User login
Feds take baseline on EHR interoperability
Office-based physicians who have electronic health records are most likely to send patient health information (PHI) in the form of referrals and laboratory results and receive it as lab results and imaging reports, according to national estimates of electronic PHI using four aspects of interoperability.
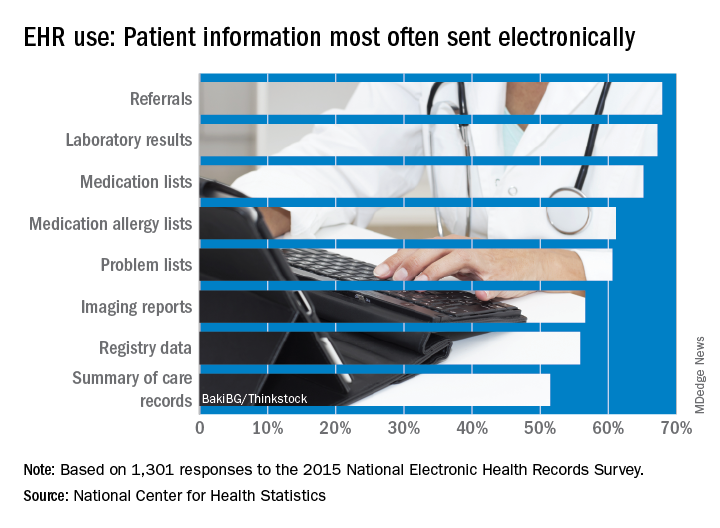
Those aspects of sharing PHI are sending, receiving, integrating, and searching. With federal estimates of EHR adoption at 78% for office-based physicians, data from the 2015 National Electronic Health Records Just over 65% are sending medication lists electronically, 61% are sending medication allergy lists, and almost 61% are sending problem lists, Ninee S. Yang, PhD, and her associates at the Centers for Disease Control and Prevention said in National Health Statistics Reports.
Communication in the other direction showed a somewhat different distribution. Of the 1,525 physicians who reported receiving PHI electronically, 79% were the recipients of laboratory results, with imaging reports well behind at 61%, followed by medication lists and referrals at 54% and summary of care records at 52%, the investigators reported.
The ability to integrate information into an EHR – reported by 959 survey respondents – is another key element of interoperability, and 73% of those physicians said that they could integrate lab results into their systems. Other types of PHI, however, did not fare as well: imaging reports came in at 50%, hospital discharge summaries at 49%, summary of care records at 41%, and emergency department notifications at 40%, Dr. Yang and her associates said.
The fourth aspect of interoperability, ability to search for PHI from sources outside the practice, was reported by 1,335 respondents in 2015, with 90% looking for medication lists, 88% for medication allergy lists, 80% for hospital discharge summaries, 59% for imaging reports, and 49% for lab results, they said.
These first national estimates of PHI type will be used as baseline data “in tracking progress outlined in the federal plan for achieving interoperability” among physicians with EHR systems, Dr. Yang and her associates wrote.
SOURCE: Yang NS et al. Natl Health Stat Report. 2018 Aug 15;(115):1-9.
Office-based physicians who have electronic health records are most likely to send patient health information (PHI) in the form of referrals and laboratory results and receive it as lab results and imaging reports, according to national estimates of electronic PHI using four aspects of interoperability.

Those aspects of sharing PHI are sending, receiving, integrating, and searching. With federal estimates of EHR adoption at 78% for office-based physicians, data from the 2015 National Electronic Health Records Just over 65% are sending medication lists electronically, 61% are sending medication allergy lists, and almost 61% are sending problem lists, Ninee S. Yang, PhD, and her associates at the Centers for Disease Control and Prevention said in National Health Statistics Reports.
Communication in the other direction showed a somewhat different distribution. Of the 1,525 physicians who reported receiving PHI electronically, 79% were the recipients of laboratory results, with imaging reports well behind at 61%, followed by medication lists and referrals at 54% and summary of care records at 52%, the investigators reported.
The ability to integrate information into an EHR – reported by 959 survey respondents – is another key element of interoperability, and 73% of those physicians said that they could integrate lab results into their systems. Other types of PHI, however, did not fare as well: imaging reports came in at 50%, hospital discharge summaries at 49%, summary of care records at 41%, and emergency department notifications at 40%, Dr. Yang and her associates said.
The fourth aspect of interoperability, ability to search for PHI from sources outside the practice, was reported by 1,335 respondents in 2015, with 90% looking for medication lists, 88% for medication allergy lists, 80% for hospital discharge summaries, 59% for imaging reports, and 49% for lab results, they said.
These first national estimates of PHI type will be used as baseline data “in tracking progress outlined in the federal plan for achieving interoperability” among physicians with EHR systems, Dr. Yang and her associates wrote.
SOURCE: Yang NS et al. Natl Health Stat Report. 2018 Aug 15;(115):1-9.
Office-based physicians who have electronic health records are most likely to send patient health information (PHI) in the form of referrals and laboratory results and receive it as lab results and imaging reports, according to national estimates of electronic PHI using four aspects of interoperability.

Those aspects of sharing PHI are sending, receiving, integrating, and searching. With federal estimates of EHR adoption at 78% for office-based physicians, data from the 2015 National Electronic Health Records Just over 65% are sending medication lists electronically, 61% are sending medication allergy lists, and almost 61% are sending problem lists, Ninee S. Yang, PhD, and her associates at the Centers for Disease Control and Prevention said in National Health Statistics Reports.
Communication in the other direction showed a somewhat different distribution. Of the 1,525 physicians who reported receiving PHI electronically, 79% were the recipients of laboratory results, with imaging reports well behind at 61%, followed by medication lists and referrals at 54% and summary of care records at 52%, the investigators reported.
The ability to integrate information into an EHR – reported by 959 survey respondents – is another key element of interoperability, and 73% of those physicians said that they could integrate lab results into their systems. Other types of PHI, however, did not fare as well: imaging reports came in at 50%, hospital discharge summaries at 49%, summary of care records at 41%, and emergency department notifications at 40%, Dr. Yang and her associates said.
The fourth aspect of interoperability, ability to search for PHI from sources outside the practice, was reported by 1,335 respondents in 2015, with 90% looking for medication lists, 88% for medication allergy lists, 80% for hospital discharge summaries, 59% for imaging reports, and 49% for lab results, they said.
These first national estimates of PHI type will be used as baseline data “in tracking progress outlined in the federal plan for achieving interoperability” among physicians with EHR systems, Dr. Yang and her associates wrote.
SOURCE: Yang NS et al. Natl Health Stat Report. 2018 Aug 15;(115):1-9.
FROM NATIONAL HEALTH STATISTICS REPORTS
Three steps are involved in assessing a vesiculopustular lesion
LAKE TAHOE, CALIF. – Evaluation of an infant with a vesiculopustular lesion entails a pragmatic approach, with three assessment steps, according to pediatric dermatologist Lawrence A. Schachner, MD.
At the annual meeting of the Society for Pediatric Dermatology, Dr. Schachner noted that the differential diagnosis of noninfectious, usually benign neonatal vesiculopustular lesions includes acropustulosis of infancy; eosinophilic pustular folliculitis; erythema toxicum neonatorum; miliaria crystallina, rubra, or profunda; transient neonatal pustular melanosis; and neonatal sucking blisters. Noninfectious lesions that can be potentially serious include acrodermatitis enteropathica, bullous pemphigoid, epidermolysis bullosa, epidermolytic hyperkeratosis, incontinentia pigmenti, Langerhans cell histiocytosis, pemphigus vulgaris, pustular psoriasis, and urticarial pigmentosa.
Step 1 for evaluating vesiculopustular lesions involves drawing out the fluid for bacterial cultures and sensitivity, Gram stains, viral culture and/or serology, said Dr. Schachner, who directs the division of pediatric dermatology at the University of Miami.
Step 2 involves snipping off the lesion’s roof for a potassium hydroxide test to send for dermatopathology or frozen pathology.
Step 3 involves scraping the lesion’s base for viral cytology and cell identification. “If we can see a predominant cell type, that’s where the action is,” said Dr. Schachner, professor of pediatrics at the university. If cytology reveals polymorphic neutrophils, differential diagnoses include transient neonatal pustular melanosis, infantile acropustulosis, bullous impetigo, and pustular psoriasis. If cytology reveals eosinophils, differential diagnoses include eosinophilic pustular folliculitis of infancy, erythema toxicum neonatorum, incontinentia pigmenti, bullous pemphigoid, drug reactions, and arthropod bites. The presence of lymphocytes on cytology, meanwhile, may suggest a differential diagnosis miliaria or acrodermatitis enteropathica.
“When in doubt about the diagnosis, biopsy,” Dr. Schachner said. “This, to me, is a pragmatic approach.”
Dr. Schachner disclosed that he is an investigator for Astellas, Ferndale Labs, Novartis, Organogenesis, Stiefel Laboratories, Berg Pharma, Medimetrics, and Lilly. He is a consultant to Beiersdorf, Lexington, TopMD, Cutanea, Hoth Therapeutics, and Mustela.
[email protected]
LAKE TAHOE, CALIF. – Evaluation of an infant with a vesiculopustular lesion entails a pragmatic approach, with three assessment steps, according to pediatric dermatologist Lawrence A. Schachner, MD.
At the annual meeting of the Society for Pediatric Dermatology, Dr. Schachner noted that the differential diagnosis of noninfectious, usually benign neonatal vesiculopustular lesions includes acropustulosis of infancy; eosinophilic pustular folliculitis; erythema toxicum neonatorum; miliaria crystallina, rubra, or profunda; transient neonatal pustular melanosis; and neonatal sucking blisters. Noninfectious lesions that can be potentially serious include acrodermatitis enteropathica, bullous pemphigoid, epidermolysis bullosa, epidermolytic hyperkeratosis, incontinentia pigmenti, Langerhans cell histiocytosis, pemphigus vulgaris, pustular psoriasis, and urticarial pigmentosa.
Step 1 for evaluating vesiculopustular lesions involves drawing out the fluid for bacterial cultures and sensitivity, Gram stains, viral culture and/or serology, said Dr. Schachner, who directs the division of pediatric dermatology at the University of Miami.
Step 2 involves snipping off the lesion’s roof for a potassium hydroxide test to send for dermatopathology or frozen pathology.
Step 3 involves scraping the lesion’s base for viral cytology and cell identification. “If we can see a predominant cell type, that’s where the action is,” said Dr. Schachner, professor of pediatrics at the university. If cytology reveals polymorphic neutrophils, differential diagnoses include transient neonatal pustular melanosis, infantile acropustulosis, bullous impetigo, and pustular psoriasis. If cytology reveals eosinophils, differential diagnoses include eosinophilic pustular folliculitis of infancy, erythema toxicum neonatorum, incontinentia pigmenti, bullous pemphigoid, drug reactions, and arthropod bites. The presence of lymphocytes on cytology, meanwhile, may suggest a differential diagnosis miliaria or acrodermatitis enteropathica.
“When in doubt about the diagnosis, biopsy,” Dr. Schachner said. “This, to me, is a pragmatic approach.”
Dr. Schachner disclosed that he is an investigator for Astellas, Ferndale Labs, Novartis, Organogenesis, Stiefel Laboratories, Berg Pharma, Medimetrics, and Lilly. He is a consultant to Beiersdorf, Lexington, TopMD, Cutanea, Hoth Therapeutics, and Mustela.
