User login
Product approved to treat hemophilia A in Japan
The Japanese Ministry of Health, Labour and Welfare has approved Jivi® (also known as damoctocog alfa pegol or antihemophilic factor [recombinant] PEGylated-aucl) for the treatment of hemophilia A.
Jivi (formerly BAY94-9027) is a DNA-derived, factor VIII concentrate approved for use in hemophilia A patients age 12 and older.
Jivi is approved for on-demand treatment and control of bleeding episodes, for perioperative management of bleeding, and as routine prophylaxis to reduce the frequency of bleeding episodes.
As prophylaxis, Jivi is typically given twice weekly, but it can also be given every 5 days or once a week, depending on patient needs.
The approval of Jivi in Japan is supported by data from the phase 2/3 PROTECT VIII trial. Some results from this trial were published in the Journal of Thrombosis and Haemostasis in 2016. Additional results are available in the U.S. prescribing information for Jivi.
PROTECT VIII enrolled previously treated adults and adolescents (ages 12 to 65) with severe hemophilia A.
In part A, researchers evaluated different dosing regimens for Jivi used as prophylaxis and on-demand treatment. An optional extension study was available to patients who completed part A.
In part B, researchers evaluated Jivi for perioperative management.
Efficacy
In part A, there were 132 patients in the intent‐to‐treat population—112 in the prophylaxis group and 20 in the on-demand group.
Patients received Jivi for 36 weeks. For the first 10 weeks, patients in the prophylaxis group received twice-weekly dosing at 25 IU/kg.
Patients with more than one bleed during this time went on to receive 30–40 IU/kg twice weekly. Patients with one or fewer bleeds were eligible for randomization to dosing every 5 days (45–60 IU/kg) or every 7 days (60 IU/kg).
The median annualized bleeding rate (ABR) was 4.1 for the patients who were treated twice weekly and were not eligible for randomization (n=13) and 1.9 for patients who were eligible for randomization but continued on twice-weekly treatment (n=11).
The median ABR was 1.9 for patients who were randomized to treatment every 5 days (n=43) and 0.96 for patients who completed prophylaxis with dosing every 7 days (32/43).
The median ABR for patients treated on demand was 24.1.
There were 388 treated bleeds in the on-demand group and 317 treated bleeds in the prophylaxis group. Overall, 73.3% of responses to treatment were considered “excellent” or “good,” 23.3% were considered “moderate,” and 3.3% were considered “poor.”
There were 17 patients who underwent 20 major surgeries in part B or the extension study and 10 patients who underwent minor surgeries in part A. Jivi provided “good” or “excellent” hemostatic control during all surgeries.
Safety
Safety data are available for 148 patients age 12 and older.
Adverse events in these patients included abdominal pain (3%), nausea (5%), vomiting (3%), injection site reactions (1%), pyrexia (5%), hypersensitivity (2%), dizziness (2%), headache (14%), insomnia (3%), cough (7%), erythema (1%), pruritus (1%), rash (2%), and flushing (1%).
A factor VIII inhibitor was reported in one adult patient, but repeat testing did not confirm the report.
One adult with asthma had a clinical hypersensitivity reaction and a transient increase of IgM anti-PEG antibody titer, which was negative upon retesting.
The Japanese Ministry of Health, Labour and Welfare has approved Jivi® (also known as damoctocog alfa pegol or antihemophilic factor [recombinant] PEGylated-aucl) for the treatment of hemophilia A.
Jivi (formerly BAY94-9027) is a DNA-derived, factor VIII concentrate approved for use in hemophilia A patients age 12 and older.
Jivi is approved for on-demand treatment and control of bleeding episodes, for perioperative management of bleeding, and as routine prophylaxis to reduce the frequency of bleeding episodes.
As prophylaxis, Jivi is typically given twice weekly, but it can also be given every 5 days or once a week, depending on patient needs.
The approval of Jivi in Japan is supported by data from the phase 2/3 PROTECT VIII trial. Some results from this trial were published in the Journal of Thrombosis and Haemostasis in 2016. Additional results are available in the U.S. prescribing information for Jivi.
PROTECT VIII enrolled previously treated adults and adolescents (ages 12 to 65) with severe hemophilia A.
In part A, researchers evaluated different dosing regimens for Jivi used as prophylaxis and on-demand treatment. An optional extension study was available to patients who completed part A.
In part B, researchers evaluated Jivi for perioperative management.
Efficacy
In part A, there were 132 patients in the intent‐to‐treat population—112 in the prophylaxis group and 20 in the on-demand group.
Patients received Jivi for 36 weeks. For the first 10 weeks, patients in the prophylaxis group received twice-weekly dosing at 25 IU/kg.
Patients with more than one bleed during this time went on to receive 30–40 IU/kg twice weekly. Patients with one or fewer bleeds were eligible for randomization to dosing every 5 days (45–60 IU/kg) or every 7 days (60 IU/kg).
The median annualized bleeding rate (ABR) was 4.1 for the patients who were treated twice weekly and were not eligible for randomization (n=13) and 1.9 for patients who were eligible for randomization but continued on twice-weekly treatment (n=11).
The median ABR was 1.9 for patients who were randomized to treatment every 5 days (n=43) and 0.96 for patients who completed prophylaxis with dosing every 7 days (32/43).
The median ABR for patients treated on demand was 24.1.
There were 388 treated bleeds in the on-demand group and 317 treated bleeds in the prophylaxis group. Overall, 73.3% of responses to treatment were considered “excellent” or “good,” 23.3% were considered “moderate,” and 3.3% were considered “poor.”
There were 17 patients who underwent 20 major surgeries in part B or the extension study and 10 patients who underwent minor surgeries in part A. Jivi provided “good” or “excellent” hemostatic control during all surgeries.
Safety
Safety data are available for 148 patients age 12 and older.
Adverse events in these patients included abdominal pain (3%), nausea (5%), vomiting (3%), injection site reactions (1%), pyrexia (5%), hypersensitivity (2%), dizziness (2%), headache (14%), insomnia (3%), cough (7%), erythema (1%), pruritus (1%), rash (2%), and flushing (1%).
A factor VIII inhibitor was reported in one adult patient, but repeat testing did not confirm the report.
One adult with asthma had a clinical hypersensitivity reaction and a transient increase of IgM anti-PEG antibody titer, which was negative upon retesting.
The Japanese Ministry of Health, Labour and Welfare has approved Jivi® (also known as damoctocog alfa pegol or antihemophilic factor [recombinant] PEGylated-aucl) for the treatment of hemophilia A.
Jivi (formerly BAY94-9027) is a DNA-derived, factor VIII concentrate approved for use in hemophilia A patients age 12 and older.
Jivi is approved for on-demand treatment and control of bleeding episodes, for perioperative management of bleeding, and as routine prophylaxis to reduce the frequency of bleeding episodes.
As prophylaxis, Jivi is typically given twice weekly, but it can also be given every 5 days or once a week, depending on patient needs.
The approval of Jivi in Japan is supported by data from the phase 2/3 PROTECT VIII trial. Some results from this trial were published in the Journal of Thrombosis and Haemostasis in 2016. Additional results are available in the U.S. prescribing information for Jivi.
PROTECT VIII enrolled previously treated adults and adolescents (ages 12 to 65) with severe hemophilia A.
In part A, researchers evaluated different dosing regimens for Jivi used as prophylaxis and on-demand treatment. An optional extension study was available to patients who completed part A.
In part B, researchers evaluated Jivi for perioperative management.
Efficacy
In part A, there were 132 patients in the intent‐to‐treat population—112 in the prophylaxis group and 20 in the on-demand group.
Patients received Jivi for 36 weeks. For the first 10 weeks, patients in the prophylaxis group received twice-weekly dosing at 25 IU/kg.
Patients with more than one bleed during this time went on to receive 30–40 IU/kg twice weekly. Patients with one or fewer bleeds were eligible for randomization to dosing every 5 days (45–60 IU/kg) or every 7 days (60 IU/kg).
The median annualized bleeding rate (ABR) was 4.1 for the patients who were treated twice weekly and were not eligible for randomization (n=13) and 1.9 for patients who were eligible for randomization but continued on twice-weekly treatment (n=11).
The median ABR was 1.9 for patients who were randomized to treatment every 5 days (n=43) and 0.96 for patients who completed prophylaxis with dosing every 7 days (32/43).
The median ABR for patients treated on demand was 24.1.
There were 388 treated bleeds in the on-demand group and 317 treated bleeds in the prophylaxis group. Overall, 73.3% of responses to treatment were considered “excellent” or “good,” 23.3% were considered “moderate,” and 3.3% were considered “poor.”
There were 17 patients who underwent 20 major surgeries in part B or the extension study and 10 patients who underwent minor surgeries in part A. Jivi provided “good” or “excellent” hemostatic control during all surgeries.
Safety
Safety data are available for 148 patients age 12 and older.
Adverse events in these patients included abdominal pain (3%), nausea (5%), vomiting (3%), injection site reactions (1%), pyrexia (5%), hypersensitivity (2%), dizziness (2%), headache (14%), insomnia (3%), cough (7%), erythema (1%), pruritus (1%), rash (2%), and flushing (1%).
A factor VIII inhibitor was reported in one adult patient, but repeat testing did not confirm the report.
One adult with asthma had a clinical hypersensitivity reaction and a transient increase of IgM anti-PEG antibody titer, which was negative upon retesting.
Polycythemia Vera and Essential Thrombocythemia
From the Columbia University Medical Center, New York, NY (Dr. Falchi), and the University of Texas MD Anderson Cancer Center, Houston, TX (Dr. Verstovsek).
ABSTRACT
- Objective: To review the clinical aspects and current practices in the management of polycythemia vera (PV) and essential thrombocythemia (ET).
- Methods: Review of the literature.
- Results: PV and ET are rare chronic myeloid disorders. The 2 most important life-limiting complications of PV and ET are thrombohemorrhagic events and myelofibrosis/acute myeloid leukemia (AML) transformation. Vascular events are at least in part preventable with counseling on risk factors, phlebotomy (for patients with PV), antiplatelet therapy, and cytoreduction with hydroxyurea, interferons, or anagrelide (for patients with ET). Ruxolitinib was recently approved for PV after hydroxyurea failure. PV/ET transformation into myelofibrosis or AML is part of the natural history of the disease and no therapy has been shown to prevent it. Treatment of leukemic transformation of myeloproliferative neoplasms (MPN LT) follows recommendations set forth for primary myelofibrosis and AML.
- Conclusion: With appropriate management, patients with PV and ET typically enjoy a long survival and near-normal quality of life. Transformation into myelofibrosis or AML cannot be prevented by current therapies, however. Treatment results with MPN LT are generally poor and novel strategies are needed to improve outcomes.
Key words: myeloproliferative neoplasms; myelofibrosis; leukemic transformation.
Polycythemia vera (PV) and essential thrombocythemia (ET), along with primary myelofibrosis (PMF), belong to the group of Philadelphia-negative myeloproliferative neoplasms (MPN). All these malignancies arise from the clonal proliferation of an aberrant hematopoietic stem cell, but are characterized by distinct clinical phenotypes [1,2]. Although the clinical course of PV and ET is indolent, it can be complicated by thrombohemorrhagic episodes and/or evolution into myelofibrosis and/or acute myeloid leukemia (AML) [3]. Since vascular events are the most frequent life-threatening complications of PV and ET, therapeutic strategies are aimed at reducing this risk. Treatment may also help control other symptoms associated with the disease [4]. No therapy has been shown to prevent evolution of PV or ET into myelofibrosis or AML. The discovery of the Janus kinase 2 (JAK2)/V617F mutation in most patients with PV and over half of those with ET (and PMF) [5,6] has opened new avenues of research and led to the development of targeted therapies, such as the JAK1/2 inhibitor ruxolitinib, for patients with MPN [7,8].
Epidemiology
PV and ET are typically diagnosed in the fifth to seventh decade of life [9]. Although these disorders are generally associated with a long clinical course, survival of patients with PV or ET may be shorter than that of the general population [10–13]. Estimating the incidence and prevalence of MPN is a challenge because most patients remain asymptomatic for long periods of time and do not seek medical attention [13]. The annual incidence rates of PV and ET are estimated at 0.01 to 2.61 and 0.21 to 2.53 per 100,000, respectively. PV occurs slightly more frequently in males, whereas ET has a predilection for females [14]. Given the long course and low mortality associated with these disorders, the prevalence rates of PV and ET are significantly higher than the respective incidence rates: up to 47 and 57 per 100,000, respectively [15–17].
Molecular Pathogenesis
In 2005 researchers discovered a gain-of-function mutation of the JAK2 gene in nearly all patients with PV and more than half of those with ET and PMF [5,6,18,19]. JAK2 is a non-receptor tyrosine kinase that plays a central role in normal hematopoiesis. Substitution of a valine for a phenylalanine at codon 617 (ie, V617F) leads to its constitutive activation and signaling through the JAK-STAT pathway [5,6,18,19]. More rarely (and exclusively in patients with PV), JAK2 mutations involve exon 12 [20–22]. The vast majority of JAK2-negative ET patients harbor mutations in either the myeloproliferative leukemia (MPL) gene, which encodes the thrombopoietin receptor [23–25], or the calreticulin (CALR) gene [26,27], which encodes for a chaperone protein that plays a role in cellular proliferation, differentiation, and apoptosis [28]. Both the MPL and CALR mutations ultimately result in the constitutive activation of the JAK-STAT pathway. Thus, JAK2, MPL, and CALR alterations are collectively referred to as driver mutations. Moreover, because these mutations affect the same oncogenic pathway (ie, JAK-STAT), they are almost always mutually exclusive in a given patient. Patients with ET (or myelofibrosis) who are wild-type for JAK2, MPL, and CALR are referred to as having “triple-negative” disease. Many recurrent non-driver mutations are also found in patients with MPN. These are not exclusive of each other (ie, patients may have many at the same time) and involve for example ten-eleven translocation-2 (TET2), additional sex combs like 1 (ASXL1), enhancer of zeste homolog 2 (EZH2), isocitrate dehydrogenase 1 and isocitrate dehydrogenase 2 (IDH1/2), and DNA methyltransferase 3A (DNMT3A) genes, among others [29]. The biologic and prognostic significance of these non-driver alterations remain to be fully defined in ET and PV.
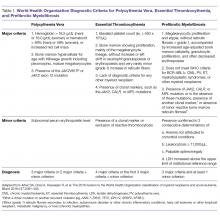
Diagnostic Criteria
Diagnostic criteria for PV and ET according to the World Health Organization (WHO) classification [30] are summarized in Table 1. Criteria for the diagnosis of prefibrotic myelofibrosis are included as well since this entity was formally recognized as separate from ET and part of the PMF spectrum in the 2016 WHO classification of myeloid tumors [30]. Clinically, both PV and ET generally remain asymptomatic for a long time. PV tends to be more symptomatic than ET and can present with debilitating constitutional symptoms (fatigue, night sweats, and weight loss), microvascular symptoms (headache, lightheadedness, acral paresthesias, erythromelalgia, atypical chest pain, and pruritus) [31], or macrovascular accidents (larger vein thrombosis, stroke, or myocardial ischemia) [32]. ET is often diagnosed incidentally, but patients can suffer from similar general symptoms and vascular complications. Causes of secondary absolute erythrocytosis (altitude, chronic hypoxemia, heavy smoking, cardiomyopathy, use of corticosteroids, erythropoietin, or other anabolic hormones, familial or congenital forms) or thrombocytosis (iron deficiency, acute blood loss, trauma or injury, acute coronary syndrome, systemic autoimmune disorders, chronic kidney failure, other malignancies, splenectomy) should be considered and appropriately excluded. Once the diagnosis is made, symptom assessment tools such as the Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) [33] or the abbreviated version, the MPN-SAF Total Symptom Score (MPN-SAF TSS) [34], are generally used to assess patients’ symptom burden and response to treatment in everyday practice.
Risk Stratification
Thrombohemorrhagic events, evolution into myelofibrosis, and leukemic transformation (LT) are the most serious complications in the course of PV or ET. Only thrombohemorrhagic events are, at least partially, preventable. Arterial or venous thrombotic complications are observed at rates of 1.8 to 10.9 per 100 patient-years in PV (arterial thrombosis being more common than venous) and 0.74 to 7.7 per 100 patient-years in ET, depending on the risk group [35] and the presence of other factors (see below).
The risk stratification of patients with PV is based on 2 factors: age ≥ 60 years and prior history of thrombosis. If either is present, the patient is assigned to the high-risk category, whereas if none is present the patient is considered at low risk [36]. In addition, high hematocrit [37] and high white blood cell (WBC) count [38], but not thrombocytosis, have been associated with the development of vascular complications. In one study, the risk of new arterial thrombosis was increased by the presence of leukoerythroblastosis, hypertension, and prior arterial thrombosis, while karyotypic abnormalities and prior venous thrombosis were predictors of new venous thrombosis [39]. Another emerging risk factor for thrombosis in patients with PV is high JAK2 allele burden (ie, the normal-to-mutated gene product ratio), although the evidence supporting this conclusion is equivocal [40].
Traditionally, in ET patients, the thrombotic risk was assessed using the same 2 factors (age ≥ 60 years and prior history of thrombosis), separating patients into low- and high-risk groups. However, the prognostication of ET patients has been refined recently with the identification of new relevant factors. In particular, the impact of JAK2 mutations on thrombotic risk has been thoroughly studied. Clinically, the presence of JAK2V617F is associated with older age, higher hemoglobin and hematocrit, lower platelet counts, more frequent need for cytoreductive treatment, and greater tendency to evolve into PV (a rare event) [41,42]. Many [41,43–46], but not all [47–51], studies suggested a correlation between JAK2 mutation and risk of both arterial and venous thrombosis. Although infrequent, a JAK2V617F homozygous state (ie, the mutation is present in both alleles) might confer an even higher thrombotic risk [52]. Moreover, the impact of the JAK2 mutation on vascular events persists over time [53], particularly in patients with high or unstable mutation burden [54]. Based on JAK2V617F’s influence on the thrombotic risk of ET patients, a new prognostic score was proposed, the International Prognostic Score for ET (IPSET)-thrombosis (Table 2). The revised version of this model is currently endorsed by the National Comprehensive Cancer Network and divides patients into 4 risk groups: high, intermediate, low, and very low. Treatment recommendations vary according to the risk group (as described below) [55].
Other thrombotic risk factors have been identified, but deemed not significant enough to be included in the model. Cardiovascular risk factors (hypercholesterolemia, hypertension, smoking, diabetes mellitus) can increase the risk of vascular events [56–59], as can splenomegaly [60] and baseline or persistent leukocytosis [61–63]. Thrombocytosis has been correlated with thrombotic risk in some studies [64–68], whereas others did not support this conclusion and/or suggested a lower rate of thrombosis and, in some cases, increased risk of bleeding in ET patients with platelet counts greater than 1000 × 103/μL (due to acquired von Willebrand syndrome) [51,61,63,68,69].
CALR mutations tend to occur in younger males with lower hemoglobin and WBC count, higher platelet count, and greater marrow megakaryocytic predominance, as compared to JAK2 mutations [26,27,70–72]. The associated incidence of thrombosis was less than 10% at 15 years in patients with CALR mutations, lower than the incidence reported for ET patients with JAK2V617F mutations [73]. The presence of the mutation per se does not appear to affect the thrombotic risk [74–76]. Information on the thrombotic risk associated with MPL mutations or a triple-negative state is scarce. In both instances, however, the risk appears to be lower than with the JAK2 mutation [73,77–79].
Venous thromboembolism (VTE) in patients with PV or ET may occur at unusual sites, such as the splanchnic or cerebral venous systems [80]. Risk factors for unusual VTE include younger age [81], female gender (especially with concomitant use of oral contraceptive pills) [82], and splenomegaly/splenectomy [83]. JAK2 mutation has also been associated with thrombosis at unusual sites. However, the prevalence of MPN or JAK2V617F in patients presenting with splanchnic VTE has varied [80]. In addition, MPN may be occult (ie, no clinical or laboratory abnormalities) in around 15% of patients [84]. Screening for JAK2V617F and underlying MPN is recommended in patients presenting with isolated unexplained splanchnic VTE. Treatment entails long-term anticoagulation therapy. JAK2V617F screening in patients with nonsplanchnic VTE is not recommended, as its prevalence in this group is low (< 3%) [85,86].
Risk-Adapted Therapy
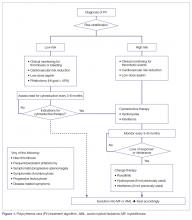
Low-Risk PV
All patients with PV should receive counseling to mitigate cardiovascular risk factors, including smoking cessation, lifestyle modifications, and lipid-lowering therapy, as indicated. Furthermore, all PV patients should receive acetylsalicylic acid (ASA) to decrease their risk for thrombosis and control vasomotor symptoms [55,87]. Aspirin 81 to 100 mg daily is the preferred regimen because it provides adequate antithrombotic effect without the associated bleeding risk of higher-dose aspirin [88]. Low-risk PV patients should also receive periodic phlebotomies to reduce and maintain their hematocrit below 45%. This recommendation is based on the results of the Cytoreductive Therapy in Polycythemia Vera (CYTO PV) randomized controlled trial. In that study, patients receiving more intense therapy to maintain the hematocrit below 45% had a lower incidence of cardiovascular-related deaths or major thrombotic events than those with hematocrit goals of 45% to 50% (2.7% versus 9.8%) [89]. Cytoreduction is an option for low-risk patients who do not tolerate phlebotomy or require frequent phlebotomy, or who have disease-related bleeding, severe symptoms, symptomatic splenomegaly, or progressive leukocytosis [38].
High-Risk PV
Patients older than 60 years and/or with a history of thrombosis should be considered for cytoreductive therapy in addition to the above measures. Frontline cytoreductive therapies include hydroxyurea or interferon (IFN)-alfa [87]. Hydroxyurea is a potent ribonucleotide reductase inhibitor that interferes with DNA repair and is the treatment of choice for most high-risk patients with PV [90]. In a small trial, hydroxyurea reduced the risk of thrombosis compared with historical controls treated with phlebotomy alone [91]. Hydroxyurea is generally well tolerated; common side effects include cytopenias, nail changes, and mucosal and/or skin ulcers. Although never formally proven to be leukemogenic, this agent should be used with caution in younger patients [87]. Indeed, in the original study, the rates of transformation were 5.9% and 1.5% for patients receiving hydroxyurea and phlebotomy alone [92], respectively, although an independent role for hydroxyurea in LT was not supported in the much larger European Collaboration on Low-dose Aspirin in Polycythemia Vera (ECLAP) study [93]. Approximately 70% of patients will have a sustained response to hydroxyurea [94], while the remaining patients become resistant to or intolerant of the drug. Resistant individuals have a higher risk of progression to acute leukemia and death [95].
IFN-alfa is a pleiotropic antitumor agent that has found application in many types of malignancies [96] and is sometimes employed as treatment for patients with newly diagnosed high-risk PV. Early studies showed responses in up to 100% of cases [97,98], albeit at the expense of a high discontinuation rate due to adverse events, such as flu-like symptoms, fatigue, and neuropsychiatric manifestations [99]. A newer formulation of the drug obtained by adding a polyethylene glycol (PEG) moiety to the native IFN-alfa molecule (PEG-IFN alfa) was shown to have a longer half-life, greater stability, less immunogenicity, and, potentially, better tolerability [100]. Pilot phase 2 trials of PEG-IFN-alfa-2a demonstrated its remarkable activity, with symptomatic and hematologic responses seen in most patients (which, in some cases, persisted beyond discontinuation), and reasonable tolerability, with long-term discontinuation rates of 20% to 30% [101–103]. In some patients, JAK2V617F became undetectable over time [104]. Results of 2 ongoing trials, MDP-RC111 (single-arm study, PEG-IFN-alfa-2a in high-risk PV or ET [NCT01259817]) and MPD-RC112 (randomized controlled trial, PEG-IFN-alfa-2a versus hydroxyurea in the same population [NCT01258856]), will shed light on the role of PEG-IFN-alfa in the management of patients with high-risk PV or ET. In two phase 2 studies of PEG-IFN-alfa-2b, complete responses were seen in 70% to 100% of patients and discontinuation occurred in around a third of cases [105,106]. A new, longer-acting formulation of PEG-IFN-alfa-2a (peg-proline INF-alfa-2b, AOP2014) is also undergoing clinical development [107,108].
The approach to treatment of PV based on thrombotic risk level is illustrated in Figure 1.
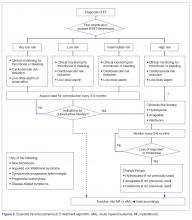
Very Low- and Low-Risk ET
Individuals with ET should undergo rigorous cardiovascular risk management and generally receive ASA to decrease their thrombotic risk and improve symptom control. Antiplatelet therapy may not be warranted in patients with documented acquired von Willebrand syndrome, with or without extreme thrombocytosis, or in those in the very low-risk category according to the IPSET-thrombosis model [55,87]. The risk/benefit ratio of antiplatelet agents in patients with ET at different thrombotic risk levels was assessed in poor-quality studies and thus remains highly uncertain. Platelet-lowering agents are sometimes recommended in patients with low-risk disease who have platelet counts ≥ 1500 × 103/μL, due to the potential risk of acquired von Willebrand syndrome and a risk of bleeding (this would require stopping ASA) [109]. Cytoreduction may also be used in low-risk patients with progressive symptoms despite ASA, symptomatic or progressive splenomegaly, and progressive leukocytosis.
Intermediate-Risk ET
This category includes patients older than 60 years, but without thrombosis or JAK2 mutations. These individuals would have been considered high risk (and thus candidates for cytoreductive therapy) according to the traditional risk stratification. Guidelines currently recommend ASA as the sole therapy for these patients, while reserving cytoreduction for those who experience thrombosis (ie, become high-risk) or have uncontrolled vasomotor or general symptoms, symptomatic splenomegaly, symptomatic thrombocytosis, or progressive leukocytosis.
High-Risk ET
For patients with ET in need of cytoreductive therapy (ie, those with prior thrombosis or older than 60 years with a JAK2V617F mutation), first-line options include hydroxyurea, IFN, and anagrelide. Hydroxyurea remains the treatment of choice in most patients [110]. In a seminal study, 114 patients with ET were randomly assigned to either observation or hydroxyurea treatment with the goal of maintaining the platelet count below 600 × 103/μL. At a median follow-up of 27 months, patients in the hydroxyurea group had a lower thrombosis rate (3.6% versus 24%, P = 0.003) and longer thrombosis-free survival, regardless of the use of antiplatelet drugs [64].
Anagrelide, a selective inhibitor of megakaryocytic differentiation and proliferation, was compared with hydroxyurea in patients with ET in 2 randomized trials. In the first (n = 809), the group receiving anagrelide had a higher risk of arterial thrombosis, major bleeding, and fibrotic evolution, but lower incidence of venous thrombosis. Hydroxyurea was better tolerated, mainly due to anagrelide-related cardiovascular adverse events [111]. As a result of this study, hydroxyurea is often preferred to anagrelide as frontline therapy for patients with newly diagnosed high-risk ET. In the second, more recent study (n = 259), however, the 2 agents proved equivalent in terms of major or minor arterial or venous thrombosis, as well as discontinuation rate [112]. The discrepancy between the 2 trials may be partly explained by the different ET diagnostic criteria used, with the latter only enrolling patients with WHO-defined true ET and the former utilizing Polycythemia Vera Study Group-ET diagnostic criteria that included patients with increases in other blood counts or varying degrees of marrow fibrosis.
Interferons were studied in ET in parallel with PV. PEG-IFN-alfa-2a proved effective in patients with ET, with responses observed in 80% of patients [103]. PEG-IFN- alfa-2b produced similar results, with responses in 70% to 90% of patients in small studies and discontinuation observed in 20% to 38% of cases [105,106,113]. Because the very long-term leukemogenic potential of hydroxyurea has remained somewhat uncertain, anagrelide or IFN might be preferable choices in younger patients.
The approach to treatment of ET based on thrombotic risk level is illustrated in Figure 2.
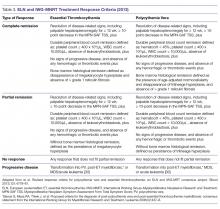
Assessing Response to Therapy
For both patients with PV and ET the endpoint of treatment set forth for clinical trials has been the achievement of a clinicohematologic response. However, studies have failed to show a correlation between response and reduction of the thrombohemorrhagic risk [114]. Therefore, proposed clinical trial response criteria were revised to include absence of hemorrhagic or thrombotic events as part of the definition of response (Table 3) [94].
Approach to Patients Refractory to or Intolerant of First-line Therapy
According to the European LeukemiaNet recommendations, an inadequate response to hydroxyurea in patients with PV (or myelofibrosis) is defined as a need for phlebotomy to maintain the hematocrit below < 45%, the platelet count > 400 × 103/μL, and a WBC count > 10,000/μL, or failure to reduce splenomegaly > 10 cm by > 50% at a dose of ≥ 2 g/day or maximum tolerated dose. Historically, treatment options for patients with PV or ET who failed first-line therapy (most commonly hydroxyurea) have included alkylating agents, such as busulfan, chlorambucil, pipobroman, and phosphorus (P)-32. However, the use of these drugs is limited by the associated risk of LT [93,115,116]. IFN (or anagrelide for ET) is often considered in patients previously treated with hydroxyurea, and vice versa.
Ruxolitinib is a JAK1 and JAK2 inhibitor currently approved for the treatment of PV patients refractory to or intolerant of hydroxyurea [7]. Following promising results of a phase 2 trial [117], ruxolitinib 10 mg twice daily was compared with best available therapy in the pivotal RESPONSE trial (n = 222). Ruxolitinib proved superior in achieving hematocrit control, reduction of spleen volume, and improvement of symptoms. Grade 3-4 hematologic toxicity was infrequent and similar in the 2 arms [118]. In addition, longer follow-up of that study suggested a lower rate of thrombotic events in patients receiving ruxolitinib (1.8 versus 8.2 per 100 patient-years) [119]. In a similarly designed randomized phase 3 study in PV patients without splenomegaly (RESPONSE-2), more patients in the ruxolitinib arm had hematocrit reduction without an increase in toxicity. Based on the results of these studies, ruxolitinib can be considered a standard of care for second-line therapy in this post-hydroxyurea patient population [120]. Ruxolitinib is also being tested in patients with high-risk ET who have become resistant to, or were intolerant of hydroxyurea, but currently has no approved indication in this setting [121,122]. Common side effects of ruxolitinib include cytopenias (especially anemia), increased risk of infections, hyperlipidemia, and increased risk of non-melanoma skin cancer.
Novel agents that have been studied in patients with PV and ET are histone deacetylase inhibitors, murine double minute 2 (MDM2, or HDM2 for their human counterpart) inhibitors (which restore the function of p53), Bcl-2 homology domain 3 mimetics such as navitoclax and venetoclax, and, for patients with ET, the telomerase inhibitor imetelstat [123].
Disease Evolution
Post-PV/Post-ET Myelofibrosis
Diagnostic criteria for post-PV and post-ET myelofibrosis are outlined in Table 4. Fibrotic transformation represents a natural evolution of the clinical course of PV or ET. It occurs in up to 15% and 9% of patients with PV and ET, respectively, in western countries [124]. The true percentage of ET patients who develop myelofibrosis is confounded by the inclusion of prefibrotic myelofibrosis cases in earlier series. The survival of patients who develop myelofibrosis is shortened compared to those who do not. In patients with PV, risk factors for myelofibrosis evolution include advanced age, leukocytosis, JAK2V617F homozygosity or higher allele burden, and hydroxyurea therapy. Once post-PV myelofibrosis has occurred, hemoglobin < 10 g/dL, platelet count < 100 × 103/μL, and WBC count > 30,000/μL are associated with worse outcomes [125]. In patients with ET, risk factors for myelofibrosis transformation include age, anemia, bone marrow hypercellularity and increased reticulin, increased lactate dehydrogenase, leukocytosis, and male gender. The management of post-PV/post-ET myelofibrosis recapitulates that of PMF.
Leukemic Transformation
The presence of more than 20% blasts in peripheral blood or bone marrow in a patient with MPN defines LT. This occurs in up to 5% to 10% of patients and may or may not be preceded by a myelofibrosis phase [126]. In cases of extramedullary transformation, a lower percentage of blasts can be seen in the bone marrow compared to the peripheral blood. The pathogenesis of LT has remained elusive, but it is believed to be associated with genetic instability, which facilitates the acquisition of additional mutations, including those of TET2, ASXL1, EZH2 DNMT3, IDH1/2, and TP53 [127].
Clinical risk factors for LT include advanced age, karyotypic abnormalities, prior therapy with alkylating agents or P-32, splenectomy, increased peripheral blood or bone marrow blasts, leukocytosis, anemia, thrombocytopenia, and cytogenetic abnormalities. Hydroxyurea, IFN, and ruxolitinib have not been shown to have leukemogenic potential thus far. Prognosis of LT is uniformly poor and patient survival rarely exceeds 6 months.
There is no standard of care for MPN LT. Treatment options range from low-intensity regimens to more aggressive AML-type induction chemotherapy. No strategy appears clearly superior to others [128]. Hematopoietic stem cell transplantation is the only therapy that provides clinically meaningful benefit to patients [129], but it is applicable only to a minority of patients with chemosensitive disease and good performance status [130]. Notable experimental approaches to MPN LT include hypomethylating agents, such as decitabine [131] or azacytidine [132], with or without ruxolitinib [133–135].
Conclusion
PV and ET are rare, chronic myeloid disorders. Patients typically experience a long clinical course and enjoy near-normal quality of life if properly managed. The 2 most important life-limiting complications of PV and ET are thrombohemorrhagic events and myelofibrosis/AML transformation. Vascular events are at least in part preventable with counseling on risk factors, phlebotomy (for patients with PV), antiplatelet therapy, and cytoreduction with hydroxyurea, IFNs, or anagrelide (for patients with ET). In addition, ruxolitinib was recently approved for PV patients after hydroxyurea failure. PV/ET transformation in myelofibrosis or AML is part of the natural history of the disease and no therapy has been shown to prevent it. Treatment follows recommendations set forth for PMF and AML, but results are generally poorer and novel strategies are needed to improve outcomes.
Corresponding author: Lorenzo Falchi, MD, Columbia University Medical Center, New York, NY.
Financial disclosures: None.
1. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC; 2008.
2. Alvarez-Larran A, Pereira A, Arellano-Rodrigo E, et al. Cytoreduction plus low-dose aspirin versus cytoreduction alone as primary prophylaxis of thrombosis in patients with high-risk essential thrombocythaemia: an observational study. Br J Haematol 2013;161:865–71.
3. Tefferi A. Polycythemia vera and essential thrombocythemia: 2013 update on diagnosis, risk-stratification, and management. Am J Hematol 2013;88:507–16.
4. Michiels JJ, Berneman Z, Schroyens W, et al. Platelet-mediated erythromelalgic, cerebral, ocular and coronary microvascular ischemic and thrombotic manifestations in patients with essential thrombocythemia and polycythemia vera: a distinct aspirin-responsive and coumadin-resistant arterial thrombophilia. Platelets 2006;17:528–44.
5. James C, Ugo V, Couedic J-PL, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005;434:1144–8.
6. Kralovics R, Passamonti F, Buser A, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders.N Engl J Med 2005;352:1779–90.
7. Verstovsek S, Mesa R, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 2012;366:799–807.
8. Harrison C, Kiladjian J, Al-Ali H, et al. JAK Inhibition with ruxolitinib versus best available therapy for myelofibrosis.N Engl J Med 2012;366:787–98.
9. Berglund S, Zettervall O. Incidence of polycythemia vera in a defined population. Eur J Haematol 1992;48:20–6.
10. Rozman C, Giralt M, Feliu E, et al. Life expectancy of patients with chronic nonleukemic myeloproliferative disorders. Cancer 1991;67:2658–63.
11. Passamonti F, Rumi E, Pungolino E, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med 2004;117:755–61.
12. Hultcrantz M, Kristinsson SY, Andersson TM, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol 2012;30:2995–3001.
13. Wolanskyj AP, Schwager SM, et al. Essential thrombocythemia beyond the first decade: life expectancy, long-term complication rates, and prognostic factors. Mayo Clin Proc 2006;81:159–66.
14. Srour SA, Devesa SS, Morton LM, et al. Incidence and patient survival of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms in the United States, 2001-12. Br J Haematol 2016;174:382–96.
15. Titmarsh GJ, Duncombe AS, McMullin MF, et al. How common are myeloproliferative neoplasms? A systematic review and meta-analysis. Am J Hematol 2014;89:581–7.
16. Moulard O, Mehta J, Fryzek J, et al. Epidemiology of myelofibrosis, essential thrombocythemia, and polycythemia vera in the European Union. Eur J Haematol 2014;92:289–97.
17. Stein BL, Gotlib J, Arcasoy M, et al. Historical views, conventional approaches, and evolving management strategies for myeloproliferative neoplasms. J Natl Compr Canc Netw 2015;13:424–34.
18. Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005;7:387–97.
19. Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005;365:1054–61.
20. Scott LM, Tong W, Levine RL, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med 2007;356:459–68.
21. Pardanani A, Lasho TL, Finke C, et al. Prevalence and clinicopathologic correlates of JAK2 exon 12 mutations in JAK2V617F-negative polycythemia vera. Leukemia 2007;21:1960–3.
22. Passamonti F, Elena C, Schnittger S, et al. Molecular and clinical features of the myeloproliferative neoplasm associated with JAK2 exon 12 mutations. Blood 2011;117:2813–6.
23. Abe M, Suzuki K, Inagaki O, et al. A novel MPL point mutation resulting in thrombopoietin-independent activation. Leukemia 2002;16:1500–6.
24. Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 2006;3:e270.
25. Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood 2006;108:3472–6.
26. Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med 2013;369:2391–405.
27. Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med 2013;369:2379–90.
28. Wang WA, Groenendyk J, Michalak M. Calreticulin signaling in health and disease. Int J Biochem Cell Biol 2012;44:842–6.
29. Saeidi K. Myeloproliferative neoplasms: Current molecular biology and genetics. Crit Rev Oncol Hematol 2016;98:375–89.
30. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127: 2391–405.
31. Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer 2007;109:68–76.
32. Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol 2005;23:2224–32.
33. Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood 2011;118:401–8.
34. Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol 2012;30:4098–103.
35. Casini A, Fontana P, Lecompte TP. Thrombotic complications of myeloproliferative neoplasms: risk assessment and risk-guided management. J Thromb Haemost 2013;11:1215–27.
36. Barbui T, Finazzi G, Falanga A. Myeloproliferative neoplasms and thrombosis. Blood 2013;122:2176-–84.
37. Pearson TC, Wetherley-Mein G. Vascular occlusive episodes and venous haematocrit in primary myeloproliferative polychytemia. Lancet 1978;2:1219–22.
38. Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood 2007;109:2446–52.
39. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia 2013;27:1874–81.
40. Barbui T, Falanga A. Molecular biomarkers of thrombosis in myeloproliferative neoplasms. Thromb Res 2016;140 Suppl 1:S71–75.
41. Campbell PJ, Scott LM, Buck G, et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet 2005;366:1945–53.
42. Tefferi A, Guglielmelli P, Larson DR, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood 2014;124:2507–13.
43. Qin Y, Wang X, Zhao C, et al. The impact of JAK2V617F mutation on different types of thrombosis risk in patients with essential thrombocythemia: a meta-analysis. Int J Hematol 2015;102:170–80.
44. Ziakas PD. Effect of JAK2 V617F on thrombotic risk in patients with essential thrombocythemia: measuring the uncertain. Haematologica 2008;93:1412–4.
45. Lussana F, Dentali F, Abbate R, et al. Screening for thrombophilia and antithrombotic prophylaxis in pregnancy: Guidelines of the Italian Society for Haemostasis and Thrombosis (SISET). Thromb Res 2009;124: e19–25.
46. Dahabreh IJ, Zoi K, Giannouli S, et al. Is JAK2 V617F mutation more than a diagnostic index? A meta-analysis of clinical outcomes in essential thrombocythemia. Leuk Res 2009;33:67–73.
