User login
Patients with OCD/hoarding differ from those with OCD/nonhoarding
More autism spectrum disorder symptoms identified as distinguishing factor
Hoarding symptoms are frequently present in patients with obsessive-compulsive disorder (OCD), and significant differences can be found between them and patients with OCD and no hoarding symptoms, a study of 407 patients shows.
The study also identified links to autism spectrum disorder (ASD) among patients with OCD/hoarding symptoms. “The most relevant outcome of this study was the association between persons with OCD/hoarding and the increased severity of autism symptoms,” wrote Yentl E. Boerema of the Amsterdam Public Health Research Institute, and associates. The study was published in the Journal of Affective Disorders.
To conduct the study, the investigators used cross-sectional baseline data from the Netherlands Obsessive Compulsive Disorder Association (NOCDA) study. Participants in the NOCDA sample were aged 18-79 years with current or remitted DSM-IV-TR criteria for OCD. Hoarding symptoms were determined via the adapted version of the Yale-Brown Obsessive Compulsive Scale. A total of 58 patients were found to have both OCD/hoarding symptoms, compared with 349 who did not, the investigators reported.
OCD/hoarding was associated with earlier age of onset (P less than .05) and more severe OCD symptoms (P less than .001), as well as higher scores on all OCD subtypes. It was associated with living without a partner (P less than .05), being less conscientious (P less than .05), and severity of autism symptoms (P less than .001). The investigators speculated that ASD factors might “be responsible for hoarding behavior, including (a) having intense or focused interests, which can lead to collecting of items, and/or (b) having a lack of seeking shared enjoyment, interests, and activity with other people.”
Meanwhile, the investigators found that coexisting OCD/hoarding was not associated with childhood trauma, posttraumatic stress disorder or attention-deficit/hyperactivity disorder – inattentive type or hyperactive type.
“Clinical implications of our findings are to have the treatment follow a more intensive and structured course (i.e., avoid surprises),” having more attention for affective education, and making more use of visual illustrations, the investigators said. “Taken together, this knowledge provides a better understanding of persons with OCD/hoarding and has the potential to improve treatment.”
The study authors reported no conflicts of interest.
More autism spectrum disorder symptoms identified as distinguishing factor
More autism spectrum disorder symptoms identified as distinguishing factor
Hoarding symptoms are frequently present in patients with obsessive-compulsive disorder (OCD), and significant differences can be found between them and patients with OCD and no hoarding symptoms, a study of 407 patients shows.
The study also identified links to autism spectrum disorder (ASD) among patients with OCD/hoarding symptoms. “The most relevant outcome of this study was the association between persons with OCD/hoarding and the increased severity of autism symptoms,” wrote Yentl E. Boerema of the Amsterdam Public Health Research Institute, and associates. The study was published in the Journal of Affective Disorders.
To conduct the study, the investigators used cross-sectional baseline data from the Netherlands Obsessive Compulsive Disorder Association (NOCDA) study. Participants in the NOCDA sample were aged 18-79 years with current or remitted DSM-IV-TR criteria for OCD. Hoarding symptoms were determined via the adapted version of the Yale-Brown Obsessive Compulsive Scale. A total of 58 patients were found to have both OCD/hoarding symptoms, compared with 349 who did not, the investigators reported.
OCD/hoarding was associated with earlier age of onset (P less than .05) and more severe OCD symptoms (P less than .001), as well as higher scores on all OCD subtypes. It was associated with living without a partner (P less than .05), being less conscientious (P less than .05), and severity of autism symptoms (P less than .001). The investigators speculated that ASD factors might “be responsible for hoarding behavior, including (a) having intense or focused interests, which can lead to collecting of items, and/or (b) having a lack of seeking shared enjoyment, interests, and activity with other people.”
Meanwhile, the investigators found that coexisting OCD/hoarding was not associated with childhood trauma, posttraumatic stress disorder or attention-deficit/hyperactivity disorder – inattentive type or hyperactive type.
“Clinical implications of our findings are to have the treatment follow a more intensive and structured course (i.e., avoid surprises),” having more attention for affective education, and making more use of visual illustrations, the investigators said. “Taken together, this knowledge provides a better understanding of persons with OCD/hoarding and has the potential to improve treatment.”
The study authors reported no conflicts of interest.
Hoarding symptoms are frequently present in patients with obsessive-compulsive disorder (OCD), and significant differences can be found between them and patients with OCD and no hoarding symptoms, a study of 407 patients shows.
The study also identified links to autism spectrum disorder (ASD) among patients with OCD/hoarding symptoms. “The most relevant outcome of this study was the association between persons with OCD/hoarding and the increased severity of autism symptoms,” wrote Yentl E. Boerema of the Amsterdam Public Health Research Institute, and associates. The study was published in the Journal of Affective Disorders.
To conduct the study, the investigators used cross-sectional baseline data from the Netherlands Obsessive Compulsive Disorder Association (NOCDA) study. Participants in the NOCDA sample were aged 18-79 years with current or remitted DSM-IV-TR criteria for OCD. Hoarding symptoms were determined via the adapted version of the Yale-Brown Obsessive Compulsive Scale. A total of 58 patients were found to have both OCD/hoarding symptoms, compared with 349 who did not, the investigators reported.
OCD/hoarding was associated with earlier age of onset (P less than .05) and more severe OCD symptoms (P less than .001), as well as higher scores on all OCD subtypes. It was associated with living without a partner (P less than .05), being less conscientious (P less than .05), and severity of autism symptoms (P less than .001). The investigators speculated that ASD factors might “be responsible for hoarding behavior, including (a) having intense or focused interests, which can lead to collecting of items, and/or (b) having a lack of seeking shared enjoyment, interests, and activity with other people.”
Meanwhile, the investigators found that coexisting OCD/hoarding was not associated with childhood trauma, posttraumatic stress disorder or attention-deficit/hyperactivity disorder – inattentive type or hyperactive type.
“Clinical implications of our findings are to have the treatment follow a more intensive and structured course (i.e., avoid surprises),” having more attention for affective education, and making more use of visual illustrations, the investigators said. “Taken together, this knowledge provides a better understanding of persons with OCD/hoarding and has the potential to improve treatment.”
The study authors reported no conflicts of interest.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
FDA halts enrollment in trial of venetoclax for multiple myeloma
The Food and Drug Administration has halted enrollment in trials of venetoclax (Venclexta) for multiple myeloma.
The move comes after a review of data from the phase 3 BELLINI trial, which pitted venetoclax against placebo in relapsed and refractory multiple myeloma patients on a background of bortezomib and low-dose dexamethasone. Venetoclax is not approved for the treatment of multiple myeloma; the agency said that patients using the drug for approved indications should continue use of the drug.
There were 41/194 deaths (21.1%) in the venetoclax arm, versus 11/97 (11.3%) in the placebo group; 13 of the deaths in the venetoclax arm (32%) and 1 death in the placebo arm (9%) were treatment related. Sepsis, pneumonia, and cardiac arrest were the most common treatment-related causes of death in the venetoclax group; 8 of the 13 deaths (62%) were due to infection.
The FDA estimated that the drug doubled the risk of death compared to placebo.
The agency warned against off-label use of venetoclax for multiple myeloma, and noted that the drug “is safe and effective for its approved uses,” which include second-line treatment of chronic lymphocytic leukemia and small lymphocytic lymphoma in adults, as well as newly-diagnosed acute myeloid leukemia in adults age 75 years or older or who have contraindications to standard chemotherapy.
There are more than 10 trials in the United States of venetoclax for multiple myeloma, and most of them have been suspended, including BELLINI.
Patients already enrolled in the trial can remain on treatment, but they must re-consent to the trial. The FDA “will be working directly with sponsors of Venclexta, as well as other investigators conducting clinical trials in patients with multiple myeloma, to determine the extent of the safety issue,” the agency said in a statement.
Abbvie, which is developing venetoclax in partnership with Roche, noted in its own press release that the drug otherwise outperformed placebo in BELLINI, both in progression-free survival (22.4 months versus 11.5 months), and in overall (82% versus 68%) and partial (59% versus 36%) response rates.
Severe grade 3-5 toxicity and serious adverse event rates were similar in the two study arms, as was the overall incidence of infections (79.8% versus 77.1%). However, the incidence of pneumonia was 20.7% with venetoclax, versus 15.6% with placebo.
“We will continue working with the FDA and worldwide regulatory agencies to determine appropriate next steps for the multiple myeloma program,” Michael Severino, MD, AbbVie vice chairman and president, said in the press release.
Venetoclax binds and inhibits the B-cell lymphoma-2 protein, which prevents some blood cancer cells from undergoing programmed cell death.
The Food and Drug Administration has halted enrollment in trials of venetoclax (Venclexta) for multiple myeloma.
The move comes after a review of data from the phase 3 BELLINI trial, which pitted venetoclax against placebo in relapsed and refractory multiple myeloma patients on a background of bortezomib and low-dose dexamethasone. Venetoclax is not approved for the treatment of multiple myeloma; the agency said that patients using the drug for approved indications should continue use of the drug.
There were 41/194 deaths (21.1%) in the venetoclax arm, versus 11/97 (11.3%) in the placebo group; 13 of the deaths in the venetoclax arm (32%) and 1 death in the placebo arm (9%) were treatment related. Sepsis, pneumonia, and cardiac arrest were the most common treatment-related causes of death in the venetoclax group; 8 of the 13 deaths (62%) were due to infection.
The FDA estimated that the drug doubled the risk of death compared to placebo.
The agency warned against off-label use of venetoclax for multiple myeloma, and noted that the drug “is safe and effective for its approved uses,” which include second-line treatment of chronic lymphocytic leukemia and small lymphocytic lymphoma in adults, as well as newly-diagnosed acute myeloid leukemia in adults age 75 years or older or who have contraindications to standard chemotherapy.
There are more than 10 trials in the United States of venetoclax for multiple myeloma, and most of them have been suspended, including BELLINI.
Patients already enrolled in the trial can remain on treatment, but they must re-consent to the trial. The FDA “will be working directly with sponsors of Venclexta, as well as other investigators conducting clinical trials in patients with multiple myeloma, to determine the extent of the safety issue,” the agency said in a statement.
Abbvie, which is developing venetoclax in partnership with Roche, noted in its own press release that the drug otherwise outperformed placebo in BELLINI, both in progression-free survival (22.4 months versus 11.5 months), and in overall (82% versus 68%) and partial (59% versus 36%) response rates.
Severe grade 3-5 toxicity and serious adverse event rates were similar in the two study arms, as was the overall incidence of infections (79.8% versus 77.1%). However, the incidence of pneumonia was 20.7% with venetoclax, versus 15.6% with placebo.
“We will continue working with the FDA and worldwide regulatory agencies to determine appropriate next steps for the multiple myeloma program,” Michael Severino, MD, AbbVie vice chairman and president, said in the press release.
Venetoclax binds and inhibits the B-cell lymphoma-2 protein, which prevents some blood cancer cells from undergoing programmed cell death.
The Food and Drug Administration has halted enrollment in trials of venetoclax (Venclexta) for multiple myeloma.
The move comes after a review of data from the phase 3 BELLINI trial, which pitted venetoclax against placebo in relapsed and refractory multiple myeloma patients on a background of bortezomib and low-dose dexamethasone. Venetoclax is not approved for the treatment of multiple myeloma; the agency said that patients using the drug for approved indications should continue use of the drug.
There were 41/194 deaths (21.1%) in the venetoclax arm, versus 11/97 (11.3%) in the placebo group; 13 of the deaths in the venetoclax arm (32%) and 1 death in the placebo arm (9%) were treatment related. Sepsis, pneumonia, and cardiac arrest were the most common treatment-related causes of death in the venetoclax group; 8 of the 13 deaths (62%) were due to infection.
The FDA estimated that the drug doubled the risk of death compared to placebo.
The agency warned against off-label use of venetoclax for multiple myeloma, and noted that the drug “is safe and effective for its approved uses,” which include second-line treatment of chronic lymphocytic leukemia and small lymphocytic lymphoma in adults, as well as newly-diagnosed acute myeloid leukemia in adults age 75 years or older or who have contraindications to standard chemotherapy.
There are more than 10 trials in the United States of venetoclax for multiple myeloma, and most of them have been suspended, including BELLINI.
Patients already enrolled in the trial can remain on treatment, but they must re-consent to the trial. The FDA “will be working directly with sponsors of Venclexta, as well as other investigators conducting clinical trials in patients with multiple myeloma, to determine the extent of the safety issue,” the agency said in a statement.
Abbvie, which is developing venetoclax in partnership with Roche, noted in its own press release that the drug otherwise outperformed placebo in BELLINI, both in progression-free survival (22.4 months versus 11.5 months), and in overall (82% versus 68%) and partial (59% versus 36%) response rates.
Severe grade 3-5 toxicity and serious adverse event rates were similar in the two study arms, as was the overall incidence of infections (79.8% versus 77.1%). However, the incidence of pneumonia was 20.7% with venetoclax, versus 15.6% with placebo.
“We will continue working with the FDA and worldwide regulatory agencies to determine appropriate next steps for the multiple myeloma program,” Michael Severino, MD, AbbVie vice chairman and president, said in the press release.
Venetoclax binds and inhibits the B-cell lymphoma-2 protein, which prevents some blood cancer cells from undergoing programmed cell death.
What does COMPASS mean for vascular surgeons?
Antithrombotic therapy with low-dose rivaroxaban plus aspirin should be considered in low–bleeding risk patients with peripheral arterial disease who are at increased risk for ischemic and/or limb events, according to an analysis of the COMPASS trial published in Current Opinion in Cardiology.
Mohamad A. Hussain, MD, of the University of Toronto and his colleagues assessed the ramifications of COMPASS to vascular surgeons. They used two clinical case studies of patients with peripheral arterial disease (PAD) to illustrate differing considerations in care.
The COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) trial showed that low-dose rivaroxaban at 2.5 mg twice daily plus daily aspirin was superior to aspirin alone in reducing major adverse cardiovascular and cerebrovascular events, as well as major adverse limb events, among patients with stable atherosclerotic vascular disease, including those with PAD. However, the risk for major bleeding was higher with rivaroxaban plus aspirin and is a serious consideration for patient treatment.
In clinical case 1, used to illustrate the pertinence of COMPASS to patient care, Dr. Hussain and his colleagues detailed a 68-year-old man presenting with a 3-month history of intermittent claudication of bilateral calves at 10 minutes of brisk walking. His comorbidities include a MI with percutaneous coronary stenting 2 years ago, diabetes mellitus, hypertension, hyperlipidemia, and chronic obstructive pulmonary disease on the basis of prior smoking.
