User login
Chest Pain Choice tool decreases health care utilization
Background: Patients who complain of chest pain make up over a quarter of annual hospital admissions, but not all chest pain is attributable to acute coronary syndrome. The one-page CPC document was developed to facilitate joint decision making between low-risk patients and providers regarding the work-up for chest pain.
Study design: Parallel, randomized, controlled trial.
Setting: Six U.S. medical centers.
Synopsis: After reviewing the CPC tool, patients with low cardiac risk who presented to the ED with chest pain were given the option either to be admitted to the hospital for cardiac testing or to not be admitted and instead follow up with their primary care doctor or a cardiologist within 3 days to determine what further cardiac work-up might be warranted.
Upon reviewing data obtained from 898 patient diaries regarding use of health care services, as well as from billing data from the medical centers, the researchers found no statistically significant difference between patients who used the CPC tool and those treated under usual care with regard to hospital readmission rates, length of stay in the ED, repeat ED visits, or clinic visits. However, at the 45-day follow-up mark, those in the CPC group had undergone fewer tests and cardiac imaging studies (decrease of 125.6 tests/100 patients; 95% confidence interval, 29.3-221.6).
Bottom line: Shared decision making between providers and patients with low cardiac risk factors that used the Chest Pain Choice tool decreased some health care utilization without worsening outcomes.
Citation: Schaffer JT et al. Impact of a shared decision-making intervention on health care utilization: A secondary analysis of the Chest Pain Choice multicenter randomized trial. Acad Emerg Med. 2018 Mar;25(3):293-300.
Dr. Ally is a hospitalist at UC San Diego Health and an assistant clinical professor at the University of California, San Diego.
Background: Patients who complain of chest pain make up over a quarter of annual hospital admissions, but not all chest pain is attributable to acute coronary syndrome. The one-page CPC document was developed to facilitate joint decision making between low-risk patients and providers regarding the work-up for chest pain.
Study design: Parallel, randomized, controlled trial.
Setting: Six U.S. medical centers.
Synopsis: After reviewing the CPC tool, patients with low cardiac risk who presented to the ED with chest pain were given the option either to be admitted to the hospital for cardiac testing or to not be admitted and instead follow up with their primary care doctor or a cardiologist within 3 days to determine what further cardiac work-up might be warranted.
Upon reviewing data obtained from 898 patient diaries regarding use of health care services, as well as from billing data from the medical centers, the researchers found no statistically significant difference between patients who used the CPC tool and those treated under usual care with regard to hospital readmission rates, length of stay in the ED, repeat ED visits, or clinic visits. However, at the 45-day follow-up mark, those in the CPC group had undergone fewer tests and cardiac imaging studies (decrease of 125.6 tests/100 patients; 95% confidence interval, 29.3-221.6).
Bottom line: Shared decision making between providers and patients with low cardiac risk factors that used the Chest Pain Choice tool decreased some health care utilization without worsening outcomes.
Citation: Schaffer JT et al. Impact of a shared decision-making intervention on health care utilization: A secondary analysis of the Chest Pain Choice multicenter randomized trial. Acad Emerg Med. 2018 Mar;25(3):293-300.
Dr. Ally is a hospitalist at UC San Diego Health and an assistant clinical professor at the University of California, San Diego.
Background: Patients who complain of chest pain make up over a quarter of annual hospital admissions, but not all chest pain is attributable to acute coronary syndrome. The one-page CPC document was developed to facilitate joint decision making between low-risk patients and providers regarding the work-up for chest pain.
Study design: Parallel, randomized, controlled trial.
Setting: Six U.S. medical centers.
Synopsis: After reviewing the CPC tool, patients with low cardiac risk who presented to the ED with chest pain were given the option either to be admitted to the hospital for cardiac testing or to not be admitted and instead follow up with their primary care doctor or a cardiologist within 3 days to determine what further cardiac work-up might be warranted.
Upon reviewing data obtained from 898 patient diaries regarding use of health care services, as well as from billing data from the medical centers, the researchers found no statistically significant difference between patients who used the CPC tool and those treated under usual care with regard to hospital readmission rates, length of stay in the ED, repeat ED visits, or clinic visits. However, at the 45-day follow-up mark, those in the CPC group had undergone fewer tests and cardiac imaging studies (decrease of 125.6 tests/100 patients; 95% confidence interval, 29.3-221.6).
Bottom line: Shared decision making between providers and patients with low cardiac risk factors that used the Chest Pain Choice tool decreased some health care utilization without worsening outcomes.
Citation: Schaffer JT et al. Impact of a shared decision-making intervention on health care utilization: A secondary analysis of the Chest Pain Choice multicenter randomized trial. Acad Emerg Med. 2018 Mar;25(3):293-300.
Dr. Ally is a hospitalist at UC San Diego Health and an assistant clinical professor at the University of California, San Diego.
Ropeg outperforms HU in PV patients of all ages
STOCKHOLM—Follow-up data suggest that ropeginterferon alfa-2b (ropeg) provides an advantage over hydroxyurea (HU) for patients with polycythemia vera (PV), regardless of their age.
Two-year results from an extension study have shown that, compared to HU, ropeg produces higher rates of complete hematologic response (CHR) and molecular response (MR) in PV patients, including patients age 60 and older.
Additionally, rates of adverse events (AEs) and serious AEs were similar between the ropeg and HU arms.
“In all, I think these data suggest that ropeginterferon alfa-2b provides a valuable, efficacious, and safe new treatment option for PV patients of all ages, including those older than 60 years,” said Jean-Jacques Kiladjian, MD, PhD, of Hôpital Saint-Louis, Université Paris Diderot in Paris, France.
Dr Kiladjian presented these results at the 23rd Congress of the European Hematology Association (EHA) as abstract S132.
The research was sponsored by AOP Orphan Pharmaceuticals AG.
Dr Kiladjian presented data from CONTINUATION-PV, an extension trial of PROUD-PV. Results from PROUD-PV were presented at the 2016 ASH Annual Meeting.
PROUD-PV enrolled 254 patients who were treatment-naive or pretreated with HU. They were randomized to receive ropeg (n=127) or HU (n=127).
CONTINUATION-PV included 95 of the patients on ropeg and 76 of the patients on HU. Dr Kiladjian noted that baseline characteristics were similar between these groups.
| Patient characteristics | ||||
| Ropeg | HU | |||
| <60 years (n=49) | ≥60 years (n=46) | <60 years (n=39) | ≥60 years (n=37) | |
| Median age (range) | 51 (30-59) | 64 (60-85) | 50 (32-59) | 66 (61-79) |
| Median duration of PV (range) | 1.9 months (0-145.5) | 1.7 months (0.1-102.4) | 1.6 months (0-91.6) | 1.7 months (0-65.6) |
| HU pretreatment | 17 (34.7%) | 14 (30.4%) | 12 (30.8%) | 13 (35.1%) |
| Mean duration of HU treatment | 11.3 months | 14.8 months | 16.0 months | 11.2 months |
| Mean JAK2V617F allele burden | 38.4% | 47.4% | 38.1% | 48.1% |
| Splenomegaly present | 2 (4.1%) | 5 (10.9%) | 4 (10.3%) | 4 (10.8%) |
| Disease-related symptoms present | 5 (10.2%) | 10 (21.7%) | 4 (10.3%) | 13 (35.1%) |
CHR
At 24 months, the rate of CHR was 70.5% (67/95) in the ropeg arm and 49.3% (33/67) in the HU arm (RR=1.42, P<0.05).
In patients younger than 60, the rate of CHR was 77.6% in the ropeg arm and 55.9% in the HU arm. In patients age 60 and older, rates of CHR were 63% and 42.4%, respectively.
Dr Kiladjian noted that CHR rates increased over time in ropeg recipients. In PROUD-PV, CHR rates were similar between the ropeg and HU arms at 12 months. However, at 24 months, the CHR rates were higher in ropeg recipients.
Ropeg recipients were also more likely than HU recipients to maintain their CHR from the first occurrence to 24 months.
In patients younger than 60, 49% of the ropeg arm and 17.9% of the HU arm maintained a CHR (P<0.001). In patients age 60 and older, rates of CHR maintenance were 37% and 18.9%, respectively.
MR and JAK2V617F allele burden
Rates of MR at 24 months were 68.1% (64/94) in the ropeg arm and 34.7% (26/75) in the HU arm (RR=1.85, P<0.01).
In patients younger than 60, the rate of MR was 77.1% in the ropeg arm and 33.3% in the HU arm (P<0.001). In patients age 60 and older, MR rates were 58.7% and 36.1%, respectively.
For patients younger than 60, the reduction in JAK2V617F allele burden at 12 months was 29.9% in the ropeg arm and 42.3% in the HU arm. At 24 months, the reductions were 54.8% and 4.5%, respectively (P<0.001).
For patients 60 and older, the reduction in JAK2V617F allele burden at 12 months was 25.2% in the ropeg arm and 37.5% in the HU arm. At 24 months, the reductions were 35.1% and 18.4%, respectively.
Safety
“I think an important point here is the safety because we assume that [ropeg] is not well tolerated in elderly patients,” Dr Kiladjian said. “So what are the results in this prospective, controlled trial? There was a comparable number of adverse events and serious adverse events in the treatment arms, irrespective of age.”
Dr Kiladjian also pointed out that the number of adverse drug reactions (ADRs) was comparable between the treatment arms for younger patients, and there was a trend toward a lower number of ADRs in the ropeg arm for the patients age 60 and older.
| Safety results at a mean of 2.7 years of treatment (up to 3.6 years) | ||||
| Ropeg | HU | |||
| <60 (n=49) | ≥60 (n=46) | <60 (n=39) | ≥60 (n=37) | |
| Patients with any AE | 44 (89.8%) | 43 (93.5%) | 36 (92.3%) | 34 (91.1%) |
| Serious AE | 3 (6.1%) | 10 (21.7%) | 4 (10.3%) | 9 (24.3%) |
| ADR | 38 (77.6%) | 29 (63%) | 29 (74.4%) | 33 (89.2%) |
| Serious ADR | 0 | 0 | 0 | 4 (10.8%) |
| Grade 3+ AE | 10 (20.4%) | 16 (34.8%) | 10 (25.6%) | 14 (37.8%) |
| Recovered from AE | 43 (87.8%) | 40 (87%) | 35 (89.7%) | 34 (91.9%) |
The 4 serious ADRs in the HU patients age 60 and older were acute leukemia, anemia, leukopenia, and granulocytopenia.
STOCKHOLM—Follow-up data suggest that ropeginterferon alfa-2b (ropeg) provides an advantage over hydroxyurea (HU) for patients with polycythemia vera (PV), regardless of their age.
Two-year results from an extension study have shown that, compared to HU, ropeg produces higher rates of complete hematologic response (CHR) and molecular response (MR) in PV patients, including patients age 60 and older.
Additionally, rates of adverse events (AEs) and serious AEs were similar between the ropeg and HU arms.
“In all, I think these data suggest that ropeginterferon alfa-2b provides a valuable, efficacious, and safe new treatment option for PV patients of all ages, including those older than 60 years,” said Jean-Jacques Kiladjian, MD, PhD, of Hôpital Saint-Louis, Université Paris Diderot in Paris, France.
Dr Kiladjian presented these results at the 23rd Congress of the European Hematology Association (EHA) as abstract S132.
The research was sponsored by AOP Orphan Pharmaceuticals AG.
Dr Kiladjian presented data from CONTINUATION-PV, an extension trial of PROUD-PV. Results from PROUD-PV were presented at the 2016 ASH Annual Meeting.
PROUD-PV enrolled 254 patients who were treatment-naive or pretreated with HU. They were randomized to receive ropeg (n=127) or HU (n=127).
CONTINUATION-PV included 95 of the patients on ropeg and 76 of the patients on HU. Dr Kiladjian noted that baseline characteristics were similar between these groups.
| Patient characteristics | ||||
| Ropeg | HU | |||
| <60 years (n=49) | ≥60 years (n=46) | <60 years (n=39) | ≥60 years (n=37) | |
| Median age (range) | 51 (30-59) | 64 (60-85) | 50 (32-59) | 66 (61-79) |
| Median duration of PV (range) | 1.9 months (0-145.5) | 1.7 months (0.1-102.4) | 1.6 months (0-91.6) | 1.7 months (0-65.6) |
| HU pretreatment | 17 (34.7%) | 14 (30.4%) | 12 (30.8%) | 13 (35.1%) |
| Mean duration of HU treatment | 11.3 months | 14.8 months | 16.0 months | 11.2 months |
| Mean JAK2V617F allele burden | 38.4% | 47.4% | 38.1% | 48.1% |
| Splenomegaly present | 2 (4.1%) | 5 (10.9%) | 4 (10.3%) | 4 (10.8%) |
| Disease-related symptoms present | 5 (10.2%) | 10 (21.7%) | 4 (10.3%) | 13 (35.1%) |
CHR
At 24 months, the rate of CHR was 70.5% (67/95) in the ropeg arm and 49.3% (33/67) in the HU arm (RR=1.42, P<0.05).
In patients younger than 60, the rate of CHR was 77.6% in the ropeg arm and 55.9% in the HU arm. In patients age 60 and older, rates of CHR were 63% and 42.4%, respectively.
Dr Kiladjian noted that CHR rates increased over time in ropeg recipients. In PROUD-PV, CHR rates were similar between the ropeg and HU arms at 12 months. However, at 24 months, the CHR rates were higher in ropeg recipients.
