User login
New Registry Offers Insight Into Opsoclonus-Myoclonus Syndrome
WASHINGTON – a rare disease affecting only 1 in 5,000,000 individuals, mostly aged 1-5 years, based on data from a new patient registry.
In partnership with the National Organization of Rare Disorders (NORD) the nonprofit OMSLife Foundation has created a patient registry to better understand the disease experience in patients, wrote Mike Michaelis, chairman of OMSLife, and his colleagues. Early data from 275 enrolled patients were presented in a poster at the NORD Rare Summit, held by the National Organization for Rare Disorders.
The registry patients were mainly born in the United States (86%) and white (74%); approximately half were female. Of 150 patients who indicated symptoms at onset, 87% reported ataxia. Additional symptoms at onset were myoclonus (61%), opsoclonus (59%), tremors (46%), sleep disturbances (45%), temper tantrums (38%), vomiting (27%), fever (13%), headache (9%) and other symptoms (13%).
The researchers reviewed associations of symptoms at onset to determine the frequency of other symptoms for each individual symptom. Ataxia was present with 89% or higher instances of the other reported symptoms. Of note, some symptoms occurred more frequently than expected, such as temper tantrums and tremors in approximately 70% of patients with sleep disturbances. Myoclonus and opsoclonus, as well as fever and vomiting, also were significantly associated with the presence of other symptoms.
Two-thirds of the registry patients (69%) were diagnosed within 3 months of symptom onset, and 83% of these were diagnosed by a neurologist. Based on the Mitchell-Pike OMS severity scale, 59% of the patients met criteria for severe disease, 34% were classified as moderate, and 7% were mild. The registry is ongoing, but the current data provide insight on the clinical picture and common symptoms of OMS, the researchers said.
OMS Life was established in 2012 to support patients, caregivers, and researchers in raising awareness of opsoclonus-myoclonus syndrome as well as funds for research.
The study was supported by the OMSLife Foundation, NORD, and Trio Health Analytics. The researchers are employed by these organizations.
WASHINGTON – a rare disease affecting only 1 in 5,000,000 individuals, mostly aged 1-5 years, based on data from a new patient registry.
In partnership with the National Organization of Rare Disorders (NORD) the nonprofit OMSLife Foundation has created a patient registry to better understand the disease experience in patients, wrote Mike Michaelis, chairman of OMSLife, and his colleagues. Early data from 275 enrolled patients were presented in a poster at the NORD Rare Summit, held by the National Organization for Rare Disorders.
The registry patients were mainly born in the United States (86%) and white (74%); approximately half were female. Of 150 patients who indicated symptoms at onset, 87% reported ataxia. Additional symptoms at onset were myoclonus (61%), opsoclonus (59%), tremors (46%), sleep disturbances (45%), temper tantrums (38%), vomiting (27%), fever (13%), headache (9%) and other symptoms (13%).
The researchers reviewed associations of symptoms at onset to determine the frequency of other symptoms for each individual symptom. Ataxia was present with 89% or higher instances of the other reported symptoms. Of note, some symptoms occurred more frequently than expected, such as temper tantrums and tremors in approximately 70% of patients with sleep disturbances. Myoclonus and opsoclonus, as well as fever and vomiting, also were significantly associated with the presence of other symptoms.
Two-thirds of the registry patients (69%) were diagnosed within 3 months of symptom onset, and 83% of these were diagnosed by a neurologist. Based on the Mitchell-Pike OMS severity scale, 59% of the patients met criteria for severe disease, 34% were classified as moderate, and 7% were mild. The registry is ongoing, but the current data provide insight on the clinical picture and common symptoms of OMS, the researchers said.
OMS Life was established in 2012 to support patients, caregivers, and researchers in raising awareness of opsoclonus-myoclonus syndrome as well as funds for research.
The study was supported by the OMSLife Foundation, NORD, and Trio Health Analytics. The researchers are employed by these organizations.
WASHINGTON – a rare disease affecting only 1 in 5,000,000 individuals, mostly aged 1-5 years, based on data from a new patient registry.
In partnership with the National Organization of Rare Disorders (NORD) the nonprofit OMSLife Foundation has created a patient registry to better understand the disease experience in patients, wrote Mike Michaelis, chairman of OMSLife, and his colleagues. Early data from 275 enrolled patients were presented in a poster at the NORD Rare Summit, held by the National Organization for Rare Disorders.
The registry patients were mainly born in the United States (86%) and white (74%); approximately half were female. Of 150 patients who indicated symptoms at onset, 87% reported ataxia. Additional symptoms at onset were myoclonus (61%), opsoclonus (59%), tremors (46%), sleep disturbances (45%), temper tantrums (38%), vomiting (27%), fever (13%), headache (9%) and other symptoms (13%).
The researchers reviewed associations of symptoms at onset to determine the frequency of other symptoms for each individual symptom. Ataxia was present with 89% or higher instances of the other reported symptoms. Of note, some symptoms occurred more frequently than expected, such as temper tantrums and tremors in approximately 70% of patients with sleep disturbances. Myoclonus and opsoclonus, as well as fever and vomiting, also were significantly associated with the presence of other symptoms.
Two-thirds of the registry patients (69%) were diagnosed within 3 months of symptom onset, and 83% of these were diagnosed by a neurologist. Based on the Mitchell-Pike OMS severity scale, 59% of the patients met criteria for severe disease, 34% were classified as moderate, and 7% were mild. The registry is ongoing, but the current data provide insight on the clinical picture and common symptoms of OMS, the researchers said.
OMS Life was established in 2012 to support patients, caregivers, and researchers in raising awareness of opsoclonus-myoclonus syndrome as well as funds for research.
The study was supported by the OMSLife Foundation, NORD, and Trio Health Analytics. The researchers are employed by these organizations.
REPORTING FROM NORD SUMMIT 2018
Key clinical point: Most patients with OMS experienced multiple symptoms at disease onset; ataxia was the most common.
Major finding: Approximately 87% of patients with OMS reported ataxia at disease onset and 59% experienced severe disease.
Study details: The data come from a registry including 275 OMS patients.
Disclosures: The study was supported by the OMSLife Foundation, NORD, and Trio Health Analytics. The researchers are employed by these organizations.
Physician Commentary: Neurology Community Responds to Diclofenac Cardiovascular Risks
In July 2018, The BMJ published a study examining the cardiovascular risks of diclofenac initiation compared with initiation of other traditional non-steroidal anti-inflammatory drugs, initiation of paracetamol, and no initiation. The results showed a 50% increase in adverse events among diclofenac initiators compared with non-initiators (as well as a 20% increase over paracetamol/ibuprofen initiators, and 30% increase over naproxen initiators). (Read the full study here). Here, I asked several of my colleagues to weigh in on the results of this study and its implications for our practices, and then I share my own thoughts on these findings:
Stewart J. Tepper, MD, FAHS
Professor of Neurology
Geisel School of Medicine at Dartmouth
There have been previous studies and meta-analyses demonstrating the cardiovascular risks of diclofenac. This very large cohort study highlights the magnitude of effects for both those patients at high risk and at low risk for cardiovascular disease. Diclofenac has many advantages for migraine treatment, such as a rapid onset of action in its liquid form, but it has higher risks for major cardiac events than most currently available nonsteroidal anti-inflammatory drugs (NSAIDs). As providers, we must be judicious in diclofenac use and informative with our patients.
Marcelo Bigal, MD, PhD
Chief Medical Officer, Purdue Pharma
It is well established that NSAIDs are associated with increased risk of poor cardiovascular outcomes. This study offers powerful evidence that the risk after frequent diclofenac use is disproportionally increased relative to other commonly used NSAIDs, such as ibuprofen or naproxen. It is relevant to discuss the implications of the findings for the treatment of migraine.
The acute treatment of migraine associated with attack-related disability should favor triptans as first line therapy, not NSAIDs. Because triptans are vasoconstrictive medications, unmet needs exist in patients at cardiovascular risk. Anti-CGRP acute migraine therapies, as well as “ditans” (5HT-1f antagonist) are under regulatory review and may address the needs of these patients. In the context of acute migraine therapy, diclofenac and NSAIDs are typically used instead of triptans, or with triptans when additional efficacy is needed. We certainly find that the use of diclofenac in these situations should be judicious, and reserved to those who clearly need it, have infrequent migraine attacks, and are otherwise healthy.
Diclofenac is also often used in the emergency department in many countries as a rescue therapy. In a series of clinical trials where we tested most commonly used drugs in this setting in Brazil, we found that efficacies were 83.6% for intravenous dipyrone, 66.7% for intramuscular diclofenac and 81.8% for intravenous chlorpromazine. We continue to believe that diclofenac is an important, non-sedative and non-opioid option for the management of headaches in the emergency department, assuming that at discharge, patients would receive proper guidance on the management of migraine without relying on frequent use of NSAIDs.
Jack Schim, MD
Co-Director, The Headache Center of Southern California
This article supports findings of prior epidemiologic studies correlating exposure to NSAIDs with increased cerebrovascular and cardiovascular risk. Prior studies have shown a dose-related response in risk associated with NSAID therapy, supporting a causal association. However, while relative risk is significantly higher in individuals with NSAID exposure, the absolute risk remains very low. The greater risk from NSAIDs continues to be to the kidneys, and to the stomach.
As with all therapies, we need to weigh the advantages and disadvantages of NSAID therapy with our headache patients. All medications carry their own risks. For acute treatment of migraines, our primary tool, triptans, are contraindicated in a significant subset of individuals, including patients with ischemic coronary artery as well as those with history of stroke or transient ischemic attack (TIA). The alternatives, NSAIDs, dopamine blocking agents, have utility and risks.
Diclofenac powder to be dissolved in water is an effective abortive for migraine for many individuals. In general, our patients have intermittent exposure, preferably not more than 2 days per week. For the appropriate individual, NSAIDS, including diclofenac, remain an important tool in the acute care armamentarium.
Rob Cowan, MD, FAAN, FAHS
Higgins Professor of Neurology and Neurosciences
Chief, Division of Headache Medicine, Dept. of Neurology and Neurosciences
Director, Stanford University School of Medicine
These kinds of large, population-based studies must be interpreted with caution. While they may emulate the protocol of prospective studies, they lack proper inclusion/exclusion criteria, particularly with respect to indication. It may be reasonable to assume that the population of diclofenac users is "sicker" than the general population and the population that is using cheaper, more accessible NSAIDs or paracetamol. Without knowing the access and economic issues in Denmark, it is difficult to weigh these variables in the study. Thus, while it is certainly an important issue to explore (the relative risks and benefits of a given medication within a class), the absence of a well-designed, prospective study precludes any definitive conclusion regarding relative safety and risk profile for Diclofenac.
+++
These are great comments by my colleagues. My impression after seeing the data and reading my colleague’s comments, is that diclofenac may be riskier than other NSAIDs in this study; but when used properly in generally healthy migraineurs, it is probably more effective than dangerous when evaluating the risk/benefit ratio. When diclofenac is used as an oral solution (Cambia), 2 days per week or less, in a patient without serious gastrointestinal, renal, cardiac or hypertensive issues, it appears to pose little risk to the patient. When given to the wrong patient, or when taken too frequently, is could be dangerous. What I really like about this preparation is that it causes fewer adverse events compared to triptans and works very quickly. It can be used when triptans have been used enough that week or if they tend to cause significant adverse events when taken. We can use diclofenac for our headache patients, but we should remain vigilant to give it cautiously and only to patients who have no contraindication to its use.
Please write to us at Neurology Reviews Migraine Resource Center ([email protected]) with your opinions.
Alan M. Rapoport, M.D.
Editor-in-Chief
Migraine Resource Center
Clinical Professor of Neurology
The David Geffen School of Medicine at UCLA
Los Angeles, California
In July 2018, The BMJ published a study examining the cardiovascular risks of diclofenac initiation compared with initiation of other traditional non-steroidal anti-inflammatory drugs, initiation of paracetamol, and no initiation. The results showed a 50% increase in adverse events among diclofenac initiators compared with non-initiators (as well as a 20% increase over paracetamol/ibuprofen initiators, and 30% increase over naproxen initiators). (Read the full study here). Here, I asked several of my colleagues to weigh in on the results of this study and its implications for our practices, and then I share my own thoughts on these findings:
Stewart J. Tepper, MD, FAHS
Professor of Neurology
Geisel School of Medicine at Dartmouth
There have been previous studies and meta-analyses demonstrating the cardiovascular risks of diclofenac. This very large cohort study highlights the magnitude of effects for both those patients at high risk and at low risk for cardiovascular disease. Diclofenac has many advantages for migraine treatment, such as a rapid onset of action in its liquid form, but it has higher risks for major cardiac events than most currently available nonsteroidal anti-inflammatory drugs (NSAIDs). As providers, we must be judicious in diclofenac use and informative with our patients.
Marcelo Bigal, MD, PhD
Chief Medical Officer, Purdue Pharma
It is well established that NSAIDs are associated with increased risk of poor cardiovascular outcomes. This study offers powerful evidence that the risk after frequent diclofenac use is disproportionally increased relative to other commonly used NSAIDs, such as ibuprofen or naproxen. It is relevant to discuss the implications of the findings for the treatment of migraine.
The acute treatment of migraine associated with attack-related disability should favor triptans as first line therapy, not NSAIDs. Because triptans are vasoconstrictive medications, unmet needs exist in patients at cardiovascular risk. Anti-CGRP acute migraine therapies, as well as “ditans” (5HT-1f antagonist) are under regulatory review and may address the needs of these patients. In the context of acute migraine therapy, diclofenac and NSAIDs are typically used instead of triptans, or with triptans when additional efficacy is needed. We certainly find that the use of diclofenac in these situations should be judicious, and reserved to those who clearly need it, have infrequent migraine attacks, and are otherwise healthy.
Diclofenac is also often used in the emergency department in many countries as a rescue therapy. In a series of clinical trials where we tested most commonly used drugs in this setting in Brazil, we found that efficacies were 83.6% for intravenous dipyrone, 66.7% for intramuscular diclofenac and 81.8% for intravenous chlorpromazine. We continue to believe that diclofenac is an important, non-sedative and non-opioid option for the management of headaches in the emergency department, assuming that at discharge, patients would receive proper guidance on the management of migraine without relying on frequent use of NSAIDs.
Jack Schim, MD
Co-Director, The Headache Center of Southern California
This article supports findings of prior epidemiologic studies correlating exposure to NSAIDs with increased cerebrovascular and cardiovascular risk. Prior studies have shown a dose-related response in risk associated with NSAID therapy, supporting a causal association. However, while relative risk is significantly higher in individuals with NSAID exposure, the absolute risk remains very low. The greater risk from NSAIDs continues to be to the kidneys, and to the stomach.
As with all therapies, we need to weigh the advantages and disadvantages of NSAID therapy with our headache patients. All medications carry their own risks. For acute treatment of migraines, our primary tool, triptans, are contraindicated in a significant subset of individuals, including patients with ischemic coronary artery as well as those with history of stroke or transient ischemic attack (TIA). The alternatives, NSAIDs, dopamine blocking agents, have utility and risks.
Diclofenac powder to be dissolved in water is an effective abortive for migraine for many individuals. In general, our patients have intermittent exposure, preferably not more than 2 days per week. For the appropriate individual, NSAIDS, including diclofenac, remain an important tool in the acute care armamentarium.
Rob Cowan, MD, FAAN, FAHS
Higgins Professor of Neurology and Neurosciences
Chief, Division of Headache Medicine, Dept. of Neurology and Neurosciences
Director, Stanford University School of Medicine
These kinds of large, population-based studies must be interpreted with caution. While they may emulate the protocol of prospective studies, they lack proper inclusion/exclusion criteria, particularly with respect to indication. It may be reasonable to assume that the population of diclofenac users is "sicker" than the general population and the population that is using cheaper, more accessible NSAIDs or paracetamol. Without knowing the access and economic issues in Denmark, it is difficult to weigh these variables in the study. Thus, while it is certainly an important issue to explore (the relative risks and benefits of a given medication within a class), the absence of a well-designed, prospective study precludes any definitive conclusion regarding relative safety and risk profile for Diclofenac.
