User login
Barrett’s esophagus risk factor profile may predict progression
Older age, male sex, smoking, longer segment length, and low-grade dysplasia were significant risk factors for progression of Barrett’s esophagus in a meta-analysis of 20 studies.
“Individuals with these features should undergo more intensive surveillance or endoscopic therapy,” Rajesh Krishnamoorthi, MD, of Mayo Clinic in Rochester, Minn., and his associates wrote in Clinical Gastroenterology and Hepatology. “Smoking is a modifiable risk factor for cancer prevention in patients with BE.”
“Currently, gastrointestinal societies’ guidelines on BE surveillance are solely based on dysplasia grade and do not take into account any of the other risk factors,” the reviewers concluded. Their findings could form the backbone of a risk score that identifies high-risk BE patients with baseline low-grade dysplasia or nondysplastic BE “who would benefit from intensive surveillance or endoscopic therapy.”
Esophageal adenocarcinoma is on the rise and fewer than one in five patients survive 5 years past diagnosis. Endoscopic surveillance for esophageal adenocarcinoma is recommended in Barrett’s esophagus, but only about one in 10 esophageal adenocarcinoma patients has a preceding BE diagnosis. “This ostensible discrepancy has raised concerns about the effectiveness of current screening and surveillance programs,” the reviewers noted. Studies also have yielded conflicting evidence about the value of endoscopic surveillance as currently performed. To help prioritize BE patients for surveillance, the reviewers searched EMBASE, MEDLINE, and Web of Science from inception through May 2016 for cohort studies of risk factors for progression of BE among patients with either no dysplasia or low-grade dysplasia.
The 20 studies covered 1,231 BE progression events among 74,943 patients. In separate pooled estimates, progression of BE correlated significantly with older age (odds ratio, 1.03; 95% CI, 1.01–1.05), male sex (OR, 2.2; 95% CI, 1.8-2.5), current or former smoking (OR, 1.5; 95% CI, 1.09-2.0), and greater BE segment length (OR, 1.3; 95% CI, 1.16-1.36). Results tended to be homogeneous among studies, said the reviewers. Low-grade dysplasia correlated strongly with progression (OR, 4.3; 95% CI, 2.6-7.0), while use of proton pump inhibitors (OR, 0.55; 95% CI, 0.32–0.96) and statins (OR, 0.48; 95% CI, 0.31-0.73) showed the opposite trend. “Alcohol use and obesity did not associate with risk of progression,” the reviewers added.
Thirteen studies in the meta-analysis were from Europe, six were from the United States, and one was from Australia. Ten were multicenter studies, 13 were deemed high-quality, three were deemed medium-quality, and four were deemed low-quality. The reviewers were unable to assess dose-response relationships for relevant factors, such as alcohol, tobacco, and medications, and not all studies accounted for potential confounding.
Only four studies included multivariate analyses to control for the confounding effects of age, sex, and BE characteristics (length and dysplasia). When the reviewers analyzed only these studies, older age and smoking no longer predicted BE progression. Use of proton pump inhibitors remained protective, and use of nonsteroidal anti-inflammatory drugs (NSAIDs) became protective, while statin use lost significance.
The reviewers disclosed no external funding sources or conflicts of interest.
SOURCE: Krishnamoorthi R, et al. Clinical Gastroenterol and Hepatol. 2017 Nov 30. doi: 10.1016/j.cgh.2017.11.044
Endoscopic surveillance is currently recommended for nondysplastic Barrett's esophagus (BE), but there are conflicting results on the effectiveness of surveillance on esophageal adenocarcinoma outcomes. This meta-analysis by Krishnamoorthi et al. found several risk factors associated with BE progression (i.e., age, male sex, smoking, BE length) among patients with nondysplastic BE or low-grade dysplasia. Current recommendations for BE surveillance intervals are solely based on dysplasia grade without consideration for other high-risk features (i.e., smoking, BE length, age). This meta-analysis demonstrates that some patients with nondysplastic BE are at a higher risk of neoplastic progression, and the AGA recommendation for BE surveillance every 3-5 years may not be suitable for all.
IMG: 2400A107.SIG Tan_Mimi_TEXAS_web
Parasa et al. recently developed a risk prediction model to stratify risk of progression in patients with nondysplastic BE based on BE length, male sex, smoking, and baseline low-grade dysplasia. Patients with one or more of these risk factors are at highest risk of neoplastic progression and may benefit from shorter surveillance intervals or endoscopic eradication therapy.
Mimi C. Tan, MD, MPH, is a postdoctoral fellow in gastroenterology and hepatology, T32 research track at Baylor College of Medicine, Houston, and an investigator at the Center for Innovations in Quality, Effectiveness, and Safety at the Michael E. DeBakey VA Medical Center, Houston. She has no conflicts.
Endoscopic surveillance is currently recommended for nondysplastic Barrett's esophagus (BE), but there are conflicting results on the effectiveness of surveillance on esophageal adenocarcinoma outcomes. This meta-analysis by Krishnamoorthi et al. found several risk factors associated with BE progression (i.e., age, male sex, smoking, BE length) among patients with nondysplastic BE or low-grade dysplasia. Current recommendations for BE surveillance intervals are solely based on dysplasia grade without consideration for other high-risk features (i.e., smoking, BE length, age). This meta-analysis demonstrates that some patients with nondysplastic BE are at a higher risk of neoplastic progression, and the AGA recommendation for BE surveillance every 3-5 years may not be suitable for all.
IMG: 2400A107.SIG Tan_Mimi_TEXAS_web
Parasa et al. recently developed a risk prediction model to stratify risk of progression in patients with nondysplastic BE based on BE length, male sex, smoking, and baseline low-grade dysplasia. Patients with one or more of these risk factors are at highest risk of neoplastic progression and may benefit from shorter surveillance intervals or endoscopic eradication therapy.
Mimi C. Tan, MD, MPH, is a postdoctoral fellow in gastroenterology and hepatology, T32 research track at Baylor College of Medicine, Houston, and an investigator at the Center for Innovations in Quality, Effectiveness, and Safety at the Michael E. DeBakey VA Medical Center, Houston. She has no conflicts.
Endoscopic surveillance is currently recommended for nondysplastic Barrett's esophagus (BE), but there are conflicting results on the effectiveness of surveillance on esophageal adenocarcinoma outcomes. This meta-analysis by Krishnamoorthi et al. found several risk factors associated with BE progression (i.e., age, male sex, smoking, BE length) among patients with nondysplastic BE or low-grade dysplasia. Current recommendations for BE surveillance intervals are solely based on dysplasia grade without consideration for other high-risk features (i.e., smoking, BE length, age). This meta-analysis demonstrates that some patients with nondysplastic BE are at a higher risk of neoplastic progression, and the AGA recommendation for BE surveillance every 3-5 years may not be suitable for all.
IMG: 2400A107.SIG Tan_Mimi_TEXAS_web
Parasa et al. recently developed a risk prediction model to stratify risk of progression in patients with nondysplastic BE based on BE length, male sex, smoking, and baseline low-grade dysplasia. Patients with one or more of these risk factors are at highest risk of neoplastic progression and may benefit from shorter surveillance intervals or endoscopic eradication therapy.
Mimi C. Tan, MD, MPH, is a postdoctoral fellow in gastroenterology and hepatology, T32 research track at Baylor College of Medicine, Houston, and an investigator at the Center for Innovations in Quality, Effectiveness, and Safety at the Michael E. DeBakey VA Medical Center, Houston. She has no conflicts.
Older age, male sex, smoking, longer segment length, and low-grade dysplasia were significant risk factors for progression of Barrett’s esophagus in a meta-analysis of 20 studies.
“Individuals with these features should undergo more intensive surveillance or endoscopic therapy,” Rajesh Krishnamoorthi, MD, of Mayo Clinic in Rochester, Minn., and his associates wrote in Clinical Gastroenterology and Hepatology. “Smoking is a modifiable risk factor for cancer prevention in patients with BE.”
“Currently, gastrointestinal societies’ guidelines on BE surveillance are solely based on dysplasia grade and do not take into account any of the other risk factors,” the reviewers concluded. Their findings could form the backbone of a risk score that identifies high-risk BE patients with baseline low-grade dysplasia or nondysplastic BE “who would benefit from intensive surveillance or endoscopic therapy.”
Esophageal adenocarcinoma is on the rise and fewer than one in five patients survive 5 years past diagnosis. Endoscopic surveillance for esophageal adenocarcinoma is recommended in Barrett’s esophagus, but only about one in 10 esophageal adenocarcinoma patients has a preceding BE diagnosis. “This ostensible discrepancy has raised concerns about the effectiveness of current screening and surveillance programs,” the reviewers noted. Studies also have yielded conflicting evidence about the value of endoscopic surveillance as currently performed. To help prioritize BE patients for surveillance, the reviewers searched EMBASE, MEDLINE, and Web of Science from inception through May 2016 for cohort studies of risk factors for progression of BE among patients with either no dysplasia or low-grade dysplasia.
The 20 studies covered 1,231 BE progression events among 74,943 patients. In separate pooled estimates, progression of BE correlated significantly with older age (odds ratio, 1.03; 95% CI, 1.01–1.05), male sex (OR, 2.2; 95% CI, 1.8-2.5), current or former smoking (OR, 1.5; 95% CI, 1.09-2.0), and greater BE segment length (OR, 1.3; 95% CI, 1.16-1.36). Results tended to be homogeneous among studies, said the reviewers. Low-grade dysplasia correlated strongly with progression (OR, 4.3; 95% CI, 2.6-7.0), while use of proton pump inhibitors (OR, 0.55; 95% CI, 0.32–0.96) and statins (OR, 0.48; 95% CI, 0.31-0.73) showed the opposite trend. “Alcohol use and obesity did not associate with risk of progression,” the reviewers added.
Thirteen studies in the meta-analysis were from Europe, six were from the United States, and one was from Australia. Ten were multicenter studies, 13 were deemed high-quality, three were deemed medium-quality, and four were deemed low-quality. The reviewers were unable to assess dose-response relationships for relevant factors, such as alcohol, tobacco, and medications, and not all studies accounted for potential confounding.
Only four studies included multivariate analyses to control for the confounding effects of age, sex, and BE characteristics (length and dysplasia). When the reviewers analyzed only these studies, older age and smoking no longer predicted BE progression. Use of proton pump inhibitors remained protective, and use of nonsteroidal anti-inflammatory drugs (NSAIDs) became protective, while statin use lost significance.
The reviewers disclosed no external funding sources or conflicts of interest.
SOURCE: Krishnamoorthi R, et al. Clinical Gastroenterol and Hepatol. 2017 Nov 30. doi: 10.1016/j.cgh.2017.11.044
Older age, male sex, smoking, longer segment length, and low-grade dysplasia were significant risk factors for progression of Barrett’s esophagus in a meta-analysis of 20 studies.
“Individuals with these features should undergo more intensive surveillance or endoscopic therapy,” Rajesh Krishnamoorthi, MD, of Mayo Clinic in Rochester, Minn., and his associates wrote in Clinical Gastroenterology and Hepatology. “Smoking is a modifiable risk factor for cancer prevention in patients with BE.”
“Currently, gastrointestinal societies’ guidelines on BE surveillance are solely based on dysplasia grade and do not take into account any of the other risk factors,” the reviewers concluded. Their findings could form the backbone of a risk score that identifies high-risk BE patients with baseline low-grade dysplasia or nondysplastic BE “who would benefit from intensive surveillance or endoscopic therapy.”
Esophageal adenocarcinoma is on the rise and fewer than one in five patients survive 5 years past diagnosis. Endoscopic surveillance for esophageal adenocarcinoma is recommended in Barrett’s esophagus, but only about one in 10 esophageal adenocarcinoma patients has a preceding BE diagnosis. “This ostensible discrepancy has raised concerns about the effectiveness of current screening and surveillance programs,” the reviewers noted. Studies also have yielded conflicting evidence about the value of endoscopic surveillance as currently performed. To help prioritize BE patients for surveillance, the reviewers searched EMBASE, MEDLINE, and Web of Science from inception through May 2016 for cohort studies of risk factors for progression of BE among patients with either no dysplasia or low-grade dysplasia.
The 20 studies covered 1,231 BE progression events among 74,943 patients. In separate pooled estimates, progression of BE correlated significantly with older age (odds ratio, 1.03; 95% CI, 1.01–1.05), male sex (OR, 2.2; 95% CI, 1.8-2.5), current or former smoking (OR, 1.5; 95% CI, 1.09-2.0), and greater BE segment length (OR, 1.3; 95% CI, 1.16-1.36). Results tended to be homogeneous among studies, said the reviewers. Low-grade dysplasia correlated strongly with progression (OR, 4.3; 95% CI, 2.6-7.0), while use of proton pump inhibitors (OR, 0.55; 95% CI, 0.32–0.96) and statins (OR, 0.48; 95% CI, 0.31-0.73) showed the opposite trend. “Alcohol use and obesity did not associate with risk of progression,” the reviewers added.
Thirteen studies in the meta-analysis were from Europe, six were from the United States, and one was from Australia. Ten were multicenter studies, 13 were deemed high-quality, three were deemed medium-quality, and four were deemed low-quality. The reviewers were unable to assess dose-response relationships for relevant factors, such as alcohol, tobacco, and medications, and not all studies accounted for potential confounding.
Only four studies included multivariate analyses to control for the confounding effects of age, sex, and BE characteristics (length and dysplasia). When the reviewers analyzed only these studies, older age and smoking no longer predicted BE progression. Use of proton pump inhibitors remained protective, and use of nonsteroidal anti-inflammatory drugs (NSAIDs) became protective, while statin use lost significance.
The reviewers disclosed no external funding sources or conflicts of interest.
SOURCE: Krishnamoorthi R, et al. Clinical Gastroenterol and Hepatol. 2017 Nov 30. doi: 10.1016/j.cgh.2017.11.044
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Male sex, older age, smoking, greater segment length, and low-grade dysplasia separately predicted progression of Barrett’s esophagus.
Major finding: Pooled odds ratios for risk ranged from 4.3 (low-grade dysplasia) to 1.03 (older age).
Study details: Systematic review and meta-analysis of 20 studies published through May 2016.
Disclosures: The reviewers disclosed no external funding sources or conflicts of interest.
Source: Krishnamoorthi R, et al. Clinical Gastroenterol and Hepatol. 2017 Nov 30.
July 2018 Digital Edition
Click here to access the July 2018 Digital Edition.
Table of Contents
- Vertebral Artery Dissection Due to Mixed Martial Arts Choke Hold
- Using Stroke Order Sets to Improve Compliance With Quality Measures for Stroke Admissions
- Screening and Treating HCV in the VA: Achieving Excellence Using Lean and System Redesign
- Transgender Care in the Primary Care Setting
- Pharmacist-Led ED Antimicrobial Surveillance
- Risks vs Benefits for SGLT2 Inhibitor Medications
- Caring Under a Microscope
- Tedizolid Use in Immunocompromised Patients

Click here to access the July 2018 Digital Edition.