Ropeg recipients were also more likely than HU recipients to maintain their CHR from the first occurrence to 24 months.
In patients younger than 60, 49% of the ropeg arm and 17.9% of the HU arm maintained a CHR (P<0.001). In patients age 60 and older, rates of CHR maintenance were 37% and 18.9%, respectively.
MR and JAK2V617F allele burden
Rates of MR at 24 months were 68.1% (64/94) in the ropeg arm and 34.7% (26/75) in the HU arm (RR=1.85, P<0.01).
In patients younger than 60, the rate of MR was 77.1% in the ropeg arm and 33.3% in the HU arm (P<0.001). In patients age 60 and older, MR rates were 58.7% and 36.1%, respectively.
For patients younger than 60, the reduction in JAK2V617F allele burden at 12 months was 29.9% in the ropeg arm and 42.3% in the HU arm. At 24 months, the reductions were 54.8% and 4.5%, respectively (P<0.001).
For patients 60 and older, the reduction in JAK2V617F allele burden at 12 months was 25.2% in the ropeg arm and 37.5% in the HU arm. At 24 months, the reductions were 35.1% and 18.4%, respectively.
Safety
“I think an important point here is the safety because we assume that [ropeg] is not well tolerated in elderly patients,” Dr Kiladjian said. “So what are the results in this prospective, controlled trial? There was a comparable number of adverse events and serious adverse events in the treatment arms, irrespective of age.”
Dr Kiladjian also pointed out that the number of adverse drug reactions (ADRs) was comparable between the treatment arms for younger patients, and there was a trend toward a lower number of ADRs in the ropeg arm for the patients age 60 and older.
| Safety results at a mean of 2.7 years of treatment (up to 3.6 years) | ||||
| Ropeg | HU | |||
| <60 (n=49) | ≥60 (n=46) | <60 (n=39) | ≥60 (n=37) | |
| Patients with any AE | 44 (89.8%) | 43 (93.5%) | 36 (92.3%) | 34 (91.1%) |
| Serious AE | 3 (6.1%) | 10 (21.7%) | 4 (10.3%) | 9 (24.3%) |
| ADR | 38 (77.6%) | 29 (63%) | 29 (74.4%) | 33 (89.2%) |
| Serious ADR | 0 | 0 | 0 | 4 (10.8%) |
| Grade 3+ AE | 10 (20.4%) | 16 (34.8%) | 10 (25.6%) | 14 (37.8%) |
| Recovered from AE | 43 (87.8%) | 40 (87%) | 35 (89.7%) | 34 (91.9%) |
The 4 serious ADRs in the HU patients age 60 and older were acute leukemia, anemia, leukopenia, and granulocytopenia.
STOCKHOLM—Follow-up data suggest that ropeginterferon alfa-2b (ropeg) provides an advantage over hydroxyurea (HU) for patients with polycythemia vera (PV), regardless of their age.
Two-year results from an extension study have shown that, compared to HU, ropeg produces higher rates of complete hematologic response (CHR) and molecular response (MR) in PV patients, including patients age 60 and older.
Additionally, rates of adverse events (AEs) and serious AEs were similar between the ropeg and HU arms.
“In all, I think these data suggest that ropeginterferon alfa-2b provides a valuable, efficacious, and safe new treatment option for PV patients of all ages, including those older than 60 years,” said Jean-Jacques Kiladjian, MD, PhD, of Hôpital Saint-Louis, Université Paris Diderot in Paris, France.
Dr Kiladjian presented these results at the 23rd Congress of the European Hematology Association (EHA) as abstract S132.
The research was sponsored by AOP Orphan Pharmaceuticals AG.
Dr Kiladjian presented data from CONTINUATION-PV, an extension trial of PROUD-PV. Results from PROUD-PV were presented at the 2016 ASH Annual Meeting.
PROUD-PV enrolled 254 patients who were treatment-naive or pretreated with HU. They were randomized to receive ropeg (n=127) or HU (n=127).
CONTINUATION-PV included 95 of the patients on ropeg and 76 of the patients on HU. Dr Kiladjian noted that baseline characteristics were similar between these groups.
| Patient characteristics | ||||
| Ropeg | HU | |||
| <60 years (n=49) | ≥60 years (n=46) | <60 years (n=39) | ≥60 years (n=37) | |
| Median age (range) | 51 (30-59) | 64 (60-85) | 50 (32-59) | 66 (61-79) |
| Median duration of PV (range) | 1.9 months (0-145.5) | 1.7 months (0.1-102.4) | 1.6 months (0-91.6) | 1.7 months (0-65.6) |
| HU pretreatment | 17 (34.7%) | 14 (30.4%) | 12 (30.8%) | 13 (35.1%) |
| Mean duration of HU treatment | 11.3 months | 14.8 months | 16.0 months | 11.2 months |
| Mean JAK2V617F allele burden | 38.4% | 47.4% | 38.1% | 48.1% |
| Splenomegaly present | 2 (4.1%) | 5 (10.9%) | 4 (10.3%) | 4 (10.8%) |
| Disease-related symptoms present | 5 (10.2%) | 10 (21.7%) | 4 (10.3%) | 13 (35.1%) |
CHR
At 24 months, the rate of CHR was 70.5% (67/95) in the ropeg arm and 49.3% (33/67) in the HU arm (RR=1.42, P<0.05).
In patients younger than 60, the rate of CHR was 77.6% in the ropeg arm and 55.9% in the HU arm. In patients age 60 and older, rates of CHR were 63% and 42.4%, respectively.
Dr Kiladjian noted that CHR rates increased over time in ropeg recipients. In PROUD-PV, CHR rates were similar between the ropeg and HU arms at 12 months. However, at 24 months, the CHR rates were higher in ropeg recipients.
Ropeg recipients were also more likely than HU recipients to maintain their CHR from the first occurrence to 24 months.
In patients younger than 60, 49% of the ropeg arm and 17.9% of the HU arm maintained a CHR (P<0.001). In patients age 60 and older, rates of CHR maintenance were 37% and 18.9%, respectively.
MR and JAK2V617F allele burden
Rates of MR at 24 months were 68.1% (64/94) in the ropeg arm and 34.7% (26/75) in the HU arm (RR=1.85, P<0.01).
In patients younger than 60, the rate of MR was 77.1% in the ropeg arm and 33.3% in the HU arm (P<0.001). In patients age 60 and older, MR rates were 58.7% and 36.1%, respectively.
For patients younger than 60, the reduction in JAK2V617F allele burden at 12 months was 29.9% in the ropeg arm and 42.3% in the HU arm. At 24 months, the reductions were 54.8% and 4.5%, respectively (P<0.001).
For patients 60 and older, the reduction in JAK2V617F allele burden at 12 months was 25.2% in the ropeg arm and 37.5% in the HU arm. At 24 months, the reductions were 35.1% and 18.4%, respectively.
Safety
“I think an important point here is the safety because we assume that [ropeg] is not well tolerated in elderly patients,” Dr Kiladjian said. “So what are the results in this prospective, controlled trial? There was a comparable number of adverse events and serious adverse events in the treatment arms, irrespective of age.”
Dr Kiladjian also pointed out that the number of adverse drug reactions (ADRs) was comparable between the treatment arms for younger patients, and there was a trend toward a lower number of ADRs in the ropeg arm for the patients age 60 and older.
| Safety results at a mean of 2.7 years of treatment (up to 3.6 years) | ||||
| Ropeg | HU | |||
| <60 (n=49) | ≥60 (n=46) | <60 (n=39) | ≥60 (n=37) | |
| Patients with any AE | 44 (89.8%) | 43 (93.5%) | 36 (92.3%) | 34 (91.1%) |
| Serious AE | 3 (6.1%) | 10 (21.7%) | 4 (10.3%) | 9 (24.3%) |
| ADR | 38 (77.6%) | 29 (63%) | 29 (74.4%) | 33 (89.2%) |
| Serious ADR | 0 | 0 | 0 | 4 (10.8%) |
| Grade 3+ AE | 10 (20.4%) | 16 (34.8%) | 10 (25.6%) | 14 (37.8%) |
| Recovered from AE | 43 (87.8%) | 40 (87%) | 35 (89.7%) | 34 (91.9%) |
The 4 serious ADRs in the HU patients age 60 and older were acute leukemia, anemia, leukopenia, and granulocytopenia.
Many CCSs not concerned about future health
A survey of more than 15,000 childhood cancer survivors (CCSs) revealed that many were unconcerned about their risk of health problems.
Thirty-one percent of CCSs said they were “not at all” or “not very” concerned about their future health, and 40% said they were “not at all” or “not very” concerned about developing new cancers.
Researchers say it isn’t clear what’s driving this lack of concern, but it is possible that some CCSs don’t know they have an increased risk of new malignancies and other health problems.
“Other possibilities include that some survivors may actually be aware of their increased risks and choose not to be concerned, or it may even be that some survivors are, indeed, following health guidelines and working with healthcare providers, leading to their lack of concern,” said Todd Gibson, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Dr Gibson and his colleagues conducted this research and reported the results in Cancer.
The researchers analyzed data on 15,620 CCSs and 3991 of their siblings who did not have a history of cancer. The data came from questionnaires administered to participants in the Childhood Cancer Survivor Study.
At baseline, the median time from CCSs’ cancer diagnosis was 17 years (interquartile range [IQR], 14-21). Their median age at baseline was 26 (IQR, 22-31 years), and the siblings’ median age was 29 (IQR, 24-35).
When respondents were asked about their level of concern regarding their future health, the answers were as follows:
- 12% of both CCSs and siblings were “not at all concerned”
- 18.7% of CCSs and 21.6% of siblings were “not very concerned”
- 23.2% of CCSs and 24.1% of siblings were “concerned”
- 21.4% of CCSs and 22.2% of siblings were “somewhat concerned”
- 24.8% of CCSs and 20.1% of siblings were “very concerned.”
When respondents were asked to rate their level of concern about developing a new cancer, the answers were as follows:
- 17.2% of CCSs and 16.3% of siblings were “not at all concerned”
- 22.8% of CCSs and 22.2% of siblings were “not very concerned”
- 21.1% of CCSs and 25.1% of siblings were “concerned”
- 18.3% of CCSs and 18.4% of siblings were “somewhat concerned”
- 20.6% of CCSs and 18.1% of siblings were “very concerned.”
When the researchers adjusted for age, sex, race/ethnicity, education, and decade of diagnosis, CCSs were only slightly more likely than siblings to report concern about future health (relative risk, 1.12) or subsequent cancer (relative risk, 1.02).
“That similarity [between CCS and sibling answers] was really the major surprise in our findings,” Dr Gibson said. “Despite the fact that survivors have such a greatly increased risk of both second cancers and other health problems, their perception of risk was not always commensurate with their actual risk.”
The researchers did find that CCSs were more likely to report concern about future health or developing cancer if they were female, non-Hispanic black or Hispanic, older, identified as having clinical anxiety, or had already experienced a grade 3/4 chronic condition or subsequent cancer.
However, it isn’t clear why some CCSs are concerned about their future health and others are not.
“At this point, we can only speculate, but the most obvious reason [for lack of concern] would be that survivors may not fully understand their risks,” Dr Gibson said. “We do know from prior studies that not all survivors are fully aware of the specific treatments they received and how those might increase their risks of late effects.”
“If, however, survivors are aware but not motivated or sufficiently aroused to be concerned, then more motivational education will have to be developed. In any case, these findings offer a teaching point that we can use to emphasize to all survivors that they need to understand their risks.”
A survey of more than 15,000 childhood cancer survivors (CCSs) revealed that many were unconcerned about their risk of health problems.
Thirty-one percent of CCSs said they were “not at all” or “not very” concerned about their future health, and 40% said they were “not at all” or “not very” concerned about developing new cancers.
Researchers say it isn’t clear what’s driving this lack of concern, but it is possible that some CCSs don’t know they have an increased risk of new malignancies and other health problems.
“Other possibilities include that some survivors may actually be aware of their increased risks and choose not to be concerned, or it may even be that some survivors are, indeed, following health guidelines and working with healthcare providers, leading to their lack of concern,” said Todd Gibson, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Dr Gibson and his colleagues conducted this research and reported the results in Cancer.
The researchers analyzed data on 15,620 CCSs and 3991 of their siblings who did not have a history of cancer. The data came from questionnaires administered to participants in the Childhood Cancer Survivor Study.
At baseline, the median time from CCSs’ cancer diagnosis was 17 years (interquartile range [IQR], 14-21). Their median age at baseline was 26 (IQR, 22-31 years), and the siblings’ median age was 29 (IQR, 24-35).
When respondents were asked about their level of concern regarding their future health, the answers were as follows:
- 12% of both CCSs and siblings were “not at all concerned”
- 18.7% of CCSs and 21.6% of siblings were “not very concerned”
- 23.2% of CCSs and 24.1% of siblings were “concerned”
- 21.4% of CCSs and 22.2% of siblings were “somewhat concerned”
- 24.8% of CCSs and 20.1% of siblings were “very concerned.”
When respondents were asked to rate their level of concern about developing a new cancer, the answers were as follows:
- 17.2% of CCSs and 16.3% of siblings were “not at all concerned”
- 22.8% of CCSs and 22.2% of siblings were “not very concerned”
- 21.1% of CCSs and 25.1% of siblings were “concerned”
- 18.3% of CCSs and 18.4% of siblings were “somewhat concerned”
- 20.6% of CCSs and 18.1% of siblings were “very concerned.”