+++
These are great comments by my colleagues. My impression after seeing the data and reading my colleague’s comments, is that diclofenac may be riskier than other NSAIDs in this study; but when used properly in generally healthy migraineurs, it is probably more effective than dangerous when evaluating the risk/benefit ratio. When diclofenac is used as an oral solution (Cambia), 2 days per week or less, in a patient without serious gastrointestinal, renal, cardiac or hypertensive issues, it appears to pose little risk to the patient. When given to the wrong patient, or when taken too frequently, is could be dangerous. What I really like about this preparation is that it causes fewer adverse events compared to triptans and works very quickly. It can be used when triptans have been used enough that week or if they tend to cause significant adverse events when taken. We can use diclofenac for our headache patients, but we should remain vigilant to give it cautiously and only to patients who have no contraindication to its use.
Please write to us at Neurology Reviews Migraine Resource Center ([email protected]) with your opinions.
Alan M. Rapoport, M.D.
Editor-in-Chief
Migraine Resource Center
Clinical Professor of Neurology
The David Geffen School of Medicine at UCLA
Los Angeles, California
In July 2018, The BMJ published a study examining the cardiovascular risks of diclofenac initiation compared with initiation of other traditional non-steroidal anti-inflammatory drugs, initiation of paracetamol, and no initiation. The results showed a 50% increase in adverse events among diclofenac initiators compared with non-initiators (as well as a 20% increase over paracetamol/ibuprofen initiators, and 30% increase over naproxen initiators). (Read the full study here). Here, I asked several of my colleagues to weigh in on the results of this study and its implications for our practices, and then I share my own thoughts on these findings:
Stewart J. Tepper, MD, FAHS
Professor of Neurology
Geisel School of Medicine at Dartmouth
There have been previous studies and meta-analyses demonstrating the cardiovascular risks of diclofenac. This very large cohort study highlights the magnitude of effects for both those patients at high risk and at low risk for cardiovascular disease. Diclofenac has many advantages for migraine treatment, such as a rapid onset of action in its liquid form, but it has higher risks for major cardiac events than most currently available nonsteroidal anti-inflammatory drugs (NSAIDs). As providers, we must be judicious in diclofenac use and informative with our patients.
Marcelo Bigal, MD, PhD
Chief Medical Officer, Purdue Pharma
It is well established that NSAIDs are associated with increased risk of poor cardiovascular outcomes. This study offers powerful evidence that the risk after frequent diclofenac use is disproportionally increased relative to other commonly used NSAIDs, such as ibuprofen or naproxen. It is relevant to discuss the implications of the findings for the treatment of migraine.
The acute treatment of migraine associated with attack-related disability should favor triptans as first line therapy, not NSAIDs. Because triptans are vasoconstrictive medications, unmet needs exist in patients at cardiovascular risk. Anti-CGRP acute migraine therapies, as well as “ditans” (5HT-1f antagonist) are under regulatory review and may address the needs of these patients. In the context of acute migraine therapy, diclofenac and NSAIDs are typically used instead of triptans, or with triptans when additional efficacy is needed. We certainly find that the use of diclofenac in these situations should be judicious, and reserved to those who clearly need it, have infrequent migraine attacks, and are otherwise healthy.
Diclofenac is also often used in the emergency department in many countries as a rescue therapy. In a series of clinical trials where we tested most commonly used drugs in this setting in Brazil, we found that efficacies were 83.6% for intravenous dipyrone, 66.7% for intramuscular diclofenac and 81.8% for intravenous chlorpromazine. We continue to believe that diclofenac is an important, non-sedative and non-opioid option for the management of headaches in the emergency department, assuming that at discharge, patients would receive proper guidance on the management of migraine without relying on frequent use of NSAIDs.
Jack Schim, MD
Co-Director, The Headache Center of Southern California
This article supports findings of prior epidemiologic studies correlating exposure to NSAIDs with increased cerebrovascular and cardiovascular risk. Prior studies have shown a dose-related response in risk associated with NSAID therapy, supporting a causal association. However, while relative risk is significantly higher in individuals with NSAID exposure, the absolute risk remains very low. The greater risk from NSAIDs continues to be to the kidneys, and to the stomach.
As with all therapies, we need to weigh the advantages and disadvantages of NSAID therapy with our headache patients. All medications carry their own risks. For acute treatment of migraines, our primary tool, triptans, are contraindicated in a significant subset of individuals, including patients with ischemic coronary artery as well as those with history of stroke or transient ischemic attack (TIA). The alternatives, NSAIDs, dopamine blocking agents, have utility and risks.
Diclofenac powder to be dissolved in water is an effective abortive for migraine for many individuals. In general, our patients have intermittent exposure, preferably not more than 2 days per week. For the appropriate individual, NSAIDS, including diclofenac, remain an important tool in the acute care armamentarium.
Rob Cowan, MD, FAAN, FAHS
Higgins Professor of Neurology and Neurosciences
Chief, Division of Headache Medicine, Dept. of Neurology and Neurosciences
Director, Stanford University School of Medicine
These kinds of large, population-based studies must be interpreted with caution. While they may emulate the protocol of prospective studies, they lack proper inclusion/exclusion criteria, particularly with respect to indication. It may be reasonable to assume that the population of diclofenac users is "sicker" than the general population and the population that is using cheaper, more accessible NSAIDs or paracetamol. Without knowing the access and economic issues in Denmark, it is difficult to weigh these variables in the study. Thus, while it is certainly an important issue to explore (the relative risks and benefits of a given medication within a class), the absence of a well-designed, prospective study precludes any definitive conclusion regarding relative safety and risk profile for Diclofenac.
+++
These are great comments by my colleagues. My impression after seeing the data and reading my colleague’s comments, is that diclofenac may be riskier than other NSAIDs in this study; but when used properly in generally healthy migraineurs, it is probably more effective than dangerous when evaluating the risk/benefit ratio. When diclofenac is used as an oral solution (Cambia), 2 days per week or less, in a patient without serious gastrointestinal, renal, cardiac or hypertensive issues, it appears to pose little risk to the patient. When given to the wrong patient, or when taken too frequently, is could be dangerous. What I really like about this preparation is that it causes fewer adverse events compared to triptans and works very quickly. It can be used when triptans have been used enough that week or if they tend to cause significant adverse events when taken. We can use diclofenac for our headache patients, but we should remain vigilant to give it cautiously and only to patients who have no contraindication to its use.
Please write to us at Neurology Reviews Migraine Resource Center ([email protected]) with your opinions.
Alan M. Rapoport, M.D.
Editor-in-Chief
Migraine Resource Center
Clinical Professor of Neurology
The David Geffen School of Medicine at UCLA
Los Angeles, California
Breast cancer in men comparable with women, gonadal therapy debated
MUNICH – Men with metastatic breast cancer (MBC) have a similar prognosis, compared with women, but gonadal suppression remains a topic of debate, according to recent trials and conference proceedings.
Male breast cancer is a rare, little-studied disease that was highlighted at the annual meeting of the European Society for Medical Oncology.
“This is a truly magnificent effort made by these authors,” said invited discussant Carolien Schroeder, MD, PhD, of the University Medical Center Groningen in the Netherlands, noting the challenges inherent to studies of niche cancer populations.
Previous studies have shown that men with MBC are usually hormone receptor positive (HR+) and older than women with MBC. Male patients also tend to present with more severe disease.
“[Male breast cancer patients] usually present with higher stages of disease; larger tumors, more nodal involvement, and more metastatic disease,” Dr. Schroeder said. “And 4%-16% may have genetic predisposition, usually due to a BRCA2 mutation.”
One retrospective study used data from the Epidemiological Strategy and Medical Economics Metastatic Breast Cancer (ESME MBC) platform to further knowledge of male patient characteristics, treatment types, and disease outcomes. ESME is a national database that stores patient information from 18 cancer centers in France.
“We have reported on one of the biggest series of men with metastatic disease, with comprehensive data on their management and outcome with different types of treatment,” said principal investigator Jean-Sébastien Frénel, MD, of the Institut de Cancérologie de l’Ouest in Nantes, France, in an interview.
The ESME study evaluated 16,701 patients with MBC: 16,552 women (99.11%) and 149 men (0.89%). Patients received at least one treatment between January 2008 and December 2014.
On average, male patients were older than females (mean: 68.1 years vs. 60.6 years), which lines up with existing data; in contrast, 78.4% of men were HR+, which is slightly lower than the widely described figure of 90%. Almost half of the HR+/human epidermal growth factor 2– (HER2–) male patients were given a frontline hormonal therapy (43%); of those, 44% received tamoxifen, 40% received an aromatase inhibitor (with or without a gonadotropin-releasing hormone analog [GnRH]), and 16% received other therapies. Outcomes were relatively similar between men and women: median progression-free survival (PFS) was 9.8 months for men, compared with 13.0 months for women. About one-quarter of HR+/HER2– men (27.6%) received frontline chemotherapy, resulting in a PFS of 6.9 months compared with 6.3 months for matched women. Overall survival was also slightly longer for men than matched women (41.8 months vs 34.9 months). In general, these statistics show that men and women received similar treatments and had similar outcomes.
“Most of the patients receiving hormonal therapy were treated with tamoxifen and the remainder received aromatase inhibitors,” Dr. Frénel said. “But few patients received aromatase inhibitors plus [GnRH] analogs despite some guidelines recommending that they should be given in combination.”
GnRH for men remains a topic of debate. Although aromatase inhibitors should be given with GnRH to avoid a negative feedback loop, gonadal suppression causes erectile dysfunction, thereby decreasing well-being. This dilemma is made worse by a lack of data on hormonal therapy for men with breast cancer.
To address this shortcoming, a prospective, randomized trial compared three different hormonal regimens for men. Male-GBG54 involved 55 men with breast cancer. For 6 months, patients received 1 of 3 treatment regimens: tamoxifen (20 mg/day), tamoxifen + GnRH (subcutaneous every 3 months), or exemestane (25 mg daily) + GnRH. Median estradiol levels were measured at 3 months and 6 months, and wellbeing was measured using questionnaires.
As expected, the results showed increased estradiol levels in the tamoxifen group and decreased levels in the GnRH group. Men were generally dissatisfied with GnRH therapy because of the erectile dysfunction it caused.
“Tamoxifen monotherapy should be kept as standard hormonal therapy for men with breast cancer. The side effects are moderate, hardly impairing sexual behavior. The combination with GnRH influenced patients’ well-being and erectile function profoundly,” lead author Mattea Reinisch, MD, of Klinikum Essen-Mitte (Germany), said in an interview.
Dr. Schroeder agreed that tamoxifen should remain the standard treatment but suggested that the benefits of gonadal suppression may outweigh the downsides. “We need efficacy data for gonadal suppression,” she said. “After all, we are advising our premenopausal breast cancer patients to undergo the gonadal suppression therapy on a daily basis because of the oncological superiority, despite the toxicity they are also experiencing.”
Dr. Schroeder again called for efficacy data and described shortcomings of Male-GBG54: “[Dr. Reinisch and her colleagues] have chosen a biological surrogate endpoint, but what we’d really like, of course, [are] the efficacy data. The quality of life data are not breast-cancer specific, and these are only data for 6 months, whereas, particularly in the metastatic setting, the compliance issue after 6 months is also relevant.”
Reflecting on the data from ESME and Male-GBG54, Dr. Schroeder said, “I think this field is maturing, and intervention trials have proven themselves to be possible in this niche population.”
Looking to the future, Dr. Schroeder suggested that male breast cancer can be studied either in separate trials from women (focusing on sex-specific targets), or in shared studies, as many disease characteristics are the same regardless of sex. She also said that worse disease in men is likely due to delayed presentation rather than biological differences between men and women.
“This leaves room for improvement,” Dr. Schroeder said. “We still need to work on the awareness of this disease.”
Discussant Dr. Schroeder disclosed financial relationships with Novartis, Roche, Genentech, and others. Male-GBG54 was funded by Claudia von Schilling Foundation.
SOURCES: Sirieix J et al. ESMO 2018, Abstract 294PD; Reinisch et al. ESMO 2018, Abstract 273PD.
MUNICH – Men with metastatic breast cancer (MBC) have a similar prognosis, compared with women, but gonadal suppression remains a topic of debate, according to recent trials and conference proceedings.
Male breast cancer is a rare, little-studied disease that was highlighted at the annual meeting of the European Society for Medical Oncology.
“This is a truly magnificent effort made by these authors,” said invited discussant Carolien Schroeder, MD, PhD, of the University Medical Center Groningen in the Netherlands, noting the challenges inherent to studies of niche cancer populations.
Previous studies have shown that men with MBC are usually hormone receptor positive (HR+) and older than women with MBC. Male patients also tend to present with more severe disease.
“[Male breast cancer patients] usually present with higher stages of disease; larger tumors, more nodal involvement, and more metastatic disease,” Dr. Schroeder said. “And 4%-16% may have genetic predisposition, usually due to a BRCA2 mutation.”
One retrospective study used data from the Epidemiological Strategy and Medical Economics Metastatic Breast Cancer (ESME MBC) platform to further knowledge of male patient characteristics, treatment types, and disease outcomes. ESME is a national database that stores patient information from 18 cancer centers in France.
“We have reported on one of the biggest series of men with metastatic disease, with comprehensive data on their management and outcome with different types of treatment,” said principal investigator Jean-Sébastien Frénel, MD, of the Institut de Cancérologie de l’Ouest in Nantes, France, in an interview.
The ESME study evaluated 16,701 patients with MBC: 16,552 women (99.11%) and 149 men (0.89%). Patients received at least one treatment between January 2008 and December 2014.
On average, male patients were older than females (mean: 68.1 years vs. 60.6 years), which lines up with existing data; in contrast, 78.4% of men were HR+, which is slightly lower than the widely described figure of 90%. Almost half of the HR+/human epidermal growth factor 2– (HER2–) male patients were given a frontline hormonal therapy (43%); of those, 44% received tamoxifen, 40% received an aromatase inhibitor (with or without a gonadotropin-releasing hormone analog [GnRH]), and 16% received other therapies. Outcomes were relatively similar between men and women: median progression-free survival (PFS) was 9.8 months for men, compared with 13.0 months for women. About one-quarter of HR+/HER2– men (27.6%) received frontline chemotherapy, resulting in a PFS of 6.9 months compared with 6.3 months for matched women. Overall survival was also slightly longer for men than matched women (41.8 months vs 34.9 months). In general, these statistics show that men and women received similar treatments and had similar outcomes.
“Most of the patients receiving hormonal therapy were treated with tamoxifen and the remainder received aromatase inhibitors,” Dr. Frénel said. “But few patients received aromatase inhibitors plus [GnRH] analogs despite some guidelines recommending that they should be given in combination.”
GnRH for men remains a topic of debate. Although aromatase inhibitors should be given with GnRH to avoid a negative feedback loop, gonadal suppression causes erectile dysfunction, thereby decreasing well-being. This dilemma is made worse by a lack of data on hormonal therapy for men with breast cancer.
To address this shortcoming, a prospective, randomized trial compared three different hormonal regimens for men. Male-GBG54 involved 55 men with breast cancer. For 6 months, patients received 1 of 3 treatment regimens: tamoxifen (20 mg/day), tamoxifen + GnRH (subcutaneous every 3 months), or exemestane (25 mg daily) + GnRH. Median estradiol levels were measured at 3 months and 6 months, and wellbeing was measured using questionnaires.
As expected, the results showed increased estradiol levels in the tamoxifen group and decreased levels in the GnRH group. Men were generally dissatisfied with GnRH therapy because of the erectile dysfunction it caused.
