User login
D-dimer and WCP can render VDUs unnecessary
Venous duplex ultrasounds (VDUs) may be over-utilized in patients suspected of having deep vein thrombosis (DVT), according to research published in Annals of Vascular Surgery.
The retrospective study indicated that D-dimer tests and the pretest Wells criteria probability (WCP) score could safely exclude DVT.
The researchers therefore believe that consistent use of D-dimer and WCP could reduce the number of unnecessary immediate VDUs, cut costs, and save time.
“Usually, waiting for a venous ultrasound would be a matter of three, four, five hours,” said study author Albeir Mousa, MD, of West Virginia University in Charleston, West Virginia.
“With the D-dimer test, it’s a few minutes, and you’re done.”
For this study, Dr. Mousa and his colleagues reviewed data on 1670 patients who presented to a high-volume tertiary care center with suspected DVT and were referred for VDU. Their average age was 62.1, and 55.7% were female.
The researchers calculated WCP scores for all 1670 patients and divided them into DVT risk groups accordingly—low- (<1), moderate- (1-2), and high-risk (≥3).
The team also divided patients according to D-dimer values—low (0.1-0.59), moderate (0.60-1.2), and high (≥1.3 mg/L FEU).
Results
The researchers found that D-dimer (with an abnormal threshold of ≥0.60 mg/L FEU) identified all patients with DVT (n=183, 11%).
The sensitivity and negative predictive values of D-dimer were both 100%, while specificity was 14.9% and the positive predictive value was 15.9%.
When the researchers used an age-adjusted D-dimer threshold (age x 0.01), the specificity increased from 14.9% to 21.3%, and the positive predictive value increased from 15.9% to 17%.
There were no DVTs among patients with low D-dimer values, but the rate of DVT significantly increased in the moderate (5.9%) and high groups (20.0%; P=0.007).
The rate of DVT increased with increasing WCP as well. The DVT rate was 6.6% in the low WCP group, 14% in the moderate group, and 29.1% in the high group (P<0.001).
The researchers also noted an increase in the rate of DVT across all levels of WCP as D-dimer levels increased.
Based on these findings, the researchers said there were 762 patients who were sent for unnecessary immediate VDUs. This included 685 patients in the low WCP group (<1) who did not undergo D-dimer testing, 51 patients who had moderate D-dimer values, and 26 who had low D-dimer values.
The researchers calculated the potential savings of using D-dimer instead of VDU in these patients, based on US 2016 dollar estimates.
A VDU cost of $1557 per person would mean a total cost of $1,186,434 for 762 patients. A D-dimer cost of $182 per person would mean a total cost of $138,684. So the total potential savings would be $1,047,750.
Dr. Mousa also noted that D-dimer testing is quicker than VDU and requires fewer resources.
“It’s a very simple sample of blood, which is sent to the lab,” he said. “And, usually in 15 or 20 minutes, you have significant information that can help you take good care of the patients. And you can send patients home quicker, with less demand on the hospital staff and the institution, yet increase patient satisfaction.”
Venous duplex ultrasounds (VDUs) may be over-utilized in patients suspected of having deep vein thrombosis (DVT), according to research published in Annals of Vascular Surgery.
The retrospective study indicated that D-dimer tests and the pretest Wells criteria probability (WCP) score could safely exclude DVT.
The researchers therefore believe that consistent use of D-dimer and WCP could reduce the number of unnecessary immediate VDUs, cut costs, and save time.
“Usually, waiting for a venous ultrasound would be a matter of three, four, five hours,” said study author Albeir Mousa, MD, of West Virginia University in Charleston, West Virginia.
“With the D-dimer test, it’s a few minutes, and you’re done.”
For this study, Dr. Mousa and his colleagues reviewed data on 1670 patients who presented to a high-volume tertiary care center with suspected DVT and were referred for VDU. Their average age was 62.1, and 55.7% were female.
The researchers calculated WCP scores for all 1670 patients and divided them into DVT risk groups accordingly—low- (<1), moderate- (1-2), and high-risk (≥3).
The team also divided patients according to D-dimer values—low (0.1-0.59), moderate (0.60-1.2), and high (≥1.3 mg/L FEU).
Results
The researchers found that D-dimer (with an abnormal threshold of ≥0.60 mg/L FEU) identified all patients with DVT (n=183, 11%).
The sensitivity and negative predictive values of D-dimer were both 100%, while specificity was 14.9% and the positive predictive value was 15.9%.
When the researchers used an age-adjusted D-dimer threshold (age x 0.01), the specificity increased from 14.9% to 21.3%, and the positive predictive value increased from 15.9% to 17%.
There were no DVTs among patients with low D-dimer values, but the rate of DVT significantly increased in the moderate (5.9%) and high groups (20.0%; P=0.007).
The rate of DVT increased with increasing WCP as well. The DVT rate was 6.6% in the low WCP group, 14% in the moderate group, and 29.1% in the high group (P<0.001).
The researchers also noted an increase in the rate of DVT across all levels of WCP as D-dimer levels increased.
Based on these findings, the researchers said there were 762 patients who were sent for unnecessary immediate VDUs. This included 685 patients in the low WCP group (<1) who did not undergo D-dimer testing, 51 patients who had moderate D-dimer values, and 26 who had low D-dimer values.
The researchers calculated the potential savings of using D-dimer instead of VDU in these patients, based on US 2016 dollar estimates.
A VDU cost of $1557 per person would mean a total cost of $1,186,434 for 762 patients. A D-dimer cost of $182 per person would mean a total cost of $138,684. So the total potential savings would be $1,047,750.
Dr. Mousa also noted that D-dimer testing is quicker than VDU and requires fewer resources.
“It’s a very simple sample of blood, which is sent to the lab,” he said. “And, usually in 15 or 20 minutes, you have significant information that can help you take good care of the patients. And you can send patients home quicker, with less demand on the hospital staff and the institution, yet increase patient satisfaction.”
Venous duplex ultrasounds (VDUs) may be over-utilized in patients suspected of having deep vein thrombosis (DVT), according to research published in Annals of Vascular Surgery.
The retrospective study indicated that D-dimer tests and the pretest Wells criteria probability (WCP) score could safely exclude DVT.
The researchers therefore believe that consistent use of D-dimer and WCP could reduce the number of unnecessary immediate VDUs, cut costs, and save time.
“Usually, waiting for a venous ultrasound would be a matter of three, four, five hours,” said study author Albeir Mousa, MD, of West Virginia University in Charleston, West Virginia.
“With the D-dimer test, it’s a few minutes, and you’re done.”
For this study, Dr. Mousa and his colleagues reviewed data on 1670 patients who presented to a high-volume tertiary care center with suspected DVT and were referred for VDU. Their average age was 62.1, and 55.7% were female.
The researchers calculated WCP scores for all 1670 patients and divided them into DVT risk groups accordingly—low- (<1), moderate- (1-2), and high-risk (≥3).
The team also divided patients according to D-dimer values—low (0.1-0.59), moderate (0.60-1.2), and high (≥1.3 mg/L FEU).
Results
The researchers found that D-dimer (with an abnormal threshold of ≥0.60 mg/L FEU) identified all patients with DVT (n=183, 11%).
The sensitivity and negative predictive values of D-dimer were both 100%, while specificity was 14.9% and the positive predictive value was 15.9%.
When the researchers used an age-adjusted D-dimer threshold (age x 0.01), the specificity increased from 14.9% to 21.3%, and the positive predictive value increased from 15.9% to 17%.
There were no DVTs among patients with low D-dimer values, but the rate of DVT significantly increased in the moderate (5.9%) and high groups (20.0%; P=0.007).
The rate of DVT increased with increasing WCP as well. The DVT rate was 6.6% in the low WCP group, 14% in the moderate group, and 29.1% in the high group (P<0.001).
The researchers also noted an increase in the rate of DVT across all levels of WCP as D-dimer levels increased.
Based on these findings, the researchers said there were 762 patients who were sent for unnecessary immediate VDUs. This included 685 patients in the low WCP group (<1) who did not undergo D-dimer testing, 51 patients who had moderate D-dimer values, and 26 who had low D-dimer values.
The researchers calculated the potential savings of using D-dimer instead of VDU in these patients, based on US 2016 dollar estimates.
A VDU cost of $1557 per person would mean a total cost of $1,186,434 for 762 patients. A D-dimer cost of $182 per person would mean a total cost of $138,684. So the total potential savings would be $1,047,750.
Dr. Mousa also noted that D-dimer testing is quicker than VDU and requires fewer resources.
“It’s a very simple sample of blood, which is sent to the lab,” he said. “And, usually in 15 or 20 minutes, you have significant information that can help you take good care of the patients. And you can send patients home quicker, with less demand on the hospital staff and the institution, yet increase patient satisfaction.”
Enzymes convert blood from type A to O more efficiently
BOSTON—New research suggests enzymes from the human gut can turn type A blood into type O more efficiently than previously studied enzymes.
Researchers found this new family of enzymes—whose name has not been made public—can remove the A antigens from red blood cells (RBCs), thereby converting type A blood to O.
Stephen Withers, PhD, of the University of British Columbia in Vancouver, Canada, presented details on the enzymes at the 256th National Meeting & Exposition of the American Chemical Society (abstract CARB105).
