User login
For borderline personality disorder, less may be more
SAN FRANCISCO – Borderline personality disorder is often treated with long-running psychotherapy, but this could be a disservice to patients. Instead, stepped-care models may offer more benefit in a shorter period of time, conserving resources and potentially expanding the reach of therapeutic interventions.
“There is this legacy of psychoanalysis, this idea that if you’ve had a condition for 20 years, it’s going to take 20 years of therapy to get rid of it – which is not true,” Joel Paris, MD, said in an interview. Dr. Paris moderated a session on stepped-care interventions for borderline personality disorder at the annual meeting of the American Psychiatric Association.
He also pointed out that psychoanalysis can prove self-sustaining: “There’s something to talk about, so it can go on and if there’s money to pay for the service, why stop?”
But psychoanalysis can have diminishing returns, and that in turn can strain resources. “Some people won’t get better or won’t go beyond a certain point. You help them to a certain extent, and then you have to be able to say, ‘That’s enough; stop there. If you get in to bad trouble, you can always come back,’ ” said Dr. Paris, professor of psychiatry at McGill University, Montreal.
That realization has led Dr. McGill to introduce a stepped-care model, which has provided a 12-week program for 15 years. Dr. Paris presented a retrospective look at the program, including 479 patients who received individual and group therapy. The dropout rate was high at 30%, but only 12% of patients returned asking for more therapy. A total of 145 patients deemed to be more chronic or who did not respond to the short-term program had the option of completing additional individual or group therapy over a period of 6-24 months.
“ There are also dropouts; maybe a quarter will not complete the program,” Dr. Paris said. “But that means 75% do, and most of them get better. There’s also good evidence that short therapy can help many people with substance abuse.”
Lois W. Choi-Kain, MD, assistant professor psychiatry at Harvard Medical School, Boston, presented evidence from several studies showing that shorter- and long-term courses of dialectical behavior therapy led to similar improvements, but that brief courses were associated with more rapid improvement. The short course consisted of weekly 1-hour individual sessions, while the longer course involved weekly 2-hour group sessions. The individual approach was associated with an 89% faster improvement in symptoms in the first 3 months (P less than .0001).
These kinds of findings underscore the need for shorter courses of therapy, at least for most patients, in order to conserve resources and broaden the availability of therapy, particularly in less affluent settings. “We saw in today’s symposium that most improvement takes place at the beginning of therapy, and you should quit while you’re ahead,” Dr. Paris said. “You should not try to do impossible things and make everybody into paradigms of mental health.”
Dr. Paris reported no relevant financial disclosures.
SAN FRANCISCO – Borderline personality disorder is often treated with long-running psychotherapy, but this could be a disservice to patients. Instead, stepped-care models may offer more benefit in a shorter period of time, conserving resources and potentially expanding the reach of therapeutic interventions.
“There is this legacy of psychoanalysis, this idea that if you’ve had a condition for 20 years, it’s going to take 20 years of therapy to get rid of it – which is not true,” Joel Paris, MD, said in an interview. Dr. Paris moderated a session on stepped-care interventions for borderline personality disorder at the annual meeting of the American Psychiatric Association.
He also pointed out that psychoanalysis can prove self-sustaining: “There’s something to talk about, so it can go on and if there’s money to pay for the service, why stop?”
But psychoanalysis can have diminishing returns, and that in turn can strain resources. “Some people won’t get better or won’t go beyond a certain point. You help them to a certain extent, and then you have to be able to say, ‘That’s enough; stop there. If you get in to bad trouble, you can always come back,’ ” said Dr. Paris, professor of psychiatry at McGill University, Montreal.
That realization has led Dr. McGill to introduce a stepped-care model, which has provided a 12-week program for 15 years. Dr. Paris presented a retrospective look at the program, including 479 patients who received individual and group therapy. The dropout rate was high at 30%, but only 12% of patients returned asking for more therapy. A total of 145 patients deemed to be more chronic or who did not respond to the short-term program had the option of completing additional individual or group therapy over a period of 6-24 months.
“ There are also dropouts; maybe a quarter will not complete the program,” Dr. Paris said. “But that means 75% do, and most of them get better. There’s also good evidence that short therapy can help many people with substance abuse.”
Lois W. Choi-Kain, MD, assistant professor psychiatry at Harvard Medical School, Boston, presented evidence from several studies showing that shorter- and long-term courses of dialectical behavior therapy led to similar improvements, but that brief courses were associated with more rapid improvement. The short course consisted of weekly 1-hour individual sessions, while the longer course involved weekly 2-hour group sessions. The individual approach was associated with an 89% faster improvement in symptoms in the first 3 months (P less than .0001).
These kinds of findings underscore the need for shorter courses of therapy, at least for most patients, in order to conserve resources and broaden the availability of therapy, particularly in less affluent settings. “We saw in today’s symposium that most improvement takes place at the beginning of therapy, and you should quit while you’re ahead,” Dr. Paris said. “You should not try to do impossible things and make everybody into paradigms of mental health.”
Dr. Paris reported no relevant financial disclosures.
SAN FRANCISCO – Borderline personality disorder is often treated with long-running psychotherapy, but this could be a disservice to patients. Instead, stepped-care models may offer more benefit in a shorter period of time, conserving resources and potentially expanding the reach of therapeutic interventions.
“There is this legacy of psychoanalysis, this idea that if you’ve had a condition for 20 years, it’s going to take 20 years of therapy to get rid of it – which is not true,” Joel Paris, MD, said in an interview. Dr. Paris moderated a session on stepped-care interventions for borderline personality disorder at the annual meeting of the American Psychiatric Association.
He also pointed out that psychoanalysis can prove self-sustaining: “There’s something to talk about, so it can go on and if there’s money to pay for the service, why stop?”
But psychoanalysis can have diminishing returns, and that in turn can strain resources. “Some people won’t get better or won’t go beyond a certain point. You help them to a certain extent, and then you have to be able to say, ‘That’s enough; stop there. If you get in to bad trouble, you can always come back,’ ” said Dr. Paris, professor of psychiatry at McGill University, Montreal.
That realization has led Dr. McGill to introduce a stepped-care model, which has provided a 12-week program for 15 years. Dr. Paris presented a retrospective look at the program, including 479 patients who received individual and group therapy. The dropout rate was high at 30%, but only 12% of patients returned asking for more therapy. A total of 145 patients deemed to be more chronic or who did not respond to the short-term program had the option of completing additional individual or group therapy over a period of 6-24 months.
“ There are also dropouts; maybe a quarter will not complete the program,” Dr. Paris said. “But that means 75% do, and most of them get better. There’s also good evidence that short therapy can help many people with substance abuse.”
Lois W. Choi-Kain, MD, assistant professor psychiatry at Harvard Medical School, Boston, presented evidence from several studies showing that shorter- and long-term courses of dialectical behavior therapy led to similar improvements, but that brief courses were associated with more rapid improvement. The short course consisted of weekly 1-hour individual sessions, while the longer course involved weekly 2-hour group sessions. The individual approach was associated with an 89% faster improvement in symptoms in the first 3 months (P less than .0001).
These kinds of findings underscore the need for shorter courses of therapy, at least for most patients, in order to conserve resources and broaden the availability of therapy, particularly in less affluent settings. “We saw in today’s symposium that most improvement takes place at the beginning of therapy, and you should quit while you’re ahead,” Dr. Paris said. “You should not try to do impossible things and make everybody into paradigms of mental health.”
Dr. Paris reported no relevant financial disclosures.
REPORTING FROM APA 2019
Vet accuracy, privacy policies of mental health apps
SAN FRANCISCO – Smartphones are nearly ubiquitous, and the apps available on them run the gamut from entertainment to social media, organization tools, and references. It should be no surprise that mental health applications are also gaining traction, as people experiencing depression, anxiety, and other conditions turn to these programs for assistance.
Psychiatrists should be aware of any mental health apps that their patients are using, and they should do a little research to determine their safety and efficacy, according to Hephsibah M. Loeb, MD, a psychiatry resident at Jefferson Medical College, Philadelphia.
“It’s our responsibility to understand the medications we’re prescribing, so I don’t think this is any different,” said Dr. Loeb, who presented a poster outlining advice for clinicians at the annual meeting of the American Psychiatric Association.
One such app, called reSET, gained approval from Food and Drug Administration for the treatment of alcohol, cocaine, marijuana, and stimulant use disorders. Another app, called PRIME-D, designed for self-management of depression, showed some benefit in an open-label study. But there are many more apps out there, and how is a clinician to know of the potential benefits and hazards?
In her poster, Dr. Loeb outlined the APA’s App Evaluation tool, which is designed to help clinicians systematically investigate an app. The APA has no standing list of reviewed apps because they are changing all the time, and new apps are introduced and removed all the time.