“Tamoxifen monotherapy should be kept as standard hormonal therapy for men with breast cancer. The side effects are moderate, hardly impairing sexual behavior. The combination with GnRH influenced patients’ well-being and erectile function profoundly,” lead author Mattea Reinisch, MD, of Klinikum Essen-Mitte (Germany), said in an interview.
Dr. Schroeder agreed that tamoxifen should remain the standard treatment but suggested that the benefits of gonadal suppression may outweigh the downsides. “We need efficacy data for gonadal suppression,” she said. “After all, we are advising our premenopausal breast cancer patients to undergo the gonadal suppression therapy on a daily basis because of the oncological superiority, despite the toxicity they are also experiencing.”
Dr. Schroeder again called for efficacy data and described shortcomings of Male-GBG54: “[Dr. Reinisch and her colleagues] have chosen a biological surrogate endpoint, but what we’d really like, of course, [are] the efficacy data. The quality of life data are not breast-cancer specific, and these are only data for 6 months, whereas, particularly in the metastatic setting, the compliance issue after 6 months is also relevant.”
Reflecting on the data from ESME and Male-GBG54, Dr. Schroeder said, “I think this field is maturing, and intervention trials have proven themselves to be possible in this niche population.”
Looking to the future, Dr. Schroeder suggested that male breast cancer can be studied either in separate trials from women (focusing on sex-specific targets), or in shared studies, as many disease characteristics are the same regardless of sex. She also said that worse disease in men is likely due to delayed presentation rather than biological differences between men and women.
“This leaves room for improvement,” Dr. Schroeder said. “We still need to work on the awareness of this disease.”
Discussant Dr. Schroeder disclosed financial relationships with Novartis, Roche, Genentech, and others. Male-GBG54 was funded by Claudia von Schilling Foundation.
SOURCES: Sirieix J et al. ESMO 2018, Abstract 294PD; Reinisch et al. ESMO 2018, Abstract 273PD.
MUNICH – Men with metastatic breast cancer (MBC) have a similar prognosis, compared with women, but gonadal suppression remains a topic of debate, according to recent trials and conference proceedings.
Male breast cancer is a rare, little-studied disease that was highlighted at the annual meeting of the European Society for Medical Oncology.
“This is a truly magnificent effort made by these authors,” said invited discussant Carolien Schroeder, MD, PhD, of the University Medical Center Groningen in the Netherlands, noting the challenges inherent to studies of niche cancer populations.
Previous studies have shown that men with MBC are usually hormone receptor positive (HR+) and older than women with MBC. Male patients also tend to present with more severe disease.
“[Male breast cancer patients] usually present with higher stages of disease; larger tumors, more nodal involvement, and more metastatic disease,” Dr. Schroeder said. “And 4%-16% may have genetic predisposition, usually due to a BRCA2 mutation.”
One retrospective study used data from the Epidemiological Strategy and Medical Economics Metastatic Breast Cancer (ESME MBC) platform to further knowledge of male patient characteristics, treatment types, and disease outcomes. ESME is a national database that stores patient information from 18 cancer centers in France.
“We have reported on one of the biggest series of men with metastatic disease, with comprehensive data on their management and outcome with different types of treatment,” said principal investigator Jean-Sébastien Frénel, MD, of the Institut de Cancérologie de l’Ouest in Nantes, France, in an interview.
The ESME study evaluated 16,701 patients with MBC: 16,552 women (99.11%) and 149 men (0.89%). Patients received at least one treatment between January 2008 and December 2014.
On average, male patients were older than females (mean: 68.1 years vs. 60.6 years), which lines up with existing data; in contrast, 78.4% of men were HR+, which is slightly lower than the widely described figure of 90%. Almost half of the HR+/human epidermal growth factor 2– (HER2–) male patients were given a frontline hormonal therapy (43%); of those, 44% received tamoxifen, 40% received an aromatase inhibitor (with or without a gonadotropin-releasing hormone analog [GnRH]), and 16% received other therapies. Outcomes were relatively similar between men and women: median progression-free survival (PFS) was 9.8 months for men, compared with 13.0 months for women. About one-quarter of HR+/HER2– men (27.6%) received frontline chemotherapy, resulting in a PFS of 6.9 months compared with 6.3 months for matched women. Overall survival was also slightly longer for men than matched women (41.8 months vs 34.9 months). In general, these statistics show that men and women received similar treatments and had similar outcomes.
“Most of the patients receiving hormonal therapy were treated with tamoxifen and the remainder received aromatase inhibitors,” Dr. Frénel said. “But few patients received aromatase inhibitors plus [GnRH] analogs despite some guidelines recommending that they should be given in combination.”
GnRH for men remains a topic of debate. Although aromatase inhibitors should be given with GnRH to avoid a negative feedback loop, gonadal suppression causes erectile dysfunction, thereby decreasing well-being. This dilemma is made worse by a lack of data on hormonal therapy for men with breast cancer.
To address this shortcoming, a prospective, randomized trial compared three different hormonal regimens for men. Male-GBG54 involved 55 men with breast cancer. For 6 months, patients received 1 of 3 treatment regimens: tamoxifen (20 mg/day), tamoxifen + GnRH (subcutaneous every 3 months), or exemestane (25 mg daily) + GnRH. Median estradiol levels were measured at 3 months and 6 months, and wellbeing was measured using questionnaires.
As expected, the results showed increased estradiol levels in the tamoxifen group and decreased levels in the GnRH group. Men were generally dissatisfied with GnRH therapy because of the erectile dysfunction it caused.
“Tamoxifen monotherapy should be kept as standard hormonal therapy for men with breast cancer. The side effects are moderate, hardly impairing sexual behavior. The combination with GnRH influenced patients’ well-being and erectile function profoundly,” lead author Mattea Reinisch, MD, of Klinikum Essen-Mitte (Germany), said in an interview.
Dr. Schroeder agreed that tamoxifen should remain the standard treatment but suggested that the benefits of gonadal suppression may outweigh the downsides. “We need efficacy data for gonadal suppression,” she said. “After all, we are advising our premenopausal breast cancer patients to undergo the gonadal suppression therapy on a daily basis because of the oncological superiority, despite the toxicity they are also experiencing.”
Dr. Schroeder again called for efficacy data and described shortcomings of Male-GBG54: “[Dr. Reinisch and her colleagues] have chosen a biological surrogate endpoint, but what we’d really like, of course, [are] the efficacy data. The quality of life data are not breast-cancer specific, and these are only data for 6 months, whereas, particularly in the metastatic setting, the compliance issue after 6 months is also relevant.”
Reflecting on the data from ESME and Male-GBG54, Dr. Schroeder said, “I think this field is maturing, and intervention trials have proven themselves to be possible in this niche population.”
Looking to the future, Dr. Schroeder suggested that male breast cancer can be studied either in separate trials from women (focusing on sex-specific targets), or in shared studies, as many disease characteristics are the same regardless of sex. She also said that worse disease in men is likely due to delayed presentation rather than biological differences between men and women.
“This leaves room for improvement,” Dr. Schroeder said. “We still need to work on the awareness of this disease.”
Discussant Dr. Schroeder disclosed financial relationships with Novartis, Roche, Genentech, and others. Male-GBG54 was funded by Claudia von Schilling Foundation.
SOURCES: Sirieix J et al. ESMO 2018, Abstract 294PD; Reinisch et al. ESMO 2018, Abstract 273PD.
REPORTING FROM ESMO 2018
Key clinical point: Men with metastatic breast cancer (MBC) have a similar prognosis, compared with women, but gonadal suppression remains a topic of debate.
Major finding: Median PFS was 9.8 months in men, compared with 13.0 months in matched women.
Study details: The ESME study was a large-scale retrospective analysis of 16,701 patients, 149 of whom were men with MBC. Male-GBG54 was a prospective, randomized trial involving 46 MBC patients who received three different endocrine therapies.
Disclosures: Discussant Dr. Schroeder disclosed financial relationships with Novartis, Roche, Genentech, and others. Male-GBG54 was funded by Claudia von Schilling Foundation.
Source: Sirieix et al. ESMO 2018, Abstract 294PD; Reinisch et al. ESMO 2018, Abstract 273PD.
Private practice gastroenterology models: Weighing the options
Editor’s note: It is my pleasure to introduce this new quarterly column in The New Gastroenterologist that will be dedicated to addressing important topics for early-career GIs who are either considering a career or starting a career as an independent GI physician in practice. This column is a collaboration between the AGA’s The New Gastroenterologist and the Digestive Health Physicians Association (DHPA), a national advocacy organization of more than 1,800 gastroenterologists in 79 member practices, which is focused exclusively on policies that promote and protect the high-quality, cost-efficient care provided to patients in the independent GI-practice setting.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Educating and training for your life’s work has likely occupied much of the past 10 years including medical school, residency, and fellowship. When it comes to deciding the next steps, the options can seem daunting.
As a long-standing private practice gastroenterologist, I think it is important for early-career GIs to understand the different private practice options and the new challenges and opportunities that private practitioners are experiencing.
According to recent data, there are approximately 12,500 clinical gastroenterologists divided among private gastroenterology practice models, hospital-based employees, and large multispecialty groups. There are nearly 6,000 private practice gastroenterologists.
There has been ongoing dialogue among all health care system stakeholders and the public regarding health care delivery, access, and financing. For the past several years, private practice advocacy groups, including DHPA, have been urging the elimination of the Medicare “site of service” differential between hospital and nonhospital settings, which typically results in substantially higher costs for hospital-based services.
In the 2015 Balanced Budget Act, Congress mandated that patient services provided in off-campus locations acquired by hospitals after Nov.1, 2015, be paid at the nonhospital rate. The 2019 Medicare Outpatient Prospective Payment System/Ambulatory Surgical Center proposed rule takes additional steps in achieving site neutrality and will likely have the effect of tamping down hospitals’ incentives to acquire independent physician practices.
This is a positive step forward in preserving the cost-efficient, high-quality care provided in the independent GI medical setting. And, with the growing health needs of an aging population and an aging GI physician workforce (nearly half of gastroenterologists are older than 55 years old), there is sure to be an ongoing high demand for providers in our specialty.
Selecting the career path that best fits your goals, ambitions, and lifestyle can be challenging. But, our professional training has taught us that the best method for arriving at the correct course of action is to first understand the questions and then seek the answers – let’s get to it.
Private practice models: What are the options?
A lot has changed since I completed my fellowship training in 1978. But the most dramatic changes have happened in the past decade, including the trend of smaller practices consolidating into larger groups.
Traditionally, physicians and patients have favored individual and very-small-group practices. Patients view small-group practices as highly personalized. They come to appreciate knowing all the physicians and staff in a practice. These long-standing “family type” relationships among patients and providers that often develop in this clinical care setting engenders in both the patient and provider a high level of satisfaction with the type and experience of care provided.
New physicians who are part of a small group practice often have the opportunity to take an earlier and more active role within the leadership of the practice. Small groups typically look to new physicians to function as “innovators” who can introduce into the practice those cutting-edge treatments and procedures learned during fellowship.
In the past decade, however, the trend toward the disappearance of solo and very-small-group practices has accelerated. Today, very small groups face several challenges. Providing all the “necessities” that are now part of today’s medical practice can be daunting. Small-sized practices are less likely to integrate ancillary services (e.g., lab and pathology services, in-office infusions, dietary and weight loss management) that are more typically seen in larger practices. Patients may find this fragmentation of care burdensome when they have to go to several providers for treatment.
The difficulties of implementing and maintaining information technology, EHRs, and patient-engagement tools are often inversely related to group size. In addition, the ability and effectiveness of a group to negotiate with hospital systems and insurance companies can be easier for larger practices, although other local factors will also come into play.
Beyond the administrative aspects of running a small group practice, your views about work-life balance should also be an important consideration when choosing a career path. Understandably, issues of call coverage and time off can be more restrictive in small groups.
Is bigger better?
The consolidation seen in hospital systems and multispecialty groups has found its way into single specialty practices. Many urban areas now have GI group practices of 10 or more physicians. There are now approximately 15 groups with 40 or more gastroenterologists, including a few GI practices with 100 or more physicians.
Increasing the size of a practice has obvious potential advantages, including less burdensome on-call requirements and a lower per-physician cost of maintaining and operating the practice. Larger groups often have dedicated software development and IT support staff. Patients are engaged and can connect with their providers through all manner of social media.
Large practice size also can make it possible to enable physicians who may choose to focus on single areas of gastroenterology. This means that a physician who wants to subspecialize in areas such as inflammatory bowel disease, hepatology, woman’s health, and advanced therapeutic endoscopy, would have the requisite large patient base, through internal practice referral, to support subspecialization. Larger groups can also integrate ancillary services into their practice such as pathology, infusion therapy, and nonhospital-based endoscopy services.
However, there can be disadvantages to choosing a larger practice. As in other larger institutions, physicians practicing in larger-sized groups may feel somewhat removed from practice management decisions. It may take several years to become a partner in a large practice – if you are more interested in the opportunity to be involved in practice decisions, a smaller group may be right for you.
New trends in practice groups
Physicians are continuously looking for ways to practice effectively and efficiently while expanding the range of services offered (think obesity management). Independent practice physicians are finding it increasingly difficult to grow and manage successful organizations while they care for their patients. Larger practices now typically include areas such as nursing, information technology, human resources, billing, and practice administration. I trained to treat patients, not run a business – there was much I’ve learned along the way. Many schools now offer joint MD/MBA programs. This may help blend the clinical, operational, and business components of practice.
In a newly developing trend, practice groups are exploring strategic partnerships with private equity/venture capital, practice management companies, national ambulatory surgery center companies, and even managed care insurance companies. This creates the opportunity to forge partnerships with these various health care–focused groups, and results in investment in GI practices seeking experienced business leadership and management while remaining independent of a health system. Already well established in dermatology, ophthalmology, and anesthesia, this phenomenon is now beginning in gastroenterology.
There are many things to consider when choosing a career path. Independent practice in gastroenterology continues as a vitally important component of care delivery, and it’s my hope that the new generation of gastroenterologists finds their journey as rewarding and personally satisfying as mine has been.
Fred B. Rosenberg, MD, is a board-certified gastroenterologist and the medical director of the North Shore Endoscopy Center in Lake Bluff, Ill., the founding president of Illinois Gastroenterology Group, and immediate past president of DHPA.
Editor’s note: It is my pleasure to introduce this new quarterly column in The New Gastroenterologist that will be dedicated to addressing important topics for early-career GIs who are either considering a career or starting a career as an independent GI physician in practice. This column is a collaboration between the AGA’s The New Gastroenterologist and the Digestive Health Physicians Association (DHPA), a national advocacy organization of more than 1,800 gastroenterologists in 79 member practices, which is focused exclusively on policies that promote and protect the high-quality, cost-efficient care provided to patients in the independent GI-practice setting.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Educating and training for your life’s work has likely occupied much of the past 10 years including medical school, residency, and fellowship. When it comes to deciding the next steps, the options can seem daunting.
As a long-standing private practice gastroenterologist, I think it is important for early-career GIs to understand the different private practice options and the new challenges and opportunities that private practitioners are experiencing.
According to recent data, there are approximately 12,500 clinical gastroenterologists divided among private gastroenterology practice models, hospital-based employees, and large multispecialty groups. There are nearly 6,000 private practice gastroenterologists.
There has been ongoing dialogue among all health care system stakeholders and the public regarding health care delivery, access, and financing. For the past several years, private practice advocacy groups, including DHPA, have been urging the elimination of the Medicare “site of service” differential between hospital and nonhospital settings, which typically results in substantially higher costs for hospital-based services.
In the 2015 Balanced Budget Act, Congress mandated that patient services provided in off-campus locations acquired by hospitals after Nov.1, 2015, be paid at the nonhospital rate. The 2019 Medicare Outpatient Prospective Payment System/Ambulatory Surgical Center proposed rule takes additional steps in achieving site neutrality and will likely have the effect of tamping down hospitals’ incentives to acquire independent physician practices.
