elevated” BP and stage 1 hypertension at low risk for CVD, the 2017 ACC/AHA guideline recommends that drug therapy be initiated for all adults only when their BP average is ≥140/90 mm Hg.1 In contrast, for patients needing secondary prevention and for those with elevated CVD risk, the guideline recommends medication in addition to lifestyle modifications once the average BP is ≥130/80 mm Hg. The recommendation to withhold drug therapy until the BP is ≥140/90 mm Hg in patients needing primary prevention is supported by a new meta-analysis of 74 trials with 306,273 participants that aimed to assess the association between BP-lowering treatment and death and CVD at various BP levels.17 In this analysis, pharmacologic treatment was associated with a reduced risk of all-cause mortality, major CVD events, and coronary heart disease if the SBP was ≥140 mm Hg.
Continue to: Treating older patients
Treating older patients
Significant controversy has existed regarding the optimal BP goal in older patients, particularly once the JNC 8 recommended relaxing the SBP goal to <150 mm Hg for pateints ≥60 years of age.6,7 This recommendation was consistent with the guideline from the American College of Physicians (ACP)/AAFP,26 which also recommended a lower SBP of <140 mm Hg in patients with a history of stroke or transient ischemic attack and those at high CV risk.26
Evidence is available, however, supporting more intensive BP goals in older independently-living ambulatory adults. A pre-planned subgroup analysis was conducted in 2636 SPRINT participants ≥75 years of age.27 Similar to the overall experience in SPRINT, lower SBP goals were associated with significant reductions in CV events, including the composite CVD primary outcome (NNT=27), heart failure (NNT=63), nonfatal heart failure (NNT=66), and all-cause mortality (NNT=41). In addition, the relative benefits were approximately equal whether the patients were the most fit, non-fit, or frail, with the absolute benefit being greatest in those who were frail (recognizing that the SPRINT participants were independently-living ambulatory adults). While the absolute rate of serious adverse events was higher in the more intensive BP goal group, there was no statistically significant difference in the incidence of hypotension, orthostatic hypotension, syncope, electrolyte abnormalities, or acute kidney injury or renal failure.
Use of lower BP goals than recommended by JNC 8 was also supported by another recent meta-analysis that compared the outcomes of intensive BP lowering (SBP <140 mm Hg) to a standard BP-lowering strategy (SBP <150 mm Hg).18 Using a random-effects model, more intensive BP lowering was associated with a significant reduction in major adverse CV events (29%), CV mortality (33%), and heart failure (37%), with no increase in serious adverse events or renal failure. Findings with the fixed-effects model used to confirm results were largely consistent, with the exception of a possible increase in renal failure.
Although the evidence supporting lower BP goals in older, ambulatory, noninstitutionalized patients is sound, it is important to consider a patient’s overall disease burden. For older adults with multiple comorbidities and limited life expectancy, as well as those who are nonambulatory or institutionalized, decisions on the intensity of BP lowering should be made using a team-based approach, weighing the risks and benefits.1
Continue to: Treating patients with diabetes
Treating patients with diabetes
The most appropriate BP goal for patients with diabetes has been the subject of much debate, with different goals recommended in different guidelines (TABLE 21,2,6). The most recent American Diabetes Association guideline recommends a BP goal <140/90 mm Hg for most patients, with lower targets (<130/80 mm Hg) for patients at high CV risk if it is achievable without undue treatment burden,28 whereas the 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for all adults with diabetes.1
The ACCORD trial. There is limited evidence to suggest which BP goal is most appropriate for patients with diabetes. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial is the only RCT specifically designed to assess the impact of intensive vs standard BP goals in patients with diabetes.29 In ACCORD, 4733 patients with type 2 diabetes were randomized to either an intensive BP-lowering group (SBP <120 mm Hg) or a standard BP-lowering group (SBP <140 mm Hg). After a mean follow-up of 4.7 years, there was no difference in the primary composite endpoint of nonfatal MI, nonfatal stroke, or death from CV causes. However, the risk of stroke was reduced (NNT=89). Interpretation of ACCORD is limited due to its factorial design and because the trial was significantly underpowered.