“The evaluation model is a set of questions that the APA developed [representing] certain issues that if a patient reports using an app, a clinician should look into in order to determine if it’s an appropriate and safe tool. For example: Who developed the app? What is its privacy policy? Is the information correct?” Dr. Loeb asked.
Privacy is of particular concern, since the patient has no way of knowing how his or her data are being handled. “If you’re putting really personal stuff in there and you don’t know where it’s going, that’s a huge risk. We have HIPAA protection in the office, but needless to say, if someone downloads something from the app store, their information is not going to be protected by HIPAA,” Dr. Loeb said.
Despite these concerns, Dr. Loeb is not a naysayer. “I think that self-help is really interesting. Presumably, there are patients we’re not even seeing in the office,” she said. In some cases, people are not patients but have a complaint and need help. “If there weren’t an app or online therapy, they might not otherwise get help.”
But whether the help they’re getting is reliable is another question. “There’s the chance that the information is perfectly accurate; there’s the chance that there’s misinformation. There’s also the chance of mis–self-diagnosis,” Dr. Loeb said.
since better clinical outcomes with apps are associated with receiving support from a trained mental health coach.
Finally, there’s one sure way to determine the quality of an app: Dr. Loeb said that a coauthor on the poster, Ann Chandy, MD, a clinical professor of psychiatry at Jefferson Medical College, uses an app herself when a patient brings it to her attention.
Dr. Loeb reported no relevant financial disclosures.
SAN FRANCISCO – Smartphones are nearly ubiquitous, and the apps available on them run the gamut from entertainment to social media, organization tools, and references. It should be no surprise that mental health applications are also gaining traction, as people experiencing depression, anxiety, and other conditions turn to these programs for assistance.
Psychiatrists should be aware of any mental health apps that their patients are using, and they should do a little research to determine their safety and efficacy, according to Hephsibah M. Loeb, MD, a psychiatry resident at Jefferson Medical College, Philadelphia.
“It’s our responsibility to understand the medications we’re prescribing, so I don’t think this is any different,” said Dr. Loeb, who presented a poster outlining advice for clinicians at the annual meeting of the American Psychiatric Association.
One such app, called reSET, gained approval from Food and Drug Administration for the treatment of alcohol, cocaine, marijuana, and stimulant use disorders. Another app, called PRIME-D, designed for self-management of depression, showed some benefit in an open-label study. But there are many more apps out there, and how is a clinician to know of the potential benefits and hazards?
In her poster, Dr. Loeb outlined the APA’s App Evaluation tool, which is designed to help clinicians systematically investigate an app. The APA has no standing list of reviewed apps because they are changing all the time, and new apps are introduced and removed all the time.
“The evaluation model is a set of questions that the APA developed [representing] certain issues that if a patient reports using an app, a clinician should look into in order to determine if it’s an appropriate and safe tool. For example: Who developed the app? What is its privacy policy? Is the information correct?” Dr. Loeb asked.
Privacy is of particular concern, since the patient has no way of knowing how his or her data are being handled. “If you’re putting really personal stuff in there and you don’t know where it’s going, that’s a huge risk. We have HIPAA protection in the office, but needless to say, if someone downloads something from the app store, their information is not going to be protected by HIPAA,” Dr. Loeb said.
Despite these concerns, Dr. Loeb is not a naysayer. “I think that self-help is really interesting. Presumably, there are patients we’re not even seeing in the office,” she said. In some cases, people are not patients but have a complaint and need help. “If there weren’t an app or online therapy, they might not otherwise get help.”
But whether the help they’re getting is reliable is another question. “There’s the chance that the information is perfectly accurate; there’s the chance that there’s misinformation. There’s also the chance of mis–self-diagnosis,” Dr. Loeb said.
since better clinical outcomes with apps are associated with receiving support from a trained mental health coach.
Finally, there’s one sure way to determine the quality of an app: Dr. Loeb said that a coauthor on the poster, Ann Chandy, MD, a clinical professor of psychiatry at Jefferson Medical College, uses an app herself when a patient brings it to her attention.
Dr. Loeb reported no relevant financial disclosures.
SAN FRANCISCO – Smartphones are nearly ubiquitous, and the apps available on them run the gamut from entertainment to social media, organization tools, and references. It should be no surprise that mental health applications are also gaining traction, as people experiencing depression, anxiety, and other conditions turn to these programs for assistance.
Psychiatrists should be aware of any mental health apps that their patients are using, and they should do a little research to determine their safety and efficacy, according to Hephsibah M. Loeb, MD, a psychiatry resident at Jefferson Medical College, Philadelphia.
“It’s our responsibility to understand the medications we’re prescribing, so I don’t think this is any different,” said Dr. Loeb, who presented a poster outlining advice for clinicians at the annual meeting of the American Psychiatric Association.
One such app, called reSET, gained approval from Food and Drug Administration for the treatment of alcohol, cocaine, marijuana, and stimulant use disorders. Another app, called PRIME-D, designed for self-management of depression, showed some benefit in an open-label study. But there are many more apps out there, and how is a clinician to know of the potential benefits and hazards?
In her poster, Dr. Loeb outlined the APA’s App Evaluation tool, which is designed to help clinicians systematically investigate an app. The APA has no standing list of reviewed apps because they are changing all the time, and new apps are introduced and removed all the time.
“The evaluation model is a set of questions that the APA developed [representing] certain issues that if a patient reports using an app, a clinician should look into in order to determine if it’s an appropriate and safe tool. For example: Who developed the app? What is its privacy policy? Is the information correct?” Dr. Loeb asked.
Privacy is of particular concern, since the patient has no way of knowing how his or her data are being handled. “If you’re putting really personal stuff in there and you don’t know where it’s going, that’s a huge risk. We have HIPAA protection in the office, but needless to say, if someone downloads something from the app store, their information is not going to be protected by HIPAA,” Dr. Loeb said.
Despite these concerns, Dr. Loeb is not a naysayer. “I think that self-help is really interesting. Presumably, there are patients we’re not even seeing in the office,” she said. In some cases, people are not patients but have a complaint and need help. “If there weren’t an app or online therapy, they might not otherwise get help.”
But whether the help they’re getting is reliable is another question. “There’s the chance that the information is perfectly accurate; there’s the chance that there’s misinformation. There’s also the chance of mis–self-diagnosis,” Dr. Loeb said.
since better clinical outcomes with apps are associated with receiving support from a trained mental health coach.
Finally, there’s one sure way to determine the quality of an app: Dr. Loeb said that a coauthor on the poster, Ann Chandy, MD, a clinical professor of psychiatry at Jefferson Medical College, uses an app herself when a patient brings it to her attention.
Dr. Loeb reported no relevant financial disclosures.
EXPERT ANALYSIS FROM APA 2019
Genetic analysis identifies prognostic markers in CLL
A genetic analysis of patients with chronic lymphocytic leukemia treated with frontline, rituximab-based regimens found that deletion 11q22 and unmutated IgVH status may predict worse prognosis.
Michaela Spunarova, MD, of Masaryk University, Brno, Czech Republic, and colleagues conducted a genetic analysis of 177 patients with chronic lymphocytic leukemia (CLL). The results of the analysis were published in Leukemia Research.
The study focused on patients with CLL with an intact TP53 gene, looking at recurrently muted genes in CLL, genomic aberrations by fluorescence in situ hybridization, and IgVH status, according to the researchers.
The team analyzed the effects of these mutations on progression-free survival (PFS) following frontline treatment with bendamustine and rituximab (BR) or fludarabine, cyclophosphamide, and rituximab (FCR) therapeutic regimens.
Dr. Spunarova and colleagues used next-generation sequencing to analyze DNA from the patient samples. Data on 11q22, 13q14, trisomy 12, and IgVH mutation status were also considered in the analyses of PFS.
After analysis, the researchers validated that unmutated IgVH status is an indicator of poor prognosis in CLL patients with wild-type TP53 treated with frontline FCR.
When looking at both BR and FCR regimens, a single 11q22 deletion, lacking an ATM mutation on the other allele, resulted in the shortest PFS, at a median of just 16 months.
“Based on our data, special attention should be given to CLL patients harboring a sole 11q22 deletion, with no ATM mutation on the other allele, who manifest particularly short PFS,” they noted.
The researchers acknowledged a key limitation of the study was the small sample size. As a result, the results should be interpreted in a careful manner.
The study was funded by the Ministry of Health of the Czech Republic. The authors reported having no conflicts of interest.
SOURCE: Spunarova M et al. Leuk Res. 2019 Jun;81:75-81.
A genetic analysis of patients with chronic lymphocytic leukemia treated with frontline, rituximab-based regimens found that deletion 11q22 and unmutated IgVH status may predict worse prognosis.
Michaela Spunarova, MD, of Masaryk University, Brno, Czech Republic, and colleagues conducted a genetic analysis of 177 patients with chronic lymphocytic leukemia (CLL). The results of the analysis were published in Leukemia Research.
