User login
Abstinence by moderate drinkers improves their AFib
NEW ORLEANS – Abstinence from alcohol on the part of moderate drinkers with atrial fibrillation resulted in clinically meaningful improvement in their arrhythmia in the randomized controlled Alcohol-AF trial, Aleksandr Voskoboinik, MBBS, reported at the annual meeting of the American College of Cardiology.
The benefits of abstinence included significant reductions in the AFib recurrence rate, total AFib burden, and symptom severity. And the payoff extended beyond the arrhythmia: The abstinent group also averaged a 12–mm Hg drop in systolic blood pressure and dropped 3 kg of body weight over the course of the 6-month trial, added Dr. Voskoboinik, a cardiologist at the Baker Heart & Diabetes Institute at the University of Melbourne.
“We would conclude that significant reduction in alcohol intake should be considered as part of the lifestyle intervention in moderate drinkers with AFib,” he said.
Some physicians already advise alcohol abstinence for moderate drinkers with AFib, but until now such recommendations were not evidence based. Instead, the guidance was based on extrapolation from the well-established harmful effects of heavy drinking and binge drinking on AFib as well as epidemiologic studies.
“We wanted to provide some evidence base for physicians to say, ‘If I have motivated patients, here’s what’s potentially achievable,’” he explained.
The key word here is “motivated.” Conducting the Alcohol-AF trial made clear that many moderate drinkers with AFib enjoy their beverages more than they hate having their arrhythmia. Indeed, out of 697 moderate-drinking patients with paroxysmal or persistent AFib who were deemed good candidates and were approached about study participation, more than 500 were excluded because they were flat out unwilling to consider abstinence. After Dr. Voskoboinik and his coinvestigators shortened the planned trial duration from 12 months to 6 because so many recruits were unwilling to abstain for a full year, 140 of the initial 697 patients were randomized to abstinence or continuation of their usual consumption. Thirty percent of them had previously undergone AFib ablation.
The Alcohol-AF study was a multicenter, prospective, open-label study conducted at six Australian hospitals. All subjects underwent comprehensive rhythm monitoring via an implantable loop recorder or an existing pacemaker. Twice daily they triggered the AliveCor mobile phone app together with Holter monitoring. Patients assigned to abstinence were counseled to abstain completely, got written and verbal advice to help with compliance, and had contact with the investigators on a monthly basis. They also underwent urine testing to monitor compliance. All study participants kept a weekly alcohol intake diary, reviewed monthly by investigators.
Participants averaged 17 standard drinks per week at enrollment. Two-thirds were primarily wine drinkers. At the 6-month mark, patients in the abstinence arm averaged two drinks per week, for an 88% reduction from baseline, and 43 of the 70, or 61%, had remained completely abstinent. The control arm averaged a 20% reduction in weekly consumption.
One of the two co-primary endpoints was recurrent AFib episodes lasting for at least 30 seconds. The rate was 53% in the abstinence group and 73% in controls. The time to first recurrence averaged 118 days in the abstinence group, compared to 86 days in controls, for a 37% prolongation through abstinence.
The other primary endpoint was total AFib burden. During the 6-month study, the abstinence group spent a mean of 5.6% of their time in AFib, compared to 8.2% in controls. The median times were 0.5% and 1.2%.
In a multivariate analysis, neither AFib type, duration, history of AFib ablation, age, nor gender predicted AFib recurrence. In fact, the only significant predictor was alcohol abstinence, which conferred a 48% reduction in risk.
Symptom severity, a secondary endpoint, showed an impressive between-group difference at follow-up. Ten percent of patients in the abstinence group had moderate or severe symptoms at follow-up as assessed via the European Heart Rhythm Association score of atrial fibrillation, compared to 32% of controls, for an absolute 22% difference. And 90% of the abstinence group had no or mild symptoms, as did 68% of controls. Nine percent of the abstinence group had an AFib-related hospitalization, as did 20% of controls.
Cardiac MRI, performed in all subjects, showed that the abstinence group experienced a significant reduction in left atrial area, from 29.5 cm2 at baseline to 27.1 cm2 at follow-up. They also experienced significant improvement in left atrial mechanical function, with their left atrial emptying fraction climbing from 42% to 50%.
The 3-kg weight loss from a baseline of 90 kg in the abstinent group represented a 0.7 kg/m2 reduction in body mass index.
In terms of the likely mechanism of benefit of alcohol abstinence in moderate drinkers, Dr. Voskoboinik noted that he and his colleagues recently demonstrated that regular moderate alcohol consumption, but not mild intake, is associated with potentially explanatory atrial electrical and structural changes, including conduction slowing, lower global bipolar atrial voltage, and an increase in complex atrial potentials (Heart Rhythm. 2019 Feb;16[2]:251-9).
Alcohol has been shown to be linked with AFib through multiple mechanisms. Alcohol has adverse effects on the atrial substrate, including promotion of left atrial remodeling, dilation, and fibrosis via oxidative stress, and alcohol has a contributory role in hypertension and obstructive sleep apnea. Alcohol can act as an acute trigger of AFib through binge drinking – the holiday heart syndrome – which causes autonomic changes, electrolyte abnormalities, and electrophysiologic effects, he continued.
Epidemiologic evidence in support of the notion that moderate drinking increases the risk of AFib includes a Swedish meta-analysis of seven prospective studies comprising 12,554 patients with AFib. The researchers concluded that the relative risk of AFib rose by about 8% with each drink per day, compared with the risk of a reference group composed of current drinkers at a rate of less than one drink per week (J Am Coll Cardiol. 2014 Jul 22;64[3]:281-9).
Although discussants were wowed by the 61% complete abstinence rate in the trial, Dr. Voskoboinik cautioned that the study participants were a highly motivated subset of the universe of moderate drinkers with AFib. And even so, “It was an incredibly challenging study to run,” he said.
“I think lifestyle change is a challenge, and with alcohol particularly so, because alcohol is so ubiquitous in our society. I think the important key message for us as physicians is to take an alcohol history and have a discussion with the patient. And we now have some data to show them. But at the end of the day, it’s up to the patient in conjunction with the physician,” he observed.
Discussant Annabelle S. Volgman, MD, noted that 85% of study participants were men, and because of important differences in AFib between men and women, she doesn’t consider the findings applicable to women.
“You need to redo the study in women,” advised Dr. Volgman, professor of medicine at Rush University, Chicago, and director of the Rush Heart Center for Women.
She suggested that an interventional trial such as this one is a great opportunity to utilize wearable device technology, such as the Apple Watch, which can detect irregular pulse rhythms as demonstrated in the Apple Heart Study. This technology is especially popular with younger individuals.
“A lot of young men and women drink a lot of alcohol,” Dr. Volgman noted.
“That’s a great idea. It gets around a problem with AliveCor, which can miss episodes while you’re sleeping,” Dr. Voskoboinik replied.
He reported having no financial conflicts regarding the investigator-initiated and -funded Alcohol-AF trial.
SOURCE: Voskoboinik A. ACC 19, Session 413-08.
NEW ORLEANS – Abstinence from alcohol on the part of moderate drinkers with atrial fibrillation resulted in clinically meaningful improvement in their arrhythmia in the randomized controlled Alcohol-AF trial, Aleksandr Voskoboinik, MBBS, reported at the annual meeting of the American College of Cardiology.
The benefits of abstinence included significant reductions in the AFib recurrence rate, total AFib burden, and symptom severity. And the payoff extended beyond the arrhythmia: The abstinent group also averaged a 12–mm Hg drop in systolic blood pressure and dropped 3 kg of body weight over the course of the 6-month trial, added Dr. Voskoboinik, a cardiologist at the Baker Heart & Diabetes Institute at the University of Melbourne.
“We would conclude that significant reduction in alcohol intake should be considered as part of the lifestyle intervention in moderate drinkers with AFib,” he said.
Some physicians already advise alcohol abstinence for moderate drinkers with AFib, but until now such recommendations were not evidence based. Instead, the guidance was based on extrapolation from the well-established harmful effects of heavy drinking and binge drinking on AFib as well as epidemiologic studies.
“We wanted to provide some evidence base for physicians to say, ‘If I have motivated patients, here’s what’s potentially achievable,’” he explained.
The key word here is “motivated.” Conducting the Alcohol-AF trial made clear that many moderate drinkers with AFib enjoy their beverages more than they hate having their arrhythmia. Indeed, out of 697 moderate-drinking patients with paroxysmal or persistent AFib who were deemed good candidates and were approached about study participation, more than 500 were excluded because they were flat out unwilling to consider abstinence. After Dr. Voskoboinik and his coinvestigators shortened the planned trial duration from 12 months to 6 because so many recruits were unwilling to abstain for a full year, 140 of the initial 697 patients were randomized to abstinence or continuation of their usual consumption. Thirty percent of them had previously undergone AFib ablation.
The Alcohol-AF study was a multicenter, prospective, open-label study conducted at six Australian hospitals. All subjects underwent comprehensive rhythm monitoring via an implantable loop recorder or an existing pacemaker. Twice daily they triggered the AliveCor mobile phone app together with Holter monitoring. Patients assigned to abstinence were counseled to abstain completely, got written and verbal advice to help with compliance, and had contact with the investigators on a monthly basis. They also underwent urine testing to monitor compliance. All study participants kept a weekly alcohol intake diary, reviewed monthly by investigators.
Participants averaged 17 standard drinks per week at enrollment. Two-thirds were primarily wine drinkers. At the 6-month mark, patients in the abstinence arm averaged two drinks per week, for an 88% reduction from baseline, and 43 of the 70, or 61%, had remained completely abstinent. The control arm averaged a 20% reduction in weekly consumption.
One of the two co-primary endpoints was recurrent AFib episodes lasting for at least 30 seconds. The rate was 53% in the abstinence group and 73% in controls. The time to first recurrence averaged 118 days in the abstinence group, compared to 86 days in controls, for a 37% prolongation through abstinence.
The other primary endpoint was total AFib burden. During the 6-month study, the abstinence group spent a mean of 5.6% of their time in AFib, compared to 8.2% in controls. The median times were 0.5% and 1.2%.
In a multivariate analysis, neither AFib type, duration, history of AFib ablation, age, nor gender predicted AFib recurrence. In fact, the only significant predictor was alcohol abstinence, which conferred a 48% reduction in risk.
Symptom severity, a secondary endpoint, showed an impressive between-group difference at follow-up. Ten percent of patients in the abstinence group had moderate or severe symptoms at follow-up as assessed via the European Heart Rhythm Association score of atrial fibrillation, compared to 32% of controls, for an absolute 22% difference. And 90% of the abstinence group had no or mild symptoms, as did 68% of controls. Nine percent of the abstinence group had an AFib-related hospitalization, as did 20% of controls.
Cardiac MRI, performed in all subjects, showed that the abstinence group experienced a significant reduction in left atrial area, from 29.5 cm2 at baseline to 27.1 cm2 at follow-up. They also experienced significant improvement in left atrial mechanical function, with their left atrial emptying fraction climbing from 42% to 50%.
The 3-kg weight loss from a baseline of 90 kg in the abstinent group represented a 0.7 kg/m2 reduction in body mass index.
In terms of the likely mechanism of benefit of alcohol abstinence in moderate drinkers, Dr. Voskoboinik noted that he and his colleagues recently demonstrated that regular moderate alcohol consumption, but not mild intake, is associated with potentially explanatory atrial electrical and structural changes, including conduction slowing, lower global bipolar atrial voltage, and an increase in complex atrial potentials (Heart Rhythm. 2019 Feb;16[2]:251-9).
Alcohol has been shown to be linked with AFib through multiple mechanisms. Alcohol has adverse effects on the atrial substrate, including promotion of left atrial remodeling, dilation, and fibrosis via oxidative stress, and alcohol has a contributory role in hypertension and obstructive sleep apnea. Alcohol can act as an acute trigger of AFib through binge drinking – the holiday heart syndrome – which causes autonomic changes, electrolyte abnormalities, and electrophysiologic effects, he continued.
Epidemiologic evidence in support of the notion that moderate drinking increases the risk of AFib includes a Swedish meta-analysis of seven prospective studies comprising 12,554 patients with AFib. The researchers concluded that the relative risk of AFib rose by about 8% with each drink per day, compared with the risk of a reference group composed of current drinkers at a rate of less than one drink per week (J Am Coll Cardiol. 2014 Jul 22;64[3]:281-9).