Systematic reviews and meta-analyses. Literature supporting lower BP goals in patients with diabetes primarily comes from systematic reviews and meta-analyses.30 In the evidence-based review performed for the 2017 ACC/AHA guidelines, more intensive treatment was associated with a decrease in fatal or nonfatal stroke.8 The results from the ACCORD trial and SPRINT are consistent,31 and a sub-study of SPRINT patients with pre-diabetes showed preservation of CV benefit.32 Also, a meta-analysis of subgroups of trial participants with diabetes showed that more intensive BP lowering in patients is associated with a decrease in major CV events.14
Treating patients with chronic kidney disease
As with diabetes and older patients, recommended goals for patients with CKD have varied (TABLE 21,2,6). The Kidney Disease Improving Global Outcomes (KDIGO) 2012 guideline recommended the same target BP as JNC 7 and the 2017 ACC/AHA guideline: ≤130/80 mm Hg in patients with CKD and urine albumin excretion ≥30 mg/24 hours (or equivalent).1,2,33 KDIGO recommended a more relaxed target (≤140/90 mm Hg), however, for patients with CKD and urine albumin excretion <30 mg/24 hours.1,33
Scant data exist from RCTs designed to assess the CV effects of intensive BP targets in patients with CKD. In SPRINT, where 28% of patients had stage 3 or 4 CKD, benefits of more intensive therapy were similar to those observed in the overall cohort.16,34 While some RCTs have assessed the effect of more intensive BP lowering on progression of CKD, they were not specifically designed or powered to address CV outcomes.35,36
Continue to: In recent meta-analyses assessing the effects...
In recent meta-analyses assessing the effects of intensive BP lowering on renal and CV events in patients with CKD, a lower BP strategy was not associated with a decrease in CV events.8,14,19 However, more intensive therapy was associated with a 17% reduced risk of composite kidney failure events and an 18% reduction in end-stage kidney disease.19 The risk of kidney failure with lower BP goals was 27% lower in patients with baseline proteinuria, but was not significant in patients who did not have proteinuria.19
Evidence supports lower BP goals, but guidelines should guide
The lower BP goals advised in the 2017 ACC/AHA guideline are supported by substantial new high-quality evidence that was not available at the time of the JNC 8 report.1 The strongest evidence for lower goals is found in patients with, or at high risk for, CVD, but other patients commonly seen by primary care providers, including those at lower CVD risk, older patients, and those with diabetes or CKD are also likely to benefit.1
Despite the debates, it is important to remember that guidelines are intended to “guide.” As stated in the guideline, “Guidelines are intended to define practices meeting the needs of patients in most, but not all, circumstances and should not replace clinical judgment.”1 They should be easy to understand and apply, and a consistent, evidence-based BP goal of <130/80 mm Hg for most patients facilitates implementation.
Although more of the US population is categorized as hypertensive under the new guideline (46% now vs 32% before), only 1.9% more require drug therapy, as the vast majority of the newly classified hypertensives are primary prevention patients for whom only lifestyle modification is recommended.37 However, to attain these goals, greater emphasis will be needed on utilizing team-based care, health information technology including electronic medical records and telehealth, performance measures, quality improvement strategies, and financial incentives.1
Finally, as emphasized in the guidelines, BP monitoring technique matters. Clinicians should not accept flawed BP measurement techniques any more than they would accept flawed results from studies performed incorrectly.
CORRESPONDENCE
Eric J. MacLaughlin, PharmD, BCPS, FASHP, FCCP, Texas Tech University Health Sciences Center,1300 S. Coulter Dr., Amarillo, TX 79106; [email protected].
ACKNOWLEDGEMENTS
The authors thank Paul K. Whelton, MB, MD, MSc, FAHA, and Robert M. Carey, MD, FAHA, for their review of this manuscript.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
2. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560-2572.
3. Wilt TJ, Kansagara D, Qaseem A; Clinical Guidelines Committee of the American College of Physicians. Hypertension limbo: balancing benefits, harms, and patient preferences before we lower the bar on blood pressure. Ann Intern Med. 2018;168:369-370.
4. American Academy of Family Physicians. AAFP decides to not endorse AHA/ACC hypertension guideline. Available at: https://www.aafp.org/news/health-of-the-public/20171212notendorseaha-accgdlne.html. Accessed January 9, 2018.
5. Gibbons GH, Shurin SB, Mensah GA, et al. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation. 2013;128:1713-1715.
6. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
7. Wright JT Jr., Fine LJ, Lackland DT, et al. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499-503.
8. Reboussin DM, Allen NB, Griswold ME, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e116-e135.
9. Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation. 2016;134:904-905.
10. Burgess SE, MacLaughlin EJ, Smith PA, et al. Blood pressure rising: differences between current clinical and recommended measurement techniques. J Am Soc Hypertens. 2011;5:484-488.
11. American College of Cardiology. ASCVD Risk Estimator Plus. Available at: http://tools.acc.org/ascvd-risk-estimator-plus/#!/calculate/estimate/. Accessed January 9, 2018.
12. The Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591-598.
13. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels - updated overview and meta-analyses of randomized trials. J Hypertens. 2016;34:613-622.
14. Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435-443.
15. Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2:775-781.
16. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
17. Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:28-36.
18. Bavishi C, Bangalore S, Messerli FH. Outcomes of intensive blood pressure lowering in older hypertensive patients. J Am Coll Cardiol. 2017;69:486-493.
19. Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949-957.
20. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957-967.
21. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med. 2017;166:419-429.
22. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
23. Lewington S, Clarke R, Qizilbash N, et al; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913.
24. Guo X, Zhang X, Guo L, et al. Association between pre-hypertension and cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Curr Hypertens Rep. 2013;15:703-716.
25. Huang Y, Cai X, Li Y, et al. Prehypertension and the risk of stroke: a meta-analysis. Neurology. 2014;82:1153-1161.
26. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430-437.
27. Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673-2682.
28. American Diabetes Association. 9. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S86-S104.
29. The ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
30. Reboldi G, Gentile G, Angeli F, et al. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J Hypertens. 2011;29:1253-1269.
31. Perkovic V, Rodgers A. Redefining blood-pressure targets—SPRINT starts the marathon. N Engl J Med. 2015;373:2175-2178.
32. Bress AP, King JB, Kreider KE, et al. Effect of intensive versus standard blood pressure treatment according to baseline prediabetes status: a post hoc analysis of a randomized trial. Diabetes Care. 2017 Aug 9.
33. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:337-414.
34. Cheung AK, Rahman M, Reboussin DM, et al. Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017;28:2812-2823.
35. Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939-946.
36. Wright JT Jr., Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421-2431.
37. Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 American College of Cardiology/American Heart Association High Blood Pressure Guideline. J Am Coll Cardiol. 2018;71:109-188.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
2. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560-2572.
3. Wilt TJ, Kansagara D, Qaseem A; Clinical Guidelines Committee of the American College of Physicians. Hypertension limbo: balancing benefits, harms, and patient preferences before we lower the bar on blood pressure. Ann Intern Med. 2018;168:369-370.
4. American Academy of Family Physicians. AAFP decides to not endorse AHA/ACC hypertension guideline. Available at: https://www.aafp.org/news/health-of-the-public/20171212notendorseaha-accgdlne.html. Accessed January 9, 2018.
5. Gibbons GH, Shurin SB, Mensah GA, et al. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation. 2013;128:1713-1715.
6. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
7. Wright JT Jr., Fine LJ, Lackland DT, et al. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499-503.
8. Reboussin DM, Allen NB, Griswold ME, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e116-e135.
9. Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation. 2016;134:904-905.
10. Burgess SE, MacLaughlin EJ, Smith PA, et al. Blood pressure rising: differences between current clinical and recommended measurement techniques. J Am Soc Hypertens. 2011;5:484-488.
11. American College of Cardiology. ASCVD Risk Estimator Plus. Available at: http://tools.acc.org/ascvd-risk-estimator-plus/#!/calculate/estimate/. Accessed January 9, 2018.
12. The Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591-598.
13. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels - updated overview and meta-analyses of randomized trials. J Hypertens. 2016;34:613-622.
14. Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435-443.
15. Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2:775-781.
16. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
17. Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:28-36.
18. Bavishi C, Bangalore S, Messerli FH. Outcomes of intensive blood pressure lowering in older hypertensive patients. J Am Coll Cardiol. 2017;69:486-493.
19. Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949-957.
20. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957-967.
21. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med. 2017;166:419-429.
22. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
23. Lewington S, Clarke R, Qizilbash N, et al; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913.
24. Guo X, Zhang X, Guo L, et al. Association between pre-hypertension and cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Curr Hypertens Rep. 2013;15:703-716.
25. Huang Y, Cai X, Li Y, et al. Prehypertension and the risk of stroke: a meta-analysis. Neurology. 2014;82:1153-1161.
26. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430-437.
27. Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673-2682.
28. American Diabetes Association. 9. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S86-S104.
29. The ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
30. Reboldi G, Gentile G, Angeli F, et al. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J Hypertens. 2011;29:1253-1269.
31. Perkovic V, Rodgers A. Redefining blood-pressure targets—SPRINT starts the marathon. N Engl J Med. 2015;373:2175-2178.
32. Bress AP, King JB, Kreider KE, et al. Effect of intensive versus standard blood pressure treatment according to baseline prediabetes status: a post hoc analysis of a randomized trial. Diabetes Care. 2017 Aug 9.
33. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:337-414.
34. Cheung AK, Rahman M, Reboussin DM, et al. Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017;28:2812-2823.
35. Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939-946.
36. Wright JT Jr., Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421-2431.
37. Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 American College of Cardiology/American Heart Association High Blood Pressure Guideline. J Am Coll Cardiol. 2018;71:109-188.
PRACTICE RECOMMENDATIONS
› Treat adults with hypertension and cardiovascular disease or those at high risk (≥10%) of an atherosclerotic cardiovascular disease (ASCVD) event to a blood pressure (BP) goal <130/80 mm Hg. A for systolic BP goal; C for diastolic BP goal.
› Treat adults with hypertension and a low risk of a cardiovascular event (ie, primary prevention and ASCVD <10%) to a BP goal <130/80 mm Hg. B for systolic BP goal; C for diastolic BP goal.