This is a positive step forward in preserving the cost-efficient, high-quality care provided in the independent GI medical setting. And, with the growing health needs of an aging population and an aging GI physician workforce (nearly half of gastroenterologists are older than 55 years old), there is sure to be an ongoing high demand for providers in our specialty.
Selecting the career path that best fits your goals, ambitions, and lifestyle can be challenging. But, our professional training has taught us that the best method for arriving at the correct course of action is to first understand the questions and then seek the answers – let’s get to it.
Private practice models: What are the options?
A lot has changed since I completed my fellowship training in 1978. But the most dramatic changes have happened in the past decade, including the trend of smaller practices consolidating into larger groups.
Traditionally, physicians and patients have favored individual and very-small-group practices. Patients view small-group practices as highly personalized. They come to appreciate knowing all the physicians and staff in a practice. These long-standing “family type” relationships among patients and providers that often develop in this clinical care setting engenders in both the patient and provider a high level of satisfaction with the type and experience of care provided.
New physicians who are part of a small group practice often have the opportunity to take an earlier and more active role within the leadership of the practice. Small groups typically look to new physicians to function as “innovators” who can introduce into the practice those cutting-edge treatments and procedures learned during fellowship.
In the past decade, however, the trend toward the disappearance of solo and very-small-group practices has accelerated. Today, very small groups face several challenges. Providing all the “necessities” that are now part of today’s medical practice can be daunting. Small-sized practices are less likely to integrate ancillary services (e.g., lab and pathology services, in-office infusions, dietary and weight loss management) that are more typically seen in larger practices. Patients may find this fragmentation of care burdensome when they have to go to several providers for treatment.
The difficulties of implementing and maintaining information technology, EHRs, and patient-engagement tools are often inversely related to group size. In addition, the ability and effectiveness of a group to negotiate with hospital systems and insurance companies can be easier for larger practices, although other local factors will also come into play.
Beyond the administrative aspects of running a small group practice, your views about work-life balance should also be an important consideration when choosing a career path. Understandably, issues of call coverage and time off can be more restrictive in small groups.
Is bigger better?
The consolidation seen in hospital systems and multispecialty groups has found its way into single specialty practices. Many urban areas now have GI group practices of 10 or more physicians. There are now approximately 15 groups with 40 or more gastroenterologists, including a few GI practices with 100 or more physicians.
Increasing the size of a practice has obvious potential advantages, including less burdensome on-call requirements and a lower per-physician cost of maintaining and operating the practice. Larger groups often have dedicated software development and IT support staff. Patients are engaged and can connect with their providers through all manner of social media.
Large practice size also can make it possible to enable physicians who may choose to focus on single areas of gastroenterology. This means that a physician who wants to subspecialize in areas such as inflammatory bowel disease, hepatology, woman’s health, and advanced therapeutic endoscopy, would have the requisite large patient base, through internal practice referral, to support subspecialization. Larger groups can also integrate ancillary services into their practice such as pathology, infusion therapy, and nonhospital-based endoscopy services.
However, there can be disadvantages to choosing a larger practice. As in other larger institutions, physicians practicing in larger-sized groups may feel somewhat removed from practice management decisions. It may take several years to become a partner in a large practice – if you are more interested in the opportunity to be involved in practice decisions, a smaller group may be right for you.
New trends in practice groups
Physicians are continuously looking for ways to practice effectively and efficiently while expanding the range of services offered (think obesity management). Independent practice physicians are finding it increasingly difficult to grow and manage successful organizations while they care for their patients. Larger practices now typically include areas such as nursing, information technology, human resources, billing, and practice administration. I trained to treat patients, not run a business – there was much I’ve learned along the way. Many schools now offer joint MD/MBA programs. This may help blend the clinical, operational, and business components of practice.
In a newly developing trend, practice groups are exploring strategic partnerships with private equity/venture capital, practice management companies, national ambulatory surgery center companies, and even managed care insurance companies. This creates the opportunity to forge partnerships with these various health care–focused groups, and results in investment in GI practices seeking experienced business leadership and management while remaining independent of a health system. Already well established in dermatology, ophthalmology, and anesthesia, this phenomenon is now beginning in gastroenterology.
There are many things to consider when choosing a career path. Independent practice in gastroenterology continues as a vitally important component of care delivery, and it’s my hope that the new generation of gastroenterologists finds their journey as rewarding and personally satisfying as mine has been.
Fred B. Rosenberg, MD, is a board-certified gastroenterologist and the medical director of the North Shore Endoscopy Center in Lake Bluff, Ill., the founding president of Illinois Gastroenterology Group, and immediate past president of DHPA.
Editor’s note: It is my pleasure to introduce this new quarterly column in The New Gastroenterologist that will be dedicated to addressing important topics for early-career GIs who are either considering a career or starting a career as an independent GI physician in practice. This column is a collaboration between the AGA’s The New Gastroenterologist and the Digestive Health Physicians Association (DHPA), a national advocacy organization of more than 1,800 gastroenterologists in 79 member practices, which is focused exclusively on policies that promote and protect the high-quality, cost-efficient care provided to patients in the independent GI-practice setting.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Educating and training for your life’s work has likely occupied much of the past 10 years including medical school, residency, and fellowship. When it comes to deciding the next steps, the options can seem daunting.
As a long-standing private practice gastroenterologist, I think it is important for early-career GIs to understand the different private practice options and the new challenges and opportunities that private practitioners are experiencing.
According to recent data, there are approximately 12,500 clinical gastroenterologists divided among private gastroenterology practice models, hospital-based employees, and large multispecialty groups. There are nearly 6,000 private practice gastroenterologists.
There has been ongoing dialogue among all health care system stakeholders and the public regarding health care delivery, access, and financing. For the past several years, private practice advocacy groups, including DHPA, have been urging the elimination of the Medicare “site of service” differential between hospital and nonhospital settings, which typically results in substantially higher costs for hospital-based services.
In the 2015 Balanced Budget Act, Congress mandated that patient services provided in off-campus locations acquired by hospitals after Nov.1, 2015, be paid at the nonhospital rate. The 2019 Medicare Outpatient Prospective Payment System/Ambulatory Surgical Center proposed rule takes additional steps in achieving site neutrality and will likely have the effect of tamping down hospitals’ incentives to acquire independent physician practices.
This is a positive step forward in preserving the cost-efficient, high-quality care provided in the independent GI medical setting. And, with the growing health needs of an aging population and an aging GI physician workforce (nearly half of gastroenterologists are older than 55 years old), there is sure to be an ongoing high demand for providers in our specialty.
Selecting the career path that best fits your goals, ambitions, and lifestyle can be challenging. But, our professional training has taught us that the best method for arriving at the correct course of action is to first understand the questions and then seek the answers – let’s get to it.
Private practice models: What are the options?
A lot has changed since I completed my fellowship training in 1978. But the most dramatic changes have happened in the past decade, including the trend of smaller practices consolidating into larger groups.
Traditionally, physicians and patients have favored individual and very-small-group practices. Patients view small-group practices as highly personalized. They come to appreciate knowing all the physicians and staff in a practice. These long-standing “family type” relationships among patients and providers that often develop in this clinical care setting engenders in both the patient and provider a high level of satisfaction with the type and experience of care provided.
New physicians who are part of a small group practice often have the opportunity to take an earlier and more active role within the leadership of the practice. Small groups typically look to new physicians to function as “innovators” who can introduce into the practice those cutting-edge treatments and procedures learned during fellowship.
In the past decade, however, the trend toward the disappearance of solo and very-small-group practices has accelerated. Today, very small groups face several challenges. Providing all the “necessities” that are now part of today’s medical practice can be daunting. Small-sized practices are less likely to integrate ancillary services (e.g., lab and pathology services, in-office infusions, dietary and weight loss management) that are more typically seen in larger practices. Patients may find this fragmentation of care burdensome when they have to go to several providers for treatment.
The difficulties of implementing and maintaining information technology, EHRs, and patient-engagement tools are often inversely related to group size. In addition, the ability and effectiveness of a group to negotiate with hospital systems and insurance companies can be easier for larger practices, although other local factors will also come into play.
Beyond the administrative aspects of running a small group practice, your views about work-life balance should also be an important consideration when choosing a career path. Understandably, issues of call coverage and time off can be more restrictive in small groups.
Is bigger better?
The consolidation seen in hospital systems and multispecialty groups has found its way into single specialty practices. Many urban areas now have GI group practices of 10 or more physicians. There are now approximately 15 groups with 40 or more gastroenterologists, including a few GI practices with 100 or more physicians.
Increasing the size of a practice has obvious potential advantages, including less burdensome on-call requirements and a lower per-physician cost of maintaining and operating the practice. Larger groups often have dedicated software development and IT support staff. Patients are engaged and can connect with their providers through all manner of social media.
Large practice size also can make it possible to enable physicians who may choose to focus on single areas of gastroenterology. This means that a physician who wants to subspecialize in areas such as inflammatory bowel disease, hepatology, woman’s health, and advanced therapeutic endoscopy, would have the requisite large patient base, through internal practice referral, to support subspecialization. Larger groups can also integrate ancillary services into their practice such as pathology, infusion therapy, and nonhospital-based endoscopy services.
However, there can be disadvantages to choosing a larger practice. As in other larger institutions, physicians practicing in larger-sized groups may feel somewhat removed from practice management decisions. It may take several years to become a partner in a large practice – if you are more interested in the opportunity to be involved in practice decisions, a smaller group may be right for you.
New trends in practice groups
Physicians are continuously looking for ways to practice effectively and efficiently while expanding the range of services offered (think obesity management). Independent practice physicians are finding it increasingly difficult to grow and manage successful organizations while they care for their patients. Larger practices now typically include areas such as nursing, information technology, human resources, billing, and practice administration. I trained to treat patients, not run a business – there was much I’ve learned along the way. Many schools now offer joint MD/MBA programs. This may help blend the clinical, operational, and business components of practice.
In a newly developing trend, practice groups are exploring strategic partnerships with private equity/venture capital, practice management companies, national ambulatory surgery center companies, and even managed care insurance companies. This creates the opportunity to forge partnerships with these various health care–focused groups, and results in investment in GI practices seeking experienced business leadership and management while remaining independent of a health system. Already well established in dermatology, ophthalmology, and anesthesia, this phenomenon is now beginning in gastroenterology.
There are many things to consider when choosing a career path. Independent practice in gastroenterology continues as a vitally important component of care delivery, and it’s my hope that the new generation of gastroenterologists finds their journey as rewarding and personally satisfying as mine has been.
Fred B. Rosenberg, MD, is a board-certified gastroenterologist and the medical director of the North Shore Endoscopy Center in Lake Bluff, Ill., the founding president of Illinois Gastroenterology Group, and immediate past president of DHPA.
Drug overdose deaths down since late 2017
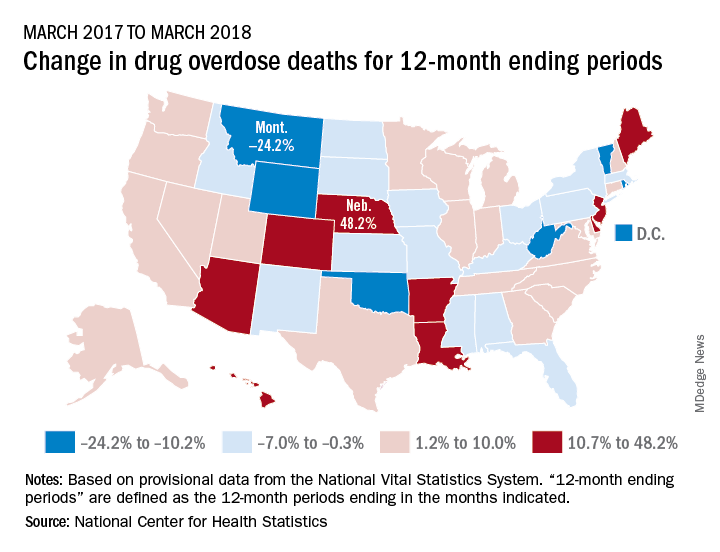
Longer-term data, however, show an increase over the year from March 2017 to March 2018, as the short-term decrease was not enough to overcome the previous year’s increase. The provisional 12-month ending count – deaths during the 12-month period ending in the month indicated – went from 66,859 in March 2017 to 68,690 in March 2018, an increase of 2.7%, the NCHS reported.
That year-long increase was not spread evenly among the states. Nebraska’s 12-month ending count jumped over 48% from March 2017 to March 2018, more than twice as much as second-place Hawaii’s 20.9%. Montana had the largest drop over that year, –24.2%, with Wyoming next at –20.7% and the District of Columbia third at –14.8%, data from the National Vital Statistics System show.
“Provisional drug overdose death data are often incomplete,” the NCHS noted, “and the degree of completeness varies by jurisdiction and 12-month ending period. Consequently, the numbers of drug overdose deaths are underestimated, based on provisional data relative to final data and are subject to random variation.”

Longer-term data, however, show an increase over the year from March 2017 to March 2018, as the short-term decrease was not enough to overcome the previous year’s increase. The provisional 12-month ending count – deaths during the 12-month period ending in the month indicated – went from 66,859 in March 2017 to 68,690 in March 2018, an increase of 2.7%, the NCHS reported.
That year-long increase was not spread evenly among the states. Nebraska’s 12-month ending count jumped over 48% from March 2017 to March 2018, more than twice as much as second-place Hawaii’s 20.9%. Montana had the largest drop over that year, –24.2%, with Wyoming next at –20.7% and the District of Columbia third at –14.8%, data from the National Vital Statistics System show.
“Provisional drug overdose death data are often incomplete,” the NCHS noted, “and the degree of completeness varies by jurisdiction and 12-month ending period. Consequently, the numbers of drug overdose deaths are underestimated, based on provisional data relative to final data and are subject to random variation.”

Longer-term data, however, show an increase over the year from March 2017 to March 2018, as the short-term decrease was not enough to overcome the previous year’s increase. The provisional 12-month ending count – deaths during the 12-month period ending in the month indicated – went from 66,859 in March 2017 to 68,690 in March 2018, an increase of 2.7%, the NCHS reported.
That year-long increase was not spread evenly among the states. Nebraska’s 12-month ending count jumped over 48% from March 2017 to March 2018, more than twice as much as second-place Hawaii’s 20.9%. Montana had the largest drop over that year, –24.2%, with Wyoming next at –20.7% and the District of Columbia third at –14.8%, data from the National Vital Statistics System show.
“Provisional drug overdose death data are often incomplete,” the NCHS noted, “and the degree of completeness varies by jurisdiction and 12-month ending period. Consequently, the numbers of drug overdose deaths are underestimated, based on provisional data relative to final data and are subject to random variation.”
Calendar
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Dec. 10-11, 12-13, 2018; Jan. 16-17, 22-23, 23-24, 2019; Feb. 20-21, 2019
Two-Day, In-Depth Coding Seminar by McVey Associates, Inc
.Become a certified GI coder with a two-day, in-depth training course provided by McVey Associates, Inc.
Tampa, FL (12/10-11), Dallas, TX (12/12-13), Houston, TX (1/16-17), New Orleans, LA (1/22-23), Pittsburgh, PA (1/23-24), 2/20 (Hartford, CT)
Jan. 17-19, 2019
2019 GI Cancers Symposium
Join colleagues from across the globe in San Francisco to discover and share groundbreaking research in treating gastrointestinal cancers.
San Francisco, CA
Feb. 7–9, 2019
Crohn’s & Colitis Congress™ (A Partnership of the Crohn’s & Colitis Foundation and American Gastroenterological Association)
Expand your knowledge, network with IBD leaders, spark innovative research and get inspired to improve patient care.