[email protected]
LAKE TAHOE, CALIF. – Evaluation of an infant with a vesiculopustular lesion entails a pragmatic approach, with three assessment steps, according to pediatric dermatologist Lawrence A. Schachner, MD.
At the annual meeting of the Society for Pediatric Dermatology, Dr. Schachner noted that the differential diagnosis of noninfectious, usually benign neonatal vesiculopustular lesions includes acropustulosis of infancy; eosinophilic pustular folliculitis; erythema toxicum neonatorum; miliaria crystallina, rubra, or profunda; transient neonatal pustular melanosis; and neonatal sucking blisters. Noninfectious lesions that can be potentially serious include acrodermatitis enteropathica, bullous pemphigoid, epidermolysis bullosa, epidermolytic hyperkeratosis, incontinentia pigmenti, Langerhans cell histiocytosis, pemphigus vulgaris, pustular psoriasis, and urticarial pigmentosa.
Step 1 for evaluating vesiculopustular lesions involves drawing out the fluid for bacterial cultures and sensitivity, Gram stains, viral culture and/or serology, said Dr. Schachner, who directs the division of pediatric dermatology at the University of Miami.
Step 2 involves snipping off the lesion’s roof for a potassium hydroxide test to send for dermatopathology or frozen pathology.
Step 3 involves scraping the lesion’s base for viral cytology and cell identification. “If we can see a predominant cell type, that’s where the action is,” said Dr. Schachner, professor of pediatrics at the university. If cytology reveals polymorphic neutrophils, differential diagnoses include transient neonatal pustular melanosis, infantile acropustulosis, bullous impetigo, and pustular psoriasis. If cytology reveals eosinophils, differential diagnoses include eosinophilic pustular folliculitis of infancy, erythema toxicum neonatorum, incontinentia pigmenti, bullous pemphigoid, drug reactions, and arthropod bites. The presence of lymphocytes on cytology, meanwhile, may suggest a differential diagnosis miliaria or acrodermatitis enteropathica.
“When in doubt about the diagnosis, biopsy,” Dr. Schachner said. “This, to me, is a pragmatic approach.”
Dr. Schachner disclosed that he is an investigator for Astellas, Ferndale Labs, Novartis, Organogenesis, Stiefel Laboratories, Berg Pharma, Medimetrics, and Lilly. He is a consultant to Beiersdorf, Lexington, TopMD, Cutanea, Hoth Therapeutics, and Mustela.
[email protected]
REPORTING FROM SPD 2018
New Valsalva maneuver for SVT beats all others
NEW ORLEANS – , according to Jeet Mehta, MD, a resident in the combined medicine/pediatrics program at the University of Kansas, Wichita.
The 2015 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines recommended vagal maneuvers as first-line treatment of supraventricular tachycardia, but added that there was no gold standard method. Since then, the situation has changed. Two well-conducted randomized clinical trials have been published that bring clarity as to the vagal maneuver of choice, Dr. Mehta reported at the annual meeting of the American College of Physicians.
He and his coinvestigators performed a meta-analysis of the three pre-2000 randomized controlled trials that compared the standard Valsalva maneuver to carotid sinus massage plus the two newer studies, both of which systematically compared a modified Valsalva maneuver with the standard version.
The clear winner in terms of efficacy was the modified Valsalva maneuver, in which patients with supraventricular tachycardia (SVT) performed a standardized strain while in a semirecumbent position, then immediately laid flat and had their legs raised to 45 degrees for 15 seconds before returning to the semirecumbent position. The purpose of this postural modification is to boost relaxation phase venous return and vagal stimulation.
In the 433-patient multicenter REVERT trial in the United Kingdom, 43% of those assigned to the modified Valsalva maneuver returned to sinus rhythm 1 minute after completing the task, compared with 17% of those randomized to the standard semirecumbent Valsalva maneuver. This resulted in significantly less need for adenosine and other treatments. Although REVERT investigators had the patients blow into a manometer at 40 mm Hg for 15 seconds, they noted that the same intensity of strain can be achieved more practically by blowing into a 10-mL syringe sufficient to just move the plunger (Lancet. 2015 Oct 31;386[10005]:1747-53).
The REVERT findings were confirmed by a second trial conducted by Turkish investigators, in which the modified Valsalva maneuver was successful in 43% of patients, compared with 11% in the standard Valsalva maneuver group (Am J Emerg Med. 2017 Nov;35[11]:1662-5).
Extrapolating from the published evidence, including a Cochrane Collaboration review (Cochrane Database Syst Rev. 2015 Feb 18;[2]:CD009502. doi: 10.1002/14651858.CD009502.pub3), Dr. Mehta and his coinvestigators ranked the likelihood of successful conversion of SVT to sinus rhythm from a high of 48% for the modified Valsalva maneuver, descending to 43% for a supine Valsalva maneuver, 36% for a standard semirecumbent Valsalva, 21% for a seated Valsalva, 19% for a standing one, and just 11% for carotid sinus massage.
“Based on evidence of high quality, we encourage that the modified Valsalva maneuver be done due its safety and low cost,” Dr. Mehta concluded.
He reported having no financial conflicts regarding his study, conducted free of commercial support.
NEW ORLEANS – , according to Jeet Mehta, MD, a resident in the combined medicine/pediatrics program at the University of Kansas, Wichita.
The 2015 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines recommended vagal maneuvers as first-line treatment of supraventricular tachycardia, but added that there was no gold standard method. Since then, the situation has changed. Two well-conducted randomized clinical trials have been published that bring clarity as to the vagal maneuver of choice, Dr. Mehta reported at the annual meeting of the American College of Physicians.
He and his coinvestigators performed a meta-analysis of the three pre-2000 randomized controlled trials that compared the standard Valsalva maneuver to carotid sinus massage plus the two newer studies, both of which systematically compared a modified Valsalva maneuver with the standard version.
The clear winner in terms of efficacy was the modified Valsalva maneuver, in which patients with supraventricular tachycardia (SVT) performed a standardized strain while in a semirecumbent position, then immediately laid flat and had their legs raised to 45 degrees for 15 seconds before returning to the semirecumbent position. The purpose of this postural modification is to boost relaxation phase venous return and vagal stimulation.
In the 433-patient multicenter REVERT trial in the United Kingdom, 43% of those assigned to the modified Valsalva maneuver returned to sinus rhythm 1 minute after completing the task, compared with 17% of those randomized to the standard semirecumbent Valsalva maneuver. This resulted in significantly less need for adenosine and other treatments. Although REVERT investigators had the patients blow into a manometer at 40 mm Hg for 15 seconds, they noted that the same intensity of strain can be achieved more practically by blowing into a 10-mL syringe sufficient to just move the plunger (Lancet. 2015 Oct 31;386[10005]:1747-53).
The REVERT findings were confirmed by a second trial conducted by Turkish investigators, in which the modified Valsalva maneuver was successful in 43% of patients, compared with 11% in the standard Valsalva maneuver group (Am J Emerg Med. 2017 Nov;35[11]:1662-5).
Extrapolating from the published evidence, including a Cochrane Collaboration review (Cochrane Database Syst Rev. 2015 Feb 18;[2]:CD009502. doi: 10.1002/14651858.CD009502.pub3), Dr. Mehta and his coinvestigators ranked the likelihood of successful conversion of SVT to sinus rhythm from a high of 48% for the modified Valsalva maneuver, descending to 43% for a supine Valsalva maneuver, 36% for a standard semirecumbent Valsalva, 21% for a seated Valsalva, 19% for a standing one, and just 11% for carotid sinus massage.
“Based on evidence of high quality, we encourage that the modified Valsalva maneuver be done due its safety and low cost,” Dr. Mehta concluded.
He reported having no financial conflicts regarding his study, conducted free of commercial support.
NEW ORLEANS – , according to Jeet Mehta, MD, a resident in the combined medicine/pediatrics program at the University of Kansas, Wichita.
The 2015 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines recommended vagal maneuvers as first-line treatment of supraventricular tachycardia, but added that there was no gold standard method. Since then, the situation has changed. Two well-conducted randomized clinical trials have been published that bring clarity as to the vagal maneuver of choice, Dr. Mehta reported at the annual meeting of the American College of Physicians.
He and his coinvestigators performed a meta-analysis of the three pre-2000 randomized controlled trials that compared the standard Valsalva maneuver to carotid sinus massage plus the two newer studies, both of which systematically compared a modified Valsalva maneuver with the standard version.
The clear winner in terms of efficacy was the modified Valsalva maneuver, in which patients with supraventricular tachycardia (SVT) performed a standardized strain while in a semirecumbent position, then immediately laid flat and had their legs raised to 45 degrees for 15 seconds before returning to the semirecumbent position. The purpose of this postural modification is to boost relaxation phase venous return and vagal stimulation.
In the 433-patient multicenter REVERT trial in the United Kingdom, 43% of those assigned to the modified Valsalva maneuver returned to sinus rhythm 1 minute after completing the task, compared with 17% of those randomized to the standard semirecumbent Valsalva maneuver. This resulted in significantly less need for adenosine and other treatments. Although REVERT investigators had the patients blow into a manometer at 40 mm Hg for 15 seconds, they noted that the same intensity of strain can be achieved more practically by blowing into a 10-mL syringe sufficient to just move the plunger (Lancet. 2015 Oct 31;386[10005]:1747-53).
The REVERT findings were confirmed by a second trial conducted by Turkish investigators, in which the modified Valsalva maneuver was successful in 43% of patients, compared with 11% in the standard Valsalva maneuver group (Am J Emerg Med. 2017 Nov;35[11]:1662-5).
Extrapolating from the published evidence, including a Cochrane Collaboration review (Cochrane Database Syst Rev. 2015 Feb 18;[2]:CD009502. doi: 10.1002/14651858.CD009502.pub3), Dr. Mehta and his coinvestigators ranked the likelihood of successful conversion of SVT to sinus rhythm from a high of 48% for the modified Valsalva maneuver, descending to 43% for a supine Valsalva maneuver, 36% for a standard semirecumbent Valsalva, 21% for a seated Valsalva, 19% for a standing one, and just 11% for carotid sinus massage.
“Based on evidence of high quality, we encourage that the modified Valsalva maneuver be done due its safety and low cost,” Dr. Mehta concluded.
He reported having no financial conflicts regarding his study, conducted free of commercial support.
REPORTING FROM ACP INTERNAL MEDICINE
Key clinical point: A simple postural modification to the standard Valsalva maneuver boosts conversion rate.
Major finding: Nearly half of patients in SVT converted to sinus rhythm in response to a modified Valsalva maneuver.
Study details: This was a meta-analysis of five randomized clinical trials of vagal maneuvers for conversion of supraventricular tachycardia to sinus rhythm.
Disclosures: The presenter reported having no financial conflicts regarding the study, conducted free of commercial support.
Signal strength may limit potency of CAR T-cell therapy
Contrary to what might be expected, chimeric antigen receptor (CAR) T cells with stronger signaling capabilities were less effective against lymphoma cells in a mouse model, investigators reported.
Intracellular signaling strength was a key determinant of T cell fate in the study, which was published in the journal Science Signaling.
By contrast, CAR signaling pathways could not be predicted solely by the costimulatory domains used to construct the receptor, investigators said.
Based on those findings, tailoring CAR design based on signal strength might improve the efficacy and reduce the toxicity of CAR T-cell therapy, according to Alexander Salter, an MD/PhD student at Fred Hutchinson Cancer Research Center, Seattle, Wash.
