User login
Age may influence choice of behavioral therapy to improve sleep in MS
SEATTLE – Future behavioral interventions for improving sleep in patients with multiple sclerosis (MS) should focus on sedentary behavior and light physical activity, according to a study presented at the annual meeting of the Consortium of Multiple Sclerosis Centers. , said the researchers.
Sleep quality generally decreases with age. Among patients with MS, the prevalence of sleep problems increases threefold with age. Although data indicate that physical activity has many benefits for patients with MS, little research has examined the relationships between physical activity, sedentary behavior, and sleep quality across the lifespan in this population.
Katie L.J. Cederberg, a doctoral student at the University of Alabama at Birmingham, and colleagues recruited 127 adults with MS representing three age groups into a study. In all, 42 participants were younger (aged 20-39 years), 44 were middle-aged (40-59 years), and 41 were older (60-79 years). Participants completed the Pittsburgh Sleep Quality Index (PSQI) and the Patient-Determined Disease Steps (PDDS) scale. Each participant also wore an accelerometer for 7 days. Ms. Cederberg and colleagues analyzed the accelerometer data to determine the time per day that participants spent in light physical activity, moderate-to-vigorous physical activity, and sedentary behavior using MS-specific cutpoints.
Compared with younger adults, older adults had significantly lower PSQI global scores and reported more frequent use of sleeping medications. Compared with middle-aged adults, older adults had significantly higher disability levels and spent significantly less time in moderate-to-vigorous physical activity. In addition, among older adults, sleep latency was negatively associated with time spent in light physical activity, and clinical disability was inversely associated with time spent in moderate-to-vigorous physical activity.
In younger adults, habitual sleep efficiency was inversely associated with time spent in sedentary behavior. The researchers found no significant associations between these variables in middle-aged adults.
SOURCE: Cederberg KLJ et al. CMSC 2019. Abstract DXA05.
SEATTLE – Future behavioral interventions for improving sleep in patients with multiple sclerosis (MS) should focus on sedentary behavior and light physical activity, according to a study presented at the annual meeting of the Consortium of Multiple Sclerosis Centers. , said the researchers.
Sleep quality generally decreases with age. Among patients with MS, the prevalence of sleep problems increases threefold with age. Although data indicate that physical activity has many benefits for patients with MS, little research has examined the relationships between physical activity, sedentary behavior, and sleep quality across the lifespan in this population.
Katie L.J. Cederberg, a doctoral student at the University of Alabama at Birmingham, and colleagues recruited 127 adults with MS representing three age groups into a study. In all, 42 participants were younger (aged 20-39 years), 44 were middle-aged (40-59 years), and 41 were older (60-79 years). Participants completed the Pittsburgh Sleep Quality Index (PSQI) and the Patient-Determined Disease Steps (PDDS) scale. Each participant also wore an accelerometer for 7 days. Ms. Cederberg and colleagues analyzed the accelerometer data to determine the time per day that participants spent in light physical activity, moderate-to-vigorous physical activity, and sedentary behavior using MS-specific cutpoints.
Compared with younger adults, older adults had significantly lower PSQI global scores and reported more frequent use of sleeping medications. Compared with middle-aged adults, older adults had significantly higher disability levels and spent significantly less time in moderate-to-vigorous physical activity. In addition, among older adults, sleep latency was negatively associated with time spent in light physical activity, and clinical disability was inversely associated with time spent in moderate-to-vigorous physical activity.
In younger adults, habitual sleep efficiency was inversely associated with time spent in sedentary behavior. The researchers found no significant associations between these variables in middle-aged adults.
SOURCE: Cederberg KLJ et al. CMSC 2019. Abstract DXA05.
SEATTLE – Future behavioral interventions for improving sleep in patients with multiple sclerosis (MS) should focus on sedentary behavior and light physical activity, according to a study presented at the annual meeting of the Consortium of Multiple Sclerosis Centers. , said the researchers.
Sleep quality generally decreases with age. Among patients with MS, the prevalence of sleep problems increases threefold with age. Although data indicate that physical activity has many benefits for patients with MS, little research has examined the relationships between physical activity, sedentary behavior, and sleep quality across the lifespan in this population.
Katie L.J. Cederberg, a doctoral student at the University of Alabama at Birmingham, and colleagues recruited 127 adults with MS representing three age groups into a study. In all, 42 participants were younger (aged 20-39 years), 44 were middle-aged (40-59 years), and 41 were older (60-79 years). Participants completed the Pittsburgh Sleep Quality Index (PSQI) and the Patient-Determined Disease Steps (PDDS) scale. Each participant also wore an accelerometer for 7 days. Ms. Cederberg and colleagues analyzed the accelerometer data to determine the time per day that participants spent in light physical activity, moderate-to-vigorous physical activity, and sedentary behavior using MS-specific cutpoints.
Compared with younger adults, older adults had significantly lower PSQI global scores and reported more frequent use of sleeping medications. Compared with middle-aged adults, older adults had significantly higher disability levels and spent significantly less time in moderate-to-vigorous physical activity. In addition, among older adults, sleep latency was negatively associated with time spent in light physical activity, and clinical disability was inversely associated with time spent in moderate-to-vigorous physical activity.
In younger adults, habitual sleep efficiency was inversely associated with time spent in sedentary behavior. The researchers found no significant associations between these variables in middle-aged adults.
SOURCE: Cederberg KLJ et al. CMSC 2019. Abstract DXA05.
REPORTING FROM CMSC 2019
Key clinical point: Future interventions could reduce sedentary behavior and encourage light physical activity in patients with multiple sclerosis.
Major finding: Older adults with MS have significantly lower sleep quality than younger adults with MS.
Study details: A prospective study of 127 adults with MS.
Disclosures: The study had no sponsor, and the researchers reported no disclosures.
Source: Cederberg KLJ et al. CMSC 2019. Abstract DXA05.
Pain, fatigue, depression, and anxiety are common in the year after MS diagnosis
SEATTLE – researchers reported at the annual meeting of the Consortium of Multiple Sclerosis Centers. In a novel study, about half of patients with MS reported clinically significant symptoms of depression or pain, and approximately 60% reported fatigue during that time.
Pain, fatigue, depression, and anxiety are common in MS, but their prevalence in the first year after diagnosis is not well understood. To examine the rates of these conditions and how often they co-occur during that period, Anna L. Kratz, PhD, associate professor of physical medicine and rehabilitation at the University of Michigan in Ann Arbor, and her research colleagues had 231 adults with MS complete validated surveys at 1, 2, 3, 6, 9, and 12 months after diagnosis to assess symptoms of these conditions.
Overall, 47.2% of patients reported clinically significant levels of depression, 38.5% reported clinically significant levels of anxiety, 50.4% reported clinically significant pain, and 62.2% reported clinically significant fatigue at any point during the year after diagnosis. “Of those who did not have clinically significant symptoms at time of diagnosis, 21.3% went on to develop clinically significant depression, 17.0% anxiety, 30.9% pain, and 34.1% fatigue,” the authors reported.
About 23% of patients did not have clinically significant symptoms for any condition, while 20% had clinically significant symptoms for one condition, 21% for two, 19% for three, and 17% for all four.
Depression and fatigue had the highest rate of comorbidity, whereas pain and anxiety had the lowest rate of comorbidity.
“Important clinical symptoms associated with MS are present at high levels in the first year post diagnosis,” Dr. Kratz and colleagues concluded. “While the rates and severity are marginally lower than have been identified in studies of individuals farther into the MS disease course, this study is a reminder that early MS intervention should incorporate interventions for these symptoms that are known to have strong associations with quality of life.”
The researchers had no disclosures.
SEATTLE – researchers reported at the annual meeting of the Consortium of Multiple Sclerosis Centers. In a novel study, about half of patients with MS reported clinically significant symptoms of depression or pain, and approximately 60% reported fatigue during that time.
Pain, fatigue, depression, and anxiety are common in MS, but their prevalence in the first year after diagnosis is not well understood. To examine the rates of these conditions and how often they co-occur during that period, Anna L. Kratz, PhD, associate professor of physical medicine and rehabilitation at the University of Michigan in Ann Arbor, and her research colleagues had 231 adults with MS complete validated surveys at 1, 2, 3, 6, 9, and 12 months after diagnosis to assess symptoms of these conditions.
Overall, 47.2% of patients reported clinically significant levels of depression, 38.5% reported clinically significant levels of anxiety, 50.4% reported clinically significant pain, and 62.2% reported clinically significant fatigue at any point during the year after diagnosis. “Of those who did not have clinically significant symptoms at time of diagnosis, 21.3% went on to develop clinically significant depression, 17.0% anxiety, 30.9% pain, and 34.1% fatigue,” the authors reported.
About 23% of patients did not have clinically significant symptoms for any condition, while 20% had clinically significant symptoms for one condition, 21% for two, 19% for three, and 17% for all four.
Depression and fatigue had the highest rate of comorbidity, whereas pain and anxiety had the lowest rate of comorbidity.
“Important clinical symptoms associated with MS are present at high levels in the first year post diagnosis,” Dr. Kratz and colleagues concluded. “While the rates and severity are marginally lower than have been identified in studies of individuals farther into the MS disease course, this study is a reminder that early MS intervention should incorporate interventions for these symptoms that are known to have strong associations with quality of life.”
The researchers had no disclosures.
SEATTLE – researchers reported at the annual meeting of the Consortium of Multiple Sclerosis Centers. In a novel study, about half of patients with MS reported clinically significant symptoms of depression or pain, and approximately 60% reported fatigue during that time.
Pain, fatigue, depression, and anxiety are common in MS, but their prevalence in the first year after diagnosis is not well understood. To examine the rates of these conditions and how often they co-occur during that period, Anna L. Kratz, PhD, associate professor of physical medicine and rehabilitation at the University of Michigan in Ann Arbor, and her research colleagues had 231 adults with MS complete validated surveys at 1, 2, 3, 6, 9, and 12 months after diagnosis to assess symptoms of these conditions.