› Treat ambulatory, community-dwelling, noninstitutionalized older patients to a systolic BP goal <130 mm Hg. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Vector-borne diseases: Trends and take-home points
Resources
Rosenberg R, Lindsey NP, Fischer M, et al. Vital Signs: Trends in reported vectorborne disease cases—United States and territories, 2004-2016. MMWR Morb Mortal Wkly Rep. 2018;67:496-501.
US Environmental Protection Agency. Repellents: protection against mosquitoes, ticks, and other arthropods. Available at: https://www.epa.gov/insect-repellents. Accessed June 6, 2018.
Centers for Disease Control and Prevention. Zika virus: prevent mosquito bites. Available at: https://www.cdc.gov/zika/prevention/prevent-mosquito-bites.html. Accessed June 6, 2018.
Resources
Rosenberg R, Lindsey NP, Fischer M, et al. Vital Signs: Trends in reported vectorborne disease cases—United States and territories, 2004-2016. MMWR Morb Mortal Wkly Rep. 2018;67:496-501.
US Environmental Protection Agency. Repellents: protection against mosquitoes, ticks, and other arthropods. Available at: https://www.epa.gov/insect-repellents. Accessed June 6, 2018.
Centers for Disease Control and Prevention. Zika virus: prevent mosquito bites. Available at: https://www.cdc.gov/zika/prevention/prevent-mosquito-bites.html. Accessed June 6, 2018.
Resources
Rosenberg R, Lindsey NP, Fischer M, et al. Vital Signs: Trends in reported vectorborne disease cases—United States and territories, 2004-2016. MMWR Morb Mortal Wkly Rep. 2018;67:496-501.
US Environmental Protection Agency. Repellents: protection against mosquitoes, ticks, and other arthropods. Available at: https://www.epa.gov/insect-repellents. Accessed June 6, 2018.
Centers for Disease Control and Prevention. Zika virus: prevent mosquito bites. Available at: https://www.cdc.gov/zika/prevention/prevent-mosquito-bites.html. Accessed June 6, 2018.
Home-based CBT significantly improved IBS symptoms
Primarily home-based cognitive-behavioral therapy improved irritable bowel syndrome symptoms at least as much as conventional CBT, cut clinician time by 60%, and significantly outperformed educational sessions in a multicenter clinical trial reported in the July issue of Gastroenterology.
Acutely, primarily home-based CBT produced a mean 61% improvement in self-reported symptoms on the IBS version of the Clinical Global Impressions Scale, versus 44% for the educational control group (P less than .05), wrote Jeffrey M. Lackner, PsyD, of the State University of New York at Buffalo and his associates. Blinded gastroenterologists reported improvements of 56% and 40%, respectively (P less than .05). The superiority of the minimal-contact CBT program held up at 6 months and equivalence tests found it “at least as effective as standard CBT,” the researchers wrote.
IBS is a major area of unmet clinical need that costs the United States some $28 billion annually. Clinicians and patients lack both reliable biomarkers and “uniformly effective” therapies, the investigators noted. In recent years, severe adverse events have greatly restricted the availability of otherwise promising Food and Drug Administration–approved therapies, such as Lotronex (alosetron hydrochlorine), which has been linked to ischemic colitis and fatal cases of ruptured bowel, and Zelnorm (tegaserod maleate), which has been associated with myocardial infarction, stroke, and unstable angina.
In contrast, face-to-face CBT is safe, efficacious, and guideline recommended for IBS. However, uptake is limited by cost, stigma, geography, and a shortage of certified providers, the researchers noted. They enrolled 436 patients with IBS based on Rome III criteria and randomly assigned them to one of three interventions. The standard CBT group received 10 weekly, 60-minute, face-to-face CBT sessions on brain-gut interactions, symptom triggers and monitoring, muscle relaxation, worry control, problem-solving, and relapse prevention. The primarily home-based CBT group covered the same topics but attended only four clinic sessions and was provided home study materials. Finally, the education group attended four sessions with background information on IBS and the role of stress, diet, and exercise.
Baseline characteristics were comparable among groups, as were dropout rates (9% overall). In all, 89% of patients completed at least 8 of 10 standard cognitive-behavioral therapy sessions or at least three of four home-based CBT or educational sessions. Six months after the interventions ended, primarily home-based CBT continued to outperform education (blinded gastroenterologist-reported improvements, 58.4% and 44.8%, respectively; P = .05 for difference between groups).
Equivalence tests indicated that the minimal-CBT intervention was at least as effective as standard CBT, and improvements were not primarily the result of concomitant medications, according to the researchers. Nonetheless, only 42% of patients who benefited from CBT achieved remission, defined as no or mild IBS symptoms on the gastroenterologist-administered Clinical Global Impressions Scale. Unremitted patients might benefit from combining CBT with medical therapies that target both “central and peripheral mechanisms of IBS,” the investigators said.