Although discussants were wowed by the 61% complete abstinence rate in the trial, Dr. Voskoboinik cautioned that the study participants were a highly motivated subset of the universe of moderate drinkers with AFib. And even so, “It was an incredibly challenging study to run,” he said.
“I think lifestyle change is a challenge, and with alcohol particularly so, because alcohol is so ubiquitous in our society. I think the important key message for us as physicians is to take an alcohol history and have a discussion with the patient. And we now have some data to show them. But at the end of the day, it’s up to the patient in conjunction with the physician,” he observed.
Discussant Annabelle S. Volgman, MD, noted that 85% of study participants were men, and because of important differences in AFib between men and women, she doesn’t consider the findings applicable to women.
“You need to redo the study in women,” advised Dr. Volgman, professor of medicine at Rush University, Chicago, and director of the Rush Heart Center for Women.
She suggested that an interventional trial such as this one is a great opportunity to utilize wearable device technology, such as the Apple Watch, which can detect irregular pulse rhythms as demonstrated in the Apple Heart Study. This technology is especially popular with younger individuals.
“A lot of young men and women drink a lot of alcohol,” Dr. Volgman noted.
“That’s a great idea. It gets around a problem with AliveCor, which can miss episodes while you’re sleeping,” Dr. Voskoboinik replied.
He reported having no financial conflicts regarding the investigator-initiated and -funded Alcohol-AF trial.
SOURCE: Voskoboinik A. ACC 19, Session 413-08.
NEW ORLEANS – Abstinence from alcohol on the part of moderate drinkers with atrial fibrillation resulted in clinically meaningful improvement in their arrhythmia in the randomized controlled Alcohol-AF trial, Aleksandr Voskoboinik, MBBS, reported at the annual meeting of the American College of Cardiology.
The benefits of abstinence included significant reductions in the AFib recurrence rate, total AFib burden, and symptom severity. And the payoff extended beyond the arrhythmia: The abstinent group also averaged a 12–mm Hg drop in systolic blood pressure and dropped 3 kg of body weight over the course of the 6-month trial, added Dr. Voskoboinik, a cardiologist at the Baker Heart & Diabetes Institute at the University of Melbourne.
“We would conclude that significant reduction in alcohol intake should be considered as part of the lifestyle intervention in moderate drinkers with AFib,” he said.
Some physicians already advise alcohol abstinence for moderate drinkers with AFib, but until now such recommendations were not evidence based. Instead, the guidance was based on extrapolation from the well-established harmful effects of heavy drinking and binge drinking on AFib as well as epidemiologic studies.
“We wanted to provide some evidence base for physicians to say, ‘If I have motivated patients, here’s what’s potentially achievable,’” he explained.
The key word here is “motivated.” Conducting the Alcohol-AF trial made clear that many moderate drinkers with AFib enjoy their beverages more than they hate having their arrhythmia. Indeed, out of 697 moderate-drinking patients with paroxysmal or persistent AFib who were deemed good candidates and were approached about study participation, more than 500 were excluded because they were flat out unwilling to consider abstinence. After Dr. Voskoboinik and his coinvestigators shortened the planned trial duration from 12 months to 6 because so many recruits were unwilling to abstain for a full year, 140 of the initial 697 patients were randomized to abstinence or continuation of their usual consumption. Thirty percent of them had previously undergone AFib ablation.
The Alcohol-AF study was a multicenter, prospective, open-label study conducted at six Australian hospitals. All subjects underwent comprehensive rhythm monitoring via an implantable loop recorder or an existing pacemaker. Twice daily they triggered the AliveCor mobile phone app together with Holter monitoring. Patients assigned to abstinence were counseled to abstain completely, got written and verbal advice to help with compliance, and had contact with the investigators on a monthly basis. They also underwent urine testing to monitor compliance. All study participants kept a weekly alcohol intake diary, reviewed monthly by investigators.
Participants averaged 17 standard drinks per week at enrollment. Two-thirds were primarily wine drinkers. At the 6-month mark, patients in the abstinence arm averaged two drinks per week, for an 88% reduction from baseline, and 43 of the 70, or 61%, had remained completely abstinent. The control arm averaged a 20% reduction in weekly consumption.
One of the two co-primary endpoints was recurrent AFib episodes lasting for at least 30 seconds. The rate was 53% in the abstinence group and 73% in controls. The time to first recurrence averaged 118 days in the abstinence group, compared to 86 days in controls, for a 37% prolongation through abstinence.
The other primary endpoint was total AFib burden. During the 6-month study, the abstinence group spent a mean of 5.6% of their time in AFib, compared to 8.2% in controls. The median times were 0.5% and 1.2%.
In a multivariate analysis, neither AFib type, duration, history of AFib ablation, age, nor gender predicted AFib recurrence. In fact, the only significant predictor was alcohol abstinence, which conferred a 48% reduction in risk.
Symptom severity, a secondary endpoint, showed an impressive between-group difference at follow-up. Ten percent of patients in the abstinence group had moderate or severe symptoms at follow-up as assessed via the European Heart Rhythm Association score of atrial fibrillation, compared to 32% of controls, for an absolute 22% difference. And 90% of the abstinence group had no or mild symptoms, as did 68% of controls. Nine percent of the abstinence group had an AFib-related hospitalization, as did 20% of controls.
Cardiac MRI, performed in all subjects, showed that the abstinence group experienced a significant reduction in left atrial area, from 29.5 cm2 at baseline to 27.1 cm2 at follow-up. They also experienced significant improvement in left atrial mechanical function, with their left atrial emptying fraction climbing from 42% to 50%.
The 3-kg weight loss from a baseline of 90 kg in the abstinent group represented a 0.7 kg/m2 reduction in body mass index.
In terms of the likely mechanism of benefit of alcohol abstinence in moderate drinkers, Dr. Voskoboinik noted that he and his colleagues recently demonstrated that regular moderate alcohol consumption, but not mild intake, is associated with potentially explanatory atrial electrical and structural changes, including conduction slowing, lower global bipolar atrial voltage, and an increase in complex atrial potentials (Heart Rhythm. 2019 Feb;16[2]:251-9).
Alcohol has been shown to be linked with AFib through multiple mechanisms. Alcohol has adverse effects on the atrial substrate, including promotion of left atrial remodeling, dilation, and fibrosis via oxidative stress, and alcohol has a contributory role in hypertension and obstructive sleep apnea. Alcohol can act as an acute trigger of AFib through binge drinking – the holiday heart syndrome – which causes autonomic changes, electrolyte abnormalities, and electrophysiologic effects, he continued.
Epidemiologic evidence in support of the notion that moderate drinking increases the risk of AFib includes a Swedish meta-analysis of seven prospective studies comprising 12,554 patients with AFib. The researchers concluded that the relative risk of AFib rose by about 8% with each drink per day, compared with the risk of a reference group composed of current drinkers at a rate of less than one drink per week (J Am Coll Cardiol. 2014 Jul 22;64[3]:281-9).
Although discussants were wowed by the 61% complete abstinence rate in the trial, Dr. Voskoboinik cautioned that the study participants were a highly motivated subset of the universe of moderate drinkers with AFib. And even so, “It was an incredibly challenging study to run,” he said.
“I think lifestyle change is a challenge, and with alcohol particularly so, because alcohol is so ubiquitous in our society. I think the important key message for us as physicians is to take an alcohol history and have a discussion with the patient. And we now have some data to show them. But at the end of the day, it’s up to the patient in conjunction with the physician,” he observed.
Discussant Annabelle S. Volgman, MD, noted that 85% of study participants were men, and because of important differences in AFib between men and women, she doesn’t consider the findings applicable to women.
“You need to redo the study in women,” advised Dr. Volgman, professor of medicine at Rush University, Chicago, and director of the Rush Heart Center for Women.
She suggested that an interventional trial such as this one is a great opportunity to utilize wearable device technology, such as the Apple Watch, which can detect irregular pulse rhythms as demonstrated in the Apple Heart Study. This technology is especially popular with younger individuals.
“A lot of young men and women drink a lot of alcohol,” Dr. Volgman noted.
“That’s a great idea. It gets around a problem with AliveCor, which can miss episodes while you’re sleeping,” Dr. Voskoboinik replied.
He reported having no financial conflicts regarding the investigator-initiated and -funded Alcohol-AF trial.
SOURCE: Voskoboinik A. ACC 19, Session 413-08.
REPORTING FROM ACC 19
Biogen, Eisai discontinue aducanumab Alzheimer’s trials
Biogen and Eisai have announced that they are discontinuing the ENGAGE and EMERGE trials, which were designed to test the efficacy and safety of aducanumab in patients with mild cognitive impairment caused by Alzheimer’s disease and mild Alzheimer’s disease dementia.
The phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group trials were canceled not because of safety concerns but because of a futility analysis conducted by an independent data monitoring committee that indicated the drug would not meet the trials’ primary endpoint, which was the slowing of cognitive and functional impairment as measured by changes in Clinical Dementia Rating–Sum of Boxes score, compared with placebo.
In addition to ENGAGE and EMERGE, the phase 2 EVOLVE safety study and the long-term extension of the phase 1b PRIME study have also been canceled. Data from the ENGAGE and EMERGE trials will be presented at future medical meetings.
Aducanumab is a human monoclonal antibody derived from B cells collected from healthy elderly subjects with no cognitive decline or those with unusually slow cognitive decline through Neurimmune’s technology platform called Reverse Translational Medicine. It was granted Fast Track designation by the Food and Drug Administration.
“This disappointing news confirms the complexity of treating Alzheimer’s disease and the need to further advance knowledge in neuroscience. We are incredibly grateful to all the Alzheimer’s disease patients, their families, and the investigators who participated in the trials and contributed greatly to this research,” Michel Vounatsos, CEO at Biogen, said in a press release.
Biogen and Eisai have announced that they are discontinuing the ENGAGE and EMERGE trials, which were designed to test the efficacy and safety of aducanumab in patients with mild cognitive impairment caused by Alzheimer’s disease and mild Alzheimer’s disease dementia.
The phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group trials were canceled not because of safety concerns but because of a futility analysis conducted by an independent data monitoring committee that indicated the drug would not meet the trials’ primary endpoint, which was the slowing of cognitive and functional impairment as measured by changes in Clinical Dementia Rating–Sum of Boxes score, compared with placebo.
In addition to ENGAGE and EMERGE, the phase 2 EVOLVE safety study and the long-term extension of the phase 1b PRIME study have also been canceled. Data from the ENGAGE and EMERGE trials will be presented at future medical meetings.
Aducanumab is a human monoclonal antibody derived from B cells collected from healthy elderly subjects with no cognitive decline or those with unusually slow cognitive decline through Neurimmune’s technology platform called Reverse Translational Medicine. It was granted Fast Track designation by the Food and Drug Administration.
“This disappointing news confirms the complexity of treating Alzheimer’s disease and the need to further advance knowledge in neuroscience. We are incredibly grateful to all the Alzheimer’s disease patients, their families, and the investigators who participated in the trials and contributed greatly to this research,” Michel Vounatsos, CEO at Biogen, said in a press release.
Biogen and Eisai have announced that they are discontinuing the ENGAGE and EMERGE trials, which were designed to test the efficacy and safety of aducanumab in patients with mild cognitive impairment caused by Alzheimer’s disease and mild Alzheimer’s disease dementia.
The phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group trials were canceled not because of safety concerns but because of a futility analysis conducted by an independent data monitoring committee that indicated the drug would not meet the trials’ primary endpoint, which was the slowing of cognitive and functional impairment as measured by changes in Clinical Dementia Rating–Sum of Boxes score, compared with placebo.
In addition to ENGAGE and EMERGE, the phase 2 EVOLVE safety study and the long-term extension of the phase 1b PRIME study have also been canceled. Data from the ENGAGE and EMERGE trials will be presented at future medical meetings.
Aducanumab is a human monoclonal antibody derived from B cells collected from healthy elderly subjects with no cognitive decline or those with unusually slow cognitive decline through Neurimmune’s technology platform called Reverse Translational Medicine. It was granted Fast Track designation by the Food and Drug Administration.
“This disappointing news confirms the complexity of treating Alzheimer’s disease and the need to further advance knowledge in neuroscience. We are incredibly grateful to all the Alzheimer’s disease patients, their families, and the investigators who participated in the trials and contributed greatly to this research,” Michel Vounatsos, CEO at Biogen, said in a press release.