In a press conference, Mr. Salter described results of the study, which used mass spectrometry to evaluate CARs encoding CD28 or 4-1BB costimulatory domains in primary human T cells.
While CARs with CD28 domains elicited more robust intracellular signaling than those with 4-1BB domains, there was considerable overlap in activation of T cell signaling pathways, Mr. Salter said.
That overlap was somewhat surprising, according to Mr. Salter, since researchers have generally assumed that CARs with CD28 and 4-1BB costimulatory domains will primarily signal through those respective pathways.
“No matter what costimulatory domain was encoded by the receptor, both CARs… activated both CD28 and 41BB signaling pathways,” Mr. Salter said.
The major determinant of efficacy in the study turned out to be not the domain used to construct the receptor, but the speed and strength of signaling, he added. In particular, the CARs that evoked stronger signals also had increased T cell dysfunction, decreasing their potency in the mouse lymphoma model.
The T cells with a CD28 CAR had very strong initial antitumor function that quickly waned in the mouse model of lymphoma; by contrast, the “slower burning” 4-1BB CAR signal led to T cells that better retained their function in vivo and were associated with longer median survival in the model, he said.
Those findings suggest tailoring CAR design based on signal strength may improve clinical efficacy and reduce toxicity.
As part of the study, Mr. Salter and his co-investigators were able to modify the CAR CD28 domain to make the signaling of the CD28 CARs less intense. “This is a modification that we think should be considered in future CAR design,” Mr. Salter said.
While the alterations in the CD28 signaling domain were able to reduce levels of cytokines produced by T cells, the study was primarily designed to look at the efficacy, noted Stanley Riddell, MD, scientific director of the Immunotherapy Integrated Research Center at Fred Hutchinson Cancer Research Center.
“Our models were not set up to address the question of toxicity, so we can’t directly say this would translate to what we would see in patients,” Dr. Riddell said during the press conference. “But I think we gleaned a lot of insights as to why cytokines are produced at greater or lesser levels with various CAR designs, and insights as to how to redesign these receptors to lower the levels of cytokines they make without compromising their ability to kill.”
Dr. Riddell is a founder, shareholder, and scientific advisor of Juno Therapeutics, and together with Mr. Salter, he has filed a patent application on the use of mutant CD28 CARs for cellular therapy. Co-author Raphael Gottardo, PhD, also with Fred Hutchinson Cancer Research Center, is a consultant for Juno Therapeutics. No other competing interests were reported.
SOURCE: Salter AI et al., Sci Signal. 2018 Aug 21;11. pii:eaat6753.
Contrary to what might be expected, chimeric antigen receptor (CAR) T cells with stronger signaling capabilities were less effective against lymphoma cells in a mouse model, investigators reported.
Intracellular signaling strength was a key determinant of T cell fate in the study, which was published in the journal Science Signaling.
By contrast, CAR signaling pathways could not be predicted solely by the costimulatory domains used to construct the receptor, investigators said.
Based on those findings, tailoring CAR design based on signal strength might improve the efficacy and reduce the toxicity of CAR T-cell therapy, according to Alexander Salter, an MD/PhD student at Fred Hutchinson Cancer Research Center, Seattle, Wash.
In a press conference, Mr. Salter described results of the study, which used mass spectrometry to evaluate CARs encoding CD28 or 4-1BB costimulatory domains in primary human T cells.
While CARs with CD28 domains elicited more robust intracellular signaling than those with 4-1BB domains, there was considerable overlap in activation of T cell signaling pathways, Mr. Salter said.
That overlap was somewhat surprising, according to Mr. Salter, since researchers have generally assumed that CARs with CD28 and 4-1BB costimulatory domains will primarily signal through those respective pathways.
“No matter what costimulatory domain was encoded by the receptor, both CARs… activated both CD28 and 41BB signaling pathways,” Mr. Salter said.
The major determinant of efficacy in the study turned out to be not the domain used to construct the receptor, but the speed and strength of signaling, he added. In particular, the CARs that evoked stronger signals also had increased T cell dysfunction, decreasing their potency in the mouse lymphoma model.
The T cells with a CD28 CAR had very strong initial antitumor function that quickly waned in the mouse model of lymphoma; by contrast, the “slower burning” 4-1BB CAR signal led to T cells that better retained their function in vivo and were associated with longer median survival in the model, he said.
Those findings suggest tailoring CAR design based on signal strength may improve clinical efficacy and reduce toxicity.
As part of the study, Mr. Salter and his co-investigators were able to modify the CAR CD28 domain to make the signaling of the CD28 CARs less intense. “This is a modification that we think should be considered in future CAR design,” Mr. Salter said.
While the alterations in the CD28 signaling domain were able to reduce levels of cytokines produced by T cells, the study was primarily designed to look at the efficacy, noted Stanley Riddell, MD, scientific director of the Immunotherapy Integrated Research Center at Fred Hutchinson Cancer Research Center.
“Our models were not set up to address the question of toxicity, so we can’t directly say this would translate to what we would see in patients,” Dr. Riddell said during the press conference. “But I think we gleaned a lot of insights as to why cytokines are produced at greater or lesser levels with various CAR designs, and insights as to how to redesign these receptors to lower the levels of cytokines they make without compromising their ability to kill.”
Dr. Riddell is a founder, shareholder, and scientific advisor of Juno Therapeutics, and together with Mr. Salter, he has filed a patent application on the use of mutant CD28 CARs for cellular therapy. Co-author Raphael Gottardo, PhD, also with Fred Hutchinson Cancer Research Center, is a consultant for Juno Therapeutics. No other competing interests were reported.
SOURCE: Salter AI et al., Sci Signal. 2018 Aug 21;11. pii:eaat6753.
Contrary to what might be expected, chimeric antigen receptor (CAR) T cells with stronger signaling capabilities were less effective against lymphoma cells in a mouse model, investigators reported.
Intracellular signaling strength was a key determinant of T cell fate in the study, which was published in the journal Science Signaling.
By contrast, CAR signaling pathways could not be predicted solely by the costimulatory domains used to construct the receptor, investigators said.
Based on those findings, tailoring CAR design based on signal strength might improve the efficacy and reduce the toxicity of CAR T-cell therapy, according to Alexander Salter, an MD/PhD student at Fred Hutchinson Cancer Research Center, Seattle, Wash.
In a press conference, Mr. Salter described results of the study, which used mass spectrometry to evaluate CARs encoding CD28 or 4-1BB costimulatory domains in primary human T cells.
While CARs with CD28 domains elicited more robust intracellular signaling than those with 4-1BB domains, there was considerable overlap in activation of T cell signaling pathways, Mr. Salter said.
That overlap was somewhat surprising, according to Mr. Salter, since researchers have generally assumed that CARs with CD28 and 4-1BB costimulatory domains will primarily signal through those respective pathways.
“No matter what costimulatory domain was encoded by the receptor, both CARs… activated both CD28 and 41BB signaling pathways,” Mr. Salter said.
The major determinant of efficacy in the study turned out to be not the domain used to construct the receptor, but the speed and strength of signaling, he added. In particular, the CARs that evoked stronger signals also had increased T cell dysfunction, decreasing their potency in the mouse lymphoma model.
The T cells with a CD28 CAR had very strong initial antitumor function that quickly waned in the mouse model of lymphoma; by contrast, the “slower burning” 4-1BB CAR signal led to T cells that better retained their function in vivo and were associated with longer median survival in the model, he said.
Those findings suggest tailoring CAR design based on signal strength may improve clinical efficacy and reduce toxicity.
As part of the study, Mr. Salter and his co-investigators were able to modify the CAR CD28 domain to make the signaling of the CD28 CARs less intense. “This is a modification that we think should be considered in future CAR design,” Mr. Salter said.
While the alterations in the CD28 signaling domain were able to reduce levels of cytokines produced by T cells, the study was primarily designed to look at the efficacy, noted Stanley Riddell, MD, scientific director of the Immunotherapy Integrated Research Center at Fred Hutchinson Cancer Research Center.
“Our models were not set up to address the question of toxicity, so we can’t directly say this would translate to what we would see in patients,” Dr. Riddell said during the press conference. “But I think we gleaned a lot of insights as to why cytokines are produced at greater or lesser levels with various CAR designs, and insights as to how to redesign these receptors to lower the levels of cytokines they make without compromising their ability to kill.”
Dr. Riddell is a founder, shareholder, and scientific advisor of Juno Therapeutics, and together with Mr. Salter, he has filed a patent application on the use of mutant CD28 CARs for cellular therapy. Co-author Raphael Gottardo, PhD, also with Fred Hutchinson Cancer Research Center, is a consultant for Juno Therapeutics. No other competing interests were reported.
SOURCE: Salter AI et al., Sci Signal. 2018 Aug 21;11. pii:eaat6753.
FROM SCIENCE SIGNALING
Key clinical point:
Major finding: T cells with a CD28 CAR had very strong initial antitumor function that quickly waned in a mouse model of lymphoma, while the 4-1BB CAR signal led to T cells that better retained their function in vivo and had a longer median survival in the model.
Study details: Analysis of CARs encoding CD28 or 4-1BB costimulatory domains in primary human T cells using mass spectrometry, plus analysis of efficacy in a mouse model of lymphoma.
Disclosures: Study authors reported disclosures related to Juno therapeutics and a patent application related to use of mutant CD28 CARs for cellular therapy.
Source: Salter AI et al., Sci Signal. 2018 Aug 21;11. pii:eaat6753.
Bertilimumab granted orphan drug status for bullous pemphigoid
The , according to an announcement issued by the company that is developing the treatment.
The company, Immune Pharmaceuticals, plans to launch a pivotal phase 2/3 study in 2019, the company said in an Aug. 20 press release. Two phase 2 studies of bertilimumab are currently underway, one in patients with bullous pemphigoid, and another in patients with ulcerative colitis. Bertilimumab is “a first-in-class, fully human monoclonal antibody that targets and lowers levels of eotaxin-1, a chemokine that plays a role in immune responses and attracts eosinophils to the site of inflammation,” the statement said.“By neutralizing eotaxin-1, bertilimumab may prevent the migration of eosinophils and other cells, thus helping to relieve associated inflammatory conditions.”
The FDA’s Orphan Drug Designation program grants orphan status to drugs and biologics, which are “defined as those intended for the safe and effective treatment, diagnosis or prevention of rare diseases/disorders that affect fewer than 200,000 people in the United States, or that affect more than 200,000 persons but are not expected to recover the costs of developing and marketing a treatment drug,” according to the agency.
The , according to an announcement issued by the company that is developing the treatment.
The company, Immune Pharmaceuticals, plans to launch a pivotal phase 2/3 study in 2019, the company said in an Aug. 20 press release. Two phase 2 studies of bertilimumab are currently underway, one in patients with bullous pemphigoid, and another in patients with ulcerative colitis. Bertilimumab is “a first-in-class, fully human monoclonal antibody that targets and lowers levels of eotaxin-1, a chemokine that plays a role in immune responses and attracts eosinophils to the site of inflammation,” the statement said.“By neutralizing eotaxin-1, bertilimumab may prevent the migration of eosinophils and other cells, thus helping to relieve associated inflammatory conditions.”
The FDA’s Orphan Drug Designation program grants orphan status to drugs and biologics, which are “defined as those intended for the safe and effective treatment, diagnosis or prevention of rare diseases/disorders that affect fewer than 200,000 people in the United States, or that affect more than 200,000 persons but are not expected to recover the costs of developing and marketing a treatment drug,” according to the agency.