47. Cho YU, Chi HS, Lee EH, et al. Comparison of clinicopathologic findings according to JAK2 V617F mutation in patients with essential thrombocythemia. Int J Hematol 2009;89:39–44.
48. Wolanskyj AP, Lasho TL, Schwager SM, et al. JAK2 mutation in essential thrombocythaemia: clinical associations and long-term prognostic relevance. Br J Haematol 2005;131:208–13.
49. Antonioli E, Guglielmelli P, Pancrazzi A, et al. Clinical implications of the JAK2 V617F mutation in essential thrombocythemia. Leukemia 2005;19:1847–9.
50. Palandri F, Catani L, Testoni N, et al. Long-term follow-up of 386 consecutive patients with essential thrombocythemia: safety of cytoreductive therapy. Am J Hematol 2009;84:215–20.
51. Chim CS, Sim JP, Chan CC, et al. Impact of JAK2V617F mutation on thrombosis and myeloid transformation in essential thrombocythemia: a multivariate analysis by Cox regression in 141 patients. Hematology 2010;15: 187–92.
52. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood 2007;110:840–6.
53. Carobbio A, Finazzi G, Antonioli E, et al. JAK2V617F allele burden and thrombosis: a direct comparison in essential thrombocythemia and polycythemia vera. Exp Hematol 2009;37:1016–21.
54. Alvarez-Larran A, Bellosillo B, Pereira A, et al. JAK2V617F monitoring in polycythemia vera and essential thrombocythemia: clinical usefulness for predicting myelofibrotic transformation and thrombotic events. Am J Hematol 2014;89:517–23.
55. Barbui T, Vannucchi AM, Buxhofer-Ausch V, et al. Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J 2015;5:e369.
56. Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood 2011;117:5857–9.
57. Alvarez-Larran A, Cervantes F, Bellosillo B, et al. Essential thrombocythemia in young individuals: frequency and risk factors for vascular events and evolution to myelofibrosis in 126 patients. Leukemia 2007;21:1218–23.
58. Jantunen R, Juvonen E, Ikkala E, et al. The predictive value of vascular risk factors and gender for the development of thrombotic complications in essential thrombocythemia. Ann Hematol 2001;80:74–8.
59. Besses C, Cervantes F, Pereira A, et al. Major vascular complications in essential thrombocythemia: a study of the predictive factors in a series of 148 patients. Leukemia 1999;13:150–4.
60. Haider M, Gangat N, Hanson C, Tefferi A. Splenomegaly and thrombosis risk in essential thrombocythemia: the mayo clinic experience. Am J Hematol 2016;91: E296–297.
61. Carobbio A, Finazzi G, Antonioli E, et al. Thrombocytosis and leukocytosis interaction in vascular complications of essential thrombocythemia. Blood 2008;112:3135–7.
62. Palandri F, Polverelli N, Catani L, et al. Impact of leukocytosis on thrombotic risk and survival in 532 patients with essential thrombocythemia: a retrospective study. Ann Hematol 2011;90:933–8.
63. Campbell PJ, MacLean C, Beer PA, et al. Correlation of blood counts with vascular complications in essential thrombocythemia: analysis of the prospective PT1 cohort. Blood 2012;120:1409–11.
64. Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med 1995;332:1132–6.
65. van Genderen PJ, Mulder PG, Waleboer M, et al. Prevention and treatment of thrombotic complications in essential thrombocythaemia: efficacy and safety of aspirin. Br J Haematol 1997;97:179–84.
66. Storen EC, Tefferi A. Long-term use of anagrelide in young patients with essential thrombocythemia. Blood 2001;97:863–6.
67. De Stefano V, Za T, Rossi E, et al. Recurrent thrombosis in patients with polycythemia vera and essential thrombocythemia: incidence, risk factors, and effect of treatments. Haematologica 2008;93:372–80.
68. Alvarez-Larran A, Cervantes F, Pereira A, et al. Observation versus antiplatelet therapy as primary prophylaxis for thrombosis in low-risk essential thrombocythemia. Blood 2010;116:1205–10.
69. Palandri F, Polverelli N, Catani L, et al. Bleeding in essential thrombocythaemia: a retrospective analysis on 565 patients. Br J Haematol 2012;156:281–4.
70. Rotunno G, Mannarelli C, Guglielmelli P, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood 2014;123:1552–5.
71. Tefferi A, Wassie EA, Lasho TL, et al. Calreticulin mutations and long-term survival in essential thrombocythemia. Leukemia 2014;28:2300–3.
72. Rumi E, Pietra D, Ferretti V, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood 2014;123:1544–51.
73. Palandri F, Latagliata R, Polverelli N, et al. Mutations and long-term outcome of 217 young patients with essential thrombocythemia or early primary myelofibrosis. Leukemia 2015;29:1344–9.
74. Fu R, Xuan M, Zhou Y, et al. Analysis of calreticulin mutations in Chinese patients with essential thrombocythemia: clinical implications in diagnosis, prognosis and treatment. Leukemia 2014;28:1912–4.
75. Tefferi A, Wassie EA, Guglielmelli P, et al. Type 1 versus Type 2 calreticulin mutations in essential thrombocythemia: a collaborative study of 1027 patients. Am J Hematol 2014;89:E121–4.
76. Pietra D, Rumi E, Ferretti VV, et al. Differential clinical effects of different mutation subtypes in CALR-mutant myeloproliferative neoplasms. Leukemia 2016;30: 431–8.
77. Rumi E, Pietra D, Guglielmelli P, et al. Acquired copy-neutral loss of heterozygosity of chromosome 1p as a molecular event associated with marrow fibrosis in MPL-mutated myeloproliferative neoplasms. Blood 2013;121:4388–95.
78. Beer PA, Campbell PJ, Scott LM, et al. MPL mutations in myeloproliferative disorders: analysis of the PT-1 cohort. Blood 2008;112:141–9.
79. Gangat N, Wassie EA, Lasho TL, et al. Mutations and thrombosis in essential thrombocythemia: prognostic interaction with age and thrombosis history. Eur J Haematol 2015;94:31–6.
80. Sekhar M, McVinnie K, Burroughs AK. Splanchnic vein thrombosis in myeloproliferative neoplasms. Br J Haematol 2013;162:730–47.
81. Stein BL, Saraf S, Sobol U, et al. Age-related differences in disease characteristics and clinical outcomes in polycythemia vera. Leuk Lymph 2013;54:1989–95.
82. Landolfi R, Di Gennaro L, Nicolazzi MA, et al. Polycythemia vera: gender-related phenotypic differences. Intern Emerg Med 2012;7:509–15.
83. Winslow ER, Brunt LM, Drebin JA, et al. Portal vein thrombosis after splenectomy. Am J Surg 2002;184:631–6.
84. Smalberg JH, Arends LR, Valla DC, et al. Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis: a meta-analysis. Blood 2012;120:4921–8.
85. Dentali F, Squizzato A, Brivio L, et al. JAK2V617F mutation for the early diagnosis of Ph- myeloproliferative neoplasms in patients with venous thromboembolism: a meta-analysis. Blood 2009;113:5617–23.
86. Pardanani A, Lasho TL, Hussein K, et al. JAK2V617F mutation screening as part of the hypercoagulable work-up in the absence of splanchnic venous thrombosis or overt myeloproliferative neoplasm: assessment of value in a series of 664 consecutive patients. Mayo Clin Proc 2008;83:457–9.
87. Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol 2011;29:761–70.
88. Landolfi R, Marchioli R, Kutti J, et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med 2004;350:114–24.
89. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med 2013;368:22–33.
90. Kiladjian JJ, Chevret S, Dosquet C, et al. Treatment of polycythemia vera with hydroxyurea and pipobroman: final results of a randomized trial initiated in 1980. J Clin Oncol 2011;29:3907–13.
91. Kaplan ME, Mack K, Goldberg JD, et al. Long-term management of polycythemia vera with hydroxyurea: a progress report. Semin Hematol 1986;23:167–71.
92. Fruchtman SM, Mack K, Kaplan ME, et al. From efficacy to safety: a Polycythemia Vera Study group report on hydroxyurea in patients with polycythemia vera. Semin Hematol 1997;34:17–23.
93. Finazzi G, Caruso V, Marchioli R, et al. Acute leukemia in polycythemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood 2005;105: 2664–70.
94. Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood 2013;121:4778–81.
95. Alvarez-Larran A, Pereira A, Cervantes F, et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood 2012;119:1363–9.
96. Stein BL, Tiu RV. Biological rationale and clinical use of interferon in the classical BCR-ABL-negative myeloproliferative neoplasms. J Interferon Cytokine Res 2013;33: 145–53.
97. Ludwig H, Cortelezzi A, Van Camp BG, et al. Treatment with recombinant interferon-alpha-2C: multiple myeloma and thrombocythaemia in myeloproliferative diseases. Oncology 1985;42 Suppl 1:19–25.
98. Silver RT. Long-term effects of the treatment of polycythemia vera with recombinant interferon-alpha. Cancer 2006;107:451–8.
99. Kiladjian JJ, Mesa RA, Hoffman R. The renaissance of interferon therapy for the treatment of myeloid malignancies. Blood 2011;117:4706–15.
100. Veronese FM, Mero A. The impact of PEGylation on biological therapies. BioDrugs 2008;22:315–29.
101. Kiladjian JJ, Cassinat B, Chevret S, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood 2008;112:3065–72.
102. Turlure P, Cambier N, Roussel M, et al. Complete hematological, molecular and histological remissions without cytoreductive treatment lasting after pegylated-interferon {alpha}-2a (peg-IFN{alpha}-2a) therapy in polycythemia vera (PV): long term results of a phase 2 trial [abstract]. Blood 2011;118(21). Abstract 280.
103. Quintas-Cardama A, Kantarjian H, Manshouri T, et al. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J Clin Oncol 2009;27:5418–24.
104. Quintas-Cardama A, Abdel-Wahab O, Manshouri T, et al. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon a-2a. Blood 2013;122:893–901.
105. Samuelsson J, Hasselbalch H, Bruserud O, et al. A phase II trial of pegylated interferon alpha-2b therapy for polycythemia vera and essential thrombocythemia: feasibility, clinical and biologic effects, and impact on quality of life. Cancer 2006;106:2397–405.
106. Jabbour E, Kantarjian H, Cortes J, et al. PEG-IFN-alpha-2b therapy in BCR-ABL-negative myeloproliferative disorders: final result of a phase 2 study. Cancer 2007; 110:2012–18.
107. Them NC, Bagienski K, Berg T, et al. Molecular responses and chromosomal aberrations in patients with polycythemia vera treated with peg-proline-interferon alpha-2b. Am J Hematol 2015;90:288–94.
108. Gisslinger H, Klade C, Georgiev P, et al. Final results from PROUD-PV a randomized controlled phase 3 trial comparing ropeginterferon alfa-2b to hydroxyurea in polycythemia vera patients [abstract]. Blood 2016;128(suppl 22). Abstract 475.
109. van Genderen PJ, van Vliet HH, Prins FJ, et al. Excessive prolongation of the bleeding time by aspirin in essential thrombocythemia is related to a decrease of large von Willebrand factor multimers in plasma. Ann Hematol 1997;75:215–20.
110. Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med 1995;332:1132–7.
111. Harrison CN, Campbell PJ, Buck G, et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med 2005;353:33–45.
112. Gisslinger H, Gotic M, Holowiecki J, et al. Anagrelide compared with hydroxyurea in WHO-classified essential thrombocythemia: the ANAHYDRET Study, a randomized controlled trial. Blood 2013;121:1720–8.
113. Alvarado Y, Cortes J, Verstovsek S, et al. Pilot study of pegylated interferon-alpha 2b in patients with essential thrombocythemia. Cancer Chemother Pharmacol 2003;51:81–6.
114. Barosi G, Tefferi A, Barbui T, ad hoc committee ‘Definition of clinically relevant outcomes for contemporarily clinical trials in Ph-neg M. Do current response criteria in classical Ph-negative myeloproliferative neoplasms capture benefit for patients? Leukemia 2012;26:1148–9.
115. Bjorkholm M, Derolf AR, Hultcrantz M, et al. Treatment-related risk factors for transformation to acute myeloid leukemia and myelodysplastic syndromes in myeloproliferative neoplasms. J Clin Oncol 2011;29:2410–5.
116. Alvarez-Larran A, Martinez-Aviles L, Hernandez-Boluda JC, et al. Busulfan in patients with polycythemia vera or essential thrombocythemia refractory or intolerant to hydroxyurea. Ann Hematol 2014;93:2037–43.
117. Verstovsek S, Passamonti F, Rambaldi A, et al. A phase 2 study of ruxolitinib, an oral JAK1 and JAK2 Inhibitor, in patients with advanced polycythemia vera who are refractory or intolerant to hydroxyurea. Cancer 2014;120: 513–20.
118. Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib in polycythemia vera resistant to or intolerant of hydroxyurea. N Engl J Med 2015; 372:426–35.
119. Verstovsek S, Vannucchi AM, Griesshammer M, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica 2016;101:821–9.
120. Passamonti F, Griesshammer M, Palandri F, et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): a randomised, open-label, phase 3b study. Lancet Oncol 2017;18:88–99.
121. Verstovsek S, Passamonti F, Rambaldi A, et al. Long-term results from a phase II open-label study of ruxolitinib in patients with essential thrombocythemia refractory to or intolerant of hydroxyurea [abstract]. Blood 2014;124. Abstract 1847.
122. Harrison CN, Mead AJ, Panchal A, et al. Ruxolitinib versus best available therapy for ET intolerant or resistant to hydroxycarbamide in a randomized trial. Blood 2017 Aug 9. pii: blood-2017-05-785790 .
123. Bose P, Verstovsek S. Drug development pipeline for myeloproliferative neoplasms: potential future impact on guidelines and management. J Natl Compr Canc Netw 2016;14:1613–24.
124. Cerquozzi S, Teffieri A. Blast transformation and fibrotic progression in polycythemia vera and essential thrombocythemia: a literature review of incidence and risk factors. Blood Cancer J 2015;Nov 13;5:e366.
125. Passamonti F, Rumi E, Caramella M, et al. A dynamic prognostic model to predict survival in post-polycythemia vera myelofibrosis. Blood 2008;111:3383–7.
126. Mesa RA, Verstovsek S, Cervantes F, et al. Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post- PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMF-BP): Consensus on terminology by the international working group for myelofibrosis research and treatment (IWG-MRT). Leuk Res 2007;31:737–40.
127. Rampal R, Mascarenhas J. Pathogenesis and management of acute myeloid leukemia that has evolved from a myeloproliferative neoplasm. Curr Opin Hematol 2014;21:65–71.
128. Chihara D, Kantarjian HM, Newberry KJ, et al. Survival outcome of patients with acute myeloid leukemia transformed from myeloproliferative neoplasms [abstract]. Blood 2016;128. Abstract 1940.
129. Tam CS, Nussenzveig RM, Popat U, et al. The natural history and treatment outcome of blast phase BCR-ABL-myeloproliferative neoplasms. Blood 2008;112:1628–37.
130. Kundranda MN, Tibes R, Mesa RA. Transformation of a chronic myeloproliferative neoplasm to acute myelogenous leukemia: does anything work? Curr Hematol Malig Rep 2012;7:78–86.
131. Badar T, Kantarjian HM, Ravandi F, et al. Therapeutic benefit of decitabine, a hypomethylating agent, in patients with high-risk primary myelofibrosis and myeloproliferative neoplasm in accelerated or blastic/acute myeloid leukemia phase. Leuk Res 2015;39:950–6.
132. Thepot S, Itzykson R, Seegers V, et al. Treatment of progression of Philadelphia-negative myeloproliferative neoplasms to myelodysplastic syndrome or acute myeloid leukemia by azacitidine: a report on 54 cases on the behalf of the Groupe Francophone des Myelodysplasies (GFM). Blood 2010;116:3735–42.
133. Pemmaraju N, Kantarjian H, Kadia T, et al. A phase I/II study of the Janus kinase (JAK)1 and 2 inhibitor ruxolitinib in patients with relapsed or refractory acute myeloid leukemia. Clin Lymphoma Myeloma Leuk 2015;15:171–6.
134. Rampal RK, Mascarenhas JO, Kosiorek HE, et al. Safety and efficacy of combined ruxolitinib and decitabine in patients with blast-phase MPN and post-MPN AML: results of a phase I study (Myeloproliferative Disorders Research Consortium 109 trial) [abstract]. Blood 2016;128. Abstract 1124.
135. Bose P, Verstovsek S, Gasior Y, et al. Phase I/II study of ruxolitinib (RUX) with decitabine (DAC) in patients with post-myeloproliferative neoplasm acute myeloid leukemia (post-MPN AML): phase I results [abstract]. Blood 2016;128. Abstract 4262.
From the Columbia University Medical Center, New York, NY (Dr. Falchi), and the University of Texas MD Anderson Cancer Center, Houston, TX (Dr. Verstovsek).
ABSTRACT
- Objective: To review the clinical aspects and current practices in the management of polycythemia vera (PV) and essential thrombocythemia (ET).
- Methods: Review of the literature.
- Results: PV and ET are rare chronic myeloid disorders. The 2 most important life-limiting complications of PV and ET are thrombohemorrhagic events and myelofibrosis/acute myeloid leukemia (AML) transformation. Vascular events are at least in part preventable with counseling on risk factors, phlebotomy (for patients with PV), antiplatelet therapy, and cytoreduction with hydroxyurea, interferons, or anagrelide (for patients with ET). Ruxolitinib was recently approved for PV after hydroxyurea failure. PV/ET transformation into myelofibrosis or AML is part of the natural history of the disease and no therapy has been shown to prevent it. Treatment of leukemic transformation of myeloproliferative neoplasms (MPN LT) follows recommendations set forth for primary myelofibrosis and AML.
- Conclusion: With appropriate management, patients with PV and ET typically enjoy a long survival and near-normal quality of life. Transformation into myelofibrosis or AML cannot be prevented by current therapies, however. Treatment results with MPN LT are generally poor and novel strategies are needed to improve outcomes.
Key words: myeloproliferative neoplasms; myelofibrosis; leukemic transformation.
Polycythemia vera (PV) and essential thrombocythemia (ET), along with primary myelofibrosis (PMF), belong to the group of Philadelphia-negative myeloproliferative neoplasms (MPN). All these malignancies arise from the clonal proliferation of an aberrant hematopoietic stem cell, but are characterized by distinct clinical phenotypes [1,2]. Although the clinical course of PV and ET is indolent, it can be complicated by thrombohemorrhagic episodes and/or evolution into myelofibrosis and/or acute myeloid leukemia (AML) [3]. Since vascular events are the most frequent life-threatening complications of PV and ET, therapeutic strategies are aimed at reducing this risk. Treatment may also help control other symptoms associated with the disease [4]. No therapy has been shown to prevent evolution of PV or ET into myelofibrosis or AML. The discovery of the Janus kinase 2 (JAK2)/V617F mutation in most patients with PV and over half of those with ET (and PMF) [5,6] has opened new avenues of research and led to the development of targeted therapies, such as the JAK1/2 inhibitor ruxolitinib, for patients with MPN [7,8].
Epidemiology
PV and ET are typically diagnosed in the fifth to seventh decade of life [9]. Although these disorders are generally associated with a long clinical course, survival of patients with PV or ET may be shorter than that of the general population [10–13]. Estimating the incidence and prevalence of MPN is a challenge because most patients remain asymptomatic for long periods of time and do not seek medical attention [13]. The annual incidence rates of PV and ET are estimated at 0.01 to 2.61 and 0.21 to 2.53 per 100,000, respectively. PV occurs slightly more frequently in males, whereas ET has a predilection for females [14]. Given the long course and low mortality associated with these disorders, the prevalence rates of PV and ET are significantly higher than the respective incidence rates: up to 47 and 57 per 100,000, respectively [15–17].
Molecular Pathogenesis
In 2005 researchers discovered a gain-of-function mutation of the JAK2 gene in nearly all patients with PV and more than half of those with ET and PMF [5,6,18,19]. JAK2 is a non-receptor tyrosine kinase that plays a central role in normal hematopoiesis. Substitution of a valine for a phenylalanine at codon 617 (ie, V617F) leads to its constitutive activation and signaling through the JAK-STAT pathway [5,6,18,19]. More rarely (and exclusively in patients with PV), JAK2 mutations involve exon 12 [20–22]. The vast majority of JAK2-negative ET patients harbor mutations in either the myeloproliferative leukemia (MPL) gene, which encodes the thrombopoietin receptor [23–25], or the calreticulin (CALR) gene [26,27], which encodes for a chaperone protein that plays a role in cellular proliferation, differentiation, and apoptosis [28]. Both the MPL and CALR mutations ultimately result in the constitutive activation of the JAK-STAT pathway. Thus, JAK2, MPL, and CALR alterations are collectively referred to as driver mutations. Moreover, because these mutations affect the same oncogenic pathway (ie, JAK-STAT), they are almost always mutually exclusive in a given patient. Patients with ET (or myelofibrosis) who are wild-type for JAK2, MPL, and CALR are referred to as having “triple-negative” disease. Many recurrent non-driver mutations are also found in patients with MPN. These are not exclusive of each other (ie, patients may have many at the same time) and involve for example ten-eleven translocation-2 (TET2), additional sex combs like 1 (ASXL1), enhancer of zeste homolog 2 (EZH2), isocitrate dehydrogenase 1 and isocitrate dehydrogenase 2 (IDH1/2), and DNA methyltransferase 3A (DNMT3A) genes, among others [29]. The biologic and prognostic significance of these non-driver alterations remain to be fully defined in ET and PV.
Diagnostic Criteria
Diagnostic criteria for PV and ET according to the World Health Organization (WHO) classification [30] are summarized in Table 1. Criteria for the diagnosis of prefibrotic myelofibrosis are included as well since this entity was formally recognized as separate from ET and part of the PMF spectrum in the 2016 WHO classification of myeloid tumors [30]. Clinically, both PV and ET generally remain asymptomatic for a long time. PV tends to be more symptomatic than ET and can present with debilitating constitutional symptoms (fatigue, night sweats, and weight loss), microvascular symptoms (headache, lightheadedness, acral paresthesias, erythromelalgia, atypical chest pain, and pruritus) [31], or macrovascular accidents (larger vein thrombosis, stroke, or myocardial ischemia) [32]. ET is often diagnosed incidentally, but patients can suffer from similar general symptoms and vascular complications. Causes of secondary absolute erythrocytosis (altitude, chronic hypoxemia, heavy smoking, cardiomyopathy, use of corticosteroids, erythropoietin, or other anabolic hormones, familial or congenital forms) or thrombocytosis (iron deficiency, acute blood loss, trauma or injury, acute coronary syndrome, systemic autoimmune disorders, chronic kidney failure, other malignancies, splenectomy) should be considered and appropriately excluded. Once the diagnosis is made, symptom assessment tools such as the Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) [33] or the abbreviated version, the MPN-SAF Total Symptom Score (MPN-SAF TSS) [34], are generally used to assess patients’ symptom burden and response to treatment in everyday practice.
Risk Stratification
Thrombohemorrhagic events, evolution into myelofibrosis, and leukemic transformation (LT) are the most serious complications in the course of PV or ET. Only thrombohemorrhagic events are, at least partially, preventable. Arterial or venous thrombotic complications are observed at rates of 1.8 to 10.9 per 100 patient-years in PV (arterial thrombosis being more common than venous) and 0.74 to 7.7 per 100 patient-years in ET, depending on the risk group [35] and the presence of other factors (see below).
The risk stratification of patients with PV is based on 2 factors: age ≥ 60 years and prior history of thrombosis. If either is present, the patient is assigned to the high-risk category, whereas if none is present the patient is considered at low risk [36]. In addition, high hematocrit [37] and high white blood cell (WBC) count [38], but not thrombocytosis, have been associated with the development of vascular complications. In one study, the risk of new arterial thrombosis was increased by the presence of leukoerythroblastosis, hypertension, and prior arterial thrombosis, while karyotypic abnormalities and prior venous thrombosis were predictors of new venous thrombosis [39]. Another emerging risk factor for thrombosis in patients with PV is high JAK2 allele burden (ie, the normal-to-mutated gene product ratio), although the evidence supporting this conclusion is equivocal [40].
Traditionally, in ET patients, the thrombotic risk was assessed using the same 2 factors (age ≥ 60 years and prior history of thrombosis), separating patients into low- and high-risk groups. However, the prognostication of ET patients has been refined recently with the identification of new relevant factors. In particular, the impact of JAK2 mutations on thrombotic risk has been thoroughly studied. Clinically, the presence of JAK2V617F is associated with older age, higher hemoglobin and hematocrit, lower platelet counts, more frequent need for cytoreductive treatment, and greater tendency to evolve into PV (a rare event) [41,42]. Many [41,43–46], but not all [47–51], studies suggested a correlation between JAK2 mutation and risk of both arterial and venous thrombosis. Although infrequent, a JAK2V617F homozygous state (ie, the mutation is present in both alleles) might confer an even higher thrombotic risk [52]. Moreover, the impact of the JAK2 mutation on vascular events persists over time [53], particularly in patients with high or unstable mutation burden [54]. Based on JAK2V617F’s influence on the thrombotic risk of ET patients, a new prognostic score was proposed, the International Prognostic Score for ET (IPSET)-thrombosis (Table 2). The revised version of this model is currently endorsed by the National Comprehensive Cancer Network and divides patients into 4 risk groups: high, intermediate, low, and very low. Treatment recommendations vary according to the risk group (as described below) [55].
Other thrombotic risk factors have been identified, but deemed not significant enough to be included in the model. Cardiovascular risk factors (hypercholesterolemia, hypertension, smoking, diabetes mellitus) can increase the risk of vascular events [56–59], as can splenomegaly [60] and baseline or persistent leukocytosis [61–63]. Thrombocytosis has been correlated with thrombotic risk in some studies [64–68], whereas others did not support this conclusion and/or suggested a lower rate of thrombosis and, in some cases, increased risk of bleeding in ET patients with platelet counts greater than 1000 × 103/μL (due to acquired von Willebrand syndrome) [51,61,63,68,69].
CALR mutations tend to occur in younger males with lower hemoglobin and WBC count, higher platelet count, and greater marrow megakaryocytic predominance, as compared to JAK2 mutations [26,27,70–72]. The associated incidence of thrombosis was less than 10% at 15 years in patients with CALR mutations, lower than the incidence reported for ET patients with JAK2V617F mutations [73]. The presence of the mutation per se does not appear to affect the thrombotic risk [74–76]. Information on the thrombotic risk associated with MPL mutations or a triple-negative state is scarce. In both instances, however, the risk appears to be lower than with the JAK2 mutation [73,77–79].
Venous thromboembolism (VTE) in patients with PV or ET may occur at unusual sites, such as the splanchnic or cerebral venous systems [80]. Risk factors for unusual VTE include younger age [81], female gender (especially with concomitant use of oral contraceptive pills) [82], and splenomegaly/splenectomy [83]. JAK2 mutation has also been associated with thrombosis at unusual sites. However, the prevalence of MPN or JAK2V617F in patients presenting with splanchnic VTE has varied [80]. In addition, MPN may be occult (ie, no clinical or laboratory abnormalities) in around 15% of patients [84]. Screening for JAK2V617F and underlying MPN is recommended in patients presenting with isolated unexplained splanchnic VTE. Treatment entails long-term anticoagulation therapy. JAK2V617F screening in patients with nonsplanchnic VTE is not recommended, as its prevalence in this group is low (< 3%) [85,86].
Risk-Adapted Therapy
Low-Risk PV
All patients with PV should receive counseling to mitigate cardiovascular risk factors, including smoking cessation, lifestyle modifications, and lipid-lowering therapy, as indicated. Furthermore, all PV patients should receive acetylsalicylic acid (ASA) to decrease their risk for thrombosis and control vasomotor symptoms [55,87]. Aspirin 81 to 100 mg daily is the preferred regimen because it provides adequate antithrombotic effect without the associated bleeding risk of higher-dose aspirin [88]. Low-risk PV patients should also receive periodic phlebotomies to reduce and maintain their hematocrit below 45%. This recommendation is based on the results of the Cytoreductive Therapy in Polycythemia Vera (CYTO PV) randomized controlled trial. In that study, patients receiving more intense therapy to maintain the hematocrit below 45% had a lower incidence of cardiovascular-related deaths or major thrombotic events than those with hematocrit goals of 45% to 50% (2.7% versus 9.8%) [89]. Cytoreduction is an option for low-risk patients who do not tolerate phlebotomy or require frequent phlebotomy, or who have disease-related bleeding, severe symptoms, symptomatic splenomegaly, or progressive leukocytosis [38].
High-Risk PV
Patients older than 60 years and/or with a history of thrombosis should be considered for cytoreductive therapy in addition to the above measures. Frontline cytoreductive therapies include hydroxyurea or interferon (IFN)-alfa [87]. Hydroxyurea is a potent ribonucleotide reductase inhibitor that interferes with DNA repair and is the treatment of choice for most high-risk patients with PV [90]. In a small trial, hydroxyurea reduced the risk of thrombosis compared with historical controls treated with phlebotomy alone [91]. Hydroxyurea is generally well tolerated; common side effects include cytopenias, nail changes, and mucosal and/or skin ulcers. Although never formally proven to be leukemogenic, this agent should be used with caution in younger patients [87]. Indeed, in the original study, the rates of transformation were 5.9% and 1.5% for patients receiving hydroxyurea and phlebotomy alone [92], respectively, although an independent role for hydroxyurea in LT was not supported in the much larger European Collaboration on Low-dose Aspirin in Polycythemia Vera (ECLAP) study [93]. Approximately 70% of patients will have a sustained response to hydroxyurea [94], while the remaining patients become resistant to or intolerant of the drug. Resistant individuals have a higher risk of progression to acute leukemia and death [95].
IFN-alfa is a pleiotropic antitumor agent that has found application in many types of malignancies [96] and is sometimes employed as treatment for patients with newly diagnosed high-risk PV. Early studies showed responses in up to 100% of cases [97,98], albeit at the expense of a high discontinuation rate due to adverse events, such as flu-like symptoms, fatigue, and neuropsychiatric manifestations [99]. A newer formulation of the drug obtained by adding a polyethylene glycol (PEG) moiety to the native IFN-alfa molecule (PEG-IFN alfa) was shown to have a longer half-life, greater stability, less immunogenicity, and, potentially, better tolerability [100]. Pilot phase 2 trials of PEG-IFN-alfa-2a demonstrated its remarkable activity, with symptomatic and hematologic responses seen in most patients (which, in some cases, persisted beyond discontinuation), and reasonable tolerability, with long-term discontinuation rates of 20% to 30% [101–103]. In some patients, JAK2V617F became undetectable over time [104]. Results of 2 ongoing trials, MDP-RC111 (single-arm study, PEG-IFN-alfa-2a in high-risk PV or ET [NCT01259817]) and MPD-RC112 (randomized controlled trial, PEG-IFN-alfa-2a versus hydroxyurea in the same population [NCT01258856]), will shed light on the role of PEG-IFN-alfa in the management of patients with high-risk PV or ET. In two phase 2 studies of PEG-IFN-alfa-2b, complete responses were seen in 70% to 100% of patients and discontinuation occurred in around a third of cases [105,106]. A new, longer-acting formulation of PEG-IFN-alfa-2a (peg-proline INF-alfa-2b, AOP2014) is also undergoing clinical development [107,108].
The approach to treatment of PV based on thrombotic risk level is illustrated in Figure 1.
Very Low- and Low-Risk ET
Individuals with ET should undergo rigorous cardiovascular risk management and generally receive ASA to decrease their thrombotic risk and improve symptom control. Antiplatelet therapy may not be warranted in patients with documented acquired von Willebrand syndrome, with or without extreme thrombocytosis, or in those in the very low-risk category according to the IPSET-thrombosis model [55,87]. The risk/benefit ratio of antiplatelet agents in patients with ET at different thrombotic risk levels was assessed in poor-quality studies and thus remains highly uncertain. Platelet-lowering agents are sometimes recommended in patients with low-risk disease who have platelet counts ≥ 1500 × 103/μL, due to the potential risk of acquired von Willebrand syndrome and a risk of bleeding (this would require stopping ASA) [109]. Cytoreduction may also be used in low-risk patients with progressive symptoms despite ASA, symptomatic or progressive splenomegaly, and progressive leukocytosis.
Intermediate-Risk ET
This category includes patients older than 60 years, but without thrombosis or JAK2 mutations. These individuals would have been considered high risk (and thus candidates for cytoreductive therapy) according to the traditional risk stratification. Guidelines currently recommend ASA as the sole therapy for these patients, while reserving cytoreduction for those who experience thrombosis (ie, become high-risk) or have uncontrolled vasomotor or general symptoms, symptomatic splenomegaly, symptomatic thrombocytosis, or progressive leukocytosis.
High-Risk ET
For patients with ET in need of cytoreductive therapy (ie, those with prior thrombosis or older than 60 years with a JAK2V617F mutation), first-line options include hydroxyurea, IFN, and anagrelide. Hydroxyurea remains the treatment of choice in most patients [110]. In a seminal study, 114 patients with ET were randomly assigned to either observation or hydroxyurea treatment with the goal of maintaining the platelet count below 600 × 103/μL. At a median follow-up of 27 months, patients in the hydroxyurea group had a lower thrombosis rate (3.6% versus 24%, P = 0.003) and longer thrombosis-free survival, regardless of the use of antiplatelet drugs [64].
Anagrelide, a selective inhibitor of megakaryocytic differentiation and proliferation, was compared with hydroxyurea in patients with ET in 2 randomized trials. In the first (n = 809), the group receiving anagrelide had a higher risk of arterial thrombosis, major bleeding, and fibrotic evolution, but lower incidence of venous thrombosis. Hydroxyurea was better tolerated, mainly due to anagrelide-related cardiovascular adverse events [111]. As a result of this study, hydroxyurea is often preferred to anagrelide as frontline therapy for patients with newly diagnosed high-risk ET. In the second, more recent study (n = 259), however, the 2 agents proved equivalent in terms of major or minor arterial or venous thrombosis, as well as discontinuation rate [112]. The discrepancy between the 2 trials may be partly explained by the different ET diagnostic criteria used, with the latter only enrolling patients with WHO-defined true ET and the former utilizing Polycythemia Vera Study Group-ET diagnostic criteria that included patients with increases in other blood counts or varying degrees of marrow fibrosis.
Interferons were studied in ET in parallel with PV. PEG-IFN-alfa-2a proved effective in patients with ET, with responses observed in 80% of patients [103]. PEG-IFN- alfa-2b produced similar results, with responses in 70% to 90% of patients in small studies and discontinuation observed in 20% to 38% of cases [105,106,113]. Because the very long-term leukemogenic potential of hydroxyurea has remained somewhat uncertain, anagrelide or IFN might be preferable choices in younger patients.
The approach to treatment of ET based on thrombotic risk level is illustrated in Figure 2.
Assessing Response to Therapy
For both patients with PV and ET the endpoint of treatment set forth for clinical trials has been the achievement of a clinicohematologic response. However, studies have failed to show a correlation between response and reduction of the thrombohemorrhagic risk [114]. Therefore, proposed clinical trial response criteria were revised to include absence of hemorrhagic or thrombotic events as part of the definition of response (Table 3) [94].
Approach to Patients Refractory to or Intolerant of First-line Therapy
According to the European LeukemiaNet recommendations, an inadequate response to hydroxyurea in patients with PV (or myelofibrosis) is defined as a need for phlebotomy to maintain the hematocrit below < 45%, the platelet count > 400 × 103/μL, and a WBC count > 10,000/μL, or failure to reduce splenomegaly > 10 cm by > 50% at a dose of ≥ 2 g/day or maximum tolerated dose. Historically, treatment options for patients with PV or ET who failed first-line therapy (most commonly hydroxyurea) have included alkylating agents, such as busulfan, chlorambucil, pipobroman, and phosphorus (P)-32. However, the use of these drugs is limited by the associated risk of LT [93,115,116]. IFN (or anagrelide for ET) is often considered in patients previously treated with hydroxyurea, and vice versa.
Ruxolitinib is a JAK1 and JAK2 inhibitor currently approved for the treatment of PV patients refractory to or intolerant of hydroxyurea [7]. Following promising results of a phase 2 trial [117], ruxolitinib 10 mg twice daily was compared with best available therapy in the pivotal RESPONSE trial (n = 222). Ruxolitinib proved superior in achieving hematocrit control, reduction of spleen volume, and improvement of symptoms. Grade 3-4 hematologic toxicity was infrequent and similar in the 2 arms [118]. In addition, longer follow-up of that study suggested a lower rate of thrombotic events in patients receiving ruxolitinib (1.8 versus 8.2 per 100 patient-years) [119]. In a similarly designed randomized phase 3 study in PV patients without splenomegaly (RESPONSE-2), more patients in the ruxolitinib arm had hematocrit reduction without an increase in toxicity. Based on the results of these studies, ruxolitinib can be considered a standard of care for second-line therapy in this post-hydroxyurea patient population [120]. Ruxolitinib is also being tested in patients with high-risk ET who have become resistant to, or were intolerant of hydroxyurea, but currently has no approved indication in this setting [121,122]. Common side effects of ruxolitinib include cytopenias (especially anemia), increased risk of infections, hyperlipidemia, and increased risk of non-melanoma skin cancer.
Novel agents that have been studied in patients with PV and ET are histone deacetylase inhibitors, murine double minute 2 (MDM2, or HDM2 for their human counterpart) inhibitors (which restore the function of p53), Bcl-2 homology domain 3 mimetics such as navitoclax and venetoclax, and, for patients with ET, the telomerase inhibitor imetelstat [123].
Disease Evolution
Post-PV/Post-ET Myelofibrosis
Diagnostic criteria for post-PV and post-ET myelofibrosis are outlined in Table 4. Fibrotic transformation represents a natural evolution of the clinical course of PV or ET. It occurs in up to 15% and 9% of patients with PV and ET, respectively, in western countries [124]. The true percentage of ET patients who develop myelofibrosis is confounded by the inclusion of prefibrotic myelofibrosis cases in earlier series. The survival of patients who develop myelofibrosis is shortened compared to those who do not. In patients with PV, risk factors for myelofibrosis evolution include advanced age, leukocytosis, JAK2V617F homozygosity or higher allele burden, and hydroxyurea therapy. Once post-PV myelofibrosis has occurred, hemoglobin < 10 g/dL, platelet count < 100 × 103/μL, and WBC count > 30,000/μL are associated with worse outcomes [125]. In patients with ET, risk factors for myelofibrosis transformation include age, anemia, bone marrow hypercellularity and increased reticulin, increased lactate dehydrogenase, leukocytosis, and male gender. The management of post-PV/post-ET myelofibrosis recapitulates that of PMF.
Leukemic Transformation
The presence of more than 20% blasts in peripheral blood or bone marrow in a patient with MPN defines LT. This occurs in up to 5% to 10% of patients and may or may not be preceded by a myelofibrosis phase [126]. In cases of extramedullary transformation, a lower percentage of blasts can be seen in the bone marrow compared to the peripheral blood. The pathogenesis of LT has remained elusive, but it is believed to be associated with genetic instability, which facilitates the acquisition of additional mutations, including those of TET2, ASXL1, EZH2 DNMT3, IDH1/2, and TP53 [127].
Clinical risk factors for LT include advanced age, karyotypic abnormalities, prior therapy with alkylating agents or P-32, splenectomy, increased peripheral blood or bone marrow blasts, leukocytosis, anemia, thrombocytopenia, and cytogenetic abnormalities. Hydroxyurea, IFN, and ruxolitinib have not been shown to have leukemogenic potential thus far. Prognosis of LT is uniformly poor and patient survival rarely exceeds 6 months.
There is no standard of care for MPN LT. Treatment options range from low-intensity regimens to more aggressive AML-type induction chemotherapy. No strategy appears clearly superior to others [128]. Hematopoietic stem cell transplantation is the only therapy that provides clinically meaningful benefit to patients [129], but it is applicable only to a minority of patients with chemosensitive disease and good performance status [130]. Notable experimental approaches to MPN LT include hypomethylating agents, such as decitabine [131] or azacytidine [132], with or without ruxolitinib [133–135].
