User login
New Survey on Burnout Coming to Members
Society for Vascular Surgery members are receiving an important email from the Mayo Clinic containing a survey from the SVS Wellness Task Force.

It is the second survey the task force has distributed, all aimed at ascertaining burnout and wellness statistics from SVS members.
“We need evidence,” said Malachi Sheahan, MD, who co-chairs the group with Dawn Coleman, MD. “We can’t make change without evidence.”
He issued a “Societal Call to Action” to SVS members at the end of a Friday session addressing burnout issues, “Promoting Physician Well-Being: Achieving Quadruple Aim.”
Dr. Sheahan disclosed statistics from the first task force survey, completed by 860 members. Collectively, members worked an average 73.5 hours a week, with five hours completing electronic medical records and 5.5 hours of administrative/scholarly activities added to 63 hours in the office.
“Eighty-nine percent feel burned out on occasion, everyone thinks they’re working too hard and when there are conflicts between work and personal life, they’re resolved in favor of the personal side only 8 percent of the time,” he said of the just-released data. He believes EMR will be the No. 1 conflict of vascular surgeons, with surgeons reporting they spend one hour charting for every one hour of patient time. “It’s just not working out,” he said.
Twenty percent said they had been sued for malpractice within the past two years, 37 percent reported being depressed within the month prior to completing the survey and the 8 percent who reported suicide ideation within the past year is double the national rate, Dr. Sheahan said. The second survey, launched Monday, focuses more on physical debility and should take fewer than 10 minutes to complete, he said. “Look for the survey, and please take it.”
He added that there are initiatives going forward that aim to change the environment and change the culture, including the SVS task force and the American Board of Surgery’s new lifelong learning initiative. “This is a call to action,” he said. “The main thing I want to say is that this is changeable. I don’t want you to think or say that we can’t do it. “We can. We just need evidence.”
Society for Vascular Surgery members are receiving an important email from the Mayo Clinic containing a survey from the SVS Wellness Task Force.

It is the second survey the task force has distributed, all aimed at ascertaining burnout and wellness statistics from SVS members.
“We need evidence,” said Malachi Sheahan, MD, who co-chairs the group with Dawn Coleman, MD. “We can’t make change without evidence.”
He issued a “Societal Call to Action” to SVS members at the end of a Friday session addressing burnout issues, “Promoting Physician Well-Being: Achieving Quadruple Aim.”
Dr. Sheahan disclosed statistics from the first task force survey, completed by 860 members. Collectively, members worked an average 73.5 hours a week, with five hours completing electronic medical records and 5.5 hours of administrative/scholarly activities added to 63 hours in the office.
“Eighty-nine percent feel burned out on occasion, everyone thinks they’re working too hard and when there are conflicts between work and personal life, they’re resolved in favor of the personal side only 8 percent of the time,” he said of the just-released data. He believes EMR will be the No. 1 conflict of vascular surgeons, with surgeons reporting they spend one hour charting for every one hour of patient time. “It’s just not working out,” he said.
Twenty percent said they had been sued for malpractice within the past two years, 37 percent reported being depressed within the month prior to completing the survey and the 8 percent who reported suicide ideation within the past year is double the national rate, Dr. Sheahan said. The second survey, launched Monday, focuses more on physical debility and should take fewer than 10 minutes to complete, he said. “Look for the survey, and please take it.”
He added that there are initiatives going forward that aim to change the environment and change the culture, including the SVS task force and the American Board of Surgery’s new lifelong learning initiative. “This is a call to action,” he said. “The main thing I want to say is that this is changeable. I don’t want you to think or say that we can’t do it. “We can. We just need evidence.”
Society for Vascular Surgery members are receiving an important email from the Mayo Clinic containing a survey from the SVS Wellness Task Force.

It is the second survey the task force has distributed, all aimed at ascertaining burnout and wellness statistics from SVS members.
“We need evidence,” said Malachi Sheahan, MD, who co-chairs the group with Dawn Coleman, MD. “We can’t make change without evidence.”
He issued a “Societal Call to Action” to SVS members at the end of a Friday session addressing burnout issues, “Promoting Physician Well-Being: Achieving Quadruple Aim.”
Dr. Sheahan disclosed statistics from the first task force survey, completed by 860 members. Collectively, members worked an average 73.5 hours a week, with five hours completing electronic medical records and 5.5 hours of administrative/scholarly activities added to 63 hours in the office.
“Eighty-nine percent feel burned out on occasion, everyone thinks they’re working too hard and when there are conflicts between work and personal life, they’re resolved in favor of the personal side only 8 percent of the time,” he said of the just-released data. He believes EMR will be the No. 1 conflict of vascular surgeons, with surgeons reporting they spend one hour charting for every one hour of patient time. “It’s just not working out,” he said.
Twenty percent said they had been sued for malpractice within the past two years, 37 percent reported being depressed within the month prior to completing the survey and the 8 percent who reported suicide ideation within the past year is double the national rate, Dr. Sheahan said. The second survey, launched Monday, focuses more on physical debility and should take fewer than 10 minutes to complete, he said. “Look for the survey, and please take it.”
He added that there are initiatives going forward that aim to change the environment and change the culture, including the SVS task force and the American Board of Surgery’s new lifelong learning initiative. “This is a call to action,” he said. “The main thing I want to say is that this is changeable. I don’t want you to think or say that we can’t do it. “We can. We just need evidence.”
Welcoming a New President, Presenting Awards
The Vascular Annual Meeting conducts important Society business at the SVS Annual Business Meeting. During Saturday’s meeting and luncheon, President R. Clement Darling III, MD, will hand over the leadership reins to President-Elect Michel S. Makaroun, MD.
The meeting is from 12 to 1:30 p.m. Saturday in Ballroom C of the Hynes Convention Center. Besides welcoming a new president, the meeting also will include acting on the 2018-2019 slate of officers.
Members also recieved updates from officers and select committees and recognized outstanding achievements and awards from the Journal of Vascular Surgery, SVS Foundation and SVS. Award winners included the first recipients of the new SVS Foundation Community Awareness and Prevention Project Practice Grant.
The following people received awards during the luncheon:
SVS Presidential Citation Award
Drs. Kellie R. Brown, for her work as Chair of the Postgraduate Education Committee; O. William Brown, Chair of the Conflict of Interest Committee; Daniel G. Clair, Chair of the Education Council; Michael C. Dalsing, Chair of the Government Relations Committee; Alan Dardik, Chair of the Research Council; Dennis R. Gable, Chair of the Public and Professional Outreach Committee; Richard J. Fowl, Chair of the Ethics and Professional Conduct Committee; Brad L. Johnson, Chair of the Quality and Performance Measures Committee; Larry Kraiss, Chair of the SVS Patient Safety Organization; Walter J. McCarthy, Chair of the History Committee; Frank B. Pomposelli, Chair of the Clinical Practice Council; Jeffrey Raines; Amy Reed, Chair of the Fellows Committee; Edith Tzeng, Chair of the Research and Education Committee; Robert Zwolak, Chair of the VA Vascular Surgeons Committee; and Roger Gregory and James S.T. Yao, for their dedicated service in capturing the history of SVS.
SVS Awards
Women’s Leadership Training Grants
Drs. Dawn Coleman, Bao-Ngoc Nguyen and Margaret Tracci
SVS Vascular Surgery Trainee Advocacy Travel Scholarship
Dr. Anahita Dua
SVS Foundation Awards
SVS Foundation and American College of Surgeons Mentored Clinical Scientist Research Career Development Award (K08)
Dr. Bao-Ngoc Nguyen
SVS Foundation E.J. Wylie Traveling Fellowship
Dr. Omid Jazaeri
SVS Foundation Clinical Research Seed Grant
Drs. Samantha D. Minc and Bjoern D. Suckow, MD, MS
SVS Foundation Resident Research Award
Dr. Kaspar M. Trocha
SVS Foundation Research Career Development Travel Award
Drs. James Brooks, Kristina Giles, and Samir Shah, MD
Vascular Cures/ SVS Foundation Wylie Scholar Award
John Byrne, MB BCh, MD (by research), FRCSI
SVS Foundation Community Awareness and Prevention Project Practice Grant
Drs. Manish Mehta, MD, MPH; Elizabeth L. Detschelt, MD; and Marcus E. Semel, MD, MPH
Vascular Research Initiatives Conference Trainee Travel Scholarship
Drs. Frank M. Davis, Catherine Go, Omar Saffaf, and Karim M. Salem
SVS Foundation Student Research Fellowship Award
Arash Fereydooni* (Yale School of Medicine), Helen Genis* (University of Toronto), Nikolai Thomas Harroun (Washington University, St.Louis), Alice Jo* (Case Western Reserve University School of Medicine), Revanth Kosaraju* (Beth Israel Deaconess Medical Center), Alexa Mordhorst* (Vancouver General Hospital/University of British Columbia), Lindsey Anne Olivere (Duke University School of Medicine), Suzannah Patterson (Brigham and Women’s Hospital/Harvard Medical School), Joel L. Ramirez* (University of California, San Francisco), Sudie Ann Robinson* (SUNY Upstate Medical University), Muzammil Hussain Syed (St. Michael’s Hospital/McMaster University), Jeffrey W. Zhao* (Northwestern Feinberg School of Medicine)
(*Awarded a Society for Vascular Surgery General Surgery Resident/Medical Student VAM Travel Scholarship)
The Vascular Annual Meeting conducts important Society business at the SVS Annual Business Meeting. During Saturday’s meeting and luncheon, President R. Clement Darling III, MD, will hand over the leadership reins to President-Elect Michel S. Makaroun, MD.
The meeting is from 12 to 1:30 p.m. Saturday in Ballroom C of the Hynes Convention Center. Besides welcoming a new president, the meeting also will include acting on the 2018-2019 slate of officers.
Members also recieved updates from officers and select committees and recognized outstanding achievements and awards from the Journal of Vascular Surgery, SVS Foundation and SVS. Award winners included the first recipients of the new SVS Foundation Community Awareness and Prevention Project Practice Grant.
The following people received awards during the luncheon:
SVS Presidential Citation Award
Drs. Kellie R. Brown, for her work as Chair of the Postgraduate Education Committee; O. William Brown, Chair of the Conflict of Interest Committee; Daniel G. Clair, Chair of the Education Council; Michael C. Dalsing, Chair of the Government Relations Committee; Alan Dardik, Chair of the Research Council; Dennis R. Gable, Chair of the Public and Professional Outreach Committee; Richard J. Fowl, Chair of the Ethics and Professional Conduct Committee; Brad L. Johnson, Chair of the Quality and Performance Measures Committee; Larry Kraiss, Chair of the SVS Patient Safety Organization; Walter J. McCarthy, Chair of the History Committee; Frank B. Pomposelli, Chair of the Clinical Practice Council; Jeffrey Raines; Amy Reed, Chair of the Fellows Committee; Edith Tzeng, Chair of the Research and Education Committee; Robert Zwolak, Chair of the VA Vascular Surgeons Committee; and Roger Gregory and James S.T. Yao, for their dedicated service in capturing the history of SVS.
SVS Awards
Women’s Leadership Training Grants
Drs. Dawn Coleman, Bao-Ngoc Nguyen and Margaret Tracci
SVS Vascular Surgery Trainee Advocacy Travel Scholarship
Dr. Anahita Dua
SVS Foundation Awards
SVS Foundation and American College of Surgeons Mentored Clinical Scientist Research Career Development Award (K08)
Dr. Bao-Ngoc Nguyen
SVS Foundation E.J. Wylie Traveling Fellowship
Dr. Omid Jazaeri
SVS Foundation Clinical Research Seed Grant
Drs. Samantha D. Minc and Bjoern D. Suckow, MD, MS
SVS Foundation Resident Research Award
Dr. Kaspar M. Trocha
SVS Foundation Research Career Development Travel Award
Drs. James Brooks, Kristina Giles, and Samir Shah, MD
Vascular Cures/ SVS Foundation Wylie Scholar Award
John Byrne, MB BCh, MD (by research), FRCSI
SVS Foundation Community Awareness and Prevention Project Practice Grant
Drs. Manish Mehta, MD, MPH; Elizabeth L. Detschelt, MD; and Marcus E. Semel, MD, MPH
Vascular Research Initiatives Conference Trainee Travel Scholarship
Drs. Frank M. Davis, Catherine Go, Omar Saffaf, and Karim M. Salem
SVS Foundation Student Research Fellowship Award
Arash Fereydooni* (Yale School of Medicine), Helen Genis* (University of Toronto), Nikolai Thomas Harroun (Washington University, St.Louis), Alice Jo* (Case Western Reserve University School of Medicine), Revanth Kosaraju* (Beth Israel Deaconess Medical Center), Alexa Mordhorst* (Vancouver General Hospital/University of British Columbia), Lindsey Anne Olivere (Duke University School of Medicine), Suzannah Patterson (Brigham and Women’s Hospital/Harvard Medical School), Joel L. Ramirez* (University of California, San Francisco), Sudie Ann Robinson* (SUNY Upstate Medical University), Muzammil Hussain Syed (St. Michael’s Hospital/McMaster University), Jeffrey W. Zhao* (Northwestern Feinberg School of Medicine)
(*Awarded a Society for Vascular Surgery General Surgery Resident/Medical Student VAM Travel Scholarship)
The Vascular Annual Meeting conducts important Society business at the SVS Annual Business Meeting. During Saturday’s meeting and luncheon, President R. Clement Darling III, MD, will hand over the leadership reins to President-Elect Michel S. Makaroun, MD.
The meeting is from 12 to 1:30 p.m. Saturday in Ballroom C of the Hynes Convention Center. Besides welcoming a new president, the meeting also will include acting on the 2018-2019 slate of officers.
Members also recieved updates from officers and select committees and recognized outstanding achievements and awards from the Journal of Vascular Surgery, SVS Foundation and SVS. Award winners included the first recipients of the new SVS Foundation Community Awareness and Prevention Project Practice Grant.
The following people received awards during the luncheon:
SVS Presidential Citation Award
Drs. Kellie R. Brown, for her work as Chair of the Postgraduate Education Committee; O. William Brown, Chair of the Conflict of Interest Committee; Daniel G. Clair, Chair of the Education Council; Michael C. Dalsing, Chair of the Government Relations Committee; Alan Dardik, Chair of the Research Council; Dennis R. Gable, Chair of the Public and Professional Outreach Committee; Richard J. Fowl, Chair of the Ethics and Professional Conduct Committee; Brad L. Johnson, Chair of the Quality and Performance Measures Committee; Larry Kraiss, Chair of the SVS Patient Safety Organization; Walter J. McCarthy, Chair of the History Committee; Frank B. Pomposelli, Chair of the Clinical Practice Council; Jeffrey Raines; Amy Reed, Chair of the Fellows Committee; Edith Tzeng, Chair of the Research and Education Committee; Robert Zwolak, Chair of the VA Vascular Surgeons Committee; and Roger Gregory and James S.T. Yao, for their dedicated service in capturing the history of SVS.
SVS Awards
Women’s Leadership Training Grants
Drs. Dawn Coleman, Bao-Ngoc Nguyen and Margaret Tracci
SVS Vascular Surgery Trainee Advocacy Travel Scholarship
Dr. Anahita Dua
SVS Foundation Awards
SVS Foundation and American College of Surgeons Mentored Clinical Scientist Research Career Development Award (K08)
Dr. Bao-Ngoc Nguyen
SVS Foundation E.J. Wylie Traveling Fellowship
Dr. Omid Jazaeri
SVS Foundation Clinical Research Seed Grant
Drs. Samantha D. Minc and Bjoern D. Suckow, MD, MS
SVS Foundation Resident Research Award
Dr. Kaspar M. Trocha
SVS Foundation Research Career Development Travel Award
Drs. James Brooks, Kristina Giles, and Samir Shah, MD
Vascular Cures/ SVS Foundation Wylie Scholar Award
John Byrne, MB BCh, MD (by research), FRCSI
SVS Foundation Community Awareness and Prevention Project Practice Grant
Drs. Manish Mehta, MD, MPH; Elizabeth L. Detschelt, MD; and Marcus E. Semel, MD, MPH
Vascular Research Initiatives Conference Trainee Travel Scholarship
Drs. Frank M. Davis, Catherine Go, Omar Saffaf, and Karim M. Salem
SVS Foundation Student Research Fellowship Award
Arash Fereydooni* (Yale School of Medicine), Helen Genis* (University of Toronto), Nikolai Thomas Harroun (Washington University, St.Louis), Alice Jo* (Case Western Reserve University School of Medicine), Revanth Kosaraju* (Beth Israel Deaconess Medical Center), Alexa Mordhorst* (Vancouver General Hospital/University of British Columbia), Lindsey Anne Olivere (Duke University School of Medicine), Suzannah Patterson (Brigham and Women’s Hospital/Harvard Medical School), Joel L. Ramirez* (University of California, San Francisco), Sudie Ann Robinson* (SUNY Upstate Medical University), Muzammil Hussain Syed (St. Michael’s Hospital/McMaster University), Jeffrey W. Zhao* (Northwestern Feinberg School of Medicine)
(*Awarded a Society for Vascular Surgery General Surgery Resident/Medical Student VAM Travel Scholarship)
A Six-Year Review of the First U.S. Vascular Simulation Course
Duty hour restrictions, changing training paradigms, and diminishing open surgical case volumes have caused dramatic shifts in the vascular trainee experience, according to Malachi Sheahan, MD, and his colleagues from Louisiana State University, New Orleans.
Dr. Sheahan and his colleagues examined the benefits of simulation courses as an augment to vascular training. They performed a 6-year review of the first simulation course established for vascular trainees in the United States.
The 3-day vascular simulation course studied was conducted at a dedicated learning center from 2012 to 2017. Attendees rated their confidence pre- and postcourse on a 6-point Likert scale ranging from 1 (none) to 6 (expert) across 8 different technical and cognitive categories. Participants were also asked to rate the value of each activity, according to Dr. Sheahan.
Assessments of each trainee were completed by the course director and sent to their program director. After 6 months, program directors and participants were surveyed on the lasting usefulness of the course. Full data were available for 98 vascular trainees: 59 categorized as Junior (PGY1-2); and 39 as Senior (PGY 3).
“Our study demonstrates that a brief, intensive simulation course can have a valuable and lasting impact on vascular resident education,” said Dr. Sheahan.
Overall, the participants rated all teaching activities as useful (4) or better, with anatomic exposures (5.8) and one-on-one suturing (5.5) rated most valuable. Both groups showed significant improvement in confidence in all measures, with Juniors improving significantly more than did Seniors in anastomoses, abdominal aortic aneurysm measurements, and tibial exposures.
Six-month follow-up with program directors found that 100% reported at least one lasting skill improvement and 85% stated that they modified their trainees curriculum based on the course assessment.
Duty hour restrictions, changing training paradigms, and diminishing open surgical case volumes have caused dramatic shifts in the vascular trainee experience, according to Malachi Sheahan, MD, and his colleagues from Louisiana State University, New Orleans.
Dr. Sheahan and his colleagues examined the benefits of simulation courses as an augment to vascular training. They performed a 6-year review of the first simulation course established for vascular trainees in the United States.
The 3-day vascular simulation course studied was conducted at a dedicated learning center from 2012 to 2017. Attendees rated their confidence pre- and postcourse on a 6-point Likert scale ranging from 1 (none) to 6 (expert) across 8 different technical and cognitive categories. Participants were also asked to rate the value of each activity, according to Dr. Sheahan.
Assessments of each trainee were completed by the course director and sent to their program director. After 6 months, program directors and participants were surveyed on the lasting usefulness of the course. Full data were available for 98 vascular trainees: 59 categorized as Junior (PGY1-2); and 39 as Senior (PGY 3).
“Our study demonstrates that a brief, intensive simulation course can have a valuable and lasting impact on vascular resident education,” said Dr. Sheahan.
Overall, the participants rated all teaching activities as useful (4) or better, with anatomic exposures (5.8) and one-on-one suturing (5.5) rated most valuable. Both groups showed significant improvement in confidence in all measures, with Juniors improving significantly more than did Seniors in anastomoses, abdominal aortic aneurysm measurements, and tibial exposures.
Six-month follow-up with program directors found that 100% reported at least one lasting skill improvement and 85% stated that they modified their trainees curriculum based on the course assessment.
Duty hour restrictions, changing training paradigms, and diminishing open surgical case volumes have caused dramatic shifts in the vascular trainee experience, according to Malachi Sheahan, MD, and his colleagues from Louisiana State University, New Orleans.
Dr. Sheahan and his colleagues examined the benefits of simulation courses as an augment to vascular training. They performed a 6-year review of the first simulation course established for vascular trainees in the United States.
The 3-day vascular simulation course studied was conducted at a dedicated learning center from 2012 to 2017. Attendees rated their confidence pre- and postcourse on a 6-point Likert scale ranging from 1 (none) to 6 (expert) across 8 different technical and cognitive categories. Participants were also asked to rate the value of each activity, according to Dr. Sheahan.
Assessments of each trainee were completed by the course director and sent to their program director. After 6 months, program directors and participants were surveyed on the lasting usefulness of the course. Full data were available for 98 vascular trainees: 59 categorized as Junior (PGY1-2); and 39 as Senior (PGY 3).
“Our study demonstrates that a brief, intensive simulation course can have a valuable and lasting impact on vascular resident education,” said Dr. Sheahan.
Overall, the participants rated all teaching activities as useful (4) or better, with anatomic exposures (5.8) and one-on-one suturing (5.5) rated most valuable. Both groups showed significant improvement in confidence in all measures, with Juniors improving significantly more than did Seniors in anastomoses, abdominal aortic aneurysm measurements, and tibial exposures.
Six-month follow-up with program directors found that 100% reported at least one lasting skill improvement and 85% stated that they modified their trainees curriculum based on the course assessment.
Crawford Critical Issues Forum Covers the National Shortage of Vascular Surgeons
This year’s E. Stanley Crawford Critical Issues Forum addressed the current status of the vascular surgery workforce, its existing geographic distribution, the potential for a worsening shortage, and proposed solutions to ensure future vascular care delivery.

As is tradition, the forum was organized and moderated by the SVS president-elect, this year Michel S. Makaroun, MD, co-director of the UPMC Heart and Vascular Institute in Pittsburgh. The session began with an overview from Dr. Makaroun on the critical nature of the growing problem.
He described how the current shortage of vascular surgeons is projected to worsen for two main reasons: First, newly trained vascular surgeons are not entering the workforce in adequate numbers to meet the increased demand presented by the nation’s aging population, even with the improvements projected from the new 0-5 training program; second, there is a large percentage of vascular surgeons currently practicing who are expected to retire in the next decade, with estimates reaching 35%-45%. He detailed the results of the 2017 survey of vascular surgeons that showed this and other concerning factors.
“We do not take vacations,” he said. “We have significant overwork.” In addition, “half of the surgeons who said they would retire, said they would retire before the age of 65.” In fact, nearly 50% of those surveyed have firm retirement plans, and nearly 20% were planning on retiring within the next five years.
“To summarize, we have a pipeline, which at maximum would within the next five years introduce 608 new vascular resident and fellow graduates into the workforce, and at the same time we’ll be losing the same number of practicing vascular surgeons. So, at best, we will stay even,” said Dr. Makaroun.
“The Current Landscape of the Vascular Surgery Recruitment Market” was addressed by Jeremy Robinson of Merritt Hawkins. He pointed out the market evidence for a large scarcity of vascular surgeons, with a huge number of positions going unfilled, more than half of new trainees receiving more than 100 offers, and bidding wars going on among employers, including offering expansive salaries and benefits to vascular surgeons as part of recruitment efforts.
In his talk, “Where Did the Vascular Surgeons Go? Seven Years of Futile Recruitment Attempts,” Gerald Goldstein, MD, of the Western Maryland Health System, addressed the problem of attracting new vascular surgeons. His talk was key in pointing out the problems of large health care facilities in poor and rural areas. “We’re not a Band-Aid station. ... we’re a big hospital, we serve a large geographic area and a large cohort of patients,” he said. Still they have failed to recruit a single vascular surgeon. He pointed out how places such as his could not compete for vascular surgeons against more desirable urban centers with more amenities and sufficient backup surgeons to provide lower workloads and improved quality of life, which includes time off, not being on call, and collaboration and teamwork. He was not optimistic about solutions and was fighting the final solution: hospital contraction of services.
“Workforce Targets: How Can We Know Whether We Have Enough Vascular Surgeons?” was discussed by Mark Friedberg, MD, of the RAND Corporation. Dr. Friedberg pointed out that it was important to define what was meant by a shortage of vascular surgeons, asking whether a surgeon/population metric was the most valuable or should it include other components, such as geographic considerations, quality of care issues, patient waiting times, and more. In addition, he asked, for any metric chosen, how was the decision made in choosing the optimal level? What is the best ratio of vascular surgeons to population, and how do you decide it?
Finally, Anton Sidawy, MD, of the George Washington University Hospital, Washington, looked at possible solutions to the problem in his talk, “Pathways to Alleviate Shortages and Maldistributions of Vascular Surgeons.” Dr. Sidawy stressed the need for a multifaceted approach, including government at the federal, state, and local levels, as well as medical schools and training programs. He particularly stressed the need for improving the distribution of vascular surgeons to underserved rural areas, such as described by Dr. Goldstein. However, this would require thinking outside the box, perhaps involving redistribution of training to more underserved areas of the country and also recruitment of more individuals from those areas. This is more likely to produce vascular surgeons willing to remain or return to such areas to practice, as has been well documented.
Another possibility is to provide incentives for older vascular surgeons to delay retirement, perhaps with lowered workload, freedom from being on-call, and other benefits.
But perhaps his most radical suggestion is the idea of rethinking the role of vascular surgeons entirely, to include the ability to perform more general surgery operations, allowing them more flexibility to take on roles in smaller communities and organizations that could not afford a narrowly defined specialist.
The forum concluded with a panel discussion in which the panelists addressed questions from the audience.
This year’s E. Stanley Crawford Critical Issues Forum addressed the current status of the vascular surgery workforce, its existing geographic distribution, the potential for a worsening shortage, and proposed solutions to ensure future vascular care delivery.

