User login
Sore on arm
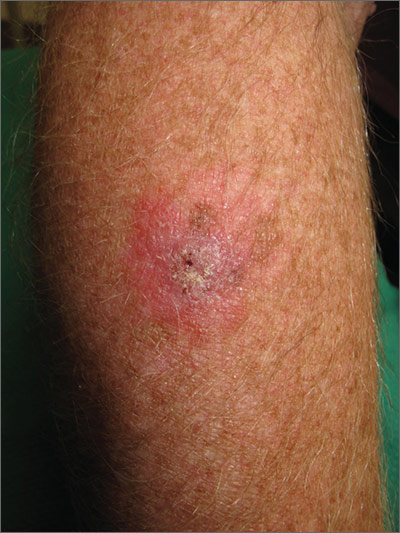
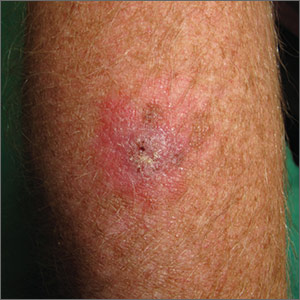
The FP suspected that this was a skin cancer with ulceration rather than a dermatitis because of the ulceration and polymorphous vessels on dermoscopy. The differential diagnosis included squamous cell carcinoma, amelanotic melanoma, and basal cell carcinoma.
The FP explained to the patient that a biopsy would be needed and suggested a broad shave biopsy because it would provide adequate tissue to the pathologist. The physician performed a broad shave biopsy with a DermaBlade. (See the Watch & Learn video on “Shave biopsy.”) The biopsy report came back as amelanotic melanoma with a depth of 1.5 mm.
While most melanomas have visible pigment, some melanomas will present without pigmentation. Based on a Breslow depth of 1.5 mm, the patient was sent to Surgical Oncology for a wide excision with 1 cm margins and sentinel lymph node biopsy. Fortunately, the sentinel lymph node biopsy did not show any metastasis. The FP advised the patient that he would require regular skin surveillance and would need to take skin care precautions when exposed to the sun.
Photo courtesy of Jonathan Karnes, MD and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1103-1111.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP suspected that this was a skin cancer with ulceration rather than a dermatitis because of the ulceration and polymorphous vessels on dermoscopy. The differential diagnosis included squamous cell carcinoma, amelanotic melanoma, and basal cell carcinoma.
The FP explained to the patient that a biopsy would be needed and suggested a broad shave biopsy because it would provide adequate tissue to the pathologist. The physician performed a broad shave biopsy with a DermaBlade. (See the Watch & Learn video on “Shave biopsy.”) The biopsy report came back as amelanotic melanoma with a depth of 1.5 mm.
While most melanomas have visible pigment, some melanomas will present without pigmentation. Based on a Breslow depth of 1.5 mm, the patient was sent to Surgical Oncology for a wide excision with 1 cm margins and sentinel lymph node biopsy. Fortunately, the sentinel lymph node biopsy did not show any metastasis. The FP advised the patient that he would require regular skin surveillance and would need to take skin care precautions when exposed to the sun.
Photo courtesy of Jonathan Karnes, MD and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1103-1111.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP suspected that this was a skin cancer with ulceration rather than a dermatitis because of the ulceration and polymorphous vessels on dermoscopy. The differential diagnosis included squamous cell carcinoma, amelanotic melanoma, and basal cell carcinoma.
The FP explained to the patient that a biopsy would be needed and suggested a broad shave biopsy because it would provide adequate tissue to the pathologist. The physician performed a broad shave biopsy with a DermaBlade. (See the Watch & Learn video on “Shave biopsy.”) The biopsy report came back as amelanotic melanoma with a depth of 1.5 mm.
While most melanomas have visible pigment, some melanomas will present without pigmentation. Based on a Breslow depth of 1.5 mm, the patient was sent to Surgical Oncology for a wide excision with 1 cm margins and sentinel lymph node biopsy. Fortunately, the sentinel lymph node biopsy did not show any metastasis. The FP advised the patient that he would require regular skin surveillance and would need to take skin care precautions when exposed to the sun.
Photo courtesy of Jonathan Karnes, MD and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1103-1111.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
For patients with end-stage liver disease, acute care incurs steep costs
End-of-life care for patients with end-stage liver disease cost more than four of the five most expensive chronic medical conditions, according to the findings of a population-based study in Canada.
During their final year of life, patients with end-stage liver disease incurred a median of $51,191 Canadian dollars in health care costs (interquartile range, $28,510-$86,659) – approximately $2,360 more than ischemic heart disease, $1,830 more than diabetes, $1,600 more than mental health disorders, and $600 more than congestive heart failure, Erin M. Kelly, MD, of the University of Ottawa, and her associates wrote in Clinical Gastroenterology and Hepatology. Only chronic renal disease cost more (median, $55,453). Most health care costs of end-stage liver disease covered the final 90 days of life and were tied to high use of hospital resources, the researchers said.
In the United States, more than 150,000 patients are hospitalized for end-stage liver disease every year at a price tag of $4 billion, Dr. Kelly and her associates noted. This price tag is expected to rise further because of epidemic levels of obesity and related nonalcoholic fatty liver disease. The shortage of livers for transplantation and the fact that many patients with cirrhosis are not transplantation candidates leave many in end-of-life care. Given the lack of population-level data on costs of this care, the researchers studied data for all individuals who died in Ontario – Canada’s largest province – between April 2010 and March 2013. The data source was the Institute for Clinical Evaluative Sciences, a nonprofit group that tracks diagnoses, health care, outcomes, and costs.
Among 264,723 decedents, 5,087 (1.9%) had a diagnosis of end-stage liver disease. These patients died a median of 15 years earlier than other patients (median age of death, 65 vs. 80 years old). During the last year of life, 99% visited the emergency department or were hospitalized, compared with 86% of other patients. Importantly, health care costs for the two groups were similar up until the final 90 days of life, when there was “a clear divergence,” the researchers said. A total of 51% of the costs of the final 12 months of care related to acute care during the final 90 days of life. Consequently, during their last 3 months, patients with end-stage liver disease cost the health care system 46% more than other individuals, the difference remained statistically significant after accounting for demographics and comorbidities, and the picture changed little after excluding transplantation patients and those with hepatocellular carcinoma.
Medical care for patients with end-stage liver disease is complex – often involving serious infections, gastrointestinal bleeding, renal dysfunction, electrolyte disturbances, and worsening encephalopathy – and often involves frequent hospital readmissions, the researchers noted. Nonetheless, the findings highlight the need to consider steps such as advanced care planning and palliative care to help keep patients with end-stage liver disease from dying in acute care settings, they concluded. Such steps “may direct services toward more appropriate sectors, while reducing costs.”
The Ontario Ministry of Health and Long-Term Care supported the work. The researchers reported having no competing interests.
SOURCE: Kelly EM et al. Clin Gastroenterol Hepatol. 2019 Jan 28. doi: 10.1016/j.cgh.2019.01.046.
End-of-life care for patients with end-stage liver disease cost more than four of the five most expensive chronic medical conditions, according to the findings of a population-based study in Canada.
During their final year of life, patients with end-stage liver disease incurred a median of $51,191 Canadian dollars in health care costs (interquartile range, $28,510-$86,659) – approximately $2,360 more than ischemic heart disease, $1,830 more than diabetes, $1,600 more than mental health disorders, and $600 more than congestive heart failure, Erin M. Kelly, MD, of the University of Ottawa, and her associates wrote in Clinical Gastroenterology and Hepatology. Only chronic renal disease cost more (median, $55,453). Most health care costs of end-stage liver disease covered the final 90 days of life and were tied to high use of hospital resources, the researchers said.
In the United States, more than 150,000 patients are hospitalized for end-stage liver disease every year at a price tag of $4 billion, Dr. Kelly and her associates noted. This price tag is expected to rise further because of epidemic levels of obesity and related nonalcoholic fatty liver disease. The shortage of livers for transplantation and the fact that many patients with cirrhosis are not transplantation candidates leave many in end-of-life care. Given the lack of population-level data on costs of this care, the researchers studied data for all individuals who died in Ontario – Canada’s largest province – between April 2010 and March 2013. The data source was the Institute for Clinical Evaluative Sciences, a nonprofit group that tracks diagnoses, health care, outcomes, and costs.
Among 264,723 decedents, 5,087 (1.9%) had a diagnosis of end-stage liver disease. These patients died a median of 15 years earlier than other patients (median age of death, 65 vs. 80 years old). During the last year of life, 99% visited the emergency department or were hospitalized, compared with 86% of other patients. Importantly, health care costs for the two groups were similar up until the final 90 days of life, when there was “a clear divergence,” the researchers said. A total of 51% of the costs of the final 12 months of care related to acute care during the final 90 days of life. Consequently, during their last 3 months, patients with end-stage liver disease cost the health care system 46% more than other individuals, the difference remained statistically significant after accounting for demographics and comorbidities, and the picture changed little after excluding transplantation patients and those with hepatocellular carcinoma.
Medical care for patients with end-stage liver disease is complex – often involving serious infections, gastrointestinal bleeding, renal dysfunction, electrolyte disturbances, and worsening encephalopathy – and often involves frequent hospital readmissions, the researchers noted. Nonetheless, the findings highlight the need to consider steps such as advanced care planning and palliative care to help keep patients with end-stage liver disease from dying in acute care settings, they concluded. Such steps “may direct services toward more appropriate sectors, while reducing costs.”
The Ontario Ministry of Health and Long-Term Care supported the work. The researchers reported having no competing interests.
SOURCE: Kelly EM et al. Clin Gastroenterol Hepatol. 2019 Jan 28. doi: 10.1016/j.cgh.2019.01.046.
End-of-life care for patients with end-stage liver disease cost more than four of the five most expensive chronic medical conditions, according to the findings of a population-based study in Canada.
During their final year of life, patients with end-stage liver disease incurred a median of $51,191 Canadian dollars in health care costs (interquartile range, $28,510-$86,659) – approximately $2,360 more than ischemic heart disease, $1,830 more than diabetes, $1,600 more than mental health disorders, and $600 more than congestive heart failure, Erin M. Kelly, MD, of the University of Ottawa, and her associates wrote in Clinical Gastroenterology and Hepatology. Only chronic renal disease cost more (median, $55,453). Most health care costs of end-stage liver disease covered the final 90 days of life and were tied to high use of hospital resources, the researchers said.
In the United States, more than 150,000 patients are hospitalized for end-stage liver disease every year at a price tag of $4 billion, Dr. Kelly and her associates noted. This price tag is expected to rise further because of epidemic levels of obesity and related nonalcoholic fatty liver disease. The shortage of livers for transplantation and the fact that many patients with cirrhosis are not transplantation candidates leave many in end-of-life care. Given the lack of population-level data on costs of this care, the researchers studied data for all individuals who died in Ontario – Canada’s largest province – between April 2010 and March 2013. The data source was the Institute for Clinical Evaluative Sciences, a nonprofit group that tracks diagnoses, health care, outcomes, and costs.
Among 264,723 decedents, 5,087 (1.9%) had a diagnosis of end-stage liver disease. These patients died a median of 15 years earlier than other patients (median age of death, 65 vs. 80 years old). During the last year of life, 99% visited the emergency department or were hospitalized, compared with 86% of other patients. Importantly, health care costs for the two groups were similar up until the final 90 days of life, when there was “a clear divergence,” the researchers said. A total of 51% of the costs of the final 12 months of care related to acute care during the final 90 days of life. Consequently, during their last 3 months, patients with end-stage liver disease cost the health care system 46% more than other individuals, the difference remained statistically significant after accounting for demographics and comorbidities, and the picture changed little after excluding transplantation patients and those with hepatocellular carcinoma.
Medical care for patients with end-stage liver disease is complex – often involving serious infections, gastrointestinal bleeding, renal dysfunction, electrolyte disturbances, and worsening encephalopathy – and often involves frequent hospital readmissions, the researchers noted. Nonetheless, the findings highlight the need to consider steps such as advanced care planning and palliative care to help keep patients with end-stage liver disease from dying in acute care settings, they concluded. Such steps “may direct services toward more appropriate sectors, while reducing costs.”
