User login
FDA requires companion diagnostics for checkpoint inhibitors in urothelial cancer
Two separate FDA-approved companion diagnostic tests are now required to determine PD-L1 levels in tumor tissue for those who are cisplatin-ineligible.
Pembrolizumab is now indicated for the treatment of patients who are not eligible for cisplatin-containing chemotherapy and whose tumors have a Combined Positive Score (CPS) for PD-L1 expression of greater than or equal to 10, or in patients who are not eligible for any platinum-containing chemotherapy regardless of PD-L1 status. The FDA approved the Dako PD-L1 IHC 22C3 PharmDx Assay for determining PD-L1 expression, through staining in tumor and immune cells, before prescribing pembrolizumab.
Atezolizumab is now indicated for the treatment of patients who are not eligible for cisplatin-containing chemotherapy, and whose tumors show PD-L1 expression through stained tumor-infiltrating immune cells covering greater than or equal to 5% of the tumor area, or patients who are not eligible for any platinum-containing therapy regardless of level of tumor PD-L1 expression. The FDA approved the Ventana PD-L1 (SP142) Assay as a companion diagnostic test to determine PD L1 expression in immune cells before prescribing atezolizumab.
PI is updated for both drugs to require use of an FDA-approved test for selection of patients being treated in the first-line setting who are cisplatin-ineligible, but second-line indications in urothelial carcinoma for both drugs remain unchanged, according to an FDA statement.
Two separate FDA-approved companion diagnostic tests are now required to determine PD-L1 levels in tumor tissue for those who are cisplatin-ineligible.
Pembrolizumab is now indicated for the treatment of patients who are not eligible for cisplatin-containing chemotherapy and whose tumors have a Combined Positive Score (CPS) for PD-L1 expression of greater than or equal to 10, or in patients who are not eligible for any platinum-containing chemotherapy regardless of PD-L1 status. The FDA approved the Dako PD-L1 IHC 22C3 PharmDx Assay for determining PD-L1 expression, through staining in tumor and immune cells, before prescribing pembrolizumab.
Atezolizumab is now indicated for the treatment of patients who are not eligible for cisplatin-containing chemotherapy, and whose tumors show PD-L1 expression through stained tumor-infiltrating immune cells covering greater than or equal to 5% of the tumor area, or patients who are not eligible for any platinum-containing therapy regardless of level of tumor PD-L1 expression. The FDA approved the Ventana PD-L1 (SP142) Assay as a companion diagnostic test to determine PD L1 expression in immune cells before prescribing atezolizumab.
PI is updated for both drugs to require use of an FDA-approved test for selection of patients being treated in the first-line setting who are cisplatin-ineligible, but second-line indications in urothelial carcinoma for both drugs remain unchanged, according to an FDA statement.
Two separate FDA-approved companion diagnostic tests are now required to determine PD-L1 levels in tumor tissue for those who are cisplatin-ineligible.
Pembrolizumab is now indicated for the treatment of patients who are not eligible for cisplatin-containing chemotherapy and whose tumors have a Combined Positive Score (CPS) for PD-L1 expression of greater than or equal to 10, or in patients who are not eligible for any platinum-containing chemotherapy regardless of PD-L1 status. The FDA approved the Dako PD-L1 IHC 22C3 PharmDx Assay for determining PD-L1 expression, through staining in tumor and immune cells, before prescribing pembrolizumab.
Atezolizumab is now indicated for the treatment of patients who are not eligible for cisplatin-containing chemotherapy, and whose tumors show PD-L1 expression through stained tumor-infiltrating immune cells covering greater than or equal to 5% of the tumor area, or patients who are not eligible for any platinum-containing therapy regardless of level of tumor PD-L1 expression. The FDA approved the Ventana PD-L1 (SP142) Assay as a companion diagnostic test to determine PD L1 expression in immune cells before prescribing atezolizumab.
PI is updated for both drugs to require use of an FDA-approved test for selection of patients being treated in the first-line setting who are cisplatin-ineligible, but second-line indications in urothelial carcinoma for both drugs remain unchanged, according to an FDA statement.
A new, simple, inexpensive DVT diagnostic aid
NEW ORLEANS – Both the neutrophil-to-lymphocyte ratio and the platelet-to-lymphocyte ratio proved to be better predictors of the presence or absence of deep vein thrombosis than the ubiquitous D-dimer test in a retrospective study, Jason Mouabbi, MD, reported at the annual meeting of the American College of Physicians.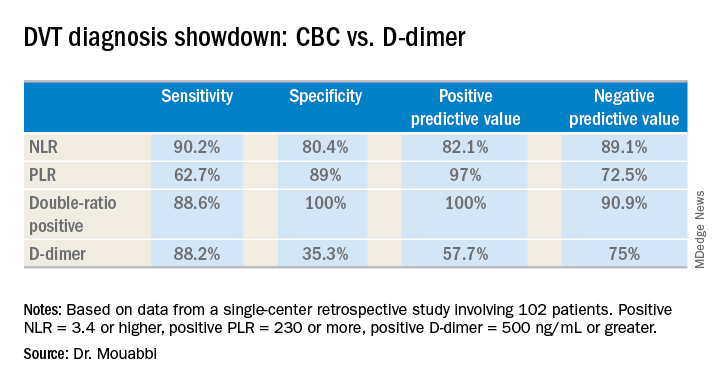
What’s more, both the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) can be readily calculated from the readout of a complete blood count (CBC) with differential. A CBC costs an average of $16, and everybody that comes through a hospital emergency department gets one. In contrast, the average charge for a D-dimer test is about $231 nationwide, and depending upon the specific test used the results can take up to a couple of hours to come back, noted Dr. Mouabbi of St. John Hospital and Medical Center in Detroit.
“The NLR and PLR ratios offer a new, powerful, affordable, simple, and readily available tool in the hands of clinicians to help them in the diagnosis of DVT,” he said. “The NLR can be useful to rule out DVT when it’s negative, whereas PLR can be useful in ruling DVT when positive.”
Investigators in a variety of fields are looking at the NLR and PLR as emerging practical, easily obtainable biomarkers for systemic inflammation. And DVT is thought to be an inflammatory process, he explained.
Dr. Mouabbi presented a single-center retrospective study of 102 matched patients who presented with lower extremity swelling and had a CBC drawn, as well as a D-dimer test, on the same day they underwent a lower extremity Doppler ultrasound evaluation. In 51 patients, the ultrasound revealed the presence of DVT and anticoagulation was started. In the other 51 patients, the ultrasound exam was negative and they weren’t anticoagulated. Since the study purpose was to assess the implications of a primary elevation of NLR and/or PLR, patients with rheumatic diseases, inflammatory bowel disease, recent surgery, chronic renal or liver disease, inherited thrombophilia, infection, or other possible secondary causes of altered ratios were excluded from the study.
A positive NLR was considered 3.4 or higher, a positive PLR was a ratio of 230 or more, and a positive D-dimer level was 500 ng/mL or greater. The NLR and PLR collectively outperformed the D-dimer test in terms of sensitivity, specificity, positive predictive value, and negative predictive value.
In addition, 89% of the DVT group were classified as “double-positive,” meaning they were both NLR and PLR positive. That combination provided the best diagnostic value of all, since none of the controls were double-positive and only 2% were PLR positive.
While the results are encouraging, before NLR and PLR can supplant D-dimer in patients with suspected DVT in clinical practice a confirmatory prospective study should be carried out, according to Dr. Mouabbi. Ideally it should include the use of the Wells score, which is part of most diagnostic algorithms as a preliminary means of categorizing DVT probability as low, moderate, or high. However, the popularity of the Wells score has fallen off in the face of reports that the results are subjective and variable. Indeed, the Wells score was included in the electronic medical record of so few participants in Dr. Mouabbi’s study that he couldn’t evaluate its utility.
He reported having no financial conflicts regarding his study, which was conducted free of commercial support.
NEW ORLEANS – Both the neutrophil-to-lymphocyte ratio and the platelet-to-lymphocyte ratio proved to be better predictors of the presence or absence of deep vein thrombosis than the ubiquitous D-dimer test in a retrospective study, Jason Mouabbi, MD, reported at the annual meeting of the American College of Physicians.
What’s more, both the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) can be readily calculated from the readout of a complete blood count (CBC) with differential. A CBC costs an average of $16, and everybody that comes through a hospital emergency department gets one. In contrast, the average charge for a D-dimer test is about $231 nationwide, and depending upon the specific test used the results can take up to a couple of hours to come back, noted Dr. Mouabbi of St. John Hospital and Medical Center in Detroit.
“The NLR and PLR ratios offer a new, powerful, affordable, simple, and readily available tool in the hands of clinicians to help them in the diagnosis of DVT,” he said. “The NLR can be useful to rule out DVT when it’s negative, whereas PLR can be useful in ruling DVT when positive.”
Investigators in a variety of fields are looking at the NLR and PLR as emerging practical, easily obtainable biomarkers for systemic inflammation. And DVT is thought to be an inflammatory process, he explained.
Dr. Mouabbi presented a single-center retrospective study of 102 matched patients who presented with lower extremity swelling and had a CBC drawn, as well as a D-dimer test, on the same day they underwent a lower extremity Doppler ultrasound evaluation. In 51 patients, the ultrasound revealed the presence of DVT and anticoagulation was started. In the other 51 patients, the ultrasound exam was negative and they weren’t anticoagulated. Since the study purpose was to assess the implications of a primary elevation of NLR and/or PLR, patients with rheumatic diseases, inflammatory bowel disease, recent surgery, chronic renal or liver disease, inherited thrombophilia, infection, or other possible secondary causes of altered ratios were excluded from the study.
A positive NLR was considered 3.4 or higher, a positive PLR was a ratio of 230 or more, and a positive D-dimer level was 500 ng/mL or greater. The NLR and PLR collectively outperformed the D-dimer test in terms of sensitivity, specificity, positive predictive value, and negative predictive value.
In addition, 89% of the DVT group were classified as “double-positive,” meaning they were both NLR and PLR positive. That combination provided the best diagnostic value of all, since none of the controls were double-positive and only 2% were PLR positive.
While the results are encouraging, before NLR and PLR can supplant D-dimer in patients with suspected DVT in clinical practice a confirmatory prospective study should be carried out, according to Dr. Mouabbi. Ideally it should include the use of the Wells score, which is part of most diagnostic algorithms as a preliminary means of categorizing DVT probability as low, moderate, or high. However, the popularity of the Wells score has fallen off in the face of reports that the results are subjective and variable. Indeed, the Wells score was included in the electronic medical record of so few participants in Dr. Mouabbi’s study that he couldn’t evaluate its utility.
He reported having no financial conflicts regarding his study, which was conducted free of commercial support.
NEW ORLEANS – Both the neutrophil-to-lymphocyte ratio and the platelet-to-lymphocyte ratio proved to be better predictors of the presence or absence of deep vein thrombosis than the ubiquitous D-dimer test in a retrospective study, Jason Mouabbi, MD, reported at the annual meeting of the American College of Physicians.
What’s more, both the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) can be readily calculated from the readout of a complete blood count (CBC) with differential. A CBC costs an average of $16, and everybody that comes through a hospital emergency department gets one. In contrast, the average charge for a D-dimer test is about $231 nationwide, and depending upon the specific test used the results can take up to a couple of hours to come back, noted Dr. Mouabbi of St. John Hospital and Medical Center in Detroit.
“The NLR and PLR ratios offer a new, powerful, affordable, simple, and readily available tool in the hands of clinicians to help them in the diagnosis of DVT,” he said. “The NLR can be useful to rule out DVT when it’s negative, whereas PLR can be useful in ruling DVT when positive.”
Investigators in a variety of fields are looking at the NLR and PLR as emerging practical, easily obtainable biomarkers for systemic inflammation. And DVT is thought to be an inflammatory process, he explained.
Dr. Mouabbi presented a single-center retrospective study of 102 matched patients who presented with lower extremity swelling and had a CBC drawn, as well as a D-dimer test, on the same day they underwent a lower extremity Doppler ultrasound evaluation. In 51 patients, the ultrasound revealed the presence of DVT and anticoagulation was started. In the other 51 patients, the ultrasound exam was negative and they weren’t anticoagulated. Since the study purpose was to assess the implications of a primary elevation of NLR and/or PLR, patients with rheumatic diseases, inflammatory bowel disease, recent surgery, chronic renal or liver disease, inherited thrombophilia, infection, or other possible secondary causes of altered ratios were excluded from the study.
