User login
What is the Diagnosis - September 2018
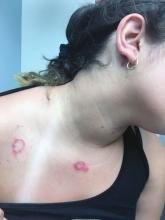
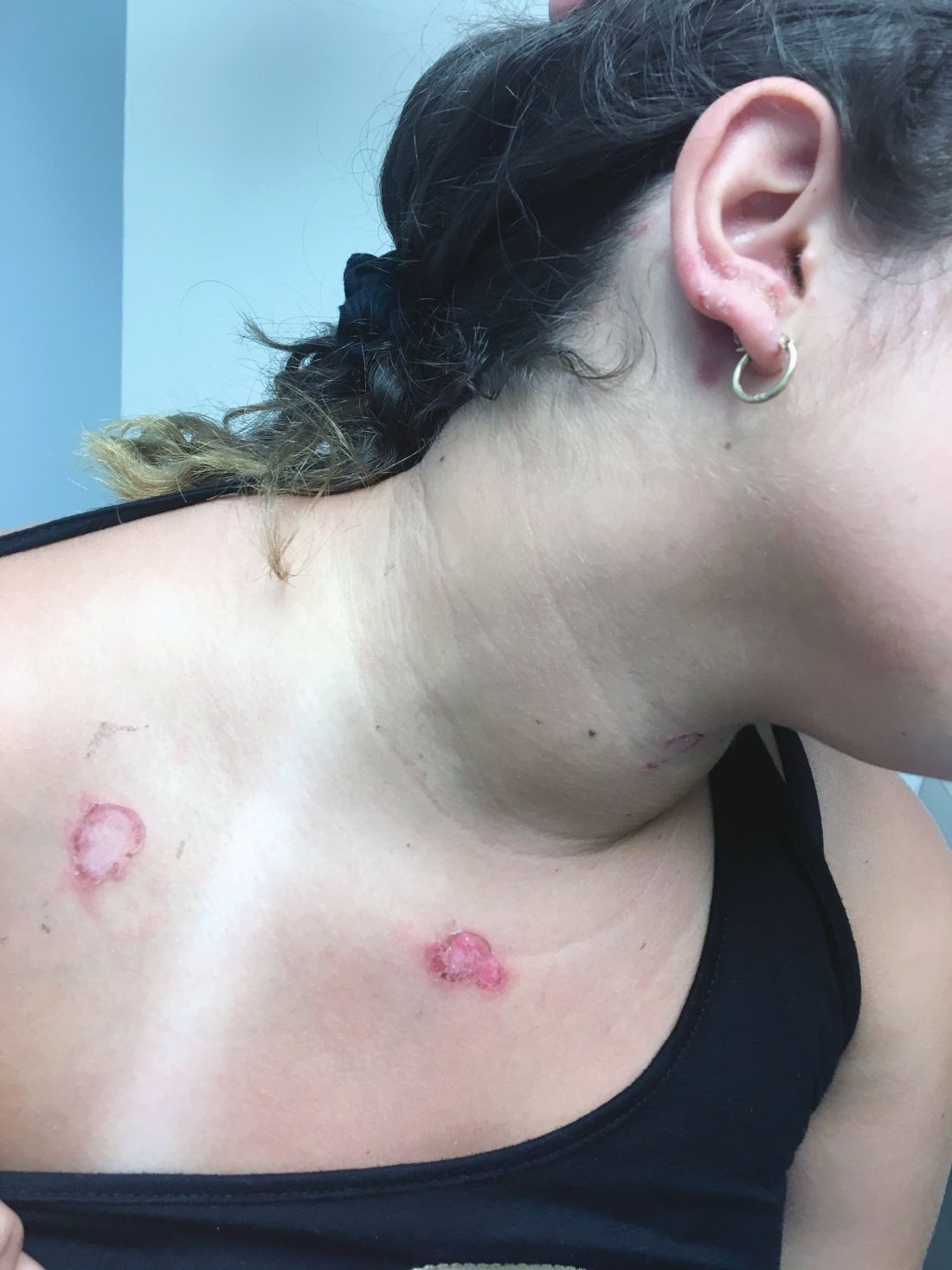
At the visit, the girl’s skin scrapings were analyzed under the microscope with potassium hydroxide (KOH) and no fungal elements were seen. A culture from one of the lesions was positive for methicillin-sensitive Staphylococcus aureus.
She was diagnosed with bullous impetigo (BI).
Impetigo is the most common superficial skin infection and can present as a nonbullous (most common) and bullous (least common) form.1 Nonbullous impetigo is usually caused the Staphylococcus aureus or Streptococcus pyogenes and tends to occur at sites of prior trauma like insect bites, scratches, atopic dermatitis, or varicella. On the other hand, bullous impetigo is caused by the local production of exfoliative toxins (ETA or ETB) by phage group II of Staphylococcus aureus. The exfoliative toxin binds to desmoglin-1, one of the desmosomal proteins of the skin, causing acantholysis at the level of the granular layer and blister formation. Different from nonbullous impetigo, bullous impetigo tends to occur in normal, undamaged skin. Lesions are more common in neonates and young infants but children also can be affected.
The characteristic lesions in bullous impetigo are small blisters that enlarge to 1-cm to 5-cm bullae that easily rupture, leaving an erythematous plaque with a collarette of scale or “double ring scale,” with minimal crust and mild erythema. They commonly occur on the face, trunk, buttocks, and intertriginous areas. The lesions heal within 4-6 weeks, leaving no scarring. Associated systemic symptoms are rare but some patients can present with weakness, fever, and diarrhea. The toxin can disseminate and cause staphylococcal scalded skin syndrome in neonates or older patients with renal failure or immunodeficiency.
The transmission of Staphylococcus aureus can occur from colonized or infected family members, children in contact sports, as well as contact with animals such as dogs, cattle, and poultry.2 Transmission from a pet rabbit also has been reported. In our patient, transmission from her pet hamster could have occurred as the areas on the body where there were lesions were areas where she was holding and cuddling her new pet.
The differential diagnosis of the type of lesions our patient presented with includes tinea corporis, and bullous tinea, which also can be transmitted by animals such as kittens. A KOH analysis ruled out this diagnosis. Tinea skin lesions tend to be more scaly than bullous impetigo lesions, which are more inflamed and crusted. Bullous arthropod reactions should be considered in the differential diagnosis as well. Bullous bite reaction lesions present with tense bullae, as they are subepidermal in nature and are pruritic. Subacute cutaneous lupus lesions present as annular scaly plaques with an erythematous border and central clearing usually in sun exposed areas similar to the distribution of our patient. Severe contact dermatitis reactions also can blister and form similar lesions as seen in our patient but with the difference that our patient didn’t complain of pruritus, which is a characteristic feature of allergic contact dermatitis. In neonates or young infants with bullous lesions other conditions such as herpes simplex infection, epidermolysis bullosa, bullous pemphigoid, linear IgA bullous dermatosis, bullous mastocytosis, and bullous erythema multiforme should be considered in the differential diagnosis.
First line treatment for impetigo consists of the use of topical application of mupirocin (Bactroban) 2% ointment, retapamulin (Altabax) 1% ointment, or fusidic acid 2% cream. A Cochrane review compared systemic versus topical treatment for impetigo concluding that topical treatment with either mupirocin or retapamulin is equally if not more effective than oral antibiotics.3 Ozenoxacin (Xepi), a new nonfluorinated topical quinolone has recently been Food and Drug Administration approved for the treatment of localized impetigo in patients 2 months of age and older.4 When there is treatment failure with topical antibiotics, widespread disease, or systemic symptoms, oral antimicrobials should be consider, such as beta-lactamase–resistant penicillin, first-generation cephalosporins, or clindamycin. The use of bleach baths and general hygiene measures for 4 months can reduce the risks of recurrence in 20% of the patients as noted by a study by Kaplan et al.5
Our patient was treated with oral cephalexin for 7 days as well as topical mupirocin with fast resolution of the lesions. Sadly, the parents gave her hamster pet away.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected]
References
1. Am Fam Physician. 2014 Aug 15;90(4):229-35.
2. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Jun;265(1-2):218-26.
3. Cochrane Database Syst Rev. 2012 Jan 18;1:CD003261.
4. Ann Pharmacother. 2018 Jun 1:1060028018786510.
5. Clin Infect Dis. 2014 Mar;58(5):679-82.
At the visit, the girl’s skin scrapings were analyzed under the microscope with potassium hydroxide (KOH) and no fungal elements were seen. A culture from one of the lesions was positive for methicillin-sensitive Staphylococcus aureus.
She was diagnosed with bullous impetigo (BI).
Impetigo is the most common superficial skin infection and can present as a nonbullous (most common) and bullous (least common) form.1 Nonbullous impetigo is usually caused the Staphylococcus aureus or Streptococcus pyogenes and tends to occur at sites of prior trauma like insect bites, scratches, atopic dermatitis, or varicella. On the other hand, bullous impetigo is caused by the local production of exfoliative toxins (ETA or ETB) by phage group II of Staphylococcus aureus. The exfoliative toxin binds to desmoglin-1, one of the desmosomal proteins of the skin, causing acantholysis at the level of the granular layer and blister formation. Different from nonbullous impetigo, bullous impetigo tends to occur in normal, undamaged skin. Lesions are more common in neonates and young infants but children also can be affected.
The characteristic lesions in bullous impetigo are small blisters that enlarge to 1-cm to 5-cm bullae that easily rupture, leaving an erythematous plaque with a collarette of scale or “double ring scale,” with minimal crust and mild erythema. They commonly occur on the face, trunk, buttocks, and intertriginous areas. The lesions heal within 4-6 weeks, leaving no scarring. Associated systemic symptoms are rare but some patients can present with weakness, fever, and diarrhea. The toxin can disseminate and cause staphylococcal scalded skin syndrome in neonates or older patients with renal failure or immunodeficiency.
The transmission of Staphylococcus aureus can occur from colonized or infected family members, children in contact sports, as well as contact with animals such as dogs, cattle, and poultry.2 Transmission from a pet rabbit also has been reported. In our patient, transmission from her pet hamster could have occurred as the areas on the body where there were lesions were areas where she was holding and cuddling her new pet.
The differential diagnosis of the type of lesions our patient presented with includes tinea corporis, and bullous tinea, which also can be transmitted by animals such as kittens. A KOH analysis ruled out this diagnosis. Tinea skin lesions tend to be more scaly than bullous impetigo lesions, which are more inflamed and crusted. Bullous arthropod reactions should be considered in the differential diagnosis as well. Bullous bite reaction lesions present with tense bullae, as they are subepidermal in nature and are pruritic. Subacute cutaneous lupus lesions present as annular scaly plaques with an erythematous border and central clearing usually in sun exposed areas similar to the distribution of our patient. Severe contact dermatitis reactions also can blister and form similar lesions as seen in our patient but with the difference that our patient didn’t complain of pruritus, which is a characteristic feature of allergic contact dermatitis. In neonates or young infants with bullous lesions other conditions such as herpes simplex infection, epidermolysis bullosa, bullous pemphigoid, linear IgA bullous dermatosis, bullous mastocytosis, and bullous erythema multiforme should be considered in the differential diagnosis.
First line treatment for impetigo consists of the use of topical application of mupirocin (Bactroban) 2% ointment, retapamulin (Altabax) 1% ointment, or fusidic acid 2% cream. A Cochrane review compared systemic versus topical treatment for impetigo concluding that topical treatment with either mupirocin or retapamulin is equally if not more effective than oral antibiotics.3 Ozenoxacin (Xepi), a new nonfluorinated topical quinolone has recently been Food and Drug Administration approved for the treatment of localized impetigo in patients 2 months of age and older.4 When there is treatment failure with topical antibiotics, widespread disease, or systemic symptoms, oral antimicrobials should be consider, such as beta-lactamase–resistant penicillin, first-generation cephalosporins, or clindamycin. The use of bleach baths and general hygiene measures for 4 months can reduce the risks of recurrence in 20% of the patients as noted by a study by Kaplan et al.5
Our patient was treated with oral cephalexin for 7 days as well as topical mupirocin with fast resolution of the lesions. Sadly, the parents gave her hamster pet away.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected]
References
1. Am Fam Physician. 2014 Aug 15;90(4):229-35.
2. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Jun;265(1-2):218-26.