Clinical case 2 was a 70-year-old woman with a small gangrenous ulcer on the dorsal part of her first toe on the left foot. She has history of coronary artery disease with coronary artery bypass graft surgery 5 years prior, diabetes mellitus, mild chronic kidney disease, and hypertension. She underwent an uneventful lower extremity bypass using a prosthetic graft and had an uncomplicated postoperative course.
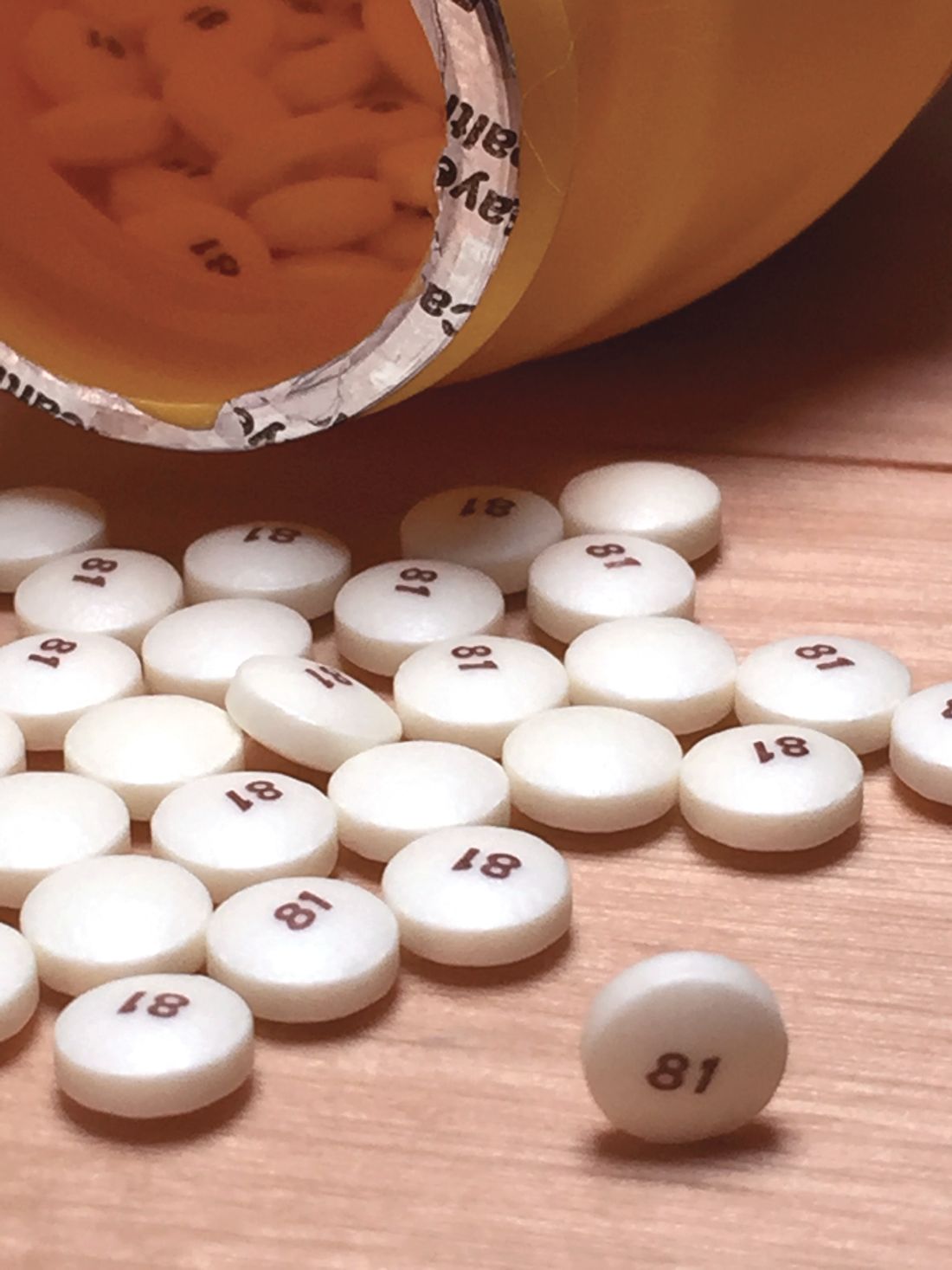
Both patients were on daily 81 mg aspirin.
In order to determine the appropriate care for these patients, the authors presented a flowchart of considerations regarding risk and benefits. Patients with symptomatic PAD who had no recent major bleeds, history of stroke, congestive heart failure, chronic kidney disease, frailty, or anemia were considered for rivaroxaban treatment, otherwise they were put on single antiplatelet therapy.
The investigators recommended that, if the patients were at high limb risk or high ischemic risk, they should be treated with either rivaroxaban plus aspirin or dual antiplatelet therapy (the latter if there was a recent MI or peripheral stenting). If the patients were not at risk, they were deemed eligible for either the rivaroxaban plus aspirin therapy or single antiplatelet therapy.
With regard to the clinical case studies, the authors discussed the rationale for putting both patients on the rivaroxaban plus aspirin therapy after an assessment of the risk/benefit profile for each patient based upon the above considerations. In both cases the bleeding risk was considered low; the ischemic risk in the first patient and the limb risk in the second patient was considered high. Although the second patient had chronic kidney disease, it was not considered severe enough to preclude such treatment.
“Future data from trials such as Vascular Outcomes Study of ASA Along With Rivaroxaban in Endovascular or Surgical Limb Revascularization For Peripheral Artery Disease [VOYAGER PAD] will provide data with regards to the role of low-dose rivaroxaban plus aspirin following peripheral artery revascularization for PAD,” the researchers concluded.
Dr. Hussain reported having no conflicts; his coauthors reported receiving funding from various pharmaceutical companies, including Bayer, which was a sponsor of the original COMPASS trial.
SOURCE: Hussain MA et al. Curr Opin Cardiol. 2019;34:178-84.
Antithrombotic therapy with low-dose rivaroxaban plus aspirin should be considered in low–bleeding risk patients with peripheral arterial disease who are at increased risk for ischemic and/or limb events, according to an analysis of the COMPASS trial published in Current Opinion in Cardiology.
Mohamad A. Hussain, MD, of the University of Toronto and his colleagues assessed the ramifications of COMPASS to vascular surgeons. They used two clinical case studies of patients with peripheral arterial disease (PAD) to illustrate differing considerations in care.
The COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) trial showed that low-dose rivaroxaban at 2.5 mg twice daily plus daily aspirin was superior to aspirin alone in reducing major adverse cardiovascular and cerebrovascular events, as well as major adverse limb events, among patients with stable atherosclerotic vascular disease, including those with PAD. However, the risk for major bleeding was higher with rivaroxaban plus aspirin and is a serious consideration for patient treatment.
In clinical case 1, used to illustrate the pertinence of COMPASS to patient care, Dr. Hussain and his colleagues detailed a 68-year-old man presenting with a 3-month history of intermittent claudication of bilateral calves at 10 minutes of brisk walking. His comorbidities include a MI with percutaneous coronary stenting 2 years ago, diabetes mellitus, hypertension, hyperlipidemia, and chronic obstructive pulmonary disease on the basis of prior smoking.
Clinical case 2 was a 70-year-old woman with a small gangrenous ulcer on the dorsal part of her first toe on the left foot. She has history of coronary artery disease with coronary artery bypass graft surgery 5 years prior, diabetes mellitus, mild chronic kidney disease, and hypertension. She underwent an uneventful lower extremity bypass using a prosthetic graft and had an uncomplicated postoperative course.
Both patients were on daily 81 mg aspirin.
In order to determine the appropriate care for these patients, the authors presented a flowchart of considerations regarding risk and benefits. Patients with symptomatic PAD who had no recent major bleeds, history of stroke, congestive heart failure, chronic kidney disease, frailty, or anemia were considered for rivaroxaban treatment, otherwise they were put on single antiplatelet therapy.
The investigators recommended that, if the patients were at high limb risk or high ischemic risk, they should be treated with either rivaroxaban plus aspirin or dual antiplatelet therapy (the latter if there was a recent MI or peripheral stenting). If the patients were not at risk, they were deemed eligible for either the rivaroxaban plus aspirin therapy or single antiplatelet therapy.
With regard to the clinical case studies, the authors discussed the rationale for putting both patients on the rivaroxaban plus aspirin therapy after an assessment of the risk/benefit profile for each patient based upon the above considerations. In both cases the bleeding risk was considered low; the ischemic risk in the first patient and the limb risk in the second patient was considered high. Although the second patient had chronic kidney disease, it was not considered severe enough to preclude such treatment.
“Future data from trials such as Vascular Outcomes Study of ASA Along With Rivaroxaban in Endovascular or Surgical Limb Revascularization For Peripheral Artery Disease [VOYAGER PAD] will provide data with regards to the role of low-dose rivaroxaban plus aspirin following peripheral artery revascularization for PAD,” the researchers concluded.
Dr. Hussain reported having no conflicts; his coauthors reported receiving funding from various pharmaceutical companies, including Bayer, which was a sponsor of the original COMPASS trial.
SOURCE: Hussain MA et al. Curr Opin Cardiol. 2019;34:178-84.
Antithrombotic therapy with low-dose rivaroxaban plus aspirin should be considered in low–bleeding risk patients with peripheral arterial disease who are at increased risk for ischemic and/or limb events, according to an analysis of the COMPASS trial published in Current Opinion in Cardiology.
Mohamad A. Hussain, MD, of the University of Toronto and his colleagues assessed the ramifications of COMPASS to vascular surgeons. They used two clinical case studies of patients with peripheral arterial disease (PAD) to illustrate differing considerations in care.
The COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) trial showed that low-dose rivaroxaban at 2.5 mg twice daily plus daily aspirin was superior to aspirin alone in reducing major adverse cardiovascular and cerebrovascular events, as well as major adverse limb events, among patients with stable atherosclerotic vascular disease, including those with PAD. However, the risk for major bleeding was higher with rivaroxaban plus aspirin and is a serious consideration for patient treatment.
In clinical case 1, used to illustrate the pertinence of COMPASS to patient care, Dr. Hussain and his colleagues detailed a 68-year-old man presenting with a 3-month history of intermittent claudication of bilateral calves at 10 minutes of brisk walking. His comorbidities include a MI with percutaneous coronary stenting 2 years ago, diabetes mellitus, hypertension, hyperlipidemia, and chronic obstructive pulmonary disease on the basis of prior smoking.
Clinical case 2 was a 70-year-old woman with a small gangrenous ulcer on the dorsal part of her first toe on the left foot. She has history of coronary artery disease with coronary artery bypass graft surgery 5 years prior, diabetes mellitus, mild chronic kidney disease, and hypertension. She underwent an uneventful lower extremity bypass using a prosthetic graft and had an uncomplicated postoperative course.
Both patients were on daily 81 mg aspirin.
In order to determine the appropriate care for these patients, the authors presented a flowchart of considerations regarding risk and benefits. Patients with symptomatic PAD who had no recent major bleeds, history of stroke, congestive heart failure, chronic kidney disease, frailty, or anemia were considered for rivaroxaban treatment, otherwise they were put on single antiplatelet therapy.
The investigators recommended that, if the patients were at high limb risk or high ischemic risk, they should be treated with either rivaroxaban plus aspirin or dual antiplatelet therapy (the latter if there was a recent MI or peripheral stenting). If the patients were not at risk, they were deemed eligible for either the rivaroxaban plus aspirin therapy or single antiplatelet therapy.
With regard to the clinical case studies, the authors discussed the rationale for putting both patients on the rivaroxaban plus aspirin therapy after an assessment of the risk/benefit profile for each patient based upon the above considerations. In both cases the bleeding risk was considered low; the ischemic risk in the first patient and the limb risk in the second patient was considered high. Although the second patient had chronic kidney disease, it was not considered severe enough to preclude such treatment.
“Future data from trials such as Vascular Outcomes Study of ASA Along With Rivaroxaban in Endovascular or Surgical Limb Revascularization For Peripheral Artery Disease [VOYAGER PAD] will provide data with regards to the role of low-dose rivaroxaban plus aspirin following peripheral artery revascularization for PAD,” the researchers concluded.
Dr. Hussain reported having no conflicts; his coauthors reported receiving funding from various pharmaceutical companies, including Bayer, which was a sponsor of the original COMPASS trial.
SOURCE: Hussain MA et al. Curr Opin Cardiol. 2019;34:178-84.
FROM CURRENT OPINION IN CARDIOLOGY
Promising New Approach for Treating Cystic Fibrosis
In cystic fibrosis (CF), a defective gene makes a defective protein that causes acidic and sticky mucus that clogs the lungs and puts patients at risk for bacterial infections. Because different people have different protein mutations, and 10% of patients with CF make no protein at all, treatments are limited. But amphotericin apparently has the potential to work regardless of the mutation and even when the protein is missing. The researchers liken it to a “molecular prosthetic,” because it restores function much as a prosthetic device replaces a limb.
In the study, supported in part by the National Heart, Lung, and Blood Institute, researchers used lung tissue from patients with CF as well as animal models. Instead of trying to correct the protein or do gene therapy, which the researchers say is not yet effective in the lung, they used a small molecule protein that can perform the channel function of the missing or defective protein.
They found that amphotericin restored pH levels, improved viscosity, and increased antibacterial activity.
Amphotericin also can be delivered directly to the lungs to avoid common adverse effects, the researchers say. Although more studies are needed, according to the National Institute of Health, “experts are hopeful.”
In cystic fibrosis (CF), a defective gene makes a defective protein that causes acidic and sticky mucus that clogs the lungs and puts patients at risk for bacterial infections. Because different people have different protein mutations, and 10% of patients with CF make no protein at all, treatments are limited. But amphotericin apparently has the potential to work regardless of the mutation and even when the protein is missing. The researchers liken it to a “molecular prosthetic,” because it restores function much as a prosthetic device replaces a limb.
In the study, supported in part by the National Heart, Lung, and Blood Institute, researchers used lung tissue from patients with CF as well as animal models. Instead of trying to correct the protein or do gene therapy, which the researchers say is not yet effective in the lung, they used a small molecule protein that can perform the channel function of the missing or defective protein.
They found that amphotericin restored pH levels, improved viscosity, and increased antibacterial activity.
Amphotericin also can be delivered directly to the lungs to avoid common adverse effects, the researchers say. Although more studies are needed, according to the National Institute of Health, “experts are hopeful.”