Overall, 47.2% of patients reported clinically significant levels of depression, 38.5% reported clinically significant levels of anxiety, 50.4% reported clinically significant pain, and 62.2% reported clinically significant fatigue at any point during the year after diagnosis. “Of those who did not have clinically significant symptoms at time of diagnosis, 21.3% went on to develop clinically significant depression, 17.0% anxiety, 30.9% pain, and 34.1% fatigue,” the authors reported.
About 23% of patients did not have clinically significant symptoms for any condition, while 20% had clinically significant symptoms for one condition, 21% for two, 19% for three, and 17% for all four.
Depression and fatigue had the highest rate of comorbidity, whereas pain and anxiety had the lowest rate of comorbidity.
“Important clinical symptoms associated with MS are present at high levels in the first year post diagnosis,” Dr. Kratz and colleagues concluded. “While the rates and severity are marginally lower than have been identified in studies of individuals farther into the MS disease course, this study is a reminder that early MS intervention should incorporate interventions for these symptoms that are known to have strong associations with quality of life.”
The researchers had no disclosures.
REPORTING FROM CMSC 2019
Key clinical point: Pain, fatigue, depression, and anxiety are common among patients with multiple sclerosis in the 12 months after diagnosis.
Major finding: About half of patients with multiple sclerosis reported clinically significant symptoms of depression or pain, and approximately 60% reported fatigue.
Study details: An analysis of data from 231 adults with multiple sclerosis who completed validated surveys at 1, 2, 3, 6, 9, and 12 months after diagnosis to assess symptoms of pain, fatigue, depression, and anxiety.
Disclosures: The researchers had no disclosures.
More patients than ever receive DMT within 1 year of MS disease onset
SEATTLE – The proportion of patients with multiple sclerosis (MS) who start a disease-modifying therapy (DMT) within 1 year of MS onset has increased in Southern Alberta, researchers reported at the annual meeting of the Consortium of Multiple Sclerosis Centers. , said Jamie Greenfield, MPH, an epidemiologist at the University of Calgary, Alta., and colleagues.
Studies suggest that early initiation of DMT may change the course of MS, but diagnostic delays and barriers to treatment “often impede this opportunity,” the investigators said at the annual meeting of the Consortium of Multiple Sclerosis Centers. To assess time to treatment initiation among patients with relapsing-remitting MS, the researchers analyzed administrative data from the Calgary MS Clinic. They determined the time to initiation of an approved first-line DMT from MS onset, first MS clinic appointment, MS diagnosis, and most recent prior MS clinic appointment. They excluded patients who started a second-line DMT, started a DMT before an MS clinic appointment, or started a DMT during a clinical trial. In addition, they excluded patients with MS onset before 1999, when government reimbursements for DMTs became available.
In all, 1,462 eligible patients started DMTs during August 1999–March 2018; 57.2% started glatiramer acetate, 28.2% interferon-beta, 12.5% dimethyl fumarate, and 2.1% teriflunomide. Average age at treatment initiation was 36 years, and 71.3% were women. Median Expanded Disability Status Scale (EDSS) score was 2.0, and about 90% were urban residents. Approximately 23% had MS diagnosed before their first visit, 37% at their first visit, and 40% after their first visit.
Median time to DMT initiations was 20.3 months from MS onset, 5.4 months from first appointment, 4.1 months from MS diagnosis, and 1.6 months from most recent prior appointment. During 2015-2017, a greater percentage of patients started a DMT within 1 year of MS onset, compared with 1999-2004 (60.9% vs. 20.4%). During 2015-2017, patients also were more likely to start DMTs within 1 year of their first appointment (88.4% vs. 65.7%), within 1 year of MS diagnosis (92.8% vs. 81.3%), and within 3 months of their most recent prior appointment (89.2% vs. 59.5%), compared with 1999-2004.
The investigators used Spearman rank correlations or Kruskal-Wallis tests to evaluate associations between baseline characteristics and time to DMT initiation. A diagnosis of MS or a relapsing-remitting MS course at the first appointment, older age at MS onset, longer MS duration at the first appointment, higher EDSS scores at the first or most recent prior appointment, and shorter time between diagnosis and first appointment, regardless of whether diagnosis occurred before or after the appointment, were associated with earlier DMT initiation.
“Treatment delays are improving. Better understanding of these delays will guide development of additional early-initiation strategies,” the authors concluded.
Ms. Greenfield had no disclosures. A coauthor reported consulting fees from Biogen, Roche, Sanofi Genzyme, and Serono.
SOURCE: Greenfield J et al. CMSC 2019, Abstract DXT25.
SEATTLE – The proportion of patients with multiple sclerosis (MS) who start a disease-modifying therapy (DMT) within 1 year of MS onset has increased in Southern Alberta, researchers reported at the annual meeting of the Consortium of Multiple Sclerosis Centers. , said Jamie Greenfield, MPH, an epidemiologist at the University of Calgary, Alta., and colleagues.
Studies suggest that early initiation of DMT may change the course of MS, but diagnostic delays and barriers to treatment “often impede this opportunity,” the investigators said at the annual meeting of the Consortium of Multiple Sclerosis Centers. To assess time to treatment initiation among patients with relapsing-remitting MS, the researchers analyzed administrative data from the Calgary MS Clinic. They determined the time to initiation of an approved first-line DMT from MS onset, first MS clinic appointment, MS diagnosis, and most recent prior MS clinic appointment. They excluded patients who started a second-line DMT, started a DMT before an MS clinic appointment, or started a DMT during a clinical trial. In addition, they excluded patients with MS onset before 1999, when government reimbursements for DMTs became available.
In all, 1,462 eligible patients started DMTs during August 1999–March 2018; 57.2% started glatiramer acetate, 28.2% interferon-beta, 12.5% dimethyl fumarate, and 2.1% teriflunomide. Average age at treatment initiation was 36 years, and 71.3% were women. Median Expanded Disability Status Scale (EDSS) score was 2.0, and about 90% were urban residents. Approximately 23% had MS diagnosed before their first visit, 37% at their first visit, and 40% after their first visit.
Median time to DMT initiations was 20.3 months from MS onset, 5.4 months from first appointment, 4.1 months from MS diagnosis, and 1.6 months from most recent prior appointment. During 2015-2017, a greater percentage of patients started a DMT within 1 year of MS onset, compared with 1999-2004 (60.9% vs. 20.4%). During 2015-2017, patients also were more likely to start DMTs within 1 year of their first appointment (88.4% vs. 65.7%), within 1 year of MS diagnosis (92.8% vs. 81.3%), and within 3 months of their most recent prior appointment (89.2% vs. 59.5%), compared with 1999-2004.
The investigators used Spearman rank correlations or Kruskal-Wallis tests to evaluate associations between baseline characteristics and time to DMT initiation. A diagnosis of MS or a relapsing-remitting MS course at the first appointment, older age at MS onset, longer MS duration at the first appointment, higher EDSS scores at the first or most recent prior appointment, and shorter time between diagnosis and first appointment, regardless of whether diagnosis occurred before or after the appointment, were associated with earlier DMT initiation.
“Treatment delays are improving. Better understanding of these delays will guide development of additional early-initiation strategies,” the authors concluded.
Ms. Greenfield had no disclosures. A coauthor reported consulting fees from Biogen, Roche, Sanofi Genzyme, and Serono.
SOURCE: Greenfield J et al. CMSC 2019, Abstract DXT25.
SEATTLE – The proportion of patients with multiple sclerosis (MS) who start a disease-modifying therapy (DMT) within 1 year of MS onset has increased in Southern Alberta, researchers reported at the annual meeting of the Consortium of Multiple Sclerosis Centers. , said Jamie Greenfield, MPH, an epidemiologist at the University of Calgary, Alta., and colleagues.
Studies suggest that early initiation of DMT may change the course of MS, but diagnostic delays and barriers to treatment “often impede this opportunity,” the investigators said at the annual meeting of the Consortium of Multiple Sclerosis Centers. To assess time to treatment initiation among patients with relapsing-remitting MS, the researchers analyzed administrative data from the Calgary MS Clinic. They determined the time to initiation of an approved first-line DMT from MS onset, first MS clinic appointment, MS diagnosis, and most recent prior MS clinic appointment. They excluded patients who started a second-line DMT, started a DMT before an MS clinic appointment, or started a DMT during a clinical trial. In addition, they excluded patients with MS onset before 1999, when government reimbursements for DMTs became available.
In all, 1,462 eligible patients started DMTs during August 1999–March 2018; 57.2% started glatiramer acetate, 28.2% interferon-beta, 12.5% dimethyl fumarate, and 2.1% teriflunomide. Average age at treatment initiation was 36 years, and 71.3% were women. Median Expanded Disability Status Scale (EDSS) score was 2.0, and about 90% were urban residents. Approximately 23% had MS diagnosed before their first visit, 37% at their first visit, and 40% after their first visit.
Median time to DMT initiations was 20.3 months from MS onset, 5.4 months from first appointment, 4.1 months from MS diagnosis, and 1.6 months from most recent prior appointment. During 2015-2017, a greater percentage of patients started a DMT within 1 year of MS onset, compared with 1999-2004 (60.9% vs. 20.4%). During 2015-2017, patients also were more likely to start DMTs within 1 year of their first appointment (88.4% vs. 65.7%), within 1 year of MS diagnosis (92.8% vs. 81.3%), and within 3 months of their most recent prior appointment (89.2% vs. 59.5%), compared with 1999-2004.