3. Cochrane Database Syst Rev. 2012 Jan 18;1:CD003261.
4. Ann Pharmacother. 2018 Jun 1:1060028018786510.
5. Clin Infect Dis. 2014 Mar;58(5):679-82.
At the visit, the girl’s skin scrapings were analyzed under the microscope with potassium hydroxide (KOH) and no fungal elements were seen. A culture from one of the lesions was positive for methicillin-sensitive Staphylococcus aureus.
She was diagnosed with bullous impetigo (BI).
Impetigo is the most common superficial skin infection and can present as a nonbullous (most common) and bullous (least common) form.1 Nonbullous impetigo is usually caused the Staphylococcus aureus or Streptococcus pyogenes and tends to occur at sites of prior trauma like insect bites, scratches, atopic dermatitis, or varicella. On the other hand, bullous impetigo is caused by the local production of exfoliative toxins (ETA or ETB) by phage group II of Staphylococcus aureus. The exfoliative toxin binds to desmoglin-1, one of the desmosomal proteins of the skin, causing acantholysis at the level of the granular layer and blister formation. Different from nonbullous impetigo, bullous impetigo tends to occur in normal, undamaged skin. Lesions are more common in neonates and young infants but children also can be affected.
The characteristic lesions in bullous impetigo are small blisters that enlarge to 1-cm to 5-cm bullae that easily rupture, leaving an erythematous plaque with a collarette of scale or “double ring scale,” with minimal crust and mild erythema. They commonly occur on the face, trunk, buttocks, and intertriginous areas. The lesions heal within 4-6 weeks, leaving no scarring. Associated systemic symptoms are rare but some patients can present with weakness, fever, and diarrhea. The toxin can disseminate and cause staphylococcal scalded skin syndrome in neonates or older patients with renal failure or immunodeficiency.
The transmission of Staphylococcus aureus can occur from colonized or infected family members, children in contact sports, as well as contact with animals such as dogs, cattle, and poultry.2 Transmission from a pet rabbit also has been reported. In our patient, transmission from her pet hamster could have occurred as the areas on the body where there were lesions were areas where she was holding and cuddling her new pet.
The differential diagnosis of the type of lesions our patient presented with includes tinea corporis, and bullous tinea, which also can be transmitted by animals such as kittens. A KOH analysis ruled out this diagnosis. Tinea skin lesions tend to be more scaly than bullous impetigo lesions, which are more inflamed and crusted. Bullous arthropod reactions should be considered in the differential diagnosis as well. Bullous bite reaction lesions present with tense bullae, as they are subepidermal in nature and are pruritic. Subacute cutaneous lupus lesions present as annular scaly plaques with an erythematous border and central clearing usually in sun exposed areas similar to the distribution of our patient. Severe contact dermatitis reactions also can blister and form similar lesions as seen in our patient but with the difference that our patient didn’t complain of pruritus, which is a characteristic feature of allergic contact dermatitis. In neonates or young infants with bullous lesions other conditions such as herpes simplex infection, epidermolysis bullosa, bullous pemphigoid, linear IgA bullous dermatosis, bullous mastocytosis, and bullous erythema multiforme should be considered in the differential diagnosis.
First line treatment for impetigo consists of the use of topical application of mupirocin (Bactroban) 2% ointment, retapamulin (Altabax) 1% ointment, or fusidic acid 2% cream. A Cochrane review compared systemic versus topical treatment for impetigo concluding that topical treatment with either mupirocin or retapamulin is equally if not more effective than oral antibiotics.3 Ozenoxacin (Xepi), a new nonfluorinated topical quinolone has recently been Food and Drug Administration approved for the treatment of localized impetigo in patients 2 months of age and older.4 When there is treatment failure with topical antibiotics, widespread disease, or systemic symptoms, oral antimicrobials should be consider, such as beta-lactamase–resistant penicillin, first-generation cephalosporins, or clindamycin. The use of bleach baths and general hygiene measures for 4 months can reduce the risks of recurrence in 20% of the patients as noted by a study by Kaplan et al.5
Our patient was treated with oral cephalexin for 7 days as well as topical mupirocin with fast resolution of the lesions. Sadly, the parents gave her hamster pet away.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected]
References
1. Am Fam Physician. 2014 Aug 15;90(4):229-35.
2. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Jun;265(1-2):218-26.
3. Cochrane Database Syst Rev. 2012 Jan 18;1:CD003261.
4. Ann Pharmacother. 2018 Jun 1:1060028018786510.
5. Clin Infect Dis. 2014 Mar;58(5):679-82.
On physical exam, the girl is in no acute distress. Her vital signs are stable, and she has no fever.
On skin examination, she has several erythematous, crusted scaly plaques with double ring of scale on the nose, ears, neck, upper chest, and few on the abdomen. On her left abdomen, there is a small blister. Her seborrheic dermatitis is well controlled with mild erythema behind her ears and minimal scale on her scalp.
Teva Announces FDA Approval of Ajovy (fremanezumab-vfrm)
Teva Pharmaceutical Industries Ltd. announced that the FDA approved Ajovy (fremanezumab-vfrm) injection for the preventive treatment of migraine in adults. Ajovy, a humanized monoclonal antibody that binds to the calcitonin gene-related peptide (CGRP) ligand and blocks its binding to the receptor, is the first and only anti-CGRP treatment for the prevention of migraine with quarterly (675 mg) and monthly (225 mg) dosing options.
“Migraine is a disabling neurological disease that affects more than 36 million people in the United States,” said Stephen Silberstein
Ajovy was evaluated in two Phase III, placebo-controlled clinical trials that enrolled patients with disabling migraine and was studied as both a stand-alone preventive treatment and in combination with oral preventive treatments. In these trials, patients experienced a reduction in monthly migraine days during a 12-week period. The most common adverse reactions (≥ 5% and greater than placebo) were injection site reactions.
Teva Pharmaceutical Industries Ltd. announced that the FDA approved Ajovy (fremanezumab-vfrm) injection for the preventive treatment of migraine in adults. Ajovy, a humanized monoclonal antibody that binds to the calcitonin gene-related peptide (CGRP) ligand and blocks its binding to the receptor, is the first and only anti-CGRP treatment for the prevention of migraine with quarterly (675 mg) and monthly (225 mg) dosing options.
“Migraine is a disabling neurological disease that affects more than 36 million people in the United States,” said Stephen Silberstein
Ajovy was evaluated in two Phase III, placebo-controlled clinical trials that enrolled patients with disabling migraine and was studied as both a stand-alone preventive treatment and in combination with oral preventive treatments. In these trials, patients experienced a reduction in monthly migraine days during a 12-week period. The most common adverse reactions (≥ 5% and greater than placebo) were injection site reactions.
Teva Pharmaceutical Industries Ltd. announced that the FDA approved Ajovy (fremanezumab-vfrm) injection for the preventive treatment of migraine in adults. Ajovy, a humanized monoclonal antibody that binds to the calcitonin gene-related peptide (CGRP) ligand and blocks its binding to the receptor, is the first and only anti-CGRP treatment for the prevention of migraine with quarterly (675 mg) and monthly (225 mg) dosing options.
“Migraine is a disabling neurological disease that affects more than 36 million people in the United States,” said Stephen Silberstein
Ajovy was evaluated in two Phase III, placebo-controlled clinical trials that enrolled patients with disabling migraine and was studied as both a stand-alone preventive treatment and in combination with oral preventive treatments. In these trials, patients experienced a reduction in monthly migraine days during a 12-week period. The most common adverse reactions (≥ 5% and greater than placebo) were injection site reactions.
NELSON trial: CT Screening reduces lung cancer deaths
TORONTO – Computed tomography screening among asymptomatic men at high risk for lung cancer reduced lung cancer deaths by a highly statistically significant 26% at 10 years and appeared to reduce lung cancer mortality risk even more in women in the population-based, NELSON trial.
The findings from this large controlled trial encompassing more than 27,000 CT scans in 15,792 individuals support those from the National Lung Screening Trials (NLST), published in 2011, and should “inform and direct future CT screening programs worldwide,” according to Harry de Koning, MD, who presented the findings at the World Conference on Lung Cancer.
Participants were randomized to CT screening at baseline, 1, 3, and 5.5 years after randomization, or to a control group that received usual care. Overall 157 lung cancer deaths occurred in the screening arm vs. 250 in the control arm. Detection rates varied between 0.8% and 1.1% across screenings (0.9% overall), and the positive predictive value of screening was 41%, Dr. de Koning of Erasmus Medical Center, Rotterdam, the Netherlands, said at the meeting sponsored by the International Association for the Study of Lung Cancer.
Notably, 69% of the 243 lung cancers detected by screening were detected at stage 1A or 1B, compared with 10%-12% being detected at stage 4 in about 50% of control patients and based on registry data in the Netherlands.
“There’s huge importance of this early detection in the screening arm,” Dr. de Koning said.
Additionally, an analysis of a subset of those with lung cancer showed a significant threefold increase in surgical treatment among the screened patients vs. those in the control arm who developed lung cancer (67.7% vs. 24.5%), he said.
CT screening reduced the risk of death from lung cancer by 9% to 41% in men over the course of the study, with an overall reduction of 26% at 10 years, and in a smaller subset of women, the rate-ratio of dying from lung cancer varied from 0.39 to 0.61 at different years of follow-up, he said, noting that this suggests a “significant and even larger reduction” in women.
Study participants were individuals aged 50-74 years in the Netherlands and Leuven, Belgium, who were considered at high risk for lung cancer based on responses to a general questionnaire. Participants’ records were linked with national registries with 100% coverage regarding cancer diagnosis and date and cause of death, and medical records for deceased lung cancer patients were reviewed by a blinded expert panel through 2013, and for the remaining study years cause of death as reported by Statistics Netherlands was used. Compliance among those randomized to the screening group was 86%, Dr. de Koning said.
“These findings show that CT screenings are an effective way to assess lung nodules in people at high risk for lung cancer, often leading to detection of suspicious nodules and subsequent surgical intervention at relatively low rates and with few false positives, and can positively increase the chances of cure in this devastating disease,” Dr. de Koning said in a press statement. “It is the second-largest trial in the world, with an even more favorable outcome than the first trial, the NLST, showed. These results should be used to inform and direct future CT screening in the world.”
During a press briefing, in response to a question about whether lung cancer screening should be offered more widely, he said that yes, countries – including the United States – should take note that “now two large-scale trials show large benefit.”
Dr. de Koning reported having no disclosures.
TORONTO – Computed tomography screening among asymptomatic men at high risk for lung cancer reduced lung cancer deaths by a highly statistically significant 26% at 10 years and appeared to reduce lung cancer mortality risk even more in women in the population-based, NELSON trial.
The findings from this large controlled trial encompassing more than 27,000 CT scans in 15,792 individuals support those from the National Lung Screening Trials (NLST), published in 2011, and should “inform and direct future CT screening programs worldwide,” according to Harry de Koning, MD, who presented the findings at the World Conference on Lung Cancer.
Participants were randomized to CT screening at baseline, 1, 3, and 5.5 years after randomization, or to a control group that received usual care. Overall 157 lung cancer deaths occurred in the screening arm vs. 250 in the control arm. Detection rates varied between 0.8% and 1.1% across screenings (0.9% overall), and the positive predictive value of screening was 41%, Dr. de Koning of Erasmus Medical Center, Rotterdam, the Netherlands, said at the meeting sponsored by the International Association for the Study of Lung Cancer.
Notably, 69% of the 243 lung cancers detected by screening were detected at stage 1A or 1B, compared with 10%-12% being detected at stage 4 in about 50% of control patients and based on registry data in the Netherlands.
“There’s huge importance of this early detection in the screening arm,” Dr. de Koning said.
Additionally, an analysis of a subset of those with lung cancer showed a significant threefold increase in surgical treatment among the screened patients vs. those in the control arm who developed lung cancer (67.7% vs. 24.5%), he said.