A positive NLR was considered 3.4 or higher, a positive PLR was a ratio of 230 or more, and a positive D-dimer level was 500 ng/mL or greater. The NLR and PLR collectively outperformed the D-dimer test in terms of sensitivity, specificity, positive predictive value, and negative predictive value.
In addition, 89% of the DVT group were classified as “double-positive,” meaning they were both NLR and PLR positive. That combination provided the best diagnostic value of all, since none of the controls were double-positive and only 2% were PLR positive.
While the results are encouraging, before NLR and PLR can supplant D-dimer in patients with suspected DVT in clinical practice a confirmatory prospective study should be carried out, according to Dr. Mouabbi. Ideally it should include the use of the Wells score, which is part of most diagnostic algorithms as a preliminary means of categorizing DVT probability as low, moderate, or high. However, the popularity of the Wells score has fallen off in the face of reports that the results are subjective and variable. Indeed, the Wells score was included in the electronic medical record of so few participants in Dr. Mouabbi’s study that he couldn’t evaluate its utility.
He reported having no financial conflicts regarding his study, which was conducted free of commercial support.
REPORTING FROM ACP INTERNAL MEDICINE
Key clinical point:
Major finding: The neutrophil-to-lymphocyte ratio was better than the D-dimer test at helping to rule out DVT, while the platelet-to-lymphocyte ratio bested the D-dimer at ruling in DVT.
Study details: A retrospective study of 102 patients with suspected DVT.
Disclosures: Dr. Mouabbi reported no financial conflicts regarding his study, which was conducted free of commercial support.
When your patients are your neighbors
“We’ll have one strawberry sugar cone, two chocolate swirls, and a mocha almond.”
“Dr. Wilkoff, I haven’t seen you in ... must be 5 years. You look great! How’s retirement going?”
“You look great too, Kim. The neighborhood has needed an ice cream parlor like yours for a long time. How’s the family?”
Alerted by the commotion of our catching up, Kim’s husband came outside to see what was going on. Looking at my wife, he said, “You know he saved our daughter’s life?”
Well, not exactly. A timely referral to a psychologist I knew was good with eating disorders had started the slow process of returning their anorectic daughter to health. I thanked him and tried to put a more historically correct spin on his story.
Most of my encounters with former patients and their parents aren’t as dramatic as this one at Kim’s Ice Cream Shack, but they always leave me with a warm, positive feeling that stays with me all day. Every now and then they include a compliment or a thank you, but most of the time the conversations are dominated by questions about how the other are doing and what our families are up to.
One of the perks of living in the town where you practice is that your patients also are your neighbors. Not every physician views this proximity as a positive, but for me, it was a gift that has kept on giving after I retired.
Our house phone number was always listed in the phone book, and I can count on the fingers of two hands how many times in 40 years that I received what I would consider an inappropriate or invasive call. Our office offered evening and weekend hours and a generous schedule of phone-in call times. But I’m convinced that it was the neighbor-to-neighbor relationship that kept the work/home balance intact. Even though I may have helped a plumber and his wife with their sick children, that professional arrangement didn’t include a free pass to call him after hours if I knew my leaking faucet could wait until the weekend was over.
By the same token, when a patient or a customer is also your neighbor there is an unspoken ethic that the service you provide must be your best effort. That’s not an admission that I was in the habit of offering substandard care to “folks from away,” but there is special motivation when your work is being scrutinized by people you’re likely to see next week at the grocery store checkout.
Office visits with neighbors often tended to take longer because there was a tendency to drift off topic and ask about a sibling’s baseball game I had read about in the paper or how the lobster catch was running that season. On the other hand, I must admit that I did my share of reporting (really it was bragging) on my own children’s accomplishments.
But now I’m reaping the benefits of those extra minutes invested in the office, because I suspect former patients and their families are more likely to want to reminisce when we meet in a restaurant or at the farmers’ market.
If you are a young physician and worrying about finding a good work/life balance, I urge you to consider living and working and then staying on in a place in which your patients also will be your neighbors. It will enrich your work experience and repay you many times over when it’s time to retire.
If you can’t find that place, at least treat your patients as though they were your neighbors.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
“We’ll have one strawberry sugar cone, two chocolate swirls, and a mocha almond.”
“Dr. Wilkoff, I haven’t seen you in ... must be 5 years. You look great! How’s retirement going?”
“You look great too, Kim. The neighborhood has needed an ice cream parlor like yours for a long time. How’s the family?”
Alerted by the commotion of our catching up, Kim’s husband came outside to see what was going on. Looking at my wife, he said, “You know he saved our daughter’s life?”
Well, not exactly. A timely referral to a psychologist I knew was good with eating disorders had started the slow process of returning their anorectic daughter to health. I thanked him and tried to put a more historically correct spin on his story.
Most of my encounters with former patients and their parents aren’t as dramatic as this one at Kim’s Ice Cream Shack, but they always leave me with a warm, positive feeling that stays with me all day. Every now and then they include a compliment or a thank you, but most of the time the conversations are dominated by questions about how the other are doing and what our families are up to.
One of the perks of living in the town where you practice is that your patients also are your neighbors. Not every physician views this proximity as a positive, but for me, it was a gift that has kept on giving after I retired.
Our house phone number was always listed in the phone book, and I can count on the fingers of two hands how many times in 40 years that I received what I would consider an inappropriate or invasive call. Our office offered evening and weekend hours and a generous schedule of phone-in call times. But I’m convinced that it was the neighbor-to-neighbor relationship that kept the work/home balance intact. Even though I may have helped a plumber and his wife with their sick children, that professional arrangement didn’t include a free pass to call him after hours if I knew my leaking faucet could wait until the weekend was over.
By the same token, when a patient or a customer is also your neighbor there is an unspoken ethic that the service you provide must be your best effort. That’s not an admission that I was in the habit of offering substandard care to “folks from away,” but there is special motivation when your work is being scrutinized by people you’re likely to see next week at the grocery store checkout.
Office visits with neighbors often tended to take longer because there was a tendency to drift off topic and ask about a sibling’s baseball game I had read about in the paper or how the lobster catch was running that season. On the other hand, I must admit that I did my share of reporting (really it was bragging) on my own children’s accomplishments.
But now I’m reaping the benefits of those extra minutes invested in the office, because I suspect former patients and their families are more likely to want to reminisce when we meet in a restaurant or at the farmers’ market.
If you are a young physician and worrying about finding a good work/life balance, I urge you to consider living and working and then staying on in a place in which your patients also will be your neighbors. It will enrich your work experience and repay you many times over when it’s time to retire.
If you can’t find that place, at least treat your patients as though they were your neighbors.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
“We’ll have one strawberry sugar cone, two chocolate swirls, and a mocha almond.”
“Dr. Wilkoff, I haven’t seen you in ... must be 5 years. You look great! How’s retirement going?”
“You look great too, Kim. The neighborhood has needed an ice cream parlor like yours for a long time. How’s the family?”
Alerted by the commotion of our catching up, Kim’s husband came outside to see what was going on. Looking at my wife, he said, “You know he saved our daughter’s life?”
Well, not exactly. A timely referral to a psychologist I knew was good with eating disorders had started the slow process of returning their anorectic daughter to health. I thanked him and tried to put a more historically correct spin on his story.
Most of my encounters with former patients and their parents aren’t as dramatic as this one at Kim’s Ice Cream Shack, but they always leave me with a warm, positive feeling that stays with me all day. Every now and then they include a compliment or a thank you, but most of the time the conversations are dominated by questions about how the other are doing and what our families are up to.
One of the perks of living in the town where you practice is that your patients also are your neighbors. Not every physician views this proximity as a positive, but for me, it was a gift that has kept on giving after I retired.
Our house phone number was always listed in the phone book, and I can count on the fingers of two hands how many times in 40 years that I received what I would consider an inappropriate or invasive call. Our office offered evening and weekend hours and a generous schedule of phone-in call times. But I’m convinced that it was the neighbor-to-neighbor relationship that kept the work/home balance intact. Even though I may have helped a plumber and his wife with their sick children, that professional arrangement didn’t include a free pass to call him after hours if I knew my leaking faucet could wait until the weekend was over.
By the same token, when a patient or a customer is also your neighbor there is an unspoken ethic that the service you provide must be your best effort. That’s not an admission that I was in the habit of offering substandard care to “folks from away,” but there is special motivation when your work is being scrutinized by people you’re likely to see next week at the grocery store checkout.
Office visits with neighbors often tended to take longer because there was a tendency to drift off topic and ask about a sibling’s baseball game I had read about in the paper or how the lobster catch was running that season. On the other hand, I must admit that I did my share of reporting (really it was bragging) on my own children’s accomplishments.
But now I’m reaping the benefits of those extra minutes invested in the office, because I suspect former patients and their families are more likely to want to reminisce when we meet in a restaurant or at the farmers’ market.
If you are a young physician and worrying about finding a good work/life balance, I urge you to consider living and working and then staying on in a place in which your patients also will be your neighbors. It will enrich your work experience and repay you many times over when it’s time to retire.
If you can’t find that place, at least treat your patients as though they were your neighbors.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
FDA alert: Artificial heart driver linked to higher mortality
Postapproval results for SynCardia Systems’ Companion 2 (C2) driver system for temporary total artificial hearts (TAH-t) have shown higher mortality and stroke rates than were seen with the previous system, the circulatory support system. As a result, the Food and Drug Administration has issued a safety alert cautioning them to weigh the risks and benefits carefully. The alert, issued on August 17, is based on a postapproval study conducted by SynCardia Systems.
Furthermore, patients and health care professionals are encouraged to report any adverse events using the FDA’s Medwatch reporting form, as well as return any devices associated with adverse events to the SynCardia Systems to help them and the FDA better understand the issue.
The C2 driver system is an external pneumatic system that activates an implanted TAH-t in eligible heart failure patients who have severe biventricular failure and are waiting for transplant. It is smaller than its predecessor, but per the device’s approved use, patients must still remain in the hospital while on the device. Since its approval in 2012, the Freedom driver system was approved in 2014, which allows patients to return home.
The full safety alert can be found on the FDA website.
Postapproval results for SynCardia Systems’ Companion 2 (C2) driver system for temporary total artificial hearts (TAH-t) have shown higher mortality and stroke rates than were seen with the previous system, the circulatory support system. As a result, the Food and Drug Administration has issued a safety alert cautioning them to weigh the risks and benefits carefully. The alert, issued on August 17, is based on a postapproval study conducted by SynCardia Systems.
Furthermore, patients and health care professionals are encouraged to report any adverse events using the FDA’s Medwatch reporting form, as well as return any devices associated with adverse events to the SynCardia Systems to help them and the FDA better understand the issue.
The C2 driver system is an external pneumatic system that activates an implanted TAH-t in eligible heart failure patients who have severe biventricular failure and are waiting for transplant. It is smaller than its predecessor, but per the device’s approved use, patients must still remain in the hospital while on the device. Since its approval in 2012, the Freedom driver system was approved in 2014, which allows patients to return home.
The full safety alert can be found on the FDA website.
Postapproval results for SynCardia Systems’ Companion 2 (C2) driver system for temporary total artificial hearts (TAH-t) have shown higher mortality and stroke rates than were seen with the previous system, the circulatory support system. As a result, the Food and Drug Administration has issued a safety alert cautioning them to weigh the risks and benefits carefully. The alert, issued on August 17, is based on a postapproval study conducted by SynCardia Systems.
Furthermore, patients and health care professionals are encouraged to report any adverse events using the FDA’s Medwatch reporting form, as well as return any devices associated with adverse events to the SynCardia Systems to help them and the FDA better understand the issue.
The C2 driver system is an external pneumatic system that activates an implanted TAH-t in eligible heart failure patients who have severe biventricular failure and are waiting for transplant. It is smaller than its predecessor, but per the device’s approved use, patients must still remain in the hospital while on the device. Since its approval in 2012, the Freedom driver system was approved in 2014, which allows patients to return home.
The full safety alert can be found on the FDA website.