The Ontario Ministry of Health and Long-Term Care supported the work. The researchers reported having no competing interests.
SOURCE: Kelly EM et al. Clin Gastroenterol Hepatol. 2019 Jan 28. doi: 10.1016/j.cgh.2019.01.046.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
DDNA19: The NASH conundrum
Zobair Younossi, MD, MPH, chairman of the department of medicine at Inova Fairfax (Va.) Medical Campus, discusses the progressive form of NAFLD -- NASH -- and its optimal treatment.

Zobair Younossi, MD, MPH, chairman of the department of medicine at Inova Fairfax (Va.) Medical Campus, discusses the progressive form of NAFLD -- NASH -- and its optimal treatment.

Zobair Younossi, MD, MPH, chairman of the department of medicine at Inova Fairfax (Va.) Medical Campus, discusses the progressive form of NAFLD -- NASH -- and its optimal treatment.

AT DIGESTIVE DISEASES: NEW ADVANCES 2019
No biological benefits from alcohol seen in rheumatoid arthritis
, according to a study published online March 20 in Arthritis Care & Research.
Joshua F. Baker, MD, of the University of Pennsylvania, Philadelphia, and his coauthors wrote that previous studies had suggested a link between moderate alcohol consumption and lower disease activity, better quality of life, and better functional status in people with rheumatoid arthritis. This link may tempt clinicians “to encourage moderate alcohol consumption among patients with RA,” the researchers wrote, and so it prompted them to examine the relationship more closely.
The researchers studied 16,762 individuals with rheumatoid arthritis in the National Databank for Rheumatic Diseases who had been asked about alcohol use and disease activity in a series of semiannual surveys, providing a total of 121,280 observations, at which 53% reported using alcohol.
Across the observations taken from the semiannual surveys, a total of 8.2% reported discontinuing alcohol consumption from one survey to the next, and 8.4% of abstainers reported initiating alcohol use. Importantly, individuals with high disease activity had a significantly shorter time to discontinuation of alcohol, and those with a moderate or high Patient Activity Scale-II (PAS-II) score were 36% more likely to stop alcohol consumption, compared with individuals who had a low PAS-II score.
Individuals who were older or obese or had more comorbidities or greater work disability were all independently more likely to discontinue alcohol use, while those less likely to give up alcohol tended to be white, male, and have higher physical and mental quality of life, higher educational level, and greater household income.
Participants with moderate or high PAS-II scores were also less likely to start consuming alcohol in comparison to those with low scores.
“Overall, these observations suggest that patients with RA are substantially less likely to use alcohol when their disease activity is high and their health and quality of life are poor,” the authors wrote. “This study also found that active drinking, recent discontinuation of drinking, and recent initiation of drinking were not associated with disease activity or death in this population when considering the reasons for the changes in behavior.”
They said this offered a different explanation for the previously observed association between alcohol use and lower disease activity by showing an effect of reverse causality rather than any biologically protective effect of alcohol.
While the study also found a strong link between discontinuation of alcohol use and increased subsequent mortality, they suggested this was also likely a function of disease activity and disability, rather than the effect of giving up alcohol.
The study was funded by grants to several authors from the Department of Veterans Affairs, the National Institutes of Health, and the Rheumatology Research Foundation. Dr. Baker reported receiving consulting fees from Bristol-Myers Squibb outside of the current work.
SOURCE: Baker J et al. Arthritis Care Res. 2019 Mar 20. doi: 10.1002/acr.23847.
, according to a study published online March 20 in Arthritis Care & Research.
Joshua F. Baker, MD, of the University of Pennsylvania, Philadelphia, and his coauthors wrote that previous studies had suggested a link between moderate alcohol consumption and lower disease activity, better quality of life, and better functional status in people with rheumatoid arthritis. This link may tempt clinicians “to encourage moderate alcohol consumption among patients with RA,” the researchers wrote, and so it prompted them to examine the relationship more closely.
The researchers studied 16,762 individuals with rheumatoid arthritis in the National Databank for Rheumatic Diseases who had been asked about alcohol use and disease activity in a series of semiannual surveys, providing a total of 121,280 observations, at which 53% reported using alcohol.
Across the observations taken from the semiannual surveys, a total of 8.2% reported discontinuing alcohol consumption from one survey to the next, and 8.4% of abstainers reported initiating alcohol use. Importantly, individuals with high disease activity had a significantly shorter time to discontinuation of alcohol, and those with a moderate or high Patient Activity Scale-II (PAS-II) score were 36% more likely to stop alcohol consumption, compared with individuals who had a low PAS-II score.
Individuals who were older or obese or had more comorbidities or greater work disability were all independently more likely to discontinue alcohol use, while those less likely to give up alcohol tended to be white, male, and have higher physical and mental quality of life, higher educational level, and greater household income.
Participants with moderate or high PAS-II scores were also less likely to start consuming alcohol in comparison to those with low scores.
“Overall, these observations suggest that patients with RA are substantially less likely to use alcohol when their disease activity is high and their health and quality of life are poor,” the authors wrote. “This study also found that active drinking, recent discontinuation of drinking, and recent initiation of drinking were not associated with disease activity or death in this population when considering the reasons for the changes in behavior.”
They said this offered a different explanation for the previously observed association between alcohol use and lower disease activity by showing an effect of reverse causality rather than any biologically protective effect of alcohol.
While the study also found a strong link between discontinuation of alcohol use and increased subsequent mortality, they suggested this was also likely a function of disease activity and disability, rather than the effect of giving up alcohol.
The study was funded by grants to several authors from the Department of Veterans Affairs, the National Institutes of Health, and the Rheumatology Research Foundation. Dr. Baker reported receiving consulting fees from Bristol-Myers Squibb outside of the current work.
SOURCE: Baker J et al. Arthritis Care Res. 2019 Mar 20. doi: 10.1002/acr.23847.
, according to a study published online March 20 in Arthritis Care & Research.
Joshua F. Baker, MD, of the University of Pennsylvania, Philadelphia, and his coauthors wrote that previous studies had suggested a link between moderate alcohol consumption and lower disease activity, better quality of life, and better functional status in people with rheumatoid arthritis. This link may tempt clinicians “to encourage moderate alcohol consumption among patients with RA,” the researchers wrote, and so it prompted them to examine the relationship more closely.
The researchers studied 16,762 individuals with rheumatoid arthritis in the National Databank for Rheumatic Diseases who had been asked about alcohol use and disease activity in a series of semiannual surveys, providing a total of 121,280 observations, at which 53% reported using alcohol.
Across the observations taken from the semiannual surveys, a total of 8.2% reported discontinuing alcohol consumption from one survey to the next, and 8.4% of abstainers reported initiating alcohol use. Importantly, individuals with high disease activity had a significantly shorter time to discontinuation of alcohol, and those with a moderate or high Patient Activity Scale-II (PAS-II) score were 36% more likely to stop alcohol consumption, compared with individuals who had a low PAS-II score.
Individuals who were older or obese or had more comorbidities or greater work disability were all independently more likely to discontinue alcohol use, while those less likely to give up alcohol tended to be white, male, and have higher physical and mental quality of life, higher educational level, and greater household income.
Participants with moderate or high PAS-II scores were also less likely to start consuming alcohol in comparison to those with low scores.
“Overall, these observations suggest that patients with RA are substantially less likely to use alcohol when their disease activity is high and their health and quality of life are poor,” the authors wrote. “This study also found that active drinking, recent discontinuation of drinking, and recent initiation of drinking were not associated with disease activity or death in this population when considering the reasons for the changes in behavior.”
They said this offered a different explanation for the previously observed association between alcohol use and lower disease activity by showing an effect of reverse causality rather than any biologically protective effect of alcohol.
While the study also found a strong link between discontinuation of alcohol use and increased subsequent mortality, they suggested this was also likely a function of disease activity and disability, rather than the effect of giving up alcohol.
The study was funded by grants to several authors from the Department of Veterans Affairs, the National Institutes of Health, and the Rheumatology Research Foundation. Dr. Baker reported receiving consulting fees from Bristol-Myers Squibb outside of the current work.
SOURCE: Baker J et al. Arthritis Care Res. 2019 Mar 20. doi: 10.1002/acr.23847.
FROM ARTHRITIS CARE & RESEARCH
Amphetamine tied to higher risk of new-onset psychosis than methylphenidate
Greater risk applies only to adolescents, young adults with ADHD treated in primary care
Adolescents and young adults with ADHD who start on amphetamine might have twice the risk of developing new-onset psychosis as do those who start on methylphenidate, a cohort study of more than 220,000 patients suggests.
“The percentage of patients who had a psychotic episode was 0.10% among patients who received methylphenidate and 0.21% among patients who received amphetamine, reported Lauren V. Moran, MD, of the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital in Boston and her colleagues. The study was published by the New England Journal of Medicine.
(aged 13-25 years) with ADHD between January 2004 and September 2015 who were prescribed methylphenidate or amphetamine (both 110,923 patients; 143,286 total person-years of follow-up). They looked for an ICD-9 or ICD-10 code for new-onset psychosis followed by a prescription for an antipsychotic medication the same day or within 60 days of the psychosis diagnosis. Hazard ratios were calculated by matching patients taking methylphenidate with patients taking amphetamine across both databases and calculating the incidence rate of psychosis in each group.
The researchers found 343 new cases of psychosis overall, with an incidence of 2.4 cases per 1,000 person-years. There were 106 episodes of psychosis among patients receiving methylphenidate (0.10%) and 237 new cases among patients receiving amphetamine (0.21%). There was an incidence rate of 1.78 cases per 1,000 person-years for methylphenidate patients and 2.83 cases per 1,000 person-years for amphetamine patients. Across both databases, the pooled hazard ratio for amphetamine use and new-onset psychosis, compared with matched patients, was 1.65 (95% confidence interval, 1.31-2.09).
“The attribution of the higher risk of psychosis to amphetamine use was supported by negative control outcome analyses, which showed that there was no difference in the risk of other psychiatric events between the two stimulant groups,” Dr. Moran and her colleagues reported. “The different biologic mechanisms of methylphenidate and amphetamine activity on neurotransmitters could explain our findings.”
Patients who were prescribed amphetamine by family medicine physicians, internists, and pediatricians were at a higher risk of developing psychosis. That risk, however, did not extend to patients prescribed amphetamine by psychiatrists, the researchers said.
“Psychosis may develop in these patients regardless of stimulant treatment. Alternatively, psychiatrists may prescribe amphetamine more cautiously than other providers and may screen for risk factors for psychosis,” Dr. Moran and her colleagues wrote.
The researchers said the study was limited by unmeasured confounders, such as substance or stimulant misuse; the rate of diversion for amphetamine; and lack of information on race, gender, or socioeconomic status. In addition, they noted, the results could not be generalized to patients with public insurance or no insurance, “which disproportionately applies to patients who are black or Hispanic.”
Dr. Moran reported receiving grants from National Institute of Mental Health (NIMH). The other authors reported grants, personal fees, and other relationships with several entities, including Boehringer Ingelheim, the Food and Drug Administration, the NIMH, and Takeda.
SOURCE: Moran LV et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1813751.
The findings by Moran et al. are consistent with other randomized controlled trials that suggest a better safety profile for methylphenidate over amphetamine. But the data cannot determine causality in this patient population, Samuele Cortese, MD, PhD, wrote in a related editorial.
“The findings of the current study should not be considered definitive. Observational studies such as this one can provide information on uncommon adverse events in real-world clinical practice that are challenging to assess in randomized trials performed over brief periods,” he said. “However, even sophisticated approaches, such as the ones used in this study to address possible biases, do not have the advantages of randomized trials in excluding confounding factors.”
It is still unclear why some patients developed psychosis, such as in cases of patients with stimulant use and had a “low” or “high” vulnerability to developing psychosis after exposure. The lack of association between psychosis and prescribing amphetamines among psychiatrists also might indicate that those clinicians identified risk factors in patients that predicted the development of psychosis and thus avoided prescribing amphetamines to these patients, he said.