A metagenomics approach
Dr. Withers said researchers have been studying the use of enzymes to modify blood as far back as 1982. However, it has been difficult to identify efficient, selective enzymes that are also safe and economical.
To assess potential enzyme candidates quickly, Dr. Withers and his colleagues used metagenomics.
“With metagenomics, you take all of the organisms from an environment and extract the sum total DNA of those organisms all mixed up together,” Dr. Withers explained.
Casting such a wide net allowed the researchers to sample the genes of millions of microorganisms without the need for individual cultures. The researchers then used E. coli to select for genes that code for enzymes that can cleave sugar residues.
“This is a way of getting that genetic information out of the environment and into the laboratory setting and then screening for the activity we are interested in,” Dr. Withers said.
Focusing on the gut
Dr. Withers and his colleagues had considered sampling DNA from mosquitoes and leeches, the types of organisms that degrade blood, but the researchers ultimately found successful candidate enzymes in the human gut microbiome.
Glycosylated proteins called mucins line the gut wall, providing sugars that serve as attachment points for gut bacteria while also feeding them as they assist in digestion. Some of the mucin sugars are similar in structure to the antigens on RBCs in type A and B blood.
Dr. Withers and his colleagues studied the enzymes the bacteria use to pluck the sugars off mucin and identified a family of enzymes that could convert type A blood to type O.
The name of this enzyme family has not been released to the public, as the patent for the enzymes has not been submitted.
“By homing in on the bacteria feeding on those sugars, we isolated the enzymes the bacteria use to pluck off the sugar molecules,” Dr. Withers explained. “We then produced quantities of those enzymes through cloning and found that they were capable of performing a similar action on blood antigens.”
The enzymes could convert type A blood to type O by removing N-Acetyl galactosamine from the surface of RBCs.
In fact, the new enzymes were 30 times more effective for converting A to O than the alpha-N-acetylgalactosaminidase from Elizabethkingia meningospeticum that was described in a 2007 Nature Biotechnology paper.
Dr. Withers said he and his colleagues needed to use 30 times less of the new enzymes. This means the new enzymes are more economical, and it is easier to remove all traces of the added enzymes, which would need to be done before transfusion.
Looking ahead
Dr. Withers noted that the new enzymes work in whole blood. So if they prove safe for use in humans, the enzymes could potentially be introduced to whole blood donations and left to make the conversion from A to O. Then, the enzymes could be removed by washing RBCs before transfusion.
Dr. Withers and his colleagues are now working to validate these enzymes and test them on a larger scale for potential clinical testing. He also plans to carry out directed evolution, a protein engineering technique that simulates natural evolution, with the goal of creating the most efficient sugar-removing enzyme.
“I am optimistic that we have a very interesting candidate to adjust donated blood to a common type,” Dr. Withers said. “Of course, it will have to go through lots of clinical trials to make sure that it doesn’t have any adverse consequences, but it is looking very promising.”
The researchers acknowledged support and funding from the Canadian Institutes of Health Research.
BOSTON—New research suggests enzymes from the human gut can turn type A blood into type O more efficiently than previously studied enzymes.
Researchers found this new family of enzymes—whose name has not been made public—can remove the A antigens from red blood cells (RBCs), thereby converting type A blood to O.
Stephen Withers, PhD, of the University of British Columbia in Vancouver, Canada, presented details on the enzymes at the 256th National Meeting & Exposition of the American Chemical Society (abstract CARB105).
A metagenomics approach
Dr. Withers said researchers have been studying the use of enzymes to modify blood as far back as 1982. However, it has been difficult to identify efficient, selective enzymes that are also safe and economical.
To assess potential enzyme candidates quickly, Dr. Withers and his colleagues used metagenomics.
“With metagenomics, you take all of the organisms from an environment and extract the sum total DNA of those organisms all mixed up together,” Dr. Withers explained.
Casting such a wide net allowed the researchers to sample the genes of millions of microorganisms without the need for individual cultures. The researchers then used E. coli to select for genes that code for enzymes that can cleave sugar residues.
“This is a way of getting that genetic information out of the environment and into the laboratory setting and then screening for the activity we are interested in,” Dr. Withers said.
Focusing on the gut
Dr. Withers and his colleagues had considered sampling DNA from mosquitoes and leeches, the types of organisms that degrade blood, but the researchers ultimately found successful candidate enzymes in the human gut microbiome.
Glycosylated proteins called mucins line the gut wall, providing sugars that serve as attachment points for gut bacteria while also feeding them as they assist in digestion. Some of the mucin sugars are similar in structure to the antigens on RBCs in type A and B blood.
Dr. Withers and his colleagues studied the enzymes the bacteria use to pluck the sugars off mucin and identified a family of enzymes that could convert type A blood to type O.
The name of this enzyme family has not been released to the public, as the patent for the enzymes has not been submitted.
“By homing in on the bacteria feeding on those sugars, we isolated the enzymes the bacteria use to pluck off the sugar molecules,” Dr. Withers explained. “We then produced quantities of those enzymes through cloning and found that they were capable of performing a similar action on blood antigens.”
The enzymes could convert type A blood to type O by removing N-Acetyl galactosamine from the surface of RBCs.
In fact, the new enzymes were 30 times more effective for converting A to O than the alpha-N-acetylgalactosaminidase from Elizabethkingia meningospeticum that was described in a 2007 Nature Biotechnology paper.
Dr. Withers said he and his colleagues needed to use 30 times less of the new enzymes. This means the new enzymes are more economical, and it is easier to remove all traces of the added enzymes, which would need to be done before transfusion.
Looking ahead
Dr. Withers noted that the new enzymes work in whole blood. So if they prove safe for use in humans, the enzymes could potentially be introduced to whole blood donations and left to make the conversion from A to O. Then, the enzymes could be removed by washing RBCs before transfusion.
Dr. Withers and his colleagues are now working to validate these enzymes and test them on a larger scale for potential clinical testing. He also plans to carry out directed evolution, a protein engineering technique that simulates natural evolution, with the goal of creating the most efficient sugar-removing enzyme.
“I am optimistic that we have a very interesting candidate to adjust donated blood to a common type,” Dr. Withers said. “Of course, it will have to go through lots of clinical trials to make sure that it doesn’t have any adverse consequences, but it is looking very promising.”
The researchers acknowledged support and funding from the Canadian Institutes of Health Research.
BOSTON—New research suggests enzymes from the human gut can turn type A blood into type O more efficiently than previously studied enzymes.
Researchers found this new family of enzymes—whose name has not been made public—can remove the A antigens from red blood cells (RBCs), thereby converting type A blood to O.
Stephen Withers, PhD, of the University of British Columbia in Vancouver, Canada, presented details on the enzymes at the 256th National Meeting & Exposition of the American Chemical Society (abstract CARB105).
A metagenomics approach
Dr. Withers said researchers have been studying the use of enzymes to modify blood as far back as 1982. However, it has been difficult to identify efficient, selective enzymes that are also safe and economical.
To assess potential enzyme candidates quickly, Dr. Withers and his colleagues used metagenomics.
“With metagenomics, you take all of the organisms from an environment and extract the sum total DNA of those organisms all mixed up together,” Dr. Withers explained.
Casting such a wide net allowed the researchers to sample the genes of millions of microorganisms without the need for individual cultures. The researchers then used E. coli to select for genes that code for enzymes that can cleave sugar residues.
“This is a way of getting that genetic information out of the environment and into the laboratory setting and then screening for the activity we are interested in,” Dr. Withers said.
Focusing on the gut
Dr. Withers and his colleagues had considered sampling DNA from mosquitoes and leeches, the types of organisms that degrade blood, but the researchers ultimately found successful candidate enzymes in the human gut microbiome.
Glycosylated proteins called mucins line the gut wall, providing sugars that serve as attachment points for gut bacteria while also feeding them as they assist in digestion. Some of the mucin sugars are similar in structure to the antigens on RBCs in type A and B blood.
Dr. Withers and his colleagues studied the enzymes the bacteria use to pluck the sugars off mucin and identified a family of enzymes that could convert type A blood to type O.
The name of this enzyme family has not been released to the public, as the patent for the enzymes has not been submitted.
“By homing in on the bacteria feeding on those sugars, we isolated the enzymes the bacteria use to pluck off the sugar molecules,” Dr. Withers explained. “We then produced quantities of those enzymes through cloning and found that they were capable of performing a similar action on blood antigens.”
The enzymes could convert type A blood to type O by removing N-Acetyl galactosamine from the surface of RBCs.
In fact, the new enzymes were 30 times more effective for converting A to O than the alpha-N-acetylgalactosaminidase from Elizabethkingia meningospeticum that was described in a 2007 Nature Biotechnology paper.
Dr. Withers said he and his colleagues needed to use 30 times less of the new enzymes. This means the new enzymes are more economical, and it is easier to remove all traces of the added enzymes, which would need to be done before transfusion.
Looking ahead
Dr. Withers noted that the new enzymes work in whole blood. So if they prove safe for use in humans, the enzymes could potentially be introduced to whole blood donations and left to make the conversion from A to O. Then, the enzymes could be removed by washing RBCs before transfusion.
Dr. Withers and his colleagues are now working to validate these enzymes and test them on a larger scale for potential clinical testing. He also plans to carry out directed evolution, a protein engineering technique that simulates natural evolution, with the goal of creating the most efficient sugar-removing enzyme.
