User login
Hypofractionated radiation has untapped potential as RCC mets therapy
Hypofractionated radiation therapy (RT) may be a more viable treatment option for oligometastatic renal cell carcinoma (RCC) than is generally recognized, a recent literature review has suggested.
Advances in stereotactic RT offer “new opportunities in RCC management” with limited toxicity, reported Francesca De Felice, PhD, of Sapienza University in Rome and her coauthor. The authors suggested that future studies investigate RT in combination with immunotherapy.
“Due to the assumption that RCC is a radioresistant tumor,” the authors wrote in Critical Reviews in Oncology/Hematology, “RT has long been considered a futile approach to manage primary disease” and is predominantly used for treatment of distant metastases with palliative intent. “This review provides highlights in current RCC strategies to potentially suggest a more tailored treatment approach in clinical daily practice.”
The investigators concluded that hypofractionated RT (greater than 3 Gy/fraction) deserves more serious consideration. “It has enormous advantages,” the authors wrote, “assuring ablative doses to the target meanwhile preserving surrounding normal tissues. Using stereotactic technique, surprising high local control rates have been achieved in several tumors (such as lung, liver, and bone), in both primary and oligometastatic setting[s].”
In five studies, single-dose RT (ranging from 8 to 24 Gy) was used to treat patients with RCC and extracranial metastases. Of the patients in these studies, 89% of them achieved local control, median overall survival (OS) ranged from 11.7 months to 21 months, and severe RT-related toxicity occurred 0%-4% of the time.
“Although [there is a] high level of data heterogeneity,” the authors wrote, “this systematic review suggested that stereotactic RT is associated with excellent local control rates and low toxicity incidence. Thus, if feasible, stereotactic RT represents an effective and safe approach to treat RCC metastasis.”
The authors cautioned that “the optimal high dose required for local tumor control has not yet been defined.”
The authors suggested that, in the future, immunotherapy in combination with RT may “produce synergistic effects, resulting in better response rate and duration, given the known immune-modulated abscopal effect of RT.” First, questions about treatment sequencing, dosing, and patient selection would need to be answered. “Further research should be aimed at these clinical needs in order to achieve the maximum benefit to RCC patient[s].”
This study did not receive specific funding.
SOURCE: Felice F et al. Crit Rev Oncol Hematol. 2018 Aug 1. doi: 10.1016/j.critrevonc.2018.06.002
Hypofractionated radiation therapy (RT) may be a more viable treatment option for oligometastatic renal cell carcinoma (RCC) than is generally recognized, a recent literature review has suggested.
Advances in stereotactic RT offer “new opportunities in RCC management” with limited toxicity, reported Francesca De Felice, PhD, of Sapienza University in Rome and her coauthor. The authors suggested that future studies investigate RT in combination with immunotherapy.
“Due to the assumption that RCC is a radioresistant tumor,” the authors wrote in Critical Reviews in Oncology/Hematology, “RT has long been considered a futile approach to manage primary disease” and is predominantly used for treatment of distant metastases with palliative intent. “This review provides highlights in current RCC strategies to potentially suggest a more tailored treatment approach in clinical daily practice.”
The investigators concluded that hypofractionated RT (greater than 3 Gy/fraction) deserves more serious consideration. “It has enormous advantages,” the authors wrote, “assuring ablative doses to the target meanwhile preserving surrounding normal tissues. Using stereotactic technique, surprising high local control rates have been achieved in several tumors (such as lung, liver, and bone), in both primary and oligometastatic setting[s].”
In five studies, single-dose RT (ranging from 8 to 24 Gy) was used to treat patients with RCC and extracranial metastases. Of the patients in these studies, 89% of them achieved local control, median overall survival (OS) ranged from 11.7 months to 21 months, and severe RT-related toxicity occurred 0%-4% of the time.
“Although [there is a] high level of data heterogeneity,” the authors wrote, “this systematic review suggested that stereotactic RT is associated with excellent local control rates and low toxicity incidence. Thus, if feasible, stereotactic RT represents an effective and safe approach to treat RCC metastasis.”
The authors cautioned that “the optimal high dose required for local tumor control has not yet been defined.”
The authors suggested that, in the future, immunotherapy in combination with RT may “produce synergistic effects, resulting in better response rate and duration, given the known immune-modulated abscopal effect of RT.” First, questions about treatment sequencing, dosing, and patient selection would need to be answered. “Further research should be aimed at these clinical needs in order to achieve the maximum benefit to RCC patient[s].”
This study did not receive specific funding.
SOURCE: Felice F et al. Crit Rev Oncol Hematol. 2018 Aug 1. doi: 10.1016/j.critrevonc.2018.06.002
Hypofractionated radiation therapy (RT) may be a more viable treatment option for oligometastatic renal cell carcinoma (RCC) than is generally recognized, a recent literature review has suggested.
Advances in stereotactic RT offer “new opportunities in RCC management” with limited toxicity, reported Francesca De Felice, PhD, of Sapienza University in Rome and her coauthor. The authors suggested that future studies investigate RT in combination with immunotherapy.
“Due to the assumption that RCC is a radioresistant tumor,” the authors wrote in Critical Reviews in Oncology/Hematology, “RT has long been considered a futile approach to manage primary disease” and is predominantly used for treatment of distant metastases with palliative intent. “This review provides highlights in current RCC strategies to potentially suggest a more tailored treatment approach in clinical daily practice.”
The investigators concluded that hypofractionated RT (greater than 3 Gy/fraction) deserves more serious consideration. “It has enormous advantages,” the authors wrote, “assuring ablative doses to the target meanwhile preserving surrounding normal tissues. Using stereotactic technique, surprising high local control rates have been achieved in several tumors (such as lung, liver, and bone), in both primary and oligometastatic setting[s].”
In five studies, single-dose RT (ranging from 8 to 24 Gy) was used to treat patients with RCC and extracranial metastases. Of the patients in these studies, 89% of them achieved local control, median overall survival (OS) ranged from 11.7 months to 21 months, and severe RT-related toxicity occurred 0%-4% of the time.
“Although [there is a] high level of data heterogeneity,” the authors wrote, “this systematic review suggested that stereotactic RT is associated with excellent local control rates and low toxicity incidence. Thus, if feasible, stereotactic RT represents an effective and safe approach to treat RCC metastasis.”
The authors cautioned that “the optimal high dose required for local tumor control has not yet been defined.”
The authors suggested that, in the future, immunotherapy in combination with RT may “produce synergistic effects, resulting in better response rate and duration, given the known immune-modulated abscopal effect of RT.” First, questions about treatment sequencing, dosing, and patient selection would need to be answered. “Further research should be aimed at these clinical needs in order to achieve the maximum benefit to RCC patient[s].”
This study did not receive specific funding.
SOURCE: Felice F et al. Crit Rev Oncol Hematol. 2018 Aug 1. doi: 10.1016/j.critrevonc.2018.06.002
FROM CRITICAL REVIEWS IN ONCOLOGY/HEMATOLOGY
Key clinical point: Hypofractionated radiation therapy (RT) is a safe and efficient treatment strategy in patients with oligometastatic renal cell carcinoma (RCC).
Major finding: In five studies, single-dose RT was used to treat patients with RCC and extracranial metastases; 89% of patients achieved local control, median overall survival (OS) was as high as 21 months, and severe RT-related toxicity occurred 0%-4% of the time.
Study details: A literature review of radiation therapy for RCC.
Disclosures: None.
Source: Felice F et al. Crit Rev Oncol Hematol. 2018 Aug 1. doi: 10.1016/j.critrevonc.2018.06.002.
Blood disorders researcher is finalist for Trailblazer Prize
Daniel Bauer, MD, PhD, a pediatric hematologist and blood disorders researcher in Boston, is one of three finalists for the inaugural Trailblazer Prize for Clinician-Scientists, which is awarded by the Foundation for the National Institutes of Health.
Dr. Bauer, of Dana-Farber/Boston Children’s Cancer and Blood Disorders Center and Harvard Medical School, was selected based on his research using genome editing to tease out the causes of blood disorders, such as sickle cell disease and beta-thalassemia.
All three finalists for the Trailblazer Prize are early career clinician-scientists whose work has the potential to or has led to innovations in patient care, according to the Foundation for the National Institutes of Health.
The other two finalists are Jaehyuk Choi, MD, PhD, of Northwestern University in Chicago and Michael Fox, MD, PhD, of Beth Israel Deaconess Medical Center in Boston.
Dr. Choi was selected for using genomics to identify mutations in skin cells that can lead to autoinflammatory diseases and cancer. Dr. Fox was selected for the development of innovative techniques to map human brain connectivity that can be used in novel treatments for Parkinson’s disease and depression.
The winner will be announced during a ceremony in Washington on Oct. 24, 2018, and will receive a $10,000 honorarium.
Daniel Bauer, MD, PhD, a pediatric hematologist and blood disorders researcher in Boston, is one of three finalists for the inaugural Trailblazer Prize for Clinician-Scientists, which is awarded by the Foundation for the National Institutes of Health.
Dr. Bauer, of Dana-Farber/Boston Children’s Cancer and Blood Disorders Center and Harvard Medical School, was selected based on his research using genome editing to tease out the causes of blood disorders, such as sickle cell disease and beta-thalassemia.
All three finalists for the Trailblazer Prize are early career clinician-scientists whose work has the potential to or has led to innovations in patient care, according to the Foundation for the National Institutes of Health.
The other two finalists are Jaehyuk Choi, MD, PhD, of Northwestern University in Chicago and Michael Fox, MD, PhD, of Beth Israel Deaconess Medical Center in Boston.
Dr. Choi was selected for using genomics to identify mutations in skin cells that can lead to autoinflammatory diseases and cancer. Dr. Fox was selected for the development of innovative techniques to map human brain connectivity that can be used in novel treatments for Parkinson’s disease and depression.
The winner will be announced during a ceremony in Washington on Oct. 24, 2018, and will receive a $10,000 honorarium.
Daniel Bauer, MD, PhD, a pediatric hematologist and blood disorders researcher in Boston, is one of three finalists for the inaugural Trailblazer Prize for Clinician-Scientists, which is awarded by the Foundation for the National Institutes of Health.
Dr. Bauer, of Dana-Farber/Boston Children’s Cancer and Blood Disorders Center and Harvard Medical School, was selected based on his research using genome editing to tease out the causes of blood disorders, such as sickle cell disease and beta-thalassemia.
All three finalists for the Trailblazer Prize are early career clinician-scientists whose work has the potential to or has led to innovations in patient care, according to the Foundation for the National Institutes of Health.
The other two finalists are Jaehyuk Choi, MD, PhD, of Northwestern University in Chicago and Michael Fox, MD, PhD, of Beth Israel Deaconess Medical Center in Boston.
Dr. Choi was selected for using genomics to identify mutations in skin cells that can lead to autoinflammatory diseases and cancer. Dr. Fox was selected for the development of innovative techniques to map human brain connectivity that can be used in novel treatments for Parkinson’s disease and depression.
The winner will be announced during a ceremony in Washington on Oct. 24, 2018, and will receive a $10,000 honorarium.
Increasing incidence of metastatic RCC raises concerns for SREs
The incidence of metastatic renal cell carcinoma (RCC) continues to rise, according to a recent study. In turn, skeletal-related events are also becoming more common.
Many patients with metastatic disease have skeletal involvement, so knowledge of skeletal-related events (SREs) is more important than ever, reported Masood Umer, MD, of Aga Khan University Hospital in Karachi, Pakistan, and his coauthors. SREs include nerve compression, hypercalcemia, impending fractures, and pathological fractures, any one of which may require medical or surgical intervention.
Beyond SREs, “bone metastases in RCC [have a] negative impact on progression-free survival and overall survival of patients treated with systemic therapies,” the authors wrote in Annals of Medicine and Surgery.
The authors conducted a literature review of skeletal metastasis in RCC, which included 947 patients, assessing incidence and discussing appropriate medical and surgical interventions.
A total of 26.7% of patients with RCC also had skeletal metastasis. The most common sites of metastasis were the proximal femur, pelvis, and spine. It was estimated that 85% of patients with metastatic RCC may experience SREs and related complications, with an average of more than two events per individual.
A multimodal approach is required, potentially involving surgical and medical interventions. For isolated bony metastases and fractures, surgery is often beneficial. Denosumab is the leading medical treatment; compared with zoledronic acid, denosumab prolongs time to first SRE by a median of approximately 8 months and reduces risk of first SRE by almost 20%. Risks of osteonecrosis are similar between agents.
The authors noted that research concerning the impact of targeted therapies on rates of bone metastasis and SREs is limited by patient exclusions in clinical trials. Granted, these agents have likely made for better outcomes.
“Advancement in targeted therapy in recent decades [has] made some improvement in treatment of SREs and has helped in improving patent’s quality of life, but still we are in need of further improvement in treatment modalities,” they concluded
This study did not receive specific funding.
SOURCE: Umer M et al. Ann Med Surg. 2018 Jan 21. doi: 10.1016/j.amsu.2018.01.002.
The incidence of metastatic renal cell carcinoma (RCC) continues to rise, according to a recent study. In turn, skeletal-related events are also becoming more common.
Many patients with metastatic disease have skeletal involvement, so knowledge of skeletal-related events (SREs) is more important than ever, reported Masood Umer, MD, of Aga Khan University Hospital in Karachi, Pakistan, and his coauthors. SREs include nerve compression, hypercalcemia, impending fractures, and pathological fractures, any one of which may require medical or surgical intervention.
Beyond SREs, “bone metastases in RCC [have a] negative impact on progression-free survival and overall survival of patients treated with systemic therapies,” the authors wrote in Annals of Medicine and Surgery.
The authors conducted a literature review of skeletal metastasis in RCC, which included 947 patients, assessing incidence and discussing appropriate medical and surgical interventions.
A total of 26.7% of patients with RCC also had skeletal metastasis. The most common sites of metastasis were the proximal femur, pelvis, and spine. It was estimated that 85% of patients with metastatic RCC may experience SREs and related complications, with an average of more than two events per individual.
A multimodal approach is required, potentially involving surgical and medical interventions. For isolated bony metastases and fractures, surgery is often beneficial. Denosumab is the leading medical treatment; compared with zoledronic acid, denosumab prolongs time to first SRE by a median of approximately 8 months and reduces risk of first SRE by almost 20%. Risks of osteonecrosis are similar between agents.
The authors noted that research concerning the impact of targeted therapies on rates of bone metastasis and SREs is limited by patient exclusions in clinical trials. Granted, these agents have likely made for better outcomes.
“Advancement in targeted therapy in recent decades [has] made some improvement in treatment of SREs and has helped in improving patent’s quality of life, but still we are in need of further improvement in treatment modalities,” they concluded
This study did not receive specific funding.
SOURCE: Umer M et al. Ann Med Surg. 2018 Jan 21. doi: 10.1016/j.amsu.2018.01.002.
The incidence of metastatic renal cell carcinoma (RCC) continues to rise, according to a recent study. In turn, skeletal-related events are also becoming more common.
Many patients with metastatic disease have skeletal involvement, so knowledge of skeletal-related events (SREs) is more important than ever, reported Masood Umer, MD, of Aga Khan University Hospital in Karachi, Pakistan, and his coauthors. SREs include nerve compression, hypercalcemia, impending fractures, and pathological fractures, any one of which may require medical or surgical intervention.
Beyond SREs, “bone metastases in RCC [have a] negative impact on progression-free survival and overall survival of patients treated with systemic therapies,” the authors wrote in Annals of Medicine and Surgery.
The authors conducted a literature review of skeletal metastasis in RCC, which included 947 patients, assessing incidence and discussing appropriate medical and surgical interventions.
A total of 26.7% of patients with RCC also had skeletal metastasis. The most common sites of metastasis were the proximal femur, pelvis, and spine. It was estimated that 85% of patients with metastatic RCC may experience SREs and related complications, with an average of more than two events per individual.
A multimodal approach is required, potentially involving surgical and medical interventions. For isolated bony metastases and fractures, surgery is often beneficial. Denosumab is the leading medical treatment; compared with zoledronic acid, denosumab prolongs time to first SRE by a median of approximately 8 months and reduces risk of first SRE by almost 20%. Risks of osteonecrosis are similar between agents.
The authors noted that research concerning the impact of targeted therapies on rates of bone metastasis and SREs is limited by patient exclusions in clinical trials. Granted, these agents have likely made for better outcomes.
“Advancement in targeted therapy in recent decades [has] made some improvement in treatment of SREs and has helped in improving patent’s quality of life, but still we are in need of further improvement in treatment modalities,” they concluded
This study did not receive specific funding.
SOURCE: Umer M et al. Ann Med Surg. 2018 Jan 21. doi: 10.1016/j.amsu.2018.01.002.