Las Vegas, NV
March 8-9, 2019
2019 Women’s Leadership Conference
The conference is specifically designed for women looking to advance their careers, further professional goals, enhance personal growth and effectively network.
Bethesda, MD
March 8-10, 2019
FORWARD Program
AGA’s Fostering Opportunities Resulting in Workforce and Research Diversity (FORWARD) Program is a new initiative funded by NIH, supporting the career entry and development for underrepresented minority physician scientists in gastroenterology. The program provides concrete leadership and skill development that includes scientific manuscript and grant writing, research development, executive coaching and more.
Bethesda, MD
March 8-10, 2019
Future Leaders Program
The Future Leaders Program provides a pathway within the organization to network, connect with mentors, develop leadership skills and learn about AGA’s governance and operations while advancing your career and supporting the profession.
Bethesda, MD
March 23–24, 2019
2019 Gut Microbiota for Health World Summit
The 2019 program will present the latest evidence on the interaction between diet, nutrition and the gut microbiome. Learn how diet and nutrition are being used in concert with traditional therapies to manage GI disorders.
Miami, FL
May 18-21, 2019
Digestive Disease Week (DDW)®
DDW® is the world’s leading educational forum for academicians, clinicians, researchers, students and trainees working in gastroenterology, hepatology, GI endoscopy, gastrointestinal surgery and related fields. Whether you work in patient care, research, education or administration, the DDW program offers something for you. DDW is co-sponsored by AGA, AASLD, ASGE and SSAT.
San Diego, CA
AWARDS APPLICATION DEADLINES
AGA-Rady Children’s Institute for Genomic Medicine Research Scholar Award in Pediatric Genomics
This award provides $90,000 per year for three years (totaling $270,000) to a young investigator, instructor, research associate or equivalent working toward an independent career in gastroenterology, hepatology or related areas. The proposed research may be basic, translational or clinical and must use genomics as an approach to enhance understanding of pediatric digestive diseases toward prevention, treatment and/or cure of such diseases. The funded research must be conducted full-time at the Rady Children’s Institute for Genomic Medicine in San Diego, California, or at Rady Children’s Hospital – San Diego.
Application Deadline: Dec. 14, 2018
AGA-Bern Schwartz Family Fund Research Scholar Award in Pancreatic Cancer
This award provides $100,000 per year for three years (total $300,000) to early career faculty (i.e., investigator, instructor, research associate or equivalent) working toward an independent career in pancreatic cancer research.
Application Deadline: Dec. 14, 2018
AGA Research Scholar Award (RSA)
This award provides $90,000 per year for three years (total $270,000) to a young investigator, instructor, research associate or equivalent working toward an independent career in gastroenterology, hepatology or related areas.
Application Deadline: Dec. 14, 2018
AGA-Shire Research Scholar Award in Functional GI and Motility Disorders
This award provides $90,000 per year for three years (total $270,000) to a young investigator, instructor, research associate or equivalent working toward an independent career in functional GI and motility disorders research.
Application Deadline: Dec. 14, 2018
AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
This award provides $90,000 per year for three years (total $270,000) to a young investigator, instructor, research associate or equivalent working toward an independent career in inflammatory bowel disease research.
Application Deadline: Dec. 14, 2018
AGA Fellow Abstract Award
This travel award provides nine $500 and one $1,000 prize to recipients who are MD, PhD or equivalent fellows presenting posters/oral sessions at Digestive Disease Week® (DDW).
Application Deadline: Feb. 15, 2019
AGA Moti L. & Kamla Rustgi International Travel Awards
This travel award provides two $750 prizes to recipients who are young basic, translational or clinical investigators residing outside North America to support travel and related expenses to attend Digestive Disease Week® (DDW).
Application Deadline: Feb. 15, 2019
AGA Student Abstract Award
This travel award provides nine $500 and one $1,000 prize to recipients who are high school, undergraduate, graduate, or medical students or residents (residents up to year three postgraduate) presenting posters/oral sessions at Digestive Disease Week® (DDW).
Application Deadline: Feb. 15, 2019
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Dec. 10-11, 12-13, 2018; Jan. 16-17, 22-23, 23-24, 2019; Feb. 20-21, 2019
Two-Day, In-Depth Coding Seminar by McVey Associates, Inc
.Become a certified GI coder with a two-day, in-depth training course provided by McVey Associates, Inc.
Tampa, FL (12/10-11), Dallas, TX (12/12-13), Houston, TX (1/16-17), New Orleans, LA (1/22-23), Pittsburgh, PA (1/23-24), 2/20 (Hartford, CT)
Jan. 17-19, 2019
2019 GI Cancers Symposium
Join colleagues from across the globe in San Francisco to discover and share groundbreaking research in treating gastrointestinal cancers.
San Francisco, CA
Feb. 7–9, 2019
Crohn’s & Colitis Congress™ (A Partnership of the Crohn’s & Colitis Foundation and American Gastroenterological Association)
Expand your knowledge, network with IBD leaders, spark innovative research and get inspired to improve patient care.
Las Vegas, NV
March 8-9, 2019
2019 Women’s Leadership Conference
The conference is specifically designed for women looking to advance their careers, further professional goals, enhance personal growth and effectively network.
Bethesda, MD
March 8-10, 2019
FORWARD Program
AGA’s Fostering Opportunities Resulting in Workforce and Research Diversity (FORWARD) Program is a new initiative funded by NIH, supporting the career entry and development for underrepresented minority physician scientists in gastroenterology. The program provides concrete leadership and skill development that includes scientific manuscript and grant writing, research development, executive coaching and more.
Bethesda, MD
March 8-10, 2019
Future Leaders Program
The Future Leaders Program provides a pathway within the organization to network, connect with mentors, develop leadership skills and learn about AGA’s governance and operations while advancing your career and supporting the profession.
Bethesda, MD
March 23–24, 2019
2019 Gut Microbiota for Health World Summit
The 2019 program will present the latest evidence on the interaction between diet, nutrition and the gut microbiome. Learn how diet and nutrition are being used in concert with traditional therapies to manage GI disorders.
Miami, FL
May 18-21, 2019
Digestive Disease Week (DDW)®
DDW® is the world’s leading educational forum for academicians, clinicians, researchers, students and trainees working in gastroenterology, hepatology, GI endoscopy, gastrointestinal surgery and related fields. Whether you work in patient care, research, education or administration, the DDW program offers something for you. DDW is co-sponsored by AGA, AASLD, ASGE and SSAT.
San Diego, CA
AWARDS APPLICATION DEADLINES
AGA-Rady Children’s Institute for Genomic Medicine Research Scholar Award in Pediatric Genomics
This award provides $90,000 per year for three years (totaling $270,000) to a young investigator, instructor, research associate or equivalent working toward an independent career in gastroenterology, hepatology or related areas. The proposed research may be basic, translational or clinical and must use genomics as an approach to enhance understanding of pediatric digestive diseases toward prevention, treatment and/or cure of such diseases. The funded research must be conducted full-time at the Rady Children’s Institute for Genomic Medicine in San Diego, California, or at Rady Children’s Hospital – San Diego.
Application Deadline: Dec. 14, 2018
AGA-Bern Schwartz Family Fund Research Scholar Award in Pancreatic Cancer
This award provides $100,000 per year for three years (total $300,000) to early career faculty (i.e., investigator, instructor, research associate or equivalent) working toward an independent career in pancreatic cancer research.
Application Deadline: Dec. 14, 2018
AGA Research Scholar Award (RSA)
This award provides $90,000 per year for three years (total $270,000) to a young investigator, instructor, research associate or equivalent working toward an independent career in gastroenterology, hepatology or related areas.
Application Deadline: Dec. 14, 2018
AGA-Shire Research Scholar Award in Functional GI and Motility Disorders
This award provides $90,000 per year for three years (total $270,000) to a young investigator, instructor, research associate or equivalent working toward an independent career in functional GI and motility disorders research.
Application Deadline: Dec. 14, 2018
AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
This award provides $90,000 per year for three years (total $270,000) to a young investigator, instructor, research associate or equivalent working toward an independent career in inflammatory bowel disease research.
Application Deadline: Dec. 14, 2018
AGA Fellow Abstract Award
This travel award provides nine $500 and one $1,000 prize to recipients who are MD, PhD or equivalent fellows presenting posters/oral sessions at Digestive Disease Week® (DDW).
Application Deadline: Feb. 15, 2019
AGA Moti L. & Kamla Rustgi International Travel Awards
This travel award provides two $750 prizes to recipients who are young basic, translational or clinical investigators residing outside North America to support travel and related expenses to attend Digestive Disease Week® (DDW).
Application Deadline: Feb. 15, 2019
AGA Student Abstract Award
This travel award provides nine $500 and one $1,000 prize to recipients who are high school, undergraduate, graduate, or medical students or residents (residents up to year three postgraduate) presenting posters/oral sessions at Digestive Disease Week® (DDW).
Application Deadline: Feb. 15, 2019
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Dec. 10-11, 12-13, 2018; Jan. 16-17, 22-23, 23-24, 2019; Feb. 20-21, 2019
Two-Day, In-Depth Coding Seminar by McVey Associates, Inc
.Become a certified GI coder with a two-day, in-depth training course provided by McVey Associates, Inc.
Tampa, FL (12/10-11), Dallas, TX (12/12-13), Houston, TX (1/16-17), New Orleans, LA (1/22-23), Pittsburgh, PA (1/23-24), 2/20 (Hartford, CT)
Jan. 17-19, 2019
2019 GI Cancers Symposium
Join colleagues from across the globe in San Francisco to discover and share groundbreaking research in treating gastrointestinal cancers.
San Francisco, CA
Feb. 7–9, 2019
Crohn’s & Colitis Congress™ (A Partnership of the Crohn’s & Colitis Foundation and American Gastroenterological Association)
Expand your knowledge, network with IBD leaders, spark innovative research and get inspired to improve patient care.
Las Vegas, NV
March 8-9, 2019
2019 Women’s Leadership Conference
The conference is specifically designed for women looking to advance their careers, further professional goals, enhance personal growth and effectively network.
Bethesda, MD
March 8-10, 2019
FORWARD Program
AGA’s Fostering Opportunities Resulting in Workforce and Research Diversity (FORWARD) Program is a new initiative funded by NIH, supporting the career entry and development for underrepresented minority physician scientists in gastroenterology. The program provides concrete leadership and skill development that includes scientific manuscript and grant writing, research development, executive coaching and more.
Bethesda, MD
March 8-10, 2019
Future Leaders Program
The Future Leaders Program provides a pathway within the organization to network, connect with mentors, develop leadership skills and learn about AGA’s governance and operations while advancing your career and supporting the profession.
Bethesda, MD
March 23–24, 2019
2019 Gut Microbiota for Health World Summit
The 2019 program will present the latest evidence on the interaction between diet, nutrition and the gut microbiome. Learn how diet and nutrition are being used in concert with traditional therapies to manage GI disorders.
Miami, FL
May 18-21, 2019
Digestive Disease Week (DDW)®
DDW® is the world’s leading educational forum for academicians, clinicians, researchers, students and trainees working in gastroenterology, hepatology, GI endoscopy, gastrointestinal surgery and related fields. Whether you work in patient care, research, education or administration, the DDW program offers something for you. DDW is co-sponsored by AGA, AASLD, ASGE and SSAT.
San Diego, CA
AWARDS APPLICATION DEADLINES
AGA-Rady Children’s Institute for Genomic Medicine Research Scholar Award in Pediatric Genomics
This award provides $90,000 per year for three years (totaling $270,000) to a young investigator, instructor, research associate or equivalent working toward an independent career in gastroenterology, hepatology or related areas. The proposed research may be basic, translational or clinical and must use genomics as an approach to enhance understanding of pediatric digestive diseases toward prevention, treatment and/or cure of such diseases. The funded research must be conducted full-time at the Rady Children’s Institute for Genomic Medicine in San Diego, California, or at Rady Children’s Hospital – San Diego.
Application Deadline: Dec. 14, 2018
AGA-Bern Schwartz Family Fund Research Scholar Award in Pancreatic Cancer
This award provides $100,000 per year for three years (total $300,000) to early career faculty (i.e., investigator, instructor, research associate or equivalent) working toward an independent career in pancreatic cancer research.
Application Deadline: Dec. 14, 2018
AGA Research Scholar Award (RSA)
This award provides $90,000 per year for three years (total $270,000) to a young investigator, instructor, research associate or equivalent working toward an independent career in gastroenterology, hepatology or related areas.
Application Deadline: Dec. 14, 2018
AGA-Shire Research Scholar Award in Functional GI and Motility Disorders
This award provides $90,000 per year for three years (total $270,000) to a young investigator, instructor, research associate or equivalent working toward an independent career in functional GI and motility disorders research.
Application Deadline: Dec. 14, 2018
AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
This award provides $90,000 per year for three years (total $270,000) to a young investigator, instructor, research associate or equivalent working toward an independent career in inflammatory bowel disease research.
Application Deadline: Dec. 14, 2018
AGA Fellow Abstract Award
This travel award provides nine $500 and one $1,000 prize to recipients who are MD, PhD or equivalent fellows presenting posters/oral sessions at Digestive Disease Week® (DDW).
Application Deadline: Feb. 15, 2019
AGA Moti L. & Kamla Rustgi International Travel Awards
This travel award provides two $750 prizes to recipients who are young basic, translational or clinical investigators residing outside North America to support travel and related expenses to attend Digestive Disease Week® (DDW).
Application Deadline: Feb. 15, 2019
AGA Student Abstract Award
This travel award provides nine $500 and one $1,000 prize to recipients who are high school, undergraduate, graduate, or medical students or residents (residents up to year three postgraduate) presenting posters/oral sessions at Digestive Disease Week® (DDW).
Application Deadline: Feb. 15, 2019
SOLAR-1 shines light on PI3K in breast cancer
MUNICH – A combination of the selective phosphoinositide 3-kinase inhibitor alpelisib and fulvestrant (Faslodex) was associated with significantly better progression-free survival than placebo plus fulvestrant in patients with hormone receptor–positive, HER2-negative breast cancer with PIK3CA mutations, investigators in the SOLAR-1 trial reported.
The addition of alpelisib, an inhibitor of the alpha isoform of the phosphoinositide 3-kinase (PI3K), to fulvestrant, a selective estrogen receptor modifier, was associated with a 35% improvement in progression-free survival (PFS), reported Fabrice André, MD, of Institut Gustave Roussy, Villejuif, France.
“Alpelisib plus fulvestrant is a potential new treatment option for patients with PIK3CA-mutant, hormone receptor–positive, HER2-negative advanced breast cancer who have progressed on prior endocrine therapy, with or without a CDK 4/6 [cyclin-dependent kinases 4/6] inhibitor,” he said in a podium presentation at the European Society for Medical Oncology Congress.
Unlike other PI3K inhibitors which have activity spanning the alpha, beta, gamma, and delta isoforms of PI3K, alpelisib is specific for only the alpha isoform, giving it a safety profile that compares favorably with other agents in its class, Dr. André said.
Results of other studies combining PI3K inhibition with endocrine therapy have not borne especially sweet fruit. For example, the SANDPIPER trial, a study of the combination of fulvestrant and the PI3K inhibitor idelalisib (Zydelig) presented at the 2018 annual meeting of the American Society of Clinical Oncology, showed a benefit of 2 months more PFS, compared with fulvestrant alone, but at the cost of serious toxicities in half of all patients treated with the combination.
In the SOLAR-1 trial, investigators randomized 572 postmenopausal women, or men (sex of patients not shown), with hormone receptor–positive, HER2-negative advanced breast cancer. Of the 572 patients, 341 were determined to have PIK3CA mutations.
To be eligible for the study, patients had to have good Eastern Cooperative Oncology Group performance status (1 or less) and have received one or more prior lines of endocrine therapy but no chemotherapy for advanced breast cancer.
Patients who had previously received fulvestrant, any PI3K inhibitor, Akt inhibitor, or mammalian target of rapamycin inhibitor were excluded.