In cystic fibrosis (CF), a defective gene makes a defective protein that causes acidic and sticky mucus that clogs the lungs and puts patients at risk for bacterial infections. Because different people have different protein mutations, and 10% of patients with CF make no protein at all, treatments are limited. But amphotericin apparently has the potential to work regardless of the mutation and even when the protein is missing. The researchers liken it to a “molecular prosthetic,” because it restores function much as a prosthetic device replaces a limb.
In the study, supported in part by the National Heart, Lung, and Blood Institute, researchers used lung tissue from patients with CF as well as animal models. Instead of trying to correct the protein or do gene therapy, which the researchers say is not yet effective in the lung, they used a small molecule protein that can perform the channel function of the missing or defective protein.
They found that amphotericin restored pH levels, improved viscosity, and increased antibacterial activity.
Amphotericin also can be delivered directly to the lungs to avoid common adverse effects, the researchers say. Although more studies are needed, according to the National Institute of Health, “experts are hopeful.”
FDA examines changing donation policies for men who have sex with men
The
At a meeting of the FDA’s Blood Products Advisory Committee, the agency shared the content of the 5-item questionnaire and reviewed the proposed study design with committee members, who were asked to comment – but not vote – on the best path forward for MSM donation policies.
The FDA is “committed to ongoing evaluation of the MSM deferral policy” and remains open to adjusting the policy based on the best available scientific evidence, said Barbee Whitaker, PhD, a lead scientist in the agency’s Office of Emerging and Transfusion Transmitted Disease
After recruiting 2,000 men who have had sex with men at least once during the past 3 months, the study will aim to identify individuals who have very recently become HIV infected, in order to assess the discriminant function of the set of behavioral questions that are proposed in the questionnaire.
The crux of the problem currently, noted Dr. Whitaker, is identifying those individuals who are very recently infected with HIV. Nucleic acid testing has tightened the window of undetectability considerably, but the current 12-month deferral window after men have had sexual contact with other men is designed to ensure safety of the blood supply.
Social justice concerns have been raised about the blanket deferral, said Dr. Whitaker; the behavioral questions in the pilot study will ask about the number of different sexual partners men have had within the past 1, 3, and 12 months and ask about the type of sexual contact (oral sex, or anal penetrative or receptive intercourse). The questionnaire also asks about sex with a partner known to be HIV positive, condom use, and use of pre-exposure prophylaxis (PrEP).
The FDA will ask for proposals to conduct the study with an eye to having sites in such cities as Washington, Atlanta, and Miami, which have high incidences of HIV, to improve chances of early detection.
The behavioral questionnaire is not seen as an immediate replacement for the 12-month deferral policy, the FDA made clear in its briefing documents and in discussion with the committee. Instead, its utility will be in the information gleaned from the pilot study and a follow-on that may include several hundred thousand individuals. These data should provide “population-based evidence upon which to base regulatory decisions to ensure blood safety,” she said.
Donation policies outside the United States
Whether a change in blood donation deferral policies for MSM would be a shortened window or a move toward a behavioral questionnaire is currently not known. Globally, a variety of practices are used for blood screening, said Mindy Goldman, MD, medical director of Canadian Blood Services, who reviewed international perspectives on blood donation for MSM.
“There’s no general consensus on donation deferrals internationally,” she said. Factors influencing policy can include epidemiology, risk analysis, modeling, and history of response to threats in the past.
However, “there’s basically a couple of main approaches” to handling deferrals for MSM, Dr. Goldman said. One is time-based deferral – the strategy used in the United States, as well as Canada, the United Kingdom, Japan, and Australia.
Japan and the U.K. have recently moved to 3-month deferral periods, a figure arrived at by doubling the window period for nucleic acid testing for HIV, roughly, Dr. Goldman said. Early data from the U.K. experience has not shown an increase in HIV rates among donors, or an increase in NAT-only positive donors, she said. An application to move from a 12-month to a 3-month deferral period is pending in Canada.
A strong advantage of time-based deferral as a risk management strategy, Dr. Goldman said, is standardization. “For us, standardization is close to godliness.”
However, she added, “another major limitation is that you’re still deferring all sexually active MSM, including those who are in a stable monogamous relationship from donating. From a justice perspective for the lowest risk population of MSM – they are still being deferred using this type of approach.”
Some nations, such as Spain and Italy, use individual risk assessment via physician-led interviews. These approaches are often not standardized. “There’s no national uniform questionnaire, so there’s less standardization, and more variability between blood centers,” Dr. Goldman said. “So you wind up trying to compare apples with oranges.”
This means the results are harder to evaluate on a national level. However, there appears to be higher residual risk, with HIV rates among first-time donors approaching those of the general population, Dr. Goldman said.
Another strategy, used in France, is a test-retest model, where blood from first-time MSM that initially tests negative for HIV is held until the individual returns for re-testing or an additional donation, with a second negative test. This approach increases operational complexity and cost, noted Dr. Goldman, and because of the short shelf life of platelets, it’s not practical for this blood component.
In general questioning and discussion after this and other background presentations, the committee could agree on one point: this isn’t an easy question.
“I’m increasingly struck by how difficult this problem is,” said committee member Roger Lewis, MD, PhD, professor at the University of California, Los Angeles, and chair of the department of emergency medicine at Harbor-UCLA Medical Center. Regarding just the problem of completing the pilot study, Dr. Lewis commented, “It sounds like it’s going to be impossible to get the data that directly answers the questions.”
Peter Marx, MD, PhD, who directs the FDA’s Center for Biologics Evaluation and Research (CBER), which oversees blood products safety, joined the discussion to acknowledge the difficulty, but underscore the social importance of a careful examination of the current MSM donation policy.
“We understand the issues here…. With all due respect to our European colleagues, there’s not enough data. That’s the point of this study; we also know that the U.S. has a very different epidemiology of HIV than the U.K. and a lot of other places,” Dr. Marx said. “The pilot study is a way to get some data where we might be able to get away from a time-based deferral. The LGBT community finds any time-based deferral discriminatory.”
Pathogen reduction technology
The committee heard a proposal for a completely different strategy during its afternoon session: pathogen reduction technology (PRT) holds promise to achieve virtual elimination of HIV and other pathogens from donated blood products.
The FDA is reviewing a variance request from the nonprofit blood donation organization Bloodworks Northwest organization to use PRT for apheresis platelet donations from MSM who would otherwise be deferred because of sexual activity within the 12-month deferral window.
James AuBuchon, MD, president of Bloodworks Northwest, explained that his organization takes in about 225,000 donations annually. The variance sought would use the FDA-approved INTERCEPT device to achieve pathogen reduction for donations that meet all requirements except the MSM deferral, and that would still undergo all relevant transfusion transmitted infection testing.
The INTERCEPT device uses amotosalen, which intercalates with DNA and RNA, inactivating it after exposure to ultraviolet A light. Amotosalen is then removed from the blood product before administration. The pathogen reduction activity doesn’t interfere with platelets or plasma, and is active against a wide range of viruses, bacteria, and fungal pathogens, explained Dr. AuBuchon, who is also a professor of hematology at the University of Washington, Seattle.
Dr. AuBuchon walked the committee through procedures designed to flag donors for PRT platelet apheresis, and to ensure these donations receive the intended PRT treatment. Platelets were chosen for this variance request, he explained, because demand outstrips supply. “We are all spending additional time and resources in recruiting a new framework and demographic, and it is exceedingly difficult to keep enough donors coming through the door,” he said. “Our platelet utilization climbs continually – it’s up 15% in the last 4 years.”
Committee members circled around the idea that all risk can’t be eliminated, even with the highly effective PRT technology. But the risk is exceedingly low, said committee chair Richard Kaufman, MD, medical director of the adult transfusion service at Brigham and Women’s Hospital, Boston. “It’s not possible to get rid of the window. We can kind of hammer down the risk by shrinking down the window by using incredibly sensitive tests. But that risk continues to exist. Pathogen reduction can take care of that residual risk…. So what’s left is really quite a low risk,” Dr. Kaufman said.
Susan Stramer, PhD, vice president of scientific affairs for the American Red Cross, concurred, noting that pathogen reduction techniques are already in use for many other blood products, particularly within the plasma industry.
Wrapping up, Dr. Kaufman asked individual committee members to summarize their position on the variance request, though the FDA had not placed a voting question before the committee. Consensus in the room was that this real-world examination of PRT could point to a path to expanding the donor pool while maintaining patient safety – a concern all agreed was paramount.
The FDA usually follows the recommendations of its committees.
The
At a meeting of the FDA’s Blood Products Advisory Committee, the agency shared the content of the 5-item questionnaire and reviewed the proposed study design with committee members, who were asked to comment – but not vote – on the best path forward for MSM donation policies.
The FDA is “committed to ongoing evaluation of the MSM deferral policy” and remains open to adjusting the policy based on the best available scientific evidence, said Barbee Whitaker, PhD, a lead scientist in the agency’s Office of Emerging and Transfusion Transmitted Disease
After recruiting 2,000 men who have had sex with men at least once during the past 3 months, the study will aim to identify individuals who have very recently become HIV infected, in order to assess the discriminant function of the set of behavioral questions that are proposed in the questionnaire.
The crux of the problem currently, noted Dr. Whitaker, is identifying those individuals who are very recently infected with HIV. Nucleic acid testing has tightened the window of undetectability considerably, but the current 12-month deferral window after men have had sexual contact with other men is designed to ensure safety of the blood supply.
Social justice concerns have been raised about the blanket deferral, said Dr. Whitaker; the behavioral questions in the pilot study will ask about the number of different sexual partners men have had within the past 1, 3, and 12 months and ask about the type of sexual contact (oral sex, or anal penetrative or receptive intercourse). The questionnaire also asks about sex with a partner known to be HIV positive, condom use, and use of pre-exposure prophylaxis (PrEP).
The FDA will ask for proposals to conduct the study with an eye to having sites in such cities as Washington, Atlanta, and Miami, which have high incidences of HIV, to improve chances of early detection.
The behavioral questionnaire is not seen as an immediate replacement for the 12-month deferral policy, the FDA made clear in its briefing documents and in discussion with the committee. Instead, its utility will be in the information gleaned from the pilot study and a follow-on that may include several hundred thousand individuals. These data should provide “population-based evidence upon which to base regulatory decisions to ensure blood safety,” she said.
Donation policies outside the United States
Whether a change in blood donation deferral policies for MSM would be a shortened window or a move toward a behavioral questionnaire is currently not known. Globally, a variety of practices are used for blood screening, said Mindy Goldman, MD, medical director of Canadian Blood Services, who reviewed international perspectives on blood donation for MSM.
“There’s no general consensus on donation deferrals internationally,” she said. Factors influencing policy can include epidemiology, risk analysis, modeling, and history of response to threats in the past.
However, “there’s basically a couple of main approaches” to handling deferrals for MSM, Dr. Goldman said. One is time-based deferral – the strategy used in the United States, as well as Canada, the United Kingdom, Japan, and Australia.
Japan and the U.K. have recently moved to 3-month deferral periods, a figure arrived at by doubling the window period for nucleic acid testing for HIV, roughly, Dr. Goldman said. Early data from the U.K. experience has not shown an increase in HIV rates among donors, or an increase in NAT-only positive donors, she said. An application to move from a 12-month to a 3-month deferral period is pending in Canada.
A strong advantage of time-based deferral as a risk management strategy, Dr. Goldman said, is standardization. “For us, standardization is close to godliness.”
However, she added, “another major limitation is that you’re still deferring all sexually active MSM, including those who are in a stable monogamous relationship from donating. From a justice perspective for the lowest risk population of MSM – they are still being deferred using this type of approach.”
Some nations, such as Spain and Italy, use individual risk assessment via physician-led interviews. These approaches are often not standardized. “There’s no national uniform questionnaire, so there’s less standardization, and more variability between blood centers,” Dr. Goldman said. “So you wind up trying to compare apples with oranges.”
This means the results are harder to evaluate on a national level. However, there appears to be higher residual risk, with HIV rates among first-time donors approaching those of the general population, Dr. Goldman said.
Another strategy, used in France, is a test-retest model, where blood from first-time MSM that initially tests negative for HIV is held until the individual returns for re-testing or an additional donation, with a second negative test. This approach increases operational complexity and cost, noted Dr. Goldman, and because of the short shelf life of platelets, it’s not practical for this blood component.
In general questioning and discussion after this and other background presentations, the committee could agree on one point: this isn’t an easy question.
“I’m increasingly struck by how difficult this problem is,” said committee member Roger Lewis, MD, PhD, professor at the University of California, Los Angeles, and chair of the department of emergency medicine at Harbor-UCLA Medical Center. Regarding just the problem of completing the pilot study, Dr. Lewis commented, “It sounds like it’s going to be impossible to get the data that directly answers the questions.”
Peter Marx, MD, PhD, who directs the FDA’s Center for Biologics Evaluation and Research (CBER), which oversees blood products safety, joined the discussion to acknowledge the difficulty, but underscore the social importance of a careful examination of the current MSM donation policy.
“We understand the issues here…. With all due respect to our European colleagues, there’s not enough data. That’s the point of this study; we also know that the U.S. has a very different epidemiology of HIV than the U.K. and a lot of other places,” Dr. Marx said. “The pilot study is a way to get some data where we might be able to get away from a time-based deferral. The LGBT community finds any time-based deferral discriminatory.”
Pathogen reduction technology
The committee heard a proposal for a completely different strategy during its afternoon session: pathogen reduction technology (PRT) holds promise to achieve virtual elimination of HIV and other pathogens from donated blood products.
The FDA is reviewing a variance request from the nonprofit blood donation organization Bloodworks Northwest organization to use PRT for apheresis platelet donations from MSM who would otherwise be deferred because of sexual activity within the 12-month deferral window.
James AuBuchon, MD, president of Bloodworks Northwest, explained that his organization takes in about 225,000 donations annually. The variance sought would use the FDA-approved INTERCEPT device to achieve pathogen reduction for donations that meet all requirements except the MSM deferral, and that would still undergo all relevant transfusion transmitted infection testing.
The INTERCEPT device uses amotosalen, which intercalates with DNA and RNA, inactivating it after exposure to ultraviolet A light. Amotosalen is then removed from the blood product before administration. The pathogen reduction activity doesn’t interfere with platelets or plasma, and is active against a wide range of viruses, bacteria, and fungal pathogens, explained Dr. AuBuchon, who is also a professor of hematology at the University of Washington, Seattle.
Dr. AuBuchon walked the committee through procedures designed to flag donors for PRT platelet apheresis, and to ensure these donations receive the intended PRT treatment. Platelets were chosen for this variance request, he explained, because demand outstrips supply. “We are all spending additional time and resources in recruiting a new framework and demographic, and it is exceedingly difficult to keep enough donors coming through the door,” he said. “Our platelet utilization climbs continually – it’s up 15% in the last 4 years.”