“I am optimistic that we have a very interesting candidate to adjust donated blood to a common type,” Dr. Withers said. “Of course, it will have to go through lots of clinical trials to make sure that it doesn’t have any adverse consequences, but it is looking very promising.”
The researchers acknowledged support and funding from the Canadian Institutes of Health Research.
AMP publishes report on DNA variants in CMNs
A new report addresses the clinical relevance of DNA variants in chronic myeloid neoplasms (CMNs).
The report is intended to aid clinical laboratory professionals with the management of most CMNs and the development of high-throughput pan-myeloid sequencing testing panels.
The authors list 34 genes they consider “critical” for sequencing tests to help standardize clinical practice and improve care of patients with CMNs.
The Association for Molecular Pathology (AMP) established a CMN Working Group to generate the report, which was published in The Journal of Molecular Diagnostics.
“The molecular pathology community has witnessed a recent explosion of scientific literature highlighting the clinical significance of small DNA variants in CMNs,” said Rebecca F. McClure, MD, a member of the AMP CMN Working Group and an associate professor at Health Sciences North/Horizon Santé-Nord in Sudbury, Ontario, Canada.
“AMP’s working group recognized a clear, unmet need for evidence-based recommendations to assist in the development of the high-quality pan-myeloid gene panels that provide relevant diagnostic and prognostic information and enable monitoring of clonal architecture.”
The increasing availability of targeted, high-throughput, next-generation sequencing panels has enabled scientists to explore the genetic heterogeneity and clinical relevance of the small DNA variants in CMNs.
However, the biological complexity and multiple forms of CMNs have led to variability in the genes included on the available panels that are used to make an accurate diagnosis, provide reliable prognostic information, and select an appropriate therapy based on DNA variant profiles present at various time points.
AMP established its CMN Working Group to review the published literature on CMNs, summarize key findings that support clinical utility, and define a set of critical gene inclusions for all high-throughput pan-myeloid sequencing testing panels.
The group proposed the following 34 genes as a minimum recommended testing list: ASXL1, BCOR, BCORL1, CALR, CBL, CEBPA, CSF3R, DNMT3A, ETV6, EZH2, FLT3, IDH1, IDH2, JAK2, KIT, KRAS, MPL, NF1, NPM1, NRAS, PHF6, PPM1D, PTPN11, RAD21, RUNX1, SETBP1, SF3B1, SMC3, SRSF2, STAG2, TET2, TP53, U2AF1, and ZRSR2.
“While the goal of the study was to distill the literature for molecular pathologists, in doing so, we also revealed recurrent mutational patterns of clonal evolution that will [help] hematologist/oncologists, researchers, and pathologists understand how to interpret the results of these panels as they reveal critical biology of the neoplasms,” said Annette S. Kim, MD, PhD, CMN Working Group Chair and an associate professor at Harvard Medical School and Brigham and Women’s Hospital in Boston, Massachusetts.
A new report addresses the clinical relevance of DNA variants in chronic myeloid neoplasms (CMNs).
The report is intended to aid clinical laboratory professionals with the management of most CMNs and the development of high-throughput pan-myeloid sequencing testing panels.
The authors list 34 genes they consider “critical” for sequencing tests to help standardize clinical practice and improve care of patients with CMNs.
The Association for Molecular Pathology (AMP) established a CMN Working Group to generate the report, which was published in The Journal of Molecular Diagnostics.
“The molecular pathology community has witnessed a recent explosion of scientific literature highlighting the clinical significance of small DNA variants in CMNs,” said Rebecca F. McClure, MD, a member of the AMP CMN Working Group and an associate professor at Health Sciences North/Horizon Santé-Nord in Sudbury, Ontario, Canada.
“AMP’s working group recognized a clear, unmet need for evidence-based recommendations to assist in the development of the high-quality pan-myeloid gene panels that provide relevant diagnostic and prognostic information and enable monitoring of clonal architecture.”
The increasing availability of targeted, high-throughput, next-generation sequencing panels has enabled scientists to explore the genetic heterogeneity and clinical relevance of the small DNA variants in CMNs.
However, the biological complexity and multiple forms of CMNs have led to variability in the genes included on the available panels that are used to make an accurate diagnosis, provide reliable prognostic information, and select an appropriate therapy based on DNA variant profiles present at various time points.
AMP established its CMN Working Group to review the published literature on CMNs, summarize key findings that support clinical utility, and define a set of critical gene inclusions for all high-throughput pan-myeloid sequencing testing panels.
The group proposed the following 34 genes as a minimum recommended testing list: ASXL1, BCOR, BCORL1, CALR, CBL, CEBPA, CSF3R, DNMT3A, ETV6, EZH2, FLT3, IDH1, IDH2, JAK2, KIT, KRAS, MPL, NF1, NPM1, NRAS, PHF6, PPM1D, PTPN11, RAD21, RUNX1, SETBP1, SF3B1, SMC3, SRSF2, STAG2, TET2, TP53, U2AF1, and ZRSR2.
“While the goal of the study was to distill the literature for molecular pathologists, in doing so, we also revealed recurrent mutational patterns of clonal evolution that will [help] hematologist/oncologists, researchers, and pathologists understand how to interpret the results of these panels as they reveal critical biology of the neoplasms,” said Annette S. Kim, MD, PhD, CMN Working Group Chair and an associate professor at Harvard Medical School and Brigham and Women’s Hospital in Boston, Massachusetts.
A new report addresses the clinical relevance of DNA variants in chronic myeloid neoplasms (CMNs).
The report is intended to aid clinical laboratory professionals with the management of most CMNs and the development of high-throughput pan-myeloid sequencing testing panels.
The authors list 34 genes they consider “critical” for sequencing tests to help standardize clinical practice and improve care of patients with CMNs.
The Association for Molecular Pathology (AMP) established a CMN Working Group to generate the report, which was published in The Journal of Molecular Diagnostics.
“The molecular pathology community has witnessed a recent explosion of scientific literature highlighting the clinical significance of small DNA variants in CMNs,” said Rebecca F. McClure, MD, a member of the AMP CMN Working Group and an associate professor at Health Sciences North/Horizon Santé-Nord in Sudbury, Ontario, Canada.
“AMP’s working group recognized a clear, unmet need for evidence-based recommendations to assist in the development of the high-quality pan-myeloid gene panels that provide relevant diagnostic and prognostic information and enable monitoring of clonal architecture.”
The increasing availability of targeted, high-throughput, next-generation sequencing panels has enabled scientists to explore the genetic heterogeneity and clinical relevance of the small DNA variants in CMNs.
However, the biological complexity and multiple forms of CMNs have led to variability in the genes included on the available panels that are used to make an accurate diagnosis, provide reliable prognostic information, and select an appropriate therapy based on DNA variant profiles present at various time points.
AMP established its CMN Working Group to review the published literature on CMNs, summarize key findings that support clinical utility, and define a set of critical gene inclusions for all high-throughput pan-myeloid sequencing testing panels.
The group proposed the following 34 genes as a minimum recommended testing list: ASXL1, BCOR, BCORL1, CALR, CBL, CEBPA, CSF3R, DNMT3A, ETV6, EZH2, FLT3, IDH1, IDH2, JAK2, KIT, KRAS, MPL, NF1, NPM1, NRAS, PHF6, PPM1D, PTPN11, RAD21, RUNX1, SETBP1, SF3B1, SMC3, SRSF2, STAG2, TET2, TP53, U2AF1, and ZRSR2.
“While the goal of the study was to distill the literature for molecular pathologists, in doing so, we also revealed recurrent mutational patterns of clonal evolution that will [help] hematologist/oncologists, researchers, and pathologists understand how to interpret the results of these panels as they reveal critical biology of the neoplasms,” said Annette S. Kim, MD, PhD, CMN Working Group Chair and an associate professor at Harvard Medical School and Brigham and Women’s Hospital in Boston, Massachusetts.
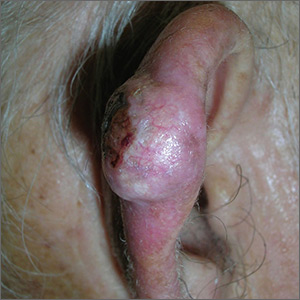
Growth on ear
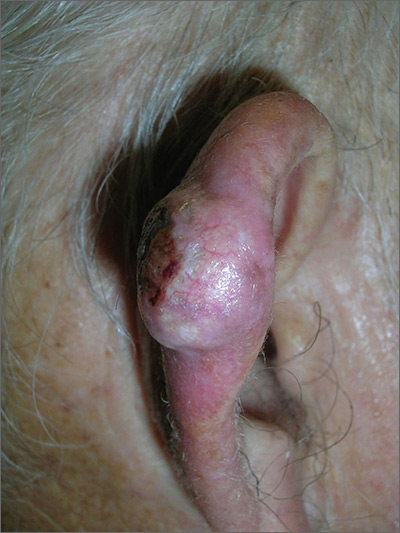
The FP suspected skin cancer, but was not sure if it was a basal cell carcinoma or a squamous cell carcinoma (SCC).