The investigators used Spearman rank correlations or Kruskal-Wallis tests to evaluate associations between baseline characteristics and time to DMT initiation. A diagnosis of MS or a relapsing-remitting MS course at the first appointment, older age at MS onset, longer MS duration at the first appointment, higher EDSS scores at the first or most recent prior appointment, and shorter time between diagnosis and first appointment, regardless of whether diagnosis occurred before or after the appointment, were associated with earlier DMT initiation.
“Treatment delays are improving. Better understanding of these delays will guide development of additional early-initiation strategies,” the authors concluded.
Ms. Greenfield had no disclosures. A coauthor reported consulting fees from Biogen, Roche, Sanofi Genzyme, and Serono.
SOURCE: Greenfield J et al. CMSC 2019, Abstract DXT25.
REPORTING FROM CMSC 2019
Key clinical point: The proportion of patients with multiple sclerosis who start disease-modifying therapies within 1 year of disease onset may be increasing.
Major finding: During 1999-2004, about 20% of patients with MS started treatment within 1 year of disease onset, compared with 60% of patients during 2015-2017, at a center in Southern Alberta.
Study details: An analysis of administrative data from 1,462 patients from the Calgary MS Clinic between 1999 and 2018.
Disclosures: Ms. Greenfield had no disclosures. A coauthor reported consulting fees from Biogen, Roche, Sanofi Genzyme, and Serono.
Source: Greenfield J et al. CMSC 2019, Abstract DXT25.
MS linked to higher rates of hoarding behavior
SEATTLE – according to a small study that appears to be the first of its kind. It is not clear how MS and hoarding may be linked, but study author Joshua Bacon, PhD, an MS researcher and associate professor at New York University, and coauthors suspect that physical limitations are an important factor.
“It is important for clinicians to identify patients who might be hoarders and/or clutterers. It is very likely that this has an impact on the trajectory of their activities of daily living,” he said in an interview prior to the presentation of the study findings at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Dr. Bacon said the study was inspired by his observation that hoarding and cluttering behavior appear to be common among patients with MS. “As I became more interested in it, it became clear there hasn’t been any work on this in the MS population.”
For the new study, Dr. Bacon and colleagues surveyed 139 consecutive patients with MS at the New York University MS Center. The patients had a mean age of 45 years and mean disease duration of 14 years; 71% were female, and 48% were non white. The researchers measured the patients on scales of hoarding behavior (Activities of Daily Living for Hoarding and the Hoarding Rating Scale) and disability (Patient-Determined Disability Steps).
The researchers found that nearly 12% showed signs of clinically significant hoarding behavior, compared with an estimated 5% of the general population (P = .0008). Researchers linked disability and Hoarding Rating Scale to the variability in degree of difficulty in performing activities of daily living (P less than .0001).
Dr. Bacon and colleagues do not believe MS is the direct cause of hoarding behaviors. “There has been no literature on this, and we do not know whether this is connected to the neurological condition,” he said. “I think it has more to do with physical capabilities.”
Patients with MS may have mobility problems that disrupt their ability to organize their homes, he said. “You can’t move things the way you can when you have normal mobility,” he said. “Things can start building up, and it is harder to get yourself out of the mess because you don’t have the wherewithal to move things out of that way.”
As a result, he said, patients may become more isolated if they become embarrassed about inviting people into their homes. To make matters worse, some patients with MS already suffer from social isolation, he said.
He added that some patients with MS may be “clutterers” who do not fit the definition of hoarders but are still affected. “Even cluttering can have an impact on quality of life. You do not have to have the disorder,” he said.
What can be done to help patients who are hoarders or clutterers? Dr. Bacon acknowledged that hoarding behavior is very difficult to treat successfully, but cluttering – a step below hoarding – may be easier to address.
“As therapists, we try to help MS patients confront the debilitating emotional distress that inevitably emerges from the loss of control as disability progresses,” he said. “A central emphasis in therapy is to turn the focus away from the neurological changes and their sequelae that cannot be changed to those facets of their lives over which they can have control and that can be nurtured and strengthened.”
No study funding was reported, and the study authors reported no relevant disclosures.
SEATTLE – according to a small study that appears to be the first of its kind. It is not clear how MS and hoarding may be linked, but study author Joshua Bacon, PhD, an MS researcher and associate professor at New York University, and coauthors suspect that physical limitations are an important factor.
“It is important for clinicians to identify patients who might be hoarders and/or clutterers. It is very likely that this has an impact on the trajectory of their activities of daily living,” he said in an interview prior to the presentation of the study findings at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Dr. Bacon said the study was inspired by his observation that hoarding and cluttering behavior appear to be common among patients with MS. “As I became more interested in it, it became clear there hasn’t been any work on this in the MS population.”
For the new study, Dr. Bacon and colleagues surveyed 139 consecutive patients with MS at the New York University MS Center. The patients had a mean age of 45 years and mean disease duration of 14 years; 71% were female, and 48% were non white. The researchers measured the patients on scales of hoarding behavior (Activities of Daily Living for Hoarding and the Hoarding Rating Scale) and disability (Patient-Determined Disability Steps).
The researchers found that nearly 12% showed signs of clinically significant hoarding behavior, compared with an estimated 5% of the general population (P = .0008). Researchers linked disability and Hoarding Rating Scale to the variability in degree of difficulty in performing activities of daily living (P less than .0001).
Dr. Bacon and colleagues do not believe MS is the direct cause of hoarding behaviors. “There has been no literature on this, and we do not know whether this is connected to the neurological condition,” he said. “I think it has more to do with physical capabilities.”
Patients with MS may have mobility problems that disrupt their ability to organize their homes, he said. “You can’t move things the way you can when you have normal mobility,” he said. “Things can start building up, and it is harder to get yourself out of the mess because you don’t have the wherewithal to move things out of that way.”
As a result, he said, patients may become more isolated if they become embarrassed about inviting people into their homes. To make matters worse, some patients with MS already suffer from social isolation, he said.
He added that some patients with MS may be “clutterers” who do not fit the definition of hoarders but are still affected. “Even cluttering can have an impact on quality of life. You do not have to have the disorder,” he said.
What can be done to help patients who are hoarders or clutterers? Dr. Bacon acknowledged that hoarding behavior is very difficult to treat successfully, but cluttering – a step below hoarding – may be easier to address.
“As therapists, we try to help MS patients confront the debilitating emotional distress that inevitably emerges from the loss of control as disability progresses,” he said. “A central emphasis in therapy is to turn the focus away from the neurological changes and their sequelae that cannot be changed to those facets of their lives over which they can have control and that can be nurtured and strengthened.”
No study funding was reported, and the study authors reported no relevant disclosures.
SEATTLE – according to a small study that appears to be the first of its kind. It is not clear how MS and hoarding may be linked, but study author Joshua Bacon, PhD, an MS researcher and associate professor at New York University, and coauthors suspect that physical limitations are an important factor.
“It is important for clinicians to identify patients who might be hoarders and/or clutterers. It is very likely that this has an impact on the trajectory of their activities of daily living,” he said in an interview prior to the presentation of the study findings at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Dr. Bacon said the study was inspired by his observation that hoarding and cluttering behavior appear to be common among patients with MS. “As I became more interested in it, it became clear there hasn’t been any work on this in the MS population.”
For the new study, Dr. Bacon and colleagues surveyed 139 consecutive patients with MS at the New York University MS Center. The patients had a mean age of 45 years and mean disease duration of 14 years; 71% were female, and 48% were non white. The researchers measured the patients on scales of hoarding behavior (Activities of Daily Living for Hoarding and the Hoarding Rating Scale) and disability (Patient-Determined Disability Steps).
The researchers found that nearly 12% showed signs of clinically significant hoarding behavior, compared with an estimated 5% of the general population (P = .0008). Researchers linked disability and Hoarding Rating Scale to the variability in degree of difficulty in performing activities of daily living (P less than .0001).
Dr. Bacon and colleagues do not believe MS is the direct cause of hoarding behaviors. “There has been no literature on this, and we do not know whether this is connected to the neurological condition,” he said. “I think it has more to do with physical capabilities.”
Patients with MS may have mobility problems that disrupt their ability to organize their homes, he said. “You can’t move things the way you can when you have normal mobility,” he said. “Things can start building up, and it is harder to get yourself out of the mess because you don’t have the wherewithal to move things out of that way.”
As a result, he said, patients may become more isolated if they become embarrassed about inviting people into their homes. To make matters worse, some patients with MS already suffer from social isolation, he said.
He added that some patients with MS may be “clutterers” who do not fit the definition of hoarders but are still affected. “Even cluttering can have an impact on quality of life. You do not have to have the disorder,” he said.
What can be done to help patients who are hoarders or clutterers? Dr. Bacon acknowledged that hoarding behavior is very difficult to treat successfully, but cluttering – a step below hoarding – may be easier to address.
“As therapists, we try to help MS patients confront the debilitating emotional distress that inevitably emerges from the loss of control as disability progresses,” he said. “A central emphasis in therapy is to turn the focus away from the neurological changes and their sequelae that cannot be changed to those facets of their lives over which they can have control and that can be nurtured and strengthened.”
No study funding was reported, and the study authors reported no relevant disclosures.
REPORTING FROM CMSC 2019
Key clinical point: Given the effects of hoarding and cluttering behavior on health and psychological well-being in the general population, these study results highlight the importance of of identifying such behavior in patients with multiple sclerosis and developing effective interventions.
Major finding: Hoarding and cluttering behavior has a significantly higher prevalence in the MS population (11.5%) than in the general population (5%).
Study details: Retrospective review of 139 consecutive patients with MS attending the New York University MS Center.
Disclosures: The authors had nothing to disclose.
What other drugs do patients take when they start MS therapy?
SEATTLE – , according to research presented at the annual meeting of the Consortium of Multiple Sclerosis Centers. The likelihood of particular comorbidities and concomitant medications varies by age and sex, researchers reported.