FROM ANNALS OF MEDICINE AND SURGERY
Key clinical point: As the incidence of metastatic renal cell carcinoma (RCC) continues to rise, knowledge of skeletal-related events and appropriate interventions is essential.
Major finding: About 85% of patients with metastatic RCC experience skeletal-related events and associated complications.
Study details: A literature review of skeletal metastasis in RCC.
Disclosures: The study did not receive specific funding.
Source: Umer M et al. Ann Med Surg. 2018 Jan 21. doi: 10.1016/j.amsu.2018.01.002.
Dr. Eric Howell joins SHM as chief operating officer
Veteran hospitalist will help define organizational goals
The Society of Hospital Medicine has announced the appointment of Eric Howell, MD, MHM, to the position of chief operating officer (COO).
“Having been involved with SHM in many capacities since first joining, I am honored to now transition to chief operating officer,” Dr. Howell said. “I always tell everyone that my goal is to make the world a better place, and I know that SHM’s staff will be able to do just that through the development and deployment of a variety of products, tools, and services to help hospitalists improve patient care.”
In his new role as COO at SHM, Dr. Howell will lead senior management’s strategic planning as well as define organizational goals to drive extensive, sustainable growth. In addition to serving as SHM’s COO, Dr. Howell will continue his role as director of the hospital medicine division of Johns Hopkins Bayview Medical Center in Baltimore and professor of medicine in the department of medicine at Johns Hopkins University, also in Baltimore. Dr. Howell joined the Johns Hopkins Bayview hospitalist program in 2000, began the Howard County (Md.) General Hospital hospitalist program in 2010, and now oversees more than 200 physicians and clinical staff providing patient care in three hospitals.
“Eric has the perfect background to take SHM, its staff, and its membership to the next level,” said Laurence Wellikson, MD, MHM, chief executive officer of SHM. “His foundational leadership in the hospital medicine movement makes him the ideal person to lead SHM forward in its quest to provide hospitalists with the tools necessary to make a noteworthy difference in their institutions and in the lives of their patients.”
Dr. Howell is also a past president of SHM, the course director for the SHM Leadership Academies, and most recently, served as the senior physician advisor to SHM’s Center for Quality Improvement, which conducts quality improvement programs for hospitalist teams. He received his electrical engineering degree from the University of Maryland, which he said has served as an instrumental piece of his background for managing and implementing change in the hospital. His research has focused on the relationship between the emergency department and medicine floors, improving communication, throughput, and patient outcomes.
Veteran hospitalist will help define organizational goals
Veteran hospitalist will help define organizational goals
The Society of Hospital Medicine has announced the appointment of Eric Howell, MD, MHM, to the position of chief operating officer (COO).
“Having been involved with SHM in many capacities since first joining, I am honored to now transition to chief operating officer,” Dr. Howell said. “I always tell everyone that my goal is to make the world a better place, and I know that SHM’s staff will be able to do just that through the development and deployment of a variety of products, tools, and services to help hospitalists improve patient care.”
In his new role as COO at SHM, Dr. Howell will lead senior management’s strategic planning as well as define organizational goals to drive extensive, sustainable growth. In addition to serving as SHM’s COO, Dr. Howell will continue his role as director of the hospital medicine division of Johns Hopkins Bayview Medical Center in Baltimore and professor of medicine in the department of medicine at Johns Hopkins University, also in Baltimore. Dr. Howell joined the Johns Hopkins Bayview hospitalist program in 2000, began the Howard County (Md.) General Hospital hospitalist program in 2010, and now oversees more than 200 physicians and clinical staff providing patient care in three hospitals.
“Eric has the perfect background to take SHM, its staff, and its membership to the next level,” said Laurence Wellikson, MD, MHM, chief executive officer of SHM. “His foundational leadership in the hospital medicine movement makes him the ideal person to lead SHM forward in its quest to provide hospitalists with the tools necessary to make a noteworthy difference in their institutions and in the lives of their patients.”
Dr. Howell is also a past president of SHM, the course director for the SHM Leadership Academies, and most recently, served as the senior physician advisor to SHM’s Center for Quality Improvement, which conducts quality improvement programs for hospitalist teams. He received his electrical engineering degree from the University of Maryland, which he said has served as an instrumental piece of his background for managing and implementing change in the hospital. His research has focused on the relationship between the emergency department and medicine floors, improving communication, throughput, and patient outcomes.
The Society of Hospital Medicine has announced the appointment of Eric Howell, MD, MHM, to the position of chief operating officer (COO).
“Having been involved with SHM in many capacities since first joining, I am honored to now transition to chief operating officer,” Dr. Howell said. “I always tell everyone that my goal is to make the world a better place, and I know that SHM’s staff will be able to do just that through the development and deployment of a variety of products, tools, and services to help hospitalists improve patient care.”
In his new role as COO at SHM, Dr. Howell will lead senior management’s strategic planning as well as define organizational goals to drive extensive, sustainable growth. In addition to serving as SHM’s COO, Dr. Howell will continue his role as director of the hospital medicine division of Johns Hopkins Bayview Medical Center in Baltimore and professor of medicine in the department of medicine at Johns Hopkins University, also in Baltimore. Dr. Howell joined the Johns Hopkins Bayview hospitalist program in 2000, began the Howard County (Md.) General Hospital hospitalist program in 2010, and now oversees more than 200 physicians and clinical staff providing patient care in three hospitals.
“Eric has the perfect background to take SHM, its staff, and its membership to the next level,” said Laurence Wellikson, MD, MHM, chief executive officer of SHM. “His foundational leadership in the hospital medicine movement makes him the ideal person to lead SHM forward in its quest to provide hospitalists with the tools necessary to make a noteworthy difference in their institutions and in the lives of their patients.”
Dr. Howell is also a past president of SHM, the course director for the SHM Leadership Academies, and most recently, served as the senior physician advisor to SHM’s Center for Quality Improvement, which conducts quality improvement programs for hospitalist teams. He received his electrical engineering degree from the University of Maryland, which he said has served as an instrumental piece of his background for managing and implementing change in the hospital. His research has focused on the relationship between the emergency department and medicine floors, improving communication, throughput, and patient outcomes.
Little overlap between surgical M&M and AHRQ on adverse events
Limited overlap in adverse events identified by surgical morbidity and mortality (M&M) conferences and by Agency for Healthcare Research and Quality patient safety indicators (PSIs) demonstrates that the two processes tend to capture different, but equally important, measures, according to study results published in the Journal of the American College of Surgeons.
Just 18 of 149 (12.1%) PSI-defined events were identified by both processes in a retrospective, observational study of complications at the UC Davis Medical Center’s department of surgery. Most events (62.4%) were identified by only the M&M review, while 25.5% were identified by only the PSIs, reported Jamie E. Anderson, MD, MPH, of the department of surgery at UC Davis Medical Center in Sacramento and coauthors.
The study authors identified 6,563 surgical hospitalizations in the year 2016, of which 647 (9.9%) had at least one event that was either submitted for review for a departmental M&M conference, identified as a PSI event from administrative data, or both. Cases in patients aged less than 18 years were excluded.
Hospital administrative data were reported using ICD-10 CM/PCS codes. Investigators identified all PSI cases, which included pressure ulcer, retained surgical item, iatrogenic pneumothorax, central venous catheter–related blood stream infection, postoperative hip fracture, perioperative hematoma or hemorrhage requiring a procedure, postoperative acute kidney injury requiring dialysis, postoperative respiratory failure, perioperative pulmonary embolism or deep venous thrombosis, postoperative sepsis, postoperative wound dehiscence, and unrecognized abdominopelvic accidental puncture or laceration.
Complications submitted to the M&M conference were reviewed for PSI-defined events, and included events from general surgery, bariatric surgery, burn, cardiothoracic, colorectal, surgical oncology, plastic, vascular, transplant, and trauma. PSI-defined events were then reviewed to verify whether they counted as “true” PSI events and further classified as a documentation error, intentional exclusion, or inherent limitation of the PSI, the authors reported.
Of 6,563 surgical hospitalizations, 647 had at least one complication identified by M&M, PSI, or both. Of these, 116 had at least one PSI-defined event identified by either M&M or PSI. The remaining hospitalizations had unrelated complications and were excluded from analysis.
Of the 116 hospitalizations, there were 149 PSI-defined events, of which 18 (12.1%) were identified by both methods. Most events (62.4%) were identified by only the M&M review, and 25.5% were identified by only the PSIs. Perioperative hemorrhage/hematoma and postoperative sepsis were most likely to be identified by both.
Of the 93 PSI-defined events captured by only M&M, 11 (11.8%) met AHRQ criteria and were considered “true” events, or “false negatives.” All 38 events identified by PSI alone were correctly identified as true PSI events, Dr. Anderson and colleagues reported.
The findings indicate that the AHRQ PSI and surgical M&M conference “should be considered complementary approaches for identifying complications,” the authors wrote.
The PSI data captured central venous catheter–related blood stream infection and pressure ulcers, but the M&M conferences did not include these outcomes. The M&M reviewed more cases of postoperative sepsis, abdominopelvic accidental laceration, and the one case of retained surgical item.
“These two processes of identifying complications have different purposes, and each approach captured different events,” they added.
The M&M conference “balances clinician education and quality improvement with an underlying theme of accountability,” they said, with increased emphasis on examining adverse events in the context of systems-based practices. PSI, on the other hand, is intended as a “resource-nonintensive means” to help hospitals identify preventable events and facilitate quality improvement, they said.
“In an era in which there are numerous mechanisms to measure surgical quality, the traditional M&M conference is still relevant for identifying and discussing surgical complications,” the authors concluded. “We believe that our center’s existing M&M case-finding process is fundamentally sound, but it could be improved by including all PSI-flagged hospitalizations in our M&M process. This may result in review of some false-positive records, but it will enable our department to address certain potentially preventable complications that are currently overlooked.”
Two of the study coauthors received salary support from the AHRQ to support the agency’s Quality Indicator Program, one of whom serves on the agency’s Quality Indicators Expert Workgroup. No other disclosures were reported.
SOURCE: Anderson J et al. J Am Coll Surg. 2018 Jul 5. doi: 10.1016/j.jamcollsurg.2018.06.008.
Limited overlap in adverse events identified by surgical morbidity and mortality (M&M) conferences and by Agency for Healthcare Research and Quality patient safety indicators (PSIs) demonstrates that the two processes tend to capture different, but equally important, measures, according to study results published in the Journal of the American College of Surgeons.
Just 18 of 149 (12.1%) PSI-defined events were identified by both processes in a retrospective, observational study of complications at the UC Davis Medical Center’s department of surgery. Most events (62.4%) were identified by only the M&M review, while 25.5% were identified by only the PSIs, reported Jamie E. Anderson, MD, MPH, of the department of surgery at UC Davis Medical Center in Sacramento and coauthors.
The study authors identified 6,563 surgical hospitalizations in the year 2016, of which 647 (9.9%) had at least one event that was either submitted for review for a departmental M&M conference, identified as a PSI event from administrative data, or both. Cases in patients aged less than 18 years were excluded.
Hospital administrative data were reported using ICD-10 CM/PCS codes. Investigators identified all PSI cases, which included pressure ulcer, retained surgical item, iatrogenic pneumothorax, central venous catheter–related blood stream infection, postoperative hip fracture, perioperative hematoma or hemorrhage requiring a procedure, postoperative acute kidney injury requiring dialysis, postoperative respiratory failure, perioperative pulmonary embolism or deep venous thrombosis, postoperative sepsis, postoperative wound dehiscence, and unrecognized abdominopelvic accidental puncture or laceration.
Complications submitted to the M&M conference were reviewed for PSI-defined events, and included events from general surgery, bariatric surgery, burn, cardiothoracic, colorectal, surgical oncology, plastic, vascular, transplant, and trauma. PSI-defined events were then reviewed to verify whether they counted as “true” PSI events and further classified as a documentation error, intentional exclusion, or inherent limitation of the PSI, the authors reported.
Of 6,563 surgical hospitalizations, 647 had at least one complication identified by M&M, PSI, or both. Of these, 116 had at least one PSI-defined event identified by either M&M or PSI. The remaining hospitalizations had unrelated complications and were excluded from analysis.
Of the 116 hospitalizations, there were 149 PSI-defined events, of which 18 (12.1%) were identified by both methods. Most events (62.4%) were identified by only the M&M review, and 25.5% were identified by only the PSIs. Perioperative hemorrhage/hematoma and postoperative sepsis were most likely to be identified by both.
Of the 93 PSI-defined events captured by only M&M, 11 (11.8%) met AHRQ criteria and were considered “true” events, or “false negatives.” All 38 events identified by PSI alone were correctly identified as true PSI events, Dr. Anderson and colleagues reported.
The findings indicate that the AHRQ PSI and surgical M&M conference “should be considered complementary approaches for identifying complications,” the authors wrote.
The PSI data captured central venous catheter–related blood stream infection and pressure ulcers, but the M&M conferences did not include these outcomes. The M&M reviewed more cases of postoperative sepsis, abdominopelvic accidental laceration, and the one case of retained surgical item.
“These two processes of identifying complications have different purposes, and each approach captured different events,” they added.
The M&M conference “balances clinician education and quality improvement with an underlying theme of accountability,” they said, with increased emphasis on examining adverse events in the context of systems-based practices. PSI, on the other hand, is intended as a “resource-nonintensive means” to help hospitals identify preventable events and facilitate quality improvement, they said.
“In an era in which there are numerous mechanisms to measure surgical quality, the traditional M&M conference is still relevant for identifying and discussing surgical complications,” the authors concluded. “We believe that our center’s existing M&M case-finding process is fundamentally sound, but it could be improved by including all PSI-flagged hospitalizations in our M&M process. This may result in review of some false-positive records, but it will enable our department to address certain potentially preventable complications that are currently overlooked.”
Two of the study coauthors received salary support from the AHRQ to support the agency’s Quality Indicator Program, one of whom serves on the agency’s Quality Indicators Expert Workgroup. No other disclosures were reported.
SOURCE: Anderson J et al. J Am Coll Surg. 2018 Jul 5. doi: 10.1016/j.jamcollsurg.2018.06.008.
Limited overlap in adverse events identified by surgical morbidity and mortality (M&M) conferences and by Agency for Healthcare Research and Quality patient safety indicators (PSIs) demonstrates that the two processes tend to capture different, but equally important, measures, according to study results published in the Journal of the American College of Surgeons.
Just 18 of 149 (12.1%) PSI-defined events were identified by both processes in a retrospective, observational study of complications at the UC Davis Medical Center’s department of surgery. Most events (62.4%) were identified by only the M&M review, while 25.5% were identified by only the PSIs, reported Jamie E. Anderson, MD, MPH, of the department of surgery at UC Davis Medical Center in Sacramento and coauthors.
The study authors identified 6,563 surgical hospitalizations in the year 2016, of which 647 (9.9%) had at least one event that was either submitted for review for a departmental M&M conference, identified as a PSI event from administrative data, or both. Cases in patients aged less than 18 years were excluded.
Hospital administrative data were reported using ICD-10 CM/PCS codes. Investigators identified all PSI cases, which included pressure ulcer, retained surgical item, iatrogenic pneumothorax, central venous catheter–related blood stream infection, postoperative hip fracture, perioperative hematoma or hemorrhage requiring a procedure, postoperative acute kidney injury requiring dialysis, postoperative respiratory failure, perioperative pulmonary embolism or deep venous thrombosis, postoperative sepsis, postoperative wound dehiscence, and unrecognized abdominopelvic accidental puncture or laceration.
Complications submitted to the M&M conference were reviewed for PSI-defined events, and included events from general surgery, bariatric surgery, burn, cardiothoracic, colorectal, surgical oncology, plastic, vascular, transplant, and trauma. PSI-defined events were then reviewed to verify whether they counted as “true” PSI events and further classified as a documentation error, intentional exclusion, or inherent limitation of the PSI, the authors reported.
Of 6,563 surgical hospitalizations, 647 had at least one complication identified by M&M, PSI, or both. Of these, 116 had at least one PSI-defined event identified by either M&M or PSI. The remaining hospitalizations had unrelated complications and were excluded from analysis.
Of the 116 hospitalizations, there were 149 PSI-defined events, of which 18 (12.1%) were identified by both methods. Most events (62.4%) were identified by only the M&M review, and 25.5% were identified by only the PSIs. Perioperative hemorrhage/hematoma and postoperative sepsis were most likely to be identified by both.
Of the 93 PSI-defined events captured by only M&M, 11 (11.8%) met AHRQ criteria and were considered “true” events, or “false negatives.” All 38 events identified by PSI alone were correctly identified as true PSI events, Dr. Anderson and colleagues reported.
The findings indicate that the AHRQ PSI and surgical M&M conference “should be considered complementary approaches for identifying complications,” the authors wrote.
