User login
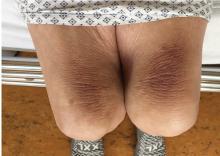
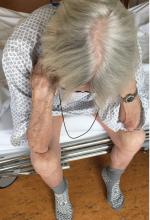
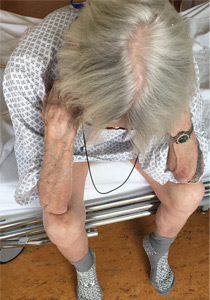
Thinker’s sign
See Mangione and Aronowitz editorials
Mechanical pressure induced by friction of the elbows on the thighs may result in proliferation of the stratum corneum and the release of hemosiderin from erythrocytes, resulting in the skin changes seen in this patient, which because of the tripod positioning are known as “thinker’s sign,” a term coined in 1963 by Rothenberg1 to describe findings in patients with chronic pulmonary disease and advanced respiratory insufficiency. It is also referred to as the Dahl sign, based on a report by Dahl of similar findings in patients with emphysema.2
- Rothenberg HJ. The thinker's sign. JAMA 1963; 184:902–903. pmid:13975358
- Dahl MV. Emphysema. Arch Dermatol 1970; 101(1):117. pmid:5416788
See Mangione and Aronowitz editorials
Mechanical pressure induced by friction of the elbows on the thighs may result in proliferation of the stratum corneum and the release of hemosiderin from erythrocytes, resulting in the skin changes seen in this patient, which because of the tripod positioning are known as “thinker’s sign,” a term coined in 1963 by Rothenberg1 to describe findings in patients with chronic pulmonary disease and advanced respiratory insufficiency. It is also referred to as the Dahl sign, based on a report by Dahl of similar findings in patients with emphysema.2
See Mangione and Aronowitz editorials
Mechanical pressure induced by friction of the elbows on the thighs may result in proliferation of the stratum corneum and the release of hemosiderin from erythrocytes, resulting in the skin changes seen in this patient, which because of the tripod positioning are known as “thinker’s sign,” a term coined in 1963 by Rothenberg1 to describe findings in patients with chronic pulmonary disease and advanced respiratory insufficiency. It is also referred to as the Dahl sign, based on a report by Dahl of similar findings in patients with emphysema.2
- Rothenberg HJ. The thinker's sign. JAMA 1963; 184:902–903. pmid:13975358
- Dahl MV. Emphysema. Arch Dermatol 1970; 101(1):117. pmid:5416788
- Rothenberg HJ. The thinker's sign. JAMA 1963; 184:902–903. pmid:13975358
- Dahl MV. Emphysema. Arch Dermatol 1970; 101(1):117. pmid:5416788
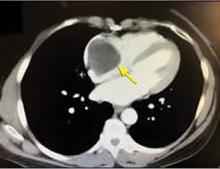
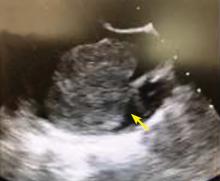
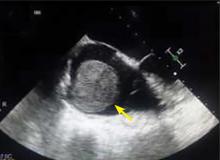
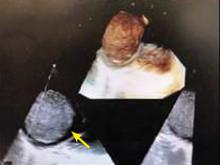
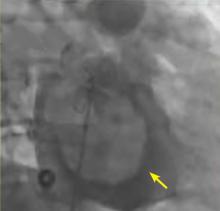
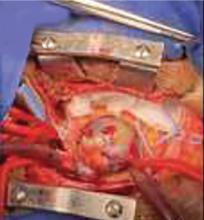
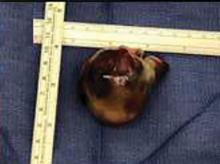
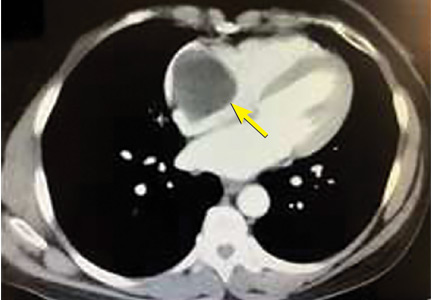
A right atrial mass
PRIMARY HEART TUMORS ARE RARE
The most common neoplasms that metastasize to the heart are malignant melanoma, lymphoma, leukemia, breast, and lung cancers. The layers of the heart affected by malignant neoplasms in order of frequency from highest to lowest are the pericardium, epicardium, myocardium, and endocardium.3
MYXOMA: A PRIMARY CARDIAC TUMOR
The most common type of primary cardiac tumor is myxoma. Most—75% to 80%—occur in the left atrium, while 15% to 20% occur in the right atrium.5 Right atrial myxomas are usually found in the intraatrial septum at the border of the fossa ovalis.6 Myxomas can occur at any age, but are most common in women between the third and sixth decades.2
The cause of atrial myxomas is currently unknown. Most cases are sporadic. However, 10% are familial, with an autosomal-dominant pattern.7
The clinical symptoms of right atrial myxoma depend on the tumor’s size, location, and mobility and on the patient’s physical activity and body position.4 Common presenting symptoms include shortness of breath, pulmonary edema, cough, hemoptysis, and fatigue. Thirty percent of patients present with constitutional symptoms.4
Auscultation may reveal a characteristic “tumor plop” early in diastole.4,7 About 35% of patients have laboratory abnormalities such as elevations in erythrocyte sedimentation rate, C-reactive protein, and globulin levels and anemia. Our patient did not.
Embolization occurs in about 10% of cases of right-sided myxoma and can result in pulmonary artery embolism or a stroke. Pulmonary artery embolism occurs with myxoma embolization to the lungs. Strokes can occur in patients who have a patent foramen ovale or atrial septal defect, through which embolism to the systemic arterial circulation can occur.
The primary treatment for myxoma is complete resection of the tumor and its base with wide safety margins. This is particularly important to prevent recurrence of the myxoma and need for repeat operations, with their risk of surgical complications.9
- Dujardin KS, Click RL, Oh JK. The role of intraoperative transesophageal echocardiography in patients undergoing cardiac mass removal. J Am Soc Echocardiogr 2000; 13(12):1080–1083. pmid:11119275
- Jang KH, Shin DH, Lee C, Jang JK, Cheong S, Yoo SY. Left atrial mass with stalk: thrombus or myxoma? J Cardiovasc Ultrasound 2010; 18(4):154–156. doi:10.4250/jcu.2010.18.4.154
- Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the heart. Circulation 2013; 128(16):1790–1794. doi:10.1161/CIRCULATIONAHA.112.000790
- Aggarwal SK, Barik R, Sarma TC, et al. Clinical presentation and investigation findings in cardiac myxomas: new insights from the developing world. Am Heart J 2007; 154(6):1102–1107. doi:10.1016/j.ahj.2007.07.032
- Diaz A, Di Salvo C, Lawrence D, Hayward M. Left atrial and right ventricular myxoma: an uncommon presentation of a rare tumour. Interact Cardiovasc Thorac Surg 2011; 12(4):622–623. doi:10.1510/icvts.2010.255661
- Reynen K. Cardiac myxomas. N Engl J Med 1995; 333(24):1610–1617. doi:10.1056/NEJM199512143332407
- Kolluru A, Desai D, Cohen GI. The etiology of atrial myxoma tumor plop. J Am Coll Cardiol 2011; 57(21):e371. doi:10.1016/j.jacc.2010.09.085
- Kassab R, Wehbe L, Badaoui G, el Asmar B, Jebara V, Ashoush R. Recurrent cerebrovascular accident: unusual and isolated manifestation of myxoma of the left atrium. J Med Liban 1999; 47(4):246–250. French. pmid:10641454
- Guhathakurta S, Riordan JP. Surgical treatment of right atrial myxoma. Tex Heart Inst J 2000; 27(1):61–63. pmid:10830633
PRIMARY HEART TUMORS ARE RARE
The most common neoplasms that metastasize to the heart are malignant melanoma, lymphoma, leukemia, breast, and lung cancers. The layers of the heart affected by malignant neoplasms in order of frequency from highest to lowest are the pericardium, epicardium, myocardium, and endocardium.3
MYXOMA: A PRIMARY CARDIAC TUMOR
The most common type of primary cardiac tumor is myxoma. Most—75% to 80%—occur in the left atrium, while 15% to 20% occur in the right atrium.5 Right atrial myxomas are usually found in the intraatrial septum at the border of the fossa ovalis.6 Myxomas can occur at any age, but are most common in women between the third and sixth decades.2
The cause of atrial myxomas is currently unknown. Most cases are sporadic. However, 10% are familial, with an autosomal-dominant pattern.7
The clinical symptoms of right atrial myxoma depend on the tumor’s size, location, and mobility and on the patient’s physical activity and body position.4 Common presenting symptoms include shortness of breath, pulmonary edema, cough, hemoptysis, and fatigue. Thirty percent of patients present with constitutional symptoms.4
Auscultation may reveal a characteristic “tumor plop” early in diastole.4,7 About 35% of patients have laboratory abnormalities such as elevations in erythrocyte sedimentation rate, C-reactive protein, and globulin levels and anemia. Our patient did not.