“This may have implications for MS treatment,” said study author Jacqueline Nicholas, MD, MPH, of Ohio Multiple Sclerosis Center in Columbus and her research colleagues. “A better understanding of the effects of comorbidities and concomitant medications on the effectiveness and safety of DMDs is needed to support clinical decision making.”
Researchers have examined comorbidities in patients with MS, but concomitant medication use among patients starting DMDs is poorly understood, the authors said.
To study this question, Dr. Nicholas and colleagues analyzed retrospective administrative claims data from IQVIA’s Real-World Data Adjudicated Claims–U.S. database from Jan. 1, 2010, to June 30, 2017. Their analysis included patients with two or more MS diagnosis claims and at least one DMD claim between Jan. 1, 2011, and June 30, 2015. Eligible patients were aged 18-63 years and had continuous eligibility with commercial insurance 1 year before and 2 years after DMD initiation. In addition, patients had no evidence of DMD use during the 1-year baseline period.
The investigators used International Classification of Diseases, 9th and 10th Revision, Clinical Modification codes and claims to evaluate patients’ comorbidities and concomitant medications during the study period.
The researchers identified 8,251 eligible patients. Patients had a mean age of 43.2 years, and 75.5% were female. Average baseline Charlson comorbidity score was 0.41. In the 2 years after DMD initiation, common comorbid diagnoses were hyperlipidemia (30.0%), hypertension (28.2%), gastrointestinal disorders (26.2%), depression (25.5%), and anxiety (20.1%).
Common concomitant medications included antibiotics (70.6%); analgesics (57.0%); corticosteroids (52.0%); antidepressants (47.7%); anticonvulsants (46.7%); anxiolytics, sedatives, or hypnotics (43.2%); spasticity medications (36.2%); and muscle relaxants (35.4%).
Most comorbidities and many medications, including bladder and antifatigue medications, were more common among patients aged 55 years and older. Hyperlipidemia, hypertension, and diabetes were more likely in males than in females. Females were more likely to have gastrointestinal disease, depression, thyroid disease, anxiety, lung disease, and arthritis. In addition, females were more likely than males to use many of the concomitant medications.
Dr. Nicholas disclosed grant support from EMD Serono. A coauthor is an employee of Health Services Consulting Corporation and received funding from EMD Serono to conduct the study. Other coauthors are employees of EMD Serono.
SEATTLE – , according to research presented at the annual meeting of the Consortium of Multiple Sclerosis Centers. The likelihood of particular comorbidities and concomitant medications varies by age and sex, researchers reported.
“This may have implications for MS treatment,” said study author Jacqueline Nicholas, MD, MPH, of Ohio Multiple Sclerosis Center in Columbus and her research colleagues. “A better understanding of the effects of comorbidities and concomitant medications on the effectiveness and safety of DMDs is needed to support clinical decision making.”
Researchers have examined comorbidities in patients with MS, but concomitant medication use among patients starting DMDs is poorly understood, the authors said.
To study this question, Dr. Nicholas and colleagues analyzed retrospective administrative claims data from IQVIA’s Real-World Data Adjudicated Claims–U.S. database from Jan. 1, 2010, to June 30, 2017. Their analysis included patients with two or more MS diagnosis claims and at least one DMD claim between Jan. 1, 2011, and June 30, 2015. Eligible patients were aged 18-63 years and had continuous eligibility with commercial insurance 1 year before and 2 years after DMD initiation. In addition, patients had no evidence of DMD use during the 1-year baseline period.
The investigators used International Classification of Diseases, 9th and 10th Revision, Clinical Modification codes and claims to evaluate patients’ comorbidities and concomitant medications during the study period.
The researchers identified 8,251 eligible patients. Patients had a mean age of 43.2 years, and 75.5% were female. Average baseline Charlson comorbidity score was 0.41. In the 2 years after DMD initiation, common comorbid diagnoses were hyperlipidemia (30.0%), hypertension (28.2%), gastrointestinal disorders (26.2%), depression (25.5%), and anxiety (20.1%).
Common concomitant medications included antibiotics (70.6%); analgesics (57.0%); corticosteroids (52.0%); antidepressants (47.7%); anticonvulsants (46.7%); anxiolytics, sedatives, or hypnotics (43.2%); spasticity medications (36.2%); and muscle relaxants (35.4%).
Most comorbidities and many medications, including bladder and antifatigue medications, were more common among patients aged 55 years and older. Hyperlipidemia, hypertension, and diabetes were more likely in males than in females. Females were more likely to have gastrointestinal disease, depression, thyroid disease, anxiety, lung disease, and arthritis. In addition, females were more likely than males to use many of the concomitant medications.
Dr. Nicholas disclosed grant support from EMD Serono. A coauthor is an employee of Health Services Consulting Corporation and received funding from EMD Serono to conduct the study. Other coauthors are employees of EMD Serono.
SEATTLE – , according to research presented at the annual meeting of the Consortium of Multiple Sclerosis Centers. The likelihood of particular comorbidities and concomitant medications varies by age and sex, researchers reported.
“This may have implications for MS treatment,” said study author Jacqueline Nicholas, MD, MPH, of Ohio Multiple Sclerosis Center in Columbus and her research colleagues. “A better understanding of the effects of comorbidities and concomitant medications on the effectiveness and safety of DMDs is needed to support clinical decision making.”
Researchers have examined comorbidities in patients with MS, but concomitant medication use among patients starting DMDs is poorly understood, the authors said.
To study this question, Dr. Nicholas and colleagues analyzed retrospective administrative claims data from IQVIA’s Real-World Data Adjudicated Claims–U.S. database from Jan. 1, 2010, to June 30, 2017. Their analysis included patients with two or more MS diagnosis claims and at least one DMD claim between Jan. 1, 2011, and June 30, 2015. Eligible patients were aged 18-63 years and had continuous eligibility with commercial insurance 1 year before and 2 years after DMD initiation. In addition, patients had no evidence of DMD use during the 1-year baseline period.
The investigators used International Classification of Diseases, 9th and 10th Revision, Clinical Modification codes and claims to evaluate patients’ comorbidities and concomitant medications during the study period.
The researchers identified 8,251 eligible patients. Patients had a mean age of 43.2 years, and 75.5% were female. Average baseline Charlson comorbidity score was 0.41. In the 2 years after DMD initiation, common comorbid diagnoses were hyperlipidemia (30.0%), hypertension (28.2%), gastrointestinal disorders (26.2%), depression (25.5%), and anxiety (20.1%).
Common concomitant medications included antibiotics (70.6%); analgesics (57.0%); corticosteroids (52.0%); antidepressants (47.7%); anticonvulsants (46.7%); anxiolytics, sedatives, or hypnotics (43.2%); spasticity medications (36.2%); and muscle relaxants (35.4%).
Most comorbidities and many medications, including bladder and antifatigue medications, were more common among patients aged 55 years and older. Hyperlipidemia, hypertension, and diabetes were more likely in males than in females. Females were more likely to have gastrointestinal disease, depression, thyroid disease, anxiety, lung disease, and arthritis. In addition, females were more likely than males to use many of the concomitant medications.
Dr. Nicholas disclosed grant support from EMD Serono. A coauthor is an employee of Health Services Consulting Corporation and received funding from EMD Serono to conduct the study. Other coauthors are employees of EMD Serono.
REPORTING FROM CMSC 2019
Key clinical point: The effect of comorbidities and concomitant medications on the effectiveness and safety of disease-modifying drugs for multiple sclerosis requires further study.
Major finding: In one analysis, common concomitant medications included antibiotics (70.6%), analgesics (57.0%), corticosteroids (52.0%), antidepressants (47.7%), and anticonvulsants (46.7%).
Study details: An analysis of retrospective administrative claims data from 8,251 patients with MS.
Disclosures: Dr. Nicholas disclosed grant support from EMD Serono. A coauthor is an employee of Health Services Consulting Corporation and received funding from EMD Serono to conduct the study. Other coauthors are employees of EMD Serono.
Anxiety and fatigue impair processing speed in MS
SEATTLE – (MS), according to data described at the annual meeting of the Consortium of Multiple Sclerosis Centers. Increased anxiety is associated with slower processing speed in the context of increasing cognitive fatigue. “This [finding] has implications on development of cognitive remediation strategies, which may aim to target patient fatigue or anxiety to improve processing speed,” said Caroline Altaras, a doctoral candidate at Yeshiva University in New York, and colleagues.
Approximately 90% of patients with MS have fatigue, which can be a highly debilitating symptom. Fatigue often is understood to include motor fatigue (difficulty maintaining physical stamina) and cognitive fatigue (difficulty maintaining mental stamina). MS-related fatigue decreases patients’ quality of life, including cognitive functioning.
Anxiety is a psychiatric comorbidity that is highly prevalent in MS and that has a bidirectional association with fatigue. Anxiety and fatigue independently impair cognitive function.
Impaired processing speed is the most common cognitive impairment among patients with MS. Ms. Altaras and colleagues conducted a study to analyze how anxiety and fatigue interact to affect processing speed in MS. They evaluated total fatigue, cognitive fatigue, and motor fatigue separately. The investigators collected data from 183 patients with MS who had been referred by physicians for neuropsychological testing at the MS Center at Holy Name Medical Center in Teaneck, New Jersey. Researchers measured patients’ anxiety and fatigue using the Hospital Anxiety and Depression Scale (HADS, a self-reported measure) and the Fatigue Scale for Motor and Cognitive Functions (FSMC), which measures cognitive fatigue and motor fatigue. Patients also took the Symbol Digit Modalities Test (SDMT), a neuropsychological measure of processing speed. Ms. Altaras and colleagues created three multivariate general linear models using SPSS 25.0 to test the hypothesized relationships, using fatigue types (cognitive, motor, and total) as separate outcomes. The investigators controlled their analyses for gender, age, and education.