The , according to an announcement issued by the company that is developing the treatment.
The company, Immune Pharmaceuticals, plans to launch a pivotal phase 2/3 study in 2019, the company said in an Aug. 20 press release. Two phase 2 studies of bertilimumab are currently underway, one in patients with bullous pemphigoid, and another in patients with ulcerative colitis. Bertilimumab is “a first-in-class, fully human monoclonal antibody that targets and lowers levels of eotaxin-1, a chemokine that plays a role in immune responses and attracts eosinophils to the site of inflammation,” the statement said.“By neutralizing eotaxin-1, bertilimumab may prevent the migration of eosinophils and other cells, thus helping to relieve associated inflammatory conditions.”
The FDA’s Orphan Drug Designation program grants orphan status to drugs and biologics, which are “defined as those intended for the safe and effective treatment, diagnosis or prevention of rare diseases/disorders that affect fewer than 200,000 people in the United States, or that affect more than 200,000 persons but are not expected to recover the costs of developing and marketing a treatment drug,” according to the agency.
Oral prednisone is not effective for OME in older children
because many participants had spontaneous resolution of their symptoms, according to results from a randomized, parallel, double-blinded, placebo-controlled trial.
“If effective, a short course of oral steroids for otitis media with effusion would have been appealing as the treatment is generally well tolerated and would avoid more burdensome and expensive interventions such as ventilation tubes or hearing aids,” Nick A. Francis, PhD, of Cardiff University, Wales, and his colleagues wrote in the Lancet.
Dr. Francis and his colleagues enrolled 389 children aged 2-8 years with symptoms of OME from 20 ear, nose, and throat outpatient medical departments into the OSTRICH trial between March 2014 and April 2016, where they were randomized to receive a 7-day course of once daily oral prednisone at 20 mg for children aged 2-5 years and 30 mg for children 6-8 years (total of 200 patients), or placebo (189 patients). Some patients had symptoms of hearing loss from their condition so 183 participants in the oral steroid group and 180 participants in the placebo group underwent a hearing test 4 weeks after treatment.
At 5 weeks, Dr. Francis and his colleagues found that 40% of 183 patients in the oral steroid group and 53% of 180 in the placebo group achieved acceptable hearing after treatment, a small nonsignificant between group difference (absolute difference, 7%; 95% confidence interval, –3 to 17). However, the number needed to treat was 14 to 1, and there was a high number of spontaneous resolutions of symptoms in the study, they said. In addition, there were no between-group differences in adverse events or quality of life.
While they do not recommend routine oral steroids for children in this setting based on limited clinical significance, the investigators suggested oral steroids may be a “reasonable candidate intervention” for treatment of children in other patient populations.
“The high rate of spontaneous resolution identified in this study will support the evidence base informing discussions about watchful waiting in children with hearing loss associated with otitis media with effusion. Given the findings of some benefit from antibiotics for otitis media with effusion in children, and limited trial evidence for a benefit from oral steroids in combination with antibiotics, a rigorous trial of oral steroids combined with antibiotics might be indicated,” Dr. Francis and his colleagues wrote.
The OSTRICH Trial was funded by the National Institute for Health Research Health Technology Assessment program. The authors reported no conflicts of interest.
SOURCE: Francis NA et al. Lancet. 2018 Aug 18. doi: 10.1016/S0140-6736(18)31490-9.
The inclusion criteria for this study were age between 2 and 8 years, which is “generally older” than pediatric patients who develop otitis media with effusion, so the study can apply only to this patient population, Michael E. Pichichero, MD, wrote in a related editorial.
“The pathophysiology of otitis media with effusion and mechanistic work in animal models support the use of systematic steroids for clearing otitis media with effusion,” Dr. Pichichero said. “Given the evidence from trials suggesting that oral steroids alone and in combination with antibiotics help clear otitis media with effusion, the issue remains an open question for children who do not meet the enrollment criteria applied in the current trial.”
Further, he noted that because two-thirds of patients had the condition for a minimum of 12 months were likely to have high viscosity fluid, so-called glue ear, a “temporary opening of the Eustachian tube with 1 week of oral steroids might not have been a fair test.” In addition, pediatric patients with otitis media have a high rate of spontaneous resolution, he said, and noted that Francis et al. addressed the evidence gap with their research.
“Apart from a clear answer about the effect of oral steroids in children aged 2-8 years with prolonged otitis media with effusion, the unique natural history data of otitis media with effusion provided in Francis and colleagues’ study will help inform discussions about whether to opt for grommet surgery or to continue with watchful waiting,” Dr. Pichichero said.
Dr. Pichichero is with the Rochester (N.Y.) General Hospital Research Institute. He reports no relevant conflicts of interest. These comments summarize his editorial on the article by Francis et al. (Lancet. 2018 Aug 18. doi: 10.1016/S0140-6736[18]31862-2 .)
The inclusion criteria for this study were age between 2 and 8 years, which is “generally older” than pediatric patients who develop otitis media with effusion, so the study can apply only to this patient population, Michael E. Pichichero, MD, wrote in a related editorial.
“The pathophysiology of otitis media with effusion and mechanistic work in animal models support the use of systematic steroids for clearing otitis media with effusion,” Dr. Pichichero said. “Given the evidence from trials suggesting that oral steroids alone and in combination with antibiotics help clear otitis media with effusion, the issue remains an open question for children who do not meet the enrollment criteria applied in the current trial.”
Further, he noted that because two-thirds of patients had the condition for a minimum of 12 months were likely to have high viscosity fluid, so-called glue ear, a “temporary opening of the Eustachian tube with 1 week of oral steroids might not have been a fair test.” In addition, pediatric patients with otitis media have a high rate of spontaneous resolution, he said, and noted that Francis et al. addressed the evidence gap with their research.
“Apart from a clear answer about the effect of oral steroids in children aged 2-8 years with prolonged otitis media with effusion, the unique natural history data of otitis media with effusion provided in Francis and colleagues’ study will help inform discussions about whether to opt for grommet surgery or to continue with watchful waiting,” Dr. Pichichero said.
Dr. Pichichero is with the Rochester (N.Y.) General Hospital Research Institute. He reports no relevant conflicts of interest. These comments summarize his editorial on the article by Francis et al. (Lancet. 2018 Aug 18. doi: 10.1016/S0140-6736[18]31862-2 .)
The inclusion criteria for this study were age between 2 and 8 years, which is “generally older” than pediatric patients who develop otitis media with effusion, so the study can apply only to this patient population, Michael E. Pichichero, MD, wrote in a related editorial.
“The pathophysiology of otitis media with effusion and mechanistic work in animal models support the use of systematic steroids for clearing otitis media with effusion,” Dr. Pichichero said. “Given the evidence from trials suggesting that oral steroids alone and in combination with antibiotics help clear otitis media with effusion, the issue remains an open question for children who do not meet the enrollment criteria applied in the current trial.”
Further, he noted that because two-thirds of patients had the condition for a minimum of 12 months were likely to have high viscosity fluid, so-called glue ear, a “temporary opening of the Eustachian tube with 1 week of oral steroids might not have been a fair test.” In addition, pediatric patients with otitis media have a high rate of spontaneous resolution, he said, and noted that Francis et al. addressed the evidence gap with their research.
“Apart from a clear answer about the effect of oral steroids in children aged 2-8 years with prolonged otitis media with effusion, the unique natural history data of otitis media with effusion provided in Francis and colleagues’ study will help inform discussions about whether to opt for grommet surgery or to continue with watchful waiting,” Dr. Pichichero said.
Dr. Pichichero is with the Rochester (N.Y.) General Hospital Research Institute. He reports no relevant conflicts of interest. These comments summarize his editorial on the article by Francis et al. (Lancet. 2018 Aug 18. doi: 10.1016/S0140-6736[18]31862-2 .)
because many participants had spontaneous resolution of their symptoms, according to results from a randomized, parallel, double-blinded, placebo-controlled trial.
“If effective, a short course of oral steroids for otitis media with effusion would have been appealing as the treatment is generally well tolerated and would avoid more burdensome and expensive interventions such as ventilation tubes or hearing aids,” Nick A. Francis, PhD, of Cardiff University, Wales, and his colleagues wrote in the Lancet.
Dr. Francis and his colleagues enrolled 389 children aged 2-8 years with symptoms of OME from 20 ear, nose, and throat outpatient medical departments into the OSTRICH trial between March 2014 and April 2016, where they were randomized to receive a 7-day course of once daily oral prednisone at 20 mg for children aged 2-5 years and 30 mg for children 6-8 years (total of 200 patients), or placebo (189 patients). Some patients had symptoms of hearing loss from their condition so 183 participants in the oral steroid group and 180 participants in the placebo group underwent a hearing test 4 weeks after treatment.
At 5 weeks, Dr. Francis and his colleagues found that 40% of 183 patients in the oral steroid group and 53% of 180 in the placebo group achieved acceptable hearing after treatment, a small nonsignificant between group difference (absolute difference, 7%; 95% confidence interval, –3 to 17). However, the number needed to treat was 14 to 1, and there was a high number of spontaneous resolutions of symptoms in the study, they said. In addition, there were no between-group differences in adverse events or quality of life.
While they do not recommend routine oral steroids for children in this setting based on limited clinical significance, the investigators suggested oral steroids may be a “reasonable candidate intervention” for treatment of children in other patient populations.
“The high rate of spontaneous resolution identified in this study will support the evidence base informing discussions about watchful waiting in children with hearing loss associated with otitis media with effusion. Given the findings of some benefit from antibiotics for otitis media with effusion in children, and limited trial evidence for a benefit from oral steroids in combination with antibiotics, a rigorous trial of oral steroids combined with antibiotics might be indicated,” Dr. Francis and his colleagues wrote.
The OSTRICH Trial was funded by the National Institute for Health Research Health Technology Assessment program. The authors reported no conflicts of interest.
SOURCE: Francis NA et al. Lancet. 2018 Aug 18. doi: 10.1016/S0140-6736(18)31490-9.
because many participants had spontaneous resolution of their symptoms, according to results from a randomized, parallel, double-blinded, placebo-controlled trial.
“If effective, a short course of oral steroids for otitis media with effusion would have been appealing as the treatment is generally well tolerated and would avoid more burdensome and expensive interventions such as ventilation tubes or hearing aids,” Nick A. Francis, PhD, of Cardiff University, Wales, and his colleagues wrote in the Lancet.
Dr. Francis and his colleagues enrolled 389 children aged 2-8 years with symptoms of OME from 20 ear, nose, and throat outpatient medical departments into the OSTRICH trial between March 2014 and April 2016, where they were randomized to receive a 7-day course of once daily oral prednisone at 20 mg for children aged 2-5 years and 30 mg for children 6-8 years (total of 200 patients), or placebo (189 patients). Some patients had symptoms of hearing loss from their condition so 183 participants in the oral steroid group and 180 participants in the placebo group underwent a hearing test 4 weeks after treatment.
At 5 weeks, Dr. Francis and his colleagues found that 40% of 183 patients in the oral steroid group and 53% of 180 in the placebo group achieved acceptable hearing after treatment, a small nonsignificant between group difference (absolute difference, 7%; 95% confidence interval, –3 to 17). However, the number needed to treat was 14 to 1, and there was a high number of spontaneous resolutions of symptoms in the study, they said. In addition, there were no between-group differences in adverse events or quality of life.