Wedding dermatology
Planning a wedding doesn’t just involve decisions on venues, vows, guests, food, and décor, but also on the betrothed couple’s appearance. Memories and photographs from this day last a lifetime, so it is understandable that people may want to and feel pressured to look their best on this important day – which along with the pressures of planning a wedding, can lead to unnecessary stress, increased cortisol, and unexpected acne and other skin issues.
Because of a complete absence of wedding skin recommendations in the dermatology literature, Winklemann R et al. recently published a paper titled “Wedding Dermatology: A proposed timeline to optimize skin clearance and the avoidance of a true dermatologic emergency” (SKIN The Journal of Cutaneous Medicine 2019 Mar;3[2]:159-60). He focused on acne, using the American Academy of Dermatology acne treatment guidelines and expert opinion, they point out that other than intralesional corticosteroids (which take 0-14 days to have an effect), the majority of acne treatments require at least 3-12 months to achieve clearance or improvement.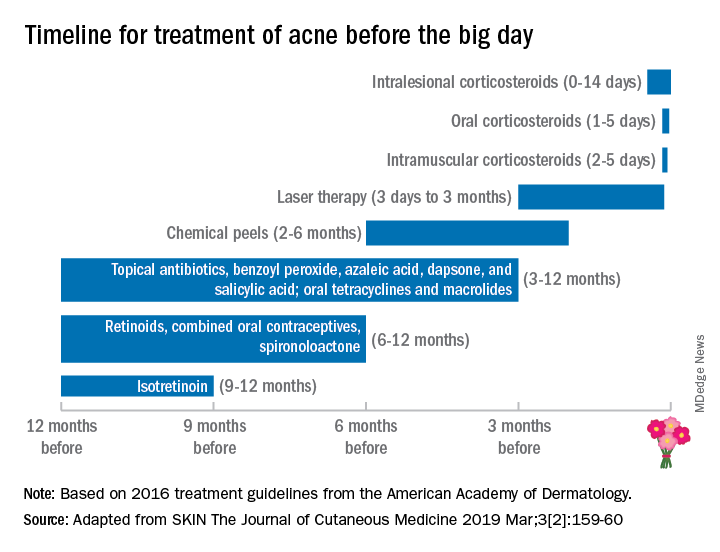
This proposed treatment timeline makes sense given that skin cell turnover on the face takes about 6-8 weeks; therefore, it may take that long for acne lesions to resolve or for treatment to have an effect.
Besides acne treatment, cosmetic treatments also have varying healing times and may require multiple sessions with time in between treatments for optimal results. For instance, treatment of photoaging with intense pulsed light or nonablative fractionated resurfacing may require three to six treatments, typically spaced 1 month apart.
Botulinum toxin treatments may take up to 2 weeks to kick in fully, then last 3-4 months. While the lead time for botulinum toxin to kick in is relatively short, I advise people not to get their first botulinum toxin 2 weeks before their wedding. Sometimes, having this treatment 4-6 weeks prior to the wedding date provides enough time for botulinum toxin to kick in – and to start wearing off to the point that the patient has the desired cosmetic effect, but still has some movement for the desired emotional facial expressions on the wedding day. Some patients also may require touch-ups, optimally at the 2-week window, once the botulinum toxin effect has fully kicked in.
With any injectable, there may be bruising and swelling that can take a week or so to heal, even when the bruises are treated with pulse dye laser used to make bruises resolve more quickly. Even a facial may result in blemishes that take a few days to 1-2 weeks to heal, especially with extractions. As such, a facial, especially a hydrafacial, may be beneficial before one’s wedding, but I would recommend having them done at least once or twice to assess an individual’s recovery time (if any) prior to the actual wedding date.
Treatment needs will vary considerably depending on the patient’s baseline skin health. As dermatologists, our patients depend greatly on us to help them look and feel their best, especially during a time when they are about to embark on a new journey like marriage. Patients need to be given realistic treatment options and time frames to achieve their goals. Whether the goal is treating acne or acne scars, starting or fine-tuning cosmetic treatments, or deciding on a skin-care regimen, the bottom line is making an appointment with the dermatologist early – 6-12 months in advance, if possible – to figure out the right plan.
Dr. Wesley and Dr. Talakoub are co-contributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Planning a wedding doesn’t just involve decisions on venues, vows, guests, food, and décor, but also on the betrothed couple’s appearance. Memories and photographs from this day last a lifetime, so it is understandable that people may want to and feel pressured to look their best on this important day – which along with the pressures of planning a wedding, can lead to unnecessary stress, increased cortisol, and unexpected acne and other skin issues.
Because of a complete absence of wedding skin recommendations in the dermatology literature, Winklemann R et al. recently published a paper titled “Wedding Dermatology: A proposed timeline to optimize skin clearance and the avoidance of a true dermatologic emergency” (SKIN The Journal of Cutaneous Medicine 2019 Mar;3[2]:159-60). He focused on acne, using the American Academy of Dermatology acne treatment guidelines and expert opinion, they point out that other than intralesional corticosteroids (which take 0-14 days to have an effect), the majority of acne treatments require at least 3-12 months to achieve clearance or improvement.
This proposed treatment timeline makes sense given that skin cell turnover on the face takes about 6-8 weeks; therefore, it may take that long for acne lesions to resolve or for treatment to have an effect.
Besides acne treatment, cosmetic treatments also have varying healing times and may require multiple sessions with time in between treatments for optimal results. For instance, treatment of photoaging with intense pulsed light or nonablative fractionated resurfacing may require three to six treatments, typically spaced 1 month apart.
Botulinum toxin treatments may take up to 2 weeks to kick in fully, then last 3-4 months. While the lead time for botulinum toxin to kick in is relatively short, I advise people not to get their first botulinum toxin 2 weeks before their wedding. Sometimes, having this treatment 4-6 weeks prior to the wedding date provides enough time for botulinum toxin to kick in – and to start wearing off to the point that the patient has the desired cosmetic effect, but still has some movement for the desired emotional facial expressions on the wedding day. Some patients also may require touch-ups, optimally at the 2-week window, once the botulinum toxin effect has fully kicked in.
With any injectable, there may be bruising and swelling that can take a week or so to heal, even when the bruises are treated with pulse dye laser used to make bruises resolve more quickly. Even a facial may result in blemishes that take a few days to 1-2 weeks to heal, especially with extractions. As such, a facial, especially a hydrafacial, may be beneficial before one’s wedding, but I would recommend having them done at least once or twice to assess an individual’s recovery time (if any) prior to the actual wedding date.
Treatment needs will vary considerably depending on the patient’s baseline skin health. As dermatologists, our patients depend greatly on us to help them look and feel their best, especially during a time when they are about to embark on a new journey like marriage. Patients need to be given realistic treatment options and time frames to achieve their goals. Whether the goal is treating acne or acne scars, starting or fine-tuning cosmetic treatments, or deciding on a skin-care regimen, the bottom line is making an appointment with the dermatologist early – 6-12 months in advance, if possible – to figure out the right plan.
Dr. Wesley and Dr. Talakoub are co-contributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Planning a wedding doesn’t just involve decisions on venues, vows, guests, food, and décor, but also on the betrothed couple’s appearance. Memories and photographs from this day last a lifetime, so it is understandable that people may want to and feel pressured to look their best on this important day – which along with the pressures of planning a wedding, can lead to unnecessary stress, increased cortisol, and unexpected acne and other skin issues.
Because of a complete absence of wedding skin recommendations in the dermatology literature, Winklemann R et al. recently published a paper titled “Wedding Dermatology: A proposed timeline to optimize skin clearance and the avoidance of a true dermatologic emergency” (SKIN The Journal of Cutaneous Medicine 2019 Mar;3[2]:159-60). He focused on acne, using the American Academy of Dermatology acne treatment guidelines and expert opinion, they point out that other than intralesional corticosteroids (which take 0-14 days to have an effect), the majority of acne treatments require at least 3-12 months to achieve clearance or improvement.
This proposed treatment timeline makes sense given that skin cell turnover on the face takes about 6-8 weeks; therefore, it may take that long for acne lesions to resolve or for treatment to have an effect.
Besides acne treatment, cosmetic treatments also have varying healing times and may require multiple sessions with time in between treatments for optimal results. For instance, treatment of photoaging with intense pulsed light or nonablative fractionated resurfacing may require three to six treatments, typically spaced 1 month apart.
Botulinum toxin treatments may take up to 2 weeks to kick in fully, then last 3-4 months. While the lead time for botulinum toxin to kick in is relatively short, I advise people not to get their first botulinum toxin 2 weeks before their wedding. Sometimes, having this treatment 4-6 weeks prior to the wedding date provides enough time for botulinum toxin to kick in – and to start wearing off to the point that the patient has the desired cosmetic effect, but still has some movement for the desired emotional facial expressions on the wedding day. Some patients also may require touch-ups, optimally at the 2-week window, once the botulinum toxin effect has fully kicked in.
With any injectable, there may be bruising and swelling that can take a week or so to heal, even when the bruises are treated with pulse dye laser used to make bruises resolve more quickly. Even a facial may result in blemishes that take a few days to 1-2 weeks to heal, especially with extractions. As such, a facial, especially a hydrafacial, may be beneficial before one’s wedding, but I would recommend having them done at least once or twice to assess an individual’s recovery time (if any) prior to the actual wedding date.
Treatment needs will vary considerably depending on the patient’s baseline skin health. As dermatologists, our patients depend greatly on us to help them look and feel their best, especially during a time when they are about to embark on a new journey like marriage. Patients need to be given realistic treatment options and time frames to achieve their goals. Whether the goal is treating acne or acne scars, starting or fine-tuning cosmetic treatments, or deciding on a skin-care regimen, the bottom line is making an appointment with the dermatologist early – 6-12 months in advance, if possible – to figure out the right plan.
Dr. Wesley and Dr. Talakoub are co-contributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
High survival in relapsed FL after primary radiotherapy
The prognosis post relapse following primary radiotherapy was found to be excellent for patients with localized follicular lymphoma (FL), according to a retrospective analysis.
But patients who experienced early relapse – at 1 year or less after diagnosis – had significantly worse survival.
While primary radiotherapy may be curative for localized FL, about 30%-50% of patients will relapse and optimal salvage therapy is not well defined, Michael S. Binkley, MD, of Stanford (Calif.) University, and his colleagues wrote in the International Journal of Radiation Oncology, Biology, Physics.
The researchers retrospectively studied 512 patients with localized FL using data from multiple centers under the direction of the International Lymphoma Radiation Oncology Group (ILROG). Clinical information was collected, including method of detection, age at relapse, location of recurrence, and response to salvage therapy.
The team defined disease recurrence as lymphoma nonresponsive to primary radiotherapy inside of the prescribed dose volume, which included having no radiographic or clinical response within 6 months of initial radiotherapy treatment.
With a median follow-up of 52 months, Dr. Binkley and his colleagues found that 29.1% of patients developed relapsed lymphoma at a median 23 months (range, 1-143 months) following primary radiotherapy.
The team reported that the 3-year overall survival rate was 91.4% for patients with lymphoma recurrence after primary radiotherapy. In total, 16 deaths occurred during follow-up: eight were lymphoma specific deaths, three were from other causes, and in five patients the cause was unknown.
Of the 149 cases of relapsed lymphoma, 93 were indolent. There were also three cases of FL grade 3B/not otherwise specified and 18 cases of diffuse large B-cell lymphoma. In 35 patients who relapsed, biopsies were not performed.
“The excellent prognosis observed for this relapse cohort emphasizes that primary radiation for localized follicular lymphoma is an excellent treatment option,” the researchers wrote.
When the researchers examined survival based on the time of relapse, they found that overall survival was “significantly worse” for patients who had relapsed 12 months or less after the date of diagnosis, at 88.7%, compared with all others at 95.8% (P = .01).
They found no difference in overall survival between patients who received immediate salvage treatment, compared with observation after relapse (P = .28). There was also no significant difference in survival between patients who relapsed in 1 year or less but underwent immediate treatment, compared to early relapsed patients who were observed (log-rank P = .34).
“[A]ny decision to offer treatment at time of relapse must be weighed with the risk of acute and late adverse effects. Greater than 60% of patients in our cohort with indolent recurrence who underwent salvage treatment received rituximab, likely contributing to the excellent outcomes,” the researchers wrote. “However, nearly 60% of patients with indolent recurrence who were observed did not have disease progression nor [did they] receive treatment within 3 years.”