The researchers found a significant interaction effect of cognitive fatigue and anxiety on SDMT score. Specifically, patients with MS and minimal anxiety and cognitive fatigue had similar SDMT performance; as anxiety increased, patients who had increased cognitive fatigue demonstrated worse performance on the SDMT. Ms. Altaras and colleagues observed that SDMT performance improved slightly with worsening anxiety when cognitive fatigue was minimal. Although total fatigue interacted significantly with anxiety to affect SDMT performance, motor fatigue did not, which suggests that the effects of total fatigue largely resulted from cognitive fatigue.
The study had no outside financial support, and the authors reported no disclosures.
SEATTLE – (MS), according to data described at the annual meeting of the Consortium of Multiple Sclerosis Centers. Increased anxiety is associated with slower processing speed in the context of increasing cognitive fatigue. “This [finding] has implications on development of cognitive remediation strategies, which may aim to target patient fatigue or anxiety to improve processing speed,” said Caroline Altaras, a doctoral candidate at Yeshiva University in New York, and colleagues.
Approximately 90% of patients with MS have fatigue, which can be a highly debilitating symptom. Fatigue often is understood to include motor fatigue (difficulty maintaining physical stamina) and cognitive fatigue (difficulty maintaining mental stamina). MS-related fatigue decreases patients’ quality of life, including cognitive functioning.
Anxiety is a psychiatric comorbidity that is highly prevalent in MS and that has a bidirectional association with fatigue. Anxiety and fatigue independently impair cognitive function.
Impaired processing speed is the most common cognitive impairment among patients with MS. Ms. Altaras and colleagues conducted a study to analyze how anxiety and fatigue interact to affect processing speed in MS. They evaluated total fatigue, cognitive fatigue, and motor fatigue separately. The investigators collected data from 183 patients with MS who had been referred by physicians for neuropsychological testing at the MS Center at Holy Name Medical Center in Teaneck, New Jersey. Researchers measured patients’ anxiety and fatigue using the Hospital Anxiety and Depression Scale (HADS, a self-reported measure) and the Fatigue Scale for Motor and Cognitive Functions (FSMC), which measures cognitive fatigue and motor fatigue. Patients also took the Symbol Digit Modalities Test (SDMT), a neuropsychological measure of processing speed. Ms. Altaras and colleagues created three multivariate general linear models using SPSS 25.0 to test the hypothesized relationships, using fatigue types (cognitive, motor, and total) as separate outcomes. The investigators controlled their analyses for gender, age, and education.
The researchers found a significant interaction effect of cognitive fatigue and anxiety on SDMT score. Specifically, patients with MS and minimal anxiety and cognitive fatigue had similar SDMT performance; as anxiety increased, patients who had increased cognitive fatigue demonstrated worse performance on the SDMT. Ms. Altaras and colleagues observed that SDMT performance improved slightly with worsening anxiety when cognitive fatigue was minimal. Although total fatigue interacted significantly with anxiety to affect SDMT performance, motor fatigue did not, which suggests that the effects of total fatigue largely resulted from cognitive fatigue.
The study had no outside financial support, and the authors reported no disclosures.
SEATTLE – (MS), according to data described at the annual meeting of the Consortium of Multiple Sclerosis Centers. Increased anxiety is associated with slower processing speed in the context of increasing cognitive fatigue. “This [finding] has implications on development of cognitive remediation strategies, which may aim to target patient fatigue or anxiety to improve processing speed,” said Caroline Altaras, a doctoral candidate at Yeshiva University in New York, and colleagues.
Approximately 90% of patients with MS have fatigue, which can be a highly debilitating symptom. Fatigue often is understood to include motor fatigue (difficulty maintaining physical stamina) and cognitive fatigue (difficulty maintaining mental stamina). MS-related fatigue decreases patients’ quality of life, including cognitive functioning.
Anxiety is a psychiatric comorbidity that is highly prevalent in MS and that has a bidirectional association with fatigue. Anxiety and fatigue independently impair cognitive function.
Impaired processing speed is the most common cognitive impairment among patients with MS. Ms. Altaras and colleagues conducted a study to analyze how anxiety and fatigue interact to affect processing speed in MS. They evaluated total fatigue, cognitive fatigue, and motor fatigue separately. The investigators collected data from 183 patients with MS who had been referred by physicians for neuropsychological testing at the MS Center at Holy Name Medical Center in Teaneck, New Jersey. Researchers measured patients’ anxiety and fatigue using the Hospital Anxiety and Depression Scale (HADS, a self-reported measure) and the Fatigue Scale for Motor and Cognitive Functions (FSMC), which measures cognitive fatigue and motor fatigue. Patients also took the Symbol Digit Modalities Test (SDMT), a neuropsychological measure of processing speed. Ms. Altaras and colleagues created three multivariate general linear models using SPSS 25.0 to test the hypothesized relationships, using fatigue types (cognitive, motor, and total) as separate outcomes. The investigators controlled their analyses for gender, age, and education.
The researchers found a significant interaction effect of cognitive fatigue and anxiety on SDMT score. Specifically, patients with MS and minimal anxiety and cognitive fatigue had similar SDMT performance; as anxiety increased, patients who had increased cognitive fatigue demonstrated worse performance on the SDMT. Ms. Altaras and colleagues observed that SDMT performance improved slightly with worsening anxiety when cognitive fatigue was minimal. Although total fatigue interacted significantly with anxiety to affect SDMT performance, motor fatigue did not, which suggests that the effects of total fatigue largely resulted from cognitive fatigue.
The study had no outside financial support, and the authors reported no disclosures.
REPORTING FROM CMSC 2019
Key clinical point: Anxiety and fatigue interact to affect processing speed in MS.
Major finding: Cognitive fatigue and anxiety interact to affect performance on the Symbol Digit Modalities Test.
Study details: A prospective study of 183 patients with MS referred for neuropsychological testing.
Disclosures: The study had no outside funding, and the investigators had no disclosures.
MS significantly affects employment and home activities
SEATTLE – according to data presented at the annual meeting of the Consortium of Multiple Sclerosis Centers. The disease appears to prevent people from achieving their full potential at work and at home, largely because of its associated fatigue, said the researchers. “The economic impact of identifying an effective treatment for this symptom of MS cannot be overstated,” said Terrie Livingston, PharmD, head of patient outcomes and solutions at EMD Serono in Wayland, Massachusetts, and colleagues.
The research results from an initiative by the North American Registry for Care and Research in MS (NARCRMS). Since December 2016, NARCRMS has prospectively collected clinical and imaging data, information about patients’ health care economics, and data about the effects of MS on daily life. To examine the economic impact of MS and to help implement health economics outcomes research (HEOR) in decision-making processes, NARCRMS established the HEOR Advisory Group in 2017. The registry created a Health-Related Productivity Questionnaire and Health Resource Utilization Questionnaire, both of which were incorporated into the existing case report forms. Patients complete these questionnaires at enrollment and at annual and exacerbation visits.
As of January 2, 2019, NARCRMS had enrolled 378 people with MS into the registry, and 368 had completed the HEOR case report forms. Among the respondents, 270 (73%) are employed either full or part time. During the week before reporting, 39 respondents (11%) reported that MS kept them from work, 93 (25%) reported that MS affected their work, 105 (29%) reported that MS stopped them from finishing household chores, and 140 (38%) reported that MS affected their household chores. Fatigue was the symptom most commonly reported to affect work and household chores. In the 3 months before reporting, 13 patients (4%) had inpatient hospital stays, 24 patients (7%) visited the ED, 71 patients (19%) visited a general practitioner, and 296 (80%) patients visited a neurologist.
The study had no sponsor. Several of the study authors reported receiving compensation from companies such as Biogen, Celgene, Genentech, Novartis, Sanofi Genzyme, and Teva.
SEATTLE – according to data presented at the annual meeting of the Consortium of Multiple Sclerosis Centers. The disease appears to prevent people from achieving their full potential at work and at home, largely because of its associated fatigue, said the researchers. “The economic impact of identifying an effective treatment for this symptom of MS cannot be overstated,” said Terrie Livingston, PharmD, head of patient outcomes and solutions at EMD Serono in Wayland, Massachusetts, and colleagues.
The research results from an initiative by the North American Registry for Care and Research in MS (NARCRMS). Since December 2016, NARCRMS has prospectively collected clinical and imaging data, information about patients’ health care economics, and data about the effects of MS on daily life. To examine the economic impact of MS and to help implement health economics outcomes research (HEOR) in decision-making processes, NARCRMS established the HEOR Advisory Group in 2017. The registry created a Health-Related Productivity Questionnaire and Health Resource Utilization Questionnaire, both of which were incorporated into the existing case report forms. Patients complete these questionnaires at enrollment and at annual and exacerbation visits.
As of January 2, 2019, NARCRMS had enrolled 378 people with MS into the registry, and 368 had completed the HEOR case report forms. Among the respondents, 270 (73%) are employed either full or part time. During the week before reporting, 39 respondents (11%) reported that MS kept them from work, 93 (25%) reported that MS affected their work, 105 (29%) reported that MS stopped them from finishing household chores, and 140 (38%) reported that MS affected their household chores. Fatigue was the symptom most commonly reported to affect work and household chores. In the 3 months before reporting, 13 patients (4%) had inpatient hospital stays, 24 patients (7%) visited the ED, 71 patients (19%) visited a general practitioner, and 296 (80%) patients visited a neurologist.
The study had no sponsor. Several of the study authors reported receiving compensation from companies such as Biogen, Celgene, Genentech, Novartis, Sanofi Genzyme, and Teva.