Embolization occurs in about 10% of cases of right-sided myxoma and can result in pulmonary artery embolism or a stroke. Pulmonary artery embolism occurs with myxoma embolization to the lungs. Strokes can occur in patients who have a patent foramen ovale or atrial septal defect, through which embolism to the systemic arterial circulation can occur.
The primary treatment for myxoma is complete resection of the tumor and its base with wide safety margins. This is particularly important to prevent recurrence of the myxoma and need for repeat operations, with their risk of surgical complications.9
PRIMARY HEART TUMORS ARE RARE
The most common neoplasms that metastasize to the heart are malignant melanoma, lymphoma, leukemia, breast, and lung cancers. The layers of the heart affected by malignant neoplasms in order of frequency from highest to lowest are the pericardium, epicardium, myocardium, and endocardium.3
MYXOMA: A PRIMARY CARDIAC TUMOR
The most common type of primary cardiac tumor is myxoma. Most—75% to 80%—occur in the left atrium, while 15% to 20% occur in the right atrium.5 Right atrial myxomas are usually found in the intraatrial septum at the border of the fossa ovalis.6 Myxomas can occur at any age, but are most common in women between the third and sixth decades.2
The cause of atrial myxomas is currently unknown. Most cases are sporadic. However, 10% are familial, with an autosomal-dominant pattern.7
The clinical symptoms of right atrial myxoma depend on the tumor’s size, location, and mobility and on the patient’s physical activity and body position.4 Common presenting symptoms include shortness of breath, pulmonary edema, cough, hemoptysis, and fatigue. Thirty percent of patients present with constitutional symptoms.4
Auscultation may reveal a characteristic “tumor plop” early in diastole.4,7 About 35% of patients have laboratory abnormalities such as elevations in erythrocyte sedimentation rate, C-reactive protein, and globulin levels and anemia. Our patient did not.
Embolization occurs in about 10% of cases of right-sided myxoma and can result in pulmonary artery embolism or a stroke. Pulmonary artery embolism occurs with myxoma embolization to the lungs. Strokes can occur in patients who have a patent foramen ovale or atrial septal defect, through which embolism to the systemic arterial circulation can occur.
The primary treatment for myxoma is complete resection of the tumor and its base with wide safety margins. This is particularly important to prevent recurrence of the myxoma and need for repeat operations, with their risk of surgical complications.9
- Dujardin KS, Click RL, Oh JK. The role of intraoperative transesophageal echocardiography in patients undergoing cardiac mass removal. J Am Soc Echocardiogr 2000; 13(12):1080–1083. pmid:11119275
- Jang KH, Shin DH, Lee C, Jang JK, Cheong S, Yoo SY. Left atrial mass with stalk: thrombus or myxoma? J Cardiovasc Ultrasound 2010; 18(4):154–156. doi:10.4250/jcu.2010.18.4.154
- Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the heart. Circulation 2013; 128(16):1790–1794. doi:10.1161/CIRCULATIONAHA.112.000790
- Aggarwal SK, Barik R, Sarma TC, et al. Clinical presentation and investigation findings in cardiac myxomas: new insights from the developing world. Am Heart J 2007; 154(6):1102–1107. doi:10.1016/j.ahj.2007.07.032
- Diaz A, Di Salvo C, Lawrence D, Hayward M. Left atrial and right ventricular myxoma: an uncommon presentation of a rare tumour. Interact Cardiovasc Thorac Surg 2011; 12(4):622–623. doi:10.1510/icvts.2010.255661
- Reynen K. Cardiac myxomas. N Engl J Med 1995; 333(24):1610–1617. doi:10.1056/NEJM199512143332407
- Kolluru A, Desai D, Cohen GI. The etiology of atrial myxoma tumor plop. J Am Coll Cardiol 2011; 57(21):e371. doi:10.1016/j.jacc.2010.09.085
- Kassab R, Wehbe L, Badaoui G, el Asmar B, Jebara V, Ashoush R. Recurrent cerebrovascular accident: unusual and isolated manifestation of myxoma of the left atrium. J Med Liban 1999; 47(4):246–250. French. pmid:10641454
- Guhathakurta S, Riordan JP. Surgical treatment of right atrial myxoma. Tex Heart Inst J 2000; 27(1):61–63. pmid:10830633
- Dujardin KS, Click RL, Oh JK. The role of intraoperative transesophageal echocardiography in patients undergoing cardiac mass removal. J Am Soc Echocardiogr 2000; 13(12):1080–1083. pmid:11119275
- Jang KH, Shin DH, Lee C, Jang JK, Cheong S, Yoo SY. Left atrial mass with stalk: thrombus or myxoma? J Cardiovasc Ultrasound 2010; 18(4):154–156. doi:10.4250/jcu.2010.18.4.154
- Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the heart. Circulation 2013; 128(16):1790–1794. doi:10.1161/CIRCULATIONAHA.112.000790
- Aggarwal SK, Barik R, Sarma TC, et al. Clinical presentation and investigation findings in cardiac myxomas: new insights from the developing world. Am Heart J 2007; 154(6):1102–1107. doi:10.1016/j.ahj.2007.07.032
- Diaz A, Di Salvo C, Lawrence D, Hayward M. Left atrial and right ventricular myxoma: an uncommon presentation of a rare tumour. Interact Cardiovasc Thorac Surg 2011; 12(4):622–623. doi:10.1510/icvts.2010.255661
- Reynen K. Cardiac myxomas. N Engl J Med 1995; 333(24):1610–1617. doi:10.1056/NEJM199512143332407
- Kolluru A, Desai D, Cohen GI. The etiology of atrial myxoma tumor plop. J Am Coll Cardiol 2011; 57(21):e371. doi:10.1016/j.jacc.2010.09.085
- Kassab R, Wehbe L, Badaoui G, el Asmar B, Jebara V, Ashoush R. Recurrent cerebrovascular accident: unusual and isolated manifestation of myxoma of the left atrium. J Med Liban 1999; 47(4):246–250. French. pmid:10641454
- Guhathakurta S, Riordan JP. Surgical treatment of right atrial myxoma. Tex Heart Inst J 2000; 27(1):61–63. pmid:10830633
New CLTI Global Guidelines Available
On May 31, new global guidelines on the best ways to treat Chronic Limb-Threatening Ischemia were co-published in the Journal of Vascular Surgery and the European Journal of Vascular and Endovascular Surgery. This comes after four years of collaboration between vascular experts from around the world. According to the SVS’ own Dr. Conte, a co-editor, the group created a unique practice guideline that reflects the spectrum of the diseases and the approaches seen worldwide. Read the guidelines in the JVS here.
On May 31, new global guidelines on the best ways to treat Chronic Limb-Threatening Ischemia were co-published in the Journal of Vascular Surgery and the European Journal of Vascular and Endovascular Surgery. This comes after four years of collaboration between vascular experts from around the world. According to the SVS’ own Dr. Conte, a co-editor, the group created a unique practice guideline that reflects the spectrum of the diseases and the approaches seen worldwide. Read the guidelines in the JVS here.
On May 31, new global guidelines on the best ways to treat Chronic Limb-Threatening Ischemia were co-published in the Journal of Vascular Surgery and the European Journal of Vascular and Endovascular Surgery. This comes after four years of collaboration between vascular experts from around the world. According to the SVS’ own Dr. Conte, a co-editor, the group created a unique practice guideline that reflects the spectrum of the diseases and the approaches seen worldwide. Read the guidelines in the JVS here.
Do patients on biologic drugs for rheumatic disease need PCP prophylaxis?
Pneumocystis jirovecii (previously carinii) pneumonia (PCP) is rare in patients taking biologic response modifiers for rheumatic disease.1–10 However, prophylaxis should be considered in patients who have granulomatosis with polyangiitis or underlying pulmonary disease, or who are concomitantly receiving glucocorticoids in high doses. There is some risk of adverse reactions to the prophylactic medicine.1,11–21 Until clear guidelines are available, the decision to initiate PCP prophylaxis and the choice of agent should be individualized.
THE BURDEN OF PCP
In a meta-analysis23 of 867 patients who developed PCP and did not have HIV infection, 20.1% had autoimmune or chronic inflammatory disease and the rest were transplant recipients or had malignancies. The mortality rate was 30.6%.
PHARMACOLOGIC RISK FACTORS FOR PCP
Treatment with glucocorticoids
Treatment with glucocorticoids is an important risk factor for PCP, independent of biologic therapy.
Calero-Bernal et al11 reported on 128 patients with non-HIV PCP, of whom 114 (89%) had received a glucocorticoid for more than 4 weeks, and 98 (76%) were currently receiving one. The mean daily dose was equivalent to 27.73 mg of prednisone per day in those on glucocorticoids only, and 21.34 mg in those receiving glucocorticoids in combination with other immunosuppressants.
Park et al,12 in a retrospective study of Korean patients treated for rheumatic disease with high-dose glucocorticoids (≥ 30 mg/day of prednisone or equivalent for more than 4 weeks), reported an incidence rate of PCP of 2.37 per 100 patient-years in those not on prophylaxis.