CT screening reduced the risk of death from lung cancer by 9% to 41% in men over the course of the study, with an overall reduction of 26% at 10 years, and in a smaller subset of women, the rate-ratio of dying from lung cancer varied from 0.39 to 0.61 at different years of follow-up, he said, noting that this suggests a “significant and even larger reduction” in women.
Study participants were individuals aged 50-74 years in the Netherlands and Leuven, Belgium, who were considered at high risk for lung cancer based on responses to a general questionnaire. Participants’ records were linked with national registries with 100% coverage regarding cancer diagnosis and date and cause of death, and medical records for deceased lung cancer patients were reviewed by a blinded expert panel through 2013, and for the remaining study years cause of death as reported by Statistics Netherlands was used. Compliance among those randomized to the screening group was 86%, Dr. de Koning said.
“These findings show that CT screenings are an effective way to assess lung nodules in people at high risk for lung cancer, often leading to detection of suspicious nodules and subsequent surgical intervention at relatively low rates and with few false positives, and can positively increase the chances of cure in this devastating disease,” Dr. de Koning said in a press statement. “It is the second-largest trial in the world, with an even more favorable outcome than the first trial, the NLST, showed. These results should be used to inform and direct future CT screening in the world.”
During a press briefing, in response to a question about whether lung cancer screening should be offered more widely, he said that yes, countries – including the United States – should take note that “now two large-scale trials show large benefit.”
Dr. de Koning reported having no disclosures.
TORONTO – Computed tomography screening among asymptomatic men at high risk for lung cancer reduced lung cancer deaths by a highly statistically significant 26% at 10 years and appeared to reduce lung cancer mortality risk even more in women in the population-based, NELSON trial.
The findings from this large controlled trial encompassing more than 27,000 CT scans in 15,792 individuals support those from the National Lung Screening Trials (NLST), published in 2011, and should “inform and direct future CT screening programs worldwide,” according to Harry de Koning, MD, who presented the findings at the World Conference on Lung Cancer.
Participants were randomized to CT screening at baseline, 1, 3, and 5.5 years after randomization, or to a control group that received usual care. Overall 157 lung cancer deaths occurred in the screening arm vs. 250 in the control arm. Detection rates varied between 0.8% and 1.1% across screenings (0.9% overall), and the positive predictive value of screening was 41%, Dr. de Koning of Erasmus Medical Center, Rotterdam, the Netherlands, said at the meeting sponsored by the International Association for the Study of Lung Cancer.
Notably, 69% of the 243 lung cancers detected by screening were detected at stage 1A or 1B, compared with 10%-12% being detected at stage 4 in about 50% of control patients and based on registry data in the Netherlands.
“There’s huge importance of this early detection in the screening arm,” Dr. de Koning said.
Additionally, an analysis of a subset of those with lung cancer showed a significant threefold increase in surgical treatment among the screened patients vs. those in the control arm who developed lung cancer (67.7% vs. 24.5%), he said.
CT screening reduced the risk of death from lung cancer by 9% to 41% in men over the course of the study, with an overall reduction of 26% at 10 years, and in a smaller subset of women, the rate-ratio of dying from lung cancer varied from 0.39 to 0.61 at different years of follow-up, he said, noting that this suggests a “significant and even larger reduction” in women.
Study participants were individuals aged 50-74 years in the Netherlands and Leuven, Belgium, who were considered at high risk for lung cancer based on responses to a general questionnaire. Participants’ records were linked with national registries with 100% coverage regarding cancer diagnosis and date and cause of death, and medical records for deceased lung cancer patients were reviewed by a blinded expert panel through 2013, and for the remaining study years cause of death as reported by Statistics Netherlands was used. Compliance among those randomized to the screening group was 86%, Dr. de Koning said.
“These findings show that CT screenings are an effective way to assess lung nodules in people at high risk for lung cancer, often leading to detection of suspicious nodules and subsequent surgical intervention at relatively low rates and with few false positives, and can positively increase the chances of cure in this devastating disease,” Dr. de Koning said in a press statement. “It is the second-largest trial in the world, with an even more favorable outcome than the first trial, the NLST, showed. These results should be used to inform and direct future CT screening in the world.”
During a press briefing, in response to a question about whether lung cancer screening should be offered more widely, he said that yes, countries – including the United States – should take note that “now two large-scale trials show large benefit.”
Dr. de Koning reported having no disclosures.
REPORTING FROM WCLC 2018
Key clinical point: CT screening among high-risk patients significantly reduces lung cancer mortality.
Major finding: CT screening in high-risk patients reduced lung cancer deaths by 26% in men, 39%-61% in women.
Study details: A population-based controlled trial of 15,792 individuals.
Disclosures: Dr. de Koning reported having no disclosures.
Dapagliflozin meets endpoint in top-line DECLARE results, AstraZeneca says
AstraZeneca announced top-line results of its phase III DECLARE-TIMI 58 cardiovascular outcomes trial for dapagliflozin (Farxiga). The 5-year international trial evaluated the cardiovascular outcomes of dapagliflozin compared with placebo in more than 17,000 adults with type 2 diabetes at high cardiovascular risk or established disease.
DECLARE met its primary safety endpoint of noninferiority for major adverse cardiovascular events for dabigatran the company said. Specifically, it achieved a statistically significant reduction in the composite endpoint of hospitalization for heart failure or cardiovascular death.
Dapagliflozin, approved in 2014, is a sodium-glucose cotransporter 2 (SGLT2) inhibitor indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes.
Full results of DECLARE will be presented on November 10 at the American Heart Association annual meeting, according to the AstraZeneca release.
AstraZeneca announced top-line results of its phase III DECLARE-TIMI 58 cardiovascular outcomes trial for dapagliflozin (Farxiga). The 5-year international trial evaluated the cardiovascular outcomes of dapagliflozin compared with placebo in more than 17,000 adults with type 2 diabetes at high cardiovascular risk or established disease.
DECLARE met its primary safety endpoint of noninferiority for major adverse cardiovascular events for dabigatran the company said. Specifically, it achieved a statistically significant reduction in the composite endpoint of hospitalization for heart failure or cardiovascular death.
Dapagliflozin, approved in 2014, is a sodium-glucose cotransporter 2 (SGLT2) inhibitor indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes.
Full results of DECLARE will be presented on November 10 at the American Heart Association annual meeting, according to the AstraZeneca release.
AstraZeneca announced top-line results of its phase III DECLARE-TIMI 58 cardiovascular outcomes trial for dapagliflozin (Farxiga). The 5-year international trial evaluated the cardiovascular outcomes of dapagliflozin compared with placebo in more than 17,000 adults with type 2 diabetes at high cardiovascular risk or established disease.
DECLARE met its primary safety endpoint of noninferiority for major adverse cardiovascular events for dabigatran the company said. Specifically, it achieved a statistically significant reduction in the composite endpoint of hospitalization for heart failure or cardiovascular death.
Dapagliflozin, approved in 2014, is a sodium-glucose cotransporter 2 (SGLT2) inhibitor indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes.
Full results of DECLARE will be presented on November 10 at the American Heart Association annual meeting, according to the AstraZeneca release.
Real-world clues for optimal sequencing of CLL novel agents
NEW YORK – Although optimal sequencing strategies in chronic lymphocytic leukemia are still unclear, real-world data suggest an alternate kinase inhibitor or venetoclax is the best approach for a patient who has received ibrutinib or idelalisib, according to John N. Allan, MD, of Cornell University, New York.
“I think for the most part, there’s enough evidence,” Dr. Allan said at the annual congress on Hematologic Malignancies held by the National Comprehensive Cancer Network.
“If you had one to two lines of therapy, it still favors the novel agents rather than the chemotherapy arms in all these studies,” said Dr. Allan, referring to some of the pivotal trials supporting approval of novel agents in chronic lymphocytic leukemia (CLL). “The earlier we get to these drugs, I believe, the better.”
While venetoclax after ibrutinib is supported by multiple studies, “vice versa is unknown, but there’s seemingly no reason to think it wouldn’t work – different mechanisms of actions, different pathways,” Dr. Allan said.
What is clear, he added, is that retreating those patients with chemoimmunotherapy is not optimal.
In support of that, he cited a multicenter retrospective analysis, which is believed to be the largest real-world experience to date of novel agents in CLL looking at post–kinase inhibitor salvage strategies (Ann Oncol. 2017 May 1;28[5]:1050-6).
Using an alternate kinase inhibitor or venetoclax resulted in superior progression-free survival versus chemoimmunotherapy at the time of initial kinase inhibitor failure in that study, which looked at treatment strategies and outcomes for 683 patients.
Ibrutinib appeared to be superior to idelalisib as a first kinase inhibitor, with significantly better progression-free survival in both frontline and relapsed/refractory settings, and in both complex karyotype and del17p patients, according to the report. Additionally, the response rate to venetoclax seemed superior to that of idelalisib in patients who discontinued ibrutinib because of progression or toxicity.
All of that supports the need for trials to test various sequencing strategies and establish clear treatment algorithms, according to Dr. Allan. “Optimal sequencing is unknown, but real-world data gives us some idea.”
For relapsed/refractory patients, ibrutinib, idelalisib, and venetoclax all have lengthened responses, improved survival, and are approved by the Food and Drug Administration, he added, noting that the toxicity profiles vary and must be understood when dosing and prescribing these agents.
More novel treatments are on the way. On Sept. 24, just days after Dr. Allan’s NCCN presentation, the FDA granted approval to duvelisib for adults with relapsed or refractory CLL or small lymphocytic lymphoma following two or more previous lines of therapy.
Dr. Allan reported financial disclosures related to AbbVie, Acerta Pharma, Genentech, Pharmacyclics, Sunesis Pharmaceuticals, and Verastem Oncology.
NEW YORK – Although optimal sequencing strategies in chronic lymphocytic leukemia are still unclear, real-world data suggest an alternate kinase inhibitor or venetoclax is the best approach for a patient who has received ibrutinib or idelalisib, according to John N. Allan, MD, of Cornell University, New York.
“I think for the most part, there’s enough evidence,” Dr. Allan said at the annual congress on Hematologic Malignancies held by the National Comprehensive Cancer Network.
“If you had one to two lines of therapy, it still favors the novel agents rather than the chemotherapy arms in all these studies,” said Dr. Allan, referring to some of the pivotal trials supporting approval of novel agents in chronic lymphocytic leukemia (CLL). “The earlier we get to these drugs, I believe, the better.”
While venetoclax after ibrutinib is supported by multiple studies, “vice versa is unknown, but there’s seemingly no reason to think it wouldn’t work – different mechanisms of actions, different pathways,” Dr. Allan said.
What is clear, he added, is that retreating those patients with chemoimmunotherapy is not optimal.
In support of that, he cited a multicenter retrospective analysis, which is believed to be the largest real-world experience to date of novel agents in CLL looking at post–kinase inhibitor salvage strategies (Ann Oncol. 2017 May 1;28[5]:1050-6).
Using an alternate kinase inhibitor or venetoclax resulted in superior progression-free survival versus chemoimmunotherapy at the time of initial kinase inhibitor failure in that study, which looked at treatment strategies and outcomes for 683 patients.
Ibrutinib appeared to be superior to idelalisib as a first kinase inhibitor, with significantly better progression-free survival in both frontline and relapsed/refractory settings, and in both complex karyotype and del17p patients, according to the report. Additionally, the response rate to venetoclax seemed superior to that of idelalisib in patients who discontinued ibrutinib because of progression or toxicity.
All of that supports the need for trials to test various sequencing strategies and establish clear treatment algorithms, according to Dr. Allan. “Optimal sequencing is unknown, but real-world data gives us some idea.”
For relapsed/refractory patients, ibrutinib, idelalisib, and venetoclax all have lengthened responses, improved survival, and are approved by the Food and Drug Administration, he added, noting that the toxicity profiles vary and must be understood when dosing and prescribing these agents.
More novel treatments are on the way. On Sept. 24, just days after Dr. Allan’s NCCN presentation, the FDA granted approval to duvelisib for adults with relapsed or refractory CLL or small lymphocytic lymphoma following two or more previous lines of therapy.