The study focused on patients with CLL with an intact TP53 gene, looking at recurrently muted genes in CLL, genomic aberrations by fluorescence in situ hybridization, and IgVH status, according to the researchers.
The team analyzed the effects of these mutations on progression-free survival (PFS) following frontline treatment with bendamustine and rituximab (BR) or fludarabine, cyclophosphamide, and rituximab (FCR) therapeutic regimens.
Dr. Spunarova and colleagues used next-generation sequencing to analyze DNA from the patient samples. Data on 11q22, 13q14, trisomy 12, and IgVH mutation status were also considered in the analyses of PFS.
After analysis, the researchers validated that unmutated IgVH status is an indicator of poor prognosis in CLL patients with wild-type TP53 treated with frontline FCR.
When looking at both BR and FCR regimens, a single 11q22 deletion, lacking an ATM mutation on the other allele, resulted in the shortest PFS, at a median of just 16 months.
“Based on our data, special attention should be given to CLL patients harboring a sole 11q22 deletion, with no ATM mutation on the other allele, who manifest particularly short PFS,” they noted.
The researchers acknowledged a key limitation of the study was the small sample size. As a result, the results should be interpreted in a careful manner.
The study was funded by the Ministry of Health of the Czech Republic. The authors reported having no conflicts of interest.
SOURCE: Spunarova M et al. Leuk Res. 2019 Jun;81:75-81.
A genetic analysis of patients with chronic lymphocytic leukemia treated with frontline, rituximab-based regimens found that deletion 11q22 and unmutated IgVH status may predict worse prognosis.
Michaela Spunarova, MD, of Masaryk University, Brno, Czech Republic, and colleagues conducted a genetic analysis of 177 patients with chronic lymphocytic leukemia (CLL). The results of the analysis were published in Leukemia Research.
The study focused on patients with CLL with an intact TP53 gene, looking at recurrently muted genes in CLL, genomic aberrations by fluorescence in situ hybridization, and IgVH status, according to the researchers.
The team analyzed the effects of these mutations on progression-free survival (PFS) following frontline treatment with bendamustine and rituximab (BR) or fludarabine, cyclophosphamide, and rituximab (FCR) therapeutic regimens.
Dr. Spunarova and colleagues used next-generation sequencing to analyze DNA from the patient samples. Data on 11q22, 13q14, trisomy 12, and IgVH mutation status were also considered in the analyses of PFS.
After analysis, the researchers validated that unmutated IgVH status is an indicator of poor prognosis in CLL patients with wild-type TP53 treated with frontline FCR.
When looking at both BR and FCR regimens, a single 11q22 deletion, lacking an ATM mutation on the other allele, resulted in the shortest PFS, at a median of just 16 months.
“Based on our data, special attention should be given to CLL patients harboring a sole 11q22 deletion, with no ATM mutation on the other allele, who manifest particularly short PFS,” they noted.
The researchers acknowledged a key limitation of the study was the small sample size. As a result, the results should be interpreted in a careful manner.
The study was funded by the Ministry of Health of the Czech Republic. The authors reported having no conflicts of interest.
SOURCE: Spunarova M et al. Leuk Res. 2019 Jun;81:75-81.
FROM LEUKEMIA RESEARCH
Refractory OCD? Consider opioids, amphetamines, caffeine
SAN FRANCISCO – An expert in obsessive-compulsive disease had some surprising advice at the American Psychiatric Association annual meeting for treatment of adults with refractory illness. He endorsed amphetamines, caffeine, and once-weekly opioids in carefully selected patients.
“These are not things that will be taught to you in your residency,” said Lorrin M. Koran, MD, professor emeritus of psychiatry and behavioral sciences at Stanford (Calif.) University, and past director of the university’s OCD clinic and research program. “These are pharmacological pearls that have come to my attention over many years. I hope at the end of this talk you’ll be more comfortable, especially [with] stimulants, because [they are] very simple and very quick.”
Opioids are a last resort but can prove effective for some patients. “I had a woman who wrote me just last week who said her son’s been on an opioid for 9 years once a week,” Dr. Koran said. “He does very well, and if he stops, he relapses within a few days. He has not become dependent or abusive. You have to screen who you give these to.
“You and I are dedicated to helping people not suffer, so we might want to take a little risk” for people who are struggling, he said.
Bolus caffeine, not a casual cup
Inspired by findings from the 1980s-90s, Dr. Koran and his colleagues randomized 12 treatment-resistant adults at Stanford’s OCD clinic to dextroamphetamine (Dexedrine) 30 mg/day and 12 others to caffeine (NoDoz pills) 300 mg/day, both in pink capsules so patients couldn’t tell them apart. “They were really sick” at baseline, Dr. Koran said, with a mean Yale Brown OCD Scale (YBOCS) score of 28 points (J Clin Psychiatry. 2009 Nov;70[11]:1530-5).
Subjects remained on their antidepressants during the study. Patients with histories of substance abuse, heart disease, schizophrenia, bipolar disorder, or panic attacks were among those excluded.
Caffeine was supposed to be the placebo. But a curious thing happened: About half of patients in both groups did remarkably well after 1 week, with a mean YBOCS drop of 41% among the six amphetamine responders and 45% among seven caffeine responders – with more improvement after 5 weeks.
“I was shocked. In clinical trials, anything 25% or more is considered a response. What patients said was, ‘Gee doc, I still get my obsessions, but I can shift my attention. I can get away from them, so I don’t have to do my compulsions, anymore,’ ” Dr. Koran said.
There were a few dose reductions for insomnia and anxiety. However, overall, YBOCS improvement did not correlate with depression and anxiety scores, so responses appeared to be independent. There were no differences between the groups in liking their treatment or how high people felt.
“I encourage you to try this” – amphetamine or caffeine bolus – “for people who have not responded to say two [treatment] trials. Meanwhile, “the data for methylphenidate are less convincing,” Dr. Koran said.
He’s wondered why caffeine helps. After all, no one has ever come into the OCD clinic and said they felt better after their morning coffee. The hypothesis is that 300 mg of caffeine all at once triggers a spike of methylxanthines in the brain, which, much like amphetamines, promotes dopamine and serotonin release. Casually sipping coffee does not have the same effect.
When all else fails
Dr. Koran and his colleagues also ran a small trial of once-weekly opiates.
Spurred again by case histories and small studies, they randomized 23 refractory adults to once-weekly morphine 30 mg (with 15-mg dose adjustment as needed at 2 weeks), lorazepam 1 mg (with 0.5-mg adjustment at 2 weeks), or placebo. Subjects had OCD for at least 3 years, and had failed at least two antidepressant trials (some had been on atypicals). Median baseline YBOCS was 29 points. Subjects remained on their baseline medications during the study (J Clin Psychiatry. 2005 Mar;66[3]:353-9).
Seven patients responded to morphine with a drop of at least 25% in their YBOCS; five had at least a 40% drop. Patients who were not taking a selective serotonin or norepinephrine reuptake inhibitor did not respond to morphine.
There were four lorazepam responders, but only one with a reduction of 40% or more. There were no placebo responders.
Opioids are the “the last thing to think of” in OCD, but when all else fails, “you could try morphine in a properly screened individual,” as long as there is no personal or family history of substance abuse.
Dr. Koran said he had no conflicts of interest.
SAN FRANCISCO – An expert in obsessive-compulsive disease had some surprising advice at the American Psychiatric Association annual meeting for treatment of adults with refractory illness. He endorsed amphetamines, caffeine, and once-weekly opioids in carefully selected patients.
“These are not things that will be taught to you in your residency,” said Lorrin M. Koran, MD, professor emeritus of psychiatry and behavioral sciences at Stanford (Calif.) University, and past director of the university’s OCD clinic and research program. “These are pharmacological pearls that have come to my attention over many years. I hope at the end of this talk you’ll be more comfortable, especially [with] stimulants, because [they are] very simple and very quick.”
Opioids are a last resort but can prove effective for some patients. “I had a woman who wrote me just last week who said her son’s been on an opioid for 9 years once a week,” Dr. Koran said. “He does very well, and if he stops, he relapses within a few days. He has not become dependent or abusive. You have to screen who you give these to.
“You and I are dedicated to helping people not suffer, so we might want to take a little risk” for people who are struggling, he said.
Bolus caffeine, not a casual cup
Inspired by findings from the 1980s-90s, Dr. Koran and his colleagues randomized 12 treatment-resistant adults at Stanford’s OCD clinic to dextroamphetamine (Dexedrine) 30 mg/day and 12 others to caffeine (NoDoz pills) 300 mg/day, both in pink capsules so patients couldn’t tell them apart. “They were really sick” at baseline, Dr. Koran said, with a mean Yale Brown OCD Scale (YBOCS) score of 28 points (J Clin Psychiatry. 2009 Nov;70[11]:1530-5).