When the researchers adjusted for age, sex, race/ethnicity, education, and decade of diagnosis, CCSs were only slightly more likely than siblings to report concern about future health (relative risk, 1.12) or subsequent cancer (relative risk, 1.02).
“That similarity [between CCS and sibling answers] was really the major surprise in our findings,” Dr Gibson said. “Despite the fact that survivors have such a greatly increased risk of both second cancers and other health problems, their perception of risk was not always commensurate with their actual risk.”
The researchers did find that CCSs were more likely to report concern about future health or developing cancer if they were female, non-Hispanic black or Hispanic, older, identified as having clinical anxiety, or had already experienced a grade 3/4 chronic condition or subsequent cancer.
However, it isn’t clear why some CCSs are concerned about their future health and others are not.
“At this point, we can only speculate, but the most obvious reason [for lack of concern] would be that survivors may not fully understand their risks,” Dr Gibson said. “We do know from prior studies that not all survivors are fully aware of the specific treatments they received and how those might increase their risks of late effects.”
“If, however, survivors are aware but not motivated or sufficiently aroused to be concerned, then more motivational education will have to be developed. In any case, these findings offer a teaching point that we can use to emphasize to all survivors that they need to understand their risks.”
A survey of more than 15,000 childhood cancer survivors (CCSs) revealed that many were unconcerned about their risk of health problems.
Thirty-one percent of CCSs said they were “not at all” or “not very” concerned about their future health, and 40% said they were “not at all” or “not very” concerned about developing new cancers.
Researchers say it isn’t clear what’s driving this lack of concern, but it is possible that some CCSs don’t know they have an increased risk of new malignancies and other health problems.
“Other possibilities include that some survivors may actually be aware of their increased risks and choose not to be concerned, or it may even be that some survivors are, indeed, following health guidelines and working with healthcare providers, leading to their lack of concern,” said Todd Gibson, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Dr Gibson and his colleagues conducted this research and reported the results in Cancer.
The researchers analyzed data on 15,620 CCSs and 3991 of their siblings who did not have a history of cancer. The data came from questionnaires administered to participants in the Childhood Cancer Survivor Study.
At baseline, the median time from CCSs’ cancer diagnosis was 17 years (interquartile range [IQR], 14-21). Their median age at baseline was 26 (IQR, 22-31 years), and the siblings’ median age was 29 (IQR, 24-35).
When respondents were asked about their level of concern regarding their future health, the answers were as follows:
- 12% of both CCSs and siblings were “not at all concerned”
- 18.7% of CCSs and 21.6% of siblings were “not very concerned”
- 23.2% of CCSs and 24.1% of siblings were “concerned”
- 21.4% of CCSs and 22.2% of siblings were “somewhat concerned”
- 24.8% of CCSs and 20.1% of siblings were “very concerned.”
When respondents were asked to rate their level of concern about developing a new cancer, the answers were as follows:
- 17.2% of CCSs and 16.3% of siblings were “not at all concerned”
- 22.8% of CCSs and 22.2% of siblings were “not very concerned”
- 21.1% of CCSs and 25.1% of siblings were “concerned”
- 18.3% of CCSs and 18.4% of siblings were “somewhat concerned”
- 20.6% of CCSs and 18.1% of siblings were “very concerned.”
When the researchers adjusted for age, sex, race/ethnicity, education, and decade of diagnosis, CCSs were only slightly more likely than siblings to report concern about future health (relative risk, 1.12) or subsequent cancer (relative risk, 1.02).
“That similarity [between CCS and sibling answers] was really the major surprise in our findings,” Dr Gibson said. “Despite the fact that survivors have such a greatly increased risk of both second cancers and other health problems, their perception of risk was not always commensurate with their actual risk.”
The researchers did find that CCSs were more likely to report concern about future health or developing cancer if they were female, non-Hispanic black or Hispanic, older, identified as having clinical anxiety, or had already experienced a grade 3/4 chronic condition or subsequent cancer.
However, it isn’t clear why some CCSs are concerned about their future health and others are not.
“At this point, we can only speculate, but the most obvious reason [for lack of concern] would be that survivors may not fully understand their risks,” Dr Gibson said. “We do know from prior studies that not all survivors are fully aware of the specific treatments they received and how those might increase their risks of late effects.”
“If, however, survivors are aware but not motivated or sufficiently aroused to be concerned, then more motivational education will have to be developed. In any case, these findings offer a teaching point that we can use to emphasize to all survivors that they need to understand their risks.”
FDA grants priority review to drug for AML
The US Food and Drug Administration (FDA) has accepted for priority review a new drug application (NDA) for glasdegib, an oral SMO inhibitor.
With this NDA, Pfizer is seeking approval for glasdegib in combination with low-dose cytarabine (LDAC) as a treatment for adults with previously untreated acute myeloid leukemia (AML).
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency intends to take action on a priority review application within 6 months of receiving it rather than the standard 10 months.
The FDA expects to make a decision on the NDA for glasdegib by December 2018.
The NDA is supported by results from the phase 2 BRIGHT 1003 study. Results from this trial were presented at the 2016 ASH Annual Meeting.
The trial was a comparison of glasdegib plus LDAC (n=88) to LDAC alone (n=44) in patients with previously untreated AML or high-risk myelodysplastic syndromes who were not eligible for intensive chemotherapy.
Results demonstrated a significant improvement in overall survival with glasdegib. The median overall survival was 8.8 months in the glasdegib arm and 4.9 months in the LDAC-alone arm.
This difference represented a 49.9% reduction in the risk of death for patients treated with glasdegib plus LDAC (hazard ratio=0.501; 95% CI: 0.334, 0.752; one-sided P-value=0.0003).
The most frequent adverse events—occurring in at least 30% of patients in the glasdegib arm and LDAC-alone arm, respectively—were anemia (45% vs 42%), febrile neutropenia (36% vs 27%), nausea (36% vs 12%), decreased appetite (32% vs 12%), fatigue (31% vs 20%), and thrombocytopenia (30% vs 27%).
The most frequently reported serious adverse events—occurring in at least 15% of patients in the glasdegib and LDAC-alone arms, respectively—were febrile neutropenia (29% vs 20%) and pneumonia (21% vs 17%).
A phase 3 trial of glasdegib in AML began enrolling earlier this year. In this trial (BRIGHT AML 1019; NCT03416179), researchers are evaluating glasdegib plus intensive or non-intensive chemotherapy in patients with newly diagnosed AML.
The US Food and Drug Administration (FDA) has accepted for priority review a new drug application (NDA) for glasdegib, an oral SMO inhibitor.
With this NDA, Pfizer is seeking approval for glasdegib in combination with low-dose cytarabine (LDAC) as a treatment for adults with previously untreated acute myeloid leukemia (AML).
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency intends to take action on a priority review application within 6 months of receiving it rather than the standard 10 months.
The FDA expects to make a decision on the NDA for glasdegib by December 2018.
The NDA is supported by results from the phase 2 BRIGHT 1003 study. Results from this trial were presented at the 2016 ASH Annual Meeting.
The trial was a comparison of glasdegib plus LDAC (n=88) to LDAC alone (n=44) in patients with previously untreated AML or high-risk myelodysplastic syndromes who were not eligible for intensive chemotherapy.
Results demonstrated a significant improvement in overall survival with glasdegib. The median overall survival was 8.8 months in the glasdegib arm and 4.9 months in the LDAC-alone arm.
This difference represented a 49.9% reduction in the risk of death for patients treated with glasdegib plus LDAC (hazard ratio=0.501; 95% CI: 0.334, 0.752; one-sided P-value=0.0003).
The most frequent adverse events—occurring in at least 30% of patients in the glasdegib arm and LDAC-alone arm, respectively—were anemia (45% vs 42%), febrile neutropenia (36% vs 27%), nausea (36% vs 12%), decreased appetite (32% vs 12%), fatigue (31% vs 20%), and thrombocytopenia (30% vs 27%).
The most frequently reported serious adverse events—occurring in at least 15% of patients in the glasdegib and LDAC-alone arms, respectively—were febrile neutropenia (29% vs 20%) and pneumonia (21% vs 17%).
A phase 3 trial of glasdegib in AML began enrolling earlier this year. In this trial (BRIGHT AML 1019; NCT03416179), researchers are evaluating glasdegib plus intensive or non-intensive chemotherapy in patients with newly diagnosed AML.
The US Food and Drug Administration (FDA) has accepted for priority review a new drug application (NDA) for glasdegib, an oral SMO inhibitor.
With this NDA, Pfizer is seeking approval for glasdegib in combination with low-dose cytarabine (LDAC) as a treatment for adults with previously untreated acute myeloid leukemia (AML).
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency intends to take action on a priority review application within 6 months of receiving it rather than the standard 10 months.
The FDA expects to make a decision on the NDA for glasdegib by December 2018.
The NDA is supported by results from the phase 2 BRIGHT 1003 study. Results from this trial were presented at the 2016 ASH Annual Meeting.
The trial was a comparison of glasdegib plus LDAC (n=88) to LDAC alone (n=44) in patients with previously untreated AML or high-risk myelodysplastic syndromes who were not eligible for intensive chemotherapy.
Results demonstrated a significant improvement in overall survival with glasdegib. The median overall survival was 8.8 months in the glasdegib arm and 4.9 months in the LDAC-alone arm.
This difference represented a 49.9% reduction in the risk of death for patients treated with glasdegib plus LDAC (hazard ratio=0.501; 95% CI: 0.334, 0.752; one-sided P-value=0.0003).
The most frequent adverse events—occurring in at least 30% of patients in the glasdegib arm and LDAC-alone arm, respectively—were anemia (45% vs 42%), febrile neutropenia (36% vs 27%), nausea (36% vs 12%), decreased appetite (32% vs 12%), fatigue (31% vs 20%), and thrombocytopenia (30% vs 27%).
The most frequently reported serious adverse events—occurring in at least 15% of patients in the glasdegib and LDAC-alone arms, respectively—were febrile neutropenia (29% vs 20%) and pneumonia (21% vs 17%).
A phase 3 trial of glasdegib in AML began enrolling earlier this year. In this trial (BRIGHT AML 1019; NCT03416179), researchers are evaluating glasdegib plus intensive or non-intensive chemotherapy in patients with newly diagnosed AML.
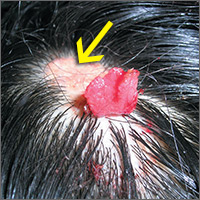
Changing growth on scalp
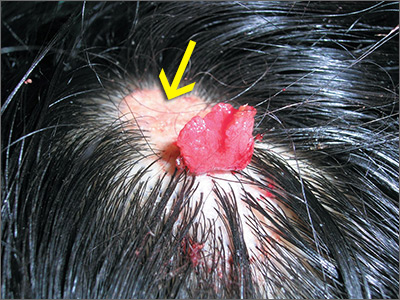
The FP thought the flat lesion (arrow) might be a nevus sebaceous (NS) and that the new area could be a malignant transformation.
The FP explained that a biopsy would be needed to learn more about the lesion. He explained that he would remove the area that was friable and bleeding along with part of the original flat lesion. After injecting the area with 1% lidocaine and epinephrine, a shave biopsy was performed using a DermaBlade. (See the Watch & Learn video on “Shave biopsy.”) The bleeding was stopped using aluminum chloride in water and some electrosurgery. The pathology results revealed syringocystadenoma papilliferum growing within an NS. This benign tumor is rare, but may develop within an NS.
The FP reassured the family that there was no skin cancer. The FP also referred the patient for full removal of the NS and any remnant of the syringocystadenoma papilliferum to avoid future growth and prevent additional bleeding.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Epidermal nevus and nevus sebaceous. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine, 2nd ed. New York, NY: McGraw-Hill; 2013:958-962.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP thought the flat lesion (arrow) might be a nevus sebaceous (NS) and that the new area could be a malignant transformation.
The FP explained that a biopsy would be needed to learn more about the lesion. He explained that he would remove the area that was friable and bleeding along with part of the original flat lesion. After injecting the area with 1% lidocaine and epinephrine, a shave biopsy was performed using a DermaBlade. (See the Watch & Learn video on “Shave biopsy.”) The bleeding was stopped using aluminum chloride in water and some electrosurgery. The pathology results revealed syringocystadenoma papilliferum growing within an NS. This benign tumor is rare, but may develop within an NS.
The FP reassured the family that there was no skin cancer. The FP also referred the patient for full removal of the NS and any remnant of the syringocystadenoma papilliferum to avoid future growth and prevent additional bleeding.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Epidermal nevus and nevus sebaceous. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine, 2nd ed. New York, NY: McGraw-Hill; 2013:958-962.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP thought the flat lesion (arrow) might be a nevus sebaceous (NS) and that the new area could be a malignant transformation.
The FP explained that a biopsy would be needed to learn more about the lesion. He explained that he would remove the area that was friable and bleeding along with part of the original flat lesion. After injecting the area with 1% lidocaine and epinephrine, a shave biopsy was performed using a DermaBlade. (See the Watch & Learn video on “Shave biopsy.”) The bleeding was stopped using aluminum chloride in water and some electrosurgery. The pathology results revealed syringocystadenoma papilliferum growing within an NS. This benign tumor is rare, but may develop within an NS.