Dr. Allan reported financial disclosures related to AbbVie, Acerta Pharma, Genentech, Pharmacyclics, Sunesis Pharmaceuticals, and Verastem Oncology.
NEW YORK – Although optimal sequencing strategies in chronic lymphocytic leukemia are still unclear, real-world data suggest an alternate kinase inhibitor or venetoclax is the best approach for a patient who has received ibrutinib or idelalisib, according to John N. Allan, MD, of Cornell University, New York.
“I think for the most part, there’s enough evidence,” Dr. Allan said at the annual congress on Hematologic Malignancies held by the National Comprehensive Cancer Network.
“If you had one to two lines of therapy, it still favors the novel agents rather than the chemotherapy arms in all these studies,” said Dr. Allan, referring to some of the pivotal trials supporting approval of novel agents in chronic lymphocytic leukemia (CLL). “The earlier we get to these drugs, I believe, the better.”
While venetoclax after ibrutinib is supported by multiple studies, “vice versa is unknown, but there’s seemingly no reason to think it wouldn’t work – different mechanisms of actions, different pathways,” Dr. Allan said.
What is clear, he added, is that retreating those patients with chemoimmunotherapy is not optimal.
In support of that, he cited a multicenter retrospective analysis, which is believed to be the largest real-world experience to date of novel agents in CLL looking at post–kinase inhibitor salvage strategies (Ann Oncol. 2017 May 1;28[5]:1050-6).
Using an alternate kinase inhibitor or venetoclax resulted in superior progression-free survival versus chemoimmunotherapy at the time of initial kinase inhibitor failure in that study, which looked at treatment strategies and outcomes for 683 patients.
Ibrutinib appeared to be superior to idelalisib as a first kinase inhibitor, with significantly better progression-free survival in both frontline and relapsed/refractory settings, and in both complex karyotype and del17p patients, according to the report. Additionally, the response rate to venetoclax seemed superior to that of idelalisib in patients who discontinued ibrutinib because of progression or toxicity.
All of that supports the need for trials to test various sequencing strategies and establish clear treatment algorithms, according to Dr. Allan. “Optimal sequencing is unknown, but real-world data gives us some idea.”
For relapsed/refractory patients, ibrutinib, idelalisib, and venetoclax all have lengthened responses, improved survival, and are approved by the Food and Drug Administration, he added, noting that the toxicity profiles vary and must be understood when dosing and prescribing these agents.
More novel treatments are on the way. On Sept. 24, just days after Dr. Allan’s NCCN presentation, the FDA granted approval to duvelisib for adults with relapsed or refractory CLL or small lymphocytic lymphoma following two or more previous lines of therapy.
Dr. Allan reported financial disclosures related to AbbVie, Acerta Pharma, Genentech, Pharmacyclics, Sunesis Pharmaceuticals, and Verastem Oncology.
EXPERT ANALYSIS FROM NCCN HEMATOLOGIC MALIGNANCIES CONGRESS
PIDS 40th anniversary and a spotlight on pediatric ID
IDWeek 2018 includes the annual meeting of the Pediatric Infectious Diseases Society, which is celebrating its 40th anniversary as a society this year.
Paul Spearman, MD, the PIDS president, specifically highlighted some of the special 40th anniversary events: The Stanley A. Plotkin Lectureship in Vaccinology will be held on Friday, Oct. 5. “This is an annual highlight for PIDS in honor of Stanley Plotkin, the founder of PIDS and [an] inspiration to all of us. We are delighted to have Gary Marshall as the speaker this year giving a talk entitled “Vaccine Hesitancy, History and Human Nature,” Dr. Spearman wrote in an article posted on the PIDS website.
He noted that the PIDS anniversary special celebration, dinner, and lecture in honor of the contributions of George McCracken and John Nelson also will be held Oct. 5.
Throughout the meeting, the latest data and practices for the treatment of infants and children will be featured. Although pediatric issues will be discussed in many sessions, there are a number of events specifically dedicated to pediatric cases.
Of particular interest, on Wednesday morning, Oral Abstract Session 31 will focus on “Infant Viral Infections.” Talks will include “Role of Maternal Antibodies in Protection Against Postnatal Cytomegalovirus Acquisition,” by Frances Saccoccio, MD, PhD, and “Effect of Nasopharyngeal Pneumococcal Carriage on RSV and hMPV Illness Severity in Infants in Nepal,” by Alastair Murray.
On Thursday morning, the symposium “Hot Topics in Pediatric Infectious Diseases” will feature talks by experts in the field detailing this year’s important clinical advances and discoveries that have the potential to impact clinical care.
The Thursday afternoon poster session “Pediatric Antimicrobial and Diagnostic Stewardship” will focus on topics such as “Variation in Antibiotic Use Among Neonates Hospitalized in United States Academically Affiliated Centers,” which will be presented by Prachi Singh, DO, and “Alternative Antibiotic Prescribing for Community Acquired Pneumonia (CAP) in Pediatric Patients in Relation to Allergy Status,” to be presented by Ankita Desai, MD.
There also will be a poster session that afternoon focused on “Maternal-Child Infections” that will feature “Risk Factors for Congenital Infection in the United States: Analysis of the Kids’ Inpatient Database (KID),” by Angela F. Veesenmeyer, MD, and “The Effect of Prenatal Screening for Chlamydia trachomatis (CT) on Chlamydial Conjunctivitis in Infants,” by Natalie Banniettis, MD.
Later that same day, “Mano-a-Mano III,” an interactive session on “Controversies in Pediatric ID,” will focus on the tuberculin skin test vs. the interferon-gamma release assay (IGRA) for TB identification, the use of vancomycin therapy for meningitis, and the value of respiratory viral panel testing.
As part of the program committee, Marsha S. Anderson, MD, FIDSA, from the University of Colorado School of Medicine, serves as this year’s IDWeek chair, for the PIDS portion of the meeting, and Roberta L. DeBiasi, MD, of the Children’s National Health System serves as vice chair.
IDWeek 2018 includes the annual meeting of the Pediatric Infectious Diseases Society, which is celebrating its 40th anniversary as a society this year.
Paul Spearman, MD, the PIDS president, specifically highlighted some of the special 40th anniversary events: The Stanley A. Plotkin Lectureship in Vaccinology will be held on Friday, Oct. 5. “This is an annual highlight for PIDS in honor of Stanley Plotkin, the founder of PIDS and [an] inspiration to all of us. We are delighted to have Gary Marshall as the speaker this year giving a talk entitled “Vaccine Hesitancy, History and Human Nature,” Dr. Spearman wrote in an article posted on the PIDS website.
He noted that the PIDS anniversary special celebration, dinner, and lecture in honor of the contributions of George McCracken and John Nelson also will be held Oct. 5.
Throughout the meeting, the latest data and practices for the treatment of infants and children will be featured. Although pediatric issues will be discussed in many sessions, there are a number of events specifically dedicated to pediatric cases.
Of particular interest, on Wednesday morning, Oral Abstract Session 31 will focus on “Infant Viral Infections.” Talks will include “Role of Maternal Antibodies in Protection Against Postnatal Cytomegalovirus Acquisition,” by Frances Saccoccio, MD, PhD, and “Effect of Nasopharyngeal Pneumococcal Carriage on RSV and hMPV Illness Severity in Infants in Nepal,” by Alastair Murray.
On Thursday morning, the symposium “Hot Topics in Pediatric Infectious Diseases” will feature talks by experts in the field detailing this year’s important clinical advances and discoveries that have the potential to impact clinical care.
The Thursday afternoon poster session “Pediatric Antimicrobial and Diagnostic Stewardship” will focus on topics such as “Variation in Antibiotic Use Among Neonates Hospitalized in United States Academically Affiliated Centers,” which will be presented by Prachi Singh, DO, and “Alternative Antibiotic Prescribing for Community Acquired Pneumonia (CAP) in Pediatric Patients in Relation to Allergy Status,” to be presented by Ankita Desai, MD.
There also will be a poster session that afternoon focused on “Maternal-Child Infections” that will feature “Risk Factors for Congenital Infection in the United States: Analysis of the Kids’ Inpatient Database (KID),” by Angela F. Veesenmeyer, MD, and “The Effect of Prenatal Screening for Chlamydia trachomatis (CT) on Chlamydial Conjunctivitis in Infants,” by Natalie Banniettis, MD.
Later that same day, “Mano-a-Mano III,” an interactive session on “Controversies in Pediatric ID,” will focus on the tuberculin skin test vs. the interferon-gamma release assay (IGRA) for TB identification, the use of vancomycin therapy for meningitis, and the value of respiratory viral panel testing.
As part of the program committee, Marsha S. Anderson, MD, FIDSA, from the University of Colorado School of Medicine, serves as this year’s IDWeek chair, for the PIDS portion of the meeting, and Roberta L. DeBiasi, MD, of the Children’s National Health System serves as vice chair.
IDWeek 2018 includes the annual meeting of the Pediatric Infectious Diseases Society, which is celebrating its 40th anniversary as a society this year.
Paul Spearman, MD, the PIDS president, specifically highlighted some of the special 40th anniversary events: The Stanley A. Plotkin Lectureship in Vaccinology will be held on Friday, Oct. 5. “This is an annual highlight for PIDS in honor of Stanley Plotkin, the founder of PIDS and [an] inspiration to all of us. We are delighted to have Gary Marshall as the speaker this year giving a talk entitled “Vaccine Hesitancy, History and Human Nature,” Dr. Spearman wrote in an article posted on the PIDS website.
He noted that the PIDS anniversary special celebration, dinner, and lecture in honor of the contributions of George McCracken and John Nelson also will be held Oct. 5.
Throughout the meeting, the latest data and practices for the treatment of infants and children will be featured. Although pediatric issues will be discussed in many sessions, there are a number of events specifically dedicated to pediatric cases.
Of particular interest, on Wednesday morning, Oral Abstract Session 31 will focus on “Infant Viral Infections.” Talks will include “Role of Maternal Antibodies in Protection Against Postnatal Cytomegalovirus Acquisition,” by Frances Saccoccio, MD, PhD, and “Effect of Nasopharyngeal Pneumococcal Carriage on RSV and hMPV Illness Severity in Infants in Nepal,” by Alastair Murray.
On Thursday morning, the symposium “Hot Topics in Pediatric Infectious Diseases” will feature talks by experts in the field detailing this year’s important clinical advances and discoveries that have the potential to impact clinical care.
The Thursday afternoon poster session “Pediatric Antimicrobial and Diagnostic Stewardship” will focus on topics such as “Variation in Antibiotic Use Among Neonates Hospitalized in United States Academically Affiliated Centers,” which will be presented by Prachi Singh, DO, and “Alternative Antibiotic Prescribing for Community Acquired Pneumonia (CAP) in Pediatric Patients in Relation to Allergy Status,” to be presented by Ankita Desai, MD.
There also will be a poster session that afternoon focused on “Maternal-Child Infections” that will feature “Risk Factors for Congenital Infection in the United States: Analysis of the Kids’ Inpatient Database (KID),” by Angela F. Veesenmeyer, MD, and “The Effect of Prenatal Screening for Chlamydia trachomatis (CT) on Chlamydial Conjunctivitis in Infants,” by Natalie Banniettis, MD.
Later that same day, “Mano-a-Mano III,” an interactive session on “Controversies in Pediatric ID,” will focus on the tuberculin skin test vs. the interferon-gamma release assay (IGRA) for TB identification, the use of vancomycin therapy for meningitis, and the value of respiratory viral panel testing.
As part of the program committee, Marsha S. Anderson, MD, FIDSA, from the University of Colorado School of Medicine, serves as this year’s IDWeek chair, for the PIDS portion of the meeting, and Roberta L. DeBiasi, MD, of the Children’s National Health System serves as vice chair.
Health Care Disparities Dominate at AVAHO Meeting
More than 500 VA hematologists, oncologists, pharmacists, nurses, social workers, and cancer registrars came to the 14th annual Association of VA Hematology/Oncology meeting held outside of Chicago, September 28 through September 30. Mark Klein, MD, succeeded Rusty Crawford, BPharm, as president and William Wachsman, MD, PhD, was named president-elect.