Other studies13,14 have also found a prednisone dose greater than 15 to 20 mg per day for more than 4 weeks or concomitant use of 2 or more disease-modifying antirheumatic drugs to be a significant risk factor.13,14
Tumor necrosis factor alpha antagonists
A US Food and Drug Administration review1 of voluntary reports of adverse drug events estimated the incidence of PCP to be 2.3 per 100,000 patient-years with infliximab and 1.6 per 100,000 patient-years with etanercept. In most cases, other immunosuppressants were used concomitantly.1
Postmarketing surveillance2 of 5,000 patients with rheumatoid arthritis showed an incidence of suspected PCP of 0.4% within the first 6 months of starting infliximab therapy.
Komano et al,15 in a case-control study of patients with rheumatoid arthritis treated with infliximab, reported that all 21 patients with PCP were also on methotrexate (median dosage 8 mg per week) and prednisolone (median dosage 7.5 mg per day).
PCP has also been reported after adalimumab use in combination with prednisone, azathioprine, and methotrexate, as well as with certolizumab, golimumab, tocilizumab, abatacept, and rituximab.3–6,24–26
Rituximab
Calero-Bernal et al11 reported that 23% of patients with non-HIV PCP who were receiving immunosuppressant drugs were on rituximab.
Alexandre et al16 performed a retrospective review of 11 cases of PCP complicating rituximab therapy for autoimmune disease, in which 10 (91%) of the patients were also on corticosteroids, with a median dosage of 30 mg of prednisone daily. A literature review of an additional 18 cases revealed similar findings.
PATIENT RISK FACTORS FOR PCP
Pulmonary disease, age, other factors
Komano et al,15 in their study of patients with rheumatoid arthritis treated with infliximab, found that 10 (48%) of 21 patients with PCP had preexisting pulmonary disease, compared with 11 (10.8%) of 102 patients without PCP (P < .001). Patients with PCP were older (mean age 64 vs 54, P < .001), were on higher median doses of prednisolone per day (7.5 vs 5 mg, P = .001), and had lower median serum immunoglobulin G (IgG) levels (944 vs 1,394 mg/dL, P < .001).15
Tadros et al13 performed a case-control study that also showed that patients with autoimmune disease who developed PCP had lower lymphocyte counts than controls on admission. Other risk factors included low CD4 counts and age older than 50.
Li et al17 found that patients with autoimmune or inflammatory disease with PCP were more likely to have low CD3, CD4, and CD8 cell counts, as well as albumin levels less than 28 g/L. They therefore suggested that lymphocyte subtyping may be a useful tool to guide PCP prophylaxis.
Granulomatosis with polyangiitis
Patients with granulomatosis with polyangiitis have a significantly higher incidence of PCP than patients with other connective tissue diseases.
Ward and Donald18 reviewed 223 cases of PCP in patients with connective tissue disease. The highest frequency (89 cases per 10,000 hospitalizations per year) was in patients with granulomatosis with polyangiitis, followed by 65 per 10,000 hospitalizations per year for patients with polyarteritis nodosa. The lowest frequency was in rheumatoid arthritis patients, at 2 per 10,000 hospitalizations per year. In decreasing order, diseases with significant associations with PCP were:
- Polyarteritis nodosa (odds ratio [OR] 10.20, 95% confidence interval [CI] 5.69–18.29)
- Granulomatosis with polyangiitis (OR 7.81, 95% CI 4.71–13.05)
- Inflammatory myopathy (OR 4.44, 95% CI 2.67–7.38)
- Systemic lupus erythematosus (OR 2.52, 95% CI 1.66–3.82).
Vallabhaneni and Chiller,26 in a meta-analysis including rheumatoid arthritis patients on biologics, did not find an increased risk of PCP (OR 1.77, 95% CI 0.42–7.47).
Park et al12 found that the highest incidences of PCP were in patients with granulomatosis with polyangiitis, microscopic polyangiitis, and systemic sclerosis. For systemic sclerosis, the main reason for giving high-dose glucocorticoids was interstitial lung disease.
Other studies19,20,28 also found an association with coexisting pulmonary disease in patients with rheumatoid arthritis.
CURRENT GUIDELINES
There are guidelines for primary and secondary prophylaxis of PCP in HIV-positive patients with CD4 counts less than 200/mm3 or a history of acquired immunodeficiency syndrome (AIDS)-defining illness.27 Additionally, patients with a CD4 cell percentage less than 14% should be considered for prophylaxis.27
Unfortunately, there are no guidelines for prophylaxis in patients taking immunosuppressants for rheumatic disease.
The recommended regimen for PCP prophylaxis in HIV-infected patients is trimethoprim-sulfamethoxazole, 1 double-strength or 1 single-strength tablet daily. Alternative regimens include 1 double-strength tablet 3 times per week, dapsone, aerosolized pentamidine, and atovaquone.27
There are also guidelines for prophylaxis in kidney transplant recipients, as well as for patients with hematologic malignancies and solid-organ malignancies, particularly those on chemotherapeutic agents and the T-cell-depleting agent alemtuzumab.29–31
Italian clinical practice guidelines for the use of tumor necrosis factor antagonists in inflammatory bowel disease recommend consideration of PCP prophylaxis in patients who are also on other immunosuppressants, particularly high-dose glucocorticoids.32
Prophylaxis has been shown to increase life expectancy and quality-adjusted life-years and to reduce cost for patients on immunosuppressive therapy for granulomatosis with polyangiitis.21 The European Society of Clinical Microbiology and Infectious Diseases recently produced consensus statements recommending PCP prophylaxis for patients on rituximab with other concomitant immunosuppressants such as the equivalent of prednisone 20 mg daily for more than 4 weeks.33 Prophylaxis was not recommended for other biologic therapies.34,35
THE RISKS OF PROPHYLAXIS
The risk of PCP should be weighed against the risk of prophylaxis in patients with rheumatic disease. Adverse reactions to sulfonamide antibiotics including disease flares have been reported in patients with systemic lupus erythematosus.36,37 Other studies have found no increased risk of flares in patients taking trimethoprim-sulfamethoxazole for PCP prophylaxis.12,38 A retrospective analysis of patients with vasculitis found no increased risk of combining methotrexate and trimethoprim-sulfamethoxazole.39
KEY POINTS
- PCP is an opportunistic infection with a high risk of death.
- PCP has been reported with biologics used as immunomodulators in rheumatic disease.
- PCP prophylaxis should be considered in patients at high risk of PCP, such as those who have granulomatosis with polyangiitis, underlying pulmonary disease or who are concomitantly taking glucocorticoids.
- US Food and Drug Administration. Safety update on TNF-alpha antagonists: infliximab and etanercept.https://wayback.archive-it.org/7993/20180127041103/https://www.fda.gov/ohrms/dockets/ac/01/briefing/3779b2_01_cber_safety_revision2.htm. Accessed May 3, 2019.
- Takeuchi T, Tatsuki Y, Nogami Y, et al. Postmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritis. Ann Rheum Dis 2008; 67(2):189–194. doi:10.1136/ard.2007.072967
- Koike T, Harigai M, Ishiguro N, et al. Safety and effectiveness of adalimumab in Japanese rheumatoid arthritis patients: postmarketing surveillance report of the first 3,000 patients. Mod Rheumatol 2012; 22(4):498–508. doi:10.1007/s10165-011-0541-5
- Bykerk V, Cush J, Winthrop K, et al. Update on the safety profile of certolizumab pegol in rheumatoid arthritis: an integrated analysis from clinical trials. Ann Rheum Dis 2015; 74(1):96–103. doi:10.1136/annrheumdis-2013-203660
- Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann Rheum Dis 2011; 70(12):2148–2151. doi:10.1136/ard.2011.151092
- Harigai M, Ishiguro N, Inokuma S, et al. Postmarketing surveillance of the safety and effectiveness of abatacept in Japanese patients with rheumatoid arthritis. Mod Rheumatol 2016; 26(4):491–498. doi:10.3109/14397595.2015.1123211
- Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of the safety and effectiveness of etanercept in Japan. J Rheumatol 2009; 36(5):898–906. doi:10.3899/jrheum.080791
- Grubbs JA, Baddley JW. Pneumocystis jirovecii pneumonia in patients receiving tumor-necrosis-factor-inhibitor therapy: implications for chemoprophylaxis. Curr Rheumatol Rep 2014; 16(10):445. doi:10.1007/s11926-014-0445-4
- US Food and Drug Administration. FDA adverse event reporting system (FAERS) public dashboard. www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm070093.htm. Accessed May 3, 2019.