Committee members circled around the idea that all risk can’t be eliminated, even with the highly effective PRT technology. But the risk is exceedingly low, said committee chair Richard Kaufman, MD, medical director of the adult transfusion service at Brigham and Women’s Hospital, Boston. “It’s not possible to get rid of the window. We can kind of hammer down the risk by shrinking down the window by using incredibly sensitive tests. But that risk continues to exist. Pathogen reduction can take care of that residual risk…. So what’s left is really quite a low risk,” Dr. Kaufman said.
Susan Stramer, PhD, vice president of scientific affairs for the American Red Cross, concurred, noting that pathogen reduction techniques are already in use for many other blood products, particularly within the plasma industry.
Wrapping up, Dr. Kaufman asked individual committee members to summarize their position on the variance request, though the FDA had not placed a voting question before the committee. Consensus in the room was that this real-world examination of PRT could point to a path to expanding the donor pool while maintaining patient safety – a concern all agreed was paramount.
The FDA usually follows the recommendations of its committees.
The
At a meeting of the FDA’s Blood Products Advisory Committee, the agency shared the content of the 5-item questionnaire and reviewed the proposed study design with committee members, who were asked to comment – but not vote – on the best path forward for MSM donation policies.
The FDA is “committed to ongoing evaluation of the MSM deferral policy” and remains open to adjusting the policy based on the best available scientific evidence, said Barbee Whitaker, PhD, a lead scientist in the agency’s Office of Emerging and Transfusion Transmitted Disease
After recruiting 2,000 men who have had sex with men at least once during the past 3 months, the study will aim to identify individuals who have very recently become HIV infected, in order to assess the discriminant function of the set of behavioral questions that are proposed in the questionnaire.
The crux of the problem currently, noted Dr. Whitaker, is identifying those individuals who are very recently infected with HIV. Nucleic acid testing has tightened the window of undetectability considerably, but the current 12-month deferral window after men have had sexual contact with other men is designed to ensure safety of the blood supply.
Social justice concerns have been raised about the blanket deferral, said Dr. Whitaker; the behavioral questions in the pilot study will ask about the number of different sexual partners men have had within the past 1, 3, and 12 months and ask about the type of sexual contact (oral sex, or anal penetrative or receptive intercourse). The questionnaire also asks about sex with a partner known to be HIV positive, condom use, and use of pre-exposure prophylaxis (PrEP).
The FDA will ask for proposals to conduct the study with an eye to having sites in such cities as Washington, Atlanta, and Miami, which have high incidences of HIV, to improve chances of early detection.
The behavioral questionnaire is not seen as an immediate replacement for the 12-month deferral policy, the FDA made clear in its briefing documents and in discussion with the committee. Instead, its utility will be in the information gleaned from the pilot study and a follow-on that may include several hundred thousand individuals. These data should provide “population-based evidence upon which to base regulatory decisions to ensure blood safety,” she said.
Donation policies outside the United States
Whether a change in blood donation deferral policies for MSM would be a shortened window or a move toward a behavioral questionnaire is currently not known. Globally, a variety of practices are used for blood screening, said Mindy Goldman, MD, medical director of Canadian Blood Services, who reviewed international perspectives on blood donation for MSM.
“There’s no general consensus on donation deferrals internationally,” she said. Factors influencing policy can include epidemiology, risk analysis, modeling, and history of response to threats in the past.
However, “there’s basically a couple of main approaches” to handling deferrals for MSM, Dr. Goldman said. One is time-based deferral – the strategy used in the United States, as well as Canada, the United Kingdom, Japan, and Australia.
Japan and the U.K. have recently moved to 3-month deferral periods, a figure arrived at by doubling the window period for nucleic acid testing for HIV, roughly, Dr. Goldman said. Early data from the U.K. experience has not shown an increase in HIV rates among donors, or an increase in NAT-only positive donors, she said. An application to move from a 12-month to a 3-month deferral period is pending in Canada.
A strong advantage of time-based deferral as a risk management strategy, Dr. Goldman said, is standardization. “For us, standardization is close to godliness.”
However, she added, “another major limitation is that you’re still deferring all sexually active MSM, including those who are in a stable monogamous relationship from donating. From a justice perspective for the lowest risk population of MSM – they are still being deferred using this type of approach.”
Some nations, such as Spain and Italy, use individual risk assessment via physician-led interviews. These approaches are often not standardized. “There’s no national uniform questionnaire, so there’s less standardization, and more variability between blood centers,” Dr. Goldman said. “So you wind up trying to compare apples with oranges.”
This means the results are harder to evaluate on a national level. However, there appears to be higher residual risk, with HIV rates among first-time donors approaching those of the general population, Dr. Goldman said.
Another strategy, used in France, is a test-retest model, where blood from first-time MSM that initially tests negative for HIV is held until the individual returns for re-testing or an additional donation, with a second negative test. This approach increases operational complexity and cost, noted Dr. Goldman, and because of the short shelf life of platelets, it’s not practical for this blood component.
In general questioning and discussion after this and other background presentations, the committee could agree on one point: this isn’t an easy question.
“I’m increasingly struck by how difficult this problem is,” said committee member Roger Lewis, MD, PhD, professor at the University of California, Los Angeles, and chair of the department of emergency medicine at Harbor-UCLA Medical Center. Regarding just the problem of completing the pilot study, Dr. Lewis commented, “It sounds like it’s going to be impossible to get the data that directly answers the questions.”
Peter Marx, MD, PhD, who directs the FDA’s Center for Biologics Evaluation and Research (CBER), which oversees blood products safety, joined the discussion to acknowledge the difficulty, but underscore the social importance of a careful examination of the current MSM donation policy.
“We understand the issues here…. With all due respect to our European colleagues, there’s not enough data. That’s the point of this study; we also know that the U.S. has a very different epidemiology of HIV than the U.K. and a lot of other places,” Dr. Marx said. “The pilot study is a way to get some data where we might be able to get away from a time-based deferral. The LGBT community finds any time-based deferral discriminatory.”
Pathogen reduction technology
The committee heard a proposal for a completely different strategy during its afternoon session: pathogen reduction technology (PRT) holds promise to achieve virtual elimination of HIV and other pathogens from donated blood products.
The FDA is reviewing a variance request from the nonprofit blood donation organization Bloodworks Northwest organization to use PRT for apheresis platelet donations from MSM who would otherwise be deferred because of sexual activity within the 12-month deferral window.
James AuBuchon, MD, president of Bloodworks Northwest, explained that his organization takes in about 225,000 donations annually. The variance sought would use the FDA-approved INTERCEPT device to achieve pathogen reduction for donations that meet all requirements except the MSM deferral, and that would still undergo all relevant transfusion transmitted infection testing.
The INTERCEPT device uses amotosalen, which intercalates with DNA and RNA, inactivating it after exposure to ultraviolet A light. Amotosalen is then removed from the blood product before administration. The pathogen reduction activity doesn’t interfere with platelets or plasma, and is active against a wide range of viruses, bacteria, and fungal pathogens, explained Dr. AuBuchon, who is also a professor of hematology at the University of Washington, Seattle.
Dr. AuBuchon walked the committee through procedures designed to flag donors for PRT platelet apheresis, and to ensure these donations receive the intended PRT treatment. Platelets were chosen for this variance request, he explained, because demand outstrips supply. “We are all spending additional time and resources in recruiting a new framework and demographic, and it is exceedingly difficult to keep enough donors coming through the door,” he said. “Our platelet utilization climbs continually – it’s up 15% in the last 4 years.”
Committee members circled around the idea that all risk can’t be eliminated, even with the highly effective PRT technology. But the risk is exceedingly low, said committee chair Richard Kaufman, MD, medical director of the adult transfusion service at Brigham and Women’s Hospital, Boston. “It’s not possible to get rid of the window. We can kind of hammer down the risk by shrinking down the window by using incredibly sensitive tests. But that risk continues to exist. Pathogen reduction can take care of that residual risk…. So what’s left is really quite a low risk,” Dr. Kaufman said.
Susan Stramer, PhD, vice president of scientific affairs for the American Red Cross, concurred, noting that pathogen reduction techniques are already in use for many other blood products, particularly within the plasma industry.
Wrapping up, Dr. Kaufman asked individual committee members to summarize their position on the variance request, though the FDA had not placed a voting question before the committee. Consensus in the room was that this real-world examination of PRT could point to a path to expanding the donor pool while maintaining patient safety – a concern all agreed was paramount.
The FDA usually follows the recommendations of its committees.
FROM AN FDA ADVISORY COMMITTEE MEETING
How to manage infected eczema in children
WAIKOLOA, HAWAII – , according to Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at the University of California, San Diego.
It’s the breach in skin integrity that gives skin flora – most commonly Staphylococcus or Streptococcus – an opening for infection.
Dr. Eichenfield shared his treatment approach in a pearl-filled interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Many times, anti-inflammatories are going to be the effective therapy,” whether topical steroids or systemic therapies. But with a secondary infection, systemic antibiotics are in order, too, but topical ones aren’t much use, he said.
Dr. Eichenfield gets a lot of questions about steroid-sparing options for very young children, since topical calcineurin inhibitors and the like aren’t approved in children under 2 years old, and insurance coverage can be a problem. He noted, however, that various guidelines support their use even in the very young, “so I tell my physicians to fight for them ... If you need a steroid-sparing agent, push for it, because it may be the right thing for your patient,” he said.
He’s finding in his area that infected eczema often is resistant to clindamycin, which has been used heavily because of concerns about methicillin-resistant Staphylococcus aureus (MRSA). But it often will “respond to what we considered to be wimpier antibiotics in the past, such as cephalosporin ... So I’ll use cephalosporin or an extended-spectrum penicillin as my first-line agent, and then I’ll culture, depending on history, to see if I have to be concerned about methicillin resistance,” he said.
Be on the lookout for cutaneous herpes and show parents pictures of what it looks like so they recognize it and know to come in right away. It is a dangerous infection, but can be shut down quickly with oral acyclovir and similar agents, Dr. Eichenfield added.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – , according to Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at the University of California, San Diego.
It’s the breach in skin integrity that gives skin flora – most commonly Staphylococcus or Streptococcus – an opening for infection.
Dr. Eichenfield shared his treatment approach in a pearl-filled interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Many times, anti-inflammatories are going to be the effective therapy,” whether topical steroids or systemic therapies. But with a secondary infection, systemic antibiotics are in order, too, but topical ones aren’t much use, he said.
Dr. Eichenfield gets a lot of questions about steroid-sparing options for very young children, since topical calcineurin inhibitors and the like aren’t approved in children under 2 years old, and insurance coverage can be a problem. He noted, however, that various guidelines support their use even in the very young, “so I tell my physicians to fight for them ... If you need a steroid-sparing agent, push for it, because it may be the right thing for your patient,” he said.
He’s finding in his area that infected eczema often is resistant to clindamycin, which has been used heavily because of concerns about methicillin-resistant Staphylococcus aureus (MRSA). But it often will “respond to what we considered to be wimpier antibiotics in the past, such as cephalosporin ... So I’ll use cephalosporin or an extended-spectrum penicillin as my first-line agent, and then I’ll culture, depending on history, to see if I have to be concerned about methicillin resistance,” he said.
Be on the lookout for cutaneous herpes and show parents pictures of what it looks like so they recognize it and know to come in right away. It is a dangerous infection, but can be shut down quickly with oral acyclovir and similar agents, Dr. Eichenfield added.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – , according to Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at the University of California, San Diego.
It’s the breach in skin integrity that gives skin flora – most commonly Staphylococcus or Streptococcus – an opening for infection.
Dr. Eichenfield shared his treatment approach in a pearl-filled interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Many times, anti-inflammatories are going to be the effective therapy,” whether topical steroids or systemic therapies. But with a secondary infection, systemic antibiotics are in order, too, but topical ones aren’t much use, he said.
Dr. Eichenfield gets a lot of questions about steroid-sparing options for very young children, since topical calcineurin inhibitors and the like aren’t approved in children under 2 years old, and insurance coverage can be a problem. He noted, however, that various guidelines support their use even in the very young, “so I tell my physicians to fight for them ... If you need a steroid-sparing agent, push for it, because it may be the right thing for your patient,” he said.
He’s finding in his area that infected eczema often is resistant to clindamycin, which has been used heavily because of concerns about methicillin-resistant Staphylococcus aureus (MRSA). But it often will “respond to what we considered to be wimpier antibiotics in the past, such as cephalosporin ... So I’ll use cephalosporin or an extended-spectrum penicillin as my first-line agent, and then I’ll culture, depending on history, to see if I have to be concerned about methicillin resistance,” he said.
Be on the lookout for cutaneous herpes and show parents pictures of what it looks like so they recognize it and know to come in right away. It is a dangerous infection, but can be shut down quickly with oral acyclovir and similar agents, Dr. Eichenfield added.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Depression: a changing concept in the age of ketamine
What does it mean to define an amalgamation of symptoms as a “psychiatric disorder?” Are psychiatric disorders an extreme variation of normative human behavior? Is human behavior simply an output phenotype of some neurologic chemical processes that become disordered in mental illness? Can depression be localized in the brain and subsequently turned on or off? If depression were to be localized in the brain, would it be an excess of a neurotransmitter, the depletion of a receptor, a malfunctioning neuron, an overactive connectome, poorly processed genetic material, or something as yet undefined? Those questions always have been present in our minds and influenced our understanding of patients, but a recent development in psychiatry raises questions about one of the few things we were historically confident about.
A part of our foundational understanding of depression was that it is not sadness, per se. One can be sad for any amount of time. It is not uncommon to feel sad for any variety of reasons, such as watching an adorable 60-second commercial for dog food.1 Those fleeting moments of sadness can even be empowering; they remind us about the things we care about and would be sad to miss.
Sadness in oneself can demonstrate the experience of empathic sadness for others. On the contrary, depression appears to have little apparent purpose, and instead results in a maladaptive way of coping that is all-consuming and often very damaging. Depression is not a mood but a state of being, something that is not defined by how one feels but who one is or has become because of the disorder. So it comes as somewhat of a surprise when we heard that ketamine could alleviate depression in minutes.2,3 As described by a ketamine expert, symptoms are relieved in “no less than an hour.”4 The surprise is not so much that a treatment would work but that improvement could be defined in such a short time frame.