Conclusion
PV and ET are rare, chronic myeloid disorders. Patients typically experience a long clinical course and enjoy near-normal quality of life if properly managed. The 2 most important life-limiting complications of PV and ET are thrombohemorrhagic events and myelofibrosis/AML transformation. Vascular events are at least in part preventable with counseling on risk factors, phlebotomy (for patients with PV), antiplatelet therapy, and cytoreduction with hydroxyurea, IFNs, or anagrelide (for patients with ET). In addition, ruxolitinib was recently approved for PV patients after hydroxyurea failure. PV/ET transformation in myelofibrosis or AML is part of the natural history of the disease and no therapy has been shown to prevent it. Treatment follows recommendations set forth for PMF and AML, but results are generally poorer and novel strategies are needed to improve outcomes.
Corresponding author: Lorenzo Falchi, MD, Columbia University Medical Center, New York, NY.
Financial disclosures: None.
From the Columbia University Medical Center, New York, NY (Dr. Falchi), and the University of Texas MD Anderson Cancer Center, Houston, TX (Dr. Verstovsek).
ABSTRACT
- Objective: To review the clinical aspects and current practices in the management of polycythemia vera (PV) and essential thrombocythemia (ET).
- Methods: Review of the literature.
- Results: PV and ET are rare chronic myeloid disorders. The 2 most important life-limiting complications of PV and ET are thrombohemorrhagic events and myelofibrosis/acute myeloid leukemia (AML) transformation. Vascular events are at least in part preventable with counseling on risk factors, phlebotomy (for patients with PV), antiplatelet therapy, and cytoreduction with hydroxyurea, interferons, or anagrelide (for patients with ET). Ruxolitinib was recently approved for PV after hydroxyurea failure. PV/ET transformation into myelofibrosis or AML is part of the natural history of the disease and no therapy has been shown to prevent it. Treatment of leukemic transformation of myeloproliferative neoplasms (MPN LT) follows recommendations set forth for primary myelofibrosis and AML.
- Conclusion: With appropriate management, patients with PV and ET typically enjoy a long survival and near-normal quality of life. Transformation into myelofibrosis or AML cannot be prevented by current therapies, however. Treatment results with MPN LT are generally poor and novel strategies are needed to improve outcomes.
Key words: myeloproliferative neoplasms; myelofibrosis; leukemic transformation.
Polycythemia vera (PV) and essential thrombocythemia (ET), along with primary myelofibrosis (PMF), belong to the group of Philadelphia-negative myeloproliferative neoplasms (MPN). All these malignancies arise from the clonal proliferation of an aberrant hematopoietic stem cell, but are characterized by distinct clinical phenotypes [1,2]. Although the clinical course of PV and ET is indolent, it can be complicated by thrombohemorrhagic episodes and/or evolution into myelofibrosis and/or acute myeloid leukemia (AML) [3]. Since vascular events are the most frequent life-threatening complications of PV and ET, therapeutic strategies are aimed at reducing this risk. Treatment may also help control other symptoms associated with the disease [4]. No therapy has been shown to prevent evolution of PV or ET into myelofibrosis or AML. The discovery of the Janus kinase 2 (JAK2)/V617F mutation in most patients with PV and over half of those with ET (and PMF) [5,6] has opened new avenues of research and led to the development of targeted therapies, such as the JAK1/2 inhibitor ruxolitinib, for patients with MPN [7,8].
Epidemiology
PV and ET are typically diagnosed in the fifth to seventh decade of life [9]. Although these disorders are generally associated with a long clinical course, survival of patients with PV or ET may be shorter than that of the general population [10–13]. Estimating the incidence and prevalence of MPN is a challenge because most patients remain asymptomatic for long periods of time and do not seek medical attention [13]. The annual incidence rates of PV and ET are estimated at 0.01 to 2.61 and 0.21 to 2.53 per 100,000, respectively. PV occurs slightly more frequently in males, whereas ET has a predilection for females [14]. Given the long course and low mortality associated with these disorders, the prevalence rates of PV and ET are significantly higher than the respective incidence rates: up to 47 and 57 per 100,000, respectively [15–17].
Molecular Pathogenesis
In 2005 researchers discovered a gain-of-function mutation of the JAK2 gene in nearly all patients with PV and more than half of those with ET and PMF [5,6,18,19]. JAK2 is a non-receptor tyrosine kinase that plays a central role in normal hematopoiesis. Substitution of a valine for a phenylalanine at codon 617 (ie, V617F) leads to its constitutive activation and signaling through the JAK-STAT pathway [5,6,18,19]. More rarely (and exclusively in patients with PV), JAK2 mutations involve exon 12 [20–22]. The vast majority of JAK2-negative ET patients harbor mutations in either the myeloproliferative leukemia (MPL) gene, which encodes the thrombopoietin receptor [23–25], or the calreticulin (CALR) gene [26,27], which encodes for a chaperone protein that plays a role in cellular proliferation, differentiation, and apoptosis [28]. Both the MPL and CALR mutations ultimately result in the constitutive activation of the JAK-STAT pathway. Thus, JAK2, MPL, and CALR alterations are collectively referred to as driver mutations. Moreover, because these mutations affect the same oncogenic pathway (ie, JAK-STAT), they are almost always mutually exclusive in a given patient. Patients with ET (or myelofibrosis) who are wild-type for JAK2, MPL, and CALR are referred to as having “triple-negative” disease. Many recurrent non-driver mutations are also found in patients with MPN. These are not exclusive of each other (ie, patients may have many at the same time) and involve for example ten-eleven translocation-2 (TET2), additional sex combs like 1 (ASXL1), enhancer of zeste homolog 2 (EZH2), isocitrate dehydrogenase 1 and isocitrate dehydrogenase 2 (IDH1/2), and DNA methyltransferase 3A (DNMT3A) genes, among others [29]. The biologic and prognostic significance of these non-driver alterations remain to be fully defined in ET and PV.
Diagnostic Criteria
Diagnostic criteria for PV and ET according to the World Health Organization (WHO) classification [30] are summarized in Table 1. Criteria for the diagnosis of prefibrotic myelofibrosis are included as well since this entity was formally recognized as separate from ET and part of the PMF spectrum in the 2016 WHO classification of myeloid tumors [30]. Clinically, both PV and ET generally remain asymptomatic for a long time. PV tends to be more symptomatic than ET and can present with debilitating constitutional symptoms (fatigue, night sweats, and weight loss), microvascular symptoms (headache, lightheadedness, acral paresthesias, erythromelalgia, atypical chest pain, and pruritus) [31], or macrovascular accidents (larger vein thrombosis, stroke, or myocardial ischemia) [32]. ET is often diagnosed incidentally, but patients can suffer from similar general symptoms and vascular complications. Causes of secondary absolute erythrocytosis (altitude, chronic hypoxemia, heavy smoking, cardiomyopathy, use of corticosteroids, erythropoietin, or other anabolic hormones, familial or congenital forms) or thrombocytosis (iron deficiency, acute blood loss, trauma or injury, acute coronary syndrome, systemic autoimmune disorders, chronic kidney failure, other malignancies, splenectomy) should be considered and appropriately excluded. Once the diagnosis is made, symptom assessment tools such as the Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) [33] or the abbreviated version, the MPN-SAF Total Symptom Score (MPN-SAF TSS) [34], are generally used to assess patients’ symptom burden and response to treatment in everyday practice.
Risk Stratification
Thrombohemorrhagic events, evolution into myelofibrosis, and leukemic transformation (LT) are the most serious complications in the course of PV or ET. Only thrombohemorrhagic events are, at least partially, preventable. Arterial or venous thrombotic complications are observed at rates of 1.8 to 10.9 per 100 patient-years in PV (arterial thrombosis being more common than venous) and 0.74 to 7.7 per 100 patient-years in ET, depending on the risk group [35] and the presence of other factors (see below).
The risk stratification of patients with PV is based on 2 factors: age ≥ 60 years and prior history of thrombosis. If either is present, the patient is assigned to the high-risk category, whereas if none is present the patient is considered at low risk [36]. In addition, high hematocrit [37] and high white blood cell (WBC) count [38], but not thrombocytosis, have been associated with the development of vascular complications. In one study, the risk of new arterial thrombosis was increased by the presence of leukoerythroblastosis, hypertension, and prior arterial thrombosis, while karyotypic abnormalities and prior venous thrombosis were predictors of new venous thrombosis [39]. Another emerging risk factor for thrombosis in patients with PV is high JAK2 allele burden (ie, the normal-to-mutated gene product ratio), although the evidence supporting this conclusion is equivocal [40].
Traditionally, in ET patients, the thrombotic risk was assessed using the same 2 factors (age ≥ 60 years and prior history of thrombosis), separating patients into low- and high-risk groups. However, the prognostication of ET patients has been refined recently with the identification of new relevant factors. In particular, the impact of JAK2 mutations on thrombotic risk has been thoroughly studied. Clinically, the presence of JAK2V617F is associated with older age, higher hemoglobin and hematocrit, lower platelet counts, more frequent need for cytoreductive treatment, and greater tendency to evolve into PV (a rare event) [41,42]. Many [41,43–46], but not all [47–51], studies suggested a correlation between JAK2 mutation and risk of both arterial and venous thrombosis. Although infrequent, a JAK2V617F homozygous state (ie, the mutation is present in both alleles) might confer an even higher thrombotic risk [52]. Moreover, the impact of the JAK2 mutation on vascular events persists over time [53], particularly in patients with high or unstable mutation burden [54]. Based on JAK2V617F’s influence on the thrombotic risk of ET patients, a new prognostic score was proposed, the International Prognostic Score for ET (IPSET)-thrombosis (Table 2). The revised version of this model is currently endorsed by the National Comprehensive Cancer Network and divides patients into 4 risk groups: high, intermediate, low, and very low. Treatment recommendations vary according to the risk group (as described below) [55].
Other thrombotic risk factors have been identified, but deemed not significant enough to be included in the model. Cardiovascular risk factors (hypercholesterolemia, hypertension, smoking, diabetes mellitus) can increase the risk of vascular events [56–59], as can splenomegaly [60] and baseline or persistent leukocytosis [61–63]. Thrombocytosis has been correlated with thrombotic risk in some studies [64–68], whereas others did not support this conclusion and/or suggested a lower rate of thrombosis and, in some cases, increased risk of bleeding in ET patients with platelet counts greater than 1000 × 103/μL (due to acquired von Willebrand syndrome) [51,61,63,68,69].
CALR mutations tend to occur in younger males with lower hemoglobin and WBC count, higher platelet count, and greater marrow megakaryocytic predominance, as compared to JAK2 mutations [26,27,70–72]. The associated incidence of thrombosis was less than 10% at 15 years in patients with CALR mutations, lower than the incidence reported for ET patients with JAK2V617F mutations [73]. The presence of the mutation per se does not appear to affect the thrombotic risk [74–76]. Information on the thrombotic risk associated with MPL mutations or a triple-negative state is scarce. In both instances, however, the risk appears to be lower than with the JAK2 mutation [73,77–79].
Venous thromboembolism (VTE) in patients with PV or ET may occur at unusual sites, such as the splanchnic or cerebral venous systems [80]. Risk factors for unusual VTE include younger age [81], female gender (especially with concomitant use of oral contraceptive pills) [82], and splenomegaly/splenectomy [83]. JAK2 mutation has also been associated with thrombosis at unusual sites. However, the prevalence of MPN or JAK2V617F in patients presenting with splanchnic VTE has varied [80]. In addition, MPN may be occult (ie, no clinical or laboratory abnormalities) in around 15% of patients [84]. Screening for JAK2V617F and underlying MPN is recommended in patients presenting with isolated unexplained splanchnic VTE. Treatment entails long-term anticoagulation therapy. JAK2V617F screening in patients with nonsplanchnic VTE is not recommended, as its prevalence in this group is low (< 3%) [85,86].
Risk-Adapted Therapy
Low-Risk PV
All patients with PV should receive counseling to mitigate cardiovascular risk factors, including smoking cessation, lifestyle modifications, and lipid-lowering therapy, as indicated. Furthermore, all PV patients should receive acetylsalicylic acid (ASA) to decrease their risk for thrombosis and control vasomotor symptoms [55,87]. Aspirin 81 to 100 mg daily is the preferred regimen because it provides adequate antithrombotic effect without the associated bleeding risk of higher-dose aspirin [88]. Low-risk PV patients should also receive periodic phlebotomies to reduce and maintain their hematocrit below 45%. This recommendation is based on the results of the Cytoreductive Therapy in Polycythemia Vera (CYTO PV) randomized controlled trial. In that study, patients receiving more intense therapy to maintain the hematocrit below 45% had a lower incidence of cardiovascular-related deaths or major thrombotic events than those with hematocrit goals of 45% to 50% (2.7% versus 9.8%) [89]. Cytoreduction is an option for low-risk patients who do not tolerate phlebotomy or require frequent phlebotomy, or who have disease-related bleeding, severe symptoms, symptomatic splenomegaly, or progressive leukocytosis [38].
High-Risk PV
Patients older than 60 years and/or with a history of thrombosis should be considered for cytoreductive therapy in addition to the above measures. Frontline cytoreductive therapies include hydroxyurea or interferon (IFN)-alfa [87]. Hydroxyurea is a potent ribonucleotide reductase inhibitor that interferes with DNA repair and is the treatment of choice for most high-risk patients with PV [90]. In a small trial, hydroxyurea reduced the risk of thrombosis compared with historical controls treated with phlebotomy alone [91]. Hydroxyurea is generally well tolerated; common side effects include cytopenias, nail changes, and mucosal and/or skin ulcers. Although never formally proven to be leukemogenic, this agent should be used with caution in younger patients [87]. Indeed, in the original study, the rates of transformation were 5.9% and 1.5% for patients receiving hydroxyurea and phlebotomy alone [92], respectively, although an independent role for hydroxyurea in LT was not supported in the much larger European Collaboration on Low-dose Aspirin in Polycythemia Vera (ECLAP) study [93]. Approximately 70% of patients will have a sustained response to hydroxyurea [94], while the remaining patients become resistant to or intolerant of the drug. Resistant individuals have a higher risk of progression to acute leukemia and death [95].
IFN-alfa is a pleiotropic antitumor agent that has found application in many types of malignancies [96] and is sometimes employed as treatment for patients with newly diagnosed high-risk PV. Early studies showed responses in up to 100% of cases [97,98], albeit at the expense of a high discontinuation rate due to adverse events, such as flu-like symptoms, fatigue, and neuropsychiatric manifestations [99]. A newer formulation of the drug obtained by adding a polyethylene glycol (PEG) moiety to the native IFN-alfa molecule (PEG-IFN alfa) was shown to have a longer half-life, greater stability, less immunogenicity, and, potentially, better tolerability [100]. Pilot phase 2 trials of PEG-IFN-alfa-2a demonstrated its remarkable activity, with symptomatic and hematologic responses seen in most patients (which, in some cases, persisted beyond discontinuation), and reasonable tolerability, with long-term discontinuation rates of 20% to 30% [101–103]. In some patients, JAK2V617F became undetectable over time [104]. Results of 2 ongoing trials, MDP-RC111 (single-arm study, PEG-IFN-alfa-2a in high-risk PV or ET [NCT01259817]) and MPD-RC112 (randomized controlled trial, PEG-IFN-alfa-2a versus hydroxyurea in the same population [NCT01258856]), will shed light on the role of PEG-IFN-alfa in the management of patients with high-risk PV or ET. In two phase 2 studies of PEG-IFN-alfa-2b, complete responses were seen in 70% to 100% of patients and discontinuation occurred in around a third of cases [105,106]. A new, longer-acting formulation of PEG-IFN-alfa-2a (peg-proline INF-alfa-2b, AOP2014) is also undergoing clinical development [107,108].
The approach to treatment of PV based on thrombotic risk level is illustrated in Figure 1.
Very Low- and Low-Risk ET
Individuals with ET should undergo rigorous cardiovascular risk management and generally receive ASA to decrease their thrombotic risk and improve symptom control. Antiplatelet therapy may not be warranted in patients with documented acquired von Willebrand syndrome, with or without extreme thrombocytosis, or in those in the very low-risk category according to the IPSET-thrombosis model [55,87]. The risk/benefit ratio of antiplatelet agents in patients with ET at different thrombotic risk levels was assessed in poor-quality studies and thus remains highly uncertain. Platelet-lowering agents are sometimes recommended in patients with low-risk disease who have platelet counts ≥ 1500 × 103/μL, due to the potential risk of acquired von Willebrand syndrome and a risk of bleeding (this would require stopping ASA) [109]. Cytoreduction may also be used in low-risk patients with progressive symptoms despite ASA, symptomatic or progressive splenomegaly, and progressive leukocytosis.
Intermediate-Risk ET
This category includes patients older than 60 years, but without thrombosis or JAK2 mutations. These individuals would have been considered high risk (and thus candidates for cytoreductive therapy) according to the traditional risk stratification. Guidelines currently recommend ASA as the sole therapy for these patients, while reserving cytoreduction for those who experience thrombosis (ie, become high-risk) or have uncontrolled vasomotor or general symptoms, symptomatic splenomegaly, symptomatic thrombocytosis, or progressive leukocytosis.
High-Risk ET
For patients with ET in need of cytoreductive therapy (ie, those with prior thrombosis or older than 60 years with a JAK2V617F mutation), first-line options include hydroxyurea, IFN, and anagrelide. Hydroxyurea remains the treatment of choice in most patients [110]. In a seminal study, 114 patients with ET were randomly assigned to either observation or hydroxyurea treatment with the goal of maintaining the platelet count below 600 × 103/μL. At a median follow-up of 27 months, patients in the hydroxyurea group had a lower thrombosis rate (3.6% versus 24%, P = 0.003) and longer thrombosis-free survival, regardless of the use of antiplatelet drugs [64].
Anagrelide, a selective inhibitor of megakaryocytic differentiation and proliferation, was compared with hydroxyurea in patients with ET in 2 randomized trials. In the first (n = 809), the group receiving anagrelide had a higher risk of arterial thrombosis, major bleeding, and fibrotic evolution, but lower incidence of venous thrombosis. Hydroxyurea was better tolerated, mainly due to anagrelide-related cardiovascular adverse events [111]. As a result of this study, hydroxyurea is often preferred to anagrelide as frontline therapy for patients with newly diagnosed high-risk ET. In the second, more recent study (n = 259), however, the 2 agents proved equivalent in terms of major or minor arterial or venous thrombosis, as well as discontinuation rate [112]. The discrepancy between the 2 trials may be partly explained by the different ET diagnostic criteria used, with the latter only enrolling patients with WHO-defined true ET and the former utilizing Polycythemia Vera Study Group-ET diagnostic criteria that included patients with increases in other blood counts or varying degrees of marrow fibrosis.
Interferons were studied in ET in parallel with PV. PEG-IFN-alfa-2a proved effective in patients with ET, with responses observed in 80% of patients [103]. PEG-IFN- alfa-2b produced similar results, with responses in 70% to 90% of patients in small studies and discontinuation observed in 20% to 38% of cases [105,106,113]. Because the very long-term leukemogenic potential of hydroxyurea has remained somewhat uncertain, anagrelide or IFN might be preferable choices in younger patients.
The approach to treatment of ET based on thrombotic risk level is illustrated in Figure 2.
Assessing Response to Therapy
For both patients with PV and ET the endpoint of treatment set forth for clinical trials has been the achievement of a clinicohematologic response. However, studies have failed to show a correlation between response and reduction of the thrombohemorrhagic risk [114]. Therefore, proposed clinical trial response criteria were revised to include absence of hemorrhagic or thrombotic events as part of the definition of response (Table 3) [94].
Approach to Patients Refractory to or Intolerant of First-line Therapy
According to the European LeukemiaNet recommendations, an inadequate response to hydroxyurea in patients with PV (or myelofibrosis) is defined as a need for phlebotomy to maintain the hematocrit below < 45%, the platelet count > 400 × 103/μL, and a WBC count > 10,000/μL, or failure to reduce splenomegaly > 10 cm by > 50% at a dose of ≥ 2 g/day or maximum tolerated dose. Historically, treatment options for patients with PV or ET who failed first-line therapy (most commonly hydroxyurea) have included alkylating agents, such as busulfan, chlorambucil, pipobroman, and phosphorus (P)-32. However, the use of these drugs is limited by the associated risk of LT [93,115,116]. IFN (or anagrelide for ET) is often considered in patients previously treated with hydroxyurea, and vice versa.
Ruxolitinib is a JAK1 and JAK2 inhibitor currently approved for the treatment of PV patients refractory to or intolerant of hydroxyurea [7]. Following promising results of a phase 2 trial [117], ruxolitinib 10 mg twice daily was compared with best available therapy in the pivotal RESPONSE trial (n = 222). Ruxolitinib proved superior in achieving hematocrit control, reduction of spleen volume, and improvement of symptoms. Grade 3-4 hematologic toxicity was infrequent and similar in the 2 arms [118]. In addition, longer follow-up of that study suggested a lower rate of thrombotic events in patients receiving ruxolitinib (1.8 versus 8.2 per 100 patient-years) [119]. In a similarly designed randomized phase 3 study in PV patients without splenomegaly (RESPONSE-2), more patients in the ruxolitinib arm had hematocrit reduction without an increase in toxicity. Based on the results of these studies, ruxolitinib can be considered a standard of care for second-line therapy in this post-hydroxyurea patient population [120]. Ruxolitinib is also being tested in patients with high-risk ET who have become resistant to, or were intolerant of hydroxyurea, but currently has no approved indication in this setting [121,122]. Common side effects of ruxolitinib include cytopenias (especially anemia), increased risk of infections, hyperlipidemia, and increased risk of non-melanoma skin cancer.
Novel agents that have been studied in patients with PV and ET are histone deacetylase inhibitors, murine double minute 2 (MDM2, or HDM2 for their human counterpart) inhibitors (which restore the function of p53), Bcl-2 homology domain 3 mimetics such as navitoclax and venetoclax, and, for patients with ET, the telomerase inhibitor imetelstat [123].
Disease Evolution
Post-PV/Post-ET Myelofibrosis
Diagnostic criteria for post-PV and post-ET myelofibrosis are outlined in Table 4. Fibrotic transformation represents a natural evolution of the clinical course of PV or ET. It occurs in up to 15% and 9% of patients with PV and ET, respectively, in western countries [124]. The true percentage of ET patients who develop myelofibrosis is confounded by the inclusion of prefibrotic myelofibrosis cases in earlier series. The survival of patients who develop myelofibrosis is shortened compared to those who do not. In patients with PV, risk factors for myelofibrosis evolution include advanced age, leukocytosis, JAK2V617F homozygosity or higher allele burden, and hydroxyurea therapy. Once post-PV myelofibrosis has occurred, hemoglobin < 10 g/dL, platelet count < 100 × 103/μL, and WBC count > 30,000/μL are associated with worse outcomes [125]. In patients with ET, risk factors for myelofibrosis transformation include age, anemia, bone marrow hypercellularity and increased reticulin, increased lactate dehydrogenase, leukocytosis, and male gender. The management of post-PV/post-ET myelofibrosis recapitulates that of PMF.
Leukemic Transformation
The presence of more than 20% blasts in peripheral blood or bone marrow in a patient with MPN defines LT. This occurs in up to 5% to 10% of patients and may or may not be preceded by a myelofibrosis phase [126]. In cases of extramedullary transformation, a lower percentage of blasts can be seen in the bone marrow compared to the peripheral blood. The pathogenesis of LT has remained elusive, but it is believed to be associated with genetic instability, which facilitates the acquisition of additional mutations, including those of TET2, ASXL1, EZH2 DNMT3, IDH1/2, and TP53 [127].
Clinical risk factors for LT include advanced age, karyotypic abnormalities, prior therapy with alkylating agents or P-32, splenectomy, increased peripheral blood or bone marrow blasts, leukocytosis, anemia, thrombocytopenia, and cytogenetic abnormalities. Hydroxyurea, IFN, and ruxolitinib have not been shown to have leukemogenic potential thus far. Prognosis of LT is uniformly poor and patient survival rarely exceeds 6 months.
There is no standard of care for MPN LT. Treatment options range from low-intensity regimens to more aggressive AML-type induction chemotherapy. No strategy appears clearly superior to others [128]. Hematopoietic stem cell transplantation is the only therapy that provides clinically meaningful benefit to patients [129], but it is applicable only to a minority of patients with chemosensitive disease and good performance status [130]. Notable experimental approaches to MPN LT include hypomethylating agents, such as decitabine [131] or azacytidine [132], with or without ruxolitinib [133–135].
Conclusion
PV and ET are rare, chronic myeloid disorders. Patients typically experience a long clinical course and enjoy near-normal quality of life if properly managed. The 2 most important life-limiting complications of PV and ET are thrombohemorrhagic events and myelofibrosis/AML transformation. Vascular events are at least in part preventable with counseling on risk factors, phlebotomy (for patients with PV), antiplatelet therapy, and cytoreduction with hydroxyurea, IFNs, or anagrelide (for patients with ET). In addition, ruxolitinib was recently approved for PV patients after hydroxyurea failure. PV/ET transformation in myelofibrosis or AML is part of the natural history of the disease and no therapy has been shown to prevent it. Treatment follows recommendations set forth for PMF and AML, but results are generally poorer and novel strategies are needed to improve outcomes.
Corresponding author: Lorenzo Falchi, MD, Columbia University Medical Center, New York, NY.
Financial disclosures: None.
1. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC; 2008.
2. Alvarez-Larran A, Pereira A, Arellano-Rodrigo E, et al. Cytoreduction plus low-dose aspirin versus cytoreduction alone as primary prophylaxis of thrombosis in patients with high-risk essential thrombocythaemia: an observational study. Br J Haematol 2013;161:865–71.
3. Tefferi A. Polycythemia vera and essential thrombocythemia: 2013 update on diagnosis, risk-stratification, and management. Am J Hematol 2013;88:507–16.
4. Michiels JJ, Berneman Z, Schroyens W, et al. Platelet-mediated erythromelalgic, cerebral, ocular and coronary microvascular ischemic and thrombotic manifestations in patients with essential thrombocythemia and polycythemia vera: a distinct aspirin-responsive and coumadin-resistant arterial thrombophilia. Platelets 2006;17:528–44.
5. James C, Ugo V, Couedic J-PL, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005;434:1144–8.
6. Kralovics R, Passamonti F, Buser A, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders.N Engl J Med 2005;352:1779–90.
7. Verstovsek S, Mesa R, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 2012;366:799–807.
8. Harrison C, Kiladjian J, Al-Ali H, et al. JAK Inhibition with ruxolitinib versus best available therapy for myelofibrosis.N Engl J Med 2012;366:787–98.
9. Berglund S, Zettervall O. Incidence of polycythemia vera in a defined population. Eur J Haematol 1992;48:20–6.
10. Rozman C, Giralt M, Feliu E, et al. Life expectancy of patients with chronic nonleukemic myeloproliferative disorders. Cancer 1991;67:2658–63.
11. Passamonti F, Rumi E, Pungolino E, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med 2004;117:755–61.
12. Hultcrantz M, Kristinsson SY, Andersson TM, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol 2012;30:2995–3001.
13. Wolanskyj AP, Schwager SM, et al. Essential thrombocythemia beyond the first decade: life expectancy, long-term complication rates, and prognostic factors. Mayo Clin Proc 2006;81:159–66.
14. Srour SA, Devesa SS, Morton LM, et al. Incidence and patient survival of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms in the United States, 2001-12. Br J Haematol 2016;174:382–96.
15. Titmarsh GJ, Duncombe AS, McMullin MF, et al. How common are myeloproliferative neoplasms? A systematic review and meta-analysis. Am J Hematol 2014;89:581–7.
16. Moulard O, Mehta J, Fryzek J, et al. Epidemiology of myelofibrosis, essential thrombocythemia, and polycythemia vera in the European Union. Eur J Haematol 2014;92:289–97.
17. Stein BL, Gotlib J, Arcasoy M, et al. Historical views, conventional approaches, and evolving management strategies for myeloproliferative neoplasms. J Natl Compr Canc Netw 2015;13:424–34.
18. Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005;7:387–97.
19. Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005;365:1054–61.
20. Scott LM, Tong W, Levine RL, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med 2007;356:459–68.
21. Pardanani A, Lasho TL, Finke C, et al. Prevalence and clinicopathologic correlates of JAK2 exon 12 mutations in JAK2V617F-negative polycythemia vera. Leukemia 2007;21:1960–3.
22. Passamonti F, Elena C, Schnittger S, et al. Molecular and clinical features of the myeloproliferative neoplasm associated with JAK2 exon 12 mutations. Blood 2011;117:2813–6.
23. Abe M, Suzuki K, Inagaki O, et al. A novel MPL point mutation resulting in thrombopoietin-independent activation. Leukemia 2002;16:1500–6.
24. Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 2006;3:e270.
25. Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood 2006;108:3472–6.
26. Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med 2013;369:2391–405.
27. Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med 2013;369:2379–90.
28. Wang WA, Groenendyk J, Michalak M. Calreticulin signaling in health and disease. Int J Biochem Cell Biol 2012;44:842–6.
29. Saeidi K. Myeloproliferative neoplasms: Current molecular biology and genetics. Crit Rev Oncol Hematol 2016;98:375–89.
30. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127: 2391–405.
31. Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer 2007;109:68–76.
32. Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol 2005;23:2224–32.
33. Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood 2011;118:401–8.
34. Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol 2012;30:4098–103.
35. Casini A, Fontana P, Lecompte TP. Thrombotic complications of myeloproliferative neoplasms: risk assessment and risk-guided management. J Thromb Haemost 2013;11:1215–27.
36. Barbui T, Finazzi G, Falanga A. Myeloproliferative neoplasms and thrombosis. Blood 2013;122:2176-–84.
37. Pearson TC, Wetherley-Mein G. Vascular occlusive episodes and venous haematocrit in primary myeloproliferative polychytemia. Lancet 1978;2:1219–22.
38. Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood 2007;109:2446–52.
39. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia 2013;27:1874–81.
40. Barbui T, Falanga A. Molecular biomarkers of thrombosis in myeloproliferative neoplasms. Thromb Res 2016;140 Suppl 1:S71–75.
41. Campbell PJ, Scott LM, Buck G, et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet 2005;366:1945–53.
42. Tefferi A, Guglielmelli P, Larson DR, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood 2014;124:2507–13.
43. Qin Y, Wang X, Zhao C, et al. The impact of JAK2V617F mutation on different types of thrombosis risk in patients with essential thrombocythemia: a meta-analysis. Int J Hematol 2015;102:170–80.
44. Ziakas PD. Effect of JAK2 V617F on thrombotic risk in patients with essential thrombocythemia: measuring the uncertain. Haematologica 2008;93:1412–4.
45. Lussana F, Dentali F, Abbate R, et al. Screening for thrombophilia and antithrombotic prophylaxis in pregnancy: Guidelines of the Italian Society for Haemostasis and Thrombosis (SISET). Thromb Res 2009;124: e19–25.
46. Dahabreh IJ, Zoi K, Giannouli S, et al. Is JAK2 V617F mutation more than a diagnostic index? A meta-analysis of clinical outcomes in essential thrombocythemia. Leuk Res 2009;33:67–73.
47. Cho YU, Chi HS, Lee EH, et al. Comparison of clinicopathologic findings according to JAK2 V617F mutation in patients with essential thrombocythemia. Int J Hematol 2009;89:39–44.
48. Wolanskyj AP, Lasho TL, Schwager SM, et al. JAK2 mutation in essential thrombocythaemia: clinical associations and long-term prognostic relevance. Br J Haematol 2005;131:208–13.
49. Antonioli E, Guglielmelli P, Pancrazzi A, et al. Clinical implications of the JAK2 V617F mutation in essential thrombocythemia. Leukemia 2005;19:1847–9.
50. Palandri F, Catani L, Testoni N, et al. Long-term follow-up of 386 consecutive patients with essential thrombocythemia: safety of cytoreductive therapy. Am J Hematol 2009;84:215–20.
51. Chim CS, Sim JP, Chan CC, et al. Impact of JAK2V617F mutation on thrombosis and myeloid transformation in essential thrombocythemia: a multivariate analysis by Cox regression in 141 patients. Hematology 2010;15: 187–92.
52. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood 2007;110:840–6.
53. Carobbio A, Finazzi G, Antonioli E, et al. JAK2V617F allele burden and thrombosis: a direct comparison in essential thrombocythemia and polycythemia vera. Exp Hematol 2009;37:1016–21.
54. Alvarez-Larran A, Bellosillo B, Pereira A, et al. JAK2V617F monitoring in polycythemia vera and essential thrombocythemia: clinical usefulness for predicting myelofibrotic transformation and thrombotic events. Am J Hematol 2014;89:517–23.
55. Barbui T, Vannucchi AM, Buxhofer-Ausch V, et al. Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J 2015;5:e369.
56. Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood 2011;117:5857–9.
57. Alvarez-Larran A, Cervantes F, Bellosillo B, et al. Essential thrombocythemia in young individuals: frequency and risk factors for vascular events and evolution to myelofibrosis in 126 patients. Leukemia 2007;21:1218–23.
58. Jantunen R, Juvonen E, Ikkala E, et al. The predictive value of vascular risk factors and gender for the development of thrombotic complications in essential thrombocythemia. Ann Hematol 2001;80:74–8.
59. Besses C, Cervantes F, Pereira A, et al. Major vascular complications in essential thrombocythemia: a study of the predictive factors in a series of 148 patients. Leukemia 1999;13:150–4.
60. Haider M, Gangat N, Hanson C, Tefferi A. Splenomegaly and thrombosis risk in essential thrombocythemia: the mayo clinic experience. Am J Hematol 2016;91: E296–297.
61. Carobbio A, Finazzi G, Antonioli E, et al. Thrombocytosis and leukocytosis interaction in vascular complications of essential thrombocythemia. Blood 2008;112:3135–7.
62. Palandri F, Polverelli N, Catani L, et al. Impact of leukocytosis on thrombotic risk and survival in 532 patients with essential thrombocythemia: a retrospective study. Ann Hematol 2011;90:933–8.
63. Campbell PJ, MacLean C, Beer PA, et al. Correlation of blood counts with vascular complications in essential thrombocythemia: analysis of the prospective PT1 cohort. Blood 2012;120:1409–11.
64. Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med 1995;332:1132–6.
65. van Genderen PJ, Mulder PG, Waleboer M, et al. Prevention and treatment of thrombotic complications in essential thrombocythaemia: efficacy and safety of aspirin. Br J Haematol 1997;97:179–84.
66. Storen EC, Tefferi A. Long-term use of anagrelide in young patients with essential thrombocythemia. Blood 2001;97:863–6.
67. De Stefano V, Za T, Rossi E, et al. Recurrent thrombosis in patients with polycythemia vera and essential thrombocythemia: incidence, risk factors, and effect of treatments. Haematologica 2008;93:372–80.
68. Alvarez-Larran A, Cervantes F, Pereira A, et al. Observation versus antiplatelet therapy as primary prophylaxis for thrombosis in low-risk essential thrombocythemia. Blood 2010;116:1205–10.
69. Palandri F, Polverelli N, Catani L, et al. Bleeding in essential thrombocythaemia: a retrospective analysis on 565 patients. Br J Haematol 2012;156:281–4.
70. Rotunno G, Mannarelli C, Guglielmelli P, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood 2014;123:1552–5.
71. Tefferi A, Wassie EA, Lasho TL, et al. Calreticulin mutations and long-term survival in essential thrombocythemia. Leukemia 2014;28:2300–3.
72. Rumi E, Pietra D, Ferretti V, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood 2014;123:1544–51.
73. Palandri F, Latagliata R, Polverelli N, et al. Mutations and long-term outcome of 217 young patients with essential thrombocythemia or early primary myelofibrosis. Leukemia 2015;29:1344–9.
74. Fu R, Xuan M, Zhou Y, et al. Analysis of calreticulin mutations in Chinese patients with essential thrombocythemia: clinical implications in diagnosis, prognosis and treatment. Leukemia 2014;28:1912–4.
75. Tefferi A, Wassie EA, Guglielmelli P, et al. Type 1 versus Type 2 calreticulin mutations in essential thrombocythemia: a collaborative study of 1027 patients. Am J Hematol 2014;89:E121–4.
76. Pietra D, Rumi E, Ferretti VV, et al. Differential clinical effects of different mutation subtypes in CALR-mutant myeloproliferative neoplasms. Leukemia 2016;30: 431–8.
77. Rumi E, Pietra D, Guglielmelli P, et al. Acquired copy-neutral loss of heterozygosity of chromosome 1p as a molecular event associated with marrow fibrosis in MPL-mutated myeloproliferative neoplasms. Blood 2013;121:4388–95.
78. Beer PA, Campbell PJ, Scott LM, et al. MPL mutations in myeloproliferative disorders: analysis of the PT-1 cohort. Blood 2008;112:141–9.
79. Gangat N, Wassie EA, Lasho TL, et al. Mutations and thrombosis in essential thrombocythemia: prognostic interaction with age and thrombosis history. Eur J Haematol 2015;94:31–6.
80. Sekhar M, McVinnie K, Burroughs AK. Splanchnic vein thrombosis in myeloproliferative neoplasms. Br J Haematol 2013;162:730–47.
81. Stein BL, Saraf S, Sobol U, et al. Age-related differences in disease characteristics and clinical outcomes in polycythemia vera. Leuk Lymph 2013;54:1989–95.
82. Landolfi R, Di Gennaro L, Nicolazzi MA, et al. Polycythemia vera: gender-related phenotypic differences. Intern Emerg Med 2012;7:509–15.
83. Winslow ER, Brunt LM, Drebin JA, et al. Portal vein thrombosis after splenectomy. Am J Surg 2002;184:631–6.
84. Smalberg JH, Arends LR, Valla DC, et al. Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis: a meta-analysis. Blood 2012;120:4921–8.
85. Dentali F, Squizzato A, Brivio L, et al. JAK2V617F mutation for the early diagnosis of Ph- myeloproliferative neoplasms in patients with venous thromboembolism: a meta-analysis. Blood 2009;113:5617–23.
86. Pardanani A, Lasho TL, Hussein K, et al. JAK2V617F mutation screening as part of the hypercoagulable work-up in the absence of splanchnic venous thrombosis or overt myeloproliferative neoplasm: assessment of value in a series of 664 consecutive patients. Mayo Clin Proc 2008;83:457–9.
87. Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol 2011;29:761–70.
88. Landolfi R, Marchioli R, Kutti J, et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med 2004;350:114–24.
89. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med 2013;368:22–33.
90. Kiladjian JJ, Chevret S, Dosquet C, et al. Treatment of polycythemia vera with hydroxyurea and pipobroman: final results of a randomized trial initiated in 1980. J Clin Oncol 2011;29:3907–13.
91. Kaplan ME, Mack K, Goldberg JD, et al. Long-term management of polycythemia vera with hydroxyurea: a progress report. Semin Hematol 1986;23:167–71.
92. Fruchtman SM, Mack K, Kaplan ME, et al. From efficacy to safety: a Polycythemia Vera Study group report on hydroxyurea in patients with polycythemia vera. Semin Hematol 1997;34:17–23.
93. Finazzi G, Caruso V, Marchioli R, et al. Acute leukemia in polycythemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood 2005;105: 2664–70.
94. Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood 2013;121:4778–81.
95. Alvarez-Larran A, Pereira A, Cervantes F, et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood 2012;119:1363–9.
96. Stein BL, Tiu RV. Biological rationale and clinical use of interferon in the classical BCR-ABL-negative myeloproliferative neoplasms. J Interferon Cytokine Res 2013;33: 145–53.
97. Ludwig H, Cortelezzi A, Van Camp BG, et al. Treatment with recombinant interferon-alpha-2C: multiple myeloma and thrombocythaemia in myeloproliferative diseases. Oncology 1985;42 Suppl 1:19–25.