The value of low-dose aspirin for prevention of preeclampsia
Low-dose aspirin for the prevention of preeclampsia has been studied for more than 25 years, often with contradictory and confusing results. Studies have enrolled patients with varying levels of risk, assessed risk differently, and used different definitions of preeclampsia as well as a variety of aspirin dosages and treatment-initiation dates. Undoubtedly, this heterogeneity has made interpretation and comparisons difficult and frustrating.
Recently, systematic reviews and meta-analyses have improved our understanding of the role of low-dose aspirin, providing solid evidence that low-dose aspirin started after the first-trimester reduces the occurrence of preeclampsia in high-risk women. Data also suggest that low-dose aspirin reduces the incidence of fetal growth restriction and preterm birth in these women.
There is reasonable evidence, moreover, that low-dose aspirin provides similar benefit in women with modest levels of risk and that it’s best to begin aspirin use at 12-14 weeks’ gestation rather than later in the second trimester. Finally,
Despite this evidence and current recommendations for low-dose aspirin use by the U.S. Preventive Services Task Force and the American College of Obstetricians and Gynecologists, its use in practice is varied. Obstetricians and other obstetrics providers are not consistently making the recommendation, and pharmacists are not consistently supporting it.
Without more consistent initiation of low-dose aspirin prophylaxis and more consistent adherence, we are losing an opportunity to reduce serious maternal morbidity and mortality. We also are underutilizing an important tool for the reduction of racial and other health disparities relating to preterm birth, maternal death, and other complications of preeclampsia.
Dr. Lockwood: Epidemiology, etiology, and clinical value of aspirin
The use of low-dose aspirin can have a high impact, considering that preeclampsia complicates 3.4% of pregnancies nationally and accounts for at least 9% of maternal deaths (BMJ. 2013 Nov;347:f6564).
Preeclampsia also has been shown in multiple long-term epidemiologic studies to be a strong risk factor for future cardiovascular disease and metabolic disorders in women – especially when it occurs in multiple pregnancies or develops preterm. Moreover, it is associated with stillbirth, intrauterine growth restriction (IUGR), and oligohydramnios in the fetus (BMJ. 2013 Nov;347:f6564).
It is important to remember that criteria for a diagnosis of preeclampsia changed in 2013 such that the detection of proteinuria is no longer required. Preeclampsia is defined today as the new onset of hypertension and proteinuria, or hypertension and end-organ dysfunction with or without proteinuria, after 20 weeks in a previously normotensive woman, according to the ACOG Task Force on Hypertension in Pregnancy.
The leading risk factor appears to be previous preeclampsia. In a systematic review and meta-analysis of 92 cohort studies that looked at the pooled relative risk of developing preeclampsia in the presence or absence of 14 commonly reported and accepted risk factors, prior preeclampsia topped the list, putting patients at an eightfold increased risk (relative risk 8.4) (BMJ. 2016 Apr 19;353:i1753).
Nulliparity (relative risk, 2.1) and multiple gestation (RR, 2.9) presented lesser risks but still were significant, and preexisting medical conditions increased risk as well. Notably, both chronic hypertension and a body mass index (BMI) greater than 30 had a fivefold increased risk (RR, 5.1), and preexisting diabetes presented more than a threefold increased risk (RR, 3.7). The review covered more than 25 million pregnancies in 27 countries.
The etiology of preeclampsia still is not completely understood. There is evidence that underlying decidual inflammation, including increased activated macrophages and decreased uterine natural killer cells (uNK), promotes shallow placentation leading to incomplete uterine spiral artery remodeling, relative placental hypoxia, and progressive release of placental antiangiogenic substances such as soluble fms-like tyrosine kinase 1 (sFlt1) and endoglin (Am J Pathol. 2013 Sep;183[3]:841-56; Reprod Sci. 2015 Nov;22[11]:1461-7). The latter result in systemic endothelial cell damage, reduced endothelial prostacyclin (PGI2), and increased platelet thromboxane A2, triggering vasospasm and increased platelet turnover that ultimately lead to the typical signs and symptoms of preeclampsia.
The research focus traditionally has been on the placenta, but more recently the uterine decidual contribution has received more attention. A recent study published in the Proceedings of the National Academy of Sciences offers evidence that affected women have defective decidualization during and after severe preeclampsia, suggesting that the defect could be detected prior to conception.
Investigators isolated endometrial cells from women at the end of a pregnancy complicated by preeclampsia and found a transcriptional signature that persisted for years. They then linked the defect to impaired cytotrophoblast invasion (Proc Natl Acad Sci. 2017;114[40]:E8468-77). This elegant and provocative study suggests that it might be possible in the future to evaluate the endometrium and try to enhance stromal cell decidualization before pregnancy.
Currently, the rationale for using aspirin to prevent preeclampsia lies with its ability to inhibit platelet production of thromboxane and block NF-kB, a protein complex that plays a role in systemic and/or decidual inflammation. There likely are numerous mechanisms of action, however, including some that improve placentation.
Among the most recent studies on timing and dosage is a systematic review and meta-analysis of 45 randomized controlled trials with 20,909 women randomized to 50-150 mg aspirin daily or to placebo or no treatment. The investigators stratified the results by gestational age at the time of aspirin initiation and found that timing matters. Women who began aspirin at or before 16 weeks had the most significant reductions in preeclampsia (RR, 0.57) and severe preeclampsia (RR, 0.47), as well as fetal growth restriction (RR, 0.56), with a dose-response effect up to 150 mg.
When aspirin was initiated after 16 weeks, there was a much smaller reduction of preeclampsia (RR, 0.81) and no effects for severe preeclampsia or IUGR. Nor was there any dose-response effect (Am J Obstet Gynecol. 2017; 216[2]:110-20.e6).
In contrast, another recent meta-analysis of individual participant data on 32,217 women recruited in 31 randomized controlled trials found no significant difference among women who were randomized before 16 weeks versus those who were randomized at 16 weeks or later (Am J Obstet Gynecol. 2017 Feb;216[2]:121-8.e2). It’s important to note that this analysis covered other antiplatelet agents as well and that it stratified outcomes by gestational age with a slightly later cutoff point.
What do official guidelines say? The USPSTF’s recommendation, issued in 2014, calls for low-dose aspirin at 81 mg/day after 12 weeks’ gestation in women who have one or more high-risk factors, and consideration of such treatment in patients with “several” moderate-risk factors (Ann Intern Med. 2014 Dec 2;161[11]:819-26). In July 2018, ACOG reaffirmed its earlier support for low-dose aspirin in a committee opinion that recommends 81 mg/day beginning at 12-28 weeks’ gestation, optimally before 16 weeks’, for women who have one or more high-risk factors or more than one moderate-risk factor (Obstet Gynecol. 2018 Jul;132[1]:e44-e52).
My own take, based on published literature, including my own research, is that low-dose aspirin reduces the frequency of preeclampsia, particularly cases occurring preterm, as well as related IUGR, by approximately 10%-20% in moderate- and high-risk women. Regarding dose and gestational age for initiation, I have split the difference of what’s reflected in the literature and in guidelines. I advise 122 mg (a tablet-and-a-half) a day, starting at 12-14 weeks’, for patients at high and moderate levels of risk. For patients who are not seen until later, low-dose aspirin can be started up to 28 weeks’ gestation.
Dr. Abbott: Messaging and education to reduce disparities
Black women are not only more likely to develop preeclampsia, but they’re also more likely to have more severe complications and worse outcomes. In one analysis, black women with preeclampsia experienced an almost threefold higher risk of maternal mortality and intrauterine fetal death than did white women with the disorder (Hypertens Pregnancy. 2015 Nov;34[4]:506-15).
At Boston Medical Center, 30% of pregnant women have a diagnosis of preeclampsia or hypertension at term. In addition to 68% identifying as Hispanic/black or black, half of the families we care for have incomes less than $20,000, and 30% are non–English speaking. Low-dose prenatal aspirin is therefore an important tool for reducing racial health disparities as well as disparities created by health literacy, economic status, and language and cultural barriers. At BMC, New England’s largest safety-net hospital, we’ve found that the factors driving health disparities often overlap.
To increase the use of low-dose aspirin for women at moderate to high risk, we marry education about aspirin’s effectiveness and safety with education about the potential severity of hypertension and preeclampsia. We counsel patients who are hospitalized at delivery with gestational or chronic hypertension, or fetal growth restriction, about how preeclampsia can be very serious – contrary to what they’ve experienced or what friends or family may have shared. We also counsel them about signs and symptoms of severe preeclampsia that warrant consulting their provider. And overall, we deliberately use the term “prenatal aspirin” so that, over time and in the broader community, it will become associated with good prenatal care and risk reduction.
To counter perceived risks and dangers that we identified through focus groups and interviews, our patient education materials state that low-dose aspirin in pregnancy will not cause increased bleeding, does not reach the baby’s blood, does not increase the risk of miscarriage, and has not been shown to have negative effects on the baby’s initial development (www.prenatalaspirin.com/education-materials). We try to engage family members whenever possible, and we recognize that the black population has historical reasons to be concerned or suspicious that aspirin might not be safe for them.
Especially for underserved patients who receive prescriptions for low-dose aspirin, we must ensure that pharmacists will dispense the medication. A national survey of pharmacists (not yet published) found that over two-thirds were unaware of the USPSTF guidelines, and that only a minority would feel comfortable dispensing low-dose aspirin during pregnancy. In our community, some pharmacists have told patients to return to their physician and inquire more. Until recently, one of the major pharmacy chains placed a warning label on aspirin bottles being dispensed to women who also had an active prescription for prenatal vitamins.
We are working both with pharmacies and with pharmacy schools to impact the education of current and future pharmacists on guidelines and recommendations for low-dose aspirin prophylaxis. In addition, when I write a prescription for prenatal aspirin, starting at 12 weeks’ whenever possible, I include the message “for the purpose of trying to reduce pregnancy complications.”
Dr. Lockwood is senior vice president at University of South Florida Health and dean of Morsani College of Medicine at the University of South Florida, Tampa. He said he had no relevant financial disclosures or conflicts of interest. Dr. Abbot is a specialist in maternal-fetal medicine, the director of obstetrics and gynecology, and assistant dean for patient safety and quality improvement education at Boston Medical Center. She also is an associate professor of obstetrics and gynecology at Boston University. She disclosed a grant from the March of Dimes. Email them at [email protected].
Low-dose aspirin for the prevention of preeclampsia has been studied for more than 25 years, often with contradictory and confusing results. Studies have enrolled patients with varying levels of risk, assessed risk differently, and used different definitions of preeclampsia as well as a variety of aspirin dosages and treatment-initiation dates. Undoubtedly, this heterogeneity has made interpretation and comparisons difficult and frustrating.
Recently, systematic reviews and meta-analyses have improved our understanding of the role of low-dose aspirin, providing solid evidence that low-dose aspirin started after the first-trimester reduces the occurrence of preeclampsia in high-risk women. Data also suggest that low-dose aspirin reduces the incidence of fetal growth restriction and preterm birth in these women.
There is reasonable evidence, moreover, that low-dose aspirin provides similar benefit in women with modest levels of risk and that it’s best to begin aspirin use at 12-14 weeks’ gestation rather than later in the second trimester. Finally,
Despite this evidence and current recommendations for low-dose aspirin use by the U.S. Preventive Services Task Force and the American College of Obstetricians and Gynecologists, its use in practice is varied. Obstetricians and other obstetrics providers are not consistently making the recommendation, and pharmacists are not consistently supporting it.
Without more consistent initiation of low-dose aspirin prophylaxis and more consistent adherence, we are losing an opportunity to reduce serious maternal morbidity and mortality. We also are underutilizing an important tool for the reduction of racial and other health disparities relating to preterm birth, maternal death, and other complications of preeclampsia.
Dr. Lockwood: Epidemiology, etiology, and clinical value of aspirin
The use of low-dose aspirin can have a high impact, considering that preeclampsia complicates 3.4% of pregnancies nationally and accounts for at least 9% of maternal deaths (BMJ. 2013 Nov;347:f6564).
Preeclampsia also has been shown in multiple long-term epidemiologic studies to be a strong risk factor for future cardiovascular disease and metabolic disorders in women – especially when it occurs in multiple pregnancies or develops preterm. Moreover, it is associated with stillbirth, intrauterine growth restriction (IUGR), and oligohydramnios in the fetus (BMJ. 2013 Nov;347:f6564).