The researchers reported having no conflicts of interest.
SOURCE: Binkley MS et al. Int J Radiat Oncol Biol Phys. 2019 Mar 8. doi: 10.1016/j.ijrobp.2019.03.004.
The prognosis post relapse following primary radiotherapy was found to be excellent for patients with localized follicular lymphoma (FL), according to a retrospective analysis.
But patients who experienced early relapse – at 1 year or less after diagnosis – had significantly worse survival.
While primary radiotherapy may be curative for localized FL, about 30%-50% of patients will relapse and optimal salvage therapy is not well defined, Michael S. Binkley, MD, of Stanford (Calif.) University, and his colleagues wrote in the International Journal of Radiation Oncology, Biology, Physics.
The researchers retrospectively studied 512 patients with localized FL using data from multiple centers under the direction of the International Lymphoma Radiation Oncology Group (ILROG). Clinical information was collected, including method of detection, age at relapse, location of recurrence, and response to salvage therapy.
The team defined disease recurrence as lymphoma nonresponsive to primary radiotherapy inside of the prescribed dose volume, which included having no radiographic or clinical response within 6 months of initial radiotherapy treatment.
With a median follow-up of 52 months, Dr. Binkley and his colleagues found that 29.1% of patients developed relapsed lymphoma at a median 23 months (range, 1-143 months) following primary radiotherapy.
The team reported that the 3-year overall survival rate was 91.4% for patients with lymphoma recurrence after primary radiotherapy. In total, 16 deaths occurred during follow-up: eight were lymphoma specific deaths, three were from other causes, and in five patients the cause was unknown.
Of the 149 cases of relapsed lymphoma, 93 were indolent. There were also three cases of FL grade 3B/not otherwise specified and 18 cases of diffuse large B-cell lymphoma. In 35 patients who relapsed, biopsies were not performed.
“The excellent prognosis observed for this relapse cohort emphasizes that primary radiation for localized follicular lymphoma is an excellent treatment option,” the researchers wrote.
When the researchers examined survival based on the time of relapse, they found that overall survival was “significantly worse” for patients who had relapsed 12 months or less after the date of diagnosis, at 88.7%, compared with all others at 95.8% (P = .01).
They found no difference in overall survival between patients who received immediate salvage treatment, compared with observation after relapse (P = .28). There was also no significant difference in survival between patients who relapsed in 1 year or less but underwent immediate treatment, compared to early relapsed patients who were observed (log-rank P = .34).
“[A]ny decision to offer treatment at time of relapse must be weighed with the risk of acute and late adverse effects. Greater than 60% of patients in our cohort with indolent recurrence who underwent salvage treatment received rituximab, likely contributing to the excellent outcomes,” the researchers wrote. “However, nearly 60% of patients with indolent recurrence who were observed did not have disease progression nor [did they] receive treatment within 3 years.”
The researchers reported having no conflicts of interest.
SOURCE: Binkley MS et al. Int J Radiat Oncol Biol Phys. 2019 Mar 8. doi: 10.1016/j.ijrobp.2019.03.004.
The prognosis post relapse following primary radiotherapy was found to be excellent for patients with localized follicular lymphoma (FL), according to a retrospective analysis.
But patients who experienced early relapse – at 1 year or less after diagnosis – had significantly worse survival.
While primary radiotherapy may be curative for localized FL, about 30%-50% of patients will relapse and optimal salvage therapy is not well defined, Michael S. Binkley, MD, of Stanford (Calif.) University, and his colleagues wrote in the International Journal of Radiation Oncology, Biology, Physics.
The researchers retrospectively studied 512 patients with localized FL using data from multiple centers under the direction of the International Lymphoma Radiation Oncology Group (ILROG). Clinical information was collected, including method of detection, age at relapse, location of recurrence, and response to salvage therapy.
The team defined disease recurrence as lymphoma nonresponsive to primary radiotherapy inside of the prescribed dose volume, which included having no radiographic or clinical response within 6 months of initial radiotherapy treatment.
With a median follow-up of 52 months, Dr. Binkley and his colleagues found that 29.1% of patients developed relapsed lymphoma at a median 23 months (range, 1-143 months) following primary radiotherapy.
The team reported that the 3-year overall survival rate was 91.4% for patients with lymphoma recurrence after primary radiotherapy. In total, 16 deaths occurred during follow-up: eight were lymphoma specific deaths, three were from other causes, and in five patients the cause was unknown.
Of the 149 cases of relapsed lymphoma, 93 were indolent. There were also three cases of FL grade 3B/not otherwise specified and 18 cases of diffuse large B-cell lymphoma. In 35 patients who relapsed, biopsies were not performed.
“The excellent prognosis observed for this relapse cohort emphasizes that primary radiation for localized follicular lymphoma is an excellent treatment option,” the researchers wrote.
When the researchers examined survival based on the time of relapse, they found that overall survival was “significantly worse” for patients who had relapsed 12 months or less after the date of diagnosis, at 88.7%, compared with all others at 95.8% (P = .01).
They found no difference in overall survival between patients who received immediate salvage treatment, compared with observation after relapse (P = .28). There was also no significant difference in survival between patients who relapsed in 1 year or less but underwent immediate treatment, compared to early relapsed patients who were observed (log-rank P = .34).
“[A]ny decision to offer treatment at time of relapse must be weighed with the risk of acute and late adverse effects. Greater than 60% of patients in our cohort with indolent recurrence who underwent salvage treatment received rituximab, likely contributing to the excellent outcomes,” the researchers wrote. “However, nearly 60% of patients with indolent recurrence who were observed did not have disease progression nor [did they] receive treatment within 3 years.”
The researchers reported having no conflicts of interest.
SOURCE: Binkley MS et al. Int J Radiat Oncol Biol Phys. 2019 Mar 8. doi: 10.1016/j.ijrobp.2019.03.004.
FROM THE INTERNATIONAL JOURNAL OF RADIATION ONCOLOGY, BIOLOGY, PHYSICS
Sleepless democracy, space herpes, and the F-bomb diet
Sleep: the key to democracy?
You might need to lie down for this one. Insufficient sleep could be affecting the very institutions and behaviors that keep society from crumbling.
Lack of sleep is proven to affect “private” behaviors, like working, but a new study has shown that exhaustion also has consequences on a national scale. Sleeping less negatively affects the “social behaviors that hold society and democracy together,” according to the study’s authors.
Willingness to vote, donate to charities, and sign petitions decreased in sleep-deprived participants. Although that last one is iffy – is anyone really willing to sign a petition, regardless of sleep intake? One thing to glean from this study is, if you’re holding a clipboard, let the most tired-looking people keep walking.
Perhaps we should start a petition to make the day before Election Day a national napping holiday.
I hear you knocking, but you can’t come in
LOTME takes you to the ends of the earth for this edition of Bacteria vs. the World. Let’s go to our correspondent, Danielle Kang, on the International Space Station.
Hi, this is Briny Baird in Berlin, Germany. The space station’s extreme environment weakens astronauts’ immune systems, but it has the opposite effect on bacteria, as Elisabeth Grohmann, PhD, of Beuth University of Applied Sciences explains.
“The bacteria [astronauts] carry become hardier – developing thick protective coatings and resistance to antibiotics – and more vigorous, multiplying and metabolizing faster.”
This is, as scientists put it, bad. To prevent an on-board bacterial bacchanal, Dr. Grohmann and her associates covered a vital piece of ISS equipment, the toilet door, with a new coating containing “silver and ruthenium, conditioned by a vitamin derivative.” After 19 months in space, the coated section of the door had 80% fewer bacteria on it than an uncoated control surface.
With long-term missions to Mars being considered, it should be safer for astronauts and their toilet doors to … go where no one has gone before. Back to the studio.
The F-bomb diet
You might think vulgarities and Homo sapiens have been an inseparable pair since the first human bashed her thumb while chipping stones into spearheads to add meat to her diet. You’d probably be right – except for blue-tinged language built on the consonant foundations of the letters F and V.
New linguistics research finds that those $#%*! letters, like truckers and Marine gunnery sergeants, are relatively modern developments. As humans moved away from finger-harming hunting and gathering and toward finger-harming farming, they developed a taste for softer foods. And their speech picked up “labiodentals” such as F and V – which are sounds made when we touch our lower lips to our upper teeth. Half the world’s languages are now riddled with soft-food profanities, er, labiodentals.
Next on the linguists’ research list: Does the paleo diet lead to the dropping of fewer F-bombs?
Herpes! In space!
Viruses can never let bacteria have all the fun. Where there’s an immune system weakened by radiation and microgravity, rest assured, our old friend the herpes virus will be waiting for us.
Yes, it looks like frequent bathroom trips won’t be the only issue future Dr. McCoys will be treating. According to a study published in Frontiers in Microbiology, four of the eight herpes viruses were discovered in the saliva and urine of more than half of the hundred or so astronauts who had samples analyzed during spaceflight.
The good news is that only six astronauts actually had symptoms emerge, all of which were minor. The bad news is that the strength, frequency, and duration of viral shedding through urine and saliva increased as more time was spent in space. Also, only one of the herpes varieties found has a vaccine. The rest will just have to be treated as symptoms emerge.
We’ll just hope Captain Kirk doesn’t come down with a case of mononucleosis while fighting the Klingons. Damn it, Jim, I’m a doctor, not a miracle worker!

Sleep: the key to democracy?
You might need to lie down for this one. Insufficient sleep could be affecting the very institutions and behaviors that keep society from crumbling.
Lack of sleep is proven to affect “private” behaviors, like working, but a new study has shown that exhaustion also has consequences on a national scale. Sleeping less negatively affects the “social behaviors that hold society and democracy together,” according to the study’s authors.
Willingness to vote, donate to charities, and sign petitions decreased in sleep-deprived participants. Although that last one is iffy – is anyone really willing to sign a petition, regardless of sleep intake? One thing to glean from this study is, if you’re holding a clipboard, let the most tired-looking people keep walking.
Perhaps we should start a petition to make the day before Election Day a national napping holiday.
I hear you knocking, but you can’t come in
LOTME takes you to the ends of the earth for this edition of Bacteria vs. the World. Let’s go to our correspondent, Danielle Kang, on the International Space Station.
Hi, this is Briny Baird in Berlin, Germany. The space station’s extreme environment weakens astronauts’ immune systems, but it has the opposite effect on bacteria, as Elisabeth Grohmann, PhD, of Beuth University of Applied Sciences explains.
“The bacteria [astronauts] carry become hardier – developing thick protective coatings and resistance to antibiotics – and more vigorous, multiplying and metabolizing faster.”
This is, as scientists put it, bad. To prevent an on-board bacterial bacchanal, Dr. Grohmann and her associates covered a vital piece of ISS equipment, the toilet door, with a new coating containing “silver and ruthenium, conditioned by a vitamin derivative.” After 19 months in space, the coated section of the door had 80% fewer bacteria on it than an uncoated control surface.
With long-term missions to Mars being considered, it should be safer for astronauts and their toilet doors to … go where no one has gone before. Back to the studio.
The F-bomb diet
You might think vulgarities and Homo sapiens have been an inseparable pair since the first human bashed her thumb while chipping stones into spearheads to add meat to her diet. You’d probably be right – except for blue-tinged language built on the consonant foundations of the letters F and V.
New linguistics research finds that those $#%*! letters, like truckers and Marine gunnery sergeants, are relatively modern developments. As humans moved away from finger-harming hunting and gathering and toward finger-harming farming, they developed a taste for softer foods. And their speech picked up “labiodentals” such as F and V – which are sounds made when we touch our lower lips to our upper teeth. Half the world’s languages are now riddled with soft-food profanities, er, labiodentals.
Next on the linguists’ research list: Does the paleo diet lead to the dropping of fewer F-bombs?
Herpes! In space!
Viruses can never let bacteria have all the fun. Where there’s an immune system weakened by radiation and microgravity, rest assured, our old friend the herpes virus will be waiting for us.
Yes, it looks like frequent bathroom trips won’t be the only issue future Dr. McCoys will be treating. According to a study published in Frontiers in Microbiology, four of the eight herpes viruses were discovered in the saliva and urine of more than half of the hundred or so astronauts who had samples analyzed during spaceflight.