98. Silver RT. Long-term effects of the treatment of polycythemia vera with recombinant interferon-alpha. Cancer 2006;107:451–8.
99. Kiladjian JJ, Mesa RA, Hoffman R. The renaissance of interferon therapy for the treatment of myeloid malignancies. Blood 2011;117:4706–15.
100. Veronese FM, Mero A. The impact of PEGylation on biological therapies. BioDrugs 2008;22:315–29.
101. Kiladjian JJ, Cassinat B, Chevret S, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood 2008;112:3065–72.
102. Turlure P, Cambier N, Roussel M, et al. Complete hematological, molecular and histological remissions without cytoreductive treatment lasting after pegylated-interferon {alpha}-2a (peg-IFN{alpha}-2a) therapy in polycythemia vera (PV): long term results of a phase 2 trial [abstract]. Blood 2011;118(21). Abstract 280.
103. Quintas-Cardama A, Kantarjian H, Manshouri T, et al. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J Clin Oncol 2009;27:5418–24.
104. Quintas-Cardama A, Abdel-Wahab O, Manshouri T, et al. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon a-2a. Blood 2013;122:893–901.
105. Samuelsson J, Hasselbalch H, Bruserud O, et al. A phase II trial of pegylated interferon alpha-2b therapy for polycythemia vera and essential thrombocythemia: feasibility, clinical and biologic effects, and impact on quality of life. Cancer 2006;106:2397–405.
106. Jabbour E, Kantarjian H, Cortes J, et al. PEG-IFN-alpha-2b therapy in BCR-ABL-negative myeloproliferative disorders: final result of a phase 2 study. Cancer 2007; 110:2012–18.
107. Them NC, Bagienski K, Berg T, et al. Molecular responses and chromosomal aberrations in patients with polycythemia vera treated with peg-proline-interferon alpha-2b. Am J Hematol 2015;90:288–94.
108. Gisslinger H, Klade C, Georgiev P, et al. Final results from PROUD-PV a randomized controlled phase 3 trial comparing ropeginterferon alfa-2b to hydroxyurea in polycythemia vera patients [abstract]. Blood 2016;128(suppl 22). Abstract 475.
109. van Genderen PJ, van Vliet HH, Prins FJ, et al. Excessive prolongation of the bleeding time by aspirin in essential thrombocythemia is related to a decrease of large von Willebrand factor multimers in plasma. Ann Hematol 1997;75:215–20.
110. Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med 1995;332:1132–7.
111. Harrison CN, Campbell PJ, Buck G, et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med 2005;353:33–45.
112. Gisslinger H, Gotic M, Holowiecki J, et al. Anagrelide compared with hydroxyurea in WHO-classified essential thrombocythemia: the ANAHYDRET Study, a randomized controlled trial. Blood 2013;121:1720–8.
113. Alvarado Y, Cortes J, Verstovsek S, et al. Pilot study of pegylated interferon-alpha 2b in patients with essential thrombocythemia. Cancer Chemother Pharmacol 2003;51:81–6.
114. Barosi G, Tefferi A, Barbui T, ad hoc committee ‘Definition of clinically relevant outcomes for contemporarily clinical trials in Ph-neg M. Do current response criteria in classical Ph-negative myeloproliferative neoplasms capture benefit for patients? Leukemia 2012;26:1148–9.
115. Bjorkholm M, Derolf AR, Hultcrantz M, et al. Treatment-related risk factors for transformation to acute myeloid leukemia and myelodysplastic syndromes in myeloproliferative neoplasms. J Clin Oncol 2011;29:2410–5.
116. Alvarez-Larran A, Martinez-Aviles L, Hernandez-Boluda JC, et al. Busulfan in patients with polycythemia vera or essential thrombocythemia refractory or intolerant to hydroxyurea. Ann Hematol 2014;93:2037–43.
117. Verstovsek S, Passamonti F, Rambaldi A, et al. A phase 2 study of ruxolitinib, an oral JAK1 and JAK2 Inhibitor, in patients with advanced polycythemia vera who are refractory or intolerant to hydroxyurea. Cancer 2014;120: 513–20.
118. Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib in polycythemia vera resistant to or intolerant of hydroxyurea. N Engl J Med 2015; 372:426–35.
119. Verstovsek S, Vannucchi AM, Griesshammer M, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica 2016;101:821–9.
120. Passamonti F, Griesshammer M, Palandri F, et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): a randomised, open-label, phase 3b study. Lancet Oncol 2017;18:88–99.
121. Verstovsek S, Passamonti F, Rambaldi A, et al. Long-term results from a phase II open-label study of ruxolitinib in patients with essential thrombocythemia refractory to or intolerant of hydroxyurea [abstract]. Blood 2014;124. Abstract 1847.
122. Harrison CN, Mead AJ, Panchal A, et al. Ruxolitinib versus best available therapy for ET intolerant or resistant to hydroxycarbamide in a randomized trial. Blood 2017 Aug 9. pii: blood-2017-05-785790 .
123. Bose P, Verstovsek S. Drug development pipeline for myeloproliferative neoplasms: potential future impact on guidelines and management. J Natl Compr Canc Netw 2016;14:1613–24.
124. Cerquozzi S, Teffieri A. Blast transformation and fibrotic progression in polycythemia vera and essential thrombocythemia: a literature review of incidence and risk factors. Blood Cancer J 2015;Nov 13;5:e366.
125. Passamonti F, Rumi E, Caramella M, et al. A dynamic prognostic model to predict survival in post-polycythemia vera myelofibrosis. Blood 2008;111:3383–7.
126. Mesa RA, Verstovsek S, Cervantes F, et al. Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post- PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMF-BP): Consensus on terminology by the international working group for myelofibrosis research and treatment (IWG-MRT). Leuk Res 2007;31:737–40.
127. Rampal R, Mascarenhas J. Pathogenesis and management of acute myeloid leukemia that has evolved from a myeloproliferative neoplasm. Curr Opin Hematol 2014;21:65–71.
128. Chihara D, Kantarjian HM, Newberry KJ, et al. Survival outcome of patients with acute myeloid leukemia transformed from myeloproliferative neoplasms [abstract]. Blood 2016;128. Abstract 1940.
129. Tam CS, Nussenzveig RM, Popat U, et al. The natural history and treatment outcome of blast phase BCR-ABL-myeloproliferative neoplasms. Blood 2008;112:1628–37.
130. Kundranda MN, Tibes R, Mesa RA. Transformation of a chronic myeloproliferative neoplasm to acute myelogenous leukemia: does anything work? Curr Hematol Malig Rep 2012;7:78–86.
131. Badar T, Kantarjian HM, Ravandi F, et al. Therapeutic benefit of decitabine, a hypomethylating agent, in patients with high-risk primary myelofibrosis and myeloproliferative neoplasm in accelerated or blastic/acute myeloid leukemia phase. Leuk Res 2015;39:950–6.
132. Thepot S, Itzykson R, Seegers V, et al. Treatment of progression of Philadelphia-negative myeloproliferative neoplasms to myelodysplastic syndrome or acute myeloid leukemia by azacitidine: a report on 54 cases on the behalf of the Groupe Francophone des Myelodysplasies (GFM). Blood 2010;116:3735–42.
133. Pemmaraju N, Kantarjian H, Kadia T, et al. A phase I/II study of the Janus kinase (JAK)1 and 2 inhibitor ruxolitinib in patients with relapsed or refractory acute myeloid leukemia. Clin Lymphoma Myeloma Leuk 2015;15:171–6.
134. Rampal RK, Mascarenhas JO, Kosiorek HE, et al. Safety and efficacy of combined ruxolitinib and decitabine in patients with blast-phase MPN and post-MPN AML: results of a phase I study (Myeloproliferative Disorders Research Consortium 109 trial) [abstract]. Blood 2016;128. Abstract 1124.
135. Bose P, Verstovsek S, Gasior Y, et al. Phase I/II study of ruxolitinib (RUX) with decitabine (DAC) in patients with post-myeloproliferative neoplasm acute myeloid leukemia (post-MPN AML): phase I results [abstract]. Blood 2016;128. Abstract 4262.
1. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC; 2008.
2. Alvarez-Larran A, Pereira A, Arellano-Rodrigo E, et al. Cytoreduction plus low-dose aspirin versus cytoreduction alone as primary prophylaxis of thrombosis in patients with high-risk essential thrombocythaemia: an observational study. Br J Haematol 2013;161:865–71.
3. Tefferi A. Polycythemia vera and essential thrombocythemia: 2013 update on diagnosis, risk-stratification, and management. Am J Hematol 2013;88:507–16.
4. Michiels JJ, Berneman Z, Schroyens W, et al. Platelet-mediated erythromelalgic, cerebral, ocular and coronary microvascular ischemic and thrombotic manifestations in patients with essential thrombocythemia and polycythemia vera: a distinct aspirin-responsive and coumadin-resistant arterial thrombophilia. Platelets 2006;17:528–44.
5. James C, Ugo V, Couedic J-PL, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005;434:1144–8.
6. Kralovics R, Passamonti F, Buser A, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders.N Engl J Med 2005;352:1779–90.
7. Verstovsek S, Mesa R, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 2012;366:799–807.
8. Harrison C, Kiladjian J, Al-Ali H, et al. JAK Inhibition with ruxolitinib versus best available therapy for myelofibrosis.N Engl J Med 2012;366:787–98.
9. Berglund S, Zettervall O. Incidence of polycythemia vera in a defined population. Eur J Haematol 1992;48:20–6.
10. Rozman C, Giralt M, Feliu E, et al. Life expectancy of patients with chronic nonleukemic myeloproliferative disorders. Cancer 1991;67:2658–63.
11. Passamonti F, Rumi E, Pungolino E, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med 2004;117:755–61.
12. Hultcrantz M, Kristinsson SY, Andersson TM, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol 2012;30:2995–3001.
13. Wolanskyj AP, Schwager SM, et al. Essential thrombocythemia beyond the first decade: life expectancy, long-term complication rates, and prognostic factors. Mayo Clin Proc 2006;81:159–66.
14. Srour SA, Devesa SS, Morton LM, et al. Incidence and patient survival of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms in the United States, 2001-12. Br J Haematol 2016;174:382–96.
15. Titmarsh GJ, Duncombe AS, McMullin MF, et al. How common are myeloproliferative neoplasms? A systematic review and meta-analysis. Am J Hematol 2014;89:581–7.
16. Moulard O, Mehta J, Fryzek J, et al. Epidemiology of myelofibrosis, essential thrombocythemia, and polycythemia vera in the European Union. Eur J Haematol 2014;92:289–97.
17. Stein BL, Gotlib J, Arcasoy M, et al. Historical views, conventional approaches, and evolving management strategies for myeloproliferative neoplasms. J Natl Compr Canc Netw 2015;13:424–34.
18. Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005;7:387–97.
19. Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005;365:1054–61.
20. Scott LM, Tong W, Levine RL, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med 2007;356:459–68.
21. Pardanani A, Lasho TL, Finke C, et al. Prevalence and clinicopathologic correlates of JAK2 exon 12 mutations in JAK2V617F-negative polycythemia vera. Leukemia 2007;21:1960–3.
22. Passamonti F, Elena C, Schnittger S, et al. Molecular and clinical features of the myeloproliferative neoplasm associated with JAK2 exon 12 mutations. Blood 2011;117:2813–6.
23. Abe M, Suzuki K, Inagaki O, et al. A novel MPL point mutation resulting in thrombopoietin-independent activation. Leukemia 2002;16:1500–6.
24. Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 2006;3:e270.
25. Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood 2006;108:3472–6.
26. Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med 2013;369:2391–405.
27. Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med 2013;369:2379–90.
28. Wang WA, Groenendyk J, Michalak M. Calreticulin signaling in health and disease. Int J Biochem Cell Biol 2012;44:842–6.
29. Saeidi K. Myeloproliferative neoplasms: Current molecular biology and genetics. Crit Rev Oncol Hematol 2016;98:375–89.
30. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127: 2391–405.
31. Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer 2007;109:68–76.
32. Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol 2005;23:2224–32.
33. Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood 2011;118:401–8.
34. Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol 2012;30:4098–103.
35. Casini A, Fontana P, Lecompte TP. Thrombotic complications of myeloproliferative neoplasms: risk assessment and risk-guided management. J Thromb Haemost 2013;11:1215–27.
36. Barbui T, Finazzi G, Falanga A. Myeloproliferative neoplasms and thrombosis. Blood 2013;122:2176-–84.
37. Pearson TC, Wetherley-Mein G. Vascular occlusive episodes and venous haematocrit in primary myeloproliferative polychytemia. Lancet 1978;2:1219–22.
38. Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood 2007;109:2446–52.
39. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia 2013;27:1874–81.
40. Barbui T, Falanga A. Molecular biomarkers of thrombosis in myeloproliferative neoplasms. Thromb Res 2016;140 Suppl 1:S71–75.
41. Campbell PJ, Scott LM, Buck G, et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet 2005;366:1945–53.
42. Tefferi A, Guglielmelli P, Larson DR, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood 2014;124:2507–13.
43. Qin Y, Wang X, Zhao C, et al. The impact of JAK2V617F mutation on different types of thrombosis risk in patients with essential thrombocythemia: a meta-analysis. Int J Hematol 2015;102:170–80.
44. Ziakas PD. Effect of JAK2 V617F on thrombotic risk in patients with essential thrombocythemia: measuring the uncertain. Haematologica 2008;93:1412–4.
45. Lussana F, Dentali F, Abbate R, et al. Screening for thrombophilia and antithrombotic prophylaxis in pregnancy: Guidelines of the Italian Society for Haemostasis and Thrombosis (SISET). Thromb Res 2009;124: e19–25.
46. Dahabreh IJ, Zoi K, Giannouli S, et al. Is JAK2 V617F mutation more than a diagnostic index? A meta-analysis of clinical outcomes in essential thrombocythemia. Leuk Res 2009;33:67–73.
47. Cho YU, Chi HS, Lee EH, et al. Comparison of clinicopathologic findings according to JAK2 V617F mutation in patients with essential thrombocythemia. Int J Hematol 2009;89:39–44.
48. Wolanskyj AP, Lasho TL, Schwager SM, et al. JAK2 mutation in essential thrombocythaemia: clinical associations and long-term prognostic relevance. Br J Haematol 2005;131:208–13.
49. Antonioli E, Guglielmelli P, Pancrazzi A, et al. Clinical implications of the JAK2 V617F mutation in essential thrombocythemia. Leukemia 2005;19:1847–9.
50. Palandri F, Catani L, Testoni N, et al. Long-term follow-up of 386 consecutive patients with essential thrombocythemia: safety of cytoreductive therapy. Am J Hematol 2009;84:215–20.
51. Chim CS, Sim JP, Chan CC, et al. Impact of JAK2V617F mutation on thrombosis and myeloid transformation in essential thrombocythemia: a multivariate analysis by Cox regression in 141 patients. Hematology 2010;15: 187–92.
52. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood 2007;110:840–6.
53. Carobbio A, Finazzi G, Antonioli E, et al. JAK2V617F allele burden and thrombosis: a direct comparison in essential thrombocythemia and polycythemia vera. Exp Hematol 2009;37:1016–21.
54. Alvarez-Larran A, Bellosillo B, Pereira A, et al. JAK2V617F monitoring in polycythemia vera and essential thrombocythemia: clinical usefulness for predicting myelofibrotic transformation and thrombotic events. Am J Hematol 2014;89:517–23.
55. Barbui T, Vannucchi AM, Buxhofer-Ausch V, et al. Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J 2015;5:e369.
56. Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood 2011;117:5857–9.
57. Alvarez-Larran A, Cervantes F, Bellosillo B, et al. Essential thrombocythemia in young individuals: frequency and risk factors for vascular events and evolution to myelofibrosis in 126 patients. Leukemia 2007;21:1218–23.
58. Jantunen R, Juvonen E, Ikkala E, et al. The predictive value of vascular risk factors and gender for the development of thrombotic complications in essential thrombocythemia. Ann Hematol 2001;80:74–8.
59. Besses C, Cervantes F, Pereira A, et al. Major vascular complications in essential thrombocythemia: a study of the predictive factors in a series of 148 patients. Leukemia 1999;13:150–4.
60. Haider M, Gangat N, Hanson C, Tefferi A. Splenomegaly and thrombosis risk in essential thrombocythemia: the mayo clinic experience. Am J Hematol 2016;91: E296–297.
61. Carobbio A, Finazzi G, Antonioli E, et al. Thrombocytosis and leukocytosis interaction in vascular complications of essential thrombocythemia. Blood 2008;112:3135–7.
62. Palandri F, Polverelli N, Catani L, et al. Impact of leukocytosis on thrombotic risk and survival in 532 patients with essential thrombocythemia: a retrospective study. Ann Hematol 2011;90:933–8.
63. Campbell PJ, MacLean C, Beer PA, et al. Correlation of blood counts with vascular complications in essential thrombocythemia: analysis of the prospective PT1 cohort. Blood 2012;120:1409–11.
64. Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med 1995;332:1132–6.
65. van Genderen PJ, Mulder PG, Waleboer M, et al. Prevention and treatment of thrombotic complications in essential thrombocythaemia: efficacy and safety of aspirin. Br J Haematol 1997;97:179–84.
66. Storen EC, Tefferi A. Long-term use of anagrelide in young patients with essential thrombocythemia. Blood 2001;97:863–6.
67. De Stefano V, Za T, Rossi E, et al. Recurrent thrombosis in patients with polycythemia vera and essential thrombocythemia: incidence, risk factors, and effect of treatments. Haematologica 2008;93:372–80.
68. Alvarez-Larran A, Cervantes F, Pereira A, et al. Observation versus antiplatelet therapy as primary prophylaxis for thrombosis in low-risk essential thrombocythemia. Blood 2010;116:1205–10.
69. Palandri F, Polverelli N, Catani L, et al. Bleeding in essential thrombocythaemia: a retrospective analysis on 565 patients. Br J Haematol 2012;156:281–4.
70. Rotunno G, Mannarelli C, Guglielmelli P, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood 2014;123:1552–5.
71. Tefferi A, Wassie EA, Lasho TL, et al. Calreticulin mutations and long-term survival in essential thrombocythemia. Leukemia 2014;28:2300–3.
72. Rumi E, Pietra D, Ferretti V, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood 2014;123:1544–51.
73. Palandri F, Latagliata R, Polverelli N, et al. Mutations and long-term outcome of 217 young patients with essential thrombocythemia or early primary myelofibrosis. Leukemia 2015;29:1344–9.
74. Fu R, Xuan M, Zhou Y, et al. Analysis of calreticulin mutations in Chinese patients with essential thrombocythemia: clinical implications in diagnosis, prognosis and treatment. Leukemia 2014;28:1912–4.
75. Tefferi A, Wassie EA, Guglielmelli P, et al. Type 1 versus Type 2 calreticulin mutations in essential thrombocythemia: a collaborative study of 1027 patients. Am J Hematol 2014;89:E121–4.
76. Pietra D, Rumi E, Ferretti VV, et al. Differential clinical effects of different mutation subtypes in CALR-mutant myeloproliferative neoplasms. Leukemia 2016;30: 431–8.
77. Rumi E, Pietra D, Guglielmelli P, et al. Acquired copy-neutral loss of heterozygosity of chromosome 1p as a molecular event associated with marrow fibrosis in MPL-mutated myeloproliferative neoplasms. Blood 2013;121:4388–95.
78. Beer PA, Campbell PJ, Scott LM, et al. MPL mutations in myeloproliferative disorders: analysis of the PT-1 cohort. Blood 2008;112:141–9.
79. Gangat N, Wassie EA, Lasho TL, et al. Mutations and thrombosis in essential thrombocythemia: prognostic interaction with age and thrombosis history. Eur J Haematol 2015;94:31–6.
80. Sekhar M, McVinnie K, Burroughs AK. Splanchnic vein thrombosis in myeloproliferative neoplasms. Br J Haematol 2013;162:730–47.
81. Stein BL, Saraf S, Sobol U, et al. Age-related differences in disease characteristics and clinical outcomes in polycythemia vera. Leuk Lymph 2013;54:1989–95.
82. Landolfi R, Di Gennaro L, Nicolazzi MA, et al. Polycythemia vera: gender-related phenotypic differences. Intern Emerg Med 2012;7:509–15.
83. Winslow ER, Brunt LM, Drebin JA, et al. Portal vein thrombosis after splenectomy. Am J Surg 2002;184:631–6.
84. Smalberg JH, Arends LR, Valla DC, et al. Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis: a meta-analysis. Blood 2012;120:4921–8.
85. Dentali F, Squizzato A, Brivio L, et al. JAK2V617F mutation for the early diagnosis of Ph- myeloproliferative neoplasms in patients with venous thromboembolism: a meta-analysis. Blood 2009;113:5617–23.
86. Pardanani A, Lasho TL, Hussein K, et al. JAK2V617F mutation screening as part of the hypercoagulable work-up in the absence of splanchnic venous thrombosis or overt myeloproliferative neoplasm: assessment of value in a series of 664 consecutive patients. Mayo Clin Proc 2008;83:457–9.
87. Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol 2011;29:761–70.
88. Landolfi R, Marchioli R, Kutti J, et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med 2004;350:114–24.
89. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med 2013;368:22–33.
90. Kiladjian JJ, Chevret S, Dosquet C, et al. Treatment of polycythemia vera with hydroxyurea and pipobroman: final results of a randomized trial initiated in 1980. J Clin Oncol 2011;29:3907–13.
91. Kaplan ME, Mack K, Goldberg JD, et al. Long-term management of polycythemia vera with hydroxyurea: a progress report. Semin Hematol 1986;23:167–71.
92. Fruchtman SM, Mack K, Kaplan ME, et al. From efficacy to safety: a Polycythemia Vera Study group report on hydroxyurea in patients with polycythemia vera. Semin Hematol 1997;34:17–23.
93. Finazzi G, Caruso V, Marchioli R, et al. Acute leukemia in polycythemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood 2005;105: 2664–70.
94. Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood 2013;121:4778–81.
95. Alvarez-Larran A, Pereira A, Cervantes F, et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood 2012;119:1363–9.
96. Stein BL, Tiu RV. Biological rationale and clinical use of interferon in the classical BCR-ABL-negative myeloproliferative neoplasms. J Interferon Cytokine Res 2013;33: 145–53.
97. Ludwig H, Cortelezzi A, Van Camp BG, et al. Treatment with recombinant interferon-alpha-2C: multiple myeloma and thrombocythaemia in myeloproliferative diseases. Oncology 1985;42 Suppl 1:19–25.
98. Silver RT. Long-term effects of the treatment of polycythemia vera with recombinant interferon-alpha. Cancer 2006;107:451–8.
99. Kiladjian JJ, Mesa RA, Hoffman R. The renaissance of interferon therapy for the treatment of myeloid malignancies. Blood 2011;117:4706–15.
100. Veronese FM, Mero A. The impact of PEGylation on biological therapies. BioDrugs 2008;22:315–29.
101. Kiladjian JJ, Cassinat B, Chevret S, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood 2008;112:3065–72.
102. Turlure P, Cambier N, Roussel M, et al. Complete hematological, molecular and histological remissions without cytoreductive treatment lasting after pegylated-interferon {alpha}-2a (peg-IFN{alpha}-2a) therapy in polycythemia vera (PV): long term results of a phase 2 trial [abstract]. Blood 2011;118(21). Abstract 280.
103. Quintas-Cardama A, Kantarjian H, Manshouri T, et al. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J Clin Oncol 2009;27:5418–24.
104. Quintas-Cardama A, Abdel-Wahab O, Manshouri T, et al. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon a-2a. Blood 2013;122:893–901.
105. Samuelsson J, Hasselbalch H, Bruserud O, et al. A phase II trial of pegylated interferon alpha-2b therapy for polycythemia vera and essential thrombocythemia: feasibility, clinical and biologic effects, and impact on quality of life. Cancer 2006;106:2397–405.
106. Jabbour E, Kantarjian H, Cortes J, et al. PEG-IFN-alpha-2b therapy in BCR-ABL-negative myeloproliferative disorders: final result of a phase 2 study. Cancer 2007; 110:2012–18.
107. Them NC, Bagienski K, Berg T, et al. Molecular responses and chromosomal aberrations in patients with polycythemia vera treated with peg-proline-interferon alpha-2b. Am J Hematol 2015;90:288–94.
108. Gisslinger H, Klade C, Georgiev P, et al. Final results from PROUD-PV a randomized controlled phase 3 trial comparing ropeginterferon alfa-2b to hydroxyurea in polycythemia vera patients [abstract]. Blood 2016;128(suppl 22). Abstract 475.
109. van Genderen PJ, van Vliet HH, Prins FJ, et al. Excessive prolongation of the bleeding time by aspirin in essential thrombocythemia is related to a decrease of large von Willebrand factor multimers in plasma. Ann Hematol 1997;75:215–20.
110. Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med 1995;332:1132–7.
111. Harrison CN, Campbell PJ, Buck G, et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med 2005;353:33–45.
112. Gisslinger H, Gotic M, Holowiecki J, et al. Anagrelide compared with hydroxyurea in WHO-classified essential thrombocythemia: the ANAHYDRET Study, a randomized controlled trial. Blood 2013;121:1720–8.
113. Alvarado Y, Cortes J, Verstovsek S, et al. Pilot study of pegylated interferon-alpha 2b in patients with essential thrombocythemia. Cancer Chemother Pharmacol 2003;51:81–6.
114. Barosi G, Tefferi A, Barbui T, ad hoc committee ‘Definition of clinically relevant outcomes for contemporarily clinical trials in Ph-neg M. Do current response criteria in classical Ph-negative myeloproliferative neoplasms capture benefit for patients? Leukemia 2012;26:1148–9.
115. Bjorkholm M, Derolf AR, Hultcrantz M, et al. Treatment-related risk factors for transformation to acute myeloid leukemia and myelodysplastic syndromes in myeloproliferative neoplasms. J Clin Oncol 2011;29:2410–5.
116. Alvarez-Larran A, Martinez-Aviles L, Hernandez-Boluda JC, et al. Busulfan in patients with polycythemia vera or essential thrombocythemia refractory or intolerant to hydroxyurea. Ann Hematol 2014;93:2037–43.
117. Verstovsek S, Passamonti F, Rambaldi A, et al. A phase 2 study of ruxolitinib, an oral JAK1 and JAK2 Inhibitor, in patients with advanced polycythemia vera who are refractory or intolerant to hydroxyurea. Cancer 2014;120: 513–20.
118. Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib in polycythemia vera resistant to or intolerant of hydroxyurea. N Engl J Med 2015; 372:426–35.
119. Verstovsek S, Vannucchi AM, Griesshammer M, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica 2016;101:821–9.
120. Passamonti F, Griesshammer M, Palandri F, et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): a randomised, open-label, phase 3b study. Lancet Oncol 2017;18:88–99.
121. Verstovsek S, Passamonti F, Rambaldi A, et al. Long-term results from a phase II open-label study of ruxolitinib in patients with essential thrombocythemia refractory to or intolerant of hydroxyurea [abstract]. Blood 2014;124. Abstract 1847.
122. Harrison CN, Mead AJ, Panchal A, et al. Ruxolitinib versus best available therapy for ET intolerant or resistant to hydroxycarbamide in a randomized trial. Blood 2017 Aug 9. pii: blood-2017-05-785790 .
123. Bose P, Verstovsek S. Drug development pipeline for myeloproliferative neoplasms: potential future impact on guidelines and management. J Natl Compr Canc Netw 2016;14:1613–24.
124. Cerquozzi S, Teffieri A. Blast transformation and fibrotic progression in polycythemia vera and essential thrombocythemia: a literature review of incidence and risk factors. Blood Cancer J 2015;Nov 13;5:e366.
125. Passamonti F, Rumi E, Caramella M, et al. A dynamic prognostic model to predict survival in post-polycythemia vera myelofibrosis. Blood 2008;111:3383–7.
126. Mesa RA, Verstovsek S, Cervantes F, et al. Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post- PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMF-BP): Consensus on terminology by the international working group for myelofibrosis research and treatment (IWG-MRT). Leuk Res 2007;31:737–40.
127. Rampal R, Mascarenhas J. Pathogenesis and management of acute myeloid leukemia that has evolved from a myeloproliferative neoplasm. Curr Opin Hematol 2014;21:65–71.
128. Chihara D, Kantarjian HM, Newberry KJ, et al. Survival outcome of patients with acute myeloid leukemia transformed from myeloproliferative neoplasms [abstract]. Blood 2016;128. Abstract 1940.
129. Tam CS, Nussenzveig RM, Popat U, et al. The natural history and treatment outcome of blast phase BCR-ABL-myeloproliferative neoplasms. Blood 2008;112:1628–37.
130. Kundranda MN, Tibes R, Mesa RA. Transformation of a chronic myeloproliferative neoplasm to acute myelogenous leukemia: does anything work? Curr Hematol Malig Rep 2012;7:78–86.
131. Badar T, Kantarjian HM, Ravandi F, et al. Therapeutic benefit of decitabine, a hypomethylating agent, in patients with high-risk primary myelofibrosis and myeloproliferative neoplasm in accelerated or blastic/acute myeloid leukemia phase. Leuk Res 2015;39:950–6.
132. Thepot S, Itzykson R, Seegers V, et al. Treatment of progression of Philadelphia-negative myeloproliferative neoplasms to myelodysplastic syndrome or acute myeloid leukemia by azacitidine: a report on 54 cases on the behalf of the Groupe Francophone des Myelodysplasies (GFM). Blood 2010;116:3735–42.
133. Pemmaraju N, Kantarjian H, Kadia T, et al. A phase I/II study of the Janus kinase (JAK)1 and 2 inhibitor ruxolitinib in patients with relapsed or refractory acute myeloid leukemia. Clin Lymphoma Myeloma Leuk 2015;15:171–6.
134. Rampal RK, Mascarenhas JO, Kosiorek HE, et al. Safety and efficacy of combined ruxolitinib and decitabine in patients with blast-phase MPN and post-MPN AML: results of a phase I study (Myeloproliferative Disorders Research Consortium 109 trial) [abstract]. Blood 2016;128. Abstract 1124.
135. Bose P, Verstovsek S, Gasior Y, et al. Phase I/II study of ruxolitinib (RUX) with decitabine (DAC) in patients with post-myeloproliferative neoplasm acute myeloid leukemia (post-MPN AML): phase I results [abstract]. Blood 2016;128. Abstract 4262.
Sustainability of Ambulatory Safety Event Reporting Improvement After Intervention Discontinuation
From Novant Health and Novant Health Medical Group, Winston-Salem, NC (Dr. C
Abstract
- Objective: An educational intervention stressing anonymous, voluntary safety event reporting together with monthly regular audit and feedback led to significantly increased reporting of safety events in a nonacademic, community practice setting during a 15-month intervention period. We assessed whether these increased reporting rates would be sustained during the 30-month period after the intervention was discontinued.
- Methods: We reviewed all patient safety events reported in our ambulatory clinics for the period 2012–2016, and selected 6 clinics that comprised the intervention collaborative and 18 specialty- and size-matched clinics (1:3 match) that comprised the comparator group. To test the changes in safety event reporting (SER) rates between the intervention and postintervention periods for the intervention collaborative, interrupted time series analysis with a control group was performed.
- Results: The SER rate peaked in the first month following the start of the intervention. Following discontinuation of regular auditing and feedback, reporting rates declined abruptly and reverted to baseline by 16 months post intervention.
- Conclusion: It is likely that sustaining enhanced reporting rates requires ongoing audit and feedback to maintain a focus on event reporting.
Keywords: patient safety; safety event reporting; voluntary reporting system; risk management; ambulatory clinic.
We have previously shown that patient safety reporting rates for a 6-practice collaborative group in our non-academic community clinics increased 10-fold after we implemented an improvement initiative consisting of an initial education session followed by provision of monthly audit and written and in-person feedback [1]. The intervention was implemented for 15 months, and after discontinuation of the intervention we have continued to monitor reporting rates. Our objective was to assess whether the increased reporting rates observed in this collaborative during the intervention period would be sustained for 30 months following the intervention.
Methods
This study’s methods have been described in detail previously [1]. For this improvement initiative, we reviewed all patient safety events reported in our ambulatory clinics for the period 2012–2016. We identified 6 clinics, the intervention collaborative, in family medicine (n = 3), pediatrics (n = 2), and general surgery (n = 1), and 18 specialty- and size-matched clinics (1:3 match), the comparator group [1]. For the intervention collaborative only, we provided an initial 1-hour educational session on safety events with a listing of all safety event types, along with a 1-page reporting form for voluntary, anonymous submission, with use of the term “safety event” rather than “ error,” to support a nonpunitive culture. After the educational session, we provided monthly audit and written and in-person feedback with peer comparison data by clinic. Monthly audit and feedback continued throughout the intervention and was discontinued postintervention. For event reporting, in our inpatient and outpatient facilities we used VIncident (Verge Solutions, Mt. Pleasant, SC) for the period 2012–2015 and RL6: Risk (RL Solutions, Toronto, ON) for 2016.
The baseline period was 15 months (January 2012–March 2013), the intervention period was 15 months (April 2013–June 2014), and the postintervention period was 30 months (July 2014–December 2016). All 24 clinics were monitored for the 60-month period.
To test the changes in the rate of safety event reporting (SER) between the pre-intervention and postintervention periods and between the intervention and the postintervention periods, interrupted time series (ITS) analysis with a control group was performed using PROC AUTOREG in SAS Enterprise Guide 6.1 (SAS Institute Inc., Cary, NC). Because SER rates are reported monthly, ITS analysis was used to control for autocorrelation, nonstationary variance, seasonality, and trends [2,3].
Results
The SER rate was assessed monthly, so the number of SER rates for each group (intervention and comparator) was 15 during the pre-intervention and intervention periods, respectively, and 30 during the postintervention period. During the pre-intervention period, the intervention collaborative’s baseline median rate of safety events reported was 1.5 per 10,000 patient encounters (Figure). Also, for the intervention collaborative, the pre-intervention baseline mean (standard deviation, SD) SER rate (per 10,000 patient encounters by month) was 1.3 (1.2), the intervention mean SER rate was 12.0 (7.3), and the postintervention rate was 3.2 (1.8). Based on the ITS analysis, there was a significant change in the SER rate between the intervention and postintervention periods for the intervention collaborative (P = 0.01).
The SER rate peaked in the first month following the start of the intervention. After discontinuation of feedback, reporting rates declined abruptly and reverted to baseline by 16 months post intervention (Figure). The postintervention SER rate was also significantly higher than the pre-intervention rate (P = 0.001).
For the comparator clinics, no significant change in SER rates occurred for the 3 time periods.
Discussion
In this initiative with a 5-year reporting window, we had previously shown that with education and prospective audit and feedback, we could achieve a 10-fold increase in patient SER rates among a multi-practice collaborative while the intervention was maintained [1]. Even though there was a modest but significant increase in the SER rate in the postintervention period for the 6-clinic intervention collaborative compared to baseline, the substantial gains seen during the course of the intervention were not maintained when monthly audit and feedback ceased and monitoring continued for 30 months.
Limitations of this study include possible selection bias resulting from including clinics felt likely to participate rather than identifying clinics in a random fashion. In addition, we did not attempt to determine the specific reasons for the decrease in reporting among these clinics.
The few studies of ambulatory SER do not adequately address the effect of intervention cessation, but researchers who implemented other ambulatory quality improvement efforts have reported that gains often deteriorate or revert to baseline without consistent, ongoing feedback [4]. Likewise, in hospital-based residency programs, a multifaceted approach that includes feedback can increase SER rates, but it is uncertain if the success of this approach can be maintained long-term without continuing feedback of some type [5–7].
There are likely many factors influencing SER in ambulatory clinics, many of which are also applicable in the hospital setting. These include ease of reporting, knowing what events to report, confidentiality of reporting, and the belief that reporting makes a difference in enhancing patient safety [8]. A strong culture of safety in ambulatory clinics may lead to enhanced voluntary SER [9], and a nonpunitive, team-based approach has been advocated to promote reporting and improve ambulatory safety [10]. Historically, our ambulatory medical group clinics have had a strong culture of safety and, with patient safety coaches present in all of our clinics, we have supported a nonpunitive, team-based approach to SER [11].
In our intervention, we made reporting safety events easy, reporters knew which events to report, events could be reported anonymously, and reporters were rewarded, at least with data feedback, for reporting. The only factor known to have changed was discontinuation of monthly feedback. Which factors are most important could not be determined by our work, but we strongly suspect that sustaining enhanced reporting rates requires ongoing audit and feedback to maintain a focus on event reporting.
Corresponding author: Herbert Clegg, MD, 108 Providence Road, Charlotte NC, 28207, [email protected].
Financial disclosures: None.
1. Clegg HW, Cardwell T, West AM, Ferrell F. Improved safety event reporting in outpatient, nonacademic practices with an anonymous, nonpunitive approach. J Clin Outcomes Manag 2015;22:66–72.
2. Newland JG, Stach LM, De Lurgio SA, et al. Impact of a prospective-audit-with-feedback antimicrobial stewardship program at a children’s hospital. J Pediatric Infect Dis Soc 2012; 1:179–86.
3. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr 2013;13 (6 Suppl):S38–44.
4. Gerber JS, Prasad PA, Fiks AG, et al. Durability of benefits of an outpatient antimicrobial stewardship intervention after discontinuation of audit and feedback. JAMA 2014;312:2569–70.
5. Steen S, Jaeger C, Price L, Griffen D. Increasing patient safety event reporting in an emergency medicine residency. BMJ Qual Improv Rep 2017;6(1).
6. Fox M, Bump G, Butler G, et al. Making residents part of the safety culture: improving error reporting and reducing harms. J Patient Saf 2017. [Epub ahead of print]
7. Dunbar AE 3rd, Cupit M, Vath RJ, et al. An improvement approach to integrate teaching teams in the reporting of safety events. Pediatrics 2017;139:e20153807.
8. Institute of Medicine. To err is human: Building a safer health system. National Academies. www.nationalacademies.org/hmd/~/media/Files/Report%20Files/1999/To-Err-is-Human/To%20Err%20is%20Human%201999%20%20report%20brief.pdf Published November 1999. Accessed August 22, 2018.
9. Miller N, Bhowmik S, Ezinwa M, et al. The relationship between safety culture and voluntary event reporting in a large regional ambulatory care group. J Patient Saf 2017. [Epub ahead of print]
10. Neuspiel DR, Stubbs EH. Patient safety in ambulatory care. Pediatr Clin North Am 2012;59:1341–54.
11. West AM, Cardwell T, Clegg HW. Improving patient safety culture through patient safety coaches in the ambulatory setting. Presented at: Institute for Healthcare Improvement Annual Summit on Improving Patient Care in the Office Practice and the Community; March 2015; Dallas, Texas.
From Novant Health and Novant Health Medical Group, Winston-Salem, NC (Dr. C
Abstract
- Objective: An educational intervention stressing anonymous, voluntary safety event reporting together with monthly regular audit and feedback led to significantly increased reporting of safety events in a nonacademic, community practice setting during a 15-month intervention period. We assessed whether these increased reporting rates would be sustained during the 30-month period after the intervention was discontinued.
- Methods: We reviewed all patient safety events reported in our ambulatory clinics for the period 2012–2016, and selected 6 clinics that comprised the intervention collaborative and 18 specialty- and size-matched clinics (1:3 match) that comprised the comparator group. To test the changes in safety event reporting (SER) rates between the intervention and postintervention periods for the intervention collaborative, interrupted time series analysis with a control group was performed.
- Results: The SER rate peaked in the first month following the start of the intervention. Following discontinuation of regular auditing and feedback, reporting rates declined abruptly and reverted to baseline by 16 months post intervention.
- Conclusion: It is likely that sustaining enhanced reporting rates requires ongoing audit and feedback to maintain a focus on event reporting.
Keywords: patient safety; safety event reporting; voluntary reporting system; risk management; ambulatory clinic.