Table of Contents
- Vertebral Artery Dissection Due to Mixed Martial Arts Choke Hold
- Using Stroke Order Sets to Improve Compliance With Quality Measures for Stroke Admissions
- Screening and Treating HCV in the VA: Achieving Excellence Using Lean and System Redesign
- Transgender Care in the Primary Care Setting
- Pharmacist-Led ED Antimicrobial Surveillance
- Risks vs Benefits for SGLT2 Inhibitor Medications
- Caring Under a Microscope
- Tedizolid Use in Immunocompromised Patients

Click here to access the July 2018 Digital Edition.
Table of Contents
- Vertebral Artery Dissection Due to Mixed Martial Arts Choke Hold
- Using Stroke Order Sets to Improve Compliance With Quality Measures for Stroke Admissions
- Screening and Treating HCV in the VA: Achieving Excellence Using Lean and System Redesign
- Transgender Care in the Primary Care Setting
- Pharmacist-Led ED Antimicrobial Surveillance
- Risks vs Benefits for SGLT2 Inhibitor Medications
- Caring Under a Microscope
- Tedizolid Use in Immunocompromised Patients

Owning a Gun: Not as Easy as it Looks
I am a nurse practitioner living in the South; I am also a concealed carry permit holder and an NRA pistol instructor who competes. I own an AR15; it is not an assault rifle—it’s just a rifle.
I often see articles about the “ease” of purchasing a gun, but this is just not true. Even with the laxer gun control of the South, obtaining a concealed carry license entails going through both the FBI and local police, a review of mental health records, a long questionnaire, and an eight-hour class that involves shooting. So, yes, I can purchase a gun in 30 minutes—but only because I’ve already been through this process.
If I wanted to purchase a gun without a permit, I would have to go to the courthouse and be fingerprinted and run through the system before I could get a one-time purchase permit. I could not get a permit if I had a mental illness, had ever been arrested or accused of domestic violence, etc.
My heart breaks every time a mass shooting, like the one at Marjory Stoneman Douglas High School, happens. Guns have been around in our area for many, many years. High school kids used to mount a shotgun in a rack on the top of their truck to hunt before school; they didn’t think of using it to hurt a person. I believe the problems we face today are multifaceted: a lack of parenting, absent fathers, people not getting the mental health services they need, and HIPAA! Mental health professionals are afraid to call authorities for fear of being sued.
I truly believe we need to stop politicizing this issue. Let’s quit blaming the guns themselves and work on real solutions. For example, parents have an obligation to lock up all firearms! Kids should never have access to guns from their own home. In my house, when we have visitors—even if they are adults—we lock our guns in our safe. Security at our schools should mimic that at courthouses, with metal detectors, armed security personnel, and limited entrance/exit areas.
Deborah Johnson, FNP-C
Kinston, NC
I am a nurse practitioner living in the South; I am also a concealed carry permit holder and an NRA pistol instructor who competes. I own an AR15; it is not an assault rifle—it’s just a rifle.
I often see articles about the “ease” of purchasing a gun, but this is just not true. Even with the laxer gun control of the South, obtaining a concealed carry license entails going through both the FBI and local police, a review of mental health records, a long questionnaire, and an eight-hour class that involves shooting. So, yes, I can purchase a gun in 30 minutes—but only because I’ve already been through this process.
If I wanted to purchase a gun without a permit, I would have to go to the courthouse and be fingerprinted and run through the system before I could get a one-time purchase permit. I could not get a permit if I had a mental illness, had ever been arrested or accused of domestic violence, etc.
My heart breaks every time a mass shooting, like the one at Marjory Stoneman Douglas High School, happens. Guns have been around in our area for many, many years. High school kids used to mount a shotgun in a rack on the top of their truck to hunt before school; they didn’t think of using it to hurt a person. I believe the problems we face today are multifaceted: a lack of parenting, absent fathers, people not getting the mental health services they need, and HIPAA! Mental health professionals are afraid to call authorities for fear of being sued.
I truly believe we need to stop politicizing this issue. Let’s quit blaming the guns themselves and work on real solutions. For example, parents have an obligation to lock up all firearms! Kids should never have access to guns from their own home. In my house, when we have visitors—even if they are adults—we lock our guns in our safe. Security at our schools should mimic that at courthouses, with metal detectors, armed security personnel, and limited entrance/exit areas.
Deborah Johnson, FNP-C
Kinston, NC
I am a nurse practitioner living in the South; I am also a concealed carry permit holder and an NRA pistol instructor who competes. I own an AR15; it is not an assault rifle—it’s just a rifle.
I often see articles about the “ease” of purchasing a gun, but this is just not true. Even with the laxer gun control of the South, obtaining a concealed carry license entails going through both the FBI and local police, a review of mental health records, a long questionnaire, and an eight-hour class that involves shooting. So, yes, I can purchase a gun in 30 minutes—but only because I’ve already been through this process.
If I wanted to purchase a gun without a permit, I would have to go to the courthouse and be fingerprinted and run through the system before I could get a one-time purchase permit. I could not get a permit if I had a mental illness, had ever been arrested or accused of domestic violence, etc.
My heart breaks every time a mass shooting, like the one at Marjory Stoneman Douglas High School, happens. Guns have been around in our area for many, many years. High school kids used to mount a shotgun in a rack on the top of their truck to hunt before school; they didn’t think of using it to hurt a person. I believe the problems we face today are multifaceted: a lack of parenting, absent fathers, people not getting the mental health services they need, and HIPAA! Mental health professionals are afraid to call authorities for fear of being sued.
I truly believe we need to stop politicizing this issue. Let’s quit blaming the guns themselves and work on real solutions. For example, parents have an obligation to lock up all firearms! Kids should never have access to guns from their own home. In my house, when we have visitors—even if they are adults—we lock our guns in our safe. Security at our schools should mimic that at courthouses, with metal detectors, armed security personnel, and limited entrance/exit areas.
Deborah Johnson, FNP-C
Kinston, NC
Fecal calprotectin levels predicted mucosal, deep healing in pediatric Crohn’s
For children with Crohn’s disease, fecal calprotectin levels below 300 mcg indicated mucosal healing, while values below 100 mcg signified deep healing in a multicenter, 151-patient study.
Sensitivity was 80% for mucosal healing and 71% for deep healing, while specificities were 81% and 92%, respectively, said Inbar Nakar of the Hebrew University of Jerusalem, with her associates. In line with prior studies, adding C-reactive protein (CRP) to fecal calprotectin improved neither sensitivity or specificity, the researchers wrote in Clinical Gastroenterology and Hepatology.
Bowel healing is a crucial goal in Crohn’s disease (CD). Because pediatric transmural healing had not been studied, the researchers analyzed data from the ImageKids study, a multicenter effort to develop magnetic resonance enterography (MRE) measures for CD patients aged 6-18 years. Participants averaged 14 years old with a standard deviation of 2 years. Assessments included MRE, complete ileocolonoscopic evaluation, CRP, and fecal calprotectin. The researchers defined mucosal healing as a Simple Endoscopic Severity Index in Crohn’s Disease score below 3, transmural healing as an MRE visual analog score below 20 mm, and deep healing as transmural plus mucosal healing.
Nearly one-third of patients had healing only in the mucosa or the bowel wall, but not both; 6% had mucosal healing but transmural inflammation, and 25% of children had transmural healing but mucosal inflammation. In addition, 14% of children had deep healing, and 55% of children had both mucosal and transmural inflammation. Those findings highlight “the discrepancy between mucosal and transmural inflammation and the importance of evaluating the disease by both ileocolonoscopy and imaging,” the researchers wrote.
Median calprotectin levels varied significantly by healing status (P less than .001). They were lowest (10 mcg/g) for deep healing, followed by either transmural or mucosal inflammation, and were highest (median, 810 mcg/g) when children had both mucosal and transmural inflammation. Calprotectin in children with deep healing had an area under the receiver operating characteristic curve value of 0.93 (95% confidence interval, 0.89- 0.98). In contrast, CRP level identified children with deep healing with an AUROC value of only 0.81 (95% CI, 0.71-0.90).
Although “calprotectin level is driven primarily by mucosal healing, [it] is still superior to CRP,” the investigators concluded. “Although a calprotectin cutoff [less than] 300 mcg/g predicted mucosal healing, a lower cutoff of [less than] 100 mcg/g may be more suitable to predict deep healing.” However, they emphasized that fecal calprotectin level is only moderately accurate in predicting mucosal or transmural healing in children with CD. They advised physicians to “be familiar with the predictive values of each cutoff before incorporating them in clinical decision making.”
An educational grant from AbbVie funded the ImageKids study. AbbVie was not otherwise involved in the study. Two coinvestigators disclosed ties to AbbVie and other pharmaceutical companies. There were no other disclosures.
SOURCE: Nakar I et al. Clin Gastroenterol Hepatol. 2018 Mar 2. doi: 10.1016/j.cgh.2018.01.024.
For children with Crohn’s disease, fecal calprotectin levels below 300 mcg indicated mucosal healing, while values below 100 mcg signified deep healing in a multicenter, 151-patient study.
Sensitivity was 80% for mucosal healing and 71% for deep healing, while specificities were 81% and 92%, respectively, said Inbar Nakar of the Hebrew University of Jerusalem, with her associates. In line with prior studies, adding C-reactive protein (CRP) to fecal calprotectin improved neither sensitivity or specificity, the researchers wrote in Clinical Gastroenterology and Hepatology.
Bowel healing is a crucial goal in Crohn’s disease (CD). Because pediatric transmural healing had not been studied, the researchers analyzed data from the ImageKids study, a multicenter effort to develop magnetic resonance enterography (MRE) measures for CD patients aged 6-18 years. Participants averaged 14 years old with a standard deviation of 2 years. Assessments included MRE, complete ileocolonoscopic evaluation, CRP, and fecal calprotectin. The researchers defined mucosal healing as a Simple Endoscopic Severity Index in Crohn’s Disease score below 3, transmural healing as an MRE visual analog score below 20 mm, and deep healing as transmural plus mucosal healing.
Nearly one-third of patients had healing only in the mucosa or the bowel wall, but not both; 6% had mucosal healing but transmural inflammation, and 25% of children had transmural healing but mucosal inflammation. In addition, 14% of children had deep healing, and 55% of children had both mucosal and transmural inflammation. Those findings highlight “the discrepancy between mucosal and transmural inflammation and the importance of evaluating the disease by both ileocolonoscopy and imaging,” the researchers wrote.
Median calprotectin levels varied significantly by healing status (P less than .001). They were lowest (10 mcg/g) for deep healing, followed by either transmural or mucosal inflammation, and were highest (median, 810 mcg/g) when children had both mucosal and transmural inflammation. Calprotectin in children with deep healing had an area under the receiver operating characteristic curve value of 0.93 (95% confidence interval, 0.89- 0.98). In contrast, CRP level identified children with deep healing with an AUROC value of only 0.81 (95% CI, 0.71-0.90).
Although “calprotectin level is driven primarily by mucosal healing, [it] is still superior to CRP,” the investigators concluded. “Although a calprotectin cutoff [less than] 300 mcg/g predicted mucosal healing, a lower cutoff of [less than] 100 mcg/g may be more suitable to predict deep healing.” However, they emphasized that fecal calprotectin level is only moderately accurate in predicting mucosal or transmural healing in children with CD. They advised physicians to “be familiar with the predictive values of each cutoff before incorporating them in clinical decision making.”
An educational grant from AbbVie funded the ImageKids study. AbbVie was not otherwise involved in the study. Two coinvestigators disclosed ties to AbbVie and other pharmaceutical companies. There were no other disclosures.
SOURCE: Nakar I et al. Clin Gastroenterol Hepatol. 2018 Mar 2. doi: 10.1016/j.cgh.2018.01.024.
For children with Crohn’s disease, fecal calprotectin levels below 300 mcg indicated mucosal healing, while values below 100 mcg signified deep healing in a multicenter, 151-patient study.
Sensitivity was 80% for mucosal healing and 71% for deep healing, while specificities were 81% and 92%, respectively, said Inbar Nakar of the Hebrew University of Jerusalem, with her associates. In line with prior studies, adding C-reactive protein (CRP) to fecal calprotectin improved neither sensitivity or specificity, the researchers wrote in Clinical Gastroenterology and Hepatology.
Bowel healing is a crucial goal in Crohn’s disease (CD). Because pediatric transmural healing had not been studied, the researchers analyzed data from the ImageKids study, a multicenter effort to develop magnetic resonance enterography (MRE) measures for CD patients aged 6-18 years. Participants averaged 14 years old with a standard deviation of 2 years. Assessments included MRE, complete ileocolonoscopic evaluation, CRP, and fecal calprotectin. The researchers defined mucosal healing as a Simple Endoscopic Severity Index in Crohn’s Disease score below 3, transmural healing as an MRE visual analog score below 20 mm, and deep healing as transmural plus mucosal healing.
Nearly one-third of patients had healing only in the mucosa or the bowel wall, but not both; 6% had mucosal healing but transmural inflammation, and 25% of children had transmural healing but mucosal inflammation. In addition, 14% of children had deep healing, and 55% of children had both mucosal and transmural inflammation. Those findings highlight “the discrepancy between mucosal and transmural inflammation and the importance of evaluating the disease by both ileocolonoscopy and imaging,” the researchers wrote.
Median calprotectin levels varied significantly by healing status (P less than .001). They were lowest (10 mcg/g) for deep healing, followed by either transmural or mucosal inflammation, and were highest (median, 810 mcg/g) when children had both mucosal and transmural inflammation. Calprotectin in children with deep healing had an area under the receiver operating characteristic curve value of 0.93 (95% confidence interval, 0.89- 0.98). In contrast, CRP level identified children with deep healing with an AUROC value of only 0.81 (95% CI, 0.71-0.90).
Although “calprotectin level is driven primarily by mucosal healing, [it] is still superior to CRP,” the investigators concluded. “Although a calprotectin cutoff [less than] 300 mcg/g predicted mucosal healing, a lower cutoff of [less than] 100 mcg/g may be more suitable to predict deep healing.” However, they emphasized that fecal calprotectin level is only moderately accurate in predicting mucosal or transmural healing in children with CD. They advised physicians to “be familiar with the predictive values of each cutoff before incorporating them in clinical decision making.”
An educational grant from AbbVie funded the ImageKids study. AbbVie was not otherwise involved in the study. Two coinvestigators disclosed ties to AbbVie and other pharmaceutical companies. There were no other disclosures.