The good news is that only six astronauts actually had symptoms emerge, all of which were minor. The bad news is that the strength, frequency, and duration of viral shedding through urine and saliva increased as more time was spent in space. Also, only one of the herpes varieties found has a vaccine. The rest will just have to be treated as symptoms emerge.
We’ll just hope Captain Kirk doesn’t come down with a case of mononucleosis while fighting the Klingons. Damn it, Jim, I’m a doctor, not a miracle worker!

Sleep: the key to democracy?
You might need to lie down for this one. Insufficient sleep could be affecting the very institutions and behaviors that keep society from crumbling.
Lack of sleep is proven to affect “private” behaviors, like working, but a new study has shown that exhaustion also has consequences on a national scale. Sleeping less negatively affects the “social behaviors that hold society and democracy together,” according to the study’s authors.
Willingness to vote, donate to charities, and sign petitions decreased in sleep-deprived participants. Although that last one is iffy – is anyone really willing to sign a petition, regardless of sleep intake? One thing to glean from this study is, if you’re holding a clipboard, let the most tired-looking people keep walking.
Perhaps we should start a petition to make the day before Election Day a national napping holiday.
I hear you knocking, but you can’t come in
LOTME takes you to the ends of the earth for this edition of Bacteria vs. the World. Let’s go to our correspondent, Danielle Kang, on the International Space Station.
Hi, this is Briny Baird in Berlin, Germany. The space station’s extreme environment weakens astronauts’ immune systems, but it has the opposite effect on bacteria, as Elisabeth Grohmann, PhD, of Beuth University of Applied Sciences explains.
“The bacteria [astronauts] carry become hardier – developing thick protective coatings and resistance to antibiotics – and more vigorous, multiplying and metabolizing faster.”
This is, as scientists put it, bad. To prevent an on-board bacterial bacchanal, Dr. Grohmann and her associates covered a vital piece of ISS equipment, the toilet door, with a new coating containing “silver and ruthenium, conditioned by a vitamin derivative.” After 19 months in space, the coated section of the door had 80% fewer bacteria on it than an uncoated control surface.
With long-term missions to Mars being considered, it should be safer for astronauts and their toilet doors to … go where no one has gone before. Back to the studio.
The F-bomb diet
You might think vulgarities and Homo sapiens have been an inseparable pair since the first human bashed her thumb while chipping stones into spearheads to add meat to her diet. You’d probably be right – except for blue-tinged language built on the consonant foundations of the letters F and V.
New linguistics research finds that those $#%*! letters, like truckers and Marine gunnery sergeants, are relatively modern developments. As humans moved away from finger-harming hunting and gathering and toward finger-harming farming, they developed a taste for softer foods. And their speech picked up “labiodentals” such as F and V – which are sounds made when we touch our lower lips to our upper teeth. Half the world’s languages are now riddled with soft-food profanities, er, labiodentals.
Next on the linguists’ research list: Does the paleo diet lead to the dropping of fewer F-bombs?
Herpes! In space!
Viruses can never let bacteria have all the fun. Where there’s an immune system weakened by radiation and microgravity, rest assured, our old friend the herpes virus will be waiting for us.
Yes, it looks like frequent bathroom trips won’t be the only issue future Dr. McCoys will be treating. According to a study published in Frontiers in Microbiology, four of the eight herpes viruses were discovered in the saliva and urine of more than half of the hundred or so astronauts who had samples analyzed during spaceflight.
The good news is that only six astronauts actually had symptoms emerge, all of which were minor. The bad news is that the strength, frequency, and duration of viral shedding through urine and saliva increased as more time was spent in space. Also, only one of the herpes varieties found has a vaccine. The rest will just have to be treated as symptoms emerge.
We’ll just hope Captain Kirk doesn’t come down with a case of mononucleosis while fighting the Klingons. Damn it, Jim, I’m a doctor, not a miracle worker!

Spring
There is little to want for living in San Diego, America’s Finest City. The weather here is 72 and sunny year round. Yet, there are shortcomings. For one, there are no Forsythia. Forsythia are the deciduous shrubs that act as the harbingers of spring, blooming brilliant yellow across cold gray damp parts of the United States right now.
I grew up in New England where Forsythia bushes flower this time of year – a most welcome sign that the winter’s worst was over. Along with the purple crocus plants popping up in the warm bits of grass that breaks through the snow, seeing the Forsythia bloom always evoked that most appealing of feelings, hope. Hope that the discomfort of winter has passed. Hope that the beauty of nature will return. A promise that this year’s cycle of life will continue.
. A future with less suffering or with more joy. And as their doctors, we can help them get there.
A newly insured patient came to see me today. She had severe psoriasis. Her face, masked with red scaly patches, was heavy with the burden of the long winter she had endured. She was itchy and flaky and so embarrassed as to struggle to make eye contact with me. When I told her that we could help her, that there are treatments for her that would clear up the psoriasis and relieve her symptoms, she started to cry. Her husband intervened, apologizing for her. “I’m sorry. She has had this for so long, and you are the first person to tell her that she can get better. You have given us hope.”
When I walked back to my office I noticed the Rhode Island flag that I have mounted. Under the stars and blue anchor on it is the word “hope.” In 1664, when the state seal was created, it was the most important of ideas. It is why the settlers of Rhode Island risked their lives to cross an ocean to start anew, why my ancestors came from Italy two centuries later, why my parents sent me to college, why I decided to try for medicine. It is what most of us give every day. Hope, the ability to see into the future and bring that feeling back to the present. The belief that whatever and wherever you are, soon it will be even better. It cannot, however, be commanded. You can’t insist a patient hope any more than you can make them love. You must first understand what they see and feel, then show them how things might be better through trust.
Throughout life, hope creates possibilities. It unites us. It motivates us. It is the destroyer of winter and of burnout and of disease. It is one of the most important gifts that we give patients, and we do it everyday. Tomorrow in your practice, notice how often you foster it. Pay attention to how your patient changes the moment you give it to them. Watch the Forsythia bloom as you reassure them that their spring will return again.
“We must accept finite disappointment, but never lose infinite hope.” – Martin Luther King Jr.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
There is little to want for living in San Diego, America’s Finest City. The weather here is 72 and sunny year round. Yet, there are shortcomings. For one, there are no Forsythia. Forsythia are the deciduous shrubs that act as the harbingers of spring, blooming brilliant yellow across cold gray damp parts of the United States right now.
I grew up in New England where Forsythia bushes flower this time of year – a most welcome sign that the winter’s worst was over. Along with the purple crocus plants popping up in the warm bits of grass that breaks through the snow, seeing the Forsythia bloom always evoked that most appealing of feelings, hope. Hope that the discomfort of winter has passed. Hope that the beauty of nature will return. A promise that this year’s cycle of life will continue.
. A future with less suffering or with more joy. And as their doctors, we can help them get there.
A newly insured patient came to see me today. She had severe psoriasis. Her face, masked with red scaly patches, was heavy with the burden of the long winter she had endured. She was itchy and flaky and so embarrassed as to struggle to make eye contact with me. When I told her that we could help her, that there are treatments for her that would clear up the psoriasis and relieve her symptoms, she started to cry. Her husband intervened, apologizing for her. “I’m sorry. She has had this for so long, and you are the first person to tell her that she can get better. You have given us hope.”
When I walked back to my office I noticed the Rhode Island flag that I have mounted. Under the stars and blue anchor on it is the word “hope.” In 1664, when the state seal was created, it was the most important of ideas. It is why the settlers of Rhode Island risked their lives to cross an ocean to start anew, why my ancestors came from Italy two centuries later, why my parents sent me to college, why I decided to try for medicine. It is what most of us give every day. Hope, the ability to see into the future and bring that feeling back to the present. The belief that whatever and wherever you are, soon it will be even better. It cannot, however, be commanded. You can’t insist a patient hope any more than you can make them love. You must first understand what they see and feel, then show them how things might be better through trust.
Throughout life, hope creates possibilities. It unites us. It motivates us. It is the destroyer of winter and of burnout and of disease. It is one of the most important gifts that we give patients, and we do it everyday. Tomorrow in your practice, notice how often you foster it. Pay attention to how your patient changes the moment you give it to them. Watch the Forsythia bloom as you reassure them that their spring will return again.
“We must accept finite disappointment, but never lose infinite hope.” – Martin Luther King Jr.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
There is little to want for living in San Diego, America’s Finest City. The weather here is 72 and sunny year round. Yet, there are shortcomings. For one, there are no Forsythia. Forsythia are the deciduous shrubs that act as the harbingers of spring, blooming brilliant yellow across cold gray damp parts of the United States right now.
I grew up in New England where Forsythia bushes flower this time of year – a most welcome sign that the winter’s worst was over. Along with the purple crocus plants popping up in the warm bits of grass that breaks through the snow, seeing the Forsythia bloom always evoked that most appealing of feelings, hope. Hope that the discomfort of winter has passed. Hope that the beauty of nature will return. A promise that this year’s cycle of life will continue.
. A future with less suffering or with more joy. And as their doctors, we can help them get there.
A newly insured patient came to see me today. She had severe psoriasis. Her face, masked with red scaly patches, was heavy with the burden of the long winter she had endured. She was itchy and flaky and so embarrassed as to struggle to make eye contact with me. When I told her that we could help her, that there are treatments for her that would clear up the psoriasis and relieve her symptoms, she started to cry. Her husband intervened, apologizing for her. “I’m sorry. She has had this for so long, and you are the first person to tell her that she can get better. You have given us hope.”
When I walked back to my office I noticed the Rhode Island flag that I have mounted. Under the stars and blue anchor on it is the word “hope.” In 1664, when the state seal was created, it was the most important of ideas. It is why the settlers of Rhode Island risked their lives to cross an ocean to start anew, why my ancestors came from Italy two centuries later, why my parents sent me to college, why I decided to try for medicine. It is what most of us give every day. Hope, the ability to see into the future and bring that feeling back to the present. The belief that whatever and wherever you are, soon it will be even better. It cannot, however, be commanded. You can’t insist a patient hope any more than you can make them love. You must first understand what they see and feel, then show them how things might be better through trust.
Throughout life, hope creates possibilities. It unites us. It motivates us. It is the destroyer of winter and of burnout and of disease. It is one of the most important gifts that we give patients, and we do it everyday. Tomorrow in your practice, notice how often you foster it. Pay attention to how your patient changes the moment you give it to them. Watch the Forsythia bloom as you reassure them that their spring will return again.
“We must accept finite disappointment, but never lose infinite hope.” – Martin Luther King Jr.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Challenges in outpatient psychiatry
The patient who shuns our recommendations can be very frustrating to treat
Editor’s Note: This is the first in a series of articles by Dr. Miller about challenges in outpatient psychiatry.
“I don’t understand why it’s so hard for me to get up and get started in the mornings,” my patient said. She was on the verge of losing her job for repeatedly being late to work.
“I really believe you’d have an easier time if you didn’t drink every night then start your mornings with that wake-up shot of gin,” I replied.
“Here we go again,” she said, exasperated with me.
It was a conversation that has been going on for years (literally) and with which we have both been quite frustrated. My patient wants to get better, she asks for my help, and then she refuses any solutions I might suggest.
Patients with addictions have their own challenges; this patient does not want to stop drinking and all my efforts to suggest that alcohol is part of the problem simply don’t matter. When it became clear that it was not simply a matter of her willingness to believe that alcohol was a problem, I made stronger suggestions. She should try a 12-step program, and when that recommendation was scorned, I insisted she go to an outpatient intensive program and pressed the name and phone number into her palm. There have been prescriptions for oral naltrexone, which initially was helpful until the patient stopped taking it, and an offer for a topiramate trial. At the suggestion of a colleague, I even offered to go with her to an intake for substance use treatment. None of my efforts, however, change the fact that this patient does not want these interventions, and our treatment plans don’t seem to converge.
Patients with addictions are often complex, and the forces in play are more than simply the agreement that a problem exists and a desire to stop. Patients who want – yet don’t want – help come in many varieties. We’ve all sat with patients who refuse to take the medications we prescribe or who come back appointment after appointment still never having turned on the lightbox we convinced them to purchase. There are patients who are miserably troubled by being overweight but are not open to changing their diet, exercising, joining a weight-loss group, taking medications, or having bariatric surgery. There are patients who insist they can’t stop smoking but won’t give any of the available treatments a try. There are those we badger to get the lab work that make it safe for them to continue on the medications we prescribe. There are moments when we may wonder what more we have to offer patients who either can’t or won’t follow our suggestions, what may have once been considered “doctor’s orders.”