It is important to remember that criteria for a diagnosis of preeclampsia changed in 2013 such that the detection of proteinuria is no longer required. Preeclampsia is defined today as the new onset of hypertension and proteinuria, or hypertension and end-organ dysfunction with or without proteinuria, after 20 weeks in a previously normotensive woman, according to the ACOG Task Force on Hypertension in Pregnancy.
The leading risk factor appears to be previous preeclampsia. In a systematic review and meta-analysis of 92 cohort studies that looked at the pooled relative risk of developing preeclampsia in the presence or absence of 14 commonly reported and accepted risk factors, prior preeclampsia topped the list, putting patients at an eightfold increased risk (relative risk 8.4) (BMJ. 2016 Apr 19;353:i1753).
Nulliparity (relative risk, 2.1) and multiple gestation (RR, 2.9) presented lesser risks but still were significant, and preexisting medical conditions increased risk as well. Notably, both chronic hypertension and a body mass index (BMI) greater than 30 had a fivefold increased risk (RR, 5.1), and preexisting diabetes presented more than a threefold increased risk (RR, 3.7). The review covered more than 25 million pregnancies in 27 countries.
The etiology of preeclampsia still is not completely understood. There is evidence that underlying decidual inflammation, including increased activated macrophages and decreased uterine natural killer cells (uNK), promotes shallow placentation leading to incomplete uterine spiral artery remodeling, relative placental hypoxia, and progressive release of placental antiangiogenic substances such as soluble fms-like tyrosine kinase 1 (sFlt1) and endoglin (Am J Pathol. 2013 Sep;183[3]:841-56; Reprod Sci. 2015 Nov;22[11]:1461-7). The latter result in systemic endothelial cell damage, reduced endothelial prostacyclin (PGI2), and increased platelet thromboxane A2, triggering vasospasm and increased platelet turnover that ultimately lead to the typical signs and symptoms of preeclampsia.
The research focus traditionally has been on the placenta, but more recently the uterine decidual contribution has received more attention. A recent study published in the Proceedings of the National Academy of Sciences offers evidence that affected women have defective decidualization during and after severe preeclampsia, suggesting that the defect could be detected prior to conception.
Investigators isolated endometrial cells from women at the end of a pregnancy complicated by preeclampsia and found a transcriptional signature that persisted for years. They then linked the defect to impaired cytotrophoblast invasion (Proc Natl Acad Sci. 2017;114[40]:E8468-77). This elegant and provocative study suggests that it might be possible in the future to evaluate the endometrium and try to enhance stromal cell decidualization before pregnancy.
Currently, the rationale for using aspirin to prevent preeclampsia lies with its ability to inhibit platelet production of thromboxane and block NF-kB, a protein complex that plays a role in systemic and/or decidual inflammation. There likely are numerous mechanisms of action, however, including some that improve placentation.
Among the most recent studies on timing and dosage is a systematic review and meta-analysis of 45 randomized controlled trials with 20,909 women randomized to 50-150 mg aspirin daily or to placebo or no treatment. The investigators stratified the results by gestational age at the time of aspirin initiation and found that timing matters. Women who began aspirin at or before 16 weeks had the most significant reductions in preeclampsia (RR, 0.57) and severe preeclampsia (RR, 0.47), as well as fetal growth restriction (RR, 0.56), with a dose-response effect up to 150 mg.
When aspirin was initiated after 16 weeks, there was a much smaller reduction of preeclampsia (RR, 0.81) and no effects for severe preeclampsia or IUGR. Nor was there any dose-response effect (Am J Obstet Gynecol. 2017; 216[2]:110-20.e6).
In contrast, another recent meta-analysis of individual participant data on 32,217 women recruited in 31 randomized controlled trials found no significant difference among women who were randomized before 16 weeks versus those who were randomized at 16 weeks or later (Am J Obstet Gynecol. 2017 Feb;216[2]:121-8.e2). It’s important to note that this analysis covered other antiplatelet agents as well and that it stratified outcomes by gestational age with a slightly later cutoff point.
What do official guidelines say? The USPSTF’s recommendation, issued in 2014, calls for low-dose aspirin at 81 mg/day after 12 weeks’ gestation in women who have one or more high-risk factors, and consideration of such treatment in patients with “several” moderate-risk factors (Ann Intern Med. 2014 Dec 2;161[11]:819-26). In July 2018, ACOG reaffirmed its earlier support for low-dose aspirin in a committee opinion that recommends 81 mg/day beginning at 12-28 weeks’ gestation, optimally before 16 weeks’, for women who have one or more high-risk factors or more than one moderate-risk factor (Obstet Gynecol. 2018 Jul;132[1]:e44-e52).
My own take, based on published literature, including my own research, is that low-dose aspirin reduces the frequency of preeclampsia, particularly cases occurring preterm, as well as related IUGR, by approximately 10%-20% in moderate- and high-risk women. Regarding dose and gestational age for initiation, I have split the difference of what’s reflected in the literature and in guidelines. I advise 122 mg (a tablet-and-a-half) a day, starting at 12-14 weeks’, for patients at high and moderate levels of risk. For patients who are not seen until later, low-dose aspirin can be started up to 28 weeks’ gestation.
Dr. Abbott: Messaging and education to reduce disparities
Black women are not only more likely to develop preeclampsia, but they’re also more likely to have more severe complications and worse outcomes. In one analysis, black women with preeclampsia experienced an almost threefold higher risk of maternal mortality and intrauterine fetal death than did white women with the disorder (Hypertens Pregnancy. 2015 Nov;34[4]:506-15).
At Boston Medical Center, 30% of pregnant women have a diagnosis of preeclampsia or hypertension at term. In addition to 68% identifying as Hispanic/black or black, half of the families we care for have incomes less than $20,000, and 30% are non–English speaking. Low-dose prenatal aspirin is therefore an important tool for reducing racial health disparities as well as disparities created by health literacy, economic status, and language and cultural barriers. At BMC, New England’s largest safety-net hospital, we’ve found that the factors driving health disparities often overlap.
To increase the use of low-dose aspirin for women at moderate to high risk, we marry education about aspirin’s effectiveness and safety with education about the potential severity of hypertension and preeclampsia. We counsel patients who are hospitalized at delivery with gestational or chronic hypertension, or fetal growth restriction, about how preeclampsia can be very serious – contrary to what they’ve experienced or what friends or family may have shared. We also counsel them about signs and symptoms of severe preeclampsia that warrant consulting their provider. And overall, we deliberately use the term “prenatal aspirin” so that, over time and in the broader community, it will become associated with good prenatal care and risk reduction.
To counter perceived risks and dangers that we identified through focus groups and interviews, our patient education materials state that low-dose aspirin in pregnancy will not cause increased bleeding, does not reach the baby’s blood, does not increase the risk of miscarriage, and has not been shown to have negative effects on the baby’s initial development (www.prenatalaspirin.com/education-materials). We try to engage family members whenever possible, and we recognize that the black population has historical reasons to be concerned or suspicious that aspirin might not be safe for them.
Especially for underserved patients who receive prescriptions for low-dose aspirin, we must ensure that pharmacists will dispense the medication. A national survey of pharmacists (not yet published) found that over two-thirds were unaware of the USPSTF guidelines, and that only a minority would feel comfortable dispensing low-dose aspirin during pregnancy. In our community, some pharmacists have told patients to return to their physician and inquire more. Until recently, one of the major pharmacy chains placed a warning label on aspirin bottles being dispensed to women who also had an active prescription for prenatal vitamins.
We are working both with pharmacies and with pharmacy schools to impact the education of current and future pharmacists on guidelines and recommendations for low-dose aspirin prophylaxis. In addition, when I write a prescription for prenatal aspirin, starting at 12 weeks’ whenever possible, I include the message “for the purpose of trying to reduce pregnancy complications.”
Dr. Lockwood is senior vice president at University of South Florida Health and dean of Morsani College of Medicine at the University of South Florida, Tampa. He said he had no relevant financial disclosures or conflicts of interest. Dr. Abbot is a specialist in maternal-fetal medicine, the director of obstetrics and gynecology, and assistant dean for patient safety and quality improvement education at Boston Medical Center. She also is an associate professor of obstetrics and gynecology at Boston University. She disclosed a grant from the March of Dimes. Email them at [email protected].
Low-dose aspirin for the prevention of preeclampsia has been studied for more than 25 years, often with contradictory and confusing results. Studies have enrolled patients with varying levels of risk, assessed risk differently, and used different definitions of preeclampsia as well as a variety of aspirin dosages and treatment-initiation dates. Undoubtedly, this heterogeneity has made interpretation and comparisons difficult and frustrating.
Recently, systematic reviews and meta-analyses have improved our understanding of the role of low-dose aspirin, providing solid evidence that low-dose aspirin started after the first-trimester reduces the occurrence of preeclampsia in high-risk women. Data also suggest that low-dose aspirin reduces the incidence of fetal growth restriction and preterm birth in these women.
There is reasonable evidence, moreover, that low-dose aspirin provides similar benefit in women with modest levels of risk and that it’s best to begin aspirin use at 12-14 weeks’ gestation rather than later in the second trimester. Finally,
Despite this evidence and current recommendations for low-dose aspirin use by the U.S. Preventive Services Task Force and the American College of Obstetricians and Gynecologists, its use in practice is varied. Obstetricians and other obstetrics providers are not consistently making the recommendation, and pharmacists are not consistently supporting it.
Without more consistent initiation of low-dose aspirin prophylaxis and more consistent adherence, we are losing an opportunity to reduce serious maternal morbidity and mortality. We also are underutilizing an important tool for the reduction of racial and other health disparities relating to preterm birth, maternal death, and other complications of preeclampsia.
Dr. Lockwood: Epidemiology, etiology, and clinical value of aspirin
The use of low-dose aspirin can have a high impact, considering that preeclampsia complicates 3.4% of pregnancies nationally and accounts for at least 9% of maternal deaths (BMJ. 2013 Nov;347:f6564).
Preeclampsia also has been shown in multiple long-term epidemiologic studies to be a strong risk factor for future cardiovascular disease and metabolic disorders in women – especially when it occurs in multiple pregnancies or develops preterm. Moreover, it is associated with stillbirth, intrauterine growth restriction (IUGR), and oligohydramnios in the fetus (BMJ. 2013 Nov;347:f6564).
It is important to remember that criteria for a diagnosis of preeclampsia changed in 2013 such that the detection of proteinuria is no longer required. Preeclampsia is defined today as the new onset of hypertension and proteinuria, or hypertension and end-organ dysfunction with or without proteinuria, after 20 weeks in a previously normotensive woman, according to the ACOG Task Force on Hypertension in Pregnancy.
The leading risk factor appears to be previous preeclampsia. In a systematic review and meta-analysis of 92 cohort studies that looked at the pooled relative risk of developing preeclampsia in the presence or absence of 14 commonly reported and accepted risk factors, prior preeclampsia topped the list, putting patients at an eightfold increased risk (relative risk 8.4) (BMJ. 2016 Apr 19;353:i1753).
Nulliparity (relative risk, 2.1) and multiple gestation (RR, 2.9) presented lesser risks but still were significant, and preexisting medical conditions increased risk as well. Notably, both chronic hypertension and a body mass index (BMI) greater than 30 had a fivefold increased risk (RR, 5.1), and preexisting diabetes presented more than a threefold increased risk (RR, 3.7). The review covered more than 25 million pregnancies in 27 countries.
The etiology of preeclampsia still is not completely understood. There is evidence that underlying decidual inflammation, including increased activated macrophages and decreased uterine natural killer cells (uNK), promotes shallow placentation leading to incomplete uterine spiral artery remodeling, relative placental hypoxia, and progressive release of placental antiangiogenic substances such as soluble fms-like tyrosine kinase 1 (sFlt1) and endoglin (Am J Pathol. 2013 Sep;183[3]:841-56; Reprod Sci. 2015 Nov;22[11]:1461-7). The latter result in systemic endothelial cell damage, reduced endothelial prostacyclin (PGI2), and increased platelet thromboxane A2, triggering vasospasm and increased platelet turnover that ultimately lead to the typical signs and symptoms of preeclampsia.