We have previously shown that patient safety reporting rates for a 6-practice collaborative group in our non-academic community clinics increased 10-fold after we implemented an improvement initiative consisting of an initial education session followed by provision of monthly audit and written and in-person feedback [1]. The intervention was implemented for 15 months, and after discontinuation of the intervention we have continued to monitor reporting rates. Our objective was to assess whether the increased reporting rates observed in this collaborative during the intervention period would be sustained for 30 months following the intervention.
Methods
This study’s methods have been described in detail previously [1]. For this improvement initiative, we reviewed all patient safety events reported in our ambulatory clinics for the period 2012–2016. We identified 6 clinics, the intervention collaborative, in family medicine (n = 3), pediatrics (n = 2), and general surgery (n = 1), and 18 specialty- and size-matched clinics (1:3 match), the comparator group [1]. For the intervention collaborative only, we provided an initial 1-hour educational session on safety events with a listing of all safety event types, along with a 1-page reporting form for voluntary, anonymous submission, with use of the term “safety event” rather than “ error,” to support a nonpunitive culture. After the educational session, we provided monthly audit and written and in-person feedback with peer comparison data by clinic. Monthly audit and feedback continued throughout the intervention and was discontinued postintervention. For event reporting, in our inpatient and outpatient facilities we used VIncident (Verge Solutions, Mt. Pleasant, SC) for the period 2012–2015 and RL6: Risk (RL Solutions, Toronto, ON) for 2016.
The baseline period was 15 months (January 2012–March 2013), the intervention period was 15 months (April 2013–June 2014), and the postintervention period was 30 months (July 2014–December 2016). All 24 clinics were monitored for the 60-month period.
To test the changes in the rate of safety event reporting (SER) between the pre-intervention and postintervention periods and between the intervention and the postintervention periods, interrupted time series (ITS) analysis with a control group was performed using PROC AUTOREG in SAS Enterprise Guide 6.1 (SAS Institute Inc., Cary, NC). Because SER rates are reported monthly, ITS analysis was used to control for autocorrelation, nonstationary variance, seasonality, and trends [2,3].
Results
The SER rate was assessed monthly, so the number of SER rates for each group (intervention and comparator) was 15 during the pre-intervention and intervention periods, respectively, and 30 during the postintervention period. During the pre-intervention period, the intervention collaborative’s baseline median rate of safety events reported was 1.5 per 10,000 patient encounters (Figure). Also, for the intervention collaborative, the pre-intervention baseline mean (standard deviation, SD) SER rate (per 10,000 patient encounters by month) was 1.3 (1.2), the intervention mean SER rate was 12.0 (7.3), and the postintervention rate was 3.2 (1.8). Based on the ITS analysis, there was a significant change in the SER rate between the intervention and postintervention periods for the intervention collaborative (P = 0.01).
The SER rate peaked in the first month following the start of the intervention. After discontinuation of feedback, reporting rates declined abruptly and reverted to baseline by 16 months post intervention (Figure). The postintervention SER rate was also significantly higher than the pre-intervention rate (P = 0.001).
For the comparator clinics, no significant change in SER rates occurred for the 3 time periods.
Discussion
In this initiative with a 5-year reporting window, we had previously shown that with education and prospective audit and feedback, we could achieve a 10-fold increase in patient SER rates among a multi-practice collaborative while the intervention was maintained [1]. Even though there was a modest but significant increase in the SER rate in the postintervention period for the 6-clinic intervention collaborative compared to baseline, the substantial gains seen during the course of the intervention were not maintained when monthly audit and feedback ceased and monitoring continued for 30 months.
Limitations of this study include possible selection bias resulting from including clinics felt likely to participate rather than identifying clinics in a random fashion. In addition, we did not attempt to determine the specific reasons for the decrease in reporting among these clinics.
The few studies of ambulatory SER do not adequately address the effect of intervention cessation, but researchers who implemented other ambulatory quality improvement efforts have reported that gains often deteriorate or revert to baseline without consistent, ongoing feedback [4]. Likewise, in hospital-based residency programs, a multifaceted approach that includes feedback can increase SER rates, but it is uncertain if the success of this approach can be maintained long-term without continuing feedback of some type [5–7].
There are likely many factors influencing SER in ambulatory clinics, many of which are also applicable in the hospital setting. These include ease of reporting, knowing what events to report, confidentiality of reporting, and the belief that reporting makes a difference in enhancing patient safety [8]. A strong culture of safety in ambulatory clinics may lead to enhanced voluntary SER [9], and a nonpunitive, team-based approach has been advocated to promote reporting and improve ambulatory safety [10]. Historically, our ambulatory medical group clinics have had a strong culture of safety and, with patient safety coaches present in all of our clinics, we have supported a nonpunitive, team-based approach to SER [11].
In our intervention, we made reporting safety events easy, reporters knew which events to report, events could be reported anonymously, and reporters were rewarded, at least with data feedback, for reporting. The only factor known to have changed was discontinuation of monthly feedback. Which factors are most important could not be determined by our work, but we strongly suspect that sustaining enhanced reporting rates requires ongoing audit and feedback to maintain a focus on event reporting.
Corresponding author: Herbert Clegg, MD, 108 Providence Road, Charlotte NC, 28207, [email protected].
Financial disclosures: None.
From Novant Health and Novant Health Medical Group, Winston-Salem, NC (Dr. C
Abstract
- Objective: An educational intervention stressing anonymous, voluntary safety event reporting together with monthly regular audit and feedback led to significantly increased reporting of safety events in a nonacademic, community practice setting during a 15-month intervention period. We assessed whether these increased reporting rates would be sustained during the 30-month period after the intervention was discontinued.
- Methods: We reviewed all patient safety events reported in our ambulatory clinics for the period 2012–2016, and selected 6 clinics that comprised the intervention collaborative and 18 specialty- and size-matched clinics (1:3 match) that comprised the comparator group. To test the changes in safety event reporting (SER) rates between the intervention and postintervention periods for the intervention collaborative, interrupted time series analysis with a control group was performed.
- Results: The SER rate peaked in the first month following the start of the intervention. Following discontinuation of regular auditing and feedback, reporting rates declined abruptly and reverted to baseline by 16 months post intervention.
- Conclusion: It is likely that sustaining enhanced reporting rates requires ongoing audit and feedback to maintain a focus on event reporting.
Keywords: patient safety; safety event reporting; voluntary reporting system; risk management; ambulatory clinic.
We have previously shown that patient safety reporting rates for a 6-practice collaborative group in our non-academic community clinics increased 10-fold after we implemented an improvement initiative consisting of an initial education session followed by provision of monthly audit and written and in-person feedback [1]. The intervention was implemented for 15 months, and after discontinuation of the intervention we have continued to monitor reporting rates. Our objective was to assess whether the increased reporting rates observed in this collaborative during the intervention period would be sustained for 30 months following the intervention.
Methods
This study’s methods have been described in detail previously [1]. For this improvement initiative, we reviewed all patient safety events reported in our ambulatory clinics for the period 2012–2016. We identified 6 clinics, the intervention collaborative, in family medicine (n = 3), pediatrics (n = 2), and general surgery (n = 1), and 18 specialty- and size-matched clinics (1:3 match), the comparator group [1]. For the intervention collaborative only, we provided an initial 1-hour educational session on safety events with a listing of all safety event types, along with a 1-page reporting form for voluntary, anonymous submission, with use of the term “safety event” rather than “ error,” to support a nonpunitive culture. After the educational session, we provided monthly audit and written and in-person feedback with peer comparison data by clinic. Monthly audit and feedback continued throughout the intervention and was discontinued postintervention. For event reporting, in our inpatient and outpatient facilities we used VIncident (Verge Solutions, Mt. Pleasant, SC) for the period 2012–2015 and RL6: Risk (RL Solutions, Toronto, ON) for 2016.
The baseline period was 15 months (January 2012–March 2013), the intervention period was 15 months (April 2013–June 2014), and the postintervention period was 30 months (July 2014–December 2016). All 24 clinics were monitored for the 60-month period.
To test the changes in the rate of safety event reporting (SER) between the pre-intervention and postintervention periods and between the intervention and the postintervention periods, interrupted time series (ITS) analysis with a control group was performed using PROC AUTOREG in SAS Enterprise Guide 6.1 (SAS Institute Inc., Cary, NC). Because SER rates are reported monthly, ITS analysis was used to control for autocorrelation, nonstationary variance, seasonality, and trends [2,3].
Results
The SER rate was assessed monthly, so the number of SER rates for each group (intervention and comparator) was 15 during the pre-intervention and intervention periods, respectively, and 30 during the postintervention period. During the pre-intervention period, the intervention collaborative’s baseline median rate of safety events reported was 1.5 per 10,000 patient encounters (Figure). Also, for the intervention collaborative, the pre-intervention baseline mean (standard deviation, SD) SER rate (per 10,000 patient encounters by month) was 1.3 (1.2), the intervention mean SER rate was 12.0 (7.3), and the postintervention rate was 3.2 (1.8). Based on the ITS analysis, there was a significant change in the SER rate between the intervention and postintervention periods for the intervention collaborative (P = 0.01).
The SER rate peaked in the first month following the start of the intervention. After discontinuation of feedback, reporting rates declined abruptly and reverted to baseline by 16 months post intervention (Figure). The postintervention SER rate was also significantly higher than the pre-intervention rate (P = 0.001).
For the comparator clinics, no significant change in SER rates occurred for the 3 time periods.
Discussion
In this initiative with a 5-year reporting window, we had previously shown that with education and prospective audit and feedback, we could achieve a 10-fold increase in patient SER rates among a multi-practice collaborative while the intervention was maintained [1]. Even though there was a modest but significant increase in the SER rate in the postintervention period for the 6-clinic intervention collaborative compared to baseline, the substantial gains seen during the course of the intervention were not maintained when monthly audit and feedback ceased and monitoring continued for 30 months.
Limitations of this study include possible selection bias resulting from including clinics felt likely to participate rather than identifying clinics in a random fashion. In addition, we did not attempt to determine the specific reasons for the decrease in reporting among these clinics.
The few studies of ambulatory SER do not adequately address the effect of intervention cessation, but researchers who implemented other ambulatory quality improvement efforts have reported that gains often deteriorate or revert to baseline without consistent, ongoing feedback [4]. Likewise, in hospital-based residency programs, a multifaceted approach that includes feedback can increase SER rates, but it is uncertain if the success of this approach can be maintained long-term without continuing feedback of some type [5–7].
There are likely many factors influencing SER in ambulatory clinics, many of which are also applicable in the hospital setting. These include ease of reporting, knowing what events to report, confidentiality of reporting, and the belief that reporting makes a difference in enhancing patient safety [8]. A strong culture of safety in ambulatory clinics may lead to enhanced voluntary SER [9], and a nonpunitive, team-based approach has been advocated to promote reporting and improve ambulatory safety [10]. Historically, our ambulatory medical group clinics have had a strong culture of safety and, with patient safety coaches present in all of our clinics, we have supported a nonpunitive, team-based approach to SER [11].
In our intervention, we made reporting safety events easy, reporters knew which events to report, events could be reported anonymously, and reporters were rewarded, at least with data feedback, for reporting. The only factor known to have changed was discontinuation of monthly feedback. Which factors are most important could not be determined by our work, but we strongly suspect that sustaining enhanced reporting rates requires ongoing audit and feedback to maintain a focus on event reporting.
Corresponding author: Herbert Clegg, MD, 108 Providence Road, Charlotte NC, 28207, [email protected].
Financial disclosures: None.
1. Clegg HW, Cardwell T, West AM, Ferrell F. Improved safety event reporting in outpatient, nonacademic practices with an anonymous, nonpunitive approach. J Clin Outcomes Manag 2015;22:66–72.
2. Newland JG, Stach LM, De Lurgio SA, et al. Impact of a prospective-audit-with-feedback antimicrobial stewardship program at a children’s hospital. J Pediatric Infect Dis Soc 2012; 1:179–86.
3. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr 2013;13 (6 Suppl):S38–44.
4. Gerber JS, Prasad PA, Fiks AG, et al. Durability of benefits of an outpatient antimicrobial stewardship intervention after discontinuation of audit and feedback. JAMA 2014;312:2569–70.
5. Steen S, Jaeger C, Price L, Griffen D. Increasing patient safety event reporting in an emergency medicine residency. BMJ Qual Improv Rep 2017;6(1).
6. Fox M, Bump G, Butler G, et al. Making residents part of the safety culture: improving error reporting and reducing harms. J Patient Saf 2017. [Epub ahead of print]
7. Dunbar AE 3rd, Cupit M, Vath RJ, et al. An improvement approach to integrate teaching teams in the reporting of safety events. Pediatrics 2017;139:e20153807.
8. Institute of Medicine. To err is human: Building a safer health system. National Academies. www.nationalacademies.org/hmd/~/media/Files/Report%20Files/1999/To-Err-is-Human/To%20Err%20is%20Human%201999%20%20report%20brief.pdf Published November 1999. Accessed August 22, 2018.
9. Miller N, Bhowmik S, Ezinwa M, et al. The relationship between safety culture and voluntary event reporting in a large regional ambulatory care group. J Patient Saf 2017. [Epub ahead of print]
10. Neuspiel DR, Stubbs EH. Patient safety in ambulatory care. Pediatr Clin North Am 2012;59:1341–54.
11. West AM, Cardwell T, Clegg HW. Improving patient safety culture through patient safety coaches in the ambulatory setting. Presented at: Institute for Healthcare Improvement Annual Summit on Improving Patient Care in the Office Practice and the Community; March 2015; Dallas, Texas.
1. Clegg HW, Cardwell T, West AM, Ferrell F. Improved safety event reporting in outpatient, nonacademic practices with an anonymous, nonpunitive approach. J Clin Outcomes Manag 2015;22:66–72.
2. Newland JG, Stach LM, De Lurgio SA, et al. Impact of a prospective-audit-with-feedback antimicrobial stewardship program at a children’s hospital. J Pediatric Infect Dis Soc 2012; 1:179–86.
3. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr 2013;13 (6 Suppl):S38–44.
4. Gerber JS, Prasad PA, Fiks AG, et al. Durability of benefits of an outpatient antimicrobial stewardship intervention after discontinuation of audit and feedback. JAMA 2014;312:2569–70.
5. Steen S, Jaeger C, Price L, Griffen D. Increasing patient safety event reporting in an emergency medicine residency. BMJ Qual Improv Rep 2017;6(1).
6. Fox M, Bump G, Butler G, et al. Making residents part of the safety culture: improving error reporting and reducing harms. J Patient Saf 2017. [Epub ahead of print]
7. Dunbar AE 3rd, Cupit M, Vath RJ, et al. An improvement approach to integrate teaching teams in the reporting of safety events. Pediatrics 2017;139:e20153807.
8. Institute of Medicine. To err is human: Building a safer health system. National Academies. www.nationalacademies.org/hmd/~/media/Files/Report%20Files/1999/To-Err-is-Human/To%20Err%20is%20Human%201999%20%20report%20brief.pdf Published November 1999. Accessed August 22, 2018.
9. Miller N, Bhowmik S, Ezinwa M, et al. The relationship between safety culture and voluntary event reporting in a large regional ambulatory care group. J Patient Saf 2017. [Epub ahead of print]
10. Neuspiel DR, Stubbs EH. Patient safety in ambulatory care. Pediatr Clin North Am 2012;59:1341–54.
11. West AM, Cardwell T, Clegg HW. Improving patient safety culture through patient safety coaches in the ambulatory setting. Presented at: Institute for Healthcare Improvement Annual Summit on Improving Patient Care in the Office Practice and the Community; March 2015; Dallas, Texas.
Utilization of Primary Care Physicians by Medical Residents: A Survey-Based Study
From the University of Michigan Medical School, Ann Arbor, MI.
Abstract
- Objective: Existing research has demonstrated overall low rates of residents establishing care with a primary care physician (PCP). We conducted a survey-based study to better understand chronic illness, PCP utilization, and prescription medication use patterns in resident physician populations.
- Methods: In 2017, we invited internal and family medicine trainees from a convenience sample of U.S. residency programs to participate in a survey. We compared the characteristics of residents who had established care with a PCP to those who had not.
- Results: The response rate was 45% (348/766 residents). The majority (n = 205, 59%) of respondents stated they had established care with a PCP primarily for routine preventative care (n = 159, 79%) and access in the event of an emergency (n = 132, 66%). However, 31% (n = 103) denied having had a wellness visit in over 3 years. Nearly a quarter of residents (n = 77, 23%) reported a chronic medical illness and 14% (n = 45) reported a preexisting mental health condition prior to residency. One-third (n = 111, 33%) reported taking a long-term prescription medication. Compared to residents who had not established care, those with a PCP (n = 205) more often reported a chronic condition (P < 0.001), seeing a subspecialist (P = 0.01), or taking long-term prescription medications (P < 0.001). One in 5 (n = 62,19%) respondents reported receiving prescriptions for an acute illness from an individual with whom they did not have a doctor-patient relationship.
- Conclusion: Medical residents have a substantial burden of chronic illness that may not be met through interactions with PCPs. Further understanding their medical needs and barriers to accessing care is necessary to ensure trainee well-being.
Keywords: Medical education-graduate, physician behavior, survey research, access to care.
Although internal medicine (IM) and family medicine (FM) residents must learn to provide high-quality primary care to their patients, little is known about whether they appropriately access such care themselves. Resident burnout and resilience has received attention [1,2], but there has been limited focus on understanding the burden of chronic medical and mental illness among residents. In particular, little is known about whether residents access primary care physicians (PCPs)—for either acute or chronic medical needs—and about resident self-medication practices.
Residency is often characterized by a life-changing geographic relocation. Even residents who do not relocate may still need to establish care with a new PCP due to health insurance or loss of access to a student clinic [3]. Establishing primary care with a new doctor typically requires scheduling a new patient visit, often with a wait time of several days to weeks [4,5]. Furthermore, lack of time, erratic schedules, and concerns about privacy and the stigma of being ill as a physician are barriers to establishing care [6-8]. Individuals who have not established primary care may experience delays in routine preventative health services, screening for chronic medical and mental health conditions, as well as access to care during acute illnesses [9,10]. Worse, they may engage in potentially unsafe practices, such as having colleagues write prescriptions for them, or even self-prescribing [8,11,12].
Existing research has demonstrated overall low rates of residents establishing care with a PCP [6–8,13]. However, these studies have either been limited to large academic centers or conducted outside the United States. Improving resident well-being may prove challenging without a clear understanding of current primary care utilization practices, the burden of chronic illness among residents, and patterns of prescription medication use and needs. Therefore, we conducted a survey-based study to understand primary care utilization and the burden of chronic illness among residents. We also assessed whether lack of primary care is associated with potentially risky behaviors, such as self-prescribing of medications.
Methods
Study Setting and Participants
The survey was distributed to current residents at IM and FM programs within the United States in 2017. Individual programs were recruited by directly contacting program directors or chief medical residents via email. Rather than contacting sites directly through standard templated emails, we identified programs both through personal contacts as well as the Electronic Residency Application Service list of accredited IM training programs. We elected to use this approach in order to increase response rates and to ensure that a sample representative of the trainee population was constructed. Programs were located in the Northeast, Midwest, South, and Pacific regions, and included small community-based programs and large academic centers.
Development of the Survey
The survey instrument was developed by the authors and reviewed by residents and PCPs at the University of Michigan to ensure relevance and comprehension of questions (The survey is available in the Appendix.). Once finalized, the survey was programmed into an online survey tool (Qualtrics, Provo, UT) and pilot-tested before being disseminated to the sampling frame. Data collected in the survey included: respondent’s utilization of a PCP, burden of chronic illness, long-term prescription medications, prescribing source, and demographic characteristics.
Each participating program distributed the survey to their residents through an email containing an anonymous hyperlink. The survey was available for completion for 4 weeks. We asked participating programs to send email reminders to encourage participation. Participants were given the option of receiving a $10 Amazon gift card after completion. All responses were recorded anonymously. The study received a “not regulated” status by the University of Michigan Institutional Review Board (HUM 00123888).
Statistical Analysis
Descriptive statistics were used to tabulate results. Respondents were encouraged, but not required, to answer all questions. Therefore, the response rate for each question was calculated using the total number of responses for that question as the denominator. Bivariable comparisons were made using Chi-squared or Fisher’s exact tests, as appropriate, for categorical data. A P value < 0.05, with 2-sided alpha, was considered statistically significant. All statistical analyses were conducted using Stata 13 SE (StataCorp, College Station, TX).
Results
Respondent Characteristics
Of the 29 programs contacted, 10 agreed to participate within the study timeframe. Of 766 potential respondents, 348 (45%) residents answered the survey (Table 1). The majority of respondents (n = 276, 82%) were from IM programs. Respondents were from all training years as follows: postgraduate year 1 residents (PGY-1, or interns; n = 130, 39%), PGY-2 residents (n = 98, 29%), PGY-3 residents (n = 93, 28%), and PGY-4 residents (n = 12, 4%). Most respondents were from the South (n = 130, 39%) and Midwest (n = 123, 37%) regions, and over half (n = 179, 54%) were female. Most respondents (n = 285, 86%) stated that they did not have children. The majority (n = 236, 71%) were completing residency in an area where they had not previously lived for more than 1 year.
Primary Care Utilization
Among the 348 respondents, 59% (n = 205) reported having established care with a PCP. An additional 6% (n = 21) had established care with an obstetrician/gynecologist for routine needs (Table 2). The 2 most common reasons for establishing care with a PCP were routine primary care needs, including contraception (n = 159, 79%), and access to a physician in the event of an acute medical need (n = 132, 66%).
Among respondents who had established care with a PCP, most (n = 188, 94%) had completed at least 1 appointment. However, among these 188 respondents, 68% (n = 127) stated that they had not made an acute visit in more than 12 months. When asked about wellness visits, almost one third of respondents (n = 103, 31%) stated that they had not been seen for a wellness visit in the past 3 years.
Burden of Chronic Illness
Most respondents (n = 223, 67%) stated that they did not have a chronic medical or mental health condition prior to residency (Table 3). However, 23% (n = 77) of respondents stated that they had been diagnosed with a chronic medical illness prior to residency, and 14% (n = 45) indicated they had been diagnosed with a mental health condition prior to residency. Almost one fifth of respondents (n = 60, 18%) reported seeing a subspecialist for a medical illness, and 33% (n = 111) reported taking a long-term prescription medication. With respect to major medical issues, the majority of residents (n = 239, 72%) denied experiencing events such as pregnancy, hospitalization, surgery, or an emergency department (ED) visit during training.
[polldaddy:10116940]
Inappropriate Prescriptions
While the majority of respondents denied writing a prescription for themselves for an acute or chronic medical condition, almost one fifth (n = 62, 19%) had received a prescription for an acute medical need from a provider outside of a clinical relationship (ie, from someone other than their PCP or specialty provider). Notably, 5% (n = 15) reported that this had occurred at least 2 or 3 times in the past 12 months (Table 4). Compared to respondents not taking long-term prescription medications, respondents who were already taking long-term prescription medications more frequently reported inappropriately receiving chronic prescriptions outside of an established clinical relationship (n = 14, 13% vs. n = 14, 6%; P = 0.05) and more often self-prescribed medications for acute needs (n = 12, 11% vs. n = 7, 3%; P = 0.005).
Comparison of Residents With and Without a PCP
Important differences were noted between residents who had a PCP versus those who did not (Table 5). For example, a higher percentage of residents with a PCP indicated they had been diagnosed with a chronic medical illness (n = 55, 28% vs. n = 22, 16%; P = 0.01) or a chronic mental health condition (n = 34, 17% vs. n = 11, 8%; P = 0.02) before residency. Additionally, a higher percentage of residents with a PCP (n = 70, 35% vs. n = 25, 18%; P = 0.001) reported experiencing medical events such as pregnancy, hospitalization, surgery, ED visit, or new diagnosis of a chronic medical illness during residency. Finally, a higher percentage of respondents with a PCP stated that they had visited a subspecialist for a medical illness (n = 44, 22% vs. n = 16,12%; P = 0.01) or were taking long-term prescription medications (n = 86, 43% vs. n = 25; 18%; P < 0.001). When comparing PGY-1 to PGY-2–PGY-4 residents, the former reported having established a medical relationship with a PCP significantly less frequently (n = 56, 43% vs. n = 142, 70%; P < 0.001).
Discussion
This survey-based study of medical residents across the United States suggests that a substantial proportion do not establish relationships with PCPs. Additionally, our data suggest that despite establishing care, few residents subsequently visited their PCP during training for wellness visits or routine care. Self-reported rates of chronic medical and mental health conditions were substantial in our sample. Furthermore, inappropriate self-prescription and the receipt of prescriptions outside of a medical relationship were also reported. These findings suggest that future studies that focus on the unique medical and mental health needs of physicians in training, as well as interventions to encourage care in this vulnerable period, are necessary.
We observed that most respondents that established primary care were female trainees. Although it is impossible to know with certainty, one hypothesis behind this discrepancy is that women routinely need to access preventative care for gynecologic needs such as pap smears, contraception, and potentially pregnancy and preconception counseling [14,15]. Similarly, residents with a chronic medical or mental health condition prior to residency established care with a local PCP at a significantly greater frequency than those without such diagnoses. While selection bias cannot be excluded, this finding suggests that illness is a driving factor in establishing care. There also appears to be an association between accessing the medical system (either for prescription medications or subspecialist care) and having established care with a PCP. Collectively, these data suggest that individuals without a compelling reason to access medical services might have barriers to accessing care in the event of medical needs or may not receive routine preventative care [9,10].
In general, we found that rates of reported inappropriate prescriptions were lower than those reported in prior studies where a comparable resident population was surveyed [8,12,16]. Inclusion of multiple institutions, differences in temporality, social desirability bias, and reporting bias might have influenced our findings in this regard. Surprisingly, we found that having a PCP did not influence likelihood of inappropriate prescription receipt, perhaps suggesting that this behavior reflects some degree of universal difficulty in accessing care. Alternatively, this finding might relate to a cultural tendency to self-prescribe among resident physicians. The fact that individuals on chronic medications more often both received and wrote inappropriate prescriptions suggests this problem might be more pronounced in individuals who take medications more often, as these residents have specific needs [12]. Future studies targeting these individuals thus appear warranted.
Our study has several limitations. First, our sample size was modest and the response rate of 45% was low. However, to our knowledge, this remains among the largest survey on this topic, and our response rate is comparable to similar trainee studies [8,11,13]. Second, we designed and created a novel survey for this study. While the questions were pilot-tested with users prior to dissemination, validation of the instrument was not performed. Third, since the study population was restricted to residents in fields that participate in primary care, our findings may not be generalizable to patterns of PCP use in other specialties [6].
These limitations aside, our study has important strengths. This is the first national study of its kind with specific questions addressing primary care access and utilization, prescription medication use and related practices, and the prevalence of medical conditions among trainees. Important differences in the rates of establishing primary care between male and female respondents, first- year and senior residents, and those with and without chronic disease suggest a need to target specific resident groups (males, interns, those without pre-existing conditions) for wellness-related interventions. Such interventions could include distribution of a list of local providers to first year residents, advanced protected time for doctor’s appointments, and safeguards to ensure health information is protected from potential supervisors. Future studies should also include residents from non-primary care oriented specialties such as surgery, emergency medicine, and anesthesiology to obtain results that are more generalizable to the resident population as a whole. Additionally, the rates of inappropriate prescriptions were not insignificant and warrant further evaluation of the driving forces behind these behaviors.
Conclusion
Medical residents have a substantial burden of chronic illness that may not be met through interactions with PCPs. More research into barriers that residents face while accessing care and an assessment of interventions to facilitate their access to care is important to promote trainee well-being. Without such direction and initiative, it may prove harder for physicians to heal themselves or those for whom they provide care.
Acknowledgments: We thank Suzanne Winter, the study coordinator, for her support with editing and formatting the manuscript, Latoya Kuhn for performing the statistical analysis and creating data tables, and Dr. Namita Sachdev and Dr. Renuka Tipirneni for providing feedback on the survey instrument. We also thank the involved programs for their participation.
Corresponding author: Vineet Chopra, NCRC 2800 Plymouth Rd., Bldg 16, 432, Ann Arbor, MI 48109, [email protected].
Financial disclosures: None.
Previous presentations: Results were presented at the Annual Michigan Medicine 2017 Internal Medicine Research Symposium.
1. Kassam A, Horton J, Shoimer I, Patten S. Predictors of well-being in resident physicians: a descriptive and psychometric study. J Grad Med Educ 2015;7:70–4.
2. Shanafelt TD, Bradley KA, Wipf JE, Back AL. Burnout and self-reported patient care in an internal medicine residency program. Ann Intern Med 2002;136:358–67.
3. Burstin HR, Swartz K, O’Neil AC, et al. The effect of change of health insurance on access to care. Inquiry 1998;35:389–97.
4. Rhodes KV, Basseyn S, Friedman AB, et al. Access to primary care appointments following 2014 insurance expansions. Ann Fam Med 2017;15:107–12.
5. Polsky D, Richards M, Basseyn S, et al. Appointment availability after increases in Medicaid payments for primary care. N Engl J Med 2015;372:537–45.
6. Gupta G, Schleinitz MD, Reinert SE, McGarry KA. Resident physician preventive health behaviors and perspectives on primary care. R I Med J (2013) 2013;96:43–7.
7. Rosen IM, Christine JD, Bellini LM, Asch DA. Health and health care among housestaff in four U.S. internal medicine residency programs. J Gen Intern Med 2000;15:116-21.
8. Campbell S, Delva D. Physician do not heal thyself. Survey of personal health practices among medical residents. Can Fam Physician 2003;49:1121–7.
9. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q 2005;83(3):457-502.
10. Weissman JS, Stern R, Fielding SL, et al. Delayed access to health care: risk factors, reasons, and consequences. Ann Intern Med 1991;114:325–31.
11. Guille C, Sen S. Prescription drug use and self-prescription among training physicians. Arch Intern Med 2012;172:371–2.
12. Roberts LW, Kim JP. Informal health care practices of residents: “curbside” consultation and self-diagnosis and treatment. Acad Psychiatry 2015;39:22-30.
13. Cohen JS, Patten S. Well-being in residency training: a survey examining resident physician satisfaction both within and outside of residency training and mental health in Alberta. BMC Med Educ 2005;5:21.
14. U.S. Preventive Services Task Force. Cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening. Published March 2012. Accessed August 21, 2018.
15. Health Resources and Services Administration. Women’s preventative services guidelines. https://www.hrsa.gov/womensguidelines2016/index.html. Updated October 2017. Accessed August 21, 2018.
16. Christie JD, Rosen IM, Bellini LM, et al. Prescription drug use and self-prescription among resident physicians. JAMA 1998;280(14):1253–5.
From the University of Michigan Medical School, Ann Arbor, MI.
Abstract
- Objective: Existing research has demonstrated overall low rates of residents establishing care with a primary care physician (PCP). We conducted a survey-based study to better understand chronic illness, PCP utilization, and prescription medication use patterns in resident physician populations.
- Methods: In 2017, we invited internal and family medicine trainees from a convenience sample of U.S. residency programs to participate in a survey. We compared the characteristics of residents who had established care with a PCP to those who had not.
- Results: The response rate was 45% (348/766 residents). The majority (n = 205, 59%) of respondents stated they had established care with a PCP primarily for routine preventative care (n = 159, 79%) and access in the event of an emergency (n = 132, 66%). However, 31% (n = 103) denied having had a wellness visit in over 3 years. Nearly a quarter of residents (n = 77, 23%) reported a chronic medical illness and 14% (n = 45) reported a preexisting mental health condition prior to residency. One-third (n = 111, 33%) reported taking a long-term prescription medication. Compared to residents who had not established care, those with a PCP (n = 205) more often reported a chronic condition (P < 0.001), seeing a subspecialist (P = 0.01), or taking long-term prescription medications (P < 0.001). One in 5 (n = 62,19%) respondents reported receiving prescriptions for an acute illness from an individual with whom they did not have a doctor-patient relationship.
- Conclusion: Medical residents have a substantial burden of chronic illness that may not be met through interactions with PCPs. Further understanding their medical needs and barriers to accessing care is necessary to ensure trainee well-being.
Keywords: Medical education-graduate, physician behavior, survey research, access to care.
Although internal medicine (IM) and family medicine (FM) residents must learn to provide high-quality primary care to their patients, little is known about whether they appropriately access such care themselves. Resident burnout and resilience has received attention [1,2], but there has been limited focus on understanding the burden of chronic medical and mental illness among residents. In particular, little is known about whether residents access primary care physicians (PCPs)—for either acute or chronic medical needs—and about resident self-medication practices.
Residency is often characterized by a life-changing geographic relocation. Even residents who do not relocate may still need to establish care with a new PCP due to health insurance or loss of access to a student clinic [3]. Establishing primary care with a new doctor typically requires scheduling a new patient visit, often with a wait time of several days to weeks [4,5]. Furthermore, lack of time, erratic schedules, and concerns about privacy and the stigma of being ill as a physician are barriers to establishing care [6-8]. Individuals who have not established primary care may experience delays in routine preventative health services, screening for chronic medical and mental health conditions, as well as access to care during acute illnesses [9,10]. Worse, they may engage in potentially unsafe practices, such as having colleagues write prescriptions for them, or even self-prescribing [8,11,12].
Existing research has demonstrated overall low rates of residents establishing care with a PCP [6–8,13]. However, these studies have either been limited to large academic centers or conducted outside the United States. Improving resident well-being may prove challenging without a clear understanding of current primary care utilization practices, the burden of chronic illness among residents, and patterns of prescription medication use and needs. Therefore, we conducted a survey-based study to understand primary care utilization and the burden of chronic illness among residents. We also assessed whether lack of primary care is associated with potentially risky behaviors, such as self-prescribing of medications.
Methods
Study Setting and Participants
The survey was distributed to current residents at IM and FM programs within the United States in 2017. Individual programs were recruited by directly contacting program directors or chief medical residents via email. Rather than contacting sites directly through standard templated emails, we identified programs both through personal contacts as well as the Electronic Residency Application Service list of accredited IM training programs. We elected to use this approach in order to increase response rates and to ensure that a sample representative of the trainee population was constructed. Programs were located in the Northeast, Midwest, South, and Pacific regions, and included small community-based programs and large academic centers.
Development of the Survey
The survey instrument was developed by the authors and reviewed by residents and PCPs at the University of Michigan to ensure relevance and comprehension of questions (The survey is available in the Appendix.). Once finalized, the survey was programmed into an online survey tool (Qualtrics, Provo, UT) and pilot-tested before being disseminated to the sampling frame. Data collected in the survey included: respondent’s utilization of a PCP, burden of chronic illness, long-term prescription medications, prescribing source, and demographic characteristics.
Each participating program distributed the survey to their residents through an email containing an anonymous hyperlink. The survey was available for completion for 4 weeks. We asked participating programs to send email reminders to encourage participation. Participants were given the option of receiving a $10 Amazon gift card after completion. All responses were recorded anonymously. The study received a “not regulated” status by the University of Michigan Institutional Review Board (HUM 00123888).
Statistical Analysis
Descriptive statistics were used to tabulate results. Respondents were encouraged, but not required, to answer all questions. Therefore, the response rate for each question was calculated using the total number of responses for that question as the denominator. Bivariable comparisons were made using Chi-squared or Fisher’s exact tests, as appropriate, for categorical data. A P value < 0.05, with 2-sided alpha, was considered statistically significant. All statistical analyses were conducted using Stata 13 SE (StataCorp, College Station, TX).
Results
Respondent Characteristics
Of the 29 programs contacted, 10 agreed to participate within the study timeframe. Of 766 potential respondents, 348 (45%) residents answered the survey (Table 1). The majority of respondents (n = 276, 82%) were from IM programs. Respondents were from all training years as follows: postgraduate year 1 residents (PGY-1, or interns; n = 130, 39%), PGY-2 residents (n = 98, 29%), PGY-3 residents (n = 93, 28%), and PGY-4 residents (n = 12, 4%). Most respondents were from the South (n = 130, 39%) and Midwest (n = 123, 37%) regions, and over half (n = 179, 54%) were female. Most respondents (n = 285, 86%) stated that they did not have children. The majority (n = 236, 71%) were completing residency in an area where they had not previously lived for more than 1 year.
Primary Care Utilization
Among the 348 respondents, 59% (n = 205) reported having established care with a PCP. An additional 6% (n = 21) had established care with an obstetrician/gynecologist for routine needs (Table 2). The 2 most common reasons for establishing care with a PCP were routine primary care needs, including contraception (n = 159, 79%), and access to a physician in the event of an acute medical need (n = 132, 66%).
Among respondents who had established care with a PCP, most (n = 188, 94%) had completed at least 1 appointment. However, among these 188 respondents, 68% (n = 127) stated that they had not made an acute visit in more than 12 months. When asked about wellness visits, almost one third of respondents (n = 103, 31%) stated that they had not been seen for a wellness visit in the past 3 years.
Burden of Chronic Illness
Most respondents (n = 223, 67%) stated that they did not have a chronic medical or mental health condition prior to residency (Table 3). However, 23% (n = 77) of respondents stated that they had been diagnosed with a chronic medical illness prior to residency, and 14% (n = 45) indicated they had been diagnosed with a mental health condition prior to residency. Almost one fifth of respondents (n = 60, 18%) reported seeing a subspecialist for a medical illness, and 33% (n = 111) reported taking a long-term prescription medication. With respect to major medical issues, the majority of residents (n = 239, 72%) denied experiencing events such as pregnancy, hospitalization, surgery, or an emergency department (ED) visit during training.
[polldaddy:10116940]
Inappropriate Prescriptions
While the majority of respondents denied writing a prescription for themselves for an acute or chronic medical condition, almost one fifth (n = 62, 19%) had received a prescription for an acute medical need from a provider outside of a clinical relationship (ie, from someone other than their PCP or specialty provider). Notably, 5% (n = 15) reported that this had occurred at least 2 or 3 times in the past 12 months (Table 4). Compared to respondents not taking long-term prescription medications, respondents who were already taking long-term prescription medications more frequently reported inappropriately receiving chronic prescriptions outside of an established clinical relationship (n = 14, 13% vs. n = 14, 6%; P = 0.05) and more often self-prescribed medications for acute needs (n = 12, 11% vs. n = 7, 3%; P = 0.005).
Comparison of Residents With and Without a PCP
Important differences were noted between residents who had a PCP versus those who did not (Table 5). For example, a higher percentage of residents with a PCP indicated they had been diagnosed with a chronic medical illness (n = 55, 28% vs. n = 22, 16%; P = 0.01) or a chronic mental health condition (n = 34, 17% vs. n = 11, 8%; P = 0.02) before residency. Additionally, a higher percentage of residents with a PCP (n = 70, 35% vs. n = 25, 18%; P = 0.001) reported experiencing medical events such as pregnancy, hospitalization, surgery, ED visit, or new diagnosis of a chronic medical illness during residency. Finally, a higher percentage of respondents with a PCP stated that they had visited a subspecialist for a medical illness (n = 44, 22% vs. n = 16,12%; P = 0.01) or were taking long-term prescription medications (n = 86, 43% vs. n = 25; 18%; P < 0.001). When comparing PGY-1 to PGY-2–PGY-4 residents, the former reported having established a medical relationship with a PCP significantly less frequently (n = 56, 43% vs. n = 142, 70%; P < 0.001).
Discussion
This survey-based study of medical residents across the United States suggests that a substantial proportion do not establish relationships with PCPs. Additionally, our data suggest that despite establishing care, few residents subsequently visited their PCP during training for wellness visits or routine care. Self-reported rates of chronic medical and mental health conditions were substantial in our sample. Furthermore, inappropriate self-prescription and the receipt of prescriptions outside of a medical relationship were also reported. These findings suggest that future studies that focus on the unique medical and mental health needs of physicians in training, as well as interventions to encourage care in this vulnerable period, are necessary.