The PSI data captured central venous catheter–related blood stream infection and pressure ulcers, but the M&M conferences did not include these outcomes. The M&M reviewed more cases of postoperative sepsis, abdominopelvic accidental laceration, and the one case of retained surgical item.
“These two processes of identifying complications have different purposes, and each approach captured different events,” they added.
The M&M conference “balances clinician education and quality improvement with an underlying theme of accountability,” they said, with increased emphasis on examining adverse events in the context of systems-based practices. PSI, on the other hand, is intended as a “resource-nonintensive means” to help hospitals identify preventable events and facilitate quality improvement, they said.
“In an era in which there are numerous mechanisms to measure surgical quality, the traditional M&M conference is still relevant for identifying and discussing surgical complications,” the authors concluded. “We believe that our center’s existing M&M case-finding process is fundamentally sound, but it could be improved by including all PSI-flagged hospitalizations in our M&M process. This may result in review of some false-positive records, but it will enable our department to address certain potentially preventable complications that are currently overlooked.”
Two of the study coauthors received salary support from the AHRQ to support the agency’s Quality Indicator Program, one of whom serves on the agency’s Quality Indicators Expert Workgroup. No other disclosures were reported.
SOURCE: Anderson J et al. J Am Coll Surg. 2018 Jul 5. doi: 10.1016/j.jamcollsurg.2018.06.008.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Key clinical point: Surgical morbidity and mortality conferences and AHRQ patient safety indicators should be considered complementary measures of adverse events because of a limited overlap in identifying adverse events.
Major finding: Eighteen of 149 (12.1%) PSI-defined events were identified by both processes; most (62.4%) were identified by only M&M review, and 25.5% by only PSI.
Study details: A retrospective observational study of all complications in 2016 at the UC Davis Medical Center department of surgery.
Disclosures: Two of the study coauthors received salary support from the AHRQ to support the agency’s Quality Indicator Program, one of whom serves on the agency’s Quality Indicators Expert Workgroup. No other disclosures were reported.
Source: Anderson J et al. J Am Coll Surg. 2018 Jul 5. doi: 10.1016/j.jamcollsurg.2018.06.008.
Is the most effective emergency contraception easily obtained at US pharmacies?
EXPERT COMMENTARY
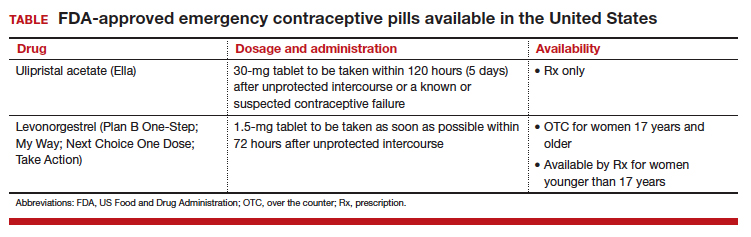
Although it is available only by prescription, ulipristal acetate provides emergency contraception that is more effective than the emergency contraception provided by levonorgestrel (LNG), which is available without a prescription (TABLE). In addition, ulipristal acetate appears more effective than LNG in obese and overweight women.1,2 Package labeling for ulipristal acetate indicates that a single 30-mg tablet should be taken orally within 5 days of unprotected sex.
According to a survey of pharmacy availability of ulipristal acetate in Hawaii, 2.6% of retail pharmacies had the drug immediately available, compared with 82.4% for LNG, and 22.8% reported the ability to order it.3 To assess pharmacy availability of ulipristal acetate on a nationwide scale, Shigesato and colleagues conducted a national “secret shopper” telephone survey in 10 cities (each with a population of at least 500,000) in all major regions of the United States.
Details of the study
Independent pharmacies (defined as having fewer than 5 locations within the city) and chain pharmacies were included in the survey. The survey callers, representing themselves as uninsured 18-year-old women attempting to fill a prescription for ulipristal acetate, followed a semistructured questionnaire and recorded the responses. They asked about the immediate availability of ulipristal acetate and LNG, the pharmacy’s ability to order ulipristal acetate if not immediately available, out-of-pocket costs, instructions for use, and the differences between ulipristal acetate and LNG. Questions were directed to whichever pharmacy staff member answered the phone; callers did not specifically ask to speak to a pharmacist.
Of the 344 pharmacies included in this analysis, 10% (33) indicated that they could fill a prescription for ulipristal acetate immediately. While availability did not vary by region, there was a difference in immediate availability by city.
Almost three-quarters of pharmacies without immediate drug availability indicated that they could order ulipristal acetate, with a median predicted time for availability of 24 hours. Of the chain pharmacies, 81% (167 of 205) reported the ability to order ulipristal acetate, compared with 55% (57 of 106) of independent pharmacies.
When asked if ulipristal acetate was different from LNG, more than one-third of pharmacy personnel contacted stated either that there was no difference between ulipristal acetate and LNG or that they were not sure of a difference.
Study strengths and weaknesses
The authors noted that the secret shopper methodology, along with having callers speak to the pharmacy staff person who answered the call (rather than asking for the pharmacist), provided data that closely approximates real-world patient experiences.
Since more pharmacies than anticipated met exclusion criteria for the study, the estimate of ulipristal acetate immediate availability was less precise than the power analysis predicted. Further, results from the 10 large, geographically diverse cities may not be representative of all similarly sized cities nationally or all areas of the United States.
As the authors point out, a low prevalence of pharmacies stock ulipristal acetate, and more than 25% are not able to order this emergency contraception. This underscores the fact that access to the most effective oral emergency contraception is limited for US women. I agree with the authors’ speculation that access to ulipristal acetate may be even lower in rural areas. In many European countries, ulipristal acetate is available without a prescription. Clinicians caring for women who may benefit from emergency contraception, particularly those using short-acting or less effective contraceptives, may wish to prescribe ulipristal acetate in advance of need.
—Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kapp N, Abitbol JL, Mathé H, et al. Effect of body weight and BMI on the efficacy of levonorgestrel emergency contraception. Contraception. 2015;91(2):97–104.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–367.
- Bullock H, Steele S, Kurata N, et al. Pharmacy access to ulipristal acetate in Hawaii: is a prescription enough? Contraception. 2016;93(5):452–454.
EXPERT COMMENTARY
Although it is available only by prescription, ulipristal acetate provides emergency contraception that is more effective than the emergency contraception provided by levonorgestrel (LNG), which is available without a prescription (TABLE). In addition, ulipristal acetate appears more effective than LNG in obese and overweight women.1,2 Package labeling for ulipristal acetate indicates that a single 30-mg tablet should be taken orally within 5 days of unprotected sex.

According to a survey of pharmacy availability of ulipristal acetate in Hawaii, 2.6% of retail pharmacies had the drug immediately available, compared with 82.4% for LNG, and 22.8% reported the ability to order it.3 To assess pharmacy availability of ulipristal acetate on a nationwide scale, Shigesato and colleagues conducted a national “secret shopper” telephone survey in 10 cities (each with a population of at least 500,000) in all major regions of the United States.
Details of the study
Independent pharmacies (defined as having fewer than 5 locations within the city) and chain pharmacies were included in the survey. The survey callers, representing themselves as uninsured 18-year-old women attempting to fill a prescription for ulipristal acetate, followed a semistructured questionnaire and recorded the responses. They asked about the immediate availability of ulipristal acetate and LNG, the pharmacy’s ability to order ulipristal acetate if not immediately available, out-of-pocket costs, instructions for use, and the differences between ulipristal acetate and LNG. Questions were directed to whichever pharmacy staff member answered the phone; callers did not specifically ask to speak to a pharmacist.
Of the 344 pharmacies included in this analysis, 10% (33) indicated that they could fill a prescription for ulipristal acetate immediately. While availability did not vary by region, there was a difference in immediate availability by city.
Almost three-quarters of pharmacies without immediate drug availability indicated that they could order ulipristal acetate, with a median predicted time for availability of 24 hours. Of the chain pharmacies, 81% (167 of 205) reported the ability to order ulipristal acetate, compared with 55% (57 of 106) of independent pharmacies.
When asked if ulipristal acetate was different from LNG, more than one-third of pharmacy personnel contacted stated either that there was no difference between ulipristal acetate and LNG or that they were not sure of a difference.
Study strengths and weaknesses
The authors noted that the secret shopper methodology, along with having callers speak to the pharmacy staff person who answered the call (rather than asking for the pharmacist), provided data that closely approximates real-world patient experiences.
Since more pharmacies than anticipated met exclusion criteria for the study, the estimate of ulipristal acetate immediate availability was less precise than the power analysis predicted. Further, results from the 10 large, geographically diverse cities may not be representative of all similarly sized cities nationally or all areas of the United States.
As the authors point out, a low prevalence of pharmacies stock ulipristal acetate, and more than 25% are not able to order this emergency contraception. This underscores the fact that access to the most effective oral emergency contraception is limited for US women. I agree with the authors’ speculation that access to ulipristal acetate may be even lower in rural areas. In many European countries, ulipristal acetate is available without a prescription. Clinicians caring for women who may benefit from emergency contraception, particularly those using short-acting or less effective contraceptives, may wish to prescribe ulipristal acetate in advance of need.
—Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Although it is available only by prescription, ulipristal acetate provides emergency contraception that is more effective than the emergency contraception provided by levonorgestrel (LNG), which is available without a prescription (TABLE). In addition, ulipristal acetate appears more effective than LNG in obese and overweight women.1,2 Package labeling for ulipristal acetate indicates that a single 30-mg tablet should be taken orally within 5 days of unprotected sex.

According to a survey of pharmacy availability of ulipristal acetate in Hawaii, 2.6% of retail pharmacies had the drug immediately available, compared with 82.4% for LNG, and 22.8% reported the ability to order it.3 To assess pharmacy availability of ulipristal acetate on a nationwide scale, Shigesato and colleagues conducted a national “secret shopper” telephone survey in 10 cities (each with a population of at least 500,000) in all major regions of the United States.
Details of the study
Independent pharmacies (defined as having fewer than 5 locations within the city) and chain pharmacies were included in the survey. The survey callers, representing themselves as uninsured 18-year-old women attempting to fill a prescription for ulipristal acetate, followed a semistructured questionnaire and recorded the responses. They asked about the immediate availability of ulipristal acetate and LNG, the pharmacy’s ability to order ulipristal acetate if not immediately available, out-of-pocket costs, instructions for use, and the differences between ulipristal acetate and LNG. Questions were directed to whichever pharmacy staff member answered the phone; callers did not specifically ask to speak to a pharmacist.
Of the 344 pharmacies included in this analysis, 10% (33) indicated that they could fill a prescription for ulipristal acetate immediately. While availability did not vary by region, there was a difference in immediate availability by city.
Almost three-quarters of pharmacies without immediate drug availability indicated that they could order ulipristal acetate, with a median predicted time for availability of 24 hours. Of the chain pharmacies, 81% (167 of 205) reported the ability to order ulipristal acetate, compared with 55% (57 of 106) of independent pharmacies.
When asked if ulipristal acetate was different from LNG, more than one-third of pharmacy personnel contacted stated either that there was no difference between ulipristal acetate and LNG or that they were not sure of a difference.
Study strengths and weaknesses
The authors noted that the secret shopper methodology, along with having callers speak to the pharmacy staff person who answered the call (rather than asking for the pharmacist), provided data that closely approximates real-world patient experiences.
Since more pharmacies than anticipated met exclusion criteria for the study, the estimate of ulipristal acetate immediate availability was less precise than the power analysis predicted. Further, results from the 10 large, geographically diverse cities may not be representative of all similarly sized cities nationally or all areas of the United States.
As the authors point out, a low prevalence of pharmacies stock ulipristal acetate, and more than 25% are not able to order this emergency contraception. This underscores the fact that access to the most effective oral emergency contraception is limited for US women. I agree with the authors’ speculation that access to ulipristal acetate may be even lower in rural areas. In many European countries, ulipristal acetate is available without a prescription. Clinicians caring for women who may benefit from emergency contraception, particularly those using short-acting or less effective contraceptives, may wish to prescribe ulipristal acetate in advance of need.
—Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kapp N, Abitbol JL, Mathé H, et al. Effect of body weight and BMI on the efficacy of levonorgestrel emergency contraception. Contraception. 2015;91(2):97–104.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–367.
- Bullock H, Steele S, Kurata N, et al. Pharmacy access to ulipristal acetate in Hawaii: is a prescription enough? Contraception. 2016;93(5):452–454.
- Kapp N, Abitbol JL, Mathé H, et al. Effect of body weight and BMI on the efficacy of levonorgestrel emergency contraception. Contraception. 2015;91(2):97–104.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–367.
- Bullock H, Steele S, Kurata N, et al. Pharmacy access to ulipristal acetate in Hawaii: is a prescription enough? Contraception. 2016;93(5):452–454.
Breastfeeding lowered later stroke risk in WHI
Postmenopausal women who breastfed their children had a lower risk of stroke compared with women who had children but never breastfed, with non-Hispanic black women showing a significantly stronger association between breastfeeding and lower stroke risk, according to results from the prospective Women’s Health Initiative Observational Study.
“Some studies have reported that breastfeeding may reduce the rates of breast cancer, ovarian cancer and risk of developing type 2 diabetes in mothers. Recent findings point to the benefits of breastfeeding on heart disease and other specific cardiovascular risk factors,” Lisette T. Jacobson, PhD, of the department of preventive medicine and public health at the University of Kansas, Wichita, said in a statement.
Dr. Jacobson and her colleagues evaluated 80,191 women from the Women’s Health Initiative (WHI) Observational Study who were aged 50-79 at baseline. The average age was 64 years, and 83% were white, 8% were non-Hispanic black, 4% were Hispanic, and 5% were another race or ethnicity. Of the women observed, 58% had breastfed and 3.4% had a stroke within an average of 13 years of follow-up. The investigators used three adjusted regression models to analyze stroke risk: Model 1 was minimally adjusted, model 2 was adjusted for nonmodifiable potential confounders, and model 3 was adjusted for modifiable lifestyle factors.
There was a 23% lower risk of stroke among all postmenopausal women who breastfed compared with those who never breastfed, with women who breastfed between 1 month and 6 months carrying a 19% lower risk of stroke. In the minimally adjusted model, non-Hispanic white women who breastfed carried a 21% lower risk, Hispanic women had an adjusted 32% lower risk, and women of other races and ethnicity had a 24% lower risk of stroke. However, women who were non-Hispanic black had a stronger association with breastfeeding and stroke reduction, with a 48% lower risk, and non-Hispanic white and non-Hispanic black women showed a stronger association between longer duration of breastfeeding and lower stroke risk when results were minimally adjusted, the investigators said. All differences were statistically significant.
The investigators noted the study’s observational nature and said they were not able to determine what caused breastfeeding’s association with lower stroke risk, with other factors potentially affecting results.
“Breastfeeding is only one of many factors that could potentially protect against stroke,” Dr. Jacobson said in the report, published online in the Journal of the American Heart Association. “Others include getting adequate exercise, choosing healthy foods, not smoking, and seeking treatment if needed to keep your blood pressure, cholesterol, and blood sugar in the normal range.”
They also noted potential limitations in the study: the WHI cohort’s low number of strokes in follow-up, lack of classification of stroke, recall bias due to the women self-reporting strokes, average age at baseline, and lack of data on pregnancy.
“Our study did not address whether racial/ethnic differences in breastfeeding contribute to disparities in stroke risk,” Dr. Jacobson said. “Additional research should consider the degree to which breastfeeding might alter racial/ethnic differences in stroke risk.”
“This is an observational, prospective cohort study that was performed very carefully, but it is important to not conclude causality in that breastfeeding results in a reduction in late life stroke,” Larry B. Goldstein, MD, said in an email interview.
Dr. Goldstein, a neurologist who has published several guidelines on primary prevention and early management of stroke with the American Heart Association, noted that although the authors addressed many confounders, studies of this type are still open to residual confounding. He said one of the factors the authors could not measure was eclampsia and preeclampsia, which inhibits breastfeeding.
“The possibility of unmeasured confounding despite how well the study was done is still there. But having said that, the recommendations for breastfeeding are strong from the American Academy of Pediatrics and from the World Health Organization,” and other studies have found an association with a reduction in later life cardiovascular disease, said Dr. Goldstein, the Ruth L. Works professor and chairman in the department of neurology at the University of Kentucky, Lexington. “But just in terms of the benefits to the mother and to the child from breastfeeding, this is another potential plus [in that] even if it doesn’t pan out, it doesn’t really change the recommendation for breastfeeding.”
Dr. Goldstein noted that finding these results in a different prospective cohort would strengthen the recommendations, as would examining whether factors such as lifestyle affected stroke risk for women.
“Showing causality is always going to be difficult,” he stressed. “There is no particular causal mechanism that’s been espoused for how this might decrease stroke risk in later life.”