The three interventions produced comparable acute and longer-term improvements on the IBS Symptom Severity Scale, which emphasizes sensory symptoms and therefore might be a less sensitive endpoint than the Clinical Global Impressions Scale, the researchers noted. Nonetheless, CBT produced some of the strongest absolute symptomatic improvements ever reported for IBS. “To put these data in context, treatment response of FDA-approved pharmacological agents using global IBS symptom improvement scales range from 17% to 40%,” the researchers wrote.
The National Institutes of Health provided funding. The investigators reported having no conflicts of interest.
SOURCE: Lackner JM et al. Gastroenterology. 2018 Apr 24. doi: 10.1053/j.gastro.2018.03.063.
Treating the myriad symptoms of irritable bowel syndrome (IBS) patients remains a great challenge in clinical practice. A bigger challenge is the management of IBS patients who are refractory to medical therapy, which commonly includes a combination of pain, bowel, and psychiatric medications. In this very well designed and executed study, Lackner and his colleagues randomized refractory IBS patients with moderate to severe symptoms to three therapeutic arms: standard cognitive-behavioral therapy (CBT), minimal-contact home-based CBT, and IBS education. The authors demonstrated that 4-session home-based CBT was as efficacious as 10 sessions of standard CBT and both were significantly more efficacious than IBS education in global improvement of IBS symptoms. The superior effect of both types of CBT was maintained over a period of 6 months post treatment.
There are several important conclusions from this pivotal trial. First, the study further cemented the therapeutic value of CBT in the management of IBS patients, especially for those patients who are refractory to the currently available medical therapy. Because of the size of the study and the rigorous design, it is probably the best evidence we currently have about the value of CBT in IBS. Second, minimal-contact home-based CBT is as effective as standard CBT in controlling the full range of IBS symptoms. The former may be preferred by IBS patients, who are not available or may not be compliant with repeated clinic visits for standard CBT sessions. Standard CBT is typically lengthy and expensive. The minimal-contact home-based CBT option has the benefit of being more accessible and less costly, and most importantly, it does so in a way that does not compromise the therapeutic value of symptom relief.
The exact duration of symptom control that can be achieved post CBT and the value of other psychological interventions in IBS patients remain to be elucidated.
Ronnie Fass, MD, is a professor of medicine at Case Western Reserve University, Cleveland, as well as the medical director of the Digestive Health Center and director of the division of gastroenterology and hepatology, head, esophageal and swallowing center at MetroHealth Medical Center, also in Cleveland. He has no conflicts of interest.
Treating the myriad symptoms of irritable bowel syndrome (IBS) patients remains a great challenge in clinical practice. A bigger challenge is the management of IBS patients who are refractory to medical therapy, which commonly includes a combination of pain, bowel, and psychiatric medications. In this very well designed and executed study, Lackner and his colleagues randomized refractory IBS patients with moderate to severe symptoms to three therapeutic arms: standard cognitive-behavioral therapy (CBT), minimal-contact home-based CBT, and IBS education. The authors demonstrated that 4-session home-based CBT was as efficacious as 10 sessions of standard CBT and both were significantly more efficacious than IBS education in global improvement of IBS symptoms. The superior effect of both types of CBT was maintained over a period of 6 months post treatment.
There are several important conclusions from this pivotal trial. First, the study further cemented the therapeutic value of CBT in the management of IBS patients, especially for those patients who are refractory to the currently available medical therapy. Because of the size of the study and the rigorous design, it is probably the best evidence we currently have about the value of CBT in IBS. Second, minimal-contact home-based CBT is as effective as standard CBT in controlling the full range of IBS symptoms. The former may be preferred by IBS patients, who are not available or may not be compliant with repeated clinic visits for standard CBT sessions. Standard CBT is typically lengthy and expensive. The minimal-contact home-based CBT option has the benefit of being more accessible and less costly, and most importantly, it does so in a way that does not compromise the therapeutic value of symptom relief.
The exact duration of symptom control that can be achieved post CBT and the value of other psychological interventions in IBS patients remain to be elucidated.
Ronnie Fass, MD, is a professor of medicine at Case Western Reserve University, Cleveland, as well as the medical director of the Digestive Health Center and director of the division of gastroenterology and hepatology, head, esophageal and swallowing center at MetroHealth Medical Center, also in Cleveland. He has no conflicts of interest.
Treating the myriad symptoms of irritable bowel syndrome (IBS) patients remains a great challenge in clinical practice. A bigger challenge is the management of IBS patients who are refractory to medical therapy, which commonly includes a combination of pain, bowel, and psychiatric medications. In this very well designed and executed study, Lackner and his colleagues randomized refractory IBS patients with moderate to severe symptoms to three therapeutic arms: standard cognitive-behavioral therapy (CBT), minimal-contact home-based CBT, and IBS education. The authors demonstrated that 4-session home-based CBT was as efficacious as 10 sessions of standard CBT and both were significantly more efficacious than IBS education in global improvement of IBS symptoms. The superior effect of both types of CBT was maintained over a period of 6 months post treatment.