The patients were randomly assigned to receive intramuscular fulvestrant 500 mg on days 1 and 15 of treatment cycle 1 and then every 28 days, plus either oral alpelisib (300 mg daily) or placebo.
Locally assessed PFS in patients with PIK3CA mutations, the primary endpoint, favored alpelisib. At a median follow-up of 20 months, the median PFS with alpelisib was 11 months, versus 5.7 months for placebo. The hazard ratio for progression with alpelisib was 0.65 (P = .00065).
A blinded independent review committee assessment of 50% of patients in the PIK3CA-mutant cohort supported the investigator-assessed findings, with a median PFS of 11.1 months with alpelisib, versus 3.7 months with placebo.
In contrast, a proof-of-concept analysis of the 231 patients without PIK3CA mutations could not show a benefit for alpelisib over placebo.
In the PIK3CA-mutant cohort, the overall response rates among patients with measurable disease were 35.7% with alpelisib versus 16.2% with placebo (P = .0002). Respective response rates among all patients were 26.6% and 12.8% (P = .0006).
Adverse events in the entire trial population included hyperglycemia leading to discontinuation of alpelisib in 6.3% of patients and rash leading to discontinuation of the drug in 3.2% of patients. There were no discontinuations for either hyperglycemia or rash in the placebo arm.
The hyperglycemia can be managed with metformin, Dr. André said.
At a briefing prior to presentation of the SOLAR-1 data in a symposium, briefing discussant Nadia Harbeck, MD, of the University of Munich Medical Center said that the trial represents a significant advance.
“We have numerous talks, also phase 3 trials, with PI3-kinase inhibitors where we saw marginal benefit, and the drugs weren’t usable in clinical practice because the tolerability was so bad. So this is now the first phase 3 data proving that if you have a targeted drug and the tumor has the target, you can actually almost double progression-free survival, and I think this will change the way we practice,” she said.
Rebecca Dent, MD, a senior consultant at the National Cancer Centre Singapore, the invited discussant at the symposium, complimented the SOLAR-1 investigators for conducting a “clinically homogeneous, biomarker-driven clinical trial reflecting new pathways to registration.”
She suggested that the significant clinical toxicities seen with PI3K inhibitors might be ameliorated with different dosing schedules, pharmacodynamic endpoints such as hyperglycemia, or potential prophylaxis.
The trial demonstrated a clinically meaningful absolute PFS benefit, the best in class, but updated data, including overall survival data, are still needed, Dr. Dent said. “We also need to see the quality-of-life data; this is what’s important for our patients. Is this improving progression-free survival, is it improving quality of life, and is it improving overall survival? Only the future will tell.”
SOLAR-1 was sponsored by Novartis. Dr. André disclosed research grants from Novartis and others. Dr. Dent disclosed advisory board, honoraria, or travel support from Novartis and others. Dr. Harbeck disclosed honoraria for consulting and lecturing for Pfizer and others.
SOURCE: Andre F et al. ESMO 2018, Abstract LBA3_PR.
MUNICH – A combination of the selective phosphoinositide 3-kinase inhibitor alpelisib and fulvestrant (Faslodex) was associated with significantly better progression-free survival than placebo plus fulvestrant in patients with hormone receptor–positive, HER2-negative breast cancer with PIK3CA mutations, investigators in the SOLAR-1 trial reported.
The addition of alpelisib, an inhibitor of the alpha isoform of the phosphoinositide 3-kinase (PI3K), to fulvestrant, a selective estrogen receptor modifier, was associated with a 35% improvement in progression-free survival (PFS), reported Fabrice André, MD, of Institut Gustave Roussy, Villejuif, France.
“Alpelisib plus fulvestrant is a potential new treatment option for patients with PIK3CA-mutant, hormone receptor–positive, HER2-negative advanced breast cancer who have progressed on prior endocrine therapy, with or without a CDK 4/6 [cyclin-dependent kinases 4/6] inhibitor,” he said in a podium presentation at the European Society for Medical Oncology Congress.
Unlike other PI3K inhibitors which have activity spanning the alpha, beta, gamma, and delta isoforms of PI3K, alpelisib is specific for only the alpha isoform, giving it a safety profile that compares favorably with other agents in its class, Dr. André said.
Results of other studies combining PI3K inhibition with endocrine therapy have not borne especially sweet fruit. For example, the SANDPIPER trial, a study of the combination of fulvestrant and the PI3K inhibitor idelalisib (Zydelig) presented at the 2018 annual meeting of the American Society of Clinical Oncology, showed a benefit of 2 months more PFS, compared with fulvestrant alone, but at the cost of serious toxicities in half of all patients treated with the combination.
In the SOLAR-1 trial, investigators randomized 572 postmenopausal women, or men (sex of patients not shown), with hormone receptor–positive, HER2-negative advanced breast cancer. Of the 572 patients, 341 were determined to have PIK3CA mutations.
To be eligible for the study, patients had to have good Eastern Cooperative Oncology Group performance status (1 or less) and have received one or more prior lines of endocrine therapy but no chemotherapy for advanced breast cancer.
Patients who had previously received fulvestrant, any PI3K inhibitor, Akt inhibitor, or mammalian target of rapamycin inhibitor were excluded.
The patients were randomly assigned to receive intramuscular fulvestrant 500 mg on days 1 and 15 of treatment cycle 1 and then every 28 days, plus either oral alpelisib (300 mg daily) or placebo.
Locally assessed PFS in patients with PIK3CA mutations, the primary endpoint, favored alpelisib. At a median follow-up of 20 months, the median PFS with alpelisib was 11 months, versus 5.7 months for placebo. The hazard ratio for progression with alpelisib was 0.65 (P = .00065).
A blinded independent review committee assessment of 50% of patients in the PIK3CA-mutant cohort supported the investigator-assessed findings, with a median PFS of 11.1 months with alpelisib, versus 3.7 months with placebo.
In contrast, a proof-of-concept analysis of the 231 patients without PIK3CA mutations could not show a benefit for alpelisib over placebo.
In the PIK3CA-mutant cohort, the overall response rates among patients with measurable disease were 35.7% with alpelisib versus 16.2% with placebo (P = .0002). Respective response rates among all patients were 26.6% and 12.8% (P = .0006).
Adverse events in the entire trial population included hyperglycemia leading to discontinuation of alpelisib in 6.3% of patients and rash leading to discontinuation of the drug in 3.2% of patients. There were no discontinuations for either hyperglycemia or rash in the placebo arm.
The hyperglycemia can be managed with metformin, Dr. André said.
At a briefing prior to presentation of the SOLAR-1 data in a symposium, briefing discussant Nadia Harbeck, MD, of the University of Munich Medical Center said that the trial represents a significant advance.
“We have numerous talks, also phase 3 trials, with PI3-kinase inhibitors where we saw marginal benefit, and the drugs weren’t usable in clinical practice because the tolerability was so bad. So this is now the first phase 3 data proving that if you have a targeted drug and the tumor has the target, you can actually almost double progression-free survival, and I think this will change the way we practice,” she said.
Rebecca Dent, MD, a senior consultant at the National Cancer Centre Singapore, the invited discussant at the symposium, complimented the SOLAR-1 investigators for conducting a “clinically homogeneous, biomarker-driven clinical trial reflecting new pathways to registration.”
She suggested that the significant clinical toxicities seen with PI3K inhibitors might be ameliorated with different dosing schedules, pharmacodynamic endpoints such as hyperglycemia, or potential prophylaxis.
The trial demonstrated a clinically meaningful absolute PFS benefit, the best in class, but updated data, including overall survival data, are still needed, Dr. Dent said. “We also need to see the quality-of-life data; this is what’s important for our patients. Is this improving progression-free survival, is it improving quality of life, and is it improving overall survival? Only the future will tell.”
SOLAR-1 was sponsored by Novartis. Dr. André disclosed research grants from Novartis and others. Dr. Dent disclosed advisory board, honoraria, or travel support from Novartis and others. Dr. Harbeck disclosed honoraria for consulting and lecturing for Pfizer and others.
SOURCE: Andre F et al. ESMO 2018, Abstract LBA3_PR.
MUNICH – A combination of the selective phosphoinositide 3-kinase inhibitor alpelisib and fulvestrant (Faslodex) was associated with significantly better progression-free survival than placebo plus fulvestrant in patients with hormone receptor–positive, HER2-negative breast cancer with PIK3CA mutations, investigators in the SOLAR-1 trial reported.
The addition of alpelisib, an inhibitor of the alpha isoform of the phosphoinositide 3-kinase (PI3K), to fulvestrant, a selective estrogen receptor modifier, was associated with a 35% improvement in progression-free survival (PFS), reported Fabrice André, MD, of Institut Gustave Roussy, Villejuif, France.
“Alpelisib plus fulvestrant is a potential new treatment option for patients with PIK3CA-mutant, hormone receptor–positive, HER2-negative advanced breast cancer who have progressed on prior endocrine therapy, with or without a CDK 4/6 [cyclin-dependent kinases 4/6] inhibitor,” he said in a podium presentation at the European Society for Medical Oncology Congress.
Unlike other PI3K inhibitors which have activity spanning the alpha, beta, gamma, and delta isoforms of PI3K, alpelisib is specific for only the alpha isoform, giving it a safety profile that compares favorably with other agents in its class, Dr. André said.
Results of other studies combining PI3K inhibition with endocrine therapy have not borne especially sweet fruit. For example, the SANDPIPER trial, a study of the combination of fulvestrant and the PI3K inhibitor idelalisib (Zydelig) presented at the 2018 annual meeting of the American Society of Clinical Oncology, showed a benefit of 2 months more PFS, compared with fulvestrant alone, but at the cost of serious toxicities in half of all patients treated with the combination.
In the SOLAR-1 trial, investigators randomized 572 postmenopausal women, or men (sex of patients not shown), with hormone receptor–positive, HER2-negative advanced breast cancer. Of the 572 patients, 341 were determined to have PIK3CA mutations.
To be eligible for the study, patients had to have good Eastern Cooperative Oncology Group performance status (1 or less) and have received one or more prior lines of endocrine therapy but no chemotherapy for advanced breast cancer.
Patients who had previously received fulvestrant, any PI3K inhibitor, Akt inhibitor, or mammalian target of rapamycin inhibitor were excluded.
The patients were randomly assigned to receive intramuscular fulvestrant 500 mg on days 1 and 15 of treatment cycle 1 and then every 28 days, plus either oral alpelisib (300 mg daily) or placebo.
Locally assessed PFS in patients with PIK3CA mutations, the primary endpoint, favored alpelisib. At a median follow-up of 20 months, the median PFS with alpelisib was 11 months, versus 5.7 months for placebo. The hazard ratio for progression with alpelisib was 0.65 (P = .00065).
A blinded independent review committee assessment of 50% of patients in the PIK3CA-mutant cohort supported the investigator-assessed findings, with a median PFS of 11.1 months with alpelisib, versus 3.7 months with placebo.
In contrast, a proof-of-concept analysis of the 231 patients without PIK3CA mutations could not show a benefit for alpelisib over placebo.
In the PIK3CA-mutant cohort, the overall response rates among patients with measurable disease were 35.7% with alpelisib versus 16.2% with placebo (P = .0002). Respective response rates among all patients were 26.6% and 12.8% (P = .0006).
Adverse events in the entire trial population included hyperglycemia leading to discontinuation of alpelisib in 6.3% of patients and rash leading to discontinuation of the drug in 3.2% of patients. There were no discontinuations for either hyperglycemia or rash in the placebo arm.
The hyperglycemia can be managed with metformin, Dr. André said.
At a briefing prior to presentation of the SOLAR-1 data in a symposium, briefing discussant Nadia Harbeck, MD, of the University of Munich Medical Center said that the trial represents a significant advance.
“We have numerous talks, also phase 3 trials, with PI3-kinase inhibitors where we saw marginal benefit, and the drugs weren’t usable in clinical practice because the tolerability was so bad. So this is now the first phase 3 data proving that if you have a targeted drug and the tumor has the target, you can actually almost double progression-free survival, and I think this will change the way we practice,” she said.
Rebecca Dent, MD, a senior consultant at the National Cancer Centre Singapore, the invited discussant at the symposium, complimented the SOLAR-1 investigators for conducting a “clinically homogeneous, biomarker-driven clinical trial reflecting new pathways to registration.”
She suggested that the significant clinical toxicities seen with PI3K inhibitors might be ameliorated with different dosing schedules, pharmacodynamic endpoints such as hyperglycemia, or potential prophylaxis.
The trial demonstrated a clinically meaningful absolute PFS benefit, the best in class, but updated data, including overall survival data, are still needed, Dr. Dent said. “We also need to see the quality-of-life data; this is what’s important for our patients. Is this improving progression-free survival, is it improving quality of life, and is it improving overall survival? Only the future will tell.”
SOLAR-1 was sponsored by Novartis. Dr. André disclosed research grants from Novartis and others. Dr. Dent disclosed advisory board, honoraria, or travel support from Novartis and others. Dr. Harbeck disclosed honoraria for consulting and lecturing for Pfizer and others.
SOURCE: Andre F et al. ESMO 2018, Abstract LBA3_PR.
REPORTING FROM ESMO 2018
Key clinical point: Alpelisib, selective for the alpha isoforms of the phosphatidylinositol 3-kinase, appears to have better tolerability than, and similar efficacy to, pan–phosphoinositide 3-kinase inhibitors.
Major finding: Median progression-free survival was 11 months with alpelisib/fulvestrant in patients with PIK3CA mutations, compared with 5.7 months for placebo/fulvestrant.
Study details: A randomized, phase 3 trial in 572 patients with hormone receptor–positive, HER2-negative advanced breast cancer.
Disclosures: SOLAR-1 was sponsored by Novartis. Dr. André disclosed research grants from Novartis and others; Dr. Dent disclosed advisory board, honoraria, or travel support from Novartis and others; Dr. Harbeck disclosed honoraria for consulting and lecturing for Pfizer and others.
Source: Andre F et al. ESMO 2018, Abstract LBA3_PR.
Quadrivalent flu vaccine okayed for 6 months and up
The expanded approval now includes persons aged 6-59 months; the quadrivalent vaccine had previously been approved for ages 5 years and up. A trivalent version of the Afluria influenza vaccine also now is indicated for people aged 6 months and up, according to an Oct. 4 communication from the FDA.
A total of 172 pediatric influenza-related deaths occurred in the United States during the 2017-2018 season, representing a new high in nonpandemic influenza seasons. About half of the pediatric influenza deaths occurred in otherwise healthy children, and about 22% of children who died were fully vaccinated, according to the Centers for Disease Control and Prevention, reporting U.S. data from 2010 to 2016.
“As we enter a new flu season, we are reminded of the enormous impact that influenza can have on public health,” Seqirus’ vice president of medical affairs Gregg Sylvester, MD, said in a press release announcing the extended indication. “Having another option to fight this disease can translate to saved lives and fewer flu-related hospitalizations this season and going forward.”
According to the CDC, the 2018-2019 influenza vaccine has been updated to provide a better match – and more protection against – viruses circulating in this influenza season. Specifically, says the CDC, the influenza B Victoria lineage and the influenza A(H3N2) components were updated.
In addition to providing protection against these two strains of influenza, trivalent vaccines for the 2018-2019 season are recommended to include protection against H1N1 influenza as well. Quadrivalent vaccines protect against a second influenza B lineage.
Most people will receive a quadrivalent vaccine this year, according to the CDC.
The expanded approval now includes persons aged 6-59 months; the quadrivalent vaccine had previously been approved for ages 5 years and up. A trivalent version of the Afluria influenza vaccine also now is indicated for people aged 6 months and up, according to an Oct. 4 communication from the FDA.