These are the patients we’ve referred to as “noncompliant.” It’s a term that recently has been questioned, and one that implies a willful refusal to abide by a physician’s decision. Often, it’s worth exploring what holds them back from following our directions. The term noncompliance implies that the person is a bad patient and it does not leave room for the idea that the patient may not agree with the diagnosis or treatment plan or is unwilling to accept the cost or risk of the prescribed solution. Sometimes, understanding what leads a patient to resist can be helpful; unwarranted fears can be addressed and logistical barriers may be overcome.
In some cases, these patient encounters may leave us feeling helpless and worried. In the worst of these cases, where a patient’s refusal to follow through with treatment suggestions may leave them vulnerable to very bad outcomes, I personally have had times when I’ve felt like I’m standing by helplessly watching as a Mack truck speeds into a brick wall.
“In my experience noncompliance is almost always a sign of deeper resistance or ambivalence that needs to be explored,” notes Neha Jain, MD, a geriatric psychiatrist at the University of Connecticut, Farmington.
Dr. Jain communicated with me about patients who don’t want to take the medications she recommends. “A typical scenario is when a patient comes to see me, but says that either they don’t believe in medications or are extraordinarily sensitive to them. I almost always offer medication as a choice and am quick to offer not taking medication as an option for those who are safe. If they refuse, I’ll review their goals and talk about why they came to see me, a primarily prescribing psychiatrist. This often leads to a deeper discussion of why they are resistant to meds, ranging from the stigma of ‘taking a crazy pill’ to what taking a medication means for their ego. For these patients I might offer a few ‘consulting’ sessions, which are really therapy sessions. Often they either become receptive to taking medicines, or they may continue with therapy alone, either with me or with someone else.”
Peter D. Kramer, MD, a psychiatrist in Providence, R.I., and author of “Ordinarily Well: The Case for Antidepressants,” says that the issues are even more complex when patients hide that they have not complied with the psychiatrist’s recommendations. “It seems to me that noncompliance that remains secret, not discussed with the therapist and then discovered incidentally or belatedly, presents an occasion to consider the success of the therapeutic alliance – in older terms, to think more about the transference.”
Dr. Kramer notes that, as our approach to psychotherapy has changed, the psychiatrist’s response to such behavior has also changed. “The prevailing focus on cognitive therapies assumes that when patients realize that a belief or behavior is illogical, they will correct it. When psychotherapy was more analytic, it focused on the reasons patients engaged in irrational and self-destructive acts repeatedly – and patients’ failure to self-correct didn’t frustrate the therapist so much. Instead you thought, ‘If education worked, I’d be out of a job.’ We deal with failures to trust. We deal with what philosophers call weakness of will. While I can’t say that I am never frustrated or surprised, I do see working with these problems as the reason I am there.”
Sometimes we learn that the patient who dismisses our suggestions has already tried the remedies we are suggesting and is just as frustrated as we are. We may be left with the unfortunate situation that nothing we do seems to foster meaningful change for the patient. In these instances it may be helpful to clarify the goals of treatment and inquire whether he feels he is making progress. We may consider trying other forms of treatment, consultation with another clinician, or more intensive therapy if the patient will agree. Other times, we may be left to rethink our treatment, consider the ways in which the patient does find the treatment helpful, and empathize with our patient’s distress while continuing to gently suggest that there might be options available whenever they feel ready.
So my patient did lose her job. She found another position with more flexible hours and, despite her heavy drinking, her life has gone mostly well. She comes to see me only rarely because the medication I prescribe helps stabilize her mood, but she stopped scheduling her regular psychotherapy sessions. Still, while she manages her life around her drinking, I worry about the toll it is taking, as there has been ample evidence that her body cannot sustain this for much longer. From what I can tell, the fact that I remain available is helpful to both the patient and her family but yes, it’s a little like standing by helplessly and watching a Mack truck race toward a brick wall.
Dr. Miller is the coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016) and has a private practice in Baltimore. Patient details were altered to preserve confidentiality.
The patient who shuns our recommendations can be very frustrating to treat
The patient who shuns our recommendations can be very frustrating to treat
Editor’s Note: This is the first in a series of articles by Dr. Miller about challenges in outpatient psychiatry.
“I don’t understand why it’s so hard for me to get up and get started in the mornings,” my patient said. She was on the verge of losing her job for repeatedly being late to work.
“I really believe you’d have an easier time if you didn’t drink every night then start your mornings with that wake-up shot of gin,” I replied.
“Here we go again,” she said, exasperated with me.
It was a conversation that has been going on for years (literally) and with which we have both been quite frustrated. My patient wants to get better, she asks for my help, and then she refuses any solutions I might suggest.
Patients with addictions have their own challenges; this patient does not want to stop drinking and all my efforts to suggest that alcohol is part of the problem simply don’t matter. When it became clear that it was not simply a matter of her willingness to believe that alcohol was a problem, I made stronger suggestions. She should try a 12-step program, and when that recommendation was scorned, I insisted she go to an outpatient intensive program and pressed the name and phone number into her palm. There have been prescriptions for oral naltrexone, which initially was helpful until the patient stopped taking it, and an offer for a topiramate trial. At the suggestion of a colleague, I even offered to go with her to an intake for substance use treatment. None of my efforts, however, change the fact that this patient does not want these interventions, and our treatment plans don’t seem to converge.
Patients with addictions are often complex, and the forces in play are more than simply the agreement that a problem exists and a desire to stop. Patients who want – yet don’t want – help come in many varieties. We’ve all sat with patients who refuse to take the medications we prescribe or who come back appointment after appointment still never having turned on the lightbox we convinced them to purchase. There are patients who are miserably troubled by being overweight but are not open to changing their diet, exercising, joining a weight-loss group, taking medications, or having bariatric surgery. There are patients who insist they can’t stop smoking but won’t give any of the available treatments a try. There are those we badger to get the lab work that make it safe for them to continue on the medications we prescribe. There are moments when we may wonder what more we have to offer patients who either can’t or won’t follow our suggestions, what may have once been considered “doctor’s orders.”
These are the patients we’ve referred to as “noncompliant.” It’s a term that recently has been questioned, and one that implies a willful refusal to abide by a physician’s decision. Often, it’s worth exploring what holds them back from following our directions. The term noncompliance implies that the person is a bad patient and it does not leave room for the idea that the patient may not agree with the diagnosis or treatment plan or is unwilling to accept the cost or risk of the prescribed solution. Sometimes, understanding what leads a patient to resist can be helpful; unwarranted fears can be addressed and logistical barriers may be overcome.
In some cases, these patient encounters may leave us feeling helpless and worried. In the worst of these cases, where a patient’s refusal to follow through with treatment suggestions may leave them vulnerable to very bad outcomes, I personally have had times when I’ve felt like I’m standing by helplessly watching as a Mack truck speeds into a brick wall.
“In my experience noncompliance is almost always a sign of deeper resistance or ambivalence that needs to be explored,” notes Neha Jain, MD, a geriatric psychiatrist at the University of Connecticut, Farmington.
Dr. Jain communicated with me about patients who don’t want to take the medications she recommends. “A typical scenario is when a patient comes to see me, but says that either they don’t believe in medications or are extraordinarily sensitive to them. I almost always offer medication as a choice and am quick to offer not taking medication as an option for those who are safe. If they refuse, I’ll review their goals and talk about why they came to see me, a primarily prescribing psychiatrist. This often leads to a deeper discussion of why they are resistant to meds, ranging from the stigma of ‘taking a crazy pill’ to what taking a medication means for their ego. For these patients I might offer a few ‘consulting’ sessions, which are really therapy sessions. Often they either become receptive to taking medicines, or they may continue with therapy alone, either with me or with someone else.”
Peter D. Kramer, MD, a psychiatrist in Providence, R.I., and author of “Ordinarily Well: The Case for Antidepressants,” says that the issues are even more complex when patients hide that they have not complied with the psychiatrist’s recommendations. “It seems to me that noncompliance that remains secret, not discussed with the therapist and then discovered incidentally or belatedly, presents an occasion to consider the success of the therapeutic alliance – in older terms, to think more about the transference.”
Dr. Kramer notes that, as our approach to psychotherapy has changed, the psychiatrist’s response to such behavior has also changed. “The prevailing focus on cognitive therapies assumes that when patients realize that a belief or behavior is illogical, they will correct it. When psychotherapy was more analytic, it focused on the reasons patients engaged in irrational and self-destructive acts repeatedly – and patients’ failure to self-correct didn’t frustrate the therapist so much. Instead you thought, ‘If education worked, I’d be out of a job.’ We deal with failures to trust. We deal with what philosophers call weakness of will. While I can’t say that I am never frustrated or surprised, I do see working with these problems as the reason I am there.”
Sometimes we learn that the patient who dismisses our suggestions has already tried the remedies we are suggesting and is just as frustrated as we are. We may be left with the unfortunate situation that nothing we do seems to foster meaningful change for the patient. In these instances it may be helpful to clarify the goals of treatment and inquire whether he feels he is making progress. We may consider trying other forms of treatment, consultation with another clinician, or more intensive therapy if the patient will agree. Other times, we may be left to rethink our treatment, consider the ways in which the patient does find the treatment helpful, and empathize with our patient’s distress while continuing to gently suggest that there might be options available whenever they feel ready.
So my patient did lose her job. She found another position with more flexible hours and, despite her heavy drinking, her life has gone mostly well. She comes to see me only rarely because the medication I prescribe helps stabilize her mood, but she stopped scheduling her regular psychotherapy sessions. Still, while she manages her life around her drinking, I worry about the toll it is taking, as there has been ample evidence that her body cannot sustain this for much longer. From what I can tell, the fact that I remain available is helpful to both the patient and her family but yes, it’s a little like standing by helplessly and watching a Mack truck race toward a brick wall.
Dr. Miller is the coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016) and has a private practice in Baltimore. Patient details were altered to preserve confidentiality.
Editor’s Note: This is the first in a series of articles by Dr. Miller about challenges in outpatient psychiatry.
“I don’t understand why it’s so hard for me to get up and get started in the mornings,” my patient said. She was on the verge of losing her job for repeatedly being late to work.
“I really believe you’d have an easier time if you didn’t drink every night then start your mornings with that wake-up shot of gin,” I replied.
“Here we go again,” she said, exasperated with me.
It was a conversation that has been going on for years (literally) and with which we have both been quite frustrated. My patient wants to get better, she asks for my help, and then she refuses any solutions I might suggest.
Patients with addictions have their own challenges; this patient does not want to stop drinking and all my efforts to suggest that alcohol is part of the problem simply don’t matter. When it became clear that it was not simply a matter of her willingness to believe that alcohol was a problem, I made stronger suggestions. She should try a 12-step program, and when that recommendation was scorned, I insisted she go to an outpatient intensive program and pressed the name and phone number into her palm. There have been prescriptions for oral naltrexone, which initially was helpful until the patient stopped taking it, and an offer for a topiramate trial. At the suggestion of a colleague, I even offered to go with her to an intake for substance use treatment. None of my efforts, however, change the fact that this patient does not want these interventions, and our treatment plans don’t seem to converge.
Patients with addictions are often complex, and the forces in play are more than simply the agreement that a problem exists and a desire to stop. Patients who want – yet don’t want – help come in many varieties. We’ve all sat with patients who refuse to take the medications we prescribe or who come back appointment after appointment still never having turned on the lightbox we convinced them to purchase. There are patients who are miserably troubled by being overweight but are not open to changing their diet, exercising, joining a weight-loss group, taking medications, or having bariatric surgery. There are patients who insist they can’t stop smoking but won’t give any of the available treatments a try. There are those we badger to get the lab work that make it safe for them to continue on the medications we prescribe. There are moments when we may wonder what more we have to offer patients who either can’t or won’t follow our suggestions, what may have once been considered “doctor’s orders.”
These are the patients we’ve referred to as “noncompliant.” It’s a term that recently has been questioned, and one that implies a willful refusal to abide by a physician’s decision. Often, it’s worth exploring what holds them back from following our directions. The term noncompliance implies that the person is a bad patient and it does not leave room for the idea that the patient may not agree with the diagnosis or treatment plan or is unwilling to accept the cost or risk of the prescribed solution. Sometimes, understanding what leads a patient to resist can be helpful; unwarranted fears can be addressed and logistical barriers may be overcome.