The research focus traditionally has been on the placenta, but more recently the uterine decidual contribution has received more attention. A recent study published in the Proceedings of the National Academy of Sciences offers evidence that affected women have defective decidualization during and after severe preeclampsia, suggesting that the defect could be detected prior to conception.
Investigators isolated endometrial cells from women at the end of a pregnancy complicated by preeclampsia and found a transcriptional signature that persisted for years. They then linked the defect to impaired cytotrophoblast invasion (Proc Natl Acad Sci. 2017;114[40]:E8468-77). This elegant and provocative study suggests that it might be possible in the future to evaluate the endometrium and try to enhance stromal cell decidualization before pregnancy.
Currently, the rationale for using aspirin to prevent preeclampsia lies with its ability to inhibit platelet production of thromboxane and block NF-kB, a protein complex that plays a role in systemic and/or decidual inflammation. There likely are numerous mechanisms of action, however, including some that improve placentation.
Among the most recent studies on timing and dosage is a systematic review and meta-analysis of 45 randomized controlled trials with 20,909 women randomized to 50-150 mg aspirin daily or to placebo or no treatment. The investigators stratified the results by gestational age at the time of aspirin initiation and found that timing matters. Women who began aspirin at or before 16 weeks had the most significant reductions in preeclampsia (RR, 0.57) and severe preeclampsia (RR, 0.47), as well as fetal growth restriction (RR, 0.56), with a dose-response effect up to 150 mg.
When aspirin was initiated after 16 weeks, there was a much smaller reduction of preeclampsia (RR, 0.81) and no effects for severe preeclampsia or IUGR. Nor was there any dose-response effect (Am J Obstet Gynecol. 2017; 216[2]:110-20.e6).
In contrast, another recent meta-analysis of individual participant data on 32,217 women recruited in 31 randomized controlled trials found no significant difference among women who were randomized before 16 weeks versus those who were randomized at 16 weeks or later (Am J Obstet Gynecol. 2017 Feb;216[2]:121-8.e2). It’s important to note that this analysis covered other antiplatelet agents as well and that it stratified outcomes by gestational age with a slightly later cutoff point.
What do official guidelines say? The USPSTF’s recommendation, issued in 2014, calls for low-dose aspirin at 81 mg/day after 12 weeks’ gestation in women who have one or more high-risk factors, and consideration of such treatment in patients with “several” moderate-risk factors (Ann Intern Med. 2014 Dec 2;161[11]:819-26). In July 2018, ACOG reaffirmed its earlier support for low-dose aspirin in a committee opinion that recommends 81 mg/day beginning at 12-28 weeks’ gestation, optimally before 16 weeks’, for women who have one or more high-risk factors or more than one moderate-risk factor (Obstet Gynecol. 2018 Jul;132[1]:e44-e52).
My own take, based on published literature, including my own research, is that low-dose aspirin reduces the frequency of preeclampsia, particularly cases occurring preterm, as well as related IUGR, by approximately 10%-20% in moderate- and high-risk women. Regarding dose and gestational age for initiation, I have split the difference of what’s reflected in the literature and in guidelines. I advise 122 mg (a tablet-and-a-half) a day, starting at 12-14 weeks’, for patients at high and moderate levels of risk. For patients who are not seen until later, low-dose aspirin can be started up to 28 weeks’ gestation.
Dr. Abbott: Messaging and education to reduce disparities
Black women are not only more likely to develop preeclampsia, but they’re also more likely to have more severe complications and worse outcomes. In one analysis, black women with preeclampsia experienced an almost threefold higher risk of maternal mortality and intrauterine fetal death than did white women with the disorder (Hypertens Pregnancy. 2015 Nov;34[4]:506-15).
At Boston Medical Center, 30% of pregnant women have a diagnosis of preeclampsia or hypertension at term. In addition to 68% identifying as Hispanic/black or black, half of the families we care for have incomes less than $20,000, and 30% are non–English speaking. Low-dose prenatal aspirin is therefore an important tool for reducing racial health disparities as well as disparities created by health literacy, economic status, and language and cultural barriers. At BMC, New England’s largest safety-net hospital, we’ve found that the factors driving health disparities often overlap.
To increase the use of low-dose aspirin for women at moderate to high risk, we marry education about aspirin’s effectiveness and safety with education about the potential severity of hypertension and preeclampsia. We counsel patients who are hospitalized at delivery with gestational or chronic hypertension, or fetal growth restriction, about how preeclampsia can be very serious – contrary to what they’ve experienced or what friends or family may have shared. We also counsel them about signs and symptoms of severe preeclampsia that warrant consulting their provider. And overall, we deliberately use the term “prenatal aspirin” so that, over time and in the broader community, it will become associated with good prenatal care and risk reduction.
To counter perceived risks and dangers that we identified through focus groups and interviews, our patient education materials state that low-dose aspirin in pregnancy will not cause increased bleeding, does not reach the baby’s blood, does not increase the risk of miscarriage, and has not been shown to have negative effects on the baby’s initial development (www.prenatalaspirin.com/education-materials). We try to engage family members whenever possible, and we recognize that the black population has historical reasons to be concerned or suspicious that aspirin might not be safe for them.
Especially for underserved patients who receive prescriptions for low-dose aspirin, we must ensure that pharmacists will dispense the medication. A national survey of pharmacists (not yet published) found that over two-thirds were unaware of the USPSTF guidelines, and that only a minority would feel comfortable dispensing low-dose aspirin during pregnancy. In our community, some pharmacists have told patients to return to their physician and inquire more. Until recently, one of the major pharmacy chains placed a warning label on aspirin bottles being dispensed to women who also had an active prescription for prenatal vitamins.
We are working both with pharmacies and with pharmacy schools to impact the education of current and future pharmacists on guidelines and recommendations for low-dose aspirin prophylaxis. In addition, when I write a prescription for prenatal aspirin, starting at 12 weeks’ whenever possible, I include the message “for the purpose of trying to reduce pregnancy complications.”
Dr. Lockwood is senior vice president at University of South Florida Health and dean of Morsani College of Medicine at the University of South Florida, Tampa. He said he had no relevant financial disclosures or conflicts of interest. Dr. Abbot is a specialist in maternal-fetal medicine, the director of obstetrics and gynecology, and assistant dean for patient safety and quality improvement education at Boston Medical Center. She also is an associate professor of obstetrics and gynecology at Boston University. She disclosed a grant from the March of Dimes. Email them at [email protected].
Aspirin has myriad benefits
Some of our readers might remember the old saying, “Take two aspirin and call me in the morning,” as advice physicians gave to patients experiencing a minor malady. Aspirin often has been called a “wonder drug” as its uses continue to expand. From its first recorded use in the Ebers papyrus as an anti-inflammatory agent, to its first use in a clinical trial showing that it induces remission of fever and joint inflammation, to the discovery that it could prevent death from heart attack, to its anticancer properties, aspirin remains one of the most researched drugs in use today. According to ClinicalTrials.gov, there are over 465 active and nearly 1,000 completed aspirin-related clinical trials around the world.
Despite its myriad benefits, aspirin has been linked to bleeding, nausea, and gastrointestinal ulcers. Additionally, more research is needed to determine the risks/benefits of daily aspirin in younger adults (under age 50 years) or older adults (over age 70 years), although the ASPREE (Aspirin in Reducing Events in the Elderly) trial, expected to be completed in 2019, is working to determine the effects of daily low-dose aspirin (100 mg) on the health of people over age 65.
It is tempting to consider aspirin one of modern medicine’s so-called silver bullets, and, for women with a history of gestational hypertension and preeclampsia, it just might be. Aspirin use, especially daily aspirin, is typically not recommended during pregnancy, and most ob.gyns. will include aspirin on the “do not take” list they give to their patients during prenatal examinations. Women at risk for developing preeclampsia are the exceptions to this general rule, and a number of clinical studies have indicated that use of low-dose aspirin can help prevent disease as well as secondary outcomes for mother (i.e., placental abruption, antepartum hemorrhage) and baby (i.e., intrauterine growth restriction, stillbirth). In addition, aspirin is an easily obtainable, low-cost preventive measure for any patient at high risk.
To discuss the value of low-dose aspirin to prevent preeclampsia and how ob.gyns. can educate their patients and other health care professionals about its benefits, we have invited Charles J. Lockwood, MD, MHCM, senior vice president of University of South Florida Health and dean of Morsani College of Medicine at the University of South Florida, Tampa, and Jodi F. Abbott, MD, MSc, MHCM, director of obstetrics and gynecology at Boston Medical Center, and associate professor of obstetrics and gynecology at Boston University, to coauthor this month’s Master Class.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Some of our readers might remember the old saying, “Take two aspirin and call me in the morning,” as advice physicians gave to patients experiencing a minor malady. Aspirin often has been called a “wonder drug” as its uses continue to expand. From its first recorded use in the Ebers papyrus as an anti-inflammatory agent, to its first use in a clinical trial showing that it induces remission of fever and joint inflammation, to the discovery that it could prevent death from heart attack, to its anticancer properties, aspirin remains one of the most researched drugs in use today. According to ClinicalTrials.gov, there are over 465 active and nearly 1,000 completed aspirin-related clinical trials around the world.
Despite its myriad benefits, aspirin has been linked to bleeding, nausea, and gastrointestinal ulcers. Additionally, more research is needed to determine the risks/benefits of daily aspirin in younger adults (under age 50 years) or older adults (over age 70 years), although the ASPREE (Aspirin in Reducing Events in the Elderly) trial, expected to be completed in 2019, is working to determine the effects of daily low-dose aspirin (100 mg) on the health of people over age 65.
It is tempting to consider aspirin one of modern medicine’s so-called silver bullets, and, for women with a history of gestational hypertension and preeclampsia, it just might be. Aspirin use, especially daily aspirin, is typically not recommended during pregnancy, and most ob.gyns. will include aspirin on the “do not take” list they give to their patients during prenatal examinations. Women at risk for developing preeclampsia are the exceptions to this general rule, and a number of clinical studies have indicated that use of low-dose aspirin can help prevent disease as well as secondary outcomes for mother (i.e., placental abruption, antepartum hemorrhage) and baby (i.e., intrauterine growth restriction, stillbirth). In addition, aspirin is an easily obtainable, low-cost preventive measure for any patient at high risk.
To discuss the value of low-dose aspirin to prevent preeclampsia and how ob.gyns. can educate their patients and other health care professionals about its benefits, we have invited Charles J. Lockwood, MD, MHCM, senior vice president of University of South Florida Health and dean of Morsani College of Medicine at the University of South Florida, Tampa, and Jodi F. Abbott, MD, MSc, MHCM, director of obstetrics and gynecology at Boston Medical Center, and associate professor of obstetrics and gynecology at Boston University, to coauthor this month’s Master Class.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Some of our readers might remember the old saying, “Take two aspirin and call me in the morning,” as advice physicians gave to patients experiencing a minor malady. Aspirin often has been called a “wonder drug” as its uses continue to expand. From its first recorded use in the Ebers papyrus as an anti-inflammatory agent, to its first use in a clinical trial showing that it induces remission of fever and joint inflammation, to the discovery that it could prevent death from heart attack, to its anticancer properties, aspirin remains one of the most researched drugs in use today. According to ClinicalTrials.gov, there are over 465 active and nearly 1,000 completed aspirin-related clinical trials around the world.
Despite its myriad benefits, aspirin has been linked to bleeding, nausea, and gastrointestinal ulcers. Additionally, more research is needed to determine the risks/benefits of daily aspirin in younger adults (under age 50 years) or older adults (over age 70 years), although the ASPREE (Aspirin in Reducing Events in the Elderly) trial, expected to be completed in 2019, is working to determine the effects of daily low-dose aspirin (100 mg) on the health of people over age 65.
It is tempting to consider aspirin one of modern medicine’s so-called silver bullets, and, for women with a history of gestational hypertension and preeclampsia, it just might be. Aspirin use, especially daily aspirin, is typically not recommended during pregnancy, and most ob.gyns. will include aspirin on the “do not take” list they give to their patients during prenatal examinations. Women at risk for developing preeclampsia are the exceptions to this general rule, and a number of clinical studies have indicated that use of low-dose aspirin can help prevent disease as well as secondary outcomes for mother (i.e., placental abruption, antepartum hemorrhage) and baby (i.e., intrauterine growth restriction, stillbirth). In addition, aspirin is an easily obtainable, low-cost preventive measure for any patient at high risk.