- Rutherford AI, Patarata E, Subesinghe S, Hyrich KL, Galloway JB. Opportunistic infections in rheumatoid arthritis patients exposed to biologic therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford) 2018; 57(6):997–1001. doi:10.1093/rheumatology/key023
- Calero-Bernal ML, Martin-Garrido I, Donazar-Ezcurra M, Limper AH, Carmona EM. Intermittent courses of corticosteroids also present a risk for Pneumocystis pneumonia in non-HIV patients. Can Respir J 2016; 2016:2464791. doi:10.1155/2016/2464791
- Park JW, Curtis JR, Moon J, Song YW, Kim S, Lee EB. Prophylactic effect of trimethoprim-sulfamethoxazole for pneumocystis pneumonia in patients with rheumatic diseases exposed to prolonged high-dose glucocorticoids. Ann Rheum Dis 2018; 77(5):644–649. doi:10.1136/annrheumdis-2017-211796
- Tadros S, Teichtahl AJ, Ciciriello S, Wicks IP. Pneumocystis jirovecii pneumonia in systemic autoimmune rheumatic disease: a case-control study. Semin Arthritis Rheum 2017; 46(6):804–809. doi:10.1016/j.semarthrit.2016.09.009
- Demoruelle MK, Kahr A, Verilhac K, Deane K, Fischer A, West S. Recent-onset systemic lupus erythematosus complicated by acute respiratory failure. Arthritis Care Res (Hoboken) 2013; 65(2):314–323. doi:10.1002/acr.21857
- Komano Y, Harigai M, Koike R, et al. Pneumocystis jiroveci pneumonia in patients with rheumatoid arthritis treated with infliximab: a retrospective review and case-control study of 21 patients. Arthritis Rheum 2009; 61(3):305–312. doi:10.1002/art.24283
- Alexandre K, Ingen-Housz-Oro S, Versini M, Sailler L, Benhamou Y. Pneumocystis jirovecii pneumonia in patients treated with rituximab for systemic diseases: report of 11 cases and review of the literature. Eur J Intern Med 2018; 50:e23–e24. doi:10.1016/j.ejim.2017.11.014
- Li Y, Ghannoum M, Deng C, et al. Pneumocystis pneumonia in patients with inflammatory or autoimmune diseases: usefulness of lymphocyte subtyping. Int J Infect Dis 2017; 57:108–115. doi:10.1016/j.ijid.2017.02.010
- Ward MM, Donald F. Pneumocystis carinii pneumonia in patients with connective tissue diseases: the role of hospital experience in diagnosis and mortality. Arthritis Rheum 1999; 42(4):780–789. doi:10.1002/1529-0131(199904)42:4<780::AID-ANR23>3.0.CO;2-M
- Katsuyama T, Saito K, Kubo S, Nawata M, Tanaka Y. Prophylaxis for Pneumocystis pneumonia in patients with rheumatoid arthritis treated with biologics, based on risk factors found in a retrospective study. Arthritis Res Ther 2014; 16(1):R43. doi:10.1186/ar4472
- Tanaka M, Sakai R, Koike R, et al. Pneumocystis jirovecii pneumonia associated with etanercept treatment in patients with rheumatoid arthritis: a retrospective review of 15 cases and analysis of risk factors. Mod Rheumatol 2012; 22(6):849–858. doi:10.1007/s10165-012-0615-z
- Chung JB, Armstrong K, Schwartz JS, Albert D. Cost-effectiveness of prophylaxis against Pneumocystis carinii pneumonia in patients with Wegener’s granulomatosis undergoing immunosuppressive therapy. Arthritis Rheum 2000; 43(8):1841–1848. doi:10.1002/1529-0131(200008)43:8<1841::AID-ANR21>3.0.CO;2-Q
- Selmi C, Generali E, Massarotti M, Bianchi G, Scire CA. New treatments for inflammatory rheumatic disease. Immunol Res 2014; 60(2–3):277–288. doi:10.1007/s12026-014-8565-5
- Liu Y, Su L, Jiang SJ, Qu H. Risk factors for mortality from Pneumocystis carinii pneumonia (PCP) in non-HIV patients: a meta-analysis. Oncotarget 2017; 8(35):59729–59739. doi:10.18632/oncotarget.19927
- Desales AL, Mendez-Navarro J, Méndez-Tovar LJ, et al. Pneumocystosis in a patient with Crohn's disease treated with combination therapy with adalimumab. J Crohns Colitis 2012; 6(4):483–487. doi:10.1016/j.crohns.2011.10.012
- Kalyoncu U, Karadag O, Akdogan A, et al. Pneumocystis carinii pneumonia in a rheumatoid arthritis patient treated with adalimumab. Scand J Infect Dis 2007; 39(5):475–478. doi:10.1080/00365540601071867
- Vallabhaneni S, Chiller TM. Fungal infections and new biologic therapies. Curr Rheumatol Rep 2016; 18(5):29. doi:10.1007/s11926-016-0572-1
- Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. www.aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed May 3, 2019.
- Kourbeti IS, Ziakas PD, Mylonakis E. Biologic therapies in rheumatoid arthritis and the risk of opportunistic infections: a meta-analysis. Clin Infect Dis 2014; 58(12):1649–1657. doi:10.1093/cid/ciu185
- Bia M, Adey DB, Bloom RD, Chan L, Kulkarni S, Tomlanovich S. KDOQI US commentary on the 2009 KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Kidney Dis 2010; 56(2):189–218. doi:10.1053/j.ajkd.2010.04.010
- Baden LR, Swaminathan S, Angarone M, et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016; 14(7):882–913. pmid:27407129
- Cooley L, Dendle C, Wolf J, et al. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J 2014; 44(12b):1350–1363. doi:10.1111/imj.12599
- Orlando A, Armuzzi A, Papi C, et al; Italian Society of Gastroenterology; Italian Group for the study of Inflammatory Bowel Disease. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) clinical practice guidelines: the use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis 2011; 43(1):1–20. doi:10.1016/j.dld.2010.07.010
- Mikulska M, Lanini S, Gudiol C, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (agents targeting lymphoid cells surface antigens [I]: CD19, CD20 and CD52). Clin Microbiol Infect 2018; 24(suppl 2):S71–S82. doi:10.1016/j.cmi.2018.02.003
- Baddley J, Cantini F, Goletti D, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (soluble immune effector molecules [I]: anti-tumor necrosis factor-alpha agents). Clin Microbiol Infect 2018; 24(suppl 2):S10–S20. doi:10.1016/j.cmi.2017.12.025
- Winthrop K, Mariette X, Silva J, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (soluble immune effector molecules [II]: agents targeting interleukins, immunoglobulins and complement factors). Clin Microbiol Infect 2018; 24(suppl 2):S21–S40. doi:10.1016/j.cmi.2018.02.002
- Petri M, Allbritton J. Antibiotic allergy in systemic lupus erythematosus: a case-control study. J Rheumatol 1992; 19(2):265–269. pmid:1629825
- Pope J, Jerome D, Fenlon D, Krizova A, Ouimet J. Frequency of adverse drug reactions in patients with systemic lupus erythematosus. J Rheumatol 2003; 30(3):480–484. pmid:12610805
- Vananuvat P, Suwannalai P, Sungkanuparph S, Limsuwan T, Ngamjanyaporn P, Janwityanujit S. Primary prophylaxis for Pneumocystis jirovecii pneumonia in patients with connective tissue diseases. Semin Arthritis Rheum 2011; 41(3):497–502. doi:10.1016/j.semarthrit.2011.05.004
- Tamaki H, Butler R, Langford C. Abstract Number: 1755: Safety of methotrexate and low-dose trimethoprim-sulfamethoxazole in patients with ANCA-associated vasculitis. www.acrabstracts.org/abstract/safety-of-methotrexate-and-low-dose-trimethoprim-sulfamethoxazole-in-patients-with-anca-associated-vasculitis. Accessed May 3, 2019.
Pneumocystis jirovecii (previously carinii) pneumonia (PCP) is rare in patients taking biologic response modifiers for rheumatic disease.1–10 However, prophylaxis should be considered in patients who have granulomatosis with polyangiitis or underlying pulmonary disease, or who are concomitantly receiving glucocorticoids in high doses. There is some risk of adverse reactions to the prophylactic medicine.1,11–21 Until clear guidelines are available, the decision to initiate PCP prophylaxis and the choice of agent should be individualized.
THE BURDEN OF PCP
In a meta-analysis23 of 867 patients who developed PCP and did not have HIV infection, 20.1% had autoimmune or chronic inflammatory disease and the rest were transplant recipients or had malignancies. The mortality rate was 30.6%.
PHARMACOLOGIC RISK FACTORS FOR PCP
Treatment with glucocorticoids
Treatment with glucocorticoids is an important risk factor for PCP, independent of biologic therapy.
Calero-Bernal et al11 reported on 128 patients with non-HIV PCP, of whom 114 (89%) had received a glucocorticoid for more than 4 weeks, and 98 (76%) were currently receiving one. The mean daily dose was equivalent to 27.73 mg of prednisone per day in those on glucocorticoids only, and 21.34 mg in those receiving glucocorticoids in combination with other immunosuppressants.
Park et al,12 in a retrospective study of Korean patients treated for rheumatic disease with high-dose glucocorticoids (≥ 30 mg/day of prednisone or equivalent for more than 4 weeks), reported an incidence rate of PCP of 2.37 per 100 patient-years in those not on prophylaxis.
Other studies13,14 have also found a prednisone dose greater than 15 to 20 mg per day for more than 4 weeks or concomitant use of 2 or more disease-modifying antirheumatic drugs to be a significant risk factor.13,14
Tumor necrosis factor alpha antagonists
A US Food and Drug Administration review1 of voluntary reports of adverse drug events estimated the incidence of PCP to be 2.3 per 100,000 patient-years with infliximab and 1.6 per 100,000 patient-years with etanercept. In most cases, other immunosuppressants were used concomitantly.1
Postmarketing surveillance2 of 5,000 patients with rheumatoid arthritis showed an incidence of suspected PCP of 0.4% within the first 6 months of starting infliximab therapy.
Komano et al,15 in a case-control study of patients with rheumatoid arthritis treated with infliximab, reported that all 21 patients with PCP were also on methotrexate (median dosage 8 mg per week) and prednisolone (median dosage 7.5 mg per day).