The FP reassured the family that there was no skin cancer. The FP also referred the patient for full removal of the NS and any remnant of the syringocystadenoma papilliferum to avoid future growth and prevent additional bleeding.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Epidermal nevus and nevus sebaceous. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine, 2nd ed. New York, NY: McGraw-Hill; 2013:958-962.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Steroids do not reduce mortality in patients with septic shock
Clinical question: Among patients with septic shock undergoing mechanical ventilation, does hydrocortisone reduce 90-day mortality?
Background: Septic shock is associated with a significant mortality risk, and there is no proven pharmacologic treatment other than fluids, vasopressors, and antimicrobials. Prior randomized, controlled trials have resulted in mixed outcomes, and meta-analyses and clinical practice guidelines also have not provided consistent guidance.
Study design: Randomized, controlled, double-blinded trial.
Setting: Medical centers in Australia, Denmark, New Zealand, Saudi Arabia, and the United Kingdom.
Synopsis: Over a 4-year period from 2013 to 2017, 3,658 patients with septic shock undergoing mechanical ventilation were randomized to receive either a continuous infusion of 200 mg/day of hydrocortisone for 7 days or placebo. The primary outcome, death within 90 days, occurred in 511 patients (27.9%) in the hydrocortisone group and in 526 patients (28.8%) in the placebo group (P = .50).
In secondary outcome analyses, patients in the hydrocortisone group had faster resolution of shock (3 vs. 4 days; P less than .001) and a shorter duration of initial mechanical ventilation (6 vs. 7 days; P less than .001), and fewer patients received blood transfusions (37.0% vs. 41.7%; P = .004). There was no difference in mortality at 28 days, recurrence of shock, number of days alive out of the ICU and hospital, recurrence of mechanical ventilation, rate of renal replacement therapy, and incidence of new-onset bacteremia or fungemia.
Bottom line: Administering hydrocortisone in patients with septic shock who are undergoing mechanical ventilation does not reduce 90-day mortality.
Citation: Venkatesh B et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018 Jan 19. doi: 10.1056/NEJMoa1705835.
Dr. Huang is associate chief of the division of hospital medicine at UC San Diego Health and an associate professor of medicine at the University of California, San Diego.
Clinical question: Among patients with septic shock undergoing mechanical ventilation, does hydrocortisone reduce 90-day mortality?
Background: Septic shock is associated with a significant mortality risk, and there is no proven pharmacologic treatment other than fluids, vasopressors, and antimicrobials. Prior randomized, controlled trials have resulted in mixed outcomes, and meta-analyses and clinical practice guidelines also have not provided consistent guidance.
Study design: Randomized, controlled, double-blinded trial.
Setting: Medical centers in Australia, Denmark, New Zealand, Saudi Arabia, and the United Kingdom.
Synopsis: Over a 4-year period from 2013 to 2017, 3,658 patients with septic shock undergoing mechanical ventilation were randomized to receive either a continuous infusion of 200 mg/day of hydrocortisone for 7 days or placebo. The primary outcome, death within 90 days, occurred in 511 patients (27.9%) in the hydrocortisone group and in 526 patients (28.8%) in the placebo group (P = .50).
In secondary outcome analyses, patients in the hydrocortisone group had faster resolution of shock (3 vs. 4 days; P less than .001) and a shorter duration of initial mechanical ventilation (6 vs. 7 days; P less than .001), and fewer patients received blood transfusions (37.0% vs. 41.7%; P = .004). There was no difference in mortality at 28 days, recurrence of shock, number of days alive out of the ICU and hospital, recurrence of mechanical ventilation, rate of renal replacement therapy, and incidence of new-onset bacteremia or fungemia.
Bottom line: Administering hydrocortisone in patients with septic shock who are undergoing mechanical ventilation does not reduce 90-day mortality.
Citation: Venkatesh B et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018 Jan 19. doi: 10.1056/NEJMoa1705835.
Dr. Huang is associate chief of the division of hospital medicine at UC San Diego Health and an associate professor of medicine at the University of California, San Diego.
Clinical question: Among patients with septic shock undergoing mechanical ventilation, does hydrocortisone reduce 90-day mortality?
Background: Septic shock is associated with a significant mortality risk, and there is no proven pharmacologic treatment other than fluids, vasopressors, and antimicrobials. Prior randomized, controlled trials have resulted in mixed outcomes, and meta-analyses and clinical practice guidelines also have not provided consistent guidance.
Study design: Randomized, controlled, double-blinded trial.
Setting: Medical centers in Australia, Denmark, New Zealand, Saudi Arabia, and the United Kingdom.
Synopsis: Over a 4-year period from 2013 to 2017, 3,658 patients with septic shock undergoing mechanical ventilation were randomized to receive either a continuous infusion of 200 mg/day of hydrocortisone for 7 days or placebo. The primary outcome, death within 90 days, occurred in 511 patients (27.9%) in the hydrocortisone group and in 526 patients (28.8%) in the placebo group (P = .50).
In secondary outcome analyses, patients in the hydrocortisone group had faster resolution of shock (3 vs. 4 days; P less than .001) and a shorter duration of initial mechanical ventilation (6 vs. 7 days; P less than .001), and fewer patients received blood transfusions (37.0% vs. 41.7%; P = .004). There was no difference in mortality at 28 days, recurrence of shock, number of days alive out of the ICU and hospital, recurrence of mechanical ventilation, rate of renal replacement therapy, and incidence of new-onset bacteremia or fungemia.
Bottom line: Administering hydrocortisone in patients with septic shock who are undergoing mechanical ventilation does not reduce 90-day mortality.
Citation: Venkatesh B et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018 Jan 19. doi: 10.1056/NEJMoa1705835.
Dr. Huang is associate chief of the division of hospital medicine at UC San Diego Health and an associate professor of medicine at the University of California, San Diego.
FDA approves encorafenib/binimetinib for advanced melanoma with BRAF mutations
The Food and Drug Administration has approved combination therapy of encorafenib (Braftovi) and binimetinib (Mektovi) for the treatment of unresectable or metastatic melanoma with BRAF V600E or BRAF V600K mutations; the FDA also has approved the THxID BRAF Kit as a companion diagnostic for this combination therapy.
The approval was based on results from the randomized, active-controlled, open-label, multicenter COLUMBUS trial, which included 517 patients. Progression-free survival, according to RECIST 1.1 criteria, was the major efficacy measure; the median progression-free survival was 14.9 months in the encorafenib/binimetinib combination arm versus 7.3 months in the vemurafenib (Zelboraf) monotherapy arm (hazard ratio, 0.54; 95% confidence interval, 0.41-0.71; P less than .0001).
Fatigue, nausea, diarrhea, vomiting, abdominal pain, and arthralgia were the most common adverse reactions. Discontinuation of therapy from adverse reactions occurred in 5% of patients receiving the combination, the FDA said in a press statement.
The full prescribing information for encorafenib and binimetinib can be found on the FDA website.
The Food and Drug Administration has approved combination therapy of encorafenib (Braftovi) and binimetinib (Mektovi) for the treatment of unresectable or metastatic melanoma with BRAF V600E or BRAF V600K mutations; the FDA also has approved the THxID BRAF Kit as a companion diagnostic for this combination therapy.
The approval was based on results from the randomized, active-controlled, open-label, multicenter COLUMBUS trial, which included 517 patients. Progression-free survival, according to RECIST 1.1 criteria, was the major efficacy measure; the median progression-free survival was 14.9 months in the encorafenib/binimetinib combination arm versus 7.3 months in the vemurafenib (Zelboraf) monotherapy arm (hazard ratio, 0.54; 95% confidence interval, 0.41-0.71; P less than .0001).
Fatigue, nausea, diarrhea, vomiting, abdominal pain, and arthralgia were the most common adverse reactions. Discontinuation of therapy from adverse reactions occurred in 5% of patients receiving the combination, the FDA said in a press statement.
The full prescribing information for encorafenib and binimetinib can be found on the FDA website.
The Food and Drug Administration has approved combination therapy of encorafenib (Braftovi) and binimetinib (Mektovi) for the treatment of unresectable or metastatic melanoma with BRAF V600E or BRAF V600K mutations; the FDA also has approved the THxID BRAF Kit as a companion diagnostic for this combination therapy.
The approval was based on results from the randomized, active-controlled, open-label, multicenter COLUMBUS trial, which included 517 patients. Progression-free survival, according to RECIST 1.1 criteria, was the major efficacy measure; the median progression-free survival was 14.9 months in the encorafenib/binimetinib combination arm versus 7.3 months in the vemurafenib (Zelboraf) monotherapy arm (hazard ratio, 0.54; 95% confidence interval, 0.41-0.71; P less than .0001).
Fatigue, nausea, diarrhea, vomiting, abdominal pain, and arthralgia were the most common adverse reactions. Discontinuation of therapy from adverse reactions occurred in 5% of patients receiving the combination, the FDA said in a press statement.
The full prescribing information for encorafenib and binimetinib can be found on the FDA website.
Think local when assessing adolescent heroin use
SAN DIEGO – Between 1999 and 2015, heroin use increased among high school students who live in Milwaukee, Chicago, and New York – trends that accompanied a rise in injection drug use in those cities.
“This implies that a subset of these students who are using heroin are probably injecting heroin as well; otherwise these trends wouldn’t be mirroring each other so well,” lead study author Sherri-Chanelle Brighthaupt said in an interview at the annual meeting of the College on Problems of Drug Dependence.
The finding comes from a trend analysis of heroin use and injection drug use in nine urban U.S. school districts drawn from Youth Risk Behavior Survey (YRBS) data from 1999-2015. The analysis was conducted using data from New York City, three Florida counties (Broward, Orange, and Miami-Dade), Dallas, Chicago, Milwaukee, and two California cities (San Diego and San Bernardino). a third-year doctoral student in the department of mental health at Johns Hopkins University, Baltimore. “National estimates may mask variation at the local level.”
The sample population studied included local-level YRBS responses from 260,952 students in grades 9-12. All responses were weighted by sex, grade, and race/ethnicity, and the researchers used logistic regression models to test for liner and quadratic trends in the pooled sample and in each city. Between 1999 and 2015, lifetime heroin use among this population increased significantly, from 2.8% to 7.4% in Milwaukee (P = .0001), from 3.1% to 4.1% in Chicago (P = .02), and from 1% to 2.5% in New York (P less than .0001). However, during the same time frame, heroin use decreased in San Bernardino from 4.6% to 1.6% (P = .0001).
The researchers also found that between 1999 and 2015, lifetime injection drug use in this age group increased significantly, from 0.8% to 2.2% in New York (P less than .0001) and from 2.5% to 2.7% in Chicago (P = .05). During the same time period, lifetime injection drug use decreased in San Bernardino, (from 2.5% to 1.9%; P = .05) and in Dallas after peaking in 2007 (from 3.6% to 1%; P = .02).
“The take-home message is to look locally,” Ms. Brighthaupt said. “Some cities may have a historically entrenched culture of heroin use, and prevention and intervention efforts should be tailored to the unique social and cultural context of the geographic region.”
The research was supported by grants from the National Institute on Drug Abuse. Ms. Brighthaupt reported having no financial disclosures.
SAN DIEGO – Between 1999 and 2015, heroin use increased among high school students who live in Milwaukee, Chicago, and New York – trends that accompanied a rise in injection drug use in those cities.
“This implies that a subset of these students who are using heroin are probably injecting heroin as well; otherwise these trends wouldn’t be mirroring each other so well,” lead study author Sherri-Chanelle Brighthaupt said in an interview at the annual meeting of the College on Problems of Drug Dependence.
The finding comes from a trend analysis of heroin use and injection drug use in nine urban U.S. school districts drawn from Youth Risk Behavior Survey (YRBS) data from 1999-2015. The analysis was conducted using data from New York City, three Florida counties (Broward, Orange, and Miami-Dade), Dallas, Chicago, Milwaukee, and two California cities (San Diego and San Bernardino). a third-year doctoral student in the department of mental health at Johns Hopkins University, Baltimore. “National estimates may mask variation at the local level.”
The sample population studied included local-level YRBS responses from 260,952 students in grades 9-12. All responses were weighted by sex, grade, and race/ethnicity, and the researchers used logistic regression models to test for liner and quadratic trends in the pooled sample and in each city. Between 1999 and 2015, lifetime heroin use among this population increased significantly, from 2.8% to 7.4% in Milwaukee (P = .0001), from 3.1% to 4.1% in Chicago (P = .02), and from 1% to 2.5% in New York (P less than .0001). However, during the same time frame, heroin use decreased in San Bernardino from 4.6% to 1.6% (P = .0001).
The researchers also found that between 1999 and 2015, lifetime injection drug use in this age group increased significantly, from 0.8% to 2.2% in New York (P less than .0001) and from 2.5% to 2.7% in Chicago (P = .05). During the same time period, lifetime injection drug use decreased in San Bernardino, (from 2.5% to 1.9%; P = .05) and in Dallas after peaking in 2007 (from 3.6% to 1%; P = .02).
“The take-home message is to look locally,” Ms. Brighthaupt said. “Some cities may have a historically entrenched culture of heroin use, and prevention and intervention efforts should be tailored to the unique social and cultural context of the geographic region.”
The research was supported by grants from the National Institute on Drug Abuse. Ms. Brighthaupt reported having no financial disclosures.
SAN DIEGO – Between 1999 and 2015, heroin use increased among high school students who live in Milwaukee, Chicago, and New York – trends that accompanied a rise in injection drug use in those cities.