SEATTLE – according to data presented at the annual meeting of the Consortium of Multiple Sclerosis Centers. The disease appears to prevent people from achieving their full potential at work and at home, largely because of its associated fatigue, said the researchers. “The economic impact of identifying an effective treatment for this symptom of MS cannot be overstated,” said Terrie Livingston, PharmD, head of patient outcomes and solutions at EMD Serono in Wayland, Massachusetts, and colleagues.
The research results from an initiative by the North American Registry for Care and Research in MS (NARCRMS). Since December 2016, NARCRMS has prospectively collected clinical and imaging data, information about patients’ health care economics, and data about the effects of MS on daily life. To examine the economic impact of MS and to help implement health economics outcomes research (HEOR) in decision-making processes, NARCRMS established the HEOR Advisory Group in 2017. The registry created a Health-Related Productivity Questionnaire and Health Resource Utilization Questionnaire, both of which were incorporated into the existing case report forms. Patients complete these questionnaires at enrollment and at annual and exacerbation visits.
As of January 2, 2019, NARCRMS had enrolled 378 people with MS into the registry, and 368 had completed the HEOR case report forms. Among the respondents, 270 (73%) are employed either full or part time. During the week before reporting, 39 respondents (11%) reported that MS kept them from work, 93 (25%) reported that MS affected their work, 105 (29%) reported that MS stopped them from finishing household chores, and 140 (38%) reported that MS affected their household chores. Fatigue was the symptom most commonly reported to affect work and household chores. In the 3 months before reporting, 13 patients (4%) had inpatient hospital stays, 24 patients (7%) visited the ED, 71 patients (19%) visited a general practitioner, and 296 (80%) patients visited a neurologist.
The study had no sponsor. Several of the study authors reported receiving compensation from companies such as Biogen, Celgene, Genentech, Novartis, Sanofi Genzyme, and Teva.
REPORTING FROM CMSC 2019
Key clinical point: Fatigue is the most common symptom preventing people with MS from completing work or chores.
Major finding: Approximately 28% of people with MS may be underemployed or unemployed.
Study details: An analysis of registry data, including questionnaires for 368 patients with MS.
Disclosures: The study had no sponsor. Dr. Livingston is an employee of EMD Serono.
Alemtuzumab increases the likelihood of disability improvement in MS
Seattle – , according to a pooled analysis presented at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Patients who achieved this outcome had improvement in several functional systems, regardless of their baseline EDSS scores. “These results suggest a broad and prolonged effect of alemtuzumab on disability improvement and a potential for changing the MS disease course,” said Samuel F. Hunter, MD, a neurologist and psychiatrist at the Advanced Neurosciences Institute in Franklin, Tenn., and colleagues.
The researchers’ findings come from their analysis of pooled data from the CARE-MS I and CARE-MS II trials. Those studies indicated that alemtuzumab improved clinical and MRI outcomes over 2 years in relapsing-remitting MS, compared with interferon beta-1a. In a 4-year extension, alemtuzumab’s efficacy was maintained, and 81% of participants continued in the study until year 6. In addition, 34% of alemtuzumab-treated patients in CARE-MS I and 43% of alemtuzumab-treated patients in CARE-MS II achieved 6-month confirmed disability improvement. The relationship between baseline disability levels and the achievement of disability improvement is not well understood, however.
Dr. Hunter and colleagues conducted a pooled analysis of CARE-MS I and CARE-MS II data to evaluate how baseline disability affects improvements in each functional system in patients treated with alemtuzumab over 6 years. In those studies, patients received two 12-mg/day courses of alemtuzumab: a 5-day course at baseline and a 3-day course 12 months later. Additional treatment with alemtuzumab or other disease-modifying therapies was provided as needed during the extension study.
The investigators defined confirmed disability improvement as a decrease of 1 or more points in EDSS score confirmed over 6 months among patients with a baseline EDSS score of 2 or higher. Improvement (i.e., a decrease of 1 or more points) or stability (i.e., no change) in each of the functional system scores was assessed in patients with confirmed disability improvement, stratified by baseline EDSS scores. Patients were grouped according to whether their baseline EDSS scores were 2.0-2.5, 3.0-3.5, 4.0-4.5, 5.0-5.5, or 6.0-6.5.
A total of 208 of 565 patients (37%) achieved 6-month confirmed disability improvement through year 6. This outcome was achieved by the highest percentages of patients with baseline EDSS scores of 4.0-4.5 (57%) and 3.0-3.5 (44%), followed by those with baseline EDSS scores of 5.0-5.5 (28%) and 2.0-2.5 (27%). No patients with baseline EDSS scores of 6.0-6.5 achieved confirmed disability improvement.
At 6 months after onset of confirmed disability improvement, patients within each baseline EDSS group showed stability or improvement in each individual functional system. The proportion of stable or improved patients was 94% or greater in the 2.0-2.5 group, 92% or greater in the 3.0-3.5 group, 88% or greater in the 4.0-4.5 group, and 75% or greater in the 5.0-5.5 group. Between 67% and 76% of patients achieved improvements in two or more functional systems. Improvements were most frequent in the pyramidal (13% to 50%), sensory (42% to 50%), and cerebellar (13% to 55%) functional systems.
Sanofi, Bayer HealthCare Pharmaceuticals supported the study. Dr. Hunter received grants and financial support from AbbVie, Actelion, Acorda, Adamas, Alkermes, Avanir, Bayer HealthCare, Biogen, Novartis, Osmotica, Questcor, Roche, Sanofi, Synthon, and Teva.
SOURCE: Hunter SF et al. CMSC 2019. Abstract DXT08.
Seattle – , according to a pooled analysis presented at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Patients who achieved this outcome had improvement in several functional systems, regardless of their baseline EDSS scores. “These results suggest a broad and prolonged effect of alemtuzumab on disability improvement and a potential for changing the MS disease course,” said Samuel F. Hunter, MD, a neurologist and psychiatrist at the Advanced Neurosciences Institute in Franklin, Tenn., and colleagues.
The researchers’ findings come from their analysis of pooled data from the CARE-MS I and CARE-MS II trials. Those studies indicated that alemtuzumab improved clinical and MRI outcomes over 2 years in relapsing-remitting MS, compared with interferon beta-1a. In a 4-year extension, alemtuzumab’s efficacy was maintained, and 81% of participants continued in the study until year 6. In addition, 34% of alemtuzumab-treated patients in CARE-MS I and 43% of alemtuzumab-treated patients in CARE-MS II achieved 6-month confirmed disability improvement. The relationship between baseline disability levels and the achievement of disability improvement is not well understood, however.
Dr. Hunter and colleagues conducted a pooled analysis of CARE-MS I and CARE-MS II data to evaluate how baseline disability affects improvements in each functional system in patients treated with alemtuzumab over 6 years. In those studies, patients received two 12-mg/day courses of alemtuzumab: a 5-day course at baseline and a 3-day course 12 months later. Additional treatment with alemtuzumab or other disease-modifying therapies was provided as needed during the extension study.
The investigators defined confirmed disability improvement as a decrease of 1 or more points in EDSS score confirmed over 6 months among patients with a baseline EDSS score of 2 or higher. Improvement (i.e., a decrease of 1 or more points) or stability (i.e., no change) in each of the functional system scores was assessed in patients with confirmed disability improvement, stratified by baseline EDSS scores. Patients were grouped according to whether their baseline EDSS scores were 2.0-2.5, 3.0-3.5, 4.0-4.5, 5.0-5.5, or 6.0-6.5.
A total of 208 of 565 patients (37%) achieved 6-month confirmed disability improvement through year 6. This outcome was achieved by the highest percentages of patients with baseline EDSS scores of 4.0-4.5 (57%) and 3.0-3.5 (44%), followed by those with baseline EDSS scores of 5.0-5.5 (28%) and 2.0-2.5 (27%). No patients with baseline EDSS scores of 6.0-6.5 achieved confirmed disability improvement.
At 6 months after onset of confirmed disability improvement, patients within each baseline EDSS group showed stability or improvement in each individual functional system. The proportion of stable or improved patients was 94% or greater in the 2.0-2.5 group, 92% or greater in the 3.0-3.5 group, 88% or greater in the 4.0-4.5 group, and 75% or greater in the 5.0-5.5 group. Between 67% and 76% of patients achieved improvements in two or more functional systems. Improvements were most frequent in the pyramidal (13% to 50%), sensory (42% to 50%), and cerebellar (13% to 55%) functional systems.
Sanofi, Bayer HealthCare Pharmaceuticals supported the study. Dr. Hunter received grants and financial support from AbbVie, Actelion, Acorda, Adamas, Alkermes, Avanir, Bayer HealthCare, Biogen, Novartis, Osmotica, Questcor, Roche, Sanofi, Synthon, and Teva.
SOURCE: Hunter SF et al. CMSC 2019. Abstract DXT08.
Seattle – , according to a pooled analysis presented at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Patients who achieved this outcome had improvement in several functional systems, regardless of their baseline EDSS scores. “These results suggest a broad and prolonged effect of alemtuzumab on disability improvement and a potential for changing the MS disease course,” said Samuel F. Hunter, MD, a neurologist and psychiatrist at the Advanced Neurosciences Institute in Franklin, Tenn., and colleagues.
The researchers’ findings come from their analysis of pooled data from the CARE-MS I and CARE-MS II trials. Those studies indicated that alemtuzumab improved clinical and MRI outcomes over 2 years in relapsing-remitting MS, compared with interferon beta-1a. In a 4-year extension, alemtuzumab’s efficacy was maintained, and 81% of participants continued in the study until year 6. In addition, 34% of alemtuzumab-treated patients in CARE-MS I and 43% of alemtuzumab-treated patients in CARE-MS II achieved 6-month confirmed disability improvement. The relationship between baseline disability levels and the achievement of disability improvement is not well understood, however.