“Currently, it is not possible to predict which patients will have psychotic episodes after stimulant treatment,” Dr. Cortese concluded. “Perhaps techniques such as machine learning applied to large data sets from randomized trials, combined with observational data, will provide predictors at the individual patient level.”
Dr. Cortese is affiliated with the Center for Innovation in Mental Health at the University of Southampton (England). These comments summarize his accompanying editorial (N Engl J Med. 2019. doi: 10.1056/NEJMe1900887 ). He reported nonfinancial relationships with the Association for Child and Adolescent Central Health and the Healthcare Convention & Exhibitors Association.
Greater risk applies only to adolescents, young adults with ADHD treated in primary care
Greater risk applies only to adolescents, young adults with ADHD treated in primary care
The findings by Moran et al. are consistent with other randomized controlled trials that suggest a better safety profile for methylphenidate over amphetamine. But the data cannot determine causality in this patient population, Samuele Cortese, MD, PhD, wrote in a related editorial.
“The findings of the current study should not be considered definitive. Observational studies such as this one can provide information on uncommon adverse events in real-world clinical practice that are challenging to assess in randomized trials performed over brief periods,” he said. “However, even sophisticated approaches, such as the ones used in this study to address possible biases, do not have the advantages of randomized trials in excluding confounding factors.”
It is still unclear why some patients developed psychosis, such as in cases of patients with stimulant use and had a “low” or “high” vulnerability to developing psychosis after exposure. The lack of association between psychosis and prescribing amphetamines among psychiatrists also might indicate that those clinicians identified risk factors in patients that predicted the development of psychosis and thus avoided prescribing amphetamines to these patients, he said.
“Currently, it is not possible to predict which patients will have psychotic episodes after stimulant treatment,” Dr. Cortese concluded. “Perhaps techniques such as machine learning applied to large data sets from randomized trials, combined with observational data, will provide predictors at the individual patient level.”
Dr. Cortese is affiliated with the Center for Innovation in Mental Health at the University of Southampton (England). These comments summarize his accompanying editorial (N Engl J Med. 2019. doi: 10.1056/NEJMe1900887 ). He reported nonfinancial relationships with the Association for Child and Adolescent Central Health and the Healthcare Convention & Exhibitors Association.
The findings by Moran et al. are consistent with other randomized controlled trials that suggest a better safety profile for methylphenidate over amphetamine. But the data cannot determine causality in this patient population, Samuele Cortese, MD, PhD, wrote in a related editorial.
“The findings of the current study should not be considered definitive. Observational studies such as this one can provide information on uncommon adverse events in real-world clinical practice that are challenging to assess in randomized trials performed over brief periods,” he said. “However, even sophisticated approaches, such as the ones used in this study to address possible biases, do not have the advantages of randomized trials in excluding confounding factors.”
It is still unclear why some patients developed psychosis, such as in cases of patients with stimulant use and had a “low” or “high” vulnerability to developing psychosis after exposure. The lack of association between psychosis and prescribing amphetamines among psychiatrists also might indicate that those clinicians identified risk factors in patients that predicted the development of psychosis and thus avoided prescribing amphetamines to these patients, he said.
“Currently, it is not possible to predict which patients will have psychotic episodes after stimulant treatment,” Dr. Cortese concluded. “Perhaps techniques such as machine learning applied to large data sets from randomized trials, combined with observational data, will provide predictors at the individual patient level.”
Dr. Cortese is affiliated with the Center for Innovation in Mental Health at the University of Southampton (England). These comments summarize his accompanying editorial (N Engl J Med. 2019. doi: 10.1056/NEJMe1900887 ). He reported nonfinancial relationships with the Association for Child and Adolescent Central Health and the Healthcare Convention & Exhibitors Association.
Adolescents and young adults with ADHD who start on amphetamine might have twice the risk of developing new-onset psychosis as do those who start on methylphenidate, a cohort study of more than 220,000 patients suggests.
“The percentage of patients who had a psychotic episode was 0.10% among patients who received methylphenidate and 0.21% among patients who received amphetamine, reported Lauren V. Moran, MD, of the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital in Boston and her colleagues. The study was published by the New England Journal of Medicine.
(aged 13-25 years) with ADHD between January 2004 and September 2015 who were prescribed methylphenidate or amphetamine (both 110,923 patients; 143,286 total person-years of follow-up). They looked for an ICD-9 or ICD-10 code for new-onset psychosis followed by a prescription for an antipsychotic medication the same day or within 60 days of the psychosis diagnosis. Hazard ratios were calculated by matching patients taking methylphenidate with patients taking amphetamine across both databases and calculating the incidence rate of psychosis in each group.
The researchers found 343 new cases of psychosis overall, with an incidence of 2.4 cases per 1,000 person-years. There were 106 episodes of psychosis among patients receiving methylphenidate (0.10%) and 237 new cases among patients receiving amphetamine (0.21%). There was an incidence rate of 1.78 cases per 1,000 person-years for methylphenidate patients and 2.83 cases per 1,000 person-years for amphetamine patients. Across both databases, the pooled hazard ratio for amphetamine use and new-onset psychosis, compared with matched patients, was 1.65 (95% confidence interval, 1.31-2.09).
“The attribution of the higher risk of psychosis to amphetamine use was supported by negative control outcome analyses, which showed that there was no difference in the risk of other psychiatric events between the two stimulant groups,” Dr. Moran and her colleagues reported. “The different biologic mechanisms of methylphenidate and amphetamine activity on neurotransmitters could explain our findings.”
Patients who were prescribed amphetamine by family medicine physicians, internists, and pediatricians were at a higher risk of developing psychosis. That risk, however, did not extend to patients prescribed amphetamine by psychiatrists, the researchers said.
“Psychosis may develop in these patients regardless of stimulant treatment. Alternatively, psychiatrists may prescribe amphetamine more cautiously than other providers and may screen for risk factors for psychosis,” Dr. Moran and her colleagues wrote.
The researchers said the study was limited by unmeasured confounders, such as substance or stimulant misuse; the rate of diversion for amphetamine; and lack of information on race, gender, or socioeconomic status. In addition, they noted, the results could not be generalized to patients with public insurance or no insurance, “which disproportionately applies to patients who are black or Hispanic.”
Dr. Moran reported receiving grants from National Institute of Mental Health (NIMH). The other authors reported grants, personal fees, and other relationships with several entities, including Boehringer Ingelheim, the Food and Drug Administration, the NIMH, and Takeda.
SOURCE: Moran LV et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1813751.
Adolescents and young adults with ADHD who start on amphetamine might have twice the risk of developing new-onset psychosis as do those who start on methylphenidate, a cohort study of more than 220,000 patients suggests.
“The percentage of patients who had a psychotic episode was 0.10% among patients who received methylphenidate and 0.21% among patients who received amphetamine, reported Lauren V. Moran, MD, of the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital in Boston and her colleagues. The study was published by the New England Journal of Medicine.
(aged 13-25 years) with ADHD between January 2004 and September 2015 who were prescribed methylphenidate or amphetamine (both 110,923 patients; 143,286 total person-years of follow-up). They looked for an ICD-9 or ICD-10 code for new-onset psychosis followed by a prescription for an antipsychotic medication the same day or within 60 days of the psychosis diagnosis. Hazard ratios were calculated by matching patients taking methylphenidate with patients taking amphetamine across both databases and calculating the incidence rate of psychosis in each group.
The researchers found 343 new cases of psychosis overall, with an incidence of 2.4 cases per 1,000 person-years. There were 106 episodes of psychosis among patients receiving methylphenidate (0.10%) and 237 new cases among patients receiving amphetamine (0.21%). There was an incidence rate of 1.78 cases per 1,000 person-years for methylphenidate patients and 2.83 cases per 1,000 person-years for amphetamine patients. Across both databases, the pooled hazard ratio for amphetamine use and new-onset psychosis, compared with matched patients, was 1.65 (95% confidence interval, 1.31-2.09).
“The attribution of the higher risk of psychosis to amphetamine use was supported by negative control outcome analyses, which showed that there was no difference in the risk of other psychiatric events between the two stimulant groups,” Dr. Moran and her colleagues reported. “The different biologic mechanisms of methylphenidate and amphetamine activity on neurotransmitters could explain our findings.”
Patients who were prescribed amphetamine by family medicine physicians, internists, and pediatricians were at a higher risk of developing psychosis. That risk, however, did not extend to patients prescribed amphetamine by psychiatrists, the researchers said.
“Psychosis may develop in these patients regardless of stimulant treatment. Alternatively, psychiatrists may prescribe amphetamine more cautiously than other providers and may screen for risk factors for psychosis,” Dr. Moran and her colleagues wrote.
The researchers said the study was limited by unmeasured confounders, such as substance or stimulant misuse; the rate of diversion for amphetamine; and lack of information on race, gender, or socioeconomic status. In addition, they noted, the results could not be generalized to patients with public insurance or no insurance, “which disproportionately applies to patients who are black or Hispanic.”
Dr. Moran reported receiving grants from National Institute of Mental Health (NIMH). The other authors reported grants, personal fees, and other relationships with several entities, including Boehringer Ingelheim, the Food and Drug Administration, the NIMH, and Takeda.
SOURCE: Moran LV et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1813751.
Cirrhosis model predicts decompensation across diverse populations
A prognostic model that uses serum albumin-bilirubin (ALBI) and Fibrosis-4 (FIB-4) scores can identify patients with cirrhosis who are at high risk of liver decompensation, according to investigators.
During validation testing, the scoring system performed well among European and Middle Eastern patients, which supports prognostic value across diverse populations, reported lead author Neil Guha, MRCP, PhD, of the University of Nottingham (U.K.) and his colleagues, who suggested that the scoring system could fix an important practice gap.
“Identification of patients [with chronic liver disease] that need intensive monitoring and timely intervention is challenging,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Robust prognostic tools using simple laboratory variables, with potential for implementation in nonspecialist settings and across different health care systems, have significant appeal.”
Although existing scoring systems have been used for decades, they have clear limitations, the investigators noted, referring to predictive ability that may be too little, too late.
“[T]hese scoring systems provide value after synthetic liver function has become significantly deranged and provide only short-term prognostic value,” the investigators wrote. “Presently, there are no scores, performed in routine clinical practice, that provide robust prognostic stratification within early, compensated cirrhosis over the medium/long term.”
To fulfill this need, the investigators developed and validated a prognostic model that incorporates data from the ALBI and FIB-4 scoring systems because these tests measure both fibrosis and function. The development phase involved 145 patients with compensated cirrhosis from Nottingham. Almost half of the cohort had liver disease because of alcohol (44.8%), while about one out of three patients had nonalcoholic fatty liver disease (29.7%). After investigators collected baseline clinical features and scores, patients were followed for a median of 4.59 years, during which time decompensation events were recorded (ascites, variceal bleeding, and encephalopathy). Decompensation occurred in about one out of five patients (19.3%) in the U.K. group, with ascites being the most common (71.4%). Using these findings, the investigators created the prognostic model, which classified patients as having either low or high risk of decompensation. In the development cohort, patients with high risk scores had a hazard ratio for decompensation of 7.10.
In the second part of the study, the investigators validated their model with two clinically distinct groups in Dublin, Ireland (prospective; n = 141), and Menoufia, Egypt (retrospective; n = 93).
In the Dublin cohort, the most common etiologies were alcohol (39.7%) and hepatitis C (29.8%). Over a maximum observational period of 6.4 years, the decompensation rate was lower than the development group, at 12.1%. Types of decompensation also differed, with variceal bleeding being the most common (47.1%). Patients with high risk scores had a higher HR for decompensation than the U.K. cohort, at 12.54.
In the Egypt group, the most common causes of liver disease were nonalcoholic fatty liver disease (47.3%) and hepatitis C (34.4%). The maximum follow-up period was 10.6 years, during which time 38.7% of patients experienced decompensation, with ascites being the most common form (57.1%). The HR of 5.10 was the lowest of all cohorts.
The investigators noted that the cohorts represented unique patient populations with different etiological patterns. “This provides reassurance that the model has generalizability for stratifying liver disease at an international level,” the investigators wrote, suggesting that ALBI and FIB-4 can be used in low-resource and community settings.