SOURCE: Nakar I et al. Clin Gastroenterol Hepatol. 2018 Mar 2. doi: 10.1016/j.cgh.2018.01.024.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Fecal calprotectin levels below 300 mcg indicated mucosal healing, while values below 100 mcg signified deep healing in children with Crohn’s disease.
Major finding: Sensitivity was 80% for mucosal healing and 71% for deep healing, while specificities were 81% and 92%, respectively.
Study details: A multicenter study of 151 patients aged 6-18 years with Crohn’s disease.
Disclosures: AbbVie funded the ImageKids study through an educational grant but otherwise was not involved in the study. Two coinvestigators disclosed ties to AbbVie and other pharmaceutical companies. There were no other disclosures.
Source: Nakar I et al. Clin Gastroenterol Hepatol. 2018 Mar 2. doi: 10.1016/j.cgh.2018.01.024.
Buckwheat Extract
Native to North and East Asia, This highly adaptable plant – the most common species of which are Fagopyrum esculentum (common buckwheat or sweet buckwheat), and F. tataricum (which grows in more mountainous regions) – has acclimated to cultivation in North America, as well.1 Increasingly popular as a healthy grain option, buckwheat flour has been touted for beneficial effects on diabetes, obesity, hypertension, hypercholesterolemia, and constipation.1 It has also gained attention for its association with some allergic reactions.
Wound Healing
In 2008, van den Berg et al. performed an in vitro investigation of the antioxidant and anti-inflammatory qualities of buckwheat honey for consideration in wound healing. American buckwheat honey from New York was found to be the source of the most salient activities, with such properties attributed to its abundant phenolic components. The researchers suggested that these phenols might impart antibacterial activity, while the low pH and high free acid content of the buckwheat honey could contribute to healing wounds.4
Antioxidant Activity
The antioxidant capacity, along with other traits, characterizing the sprouts of common buckwheat (F. esculentum) and tartary buckwheat (F. tataricum) was evaluated by Liu et al. in 2008. Rutin is the main flavonoid found in both species, with fivefold higher levels identified in tartary buckwheat in this study. Ethanol extracts of tartary buckwheat also exhibited greater free radical scavenging activity and superoxide scavenging activity, compared with common buckwheat. Both buckwheat species displayed antioxidant activity on human hepatoma HepG2 cells, with tartary buckwheat more effective in diminishing cellular oxidative stress, which the authors attributed to its greater rutin and quercetin levels.5
Zhou et al. studied the protective effects of buckwheat honey on hydroxyl radical-induced DNA damage in 2012, finding that all studied honeys more effectively protected DNA in non–site specific rather than site-specific systems.6
Photoprotection
In a 2005 screening of 47 antioxidant substances and study of their effects on UV-induced lipid peroxidation, Trommer and Neubert reported that buckwheat extract significantly lowered radiation levels, as did extracts of St. John’s Wort, melissa, and sage. They concluded that their in vitro findings supported the inclusion of such ingredients in photoprotective cosmetic formulations or sunscreens pending the results of in vivo experiments with these compounds.7
In 2006, Hinneburg et al. evaluated the antioxidant and photoprotective activity of a buckwheat herb extract, also comparing its photoprotective characteristics to those of a commercial UV absorber. In an assay with 1,1-diphenyl-2-picryl-hydrazyl radical (DPPH), buckwheat extract exhibited significantly more antioxidant activity than did pure rutin, with buckwheat observed to more effectively block UV-induced peroxidation of linoleic acid as compared with rutin and the commercial UV absorber. The researchers concluded that including antioxidants such as buckwheat extract in photoprotective formulations may serve to maximize skin protection in such products.8
Buckwheat Sensitivity
Conclusion
Because it is a popular component in many diets around the world, especially Japan, Korea, Russia, and Poland, as well as other Asian and European countries, South Africa, Australia, and North America,4 it is reasonable to expect that we’ll see more research on buckwheat. For now, there are indications to suggest that more investigations are warranted to determine whether this botanical agent will have a meaningful role in the dermatologic armamentarium.
References
1. Li SQ et al. Crit Rev Food Sci Nutr. 2001 Sep;41(6):451-64.
2. Dattner AM. Dermatol Ther. 2003;16(2):106-13.
3. Hinneburg I et al. J Agric Food Chem. 2005 Jan 12;53(1):3-7.
4. van den Berg AJ et al. J Wound Care. 2008 Apr;17(4):172-4, 176-8.
5. Liu CL et al. J Agric Food Chem. 2008 Jan 9;56(1):173-8.
6. Zhou J et al. Food Chem Toxicol. 2012 Aug;50(8):2766-73.
7. Trommer H et al. J Pharm Pharm Sci. 2005 Sep 15;8(3):494-506.
8. Hinneburg I et al. Pharmazie. 2006 Mar;61(3):237-40.
9. Geiselhart S et al. Clin Exp Allergy. 2018 Feb;48(2):217-24.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014); she also authored a New York Times Best Seller for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance Therapeutics. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
Native to North and East Asia, This highly adaptable plant – the most common species of which are Fagopyrum esculentum (common buckwheat or sweet buckwheat), and F. tataricum (which grows in more mountainous regions) – has acclimated to cultivation in North America, as well.1 Increasingly popular as a healthy grain option, buckwheat flour has been touted for beneficial effects on diabetes, obesity, hypertension, hypercholesterolemia, and constipation.1 It has also gained attention for its association with some allergic reactions.
Wound Healing
In 2008, van den Berg et al. performed an in vitro investigation of the antioxidant and anti-inflammatory qualities of buckwheat honey for consideration in wound healing. American buckwheat honey from New York was found to be the source of the most salient activities, with such properties attributed to its abundant phenolic components. The researchers suggested that these phenols might impart antibacterial activity, while the low pH and high free acid content of the buckwheat honey could contribute to healing wounds.4
Antioxidant Activity
The antioxidant capacity, along with other traits, characterizing the sprouts of common buckwheat (F. esculentum) and tartary buckwheat (F. tataricum) was evaluated by Liu et al. in 2008. Rutin is the main flavonoid found in both species, with fivefold higher levels identified in tartary buckwheat in this study. Ethanol extracts of tartary buckwheat also exhibited greater free radical scavenging activity and superoxide scavenging activity, compared with common buckwheat. Both buckwheat species displayed antioxidant activity on human hepatoma HepG2 cells, with tartary buckwheat more effective in diminishing cellular oxidative stress, which the authors attributed to its greater rutin and quercetin levels.5
Zhou et al. studied the protective effects of buckwheat honey on hydroxyl radical-induced DNA damage in 2012, finding that all studied honeys more effectively protected DNA in non–site specific rather than site-specific systems.6
Photoprotection
In a 2005 screening of 47 antioxidant substances and study of their effects on UV-induced lipid peroxidation, Trommer and Neubert reported that buckwheat extract significantly lowered radiation levels, as did extracts of St. John’s Wort, melissa, and sage. They concluded that their in vitro findings supported the inclusion of such ingredients in photoprotective cosmetic formulations or sunscreens pending the results of in vivo experiments with these compounds.7
In 2006, Hinneburg et al. evaluated the antioxidant and photoprotective activity of a buckwheat herb extract, also comparing its photoprotective characteristics to those of a commercial UV absorber. In an assay with 1,1-diphenyl-2-picryl-hydrazyl radical (DPPH), buckwheat extract exhibited significantly more antioxidant activity than did pure rutin, with buckwheat observed to more effectively block UV-induced peroxidation of linoleic acid as compared with rutin and the commercial UV absorber. The researchers concluded that including antioxidants such as buckwheat extract in photoprotective formulations may serve to maximize skin protection in such products.8
Buckwheat Sensitivity
Conclusion
Because it is a popular component in many diets around the world, especially Japan, Korea, Russia, and Poland, as well as other Asian and European countries, South Africa, Australia, and North America,4 it is reasonable to expect that we’ll see more research on buckwheat. For now, there are indications to suggest that more investigations are warranted to determine whether this botanical agent will have a meaningful role in the dermatologic armamentarium.
References
1. Li SQ et al. Crit Rev Food Sci Nutr. 2001 Sep;41(6):451-64.
2. Dattner AM. Dermatol Ther. 2003;16(2):106-13.
3. Hinneburg I et al. J Agric Food Chem. 2005 Jan 12;53(1):3-7.
4. van den Berg AJ et al. J Wound Care. 2008 Apr;17(4):172-4, 176-8.
5. Liu CL et al. J Agric Food Chem. 2008 Jan 9;56(1):173-8.
6. Zhou J et al. Food Chem Toxicol. 2012 Aug;50(8):2766-73.
7. Trommer H et al. J Pharm Pharm Sci. 2005 Sep 15;8(3):494-506.
8. Hinneburg I et al. Pharmazie. 2006 Mar;61(3):237-40.
9. Geiselhart S et al. Clin Exp Allergy. 2018 Feb;48(2):217-24.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014); she also authored a New York Times Best Seller for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance Therapeutics. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
Native to North and East Asia, This highly adaptable plant – the most common species of which are Fagopyrum esculentum (common buckwheat or sweet buckwheat), and F. tataricum (which grows in more mountainous regions) – has acclimated to cultivation in North America, as well.1 Increasingly popular as a healthy grain option, buckwheat flour has been touted for beneficial effects on diabetes, obesity, hypertension, hypercholesterolemia, and constipation.1 It has also gained attention for its association with some allergic reactions.
Wound Healing
In 2008, van den Berg et al. performed an in vitro investigation of the antioxidant and anti-inflammatory qualities of buckwheat honey for consideration in wound healing. American buckwheat honey from New York was found to be the source of the most salient activities, with such properties attributed to its abundant phenolic components. The researchers suggested that these phenols might impart antibacterial activity, while the low pH and high free acid content of the buckwheat honey could contribute to healing wounds.4
Antioxidant Activity
The antioxidant capacity, along with other traits, characterizing the sprouts of common buckwheat (F. esculentum) and tartary buckwheat (F. tataricum) was evaluated by Liu et al. in 2008. Rutin is the main flavonoid found in both species, with fivefold higher levels identified in tartary buckwheat in this study. Ethanol extracts of tartary buckwheat also exhibited greater free radical scavenging activity and superoxide scavenging activity, compared with common buckwheat. Both buckwheat species displayed antioxidant activity on human hepatoma HepG2 cells, with tartary buckwheat more effective in diminishing cellular oxidative stress, which the authors attributed to its greater rutin and quercetin levels.5
Zhou et al. studied the protective effects of buckwheat honey on hydroxyl radical-induced DNA damage in 2012, finding that all studied honeys more effectively protected DNA in non–site specific rather than site-specific systems.6
Photoprotection
In a 2005 screening of 47 antioxidant substances and study of their effects on UV-induced lipid peroxidation, Trommer and Neubert reported that buckwheat extract significantly lowered radiation levels, as did extracts of St. John’s Wort, melissa, and sage. They concluded that their in vitro findings supported the inclusion of such ingredients in photoprotective cosmetic formulations or sunscreens pending the results of in vivo experiments with these compounds.7
In 2006, Hinneburg et al. evaluated the antioxidant and photoprotective activity of a buckwheat herb extract, also comparing its photoprotective characteristics to those of a commercial UV absorber. In an assay with 1,1-diphenyl-2-picryl-hydrazyl radical (DPPH), buckwheat extract exhibited significantly more antioxidant activity than did pure rutin, with buckwheat observed to more effectively block UV-induced peroxidation of linoleic acid as compared with rutin and the commercial UV absorber. The researchers concluded that including antioxidants such as buckwheat extract in photoprotective formulations may serve to maximize skin protection in such products.8
Buckwheat Sensitivity
Conclusion
Because it is a popular component in many diets around the world, especially Japan, Korea, Russia, and Poland, as well as other Asian and European countries, South Africa, Australia, and North America,4 it is reasonable to expect that we’ll see more research on buckwheat. For now, there are indications to suggest that more investigations are warranted to determine whether this botanical agent will have a meaningful role in the dermatologic armamentarium.
References
1. Li SQ et al. Crit Rev Food Sci Nutr. 2001 Sep;41(6):451-64.
2. Dattner AM. Dermatol Ther. 2003;16(2):106-13.
3. Hinneburg I et al. J Agric Food Chem. 2005 Jan 12;53(1):3-7.
4. van den Berg AJ et al. J Wound Care. 2008 Apr;17(4):172-4, 176-8.
5. Liu CL et al. J Agric Food Chem. 2008 Jan 9;56(1):173-8.
6. Zhou J et al. Food Chem Toxicol. 2012 Aug;50(8):2766-73.
7. Trommer H et al. J Pharm Pharm Sci. 2005 Sep 15;8(3):494-506.
8. Hinneburg I et al. Pharmazie. 2006 Mar;61(3):237-40.
9. Geiselhart S et al. Clin Exp Allergy. 2018 Feb;48(2):217-24.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014); she also authored a New York Times Best Seller for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance Therapeutics. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
Pediatric Dermatology: Summer 2018
Falls: Still a Deadly Danger for Elderly
Falls have long been a major hazard for older adults—accounting for the largest percentage of deaths from unintentional injuries. Each year, about 1 in 4 older adults in the US reports falling, and an estimated 3 million emergency department visits every year are related to falls.
According to the CDC, things are getting worse. Overall, deaths due to falls among older adults increased 31% from 2007- 2016, a rate of 3% per year. The rate increased in every demographic category except among American Indians/Alaska Natives, with the largest increase (4%) among people aged ≥ 85 years. Nationwide, nearly 30,000 older adults died from fall-related causes in 2016.
But falls are not an inevitable part of aging, the CDC reminds. Advanced age is a well-known independent risk factor; other risk factors include reduced activity, chronic conditions (including incontinence), prescription medications (which may act synergistically on the central nervous system), and changes in gait and balance. The CDC says health care providers can address the problem by asking patients about falls, assessing gait and balance, reviewing medications, and prescribing interventions such as strength and balance exercises or physical therapy.
Initiatives such as CDC’s STEADI (Stopping Elderly Accidents, Deaths, and Injuries) can help with risk assessment, patient education, and interventions (https://www.cdc.gov/steadi).
Falls have long been a major hazard for older adults—accounting for the largest percentage of deaths from unintentional injuries. Each year, about 1 in 4 older adults in the US reports falling, and an estimated 3 million emergency department visits every year are related to falls.
According to the CDC, things are getting worse. Overall, deaths due to falls among older adults increased 31% from 2007- 2016, a rate of 3% per year. The rate increased in every demographic category except among American Indians/Alaska Natives, with the largest increase (4%) among people aged ≥ 85 years. Nationwide, nearly 30,000 older adults died from fall-related causes in 2016.