A key focus of the meeting was health care disparities and providing cancer care to underserved populations. An entire track of the meeting was devoted to patients with serious mental illness (SMI), including discussions of the risk of suicide Also a focus was the challenge of providing optimal treatments for patients who may not be adherent because they do not have access to support networks or steady housing.
According to Jessica B. Geller, PhD, MS, and Jessica L. Brand, PhD, a patient with a SMI diagnosis should not preclude considering aggressive treatment but requires a close level of care. Cancer care of patients with SMI requires close collaboration with mental health and pharmacy providers to manage these complex patients. According to Elizabeth Holyman, PsyD, care providers should give serious attention to complaints despite difficult behavior, treat pain, use active listening and provide interpersonal supports, and assume people with SMI have similar needs to the non-SMI.
For both patients with SMI and the entire veteran population, a cancer diagnosis can raise the risk of suicide, explained Catherine Rotolo, LISW-S. Suicidal ideation occurs in nearly 9% of people with cancer, most frequently in the first 3 months after diagnosis, and remains a significant cause of mortality for cancer patients. For patients with cancer, the rate of suicide—already elevated for veterans—is twice that of the baseline rate.
In a keynote address, Lisa Margolies, executive director of the LGBT Cancer Network, cautioned care providers not only to provide a welcoming environment, but also avoid making any assumptions about sexual behavior. “Do ask, we want to tell,” she told attendees, “Make it a part of your regular questions.” According to Margolies, there are more than 1 million LGBT veterans; they use the VA at higher rates, and the best estimate is that there are about 150,000 transgender veterans. According to Margolies, many do not tell their health care providers about their identity; 24% of lesbian, gay, bisexual, and transgender (LGBT) adults withheld information about sexual practices; 9.4% of men who identified as straight had sex with another man within the previous year. In another survey, more than 77% of lesbians had at least 1 sexual experience with a man—8% in the previous year. Margolies’ key point: avoid assumptions about a patient’s identity and simply take a comprehensive medical history so that you better understand the individual in front of you.
Another track of sessions focused on the challenges women face receiving care in the VA. Many female veterans, especially survivors of military sexual trauma (MST), are not comfortable going to the VA for care or being around groups of male veterans. Col (ret) Mona Pearl Treyball, PhD, RN, pointed out that up to 70% of women veterans experienced MST; most never reported it. That point was reinforced by Chad Hamilton, MD, who noted that among veteran women “Significantly higher proportion of users, compared to nonusers reported avoiding VA because of past sexual trauma (19% of users vs 8% of nonusers).
Yeun-hee Anna Park reported on the High Risk Breast Cancer Screening Program, a 10-site pilot project to assess breast cancer risk. The quality improvement program sought to enhance screening for high-risk breast cancer and increase use of chemoprevention and genetic counseling in accordance with national guidelines. In the pilot, women veterans were at increased risk of breast cancer compared with that of the general population (46% vs 13%, respectively), based on a high rate of prior breast biopsies or positive family history. Moreover, posttraumatic stress disorder rates were nearly 3 times the national average. In the program, use of chemoprevention was nearly 2 times the national average.
Female patients undergoing treatment for cancer also face distinct risks related to fertility, bone health, and vasomotor symptoms of menopause. According to Tyler Fenton, PharmD, some cancer treatment approaches involving chemotherapy, radiation, and/or surgery carry a risk of ovarian failure and the accompanying symptoms of premature menopause. Dr. Fenton noted, that menopausal symptoms such as hot flashes are reported to occur in as many as 73% of breast cancer survivors, and 42% of female patients with cancer of reproductive age may develop premature ovarian failure as a result of their chemotherapy.
More than 500 VA hematologists, oncologists, pharmacists, nurses, social workers, and cancer registrars came to the 14th annual Association of VA Hematology/Oncology meeting held outside of Chicago, September 28 through September 30. Mark Klein, MD, succeeded Rusty Crawford, BPharm, as president and William Wachsman, MD, PhD, was named president-elect.
A key focus of the meeting was health care disparities and providing cancer care to underserved populations. An entire track of the meeting was devoted to patients with serious mental illness (SMI), including discussions of the risk of suicide Also a focus was the challenge of providing optimal treatments for patients who may not be adherent because they do not have access to support networks or steady housing.
According to Jessica B. Geller, PhD, MS, and Jessica L. Brand, PhD, a patient with a SMI diagnosis should not preclude considering aggressive treatment but requires a close level of care. Cancer care of patients with SMI requires close collaboration with mental health and pharmacy providers to manage these complex patients. According to Elizabeth Holyman, PsyD, care providers should give serious attention to complaints despite difficult behavior, treat pain, use active listening and provide interpersonal supports, and assume people with SMI have similar needs to the non-SMI.
For both patients with SMI and the entire veteran population, a cancer diagnosis can raise the risk of suicide, explained Catherine Rotolo, LISW-S. Suicidal ideation occurs in nearly 9% of people with cancer, most frequently in the first 3 months after diagnosis, and remains a significant cause of mortality for cancer patients. For patients with cancer, the rate of suicide—already elevated for veterans—is twice that of the baseline rate.
In a keynote address, Lisa Margolies, executive director of the LGBT Cancer Network, cautioned care providers not only to provide a welcoming environment, but also avoid making any assumptions about sexual behavior. “Do ask, we want to tell,” she told attendees, “Make it a part of your regular questions.” According to Margolies, there are more than 1 million LGBT veterans; they use the VA at higher rates, and the best estimate is that there are about 150,000 transgender veterans. According to Margolies, many do not tell their health care providers about their identity; 24% of lesbian, gay, bisexual, and transgender (LGBT) adults withheld information about sexual practices; 9.4% of men who identified as straight had sex with another man within the previous year. In another survey, more than 77% of lesbians had at least 1 sexual experience with a man—8% in the previous year. Margolies’ key point: avoid assumptions about a patient’s identity and simply take a comprehensive medical history so that you better understand the individual in front of you.
Another track of sessions focused on the challenges women face receiving care in the VA. Many female veterans, especially survivors of military sexual trauma (MST), are not comfortable going to the VA for care or being around groups of male veterans. Col (ret) Mona Pearl Treyball, PhD, RN, pointed out that up to 70% of women veterans experienced MST; most never reported it. That point was reinforced by Chad Hamilton, MD, who noted that among veteran women “Significantly higher proportion of users, compared to nonusers reported avoiding VA because of past sexual trauma (19% of users vs 8% of nonusers).
Yeun-hee Anna Park reported on the High Risk Breast Cancer Screening Program, a 10-site pilot project to assess breast cancer risk. The quality improvement program sought to enhance screening for high-risk breast cancer and increase use of chemoprevention and genetic counseling in accordance with national guidelines. In the pilot, women veterans were at increased risk of breast cancer compared with that of the general population (46% vs 13%, respectively), based on a high rate of prior breast biopsies or positive family history. Moreover, posttraumatic stress disorder rates were nearly 3 times the national average. In the program, use of chemoprevention was nearly 2 times the national average.
Female patients undergoing treatment for cancer also face distinct risks related to fertility, bone health, and vasomotor symptoms of menopause. According to Tyler Fenton, PharmD, some cancer treatment approaches involving chemotherapy, radiation, and/or surgery carry a risk of ovarian failure and the accompanying symptoms of premature menopause. Dr. Fenton noted, that menopausal symptoms such as hot flashes are reported to occur in as many as 73% of breast cancer survivors, and 42% of female patients with cancer of reproductive age may develop premature ovarian failure as a result of their chemotherapy.
More than 500 VA hematologists, oncologists, pharmacists, nurses, social workers, and cancer registrars came to the 14th annual Association of VA Hematology/Oncology meeting held outside of Chicago, September 28 through September 30. Mark Klein, MD, succeeded Rusty Crawford, BPharm, as president and William Wachsman, MD, PhD, was named president-elect.
A key focus of the meeting was health care disparities and providing cancer care to underserved populations. An entire track of the meeting was devoted to patients with serious mental illness (SMI), including discussions of the risk of suicide Also a focus was the challenge of providing optimal treatments for patients who may not be adherent because they do not have access to support networks or steady housing.
According to Jessica B. Geller, PhD, MS, and Jessica L. Brand, PhD, a patient with a SMI diagnosis should not preclude considering aggressive treatment but requires a close level of care. Cancer care of patients with SMI requires close collaboration with mental health and pharmacy providers to manage these complex patients. According to Elizabeth Holyman, PsyD, care providers should give serious attention to complaints despite difficult behavior, treat pain, use active listening and provide interpersonal supports, and assume people with SMI have similar needs to the non-SMI.
For both patients with SMI and the entire veteran population, a cancer diagnosis can raise the risk of suicide, explained Catherine Rotolo, LISW-S. Suicidal ideation occurs in nearly 9% of people with cancer, most frequently in the first 3 months after diagnosis, and remains a significant cause of mortality for cancer patients. For patients with cancer, the rate of suicide—already elevated for veterans—is twice that of the baseline rate.
In a keynote address, Lisa Margolies, executive director of the LGBT Cancer Network, cautioned care providers not only to provide a welcoming environment, but also avoid making any assumptions about sexual behavior. “Do ask, we want to tell,” she told attendees, “Make it a part of your regular questions.” According to Margolies, there are more than 1 million LGBT veterans; they use the VA at higher rates, and the best estimate is that there are about 150,000 transgender veterans. According to Margolies, many do not tell their health care providers about their identity; 24% of lesbian, gay, bisexual, and transgender (LGBT) adults withheld information about sexual practices; 9.4% of men who identified as straight had sex with another man within the previous year. In another survey, more than 77% of lesbians had at least 1 sexual experience with a man—8% in the previous year. Margolies’ key point: avoid assumptions about a patient’s identity and simply take a comprehensive medical history so that you better understand the individual in front of you.
Another track of sessions focused on the challenges women face receiving care in the VA. Many female veterans, especially survivors of military sexual trauma (MST), are not comfortable going to the VA for care or being around groups of male veterans. Col (ret) Mona Pearl Treyball, PhD, RN, pointed out that up to 70% of women veterans experienced MST; most never reported it. That point was reinforced by Chad Hamilton, MD, who noted that among veteran women “Significantly higher proportion of users, compared to nonusers reported avoiding VA because of past sexual trauma (19% of users vs 8% of nonusers).
Yeun-hee Anna Park reported on the High Risk Breast Cancer Screening Program, a 10-site pilot project to assess breast cancer risk. The quality improvement program sought to enhance screening for high-risk breast cancer and increase use of chemoprevention and genetic counseling in accordance with national guidelines. In the pilot, women veterans were at increased risk of breast cancer compared with that of the general population (46% vs 13%, respectively), based on a high rate of prior breast biopsies or positive family history. Moreover, posttraumatic stress disorder rates were nearly 3 times the national average. In the program, use of chemoprevention was nearly 2 times the national average.
Female patients undergoing treatment for cancer also face distinct risks related to fertility, bone health, and vasomotor symptoms of menopause. According to Tyler Fenton, PharmD, some cancer treatment approaches involving chemotherapy, radiation, and/or surgery carry a risk of ovarian failure and the accompanying symptoms of premature menopause. Dr. Fenton noted, that menopausal symptoms such as hot flashes are reported to occur in as many as 73% of breast cancer survivors, and 42% of female patients with cancer of reproductive age may develop premature ovarian failure as a result of their chemotherapy.
CAR T may have curative potential in Hodgkin lymphoma
NEW YORK – Although the data set is small and not yet mature, chimeric antigen receptor (CAR) T-cell therapy appears to be a promising approach for Hodgkin lymphoma, according to Philippe Armand, MD, PhD, of Dana-Farber/Brigham and Women’s Cancer Center and the Massachusetts General Hospital Cancer Center in Boston.
While based on a handful of patients, the data do suggest this approach may play a role by targeting CD30 or Epstein Barr virus (EBV), Dr. Armand said at the NCCN Annual Congress: Hematologic Malignancies.