“A frequently leveled criticism of algorithms such as ALBI-FIB-4 is that they are too complicated to be applied routinely in the clinical setting,” the investigators wrote. “To overcome this problem we developed a simple online calculator which can be accessed using the following link: https://jscalc.io/calc/gdEJj89Wz5PirkSL.”
“We have shown that routinely available laboratory variables, combined in a novel algorithm, ALBI-FIB-4, can stratify patients with cirrhosis for future risk of liver decompensation,” the investigators concluded. “The ability to do this in the context of early, compensated cirrhosis with preserved liver synthetic function whilst also predicting long-term clinical outcomes has clinical utility for international health care systems.”
The study was funded by National Institute for Health Research (NIHR) Nottingham Digestive Diseases Biomedical Research Centre based at Nottingham University Hospitals NHS Trust and the University of Nottingham. The investigators declared no conflicts of interest.
SOURCE: Guha N et al. CGH. 2019 Feb 1. doi: 10.1016/j.cgh.2019.01.042.
A prognostic model that uses serum albumin-bilirubin (ALBI) and Fibrosis-4 (FIB-4) scores can identify patients with cirrhosis who are at high risk of liver decompensation, according to investigators.
During validation testing, the scoring system performed well among European and Middle Eastern patients, which supports prognostic value across diverse populations, reported lead author Neil Guha, MRCP, PhD, of the University of Nottingham (U.K.) and his colleagues, who suggested that the scoring system could fix an important practice gap.
“Identification of patients [with chronic liver disease] that need intensive monitoring and timely intervention is challenging,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Robust prognostic tools using simple laboratory variables, with potential for implementation in nonspecialist settings and across different health care systems, have significant appeal.”
Although existing scoring systems have been used for decades, they have clear limitations, the investigators noted, referring to predictive ability that may be too little, too late.
“[T]hese scoring systems provide value after synthetic liver function has become significantly deranged and provide only short-term prognostic value,” the investigators wrote. “Presently, there are no scores, performed in routine clinical practice, that provide robust prognostic stratification within early, compensated cirrhosis over the medium/long term.”
To fulfill this need, the investigators developed and validated a prognostic model that incorporates data from the ALBI and FIB-4 scoring systems because these tests measure both fibrosis and function. The development phase involved 145 patients with compensated cirrhosis from Nottingham. Almost half of the cohort had liver disease because of alcohol (44.8%), while about one out of three patients had nonalcoholic fatty liver disease (29.7%). After investigators collected baseline clinical features and scores, patients were followed for a median of 4.59 years, during which time decompensation events were recorded (ascites, variceal bleeding, and encephalopathy). Decompensation occurred in about one out of five patients (19.3%) in the U.K. group, with ascites being the most common (71.4%). Using these findings, the investigators created the prognostic model, which classified patients as having either low or high risk of decompensation. In the development cohort, patients with high risk scores had a hazard ratio for decompensation of 7.10.
In the second part of the study, the investigators validated their model with two clinically distinct groups in Dublin, Ireland (prospective; n = 141), and Menoufia, Egypt (retrospective; n = 93).
In the Dublin cohort, the most common etiologies were alcohol (39.7%) and hepatitis C (29.8%). Over a maximum observational period of 6.4 years, the decompensation rate was lower than the development group, at 12.1%. Types of decompensation also differed, with variceal bleeding being the most common (47.1%). Patients with high risk scores had a higher HR for decompensation than the U.K. cohort, at 12.54.
In the Egypt group, the most common causes of liver disease were nonalcoholic fatty liver disease (47.3%) and hepatitis C (34.4%). The maximum follow-up period was 10.6 years, during which time 38.7% of patients experienced decompensation, with ascites being the most common form (57.1%). The HR of 5.10 was the lowest of all cohorts.
The investigators noted that the cohorts represented unique patient populations with different etiological patterns. “This provides reassurance that the model has generalizability for stratifying liver disease at an international level,” the investigators wrote, suggesting that ALBI and FIB-4 can be used in low-resource and community settings.
“A frequently leveled criticism of algorithms such as ALBI-FIB-4 is that they are too complicated to be applied routinely in the clinical setting,” the investigators wrote. “To overcome this problem we developed a simple online calculator which can be accessed using the following link: https://jscalc.io/calc/gdEJj89Wz5PirkSL.”
“We have shown that routinely available laboratory variables, combined in a novel algorithm, ALBI-FIB-4, can stratify patients with cirrhosis for future risk of liver decompensation,” the investigators concluded. “The ability to do this in the context of early, compensated cirrhosis with preserved liver synthetic function whilst also predicting long-term clinical outcomes has clinical utility for international health care systems.”
The study was funded by National Institute for Health Research (NIHR) Nottingham Digestive Diseases Biomedical Research Centre based at Nottingham University Hospitals NHS Trust and the University of Nottingham. The investigators declared no conflicts of interest.
SOURCE: Guha N et al. CGH. 2019 Feb 1. doi: 10.1016/j.cgh.2019.01.042.
A prognostic model that uses serum albumin-bilirubin (ALBI) and Fibrosis-4 (FIB-4) scores can identify patients with cirrhosis who are at high risk of liver decompensation, according to investigators.
During validation testing, the scoring system performed well among European and Middle Eastern patients, which supports prognostic value across diverse populations, reported lead author Neil Guha, MRCP, PhD, of the University of Nottingham (U.K.) and his colleagues, who suggested that the scoring system could fix an important practice gap.
“Identification of patients [with chronic liver disease] that need intensive monitoring and timely intervention is challenging,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Robust prognostic tools using simple laboratory variables, with potential for implementation in nonspecialist settings and across different health care systems, have significant appeal.”
Although existing scoring systems have been used for decades, they have clear limitations, the investigators noted, referring to predictive ability that may be too little, too late.
“[T]hese scoring systems provide value after synthetic liver function has become significantly deranged and provide only short-term prognostic value,” the investigators wrote. “Presently, there are no scores, performed in routine clinical practice, that provide robust prognostic stratification within early, compensated cirrhosis over the medium/long term.”
To fulfill this need, the investigators developed and validated a prognostic model that incorporates data from the ALBI and FIB-4 scoring systems because these tests measure both fibrosis and function. The development phase involved 145 patients with compensated cirrhosis from Nottingham. Almost half of the cohort had liver disease because of alcohol (44.8%), while about one out of three patients had nonalcoholic fatty liver disease (29.7%). After investigators collected baseline clinical features and scores, patients were followed for a median of 4.59 years, during which time decompensation events were recorded (ascites, variceal bleeding, and encephalopathy). Decompensation occurred in about one out of five patients (19.3%) in the U.K. group, with ascites being the most common (71.4%). Using these findings, the investigators created the prognostic model, which classified patients as having either low or high risk of decompensation. In the development cohort, patients with high risk scores had a hazard ratio for decompensation of 7.10.
In the second part of the study, the investigators validated their model with two clinically distinct groups in Dublin, Ireland (prospective; n = 141), and Menoufia, Egypt (retrospective; n = 93).
In the Dublin cohort, the most common etiologies were alcohol (39.7%) and hepatitis C (29.8%). Over a maximum observational period of 6.4 years, the decompensation rate was lower than the development group, at 12.1%. Types of decompensation also differed, with variceal bleeding being the most common (47.1%). Patients with high risk scores had a higher HR for decompensation than the U.K. cohort, at 12.54.
In the Egypt group, the most common causes of liver disease were nonalcoholic fatty liver disease (47.3%) and hepatitis C (34.4%). The maximum follow-up period was 10.6 years, during which time 38.7% of patients experienced decompensation, with ascites being the most common form (57.1%). The HR of 5.10 was the lowest of all cohorts.
The investigators noted that the cohorts represented unique patient populations with different etiological patterns. “This provides reassurance that the model has generalizability for stratifying liver disease at an international level,” the investigators wrote, suggesting that ALBI and FIB-4 can be used in low-resource and community settings.
“A frequently leveled criticism of algorithms such as ALBI-FIB-4 is that they are too complicated to be applied routinely in the clinical setting,” the investigators wrote. “To overcome this problem we developed a simple online calculator which can be accessed using the following link: https://jscalc.io/calc/gdEJj89Wz5PirkSL.”
“We have shown that routinely available laboratory variables, combined in a novel algorithm, ALBI-FIB-4, can stratify patients with cirrhosis for future risk of liver decompensation,” the investigators concluded. “The ability to do this in the context of early, compensated cirrhosis with preserved liver synthetic function whilst also predicting long-term clinical outcomes has clinical utility for international health care systems.”
The study was funded by National Institute for Health Research (NIHR) Nottingham Digestive Diseases Biomedical Research Centre based at Nottingham University Hospitals NHS Trust and the University of Nottingham. The investigators declared no conflicts of interest.
SOURCE: Guha N et al. CGH. 2019 Feb 1. doi: 10.1016/j.cgh.2019.01.042.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
FDA committee advises status quo for blood supply Zika testing
Most members of a Food and Drug Administration advisory committee considered that data support maintaining current testing protocols for Zika virus in the blood donor pool. However, committee discussion entertained the idea of revisiting testing strategies after another year or 2 of Zika virus epidemiological data are available.
In its last guidance regarding Zika virus testing, issued in July 2018, the FDA recommended that either minipool nucleic acid testing (MP NAT) or individual donor (ID) NAT be used to screen for Zika virus. Current guidance still requires conversion to all-ID NAT “when certain threshold conditions are met that indicate an increased risk of suspected mosquito-borne transmission in a defined geographic collection area.”
In the first of three separate votes, 11 of 15 voting members of the FDA’s Blood Products Advisory Committee (BPAC) answered in the affirmative to the question of whether available data support continuing the status quo for Zika testing. Committee members then were asked to weigh whether current data support scaling back to a regional testing strategy targeting at-risk areas. Here, six committee members answered in the affirmative, and nine in the negative.
Just one committee member, F. Blaine Hollinger, MD, voted in favor of the third option, elimination of all Zika virus testing without reintroducing donor screening for risk factors in risk-free areas pending another outbreak in the United States. Dr. Hollinger is a professor of virology and microbiology at Baylor College of Medicine, Houston.
The committee as whole wasn’t swayed by a line of questioning put forward by chairman Richard Kaufman, MD. “I will be the devil’s advocate a little bit: We learned that there have been zero confirmed positives from blood donors for the past year. Would anyone be comfortable with just stopping screening of donors?” asked Dr. Kaufman, medical director of the adult transfusion service at Brigham and Women’s Hospital, Boston.
A wide-ranging morning of presentations put data regarding historical trends and current global Zika hot spots in front of the committee. Current upticks in infection rates in northwest Mexico and in some states in India were areas of concern, given North American travel patterns, noted speaker Marc Fisher, MD, of the Center for Disease Control and Prevention’s Arboviral Disease Branch (Fort Collins, Colo.) “We’re going to see sporadic outbreaks; it’s hard to predict the future,” he said. “The new outbreak in India raises concerns.”
Briefing information from the FDA explained that Zika virus local transmission peaked in the United States in late summer of 2016. More than 5,000 cases were reported in the United States and over 36,000 in Puerto Rico. This has plummeted to 220 in 2018, with about two-thirds of these cases occurring in the territories, mostly (97%) from Puerto Rico across all 3 years.
Zika viremic blood donors dropped by an order of magnitude yearly, totaling 363 in 2016, 38 in 2017, and just 3 in 2018. Of the 363 detected in 2016, 96% came from Puerto Rico or Florida, noted Dr. Fisher.
The number of suspected and confirmed cases in the Americas overall has also dropped from over 650,000 in 2016 to under 30,000 in 2018, with most cases in 2018 being suspected rather than laboratory confirmed. In contrast to testing conducted in North America, few cases in much of Central and South America were laboratory confirmed.
Asymptomatic infections have occurred in blood donors, said the FDA, with 1.8% of blood donations in Puerto Rico testing positive for Zika virus during the peak of the outbreak. Transmission by transfusion is thought to have occurred in Brazil.
Although Zika virus infections have plummeted in the United States and worldwide, prevalence and rates of local transmission are unpredictable, said the FDA, which pointed to sporadic increases in autochthonous transmission of viruses such as dengue and chikungunya that are carried by the same mosquito vector as Zika.