To discuss the value of low-dose aspirin to prevent preeclampsia and how ob.gyns. can educate their patients and other health care professionals about its benefits, we have invited Charles J. Lockwood, MD, MHCM, senior vice president of University of South Florida Health and dean of Morsani College of Medicine at the University of South Florida, Tampa, and Jodi F. Abbott, MD, MSc, MHCM, director of obstetrics and gynecology at Boston Medical Center, and associate professor of obstetrics and gynecology at Boston University, to coauthor this month’s Master Class.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Prenatal marijuana use higher in women with severe nausea and vomiting
Pregnant women who experience severe nausea and vomiting have nearly fourfold greater odds of prenatal marijuana use compared with women not experiencing nausea and vomiting, according to data from 220,510 first-trimester screenings.
In a research letter published in JAMA Internal Medicine, researchers reported the results of a health care system data analysis, which found a 3.80-fold greater prevalence of prenatal marijuana use among women with severe nausea and vomiting in pregnancy, compared with those who did not experience nausea and vomiting.
Among women with mild nausea and vomiting in pregnancy, there was still a significant twofold higher prevalence of marijuana use.
“Use of marijuana, an antiemetic, is increasing among pregnant women, and data from two small surveys indicate that women self-report using marijuana to alleviate nausea and vomiting in pregnancy (NVP),” wrote Kelly C. Young-Wolff, PhD, of the division of research at Kaiser Permanente Northern California, Oakland, and her coauthors.
In this study, 2% of the women experienced severe and 15% experienced mild nausea and vomiting during pregnancy.
The overall prevalence of marijuana use – assessed either by self-report or toxicological test findings – was 5.3%, with 0.7% positive on self-report only, 3.1% positive on toxicologic testing only, and 1.5% positive on both.
The authors said the findings supported the hypothesis that pregnant women were using marijuana to self-medicate for NVP. However, they also noted that clinicians may diagnose NVP more frequently among women who report using marijuana to treat it.
Dr. Young-Wolff and her coauthors said that they would not have been able to distinguish prenatal marijuana use from use before the women knew they were pregnant, “and misclassification is possible given variability in the time that marijuana is detectable in urine.
“The health effects of prenatal marijuana use are unclear, and national guidelines recommend that pregnant women discontinue use,” the authors wrote. “Patients with NVP should be screened for marijuana use and educated about effective and safe NVP treatments.”
The study was supported by the National Institute on Drug Abuse and the National Institute of Mental Health. No conflicts of interest were declared.
SOURCE: Young-Wolff K et al. JAMA Intern Med. 2018 Aug 20. doi: 10.1001/jamainternmed.2018.3581.
Pregnant women who experience severe nausea and vomiting have nearly fourfold greater odds of prenatal marijuana use compared with women not experiencing nausea and vomiting, according to data from 220,510 first-trimester screenings.
In a research letter published in JAMA Internal Medicine, researchers reported the results of a health care system data analysis, which found a 3.80-fold greater prevalence of prenatal marijuana use among women with severe nausea and vomiting in pregnancy, compared with those who did not experience nausea and vomiting.
Among women with mild nausea and vomiting in pregnancy, there was still a significant twofold higher prevalence of marijuana use.
“Use of marijuana, an antiemetic, is increasing among pregnant women, and data from two small surveys indicate that women self-report using marijuana to alleviate nausea and vomiting in pregnancy (NVP),” wrote Kelly C. Young-Wolff, PhD, of the division of research at Kaiser Permanente Northern California, Oakland, and her coauthors.
In this study, 2% of the women experienced severe and 15% experienced mild nausea and vomiting during pregnancy.
The overall prevalence of marijuana use – assessed either by self-report or toxicological test findings – was 5.3%, with 0.7% positive on self-report only, 3.1% positive on toxicologic testing only, and 1.5% positive on both.
The authors said the findings supported the hypothesis that pregnant women were using marijuana to self-medicate for NVP. However, they also noted that clinicians may diagnose NVP more frequently among women who report using marijuana to treat it.
Dr. Young-Wolff and her coauthors said that they would not have been able to distinguish prenatal marijuana use from use before the women knew they were pregnant, “and misclassification is possible given variability in the time that marijuana is detectable in urine.
“The health effects of prenatal marijuana use are unclear, and national guidelines recommend that pregnant women discontinue use,” the authors wrote. “Patients with NVP should be screened for marijuana use and educated about effective and safe NVP treatments.”
The study was supported by the National Institute on Drug Abuse and the National Institute of Mental Health. No conflicts of interest were declared.
SOURCE: Young-Wolff K et al. JAMA Intern Med. 2018 Aug 20. doi: 10.1001/jamainternmed.2018.3581.
Pregnant women who experience severe nausea and vomiting have nearly fourfold greater odds of prenatal marijuana use compared with women not experiencing nausea and vomiting, according to data from 220,510 first-trimester screenings.
In a research letter published in JAMA Internal Medicine, researchers reported the results of a health care system data analysis, which found a 3.80-fold greater prevalence of prenatal marijuana use among women with severe nausea and vomiting in pregnancy, compared with those who did not experience nausea and vomiting.
Among women with mild nausea and vomiting in pregnancy, there was still a significant twofold higher prevalence of marijuana use.
“Use of marijuana, an antiemetic, is increasing among pregnant women, and data from two small surveys indicate that women self-report using marijuana to alleviate nausea and vomiting in pregnancy (NVP),” wrote Kelly C. Young-Wolff, PhD, of the division of research at Kaiser Permanente Northern California, Oakland, and her coauthors.
In this study, 2% of the women experienced severe and 15% experienced mild nausea and vomiting during pregnancy.
The overall prevalence of marijuana use – assessed either by self-report or toxicological test findings – was 5.3%, with 0.7% positive on self-report only, 3.1% positive on toxicologic testing only, and 1.5% positive on both.
The authors said the findings supported the hypothesis that pregnant women were using marijuana to self-medicate for NVP. However, they also noted that clinicians may diagnose NVP more frequently among women who report using marijuana to treat it.
Dr. Young-Wolff and her coauthors said that they would not have been able to distinguish prenatal marijuana use from use before the women knew they were pregnant, “and misclassification is possible given variability in the time that marijuana is detectable in urine.
“The health effects of prenatal marijuana use are unclear, and national guidelines recommend that pregnant women discontinue use,” the authors wrote. “Patients with NVP should be screened for marijuana use and educated about effective and safe NVP treatments.”
The study was supported by the National Institute on Drug Abuse and the National Institute of Mental Health. No conflicts of interest were declared.
SOURCE: Young-Wolff K et al. JAMA Intern Med. 2018 Aug 20. doi: 10.1001/jamainternmed.2018.3581.
FROM JAMA INTERNAL MEDICINE
Key clinical point:
Major finding: Women with severe nausea and vomiting in pregnancy have nearly fourfold higher odds of prenatal marijuana use.
Study details: Analysis of health insurance data from 220,510 prenatal screenings.
Disclosures: The study was supported by the National Institute on Drug Abuse and the National Institute of Mental Health. No conflicts of interest were declared.
Source: Young-Wolff K et al. JAMA Intern Med. 2018 Aug 20. doi: 10.1001/jamainternmed.2018.3581.
The human mind: Wasted or cultivated?
Some of us seem completely blank, but our minds are predisposed to constantly engage in various mental activities. Our minds can problem solve, fantasize, remember past events, get preoccupied with a book or movie, pay attention to social relationships and/or pay attention to our relationship with ourselves, focus on what we are doing in the moment, and more.
Many of us understand that our minds sometime operate on more than one level, for example, I can be talking or listening to someone in the “front of my mind” and be thinking something seemingly unrelated in the “back of my mind.” Accordingly, Sometimes we try to take a break from this never-ending mental activity by going to sleep or even taking drugs to “turn our minds off.”
Another important aspect of our minds is “will.” I like to think that most of us are familiar with the concept of “will” and put ours to good use. We intentionally or unintentionally use our wills in various ways – to improve ourselves; have fun; dawdle our lives away on imaginary activities; accomplish something tangible (for example, write a paper or wash the kitchen floor); pay attention to what we are doing in the moment; be healthy; and practice cultivating a “sound mind.”
Many of us would agree that to be of sound mind means having the ability to be comfortable with ourselves; to have some regulation of our emotions and affects; not to worry too much, but just enough to stay motivated; and to apply our minds toward accomplishing a goal we have chosen for ourselves. A sound mind also involves having a good memory, being aware and awake, being able to sort things out and adapt to various difficulties life throws at us, solving problems creatively and effectively, being able to relax, and being able to have a purpose in our lives that we have some success in fulfilling.
As a psychiatrist, I often have been curious about how people use their minds and how they direct their mental activities, or, if they do not direct their mental activities at all and simply meander through their mental lives not doing much thinking about thinking (many of us do both). Further, what is even more curious to me is how we psychiatrists use our minds; after all, we are supposed to be the experts. Certainly, with the advent of psychoanalysis, cognitive-behavioral therapy and other techniques, we are teaching our patients, and, hopefully, learning ourselves about how to manage our minds and what they preoccupy themselves with thinking. Of course, there were the methods of Abraham A. Low, MD, about “will training,” which I consider the first iteration of CBT. Prior to psychoanalysis and Low’s ideas, there have always been various forms of mindfulness.
So, my questions to my fellow psychiatrists are: What are your doing with your mind? Are you wasting it, cultivating it, or doing a little bit of both?
Dr. Bell is staff psychiatrist at Jackson Park Hospital Surgical-Medical/Psychiatric Inpatient Unit; clinical professor emeritus, department of psychiatry, University of Illinois at Chicago; and former director of the Institute for Juvenile Research (the birthplace of child psychiatry), all in Chicago. He serves as chair of psychiatry at Windsor University, St. Kitts.
Some of us seem completely blank, but our minds are predisposed to constantly engage in various mental activities. Our minds can problem solve, fantasize, remember past events, get preoccupied with a book or movie, pay attention to social relationships and/or pay attention to our relationship with ourselves, focus on what we are doing in the moment, and more.
Many of us understand that our minds sometime operate on more than one level, for example, I can be talking or listening to someone in the “front of my mind” and be thinking something seemingly unrelated in the “back of my mind.” Accordingly, Sometimes we try to take a break from this never-ending mental activity by going to sleep or even taking drugs to “turn our minds off.”
Another important aspect of our minds is “will.” I like to think that most of us are familiar with the concept of “will” and put ours to good use. We intentionally or unintentionally use our wills in various ways – to improve ourselves; have fun; dawdle our lives away on imaginary activities; accomplish something tangible (for example, write a paper or wash the kitchen floor); pay attention to what we are doing in the moment; be healthy; and practice cultivating a “sound mind.”
Many of us would agree that to be of sound mind means having the ability to be comfortable with ourselves; to have some regulation of our emotions and affects; not to worry too much, but just enough to stay motivated; and to apply our minds toward accomplishing a goal we have chosen for ourselves. A sound mind also involves having a good memory, being aware and awake, being able to sort things out and adapt to various difficulties life throws at us, solving problems creatively and effectively, being able to relax, and being able to have a purpose in our lives that we have some success in fulfilling.
As a psychiatrist, I often have been curious about how people use their minds and how they direct their mental activities, or, if they do not direct their mental activities at all and simply meander through their mental lives not doing much thinking about thinking (many of us do both). Further, what is even more curious to me is how we psychiatrists use our minds; after all, we are supposed to be the experts. Certainly, with the advent of psychoanalysis, cognitive-behavioral therapy and other techniques, we are teaching our patients, and, hopefully, learning ourselves about how to manage our minds and what they preoccupy themselves with thinking. Of course, there were the methods of Abraham A. Low, MD, about “will training,” which I consider the first iteration of CBT. Prior to psychoanalysis and Low’s ideas, there have always been various forms of mindfulness.
So, my questions to my fellow psychiatrists are: What are your doing with your mind? Are you wasting it, cultivating it, or doing a little bit of both?