PCP has also been reported after adalimumab use in combination with prednisone, azathioprine, and methotrexate, as well as with certolizumab, golimumab, tocilizumab, abatacept, and rituximab.3–6,24–26
Rituximab
Calero-Bernal et al11 reported that 23% of patients with non-HIV PCP who were receiving immunosuppressant drugs were on rituximab.
Alexandre et al16 performed a retrospective review of 11 cases of PCP complicating rituximab therapy for autoimmune disease, in which 10 (91%) of the patients were also on corticosteroids, with a median dosage of 30 mg of prednisone daily. A literature review of an additional 18 cases revealed similar findings.
PATIENT RISK FACTORS FOR PCP
Pulmonary disease, age, other factors
Komano et al,15 in their study of patients with rheumatoid arthritis treated with infliximab, found that 10 (48%) of 21 patients with PCP had preexisting pulmonary disease, compared with 11 (10.8%) of 102 patients without PCP (P < .001). Patients with PCP were older (mean age 64 vs 54, P < .001), were on higher median doses of prednisolone per day (7.5 vs 5 mg, P = .001), and had lower median serum immunoglobulin G (IgG) levels (944 vs 1,394 mg/dL, P < .001).15
Tadros et al13 performed a case-control study that also showed that patients with autoimmune disease who developed PCP had lower lymphocyte counts than controls on admission. Other risk factors included low CD4 counts and age older than 50.
Li et al17 found that patients with autoimmune or inflammatory disease with PCP were more likely to have low CD3, CD4, and CD8 cell counts, as well as albumin levels less than 28 g/L. They therefore suggested that lymphocyte subtyping may be a useful tool to guide PCP prophylaxis.
Granulomatosis with polyangiitis
Patients with granulomatosis with polyangiitis have a significantly higher incidence of PCP than patients with other connective tissue diseases.
Ward and Donald18 reviewed 223 cases of PCP in patients with connective tissue disease. The highest frequency (89 cases per 10,000 hospitalizations per year) was in patients with granulomatosis with polyangiitis, followed by 65 per 10,000 hospitalizations per year for patients with polyarteritis nodosa. The lowest frequency was in rheumatoid arthritis patients, at 2 per 10,000 hospitalizations per year. In decreasing order, diseases with significant associations with PCP were:
- Polyarteritis nodosa (odds ratio [OR] 10.20, 95% confidence interval [CI] 5.69–18.29)
- Granulomatosis with polyangiitis (OR 7.81, 95% CI 4.71–13.05)
- Inflammatory myopathy (OR 4.44, 95% CI 2.67–7.38)
- Systemic lupus erythematosus (OR 2.52, 95% CI 1.66–3.82).
Vallabhaneni and Chiller,26 in a meta-analysis including rheumatoid arthritis patients on biologics, did not find an increased risk of PCP (OR 1.77, 95% CI 0.42–7.47).
Park et al12 found that the highest incidences of PCP were in patients with granulomatosis with polyangiitis, microscopic polyangiitis, and systemic sclerosis. For systemic sclerosis, the main reason for giving high-dose glucocorticoids was interstitial lung disease.
Other studies19,20,28 also found an association with coexisting pulmonary disease in patients with rheumatoid arthritis.
CURRENT GUIDELINES
There are guidelines for primary and secondary prophylaxis of PCP in HIV-positive patients with CD4 counts less than 200/mm3 or a history of acquired immunodeficiency syndrome (AIDS)-defining illness.27 Additionally, patients with a CD4 cell percentage less than 14% should be considered for prophylaxis.27
Unfortunately, there are no guidelines for prophylaxis in patients taking immunosuppressants for rheumatic disease.
The recommended regimen for PCP prophylaxis in HIV-infected patients is trimethoprim-sulfamethoxazole, 1 double-strength or 1 single-strength tablet daily. Alternative regimens include 1 double-strength tablet 3 times per week, dapsone, aerosolized pentamidine, and atovaquone.27
There are also guidelines for prophylaxis in kidney transplant recipients, as well as for patients with hematologic malignancies and solid-organ malignancies, particularly those on chemotherapeutic agents and the T-cell-depleting agent alemtuzumab.29–31
Italian clinical practice guidelines for the use of tumor necrosis factor antagonists in inflammatory bowel disease recommend consideration of PCP prophylaxis in patients who are also on other immunosuppressants, particularly high-dose glucocorticoids.32
Prophylaxis has been shown to increase life expectancy and quality-adjusted life-years and to reduce cost for patients on immunosuppressive therapy for granulomatosis with polyangiitis.21 The European Society of Clinical Microbiology and Infectious Diseases recently produced consensus statements recommending PCP prophylaxis for patients on rituximab with other concomitant immunosuppressants such as the equivalent of prednisone 20 mg daily for more than 4 weeks.33 Prophylaxis was not recommended for other biologic therapies.34,35
THE RISKS OF PROPHYLAXIS
The risk of PCP should be weighed against the risk of prophylaxis in patients with rheumatic disease. Adverse reactions to sulfonamide antibiotics including disease flares have been reported in patients with systemic lupus erythematosus.36,37 Other studies have found no increased risk of flares in patients taking trimethoprim-sulfamethoxazole for PCP prophylaxis.12,38 A retrospective analysis of patients with vasculitis found no increased risk of combining methotrexate and trimethoprim-sulfamethoxazole.39
KEY POINTS
- PCP is an opportunistic infection with a high risk of death.
- PCP has been reported with biologics used as immunomodulators in rheumatic disease.
- PCP prophylaxis should be considered in patients at high risk of PCP, such as those who have granulomatosis with polyangiitis, underlying pulmonary disease or who are concomitantly taking glucocorticoids.
Pneumocystis jirovecii (previously carinii) pneumonia (PCP) is rare in patients taking biologic response modifiers for rheumatic disease.1–10 However, prophylaxis should be considered in patients who have granulomatosis with polyangiitis or underlying pulmonary disease, or who are concomitantly receiving glucocorticoids in high doses. There is some risk of adverse reactions to the prophylactic medicine.1,11–21 Until clear guidelines are available, the decision to initiate PCP prophylaxis and the choice of agent should be individualized.
THE BURDEN OF PCP
In a meta-analysis23 of 867 patients who developed PCP and did not have HIV infection, 20.1% had autoimmune or chronic inflammatory disease and the rest were transplant recipients or had malignancies. The mortality rate was 30.6%.
PHARMACOLOGIC RISK FACTORS FOR PCP
Treatment with glucocorticoids
Treatment with glucocorticoids is an important risk factor for PCP, independent of biologic therapy.
Calero-Bernal et al11 reported on 128 patients with non-HIV PCP, of whom 114 (89%) had received a glucocorticoid for more than 4 weeks, and 98 (76%) were currently receiving one. The mean daily dose was equivalent to 27.73 mg of prednisone per day in those on glucocorticoids only, and 21.34 mg in those receiving glucocorticoids in combination with other immunosuppressants.
Park et al,12 in a retrospective study of Korean patients treated for rheumatic disease with high-dose glucocorticoids (≥ 30 mg/day of prednisone or equivalent for more than 4 weeks), reported an incidence rate of PCP of 2.37 per 100 patient-years in those not on prophylaxis.
Other studies13,14 have also found a prednisone dose greater than 15 to 20 mg per day for more than 4 weeks or concomitant use of 2 or more disease-modifying antirheumatic drugs to be a significant risk factor.13,14
Tumor necrosis factor alpha antagonists
A US Food and Drug Administration review1 of voluntary reports of adverse drug events estimated the incidence of PCP to be 2.3 per 100,000 patient-years with infliximab and 1.6 per 100,000 patient-years with etanercept. In most cases, other immunosuppressants were used concomitantly.1
Postmarketing surveillance2 of 5,000 patients with rheumatoid arthritis showed an incidence of suspected PCP of 0.4% within the first 6 months of starting infliximab therapy.
Komano et al,15 in a case-control study of patients with rheumatoid arthritis treated with infliximab, reported that all 21 patients with PCP were also on methotrexate (median dosage 8 mg per week) and prednisolone (median dosage 7.5 mg per day).
PCP has also been reported after adalimumab use in combination with prednisone, azathioprine, and methotrexate, as well as with certolizumab, golimumab, tocilizumab, abatacept, and rituximab.3–6,24–26
Rituximab
Calero-Bernal et al11 reported that 23% of patients with non-HIV PCP who were receiving immunosuppressant drugs were on rituximab.
Alexandre et al16 performed a retrospective review of 11 cases of PCP complicating rituximab therapy for autoimmune disease, in which 10 (91%) of the patients were also on corticosteroids, with a median dosage of 30 mg of prednisone daily. A literature review of an additional 18 cases revealed similar findings.
PATIENT RISK FACTORS FOR PCP
Pulmonary disease, age, other factors
Komano et al,15 in their study of patients with rheumatoid arthritis treated with infliximab, found that 10 (48%) of 21 patients with PCP had preexisting pulmonary disease, compared with 11 (10.8%) of 102 patients without PCP (P < .001). Patients with PCP were older (mean age 64 vs 54, P < .001), were on higher median doses of prednisolone per day (7.5 vs 5 mg, P = .001), and had lower median serum immunoglobulin G (IgG) levels (944 vs 1,394 mg/dL, P < .001).15
Tadros et al13 performed a case-control study that also showed that patients with autoimmune disease who developed PCP had lower lymphocyte counts than controls on admission. Other risk factors included low CD4 counts and age older than 50.