As is tradition, the forum was organized and moderated by the SVS president-elect, this year Michel S. Makaroun, MD, co-director of the UPMC Heart and Vascular Institute in Pittsburgh. The session began with an overview from Dr. Makaroun on the critical nature of the growing problem.
He described how the current shortage of vascular surgeons is projected to worsen for two main reasons: First, newly trained vascular surgeons are not entering the workforce in adequate numbers to meet the increased demand presented by the nation’s aging population, even with the improvements projected from the new 0-5 training program; second, there is a large percentage of vascular surgeons currently practicing who are expected to retire in the next decade, with estimates reaching 35%-45%. He detailed the results of the 2017 survey of vascular surgeons that showed this and other concerning factors.
“We do not take vacations,” he said. “We have significant overwork.” In addition, “half of the surgeons who said they would retire, said they would retire before the age of 65.” In fact, nearly 50% of those surveyed have firm retirement plans, and nearly 20% were planning on retiring within the next five years.
“To summarize, we have a pipeline, which at maximum would within the next five years introduce 608 new vascular resident and fellow graduates into the workforce, and at the same time we’ll be losing the same number of practicing vascular surgeons. So, at best, we will stay even,” said Dr. Makaroun.
“The Current Landscape of the Vascular Surgery Recruitment Market” was addressed by Jeremy Robinson of Merritt Hawkins. He pointed out the market evidence for a large scarcity of vascular surgeons, with a huge number of positions going unfilled, more than half of new trainees receiving more than 100 offers, and bidding wars going on among employers, including offering expansive salaries and benefits to vascular surgeons as part of recruitment efforts.
In his talk, “Where Did the Vascular Surgeons Go? Seven Years of Futile Recruitment Attempts,” Gerald Goldstein, MD, of the Western Maryland Health System, addressed the problem of attracting new vascular surgeons. His talk was key in pointing out the problems of large health care facilities in poor and rural areas. “We’re not a Band-Aid station. ... we’re a big hospital, we serve a large geographic area and a large cohort of patients,” he said. Still they have failed to recruit a single vascular surgeon. He pointed out how places such as his could not compete for vascular surgeons against more desirable urban centers with more amenities and sufficient backup surgeons to provide lower workloads and improved quality of life, which includes time off, not being on call, and collaboration and teamwork. He was not optimistic about solutions and was fighting the final solution: hospital contraction of services.
“Workforce Targets: How Can We Know Whether We Have Enough Vascular Surgeons?” was discussed by Mark Friedberg, MD, of the RAND Corporation. Dr. Friedberg pointed out that it was important to define what was meant by a shortage of vascular surgeons, asking whether a surgeon/population metric was the most valuable or should it include other components, such as geographic considerations, quality of care issues, patient waiting times, and more. In addition, he asked, for any metric chosen, how was the decision made in choosing the optimal level? What is the best ratio of vascular surgeons to population, and how do you decide it?
Finally, Anton Sidawy, MD, of the George Washington University Hospital, Washington, looked at possible solutions to the problem in his talk, “Pathways to Alleviate Shortages and Maldistributions of Vascular Surgeons.” Dr. Sidawy stressed the need for a multifaceted approach, including government at the federal, state, and local levels, as well as medical schools and training programs. He particularly stressed the need for improving the distribution of vascular surgeons to underserved rural areas, such as described by Dr. Goldstein. However, this would require thinking outside the box, perhaps involving redistribution of training to more underserved areas of the country and also recruitment of more individuals from those areas. This is more likely to produce vascular surgeons willing to remain or return to such areas to practice, as has been well documented.
Another possibility is to provide incentives for older vascular surgeons to delay retirement, perhaps with lowered workload, freedom from being on-call, and other benefits.
But perhaps his most radical suggestion is the idea of rethinking the role of vascular surgeons entirely, to include the ability to perform more general surgery operations, allowing them more flexibility to take on roles in smaller communities and organizations that could not afford a narrowly defined specialist.
The forum concluded with a panel discussion in which the panelists addressed questions from the audience.
This year’s E. Stanley Crawford Critical Issues Forum addressed the current status of the vascular surgery workforce, its existing geographic distribution, the potential for a worsening shortage, and proposed solutions to ensure future vascular care delivery.

As is tradition, the forum was organized and moderated by the SVS president-elect, this year Michel S. Makaroun, MD, co-director of the UPMC Heart and Vascular Institute in Pittsburgh. The session began with an overview from Dr. Makaroun on the critical nature of the growing problem.
He described how the current shortage of vascular surgeons is projected to worsen for two main reasons: First, newly trained vascular surgeons are not entering the workforce in adequate numbers to meet the increased demand presented by the nation’s aging population, even with the improvements projected from the new 0-5 training program; second, there is a large percentage of vascular surgeons currently practicing who are expected to retire in the next decade, with estimates reaching 35%-45%. He detailed the results of the 2017 survey of vascular surgeons that showed this and other concerning factors.
“We do not take vacations,” he said. “We have significant overwork.” In addition, “half of the surgeons who said they would retire, said they would retire before the age of 65.” In fact, nearly 50% of those surveyed have firm retirement plans, and nearly 20% were planning on retiring within the next five years.
“To summarize, we have a pipeline, which at maximum would within the next five years introduce 608 new vascular resident and fellow graduates into the workforce, and at the same time we’ll be losing the same number of practicing vascular surgeons. So, at best, we will stay even,” said Dr. Makaroun.
“The Current Landscape of the Vascular Surgery Recruitment Market” was addressed by Jeremy Robinson of Merritt Hawkins. He pointed out the market evidence for a large scarcity of vascular surgeons, with a huge number of positions going unfilled, more than half of new trainees receiving more than 100 offers, and bidding wars going on among employers, including offering expansive salaries and benefits to vascular surgeons as part of recruitment efforts.
In his talk, “Where Did the Vascular Surgeons Go? Seven Years of Futile Recruitment Attempts,” Gerald Goldstein, MD, of the Western Maryland Health System, addressed the problem of attracting new vascular surgeons. His talk was key in pointing out the problems of large health care facilities in poor and rural areas. “We’re not a Band-Aid station. ... we’re a big hospital, we serve a large geographic area and a large cohort of patients,” he said. Still they have failed to recruit a single vascular surgeon. He pointed out how places such as his could not compete for vascular surgeons against more desirable urban centers with more amenities and sufficient backup surgeons to provide lower workloads and improved quality of life, which includes time off, not being on call, and collaboration and teamwork. He was not optimistic about solutions and was fighting the final solution: hospital contraction of services.
“Workforce Targets: How Can We Know Whether We Have Enough Vascular Surgeons?” was discussed by Mark Friedberg, MD, of the RAND Corporation. Dr. Friedberg pointed out that it was important to define what was meant by a shortage of vascular surgeons, asking whether a surgeon/population metric was the most valuable or should it include other components, such as geographic considerations, quality of care issues, patient waiting times, and more. In addition, he asked, for any metric chosen, how was the decision made in choosing the optimal level? What is the best ratio of vascular surgeons to population, and how do you decide it?
Finally, Anton Sidawy, MD, of the George Washington University Hospital, Washington, looked at possible solutions to the problem in his talk, “Pathways to Alleviate Shortages and Maldistributions of Vascular Surgeons.” Dr. Sidawy stressed the need for a multifaceted approach, including government at the federal, state, and local levels, as well as medical schools and training programs. He particularly stressed the need for improving the distribution of vascular surgeons to underserved rural areas, such as described by Dr. Goldstein. However, this would require thinking outside the box, perhaps involving redistribution of training to more underserved areas of the country and also recruitment of more individuals from those areas. This is more likely to produce vascular surgeons willing to remain or return to such areas to practice, as has been well documented.
Another possibility is to provide incentives for older vascular surgeons to delay retirement, perhaps with lowered workload, freedom from being on-call, and other benefits.
But perhaps his most radical suggestion is the idea of rethinking the role of vascular surgeons entirely, to include the ability to perform more general surgery operations, allowing them more flexibility to take on roles in smaller communities and organizations that could not afford a narrowly defined specialist.
The forum concluded with a panel discussion in which the panelists addressed questions from the audience.
Cost led to missed care for 4.5% of Americans in 2017
The percentage of Americans who went without medical care due to cost rose to 4.5% in 2017, reversing a 6-year trend, the National Center for Health Statistics reported.
The rate was 4.4% in 2016, which represented a slowdown in what had been steady decline over the previous 5 years, according to data from the National Health Interview Survey. Declining rates corresponded with the implementation of early provisions of the Affordable Care Act in 2010.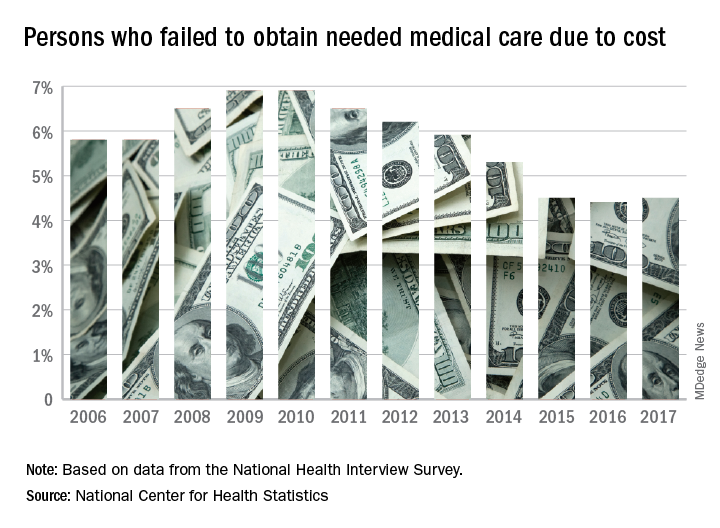
The 2017 rate varied considerably by age group. Not surprisingly, more working-age people – those aged 18-64 years – reported that they did not seek medical care at some point in the previous 12 months due to cost (6.1%). The rate was 1.2% for those under 18 years and 2.7% for the Medicare eligible – those aged 65 years and older.
For 2016, the rates were 6.2% for those aged 18-64 years, 1.2% for the under-18 group, and 2.1% for the 65+ group, the data show.
Among females of all ages in 2017, 4.8% failed to get needed care at some point in the previous year, compared with 4.1% of men. Those numbers were unchanged from 2016 but down from 4.9% for females in 2015 and up from 4.0% for males that year, the NCHS said.
In 2017, the rate also varied by race/ethnicity – 4.1% for whites, 5.3% for Hispanics, 6.1% for blacks – and by location – 4.1% for large metropolitan areas, 4.9% for small metro areas, and 5.5% for rural locales, according to the early release of survey data.
The percentage of Americans who went without medical care due to cost rose to 4.5% in 2017, reversing a 6-year trend, the National Center for Health Statistics reported.
The rate was 4.4% in 2016, which represented a slowdown in what had been steady decline over the previous 5 years, according to data from the National Health Interview Survey. Declining rates corresponded with the implementation of early provisions of the Affordable Care Act in 2010.
The 2017 rate varied considerably by age group. Not surprisingly, more working-age people – those aged 18-64 years – reported that they did not seek medical care at some point in the previous 12 months due to cost (6.1%). The rate was 1.2% for those under 18 years and 2.7% for the Medicare eligible – those aged 65 years and older.
For 2016, the rates were 6.2% for those aged 18-64 years, 1.2% for the under-18 group, and 2.1% for the 65+ group, the data show.
Among females of all ages in 2017, 4.8% failed to get needed care at some point in the previous year, compared with 4.1% of men. Those numbers were unchanged from 2016 but down from 4.9% for females in 2015 and up from 4.0% for males that year, the NCHS said.
In 2017, the rate also varied by race/ethnicity – 4.1% for whites, 5.3% for Hispanics, 6.1% for blacks – and by location – 4.1% for large metropolitan areas, 4.9% for small metro areas, and 5.5% for rural locales, according to the early release of survey data.
The percentage of Americans who went without medical care due to cost rose to 4.5% in 2017, reversing a 6-year trend, the National Center for Health Statistics reported.
The rate was 4.4% in 2016, which represented a slowdown in what had been steady decline over the previous 5 years, according to data from the National Health Interview Survey. Declining rates corresponded with the implementation of early provisions of the Affordable Care Act in 2010.
The 2017 rate varied considerably by age group. Not surprisingly, more working-age people – those aged 18-64 years – reported that they did not seek medical care at some point in the previous 12 months due to cost (6.1%). The rate was 1.2% for those under 18 years and 2.7% for the Medicare eligible – those aged 65 years and older.
For 2016, the rates were 6.2% for those aged 18-64 years, 1.2% for the under-18 group, and 2.1% for the 65+ group, the data show.
Among females of all ages in 2017, 4.8% failed to get needed care at some point in the previous year, compared with 4.1% of men. Those numbers were unchanged from 2016 but down from 4.9% for females in 2015 and up from 4.0% for males that year, the NCHS said.
In 2017, the rate also varied by race/ethnicity – 4.1% for whites, 5.3% for Hispanics, 6.1% for blacks – and by location – 4.1% for large metropolitan areas, 4.9% for small metro areas, and 5.5% for rural locales, according to the early release of survey data.
Glucocorticosteroid use raises sarcopenia risk in RA
AMSTERDAM – Patients with RA have a higher risk of developing sarcopenia if they are treated with glucocorticosteroids, study findings suggested.
“The strength of our study is that it is a prospective, longitudinal study and this study is the first, to our knowledge, to look at risk factors for sarcopenia limited to RA patients,” Yutaro Yamada, MD, said in an interview at the European Congress of Rheumatology.
Dr. Yamada, of the department of orthopedic surgery at Osaka City University, Japan, said that there is a twofold rationale for looking at risk factors for sarcopenia in RA patients. First, RA causes chronic inflammation and this in turn is thought to lead to a metabolic state that then results in muscle loss. Furthermore, joint dysfunction and subsequent disuse likely contribute to weakening muscles. Second, glucocorticosteroids, which are commonly used to treat patients with RA, have themselves been associated with a low muscle area in prior research.
In 2016, the CHIKARA study was initiated “to clarify the correlation between RA disease activity and sarcopenia,” Dr. Yamada and his associates reported in a poster presentation. They recruited 100 patients, of whom 78% were women, and recorded body weight, muscle mass, fat mass, and predicted bone mass using a body composition analyzer at entry and 1 year later. Laboratory data and disease activity parameters were also assessed, along with radiologic findings and ability to perform activities of daily living. Patients’ treatment was also recorded.
At baseline, the mean age of study participants was 68 years with a mean disease duration of 5.5 years. The majority (86%) had been treated with methotrexate, with 26% also using glucocorticosteroids, and 30% using biologic agents.
Just over one-quarter (28%) were diagnosed with sarcopenia over the course of 1 year. Sarcopenia was defined by criteria agreed by the Asia Working Group on Sarcopenia (Am Med Dir Assoc. 2014;15[2]:95-101) that set thresholds for low muscle mass, low muscle strength, and low physical performance.
Comparing the 9 patients who did develop sarcopenia with the 86 who did not, the researchers found that sarcopenia patients tended to be younger (66.7 vs. 80.2 years), although the difference was not statistically significant. Interestingly, however, more than half (55.6%) of patients who developed sarcopenia were using glucocorticosteroids, compared with 22% of those who were not (P = .029). Of note, the average glucocorticosteroid dose was just 2 mg/day. Patients who developed sarcopenia were also observed to have a lower fat mass (11.7 vs. 15.8, P = .058).
A multiple logistic regression analysis found that using a glucocorticosteroid dose of 2 mg/day or more and lower body fat mass (odds ratio, 0.78; 95% CI 0.61-0.98; P = 0.037) were significant variables for the onset of sarcopenia.
Dr. Yamada noted that 2 mg/day is “a relatively low dose” and so avoiding glucocorticosteroid therapy in those at risk of sarcopenia, particularly those with a low fat mass, may be something to consider.
The study did not receive commercial funding, and Dr. Yamada had no conflicts of interest to disclose.
SOURCE: Yamada Y et al. Ann Rheum Dis. 2018;77(Suppl 2):308-9. Abstract THU0181.
AMSTERDAM – Patients with RA have a higher risk of developing sarcopenia if they are treated with glucocorticosteroids, study findings suggested.
“The strength of our study is that it is a prospective, longitudinal study and this study is the first, to our knowledge, to look at risk factors for sarcopenia limited to RA patients,” Yutaro Yamada, MD, said in an interview at the European Congress of Rheumatology.
Dr. Yamada, of the department of orthopedic surgery at Osaka City University, Japan, said that there is a twofold rationale for looking at risk factors for sarcopenia in RA patients. First, RA causes chronic inflammation and this in turn is thought to lead to a metabolic state that then results in muscle loss. Furthermore, joint dysfunction and subsequent disuse likely contribute to weakening muscles. Second, glucocorticosteroids, which are commonly used to treat patients with RA, have themselves been associated with a low muscle area in prior research.
In 2016, the CHIKARA study was initiated “to clarify the correlation between RA disease activity and sarcopenia,” Dr. Yamada and his associates reported in a poster presentation. They recruited 100 patients, of whom 78% were women, and recorded body weight, muscle mass, fat mass, and predicted bone mass using a body composition analyzer at entry and 1 year later. Laboratory data and disease activity parameters were also assessed, along with radiologic findings and ability to perform activities of daily living. Patients’ treatment was also recorded.
At baseline, the mean age of study participants was 68 years with a mean disease duration of 5.5 years. The majority (86%) had been treated with methotrexate, with 26% also using glucocorticosteroids, and 30% using biologic agents.
Just over one-quarter (28%) were diagnosed with sarcopenia over the course of 1 year. Sarcopenia was defined by criteria agreed by the Asia Working Group on Sarcopenia (Am Med Dir Assoc. 2014;15[2]:95-101) that set thresholds for low muscle mass, low muscle strength, and low physical performance.
Comparing the 9 patients who did develop sarcopenia with the 86 who did not, the researchers found that sarcopenia patients tended to be younger (66.7 vs. 80.2 years), although the difference was not statistically significant. Interestingly, however, more than half (55.6%) of patients who developed sarcopenia were using glucocorticosteroids, compared with 22% of those who were not (P = .029). Of note, the average glucocorticosteroid dose was just 2 mg/day. Patients who developed sarcopenia were also observed to have a lower fat mass (11.7 vs. 15.8, P = .058).
A multiple logistic regression analysis found that using a glucocorticosteroid dose of 2 mg/day or more and lower body fat mass (odds ratio, 0.78; 95% CI 0.61-0.98; P = 0.037) were significant variables for the onset of sarcopenia.
Dr. Yamada noted that 2 mg/day is “a relatively low dose” and so avoiding glucocorticosteroid therapy in those at risk of sarcopenia, particularly those with a low fat mass, may be something to consider.
The study did not receive commercial funding, and Dr. Yamada had no conflicts of interest to disclose.
SOURCE: Yamada Y et al. Ann Rheum Dis. 2018;77(Suppl 2):308-9. Abstract THU0181.
AMSTERDAM – Patients with RA have a higher risk of developing sarcopenia if they are treated with glucocorticosteroids, study findings suggested.
“The strength of our study is that it is a prospective, longitudinal study and this study is the first, to our knowledge, to look at risk factors for sarcopenia limited to RA patients,” Yutaro Yamada, MD, said in an interview at the European Congress of Rheumatology.
Dr. Yamada, of the department of orthopedic surgery at Osaka City University, Japan, said that there is a twofold rationale for looking at risk factors for sarcopenia in RA patients. First, RA causes chronic inflammation and this in turn is thought to lead to a metabolic state that then results in muscle loss. Furthermore, joint dysfunction and subsequent disuse likely contribute to weakening muscles. Second, glucocorticosteroids, which are commonly used to treat patients with RA, have themselves been associated with a low muscle area in prior research.
In 2016, the CHIKARA study was initiated “to clarify the correlation between RA disease activity and sarcopenia,” Dr. Yamada and his associates reported in a poster presentation. They recruited 100 patients, of whom 78% were women, and recorded body weight, muscle mass, fat mass, and predicted bone mass using a body composition analyzer at entry and 1 year later. Laboratory data and disease activity parameters were also assessed, along with radiologic findings and ability to perform activities of daily living. Patients’ treatment was also recorded.
At baseline, the mean age of study participants was 68 years with a mean disease duration of 5.5 years. The majority (86%) had been treated with methotrexate, with 26% also using glucocorticosteroids, and 30% using biologic agents.
Just over one-quarter (28%) were diagnosed with sarcopenia over the course of 1 year. Sarcopenia was defined by criteria agreed by the Asia Working Group on Sarcopenia (Am Med Dir Assoc. 2014;15[2]:95-101) that set thresholds for low muscle mass, low muscle strength, and low physical performance.
Comparing the 9 patients who did develop sarcopenia with the 86 who did not, the researchers found that sarcopenia patients tended to be younger (66.7 vs. 80.2 years), although the difference was not statistically significant. Interestingly, however, more than half (55.6%) of patients who developed sarcopenia were using glucocorticosteroids, compared with 22% of those who were not (P = .029). Of note, the average glucocorticosteroid dose was just 2 mg/day. Patients who developed sarcopenia were also observed to have a lower fat mass (11.7 vs. 15.8, P = .058).
A multiple logistic regression analysis found that using a glucocorticosteroid dose of 2 mg/day or more and lower body fat mass (odds ratio, 0.78; 95% CI 0.61-0.98; P = 0.037) were significant variables for the onset of sarcopenia.
Dr. Yamada noted that 2 mg/day is “a relatively low dose” and so avoiding glucocorticosteroid therapy in those at risk of sarcopenia, particularly those with a low fat mass, may be something to consider.
The study did not receive commercial funding, and Dr. Yamada had no conflicts of interest to disclose.
SOURCE: Yamada Y et al. Ann Rheum Dis. 2018;77(Suppl 2):308-9. Abstract THU0181.
REPORTING FROM THE EULAR 2018 CONGRESS
Key clinical point: Patients with RA and a lean body mass have a heightened risk of developing sarcopenia if they are treated with glucocorticosteroids.
Major finding: Using glucocorticosteroids at doses of 2 mg/day or more significantly increased the odds of developing sarcopenia (odds ratio, 8.0; 95% confidence interval, 1.17-54.8; P = .034).
Study details: The CHIKARA study – a prospective, observational study involving 100 patients with RA.
Disclosures: The study did not receive commercial funding, and Dr. Yamada had no conflicts of interest to disclose.
Source: Yamada Y et al. Ann Rheum Dis. 2018;77(Suppl 2):308-9. Abstract THU0181.
How to differentiate maternal from fetal heart rate patterns on electronic fetal monitoring
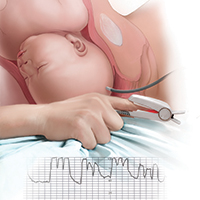
Continuous electronic fetal heart rate monitoring (EFM) is used in the vast majority of all labors in the United States. With the use of EFM categories and definitions from the American College of Obstetricians and Gynecologists, the National Institutes of Health, and the Society for Maternal-Fetal Medicine, clinicians can now better define and communicate tracing assessments. Except for reducing neonatal seizure activity, however, EFM use during labor has not been demonstrated to significantly improve fetal and neonatal outcomes, yet EFM is associated with an increase in cesarean deliveries and instrument-assisted vaginal births.1
The negative predictive value of EFM for fetal hypoxia/acidosis is high, but its positive predictive value is only 30%, and the false-positive rate is as high as 60%.2 Although a false-positive assessment may result in a potentially unnecessary operative vaginal or cesarean delivery, a falsely reassuring strip may produce devastating consequences in the newborn and, not infrequently, medical malpractice liability. One etiology associated with falsely reassuring assessments is that of EFM monitoring of the maternal heart rate and the failure to recognize the tracing as maternal.
In this article, I discuss the mechanisms and periods of labor that often are associated with the maternal heart rate masquerading as the fetal heart rate. I review common EFM patterns associated with the maternal heart rate so as to aid in recognizing the maternal heart rate. In addition, I provide 3 case scenarios that illustrate the simple yet critical steps that clinicians can take to remedy the situation. Being aware of the potential for a maternal heart rate recording, investigating the EFM signals, and correcting the monitoring can help prevent significant morbidity.
CASE 1 EFM shows seesaw decelerations and returns to baseline rate
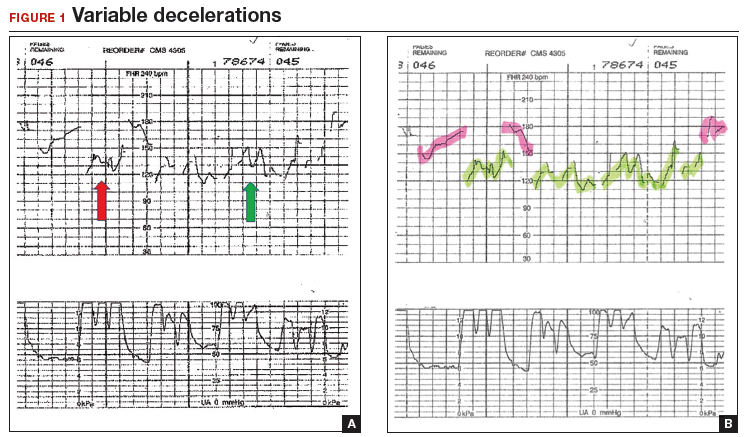
A 29-year-old woman (G3P2) at 39 weeks’ gestation was admitted to the hospital with spontaneous labor. Continuous EFM external monitoring was initiated. After membranes spontaneously ruptured at 4 cm dilation, an epidural was placed. Throughout the active phase of labor, the fetus demonstrated intermittent mild variable decelerations, and the fetal heart rate baseline increased to 180 beats per minute (BPM). With complete dilation, the patient initiated pushing. During the first several pushes, the EFM demonstrated an initial heart rate deceleration, and a loss of signal, but the heart rate returned to a baseline rate of 150 BPM. With the patient’s continued pushing efforts, the EFM baseline increased to 180 BPM, with evidence of variable decelerations to a nadir of 120 BPM, although with some signal gaps (FIGURE 1, red arrow). The tracing then appeared to have a baseline of 120 BPM with variability or accelerations (FIGURE 1, green arrow) before shifting again to 170 to 180 BPM.
What was happening?
Why does the EFM record the maternal heart rate?
Most commonly, EFM recording of the maternal heart rate occurs during the second stage of labor. Early in labor, the normal fetal heart rate (110–160 BPM) typically exceeds the basal maternal heart rate. However, in the presence of chorioamnionitis and maternal fever or with the stress of maternal pushing, the maternal heart rate frequently approaches or exceeds that of the fetal heart rate. The maximum maternal heart rate can be estimated as 220 BPM minus the maternal age. Thus, the heart rate in a 20-year-old gravida may reach rates of 160 to 180 BPM, equivalent to 80% to 90% of her maximum heart rate during second-stage pushing.
The external Doppler fetal monitor, having a somewhat narrow acoustic window, may lose the focus on the fetal heart as a result of descent of the baby, the abdominal shape-altering effect of uterine contractions, and the patient’s pushing. During the second stage, the EFM may record the maternal heart rate from the uterine arteries. Although some clinicians claim to differentiate the maternal from the fetal heart rate by the “whooshing” maternal uterine artery signal as compared with the “thumping” fetal heart rate signal, this auditory assessment is unproven and likely unreliable.
CASE 1 Problem recognized and addressed
In this case, the obstetrician recognized that “slipping” from the fetal to the maternal heart rate recording occurred with the onset of maternal pushing. After the pushing ceased, the maternal heart rate slipped back to the fetal heart rate. With the next several contractions, only the maternal heart rate was recorded. A fetal scalp electrode was then placed, and fetal variable decelerations were recognized. In view of the category II EFM recording, a vacuum procedure was performed from +3 station and a female infant was delivered. She had Apgar scores of 6 and 8 at 1 and 5 minutes, respectively, and she did well in the nursery.
Read what happened in Case 2 when the EFM demonstrated breaks in the tracing
CASE 2 EFM tracings belie the clinical situation
A 20-year-old woman (G1P0) presented for induction of labor at 41 weeks’ gestation. Continuous EFM recording was initiated, and the patient was given dinoprostone and, subsequently, oxytocin. Rupture of membranes at 3 cm demonstrated a small amount of fluid with thick meconium. The patient progressed to complete dilation and developed a temperature of 38.5°C; the EFM baseline increased to 180 BPM. Throughout the first hour of the second stage of labor, the EFM demonstrated breaks in the tracing and a heart rate of 130 to 150 BPM with each pushing effort (FIGURE 2A). The Doppler monitor was subsequently adjusted to focus on the fetal heart and repetitive late decelerations were observed (FIGURE 2B). An emergent cesarean delivery was performed. A depressed newborn male was delivered, with Apgar scores of 2 and 4 at 1 and 5 minutes, respectively, and significant metabolic acidosis.
What happened?
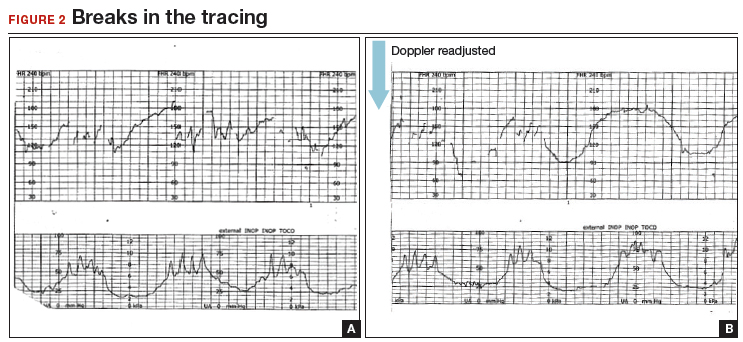
Fetal versus maternal responses to pushing
The fetal variable deceleration pattern is well recognized by clinicians. As a result of umbilical cord occlusion (due to compression, stretching, or twisting of the cord), fetal variable decelerations have a typical pattern. An initial acceleration shoulder resulting from umbilical vein occlusion (due to reduced venous return) is followed by an umbilical artery occlusion–induced sharp deceleration. The relief of the occlusion allows the sharp return toward baseline with the secondary shoulder overshoot.
In some cases, partial umbilical cord occlusion that affects only the fetal umbilical vein may result in an acceleration, although these usually resolve or evolve into variable decelerations within 30 minutes. By contrast, the maternal heart rate typically increases with contractions and with maternal pushing efforts. Thus, a repetitive pattern of heart rate accelerations with each contraction should warn of a possible maternal heart rate recording.
How maternal heart rate responds to pushing. Maternal pushing is a Valsalva maneuver. Although there are 4 classic cardiovascular phases of Valsalva responses, the typical maternal pushing effort results in an increase in the maternal heart rate. With the common sequence of three 10-second pushes during each contraction, the maternal heart rate often exhibits 3 acceleration and deceleration responses. The maternal heart rate tracing looks similar to the shape of the Three Sisters mountain peaks in Oregon (FIGURE 3). Due to Valsalva physiology, the 3 peaks of the Sisters mirror the 3 uterine wave form peaks, although with a 5- to 10-second delay in the heart rate responses (mountain peaks) from the pushing efforts.
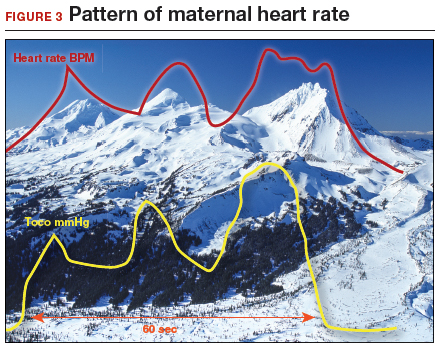
Pre- and postcontraction changes offer clues. Several classic findings aid in differentiating the maternal from the fetal heart rate. If the tracing is maternal, typically the heart rate gradually decreases following the end of the contraction/pushing and continues to decrease until the start of the next contraction/pushing, at which time it increases. During the push, the Three Sisters wave form, with the 5- to 10-second offset, should alert the clinician to possible maternal heart rate recordings. By contrast, the fetal heart rate variable deceleration typically increases following the end of the maternal contraction/pushing and is either stable or increases further (variable with slow recovery) prior to the next uterine contraction/pushing effort. These differences in the patterns of precontraction and postcontraction changes can be very valuable in differentiating periods of maternal versus fetal heart rate recordings.
With “slipping” between fetal and maternal recording, it is not uncommon to record fetal heart rate between contractions, slip to the maternal heart rate during the pushing effort, and return again to the fetal heart rate with the end of the contraction. When confounded with the potential for other EFM artifacts, including doubling of a low maternal or fetal heart rate, or halving of a tachycardic signal, it is not surprising that it is challenging to recognize an EFM maternal heart rate recording.
CASE 2 Check the monitor for accurate focus
A retrospective analysis of this case revealed that the maternal heart rate was recorded with each contraction throughout the second stage. The actual fetal heart rate pattern of decelerations was revealed with the refocusing of the Doppler monitor.
Read how subtle slipping manifested in the EFM tracing of Case 3
CASE 3 Low fetal heart rate and variability during contractions
A 22-year-old woman (G2P1) in spontaneous labor at term progressed to complete dilation. Fetal heart rate accelerations occurred for approximately 30 minutes. With the advent of pushing, the fetal heart rate showed a rate of 130 to 140 BPM and mild decelerations with each contraction (FIGURE 4A). As the second stage progressed, the tracing demonstrated an undulating baseline heart rate between 100 and 130 BPM with possible variability during contractions (FIGURE 4B). This pattern continued for an additional 60 minutes. At vaginal delivery, the ObGyn was surprised to deliver a depressed newborn with Apgar scores of 1 and 3 at 1 and 5 minutes, respectively.
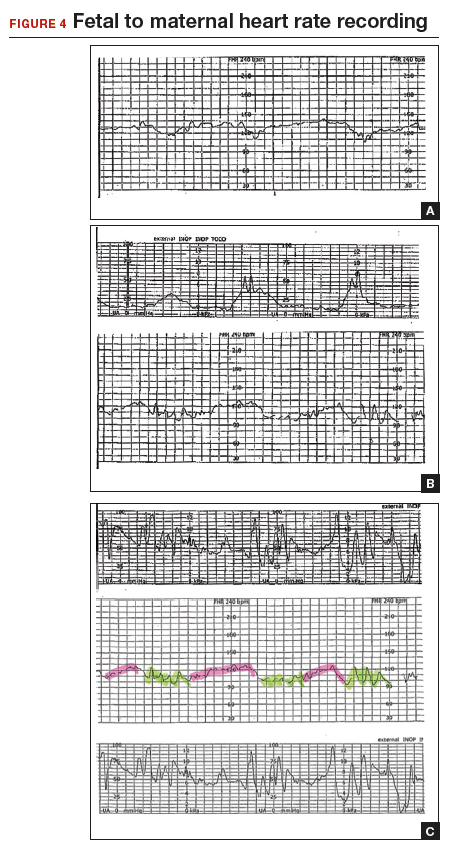
Slipping from the fetal to the maternal heart rate may be imperceptible
In contrast to the breaks in the tracings seen in Case 1 and Case 2, the EFM tracing in Case 3 appears continuous. Yet, slipping from the fetal to the maternal recording was occurring.
As seen in FIGURE 4C, the maternal heart rate with variability was recorded during pushing efforts, and the fetal heart rate was seen rising back toward a baseline between contractions. Note that the fetal heart rate did not reach a level baseline, but rather decelerated with the next contraction. The slipping to the maternal heart rate occurred without a perceptible break in the recording, making this tracing extremely difficult to interpret.
CASE 3 Be ever vigilant
The lack of recognition that the EFM recording had slipped to the maternal heart rate resulted in fetal and newborn hypoxia and acidosis, accounting for the infant’s low Apgar scores.
Read how using 3 steps can help you distinguish fetal from maternal heart rate patterns
Follow 3 steps to discern fetal vs maternal heart rate
These cases illustrate the difficulties in recognizing maternal heart rate patterns on the fetal monitor tracing. The 3 simple steps described below can aid in differentiating maternal from fetal heart rate patterns.
1 Be aware and alert
Recognize that EFM monitoring of the maternal heart rate may occur during periods of monitoring, particularly in second-stage labor. Often, the recorded tracing is a mix of fetal and maternal patterns. Remember that the maternal heart rate may increase markedly during the second stage and rise even higher during pushing efforts. When presented with a tracing that ostensibly represents the fetus, it may be challenging for the clinician to question that assumption. Thus, be aware that tracings may not represent what they seem to be.
Often, clinicians view only the 10-minute portion of the tracing displayed on the monitor screen. I recommend, however, that clinicians review the tracing over the past 30 to 60 minutes, or since their last EFM assessment, for an understanding of the recent fetal baseline heart rate and decelerations.
2 Investigate
Although it is sometimes challenging to recognize EFM maternal heart rate recordings, this is relatively easy to investigate. Even without a pulse oximeter in place, carefully examine the EFM recording for maternal signs to determine if the maternal heart rate is within the range of the recording. You can confirm that the recording is maternal through 1 of 3 easy measures:
- First, check the maternal radial pulse and correlate it with the heart rate baseline.
- Second, place a maternal electrocardiographic (EKG) heart rate monitor.
- Last, and often the simplest approach for continuous tracings, place a finger pulse oximeter to provide a continuous maternal pulse reading. Should the maternal heart rate superimpose on the EFM recording, maternal patterns are likely being detected. However, since the pulse oximeter and EFM Doppler devices use different technologies, they will provide similar—but not precisely identical—heart rate numerical readings if both are assessing the maternal heart rate. In that case, take steps to assure that the EFM truly is recording the fetal heart rate.
3 Treat and correct
If the EFM is recording a maternal signal or if a significant question remains, place a fetal scalp electrode (unless contraindicated), as this may likely occur during the second stage. Alternatively, place a maternal surface fetal EKG monitor, or use ultrasonography to visually assess the fetal heart rate in real time.
Key point summary
The use of a maternal finger pulse oximeter, combined with a careful assessment of the EFM tracing, and/or a fetal scalp electrode are appropriate measures for confirming a fetal heart rate recording.
The 3 steps described (be aware and alert, investigate, treat and correct) can help you effectively monitor the fetal heart rate and avoid the potentially dangerous outcomes that might occur when the maternal heart rate masquerades as the fetal heart rate.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Alfirevic Z, Devane D, Gyte GM, Cuthbert A. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2017; doi:10.1002/14651858.CD006066.pub3.
- Pinas A, Chandraharan E. Continuous cardiotocography during labour: analysis, classification and management. Best Pract Res Clin Obstet Gynaecol. 2016;30:33–47.
Continuous electronic fetal heart rate monitoring (EFM) is used in the vast majority of all labors in the United States. With the use of EFM categories and definitions from the American College of Obstetricians and Gynecologists, the National Institutes of Health, and the Society for Maternal-Fetal Medicine, clinicians can now better define and communicate tracing assessments. Except for reducing neonatal seizure activity, however, EFM use during labor has not been demonstrated to significantly improve fetal and neonatal outcomes, yet EFM is associated with an increase in cesarean deliveries and instrument-assisted vaginal births.1
The negative predictive value of EFM for fetal hypoxia/acidosis is high, but its positive predictive value is only 30%, and the false-positive rate is as high as 60%.2 Although a false-positive assessment may result in a potentially unnecessary operative vaginal or cesarean delivery, a falsely reassuring strip may produce devastating consequences in the newborn and, not infrequently, medical malpractice liability. One etiology associated with falsely reassuring assessments is that of EFM monitoring of the maternal heart rate and the failure to recognize the tracing as maternal.
In this article, I discuss the mechanisms and periods of labor that often are associated with the maternal heart rate masquerading as the fetal heart rate. I review common EFM patterns associated with the maternal heart rate so as to aid in recognizing the maternal heart rate. In addition, I provide 3 case scenarios that illustrate the simple yet critical steps that clinicians can take to remedy the situation. Being aware of the potential for a maternal heart rate recording, investigating the EFM signals, and correcting the monitoring can help prevent significant morbidity.
CASE 1 EFM shows seesaw decelerations and returns to baseline rate
A 29-year-old woman (G3P2) at 39 weeks’ gestation was admitted to the hospital with spontaneous labor. Continuous EFM external monitoring was initiated. After membranes spontaneously ruptured at 4 cm dilation, an epidural was placed. Throughout the active phase of labor, the fetus demonstrated intermittent mild variable decelerations, and the fetal heart rate baseline increased to 180 beats per minute (BPM). With complete dilation, the patient initiated pushing. During the first several pushes, the EFM demonstrated an initial heart rate deceleration, and a loss of signal, but the heart rate returned to a baseline rate of 150 BPM. With the patient’s continued pushing efforts, the EFM baseline increased to 180 BPM, with evidence of variable decelerations to a nadir of 120 BPM, although with some signal gaps (FIGURE 1, red arrow). The tracing then appeared to have a baseline of 120 BPM with variability or accelerations (FIGURE 1, green arrow) before shifting again to 170 to 180 BPM.
What was happening?