“Most importantly perhaps, like its experience outside of Hodgkin lymphoma, it may really have curative potential, based on the long [complete response] rates that have been already exhibited,” he said.
Much of the published clinical experience to date is with CD30-directed CAR Ts, Dr. Armand said, noting that in Hodgkin lymphoma, results so far show promise for this particular approach.
In a recent phase 1 dose escalation study, nine patients with relapsed/refractory Hodgkin lymphoma or anaplastic large-cell lymphoma (ALCL) received infusions of autologous T cells modified to express CD30-specific CAR T cells encoding the CD28 costimulatory domain, with no conditioning regimen.
Out of seven relapsed Hodgkin lymphoma patients, one had a complete response (CR) lasting beyond 2.5 years following a second infusion. Another patient had a CR persisting almost 2 years, and three patients had transient stable disease. One of the two ALCL patients had a CR lasting 9 months after a fourth infusion.
No toxicities attributable to the therapy were seen, according to the investigators.
The CD30 CAR T cells are being evaluated with a conditioning regimen in the phase 1 RELY-30 trial. Preliminary results presented at the 2018 European Society for Blood and Marrow Transplantation meeting in Lisbon showed better expansion of CAR T cells and responses in three out of five patients, including two CRs, according to Dr. Armand.
A CD30-directed CAR T-cell therapy with a 4-1BB costimulatory domain has also been tested in a small group of Hodgkin patients with a response rate of 35% – including some CRs – with an apparent lower response rate in patients with extranodal involvement. Dr. Armand noted that those findings need to be validated in additional studies.
Among non CD-30 targeted products, a CD19 CAR-T approach has been tried in Hodgkin lymphoma, though preliminary results suggest only transient activity, according to the presenter.
One interesting approach has been the targeting of EBV, he added. Recently reported results showed that two doses of T cells with specificity for EBV-derived tumor antigens induced clinical responses in patients with EBV-positive Hodgkin lymphoma.
The cells were engineered to express dominant-negative TGF-beta receptor type 2, according to the report. “We know that TGF-beta provides a strong immunosuppressant signal in the tumor microenvironment,” Dr. Armand said, noting that some of the responses in the seven evaluable patients lasted 4 years or more.
Dr. Armand reported financial disclosures related to Adaptive Biotechnologies/Sequenta, Affimed, Bristol-Myers Squibb, Merck, and Roche.
NEW YORK – Although the data set is small and not yet mature, chimeric antigen receptor (CAR) T-cell therapy appears to be a promising approach for Hodgkin lymphoma, according to Philippe Armand, MD, PhD, of Dana-Farber/Brigham and Women’s Cancer Center and the Massachusetts General Hospital Cancer Center in Boston.
While based on a handful of patients, the data do suggest this approach may play a role by targeting CD30 or Epstein Barr virus (EBV), Dr. Armand said at the NCCN Annual Congress: Hematologic Malignancies.
“Most importantly perhaps, like its experience outside of Hodgkin lymphoma, it may really have curative potential, based on the long [complete response] rates that have been already exhibited,” he said.
Much of the published clinical experience to date is with CD30-directed CAR Ts, Dr. Armand said, noting that in Hodgkin lymphoma, results so far show promise for this particular approach.
In a recent phase 1 dose escalation study, nine patients with relapsed/refractory Hodgkin lymphoma or anaplastic large-cell lymphoma (ALCL) received infusions of autologous T cells modified to express CD30-specific CAR T cells encoding the CD28 costimulatory domain, with no conditioning regimen.
Out of seven relapsed Hodgkin lymphoma patients, one had a complete response (CR) lasting beyond 2.5 years following a second infusion. Another patient had a CR persisting almost 2 years, and three patients had transient stable disease. One of the two ALCL patients had a CR lasting 9 months after a fourth infusion.
No toxicities attributable to the therapy were seen, according to the investigators.
The CD30 CAR T cells are being evaluated with a conditioning regimen in the phase 1 RELY-30 trial. Preliminary results presented at the 2018 European Society for Blood and Marrow Transplantation meeting in Lisbon showed better expansion of CAR T cells and responses in three out of five patients, including two CRs, according to Dr. Armand.
A CD30-directed CAR T-cell therapy with a 4-1BB costimulatory domain has also been tested in a small group of Hodgkin patients with a response rate of 35% – including some CRs – with an apparent lower response rate in patients with extranodal involvement. Dr. Armand noted that those findings need to be validated in additional studies.
Among non CD-30 targeted products, a CD19 CAR-T approach has been tried in Hodgkin lymphoma, though preliminary results suggest only transient activity, according to the presenter.
One interesting approach has been the targeting of EBV, he added. Recently reported results showed that two doses of T cells with specificity for EBV-derived tumor antigens induced clinical responses in patients with EBV-positive Hodgkin lymphoma.
The cells were engineered to express dominant-negative TGF-beta receptor type 2, according to the report. “We know that TGF-beta provides a strong immunosuppressant signal in the tumor microenvironment,” Dr. Armand said, noting that some of the responses in the seven evaluable patients lasted 4 years or more.
Dr. Armand reported financial disclosures related to Adaptive Biotechnologies/Sequenta, Affimed, Bristol-Myers Squibb, Merck, and Roche.
NEW YORK – Although the data set is small and not yet mature, chimeric antigen receptor (CAR) T-cell therapy appears to be a promising approach for Hodgkin lymphoma, according to Philippe Armand, MD, PhD, of Dana-Farber/Brigham and Women’s Cancer Center and the Massachusetts General Hospital Cancer Center in Boston.
While based on a handful of patients, the data do suggest this approach may play a role by targeting CD30 or Epstein Barr virus (EBV), Dr. Armand said at the NCCN Annual Congress: Hematologic Malignancies.
“Most importantly perhaps, like its experience outside of Hodgkin lymphoma, it may really have curative potential, based on the long [complete response] rates that have been already exhibited,” he said.
Much of the published clinical experience to date is with CD30-directed CAR Ts, Dr. Armand said, noting that in Hodgkin lymphoma, results so far show promise for this particular approach.
In a recent phase 1 dose escalation study, nine patients with relapsed/refractory Hodgkin lymphoma or anaplastic large-cell lymphoma (ALCL) received infusions of autologous T cells modified to express CD30-specific CAR T cells encoding the CD28 costimulatory domain, with no conditioning regimen.
Out of seven relapsed Hodgkin lymphoma patients, one had a complete response (CR) lasting beyond 2.5 years following a second infusion. Another patient had a CR persisting almost 2 years, and three patients had transient stable disease. One of the two ALCL patients had a CR lasting 9 months after a fourth infusion.
No toxicities attributable to the therapy were seen, according to the investigators.
The CD30 CAR T cells are being evaluated with a conditioning regimen in the phase 1 RELY-30 trial. Preliminary results presented at the 2018 European Society for Blood and Marrow Transplantation meeting in Lisbon showed better expansion of CAR T cells and responses in three out of five patients, including two CRs, according to Dr. Armand.
A CD30-directed CAR T-cell therapy with a 4-1BB costimulatory domain has also been tested in a small group of Hodgkin patients with a response rate of 35% – including some CRs – with an apparent lower response rate in patients with extranodal involvement. Dr. Armand noted that those findings need to be validated in additional studies.
Among non CD-30 targeted products, a CD19 CAR-T approach has been tried in Hodgkin lymphoma, though preliminary results suggest only transient activity, according to the presenter.
One interesting approach has been the targeting of EBV, he added. Recently reported results showed that two doses of T cells with specificity for EBV-derived tumor antigens induced clinical responses in patients with EBV-positive Hodgkin lymphoma.
The cells were engineered to express dominant-negative TGF-beta receptor type 2, according to the report. “We know that TGF-beta provides a strong immunosuppressant signal in the tumor microenvironment,” Dr. Armand said, noting that some of the responses in the seven evaluable patients lasted 4 years or more.
Dr. Armand reported financial disclosures related to Adaptive Biotechnologies/Sequenta, Affimed, Bristol-Myers Squibb, Merck, and Roche.
EXPERT ANALYSIS FROM NCCN ANNUAL CONGRESS: HEMATOLOGIC MALIGNANCIES
FAST-FFR: Noninvasive FFR nearly as good as wire
SAN DIEGO – A less-invasive way to measure fractional flow reserve using angiography had a sensitivity of 94% and specificity of 91%, compared with standard wire-based techniques, according to a report at the Transcatheter Cardiovascular Therapeutics annual meeting.
The method may provide an easier and potentially faster method for performing physiology-guided assessment of the overall coronary angiogram than with coronary pressure wire–based FFR, William Fearon, MD, said in presenting results of the FAST-FFR trial.
The added bonus of the FFRangio system, from the Israeli company CathWorks, is that it automatically produces a 3-D reconstruction of the entire coronary tree and can calculate FFR values for all occlusions. It requires high-quality angiograms, which are transferred to a proprietary counsel; the system estimates resistance and flow across stenoses using an algorithm. After a few cases, the process takes less than 5 minutes. CathWorks is working on Food and Drug Administration clearance, Dr. Fearon reported.
Several other companies are also developing noninvasive ways to measure FFR, the pressure gradient across lesions. It helps clinicians make the call on revascularization, since angiograms can be deceiving; occlusions that look bad might not really be causing a problem, and vice-versa.
FFR is recommended in assessment guidelines, but it’s not used much. The problem is that traditional measurement requires threading wires down coronary arteries; the technique is a bit risky and takes time and training. It also has to be repeated for each lesion.
The new system “has the potential to eventually replace wire-based FFR measurement and substantially increase physiologic coronary lesion assessment in the catheterization laboratory, thereby leading to improved patient outcomes,” said Dr. Fearon, professor of cardiology at Stanford (Calif.) University.
He said that he thought FFRangio could change practice, and several panelists agreed. “This is a real advance in the field. The use of FFR is not as great as it should be. I hope this will improve the ability of the assessor to identify ischemic lesions. I think that’s what’s going to happen. This is going to drive us forward,” said Mark Reisman, MD, head of cardiology and director of the Center for Emerging Cardiovascular Therapies at the University of Washington, Seattle.
The FAST-FFR (FFRangio Accuracy versus Standard FFR) study was conducted at 10 centers in the United States, Europe, and Israel; FFRangio was used to obtain FFRs in 319 vessels among 301 patients; the results were checked against FFR measured by wire. FFRangio operators were blinded to wire results.
The mean FFR was 0.81, and 43% of vessels had an FFR of 0.80 or less, signaling a possible abnormality. The diagnostic accuracy of FFRangio against wire measurement was 92%, and 87% when only gray-zone values between 0.75-0.85 were considered. Correlation with wire measurements was good (r = 0.80; P less than 0.001). Mismatches with the wire were more likely in the right coronary artery. The results were published online at the time of the presentation at TCT, sponsored by the Cardiovascular Research Foundation (Circulation. 2018 Sept 24. doi: 10.1161/circulationaha.118.037350).
“The main issue with this is that we need to do optimal angiographies. We should be doing them on a routine basis, but oftentimes, cardiologists want to be quick; they get lazy. But you do need to fill the entire vessel with contrast,” Dr. Fearon said.
“One of the nice things is you can rotate” the 3-D coronary artery tree reconstruction. “You can get a better idea of the relationship between the lesion and side branches, the length of the lesion, and a lot of additional information you don’t have on the angiogram [alone]. Then, you can pull back the cursor and measure FFR all along the vessel and different branches, all based on one angiography,” he said.
The majority of patients in the study were overweight or obese with complex coronary anatomy, as in daily practice. The investigation was limited to lesions amenable to wire measurement, so it didn’t include left main disease, low ejection fraction, and in-stent restenosis, although assessment may be possible.
Dr. Fearon didn’t know how much FFRangio will cost if it’s cleared or how CathWorks will be made available.
The work was funded by CathWorks. Dr. Fearon disclosed institutional research support from the company. One of the investigators was a cofounder of the company, with shares and intellectual property rights. The TCT meeting is sponsored by the Cardiovascular Research Foundation.