Li et al17 found that patients with autoimmune or inflammatory disease with PCP were more likely to have low CD3, CD4, and CD8 cell counts, as well as albumin levels less than 28 g/L. They therefore suggested that lymphocyte subtyping may be a useful tool to guide PCP prophylaxis.
Granulomatosis with polyangiitis
Patients with granulomatosis with polyangiitis have a significantly higher incidence of PCP than patients with other connective tissue diseases.
Ward and Donald18 reviewed 223 cases of PCP in patients with connective tissue disease. The highest frequency (89 cases per 10,000 hospitalizations per year) was in patients with granulomatosis with polyangiitis, followed by 65 per 10,000 hospitalizations per year for patients with polyarteritis nodosa. The lowest frequency was in rheumatoid arthritis patients, at 2 per 10,000 hospitalizations per year. In decreasing order, diseases with significant associations with PCP were:
- Polyarteritis nodosa (odds ratio [OR] 10.20, 95% confidence interval [CI] 5.69–18.29)
- Granulomatosis with polyangiitis (OR 7.81, 95% CI 4.71–13.05)
- Inflammatory myopathy (OR 4.44, 95% CI 2.67–7.38)
- Systemic lupus erythematosus (OR 2.52, 95% CI 1.66–3.82).
Vallabhaneni and Chiller,26 in a meta-analysis including rheumatoid arthritis patients on biologics, did not find an increased risk of PCP (OR 1.77, 95% CI 0.42–7.47).
Park et al12 found that the highest incidences of PCP were in patients with granulomatosis with polyangiitis, microscopic polyangiitis, and systemic sclerosis. For systemic sclerosis, the main reason for giving high-dose glucocorticoids was interstitial lung disease.
Other studies19,20,28 also found an association with coexisting pulmonary disease in patients with rheumatoid arthritis.
CURRENT GUIDELINES
There are guidelines for primary and secondary prophylaxis of PCP in HIV-positive patients with CD4 counts less than 200/mm3 or a history of acquired immunodeficiency syndrome (AIDS)-defining illness.27 Additionally, patients with a CD4 cell percentage less than 14% should be considered for prophylaxis.27
Unfortunately, there are no guidelines for prophylaxis in patients taking immunosuppressants for rheumatic disease.
The recommended regimen for PCP prophylaxis in HIV-infected patients is trimethoprim-sulfamethoxazole, 1 double-strength or 1 single-strength tablet daily. Alternative regimens include 1 double-strength tablet 3 times per week, dapsone, aerosolized pentamidine, and atovaquone.27
There are also guidelines for prophylaxis in kidney transplant recipients, as well as for patients with hematologic malignancies and solid-organ malignancies, particularly those on chemotherapeutic agents and the T-cell-depleting agent alemtuzumab.29–31
Italian clinical practice guidelines for the use of tumor necrosis factor antagonists in inflammatory bowel disease recommend consideration of PCP prophylaxis in patients who are also on other immunosuppressants, particularly high-dose glucocorticoids.32
Prophylaxis has been shown to increase life expectancy and quality-adjusted life-years and to reduce cost for patients on immunosuppressive therapy for granulomatosis with polyangiitis.21 The European Society of Clinical Microbiology and Infectious Diseases recently produced consensus statements recommending PCP prophylaxis for patients on rituximab with other concomitant immunosuppressants such as the equivalent of prednisone 20 mg daily for more than 4 weeks.33 Prophylaxis was not recommended for other biologic therapies.34,35
THE RISKS OF PROPHYLAXIS
The risk of PCP should be weighed against the risk of prophylaxis in patients with rheumatic disease. Adverse reactions to sulfonamide antibiotics including disease flares have been reported in patients with systemic lupus erythematosus.36,37 Other studies have found no increased risk of flares in patients taking trimethoprim-sulfamethoxazole for PCP prophylaxis.12,38 A retrospective analysis of patients with vasculitis found no increased risk of combining methotrexate and trimethoprim-sulfamethoxazole.39
KEY POINTS
- PCP is an opportunistic infection with a high risk of death.
- PCP has been reported with biologics used as immunomodulators in rheumatic disease.
- PCP prophylaxis should be considered in patients at high risk of PCP, such as those who have granulomatosis with polyangiitis, underlying pulmonary disease or who are concomitantly taking glucocorticoids.
- US Food and Drug Administration. Safety update on TNF-alpha antagonists: infliximab and etanercept.https://wayback.archive-it.org/7993/20180127041103/https://www.fda.gov/ohrms/dockets/ac/01/briefing/3779b2_01_cber_safety_revision2.htm. Accessed May 3, 2019.
- Takeuchi T, Tatsuki Y, Nogami Y, et al. Postmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritis. Ann Rheum Dis 2008; 67(2):189–194. doi:10.1136/ard.2007.072967
- Koike T, Harigai M, Ishiguro N, et al. Safety and effectiveness of adalimumab in Japanese rheumatoid arthritis patients: postmarketing surveillance report of the first 3,000 patients. Mod Rheumatol 2012; 22(4):498–508. doi:10.1007/s10165-011-0541-5
- Bykerk V, Cush J, Winthrop K, et al. Update on the safety profile of certolizumab pegol in rheumatoid arthritis: an integrated analysis from clinical trials. Ann Rheum Dis 2015; 74(1):96–103. doi:10.1136/annrheumdis-2013-203660
- Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann Rheum Dis 2011; 70(12):2148–2151. doi:10.1136/ard.2011.151092
- Harigai M, Ishiguro N, Inokuma S, et al. Postmarketing surveillance of the safety and effectiveness of abatacept in Japanese patients with rheumatoid arthritis. Mod Rheumatol 2016; 26(4):491–498. doi:10.3109/14397595.2015.1123211
- Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of the safety and effectiveness of etanercept in Japan. J Rheumatol 2009; 36(5):898–906. doi:10.3899/jrheum.080791
- Grubbs JA, Baddley JW. Pneumocystis jirovecii pneumonia in patients receiving tumor-necrosis-factor-inhibitor therapy: implications for chemoprophylaxis. Curr Rheumatol Rep 2014; 16(10):445. doi:10.1007/s11926-014-0445-4
- US Food and Drug Administration. FDA adverse event reporting system (FAERS) public dashboard. www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm070093.htm. Accessed May 3, 2019.
- Rutherford AI, Patarata E, Subesinghe S, Hyrich KL, Galloway JB. Opportunistic infections in rheumatoid arthritis patients exposed to biologic therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford) 2018; 57(6):997–1001. doi:10.1093/rheumatology/key023
- Calero-Bernal ML, Martin-Garrido I, Donazar-Ezcurra M, Limper AH, Carmona EM. Intermittent courses of corticosteroids also present a risk for Pneumocystis pneumonia in non-HIV patients. Can Respir J 2016; 2016:2464791. doi:10.1155/2016/2464791
- Park JW, Curtis JR, Moon J, Song YW, Kim S, Lee EB. Prophylactic effect of trimethoprim-sulfamethoxazole for pneumocystis pneumonia in patients with rheumatic diseases exposed to prolonged high-dose glucocorticoids. Ann Rheum Dis 2018; 77(5):644–649. doi:10.1136/annrheumdis-2017-211796
- Tadros S, Teichtahl AJ, Ciciriello S, Wicks IP. Pneumocystis jirovecii pneumonia in systemic autoimmune rheumatic disease: a case-control study. Semin Arthritis Rheum 2017; 46(6):804–809. doi:10.1016/j.semarthrit.2016.09.009
- Demoruelle MK, Kahr A, Verilhac K, Deane K, Fischer A, West S. Recent-onset systemic lupus erythematosus complicated by acute respiratory failure. Arthritis Care Res (Hoboken) 2013; 65(2):314–323. doi:10.1002/acr.21857
- Komano Y, Harigai M, Koike R, et al. Pneumocystis jiroveci pneumonia in patients with rheumatoid arthritis treated with infliximab: a retrospective review and case-control study of 21 patients. Arthritis Rheum 2009; 61(3):305–312. doi:10.1002/art.24283
- Alexandre K, Ingen-Housz-Oro S, Versini M, Sailler L, Benhamou Y. Pneumocystis jirovecii pneumonia in patients treated with rituximab for systemic diseases: report of 11 cases and review of the literature. Eur J Intern Med 2018; 50:e23–e24. doi:10.1016/j.ejim.2017.11.014
- Li Y, Ghannoum M, Deng C, et al. Pneumocystis pneumonia in patients with inflammatory or autoimmune diseases: usefulness of lymphocyte subtyping. Int J Infect Dis 2017; 57:108–115. doi:10.1016/j.ijid.2017.02.010
- Ward MM, Donald F. Pneumocystis carinii pneumonia in patients with connective tissue diseases: the role of hospital experience in diagnosis and mortality. Arthritis Rheum 1999; 42(4):780–789. doi:10.1002/1529-0131(199904)42:4<780::AID-ANR23>3.0.CO;2-M
- Katsuyama T, Saito K, Kubo S, Nawata M, Tanaka Y. Prophylaxis for Pneumocystis pneumonia in patients with rheumatoid arthritis treated with biologics, based on risk factors found in a retrospective study. Arthritis Res Ther 2014; 16(1):R43. doi:10.1186/ar4472
- Tanaka M, Sakai R, Koike R, et al. Pneumocystis jirovecii pneumonia associated with etanercept treatment in patients with rheumatoid arthritis: a retrospective review of 15 cases and analysis of risk factors. Mod Rheumatol 2012; 22(6):849–858. doi:10.1007/s10165-012-0615-z
- Chung JB, Armstrong K, Schwartz JS, Albert D. Cost-effectiveness of prophylaxis against Pneumocystis carinii pneumonia in patients with Wegener’s granulomatosis undergoing immunosuppressive therapy. Arthritis Rheum 2000; 43(8):1841–1848. doi:10.1002/1529-0131(200008)43:8<1841::AID-ANR21>3.0.CO;2-Q
- Selmi C, Generali E, Massarotti M, Bianchi G, Scire CA. New treatments for inflammatory rheumatic disease. Immunol Res 2014; 60(2–3):277–288. doi:10.1007/s12026-014-8565-5
- Liu Y, Su L, Jiang SJ, Qu H. Risk factors for mortality from Pneumocystis carinii pneumonia (PCP) in non-HIV patients: a meta-analysis. Oncotarget 2017; 8(35):59729–59739. doi:10.18632/oncotarget.19927
- Desales AL, Mendez-Navarro J, Méndez-Tovar LJ, et al. Pneumocystosis in a patient with Crohn's disease treated with combination therapy with adalimumab. J Crohns Colitis 2012; 6(4):483–487. doi:10.1016/j.crohns.2011.10.012
- Kalyoncu U, Karadag O, Akdogan A, et al. Pneumocystis carinii pneumonia in a rheumatoid arthritis patient treated with adalimumab. Scand J Infect Dis 2007; 39(5):475–478. doi:10.1080/00365540601071867
- Vallabhaneni S, Chiller TM. Fungal infections and new biologic therapies. Curr Rheumatol Rep 2016; 18(5):29. doi:10.1007/s11926-016-0572-1
- Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. www.aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed May 3, 2019.