Psychiatry has debated the definition of depression for its entire existence. There are many ways to tackle the concept of depression. A lot of the debate has been about the causes of depression. One example of the continued evolution of our understanding of depression is our prior categorization of depression as “exogenous” or “endogenous.”5 Exogenous depression was described as happening in the context of social stressors and as best treated with therapy. Endogenous depression was a supposedly truer form of depression as a disorder and was more biologically based. Patients suffering from endogenous depression were thought to have chemical abnormalities in the brain that could be alleviated by tricyclic antidepressants and subsequently SSRIs. Like many prior debates about depression, this one appears to be little discussed nowadays. A review of the use of the term “endogenous depression” in books shows an onset in the 1930s, a peak in the 1980s, and a rapid decline since.6
More recently, psychiatrists have defined depression using the DSM-5 criteria. Depression is thought to be the presence of at least five out of nine symptoms listed in the manual for a period of 2 weeks that cause significant distress or impairment.7 The DSM attempts to address criticism by providing information on its limitations and best use, and encourages clinical interpretation of symptoms. The DSM does not portray itself as a gold standard but rather as a tool for treatment planning and effective communication between peers. Furthermore, the National Institute on Mental Health is promoting an alternative understanding of depression using its own Research Domain Criteria, which attempt to provide a more objective understanding of the disorder based on biological rather than subjective correlates.
The growing literature on ketamine partly hinges on the belief that depression is something that can be redefined and changed at any moment. Many trials ask patients whether their depression remains in remission in the subsequent hours, days, and weeks following administration of the drug. However, one wonders if that is even possible. If a patient’s depression is alleviated in an hour, was it really clinical depression? Is it truly in remission? Contrary to our previous understanding, is depression, in reality, a switch that can be turned off by an infusion of an N-methyl-D-aspartate (NMDA)–receptor antagonist? Without minimizing the suffering of patients seeking ketamine or the relief provided to patients who benefit from the treatment, we simply are pointing out that the definition of depression did not account for this reported phenomenon of relatively instantaneous relief. The seemingly miraculous effects of ketamine suggest a new paradigm where any intervention – whether chemical, social, or psychological – could turn off the devastating effects of depression in an instant.
After all, the most widely used scale of depression, the nine-item Patient Health Questionnaire (PHQ-9) asks patients, “Over the last 2 weeks, how often have you been bothered by any of the following problems?” The highest answer one can give is “Nearly every day.” Are we incorrect to think that if one were suicidal every minute of the past 2 weeks, one would still score, nearly every day, even if one’s symptoms were relieved for the past hour? Thus, a maximum score of 27 would remain a 27 no matter what happened in the past hour.
We do realize that we are being overly literal. Ketamine makes some people feel better quickly, and researchers try to capture that effect by asking patients about their symptoms within short intervals. Furthermore, one has to start somewhere. After the infusion is a reasonable time to ask patients how they feel. We are also cognizant that many ketamine researchers do more long-term follow-ups and/or have recommended longer-term studies. Nonetheless,
Expanding our definition of depression to encompass experiences with short time frames may have unintended consequences. As living circumstances rarely change in minutes, the emphasis on rapid recovery makes the patients more in control of their reported experiences and thus their diagnoses. One cannot assess a patient’s impairment or disability from minute to minute. One is left with emphasizing the patient’s subjective symptoms and deemphasizing their relationships, goals, and daily functioning. How could one measure eating habits, hygiene, or participation in hobbies every hour? Another consequence of this reduced time frame is the expansion of a diagnosis that no longer requires the presence of symptoms for 2 weeks. Considering the already vast number of people diagnosed with depression,9,10 this small change may further expand the number diagnosed with a mood disorder. Perhaps to many practitioners and patients these arguments seem obtuse and fastidious, but there is a core failure in modern psychiatry to clearly differentiate the human condition from mental illness. Said failure has vast implications for psychiatric epidemiology, the sociological understanding of psychic suffering and suicide, as well as the overprescribing of psychotropic medications.
Ketamine is an exciting prospect to many psychiatrists who feel like we have had little advancement and few novel treatments in a long time; advertised breakthroughs in the treatment of depression since fluoxetine have not been particularly impressive. Furthermore, the concerns about potential ketamine abuse are not theoretical but a very real problem in some parts of the world.11,12 The concerns about abuse are worsened considering recent evidence that suggests that ketamine’s effect may be driven by its opiate rather than NMDA effects.13 While some have discussed those concerns, we think that the field also needs to address the fact that the debate about ketamine is also changing our definition of depression.
References
1. https://www.youtube.com/watch?v=MpcUN6XvGmk.
2. J Clin Psychiatry. 2016 Jun;77(6):e719-25.
3. Emerg (Tehran). 2014 Winter;2(1):36-9.
4. “Is esketamine the game-changer for depression we want?” Rolling Stone. 2019 Mar 11.
5. Psychol Med. 1971;1(3):191-6.
6. https://books.google.com/ngrams.
7. Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5). American Psychiatric Association, 2013.
8. Am J Psychiatry. 2014 Apr;171(4):395-7.
9. “Many people taking antidepressants discover they cannot quit.” New York Times. 2018 Apr 7.
10. “Antidepressants show greatest increase in number of prescription items dispensed.” National Health Service. 2015 Jul 5.
11. “The ketamine connection.” BBC News. 2015 Jul 10.
12. Front Psychiatry. 2018 Jul 17. doi: 10.33389/fpsyt.2018.00313.
13. Am J Psychiatry. 2018 Dec 1;175(2):1205-15.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. Among his writings is Coercion and the critical psychiatrist, chapter 7 in the new book “Critical Psychiatry: Controversies and Clinical Implications” (Cham, Switzerland: Springer Nature Switzerland, 2019, pp. 155-77). Dr. Lehman is an associate professor of psychiatry at UCSD. He is codirector of all Acute and Intensive Psychiatric Treatment at the VA Medical Center in San Diego, where he practices clinical psychiatry. He also is the course director for the UCSD third-year medical student psychiatry clerkship.
*This article was updated 3/25/2019.
What does it mean to define an amalgamation of symptoms as a “psychiatric disorder?” Are psychiatric disorders an extreme variation of normative human behavior? Is human behavior simply an output phenotype of some neurologic chemical processes that become disordered in mental illness? Can depression be localized in the brain and subsequently turned on or off? If depression were to be localized in the brain, would it be an excess of a neurotransmitter, the depletion of a receptor, a malfunctioning neuron, an overactive connectome, poorly processed genetic material, or something as yet undefined? Those questions always have been present in our minds and influenced our understanding of patients, but a recent development in psychiatry raises questions about one of the few things we were historically confident about.
A part of our foundational understanding of depression was that it is not sadness, per se. One can be sad for any amount of time. It is not uncommon to feel sad for any variety of reasons, such as watching an adorable 60-second commercial for dog food.1 Those fleeting moments of sadness can even be empowering; they remind us about the things we care about and would be sad to miss.
Sadness in oneself can demonstrate the experience of empathic sadness for others. On the contrary, depression appears to have little apparent purpose, and instead results in a maladaptive way of coping that is all-consuming and often very damaging. Depression is not a mood but a state of being, something that is not defined by how one feels but who one is or has become because of the disorder. So it comes as somewhat of a surprise when we heard that ketamine could alleviate depression in minutes.2,3 As described by a ketamine expert, symptoms are relieved in “no less than an hour.”4 The surprise is not so much that a treatment would work but that improvement could be defined in such a short time frame.
Psychiatry has debated the definition of depression for its entire existence. There are many ways to tackle the concept of depression. A lot of the debate has been about the causes of depression. One example of the continued evolution of our understanding of depression is our prior categorization of depression as “exogenous” or “endogenous.”5 Exogenous depression was described as happening in the context of social stressors and as best treated with therapy. Endogenous depression was a supposedly truer form of depression as a disorder and was more biologically based. Patients suffering from endogenous depression were thought to have chemical abnormalities in the brain that could be alleviated by tricyclic antidepressants and subsequently SSRIs. Like many prior debates about depression, this one appears to be little discussed nowadays. A review of the use of the term “endogenous depression” in books shows an onset in the 1930s, a peak in the 1980s, and a rapid decline since.6
More recently, psychiatrists have defined depression using the DSM-5 criteria. Depression is thought to be the presence of at least five out of nine symptoms listed in the manual for a period of 2 weeks that cause significant distress or impairment.7 The DSM attempts to address criticism by providing information on its limitations and best use, and encourages clinical interpretation of symptoms. The DSM does not portray itself as a gold standard but rather as a tool for treatment planning and effective communication between peers. Furthermore, the National Institute on Mental Health is promoting an alternative understanding of depression using its own Research Domain Criteria, which attempt to provide a more objective understanding of the disorder based on biological rather than subjective correlates.
The growing literature on ketamine partly hinges on the belief that depression is something that can be redefined and changed at any moment. Many trials ask patients whether their depression remains in remission in the subsequent hours, days, and weeks following administration of the drug. However, one wonders if that is even possible. If a patient’s depression is alleviated in an hour, was it really clinical depression? Is it truly in remission? Contrary to our previous understanding, is depression, in reality, a switch that can be turned off by an infusion of an N-methyl-D-aspartate (NMDA)–receptor antagonist? Without minimizing the suffering of patients seeking ketamine or the relief provided to patients who benefit from the treatment, we simply are pointing out that the definition of depression did not account for this reported phenomenon of relatively instantaneous relief. The seemingly miraculous effects of ketamine suggest a new paradigm where any intervention – whether chemical, social, or psychological – could turn off the devastating effects of depression in an instant.
After all, the most widely used scale of depression, the nine-item Patient Health Questionnaire (PHQ-9) asks patients, “Over the last 2 weeks, how often have you been bothered by any of the following problems?” The highest answer one can give is “Nearly every day.” Are we incorrect to think that if one were suicidal every minute of the past 2 weeks, one would still score, nearly every day, even if one’s symptoms were relieved for the past hour? Thus, a maximum score of 27 would remain a 27 no matter what happened in the past hour.
We do realize that we are being overly literal. Ketamine makes some people feel better quickly, and researchers try to capture that effect by asking patients about their symptoms within short intervals. Furthermore, one has to start somewhere. After the infusion is a reasonable time to ask patients how they feel. We are also cognizant that many ketamine researchers do more long-term follow-ups and/or have recommended longer-term studies. Nonetheless,
Expanding our definition of depression to encompass experiences with short time frames may have unintended consequences. As living circumstances rarely change in minutes, the emphasis on rapid recovery makes the patients more in control of their reported experiences and thus their diagnoses. One cannot assess a patient’s impairment or disability from minute to minute. One is left with emphasizing the patient’s subjective symptoms and deemphasizing their relationships, goals, and daily functioning. How could one measure eating habits, hygiene, or participation in hobbies every hour? Another consequence of this reduced time frame is the expansion of a diagnosis that no longer requires the presence of symptoms for 2 weeks. Considering the already vast number of people diagnosed with depression,9,10 this small change may further expand the number diagnosed with a mood disorder. Perhaps to many practitioners and patients these arguments seem obtuse and fastidious, but there is a core failure in modern psychiatry to clearly differentiate the human condition from mental illness. Said failure has vast implications for psychiatric epidemiology, the sociological understanding of psychic suffering and suicide, as well as the overprescribing of psychotropic medications.
Ketamine is an exciting prospect to many psychiatrists who feel like we have had little advancement and few novel treatments in a long time; advertised breakthroughs in the treatment of depression since fluoxetine have not been particularly impressive. Furthermore, the concerns about potential ketamine abuse are not theoretical but a very real problem in some parts of the world.11,12 The concerns about abuse are worsened considering recent evidence that suggests that ketamine’s effect may be driven by its opiate rather than NMDA effects.13 While some have discussed those concerns, we think that the field also needs to address the fact that the debate about ketamine is also changing our definition of depression.
References
1. https://www.youtube.com/watch?v=MpcUN6XvGmk.
2. J Clin Psychiatry. 2016 Jun;77(6):e719-25.
3. Emerg (Tehran). 2014 Winter;2(1):36-9.
4. “Is esketamine the game-changer for depression we want?” Rolling Stone. 2019 Mar 11.
5. Psychol Med. 1971;1(3):191-6.
6. https://books.google.com/ngrams.
7. Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5). American Psychiatric Association, 2013.
8. Am J Psychiatry. 2014 Apr;171(4):395-7.
9. “Many people taking antidepressants discover they cannot quit.” New York Times. 2018 Apr 7.
10. “Antidepressants show greatest increase in number of prescription items dispensed.” National Health Service. 2015 Jul 5.
11. “The ketamine connection.” BBC News. 2015 Jul 10.
12. Front Psychiatry. 2018 Jul 17. doi: 10.33389/fpsyt.2018.00313.
13. Am J Psychiatry. 2018 Dec 1;175(2):1205-15.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. Among his writings is Coercion and the critical psychiatrist, chapter 7 in the new book “Critical Psychiatry: Controversies and Clinical Implications” (Cham, Switzerland: Springer Nature Switzerland, 2019, pp. 155-77). Dr. Lehman is an associate professor of psychiatry at UCSD. He is codirector of all Acute and Intensive Psychiatric Treatment at the VA Medical Center in San Diego, where he practices clinical psychiatry. He also is the course director for the UCSD third-year medical student psychiatry clerkship.
*This article was updated 3/25/2019.
What does it mean to define an amalgamation of symptoms as a “psychiatric disorder?” Are psychiatric disorders an extreme variation of normative human behavior? Is human behavior simply an output phenotype of some neurologic chemical processes that become disordered in mental illness? Can depression be localized in the brain and subsequently turned on or off? If depression were to be localized in the brain, would it be an excess of a neurotransmitter, the depletion of a receptor, a malfunctioning neuron, an overactive connectome, poorly processed genetic material, or something as yet undefined? Those questions always have been present in our minds and influenced our understanding of patients, but a recent development in psychiatry raises questions about one of the few things we were historically confident about.
A part of our foundational understanding of depression was that it is not sadness, per se. One can be sad for any amount of time. It is not uncommon to feel sad for any variety of reasons, such as watching an adorable 60-second commercial for dog food.1 Those fleeting moments of sadness can even be empowering; they remind us about the things we care about and would be sad to miss.
Sadness in oneself can demonstrate the experience of empathic sadness for others. On the contrary, depression appears to have little apparent purpose, and instead results in a maladaptive way of coping that is all-consuming and often very damaging. Depression is not a mood but a state of being, something that is not defined by how one feels but who one is or has become because of the disorder. So it comes as somewhat of a surprise when we heard that ketamine could alleviate depression in minutes.2,3 As described by a ketamine expert, symptoms are relieved in “no less than an hour.”4 The surprise is not so much that a treatment would work but that improvement could be defined in such a short time frame.