Subjects remained on their antidepressants during the study. Patients with histories of substance abuse, heart disease, schizophrenia, bipolar disorder, or panic attacks were among those excluded.
Caffeine was supposed to be the placebo. But a curious thing happened: About half of patients in both groups did remarkably well after 1 week, with a mean YBOCS drop of 41% among the six amphetamine responders and 45% among seven caffeine responders – with more improvement after 5 weeks.
“I was shocked. In clinical trials, anything 25% or more is considered a response. What patients said was, ‘Gee doc, I still get my obsessions, but I can shift my attention. I can get away from them, so I don’t have to do my compulsions, anymore,’ ” Dr. Koran said.
There were a few dose reductions for insomnia and anxiety. However, overall, YBOCS improvement did not correlate with depression and anxiety scores, so responses appeared to be independent. There were no differences between the groups in liking their treatment or how high people felt.
“I encourage you to try this” – amphetamine or caffeine bolus – “for people who have not responded to say two [treatment] trials. Meanwhile, “the data for methylphenidate are less convincing,” Dr. Koran said.
He’s wondered why caffeine helps. After all, no one has ever come into the OCD clinic and said they felt better after their morning coffee. The hypothesis is that 300 mg of caffeine all at once triggers a spike of methylxanthines in the brain, which, much like amphetamines, promotes dopamine and serotonin release. Casually sipping coffee does not have the same effect.
When all else fails
Dr. Koran and his colleagues also ran a small trial of once-weekly opiates.
Spurred again by case histories and small studies, they randomized 23 refractory adults to once-weekly morphine 30 mg (with 15-mg dose adjustment as needed at 2 weeks), lorazepam 1 mg (with 0.5-mg adjustment at 2 weeks), or placebo. Subjects had OCD for at least 3 years, and had failed at least two antidepressant trials (some had been on atypicals). Median baseline YBOCS was 29 points. Subjects remained on their baseline medications during the study (J Clin Psychiatry. 2005 Mar;66[3]:353-9).
Seven patients responded to morphine with a drop of at least 25% in their YBOCS; five had at least a 40% drop. Patients who were not taking a selective serotonin or norepinephrine reuptake inhibitor did not respond to morphine.
There were four lorazepam responders, but only one with a reduction of 40% or more. There were no placebo responders.
Opioids are the “the last thing to think of” in OCD, but when all else fails, “you could try morphine in a properly screened individual,” as long as there is no personal or family history of substance abuse.
Dr. Koran said he had no conflicts of interest.
SAN FRANCISCO – An expert in obsessive-compulsive disease had some surprising advice at the American Psychiatric Association annual meeting for treatment of adults with refractory illness. He endorsed amphetamines, caffeine, and once-weekly opioids in carefully selected patients.
“These are not things that will be taught to you in your residency,” said Lorrin M. Koran, MD, professor emeritus of psychiatry and behavioral sciences at Stanford (Calif.) University, and past director of the university’s OCD clinic and research program. “These are pharmacological pearls that have come to my attention over many years. I hope at the end of this talk you’ll be more comfortable, especially [with] stimulants, because [they are] very simple and very quick.”
Opioids are a last resort but can prove effective for some patients. “I had a woman who wrote me just last week who said her son’s been on an opioid for 9 years once a week,” Dr. Koran said. “He does very well, and if he stops, he relapses within a few days. He has not become dependent or abusive. You have to screen who you give these to.
“You and I are dedicated to helping people not suffer, so we might want to take a little risk” for people who are struggling, he said.
Bolus caffeine, not a casual cup
Inspired by findings from the 1980s-90s, Dr. Koran and his colleagues randomized 12 treatment-resistant adults at Stanford’s OCD clinic to dextroamphetamine (Dexedrine) 30 mg/day and 12 others to caffeine (NoDoz pills) 300 mg/day, both in pink capsules so patients couldn’t tell them apart. “They were really sick” at baseline, Dr. Koran said, with a mean Yale Brown OCD Scale (YBOCS) score of 28 points (J Clin Psychiatry. 2009 Nov;70[11]:1530-5).
Subjects remained on their antidepressants during the study. Patients with histories of substance abuse, heart disease, schizophrenia, bipolar disorder, or panic attacks were among those excluded.
Caffeine was supposed to be the placebo. But a curious thing happened: About half of patients in both groups did remarkably well after 1 week, with a mean YBOCS drop of 41% among the six amphetamine responders and 45% among seven caffeine responders – with more improvement after 5 weeks.
“I was shocked. In clinical trials, anything 25% or more is considered a response. What patients said was, ‘Gee doc, I still get my obsessions, but I can shift my attention. I can get away from them, so I don’t have to do my compulsions, anymore,’ ” Dr. Koran said.
There were a few dose reductions for insomnia and anxiety. However, overall, YBOCS improvement did not correlate with depression and anxiety scores, so responses appeared to be independent. There were no differences between the groups in liking their treatment or how high people felt.
“I encourage you to try this” – amphetamine or caffeine bolus – “for people who have not responded to say two [treatment] trials. Meanwhile, “the data for methylphenidate are less convincing,” Dr. Koran said.
He’s wondered why caffeine helps. After all, no one has ever come into the OCD clinic and said they felt better after their morning coffee. The hypothesis is that 300 mg of caffeine all at once triggers a spike of methylxanthines in the brain, which, much like amphetamines, promotes dopamine and serotonin release. Casually sipping coffee does not have the same effect.
When all else fails
Dr. Koran and his colleagues also ran a small trial of once-weekly opiates.
Spurred again by case histories and small studies, they randomized 23 refractory adults to once-weekly morphine 30 mg (with 15-mg dose adjustment as needed at 2 weeks), lorazepam 1 mg (with 0.5-mg adjustment at 2 weeks), or placebo. Subjects had OCD for at least 3 years, and had failed at least two antidepressant trials (some had been on atypicals). Median baseline YBOCS was 29 points. Subjects remained on their baseline medications during the study (J Clin Psychiatry. 2005 Mar;66[3]:353-9).
Seven patients responded to morphine with a drop of at least 25% in their YBOCS; five had at least a 40% drop. Patients who were not taking a selective serotonin or norepinephrine reuptake inhibitor did not respond to morphine.
There were four lorazepam responders, but only one with a reduction of 40% or more. There were no placebo responders.
Opioids are the “the last thing to think of” in OCD, but when all else fails, “you could try morphine in a properly screened individual,” as long as there is no personal or family history of substance abuse.
Dr. Koran said he had no conflicts of interest.
REPORTING FROM APA 2019
Subsegmental PEs overtreated despite link with patient harm
Background: CT pulmonary angiography (CTPA) often detects distal, subsegmental pulmonary embolisms (SSPE) for which there is unclear clinical significance. For these isolated SSPEs, the 2016 CHEST guidelines recommend clinical surveillance in lieu of treatment. Such clinical surveillance has not been associated with an increased recurrence of venous thromboembolism (VTE) over 3 months.
Study design: Retrospective review.
Setting: Tertiary care center in Quebec.
Synopsis: A review of all CTPAs at McGill University in Montreal, from 2014-2016 yielded 222 acute pulmonary emboli (PEs), 71 of which were SSPEs without associated Doppler imaging positive for deep vein thrombosis. Of those 71, 62 (87%) were systemically anticoagulated, compared with 135/143 (94%) of the more proximal PEs. The adverse events of both groups of anticoagulated patients were common and similar. Over the following 3 months, 26 patients in the SSPE group visited the ED or were readmitted (42%; 95% confidence interval, 30%-55%), 21 had a drop in hemoglobin level of 2 g/dL or greater and/or received a blood transfusion (34%; 95% CI, 22%-47%), and 10 died from causes unrelated to VTE (16%; 95% CI, 8%-28%). Limitations of this study included the small number of participants and short time to follow-up.
Bottom line: Although SSPEs have unknown clinical significance, they are being treated with systemic anticoagulation at a similar rate to more proximal PEs and are associated with patient harm.
Citation: Raslan IA et al. Rates of overtreatment and treatment-related adverse effects among patients with subsegmental pulmonary embolism. JAMA Intern Med. 2018 Sep 1;178(9):1272-4.
Dr. Shaw is an assistant professor in the division of hospital medicine, University of New Mexico.
Background: CT pulmonary angiography (CTPA) often detects distal, subsegmental pulmonary embolisms (SSPE) for which there is unclear clinical significance. For these isolated SSPEs, the 2016 CHEST guidelines recommend clinical surveillance in lieu of treatment. Such clinical surveillance has not been associated with an increased recurrence of venous thromboembolism (VTE) over 3 months.
Study design: Retrospective review.
Setting: Tertiary care center in Quebec.