We observed that most respondents that established primary care were female trainees. Although it is impossible to know with certainty, one hypothesis behind this discrepancy is that women routinely need to access preventative care for gynecologic needs such as pap smears, contraception, and potentially pregnancy and preconception counseling [14,15]. Similarly, residents with a chronic medical or mental health condition prior to residency established care with a local PCP at a significantly greater frequency than those without such diagnoses. While selection bias cannot be excluded, this finding suggests that illness is a driving factor in establishing care. There also appears to be an association between accessing the medical system (either for prescription medications or subspecialist care) and having established care with a PCP. Collectively, these data suggest that individuals without a compelling reason to access medical services might have barriers to accessing care in the event of medical needs or may not receive routine preventative care [9,10].
In general, we found that rates of reported inappropriate prescriptions were lower than those reported in prior studies where a comparable resident population was surveyed [8,12,16]. Inclusion of multiple institutions, differences in temporality, social desirability bias, and reporting bias might have influenced our findings in this regard. Surprisingly, we found that having a PCP did not influence likelihood of inappropriate prescription receipt, perhaps suggesting that this behavior reflects some degree of universal difficulty in accessing care. Alternatively, this finding might relate to a cultural tendency to self-prescribe among resident physicians. The fact that individuals on chronic medications more often both received and wrote inappropriate prescriptions suggests this problem might be more pronounced in individuals who take medications more often, as these residents have specific needs [12]. Future studies targeting these individuals thus appear warranted.
Our study has several limitations. First, our sample size was modest and the response rate of 45% was low. However, to our knowledge, this remains among the largest survey on this topic, and our response rate is comparable to similar trainee studies [8,11,13]. Second, we designed and created a novel survey for this study. While the questions were pilot-tested with users prior to dissemination, validation of the instrument was not performed. Third, since the study population was restricted to residents in fields that participate in primary care, our findings may not be generalizable to patterns of PCP use in other specialties [6].
These limitations aside, our study has important strengths. This is the first national study of its kind with specific questions addressing primary care access and utilization, prescription medication use and related practices, and the prevalence of medical conditions among trainees. Important differences in the rates of establishing primary care between male and female respondents, first- year and senior residents, and those with and without chronic disease suggest a need to target specific resident groups (males, interns, those without pre-existing conditions) for wellness-related interventions. Such interventions could include distribution of a list of local providers to first year residents, advanced protected time for doctor’s appointments, and safeguards to ensure health information is protected from potential supervisors. Future studies should also include residents from non-primary care oriented specialties such as surgery, emergency medicine, and anesthesiology to obtain results that are more generalizable to the resident population as a whole. Additionally, the rates of inappropriate prescriptions were not insignificant and warrant further evaluation of the driving forces behind these behaviors.
Conclusion
Medical residents have a substantial burden of chronic illness that may not be met through interactions with PCPs. More research into barriers that residents face while accessing care and an assessment of interventions to facilitate their access to care is important to promote trainee well-being. Without such direction and initiative, it may prove harder for physicians to heal themselves or those for whom they provide care.
Acknowledgments: We thank Suzanne Winter, the study coordinator, for her support with editing and formatting the manuscript, Latoya Kuhn for performing the statistical analysis and creating data tables, and Dr. Namita Sachdev and Dr. Renuka Tipirneni for providing feedback on the survey instrument. We also thank the involved programs for their participation.
Corresponding author: Vineet Chopra, NCRC 2800 Plymouth Rd., Bldg 16, 432, Ann Arbor, MI 48109, [email protected].
Financial disclosures: None.
Previous presentations: Results were presented at the Annual Michigan Medicine 2017 Internal Medicine Research Symposium.
From the University of Michigan Medical School, Ann Arbor, MI.
Abstract
- Objective: Existing research has demonstrated overall low rates of residents establishing care with a primary care physician (PCP). We conducted a survey-based study to better understand chronic illness, PCP utilization, and prescription medication use patterns in resident physician populations.
- Methods: In 2017, we invited internal and family medicine trainees from a convenience sample of U.S. residency programs to participate in a survey. We compared the characteristics of residents who had established care with a PCP to those who had not.
- Results: The response rate was 45% (348/766 residents). The majority (n = 205, 59%) of respondents stated they had established care with a PCP primarily for routine preventative care (n = 159, 79%) and access in the event of an emergency (n = 132, 66%). However, 31% (n = 103) denied having had a wellness visit in over 3 years. Nearly a quarter of residents (n = 77, 23%) reported a chronic medical illness and 14% (n = 45) reported a preexisting mental health condition prior to residency. One-third (n = 111, 33%) reported taking a long-term prescription medication. Compared to residents who had not established care, those with a PCP (n = 205) more often reported a chronic condition (P < 0.001), seeing a subspecialist (P = 0.01), or taking long-term prescription medications (P < 0.001). One in 5 (n = 62,19%) respondents reported receiving prescriptions for an acute illness from an individual with whom they did not have a doctor-patient relationship.
- Conclusion: Medical residents have a substantial burden of chronic illness that may not be met through interactions with PCPs. Further understanding their medical needs and barriers to accessing care is necessary to ensure trainee well-being.
Keywords: Medical education-graduate, physician behavior, survey research, access to care.
Although internal medicine (IM) and family medicine (FM) residents must learn to provide high-quality primary care to their patients, little is known about whether they appropriately access such care themselves. Resident burnout and resilience has received attention [1,2], but there has been limited focus on understanding the burden of chronic medical and mental illness among residents. In particular, little is known about whether residents access primary care physicians (PCPs)—for either acute or chronic medical needs—and about resident self-medication practices.
Residency is often characterized by a life-changing geographic relocation. Even residents who do not relocate may still need to establish care with a new PCP due to health insurance or loss of access to a student clinic [3]. Establishing primary care with a new doctor typically requires scheduling a new patient visit, often with a wait time of several days to weeks [4,5]. Furthermore, lack of time, erratic schedules, and concerns about privacy and the stigma of being ill as a physician are barriers to establishing care [6-8]. Individuals who have not established primary care may experience delays in routine preventative health services, screening for chronic medical and mental health conditions, as well as access to care during acute illnesses [9,10]. Worse, they may engage in potentially unsafe practices, such as having colleagues write prescriptions for them, or even self-prescribing [8,11,12].
Existing research has demonstrated overall low rates of residents establishing care with a PCP [6–8,13]. However, these studies have either been limited to large academic centers or conducted outside the United States. Improving resident well-being may prove challenging without a clear understanding of current primary care utilization practices, the burden of chronic illness among residents, and patterns of prescription medication use and needs. Therefore, we conducted a survey-based study to understand primary care utilization and the burden of chronic illness among residents. We also assessed whether lack of primary care is associated with potentially risky behaviors, such as self-prescribing of medications.
Methods
Study Setting and Participants
The survey was distributed to current residents at IM and FM programs within the United States in 2017. Individual programs were recruited by directly contacting program directors or chief medical residents via email. Rather than contacting sites directly through standard templated emails, we identified programs both through personal contacts as well as the Electronic Residency Application Service list of accredited IM training programs. We elected to use this approach in order to increase response rates and to ensure that a sample representative of the trainee population was constructed. Programs were located in the Northeast, Midwest, South, and Pacific regions, and included small community-based programs and large academic centers.
Development of the Survey
The survey instrument was developed by the authors and reviewed by residents and PCPs at the University of Michigan to ensure relevance and comprehension of questions (The survey is available in the Appendix.). Once finalized, the survey was programmed into an online survey tool (Qualtrics, Provo, UT) and pilot-tested before being disseminated to the sampling frame. Data collected in the survey included: respondent’s utilization of a PCP, burden of chronic illness, long-term prescription medications, prescribing source, and demographic characteristics.
Each participating program distributed the survey to their residents through an email containing an anonymous hyperlink. The survey was available for completion for 4 weeks. We asked participating programs to send email reminders to encourage participation. Participants were given the option of receiving a $10 Amazon gift card after completion. All responses were recorded anonymously. The study received a “not regulated” status by the University of Michigan Institutional Review Board (HUM 00123888).
Statistical Analysis
Descriptive statistics were used to tabulate results. Respondents were encouraged, but not required, to answer all questions. Therefore, the response rate for each question was calculated using the total number of responses for that question as the denominator. Bivariable comparisons were made using Chi-squared or Fisher’s exact tests, as appropriate, for categorical data. A P value < 0.05, with 2-sided alpha, was considered statistically significant. All statistical analyses were conducted using Stata 13 SE (StataCorp, College Station, TX).
Results
Respondent Characteristics
Of the 29 programs contacted, 10 agreed to participate within the study timeframe. Of 766 potential respondents, 348 (45%) residents answered the survey (Table 1). The majority of respondents (n = 276, 82%) were from IM programs. Respondents were from all training years as follows: postgraduate year 1 residents (PGY-1, or interns; n = 130, 39%), PGY-2 residents (n = 98, 29%), PGY-3 residents (n = 93, 28%), and PGY-4 residents (n = 12, 4%). Most respondents were from the South (n = 130, 39%) and Midwest (n = 123, 37%) regions, and over half (n = 179, 54%) were female. Most respondents (n = 285, 86%) stated that they did not have children. The majority (n = 236, 71%) were completing residency in an area where they had not previously lived for more than 1 year.
Primary Care Utilization
Among the 348 respondents, 59% (n = 205) reported having established care with a PCP. An additional 6% (n = 21) had established care with an obstetrician/gynecologist for routine needs (Table 2). The 2 most common reasons for establishing care with a PCP were routine primary care needs, including contraception (n = 159, 79%), and access to a physician in the event of an acute medical need (n = 132, 66%).
Among respondents who had established care with a PCP, most (n = 188, 94%) had completed at least 1 appointment. However, among these 188 respondents, 68% (n = 127) stated that they had not made an acute visit in more than 12 months. When asked about wellness visits, almost one third of respondents (n = 103, 31%) stated that they had not been seen for a wellness visit in the past 3 years.
Burden of Chronic Illness
Most respondents (n = 223, 67%) stated that they did not have a chronic medical or mental health condition prior to residency (Table 3). However, 23% (n = 77) of respondents stated that they had been diagnosed with a chronic medical illness prior to residency, and 14% (n = 45) indicated they had been diagnosed with a mental health condition prior to residency. Almost one fifth of respondents (n = 60, 18%) reported seeing a subspecialist for a medical illness, and 33% (n = 111) reported taking a long-term prescription medication. With respect to major medical issues, the majority of residents (n = 239, 72%) denied experiencing events such as pregnancy, hospitalization, surgery, or an emergency department (ED) visit during training.
[polldaddy:10116940]
Inappropriate Prescriptions
While the majority of respondents denied writing a prescription for themselves for an acute or chronic medical condition, almost one fifth (n = 62, 19%) had received a prescription for an acute medical need from a provider outside of a clinical relationship (ie, from someone other than their PCP or specialty provider). Notably, 5% (n = 15) reported that this had occurred at least 2 or 3 times in the past 12 months (Table 4). Compared to respondents not taking long-term prescription medications, respondents who were already taking long-term prescription medications more frequently reported inappropriately receiving chronic prescriptions outside of an established clinical relationship (n = 14, 13% vs. n = 14, 6%; P = 0.05) and more often self-prescribed medications for acute needs (n = 12, 11% vs. n = 7, 3%; P = 0.005).
Comparison of Residents With and Without a PCP
Important differences were noted between residents who had a PCP versus those who did not (Table 5). For example, a higher percentage of residents with a PCP indicated they had been diagnosed with a chronic medical illness (n = 55, 28% vs. n = 22, 16%; P = 0.01) or a chronic mental health condition (n = 34, 17% vs. n = 11, 8%; P = 0.02) before residency. Additionally, a higher percentage of residents with a PCP (n = 70, 35% vs. n = 25, 18%; P = 0.001) reported experiencing medical events such as pregnancy, hospitalization, surgery, ED visit, or new diagnosis of a chronic medical illness during residency. Finally, a higher percentage of respondents with a PCP stated that they had visited a subspecialist for a medical illness (n = 44, 22% vs. n = 16,12%; P = 0.01) or were taking long-term prescription medications (n = 86, 43% vs. n = 25; 18%; P < 0.001). When comparing PGY-1 to PGY-2–PGY-4 residents, the former reported having established a medical relationship with a PCP significantly less frequently (n = 56, 43% vs. n = 142, 70%; P < 0.001).
Discussion
This survey-based study of medical residents across the United States suggests that a substantial proportion do not establish relationships with PCPs. Additionally, our data suggest that despite establishing care, few residents subsequently visited their PCP during training for wellness visits or routine care. Self-reported rates of chronic medical and mental health conditions were substantial in our sample. Furthermore, inappropriate self-prescription and the receipt of prescriptions outside of a medical relationship were also reported. These findings suggest that future studies that focus on the unique medical and mental health needs of physicians in training, as well as interventions to encourage care in this vulnerable period, are necessary.
We observed that most respondents that established primary care were female trainees. Although it is impossible to know with certainty, one hypothesis behind this discrepancy is that women routinely need to access preventative care for gynecologic needs such as pap smears, contraception, and potentially pregnancy and preconception counseling [14,15]. Similarly, residents with a chronic medical or mental health condition prior to residency established care with a local PCP at a significantly greater frequency than those without such diagnoses. While selection bias cannot be excluded, this finding suggests that illness is a driving factor in establishing care. There also appears to be an association between accessing the medical system (either for prescription medications or subspecialist care) and having established care with a PCP. Collectively, these data suggest that individuals without a compelling reason to access medical services might have barriers to accessing care in the event of medical needs or may not receive routine preventative care [9,10].
In general, we found that rates of reported inappropriate prescriptions were lower than those reported in prior studies where a comparable resident population was surveyed [8,12,16]. Inclusion of multiple institutions, differences in temporality, social desirability bias, and reporting bias might have influenced our findings in this regard. Surprisingly, we found that having a PCP did not influence likelihood of inappropriate prescription receipt, perhaps suggesting that this behavior reflects some degree of universal difficulty in accessing care. Alternatively, this finding might relate to a cultural tendency to self-prescribe among resident physicians. The fact that individuals on chronic medications more often both received and wrote inappropriate prescriptions suggests this problem might be more pronounced in individuals who take medications more often, as these residents have specific needs [12]. Future studies targeting these individuals thus appear warranted.
Our study has several limitations. First, our sample size was modest and the response rate of 45% was low. However, to our knowledge, this remains among the largest survey on this topic, and our response rate is comparable to similar trainee studies [8,11,13]. Second, we designed and created a novel survey for this study. While the questions were pilot-tested with users prior to dissemination, validation of the instrument was not performed. Third, since the study population was restricted to residents in fields that participate in primary care, our findings may not be generalizable to patterns of PCP use in other specialties [6].
These limitations aside, our study has important strengths. This is the first national study of its kind with specific questions addressing primary care access and utilization, prescription medication use and related practices, and the prevalence of medical conditions among trainees. Important differences in the rates of establishing primary care between male and female respondents, first- year and senior residents, and those with and without chronic disease suggest a need to target specific resident groups (males, interns, those without pre-existing conditions) for wellness-related interventions. Such interventions could include distribution of a list of local providers to first year residents, advanced protected time for doctor’s appointments, and safeguards to ensure health information is protected from potential supervisors. Future studies should also include residents from non-primary care oriented specialties such as surgery, emergency medicine, and anesthesiology to obtain results that are more generalizable to the resident population as a whole. Additionally, the rates of inappropriate prescriptions were not insignificant and warrant further evaluation of the driving forces behind these behaviors.
Conclusion
Medical residents have a substantial burden of chronic illness that may not be met through interactions with PCPs. More research into barriers that residents face while accessing care and an assessment of interventions to facilitate their access to care is important to promote trainee well-being. Without such direction and initiative, it may prove harder for physicians to heal themselves or those for whom they provide care.
Acknowledgments: We thank Suzanne Winter, the study coordinator, for her support with editing and formatting the manuscript, Latoya Kuhn for performing the statistical analysis and creating data tables, and Dr. Namita Sachdev and Dr. Renuka Tipirneni for providing feedback on the survey instrument. We also thank the involved programs for their participation.
Corresponding author: Vineet Chopra, NCRC 2800 Plymouth Rd., Bldg 16, 432, Ann Arbor, MI 48109, [email protected].
Financial disclosures: None.
Previous presentations: Results were presented at the Annual Michigan Medicine 2017 Internal Medicine Research Symposium.
1. Kassam A, Horton J, Shoimer I, Patten S. Predictors of well-being in resident physicians: a descriptive and psychometric study. J Grad Med Educ 2015;7:70–4.
2. Shanafelt TD, Bradley KA, Wipf JE, Back AL. Burnout and self-reported patient care in an internal medicine residency program. Ann Intern Med 2002;136:358–67.
3. Burstin HR, Swartz K, O’Neil AC, et al. The effect of change of health insurance on access to care. Inquiry 1998;35:389–97.
4. Rhodes KV, Basseyn S, Friedman AB, et al. Access to primary care appointments following 2014 insurance expansions. Ann Fam Med 2017;15:107–12.
5. Polsky D, Richards M, Basseyn S, et al. Appointment availability after increases in Medicaid payments for primary care. N Engl J Med 2015;372:537–45.
6. Gupta G, Schleinitz MD, Reinert SE, McGarry KA. Resident physician preventive health behaviors and perspectives on primary care. R I Med J (2013) 2013;96:43–7.
7. Rosen IM, Christine JD, Bellini LM, Asch DA. Health and health care among housestaff in four U.S. internal medicine residency programs. J Gen Intern Med 2000;15:116-21.
8. Campbell S, Delva D. Physician do not heal thyself. Survey of personal health practices among medical residents. Can Fam Physician 2003;49:1121–7.
9. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q 2005;83(3):457-502.
10. Weissman JS, Stern R, Fielding SL, et al. Delayed access to health care: risk factors, reasons, and consequences. Ann Intern Med 1991;114:325–31.
11. Guille C, Sen S. Prescription drug use and self-prescription among training physicians. Arch Intern Med 2012;172:371–2.
12. Roberts LW, Kim JP. Informal health care practices of residents: “curbside” consultation and self-diagnosis and treatment. Acad Psychiatry 2015;39:22-30.
13. Cohen JS, Patten S. Well-being in residency training: a survey examining resident physician satisfaction both within and outside of residency training and mental health in Alberta. BMC Med Educ 2005;5:21.
14. U.S. Preventive Services Task Force. Cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening. Published March 2012. Accessed August 21, 2018.
15. Health Resources and Services Administration. Women’s preventative services guidelines. https://www.hrsa.gov/womensguidelines2016/index.html. Updated October 2017. Accessed August 21, 2018.
16. Christie JD, Rosen IM, Bellini LM, et al. Prescription drug use and self-prescription among resident physicians. JAMA 1998;280(14):1253–5.
1. Kassam A, Horton J, Shoimer I, Patten S. Predictors of well-being in resident physicians: a descriptive and psychometric study. J Grad Med Educ 2015;7:70–4.
2. Shanafelt TD, Bradley KA, Wipf JE, Back AL. Burnout and self-reported patient care in an internal medicine residency program. Ann Intern Med 2002;136:358–67.
3. Burstin HR, Swartz K, O’Neil AC, et al. The effect of change of health insurance on access to care. Inquiry 1998;35:389–97.
4. Rhodes KV, Basseyn S, Friedman AB, et al. Access to primary care appointments following 2014 insurance expansions. Ann Fam Med 2017;15:107–12.
5. Polsky D, Richards M, Basseyn S, et al. Appointment availability after increases in Medicaid payments for primary care. N Engl J Med 2015;372:537–45.
6. Gupta G, Schleinitz MD, Reinert SE, McGarry KA. Resident physician preventive health behaviors and perspectives on primary care. R I Med J (2013) 2013;96:43–7.
7. Rosen IM, Christine JD, Bellini LM, Asch DA. Health and health care among housestaff in four U.S. internal medicine residency programs. J Gen Intern Med 2000;15:116-21.
8. Campbell S, Delva D. Physician do not heal thyself. Survey of personal health practices among medical residents. Can Fam Physician 2003;49:1121–7.
9. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q 2005;83(3):457-502.
10. Weissman JS, Stern R, Fielding SL, et al. Delayed access to health care: risk factors, reasons, and consequences. Ann Intern Med 1991;114:325–31.
11. Guille C, Sen S. Prescription drug use and self-prescription among training physicians. Arch Intern Med 2012;172:371–2.
12. Roberts LW, Kim JP. Informal health care practices of residents: “curbside” consultation and self-diagnosis and treatment. Acad Psychiatry 2015;39:22-30.
13. Cohen JS, Patten S. Well-being in residency training: a survey examining resident physician satisfaction both within and outside of residency training and mental health in Alberta. BMC Med Educ 2005;5:21.
14. U.S. Preventive Services Task Force. Cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening. Published March 2012. Accessed August 21, 2018.
15. Health Resources and Services Administration. Women’s preventative services guidelines. https://www.hrsa.gov/womensguidelines2016/index.html. Updated October 2017. Accessed August 21, 2018.
16. Christie JD, Rosen IM, Bellini LM, et al. Prescription drug use and self-prescription among resident physicians. JAMA 1998;280(14):1253–5.
Nocturnal Dexmedetomidine for Prevention of Delirium in the ICU
Study Overview
Objective. To determine if nocturnal dexmedetomidine prevents delirium and improves sleep in critically ill patients.
Design. Two-center, double-blind, placebo-controlled, randomized, trial.
Setting and participants. This study was conducted in the intensive care units (ICU) at 2 centers in North America between 2013 and 2016. Adults admitted to the ICU and receiving intermittent or continuous sedatives and expected to require at least 48 hours of ICU care were included in the study. Exclusion criteria were presence of delirium, severe dementia, acute neurologic injury, severe bradycardia, hepatic encephalopathy, end-stage liver disease, and expected death within 24 hours.
Intervention. Patients were randomized 1:1 to receive nocturnal dexmedetomidine (0.2–0.7 mcg/kg/hr) or dextrose 5% in water. Patients, clinicians, bedside nurses, and all study personnel were blinded to study drug assignment throughout the study. All sedatives were halved before the study drug was administered each evening. As-needed intravenous midazolam was used while titrating up the study drug. Study drug was administered nightly until either ICU discharge or an adverse event occurred. Decisions regarding use of other analgesic and sedative therapy, including opioids, oral benzodiazepines, acetaminophen, and nonsteroidal anti-inflammatory drugs, were left to the discretion of the clinician. Sleep-promoting agents such as melatonin or trazodone were not allowed.
Main outcome measures. The primary outcome was the proportion of patients who remained free of delirium during their critical illness. Secondary outcomes included ICU days spent without delirium; duration of delirium; sleep quality; proportion of patients who ever developed coma; proportion of nocturnal hours spent at each Richmond Agitation and Sedation Scale (RASS) score; maximal nocturnal pain levels; antipsychotic, corticosteroid, and oral analgesic use; days of mechanical ventilation; ICU and hospital stay duration; and ICU and hospital mortality.
Main results. 100 patients were randomized, with 50 patients in each group. 89% of patients were mechanically ventilated, and the Prediction of Delirium in ICU (PRE-DELIRIC) score [1] was 54 in the dexmedetomidine group and 51 in the placebo group. Continuous propofol and fentanyl infusion at randomization was used in 49% and 80%, respectively. Duration of median ICU stay was 10 days in the dexmedetomidine group and 9 days in the placebo group. More patients in the dexmedetomidine group (40 of 50 patients [80%]) than in the placebo group (27 of 50 patients [54%]) remained free of delirium (relative risk [RR], 0.44, 95% confidence interval {CI} 0.23 to 0.82; P = 0.006). The median (interquartile range [IQR]) duration of the first episode of delirium was similar between the dexmedetomidine (IQR 2.0 [0.6–2.7] days) and placebo (2.2 [0.7–3.2] days) groups (P = 0.73). The average Leeds Sleep Evaluation Questionnaire score also was similar (mean difference, 0.02, 95% CI 0.42 to 1.92) between the 2 groups. Incidence of hypotension or bradycardia did not differ significantly between the groups.
Conclusion. Nocturnal administration of low-dose dexmedetomidine in critically ill adults reduces the incidence of delirium during the ICU stay, and patient-reported sleep quality appears unchanged.
Commentary
Delirium is a sudden state of confusion and/or disturbance of consciousness and cognition that is believed to result from acute brain dysfunction, including neurochemical disequilibrium. It often occurs in association with a general medical condition, such as various types of shock, sepsis, surgery, anesthesia, or electrolyte imbalance. Studies have shown that delirium is associated with increased mortality in critically ill patients [2]. Most ICUs use a systematic assessment tool for early detection of delirium, such as the Confusion Assessment Method for the ICU (CAM-ICU), the Intensive Care Delirium Screening Checklist (ICDSC), or the DSM-IV TR score system. The CAM-ICU is the most frequently used tool to evaluate for the presence of delirium in critically ill patients; it is scored as positive if the patient manifests both an acute change in mental status and inattention, and has either a RASS greater than 0 or disorganized thinking [3].
The level of evidence regarding delirium prevention is low. Ear plugs, eye masks, educational staff, supportive reorientation, and music have been studied as nonpharmacologic methods for preventing delirium [4]. From a pharmacologic standpoint, the dopamine D2 antagonist haloperidol has been explored as a therapy for both treating and preventing delirium, since the condition is thought to be associated with anticholinergic and excessive dopaminergic mechanisms. A randomized controlled study in 142 patients who received haloperidol 2.5 mg intravenously every 8 hours found that the duration of delirium did not differ between the haloperidol and the placebo groups [5]. The most feared adverse effects of haloperidol, such as akathisia, muscle stiffness, arrhythmia, or QT prolongation, did not occur more frequently in the haloperidol group. Similar results have been reported by Al-Qadheeb et al [6]. Pharmacologic prophylaxis of delirium using atypical antipsychotics such as quetiapine has also been explored, but the level of evidence for this intervention remains very low. Current American College of Critical Care Medicine guidelines recommend nonpharmacologic management and do not firmly recommend any pharmacologic prevention for ICU delirium [7].
Dexmedetomidine is a selective alpha-2 adrenergic receptor agonist that acts at the locus ceruleus, providing sedation and analgesia. Studies assessing the choice of sedation in the ICU found that the use of dexmedetomidine or propofol, compared to benzodiazepines, is associated with a lower rate of delirium occurrence, especially in mechanically ventilated patients [8,9]. Dexmedetomidine offers several potential advantages over other sedative drugs: it has little effect on cognition, has minimal anticholinergic effect, and may restore a natural sleep pattern. While propofol causes hypotension, respiratory depression, and deeper sedation, dexmedetomidine is associated with lighter sedation, a minimal effect on respiratory drive, and a milder hemodynamic effect. In a randomized controlled trial involving post-surgery ICU patients, dexmedetomidine partially restored a normal sleep pattern (eg, increased percentage of stage 2 non-rapid eye movement sleep), prolonged total sleep time, improved sleep efficiency, and increased sleep quality [10]; by improving overall sleep quality, dexmedetomidine potentially may prevent delirium. Another study that randomly assigned 700 ICU patients who underwent noncardiac surgery to dexmedetomidine infusion (0.1 mcg/kg/hr from ICU admission on the day of surgery until the following morning) or placebo reported a significantly reduced incidence of delirium in the dexmedetomidine group [11]. On the other hand, a 2015 Cochrane meta-analysis that included 7 randomized controlled studies did not find a significant risk reduction of delirium with dexmedetomidine [12].
The current study by Skrobik et al was a randomized, placebo-controlled trial that examined the role of nocturnal dexmedetomidine in ICU delirium prevention in 100 ICU patients. Nocturnal administration of low-dose dexmedetomidine led to a statistically significant reduction in delirium incidence compared to placebo (RR of delirium, 0.44, 95% CI 0.23 to 0.82, which is similar to that suggested by previous studies). This study adds additional evidence regarding the use of dexmedetomidine for pharmacologic delirium prevention. It included many mechanically ventilated patients (89% of study population), strengthening the applicability of the result. Mechanical ventilation is a known risk factor for ICU delirium, and therefore this is an important population to study; previous trials largely included patients who were not mechanically ventilated. This study also supports the safety of dexmedetomidine infusion, especially in lower doses in critically ill patients, without significantly increasing the incidence of adverse events (mainly hypotension and bradycardia). The study protocol closely approximated real practice by allowing other analgesics, including opioids, and therefore suggests safety and real world applicability.
There are several confounding issues in this study. The study was blinded, and there was concern that the bedside nurses may have been able to identify the study drug based on the effects on heart rate. In addition, 50% of patients received antipsychotics. While baseline RASS score was significantly different between the 2 groups, patients in the dexmedetomidine group reached a deeper level of sedation during the study. Also, the protocol mandated halving the pre-existing sedative on the night of study drug initiation, which could have led to inadequate sedation in the placebo group. Placebo patients received propofol for a similar duration but at a higher dose compared to dexmedetomidine patients, and midazolam and fentanyl infusion was used in a similar pattern between the groups. The high exclusion rate (71%) limits the ability to generalize the results to all ICU patients.
Applications for Clinical Practice
ICU delirium is an important complication of critical illness and is potentially preventable. Benzodiazepines are associated with an increased risk of delirium, while there has been increasing interest in dexmedetomidine, a selective alpha-2 adrenergic receptor agonist, because of its potential for delirium prevention. Evidence to date does not strongly support routine use of pharmacologic prevention of delirium; however, dexmedetomidine may be an option for sedation, as opposed to benzodiazepines or propofol, in selected patients and may potentially prevent delirium.
—Minkyung Kwon, MD, Neal Patel, MD, and Vichaya Arunthari, MD, Pulmonary and Critical Care Medicine, Mayo Clinic Florida, Jacksonville, FL
1. van den Boogaard M, Pickkers P, Slooter AJ, et al. Development and validation of PRE-DELIRIC (PREdiction of DELIRium in ICu patients) delirium prediction model for intensive care patients: observational multicentre study. BMJ 2012;344:e420.
2. Slooter AJ, Van De Leur RR, Zaal IJ. Delirium in critically ill patients. Handb Clin Neurol 2017;141:449–66.
3. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001;286:2703–10.
4. Abraha I, Trotta F, Rimland JM, et al. Efficacy of non-pharmacological interventions to prevent and treat delirium in older patients: a systematic overview. The SENATOR project ONTOP Series. PLoS One 2015;10:e0123090.
5. Page VJ, Ely EW, Gates S, et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2013;1:515–23.
6. Al-Qadheeb NS, Skrobik Y, Schumaker G, et al. Preventing ICU subsyndromal delirium conversion to delirium with low-dose IV haloperidol: a double-blind, placebo-controlled pilot study. Crit Care Med 2016;44:583–91.
7. Barr J, Fraser GL, Puntillo K, et al; American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41:263–306.
8. Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 2009;301:489–99.
9. Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 2007;298:2644–53.
10. Wu XH, Cui F, Zhang C, et al. Low-dose dexmedetomidine improves sleep quality pattern in elderly patients after noncardiac surgery in the intensive care unit: a pilot randomized controlled trial. Anesthesiology 2016;125:979–91.
11. Su X, Meng Z-T, Wu X-H, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet 2016;388:1893–1902.
12. Chen K, Lu Z, Xin YC, et al. Alpha-2 agonists for long-term sedation during mechanical ventilation in critically ill patients. Cochrane Database Syst Rev 2015;1:CD010269.
Study Overview
Objective. To determine if nocturnal dexmedetomidine prevents delirium and improves sleep in critically ill patients.
Design. Two-center, double-blind, placebo-controlled, randomized, trial.
Setting and participants. This study was conducted in the intensive care units (ICU) at 2 centers in North America between 2013 and 2016. Adults admitted to the ICU and receiving intermittent or continuous sedatives and expected to require at least 48 hours of ICU care were included in the study. Exclusion criteria were presence of delirium, severe dementia, acute neurologic injury, severe bradycardia, hepatic encephalopathy, end-stage liver disease, and expected death within 24 hours.
Intervention. Patients were randomized 1:1 to receive nocturnal dexmedetomidine (0.2–0.7 mcg/kg/hr) or dextrose 5% in water. Patients, clinicians, bedside nurses, and all study personnel were blinded to study drug assignment throughout the study. All sedatives were halved before the study drug was administered each evening. As-needed intravenous midazolam was used while titrating up the study drug. Study drug was administered nightly until either ICU discharge or an adverse event occurred. Decisions regarding use of other analgesic and sedative therapy, including opioids, oral benzodiazepines, acetaminophen, and nonsteroidal anti-inflammatory drugs, were left to the discretion of the clinician. Sleep-promoting agents such as melatonin or trazodone were not allowed.
Main outcome measures. The primary outcome was the proportion of patients who remained free of delirium during their critical illness. Secondary outcomes included ICU days spent without delirium; duration of delirium; sleep quality; proportion of patients who ever developed coma; proportion of nocturnal hours spent at each Richmond Agitation and Sedation Scale (RASS) score; maximal nocturnal pain levels; antipsychotic, corticosteroid, and oral analgesic use; days of mechanical ventilation; ICU and hospital stay duration; and ICU and hospital mortality.
Main results. 100 patients were randomized, with 50 patients in each group. 89% of patients were mechanically ventilated, and the Prediction of Delirium in ICU (PRE-DELIRIC) score [1] was 54 in the dexmedetomidine group and 51 in the placebo group. Continuous propofol and fentanyl infusion at randomization was used in 49% and 80%, respectively. Duration of median ICU stay was 10 days in the dexmedetomidine group and 9 days in the placebo group. More patients in the dexmedetomidine group (40 of 50 patients [80%]) than in the placebo group (27 of 50 patients [54%]) remained free of delirium (relative risk [RR], 0.44, 95% confidence interval {CI} 0.23 to 0.82; P = 0.006). The median (interquartile range [IQR]) duration of the first episode of delirium was similar between the dexmedetomidine (IQR 2.0 [0.6–2.7] days) and placebo (2.2 [0.7–3.2] days) groups (P = 0.73). The average Leeds Sleep Evaluation Questionnaire score also was similar (mean difference, 0.02, 95% CI 0.42 to 1.92) between the 2 groups. Incidence of hypotension or bradycardia did not differ significantly between the groups.
Conclusion. Nocturnal administration of low-dose dexmedetomidine in critically ill adults reduces the incidence of delirium during the ICU stay, and patient-reported sleep quality appears unchanged.
Commentary
Delirium is a sudden state of confusion and/or disturbance of consciousness and cognition that is believed to result from acute brain dysfunction, including neurochemical disequilibrium. It often occurs in association with a general medical condition, such as various types of shock, sepsis, surgery, anesthesia, or electrolyte imbalance. Studies have shown that delirium is associated with increased mortality in critically ill patients [2]. Most ICUs use a systematic assessment tool for early detection of delirium, such as the Confusion Assessment Method for the ICU (CAM-ICU), the Intensive Care Delirium Screening Checklist (ICDSC), or the DSM-IV TR score system. The CAM-ICU is the most frequently used tool to evaluate for the presence of delirium in critically ill patients; it is scored as positive if the patient manifests both an acute change in mental status and inattention, and has either a RASS greater than 0 or disorganized thinking [3].
The level of evidence regarding delirium prevention is low. Ear plugs, eye masks, educational staff, supportive reorientation, and music have been studied as nonpharmacologic methods for preventing delirium [4]. From a pharmacologic standpoint, the dopamine D2 antagonist haloperidol has been explored as a therapy for both treating and preventing delirium, since the condition is thought to be associated with anticholinergic and excessive dopaminergic mechanisms. A randomized controlled study in 142 patients who received haloperidol 2.5 mg intravenously every 8 hours found that the duration of delirium did not differ between the haloperidol and the placebo groups [5]. The most feared adverse effects of haloperidol, such as akathisia, muscle stiffness, arrhythmia, or QT prolongation, did not occur more frequently in the haloperidol group. Similar results have been reported by Al-Qadheeb et al [6]. Pharmacologic prophylaxis of delirium using atypical antipsychotics such as quetiapine has also been explored, but the level of evidence for this intervention remains very low. Current American College of Critical Care Medicine guidelines recommend nonpharmacologic management and do not firmly recommend any pharmacologic prevention for ICU delirium [7].
Dexmedetomidine is a selective alpha-2 adrenergic receptor agonist that acts at the locus ceruleus, providing sedation and analgesia. Studies assessing the choice of sedation in the ICU found that the use of dexmedetomidine or propofol, compared to benzodiazepines, is associated with a lower rate of delirium occurrence, especially in mechanically ventilated patients [8,9]. Dexmedetomidine offers several potential advantages over other sedative drugs: it has little effect on cognition, has minimal anticholinergic effect, and may restore a natural sleep pattern. While propofol causes hypotension, respiratory depression, and deeper sedation, dexmedetomidine is associated with lighter sedation, a minimal effect on respiratory drive, and a milder hemodynamic effect. In a randomized controlled trial involving post-surgery ICU patients, dexmedetomidine partially restored a normal sleep pattern (eg, increased percentage of stage 2 non-rapid eye movement sleep), prolonged total sleep time, improved sleep efficiency, and increased sleep quality [10]; by improving overall sleep quality, dexmedetomidine potentially may prevent delirium. Another study that randomly assigned 700 ICU patients who underwent noncardiac surgery to dexmedetomidine infusion (0.1 mcg/kg/hr from ICU admission on the day of surgery until the following morning) or placebo reported a significantly reduced incidence of delirium in the dexmedetomidine group [11]. On the other hand, a 2015 Cochrane meta-analysis that included 7 randomized controlled studies did not find a significant risk reduction of delirium with dexmedetomidine [12].
The current study by Skrobik et al was a randomized, placebo-controlled trial that examined the role of nocturnal dexmedetomidine in ICU delirium prevention in 100 ICU patients. Nocturnal administration of low-dose dexmedetomidine led to a statistically significant reduction in delirium incidence compared to placebo (RR of delirium, 0.44, 95% CI 0.23 to 0.82, which is similar to that suggested by previous studies). This study adds additional evidence regarding the use of dexmedetomidine for pharmacologic delirium prevention. It included many mechanically ventilated patients (89% of study population), strengthening the applicability of the result. Mechanical ventilation is a known risk factor for ICU delirium, and therefore this is an important population to study; previous trials largely included patients who were not mechanically ventilated. This study also supports the safety of dexmedetomidine infusion, especially in lower doses in critically ill patients, without significantly increasing the incidence of adverse events (mainly hypotension and bradycardia). The study protocol closely approximated real practice by allowing other analgesics, including opioids, and therefore suggests safety and real world applicability.
There are several confounding issues in this study. The study was blinded, and there was concern that the bedside nurses may have been able to identify the study drug based on the effects on heart rate. In addition, 50% of patients received antipsychotics. While baseline RASS score was significantly different between the 2 groups, patients in the dexmedetomidine group reached a deeper level of sedation during the study. Also, the protocol mandated halving the pre-existing sedative on the night of study drug initiation, which could have led to inadequate sedation in the placebo group. Placebo patients received propofol for a similar duration but at a higher dose compared to dexmedetomidine patients, and midazolam and fentanyl infusion was used in a similar pattern between the groups. The high exclusion rate (71%) limits the ability to generalize the results to all ICU patients.
Applications for Clinical Practice
ICU delirium is an important complication of critical illness and is potentially preventable. Benzodiazepines are associated with an increased risk of delirium, while there has been increasing interest in dexmedetomidine, a selective alpha-2 adrenergic receptor agonist, because of its potential for delirium prevention. Evidence to date does not strongly support routine use of pharmacologic prevention of delirium; however, dexmedetomidine may be an option for sedation, as opposed to benzodiazepines or propofol, in selected patients and may potentially prevent delirium.
—Minkyung Kwon, MD, Neal Patel, MD, and Vichaya Arunthari, MD, Pulmonary and Critical Care Medicine, Mayo Clinic Florida, Jacksonville, FL
Study Overview
Objective. To determine if nocturnal dexmedetomidine prevents delirium and improves sleep in critically ill patients.
Design. Two-center, double-blind, placebo-controlled, randomized, trial.
Setting and participants. This study was conducted in the intensive care units (ICU) at 2 centers in North America between 2013 and 2016. Adults admitted to the ICU and receiving intermittent or continuous sedatives and expected to require at least 48 hours of ICU care were included in the study. Exclusion criteria were presence of delirium, severe dementia, acute neurologic injury, severe bradycardia, hepatic encephalopathy, end-stage liver disease, and expected death within 24 hours.