Psychiatry has debated the definition of depression for its entire existence. There are many ways to tackle the concept of depression. A lot of the debate has been about the causes of depression. One example of the continued evolution of our understanding of depression is our prior categorization of depression as “exogenous” or “endogenous.”5 Exogenous depression was described as happening in the context of social stressors and as best treated with therapy. Endogenous depression was a supposedly truer form of depression as a disorder and was more biologically based. Patients suffering from endogenous depression were thought to have chemical abnormalities in the brain that could be alleviated by tricyclic antidepressants and subsequently SSRIs. Like many prior debates about depression, this one appears to be little discussed nowadays. A review of the use of the term “endogenous depression” in books shows an onset in the 1930s, a peak in the 1980s, and a rapid decline since.6
More recently, psychiatrists have defined depression using the DSM-5 criteria. Depression is thought to be the presence of at least five out of nine symptoms listed in the manual for a period of 2 weeks that cause significant distress or impairment.7 The DSM attempts to address criticism by providing information on its limitations and best use, and encourages clinical interpretation of symptoms. The DSM does not portray itself as a gold standard but rather as a tool for treatment planning and effective communication between peers. Furthermore, the National Institute on Mental Health is promoting an alternative understanding of depression using its own Research Domain Criteria, which attempt to provide a more objective understanding of the disorder based on biological rather than subjective correlates.
The growing literature on ketamine partly hinges on the belief that depression is something that can be redefined and changed at any moment. Many trials ask patients whether their depression remains in remission in the subsequent hours, days, and weeks following administration of the drug. However, one wonders if that is even possible. If a patient’s depression is alleviated in an hour, was it really clinical depression? Is it truly in remission? Contrary to our previous understanding, is depression, in reality, a switch that can be turned off by an infusion of an N-methyl-D-aspartate (NMDA)–receptor antagonist? Without minimizing the suffering of patients seeking ketamine or the relief provided to patients who benefit from the treatment, we simply are pointing out that the definition of depression did not account for this reported phenomenon of relatively instantaneous relief. The seemingly miraculous effects of ketamine suggest a new paradigm where any intervention – whether chemical, social, or psychological – could turn off the devastating effects of depression in an instant.
After all, the most widely used scale of depression, the nine-item Patient Health Questionnaire (PHQ-9) asks patients, “Over the last 2 weeks, how often have you been bothered by any of the following problems?” The highest answer one can give is “Nearly every day.” Are we incorrect to think that if one were suicidal every minute of the past 2 weeks, one would still score, nearly every day, even if one’s symptoms were relieved for the past hour? Thus, a maximum score of 27 would remain a 27 no matter what happened in the past hour.
We do realize that we are being overly literal. Ketamine makes some people feel better quickly, and researchers try to capture that effect by asking patients about their symptoms within short intervals. Furthermore, one has to start somewhere. After the infusion is a reasonable time to ask patients how they feel. We are also cognizant that many ketamine researchers do more long-term follow-ups and/or have recommended longer-term studies. Nonetheless,
Expanding our definition of depression to encompass experiences with short time frames may have unintended consequences. As living circumstances rarely change in minutes, the emphasis on rapid recovery makes the patients more in control of their reported experiences and thus their diagnoses. One cannot assess a patient’s impairment or disability from minute to minute. One is left with emphasizing the patient’s subjective symptoms and deemphasizing their relationships, goals, and daily functioning. How could one measure eating habits, hygiene, or participation in hobbies every hour? Another consequence of this reduced time frame is the expansion of a diagnosis that no longer requires the presence of symptoms for 2 weeks. Considering the already vast number of people diagnosed with depression,9,10 this small change may further expand the number diagnosed with a mood disorder. Perhaps to many practitioners and patients these arguments seem obtuse and fastidious, but there is a core failure in modern psychiatry to clearly differentiate the human condition from mental illness. Said failure has vast implications for psychiatric epidemiology, the sociological understanding of psychic suffering and suicide, as well as the overprescribing of psychotropic medications.
Ketamine is an exciting prospect to many psychiatrists who feel like we have had little advancement and few novel treatments in a long time; advertised breakthroughs in the treatment of depression since fluoxetine have not been particularly impressive. Furthermore, the concerns about potential ketamine abuse are not theoretical but a very real problem in some parts of the world.11,12 The concerns about abuse are worsened considering recent evidence that suggests that ketamine’s effect may be driven by its opiate rather than NMDA effects.13 While some have discussed those concerns, we think that the field also needs to address the fact that the debate about ketamine is also changing our definition of depression.
References
1. https://www.youtube.com/watch?v=MpcUN6XvGmk.
2. J Clin Psychiatry. 2016 Jun;77(6):e719-25.
3. Emerg (Tehran). 2014 Winter;2(1):36-9.
4. “Is esketamine the game-changer for depression we want?” Rolling Stone. 2019 Mar 11.
5. Psychol Med. 1971;1(3):191-6.
6. https://books.google.com/ngrams.
7. Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5). American Psychiatric Association, 2013.
8. Am J Psychiatry. 2014 Apr;171(4):395-7.
9. “Many people taking antidepressants discover they cannot quit.” New York Times. 2018 Apr 7.
10. “Antidepressants show greatest increase in number of prescription items dispensed.” National Health Service. 2015 Jul 5.
11. “The ketamine connection.” BBC News. 2015 Jul 10.
12. Front Psychiatry. 2018 Jul 17. doi: 10.33389/fpsyt.2018.00313.
13. Am J Psychiatry. 2018 Dec 1;175(2):1205-15.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. Among his writings is Coercion and the critical psychiatrist, chapter 7 in the new book “Critical Psychiatry: Controversies and Clinical Implications” (Cham, Switzerland: Springer Nature Switzerland, 2019, pp. 155-77). Dr. Lehman is an associate professor of psychiatry at UCSD. He is codirector of all Acute and Intensive Psychiatric Treatment at the VA Medical Center in San Diego, where he practices clinical psychiatry. He also is the course director for the UCSD third-year medical student psychiatry clerkship.
*This article was updated 3/25/2019.
ARIEL3 analysis: Rucaparib PFS benefit in recurrent OC occurs across age groups
HONOLULU – The improved progression-free survival and reduced risk of disease progression seen with maintenance rucaparib versus placebo in women with recurrent ovarian cancer in the pivotal ARIEL3 study occurred across age subgroups, according to a post hoc exploratory analysis of data from the phase 3 study.
In general, the safety profile of the first-in-class poly (ADP-ribose) polymerase (PARP) inhibitor was consistent across age groups, as well, but rates of dose modifications and treatment discontinuations varied slightly by age subgroup in the rucaparib (Rubraca)and placebo arms, Jonathan A. Ledermann, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
No clear trend emerged with respect to those differences, said Dr. Ledermann, a professor of medical oncology at University College of London Cancer Institute and UCL Hospitals.
Initial results from the randomized, placebo-controlled study were published in 2017 and showed a significant progression-free survival (PFS) benefit with 600 mg of maintenance rucaparib vs. placebo (10.8 vs. 5.4 months, respectively, in the intention-to-treat population). For the current analysis, outcomes were assessed for the ITT population across three baseline age-based subgroups: those under age 65 years, those aged 65-74 years, and those 75 years or older.
Investigator-assessed PFS – the primary endpoint of ARIEL3 – for the rucaparib vs. placebo groups was 11.1 vs. 5.4 months among 237 and 117 patients under age 65 years in the groups, respectively (hazard ratio, 0.33); 8.3 vs. 5.3 for 113 and 64 patients aged 65-74 years, respectively (HR, 0.43); and 9.2 and 5.5 months in 25 and 8 patients aged 75 and older (HR, 0.47), he said.
“The hazard ratios are comparable across all three age cohorts,” Dr. Ledermann said.
Treatment-emergent adverse events (TEAEs) of any grade occurring in at least 35% of patients included nausea, asthenia, vomiting, dysgeusia, constipation, anemia, ALT/AST increase, diarrhea, abdominal pain, thrombocytopenia, and pruritus.
The rates of these events were similar across age groups, although slight, nonsignificant numerical increases in grade 3 toxicities occurred with increasing age, he noted.
“If we look at dose interruption and dose reduction rates – again, broadly similar, [but with] a slight trend toward an increase in older age groups,” he said.
Dose modifications related to TEAEs, however, occurred in 65.5% of rucaparib and 9.4% of placebo patients aged under 65 years, compared with 82.3% and 12.5% of those aged 65-74, and 83.3% and 12.5% of those aged 75 or older, respectively. Discontinuations because of TEAEs occurred in 13.6% and 1.7% in those under age 65 years vs. 19.5% and 3.1% in those aged 65-74 years, and 20.8% and 0% of those aged 75 years or older in the groups, respectively.
Of note, the age subgroups and the treatment and placebo groups were well balanced except more of those under age 65 years had a deleterious germline or somatic BRCA mutation (96 and 49 in the rucaparib and placebo arms, respectively) than did patients aged 65-74 years (29 and 15, respectively), and patients aged 75 years and older (5 and 2, respectively).
ARIEL3 was funded by Clovis Oncology. Dr. Ledermann reported financial relationships (consulting, grant receipt, honorarial reimbursement, and/or speakers bureau fees) with AstraZeneca, Clovis Oncology, Pfizer, Seattle Genetics, Roche, and MSD/Merck.
SOURCE: Ledermann J et al. SGO 2019, Abstract 4.
HONOLULU – The improved progression-free survival and reduced risk of disease progression seen with maintenance rucaparib versus placebo in women with recurrent ovarian cancer in the pivotal ARIEL3 study occurred across age subgroups, according to a post hoc exploratory analysis of data from the phase 3 study.
In general, the safety profile of the first-in-class poly (ADP-ribose) polymerase (PARP) inhibitor was consistent across age groups, as well, but rates of dose modifications and treatment discontinuations varied slightly by age subgroup in the rucaparib (Rubraca)and placebo arms, Jonathan A. Ledermann, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
No clear trend emerged with respect to those differences, said Dr. Ledermann, a professor of medical oncology at University College of London Cancer Institute and UCL Hospitals.
Initial results from the randomized, placebo-controlled study were published in 2017 and showed a significant progression-free survival (PFS) benefit with 600 mg of maintenance rucaparib vs. placebo (10.8 vs. 5.4 months, respectively, in the intention-to-treat population). For the current analysis, outcomes were assessed for the ITT population across three baseline age-based subgroups: those under age 65 years, those aged 65-74 years, and those 75 years or older.
Investigator-assessed PFS – the primary endpoint of ARIEL3 – for the rucaparib vs. placebo groups was 11.1 vs. 5.4 months among 237 and 117 patients under age 65 years in the groups, respectively (hazard ratio, 0.33); 8.3 vs. 5.3 for 113 and 64 patients aged 65-74 years, respectively (HR, 0.43); and 9.2 and 5.5 months in 25 and 8 patients aged 75 and older (HR, 0.47), he said.
“The hazard ratios are comparable across all three age cohorts,” Dr. Ledermann said.
Treatment-emergent adverse events (TEAEs) of any grade occurring in at least 35% of patients included nausea, asthenia, vomiting, dysgeusia, constipation, anemia, ALT/AST increase, diarrhea, abdominal pain, thrombocytopenia, and pruritus.
The rates of these events were similar across age groups, although slight, nonsignificant numerical increases in grade 3 toxicities occurred with increasing age, he noted.
“If we look at dose interruption and dose reduction rates – again, broadly similar, [but with] a slight trend toward an increase in older age groups,” he said.
Dose modifications related to TEAEs, however, occurred in 65.5% of rucaparib and 9.4% of placebo patients aged under 65 years, compared with 82.3% and 12.5% of those aged 65-74, and 83.3% and 12.5% of those aged 75 or older, respectively. Discontinuations because of TEAEs occurred in 13.6% and 1.7% in those under age 65 years vs. 19.5% and 3.1% in those aged 65-74 years, and 20.8% and 0% of those aged 75 years or older in the groups, respectively.
Of note, the age subgroups and the treatment and placebo groups were well balanced except more of those under age 65 years had a deleterious germline or somatic BRCA mutation (96 and 49 in the rucaparib and placebo arms, respectively) than did patients aged 65-74 years (29 and 15, respectively), and patients aged 75 years and older (5 and 2, respectively).
ARIEL3 was funded by Clovis Oncology. Dr. Ledermann reported financial relationships (consulting, grant receipt, honorarial reimbursement, and/or speakers bureau fees) with AstraZeneca, Clovis Oncology, Pfizer, Seattle Genetics, Roche, and MSD/Merck.
SOURCE: Ledermann J et al. SGO 2019, Abstract 4.
HONOLULU – The improved progression-free survival and reduced risk of disease progression seen with maintenance rucaparib versus placebo in women with recurrent ovarian cancer in the pivotal ARIEL3 study occurred across age subgroups, according to a post hoc exploratory analysis of data from the phase 3 study.
In general, the safety profile of the first-in-class poly (ADP-ribose) polymerase (PARP) inhibitor was consistent across age groups, as well, but rates of dose modifications and treatment discontinuations varied slightly by age subgroup in the rucaparib (Rubraca)and placebo arms, Jonathan A. Ledermann, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
No clear trend emerged with respect to those differences, said Dr. Ledermann, a professor of medical oncology at University College of London Cancer Institute and UCL Hospitals.
Initial results from the randomized, placebo-controlled study were published in 2017 and showed a significant progression-free survival (PFS) benefit with 600 mg of maintenance rucaparib vs. placebo (10.8 vs. 5.4 months, respectively, in the intention-to-treat population). For the current analysis, outcomes were assessed for the ITT population across three baseline age-based subgroups: those under age 65 years, those aged 65-74 years, and those 75 years or older.
Investigator-assessed PFS – the primary endpoint of ARIEL3 – for the rucaparib vs. placebo groups was 11.1 vs. 5.4 months among 237 and 117 patients under age 65 years in the groups, respectively (hazard ratio, 0.33); 8.3 vs. 5.3 for 113 and 64 patients aged 65-74 years, respectively (HR, 0.43); and 9.2 and 5.5 months in 25 and 8 patients aged 75 and older (HR, 0.47), he said.
“The hazard ratios are comparable across all three age cohorts,” Dr. Ledermann said.
Treatment-emergent adverse events (TEAEs) of any grade occurring in at least 35% of patients included nausea, asthenia, vomiting, dysgeusia, constipation, anemia, ALT/AST increase, diarrhea, abdominal pain, thrombocytopenia, and pruritus.
The rates of these events were similar across age groups, although slight, nonsignificant numerical increases in grade 3 toxicities occurred with increasing age, he noted.