SAN DIEGO – A less-invasive way to measure fractional flow reserve using angiography had a sensitivity of 94% and specificity of 91%, compared with standard wire-based techniques, according to a report at the Transcatheter Cardiovascular Therapeutics annual meeting.
The method may provide an easier and potentially faster method for performing physiology-guided assessment of the overall coronary angiogram than with coronary pressure wire–based FFR, William Fearon, MD, said in presenting results of the FAST-FFR trial.
The added bonus of the FFRangio system, from the Israeli company CathWorks, is that it automatically produces a 3-D reconstruction of the entire coronary tree and can calculate FFR values for all occlusions. It requires high-quality angiograms, which are transferred to a proprietary counsel; the system estimates resistance and flow across stenoses using an algorithm. After a few cases, the process takes less than 5 minutes. CathWorks is working on Food and Drug Administration clearance, Dr. Fearon reported.
Several other companies are also developing noninvasive ways to measure FFR, the pressure gradient across lesions. It helps clinicians make the call on revascularization, since angiograms can be deceiving; occlusions that look bad might not really be causing a problem, and vice-versa.
FFR is recommended in assessment guidelines, but it’s not used much. The problem is that traditional measurement requires threading wires down coronary arteries; the technique is a bit risky and takes time and training. It also has to be repeated for each lesion.
The new system “has the potential to eventually replace wire-based FFR measurement and substantially increase physiologic coronary lesion assessment in the catheterization laboratory, thereby leading to improved patient outcomes,” said Dr. Fearon, professor of cardiology at Stanford (Calif.) University.
He said that he thought FFRangio could change practice, and several panelists agreed. “This is a real advance in the field. The use of FFR is not as great as it should be. I hope this will improve the ability of the assessor to identify ischemic lesions. I think that’s what’s going to happen. This is going to drive us forward,” said Mark Reisman, MD, head of cardiology and director of the Center for Emerging Cardiovascular Therapies at the University of Washington, Seattle.
The FAST-FFR (FFRangio Accuracy versus Standard FFR) study was conducted at 10 centers in the United States, Europe, and Israel; FFRangio was used to obtain FFRs in 319 vessels among 301 patients; the results were checked against FFR measured by wire. FFRangio operators were blinded to wire results.
The mean FFR was 0.81, and 43% of vessels had an FFR of 0.80 or less, signaling a possible abnormality. The diagnostic accuracy of FFRangio against wire measurement was 92%, and 87% when only gray-zone values between 0.75-0.85 were considered. Correlation with wire measurements was good (r = 0.80; P less than 0.001). Mismatches with the wire were more likely in the right coronary artery. The results were published online at the time of the presentation at TCT, sponsored by the Cardiovascular Research Foundation (Circulation. 2018 Sept 24. doi: 10.1161/circulationaha.118.037350).
“The main issue with this is that we need to do optimal angiographies. We should be doing them on a routine basis, but oftentimes, cardiologists want to be quick; they get lazy. But you do need to fill the entire vessel with contrast,” Dr. Fearon said.
“One of the nice things is you can rotate” the 3-D coronary artery tree reconstruction. “You can get a better idea of the relationship between the lesion and side branches, the length of the lesion, and a lot of additional information you don’t have on the angiogram [alone]. Then, you can pull back the cursor and measure FFR all along the vessel and different branches, all based on one angiography,” he said.
The majority of patients in the study were overweight or obese with complex coronary anatomy, as in daily practice. The investigation was limited to lesions amenable to wire measurement, so it didn’t include left main disease, low ejection fraction, and in-stent restenosis, although assessment may be possible.
Dr. Fearon didn’t know how much FFRangio will cost if it’s cleared or how CathWorks will be made available.
The work was funded by CathWorks. Dr. Fearon disclosed institutional research support from the company. One of the investigators was a cofounder of the company, with shares and intellectual property rights. The TCT meeting is sponsored by the Cardiovascular Research Foundation.
SAN DIEGO – A less-invasive way to measure fractional flow reserve using angiography had a sensitivity of 94% and specificity of 91%, compared with standard wire-based techniques, according to a report at the Transcatheter Cardiovascular Therapeutics annual meeting.
The method may provide an easier and potentially faster method for performing physiology-guided assessment of the overall coronary angiogram than with coronary pressure wire–based FFR, William Fearon, MD, said in presenting results of the FAST-FFR trial.
The added bonus of the FFRangio system, from the Israeli company CathWorks, is that it automatically produces a 3-D reconstruction of the entire coronary tree and can calculate FFR values for all occlusions. It requires high-quality angiograms, which are transferred to a proprietary counsel; the system estimates resistance and flow across stenoses using an algorithm. After a few cases, the process takes less than 5 minutes. CathWorks is working on Food and Drug Administration clearance, Dr. Fearon reported.
Several other companies are also developing noninvasive ways to measure FFR, the pressure gradient across lesions. It helps clinicians make the call on revascularization, since angiograms can be deceiving; occlusions that look bad might not really be causing a problem, and vice-versa.
FFR is recommended in assessment guidelines, but it’s not used much. The problem is that traditional measurement requires threading wires down coronary arteries; the technique is a bit risky and takes time and training. It also has to be repeated for each lesion.
The new system “has the potential to eventually replace wire-based FFR measurement and substantially increase physiologic coronary lesion assessment in the catheterization laboratory, thereby leading to improved patient outcomes,” said Dr. Fearon, professor of cardiology at Stanford (Calif.) University.
He said that he thought FFRangio could change practice, and several panelists agreed. “This is a real advance in the field. The use of FFR is not as great as it should be. I hope this will improve the ability of the assessor to identify ischemic lesions. I think that’s what’s going to happen. This is going to drive us forward,” said Mark Reisman, MD, head of cardiology and director of the Center for Emerging Cardiovascular Therapies at the University of Washington, Seattle.
The FAST-FFR (FFRangio Accuracy versus Standard FFR) study was conducted at 10 centers in the United States, Europe, and Israel; FFRangio was used to obtain FFRs in 319 vessels among 301 patients; the results were checked against FFR measured by wire. FFRangio operators were blinded to wire results.
The mean FFR was 0.81, and 43% of vessels had an FFR of 0.80 or less, signaling a possible abnormality. The diagnostic accuracy of FFRangio against wire measurement was 92%, and 87% when only gray-zone values between 0.75-0.85 were considered. Correlation with wire measurements was good (r = 0.80; P less than 0.001). Mismatches with the wire were more likely in the right coronary artery. The results were published online at the time of the presentation at TCT, sponsored by the Cardiovascular Research Foundation (Circulation. 2018 Sept 24. doi: 10.1161/circulationaha.118.037350).
“The main issue with this is that we need to do optimal angiographies. We should be doing them on a routine basis, but oftentimes, cardiologists want to be quick; they get lazy. But you do need to fill the entire vessel with contrast,” Dr. Fearon said.
“One of the nice things is you can rotate” the 3-D coronary artery tree reconstruction. “You can get a better idea of the relationship between the lesion and side branches, the length of the lesion, and a lot of additional information you don’t have on the angiogram [alone]. Then, you can pull back the cursor and measure FFR all along the vessel and different branches, all based on one angiography,” he said.
The majority of patients in the study were overweight or obese with complex coronary anatomy, as in daily practice. The investigation was limited to lesions amenable to wire measurement, so it didn’t include left main disease, low ejection fraction, and in-stent restenosis, although assessment may be possible.
Dr. Fearon didn’t know how much FFRangio will cost if it’s cleared or how CathWorks will be made available.
The work was funded by CathWorks. Dr. Fearon disclosed institutional research support from the company. One of the investigators was a cofounder of the company, with shares and intellectual property rights. The TCT meeting is sponsored by the Cardiovascular Research Foundation.
REPORTING FROM TCT 2018
Key clinical point:
Major finding: FFRangio, a less-invasive way to measure fractional flow reserve using angiograms, had a sensitivity of 94% and specificity of 91%, compared with standard, wire-based techniques.
Study details: The prospective, multicenter FAST-FFR compared FFRangio with pressure wire–derived fractional flow reserve in 301 subjects.
Disclosures: The work was funded by FFRangio maker, CathWorks. Dr. Fearon disclosed institutional research support from the company. One of the authors was a cofounder of the company, with shares and intellectual property rights.
All children deserve support for their gender identities
The Atlantic published the article “When Children Say They’re Trans” by Jesse Singal in its July/August edition not too long ago. In this article, the author wrote about the increasing availability of treatments for affirming one’s gender identity and the rising concerns about the risks surrounding those treatments.
A key issue in the article is the concept of desistance. Desistance is a phenomenon in which individuals no longer feel that their gender identities are incongruent with their physical appearance. Highly related to desistance is detransitioning, a phenomenon in which transgender individuals no longer take the steps (e.g., hormone therapy) to affirm their gender identity. Singal highlights the concern surrounding starting medical treatments to affirm an individual’s gender identity, considering that the changes are irreversible and that it is possible for children to change their minds. Implied in the article is a call for a cautious approach for treating children who identify as transgender because it will be difficult to predict what one’s final gender identity is; however, I believe that a better approach is to support the child in the journey in affirming the gender identity.
The evidence on the rate of desistance may not be accurate
One argument for the cautious approach is the often cited statistic that 80% of children with gender nonconforming behaviors do not identify as transgender when they are adults. This is derived from four published studies that track the gender identity of individuals with gender nonconforming behaviors in childhood.1-4 These estimates may not be accurate, mainly due to these studies’ methodological shortcomings. For example, those who were lost to follow-up were assumed to be cisgender as adults and no efforts were made to verify these individuals’ gender identity.2-4 I do not intend to thoroughly critique these studies in this column. This is best left to peer-reviewed commentaries (a good example is one written by Newhook et al. 2018).5 I worry, however, that some clinicians may dismiss a child’s gender identity based on these studies and recommend to the parents to delay supporting a transition until the child “knows for sure.” The problem with this approach is that it may worsen the health and well-being of transgender youth, as there is growing evidence that transgender children who are supported by their parents are less likely to have mental health problems.6,7
The reasons for desistance are far more complicated
The common narrative of desistance is that the individuals simply change their minds because they were “confused” during adolescence. However, the truth is more complicated. Children can identify their own gender as early as 2 years old;8 however, when a child’s gender identity matches the assigned sex at birth, this is often reinforced. In contrast, if a child’s gender identity does not match the assigned sex at birth, it often is challenged by peers and adults. This challenge by peers, their families, and medical providers may be one of the reasons why transitioning is so difficult for many transgender youth – and many do give up.3,9 In these cases, some people wait for years, if not decades, to come out again and start transitioning when they finally feel supported and safe – even in their 90s! Other transgender people realize that their gender identity is not on the binary (neither male nor female), so they no longer need cross-sex hormones or surgeries to affirm their gender identity. Finally, others are concerned about the side effects, such as infertility, and feel that the risks for those side effects are not worth it, so they find other, nonmedical or nonsurgical ways to affirm their gender identity or manage their gender dysphoria.
Positive outcomes are more common
Reports of youth detransitioning highlight many physicians’ fears of making a mistake; however, these reports obscure the more common – and positive – outcomes for transgender individuals who took steps to affirm their gender identity. The Report of the 2011 Transition Survey shows that 97% were satisfied with being on hormone therapy and 90% were satisfied with obtaining bottom surgery.10 Furthermore, there is growing evidence showing that such treatments are associated with better health.11 A study by de Vries et al. found that transgender youth who transitioned in adolescence had less depression and better adjustment as adults.12 Finally, there is a lack of evidence supporting the concept that someone whose gender identity is fluid over time is any less healthy than those whose gender identity is static over time. Rare outcomes should never be dismissed; however, providers should not use rare events as the primary driver for discouraging evidence-based treatment.
The key is support
I believe that every child’s gender identity should be supported and affirmed. Clinicians can provide this support and affirmation through the following actions:
- At the first visit, clinicians should ask what the child’s hopes and expectations are for pursuing gender-affirming medical treatments.
- Clinicians should allow the child the opportunity to describe and process their gender identity instead of assuming that they are on the binary.