While they do not recommend routine oral steroids for children in this setting based on limited clinical significance, the investigators suggested oral steroids may be a “reasonable candidate intervention” for treatment of children in other patient populations.
“The high rate of spontaneous resolution identified in this study will support the evidence base informing discussions about watchful waiting in children with hearing loss associated with otitis media with effusion. Given the findings of some benefit from antibiotics for otitis media with effusion in children, and limited trial evidence for a benefit from oral steroids in combination with antibiotics, a rigorous trial of oral steroids combined with antibiotics might be indicated,” Dr. Francis and his colleagues wrote.
The OSTRICH Trial was funded by the National Institute for Health Research Health Technology Assessment program. The authors reported no conflicts of interest.
SOURCE: Francis NA et al. Lancet. 2018 Aug 18. doi: 10.1016/S0140-6736(18)31490-9.
FROM THE LANCET
Key clinical point: Oral prednisone was well tolerated by children with otitis media with effusion, but there was a high rate of spontaneous resolution in the trial.
Major finding: Acceptable hearing was achieved in 73 of 183 children in the oral steroid group and 59 of 180 children in the placebo group, with a number needed to treat of 14.
Study details: A randomized, parallel, double-blinded, placebo-controlled trial of 389 children aged 2-8 years with symptoms of otitis media with effusion.
Disclosures: The OSTRICH Trial was funded by the National Institute for Health Research Health Technology Assessment program. The authors report no conflicts of interest.
Source: Francis NA et al. Lancet. 2018 Aug 18. doi: 10.1016/S0140-6736(18)31490-9.
CMS finalizes CAR T-cell therapy inpatient payments
Medical associations are expressing disappointment at the new payment scheme put forward by the Centers for Medicare & Medicaid Services for inpatient administration of two chimeric antigen receptor (CAR) T-cell therapies, calling the reimbursement insufficient for use of the expensive medications.
Under its Aug. 17 final rule, CMS will now categorize CAR T-cell therapies under the umbrella of the renamed Medicare Severity–Diagnosis Related Groups (MS-DRG) 016 – Autologous Bone Marrow Transplant with CC/MCC or T-cell Immunotherapy – and assign ICD-10-PCS procedure codes XW033C3 and XW043C3 to the use of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) in the inpatient setting for fiscal year 2019, which begins in October 2018.
CMS also approved a temporary New Technology Add-On Payment (NTAP) for use of the therapies with a maximum threshold of $186,500, according to the rule.
According to the American Society of Hematology (ASH), this payment structure is an improvement, but it hardly covers the cost of the products, nor does it account for full hospitalization costs. ASH noted that the revised MS-DRG 016 has a base payment rate of $36,000 and that the maximum NTAP payment ($186,500) is only about half of the cost for a CAR T-cell product.
“ASH is concerned that this final policy may impede access to care to this cutting-edge therapy because hospitals and academic medical centers that provide this personalized treatment will simply not be able to withstand the negative financial impact,” the society said in a statement. “While this final policy represents an improvement over current CAR T therapy reimbursement rates, ASH believes patient access to care will be jeopardized as providers and hospitals will not be able to afford to deliver the therapy at this reimbursement rate, particularly as other CAR T products receive FDA [Food and Drug Administration] approval.”
ASH and the American Society for Blood and Marrow Transplantation (ASBMT) had strongly urged CMS to develop a site-neutral, equitable payment structure that would have allowed providers to recover more product acquisition costs from CAR T-cell therapies. In its final rule, CMS stated that it was too early to develop a novel payment structure for CAR T-cell treatments and that more research is needed before such changes are made. The agency noted that in May CMS opened a national coverage determination analysis on CAR T-cell therapy for Medicare patients with advanced cancer, which is expected to be completed by May 2019.
“[CMS] is soliciting public comment … on key design considerations for developing a potential model that would test private market strategies and introduce competition to improve quality of care for beneficiaries,” the agency said in the rule. “Given the relative newness of CAR T-cell therapy, the potential model, and our request for feedback on this model approach, we believe it would be premature to adopt changes to our existing payment mechanisms.”
The payment outline by CMS is essentially the bare minimum it could have extended to CAR T-cell therapies for 2019, said Stephanie Farnia, director of health policy and strategic relations for the ASBMT.
“[ASBMT] and a number of stakeholders have been very clear in our comment letters that that would not be enough and the reasons why,” Ms. Farnia said in an interview. “It’s not going to be sufficient to cover the cost of care or the product.”
The rule also fails to address the cancer centers that are exempt from the DRG payment system, Ms. Farnia said. Eleven centers are excluded from the payment system because of past legislation that excludes exclusive cancer hospitals that do not provide noncancer services. The exempt cancer centers cannot receive additional money for new or expensive drugs and therefore will not gain any financial relief from the CAR T-cell therapy payment changes in the CMS final rule.
ASH officials plan to follow up with congressional leaders to identify ways to improve future CAR T-cell therapy payments, including a potential legislative solution. An ASH spokesperson declined to elaborate on its ideal legislative remedy.
Hospital administrators and physicians will need to have difficult conversations in the upcoming year about whether treating patients with CAR T-cell therapies is worth the cost deficits, Ms. Farnia said.
“Everyone was really counting on it being a different reimbursement scenario for the upcoming fiscal year, and it is, but again, it’s that bare minimum difference,” Ms. Farnia said. “I think a number of programs are going to be taking a look at their financial experience thus far and comparing that to the reimbursement and deciding on if they [should] continue to offer it and how to do that.”
In April 2018, CMS announced payment rates for outpatient administration of the two drugs, settling on $395,380 for axicabtagene ciloleucel and $500,839 for tisagenlecleucel. The two medications have list prices of $373,000 and $475,000, respectively.
However, physicians have raised concerns that even if the drugs are first administered in the outpatient setting, inpatient care is likely to occur with CAR T-cell therapies because some patients will need to be admitted in order to be monitored for serious side effects. In such cases, all payments will become part of the inpatient stay under CMS’s 3-day payment window rule.
Medical associations are expressing disappointment at the new payment scheme put forward by the Centers for Medicare & Medicaid Services for inpatient administration of two chimeric antigen receptor (CAR) T-cell therapies, calling the reimbursement insufficient for use of the expensive medications.
Under its Aug. 17 final rule, CMS will now categorize CAR T-cell therapies under the umbrella of the renamed Medicare Severity–Diagnosis Related Groups (MS-DRG) 016 – Autologous Bone Marrow Transplant with CC/MCC or T-cell Immunotherapy – and assign ICD-10-PCS procedure codes XW033C3 and XW043C3 to the use of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) in the inpatient setting for fiscal year 2019, which begins in October 2018.
CMS also approved a temporary New Technology Add-On Payment (NTAP) for use of the therapies with a maximum threshold of $186,500, according to the rule.
According to the American Society of Hematology (ASH), this payment structure is an improvement, but it hardly covers the cost of the products, nor does it account for full hospitalization costs. ASH noted that the revised MS-DRG 016 has a base payment rate of $36,000 and that the maximum NTAP payment ($186,500) is only about half of the cost for a CAR T-cell product.
“ASH is concerned that this final policy may impede access to care to this cutting-edge therapy because hospitals and academic medical centers that provide this personalized treatment will simply not be able to withstand the negative financial impact,” the society said in a statement. “While this final policy represents an improvement over current CAR T therapy reimbursement rates, ASH believes patient access to care will be jeopardized as providers and hospitals will not be able to afford to deliver the therapy at this reimbursement rate, particularly as other CAR T products receive FDA [Food and Drug Administration] approval.”
ASH and the American Society for Blood and Marrow Transplantation (ASBMT) had strongly urged CMS to develop a site-neutral, equitable payment structure that would have allowed providers to recover more product acquisition costs from CAR T-cell therapies. In its final rule, CMS stated that it was too early to develop a novel payment structure for CAR T-cell treatments and that more research is needed before such changes are made. The agency noted that in May CMS opened a national coverage determination analysis on CAR T-cell therapy for Medicare patients with advanced cancer, which is expected to be completed by May 2019.
“[CMS] is soliciting public comment … on key design considerations for developing a potential model that would test private market strategies and introduce competition to improve quality of care for beneficiaries,” the agency said in the rule. “Given the relative newness of CAR T-cell therapy, the potential model, and our request for feedback on this model approach, we believe it would be premature to adopt changes to our existing payment mechanisms.”
The payment outline by CMS is essentially the bare minimum it could have extended to CAR T-cell therapies for 2019, said Stephanie Farnia, director of health policy and strategic relations for the ASBMT.
“[ASBMT] and a number of stakeholders have been very clear in our comment letters that that would not be enough and the reasons why,” Ms. Farnia said in an interview. “It’s not going to be sufficient to cover the cost of care or the product.”
The rule also fails to address the cancer centers that are exempt from the DRG payment system, Ms. Farnia said. Eleven centers are excluded from the payment system because of past legislation that excludes exclusive cancer hospitals that do not provide noncancer services. The exempt cancer centers cannot receive additional money for new or expensive drugs and therefore will not gain any financial relief from the CAR T-cell therapy payment changes in the CMS final rule.
ASH officials plan to follow up with congressional leaders to identify ways to improve future CAR T-cell therapy payments, including a potential legislative solution. An ASH spokesperson declined to elaborate on its ideal legislative remedy.
Hospital administrators and physicians will need to have difficult conversations in the upcoming year about whether treating patients with CAR T-cell therapies is worth the cost deficits, Ms. Farnia said.
“Everyone was really counting on it being a different reimbursement scenario for the upcoming fiscal year, and it is, but again, it’s that bare minimum difference,” Ms. Farnia said. “I think a number of programs are going to be taking a look at their financial experience thus far and comparing that to the reimbursement and deciding on if they [should] continue to offer it and how to do that.”
In April 2018, CMS announced payment rates for outpatient administration of the two drugs, settling on $395,380 for axicabtagene ciloleucel and $500,839 for tisagenlecleucel. The two medications have list prices of $373,000 and $475,000, respectively.
However, physicians have raised concerns that even if the drugs are first administered in the outpatient setting, inpatient care is likely to occur with CAR T-cell therapies because some patients will need to be admitted in order to be monitored for serious side effects. In such cases, all payments will become part of the inpatient stay under CMS’s 3-day payment window rule.
Medical associations are expressing disappointment at the new payment scheme put forward by the Centers for Medicare & Medicaid Services for inpatient administration of two chimeric antigen receptor (CAR) T-cell therapies, calling the reimbursement insufficient for use of the expensive medications.
Under its Aug. 17 final rule, CMS will now categorize CAR T-cell therapies under the umbrella of the renamed Medicare Severity–Diagnosis Related Groups (MS-DRG) 016 – Autologous Bone Marrow Transplant with CC/MCC or T-cell Immunotherapy – and assign ICD-10-PCS procedure codes XW033C3 and XW043C3 to the use of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) in the inpatient setting for fiscal year 2019, which begins in October 2018.
CMS also approved a temporary New Technology Add-On Payment (NTAP) for use of the therapies with a maximum threshold of $186,500, according to the rule.
According to the American Society of Hematology (ASH), this payment structure is an improvement, but it hardly covers the cost of the products, nor does it account for full hospitalization costs. ASH noted that the revised MS-DRG 016 has a base payment rate of $36,000 and that the maximum NTAP payment ($186,500) is only about half of the cost for a CAR T-cell product.