This study is funded by Frontiers: The Heartland Institute for Clinical and Translational Research and the Wichita Center for Graduate Medical Education–Kansas Bioscience Authority. The WHI program is funded by the National Heart, Lung, and Blood Institute, the National Institutes of Health, and the U.S. Department of Health and Human Services. The authors reported having no conflicts of interest.
SOURCE: Jacobson LT et al. J Am Heart Assoc. 2018 Aug 22. doi:10.1161/JAHA.118.008739.
This is an important study for pediatricians who counsel breastfeeding mothers and families on the benefits of breastfeeding for mothers and their families.
The current study is important because of its large scale and the fact that it shows an association between any breastfeeding longer than 1 month and protection against stroke, especially for the non-Hispanic black population. These women face higher risks of cardiovascular disease, including hypertension and heart disease, and also higher risks from obesity and hypertension. Longer duration of breastfeeding showed an association with decreased risk of stroke for both non-Hispanic white women and non-Hispanic black women in this study.
On the basis of this study, pediatricians can include potential protection against strokes, as part of the list of protective effects when counseling mothers, either prenatally or in the postpartum setting. Women of childbearing age are not at high risk for stroke, but breastfeeding is a healthy life choice that has significant benefits not just during the period of direct breastfeeding but for years afterward. This study also emphasizes that the benefits of breastfeeding are often dose related. In other words, the longer the mother breastfeeds, the greater the health benefits are for her and for her child.
It would be helpful to have further long-term prospective studies that collect information about breastfeeding at the time that the mother is breastfeeding and then throughout her lifespan. That way, the risk of stroke as well as other cardiovascular risks and cancer risks could be more precisely delineated without the potential for recall bias.
Joan Younger Meek, MD, is chair of the American Academy of Pediatrics Section on Breastfeeding and associate dean for graduate medical education at Florida State University, Orlando. These comments were excerpted from an email interview. She has no relevant conflicts of interest.
This is an important study for pediatricians who counsel breastfeeding mothers and families on the benefits of breastfeeding for mothers and their families.
The current study is important because of its large scale and the fact that it shows an association between any breastfeeding longer than 1 month and protection against stroke, especially for the non-Hispanic black population. These women face higher risks of cardiovascular disease, including hypertension and heart disease, and also higher risks from obesity and hypertension. Longer duration of breastfeeding showed an association with decreased risk of stroke for both non-Hispanic white women and non-Hispanic black women in this study.
On the basis of this study, pediatricians can include potential protection against strokes, as part of the list of protective effects when counseling mothers, either prenatally or in the postpartum setting. Women of childbearing age are not at high risk for stroke, but breastfeeding is a healthy life choice that has significant benefits not just during the period of direct breastfeeding but for years afterward. This study also emphasizes that the benefits of breastfeeding are often dose related. In other words, the longer the mother breastfeeds, the greater the health benefits are for her and for her child.
It would be helpful to have further long-term prospective studies that collect information about breastfeeding at the time that the mother is breastfeeding and then throughout her lifespan. That way, the risk of stroke as well as other cardiovascular risks and cancer risks could be more precisely delineated without the potential for recall bias.
Joan Younger Meek, MD, is chair of the American Academy of Pediatrics Section on Breastfeeding and associate dean for graduate medical education at Florida State University, Orlando. These comments were excerpted from an email interview. She has no relevant conflicts of interest.
This is an important study for pediatricians who counsel breastfeeding mothers and families on the benefits of breastfeeding for mothers and their families.
The current study is important because of its large scale and the fact that it shows an association between any breastfeeding longer than 1 month and protection against stroke, especially for the non-Hispanic black population. These women face higher risks of cardiovascular disease, including hypertension and heart disease, and also higher risks from obesity and hypertension. Longer duration of breastfeeding showed an association with decreased risk of stroke for both non-Hispanic white women and non-Hispanic black women in this study.
On the basis of this study, pediatricians can include potential protection against strokes, as part of the list of protective effects when counseling mothers, either prenatally or in the postpartum setting. Women of childbearing age are not at high risk for stroke, but breastfeeding is a healthy life choice that has significant benefits not just during the period of direct breastfeeding but for years afterward. This study also emphasizes that the benefits of breastfeeding are often dose related. In other words, the longer the mother breastfeeds, the greater the health benefits are for her and for her child.
It would be helpful to have further long-term prospective studies that collect information about breastfeeding at the time that the mother is breastfeeding and then throughout her lifespan. That way, the risk of stroke as well as other cardiovascular risks and cancer risks could be more precisely delineated without the potential for recall bias.
Joan Younger Meek, MD, is chair of the American Academy of Pediatrics Section on Breastfeeding and associate dean for graduate medical education at Florida State University, Orlando. These comments were excerpted from an email interview. She has no relevant conflicts of interest.
Postmenopausal women who breastfed their children had a lower risk of stroke compared with women who had children but never breastfed, with non-Hispanic black women showing a significantly stronger association between breastfeeding and lower stroke risk, according to results from the prospective Women’s Health Initiative Observational Study.
“Some studies have reported that breastfeeding may reduce the rates of breast cancer, ovarian cancer and risk of developing type 2 diabetes in mothers. Recent findings point to the benefits of breastfeeding on heart disease and other specific cardiovascular risk factors,” Lisette T. Jacobson, PhD, of the department of preventive medicine and public health at the University of Kansas, Wichita, said in a statement.
Dr. Jacobson and her colleagues evaluated 80,191 women from the Women’s Health Initiative (WHI) Observational Study who were aged 50-79 at baseline. The average age was 64 years, and 83% were white, 8% were non-Hispanic black, 4% were Hispanic, and 5% were another race or ethnicity. Of the women observed, 58% had breastfed and 3.4% had a stroke within an average of 13 years of follow-up. The investigators used three adjusted regression models to analyze stroke risk: Model 1 was minimally adjusted, model 2 was adjusted for nonmodifiable potential confounders, and model 3 was adjusted for modifiable lifestyle factors.
There was a 23% lower risk of stroke among all postmenopausal women who breastfed compared with those who never breastfed, with women who breastfed between 1 month and 6 months carrying a 19% lower risk of stroke. In the minimally adjusted model, non-Hispanic white women who breastfed carried a 21% lower risk, Hispanic women had an adjusted 32% lower risk, and women of other races and ethnicity had a 24% lower risk of stroke. However, women who were non-Hispanic black had a stronger association with breastfeeding and stroke reduction, with a 48% lower risk, and non-Hispanic white and non-Hispanic black women showed a stronger association between longer duration of breastfeeding and lower stroke risk when results were minimally adjusted, the investigators said. All differences were statistically significant.
The investigators noted the study’s observational nature and said they were not able to determine what caused breastfeeding’s association with lower stroke risk, with other factors potentially affecting results.
“Breastfeeding is only one of many factors that could potentially protect against stroke,” Dr. Jacobson said in the report, published online in the Journal of the American Heart Association. “Others include getting adequate exercise, choosing healthy foods, not smoking, and seeking treatment if needed to keep your blood pressure, cholesterol, and blood sugar in the normal range.”
They also noted potential limitations in the study: the WHI cohort’s low number of strokes in follow-up, lack of classification of stroke, recall bias due to the women self-reporting strokes, average age at baseline, and lack of data on pregnancy.
“Our study did not address whether racial/ethnic differences in breastfeeding contribute to disparities in stroke risk,” Dr. Jacobson said. “Additional research should consider the degree to which breastfeeding might alter racial/ethnic differences in stroke risk.”
“This is an observational, prospective cohort study that was performed very carefully, but it is important to not conclude causality in that breastfeeding results in a reduction in late life stroke,” Larry B. Goldstein, MD, said in an email interview.
Dr. Goldstein, a neurologist who has published several guidelines on primary prevention and early management of stroke with the American Heart Association, noted that although the authors addressed many confounders, studies of this type are still open to residual confounding. He said one of the factors the authors could not measure was eclampsia and preeclampsia, which inhibits breastfeeding.
“The possibility of unmeasured confounding despite how well the study was done is still there. But having said that, the recommendations for breastfeeding are strong from the American Academy of Pediatrics and from the World Health Organization,” and other studies have found an association with a reduction in later life cardiovascular disease, said Dr. Goldstein, the Ruth L. Works professor and chairman in the department of neurology at the University of Kentucky, Lexington. “But just in terms of the benefits to the mother and to the child from breastfeeding, this is another potential plus [in that] even if it doesn’t pan out, it doesn’t really change the recommendation for breastfeeding.”
Dr. Goldstein noted that finding these results in a different prospective cohort would strengthen the recommendations, as would examining whether factors such as lifestyle affected stroke risk for women.
“Showing causality is always going to be difficult,” he stressed. “There is no particular causal mechanism that’s been espoused for how this might decrease stroke risk in later life.”
This study is funded by Frontiers: The Heartland Institute for Clinical and Translational Research and the Wichita Center for Graduate Medical Education–Kansas Bioscience Authority. The WHI program is funded by the National Heart, Lung, and Blood Institute, the National Institutes of Health, and the U.S. Department of Health and Human Services. The authors reported having no conflicts of interest.
SOURCE: Jacobson LT et al. J Am Heart Assoc. 2018 Aug 22. doi:10.1161/JAHA.118.008739.
Postmenopausal women who breastfed their children had a lower risk of stroke compared with women who had children but never breastfed, with non-Hispanic black women showing a significantly stronger association between breastfeeding and lower stroke risk, according to results from the prospective Women’s Health Initiative Observational Study.
“Some studies have reported that breastfeeding may reduce the rates of breast cancer, ovarian cancer and risk of developing type 2 diabetes in mothers. Recent findings point to the benefits of breastfeeding on heart disease and other specific cardiovascular risk factors,” Lisette T. Jacobson, PhD, of the department of preventive medicine and public health at the University of Kansas, Wichita, said in a statement.
Dr. Jacobson and her colleagues evaluated 80,191 women from the Women’s Health Initiative (WHI) Observational Study who were aged 50-79 at baseline. The average age was 64 years, and 83% were white, 8% were non-Hispanic black, 4% were Hispanic, and 5% were another race or ethnicity. Of the women observed, 58% had breastfed and 3.4% had a stroke within an average of 13 years of follow-up. The investigators used three adjusted regression models to analyze stroke risk: Model 1 was minimally adjusted, model 2 was adjusted for nonmodifiable potential confounders, and model 3 was adjusted for modifiable lifestyle factors.
There was a 23% lower risk of stroke among all postmenopausal women who breastfed compared with those who never breastfed, with women who breastfed between 1 month and 6 months carrying a 19% lower risk of stroke. In the minimally adjusted model, non-Hispanic white women who breastfed carried a 21% lower risk, Hispanic women had an adjusted 32% lower risk, and women of other races and ethnicity had a 24% lower risk of stroke. However, women who were non-Hispanic black had a stronger association with breastfeeding and stroke reduction, with a 48% lower risk, and non-Hispanic white and non-Hispanic black women showed a stronger association between longer duration of breastfeeding and lower stroke risk when results were minimally adjusted, the investigators said. All differences were statistically significant.
The investigators noted the study’s observational nature and said they were not able to determine what caused breastfeeding’s association with lower stroke risk, with other factors potentially affecting results.
“Breastfeeding is only one of many factors that could potentially protect against stroke,” Dr. Jacobson said in the report, published online in the Journal of the American Heart Association. “Others include getting adequate exercise, choosing healthy foods, not smoking, and seeking treatment if needed to keep your blood pressure, cholesterol, and blood sugar in the normal range.”
They also noted potential limitations in the study: the WHI cohort’s low number of strokes in follow-up, lack of classification of stroke, recall bias due to the women self-reporting strokes, average age at baseline, and lack of data on pregnancy.
“Our study did not address whether racial/ethnic differences in breastfeeding contribute to disparities in stroke risk,” Dr. Jacobson said. “Additional research should consider the degree to which breastfeeding might alter racial/ethnic differences in stroke risk.”
“This is an observational, prospective cohort study that was performed very carefully, but it is important to not conclude causality in that breastfeeding results in a reduction in late life stroke,” Larry B. Goldstein, MD, said in an email interview.
Dr. Goldstein, a neurologist who has published several guidelines on primary prevention and early management of stroke with the American Heart Association, noted that although the authors addressed many confounders, studies of this type are still open to residual confounding. He said one of the factors the authors could not measure was eclampsia and preeclampsia, which inhibits breastfeeding.
“The possibility of unmeasured confounding despite how well the study was done is still there. But having said that, the recommendations for breastfeeding are strong from the American Academy of Pediatrics and from the World Health Organization,” and other studies have found an association with a reduction in later life cardiovascular disease, said Dr. Goldstein, the Ruth L. Works professor and chairman in the department of neurology at the University of Kentucky, Lexington. “But just in terms of the benefits to the mother and to the child from breastfeeding, this is another potential plus [in that] even if it doesn’t pan out, it doesn’t really change the recommendation for breastfeeding.”
Dr. Goldstein noted that finding these results in a different prospective cohort would strengthen the recommendations, as would examining whether factors such as lifestyle affected stroke risk for women.
“Showing causality is always going to be difficult,” he stressed. “There is no particular causal mechanism that’s been espoused for how this might decrease stroke risk in later life.”
This study is funded by Frontiers: The Heartland Institute for Clinical and Translational Research and the Wichita Center for Graduate Medical Education–Kansas Bioscience Authority. The WHI program is funded by the National Heart, Lung, and Blood Institute, the National Institutes of Health, and the U.S. Department of Health and Human Services. The authors reported having no conflicts of interest.
SOURCE: Jacobson LT et al. J Am Heart Assoc. 2018 Aug 22. doi:10.1161/JAHA.118.008739.
FROM JOURNAL OF THE AMERICAN HEART ASSOCIATION
Key clinical point:
Major finding: Women who had ever breastfed had a 23% decreased risk of stroke, while breastfeeding between 1 month and 6 months carried a 19% lower risk of stroke.
Study details: A longitudinal national health study of 80,191 postmenopausal women in the Women’s Health Initiative.
Disclosures: This study is funded by the Heartland Institute for Clinical and Translational Research and the Wichita Center for Graduate Medical Education–Kansas Bioscience Authority. The authors reported having no conflicts of interest.
Source: Jacobson LT et al. J Am Heart Assoc. 2018 Aug 22. doi: 10.1161/JAHA.118.008739.
Morcellation at the time of vaginal hysterectomy

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:
This video is brought to you by
Neural-tube defect signal from dolutegravir HIV treatment raises concerns
AMSTERDAM – Over the past couple of years, integrase inhibitors have become the preferred anchor drug worldwide for HIV-treatment regimens. But in May 2018, researchers first reported an unexpected signal that one drug from the class, dolutegravir, showed a statistically significant link with an increased rate of neural-tube defects in neonates born to women in Botswana who had received dolutegravir at the time they conceived.
The data showed a 0.94% incidence of a neonate born with a neural-tube defect (NTD) among 426 HIV-infected women who were taking dolutegravir when they became pregnant. While this surprising finding remains preliminary because of limited number of women studied so far, and although the magnitude of the apparent effect fell somewhat after factoring in no further infants born with an NTD among 170 additional exposed women, the suggestion of an important teratogenic effect from dolutegravir led to a special session during the 22nd International AIDS Conference. The overwhelming consensus from this session seemed to be that the possible excess of NTDs linked with treatment with an integrase strand transfer inhibitor (INSTI) at the start of pregnancy was concerning enough to suggest caution and extra counseling for women of childbearing potential, but it was by no means a reason to derail the worldwide shift to the INSTI drug class as the core agent for treating HIV.
“Dolutegravir has been a beacon of hope for treating HIV,” said Maggie Little, PhD, a professor of philosophy and medical ethicist at Georgetown University in Washington. “Dolutegravir offers substantial benefits to quality of life in addition to reducing women’s mortality.” The new finding of excess NTDs “appears to pit pregnant women against their children. But the numbers never tell us the answer; it’s not arithmetic.” The appropriate public health response should focus on “supporting meaningful choice by women,” Dr. Little said during a talk at the session. “Policies must be made in ongoing consultation with communities of women who live with HIV.”
Rise of the INSTIs
The International AIDS Conference showcased the contrast between the benefits of the INSTIs and their possible perils.
Well before news of the NTD signal came out, the conference program featured a plenary talk from Pedro Cahn, MD, PhD, entitled “Moving into the Integrase Era.” During his talk, Dr. Cahn proclaimed that HIV treatment is “moving toward the integrase world,” and recently featured “unprecedented rollout” in low-income countries. In addition to dolutegravir (Tivicay) the INSTI class includes raltegravir (Isentress), elvitegravir (Vitekta), and bictegravir (Symtuza).