In some cases, these patient encounters may leave us feeling helpless and worried. In the worst of these cases, where a patient’s refusal to follow through with treatment suggestions may leave them vulnerable to very bad outcomes, I personally have had times when I’ve felt like I’m standing by helplessly watching as a Mack truck speeds into a brick wall.
“In my experience noncompliance is almost always a sign of deeper resistance or ambivalence that needs to be explored,” notes Neha Jain, MD, a geriatric psychiatrist at the University of Connecticut, Farmington.
Dr. Jain communicated with me about patients who don’t want to take the medications she recommends. “A typical scenario is when a patient comes to see me, but says that either they don’t believe in medications or are extraordinarily sensitive to them. I almost always offer medication as a choice and am quick to offer not taking medication as an option for those who are safe. If they refuse, I’ll review their goals and talk about why they came to see me, a primarily prescribing psychiatrist. This often leads to a deeper discussion of why they are resistant to meds, ranging from the stigma of ‘taking a crazy pill’ to what taking a medication means for their ego. For these patients I might offer a few ‘consulting’ sessions, which are really therapy sessions. Often they either become receptive to taking medicines, or they may continue with therapy alone, either with me or with someone else.”
Peter D. Kramer, MD, a psychiatrist in Providence, R.I., and author of “Ordinarily Well: The Case for Antidepressants,” says that the issues are even more complex when patients hide that they have not complied with the psychiatrist’s recommendations. “It seems to me that noncompliance that remains secret, not discussed with the therapist and then discovered incidentally or belatedly, presents an occasion to consider the success of the therapeutic alliance – in older terms, to think more about the transference.”
Dr. Kramer notes that, as our approach to psychotherapy has changed, the psychiatrist’s response to such behavior has also changed. “The prevailing focus on cognitive therapies assumes that when patients realize that a belief or behavior is illogical, they will correct it. When psychotherapy was more analytic, it focused on the reasons patients engaged in irrational and self-destructive acts repeatedly – and patients’ failure to self-correct didn’t frustrate the therapist so much. Instead you thought, ‘If education worked, I’d be out of a job.’ We deal with failures to trust. We deal with what philosophers call weakness of will. While I can’t say that I am never frustrated or surprised, I do see working with these problems as the reason I am there.”
Sometimes we learn that the patient who dismisses our suggestions has already tried the remedies we are suggesting and is just as frustrated as we are. We may be left with the unfortunate situation that nothing we do seems to foster meaningful change for the patient. In these instances it may be helpful to clarify the goals of treatment and inquire whether he feels he is making progress. We may consider trying other forms of treatment, consultation with another clinician, or more intensive therapy if the patient will agree. Other times, we may be left to rethink our treatment, consider the ways in which the patient does find the treatment helpful, and empathize with our patient’s distress while continuing to gently suggest that there might be options available whenever they feel ready.
So my patient did lose her job. She found another position with more flexible hours and, despite her heavy drinking, her life has gone mostly well. She comes to see me only rarely because the medication I prescribe helps stabilize her mood, but she stopped scheduling her regular psychotherapy sessions. Still, while she manages her life around her drinking, I worry about the toll it is taking, as there has been ample evidence that her body cannot sustain this for much longer. From what I can tell, the fact that I remain available is helpful to both the patient and her family but yes, it’s a little like standing by helplessly and watching a Mack truck race toward a brick wall.
Dr. Miller is the coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016) and has a private practice in Baltimore. Patient details were altered to preserve confidentiality.
When to Start Dialysis
Q) I sent a patient with a glomerular filtration rate (GFR) of 15 mL/min to nephrology to start dialysis. He came back to me and said they don’t start dialysis at 15. When do you start? Why?
There is considerable variation in the timing of dialysis initiation. Research suggests that sometimes earlier is not better.
IDEAL, a randomized controlled trial conducted in Australia and New Zealand, evaluated the advantages and disadvantages of earlier versus later dialysis initiation.1 Patients were randomly assigned to start any type of dialysis when their GFR was 8 or 11 mL/min. The results indicated that starting dialysis in a patient with a higher GFR did not lower the mortality or morbidity rate but did increase costs and complications (mostly for vascular access).1
Based on these findings, most of us start dialysis in a patient who has a GFR < 10 mL/min and symptoms of kidney failure. These include a metallic taste in mouth, weight gain (usually due to edema) or loss (cachexia), feeling “poorly,” hard-to-control hypertension, shortness of breath, confusion (uremic brain), odor, skin color changes, and insomnia. Symptomatic patients can be started on dialysis at a higher GFR (usually ≤ 18 mL/min), but there are many hoops to jump through with Medicare.
However, IDEAL was conducted outside the United States and included very few elderly (age > 75) patients with chronic kidney disease. In 2018, Kurella and colleagues published a study that analyzed age and kidney function in a US veteran population.2 Their results showed that age should be included in the “when to start dialysis” calculation. For older veterans, starting dialysis earlier—at a GFR of 10 mL/min—increased survival. However, the researchers pointed out that in this age group, survival is in months (not years) and does not necessarily equate to quality of life.
In conclusion, there is no compelling evidence that initiation of dialysis based solely on measurement of kidney function leads to improvement in clinical outcomes. In otherwise asymptomatic patients, there is no reason to begin dialysis based solely on GFR; age and fragility need to be considered in the equation. Earlier is not always better, and for the elderly patient with multiple comorbidities, dialysis is not always a better choice. —TH
Tricia Howard, MHS, PA-C, DFAAPA
Georgia Regional Medical Team, Savannah
1. Cooper BA, Branley P, Bulfone L, et al; for the IDEAL Trial. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609-619.
2. Kurella Tamura M, Desai M, Kapphahn KI, et al. Dialysis versus medical management at different ages and levels of kidney function in veterans with advanced CKD. J Am Soc Nephrol. 2018;29(8):2169-2177.
Q) I sent a patient with a glomerular filtration rate (GFR) of 15 mL/min to nephrology to start dialysis. He came back to me and said they don’t start dialysis at 15. When do you start? Why?
There is considerable variation in the timing of dialysis initiation. Research suggests that sometimes earlier is not better.
IDEAL, a randomized controlled trial conducted in Australia and New Zealand, evaluated the advantages and disadvantages of earlier versus later dialysis initiation.1 Patients were randomly assigned to start any type of dialysis when their GFR was 8 or 11 mL/min. The results indicated that starting dialysis in a patient with a higher GFR did not lower the mortality or morbidity rate but did increase costs and complications (mostly for vascular access).1
Based on these findings, most of us start dialysis in a patient who has a GFR < 10 mL/min and symptoms of kidney failure. These include a metallic taste in mouth, weight gain (usually due to edema) or loss (cachexia), feeling “poorly,” hard-to-control hypertension, shortness of breath, confusion (uremic brain), odor, skin color changes, and insomnia. Symptomatic patients can be started on dialysis at a higher GFR (usually ≤ 18 mL/min), but there are many hoops to jump through with Medicare.
However, IDEAL was conducted outside the United States and included very few elderly (age > 75) patients with chronic kidney disease. In 2018, Kurella and colleagues published a study that analyzed age and kidney function in a US veteran population.2 Their results showed that age should be included in the “when to start dialysis” calculation. For older veterans, starting dialysis earlier—at a GFR of 10 mL/min—increased survival. However, the researchers pointed out that in this age group, survival is in months (not years) and does not necessarily equate to quality of life.
In conclusion, there is no compelling evidence that initiation of dialysis based solely on measurement of kidney function leads to improvement in clinical outcomes. In otherwise asymptomatic patients, there is no reason to begin dialysis based solely on GFR; age and fragility need to be considered in the equation. Earlier is not always better, and for the elderly patient with multiple comorbidities, dialysis is not always a better choice. —TH
Tricia Howard, MHS, PA-C, DFAAPA
Georgia Regional Medical Team, Savannah
Q) I sent a patient with a glomerular filtration rate (GFR) of 15 mL/min to nephrology to start dialysis. He came back to me and said they don’t start dialysis at 15. When do you start? Why?
There is considerable variation in the timing of dialysis initiation. Research suggests that sometimes earlier is not better.
IDEAL, a randomized controlled trial conducted in Australia and New Zealand, evaluated the advantages and disadvantages of earlier versus later dialysis initiation.1 Patients were randomly assigned to start any type of dialysis when their GFR was 8 or 11 mL/min. The results indicated that starting dialysis in a patient with a higher GFR did not lower the mortality or morbidity rate but did increase costs and complications (mostly for vascular access).1
Based on these findings, most of us start dialysis in a patient who has a GFR < 10 mL/min and symptoms of kidney failure. These include a metallic taste in mouth, weight gain (usually due to edema) or loss (cachexia), feeling “poorly,” hard-to-control hypertension, shortness of breath, confusion (uremic brain), odor, skin color changes, and insomnia. Symptomatic patients can be started on dialysis at a higher GFR (usually ≤ 18 mL/min), but there are many hoops to jump through with Medicare.
However, IDEAL was conducted outside the United States and included very few elderly (age > 75) patients with chronic kidney disease. In 2018, Kurella and colleagues published a study that analyzed age and kidney function in a US veteran population.2 Their results showed that age should be included in the “when to start dialysis” calculation. For older veterans, starting dialysis earlier—at a GFR of 10 mL/min—increased survival. However, the researchers pointed out that in this age group, survival is in months (not years) and does not necessarily equate to quality of life.
In conclusion, there is no compelling evidence that initiation of dialysis based solely on measurement of kidney function leads to improvement in clinical outcomes. In otherwise asymptomatic patients, there is no reason to begin dialysis based solely on GFR; age and fragility need to be considered in the equation. Earlier is not always better, and for the elderly patient with multiple comorbidities, dialysis is not always a better choice. —TH
Tricia Howard, MHS, PA-C, DFAAPA
Georgia Regional Medical Team, Savannah
1. Cooper BA, Branley P, Bulfone L, et al; for the IDEAL Trial. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609-619.
2. Kurella Tamura M, Desai M, Kapphahn KI, et al. Dialysis versus medical management at different ages and levels of kidney function in veterans with advanced CKD. J Am Soc Nephrol. 2018;29(8):2169-2177.
1. Cooper BA, Branley P, Bulfone L, et al; for the IDEAL Trial. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609-619.
2. Kurella Tamura M, Desai M, Kapphahn KI, et al. Dialysis versus medical management at different ages and levels of kidney function in veterans with advanced CKD. J Am Soc Nephrol. 2018;29(8):2169-2177.
Industry-funded rheumatology RCTs are higher quality
MAUI, HAWAII – Industry-funded randomized, controlled clinical trials published in the three top-rated rheumatology journals during the past 20 years are of significantly higher overall quality than the nonindustry-funded ones, Michael Putman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
Dr. Putman, a second-year rheumatology fellow at Northwestern University, Chicago, analyzed all randomized, controlled trials (RCTs) of pharmacotherapy featuring a comparator – either placebo or an active agent – published in 1998, 2008, and 2018 in Annals of the Rheumatic Diseases, Rheumatology, and Arthritis & Rheumatology.
His main takeaway: “Rheumatologic interventions seem to work pretty well. The mean absolute risk reduction in the trials is 17.5%, so the average number of patients who need to be treated with a rheumatologic intervention is about five. This is why it’s such a great specialty to be a part of: A lot of our patients get better.”
He created an RCT quality rating scale that captured the strength of study design, methodology, and findings based upon whether a randomized trial used a double-blind design; identified a prespecified primary outcome; and featured patient-reported outcomes, power calculations, sensitivity analysis, adjustment for multiple hypotheses, and intention-to-treat analysis. He then applied the rating scale to the 85 published RCTs in the three study years.
Of note, 84% of the trials published in 2018 were industry funded, up from 74% in 2008 and 1998.
“Industry funds the vast majority of studies. Industry studies are significantly more likely to be appropriately double blinded, report patient-reported outcome measures, use intention to treat, and they have a higher overall quality,” according to Dr. Putman.
Indeed, the industry-funded studies averaged a 66% score on his quality grading scale, compared with 45% for nonindustry-funded studies.
Utilization of most of the quality metrics remained stable over time. The exceptions: Incorporation of intent-to-treat analysis increased from 58% in 1998 to 87% in 2018, and sensitivity analysis was employed in just 5% of the trials published in 1998, compared with 37% in 2008 and 26% in 2018.