“ASH is concerned that this final policy may impede access to care to this cutting-edge therapy because hospitals and academic medical centers that provide this personalized treatment will simply not be able to withstand the negative financial impact,” the society said in a statement. “While this final policy represents an improvement over current CAR T therapy reimbursement rates, ASH believes patient access to care will be jeopardized as providers and hospitals will not be able to afford to deliver the therapy at this reimbursement rate, particularly as other CAR T products receive FDA [Food and Drug Administration] approval.”
ASH and the American Society for Blood and Marrow Transplantation (ASBMT) had strongly urged CMS to develop a site-neutral, equitable payment structure that would have allowed providers to recover more product acquisition costs from CAR T-cell therapies. In its final rule, CMS stated that it was too early to develop a novel payment structure for CAR T-cell treatments and that more research is needed before such changes are made. The agency noted that in May CMS opened a national coverage determination analysis on CAR T-cell therapy for Medicare patients with advanced cancer, which is expected to be completed by May 2019.
“[CMS] is soliciting public comment … on key design considerations for developing a potential model that would test private market strategies and introduce competition to improve quality of care for beneficiaries,” the agency said in the rule. “Given the relative newness of CAR T-cell therapy, the potential model, and our request for feedback on this model approach, we believe it would be premature to adopt changes to our existing payment mechanisms.”
The payment outline by CMS is essentially the bare minimum it could have extended to CAR T-cell therapies for 2019, said Stephanie Farnia, director of health policy and strategic relations for the ASBMT.
“[ASBMT] and a number of stakeholders have been very clear in our comment letters that that would not be enough and the reasons why,” Ms. Farnia said in an interview. “It’s not going to be sufficient to cover the cost of care or the product.”
The rule also fails to address the cancer centers that are exempt from the DRG payment system, Ms. Farnia said. Eleven centers are excluded from the payment system because of past legislation that excludes exclusive cancer hospitals that do not provide noncancer services. The exempt cancer centers cannot receive additional money for new or expensive drugs and therefore will not gain any financial relief from the CAR T-cell therapy payment changes in the CMS final rule.
ASH officials plan to follow up with congressional leaders to identify ways to improve future CAR T-cell therapy payments, including a potential legislative solution. An ASH spokesperson declined to elaborate on its ideal legislative remedy.
Hospital administrators and physicians will need to have difficult conversations in the upcoming year about whether treating patients with CAR T-cell therapies is worth the cost deficits, Ms. Farnia said.
“Everyone was really counting on it being a different reimbursement scenario for the upcoming fiscal year, and it is, but again, it’s that bare minimum difference,” Ms. Farnia said. “I think a number of programs are going to be taking a look at their financial experience thus far and comparing that to the reimbursement and deciding on if they [should] continue to offer it and how to do that.”
In April 2018, CMS announced payment rates for outpatient administration of the two drugs, settling on $395,380 for axicabtagene ciloleucel and $500,839 for tisagenlecleucel. The two medications have list prices of $373,000 and $475,000, respectively.
However, physicians have raised concerns that even if the drugs are first administered in the outpatient setting, inpatient care is likely to occur with CAR T-cell therapies because some patients will need to be admitted in order to be monitored for serious side effects. In such cases, all payments will become part of the inpatient stay under CMS’s 3-day payment window rule.
Cervical cancer screening recommendations vary by age and risk
Screen women for cervical cancer with basic cytology starting at age 21 years, and consider adding high-risk human papillomavirus (hrHPV) testing alone or with cytology for women aged 30 years and older, the U.S. Preventive Services Task Force recommended in an updated statement on cervical cancer screening .
The statement, accompanying evidence report, and a modeling study were published online in JAMA.
Cervical cancer deaths in the United States have declined from 2.8 deaths per 100,000 women in 2000 to 2.3 deaths per 100,000 women in 2015 because of the adoption of widespread screening, according to Susan J. Curry, PhD., of the University of Iowa, Iowa City, and her colleagues in the USPSTF (JAMA. 2018 Aug 21. doi: 10.1001/jama.2018.10897.
Based on the latest evidence and the modeling study, the USPSTF gives an A recommendation to screening women aged 21-29 years for cervical cancer every 3 years with cervical cytology alone. The task force also gives an A to screening women aged 30-65 years every 5 years with either hrHPV testing alone or in combination with cytology.
The task force recommends against screening (D recommendation) for women younger than 21 years, older than 65 years with a history of screening and low cervical cancer risk, and women who have had hysterectomies with removal of the cervix and no history of cervical cancer risk.
To update the previous recommendations issued in 2012, the task force reviewed the latest evidence and commissioned a modeling study to help determine the best screening strategies in terms of age, screening intervals, and risks vs. benefits.
In the model, researchers assessed 19 strategies for cervical cancer screening based on a hypothetical cohort of women who began screening at 21 years of age.
Overall, the different strategies were similar in effectiveness, but primary hrHPV testing and alternative cotesting were slightly more effective: Cervical cancer deaths ranged from 0.23 to 0.29 deaths per 1,000 women in strategies involving hrHPV testing or cotesting, vs. 0.30 to 0.76 deaths per 1,000 women for strategies based on the current guidelines.
In addition, switching the age of hrHPV testing from 25 years to 30 years and using a 5-year screening interval showed the most effectiveness in terms of risks vs. harms, wrote Jane K. Kim, PhD, of Harvard University, Boston, and her colleagues (JAMA. 2018 Aug 21. doi: 10.1001/jama.2017.19872). “Switching from cytology to 5-year primary hrHPV testing at age 30 years (strategy 14) was associated with a ratio of 640 colposcopies per cancer case averted; earlier switch ages required a greater number of colposcopies per cancer case averted.”
The recommendations also were supported by an evidence report including eight randomized, controlled trials of 410,556 women, five cohort studies of 402,615 women, and a meta-analysis of individual participant data including 176,464 women.
The evidence report sought to address the benefits and harms of cervical cancer screening using hrHPV screening alone as the primary screening method or paired with cytology (cotesting), compared with primary screening using cytology alone.
Overall, both hrHPV and hrHPV plus cytology were associated with higher rates of false-positives and colposcopy compared with cytology alone, “which could lead to more treatments with potential harms,” wrote Joy Melnikow, MD, of the University of California, Davis, and her colleagues (JAMA. 2018 Aug 21. doi: 10.1001/jama.2018.10400.
In addition, hrHPV testing yielded higher rates of positive cervical intraepithelial neoplasia, compared with cytology alone as initial screening.
However, further research is needed to address the impact of any cervical cancer screening strategies in populations with limited access to health care and screening, the researchers noted.
The updated USPSTF recommendations are largely in line with those issued by leading women’s health organizations including the American College of Obstetricians and Gynecologists, ASCCP, and the Society for Gynecologic Oncology, according to a joint statement.
“With a number of screening options now available, the new guidelines emphasize the importance of the patient-provider shared decision-making process to assist women in making an informed choice about which screening method is most suitable for them,” according to the statement, “However, more importantly, there needs to be a continued effort to ensure all women are adequately screened because a significant number of women in the country are not. It’s also essential for women to have access to all of the tests and that they are appropriately covered by insurance companies.
“We hope the USPSTF recommendations foster more discussions between patients and providers about cervical cancer screening, promote opportunities for patient education on the benefits and safety of HPV vaccination for cervical cancer prevention and encourage providers to offer HPV vaccines in their offices,” the statement noted.
The USPSTF research was funded by the Agency for Healthcare Research and Quality. The researchers for the modeling report were supported in part by a National Cancer Institute grant. The researchers had no relevant financial conflicts to disclose.
SOURCES: Kim J et al. JAMA. 2018 Aug 21. doi: 10.1001/jama.2017.19872; Melnikow J et al. JAMA. 2018 Aug 21. doi: 10.1001/jama.2018.10400; Curry S et al. JAMA. 2018 Aug 21. doi: 10.1001/jama.2018.10897.
In 2016, the Society for Gynecologic Oncology (SGO) recommended screening with the newly approved hrHPV test for women aged 25 years and older, with rescreening 3 years later if the test was negative, George F. Sawaya, MD, wrote in an accompanying editorial published in JAMA Internal Medicine. The new recommendations from the U.S. Preventive Services Task Force do not endorse a single triage strategy, and do not consider costs, he said.
“Although the USPSTF sets the standard for evidence-based recommendations and acknowledges the critical value of high-quality evidence in making recommendations, it might reasonably be asked, where is the evidence of value in cervical cancer screening?” Dr. Sawaya wrote.
The updated USPSTF recommendations differ from the SGO recommendation by changing the starting age for hrHPV testing to 30 years from 25, and rescreening at 5-year intervals.
“The USPSTF recommendation that HPV testing not begin until age 30 years seems prudent,” Dr. Sawaya said, in light of the evidence report and modeling analysis of harms and benefits. He noted that the evidence reviewed by the task force showed that HPV testing and cotesting resulting in a small amount of life-years gained compared with no testing, but with the trade-off of more follow-up tests and colposcopies.
“From the perspective of society, it has been proposed that cost-effectiveness analyses be an essential part of the guideline process,” Dr. Sawaya noted. “To assist in policy decisions that many professional societies will soon face, a study that I am leading is seeking to use cost-effectiveness analyses to determine the range of reasonable options for cervical cancer screening. Such analyses may inform future screening recommendations.”
Dr. Sawaya is affiliated with the University of California, San Francisco. These comments are taken from an editorial accompanying USPSTF recommendations on cervical cancer screening (JAMA Intern Med. 2018 Aug 21. doi: 10.1001/jamainternmed.2018.4282). He disclosed serving as the principal investigator of a National Cancer Institute study on cost-effectiveness analyses to determine reasonable options for cervical cancer screening. He also served as a member of the U.S. Preventive Services Task Force from 2004 to 2008.
In 2016, the Society for Gynecologic Oncology (SGO) recommended screening with the newly approved hrHPV test for women aged 25 years and older, with rescreening 3 years later if the test was negative, George F. Sawaya, MD, wrote in an accompanying editorial published in JAMA Internal Medicine. The new recommendations from the U.S. Preventive Services Task Force do not endorse a single triage strategy, and do not consider costs, he said.
“Although the USPSTF sets the standard for evidence-based recommendations and acknowledges the critical value of high-quality evidence in making recommendations, it might reasonably be asked, where is the evidence of value in cervical cancer screening?” Dr. Sawaya wrote.
The updated USPSTF recommendations differ from the SGO recommendation by changing the starting age for hrHPV testing to 30 years from 25, and rescreening at 5-year intervals.
“The USPSTF recommendation that HPV testing not begin until age 30 years seems prudent,” Dr. Sawaya said, in light of the evidence report and modeling analysis of harms and benefits. He noted that the evidence reviewed by the task force showed that HPV testing and cotesting resulting in a small amount of life-years gained compared with no testing, but with the trade-off of more follow-up tests and colposcopies.
“From the perspective of society, it has been proposed that cost-effectiveness analyses be an essential part of the guideline process,” Dr. Sawaya noted. “To assist in policy decisions that many professional societies will soon face, a study that I am leading is seeking to use cost-effectiveness analyses to determine the range of reasonable options for cervical cancer screening. Such analyses may inform future screening recommendations.”