But falls are not an inevitable part of aging, the CDC reminds. Advanced age is a well-known independent risk factor; other risk factors include reduced activity, chronic conditions (including incontinence), prescription medications (which may act synergistically on the central nervous system), and changes in gait and balance. The CDC says health care providers can address the problem by asking patients about falls, assessing gait and balance, reviewing medications, and prescribing interventions such as strength and balance exercises or physical therapy.
Initiatives such as CDC’s STEADI (Stopping Elderly Accidents, Deaths, and Injuries) can help with risk assessment, patient education, and interventions (https://www.cdc.gov/steadi).
Falls have long been a major hazard for older adults—accounting for the largest percentage of deaths from unintentional injuries. Each year, about 1 in 4 older adults in the US reports falling, and an estimated 3 million emergency department visits every year are related to falls.
According to the CDC, things are getting worse. Overall, deaths due to falls among older adults increased 31% from 2007- 2016, a rate of 3% per year. The rate increased in every demographic category except among American Indians/Alaska Natives, with the largest increase (4%) among people aged ≥ 85 years. Nationwide, nearly 30,000 older adults died from fall-related causes in 2016.
But falls are not an inevitable part of aging, the CDC reminds. Advanced age is a well-known independent risk factor; other risk factors include reduced activity, chronic conditions (including incontinence), prescription medications (which may act synergistically on the central nervous system), and changes in gait and balance. The CDC says health care providers can address the problem by asking patients about falls, assessing gait and balance, reviewing medications, and prescribing interventions such as strength and balance exercises or physical therapy.
Initiatives such as CDC’s STEADI (Stopping Elderly Accidents, Deaths, and Injuries) can help with risk assessment, patient education, and interventions (https://www.cdc.gov/steadi).
Updating the core competencies in hospital medicine—2017 revision: Introduction and methodology
In 2006, the Society of Hospital Medicine (SHM) first published The Core Competencies in Hospital Medicine: A Framework for Curricular Development (henceforth described as the Core Competencies) to help define the role and expectations of hospitalists.1,2 The Core Competencies provided a framework for evaluating clinical skills and professional expertise within a rapidly developing field and highlighted opportunities for growth. Since the initial development and publication of the Core Competencies, changes in the healthcare landscape and hospitalist practice environment have prompted this revision.
Over the past decade, the field of hospital medicine has experienced exponential growth. In 2005, just over 16,000 hospitalists were practicing in the United States. By 2015, that number had increased to an estimated 44,000 hospitalists, accounting for approximately 6% of the physician workforce.3 Hospitalists have expanded the scope of hospital medicine in many ways. In their roles, hospitalists lead and participate in hospital-based care models that emphasize interprofessional collaboration and a focus on the delivery of high-quality and cost-effective care across a variety of clinical domains (eg, the Choosing Wisely initiative).4 They are also engaged in patient safety and quality initiatives that are increasingly being used as benchmarks to rate hospitals and as factors for hospital payment (eg, Hospital Inpatient Value-Based Purchasing Program).5 In fact, the American Board of Internal Medicine (ABIM) created a Focused Practice in Hospital Medicine Maintenance of Certification program in response to the growing number of internists choosing to concentrate their practice in the hospital setting. This decision by the ABIM underscores the value that hospitalists bring to improving patient care in the hospital setting. The ABIM also recognizes the Core Competencies as a curricular framework for a focused practice in hospital medicine.6
Changes within the educational environment have demanded attentive and active participation by many hospitalists. For example, in 2012, the Accreditation Council for Graduate Medical Education (ACGME) introduced the Milestones Project, a new outcomes-based framework designed to more effectively assess learner performance across the 6 core competencies.7 These milestones assessments create intentional opportunities to guide the development of physicians during their training, including in the inpatient environments in which hospitalists practice. Where applicable, existing Core Competencies learning objectives were compared with external sources such as the individual ACGME performance milestones for this revision.
THE CORE COMPETENCIES
The Core Competencies focus on adult hospital medicine. The Pediatric Hospital Medicine Core Competencies are published separately.8 Importantly, the Core Competencies document is not intended to define an absolute set of clinical, procedural, or system-based topics described in textbooks or used by graduate medical education training programs. It does not define or limit the scope of the practice of hospital medicine. Rather, the Core Competencies serve as measurable learning objectives that encourage teaching faculty, practicing hospitalists, and administrators to develop individual skill sets and programs to improve patient care contextualized to the needs of an individual, care setting, or institution. To permit this flexibility, individual chapter-specific objectives are intentionally general in nature. Finally, the Core Competencies document is not a set of practice guidelines, nor does it offer any representation of a “standard of care.” Readers are encouraged to explore the article by McKean et al.9 to review examples of application of the Core Competencies and suggestions for curricular development.
The purpose of this article is to describe the criteria for inclusion of new chapters in the Core Competencies and the methodology of the review and revision process. It outlines the process of initial review and editing of the existing chapters; needs assessment for new topics; new chapter production; and the process of review and revision of individual chapters to create the complete document. The revised Core Competencies document is available online at http://www.journalofhospitalmedicine.com/jhospmed/issue/134981/journal-hospital-medicine-124-suppl-1.
REVIEW AND REVISION PROCESS
In 2012, the Society of Hospital Medicine (SHM) Education Committee created a Core Competencies Task Force (CCTF) in response to the SHM Board of Directors’ charge that it review and update the initial Core Competencies document. The CCTF comprised of 5 physician SHM Education Committee members and one SHM staff representative. CCTF membership included hospitalists with an interest and familiarity with the Core Competencies document. The SHM Education Committee nominated the CCTF chair, who determined the optimal size, qualifications, and composition of the task force with approval from the Committee. The CCTF communicated through frequent conference calls and via e-mail correspondence to conduct an initial review of the existing chapters and to perform a needs assessment for new topics.
Individual Chapter Review
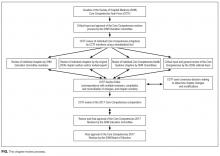
The SHM Education Committee provided critical input and approved the chapter review process designed by the CCTF (Figure). The CCTF reviewed each chapter of the Core Competencies document to assess its continuing relevance to the field of hospital medicine with a standardized tool (Appendix 1). The process required that at least 2 CCTF members reviewed each chapter. Preliminary reviewers assessed the current relevance of each chapter, determined whether individual learning objectives required additional investigation or modification, and developed new learning objectives to fill any educational gaps. All CCTF members then discussed assimilated feedback from the initial CCTF review, using consensus decision making to determine chapter changes and modifications. The CCTF found each of the existing chapters to be relevant to the field and identified none for removal.
The CCTF rewrote all chapters. It then disseminated proposed chapter changes to a panel of diverse independent reviewers to solicit suggestions and comments to ensure a multidisciplinary and balanced review process. Independent reviewers included authors of the original Core Competencies chapters, invited content experts, and members of the SHM Education Committee. When appropriate, corresponding SHM Committees reviewed individual chapters for updates and revisions. For example, the SHM Hospital Quality and Patient Safety Committee reviewed the chapters on patient safety and quality improvement, and the SHM Practice Management Committee reviewed the chapter on management practices. Four CCTF section editors managed an independent portfolio of chapters. Each CCTF section editor assimilated the various draft versions, corresponded with individual reviewers when necessary, and compiled the changes into a subsequent draft. This process ensured that the final version of every chapter reflected the thoughtful input from all parties involved in the review. Throughout the process, the CCTF used consensus decision making to adjudicate chapter changes and modifications. The 2006 Core Competencies Editorial team also reviewed the revision and provided critical input. The SHM Education Committee and the SHM Board of Directors reviewed and approved the final version of the Core Competencies document.
Needs Assessment and Selection of New Core Competency Chapters
The CCTF issued a call for new topics to the members of the SHM Education Committee for inclusion in the Core Competencies. Topics were also identified from the following sources: the top 100 adult medical diagnoses at hospital discharge in the Healthcare Cost and Utilization Project database in 2010; topics in hospital medicine textbooks; curricula presented at the 3 most recent SHM annual meetings; and responses from SHM annual meeting surveys. Table 1 lists the topics considered for addition.
Members of the SHM Education Committee rated each of the potential topics considered for inclusion based on the following characteristics: relevance to the field of hospital medicine; intersection of the topic with medical subspecialties; and its appropriateness as a separate, stand-alone chapter. In addition, topics more frequently encountered by hospitalists, those deemed clinically important with a known risk of complications or management inconsistencies, and those with significant opportunities for quality improvement initiatives carried more weight. Syncope and hyponatremia were the only 2 clinical conditions identified that met all of the inclusion criteria. No additional topics met the criteria for new chapter development in the Procedures or Healthcare Systems sections. The SHM Education Committee identified the use of point-of-care ultrasonography as an important advancement in the field. Where appropriate, the individual procedure chapters now include a new competency-based objective highlighting its role. In addition, a separate SHM task force is working to develop a practice guideline for the use of point-of-care ultrasonography by hospitalists.
Contributors
The SHM Education Committee determined authorship for the new chapters (syncope and hyponatremia). It assigned 2 CCTF members with content expertise and familiarity with the Core Competencies to each author one chapter. Given the limited number of new chapters, it made a decision to develop the content internally rather than through an open-call for authorship nominations to practicing SHM members. The authors made an effort to maintain consistency with the educational theory used to develop the initial Core Competencies. Each of the new topics underwent rigorous review as previously described, including additional independent reviews by hospitalists with content expertise in these areas.
CHAPTER FORMAT AND CONTENT CHANGES
Following the same format as the earlier version, the 2017 Core Competencies revision contains 53 chapters, divided into 3 sections—Clinical Conditions, Procedures, and Healthcare Systems (Table 2) —all integral components of the practice of hospital medicine. The design allows individual chapters to stand alone. However, each chapter should be considered in the context of the entire document because a particular concept may be only briefly discussed in one chapter, but described in greater depth in another given the potential overlap across topics.
The chapters maintain the same content structure as the original version. Each chapter begins with an introductory paragraph followed by a list of competency-based objectives grouped in subsections according to the educational theory of learning domains: cognitive (knowledge), psychomotor (skills), and affective (attitudes).10 In addition, a subsection for System Organization and Improvement is included in the Clinical Conditions and Procedure chapters to emphasize the importance of interprofessional collaboration for optimal patient care. These subsections were not included in the Healthcare Systems chapters, as system organization and improvement is intrinsic to these subjects.
The introductory paragraph provides background information and describes how the chapter remains relevant to the current practice of hospital medicine. Individual competency-based objectives outline a relevant concept and expected level of proficiency as defined by Bloom’s taxonomy.10 New objectives reflect changes in the healthcare landscape over the past decade or further enhance each chapter’s concepts. Chapter authors made an effort to develop chapter and learning objective concepts that are consistent with external resources such as the ACGME Milestones Project and practice guideline objectives developed by a variety of professional organizations.
SUMMARY AND FUTURE DIRECTIONS
The Core Competencies document serves as a resource for hospitalists and hospital medicine programs to evaluate, develop, and improve individual and collective skills and the practice environment. The Core Competencies also provide a framework for medical school clerkship directors and residency and fellowship program directors, as well as course directors of Continuing Medical Education programs, to develop curricula to enhance educational experiences for trainees and hospital medicine providers. The updates in every chapter in this revision to the Core Competencies reflects the changes in the healthcare landscape and hospitalist practice environment over the past decade, and we encourage readers to revisit the entire compendium. Table 3 highlights some of the salient changes in this revision.
Hospital medicine continues to evolve as a specialty. The Core Competencies define hospitalists as agents of change and foster the development of a culture of safe and effective patient care within the hospital environment. Although the CCTF hopes that the Core Competencies will preserve their relevance over time, it recognizes the importance of their periodic reevaluation and adaptation. Additionally, SHM developed the Core Competencies primarily for physicians practicing as hospitalists. As the number of physician assistants and nurse practitioners engaged in the practice of hospital medicine increases, and hospital medicine expands into nontraditional specialties such as surgical comanagement, it may be necessary to consider the development of additional or separate Hospital Medicine Core Competencies tailored to the needs of these subsets of clinicians.
Acknowledgments
The authors and the CCTF are immensely grateful to Nick Marzano for project coordination and Abbie Young for her assistance with medical editing and chapter formatting. We extend our sincerest appreciation and gratitude to the index team of authors and editors whose efforts laid the foundation for this body of work. The initial development and this revision of the Core Competencies would not have been possible without the support and assistance of the SHM staff, the SHM Education Committee, and the scores of contributors and reviewers who participated in its creation (complete list of individuals is available in Appendix 2). We thank everyone for his or her invaluable input and effort.
Disclosures
The Society of Hospital Medicine (SHM) provided administrative support for project coordination. SHM, or any of its representatives, had no role in the development of topic areas, refinement, or vetting of the topic list. No member of the Core Competencies Task Force or the SHM Education Committee received compensation for their participation in revising the Core Competencies. The authors report no conflicts of inte
1. The core competencies in hospital medicine: a framework for curriculum development by the society of hospital medicine. J Hosp Med. 2006;1 Suppl 1:2-95.
2. Dressler DD, Pistoria MJ, Budnitz TL, McKean SCW, Amin AN. Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1(1):48-56.
3. Hospital Medicine News, Society of Hospital Medicine. http://www.hospitalmedicine.org/press. Accessed June 16, 2016.
4. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492.
5. Conway PH. Value-driven health care: implications for hospitals and hospitalists. J Hosp Med. 2009;4(8):507-511.
6. American Board of Internal Medicine. Questions and Answers Regarding ABIM’s Maintenance of Certification in Internal Medicine with a Focused Practice in Hospital Medicine Program. 2009. http://www.abim.org/news/focused-practice-hospital-medicine-questions-answers.aspx. Accessed November 11, 2016.
7. The Internal Medicine Milestone Project. http://www.acgme.org/acgmeweb/portals/0/pdfs/milestones/internalmedicinemilestones.pdf. Accessed February 29, 2016.
8. Stucky ER, Ottolini MC, Maniscalco J. Pediatric hospital medicine core competencies: development and methodology. J Hosp Med. 2010;5(6):339-343.
9. McKean SC, Budnitz TL, Dressler DD, Amin AN, Pistoria MJ. How to use the core competencies in hospital medicine: a framework for curriculum development. J Hosp Med. 2006;1 Suppl 1:57-67.
10. Anderson LW, Krathwohl DR (eds). A Taxonomy for Learning, Teaching and Assessing: A Revision of Bloom’s Taxonomy of Educational Outcomes. Complete edition. New York, NY: Longman; 2001.
In 2006, the Society of Hospital Medicine (SHM) first published The Core Competencies in Hospital Medicine: A Framework for Curricular Development (henceforth described as the Core Competencies) to help define the role and expectations of hospitalists.1,2 The Core Competencies provided a framework for evaluating clinical skills and professional expertise within a rapidly developing field and highlighted opportunities for growth. Since the initial development and publication of the Core Competencies, changes in the healthcare landscape and hospitalist practice environment have prompted this revision.