Some of the committee’s discussion centered around finding a way to carve out protection for those most harmed by Zika virus – pregnant women and their fetuses. Martin Schreiber, MD, professor of surgery at Oregon Health and Sciences University, Portland, proposed a point-of-care testing strategy in which only blood destined for pregnant women would be tested for Zika virus. Dr. Schreiber, a trauma surgeon, put forward the rationale that Zika virus causes harm almost exclusively to fetuses, except for rare cases of Guillain-Barré syndrome.
In response, Dr. Kaufman pointed out that with rare exceptions for some bacterial testing, all testing is done from samples taken at the point of donation. The supply chain for donor blood is not set up to accommodate point-of-care testing, he said.
Answering questions about another targeted strategy – maintaining a separate, Zika-tested supply of blood for pregnant women – Susan Stramer, PhD, vice president of scientific affairs for the American Red Cross, said, “Most hospitals do not want, and are very adamant against, carrying a dual inventory.”
Ultimately, the committee’s discussion swung toward the realization that it may be too soon after the recent spike in U.S. Zika cases to plot the best course for ongoing testing strategies. “We are at the tail end of a waning epidemic. ... I think it would probably be a pretty easy question for the committee and for the agency if we actually had some way of having a crystal ball and knowing that the current trend was likely to continue,” said Roger Lewis, MD, PhD, professor at the University of California, Los Angeles, and chair of the department of emergency medicine at Harbor-UCLA Medical Center.
“I think that is not the question,” he went on. “I think the question is, What is the optimal strategy if we have no idea if that tail is going to continue in this current trend. ... And that maybe the committee ought to be thinking about what is the right strategy for the next 2 years – with an underlying assumption that this is a question that can be brought back as we learn more about how this disease behaves.”
The FDA usually follows the recommendations of its advisory committees.
Most members of a Food and Drug Administration advisory committee considered that data support maintaining current testing protocols for Zika virus in the blood donor pool. However, committee discussion entertained the idea of revisiting testing strategies after another year or 2 of Zika virus epidemiological data are available.
In its last guidance regarding Zika virus testing, issued in July 2018, the FDA recommended that either minipool nucleic acid testing (MP NAT) or individual donor (ID) NAT be used to screen for Zika virus. Current guidance still requires conversion to all-ID NAT “when certain threshold conditions are met that indicate an increased risk of suspected mosquito-borne transmission in a defined geographic collection area.”
In the first of three separate votes, 11 of 15 voting members of the FDA’s Blood Products Advisory Committee (BPAC) answered in the affirmative to the question of whether available data support continuing the status quo for Zika testing. Committee members then were asked to weigh whether current data support scaling back to a regional testing strategy targeting at-risk areas. Here, six committee members answered in the affirmative, and nine in the negative.
Just one committee member, F. Blaine Hollinger, MD, voted in favor of the third option, elimination of all Zika virus testing without reintroducing donor screening for risk factors in risk-free areas pending another outbreak in the United States. Dr. Hollinger is a professor of virology and microbiology at Baylor College of Medicine, Houston.
The committee as whole wasn’t swayed by a line of questioning put forward by chairman Richard Kaufman, MD. “I will be the devil’s advocate a little bit: We learned that there have been zero confirmed positives from blood donors for the past year. Would anyone be comfortable with just stopping screening of donors?” asked Dr. Kaufman, medical director of the adult transfusion service at Brigham and Women’s Hospital, Boston.
A wide-ranging morning of presentations put data regarding historical trends and current global Zika hot spots in front of the committee. Current upticks in infection rates in northwest Mexico and in some states in India were areas of concern, given North American travel patterns, noted speaker Marc Fisher, MD, of the Center for Disease Control and Prevention’s Arboviral Disease Branch (Fort Collins, Colo.) “We’re going to see sporadic outbreaks; it’s hard to predict the future,” he said. “The new outbreak in India raises concerns.”
Briefing information from the FDA explained that Zika virus local transmission peaked in the United States in late summer of 2016. More than 5,000 cases were reported in the United States and over 36,000 in Puerto Rico. This has plummeted to 220 in 2018, with about two-thirds of these cases occurring in the territories, mostly (97%) from Puerto Rico across all 3 years.
Zika viremic blood donors dropped by an order of magnitude yearly, totaling 363 in 2016, 38 in 2017, and just 3 in 2018. Of the 363 detected in 2016, 96% came from Puerto Rico or Florida, noted Dr. Fisher.
The number of suspected and confirmed cases in the Americas overall has also dropped from over 650,000 in 2016 to under 30,000 in 2018, with most cases in 2018 being suspected rather than laboratory confirmed. In contrast to testing conducted in North America, few cases in much of Central and South America were laboratory confirmed.
Asymptomatic infections have occurred in blood donors, said the FDA, with 1.8% of blood donations in Puerto Rico testing positive for Zika virus during the peak of the outbreak. Transmission by transfusion is thought to have occurred in Brazil.
Although Zika virus infections have plummeted in the United States and worldwide, prevalence and rates of local transmission are unpredictable, said the FDA, which pointed to sporadic increases in autochthonous transmission of viruses such as dengue and chikungunya that are carried by the same mosquito vector as Zika.
Some of the committee’s discussion centered around finding a way to carve out protection for those most harmed by Zika virus – pregnant women and their fetuses. Martin Schreiber, MD, professor of surgery at Oregon Health and Sciences University, Portland, proposed a point-of-care testing strategy in which only blood destined for pregnant women would be tested for Zika virus. Dr. Schreiber, a trauma surgeon, put forward the rationale that Zika virus causes harm almost exclusively to fetuses, except for rare cases of Guillain-Barré syndrome.
In response, Dr. Kaufman pointed out that with rare exceptions for some bacterial testing, all testing is done from samples taken at the point of donation. The supply chain for donor blood is not set up to accommodate point-of-care testing, he said.
Answering questions about another targeted strategy – maintaining a separate, Zika-tested supply of blood for pregnant women – Susan Stramer, PhD, vice president of scientific affairs for the American Red Cross, said, “Most hospitals do not want, and are very adamant against, carrying a dual inventory.”
Ultimately, the committee’s discussion swung toward the realization that it may be too soon after the recent spike in U.S. Zika cases to plot the best course for ongoing testing strategies. “We are at the tail end of a waning epidemic. ... I think it would probably be a pretty easy question for the committee and for the agency if we actually had some way of having a crystal ball and knowing that the current trend was likely to continue,” said Roger Lewis, MD, PhD, professor at the University of California, Los Angeles, and chair of the department of emergency medicine at Harbor-UCLA Medical Center.
“I think that is not the question,” he went on. “I think the question is, What is the optimal strategy if we have no idea if that tail is going to continue in this current trend. ... And that maybe the committee ought to be thinking about what is the right strategy for the next 2 years – with an underlying assumption that this is a question that can be brought back as we learn more about how this disease behaves.”
The FDA usually follows the recommendations of its advisory committees.
Most members of a Food and Drug Administration advisory committee considered that data support maintaining current testing protocols for Zika virus in the blood donor pool. However, committee discussion entertained the idea of revisiting testing strategies after another year or 2 of Zika virus epidemiological data are available.
In its last guidance regarding Zika virus testing, issued in July 2018, the FDA recommended that either minipool nucleic acid testing (MP NAT) or individual donor (ID) NAT be used to screen for Zika virus. Current guidance still requires conversion to all-ID NAT “when certain threshold conditions are met that indicate an increased risk of suspected mosquito-borne transmission in a defined geographic collection area.”
In the first of three separate votes, 11 of 15 voting members of the FDA’s Blood Products Advisory Committee (BPAC) answered in the affirmative to the question of whether available data support continuing the status quo for Zika testing. Committee members then were asked to weigh whether current data support scaling back to a regional testing strategy targeting at-risk areas. Here, six committee members answered in the affirmative, and nine in the negative.
Just one committee member, F. Blaine Hollinger, MD, voted in favor of the third option, elimination of all Zika virus testing without reintroducing donor screening for risk factors in risk-free areas pending another outbreak in the United States. Dr. Hollinger is a professor of virology and microbiology at Baylor College of Medicine, Houston.
The committee as whole wasn’t swayed by a line of questioning put forward by chairman Richard Kaufman, MD. “I will be the devil’s advocate a little bit: We learned that there have been zero confirmed positives from blood donors for the past year. Would anyone be comfortable with just stopping screening of donors?” asked Dr. Kaufman, medical director of the adult transfusion service at Brigham and Women’s Hospital, Boston.
A wide-ranging morning of presentations put data regarding historical trends and current global Zika hot spots in front of the committee. Current upticks in infection rates in northwest Mexico and in some states in India were areas of concern, given North American travel patterns, noted speaker Marc Fisher, MD, of the Center for Disease Control and Prevention’s Arboviral Disease Branch (Fort Collins, Colo.) “We’re going to see sporadic outbreaks; it’s hard to predict the future,” he said. “The new outbreak in India raises concerns.”
Briefing information from the FDA explained that Zika virus local transmission peaked in the United States in late summer of 2016. More than 5,000 cases were reported in the United States and over 36,000 in Puerto Rico. This has plummeted to 220 in 2018, with about two-thirds of these cases occurring in the territories, mostly (97%) from Puerto Rico across all 3 years.
Zika viremic blood donors dropped by an order of magnitude yearly, totaling 363 in 2016, 38 in 2017, and just 3 in 2018. Of the 363 detected in 2016, 96% came from Puerto Rico or Florida, noted Dr. Fisher.
The number of suspected and confirmed cases in the Americas overall has also dropped from over 650,000 in 2016 to under 30,000 in 2018, with most cases in 2018 being suspected rather than laboratory confirmed. In contrast to testing conducted in North America, few cases in much of Central and South America were laboratory confirmed.
Asymptomatic infections have occurred in blood donors, said the FDA, with 1.8% of blood donations in Puerto Rico testing positive for Zika virus during the peak of the outbreak. Transmission by transfusion is thought to have occurred in Brazil.
Although Zika virus infections have plummeted in the United States and worldwide, prevalence and rates of local transmission are unpredictable, said the FDA, which pointed to sporadic increases in autochthonous transmission of viruses such as dengue and chikungunya that are carried by the same mosquito vector as Zika.
Some of the committee’s discussion centered around finding a way to carve out protection for those most harmed by Zika virus – pregnant women and their fetuses. Martin Schreiber, MD, professor of surgery at Oregon Health and Sciences University, Portland, proposed a point-of-care testing strategy in which only blood destined for pregnant women would be tested for Zika virus. Dr. Schreiber, a trauma surgeon, put forward the rationale that Zika virus causes harm almost exclusively to fetuses, except for rare cases of Guillain-Barré syndrome.
In response, Dr. Kaufman pointed out that with rare exceptions for some bacterial testing, all testing is done from samples taken at the point of donation. The supply chain for donor blood is not set up to accommodate point-of-care testing, he said.
Answering questions about another targeted strategy – maintaining a separate, Zika-tested supply of blood for pregnant women – Susan Stramer, PhD, vice president of scientific affairs for the American Red Cross, said, “Most hospitals do not want, and are very adamant against, carrying a dual inventory.”
Ultimately, the committee’s discussion swung toward the realization that it may be too soon after the recent spike in U.S. Zika cases to plot the best course for ongoing testing strategies. “We are at the tail end of a waning epidemic. ... I think it would probably be a pretty easy question for the committee and for the agency if we actually had some way of having a crystal ball and knowing that the current trend was likely to continue,” said Roger Lewis, MD, PhD, professor at the University of California, Los Angeles, and chair of the department of emergency medicine at Harbor-UCLA Medical Center.
“I think that is not the question,” he went on. “I think the question is, What is the optimal strategy if we have no idea if that tail is going to continue in this current trend. ... And that maybe the committee ought to be thinking about what is the right strategy for the next 2 years – with an underlying assumption that this is a question that can be brought back as we learn more about how this disease behaves.”
The FDA usually follows the recommendations of its advisory committees.
