User login
Psychosis as a common thread across psychiatric disorders
Ask a psychiatrist to name a psychotic disorder, and the answer will most likely be “schizophrenia.” But if you closely examine the symptom structure of DSM-5 psychiatric disorders, you will note the presence of psychosis in almost all of them.
Fixed false beliefs and impaired reality testing are core features of psychosis. Those are certainly prominent in severe psychoses such as schizophrenia, schizoaffective disorder, or delusional disorder. But psychosis is actually a continuum of varying severity across most psychiatric disorders, although they carry different diagnostic labels. Irrational false beliefs and impaired functioning due to poor reality testing are embedded among many DSM-5 disorders. Hallucinations are less common; they are perceptual aberrations, not thought abnormalities, although they can trigger delusional explanations as to their causation.
Consider the following:
- Bipolar disorder. A large proportion of patients with bipolar disorder manifest delusions, usually grandiose, but often paranoid or referential.
- Major depressive disorder (MDD). Although regarded as a “pure mood disorder,” the core symptoms of MDD—self-deprecation and sense of worthlessness—as well as the poor reality testing of suicidal thoughts (that death is a better option than living) are psychotic false beliefs.
- Anxiety and panic disorder. The central symptom in anxiety and panic attacks is a belief in impending doom and/or death. The fear in anxiety disorders is actually based on a false belief (eg, if I get on the plane, it will crash, and I will die). Thus, technically an irrational/psychotic thought process underpins the terror and fear of anxiety disorders.
- Borderline personality disorder. Frank psychotic symptoms, such as paranoid beliefs, are known to be a component of borderline personality disorder symptoms. Although these symptoms tend to be brief and episodic, they can have a deleterious effect on the person’s coping and relationships.
- Other personality disorders. While many individuals with narcissistic personality disorder are functional, their exaggerated sense of self-importance, entitlement, and self-aggrandizement certainly qualifies as a fixed false belief. Patients with other personality disorders, such as schizotypal and paranoid, are known to harbor false beliefs or magical thinking.
- Body dysmorphic disorder. False beliefs about one’s appearance (such as blemishes or asymmetry) are at the center of this disorder, and it meets the litmus test of a psychosis.
- Anorexia nervosa. This disorder is well known to be characterized by a fixed false belief that one is “fat,” even when the patient’s body borders on being cachectic in appearance according to objective observers.
- Autism. This spectrum of diseases includes false beliefs that drive the ritualistic or odd behaviors.
- Obsessive-compulsive disorder. Although obsessions are usually ego-dystonic, in severe cases, they become ego-syntonic, similar to delusions. On the other hand, compulsions are often driven by a false belief, such as believing that one’s hands are dirty and must be washed incessantly, or that the locks on the door must be rechecked repeatedly because an intruder may break into the house and harm the inhabitants.
- Neurodegenerative syndromes. Neurodegenerative syndromes are neuropsychiatric disorders that very frequently include psychotic symptoms, such as paranoid delusions, delusions of marital infidelity, Capgras syndrome, or folie à deux. These disorders include Alzheimer’s disease, Parkinson’s disease, Lewy body dementia, frontal temporal dementia, metachromatic leukodystrophy, Huntington’s chorea, temporal lobe epilepsy, stroke, xenomelia, reduplicative phenomena, etc. This reflects the common emergence of faulty thinking with disintegration of neural tissue, both gray and white matter.
Continue to: So it should not be...
So it should not be surprising that antipsychotic medications, especially second-generation agents, have been shown to be helpful as monotherapy or adjunctive therapy in practically all the above psychiatric disorders, whether on-label or off-label.
Finally, it should also be noted that a case has been made for the existence of one dimension in all mental disorders manifesting in multiple psychopathologies.1 It is possible that a continuum of delusional thinking is a common thread across many psychiatric disorders due to this putative shared dimension. The milder form of this dimension may also explain the presence of pre-psychotic thinking in a significant proportion of the general population who do not seek psychiatric help.2 Just think of how many people you befriend, socialize with, and regard as perfectly “normal” endorse wild superstitions and astrological predictions, or believe in various conspiracy theories that have no basis in reality.
To comment on this editorial or other topics of interest: [email protected].
1. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
2. van Os J, Linscott RJ, Myin-Germeys I, et al. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39(2):179-195.
Ask a psychiatrist to name a psychotic disorder, and the answer will most likely be “schizophrenia.” But if you closely examine the symptom structure of DSM-5 psychiatric disorders, you will note the presence of psychosis in almost all of them.
Fixed false beliefs and impaired reality testing are core features of psychosis. Those are certainly prominent in severe psychoses such as schizophrenia, schizoaffective disorder, or delusional disorder. But psychosis is actually a continuum of varying severity across most psychiatric disorders, although they carry different diagnostic labels. Irrational false beliefs and impaired functioning due to poor reality testing are embedded among many DSM-5 disorders. Hallucinations are less common; they are perceptual aberrations, not thought abnormalities, although they can trigger delusional explanations as to their causation.
Consider the following:
- Bipolar disorder. A large proportion of patients with bipolar disorder manifest delusions, usually grandiose, but often paranoid or referential.
- Major depressive disorder (MDD). Although regarded as a “pure mood disorder,” the core symptoms of MDD—self-deprecation and sense of worthlessness—as well as the poor reality testing of suicidal thoughts (that death is a better option than living) are psychotic false beliefs.
- Anxiety and panic disorder. The central symptom in anxiety and panic attacks is a belief in impending doom and/or death. The fear in anxiety disorders is actually based on a false belief (eg, if I get on the plane, it will crash, and I will die). Thus, technically an irrational/psychotic thought process underpins the terror and fear of anxiety disorders.
- Borderline personality disorder. Frank psychotic symptoms, such as paranoid beliefs, are known to be a component of borderline personality disorder symptoms. Although these symptoms tend to be brief and episodic, they can have a deleterious effect on the person’s coping and relationships.
- Other personality disorders. While many individuals with narcissistic personality disorder are functional, their exaggerated sense of self-importance, entitlement, and self-aggrandizement certainly qualifies as a fixed false belief. Patients with other personality disorders, such as schizotypal and paranoid, are known to harbor false beliefs or magical thinking.
- Body dysmorphic disorder. False beliefs about one’s appearance (such as blemishes or asymmetry) are at the center of this disorder, and it meets the litmus test of a psychosis.
- Anorexia nervosa. This disorder is well known to be characterized by a fixed false belief that one is “fat,” even when the patient’s body borders on being cachectic in appearance according to objective observers.
- Autism. This spectrum of diseases includes false beliefs that drive the ritualistic or odd behaviors.
- Obsessive-compulsive disorder. Although obsessions are usually ego-dystonic, in severe cases, they become ego-syntonic, similar to delusions. On the other hand, compulsions are often driven by a false belief, such as believing that one’s hands are dirty and must be washed incessantly, or that the locks on the door must be rechecked repeatedly because an intruder may break into the house and harm the inhabitants.
- Neurodegenerative syndromes. Neurodegenerative syndromes are neuropsychiatric disorders that very frequently include psychotic symptoms, such as paranoid delusions, delusions of marital infidelity, Capgras syndrome, or folie à deux. These disorders include Alzheimer’s disease, Parkinson’s disease, Lewy body dementia, frontal temporal dementia, metachromatic leukodystrophy, Huntington’s chorea, temporal lobe epilepsy, stroke, xenomelia, reduplicative phenomena, etc. This reflects the common emergence of faulty thinking with disintegration of neural tissue, both gray and white matter.
Continue to: So it should not be...
So it should not be surprising that antipsychotic medications, especially second-generation agents, have been shown to be helpful as monotherapy or adjunctive therapy in practically all the above psychiatric disorders, whether on-label or off-label.
Finally, it should also be noted that a case has been made for the existence of one dimension in all mental disorders manifesting in multiple psychopathologies.1 It is possible that a continuum of delusional thinking is a common thread across many psychiatric disorders due to this putative shared dimension. The milder form of this dimension may also explain the presence of pre-psychotic thinking in a significant proportion of the general population who do not seek psychiatric help.2 Just think of how many people you befriend, socialize with, and regard as perfectly “normal” endorse wild superstitions and astrological predictions, or believe in various conspiracy theories that have no basis in reality.
To comment on this editorial or other topics of interest: [email protected].
Ask a psychiatrist to name a psychotic disorder, and the answer will most likely be “schizophrenia.” But if you closely examine the symptom structure of DSM-5 psychiatric disorders, you will note the presence of psychosis in almost all of them.
Fixed false beliefs and impaired reality testing are core features of psychosis. Those are certainly prominent in severe psychoses such as schizophrenia, schizoaffective disorder, or delusional disorder. But psychosis is actually a continuum of varying severity across most psychiatric disorders, although they carry different diagnostic labels. Irrational false beliefs and impaired functioning due to poor reality testing are embedded among many DSM-5 disorders. Hallucinations are less common; they are perceptual aberrations, not thought abnormalities, although they can trigger delusional explanations as to their causation.
Consider the following:
- Bipolar disorder. A large proportion of patients with bipolar disorder manifest delusions, usually grandiose, but often paranoid or referential.
- Major depressive disorder (MDD). Although regarded as a “pure mood disorder,” the core symptoms of MDD—self-deprecation and sense of worthlessness—as well as the poor reality testing of suicidal thoughts (that death is a better option than living) are psychotic false beliefs.
- Anxiety and panic disorder. The central symptom in anxiety and panic attacks is a belief in impending doom and/or death. The fear in anxiety disorders is actually based on a false belief (eg, if I get on the plane, it will crash, and I will die). Thus, technically an irrational/psychotic thought process underpins the terror and fear of anxiety disorders.
- Borderline personality disorder. Frank psychotic symptoms, such as paranoid beliefs, are known to be a component of borderline personality disorder symptoms. Although these symptoms tend to be brief and episodic, they can have a deleterious effect on the person’s coping and relationships.
- Other personality disorders. While many individuals with narcissistic personality disorder are functional, their exaggerated sense of self-importance, entitlement, and self-aggrandizement certainly qualifies as a fixed false belief. Patients with other personality disorders, such as schizotypal and paranoid, are known to harbor false beliefs or magical thinking.
- Body dysmorphic disorder. False beliefs about one’s appearance (such as blemishes or asymmetry) are at the center of this disorder, and it meets the litmus test of a psychosis.
- Anorexia nervosa. This disorder is well known to be characterized by a fixed false belief that one is “fat,” even when the patient’s body borders on being cachectic in appearance according to objective observers.
- Autism. This spectrum of diseases includes false beliefs that drive the ritualistic or odd behaviors.
- Obsessive-compulsive disorder. Although obsessions are usually ego-dystonic, in severe cases, they become ego-syntonic, similar to delusions. On the other hand, compulsions are often driven by a false belief, such as believing that one’s hands are dirty and must be washed incessantly, or that the locks on the door must be rechecked repeatedly because an intruder may break into the house and harm the inhabitants.
- Neurodegenerative syndromes. Neurodegenerative syndromes are neuropsychiatric disorders that very frequently include psychotic symptoms, such as paranoid delusions, delusions of marital infidelity, Capgras syndrome, or folie à deux. These disorders include Alzheimer’s disease, Parkinson’s disease, Lewy body dementia, frontal temporal dementia, metachromatic leukodystrophy, Huntington’s chorea, temporal lobe epilepsy, stroke, xenomelia, reduplicative phenomena, etc. This reflects the common emergence of faulty thinking with disintegration of neural tissue, both gray and white matter.
Continue to: So it should not be...
So it should not be surprising that antipsychotic medications, especially second-generation agents, have been shown to be helpful as monotherapy or adjunctive therapy in practically all the above psychiatric disorders, whether on-label or off-label.
Finally, it should also be noted that a case has been made for the existence of one dimension in all mental disorders manifesting in multiple psychopathologies.1 It is possible that a continuum of delusional thinking is a common thread across many psychiatric disorders due to this putative shared dimension. The milder form of this dimension may also explain the presence of pre-psychotic thinking in a significant proportion of the general population who do not seek psychiatric help.2 Just think of how many people you befriend, socialize with, and regard as perfectly “normal” endorse wild superstitions and astrological predictions, or believe in various conspiracy theories that have no basis in reality.
To comment on this editorial or other topics of interest: [email protected].
1. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
2. van Os J, Linscott RJ, Myin-Germeys I, et al. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39(2):179-195.
1. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
2. van Os J, Linscott RJ, Myin-Germeys I, et al. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39(2):179-195.
Career Choices: Academic psychiatry
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he or she has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Saeed Ahmed, MD, Chief Resident at Nassau University Medical Center, East Meadow, New York, talked with Donald W. Black, MD, Professor of Psychiatry, Department of Psychiatry, University of Iowa Roy J. and Lucille A. Carver College of Medicine, Iowa City, Iowa. Dr. Black is also Editor-in-Chief of
Dr. Ahmed: What made you choose the academic track, and how did your training lead you towards this path?
Dr. Black: I had always been interested in the idea of working at a medical school, and enjoyed writing and speaking. I was exposed to clinical research as a resident, and that confirmed my interest in academia, because I could envision combining all my interests, along with patient care. I always thought that patients were a major source of ideas for research and writing.
Dr. Ahmed: What are some of the pros and cons of working in academia?
Dr. Black: The pros include being able to influence future physicians through my teaching and writing; being able to pursue important research; and not being isolated from peers. Other advantages are being largely protected from utilization review; having more free time than peers in the private sector, who have difficulty finding coverage; and having defined benefits and a steady salary. I also share call with many peers.
When it comes to the cons, salaries are lower than in the private sector. The cons also include not being my own boss, and sometimes having to bend to the whims of an institution or supervisor.
Continue to: Dr. Ahmed...
Dr. Ahmed: Are you required to conduct research?
Dr. Black: Yes. This is one of the best aspects of my job: being able to make clinical discoveries that I can disseminate through writing and speaking. Over time, this has become increasingly challenging due to the difficulty of obtaining research funding from foundations or the federal government. This has become highly problematic, particularly for clinical researchers, because the National Institutes of Health has clearly been favoring neuroscience.
Dr. Ahmed: What is your typical day like?
Dr. Black: Because of the many hats I wear (or have worn), each day is different from the other. I combine patient care with research, writing, speaking, teaching, and administration. As a tenure-track faculty member, I am expected to write grants, conduct research, and publish. My clinical-track peers primarily provide patient care and teach students and residents.
Dr. Ahmed: What is unique about working in a training institute vs private practice?
Continue to: Dr. Black...
Dr. Black: As an academic psychiatrist, I feel I have the best of both worlds: patient care combined with opportunities my private practice colleagues do not have. Because I have published widely, and have developed a reputation, I am frequently invited to speak at meetings throughout the United States, and sometimes internationally. Travel is a perk of academia, and as someone who loves travel, that is important.
Dr. Ahmed: Where do you see psychiatry going?
Dr. Black: Psychiatry will always be an important specialty because no one else truly cares about patients with psychiatric illnesses. Mental illness will not go away, and society needs highly trained individuals to provide care. There are many “me too” clinicians who now share in caring for patients with psychiatric illnesses, but psychiatrists will always have the most training, and are in a position to provide supervision to others and to direct mental health care teams.
Dr. Ahmed: What advice do you have for residents contemplating a career in academic psychiatry?
Dr. Black: Because most medical schools now have both tenure and clinical tracks, no one needs to feel left out. Those who are interested in scholarly activities will gravitate to the tenure tract, and all that requires in terms of grants and papers, while those who are primarily interested in patient care and teaching will choose the clinical track.
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he or she has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Saeed Ahmed, MD, Chief Resident at Nassau University Medical Center, East Meadow, New York, talked with Donald W. Black, MD, Professor of Psychiatry, Department of Psychiatry, University of Iowa Roy J. and Lucille A. Carver College of Medicine, Iowa City, Iowa. Dr. Black is also Editor-in-Chief of
Dr. Ahmed: What made you choose the academic track, and how did your training lead you towards this path?
Dr. Black: I had always been interested in the idea of working at a medical school, and enjoyed writing and speaking. I was exposed to clinical research as a resident, and that confirmed my interest in academia, because I could envision combining all my interests, along with patient care. I always thought that patients were a major source of ideas for research and writing.
Dr. Ahmed: What are some of the pros and cons of working in academia?
Dr. Black: The pros include being able to influence future physicians through my teaching and writing; being able to pursue important research; and not being isolated from peers. Other advantages are being largely protected from utilization review; having more free time than peers in the private sector, who have difficulty finding coverage; and having defined benefits and a steady salary. I also share call with many peers.
When it comes to the cons, salaries are lower than in the private sector. The cons also include not being my own boss, and sometimes having to bend to the whims of an institution or supervisor.
Continue to: Dr. Ahmed...
Dr. Ahmed: Are you required to conduct research?
Dr. Black: Yes. This is one of the best aspects of my job: being able to make clinical discoveries that I can disseminate through writing and speaking. Over time, this has become increasingly challenging due to the difficulty of obtaining research funding from foundations or the federal government. This has become highly problematic, particularly for clinical researchers, because the National Institutes of Health has clearly been favoring neuroscience.
Dr. Ahmed: What is your typical day like?
Dr. Black: Because of the many hats I wear (or have worn), each day is different from the other. I combine patient care with research, writing, speaking, teaching, and administration. As a tenure-track faculty member, I am expected to write grants, conduct research, and publish. My clinical-track peers primarily provide patient care and teach students and residents.
Dr. Ahmed: What is unique about working in a training institute vs private practice?
Continue to: Dr. Black...
Dr. Black: As an academic psychiatrist, I feel I have the best of both worlds: patient care combined with opportunities my private practice colleagues do not have. Because I have published widely, and have developed a reputation, I am frequently invited to speak at meetings throughout the United States, and sometimes internationally. Travel is a perk of academia, and as someone who loves travel, that is important.
Dr. Ahmed: Where do you see psychiatry going?
Dr. Black: Psychiatry will always be an important specialty because no one else truly cares about patients with psychiatric illnesses. Mental illness will not go away, and society needs highly trained individuals to provide care. There are many “me too” clinicians who now share in caring for patients with psychiatric illnesses, but psychiatrists will always have the most training, and are in a position to provide supervision to others and to direct mental health care teams.
Dr. Ahmed: What advice do you have for residents contemplating a career in academic psychiatry?
Dr. Black: Because most medical schools now have both tenure and clinical tracks, no one needs to feel left out. Those who are interested in scholarly activities will gravitate to the tenure tract, and all that requires in terms of grants and papers, while those who are primarily interested in patient care and teaching will choose the clinical track.
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he or she has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Saeed Ahmed, MD, Chief Resident at Nassau University Medical Center, East Meadow, New York, talked with Donald W. Black, MD, Professor of Psychiatry, Department of Psychiatry, University of Iowa Roy J. and Lucille A. Carver College of Medicine, Iowa City, Iowa. Dr. Black is also Editor-in-Chief of
Dr. Ahmed: What made you choose the academic track, and how did your training lead you towards this path?
Dr. Black: I had always been interested in the idea of working at a medical school, and enjoyed writing and speaking. I was exposed to clinical research as a resident, and that confirmed my interest in academia, because I could envision combining all my interests, along with patient care. I always thought that patients were a major source of ideas for research and writing.
Dr. Ahmed: What are some of the pros and cons of working in academia?
Dr. Black: The pros include being able to influence future physicians through my teaching and writing; being able to pursue important research; and not being isolated from peers. Other advantages are being largely protected from utilization review; having more free time than peers in the private sector, who have difficulty finding coverage; and having defined benefits and a steady salary. I also share call with many peers.
When it comes to the cons, salaries are lower than in the private sector. The cons also include not being my own boss, and sometimes having to bend to the whims of an institution or supervisor.
Continue to: Dr. Ahmed...
Dr. Ahmed: Are you required to conduct research?
Dr. Black: Yes. This is one of the best aspects of my job: being able to make clinical discoveries that I can disseminate through writing and speaking. Over time, this has become increasingly challenging due to the difficulty of obtaining research funding from foundations or the federal government. This has become highly problematic, particularly for clinical researchers, because the National Institutes of Health has clearly been favoring neuroscience.
Dr. Ahmed: What is your typical day like?
Dr. Black: Because of the many hats I wear (or have worn), each day is different from the other. I combine patient care with research, writing, speaking, teaching, and administration. As a tenure-track faculty member, I am expected to write grants, conduct research, and publish. My clinical-track peers primarily provide patient care and teach students and residents.
Dr. Ahmed: What is unique about working in a training institute vs private practice?
Continue to: Dr. Black...
Dr. Black: As an academic psychiatrist, I feel I have the best of both worlds: patient care combined with opportunities my private practice colleagues do not have. Because I have published widely, and have developed a reputation, I am frequently invited to speak at meetings throughout the United States, and sometimes internationally. Travel is a perk of academia, and as someone who loves travel, that is important.
Dr. Ahmed: Where do you see psychiatry going?
Dr. Black: Psychiatry will always be an important specialty because no one else truly cares about patients with psychiatric illnesses. Mental illness will not go away, and society needs highly trained individuals to provide care. There are many “me too” clinicians who now share in caring for patients with psychiatric illnesses, but psychiatrists will always have the most training, and are in a position to provide supervision to others and to direct mental health care teams.
Dr. Ahmed: What advice do you have for residents contemplating a career in academic psychiatry?
Dr. Black: Because most medical schools now have both tenure and clinical tracks, no one needs to feel left out. Those who are interested in scholarly activities will gravitate to the tenure tract, and all that requires in terms of grants and papers, while those who are primarily interested in patient care and teaching will choose the clinical track.
Factors that change our brains; The APA’s stance on neuroimaging
Factors that change our brains
I greatly enjoyed Dr. Nasrallah’s editorial, “Your patient’s brain is different at every visit” (From the Editor,
In reading this editorial, it is clear that a myriad of factors we consider and address with our patients during each visit underly intricate neurobiologic mechanisms and processes that ever deepen our understanding of the brain. In discussing the changes taking place in our patients, I can’t help but wonder what changes are also occurring in our brains (as Dr. Nasrallah noted). What would be the resulting impact of these changes in our next patient interaction and/or subsequent interaction(s) with the same patient? Looking through the editorial’s bullet points, many (if not all) of the factors contributing to brain changes apply equally and naturally to clinicians as well as patients. In this light, the editorial serves not only as a broad guideline for patient psychoeducation but also as a reminder of wellness and well-being for clinicians.
As a “fresh-out-of-training” psychiatrist, I can definitely work on several of the factors, such as diet and exercise. Trainees and residents can be more susceptible to overlook and befall some of these factors and changes, and may already be basing the clinical advice they give to their patients on these same factors and changes. As a child psychiatrist, I value the importance of modeling healthy behaviors for my patients, and their families and with coworkers or colleagues. In accordance with the impact these factors have on our brains, it’s important to emphasize what we can do to further strengthen rapport and therapeutic value through modeling. I strive to model the desired behaviors, attitudes, and dynamics that are the external, observable manifestation or symptomology of what takes place in my brain. To do so, I understand I need to be mindful in proactively managing the contributing factors, such as those listed in Dr. Nasrallah’s editorial. I imagine patients and their families would easily notice if we are in suboptimal physical and/or mental health that results in us not being prompt, fully engaged, or receptive. I believe that attending to these facets during training falls under the umbrella of professionalism. Being a professional in our field often entails practicing what we preach. So, I’m grateful that what we preach is informed by our field’s exciting research, continued advancements, and expertise that benefits our patients and us professionally and personally.