Why does the EFM record the maternal heart rate?
Most commonly, EFM recording of the maternal heart rate occurs during the second stage of labor. Early in labor, the normal fetal heart rate (110–160 BPM) typically exceeds the basal maternal heart rate. However, in the presence of chorioamnionitis and maternal fever or with the stress of maternal pushing, the maternal heart rate frequently approaches or exceeds that of the fetal heart rate. The maximum maternal heart rate can be estimated as 220 BPM minus the maternal age. Thus, the heart rate in a 20-year-old gravida may reach rates of 160 to 180 BPM, equivalent to 80% to 90% of her maximum heart rate during second-stage pushing.
The external Doppler fetal monitor, having a somewhat narrow acoustic window, may lose the focus on the fetal heart as a result of descent of the baby, the abdominal shape-altering effect of uterine contractions, and the patient’s pushing. During the second stage, the EFM may record the maternal heart rate from the uterine arteries. Although some clinicians claim to differentiate the maternal from the fetal heart rate by the “whooshing” maternal uterine artery signal as compared with the “thumping” fetal heart rate signal, this auditory assessment is unproven and likely unreliable.
CASE 1 Problem recognized and addressed
In this case, the obstetrician recognized that “slipping” from the fetal to the maternal heart rate recording occurred with the onset of maternal pushing. After the pushing ceased, the maternal heart rate slipped back to the fetal heart rate. With the next several contractions, only the maternal heart rate was recorded. A fetal scalp electrode was then placed, and fetal variable decelerations were recognized. In view of the category II EFM recording, a vacuum procedure was performed from +3 station and a female infant was delivered. She had Apgar scores of 6 and 8 at 1 and 5 minutes, respectively, and she did well in the nursery.
Read what happened in Case 2 when the EFM demonstrated breaks in the tracing
CASE 2 EFM tracings belie the clinical situation
A 20-year-old woman (G1P0) presented for induction of labor at 41 weeks’ gestation. Continuous EFM recording was initiated, and the patient was given dinoprostone and, subsequently, oxytocin. Rupture of membranes at 3 cm demonstrated a small amount of fluid with thick meconium. The patient progressed to complete dilation and developed a temperature of 38.5°C; the EFM baseline increased to 180 BPM. Throughout the first hour of the second stage of labor, the EFM demonstrated breaks in the tracing and a heart rate of 130 to 150 BPM with each pushing effort (FIGURE 2A). The Doppler monitor was subsequently adjusted to focus on the fetal heart and repetitive late decelerations were observed (FIGURE 2B). An emergent cesarean delivery was performed. A depressed newborn male was delivered, with Apgar scores of 2 and 4 at 1 and 5 minutes, respectively, and significant metabolic acidosis.
What happened?

Fetal versus maternal responses to pushing
The fetal variable deceleration pattern is well recognized by clinicians. As a result of umbilical cord occlusion (due to compression, stretching, or twisting of the cord), fetal variable decelerations have a typical pattern. An initial acceleration shoulder resulting from umbilical vein occlusion (due to reduced venous return) is followed by an umbilical artery occlusion–induced sharp deceleration. The relief of the occlusion allows the sharp return toward baseline with the secondary shoulder overshoot.
In some cases, partial umbilical cord occlusion that affects only the fetal umbilical vein may result in an acceleration, although these usually resolve or evolve into variable decelerations within 30 minutes. By contrast, the maternal heart rate typically increases with contractions and with maternal pushing efforts. Thus, a repetitive pattern of heart rate accelerations with each contraction should warn of a possible maternal heart rate recording.
How maternal heart rate responds to pushing. Maternal pushing is a Valsalva maneuver. Although there are 4 classic cardiovascular phases of Valsalva responses, the typical maternal pushing effort results in an increase in the maternal heart rate. With the common sequence of three 10-second pushes during each contraction, the maternal heart rate often exhibits 3 acceleration and deceleration responses. The maternal heart rate tracing looks similar to the shape of the Three Sisters mountain peaks in Oregon (FIGURE 3). Due to Valsalva physiology, the 3 peaks of the Sisters mirror the 3 uterine wave form peaks, although with a 5- to 10-second delay in the heart rate responses (mountain peaks) from the pushing efforts.

Pre- and postcontraction changes offer clues. Several classic findings aid in differentiating the maternal from the fetal heart rate. If the tracing is maternal, typically the heart rate gradually decreases following the end of the contraction/pushing and continues to decrease until the start of the next contraction/pushing, at which time it increases. During the push, the Three Sisters wave form, with the 5- to 10-second offset, should alert the clinician to possible maternal heart rate recordings. By contrast, the fetal heart rate variable deceleration typically increases following the end of the maternal contraction/pushing and is either stable or increases further (variable with slow recovery) prior to the next uterine contraction/pushing effort. These differences in the patterns of precontraction and postcontraction changes can be very valuable in differentiating periods of maternal versus fetal heart rate recordings.
With “slipping” between fetal and maternal recording, it is not uncommon to record fetal heart rate between contractions, slip to the maternal heart rate during the pushing effort, and return again to the fetal heart rate with the end of the contraction. When confounded with the potential for other EFM artifacts, including doubling of a low maternal or fetal heart rate, or halving of a tachycardic signal, it is not surprising that it is challenging to recognize an EFM maternal heart rate recording.
CASE 2 Check the monitor for accurate focus
A retrospective analysis of this case revealed that the maternal heart rate was recorded with each contraction throughout the second stage. The actual fetal heart rate pattern of decelerations was revealed with the refocusing of the Doppler monitor.
Read how subtle slipping manifested in the EFM tracing of Case 3
CASE 3 Low fetal heart rate and variability during contractions
A 22-year-old woman (G2P1) in spontaneous labor at term progressed to complete dilation. Fetal heart rate accelerations occurred for approximately 30 minutes. With the advent of pushing, the fetal heart rate showed a rate of 130 to 140 BPM and mild decelerations with each contraction (FIGURE 4A). As the second stage progressed, the tracing demonstrated an undulating baseline heart rate between 100 and 130 BPM with possible variability during contractions (FIGURE 4B). This pattern continued for an additional 60 minutes. At vaginal delivery, the ObGyn was surprised to deliver a depressed newborn with Apgar scores of 1 and 3 at 1 and 5 minutes, respectively.

Slipping from the fetal to the maternal heart rate may be imperceptible
In contrast to the breaks in the tracings seen in Case 1 and Case 2, the EFM tracing in Case 3 appears continuous. Yet, slipping from the fetal to the maternal recording was occurring.
As seen in FIGURE 4C, the maternal heart rate with variability was recorded during pushing efforts, and the fetal heart rate was seen rising back toward a baseline between contractions. Note that the fetal heart rate did not reach a level baseline, but rather decelerated with the next contraction. The slipping to the maternal heart rate occurred without a perceptible break in the recording, making this tracing extremely difficult to interpret.
CASE 3 Be ever vigilant
The lack of recognition that the EFM recording had slipped to the maternal heart rate resulted in fetal and newborn hypoxia and acidosis, accounting for the infant’s low Apgar scores.
Read how using 3 steps can help you distinguish fetal from maternal heart rate patterns
Follow 3 steps to discern fetal vs maternal heart rate
These cases illustrate the difficulties in recognizing maternal heart rate patterns on the fetal monitor tracing. The 3 simple steps described below can aid in differentiating maternal from fetal heart rate patterns.
1 Be aware and alert
Recognize that EFM monitoring of the maternal heart rate may occur during periods of monitoring, particularly in second-stage labor. Often, the recorded tracing is a mix of fetal and maternal patterns. Remember that the maternal heart rate may increase markedly during the second stage and rise even higher during pushing efforts. When presented with a tracing that ostensibly represents the fetus, it may be challenging for the clinician to question that assumption. Thus, be aware that tracings may not represent what they seem to be.
Often, clinicians view only the 10-minute portion of the tracing displayed on the monitor screen. I recommend, however, that clinicians review the tracing over the past 30 to 60 minutes, or since their last EFM assessment, for an understanding of the recent fetal baseline heart rate and decelerations.
2 Investigate
Although it is sometimes challenging to recognize EFM maternal heart rate recordings, this is relatively easy to investigate. Even without a pulse oximeter in place, carefully examine the EFM recording for maternal signs to determine if the maternal heart rate is within the range of the recording. You can confirm that the recording is maternal through 1 of 3 easy measures:
- First, check the maternal radial pulse and correlate it with the heart rate baseline.
- Second, place a maternal electrocardiographic (EKG) heart rate monitor.
- Last, and often the simplest approach for continuous tracings, place a finger pulse oximeter to provide a continuous maternal pulse reading. Should the maternal heart rate superimpose on the EFM recording, maternal patterns are likely being detected. However, since the pulse oximeter and EFM Doppler devices use different technologies, they will provide similar—but not precisely identical—heart rate numerical readings if both are assessing the maternal heart rate. In that case, take steps to assure that the EFM truly is recording the fetal heart rate.
3 Treat and correct
If the EFM is recording a maternal signal or if a significant question remains, place a fetal scalp electrode (unless contraindicated), as this may likely occur during the second stage. Alternatively, place a maternal surface fetal EKG monitor, or use ultrasonography to visually assess the fetal heart rate in real time.
Key point summary
The use of a maternal finger pulse oximeter, combined with a careful assessment of the EFM tracing, and/or a fetal scalp electrode are appropriate measures for confirming a fetal heart rate recording.
The 3 steps described (be aware and alert, investigate, treat and correct) can help you effectively monitor the fetal heart rate and avoid the potentially dangerous outcomes that might occur when the maternal heart rate masquerades as the fetal heart rate.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Continuous electronic fetal heart rate monitoring (EFM) is used in the vast majority of all labors in the United States. With the use of EFM categories and definitions from the American College of Obstetricians and Gynecologists, the National Institutes of Health, and the Society for Maternal-Fetal Medicine, clinicians can now better define and communicate tracing assessments. Except for reducing neonatal seizure activity, however, EFM use during labor has not been demonstrated to significantly improve fetal and neonatal outcomes, yet EFM is associated with an increase in cesarean deliveries and instrument-assisted vaginal births.1
The negative predictive value of EFM for fetal hypoxia/acidosis is high, but its positive predictive value is only 30%, and the false-positive rate is as high as 60%.2 Although a false-positive assessment may result in a potentially unnecessary operative vaginal or cesarean delivery, a falsely reassuring strip may produce devastating consequences in the newborn and, not infrequently, medical malpractice liability. One etiology associated with falsely reassuring assessments is that of EFM monitoring of the maternal heart rate and the failure to recognize the tracing as maternal.
In this article, I discuss the mechanisms and periods of labor that often are associated with the maternal heart rate masquerading as the fetal heart rate. I review common EFM patterns associated with the maternal heart rate so as to aid in recognizing the maternal heart rate. In addition, I provide 3 case scenarios that illustrate the simple yet critical steps that clinicians can take to remedy the situation. Being aware of the potential for a maternal heart rate recording, investigating the EFM signals, and correcting the monitoring can help prevent significant morbidity.
CASE 1 EFM shows seesaw decelerations and returns to baseline rate
A 29-year-old woman (G3P2) at 39 weeks’ gestation was admitted to the hospital with spontaneous labor. Continuous EFM external monitoring was initiated. After membranes spontaneously ruptured at 4 cm dilation, an epidural was placed. Throughout the active phase of labor, the fetus demonstrated intermittent mild variable decelerations, and the fetal heart rate baseline increased to 180 beats per minute (BPM). With complete dilation, the patient initiated pushing. During the first several pushes, the EFM demonstrated an initial heart rate deceleration, and a loss of signal, but the heart rate returned to a baseline rate of 150 BPM. With the patient’s continued pushing efforts, the EFM baseline increased to 180 BPM, with evidence of variable decelerations to a nadir of 120 BPM, although with some signal gaps (FIGURE 1, red arrow). The tracing then appeared to have a baseline of 120 BPM with variability or accelerations (FIGURE 1, green arrow) before shifting again to 170 to 180 BPM.
What was happening?