“This implies that a subset of these students who are using heroin are probably injecting heroin as well; otherwise these trends wouldn’t be mirroring each other so well,” lead study author Sherri-Chanelle Brighthaupt said in an interview at the annual meeting of the College on Problems of Drug Dependence.
The finding comes from a trend analysis of heroin use and injection drug use in nine urban U.S. school districts drawn from Youth Risk Behavior Survey (YRBS) data from 1999-2015. The analysis was conducted using data from New York City, three Florida counties (Broward, Orange, and Miami-Dade), Dallas, Chicago, Milwaukee, and two California cities (San Diego and San Bernardino). a third-year doctoral student in the department of mental health at Johns Hopkins University, Baltimore. “National estimates may mask variation at the local level.”
The sample population studied included local-level YRBS responses from 260,952 students in grades 9-12. All responses were weighted by sex, grade, and race/ethnicity, and the researchers used logistic regression models to test for liner and quadratic trends in the pooled sample and in each city. Between 1999 and 2015, lifetime heroin use among this population increased significantly, from 2.8% to 7.4% in Milwaukee (P = .0001), from 3.1% to 4.1% in Chicago (P = .02), and from 1% to 2.5% in New York (P less than .0001). However, during the same time frame, heroin use decreased in San Bernardino from 4.6% to 1.6% (P = .0001).
The researchers also found that between 1999 and 2015, lifetime injection drug use in this age group increased significantly, from 0.8% to 2.2% in New York (P less than .0001) and from 2.5% to 2.7% in Chicago (P = .05). During the same time period, lifetime injection drug use decreased in San Bernardino, (from 2.5% to 1.9%; P = .05) and in Dallas after peaking in 2007 (from 3.6% to 1%; P = .02).
“The take-home message is to look locally,” Ms. Brighthaupt said. “Some cities may have a historically entrenched culture of heroin use, and prevention and intervention efforts should be tailored to the unique social and cultural context of the geographic region.”
The research was supported by grants from the National Institute on Drug Abuse. Ms. Brighthaupt reported having no financial disclosures.
AT CPDD 2018
Key clinical point: Trends in heroin use and injection drug use follow one another over time.
Major finding: Between 1999 and 2015, lifetime heroin use increased significantly, from 2.8% to 7.4% in Milwaukee (P = .0001), from 3.1% to 4.1% in Chicago (P = .02), and from 1% to 2.5% in New York (P less than .0001).
Study details: An analysis of local-level Youth Risk Behavior Survey responses from 260,952 students in grades 9-12.
Disclosures: The research was supported by grants from the National Institute on Drug Abuse. Ms. Brighthaupt reported having no financial disclosures.
Does hormone therapy increase breast cancer risk in BRCA1 mutation carriers?
EXPERT COMMENTARY
Prophylactic bilateral oophorectomy (BO) reduces the risk of future ovarian cancer in women who have BRCA1 gene mutations. Women in this high-risk population may be reluctant, however, to use menopausal hormone therapy (HT) to mitigate the symptoms of surgical menopause because of concerns that it might elevate their risk of breast cancer.
To determine the relationship between HT use and BRCA1-associated breast cancer, Kotsopoulos and colleagues conducted a multicenter international cohort study. They prospectively followed women with BRCA1 mutations who had undergone BO and had intact breasts and no history of breast cancer.
Details of the study
The study included women who had a BRCA1 mutation and considered HT use following BO. Women were excluded from the analysis if they had a prior diagnosis of breast cancer or had BO prior to study enrollment. Study participants completed a questionnaire at baseline and a follow-up questionnaire every 2 years thereafter. The primary end point was invasive breast cancer.
Among 872 participating BRCA1 carriers, 43% (n = 377) used HT following BO. Mean duration of HT use following BO was 3.9 years, with 69% of users taking estrogen therapy alone (ET) and 19% using estrogen plus progestogen therapy (EPT). Those who used HT were younger at the time of BO compared with women who never used HT (mean age, 43.0 vs 48.4 years).
During follow-up (mean, 7.6 years; range, 0.4–22.1), invasive breast cancer was diagnosed in similar proportions of HT users and nonusers—10.3% and 10.7%, respectively (P = .86). The hazard ratio was 0.97 (95% confidence interval, 0.62–1.52; P = .89) for ever use of any type of hormone therapy versus no use.
When the type of HT used was examined, the 10-year actuarial risk of breast cancer was significantly lower with ET than with EPT (12% vs 22%, respectively; P = .04); this difference was more marked for women who underwent BO prior to age 45 (9% vs 24%; P = .009).
Study strengths and weaknesses
This investigation had several strengths, including the large number of BRCA1 mutation carriers studied, the relatively long follow-up, and the detailed exposure data obtained.
The use of self-administered questionnaires for collecting information on lifetime HT use and breast cancer diagnoses may be a limitation. In addition, the HT route, regimen, and dose were not considered in the analysis, and the effect of intrauterine devices as progestational endometrial protection was not evaluated. Finally, the relationship between HT and breast cancer risk in women with intact ovaries was not evaluated.
Because women with BRCA1 mutations have an elevated risk of ovarian cancer, risk-reducing gynecologic surgery is recommended for these women who have completed childbearing. In young women, BO without HT is associated with severe vasomotor symptoms, osteoporosis, cardiovascular disease, and cognitive decline. The clear reduction in breast cancer risk associated with ET (vs EPT) following BO suggests that in BRCA1 carriers who have completed childbearing, hysterectomy (which precludes the need for progestogen therapy) should be considered as part of risk-reducing gynecologic surgery. Further, the findings of this prospective study in high-risk women parallels the findings of the large randomized Women's Health Initiative trial (performed in the general population of menopausal women), which found that ET (conjugated equine estrogen) reduces the risk.1
-- Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310(13):1353-1368.
EXPERT COMMENTARY
Prophylactic bilateral oophorectomy (BO) reduces the risk of future ovarian cancer in women who have BRCA1 gene mutations. Women in this high-risk population may be reluctant, however, to use menopausal hormone therapy (HT) to mitigate the symptoms of surgical menopause because of concerns that it might elevate their risk of breast cancer.
To determine the relationship between HT use and BRCA1-associated breast cancer, Kotsopoulos and colleagues conducted a multicenter international cohort study. They prospectively followed women with BRCA1 mutations who had undergone BO and had intact breasts and no history of breast cancer.
Details of the study
The study included women who had a BRCA1 mutation and considered HT use following BO. Women were excluded from the analysis if they had a prior diagnosis of breast cancer or had BO prior to study enrollment. Study participants completed a questionnaire at baseline and a follow-up questionnaire every 2 years thereafter. The primary end point was invasive breast cancer.
Among 872 participating BRCA1 carriers, 43% (n = 377) used HT following BO. Mean duration of HT use following BO was 3.9 years, with 69% of users taking estrogen therapy alone (ET) and 19% using estrogen plus progestogen therapy (EPT). Those who used HT were younger at the time of BO compared with women who never used HT (mean age, 43.0 vs 48.4 years).
During follow-up (mean, 7.6 years; range, 0.4–22.1), invasive breast cancer was diagnosed in similar proportions of HT users and nonusers—10.3% and 10.7%, respectively (P = .86). The hazard ratio was 0.97 (95% confidence interval, 0.62–1.52; P = .89) for ever use of any type of hormone therapy versus no use.
When the type of HT used was examined, the 10-year actuarial risk of breast cancer was significantly lower with ET than with EPT (12% vs 22%, respectively; P = .04); this difference was more marked for women who underwent BO prior to age 45 (9% vs 24%; P = .009).
Study strengths and weaknesses
This investigation had several strengths, including the large number of BRCA1 mutation carriers studied, the relatively long follow-up, and the detailed exposure data obtained.
The use of self-administered questionnaires for collecting information on lifetime HT use and breast cancer diagnoses may be a limitation. In addition, the HT route, regimen, and dose were not considered in the analysis, and the effect of intrauterine devices as progestational endometrial protection was not evaluated. Finally, the relationship between HT and breast cancer risk in women with intact ovaries was not evaluated.
Because women with BRCA1 mutations have an elevated risk of ovarian cancer, risk-reducing gynecologic surgery is recommended for these women who have completed childbearing. In young women, BO without HT is associated with severe vasomotor symptoms, osteoporosis, cardiovascular disease, and cognitive decline. The clear reduction in breast cancer risk associated with ET (vs EPT) following BO suggests that in BRCA1 carriers who have completed childbearing, hysterectomy (which precludes the need for progestogen therapy) should be considered as part of risk-reducing gynecologic surgery. Further, the findings of this prospective study in high-risk women parallels the findings of the large randomized Women's Health Initiative trial (performed in the general population of menopausal women), which found that ET (conjugated equine estrogen) reduces the risk.1
-- Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Prophylactic bilateral oophorectomy (BO) reduces the risk of future ovarian cancer in women who have BRCA1 gene mutations. Women in this high-risk population may be reluctant, however, to use menopausal hormone therapy (HT) to mitigate the symptoms of surgical menopause because of concerns that it might elevate their risk of breast cancer.
To determine the relationship between HT use and BRCA1-associated breast cancer, Kotsopoulos and colleagues conducted a multicenter international cohort study. They prospectively followed women with BRCA1 mutations who had undergone BO and had intact breasts and no history of breast cancer.
Details of the study
The study included women who had a BRCA1 mutation and considered HT use following BO. Women were excluded from the analysis if they had a prior diagnosis of breast cancer or had BO prior to study enrollment. Study participants completed a questionnaire at baseline and a follow-up questionnaire every 2 years thereafter. The primary end point was invasive breast cancer.
Among 872 participating BRCA1 carriers, 43% (n = 377) used HT following BO. Mean duration of HT use following BO was 3.9 years, with 69% of users taking estrogen therapy alone (ET) and 19% using estrogen plus progestogen therapy (EPT). Those who used HT were younger at the time of BO compared with women who never used HT (mean age, 43.0 vs 48.4 years).
During follow-up (mean, 7.6 years; range, 0.4–22.1), invasive breast cancer was diagnosed in similar proportions of HT users and nonusers—10.3% and 10.7%, respectively (P = .86). The hazard ratio was 0.97 (95% confidence interval, 0.62–1.52; P = .89) for ever use of any type of hormone therapy versus no use.
When the type of HT used was examined, the 10-year actuarial risk of breast cancer was significantly lower with ET than with EPT (12% vs 22%, respectively; P = .04); this difference was more marked for women who underwent BO prior to age 45 (9% vs 24%; P = .009).
Study strengths and weaknesses
This investigation had several strengths, including the large number of BRCA1 mutation carriers studied, the relatively long follow-up, and the detailed exposure data obtained.
The use of self-administered questionnaires for collecting information on lifetime HT use and breast cancer diagnoses may be a limitation. In addition, the HT route, regimen, and dose were not considered in the analysis, and the effect of intrauterine devices as progestational endometrial protection was not evaluated. Finally, the relationship between HT and breast cancer risk in women with intact ovaries was not evaluated.
Because women with BRCA1 mutations have an elevated risk of ovarian cancer, risk-reducing gynecologic surgery is recommended for these women who have completed childbearing. In young women, BO without HT is associated with severe vasomotor symptoms, osteoporosis, cardiovascular disease, and cognitive decline. The clear reduction in breast cancer risk associated with ET (vs EPT) following BO suggests that in BRCA1 carriers who have completed childbearing, hysterectomy (which precludes the need for progestogen therapy) should be considered as part of risk-reducing gynecologic surgery. Further, the findings of this prospective study in high-risk women parallels the findings of the large randomized Women's Health Initiative trial (performed in the general population of menopausal women), which found that ET (conjugated equine estrogen) reduces the risk.1
-- Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310(13):1353-1368.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310(13):1353-1368.
Are we ready for primary HPV testing for the prevention of cervical cancer?
Cervical cancer screening represents one of the great public health successes of the 20th Century. Two physician-scientists, George Papanicolaou, MD, PhD (1883–1962), and Harald zur Hausen, MD (1936–), made extraordinary contributions to the evolution of effective cervical cancer screening programs. Dr. Papanicolaou led development of the iconic Pap smear, creating techniques for collecting specimens and using cytologic techniques to identify cervical cancer and its precursors, and Dr. zur Hausen discovered the association of human papillomavirus (HPV) infection with cervical cancer.1,2
Although it is but a distant memory, in the 1930s cervical and uterine cancer caused more deaths among women than breast, lung, or ovarian cancer. The successful deployment of Pap smear screening resulted in a decrease in cervical cancer rates in developed countries. Cervical cancer deaths remain common in many parts of the world, however. Cervical cancer screening programs can reduce cervical cancer incidence by greater than 80%.3 In the United States between 1973 and 2006, the invasive cervical cancer age-adjusted incidence rates dropped from 10.28 to 3.97 per 100,000 women.4
HPV causes cervical cancer
Dr. zur Hausen dedicated his career to identifying viral causes of human cancer. In his Nobel Laureate autobiography, he reported that during his 2-year rotating residency, he loved his obstetrics and gynecology experience, but found it “physically highly demanding” and decided to focus his career in microbiology and immunology.5 After proving that herpes simplex virus did not cause cervical cancer he began to explore the role of HPV in the disease process. He first identified HPV types 6 and 11 and showed that these agents caused genital warts. He then used low-stringency hybridization techniques to identify HPV types 16 and 18 in specimens of cervical cancer. Later, he and his colleagues proved that two HPV proteins, E6 and E7, interfere with the function of cell cycle control proteins p53 and retinoblastoma protein, resulting in dysregulated cell growth and cancer.2 These findings permitted the development of both HPV vaccines and nucleic acid–based tests to identify high-risk oncogenic HPV (hrHPV) in cells and tissue specimens.