Philip Yen-Tsun Liu, MD
Child and adolescent psychiatrist
innovaTel Telepsychiatry
San Antonio, Texas
Dr. Nasrallah responds
I would like to thank Dr. Liu for his thoughtful response to my editorial. He seems to be very cognizant of the fact that experiential neuroplasticity and brain tissue remodeling occurs in both the patient and physician. I admire his focus on psychoeducation, wellness, and professionalism. He is right that we as psychiatrists (and nurse practitioners) must be role models for our patients in multiple ways, because it may help enhance clinical outcomes and have a positive impact on their brains.
I would also like to point Dr. Liu to the editorial “The most powerful placebo is not a pill” (From the Editor,
Henry A. Nasrallah, MD
Editor-in-Chief
Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
The APA’s stance on neuroimaging
Can anyone in the modern world argue that the brain is irrelevant to psychiatry? Yet surprisingly, in September 2018, the American Psychiatric Association (APA) officially declared that neuroimaging of the brain has no clinical value in psychiatry.1
Unfortunately, the APA focused almost exclusively on functional magnetic resonance imaging (fMRI) and neglected an extensive library of studies of single-photon emission computed tomography (SPECT) and positron emission tomography (PET). The APA’s position on neuroimaging is as follows1,2:
- A neuroimaging finding must have a sensitivity and specificity (S/sp) of no less than 80%.
- The psychiatric imaging literature does not support using neuroimaging in psychiatric diagnostics or treatment.
- Neuroimaging has not had a significant impact on the diagnosis and treatment of psychiatric disorders.
The APA set unrealistic standards for biomarkers in a field that lacks pathologic markers of specific disease entities.3 Moreover, numerous widely used tests fall below the APA’s unrealistic S/sp cutoff, including the Hamilton Depression Rating Scale,4 Zung Depression Scale,5 the clock drawing test,6 and even the chest X-ray.3 Curiously, numerous replicated SPECT and PET studies were not included in the APA’s analysis.1-3 For example, in a study of 196 veterans, posttraumatic stress disorder was distinguished from traumatic brain injury with an S/sp of 0.92/0.85.7,8 Also, fluorodeoxyglucose (FDG)-PET has an S/sp of 0.84/0.74 in differentiating patients with Alzheimer’s disease from controls, while perfusion SPECT, using multi-detector cameras, has an S/sp of 0.93/0.84.3,9 Moreover, both FDG-PET and SPECT can differentiate other forms of dementia from Alzheimer’s disease, yielding an additional benefit compared to amyloid imaging alone.2,9 As President of the International Society of Applied Neuroimaging, I suggest neuroimaging should not be feared. Neuroimaging does not replace the diagnostician; rather, it aids him/her in a complex case.
Theodore A. Henderson, MD, PhD
President
Neuro-Luminance Brain Health Centers, Inc.
Denver, Colorado
Director
The Synaptic Space
Vice President
The Neuro-Laser Foundation
President
International Society of Applied Neuroimaging
Centennial, Colorado
Disclosure
The author has no ownership in, and receives no remuneration from, any neuroimaging company.
References
1. First MB, Drevets WC, Carter C, et al. Clinical applications of neuroimaging in psychiatric disorders. Am J Psychiatry. 2018:175:
2. First MB, Drevets WC, Carter C, et al. Data supplement for Clinical applications of neuroimaging in psychiatric disorders. Am J Psychiatry. 2018;175(suppl).
3. Henderson TA. Brain SPECT imaging in neuropsychiatric diagnosis and monitoring. EPatient. http://nmpangea.com/2018/10/09/738/. Published 2018. Accessed May 31, 2019.
4. Bagby RM, Ryder AG, Schuller DR, et al. The Hamilton Depression Rating Scale: has the gold standard become a lead weight? Am J Psychiatry. 2004;161(12):2163-2177.
5. Biggs JT, Wylie LT, Ziegler VE. Validity of the Zung Self-rating Depression Scale. Br J Psychiatry. 1978;132:381-385.
6. Seigerschmidt E, Mösch E, Siemen M, et al. The clock drawing test and questionable dementia: reliability and validity. Int J Geriatr Psychiatry. 2002;17(11):1048-1054.
7. Raji CA, Willeumier K, Taylor D, et al. Functional neuroimaging with default mode network regions distinguishes PTSD from TBI in a military veteran population. Brain Imaging Behav. 2015;9(3):527-534.
8. Amen DG, Raji CA, Willeumier K, et al. Functional neuroimaging distinguishes posttraumatic stress disorder from traumatic brain injury in focused and large community datasets. PLoS One. 2015;10(7):e0129659. doi: 10.1371/journal.pone.0129659.
9. Henderson TA. The diagnosis and evaluation of dementia and mild cognitive impairment with emphasis on SPECT perfusion neuroimaging. CNS Spectr. 2012;17(4):176-206.
Factors that change our brains
I greatly enjoyed Dr. Nasrallah’s editorial, “Your patient’s brain is different at every visit” (From the Editor,
In reading this editorial, it is clear that a myriad of factors we consider and address with our patients during each visit underly intricate neurobiologic mechanisms and processes that ever deepen our understanding of the brain. In discussing the changes taking place in our patients, I can’t help but wonder what changes are also occurring in our brains (as Dr. Nasrallah noted). What would be the resulting impact of these changes in our next patient interaction and/or subsequent interaction(s) with the same patient? Looking through the editorial’s bullet points, many (if not all) of the factors contributing to brain changes apply equally and naturally to clinicians as well as patients. In this light, the editorial serves not only as a broad guideline for patient psychoeducation but also as a reminder of wellness and well-being for clinicians.
As a “fresh-out-of-training” psychiatrist, I can definitely work on several of the factors, such as diet and exercise. Trainees and residents can be more susceptible to overlook and befall some of these factors and changes, and may already be basing the clinical advice they give to their patients on these same factors and changes. As a child psychiatrist, I value the importance of modeling healthy behaviors for my patients, and their families and with coworkers or colleagues. In accordance with the impact these factors have on our brains, it’s important to emphasize what we can do to further strengthen rapport and therapeutic value through modeling. I strive to model the desired behaviors, attitudes, and dynamics that are the external, observable manifestation or symptomology of what takes place in my brain. To do so, I understand I need to be mindful in proactively managing the contributing factors, such as those listed in Dr. Nasrallah’s editorial. I imagine patients and their families would easily notice if we are in suboptimal physical and/or mental health that results in us not being prompt, fully engaged, or receptive. I believe that attending to these facets during training falls under the umbrella of professionalism. Being a professional in our field often entails practicing what we preach. So, I’m grateful that what we preach is informed by our field’s exciting research, continued advancements, and expertise that benefits our patients and us professionally and personally.
Philip Yen-Tsun Liu, MD
Child and adolescent psychiatrist
innovaTel Telepsychiatry
San Antonio, Texas
Dr. Nasrallah responds
I would like to thank Dr. Liu for his thoughtful response to my editorial. He seems to be very cognizant of the fact that experiential neuroplasticity and brain tissue remodeling occurs in both the patient and physician. I admire his focus on psychoeducation, wellness, and professionalism. He is right that we as psychiatrists (and nurse practitioners) must be role models for our patients in multiple ways, because it may help enhance clinical outcomes and have a positive impact on their brains.
I would also like to point Dr. Liu to the editorial “The most powerful placebo is not a pill” (From the Editor,
Henry A. Nasrallah, MD
Editor-in-Chief
Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
The APA’s stance on neuroimaging
Can anyone in the modern world argue that the brain is irrelevant to psychiatry? Yet surprisingly, in September 2018, the American Psychiatric Association (APA) officially declared that neuroimaging of the brain has no clinical value in psychiatry.1
Unfortunately, the APA focused almost exclusively on functional magnetic resonance imaging (fMRI) and neglected an extensive library of studies of single-photon emission computed tomography (SPECT) and positron emission tomography (PET). The APA’s position on neuroimaging is as follows1,2:
- A neuroimaging finding must have a sensitivity and specificity (S/sp) of no less than 80%.
- The psychiatric imaging literature does not support using neuroimaging in psychiatric diagnostics or treatment.
- Neuroimaging has not had a significant impact on the diagnosis and treatment of psychiatric disorders.
The APA set unrealistic standards for biomarkers in a field that lacks pathologic markers of specific disease entities.3 Moreover, numerous widely used tests fall below the APA’s unrealistic S/sp cutoff, including the Hamilton Depression Rating Scale,4 Zung Depression Scale,5 the clock drawing test,6 and even the chest X-ray.3 Curiously, numerous replicated SPECT and PET studies were not included in the APA’s analysis.1-3 For example, in a study of 196 veterans, posttraumatic stress disorder was distinguished from traumatic brain injury with an S/sp of 0.92/0.85.7,8 Also, fluorodeoxyglucose (FDG)-PET has an S/sp of 0.84/0.74 in differentiating patients with Alzheimer’s disease from controls, while perfusion SPECT, using multi-detector cameras, has an S/sp of 0.93/0.84.3,9 Moreover, both FDG-PET and SPECT can differentiate other forms of dementia from Alzheimer’s disease, yielding an additional benefit compared to amyloid imaging alone.2,9 As President of the International Society of Applied Neuroimaging, I suggest neuroimaging should not be feared. Neuroimaging does not replace the diagnostician; rather, it aids him/her in a complex case.
Theodore A. Henderson, MD, PhD
President
Neuro-Luminance Brain Health Centers, Inc.
Denver, Colorado
Director
The Synaptic Space
Vice President
The Neuro-Laser Foundation
President
International Society of Applied Neuroimaging
Centennial, Colorado
Disclosure
The author has no ownership in, and receives no remuneration from, any neuroimaging company.
References
1. First MB, Drevets WC, Carter C, et al. Clinical applications of neuroimaging in psychiatric disorders. Am J Psychiatry. 2018:175:
2. First MB, Drevets WC, Carter C, et al. Data supplement for Clinical applications of neuroimaging in psychiatric disorders. Am J Psychiatry. 2018;175(suppl).
3. Henderson TA. Brain SPECT imaging in neuropsychiatric diagnosis and monitoring. EPatient. http://nmpangea.com/2018/10/09/738/. Published 2018. Accessed May 31, 2019.
4. Bagby RM, Ryder AG, Schuller DR, et al. The Hamilton Depression Rating Scale: has the gold standard become a lead weight? Am J Psychiatry. 2004;161(12):2163-2177.
5. Biggs JT, Wylie LT, Ziegler VE. Validity of the Zung Self-rating Depression Scale. Br J Psychiatry. 1978;132:381-385.
6. Seigerschmidt E, Mösch E, Siemen M, et al. The clock drawing test and questionable dementia: reliability and validity. Int J Geriatr Psychiatry. 2002;17(11):1048-1054.
7. Raji CA, Willeumier K, Taylor D, et al. Functional neuroimaging with default mode network regions distinguishes PTSD from TBI in a military veteran population. Brain Imaging Behav. 2015;9(3):527-534.
8. Amen DG, Raji CA, Willeumier K, et al. Functional neuroimaging distinguishes posttraumatic stress disorder from traumatic brain injury in focused and large community datasets. PLoS One. 2015;10(7):e0129659. doi: 10.1371/journal.pone.0129659.
9. Henderson TA. The diagnosis and evaluation of dementia and mild cognitive impairment with emphasis on SPECT perfusion neuroimaging. CNS Spectr. 2012;17(4):176-206.
Factors that change our brains
I greatly enjoyed Dr. Nasrallah’s editorial, “Your patient’s brain is different at every visit” (From the Editor,
In reading this editorial, it is clear that a myriad of factors we consider and address with our patients during each visit underly intricate neurobiologic mechanisms and processes that ever deepen our understanding of the brain. In discussing the changes taking place in our patients, I can’t help but wonder what changes are also occurring in our brains (as Dr. Nasrallah noted). What would be the resulting impact of these changes in our next patient interaction and/or subsequent interaction(s) with the same patient? Looking through the editorial’s bullet points, many (if not all) of the factors contributing to brain changes apply equally and naturally to clinicians as well as patients. In this light, the editorial serves not only as a broad guideline for patient psychoeducation but also as a reminder of wellness and well-being for clinicians.
As a “fresh-out-of-training” psychiatrist, I can definitely work on several of the factors, such as diet and exercise. Trainees and residents can be more susceptible to overlook and befall some of these factors and changes, and may already be basing the clinical advice they give to their patients on these same factors and changes. As a child psychiatrist, I value the importance of modeling healthy behaviors for my patients, and their families and with coworkers or colleagues. In accordance with the impact these factors have on our brains, it’s important to emphasize what we can do to further strengthen rapport and therapeutic value through modeling. I strive to model the desired behaviors, attitudes, and dynamics that are the external, observable manifestation or symptomology of what takes place in my brain. To do so, I understand I need to be mindful in proactively managing the contributing factors, such as those listed in Dr. Nasrallah’s editorial. I imagine patients and their families would easily notice if we are in suboptimal physical and/or mental health that results in us not being prompt, fully engaged, or receptive. I believe that attending to these facets during training falls under the umbrella of professionalism. Being a professional in our field often entails practicing what we preach. So, I’m grateful that what we preach is informed by our field’s exciting research, continued advancements, and expertise that benefits our patients and us professionally and personally.
Philip Yen-Tsun Liu, MD
Child and adolescent psychiatrist
innovaTel Telepsychiatry
San Antonio, Texas
Dr. Nasrallah responds
I would like to thank Dr. Liu for his thoughtful response to my editorial. He seems to be very cognizant of the fact that experiential neuroplasticity and brain tissue remodeling occurs in both the patient and physician. I admire his focus on psychoeducation, wellness, and professionalism. He is right that we as psychiatrists (and nurse practitioners) must be role models for our patients in multiple ways, because it may help enhance clinical outcomes and have a positive impact on their brains.
I would also like to point Dr. Liu to the editorial “The most powerful placebo is not a pill” (From the Editor,
Henry A. Nasrallah, MD
Editor-in-Chief
Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
The APA’s stance on neuroimaging
Can anyone in the modern world argue that the brain is irrelevant to psychiatry? Yet surprisingly, in September 2018, the American Psychiatric Association (APA) officially declared that neuroimaging of the brain has no clinical value in psychiatry.1
Unfortunately, the APA focused almost exclusively on functional magnetic resonance imaging (fMRI) and neglected an extensive library of studies of single-photon emission computed tomography (SPECT) and positron emission tomography (PET). The APA’s position on neuroimaging is as follows1,2:
- A neuroimaging finding must have a sensitivity and specificity (S/sp) of no less than 80%.
- The psychiatric imaging literature does not support using neuroimaging in psychiatric diagnostics or treatment.
- Neuroimaging has not had a significant impact on the diagnosis and treatment of psychiatric disorders.
The APA set unrealistic standards for biomarkers in a field that lacks pathologic markers of specific disease entities.3 Moreover, numerous widely used tests fall below the APA’s unrealistic S/sp cutoff, including the Hamilton Depression Rating Scale,4 Zung Depression Scale,5 the clock drawing test,6 and even the chest X-ray.3 Curiously, numerous replicated SPECT and PET studies were not included in the APA’s analysis.1-3 For example, in a study of 196 veterans, posttraumatic stress disorder was distinguished from traumatic brain injury with an S/sp of 0.92/0.85.7,8 Also, fluorodeoxyglucose (FDG)-PET has an S/sp of 0.84/0.74 in differentiating patients with Alzheimer’s disease from controls, while perfusion SPECT, using multi-detector cameras, has an S/sp of 0.93/0.84.3,9 Moreover, both FDG-PET and SPECT can differentiate other forms of dementia from Alzheimer’s disease, yielding an additional benefit compared to amyloid imaging alone.2,9 As President of the International Society of Applied Neuroimaging, I suggest neuroimaging should not be feared. Neuroimaging does not replace the diagnostician; rather, it aids him/her in a complex case.
Theodore A. Henderson, MD, PhD
President
Neuro-Luminance Brain Health Centers, Inc.
Denver, Colorado
Director
The Synaptic Space
Vice President
The Neuro-Laser Foundation
President
International Society of Applied Neuroimaging
Centennial, Colorado
Disclosure
The author has no ownership in, and receives no remuneration from, any neuroimaging company.
References
1. First MB, Drevets WC, Carter C, et al. Clinical applications of neuroimaging in psychiatric disorders. Am J Psychiatry. 2018:175:
2. First MB, Drevets WC, Carter C, et al. Data supplement for Clinical applications of neuroimaging in psychiatric disorders. Am J Psychiatry. 2018;175(suppl).
3. Henderson TA. Brain SPECT imaging in neuropsychiatric diagnosis and monitoring. EPatient. http://nmpangea.com/2018/10/09/738/. Published 2018. Accessed May 31, 2019.
4. Bagby RM, Ryder AG, Schuller DR, et al. The Hamilton Depression Rating Scale: has the gold standard become a lead weight? Am J Psychiatry. 2004;161(12):2163-2177.
5. Biggs JT, Wylie LT, Ziegler VE. Validity of the Zung Self-rating Depression Scale. Br J Psychiatry. 1978;132:381-385.
6. Seigerschmidt E, Mösch E, Siemen M, et al. The clock drawing test and questionable dementia: reliability and validity. Int J Geriatr Psychiatry. 2002;17(11):1048-1054.
7. Raji CA, Willeumier K, Taylor D, et al. Functional neuroimaging with default mode network regions distinguishes PTSD from TBI in a military veteran population. Brain Imaging Behav. 2015;9(3):527-534.
8. Amen DG, Raji CA, Willeumier K, et al. Functional neuroimaging distinguishes posttraumatic stress disorder from traumatic brain injury in focused and large community datasets. PLoS One. 2015;10(7):e0129659. doi: 10.1371/journal.pone.0129659.
9. Henderson TA. The diagnosis and evaluation of dementia and mild cognitive impairment with emphasis on SPECT perfusion neuroimaging. CNS Spectr. 2012;17(4):176-206.
Nothing to sneeze at: Upper respiratory infections and mood disorders
Acute upper respiratory infections (URIs) often lead to mild illnesses, but they can be severely destabilizing for individuals with mood disorders. Additionally, the medications patients often take to target symptoms of the common cold or influenza can interact with psychiatric medications to produce dangerous adverse events or induce further mood symptoms. In this article, we describe the relationship between URIs and mood disorders, the psychiatric diagnostic challenges that arise when evaluating a patient with a URI, and treatment approaches that emphasize psychoeducation and watchful waiting, when appropriate.
A bidirectional relationship
Acute upper respiratory infections are the most common human illnesses, affecting almost 25 million people annually in the United States.1 The common cold is caused by >200 different viruses; rhinovirus and coronavirus are the most common. Influenza, which also attacks the upper respiratory tract, is caused by strains of influenza A, B, or C virus.2 The common cold may present initially with mild symptoms of headache, sneezing, chills, and sore throat, and then progress to nasal discharge, congestion, cough, and malaise. When influenza strikes, patients may have a sudden onset of fever, headache, cough, sore throat, myalgia, congestion, weakness, anorexia, and gastrointestinal (GI) symptoms. Production of URI symptoms results from viral cytopathic activity along with immune activation of inflammatory pathways.2,3 The incidence of colds is inversely correlated with age; adults average 2 to 4 colds per year.4,5 Cold symptoms peak at 1 to 3 days and typically last 7 to 10 days, but can persist up to 3 weeks.6 With influenza, fever and other systemic symptoms last for 3 days but can persist up to 8 days, while cough and lethargy can persist for another 2 weeks.7
Upper respiratory infections have the potential to disrupt mood. Large studies of psychiatrically-healthy undergraduate students have found that compared with healthy controls, participants with URIs endorsed a negative affect within the first week of viral illness,8 and that the number and intensity of URI symptoms caused by cold viruses were correlated with the degree of their negative affect.9 A few case reports have documented instances of individuals with no previous personal or family psychiatric history developing full manic episodes in the setting of influenza.10-12 One case report described an influenza-induced manic episode in a patient with pre-existing psychiatric illness.13 There are no published case reports of common cold viruses inducing a full depressive or manic episode. If cold symptom severity correlates with negative affect among individuals with no psychiatric illness, and if influenza can induce manic episodes, then it is reasonable to expect that patients with pre-existing mood disorders could have an elevated risk for mood disturbances when they experience a URI (Box).
Box
Ms. E is a 35-year-old financial analyst with bipolar disorder type I and alcohol use disorder in sustained remission. She had been euthymic for the last 3 years, receiving weekly psychotherapy and taking lamotrigine, 350 mg/d, lithium ER, 900 mg/d (lithium level: 1.0 mmol/L), lurasidone, 60 mg/d, and clonazepam, 1 mg/d. At her most recent quarterly outpatient psychiatrist visit, she says her depression had returned. She reports 1 week of crying spells, initial and middle insomnia, anhedonia, feelings of worthlessness, fatigue, poor concentration, and poor appetite. She denies having suicidal ideation or manic or psychotic symptoms, and she continues to abstain from alcohol, illicit drugs, and tobacco. She has been fully adherent to her medication regimen and has not added any new medications or made any dietary changes since her last visit. She is puzzled as to what brought on this depression recurrence and says she feels defeated by the bipolar illness, a condition she had worked tirelessly to manage. When asked about changes in her health, she reports that about 1.5 weeks ago she developed a cough, nasal congestion, rhinorrhea, and fatigue. Because of her annual goal to run a marathon, she continues to train, albeit at a slower pace, and has not had much time to rest because of her demanding job.
The psychiatrist explains to Ms. E that an upper respiratory infection (URI) can sometimes induce depressive symptoms. Given the patient’s lengthy period of euthymia and the absence of new medicines, dietary changes, or drug/alcohol intake, the psychiatrist suspects that the cause of her mood episode recurrence is related to the URI. Hearing this is a relief for Ms. E. She and the psychiatrist decide to refrain from making any medication changes with the expectation that the URI would soon resolve because it had already persisted for 1.5 weeks. The psychiatrist tells Ms. E that if it does not and her symptoms worsen, she should call him to discuss treatment options. The psychiatrist also encourages Ms. E to take a temporary break from training and allow her body to rest.
Three weeks later, Ms. E returns and reports that both the URI symptoms and the depressive symptoms lifted a few days after her last visit.