A total of 172 pediatric influenza-related deaths occurred in the United States during the 2017-2018 season, representing a new high in nonpandemic influenza seasons. About half of the pediatric influenza deaths occurred in otherwise healthy children, and about 22% of children who died were fully vaccinated, according to the Centers for Disease Control and Prevention, reporting U.S. data from 2010 to 2016.
“As we enter a new flu season, we are reminded of the enormous impact that influenza can have on public health,” Seqirus’ vice president of medical affairs Gregg Sylvester, MD, said in a press release announcing the extended indication. “Having another option to fight this disease can translate to saved lives and fewer flu-related hospitalizations this season and going forward.”
According to the CDC, the 2018-2019 influenza vaccine has been updated to provide a better match – and more protection against – viruses circulating in this influenza season. Specifically, says the CDC, the influenza B Victoria lineage and the influenza A(H3N2) components were updated.
In addition to providing protection against these two strains of influenza, trivalent vaccines for the 2018-2019 season are recommended to include protection against H1N1 influenza as well. Quadrivalent vaccines protect against a second influenza B lineage.
Most people will receive a quadrivalent vaccine this year, according to the CDC.
The expanded approval now includes persons aged 6-59 months; the quadrivalent vaccine had previously been approved for ages 5 years and up. A trivalent version of the Afluria influenza vaccine also now is indicated for people aged 6 months and up, according to an Oct. 4 communication from the FDA.
A total of 172 pediatric influenza-related deaths occurred in the United States during the 2017-2018 season, representing a new high in nonpandemic influenza seasons. About half of the pediatric influenza deaths occurred in otherwise healthy children, and about 22% of children who died were fully vaccinated, according to the Centers for Disease Control and Prevention, reporting U.S. data from 2010 to 2016.
“As we enter a new flu season, we are reminded of the enormous impact that influenza can have on public health,” Seqirus’ vice president of medical affairs Gregg Sylvester, MD, said in a press release announcing the extended indication. “Having another option to fight this disease can translate to saved lives and fewer flu-related hospitalizations this season and going forward.”
According to the CDC, the 2018-2019 influenza vaccine has been updated to provide a better match – and more protection against – viruses circulating in this influenza season. Specifically, says the CDC, the influenza B Victoria lineage and the influenza A(H3N2) components were updated.
In addition to providing protection against these two strains of influenza, trivalent vaccines for the 2018-2019 season are recommended to include protection against H1N1 influenza as well. Quadrivalent vaccines protect against a second influenza B lineage.
Most people will receive a quadrivalent vaccine this year, according to the CDC.
VTE risk may be high throughout disease course in uterine serous carcinoma
Uterine serous carcinoma patients may have a high risk of venous thromboembolism (VTE), not just in the postoperative period, but throughout the natural history of the disease, results of a retrospective analysis suggest.
Most patients developed VTE either before staging surgery or more than 6 months postoperatively, according to results of the analysis reported in Obstetrics & Gynecology.
Nearly one-third (31%) of the women developed VTE while receiving chemotherapy, reported Gregory M. Gressel, MD, a gynecologic oncology fellow at Montefiore Medical Center, New York, and his coinvestigators.
The risk was highest in women with cardiovascular disease, hypertension, and stage III and IV disease, they said.
“Although this is a retrospective study, it generates the hypothesis that venous thromboembolism prophylaxis may be beneficial in women with active uterine serous carcinoma, at least while receiving treatment such as neoadjuvant or adjuvant chemotherapy,” Dr. Gressel and his coauthors noted.
Historically, clinical practice guidelines have focused on risk stratification in the perioperative period due to the strong association between cancer-related VTE and surgery, the authors wrote.
To better assess the timing and risk factors associated with clot development, Dr. Gressel and his colleagues abstracted clinical data from the medical records of 413 patients with uterine serous carcinoma between 1999 and 2016 at one center in New York, about half of whom identified as black and one-quarter as Hispanic.
Eighty-four percent of the patients were diagnosed with VTE before or after the 6-week postoperative window when thromboprophylaxis typically is recommended, and 31% developed clots during chemotherapy, the investigators reported. The median time to clot development was 7.2 months after diagnosis, and, after excluding patients who developed clots preoperatively or during chemotherapy, the investigators found the median time from surgery to VTE was 13.2 months.
Patients with stage III and IV disease were, respectively, 2.6 and 4 times more likely to develop thrombosis, compared with patients with stage I disease. Conversely, age, body mass index, and race were not associated with VTE diagnosis.
Patients who developed VTE on chemotherapy had a median Khorana score of 1, which corresponds to an intermediate risk of VTE, the investigators said, adding that pharmacologic prophylaxis is recommended only in patients with scores of 3 or higher.
“Ours is not the first report to posit that currently available venous thromboembolism risk stratification tools are of limited utility in gynecologic oncology patients,” said Dr. Gressel and his coauthors.
However, larger prospective studies are needed, not only to look at the utility of Khorana scoring in this high-risk histologic subtype, they said, but also to test their hypothesis that VTE prophylaxis may be beneficial during chemotherapy or other active treatment.
Dr. Gressel and his colleagues reported no conflicts of interest. The study was supported by the National Institutes of Health and a grant from the National Center for Advancing Translational Science.
SOURCE: Gressel GM et al. Obstet Gynecol. 2018 Oct 5;132:1130-6.
It has been reported in previous literature that the risk of developing VTE is typically within 48 hours of surgical staging, and guidelines have focused on risk factors to identify patients needing extended thromboprophylaxis after surgery. Therefore, Gressel et al. set forth to identify risk factors and timing of development of VTE in women with uterine serous carcinoma.
The study retrospectively evaluated 413 women with uterine serous cancer from 1999 to 2016. Of these, 70 women (about 17%) were diagnosed with a VTE. There was not a statistically significant association between age, body mass index, race, or surgical approach with risk of VTE. However, stage and/or having two or more medical comorbidities were significant. Compared with stage I patients, stage III and IV patients had a 2.6- and fourfold increase in risk, respectively. Not surprisingly, hypertension and cardiovascular disease (CVD) were independently associated with risk of VTE development; odds ratio 2.97 and 1.87, respectively. Interestingly, in the women diagnosed with VTE, 84% were outside the 6-week postoperative period and 31% occurred during chemotherapy treatment. The median time to develop VTE was 13.2 months and occurred sooner in women with advanced stage.
In this study, the majority of patients developed a VTE outside of the standard 28-day thromboprophylaxis window. This finding suggests that there is a subset of patients who would benefit from longer duration of thromboprophylaxis. Perhaps patients with hypertension, CVD, and/or advanced stage malignancy would benefit from thromboprophylaxis throughout their treatment.
Based on the data presented, at this time, we cannot recommend treating patients with the above risk factors during the entirety of their care. Although further studies and even a nomogram may help determine who is likely to benefit from prolonged prophylaxis, it is unknown if doing so would result in significant decreased morbidity or increased adverse events (e.g. bleeding, thrombocytopenia). However, this should remain a topic for further evaluation and providers should remain vigilant for prevention and diagnosis of VTE in at-risk patients by adhering to validated risk assessment tools and clinical evaluation.
Antonio Castaneda, MD, is a gynecologic oncology fellow at The Ohio State University in Columbus. He said he had no relevant financial disclosures.
It has been reported in previous literature that the risk of developing VTE is typically within 48 hours of surgical staging, and guidelines have focused on risk factors to identify patients needing extended thromboprophylaxis after surgery. Therefore, Gressel et al. set forth to identify risk factors and timing of development of VTE in women with uterine serous carcinoma.
The study retrospectively evaluated 413 women with uterine serous cancer from 1999 to 2016. Of these, 70 women (about 17%) were diagnosed with a VTE. There was not a statistically significant association between age, body mass index, race, or surgical approach with risk of VTE. However, stage and/or having two or more medical comorbidities were significant. Compared with stage I patients, stage III and IV patients had a 2.6- and fourfold increase in risk, respectively. Not surprisingly, hypertension and cardiovascular disease (CVD) were independently associated with risk of VTE development; odds ratio 2.97 and 1.87, respectively. Interestingly, in the women diagnosed with VTE, 84% were outside the 6-week postoperative period and 31% occurred during chemotherapy treatment. The median time to develop VTE was 13.2 months and occurred sooner in women with advanced stage.
In this study, the majority of patients developed a VTE outside of the standard 28-day thromboprophylaxis window. This finding suggests that there is a subset of patients who would benefit from longer duration of thromboprophylaxis. Perhaps patients with hypertension, CVD, and/or advanced stage malignancy would benefit from thromboprophylaxis throughout their treatment.
Based on the data presented, at this time, we cannot recommend treating patients with the above risk factors during the entirety of their care. Although further studies and even a nomogram may help determine who is likely to benefit from prolonged prophylaxis, it is unknown if doing so would result in significant decreased morbidity or increased adverse events (e.g. bleeding, thrombocytopenia). However, this should remain a topic for further evaluation and providers should remain vigilant for prevention and diagnosis of VTE in at-risk patients by adhering to validated risk assessment tools and clinical evaluation.
Antonio Castaneda, MD, is a gynecologic oncology fellow at The Ohio State University in Columbus. He said he had no relevant financial disclosures.
It has been reported in previous literature that the risk of developing VTE is typically within 48 hours of surgical staging, and guidelines have focused on risk factors to identify patients needing extended thromboprophylaxis after surgery. Therefore, Gressel et al. set forth to identify risk factors and timing of development of VTE in women with uterine serous carcinoma.
The study retrospectively evaluated 413 women with uterine serous cancer from 1999 to 2016. Of these, 70 women (about 17%) were diagnosed with a VTE. There was not a statistically significant association between age, body mass index, race, or surgical approach with risk of VTE. However, stage and/or having two or more medical comorbidities were significant. Compared with stage I patients, stage III and IV patients had a 2.6- and fourfold increase in risk, respectively. Not surprisingly, hypertension and cardiovascular disease (CVD) were independently associated with risk of VTE development; odds ratio 2.97 and 1.87, respectively. Interestingly, in the women diagnosed with VTE, 84% were outside the 6-week postoperative period and 31% occurred during chemotherapy treatment. The median time to develop VTE was 13.2 months and occurred sooner in women with advanced stage.
In this study, the majority of patients developed a VTE outside of the standard 28-day thromboprophylaxis window. This finding suggests that there is a subset of patients who would benefit from longer duration of thromboprophylaxis. Perhaps patients with hypertension, CVD, and/or advanced stage malignancy would benefit from thromboprophylaxis throughout their treatment.
Based on the data presented, at this time, we cannot recommend treating patients with the above risk factors during the entirety of their care. Although further studies and even a nomogram may help determine who is likely to benefit from prolonged prophylaxis, it is unknown if doing so would result in significant decreased morbidity or increased adverse events (e.g. bleeding, thrombocytopenia). However, this should remain a topic for further evaluation and providers should remain vigilant for prevention and diagnosis of VTE in at-risk patients by adhering to validated risk assessment tools and clinical evaluation.
Antonio Castaneda, MD, is a gynecologic oncology fellow at The Ohio State University in Columbus. He said he had no relevant financial disclosures.
Uterine serous carcinoma patients may have a high risk of venous thromboembolism (VTE), not just in the postoperative period, but throughout the natural history of the disease, results of a retrospective analysis suggest.
Most patients developed VTE either before staging surgery or more than 6 months postoperatively, according to results of the analysis reported in Obstetrics & Gynecology.
Nearly one-third (31%) of the women developed VTE while receiving chemotherapy, reported Gregory M. Gressel, MD, a gynecologic oncology fellow at Montefiore Medical Center, New York, and his coinvestigators.
The risk was highest in women with cardiovascular disease, hypertension, and stage III and IV disease, they said.
“Although this is a retrospective study, it generates the hypothesis that venous thromboembolism prophylaxis may be beneficial in women with active uterine serous carcinoma, at least while receiving treatment such as neoadjuvant or adjuvant chemotherapy,” Dr. Gressel and his coauthors noted.
Historically, clinical practice guidelines have focused on risk stratification in the perioperative period due to the strong association between cancer-related VTE and surgery, the authors wrote.
To better assess the timing and risk factors associated with clot development, Dr. Gressel and his colleagues abstracted clinical data from the medical records of 413 patients with uterine serous carcinoma between 1999 and 2016 at one center in New York, about half of whom identified as black and one-quarter as Hispanic.
Eighty-four percent of the patients were diagnosed with VTE before or after the 6-week postoperative window when thromboprophylaxis typically is recommended, and 31% developed clots during chemotherapy, the investigators reported. The median time to clot development was 7.2 months after diagnosis, and, after excluding patients who developed clots preoperatively or during chemotherapy, the investigators found the median time from surgery to VTE was 13.2 months.
Patients with stage III and IV disease were, respectively, 2.6 and 4 times more likely to develop thrombosis, compared with patients with stage I disease. Conversely, age, body mass index, and race were not associated with VTE diagnosis.
Patients who developed VTE on chemotherapy had a median Khorana score of 1, which corresponds to an intermediate risk of VTE, the investigators said, adding that pharmacologic prophylaxis is recommended only in patients with scores of 3 or higher.
“Ours is not the first report to posit that currently available venous thromboembolism risk stratification tools are of limited utility in gynecologic oncology patients,” said Dr. Gressel and his coauthors.
However, larger prospective studies are needed, not only to look at the utility of Khorana scoring in this high-risk histologic subtype, they said, but also to test their hypothesis that VTE prophylaxis may be beneficial during chemotherapy or other active treatment.
Dr. Gressel and his colleagues reported no conflicts of interest. The study was supported by the National Institutes of Health and a grant from the National Center for Advancing Translational Science.
SOURCE: Gressel GM et al. Obstet Gynecol. 2018 Oct 5;132:1130-6.
Uterine serous carcinoma patients may have a high risk of venous thromboembolism (VTE), not just in the postoperative period, but throughout the natural history of the disease, results of a retrospective analysis suggest.
Most patients developed VTE either before staging surgery or more than 6 months postoperatively, according to results of the analysis reported in Obstetrics & Gynecology.
Nearly one-third (31%) of the women developed VTE while receiving chemotherapy, reported Gregory M. Gressel, MD, a gynecologic oncology fellow at Montefiore Medical Center, New York, and his coinvestigators.
The risk was highest in women with cardiovascular disease, hypertension, and stage III and IV disease, they said.
“Although this is a retrospective study, it generates the hypothesis that venous thromboembolism prophylaxis may be beneficial in women with active uterine serous carcinoma, at least while receiving treatment such as neoadjuvant or adjuvant chemotherapy,” Dr. Gressel and his coauthors noted.
Historically, clinical practice guidelines have focused on risk stratification in the perioperative period due to the strong association between cancer-related VTE and surgery, the authors wrote.
To better assess the timing and risk factors associated with clot development, Dr. Gressel and his colleagues abstracted clinical data from the medical records of 413 patients with uterine serous carcinoma between 1999 and 2016 at one center in New York, about half of whom identified as black and one-quarter as Hispanic.
Eighty-four percent of the patients were diagnosed with VTE before or after the 6-week postoperative window when thromboprophylaxis typically is recommended, and 31% developed clots during chemotherapy, the investigators reported. The median time to clot development was 7.2 months after diagnosis, and, after excluding patients who developed clots preoperatively or during chemotherapy, the investigators found the median time from surgery to VTE was 13.2 months.
Patients with stage III and IV disease were, respectively, 2.6 and 4 times more likely to develop thrombosis, compared with patients with stage I disease. Conversely, age, body mass index, and race were not associated with VTE diagnosis.
Patients who developed VTE on chemotherapy had a median Khorana score of 1, which corresponds to an intermediate risk of VTE, the investigators said, adding that pharmacologic prophylaxis is recommended only in patients with scores of 3 or higher.
“Ours is not the first report to posit that currently available venous thromboembolism risk stratification tools are of limited utility in gynecologic oncology patients,” said Dr. Gressel and his coauthors.