Dr. Cahn attributed the first-line status of the INSTIs to several factors: their higher antiviral activity, compared with every other anti-HIV drug including proven superior efficacy to efavirenz (Sustiva) – the former core drug for antiretroviral regimens, rapid viral suppression, good tolerability with a low rate of treatment discontinuations, good recovery of CD4 cells, a relatively high genetic barrier to selection of HIV resistance mutations with relatively few resistant mutations seen when used in combination regimen’s in treatment-naive patients, and few drug-drug interactions, By mid-2018, dolutegravir or another INSTI had been named part of a first-line HIV treatment regimen by several countries and by the World Health Organization; according to WHO data, by mid-2018 more than half the low- and middle-income countries of the world had endorsed an INSTI-containing regimen including Botswana, Brazil, Kenya, Nigeria, and Uganda, said Dr. Cahn, scientific director of the Huésbed Foundation in Buenos Aires.
One example of the success that dolutegravir has recently shown as first-line treatment came in data reported at the Conference from Brazil. where a three-drug regimen containing dolutegravir plus lamivudine (3TC; Epivir) and tenofovir (TDF; Viread) replaced a triple regimen of efavirenz plus 3TC and TDF as recommended first-line treatment in 2017. Data collected by the Brazilian Ministry of Health during January 2014-June 2017 identified 103,240 people at least 15 years old who received treatment for HIV. The review showed that 85% of people treated with a dolutegravir-containing regimen had successful viral suppression to an undetectable level, compared with 78% of people on the same regimen but with efavirenz instead of dolutegravir, Mariana V. Meireles reported at the conference. Other triple-drug regimens had even lower rates of viral suppression. After researchers controlled for the age, sex, level of adherence, and baseline viral load and CD4 cell count the people who received the dolutegravir-containing regimen had at least a 42% higher rate of undetectable virus compared with any other regimen used by Brazilian patients, said Ms. Meireles, a researcher with the Brazilian Ministry of Health in Brasilia.
Dr. Cahn acknowledged the current concern and uncertainty about INSTIs and NTDs. “Caution and effective contraception are recommended for dolutegravir. The risks and benefits should be compared with other [treatment] options. Women have the right to make informed choices,” he said. Dr. Cahn also highlighted that safety analyses need data from additional early-pregnancy exposures to clearly rule in or rule out a teratogenic effect from dolutegravir. And he stressed that, whether or not the possible NTD link is a class effect remains to be assessed as data from early-pregnancy exposures of women on other INSTIs are currently much more limited than they are those for dolutegravir. He also raised a question voiced by others: Is the effect from dolutegravir somehow mediated by folic acid levels, a dietary component that protects against NTDs? Botswana, the country that generated the NTD data, doesn’t fortify wheat flour or any other food with folic acid, as occurs in the United States, noted Rebecca M. Zash, MD, the researcher who led the Botswana study.
The NTD data
The signal for an NTD link to dolutegravir came from a study run in Botswana designed for a totally different, albeit related purpose. The Tsepamo study launched in 2014 with the goal of assessing the safety of efavirenz-based HIV regimens when used by pregnant women. The study has run at eight of the country’s largest maternity wards, where nearly half of Botswana’s deliveries occur. The midwives at those locations collected data on all women at their clinics, and once Botswana adopted dolutegravir as its anchor drug of choice for treating people infected with HIV in 2017 significant numbers of the women in the Tsepamo study received dolutegravir. Through May 1, 2018, the study had enrolled more than 89,000 women who had 88,755 live births, including nearly 22,000 women infected with HIV (and more than 66,000 without infection), nearly 12,00 of those infected with HIV who received some type of antiretroviral therapy, 5,787 on efavirenz at the time of conception, 2,812 women who started on dolutegravir treatment during pregnancy, and 426 women who were on dolutegravir at the time of conception, said Dr. Zash, an infectious diseases physician at Beth Israel Deaconess Hospital in Boston and codirector of the Reproductive Health for HIV-Infected Populations Study Working Group at the Harvard University Center for AIDS Research in Cambridge, Mass.
A recently published analysis by Dr. Zash and her associates found no difference in the incidence rate of adverse birth outcomes among women who started on either efavirenz or dolutegravir during pregnancy. This analysis also showed that women infected with HIV overall had “mildly increased” rates of both total adverse birth outcomes and severe adverse birth outcomes compared with women without HIV infection (Lancet Glob Health. 2018 Jul;6[7]:e804-e10).
When the researchers looked at NTDs among neonates born to women exposed at conception, they saw a different picture. The entire cohort of nearly 89,000 live births included 86 neonates with an NTD, a 0.1% rate. This included 4 of the 426 births from mothers on dolutegravir at conception, a 0.94% rate, significantly higher than the overall rate. Other comparator NTD rates included a 0,12% incidence among mothers on any anti-HIV drug other than dolutegravir at conception, a 0.09% rate among mothers who were not infected with HIV, and a 0.05% rate among mothers on efavirenz at conception, Dr. Zash and her associates reported in a publication that appeared coincident with her talk at the conference (N Engl J Med. 2018 Jul 24.doi: 10.1056/NEJMc1807653). The NTDs linked with dolutegravir use involved four distinct types of NTD, a finding Dr. Zash called “unusual,” but not unique among teratogens.
During her talk, Dr. Zash further updated the dolutegravir numbers based on extended follow-up of the Botswana cohort during May 1-July 15, during which time two NTDs occurred, one involving an uninfected mother and the second from a mother who started on dolutegravir at 8 weeks’ gestational age, after the time when NTDs occur. Further follow-up also added 170 more neonates born to women exposed to dolutegravir at conception, bringing the total now to 596 births with 4 NTDs or a rate of 0.67%, still significantly elevated, compared with other exposure groups. The Tsepamo study continues, with an additional 10 sites planned to soon join that will boost maternity coverage to 72% of Botswana’s annual births. The next planned analysis is in March 2019, and by then the number of neonates born to women with early dolutegravir exposure should more than double, Dr. Zash predicted.
Modeling the risks and benefits
Identifying a possible excess of NTDs with dolutegravir treatment in adolescent girls and young women doesn’t, of course, tell the whole risk-benefit story for dolutegravir and possibly the other INSTIs. Caitlin Dugdale, MD, an infectious diseases physician at Massachusetts General Hospital in Boston, reported a model she developed to better define the pluses and minuses of dolutegravir treatment, compared with efavirenz. The model used projections for women in South Africa of child-bearing potential infected by HIV over the next 5 years and used data on drug efficacy and harms based on published reports. For example, the ability of the two drugs to produce undetectable viral loads was assumed by the model to be 94% after 48 weeks on treatment with dolutegravir and 86% with efavirenz, based on the rates reported in the phase 3, randomized comparison of dolutegravir- and efavirenz-based regimens in the SINGLE trial, with adjustments for factors such as protocol deviations and mortality that were accounted for in other parts of the model, Dr. Dugdale said. She used estimates for NTD incidence based on the published numbers reported by Dr. Zash.
The results showed that, over the next 5 years, based on just the existing and projected rates of HIV infection, treating all infected women and children with a dolutegravir-based regimen instead of a regimen anchored by efavirenz would result in the benefits of 28,400 fewer deaths among women, 52,800 fewer sexual transmissions of HIV, 5,000 fewer pediatric HIV transmissions, and 1,600 fewer pediatric deaths unrelated to an NTD. On the minus side relying on dolutegravir instead of efavirenz was projected to cause an excess of 10,000 neonates born with an NTD, 8,400 excess pediatric deaths, and overall 5,400 fewer children alive and free from HIV. These projections were based on 3.5 million women on first-line treatment with antiretroviral therapy and 1.1 million children born with HIV exposure.
Dr. Dugdale drew particular attention to the comparison between 28,400 fewer deaths among women when treated with dolutegravir at the cost of 8,400 excess pediatric deaths, but cautioned that this creates “a difficult trade-off to balance.” Findings from the model and other information on HIV treatment options should enter into the decision making of each HIV-infected woman who could become pregnant, she said. It’s important that patients view the risks and benefits not just on a population level but on an individual, personal level, Dr. Dugdale said in a video interview. “The individual woman must balance the risks and benefits for herself and her child.”
“Patients need to decide what is important to them,” agreed Dr. Zash during the conference.
The NTD findings also underscored the importance of better contraception options for HIV-infected women. “This is an opportunity to improve reproductive health and contraception for women, especially in resource-poor countries,” commented Elaine J. Abrams, MD, professor of epidemiology and pediatrics at Columbia University in New York, who cochaired the conference session.
Another lesson from the NTD findings is the importance of tracking the safety of new drugs used when women become pregnant and during pregnancy. The dolutegravir arm of the Tsepamo study “was almost by accident,” noted the Georgetown medical ethicist, Dr. Little. “Every new treatment should be examined in pregnant women and infants,” she added. “Studies like this should not be left to chance. Women deserve an evidence base for medication use across the lifespan including during pregnancy and periconception.”
Dr. Little, Ms. Meireles, Dr. Zash, and Dr. Dugdale had no disclosures. Dr. Cahn has been an adviser to or speaker for AbbVie, Merck, and ViiV and has received research funding from AbbVie, Merck, ViiV, and Richmond. Dr. Abrams has been an adviser to Merck and ViiV. Viiv is the company that markets dolutegravir.
AMSTERDAM – Over the past couple of years, integrase inhibitors have become the preferred anchor drug worldwide for HIV-treatment regimens. But in May 2018, researchers first reported an unexpected signal that one drug from the class, dolutegravir, showed a statistically significant link with an increased rate of neural-tube defects in neonates born to women in Botswana who had received dolutegravir at the time they conceived.
The data showed a 0.94% incidence of a neonate born with a neural-tube defect (NTD) among 426 HIV-infected women who were taking dolutegravir when they became pregnant. While this surprising finding remains preliminary because of limited number of women studied so far, and although the magnitude of the apparent effect fell somewhat after factoring in no further infants born with an NTD among 170 additional exposed women, the suggestion of an important teratogenic effect from dolutegravir led to a special session during the 22nd International AIDS Conference. The overwhelming consensus from this session seemed to be that the possible excess of NTDs linked with treatment with an integrase strand transfer inhibitor (INSTI) at the start of pregnancy was concerning enough to suggest caution and extra counseling for women of childbearing potential, but it was by no means a reason to derail the worldwide shift to the INSTI drug class as the core agent for treating HIV.
“Dolutegravir has been a beacon of hope for treating HIV,” said Maggie Little, PhD, a professor of philosophy and medical ethicist at Georgetown University in Washington. “Dolutegravir offers substantial benefits to quality of life in addition to reducing women’s mortality.” The new finding of excess NTDs “appears to pit pregnant women against their children. But the numbers never tell us the answer; it’s not arithmetic.” The appropriate public health response should focus on “supporting meaningful choice by women,” Dr. Little said during a talk at the session. “Policies must be made in ongoing consultation with communities of women who live with HIV.”
Rise of the INSTIs
The International AIDS Conference showcased the contrast between the benefits of the INSTIs and their possible perils.
Well before news of the NTD signal came out, the conference program featured a plenary talk from Pedro Cahn, MD, PhD, entitled “Moving into the Integrase Era.” During his talk, Dr. Cahn proclaimed that HIV treatment is “moving toward the integrase world,” and recently featured “unprecedented rollout” in low-income countries. In addition to dolutegravir (Tivicay) the INSTI class includes raltegravir (Isentress), elvitegravir (Vitekta), and bictegravir (Symtuza).
Dr. Cahn attributed the first-line status of the INSTIs to several factors: their higher antiviral activity, compared with every other anti-HIV drug including proven superior efficacy to efavirenz (Sustiva) – the former core drug for antiretroviral regimens, rapid viral suppression, good tolerability with a low rate of treatment discontinuations, good recovery of CD4 cells, a relatively high genetic barrier to selection of HIV resistance mutations with relatively few resistant mutations seen when used in combination regimen’s in treatment-naive patients, and few drug-drug interactions, By mid-2018, dolutegravir or another INSTI had been named part of a first-line HIV treatment regimen by several countries and by the World Health Organization; according to WHO data, by mid-2018 more than half the low- and middle-income countries of the world had endorsed an INSTI-containing regimen including Botswana, Brazil, Kenya, Nigeria, and Uganda, said Dr. Cahn, scientific director of the Huésbed Foundation in Buenos Aires.
One example of the success that dolutegravir has recently shown as first-line treatment came in data reported at the Conference from Brazil. where a three-drug regimen containing dolutegravir plus lamivudine (3TC; Epivir) and tenofovir (TDF; Viread) replaced a triple regimen of efavirenz plus 3TC and TDF as recommended first-line treatment in 2017. Data collected by the Brazilian Ministry of Health during January 2014-June 2017 identified 103,240 people at least 15 years old who received treatment for HIV. The review showed that 85% of people treated with a dolutegravir-containing regimen had successful viral suppression to an undetectable level, compared with 78% of people on the same regimen but with efavirenz instead of dolutegravir, Mariana V. Meireles reported at the conference. Other triple-drug regimens had even lower rates of viral suppression. After researchers controlled for the age, sex, level of adherence, and baseline viral load and CD4 cell count the people who received the dolutegravir-containing regimen had at least a 42% higher rate of undetectable virus compared with any other regimen used by Brazilian patients, said Ms. Meireles, a researcher with the Brazilian Ministry of Health in Brasilia.
Dr. Cahn acknowledged the current concern and uncertainty about INSTIs and NTDs. “Caution and effective contraception are recommended for dolutegravir. The risks and benefits should be compared with other [treatment] options. Women have the right to make informed choices,” he said. Dr. Cahn also highlighted that safety analyses need data from additional early-pregnancy exposures to clearly rule in or rule out a teratogenic effect from dolutegravir. And he stressed that, whether or not the possible NTD link is a class effect remains to be assessed as data from early-pregnancy exposures of women on other INSTIs are currently much more limited than they are those for dolutegravir. He also raised a question voiced by others: Is the effect from dolutegravir somehow mediated by folic acid levels, a dietary component that protects against NTDs? Botswana, the country that generated the NTD data, doesn’t fortify wheat flour or any other food with folic acid, as occurs in the United States, noted Rebecca M. Zash, MD, the researcher who led the Botswana study.
The NTD data
The signal for an NTD link to dolutegravir came from a study run in Botswana designed for a totally different, albeit related purpose. The Tsepamo study launched in 2014 with the goal of assessing the safety of efavirenz-based HIV regimens when used by pregnant women. The study has run at eight of the country’s largest maternity wards, where nearly half of Botswana’s deliveries occur. The midwives at those locations collected data on all women at their clinics, and once Botswana adopted dolutegravir as its anchor drug of choice for treating people infected with HIV in 2017 significant numbers of the women in the Tsepamo study received dolutegravir. Through May 1, 2018, the study had enrolled more than 89,000 women who had 88,755 live births, including nearly 22,000 women infected with HIV (and more than 66,000 without infection), nearly 12,00 of those infected with HIV who received some type of antiretroviral therapy, 5,787 on efavirenz at the time of conception, 2,812 women who started on dolutegravir treatment during pregnancy, and 426 women who were on dolutegravir at the time of conception, said Dr. Zash, an infectious diseases physician at Beth Israel Deaconess Hospital in Boston and codirector of the Reproductive Health for HIV-Infected Populations Study Working Group at the Harvard University Center for AIDS Research in Cambridge, Mass.
A recently published analysis by Dr. Zash and her associates found no difference in the incidence rate of adverse birth outcomes among women who started on either efavirenz or dolutegravir during pregnancy. This analysis also showed that women infected with HIV overall had “mildly increased” rates of both total adverse birth outcomes and severe adverse birth outcomes compared with women without HIV infection (Lancet Glob Health. 2018 Jul;6[7]:e804-e10).
When the researchers looked at NTDs among neonates born to women exposed at conception, they saw a different picture. The entire cohort of nearly 89,000 live births included 86 neonates with an NTD, a 0.1% rate. This included 4 of the 426 births from mothers on dolutegravir at conception, a 0.94% rate, significantly higher than the overall rate. Other comparator NTD rates included a 0,12% incidence among mothers on any anti-HIV drug other than dolutegravir at conception, a 0.09% rate among mothers who were not infected with HIV, and a 0.05% rate among mothers on efavirenz at conception, Dr. Zash and her associates reported in a publication that appeared coincident with her talk at the conference (N Engl J Med. 2018 Jul 24.doi: 10.1056/NEJMc1807653). The NTDs linked with dolutegravir use involved four distinct types of NTD, a finding Dr. Zash called “unusual,” but not unique among teratogens.
During her talk, Dr. Zash further updated the dolutegravir numbers based on extended follow-up of the Botswana cohort during May 1-July 15, during which time two NTDs occurred, one involving an uninfected mother and the second from a mother who started on dolutegravir at 8 weeks’ gestational age, after the time when NTDs occur. Further follow-up also added 170 more neonates born to women exposed to dolutegravir at conception, bringing the total now to 596 births with 4 NTDs or a rate of 0.67%, still significantly elevated, compared with other exposure groups. The Tsepamo study continues, with an additional 10 sites planned to soon join that will boost maternity coverage to 72% of Botswana’s annual births. The next planned analysis is in March 2019, and by then the number of neonates born to women with early dolutegravir exposure should more than double, Dr. Zash predicted.