Mood disorders may also be a risk factor for contracting URIs. Patients with mood disorders are more likely than healthy controls to be seropositive for markers of influenza A, influenza B, and coronavirus, and those with a history of suicide attempts are more likely to be seropositive for markers of influenza B.14 In a community sample of German adults age 18 to 65, those with mood disorders had a 35% higher likelihood of having had a cold within the last 12 months compared with those without a mood disorder.15 A survey of Korean employees found the odds of having had a cold in the last 4 months were up to 2.5 times greater for individuals with elevated scores on a depression symptom severity scale compared with those with lower scores.16 Because these studies were retrospective, recall bias may have impacted the results, as patients who are depressed are more likely to recall negative recent events.17
Proposed mechanisms
Researchers have proposed several mechanisms to explain the association of URIs with mood episodes. Mood disorders, such as bipolar disorder and major depressive disorder (MDD), are associated with chronic dysregulation of the innate immune system, which leads to elevated levels of cortisol and pro-inflammatory cytokines.18,19 Men with chronic low-grade inflammation are more vulnerable to all types of infection, including those that cause respiratory illnesses.20 High levels of stress,21 a negative affective style,22 and depression23 have all been associated with reduced antibody response and/or cellular-mediated immunity following vaccination, which suggests a possible mechanism for the vulnerability to infection found in individuals with mood disorders. On the other hand, after influenza vaccination, patients with depression produce a greater and more prolonged release of the cytokine interleukin 6, which perpetuates the state of chronic low-grade inflammation.24 Additionally, patients with mood disorders may engage in behaviors that reduce immune functioning, such as using illicit substances, drinking alcohol, smoking cigarettes, consuming an unhealthy diet, or living a sedentary lifestyle.
Conversely, there are several mechanisms by which a URI could induce a mood episode in a patient with a mood disorder. Animal studies have shown that a non-CNS viral infection can lead to depressive behavior by inducing peripheral interferon-beta release. This signaling protein binds to a receptor on the endothelial cells of the blood-brain barrier, inducing the release of additional cytokines that affect neuronal functioning.25 Among patients receiving interferon treatments for hepatitis C, a history of depression increased their likelihood of becoming depressed during their treatment course, which suggests people with mood disorders have a sensitivity to peripheral cytokines.26
Sleep interruptions from nighttime coughing or nasal congestion can increase the risk of a recurrence of hypomania or mania in patients with bipolar disorder,27 or a recurrence of depression in a patient with MDD.28 The stress that comes with missed work days or the inability to take care of other personal responsibilities due to a URI may increase the risk of becoming depressed in a patient with bipolar disorder or MDD. When present, GI symptoms such as vomiting and diarrhea can reduce the absorption of psychotropic medications and increase the risk of a mood recurrence. Finally, the treatments used for URIs may also contribute to mood instability. Case reports have described instances where patients with URIs developed mania or depression when exposed to medications such as intranasal corticosteroids,29 nasal decongestants,30,31 and anti-influenza treatments.32,33
Continue to: A diagnostic challenge
A diagnostic challenge
Making the diagnosis of a major depressive episode can be challenging in patients who present with a URI, particularly in those who are highly vigilant for relapse and seek care soon after mood symptoms emerge. Many symptoms overlap between the conditions, including insomnia, hypersomnia, reduced interest, anhedonia, fatigue, impaired concentration, and anorexia. Symptoms that are more specific for a major depressive episode include depressed mood, pathologic guilt, worthlessness, and suicidal ideation. Of course, a major depressive episode and a URI are not mutually exclusive and can occur simultaneously. However, incorrectly diagnosing recurrence of a major depressive episode in a euthymic patient who has a URI could lead to unnecessary changes to psychiatric treatment.
Psychoeducation is key
Teach patients about the bidirectional relationship between URIs and mood symptoms to reduce anxiety and confusion about the cause of the return of mood symptoms. Telling patients that they can expect their mood symptoms to be of short duration and self-limiting due to the URI can provide helpful reassurance.
Because it is possible that the mood symptoms will be transient, increasing psychotropic doses or adding a new psychotropic medication may not be necessary. The decision to initiate such changes should be made collaboratively with patients and should be based on the severity and duration of the patient’s mood symptoms. Symptoms that may warrant a medication change include psychosis, suicidal ideation, or mania. If a patient taking lithium becomes dehydrated because of excessive vomiting, diarrhea, or anorexia, temporarily reducing the dose or stopping the medication until the patient is hydrated may be appropriate.
When a patient presents with a URI, make basic URI treatment recommendations, including rest, hydration, and the use of over-the-counter (OTC) anti-cold medications and zinc.34 Encourage patients with suspected influenza to visit their primary care physician so that they may receive an anti-influenza medication. However, also remind patients about the psychiatric risks associated with some of these treatments and their potential interactions with psychotropics (Table). For example, many OTC cold formulations contain dextromethorphan or chlorpheniramine, both of which have weak serotonin reuptake properties and should not be combined with a monoamine oxidase inhibitor. Such cold formulations may also contain non-steroidal anti-inflammatory agents, which could elevate lithium levels. Codeine, which is often prescribed to suppress the coughing reflex, can lead a patient with a history of substance use to relapse on their drug of choice.
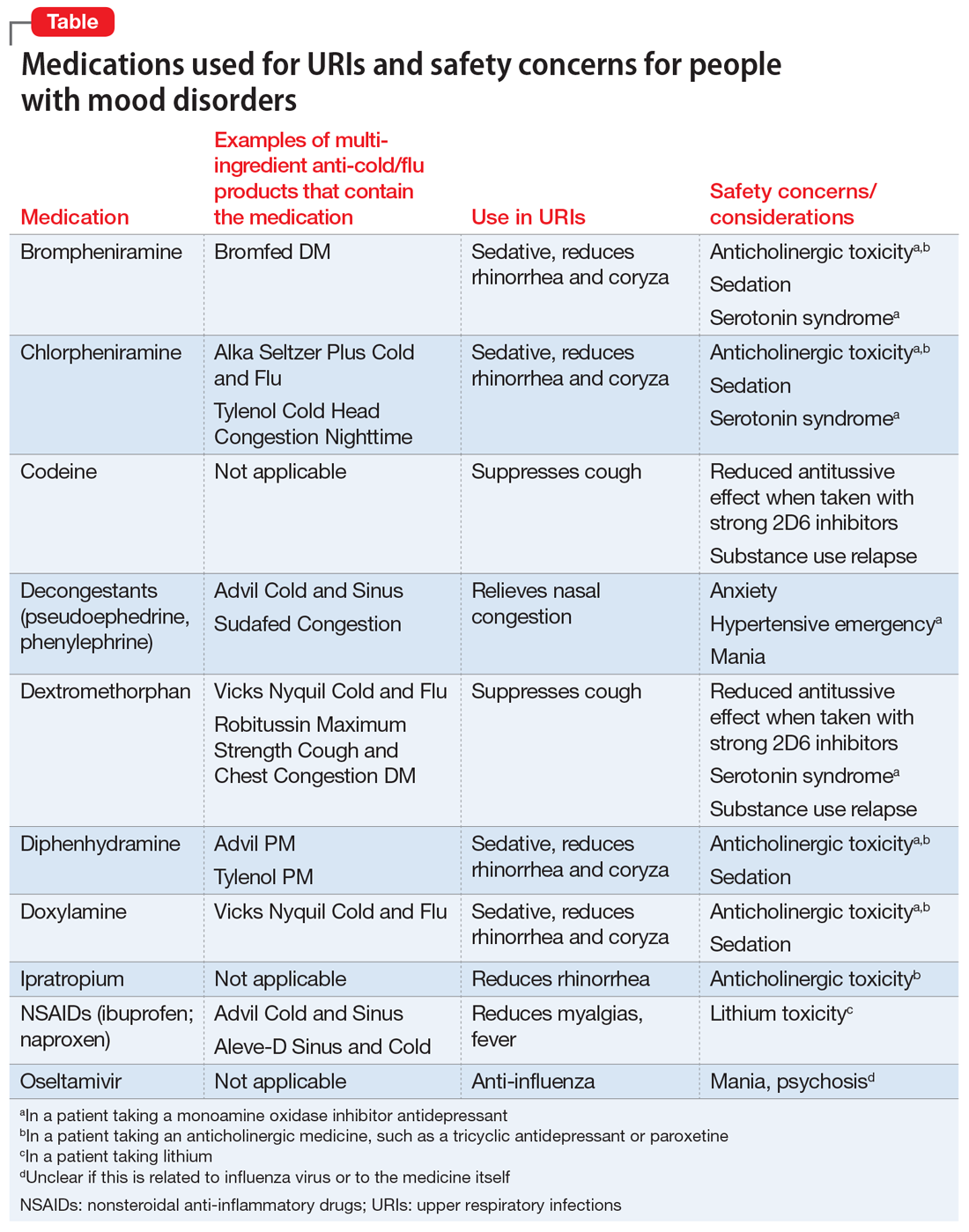
Also recommend lifestyle modifications to help patients reduce their risk of infection. These includes frequent hand washing, avoiding or limiting alcohol use, avoiding cigarettes, exercising regularly, consuming a Mediterranean diet, and receiving scheduled immunizations. To avoid contracting a URI and infecting patients, wash your hands or use an alcohol-based cleanser after shaking hands with patients. Finally, if a patient does not have a primary care physician, encourage him/her to find one to help manage subsequent infections.
Continue to: Bottom Line
Bottom Line
Patients with mood disorders may have an increased risk of developing an upper respiratory infection (URI), which can worsen their mood. Clinicians must make psychotropic treatment changes cautiously and guide patients to select safe over-the-counter medications for relief of URI symptoms.
Related Resources
- Centers for Disease Control and Prevention. Cold versus flu. www.cdc.gov/flu/about/qa/coldflu.htm.
- Centers for Disease Control and Prevention. Nonspecific upper respiratory tract infection. www.cdc.gov/getsmart/community/materials-references/print-materials/hcp/adult-tract-infection.pdf.
Drug Brand Names
Clonazepam • Klonopin
Ipratropium • Atrovent
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oseltamivir • Tamiflu
Paroxetine • Paxil
1. Gonzales R, Malone DC, Maselli JH, et al. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757-762.
2. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
3. Passioti M, Maggina P, Megremis S, et al. The common cold: potential for future prevention or cure. Curr Allergy Asthma Rep. 2014;14(2):413.
4. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164-169.
5. Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16(2):351-373.
6. Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361(9351):51-59.
7. Paules C, Subbarao K. Influenza. Lancet. 2017;390(10095):697-708.
8. Hall S, Smith A. Investigation of the effects and aftereffects of naturally occurring upper respiratory tract illnesses on mood and performance. Physiol Behav. 1996;59(3):569-577.
9. Smith A, Thomas M, Kent J, et al. Effects of the common cold on mood and performance. Psychoneuroendocrinology. 1998;23(7):733-739.
10. Ayub S, Kanner J, Riddle M, et al. Influenza-induced mania. J Neuropsychiatry Clin Neurosci. 2016;28(1):e17-e18.
11. Maurizi CP. Influenza and mania: a possible connection with the locus ceruleus. South Med J. 1985;78(2):207-209.
12. Steinberg D, Hirsch SR, Marston SD, et al. Influenza infection causing manic psychosis. Br J Psychiatry. 1972;120(558):531-535.
13. Ishitobi M, Shukunami K, Murata T, et al. Hypomanic switching during influenza infection without intracranial infection in an adolescent patient with bipolar disorder. Pediatr Emerg Care. 2011;27(7):652-653.
14. Okusaga O, Yolken RH, Langenberg P, et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011;130(1-2):220-225.
15. Adam Y, Meinlschmidt G, Lieb R. Associations between mental disorders and the common cold in adults: a population-based cross-sectional study. J Psychosom Res. 2013;74(1):69-73.
16. Kim HC, Park SG, Leem JH, et al. Depressive symptoms as a risk factor for the common cold among employees: a 4-month follow-up study. J Psychosom Res. 2011;71(3):194-196.
17. Dalgleish T, Werner-Seidler A. Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends Cogn Sci. 2014;18(11):596-604.
18. Rosenblat JD, McIntyre RS. Bipolar disorder and inflammation. Psychiatr Clin North Am. 2016;39(1):125-137.
19. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075-1091.
20. Kaspersen KA, Dinh KM, Erikstrup LT, et al. Low-grade inflammation is associated with susceptibility to infection in healthy men: results from the Danish Blood Donor Study (DBDS). PLoS One. 2016;11(10):e0164220.
21. Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93(7):3043-3047.
22. Rosenkranz MA, Jackson DC, Dalton KM, et al. Affective style and in vivo immune response: neurobehavioral mechanisms. Proc Natl Acad Sci U S A. 2003;100(19):11148-1152.
23. Irwin MR, Levin MJ, Laudenslager ML, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis. 2013;56(8):1085-1093.
24. Glaser R, Robles TF, Sheridan J, et al. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60(10):1009-1014.
25. Blank T, Detje CN, Spiess A, et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity. 2016;44(4):901-912.
26. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
27. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
28. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165(12):1543-1550.
29. Saraga M. A manic episode in a patient with stable bipolar disorder triggered by intranasal mometasone furoate. Ther Adv Psychopharmacol. 2014;4(1):48-49.
30. Kandeger A, Tekdemir R, Sen B, et al. A case report of patient who had two manic episodes with psychotic features induced by nasal decongestant. European Psychiatry. 2017;41(Suppl):S428.
31. Waters BG, Lapierre YD. Secondary mania associated with sympathomimetic drug use. Am J Psychiatry. 1981;138(6):837-838.
32. Ho LN, Chung JP, Choy KL. Oseltamivir-induced mania in a patient with H1N1. Am J Psychiatry. 2010;167(3):350.
33. Jeon SW, Han C. Psychiatric symptoms in a patient with influenza A (H1N1) treated with oseltamivir (Tamiflu): a case report. Clin Psychopharmacol Neurosci. 2015;13(2):209-211.
34. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186(3):190-199.
Acute upper respiratory infections (URIs) often lead to mild illnesses, but they can be severely destabilizing for individuals with mood disorders. Additionally, the medications patients often take to target symptoms of the common cold or influenza can interact with psychiatric medications to produce dangerous adverse events or induce further mood symptoms. In this article, we describe the relationship between URIs and mood disorders, the psychiatric diagnostic challenges that arise when evaluating a patient with a URI, and treatment approaches that emphasize psychoeducation and watchful waiting, when appropriate.
A bidirectional relationship
Acute upper respiratory infections are the most common human illnesses, affecting almost 25 million people annually in the United States.1 The common cold is caused by >200 different viruses; rhinovirus and coronavirus are the most common. Influenza, which also attacks the upper respiratory tract, is caused by strains of influenza A, B, or C virus.2 The common cold may present initially with mild symptoms of headache, sneezing, chills, and sore throat, and then progress to nasal discharge, congestion, cough, and malaise. When influenza strikes, patients may have a sudden onset of fever, headache, cough, sore throat, myalgia, congestion, weakness, anorexia, and gastrointestinal (GI) symptoms. Production of URI symptoms results from viral cytopathic activity along with immune activation of inflammatory pathways.2,3 The incidence of colds is inversely correlated with age; adults average 2 to 4 colds per year.4,5 Cold symptoms peak at 1 to 3 days and typically last 7 to 10 days, but can persist up to 3 weeks.6 With influenza, fever and other systemic symptoms last for 3 days but can persist up to 8 days, while cough and lethargy can persist for another 2 weeks.7
Upper respiratory infections have the potential to disrupt mood. Large studies of psychiatrically-healthy undergraduate students have found that compared with healthy controls, participants with URIs endorsed a negative affect within the first week of viral illness,8 and that the number and intensity of URI symptoms caused by cold viruses were correlated with the degree of their negative affect.9 A few case reports have documented instances of individuals with no previous personal or family psychiatric history developing full manic episodes in the setting of influenza.10-12 One case report described an influenza-induced manic episode in a patient with pre-existing psychiatric illness.13 There are no published case reports of common cold viruses inducing a full depressive or manic episode. If cold symptom severity correlates with negative affect among individuals with no psychiatric illness, and if influenza can induce manic episodes, then it is reasonable to expect that patients with pre-existing mood disorders could have an elevated risk for mood disturbances when they experience a URI (Box).
Box
Ms. E is a 35-year-old financial analyst with bipolar disorder type I and alcohol use disorder in sustained remission. She had been euthymic for the last 3 years, receiving weekly psychotherapy and taking lamotrigine, 350 mg/d, lithium ER, 900 mg/d (lithium level: 1.0 mmol/L), lurasidone, 60 mg/d, and clonazepam, 1 mg/d. At her most recent quarterly outpatient psychiatrist visit, she says her depression had returned. She reports 1 week of crying spells, initial and middle insomnia, anhedonia, feelings of worthlessness, fatigue, poor concentration, and poor appetite. She denies having suicidal ideation or manic or psychotic symptoms, and she continues to abstain from alcohol, illicit drugs, and tobacco. She has been fully adherent to her medication regimen and has not added any new medications or made any dietary changes since her last visit. She is puzzled as to what brought on this depression recurrence and says she feels defeated by the bipolar illness, a condition she had worked tirelessly to manage. When asked about changes in her health, she reports that about 1.5 weeks ago she developed a cough, nasal congestion, rhinorrhea, and fatigue. Because of her annual goal to run a marathon, she continues to train, albeit at a slower pace, and has not had much time to rest because of her demanding job.
The psychiatrist explains to Ms. E that an upper respiratory infection (URI) can sometimes induce depressive symptoms. Given the patient’s lengthy period of euthymia and the absence of new medicines, dietary changes, or drug/alcohol intake, the psychiatrist suspects that the cause of her mood episode recurrence is related to the URI. Hearing this is a relief for Ms. E. She and the psychiatrist decide to refrain from making any medication changes with the expectation that the URI would soon resolve because it had already persisted for 1.5 weeks. The psychiatrist tells Ms. E that if it does not and her symptoms worsen, she should call him to discuss treatment options. The psychiatrist also encourages Ms. E to take a temporary break from training and allow her body to rest.
Three weeks later, Ms. E returns and reports that both the URI symptoms and the depressive symptoms lifted a few days after her last visit.
Mood disorders may also be a risk factor for contracting URIs. Patients with mood disorders are more likely than healthy controls to be seropositive for markers of influenza A, influenza B, and coronavirus, and those with a history of suicide attempts are more likely to be seropositive for markers of influenza B.14 In a community sample of German adults age 18 to 65, those with mood disorders had a 35% higher likelihood of having had a cold within the last 12 months compared with those without a mood disorder.15 A survey of Korean employees found the odds of having had a cold in the last 4 months were up to 2.5 times greater for individuals with elevated scores on a depression symptom severity scale compared with those with lower scores.16 Because these studies were retrospective, recall bias may have impacted the results, as patients who are depressed are more likely to recall negative recent events.17
Proposed mechanisms
Researchers have proposed several mechanisms to explain the association of URIs with mood episodes. Mood disorders, such as bipolar disorder and major depressive disorder (MDD), are associated with chronic dysregulation of the innate immune system, which leads to elevated levels of cortisol and pro-inflammatory cytokines.18,19 Men with chronic low-grade inflammation are more vulnerable to all types of infection, including those that cause respiratory illnesses.20 High levels of stress,21 a negative affective style,22 and depression23 have all been associated with reduced antibody response and/or cellular-mediated immunity following vaccination, which suggests a possible mechanism for the vulnerability to infection found in individuals with mood disorders. On the other hand, after influenza vaccination, patients with depression produce a greater and more prolonged release of the cytokine interleukin 6, which perpetuates the state of chronic low-grade inflammation.24 Additionally, patients with mood disorders may engage in behaviors that reduce immune functioning, such as using illicit substances, drinking alcohol, smoking cigarettes, consuming an unhealthy diet, or living a sedentary lifestyle.
Conversely, there are several mechanisms by which a URI could induce a mood episode in a patient with a mood disorder. Animal studies have shown that a non-CNS viral infection can lead to depressive behavior by inducing peripheral interferon-beta release. This signaling protein binds to a receptor on the endothelial cells of the blood-brain barrier, inducing the release of additional cytokines that affect neuronal functioning.25 Among patients receiving interferon treatments for hepatitis C, a history of depression increased their likelihood of becoming depressed during their treatment course, which suggests people with mood disorders have a sensitivity to peripheral cytokines.26
Sleep interruptions from nighttime coughing or nasal congestion can increase the risk of a recurrence of hypomania or mania in patients with bipolar disorder,27 or a recurrence of depression in a patient with MDD.28 The stress that comes with missed work days or the inability to take care of other personal responsibilities due to a URI may increase the risk of becoming depressed in a patient with bipolar disorder or MDD. When present, GI symptoms such as vomiting and diarrhea can reduce the absorption of psychotropic medications and increase the risk of a mood recurrence. Finally, the treatments used for URIs may also contribute to mood instability. Case reports have described instances where patients with URIs developed mania or depression when exposed to medications such as intranasal corticosteroids,29 nasal decongestants,30,31 and anti-influenza treatments.32,33
Continue to: A diagnostic challenge
A diagnostic challenge
Making the diagnosis of a major depressive episode can be challenging in patients who present with a URI, particularly in those who are highly vigilant for relapse and seek care soon after mood symptoms emerge. Many symptoms overlap between the conditions, including insomnia, hypersomnia, reduced interest, anhedonia, fatigue, impaired concentration, and anorexia. Symptoms that are more specific for a major depressive episode include depressed mood, pathologic guilt, worthlessness, and suicidal ideation. Of course, a major depressive episode and a URI are not mutually exclusive and can occur simultaneously. However, incorrectly diagnosing recurrence of a major depressive episode in a euthymic patient who has a URI could lead to unnecessary changes to psychiatric treatment.
Psychoeducation is key
Teach patients about the bidirectional relationship between URIs and mood symptoms to reduce anxiety and confusion about the cause of the return of mood symptoms. Telling patients that they can expect their mood symptoms to be of short duration and self-limiting due to the URI can provide helpful reassurance.
Because it is possible that the mood symptoms will be transient, increasing psychotropic doses or adding a new psychotropic medication may not be necessary. The decision to initiate such changes should be made collaboratively with patients and should be based on the severity and duration of the patient’s mood symptoms. Symptoms that may warrant a medication change include psychosis, suicidal ideation, or mania. If a patient taking lithium becomes dehydrated because of excessive vomiting, diarrhea, or anorexia, temporarily reducing the dose or stopping the medication until the patient is hydrated may be appropriate.
When a patient presents with a URI, make basic URI treatment recommendations, including rest, hydration, and the use of over-the-counter (OTC) anti-cold medications and zinc.34 Encourage patients with suspected influenza to visit their primary care physician so that they may receive an anti-influenza medication. However, also remind patients about the psychiatric risks associated with some of these treatments and their potential interactions with psychotropics (Table). For example, many OTC cold formulations contain dextromethorphan or chlorpheniramine, both of which have weak serotonin reuptake properties and should not be combined with a monoamine oxidase inhibitor. Such cold formulations may also contain non-steroidal anti-inflammatory agents, which could elevate lithium levels. Codeine, which is often prescribed to suppress the coughing reflex, can lead a patient with a history of substance use to relapse on their drug of choice.

Also recommend lifestyle modifications to help patients reduce their risk of infection. These includes frequent hand washing, avoiding or limiting alcohol use, avoiding cigarettes, exercising regularly, consuming a Mediterranean diet, and receiving scheduled immunizations. To avoid contracting a URI and infecting patients, wash your hands or use an alcohol-based cleanser after shaking hands with patients. Finally, if a patient does not have a primary care physician, encourage him/her to find one to help manage subsequent infections.