MedPAC puts Part B reference pricing, binding arbitration on the table
Much of the presentation, offered during the commission’s March meeting, was general ideas with more work to come in terms of fleshing out the details. An ambitious goal of having something ready for the commission’s June 2019 report to Congress was set.
The policy recommendations for reference pricing, to be used when multiple similar drugs are available, and binding arbitration, to be used on new entrants to the market with limited or no competition, are being designed to work with the previously recommended drug value program, but could be implemented on their own.
In general, the reference pricing policy would set a maximum payment rate for a group of drugs with similar health effects based on the minimum, median, or other point along the range of prices for all drugs in that group. Providers would be incentivized to choose a lower-cost alternative when clinically appropriate.
Beneficiaries who still want access to a higher-cost drug would be on the hook for the difference through cost-sharing mechanisms.
MedPAC staff presented two options for setting the reference price. One would be to establish the price based on internal Medicare data. The other would take international pricing into consideration.
Binding arbitration, which is already a component of the drug value program, would be expanded. In the program described by staff, Medicare and the manufacturer would each come to the table with a price and the arbitrator (either an individual or a panel) would set one price.
Potential cost savings from one or both programs was not addressed
“It seems like an important thing for us to understand in order to know the potential impact ... through these two levers that work on different parts of the spend problem,” said Commissioner Dana Safran, head of measurement for the health care venture formed by Amazon, Berkshire Hathaway, and JPMorgan Chase.
Staff said it would work on making that determination.
Commissioners raised additional questions on operational details.
Marjorie Ginsburg, founding executive director of the Center for Healthcare Decisions Inc. in Sacramento, Calif., questioned what would happen if a manufacturer declined to participate in the arbitration process and whether that would mean Medicare would not cover a drug in that circumstance.
Jay Crosson, MD, noted that “Congress would have to … figure out how to deal with that circumstance. ... We would not want to end up with a system that would deny coverage” of effective medications for Medicare beneficiaries.
Another area affecting both issues was the potential for cross subsidization of drugs.
Jonathan Perlin, MD, president of clinical services and chief medical officer of HCA Healthcare of Nashville, Tenn., questioned whether this could open a door for a provider buying at a cheaper government price and using the drugs across patients not from Medicare or whether it could lead to higher prices being charged to commercial payers.
MedPAC staff member Kim Neuman said that “there would need to be some back end reconciliations that would happen to ensure that the stock that was then administered to Medicare patients was provided at a price that was no higher than that ceiling. ... We haven’t scoped out implications for other payers.”
Commissioner Kathy Buto, independent consultant and former vice president of global health policy at Johnson & Johnson, inquired about whether a drug would be made available upon launch while reference pricing or arbitration processes were in progress.
Commissioners also inquired as to how the reference pricing aspects will be operationalized into conversations between the doctor and the patient.
Ms. Buto also cautioned that using a reference pricing scheme could alter the dynamic of pricing competition that has companies competing against a reference price rather than doing what they can to lower prices beyond that.
Much of the presentation, offered during the commission’s March meeting, was general ideas with more work to come in terms of fleshing out the details. An ambitious goal of having something ready for the commission’s June 2019 report to Congress was set.
The policy recommendations for reference pricing, to be used when multiple similar drugs are available, and binding arbitration, to be used on new entrants to the market with limited or no competition, are being designed to work with the previously recommended drug value program, but could be implemented on their own.
In general, the reference pricing policy would set a maximum payment rate for a group of drugs with similar health effects based on the minimum, median, or other point along the range of prices for all drugs in that group. Providers would be incentivized to choose a lower-cost alternative when clinically appropriate.
Beneficiaries who still want access to a higher-cost drug would be on the hook for the difference through cost-sharing mechanisms.
MedPAC staff presented two options for setting the reference price. One would be to establish the price based on internal Medicare data. The other would take international pricing into consideration.
Binding arbitration, which is already a component of the drug value program, would be expanded. In the program described by staff, Medicare and the manufacturer would each come to the table with a price and the arbitrator (either an individual or a panel) would set one price.
Potential cost savings from one or both programs was not addressed
“It seems like an important thing for us to understand in order to know the potential impact ... through these two levers that work on different parts of the spend problem,” said Commissioner Dana Safran, head of measurement for the health care venture formed by Amazon, Berkshire Hathaway, and JPMorgan Chase.
Staff said it would work on making that determination.
Commissioners raised additional questions on operational details.
Marjorie Ginsburg, founding executive director of the Center for Healthcare Decisions Inc. in Sacramento, Calif., questioned what would happen if a manufacturer declined to participate in the arbitration process and whether that would mean Medicare would not cover a drug in that circumstance.
Jay Crosson, MD, noted that “Congress would have to … figure out how to deal with that circumstance. ... We would not want to end up with a system that would deny coverage” of effective medications for Medicare beneficiaries.
Another area affecting both issues was the potential for cross subsidization of drugs.
Jonathan Perlin, MD, president of clinical services and chief medical officer of HCA Healthcare of Nashville, Tenn., questioned whether this could open a door for a provider buying at a cheaper government price and using the drugs across patients not from Medicare or whether it could lead to higher prices being charged to commercial payers.
MedPAC staff member Kim Neuman said that “there would need to be some back end reconciliations that would happen to ensure that the stock that was then administered to Medicare patients was provided at a price that was no higher than that ceiling. ... We haven’t scoped out implications for other payers.”
Commissioner Kathy Buto, independent consultant and former vice president of global health policy at Johnson & Johnson, inquired about whether a drug would be made available upon launch while reference pricing or arbitration processes were in progress.
Commissioners also inquired as to how the reference pricing aspects will be operationalized into conversations between the doctor and the patient.
Ms. Buto also cautioned that using a reference pricing scheme could alter the dynamic of pricing competition that has companies competing against a reference price rather than doing what they can to lower prices beyond that.
Much of the presentation, offered during the commission’s March meeting, was general ideas with more work to come in terms of fleshing out the details. An ambitious goal of having something ready for the commission’s June 2019 report to Congress was set.
The policy recommendations for reference pricing, to be used when multiple similar drugs are available, and binding arbitration, to be used on new entrants to the market with limited or no competition, are being designed to work with the previously recommended drug value program, but could be implemented on their own.
In general, the reference pricing policy would set a maximum payment rate for a group of drugs with similar health effects based on the minimum, median, or other point along the range of prices for all drugs in that group. Providers would be incentivized to choose a lower-cost alternative when clinically appropriate.
Beneficiaries who still want access to a higher-cost drug would be on the hook for the difference through cost-sharing mechanisms.
MedPAC staff presented two options for setting the reference price. One would be to establish the price based on internal Medicare data. The other would take international pricing into consideration.
Binding arbitration, which is already a component of the drug value program, would be expanded. In the program described by staff, Medicare and the manufacturer would each come to the table with a price and the arbitrator (either an individual or a panel) would set one price.
Potential cost savings from one or both programs was not addressed
“It seems like an important thing for us to understand in order to know the potential impact ... through these two levers that work on different parts of the spend problem,” said Commissioner Dana Safran, head of measurement for the health care venture formed by Amazon, Berkshire Hathaway, and JPMorgan Chase.
Staff said it would work on making that determination.
Commissioners raised additional questions on operational details.
Marjorie Ginsburg, founding executive director of the Center for Healthcare Decisions Inc. in Sacramento, Calif., questioned what would happen if a manufacturer declined to participate in the arbitration process and whether that would mean Medicare would not cover a drug in that circumstance.
Jay Crosson, MD, noted that “Congress would have to … figure out how to deal with that circumstance. ... We would not want to end up with a system that would deny coverage” of effective medications for Medicare beneficiaries.
Another area affecting both issues was the potential for cross subsidization of drugs.
Jonathan Perlin, MD, president of clinical services and chief medical officer of HCA Healthcare of Nashville, Tenn., questioned whether this could open a door for a provider buying at a cheaper government price and using the drugs across patients not from Medicare or whether it could lead to higher prices being charged to commercial payers.
MedPAC staff member Kim Neuman said that “there would need to be some back end reconciliations that would happen to ensure that the stock that was then administered to Medicare patients was provided at a price that was no higher than that ceiling. ... We haven’t scoped out implications for other payers.”
Commissioner Kathy Buto, independent consultant and former vice president of global health policy at Johnson & Johnson, inquired about whether a drug would be made available upon launch while reference pricing or arbitration processes were in progress.
Commissioners also inquired as to how the reference pricing aspects will be operationalized into conversations between the doctor and the patient.
Ms. Buto also cautioned that using a reference pricing scheme could alter the dynamic of pricing competition that has companies competing against a reference price rather than doing what they can to lower prices beyond that.
REPORTING FROM A MEDPAC MEETING
Home oxygen therapy for children: New guidelines combine limited evidence, expert experience
Based on the very limited evidence available, an expert panel convened by the .
The guideline authors not only addressed specific indications for chronic lung and pulmonary vascular diseases, but also defined hypoxemia in children – noting that Medicare and Medicaid coverage determinations for home oxygen therapy in children are based on decades-old studies that lacked pediatric patients – and offer expert advice on how to wean and discontinue oxygen, when warranted.
The disease-specific recommendations on whether or not to prescribe home oxygen therapy are characterized either as strong, meaning that it’s the right course of action for at least 95% of patients; or conditional, meaning it might not be right for a “sizable minority” of patients, authors explained in the guideline.
Home oxygen therapy gets a strong recommendation, for example, in patients with cystic fibrosis complicated by severe chronic hypoxemia, but gets a conditional recommendation for sickle cell disease with severe chronic hypoxemia, according to the guideline, published in the American Journal of Respiratory and Critical Care Medicine.
Regardless of strong or conditional, the recommendations were largely based on “very low-quality evidence,” according to ad hoc subcommittee of the ATS Assembly on Pediatrics, cochaired by Don Hayes Jr., MD, of Nationwide Children’s Hospital, Columbus, Ohio, and Robin R. Deterding, MD, of Children’s Hospital Colorado, Denver.
“Despite widespread use of home oxygen therapy for various lung and pulmonary vascular diseases, there is a striking paucity of data regarding its implementation, efficacy, monitoring, and discontinuation,” Dr. Hayes, Dr. Deterding, and 20 additional committee members wrote in their report.
Accordingly, the panel sought to add expert opinion and experience to the limited evidence, in the hope that it would aid clinicians in the management of complex pediatric patients, they said.
One new tool they provide, toward that end, is a definition of hypoxemia in children based on oxygen saturation as quantified by pulse oximetry (SpO2).
Based on a review of 31 selected studies measuring oxygenation in healthy children, the expert panel defined hypoxemia (at or near sea level) as SpO2 of 90% or lower for 5% of the recording time in children under 1 year old, and an SpO2 of 93% or lower in older children; or alternately, as three independent measurements of SpO2 less than or equal to 90% in the younger children and 93% in the older children.
By contrast, an SpO2 of less than 88% is one of the indications for funding home oxygen therapy as determined by the Centers for Medicare & Medicaid Services for both pediatric and adult patients, according to the committee.
The CMS indications derived from “seminal studies” showing that continuous oxygen therapy reduced mortality in adults with chronic obstructive pulmonary disease, they said in the guideline document.
“Despite the lack of pediatric patients in these historic studies performed over 35 years ago, the CMS coverage determination for [home oxygen therapy] is the same for pediatric patients of all ages compared with adult patients,” they wrote in the report.
The committee unanimously agreed that 2 weeks of low SpO2 was “sufficient evidence” to indicate chronic hypoxemia, their report says.
Dr. Hayes reported no relationships with relevant commercial interests, while Dr. Deterding provided disclosures related to Boehringer Ingelheim, Novartis, and Elsevier Publishing, among others. Fellow committee members provided disclosures related to Shire Pharmaceuticals, United Therapeutics, and others as listed in the clinical practice guideline document.
SOURCE: Hayes D Jr. et al. J Respir Crit Care Med. 2019 Feb 1;199(3):e5-e23. doi: 10.1164/rccm.201812-2276ST.
It is unfortunate that over the course of a decade, the evidence base supporting home oxygen therapy in children has not substantially changed, according to Ian Balfour-Lynn, MD, a member of the American Thoracic Society (ATS) committee that developed the clinical practice guideline.