- Kourbeti IS, Ziakas PD, Mylonakis E. Biologic therapies in rheumatoid arthritis and the risk of opportunistic infections: a meta-analysis. Clin Infect Dis 2014; 58(12):1649–1657. doi:10.1093/cid/ciu185
- Bia M, Adey DB, Bloom RD, Chan L, Kulkarni S, Tomlanovich S. KDOQI US commentary on the 2009 KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Kidney Dis 2010; 56(2):189–218. doi:10.1053/j.ajkd.2010.04.010
- Baden LR, Swaminathan S, Angarone M, et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016; 14(7):882–913. pmid:27407129
- Cooley L, Dendle C, Wolf J, et al. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J 2014; 44(12b):1350–1363. doi:10.1111/imj.12599
- Orlando A, Armuzzi A, Papi C, et al; Italian Society of Gastroenterology; Italian Group for the study of Inflammatory Bowel Disease. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) clinical practice guidelines: the use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis 2011; 43(1):1–20. doi:10.1016/j.dld.2010.07.010
- Mikulska M, Lanini S, Gudiol C, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (agents targeting lymphoid cells surface antigens [I]: CD19, CD20 and CD52). Clin Microbiol Infect 2018; 24(suppl 2):S71–S82. doi:10.1016/j.cmi.2018.02.003
- Baddley J, Cantini F, Goletti D, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (soluble immune effector molecules [I]: anti-tumor necrosis factor-alpha agents). Clin Microbiol Infect 2018; 24(suppl 2):S10–S20. doi:10.1016/j.cmi.2017.12.025
- Winthrop K, Mariette X, Silva J, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (soluble immune effector molecules [II]: agents targeting interleukins, immunoglobulins and complement factors). Clin Microbiol Infect 2018; 24(suppl 2):S21–S40. doi:10.1016/j.cmi.2018.02.002
- Petri M, Allbritton J. Antibiotic allergy in systemic lupus erythematosus: a case-control study. J Rheumatol 1992; 19(2):265–269. pmid:1629825
- Pope J, Jerome D, Fenlon D, Krizova A, Ouimet J. Frequency of adverse drug reactions in patients with systemic lupus erythematosus. J Rheumatol 2003; 30(3):480–484. pmid:12610805
- Vananuvat P, Suwannalai P, Sungkanuparph S, Limsuwan T, Ngamjanyaporn P, Janwityanujit S. Primary prophylaxis for Pneumocystis jirovecii pneumonia in patients with connective tissue diseases. Semin Arthritis Rheum 2011; 41(3):497–502. doi:10.1016/j.semarthrit.2011.05.004
- Tamaki H, Butler R, Langford C. Abstract Number: 1755: Safety of methotrexate and low-dose trimethoprim-sulfamethoxazole in patients with ANCA-associated vasculitis. www.acrabstracts.org/abstract/safety-of-methotrexate-and-low-dose-trimethoprim-sulfamethoxazole-in-patients-with-anca-associated-vasculitis. Accessed May 3, 2019.
- US Food and Drug Administration. Safety update on TNF-alpha antagonists: infliximab and etanercept.https://wayback.archive-it.org/7993/20180127041103/https://www.fda.gov/ohrms/dockets/ac/01/briefing/3779b2_01_cber_safety_revision2.htm. Accessed May 3, 2019.
- Takeuchi T, Tatsuki Y, Nogami Y, et al. Postmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritis. Ann Rheum Dis 2008; 67(2):189–194. doi:10.1136/ard.2007.072967
- Koike T, Harigai M, Ishiguro N, et al. Safety and effectiveness of adalimumab in Japanese rheumatoid arthritis patients: postmarketing surveillance report of the first 3,000 patients. Mod Rheumatol 2012; 22(4):498–508. doi:10.1007/s10165-011-0541-5
- Bykerk V, Cush J, Winthrop K, et al. Update on the safety profile of certolizumab pegol in rheumatoid arthritis: an integrated analysis from clinical trials. Ann Rheum Dis 2015; 74(1):96–103. doi:10.1136/annrheumdis-2013-203660
- Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann Rheum Dis 2011; 70(12):2148–2151. doi:10.1136/ard.2011.151092
- Harigai M, Ishiguro N, Inokuma S, et al. Postmarketing surveillance of the safety and effectiveness of abatacept in Japanese patients with rheumatoid arthritis. Mod Rheumatol 2016; 26(4):491–498. doi:10.3109/14397595.2015.1123211
- Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of the safety and effectiveness of etanercept in Japan. J Rheumatol 2009; 36(5):898–906. doi:10.3899/jrheum.080791
- Grubbs JA, Baddley JW. Pneumocystis jirovecii pneumonia in patients receiving tumor-necrosis-factor-inhibitor therapy: implications for chemoprophylaxis. Curr Rheumatol Rep 2014; 16(10):445. doi:10.1007/s11926-014-0445-4
- US Food and Drug Administration. FDA adverse event reporting system (FAERS) public dashboard. www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm070093.htm. Accessed May 3, 2019.