Synopsis: A review of all CTPAs at McGill University in Montreal, from 2014-2016 yielded 222 acute pulmonary emboli (PEs), 71 of which were SSPEs without associated Doppler imaging positive for deep vein thrombosis. Of those 71, 62 (87%) were systemically anticoagulated, compared with 135/143 (94%) of the more proximal PEs. The adverse events of both groups of anticoagulated patients were common and similar. Over the following 3 months, 26 patients in the SSPE group visited the ED or were readmitted (42%; 95% confidence interval, 30%-55%), 21 had a drop in hemoglobin level of 2 g/dL or greater and/or received a blood transfusion (34%; 95% CI, 22%-47%), and 10 died from causes unrelated to VTE (16%; 95% CI, 8%-28%). Limitations of this study included the small number of participants and short time to follow-up.
Bottom line: Although SSPEs have unknown clinical significance, they are being treated with systemic anticoagulation at a similar rate to more proximal PEs and are associated with patient harm.
Citation: Raslan IA et al. Rates of overtreatment and treatment-related adverse effects among patients with subsegmental pulmonary embolism. JAMA Intern Med. 2018 Sep 1;178(9):1272-4.
Dr. Shaw is an assistant professor in the division of hospital medicine, University of New Mexico.
Background: CT pulmonary angiography (CTPA) often detects distal, subsegmental pulmonary embolisms (SSPE) for which there is unclear clinical significance. For these isolated SSPEs, the 2016 CHEST guidelines recommend clinical surveillance in lieu of treatment. Such clinical surveillance has not been associated with an increased recurrence of venous thromboembolism (VTE) over 3 months.
Study design: Retrospective review.
Setting: Tertiary care center in Quebec.
Synopsis: A review of all CTPAs at McGill University in Montreal, from 2014-2016 yielded 222 acute pulmonary emboli (PEs), 71 of which were SSPEs without associated Doppler imaging positive for deep vein thrombosis. Of those 71, 62 (87%) were systemically anticoagulated, compared with 135/143 (94%) of the more proximal PEs. The adverse events of both groups of anticoagulated patients were common and similar. Over the following 3 months, 26 patients in the SSPE group visited the ED or were readmitted (42%; 95% confidence interval, 30%-55%), 21 had a drop in hemoglobin level of 2 g/dL or greater and/or received a blood transfusion (34%; 95% CI, 22%-47%), and 10 died from causes unrelated to VTE (16%; 95% CI, 8%-28%). Limitations of this study included the small number of participants and short time to follow-up.
Bottom line: Although SSPEs have unknown clinical significance, they are being treated with systemic anticoagulation at a similar rate to more proximal PEs and are associated with patient harm.
Citation: Raslan IA et al. Rates of overtreatment and treatment-related adverse effects among patients with subsegmental pulmonary embolism. JAMA Intern Med. 2018 Sep 1;178(9):1272-4.
Dr. Shaw is an assistant professor in the division of hospital medicine, University of New Mexico.
Study finds inconsistent links with aspirin, nonaspirin NSAIDs and reduced skin cancer risk
Use of aspirin or nonaspirin NSAIDs was not associated with a reduced risk of basal cell carcinoma (BCC) or squamous cell carcinoma (SCC), in a large, prospective cohort study of Australian residents.
“Overall, we observed weak and inconsistent inverse associations between use of these medications and incidence of either BCC or SCC,” wrote Nirmala Pandeya, PhD, of the University of Queensland (Australia) and coauthors. “While we did observe a modest reduction in use,” they added. The study was published in the British Journal of Dermatology.
While reviews of observational studies have suggested that NSAIDs may have “a potential benefit” in reducing the incidence of BCC and SCC, the results have varied, they noted.
To investigate the potential chemopreventive effects of NSAID use on skin cancer, the investigators used data from the QSkin Sun and Health Study, a prospective cohort of 43,764 residents of Queensland, Australia. Those eligible for the study had a white ethnic background and no history of melanoma; 34,630 participants were available for analysis, their median age was 57 years, and 55% were women
Almost 15,600 (45%) were classified as “high risk” because they had had at least one skin cancer excision or more than five actinic lesions treated; 18,828 participants were classified as “average to low risk;” and data were unavailable for 206 participants. One‐third of the participants in the high-risk group (5,398) used aspirin; of these individuals, 39% (2,132) used aspirin more than once a week (defined as “frequent” users). Also, 60% (9,236) used NSAIDs, and of those, 24% (2,229) were frequent users.
During a median follow-up of 3 years, 3,421 of those in the study (10%) developed one or more BCC, and 1,470 (4%) developed one or more SCC.
Compared with never users, frequent NSAID use in the high-risk group was modestly associated with a reduced risk of BCC (hazard ratio, 0.84; 95% confidence interval, 0.71-0.99), but not with SCC. Aspirin use was weakly associated with a reduced risk of SCC (HR, 0.77; 95% CI, 0.64-0.93) but only among infrequent users and was not associated with BCC risk. In the average- to low-risk group, there was no association with either NSAIDs or aspirin and BCC or SCC occurrence.
The authors noted limitations of their study, including its reliance on self-reported NSAID use and a lack of detail in regard to usage dose and duration. In addition, though the investigators controlled for all likely confounders, “the possibility of some residual confounding cannot be excluded.”
The QSkin Study was funded by a grant from the National Health and Medical Research Council of Australia (NHMRC). The authors declared no conflicts of interest.
SOURCE: Pandeya N et al. Br J Dermatol. 2019 Mar 28. doi: 10.1111/bjd.17938.
Use of aspirin or nonaspirin NSAIDs was not associated with a reduced risk of basal cell carcinoma (BCC) or squamous cell carcinoma (SCC), in a large, prospective cohort study of Australian residents.
“Overall, we observed weak and inconsistent inverse associations between use of these medications and incidence of either BCC or SCC,” wrote Nirmala Pandeya, PhD, of the University of Queensland (Australia) and coauthors. “While we did observe a modest reduction in use,” they added. The study was published in the British Journal of Dermatology.
While reviews of observational studies have suggested that NSAIDs may have “a potential benefit” in reducing the incidence of BCC and SCC, the results have varied, they noted.
To investigate the potential chemopreventive effects of NSAID use on skin cancer, the investigators used data from the QSkin Sun and Health Study, a prospective cohort of 43,764 residents of Queensland, Australia. Those eligible for the study had a white ethnic background and no history of melanoma; 34,630 participants were available for analysis, their median age was 57 years, and 55% were women
Almost 15,600 (45%) were classified as “high risk” because they had had at least one skin cancer excision or more than five actinic lesions treated; 18,828 participants were classified as “average to low risk;” and data were unavailable for 206 participants. One‐third of the participants in the high-risk group (5,398) used aspirin; of these individuals, 39% (2,132) used aspirin more than once a week (defined as “frequent” users). Also, 60% (9,236) used NSAIDs, and of those, 24% (2,229) were frequent users.
During a median follow-up of 3 years, 3,421 of those in the study (10%) developed one or more BCC, and 1,470 (4%) developed one or more SCC.
Compared with never users, frequent NSAID use in the high-risk group was modestly associated with a reduced risk of BCC (hazard ratio, 0.84; 95% confidence interval, 0.71-0.99), but not with SCC. Aspirin use was weakly associated with a reduced risk of SCC (HR, 0.77; 95% CI, 0.64-0.93) but only among infrequent users and was not associated with BCC risk. In the average- to low-risk group, there was no association with either NSAIDs or aspirin and BCC or SCC occurrence.
The authors noted limitations of their study, including its reliance on self-reported NSAID use and a lack of detail in regard to usage dose and duration. In addition, though the investigators controlled for all likely confounders, “the possibility of some residual confounding cannot be excluded.”
The QSkin Study was funded by a grant from the National Health and Medical Research Council of Australia (NHMRC). The authors declared no conflicts of interest.
SOURCE: Pandeya N et al. Br J Dermatol. 2019 Mar 28. doi: 10.1111/bjd.17938.
Use of aspirin or nonaspirin NSAIDs was not associated with a reduced risk of basal cell carcinoma (BCC) or squamous cell carcinoma (SCC), in a large, prospective cohort study of Australian residents.
“Overall, we observed weak and inconsistent inverse associations between use of these medications and incidence of either BCC or SCC,” wrote Nirmala Pandeya, PhD, of the University of Queensland (Australia) and coauthors. “While we did observe a modest reduction in use,” they added. The study was published in the British Journal of Dermatology.
While reviews of observational studies have suggested that NSAIDs may have “a potential benefit” in reducing the incidence of BCC and SCC, the results have varied, they noted.
To investigate the potential chemopreventive effects of NSAID use on skin cancer, the investigators used data from the QSkin Sun and Health Study, a prospective cohort of 43,764 residents of Queensland, Australia. Those eligible for the study had a white ethnic background and no history of melanoma; 34,630 participants were available for analysis, their median age was 57 years, and 55% were women
Almost 15,600 (45%) were classified as “high risk” because they had had at least one skin cancer excision or more than five actinic lesions treated; 18,828 participants were classified as “average to low risk;” and data were unavailable for 206 participants. One‐third of the participants in the high-risk group (5,398) used aspirin; of these individuals, 39% (2,132) used aspirin more than once a week (defined as “frequent” users). Also, 60% (9,236) used NSAIDs, and of those, 24% (2,229) were frequent users.