Modeling the risks and benefits
Identifying a possible excess of NTDs with dolutegravir treatment in adolescent girls and young women doesn’t, of course, tell the whole risk-benefit story for dolutegravir and possibly the other INSTIs. Caitlin Dugdale, MD, an infectious diseases physician at Massachusetts General Hospital in Boston, reported a model she developed to better define the pluses and minuses of dolutegravir treatment, compared with efavirenz. The model used projections for women in South Africa of child-bearing potential infected by HIV over the next 5 years and used data on drug efficacy and harms based on published reports. For example, the ability of the two drugs to produce undetectable viral loads was assumed by the model to be 94% after 48 weeks on treatment with dolutegravir and 86% with efavirenz, based on the rates reported in the phase 3, randomized comparison of dolutegravir- and efavirenz-based regimens in the SINGLE trial, with adjustments for factors such as protocol deviations and mortality that were accounted for in other parts of the model, Dr. Dugdale said. She used estimates for NTD incidence based on the published numbers reported by Dr. Zash.
The results showed that, over the next 5 years, based on just the existing and projected rates of HIV infection, treating all infected women and children with a dolutegravir-based regimen instead of a regimen anchored by efavirenz would result in the benefits of 28,400 fewer deaths among women, 52,800 fewer sexual transmissions of HIV, 5,000 fewer pediatric HIV transmissions, and 1,600 fewer pediatric deaths unrelated to an NTD. On the minus side relying on dolutegravir instead of efavirenz was projected to cause an excess of 10,000 neonates born with an NTD, 8,400 excess pediatric deaths, and overall 5,400 fewer children alive and free from HIV. These projections were based on 3.5 million women on first-line treatment with antiretroviral therapy and 1.1 million children born with HIV exposure.
Dr. Dugdale drew particular attention to the comparison between 28,400 fewer deaths among women when treated with dolutegravir at the cost of 8,400 excess pediatric deaths, but cautioned that this creates “a difficult trade-off to balance.” Findings from the model and other information on HIV treatment options should enter into the decision making of each HIV-infected woman who could become pregnant, she said. It’s important that patients view the risks and benefits not just on a population level but on an individual, personal level, Dr. Dugdale said in a video interview. “The individual woman must balance the risks and benefits for herself and her child.”
“Patients need to decide what is important to them,” agreed Dr. Zash during the conference.
The NTD findings also underscored the importance of better contraception options for HIV-infected women. “This is an opportunity to improve reproductive health and contraception for women, especially in resource-poor countries,” commented Elaine J. Abrams, MD, professor of epidemiology and pediatrics at Columbia University in New York, who cochaired the conference session.
Another lesson from the NTD findings is the importance of tracking the safety of new drugs used when women become pregnant and during pregnancy. The dolutegravir arm of the Tsepamo study “was almost by accident,” noted the Georgetown medical ethicist, Dr. Little. “Every new treatment should be examined in pregnant women and infants,” she added. “Studies like this should not be left to chance. Women deserve an evidence base for medication use across the lifespan including during pregnancy and periconception.”
Dr. Little, Ms. Meireles, Dr. Zash, and Dr. Dugdale had no disclosures. Dr. Cahn has been an adviser to or speaker for AbbVie, Merck, and ViiV and has received research funding from AbbVie, Merck, ViiV, and Richmond. Dr. Abrams has been an adviser to Merck and ViiV. Viiv is the company that markets dolutegravir.
AMSTERDAM – Over the past couple of years, integrase inhibitors have become the preferred anchor drug worldwide for HIV-treatment regimens. But in May 2018, researchers first reported an unexpected signal that one drug from the class, dolutegravir, showed a statistically significant link with an increased rate of neural-tube defects in neonates born to women in Botswana who had received dolutegravir at the time they conceived.
The data showed a 0.94% incidence of a neonate born with a neural-tube defect (NTD) among 426 HIV-infected women who were taking dolutegravir when they became pregnant. While this surprising finding remains preliminary because of limited number of women studied so far, and although the magnitude of the apparent effect fell somewhat after factoring in no further infants born with an NTD among 170 additional exposed women, the suggestion of an important teratogenic effect from dolutegravir led to a special session during the 22nd International AIDS Conference. The overwhelming consensus from this session seemed to be that the possible excess of NTDs linked with treatment with an integrase strand transfer inhibitor (INSTI) at the start of pregnancy was concerning enough to suggest caution and extra counseling for women of childbearing potential, but it was by no means a reason to derail the worldwide shift to the INSTI drug class as the core agent for treating HIV.
“Dolutegravir has been a beacon of hope for treating HIV,” said Maggie Little, PhD, a professor of philosophy and medical ethicist at Georgetown University in Washington. “Dolutegravir offers substantial benefits to quality of life in addition to reducing women’s mortality.” The new finding of excess NTDs “appears to pit pregnant women against their children. But the numbers never tell us the answer; it’s not arithmetic.” The appropriate public health response should focus on “supporting meaningful choice by women,” Dr. Little said during a talk at the session. “Policies must be made in ongoing consultation with communities of women who live with HIV.”
Rise of the INSTIs
The International AIDS Conference showcased the contrast between the benefits of the INSTIs and their possible perils.
Well before news of the NTD signal came out, the conference program featured a plenary talk from Pedro Cahn, MD, PhD, entitled “Moving into the Integrase Era.” During his talk, Dr. Cahn proclaimed that HIV treatment is “moving toward the integrase world,” and recently featured “unprecedented rollout” in low-income countries. In addition to dolutegravir (Tivicay) the INSTI class includes raltegravir (Isentress), elvitegravir (Vitekta), and bictegravir (Symtuza).
Dr. Cahn attributed the first-line status of the INSTIs to several factors: their higher antiviral activity, compared with every other anti-HIV drug including proven superior efficacy to efavirenz (Sustiva) – the former core drug for antiretroviral regimens, rapid viral suppression, good tolerability with a low rate of treatment discontinuations, good recovery of CD4 cells, a relatively high genetic barrier to selection of HIV resistance mutations with relatively few resistant mutations seen when used in combination regimen’s in treatment-naive patients, and few drug-drug interactions, By mid-2018, dolutegravir or another INSTI had been named part of a first-line HIV treatment regimen by several countries and by the World Health Organization; according to WHO data, by mid-2018 more than half the low- and middle-income countries of the world had endorsed an INSTI-containing regimen including Botswana, Brazil, Kenya, Nigeria, and Uganda, said Dr. Cahn, scientific director of the Huésbed Foundation in Buenos Aires.
One example of the success that dolutegravir has recently shown as first-line treatment came in data reported at the Conference from Brazil. where a three-drug regimen containing dolutegravir plus lamivudine (3TC; Epivir) and tenofovir (TDF; Viread) replaced a triple regimen of efavirenz plus 3TC and TDF as recommended first-line treatment in 2017. Data collected by the Brazilian Ministry of Health during January 2014-June 2017 identified 103,240 people at least 15 years old who received treatment for HIV. The review showed that 85% of people treated with a dolutegravir-containing regimen had successful viral suppression to an undetectable level, compared with 78% of people on the same regimen but with efavirenz instead of dolutegravir, Mariana V. Meireles reported at the conference. Other triple-drug regimens had even lower rates of viral suppression. After researchers controlled for the age, sex, level of adherence, and baseline viral load and CD4 cell count the people who received the dolutegravir-containing regimen had at least a 42% higher rate of undetectable virus compared with any other regimen used by Brazilian patients, said Ms. Meireles, a researcher with the Brazilian Ministry of Health in Brasilia.
Dr. Cahn acknowledged the current concern and uncertainty about INSTIs and NTDs. “Caution and effective contraception are recommended for dolutegravir. The risks and benefits should be compared with other [treatment] options. Women have the right to make informed choices,” he said. Dr. Cahn also highlighted that safety analyses need data from additional early-pregnancy exposures to clearly rule in or rule out a teratogenic effect from dolutegravir. And he stressed that, whether or not the possible NTD link is a class effect remains to be assessed as data from early-pregnancy exposures of women on other INSTIs are currently much more limited than they are those for dolutegravir. He also raised a question voiced by others: Is the effect from dolutegravir somehow mediated by folic acid levels, a dietary component that protects against NTDs? Botswana, the country that generated the NTD data, doesn’t fortify wheat flour or any other food with folic acid, as occurs in the United States, noted Rebecca M. Zash, MD, the researcher who led the Botswana study.
The NTD data
The signal for an NTD link to dolutegravir came from a study run in Botswana designed for a totally different, albeit related purpose. The Tsepamo study launched in 2014 with the goal of assessing the safety of efavirenz-based HIV regimens when used by pregnant women. The study has run at eight of the country’s largest maternity wards, where nearly half of Botswana’s deliveries occur. The midwives at those locations collected data on all women at their clinics, and once Botswana adopted dolutegravir as its anchor drug of choice for treating people infected with HIV in 2017 significant numbers of the women in the Tsepamo study received dolutegravir. Through May 1, 2018, the study had enrolled more than 89,000 women who had 88,755 live births, including nearly 22,000 women infected with HIV (and more than 66,000 without infection), nearly 12,00 of those infected with HIV who received some type of antiretroviral therapy, 5,787 on efavirenz at the time of conception, 2,812 women who started on dolutegravir treatment during pregnancy, and 426 women who were on dolutegravir at the time of conception, said Dr. Zash, an infectious diseases physician at Beth Israel Deaconess Hospital in Boston and codirector of the Reproductive Health for HIV-Infected Populations Study Working Group at the Harvard University Center for AIDS Research in Cambridge, Mass.
A recently published analysis by Dr. Zash and her associates found no difference in the incidence rate of adverse birth outcomes among women who started on either efavirenz or dolutegravir during pregnancy. This analysis also showed that women infected with HIV overall had “mildly increased” rates of both total adverse birth outcomes and severe adverse birth outcomes compared with women without HIV infection (Lancet Glob Health. 2018 Jul;6[7]:e804-e10).
When the researchers looked at NTDs among neonates born to women exposed at conception, they saw a different picture. The entire cohort of nearly 89,000 live births included 86 neonates with an NTD, a 0.1% rate. This included 4 of the 426 births from mothers on dolutegravir at conception, a 0.94% rate, significantly higher than the overall rate. Other comparator NTD rates included a 0,12% incidence among mothers on any anti-HIV drug other than dolutegravir at conception, a 0.09% rate among mothers who were not infected with HIV, and a 0.05% rate among mothers on efavirenz at conception, Dr. Zash and her associates reported in a publication that appeared coincident with her talk at the conference (N Engl J Med. 2018 Jul 24.doi: 10.1056/NEJMc1807653). The NTDs linked with dolutegravir use involved four distinct types of NTD, a finding Dr. Zash called “unusual,” but not unique among teratogens.
During her talk, Dr. Zash further updated the dolutegravir numbers based on extended follow-up of the Botswana cohort during May 1-July 15, during which time two NTDs occurred, one involving an uninfected mother and the second from a mother who started on dolutegravir at 8 weeks’ gestational age, after the time when NTDs occur. Further follow-up also added 170 more neonates born to women exposed to dolutegravir at conception, bringing the total now to 596 births with 4 NTDs or a rate of 0.67%, still significantly elevated, compared with other exposure groups. The Tsepamo study continues, with an additional 10 sites planned to soon join that will boost maternity coverage to 72% of Botswana’s annual births. The next planned analysis is in March 2019, and by then the number of neonates born to women with early dolutegravir exposure should more than double, Dr. Zash predicted.
Modeling the risks and benefits
Identifying a possible excess of NTDs with dolutegravir treatment in adolescent girls and young women doesn’t, of course, tell the whole risk-benefit story for dolutegravir and possibly the other INSTIs. Caitlin Dugdale, MD, an infectious diseases physician at Massachusetts General Hospital in Boston, reported a model she developed to better define the pluses and minuses of dolutegravir treatment, compared with efavirenz. The model used projections for women in South Africa of child-bearing potential infected by HIV over the next 5 years and used data on drug efficacy and harms based on published reports. For example, the ability of the two drugs to produce undetectable viral loads was assumed by the model to be 94% after 48 weeks on treatment with dolutegravir and 86% with efavirenz, based on the rates reported in the phase 3, randomized comparison of dolutegravir- and efavirenz-based regimens in the SINGLE trial, with adjustments for factors such as protocol deviations and mortality that were accounted for in other parts of the model, Dr. Dugdale said. She used estimates for NTD incidence based on the published numbers reported by Dr. Zash.
The results showed that, over the next 5 years, based on just the existing and projected rates of HIV infection, treating all infected women and children with a dolutegravir-based regimen instead of a regimen anchored by efavirenz would result in the benefits of 28,400 fewer deaths among women, 52,800 fewer sexual transmissions of HIV, 5,000 fewer pediatric HIV transmissions, and 1,600 fewer pediatric deaths unrelated to an NTD. On the minus side relying on dolutegravir instead of efavirenz was projected to cause an excess of 10,000 neonates born with an NTD, 8,400 excess pediatric deaths, and overall 5,400 fewer children alive and free from HIV. These projections were based on 3.5 million women on first-line treatment with antiretroviral therapy and 1.1 million children born with HIV exposure.
Dr. Dugdale drew particular attention to the comparison between 28,400 fewer deaths among women when treated with dolutegravir at the cost of 8,400 excess pediatric deaths, but cautioned that this creates “a difficult trade-off to balance.” Findings from the model and other information on HIV treatment options should enter into the decision making of each HIV-infected woman who could become pregnant, she said. It’s important that patients view the risks and benefits not just on a population level but on an individual, personal level, Dr. Dugdale said in a video interview. “The individual woman must balance the risks and benefits for herself and her child.”
“Patients need to decide what is important to them,” agreed Dr. Zash during the conference.
The NTD findings also underscored the importance of better contraception options for HIV-infected women. “This is an opportunity to improve reproductive health and contraception for women, especially in resource-poor countries,” commented Elaine J. Abrams, MD, professor of epidemiology and pediatrics at Columbia University in New York, who cochaired the conference session.
Another lesson from the NTD findings is the importance of tracking the safety of new drugs used when women become pregnant and during pregnancy. The dolutegravir arm of the Tsepamo study “was almost by accident,” noted the Georgetown medical ethicist, Dr. Little. “Every new treatment should be examined in pregnant women and infants,” she added. “Studies like this should not be left to chance. Women deserve an evidence base for medication use across the lifespan including during pregnancy and periconception.”
Dr. Little, Ms. Meireles, Dr. Zash, and Dr. Dugdale had no disclosures. Dr. Cahn has been an adviser to or speaker for AbbVie, Merck, and ViiV and has received research funding from AbbVie, Merck, ViiV, and Richmond. Dr. Abrams has been an adviser to Merck and ViiV. Viiv is the company that markets dolutegravir.
REPORTING FROM AIDS 2018
Mental illness and the criminal justice system: Reducing the risks
NEW YORK – The overrepresentation of people with serious mental illness (SMI) in the criminal justice system has led to creation of a resource from the Judges’ and Psychiatrists’ Leadership Initiative (JPLI) aimed at helping psychiatry and law enforcement address the problem.
The resource, “Supporting People with Serious Mental Illnesses and Reducing Their Risk of Contact with the Criminal Justice System: A Primer for Psychiatrists,” released last year, was designed to provide psychiatrists with specific knowledge and tools, according to Michael Champion, MD, forensic chief at the Hawaii State Department of Health, Adult Mental Health Division, Honolulu, and a member of the JPLI executive leadership team.
In developing the primer, the JPLI, which was created about 10 years ago by the American Psychiatric Association Foundation in partnership with the Council of State Governments Justice Center in response to the growing problem of such overrepresentation, sought to teach psychiatrists about what the criminal justice literature has dubbed “criminogenic risk” and to explore strategies to address those risks in community treatment settings, Dr. Champion said at the annual meeting of the American Psychiatric Association.
“The fact is that one in three Americans has a criminal record, and people with serious mental illness and criminal justice involvement are frequently part of our patient population – particularly in the public mental health sector,” Dr. Champion said. “Part of the challenge is that psychiatrists ... aren’t typically trained in these principles ... so the JPLI saw that this as an area that we could try to make some traction in and try to make a difference.”
The JPLI’s goals in publishing this resource are to reduce the risk of patient involvement in the criminal justice system, and to improve clinical and recovery outcomes by educating community psychiatrists about Risk-Need-Responsivity (RNR) principles. The JPLI also seeks to provide strategies for collaborating with criminal justice partners, incorporating criminal justice history into screening and assessment, and integrating criminogenic risk needs of patients into comprehensive treatment plans, Dr. Champion said.