There are several important conclusions from this pivotal trial. First, the study further cemented the therapeutic value of CBT in the management of IBS patients, especially for those patients who are refractory to the currently available medical therapy. Because of the size of the study and the rigorous design, it is probably the best evidence we currently have about the value of CBT in IBS. Second, minimal-contact home-based CBT is as effective as standard CBT in controlling the full range of IBS symptoms. The former may be preferred by IBS patients, who are not available or may not be compliant with repeated clinic visits for standard CBT sessions. Standard CBT is typically lengthy and expensive. The minimal-contact home-based CBT option has the benefit of being more accessible and less costly, and most importantly, it does so in a way that does not compromise the therapeutic value of symptom relief.
The exact duration of symptom control that can be achieved post CBT and the value of other psychological interventions in IBS patients remain to be elucidated.
Ronnie Fass, MD, is a professor of medicine at Case Western Reserve University, Cleveland, as well as the medical director of the Digestive Health Center and director of the division of gastroenterology and hepatology, head, esophageal and swallowing center at MetroHealth Medical Center, also in Cleveland. He has no conflicts of interest.
Primarily home-based cognitive-behavioral therapy improved irritable bowel syndrome symptoms at least as much as conventional CBT, cut clinician time by 60%, and significantly outperformed educational sessions in a multicenter clinical trial reported in the July issue of Gastroenterology.
Acutely, primarily home-based CBT produced a mean 61% improvement in self-reported symptoms on the IBS version of the Clinical Global Impressions Scale, versus 44% for the educational control group (P less than .05), wrote Jeffrey M. Lackner, PsyD, of the State University of New York at Buffalo and his associates. Blinded gastroenterologists reported improvements of 56% and 40%, respectively (P less than .05). The superiority of the minimal-contact CBT program held up at 6 months and equivalence tests found it “at least as effective as standard CBT,” the researchers wrote.
IBS is a major area of unmet clinical need that costs the United States some $28 billion annually. Clinicians and patients lack both reliable biomarkers and “uniformly effective” therapies, the investigators noted. In recent years, severe adverse events have greatly restricted the availability of otherwise promising Food and Drug Administration–approved therapies, such as Lotronex (alosetron hydrochlorine), which has been linked to ischemic colitis and fatal cases of ruptured bowel, and Zelnorm (tegaserod maleate), which has been associated with myocardial infarction, stroke, and unstable angina.
In contrast, face-to-face CBT is safe, efficacious, and guideline recommended for IBS. However, uptake is limited by cost, stigma, geography, and a shortage of certified providers, the researchers noted. They enrolled 436 patients with IBS based on Rome III criteria and randomly assigned them to one of three interventions. The standard CBT group received 10 weekly, 60-minute, face-to-face CBT sessions on brain-gut interactions, symptom triggers and monitoring, muscle relaxation, worry control, problem-solving, and relapse prevention. The primarily home-based CBT group covered the same topics but attended only four clinic sessions and was provided home study materials. Finally, the education group attended four sessions with background information on IBS and the role of stress, diet, and exercise.
Baseline characteristics were comparable among groups, as were dropout rates (9% overall). In all, 89% of patients completed at least 8 of 10 standard cognitive-behavioral therapy sessions or at least three of four home-based CBT or educational sessions. Six months after the interventions ended, primarily home-based CBT continued to outperform education (blinded gastroenterologist-reported improvements, 58.4% and 44.8%, respectively; P = .05 for difference between groups).
Equivalence tests indicated that the minimal-CBT intervention was at least as effective as standard CBT, and improvements were not primarily the result of concomitant medications, according to the researchers. Nonetheless, only 42% of patients who benefited from CBT achieved remission, defined as no or mild IBS symptoms on the gastroenterologist-administered Clinical Global Impressions Scale. Unremitted patients might benefit from combining CBT with medical therapies that target both “central and peripheral mechanisms of IBS,” the investigators said.
The three interventions produced comparable acute and longer-term improvements on the IBS Symptom Severity Scale, which emphasizes sensory symptoms and therefore might be a less sensitive endpoint than the Clinical Global Impressions Scale, the researchers noted. Nonetheless, CBT produced some of the strongest absolute symptomatic improvements ever reported for IBS. “To put these data in context, treatment response of FDA-approved pharmacological agents using global IBS symptom improvement scales range from 17% to 40%,” the researchers wrote.
The National Institutes of Health provided funding. The investigators reported having no conflicts of interest.
SOURCE: Lackner JM et al. Gastroenterology. 2018 Apr 24. doi: 10.1053/j.gastro.2018.03.063.