Over the past decade, the field of hospital medicine has experienced exponential growth. In 2005, just over 16,000 hospitalists were practicing in the United States. By 2015, that number had increased to an estimated 44,000 hospitalists, accounting for approximately 6% of the physician workforce.3 Hospitalists have expanded the scope of hospital medicine in many ways. In their roles, hospitalists lead and participate in hospital-based care models that emphasize interprofessional collaboration and a focus on the delivery of high-quality and cost-effective care across a variety of clinical domains (eg, the Choosing Wisely initiative).4 They are also engaged in patient safety and quality initiatives that are increasingly being used as benchmarks to rate hospitals and as factors for hospital payment (eg, Hospital Inpatient Value-Based Purchasing Program).5 In fact, the American Board of Internal Medicine (ABIM) created a Focused Practice in Hospital Medicine Maintenance of Certification program in response to the growing number of internists choosing to concentrate their practice in the hospital setting. This decision by the ABIM underscores the value that hospitalists bring to improving patient care in the hospital setting. The ABIM also recognizes the Core Competencies as a curricular framework for a focused practice in hospital medicine.6
Changes within the educational environment have demanded attentive and active participation by many hospitalists. For example, in 2012, the Accreditation Council for Graduate Medical Education (ACGME) introduced the Milestones Project, a new outcomes-based framework designed to more effectively assess learner performance across the 6 core competencies.7 These milestones assessments create intentional opportunities to guide the development of physicians during their training, including in the inpatient environments in which hospitalists practice. Where applicable, existing Core Competencies learning objectives were compared with external sources such as the individual ACGME performance milestones for this revision.
THE CORE COMPETENCIES
The Core Competencies focus on adult hospital medicine. The Pediatric Hospital Medicine Core Competencies are published separately.8 Importantly, the Core Competencies document is not intended to define an absolute set of clinical, procedural, or system-based topics described in textbooks or used by graduate medical education training programs. It does not define or limit the scope of the practice of hospital medicine. Rather, the Core Competencies serve as measurable learning objectives that encourage teaching faculty, practicing hospitalists, and administrators to develop individual skill sets and programs to improve patient care contextualized to the needs of an individual, care setting, or institution. To permit this flexibility, individual chapter-specific objectives are intentionally general in nature. Finally, the Core Competencies document is not a set of practice guidelines, nor does it offer any representation of a “standard of care.” Readers are encouraged to explore the article by McKean et al.9 to review examples of application of the Core Competencies and suggestions for curricular development.
The purpose of this article is to describe the criteria for inclusion of new chapters in the Core Competencies and the methodology of the review and revision process. It outlines the process of initial review and editing of the existing chapters; needs assessment for new topics; new chapter production; and the process of review and revision of individual chapters to create the complete document. The revised Core Competencies document is available online at http://www.journalofhospitalmedicine.com/jhospmed/issue/134981/journal-hospital-medicine-124-suppl-1.
REVIEW AND REVISION PROCESS
In 2012, the Society of Hospital Medicine (SHM) Education Committee created a Core Competencies Task Force (CCTF) in response to the SHM Board of Directors’ charge that it review and update the initial Core Competencies document. The CCTF comprised of 5 physician SHM Education Committee members and one SHM staff representative. CCTF membership included hospitalists with an interest and familiarity with the Core Competencies document. The SHM Education Committee nominated the CCTF chair, who determined the optimal size, qualifications, and composition of the task force with approval from the Committee. The CCTF communicated through frequent conference calls and via e-mail correspondence to conduct an initial review of the existing chapters and to perform a needs assessment for new topics.
Individual Chapter Review
The SHM Education Committee provided critical input and approved the chapter review process designed by the CCTF (Figure). The CCTF reviewed each chapter of the Core Competencies document to assess its continuing relevance to the field of hospital medicine with a standardized tool (Appendix 1). The process required that at least 2 CCTF members reviewed each chapter. Preliminary reviewers assessed the current relevance of each chapter, determined whether individual learning objectives required additional investigation or modification, and developed new learning objectives to fill any educational gaps. All CCTF members then discussed assimilated feedback from the initial CCTF review, using consensus decision making to determine chapter changes and modifications. The CCTF found each of the existing chapters to be relevant to the field and identified none for removal.
The CCTF rewrote all chapters. It then disseminated proposed chapter changes to a panel of diverse independent reviewers to solicit suggestions and comments to ensure a multidisciplinary and balanced review process. Independent reviewers included authors of the original Core Competencies chapters, invited content experts, and members of the SHM Education Committee. When appropriate, corresponding SHM Committees reviewed individual chapters for updates and revisions. For example, the SHM Hospital Quality and Patient Safety Committee reviewed the chapters on patient safety and quality improvement, and the SHM Practice Management Committee reviewed the chapter on management practices. Four CCTF section editors managed an independent portfolio of chapters. Each CCTF section editor assimilated the various draft versions, corresponded with individual reviewers when necessary, and compiled the changes into a subsequent draft. This process ensured that the final version of every chapter reflected the thoughtful input from all parties involved in the review. Throughout the process, the CCTF used consensus decision making to adjudicate chapter changes and modifications. The 2006 Core Competencies Editorial team also reviewed the revision and provided critical input. The SHM Education Committee and the SHM Board of Directors reviewed and approved the final version of the Core Competencies document.
Needs Assessment and Selection of New Core Competency Chapters
The CCTF issued a call for new topics to the members of the SHM Education Committee for inclusion in the Core Competencies. Topics were also identified from the following sources: the top 100 adult medical diagnoses at hospital discharge in the Healthcare Cost and Utilization Project database in 2010; topics in hospital medicine textbooks; curricula presented at the 3 most recent SHM annual meetings; and responses from SHM annual meeting surveys. Table 1 lists the topics considered for addition.
Members of the SHM Education Committee rated each of the potential topics considered for inclusion based on the following characteristics: relevance to the field of hospital medicine; intersection of the topic with medical subspecialties; and its appropriateness as a separate, stand-alone chapter. In addition, topics more frequently encountered by hospitalists, those deemed clinically important with a known risk of complications or management inconsistencies, and those with significant opportunities for quality improvement initiatives carried more weight. Syncope and hyponatremia were the only 2 clinical conditions identified that met all of the inclusion criteria. No additional topics met the criteria for new chapter development in the Procedures or Healthcare Systems sections. The SHM Education Committee identified the use of point-of-care ultrasonography as an important advancement in the field. Where appropriate, the individual procedure chapters now include a new competency-based objective highlighting its role. In addition, a separate SHM task force is working to develop a practice guideline for the use of point-of-care ultrasonography by hospitalists.
Contributors
The SHM Education Committee determined authorship for the new chapters (syncope and hyponatremia). It assigned 2 CCTF members with content expertise and familiarity with the Core Competencies to each author one chapter. Given the limited number of new chapters, it made a decision to develop the content internally rather than through an open-call for authorship nominations to practicing SHM members. The authors made an effort to maintain consistency with the educational theory used to develop the initial Core Competencies. Each of the new topics underwent rigorous review as previously described, including additional independent reviews by hospitalists with content expertise in these areas.
CHAPTER FORMAT AND CONTENT CHANGES
Following the same format as the earlier version, the 2017 Core Competencies revision contains 53 chapters, divided into 3 sections—Clinical Conditions, Procedures, and Healthcare Systems (Table 2) —all integral components of the practice of hospital medicine. The design allows individual chapters to stand alone. However, each chapter should be considered in the context of the entire document because a particular concept may be only briefly discussed in one chapter, but described in greater depth in another given the potential overlap across topics.
The chapters maintain the same content structure as the original version. Each chapter begins with an introductory paragraph followed by a list of competency-based objectives grouped in subsections according to the educational theory of learning domains: cognitive (knowledge), psychomotor (skills), and affective (attitudes).10 In addition, a subsection for System Organization and Improvement is included in the Clinical Conditions and Procedure chapters to emphasize the importance of interprofessional collaboration for optimal patient care. These subsections were not included in the Healthcare Systems chapters, as system organization and improvement is intrinsic to these subjects.
The introductory paragraph provides background information and describes how the chapter remains relevant to the current practice of hospital medicine. Individual competency-based objectives outline a relevant concept and expected level of proficiency as defined by Bloom’s taxonomy.10 New objectives reflect changes in the healthcare landscape over the past decade or further enhance each chapter’s concepts. Chapter authors made an effort to develop chapter and learning objective concepts that are consistent with external resources such as the ACGME Milestones Project and practice guideline objectives developed by a variety of professional organizations.
SUMMARY AND FUTURE DIRECTIONS
The Core Competencies document serves as a resource for hospitalists and hospital medicine programs to evaluate, develop, and improve individual and collective skills and the practice environment. The Core Competencies also provide a framework for medical school clerkship directors and residency and fellowship program directors, as well as course directors of Continuing Medical Education programs, to develop curricula to enhance educational experiences for trainees and hospital medicine providers. The updates in every chapter in this revision to the Core Competencies reflects the changes in the healthcare landscape and hospitalist practice environment over the past decade, and we encourage readers to revisit the entire compendium. Table 3 highlights some of the salient changes in this revision.
Hospital medicine continues to evolve as a specialty. The Core Competencies define hospitalists as agents of change and foster the development of a culture of safe and effective patient care within the hospital environment. Although the CCTF hopes that the Core Competencies will preserve their relevance over time, it recognizes the importance of their periodic reevaluation and adaptation. Additionally, SHM developed the Core Competencies primarily for physicians practicing as hospitalists. As the number of physician assistants and nurse practitioners engaged in the practice of hospital medicine increases, and hospital medicine expands into nontraditional specialties such as surgical comanagement, it may be necessary to consider the development of additional or separate Hospital Medicine Core Competencies tailored to the needs of these subsets of clinicians.
Acknowledgments
The authors and the CCTF are immensely grateful to Nick Marzano for project coordination and Abbie Young for her assistance with medical editing and chapter formatting. We extend our sincerest appreciation and gratitude to the index team of authors and editors whose efforts laid the foundation for this body of work. The initial development and this revision of the Core Competencies would not have been possible without the support and assistance of the SHM staff, the SHM Education Committee, and the scores of contributors and reviewers who participated in its creation (complete list of individuals is available in Appendix 2). We thank everyone for his or her invaluable input and effort.
Disclosures
The Society of Hospital Medicine (SHM) provided administrative support for project coordination. SHM, or any of its representatives, had no role in the development of topic areas, refinement, or vetting of the topic list. No member of the Core Competencies Task Force or the SHM Education Committee received compensation for their participation in revising the Core Competencies. The authors report no conflicts of inte
In 2006, the Society of Hospital Medicine (SHM) first published The Core Competencies in Hospital Medicine: A Framework for Curricular Development (henceforth described as the Core Competencies) to help define the role and expectations of hospitalists.1,2 The Core Competencies provided a framework for evaluating clinical skills and professional expertise within a rapidly developing field and highlighted opportunities for growth. Since the initial development and publication of the Core Competencies, changes in the healthcare landscape and hospitalist practice environment have prompted this revision.
Over the past decade, the field of hospital medicine has experienced exponential growth. In 2005, just over 16,000 hospitalists were practicing in the United States. By 2015, that number had increased to an estimated 44,000 hospitalists, accounting for approximately 6% of the physician workforce.3 Hospitalists have expanded the scope of hospital medicine in many ways. In their roles, hospitalists lead and participate in hospital-based care models that emphasize interprofessional collaboration and a focus on the delivery of high-quality and cost-effective care across a variety of clinical domains (eg, the Choosing Wisely initiative).4 They are also engaged in patient safety and quality initiatives that are increasingly being used as benchmarks to rate hospitals and as factors for hospital payment (eg, Hospital Inpatient Value-Based Purchasing Program).5 In fact, the American Board of Internal Medicine (ABIM) created a Focused Practice in Hospital Medicine Maintenance of Certification program in response to the growing number of internists choosing to concentrate their practice in the hospital setting. This decision by the ABIM underscores the value that hospitalists bring to improving patient care in the hospital setting. The ABIM also recognizes the Core Competencies as a curricular framework for a focused practice in hospital medicine.6
Changes within the educational environment have demanded attentive and active participation by many hospitalists. For example, in 2012, the Accreditation Council for Graduate Medical Education (ACGME) introduced the Milestones Project, a new outcomes-based framework designed to more effectively assess learner performance across the 6 core competencies.7 These milestones assessments create intentional opportunities to guide the development of physicians during their training, including in the inpatient environments in which hospitalists practice. Where applicable, existing Core Competencies learning objectives were compared with external sources such as the individual ACGME performance milestones for this revision.
THE CORE COMPETENCIES
The Core Competencies focus on adult hospital medicine. The Pediatric Hospital Medicine Core Competencies are published separately.8 Importantly, the Core Competencies document is not intended to define an absolute set of clinical, procedural, or system-based topics described in textbooks or used by graduate medical education training programs. It does not define or limit the scope of the practice of hospital medicine. Rather, the Core Competencies serve as measurable learning objectives that encourage teaching faculty, practicing hospitalists, and administrators to develop individual skill sets and programs to improve patient care contextualized to the needs of an individual, care setting, or institution. To permit this flexibility, individual chapter-specific objectives are intentionally general in nature. Finally, the Core Competencies document is not a set of practice guidelines, nor does it offer any representation of a “standard of care.” Readers are encouraged to explore the article by McKean et al.9 to review examples of application of the Core Competencies and suggestions for curricular development.
The purpose of this article is to describe the criteria for inclusion of new chapters in the Core Competencies and the methodology of the review and revision process. It outlines the process of initial review and editing of the existing chapters; needs assessment for new topics; new chapter production; and the process of review and revision of individual chapters to create the complete document. The revised Core Competencies document is available online at http://www.journalofhospitalmedicine.com/jhospmed/issue/134981/journal-hospital-medicine-124-suppl-1.
REVIEW AND REVISION PROCESS
In 2012, the Society of Hospital Medicine (SHM) Education Committee created a Core Competencies Task Force (CCTF) in response to the SHM Board of Directors’ charge that it review and update the initial Core Competencies document. The CCTF comprised of 5 physician SHM Education Committee members and one SHM staff representative. CCTF membership included hospitalists with an interest and familiarity with the Core Competencies document. The SHM Education Committee nominated the CCTF chair, who determined the optimal size, qualifications, and composition of the task force with approval from the Committee. The CCTF communicated through frequent conference calls and via e-mail correspondence to conduct an initial review of the existing chapters and to perform a needs assessment for new topics.