“If we look at dose interruption and dose reduction rates – again, broadly similar, [but with] a slight trend toward an increase in older age groups,” he said.
Dose modifications related to TEAEs, however, occurred in 65.5% of rucaparib and 9.4% of placebo patients aged under 65 years, compared with 82.3% and 12.5% of those aged 65-74, and 83.3% and 12.5% of those aged 75 or older, respectively. Discontinuations because of TEAEs occurred in 13.6% and 1.7% in those under age 65 years vs. 19.5% and 3.1% in those aged 65-74 years, and 20.8% and 0% of those aged 75 years or older in the groups, respectively.
Of note, the age subgroups and the treatment and placebo groups were well balanced except more of those under age 65 years had a deleterious germline or somatic BRCA mutation (96 and 49 in the rucaparib and placebo arms, respectively) than did patients aged 65-74 years (29 and 15, respectively), and patients aged 75 years and older (5 and 2, respectively).
ARIEL3 was funded by Clovis Oncology. Dr. Ledermann reported financial relationships (consulting, grant receipt, honorarial reimbursement, and/or speakers bureau fees) with AstraZeneca, Clovis Oncology, Pfizer, Seattle Genetics, Roche, and MSD/Merck.
SOURCE: Ledermann J et al. SGO 2019, Abstract 4.
REPORTING FROM SGO 2019
Low-flow, low-gradient aortic stenosis with preserved LVEF: a special situation
SNOWMASS, COLO. – A patient who presents with symptomatic low-flow, low-gradient severe aortic stenosis, hypertension, and preserved left ventricular ejection fraction (LVEF) is often referred straightaway for consideration of aortic valve replacement. Not so fast – these patients actually constitute a special case for whom two essential questions must be answered before proceeding to that stage, Rick A. Nishimura, MD, said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The first question is, What happens to the patient’s symptoms upon control of the hypertension?
“Almost all of these patients with low-flow severe aortic stenosis with preserved ejection fraction are going to be hypertensive. Treat the hypertension first. If they become asymptomatic, you don’t need to intervene. The aortic stenosis wasn’t causing their symptoms. You can afford to continue to watch them,” according to Dr. Nishimura, professor of cardiovascular diseases and hypertension at the Mayo Clinic in Rochester, Minn.
An aortic valve area of less than 1.0 cm2 is a prerequisite for surgical or transcatheter aortic valve replacement. So the second key question is this, Does the patient have truly severe aortic stenosis (AS), or is it instead a case of pseudo-AS in which the small aortic valve area noted on echocardiography is caused by low flow secondary to a small left ventricle with a low stroke volume?
“If you increase the flow and remeasure the aortic valve area, you’ll find that a lot of these patients don’t have a really small aortic valve area of less than 1.0 cm2. You might find the aortic valve area pops up to 1.4-1.6 cm2,” he explained.
These patients with symptomatic low-flow, low-gradient severe AS with preserved LVEF are quite common.
“I don’t know why, but we’re seeing more and more of these patients. I think 10 years ago we just kind of ignored them. We thought we’d made a mistake in our calculations. But in fact if you’re very meticulous about your calculations, 30%-40% of your aortic stenosis patients fit into this category,” the cardiologist said.
Moreover, if these patients undergo aortic valve replacement when their symptoms stemmed from poorly controlled hypertension and/or pseudo-AS, they are not going to benefit from this major intervention, he added.
This issue was addressed, albeit briefly and obliquely, in the American Heart Association/ACC guidelines for management of patients with valvular heart disease, for which Dr. Nishimura served as first author and cochair of the writing committee (Circulation. 2014 Jun 10;129[23]:e521-643) as well as for the 2017 focused update of the guidelines.
The guidelines give a IIa recommendation to aortic valve replacement as “reasonable” in “symptomatic patients with low-flow/low-gradient severe AS (stage D3) with an LVEF 50% or greater, a calcified aortic valve with significantly reduced leaflet motion, and a valve area 1.0 cm2 or less only if clinical, hemodynamic, and anatomic data support valve obstruction as the most likely cause of symptoms and data recorded when the patient is normotensive.”
Dr. Nishimura chose the 50th annual meeting of the storied ACC Snowmass conference to elaborate upon that brief guidance. He explained that these patients with low-flow, low-gradient symptomatic “severe” AS with preserved LVEF and hypertension have two resistors in a series.
“You have a resistor at the aortic valve area but probably a greater resistor in the systemic circulation. They have high resistance at the arterial level and diastolic dysfunction due to ventricular-vascular coupling,” the cardiologist continued.
Checking for pseudo-AS in these patients is a matter of boosting their low transvalvular flow. This can be accomplished by increasing their cardiac output via monitored exercise or by pharmacologic afterload reduction.
“We’re exercising these patients in the cath lab, but you could also do it in the echocardiographic laboratory. With exercise, if cardiac output increases and the aortic valve area increases without significant change in the aortic valve mean gradient, the patient probably doesn’t have truly severe AS,” according to Dr. Nishimura.
One reason referral centers are seeing a lot more of these patients during the last decade is an influential study by Canadian investigators entitled “Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival.” Those investigators warned “this condition may often be misdiagnosed, which leads to a neglect and/or underestimation of symptoms and an inappropriate delay of aortic valve replacement surgery” (Circulation. 2007 Jun 5;115(22):2856-64).
This report led to a great deal of interest in performing aortic valve replacement in such patients during a period when transcatheter replacement was really taking off.
When an audience member asked how commonly such patients have undergone inappropriate aortic valve replacement, Michael J. Mack, MD, took the question.
“I don’t think it’s a huge number,” said Dr. Mack, medical director of cardiovascular surgery at the Baylor Health Care System in Plano, Tex. “This is the patient group we wring our hands about most. We know they don’t do as well with aortic valve replacement as patients with high-gradient AS with a low or normal ejection fraction. We’re loathe to treat them. I think most centers are.”
SNOWMASS, COLO. – A patient who presents with symptomatic low-flow, low-gradient severe aortic stenosis, hypertension, and preserved left ventricular ejection fraction (LVEF) is often referred straightaway for consideration of aortic valve replacement. Not so fast – these patients actually constitute a special case for whom two essential questions must be answered before proceeding to that stage, Rick A. Nishimura, MD, said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The first question is, What happens to the patient’s symptoms upon control of the hypertension?
“Almost all of these patients with low-flow severe aortic stenosis with preserved ejection fraction are going to be hypertensive. Treat the hypertension first. If they become asymptomatic, you don’t need to intervene. The aortic stenosis wasn’t causing their symptoms. You can afford to continue to watch them,” according to Dr. Nishimura, professor of cardiovascular diseases and hypertension at the Mayo Clinic in Rochester, Minn.
An aortic valve area of less than 1.0 cm2 is a prerequisite for surgical or transcatheter aortic valve replacement. So the second key question is this, Does the patient have truly severe aortic stenosis (AS), or is it instead a case of pseudo-AS in which the small aortic valve area noted on echocardiography is caused by low flow secondary to a small left ventricle with a low stroke volume?
“If you increase the flow and remeasure the aortic valve area, you’ll find that a lot of these patients don’t have a really small aortic valve area of less than 1.0 cm2. You might find the aortic valve area pops up to 1.4-1.6 cm2,” he explained.
These patients with symptomatic low-flow, low-gradient severe AS with preserved LVEF are quite common.
“I don’t know why, but we’re seeing more and more of these patients. I think 10 years ago we just kind of ignored them. We thought we’d made a mistake in our calculations. But in fact if you’re very meticulous about your calculations, 30%-40% of your aortic stenosis patients fit into this category,” the cardiologist said.
Moreover, if these patients undergo aortic valve replacement when their symptoms stemmed from poorly controlled hypertension and/or pseudo-AS, they are not going to benefit from this major intervention, he added.
This issue was addressed, albeit briefly and obliquely, in the American Heart Association/ACC guidelines for management of patients with valvular heart disease, for which Dr. Nishimura served as first author and cochair of the writing committee (Circulation. 2014 Jun 10;129[23]:e521-643) as well as for the 2017 focused update of the guidelines.
The guidelines give a IIa recommendation to aortic valve replacement as “reasonable” in “symptomatic patients with low-flow/low-gradient severe AS (stage D3) with an LVEF 50% or greater, a calcified aortic valve with significantly reduced leaflet motion, and a valve area 1.0 cm2 or less only if clinical, hemodynamic, and anatomic data support valve obstruction as the most likely cause of symptoms and data recorded when the patient is normotensive.”
Dr. Nishimura chose the 50th annual meeting of the storied ACC Snowmass conference to elaborate upon that brief guidance. He explained that these patients with low-flow, low-gradient symptomatic “severe” AS with preserved LVEF and hypertension have two resistors in a series.
“You have a resistor at the aortic valve area but probably a greater resistor in the systemic circulation. They have high resistance at the arterial level and diastolic dysfunction due to ventricular-vascular coupling,” the cardiologist continued.
Checking for pseudo-AS in these patients is a matter of boosting their low transvalvular flow. This can be accomplished by increasing their cardiac output via monitored exercise or by pharmacologic afterload reduction.
“We’re exercising these patients in the cath lab, but you could also do it in the echocardiographic laboratory. With exercise, if cardiac output increases and the aortic valve area increases without significant change in the aortic valve mean gradient, the patient probably doesn’t have truly severe AS,” according to Dr. Nishimura.
One reason referral centers are seeing a lot more of these patients during the last decade is an influential study by Canadian investigators entitled “Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival.” Those investigators warned “this condition may often be misdiagnosed, which leads to a neglect and/or underestimation of symptoms and an inappropriate delay of aortic valve replacement surgery” (Circulation. 2007 Jun 5;115(22):2856-64).
This report led to a great deal of interest in performing aortic valve replacement in such patients during a period when transcatheter replacement was really taking off.
When an audience member asked how commonly such patients have undergone inappropriate aortic valve replacement, Michael J. Mack, MD, took the question.
“I don’t think it’s a huge number,” said Dr. Mack, medical director of cardiovascular surgery at the Baylor Health Care System in Plano, Tex. “This is the patient group we wring our hands about most. We know they don’t do as well with aortic valve replacement as patients with high-gradient AS with a low or normal ejection fraction. We’re loathe to treat them. I think most centers are.”
SNOWMASS, COLO. – A patient who presents with symptomatic low-flow, low-gradient severe aortic stenosis, hypertension, and preserved left ventricular ejection fraction (LVEF) is often referred straightaway for consideration of aortic valve replacement. Not so fast – these patients actually constitute a special case for whom two essential questions must be answered before proceeding to that stage, Rick A. Nishimura, MD, said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The first question is, What happens to the patient’s symptoms upon control of the hypertension?
“Almost all of these patients with low-flow severe aortic stenosis with preserved ejection fraction are going to be hypertensive. Treat the hypertension first. If they become asymptomatic, you don’t need to intervene. The aortic stenosis wasn’t causing their symptoms. You can afford to continue to watch them,” according to Dr. Nishimura, professor of cardiovascular diseases and hypertension at the Mayo Clinic in Rochester, Minn.
An aortic valve area of less than 1.0 cm2 is a prerequisite for surgical or transcatheter aortic valve replacement. So the second key question is this, Does the patient have truly severe aortic stenosis (AS), or is it instead a case of pseudo-AS in which the small aortic valve area noted on echocardiography is caused by low flow secondary to a small left ventricle with a low stroke volume?
“If you increase the flow and remeasure the aortic valve area, you’ll find that a lot of these patients don’t have a really small aortic valve area of less than 1.0 cm2. You might find the aortic valve area pops up to 1.4-1.6 cm2,” he explained.
These patients with symptomatic low-flow, low-gradient severe AS with preserved LVEF are quite common.
“I don’t know why, but we’re seeing more and more of these patients. I think 10 years ago we just kind of ignored them. We thought we’d made a mistake in our calculations. But in fact if you’re very meticulous about your calculations, 30%-40% of your aortic stenosis patients fit into this category,” the cardiologist said.
Moreover, if these patients undergo aortic valve replacement when their symptoms stemmed from poorly controlled hypertension and/or pseudo-AS, they are not going to benefit from this major intervention, he added.
This issue was addressed, albeit briefly and obliquely, in the American Heart Association/ACC guidelines for management of patients with valvular heart disease, for which Dr. Nishimura served as first author and cochair of the writing committee (Circulation. 2014 Jun 10;129[23]:e521-643) as well as for the 2017 focused update of the guidelines.
The guidelines give a IIa recommendation to aortic valve replacement as “reasonable” in “symptomatic patients with low-flow/low-gradient severe AS (stage D3) with an LVEF 50% or greater, a calcified aortic valve with significantly reduced leaflet motion, and a valve area 1.0 cm2 or less only if clinical, hemodynamic, and anatomic data support valve obstruction as the most likely cause of symptoms and data recorded when the patient is normotensive.”
Dr. Nishimura chose the 50th annual meeting of the storied ACC Snowmass conference to elaborate upon that brief guidance. He explained that these patients with low-flow, low-gradient symptomatic “severe” AS with preserved LVEF and hypertension have two resistors in a series.
“You have a resistor at the aortic valve area but probably a greater resistor in the systemic circulation. They have high resistance at the arterial level and diastolic dysfunction due to ventricular-vascular coupling,” the cardiologist continued.
Checking for pseudo-AS in these patients is a matter of boosting their low transvalvular flow. This can be accomplished by increasing their cardiac output via monitored exercise or by pharmacologic afterload reduction.
“We’re exercising these patients in the cath lab, but you could also do it in the echocardiographic laboratory. With exercise, if cardiac output increases and the aortic valve area increases without significant change in the aortic valve mean gradient, the patient probably doesn’t have truly severe AS,” according to Dr. Nishimura.
One reason referral centers are seeing a lot more of these patients during the last decade is an influential study by Canadian investigators entitled “Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival.” Those investigators warned “this condition may often be misdiagnosed, which leads to a neglect and/or underestimation of symptoms and an inappropriate delay of aortic valve replacement surgery” (Circulation. 2007 Jun 5;115(22):2856-64).
This report led to a great deal of interest in performing aortic valve replacement in such patients during a period when transcatheter replacement was really taking off.
When an audience member asked how commonly such patients have undergone inappropriate aortic valve replacement, Michael J. Mack, MD, took the question.
“I don’t think it’s a huge number,” said Dr. Mack, medical director of cardiovascular surgery at the Baylor Health Care System in Plano, Tex. “This is the patient group we wring our hands about most. We know they don’t do as well with aortic valve replacement as patients with high-gradient AS with a low or normal ejection fraction. We’re loathe to treat them. I think most centers are.”