The ATS clinical practice guideline on home oxygen therapy for children echoes conclusions reached in a 2009 guideline published by the British Thoracic Society (BTS), he wrote in The Lancet Respiratory Medicine.
Dr. Balfour-Lynn, who chaired the BTS guideline committee, said new research is sorely needed, particularly in the prevention of preterm births, which he said constitute the commonest cause of home oxygen need among children, according to the Lancet report.
In addition, a large prospective trial is needed to evaluate strategies for weaning or discontinuing oxygen, he said, noting that the ATS recommendations on weaning were almost entirely based on the expert panel’s combined clinical experience.
Dr. Balfour-Lynn is a consultant in pediatric respiratory medicine at Royal Brompton Hospital, London. This summary of his opinions is based on his comments in a report that appeared March 8 in The Lancet Respiratory Medicine . He reported no relationships with commercial interests relevant to his work on the ATS clinical practice guideline.
It is unfortunate that over the course of a decade, the evidence base supporting home oxygen therapy in children has not substantially changed, according to Ian Balfour-Lynn, MD, a member of the American Thoracic Society (ATS) committee that developed the clinical practice guideline.
The ATS clinical practice guideline on home oxygen therapy for children echoes conclusions reached in a 2009 guideline published by the British Thoracic Society (BTS), he wrote in The Lancet Respiratory Medicine.
Dr. Balfour-Lynn, who chaired the BTS guideline committee, said new research is sorely needed, particularly in the prevention of preterm births, which he said constitute the commonest cause of home oxygen need among children, according to the Lancet report.
In addition, a large prospective trial is needed to evaluate strategies for weaning or discontinuing oxygen, he said, noting that the ATS recommendations on weaning were almost entirely based on the expert panel’s combined clinical experience.
Dr. Balfour-Lynn is a consultant in pediatric respiratory medicine at Royal Brompton Hospital, London. This summary of his opinions is based on his comments in a report that appeared March 8 in The Lancet Respiratory Medicine . He reported no relationships with commercial interests relevant to his work on the ATS clinical practice guideline.
It is unfortunate that over the course of a decade, the evidence base supporting home oxygen therapy in children has not substantially changed, according to Ian Balfour-Lynn, MD, a member of the American Thoracic Society (ATS) committee that developed the clinical practice guideline.
The ATS clinical practice guideline on home oxygen therapy for children echoes conclusions reached in a 2009 guideline published by the British Thoracic Society (BTS), he wrote in The Lancet Respiratory Medicine.
Dr. Balfour-Lynn, who chaired the BTS guideline committee, said new research is sorely needed, particularly in the prevention of preterm births, which he said constitute the commonest cause of home oxygen need among children, according to the Lancet report.
In addition, a large prospective trial is needed to evaluate strategies for weaning or discontinuing oxygen, he said, noting that the ATS recommendations on weaning were almost entirely based on the expert panel’s combined clinical experience.
Dr. Balfour-Lynn is a consultant in pediatric respiratory medicine at Royal Brompton Hospital, London. This summary of his opinions is based on his comments in a report that appeared March 8 in The Lancet Respiratory Medicine . He reported no relationships with commercial interests relevant to his work on the ATS clinical practice guideline.
Based on the very limited evidence available, an expert panel convened by the .
The guideline authors not only addressed specific indications for chronic lung and pulmonary vascular diseases, but also defined hypoxemia in children – noting that Medicare and Medicaid coverage determinations for home oxygen therapy in children are based on decades-old studies that lacked pediatric patients – and offer expert advice on how to wean and discontinue oxygen, when warranted.
The disease-specific recommendations on whether or not to prescribe home oxygen therapy are characterized either as strong, meaning that it’s the right course of action for at least 95% of patients; or conditional, meaning it might not be right for a “sizable minority” of patients, authors explained in the guideline.
Home oxygen therapy gets a strong recommendation, for example, in patients with cystic fibrosis complicated by severe chronic hypoxemia, but gets a conditional recommendation for sickle cell disease with severe chronic hypoxemia, according to the guideline, published in the American Journal of Respiratory and Critical Care Medicine.
Regardless of strong or conditional, the recommendations were largely based on “very low-quality evidence,” according to ad hoc subcommittee of the ATS Assembly on Pediatrics, cochaired by Don Hayes Jr., MD, of Nationwide Children’s Hospital, Columbus, Ohio, and Robin R. Deterding, MD, of Children’s Hospital Colorado, Denver.
“Despite widespread use of home oxygen therapy for various lung and pulmonary vascular diseases, there is a striking paucity of data regarding its implementation, efficacy, monitoring, and discontinuation,” Dr. Hayes, Dr. Deterding, and 20 additional committee members wrote in their report.
Accordingly, the panel sought to add expert opinion and experience to the limited evidence, in the hope that it would aid clinicians in the management of complex pediatric patients, they said.
One new tool they provide, toward that end, is a definition of hypoxemia in children based on oxygen saturation as quantified by pulse oximetry (SpO2).
Based on a review of 31 selected studies measuring oxygenation in healthy children, the expert panel defined hypoxemia (at or near sea level) as SpO2 of 90% or lower for 5% of the recording time in children under 1 year old, and an SpO2 of 93% or lower in older children; or alternately, as three independent measurements of SpO2 less than or equal to 90% in the younger children and 93% in the older children.
By contrast, an SpO2 of less than 88% is one of the indications for funding home oxygen therapy as determined by the Centers for Medicare & Medicaid Services for both pediatric and adult patients, according to the committee.
The CMS indications derived from “seminal studies” showing that continuous oxygen therapy reduced mortality in adults with chronic obstructive pulmonary disease, they said in the guideline document.
“Despite the lack of pediatric patients in these historic studies performed over 35 years ago, the CMS coverage determination for [home oxygen therapy] is the same for pediatric patients of all ages compared with adult patients,” they wrote in the report.
The committee unanimously agreed that 2 weeks of low SpO2 was “sufficient evidence” to indicate chronic hypoxemia, their report says.
Dr. Hayes reported no relationships with relevant commercial interests, while Dr. Deterding provided disclosures related to Boehringer Ingelheim, Novartis, and Elsevier Publishing, among others. Fellow committee members provided disclosures related to Shire Pharmaceuticals, United Therapeutics, and others as listed in the clinical practice guideline document.
SOURCE: Hayes D Jr. et al. J Respir Crit Care Med. 2019 Feb 1;199(3):e5-e23. doi: 10.1164/rccm.201812-2276ST.
Based on the very limited evidence available, an expert panel convened by the .
The guideline authors not only addressed specific indications for chronic lung and pulmonary vascular diseases, but also defined hypoxemia in children – noting that Medicare and Medicaid coverage determinations for home oxygen therapy in children are based on decades-old studies that lacked pediatric patients – and offer expert advice on how to wean and discontinue oxygen, when warranted.
The disease-specific recommendations on whether or not to prescribe home oxygen therapy are characterized either as strong, meaning that it’s the right course of action for at least 95% of patients; or conditional, meaning it might not be right for a “sizable minority” of patients, authors explained in the guideline.
Home oxygen therapy gets a strong recommendation, for example, in patients with cystic fibrosis complicated by severe chronic hypoxemia, but gets a conditional recommendation for sickle cell disease with severe chronic hypoxemia, according to the guideline, published in the American Journal of Respiratory and Critical Care Medicine.
Regardless of strong or conditional, the recommendations were largely based on “very low-quality evidence,” according to ad hoc subcommittee of the ATS Assembly on Pediatrics, cochaired by Don Hayes Jr., MD, of Nationwide Children’s Hospital, Columbus, Ohio, and Robin R. Deterding, MD, of Children’s Hospital Colorado, Denver.
“Despite widespread use of home oxygen therapy for various lung and pulmonary vascular diseases, there is a striking paucity of data regarding its implementation, efficacy, monitoring, and discontinuation,” Dr. Hayes, Dr. Deterding, and 20 additional committee members wrote in their report.
Accordingly, the panel sought to add expert opinion and experience to the limited evidence, in the hope that it would aid clinicians in the management of complex pediatric patients, they said.
One new tool they provide, toward that end, is a definition of hypoxemia in children based on oxygen saturation as quantified by pulse oximetry (SpO2).
Based on a review of 31 selected studies measuring oxygenation in healthy children, the expert panel defined hypoxemia (at or near sea level) as SpO2 of 90% or lower for 5% of the recording time in children under 1 year old, and an SpO2 of 93% or lower in older children; or alternately, as three independent measurements of SpO2 less than or equal to 90% in the younger children and 93% in the older children.
By contrast, an SpO2 of less than 88% is one of the indications for funding home oxygen therapy as determined by the Centers for Medicare & Medicaid Services for both pediatric and adult patients, according to the committee.
The CMS indications derived from “seminal studies” showing that continuous oxygen therapy reduced mortality in adults with chronic obstructive pulmonary disease, they said in the guideline document.
“Despite the lack of pediatric patients in these historic studies performed over 35 years ago, the CMS coverage determination for [home oxygen therapy] is the same for pediatric patients of all ages compared with adult patients,” they wrote in the report.
The committee unanimously agreed that 2 weeks of low SpO2 was “sufficient evidence” to indicate chronic hypoxemia, their report says.
Dr. Hayes reported no relationships with relevant commercial interests, while Dr. Deterding provided disclosures related to Boehringer Ingelheim, Novartis, and Elsevier Publishing, among others. Fellow committee members provided disclosures related to Shire Pharmaceuticals, United Therapeutics, and others as listed in the clinical practice guideline document.
SOURCE: Hayes D Jr. et al. J Respir Crit Care Med. 2019 Feb 1;199(3):e5-e23. doi: 10.1164/rccm.201812-2276ST.
FROM THE AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE
Telerehabilitation is noninferior to in-clinic rehabilitation for poststroke arm function
HONOLULU – according to research presented at the International Stroke Conference sponsored by the American Heart Association. Telerehabilitation also provides patient education as effectively as in-clinic rehabilitation, said Steven C. Cramer, MD, professor of neurology at the University of California, Irvine.
Stroke is a leading cause of disability, and more than 80% of patients with stroke have motor deficits when they present to the ED. Research indicates that high doses of rehabilitation therapy improve brain and motor function. However, many patients get low amounts of rehabilitation because of obstacles such as travel difficulties and shortages of therapy providers. “We reasoned that telerehabilitation is ideally suited to efficiently provide a large dose of useful, high-quality rehab therapy after stroke,” Dr. Cramer said.
Participants received supervised and unsupervised therapy
He and his colleagues enrolled patients who had experienced a stroke during the previous 4-36 weeks and who had arm motor deficits into their study. Eligible participants were adults, had experienced ischemic stroke or intracerebral hemorrhage, and had an arm Fugl-Meyer score between 22 and 56 out of 66.
Dr. Cramer’s group randomized 124 participants at 11 National Institutes of Health StrokeNet sites to 6 weeks of intensive arm rehabilitation therapy, plus stroke education, delivered in clinic or at home by a telehealth system. For both groups, treatment included 36 sessions that each lasted for 70 minutes. Half of the sessions were supervised and half were not. All sessions included at least 15 minutes of arm exercises and at least 15 minutes of functional training. Unsupervised sessions also included at least 5 minutes of stroke education on topics such as prevention, risk factors, recognition, and treatment. Participants in the in-clinic group worked with therapists in the clinic on supervised days and at home with a personalized booklet on unsupervised days. Participants in the telerehabilitation group played specially designed and individually tailored computer games at home on all days and had video conferences with therapists on supervised days. The treatment groups included approximately equal numbers of patients; treatment duration, intensity, and frequency were matched between groups.
The investigators hypothesized that telerehabilitation was not inferior to in-clinic rehabilitation. The study’s primary endpoint was change in Fugl-Meyer score from baseline to 30 days after the end of therapy. Secondary end points included Box and Blocks score (that is, a measure of arm function), Stroke Impact Scale–hand, and gains in stroke knowledge. The researchers defined the noninferiority margin as 30% of the gains of the in-clinic group. End points were evaluated by blinded assessors.