Why does the EFM record the maternal heart rate?
Most commonly, EFM recording of the maternal heart rate occurs during the second stage of labor. Early in labor, the normal fetal heart rate (110–160 BPM) typically exceeds the basal maternal heart rate. However, in the presence of chorioamnionitis and maternal fever or with the stress of maternal pushing, the maternal heart rate frequently approaches or exceeds that of the fetal heart rate. The maximum maternal heart rate can be estimated as 220 BPM minus the maternal age. Thus, the heart rate in a 20-year-old gravida may reach rates of 160 to 180 BPM, equivalent to 80% to 90% of her maximum heart rate during second-stage pushing.
The external Doppler fetal monitor, having a somewhat narrow acoustic window, may lose the focus on the fetal heart as a result of descent of the baby, the abdominal shape-altering effect of uterine contractions, and the patient’s pushing. During the second stage, the EFM may record the maternal heart rate from the uterine arteries. Although some clinicians claim to differentiate the maternal from the fetal heart rate by the “whooshing” maternal uterine artery signal as compared with the “thumping” fetal heart rate signal, this auditory assessment is unproven and likely unreliable.
CASE 1 Problem recognized and addressed
In this case, the obstetrician recognized that “slipping” from the fetal to the maternal heart rate recording occurred with the onset of maternal pushing. After the pushing ceased, the maternal heart rate slipped back to the fetal heart rate. With the next several contractions, only the maternal heart rate was recorded. A fetal scalp electrode was then placed, and fetal variable decelerations were recognized. In view of the category II EFM recording, a vacuum procedure was performed from +3 station and a female infant was delivered. She had Apgar scores of 6 and 8 at 1 and 5 minutes, respectively, and she did well in the nursery.
Read what happened in Case 2 when the EFM demonstrated breaks in the tracing
CASE 2 EFM tracings belie the clinical situation
A 20-year-old woman (G1P0) presented for induction of labor at 41 weeks’ gestation. Continuous EFM recording was initiated, and the patient was given dinoprostone and, subsequently, oxytocin. Rupture of membranes at 3 cm demonstrated a small amount of fluid with thick meconium. The patient progressed to complete dilation and developed a temperature of 38.5°C; the EFM baseline increased to 180 BPM. Throughout the first hour of the second stage of labor, the EFM demonstrated breaks in the tracing and a heart rate of 130 to 150 BPM with each pushing effort (FIGURE 2A). The Doppler monitor was subsequently adjusted to focus on the fetal heart and repetitive late decelerations were observed (FIGURE 2B). An emergent cesarean delivery was performed. A depressed newborn male was delivered, with Apgar scores of 2 and 4 at 1 and 5 minutes, respectively, and significant metabolic acidosis.
What happened?

Fetal versus maternal responses to pushing
The fetal variable deceleration pattern is well recognized by clinicians. As a result of umbilical cord occlusion (due to compression, stretching, or twisting of the cord), fetal variable decelerations have a typical pattern. An initial acceleration shoulder resulting from umbilical vein occlusion (due to reduced venous return) is followed by an umbilical artery occlusion–induced sharp deceleration. The relief of the occlusion allows the sharp return toward baseline with the secondary shoulder overshoot.
In some cases, partial umbilical cord occlusion that affects only the fetal umbilical vein may result in an acceleration, although these usually resolve or evolve into variable decelerations within 30 minutes. By contrast, the maternal heart rate typically increases with contractions and with maternal pushing efforts. Thus, a repetitive pattern of heart rate accelerations with each contraction should warn of a possible maternal heart rate recording.
How maternal heart rate responds to pushing. Maternal pushing is a Valsalva maneuver. Although there are 4 classic cardiovascular phases of Valsalva responses, the typical maternal pushing effort results in an increase in the maternal heart rate. With the common sequence of three 10-second pushes during each contraction, the maternal heart rate often exhibits 3 acceleration and deceleration responses. The maternal heart rate tracing looks similar to the shape of the Three Sisters mountain peaks in Oregon (FIGURE 3). Due to Valsalva physiology, the 3 peaks of the Sisters mirror the 3 uterine wave form peaks, although with a 5- to 10-second delay in the heart rate responses (mountain peaks) from the pushing efforts.

Pre- and postcontraction changes offer clues. Several classic findings aid in differentiating the maternal from the fetal heart rate. If the tracing is maternal, typically the heart rate gradually decreases following the end of the contraction/pushing and continues to decrease until the start of the next contraction/pushing, at which time it increases. During the push, the Three Sisters wave form, with the 5- to 10-second offset, should alert the clinician to possible maternal heart rate recordings. By contrast, the fetal heart rate variable deceleration typically increases following the end of the maternal contraction/pushing and is either stable or increases further (variable with slow recovery) prior to the next uterine contraction/pushing effort. These differences in the patterns of precontraction and postcontraction changes can be very valuable in differentiating periods of maternal versus fetal heart rate recordings.
With “slipping” between fetal and maternal recording, it is not uncommon to record fetal heart rate between contractions, slip to the maternal heart rate during the pushing effort, and return again to the fetal heart rate with the end of the contraction. When confounded with the potential for other EFM artifacts, including doubling of a low maternal or fetal heart rate, or halving of a tachycardic signal, it is not surprising that it is challenging to recognize an EFM maternal heart rate recording.
CASE 2 Check the monitor for accurate focus
A retrospective analysis of this case revealed that the maternal heart rate was recorded with each contraction throughout the second stage. The actual fetal heart rate pattern of decelerations was revealed with the refocusing of the Doppler monitor.
Read how subtle slipping manifested in the EFM tracing of Case 3
CASE 3 Low fetal heart rate and variability during contractions
A 22-year-old woman (G2P1) in spontaneous labor at term progressed to complete dilation. Fetal heart rate accelerations occurred for approximately 30 minutes. With the advent of pushing, the fetal heart rate showed a rate of 130 to 140 BPM and mild decelerations with each contraction (FIGURE 4A). As the second stage progressed, the tracing demonstrated an undulating baseline heart rate between 100 and 130 BPM with possible variability during contractions (FIGURE 4B). This pattern continued for an additional 60 minutes. At vaginal delivery, the ObGyn was surprised to deliver a depressed newborn with Apgar scores of 1 and 3 at 1 and 5 minutes, respectively.

Slipping from the fetal to the maternal heart rate may be imperceptible
In contrast to the breaks in the tracings seen in Case 1 and Case 2, the EFM tracing in Case 3 appears continuous. Yet, slipping from the fetal to the maternal recording was occurring.
As seen in FIGURE 4C, the maternal heart rate with variability was recorded during pushing efforts, and the fetal heart rate was seen rising back toward a baseline between contractions. Note that the fetal heart rate did not reach a level baseline, but rather decelerated with the next contraction. The slipping to the maternal heart rate occurred without a perceptible break in the recording, making this tracing extremely difficult to interpret.
CASE 3 Be ever vigilant
The lack of recognition that the EFM recording had slipped to the maternal heart rate resulted in fetal and newborn hypoxia and acidosis, accounting for the infant’s low Apgar scores.
Read how using 3 steps can help you distinguish fetal from maternal heart rate patterns
Follow 3 steps to discern fetal vs maternal heart rate
These cases illustrate the difficulties in recognizing maternal heart rate patterns on the fetal monitor tracing. The 3 simple steps described below can aid in differentiating maternal from fetal heart rate patterns.
1 Be aware and alert
Recognize that EFM monitoring of the maternal heart rate may occur during periods of monitoring, particularly in second-stage labor. Often, the recorded tracing is a mix of fetal and maternal patterns. Remember that the maternal heart rate may increase markedly during the second stage and rise even higher during pushing efforts. When presented with a tracing that ostensibly represents the fetus, it may be challenging for the clinician to question that assumption. Thus, be aware that tracings may not represent what they seem to be.
Often, clinicians view only the 10-minute portion of the tracing displayed on the monitor screen. I recommend, however, that clinicians review the tracing over the past 30 to 60 minutes, or since their last EFM assessment, for an understanding of the recent fetal baseline heart rate and decelerations.
2 Investigate
Although it is sometimes challenging to recognize EFM maternal heart rate recordings, this is relatively easy to investigate. Even without a pulse oximeter in place, carefully examine the EFM recording for maternal signs to determine if the maternal heart rate is within the range of the recording. You can confirm that the recording is maternal through 1 of 3 easy measures:
- First, check the maternal radial pulse and correlate it with the heart rate baseline.
- Second, place a maternal electrocardiographic (EKG) heart rate monitor.
- Last, and often the simplest approach for continuous tracings, place a finger pulse oximeter to provide a continuous maternal pulse reading. Should the maternal heart rate superimpose on the EFM recording, maternal patterns are likely being detected. However, since the pulse oximeter and EFM Doppler devices use different technologies, they will provide similar—but not precisely identical—heart rate numerical readings if both are assessing the maternal heart rate. In that case, take steps to assure that the EFM truly is recording the fetal heart rate.
3 Treat and correct
If the EFM is recording a maternal signal or if a significant question remains, place a fetal scalp electrode (unless contraindicated), as this may likely occur during the second stage. Alternatively, place a maternal surface fetal EKG monitor, or use ultrasonography to visually assess the fetal heart rate in real time.
Key point summary
The use of a maternal finger pulse oximeter, combined with a careful assessment of the EFM tracing, and/or a fetal scalp electrode are appropriate measures for confirming a fetal heart rate recording.
The 3 steps described (be aware and alert, investigate, treat and correct) can help you effectively monitor the fetal heart rate and avoid the potentially dangerous outcomes that might occur when the maternal heart rate masquerades as the fetal heart rate.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Alfirevic Z, Devane D, Gyte GM, Cuthbert A. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2017; doi:10.1002/14651858.CD006066.pub3.
- Pinas A, Chandraharan E. Continuous cardiotocography during labour: analysis, classification and management. Best Pract Res Clin Obstet Gynaecol. 2016;30:33–47.
- Alfirevic Z, Devane D, Gyte GM, Cuthbert A. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2017; doi:10.1002/14651858.CD006066.pub3.
- Pinas A, Chandraharan E. Continuous cardiotocography during labour: analysis, classification and management. Best Pract Res Clin Obstet Gynaecol. 2016;30:33–47.
2018 Update on infectious disease
In this Update I highlight 5 interesting investigations on infectious diseases. The first addresses the value of applying prophylactically a negative-pressure wound dressing to prevent surgical site infection (SSI) in obese women having cesarean delivery (CD). The second report assesses the effectiveness of a preoperative vaginal wash in reducing the frequency of postcesarean endometritis. The third investigation examines the role of systemic antibiotics, combined with surgical drainage, for patients who have subcutaneous abscesses ranging in size up to 5 cm. The fourth study presents new information about the major risk factors for Clostridium difficile infections in obstetric patients. The final study presents valuable sobering new data about the risks of congenital Zika virus infection.
Negative-pressure wound therapy after CD shows some benefit in preventing SSI
Yu L, Kronen RJ, Simon LE, Stoll CR, Colditz GA, Tuuli MG. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(2):200-210.e1.
Yu and colleagues sought to determine if the prophylactic use of negative-pressure devices, compared with standard wound dressing, was effective in reducing the frequency of SSI after CD.
The authors searched multiple databases and initially identified 161 randomized controlled trials and cohort studies for further assessment. After applying rigorous exclusion criteria, they ultimately selected 9 studies for systematic review and meta-analysis. Six studies were randomized controlled trials (RCTs), 2 were retrospective cohort studies, and 1 was a prospective cohort study. Five studies were considered high quality; 4 were of low quality.
Details of the study
Several types of negative-pressure devices were used, but the 2 most common were the Prevena incision management system (KCI, San Antonio, Texas) and PICO negative- pressure wound therapy (Smith & Nephew, St. Petersburg, Florida). The majority of patients in all groups were at high risk for wound complications because of obesity.
The primary outcome of interest was the frequency of SSI. Secondary outcomes included dehiscence, seroma, endometritis, a composite measure for all wound complications, and hospital readmission.
The absolute risk of SSI in the intervention group was 5% (95% confidence interval [CI], 2.0%-7.0%) compared with 11% (95% CI, 7.0%-16.0%) in the standard dressing group. The pooled risk ratio was 0.45 (95% CI, 0.31-0.66). The absolute risk reduction was 6% (95% CI, -10.0% to -3.0%), and the number needed to treat was 17.
There were no significant differences in the rate of any of the secondary outcomes other than the composite of all wound complications. This difference was largely accounted for by the difference in the rate of SSI.
How negative-pressure devices aid wound healing
Yu and colleagues explained that negative-pressure devices exert their beneficial effects in various ways, including:
- shrinking the wound
- inducing cellular stretch
- removing extracellular fluids
- creating a favorable environment for healing
- promoting angiogenesis and neurogenesis.
Multiple studies in nonobstetric patients have shown that prophylactic use of negative-pressure devices is beneficial in reducing the rate of SSI.1 Yu and colleagues' systematic review and meta-analysis confirms those findings in a high-risk population of women having CD.
Study limitations
Before routinely adopting the use of negative-pressure devices for all women having CD, however, obstetricians should consider the following caveats:
- The investigations included in the study by Yu and colleagues did not consistently distinguish between scheduled versus unscheduled CDs.
- The reports did not systematically consider other major risk factors for wound complications besides obesity, and they did not control for these confounders in the statistical analyses.
- The studies included in the meta-analysis did not provide full descriptions of other measures that might influence the rate of SSIs, such as timing and selection of prophylactic antibiotics, selection of suture material, preoperative skin preparation, and closure techniques for the deep subcutaneous tissue and skin.
- None of the included studies systematically considered the cost-effectiveness of the negative-pressure devices. This is an important consideration given that the acquisition cost of these devices ranges from $200 to $500.
Results of the systematic review and meta-analysis by Yu and colleagues suggest that prophylactic negative-pressure wound therapy in high-risk mostly obese women after CD reduces SSI and overall wound complications. The study's limitations, however, must be kept in mind, and more data are needed. It would be most helpful if a large, well-designed RCT was conducted and included 2 groups with comparable multiple major risk factors for wound complications, and in which all women received the following important interventions2-4:
- removal of hair in the surgical site with a clipper, not a razor
- cleansing of the skin with a chlorhexidine rather than an iodophor solution
- closure of the deep subcutaneous tissue if the total subcutaneous layer exceeds 2 cm in depth
- closure of the skin with suture rather than staples
- administration of antibiotic prophylaxis, ideally with a combination of cefazolin plus azithromycin, prior to the surgical incision.
Read about vaginal cleansing’s effect on post-CD endometritis
Vaginal cleansing before CD lowers risk of postop endometritis
Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(3):527-538.
Caissutti and colleagues aimed to determine if cleansing the vagina with an antiseptic solution prior to surgery reduced the frequency of postcesarean endometritis. They included 16 RCTs (4,837 patients) in their systematic review and meta-analysis. The primary outcome was the frequency of postoperative endometritis.
Details of the study
The studies were conducted in several countries and included patients of various socioeconomic classes. Six trials included only patients having a scheduled CD; 9 included both scheduled and unscheduled cesareans; and 1 included only unscheduled cesareans. In 11 studies, povidone-iodine was the antiseptic solution used. Two trials used chlorhexidine diacetate 0.2%, and 1 used chlorhexidine diacetate 0.4%. One trial used metronidazole 0.5% gel, and another used the antiseptic cetrimide, which is a mixture of different quaternary ammonium salts, including cetrimonium bromide.
In all trials, patients received prophylactic antibiotics. The antibiotics were administered prior to the surgical incision in 6 trials; they were given after the umbilical cord was clamped in 6 trials. In 2 trials, the antibiotics were given at varying times, and in the final 2 trials, the timing of antibiotic administration was not reported. Of note, no trials described the method of placenta removal, a factor of considerable significance in influencing the rate of postoperative endometritis.5,6
Endometritis frequency reduced with vaginal cleansing; benefit greater in certain groups. Overall, in the 15 trials in which vaginal cleansing was compared with placebo or with no treatment, women in the treatment group had a significantly lower rate of endometritis (4.5% compared with 8.8%; relative risk [RR], 0.52; 95% CI, 0.37-0.72). When only women in labor were considered, the frequency of endometritis was 8.1% in the intervention group compared with 13.8% in the control group (RR, 0.52; 95% CI, 0.28-0.97). In the women who were not in labor, the difference in the incidence of endometritis was not statistically significant (3.5% vs 6.6%; RR, 0.62; 95% CI, 0.34-1.15).
In the subgroup analysis of women with ruptured membranes at the time of surgery, the incidence of endometritis was 4.3% in the treatment group compared with 20.1% in the control group (RR, 0.23; 95% CI, 0.10-0.52). In women with intact membranes at the time of surgery, the incidence of endometritis was not significantly reduced in the treatment group.
Interestingly, in the subgroup analysis of the 10 trials that used povidone-iodine, the reduction in the frequency of postcesarean endometritis was statistically significant (2.8% vs 6.3%; RR, 0.42; 95% CI, 0.25-0.71). However, this same protective effect was not observed in the women treated with chlorhexidine. In the 1 trial that directly compared povidone-iodine with chlorhexidine, there was no statistically significant difference in outcome.
Simple intervention, solid benefit
Endometritis is the most common complication following CD. The infection is polymicrobial, with mixed aerobic and anaerobic organisms. The principal risk factors for postcesarean endometritis are low socioeconomic status, extended duration of labor and ruptured membranes, multiple vaginal examinations, internal fetal monitoring, and pre-existing vaginal infections (principally, bacterial vaginosis and group B streptococcal colonization).
Two interventions are clearly of value in reducing the incidence of endometritis: administration of prophylactic antibiotics prior to the surgical incision and removal of the placenta by traction on the cord as opposed to manual extraction.5,6
The assessment by Caissutti and colleagues confirms that a third measure — preoperative vaginal cleansing — also helps reduce the incidence of postcesarean endometritis. The principal benefit is seen in women who have been in labor with ruptured membranes, although certainly it is not harmful in lower-risk patients. The intervention is simple and straightforward: a 30-second vaginal wash with a povidone-iodine solution just prior to surgery.
From my perspective, the interesting unanswered question is why a chlorhexidine solution with low alcohol content was not more effective than povidone-iodine, given that a chlorhexidine abdominal wash is superior to povidone-iodine in preventing wound infection after cesarean delivery.7 Until additional studies confirm the effectiveness of vaginal cleansing with chlorhexidine, I recommend the routine use of the povidone-iodine solution in all women having CD.
Read about management approaches for skin abscesses
Treat smaller skin abscesses with antibiotics after surgical drainage? Yes.
Daum RS, Miller LG, Immergluck L, et al; for the DMID 07-0051 Team. A placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl J Med. 2017;376(26):2545-2555.
For treatment of subcutaneous abscesses that were 5 cm or smaller in diameter, investigators sought to determine if surgical drainage alone was equivalent to surgical drainage plus systemic antibiotics. After their abscess was drained, patients were randomly assigned to receive either clindamycin (300 mg 3 times daily) or trimethoprim-sulfamethoxazole (80 mg/400 mg twice daily) or placebo for 10 days. The primary outcome was clinical cure 7 to 10 days after treatment.
Details of the study
Daum and colleagues enrolled 786 participants (505 adults, 281 children) in the prospective double-blind study. Staphylococcus aureus was isolated from 527 patients (67.0%); methicillin-resistant S aureus (MRSA) was isolated from 388 (49.4%). The cure rate was similar in patients in the clindamycin group (83.1%) and the trimethoprim-sulfamethoxazole group (81.7%), and the cure rate in each antibiotic group was significantly higher than that in the placebo group (68.9%; P<.001 for both comparisons). The difference in treatment effect was specifically limited to patients who had S aureus isolated from their lesions.
Findings at follow-up. At 1 month of follow-up, new infections were less common in the clindamycin group (6.8%) than in the trimethoprim-sulfamethoxazole group (13.5%; P = .03) or the placebo group (12.4%; P = .06). However, the highest frequency of adverse effects occurred in the patients who received clindamycin (21.9% vs 11.1% vs 12.5%). No adverse effects were judged to be serious, and all resolved without sequela.
Controversy remains on antibiotic use after drainage
This study is important for 2 major reasons. First, soft tissue infections are quite commonand can evolve into serious problems, especially when the offending pathogen is MRSA. Second, controversy exists about whether systemic antibiotics are indicated if the subcutaneous abscess is relatively small and is adequately drained. For example, Talan and colleagues demonstrated that, in settings with a high prevalence of MRSA, surgical drainage combined with trimethoprim-sulfamethoxazole (1 double-strength tablet orally twice daily) was superior to drainage plus placebo.8 However, Daum and Gold recently debated the issue of drainage plus antibiotics in a case vignette and reached opposite conclusions.9
In my opinion, this investigation by Daum and colleagues supports a role for consistent use of systemic antibiotics following surgical drainage of clinically significant subcutaneous abscesses that have a 5 cm or smaller diameter. Several oral antibiotics are effective against S aureus, including MRSA.10 These drugs include trimethoprim-sulfamethoxazole (1 double-strength tablet orally twice daily), clindamycin (300-450 mg 3 times daily), doxycycline (100 mg twice daily), and minocycline (200 mg initially, then 100 mg every 12 hours).
Of these drugs, I prefer trimethoprim-sulfamethoxazole, provided that the patient does not have an allergy to sulfonamides. Trimethoprim-sulfamethoxazole is significantly less expensive than the other 3 drugs and usually is better tolerated. In particular, compared with clindamycin, trimethoprim-sulfamethoxazole is less likely to cause antibiotic-associated diarrhea, including Clostridium difficile infection. Trimethoprim-sulfamethoxazole should not be used in the first trimester of pregnancy because of concerns about fetal teratogenicity.
Read how to avoid C difficile infections in pregnant patients
Antibiotic use, common in the obstetric population, raises risk for C difficile infection
Ruiter-Ligeti J, Vincent S, Czuzoj-Shulman N, Abenhaim HA. Risk factors, incidence, and morbidity associated with obstetric Clostridium difficile infection. Obstet Gynecol. 2018;131(2):387-391.
The objective of this investigation was to identify risk factors for Clostridium difficile infection (previously termed pseudomembranous enterocolitis) in obstetric patients. The authors performed a retrospective cohort study using information from a large database maintained by the Agency for Healthcare Research and Quality. This database provides information about inpatient hospital stays in the United States, and it is the largest repository of its kind. It includes data from a sample of 1,000 US hospitals.
Details of the study
Ruiter-Ligeti and colleagues reviewed 13,881,592 births during 1999-2013 and identified 2,757 (0.02%) admissions for delivery complicated by C difficile infection, a rate of 20 admissions per 100,000 deliveries per year (95% CI, 19.13-20.62). The rate of admissions with this diagnosis doubled from 1999 (15 per 100,000) to 2013 (30 per 100,000, P<.001).
Among these obstetric patients, the principal risk factors for C difficile infection were older age, multiple gestation, long-term antibiotic use (not precisely defined), and concurrent diagnosis of inflammatory bowel disease. In addition, patients with pyelonephritis, perineal or cesarean wound infections, or pneumonia also were at increased risk, presumably because those patients required longer courses of broad-spectrum antibiotics.
Of additional note, when compared with women who did not have C difficile infection, patients with infection were more likely to develop a thromboembolic event (38.4 per 1,000), paralytic ileus (58.0 per 1,000), sepsis (46.4 per 1,000), and death (8.0 per 1,000).
Be on guard for C difficile infection in antibiotic-treated obstetric patients
C difficile infection is an uncommon but potentially very serious complication of antibiotic therapy. Given that approximately half of all women admitted for delivery are exposed to antibiotics because of prophylaxis for group B streptococcus infection, prophylaxis for CD, and treatment of chorioamnionitis and puerperal endometritis, clinicians constantly need to be vigilant for this complication.11
Affected patients typically present with frequent loose, watery stools and lower abdominal cramping. In severe cases, blood may be present in the stool, and signs of intestinal distention and even acute peritonitis may be evident. The diagnosis can be established by documenting a positive culture or polymerase chain reaction (PCR) assay for C difficile and a positive cytotoxin assay for toxins A and/or B. In addition, if endoscopy is performed, the characteristic gray membranous plaques can be visualized on the rectal and colonic mucosa.11
Discontinue antibiotic therapy. The first step in managing affected patients is to stop all antibiotics, if possible, or at least the one most likely to be the causative agent of C difficile infection. Patients with relatively mild clinical findings should be treated with oral metronidazole, 500 mg every 8 hours for 10 to 14 days. Patients with severe findings should be treated with oral vancomycin, 500 mg every 6 hours, plus IV metronidazole, 500 mg every 8 hours. The more seriously ill patient must be observed carefully for signs of bowel obstruction, intestinal perforation, peritonitis, and sepsis.
Clearly, clinicians should make every effort to prevent C difficile infection in the first place. The following preventive measures are essential:
- Avoid the use of extremely broad-spectrum antibiotics for prophylaxis for CD.
- When using therapeutic antibiotics, keep the spectrum as narrow as possible, consistent with adequately treating the pathogens causing the infection.
- Administer antibiotics for the shortest time possible, consistent with achieving a clinical cure or providing appropriate prophylaxis for surgical procedures (usually, a maximum of 3 doses).
- If a patient receiving antibiotics experiences more than 3 loose stools in 24 hours, either discontinue all antibiotics or substitute another drug for the most likely offending agent, depending on the clinical situation.
- If, after stopping or changing antibiotics, the clinical findings do not resolve promptly, perform a culture or PCR assay for C difficile and assays for the C difficile toxin. Treat as outlined above if these tests are positive.
Read about pregnancy outcomes and trimester of maternal Zika infection
Danger for birth defects with maternal Zika infection present in all trimesters, but greatest in first
Hoen B, Schaub B, Funk AL, et al. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med. 2018;378(11):985-994.
To estimate the risk of congenital neurologic defects associated with Zika virus infection, Hoen and colleagues conducted a prospective cohort study of pregnant women with symptomatic Zika virus infection who were enrolled during March through November 2016 in French Guiana, Guadeloupe, and Martinique. All women had Zika virus infection confirmed by PCR assay.
Details of the study
The investigators reviewed 546 pregnancies, which resulted in the birth of 555 fetuses and infants. Thirty-nine fetuses and neonates (7%; 95% CI, 5.0-9.5) had neurologic and ocular findings known to be associated with Zika virus infection. Of these, 10 pregnancies were terminated, 1 fetus was stillborn, and 28 were live-born.
Microcephaly (defined as head circumference more than 2 SD below the mean) was present in 32 fetuses and infants (5.8%); 9 had severe microcephaly, defined as head circumference more than 3 SD below the mean. Neurologic and ocular abnormalities were more common when maternal infection occurred during the first trimester (24 of 189 fetuses and infants, 12.7%) compared with infection during the second trimester (9 of 252, 3.6%) or third trimester (6 of 114, 5.3%) (P = .001).
Studies report similar rates of fetal injury
Zika virus infection primarily is caused by a bite from the Aedes aegypti mosquito. The infection also can be transmitted by sexual contact, laboratory accident, and blood transfusion. Eighty percent of infected persons are asymptomatic. In symptomatic patients, the most common clinical manifestations are low-grade fever, a disseminated maculopapular rash, arthralgias, swelling of the hands and feet, and nonpurulent conjunctivitis.
The most ominous manifestation of congenital Zika virus infection is microcephaly. Other important manifestations include lissencephaly, pachygyria, cortical atrophy, ventriculomegaly, subcortical calcifications, ocular abnormalities, and arthrogryposis. Although most of these abnormalities are immediately visible in the neonate, some may not appear until the child is older.
The present study is an excellent complement to 2 recent reports that defined the risk of Zika virus-related fetal injury in patients in the United States and its territories. Based on an analysis of data from the US Zika Pregnancy Registry, Honein and colleagues reported an overall rate of congenital infection of 6%.12 The rate of fetal injury was 11% when the mother was infected in the first trimester and 0% when the infection occurred in the second or third trimester. The overall rate of infection and the first trimester rate of infection were similar to those reported by Hoen and colleagues.
Conversely, Shapiro-Mendoza and colleagues evaluated rates of infection in US territories (American Samoa, Puerto Rico, and the US Virgin Islands) and observed cases of fetal injury associated with second- and third-trimester maternal infection.13 These authors reported an overall rate of infection of 5% and an 8% rate of infection with first-trimester maternal infection. When maternal infection occurred in the second and third trimesters, the rates of fetal injury were 5% and 4%, respectively, figures almost identical to those reported by Hoen and colleagues. Of note, the investigations by Honein and Shapiro-Mendoza included women with both symptomatic and asymptomatic infection.
Taken together, the studies discussed provide 2 clear take-home messages:
- Both symptomatic and asymptomatic maternal infection pose a significant risk of injury to the fetus and neonate.
- Although the risk of fetal injury is greatest when maternal infection occurs in the first trimester, exposure in the second and third trimesters is still dangerous. The Zika virus is quite pathogenic and can cause debilitating injury to the developing fetus at any stage of gestation.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Hyldig N, Birke-Sorensen H, Kruse M, et al. Meta-analysis of negative-pressure wound therapy for closed surgical incisions. Br J Surg. 2016;103(5):477–486.
- Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: evidence-based guidance. OBG Manag. 2016;28(11):41–47.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: 5 more evidence-based measures to consider. OBG Manag. 2016;28(12):18–22.
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176(6):1250–1254.
- Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med. 2016;374(9):823–832.
- Wilbur MB, Daum RS, Gold HS. Skin abscess. N Engl J Med. 2016;374(9): 882–884.
- Singer AJ, Talan DA. Management of skin abscesses in the era of methicillin-resistant Staphylococcus aureus. N Engl J Med. 2014;370(11):1039–1047.
- Unger JA, Whimbey E, Gravett MG, Eschenbach DA. The emergence of Clostridium difficile infection among peripartum women: a case-control study of a C difficile outbreak on an obstetrical service. Infect Dis Obstet Gynecol. 2011;267249. doi:10.1155/2011/267249.
- Honein MA, Dawson AL, Petersen EE, et al; US Zika Pregnancy Registry Collaboration. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA. 2017;317(1):59–68.
- Shapiro-Mendoza CK, Rice ME, Galang RR, et al; Zika Pregnancy and Infant Registries Working Group. Pregnancy outcomes after maternal Zika virus infection during pregnancy — US territories. January 1, 2016-April 25, 2017. MMWR Morb Mortal Wkly Rep. 2017;66(23):615–621.
In this Update I highlight 5 interesting investigations on infectious diseases. The first addresses the value of applying prophylactically a negative-pressure wound dressing to prevent surgical site infection (SSI) in obese women having cesarean delivery (CD). The second report assesses the effectiveness of a preoperative vaginal wash in reducing the frequency of postcesarean endometritis. The third investigation examines the role of systemic antibiotics, combined with surgical drainage, for patients who have subcutaneous abscesses ranging in size up to 5 cm. The fourth study presents new information about the major risk factors for Clostridium difficile infections in obstetric patients. The final study presents valuable sobering new data about the risks of congenital Zika virus infection.
Negative-pressure wound therapy after CD shows some benefit in preventing SSI
Yu L, Kronen RJ, Simon LE, Stoll CR, Colditz GA, Tuuli MG. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(2):200-210.e1.
Yu and colleagues sought to determine if the prophylactic use of negative-pressure devices, compared with standard wound dressing, was effective in reducing the frequency of SSI after CD.
The authors searched multiple databases and initially identified 161 randomized controlled trials and cohort studies for further assessment. After applying rigorous exclusion criteria, they ultimately selected 9 studies for systematic review and meta-analysis. Six studies were randomized controlled trials (RCTs), 2 were retrospective cohort studies, and 1 was a prospective cohort study. Five studies were considered high quality; 4 were of low quality.
Details of the study
Several types of negative-pressure devices were used, but the 2 most common were the Prevena incision management system (KCI, San Antonio, Texas) and PICO negative- pressure wound therapy (Smith & Nephew, St. Petersburg, Florida). The majority of patients in all groups were at high risk for wound complications because of obesity.
The primary outcome of interest was the frequency of SSI. Secondary outcomes included dehiscence, seroma, endometritis, a composite measure for all wound complications, and hospital readmission.
The absolute risk of SSI in the intervention group was 5% (95% confidence interval [CI], 2.0%-7.0%) compared with 11% (95% CI, 7.0%-16.0%) in the standard dressing group. The pooled risk ratio was 0.45 (95% CI, 0.31-0.66). The absolute risk reduction was 6% (95% CI, -10.0% to -3.0%), and the number needed to treat was 17.
There were no significant differences in the rate of any of the secondary outcomes other than the composite of all wound complications. This difference was largely accounted for by the difference in the rate of SSI.