Continue to: Bottom Line
Bottom Line
Patients with mood disorders may have an increased risk of developing an upper respiratory infection (URI), which can worsen their mood. Clinicians must make psychotropic treatment changes cautiously and guide patients to select safe over-the-counter medications for relief of URI symptoms.
Related Resources
- Centers for Disease Control and Prevention. Cold versus flu. www.cdc.gov/flu/about/qa/coldflu.htm.
- Centers for Disease Control and Prevention. Nonspecific upper respiratory tract infection. www.cdc.gov/getsmart/community/materials-references/print-materials/hcp/adult-tract-infection.pdf.
Drug Brand Names
Clonazepam • Klonopin
Ipratropium • Atrovent
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oseltamivir • Tamiflu
Paroxetine • Paxil
Acute upper respiratory infections (URIs) often lead to mild illnesses, but they can be severely destabilizing for individuals with mood disorders. Additionally, the medications patients often take to target symptoms of the common cold or influenza can interact with psychiatric medications to produce dangerous adverse events or induce further mood symptoms. In this article, we describe the relationship between URIs and mood disorders, the psychiatric diagnostic challenges that arise when evaluating a patient with a URI, and treatment approaches that emphasize psychoeducation and watchful waiting, when appropriate.
A bidirectional relationship
Acute upper respiratory infections are the most common human illnesses, affecting almost 25 million people annually in the United States.1 The common cold is caused by >200 different viruses; rhinovirus and coronavirus are the most common. Influenza, which also attacks the upper respiratory tract, is caused by strains of influenza A, B, or C virus.2 The common cold may present initially with mild symptoms of headache, sneezing, chills, and sore throat, and then progress to nasal discharge, congestion, cough, and malaise. When influenza strikes, patients may have a sudden onset of fever, headache, cough, sore throat, myalgia, congestion, weakness, anorexia, and gastrointestinal (GI) symptoms. Production of URI symptoms results from viral cytopathic activity along with immune activation of inflammatory pathways.2,3 The incidence of colds is inversely correlated with age; adults average 2 to 4 colds per year.4,5 Cold symptoms peak at 1 to 3 days and typically last 7 to 10 days, but can persist up to 3 weeks.6 With influenza, fever and other systemic symptoms last for 3 days but can persist up to 8 days, while cough and lethargy can persist for another 2 weeks.7
Upper respiratory infections have the potential to disrupt mood. Large studies of psychiatrically-healthy undergraduate students have found that compared with healthy controls, participants with URIs endorsed a negative affect within the first week of viral illness,8 and that the number and intensity of URI symptoms caused by cold viruses were correlated with the degree of their negative affect.9 A few case reports have documented instances of individuals with no previous personal or family psychiatric history developing full manic episodes in the setting of influenza.10-12 One case report described an influenza-induced manic episode in a patient with pre-existing psychiatric illness.13 There are no published case reports of common cold viruses inducing a full depressive or manic episode. If cold symptom severity correlates with negative affect among individuals with no psychiatric illness, and if influenza can induce manic episodes, then it is reasonable to expect that patients with pre-existing mood disorders could have an elevated risk for mood disturbances when they experience a URI (Box).
Box
Ms. E is a 35-year-old financial analyst with bipolar disorder type I and alcohol use disorder in sustained remission. She had been euthymic for the last 3 years, receiving weekly psychotherapy and taking lamotrigine, 350 mg/d, lithium ER, 900 mg/d (lithium level: 1.0 mmol/L), lurasidone, 60 mg/d, and clonazepam, 1 mg/d. At her most recent quarterly outpatient psychiatrist visit, she says her depression had returned. She reports 1 week of crying spells, initial and middle insomnia, anhedonia, feelings of worthlessness, fatigue, poor concentration, and poor appetite. She denies having suicidal ideation or manic or psychotic symptoms, and she continues to abstain from alcohol, illicit drugs, and tobacco. She has been fully adherent to her medication regimen and has not added any new medications or made any dietary changes since her last visit. She is puzzled as to what brought on this depression recurrence and says she feels defeated by the bipolar illness, a condition she had worked tirelessly to manage. When asked about changes in her health, she reports that about 1.5 weeks ago she developed a cough, nasal congestion, rhinorrhea, and fatigue. Because of her annual goal to run a marathon, she continues to train, albeit at a slower pace, and has not had much time to rest because of her demanding job.
The psychiatrist explains to Ms. E that an upper respiratory infection (URI) can sometimes induce depressive symptoms. Given the patient’s lengthy period of euthymia and the absence of new medicines, dietary changes, or drug/alcohol intake, the psychiatrist suspects that the cause of her mood episode recurrence is related to the URI. Hearing this is a relief for Ms. E. She and the psychiatrist decide to refrain from making any medication changes with the expectation that the URI would soon resolve because it had already persisted for 1.5 weeks. The psychiatrist tells Ms. E that if it does not and her symptoms worsen, she should call him to discuss treatment options. The psychiatrist also encourages Ms. E to take a temporary break from training and allow her body to rest.
Three weeks later, Ms. E returns and reports that both the URI symptoms and the depressive symptoms lifted a few days after her last visit.
Mood disorders may also be a risk factor for contracting URIs. Patients with mood disorders are more likely than healthy controls to be seropositive for markers of influenza A, influenza B, and coronavirus, and those with a history of suicide attempts are more likely to be seropositive for markers of influenza B.14 In a community sample of German adults age 18 to 65, those with mood disorders had a 35% higher likelihood of having had a cold within the last 12 months compared with those without a mood disorder.15 A survey of Korean employees found the odds of having had a cold in the last 4 months were up to 2.5 times greater for individuals with elevated scores on a depression symptom severity scale compared with those with lower scores.16 Because these studies were retrospective, recall bias may have impacted the results, as patients who are depressed are more likely to recall negative recent events.17
Proposed mechanisms
Researchers have proposed several mechanisms to explain the association of URIs with mood episodes. Mood disorders, such as bipolar disorder and major depressive disorder (MDD), are associated with chronic dysregulation of the innate immune system, which leads to elevated levels of cortisol and pro-inflammatory cytokines.18,19 Men with chronic low-grade inflammation are more vulnerable to all types of infection, including those that cause respiratory illnesses.20 High levels of stress,21 a negative affective style,22 and depression23 have all been associated with reduced antibody response and/or cellular-mediated immunity following vaccination, which suggests a possible mechanism for the vulnerability to infection found in individuals with mood disorders. On the other hand, after influenza vaccination, patients with depression produce a greater and more prolonged release of the cytokine interleukin 6, which perpetuates the state of chronic low-grade inflammation.24 Additionally, patients with mood disorders may engage in behaviors that reduce immune functioning, such as using illicit substances, drinking alcohol, smoking cigarettes, consuming an unhealthy diet, or living a sedentary lifestyle.
Conversely, there are several mechanisms by which a URI could induce a mood episode in a patient with a mood disorder. Animal studies have shown that a non-CNS viral infection can lead to depressive behavior by inducing peripheral interferon-beta release. This signaling protein binds to a receptor on the endothelial cells of the blood-brain barrier, inducing the release of additional cytokines that affect neuronal functioning.25 Among patients receiving interferon treatments for hepatitis C, a history of depression increased their likelihood of becoming depressed during their treatment course, which suggests people with mood disorders have a sensitivity to peripheral cytokines.26
Sleep interruptions from nighttime coughing or nasal congestion can increase the risk of a recurrence of hypomania or mania in patients with bipolar disorder,27 or a recurrence of depression in a patient with MDD.28 The stress that comes with missed work days or the inability to take care of other personal responsibilities due to a URI may increase the risk of becoming depressed in a patient with bipolar disorder or MDD. When present, GI symptoms such as vomiting and diarrhea can reduce the absorption of psychotropic medications and increase the risk of a mood recurrence. Finally, the treatments used for URIs may also contribute to mood instability. Case reports have described instances where patients with URIs developed mania or depression when exposed to medications such as intranasal corticosteroids,29 nasal decongestants,30,31 and anti-influenza treatments.32,33
Continue to: A diagnostic challenge
A diagnostic challenge
Making the diagnosis of a major depressive episode can be challenging in patients who present with a URI, particularly in those who are highly vigilant for relapse and seek care soon after mood symptoms emerge. Many symptoms overlap between the conditions, including insomnia, hypersomnia, reduced interest, anhedonia, fatigue, impaired concentration, and anorexia. Symptoms that are more specific for a major depressive episode include depressed mood, pathologic guilt, worthlessness, and suicidal ideation. Of course, a major depressive episode and a URI are not mutually exclusive and can occur simultaneously. However, incorrectly diagnosing recurrence of a major depressive episode in a euthymic patient who has a URI could lead to unnecessary changes to psychiatric treatment.
Psychoeducation is key
Teach patients about the bidirectional relationship between URIs and mood symptoms to reduce anxiety and confusion about the cause of the return of mood symptoms. Telling patients that they can expect their mood symptoms to be of short duration and self-limiting due to the URI can provide helpful reassurance.
Because it is possible that the mood symptoms will be transient, increasing psychotropic doses or adding a new psychotropic medication may not be necessary. The decision to initiate such changes should be made collaboratively with patients and should be based on the severity and duration of the patient’s mood symptoms. Symptoms that may warrant a medication change include psychosis, suicidal ideation, or mania. If a patient taking lithium becomes dehydrated because of excessive vomiting, diarrhea, or anorexia, temporarily reducing the dose or stopping the medication until the patient is hydrated may be appropriate.
When a patient presents with a URI, make basic URI treatment recommendations, including rest, hydration, and the use of over-the-counter (OTC) anti-cold medications and zinc.34 Encourage patients with suspected influenza to visit their primary care physician so that they may receive an anti-influenza medication. However, also remind patients about the psychiatric risks associated with some of these treatments and their potential interactions with psychotropics (Table). For example, many OTC cold formulations contain dextromethorphan or chlorpheniramine, both of which have weak serotonin reuptake properties and should not be combined with a monoamine oxidase inhibitor. Such cold formulations may also contain non-steroidal anti-inflammatory agents, which could elevate lithium levels. Codeine, which is often prescribed to suppress the coughing reflex, can lead a patient with a history of substance use to relapse on their drug of choice.

Also recommend lifestyle modifications to help patients reduce their risk of infection. These includes frequent hand washing, avoiding or limiting alcohol use, avoiding cigarettes, exercising regularly, consuming a Mediterranean diet, and receiving scheduled immunizations. To avoid contracting a URI and infecting patients, wash your hands or use an alcohol-based cleanser after shaking hands with patients. Finally, if a patient does not have a primary care physician, encourage him/her to find one to help manage subsequent infections.
Continue to: Bottom Line
Bottom Line
Patients with mood disorders may have an increased risk of developing an upper respiratory infection (URI), which can worsen their mood. Clinicians must make psychotropic treatment changes cautiously and guide patients to select safe over-the-counter medications for relief of URI symptoms.
Related Resources
- Centers for Disease Control and Prevention. Cold versus flu. www.cdc.gov/flu/about/qa/coldflu.htm.
- Centers for Disease Control and Prevention. Nonspecific upper respiratory tract infection. www.cdc.gov/getsmart/community/materials-references/print-materials/hcp/adult-tract-infection.pdf.
Drug Brand Names
Clonazepam • Klonopin
Ipratropium • Atrovent
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oseltamivir • Tamiflu
Paroxetine • Paxil
1. Gonzales R, Malone DC, Maselli JH, et al. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757-762.
2. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
3. Passioti M, Maggina P, Megremis S, et al. The common cold: potential for future prevention or cure. Curr Allergy Asthma Rep. 2014;14(2):413.
4. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164-169.
5. Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16(2):351-373.
6. Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361(9351):51-59.
7. Paules C, Subbarao K. Influenza. Lancet. 2017;390(10095):697-708.
8. Hall S, Smith A. Investigation of the effects and aftereffects of naturally occurring upper respiratory tract illnesses on mood and performance. Physiol Behav. 1996;59(3):569-577.
9. Smith A, Thomas M, Kent J, et al. Effects of the common cold on mood and performance. Psychoneuroendocrinology. 1998;23(7):733-739.
10. Ayub S, Kanner J, Riddle M, et al. Influenza-induced mania. J Neuropsychiatry Clin Neurosci. 2016;28(1):e17-e18.
11. Maurizi CP. Influenza and mania: a possible connection with the locus ceruleus. South Med J. 1985;78(2):207-209.
12. Steinberg D, Hirsch SR, Marston SD, et al. Influenza infection causing manic psychosis. Br J Psychiatry. 1972;120(558):531-535.
13. Ishitobi M, Shukunami K, Murata T, et al. Hypomanic switching during influenza infection without intracranial infection in an adolescent patient with bipolar disorder. Pediatr Emerg Care. 2011;27(7):652-653.
14. Okusaga O, Yolken RH, Langenberg P, et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011;130(1-2):220-225.
15. Adam Y, Meinlschmidt G, Lieb R. Associations between mental disorders and the common cold in adults: a population-based cross-sectional study. J Psychosom Res. 2013;74(1):69-73.
16. Kim HC, Park SG, Leem JH, et al. Depressive symptoms as a risk factor for the common cold among employees: a 4-month follow-up study. J Psychosom Res. 2011;71(3):194-196.
17. Dalgleish T, Werner-Seidler A. Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends Cogn Sci. 2014;18(11):596-604.
18. Rosenblat JD, McIntyre RS. Bipolar disorder and inflammation. Psychiatr Clin North Am. 2016;39(1):125-137.
19. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075-1091.
20. Kaspersen KA, Dinh KM, Erikstrup LT, et al. Low-grade inflammation is associated with susceptibility to infection in healthy men: results from the Danish Blood Donor Study (DBDS). PLoS One. 2016;11(10):e0164220.
21. Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93(7):3043-3047.
22. Rosenkranz MA, Jackson DC, Dalton KM, et al. Affective style and in vivo immune response: neurobehavioral mechanisms. Proc Natl Acad Sci U S A. 2003;100(19):11148-1152.
23. Irwin MR, Levin MJ, Laudenslager ML, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis. 2013;56(8):1085-1093.
24. Glaser R, Robles TF, Sheridan J, et al. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60(10):1009-1014.
25. Blank T, Detje CN, Spiess A, et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity. 2016;44(4):901-912.
26. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
27. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
28. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165(12):1543-1550.
29. Saraga M. A manic episode in a patient with stable bipolar disorder triggered by intranasal mometasone furoate. Ther Adv Psychopharmacol. 2014;4(1):48-49.
30. Kandeger A, Tekdemir R, Sen B, et al. A case report of patient who had two manic episodes with psychotic features induced by nasal decongestant. European Psychiatry. 2017;41(Suppl):S428.
31. Waters BG, Lapierre YD. Secondary mania associated with sympathomimetic drug use. Am J Psychiatry. 1981;138(6):837-838.
32. Ho LN, Chung JP, Choy KL. Oseltamivir-induced mania in a patient with H1N1. Am J Psychiatry. 2010;167(3):350.
33. Jeon SW, Han C. Psychiatric symptoms in a patient with influenza A (H1N1) treated with oseltamivir (Tamiflu): a case report. Clin Psychopharmacol Neurosci. 2015;13(2):209-211.
34. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186(3):190-199.
1. Gonzales R, Malone DC, Maselli JH, et al. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757-762.
2. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
3. Passioti M, Maggina P, Megremis S, et al. The common cold: potential for future prevention or cure. Curr Allergy Asthma Rep. 2014;14(2):413.
4. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164-169.
5. Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16(2):351-373.
6. Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361(9351):51-59.
7. Paules C, Subbarao K. Influenza. Lancet. 2017;390(10095):697-708.
8. Hall S, Smith A. Investigation of the effects and aftereffects of naturally occurring upper respiratory tract illnesses on mood and performance. Physiol Behav. 1996;59(3):569-577.
9. Smith A, Thomas M, Kent J, et al. Effects of the common cold on mood and performance. Psychoneuroendocrinology. 1998;23(7):733-739.
10. Ayub S, Kanner J, Riddle M, et al. Influenza-induced mania. J Neuropsychiatry Clin Neurosci. 2016;28(1):e17-e18.
11. Maurizi CP. Influenza and mania: a possible connection with the locus ceruleus. South Med J. 1985;78(2):207-209.
12. Steinberg D, Hirsch SR, Marston SD, et al. Influenza infection causing manic psychosis. Br J Psychiatry. 1972;120(558):531-535.
13. Ishitobi M, Shukunami K, Murata T, et al. Hypomanic switching during influenza infection without intracranial infection in an adolescent patient with bipolar disorder. Pediatr Emerg Care. 2011;27(7):652-653.
14. Okusaga O, Yolken RH, Langenberg P, et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011;130(1-2):220-225.
15. Adam Y, Meinlschmidt G, Lieb R. Associations between mental disorders and the common cold in adults: a population-based cross-sectional study. J Psychosom Res. 2013;74(1):69-73.
16. Kim HC, Park SG, Leem JH, et al. Depressive symptoms as a risk factor for the common cold among employees: a 4-month follow-up study. J Psychosom Res. 2011;71(3):194-196.
17. Dalgleish T, Werner-Seidler A. Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends Cogn Sci. 2014;18(11):596-604.
18. Rosenblat JD, McIntyre RS. Bipolar disorder and inflammation. Psychiatr Clin North Am. 2016;39(1):125-137.
19. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075-1091.
20. Kaspersen KA, Dinh KM, Erikstrup LT, et al. Low-grade inflammation is associated with susceptibility to infection in healthy men: results from the Danish Blood Donor Study (DBDS). PLoS One. 2016;11(10):e0164220.
21. Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93(7):3043-3047.
22. Rosenkranz MA, Jackson DC, Dalton KM, et al. Affective style and in vivo immune response: neurobehavioral mechanisms. Proc Natl Acad Sci U S A. 2003;100(19):11148-1152.
23. Irwin MR, Levin MJ, Laudenslager ML, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis. 2013;56(8):1085-1093.
24. Glaser R, Robles TF, Sheridan J, et al. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60(10):1009-1014.
25. Blank T, Detje CN, Spiess A, et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity. 2016;44(4):901-912.
26. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
27. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
28. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165(12):1543-1550.
29. Saraga M. A manic episode in a patient with stable bipolar disorder triggered by intranasal mometasone furoate. Ther Adv Psychopharmacol. 2014;4(1):48-49.
30. Kandeger A, Tekdemir R, Sen B, et al. A case report of patient who had two manic episodes with psychotic features induced by nasal decongestant. European Psychiatry. 2017;41(Suppl):S428.
31. Waters BG, Lapierre YD. Secondary mania associated with sympathomimetic drug use. Am J Psychiatry. 1981;138(6):837-838.
32. Ho LN, Chung JP, Choy KL. Oseltamivir-induced mania in a patient with H1N1. Am J Psychiatry. 2010;167(3):350.
33. Jeon SW, Han C. Psychiatric symptoms in a patient with influenza A (H1N1) treated with oseltamivir (Tamiflu): a case report. Clin Psychopharmacol Neurosci. 2015;13(2):209-211.
34. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186(3):190-199.
Stigma in dementia: It’s time to talk about it
Dementia is a family of disorders characterized by a decline in multiple cognitive abilities that significantly interferes with an individual’s functioning. An estimated 50 million people are living with a dementia worldwide.1 Alzheimer’s disease (AD) is the leading cause of dementia, accounting for approximately two-thirds of dementia cases.1 These numbers are expected to increase dramatically in the upcoming decades.
Sociologist Erving Goffman defined stigma as “an attribute, behaviour, or reputation which is socially discrediting in a particular way: it causes an individual to be mentally classified by others in an undesirable, rejected stereotype rather than in an accepted, normal one.”2 Goffman2 defined 3 broad categories of stigma: public, self, and courtesy (Table 12).

Considerable evidence shows that the combined impact of having dementia and the negative response to the diagnosis significantly undermines an individual’s psychosocial well-being and quality of life.3 Persons with dementia (PwD) commonly report a loss of identity and self-worth, and stigma appears to deepen this distress.3 Stigma also negatively affects individuals associated with PwD, including family members and professionals. In this article, we discuss the impact of dementia-related stigma, and steps you can take to address it, including implementing person-centered clinical practices, promoting anti-stigma messaging campaigns, and advocating for public policy action to improve the lives of PwD and their families.
A pervasive problem
Although the Alzheimer’s Society International and the World Health Organization acknowledge that stigma has a central role in defining the experience of AD, how stigma may present, how clinicians and researchers can recognize and measure stigma, and how to best combat it have been understudied.3-5 A recent systematic literature review examined worldwide evidence on dementia-related stigma over the past decade.6 Hermann et al6 found that health care providers and the general public may hold stigmatizing attitudes toward PwD, and that stigma may be particularly harsh among racial and ethnic minorities, although the literature is scarce in this area. Cultural factors may also worsen stigma, and stigma may be associated with reduced awareness of dementia services and reduced help-seeking among minority groups.7,8 Studies show that stigmatizing attitudes are more pronounced in people with limited knowledge of dementia, in those with little contact with PwD, in men, in younger individuals, and in the context of cultural interpretations of dementia.6 Health care providers can also sometimes contribute to the perpetuation of stigma.6
In terms of standardized scales or instruments for evaluating dementia-related stigma, there is no uniformly accepted “gold standard” measure, which makes it difficult to compare studies.6 In order to effectively study efforts to reduce stigma, researchers need to identify and establish a consensus on rating scales for evaluating stigma among PwD, caregivers, and the general public. Three instruments that may be used for this purpose are the Family Stigma in Alzheimer’s Disease Scale (FS-ADS),9 the Stigma Scale for Chronic Illness (SSCI),10 and the Perceptions Regarding Investigational Screening for Memory in Primary Care (PRISM-PC).11
The detrimental effects of stigma
Burgener et al12 reported that personal stigma impacted functioning and quality of life in PwD. Higher levels of stigma were associated with higher anxiety, depression, and behavioral symptoms and lower self-esteem, social support, participation in activities, personal control, and physical health.12 Personal characteristics that may affect stigma include gender, location (rural vs urban), ethnicity, education level, and living arrangements (alone vs with family).12
In a subset of PwD with early-stage memory loss (n = 22), Burgener and Buckwalter13 found that 42% of participants were reluctant to reveal their diagnosis to others, with some fearing they would no longer be allowed to live alone and would be “sent to a facility.” In addition, 46% indicated they did not want “to be talked about like they were not there.” More than 50% of participants reported changes in their social network after receiving the diagnosis, including reducing activities and limiting types of contacts (ie, telephone only) or interacting only when “people come to me.” Participants were most comfortable with good friends “who understand” and persons within their faith communities. When asked about how they were treated by family members, >50% of participants described being treated differently, including loss of financial independence, more limited contact, and being “treated like a baby” by their children, who in general were uncomfortable talking about the diagnosis.
Continue to: In a recent study...