Intervention. Patients were randomized 1:1 to receive nocturnal dexmedetomidine (0.2–0.7 mcg/kg/hr) or dextrose 5% in water. Patients, clinicians, bedside nurses, and all study personnel were blinded to study drug assignment throughout the study. All sedatives were halved before the study drug was administered each evening. As-needed intravenous midazolam was used while titrating up the study drug. Study drug was administered nightly until either ICU discharge or an adverse event occurred. Decisions regarding use of other analgesic and sedative therapy, including opioids, oral benzodiazepines, acetaminophen, and nonsteroidal anti-inflammatory drugs, were left to the discretion of the clinician. Sleep-promoting agents such as melatonin or trazodone were not allowed.
Main outcome measures. The primary outcome was the proportion of patients who remained free of delirium during their critical illness. Secondary outcomes included ICU days spent without delirium; duration of delirium; sleep quality; proportion of patients who ever developed coma; proportion of nocturnal hours spent at each Richmond Agitation and Sedation Scale (RASS) score; maximal nocturnal pain levels; antipsychotic, corticosteroid, and oral analgesic use; days of mechanical ventilation; ICU and hospital stay duration; and ICU and hospital mortality.
Main results. 100 patients were randomized, with 50 patients in each group. 89% of patients were mechanically ventilated, and the Prediction of Delirium in ICU (PRE-DELIRIC) score [1] was 54 in the dexmedetomidine group and 51 in the placebo group. Continuous propofol and fentanyl infusion at randomization was used in 49% and 80%, respectively. Duration of median ICU stay was 10 days in the dexmedetomidine group and 9 days in the placebo group. More patients in the dexmedetomidine group (40 of 50 patients [80%]) than in the placebo group (27 of 50 patients [54%]) remained free of delirium (relative risk [RR], 0.44, 95% confidence interval {CI} 0.23 to 0.82; P = 0.006). The median (interquartile range [IQR]) duration of the first episode of delirium was similar between the dexmedetomidine (IQR 2.0 [0.6–2.7] days) and placebo (2.2 [0.7–3.2] days) groups (P = 0.73). The average Leeds Sleep Evaluation Questionnaire score also was similar (mean difference, 0.02, 95% CI 0.42 to 1.92) between the 2 groups. Incidence of hypotension or bradycardia did not differ significantly between the groups.
Conclusion. Nocturnal administration of low-dose dexmedetomidine in critically ill adults reduces the incidence of delirium during the ICU stay, and patient-reported sleep quality appears unchanged.
Commentary
Delirium is a sudden state of confusion and/or disturbance of consciousness and cognition that is believed to result from acute brain dysfunction, including neurochemical disequilibrium. It often occurs in association with a general medical condition, such as various types of shock, sepsis, surgery, anesthesia, or electrolyte imbalance. Studies have shown that delirium is associated with increased mortality in critically ill patients [2]. Most ICUs use a systematic assessment tool for early detection of delirium, such as the Confusion Assessment Method for the ICU (CAM-ICU), the Intensive Care Delirium Screening Checklist (ICDSC), or the DSM-IV TR score system. The CAM-ICU is the most frequently used tool to evaluate for the presence of delirium in critically ill patients; it is scored as positive if the patient manifests both an acute change in mental status and inattention, and has either a RASS greater than 0 or disorganized thinking [3].
The level of evidence regarding delirium prevention is low. Ear plugs, eye masks, educational staff, supportive reorientation, and music have been studied as nonpharmacologic methods for preventing delirium [4]. From a pharmacologic standpoint, the dopamine D2 antagonist haloperidol has been explored as a therapy for both treating and preventing delirium, since the condition is thought to be associated with anticholinergic and excessive dopaminergic mechanisms. A randomized controlled study in 142 patients who received haloperidol 2.5 mg intravenously every 8 hours found that the duration of delirium did not differ between the haloperidol and the placebo groups [5]. The most feared adverse effects of haloperidol, such as akathisia, muscle stiffness, arrhythmia, or QT prolongation, did not occur more frequently in the haloperidol group. Similar results have been reported by Al-Qadheeb et al [6]. Pharmacologic prophylaxis of delirium using atypical antipsychotics such as quetiapine has also been explored, but the level of evidence for this intervention remains very low. Current American College of Critical Care Medicine guidelines recommend nonpharmacologic management and do not firmly recommend any pharmacologic prevention for ICU delirium [7].
Dexmedetomidine is a selective alpha-2 adrenergic receptor agonist that acts at the locus ceruleus, providing sedation and analgesia. Studies assessing the choice of sedation in the ICU found that the use of dexmedetomidine or propofol, compared to benzodiazepines, is associated with a lower rate of delirium occurrence, especially in mechanically ventilated patients [8,9]. Dexmedetomidine offers several potential advantages over other sedative drugs: it has little effect on cognition, has minimal anticholinergic effect, and may restore a natural sleep pattern. While propofol causes hypotension, respiratory depression, and deeper sedation, dexmedetomidine is associated with lighter sedation, a minimal effect on respiratory drive, and a milder hemodynamic effect. In a randomized controlled trial involving post-surgery ICU patients, dexmedetomidine partially restored a normal sleep pattern (eg, increased percentage of stage 2 non-rapid eye movement sleep), prolonged total sleep time, improved sleep efficiency, and increased sleep quality [10]; by improving overall sleep quality, dexmedetomidine potentially may prevent delirium. Another study that randomly assigned 700 ICU patients who underwent noncardiac surgery to dexmedetomidine infusion (0.1 mcg/kg/hr from ICU admission on the day of surgery until the following morning) or placebo reported a significantly reduced incidence of delirium in the dexmedetomidine group [11]. On the other hand, a 2015 Cochrane meta-analysis that included 7 randomized controlled studies did not find a significant risk reduction of delirium with dexmedetomidine [12].
The current study by Skrobik et al was a randomized, placebo-controlled trial that examined the role of nocturnal dexmedetomidine in ICU delirium prevention in 100 ICU patients. Nocturnal administration of low-dose dexmedetomidine led to a statistically significant reduction in delirium incidence compared to placebo (RR of delirium, 0.44, 95% CI 0.23 to 0.82, which is similar to that suggested by previous studies). This study adds additional evidence regarding the use of dexmedetomidine for pharmacologic delirium prevention. It included many mechanically ventilated patients (89% of study population), strengthening the applicability of the result. Mechanical ventilation is a known risk factor for ICU delirium, and therefore this is an important population to study; previous trials largely included patients who were not mechanically ventilated. This study also supports the safety of dexmedetomidine infusion, especially in lower doses in critically ill patients, without significantly increasing the incidence of adverse events (mainly hypotension and bradycardia). The study protocol closely approximated real practice by allowing other analgesics, including opioids, and therefore suggests safety and real world applicability.
There are several confounding issues in this study. The study was blinded, and there was concern that the bedside nurses may have been able to identify the study drug based on the effects on heart rate. In addition, 50% of patients received antipsychotics. While baseline RASS score was significantly different between the 2 groups, patients in the dexmedetomidine group reached a deeper level of sedation during the study. Also, the protocol mandated halving the pre-existing sedative on the night of study drug initiation, which could have led to inadequate sedation in the placebo group. Placebo patients received propofol for a similar duration but at a higher dose compared to dexmedetomidine patients, and midazolam and fentanyl infusion was used in a similar pattern between the groups. The high exclusion rate (71%) limits the ability to generalize the results to all ICU patients.
Applications for Clinical Practice
ICU delirium is an important complication of critical illness and is potentially preventable. Benzodiazepines are associated with an increased risk of delirium, while there has been increasing interest in dexmedetomidine, a selective alpha-2 adrenergic receptor agonist, because of its potential for delirium prevention. Evidence to date does not strongly support routine use of pharmacologic prevention of delirium; however, dexmedetomidine may be an option for sedation, as opposed to benzodiazepines or propofol, in selected patients and may potentially prevent delirium.
—Minkyung Kwon, MD, Neal Patel, MD, and Vichaya Arunthari, MD, Pulmonary and Critical Care Medicine, Mayo Clinic Florida, Jacksonville, FL
1. van den Boogaard M, Pickkers P, Slooter AJ, et al. Development and validation of PRE-DELIRIC (PREdiction of DELIRium in ICu patients) delirium prediction model for intensive care patients: observational multicentre study. BMJ 2012;344:e420.
2. Slooter AJ, Van De Leur RR, Zaal IJ. Delirium in critically ill patients. Handb Clin Neurol 2017;141:449–66.
3. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001;286:2703–10.
4. Abraha I, Trotta F, Rimland JM, et al. Efficacy of non-pharmacological interventions to prevent and treat delirium in older patients: a systematic overview. The SENATOR project ONTOP Series. PLoS One 2015;10:e0123090.
5. Page VJ, Ely EW, Gates S, et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2013;1:515–23.
6. Al-Qadheeb NS, Skrobik Y, Schumaker G, et al. Preventing ICU subsyndromal delirium conversion to delirium with low-dose IV haloperidol: a double-blind, placebo-controlled pilot study. Crit Care Med 2016;44:583–91.
7. Barr J, Fraser GL, Puntillo K, et al; American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41:263–306.
8. Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 2009;301:489–99.
9. Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 2007;298:2644–53.
10. Wu XH, Cui F, Zhang C, et al. Low-dose dexmedetomidine improves sleep quality pattern in elderly patients after noncardiac surgery in the intensive care unit: a pilot randomized controlled trial. Anesthesiology 2016;125:979–91.
11. Su X, Meng Z-T, Wu X-H, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet 2016;388:1893–1902.
12. Chen K, Lu Z, Xin YC, et al. Alpha-2 agonists for long-term sedation during mechanical ventilation in critically ill patients. Cochrane Database Syst Rev 2015;1:CD010269.
1. van den Boogaard M, Pickkers P, Slooter AJ, et al. Development and validation of PRE-DELIRIC (PREdiction of DELIRium in ICu patients) delirium prediction model for intensive care patients: observational multicentre study. BMJ 2012;344:e420.
2. Slooter AJ, Van De Leur RR, Zaal IJ. Delirium in critically ill patients. Handb Clin Neurol 2017;141:449–66.
3. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001;286:2703–10.
4. Abraha I, Trotta F, Rimland JM, et al. Efficacy of non-pharmacological interventions to prevent and treat delirium in older patients: a systematic overview. The SENATOR project ONTOP Series. PLoS One 2015;10:e0123090.
5. Page VJ, Ely EW, Gates S, et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2013;1:515–23.
6. Al-Qadheeb NS, Skrobik Y, Schumaker G, et al. Preventing ICU subsyndromal delirium conversion to delirium with low-dose IV haloperidol: a double-blind, placebo-controlled pilot study. Crit Care Med 2016;44:583–91.
7. Barr J, Fraser GL, Puntillo K, et al; American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41:263–306.
8. Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 2009;301:489–99.
9. Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 2007;298:2644–53.
10. Wu XH, Cui F, Zhang C, et al. Low-dose dexmedetomidine improves sleep quality pattern in elderly patients after noncardiac surgery in the intensive care unit: a pilot randomized controlled trial. Anesthesiology 2016;125:979–91.
11. Su X, Meng Z-T, Wu X-H, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet 2016;388:1893–1902.
12. Chen K, Lu Z, Xin YC, et al. Alpha-2 agonists for long-term sedation during mechanical ventilation in critically ill patients. Cochrane Database Syst Rev 2015;1:CD010269.
Dramatic response from pembrolizumab in patient with Lynch syndrome
A woman with two distinct primary tumors achieved complete regression of both after pembrolizumab therapy, investigators report.
This is the first documented instance of a checkpoint inhibitor leading to this kind of simultaneous regression, reported Benjamin Musher, MD, of Baylor College of Medicine, Houston, and his coauthors.
The patient was a 55-year-old woman with a family history of gastric, uterine, and colon cancer. After presenting with weight loss, fatigue, and abdominal pain, colonoscopy showed a 5-cm mucosal lesion. A subsequent PET-CT scan revealed a 12-cm hepatic mass with multiple other liver masses, and bulky lymph nodes nearby. Site biopsies showed two primary cancer types: colonic adenocarcinoma and intrahepatic cholangiocarcinoma.
“Additional staining ... for proteins that repair mismatched DNA showed an absence of MLH1 expression. Sequencing of the patient’s DNA revealed a deleterious mutation in MLH1, which confirmed that Lynch syndrome caused both types of cancer,” the authors wrote in Annals of Internal Medicine.
Lynch syndrome (also called hereditary nonpolyposis colorectal cancer), is an autosomal dominant condition that incurs a high risk of colorectal, pancreatic, bile duct, ovarian, gastric, and uterine cancer. Patients may present with more than one type of cancer simultaneously, as occurred in this case study.
Following diagnosis, the patient started pembrolizumab monotherapy (200 mg IV Q3W). After 16 months the tumors were undetectable by colonoscopy or PET-CT; 2 months later the patient was free of cancer symptoms.
“To our knowledge, our case report is the first to document complete regression of 2 simultaneous types of cancer after treatment with an immune checkpoint inhibitor,” the authors wrote.
But why the dramatic response?
“The types of cancer that develop because of a mismatch repair deficiency contain more mutations than most other kinds of cancer,” the authors explained. These highly mutated cells are recognized by the host immune system, but responses are limited, in part, by programmed cell death proteins. “These events make checkpoint inhibition an attractive and potentially effective treatment for mismatch repair deficient cancer.
“We believe that this case emphasizes the importance of eliciting a thorough family history in patients with cancer and considering the presence of multiple types of primary cancer in a patient with an extensive family history of cancer,” the authors concluded. “It also shows the value of identifying mismatch repair deficiency, [in which] immunotherapy can yield dramatic and durable benefit.”
Dr. Musher reported compensation from LOKON pharmaceuticals.
SOURCE: Musher et al. Ann Intern Med. 2018 Sep 24. doi: 10.7326/L18-0360.
A woman with two distinct primary tumors achieved complete regression of both after pembrolizumab therapy, investigators report.
This is the first documented instance of a checkpoint inhibitor leading to this kind of simultaneous regression, reported Benjamin Musher, MD, of Baylor College of Medicine, Houston, and his coauthors.
The patient was a 55-year-old woman with a family history of gastric, uterine, and colon cancer. After presenting with weight loss, fatigue, and abdominal pain, colonoscopy showed a 5-cm mucosal lesion. A subsequent PET-CT scan revealed a 12-cm hepatic mass with multiple other liver masses, and bulky lymph nodes nearby. Site biopsies showed two primary cancer types: colonic adenocarcinoma and intrahepatic cholangiocarcinoma.
“Additional staining ... for proteins that repair mismatched DNA showed an absence of MLH1 expression. Sequencing of the patient’s DNA revealed a deleterious mutation in MLH1, which confirmed that Lynch syndrome caused both types of cancer,” the authors wrote in Annals of Internal Medicine.
Lynch syndrome (also called hereditary nonpolyposis colorectal cancer), is an autosomal dominant condition that incurs a high risk of colorectal, pancreatic, bile duct, ovarian, gastric, and uterine cancer. Patients may present with more than one type of cancer simultaneously, as occurred in this case study.
Following diagnosis, the patient started pembrolizumab monotherapy (200 mg IV Q3W). After 16 months the tumors were undetectable by colonoscopy or PET-CT; 2 months later the patient was free of cancer symptoms.
“To our knowledge, our case report is the first to document complete regression of 2 simultaneous types of cancer after treatment with an immune checkpoint inhibitor,” the authors wrote.
But why the dramatic response?
“The types of cancer that develop because of a mismatch repair deficiency contain more mutations than most other kinds of cancer,” the authors explained. These highly mutated cells are recognized by the host immune system, but responses are limited, in part, by programmed cell death proteins. “These events make checkpoint inhibition an attractive and potentially effective treatment for mismatch repair deficient cancer.
“We believe that this case emphasizes the importance of eliciting a thorough family history in patients with cancer and considering the presence of multiple types of primary cancer in a patient with an extensive family history of cancer,” the authors concluded. “It also shows the value of identifying mismatch repair deficiency, [in which] immunotherapy can yield dramatic and durable benefit.”
Dr. Musher reported compensation from LOKON pharmaceuticals.
SOURCE: Musher et al. Ann Intern Med. 2018 Sep 24. doi: 10.7326/L18-0360.
A woman with two distinct primary tumors achieved complete regression of both after pembrolizumab therapy, investigators report.
This is the first documented instance of a checkpoint inhibitor leading to this kind of simultaneous regression, reported Benjamin Musher, MD, of Baylor College of Medicine, Houston, and his coauthors.
The patient was a 55-year-old woman with a family history of gastric, uterine, and colon cancer. After presenting with weight loss, fatigue, and abdominal pain, colonoscopy showed a 5-cm mucosal lesion. A subsequent PET-CT scan revealed a 12-cm hepatic mass with multiple other liver masses, and bulky lymph nodes nearby. Site biopsies showed two primary cancer types: colonic adenocarcinoma and intrahepatic cholangiocarcinoma.
“Additional staining ... for proteins that repair mismatched DNA showed an absence of MLH1 expression. Sequencing of the patient’s DNA revealed a deleterious mutation in MLH1, which confirmed that Lynch syndrome caused both types of cancer,” the authors wrote in Annals of Internal Medicine.
Lynch syndrome (also called hereditary nonpolyposis colorectal cancer), is an autosomal dominant condition that incurs a high risk of colorectal, pancreatic, bile duct, ovarian, gastric, and uterine cancer. Patients may present with more than one type of cancer simultaneously, as occurred in this case study.
Following diagnosis, the patient started pembrolizumab monotherapy (200 mg IV Q3W). After 16 months the tumors were undetectable by colonoscopy or PET-CT; 2 months later the patient was free of cancer symptoms.
“To our knowledge, our case report is the first to document complete regression of 2 simultaneous types of cancer after treatment with an immune checkpoint inhibitor,” the authors wrote.
But why the dramatic response?
“The types of cancer that develop because of a mismatch repair deficiency contain more mutations than most other kinds of cancer,” the authors explained. These highly mutated cells are recognized by the host immune system, but responses are limited, in part, by programmed cell death proteins. “These events make checkpoint inhibition an attractive and potentially effective treatment for mismatch repair deficient cancer.
“We believe that this case emphasizes the importance of eliciting a thorough family history in patients with cancer and considering the presence of multiple types of primary cancer in a patient with an extensive family history of cancer,” the authors concluded. “It also shows the value of identifying mismatch repair deficiency, [in which] immunotherapy can yield dramatic and durable benefit.”
Dr. Musher reported compensation from LOKON pharmaceuticals.
SOURCE: Musher et al. Ann Intern Med. 2018 Sep 24. doi: 10.7326/L18-0360.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Pembrolizumab may provide complete tumor regression in patients with mismatch repair deficiency (Lynch syndrome) who have more than one type of cancer.
Major finding: A woman with primary colonic adenocarcinoma and primary intrahepatic cholangiocarcinoma achieved complete regression of both tumor types after pembrolizumab therapy.
Study details: A case study of a 55-year-old woman with Lynch syndrome who had a family history of gastric, uterine, and colon cancer.
Disclosures: Dr. Musher reported receiving compensation from Lokon.
Source: Musher et al. Ann Intern Med. 2018 Sep 24. doi: 10.7326/L18-0360.
Laser tattoo removal plume ‘probably safer’ than laser hair removal plume
SAN DIEGO – results from a novel study demonstrated.
“The laser plume is known to contain possible hazards,” Yakir Levin, MD, PhD, said at the annual Masters of Aesthetics Symposium. “Intact human papillomavirus DNA has been demonstrated in the CO2 laser plume of common wart treatments,” he noted, and transmission of bovine papillomavirus has been shown in a bovine model of CO2 laser treatment (Arch Dermatol 2002;138[10]:1303-7). “In addition, aerosolized human cells have been demonstrated in laser tattoo removal.”
In a more recent study, Gary S. Chuang, MD, and his colleagues demonstrated hazards in the laser hair removal plume (JAMA Dermatol 2016;152[12]:1320-6). “These include ultrafine particles that become lodged in pulmonary alveoli and cause long-term respiratory problems, as well as volatile organic compounds, which can be carcinogens and environmental toxins,” said Dr. Levin of the Massachusetts General Hospital department of dermatology and the Wellman Center for Photomedicine, both in Boston. “They showed that this can be improved but not cured by proper use of a smoke evacuator; they also emphasized the importance of wearing a mask.”
Dr. Levin and his colleagues chose to study laser tattoo removal plume because more than 40 million Americans have tattoos, especially younger adults. In addition, 17% regret having their tattoo and 11% are undergoing or have undergone tattoo removal procedures. In what is believed to be the first study of its kind, the researchers performed a study in ex vivo pig skin and in humans undergoing routine laser tattoo removal. They measured the concentration of nanoparticles as well as the presence of heavy metals, volatile organic compounds, and airborne bacteria.
For the swine study, the excised pig skin was tattooed with several differently colored inks. Dr. Levin and his colleagues found that the concentration of airborne nanoparticles measured during laser tattoo removal was elevated and varied with different inks and different lasers used. Fine metals were measured in mcg/m3 air and were below safe occupational exposure limits. The same effect was seen for volatile organic compounds.
Next, the researchers analyzed the laser plume in humans undergoing removal of blue, black, and multicolored tattoos. “Here, the results were a little bit different,” Dr. Levin said. “Airborne particle concentrations were higher in the dermatologist’s breathing zone and near the tattoo removal site than in the remainder of the room or outside of the room. However, concentrations were 30 times lower for human skin than for pig skin. That’s because the pig study was somewhat artificial in that the tattoos were done when the pig was dead.”
Metals were detected in the plume in the human study, but they were all below occupational exposure limits. The same effect was seen for volatile organic compounds.
Dr. Levin said that airborne nanoparticle concentrations for laser tattoo removal of ex vivo tattooed swine skin were comparable to those reported for hair removal, while airborne nanoparticle concentrations for laser removal of in vivo human skin were much lower than those reported for laser hair removal. “So it’s probably true that the potential health hazards from laser tattoo removal are lower than for laser hair removal, but we did not study viral particles or the presence of viable human cells in the plume,” he said.
Current methods to limit laser plume exposure include suction of the plume with a smoke evacuator, use of a barrier device placed over the skin, and wearing a face mask constructed to filter nanoparticles, such as an N95 mask.
Other safety issues to consider
Dr. Levin discussed additional safety considerations in performing laser treatments.
“We want to protect the epidermis from injury during the laser exposure, which is currently done with spray cooling, air cooling, and/or contact cooling,” he said. “We want to limit the pain experienced by patients throughout the laser treatment before and after the brief laser exposure. This is often accomplished with the use of ice packs or air cooling. We also want to avoid double pulsing and skip areas. This can sometimes but not always be achieved by paying close attention to clinical endpoints.”
He and his associates are currently developing a device to accomplish all of those safety goals with a multilayer approach. “One of the layers would be a hydrogel, which serves to protect the epidermis and to provide pain relief throughout the laser treatment,” he said. “Above that layer is an indicator layer that is not aqueous, and on top of that is a fine layer of particles. The idea is, if you’re looking at this from above, when you fire the laser, you would see a change of color or some other indicator to show you exactly where you fired the laser. Finally, the multilayer patch also serves to obstruct the laser plume.”
Dr. Levin acknowledged research support from the American Society for Dermatologic Surgery’s Fredric S. Brandt, MD, Innovations in Aesthetics Fellowship Fund and assistance from the Health Hazard Evaluation Program of National Institute for Occupational Safety and Health.
SAN DIEGO – results from a novel study demonstrated.
“The laser plume is known to contain possible hazards,” Yakir Levin, MD, PhD, said at the annual Masters of Aesthetics Symposium. “Intact human papillomavirus DNA has been demonstrated in the CO2 laser plume of common wart treatments,” he noted, and transmission of bovine papillomavirus has been shown in a bovine model of CO2 laser treatment (Arch Dermatol 2002;138[10]:1303-7). “In addition, aerosolized human cells have been demonstrated in laser tattoo removal.”
In a more recent study, Gary S. Chuang, MD, and his colleagues demonstrated hazards in the laser hair removal plume (JAMA Dermatol 2016;152[12]:1320-6). “These include ultrafine particles that become lodged in pulmonary alveoli and cause long-term respiratory problems, as well as volatile organic compounds, which can be carcinogens and environmental toxins,” said Dr. Levin of the Massachusetts General Hospital department of dermatology and the Wellman Center for Photomedicine, both in Boston. “They showed that this can be improved but not cured by proper use of a smoke evacuator; they also emphasized the importance of wearing a mask.”
Dr. Levin and his colleagues chose to study laser tattoo removal plume because more than 40 million Americans have tattoos, especially younger adults. In addition, 17% regret having their tattoo and 11% are undergoing or have undergone tattoo removal procedures. In what is believed to be the first study of its kind, the researchers performed a study in ex vivo pig skin and in humans undergoing routine laser tattoo removal. They measured the concentration of nanoparticles as well as the presence of heavy metals, volatile organic compounds, and airborne bacteria.
For the swine study, the excised pig skin was tattooed with several differently colored inks. Dr. Levin and his colleagues found that the concentration of airborne nanoparticles measured during laser tattoo removal was elevated and varied with different inks and different lasers used. Fine metals were measured in mcg/m3 air and were below safe occupational exposure limits. The same effect was seen for volatile organic compounds.
Next, the researchers analyzed the laser plume in humans undergoing removal of blue, black, and multicolored tattoos. “Here, the results were a little bit different,” Dr. Levin said. “Airborne particle concentrations were higher in the dermatologist’s breathing zone and near the tattoo removal site than in the remainder of the room or outside of the room. However, concentrations were 30 times lower for human skin than for pig skin. That’s because the pig study was somewhat artificial in that the tattoos were done when the pig was dead.”
Metals were detected in the plume in the human study, but they were all below occupational exposure limits. The same effect was seen for volatile organic compounds.
Dr. Levin said that airborne nanoparticle concentrations for laser tattoo removal of ex vivo tattooed swine skin were comparable to those reported for hair removal, while airborne nanoparticle concentrations for laser removal of in vivo human skin were much lower than those reported for laser hair removal. “So it’s probably true that the potential health hazards from laser tattoo removal are lower than for laser hair removal, but we did not study viral particles or the presence of viable human cells in the plume,” he said.
Current methods to limit laser plume exposure include suction of the plume with a smoke evacuator, use of a barrier device placed over the skin, and wearing a face mask constructed to filter nanoparticles, such as an N95 mask.
Other safety issues to consider
Dr. Levin discussed additional safety considerations in performing laser treatments.
“We want to protect the epidermis from injury during the laser exposure, which is currently done with spray cooling, air cooling, and/or contact cooling,” he said. “We want to limit the pain experienced by patients throughout the laser treatment before and after the brief laser exposure. This is often accomplished with the use of ice packs or air cooling. We also want to avoid double pulsing and skip areas. This can sometimes but not always be achieved by paying close attention to clinical endpoints.”
He and his associates are currently developing a device to accomplish all of those safety goals with a multilayer approach. “One of the layers would be a hydrogel, which serves to protect the epidermis and to provide pain relief throughout the laser treatment,” he said. “Above that layer is an indicator layer that is not aqueous, and on top of that is a fine layer of particles. The idea is, if you’re looking at this from above, when you fire the laser, you would see a change of color or some other indicator to show you exactly where you fired the laser. Finally, the multilayer patch also serves to obstruct the laser plume.”
Dr. Levin acknowledged research support from the American Society for Dermatologic Surgery’s Fredric S. Brandt, MD, Innovations in Aesthetics Fellowship Fund and assistance from the Health Hazard Evaluation Program of National Institute for Occupational Safety and Health.
SAN DIEGO – results from a novel study demonstrated.
“The laser plume is known to contain possible hazards,” Yakir Levin, MD, PhD, said at the annual Masters of Aesthetics Symposium. “Intact human papillomavirus DNA has been demonstrated in the CO2 laser plume of common wart treatments,” he noted, and transmission of bovine papillomavirus has been shown in a bovine model of CO2 laser treatment (Arch Dermatol 2002;138[10]:1303-7). “In addition, aerosolized human cells have been demonstrated in laser tattoo removal.”
In a more recent study, Gary S. Chuang, MD, and his colleagues demonstrated hazards in the laser hair removal plume (JAMA Dermatol 2016;152[12]:1320-6). “These include ultrafine particles that become lodged in pulmonary alveoli and cause long-term respiratory problems, as well as volatile organic compounds, which can be carcinogens and environmental toxins,” said Dr. Levin of the Massachusetts General Hospital department of dermatology and the Wellman Center for Photomedicine, both in Boston. “They showed that this can be improved but not cured by proper use of a smoke evacuator; they also emphasized the importance of wearing a mask.”
Dr. Levin and his colleagues chose to study laser tattoo removal plume because more than 40 million Americans have tattoos, especially younger adults. In addition, 17% regret having their tattoo and 11% are undergoing or have undergone tattoo removal procedures. In what is believed to be the first study of its kind, the researchers performed a study in ex vivo pig skin and in humans undergoing routine laser tattoo removal. They measured the concentration of nanoparticles as well as the presence of heavy metals, volatile organic compounds, and airborne bacteria.
For the swine study, the excised pig skin was tattooed with several differently colored inks. Dr. Levin and his colleagues found that the concentration of airborne nanoparticles measured during laser tattoo removal was elevated and varied with different inks and different lasers used. Fine metals were measured in mcg/m3 air and were below safe occupational exposure limits. The same effect was seen for volatile organic compounds.
Next, the researchers analyzed the laser plume in humans undergoing removal of blue, black, and multicolored tattoos. “Here, the results were a little bit different,” Dr. Levin said. “Airborne particle concentrations were higher in the dermatologist’s breathing zone and near the tattoo removal site than in the remainder of the room or outside of the room. However, concentrations were 30 times lower for human skin than for pig skin. That’s because the pig study was somewhat artificial in that the tattoos were done when the pig was dead.”
Metals were detected in the plume in the human study, but they were all below occupational exposure limits. The same effect was seen for volatile organic compounds.
Dr. Levin said that airborne nanoparticle concentrations for laser tattoo removal of ex vivo tattooed swine skin were comparable to those reported for hair removal, while airborne nanoparticle concentrations for laser removal of in vivo human skin were much lower than those reported for laser hair removal. “So it’s probably true that the potential health hazards from laser tattoo removal are lower than for laser hair removal, but we did not study viral particles or the presence of viable human cells in the plume,” he said.
Current methods to limit laser plume exposure include suction of the plume with a smoke evacuator, use of a barrier device placed over the skin, and wearing a face mask constructed to filter nanoparticles, such as an N95 mask.
Other safety issues to consider
Dr. Levin discussed additional safety considerations in performing laser treatments.
“We want to protect the epidermis from injury during the laser exposure, which is currently done with spray cooling, air cooling, and/or contact cooling,” he said. “We want to limit the pain experienced by patients throughout the laser treatment before and after the brief laser exposure. This is often accomplished with the use of ice packs or air cooling. We also want to avoid double pulsing and skip areas. This can sometimes but not always be achieved by paying close attention to clinical endpoints.”
He and his associates are currently developing a device to accomplish all of those safety goals with a multilayer approach. “One of the layers would be a hydrogel, which serves to protect the epidermis and to provide pain relief throughout the laser treatment,” he said. “Above that layer is an indicator layer that is not aqueous, and on top of that is a fine layer of particles. The idea is, if you’re looking at this from above, when you fire the laser, you would see a change of color or some other indicator to show you exactly where you fired the laser. Finally, the multilayer patch also serves to obstruct the laser plume.”
Dr. Levin acknowledged research support from the American Society for Dermatologic Surgery’s Fredric S. Brandt, MD, Innovations in Aesthetics Fellowship Fund and assistance from the Health Hazard Evaluation Program of National Institute for Occupational Safety and Health.
REPORTING FROM MOAS 2018
Trump administration proposes weakening rules governing organ transplant centers
This article was produced in collaboration with the Houston Chronicle.
The Trump administration on Sept. 17 proposed eliminating a decade-old regulation that puts hospitals at risk of losing their Medicare funding if too many of their patients die or suffer organ failure after receiving transplants.
The rule the government is proposing to scrap is the same one that led the Centers for Medicare & Medicaid Services to cut off funding in August for heart transplants at Baylor St. Luke’s Medical Center in Houston after an investigation by ProPublica and the Houston Chronicle revealed an outsized number of patient deaths and complications in recent years.
The proposal was unveiled Sept. 17 as part of the White House’s push to “cut the red tape” and do away with “burdensome regulation” that officials said put paperwork ahead of patients. In a speech announcing the proposed changes, CMS Administrator Seema Verma said the agency’s existing policies have “put lives in danger.”
“We are proposing to remove those inefficiencies to reduce the amount of time patients have to wait, so that they can begin healing,” Ms. Verma said.
The proposal, now subject to public comment and revision before it is finalized, surprised many transplant physicians who have long called for relaxed federal oversight. They’ve argued that the rules requiring that hospitals meet certain survival thresholds for transplants discourage them from taking on risky patients or accepting less-than-perfect organs, lengthening the time patients spend on the waiting list.
The regulation was put in place in 2007 after a series of scandals at transplant programs revealed lax federal oversight. Several transplant programs had compiled abysmal patient survival statistics for years while continuing to receive Medicare funding.
Even though it has the authority to do so, Medicare rarely terminates programs for poor outcomes. It is far more common for the government to force underperforming programs into systems-improvement agreements, in which hospitals agree to make certain changes and be subject to stepped-up oversight.
Medicare bypassed that process and cut off funding for heart transplants at St. Luke’s in August after the hospital’s 1-year patient survival rate fell below national norms from 2014 to mid-2016. A few St. Luke’s cardiologists grew so concerned that they started sending some of their patients to other hospitals for transplants.
St. Luke’s has appealed its Medicare termination, saying, “we do not believe CMS’ recent decisions reflect our ongoing progress and accomplishments to improve the quality of our care.” A spokeswoman said officials are still reviewing the Medicare proposal and declined to comment.
In a statement to reporters Sept. 21, CMS said it would continue to monitoring transplant programs and is strengthening its process for inspecting transplant programs to ensure they provide safe, quality care.
“CMS will continue to collect the data on each transplant program’s performance with regards to patient and graft survival,” the agency said in the statement. “These data, rather than being a stand-alone measure, will be used as a component of the survey process to further inform and direct the survey.”
If the proposed regulatory change had been in place previously, it’s not clear whether St. Luke’s would have faced punitive action from Medicare.
“I am probably in the minority in the transplant community, but I think, based on what is proposed, this is a bad idea,” said Laura J. Aguiar, an Arizona-based transplant consultant who has spent years helping programs improve their outcomes to stave off Medicare penalties. “I have been around long enough to remember that there were very valid reasons why CMS, in the George W. Bush administration, took the steps they took in implementing all of this.”
Since the rules were put in place 11 years ago, the percentage of patients who survive at least 1 year after receiving heart, kidney, lung, and other organ transplants has increased nationally. But some experts say those gains have come at a cost.
Jesse D. Schold, PhD, a researcher in quantitative health sciences at the Cleveland Clinic, has spent years chronicling what he calls the “unintended consequences” of holding transplant programs accountable for poor outcomes. Even though CMS relies on data that has been adjusted to ensure that programs aren’t punished for treating sicker patients or accepting riskier donor organs, Dr. Schold said the rules have created a perception that programs need to turn away some ailing patients and reject less-than-ideal organs in order to meet outcome targets.
As a result, Dr. Schold said, some potentially viable donor organs are discarded while thousands of patients die on waiting lists each year. Dr. Schold said he was surprised to learn a study he coauthored was among the research cited by CMS as justification for the policy change, which he said would be a “significant net positive” for patients.
“I don’t want anything to in any way imply that I’m a fan of the current administration,” Dr. Schold said, referencing the Trump administration’s aggressive and often controversial efforts to slash regulations. “However, in this case, I think it is something that is beneficial.”
Joel Adler, MD, a liver surgeon at the University of Wisconsin–Madison, whose research also was cited by CMS, said a major weakness of the current regulation is that it focuses only on the survival of patients fortunate enough to have received a transplant. Medicare, he said, does not take into account the percentage of patients who die awaiting a transplant. That can discourage programs from accepting organs for patients who might be less likely to survive afterward.
Despite identifying problems with the current rules, Dr. Adler and his coauthor did not propose eliminating Medicare’s standards, but they instead suggested ways to improve outcome measures and create incentives for programs to perform more, not fewer, transplants.
“Should we get rid of (the regulation) entirely?” Dr. Adler asked. “I don’t know. I suspect we’ll swing back to somewhere in the middle, because there has to be some mechanism of capturing things when they are really, really bad. That has to exist.”
Medicare isn’t the only organization that oversees transplant centers. The United Network for Organ Sharing, a federal contractor that operates the national waiting list for organs, can put programs on probation or even revoke their good standing for continued poor results, though it rarely takes such actions. Similarly, another federal contractor, the Scientific Registry of Transplant Recipients, analyzes transplant outcomes and publicly reports which centers have mortality rates that are higher than expected based on the characteristics of their patients.
Even if the proposal is approved, it would not mean CMS would stop regulating transplant programs. Last year, for example, CMS cut off funding to the Medical University Hospital in Charleston, South Carolina, after the program failed to perform the minimum number of heart transplants required by CMS to maintain certification. That provision, also added during the reforms of 2007, would not be affected by the changes proposed Sept. 17.
The Charleston hospital, South Carolina’s only heart transplant program, made necessary changes and regained Medicare certification this year.
Alexander Aussi, a San Antonio–based consultant who specializes in helping transplant programs satisfy regulatory requirements, said he understands the desire to reduce the number of rules and regulations that transplant centers must follow. But he said he fears that the CMS proposal would return the transplant field to an earlier era when “some programs were cowboyish about their outcomes.”
“I cannot tell you in good conscience that if you remove those guidelines and metrics … that you’re going to have better outcomes,” Mr. Aussi said. “On the contrary, I believe you’re going to have a lot of transplant programs reversing course.”
Ms. Aguiar, the Arizona-based consultant, shares those concerns. Even without strict CMS oversight, she said, many transplant programs will continue providing high-quality care; but some won’t.
“There will be others that will take the removal of these requirements as a blessing to go back to the bad old days,” she said, “and it is patients who will end up paying the price for it.”
Mike Hixenbaugh is an investigative reporter at the Houston Chronicle. Email him at [email protected] and follow him on Twitter at @Mike_Hixenbaugh. Charles Ornstein is a senior editor at ProPublica, overseeing the Local Reporting Network. Follow him on Twitter at @charlesornstein.
This article was produced in collaboration with the Houston Chronicle.
The Trump administration on Sept. 17 proposed eliminating a decade-old regulation that puts hospitals at risk of losing their Medicare funding if too many of their patients die or suffer organ failure after receiving transplants.
The rule the government is proposing to scrap is the same one that led the Centers for Medicare & Medicaid Services to cut off funding in August for heart transplants at Baylor St. Luke’s Medical Center in Houston after an investigation by ProPublica and the Houston Chronicle revealed an outsized number of patient deaths and complications in recent years.
The proposal was unveiled Sept. 17 as part of the White House’s push to “cut the red tape” and do away with “burdensome regulation” that officials said put paperwork ahead of patients. In a speech announcing the proposed changes, CMS Administrator Seema Verma said the agency’s existing policies have “put lives in danger.”
“We are proposing to remove those inefficiencies to reduce the amount of time patients have to wait, so that they can begin healing,” Ms. Verma said.
The proposal, now subject to public comment and revision before it is finalized, surprised many transplant physicians who have long called for relaxed federal oversight. They’ve argued that the rules requiring that hospitals meet certain survival thresholds for transplants discourage them from taking on risky patients or accepting less-than-perfect organs, lengthening the time patients spend on the waiting list.
The regulation was put in place in 2007 after a series of scandals at transplant programs revealed lax federal oversight. Several transplant programs had compiled abysmal patient survival statistics for years while continuing to receive Medicare funding.
Even though it has the authority to do so, Medicare rarely terminates programs for poor outcomes. It is far more common for the government to force underperforming programs into systems-improvement agreements, in which hospitals agree to make certain changes and be subject to stepped-up oversight.