Dr. Sawaya is affiliated with the University of California, San Francisco. These comments are taken from an editorial accompanying USPSTF recommendations on cervical cancer screening (JAMA Intern Med. 2018 Aug 21. doi: 10.1001/jamainternmed.2018.4282). He disclosed serving as the principal investigator of a National Cancer Institute study on cost-effectiveness analyses to determine reasonable options for cervical cancer screening. He also served as a member of the U.S. Preventive Services Task Force from 2004 to 2008.
In 2016, the Society for Gynecologic Oncology (SGO) recommended screening with the newly approved hrHPV test for women aged 25 years and older, with rescreening 3 years later if the test was negative, George F. Sawaya, MD, wrote in an accompanying editorial published in JAMA Internal Medicine. The new recommendations from the U.S. Preventive Services Task Force do not endorse a single triage strategy, and do not consider costs, he said.
“Although the USPSTF sets the standard for evidence-based recommendations and acknowledges the critical value of high-quality evidence in making recommendations, it might reasonably be asked, where is the evidence of value in cervical cancer screening?” Dr. Sawaya wrote.
The updated USPSTF recommendations differ from the SGO recommendation by changing the starting age for hrHPV testing to 30 years from 25, and rescreening at 5-year intervals.
“The USPSTF recommendation that HPV testing not begin until age 30 years seems prudent,” Dr. Sawaya said, in light of the evidence report and modeling analysis of harms and benefits. He noted that the evidence reviewed by the task force showed that HPV testing and cotesting resulting in a small amount of life-years gained compared with no testing, but with the trade-off of more follow-up tests and colposcopies.
“From the perspective of society, it has been proposed that cost-effectiveness analyses be an essential part of the guideline process,” Dr. Sawaya noted. “To assist in policy decisions that many professional societies will soon face, a study that I am leading is seeking to use cost-effectiveness analyses to determine the range of reasonable options for cervical cancer screening. Such analyses may inform future screening recommendations.”
Dr. Sawaya is affiliated with the University of California, San Francisco. These comments are taken from an editorial accompanying USPSTF recommendations on cervical cancer screening (JAMA Intern Med. 2018 Aug 21. doi: 10.1001/jamainternmed.2018.4282). He disclosed serving as the principal investigator of a National Cancer Institute study on cost-effectiveness analyses to determine reasonable options for cervical cancer screening. He also served as a member of the U.S. Preventive Services Task Force from 2004 to 2008.
Screen women for cervical cancer with basic cytology starting at age 21 years, and consider adding high-risk human papillomavirus (hrHPV) testing alone or with cytology for women aged 30 years and older, the U.S. Preventive Services Task Force recommended in an updated statement on cervical cancer screening .
The statement, accompanying evidence report, and a modeling study were published online in JAMA.
Cervical cancer deaths in the United States have declined from 2.8 deaths per 100,000 women in 2000 to 2.3 deaths per 100,000 women in 2015 because of the adoption of widespread screening, according to Susan J. Curry, PhD., of the University of Iowa, Iowa City, and her colleagues in the USPSTF (JAMA. 2018 Aug 21. doi: 10.1001/jama.2018.10897.
Based on the latest evidence and the modeling study, the USPSTF gives an A recommendation to screening women aged 21-29 years for cervical cancer every 3 years with cervical cytology alone. The task force also gives an A to screening women aged 30-65 years every 5 years with either hrHPV testing alone or in combination with cytology.
The task force recommends against screening (D recommendation) for women younger than 21 years, older than 65 years with a history of screening and low cervical cancer risk, and women who have had hysterectomies with removal of the cervix and no history of cervical cancer risk.
To update the previous recommendations issued in 2012, the task force reviewed the latest evidence and commissioned a modeling study to help determine the best screening strategies in terms of age, screening intervals, and risks vs. benefits.
In the model, researchers assessed 19 strategies for cervical cancer screening based on a hypothetical cohort of women who began screening at 21 years of age.
Overall, the different strategies were similar in effectiveness, but primary hrHPV testing and alternative cotesting were slightly more effective: Cervical cancer deaths ranged from 0.23 to 0.29 deaths per 1,000 women in strategies involving hrHPV testing or cotesting, vs. 0.30 to 0.76 deaths per 1,000 women for strategies based on the current guidelines.
In addition, switching the age of hrHPV testing from 25 years to 30 years and using a 5-year screening interval showed the most effectiveness in terms of risks vs. harms, wrote Jane K. Kim, PhD, of Harvard University, Boston, and her colleagues (JAMA. 2018 Aug 21. doi: 10.1001/jama.2017.19872). “Switching from cytology to 5-year primary hrHPV testing at age 30 years (strategy 14) was associated with a ratio of 640 colposcopies per cancer case averted; earlier switch ages required a greater number of colposcopies per cancer case averted.”
The recommendations also were supported by an evidence report including eight randomized, controlled trials of 410,556 women, five cohort studies of 402,615 women, and a meta-analysis of individual participant data including 176,464 women.
The evidence report sought to address the benefits and harms of cervical cancer screening using hrHPV screening alone as the primary screening method or paired with cytology (cotesting), compared with primary screening using cytology alone.
Overall, both hrHPV and hrHPV plus cytology were associated with higher rates of false-positives and colposcopy compared with cytology alone, “which could lead to more treatments with potential harms,” wrote Joy Melnikow, MD, of the University of California, Davis, and her colleagues (JAMA. 2018 Aug 21. doi: 10.1001/jama.2018.10400.
In addition, hrHPV testing yielded higher rates of positive cervical intraepithelial neoplasia, compared with cytology alone as initial screening.
However, further research is needed to address the impact of any cervical cancer screening strategies in populations with limited access to health care and screening, the researchers noted.
The updated USPSTF recommendations are largely in line with those issued by leading women’s health organizations including the American College of Obstetricians and Gynecologists, ASCCP, and the Society for Gynecologic Oncology, according to a joint statement.
“With a number of screening options now available, the new guidelines emphasize the importance of the patient-provider shared decision-making process to assist women in making an informed choice about which screening method is most suitable for them,” according to the statement, “However, more importantly, there needs to be a continued effort to ensure all women are adequately screened because a significant number of women in the country are not. It’s also essential for women to have access to all of the tests and that they are appropriately covered by insurance companies.
“We hope the USPSTF recommendations foster more discussions between patients and providers about cervical cancer screening, promote opportunities for patient education on the benefits and safety of HPV vaccination for cervical cancer prevention and encourage providers to offer HPV vaccines in their offices,” the statement noted.
The USPSTF research was funded by the Agency for Healthcare Research and Quality. The researchers for the modeling report were supported in part by a National Cancer Institute grant. The researchers had no relevant financial conflicts to disclose.
SOURCES: Kim J et al. JAMA. 2018 Aug 21. doi: 10.1001/jama.2017.19872; Melnikow J et al. JAMA. 2018 Aug 21. doi: 10.1001/jama.2018.10400; Curry S et al. JAMA. 2018 Aug 21. doi: 10.1001/jama.2018.10897.
Screen women for cervical cancer with basic cytology starting at age 21 years, and consider adding high-risk human papillomavirus (hrHPV) testing alone or with cytology for women aged 30 years and older, the U.S. Preventive Services Task Force recommended in an updated statement on cervical cancer screening .
The statement, accompanying evidence report, and a modeling study were published online in JAMA.
Cervical cancer deaths in the United States have declined from 2.8 deaths per 100,000 women in 2000 to 2.3 deaths per 100,000 women in 2015 because of the adoption of widespread screening, according to Susan J. Curry, PhD., of the University of Iowa, Iowa City, and her colleagues in the USPSTF (JAMA. 2018 Aug 21. doi: 10.1001/jama.2018.10897.
Based on the latest evidence and the modeling study, the USPSTF gives an A recommendation to screening women aged 21-29 years for cervical cancer every 3 years with cervical cytology alone. The task force also gives an A to screening women aged 30-65 years every 5 years with either hrHPV testing alone or in combination with cytology.
The task force recommends against screening (D recommendation) for women younger than 21 years, older than 65 years with a history of screening and low cervical cancer risk, and women who have had hysterectomies with removal of the cervix and no history of cervical cancer risk.
To update the previous recommendations issued in 2012, the task force reviewed the latest evidence and commissioned a modeling study to help determine the best screening strategies in terms of age, screening intervals, and risks vs. benefits.
In the model, researchers assessed 19 strategies for cervical cancer screening based on a hypothetical cohort of women who began screening at 21 years of age.
Overall, the different strategies were similar in effectiveness, but primary hrHPV testing and alternative cotesting were slightly more effective: Cervical cancer deaths ranged from 0.23 to 0.29 deaths per 1,000 women in strategies involving hrHPV testing or cotesting, vs. 0.30 to 0.76 deaths per 1,000 women for strategies based on the current guidelines.
In addition, switching the age of hrHPV testing from 25 years to 30 years and using a 5-year screening interval showed the most effectiveness in terms of risks vs. harms, wrote Jane K. Kim, PhD, of Harvard University, Boston, and her colleagues (JAMA. 2018 Aug 21. doi: 10.1001/jama.2017.19872). “Switching from cytology to 5-year primary hrHPV testing at age 30 years (strategy 14) was associated with a ratio of 640 colposcopies per cancer case averted; earlier switch ages required a greater number of colposcopies per cancer case averted.”
The recommendations also were supported by an evidence report including eight randomized, controlled trials of 410,556 women, five cohort studies of 402,615 women, and a meta-analysis of individual participant data including 176,464 women.
The evidence report sought to address the benefits and harms of cervical cancer screening using hrHPV screening alone as the primary screening method or paired with cytology (cotesting), compared with primary screening using cytology alone.
Overall, both hrHPV and hrHPV plus cytology were associated with higher rates of false-positives and colposcopy compared with cytology alone, “which could lead to more treatments with potential harms,” wrote Joy Melnikow, MD, of the University of California, Davis, and her colleagues (JAMA. 2018 Aug 21. doi: 10.1001/jama.2018.10400.
In addition, hrHPV testing yielded higher rates of positive cervical intraepithelial neoplasia, compared with cytology alone as initial screening.
However, further research is needed to address the impact of any cervical cancer screening strategies in populations with limited access to health care and screening, the researchers noted.
The updated USPSTF recommendations are largely in line with those issued by leading women’s health organizations including the American College of Obstetricians and Gynecologists, ASCCP, and the Society for Gynecologic Oncology, according to a joint statement.
“With a number of screening options now available, the new guidelines emphasize the importance of the patient-provider shared decision-making process to assist women in making an informed choice about which screening method is most suitable for them,” according to the statement, “However, more importantly, there needs to be a continued effort to ensure all women are adequately screened because a significant number of women in the country are not. It’s also essential for women to have access to all of the tests and that they are appropriately covered by insurance companies.
“We hope the USPSTF recommendations foster more discussions between patients and providers about cervical cancer screening, promote opportunities for patient education on the benefits and safety of HPV vaccination for cervical cancer prevention and encourage providers to offer HPV vaccines in their offices,” the statement noted.
The USPSTF research was funded by the Agency for Healthcare Research and Quality. The researchers for the modeling report were supported in part by a National Cancer Institute grant. The researchers had no relevant financial conflicts to disclose.
SOURCES: Kim J et al. JAMA. 2018 Aug 21. doi: 10.1001/jama.2017.19872; Melnikow J et al. JAMA. 2018 Aug 21. doi: 10.1001/jama.2018.10400; Curry S et al. JAMA. 2018 Aug 21. doi: 10.1001/jama.2018.10897.