The growth was somewhat pearly with telangiectasias, but there was also a prominent area of keratin on the posterior aspect of the lesion. After injecting local anesthesia with 1% lidocaine and epinephrine, the physician performed a shave biopsy on one edge of the tumor. He was careful to avoid cutting the cartilage and decided that the diagnosis would be obtained without trying to remove the whole tumor. The bleeding did not stop with chemical hemostasis alone, so electrosurgery was used. The biopsy result showed an SCC of the keratoacanthoma type.
The patient was referred for Mohs surgery because it was important to get clear margins, while attempting to preserve the ear anatomy. Also, SCC of the ear has a higher risk for metastasis, so a thorough exam of the head and neck for lymphadenopathy was performed. Fortunately, this SCC was well-differentiated and no metastases were clinically detected.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Guzman A, Usatine R. Keratocanthoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:977-980.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP suspected skin cancer, but was not sure if it was a basal cell carcinoma or a squamous cell carcinoma (SCC).
The growth was somewhat pearly with telangiectasias, but there was also a prominent area of keratin on the posterior aspect of the lesion. After injecting local anesthesia with 1% lidocaine and epinephrine, the physician performed a shave biopsy on one edge of the tumor. He was careful to avoid cutting the cartilage and decided that the diagnosis would be obtained without trying to remove the whole tumor. The bleeding did not stop with chemical hemostasis alone, so electrosurgery was used. The biopsy result showed an SCC of the keratoacanthoma type.
The patient was referred for Mohs surgery because it was important to get clear margins, while attempting to preserve the ear anatomy. Also, SCC of the ear has a higher risk for metastasis, so a thorough exam of the head and neck for lymphadenopathy was performed. Fortunately, this SCC was well-differentiated and no metastases were clinically detected.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Guzman A, Usatine R. Keratocanthoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:977-980.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP suspected skin cancer, but was not sure if it was a basal cell carcinoma or a squamous cell carcinoma (SCC).
The growth was somewhat pearly with telangiectasias, but there was also a prominent area of keratin on the posterior aspect of the lesion. After injecting local anesthesia with 1% lidocaine and epinephrine, the physician performed a shave biopsy on one edge of the tumor. He was careful to avoid cutting the cartilage and decided that the diagnosis would be obtained without trying to remove the whole tumor. The bleeding did not stop with chemical hemostasis alone, so electrosurgery was used. The biopsy result showed an SCC of the keratoacanthoma type.
The patient was referred for Mohs surgery because it was important to get clear margins, while attempting to preserve the ear anatomy. Also, SCC of the ear has a higher risk for metastasis, so a thorough exam of the head and neck for lymphadenopathy was performed. Fortunately, this SCC was well-differentiated and no metastases were clinically detected.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Guzman A, Usatine R. Keratocanthoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:977-980.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Providing culturally competent postpartum care for South Asian women
As do women from a wide range of cultures, South Asian (SA) women frequently report feelings of shame associated with receiving a psychiatric diagnosis during the postpartum period because they fear it may reflect poorly on their ability to be good mothers or negatively impact their family’s
Be aware of psychosomatic presentations. SA mothers who develop postpartum psychiatric symptoms might not present with complaints of dysphoria, crying, low energy, or suicidal thoughts. They may instead describe psychosomatic symptoms such as headaches and body pains.
Consider their hesitation to use psychiatric terms. SA women may not be comfortable using psychiatric terms such as depression or anxiety. Instead, they might respond more positively when their preferred descriptive terms (ie, sadness, worry, stress) are used by the clinicians who treat them.
Engage the partner and/or family. SA women may emphasize that they are part of a family unit, rather than regarding themselves as individuals. Thus, including family members in the treatment plan may help improve adherence.
Screen for suicide risk. Evidence suggests that young SA women have a higher rate of suicide and suicide attempts than young SA men or non-SA women.1,2 Further, they may be less willing to speak openly about it.
Ask questions about cultural or traditional forms of treatment. SA mothers, particularly those who are breastfeeding, might be wary of Western medicine and may be familiar with traditional Indian medicine practices such as herbal, homeopathic, or Ayurvedic approaches.3 These interventions may include a specified diet, use of herbal treatments, exercise, and lifestyle recommendations.When taking the patient’s history, find out which treatments she is currently using, and discuss whether she can safely continue to use them.
Do not mistake poor eye contact for lack of engagement. Because SA patients may view a physician as a source of authority, they might regard direct eye contact with a physician as being somewhat disrespectful, and they may avoid eye contact altogether.
Continue to: Maintain an active approach
Maintain an active approach. SA women may prefer to view the physician as an expert, rather than a partner with whom to develop a collaborative relationship. Thus, they may feel more comfortable with direct feedback rather than a passive or reflective approach.
Suggest a postpartum support group. In a U.K. study of 17 SA postpartum women, age 20 to 45, group therapy improved health outcomes and overall satisfaction.4 It may be particularly helpful to SA patients if group therapy is facilitated by a culturally sensitive moderator.
Help patients overcome logistical barriers. Lack of transportation, childcare difficulties, and financial limitations are common deterrents to treatment. These barriers may be particularly challenging for SA women of lower socioeconomic status. Postpartum mothers who feel overtasked with caring for their children and undertaking household duties may feel less able to complete therapy.
Screen for adherence. Although SA patients may view clinicians as authority figures, adherence with medications or treatment plans should not be assumed. Many patients may quietly avoid treatments or recommendations instead of discussing their ambivalence with their clinicians.
1. Anand AS, Cochrane R. The mental health status of South Asian women in Britain. A review of the UK literature. Psychol Dev Soc J. 2005;17(2):195-214.
2. Bhugra D, Desai M. Attempted suicide in South Asian women. Advances in Psychiatric Treatment. 2002;8(6):418-423.
3. Chopra A, Doiphode VV. Ayurvedic medicine. Core concept, therapeutic principles, and current relevance. Med Clin North Am. 2002;86(1):75-89,vii.
4. Masood Y, Lovell K, Lunat F, et al. Group psychological intervention for postnatal depression: a nested qualitative study with British South Asian women. BMC Womens Health. 2015;25(15):109.
As do women from a wide range of cultures, South Asian (SA) women frequently report feelings of shame associated with receiving a psychiatric diagnosis during the postpartum period because they fear it may reflect poorly on their ability to be good mothers or negatively impact their family’s
Be aware of psychosomatic presentations. SA mothers who develop postpartum psychiatric symptoms might not present with complaints of dysphoria, crying, low energy, or suicidal thoughts. They may instead describe psychosomatic symptoms such as headaches and body pains.
Consider their hesitation to use psychiatric terms. SA women may not be comfortable using psychiatric terms such as depression or anxiety. Instead, they might respond more positively when their preferred descriptive terms (ie, sadness, worry, stress) are used by the clinicians who treat them.
Engage the partner and/or family. SA women may emphasize that they are part of a family unit, rather than regarding themselves as individuals. Thus, including family members in the treatment plan may help improve adherence.
Screen for suicide risk. Evidence suggests that young SA women have a higher rate of suicide and suicide attempts than young SA men or non-SA women.1,2 Further, they may be less willing to speak openly about it.
Ask questions about cultural or traditional forms of treatment. SA mothers, particularly those who are breastfeeding, might be wary of Western medicine and may be familiar with traditional Indian medicine practices such as herbal, homeopathic, or Ayurvedic approaches.3 These interventions may include a specified diet, use of herbal treatments, exercise, and lifestyle recommendations.When taking the patient’s history, find out which treatments she is currently using, and discuss whether she can safely continue to use them.
Do not mistake poor eye contact for lack of engagement. Because SA patients may view a physician as a source of authority, they might regard direct eye contact with a physician as being somewhat disrespectful, and they may avoid eye contact altogether.
Continue to: Maintain an active approach
Maintain an active approach. SA women may prefer to view the physician as an expert, rather than a partner with whom to develop a collaborative relationship. Thus, they may feel more comfortable with direct feedback rather than a passive or reflective approach.
Suggest a postpartum support group. In a U.K. study of 17 SA postpartum women, age 20 to 45, group therapy improved health outcomes and overall satisfaction.4 It may be particularly helpful to SA patients if group therapy is facilitated by a culturally sensitive moderator.
Help patients overcome logistical barriers. Lack of transportation, childcare difficulties, and financial limitations are common deterrents to treatment. These barriers may be particularly challenging for SA women of lower socioeconomic status. Postpartum mothers who feel overtasked with caring for their children and undertaking household duties may feel less able to complete therapy.
Screen for adherence. Although SA patients may view clinicians as authority figures, adherence with medications or treatment plans should not be assumed. Many patients may quietly avoid treatments or recommendations instead of discussing their ambivalence with their clinicians.
As do women from a wide range of cultures, South Asian (SA) women frequently report feelings of shame associated with receiving a psychiatric diagnosis during the postpartum period because they fear it may reflect poorly on their ability to be good mothers or negatively impact their family’s
Be aware of psychosomatic presentations. SA mothers who develop postpartum psychiatric symptoms might not present with complaints of dysphoria, crying, low energy, or suicidal thoughts. They may instead describe psychosomatic symptoms such as headaches and body pains.
Consider their hesitation to use psychiatric terms. SA women may not be comfortable using psychiatric terms such as depression or anxiety. Instead, they might respond more positively when their preferred descriptive terms (ie, sadness, worry, stress) are used by the clinicians who treat them.
Engage the partner and/or family. SA women may emphasize that they are part of a family unit, rather than regarding themselves as individuals. Thus, including family members in the treatment plan may help improve adherence.