Dr. Bell is staff psychiatrist at Jackson Park Hospital Surgical-Medical/Psychiatric Inpatient Unit; clinical professor emeritus, department of psychiatry, University of Illinois at Chicago; and former director of the Institute for Juvenile Research (the birthplace of child psychiatry), all in Chicago. He serves as chair of psychiatry at Windsor University, St. Kitts.
Some of us seem completely blank, but our minds are predisposed to constantly engage in various mental activities. Our minds can problem solve, fantasize, remember past events, get preoccupied with a book or movie, pay attention to social relationships and/or pay attention to our relationship with ourselves, focus on what we are doing in the moment, and more.
Many of us understand that our minds sometime operate on more than one level, for example, I can be talking or listening to someone in the “front of my mind” and be thinking something seemingly unrelated in the “back of my mind.” Accordingly, Sometimes we try to take a break from this never-ending mental activity by going to sleep or even taking drugs to “turn our minds off.”
Another important aspect of our minds is “will.” I like to think that most of us are familiar with the concept of “will” and put ours to good use. We intentionally or unintentionally use our wills in various ways – to improve ourselves; have fun; dawdle our lives away on imaginary activities; accomplish something tangible (for example, write a paper or wash the kitchen floor); pay attention to what we are doing in the moment; be healthy; and practice cultivating a “sound mind.”
Many of us would agree that to be of sound mind means having the ability to be comfortable with ourselves; to have some regulation of our emotions and affects; not to worry too much, but just enough to stay motivated; and to apply our minds toward accomplishing a goal we have chosen for ourselves. A sound mind also involves having a good memory, being aware and awake, being able to sort things out and adapt to various difficulties life throws at us, solving problems creatively and effectively, being able to relax, and being able to have a purpose in our lives that we have some success in fulfilling.
As a psychiatrist, I often have been curious about how people use their minds and how they direct their mental activities, or, if they do not direct their mental activities at all and simply meander through their mental lives not doing much thinking about thinking (many of us do both). Further, what is even more curious to me is how we psychiatrists use our minds; after all, we are supposed to be the experts. Certainly, with the advent of psychoanalysis, cognitive-behavioral therapy and other techniques, we are teaching our patients, and, hopefully, learning ourselves about how to manage our minds and what they preoccupy themselves with thinking. Of course, there were the methods of Abraham A. Low, MD, about “will training,” which I consider the first iteration of CBT. Prior to psychoanalysis and Low’s ideas, there have always been various forms of mindfulness.
So, my questions to my fellow psychiatrists are: What are your doing with your mind? Are you wasting it, cultivating it, or doing a little bit of both?
Dr. Bell is staff psychiatrist at Jackson Park Hospital Surgical-Medical/Psychiatric Inpatient Unit; clinical professor emeritus, department of psychiatry, University of Illinois at Chicago; and former director of the Institute for Juvenile Research (the birthplace of child psychiatry), all in Chicago. He serves as chair of psychiatry at Windsor University, St. Kitts.
AAN and others update practice guidelines for prolonged disorders of consciousness
An updated set of practice guidelines developed by the American Academy of Neurology recommends that patients with prolonged disorders of consciousness, such as a vegetative state or minimally conscious state, first undergo treatment for other outside symptoms and conditions to increase the likelihood of initial accurate diagnosis. The AAN also recommends that patients be evaluated by multidisciplinary specialists using standardized neurobehavioral assessments.
“People are sometimes misdiagnosed due to underlying impairments that can mask awareness,” guidelines first author Joseph T. Giacino, PhD, of Harvard Medical School, Boston, and Spaulding Rehabilitation Hospital, Charlestown, Mass., stated in a press release about the guidelines. “An inaccurate diagnosis can lead to inappropriate care decisions and poor health outcomes. Misdiagnosis may result in premature or inappropriate treatment withdrawal, failure to recommend beneficial rehabilitative treatments, and worse outcome. That is why an early and accurate diagnosis is so important.”
The practice guidelines, published Aug. 8 in Neurology, update the 1995 recommendations from the AAN on persistent vegetative state (VS) as well as a 2002 case definition of minimally conscious state (MCS) developed by the AAN, American Congress of Rehabilitation Medicine, and the National Institute on Disability, Independent Living, and Rehabilitation Research. In the new document, these same organizations made 18 recommendations about prolonged disorders of consciousness (DoC) based on three levels of evidence: Level A evidence was defined as the strongest recommendation, Level B evidence consisted of recommendations with a confident rationale and a “favorable benefit-risk profile,” and Level C evidence was the lowest level of recommendation that was still useful to clinical practice. Evidence was grouped into four different classifications based on the modified Grading of Recommendations Assessment, Development, and Evaluation process in a systematic review and based on “strong related evidence, established principles of care, and inferences.”
In the guidelines, the committee made the following recommendations:
- Medically stable patients with DoC should be moved to multidisciplinary care settings where specialists can “optimize diagnostic evaluation, prognostication, and subsequent management, including effective medical monitoring and rehabilitative care” (Level B).
- Patients should receive care for confounding conditions, receive serial standardized assessments, and undergo care that “optimizes arousal” to maximize initial accurate diagnoses (Level B).
- Clinicians should communicate to families of patients with prolonged DoC that adult patients in an MCS caused by traumatic injury tend to have “more favorable outcomes,” compared with patients who are in a VS and patients with unresponsive wakefulness syndrome (UWS) caused by a nontraumatic injury (Level B).
- Clinicians must discuss long-term care with families of patients with prolonged DoC and a poor prognosis (Level A), indicate that not all patients of this type have a poor prognosis, administer Coma Recovery Scale–Revised, and perform imaging, such as structural MRI and single-photon emission CT, to determine prognosis in these patients (Level B).
- Regarding discussion of long-term care with families of children with prolonged DoC, clinicians should acknowledge that prognostic assessment, treatment, and natural history of recovery is not well-defined for children with prolonged DoC (Level B).
- In patients with traumatic VS, UWS, or MCS, amantadine should be prescribed (100-200 mg) between 4 weeks and 16 weeks after injury to lower the risk of disability and increase the likelihood of functional recovery (Level B).
- Clinicians should always assess and treat pain as well as discuss “evidence supporting treatment approaches” (Level B).
In addition, the subcommittee recommended changing the term permanent VS to chronic VS/UWS, citing Level B evidence. “Continued use of the term permanent VS is not justified. Use of this term implies irreversibility, which is not supported by the current research and has implications for family counseling, decision making, and the ethics of the field,” Dr. Giacino and his colleagues wrote.
In a separate summary of the guidelines, Dr. Giacino and his colleagues expressed concern about the lack of moderate or strong evidence for diagnostic assessment procedures in the literature, which they partially attributed to the inclusion of patients out 28 days or less from their injury in the systematic review for the guidelines. They further noted the lack of a gold-standard diagnostic approach for these patients, a lack of masking in diagnostic studies and tracking of recovery milestones and long-term functional outcomes for patients, and the limitations of the mainly retrospective analyses of outcomes in studies that they included. In addition, Dr. Giacino and his colleagues noted a lack of therapeutic studies with patients in inpatient rehabilitation centers and a “tendency by insurers to preferentially authorize rehabilitative care in lower-cost settings.” They excluded studies that had less than 20 patients, no control group, and were not “methodologically sound.”
Shorter lengths of stay in inpatient rehabilitation at academic medical centers have also led to problems in recruiting for placebo-controlled clinical trials, they noted.
“Under these circumstances, family members are often reticent to enroll patients with prolonged DoC in a placebo-controlled trial in view of the 50% likelihood of assignment to the placebo arm, preventing any possibility of active treatment during rehabilitation apart from routine physical, occupational, and speech therapies,” the authors wrote.
Several of the guidelines’ 16 authors disclosed ties to publishing houses and commercial or government entities, and participate in other activities related to the content of the published guidelines. Please see the full study for a complete list of disclosures.
The American Academy of Neurology Guideline on Disorders of Consciousness should be lauded for its focus on rehabilitation of this population, but it misses an opportunity to address “the broader ethical implications for patient care and institutional reform,” Joseph J. Fins, MD, and James L. Bernat, MD, wrote in a related editorial.
The authors commended the guidelines for recommending a change in reclassifying permanent vegetative state (VS) as “chronic vegetative state” but noted that the designation may be too broad, considering it encompasses patients who were misdiagnosed, patients who improved after treatment, patients with cognitive-motor disassociation, and patients who have undergone late improvements to achieve some level of consciousness. Defining these patients to clarify their prognoses will be important in determining which patients with VS are able to make late improvements, they said.
“While this redesignation seems warranted on clinical and epidemiologic grounds, it will create repercussions beyond the house of medicine given that the right to refuse life-sustaining treatment initially was predicated upon the irreversibility of the VS,” the authors wrote.
Dr. Fins and Dr. Bernat also acknowledged the seemingly contradictory recommendation of systematizing the care of patients with prolonged disorders of consciousness given that the infrastructure to provide this care is unavailable and unaffordable for many patients.
“Now that the Guideline has stipulated benchmarks for practice, practitioners and institutions need to meet this standard of care, and payers must ensure that these services are covered. It is acceptable neither to plead ignorance of these conditions nor to assert that nothing can be done to help ameliorate the burden of severe brain injury,” the authors wrote. “Given the utility of greater specialized care in diagnosis, treatment, and rehabilitation, and the equal importance of avoiding medical complications that can impede recovery, our society must provide the infrastructure and resources needed to offer quality care.”
Dr. Fins is with the division of medical ethics and the Consortium for the Advanced Study of Brain Injury at Cornell University, New York. Dr. Bernat is with the departments of neurology and medicine at the Geisel School of Medicine at Dartmouth in Hanover, N.H. They receive royalties from several published books that are relevant to the content of the guidelines.
The American Academy of Neurology Guideline on Disorders of Consciousness should be lauded for its focus on rehabilitation of this population, but it misses an opportunity to address “the broader ethical implications for patient care and institutional reform,” Joseph J. Fins, MD, and James L. Bernat, MD, wrote in a related editorial.
The authors commended the guidelines for recommending a change in reclassifying permanent vegetative state (VS) as “chronic vegetative state” but noted that the designation may be too broad, considering it encompasses patients who were misdiagnosed, patients who improved after treatment, patients with cognitive-motor disassociation, and patients who have undergone late improvements to achieve some level of consciousness. Defining these patients to clarify their prognoses will be important in determining which patients with VS are able to make late improvements, they said.
“While this redesignation seems warranted on clinical and epidemiologic grounds, it will create repercussions beyond the house of medicine given that the right to refuse life-sustaining treatment initially was predicated upon the irreversibility of the VS,” the authors wrote.
Dr. Fins and Dr. Bernat also acknowledged the seemingly contradictory recommendation of systematizing the care of patients with prolonged disorders of consciousness given that the infrastructure to provide this care is unavailable and unaffordable for many patients.
“Now that the Guideline has stipulated benchmarks for practice, practitioners and institutions need to meet this standard of care, and payers must ensure that these services are covered. It is acceptable neither to plead ignorance of these conditions nor to assert that nothing can be done to help ameliorate the burden of severe brain injury,” the authors wrote. “Given the utility of greater specialized care in diagnosis, treatment, and rehabilitation, and the equal importance of avoiding medical complications that can impede recovery, our society must provide the infrastructure and resources needed to offer quality care.”
Dr. Fins is with the division of medical ethics and the Consortium for the Advanced Study of Brain Injury at Cornell University, New York. Dr. Bernat is with the departments of neurology and medicine at the Geisel School of Medicine at Dartmouth in Hanover, N.H. They receive royalties from several published books that are relevant to the content of the guidelines.
The American Academy of Neurology Guideline on Disorders of Consciousness should be lauded for its focus on rehabilitation of this population, but it misses an opportunity to address “the broader ethical implications for patient care and institutional reform,” Joseph J. Fins, MD, and James L. Bernat, MD, wrote in a related editorial.
The authors commended the guidelines for recommending a change in reclassifying permanent vegetative state (VS) as “chronic vegetative state” but noted that the designation may be too broad, considering it encompasses patients who were misdiagnosed, patients who improved after treatment, patients with cognitive-motor disassociation, and patients who have undergone late improvements to achieve some level of consciousness. Defining these patients to clarify their prognoses will be important in determining which patients with VS are able to make late improvements, they said.