Primarily home-based cognitive-behavioral therapy improved irritable bowel syndrome symptoms at least as much as conventional CBT, cut clinician time by 60%, and significantly outperformed educational sessions in a multicenter clinical trial reported in the July issue of Gastroenterology.
Acutely, primarily home-based CBT produced a mean 61% improvement in self-reported symptoms on the IBS version of the Clinical Global Impressions Scale, versus 44% for the educational control group (P less than .05), wrote Jeffrey M. Lackner, PsyD, of the State University of New York at Buffalo and his associates. Blinded gastroenterologists reported improvements of 56% and 40%, respectively (P less than .05). The superiority of the minimal-contact CBT program held up at 6 months and equivalence tests found it “at least as effective as standard CBT,” the researchers wrote.
IBS is a major area of unmet clinical need that costs the United States some $28 billion annually. Clinicians and patients lack both reliable biomarkers and “uniformly effective” therapies, the investigators noted. In recent years, severe adverse events have greatly restricted the availability of otherwise promising Food and Drug Administration–approved therapies, such as Lotronex (alosetron hydrochlorine), which has been linked to ischemic colitis and fatal cases of ruptured bowel, and Zelnorm (tegaserod maleate), which has been associated with myocardial infarction, stroke, and unstable angina.
In contrast, face-to-face CBT is safe, efficacious, and guideline recommended for IBS. However, uptake is limited by cost, stigma, geography, and a shortage of certified providers, the researchers noted. They enrolled 436 patients with IBS based on Rome III criteria and randomly assigned them to one of three interventions. The standard CBT group received 10 weekly, 60-minute, face-to-face CBT sessions on brain-gut interactions, symptom triggers and monitoring, muscle relaxation, worry control, problem-solving, and relapse prevention. The primarily home-based CBT group covered the same topics but attended only four clinic sessions and was provided home study materials. Finally, the education group attended four sessions with background information on IBS and the role of stress, diet, and exercise.
Baseline characteristics were comparable among groups, as were dropout rates (9% overall). In all, 89% of patients completed at least 8 of 10 standard cognitive-behavioral therapy sessions or at least three of four home-based CBT or educational sessions. Six months after the interventions ended, primarily home-based CBT continued to outperform education (blinded gastroenterologist-reported improvements, 58.4% and 44.8%, respectively; P = .05 for difference between groups).
Equivalence tests indicated that the minimal-CBT intervention was at least as effective as standard CBT, and improvements were not primarily the result of concomitant medications, according to the researchers. Nonetheless, only 42% of patients who benefited from CBT achieved remission, defined as no or mild IBS symptoms on the gastroenterologist-administered Clinical Global Impressions Scale. Unremitted patients might benefit from combining CBT with medical therapies that target both “central and peripheral mechanisms of IBS,” the investigators said.
The three interventions produced comparable acute and longer-term improvements on the IBS Symptom Severity Scale, which emphasizes sensory symptoms and therefore might be a less sensitive endpoint than the Clinical Global Impressions Scale, the researchers noted. Nonetheless, CBT produced some of the strongest absolute symptomatic improvements ever reported for IBS. “To put these data in context, treatment response of FDA-approved pharmacological agents using global IBS symptom improvement scales range from 17% to 40%,” the researchers wrote.
The National Institutes of Health provided funding. The investigators reported having no conflicts of interest.
SOURCE: Lackner JM et al. Gastroenterology. 2018 Apr 24. doi: 10.1053/j.gastro.2018.03.063.
FROM GASTROENTEROLOGY
Key clinical point: Primarily home-based CBT significantly reduced self-reported and gastroenterologist-assessed symptoms of IBS.
Major finding: The intervention required 60% less clinician time and was at least as effective as 10 sessions of conventional CBT, according to responses to the Clinical Global Impressions Improvement Scale. CBT also significantly outperformed the education control (P less than .05).
Study details: Two-center, single-blinded randomized trial of 436 patients with IBS per Rome III criteria.
Disclosures: The National Institutes of Health provided funding. The researchers reported having no conflicts of interest.
Source: Lackner JM et al. Gastroenterology. 2018 Apr 24. doi: 10.1053/j.gastro.2018.03.063.
Consolidation of health care dollars
Research shows the ocean’s cod population is diminishing to dangerously low levels. In response, several countries (the United States, Iceland, and others) have instituted a resource allocation system termed “catch share,” where each fisherman is allotted an annual number of fish. Shares can be leased, bought, and traded. Consequently, there has been horizontal and vertical consolidation within the industry and huge fishing corporations have emerged while independent small-boat fishermen have virtually disappeared. Once consolidation occurred, venture capital entered the market. Parallels to what is happening to independent medical practices should not be ignored.