Criminogenic risk and RNR
Many factors contribute to the involvement of people with serious mental illnesses in the criminal justice system, including higher rates of arrest, longer stays, recidivism, and limited access to health care, said Fred C. Osher, MD, former director of health systems and services policy for the Council of State Governments Justice Center.
“We used to think that ... if we could just get folks the health care that they need, they wouldn’t get involved with the criminal justice system. It turns out that that’s a gross oversimplification, in that their needs are terribly complex, and while treatment is a necessary component, it isn’t often sufficient for a large number of individuals,” said Dr. Osher, now a member of the JPLI executive leadership team.
Criminogenic risk – the likelihood that a person who has been arrested and jailed will commit a new crime after release or return to custody – helps explain why that is the case, he said, adding: “We have ways in which we can understand those risks.”
The risks are measured via static factors (unchanging conditions such as criminal history, age at first arrest, current age, and gender) and dynamic factors, he explained.
he said, noting that the research has shown there are eight specific criminogenic risk factors: substance abuse, history of antisocial behavior, antisocial personality pattern, antisocial cognition, antisocial associates, family and/or marital discord, poor school and/or work output, and having few leisure/recreation outlets.
Notably, mental illness is not a part of that list, he said.
“The reason for that is it’s not explanatory in and of itself,” he added.
However, research shows that people with mental illness have more of these dynamic risk factors, and research by Jennifer L. Skeem, PhD, and others shows that those with mental illness were coming back to jail not for new criminal activity, but for failing to comply with their conditions of release.
“These risks, then, have been brought into a paradigm that is central to our criminal justice operations, and it’s called the Risk-Need-Responsivity model,” Dr. Osher said. “This paradigm is what allows a criminal justice system to think about how to prioritize the resources – to think about who really needs to be wrapped tight, who needs to have close supervision, frequent reporting, lots of contact.”
The risk principle in the RNR model says that resources should be focused on high-risk cases, with limited supervision in lower-risk cases. This is based on experience demonstrating that recidivism is lower in high-risk individuals with close supervision but higher in low-risk individuals with close supervision.
The needs principle suggests that dynamic needs are “the issues that get folks in trouble,” he said.
“So, if we’re going to intervene, if we’re going to provide programming, if we’re going to try and help that individual stay out of jail or prison, we need to address these criminogenic needs,” he said, adding that the “big four” are related to their antisocial thinking and personality and friends.
Targeted interventions can help those individuals make better choices going forward, he noted.
The responsivity principle is an acknowledgment that individuals have different ways of learning, different cultural factors and backgrounds that influence them, and social determinants that are important to understand if they predict the ability to stay out of trouble.
“This is where mental illness fits in,” Dr. Osher said. “It’s absolutely important that we understand that.”
Examples would be patients with severe major depressive disorder who need their depression treated before they can participate in a group treatment setting designed to address criminogenic risks.
Dynamic risk factors are best treated with cognitive-behavioral interventions, Dr. Osher said, noting that the most effective interventions provide opportunities for participants to practice new behavior patterns and skills with feedback from program staff.
In many states, those interventions are being provided by criminal justice personnel, including probation officers, partly because of “an absence of [psychiatrists’] understanding, willingness, or ability to step forward.” The JPLI primer is designed to “really amp up our own excitement about, and willingness to learn how to develop interventions to help that individual stay out of trouble,” and it includes detailed descriptions of numerous well-researched, standardized, manualized interventions that people can access that make it less likely for them to have criminal justice access going forward, he said.
Those include programs such as “Thinking for a Change,” “Reasoning and Rehabilitation,” “Moral Reconation Therapy,” and “Interactive Journaling.”
A focus on the Sequential Intercept Model, which describes how individuals move through the criminal justice system, illustrates multiple points where psychiatrists can “do things better and differently to intervene,” he said, noting that the primer includes a framework for prioritizing the target population, and validated screening and assessment tools, including tools to help corrections officers identify mental health/substance abuse/criminogenic issues at the time individuals are booked into jail so they can be referred for appropriate interventions.
Achieving positive public health and safety outcomes requires changes to policy and practice, Dr. Osher said.
The JPLI primer is a step toward making such changes, and with it comes a set of four principles:
1. Conduct universal risk, substance use, and mental health screening at booking, and full assessments as appropriate, he said, noting that “13 million times this year (9 million unduplicated count), 2 million folks with serious mental illness are going to be arrested and brought to jail. Let’s make sure they get assessed, identified, and then a plan can be made.”
2. Get relevant information into the hands of decision makers in time to inform pretrial release decisions. For example, knowing if someone is eligible for a mental health court could lead to that person’s receiving necessary support and supervision, he said.
3. Use assessment information to connect people to appropriate jail-based services and post-release services and supervision, and ensure that there is communication between the two.
4. Ensure services and supervision are evidence based and hold systems accountable by measuring outcomes.
In addition, the goal is to partner with the criminal justice system through information-sharing agreements and integrating dynamic criminogenic risk factors into treatment plans, he said.
The intercepts
To demonstrate ways in which psychiatrists can intervene over the course of a patients’ journey toward involvement in the criminal justice system, Stephanie Le Melle, MD, provided a case example involving a 30-year-old African American man diagnosed with schizophrenia at age 18 years.
As a child, “Joe” was neglected and abused; both parents had a history of mental illness and substance use. He experienced homelessness, never finished high school, and was hospitalized or visited the emergency department more than 15 times after going off medications or because of intoxication.
His history with the legal system involved a first arrest at age 14 years for gang-related fighting and assault (after being bullied as a child and seeking safety in a gang), followed by 3 years in juvenile detention. He was released with supervision at age 17 years, was arrested several times after that for public intoxication and loitering, and was held for several days or weeks each time – then released with time served or summons paid. His first hospitalization occurred at age 18, when he was diagnosed with psychosis.
Subsequent experiences included treatment in a community mental health program at age 25 for heroin use and drinking. However, he was denied admission to a substance abuse program because of his history of psychosis and violence. After stopping his medications because of side effects, he tried to buy heroin, got into a fight, and was arrested for assault with a pocket knife. He resisted arrest and was tasered, handcuffed, and taken to prison, where he was held because he could not afford bail. Involvement in gang activity while in prison led to sanctions, including time in solitary confinement.
During all of his time in the criminal justice system, Joe refused treatment, because he was afraid he’d be considered “crazy” and would be preyed upon even more by other inmates. After about 3 years, he was released to a Forensic Assertive Community Treatment team for 2 years and completed that program, and is now receiving treatment in the community. He lives alone in supported housing and has Supplemental Security Income. He does not engage in clinic-related activities and has a lack of trust in the clinical team. He often is agitated and disruptive in the clinic. Staff members have concerns about his history of violence and drug use, and were reluctant to bring him into the program.
“Going back to ... the sequential intercept model, we can think about things, as psychiatrists, that we could have done for Joe all along the way to help him not get into the criminal justice system in the first place,” said Dr. Le Melle, director of public psychiatry education at Columbia University/New York State Psychiatric Institute, New York.
This is a framework for thinking through treatment for a patient like Joe:
Intercept 0 (community services). At this early stage, Joe would have been screened for adverse childhood experiences, and that could have led to trauma treatment, substance abuse treatment, and educational and vocational services. Awareness of his family illness, discord, and poverty would have led to parenting interventions, early school involvement, and promotion of meaningful activities, she said.
“These are things, again, that we can address as clinicians ... to intervene with families and with schools and communities to try to give young people an opportunity to not get into the criminal justice system,” she said, adding that providing early co-occurring treatment for mental health and substance use is particularly important.
Intercept 1 (law enforcement) also is a stage during which a psychiatrist can intervene by giving pertinent information when 911 is called by providing police or corrections with contact information for follow-up. For Joe, psychiatrist involvement at this intercept could have allowed for treatment recommendations or assessment for diversion programs, and in fact, at some point during his care, did allow for communication about his treatment needs, Dr. Le Melle said.
In general, psychiatrists also can participate at this stage through provision of crisis intervention team training for first responders or by being part of a co-response team, she said.
Intercept 2 (initial detention/initial court hearings). Attending court on behalf of a patient can make a real difference in outcomes, she noted.
“Judges want to know that someone is out there who can help, and they want to know that there’s a team of people who can intervene and try to get someone out of the criminal justice system,” she said.
At this stage, psychiatrists can help by recommending a treatment plan for a diversion program, and – within HIPAA guidelines – can share pertinent information about treatment needs and preferences.
Intercept 3 (jails/courts). At this in-the-system stage, information shared between corrections and community behavioral health would have led to Joe’s transfer to a mental health/observation unit; he would have been offered mental health treatment and been started on substance use treatment; and he would have participated in motivational treatment and cognitive-behavioral therapy targeting his criminogenic needs, she said.
Meeting with individuals while they are incarcerated can be helpful for “keeping them grounded.”
This also is a stage where psychiatrists could help individuals prepare for release by getting them into a GED program or other training.
Intercept 4 (reentry). With appropriate intervention at this stage, Joe would have his benefits, such as Medicaid and Supplemental Security Income, reinstated prior to reentry to the community. Also, his psychiatrist and treatment program would be contacted. He would be welcomed back into treatment, and he would have assistance finding a permanent place to live with services provided in the community.
Intercept 5 (community corrections). At this stage, community behavioral health clinicians would maintain awareness of their biases and fears about people involved in the criminal justice system and avoid making assumptions about Joe. His risks, needs, and priorities would be assessed and addressed, and he would be asked about his experiences with the system and about what could be done to help him avoid incarceration in the future.
He would receive help in incorporating alternative behaviors and thinking to address dynamic criminogenic risk, and evidence-based practices would be used in treatment.
The sequential intercept model reflects the fact that the criminal justice system and the people it serves are part of the community, Dr. Le Melle said.
“The community and the behavioral health system and the criminal justice system are partners in our shared mission of public safety and public health, so we are one and we can’t expect that our responsibility for providing people with the best care and services ends if someone is in the criminal justice system,” she said.
Dr. Champion, Dr. Osher, and Dr. Le Melle reported having no disclosures.
NEW YORK – The overrepresentation of people with serious mental illness (SMI) in the criminal justice system has led to creation of a resource from the Judges’ and Psychiatrists’ Leadership Initiative (JPLI) aimed at helping psychiatry and law enforcement address the problem.
The resource, “Supporting People with Serious Mental Illnesses and Reducing Their Risk of Contact with the Criminal Justice System: A Primer for Psychiatrists,” released last year, was designed to provide psychiatrists with specific knowledge and tools, according to Michael Champion, MD, forensic chief at the Hawaii State Department of Health, Adult Mental Health Division, Honolulu, and a member of the JPLI executive leadership team.
In developing the primer, the JPLI, which was created about 10 years ago by the American Psychiatric Association Foundation in partnership with the Council of State Governments Justice Center in response to the growing problem of such overrepresentation, sought to teach psychiatrists about what the criminal justice literature has dubbed “criminogenic risk” and to explore strategies to address those risks in community treatment settings, Dr. Champion said at the annual meeting of the American Psychiatric Association.
“The fact is that one in three Americans has a criminal record, and people with serious mental illness and criminal justice involvement are frequently part of our patient population – particularly in the public mental health sector,” Dr. Champion said. “Part of the challenge is that psychiatrists ... aren’t typically trained in these principles ... so the JPLI saw that this as an area that we could try to make some traction in and try to make a difference.”
The JPLI’s goals in publishing this resource are to reduce the risk of patient involvement in the criminal justice system, and to improve clinical and recovery outcomes by educating community psychiatrists about Risk-Need-Responsivity (RNR) principles. The JPLI also seeks to provide strategies for collaborating with criminal justice partners, incorporating criminal justice history into screening and assessment, and integrating criminogenic risk needs of patients into comprehensive treatment plans, Dr. Champion said.
Criminogenic risk and RNR
Many factors contribute to the involvement of people with serious mental illnesses in the criminal justice system, including higher rates of arrest, longer stays, recidivism, and limited access to health care, said Fred C. Osher, MD, former director of health systems and services policy for the Council of State Governments Justice Center.
“We used to think that ... if we could just get folks the health care that they need, they wouldn’t get involved with the criminal justice system. It turns out that that’s a gross oversimplification, in that their needs are terribly complex, and while treatment is a necessary component, it isn’t often sufficient for a large number of individuals,” said Dr. Osher, now a member of the JPLI executive leadership team.
Criminogenic risk – the likelihood that a person who has been arrested and jailed will commit a new crime after release or return to custody – helps explain why that is the case, he said, adding: “We have ways in which we can understand those risks.”
The risks are measured via static factors (unchanging conditions such as criminal history, age at first arrest, current age, and gender) and dynamic factors, he explained.
he said, noting that the research has shown there are eight specific criminogenic risk factors: substance abuse, history of antisocial behavior, antisocial personality pattern, antisocial cognition, antisocial associates, family and/or marital discord, poor school and/or work output, and having few leisure/recreation outlets.
Notably, mental illness is not a part of that list, he said.
“The reason for that is it’s not explanatory in and of itself,” he added.
However, research shows that people with mental illness have more of these dynamic risk factors, and research by Jennifer L. Skeem, PhD, and others shows that those with mental illness were coming back to jail not for new criminal activity, but for failing to comply with their conditions of release.
“These risks, then, have been brought into a paradigm that is central to our criminal justice operations, and it’s called the Risk-Need-Responsivity model,” Dr. Osher said. “This paradigm is what allows a criminal justice system to think about how to prioritize the resources – to think about who really needs to be wrapped tight, who needs to have close supervision, frequent reporting, lots of contact.”
The risk principle in the RNR model says that resources should be focused on high-risk cases, with limited supervision in lower-risk cases. This is based on experience demonstrating that recidivism is lower in high-risk individuals with close supervision but higher in low-risk individuals with close supervision.
The needs principle suggests that dynamic needs are “the issues that get folks in trouble,” he said.
“So, if we’re going to intervene, if we’re going to provide programming, if we’re going to try and help that individual stay out of jail or prison, we need to address these criminogenic needs,” he said, adding that the “big four” are related to their antisocial thinking and personality and friends.
Targeted interventions can help those individuals make better choices going forward, he noted.
The responsivity principle is an acknowledgment that individuals have different ways of learning, different cultural factors and backgrounds that influence them, and social determinants that are important to understand if they predict the ability to stay out of trouble.
“This is where mental illness fits in,” Dr. Osher said. “It’s absolutely important that we understand that.”
Examples would be patients with severe major depressive disorder who need their depression treated before they can participate in a group treatment setting designed to address criminogenic risks.
Dynamic risk factors are best treated with cognitive-behavioral interventions, Dr. Osher said, noting that the most effective interventions provide opportunities for participants to practice new behavior patterns and skills with feedback from program staff.
In many states, those interventions are being provided by criminal justice personnel, including probation officers, partly because of “an absence of [psychiatrists’] understanding, willingness, or ability to step forward.” The JPLI primer is designed to “really amp up our own excitement about, and willingness to learn how to develop interventions to help that individual stay out of trouble,” and it includes detailed descriptions of numerous well-researched, standardized, manualized interventions that people can access that make it less likely for them to have criminal justice access going forward, he said.
Those include programs such as “Thinking for a Change,” “Reasoning and Rehabilitation,” “Moral Reconation Therapy,” and “Interactive Journaling.”
A focus on the Sequential Intercept Model, which describes how individuals move through the criminal justice system, illustrates multiple points where psychiatrists can “do things better and differently to intervene,” he said, noting that the primer includes a framework for prioritizing the target population, and validated screening and assessment tools, including tools to help corrections officers identify mental health/substance abuse/criminogenic issues at the time individuals are booked into jail so they can be referred for appropriate interventions.
Achieving positive public health and safety outcomes requires changes to policy and practice, Dr. Osher said.
The JPLI primer is a step toward making such changes, and with it comes a set of four principles:
1. Conduct universal risk, substance use, and mental health screening at booking, and full assessments as appropriate, he said, noting that “13 million times this year (9 million unduplicated count), 2 million folks with serious mental illness are going to be arrested and brought to jail. Let’s make sure they get assessed, identified, and then a plan can be made.”
2. Get relevant information into the hands of decision makers in time to inform pretrial release decisions. For example, knowing if someone is eligible for a mental health court could lead to that person’s receiving necessary support and supervision, he said.
3. Use assessment information to connect people to appropriate jail-based services and post-release services and supervision, and ensure that there is communication between the two.
4. Ensure services and supervision are evidence based and hold systems accountable by measuring outcomes.
In addition, the goal is to partner with the criminal justice system through information-sharing agreements and integrating dynamic criminogenic risk factors into treatment plans, he said.