The most important change over the past 2 decades, in his view, has been the shrinking proportion of RCTs featuring an active-drug, head-to-head comparator arm. In 1998, 42% of studies featured that design; for example, comparing methotrexate to sulfasalazine. By 2018, that figure had dropped to just 13%.
“Most of our trials today compare an active compound, such an interleukin-17 inhibitor, to a placebo. I think that’s a big change in how we do things,” Dr. Putman observed. “With 84% of our studies being funded by industry, the incentives in medicine right now don’t support active comparator research. It’s harder to show a difference between two things that work than it is to show a difference between something and nothing.”
However, he’d welcome a revival of head-to-head active comparator trials.
“I’d really love to have that happen,” he said. “We have basic questions we haven’t answered yet about a lot of our basic drugs: Like in myositis, should you start with Imuran [azathioprine], CellCept [mycophenolate mofetil], or methotrexate?”
Another striking change over time has been the dwindling proportion of published trials with a statistically significant finding for the primary outcome: 79% in 1998, 46% in 2008, and 36% last year. Dr. Putman suspects the explanation lies in the steady improvement in the effectiveness of standard background therapy for many conditions, which makes it tougher to show a striking difference between the add-on study drug and add-on placebo.
“We’re a victim of our own success,” he commented.
In any event, many key secondary outcomes in the RCTs were positive, even when the primary endpoint wasn’t, according to Dr. Putman, and there was a notable dearth of completely negative clinical RCTs published in the three top journals.
“The more cynical interpretation is there’s an incredible amount of publication bias, where we’re only publishing studies that show an effect and the journals or investigators are censoring the ones that don’t. The more charitable explanation, which is probably also true, is that by the time you get to putting on an RCT you kind of think, ‘This thing works.’ You’re not testing random stuff, so your pretest probability of a drug being effective when it enters into an RCT is probably shifted toward effectiveness,” Dr. Putman speculated.
He reported having no financial conflicts regarding his study.
MAUI, HAWAII – Industry-funded randomized, controlled clinical trials published in the three top-rated rheumatology journals during the past 20 years are of significantly higher overall quality than the nonindustry-funded ones, Michael Putman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
Dr. Putman, a second-year rheumatology fellow at Northwestern University, Chicago, analyzed all randomized, controlled trials (RCTs) of pharmacotherapy featuring a comparator – either placebo or an active agent – published in 1998, 2008, and 2018 in Annals of the Rheumatic Diseases, Rheumatology, and Arthritis & Rheumatology.
His main takeaway: “Rheumatologic interventions seem to work pretty well. The mean absolute risk reduction in the trials is 17.5%, so the average number of patients who need to be treated with a rheumatologic intervention is about five. This is why it’s such a great specialty to be a part of: A lot of our patients get better.”
He created an RCT quality rating scale that captured the strength of study design, methodology, and findings based upon whether a randomized trial used a double-blind design; identified a prespecified primary outcome; and featured patient-reported outcomes, power calculations, sensitivity analysis, adjustment for multiple hypotheses, and intention-to-treat analysis. He then applied the rating scale to the 85 published RCTs in the three study years.
Of note, 84% of the trials published in 2018 were industry funded, up from 74% in 2008 and 1998.
“Industry funds the vast majority of studies. Industry studies are significantly more likely to be appropriately double blinded, report patient-reported outcome measures, use intention to treat, and they have a higher overall quality,” according to Dr. Putman.
Indeed, the industry-funded studies averaged a 66% score on his quality grading scale, compared with 45% for nonindustry-funded studies.
Utilization of most of the quality metrics remained stable over time. The exceptions: Incorporation of intent-to-treat analysis increased from 58% in 1998 to 87% in 2018, and sensitivity analysis was employed in just 5% of the trials published in 1998, compared with 37% in 2008 and 26% in 2018.
The most important change over the past 2 decades, in his view, has been the shrinking proportion of RCTs featuring an active-drug, head-to-head comparator arm. In 1998, 42% of studies featured that design; for example, comparing methotrexate to sulfasalazine. By 2018, that figure had dropped to just 13%.
“Most of our trials today compare an active compound, such an interleukin-17 inhibitor, to a placebo. I think that’s a big change in how we do things,” Dr. Putman observed. “With 84% of our studies being funded by industry, the incentives in medicine right now don’t support active comparator research. It’s harder to show a difference between two things that work than it is to show a difference between something and nothing.”
However, he’d welcome a revival of head-to-head active comparator trials.
“I’d really love to have that happen,” he said. “We have basic questions we haven’t answered yet about a lot of our basic drugs: Like in myositis, should you start with Imuran [azathioprine], CellCept [mycophenolate mofetil], or methotrexate?”
Another striking change over time has been the dwindling proportion of published trials with a statistically significant finding for the primary outcome: 79% in 1998, 46% in 2008, and 36% last year. Dr. Putman suspects the explanation lies in the steady improvement in the effectiveness of standard background therapy for many conditions, which makes it tougher to show a striking difference between the add-on study drug and add-on placebo.
“We’re a victim of our own success,” he commented.
In any event, many key secondary outcomes in the RCTs were positive, even when the primary endpoint wasn’t, according to Dr. Putman, and there was a notable dearth of completely negative clinical RCTs published in the three top journals.
“The more cynical interpretation is there’s an incredible amount of publication bias, where we’re only publishing studies that show an effect and the journals or investigators are censoring the ones that don’t. The more charitable explanation, which is probably also true, is that by the time you get to putting on an RCT you kind of think, ‘This thing works.’ You’re not testing random stuff, so your pretest probability of a drug being effective when it enters into an RCT is probably shifted toward effectiveness,” Dr. Putman speculated.
He reported having no financial conflicts regarding his study.
MAUI, HAWAII – Industry-funded randomized, controlled clinical trials published in the three top-rated rheumatology journals during the past 20 years are of significantly higher overall quality than the nonindustry-funded ones, Michael Putman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
Dr. Putman, a second-year rheumatology fellow at Northwestern University, Chicago, analyzed all randomized, controlled trials (RCTs) of pharmacotherapy featuring a comparator – either placebo or an active agent – published in 1998, 2008, and 2018 in Annals of the Rheumatic Diseases, Rheumatology, and Arthritis & Rheumatology.
His main takeaway: “Rheumatologic interventions seem to work pretty well. The mean absolute risk reduction in the trials is 17.5%, so the average number of patients who need to be treated with a rheumatologic intervention is about five. This is why it’s such a great specialty to be a part of: A lot of our patients get better.”
He created an RCT quality rating scale that captured the strength of study design, methodology, and findings based upon whether a randomized trial used a double-blind design; identified a prespecified primary outcome; and featured patient-reported outcomes, power calculations, sensitivity analysis, adjustment for multiple hypotheses, and intention-to-treat analysis. He then applied the rating scale to the 85 published RCTs in the three study years.
Of note, 84% of the trials published in 2018 were industry funded, up from 74% in 2008 and 1998.
“Industry funds the vast majority of studies. Industry studies are significantly more likely to be appropriately double blinded, report patient-reported outcome measures, use intention to treat, and they have a higher overall quality,” according to Dr. Putman.
Indeed, the industry-funded studies averaged a 66% score on his quality grading scale, compared with 45% for nonindustry-funded studies.
Utilization of most of the quality metrics remained stable over time. The exceptions: Incorporation of intent-to-treat analysis increased from 58% in 1998 to 87% in 2018, and sensitivity analysis was employed in just 5% of the trials published in 1998, compared with 37% in 2008 and 26% in 2018.
The most important change over the past 2 decades, in his view, has been the shrinking proportion of RCTs featuring an active-drug, head-to-head comparator arm. In 1998, 42% of studies featured that design; for example, comparing methotrexate to sulfasalazine. By 2018, that figure had dropped to just 13%.
“Most of our trials today compare an active compound, such an interleukin-17 inhibitor, to a placebo. I think that’s a big change in how we do things,” Dr. Putman observed. “With 84% of our studies being funded by industry, the incentives in medicine right now don’t support active comparator research. It’s harder to show a difference between two things that work than it is to show a difference between something and nothing.”
However, he’d welcome a revival of head-to-head active comparator trials.
“I’d really love to have that happen,” he said. “We have basic questions we haven’t answered yet about a lot of our basic drugs: Like in myositis, should you start with Imuran [azathioprine], CellCept [mycophenolate mofetil], or methotrexate?”
Another striking change over time has been the dwindling proportion of published trials with a statistically significant finding for the primary outcome: 79% in 1998, 46% in 2008, and 36% last year. Dr. Putman suspects the explanation lies in the steady improvement in the effectiveness of standard background therapy for many conditions, which makes it tougher to show a striking difference between the add-on study drug and add-on placebo.
“We’re a victim of our own success,” he commented.
In any event, many key secondary outcomes in the RCTs were positive, even when the primary endpoint wasn’t, according to Dr. Putman, and there was a notable dearth of completely negative clinical RCTs published in the three top journals.
“The more cynical interpretation is there’s an incredible amount of publication bias, where we’re only publishing studies that show an effect and the journals or investigators are censoring the ones that don’t. The more charitable explanation, which is probably also true, is that by the time you get to putting on an RCT you kind of think, ‘This thing works.’ You’re not testing random stuff, so your pretest probability of a drug being effective when it enters into an RCT is probably shifted toward effectiveness,” Dr. Putman speculated.
He reported having no financial conflicts regarding his study.
REPORTING FROM RWCS 2019
Acne take home messages from the AAD annual meeting
WASHINGTON – In an interview at the close of the annual meeting of the American Academy of Dermatology, .
Both Dr. Harper, who practices in Birmingham, Ala., and is the immediate past president of the American Acne & Rosacea Society, and Dr. Keri, of the department of dermatology at the University of Miami and the Miami VA Healthcare System, spoke during several acne sessions. Among the topics they discussed during the interview were a relatively recent meta-analysis that provides reassuring information about depression and isotretinoin, how to start patients on spironolactone, and the use of antibiotics – and benzoyl peroxide.
They emphasized the importance of not withholding treatment for patients who need it and the psychosocial impact of acne. “Patients need to get to the treatment they need ... faster,” Dr. Harper said. “We want to treat sooner, and we want to prevent scarring,” Dr. Keri added.
Dr. Keri disclosed relationships with Hoffmann–La Roche, Ortho Dermatologics, and Pierre Fabre Dermatologie. Dr. Harper has no relevant financial disclosures.
WASHINGTON – In an interview at the close of the annual meeting of the American Academy of Dermatology, .
Both Dr. Harper, who practices in Birmingham, Ala., and is the immediate past president of the American Acne & Rosacea Society, and Dr. Keri, of the department of dermatology at the University of Miami and the Miami VA Healthcare System, spoke during several acne sessions. Among the topics they discussed during the interview were a relatively recent meta-analysis that provides reassuring information about depression and isotretinoin, how to start patients on spironolactone, and the use of antibiotics – and benzoyl peroxide.
They emphasized the importance of not withholding treatment for patients who need it and the psychosocial impact of acne. “Patients need to get to the treatment they need ... faster,” Dr. Harper said. “We want to treat sooner, and we want to prevent scarring,” Dr. Keri added.
Dr. Keri disclosed relationships with Hoffmann–La Roche, Ortho Dermatologics, and Pierre Fabre Dermatologie. Dr. Harper has no relevant financial disclosures.
WASHINGTON – In an interview at the close of the annual meeting of the American Academy of Dermatology, .
Both Dr. Harper, who practices in Birmingham, Ala., and is the immediate past president of the American Acne & Rosacea Society, and Dr. Keri, of the department of dermatology at the University of Miami and the Miami VA Healthcare System, spoke during several acne sessions. Among the topics they discussed during the interview were a relatively recent meta-analysis that provides reassuring information about depression and isotretinoin, how to start patients on spironolactone, and the use of antibiotics – and benzoyl peroxide.
They emphasized the importance of not withholding treatment for patients who need it and the psychosocial impact of acne. “Patients need to get to the treatment they need ... faster,” Dr. Harper said. “We want to treat sooner, and we want to prevent scarring,” Dr. Keri added.
Dr. Keri disclosed relationships with Hoffmann–La Roche, Ortho Dermatologics, and Pierre Fabre Dermatologie. Dr. Harper has no relevant financial disclosures.