Individual Chapter Review
The SHM Education Committee provided critical input and approved the chapter review process designed by the CCTF (Figure). The CCTF reviewed each chapter of the Core Competencies document to assess its continuing relevance to the field of hospital medicine with a standardized tool (Appendix 1). The process required that at least 2 CCTF members reviewed each chapter. Preliminary reviewers assessed the current relevance of each chapter, determined whether individual learning objectives required additional investigation or modification, and developed new learning objectives to fill any educational gaps. All CCTF members then discussed assimilated feedback from the initial CCTF review, using consensus decision making to determine chapter changes and modifications. The CCTF found each of the existing chapters to be relevant to the field and identified none for removal.
The CCTF rewrote all chapters. It then disseminated proposed chapter changes to a panel of diverse independent reviewers to solicit suggestions and comments to ensure a multidisciplinary and balanced review process. Independent reviewers included authors of the original Core Competencies chapters, invited content experts, and members of the SHM Education Committee. When appropriate, corresponding SHM Committees reviewed individual chapters for updates and revisions. For example, the SHM Hospital Quality and Patient Safety Committee reviewed the chapters on patient safety and quality improvement, and the SHM Practice Management Committee reviewed the chapter on management practices. Four CCTF section editors managed an independent portfolio of chapters. Each CCTF section editor assimilated the various draft versions, corresponded with individual reviewers when necessary, and compiled the changes into a subsequent draft. This process ensured that the final version of every chapter reflected the thoughtful input from all parties involved in the review. Throughout the process, the CCTF used consensus decision making to adjudicate chapter changes and modifications. The 2006 Core Competencies Editorial team also reviewed the revision and provided critical input. The SHM Education Committee and the SHM Board of Directors reviewed and approved the final version of the Core Competencies document.
Needs Assessment and Selection of New Core Competency Chapters
The CCTF issued a call for new topics to the members of the SHM Education Committee for inclusion in the Core Competencies. Topics were also identified from the following sources: the top 100 adult medical diagnoses at hospital discharge in the Healthcare Cost and Utilization Project database in 2010; topics in hospital medicine textbooks; curricula presented at the 3 most recent SHM annual meetings; and responses from SHM annual meeting surveys. Table 1 lists the topics considered for addition.
Members of the SHM Education Committee rated each of the potential topics considered for inclusion based on the following characteristics: relevance to the field of hospital medicine; intersection of the topic with medical subspecialties; and its appropriateness as a separate, stand-alone chapter. In addition, topics more frequently encountered by hospitalists, those deemed clinically important with a known risk of complications or management inconsistencies, and those with significant opportunities for quality improvement initiatives carried more weight. Syncope and hyponatremia were the only 2 clinical conditions identified that met all of the inclusion criteria. No additional topics met the criteria for new chapter development in the Procedures or Healthcare Systems sections. The SHM Education Committee identified the use of point-of-care ultrasonography as an important advancement in the field. Where appropriate, the individual procedure chapters now include a new competency-based objective highlighting its role. In addition, a separate SHM task force is working to develop a practice guideline for the use of point-of-care ultrasonography by hospitalists.
Contributors
The SHM Education Committee determined authorship for the new chapters (syncope and hyponatremia). It assigned 2 CCTF members with content expertise and familiarity with the Core Competencies to each author one chapter. Given the limited number of new chapters, it made a decision to develop the content internally rather than through an open-call for authorship nominations to practicing SHM members. The authors made an effort to maintain consistency with the educational theory used to develop the initial Core Competencies. Each of the new topics underwent rigorous review as previously described, including additional independent reviews by hospitalists with content expertise in these areas.
CHAPTER FORMAT AND CONTENT CHANGES
Following the same format as the earlier version, the 2017 Core Competencies revision contains 53 chapters, divided into 3 sections—Clinical Conditions, Procedures, and Healthcare Systems (Table 2) —all integral components of the practice of hospital medicine. The design allows individual chapters to stand alone. However, each chapter should be considered in the context of the entire document because a particular concept may be only briefly discussed in one chapter, but described in greater depth in another given the potential overlap across topics.
The chapters maintain the same content structure as the original version. Each chapter begins with an introductory paragraph followed by a list of competency-based objectives grouped in subsections according to the educational theory of learning domains: cognitive (knowledge), psychomotor (skills), and affective (attitudes).10 In addition, a subsection for System Organization and Improvement is included in the Clinical Conditions and Procedure chapters to emphasize the importance of interprofessional collaboration for optimal patient care. These subsections were not included in the Healthcare Systems chapters, as system organization and improvement is intrinsic to these subjects.
The introductory paragraph provides background information and describes how the chapter remains relevant to the current practice of hospital medicine. Individual competency-based objectives outline a relevant concept and expected level of proficiency as defined by Bloom’s taxonomy.10 New objectives reflect changes in the healthcare landscape over the past decade or further enhance each chapter’s concepts. Chapter authors made an effort to develop chapter and learning objective concepts that are consistent with external resources such as the ACGME Milestones Project and practice guideline objectives developed by a variety of professional organizations.
SUMMARY AND FUTURE DIRECTIONS
The Core Competencies document serves as a resource for hospitalists and hospital medicine programs to evaluate, develop, and improve individual and collective skills and the practice environment. The Core Competencies also provide a framework for medical school clerkship directors and residency and fellowship program directors, as well as course directors of Continuing Medical Education programs, to develop curricula to enhance educational experiences for trainees and hospital medicine providers. The updates in every chapter in this revision to the Core Competencies reflects the changes in the healthcare landscape and hospitalist practice environment over the past decade, and we encourage readers to revisit the entire compendium. Table 3 highlights some of the salient changes in this revision.
Hospital medicine continues to evolve as a specialty. The Core Competencies define hospitalists as agents of change and foster the development of a culture of safe and effective patient care within the hospital environment. Although the CCTF hopes that the Core Competencies will preserve their relevance over time, it recognizes the importance of their periodic reevaluation and adaptation. Additionally, SHM developed the Core Competencies primarily for physicians practicing as hospitalists. As the number of physician assistants and nurse practitioners engaged in the practice of hospital medicine increases, and hospital medicine expands into nontraditional specialties such as surgical comanagement, it may be necessary to consider the development of additional or separate Hospital Medicine Core Competencies tailored to the needs of these subsets of clinicians.
Acknowledgments
The authors and the CCTF are immensely grateful to Nick Marzano for project coordination and Abbie Young for her assistance with medical editing and chapter formatting. We extend our sincerest appreciation and gratitude to the index team of authors and editors whose efforts laid the foundation for this body of work. The initial development and this revision of the Core Competencies would not have been possible without the support and assistance of the SHM staff, the SHM Education Committee, and the scores of contributors and reviewers who participated in its creation (complete list of individuals is available in Appendix 2). We thank everyone for his or her invaluable input and effort.
Disclosures
The Society of Hospital Medicine (SHM) provided administrative support for project coordination. SHM, or any of its representatives, had no role in the development of topic areas, refinement, or vetting of the topic list. No member of the Core Competencies Task Force or the SHM Education Committee received compensation for their participation in revising the Core Competencies. The authors report no conflicts of inte
1. The core competencies in hospital medicine: a framework for curriculum development by the society of hospital medicine. J Hosp Med. 2006;1 Suppl 1:2-95.
2. Dressler DD, Pistoria MJ, Budnitz TL, McKean SCW, Amin AN. Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1(1):48-56.
3. Hospital Medicine News, Society of Hospital Medicine. http://www.hospitalmedicine.org/press. Accessed June 16, 2016.
4. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492.
5. Conway PH. Value-driven health care: implications for hospitals and hospitalists. J Hosp Med. 2009;4(8):507-511.
6. American Board of Internal Medicine. Questions and Answers Regarding ABIM’s Maintenance of Certification in Internal Medicine with a Focused Practice in Hospital Medicine Program. 2009. http://www.abim.org/news/focused-practice-hospital-medicine-questions-answers.aspx. Accessed November 11, 2016.
7. The Internal Medicine Milestone Project. http://www.acgme.org/acgmeweb/portals/0/pdfs/milestones/internalmedicinemilestones.pdf. Accessed February 29, 2016.
8. Stucky ER, Ottolini MC, Maniscalco J. Pediatric hospital medicine core competencies: development and methodology. J Hosp Med. 2010;5(6):339-343.
9. McKean SC, Budnitz TL, Dressler DD, Amin AN, Pistoria MJ. How to use the core competencies in hospital medicine: a framework for curriculum development. J Hosp Med. 2006;1 Suppl 1:57-67.
10. Anderson LW, Krathwohl DR (eds). A Taxonomy for Learning, Teaching and Assessing: A Revision of Bloom’s Taxonomy of Educational Outcomes. Complete edition. New York, NY: Longman; 2001.
1. The core competencies in hospital medicine: a framework for curriculum development by the society of hospital medicine. J Hosp Med. 2006;1 Suppl 1:2-95.
2. Dressler DD, Pistoria MJ, Budnitz TL, McKean SCW, Amin AN. Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1(1):48-56.
3. Hospital Medicine News, Society of Hospital Medicine. http://www.hospitalmedicine.org/press. Accessed June 16, 2016.
4. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492.
5. Conway PH. Value-driven health care: implications for hospitals and hospitalists. J Hosp Med. 2009;4(8):507-511.
6. American Board of Internal Medicine. Questions and Answers Regarding ABIM’s Maintenance of Certification in Internal Medicine with a Focused Practice in Hospital Medicine Program. 2009. http://www.abim.org/news/focused-practice-hospital-medicine-questions-answers.aspx. Accessed November 11, 2016.
7. The Internal Medicine Milestone Project. http://www.acgme.org/acgmeweb/portals/0/pdfs/milestones/internalmedicinemilestones.pdf. Accessed February 29, 2016.
8. Stucky ER, Ottolini MC, Maniscalco J. Pediatric hospital medicine core competencies: development and methodology. J Hosp Med. 2010;5(6):339-343.
9. McKean SC, Budnitz TL, Dressler DD, Amin AN, Pistoria MJ. How to use the core competencies in hospital medicine: a framework for curriculum development. J Hosp Med. 2006;1 Suppl 1:57-67.
10. Anderson LW, Krathwohl DR (eds). A Taxonomy for Learning, Teaching and Assessing: A Revision of Bloom’s Taxonomy of Educational Outcomes. Complete edition. New York, NY: Longman; 2001.
© 2017 Society of Hospital Medicine
Reducing the Risk of PML During MS Treatment
Regular screening for PML is recommended for patients treated with natalizumab.
NASHVILLE—For patients with multiple sclerosis (MS) who receive treatment with natalizumab, progressive multifocal leukoencephalopathy (PML) can be avoided, according to research presented at the 2018 CMSC Annual Meeting. “You can actually prevent this disease from occurring because we have risk-limiting strategies in many circumstances,” said Joseph R. Berger, MD, Professor of Neurology at the Hospital of the University of Pennsylvania in Philadelphia.
Long-Term Use of Natalizumab Is Associated With Increased PML Risk
Unlike other conditions such as HIV, MS itself is not linked to a higher risk of PML, said Dr. Berger. Instead, it is the medications that introduce the risk, he said, with at least three, and possibly four, drugs posing a risk to patients.
“We know that the risk with natalizumab is incredibly high in the context of John Cunningham virus [JCV] antibody positivity and prolonged therapy,” Dr. Berger said. “You can safely give natalizumab for a short period of time when treating patients with aggressive MS. I will frequently employ that strategy even in the context of JCV antibody positivity.” There is no risk of PML when natalizumab is used for under eight months, Dr. Berger said.“If you leave people on the drug indefinitely, there is a substantial risk of developing PML,” said Dr. Berger. “Individuals who have been on the drug for two years, who have seen prior immunosuppressant therapy, who are JCV antibody positive—that group of individuals develops PML at rates of one in 50 to one in 100.” These levels are “even higher than those in the HIV population before the rise of antiretroviral medications,” he added.
As of November 30, 2017, 177,800 patients have received natalizumab in the postmarketing setting, and 756 cases of PML have been reported as of December 7, 2017. All but three of those cases were in patients with MS, and the overall incidence was 4.19 out of 1,000.
Dr. Berger recommended regular screening MRIs for PML in patients taking natalizumab and advised physicians to be on alert for the appearance of new neurologic symptoms or a new or increasing JCV antibody index.
Drugs With Low and Unknown Risks
Two other MS drugs, fingolimod and dimethyl fumarate, entail a low risk of PML. The numbers of PML cases reported for these drugs are 19 and five, respectively, as of February 2018, said Dr. Berger. Two of the patients receiving fingolimod who developed PML had had earlier exposure to natalizumab.
For JC antibody positive patients receiving dimethyl fumarate, the risk of PML may be eliminated when the treatment is discontinued and their lymphocyte count decreases to a level below 500 per µL Dr. Berger said.
“Unfortunately for fingolimod, we do not have a defined risk-mitigation strategy,” he said. However, researchers have noticed that PML has occurred more often in older people on fingolimod, possibly because of the aging of the immune system.
Alemtuzumab, ocrelizumab (with rituximab as proxy), and teriflunomide (with leflunomide as proxy), entail an unknown risk of PML, according to Dr. Berger. There have been three cases of PML associated with ocrelizumab and one associated with teriflunomide, but all were carry-overs from natalizumab or fingolimod exposure or occurred after natalizumab exposure.
What should a physician do if a patient develops PML? Stopping the drug and restoring the immune system are crucial steps, Dr. Berger said. Although evidence indicates that plasma exchange clears natalizumab, “there is no study that demonstrates that it is in the patient’s best interest,” said Dr. Berger. A retrospective study found no improvement in morbidity or mortality after plasma exchange.
Multiple strategies to treat PML, including immunizations and inhibitors of DNA replication, have failed to make an impact so far, said Dr. Berger. These strategies did not show benefits in clinical trials, and evidence does not support them.
—Randy Dotinga
Suggested Reading
Berger JR. Classifying PML risk with disease modifying therapies. Mult Scler Relat Disord. 2017;12:59-63.
Khatri BO, Man S, Giovannoni G, et al. Effect of plasma exchange in accelerating natalizumab clearance and restoring leukocyte function. Neurology. 2009;72(5):402-409.
Landi D, De Rossi N, Zagaglia S, et al. No evidence of beneficial effects of plasmapheresis in natalizumab-associated PML. Neurology. 2017;88(12)
Regular screening for PML is recommended for patients treated with natalizumab.
Regular screening for PML is recommended for patients treated with natalizumab.
NASHVILLE—For patients with multiple sclerosis (MS) who receive treatment with natalizumab, progressive multifocal leukoencephalopathy (PML) can be avoided, according to research presented at the 2018 CMSC Annual Meeting. “You can actually prevent this disease from occurring because we have risk-limiting strategies in many circumstances,” said Joseph R. Berger, MD, Professor of Neurology at the Hospital of the University of Pennsylvania in Philadelphia.