Patients had clinically meaningful gains
Participants’ average age was 61 years; the mean baseline arm Fugl-Meyer score was 42. Stroke onset had occurred at a mean of 4.5 months previously, and most strokes were ischemic. In all, 10 participants dropped out of the study. The rate of compliance was 98.3% in the telerehabilitation group and 93.0% in the in-clinic group.
The change in Fugl-Meyer score from baseline to 30 days post therapy was 8.36 points in the in-clinic group and 7.86 points in the telerehabilitation group. The changes in this score were higher than the minimal clinically important difference. The difference between groups, adjusted for covariance, was approximately 0. In addition, the 95% confidence interval for the change in score in the telerehabilitation group was within the noninferiority margin. “We can say that telerehabilitation is not inferior” to in-clinic therapy, said Dr. Cramer.
Telerehabilitation also was noninferior to in-clinic rehabilitation on the Box and Blocks score, and gains in stroke knowledge were significant and comparable in both groups. “Interestingly, the arm motor gains did not differ whether the subjects had aphasia or not,” said Dr. Cramer.
The investigators measured activity-inherent motivation (that is, how much a patient likes rehabilitation) using the Physical Activity Enjoyment Scale. Scores were higher in the in-clinic group, compared with the telerehabilitation group. “People like going to sit with a live human, and they like the longer time with the live human. This is something for us to study further and understand,” said Dr. Cramer.
Dr. Cramer and colleagues observed six serious adverse events in the in-clinic group and one in the telerehabilitation group, such as pneumonia or palpitations, all of which were deemed unrelated to therapy. Adverse events related to therapy (for example, shoulder pain and fatigue) were equally distributed between the two groups.
“What we were trying to do with home-based telehealth does not compete with or replace traditional rehab medicine. It is expanding tools for occupational and physical therapists, for nurses and physicians,” said Dr. Cramer.
Future studies could examine the efficacy of telerehabilitation in the treatment of language deficits, leg weakness, micturition, and dysphagia. “We might also study telehealth such as this to see how we can improve access and lower the cost of poststroke rehab care,” he concluded.
The study was funded by the Eunice Kennedy Shriver National Institute Of Child Health & Human Development and several grants from the National Institute of Neurological Disorders and Stroke. Dr. Cramer has an ownership interest in TRCare, a company that plans to market a telerehabilitation system and was not involved in the study. In addition, he is a consultant or advisor for MicroTransponder, Dart Neuroscience, Neurolutions, Regenera, Abbvie, SanBio, and TRCare.
SOURCE: Cramer SC et al. ISC 2019, Abstract LB23.
HONOLULU – according to research presented at the International Stroke Conference sponsored by the American Heart Association. Telerehabilitation also provides patient education as effectively as in-clinic rehabilitation, said Steven C. Cramer, MD, professor of neurology at the University of California, Irvine.
Stroke is a leading cause of disability, and more than 80% of patients with stroke have motor deficits when they present to the ED. Research indicates that high doses of rehabilitation therapy improve brain and motor function. However, many patients get low amounts of rehabilitation because of obstacles such as travel difficulties and shortages of therapy providers. “We reasoned that telerehabilitation is ideally suited to efficiently provide a large dose of useful, high-quality rehab therapy after stroke,” Dr. Cramer said.
Participants received supervised and unsupervised therapy
He and his colleagues enrolled patients who had experienced a stroke during the previous 4-36 weeks and who had arm motor deficits into their study. Eligible participants were adults, had experienced ischemic stroke or intracerebral hemorrhage, and had an arm Fugl-Meyer score between 22 and 56 out of 66.
Dr. Cramer’s group randomized 124 participants at 11 National Institutes of Health StrokeNet sites to 6 weeks of intensive arm rehabilitation therapy, plus stroke education, delivered in clinic or at home by a telehealth system. For both groups, treatment included 36 sessions that each lasted for 70 minutes. Half of the sessions were supervised and half were not. All sessions included at least 15 minutes of arm exercises and at least 15 minutes of functional training. Unsupervised sessions also included at least 5 minutes of stroke education on topics such as prevention, risk factors, recognition, and treatment. Participants in the in-clinic group worked with therapists in the clinic on supervised days and at home with a personalized booklet on unsupervised days. Participants in the telerehabilitation group played specially designed and individually tailored computer games at home on all days and had video conferences with therapists on supervised days. The treatment groups included approximately equal numbers of patients; treatment duration, intensity, and frequency were matched between groups.
The investigators hypothesized that telerehabilitation was not inferior to in-clinic rehabilitation. The study’s primary endpoint was change in Fugl-Meyer score from baseline to 30 days after the end of therapy. Secondary end points included Box and Blocks score (that is, a measure of arm function), Stroke Impact Scale–hand, and gains in stroke knowledge. The researchers defined the noninferiority margin as 30% of the gains of the in-clinic group. End points were evaluated by blinded assessors.
Patients had clinically meaningful gains
Participants’ average age was 61 years; the mean baseline arm Fugl-Meyer score was 42. Stroke onset had occurred at a mean of 4.5 months previously, and most strokes were ischemic. In all, 10 participants dropped out of the study. The rate of compliance was 98.3% in the telerehabilitation group and 93.0% in the in-clinic group.
The change in Fugl-Meyer score from baseline to 30 days post therapy was 8.36 points in the in-clinic group and 7.86 points in the telerehabilitation group. The changes in this score were higher than the minimal clinically important difference. The difference between groups, adjusted for covariance, was approximately 0. In addition, the 95% confidence interval for the change in score in the telerehabilitation group was within the noninferiority margin. “We can say that telerehabilitation is not inferior” to in-clinic therapy, said Dr. Cramer.
Telerehabilitation also was noninferior to in-clinic rehabilitation on the Box and Blocks score, and gains in stroke knowledge were significant and comparable in both groups. “Interestingly, the arm motor gains did not differ whether the subjects had aphasia or not,” said Dr. Cramer.
The investigators measured activity-inherent motivation (that is, how much a patient likes rehabilitation) using the Physical Activity Enjoyment Scale. Scores were higher in the in-clinic group, compared with the telerehabilitation group. “People like going to sit with a live human, and they like the longer time with the live human. This is something for us to study further and understand,” said Dr. Cramer.
Dr. Cramer and colleagues observed six serious adverse events in the in-clinic group and one in the telerehabilitation group, such as pneumonia or palpitations, all of which were deemed unrelated to therapy. Adverse events related to therapy (for example, shoulder pain and fatigue) were equally distributed between the two groups.
“What we were trying to do with home-based telehealth does not compete with or replace traditional rehab medicine. It is expanding tools for occupational and physical therapists, for nurses and physicians,” said Dr. Cramer.
Future studies could examine the efficacy of telerehabilitation in the treatment of language deficits, leg weakness, micturition, and dysphagia. “We might also study telehealth such as this to see how we can improve access and lower the cost of poststroke rehab care,” he concluded.
The study was funded by the Eunice Kennedy Shriver National Institute Of Child Health & Human Development and several grants from the National Institute of Neurological Disorders and Stroke. Dr. Cramer has an ownership interest in TRCare, a company that plans to market a telerehabilitation system and was not involved in the study. In addition, he is a consultant or advisor for MicroTransponder, Dart Neuroscience, Neurolutions, Regenera, Abbvie, SanBio, and TRCare.
SOURCE: Cramer SC et al. ISC 2019, Abstract LB23.
HONOLULU – according to research presented at the International Stroke Conference sponsored by the American Heart Association. Telerehabilitation also provides patient education as effectively as in-clinic rehabilitation, said Steven C. Cramer, MD, professor of neurology at the University of California, Irvine.
Stroke is a leading cause of disability, and more than 80% of patients with stroke have motor deficits when they present to the ED. Research indicates that high doses of rehabilitation therapy improve brain and motor function. However, many patients get low amounts of rehabilitation because of obstacles such as travel difficulties and shortages of therapy providers. “We reasoned that telerehabilitation is ideally suited to efficiently provide a large dose of useful, high-quality rehab therapy after stroke,” Dr. Cramer said.
Participants received supervised and unsupervised therapy
He and his colleagues enrolled patients who had experienced a stroke during the previous 4-36 weeks and who had arm motor deficits into their study. Eligible participants were adults, had experienced ischemic stroke or intracerebral hemorrhage, and had an arm Fugl-Meyer score between 22 and 56 out of 66.
Dr. Cramer’s group randomized 124 participants at 11 National Institutes of Health StrokeNet sites to 6 weeks of intensive arm rehabilitation therapy, plus stroke education, delivered in clinic or at home by a telehealth system. For both groups, treatment included 36 sessions that each lasted for 70 minutes. Half of the sessions were supervised and half were not. All sessions included at least 15 minutes of arm exercises and at least 15 minutes of functional training. Unsupervised sessions also included at least 5 minutes of stroke education on topics such as prevention, risk factors, recognition, and treatment. Participants in the in-clinic group worked with therapists in the clinic on supervised days and at home with a personalized booklet on unsupervised days. Participants in the telerehabilitation group played specially designed and individually tailored computer games at home on all days and had video conferences with therapists on supervised days. The treatment groups included approximately equal numbers of patients; treatment duration, intensity, and frequency were matched between groups.
The investigators hypothesized that telerehabilitation was not inferior to in-clinic rehabilitation. The study’s primary endpoint was change in Fugl-Meyer score from baseline to 30 days after the end of therapy. Secondary end points included Box and Blocks score (that is, a measure of arm function), Stroke Impact Scale–hand, and gains in stroke knowledge. The researchers defined the noninferiority margin as 30% of the gains of the in-clinic group. End points were evaluated by blinded assessors.
Patients had clinically meaningful gains
Participants’ average age was 61 years; the mean baseline arm Fugl-Meyer score was 42. Stroke onset had occurred at a mean of 4.5 months previously, and most strokes were ischemic. In all, 10 participants dropped out of the study. The rate of compliance was 98.3% in the telerehabilitation group and 93.0% in the in-clinic group.
The change in Fugl-Meyer score from baseline to 30 days post therapy was 8.36 points in the in-clinic group and 7.86 points in the telerehabilitation group. The changes in this score were higher than the minimal clinically important difference. The difference between groups, adjusted for covariance, was approximately 0. In addition, the 95% confidence interval for the change in score in the telerehabilitation group was within the noninferiority margin. “We can say that telerehabilitation is not inferior” to in-clinic therapy, said Dr. Cramer.
Telerehabilitation also was noninferior to in-clinic rehabilitation on the Box and Blocks score, and gains in stroke knowledge were significant and comparable in both groups. “Interestingly, the arm motor gains did not differ whether the subjects had aphasia or not,” said Dr. Cramer.
The investigators measured activity-inherent motivation (that is, how much a patient likes rehabilitation) using the Physical Activity Enjoyment Scale. Scores were higher in the in-clinic group, compared with the telerehabilitation group. “People like going to sit with a live human, and they like the longer time with the live human. This is something for us to study further and understand,” said Dr. Cramer.
Dr. Cramer and colleagues observed six serious adverse events in the in-clinic group and one in the telerehabilitation group, such as pneumonia or palpitations, all of which were deemed unrelated to therapy. Adverse events related to therapy (for example, shoulder pain and fatigue) were equally distributed between the two groups.
“What we were trying to do with home-based telehealth does not compete with or replace traditional rehab medicine. It is expanding tools for occupational and physical therapists, for nurses and physicians,” said Dr. Cramer.
Future studies could examine the efficacy of telerehabilitation in the treatment of language deficits, leg weakness, micturition, and dysphagia. “We might also study telehealth such as this to see how we can improve access and lower the cost of poststroke rehab care,” he concluded.
The study was funded by the Eunice Kennedy Shriver National Institute Of Child Health & Human Development and several grants from the National Institute of Neurological Disorders and Stroke. Dr. Cramer has an ownership interest in TRCare, a company that plans to market a telerehabilitation system and was not involved in the study. In addition, he is a consultant or advisor for MicroTransponder, Dart Neuroscience, Neurolutions, Regenera, Abbvie, SanBio, and TRCare.
SOURCE: Cramer SC et al. ISC 2019, Abstract LB23.
REPORTING FROM ISC 2019