HPV vaccination
Dr. zur Hausen was an energetic and vocal advocate for the development and widescale deployment of HPV vaccines, including vaccination of males and females.6 Initially his ideas were rejected by the pharmaceutical industry, but eventually, with advances in virology and vaccine development, multiple companies pursued the development of HPV vaccines, the first cancer prevention vaccines. The best approach to cervical cancer prevention is intensive population-wide HPV vaccination of both boys and girls before exposure to the HPV virus. Beyond its beneficial effect on the incidence of cervical cancer, HPV vaccination also reduces the population incidence of anal, vulvar, and oropharyngeal cancer.7 Prevention of oropharyngeal cancer is especially important for men, supporting the recommendation for vaccination of all boys.8
Population-wide HPV vaccination will result in a lower prevalence of cervical cancer precursors and reduce the sensitivity of cytology, thereby making primary HPV screening more attractive.9 Based on one modelling study, universal HPV vaccination can reduce cervical cancer rates by greater than 50% over current levels, and introduction of primary HPV screening will reduce cervical cancer rates by an additional 20%.10 In an era of widespread vaccination for HPV, screening for cervical cancer should be intensified for nonvaccinated women.10
Read about Primary cervical cancer screening with cytology
Primary cervical cancer screening with cytology
Primary screening with cervical cytology alone remains an option supported by many authorities and professional society guidelines.11 Most studies report that HPV testing has greater sensitivity than cervical cytology alone, especially for the detection of adenocarcinoma of the cervix.12 In one Canadian study, 10,154 women were randomly assigned to HPV or cervical cytology testing. The sensitivity of HPV testing and cervical cytology for detecting cervical intraepithelial neoplasia grade 2 or 3 was 95% and 55%, respectively, with a specificity of 94% and 97%, respectively.13 When used together the sensitivity and specificity of cotesting was 100% and 93%, respectively, but resulted in an increased number of colposcopies, which may be costly and add stress for the patient. Many countries are beginning to move away from cervical cancer screening with cytology or cotesting to programs built upon a foundation of primary HPV testing.
Primary cervical cancer screening with HPV testing
The knowledge that hrHPV is a more sensitive test for cervical cancer and its precursors, as well as the relatively lower sensitivity of cytology, is the foundation for transitioning from primary screening with cervical cytology to primary screening with HPV testing. In the Netherlands14 and Australia15,16 HPV testing with reflex cytology is the nationwide approach to cervical cancer screening. The basic components of the Dutch primary HPV screening program, as explained by Dr. Lai van Zulyan Mandres, are14:
- Samples are collected by a general practitioner and sent to one of 5 central testing facilities for DNA testing for hrHPV.
- If all previous samples tested negative, the screening occurs at ages 30, 35, 40, 50, and 60 years, a minimum of 5 screens per woman.
- If there is a history of a previously positive hrHPV, the screening is intensified, with additional specimens collected at ages 45, 55, and 60 years.
- If the sample is hrHPV negative, the patient continues screening at the standard intervals. No cytology testing is performed.
- If the sample is hrHPV positive, reflex cytology is performed using the original collected sample. If the cytology shows no intraepithelial lesion or malignancy (NILM), another specimen is obtained for cytology within 6 months. If the second cytology specimen shows atypical squamous cells of undetermined significance (ASCUS) or a more worrisome cytology finding, the patient is sent for colposcopy. If two NILM cytology specimens have been obtained, the patient resumes primary hrHPV screening every 5 years.
- If the specimen is hrHPV positive and cytology is ASCUS or more worrisome the patient is referred for colposcopy (FIGURE).14 The Dutch estimate that primary hrHPV screening will reduce the number of cervical cytology specimens by 90% annually.

Australia also has implemented nationwide primary HPV testing for cervical cancer screening. This change was implemented following a 10-year program of universal school-based vaccination of girls and boys, and biennial cytology screening for all women. The Australian screening program initiates hrHPV testing at age 25 years and thereafter every 5 years until age 74. If the hrHPV test is positive, reflex testing for HPV types 16 and 18 are performed on the original specimen along with cervical cytology. Women who test positive for HPV 16 or 18 are immediately referred for colposcopy. If the hrHPV test is positive and reflex testing for HPV 16 and 18 is negative, cervical cytology demonstrating ASCUS, low- or high-grade squamous intraepithelial lesions, or more worrisome results trigger a referral for colposcopy. The Australian program supports testing of self-collected vaginal samples for women who are underscreened or have never been screened.15,16
Read about Pros and cons of switching approaches
Pros and cons of switching approaches
Deployment of new technology often yields benefits and challenges. A putative benefit of primary HPV screening is a reduction in health care costs without an increase in cervical cancer deaths. Another benefit of primary HPV screening is that it may enable self-collection of specimens for analysis, thereby increasing access to cervical cancer screening for underserved and marginalized populations of women who are not currently participating in cervical cancer screening programs.17 One challenge is that many women are unaware that hrHPV is the cause of most invasive cervical cancers. The detection of hrHPV in a woman in a long-term relationship who was previously negative for hrHPV may cause the emotions of surprise, fear, anxiety, and anger, thereby stressing the relationship.18
Another concern is that many women are worried about no longer receiving the familiar “Pap smear” cancer screening test in which they have tremendous faith. When Australia transitioned to primary HPV screening, more than 70,000 women signed a petition to “save women’s lives” by permitting continued access to the cervical cytology testing.19 Primary HPV testing may result in a transient increase in the number of women referred for colposcopy, potentially overwhelming the capacity of the health care system to deliver this vital service.20,21 The HPV types that most often cause cervical cancer may vary among countries. For example, in Thailand, HPV 52 and 58 are frequently detected in women with high-grade squamous lesions, and including these subtypes in reflex genotyping may be of regional benefit.22
Primary cervical cancer screening with HPV testing: When will it be used widely in the United States?
In contrast to the United States, the Netherlands is a small, densely populated country that has a highly integrated health system with centralized laboratory centers, a nationwide electronic health record, and clinicians organized to perform as an integrated team. These features ensure that all lifetime tests results are available in one record, that HPV testing is highly standardized, and that clinicians will follow a prescribed care pathway. The Netherlands’ health system is organized to support the successful transition, in a single step, to primary HPV testing. The United States is the third most populous country in the world, following China and India, with a diverse approach to health care, a highly mobile population, no single interoperable electronic health record, and minimal central control of clinical practice. The United States is not organized to make a “big bang” transition to primary HPV cervical cancer screening. It is likely that the introduction of primary HPV screening will occur first in highly integrated health systems that control the clinical, laboratory, and electronic records of a large population.
The results of the ATHENA study provide a clear clinical algorithm for implementing a primary HPV screening program for cervical cancer in the United States.23–25 Samples are collected for hrHPV testing at a specified interval, 3 or 5 years, beginning at age 25 years. Women younger than age 25 years should be screened with cytology alone. Detection of hrHPV results in reflex viral typing for HPV 16 and 18. Women with samples positive for HPV 16 and 18 are immediately referred for colposcopy. Samples positive for hrHPV and negative for HPV 16 and 18 have reflex cytology testing performed on the original HPV specimen. If cytology testing reports NILM, repeat cotesting is performed in one year. If cytology testing reports ASCUS or a more concerning result, the woman is referred for colposcopy.
Malcolm Gladwell, in his book The Tipping Point, identified 3 processes that help push an innovative new approach from obscurity into widespread use.26 First, authoritative voices that can catalyze change need to consistently communicate their shared vision for the future. Second, there must be a clear message that galvanizes the many to change their approach. Third, the historical context must be supportive of the change. Over the next decade we are likely to hit a tipping point and transition from cervical cancer screening that relies on cervical cytology to an approach that prioritizes hrHPV testing. When that change will occur in the United States is unclear. But our colleagues in other countries already have transitioned to primary hrHPV testing for cervical cancer screening.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Hinsey JC. George Nicholas Papanicolaou, May 13, 1883–February 19, 1962. Acta Cytol. 1962;6:483–486.
- zur Hausen H. Papillomaviruses in human cancers. Proc Assoc Am Physicians. 1999;111(6):581–587.
- International Agency for Research on Cancer. IARC Handbooks of Cancer Prevention, Vol 10: Cervix Cancer Screening. Lyon, France: IARC Press; 2005.
- Adegoke O, Kulasingam S, Virnig B. Cervical cancer trends in the United States: a 35-year population-based analysis. J Women’s Health (Larchmt). 2012;21(10):1031–1037.
- Harold zur Hausen-Biographical. Nobelprize.org website. https://www.nobelprize.org/nobel_prizes/medicine/laureates/2008/hausen-bio.html. Accessed June 19, 2018.
- Michels KB, zur Hausen H. HPV vaccine for all. Lancet. 2009;374(9686):268–270.
- Hansen BT, Campbell S, Nygaard M. Long-term incidence of HPV-related cancers, and cases preventable by HPV vaccination: a registry-based study in Norway. BMJ Open. 2018;8(2):e019005.
- Barbieri RL. Advances in protection against oncogenic human papillomaviruses: the 9-valent vaccine. OBG Manag. 2015;27(5):6–8.
- Massad LS. Anticipating the impact of human papillomavirus vaccination on US cervical cancer prevention strategies. J Low Genit Tract Dis. 2018;22(2):123–125.
- Castanon A, Landy R, Pesola F, Windridge P, Sasieni P. Prediction of cervical cancer incidence in England, UK, up to 2040, under four scenarios: a modeling study. Lancet Public Health. 2018;3(1):e34–e43.
- Moyer VA; U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–891.
- Moukarzel LA, Angarita AM, VandenBussche C, et al. Preinvasive and invasive cervical adenocarcinoma: preceding low-risk or negative Pap result increases time to diagnosis. J Low Genital Tract Dis. 2017;21(2):91–96.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579–1588.
- van Zuylen-Manders L. Primary HPV screening: The Dutch experience. http://www.britishcytology.org.uk/resources/Primary_HPV_screening_The_Dutch_experience.pdf. Accessed June 19, 2018.
- Hammond I, Canfell K, Saville M. A new era for cervical cancer screening in Australia: watch this space! Aust N Z J Obstet Gynaecol. 2017;57(5):499–501.
- Canfell K, Saville M, Caruana M, et al. Protocol for Compass: a randomised controlled trial of primary HPV testing versus cytology screening for cervical cancer in HPV-unvaccinated and vaccinated women aged 25-69 years living in Australia. BMJ Open. 2018;8(1):e016700.
- Wood B, Lofters A, Vahabi M. Strategies to reach marginalized women for cervical cancer screening: a qualitative study of stakeholder perspectives. Curr Oncol. 2018;25(1):e8–e16.
- Patel H, Moss EL, Sherman SM. HPV primary cervical cancer screening in England: women’s awareness and attitudes. Psychooncology. 2018;27(6):1559–1564.
- Obermair HM, Dodd RH, Bonner C, Jansen J, McCaffery K. “It has saved thousands of lives, so why change it?” Content analysis of objections to cervical cancer screening programme changes in Australia. BMJ Open. 2018;8(2):e019171.
- Hall MT, Simms KT, Lew JB, Smith MA, Saville M, Canfell K. Projected future impact of HPV vaccination and primary HPV screening on cervical cancer rates from 2017-2035: Example from Australia. PLoS One. 2018;13(2):e0185332.
- Rebolj M, Bonde J, Preisler S, Ejegod D, Rygaard C, Lynge E. Human papillomavirus assays and cytology in primary cervical screening of women aged 30 years and above. PLoS One. 2016;11(1):e0147326.
- Khunamornpong S, Settakorn J, Sukpan K, Suprasert P, Srisomboon J, Intaraphet S, Siriaunkgul S. Genotyping for human papillomavirus (HPV) 16/18/52/58 has a higher performance than HPV16/18 genotyping in triaging women with positive high-risk HPV test in Northern Thailand. PLoS One. 2016;11(6):e0158184.
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125(2):330–337.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- Gladwell M. The Tipping Point: How Little Things Can Make a Big Difference. New York, New York: Little Brown; 2000.
Cervical cancer screening represents one of the great public health successes of the 20th Century. Two physician-scientists, George Papanicolaou, MD, PhD (1883–1962), and Harald zur Hausen, MD (1936–), made extraordinary contributions to the evolution of effective cervical cancer screening programs. Dr. Papanicolaou led development of the iconic Pap smear, creating techniques for collecting specimens and using cytologic techniques to identify cervical cancer and its precursors, and Dr. zur Hausen discovered the association of human papillomavirus (HPV) infection with cervical cancer.1,2
Although it is but a distant memory, in the 1930s cervical and uterine cancer caused more deaths among women than breast, lung, or ovarian cancer. The successful deployment of Pap smear screening resulted in a decrease in cervical cancer rates in developed countries. Cervical cancer deaths remain common in many parts of the world, however. Cervical cancer screening programs can reduce cervical cancer incidence by greater than 80%.3 In the United States between 1973 and 2006, the invasive cervical cancer age-adjusted incidence rates dropped from 10.28 to 3.97 per 100,000 women.4
HPV causes cervical cancer
Dr. zur Hausen dedicated his career to identifying viral causes of human cancer. In his Nobel Laureate autobiography, he reported that during his 2-year rotating residency, he loved his obstetrics and gynecology experience, but found it “physically highly demanding” and decided to focus his career in microbiology and immunology.5 After proving that herpes simplex virus did not cause cervical cancer he began to explore the role of HPV in the disease process. He first identified HPV types 6 and 11 and showed that these agents caused genital warts. He then used low-stringency hybridization techniques to identify HPV types 16 and 18 in specimens of cervical cancer. Later, he and his colleagues proved that two HPV proteins, E6 and E7, interfere with the function of cell cycle control proteins p53 and retinoblastoma protein, resulting in dysregulated cell growth and cancer.2 These findings permitted the development of both HPV vaccines and nucleic acid–based tests to identify high-risk oncogenic HPV (hrHPV) in cells and tissue specimens.