Dr. Hunter and colleagues conducted a pooled analysis of CARE-MS I and CARE-MS II data to evaluate how baseline disability affects improvements in each functional system in patients treated with alemtuzumab over 6 years. In those studies, patients received two 12-mg/day courses of alemtuzumab: a 5-day course at baseline and a 3-day course 12 months later. Additional treatment with alemtuzumab or other disease-modifying therapies was provided as needed during the extension study.
The investigators defined confirmed disability improvement as a decrease of 1 or more points in EDSS score confirmed over 6 months among patients with a baseline EDSS score of 2 or higher. Improvement (i.e., a decrease of 1 or more points) or stability (i.e., no change) in each of the functional system scores was assessed in patients with confirmed disability improvement, stratified by baseline EDSS scores. Patients were grouped according to whether their baseline EDSS scores were 2.0-2.5, 3.0-3.5, 4.0-4.5, 5.0-5.5, or 6.0-6.5.
A total of 208 of 565 patients (37%) achieved 6-month confirmed disability improvement through year 6. This outcome was achieved by the highest percentages of patients with baseline EDSS scores of 4.0-4.5 (57%) and 3.0-3.5 (44%), followed by those with baseline EDSS scores of 5.0-5.5 (28%) and 2.0-2.5 (27%). No patients with baseline EDSS scores of 6.0-6.5 achieved confirmed disability improvement.
At 6 months after onset of confirmed disability improvement, patients within each baseline EDSS group showed stability or improvement in each individual functional system. The proportion of stable or improved patients was 94% or greater in the 2.0-2.5 group, 92% or greater in the 3.0-3.5 group, 88% or greater in the 4.0-4.5 group, and 75% or greater in the 5.0-5.5 group. Between 67% and 76% of patients achieved improvements in two or more functional systems. Improvements were most frequent in the pyramidal (13% to 50%), sensory (42% to 50%), and cerebellar (13% to 55%) functional systems.
Sanofi, Bayer HealthCare Pharmaceuticals supported the study. Dr. Hunter received grants and financial support from AbbVie, Actelion, Acorda, Adamas, Alkermes, Avanir, Bayer HealthCare, Biogen, Novartis, Osmotica, Questcor, Roche, Sanofi, Synthon, and Teva.
SOURCE: Hunter SF et al. CMSC 2019. Abstract DXT08.
REPORTING FROM CMSC 2019
Costs of oral cancer drugs rising faster than inflation
The cost of oral cancer drugs increased by almost 6% over inflation from 2010 to 2018, leading to increases in out-of-pocket costs for Medicare patients despite reductions in the Part D coverage gap, according to an analysis of formulary and pricing data.
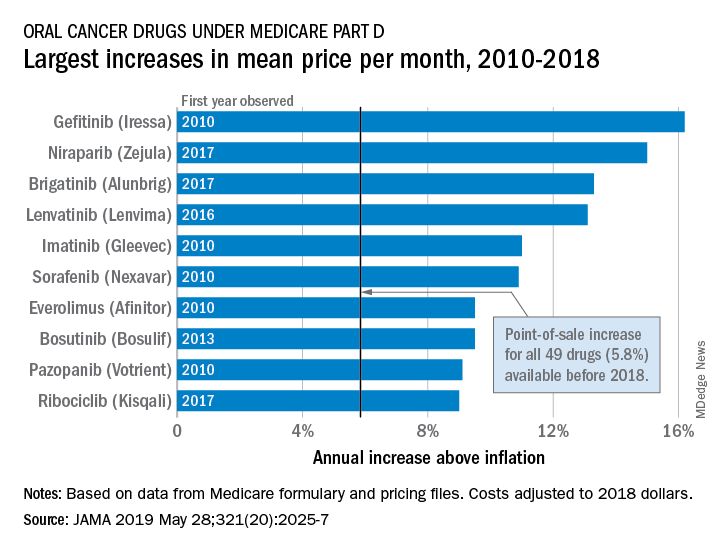
Point-of-sale prices for the 49 oral cancer drugs available before 2018 rose by 40.4% from 2010 to 2018 – an annual increase of 5.8% above the rate of inflation, Stacie B. Dusetzina, PhD, of Vanderbilt University, Nashville, Tenn., and associates reported in JAMA. Another five drugs with data that first became available in 2018 were not included in the cost-increase calculations.
The largest overall increase, 306% (16.2% above inflation per year), belonged to gefitinib (Iressa). Its point-of-sale price rose from $1,960 a month in 2010 to $7,960 in 2018. The award for largest reduction – only two others had a decrease – goes to the only generic available, imatinib. Its monthly cost dropped 44% (–28.1% below inflation per year) from $8,570 in 2016 to $4,822 in 2018, the investigators said (JAMA 2019 May 28;321[20]:2025-7).
Annual out-of-pocket spending for the 13 drugs available for the entire study period increased from $8,794 in 2010 to an expected $10,470 in 2019. Medicare patients’ out-of-pocket costs for those 13 cancer drugs in 2019 are expected to range from $7,220 for lapatinib to $15,472 for lenalidomide, they reported.
“Savings expected through closing the Part D coverage gap or through other policy changes, such as point-of-sale rebates, will be unlikely to offer financial protections to patients needing anticancer drugs. Moreover, because beneficiaries pay a percentage of the drug’s price and have no out-of-pocket spending limits on Part D, even large price decreases may not provide sufficient financial relief to patients requiring long-term anticancer drug use,” Dr. Dusetzina and associates wrote.
The study was supported by the Commonwealth Fund and the Leukemia and Lymphoma Society. The investigators reported receiving grant funding from the study funders.
The cost of oral cancer drugs increased by almost 6% over inflation from 2010 to 2018, leading to increases in out-of-pocket costs for Medicare patients despite reductions in the Part D coverage gap, according to an analysis of formulary and pricing data.

Point-of-sale prices for the 49 oral cancer drugs available before 2018 rose by 40.4% from 2010 to 2018 – an annual increase of 5.8% above the rate of inflation, Stacie B. Dusetzina, PhD, of Vanderbilt University, Nashville, Tenn., and associates reported in JAMA. Another five drugs with data that first became available in 2018 were not included in the cost-increase calculations.
The largest overall increase, 306% (16.2% above inflation per year), belonged to gefitinib (Iressa). Its point-of-sale price rose from $1,960 a month in 2010 to $7,960 in 2018. The award for largest reduction – only two others had a decrease – goes to the only generic available, imatinib. Its monthly cost dropped 44% (–28.1% below inflation per year) from $8,570 in 2016 to $4,822 in 2018, the investigators said (JAMA 2019 May 28;321[20]:2025-7).
Annual out-of-pocket spending for the 13 drugs available for the entire study period increased from $8,794 in 2010 to an expected $10,470 in 2019. Medicare patients’ out-of-pocket costs for those 13 cancer drugs in 2019 are expected to range from $7,220 for lapatinib to $15,472 for lenalidomide, they reported.
“Savings expected through closing the Part D coverage gap or through other policy changes, such as point-of-sale rebates, will be unlikely to offer financial protections to patients needing anticancer drugs. Moreover, because beneficiaries pay a percentage of the drug’s price and have no out-of-pocket spending limits on Part D, even large price decreases may not provide sufficient financial relief to patients requiring long-term anticancer drug use,” Dr. Dusetzina and associates wrote.
The study was supported by the Commonwealth Fund and the Leukemia and Lymphoma Society. The investigators reported receiving grant funding from the study funders.
The cost of oral cancer drugs increased by almost 6% over inflation from 2010 to 2018, leading to increases in out-of-pocket costs for Medicare patients despite reductions in the Part D coverage gap, according to an analysis of formulary and pricing data.

Point-of-sale prices for the 49 oral cancer drugs available before 2018 rose by 40.4% from 2010 to 2018 – an annual increase of 5.8% above the rate of inflation, Stacie B. Dusetzina, PhD, of Vanderbilt University, Nashville, Tenn., and associates reported in JAMA. Another five drugs with data that first became available in 2018 were not included in the cost-increase calculations.
The largest overall increase, 306% (16.2% above inflation per year), belonged to gefitinib (Iressa). Its point-of-sale price rose from $1,960 a month in 2010 to $7,960 in 2018. The award for largest reduction – only two others had a decrease – goes to the only generic available, imatinib. Its monthly cost dropped 44% (–28.1% below inflation per year) from $8,570 in 2016 to $4,822 in 2018, the investigators said (JAMA 2019 May 28;321[20]:2025-7).
Annual out-of-pocket spending for the 13 drugs available for the entire study period increased from $8,794 in 2010 to an expected $10,470 in 2019. Medicare patients’ out-of-pocket costs for those 13 cancer drugs in 2019 are expected to range from $7,220 for lapatinib to $15,472 for lenalidomide, they reported.
“Savings expected through closing the Part D coverage gap or through other policy changes, such as point-of-sale rebates, will be unlikely to offer financial protections to patients needing anticancer drugs. Moreover, because beneficiaries pay a percentage of the drug’s price and have no out-of-pocket spending limits on Part D, even large price decreases may not provide sufficient financial relief to patients requiring long-term anticancer drug use,” Dr. Dusetzina and associates wrote.
The study was supported by the Commonwealth Fund and the Leukemia and Lymphoma Society. The investigators reported receiving grant funding from the study funders.
FROM JAMA
Team sports may mitigate tough childhoods
Individuals who experienced adverse childhood experiences but also played team sports as teens were less likely to have mental health problems in adulthood than those with childhood challenges who did not play sports, based on data from nearly 5,000 individuals.
Physical and mental health problems are more prominent throughout life among those exposed to adverse childhood experiences (ACEs), and physical activity in general and team sports in particular have been shown to improve mental health, wrote Molly C. Easterlin, MD, of the University of California, Los Angeles, and colleagues.