“While this redesignation seems warranted on clinical and epidemiologic grounds, it will create repercussions beyond the house of medicine given that the right to refuse life-sustaining treatment initially was predicated upon the irreversibility of the VS,” the authors wrote.
Dr. Fins and Dr. Bernat also acknowledged the seemingly contradictory recommendation of systematizing the care of patients with prolonged disorders of consciousness given that the infrastructure to provide this care is unavailable and unaffordable for many patients.
“Now that the Guideline has stipulated benchmarks for practice, practitioners and institutions need to meet this standard of care, and payers must ensure that these services are covered. It is acceptable neither to plead ignorance of these conditions nor to assert that nothing can be done to help ameliorate the burden of severe brain injury,” the authors wrote. “Given the utility of greater specialized care in diagnosis, treatment, and rehabilitation, and the equal importance of avoiding medical complications that can impede recovery, our society must provide the infrastructure and resources needed to offer quality care.”
Dr. Fins is with the division of medical ethics and the Consortium for the Advanced Study of Brain Injury at Cornell University, New York. Dr. Bernat is with the departments of neurology and medicine at the Geisel School of Medicine at Dartmouth in Hanover, N.H. They receive royalties from several published books that are relevant to the content of the guidelines.
An updated set of practice guidelines developed by the American Academy of Neurology recommends that patients with prolonged disorders of consciousness, such as a vegetative state or minimally conscious state, first undergo treatment for other outside symptoms and conditions to increase the likelihood of initial accurate diagnosis. The AAN also recommends that patients be evaluated by multidisciplinary specialists using standardized neurobehavioral assessments.
“People are sometimes misdiagnosed due to underlying impairments that can mask awareness,” guidelines first author Joseph T. Giacino, PhD, of Harvard Medical School, Boston, and Spaulding Rehabilitation Hospital, Charlestown, Mass., stated in a press release about the guidelines. “An inaccurate diagnosis can lead to inappropriate care decisions and poor health outcomes. Misdiagnosis may result in premature or inappropriate treatment withdrawal, failure to recommend beneficial rehabilitative treatments, and worse outcome. That is why an early and accurate diagnosis is so important.”
The practice guidelines, published Aug. 8 in Neurology, update the 1995 recommendations from the AAN on persistent vegetative state (VS) as well as a 2002 case definition of minimally conscious state (MCS) developed by the AAN, American Congress of Rehabilitation Medicine, and the National Institute on Disability, Independent Living, and Rehabilitation Research. In the new document, these same organizations made 18 recommendations about prolonged disorders of consciousness (DoC) based on three levels of evidence: Level A evidence was defined as the strongest recommendation, Level B evidence consisted of recommendations with a confident rationale and a “favorable benefit-risk profile,” and Level C evidence was the lowest level of recommendation that was still useful to clinical practice. Evidence was grouped into four different classifications based on the modified Grading of Recommendations Assessment, Development, and Evaluation process in a systematic review and based on “strong related evidence, established principles of care, and inferences.”
In the guidelines, the committee made the following recommendations:
- Medically stable patients with DoC should be moved to multidisciplinary care settings where specialists can “optimize diagnostic evaluation, prognostication, and subsequent management, including effective medical monitoring and rehabilitative care” (Level B).
- Patients should receive care for confounding conditions, receive serial standardized assessments, and undergo care that “optimizes arousal” to maximize initial accurate diagnoses (Level B).
- Clinicians should communicate to families of patients with prolonged DoC that adult patients in an MCS caused by traumatic injury tend to have “more favorable outcomes,” compared with patients who are in a VS and patients with unresponsive wakefulness syndrome (UWS) caused by a nontraumatic injury (Level B).
- Clinicians must discuss long-term care with families of patients with prolonged DoC and a poor prognosis (Level A), indicate that not all patients of this type have a poor prognosis, administer Coma Recovery Scale–Revised, and perform imaging, such as structural MRI and single-photon emission CT, to determine prognosis in these patients (Level B).
- Regarding discussion of long-term care with families of children with prolonged DoC, clinicians should acknowledge that prognostic assessment, treatment, and natural history of recovery is not well-defined for children with prolonged DoC (Level B).
- In patients with traumatic VS, UWS, or MCS, amantadine should be prescribed (100-200 mg) between 4 weeks and 16 weeks after injury to lower the risk of disability and increase the likelihood of functional recovery (Level B).
- Clinicians should always assess and treat pain as well as discuss “evidence supporting treatment approaches” (Level B).
In addition, the subcommittee recommended changing the term permanent VS to chronic VS/UWS, citing Level B evidence. “Continued use of the term permanent VS is not justified. Use of this term implies irreversibility, which is not supported by the current research and has implications for family counseling, decision making, and the ethics of the field,” Dr. Giacino and his colleagues wrote.
In a separate summary of the guidelines, Dr. Giacino and his colleagues expressed concern about the lack of moderate or strong evidence for diagnostic assessment procedures in the literature, which they partially attributed to the inclusion of patients out 28 days or less from their injury in the systematic review for the guidelines. They further noted the lack of a gold-standard diagnostic approach for these patients, a lack of masking in diagnostic studies and tracking of recovery milestones and long-term functional outcomes for patients, and the limitations of the mainly retrospective analyses of outcomes in studies that they included. In addition, Dr. Giacino and his colleagues noted a lack of therapeutic studies with patients in inpatient rehabilitation centers and a “tendency by insurers to preferentially authorize rehabilitative care in lower-cost settings.” They excluded studies that had less than 20 patients, no control group, and were not “methodologically sound.”
Shorter lengths of stay in inpatient rehabilitation at academic medical centers have also led to problems in recruiting for placebo-controlled clinical trials, they noted.
“Under these circumstances, family members are often reticent to enroll patients with prolonged DoC in a placebo-controlled trial in view of the 50% likelihood of assignment to the placebo arm, preventing any possibility of active treatment during rehabilitation apart from routine physical, occupational, and speech therapies,” the authors wrote.
Several of the guidelines’ 16 authors disclosed ties to publishing houses and commercial or government entities, and participate in other activities related to the content of the published guidelines. Please see the full study for a complete list of disclosures.
An updated set of practice guidelines developed by the American Academy of Neurology recommends that patients with prolonged disorders of consciousness, such as a vegetative state or minimally conscious state, first undergo treatment for other outside symptoms and conditions to increase the likelihood of initial accurate diagnosis. The AAN also recommends that patients be evaluated by multidisciplinary specialists using standardized neurobehavioral assessments.
“People are sometimes misdiagnosed due to underlying impairments that can mask awareness,” guidelines first author Joseph T. Giacino, PhD, of Harvard Medical School, Boston, and Spaulding Rehabilitation Hospital, Charlestown, Mass., stated in a press release about the guidelines. “An inaccurate diagnosis can lead to inappropriate care decisions and poor health outcomes. Misdiagnosis may result in premature or inappropriate treatment withdrawal, failure to recommend beneficial rehabilitative treatments, and worse outcome. That is why an early and accurate diagnosis is so important.”
The practice guidelines, published Aug. 8 in Neurology, update the 1995 recommendations from the AAN on persistent vegetative state (VS) as well as a 2002 case definition of minimally conscious state (MCS) developed by the AAN, American Congress of Rehabilitation Medicine, and the National Institute on Disability, Independent Living, and Rehabilitation Research. In the new document, these same organizations made 18 recommendations about prolonged disorders of consciousness (DoC) based on three levels of evidence: Level A evidence was defined as the strongest recommendation, Level B evidence consisted of recommendations with a confident rationale and a “favorable benefit-risk profile,” and Level C evidence was the lowest level of recommendation that was still useful to clinical practice. Evidence was grouped into four different classifications based on the modified Grading of Recommendations Assessment, Development, and Evaluation process in a systematic review and based on “strong related evidence, established principles of care, and inferences.”
In the guidelines, the committee made the following recommendations:
- Medically stable patients with DoC should be moved to multidisciplinary care settings where specialists can “optimize diagnostic evaluation, prognostication, and subsequent management, including effective medical monitoring and rehabilitative care” (Level B).
- Patients should receive care for confounding conditions, receive serial standardized assessments, and undergo care that “optimizes arousal” to maximize initial accurate diagnoses (Level B).
- Clinicians should communicate to families of patients with prolonged DoC that adult patients in an MCS caused by traumatic injury tend to have “more favorable outcomes,” compared with patients who are in a VS and patients with unresponsive wakefulness syndrome (UWS) caused by a nontraumatic injury (Level B).
- Clinicians must discuss long-term care with families of patients with prolonged DoC and a poor prognosis (Level A), indicate that not all patients of this type have a poor prognosis, administer Coma Recovery Scale–Revised, and perform imaging, such as structural MRI and single-photon emission CT, to determine prognosis in these patients (Level B).
- Regarding discussion of long-term care with families of children with prolonged DoC, clinicians should acknowledge that prognostic assessment, treatment, and natural history of recovery is not well-defined for children with prolonged DoC (Level B).
- In patients with traumatic VS, UWS, or MCS, amantadine should be prescribed (100-200 mg) between 4 weeks and 16 weeks after injury to lower the risk of disability and increase the likelihood of functional recovery (Level B).
- Clinicians should always assess and treat pain as well as discuss “evidence supporting treatment approaches” (Level B).
In addition, the subcommittee recommended changing the term permanent VS to chronic VS/UWS, citing Level B evidence. “Continued use of the term permanent VS is not justified. Use of this term implies irreversibility, which is not supported by the current research and has implications for family counseling, decision making, and the ethics of the field,” Dr. Giacino and his colleagues wrote.
In a separate summary of the guidelines, Dr. Giacino and his colleagues expressed concern about the lack of moderate or strong evidence for diagnostic assessment procedures in the literature, which they partially attributed to the inclusion of patients out 28 days or less from their injury in the systematic review for the guidelines. They further noted the lack of a gold-standard diagnostic approach for these patients, a lack of masking in diagnostic studies and tracking of recovery milestones and long-term functional outcomes for patients, and the limitations of the mainly retrospective analyses of outcomes in studies that they included. In addition, Dr. Giacino and his colleagues noted a lack of therapeutic studies with patients in inpatient rehabilitation centers and a “tendency by insurers to preferentially authorize rehabilitative care in lower-cost settings.” They excluded studies that had less than 20 patients, no control group, and were not “methodologically sound.”
Shorter lengths of stay in inpatient rehabilitation at academic medical centers have also led to problems in recruiting for placebo-controlled clinical trials, they noted.
“Under these circumstances, family members are often reticent to enroll patients with prolonged DoC in a placebo-controlled trial in view of the 50% likelihood of assignment to the placebo arm, preventing any possibility of active treatment during rehabilitation apart from routine physical, occupational, and speech therapies,” the authors wrote.
Several of the guidelines’ 16 authors disclosed ties to publishing houses and commercial or government entities, and participate in other activities related to the content of the published guidelines. Please see the full study for a complete list of disclosures.
FROM NEUROLOGY
See VAM 2018 Presentations with VAM on Demand
VAM on Demand, a library of hundreds of presentations from the 2018 Vascular Annual Meeting is now available. Those who attended VAM can review sessions at their own pace and watch others that they missed. Those who did not attend now get the chance to learn from the sessions offered. Cost is $199 for VAM attendees and $499 for non-attendees. Access, including the ability to download materials, is good for one year. Learn more and purchase VAM on Demand here. Contact the SVS Education Department for more information at 312-334-2300, or at [email protected].
VAM on Demand, a library of hundreds of presentations from the 2018 Vascular Annual Meeting is now available. Those who attended VAM can review sessions at their own pace and watch others that they missed. Those who did not attend now get the chance to learn from the sessions offered. Cost is $199 for VAM attendees and $499 for non-attendees. Access, including the ability to download materials, is good for one year. Learn more and purchase VAM on Demand here. Contact the SVS Education Department for more information at 312-334-2300, or at [email protected].
VAM on Demand, a library of hundreds of presentations from the 2018 Vascular Annual Meeting is now available. Those who attended VAM can review sessions at their own pace and watch others that they missed. Those who did not attend now get the chance to learn from the sessions offered. Cost is $199 for VAM attendees and $499 for non-attendees. Access, including the ability to download materials, is good for one year. Learn more and purchase VAM on Demand here. Contact the SVS Education Department for more information at 312-334-2300, or at [email protected].