FROM JAMA
FDA grants orphan designation to DHODH inhibitor for AML
ASLAN003 is a small molecule inhibitor of the human dihydroorotate dehydrogenase (DHODH) enzyme. This second-generation DHODH inhibitor is being developed by Aslan Pharmaceuticals. The company is currently conducting a phase 2 trial (NCT03451084) of ASLAN003 in patients with newly diagnosed or relapsed/refractory AML. Aslan expects to report interim data from this trial in the second half of 2018.
Aslan has already completed a phase 1 trial (NCT01992367) of ASLAN003 in healthy volunteers. The results suggested that ASLAN003 has an “excellent” pharmacokinetic profile, according to Aslan, and the drug was considered well tolerated in the volunteers.
ASLAN003 has also demonstrated “potent” inhibition of DHODH, according to the drug sponsor. In fact, the company said the binding affinity of ASLAN003 to DHODH has proven to be up to two orders of magnitude stronger than first-generation DHODH inhibitors, such as leflunomide and teriflunomide, but it has less toxicity.
In addition, ASLAN003 has been shown to differentiate blast cells into granulocytes in AML cell lines that do not respond to all-trans retinoic acid. These results were published in Cell in 2016.
ASLAN003 is a small molecule inhibitor of the human dihydroorotate dehydrogenase (DHODH) enzyme. This second-generation DHODH inhibitor is being developed by Aslan Pharmaceuticals. The company is currently conducting a phase 2 trial (NCT03451084) of ASLAN003 in patients with newly diagnosed or relapsed/refractory AML. Aslan expects to report interim data from this trial in the second half of 2018.
Aslan has already completed a phase 1 trial (NCT01992367) of ASLAN003 in healthy volunteers. The results suggested that ASLAN003 has an “excellent” pharmacokinetic profile, according to Aslan, and the drug was considered well tolerated in the volunteers.
ASLAN003 has also demonstrated “potent” inhibition of DHODH, according to the drug sponsor. In fact, the company said the binding affinity of ASLAN003 to DHODH has proven to be up to two orders of magnitude stronger than first-generation DHODH inhibitors, such as leflunomide and teriflunomide, but it has less toxicity.
In addition, ASLAN003 has been shown to differentiate blast cells into granulocytes in AML cell lines that do not respond to all-trans retinoic acid. These results were published in Cell in 2016.
ASLAN003 is a small molecule inhibitor of the human dihydroorotate dehydrogenase (DHODH) enzyme. This second-generation DHODH inhibitor is being developed by Aslan Pharmaceuticals. The company is currently conducting a phase 2 trial (NCT03451084) of ASLAN003 in patients with newly diagnosed or relapsed/refractory AML. Aslan expects to report interim data from this trial in the second half of 2018.
Aslan has already completed a phase 1 trial (NCT01992367) of ASLAN003 in healthy volunteers. The results suggested that ASLAN003 has an “excellent” pharmacokinetic profile, according to Aslan, and the drug was considered well tolerated in the volunteers.
ASLAN003 has also demonstrated “potent” inhibition of DHODH, according to the drug sponsor. In fact, the company said the binding affinity of ASLAN003 to DHODH has proven to be up to two orders of magnitude stronger than first-generation DHODH inhibitors, such as leflunomide and teriflunomide, but it has less toxicity.
In addition, ASLAN003 has been shown to differentiate blast cells into granulocytes in AML cell lines that do not respond to all-trans retinoic acid. These results were published in Cell in 2016.
A systems-based charter on physician well-being
Don’t blame the burned-out clinician
“You can teach a canary in a coal mine to meditate, but it is still going to die.”
I have seen the canary sentiment above – used as a metaphor for health care and burnout – pop up a few times on Twitter, attributed to a few different thoughtful doctors, including Jenny Ramsey, MD, of the Cleveland Clinic (at Hospital Medicine 2018); Lucy Kalanithi, MD, a clinical assistant professor of medicine at Stanford (Calif.) University and widow of Paul Kalanithi, MD, of “When Breath Becomes Air” fame; and Stuart Slavin, MD, associate dean for curriculum and a professor of pediatrics at Saint Louis University.
To be honest, I am rather burned out on reading about physician burnout at this point. Nevertheless, I love the canary idea; it is such a perfect visual of the current problem facing physicians.
I was thinking about the meditating canary when I read the new “Charter on Physician Well-Being,” published in JAMA and already endorsed by most major medical organizations/acronyms, including SHM, ACP, SGIM, AMA, AAMC, AAIM, ABIM, ACCME, APA, and the IHI. This physician well-being charter was created by the Collaborative for Healing and Renewal in Medicine, a group that includes leading medical centers and organizations.
What makes this different from previous attempts at addressing burnout? The charter takes a systems-based approach to physician well-being. Aha, of course! As the patient safety movement realized more than 2 decades ago, real progress would only be made when we stopped focusing our attention, blame, and interventions on individuals and instead looked at systems; now, the physician well-being movement has officially made the same bold proclamation.
It is not the fault of the burned-out physician who apparently just needs to be hammered over the head with better coping skills – just as the majority of medical errors would not be fixed by continuing to tell physicians that they screwed up and should figure out how not to do that again!
We need to make real changes to the system. For example, one of the charter’s authors, Colin P. West, MD, PhD, highlighted why it is important that organizations commit to optimizing highly functioning interprofessional teams: “Can you imagine @KingJames [LeBron James] or @Oprah applying their unique skills AND personally seating the crowd, collecting stats, assessing satisfaction, etc.? So why do we?”
The authors also call for organizations to commit to reducing time spent on documentation and administration. Hallelujah!
Now the question is whether this charter will actually have any teeth or whether it will have the same fate as our canary, slowly fading away, never to be heard from again?
Read the full post at hospitalleader.org.
Dr. Moriates is the assistant dean for health care value and an associate professor of internal medicine at the University of Texas, Austin.
Also in The Hospital Leader
“What’s a Cost, Charge, and Price?” by Brad Flansbaum, DO, MPH, MHM
“There Is a ‘You’ in Team,” by Tracy Cardin, ACNP-BC, SFHM
“‘Harper’s Index’ of Hospital Medicine 2018,” by Jordan Messler, MD, SFHM
Don’t blame the burned-out clinician
Don’t blame the burned-out clinician
“You can teach a canary in a coal mine to meditate, but it is still going to die.”
I have seen the canary sentiment above – used as a metaphor for health care and burnout – pop up a few times on Twitter, attributed to a few different thoughtful doctors, including Jenny Ramsey, MD, of the Cleveland Clinic (at Hospital Medicine 2018); Lucy Kalanithi, MD, a clinical assistant professor of medicine at Stanford (Calif.) University and widow of Paul Kalanithi, MD, of “When Breath Becomes Air” fame; and Stuart Slavin, MD, associate dean for curriculum and a professor of pediatrics at Saint Louis University.
To be honest, I am rather burned out on reading about physician burnout at this point. Nevertheless, I love the canary idea; it is such a perfect visual of the current problem facing physicians.
I was thinking about the meditating canary when I read the new “Charter on Physician Well-Being,” published in JAMA and already endorsed by most major medical organizations/acronyms, including SHM, ACP, SGIM, AMA, AAMC, AAIM, ABIM, ACCME, APA, and the IHI. This physician well-being charter was created by the Collaborative for Healing and Renewal in Medicine, a group that includes leading medical centers and organizations.
What makes this different from previous attempts at addressing burnout? The charter takes a systems-based approach to physician well-being. Aha, of course! As the patient safety movement realized more than 2 decades ago, real progress would only be made when we stopped focusing our attention, blame, and interventions on individuals and instead looked at systems; now, the physician well-being movement has officially made the same bold proclamation.
It is not the fault of the burned-out physician who apparently just needs to be hammered over the head with better coping skills – just as the majority of medical errors would not be fixed by continuing to tell physicians that they screwed up and should figure out how not to do that again!
We need to make real changes to the system. For example, one of the charter’s authors, Colin P. West, MD, PhD, highlighted why it is important that organizations commit to optimizing highly functioning interprofessional teams: “Can you imagine @KingJames [LeBron James] or @Oprah applying their unique skills AND personally seating the crowd, collecting stats, assessing satisfaction, etc.? So why do we?”
The authors also call for organizations to commit to reducing time spent on documentation and administration. Hallelujah!
Now the question is whether this charter will actually have any teeth or whether it will have the same fate as our canary, slowly fading away, never to be heard from again?
Read the full post at hospitalleader.org.
Dr. Moriates is the assistant dean for health care value and an associate professor of internal medicine at the University of Texas, Austin.
Also in The Hospital Leader
“What’s a Cost, Charge, and Price?” by Brad Flansbaum, DO, MPH, MHM
“There Is a ‘You’ in Team,” by Tracy Cardin, ACNP-BC, SFHM
“‘Harper’s Index’ of Hospital Medicine 2018,” by Jordan Messler, MD, SFHM
“You can teach a canary in a coal mine to meditate, but it is still going to die.”
I have seen the canary sentiment above – used as a metaphor for health care and burnout – pop up a few times on Twitter, attributed to a few different thoughtful doctors, including Jenny Ramsey, MD, of the Cleveland Clinic (at Hospital Medicine 2018); Lucy Kalanithi, MD, a clinical assistant professor of medicine at Stanford (Calif.) University and widow of Paul Kalanithi, MD, of “When Breath Becomes Air” fame; and Stuart Slavin, MD, associate dean for curriculum and a professor of pediatrics at Saint Louis University.
To be honest, I am rather burned out on reading about physician burnout at this point. Nevertheless, I love the canary idea; it is such a perfect visual of the current problem facing physicians.
I was thinking about the meditating canary when I read the new “Charter on Physician Well-Being,” published in JAMA and already endorsed by most major medical organizations/acronyms, including SHM, ACP, SGIM, AMA, AAMC, AAIM, ABIM, ACCME, APA, and the IHI. This physician well-being charter was created by the Collaborative for Healing and Renewal in Medicine, a group that includes leading medical centers and organizations.
What makes this different from previous attempts at addressing burnout? The charter takes a systems-based approach to physician well-being. Aha, of course! As the patient safety movement realized more than 2 decades ago, real progress would only be made when we stopped focusing our attention, blame, and interventions on individuals and instead looked at systems; now, the physician well-being movement has officially made the same bold proclamation.
It is not the fault of the burned-out physician who apparently just needs to be hammered over the head with better coping skills – just as the majority of medical errors would not be fixed by continuing to tell physicians that they screwed up and should figure out how not to do that again!
We need to make real changes to the system. For example, one of the charter’s authors, Colin P. West, MD, PhD, highlighted why it is important that organizations commit to optimizing highly functioning interprofessional teams: “Can you imagine @KingJames [LeBron James] or @Oprah applying their unique skills AND personally seating the crowd, collecting stats, assessing satisfaction, etc.? So why do we?”
The authors also call for organizations to commit to reducing time spent on documentation and administration. Hallelujah!
Now the question is whether this charter will actually have any teeth or whether it will have the same fate as our canary, slowly fading away, never to be heard from again?
Read the full post at hospitalleader.org.
Dr. Moriates is the assistant dean for health care value and an associate professor of internal medicine at the University of Texas, Austin.
Also in The Hospital Leader
“What’s a Cost, Charge, and Price?” by Brad Flansbaum, DO, MPH, MHM
“There Is a ‘You’ in Team,” by Tracy Cardin, ACNP-BC, SFHM
“‘Harper’s Index’ of Hospital Medicine 2018,” by Jordan Messler, MD, SFHM