How negative-pressure devices aid wound healing
Yu and colleagues explained that negative-pressure devices exert their beneficial effects in various ways, including:
- shrinking the wound
- inducing cellular stretch
- removing extracellular fluids
- creating a favorable environment for healing
- promoting angiogenesis and neurogenesis.
Multiple studies in nonobstetric patients have shown that prophylactic use of negative-pressure devices is beneficial in reducing the rate of SSI.1 Yu and colleagues' systematic review and meta-analysis confirms those findings in a high-risk population of women having CD.
Study limitations
Before routinely adopting the use of negative-pressure devices for all women having CD, however, obstetricians should consider the following caveats:
- The investigations included in the study by Yu and colleagues did not consistently distinguish between scheduled versus unscheduled CDs.
- The reports did not systematically consider other major risk factors for wound complications besides obesity, and they did not control for these confounders in the statistical analyses.
- The studies included in the meta-analysis did not provide full descriptions of other measures that might influence the rate of SSIs, such as timing and selection of prophylactic antibiotics, selection of suture material, preoperative skin preparation, and closure techniques for the deep subcutaneous tissue and skin.
- None of the included studies systematically considered the cost-effectiveness of the negative-pressure devices. This is an important consideration given that the acquisition cost of these devices ranges from $200 to $500.
Results of the systematic review and meta-analysis by Yu and colleagues suggest that prophylactic negative-pressure wound therapy in high-risk mostly obese women after CD reduces SSI and overall wound complications. The study's limitations, however, must be kept in mind, and more data are needed. It would be most helpful if a large, well-designed RCT was conducted and included 2 groups with comparable multiple major risk factors for wound complications, and in which all women received the following important interventions2-4:
- removal of hair in the surgical site with a clipper, not a razor
- cleansing of the skin with a chlorhexidine rather than an iodophor solution
- closure of the deep subcutaneous tissue if the total subcutaneous layer exceeds 2 cm in depth
- closure of the skin with suture rather than staples
- administration of antibiotic prophylaxis, ideally with a combination of cefazolin plus azithromycin, prior to the surgical incision.
Read about vaginal cleansing’s effect on post-CD endometritis
Vaginal cleansing before CD lowers risk of postop endometritis
Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(3):527-538.
Caissutti and colleagues aimed to determine if cleansing the vagina with an antiseptic solution prior to surgery reduced the frequency of postcesarean endometritis. They included 16 RCTs (4,837 patients) in their systematic review and meta-analysis. The primary outcome was the frequency of postoperative endometritis.
Details of the study
The studies were conducted in several countries and included patients of various socioeconomic classes. Six trials included only patients having a scheduled CD; 9 included both scheduled and unscheduled cesareans; and 1 included only unscheduled cesareans. In 11 studies, povidone-iodine was the antiseptic solution used. Two trials used chlorhexidine diacetate 0.2%, and 1 used chlorhexidine diacetate 0.4%. One trial used metronidazole 0.5% gel, and another used the antiseptic cetrimide, which is a mixture of different quaternary ammonium salts, including cetrimonium bromide.
In all trials, patients received prophylactic antibiotics. The antibiotics were administered prior to the surgical incision in 6 trials; they were given after the umbilical cord was clamped in 6 trials. In 2 trials, the antibiotics were given at varying times, and in the final 2 trials, the timing of antibiotic administration was not reported. Of note, no trials described the method of placenta removal, a factor of considerable significance in influencing the rate of postoperative endometritis.5,6
Endometritis frequency reduced with vaginal cleansing; benefit greater in certain groups. Overall, in the 15 trials in which vaginal cleansing was compared with placebo or with no treatment, women in the treatment group had a significantly lower rate of endometritis (4.5% compared with 8.8%; relative risk [RR], 0.52; 95% CI, 0.37-0.72). When only women in labor were considered, the frequency of endometritis was 8.1% in the intervention group compared with 13.8% in the control group (RR, 0.52; 95% CI, 0.28-0.97). In the women who were not in labor, the difference in the incidence of endometritis was not statistically significant (3.5% vs 6.6%; RR, 0.62; 95% CI, 0.34-1.15).
In the subgroup analysis of women with ruptured membranes at the time of surgery, the incidence of endometritis was 4.3% in the treatment group compared with 20.1% in the control group (RR, 0.23; 95% CI, 0.10-0.52). In women with intact membranes at the time of surgery, the incidence of endometritis was not significantly reduced in the treatment group.
Interestingly, in the subgroup analysis of the 10 trials that used povidone-iodine, the reduction in the frequency of postcesarean endometritis was statistically significant (2.8% vs 6.3%; RR, 0.42; 95% CI, 0.25-0.71). However, this same protective effect was not observed in the women treated with chlorhexidine. In the 1 trial that directly compared povidone-iodine with chlorhexidine, there was no statistically significant difference in outcome.
Simple intervention, solid benefit
Endometritis is the most common complication following CD. The infection is polymicrobial, with mixed aerobic and anaerobic organisms. The principal risk factors for postcesarean endometritis are low socioeconomic status, extended duration of labor and ruptured membranes, multiple vaginal examinations, internal fetal monitoring, and pre-existing vaginal infections (principally, bacterial vaginosis and group B streptococcal colonization).
Two interventions are clearly of value in reducing the incidence of endometritis: administration of prophylactic antibiotics prior to the surgical incision and removal of the placenta by traction on the cord as opposed to manual extraction.5,6
The assessment by Caissutti and colleagues confirms that a third measure — preoperative vaginal cleansing — also helps reduce the incidence of postcesarean endometritis. The principal benefit is seen in women who have been in labor with ruptured membranes, although certainly it is not harmful in lower-risk patients. The intervention is simple and straightforward: a 30-second vaginal wash with a povidone-iodine solution just prior to surgery.
From my perspective, the interesting unanswered question is why a chlorhexidine solution with low alcohol content was not more effective than povidone-iodine, given that a chlorhexidine abdominal wash is superior to povidone-iodine in preventing wound infection after cesarean delivery.7 Until additional studies confirm the effectiveness of vaginal cleansing with chlorhexidine, I recommend the routine use of the povidone-iodine solution in all women having CD.
Read about management approaches for skin abscesses
Treat smaller skin abscesses with antibiotics after surgical drainage? Yes.
Daum RS, Miller LG, Immergluck L, et al; for the DMID 07-0051 Team. A placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl J Med. 2017;376(26):2545-2555.
For treatment of subcutaneous abscesses that were 5 cm or smaller in diameter, investigators sought to determine if surgical drainage alone was equivalent to surgical drainage plus systemic antibiotics. After their abscess was drained, patients were randomly assigned to receive either clindamycin (300 mg 3 times daily) or trimethoprim-sulfamethoxazole (80 mg/400 mg twice daily) or placebo for 10 days. The primary outcome was clinical cure 7 to 10 days after treatment.
Details of the study
Daum and colleagues enrolled 786 participants (505 adults, 281 children) in the prospective double-blind study. Staphylococcus aureus was isolated from 527 patients (67.0%); methicillin-resistant S aureus (MRSA) was isolated from 388 (49.4%). The cure rate was similar in patients in the clindamycin group (83.1%) and the trimethoprim-sulfamethoxazole group (81.7%), and the cure rate in each antibiotic group was significantly higher than that in the placebo group (68.9%; P<.001 for both comparisons). The difference in treatment effect was specifically limited to patients who had S aureus isolated from their lesions.
Findings at follow-up. At 1 month of follow-up, new infections were less common in the clindamycin group (6.8%) than in the trimethoprim-sulfamethoxazole group (13.5%; P = .03) or the placebo group (12.4%; P = .06). However, the highest frequency of adverse effects occurred in the patients who received clindamycin (21.9% vs 11.1% vs 12.5%). No adverse effects were judged to be serious, and all resolved without sequela.
Controversy remains on antibiotic use after drainage
This study is important for 2 major reasons. First, soft tissue infections are quite commonand can evolve into serious problems, especially when the offending pathogen is MRSA. Second, controversy exists about whether systemic antibiotics are indicated if the subcutaneous abscess is relatively small and is adequately drained. For example, Talan and colleagues demonstrated that, in settings with a high prevalence of MRSA, surgical drainage combined with trimethoprim-sulfamethoxazole (1 double-strength tablet orally twice daily) was superior to drainage plus placebo.8 However, Daum and Gold recently debated the issue of drainage plus antibiotics in a case vignette and reached opposite conclusions.9
In my opinion, this investigation by Daum and colleagues supports a role for consistent use of systemic antibiotics following surgical drainage of clinically significant subcutaneous abscesses that have a 5 cm or smaller diameter. Several oral antibiotics are effective against S aureus, including MRSA.10 These drugs include trimethoprim-sulfamethoxazole (1 double-strength tablet orally twice daily), clindamycin (300-450 mg 3 times daily), doxycycline (100 mg twice daily), and minocycline (200 mg initially, then 100 mg every 12 hours).
Of these drugs, I prefer trimethoprim-sulfamethoxazole, provided that the patient does not have an allergy to sulfonamides. Trimethoprim-sulfamethoxazole is significantly less expensive than the other 3 drugs and usually is better tolerated. In particular, compared with clindamycin, trimethoprim-sulfamethoxazole is less likely to cause antibiotic-associated diarrhea, including Clostridium difficile infection. Trimethoprim-sulfamethoxazole should not be used in the first trimester of pregnancy because of concerns about fetal teratogenicity.
Read how to avoid C difficile infections in pregnant patients
Antibiotic use, common in the obstetric population, raises risk for C difficile infection
Ruiter-Ligeti J, Vincent S, Czuzoj-Shulman N, Abenhaim HA. Risk factors, incidence, and morbidity associated with obstetric Clostridium difficile infection. Obstet Gynecol. 2018;131(2):387-391.
The objective of this investigation was to identify risk factors for Clostridium difficile infection (previously termed pseudomembranous enterocolitis) in obstetric patients. The authors performed a retrospective cohort study using information from a large database maintained by the Agency for Healthcare Research and Quality. This database provides information about inpatient hospital stays in the United States, and it is the largest repository of its kind. It includes data from a sample of 1,000 US hospitals.
Details of the study
Ruiter-Ligeti and colleagues reviewed 13,881,592 births during 1999-2013 and identified 2,757 (0.02%) admissions for delivery complicated by C difficile infection, a rate of 20 admissions per 100,000 deliveries per year (95% CI, 19.13-20.62). The rate of admissions with this diagnosis doubled from 1999 (15 per 100,000) to 2013 (30 per 100,000, P<.001).
Among these obstetric patients, the principal risk factors for C difficile infection were older age, multiple gestation, long-term antibiotic use (not precisely defined), and concurrent diagnosis of inflammatory bowel disease. In addition, patients with pyelonephritis, perineal or cesarean wound infections, or pneumonia also were at increased risk, presumably because those patients required longer courses of broad-spectrum antibiotics.
Of additional note, when compared with women who did not have C difficile infection, patients with infection were more likely to develop a thromboembolic event (38.4 per 1,000), paralytic ileus (58.0 per 1,000), sepsis (46.4 per 1,000), and death (8.0 per 1,000).
Be on guard for C difficile infection in antibiotic-treated obstetric patients
C difficile infection is an uncommon but potentially very serious complication of antibiotic therapy. Given that approximately half of all women admitted for delivery are exposed to antibiotics because of prophylaxis for group B streptococcus infection, prophylaxis for CD, and treatment of chorioamnionitis and puerperal endometritis, clinicians constantly need to be vigilant for this complication.11
Affected patients typically present with frequent loose, watery stools and lower abdominal cramping. In severe cases, blood may be present in the stool, and signs of intestinal distention and even acute peritonitis may be evident. The diagnosis can be established by documenting a positive culture or polymerase chain reaction (PCR) assay for C difficile and a positive cytotoxin assay for toxins A and/or B. In addition, if endoscopy is performed, the characteristic gray membranous plaques can be visualized on the rectal and colonic mucosa.11
Discontinue antibiotic therapy. The first step in managing affected patients is to stop all antibiotics, if possible, or at least the one most likely to be the causative agent of C difficile infection. Patients with relatively mild clinical findings should be treated with oral metronidazole, 500 mg every 8 hours for 10 to 14 days. Patients with severe findings should be treated with oral vancomycin, 500 mg every 6 hours, plus IV metronidazole, 500 mg every 8 hours. The more seriously ill patient must be observed carefully for signs of bowel obstruction, intestinal perforation, peritonitis, and sepsis.
Clearly, clinicians should make every effort to prevent C difficile infection in the first place. The following preventive measures are essential:
- Avoid the use of extremely broad-spectrum antibiotics for prophylaxis for CD.
- When using therapeutic antibiotics, keep the spectrum as narrow as possible, consistent with adequately treating the pathogens causing the infection.
- Administer antibiotics for the shortest time possible, consistent with achieving a clinical cure or providing appropriate prophylaxis for surgical procedures (usually, a maximum of 3 doses).
- If a patient receiving antibiotics experiences more than 3 loose stools in 24 hours, either discontinue all antibiotics or substitute another drug for the most likely offending agent, depending on the clinical situation.
- If, after stopping or changing antibiotics, the clinical findings do not resolve promptly, perform a culture or PCR assay for C difficile and assays for the C difficile toxin. Treat as outlined above if these tests are positive.
Read about pregnancy outcomes and trimester of maternal Zika infection
Danger for birth defects with maternal Zika infection present in all trimesters, but greatest in first
Hoen B, Schaub B, Funk AL, et al. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med. 2018;378(11):985-994.
To estimate the risk of congenital neurologic defects associated with Zika virus infection, Hoen and colleagues conducted a prospective cohort study of pregnant women with symptomatic Zika virus infection who were enrolled during March through November 2016 in French Guiana, Guadeloupe, and Martinique. All women had Zika virus infection confirmed by PCR assay.
Details of the study
The investigators reviewed 546 pregnancies, which resulted in the birth of 555 fetuses and infants. Thirty-nine fetuses and neonates (7%; 95% CI, 5.0-9.5) had neurologic and ocular findings known to be associated with Zika virus infection. Of these, 10 pregnancies were terminated, 1 fetus was stillborn, and 28 were live-born.
Microcephaly (defined as head circumference more than 2 SD below the mean) was present in 32 fetuses and infants (5.8%); 9 had severe microcephaly, defined as head circumference more than 3 SD below the mean. Neurologic and ocular abnormalities were more common when maternal infection occurred during the first trimester (24 of 189 fetuses and infants, 12.7%) compared with infection during the second trimester (9 of 252, 3.6%) or third trimester (6 of 114, 5.3%) (P = .001).
Studies report similar rates of fetal injury
Zika virus infection primarily is caused by a bite from the Aedes aegypti mosquito. The infection also can be transmitted by sexual contact, laboratory accident, and blood transfusion. Eighty percent of infected persons are asymptomatic. In symptomatic patients, the most common clinical manifestations are low-grade fever, a disseminated maculopapular rash, arthralgias, swelling of the hands and feet, and nonpurulent conjunctivitis.
The most ominous manifestation of congenital Zika virus infection is microcephaly. Other important manifestations include lissencephaly, pachygyria, cortical atrophy, ventriculomegaly, subcortical calcifications, ocular abnormalities, and arthrogryposis. Although most of these abnormalities are immediately visible in the neonate, some may not appear until the child is older.
The present study is an excellent complement to 2 recent reports that defined the risk of Zika virus-related fetal injury in patients in the United States and its territories. Based on an analysis of data from the US Zika Pregnancy Registry, Honein and colleagues reported an overall rate of congenital infection of 6%.12 The rate of fetal injury was 11% when the mother was infected in the first trimester and 0% when the infection occurred in the second or third trimester. The overall rate of infection and the first trimester rate of infection were similar to those reported by Hoen and colleagues.
Conversely, Shapiro-Mendoza and colleagues evaluated rates of infection in US territories (American Samoa, Puerto Rico, and the US Virgin Islands) and observed cases of fetal injury associated with second- and third-trimester maternal infection.13 These authors reported an overall rate of infection of 5% and an 8% rate of infection with first-trimester maternal infection. When maternal infection occurred in the second and third trimesters, the rates of fetal injury were 5% and 4%, respectively, figures almost identical to those reported by Hoen and colleagues. Of note, the investigations by Honein and Shapiro-Mendoza included women with both symptomatic and asymptomatic infection.
Taken together, the studies discussed provide 2 clear take-home messages:
- Both symptomatic and asymptomatic maternal infection pose a significant risk of injury to the fetus and neonate.
- Although the risk of fetal injury is greatest when maternal infection occurs in the first trimester, exposure in the second and third trimesters is still dangerous. The Zika virus is quite pathogenic and can cause debilitating injury to the developing fetus at any stage of gestation.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this Update I highlight 5 interesting investigations on infectious diseases. The first addresses the value of applying prophylactically a negative-pressure wound dressing to prevent surgical site infection (SSI) in obese women having cesarean delivery (CD). The second report assesses the effectiveness of a preoperative vaginal wash in reducing the frequency of postcesarean endometritis. The third investigation examines the role of systemic antibiotics, combined with surgical drainage, for patients who have subcutaneous abscesses ranging in size up to 5 cm. The fourth study presents new information about the major risk factors for Clostridium difficile infections in obstetric patients. The final study presents valuable sobering new data about the risks of congenital Zika virus infection.
Negative-pressure wound therapy after CD shows some benefit in preventing SSI
Yu L, Kronen RJ, Simon LE, Stoll CR, Colditz GA, Tuuli MG. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(2):200-210.e1.
Yu and colleagues sought to determine if the prophylactic use of negative-pressure devices, compared with standard wound dressing, was effective in reducing the frequency of SSI after CD.
The authors searched multiple databases and initially identified 161 randomized controlled trials and cohort studies for further assessment. After applying rigorous exclusion criteria, they ultimately selected 9 studies for systematic review and meta-analysis. Six studies were randomized controlled trials (RCTs), 2 were retrospective cohort studies, and 1 was a prospective cohort study. Five studies were considered high quality; 4 were of low quality.
Details of the study
Several types of negative-pressure devices were used, but the 2 most common were the Prevena incision management system (KCI, San Antonio, Texas) and PICO negative- pressure wound therapy (Smith & Nephew, St. Petersburg, Florida). The majority of patients in all groups were at high risk for wound complications because of obesity.
The primary outcome of interest was the frequency of SSI. Secondary outcomes included dehiscence, seroma, endometritis, a composite measure for all wound complications, and hospital readmission.
The absolute risk of SSI in the intervention group was 5% (95% confidence interval [CI], 2.0%-7.0%) compared with 11% (95% CI, 7.0%-16.0%) in the standard dressing group. The pooled risk ratio was 0.45 (95% CI, 0.31-0.66). The absolute risk reduction was 6% (95% CI, -10.0% to -3.0%), and the number needed to treat was 17.
There were no significant differences in the rate of any of the secondary outcomes other than the composite of all wound complications. This difference was largely accounted for by the difference in the rate of SSI.