Screen for suicide risk. Evidence suggests that young SA women have a higher rate of suicide and suicide attempts than young SA men or non-SA women.1,2 Further, they may be less willing to speak openly about it.
Ask questions about cultural or traditional forms of treatment. SA mothers, particularly those who are breastfeeding, might be wary of Western medicine and may be familiar with traditional Indian medicine practices such as herbal, homeopathic, or Ayurvedic approaches.3 These interventions may include a specified diet, use of herbal treatments, exercise, and lifestyle recommendations.When taking the patient’s history, find out which treatments she is currently using, and discuss whether she can safely continue to use them.
Do not mistake poor eye contact for lack of engagement. Because SA patients may view a physician as a source of authority, they might regard direct eye contact with a physician as being somewhat disrespectful, and they may avoid eye contact altogether.
Continue to: Maintain an active approach
Maintain an active approach. SA women may prefer to view the physician as an expert, rather than a partner with whom to develop a collaborative relationship. Thus, they may feel more comfortable with direct feedback rather than a passive or reflective approach.
Suggest a postpartum support group. In a U.K. study of 17 SA postpartum women, age 20 to 45, group therapy improved health outcomes and overall satisfaction.4 It may be particularly helpful to SA patients if group therapy is facilitated by a culturally sensitive moderator.
Help patients overcome logistical barriers. Lack of transportation, childcare difficulties, and financial limitations are common deterrents to treatment. These barriers may be particularly challenging for SA women of lower socioeconomic status. Postpartum mothers who feel overtasked with caring for their children and undertaking household duties may feel less able to complete therapy.
Screen for adherence. Although SA patients may view clinicians as authority figures, adherence with medications or treatment plans should not be assumed. Many patients may quietly avoid treatments or recommendations instead of discussing their ambivalence with their clinicians.
1. Anand AS, Cochrane R. The mental health status of South Asian women in Britain. A review of the UK literature. Psychol Dev Soc J. 2005;17(2):195-214.
2. Bhugra D, Desai M. Attempted suicide in South Asian women. Advances in Psychiatric Treatment. 2002;8(6):418-423.
3. Chopra A, Doiphode VV. Ayurvedic medicine. Core concept, therapeutic principles, and current relevance. Med Clin North Am. 2002;86(1):75-89,vii.
4. Masood Y, Lovell K, Lunat F, et al. Group psychological intervention for postnatal depression: a nested qualitative study with British South Asian women. BMC Womens Health. 2015;25(15):109.
1. Anand AS, Cochrane R. The mental health status of South Asian women in Britain. A review of the UK literature. Psychol Dev Soc J. 2005;17(2):195-214.
2. Bhugra D, Desai M. Attempted suicide in South Asian women. Advances in Psychiatric Treatment. 2002;8(6):418-423.
3. Chopra A, Doiphode VV. Ayurvedic medicine. Core concept, therapeutic principles, and current relevance. Med Clin North Am. 2002;86(1):75-89,vii.
4. Masood Y, Lovell K, Lunat F, et al. Group psychological intervention for postnatal depression: a nested qualitative study with British South Asian women. BMC Womens Health. 2015;25(15):109.
Hernia registries need uniform data collection
Hernia registries have proliferated in recent years but would contribute more to the evaluation of treatments and outcomes of hernia repair if the data quality was uniform, according to a study of one U.S.-based and six European registries.
The CORE (Comparison of Hernia Registries in Europe) project was initiated in 2015 by a group of to survey the defining characteristics of each registry and compare their features. Each registry has a unique profile of data collected, basis of participation, and financial support.
“Despite the differences in the way data are collected for each of the listed hernia registries, the data are indispensable in clinical research. As a consequence of the numerous innovations in hernia surgery (surgical procedures, meshes, fixation devices), hardly any other area of surgical study has such a high need for clinical trials and data collection, comparison and analysis. Registries play a vital role in this innovation process,” the CORE investigators wrote.
The project collected information about the Danish Hernia Database (DHDB, the Netherlands), Swedish Hernia Registry (SHR), Herniamed (Germany, Switzerland, Austria), EuraHS (Belgium), Club Hernie (CH, France), EVEREG (Spain) and the Americas Hernia Society Quality Collaborative (AHSQC, the United States). Representatives of each registry provided details of the size of database, the types of cases contained in the registry, the terms of participation, operative data collected, and registry sponsors.
The DHDB and the SHR have the longest histories (created 1992 and 1998, respectively) and contain the largest number of cases (more than 200,000). The SHR has data from more than 95% of all national inguinal cases, and about 15% of ventral and parastomal cases). The DHDB covers 90% of all inguinal cases and 80% of all ventral and other types of hernia. These two registries are publicly funded, nonprofit institutions.
The other registries are of more recent origin (2007-2015), cover a lower percentage of the hernia cases in each country, but nonetheless have accumulated a large number of cases (for example, Herniamed has data on more than 290,000 inguinal cases and almost 200,000 ventral and other types of hernias). These registries are industry funded and participation by surgical centers is voluntary.
These seven registry differ primarily in the kinds of data they collect. The DHDB collects far less data than do the others on complications and, in particular, no data on mesh complications or pain. And although each of the other registries covers a long list of complications, the lists are far from identical, according to the CORE project investigators.
Follow-up protocol also varies considerably among the registries. The CORE investigators note that the “limitation of all data analysis from registries is always selection and input bias,” and the potential of combining the data from all of the registries into one database could only be accomplished if the uniform quality and consistency in data collection were assured.
The CORE project was initiated with registry representatives and each was were responsible for the information about their registry. Conflicts are reported for each contributor on the Hernia website.
SOURCE: Kyle-Leinhase I et al. Hernia 2018;22(4):561-75.
Hernia registries have proliferated in recent years but would contribute more to the evaluation of treatments and outcomes of hernia repair if the data quality was uniform, according to a study of one U.S.-based and six European registries.
The CORE (Comparison of Hernia Registries in Europe) project was initiated in 2015 by a group of to survey the defining characteristics of each registry and compare their features. Each registry has a unique profile of data collected, basis of participation, and financial support.
“Despite the differences in the way data are collected for each of the listed hernia registries, the data are indispensable in clinical research. As a consequence of the numerous innovations in hernia surgery (surgical procedures, meshes, fixation devices), hardly any other area of surgical study has such a high need for clinical trials and data collection, comparison and analysis. Registries play a vital role in this innovation process,” the CORE investigators wrote.
The project collected information about the Danish Hernia Database (DHDB, the Netherlands), Swedish Hernia Registry (SHR), Herniamed (Germany, Switzerland, Austria), EuraHS (Belgium), Club Hernie (CH, France), EVEREG (Spain) and the Americas Hernia Society Quality Collaborative (AHSQC, the United States). Representatives of each registry provided details of the size of database, the types of cases contained in the registry, the terms of participation, operative data collected, and registry sponsors.
The DHDB and the SHR have the longest histories (created 1992 and 1998, respectively) and contain the largest number of cases (more than 200,000). The SHR has data from more than 95% of all national inguinal cases, and about 15% of ventral and parastomal cases). The DHDB covers 90% of all inguinal cases and 80% of all ventral and other types of hernia. These two registries are publicly funded, nonprofit institutions.
The other registries are of more recent origin (2007-2015), cover a lower percentage of the hernia cases in each country, but nonetheless have accumulated a large number of cases (for example, Herniamed has data on more than 290,000 inguinal cases and almost 200,000 ventral and other types of hernias). These registries are industry funded and participation by surgical centers is voluntary.
These seven registry differ primarily in the kinds of data they collect. The DHDB collects far less data than do the others on complications and, in particular, no data on mesh complications or pain. And although each of the other registries covers a long list of complications, the lists are far from identical, according to the CORE project investigators.
Follow-up protocol also varies considerably among the registries. The CORE investigators note that the “limitation of all data analysis from registries is always selection and input bias,” and the potential of combining the data from all of the registries into one database could only be accomplished if the uniform quality and consistency in data collection were assured.
The CORE project was initiated with registry representatives and each was were responsible for the information about their registry. Conflicts are reported for each contributor on the Hernia website.
SOURCE: Kyle-Leinhase I et al. Hernia 2018;22(4):561-75.
Hernia registries have proliferated in recent years but would contribute more to the evaluation of treatments and outcomes of hernia repair if the data quality was uniform, according to a study of one U.S.-based and six European registries.
The CORE (Comparison of Hernia Registries in Europe) project was initiated in 2015 by a group of to survey the defining characteristics of each registry and compare their features. Each registry has a unique profile of data collected, basis of participation, and financial support.
“Despite the differences in the way data are collected for each of the listed hernia registries, the data are indispensable in clinical research. As a consequence of the numerous innovations in hernia surgery (surgical procedures, meshes, fixation devices), hardly any other area of surgical study has such a high need for clinical trials and data collection, comparison and analysis. Registries play a vital role in this innovation process,” the CORE investigators wrote.
The project collected information about the Danish Hernia Database (DHDB, the Netherlands), Swedish Hernia Registry (SHR), Herniamed (Germany, Switzerland, Austria), EuraHS (Belgium), Club Hernie (CH, France), EVEREG (Spain) and the Americas Hernia Society Quality Collaborative (AHSQC, the United States). Representatives of each registry provided details of the size of database, the types of cases contained in the registry, the terms of participation, operative data collected, and registry sponsors.