Medicare bypassed that process and cut off funding for heart transplants at St. Luke’s in August after the hospital’s 1-year patient survival rate fell below national norms from 2014 to mid-2016. A few St. Luke’s cardiologists grew so concerned that they started sending some of their patients to other hospitals for transplants.
St. Luke’s has appealed its Medicare termination, saying, “we do not believe CMS’ recent decisions reflect our ongoing progress and accomplishments to improve the quality of our care.” A spokeswoman said officials are still reviewing the Medicare proposal and declined to comment.
In a statement to reporters Sept. 21, CMS said it would continue to monitoring transplant programs and is strengthening its process for inspecting transplant programs to ensure they provide safe, quality care.
“CMS will continue to collect the data on each transplant program’s performance with regards to patient and graft survival,” the agency said in the statement. “These data, rather than being a stand-alone measure, will be used as a component of the survey process to further inform and direct the survey.”
If the proposed regulatory change had been in place previously, it’s not clear whether St. Luke’s would have faced punitive action from Medicare.
“I am probably in the minority in the transplant community, but I think, based on what is proposed, this is a bad idea,” said Laura J. Aguiar, an Arizona-based transplant consultant who has spent years helping programs improve their outcomes to stave off Medicare penalties. “I have been around long enough to remember that there were very valid reasons why CMS, in the George W. Bush administration, took the steps they took in implementing all of this.”
Since the rules were put in place 11 years ago, the percentage of patients who survive at least 1 year after receiving heart, kidney, lung, and other organ transplants has increased nationally. But some experts say those gains have come at a cost.
Jesse D. Schold, PhD, a researcher in quantitative health sciences at the Cleveland Clinic, has spent years chronicling what he calls the “unintended consequences” of holding transplant programs accountable for poor outcomes. Even though CMS relies on data that has been adjusted to ensure that programs aren’t punished for treating sicker patients or accepting riskier donor organs, Dr. Schold said the rules have created a perception that programs need to turn away some ailing patients and reject less-than-ideal organs in order to meet outcome targets.
As a result, Dr. Schold said, some potentially viable donor organs are discarded while thousands of patients die on waiting lists each year. Dr. Schold said he was surprised to learn a study he coauthored was among the research cited by CMS as justification for the policy change, which he said would be a “significant net positive” for patients.
“I don’t want anything to in any way imply that I’m a fan of the current administration,” Dr. Schold said, referencing the Trump administration’s aggressive and often controversial efforts to slash regulations. “However, in this case, I think it is something that is beneficial.”
Joel Adler, MD, a liver surgeon at the University of Wisconsin–Madison, whose research also was cited by CMS, said a major weakness of the current regulation is that it focuses only on the survival of patients fortunate enough to have received a transplant. Medicare, he said, does not take into account the percentage of patients who die awaiting a transplant. That can discourage programs from accepting organs for patients who might be less likely to survive afterward.
Despite identifying problems with the current rules, Dr. Adler and his coauthor did not propose eliminating Medicare’s standards, but they instead suggested ways to improve outcome measures and create incentives for programs to perform more, not fewer, transplants.
“Should we get rid of (the regulation) entirely?” Dr. Adler asked. “I don’t know. I suspect we’ll swing back to somewhere in the middle, because there has to be some mechanism of capturing things when they are really, really bad. That has to exist.”
Medicare isn’t the only organization that oversees transplant centers. The United Network for Organ Sharing, a federal contractor that operates the national waiting list for organs, can put programs on probation or even revoke their good standing for continued poor results, though it rarely takes such actions. Similarly, another federal contractor, the Scientific Registry of Transplant Recipients, analyzes transplant outcomes and publicly reports which centers have mortality rates that are higher than expected based on the characteristics of their patients.
Even if the proposal is approved, it would not mean CMS would stop regulating transplant programs. Last year, for example, CMS cut off funding to the Medical University Hospital in Charleston, South Carolina, after the program failed to perform the minimum number of heart transplants required by CMS to maintain certification. That provision, also added during the reforms of 2007, would not be affected by the changes proposed Sept. 17.
The Charleston hospital, South Carolina’s only heart transplant program, made necessary changes and regained Medicare certification this year.
Alexander Aussi, a San Antonio–based consultant who specializes in helping transplant programs satisfy regulatory requirements, said he understands the desire to reduce the number of rules and regulations that transplant centers must follow. But he said he fears that the CMS proposal would return the transplant field to an earlier era when “some programs were cowboyish about their outcomes.”
“I cannot tell you in good conscience that if you remove those guidelines and metrics … that you’re going to have better outcomes,” Mr. Aussi said. “On the contrary, I believe you’re going to have a lot of transplant programs reversing course.”
Ms. Aguiar, the Arizona-based consultant, shares those concerns. Even without strict CMS oversight, she said, many transplant programs will continue providing high-quality care; but some won’t.
“There will be others that will take the removal of these requirements as a blessing to go back to the bad old days,” she said, “and it is patients who will end up paying the price for it.”
Mike Hixenbaugh is an investigative reporter at the Houston Chronicle. Email him at [email protected] and follow him on Twitter at @Mike_Hixenbaugh. Charles Ornstein is a senior editor at ProPublica, overseeing the Local Reporting Network. Follow him on Twitter at @charlesornstein.
This article was produced in collaboration with the Houston Chronicle.
The Trump administration on Sept. 17 proposed eliminating a decade-old regulation that puts hospitals at risk of losing their Medicare funding if too many of their patients die or suffer organ failure after receiving transplants.
The rule the government is proposing to scrap is the same one that led the Centers for Medicare & Medicaid Services to cut off funding in August for heart transplants at Baylor St. Luke’s Medical Center in Houston after an investigation by ProPublica and the Houston Chronicle revealed an outsized number of patient deaths and complications in recent years.
The proposal was unveiled Sept. 17 as part of the White House’s push to “cut the red tape” and do away with “burdensome regulation” that officials said put paperwork ahead of patients. In a speech announcing the proposed changes, CMS Administrator Seema Verma said the agency’s existing policies have “put lives in danger.”
“We are proposing to remove those inefficiencies to reduce the amount of time patients have to wait, so that they can begin healing,” Ms. Verma said.
The proposal, now subject to public comment and revision before it is finalized, surprised many transplant physicians who have long called for relaxed federal oversight. They’ve argued that the rules requiring that hospitals meet certain survival thresholds for transplants discourage them from taking on risky patients or accepting less-than-perfect organs, lengthening the time patients spend on the waiting list.
The regulation was put in place in 2007 after a series of scandals at transplant programs revealed lax federal oversight. Several transplant programs had compiled abysmal patient survival statistics for years while continuing to receive Medicare funding.
Even though it has the authority to do so, Medicare rarely terminates programs for poor outcomes. It is far more common for the government to force underperforming programs into systems-improvement agreements, in which hospitals agree to make certain changes and be subject to stepped-up oversight.
Medicare bypassed that process and cut off funding for heart transplants at St. Luke’s in August after the hospital’s 1-year patient survival rate fell below national norms from 2014 to mid-2016. A few St. Luke’s cardiologists grew so concerned that they started sending some of their patients to other hospitals for transplants.
St. Luke’s has appealed its Medicare termination, saying, “we do not believe CMS’ recent decisions reflect our ongoing progress and accomplishments to improve the quality of our care.” A spokeswoman said officials are still reviewing the Medicare proposal and declined to comment.
In a statement to reporters Sept. 21, CMS said it would continue to monitoring transplant programs and is strengthening its process for inspecting transplant programs to ensure they provide safe, quality care.
“CMS will continue to collect the data on each transplant program’s performance with regards to patient and graft survival,” the agency said in the statement. “These data, rather than being a stand-alone measure, will be used as a component of the survey process to further inform and direct the survey.”
If the proposed regulatory change had been in place previously, it’s not clear whether St. Luke’s would have faced punitive action from Medicare.
“I am probably in the minority in the transplant community, but I think, based on what is proposed, this is a bad idea,” said Laura J. Aguiar, an Arizona-based transplant consultant who has spent years helping programs improve their outcomes to stave off Medicare penalties. “I have been around long enough to remember that there were very valid reasons why CMS, in the George W. Bush administration, took the steps they took in implementing all of this.”
Since the rules were put in place 11 years ago, the percentage of patients who survive at least 1 year after receiving heart, kidney, lung, and other organ transplants has increased nationally. But some experts say those gains have come at a cost.
Jesse D. Schold, PhD, a researcher in quantitative health sciences at the Cleveland Clinic, has spent years chronicling what he calls the “unintended consequences” of holding transplant programs accountable for poor outcomes. Even though CMS relies on data that has been adjusted to ensure that programs aren’t punished for treating sicker patients or accepting riskier donor organs, Dr. Schold said the rules have created a perception that programs need to turn away some ailing patients and reject less-than-ideal organs in order to meet outcome targets.
As a result, Dr. Schold said, some potentially viable donor organs are discarded while thousands of patients die on waiting lists each year. Dr. Schold said he was surprised to learn a study he coauthored was among the research cited by CMS as justification for the policy change, which he said would be a “significant net positive” for patients.
“I don’t want anything to in any way imply that I’m a fan of the current administration,” Dr. Schold said, referencing the Trump administration’s aggressive and often controversial efforts to slash regulations. “However, in this case, I think it is something that is beneficial.”
Joel Adler, MD, a liver surgeon at the University of Wisconsin–Madison, whose research also was cited by CMS, said a major weakness of the current regulation is that it focuses only on the survival of patients fortunate enough to have received a transplant. Medicare, he said, does not take into account the percentage of patients who die awaiting a transplant. That can discourage programs from accepting organs for patients who might be less likely to survive afterward.
Despite identifying problems with the current rules, Dr. Adler and his coauthor did not propose eliminating Medicare’s standards, but they instead suggested ways to improve outcome measures and create incentives for programs to perform more, not fewer, transplants.
“Should we get rid of (the regulation) entirely?” Dr. Adler asked. “I don’t know. I suspect we’ll swing back to somewhere in the middle, because there has to be some mechanism of capturing things when they are really, really bad. That has to exist.”
Medicare isn’t the only organization that oversees transplant centers. The United Network for Organ Sharing, a federal contractor that operates the national waiting list for organs, can put programs on probation or even revoke their good standing for continued poor results, though it rarely takes such actions. Similarly, another federal contractor, the Scientific Registry of Transplant Recipients, analyzes transplant outcomes and publicly reports which centers have mortality rates that are higher than expected based on the characteristics of their patients.
Even if the proposal is approved, it would not mean CMS would stop regulating transplant programs. Last year, for example, CMS cut off funding to the Medical University Hospital in Charleston, South Carolina, after the program failed to perform the minimum number of heart transplants required by CMS to maintain certification. That provision, also added during the reforms of 2007, would not be affected by the changes proposed Sept. 17.
The Charleston hospital, South Carolina’s only heart transplant program, made necessary changes and regained Medicare certification this year.
Alexander Aussi, a San Antonio–based consultant who specializes in helping transplant programs satisfy regulatory requirements, said he understands the desire to reduce the number of rules and regulations that transplant centers must follow. But he said he fears that the CMS proposal would return the transplant field to an earlier era when “some programs were cowboyish about their outcomes.”
“I cannot tell you in good conscience that if you remove those guidelines and metrics … that you’re going to have better outcomes,” Mr. Aussi said. “On the contrary, I believe you’re going to have a lot of transplant programs reversing course.”
Ms. Aguiar, the Arizona-based consultant, shares those concerns. Even without strict CMS oversight, she said, many transplant programs will continue providing high-quality care; but some won’t.
“There will be others that will take the removal of these requirements as a blessing to go back to the bad old days,” she said, “and it is patients who will end up paying the price for it.”
Mike Hixenbaugh is an investigative reporter at the Houston Chronicle. Email him at [email protected] and follow him on Twitter at @Mike_Hixenbaugh. Charles Ornstein is a senior editor at ProPublica, overseeing the Local Reporting Network. Follow him on Twitter at @charlesornstein.
Prosthesis-patient mismatch post TAVR ups death risk 19%
SAN DIEGO – Severe prosthesis-patient mismatch (PPM) after transcatheter aortic valve replacement (TAVR) increases risk of adverse outcomes and may be preventable in some cases with careful preprocedural planning, suggests a registry-based retrospective cohort study of 62,125 patients treated in the contemporary era.
The study – the largest to date of this patient population – determined that about one in every eight patients undergoing TAVR ultimately had a severe mismatch between the hemodynamics of the valve prosthesis and the requirements for cardiac output. Compared with counterparts that have moderate or no PPM, these patients with severe PPM had a 12% higher adjusted risk of heart failure rehospitalization and a 19% higher adjusted risk of death, according to results reported at the Transcatheter Cardiovascular Therapeutics annual meeting and simultaneously published online (J Am Coll Cardiol. 2018 Sep 23. doi: 10.1016/j.jacc.2018.09.001).
Notably, some of the predictors of severe PPM, such as use of smaller-diameter valves and performance of a valve-in-valve procedure, were potentially modifiable.
“Our findings suggest that efforts should be made to identify this problem and limit the risk for PPM after TAVR,” concluded lead investigator Howard C. Herrmann, MD, a professor at the University of Pennsylvania and director of the cardiac catheterization laboratories, Hospital of the University of Pennsylvania, both in Philadelphia. “Awareness is really the first step in trying to fix it.”
“We spend a lot of time in the heart-team meetings looking at the CT scans for annular dimensions and the vascular access, but we don’t really talk too much about severe PPM or the risk of that,” he elaborated. “This [study] allows us to start to predict it, based on patient factors and what prosthesis we might be choosing for a patient, and it allows us to have that conversation and think about alternatives.
“There are alternatives to try to avoid PPM, everything from which prosthesis we choose to the size of the prosthesis, to whether we fracture a patient’s valve if we are doing a valve-in-valve procedure. In the future, in some situations, we might even choose a low-risk or low-intermediate-risk patient for surgery with an enlargement operation in order to get a larger effective orifice area. So there are choices that we can make, and we should start thinking about that in the heart-team approach.”
Findings in context
The new study reinforces the message “that hemodynamics matter,” he said. “To the extent that we can get larger valves in and get better results from those valves, it will reduce the frequency of PPM. That’s something as operators we don’t spend as much time focusing on, and this will refocus our attention in trying to prevent PPM by being more diligent in terms of prosthesis choice and some operator characteristics, to try to reduce the gradients and improve the effective orifice areas as much as we can.”
Panelist Jeffrey J. Popma, MD, an interventional cardiologist at Beth Israel Deaconess Medical Center, Boston, noted that he and his colleagues have observed similar trends in their smaller studies but had difficulty teasing out contributors. “It really goes back to the preprocedural planning about what valve you can get in, and larger orifice area is certainly better,” he concurred. “So I do think that this is a phenomenal addition.”
Study details
For the study, Dr. Herrmann and his colleagues analyzed 2014-2107 data from the STS/ACC Transcatheter Valve Therapy Registry, a national surveillance and quality improvement system. They identified enrollees aged 65 years or older at the time of their TAVR procedure who had fee-for-service Medicare and linked them to Centers for Medicare & Medicaid Services claims data to assess outcomes.
Overall, 12.1% of patients had severe PPM, defined as an effective valve orifice area indexed to body surface area of less than 0.65 cm2/m2 on discharge echocardiography, and another 24.6% had moderate PPM, Dr. Herrmann reported at the meeting, sponsored by the Cardiovascular Research Foundation.
The strongest multivariate predictors of severe PPM were small prosthetic valve size (up to 23 mm in diameter) (odds ratio, 2.77), a valve-in-valve procedure (OR, 2.78), larger body surface area (OR, 1.71 per 0.2-U increase), and female sex (OR, 1.46). Odds also increased with decreasing age and were elevated for patients of nonwhite/Hispanic race and those having a lower ejection fraction, atrial fibrillation or flutter, or severe mitral or tricuspid regurgitation.
It was not possible to assess specific valves as predictors of mismatch because the registry prohibits comparisons across manufacturers, according to Dr. Herrmann.
One-year mortality, the study’s primary endpoint, was 17.2% in patients with severe PPM, compared with 15.8% in patients with moderate or no PPM (adjusted hazard ratio, 1.19; P less than .001). Findings were similar across subgroups.
The 1-year rate of heart failure rehospitalization was 14.7% in patients with severe PPM, compared with 12.2% in patients with moderate or no PPM (AHR, 1.12; P = .017).
“I would point out that these [outcome] curves are divergent at 1 year,” Dr. Herrmann noted. “So if we look at low-intermediate-risk and low-risk patients and younger patients, who may be more active and who see the effects of PPM more commonly and who are going to be living more than 1 year, we are going to have to consider this going forward in a more important way.”
Severe PPM did not significantly influence the rate of stroke (which stood at about 4% in each group) or worsen quality of life score at 1 year.
Dr. Herrmann disclosed receiving institutional grant/research support from Abbott Vascular, Bayer, Boston Scientific, Corvia Medical, Edwards Lifesciences, Medtronic, and St. Jude Medical, as well as consulting fees/honoraria from Edwards, Medtronic, and Siemens Healthineers.
SAN DIEGO – Severe prosthesis-patient mismatch (PPM) after transcatheter aortic valve replacement (TAVR) increases risk of adverse outcomes and may be preventable in some cases with careful preprocedural planning, suggests a registry-based retrospective cohort study of 62,125 patients treated in the contemporary era.
The study – the largest to date of this patient population – determined that about one in every eight patients undergoing TAVR ultimately had a severe mismatch between the hemodynamics of the valve prosthesis and the requirements for cardiac output. Compared with counterparts that have moderate or no PPM, these patients with severe PPM had a 12% higher adjusted risk of heart failure rehospitalization and a 19% higher adjusted risk of death, according to results reported at the Transcatheter Cardiovascular Therapeutics annual meeting and simultaneously published online (J Am Coll Cardiol. 2018 Sep 23. doi: 10.1016/j.jacc.2018.09.001).
Notably, some of the predictors of severe PPM, such as use of smaller-diameter valves and performance of a valve-in-valve procedure, were potentially modifiable.
“Our findings suggest that efforts should be made to identify this problem and limit the risk for PPM after TAVR,” concluded lead investigator Howard C. Herrmann, MD, a professor at the University of Pennsylvania and director of the cardiac catheterization laboratories, Hospital of the University of Pennsylvania, both in Philadelphia. “Awareness is really the first step in trying to fix it.”
“We spend a lot of time in the heart-team meetings looking at the CT scans for annular dimensions and the vascular access, but we don’t really talk too much about severe PPM or the risk of that,” he elaborated. “This [study] allows us to start to predict it, based on patient factors and what prosthesis we might be choosing for a patient, and it allows us to have that conversation and think about alternatives.
“There are alternatives to try to avoid PPM, everything from which prosthesis we choose to the size of the prosthesis, to whether we fracture a patient’s valve if we are doing a valve-in-valve procedure. In the future, in some situations, we might even choose a low-risk or low-intermediate-risk patient for surgery with an enlargement operation in order to get a larger effective orifice area. So there are choices that we can make, and we should start thinking about that in the heart-team approach.”
Findings in context
The new study reinforces the message “that hemodynamics matter,” he said. “To the extent that we can get larger valves in and get better results from those valves, it will reduce the frequency of PPM. That’s something as operators we don’t spend as much time focusing on, and this will refocus our attention in trying to prevent PPM by being more diligent in terms of prosthesis choice and some operator characteristics, to try to reduce the gradients and improve the effective orifice areas as much as we can.”
Panelist Jeffrey J. Popma, MD, an interventional cardiologist at Beth Israel Deaconess Medical Center, Boston, noted that he and his colleagues have observed similar trends in their smaller studies but had difficulty teasing out contributors. “It really goes back to the preprocedural planning about what valve you can get in, and larger orifice area is certainly better,” he concurred. “So I do think that this is a phenomenal addition.”
Study details
For the study, Dr. Herrmann and his colleagues analyzed 2014-2107 data from the STS/ACC Transcatheter Valve Therapy Registry, a national surveillance and quality improvement system. They identified enrollees aged 65 years or older at the time of their TAVR procedure who had fee-for-service Medicare and linked them to Centers for Medicare & Medicaid Services claims data to assess outcomes.
Overall, 12.1% of patients had severe PPM, defined as an effective valve orifice area indexed to body surface area of less than 0.65 cm2/m2 on discharge echocardiography, and another 24.6% had moderate PPM, Dr. Herrmann reported at the meeting, sponsored by the Cardiovascular Research Foundation.
The strongest multivariate predictors of severe PPM were small prosthetic valve size (up to 23 mm in diameter) (odds ratio, 2.77), a valve-in-valve procedure (OR, 2.78), larger body surface area (OR, 1.71 per 0.2-U increase), and female sex (OR, 1.46). Odds also increased with decreasing age and were elevated for patients of nonwhite/Hispanic race and those having a lower ejection fraction, atrial fibrillation or flutter, or severe mitral or tricuspid regurgitation.
It was not possible to assess specific valves as predictors of mismatch because the registry prohibits comparisons across manufacturers, according to Dr. Herrmann.
One-year mortality, the study’s primary endpoint, was 17.2% in patients with severe PPM, compared with 15.8% in patients with moderate or no PPM (adjusted hazard ratio, 1.19; P less than .001). Findings were similar across subgroups.
The 1-year rate of heart failure rehospitalization was 14.7% in patients with severe PPM, compared with 12.2% in patients with moderate or no PPM (AHR, 1.12; P = .017).
“I would point out that these [outcome] curves are divergent at 1 year,” Dr. Herrmann noted. “So if we look at low-intermediate-risk and low-risk patients and younger patients, who may be more active and who see the effects of PPM more commonly and who are going to be living more than 1 year, we are going to have to consider this going forward in a more important way.”
Severe PPM did not significantly influence the rate of stroke (which stood at about 4% in each group) or worsen quality of life score at 1 year.
Dr. Herrmann disclosed receiving institutional grant/research support from Abbott Vascular, Bayer, Boston Scientific, Corvia Medical, Edwards Lifesciences, Medtronic, and St. Jude Medical, as well as consulting fees/honoraria from Edwards, Medtronic, and Siemens Healthineers.
SAN DIEGO – Severe prosthesis-patient mismatch (PPM) after transcatheter aortic valve replacement (TAVR) increases risk of adverse outcomes and may be preventable in some cases with careful preprocedural planning, suggests a registry-based retrospective cohort study of 62,125 patients treated in the contemporary era.
The study – the largest to date of this patient population – determined that about one in every eight patients undergoing TAVR ultimately had a severe mismatch between the hemodynamics of the valve prosthesis and the requirements for cardiac output. Compared with counterparts that have moderate or no PPM, these patients with severe PPM had a 12% higher adjusted risk of heart failure rehospitalization and a 19% higher adjusted risk of death, according to results reported at the Transcatheter Cardiovascular Therapeutics annual meeting and simultaneously published online (J Am Coll Cardiol. 2018 Sep 23. doi: 10.1016/j.jacc.2018.09.001).
Notably, some of the predictors of severe PPM, such as use of smaller-diameter valves and performance of a valve-in-valve procedure, were potentially modifiable.
“Our findings suggest that efforts should be made to identify this problem and limit the risk for PPM after TAVR,” concluded lead investigator Howard C. Herrmann, MD, a professor at the University of Pennsylvania and director of the cardiac catheterization laboratories, Hospital of the University of Pennsylvania, both in Philadelphia. “Awareness is really the first step in trying to fix it.”
“We spend a lot of time in the heart-team meetings looking at the CT scans for annular dimensions and the vascular access, but we don’t really talk too much about severe PPM or the risk of that,” he elaborated. “This [study] allows us to start to predict it, based on patient factors and what prosthesis we might be choosing for a patient, and it allows us to have that conversation and think about alternatives.
“There are alternatives to try to avoid PPM, everything from which prosthesis we choose to the size of the prosthesis, to whether we fracture a patient’s valve if we are doing a valve-in-valve procedure. In the future, in some situations, we might even choose a low-risk or low-intermediate-risk patient for surgery with an enlargement operation in order to get a larger effective orifice area. So there are choices that we can make, and we should start thinking about that in the heart-team approach.”
Findings in context
The new study reinforces the message “that hemodynamics matter,” he said. “To the extent that we can get larger valves in and get better results from those valves, it will reduce the frequency of PPM. That’s something as operators we don’t spend as much time focusing on, and this will refocus our attention in trying to prevent PPM by being more diligent in terms of prosthesis choice and some operator characteristics, to try to reduce the gradients and improve the effective orifice areas as much as we can.”
Panelist Jeffrey J. Popma, MD, an interventional cardiologist at Beth Israel Deaconess Medical Center, Boston, noted that he and his colleagues have observed similar trends in their smaller studies but had difficulty teasing out contributors. “It really goes back to the preprocedural planning about what valve you can get in, and larger orifice area is certainly better,” he concurred. “So I do think that this is a phenomenal addition.”
Study details
For the study, Dr. Herrmann and his colleagues analyzed 2014-2107 data from the STS/ACC Transcatheter Valve Therapy Registry, a national surveillance and quality improvement system. They identified enrollees aged 65 years or older at the time of their TAVR procedure who had fee-for-service Medicare and linked them to Centers for Medicare & Medicaid Services claims data to assess outcomes.
Overall, 12.1% of patients had severe PPM, defined as an effective valve orifice area indexed to body surface area of less than 0.65 cm2/m2 on discharge echocardiography, and another 24.6% had moderate PPM, Dr. Herrmann reported at the meeting, sponsored by the Cardiovascular Research Foundation.
The strongest multivariate predictors of severe PPM were small prosthetic valve size (up to 23 mm in diameter) (odds ratio, 2.77), a valve-in-valve procedure (OR, 2.78), larger body surface area (OR, 1.71 per 0.2-U increase), and female sex (OR, 1.46). Odds also increased with decreasing age and were elevated for patients of nonwhite/Hispanic race and those having a lower ejection fraction, atrial fibrillation or flutter, or severe mitral or tricuspid regurgitation.
It was not possible to assess specific valves as predictors of mismatch because the registry prohibits comparisons across manufacturers, according to Dr. Herrmann.
One-year mortality, the study’s primary endpoint, was 17.2% in patients with severe PPM, compared with 15.8% in patients with moderate or no PPM (adjusted hazard ratio, 1.19; P less than .001). Findings were similar across subgroups.
The 1-year rate of heart failure rehospitalization was 14.7% in patients with severe PPM, compared with 12.2% in patients with moderate or no PPM (AHR, 1.12; P = .017).
“I would point out that these [outcome] curves are divergent at 1 year,” Dr. Herrmann noted. “So if we look at low-intermediate-risk and low-risk patients and younger patients, who may be more active and who see the effects of PPM more commonly and who are going to be living more than 1 year, we are going to have to consider this going forward in a more important way.”
Severe PPM did not significantly influence the rate of stroke (which stood at about 4% in each group) or worsen quality of life score at 1 year.
Dr. Herrmann disclosed receiving institutional grant/research support from Abbott Vascular, Bayer, Boston Scientific, Corvia Medical, Edwards Lifesciences, Medtronic, and St. Jude Medical, as well as consulting fees/honoraria from Edwards, Medtronic, and Siemens Healthineers.
REPORTING FROM TCT 2018
Key clinical point:
Major finding: Patients with severe PPM after TAVR had elevated risks of heart failure rehospitalization (adjusted hazard ratio, 1.12) and death (AHR, 1.19).
Study details: A retrospective cohort study of 62,125 patients aged 65 years or older who underwent TAVR and were captured in the national STS/ACC Transcatheter Valve Therapy Registry.
Disclosures: Dr. Herrmann disclosed receiving institutional grant/research support from Abbott Vascular, Bayer, Boston Scientific, Corvia Medical, Edwards Lifesciences, Medtronic, and St. Jude Medical, as well as consulting fees/honoraria from Edwards, Medtronic, and Siemens Healthineers.
Novel device improves mitral regurgitation 30% in REDUCE-FMR
SAN DIEGO – In patients with heart failure and functional mitral regurgitation, implantation of an investigational device led to reduced MR and improved left ventricular remodeling at 1 year, compared with patients who received sham treatment in the REDUCE-FMR trial.
The device showed promise in this trial, despite a small sample size, and its nature makes it possible to follow up with other procedures if the disease progresses. “The advantage of this technique is that all other options are still open,” Horst Sievert, MD, director of the CardioVascular Center in Frankfurt, said during a press conference at the Transcatheter Cardiovascular Therapeutics annual meeting.
The Carillon Mitral Counter System includes two anchors, one in the great cardiac vein and one in the coronary sinus, connected by a shaping ribbon. The tension of the ribbon bolsters the mitral annulus, which in turn reduces mitral regurgitation.
REDUCE-FMR recruited 120 patients from centers in eight countries and randomized 87 to the Carillon device (73 implanted) and 33 to a sham procedure. Sham patients were sedated and received a coronary sinus angiogram. Patients were included if they had dilated ischemic or nonischemic cardiomyopathy and moderate to severe functional MR, among other requirements. Exclusion criteria included existing coronary artery stents in the implant target zone, severe mitral annular calcification, and significant organic mitral valve pathology.
The primary endpoint was the mean reduction of regurgitant volume at 1 year. The treated patients had a 22% reduction of 7.1 mL, while the sham group on average had an 8% increase of 3.3 mL (P = .03). In the as-treated subpopulation, which comprised 45 patients in the treatment group and 13 controls, the values were –7.5 mL and +3.3 mL (P = .02). A per-protocol analysis, which excluded patients who did not meet protocol criteria, led to an amplification of the effect when the study design was adhered to (–12.5 mL vs. +1.3 mL), though this result did not achieve statistical significance owing to the small sample size.
For the safety endpoints, the researchers examined the frequency of major adverse events (MAE), including death, myocardial infarction, cardiac perforation, device embolism, and surgery or percutaneous coronary intervention related to the device at 1 year. In the treatment group, 16.1% experienced a MAE, compared with 18.2% of control patients, a statistically nonsignificant difference.
A secondary efficacy endpoint of change in left ventricular end-diastolic volume showed improvements in the treatment group at 6 months (–12.4 mL) and 12 months (–8.6 mL), compared with increases in the sham group at 6 months (+5.4 mL) and 12 months (+6.5 mL). A similar trend occurred in left ventricular end-systolic volume (–7.8 mL and –4.8 mL; +3.4 mL and +6.1 mL, respectively).
The study was conducted in a patient population similar to that of the COAPT trial, which examined implantation of Abbott’s MitraClip. That study, presented here at TCT 2018 and simultaneously published in the New England Journal of Medicine, also examined patients with heart failure and secondary MR.
However, in REDUCE-FMR, many of the patients had milder heart failure than the researchers had expected: 44.8% in the treatment group had NYHA class II, as did 48.5% in the sham group. That surprise may help identify an appropriate patient population. “I think this device may have a nice spot in between medical therapy and MitraClip implantation, because we have, by chance, a patient population with mild heart insufficiency and mild MR,” said Dr. Sievert.
The two devices also showed different physiologic effects, Michael Mack, MD, said at a press conference. “One subtle difference is that, in this trial, the difference is due to both positive left ventricular remodeling in the treatment arm and continued progression in the sham control. In COAPT, the difference in improvement that we saw was totally due to prevention of progression of disease. We just stabilized the disease to where it was at. So that’s an intriguing difference here, that you actually were able to demonstrate positive left ventricular remodeling,” noted Dr. Mack, medical director for cardiovascular surgery at Baylor Scott & White Medical Center, Plano, Tex. He was a coinvestigator in the COAPT trial.
REDUCE-FMR was funded by Cardiac Dimensions. Dr. Sievert has received consulting fees, travel expenses, and study honoraria from Cardiac Dimensions, and 35 other companies. Dr. Mack has received grant support or had a research contract with Abbott Vascular, Medtronic, and Edwards Lifesciences.
SAN DIEGO – In patients with heart failure and functional mitral regurgitation, implantation of an investigational device led to reduced MR and improved left ventricular remodeling at 1 year, compared with patients who received sham treatment in the REDUCE-FMR trial.
The device showed promise in this trial, despite a small sample size, and its nature makes it possible to follow up with other procedures if the disease progresses. “The advantage of this technique is that all other options are still open,” Horst Sievert, MD, director of the CardioVascular Center in Frankfurt, said during a press conference at the Transcatheter Cardiovascular Therapeutics annual meeting.
The Carillon Mitral Counter System includes two anchors, one in the great cardiac vein and one in the coronary sinus, connected by a shaping ribbon. The tension of the ribbon bolsters the mitral annulus, which in turn reduces mitral regurgitation.
REDUCE-FMR recruited 120 patients from centers in eight countries and randomized 87 to the Carillon device (73 implanted) and 33 to a sham procedure. Sham patients were sedated and received a coronary sinus angiogram. Patients were included if they had dilated ischemic or nonischemic cardiomyopathy and moderate to severe functional MR, among other requirements. Exclusion criteria included existing coronary artery stents in the implant target zone, severe mitral annular calcification, and significant organic mitral valve pathology.
The primary endpoint was the mean reduction of regurgitant volume at 1 year. The treated patients had a 22% reduction of 7.1 mL, while the sham group on average had an 8% increase of 3.3 mL (P = .03). In the as-treated subpopulation, which comprised 45 patients in the treatment group and 13 controls, the values were –7.5 mL and +3.3 mL (P = .02). A per-protocol analysis, which excluded patients who did not meet protocol criteria, led to an amplification of the effect when the study design was adhered to (–12.5 mL vs. +1.3 mL), though this result did not achieve statistical significance owing to the small sample size.
For the safety endpoints, the researchers examined the frequency of major adverse events (MAE), including death, myocardial infarction, cardiac perforation, device embolism, and surgery or percutaneous coronary intervention related to the device at 1 year. In the treatment group, 16.1% experienced a MAE, compared with 18.2% of control patients, a statistically nonsignificant difference.
A secondary efficacy endpoint of change in left ventricular end-diastolic volume showed improvements in the treatment group at 6 months (–12.4 mL) and 12 months (–8.6 mL), compared with increases in the sham group at 6 months (+5.4 mL) and 12 months (+6.5 mL). A similar trend occurred in left ventricular end-systolic volume (–7.8 mL and –4.8 mL; +3.4 mL and +6.1 mL, respectively).
The study was conducted in a patient population similar to that of the COAPT trial, which examined implantation of Abbott’s MitraClip. That study, presented here at TCT 2018 and simultaneously published in the New England Journal of Medicine, also examined patients with heart failure and secondary MR.
However, in REDUCE-FMR, many of the patients had milder heart failure than the researchers had expected: 44.8% in the treatment group had NYHA class II, as did 48.5% in the sham group. That surprise may help identify an appropriate patient population. “I think this device may have a nice spot in between medical therapy and MitraClip implantation, because we have, by chance, a patient population with mild heart insufficiency and mild MR,” said Dr. Sievert.
The two devices also showed different physiologic effects, Michael Mack, MD, said at a press conference. “One subtle difference is that, in this trial, the difference is due to both positive left ventricular remodeling in the treatment arm and continued progression in the sham control. In COAPT, the difference in improvement that we saw was totally due to prevention of progression of disease. We just stabilized the disease to where it was at. So that’s an intriguing difference here, that you actually were able to demonstrate positive left ventricular remodeling,” noted Dr. Mack, medical director for cardiovascular surgery at Baylor Scott & White Medical Center, Plano, Tex. He was a coinvestigator in the COAPT trial.
REDUCE-FMR was funded by Cardiac Dimensions. Dr. Sievert has received consulting fees, travel expenses, and study honoraria from Cardiac Dimensions, and 35 other companies. Dr. Mack has received grant support or had a research contract with Abbott Vascular, Medtronic, and Edwards Lifesciences.
SAN DIEGO – In patients with heart failure and functional mitral regurgitation, implantation of an investigational device led to reduced MR and improved left ventricular remodeling at 1 year, compared with patients who received sham treatment in the REDUCE-FMR trial.
The device showed promise in this trial, despite a small sample size, and its nature makes it possible to follow up with other procedures if the disease progresses. “The advantage of this technique is that all other options are still open,” Horst Sievert, MD, director of the CardioVascular Center in Frankfurt, said during a press conference at the Transcatheter Cardiovascular Therapeutics annual meeting.
The Carillon Mitral Counter System includes two anchors, one in the great cardiac vein and one in the coronary sinus, connected by a shaping ribbon. The tension of the ribbon bolsters the mitral annulus, which in turn reduces mitral regurgitation.
REDUCE-FMR recruited 120 patients from centers in eight countries and randomized 87 to the Carillon device (73 implanted) and 33 to a sham procedure. Sham patients were sedated and received a coronary sinus angiogram. Patients were included if they had dilated ischemic or nonischemic cardiomyopathy and moderate to severe functional MR, among other requirements. Exclusion criteria included existing coronary artery stents in the implant target zone, severe mitral annular calcification, and significant organic mitral valve pathology.
The primary endpoint was the mean reduction of regurgitant volume at 1 year. The treated patients had a 22% reduction of 7.1 mL, while the sham group on average had an 8% increase of 3.3 mL (P = .03). In the as-treated subpopulation, which comprised 45 patients in the treatment group and 13 controls, the values were –7.5 mL and +3.3 mL (P = .02). A per-protocol analysis, which excluded patients who did not meet protocol criteria, led to an amplification of the effect when the study design was adhered to (–12.5 mL vs. +1.3 mL), though this result did not achieve statistical significance owing to the small sample size.
For the safety endpoints, the researchers examined the frequency of major adverse events (MAE), including death, myocardial infarction, cardiac perforation, device embolism, and surgery or percutaneous coronary intervention related to the device at 1 year. In the treatment group, 16.1% experienced a MAE, compared with 18.2% of control patients, a statistically nonsignificant difference.
A secondary efficacy endpoint of change in left ventricular end-diastolic volume showed improvements in the treatment group at 6 months (–12.4 mL) and 12 months (–8.6 mL), compared with increases in the sham group at 6 months (+5.4 mL) and 12 months (+6.5 mL). A similar trend occurred in left ventricular end-systolic volume (–7.8 mL and –4.8 mL; +3.4 mL and +6.1 mL, respectively).
The study was conducted in a patient population similar to that of the COAPT trial, which examined implantation of Abbott’s MitraClip. That study, presented here at TCT 2018 and simultaneously published in the New England Journal of Medicine, also examined patients with heart failure and secondary MR.
However, in REDUCE-FMR, many of the patients had milder heart failure than the researchers had expected: 44.8% in the treatment group had NYHA class II, as did 48.5% in the sham group. That surprise may help identify an appropriate patient population. “I think this device may have a nice spot in between medical therapy and MitraClip implantation, because we have, by chance, a patient population with mild heart insufficiency and mild MR,” said Dr. Sievert.
The two devices also showed different physiologic effects, Michael Mack, MD, said at a press conference. “One subtle difference is that, in this trial, the difference is due to both positive left ventricular remodeling in the treatment arm and continued progression in the sham control. In COAPT, the difference in improvement that we saw was totally due to prevention of progression of disease. We just stabilized the disease to where it was at. So that’s an intriguing difference here, that you actually were able to demonstrate positive left ventricular remodeling,” noted Dr. Mack, medical director for cardiovascular surgery at Baylor Scott & White Medical Center, Plano, Tex. He was a coinvestigator in the COAPT trial.
REDUCE-FMR was funded by Cardiac Dimensions. Dr. Sievert has received consulting fees, travel expenses, and study honoraria from Cardiac Dimensions, and 35 other companies. Dr. Mack has received grant support or had a research contract with Abbott Vascular, Medtronic, and Edwards Lifesciences.
REPORTING FROM TCT 2018
Key clinical point: The device led to improvement in mitral regurgitation as well as ventricular remodeling.
Major finding:
Study details: REDUCE-FMR, a randomized, sham controlled trial of 120 patients from 8 countries.
Disclosures: REDUCE-FMR was funded by Cardiac Dimensions. Dr. Sievert has received consulting fees, travel expenses, and study honoraria from Cardiac Dimensions, and 35 other companies. Dr. Mack has received grant support or had a research contract with Abbott Vascular, Medtronic, and Edwards Lifesciences.