How negative-pressure devices aid wound healing
Yu and colleagues explained that negative-pressure devices exert their beneficial effects in various ways, including:
- shrinking the wound
- inducing cellular stretch
- removing extracellular fluids
- creating a favorable environment for healing
- promoting angiogenesis and neurogenesis.
Multiple studies in nonobstetric patients have shown that prophylactic use of negative-pressure devices is beneficial in reducing the rate of SSI.1 Yu and colleagues' systematic review and meta-analysis confirms those findings in a high-risk population of women having CD.
Study limitations
Before routinely adopting the use of negative-pressure devices for all women having CD, however, obstetricians should consider the following caveats:
- The investigations included in the study by Yu and colleagues did not consistently distinguish between scheduled versus unscheduled CDs.
- The reports did not systematically consider other major risk factors for wound complications besides obesity, and they did not control for these confounders in the statistical analyses.
- The studies included in the meta-analysis did not provide full descriptions of other measures that might influence the rate of SSIs, such as timing and selection of prophylactic antibiotics, selection of suture material, preoperative skin preparation, and closure techniques for the deep subcutaneous tissue and skin.
- None of the included studies systematically considered the cost-effectiveness of the negative-pressure devices. This is an important consideration given that the acquisition cost of these devices ranges from $200 to $500.
Results of the systematic review and meta-analysis by Yu and colleagues suggest that prophylactic negative-pressure wound therapy in high-risk mostly obese women after CD reduces SSI and overall wound complications. The study's limitations, however, must be kept in mind, and more data are needed. It would be most helpful if a large, well-designed RCT was conducted and included 2 groups with comparable multiple major risk factors for wound complications, and in which all women received the following important interventions2-4:
- removal of hair in the surgical site with a clipper, not a razor
- cleansing of the skin with a chlorhexidine rather than an iodophor solution
- closure of the deep subcutaneous tissue if the total subcutaneous layer exceeds 2 cm in depth
- closure of the skin with suture rather than staples
- administration of antibiotic prophylaxis, ideally with a combination of cefazolin plus azithromycin, prior to the surgical incision.
Read about vaginal cleansing’s effect on post-CD endometritis
Vaginal cleansing before CD lowers risk of postop endometritis
Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(3):527-538.
Caissutti and colleagues aimed to determine if cleansing the vagina with an antiseptic solution prior to surgery reduced the frequency of postcesarean endometritis. They included 16 RCTs (4,837 patients) in their systematic review and meta-analysis. The primary outcome was the frequency of postoperative endometritis.
Details of the study
The studies were conducted in several countries and included patients of various socioeconomic classes. Six trials included only patients having a scheduled CD; 9 included both scheduled and unscheduled cesareans; and 1 included only unscheduled cesareans. In 11 studies, povidone-iodine was the antiseptic solution used. Two trials used chlorhexidine diacetate 0.2%, and 1 used chlorhexidine diacetate 0.4%. One trial used metronidazole 0.5% gel, and another used the antiseptic cetrimide, which is a mixture of different quaternary ammonium salts, including cetrimonium bromide.
In all trials, patients received prophylactic antibiotics. The antibiotics were administered prior to the surgical incision in 6 trials; they were given after the umbilical cord was clamped in 6 trials. In 2 trials, the antibiotics were given at varying times, and in the final 2 trials, the timing of antibiotic administration was not reported. Of note, no trials described the method of placenta removal, a factor of considerable significance in influencing the rate of postoperative endometritis.5,6
Endometritis frequency reduced with vaginal cleansing; benefit greater in certain groups. Overall, in the 15 trials in which vaginal cleansing was compared with placebo or with no treatment, women in the treatment group had a significantly lower rate of endometritis (4.5% compared with 8.8%; relative risk [RR], 0.52; 95% CI, 0.37-0.72). When only women in labor were considered, the frequency of endometritis was 8.1% in the intervention group compared with 13.8% in the control group (RR, 0.52; 95% CI, 0.28-0.97). In the women who were not in labor, the difference in the incidence of endometritis was not statistically significant (3.5% vs 6.6%; RR, 0.62; 95% CI, 0.34-1.15).
In the subgroup analysis of women with ruptured membranes at the time of surgery, the incidence of endometritis was 4.3% in the treatment group compared with 20.1% in the control group (RR, 0.23; 95% CI, 0.10-0.52). In women with intact membranes at the time of surgery, the incidence of endometritis was not significantly reduced in the treatment group.
Interestingly, in the subgroup analysis of the 10 trials that used povidone-iodine, the reduction in the frequency of postcesarean endometritis was statistically significant (2.8% vs 6.3%; RR, 0.42; 95% CI, 0.25-0.71). However, this same protective effect was not observed in the women treated with chlorhexidine. In the 1 trial that directly compared povidone-iodine with chlorhexidine, there was no statistically significant difference in outcome.
Simple intervention, solid benefit
Endometritis is the most common complication following CD. The infection is polymicrobial, with mixed aerobic and anaerobic organisms. The principal risk factors for postcesarean endometritis are low socioeconomic status, extended duration of labor and ruptured membranes, multiple vaginal examinations, internal fetal monitoring, and pre-existing vaginal infections (principally, bacterial vaginosis and group B streptococcal colonization).
Two interventions are clearly of value in reducing the incidence of endometritis: administration of prophylactic antibiotics prior to the surgical incision and removal of the placenta by traction on the cord as opposed to manual extraction.5,6
The assessment by Caissutti and colleagues confirms that a third measure — preoperative vaginal cleansing — also helps reduce the incidence of postcesarean endometritis. The principal benefit is seen in women who have been in labor with ruptured membranes, although certainly it is not harmful in lower-risk patients. The intervention is simple and straightforward: a 30-second vaginal wash with a povidone-iodine solution just prior to surgery.
From my perspective, the interesting unanswered question is why a chlorhexidine solution with low alcohol content was not more effective than povidone-iodine, given that a chlorhexidine abdominal wash is superior to povidone-iodine in preventing wound infection after cesarean delivery.7 Until additional studies confirm the effectiveness of vaginal cleansing with chlorhexidine, I recommend the routine use of the povidone-iodine solution in all women having CD.
Read about management approaches for skin abscesses
Treat smaller skin abscesses with antibiotics after surgical drainage? Yes.
Daum RS, Miller LG, Immergluck L, et al; for the DMID 07-0051 Team. A placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl J Med. 2017;376(26):2545-2555.
For treatment of subcutaneous abscesses that were 5 cm or smaller in diameter, investigators sought to determine if surgical drainage alone was equivalent to surgical drainage plus systemic antibiotics. After their abscess was drained, patients were randomly assigned to receive either clindamycin (300 mg 3 times daily) or trimethoprim-sulfamethoxazole (80 mg/400 mg twice daily) or placebo for 10 days. The primary outcome was clinical cure 7 to 10 days after treatment.
Details of the study
Daum and colleagues enrolled 786 participants (505 adults, 281 children) in the prospective double-blind study. Staphylococcus aureus was isolated from 527 patients (67.0%); methicillin-resistant S aureus (MRSA) was isolated from 388 (49.4%). The cure rate was similar in patients in the clindamycin group (83.1%) and the trimethoprim-sulfamethoxazole group (81.7%), and the cure rate in each antibiotic group was significantly higher than that in the placebo group (68.9%; P<.001 for both comparisons). The difference in treatment effect was specifically limited to patients who had S aureus isolated from their lesions.
Findings at follow-up. At 1 month of follow-up, new infections were less common in the clindamycin group (6.8%) than in the trimethoprim-sulfamethoxazole group (13.5%; P = .03) or the placebo group (12.4%; P = .06). However, the highest frequency of adverse effects occurred in the patients who received clindamycin (21.9% vs 11.1% vs 12.5%). No adverse effects were judged to be serious, and all resolved without sequela.
Controversy remains on antibiotic use after drainage
This study is important for 2 major reasons. First, soft tissue infections are quite commonand can evolve into serious problems, especially when the offending pathogen is MRSA. Second, controversy exists about whether systemic antibiotics are indicated if the subcutaneous abscess is relatively small and is adequately drained. For example, Talan and colleagues demonstrated that, in settings with a high prevalence of MRSA, surgical drainage combined with trimethoprim-sulfamethoxazole (1 double-strength tablet orally twice daily) was superior to drainage plus placebo.8 However, Daum and Gold recently debated the issue of drainage plus antibiotics in a case vignette and reached opposite conclusions.9
In my opinion, this investigation by Daum and colleagues supports a role for consistent use of systemic antibiotics following surgical drainage of clinically significant subcutaneous abscesses that have a 5 cm or smaller diameter. Several oral antibiotics are effective against S aureus, including MRSA.10 These drugs include trimethoprim-sulfamethoxazole (1 double-strength tablet orally twice daily), clindamycin (300-450 mg 3 times daily), doxycycline (100 mg twice daily), and minocycline (200 mg initially, then 100 mg every 12 hours).
Of these drugs, I prefer trimethoprim-sulfamethoxazole, provided that the patient does not have an allergy to sulfonamides. Trimethoprim-sulfamethoxazole is significantly less expensive than the other 3 drugs and usually is better tolerated. In particular, compared with clindamycin, trimethoprim-sulfamethoxazole is less likely to cause antibiotic-associated diarrhea, including Clostridium difficile infection. Trimethoprim-sulfamethoxazole should not be used in the first trimester of pregnancy because of concerns about fetal teratogenicity.
Read how to avoid C difficile infections in pregnant patients
Antibiotic use, common in the obstetric population, raises risk for C difficile infection
Ruiter-Ligeti J, Vincent S, Czuzoj-Shulman N, Abenhaim HA. Risk factors, incidence, and morbidity associated with obstetric Clostridium difficile infection. Obstet Gynecol. 2018;131(2):387-391.
The objective of this investigation was to identify risk factors for Clostridium difficile infection (previously termed pseudomembranous enterocolitis) in obstetric patients. The authors performed a retrospective cohort study using information from a large database maintained by the Agency for Healthcare Research and Quality. This database provides information about inpatient hospital stays in the United States, and it is the largest repository of its kind. It includes data from a sample of 1,000 US hospitals.
Details of the study
Ruiter-Ligeti and colleagues reviewed 13,881,592 births during 1999-2013 and identified 2,757 (0.02%) admissions for delivery complicated by C difficile infection, a rate of 20 admissions per 100,000 deliveries per year (95% CI, 19.13-20.62). The rate of admissions with this diagnosis doubled from 1999 (15 per 100,000) to 2013 (30 per 100,000, P<.001).
Among these obstetric patients, the principal risk factors for C difficile infection were older age, multiple gestation, long-term antibiotic use (not precisely defined), and concurrent diagnosis of inflammatory bowel disease. In addition, patients with pyelonephritis, perineal or cesarean wound infections, or pneumonia also were at increased risk, presumably because those patients required longer courses of broad-spectrum antibiotics.
Of additional note, when compared with women who did not have C difficile infection, patients with infection were more likely to develop a thromboembolic event (38.4 per 1,000), paralytic ileus (58.0 per 1,000), sepsis (46.4 per 1,000), and death (8.0 per 1,000).
Be on guard for C difficile infection in antibiotic-treated obstetric patients
C difficile infection is an uncommon but potentially very serious complication of antibiotic therapy. Given that approximately half of all women admitted for delivery are exposed to antibiotics because of prophylaxis for group B streptococcus infection, prophylaxis for CD, and treatment of chorioamnionitis and puerperal endometritis, clinicians constantly need to be vigilant for this complication.11
Affected patients typically present with frequent loose, watery stools and lower abdominal cramping. In severe cases, blood may be present in the stool, and signs of intestinal distention and even acute peritonitis may be evident. The diagnosis can be established by documenting a positive culture or polymerase chain reaction (PCR) assay for C difficile and a positive cytotoxin assay for toxins A and/or B. In addition, if endoscopy is performed, the characteristic gray membranous plaques can be visualized on the rectal and colonic mucosa.11
Discontinue antibiotic therapy. The first step in managing affected patients is to stop all antibiotics, if possible, or at least the one most likely to be the causative agent of C difficile infection. Patients with relatively mild clinical findings should be treated with oral metronidazole, 500 mg every 8 hours for 10 to 14 days. Patients with severe findings should be treated with oral vancomycin, 500 mg every 6 hours, plus IV metronidazole, 500 mg every 8 hours. The more seriously ill patient must be observed carefully for signs of bowel obstruction, intestinal perforation, peritonitis, and sepsis.
Clearly, clinicians should make every effort to prevent C difficile infection in the first place. The following preventive measures are essential:
- Avoid the use of extremely broad-spectrum antibiotics for prophylaxis for CD.
- When using therapeutic antibiotics, keep the spectrum as narrow as possible, consistent with adequately treating the pathogens causing the infection.
- Administer antibiotics for the shortest time possible, consistent with achieving a clinical cure or providing appropriate prophylaxis for surgical procedures (usually, a maximum of 3 doses).
- If a patient receiving antibiotics experiences more than 3 loose stools in 24 hours, either discontinue all antibiotics or substitute another drug for the most likely offending agent, depending on the clinical situation.
- If, after stopping or changing antibiotics, the clinical findings do not resolve promptly, perform a culture or PCR assay for C difficile and assays for the C difficile toxin. Treat as outlined above if these tests are positive.
Read about pregnancy outcomes and trimester of maternal Zika infection
Danger for birth defects with maternal Zika infection present in all trimesters, but greatest in first
Hoen B, Schaub B, Funk AL, et al. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med. 2018;378(11):985-994.
To estimate the risk of congenital neurologic defects associated with Zika virus infection, Hoen and colleagues conducted a prospective cohort study of pregnant women with symptomatic Zika virus infection who were enrolled during March through November 2016 in French Guiana, Guadeloupe, and Martinique. All women had Zika virus infection confirmed by PCR assay.
Details of the study
The investigators reviewed 546 pregnancies, which resulted in the birth of 555 fetuses and infants. Thirty-nine fetuses and neonates (7%; 95% CI, 5.0-9.5) had neurologic and ocular findings known to be associated with Zika virus infection. Of these, 10 pregnancies were terminated, 1 fetus was stillborn, and 28 were live-born.
Microcephaly (defined as head circumference more than 2 SD below the mean) was present in 32 fetuses and infants (5.8%); 9 had severe microcephaly, defined as head circumference more than 3 SD below the mean. Neurologic and ocular abnormalities were more common when maternal infection occurred during the first trimester (24 of 189 fetuses and infants, 12.7%) compared with infection during the second trimester (9 of 252, 3.6%) or third trimester (6 of 114, 5.3%) (P = .001).
Studies report similar rates of fetal injury
Zika virus infection primarily is caused by a bite from the Aedes aegypti mosquito. The infection also can be transmitted by sexual contact, laboratory accident, and blood transfusion. Eighty percent of infected persons are asymptomatic. In symptomatic patients, the most common clinical manifestations are low-grade fever, a disseminated maculopapular rash, arthralgias, swelling of the hands and feet, and nonpurulent conjunctivitis.
The most ominous manifestation of congenital Zika virus infection is microcephaly. Other important manifestations include lissencephaly, pachygyria, cortical atrophy, ventriculomegaly, subcortical calcifications, ocular abnormalities, and arthrogryposis. Although most of these abnormalities are immediately visible in the neonate, some may not appear until the child is older.
The present study is an excellent complement to 2 recent reports that defined the risk of Zika virus-related fetal injury in patients in the United States and its territories. Based on an analysis of data from the US Zika Pregnancy Registry, Honein and colleagues reported an overall rate of congenital infection of 6%.12 The rate of fetal injury was 11% when the mother was infected in the first trimester and 0% when the infection occurred in the second or third trimester. The overall rate of infection and the first trimester rate of infection were similar to those reported by Hoen and colleagues.
Conversely, Shapiro-Mendoza and colleagues evaluated rates of infection in US territories (American Samoa, Puerto Rico, and the US Virgin Islands) and observed cases of fetal injury associated with second- and third-trimester maternal infection.13 These authors reported an overall rate of infection of 5% and an 8% rate of infection with first-trimester maternal infection. When maternal infection occurred in the second and third trimesters, the rates of fetal injury were 5% and 4%, respectively, figures almost identical to those reported by Hoen and colleagues. Of note, the investigations by Honein and Shapiro-Mendoza included women with both symptomatic and asymptomatic infection.
Taken together, the studies discussed provide 2 clear take-home messages:
- Both symptomatic and asymptomatic maternal infection pose a significant risk of injury to the fetus and neonate.
- Although the risk of fetal injury is greatest when maternal infection occurs in the first trimester, exposure in the second and third trimesters is still dangerous. The Zika virus is quite pathogenic and can cause debilitating injury to the developing fetus at any stage of gestation.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Hyldig N, Birke-Sorensen H, Kruse M, et al. Meta-analysis of negative-pressure wound therapy for closed surgical incisions. Br J Surg. 2016;103(5):477–486.
- Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: evidence-based guidance. OBG Manag. 2016;28(11):41–47.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: 5 more evidence-based measures to consider. OBG Manag. 2016;28(12):18–22.
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176(6):1250–1254.
- Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med. 2016;374(9):823–832.
- Wilbur MB, Daum RS, Gold HS. Skin abscess. N Engl J Med. 2016;374(9): 882–884.
- Singer AJ, Talan DA. Management of skin abscesses in the era of methicillin-resistant Staphylococcus aureus. N Engl J Med. 2014;370(11):1039–1047.
- Unger JA, Whimbey E, Gravett MG, Eschenbach DA. The emergence of Clostridium difficile infection among peripartum women: a case-control study of a C difficile outbreak on an obstetrical service. Infect Dis Obstet Gynecol. 2011;267249. doi:10.1155/2011/267249.
- Honein MA, Dawson AL, Petersen EE, et al; US Zika Pregnancy Registry Collaboration. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA. 2017;317(1):59–68.
- Shapiro-Mendoza CK, Rice ME, Galang RR, et al; Zika Pregnancy and Infant Registries Working Group. Pregnancy outcomes after maternal Zika virus infection during pregnancy — US territories. January 1, 2016-April 25, 2017. MMWR Morb Mortal Wkly Rep. 2017;66(23):615–621.
- Hyldig N, Birke-Sorensen H, Kruse M, et al. Meta-analysis of negative-pressure wound therapy for closed surgical incisions. Br J Surg. 2016;103(5):477–486.
- Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: evidence-based guidance. OBG Manag. 2016;28(11):41–47.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: 5 more evidence-based measures to consider. OBG Manag. 2016;28(12):18–22.
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176(6):1250–1254.
- Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med. 2016;374(9):823–832.
- Wilbur MB, Daum RS, Gold HS. Skin abscess. N Engl J Med. 2016;374(9): 882–884.
- Singer AJ, Talan DA. Management of skin abscesses in the era of methicillin-resistant Staphylococcus aureus. N Engl J Med. 2014;370(11):1039–1047.
- Unger JA, Whimbey E, Gravett MG, Eschenbach DA. The emergence of Clostridium difficile infection among peripartum women: a case-control study of a C difficile outbreak on an obstetrical service. Infect Dis Obstet Gynecol. 2011;267249. doi:10.1155/2011/267249.
- Honein MA, Dawson AL, Petersen EE, et al; US Zika Pregnancy Registry Collaboration. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA. 2017;317(1):59–68.
- Shapiro-Mendoza CK, Rice ME, Galang RR, et al; Zika Pregnancy and Infant Registries Working Group. Pregnancy outcomes after maternal Zika virus infection during pregnancy — US territories. January 1, 2016-April 25, 2017. MMWR Morb Mortal Wkly Rep. 2017;66(23):615–621.
IN THIS ARTICLE
- Vaginal cleansing before CD lowers risk of postop endometritis
- Treat smaller skin abscesses with antibiotics after surgical drainage? Yes.
- Antibiotic use, common in the obstetric population, raises risk for C difficile infection
- Danger for birth defects with maternal Zika infection present in all trimesters, but greatest in first
Human trafficking: How ObGyns can—and should—be helping survivors

Despite increasing media coverage of human trafficking and the gravity of its many ramifications, I am struck by how often trainees and other clinicians present to me patients for which trafficking is a real potential concern—yet who give me a blank expression when I ask if anyone has screened these patients for being victims of trafficking. I suspect that few of us anticipated, during medical training, that we would be providing care to women who are enslaved.
How large is the problem?
It is impossible to comprehend the true scope of human trafficking. Estimates are that 20.9 million men, women, and children globally are forced into work that they are not free to leave.1
Although human trafficking is recognized as a global phenomenon, its prevalence in the United States is significant enough that it should prompt the health care community to engage in helping identify and assist victims/survivors: From January until June of 2017, the National Human Trafficking Hotline received 13,807 telephone calls, resulting in reporting of 4,460 cases.2 Indeed, from 2015 to 2016 there was a 35.7% increase in the number of hotline cases reported, for a total of 7,572 (6,340—more than 80%—of which regarded females). California had the most cases reported (1,323), followed by Texas (670) and Florida (550); those 3 states also reported an increase in trafficking crime. Vermont (5), Rhode Island (9), and Alaska (10) reported the fewest calls.3
How is trafficking defined?
The United Nations Office on Drugs and Crime defines “trafficking in persons” as:
… recruitment, transportation, transfer, harbouring or receipt of persons, by means of the threat or use of force or other forms of coercion, of abduction, of fraud, of deception, of the abuse of power or of a position of vulnerability or of the giving or receiving of payments or benefits to achieve the consent of a person having control over another person, for the purpose of exploitation. Exploitation shall include, at a minimum, the exploitation of the prostitution of others or other forms of sexual exploitation, forced labour or services, slavery or practices similar to slavery, servitude or the removal of organs.4
Traffickers prey on potentially vulnerable people. Girls and young women who have experienced poverty, homelessness, childhood sexual abuse, substance abuse, gender nonconformity, mental illness, or developmental delay are at particular risk.5 Children who have had interactions with Child Protective Services, come from a dysfunctional family, or have lived in a community with high crime, political or social unrest, corruption, or gender bias and discrimination are also at increased risk.6
Read about clues that raise clinical suspicion
Clues that raise clinical suspicion
A number of potential signs should make providers suspicious about potential human trafficking. Some of those signs are similar to the red flags we see in intimate partner violence, such as:
- having a difficult time talking to the patient alone
- having the accompanying person answer the patient’s questions
- body language that suggests fear, anxiety, or distrust (eg, shifting positions, looking away, appearing withdrawn)
- physical examination inconsistent with the history
- physical injury (especially multiple injuries or injuries in various stages of healing)
- refusal of interpreter services.
Trafficked girls or women may appear overly familiar with sex, have unexpected material possessions, or appear to be giving scripted or memorized answers to queries.7 Traffickers often confiscate their victims’ personal identification. They try to prevent victims from knowing their geographic locales: Patients might not have any documentation or awareness of exact surroundings (eg, their home address). Patients may be wearing clothes considered inappropriate for the weather or venue. They may have tattoos that are marks of branding.8
Medical consequences of being trafficked are obvious, numerous, and serious
Many medical sequelae that result from trafficking are obvious, given the nature of work that victims are forced to do. For example, overcrowding can lead to infectious disease, such as tuberculosis.9 Inadequate access to preventive or basic medical services can result in weight loss, poor dentition, and untreated chronic medical conditions.
If victims are experiencing physical or sexual abuse, they can present with evidence of blunt trauma, ligature marks, skin burns, wounds inflicted by weapons, and vaginal lacerations.10 A study found that 63% of survivors reported at least 10 somatic symptoms, including headache, fatigue, dizziness, back pain, abdominal or pelvic pain, memory loss, and symptoms of genital infectious disease.11
Girls and women being trafficked for sex may experience many of the sequelae of unprotected intercourse: irregular bleeding, unintended pregnancy, unwanted or unsafe pregnancy termination, vaginal trauma, and sexually transmitted infection (STI).12 In a study of trafficking survivors, 38% were HIV-positive.13
Trafficking survivors can suffer myriad mental health conditions, with high rates of depression, anxiety, posttraumatic stress, and suicidal ideation.14 A study of 387 survivors found that 12% had attempted to harm themselves or commit suicide the month before they were interviewed.15
Substance abuse is also a common problem among trafficking victims.16 One survivor interviewed in a recent study said:
It was much more difficult to work sober because I was dealing with emotions or the pain that I was feeling during intercourse, because when you have sex with people 8, 9, 10 times a day, even more than that, it starts to hurt a lot. And being high made it easier to deal with that and also it made it easier for me to get away from my body while it was happening, place my brain somewhere else.17
Because of the substantial risk of mental health problems, including substance abuse, among trafficking survivors, the physical exam of a patient should include careful assessment of demeanor and mental health status. Of course, comprehensive inspection for signs of physical or blunt trauma is paramount.
Read about Patient and staff safety during the visit
Patient and staff safety during the visit
Providers should be aware of potential safety concerns, both for the patient and for the staff. Creative strategies should be utilized to screen the patient in private. The use of interpreter services—either in person or over the telephone—should be presented and facilitated as being a routine part of practice. Any person who accompanies the patient should be asked to leave the examining room, either as a statement of practice routine or under the guise of having him (or her) step out to obtain paperwork or provide documentation.
Care of victims
Trauma-informed care should be a guiding principle for trafficking survivors. This involves empowering the patient, who may feel victimized again if asked to undress and undergo multiple physical examinations. Macias-Konstantopoulos noted: “A trauma-informed approach to care acknowledges the pervasiveness and effect of trauma across the life span of the individual, recognizes the vulnerabilities and emotional triggers of trauma survivors, minimizes repeated traumatization and fosters physical, psychological, and emotional safety, recovery, health and well-being.”18
The patient should be counseled that she has control over her body and can guide different aspects of the examination. For example the provider should discuss: 1) the amount of clothing deemed optimal for an examination, 2) the availability of a support person during the exam (for instance, a nurse or a social worker) if the patient requests one, and 3) utilization of whatever strategies the patient deems optimal for her to be most comfortable during the exam (such as leaving the door slightly ajar or having a mutually agreed-on signal to interrupt the exam).
Routine health care maintenance should be offered, including an assessment of overall physical and dental health and screening for STI and mental health. Screening for substances of abuse should be considered. If indicated, emergency contraception, postexposure HIV prophylaxis, immunizations, and empiric antibiotics for STI should be offered.19
Screening when indicated by evidence, suspicion, or concern
Unlike the case with intimate partner violence, experts do not recommend universal screening for human trafficking. Clinicians should be comfortable, however, trying to elicit that history when a concern arises, either because of identified risk factors, red flags, or concerns that arise from the findings of the history or physical. Ideally, clinicians should consider becoming comfortable choosing a few screening questions to regularly incorporate into their assessment. The US Department of Health & Human Services (HHS) offers a list of questions that can be utilized (TABLE).20

In January 2018, the Office on Trafficking in Persons, a unit of the HHS Administration for Children and Families, released an “Adult Human Trafficking Screening Tool and Guide.” The document includes 2 excellent tools21 that clinicians can utilize to identify patients who should be screened and how to identify and assist survivors (FIGURE 1 and FIGURE 2).