During a median follow-up of 3 years, 3,421 of those in the study (10%) developed one or more BCC, and 1,470 (4%) developed one or more SCC.
Compared with never users, frequent NSAID use in the high-risk group was modestly associated with a reduced risk of BCC (hazard ratio, 0.84; 95% confidence interval, 0.71-0.99), but not with SCC. Aspirin use was weakly associated with a reduced risk of SCC (HR, 0.77; 95% CI, 0.64-0.93) but only among infrequent users and was not associated with BCC risk. In the average- to low-risk group, there was no association with either NSAIDs or aspirin and BCC or SCC occurrence.
The authors noted limitations of their study, including its reliance on self-reported NSAID use and a lack of detail in regard to usage dose and duration. In addition, though the investigators controlled for all likely confounders, “the possibility of some residual confounding cannot be excluded.”
The QSkin Study was funded by a grant from the National Health and Medical Research Council of Australia (NHMRC). The authors declared no conflicts of interest.
SOURCE: Pandeya N et al. Br J Dermatol. 2019 Mar 28. doi: 10.1111/bjd.17938.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Molecular profiling shows promise for treating unusual skin rashes
CHICAGO – Although at a relatively early stage of research, according to research that was described at the annual meeting of the Society for Investigative Dermatology.
“We now have several cases that suggest single-cell molecular profiling can provide treatment guidance for atypical rashes, providing an opportunity for a rational treatment choice rather than just improvising in a difficult population,” reported Raymond Cho, MD, PhD, of the department of dermatology at the University of California, San Francisco.
Based on a growing cohort of patients with atypical rashes, one goal is to develop “a library of molecular fingerprints” for classifying rashes that are atypical when defined by morphology, histopathology, or therapeutic response, according to Dr. Cho.
“The big focus now is on expanding this patient cohort. We want to move from anecdotal cases to a larger patient population with which we can statistically prove that we can nominate the best first-line therapy through this approach,” he explained.
In describing work he is performing in collaboration with Jeffrey Cheng, MD, also with the department of dermatology at UCSF, Dr. Cho said the profiles are based on RNA sequencing from single immune cells and epitope measurements. Work already performed in rashes of known etiology supports the approach. For example, the profile for atopic dermatitis includes elevated expression of interleukin (IL)-4 and IL-13, whereas that of psoriasis includes elevated expression of IL-17, which fit with the expected molecular signatures of these diseases.
To be considered for inclusion in the cohort of atypical rashes, patients are required to have an idiopathic skin lesion of at least 6 months’ duration with at least two atypical features defined by such characteristics as morphology or location. Many of these patients have already consulted with multiple providers, have undergone multiple biopsies without a diagnosis, and have failed common treatments, such as steroids.
Examples selected from this cohort have already supported the premise that molecular profiles are relevant to treatment choice. Dr. Cho described one patient with unremitting generalized pruritus and another with nodular lesions on the legs. Both had symptoms of long duration that had failed multiple treatments.
In both cases, immune cell profiling identified lesions high in IL-13 expression. Both achieved complete or near complete resolution of their rash and symptoms when treated with dupilumab, a biologic that targets the IL-13 pathway. In one patient with a large symptom burden, Dr. Cho described the response as “remarkable.”
There are more than 40 patients in the expanding cohort, according to Dr. Cho, who emphasized that this work is timely because of “the armamentarium of immunomodulatory drugs that are coming on line.” He said this type of drug development in dermatology is the basis for a potential paradigm shift.
“Personalized therapy has been used in clinical oncology for almost 10 years now, but this is an approach that needs to find a home in our specialty as well,” Dr. Cho said. He cited data suggesting that nearly 15% of rashes are atypical and represent a major source of frustration to both patients and clinicians when conventional treatments fail.
Asked about cost, he acknowledged that the molecular profiling that he and Dr. Cheng are performing is expensive at the current time, but “we are hopeful that we can find cheaper markers and technologies” to bring this cost down. However, he noted that undiagnosed rashes consume a great deal of time of effort from clinicians while generating significant morbidity for patients, which is justifying novel strategies to find effective therapies.
“These are not happy patients,” Dr. Cho said. Although there are technical challenges for building a molecular library that has practical utility across the substantial heterogeneity of idiopathic rashes, he suggested that a larger patient sample is considered one of the important steps toward overcoming hurdles.
Dr. Cho reports no potential conflicts of interest.
CHICAGO – Although at a relatively early stage of research, according to research that was described at the annual meeting of the Society for Investigative Dermatology.
“We now have several cases that suggest single-cell molecular profiling can provide treatment guidance for atypical rashes, providing an opportunity for a rational treatment choice rather than just improvising in a difficult population,” reported Raymond Cho, MD, PhD, of the department of dermatology at the University of California, San Francisco.
Based on a growing cohort of patients with atypical rashes, one goal is to develop “a library of molecular fingerprints” for classifying rashes that are atypical when defined by morphology, histopathology, or therapeutic response, according to Dr. Cho.
“The big focus now is on expanding this patient cohort. We want to move from anecdotal cases to a larger patient population with which we can statistically prove that we can nominate the best first-line therapy through this approach,” he explained.
In describing work he is performing in collaboration with Jeffrey Cheng, MD, also with the department of dermatology at UCSF, Dr. Cho said the profiles are based on RNA sequencing from single immune cells and epitope measurements. Work already performed in rashes of known etiology supports the approach. For example, the profile for atopic dermatitis includes elevated expression of interleukin (IL)-4 and IL-13, whereas that of psoriasis includes elevated expression of IL-17, which fit with the expected molecular signatures of these diseases.
To be considered for inclusion in the cohort of atypical rashes, patients are required to have an idiopathic skin lesion of at least 6 months’ duration with at least two atypical features defined by such characteristics as morphology or location. Many of these patients have already consulted with multiple providers, have undergone multiple biopsies without a diagnosis, and have failed common treatments, such as steroids.
Examples selected from this cohort have already supported the premise that molecular profiles are relevant to treatment choice. Dr. Cho described one patient with unremitting generalized pruritus and another with nodular lesions on the legs. Both had symptoms of long duration that had failed multiple treatments.
In both cases, immune cell profiling identified lesions high in IL-13 expression. Both achieved complete or near complete resolution of their rash and symptoms when treated with dupilumab, a biologic that targets the IL-13 pathway. In one patient with a large symptom burden, Dr. Cho described the response as “remarkable.”
There are more than 40 patients in the expanding cohort, according to Dr. Cho, who emphasized that this work is timely because of “the armamentarium of immunomodulatory drugs that are coming on line.” He said this type of drug development in dermatology is the basis for a potential paradigm shift.
“Personalized therapy has been used in clinical oncology for almost 10 years now, but this is an approach that needs to find a home in our specialty as well,” Dr. Cho said. He cited data suggesting that nearly 15% of rashes are atypical and represent a major source of frustration to both patients and clinicians when conventional treatments fail.
Asked about cost, he acknowledged that the molecular profiling that he and Dr. Cheng are performing is expensive at the current time, but “we are hopeful that we can find cheaper markers and technologies” to bring this cost down. However, he noted that undiagnosed rashes consume a great deal of time of effort from clinicians while generating significant morbidity for patients, which is justifying novel strategies to find effective therapies.
“These are not happy patients,” Dr. Cho said. Although there are technical challenges for building a molecular library that has practical utility across the substantial heterogeneity of idiopathic rashes, he suggested that a larger patient sample is considered one of the important steps toward overcoming hurdles.
Dr. Cho reports no potential conflicts of interest.
CHICAGO – Although at a relatively early stage of research, according to research that was described at the annual meeting of the Society for Investigative Dermatology.
“We now have several cases that suggest single-cell molecular profiling can provide treatment guidance for atypical rashes, providing an opportunity for a rational treatment choice rather than just improvising in a difficult population,” reported Raymond Cho, MD, PhD, of the department of dermatology at the University of California, San Francisco.
Based on a growing cohort of patients with atypical rashes, one goal is to develop “a library of molecular fingerprints” for classifying rashes that are atypical when defined by morphology, histopathology, or therapeutic response, according to Dr. Cho.
“The big focus now is on expanding this patient cohort. We want to move from anecdotal cases to a larger patient population with which we can statistically prove that we can nominate the best first-line therapy through this approach,” he explained.
In describing work he is performing in collaboration with Jeffrey Cheng, MD, also with the department of dermatology at UCSF, Dr. Cho said the profiles are based on RNA sequencing from single immune cells and epitope measurements. Work already performed in rashes of known etiology supports the approach. For example, the profile for atopic dermatitis includes elevated expression of interleukin (IL)-4 and IL-13, whereas that of psoriasis includes elevated expression of IL-17, which fit with the expected molecular signatures of these diseases.