The DHDB and the SHR have the longest histories (created 1992 and 1998, respectively) and contain the largest number of cases (more than 200,000). The SHR has data from more than 95% of all national inguinal cases, and about 15% of ventral and parastomal cases). The DHDB covers 90% of all inguinal cases and 80% of all ventral and other types of hernia. These two registries are publicly funded, nonprofit institutions.
The other registries are of more recent origin (2007-2015), cover a lower percentage of the hernia cases in each country, but nonetheless have accumulated a large number of cases (for example, Herniamed has data on more than 290,000 inguinal cases and almost 200,000 ventral and other types of hernias). These registries are industry funded and participation by surgical centers is voluntary.
These seven registry differ primarily in the kinds of data they collect. The DHDB collects far less data than do the others on complications and, in particular, no data on mesh complications or pain. And although each of the other registries covers a long list of complications, the lists are far from identical, according to the CORE project investigators.
Follow-up protocol also varies considerably among the registries. The CORE investigators note that the “limitation of all data analysis from registries is always selection and input bias,” and the potential of combining the data from all of the registries into one database could only be accomplished if the uniform quality and consistency in data collection were assured.
The CORE project was initiated with registry representatives and each was were responsible for the information about their registry. Conflicts are reported for each contributor on the Hernia website.
SOURCE: Kyle-Leinhase I et al. Hernia 2018;22(4):561-75.
FROM HERNIA
Should PCPs take over chronic HCV treatment?
ESTES PARK, COLO. – Direct-acting antiviral (DAA) therapy for chronic hepatitis C virus (HCV) infection has progressed to the point that it’s time for primary care physicians to take on the treatment of all affected patients who don’t have advanced fibrosis or other complex forms of the disease, Michael Kriss, MD, asserted at a conference on internal medicine sponsored by the University of Colorado.
declared Dr. Kriss, a gastroenterologist at the university.
Referral to a specialist should be reserved for those more complex patients with stage 3 or 4 fibrosis, hepatocellular carcinoma, coinfection with HIV or hepatitis B virus, end-stage organ disease being considered for transplantation, prior exposure to an NS5A inhibitor, or concomitant secondary liver disease, such as nonalcoholic steatohepatitis, he continued.
Treatment of chronic HCV now entails just 8-12 weeks of DAA therapy, with a cure rate greater than 95% and almost no side effects. The costs have come down, too, because of increased competition.
The epidemiology of HCV infection is changing. The opioid epidemic is driving an increased incidence of acute infection, with nearly 34,000 new cases per year, most of which will go on to chronic infection. Moreover, 93% of acutely infected individuals and half of the estimated 3.5 million Americans with chronic HCV are unaware they are infected. The scope of the problem is too big for gastroenterologists and infectious disease specialists to handle alone, according to Dr. Kriss.
Contemporary treatment of HCV involves a 6-step program that’s simpler than that of many of the diseases primary care physicians currently manage.
1) Confirm chronicity by two measurements of HCV RNA obtained 6 months apart.
2) Stage the patient’s fibrosis.
3) Order a baseline cross-sectional ultrasound to exclude cirrhosis or hepatocellular carcinoma, since the fibrosis staging methods aren’t foolproof.
4) Make sure the patient is still positive for HCV RNA prior to therapy.
5) Commence DAA therapy. The simplest regimen is 8 weeks of glecaprevir/pibrentasvir (Mavyret), provided it’s approved by the patient’s insurance.
6) Get a repeat HCV RNA 12 weeks after conclusion of treatment to confirm there is a sustained virologic response.
The critical part is step 2 because that’s how primary care physicians will decide which patients to treat in their office and which to refer to a specialist. Many biomarkers are available, some proprietary and others gratis. Dr. Kriss is particularly partial to the free Fibrosis-4 (Fib-4) calculator, which provides an estimate of the amount of liver scarring based upon the patient’s age, liver enzyme levels, and platelet count.
“This is really a valuable, extremely well-validated tool that any of you can use in your clinic,” he said.
The American Association for the Study of Liver Disease/Infectious Diseases Society of America hepatitis C guidelines state that the nonproprietary biomarker assays are equivalent to transient elastography for staging fibrosis. Those guidelines are an “outstanding” and extremely user-friendly resource for primary care physicians interested in taking on the treatment of chronic HCV, Dr. Kriss added.
In selecting a DAA, another key resource is the HEP Drug Interactions website, which enables physicians to identify potentially problematic drug-drug interactions. The two most important interactions involve statins or proton pump inhibitors.
Recent studies have shown that the benefits of achieving a sustained virologic response include a clinically meaningful regression of advanced fibrosis at 1 year after treatment in roughly half of patients (Dig Dis Sci. 2018 Feb;63[2]:486-92) and a reduced risk of developing hepatocellular carcinoma (Hepatology. 2018 Jun;67[6]:2244-53).
Models of remote consultation pathways to link primary care providers with consultant HCV specialists are being developed. In a study of 600 patients at federally qualified community health centers in Washington, the risk-adjusted cure rate was 90.4% among patients assigned to a nurse practitioner, 87.6% if DAA therapy was prescribed by a primary care physician, and 84.8% if treated by a specialist. Treatment visit adherence followed the same pattern and correlated with cure rates (Ann Intern Med. 2017 Sep 5;167[5]:311-8).
“Patients might be better off seeing someone they know instead of somebody like me who they’re only going to see one time,” Dr. Kriss observed.
Turning to a couple of controversial expansions of DAA into new populations, Dr. Kriss noted that the AASLD/IDSA guidelines state that active intravenous drug use should not be considered a contraindication to HCV therapy.
“Scaling up HCV treatment in persons who inject drugs is necessary to positively impact the HCV epidemic in the U.S. and globally,” according to the guidelines.
This message was recently underscored by the positive results of the seven-country SIMPLIFY trial, in which 12 weeks of velpatasvir-sofosbuvir (Epclusa) in a population of recent IV drug users achieved a 97% cure rate, with only one reinfection in this high-risk population (Lancet Gastroenterol Hepatol. 2018 Mar;3[3]:153-61).
The hottest controversy in hepatology has been whether to place HCV-positive donor organs in HCV-negative recipients.
“This is intentionally giving hepatitis C to the recipient knowing that he can be cured. Because treatment cure rates are so high, I predict this will become the standard of care in the next year or two,” Dr. Kriss said.
He reported having no financial conflicts regarding his presentation.
.
ESTES PARK, COLO. – Direct-acting antiviral (DAA) therapy for chronic hepatitis C virus (HCV) infection has progressed to the point that it’s time for primary care physicians to take on the treatment of all affected patients who don’t have advanced fibrosis or other complex forms of the disease, Michael Kriss, MD, asserted at a conference on internal medicine sponsored by the University of Colorado.
declared Dr. Kriss, a gastroenterologist at the university.
Referral to a specialist should be reserved for those more complex patients with stage 3 or 4 fibrosis, hepatocellular carcinoma, coinfection with HIV or hepatitis B virus, end-stage organ disease being considered for transplantation, prior exposure to an NS5A inhibitor, or concomitant secondary liver disease, such as nonalcoholic steatohepatitis, he continued.
Treatment of chronic HCV now entails just 8-12 weeks of DAA therapy, with a cure rate greater than 95% and almost no side effects. The costs have come down, too, because of increased competition.
The epidemiology of HCV infection is changing. The opioid epidemic is driving an increased incidence of acute infection, with nearly 34,000 new cases per year, most of which will go on to chronic infection. Moreover, 93% of acutely infected individuals and half of the estimated 3.5 million Americans with chronic HCV are unaware they are infected. The scope of the problem is too big for gastroenterologists and infectious disease specialists to handle alone, according to Dr. Kriss.
Contemporary treatment of HCV involves a 6-step program that’s simpler than that of many of the diseases primary care physicians currently manage.
1) Confirm chronicity by two measurements of HCV RNA obtained 6 months apart.
2) Stage the patient’s fibrosis.
3) Order a baseline cross-sectional ultrasound to exclude cirrhosis or hepatocellular carcinoma, since the fibrosis staging methods aren’t foolproof.
4) Make sure the patient is still positive for HCV RNA prior to therapy.
5) Commence DAA therapy. The simplest regimen is 8 weeks of glecaprevir/pibrentasvir (Mavyret), provided it’s approved by the patient’s insurance.
6) Get a repeat HCV RNA 12 weeks after conclusion of treatment to confirm there is a sustained virologic response.
The critical part is step 2 because that’s how primary care physicians will decide which patients to treat in their office and which to refer to a specialist. Many biomarkers are available, some proprietary and others gratis. Dr. Kriss is particularly partial to the free Fibrosis-4 (Fib-4) calculator, which provides an estimate of the amount of liver scarring based upon the patient’s age, liver enzyme levels, and platelet count.
“This is really a valuable, extremely well-validated tool that any of you can use in your clinic,” he said.
The American Association for the Study of Liver Disease/Infectious Diseases Society of America hepatitis C guidelines state that the nonproprietary biomarker assays are equivalent to transient elastography for staging fibrosis. Those guidelines are an “outstanding” and extremely user-friendly resource for primary care physicians interested in taking on the treatment of chronic HCV, Dr. Kriss added.