- Clinicians must recognize the varied reasons for desistance – stigma, discrimination, shame, or need to fit within a gender binary – and find ways to address those factors.
- Clinicians should have a thorough discussion with patients and their families about the risks of not supporting the child’s gender identity versus the risk of medical or surgical treatments used to affirm one’s gender identity and process with the child and the family where the values and wishes are within the context of those risks.
- Most importantly, clinicians should emphasize support for whatever decisions the child makes to affirm their gender identity. Providing support is essential in promoting the health and well-being of any child.
Dr. Montano is assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
References
1. Dev Psychol. 2008;44(1):34-45.
2. J Am Acad Child Adolesc Psychiatry. 2013 Jun;52(6):582-90.
3. Clin Child Psychol Psychiatry. 2011 Oct;16(4):499-516.
4. J Am Acad Child Adolesc Psychiatry. 2008 Dec;47(12):1413-23.
5. International Journal of Transgenderism. 2018;19(2):212-24.
6. Pediatrics. 2016 Mar;137(3):e20153223.
7. J Sex Marital Ther. 2010;36(1):6-23.
8. “Adolescence,” 11th ed. (New York: McGraw-Hill Education; 2016).
9. Graduate Journal of Social Science. 2010;7(2):26-43.
10. “Affirming Gender, Affirming Lives: A Report of the 2011 Transition Survey,” Gender Advocacy Training & Education, 2012.
11. Transgend Health. 2016 Jan;1(1):21-31.
12. Pediatrics. 2014 Oct;134(4):696-704.
The Atlantic published the article “When Children Say They’re Trans” by Jesse Singal in its July/August edition not too long ago. In this article, the author wrote about the increasing availability of treatments for affirming one’s gender identity and the rising concerns about the risks surrounding those treatments.
A key issue in the article is the concept of desistance. Desistance is a phenomenon in which individuals no longer feel that their gender identities are incongruent with their physical appearance. Highly related to desistance is detransitioning, a phenomenon in which transgender individuals no longer take the steps (e.g., hormone therapy) to affirm their gender identity. Singal highlights the concern surrounding starting medical treatments to affirm an individual’s gender identity, considering that the changes are irreversible and that it is possible for children to change their minds. Implied in the article is a call for a cautious approach for treating children who identify as transgender because it will be difficult to predict what one’s final gender identity is; however, I believe that a better approach is to support the child in the journey in affirming the gender identity.
The evidence on the rate of desistance may not be accurate
One argument for the cautious approach is the often cited statistic that 80% of children with gender nonconforming behaviors do not identify as transgender when they are adults. This is derived from four published studies that track the gender identity of individuals with gender nonconforming behaviors in childhood.1-4 These estimates may not be accurate, mainly due to these studies’ methodological shortcomings. For example, those who were lost to follow-up were assumed to be cisgender as adults and no efforts were made to verify these individuals’ gender identity.2-4 I do not intend to thoroughly critique these studies in this column. This is best left to peer-reviewed commentaries (a good example is one written by Newhook et al. 2018).5 I worry, however, that some clinicians may dismiss a child’s gender identity based on these studies and recommend to the parents to delay supporting a transition until the child “knows for sure.” The problem with this approach is that it may worsen the health and well-being of transgender youth, as there is growing evidence that transgender children who are supported by their parents are less likely to have mental health problems.6,7
The reasons for desistance are far more complicated
The common narrative of desistance is that the individuals simply change their minds because they were “confused” during adolescence. However, the truth is more complicated. Children can identify their own gender as early as 2 years old;8 however, when a child’s gender identity matches the assigned sex at birth, this is often reinforced. In contrast, if a child’s gender identity does not match the assigned sex at birth, it often is challenged by peers and adults. This challenge by peers, their families, and medical providers may be one of the reasons why transitioning is so difficult for many transgender youth – and many do give up.3,9 In these cases, some people wait for years, if not decades, to come out again and start transitioning when they finally feel supported and safe – even in their 90s! Other transgender people realize that their gender identity is not on the binary (neither male nor female), so they no longer need cross-sex hormones or surgeries to affirm their gender identity. Finally, others are concerned about the side effects, such as infertility, and feel that the risks for those side effects are not worth it, so they find other, nonmedical or nonsurgical ways to affirm their gender identity or manage their gender dysphoria.
Positive outcomes are more common
Reports of youth detransitioning highlight many physicians’ fears of making a mistake; however, these reports obscure the more common – and positive – outcomes for transgender individuals who took steps to affirm their gender identity. The Report of the 2011 Transition Survey shows that 97% were satisfied with being on hormone therapy and 90% were satisfied with obtaining bottom surgery.10 Furthermore, there is growing evidence showing that such treatments are associated with better health.11 A study by de Vries et al. found that transgender youth who transitioned in adolescence had less depression and better adjustment as adults.12 Finally, there is a lack of evidence supporting the concept that someone whose gender identity is fluid over time is any less healthy than those whose gender identity is static over time. Rare outcomes should never be dismissed; however, providers should not use rare events as the primary driver for discouraging evidence-based treatment.
The key is support
I believe that every child’s gender identity should be supported and affirmed. Clinicians can provide this support and affirmation through the following actions:
- At the first visit, clinicians should ask what the child’s hopes and expectations are for pursuing gender-affirming medical treatments.
- Clinicians should allow the child the opportunity to describe and process their gender identity instead of assuming that they are on the binary.
- Clinicians must recognize the varied reasons for desistance – stigma, discrimination, shame, or need to fit within a gender binary – and find ways to address those factors.
- Clinicians should have a thorough discussion with patients and their families about the risks of not supporting the child’s gender identity versus the risk of medical or surgical treatments used to affirm one’s gender identity and process with the child and the family where the values and wishes are within the context of those risks.
- Most importantly, clinicians should emphasize support for whatever decisions the child makes to affirm their gender identity. Providing support is essential in promoting the health and well-being of any child.
Dr. Montano is assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
References
1. Dev Psychol. 2008;44(1):34-45.
2. J Am Acad Child Adolesc Psychiatry. 2013 Jun;52(6):582-90.
3. Clin Child Psychol Psychiatry. 2011 Oct;16(4):499-516.
4. J Am Acad Child Adolesc Psychiatry. 2008 Dec;47(12):1413-23.
5. International Journal of Transgenderism. 2018;19(2):212-24.
6. Pediatrics. 2016 Mar;137(3):e20153223.
7. J Sex Marital Ther. 2010;36(1):6-23.
8. “Adolescence,” 11th ed. (New York: McGraw-Hill Education; 2016).
9. Graduate Journal of Social Science. 2010;7(2):26-43.
10. “Affirming Gender, Affirming Lives: A Report of the 2011 Transition Survey,” Gender Advocacy Training & Education, 2012.
11. Transgend Health. 2016 Jan;1(1):21-31.
12. Pediatrics. 2014 Oct;134(4):696-704.
The Atlantic published the article “When Children Say They’re Trans” by Jesse Singal in its July/August edition not too long ago. In this article, the author wrote about the increasing availability of treatments for affirming one’s gender identity and the rising concerns about the risks surrounding those treatments.
A key issue in the article is the concept of desistance. Desistance is a phenomenon in which individuals no longer feel that their gender identities are incongruent with their physical appearance. Highly related to desistance is detransitioning, a phenomenon in which transgender individuals no longer take the steps (e.g., hormone therapy) to affirm their gender identity. Singal highlights the concern surrounding starting medical treatments to affirm an individual’s gender identity, considering that the changes are irreversible and that it is possible for children to change their minds. Implied in the article is a call for a cautious approach for treating children who identify as transgender because it will be difficult to predict what one’s final gender identity is; however, I believe that a better approach is to support the child in the journey in affirming the gender identity.
The evidence on the rate of desistance may not be accurate
One argument for the cautious approach is the often cited statistic that 80% of children with gender nonconforming behaviors do not identify as transgender when they are adults. This is derived from four published studies that track the gender identity of individuals with gender nonconforming behaviors in childhood.1-4 These estimates may not be accurate, mainly due to these studies’ methodological shortcomings. For example, those who were lost to follow-up were assumed to be cisgender as adults and no efforts were made to verify these individuals’ gender identity.2-4 I do not intend to thoroughly critique these studies in this column. This is best left to peer-reviewed commentaries (a good example is one written by Newhook et al. 2018).5 I worry, however, that some clinicians may dismiss a child’s gender identity based on these studies and recommend to the parents to delay supporting a transition until the child “knows for sure.” The problem with this approach is that it may worsen the health and well-being of transgender youth, as there is growing evidence that transgender children who are supported by their parents are less likely to have mental health problems.6,7
The reasons for desistance are far more complicated
The common narrative of desistance is that the individuals simply change their minds because they were “confused” during adolescence. However, the truth is more complicated. Children can identify their own gender as early as 2 years old;8 however, when a child’s gender identity matches the assigned sex at birth, this is often reinforced. In contrast, if a child’s gender identity does not match the assigned sex at birth, it often is challenged by peers and adults. This challenge by peers, their families, and medical providers may be one of the reasons why transitioning is so difficult for many transgender youth – and many do give up.3,9 In these cases, some people wait for years, if not decades, to come out again and start transitioning when they finally feel supported and safe – even in their 90s! Other transgender people realize that their gender identity is not on the binary (neither male nor female), so they no longer need cross-sex hormones or surgeries to affirm their gender identity. Finally, others are concerned about the side effects, such as infertility, and feel that the risks for those side effects are not worth it, so they find other, nonmedical or nonsurgical ways to affirm their gender identity or manage their gender dysphoria.
Positive outcomes are more common
Reports of youth detransitioning highlight many physicians’ fears of making a mistake; however, these reports obscure the more common – and positive – outcomes for transgender individuals who took steps to affirm their gender identity. The Report of the 2011 Transition Survey shows that 97% were satisfied with being on hormone therapy and 90% were satisfied with obtaining bottom surgery.10 Furthermore, there is growing evidence showing that such treatments are associated with better health.11 A study by de Vries et al. found that transgender youth who transitioned in adolescence had less depression and better adjustment as adults.12 Finally, there is a lack of evidence supporting the concept that someone whose gender identity is fluid over time is any less healthy than those whose gender identity is static over time. Rare outcomes should never be dismissed; however, providers should not use rare events as the primary driver for discouraging evidence-based treatment.
The key is support
I believe that every child’s gender identity should be supported and affirmed. Clinicians can provide this support and affirmation through the following actions:
- At the first visit, clinicians should ask what the child’s hopes and expectations are for pursuing gender-affirming medical treatments.
- Clinicians should allow the child the opportunity to describe and process their gender identity instead of assuming that they are on the binary.
- Clinicians must recognize the varied reasons for desistance – stigma, discrimination, shame, or need to fit within a gender binary – and find ways to address those factors.
- Clinicians should have a thorough discussion with patients and their families about the risks of not supporting the child’s gender identity versus the risk of medical or surgical treatments used to affirm one’s gender identity and process with the child and the family where the values and wishes are within the context of those risks.
- Most importantly, clinicians should emphasize support for whatever decisions the child makes to affirm their gender identity. Providing support is essential in promoting the health and well-being of any child.
Dr. Montano is assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
References
1. Dev Psychol. 2008;44(1):34-45.
2. J Am Acad Child Adolesc Psychiatry. 2013 Jun;52(6):582-90.
3. Clin Child Psychol Psychiatry. 2011 Oct;16(4):499-516.
4. J Am Acad Child Adolesc Psychiatry. 2008 Dec;47(12):1413-23.
5. International Journal of Transgenderism. 2018;19(2):212-24.
6. Pediatrics. 2016 Mar;137(3):e20153223.
7. J Sex Marital Ther. 2010;36(1):6-23.
8. “Adolescence,” 11th ed. (New York: McGraw-Hill Education; 2016).
9. Graduate Journal of Social Science. 2010;7(2):26-43.
10. “Affirming Gender, Affirming Lives: A Report of the 2011 Transition Survey,” Gender Advocacy Training & Education, 2012.
11. Transgend Health. 2016 Jan;1(1):21-31.
12. Pediatrics. 2014 Oct;134(4):696-704.