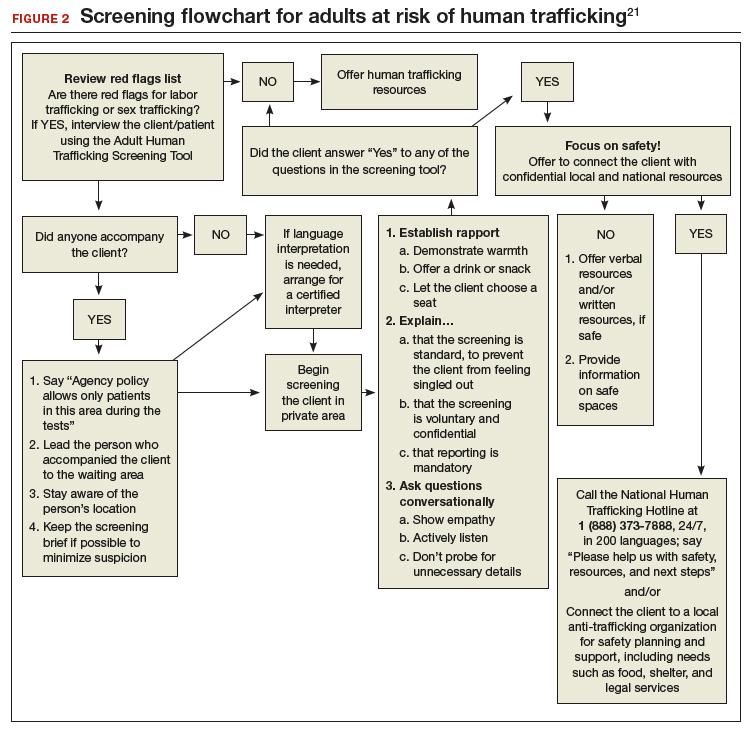
Clinicians, in their encounters with patients, are particularly well-positioned to intersect with, and identify, survivors. Regrettably, such opportunities are often missed—and victims thus remain unidentified and trapped in their circumstances. A study revealed that one-half of survivors who were interviewed reported seeing a physician while they were being trafficked.22 Even more alarming, another study showed that 87.8% of survivors had received health care during their captivity.23 It is dismaying to know that these patients left those health care settings without receiving the assistance they truly need and with their true circumstances remaining unidentified.
Read about Finding assistance and support
Finding assistance and support
Centers in the United States now provide trauma-informed care for trafficking survivors in a confidential setting (see “Specialized care is increasingly available”).24 A physician who works at a center in New York City noted: “Our survivors told us that more than fear or pain, the feelings that sat with them most often were worthlessness and invisibility. We can do better as physicians and as educators to expose this epidemic and care for its victims.”24
Here is a sampling of the growing number of centers in the United States that provide trauma-centered care for survivors of human trafficking:
- Survivor Clinic at New York Presbyterian Hospital-Weill Cornell Medical College, New York, New York
- EMPOWER Clinic for Survivors of Sex Trafficking and Sexual Violence at NYU Langone Health, New York, New York
- Freedom Clinic at Massachusetts General Hospital, Boston
- The Hope Through Health Clinic, Austin, Texas
- Pacific Survivor Center, Honolulu, Hawaii
Most clinicians practice in settings that do not have easy access to such subspecialized centers, however. For them, the National Human Trafficking Hotline can be an invaluable resource (see “Hotline is a valuable resource”).25 Law enforcement and social services colleagues also can be useful allies.
Uncertain how you can help a patient who is a victim of human trafficking? For assistance and support, contact the National Human Trafficking Hotline--24 hours a day, 7 days a week, and in 200 languages--in any of 3 ways:
- By telephone: (888) 373-7888
- By text: 233733
- On the web: https://humantraffickinghotline.orga
aIncludes a search field that clinicians can use to look up the nearest resources for additional assistance.
Let’s turn our concern and awareness into results
We, as providers of women’s health care, are uniquely positioned to help these most vulnerable of people, many of whom have been stripped of personal documents and denied access to financial resources and community support. As a medical community, we should strive to combat this tragic epidemic, 1 patient at a time.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- International Labour Organization. New ILO Global Estimate of Forced Labour: 20.9 million victims. http://www.ilo.org/global/about-the-ilo/newsroom/news/WCMS_182109/lang--en/index.htm. Published June 2012. Accessed May 30, 2018.
- National Human Trafficking Hotline. Hotline statistics. https://humantraffickinghotline.org/states. Accessed May 30, 2018.
- Cone A. Report: Human trafficking in U.S. rose 35.7 percent in one year. United Press International (UPI). https://www.upi.com/Report-Human-trafficking-in-US-rose-357-percent-in-one-year/5571486328579. Published February 5, 2017. Accessed May 30, 2018.
- United Nations Office on Drugs and Crime. Human trafficking. http://www.unodc.org/unodc/en/human-trafficking/what-is-human-trafficking.html. Accessed May 30, 2018.
- Risk factors for and consequences of commercial sexual exploitation and sex trafficking of minors. In Clayton E, Krugman R, Simon P, eds; Committee on the Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States; Board on Children, Youth, and Families; Committee on Law and Justice; Institute of Medicine; National Research Council. Confronting Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States. Washington, DC: The National Academies Press; 2013.
- Greenbaum J, Crawford-Jakubiak JE. Committee on Child Abuse and Neglect. Child sex trafficking and commercial sexual exploitation: health care needs of victims. Pediatrics. 2015:135(3);566–574.
- Alpert E, Ahn R, Albright E, Purcell G, Burke T, Macias-Konstantanopoulos W. Human Trafficking: Guidebook on Identification, Assessment, and Response in the Health Care Setting. Waltham, MA: Massachusetts General Hospital and Massachusetts Medical Society; 2014. http://www.massmed.org/Patient-Care/Health-Topics/Violence-Prevention-and-Intervention/Human-Trafficking-(pdf). Accessed May 30, 2018.
- National Human Trafficking Training and Technical Assistance Center. Adult human trafficking screening tool and guide. http://www.acf.hhs.gov/sites/default/files/otip/adult_human_trafficking_screening_tool_and_guide.pdf. Published January 2018. Accessed May 30, 2018.
- Steele S. Human trafficking, labor brokering, and mining in southern Africa: responding to a decentralized and hidden public health disaster. Int J Health Serv. 2013;43(4):665–680.
- Becker HJ, Bechtel K. Recognizing victims of human trafficking in the pediatric emergency department. Pediatr Emerg Care. 2015;31(2):144–147.
- Zimmerman C, Hossain M, Yun K, et al. The health of trafficked women: a survey of women entering postrafficking services in Europe. Am J Public Health. 2008;98(1):55–59.
- Tracy EE, Macias-Konstantopoulos W. Identifying and assisting sexually exploited and trafficked patients seeking women’s health care services. Obstet Gynecol. 2017;130(2):443–453.
- Silverman JG, Decker MR, Gupta J, Maheshwari A, Willis BM, Raj A. HIV prevalence and predictors of infection in sex-trafficked Nepalese girls and women. JAMA. 2007;298(5):536–542.
- Rafferty Y. Child trafficking and commercial sexual exploitation: a review of promising prevention policies and programs. Am J Orthopsychiatry. 2013;83(4):559–575.
- Kiss L, Yun K, Pocock N, Zimmerman C. Exploitation, violence, and suicide risk among child and adolescent survivors of human trafficking in the Greater Mekong Subregion. JAMA Pediatr. 2015;169(9):e152278.
- Stoklosa H, MacGibbon M, Stoklosa J. Human trafficking, mental illness, and addiction: avoiding diagnostic overshadowing. AMA J Ethics. 2017;19(1):23–34.
- Ravi A, Pfeiffer MR, Rosner Z, Shea JA. Trafficking and trauma: insight and advice for the healthcare system from sex-trafficked women incarcerated on Rikers Island. Med Care. 2017;55(12):1017–1022.
- Macias-Konstantopoulos W. Human trafficking: the role of medicine in interrupting the cycle of abuse and violence. Ann Intern Med. 2016:165(8):582–588.
- Chung RJ, English A. Commercial sexual exploitation and sex trafficking of adolescents. Curr Opin Pediatr. 2015;27(4):427–433.
- Resources: Screening tool for victims of human trafficking. Washington, DC: US Department of Health and Human Services. https://www.justice.gov/sites/default/files/usao-ndia/legacy/2011/10/14/health_screen_questions.pdf. Accessed May 30, 2018.
- US Department of Health and Human Services. Adult human trafficking screening tool and guide. January 2018. https://www.acf.hhs.gov/sites/default/files/otip/adult_human_trafficking_screening_tool_and_guide.pdf. Accessed May 30, 2018.
- Baldwin SB, Eisenman DP, Sayles JN, Ryan G, Chuang KS. Identification of human trafficking victims in health care settings. Health Hum Rights. 2011;13(1):e36–e49.
- Lederer LJ, Wetzel CA. The health consequences of sex trafficking and their implications for identifying victims in health-care facilities. Ann Health Law. 2014;23:61–91.
- Geynisman-Tan JM, Taylor JS, Edersheim T, Taubel D. All the darkness we don’t see. Am J Obstet Gynecol. 2017;216(2):135.e1–e5.
- National Human Trafficking Hotline. https://humantraffickinghotline.org. Accessed May 30, 2018.

Despite increasing media coverage of human trafficking and the gravity of its many ramifications, I am struck by how often trainees and other clinicians present to me patients for which trafficking is a real potential concern—yet who give me a blank expression when I ask if anyone has screened these patients for being victims of trafficking. I suspect that few of us anticipated, during medical training, that we would be providing care to women who are enslaved.
How large is the problem?
It is impossible to comprehend the true scope of human trafficking. Estimates are that 20.9 million men, women, and children globally are forced into work that they are not free to leave.1
Although human trafficking is recognized as a global phenomenon, its prevalence in the United States is significant enough that it should prompt the health care community to engage in helping identify and assist victims/survivors: From January until June of 2017, the National Human Trafficking Hotline received 13,807 telephone calls, resulting in reporting of 4,460 cases.2 Indeed, from 2015 to 2016 there was a 35.7% increase in the number of hotline cases reported, for a total of 7,572 (6,340—more than 80%—of which regarded females). California had the most cases reported (1,323), followed by Texas (670) and Florida (550); those 3 states also reported an increase in trafficking crime. Vermont (5), Rhode Island (9), and Alaska (10) reported the fewest calls.3
How is trafficking defined?
The United Nations Office on Drugs and Crime defines “trafficking in persons” as:
… recruitment, transportation, transfer, harbouring or receipt of persons, by means of the threat or use of force or other forms of coercion, of abduction, of fraud, of deception, of the abuse of power or of a position of vulnerability or of the giving or receiving of payments or benefits to achieve the consent of a person having control over another person, for the purpose of exploitation. Exploitation shall include, at a minimum, the exploitation of the prostitution of others or other forms of sexual exploitation, forced labour or services, slavery or practices similar to slavery, servitude or the removal of organs.4
Traffickers prey on potentially vulnerable people. Girls and young women who have experienced poverty, homelessness, childhood sexual abuse, substance abuse, gender nonconformity, mental illness, or developmental delay are at particular risk.5 Children who have had interactions with Child Protective Services, come from a dysfunctional family, or have lived in a community with high crime, political or social unrest, corruption, or gender bias and discrimination are also at increased risk.6
Read about clues that raise clinical suspicion
Clues that raise clinical suspicion
A number of potential signs should make providers suspicious about potential human trafficking. Some of those signs are similar to the red flags we see in intimate partner violence, such as:
- having a difficult time talking to the patient alone
- having the accompanying person answer the patient’s questions
- body language that suggests fear, anxiety, or distrust (eg, shifting positions, looking away, appearing withdrawn)
- physical examination inconsistent with the history
- physical injury (especially multiple injuries or injuries in various stages of healing)
- refusal of interpreter services.
Trafficked girls or women may appear overly familiar with sex, have unexpected material possessions, or appear to be giving scripted or memorized answers to queries.7 Traffickers often confiscate their victims’ personal identification. They try to prevent victims from knowing their geographic locales: Patients might not have any documentation or awareness of exact surroundings (eg, their home address). Patients may be wearing clothes considered inappropriate for the weather or venue. They may have tattoos that are marks of branding.8
Medical consequences of being trafficked are obvious, numerous, and serious
Many medical sequelae that result from trafficking are obvious, given the nature of work that victims are forced to do. For example, overcrowding can lead to infectious disease, such as tuberculosis.9 Inadequate access to preventive or basic medical services can result in weight loss, poor dentition, and untreated chronic medical conditions.
If victims are experiencing physical or sexual abuse, they can present with evidence of blunt trauma, ligature marks, skin burns, wounds inflicted by weapons, and vaginal lacerations.10 A study found that 63% of survivors reported at least 10 somatic symptoms, including headache, fatigue, dizziness, back pain, abdominal or pelvic pain, memory loss, and symptoms of genital infectious disease.11
Girls and women being trafficked for sex may experience many of the sequelae of unprotected intercourse: irregular bleeding, unintended pregnancy, unwanted or unsafe pregnancy termination, vaginal trauma, and sexually transmitted infection (STI).12 In a study of trafficking survivors, 38% were HIV-positive.13
Trafficking survivors can suffer myriad mental health conditions, with high rates of depression, anxiety, posttraumatic stress, and suicidal ideation.14 A study of 387 survivors found that 12% had attempted to harm themselves or commit suicide the month before they were interviewed.15
Substance abuse is also a common problem among trafficking victims.16 One survivor interviewed in a recent study said:
It was much more difficult to work sober because I was dealing with emotions or the pain that I was feeling during intercourse, because when you have sex with people 8, 9, 10 times a day, even more than that, it starts to hurt a lot. And being high made it easier to deal with that and also it made it easier for me to get away from my body while it was happening, place my brain somewhere else.17
Because of the substantial risk of mental health problems, including substance abuse, among trafficking survivors, the physical exam of a patient should include careful assessment of demeanor and mental health status. Of course, comprehensive inspection for signs of physical or blunt trauma is paramount.
Read about Patient and staff safety during the visit
Patient and staff safety during the visit
Providers should be aware of potential safety concerns, both for the patient and for the staff. Creative strategies should be utilized to screen the patient in private. The use of interpreter services—either in person or over the telephone—should be presented and facilitated as being a routine part of practice. Any person who accompanies the patient should be asked to leave the examining room, either as a statement of practice routine or under the guise of having him (or her) step out to obtain paperwork or provide documentation.
Care of victims
Trauma-informed care should be a guiding principle for trafficking survivors. This involves empowering the patient, who may feel victimized again if asked to undress and undergo multiple physical examinations. Macias-Konstantopoulos noted: “A trauma-informed approach to care acknowledges the pervasiveness and effect of trauma across the life span of the individual, recognizes the vulnerabilities and emotional triggers of trauma survivors, minimizes repeated traumatization and fosters physical, psychological, and emotional safety, recovery, health and well-being.”18
The patient should be counseled that she has control over her body and can guide different aspects of the examination. For example the provider should discuss: 1) the amount of clothing deemed optimal for an examination, 2) the availability of a support person during the exam (for instance, a nurse or a social worker) if the patient requests one, and 3) utilization of whatever strategies the patient deems optimal for her to be most comfortable during the exam (such as leaving the door slightly ajar or having a mutually agreed-on signal to interrupt the exam).
Routine health care maintenance should be offered, including an assessment of overall physical and dental health and screening for STI and mental health. Screening for substances of abuse should be considered. If indicated, emergency contraception, postexposure HIV prophylaxis, immunizations, and empiric antibiotics for STI should be offered.19
Screening when indicated by evidence, suspicion, or concern
Unlike the case with intimate partner violence, experts do not recommend universal screening for human trafficking. Clinicians should be comfortable, however, trying to elicit that history when a concern arises, either because of identified risk factors, red flags, or concerns that arise from the findings of the history or physical. Ideally, clinicians should consider becoming comfortable choosing a few screening questions to regularly incorporate into their assessment. The US Department of Health & Human Services (HHS) offers a list of questions that can be utilized (TABLE).20

In January 2018, the Office on Trafficking in Persons, a unit of the HHS Administration for Children and Families, released an “Adult Human Trafficking Screening Tool and Guide.” The document includes 2 excellent tools21 that clinicians can utilize to identify patients who should be screened and how to identify and assist survivors (FIGURE 1 and FIGURE 2).


Clinicians, in their encounters with patients, are particularly well-positioned to intersect with, and identify, survivors. Regrettably, such opportunities are often missed—and victims thus remain unidentified and trapped in their circumstances. A study revealed that one-half of survivors who were interviewed reported seeing a physician while they were being trafficked.22 Even more alarming, another study showed that 87.8% of survivors had received health care during their captivity.23 It is dismaying to know that these patients left those health care settings without receiving the assistance they truly need and with their true circumstances remaining unidentified.
Read about Finding assistance and support
Finding assistance and support
Centers in the United States now provide trauma-informed care for trafficking survivors in a confidential setting (see “Specialized care is increasingly available”).24 A physician who works at a center in New York City noted: “Our survivors told us that more than fear or pain, the feelings that sat with them most often were worthlessness and invisibility. We can do better as physicians and as educators to expose this epidemic and care for its victims.”24
Here is a sampling of the growing number of centers in the United States that provide trauma-centered care for survivors of human trafficking:
- Survivor Clinic at New York Presbyterian Hospital-Weill Cornell Medical College, New York, New York
- EMPOWER Clinic for Survivors of Sex Trafficking and Sexual Violence at NYU Langone Health, New York, New York
- Freedom Clinic at Massachusetts General Hospital, Boston
- The Hope Through Health Clinic, Austin, Texas
- Pacific Survivor Center, Honolulu, Hawaii
Most clinicians practice in settings that do not have easy access to such subspecialized centers, however. For them, the National Human Trafficking Hotline can be an invaluable resource (see “Hotline is a valuable resource”).25 Law enforcement and social services colleagues also can be useful allies.
Uncertain how you can help a patient who is a victim of human trafficking? For assistance and support, contact the National Human Trafficking Hotline--24 hours a day, 7 days a week, and in 200 languages--in any of 3 ways:
- By telephone: (888) 373-7888
- By text: 233733
- On the web: https://humantraffickinghotline.orga
aIncludes a search field that clinicians can use to look up the nearest resources for additional assistance.
Let’s turn our concern and awareness into results
We, as providers of women’s health care, are uniquely positioned to help these most vulnerable of people, many of whom have been stripped of personal documents and denied access to financial resources and community support. As a medical community, we should strive to combat this tragic epidemic, 1 patient at a time.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

Despite increasing media coverage of human trafficking and the gravity of its many ramifications, I am struck by how often trainees and other clinicians present to me patients for which trafficking is a real potential concern—yet who give me a blank expression when I ask if anyone has screened these patients for being victims of trafficking. I suspect that few of us anticipated, during medical training, that we would be providing care to women who are enslaved.
How large is the problem?
It is impossible to comprehend the true scope of human trafficking. Estimates are that 20.9 million men, women, and children globally are forced into work that they are not free to leave.1
Although human trafficking is recognized as a global phenomenon, its prevalence in the United States is significant enough that it should prompt the health care community to engage in helping identify and assist victims/survivors: From January until June of 2017, the National Human Trafficking Hotline received 13,807 telephone calls, resulting in reporting of 4,460 cases.2 Indeed, from 2015 to 2016 there was a 35.7% increase in the number of hotline cases reported, for a total of 7,572 (6,340—more than 80%—of which regarded females). California had the most cases reported (1,323), followed by Texas (670) and Florida (550); those 3 states also reported an increase in trafficking crime. Vermont (5), Rhode Island (9), and Alaska (10) reported the fewest calls.3
How is trafficking defined?
The United Nations Office on Drugs and Crime defines “trafficking in persons” as:
… recruitment, transportation, transfer, harbouring or receipt of persons, by means of the threat or use of force or other forms of coercion, of abduction, of fraud, of deception, of the abuse of power or of a position of vulnerability or of the giving or receiving of payments or benefits to achieve the consent of a person having control over another person, for the purpose of exploitation. Exploitation shall include, at a minimum, the exploitation of the prostitution of others or other forms of sexual exploitation, forced labour or services, slavery or practices similar to slavery, servitude or the removal of organs.4
Traffickers prey on potentially vulnerable people. Girls and young women who have experienced poverty, homelessness, childhood sexual abuse, substance abuse, gender nonconformity, mental illness, or developmental delay are at particular risk.5 Children who have had interactions with Child Protective Services, come from a dysfunctional family, or have lived in a community with high crime, political or social unrest, corruption, or gender bias and discrimination are also at increased risk.6
Read about clues that raise clinical suspicion
Clues that raise clinical suspicion
A number of potential signs should make providers suspicious about potential human trafficking. Some of those signs are similar to the red flags we see in intimate partner violence, such as:
- having a difficult time talking to the patient alone
- having the accompanying person answer the patient’s questions
- body language that suggests fear, anxiety, or distrust (eg, shifting positions, looking away, appearing withdrawn)
- physical examination inconsistent with the history
- physical injury (especially multiple injuries or injuries in various stages of healing)
- refusal of interpreter services.
Trafficked girls or women may appear overly familiar with sex, have unexpected material possessions, or appear to be giving scripted or memorized answers to queries.7 Traffickers often confiscate their victims’ personal identification. They try to prevent victims from knowing their geographic locales: Patients might not have any documentation or awareness of exact surroundings (eg, their home address). Patients may be wearing clothes considered inappropriate for the weather or venue. They may have tattoos that are marks of branding.8
Medical consequences of being trafficked are obvious, numerous, and serious
Many medical sequelae that result from trafficking are obvious, given the nature of work that victims are forced to do. For example, overcrowding can lead to infectious disease, such as tuberculosis.9 Inadequate access to preventive or basic medical services can result in weight loss, poor dentition, and untreated chronic medical conditions.
If victims are experiencing physical or sexual abuse, they can present with evidence of blunt trauma, ligature marks, skin burns, wounds inflicted by weapons, and vaginal lacerations.10 A study found that 63% of survivors reported at least 10 somatic symptoms, including headache, fatigue, dizziness, back pain, abdominal or pelvic pain, memory loss, and symptoms of genital infectious disease.11
Girls and women being trafficked for sex may experience many of the sequelae of unprotected intercourse: irregular bleeding, unintended pregnancy, unwanted or unsafe pregnancy termination, vaginal trauma, and sexually transmitted infection (STI).12 In a study of trafficking survivors, 38% were HIV-positive.13
Trafficking survivors can suffer myriad mental health conditions, with high rates of depression, anxiety, posttraumatic stress, and suicidal ideation.14 A study of 387 survivors found that 12% had attempted to harm themselves or commit suicide the month before they were interviewed.15
Substance abuse is also a common problem among trafficking victims.16 One survivor interviewed in a recent study said:
It was much more difficult to work sober because I was dealing with emotions or the pain that I was feeling during intercourse, because when you have sex with people 8, 9, 10 times a day, even more than that, it starts to hurt a lot. And being high made it easier to deal with that and also it made it easier for me to get away from my body while it was happening, place my brain somewhere else.17
Because of the substantial risk of mental health problems, including substance abuse, among trafficking survivors, the physical exam of a patient should include careful assessment of demeanor and mental health status. Of course, comprehensive inspection for signs of physical or blunt trauma is paramount.
Read about Patient and staff safety during the visit
Patient and staff safety during the visit
Providers should be aware of potential safety concerns, both for the patient and for the staff. Creative strategies should be utilized to screen the patient in private. The use of interpreter services—either in person or over the telephone—should be presented and facilitated as being a routine part of practice. Any person who accompanies the patient should be asked to leave the examining room, either as a statement of practice routine or under the guise of having him (or her) step out to obtain paperwork or provide documentation.
Care of victims
Trauma-informed care should be a guiding principle for trafficking survivors. This involves empowering the patient, who may feel victimized again if asked to undress and undergo multiple physical examinations. Macias-Konstantopoulos noted: “A trauma-informed approach to care acknowledges the pervasiveness and effect of trauma across the life span of the individual, recognizes the vulnerabilities and emotional triggers of trauma survivors, minimizes repeated traumatization and fosters physical, psychological, and emotional safety, recovery, health and well-being.”18
The patient should be counseled that she has control over her body and can guide different aspects of the examination. For example the provider should discuss: 1) the amount of clothing deemed optimal for an examination, 2) the availability of a support person during the exam (for instance, a nurse or a social worker) if the patient requests one, and 3) utilization of whatever strategies the patient deems optimal for her to be most comfortable during the exam (such as leaving the door slightly ajar or having a mutually agreed-on signal to interrupt the exam).
Routine health care maintenance should be offered, including an assessment of overall physical and dental health and screening for STI and mental health. Screening for substances of abuse should be considered. If indicated, emergency contraception, postexposure HIV prophylaxis, immunizations, and empiric antibiotics for STI should be offered.19
Screening when indicated by evidence, suspicion, or concern
Unlike the case with intimate partner violence, experts do not recommend universal screening for human trafficking. Clinicians should be comfortable, however, trying to elicit that history when a concern arises, either because of identified risk factors, red flags, or concerns that arise from the findings of the history or physical. Ideally, clinicians should consider becoming comfortable choosing a few screening questions to regularly incorporate into their assessment. The US Department of Health & Human Services (HHS) offers a list of questions that can be utilized (TABLE).20

In January 2018, the Office on Trafficking in Persons, a unit of the HHS Administration for Children and Families, released an “Adult Human Trafficking Screening Tool and Guide.” The document includes 2 excellent tools21 that clinicians can utilize to identify patients who should be screened and how to identify and assist survivors (FIGURE 1 and FIGURE 2).