REPORTING FROM ACC SNOWMASS 2019
Hospitalists and PTs: Building strong relationships
Optimizing discharge disposition and longitudinal recovery
Sanctimonious, self-righteous, discharge saboteurs. These are just a few descriptors I’ve heard hospitalists use to describe my physical therapy (PT) colleagues.
These charged comments come mostly after a hospitalist reads therapy notes and encounters a contradiction to their chosen discharge location for a patient.
I recently met with hospitalists from four different hospitals. They echoed the frustrations of their physician colleagues across the country. The PTs they work with write “the patient requires 24-hour supervision and 3 hours of therapy a day,” or “the patient is unsafe to go home and needs continued therapy at an inpatient rehabilitation center.” The hospitalists in turn want to know “If I discharge the patient home am I liable if the patient falls or has some other negative outcome?” The frustration hospitalists experience is palpable and understandable as their attempts to support a home recovery are often contradicted.
Outside the four walls
The transition from fee-for-service to value-based care now calls upon hospitalists to be innovators in managing patients in alternative payment models, such as accountable care organizations, bundled payment programs, and Medicare Advantage plans. Each model looks to support a home recovery whenever possible and prevent readmissions.
Case managers for Medicare Advantage programs routinely review PT notes to inform hospital discharge disposition and post-acute authorization for skilled nursing facility (SNF) admissions and days in SNF. Hospitalists, working with care managers, can follow suit to succeed in alternative payment models. They have the advantage of in-person access to PT colleagues for elaboration and push-back as necessary. For hospitalists, working collaboratively with PTs is crucial to improving the value of care provided as patients transition beyond the four walls of the hospital.
The evolution of PT in acute care
Prior to diagnosis-related groups (DRGs), PTs were profit centers for hospitals – rehabilitation departments were well staffed and easily accommodated consults and requests for mobility.
With the advent of DRGs, physical therapy became a cost center, and rehabilitation staffs were reduced. PTs became overextended, were less available for consultations for mobilization, and patients suffered the deleterious effects of immobility. With reduced staffing and a rush to get patients out of the hospital, acute PT practice morphed into evaluating functional status and determining discharge destination.
Now, as members of an aligned health care team, PTs need to facilitate a safe home discharge whenever possible and determine what skilled services a patient needs post-acute stay, not where they should receive them.
Discharge disposition and longitudinal recovery
PTs, as experts in function, have a series of “special tests” at their disposal beyond pain, range of motion, and strength assessments. These include: Activity Measure for Post-Acute Care (AM-PAC) or “6-Clicks” Mobility Score, Timed Up and Go, Six-Minute Walk Test, Tinetti, Berg Balance Scale, Modified Barthel Index, Five Times Chair Rise, and Thirty-Second Chair Rise. These are all objective measures of function that can be used to inform discharge disposition and guide longitudinal recovery.
To elaborate on one tool, the 6-Clicks Mobility Score is a validated test that allows PTs to assess basic mobility.1,2 It rates six functional tasks (hence 6 clicks) that include: turning over in bed, moving from lying to sitting, moving to/from bed to chair, transitioning from sitting to standing from a chair, walking in a hospital room, and climbing three to five steps. These functional tasks are scored based on the amount of assistance needed. The scores, in turn, have been shown to support discharge destination planning.1 In addition to informing discharge destination decisions, hospitalists and the rest of the health care team can use 6-Clicks to estimate prolonged hospital stays, readmissions, and emergency department (ED) visits.3
Of course, discharge disposition is influenced by many factors in addition to functional status. Hospitalists are the obvious choice to lead the health care team in interpreting relevant data and test results, and to communicate these results to patients and caregivers so together they can decide the most appropriate discharge destination.
I envision a conversation between a fully informed hospitalist and a patient as follows: “Based on your past history, your living situation, all of your test results including labs, x-rays and the functional tests performed by your PT, your potential for a full recovery is good. You have a moderate decline in function with a high likelihood of returning home in the next 7-10 days. I recommend you go to a SNF for high-intensity rehabilitation for 7 days and that the SNF order PT and OT twice a day and walks with nursing every evening.”
This fully informed conversation can only take place if hospitalists are provided clear, concise documentation, including results of objective functional testing, by their physical therapy colleagues.
In conclusion, PTs working in the acute setting need to use validated tests to objectively assess function and educate their hospitalist colleagues on the meaning of these tests. Hospitalists in turn can incorporate these assessments into a discussion of discharge disposition and longitudinal recovery with patients. In this way, hospitalists and physical therapists can work together to achieve patient-centered, high-value care during and following a hospitalization.
Ms. Tammany is SVP of clinical strategy & innovation for Remedy Partners, Norwalk, Conn.
References
1. Jette DU et al. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014 Sep;94(9):1252-61.
2. Jette DU et al. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014 Mar;94(3):379-91.
3. Menendez ME et al. Does “6-Clicks” Day 1 Postoperative Mobility Score Predict Discharge Disposition After Total Hip and Knee Arthroplasties?” J Arthroplasty. 2016 Sep;31(9):1916-20.
Optimizing discharge disposition and longitudinal recovery
Optimizing discharge disposition and longitudinal recovery
Sanctimonious, self-righteous, discharge saboteurs. These are just a few descriptors I’ve heard hospitalists use to describe my physical therapy (PT) colleagues.
These charged comments come mostly after a hospitalist reads therapy notes and encounters a contradiction to their chosen discharge location for a patient.
I recently met with hospitalists from four different hospitals. They echoed the frustrations of their physician colleagues across the country. The PTs they work with write “the patient requires 24-hour supervision and 3 hours of therapy a day,” or “the patient is unsafe to go home and needs continued therapy at an inpatient rehabilitation center.” The hospitalists in turn want to know “If I discharge the patient home am I liable if the patient falls or has some other negative outcome?” The frustration hospitalists experience is palpable and understandable as their attempts to support a home recovery are often contradicted.
Outside the four walls
The transition from fee-for-service to value-based care now calls upon hospitalists to be innovators in managing patients in alternative payment models, such as accountable care organizations, bundled payment programs, and Medicare Advantage plans. Each model looks to support a home recovery whenever possible and prevent readmissions.
Case managers for Medicare Advantage programs routinely review PT notes to inform hospital discharge disposition and post-acute authorization for skilled nursing facility (SNF) admissions and days in SNF. Hospitalists, working with care managers, can follow suit to succeed in alternative payment models. They have the advantage of in-person access to PT colleagues for elaboration and push-back as necessary. For hospitalists, working collaboratively with PTs is crucial to improving the value of care provided as patients transition beyond the four walls of the hospital.
The evolution of PT in acute care
Prior to diagnosis-related groups (DRGs), PTs were profit centers for hospitals – rehabilitation departments were well staffed and easily accommodated consults and requests for mobility.
With the advent of DRGs, physical therapy became a cost center, and rehabilitation staffs were reduced. PTs became overextended, were less available for consultations for mobilization, and patients suffered the deleterious effects of immobility. With reduced staffing and a rush to get patients out of the hospital, acute PT practice morphed into evaluating functional status and determining discharge destination.
Now, as members of an aligned health care team, PTs need to facilitate a safe home discharge whenever possible and determine what skilled services a patient needs post-acute stay, not where they should receive them.
Discharge disposition and longitudinal recovery
PTs, as experts in function, have a series of “special tests” at their disposal beyond pain, range of motion, and strength assessments. These include: Activity Measure for Post-Acute Care (AM-PAC) or “6-Clicks” Mobility Score, Timed Up and Go, Six-Minute Walk Test, Tinetti, Berg Balance Scale, Modified Barthel Index, Five Times Chair Rise, and Thirty-Second Chair Rise. These are all objective measures of function that can be used to inform discharge disposition and guide longitudinal recovery.
To elaborate on one tool, the 6-Clicks Mobility Score is a validated test that allows PTs to assess basic mobility.1,2 It rates six functional tasks (hence 6 clicks) that include: turning over in bed, moving from lying to sitting, moving to/from bed to chair, transitioning from sitting to standing from a chair, walking in a hospital room, and climbing three to five steps. These functional tasks are scored based on the amount of assistance needed. The scores, in turn, have been shown to support discharge destination planning.1 In addition to informing discharge destination decisions, hospitalists and the rest of the health care team can use 6-Clicks to estimate prolonged hospital stays, readmissions, and emergency department (ED) visits.3
Of course, discharge disposition is influenced by many factors in addition to functional status. Hospitalists are the obvious choice to lead the health care team in interpreting relevant data and test results, and to communicate these results to patients and caregivers so together they can decide the most appropriate discharge destination.
I envision a conversation between a fully informed hospitalist and a patient as follows: “Based on your past history, your living situation, all of your test results including labs, x-rays and the functional tests performed by your PT, your potential for a full recovery is good. You have a moderate decline in function with a high likelihood of returning home in the next 7-10 days. I recommend you go to a SNF for high-intensity rehabilitation for 7 days and that the SNF order PT and OT twice a day and walks with nursing every evening.”
This fully informed conversation can only take place if hospitalists are provided clear, concise documentation, including results of objective functional testing, by their physical therapy colleagues.
In conclusion, PTs working in the acute setting need to use validated tests to objectively assess function and educate their hospitalist colleagues on the meaning of these tests. Hospitalists in turn can incorporate these assessments into a discussion of discharge disposition and longitudinal recovery with patients. In this way, hospitalists and physical therapists can work together to achieve patient-centered, high-value care during and following a hospitalization.
Ms. Tammany is SVP of clinical strategy & innovation for Remedy Partners, Norwalk, Conn.
References
1. Jette DU et al. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014 Sep;94(9):1252-61.
2. Jette DU et al. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014 Mar;94(3):379-91.
3. Menendez ME et al. Does “6-Clicks” Day 1 Postoperative Mobility Score Predict Discharge Disposition After Total Hip and Knee Arthroplasties?” J Arthroplasty. 2016 Sep;31(9):1916-20.
Sanctimonious, self-righteous, discharge saboteurs. These are just a few descriptors I’ve heard hospitalists use to describe my physical therapy (PT) colleagues.
These charged comments come mostly after a hospitalist reads therapy notes and encounters a contradiction to their chosen discharge location for a patient.
I recently met with hospitalists from four different hospitals. They echoed the frustrations of their physician colleagues across the country. The PTs they work with write “the patient requires 24-hour supervision and 3 hours of therapy a day,” or “the patient is unsafe to go home and needs continued therapy at an inpatient rehabilitation center.” The hospitalists in turn want to know “If I discharge the patient home am I liable if the patient falls or has some other negative outcome?” The frustration hospitalists experience is palpable and understandable as their attempts to support a home recovery are often contradicted.
Outside the four walls
The transition from fee-for-service to value-based care now calls upon hospitalists to be innovators in managing patients in alternative payment models, such as accountable care organizations, bundled payment programs, and Medicare Advantage plans. Each model looks to support a home recovery whenever possible and prevent readmissions.
Case managers for Medicare Advantage programs routinely review PT notes to inform hospital discharge disposition and post-acute authorization for skilled nursing facility (SNF) admissions and days in SNF. Hospitalists, working with care managers, can follow suit to succeed in alternative payment models. They have the advantage of in-person access to PT colleagues for elaboration and push-back as necessary. For hospitalists, working collaboratively with PTs is crucial to improving the value of care provided as patients transition beyond the four walls of the hospital.
The evolution of PT in acute care
Prior to diagnosis-related groups (DRGs), PTs were profit centers for hospitals – rehabilitation departments were well staffed and easily accommodated consults and requests for mobility.
With the advent of DRGs, physical therapy became a cost center, and rehabilitation staffs were reduced. PTs became overextended, were less available for consultations for mobilization, and patients suffered the deleterious effects of immobility. With reduced staffing and a rush to get patients out of the hospital, acute PT practice morphed into evaluating functional status and determining discharge destination.
Now, as members of an aligned health care team, PTs need to facilitate a safe home discharge whenever possible and determine what skilled services a patient needs post-acute stay, not where they should receive them.
Discharge disposition and longitudinal recovery
PTs, as experts in function, have a series of “special tests” at their disposal beyond pain, range of motion, and strength assessments. These include: Activity Measure for Post-Acute Care (AM-PAC) or “6-Clicks” Mobility Score, Timed Up and Go, Six-Minute Walk Test, Tinetti, Berg Balance Scale, Modified Barthel Index, Five Times Chair Rise, and Thirty-Second Chair Rise. These are all objective measures of function that can be used to inform discharge disposition and guide longitudinal recovery.
To elaborate on one tool, the 6-Clicks Mobility Score is a validated test that allows PTs to assess basic mobility.1,2 It rates six functional tasks (hence 6 clicks) that include: turning over in bed, moving from lying to sitting, moving to/from bed to chair, transitioning from sitting to standing from a chair, walking in a hospital room, and climbing three to five steps. These functional tasks are scored based on the amount of assistance needed. The scores, in turn, have been shown to support discharge destination planning.1 In addition to informing discharge destination decisions, hospitalists and the rest of the health care team can use 6-Clicks to estimate prolonged hospital stays, readmissions, and emergency department (ED) visits.3
Of course, discharge disposition is influenced by many factors in addition to functional status. Hospitalists are the obvious choice to lead the health care team in interpreting relevant data and test results, and to communicate these results to patients and caregivers so together they can decide the most appropriate discharge destination.
I envision a conversation between a fully informed hospitalist and a patient as follows: “Based on your past history, your living situation, all of your test results including labs, x-rays and the functional tests performed by your PT, your potential for a full recovery is good. You have a moderate decline in function with a high likelihood of returning home in the next 7-10 days. I recommend you go to a SNF for high-intensity rehabilitation for 7 days and that the SNF order PT and OT twice a day and walks with nursing every evening.”
This fully informed conversation can only take place if hospitalists are provided clear, concise documentation, including results of objective functional testing, by their physical therapy colleagues.
In conclusion, PTs working in the acute setting need to use validated tests to objectively assess function and educate their hospitalist colleagues on the meaning of these tests. Hospitalists in turn can incorporate these assessments into a discussion of discharge disposition and longitudinal recovery with patients. In this way, hospitalists and physical therapists can work together to achieve patient-centered, high-value care during and following a hospitalization.
Ms. Tammany is SVP of clinical strategy & innovation for Remedy Partners, Norwalk, Conn.
References
1. Jette DU et al. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014 Sep;94(9):1252-61.
2. Jette DU et al. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014 Mar;94(3):379-91.
3. Menendez ME et al. Does “6-Clicks” Day 1 Postoperative Mobility Score Predict Discharge Disposition After Total Hip and Knee Arthroplasties?” J Arthroplasty. 2016 Sep;31(9):1916-20.