In selecting a DAA, another key resource is the HEP Drug Interactions website, which enables physicians to identify potentially problematic drug-drug interactions. The two most important interactions involve statins or proton pump inhibitors.
Recent studies have shown that the benefits of achieving a sustained virologic response include a clinically meaningful regression of advanced fibrosis at 1 year after treatment in roughly half of patients (Dig Dis Sci. 2018 Feb;63[2]:486-92) and a reduced risk of developing hepatocellular carcinoma (Hepatology. 2018 Jun;67[6]:2244-53).
Models of remote consultation pathways to link primary care providers with consultant HCV specialists are being developed. In a study of 600 patients at federally qualified community health centers in Washington, the risk-adjusted cure rate was 90.4% among patients assigned to a nurse practitioner, 87.6% if DAA therapy was prescribed by a primary care physician, and 84.8% if treated by a specialist. Treatment visit adherence followed the same pattern and correlated with cure rates (Ann Intern Med. 2017 Sep 5;167[5]:311-8).
“Patients might be better off seeing someone they know instead of somebody like me who they’re only going to see one time,” Dr. Kriss observed.
Turning to a couple of controversial expansions of DAA into new populations, Dr. Kriss noted that the AASLD/IDSA guidelines state that active intravenous drug use should not be considered a contraindication to HCV therapy.
“Scaling up HCV treatment in persons who inject drugs is necessary to positively impact the HCV epidemic in the U.S. and globally,” according to the guidelines.
This message was recently underscored by the positive results of the seven-country SIMPLIFY trial, in which 12 weeks of velpatasvir-sofosbuvir (Epclusa) in a population of recent IV drug users achieved a 97% cure rate, with only one reinfection in this high-risk population (Lancet Gastroenterol Hepatol. 2018 Mar;3[3]:153-61).
The hottest controversy in hepatology has been whether to place HCV-positive donor organs in HCV-negative recipients.
“This is intentionally giving hepatitis C to the recipient knowing that he can be cured. Because treatment cure rates are so high, I predict this will become the standard of care in the next year or two,” Dr. Kriss said.
He reported having no financial conflicts regarding his presentation.
.
ESTES PARK, COLO. – Direct-acting antiviral (DAA) therapy for chronic hepatitis C virus (HCV) infection has progressed to the point that it’s time for primary care physicians to take on the treatment of all affected patients who don’t have advanced fibrosis or other complex forms of the disease, Michael Kriss, MD, asserted at a conference on internal medicine sponsored by the University of Colorado.
declared Dr. Kriss, a gastroenterologist at the university.
Referral to a specialist should be reserved for those more complex patients with stage 3 or 4 fibrosis, hepatocellular carcinoma, coinfection with HIV or hepatitis B virus, end-stage organ disease being considered for transplantation, prior exposure to an NS5A inhibitor, or concomitant secondary liver disease, such as nonalcoholic steatohepatitis, he continued.
Treatment of chronic HCV now entails just 8-12 weeks of DAA therapy, with a cure rate greater than 95% and almost no side effects. The costs have come down, too, because of increased competition.
The epidemiology of HCV infection is changing. The opioid epidemic is driving an increased incidence of acute infection, with nearly 34,000 new cases per year, most of which will go on to chronic infection. Moreover, 93% of acutely infected individuals and half of the estimated 3.5 million Americans with chronic HCV are unaware they are infected. The scope of the problem is too big for gastroenterologists and infectious disease specialists to handle alone, according to Dr. Kriss.
Contemporary treatment of HCV involves a 6-step program that’s simpler than that of many of the diseases primary care physicians currently manage.
1) Confirm chronicity by two measurements of HCV RNA obtained 6 months apart.
2) Stage the patient’s fibrosis.
3) Order a baseline cross-sectional ultrasound to exclude cirrhosis or hepatocellular carcinoma, since the fibrosis staging methods aren’t foolproof.
4) Make sure the patient is still positive for HCV RNA prior to therapy.
5) Commence DAA therapy. The simplest regimen is 8 weeks of glecaprevir/pibrentasvir (Mavyret), provided it’s approved by the patient’s insurance.
6) Get a repeat HCV RNA 12 weeks after conclusion of treatment to confirm there is a sustained virologic response.
The critical part is step 2 because that’s how primary care physicians will decide which patients to treat in their office and which to refer to a specialist. Many biomarkers are available, some proprietary and others gratis. Dr. Kriss is particularly partial to the free Fibrosis-4 (Fib-4) calculator, which provides an estimate of the amount of liver scarring based upon the patient’s age, liver enzyme levels, and platelet count.
“This is really a valuable, extremely well-validated tool that any of you can use in your clinic,” he said.
The American Association for the Study of Liver Disease/Infectious Diseases Society of America hepatitis C guidelines state that the nonproprietary biomarker assays are equivalent to transient elastography for staging fibrosis. Those guidelines are an “outstanding” and extremely user-friendly resource for primary care physicians interested in taking on the treatment of chronic HCV, Dr. Kriss added.
In selecting a DAA, another key resource is the HEP Drug Interactions website, which enables physicians to identify potentially problematic drug-drug interactions. The two most important interactions involve statins or proton pump inhibitors.
Recent studies have shown that the benefits of achieving a sustained virologic response include a clinically meaningful regression of advanced fibrosis at 1 year after treatment in roughly half of patients (Dig Dis Sci. 2018 Feb;63[2]:486-92) and a reduced risk of developing hepatocellular carcinoma (Hepatology. 2018 Jun;67[6]:2244-53).
Models of remote consultation pathways to link primary care providers with consultant HCV specialists are being developed. In a study of 600 patients at federally qualified community health centers in Washington, the risk-adjusted cure rate was 90.4% among patients assigned to a nurse practitioner, 87.6% if DAA therapy was prescribed by a primary care physician, and 84.8% if treated by a specialist. Treatment visit adherence followed the same pattern and correlated with cure rates (Ann Intern Med. 2017 Sep 5;167[5]:311-8).
“Patients might be better off seeing someone they know instead of somebody like me who they’re only going to see one time,” Dr. Kriss observed.
Turning to a couple of controversial expansions of DAA into new populations, Dr. Kriss noted that the AASLD/IDSA guidelines state that active intravenous drug use should not be considered a contraindication to HCV therapy.
“Scaling up HCV treatment in persons who inject drugs is necessary to positively impact the HCV epidemic in the U.S. and globally,” according to the guidelines.
This message was recently underscored by the positive results of the seven-country SIMPLIFY trial, in which 12 weeks of velpatasvir-sofosbuvir (Epclusa) in a population of recent IV drug users achieved a 97% cure rate, with only one reinfection in this high-risk population (Lancet Gastroenterol Hepatol. 2018 Mar;3[3]:153-61).
The hottest controversy in hepatology has been whether to place HCV-positive donor organs in HCV-negative recipients.
“This is intentionally giving hepatitis C to the recipient knowing that he can be cured. Because treatment cure rates are so high, I predict this will become the standard of care in the next year or two,” Dr. Kriss said.
He reported having no financial conflicts regarding his presentation.
.
REPORTING FROM COLORADO IM
Marzeptacog alfa reduces bleeding episodes in hemophilia with inhibitors
The activated factor VIIa variant according to researchers.
To date, the trial has enrolled five patients with hemophilia A or B and inhibitors. Catalyst would not disclose how many patients have hemophilia A and how many have hemophilia B. Three patients have completed dosing with marzeptacog alfa in a phase 2/3 study. None of these patients experienced bleeding during treatment, and none have developed antidrug antibodies or reported injection site reactions. As for the other two patients enrolled in this study, one withdrew consent, and one died of an adverse event unrelated to marzeptacog alfa.
Howard Levy, chief medical officer of Catalyst Biosciences, which has been developing this drug and sponsored the trial, presented these data at the 2018 Hemophilia Drug Development Summit in Boston.
The goal of this ongoing trial is to determine whether daily subcutaneous injections of marzeptacog alfa can eliminate or minimize spontaneous bleeding episodes. The primary endpoint is a reduction in annualized bleed rate (ABR), compared with each individual’s recorded historical ABR.
One patient with a historic ABR of 26.7 experienced a bleed on day 46 when receiving marzeptacog alfa at 30 mcg/kg but then had no bleeds after 50 days of treatment with marzeptacog alfa at 60 mcg/kg. This patient did experience a bleed 16 days after the end of dosing at 60 mcg/kg.
A second patient with a historic ABR of 16.6 had no bleeds when receiving marzeptacog alfa at 30 mcg/kg for 50 days.
And a third patient with a historic ABR of 15.9 had no bleeds when receiving marzeptacog alfa at 30 mcg/kg for 44 days.
“The data from these 3 individuals support the efficacy of [marzeptacog alfa] to reduce annualized bleed rates after daily subcutaneous injections,” said Nassim Usman, PhD, chief executive officer of Catalyst Biosciences. “Importantly, to date, we have not observed any injection site reactions nor any antidrug antibodies after more than 200 subcutaneous doses of [marzeptacog alfa].”
A fourth patient with a historic ABR of 18.3 had a fatal hemorrhagic stroke on day 11 that was considered unrelated to marzeptacog alfa. The patient had previously treated hypertension that was going untreated at the time of death.
A fifth patient with a historic ABR of 12.2 withdrew consent.