In a recent study by Harper et al,14 stigma was prevalent in the experience of PwD. One participant disclosed:
“I think there is [are] people I know who don’t ask me to go places or do things ’cause I have a dementia…I think lots of people don’t know what dementia is and I think it scares them ’cause they think of it as crazy. It hurts…”
Another participant said:
“I have had friends for over thirty years. They have turned their backs on me…we used to go for walks and they would phone me and go for coffee. Now I don’t hear from any of them…those aren’t true friends…true friends will stand behind you, not in front of you. That’s why I am not happy.”
Overall, quantitative and qualitative findings indicate multiple, detrimental effects of personal stigma on PwD. These effects fit well with measures of self-stigma, including social rejection (eg, being treated differently, participating in fewer activities, and having fewer friends), internalized shame (eg, being treated like a child, having fewer responsibilities, others acting as if dementia is “contagious”), and social isolation (eg, being less outgoing, feeling more comfortable in small groups, having limited social contacts).15
Continue to: Receiving a diagnosis of dementia...
Receiving a diagnosis of dementia presents patients and their families with psychological and social challenges.16 Many of these challenges are the consequence of stigma. A broad range of efforts are underway worldwide to reduce dementia-related stigma. These efforts include programs to promote public awareness and education, campaigns to develop inclusive social policies, and skills-based training initiatives to promote delivery of patient-centered care by clinicians and educators.3,17,18 Many of these efforts share a common focus on promoting the “dignity” and “personhood” of PwD in order to disrupt stereotypes or fixed, oversimplified beliefs associated with dementia.
Implementing person-centered clinical care
In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Health care communications that call attention to stereotypes may allow PwD to identify stereotypes as well as inaccuracies in those stereotypes. Interventions that validate the value of diversity can help PwD accept the ways in which they may not conform to social norms. This could include language such as “There is no one way to have Alzheimer’s disease. A person’s experience can differ from what others might experience or expect, and that’s okay.” In addition, the use of language that is accurate, respectful, inclusive, and empowering can support PwD and their caregivers.19,20 For example, referring to PwD as “individuals living with dementia” rather than “those who are demented” conveys respect and appreciation for personhood. Other clinicians have provided additional practical suggestions.21
Anti-stigma messaging campaigns
The mass media is a common source of stereotypes about AD and other dementias. They typically present a “worst-case” scenario that promotes ageism, gerontophobia, and negative emotions, which may worsen stigma and discrimination towards PwD and the people who care for them. However, public messaging campaigns are emerging to counter negative messages and stereotypes in the mass media. Projects such as Typical Day, People with Dementia, and other online anti-stigma messaging campaigns allow a broad audience to gain a more nuanced understanding of the lives of PwD and their caregivers. These projects are rich resources that offer education and personal stories that can counter common stereotypes about dementia.
Typical Day is a photography project developed and maintained by clinicians and researchers at the University of Pennsylvania. Since early 2017, the project has provided a forum for individuals with mild cognitive impairment or dementia to document their lives and show what it means to them to live with dementia. Participants in the project photo-document the people, places, and objects that define their daily lives. They review and explain these photos with researchers at Penn Memory Center, who help them tell their stories. The participants’ stories, the photos they capture, and their portraits are available at www.mytypicalday.org.
People of Dementia. Storytelling is a powerful way to raise awareness of and reduce the stigma associated with dementia. For PwD, telling their stories can be an effective and therapeutic way to communicate their emotions and deliver an important message. In the blog People of Dementia (www.peopleofdementia.com),22,23 PwD highlight who they were before the disease and how things have changed, with family members highlighting the challenges of caring for a person with dementia.
Continue to: The common thread is...
The common thread is the enduring “person” behind the exterior that is obscured by dementia. By allowing the audience to form a connection with who the individual was prior to the disease, and understanding the changes that have come as a result of dementia to both PwD and their support network, readers gain a greater appreciation of those affected by dementia. Between May 1, 2017 and May 31, 2019, the blog had more than 3,860 visitors. In an accompanying online survey (N = 57), 79% of respondents agreed/strongly agreed that after visiting the People of Dementia blog, they had a better understanding of the changes that occur as a result of cognitive impairment/dementia (Figure 1). Almost two-thirds of respondents (65%) agreed/strongly agreed that they felt more comfortable interacting with PwD (Figure 2). Additionally, 60% of respondents agreed/strongly agreed that they were more encouraged to work with PwD, and 90% agreed/strongly agreed that they had a greater appreciation of the challenges of being a caregiver for PwD. Overall, these findings suggest that the People of Dementia blog is useful for engaging the public and promoting a better understanding of dementia.
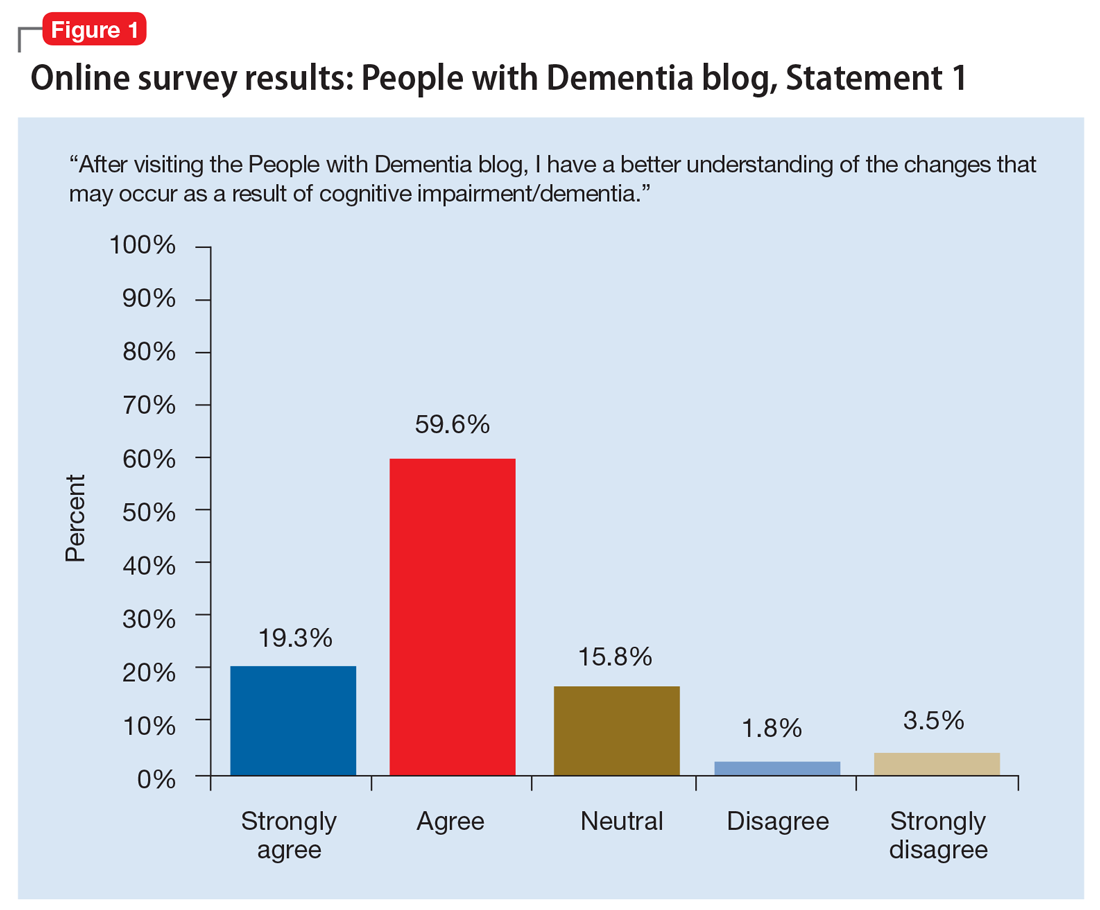
Work for policy changes
Clinicians can support public policy through education and advocacy both in the delivery of care and as spokespersons and stakeholders in their local communities. Public policies are important for providing access to medical and social services to meet the needs of PwD and their caregivers. The absence—real or perceived—of sufficient resources exacerbates dementia-related stigma. In addition to facilitating access to resources, national dementia strategies or legal frameworks, such as the National Alzheimer’s Project Act in the United States, include policy initiatives to identify and promote communication approaches that are effective and sensitive with respect to people living with dementia and their caregivers.
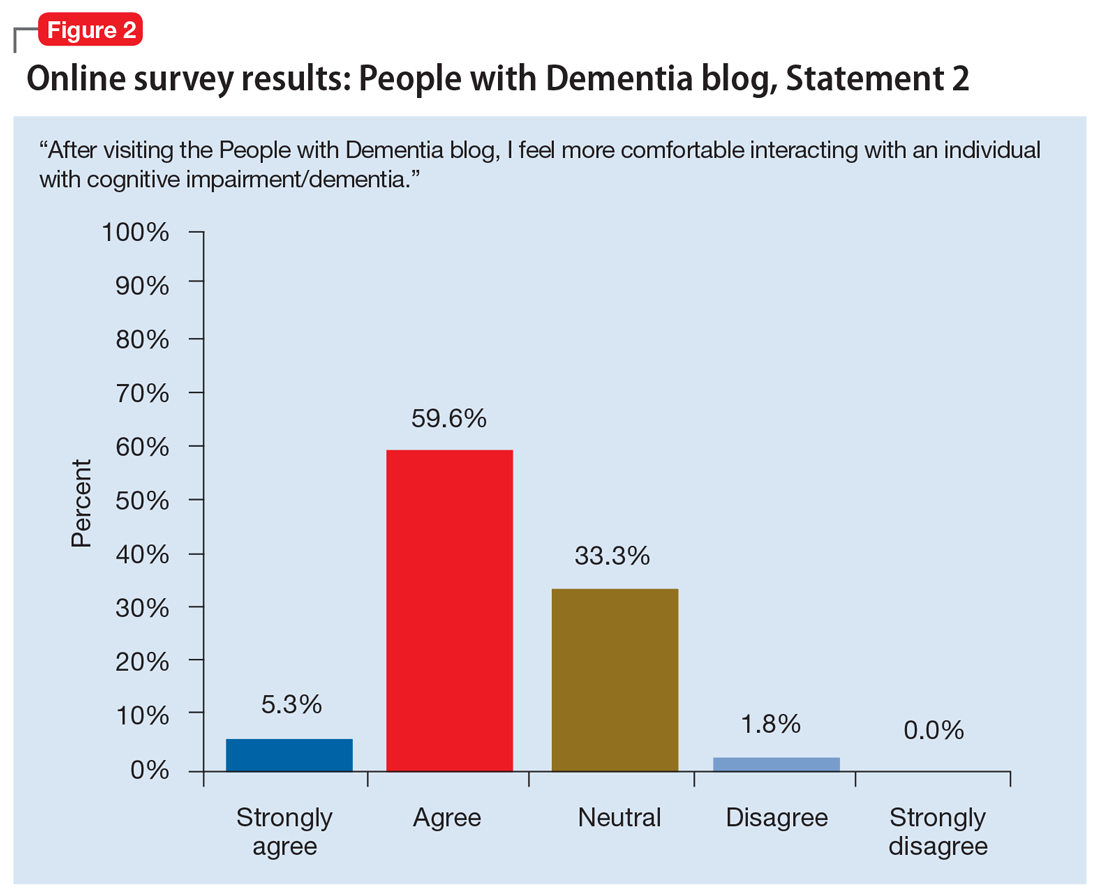
State and local legislators and patient advocates are leading policy efforts to reduce dementia-related stigma. For example, Colorado recently changed statutory references from being specific to diseases that cause dementia to the broader, more inclusive phrase “dementia diseases and related disabilities.”18 In addition to making funds available to support caregiving services for PwD, this legislative change added training for first responders to better meet the needs of missing PwD, and shifted the terminology used to diagnose and communicate about diseases causing dementia. The shift in language added new terminology that was chosen for being more person-centered to replace prior references to “senior senility,” “senility,” and other terms with pejorative meanings.
In Canada, a National Dementia Strategy will commit the Canadian government to action with definitive timelines, targets, reporting structures, and measurable outcomes.24
Table 2 summarizes approaches to a
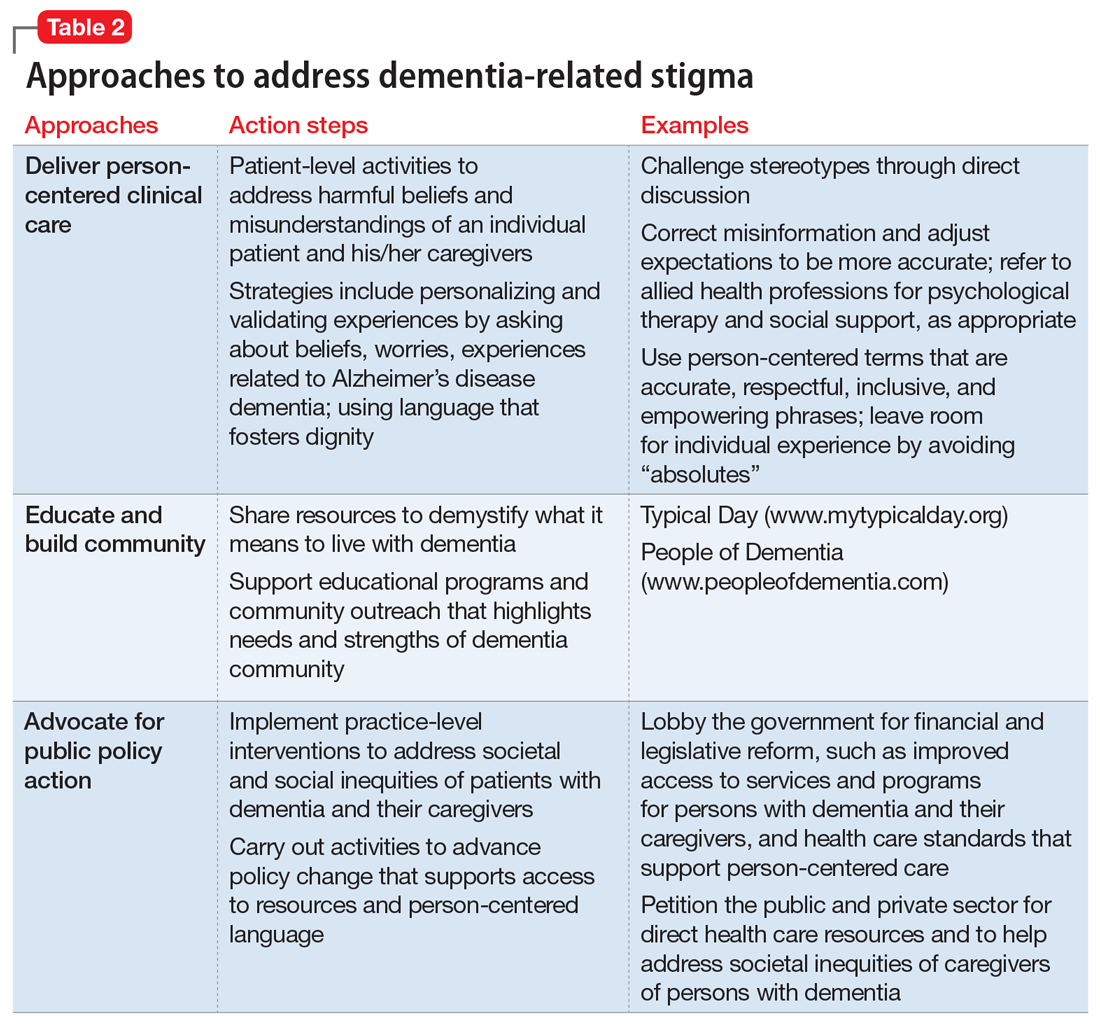
Continue to: An open discussion
An open discussion
Larger studies and testing of diverse approaches are needed to better understand whether intergenerational initiatives or other approaches can genuinely modify stigmatizing attitudes in various dementia populations, especially considering language, health literacy, cultural preferences, and other needs. The identified effects on physical and mental health, quality of life, self-esteem, and behavioral symptoms further support the extensive, negative effects of self-stigma on PwD, and emphasize the need to develop and test interventions to ameliorate these effects.
We presented at a Stigma Symposium at the 2018 Gerontological Society of America Annual Scientific Meeting in Boston, Massachusetts.25 Attendees of this conference shared our concerns about the detrimental effects of stigma. The main question we were asked was “What can we do to reduce stigma?” Perhaps the most immediate response is that in order to move the stigma dial, clinicians need to recognize that stigma has multiple, broad-reaching, and negative effects on PwD and their families.6 Bringing the discussion into the open and targeting stigma at multiple levels needs to be addressed by clinicians, researchers, administrators, and society at large.
Bottom Line
Stigma has multiple, broad-reaching, and negative effects on persons with dementia and their families. In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Anti-stigma messaging campaigns and public policy changes also can be used to address societal and social inequities of patients with dementia and their caregivers.
Related Resources
- Khoury R, Shach R, Nair A, et al. Can lifestyle modifications delay or prevent Alzheimer’s disease? Current Psychiatry. 2019;18(1):29-36,38.
- Burke AD, Burke WJ. Antipsychotics for patients with dementia: The road less traveled. Current Psychiatry. 2018;17(10):26-32,35-37.
1. World Health Organization. Towards a dementia plan: a WHO guide. https://www.who.int/mental_health/neurology/dementia/policy_guidance/en/. Published 2018. Accessed May 28, 2019.
2. Goffman E. Stigma. New York, NY: Prentice-Hall; 1963:1-123.
3. Alzheimer’s Disease International. World Alzheimer Report 2012: overcoming the stigma of dementia. https://www.alz.co.uk/research/WorldAlzheimerReport2012.pdf. Published 2012. Accessed May 28, 2019.
4. Blay SL, Peluso ETP. Public stigma: the community’s tolerance of Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(2):163-171.
5. Piver LC, Nubukpo P, Faure A, et al. Describing perceived stigma against Alzheimer’s disease in a general population in France: the STIG-MA survey. Int J Geriatr Psychiatry. 2013;28(9):933-938.
6. Herrmann LK, Welter E, Leverenz J, et al. A systematic review of dementia-related stigma research: can we move the stigma dial? Am J Geriatr Psychiatry. 2018;26(3):316-331.
7. Eng KJ, Woo BKP. Knowledge of dementia community resources and stigma among Chinese American immigrants. Gen Hosp Psychiatry. 2015;37(1):e3-e4. doi:10.1016/j.genhosppsych.2014.11.003.
8. Jang Y, Kim G, Chiriboga D. Knowledge of Alzheimer’s disease, feelings of shame, and awareness of services among Korean American elders. J Aging Health. 2010;22(4):419-433.
9. Werner P, Goldstein D, Heinik J. Development and validity of the Family Stigma in Alzheimer’s disease scale (FS-ADS). Alzheimer Disease & Associated Disorders. 2011;25(1):42-48.
10. Rao D, Choi SW, Victorson D, et al. Measuring stigma across neurological conditions: the development of the stigma scale for chronic illness (SSCI). Qual Life Res. 2009;18(5):585-595.
11. Boustani M, Perkins AJ, Monahan P, et al. Measuring primary care patients’ attitudes about dementia screening. Int J Geriatr Psychiatry. 2008;23(8):812-820.
12. Burgener SC, Buckwalter K, Perkounkova Y, et al. Perceived stigma in persons with early-stage dementia: longitudinal findings: Part 2. Dementia. 2015;14(5):609-632.
13. Burgener SC, Buckwalter K. The effects of perceived stigma on persons with dementia and their family caregivers. In: Symposium on Stigma: It’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
14. Harper L, Dobbs B, Royan H, et al. The experience of stigma in care partners of people with dementia – results from an exploratory study. In Symposium on stigma: it’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
15. Burgener S, Berger B. Measuring perceived stigma in persons with progressive neurological disease: Alzheimer’s dementia and Parkinson disease. Dementia. 2008;7(1):31-53.
16. Stites SD, Milne R, Karlawish J. Advances in Alzheimer’s imaging are changing the experience of Alzheimer’s disease. Alzheimer’s & Dementia. 2018;10;285-300.
17. Anderson LA, Egge R. Expanding efforts to address Alzheimer’s disease: the Healthy Brain Initiative. Alzheimer’s Dement. 2014;10(50):S453-S456.
18. Alzheimer’s Association National Plan Milestone Workgroup. Report on the milestones for the US National plan to address Alzheimer’s disease. Alzheimer’s Dementia. 2014;10(Suppl 5);S430-S452. doi:10.1016/j/jalz.2014.08.103.
19. Kirkman AM. Dementia in the news: the media coverage of Alzheimer’s disease. Australasian Journal on Ageing. 2006;25(2):74-79.
20. Swaffer, K. Dementia: stigma, language, and dementia-friendly. Dementia. 2014;13(6):709-716.
21. Stites SD, Karlawish J. Stigma of Alzheimer’s disease dementia: considerations for practice. Practical Neurology. https://practicalneurology.com/articles/2018-june/stigma-of-alzheimers-disease-dementia. Published June 2018. Accessed May 28, 2019.
22. Jamieson J, Dobbs B, Charles L, et al. Forgetful, but not forgotten people of dementia: a novel, technology focused project with a humanistic touch. Geriatric Grand Rounds; October 10, 2017. Edmonton, Alberta, Canada.
23. Dobbs B, Charles L, Chan K, et al. People of Dementia. CGS 37th Annual Scientific Meeting: Integrating Care, Making an Impact. Can Geriatr J. 2017;20(3):220.
24. Government of Canada. Conference report: National Dementia Conference. https://www.canada.ca/en/services/health/publications/diseases-conditions/national-dementia-conference-report.html. Government of Canada. Published August 2018. Accessed May 28, 2019.
25. The Gerontological Society of America. Program Abstracts from the GSA 2018 Annual Scientific Meeting “The Purposes of Longer Lives.” Innovation in Aging. 2018;2(Suppl 1):143.
Dementia is a family of disorders characterized by a decline in multiple cognitive abilities that significantly interferes with an individual’s functioning. An estimated 50 million people are living with a dementia worldwide.1 Alzheimer’s disease (AD) is the leading cause of dementia, accounting for approximately two-thirds of dementia cases.1 These numbers are expected to increase dramatically in the upcoming decades.
Sociologist Erving Goffman defined stigma as “an attribute, behaviour, or reputation which is socially discrediting in a particular way: it causes an individual to be mentally classified by others in an undesirable, rejected stereotype rather than in an accepted, normal one.”2 Goffman2 defined 3 broad categories of stigma: public, self, and courtesy (Table 12).

Considerable evidence shows that the combined impact of having dementia and the negative response to the diagnosis significantly undermines an individual’s psychosocial well-being and quality of life.3 Persons with dementia (PwD) commonly report a loss of identity and self-worth, and stigma appears to deepen this distress.3 Stigma also negatively affects individuals associated with PwD, including family members and professionals. In this article, we discuss the impact of dementia-related stigma, and steps you can take to address it, including implementing person-centered clinical practices, promoting anti-stigma messaging campaigns, and advocating for public policy action to improve the lives of PwD and their families.