The intercepts
To demonstrate ways in which psychiatrists can intervene over the course of a patients’ journey toward involvement in the criminal justice system, Stephanie Le Melle, MD, provided a case example involving a 30-year-old African American man diagnosed with schizophrenia at age 18 years.
As a child, “Joe” was neglected and abused; both parents had a history of mental illness and substance use. He experienced homelessness, never finished high school, and was hospitalized or visited the emergency department more than 15 times after going off medications or because of intoxication.
His history with the legal system involved a first arrest at age 14 years for gang-related fighting and assault (after being bullied as a child and seeking safety in a gang), followed by 3 years in juvenile detention. He was released with supervision at age 17 years, was arrested several times after that for public intoxication and loitering, and was held for several days or weeks each time – then released with time served or summons paid. His first hospitalization occurred at age 18, when he was diagnosed with psychosis.
Subsequent experiences included treatment in a community mental health program at age 25 for heroin use and drinking. However, he was denied admission to a substance abuse program because of his history of psychosis and violence. After stopping his medications because of side effects, he tried to buy heroin, got into a fight, and was arrested for assault with a pocket knife. He resisted arrest and was tasered, handcuffed, and taken to prison, where he was held because he could not afford bail. Involvement in gang activity while in prison led to sanctions, including time in solitary confinement.
During all of his time in the criminal justice system, Joe refused treatment, because he was afraid he’d be considered “crazy” and would be preyed upon even more by other inmates. After about 3 years, he was released to a Forensic Assertive Community Treatment team for 2 years and completed that program, and is now receiving treatment in the community. He lives alone in supported housing and has Supplemental Security Income. He does not engage in clinic-related activities and has a lack of trust in the clinical team. He often is agitated and disruptive in the clinic. Staff members have concerns about his history of violence and drug use, and were reluctant to bring him into the program.
“Going back to ... the sequential intercept model, we can think about things, as psychiatrists, that we could have done for Joe all along the way to help him not get into the criminal justice system in the first place,” said Dr. Le Melle, director of public psychiatry education at Columbia University/New York State Psychiatric Institute, New York.
This is a framework for thinking through treatment for a patient like Joe:
Intercept 0 (community services). At this early stage, Joe would have been screened for adverse childhood experiences, and that could have led to trauma treatment, substance abuse treatment, and educational and vocational services. Awareness of his family illness, discord, and poverty would have led to parenting interventions, early school involvement, and promotion of meaningful activities, she said.
“These are things, again, that we can address as clinicians ... to intervene with families and with schools and communities to try to give young people an opportunity to not get into the criminal justice system,” she said, adding that providing early co-occurring treatment for mental health and substance use is particularly important.
Intercept 1 (law enforcement) also is a stage during which a psychiatrist can intervene by giving pertinent information when 911 is called by providing police or corrections with contact information for follow-up. For Joe, psychiatrist involvement at this intercept could have allowed for treatment recommendations or assessment for diversion programs, and in fact, at some point during his care, did allow for communication about his treatment needs, Dr. Le Melle said.
In general, psychiatrists also can participate at this stage through provision of crisis intervention team training for first responders or by being part of a co-response team, she said.
Intercept 2 (initial detention/initial court hearings). Attending court on behalf of a patient can make a real difference in outcomes, she noted.
“Judges want to know that someone is out there who can help, and they want to know that there’s a team of people who can intervene and try to get someone out of the criminal justice system,” she said.
At this stage, psychiatrists can help by recommending a treatment plan for a diversion program, and – within HIPAA guidelines – can share pertinent information about treatment needs and preferences.
Intercept 3 (jails/courts). At this in-the-system stage, information shared between corrections and community behavioral health would have led to Joe’s transfer to a mental health/observation unit; he would have been offered mental health treatment and been started on substance use treatment; and he would have participated in motivational treatment and cognitive-behavioral therapy targeting his criminogenic needs, she said.
Meeting with individuals while they are incarcerated can be helpful for “keeping them grounded.”
This also is a stage where psychiatrists could help individuals prepare for release by getting them into a GED program or other training.
Intercept 4 (reentry). With appropriate intervention at this stage, Joe would have his benefits, such as Medicaid and Supplemental Security Income, reinstated prior to reentry to the community. Also, his psychiatrist and treatment program would be contacted. He would be welcomed back into treatment, and he would have assistance finding a permanent place to live with services provided in the community.
Intercept 5 (community corrections). At this stage, community behavioral health clinicians would maintain awareness of their biases and fears about people involved in the criminal justice system and avoid making assumptions about Joe. His risks, needs, and priorities would be assessed and addressed, and he would be asked about his experiences with the system and about what could be done to help him avoid incarceration in the future.
He would receive help in incorporating alternative behaviors and thinking to address dynamic criminogenic risk, and evidence-based practices would be used in treatment.
The sequential intercept model reflects the fact that the criminal justice system and the people it serves are part of the community, Dr. Le Melle said.
“The community and the behavioral health system and the criminal justice system are partners in our shared mission of public safety and public health, so we are one and we can’t expect that our responsibility for providing people with the best care and services ends if someone is in the criminal justice system,” she said.
Dr. Champion, Dr. Osher, and Dr. Le Melle reported having no disclosures.
NEW YORK – The overrepresentation of people with serious mental illness (SMI) in the criminal justice system has led to creation of a resource from the Judges’ and Psychiatrists’ Leadership Initiative (JPLI) aimed at helping psychiatry and law enforcement address the problem.
The resource, “Supporting People with Serious Mental Illnesses and Reducing Their Risk of Contact with the Criminal Justice System: A Primer for Psychiatrists,” released last year, was designed to provide psychiatrists with specific knowledge and tools, according to Michael Champion, MD, forensic chief at the Hawaii State Department of Health, Adult Mental Health Division, Honolulu, and a member of the JPLI executive leadership team.
In developing the primer, the JPLI, which was created about 10 years ago by the American Psychiatric Association Foundation in partnership with the Council of State Governments Justice Center in response to the growing problem of such overrepresentation, sought to teach psychiatrists about what the criminal justice literature has dubbed “criminogenic risk” and to explore strategies to address those risks in community treatment settings, Dr. Champion said at the annual meeting of the American Psychiatric Association.
“The fact is that one in three Americans has a criminal record, and people with serious mental illness and criminal justice involvement are frequently part of our patient population – particularly in the public mental health sector,” Dr. Champion said. “Part of the challenge is that psychiatrists ... aren’t typically trained in these principles ... so the JPLI saw that this as an area that we could try to make some traction in and try to make a difference.”
The JPLI’s goals in publishing this resource are to reduce the risk of patient involvement in the criminal justice system, and to improve clinical and recovery outcomes by educating community psychiatrists about Risk-Need-Responsivity (RNR) principles. The JPLI also seeks to provide strategies for collaborating with criminal justice partners, incorporating criminal justice history into screening and assessment, and integrating criminogenic risk needs of patients into comprehensive treatment plans, Dr. Champion said.
Criminogenic risk and RNR
Many factors contribute to the involvement of people with serious mental illnesses in the criminal justice system, including higher rates of arrest, longer stays, recidivism, and limited access to health care, said Fred C. Osher, MD, former director of health systems and services policy for the Council of State Governments Justice Center.
“We used to think that ... if we could just get folks the health care that they need, they wouldn’t get involved with the criminal justice system. It turns out that that’s a gross oversimplification, in that their needs are terribly complex, and while treatment is a necessary component, it isn’t often sufficient for a large number of individuals,” said Dr. Osher, now a member of the JPLI executive leadership team.
Criminogenic risk – the likelihood that a person who has been arrested and jailed will commit a new crime after release or return to custody – helps explain why that is the case, he said, adding: “We have ways in which we can understand those risks.”
The risks are measured via static factors (unchanging conditions such as criminal history, age at first arrest, current age, and gender) and dynamic factors, he explained.
he said, noting that the research has shown there are eight specific criminogenic risk factors: substance abuse, history of antisocial behavior, antisocial personality pattern, antisocial cognition, antisocial associates, family and/or marital discord, poor school and/or work output, and having few leisure/recreation outlets.
Notably, mental illness is not a part of that list, he said.
“The reason for that is it’s not explanatory in and of itself,” he added.
However, research shows that people with mental illness have more of these dynamic risk factors, and research by Jennifer L. Skeem, PhD, and others shows that those with mental illness were coming back to jail not for new criminal activity, but for failing to comply with their conditions of release.
“These risks, then, have been brought into a paradigm that is central to our criminal justice operations, and it’s called the Risk-Need-Responsivity model,” Dr. Osher said. “This paradigm is what allows a criminal justice system to think about how to prioritize the resources – to think about who really needs to be wrapped tight, who needs to have close supervision, frequent reporting, lots of contact.”
The risk principle in the RNR model says that resources should be focused on high-risk cases, with limited supervision in lower-risk cases. This is based on experience demonstrating that recidivism is lower in high-risk individuals with close supervision but higher in low-risk individuals with close supervision.
The needs principle suggests that dynamic needs are “the issues that get folks in trouble,” he said.
“So, if we’re going to intervene, if we’re going to provide programming, if we’re going to try and help that individual stay out of jail or prison, we need to address these criminogenic needs,” he said, adding that the “big four” are related to their antisocial thinking and personality and friends.
Targeted interventions can help those individuals make better choices going forward, he noted.
The responsivity principle is an acknowledgment that individuals have different ways of learning, different cultural factors and backgrounds that influence them, and social determinants that are important to understand if they predict the ability to stay out of trouble.
“This is where mental illness fits in,” Dr. Osher said. “It’s absolutely important that we understand that.”
Examples would be patients with severe major depressive disorder who need their depression treated before they can participate in a group treatment setting designed to address criminogenic risks.
Dynamic risk factors are best treated with cognitive-behavioral interventions, Dr. Osher said, noting that the most effective interventions provide opportunities for participants to practice new behavior patterns and skills with feedback from program staff.
In many states, those interventions are being provided by criminal justice personnel, including probation officers, partly because of “an absence of [psychiatrists’] understanding, willingness, or ability to step forward.” The JPLI primer is designed to “really amp up our own excitement about, and willingness to learn how to develop interventions to help that individual stay out of trouble,” and it includes detailed descriptions of numerous well-researched, standardized, manualized interventions that people can access that make it less likely for them to have criminal justice access going forward, he said.
Those include programs such as “Thinking for a Change,” “Reasoning and Rehabilitation,” “Moral Reconation Therapy,” and “Interactive Journaling.”
A focus on the Sequential Intercept Model, which describes how individuals move through the criminal justice system, illustrates multiple points where psychiatrists can “do things better and differently to intervene,” he said, noting that the primer includes a framework for prioritizing the target population, and validated screening and assessment tools, including tools to help corrections officers identify mental health/substance abuse/criminogenic issues at the time individuals are booked into jail so they can be referred for appropriate interventions.
Achieving positive public health and safety outcomes requires changes to policy and practice, Dr. Osher said.
The JPLI primer is a step toward making such changes, and with it comes a set of four principles:
1. Conduct universal risk, substance use, and mental health screening at booking, and full assessments as appropriate, he said, noting that “13 million times this year (9 million unduplicated count), 2 million folks with serious mental illness are going to be arrested and brought to jail. Let’s make sure they get assessed, identified, and then a plan can be made.”
2. Get relevant information into the hands of decision makers in time to inform pretrial release decisions. For example, knowing if someone is eligible for a mental health court could lead to that person’s receiving necessary support and supervision, he said.
3. Use assessment information to connect people to appropriate jail-based services and post-release services and supervision, and ensure that there is communication between the two.
4. Ensure services and supervision are evidence based and hold systems accountable by measuring outcomes.
In addition, the goal is to partner with the criminal justice system through information-sharing agreements and integrating dynamic criminogenic risk factors into treatment plans, he said.
The intercepts
To demonstrate ways in which psychiatrists can intervene over the course of a patients’ journey toward involvement in the criminal justice system, Stephanie Le Melle, MD, provided a case example involving a 30-year-old African American man diagnosed with schizophrenia at age 18 years.
As a child, “Joe” was neglected and abused; both parents had a history of mental illness and substance use. He experienced homelessness, never finished high school, and was hospitalized or visited the emergency department more than 15 times after going off medications or because of intoxication.
His history with the legal system involved a first arrest at age 14 years for gang-related fighting and assault (after being bullied as a child and seeking safety in a gang), followed by 3 years in juvenile detention. He was released with supervision at age 17 years, was arrested several times after that for public intoxication and loitering, and was held for several days or weeks each time – then released with time served or summons paid. His first hospitalization occurred at age 18, when he was diagnosed with psychosis.
Subsequent experiences included treatment in a community mental health program at age 25 for heroin use and drinking. However, he was denied admission to a substance abuse program because of his history of psychosis and violence. After stopping his medications because of side effects, he tried to buy heroin, got into a fight, and was arrested for assault with a pocket knife. He resisted arrest and was tasered, handcuffed, and taken to prison, where he was held because he could not afford bail. Involvement in gang activity while in prison led to sanctions, including time in solitary confinement.
During all of his time in the criminal justice system, Joe refused treatment, because he was afraid he’d be considered “crazy” and would be preyed upon even more by other inmates. After about 3 years, he was released to a Forensic Assertive Community Treatment team for 2 years and completed that program, and is now receiving treatment in the community. He lives alone in supported housing and has Supplemental Security Income. He does not engage in clinic-related activities and has a lack of trust in the clinical team. He often is agitated and disruptive in the clinic. Staff members have concerns about his history of violence and drug use, and were reluctant to bring him into the program.
“Going back to ... the sequential intercept model, we can think about things, as psychiatrists, that we could have done for Joe all along the way to help him not get into the criminal justice system in the first place,” said Dr. Le Melle, director of public psychiatry education at Columbia University/New York State Psychiatric Institute, New York.
This is a framework for thinking through treatment for a patient like Joe:
Intercept 0 (community services). At this early stage, Joe would have been screened for adverse childhood experiences, and that could have led to trauma treatment, substance abuse treatment, and educational and vocational services. Awareness of his family illness, discord, and poverty would have led to parenting interventions, early school involvement, and promotion of meaningful activities, she said.
“These are things, again, that we can address as clinicians ... to intervene with families and with schools and communities to try to give young people an opportunity to not get into the criminal justice system,” she said, adding that providing early co-occurring treatment for mental health and substance use is particularly important.
Intercept 1 (law enforcement) also is a stage during which a psychiatrist can intervene by giving pertinent information when 911 is called by providing police or corrections with contact information for follow-up. For Joe, psychiatrist involvement at this intercept could have allowed for treatment recommendations or assessment for diversion programs, and in fact, at some point during his care, did allow for communication about his treatment needs, Dr. Le Melle said.
In general, psychiatrists also can participate at this stage through provision of crisis intervention team training for first responders or by being part of a co-response team, she said.
Intercept 2 (initial detention/initial court hearings). Attending court on behalf of a patient can make a real difference in outcomes, she noted.
“Judges want to know that someone is out there who can help, and they want to know that there’s a team of people who can intervene and try to get someone out of the criminal justice system,” she said.
At this stage, psychiatrists can help by recommending a treatment plan for a diversion program, and – within HIPAA guidelines – can share pertinent information about treatment needs and preferences.
Intercept 3 (jails/courts). At this in-the-system stage, information shared between corrections and community behavioral health would have led to Joe’s transfer to a mental health/observation unit; he would have been offered mental health treatment and been started on substance use treatment; and he would have participated in motivational treatment and cognitive-behavioral therapy targeting his criminogenic needs, she said.
Meeting with individuals while they are incarcerated can be helpful for “keeping them grounded.”
This also is a stage where psychiatrists could help individuals prepare for release by getting them into a GED program or other training.
Intercept 4 (reentry). With appropriate intervention at this stage, Joe would have his benefits, such as Medicaid and Supplemental Security Income, reinstated prior to reentry to the community. Also, his psychiatrist and treatment program would be contacted. He would be welcomed back into treatment, and he would have assistance finding a permanent place to live with services provided in the community.
Intercept 5 (community corrections). At this stage, community behavioral health clinicians would maintain awareness of their biases and fears about people involved in the criminal justice system and avoid making assumptions about Joe. His risks, needs, and priorities would be assessed and addressed, and he would be asked about his experiences with the system and about what could be done to help him avoid incarceration in the future.
He would receive help in incorporating alternative behaviors and thinking to address dynamic criminogenic risk, and evidence-based practices would be used in treatment.
The sequential intercept model reflects the fact that the criminal justice system and the people it serves are part of the community, Dr. Le Melle said.
“The community and the behavioral health system and the criminal justice system are partners in our shared mission of public safety and public health, so we are one and we can’t expect that our responsibility for providing people with the best care and services ends if someone is in the criminal justice system,” she said.
Dr. Champion, Dr. Osher, and Dr. Le Melle reported having no disclosures.
REPORTING FROM APA 2018