HPV vaccination
Dr. zur Hausen was an energetic and vocal advocate for the development and widescale deployment of HPV vaccines, including vaccination of males and females.6 Initially his ideas were rejected by the pharmaceutical industry, but eventually, with advances in virology and vaccine development, multiple companies pursued the development of HPV vaccines, the first cancer prevention vaccines. The best approach to cervical cancer prevention is intensive population-wide HPV vaccination of both boys and girls before exposure to the HPV virus. Beyond its beneficial effect on the incidence of cervical cancer, HPV vaccination also reduces the population incidence of anal, vulvar, and oropharyngeal cancer.7 Prevention of oropharyngeal cancer is especially important for men, supporting the recommendation for vaccination of all boys.8
Population-wide HPV vaccination will result in a lower prevalence of cervical cancer precursors and reduce the sensitivity of cytology, thereby making primary HPV screening more attractive.9 Based on one modelling study, universal HPV vaccination can reduce cervical cancer rates by greater than 50% over current levels, and introduction of primary HPV screening will reduce cervical cancer rates by an additional 20%.10 In an era of widespread vaccination for HPV, screening for cervical cancer should be intensified for nonvaccinated women.10
Read about Primary cervical cancer screening with cytology
Primary cervical cancer screening with cytology
Primary screening with cervical cytology alone remains an option supported by many authorities and professional society guidelines.11 Most studies report that HPV testing has greater sensitivity than cervical cytology alone, especially for the detection of adenocarcinoma of the cervix.12 In one Canadian study, 10,154 women were randomly assigned to HPV or cervical cytology testing. The sensitivity of HPV testing and cervical cytology for detecting cervical intraepithelial neoplasia grade 2 or 3 was 95% and 55%, respectively, with a specificity of 94% and 97%, respectively.13 When used together the sensitivity and specificity of cotesting was 100% and 93%, respectively, but resulted in an increased number of colposcopies, which may be costly and add stress for the patient. Many countries are beginning to move away from cervical cancer screening with cytology or cotesting to programs built upon a foundation of primary HPV testing.
Primary cervical cancer screening with HPV testing
The knowledge that hrHPV is a more sensitive test for cervical cancer and its precursors, as well as the relatively lower sensitivity of cytology, is the foundation for transitioning from primary screening with cervical cytology to primary screening with HPV testing. In the Netherlands14 and Australia15,16 HPV testing with reflex cytology is the nationwide approach to cervical cancer screening. The basic components of the Dutch primary HPV screening program, as explained by Dr. Lai van Zulyan Mandres, are14:
- Samples are collected by a general practitioner and sent to one of 5 central testing facilities for DNA testing for hrHPV.
- If all previous samples tested negative, the screening occurs at ages 30, 35, 40, 50, and 60 years, a minimum of 5 screens per woman.
- If there is a history of a previously positive hrHPV, the screening is intensified, with additional specimens collected at ages 45, 55, and 60 years.
- If the sample is hrHPV negative, the patient continues screening at the standard intervals. No cytology testing is performed.
- If the sample is hrHPV positive, reflex cytology is performed using the original collected sample. If the cytology shows no intraepithelial lesion or malignancy (NILM), another specimen is obtained for cytology within 6 months. If the second cytology specimen shows atypical squamous cells of undetermined significance (ASCUS) or a more worrisome cytology finding, the patient is sent for colposcopy. If two NILM cytology specimens have been obtained, the patient resumes primary hrHPV screening every 5 years.
- If the specimen is hrHPV positive and cytology is ASCUS or more worrisome the patient is referred for colposcopy (FIGURE).14 The Dutch estimate that primary hrHPV screening will reduce the number of cervical cytology specimens by 90% annually.

Australia also has implemented nationwide primary HPV testing for cervical cancer screening. This change was implemented following a 10-year program of universal school-based vaccination of girls and boys, and biennial cytology screening for all women. The Australian screening program initiates hrHPV testing at age 25 years and thereafter every 5 years until age 74. If the hrHPV test is positive, reflex testing for HPV types 16 and 18 are performed on the original specimen along with cervical cytology. Women who test positive for HPV 16 or 18 are immediately referred for colposcopy. If the hrHPV test is positive and reflex testing for HPV 16 and 18 is negative, cervical cytology demonstrating ASCUS, low- or high-grade squamous intraepithelial lesions, or more worrisome results trigger a referral for colposcopy. The Australian program supports testing of self-collected vaginal samples for women who are underscreened or have never been screened.15,16
Read about Pros and cons of switching approaches
Pros and cons of switching approaches
Deployment of new technology often yields benefits and challenges. A putative benefit of primary HPV screening is a reduction in health care costs without an increase in cervical cancer deaths. Another benefit of primary HPV screening is that it may enable self-collection of specimens for analysis, thereby increasing access to cervical cancer screening for underserved and marginalized populations of women who are not currently participating in cervical cancer screening programs.17 One challenge is that many women are unaware that hrHPV is the cause of most invasive cervical cancers. The detection of hrHPV in a woman in a long-term relationship who was previously negative for hrHPV may cause the emotions of surprise, fear, anxiety, and anger, thereby stressing the relationship.18
Another concern is that many women are worried about no longer receiving the familiar “Pap smear” cancer screening test in which they have tremendous faith. When Australia transitioned to primary HPV screening, more than 70,000 women signed a petition to “save women’s lives” by permitting continued access to the cervical cytology testing.19 Primary HPV testing may result in a transient increase in the number of women referred for colposcopy, potentially overwhelming the capacity of the health care system to deliver this vital service.20,21 The HPV types that most often cause cervical cancer may vary among countries. For example, in Thailand, HPV 52 and 58 are frequently detected in women with high-grade squamous lesions, and including these subtypes in reflex genotyping may be of regional benefit.22
Primary cervical cancer screening with HPV testing: When will it be used widely in the United States?
In contrast to the United States, the Netherlands is a small, densely populated country that has a highly integrated health system with centralized laboratory centers, a nationwide electronic health record, and clinicians organized to perform as an integrated team. These features ensure that all lifetime tests results are available in one record, that HPV testing is highly standardized, and that clinicians will follow a prescribed care pathway. The Netherlands’ health system is organized to support the successful transition, in a single step, to primary HPV testing. The United States is the third most populous country in the world, following China and India, with a diverse approach to health care, a highly mobile population, no single interoperable electronic health record, and minimal central control of clinical practice. The United States is not organized to make a “big bang” transition to primary HPV cervical cancer screening. It is likely that the introduction of primary HPV screening will occur first in highly integrated health systems that control the clinical, laboratory, and electronic records of a large population.
The results of the ATHENA study provide a clear clinical algorithm for implementing a primary HPV screening program for cervical cancer in the United States.23–25 Samples are collected for hrHPV testing at a specified interval, 3 or 5 years, beginning at age 25 years. Women younger than age 25 years should be screened with cytology alone. Detection of hrHPV results in reflex viral typing for HPV 16 and 18. Women with samples positive for HPV 16 and 18 are immediately referred for colposcopy. Samples positive for hrHPV and negative for HPV 16 and 18 have reflex cytology testing performed on the original HPV specimen. If cytology testing reports NILM, repeat cotesting is performed in one year. If cytology testing reports ASCUS or a more concerning result, the woman is referred for colposcopy.
Malcolm Gladwell, in his book The Tipping Point, identified 3 processes that help push an innovative new approach from obscurity into widespread use.26 First, authoritative voices that can catalyze change need to consistently communicate their shared vision for the future. Second, there must be a clear message that galvanizes the many to change their approach. Third, the historical context must be supportive of the change. Over the next decade we are likely to hit a tipping point and transition from cervical cancer screening that relies on cervical cytology to an approach that prioritizes hrHPV testing. When that change will occur in the United States is unclear. But our colleagues in other countries already have transitioned to primary hrHPV testing for cervical cancer screening.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Cervical cancer screening represents one of the great public health successes of the 20th Century. Two physician-scientists, George Papanicolaou, MD, PhD (1883–1962), and Harald zur Hausen, MD (1936–), made extraordinary contributions to the evolution of effective cervical cancer screening programs. Dr. Papanicolaou led development of the iconic Pap smear, creating techniques for collecting specimens and using cytologic techniques to identify cervical cancer and its precursors, and Dr. zur Hausen discovered the association of human papillomavirus (HPV) infection with cervical cancer.1,2
Although it is but a distant memory, in the 1930s cervical and uterine cancer caused more deaths among women than breast, lung, or ovarian cancer. The successful deployment of Pap smear screening resulted in a decrease in cervical cancer rates in developed countries. Cervical cancer deaths remain common in many parts of the world, however. Cervical cancer screening programs can reduce cervical cancer incidence by greater than 80%.3 In the United States between 1973 and 2006, the invasive cervical cancer age-adjusted incidence rates dropped from 10.28 to 3.97 per 100,000 women.4
HPV causes cervical cancer
Dr. zur Hausen dedicated his career to identifying viral causes of human cancer. In his Nobel Laureate autobiography, he reported that during his 2-year rotating residency, he loved his obstetrics and gynecology experience, but found it “physically highly demanding” and decided to focus his career in microbiology and immunology.5 After proving that herpes simplex virus did not cause cervical cancer he began to explore the role of HPV in the disease process. He first identified HPV types 6 and 11 and showed that these agents caused genital warts. He then used low-stringency hybridization techniques to identify HPV types 16 and 18 in specimens of cervical cancer. Later, he and his colleagues proved that two HPV proteins, E6 and E7, interfere with the function of cell cycle control proteins p53 and retinoblastoma protein, resulting in dysregulated cell growth and cancer.2 These findings permitted the development of both HPV vaccines and nucleic acid–based tests to identify high-risk oncogenic HPV (hrHPV) in cells and tissue specimens.
HPV vaccination
Dr. zur Hausen was an energetic and vocal advocate for the development and widescale deployment of HPV vaccines, including vaccination of males and females.6 Initially his ideas were rejected by the pharmaceutical industry, but eventually, with advances in virology and vaccine development, multiple companies pursued the development of HPV vaccines, the first cancer prevention vaccines. The best approach to cervical cancer prevention is intensive population-wide HPV vaccination of both boys and girls before exposure to the HPV virus. Beyond its beneficial effect on the incidence of cervical cancer, HPV vaccination also reduces the population incidence of anal, vulvar, and oropharyngeal cancer.7 Prevention of oropharyngeal cancer is especially important for men, supporting the recommendation for vaccination of all boys.8
Population-wide HPV vaccination will result in a lower prevalence of cervical cancer precursors and reduce the sensitivity of cytology, thereby making primary HPV screening more attractive.9 Based on one modelling study, universal HPV vaccination can reduce cervical cancer rates by greater than 50% over current levels, and introduction of primary HPV screening will reduce cervical cancer rates by an additional 20%.10 In an era of widespread vaccination for HPV, screening for cervical cancer should be intensified for nonvaccinated women.10
Read about Primary cervical cancer screening with cytology
Primary cervical cancer screening with cytology
Primary screening with cervical cytology alone remains an option supported by many authorities and professional society guidelines.11 Most studies report that HPV testing has greater sensitivity than cervical cytology alone, especially for the detection of adenocarcinoma of the cervix.12 In one Canadian study, 10,154 women were randomly assigned to HPV or cervical cytology testing. The sensitivity of HPV testing and cervical cytology for detecting cervical intraepithelial neoplasia grade 2 or 3 was 95% and 55%, respectively, with a specificity of 94% and 97%, respectively.13 When used together the sensitivity and specificity of cotesting was 100% and 93%, respectively, but resulted in an increased number of colposcopies, which may be costly and add stress for the patient. Many countries are beginning to move away from cervical cancer screening with cytology or cotesting to programs built upon a foundation of primary HPV testing.
Primary cervical cancer screening with HPV testing
The knowledge that hrHPV is a more sensitive test for cervical cancer and its precursors, as well as the relatively lower sensitivity of cytology, is the foundation for transitioning from primary screening with cervical cytology to primary screening with HPV testing. In the Netherlands14 and Australia15,16 HPV testing with reflex cytology is the nationwide approach to cervical cancer screening. The basic components of the Dutch primary HPV screening program, as explained by Dr. Lai van Zulyan Mandres, are14:
- Samples are collected by a general practitioner and sent to one of 5 central testing facilities for DNA testing for hrHPV.
- If all previous samples tested negative, the screening occurs at ages 30, 35, 40, 50, and 60 years, a minimum of 5 screens per woman.
- If there is a history of a previously positive hrHPV, the screening is intensified, with additional specimens collected at ages 45, 55, and 60 years.