A pervasive problem
Although the Alzheimer’s Society International and the World Health Organization acknowledge that stigma has a central role in defining the experience of AD, how stigma may present, how clinicians and researchers can recognize and measure stigma, and how to best combat it have been understudied.3-5 A recent systematic literature review examined worldwide evidence on dementia-related stigma over the past decade.6 Hermann et al6 found that health care providers and the general public may hold stigmatizing attitudes toward PwD, and that stigma may be particularly harsh among racial and ethnic minorities, although the literature is scarce in this area. Cultural factors may also worsen stigma, and stigma may be associated with reduced awareness of dementia services and reduced help-seeking among minority groups.7,8 Studies show that stigmatizing attitudes are more pronounced in people with limited knowledge of dementia, in those with little contact with PwD, in men, in younger individuals, and in the context of cultural interpretations of dementia.6 Health care providers can also sometimes contribute to the perpetuation of stigma.6
In terms of standardized scales or instruments for evaluating dementia-related stigma, there is no uniformly accepted “gold standard” measure, which makes it difficult to compare studies.6 In order to effectively study efforts to reduce stigma, researchers need to identify and establish a consensus on rating scales for evaluating stigma among PwD, caregivers, and the general public. Three instruments that may be used for this purpose are the Family Stigma in Alzheimer’s Disease Scale (FS-ADS),9 the Stigma Scale for Chronic Illness (SSCI),10 and the Perceptions Regarding Investigational Screening for Memory in Primary Care (PRISM-PC).11
The detrimental effects of stigma
Burgener et al12 reported that personal stigma impacted functioning and quality of life in PwD. Higher levels of stigma were associated with higher anxiety, depression, and behavioral symptoms and lower self-esteem, social support, participation in activities, personal control, and physical health.12 Personal characteristics that may affect stigma include gender, location (rural vs urban), ethnicity, education level, and living arrangements (alone vs with family).12
In a subset of PwD with early-stage memory loss (n = 22), Burgener and Buckwalter13 found that 42% of participants were reluctant to reveal their diagnosis to others, with some fearing they would no longer be allowed to live alone and would be “sent to a facility.” In addition, 46% indicated they did not want “to be talked about like they were not there.” More than 50% of participants reported changes in their social network after receiving the diagnosis, including reducing activities and limiting types of contacts (ie, telephone only) or interacting only when “people come to me.” Participants were most comfortable with good friends “who understand” and persons within their faith communities. When asked about how they were treated by family members, >50% of participants described being treated differently, including loss of financial independence, more limited contact, and being “treated like a baby” by their children, who in general were uncomfortable talking about the diagnosis.
Continue to: In a recent study...
In a recent study by Harper et al,14 stigma was prevalent in the experience of PwD. One participant disclosed:
“I think there is [are] people I know who don’t ask me to go places or do things ’cause I have a dementia…I think lots of people don’t know what dementia is and I think it scares them ’cause they think of it as crazy. It hurts…”
Another participant said:
“I have had friends for over thirty years. They have turned their backs on me…we used to go for walks and they would phone me and go for coffee. Now I don’t hear from any of them…those aren’t true friends…true friends will stand behind you, not in front of you. That’s why I am not happy.”
Overall, quantitative and qualitative findings indicate multiple, detrimental effects of personal stigma on PwD. These effects fit well with measures of self-stigma, including social rejection (eg, being treated differently, participating in fewer activities, and having fewer friends), internalized shame (eg, being treated like a child, having fewer responsibilities, others acting as if dementia is “contagious”), and social isolation (eg, being less outgoing, feeling more comfortable in small groups, having limited social contacts).15
Continue to: Receiving a diagnosis of dementia...
Receiving a diagnosis of dementia presents patients and their families with psychological and social challenges.16 Many of these challenges are the consequence of stigma. A broad range of efforts are underway worldwide to reduce dementia-related stigma. These efforts include programs to promote public awareness and education, campaigns to develop inclusive social policies, and skills-based training initiatives to promote delivery of patient-centered care by clinicians and educators.3,17,18 Many of these efforts share a common focus on promoting the “dignity” and “personhood” of PwD in order to disrupt stereotypes or fixed, oversimplified beliefs associated with dementia.
Implementing person-centered clinical care
In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Health care communications that call attention to stereotypes may allow PwD to identify stereotypes as well as inaccuracies in those stereotypes. Interventions that validate the value of diversity can help PwD accept the ways in which they may not conform to social norms. This could include language such as “There is no one way to have Alzheimer’s disease. A person’s experience can differ from what others might experience or expect, and that’s okay.” In addition, the use of language that is accurate, respectful, inclusive, and empowering can support PwD and their caregivers.19,20 For example, referring to PwD as “individuals living with dementia” rather than “those who are demented” conveys respect and appreciation for personhood. Other clinicians have provided additional practical suggestions.21
Anti-stigma messaging campaigns
The mass media is a common source of stereotypes about AD and other dementias. They typically present a “worst-case” scenario that promotes ageism, gerontophobia, and negative emotions, which may worsen stigma and discrimination towards PwD and the people who care for them. However, public messaging campaigns are emerging to counter negative messages and stereotypes in the mass media. Projects such as Typical Day, People with Dementia, and other online anti-stigma messaging campaigns allow a broad audience to gain a more nuanced understanding of the lives of PwD and their caregivers. These projects are rich resources that offer education and personal stories that can counter common stereotypes about dementia.
Typical Day is a photography project developed and maintained by clinicians and researchers at the University of Pennsylvania. Since early 2017, the project has provided a forum for individuals with mild cognitive impairment or dementia to document their lives and show what it means to them to live with dementia. Participants in the project photo-document the people, places, and objects that define their daily lives. They review and explain these photos with researchers at Penn Memory Center, who help them tell their stories. The participants’ stories, the photos they capture, and their portraits are available at www.mytypicalday.org.
People of Dementia. Storytelling is a powerful way to raise awareness of and reduce the stigma associated with dementia. For PwD, telling their stories can be an effective and therapeutic way to communicate their emotions and deliver an important message. In the blog People of Dementia (www.peopleofdementia.com),22,23 PwD highlight who they were before the disease and how things have changed, with family members highlighting the challenges of caring for a person with dementia.
Continue to: The common thread is...
The common thread is the enduring “person” behind the exterior that is obscured by dementia. By allowing the audience to form a connection with who the individual was prior to the disease, and understanding the changes that have come as a result of dementia to both PwD and their support network, readers gain a greater appreciation of those affected by dementia. Between May 1, 2017 and May 31, 2019, the blog had more than 3,860 visitors. In an accompanying online survey (N = 57), 79% of respondents agreed/strongly agreed that after visiting the People of Dementia blog, they had a better understanding of the changes that occur as a result of cognitive impairment/dementia (Figure 1). Almost two-thirds of respondents (65%) agreed/strongly agreed that they felt more comfortable interacting with PwD (Figure 2). Additionally, 60% of respondents agreed/strongly agreed that they were more encouraged to work with PwD, and 90% agreed/strongly agreed that they had a greater appreciation of the challenges of being a caregiver for PwD. Overall, these findings suggest that the People of Dementia blog is useful for engaging the public and promoting a better understanding of dementia.

Work for policy changes
Clinicians can support public policy through education and advocacy both in the delivery of care and as spokespersons and stakeholders in their local communities. Public policies are important for providing access to medical and social services to meet the needs of PwD and their caregivers. The absence—real or perceived—of sufficient resources exacerbates dementia-related stigma. In addition to facilitating access to resources, national dementia strategies or legal frameworks, such as the National Alzheimer’s Project Act in the United States, include policy initiatives to identify and promote communication approaches that are effective and sensitive with respect to people living with dementia and their caregivers.

State and local legislators and patient advocates are leading policy efforts to reduce dementia-related stigma. For example, Colorado recently changed statutory references from being specific to diseases that cause dementia to the broader, more inclusive phrase “dementia diseases and related disabilities.”18 In addition to making funds available to support caregiving services for PwD, this legislative change added training for first responders to better meet the needs of missing PwD, and shifted the terminology used to diagnose and communicate about diseases causing dementia. The shift in language added new terminology that was chosen for being more person-centered to replace prior references to “senior senility,” “senility,” and other terms with pejorative meanings.
In Canada, a National Dementia Strategy will commit the Canadian government to action with definitive timelines, targets, reporting structures, and measurable outcomes.24
Table 2 summarizes approaches to a

Continue to: An open discussion
An open discussion
Larger studies and testing of diverse approaches are needed to better understand whether intergenerational initiatives or other approaches can genuinely modify stigmatizing attitudes in various dementia populations, especially considering language, health literacy, cultural preferences, and other needs. The identified effects on physical and mental health, quality of life, self-esteem, and behavioral symptoms further support the extensive, negative effects of self-stigma on PwD, and emphasize the need to develop and test interventions to ameliorate these effects.
We presented at a Stigma Symposium at the 2018 Gerontological Society of America Annual Scientific Meeting in Boston, Massachusetts.25 Attendees of this conference shared our concerns about the detrimental effects of stigma. The main question we were asked was “What can we do to reduce stigma?” Perhaps the most immediate response is that in order to move the stigma dial, clinicians need to recognize that stigma has multiple, broad-reaching, and negative effects on PwD and their families.6 Bringing the discussion into the open and targeting stigma at multiple levels needs to be addressed by clinicians, researchers, administrators, and society at large.
Bottom Line
Stigma has multiple, broad-reaching, and negative effects on persons with dementia and their families. In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Anti-stigma messaging campaigns and public policy changes also can be used to address societal and social inequities of patients with dementia and their caregivers.
Related Resources
- Khoury R, Shach R, Nair A, et al. Can lifestyle modifications delay or prevent Alzheimer’s disease? Current Psychiatry. 2019;18(1):29-36,38.
- Burke AD, Burke WJ. Antipsychotics for patients with dementia: The road less traveled. Current Psychiatry. 2018;17(10):26-32,35-37.
Dementia is a family of disorders characterized by a decline in multiple cognitive abilities that significantly interferes with an individual’s functioning. An estimated 50 million people are living with a dementia worldwide.1 Alzheimer’s disease (AD) is the leading cause of dementia, accounting for approximately two-thirds of dementia cases.1 These numbers are expected to increase dramatically in the upcoming decades.
Sociologist Erving Goffman defined stigma as “an attribute, behaviour, or reputation which is socially discrediting in a particular way: it causes an individual to be mentally classified by others in an undesirable, rejected stereotype rather than in an accepted, normal one.”2 Goffman2 defined 3 broad categories of stigma: public, self, and courtesy (Table 12).

Considerable evidence shows that the combined impact of having dementia and the negative response to the diagnosis significantly undermines an individual’s psychosocial well-being and quality of life.3 Persons with dementia (PwD) commonly report a loss of identity and self-worth, and stigma appears to deepen this distress.3 Stigma also negatively affects individuals associated with PwD, including family members and professionals. In this article, we discuss the impact of dementia-related stigma, and steps you can take to address it, including implementing person-centered clinical practices, promoting anti-stigma messaging campaigns, and advocating for public policy action to improve the lives of PwD and their families.
A pervasive problem
Although the Alzheimer’s Society International and the World Health Organization acknowledge that stigma has a central role in defining the experience of AD, how stigma may present, how clinicians and researchers can recognize and measure stigma, and how to best combat it have been understudied.3-5 A recent systematic literature review examined worldwide evidence on dementia-related stigma over the past decade.6 Hermann et al6 found that health care providers and the general public may hold stigmatizing attitudes toward PwD, and that stigma may be particularly harsh among racial and ethnic minorities, although the literature is scarce in this area. Cultural factors may also worsen stigma, and stigma may be associated with reduced awareness of dementia services and reduced help-seeking among minority groups.7,8 Studies show that stigmatizing attitudes are more pronounced in people with limited knowledge of dementia, in those with little contact with PwD, in men, in younger individuals, and in the context of cultural interpretations of dementia.6 Health care providers can also sometimes contribute to the perpetuation of stigma.6
In terms of standardized scales or instruments for evaluating dementia-related stigma, there is no uniformly accepted “gold standard” measure, which makes it difficult to compare studies.6 In order to effectively study efforts to reduce stigma, researchers need to identify and establish a consensus on rating scales for evaluating stigma among PwD, caregivers, and the general public. Three instruments that may be used for this purpose are the Family Stigma in Alzheimer’s Disease Scale (FS-ADS),9 the Stigma Scale for Chronic Illness (SSCI),10 and the Perceptions Regarding Investigational Screening for Memory in Primary Care (PRISM-PC).11
The detrimental effects of stigma
Burgener et al12 reported that personal stigma impacted functioning and quality of life in PwD. Higher levels of stigma were associated with higher anxiety, depression, and behavioral symptoms and lower self-esteem, social support, participation in activities, personal control, and physical health.12 Personal characteristics that may affect stigma include gender, location (rural vs urban), ethnicity, education level, and living arrangements (alone vs with family).12
In a subset of PwD with early-stage memory loss (n = 22), Burgener and Buckwalter13 found that 42% of participants were reluctant to reveal their diagnosis to others, with some fearing they would no longer be allowed to live alone and would be “sent to a facility.” In addition, 46% indicated they did not want “to be talked about like they were not there.” More than 50% of participants reported changes in their social network after receiving the diagnosis, including reducing activities and limiting types of contacts (ie, telephone only) or interacting only when “people come to me.” Participants were most comfortable with good friends “who understand” and persons within their faith communities. When asked about how they were treated by family members, >50% of participants described being treated differently, including loss of financial independence, more limited contact, and being “treated like a baby” by their children, who in general were uncomfortable talking about the diagnosis.
Continue to: In a recent study...
In a recent study by Harper et al,14 stigma was prevalent in the experience of PwD. One participant disclosed:
“I think there is [are] people I know who don’t ask me to go places or do things ’cause I have a dementia…I think lots of people don’t know what dementia is and I think it scares them ’cause they think of it as crazy. It hurts…”
Another participant said:
“I have had friends for over thirty years. They have turned their backs on me…we used to go for walks and they would phone me and go for coffee. Now I don’t hear from any of them…those aren’t true friends…true friends will stand behind you, not in front of you. That’s why I am not happy.”
Overall, quantitative and qualitative findings indicate multiple, detrimental effects of personal stigma on PwD. These effects fit well with measures of self-stigma, including social rejection (eg, being treated differently, participating in fewer activities, and having fewer friends), internalized shame (eg, being treated like a child, having fewer responsibilities, others acting as if dementia is “contagious”), and social isolation (eg, being less outgoing, feeling more comfortable in small groups, having limited social contacts).15
Continue to: Receiving a diagnosis of dementia...
Receiving a diagnosis of dementia presents patients and their families with psychological and social challenges.16 Many of these challenges are the consequence of stigma. A broad range of efforts are underway worldwide to reduce dementia-related stigma. These efforts include programs to promote public awareness and education, campaigns to develop inclusive social policies, and skills-based training initiatives to promote delivery of patient-centered care by clinicians and educators.3,17,18 Many of these efforts share a common focus on promoting the “dignity” and “personhood” of PwD in order to disrupt stereotypes or fixed, oversimplified beliefs associated with dementia.
Implementing person-centered clinical care
In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Health care communications that call attention to stereotypes may allow PwD to identify stereotypes as well as inaccuracies in those stereotypes. Interventions that validate the value of diversity can help PwD accept the ways in which they may not conform to social norms. This could include language such as “There is no one way to have Alzheimer’s disease. A person’s experience can differ from what others might experience or expect, and that’s okay.” In addition, the use of language that is accurate, respectful, inclusive, and empowering can support PwD and their caregivers.19,20 For example, referring to PwD as “individuals living with dementia” rather than “those who are demented” conveys respect and appreciation for personhood. Other clinicians have provided additional practical suggestions.21
Anti-stigma messaging campaigns
The mass media is a common source of stereotypes about AD and other dementias. They typically present a “worst-case” scenario that promotes ageism, gerontophobia, and negative emotions, which may worsen stigma and discrimination towards PwD and the people who care for them. However, public messaging campaigns are emerging to counter negative messages and stereotypes in the mass media. Projects such as Typical Day, People with Dementia, and other online anti-stigma messaging campaigns allow a broad audience to gain a more nuanced understanding of the lives of PwD and their caregivers. These projects are rich resources that offer education and personal stories that can counter common stereotypes about dementia.
Typical Day is a photography project developed and maintained by clinicians and researchers at the University of Pennsylvania. Since early 2017, the project has provided a forum for individuals with mild cognitive impairment or dementia to document their lives and show what it means to them to live with dementia. Participants in the project photo-document the people, places, and objects that define their daily lives. They review and explain these photos with researchers at Penn Memory Center, who help them tell their stories. The participants’ stories, the photos they capture, and their portraits are available at www.mytypicalday.org.
People of Dementia. Storytelling is a powerful way to raise awareness of and reduce the stigma associated with dementia. For PwD, telling their stories can be an effective and therapeutic way to communicate their emotions and deliver an important message. In the blog People of Dementia (www.peopleofdementia.com),22,23 PwD highlight who they were before the disease and how things have changed, with family members highlighting the challenges of caring for a person with dementia.
Continue to: The common thread is...
The common thread is the enduring “person” behind the exterior that is obscured by dementia. By allowing the audience to form a connection with who the individual was prior to the disease, and understanding the changes that have come as a result of dementia to both PwD and their support network, readers gain a greater appreciation of those affected by dementia. Between May 1, 2017 and May 31, 2019, the blog had more than 3,860 visitors. In an accompanying online survey (N = 57), 79% of respondents agreed/strongly agreed that after visiting the People of Dementia blog, they had a better understanding of the changes that occur as a result of cognitive impairment/dementia (Figure 1). Almost two-thirds of respondents (65%) agreed/strongly agreed that they felt more comfortable interacting with PwD (Figure 2). Additionally, 60% of respondents agreed/strongly agreed that they were more encouraged to work with PwD, and 90% agreed/strongly agreed that they had a greater appreciation of the challenges of being a caregiver for PwD. Overall, these findings suggest that the People of Dementia blog is useful for engaging the public and promoting a better understanding of dementia.

Work for policy changes
Clinicians can support public policy through education and advocacy both in the delivery of care and as spokespersons and stakeholders in their local communities. Public policies are important for providing access to medical and social services to meet the needs of PwD and their caregivers. The absence—real or perceived—of sufficient resources exacerbates dementia-related stigma. In addition to facilitating access to resources, national dementia strategies or legal frameworks, such as the National Alzheimer’s Project Act in the United States, include policy initiatives to identify and promote communication approaches that are effective and sensitive with respect to people living with dementia and their caregivers.

State and local legislators and patient advocates are leading policy efforts to reduce dementia-related stigma. For example, Colorado recently changed statutory references from being specific to diseases that cause dementia to the broader, more inclusive phrase “dementia diseases and related disabilities.”18 In addition to making funds available to support caregiving services for PwD, this legislative change added training for first responders to better meet the needs of missing PwD, and shifted the terminology used to diagnose and communicate about diseases causing dementia. The shift in language added new terminology that was chosen for being more person-centered to replace prior references to “senior senility,” “senility,” and other terms with pejorative meanings.
In Canada, a National Dementia Strategy will commit the Canadian government to action with definitive timelines, targets, reporting structures, and measurable outcomes.24
Table 2 summarizes approaches to a

Continue to: An open discussion
An open discussion
Larger studies and testing of diverse approaches are needed to better understand whether intergenerational initiatives or other approaches can genuinely modify stigmatizing attitudes in various dementia populations, especially considering language, health literacy, cultural preferences, and other needs. The identified effects on physical and mental health, quality of life, self-esteem, and behavioral symptoms further support the extensive, negative effects of self-stigma on PwD, and emphasize the need to develop and test interventions to ameliorate these effects.
We presented at a Stigma Symposium at the 2018 Gerontological Society of America Annual Scientific Meeting in Boston, Massachusetts.25 Attendees of this conference shared our concerns about the detrimental effects of stigma. The main question we were asked was “What can we do to reduce stigma?” Perhaps the most immediate response is that in order to move the stigma dial, clinicians need to recognize that stigma has multiple, broad-reaching, and negative effects on PwD and their families.6 Bringing the discussion into the open and targeting stigma at multiple levels needs to be addressed by clinicians, researchers, administrators, and society at large.
Bottom Line
Stigma has multiple, broad-reaching, and negative effects on persons with dementia and their families. In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Anti-stigma messaging campaigns and public policy changes also can be used to address societal and social inequities of patients with dementia and their caregivers.
Related Resources
- Khoury R, Shach R, Nair A, et al. Can lifestyle modifications delay or prevent Alzheimer’s disease? Current Psychiatry. 2019;18(1):29-36,38.
- Burke AD, Burke WJ. Antipsychotics for patients with dementia: The road less traveled. Current Psychiatry. 2018;17(10):26-32,35-37.
1. World Health Organization. Towards a dementia plan: a WHO guide. https://www.who.int/mental_health/neurology/dementia/policy_guidance/en/. Published 2018. Accessed May 28, 2019.
2. Goffman E. Stigma. New York, NY: Prentice-Hall; 1963:1-123.
3. Alzheimer’s Disease International. World Alzheimer Report 2012: overcoming the stigma of dementia. https://www.alz.co.uk/research/WorldAlzheimerReport2012.pdf. Published 2012. Accessed May 28, 2019.
4. Blay SL, Peluso ETP. Public stigma: the community’s tolerance of Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(2):163-171.
5. Piver LC, Nubukpo P, Faure A, et al. Describing perceived stigma against Alzheimer’s disease in a general population in France: the STIG-MA survey. Int J Geriatr Psychiatry. 2013;28(9):933-938.
6. Herrmann LK, Welter E, Leverenz J, et al. A systematic review of dementia-related stigma research: can we move the stigma dial? Am J Geriatr Psychiatry. 2018;26(3):316-331.
7. Eng KJ, Woo BKP. Knowledge of dementia community resources and stigma among Chinese American immigrants. Gen Hosp Psychiatry. 2015;37(1):e3-e4. doi:10.1016/j.genhosppsych.2014.11.003.
8. Jang Y, Kim G, Chiriboga D. Knowledge of Alzheimer’s disease, feelings of shame, and awareness of services among Korean American elders. J Aging Health. 2010;22(4):419-433.
9. Werner P, Goldstein D, Heinik J. Development and validity of the Family Stigma in Alzheimer’s disease scale (FS-ADS). Alzheimer Disease & Associated Disorders. 2011;25(1):42-48.
10. Rao D, Choi SW, Victorson D, et al. Measuring stigma across neurological conditions: the development of the stigma scale for chronic illness (SSCI). Qual Life Res. 2009;18(5):585-595.
11. Boustani M, Perkins AJ, Monahan P, et al. Measuring primary care patients’ attitudes about dementia screening. Int J Geriatr Psychiatry. 2008;23(8):812-820.
12. Burgener SC, Buckwalter K, Perkounkova Y, et al. Perceived stigma in persons with early-stage dementia: longitudinal findings: Part 2. Dementia. 2015;14(5):609-632.
13. Burgener SC, Buckwalter K. The effects of perceived stigma on persons with dementia and their family caregivers. In: Symposium on Stigma: It’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
14. Harper L, Dobbs B, Royan H, et al. The experience of stigma in care partners of people with dementia – results from an exploratory study. In Symposium on stigma: it’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
15. Burgener S, Berger B. Measuring perceived stigma in persons with progressive neurological disease: Alzheimer’s dementia and Parkinson disease. Dementia. 2008;7(1):31-53.