The activated factor VIIa variant according to researchers.
To date, the trial has enrolled five patients with hemophilia A or B and inhibitors. Catalyst would not disclose how many patients have hemophilia A and how many have hemophilia B. Three patients have completed dosing with marzeptacog alfa in a phase 2/3 study. None of these patients experienced bleeding during treatment, and none have developed antidrug antibodies or reported injection site reactions. As for the other two patients enrolled in this study, one withdrew consent, and one died of an adverse event unrelated to marzeptacog alfa.
Howard Levy, chief medical officer of Catalyst Biosciences, which has been developing this drug and sponsored the trial, presented these data at the 2018 Hemophilia Drug Development Summit in Boston.
The goal of this ongoing trial is to determine whether daily subcutaneous injections of marzeptacog alfa can eliminate or minimize spontaneous bleeding episodes. The primary endpoint is a reduction in annualized bleed rate (ABR), compared with each individual’s recorded historical ABR.
One patient with a historic ABR of 26.7 experienced a bleed on day 46 when receiving marzeptacog alfa at 30 mcg/kg but then had no bleeds after 50 days of treatment with marzeptacog alfa at 60 mcg/kg. This patient did experience a bleed 16 days after the end of dosing at 60 mcg/kg.
A second patient with a historic ABR of 16.6 had no bleeds when receiving marzeptacog alfa at 30 mcg/kg for 50 days.
And a third patient with a historic ABR of 15.9 had no bleeds when receiving marzeptacog alfa at 30 mcg/kg for 44 days.
“The data from these 3 individuals support the efficacy of [marzeptacog alfa] to reduce annualized bleed rates after daily subcutaneous injections,” said Nassim Usman, PhD, chief executive officer of Catalyst Biosciences. “Importantly, to date, we have not observed any injection site reactions nor any antidrug antibodies after more than 200 subcutaneous doses of [marzeptacog alfa].”
A fourth patient with a historic ABR of 18.3 had a fatal hemorrhagic stroke on day 11 that was considered unrelated to marzeptacog alfa. The patient had previously treated hypertension that was going untreated at the time of death.
A fifth patient with a historic ABR of 12.2 withdrew consent.
The activated factor VIIa variant according to researchers.
To date, the trial has enrolled five patients with hemophilia A or B and inhibitors. Catalyst would not disclose how many patients have hemophilia A and how many have hemophilia B. Three patients have completed dosing with marzeptacog alfa in a phase 2/3 study. None of these patients experienced bleeding during treatment, and none have developed antidrug antibodies or reported injection site reactions. As for the other two patients enrolled in this study, one withdrew consent, and one died of an adverse event unrelated to marzeptacog alfa.
Howard Levy, chief medical officer of Catalyst Biosciences, which has been developing this drug and sponsored the trial, presented these data at the 2018 Hemophilia Drug Development Summit in Boston.
The goal of this ongoing trial is to determine whether daily subcutaneous injections of marzeptacog alfa can eliminate or minimize spontaneous bleeding episodes. The primary endpoint is a reduction in annualized bleed rate (ABR), compared with each individual’s recorded historical ABR.
One patient with a historic ABR of 26.7 experienced a bleed on day 46 when receiving marzeptacog alfa at 30 mcg/kg but then had no bleeds after 50 days of treatment with marzeptacog alfa at 60 mcg/kg. This patient did experience a bleed 16 days after the end of dosing at 60 mcg/kg.
A second patient with a historic ABR of 16.6 had no bleeds when receiving marzeptacog alfa at 30 mcg/kg for 50 days.
And a third patient with a historic ABR of 15.9 had no bleeds when receiving marzeptacog alfa at 30 mcg/kg for 44 days.
“The data from these 3 individuals support the efficacy of [marzeptacog alfa] to reduce annualized bleed rates after daily subcutaneous injections,” said Nassim Usman, PhD, chief executive officer of Catalyst Biosciences. “Importantly, to date, we have not observed any injection site reactions nor any antidrug antibodies after more than 200 subcutaneous doses of [marzeptacog alfa].”
A fourth patient with a historic ABR of 18.3 had a fatal hemorrhagic stroke on day 11 that was considered unrelated to marzeptacog alfa. The patient had previously treated hypertension that was going untreated at the time of death.
A fifth patient with a historic ABR of 12.2 withdrew consent.
How Does Provider Attire Impact Perceived Care and Infection Risk in the Clinical Setting?
Schools’ cell phone policies: What’s best for students?
Schools across the country – or around the world, for that matter – are grappling with policies and regulations tied to their students’ use of cell phones during school hours.
These policies run the gamut, from allowing students to use smartphones as learning tools to requiring them to keep the devices turned off. One public high school’s action on cell phones, initiated this year by a parent, has prompted one parent’s thumbs-up. This school is phasing in a lock-up-your-cell-phone policy.
NPR reporter Jennifer Ludden said that when the idea was first floated toward the end of the last school year, it provoked a parental outcry at her sons’ school. “My generation is used to 24/7 access to our kids, wherever they are. I confess, I’ve texted mine at school. It was about a doctor’s appointment,” Ms. Ludden says.
Meanwhile, she says, some asked: “ ‘What if there’s an emergency?’ It’s a fair question. We’ve all heard about students hiding from a gunman, posting updates, and texting to let friends and family know they’re safe,” she says.
Yet, evidence suggests that a cell-free classroom is a safer classroom, without the distraction of that screen to divert attention instructions that come in the wake of a school emergency. And, in a chilling mental image, a phone’s ringtone or vibration on a desk could be a beacon for a shooter.
Putting aside the horrific potential of school violence, a no-phone policy could have other tangible benefits that would help students in real life. The rejigging of adolescent brains away from the umbilical cord of their phone would be welcome. Imagine social interactions, instead of that face-down, thumbs-poised posture that is everywhere! And it could also help curb the electronic version of passing notes during tests.
Ms. Ludden mentions another benefit. “ Invaluable time to relax, and connect, without phones. I’m grateful to see some schools investing in that,” Ms. Ludden says.
Click here to listen to Ms. Ludden’s take.
Schools across the country – or around the world, for that matter – are grappling with policies and regulations tied to their students’ use of cell phones during school hours.
These policies run the gamut, from allowing students to use smartphones as learning tools to requiring them to keep the devices turned off. One public high school’s action on cell phones, initiated this year by a parent, has prompted one parent’s thumbs-up. This school is phasing in a lock-up-your-cell-phone policy.
NPR reporter Jennifer Ludden said that when the idea was first floated toward the end of the last school year, it provoked a parental outcry at her sons’ school. “My generation is used to 24/7 access to our kids, wherever they are. I confess, I’ve texted mine at school. It was about a doctor’s appointment,” Ms. Ludden says.
Meanwhile, she says, some asked: “ ‘What if there’s an emergency?’ It’s a fair question. We’ve all heard about students hiding from a gunman, posting updates, and texting to let friends and family know they’re safe,” she says.
Yet, evidence suggests that a cell-free classroom is a safer classroom, without the distraction of that screen to divert attention instructions that come in the wake of a school emergency. And, in a chilling mental image, a phone’s ringtone or vibration on a desk could be a beacon for a shooter.
Putting aside the horrific potential of school violence, a no-phone policy could have other tangible benefits that would help students in real life. The rejigging of adolescent brains away from the umbilical cord of their phone would be welcome. Imagine social interactions, instead of that face-down, thumbs-poised posture that is everywhere! And it could also help curb the electronic version of passing notes during tests.
Ms. Ludden mentions another benefit. “ Invaluable time to relax, and connect, without phones. I’m grateful to see some schools investing in that,” Ms. Ludden says.
Click here to listen to Ms. Ludden’s take.
Schools across the country – or around the world, for that matter – are grappling with policies and regulations tied to their students’ use of cell phones during school hours.
These policies run the gamut, from allowing students to use smartphones as learning tools to requiring them to keep the devices turned off. One public high school’s action on cell phones, initiated this year by a parent, has prompted one parent’s thumbs-up. This school is phasing in a lock-up-your-cell-phone policy.
NPR reporter Jennifer Ludden said that when the idea was first floated toward the end of the last school year, it provoked a parental outcry at her sons’ school. “My generation is used to 24/7 access to our kids, wherever they are. I confess, I’ve texted mine at school. It was about a doctor’s appointment,” Ms. Ludden says.
Meanwhile, she says, some asked: “ ‘What if there’s an emergency?’ It’s a fair question. We’ve all heard about students hiding from a gunman, posting updates, and texting to let friends and family know they’re safe,” she says.
Yet, evidence suggests that a cell-free classroom is a safer classroom, without the distraction of that screen to divert attention instructions that come in the wake of a school emergency. And, in a chilling mental image, a phone’s ringtone or vibration on a desk could be a beacon for a shooter.
Putting aside the horrific potential of school violence, a no-phone policy could have other tangible benefits that would help students in real life. The rejigging of adolescent brains away from the umbilical cord of their phone would be welcome. Imagine social interactions, instead of that face-down, thumbs-poised posture that is everywhere! And it could also help curb the electronic version of passing notes during tests.
Ms. Ludden mentions another benefit. “ Invaluable time to relax, and connect, without phones. I’m grateful to see some schools investing in that,” Ms. Ludden says.
Click here to listen to Ms. Ludden’s take.