Clinicians, in their encounters with patients, are particularly well-positioned to intersect with, and identify, survivors. Regrettably, such opportunities are often missed—and victims thus remain unidentified and trapped in their circumstances. A study revealed that one-half of survivors who were interviewed reported seeing a physician while they were being trafficked.22 Even more alarming, another study showed that 87.8% of survivors had received health care during their captivity.23 It is dismaying to know that these patients left those health care settings without receiving the assistance they truly need and with their true circumstances remaining unidentified.
Read about Finding assistance and support
Finding assistance and support
Centers in the United States now provide trauma-informed care for trafficking survivors in a confidential setting (see “Specialized care is increasingly available”).24 A physician who works at a center in New York City noted: “Our survivors told us that more than fear or pain, the feelings that sat with them most often were worthlessness and invisibility. We can do better as physicians and as educators to expose this epidemic and care for its victims.”24
Here is a sampling of the growing number of centers in the United States that provide trauma-centered care for survivors of human trafficking:
- Survivor Clinic at New York Presbyterian Hospital-Weill Cornell Medical College, New York, New York
- EMPOWER Clinic for Survivors of Sex Trafficking and Sexual Violence at NYU Langone Health, New York, New York
- Freedom Clinic at Massachusetts General Hospital, Boston
- The Hope Through Health Clinic, Austin, Texas
- Pacific Survivor Center, Honolulu, Hawaii
Most clinicians practice in settings that do not have easy access to such subspecialized centers, however. For them, the National Human Trafficking Hotline can be an invaluable resource (see “Hotline is a valuable resource”).25 Law enforcement and social services colleagues also can be useful allies.
Uncertain how you can help a patient who is a victim of human trafficking? For assistance and support, contact the National Human Trafficking Hotline--24 hours a day, 7 days a week, and in 200 languages--in any of 3 ways:
- By telephone: (888) 373-7888
- By text: 233733
- On the web: https://humantraffickinghotline.orga
aIncludes a search field that clinicians can use to look up the nearest resources for additional assistance.
Let’s turn our concern and awareness into results
We, as providers of women’s health care, are uniquely positioned to help these most vulnerable of people, many of whom have been stripped of personal documents and denied access to financial resources and community support. As a medical community, we should strive to combat this tragic epidemic, 1 patient at a time.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- International Labour Organization. New ILO Global Estimate of Forced Labour: 20.9 million victims. http://www.ilo.org/global/about-the-ilo/newsroom/news/WCMS_182109/lang--en/index.htm. Published June 2012. Accessed May 30, 2018.
- National Human Trafficking Hotline. Hotline statistics. https://humantraffickinghotline.org/states. Accessed May 30, 2018.
- Cone A. Report: Human trafficking in U.S. rose 35.7 percent in one year. United Press International (UPI). https://www.upi.com/Report-Human-trafficking-in-US-rose-357-percent-in-one-year/5571486328579. Published February 5, 2017. Accessed May 30, 2018.
- United Nations Office on Drugs and Crime. Human trafficking. http://www.unodc.org/unodc/en/human-trafficking/what-is-human-trafficking.html. Accessed May 30, 2018.
- Risk factors for and consequences of commercial sexual exploitation and sex trafficking of minors. In Clayton E, Krugman R, Simon P, eds; Committee on the Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States; Board on Children, Youth, and Families; Committee on Law and Justice; Institute of Medicine; National Research Council. Confronting Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States. Washington, DC: The National Academies Press; 2013.
- Greenbaum J, Crawford-Jakubiak JE. Committee on Child Abuse and Neglect. Child sex trafficking and commercial sexual exploitation: health care needs of victims. Pediatrics. 2015:135(3);566–574.
- Alpert E, Ahn R, Albright E, Purcell G, Burke T, Macias-Konstantanopoulos W. Human Trafficking: Guidebook on Identification, Assessment, and Response in the Health Care Setting. Waltham, MA: Massachusetts General Hospital and Massachusetts Medical Society; 2014. http://www.massmed.org/Patient-Care/Health-Topics/Violence-Prevention-and-Intervention/Human-Trafficking-(pdf). Accessed May 30, 2018.
- National Human Trafficking Training and Technical Assistance Center. Adult human trafficking screening tool and guide. http://www.acf.hhs.gov/sites/default/files/otip/adult_human_trafficking_screening_tool_and_guide.pdf. Published January 2018. Accessed May 30, 2018.
- Steele S. Human trafficking, labor brokering, and mining in southern Africa: responding to a decentralized and hidden public health disaster. Int J Health Serv. 2013;43(4):665–680.
- Becker HJ, Bechtel K. Recognizing victims of human trafficking in the pediatric emergency department. Pediatr Emerg Care. 2015;31(2):144–147.
- Zimmerman C, Hossain M, Yun K, et al. The health of trafficked women: a survey of women entering postrafficking services in Europe. Am J Public Health. 2008;98(1):55–59.
- Tracy EE, Macias-Konstantopoulos W. Identifying and assisting sexually exploited and trafficked patients seeking women’s health care services. Obstet Gynecol. 2017;130(2):443–453.
- Silverman JG, Decker MR, Gupta J, Maheshwari A, Willis BM, Raj A. HIV prevalence and predictors of infection in sex-trafficked Nepalese girls and women. JAMA. 2007;298(5):536–542.
- Rafferty Y. Child trafficking and commercial sexual exploitation: a review of promising prevention policies and programs. Am J Orthopsychiatry. 2013;83(4):559–575.
- Kiss L, Yun K, Pocock N, Zimmerman C. Exploitation, violence, and suicide risk among child and adolescent survivors of human trafficking in the Greater Mekong Subregion. JAMA Pediatr. 2015;169(9):e152278.
- Stoklosa H, MacGibbon M, Stoklosa J. Human trafficking, mental illness, and addiction: avoiding diagnostic overshadowing. AMA J Ethics. 2017;19(1):23–34.
- Ravi A, Pfeiffer MR, Rosner Z, Shea JA. Trafficking and trauma: insight and advice for the healthcare system from sex-trafficked women incarcerated on Rikers Island. Med Care. 2017;55(12):1017–1022.
- Macias-Konstantopoulos W. Human trafficking: the role of medicine in interrupting the cycle of abuse and violence. Ann Intern Med. 2016:165(8):582–588.
- Chung RJ, English A. Commercial sexual exploitation and sex trafficking of adolescents. Curr Opin Pediatr. 2015;27(4):427–433.
- Resources: Screening tool for victims of human trafficking. Washington, DC: US Department of Health and Human Services. https://www.justice.gov/sites/default/files/usao-ndia/legacy/2011/10/14/health_screen_questions.pdf. Accessed May 30, 2018.
- US Department of Health and Human Services. Adult human trafficking screening tool and guide. January 2018. https://www.acf.hhs.gov/sites/default/files/otip/adult_human_trafficking_screening_tool_and_guide.pdf. Accessed May 30, 2018.
- Baldwin SB, Eisenman DP, Sayles JN, Ryan G, Chuang KS. Identification of human trafficking victims in health care settings. Health Hum Rights. 2011;13(1):e36–e49.
- Lederer LJ, Wetzel CA. The health consequences of sex trafficking and their implications for identifying victims in health-care facilities. Ann Health Law. 2014;23:61–91.
- Geynisman-Tan JM, Taylor JS, Edersheim T, Taubel D. All the darkness we don’t see. Am J Obstet Gynecol. 2017;216(2):135.e1–e5.
- National Human Trafficking Hotline. https://humantraffickinghotline.org. Accessed May 30, 2018.
- International Labour Organization. New ILO Global Estimate of Forced Labour: 20.9 million victims. http://www.ilo.org/global/about-the-ilo/newsroom/news/WCMS_182109/lang--en/index.htm. Published June 2012. Accessed May 30, 2018.
- National Human Trafficking Hotline. Hotline statistics. https://humantraffickinghotline.org/states. Accessed May 30, 2018.
- Cone A. Report: Human trafficking in U.S. rose 35.7 percent in one year. United Press International (UPI). https://www.upi.com/Report-Human-trafficking-in-US-rose-357-percent-in-one-year/5571486328579. Published February 5, 2017. Accessed May 30, 2018.
- United Nations Office on Drugs and Crime. Human trafficking. http://www.unodc.org/unodc/en/human-trafficking/what-is-human-trafficking.html. Accessed May 30, 2018.
- Risk factors for and consequences of commercial sexual exploitation and sex trafficking of minors. In Clayton E, Krugman R, Simon P, eds; Committee on the Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States; Board on Children, Youth, and Families; Committee on Law and Justice; Institute of Medicine; National Research Council. Confronting Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States. Washington, DC: The National Academies Press; 2013.
- Greenbaum J, Crawford-Jakubiak JE. Committee on Child Abuse and Neglect. Child sex trafficking and commercial sexual exploitation: health care needs of victims. Pediatrics. 2015:135(3);566–574.
- Alpert E, Ahn R, Albright E, Purcell G, Burke T, Macias-Konstantanopoulos W. Human Trafficking: Guidebook on Identification, Assessment, and Response in the Health Care Setting. Waltham, MA: Massachusetts General Hospital and Massachusetts Medical Society; 2014. http://www.massmed.org/Patient-Care/Health-Topics/Violence-Prevention-and-Intervention/Human-Trafficking-(pdf). Accessed May 30, 2018.
- National Human Trafficking Training and Technical Assistance Center. Adult human trafficking screening tool and guide. http://www.acf.hhs.gov/sites/default/files/otip/adult_human_trafficking_screening_tool_and_guide.pdf. Published January 2018. Accessed May 30, 2018.
- Steele S. Human trafficking, labor brokering, and mining in southern Africa: responding to a decentralized and hidden public health disaster. Int J Health Serv. 2013;43(4):665–680.
- Becker HJ, Bechtel K. Recognizing victims of human trafficking in the pediatric emergency department. Pediatr Emerg Care. 2015;31(2):144–147.
- Zimmerman C, Hossain M, Yun K, et al. The health of trafficked women: a survey of women entering postrafficking services in Europe. Am J Public Health. 2008;98(1):55–59.
- Tracy EE, Macias-Konstantopoulos W. Identifying and assisting sexually exploited and trafficked patients seeking women’s health care services. Obstet Gynecol. 2017;130(2):443–453.
- Silverman JG, Decker MR, Gupta J, Maheshwari A, Willis BM, Raj A. HIV prevalence and predictors of infection in sex-trafficked Nepalese girls and women. JAMA. 2007;298(5):536–542.
- Rafferty Y. Child trafficking and commercial sexual exploitation: a review of promising prevention policies and programs. Am J Orthopsychiatry. 2013;83(4):559–575.
- Kiss L, Yun K, Pocock N, Zimmerman C. Exploitation, violence, and suicide risk among child and adolescent survivors of human trafficking in the Greater Mekong Subregion. JAMA Pediatr. 2015;169(9):e152278.
- Stoklosa H, MacGibbon M, Stoklosa J. Human trafficking, mental illness, and addiction: avoiding diagnostic overshadowing. AMA J Ethics. 2017;19(1):23–34.
- Ravi A, Pfeiffer MR, Rosner Z, Shea JA. Trafficking and trauma: insight and advice for the healthcare system from sex-trafficked women incarcerated on Rikers Island. Med Care. 2017;55(12):1017–1022.
- Macias-Konstantopoulos W. Human trafficking: the role of medicine in interrupting the cycle of abuse and violence. Ann Intern Med. 2016:165(8):582–588.
- Chung RJ, English A. Commercial sexual exploitation and sex trafficking of adolescents. Curr Opin Pediatr. 2015;27(4):427–433.
- Resources: Screening tool for victims of human trafficking. Washington, DC: US Department of Health and Human Services. https://www.justice.gov/sites/default/files/usao-ndia/legacy/2011/10/14/health_screen_questions.pdf. Accessed May 30, 2018.
- US Department of Health and Human Services. Adult human trafficking screening tool and guide. January 2018. https://www.acf.hhs.gov/sites/default/files/otip/adult_human_trafficking_screening_tool_and_guide.pdf. Accessed May 30, 2018.
- Baldwin SB, Eisenman DP, Sayles JN, Ryan G, Chuang KS. Identification of human trafficking victims in health care settings. Health Hum Rights. 2011;13(1):e36–e49.
- Lederer LJ, Wetzel CA. The health consequences of sex trafficking and their implications for identifying victims in health-care facilities. Ann Health Law. 2014;23:61–91.
- Geynisman-Tan JM, Taylor JS, Edersheim T, Taubel D. All the darkness we don’t see. Am J Obstet Gynecol. 2017;216(2):135.e1–e5.
- National Human Trafficking Hotline. https://humantraffickinghotline.org. Accessed May 30, 2018.
IN THIS ARTICLE
- Clues to raise suspicion
- Medical consequences of trafficking
- Screening algorithm
Supreme Court supports anti-abortion centers in free speech case
The ruling protects people from being forced to express a message that violates their beliefs, said Michael Farris, president for Alliance Defending Freedom and counsel for the National Institute of Family and Life Advocates (NIFLA), the lead plaintiff.
“In this case, the government used its power to force pro-life pregnancy centers to provide free advertising for abortion,” Mr. Farris said in a statement. “The Supreme Court said that the government can’t do that and that it must respect pro-life beliefs. Tolerance and respect for good-faith differences of opinion are essential in a diverse society like ours. They enable us to coexist peacefully with one another. If we want to have freedom for ourselves, we have to extend it to others.”
California Attorney General Xavier Becerra expressed disappointment at the Supreme Court’s decision, saying the opinion complicates the state’s efforts to empower women with information about their health care.
“When it comes to making their health decisions, all California women – regardless of their economic background or zip code – deserve access to critical and nonbiased information to make their own informed decisions,” Mr. Becerra said in a statement. The “ruling is unfortunate, but our work to ensure that Californians receive accurate information about their health care options will continue.”
The legal challenge began after California passed its 2016 Reproductive Freedom, Accountability, Comprehensive Care, and Transparency (FACT) Act, which requires that pregnancy centers – many of which are anti-abortion – post notices about where patients can find free or low-cost abortion services. Another part of the law requires unlicensed pregnancy centers to disclose to women that they are not a licensed medical facility and have no medical professionals on staff.
The plaintiffs, led by the nonprofit NIFLA, argued that the law violated the First Amendment by requiring speech and because the measure unfairly targets centers that discourage abortions. California officials contended that the law was needed to address concerns that nonprofit organizations, often religious, were misrepresenting themselves as full-service reproductive health clinics and misleading women about their options.
However, in the court’s majority opinion, Associate Justice Clarence Thomas wrote that California can inform women about abortion services through other means, such as advertising, rather than burdening the plaintiffs with compelled speech.
“California cannot co-opt the licensed facilities to deliver its message for it,” he wrote. The “First Amendment does not permit the state to sacrifice speech for efficiency.”
In a concurring opinion, Associate Justice Anthony Kennedy wrote that the California law is a paradigmatic example of the serious threat presented when government seeks to impose its own message in the place of individual speech, thought, and expression.
“Governments must not be allowed to force persons to express a message contrary to their deepest convictions,” Justice Kennedy wrote. “Freedom of speech secures freedom of thought and belief. This law imperils those liberties.”
In a dissenting opinion, Associate Justice Stephen Breyer wrote that the high court’s majority stance contradicts a previous decision in which justices required physicians who performed abortions to give information about adoption services.
“If a state can lawfully require a doctor to tell a woman seeking an abortion about adoption services, why should it not be able, as here, to require a medical counselor to tell a woman seeking prenatal care or other reproductive health care about childbirth and abortion services?” he asked. “As the question suggests, there is no convincing reason to distinguish between information about adoption and information about abortion in this context.”
The Supreme Court’s decision has broad implications in the health care setting and other sectors that physicians and other professionals should celebrate, said Robert McNamara, a senior attorney for the Institute for Justice in Arlington, Va. The professional-speech doctrine that the Supreme Court rejected in the case posed a serious danger to the free-speech rights of health providers, Mr. McNamara said in an interview.
However, Heather Shumaker, senior counsel for reproductive rights and health at the National Women’s Law Center, said the ruling is detrimental to patients’ health care and chills their access to truthful, accurate medical information.
“Throughout the country, anti-abortion counseling centers provide false, misleading, or incomplete information, and frighten and coerce women to make certain decisions about their health care options,” Ms. Shumaker said in an interview. “This deception endangers women’s health and future fertility, and particularly burdens women of color and women struggling to make ends meet. It is devastating that [the] decision will make access to full reproductive health care more difficult.”
The American College of Obstetricians and Gynecologists (ACOG) also expressed disappointment at the Supreme Court’s ruling.
“Pregnant women who seek medical guidance must be able to trust that information being provided to them is truthful, medically accurate, and enables them to make informed decisions about their care,” ACOG President Lisa Hollier, MD, said in a statement. “Inaccurate and untruthful information can delay care and increase risk of medical complications.”
The ruling protects people from being forced to express a message that violates their beliefs, said Michael Farris, president for Alliance Defending Freedom and counsel for the National Institute of Family and Life Advocates (NIFLA), the lead plaintiff.
“In this case, the government used its power to force pro-life pregnancy centers to provide free advertising for abortion,” Mr. Farris said in a statement. “The Supreme Court said that the government can’t do that and that it must respect pro-life beliefs. Tolerance and respect for good-faith differences of opinion are essential in a diverse society like ours. They enable us to coexist peacefully with one another. If we want to have freedom for ourselves, we have to extend it to others.”
California Attorney General Xavier Becerra expressed disappointment at the Supreme Court’s decision, saying the opinion complicates the state’s efforts to empower women with information about their health care.
“When it comes to making their health decisions, all California women – regardless of their economic background or zip code – deserve access to critical and nonbiased information to make their own informed decisions,” Mr. Becerra said in a statement. The “ruling is unfortunate, but our work to ensure that Californians receive accurate information about their health care options will continue.”
The legal challenge began after California passed its 2016 Reproductive Freedom, Accountability, Comprehensive Care, and Transparency (FACT) Act, which requires that pregnancy centers – many of which are anti-abortion – post notices about where patients can find free or low-cost abortion services. Another part of the law requires unlicensed pregnancy centers to disclose to women that they are not a licensed medical facility and have no medical professionals on staff.
The plaintiffs, led by the nonprofit NIFLA, argued that the law violated the First Amendment by requiring speech and because the measure unfairly targets centers that discourage abortions. California officials contended that the law was needed to address concerns that nonprofit organizations, often religious, were misrepresenting themselves as full-service reproductive health clinics and misleading women about their options.
However, in the court’s majority opinion, Associate Justice Clarence Thomas wrote that California can inform women about abortion services through other means, such as advertising, rather than burdening the plaintiffs with compelled speech.
“California cannot co-opt the licensed facilities to deliver its message for it,” he wrote. The “First Amendment does not permit the state to sacrifice speech for efficiency.”
In a concurring opinion, Associate Justice Anthony Kennedy wrote that the California law is a paradigmatic example of the serious threat presented when government seeks to impose its own message in the place of individual speech, thought, and expression.
“Governments must not be allowed to force persons to express a message contrary to their deepest convictions,” Justice Kennedy wrote. “Freedom of speech secures freedom of thought and belief. This law imperils those liberties.”
In a dissenting opinion, Associate Justice Stephen Breyer wrote that the high court’s majority stance contradicts a previous decision in which justices required physicians who performed abortions to give information about adoption services.
“If a state can lawfully require a doctor to tell a woman seeking an abortion about adoption services, why should it not be able, as here, to require a medical counselor to tell a woman seeking prenatal care or other reproductive health care about childbirth and abortion services?” he asked. “As the question suggests, there is no convincing reason to distinguish between information about adoption and information about abortion in this context.”
The Supreme Court’s decision has broad implications in the health care setting and other sectors that physicians and other professionals should celebrate, said Robert McNamara, a senior attorney for the Institute for Justice in Arlington, Va. The professional-speech doctrine that the Supreme Court rejected in the case posed a serious danger to the free-speech rights of health providers, Mr. McNamara said in an interview.
However, Heather Shumaker, senior counsel for reproductive rights and health at the National Women’s Law Center, said the ruling is detrimental to patients’ health care and chills their access to truthful, accurate medical information.
“Throughout the country, anti-abortion counseling centers provide false, misleading, or incomplete information, and frighten and coerce women to make certain decisions about their health care options,” Ms. Shumaker said in an interview. “This deception endangers women’s health and future fertility, and particularly burdens women of color and women struggling to make ends meet. It is devastating that [the] decision will make access to full reproductive health care more difficult.”
The American College of Obstetricians and Gynecologists (ACOG) also expressed disappointment at the Supreme Court’s ruling.
“Pregnant women who seek medical guidance must be able to trust that information being provided to them is truthful, medically accurate, and enables them to make informed decisions about their care,” ACOG President Lisa Hollier, MD, said in a statement. “Inaccurate and untruthful information can delay care and increase risk of medical complications.”
The ruling protects people from being forced to express a message that violates their beliefs, said Michael Farris, president for Alliance Defending Freedom and counsel for the National Institute of Family and Life Advocates (NIFLA), the lead plaintiff.
“In this case, the government used its power to force pro-life pregnancy centers to provide free advertising for abortion,” Mr. Farris said in a statement. “The Supreme Court said that the government can’t do that and that it must respect pro-life beliefs. Tolerance and respect for good-faith differences of opinion are essential in a diverse society like ours. They enable us to coexist peacefully with one another. If we want to have freedom for ourselves, we have to extend it to others.”
California Attorney General Xavier Becerra expressed disappointment at the Supreme Court’s decision, saying the opinion complicates the state’s efforts to empower women with information about their health care.
“When it comes to making their health decisions, all California women – regardless of their economic background or zip code – deserve access to critical and nonbiased information to make their own informed decisions,” Mr. Becerra said in a statement. The “ruling is unfortunate, but our work to ensure that Californians receive accurate information about their health care options will continue.”
The legal challenge began after California passed its 2016 Reproductive Freedom, Accountability, Comprehensive Care, and Transparency (FACT) Act, which requires that pregnancy centers – many of which are anti-abortion – post notices about where patients can find free or low-cost abortion services. Another part of the law requires unlicensed pregnancy centers to disclose to women that they are not a licensed medical facility and have no medical professionals on staff.
The plaintiffs, led by the nonprofit NIFLA, argued that the law violated the First Amendment by requiring speech and because the measure unfairly targets centers that discourage abortions. California officials contended that the law was needed to address concerns that nonprofit organizations, often religious, were misrepresenting themselves as full-service reproductive health clinics and misleading women about their options.
However, in the court’s majority opinion, Associate Justice Clarence Thomas wrote that California can inform women about abortion services through other means, such as advertising, rather than burdening the plaintiffs with compelled speech.
“California cannot co-opt the licensed facilities to deliver its message for it,” he wrote. The “First Amendment does not permit the state to sacrifice speech for efficiency.”
In a concurring opinion, Associate Justice Anthony Kennedy wrote that the California law is a paradigmatic example of the serious threat presented when government seeks to impose its own message in the place of individual speech, thought, and expression.
“Governments must not be allowed to force persons to express a message contrary to their deepest convictions,” Justice Kennedy wrote. “Freedom of speech secures freedom of thought and belief. This law imperils those liberties.”
In a dissenting opinion, Associate Justice Stephen Breyer wrote that the high court’s majority stance contradicts a previous decision in which justices required physicians who performed abortions to give information about adoption services.
“If a state can lawfully require a doctor to tell a woman seeking an abortion about adoption services, why should it not be able, as here, to require a medical counselor to tell a woman seeking prenatal care or other reproductive health care about childbirth and abortion services?” he asked. “As the question suggests, there is no convincing reason to distinguish between information about adoption and information about abortion in this context.”
The Supreme Court’s decision has broad implications in the health care setting and other sectors that physicians and other professionals should celebrate, said Robert McNamara, a senior attorney for the Institute for Justice in Arlington, Va. The professional-speech doctrine that the Supreme Court rejected in the case posed a serious danger to the free-speech rights of health providers, Mr. McNamara said in an interview.
However, Heather Shumaker, senior counsel for reproductive rights and health at the National Women’s Law Center, said the ruling is detrimental to patients’ health care and chills their access to truthful, accurate medical information.
“Throughout the country, anti-abortion counseling centers provide false, misleading, or incomplete information, and frighten and coerce women to make certain decisions about their health care options,” Ms. Shumaker said in an interview. “This deception endangers women’s health and future fertility, and particularly burdens women of color and women struggling to make ends meet. It is devastating that [the] decision will make access to full reproductive health care more difficult.”
The American College of Obstetricians and Gynecologists (ACOG) also expressed disappointment at the Supreme Court’s ruling.
“Pregnant women who seek medical guidance must be able to trust that information being provided to them is truthful, medically accurate, and enables them to make informed decisions about their care,” ACOG President Lisa Hollier, MD, said in a statement. “Inaccurate and untruthful information can delay care and increase risk of medical complications.”