We have closed the book on DDW® 2018. Researchers presented new and innovative studies that will directly affect our practices. I was honored to give the “Best of AGA – DDW” lecture where I chose only seven of hundreds of abstracts to present. All DDW lectures are located at https://watch.ondemand.org/ddw. GI & Hepatology News will highlight several high-impact presentations in this and subsequent issues.
This month, our cover stories include a new ACS recommendation to drop the age of first colon cancer screening to 45 (see perspective by John M. Inadomi, MD, AGAF). Two of our most intractable disorders (NAFLD and IBS) have new therapies in the pipeline. From the AGA journals we have articles on Barrett’s surveillance, diet, cognitive-behavioral therapy for IBS, and better monitoring methods for Crohn’s disease.
July begins a new fiscal year for many of us. For many health systems, this last year saw diminishing clinical margins, increased regulations, dramatic alterations in pharmaceutical funds flow, and price pressures that are increasing. I sit on the board of a large nonprofit (nonacademic) Minnesota health system, and I am a member of key financial committees within Michigan Medicine. The learnings and contrasts from each are immense. Health care delivery in both systems is based on high fixed costs and margins that require cost reductions in the 3%-5% range per year to remain viable. Implications for physicians in all settings are immense. That said, there are solutions as you will see in coming issues.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Research shows the ocean’s cod population is diminishing to dangerously low levels. In response, several countries (the United States, Iceland, and others) have instituted a resource allocation system termed “catch share,” where each fisherman is allotted an annual number of fish. Shares can be leased, bought, and traded. Consequently, there has been horizontal and vertical consolidation within the industry and huge fishing corporations have emerged while independent small-boat fishermen have virtually disappeared. Once consolidation occurred, venture capital entered the market. Parallels to what is happening to independent medical practices should not be ignored.
We have closed the book on DDW® 2018. Researchers presented new and innovative studies that will directly affect our practices. I was honored to give the “Best of AGA – DDW” lecture where I chose only seven of hundreds of abstracts to present. All DDW lectures are located at https://watch.ondemand.org/ddw. GI & Hepatology News will highlight several high-impact presentations in this and subsequent issues.
This month, our cover stories include a new ACS recommendation to drop the age of first colon cancer screening to 45 (see perspective by John M. Inadomi, MD, AGAF). Two of our most intractable disorders (NAFLD and IBS) have new therapies in the pipeline. From the AGA journals we have articles on Barrett’s surveillance, diet, cognitive-behavioral therapy for IBS, and better monitoring methods for Crohn’s disease.
July begins a new fiscal year for many of us. For many health systems, this last year saw diminishing clinical margins, increased regulations, dramatic alterations in pharmaceutical funds flow, and price pressures that are increasing. I sit on the board of a large nonprofit (nonacademic) Minnesota health system, and I am a member of key financial committees within Michigan Medicine. The learnings and contrasts from each are immense. Health care delivery in both systems is based on high fixed costs and margins that require cost reductions in the 3%-5% range per year to remain viable. Implications for physicians in all settings are immense. That said, there are solutions as you will see in coming issues.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Research shows the ocean’s cod population is diminishing to dangerously low levels. In response, several countries (the United States, Iceland, and others) have instituted a resource allocation system termed “catch share,” where each fisherman is allotted an annual number of fish. Shares can be leased, bought, and traded. Consequently, there has been horizontal and vertical consolidation within the industry and huge fishing corporations have emerged while independent small-boat fishermen have virtually disappeared. Once consolidation occurred, venture capital entered the market. Parallels to what is happening to independent medical practices should not be ignored.
We have closed the book on DDW® 2018. Researchers presented new and innovative studies that will directly affect our practices. I was honored to give the “Best of AGA – DDW” lecture where I chose only seven of hundreds of abstracts to present. All DDW lectures are located at https://watch.ondemand.org/ddw. GI & Hepatology News will highlight several high-impact presentations in this and subsequent issues.
This month, our cover stories include a new ACS recommendation to drop the age of first colon cancer screening to 45 (see perspective by John M. Inadomi, MD, AGAF). Two of our most intractable disorders (NAFLD and IBS) have new therapies in the pipeline. From the AGA journals we have articles on Barrett’s surveillance, diet, cognitive-behavioral therapy for IBS, and better monitoring methods for Crohn’s disease.
July begins a new fiscal year for many of us. For many health systems, this last year saw diminishing clinical margins, increased regulations, dramatic alterations in pharmaceutical funds flow, and price pressures that are increasing. I sit on the board of a large nonprofit (nonacademic) Minnesota health system, and I am a member of key financial committees within Michigan Medicine. The learnings and contrasts from each are immense. Health care delivery in both systems is based on high fixed costs and margins that require cost reductions in the 3%-5% range per year to remain viable. Implications for physicians in all settings are immense. That said, there are solutions as you will see in coming issues.
John I. Allen, MD, MBA, AGAF
Editor in Chief