16. Stites SD, Milne R, Karlawish J. Advances in Alzheimer’s imaging are changing the experience of Alzheimer’s disease. Alzheimer’s & Dementia. 2018;10;285-300.
17. Anderson LA, Egge R. Expanding efforts to address Alzheimer’s disease: the Healthy Brain Initiative. Alzheimer’s Dement. 2014;10(50):S453-S456.
18. Alzheimer’s Association National Plan Milestone Workgroup. Report on the milestones for the US National plan to address Alzheimer’s disease. Alzheimer’s Dementia. 2014;10(Suppl 5);S430-S452. doi:10.1016/j/jalz.2014.08.103.
19. Kirkman AM. Dementia in the news: the media coverage of Alzheimer’s disease. Australasian Journal on Ageing. 2006;25(2):74-79.
20. Swaffer, K. Dementia: stigma, language, and dementia-friendly. Dementia. 2014;13(6):709-716.
21. Stites SD, Karlawish J. Stigma of Alzheimer’s disease dementia: considerations for practice. Practical Neurology. https://practicalneurology.com/articles/2018-june/stigma-of-alzheimers-disease-dementia. Published June 2018. Accessed May 28, 2019.
22. Jamieson J, Dobbs B, Charles L, et al. Forgetful, but not forgotten people of dementia: a novel, technology focused project with a humanistic touch. Geriatric Grand Rounds; October 10, 2017. Edmonton, Alberta, Canada.
23. Dobbs B, Charles L, Chan K, et al. People of Dementia. CGS 37th Annual Scientific Meeting: Integrating Care, Making an Impact. Can Geriatr J. 2017;20(3):220.
24. Government of Canada. Conference report: National Dementia Conference. https://www.canada.ca/en/services/health/publications/diseases-conditions/national-dementia-conference-report.html. Government of Canada. Published August 2018. Accessed May 28, 2019.
25. The Gerontological Society of America. Program Abstracts from the GSA 2018 Annual Scientific Meeting “The Purposes of Longer Lives.” Innovation in Aging. 2018;2(Suppl 1):143.
1. World Health Organization. Towards a dementia plan: a WHO guide. https://www.who.int/mental_health/neurology/dementia/policy_guidance/en/. Published 2018. Accessed May 28, 2019.
2. Goffman E. Stigma. New York, NY: Prentice-Hall; 1963:1-123.
3. Alzheimer’s Disease International. World Alzheimer Report 2012: overcoming the stigma of dementia. https://www.alz.co.uk/research/WorldAlzheimerReport2012.pdf. Published 2012. Accessed May 28, 2019.
4. Blay SL, Peluso ETP. Public stigma: the community’s tolerance of Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(2):163-171.
5. Piver LC, Nubukpo P, Faure A, et al. Describing perceived stigma against Alzheimer’s disease in a general population in France: the STIG-MA survey. Int J Geriatr Psychiatry. 2013;28(9):933-938.
6. Herrmann LK, Welter E, Leverenz J, et al. A systematic review of dementia-related stigma research: can we move the stigma dial? Am J Geriatr Psychiatry. 2018;26(3):316-331.
7. Eng KJ, Woo BKP. Knowledge of dementia community resources and stigma among Chinese American immigrants. Gen Hosp Psychiatry. 2015;37(1):e3-e4. doi:10.1016/j.genhosppsych.2014.11.003.
8. Jang Y, Kim G, Chiriboga D. Knowledge of Alzheimer’s disease, feelings of shame, and awareness of services among Korean American elders. J Aging Health. 2010;22(4):419-433.
9. Werner P, Goldstein D, Heinik J. Development and validity of the Family Stigma in Alzheimer’s disease scale (FS-ADS). Alzheimer Disease & Associated Disorders. 2011;25(1):42-48.
10. Rao D, Choi SW, Victorson D, et al. Measuring stigma across neurological conditions: the development of the stigma scale for chronic illness (SSCI). Qual Life Res. 2009;18(5):585-595.
11. Boustani M, Perkins AJ, Monahan P, et al. Measuring primary care patients’ attitudes about dementia screening. Int J Geriatr Psychiatry. 2008;23(8):812-820.
12. Burgener SC, Buckwalter K, Perkounkova Y, et al. Perceived stigma in persons with early-stage dementia: longitudinal findings: Part 2. Dementia. 2015;14(5):609-632.
13. Burgener SC, Buckwalter K. The effects of perceived stigma on persons with dementia and their family caregivers. In: Symposium on Stigma: It’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
14. Harper L, Dobbs B, Royan H, et al. The experience of stigma in care partners of people with dementia – results from an exploratory study. In Symposium on stigma: it’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
15. Burgener S, Berger B. Measuring perceived stigma in persons with progressive neurological disease: Alzheimer’s dementia and Parkinson disease. Dementia. 2008;7(1):31-53.
16. Stites SD, Milne R, Karlawish J. Advances in Alzheimer’s imaging are changing the experience of Alzheimer’s disease. Alzheimer’s & Dementia. 2018;10;285-300.
17. Anderson LA, Egge R. Expanding efforts to address Alzheimer’s disease: the Healthy Brain Initiative. Alzheimer’s Dement. 2014;10(50):S453-S456.
18. Alzheimer’s Association National Plan Milestone Workgroup. Report on the milestones for the US National plan to address Alzheimer’s disease. Alzheimer’s Dementia. 2014;10(Suppl 5);S430-S452. doi:10.1016/j/jalz.2014.08.103.
19. Kirkman AM. Dementia in the news: the media coverage of Alzheimer’s disease. Australasian Journal on Ageing. 2006;25(2):74-79.
20. Swaffer, K. Dementia: stigma, language, and dementia-friendly. Dementia. 2014;13(6):709-716.
21. Stites SD, Karlawish J. Stigma of Alzheimer’s disease dementia: considerations for practice. Practical Neurology. https://practicalneurology.com/articles/2018-june/stigma-of-alzheimers-disease-dementia. Published June 2018. Accessed May 28, 2019.
22. Jamieson J, Dobbs B, Charles L, et al. Forgetful, but not forgotten people of dementia: a novel, technology focused project with a humanistic touch. Geriatric Grand Rounds; October 10, 2017. Edmonton, Alberta, Canada.
23. Dobbs B, Charles L, Chan K, et al. People of Dementia. CGS 37th Annual Scientific Meeting: Integrating Care, Making an Impact. Can Geriatr J. 2017;20(3):220.
24. Government of Canada. Conference report: National Dementia Conference. https://www.canada.ca/en/services/health/publications/diseases-conditions/national-dementia-conference-report.html. Government of Canada. Published August 2018. Accessed May 28, 2019.
25. The Gerontological Society of America. Program Abstracts from the GSA 2018 Annual Scientific Meeting “The Purposes of Longer Lives.” Innovation in Aging. 2018;2(Suppl 1):143.
Water safety: Drowning isn’t the only concern
References
1. CDC Childhood Injury Report: Patterns of Unintentional Injuries among 0-19 Year Olds in the United States, 2000-2006. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/safechild/pdf/cdc-childhoodinjury.pdf. Accessed June 26, 2019.
2. World Health Organization. Global Report on Drowning: Preventing a Leading Killer. https://apps.who.int/iris/bitstream/handle/10665/143893/9789241564786_eng.pdf;jsessionid=0B51AAEB51E29A603A0CABB41FCD96B5?sequence=1. Accessed June 26, 2019.
3. Vanden Esschert K, Haileyesus T, Tarrier AL, et al. Pool chemical injuries in public and residential settings—United States, 2008–2017, and New York, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:433–438.
4. Healthy Swimming. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/healthywater/swimming/index.html. Accessed June 26, 2019.
References
1. CDC Childhood Injury Report: Patterns of Unintentional Injuries among 0-19 Year Olds in the United States, 2000-2006. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/safechild/pdf/cdc-childhoodinjury.pdf. Accessed June 26, 2019.
2. World Health Organization. Global Report on Drowning: Preventing a Leading Killer. https://apps.who.int/iris/bitstream/handle/10665/143893/9789241564786_eng.pdf;jsessionid=0B51AAEB51E29A603A0CABB41FCD96B5?sequence=1. Accessed June 26, 2019.
3. Vanden Esschert K, Haileyesus T, Tarrier AL, et al. Pool chemical injuries in public and residential settings—United States, 2008–2017, and New York, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:433–438.
4. Healthy Swimming. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/healthywater/swimming/index.html. Accessed June 26, 2019.
References
1. CDC Childhood Injury Report: Patterns of Unintentional Injuries among 0-19 Year Olds in the United States, 2000-2006. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/safechild/pdf/cdc-childhoodinjury.pdf. Accessed June 26, 2019.
2. World Health Organization. Global Report on Drowning: Preventing a Leading Killer. https://apps.who.int/iris/bitstream/handle/10665/143893/9789241564786_eng.pdf;jsessionid=0B51AAEB51E29A603A0CABB41FCD96B5?sequence=1. Accessed June 26, 2019.
3. Vanden Esschert K, Haileyesus T, Tarrier AL, et al. Pool chemical injuries in public and residential settings—United States, 2008–2017, and New York, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:433–438.
4. Healthy Swimming. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/healthywater/swimming/index.html. Accessed June 26, 2019.
Wellness seminars won’t fix burnout
“Burnout” has been defined as long-term, unresolvable job stress that leads to exhaustion, depression, and in some tragic circumstances, suicide. One of our lead articles this month concerns an attempt to place a financial cost on physician burnout. More important, I think, is the toll burnout takes on an individual, their family, and their patients. In my role as Chief Clinical Officer of the University of Michigan Medical Group (our faculty and other clinical providers), I struggle to balance productivity demands with the increasing damage such demands are doing to our clinicians. Few primary care physicians at Michigan Medicine work full-time as clinicians (defined as 32 hours patient facing time per week for 46 weeks). Almost all request part-time status if they do not have protected, grant-funded time. They simply cannot keep up with the documentation required in our electronic health record, combined with our “patient-friendly” access via the electronic portal. One-third of the private practice group I helped build was part-time when I left in 2012, and it is not unusual to hear complaints about burnout from my ex-partners.
Let’s be clear, burnout is not going to be solved by increasing the resilience of our physicians or sending us to wellness seminars. That approach is a direct blame-the-victim paradigm. Physicians are burned out because of the constant assault on the core reasons we entered medicine – to help people (this assault has been termed “moral injury”). BPAs (best practice alerts), coding requirements, inbox demands, prior authorizations (see the practice management section of this issue), electronic-order entry, and most other practice enhancement tools rely on the willingness of physicians to sacrifice more time and energy and sit in front of a computer screen.
Salvation of our health care system will not come from mass retirements (although that is happening), concierge practices, part-time status, or other individual responses to this crisis. We will need a fundamental reorganization of our practice, where we (physicians) reduce our work to activities for which we trained combined with a shift of nonphysician work to others; better technology, virtual visits, and ancillary personnel. Patient expectations must be realistic and legal protections need strengthening. The politics of health care has focused on funds flow and ideology. We need a stronger voice that articulates the daily microaggressions that we each endure as we try to live Oslerian physician ideals.
John I. Allen, MD, MBA, AGAF
Editor in Chief
“Burnout” has been defined as long-term, unresolvable job stress that leads to exhaustion, depression, and in some tragic circumstances, suicide. One of our lead articles this month concerns an attempt to place a financial cost on physician burnout. More important, I think, is the toll burnout takes on an individual, their family, and their patients. In my role as Chief Clinical Officer of the University of Michigan Medical Group (our faculty and other clinical providers), I struggle to balance productivity demands with the increasing damage such demands are doing to our clinicians. Few primary care physicians at Michigan Medicine work full-time as clinicians (defined as 32 hours patient facing time per week for 46 weeks). Almost all request part-time status if they do not have protected, grant-funded time. They simply cannot keep up with the documentation required in our electronic health record, combined with our “patient-friendly” access via the electronic portal. One-third of the private practice group I helped build was part-time when I left in 2012, and it is not unusual to hear complaints about burnout from my ex-partners.
Let’s be clear, burnout is not going to be solved by increasing the resilience of our physicians or sending us to wellness seminars. That approach is a direct blame-the-victim paradigm. Physicians are burned out because of the constant assault on the core reasons we entered medicine – to help people (this assault has been termed “moral injury”). BPAs (best practice alerts), coding requirements, inbox demands, prior authorizations (see the practice management section of this issue), electronic-order entry, and most other practice enhancement tools rely on the willingness of physicians to sacrifice more time and energy and sit in front of a computer screen.
Salvation of our health care system will not come from mass retirements (although that is happening), concierge practices, part-time status, or other individual responses to this crisis. We will need a fundamental reorganization of our practice, where we (physicians) reduce our work to activities for which we trained combined with a shift of nonphysician work to others; better technology, virtual visits, and ancillary personnel. Patient expectations must be realistic and legal protections need strengthening. The politics of health care has focused on funds flow and ideology. We need a stronger voice that articulates the daily microaggressions that we each endure as we try to live Oslerian physician ideals.
John I. Allen, MD, MBA, AGAF
Editor in Chief
“Burnout” has been defined as long-term, unresolvable job stress that leads to exhaustion, depression, and in some tragic circumstances, suicide. One of our lead articles this month concerns an attempt to place a financial cost on physician burnout. More important, I think, is the toll burnout takes on an individual, their family, and their patients. In my role as Chief Clinical Officer of the University of Michigan Medical Group (our faculty and other clinical providers), I struggle to balance productivity demands with the increasing damage such demands are doing to our clinicians. Few primary care physicians at Michigan Medicine work full-time as clinicians (defined as 32 hours patient facing time per week for 46 weeks). Almost all request part-time status if they do not have protected, grant-funded time. They simply cannot keep up with the documentation required in our electronic health record, combined with our “patient-friendly” access via the electronic portal. One-third of the private practice group I helped build was part-time when I left in 2012, and it is not unusual to hear complaints about burnout from my ex-partners.
Let’s be clear, burnout is not going to be solved by increasing the resilience of our physicians or sending us to wellness seminars. That approach is a direct blame-the-victim paradigm. Physicians are burned out because of the constant assault on the core reasons we entered medicine – to help people (this assault has been termed “moral injury”). BPAs (best practice alerts), coding requirements, inbox demands, prior authorizations (see the practice management section of this issue), electronic-order entry, and most other practice enhancement tools rely on the willingness of physicians to sacrifice more time and energy and sit in front of a computer screen.
Salvation of our health care system will not come from mass retirements (although that is happening), concierge practices, part-time status, or other individual responses to this crisis. We will need a fundamental reorganization of our practice, where we (physicians) reduce our work to activities for which we trained combined with a shift of nonphysician work to others; better technology, virtual visits, and ancillary personnel. Patient expectations must be realistic and legal protections need strengthening. The politics of health care has focused on funds flow and ideology. We need a stronger voice that articulates the daily microaggressions that we each endure as we try to live Oslerian physician ideals.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Leadership & Professional Development: Sponsored—Catapulting Underrepresented Talent off the Cusp and into the Game
“When you’ve worked hard, and done well, and walked through that doorway of opportunity, you do not slam it shut behind you. You reach back and you give other folks the same chances that helped you succeed.” —Michelle Obama
We are at a point in time where awareness around the existing disparities in gender equity in academic medicine couldn’t be higher. It is time for us to take this knowledge and move swiftly into action. What’s one of the best ways to do this? Become a sponsor or be sponsored. “Sponsorship can effectively catapult nascent talent from unknown to rising-star status.”1
Catapult—an excellent and fitting word to describe the effect sponsorship can have on careers. Women start out behind and often remain behind men, even with mentoring.2 With the catapult of sponsorship, however, high-level career advancement is attainable. Studies show that sponsorship is significantly associated with success: 72.5% of men and 59.0% of women who reported sponsorship were successful, compared with 57.7% and 44.8% who did not report sponsorship.3 For women and underrepresented minorities, sponsorship is especially important and can “dramatically overcome many of the tripwires to achievement.”4
Sponsorship is a two-way proposition—and both the sponsor and protégé have responsibility to make the relationship successful. Want to be sponsored? Here’s what to do: (1) Broadcast your achievements. You don’t have to be a braggart, but you don’t need to be humble—celebrate and share your achievements within and outside your network. (2) Seek out leaders of different backgrounds—sponsors don’t need to be just like you. Varied viewpoints bring broader perspectives to the challenges ahead as you climb the leadership ladder. (3) Clearly spell out your leadership goals for yourself and a potential sponsor. Then work to achieve your shared goals in a timely way.
Consider how you can be a sponsor, particularly for junior faculty and those from under-represented groups. Ask yourself: Who have you sponsored this week? Whose success have you celebrated this quarter? Who will you nominate for an award or recognition this year?
Sponsorship is an essential component of good leadership. Individual leaders and academic health centers (AHCs) must take a step forward toward equity by making sponsorship an expectation and strategic priority. Set the expectation that senior leaders will act as sponsors, set clear goals to work toward (ie, more female chairs, increasing recruitment and retention of underrepresented minorities, etc.), and track metrics.2 While “pay it forward” may seem cliché, sponsorship can truly be a remarkable opportunity for growth for both the sponsor and the protégé, and a winning proposition for the institution.
Disclosures
Dr. Spector reports other from I-PASS Patient Safety Institute, outside the submitted work; and she is a co-founder and holds equity in the I-PASS Patient Safety Institute and the Executive Director of Executive Leadership in Academic Medicine. Ms. Overholser has nothing to disclose.
1. Sponsorship: A Path to the Academic Medicine C-suite for Women Faculty? Elizabeth L. Travis, PhD, Leilani Doty, PhD, and Deborah L. Helitzer, ScD. Acad Med. 2013;88(10):1414-1417. doi: 10.1097/ACM.0b013e3182a35456. PubMed
2. Foust-Cummings, Dinolfo S, Kohler K. Sponsoring Women to Success. https://www.catalyst.org/research/sponsoring-women-to-success/. Accessed May 10, 2019.
3. Patton EW, Griffith KA, Jones RD, Stewart A, Ubel PA, Jagsi R. Differences in mentor-mentee sponsorship in male vs female recipients of national institutes of health grants. JAMA Intern Med. 2017;177(4):580-582. doi: 10.1001/jamainternmed.2016.9391. PubMed
4. Hewlett SA. Celebrating Sponsors -- and Sponsorship. Inc. https://www.inc.com/sylvia-ann-hewlett/celebrating-sponsors-and-sponsorship.html. Accessed May 10, 2019.
“When you’ve worked hard, and done well, and walked through that doorway of opportunity, you do not slam it shut behind you. You reach back and you give other folks the same chances that helped you succeed.” —Michelle Obama
We are at a point in time where awareness around the existing disparities in gender equity in academic medicine couldn’t be higher. It is time for us to take this knowledge and move swiftly into action. What’s one of the best ways to do this? Become a sponsor or be sponsored. “Sponsorship can effectively catapult nascent talent from unknown to rising-star status.”1
Catapult—an excellent and fitting word to describe the effect sponsorship can have on careers. Women start out behind and often remain behind men, even with mentoring.2 With the catapult of sponsorship, however, high-level career advancement is attainable. Studies show that sponsorship is significantly associated with success: 72.5% of men and 59.0% of women who reported sponsorship were successful, compared with 57.7% and 44.8% who did not report sponsorship.3 For women and underrepresented minorities, sponsorship is especially important and can “dramatically overcome many of the tripwires to achievement.”4
Sponsorship is a two-way proposition—and both the sponsor and protégé have responsibility to make the relationship successful. Want to be sponsored? Here’s what to do: (1) Broadcast your achievements. You don’t have to be a braggart, but you don’t need to be humble—celebrate and share your achievements within and outside your network. (2) Seek out leaders of different backgrounds—sponsors don’t need to be just like you. Varied viewpoints bring broader perspectives to the challenges ahead as you climb the leadership ladder. (3) Clearly spell out your leadership goals for yourself and a potential sponsor. Then work to achieve your shared goals in a timely way.
Consider how you can be a sponsor, particularly for junior faculty and those from under-represented groups. Ask yourself: Who have you sponsored this week? Whose success have you celebrated this quarter? Who will you nominate for an award or recognition this year?
Sponsorship is an essential component of good leadership. Individual leaders and academic health centers (AHCs) must take a step forward toward equity by making sponsorship an expectation and strategic priority. Set the expectation that senior leaders will act as sponsors, set clear goals to work toward (ie, more female chairs, increasing recruitment and retention of underrepresented minorities, etc.), and track metrics.2 While “pay it forward” may seem cliché, sponsorship can truly be a remarkable opportunity for growth for both the sponsor and the protégé, and a winning proposition for the institution.
Disclosures
Dr. Spector reports other from I-PASS Patient Safety Institute, outside the submitted work; and she is a co-founder and holds equity in the I-PASS Patient Safety Institute and the Executive Director of Executive Leadership in Academic Medicine. Ms. Overholser has nothing to disclose.
“When you’ve worked hard, and done well, and walked through that doorway of opportunity, you do not slam it shut behind you. You reach back and you give other folks the same chances that helped you succeed.” —Michelle Obama
We are at a point in time where awareness around the existing disparities in gender equity in academic medicine couldn’t be higher. It is time for us to take this knowledge and move swiftly into action. What’s one of the best ways to do this? Become a sponsor or be sponsored. “Sponsorship can effectively catapult nascent talent from unknown to rising-star status.”1
Catapult—an excellent and fitting word to describe the effect sponsorship can have on careers. Women start out behind and often remain behind men, even with mentoring.2 With the catapult of sponsorship, however, high-level career advancement is attainable. Studies show that sponsorship is significantly associated with success: 72.5% of men and 59.0% of women who reported sponsorship were successful, compared with 57.7% and 44.8% who did not report sponsorship.3 For women and underrepresented minorities, sponsorship is especially important and can “dramatically overcome many of the tripwires to achievement.”4
Sponsorship is a two-way proposition—and both the sponsor and protégé have responsibility to make the relationship successful. Want to be sponsored? Here’s what to do: (1) Broadcast your achievements. You don’t have to be a braggart, but you don’t need to be humble—celebrate and share your achievements within and outside your network. (2) Seek out leaders of different backgrounds—sponsors don’t need to be just like you. Varied viewpoints bring broader perspectives to the challenges ahead as you climb the leadership ladder. (3) Clearly spell out your leadership goals for yourself and a potential sponsor. Then work to achieve your shared goals in a timely way.
Consider how you can be a sponsor, particularly for junior faculty and those from under-represented groups. Ask yourself: Who have you sponsored this week? Whose success have you celebrated this quarter? Who will you nominate for an award or recognition this year?
Sponsorship is an essential component of good leadership. Individual leaders and academic health centers (AHCs) must take a step forward toward equity by making sponsorship an expectation and strategic priority. Set the expectation that senior leaders will act as sponsors, set clear goals to work toward (ie, more female chairs, increasing recruitment and retention of underrepresented minorities, etc.), and track metrics.2 While “pay it forward” may seem cliché, sponsorship can truly be a remarkable opportunity for growth for both the sponsor and the protégé, and a winning proposition for the institution.