In a study published in JAMA Pediatrics, the researchers used data from the National Longitudinal Study of Adolescent to Adult Health to compare the development of depression, anxiety, or depressive symptoms among those with childhood ACEs who did and did not participate in team sports in adolescence.
Overall, team sports participation was significantly associated with reduced odds of depression (adjusted odds ratio, 0.76), anxiety (aOR, 0.70), and depressive symptoms (aOR, 0.85) in young adulthood for individuals with ACEs, compared with those with ACEs who did not play team sports.
Of 9,668 adolescents in the study, 4,888 individuals reported one or more ACEs and 2,084 reported two or more ACEs. The researchers compared data from the 1994-1995 school year when participants were in grades 7-12 and in 2008 to assess their mental health as young adults (aged 24-32 years).
No significant differences in associations appeared between sports participation and mental health between males and females.
The results were limited by several factors including the study design that did not allow for causality and the potential social desirability bias that might lead to underreporting ACEs, Dr. Easterlin and associates noted.
Nonetheless, “given that participation in team sports was associated with improved adult mental health among those with ACEs, and parents might consider enrolling their children with ACEs in team sports,” they wrote.
Dr. Easterlin is supported by the Cedars-Sinai Medical Center via the UCLA National Clinician Scholars Program. The authors reported no conflicts of interest.
SOURCE: Easterlin MC et al. JAMA Pediatr. 2019 May 28. doi: 10.1001/jamapediatrics.2019.1212.
Approximately half of children suffer an adverse childhood experience (ACE) that can negatively affect their mental health throughout life, and “team sports can be an avenue to interrupt these negative sequelae and address the important public health burden of depression,” wrote Amanda E. Paluch, PhD; Nia Heard-Garris, MD, MSc; and Mercedes R. Carnethon, PhD.
However, a significant socioeconomic disparity in team sports for children continues to grow in the United States, driven in part by a youth sports industry and culture that caters to high-income families looking to improve their children’s performance. “Although unintentional, these expenses leave behind lower-income children,” many of whom may be at increased risk for ACEs, the editorialists noted. Many inexpensive, community-based recreation leagues, especially in low-income areas, are often underfunded and unable to update facilities and attract more participants.
The benefits of team sports appear to go beyond the physical, as the study by Easterlin et al. suggests that feeling accepted and connected as part of a team has an impact on mental health. Also, the winning and losing of sports helps build emotional resilience that carries over to other areas of life, the editorialists added.
“Optimizing the opportunities for sports during adolescence requires relatively few resources and is a low-cost way to improve quality of life and reduce the population burden of mental health disorders, especially for adolescents and young adults with histories of ACEs,” they concluded.
Dr. Paluch and Dr. Carnethon are affiliated with the department of preventive medicine and Dr. Heard-Garris is affiliated with the department of pediatrics at Northwestern University, Chicago. They commented on the study by Easterlin et al (JAMA Pediatr. 2019 May 28. doi:10.1001/jamapediatrics.2019.1209). They reported no conflicts of interest.
Approximately half of children suffer an adverse childhood experience (ACE) that can negatively affect their mental health throughout life, and “team sports can be an avenue to interrupt these negative sequelae and address the important public health burden of depression,” wrote Amanda E. Paluch, PhD; Nia Heard-Garris, MD, MSc; and Mercedes R. Carnethon, PhD.
However, a significant socioeconomic disparity in team sports for children continues to grow in the United States, driven in part by a youth sports industry and culture that caters to high-income families looking to improve their children’s performance. “Although unintentional, these expenses leave behind lower-income children,” many of whom may be at increased risk for ACEs, the editorialists noted. Many inexpensive, community-based recreation leagues, especially in low-income areas, are often underfunded and unable to update facilities and attract more participants.
The benefits of team sports appear to go beyond the physical, as the study by Easterlin et al. suggests that feeling accepted and connected as part of a team has an impact on mental health. Also, the winning and losing of sports helps build emotional resilience that carries over to other areas of life, the editorialists added.
“Optimizing the opportunities for sports during adolescence requires relatively few resources and is a low-cost way to improve quality of life and reduce the population burden of mental health disorders, especially for adolescents and young adults with histories of ACEs,” they concluded.
Dr. Paluch and Dr. Carnethon are affiliated with the department of preventive medicine and Dr. Heard-Garris is affiliated with the department of pediatrics at Northwestern University, Chicago. They commented on the study by Easterlin et al (JAMA Pediatr. 2019 May 28. doi:10.1001/jamapediatrics.2019.1209). They reported no conflicts of interest.
Approximately half of children suffer an adverse childhood experience (ACE) that can negatively affect their mental health throughout life, and “team sports can be an avenue to interrupt these negative sequelae and address the important public health burden of depression,” wrote Amanda E. Paluch, PhD; Nia Heard-Garris, MD, MSc; and Mercedes R. Carnethon, PhD.
However, a significant socioeconomic disparity in team sports for children continues to grow in the United States, driven in part by a youth sports industry and culture that caters to high-income families looking to improve their children’s performance. “Although unintentional, these expenses leave behind lower-income children,” many of whom may be at increased risk for ACEs, the editorialists noted. Many inexpensive, community-based recreation leagues, especially in low-income areas, are often underfunded and unable to update facilities and attract more participants.
The benefits of team sports appear to go beyond the physical, as the study by Easterlin et al. suggests that feeling accepted and connected as part of a team has an impact on mental health. Also, the winning and losing of sports helps build emotional resilience that carries over to other areas of life, the editorialists added.
“Optimizing the opportunities for sports during adolescence requires relatively few resources and is a low-cost way to improve quality of life and reduce the population burden of mental health disorders, especially for adolescents and young adults with histories of ACEs,” they concluded.
Dr. Paluch and Dr. Carnethon are affiliated with the department of preventive medicine and Dr. Heard-Garris is affiliated with the department of pediatrics at Northwestern University, Chicago. They commented on the study by Easterlin et al (JAMA Pediatr. 2019 May 28. doi:10.1001/jamapediatrics.2019.1209). They reported no conflicts of interest.
Individuals who experienced adverse childhood experiences but also played team sports as teens were less likely to have mental health problems in adulthood than those with childhood challenges who did not play sports, based on data from nearly 5,000 individuals.
Physical and mental health problems are more prominent throughout life among those exposed to adverse childhood experiences (ACEs), and physical activity in general and team sports in particular have been shown to improve mental health, wrote Molly C. Easterlin, MD, of the University of California, Los Angeles, and colleagues.
In a study published in JAMA Pediatrics, the researchers used data from the National Longitudinal Study of Adolescent to Adult Health to compare the development of depression, anxiety, or depressive symptoms among those with childhood ACEs who did and did not participate in team sports in adolescence.
Overall, team sports participation was significantly associated with reduced odds of depression (adjusted odds ratio, 0.76), anxiety (aOR, 0.70), and depressive symptoms (aOR, 0.85) in young adulthood for individuals with ACEs, compared with those with ACEs who did not play team sports.
Of 9,668 adolescents in the study, 4,888 individuals reported one or more ACEs and 2,084 reported two or more ACEs. The researchers compared data from the 1994-1995 school year when participants were in grades 7-12 and in 2008 to assess their mental health as young adults (aged 24-32 years).
No significant differences in associations appeared between sports participation and mental health between males and females.
The results were limited by several factors including the study design that did not allow for causality and the potential social desirability bias that might lead to underreporting ACEs, Dr. Easterlin and associates noted.
Nonetheless, “given that participation in team sports was associated with improved adult mental health among those with ACEs, and parents might consider enrolling their children with ACEs in team sports,” they wrote.
Dr. Easterlin is supported by the Cedars-Sinai Medical Center via the UCLA National Clinician Scholars Program. The authors reported no conflicts of interest.
SOURCE: Easterlin MC et al. JAMA Pediatr. 2019 May 28. doi: 10.1001/jamapediatrics.2019.1212.
Individuals who experienced adverse childhood experiences but also played team sports as teens were less likely to have mental health problems in adulthood than those with childhood challenges who did not play sports, based on data from nearly 5,000 individuals.
Physical and mental health problems are more prominent throughout life among those exposed to adverse childhood experiences (ACEs), and physical activity in general and team sports in particular have been shown to improve mental health, wrote Molly C. Easterlin, MD, of the University of California, Los Angeles, and colleagues.
In a study published in JAMA Pediatrics, the researchers used data from the National Longitudinal Study of Adolescent to Adult Health to compare the development of depression, anxiety, or depressive symptoms among those with childhood ACEs who did and did not participate in team sports in adolescence.
Overall, team sports participation was significantly associated with reduced odds of depression (adjusted odds ratio, 0.76), anxiety (aOR, 0.70), and depressive symptoms (aOR, 0.85) in young adulthood for individuals with ACEs, compared with those with ACEs who did not play team sports.
Of 9,668 adolescents in the study, 4,888 individuals reported one or more ACEs and 2,084 reported two or more ACEs. The researchers compared data from the 1994-1995 school year when participants were in grades 7-12 and in 2008 to assess their mental health as young adults (aged 24-32 years).
No significant differences in associations appeared between sports participation and mental health between males and females.
The results were limited by several factors including the study design that did not allow for causality and the potential social desirability bias that might lead to underreporting ACEs, Dr. Easterlin and associates noted.
Nonetheless, “given that participation in team sports was associated with improved adult mental health among those with ACEs, and parents might consider enrolling their children with ACEs in team sports,” they wrote.
Dr. Easterlin is supported by the Cedars-Sinai Medical Center via the UCLA National Clinician Scholars Program. The authors reported no conflicts of interest.
SOURCE: Easterlin MC et al. JAMA Pediatr. 2019 May 28. doi: 10.1001/jamapediatrics.2019.1212.
FROM JAMA PEDIATRICS