To be considered for inclusion in the cohort of atypical rashes, patients are required to have an idiopathic skin lesion of at least 6 months’ duration with at least two atypical features defined by such characteristics as morphology or location. Many of these patients have already consulted with multiple providers, have undergone multiple biopsies without a diagnosis, and have failed common treatments, such as steroids.
Examples selected from this cohort have already supported the premise that molecular profiles are relevant to treatment choice. Dr. Cho described one patient with unremitting generalized pruritus and another with nodular lesions on the legs. Both had symptoms of long duration that had failed multiple treatments.
In both cases, immune cell profiling identified lesions high in IL-13 expression. Both achieved complete or near complete resolution of their rash and symptoms when treated with dupilumab, a biologic that targets the IL-13 pathway. In one patient with a large symptom burden, Dr. Cho described the response as “remarkable.”
There are more than 40 patients in the expanding cohort, according to Dr. Cho, who emphasized that this work is timely because of “the armamentarium of immunomodulatory drugs that are coming on line.” He said this type of drug development in dermatology is the basis for a potential paradigm shift.
“Personalized therapy has been used in clinical oncology for almost 10 years now, but this is an approach that needs to find a home in our specialty as well,” Dr. Cho said. He cited data suggesting that nearly 15% of rashes are atypical and represent a major source of frustration to both patients and clinicians when conventional treatments fail.
Asked about cost, he acknowledged that the molecular profiling that he and Dr. Cheng are performing is expensive at the current time, but “we are hopeful that we can find cheaper markers and technologies” to bring this cost down. However, he noted that undiagnosed rashes consume a great deal of time of effort from clinicians while generating significant morbidity for patients, which is justifying novel strategies to find effective therapies.
“These are not happy patients,” Dr. Cho said. Although there are technical challenges for building a molecular library that has practical utility across the substantial heterogeneity of idiopathic rashes, he suggested that a larger patient sample is considered one of the important steps toward overcoming hurdles.
Dr. Cho reports no potential conflicts of interest.
EXPERT ANALYSIS FROM SID 2019
Flu Virus May Have an Achilles Heel
The flu virus uses a hemagglutinin (HA) protein to enter and infect cells. The “head” of the protein was thought to be safe from antibody attacks.
Turns out, it has a previously unsuspected chink in its armor. And researchers from National Institute of Allergy and Infectious Diseases may have found an “unexpected new target” for antiflu therapies. They discovered a naturally occurring human antibody (FluA-20) that—to their surprise—binds to the head of the HA protein at a site that was not thought to be vulnerable.
Using FluA-20 isolated from a patient who had received many influenza immunizations, the researchers showed that FluA-20 “reaches into” an otherwise inaccessible part of the HA trimer molecule and “rapidly disrupts” its integrity. In other words, FluA-20 causes it to fall apart, preventing the spread of virus.
Although the researchers also discovered that the window of opportunity is narrow (the region is only briefly exposed to antibody attack), unlike the rest of HA’s head, the open-access region varies little among influenza strains. The critical HA residues recognized by FluA-20, the researchers say, remain conserved across most subtypes of influenza A virus, which explains the antibody’s “extraordinary breadth.” In mouse studies, when used as prophylaxis or therapy, it protected against H1N1, N3N2, H5N1, and H7N9 subtypes.
In theory, the researchers say, direct strikes with antibody-based therapeutics against that part of the HA protein could be effective with many strains of influenza A virus, and—also theoretically—other influenza strains.
The flu virus uses a hemagglutinin (HA) protein to enter and infect cells. The “head” of the protein was thought to be safe from antibody attacks.
Turns out, it has a previously unsuspected chink in its armor. And researchers from National Institute of Allergy and Infectious Diseases may have found an “unexpected new target” for antiflu therapies. They discovered a naturally occurring human antibody (FluA-20) that—to their surprise—binds to the head of the HA protein at a site that was not thought to be vulnerable.
Using FluA-20 isolated from a patient who had received many influenza immunizations, the researchers showed that FluA-20 “reaches into” an otherwise inaccessible part of the HA trimer molecule and “rapidly disrupts” its integrity. In other words, FluA-20 causes it to fall apart, preventing the spread of virus.
Although the researchers also discovered that the window of opportunity is narrow (the region is only briefly exposed to antibody attack), unlike the rest of HA’s head, the open-access region varies little among influenza strains. The critical HA residues recognized by FluA-20, the researchers say, remain conserved across most subtypes of influenza A virus, which explains the antibody’s “extraordinary breadth.” In mouse studies, when used as prophylaxis or therapy, it protected against H1N1, N3N2, H5N1, and H7N9 subtypes.
In theory, the researchers say, direct strikes with antibody-based therapeutics against that part of the HA protein could be effective with many strains of influenza A virus, and—also theoretically—other influenza strains.
The flu virus uses a hemagglutinin (HA) protein to enter and infect cells. The “head” of the protein was thought to be safe from antibody attacks.
Turns out, it has a previously unsuspected chink in its armor. And researchers from National Institute of Allergy and Infectious Diseases may have found an “unexpected new target” for antiflu therapies. They discovered a naturally occurring human antibody (FluA-20) that—to their surprise—binds to the head of the HA protein at a site that was not thought to be vulnerable.
Using FluA-20 isolated from a patient who had received many influenza immunizations, the researchers showed that FluA-20 “reaches into” an otherwise inaccessible part of the HA trimer molecule and “rapidly disrupts” its integrity. In other words, FluA-20 causes it to fall apart, preventing the spread of virus.
Although the researchers also discovered that the window of opportunity is narrow (the region is only briefly exposed to antibody attack), unlike the rest of HA’s head, the open-access region varies little among influenza strains. The critical HA residues recognized by FluA-20, the researchers say, remain conserved across most subtypes of influenza A virus, which explains the antibody’s “extraordinary breadth.” In mouse studies, when used as prophylaxis or therapy, it protected against H1N1, N3N2, H5N1, and H7N9 subtypes.
In theory, the researchers say, direct strikes with antibody-based therapeutics against that part of the HA protein could be effective with many strains of influenza A virus, and—also theoretically—other influenza strains.
When adolescents visit the ED, 10% leave with an opioid
although there was a small but significant decrease in prescriptions over that time, according to an analysis of two nationwide ambulatory care surveys.
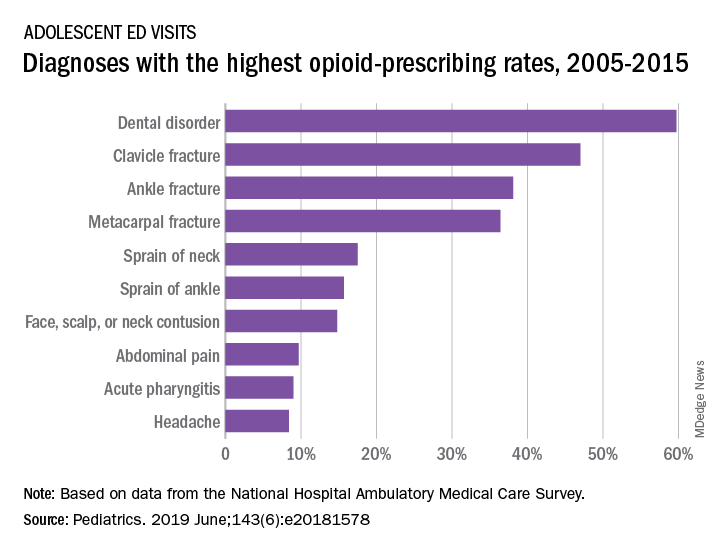
For adolescents aged 13-17 years, 10.4% of ED visits were associated with a prescription for an opioid versus 1.6% among outpatient visits. There was a slight but significant decrease in the rate of opioid prescriptions in the ED setting over the study period, with an odds ratio of 0.95 (95% confidence interval, 0.92-0.97), but there was no significant change in the trend over time in the outpatient setting (OR, 1.02; 95% CI, 0.99-1.09), Joel D. Hudgins, MD, and associates reported in Pediatrics.
“Opioid prescribing in ambulatory care visits is particularly high in the ED setting and … certain diagnoses appear to be routinely treated with an opioid,” said Dr. Hudgins and associates from Boston Children’s Hospital.
The highest rates of opioid prescribing among adolescents visiting the ED involved dental disorders (60%) and acute injuries such as fractures of the clavicle (47%), ankle (38%), and metacarpals (36%). “However, when considering the total volume of opioid prescriptions dispensed [over 7.8 million during 2005-2015], certain common conditions, including abdominal pain, acute pharyngitis, urinary tract infection, and headache, contributed large numbers of prescriptions as well,” they added.