Disclosures
Dr. Spector reports other from I-PASS Patient Safety Institute, outside the submitted work; and she is a co-founder and holds equity in the I-PASS Patient Safety Institute and the Executive Director of Executive Leadership in Academic Medicine. Ms. Overholser has nothing to disclose.
1. Sponsorship: A Path to the Academic Medicine C-suite for Women Faculty? Elizabeth L. Travis, PhD, Leilani Doty, PhD, and Deborah L. Helitzer, ScD. Acad Med. 2013;88(10):1414-1417. doi: 10.1097/ACM.0b013e3182a35456. PubMed
2. Foust-Cummings, Dinolfo S, Kohler K. Sponsoring Women to Success. https://www.catalyst.org/research/sponsoring-women-to-success/. Accessed May 10, 2019.
3. Patton EW, Griffith KA, Jones RD, Stewart A, Ubel PA, Jagsi R. Differences in mentor-mentee sponsorship in male vs female recipients of national institutes of health grants. JAMA Intern Med. 2017;177(4):580-582. doi: 10.1001/jamainternmed.2016.9391. PubMed
4. Hewlett SA. Celebrating Sponsors -- and Sponsorship. Inc. https://www.inc.com/sylvia-ann-hewlett/celebrating-sponsors-and-sponsorship.html. Accessed May 10, 2019.
1. Sponsorship: A Path to the Academic Medicine C-suite for Women Faculty? Elizabeth L. Travis, PhD, Leilani Doty, PhD, and Deborah L. Helitzer, ScD. Acad Med. 2013;88(10):1414-1417. doi: 10.1097/ACM.0b013e3182a35456. PubMed
2. Foust-Cummings, Dinolfo S, Kohler K. Sponsoring Women to Success. https://www.catalyst.org/research/sponsoring-women-to-success/. Accessed May 10, 2019.
3. Patton EW, Griffith KA, Jones RD, Stewart A, Ubel PA, Jagsi R. Differences in mentor-mentee sponsorship in male vs female recipients of national institutes of health grants. JAMA Intern Med. 2017;177(4):580-582. doi: 10.1001/jamainternmed.2016.9391. PubMed
4. Hewlett SA. Celebrating Sponsors -- and Sponsorship. Inc. https://www.inc.com/sylvia-ann-hewlett/celebrating-sponsors-and-sponsorship.html. Accessed May 10, 2019.
© 2019 Society of Hospital Medicine
Study eyes narcolepsy’s impact on patient quality of life
SAN ANTONIO – Narcolepsy adversely impacts one’s health-related quality of life in a variety of ways, from elevated levels of depression to negative social stigma, results from a mixed methods study suggest.
“Despite established pharmacological treatments to reduce narcolepsy symptoms, health-related quality of life remains poor,” the study’s first author, Jason C. Ong, PhD, said at the annual meeting of the Associated Professional Sleep Societies. “The impact these symptoms have on functioning, the disease burden, and psychosocial functioning in particular is very important. Psychosocial functioning is particularly poor.”
Previous research has shown that people with narcolepsy have two- to four times the rate of psychiatric comorbidities and that health-related stigma is a predictor of depression and poor functioning, said Dr. Ong, a psychologist with the Center for Circadian and Sleep Medicine at the Northwestern University Feinberg School of Medicine, Chicago. In an effort to assess current practices for addressing the psychosocial needs of persons with narcolepsy and to identify potential strategies that could be used to develop a psychosocial intervention, he and his associates conducted a mixed methods study to examine how narcolepsy symptoms impact health-related quality of life and the appropriateness of different health-related quality of life measures for the disorder. “Our long-term goal is to see if we can use this information to help inform the feasibility of a psychosocial intervention to improve health-related quality of life,” he said.
For the study, 29 adults with an established diagnosis of narcolepsy completed online versions of the Patient Health Questionnaire-9 (PHQ-9), the Patient Reported Outcomes Measurement Information System (PROMIS), the 36-item Short Form Survey (SF-36), and the Epworth Sleepiness Scale (ESS). They also participated in a focus group, which consisted of questions pertaining to quality of life for persons with narcolepsy, current practices for addressing psychosocial health of affected individuals, and suggestions for developing a psychosocial intervention. The researchers used thematic analysis to reduce the qualitative data to key themes.
Most of the study participants (93%) were female, 90% were white, their mean age was 31, and their mean time since narcolepsy diagnosis was 4.3 years. Clinically significant elevations on the PROMIS scale, defined as a t-score of greater than 60, were reported for depression (t-score of 64.8), anxiety (66.3), fatigue (68.3), and sleep impairment (66.9). Elevations in depressive symptoms were reported on the PHQ-9 (a mean of 15.79), “which corresponds to moderately severe levels,” Dr. Ong said. “The ESS was highly elevated and fit well with the scales for sleep impairment as well as fatigue on the PROMIS. Overall, there was nice congruence across these measures.”
On the SF-36, the researchers observed that there were deficits in physical and emotional aspects of role limitations, and in energy/fatigue. “One thing we did find was a significant difference in general functioning, where patients with type 1 narcolepsy were worse off than those with type 2 narcolepsy (P less than .05).”
Qualitative data from focus groups revealed several key themes, including the perception that narcolepsy is poorly understood by the public and health care providers.
“People have the perception that if you have narcolepsy, you just feel fine and then you fall asleep,” Dr. Ong said. “They don’t understand that it’s a constant thing. Negative social stigma was also common. As a result, we found a lot of negative impact on self-esteem and self-efficacy. People talked about being hesitant to tell other people about their diagnosis, feeling that they’re ashamed of having narcolepsy. They felt less capable. One person said, ‘I get tired trying to explain why I’m tired.’”
Another common theme that emerged was the challenge of optimal treatment for their narcolepsy. Most patients met with sleep doctors or clinics every 3-6 months. “They said that this was generally good for discussing medications and symptom management, but there didn’t seem to be much time to talk about psychosocial aspects,” Dr. Ong said. “That seemed to be one area of need. There was also a strong dissatisfaction with mental health providers. People talked about how their mental health provider really didn’t understand narcolepsy. It did seem to reduce rapport and the ability to trust their therapist. Some talked about the challenges of accessibility. In some cases, people said their narcolepsy symptoms created challenges with appointment attendance.”
In terms of preferences for a psychosocial intervention, respondents generally “preferred some kind of online or Internet delivery,” he said. “They prefer a team approach with a clinician who’s knowledgeable about both sleep and mental health.”
Dr. Ong acknowledged certain limitations of the study, including its small sample size and the fact that it was not adequately powered to detect differences between type 1 and type 2 narcolepsy.
The study was funded by a grant from Wake Up Narcolepsy. Dr. Ong reported having no relevant financial disclosures.
SOURCE: Ong J et al., SLEEP 2019, abstract 0624.
SAN ANTONIO – Narcolepsy adversely impacts one’s health-related quality of life in a variety of ways, from elevated levels of depression to negative social stigma, results from a mixed methods study suggest.
“Despite established pharmacological treatments to reduce narcolepsy symptoms, health-related quality of life remains poor,” the study’s first author, Jason C. Ong, PhD, said at the annual meeting of the Associated Professional Sleep Societies. “The impact these symptoms have on functioning, the disease burden, and psychosocial functioning in particular is very important. Psychosocial functioning is particularly poor.”
Previous research has shown that people with narcolepsy have two- to four times the rate of psychiatric comorbidities and that health-related stigma is a predictor of depression and poor functioning, said Dr. Ong, a psychologist with the Center for Circadian and Sleep Medicine at the Northwestern University Feinberg School of Medicine, Chicago. In an effort to assess current practices for addressing the psychosocial needs of persons with narcolepsy and to identify potential strategies that could be used to develop a psychosocial intervention, he and his associates conducted a mixed methods study to examine how narcolepsy symptoms impact health-related quality of life and the appropriateness of different health-related quality of life measures for the disorder. “Our long-term goal is to see if we can use this information to help inform the feasibility of a psychosocial intervention to improve health-related quality of life,” he said.
For the study, 29 adults with an established diagnosis of narcolepsy completed online versions of the Patient Health Questionnaire-9 (PHQ-9), the Patient Reported Outcomes Measurement Information System (PROMIS), the 36-item Short Form Survey (SF-36), and the Epworth Sleepiness Scale (ESS). They also participated in a focus group, which consisted of questions pertaining to quality of life for persons with narcolepsy, current practices for addressing psychosocial health of affected individuals, and suggestions for developing a psychosocial intervention. The researchers used thematic analysis to reduce the qualitative data to key themes.
Most of the study participants (93%) were female, 90% were white, their mean age was 31, and their mean time since narcolepsy diagnosis was 4.3 years. Clinically significant elevations on the PROMIS scale, defined as a t-score of greater than 60, were reported for depression (t-score of 64.8), anxiety (66.3), fatigue (68.3), and sleep impairment (66.9). Elevations in depressive symptoms were reported on the PHQ-9 (a mean of 15.79), “which corresponds to moderately severe levels,” Dr. Ong said. “The ESS was highly elevated and fit well with the scales for sleep impairment as well as fatigue on the PROMIS. Overall, there was nice congruence across these measures.”
On the SF-36, the researchers observed that there were deficits in physical and emotional aspects of role limitations, and in energy/fatigue. “One thing we did find was a significant difference in general functioning, where patients with type 1 narcolepsy were worse off than those with type 2 narcolepsy (P less than .05).”
Qualitative data from focus groups revealed several key themes, including the perception that narcolepsy is poorly understood by the public and health care providers.
“People have the perception that if you have narcolepsy, you just feel fine and then you fall asleep,” Dr. Ong said. “They don’t understand that it’s a constant thing. Negative social stigma was also common. As a result, we found a lot of negative impact on self-esteem and self-efficacy. People talked about being hesitant to tell other people about their diagnosis, feeling that they’re ashamed of having narcolepsy. They felt less capable. One person said, ‘I get tired trying to explain why I’m tired.’”
Another common theme that emerged was the challenge of optimal treatment for their narcolepsy. Most patients met with sleep doctors or clinics every 3-6 months. “They said that this was generally good for discussing medications and symptom management, but there didn’t seem to be much time to talk about psychosocial aspects,” Dr. Ong said. “That seemed to be one area of need. There was also a strong dissatisfaction with mental health providers. People talked about how their mental health provider really didn’t understand narcolepsy. It did seem to reduce rapport and the ability to trust their therapist. Some talked about the challenges of accessibility. In some cases, people said their narcolepsy symptoms created challenges with appointment attendance.”
In terms of preferences for a psychosocial intervention, respondents generally “preferred some kind of online or Internet delivery,” he said. “They prefer a team approach with a clinician who’s knowledgeable about both sleep and mental health.”
Dr. Ong acknowledged certain limitations of the study, including its small sample size and the fact that it was not adequately powered to detect differences between type 1 and type 2 narcolepsy.
The study was funded by a grant from Wake Up Narcolepsy. Dr. Ong reported having no relevant financial disclosures.
SOURCE: Ong J et al., SLEEP 2019, abstract 0624.
SAN ANTONIO – Narcolepsy adversely impacts one’s health-related quality of life in a variety of ways, from elevated levels of depression to negative social stigma, results from a mixed methods study suggest.
“Despite established pharmacological treatments to reduce narcolepsy symptoms, health-related quality of life remains poor,” the study’s first author, Jason C. Ong, PhD, said at the annual meeting of the Associated Professional Sleep Societies. “The impact these symptoms have on functioning, the disease burden, and psychosocial functioning in particular is very important. Psychosocial functioning is particularly poor.”
Previous research has shown that people with narcolepsy have two- to four times the rate of psychiatric comorbidities and that health-related stigma is a predictor of depression and poor functioning, said Dr. Ong, a psychologist with the Center for Circadian and Sleep Medicine at the Northwestern University Feinberg School of Medicine, Chicago. In an effort to assess current practices for addressing the psychosocial needs of persons with narcolepsy and to identify potential strategies that could be used to develop a psychosocial intervention, he and his associates conducted a mixed methods study to examine how narcolepsy symptoms impact health-related quality of life and the appropriateness of different health-related quality of life measures for the disorder. “Our long-term goal is to see if we can use this information to help inform the feasibility of a psychosocial intervention to improve health-related quality of life,” he said.
For the study, 29 adults with an established diagnosis of narcolepsy completed online versions of the Patient Health Questionnaire-9 (PHQ-9), the Patient Reported Outcomes Measurement Information System (PROMIS), the 36-item Short Form Survey (SF-36), and the Epworth Sleepiness Scale (ESS). They also participated in a focus group, which consisted of questions pertaining to quality of life for persons with narcolepsy, current practices for addressing psychosocial health of affected individuals, and suggestions for developing a psychosocial intervention. The researchers used thematic analysis to reduce the qualitative data to key themes.
Most of the study participants (93%) were female, 90% were white, their mean age was 31, and their mean time since narcolepsy diagnosis was 4.3 years. Clinically significant elevations on the PROMIS scale, defined as a t-score of greater than 60, were reported for depression (t-score of 64.8), anxiety (66.3), fatigue (68.3), and sleep impairment (66.9). Elevations in depressive symptoms were reported on the PHQ-9 (a mean of 15.79), “which corresponds to moderately severe levels,” Dr. Ong said. “The ESS was highly elevated and fit well with the scales for sleep impairment as well as fatigue on the PROMIS. Overall, there was nice congruence across these measures.”
On the SF-36, the researchers observed that there were deficits in physical and emotional aspects of role limitations, and in energy/fatigue. “One thing we did find was a significant difference in general functioning, where patients with type 1 narcolepsy were worse off than those with type 2 narcolepsy (P less than .05).”
Qualitative data from focus groups revealed several key themes, including the perception that narcolepsy is poorly understood by the public and health care providers.
“People have the perception that if you have narcolepsy, you just feel fine and then you fall asleep,” Dr. Ong said. “They don’t understand that it’s a constant thing. Negative social stigma was also common. As a result, we found a lot of negative impact on self-esteem and self-efficacy. People talked about being hesitant to tell other people about their diagnosis, feeling that they’re ashamed of having narcolepsy. They felt less capable. One person said, ‘I get tired trying to explain why I’m tired.’”
Another common theme that emerged was the challenge of optimal treatment for their narcolepsy. Most patients met with sleep doctors or clinics every 3-6 months. “They said that this was generally good for discussing medications and symptom management, but there didn’t seem to be much time to talk about psychosocial aspects,” Dr. Ong said. “That seemed to be one area of need. There was also a strong dissatisfaction with mental health providers. People talked about how their mental health provider really didn’t understand narcolepsy. It did seem to reduce rapport and the ability to trust their therapist. Some talked about the challenges of accessibility. In some cases, people said their narcolepsy symptoms created challenges with appointment attendance.”
In terms of preferences for a psychosocial intervention, respondents generally “preferred some kind of online or Internet delivery,” he said. “They prefer a team approach with a clinician who’s knowledgeable about both sleep and mental health.”
Dr. Ong acknowledged certain limitations of the study, including its small sample size and the fact that it was not adequately powered to detect differences between type 1 and type 2 narcolepsy.
The study was funded by a grant from Wake Up Narcolepsy. Dr. Ong reported having no relevant financial disclosures.
SOURCE: Ong J et al., SLEEP 2019, abstract 0624.
REPORTING FROM SLEEP 2019
Study: Why urban sickle cell patients quit hydroxyurea
FORT LAUDERDALE, Fla. – A study of sickle cell patients at a clinic in the Bronx found that upwards of 75% of them get a prescription for hydroxyurea to improve hemoglobin levels, but that one-third have discontinued use for various reasons, according to results reported at the 13th annual Foundation for Sickle Cell Disease Research symposium here.
“The results identify variability in reported side effects and reasons for discontinuation, and highlight the importance of clear communication between providers and patients to discuss the benefits and challenges of hydroxyurea,” said Caterina Minniti, MD, professor of clinical medicine and pediatrics at Einstein College of Medicine and director of the Sickle Cell Center for Adults at Montefiore Hospital, Bronx, N.Y. The study analyzed self-reporting surveys completed by 224 adult outpatients in the Montefiore sickle cell clinic, and then verified the data in the electronic medical record, Dr. Minniti said. She noted, “Our population is unique in the Bronx in that we have a high percentage of Hispanic patients.” They comprised 24.1% of the study population.
“We found that 77.2% of the patients have ever been prescribed hydroxyurea,” she said. “That was really great.” Also, 91% of those with severe genotypes of SCD had been prescribed the drug; 68% of them were still taking hydroxyurea at the time of the survey, she said. Among patients with the mild genotype, 42.1% had been prescribed hydroxyurea and half were still on it when they completed their surveys.
When the survey evaluated how long patients had been taking the drug, she said, “That’s where I start to get concerned.” About half – 48.6% – had taken the drug for one to five years, “which is a very short period of time,” Dr. Minniti said. Another 15% were on hydroxyurea for less than a year, 23% for 5 to 10 years and 19% for 10 years or more.
The study drilled down into reasons why patients discontinued the drug. Side effects were cited by 24.6% (n=15). They include fatigue, hair loss, and GI upset. Other reasons include perceived ineffectiveness (16.4%, n=10); physician direction (14.8%, n=9), and reproductive health and ulcer formation (each at 8.2%, n=5).
“Many patients perceive ineffectiveness of hydroxyurea in the short term, but the benefits of hydoxyurea stem from chronic use over the long term,” Dr. Minniti said. She noted that some patients discontinued the drug for legitimate medical indications, “such as pregnancy and breast feeding, but were not restarted afterward.”
Dr. Minniti disclosed relationships with Novartis, Global Blood Therapeutics, Teutona, Bluebird Bio, GBT and Bayer.
SOURCE: Minniti C, et al. Abstract no. JSCDH-D-19-00058. Foundation for Sickle Cell Disease Research Symposium; Fort Lauderdale, Fla.; June 9, 2019.
FORT LAUDERDALE, Fla. – A study of sickle cell patients at a clinic in the Bronx found that upwards of 75% of them get a prescription for hydroxyurea to improve hemoglobin levels, but that one-third have discontinued use for various reasons, according to results reported at the 13th annual Foundation for Sickle Cell Disease Research symposium here.
“The results identify variability in reported side effects and reasons for discontinuation, and highlight the importance of clear communication between providers and patients to discuss the benefits and challenges of hydroxyurea,” said Caterina Minniti, MD, professor of clinical medicine and pediatrics at Einstein College of Medicine and director of the Sickle Cell Center for Adults at Montefiore Hospital, Bronx, N.Y. The study analyzed self-reporting surveys completed by 224 adult outpatients in the Montefiore sickle cell clinic, and then verified the data in the electronic medical record, Dr. Minniti said. She noted, “Our population is unique in the Bronx in that we have a high percentage of Hispanic patients.” They comprised 24.1% of the study population.
“We found that 77.2% of the patients have ever been prescribed hydroxyurea,” she said. “That was really great.” Also, 91% of those with severe genotypes of SCD had been prescribed the drug; 68% of them were still taking hydroxyurea at the time of the survey, she said. Among patients with the mild genotype, 42.1% had been prescribed hydroxyurea and half were still on it when they completed their surveys.
When the survey evaluated how long patients had been taking the drug, she said, “That’s where I start to get concerned.” About half – 48.6% – had taken the drug for one to five years, “which is a very short period of time,” Dr. Minniti said. Another 15% were on hydroxyurea for less than a year, 23% for 5 to 10 years and 19% for 10 years or more.
The study drilled down into reasons why patients discontinued the drug. Side effects were cited by 24.6% (n=15). They include fatigue, hair loss, and GI upset. Other reasons include perceived ineffectiveness (16.4%, n=10); physician direction (14.8%, n=9), and reproductive health and ulcer formation (each at 8.2%, n=5).
“Many patients perceive ineffectiveness of hydroxyurea in the short term, but the benefits of hydoxyurea stem from chronic use over the long term,” Dr. Minniti said. She noted that some patients discontinued the drug for legitimate medical indications, “such as pregnancy and breast feeding, but were not restarted afterward.”
Dr. Minniti disclosed relationships with Novartis, Global Blood Therapeutics, Teutona, Bluebird Bio, GBT and Bayer.
SOURCE: Minniti C, et al. Abstract no. JSCDH-D-19-00058. Foundation for Sickle Cell Disease Research Symposium; Fort Lauderdale, Fla.; June 9, 2019.
FORT LAUDERDALE, Fla. – A study of sickle cell patients at a clinic in the Bronx found that upwards of 75% of them get a prescription for hydroxyurea to improve hemoglobin levels, but that one-third have discontinued use for various reasons, according to results reported at the 13th annual Foundation for Sickle Cell Disease Research symposium here.
“The results identify variability in reported side effects and reasons for discontinuation, and highlight the importance of clear communication between providers and patients to discuss the benefits and challenges of hydroxyurea,” said Caterina Minniti, MD, professor of clinical medicine and pediatrics at Einstein College of Medicine and director of the Sickle Cell Center for Adults at Montefiore Hospital, Bronx, N.Y. The study analyzed self-reporting surveys completed by 224 adult outpatients in the Montefiore sickle cell clinic, and then verified the data in the electronic medical record, Dr. Minniti said. She noted, “Our population is unique in the Bronx in that we have a high percentage of Hispanic patients.” They comprised 24.1% of the study population.
“We found that 77.2% of the patients have ever been prescribed hydroxyurea,” she said. “That was really great.” Also, 91% of those with severe genotypes of SCD had been prescribed the drug; 68% of them were still taking hydroxyurea at the time of the survey, she said. Among patients with the mild genotype, 42.1% had been prescribed hydroxyurea and half were still on it when they completed their surveys.
When the survey evaluated how long patients had been taking the drug, she said, “That’s where I start to get concerned.” About half – 48.6% – had taken the drug for one to five years, “which is a very short period of time,” Dr. Minniti said. Another 15% were on hydroxyurea for less than a year, 23% for 5 to 10 years and 19% for 10 years or more.
The study drilled down into reasons why patients discontinued the drug. Side effects were cited by 24.6% (n=15). They include fatigue, hair loss, and GI upset. Other reasons include perceived ineffectiveness (16.4%, n=10); physician direction (14.8%, n=9), and reproductive health and ulcer formation (each at 8.2%, n=5).
“Many patients perceive ineffectiveness of hydroxyurea in the short term, but the benefits of hydoxyurea stem from chronic use over the long term,” Dr. Minniti said. She noted that some patients discontinued the drug for legitimate medical indications, “such as pregnancy and breast feeding, but were not restarted afterward.”
Dr. Minniti disclosed relationships with Novartis, Global Blood Therapeutics, Teutona, Bluebird Bio, GBT and Bayer.
SOURCE: Minniti C, et al. Abstract no. JSCDH-D-19-00058. Foundation for Sickle Cell Disease Research Symposium; Fort Lauderdale, Fla.; June 9, 2019.
REPORTING FROM THE ANNUAL SICKLE CELL DISEASE RESEARCH AND EDUCATIONAL SYMPOSIUM