- If the sample is hrHPV negative, the patient continues screening at the standard intervals. No cytology testing is performed.
- If the sample is hrHPV positive, reflex cytology is performed using the original collected sample. If the cytology shows no intraepithelial lesion or malignancy (NILM), another specimen is obtained for cytology within 6 months. If the second cytology specimen shows atypical squamous cells of undetermined significance (ASCUS) or a more worrisome cytology finding, the patient is sent for colposcopy. If two NILM cytology specimens have been obtained, the patient resumes primary hrHPV screening every 5 years.
- If the specimen is hrHPV positive and cytology is ASCUS or more worrisome the patient is referred for colposcopy (FIGURE).14 The Dutch estimate that primary hrHPV screening will reduce the number of cervical cytology specimens by 90% annually.

Australia also has implemented nationwide primary HPV testing for cervical cancer screening. This change was implemented following a 10-year program of universal school-based vaccination of girls and boys, and biennial cytology screening for all women. The Australian screening program initiates hrHPV testing at age 25 years and thereafter every 5 years until age 74. If the hrHPV test is positive, reflex testing for HPV types 16 and 18 are performed on the original specimen along with cervical cytology. Women who test positive for HPV 16 or 18 are immediately referred for colposcopy. If the hrHPV test is positive and reflex testing for HPV 16 and 18 is negative, cervical cytology demonstrating ASCUS, low- or high-grade squamous intraepithelial lesions, or more worrisome results trigger a referral for colposcopy. The Australian program supports testing of self-collected vaginal samples for women who are underscreened or have never been screened.15,16
Read about Pros and cons of switching approaches
Pros and cons of switching approaches
Deployment of new technology often yields benefits and challenges. A putative benefit of primary HPV screening is a reduction in health care costs without an increase in cervical cancer deaths. Another benefit of primary HPV screening is that it may enable self-collection of specimens for analysis, thereby increasing access to cervical cancer screening for underserved and marginalized populations of women who are not currently participating in cervical cancer screening programs.17 One challenge is that many women are unaware that hrHPV is the cause of most invasive cervical cancers. The detection of hrHPV in a woman in a long-term relationship who was previously negative for hrHPV may cause the emotions of surprise, fear, anxiety, and anger, thereby stressing the relationship.18
Another concern is that many women are worried about no longer receiving the familiar “Pap smear” cancer screening test in which they have tremendous faith. When Australia transitioned to primary HPV screening, more than 70,000 women signed a petition to “save women’s lives” by permitting continued access to the cervical cytology testing.19 Primary HPV testing may result in a transient increase in the number of women referred for colposcopy, potentially overwhelming the capacity of the health care system to deliver this vital service.20,21 The HPV types that most often cause cervical cancer may vary among countries. For example, in Thailand, HPV 52 and 58 are frequently detected in women with high-grade squamous lesions, and including these subtypes in reflex genotyping may be of regional benefit.22
Primary cervical cancer screening with HPV testing: When will it be used widely in the United States?
In contrast to the United States, the Netherlands is a small, densely populated country that has a highly integrated health system with centralized laboratory centers, a nationwide electronic health record, and clinicians organized to perform as an integrated team. These features ensure that all lifetime tests results are available in one record, that HPV testing is highly standardized, and that clinicians will follow a prescribed care pathway. The Netherlands’ health system is organized to support the successful transition, in a single step, to primary HPV testing. The United States is the third most populous country in the world, following China and India, with a diverse approach to health care, a highly mobile population, no single interoperable electronic health record, and minimal central control of clinical practice. The United States is not organized to make a “big bang” transition to primary HPV cervical cancer screening. It is likely that the introduction of primary HPV screening will occur first in highly integrated health systems that control the clinical, laboratory, and electronic records of a large population.
The results of the ATHENA study provide a clear clinical algorithm for implementing a primary HPV screening program for cervical cancer in the United States.23–25 Samples are collected for hrHPV testing at a specified interval, 3 or 5 years, beginning at age 25 years. Women younger than age 25 years should be screened with cytology alone. Detection of hrHPV results in reflex viral typing for HPV 16 and 18. Women with samples positive for HPV 16 and 18 are immediately referred for colposcopy. Samples positive for hrHPV and negative for HPV 16 and 18 have reflex cytology testing performed on the original HPV specimen. If cytology testing reports NILM, repeat cotesting is performed in one year. If cytology testing reports ASCUS or a more concerning result, the woman is referred for colposcopy.
Malcolm Gladwell, in his book The Tipping Point, identified 3 processes that help push an innovative new approach from obscurity into widespread use.26 First, authoritative voices that can catalyze change need to consistently communicate their shared vision for the future. Second, there must be a clear message that galvanizes the many to change their approach. Third, the historical context must be supportive of the change. Over the next decade we are likely to hit a tipping point and transition from cervical cancer screening that relies on cervical cytology to an approach that prioritizes hrHPV testing. When that change will occur in the United States is unclear. But our colleagues in other countries already have transitioned to primary hrHPV testing for cervical cancer screening.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Hinsey JC. George Nicholas Papanicolaou, May 13, 1883–February 19, 1962. Acta Cytol. 1962;6:483–486.
- zur Hausen H. Papillomaviruses in human cancers. Proc Assoc Am Physicians. 1999;111(6):581–587.
- International Agency for Research on Cancer. IARC Handbooks of Cancer Prevention, Vol 10: Cervix Cancer Screening. Lyon, France: IARC Press; 2005.
- Adegoke O, Kulasingam S, Virnig B. Cervical cancer trends in the United States: a 35-year population-based analysis. J Women’s Health (Larchmt). 2012;21(10):1031–1037.
- Harold zur Hausen-Biographical. Nobelprize.org website. https://www.nobelprize.org/nobel_prizes/medicine/laureates/2008/hausen-bio.html. Accessed June 19, 2018.
- Michels KB, zur Hausen H. HPV vaccine for all. Lancet. 2009;374(9686):268–270.
- Hansen BT, Campbell S, Nygaard M. Long-term incidence of HPV-related cancers, and cases preventable by HPV vaccination: a registry-based study in Norway. BMJ Open. 2018;8(2):e019005.
- Barbieri RL. Advances in protection against oncogenic human papillomaviruses: the 9-valent vaccine. OBG Manag. 2015;27(5):6–8.
- Massad LS. Anticipating the impact of human papillomavirus vaccination on US cervical cancer prevention strategies. J Low Genit Tract Dis. 2018;22(2):123–125.
- Castanon A, Landy R, Pesola F, Windridge P, Sasieni P. Prediction of cervical cancer incidence in England, UK, up to 2040, under four scenarios: a modeling study. Lancet Public Health. 2018;3(1):e34–e43.
- Moyer VA; U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–891.
- Moukarzel LA, Angarita AM, VandenBussche C, et al. Preinvasive and invasive cervical adenocarcinoma: preceding low-risk or negative Pap result increases time to diagnosis. J Low Genital Tract Dis. 2017;21(2):91–96.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579–1588.
- van Zuylen-Manders L. Primary HPV screening: The Dutch experience. http://www.britishcytology.org.uk/resources/Primary_HPV_screening_The_Dutch_experience.pdf. Accessed June 19, 2018.
- Hammond I, Canfell K, Saville M. A new era for cervical cancer screening in Australia: watch this space! Aust N Z J Obstet Gynaecol. 2017;57(5):499–501.
- Canfell K, Saville M, Caruana M, et al. Protocol for Compass: a randomised controlled trial of primary HPV testing versus cytology screening for cervical cancer in HPV-unvaccinated and vaccinated women aged 25-69 years living in Australia. BMJ Open. 2018;8(1):e016700.
- Wood B, Lofters A, Vahabi M. Strategies to reach marginalized women for cervical cancer screening: a qualitative study of stakeholder perspectives. Curr Oncol. 2018;25(1):e8–e16.
- Patel H, Moss EL, Sherman SM. HPV primary cervical cancer screening in England: women’s awareness and attitudes. Psychooncology. 2018;27(6):1559–1564.
- Obermair HM, Dodd RH, Bonner C, Jansen J, McCaffery K. “It has saved thousands of lives, so why change it?” Content analysis of objections to cervical cancer screening programme changes in Australia. BMJ Open. 2018;8(2):e019171.
- Hall MT, Simms KT, Lew JB, Smith MA, Saville M, Canfell K. Projected future impact of HPV vaccination and primary HPV screening on cervical cancer rates from 2017-2035: Example from Australia. PLoS One. 2018;13(2):e0185332.
- Rebolj M, Bonde J, Preisler S, Ejegod D, Rygaard C, Lynge E. Human papillomavirus assays and cytology in primary cervical screening of women aged 30 years and above. PLoS One. 2016;11(1):e0147326.
- Khunamornpong S, Settakorn J, Sukpan K, Suprasert P, Srisomboon J, Intaraphet S, Siriaunkgul S. Genotyping for human papillomavirus (HPV) 16/18/52/58 has a higher performance than HPV16/18 genotyping in triaging women with positive high-risk HPV test in Northern Thailand. PLoS One. 2016;11(6):e0158184.
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125(2):330–337.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- Gladwell M. The Tipping Point: How Little Things Can Make a Big Difference. New York, New York: Little Brown; 2000.
- Hinsey JC. George Nicholas Papanicolaou, May 13, 1883–February 19, 1962. Acta Cytol. 1962;6:483–486.
- zur Hausen H. Papillomaviruses in human cancers. Proc Assoc Am Physicians. 1999;111(6):581–587.
- International Agency for Research on Cancer. IARC Handbooks of Cancer Prevention, Vol 10: Cervix Cancer Screening. Lyon, France: IARC Press; 2005.
- Adegoke O, Kulasingam S, Virnig B. Cervical cancer trends in the United States: a 35-year population-based analysis. J Women’s Health (Larchmt). 2012;21(10):1031–1037.
- Harold zur Hausen-Biographical. Nobelprize.org website. https://www.nobelprize.org/nobel_prizes/medicine/laureates/2008/hausen-bio.html. Accessed June 19, 2018.
- Michels KB, zur Hausen H. HPV vaccine for all. Lancet. 2009;374(9686):268–270.
- Hansen BT, Campbell S, Nygaard M. Long-term incidence of HPV-related cancers, and cases preventable by HPV vaccination: a registry-based study in Norway. BMJ Open. 2018;8(2):e019005.
- Barbieri RL. Advances in protection against oncogenic human papillomaviruses: the 9-valent vaccine. OBG Manag. 2015;27(5):6–8.
- Massad LS. Anticipating the impact of human papillomavirus vaccination on US cervical cancer prevention strategies. J Low Genit Tract Dis. 2018;22(2):123–125.
- Castanon A, Landy R, Pesola F, Windridge P, Sasieni P. Prediction of cervical cancer incidence in England, UK, up to 2040, under four scenarios: a modeling study. Lancet Public Health. 2018;3(1):e34–e43.
- Moyer VA; U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–891.
- Moukarzel LA, Angarita AM, VandenBussche C, et al. Preinvasive and invasive cervical adenocarcinoma: preceding low-risk or negative Pap result increases time to diagnosis. J Low Genital Tract Dis. 2017;21(2):91–96.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579–1588.
- van Zuylen-Manders L. Primary HPV screening: The Dutch experience. http://www.britishcytology.org.uk/resources/Primary_HPV_screening_The_Dutch_experience.pdf. Accessed June 19, 2018.
- Hammond I, Canfell K, Saville M. A new era for cervical cancer screening in Australia: watch this space! Aust N Z J Obstet Gynaecol. 2017;57(5):499–501.
- Canfell K, Saville M, Caruana M, et al. Protocol for Compass: a randomised controlled trial of primary HPV testing versus cytology screening for cervical cancer in HPV-unvaccinated and vaccinated women aged 25-69 years living in Australia. BMJ Open. 2018;8(1):e016700.
- Wood B, Lofters A, Vahabi M. Strategies to reach marginalized women for cervical cancer screening: a qualitative study of stakeholder perspectives. Curr Oncol. 2018;25(1):e8–e16.
- Patel H, Moss EL, Sherman SM. HPV primary cervical cancer screening in England: women’s awareness and attitudes. Psychooncology. 2018;27(6):1559–1564.
- Obermair HM, Dodd RH, Bonner C, Jansen J, McCaffery K. “It has saved thousands of lives, so why change it?” Content analysis of objections to cervical cancer screening programme changes in Australia. BMJ Open. 2018;8(2):e019171.
- Hall MT, Simms KT, Lew JB, Smith MA, Saville M, Canfell K. Projected future impact of HPV vaccination and primary HPV screening on cervical cancer rates from 2017-2035: Example from Australia. PLoS One. 2018;13(2):e0185332.
- Rebolj M, Bonde J, Preisler S, Ejegod D, Rygaard C, Lynge E. Human papillomavirus assays and cytology in primary cervical screening of women aged 30 years and above. PLoS One. 2016;11(1):e0147326.
- Khunamornpong S, Settakorn J, Sukpan K, Suprasert P, Srisomboon J, Intaraphet S, Siriaunkgul S. Genotyping for human papillomavirus (HPV) 16/18/52/58 has a higher performance than HPV16/18 genotyping in triaging women with positive high-risk HPV test in Northern Thailand. PLoS One. 2016;11(6):e0158184.
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125(2):330–337.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- Gladwell M. The Tipping Point: How Little Things Can Make a Big Difference. New York, New York: Little Brown; 2000.