However, larger prospective studies are needed, not only to look at the utility of Khorana scoring in this high-risk histologic subtype, they said, but also to test their hypothesis that VTE prophylaxis may be beneficial during chemotherapy or other active treatment.
Dr. Gressel and his colleagues reported no conflicts of interest. The study was supported by the National Institutes of Health and a grant from the National Center for Advancing Translational Science.
SOURCE: Gressel GM et al. Obstet Gynecol. 2018 Oct 5;132:1130-6.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point:
Major finding: Eighty-four percent of VTEs were diagnosed before or after the 6-week postoperative window, and 31% developed during chemotherapy.
Study details: Retrospective study of 431 women diagnosed with uterine serous carcinoma between 1999 and 2016 at one center in New York.
Disclosures: Dr. Gressel and his coauthors reported no conflicts of interest. The study was supported by the National Institutes of Health and a grant from the National Center for Advancing Translational Science.
Source: Gressel GM et al. Obstet Gynecol. 2018 Oct 5;132:1130-6.
Drug-free inactive disease a feasible goal in JIA
Recent recommendations from an international task force advocating that have been backed up by research that finds the goal is a feasible one.
Published online in the Annals of the Rheumatic Diseases, the investigator-initiated, multicenter, randomized BEST for Kids study found that, regardless of initial specific treatments, after 24 months of treatment to target aimed at drug-free inactive disease, 71% of recent-onset patients with JIA had inactive disease (at a median onset of 9 months), and 39% were drug free.
The investigators, led by Petra Hissink Muller, MD, department of pediatrics, Leiden (the Netherlands) University Medical Center said previous studies of JIA supported the “window of opportunity” hypothesis in which disease optimally responds to treatment.
“In JIA, continuous treatment-to-target therapy in a tight control setting, with treatment adjustments based on frequent evaluations of disease activity, has not yet been studied. Recent recommendations agree that treatment to target should be implemented in daily practice,” they wrote.
The aim of the BEST for Kids study was to investigate which of three frequently used treatment-to-target strategies was most effective and safe.
Overall, 94 children (67% girls) with a median age of 9.1 years with new-onset (oligoarticular, juvenile psoriatic arthritis, or rheumatoid factor–negative polyarticular) JIA, without previous disease-modifying antirheumatic drug (DMARD) therapy and symptom duration of less than 18 months were enrolled in the study.
Median symptom duration was 7.5 months and median duration between diagnosis and inclusion in the study was 6 weeks. The investigators noted that two patients left the study during follow-up because of a revised diagnosis.
All study participants were randomized into three strategy arms:
- Initial treatment with conventional synthetic DMARD monotherapy (methotrexate [MTX] or sulfasalazine if preferred by treating physician), n = 32.
- Initial treatment with MTX and 6 weeks of tapered prednisolone (‘bridging therapy), n = 32).
- Initial treatment with MTX and etanercept, n = 30.
In case of inactive disease for at least 3 (oligoarticular disease) or 6 (polyarticular disease) consecutive months, DMARDs were tapered and stopped in all three arms. For combination therapy, etanercept was tapered to once every 2 weeks, followed by a 50% dose reduction, then halted. The dose of MTX or sulfasalazine was reduced by 25% per week to zero.
In case of a disease flare, the last discontinued drug and/or the last effective dose was reintroduced. The protocol did not allow prednisolone to be restarted, whereas etanercept could be restarted but not discontinued for a second time.
The primary outcome of the trial was time to inactive disease and time to flare (defined as the time between first moment of drug-free inactive disease (DFID) and the first arthritis flare as judged by the treating physician) after tapering and stopping all DMARD therapy. Secondary outcomes were adapted ACR Pediatric (ACR Pedi) 30/50/70/90 scores, functional ability, and adverse events.
Results showed that after 24 months inactive disease was achieved by more than 70% of the patients (71%, arm 1; 70%, arm 2; and 72%, arm 3).
Furthermore, drug-free inactive disease (DFID) was achieved by 59% of the cohort (54 of 92 patients; 45% of arm 1, 31% of arm 2, and 41% of arm 3), although the authors noted that early flares did occur in 14 patients and were successfully retreated, resulting in 39% of the patients in DFID at the 2-year study endpoint.
In the treatment of JIA,“we showed that tapering and discontinuation of treatment is a realistic goal. On the other hand, treatment to target resulted in a relatively high use of [biologic] DMARDs, greater than 50% of patients in all arms,” the investigators wrote.
Median time to inactive disease was 9 months in all arms (P = .30), and time to flare was also not significantly different among the groups (overall 3 months [3.0-6.8]; P = .7). The investigators noted that while overall flare rates (26%) were lower than the 37%-60% mentioned in previous cohorts, this finding could be a result of the limited follow-up time.
After 3 months of treatment, more patients who started with MTX and etanercept (arm 3) had achieved rapid improvement as determined by ACR Pedi 70 scores. However, the investigators noted that, because of treatment adjustments in cases of active disease, which were needed more often in arms 1 and 2 than in arm 3, ACR Pedi improvement scores were met in similar percentages of patients over time across the arms.
Adverse events were similar across treatment arms and were generally mild, involving mostly gastrointestinal complaints, upper respiratory tract and other infections, and general malaise.
Overall, the investigators concluded that DFID was a “feasible goal” in the treatment of children with JIA.
Limitations of the study included breaches of treatment protocol by physicians in patients across the three arms.“The physicians did not follow protocol for various reasons, mainly reluctance to intensify therapy based on shared decision making,” they wrote.
Another limitation was the small sample size, which the investigators said could obscure differences between groups that in a larger population might have become clear.
“Long-term follow-up of the BEST for Kids cohort, including radiology results, is initiated to investigate possible lasting positive results of treatment to target in JIA,” they said.
SOURCE: Hissink Muller P et al. Ann Rheum Dis. Oct 12. doi: 10.1136/annrheumdis-2018-213902.
Recent recommendations from an international task force advocating that have been backed up by research that finds the goal is a feasible one.
Published online in the Annals of the Rheumatic Diseases, the investigator-initiated, multicenter, randomized BEST for Kids study found that, regardless of initial specific treatments, after 24 months of treatment to target aimed at drug-free inactive disease, 71% of recent-onset patients with JIA had inactive disease (at a median onset of 9 months), and 39% were drug free.
The investigators, led by Petra Hissink Muller, MD, department of pediatrics, Leiden (the Netherlands) University Medical Center said previous studies of JIA supported the “window of opportunity” hypothesis in which disease optimally responds to treatment.
“In JIA, continuous treatment-to-target therapy in a tight control setting, with treatment adjustments based on frequent evaluations of disease activity, has not yet been studied. Recent recommendations agree that treatment to target should be implemented in daily practice,” they wrote.
The aim of the BEST for Kids study was to investigate which of three frequently used treatment-to-target strategies was most effective and safe.
Overall, 94 children (67% girls) with a median age of 9.1 years with new-onset (oligoarticular, juvenile psoriatic arthritis, or rheumatoid factor–negative polyarticular) JIA, without previous disease-modifying antirheumatic drug (DMARD) therapy and symptom duration of less than 18 months were enrolled in the study.
Median symptom duration was 7.5 months and median duration between diagnosis and inclusion in the study was 6 weeks. The investigators noted that two patients left the study during follow-up because of a revised diagnosis.
All study participants were randomized into three strategy arms:
- Initial treatment with conventional synthetic DMARD monotherapy (methotrexate [MTX] or sulfasalazine if preferred by treating physician), n = 32.
- Initial treatment with MTX and 6 weeks of tapered prednisolone (‘bridging therapy), n = 32).
- Initial treatment with MTX and etanercept, n = 30.
In case of inactive disease for at least 3 (oligoarticular disease) or 6 (polyarticular disease) consecutive months, DMARDs were tapered and stopped in all three arms. For combination therapy, etanercept was tapered to once every 2 weeks, followed by a 50% dose reduction, then halted. The dose of MTX or sulfasalazine was reduced by 25% per week to zero.
In case of a disease flare, the last discontinued drug and/or the last effective dose was reintroduced. The protocol did not allow prednisolone to be restarted, whereas etanercept could be restarted but not discontinued for a second time.
The primary outcome of the trial was time to inactive disease and time to flare (defined as the time between first moment of drug-free inactive disease (DFID) and the first arthritis flare as judged by the treating physician) after tapering and stopping all DMARD therapy. Secondary outcomes were adapted ACR Pediatric (ACR Pedi) 30/50/70/90 scores, functional ability, and adverse events.
Results showed that after 24 months inactive disease was achieved by more than 70% of the patients (71%, arm 1; 70%, arm 2; and 72%, arm 3).
Furthermore, drug-free inactive disease (DFID) was achieved by 59% of the cohort (54 of 92 patients; 45% of arm 1, 31% of arm 2, and 41% of arm 3), although the authors noted that early flares did occur in 14 patients and were successfully retreated, resulting in 39% of the patients in DFID at the 2-year study endpoint.
In the treatment of JIA,“we showed that tapering and discontinuation of treatment is a realistic goal. On the other hand, treatment to target resulted in a relatively high use of [biologic] DMARDs, greater than 50% of patients in all arms,” the investigators wrote.
Median time to inactive disease was 9 months in all arms (P = .30), and time to flare was also not significantly different among the groups (overall 3 months [3.0-6.8]; P = .7). The investigators noted that while overall flare rates (26%) were lower than the 37%-60% mentioned in previous cohorts, this finding could be a result of the limited follow-up time.
After 3 months of treatment, more patients who started with MTX and etanercept (arm 3) had achieved rapid improvement as determined by ACR Pedi 70 scores. However, the investigators noted that, because of treatment adjustments in cases of active disease, which were needed more often in arms 1 and 2 than in arm 3, ACR Pedi improvement scores were met in similar percentages of patients over time across the arms.
Adverse events were similar across treatment arms and were generally mild, involving mostly gastrointestinal complaints, upper respiratory tract and other infections, and general malaise.
Overall, the investigators concluded that DFID was a “feasible goal” in the treatment of children with JIA.
Limitations of the study included breaches of treatment protocol by physicians in patients across the three arms.“The physicians did not follow protocol for various reasons, mainly reluctance to intensify therapy based on shared decision making,” they wrote.
Another limitation was the small sample size, which the investigators said could obscure differences between groups that in a larger population might have become clear.
“Long-term follow-up of the BEST for Kids cohort, including radiology results, is initiated to investigate possible lasting positive results of treatment to target in JIA,” they said.
SOURCE: Hissink Muller P et al. Ann Rheum Dis. Oct 12. doi: 10.1136/annrheumdis-2018-213902.
Recent recommendations from an international task force advocating that have been backed up by research that finds the goal is a feasible one.
Published online in the Annals of the Rheumatic Diseases, the investigator-initiated, multicenter, randomized BEST for Kids study found that, regardless of initial specific treatments, after 24 months of treatment to target aimed at drug-free inactive disease, 71% of recent-onset patients with JIA had inactive disease (at a median onset of 9 months), and 39% were drug free.
The investigators, led by Petra Hissink Muller, MD, department of pediatrics, Leiden (the Netherlands) University Medical Center said previous studies of JIA supported the “window of opportunity” hypothesis in which disease optimally responds to treatment.
“In JIA, continuous treatment-to-target therapy in a tight control setting, with treatment adjustments based on frequent evaluations of disease activity, has not yet been studied. Recent recommendations agree that treatment to target should be implemented in daily practice,” they wrote.
The aim of the BEST for Kids study was to investigate which of three frequently used treatment-to-target strategies was most effective and safe.
Overall, 94 children (67% girls) with a median age of 9.1 years with new-onset (oligoarticular, juvenile psoriatic arthritis, or rheumatoid factor–negative polyarticular) JIA, without previous disease-modifying antirheumatic drug (DMARD) therapy and symptom duration of less than 18 months were enrolled in the study.
Median symptom duration was 7.5 months and median duration between diagnosis and inclusion in the study was 6 weeks. The investigators noted that two patients left the study during follow-up because of a revised diagnosis.
All study participants were randomized into three strategy arms:
- Initial treatment with conventional synthetic DMARD monotherapy (methotrexate [MTX] or sulfasalazine if preferred by treating physician), n = 32.
- Initial treatment with MTX and 6 weeks of tapered prednisolone (‘bridging therapy), n = 32).
- Initial treatment with MTX and etanercept, n = 30.
In case of inactive disease for at least 3 (oligoarticular disease) or 6 (polyarticular disease) consecutive months, DMARDs were tapered and stopped in all three arms. For combination therapy, etanercept was tapered to once every 2 weeks, followed by a 50% dose reduction, then halted. The dose of MTX or sulfasalazine was reduced by 25% per week to zero.
In case of a disease flare, the last discontinued drug and/or the last effective dose was reintroduced. The protocol did not allow prednisolone to be restarted, whereas etanercept could be restarted but not discontinued for a second time.
The primary outcome of the trial was time to inactive disease and time to flare (defined as the time between first moment of drug-free inactive disease (DFID) and the first arthritis flare as judged by the treating physician) after tapering and stopping all DMARD therapy. Secondary outcomes were adapted ACR Pediatric (ACR Pedi) 30/50/70/90 scores, functional ability, and adverse events.
Results showed that after 24 months inactive disease was achieved by more than 70% of the patients (71%, arm 1; 70%, arm 2; and 72%, arm 3).
Furthermore, drug-free inactive disease (DFID) was achieved by 59% of the cohort (54 of 92 patients; 45% of arm 1, 31% of arm 2, and 41% of arm 3), although the authors noted that early flares did occur in 14 patients and were successfully retreated, resulting in 39% of the patients in DFID at the 2-year study endpoint.
In the treatment of JIA,“we showed that tapering and discontinuation of treatment is a realistic goal. On the other hand, treatment to target resulted in a relatively high use of [biologic] DMARDs, greater than 50% of patients in all arms,” the investigators wrote.
Median time to inactive disease was 9 months in all arms (P = .30), and time to flare was also not significantly different among the groups (overall 3 months [3.0-6.8]; P = .7). The investigators noted that while overall flare rates (26%) were lower than the 37%-60% mentioned in previous cohorts, this finding could be a result of the limited follow-up time.
After 3 months of treatment, more patients who started with MTX and etanercept (arm 3) had achieved rapid improvement as determined by ACR Pedi 70 scores. However, the investigators noted that, because of treatment adjustments in cases of active disease, which were needed more often in arms 1 and 2 than in arm 3, ACR Pedi improvement scores were met in similar percentages of patients over time across the arms.
Adverse events were similar across treatment arms and were generally mild, involving mostly gastrointestinal complaints, upper respiratory tract and other infections, and general malaise.
Overall, the investigators concluded that DFID was a “feasible goal” in the treatment of children with JIA.
Limitations of the study included breaches of treatment protocol by physicians in patients across the three arms.“The physicians did not follow protocol for various reasons, mainly reluctance to intensify therapy based on shared decision making,” they wrote.
Another limitation was the small sample size, which the investigators said could obscure differences between groups that in a larger population might have become clear.
“Long-term follow-up of the BEST for Kids cohort, including radiology results, is initiated to investigate possible lasting positive results of treatment to target in JIA,” they said.
SOURCE: Hissink Muller P et al. Ann Rheum Dis. Oct 12. doi: 10.1136/annrheumdis-2018-213902.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Drug-free inactive disease is a feasible goal in the treatment of children with JIA.
Major finding: After 24 months of treatment to target aimed at achieving drug-free inactive disease, 71% of recent-onset patients with JIA had inactive disease (median onset 9 months), and 39% were drug free.
Study details: A randomized, single-blinded study that assessed three treatment strategies in 94 patients with JIA who were DMARD naive.
Disclosures: The study was investigator initiated but received financial support from Pfizer. No competing interests were declared by the authors.
Source: Hissink Muller P et al. Ann Rheum Dis. 2018 Oct 12. doi: 10.1136/annrheumdis-2018-213902.