The study involved data from the National Hospital Ambulatory Medical Care Survey (hospital-based EDs) and the National Ambulatory Medical Care Survey (office-based practices), which both are conducted annually by the National Center for Health Statistics.
The senior investigator is supported by an award from the Burroughs Wellcome Fund by the Harvard-MIT Center for Regulatory Science. The authors said that they have no relevant financial relationships.
SOURCE: Hudgins JD et al. Pediatrics. 2019 June. doi: 10.1542/peds.2018-1578.
although there was a small but significant decrease in prescriptions over that time, according to an analysis of two nationwide ambulatory care surveys.

For adolescents aged 13-17 years, 10.4% of ED visits were associated with a prescription for an opioid versus 1.6% among outpatient visits. There was a slight but significant decrease in the rate of opioid prescriptions in the ED setting over the study period, with an odds ratio of 0.95 (95% confidence interval, 0.92-0.97), but there was no significant change in the trend over time in the outpatient setting (OR, 1.02; 95% CI, 0.99-1.09), Joel D. Hudgins, MD, and associates reported in Pediatrics.
“Opioid prescribing in ambulatory care visits is particularly high in the ED setting and … certain diagnoses appear to be routinely treated with an opioid,” said Dr. Hudgins and associates from Boston Children’s Hospital.
The highest rates of opioid prescribing among adolescents visiting the ED involved dental disorders (60%) and acute injuries such as fractures of the clavicle (47%), ankle (38%), and metacarpals (36%). “However, when considering the total volume of opioid prescriptions dispensed [over 7.8 million during 2005-2015], certain common conditions, including abdominal pain, acute pharyngitis, urinary tract infection, and headache, contributed large numbers of prescriptions as well,” they added.
The study involved data from the National Hospital Ambulatory Medical Care Survey (hospital-based EDs) and the National Ambulatory Medical Care Survey (office-based practices), which both are conducted annually by the National Center for Health Statistics.
The senior investigator is supported by an award from the Burroughs Wellcome Fund by the Harvard-MIT Center for Regulatory Science. The authors said that they have no relevant financial relationships.
SOURCE: Hudgins JD et al. Pediatrics. 2019 June. doi: 10.1542/peds.2018-1578.
although there was a small but significant decrease in prescriptions over that time, according to an analysis of two nationwide ambulatory care surveys.

For adolescents aged 13-17 years, 10.4% of ED visits were associated with a prescription for an opioid versus 1.6% among outpatient visits. There was a slight but significant decrease in the rate of opioid prescriptions in the ED setting over the study period, with an odds ratio of 0.95 (95% confidence interval, 0.92-0.97), but there was no significant change in the trend over time in the outpatient setting (OR, 1.02; 95% CI, 0.99-1.09), Joel D. Hudgins, MD, and associates reported in Pediatrics.
“Opioid prescribing in ambulatory care visits is particularly high in the ED setting and … certain diagnoses appear to be routinely treated with an opioid,” said Dr. Hudgins and associates from Boston Children’s Hospital.
The highest rates of opioid prescribing among adolescents visiting the ED involved dental disorders (60%) and acute injuries such as fractures of the clavicle (47%), ankle (38%), and metacarpals (36%). “However, when considering the total volume of opioid prescriptions dispensed [over 7.8 million during 2005-2015], certain common conditions, including abdominal pain, acute pharyngitis, urinary tract infection, and headache, contributed large numbers of prescriptions as well,” they added.
The study involved data from the National Hospital Ambulatory Medical Care Survey (hospital-based EDs) and the National Ambulatory Medical Care Survey (office-based practices), which both are conducted annually by the National Center for Health Statistics.
The senior investigator is supported by an award from the Burroughs Wellcome Fund by the Harvard-MIT Center for Regulatory Science. The authors said that they have no relevant financial relationships.
SOURCE: Hudgins JD et al. Pediatrics. 2019 June. doi: 10.1542/peds.2018-1578.
FROM PEDIATRICS
Social Media and Suicide
The potential harms of excessive Internet use are serious enough for the medical community to debate whether it should be included as a disorder associated with addiction. One question is Where do you draw the line between excessive use—considered nonpathologic behavior—and addiction?
Researchers surveyed 374 university students about social network habits, testing for obsession, lack of personal control, and excessive use. The questionnaire included questions such as “I feel a great need to stay connected to social media” and “I feel anxious when I cannot connect to social media.” The researchers also used a questionnaire about suicidal ideation.
More than half the students reported that WhatsApp is their most important social network, followed by Facebook. The respondents used social media for an average of nearly 7 hours a day. They used social media mainly for contact with friends, entertainment, conversing with a partner, maintaining contact with colleagues for academic matters, and contact with family.
The researchers divided the participants into 3 groups, based on their risk of addiction. The majority were considered “moderate risk.” Approximately 10% were considered “high risk.” The high-risk students spent roughly 11 hours a day on social media compared with the low-risk students who spent about 4 hours. Greater risk also implied more depressive symptoms, more mobile use, and less positive suicidal ideation.
Almost 4 in 10 students had thoughts and wishes about their death at least once in the 2 weeks before the survey. Interestingly, however, the researchers found no relationship between suicidal ideation and addictive behavior. But adding depression did make a difference. Unlike excessive use, addictive behavior was significantly related to depression and suicidal ideation.
The researchers cite other studies that have found addiction to social networks predicts depression and can worsen symptoms. But they also say their findings confirm other research that suggests social media communication can be protective for people who have suicidal thoughts. What looks like addiction may be “an act of escape” from unpleasant thoughts and feelings. Social media, they say, can be a “refuge.”
The potential harms of excessive Internet use are serious enough for the medical community to debate whether it should be included as a disorder associated with addiction. One question is Where do you draw the line between excessive use—considered nonpathologic behavior—and addiction?
Researchers surveyed 374 university students about social network habits, testing for obsession, lack of personal control, and excessive use. The questionnaire included questions such as “I feel a great need to stay connected to social media” and “I feel anxious when I cannot connect to social media.” The researchers also used a questionnaire about suicidal ideation.
More than half the students reported that WhatsApp is their most important social network, followed by Facebook. The respondents used social media for an average of nearly 7 hours a day. They used social media mainly for contact with friends, entertainment, conversing with a partner, maintaining contact with colleagues for academic matters, and contact with family.
The researchers divided the participants into 3 groups, based on their risk of addiction. The majority were considered “moderate risk.” Approximately 10% were considered “high risk.” The high-risk students spent roughly 11 hours a day on social media compared with the low-risk students who spent about 4 hours. Greater risk also implied more depressive symptoms, more mobile use, and less positive suicidal ideation.
Almost 4 in 10 students had thoughts and wishes about their death at least once in the 2 weeks before the survey. Interestingly, however, the researchers found no relationship between suicidal ideation and addictive behavior. But adding depression did make a difference. Unlike excessive use, addictive behavior was significantly related to depression and suicidal ideation.
The researchers cite other studies that have found addiction to social networks predicts depression and can worsen symptoms. But they also say their findings confirm other research that suggests social media communication can be protective for people who have suicidal thoughts. What looks like addiction may be “an act of escape” from unpleasant thoughts and feelings. Social media, they say, can be a “refuge.”
The potential harms of excessive Internet use are serious enough for the medical community to debate whether it should be included as a disorder associated with addiction. One question is Where do you draw the line between excessive use—considered nonpathologic behavior—and addiction?
Researchers surveyed 374 university students about social network habits, testing for obsession, lack of personal control, and excessive use. The questionnaire included questions such as “I feel a great need to stay connected to social media” and “I feel anxious when I cannot connect to social media.” The researchers also used a questionnaire about suicidal ideation.
More than half the students reported that WhatsApp is their most important social network, followed by Facebook. The respondents used social media for an average of nearly 7 hours a day. They used social media mainly for contact with friends, entertainment, conversing with a partner, maintaining contact with colleagues for academic matters, and contact with family.
The researchers divided the participants into 3 groups, based on their risk of addiction. The majority were considered “moderate risk.” Approximately 10% were considered “high risk.” The high-risk students spent roughly 11 hours a day on social media compared with the low-risk students who spent about 4 hours. Greater risk also implied more depressive symptoms, more mobile use, and less positive suicidal ideation.
Almost 4 in 10 students had thoughts and wishes about their death at least once in the 2 weeks before the survey. Interestingly, however, the researchers found no relationship between suicidal ideation and addictive behavior. But adding depression did make a difference. Unlike excessive use, addictive behavior was significantly related to depression and suicidal ideation.
The researchers cite other studies that have found addiction to social networks predicts depression and can worsen symptoms. But they also say their findings confirm other research that suggests social media communication can be protective for people who have suicidal thoughts. What looks like addiction may be “an act of escape” from unpleasant thoughts and feelings. Social media, they say, can be a “refuge.”