Long-Term Use of Natalizumab Is Associated With Increased PML Risk
Unlike other conditions such as HIV, MS itself is not linked to a higher risk of PML, said Dr. Berger. Instead, it is the medications that introduce the risk, he said, with at least three, and possibly four, drugs posing a risk to patients.
“We know that the risk with natalizumab is incredibly high in the context of John Cunningham virus [JCV] antibody positivity and prolonged therapy,” Dr. Berger said. “You can safely give natalizumab for a short period of time when treating patients with aggressive MS. I will frequently employ that strategy even in the context of JCV antibody positivity.” There is no risk of PML when natalizumab is used for under eight months, Dr. Berger said.“If you leave people on the drug indefinitely, there is a substantial risk of developing PML,” said Dr. Berger. “Individuals who have been on the drug for two years, who have seen prior immunosuppressant therapy, who are JCV antibody positive—that group of individuals develops PML at rates of one in 50 to one in 100.” These levels are “even higher than those in the HIV population before the rise of antiretroviral medications,” he added.
As of November 30, 2017, 177,800 patients have received natalizumab in the postmarketing setting, and 756 cases of PML have been reported as of December 7, 2017. All but three of those cases were in patients with MS, and the overall incidence was 4.19 out of 1,000.
Dr. Berger recommended regular screening MRIs for PML in patients taking natalizumab and advised physicians to be on alert for the appearance of new neurologic symptoms or a new or increasing JCV antibody index.
Drugs With Low and Unknown Risks
Two other MS drugs, fingolimod and dimethyl fumarate, entail a low risk of PML. The numbers of PML cases reported for these drugs are 19 and five, respectively, as of February 2018, said Dr. Berger. Two of the patients receiving fingolimod who developed PML had had earlier exposure to natalizumab.
For JC antibody positive patients receiving dimethyl fumarate, the risk of PML may be eliminated when the treatment is discontinued and their lymphocyte count decreases to a level below 500 per µL Dr. Berger said.
“Unfortunately for fingolimod, we do not have a defined risk-mitigation strategy,” he said. However, researchers have noticed that PML has occurred more often in older people on fingolimod, possibly because of the aging of the immune system.
Alemtuzumab, ocrelizumab (with rituximab as proxy), and teriflunomide (with leflunomide as proxy), entail an unknown risk of PML, according to Dr. Berger. There have been three cases of PML associated with ocrelizumab and one associated with teriflunomide, but all were carry-overs from natalizumab or fingolimod exposure or occurred after natalizumab exposure.
What should a physician do if a patient develops PML? Stopping the drug and restoring the immune system are crucial steps, Dr. Berger said. Although evidence indicates that plasma exchange clears natalizumab, “there is no study that demonstrates that it is in the patient’s best interest,” said Dr. Berger. A retrospective study found no improvement in morbidity or mortality after plasma exchange.
Multiple strategies to treat PML, including immunizations and inhibitors of DNA replication, have failed to make an impact so far, said Dr. Berger. These strategies did not show benefits in clinical trials, and evidence does not support them.
—Randy Dotinga
Suggested Reading
Berger JR. Classifying PML risk with disease modifying therapies. Mult Scler Relat Disord. 2017;12:59-63.
Khatri BO, Man S, Giovannoni G, et al. Effect of plasma exchange in accelerating natalizumab clearance and restoring leukocyte function. Neurology. 2009;72(5):402-409.
Landi D, De Rossi N, Zagaglia S, et al. No evidence of beneficial effects of plasmapheresis in natalizumab-associated PML. Neurology. 2017;88(12)
NASHVILLE—For patients with multiple sclerosis (MS) who receive treatment with natalizumab, progressive multifocal leukoencephalopathy (PML) can be avoided, according to research presented at the 2018 CMSC Annual Meeting. “You can actually prevent this disease from occurring because we have risk-limiting strategies in many circumstances,” said Joseph R. Berger, MD, Professor of Neurology at the Hospital of the University of Pennsylvania in Philadelphia.
Long-Term Use of Natalizumab Is Associated With Increased PML Risk
Unlike other conditions such as HIV, MS itself is not linked to a higher risk of PML, said Dr. Berger. Instead, it is the medications that introduce the risk, he said, with at least three, and possibly four, drugs posing a risk to patients.
“We know that the risk with natalizumab is incredibly high in the context of John Cunningham virus [JCV] antibody positivity and prolonged therapy,” Dr. Berger said. “You can safely give natalizumab for a short period of time when treating patients with aggressive MS. I will frequently employ that strategy even in the context of JCV antibody positivity.” There is no risk of PML when natalizumab is used for under eight months, Dr. Berger said.“If you leave people on the drug indefinitely, there is a substantial risk of developing PML,” said Dr. Berger. “Individuals who have been on the drug for two years, who have seen prior immunosuppressant therapy, who are JCV antibody positive—that group of individuals develops PML at rates of one in 50 to one in 100.” These levels are “even higher than those in the HIV population before the rise of antiretroviral medications,” he added.
As of November 30, 2017, 177,800 patients have received natalizumab in the postmarketing setting, and 756 cases of PML have been reported as of December 7, 2017. All but three of those cases were in patients with MS, and the overall incidence was 4.19 out of 1,000.
Dr. Berger recommended regular screening MRIs for PML in patients taking natalizumab and advised physicians to be on alert for the appearance of new neurologic symptoms or a new or increasing JCV antibody index.
Drugs With Low and Unknown Risks
Two other MS drugs, fingolimod and dimethyl fumarate, entail a low risk of PML. The numbers of PML cases reported for these drugs are 19 and five, respectively, as of February 2018, said Dr. Berger. Two of the patients receiving fingolimod who developed PML had had earlier exposure to natalizumab.
For JC antibody positive patients receiving dimethyl fumarate, the risk of PML may be eliminated when the treatment is discontinued and their lymphocyte count decreases to a level below 500 per µL Dr. Berger said.
“Unfortunately for fingolimod, we do not have a defined risk-mitigation strategy,” he said. However, researchers have noticed that PML has occurred more often in older people on fingolimod, possibly because of the aging of the immune system.
Alemtuzumab, ocrelizumab (with rituximab as proxy), and teriflunomide (with leflunomide as proxy), entail an unknown risk of PML, according to Dr. Berger. There have been three cases of PML associated with ocrelizumab and one associated with teriflunomide, but all were carry-overs from natalizumab or fingolimod exposure or occurred after natalizumab exposure.
What should a physician do if a patient develops PML? Stopping the drug and restoring the immune system are crucial steps, Dr. Berger said. Although evidence indicates that plasma exchange clears natalizumab, “there is no study that demonstrates that it is in the patient’s best interest,” said Dr. Berger. A retrospective study found no improvement in morbidity or mortality after plasma exchange.
Multiple strategies to treat PML, including immunizations and inhibitors of DNA replication, have failed to make an impact so far, said Dr. Berger. These strategies did not show benefits in clinical trials, and evidence does not support them.
—Randy Dotinga
Suggested Reading
Berger JR. Classifying PML risk with disease modifying therapies. Mult Scler Relat Disord. 2017;12:59-63.
Khatri BO, Man S, Giovannoni G, et al. Effect of plasma exchange in accelerating natalizumab clearance and restoring leukocyte function. Neurology. 2009;72(5):402-409.
Landi D, De Rossi N, Zagaglia S, et al. No evidence of beneficial effects of plasmapheresis in natalizumab-associated PML. Neurology. 2017;88(12)
Patisiran May Reduce Neuropathy in Patients With hATTR Amyloidosis
The RNA interference therapy suppresses transthyretin production and reduces neuropathy impairment, compared with placebo.
LOS ANGELES—Patisiran, an investigational RNA interference therapy that suppresses the production of transthyretin (TTR) protein, significantly improves polyneuropathy in patients with hereditary TTR-mediated (hATTR) amyloidosis, according to phase III trial results described at the 70th Annual Meeting of the American Academy of Neurology.
After 18 months of treatment with patisiran, patients’ scores on a measure of neuropathy impairment had improved from baseline, whereas scores progressively worsened among patients who received placebo.
“The results are amazing,” said principal investigator David Adams, MD, PhD, Head of the Department of Neurology at Centre Hospitalier Universitaire Bicêtre in Paris. “The hope is to stop the progression of the disease and eventually to reverse it.”
Patients Often Present With Polyneuropathy
Formerly known as familial amyloidotic polyneuropathy, hATTR amyloidosis is a rare, multisystemic, progressive, life-threatening disease caused by mutations in the TTR gene. The mutations may cause misfolded TTR protein to accumulate as amyloid fibrils in the nerves, heart, and gastrointestinal tract. There are more than 120 known TTR mutations, and people with the most common mutation, Val30Met, often present with polyneuropathy. Patients with hATTR amyloidosis also may present with cardiomyopathy or a mixed phenotype. The median age of disease onset is 39.
In addition, hATTR amyloidosis may cause CNS symptoms (eg, progressive dementia, headache, ataxia, and seizures), autonomic neuropathy (eg, orthostatic hypotension, urinary retention, and sexual dysfunction), and peripheral sensorimotor neuropathy (eg, neuropathic pain, altered sensitivity, muscle weakness, and impaired balance). Current treatment options include liver transplantation.
Researchers are studying whether small interfering RNAs that bind to TTR messenger RNA and prevent production of TTR protein may benefit patients with hATTR amyloidosis. Alnylam Pharmaceuticals, based in Cambridge, Massachusetts, is developing patisiran, a lipid nanoparticle formulation of small interfering RNA designed to knock down the production of mutant and wild-type TTR protein in the liver. Phase I and II trials found that patisiran was generally well tolerated and resulted in dose-dependent suppression of TTR production.
The APOLLO Trial
To evaluate the efficacy and safety of patisiran in patients with hATTR amyloidosis with polyneuropathy, Dr. Adams and colleagues conducted the phase III, randomized, double-blind, placebo-controlled APOLLO study. Eligible patients were between ages 18 and 85 with hATTR amyloidosis, investigator-estimated survival of at least two years, a Neuropathy Impairment Score (NIS) of between 5 and 130, and a Polyneuropathy Disability score of IIIb or less. The investigators randomized patients 2:1 to receive IV patisiran 0.3 mg/kg or placebo every three weeks. To reduce the likelihood of infusion-related reactions, patients received premedication with dexamethasone, oral acetaminophen, an H2 blocker, and an H1 blocker at least 60 minutes before each study drug infusion.
The primary end point was change from baseline on the modified NIS+7, a composite measure of motor strength, sensation, reflexes, nerve conduction, and autonomic function, at 18 months. Secondary end points included the effect of patisiran on Norfolk Quality of Life–Diabetic Neuropathy score, nutritional status (as evaluated by modified BMI), motor function (as measured by NIS-weakness and the timed 10-meter walk test), and autonomic symptoms (as measured by the Composite Autonomic Symptom Score-31 [COMPASS-31]). Exploratory measures include assessment of cardiac function and pathologic evaluation to assess nerve fiber innervation and amyloid burden.
Assessing Change From Baseline
The researchers enrolled 225 patients from 44 sites in 19 countries between December 2013 and January 2016. Patients’ mean age was 61, and patients had been diagnosed with hATTR amyloidosis for an average of about 2.5 years. In all, 148 patients were randomized to receive patisiran, and 77 were randomized to receive placebo. Study completion rates were 71.4% in the placebo group and 93.2% in the patisiran group.
At 18 months, mean serum TTR knockdown from baseline was 84.3% among patients who received patisiran, compared with 4.8% among patients who received placebo.
At nine months, least squares mean change from baseline in modified NIS+7 was –2.04 points in the patisiran group, versus 13.95 points in the placebo group.
At 18 months, least squares mean change from baseline in modified NIS+7 was –6.03 points among patients who received patisiran, compared with 27.96 points among patients who received placebo. The difference between groups was statistically significant. The proportion of patients with improvement in modified NIS+7 was 56.1% in the patisiran group and 3.9% in the placebo group (odds ratio, 39.9).
“Regardless of the state of the disease and the severity of the NIS at baseline, you have the same effect,” Dr. Adams said.
The positive treatment effect also was observed in patients with various TTR genotypes and in patients with cardiac involvement.
All secondary end points favored treatment with patisiran, indicating “significant improvement in quality of life, reduction in disease symptoms and disability, and improvement in nutritional status, strength, and ambulation seen with patisiran, relative to placebo,” Dr. Adams said. In the subpopulation with cardiac involvement, patisiran significantly improved cardiac end points such as mean left ventricular wall thickness and global longitudinal strain.
In a post hoc analysis, patisiran resulted in a 50% reduction in a composite rate of all-cause hospitalization and mortality and an approximately 45% reduction in a composite rate of cardiac hospitalization and all-cause mortality.
Therapy Is Under FDA Review
There were 13 deaths in the trial. None of the deaths were due to the study drug, and the rate of deaths was higher in the placebo group than in the patisiran group (7.8% vs 4.7%). The majority of adverse events were mild or moderate and included peripheral edema (29.7% of patients in the patisiran group vs 22.1% of patients in the placebo group) and infusion-related reactions (18.9% of patients in the patisiran group vs 9.1% of patients in the placebo group). Infusion-related reaction led one patient to discontinue the trial. There were no severe, life-threatening, or serious infusion-related reactions.
Adverse events that were reported more often in the placebo group than in the patisiran group included fall (28.6% vs 16.9%), urinary tract infection (18.2% vs 12.8%), nausea (20.8% vs 14.9%), muscular weakness (14.3% vs 3.4%), anemia (10.4% vs 2%), and syncope (10.4% vs 2%).
The researchers observed no safety signals regarding cataracts, hyperglycemia, infection, osteopenia or osteoporosis, liver function tests, hematology, or renal dysfunction related to patisiran. Safety in the cardiac subpopulation was comparable to that in the overall study population.
Patients who completed the APOLLO study were eligible for patisiran treatment in an open-label extension study, and 99% of eligible patients enrolled in this extension. Patisiran is under review by the FDA as a breakthrough therapy for hATTR amyloidosis. The FDA plans to complete its review by August 11, 2018.
—Jake Remaly
Suggested Reading
Adams D, Suhr OB, Dyck PJ, et al. Trial design and rationale for APOLLO, a phase 3, placebo-controlled study of patisiran in patients with hereditary ATTR amyloidosis with polyneuropathy. BMC Neurol. 2017;17(1):181.
Coelho T, Adams D, Silva A, et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369(9):819-829.
Conceição I, González-Duarte A, Obici L, et al. “Red-flag” symptom clusters in transthyretin familial amyloid polyneuropathy. J Peripher Nerv Syst. 2016;21(1):5-9.
Suhr OB, Coelho T, Buades J, et al. Efficacy and safety of patisiran for familial amyloidotic polyneuropathy: a phase II multi-dose study. Orphanet J Rare Dis. 2015;10:109.
The RNA interference therapy suppresses transthyretin production and reduces neuropathy impairment, compared with placebo.
The RNA interference therapy suppresses transthyretin production and reduces neuropathy impairment, compared with placebo.
LOS ANGELES—Patisiran, an investigational RNA interference therapy that suppresses the production of transthyretin (TTR) protein, significantly improves polyneuropathy in patients with hereditary TTR-mediated (hATTR) amyloidosis, according to phase III trial results described at the 70th Annual Meeting of the American Academy of Neurology.
After 18 months of treatment with patisiran, patients’ scores on a measure of neuropathy impairment had improved from baseline, whereas scores progressively worsened among patients who received placebo.
“The results are amazing,” said principal investigator David Adams, MD, PhD, Head of the Department of Neurology at Centre Hospitalier Universitaire Bicêtre in Paris. “The hope is to stop the progression of the disease and eventually to reverse it.”
Patients Often Present With Polyneuropathy
Formerly known as familial amyloidotic polyneuropathy, hATTR amyloidosis is a rare, multisystemic, progressive, life-threatening disease caused by mutations in the TTR gene. The mutations may cause misfolded TTR protein to accumulate as amyloid fibrils in the nerves, heart, and gastrointestinal tract. There are more than 120 known TTR mutations, and people with the most common mutation, Val30Met, often present with polyneuropathy. Patients with hATTR amyloidosis also may present with cardiomyopathy or a mixed phenotype. The median age of disease onset is 39.
In addition, hATTR amyloidosis may cause CNS symptoms (eg, progressive dementia, headache, ataxia, and seizures), autonomic neuropathy (eg, orthostatic hypotension, urinary retention, and sexual dysfunction), and peripheral sensorimotor neuropathy (eg, neuropathic pain, altered sensitivity, muscle weakness, and impaired balance). Current treatment options include liver transplantation.
Researchers are studying whether small interfering RNAs that bind to TTR messenger RNA and prevent production of TTR protein may benefit patients with hATTR amyloidosis. Alnylam Pharmaceuticals, based in Cambridge, Massachusetts, is developing patisiran, a lipid nanoparticle formulation of small interfering RNA designed to knock down the production of mutant and wild-type TTR protein in the liver. Phase I and II trials found that patisiran was generally well tolerated and resulted in dose-dependent suppression of TTR production.
The APOLLO Trial
To evaluate the efficacy and safety of patisiran in patients with hATTR amyloidosis with polyneuropathy, Dr. Adams and colleagues conducted the phase III, randomized, double-blind, placebo-controlled APOLLO study. Eligible patients were between ages 18 and 85 with hATTR amyloidosis, investigator-estimated survival of at least two years, a Neuropathy Impairment Score (NIS) of between 5 and 130, and a Polyneuropathy Disability score of IIIb or less. The investigators randomized patients 2:1 to receive IV patisiran 0.3 mg/kg or placebo every three weeks. To reduce the likelihood of infusion-related reactions, patients received premedication with dexamethasone, oral acetaminophen, an H2 blocker, and an H1 blocker at least 60 minutes before each study drug infusion.
The primary end point was change from baseline on the modified NIS+7, a composite measure of motor strength, sensation, reflexes, nerve conduction, and autonomic function, at 18 months. Secondary end points included the effect of patisiran on Norfolk Quality of Life–Diabetic Neuropathy score, nutritional status (as evaluated by modified BMI), motor function (as measured by NIS-weakness and the timed 10-meter walk test), and autonomic symptoms (as measured by the Composite Autonomic Symptom Score-31 [COMPASS-31]). Exploratory measures include assessment of cardiac function and pathologic evaluation to assess nerve fiber innervation and amyloid burden.
Assessing Change From Baseline
The researchers enrolled 225 patients from 44 sites in 19 countries between December 2013 and January 2016. Patients’ mean age was 61, and patients had been diagnosed with hATTR amyloidosis for an average of about 2.5 years. In all, 148 patients were randomized to receive patisiran, and 77 were randomized to receive placebo. Study completion rates were 71.4% in the placebo group and 93.2% in the patisiran group.
At 18 months, mean serum TTR knockdown from baseline was 84.3% among patients who received patisiran, compared with 4.8% among patients who received placebo.
At nine months, least squares mean change from baseline in modified NIS+7 was –2.04 points in the patisiran group, versus 13.95 points in the placebo group.
At 18 months, least squares mean change from baseline in modified NIS+7 was –6.03 points among patients who received patisiran, compared with 27.96 points among patients who received placebo. The difference between groups was statistically significant. The proportion of patients with improvement in modified NIS+7 was 56.1% in the patisiran group and 3.9% in the placebo group (odds ratio, 39.9).
“Regardless of the state of the disease and the severity of the NIS at baseline, you have the same effect,” Dr. Adams said.
The positive treatment effect also was observed in patients with various TTR genotypes and in patients with cardiac involvement.
All secondary end points favored treatment with patisiran, indicating “significant improvement in quality of life, reduction in disease symptoms and disability, and improvement in nutritional status, strength, and ambulation seen with patisiran, relative to placebo,” Dr. Adams said. In the subpopulation with cardiac involvement, patisiran significantly improved cardiac end points such as mean left ventricular wall thickness and global longitudinal strain.
In a post hoc analysis, patisiran resulted in a 50% reduction in a composite rate of all-cause hospitalization and mortality and an approximately 45% reduction in a composite rate of cardiac hospitalization and all-cause mortality.
Therapy Is Under FDA Review
There were 13 deaths in the trial. None of the deaths were due to the study drug, and the rate of deaths was higher in the placebo group than in the patisiran group (7.8% vs 4.7%). The majority of adverse events were mild or moderate and included peripheral edema (29.7% of patients in the patisiran group vs 22.1% of patients in the placebo group) and infusion-related reactions (18.9% of patients in the patisiran group vs 9.1% of patients in the placebo group). Infusion-related reaction led one patient to discontinue the trial. There were no severe, life-threatening, or serious infusion-related reactions.
Adverse events that were reported more often in the placebo group than in the patisiran group included fall (28.6% vs 16.9%), urinary tract infection (18.2% vs 12.8%), nausea (20.8% vs 14.9%), muscular weakness (14.3% vs 3.4%), anemia (10.4% vs 2%), and syncope (10.4% vs 2%).
The researchers observed no safety signals regarding cataracts, hyperglycemia, infection, osteopenia or osteoporosis, liver function tests, hematology, or renal dysfunction related to patisiran. Safety in the cardiac subpopulation was comparable to that in the overall study population.
Patients who completed the APOLLO study were eligible for patisiran treatment in an open-label extension study, and 99% of eligible patients enrolled in this extension. Patisiran is under review by the FDA as a breakthrough therapy for hATTR amyloidosis. The FDA plans to complete its review by August 11, 2018.
—Jake Remaly
Suggested Reading
Adams D, Suhr OB, Dyck PJ, et al. Trial design and rationale for APOLLO, a phase 3, placebo-controlled study of patisiran in patients with hereditary ATTR amyloidosis with polyneuropathy. BMC Neurol. 2017;17(1):181.
Coelho T, Adams D, Silva A, et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369(9):819-829.
Conceição I, González-Duarte A, Obici L, et al. “Red-flag” symptom clusters in transthyretin familial amyloid polyneuropathy. J Peripher Nerv Syst. 2016;21(1):5-9.
Suhr OB, Coelho T, Buades J, et al. Efficacy and safety of patisiran for familial amyloidotic polyneuropathy: a phase II multi-dose study. Orphanet J Rare Dis. 2015;10:109.
LOS ANGELES—Patisiran, an investigational RNA interference therapy that suppresses the production of transthyretin (TTR) protein, significantly improves polyneuropathy in patients with hereditary TTR-mediated (hATTR) amyloidosis, according to phase III trial results described at the 70th Annual Meeting of the American Academy of Neurology.
After 18 months of treatment with patisiran, patients’ scores on a measure of neuropathy impairment had improved from baseline, whereas scores progressively worsened among patients who received placebo.
“The results are amazing,” said principal investigator David Adams, MD, PhD, Head of the Department of Neurology at Centre Hospitalier Universitaire Bicêtre in Paris. “The hope is to stop the progression of the disease and eventually to reverse it.”
Patients Often Present With Polyneuropathy
Formerly known as familial amyloidotic polyneuropathy, hATTR amyloidosis is a rare, multisystemic, progressive, life-threatening disease caused by mutations in the TTR gene. The mutations may cause misfolded TTR protein to accumulate as amyloid fibrils in the nerves, heart, and gastrointestinal tract. There are more than 120 known TTR mutations, and people with the most common mutation, Val30Met, often present with polyneuropathy. Patients with hATTR amyloidosis also may present with cardiomyopathy or a mixed phenotype. The median age of disease onset is 39.
In addition, hATTR amyloidosis may cause CNS symptoms (eg, progressive dementia, headache, ataxia, and seizures), autonomic neuropathy (eg, orthostatic hypotension, urinary retention, and sexual dysfunction), and peripheral sensorimotor neuropathy (eg, neuropathic pain, altered sensitivity, muscle weakness, and impaired balance). Current treatment options include liver transplantation.
Researchers are studying whether small interfering RNAs that bind to TTR messenger RNA and prevent production of TTR protein may benefit patients with hATTR amyloidosis. Alnylam Pharmaceuticals, based in Cambridge, Massachusetts, is developing patisiran, a lipid nanoparticle formulation of small interfering RNA designed to knock down the production of mutant and wild-type TTR protein in the liver. Phase I and II trials found that patisiran was generally well tolerated and resulted in dose-dependent suppression of TTR production.
The APOLLO Trial
To evaluate the efficacy and safety of patisiran in patients with hATTR amyloidosis with polyneuropathy, Dr. Adams and colleagues conducted the phase III, randomized, double-blind, placebo-controlled APOLLO study. Eligible patients were between ages 18 and 85 with hATTR amyloidosis, investigator-estimated survival of at least two years, a Neuropathy Impairment Score (NIS) of between 5 and 130, and a Polyneuropathy Disability score of IIIb or less. The investigators randomized patients 2:1 to receive IV patisiran 0.3 mg/kg or placebo every three weeks. To reduce the likelihood of infusion-related reactions, patients received premedication with dexamethasone, oral acetaminophen, an H2 blocker, and an H1 blocker at least 60 minutes before each study drug infusion.
The primary end point was change from baseline on the modified NIS+7, a composite measure of motor strength, sensation, reflexes, nerve conduction, and autonomic function, at 18 months. Secondary end points included the effect of patisiran on Norfolk Quality of Life–Diabetic Neuropathy score, nutritional status (as evaluated by modified BMI), motor function (as measured by NIS-weakness and the timed 10-meter walk test), and autonomic symptoms (as measured by the Composite Autonomic Symptom Score-31 [COMPASS-31]). Exploratory measures include assessment of cardiac function and pathologic evaluation to assess nerve fiber innervation and amyloid burden.
Assessing Change From Baseline
The researchers enrolled 225 patients from 44 sites in 19 countries between December 2013 and January 2016. Patients’ mean age was 61, and patients had been diagnosed with hATTR amyloidosis for an average of about 2.5 years. In all, 148 patients were randomized to receive patisiran, and 77 were randomized to receive placebo. Study completion rates were 71.4% in the placebo group and 93.2% in the patisiran group.
At 18 months, mean serum TTR knockdown from baseline was 84.3% among patients who received patisiran, compared with 4.8% among patients who received placebo.
At nine months, least squares mean change from baseline in modified NIS+7 was –2.04 points in the patisiran group, versus 13.95 points in the placebo group.
At 18 months, least squares mean change from baseline in modified NIS+7 was –6.03 points among patients who received patisiran, compared with 27.96 points among patients who received placebo. The difference between groups was statistically significant. The proportion of patients with improvement in modified NIS+7 was 56.1% in the patisiran group and 3.9% in the placebo group (odds ratio, 39.9).
“Regardless of the state of the disease and the severity of the NIS at baseline, you have the same effect,” Dr. Adams said.
The positive treatment effect also was observed in patients with various TTR genotypes and in patients with cardiac involvement.
All secondary end points favored treatment with patisiran, indicating “significant improvement in quality of life, reduction in disease symptoms and disability, and improvement in nutritional status, strength, and ambulation seen with patisiran, relative to placebo,” Dr. Adams said. In the subpopulation with cardiac involvement, patisiran significantly improved cardiac end points such as mean left ventricular wall thickness and global longitudinal strain.
In a post hoc analysis, patisiran resulted in a 50% reduction in a composite rate of all-cause hospitalization and mortality and an approximately 45% reduction in a composite rate of cardiac hospitalization and all-cause mortality.
Therapy Is Under FDA Review
There were 13 deaths in the trial. None of the deaths were due to the study drug, and the rate of deaths was higher in the placebo group than in the patisiran group (7.8% vs 4.7%). The majority of adverse events were mild or moderate and included peripheral edema (29.7% of patients in the patisiran group vs 22.1% of patients in the placebo group) and infusion-related reactions (18.9% of patients in the patisiran group vs 9.1% of patients in the placebo group). Infusion-related reaction led one patient to discontinue the trial. There were no severe, life-threatening, or serious infusion-related reactions.
Adverse events that were reported more often in the placebo group than in the patisiran group included fall (28.6% vs 16.9%), urinary tract infection (18.2% vs 12.8%), nausea (20.8% vs 14.9%), muscular weakness (14.3% vs 3.4%), anemia (10.4% vs 2%), and syncope (10.4% vs 2%).
The researchers observed no safety signals regarding cataracts, hyperglycemia, infection, osteopenia or osteoporosis, liver function tests, hematology, or renal dysfunction related to patisiran. Safety in the cardiac subpopulation was comparable to that in the overall study population.
Patients who completed the APOLLO study were eligible for patisiran treatment in an open-label extension study, and 99% of eligible patients enrolled in this extension. Patisiran is under review by the FDA as a breakthrough therapy for hATTR amyloidosis. The FDA plans to complete its review by August 11, 2018.
—Jake Remaly
Suggested Reading
Adams D, Suhr OB, Dyck PJ, et al. Trial design and rationale for APOLLO, a phase 3, placebo-controlled study of patisiran in patients with hereditary ATTR amyloidosis with polyneuropathy. BMC Neurol. 2017;17(1):181.
Coelho T, Adams D, Silva A, et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369(9):819-829.
Conceição I, González-Duarte A, Obici L, et al. “Red-flag” symptom clusters in transthyretin familial amyloid polyneuropathy. J Peripher Nerv Syst. 2016;21(1):5-9.
Suhr OB, Coelho T, Buades J, et al. Efficacy and safety of patisiran for familial amyloidotic polyneuropathy: a phase II multi-dose study. Orphanet J Rare Dis. 2015;10:109.