- Rutherford AI, Patarata E, Subesinghe S, Hyrich KL, Galloway JB. Opportunistic infections in rheumatoid arthritis patients exposed to biologic therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford) 2018; 57(6):997–1001. doi:10.1093/rheumatology/key023
- Calero-Bernal ML, Martin-Garrido I, Donazar-Ezcurra M, Limper AH, Carmona EM. Intermittent courses of corticosteroids also present a risk for Pneumocystis pneumonia in non-HIV patients. Can Respir J 2016; 2016:2464791. doi:10.1155/2016/2464791
- Park JW, Curtis JR, Moon J, Song YW, Kim S, Lee EB. Prophylactic effect of trimethoprim-sulfamethoxazole for pneumocystis pneumonia in patients with rheumatic diseases exposed to prolonged high-dose glucocorticoids. Ann Rheum Dis 2018; 77(5):644–649. doi:10.1136/annrheumdis-2017-211796
- Tadros S, Teichtahl AJ, Ciciriello S, Wicks IP. Pneumocystis jirovecii pneumonia in systemic autoimmune rheumatic disease: a case-control study. Semin Arthritis Rheum 2017; 46(6):804–809. doi:10.1016/j.semarthrit.2016.09.009
- Demoruelle MK, Kahr A, Verilhac K, Deane K, Fischer A, West S. Recent-onset systemic lupus erythematosus complicated by acute respiratory failure. Arthritis Care Res (Hoboken) 2013; 65(2):314–323. doi:10.1002/acr.21857
- Komano Y, Harigai M, Koike R, et al. Pneumocystis jiroveci pneumonia in patients with rheumatoid arthritis treated with infliximab: a retrospective review and case-control study of 21 patients. Arthritis Rheum 2009; 61(3):305–312. doi:10.1002/art.24283
- Alexandre K, Ingen-Housz-Oro S, Versini M, Sailler L, Benhamou Y. Pneumocystis jirovecii pneumonia in patients treated with rituximab for systemic diseases: report of 11 cases and review of the literature. Eur J Intern Med 2018; 50:e23–e24. doi:10.1016/j.ejim.2017.11.014
- Li Y, Ghannoum M, Deng C, et al. Pneumocystis pneumonia in patients with inflammatory or autoimmune diseases: usefulness of lymphocyte subtyping. Int J Infect Dis 2017; 57:108–115. doi:10.1016/j.ijid.2017.02.010
- Ward MM, Donald F. Pneumocystis carinii pneumonia in patients with connective tissue diseases: the role of hospital experience in diagnosis and mortality. Arthritis Rheum 1999; 42(4):780–789. doi:10.1002/1529-0131(199904)42:4<780::AID-ANR23>3.0.CO;2-M
- Katsuyama T, Saito K, Kubo S, Nawata M, Tanaka Y. Prophylaxis for Pneumocystis pneumonia in patients with rheumatoid arthritis treated with biologics, based on risk factors found in a retrospective study. Arthritis Res Ther 2014; 16(1):R43. doi:10.1186/ar4472
- Tanaka M, Sakai R, Koike R, et al. Pneumocystis jirovecii pneumonia associated with etanercept treatment in patients with rheumatoid arthritis: a retrospective review of 15 cases and analysis of risk factors. Mod Rheumatol 2012; 22(6):849–858. doi:10.1007/s10165-012-0615-z
- Chung JB, Armstrong K, Schwartz JS, Albert D. Cost-effectiveness of prophylaxis against Pneumocystis carinii pneumonia in patients with Wegener’s granulomatosis undergoing immunosuppressive therapy. Arthritis Rheum 2000; 43(8):1841–1848. doi:10.1002/1529-0131(200008)43:8<1841::AID-ANR21>3.0.CO;2-Q
- Selmi C, Generali E, Massarotti M, Bianchi G, Scire CA. New treatments for inflammatory rheumatic disease. Immunol Res 2014; 60(2–3):277–288. doi:10.1007/s12026-014-8565-5
- Liu Y, Su L, Jiang SJ, Qu H. Risk factors for mortality from Pneumocystis carinii pneumonia (PCP) in non-HIV patients: a meta-analysis. Oncotarget 2017; 8(35):59729–59739. doi:10.18632/oncotarget.19927
- Desales AL, Mendez-Navarro J, Méndez-Tovar LJ, et al. Pneumocystosis in a patient with Crohn's disease treated with combination therapy with adalimumab. J Crohns Colitis 2012; 6(4):483–487. doi:10.1016/j.crohns.2011.10.012
- Kalyoncu U, Karadag O, Akdogan A, et al. Pneumocystis carinii pneumonia in a rheumatoid arthritis patient treated with adalimumab. Scand J Infect Dis 2007; 39(5):475–478. doi:10.1080/00365540601071867
- Vallabhaneni S, Chiller TM. Fungal infections and new biologic therapies. Curr Rheumatol Rep 2016; 18(5):29. doi:10.1007/s11926-016-0572-1
- Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. www.aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed May 3, 2019.
- Kourbeti IS, Ziakas PD, Mylonakis E. Biologic therapies in rheumatoid arthritis and the risk of opportunistic infections: a meta-analysis. Clin Infect Dis 2014; 58(12):1649–1657. doi:10.1093/cid/ciu185
- Bia M, Adey DB, Bloom RD, Chan L, Kulkarni S, Tomlanovich S. KDOQI US commentary on the 2009 KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Kidney Dis 2010; 56(2):189–218. doi:10.1053/j.ajkd.2010.04.010
- Baden LR, Swaminathan S, Angarone M, et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016; 14(7):882–913. pmid:27407129
- Cooley L, Dendle C, Wolf J, et al. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J 2014; 44(12b):1350–1363. doi:10.1111/imj.12599
- Orlando A, Armuzzi A, Papi C, et al; Italian Society of Gastroenterology; Italian Group for the study of Inflammatory Bowel Disease. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) clinical practice guidelines: the use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis 2011; 43(1):1–20. doi:10.1016/j.dld.2010.07.010
- Mikulska M, Lanini S, Gudiol C, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (agents targeting lymphoid cells surface antigens [I]: CD19, CD20 and CD52). Clin Microbiol Infect 2018; 24(suppl 2):S71–S82. doi:10.1016/j.cmi.2018.02.003
- Baddley J, Cantini F, Goletti D, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (soluble immune effector molecules [I]: anti-tumor necrosis factor-alpha agents). Clin Microbiol Infect 2018; 24(suppl 2):S10–S20. doi:10.1016/j.cmi.2017.12.025
- Winthrop K, Mariette X, Silva J, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (soluble immune effector molecules [II]: agents targeting interleukins, immunoglobulins and complement factors). Clin Microbiol Infect 2018; 24(suppl 2):S21–S40. doi:10.1016/j.cmi.2018.02.002
- Petri M, Allbritton J. Antibiotic allergy in systemic lupus erythematosus: a case-control study. J Rheumatol 1992; 19(2):265–269. pmid:1629825
- Pope J, Jerome D, Fenlon D, Krizova A, Ouimet J. Frequency of adverse drug reactions in patients with systemic lupus erythematosus. J Rheumatol 2003; 30(3):480–484. pmid:12610805
- Vananuvat P, Suwannalai P, Sungkanuparph S, Limsuwan T, Ngamjanyaporn P, Janwityanujit S. Primary prophylaxis for Pneumocystis jirovecii pneumonia in patients with connective tissue diseases. Semin Arthritis Rheum 2011; 41(3):497–502. doi:10.1016/j.semarthrit.2011.05.004
- Tamaki H, Butler R, Langford C. Abstract Number: 1755: Safety of methotrexate and low-dose trimethoprim-sulfamethoxazole in patients with ANCA-associated vasculitis. www.acrabstracts.org/abstract/safety-of-methotrexate-and-low-dose-trimethoprim-sulfamethoxazole-in-patients-with-anca-associated-vasculitis. Accessed May 3, 2019.
You can observe a lot by watching
"I have trained myself to see what others overlook."
—Sherlock Holmes1
The article by Grandjean and Huber in this issue2 is a timely reminder of the importance of skilled observation in medical care. Osler3 considered observation to represent “the whole art of medicine,” but warned that “for some men it is quite as difficult to record an observation in brief and plain language.” This insight captures not only the never-ending feud between written and visual communication, but also the higher efficiency of images. Leonardo da Vinci, a visual thinker with a touch of dyslexia,4 often boasted in colorful terms about the superiority of the visual. Next to his amazing rendition of a bovine heart he scribbled, “[Writer] how could you describe this heart in words without filling a whole book? So, don’t bother with words unless you are speaking to the blind…you will always be overruled by the painter.”5
See related article and editorial
Ironically, physicians have often preferred the written over the visual. Oliver Wendell Holmes Sr., professor of anatomy at Harvard Medical School and renowned essayist, once wrote a scathing review of a new anatomy textbook that, according to him, had just too many pictures. “Let a student have illustrations,” he thundered “and just so surely will he use them at the expense of the text.”6 The book was Gray’s Anatomy, but Holmes’ tirade exemplifies the conundrum of our profession: to become physicians we must read (and memorize) lots of written text, with little emphasis on how much more efficiently information might be conveyed through a single picture.
This trend is probably worsening. When I first came to the United States 43 years ago, I was amazed at how many of my professors immediately grabbed a sheet of paper and started drawing their explanations to my questions. But I have not seen much of this lately, and that is a pity, since pictures are undoubtedly a better way of communicating.
OBSERVING A PATIENT WITH COPD
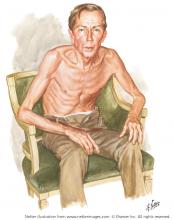
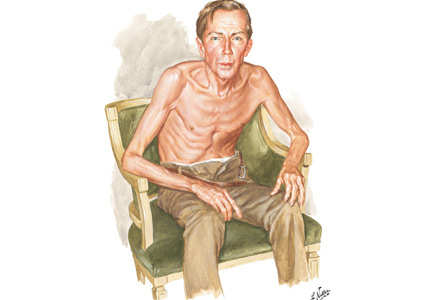
Netter’s patient is also exhaling through pursed lips. This reduces the respiratory rate and carbon dioxide level, while improving distribution of ventilation,9,10 oxygen saturation, tidal volume, inspiratory muscle strength, and diaphragmatic efficiency.11,12 Since less inspiratory force is required for each breath, dyspnea is also improved.13,14 Diagnostically, pursed‑lip breathing increases the probability of chronic obstructive pulmonary disease (COPD), with a likelihood ratio of 5.05.15
The man in The Pink Puffer is using accessory respiratory muscles, which not only represents one of the earliest signs of airway obstruction, but also reflects severe disease. In fact, use of accessory respiratory muscles occurs in more than 90% of COPD patients admitted for acute exacerbations.7
Lastly, Netter’s patient exhibits inspiratory retraction of supraclavicular fossae and interspaces (tirage), which indicates increased airway resistance and reduced forced expiratory volume in 1 second (FEV1).16,17 A clavicular “lift” of more than 5 mm correlates with an FEV1 of 0.6 L.18
But what is odd about this patient is what Netter did not portray: clubbing. This goes against the conventional wisdom of the time but is actually correct, since we now know that clubbing is more a feature of chronic bronchitis than emphysema.19 In fact, if present in a “pink puffer,” it should suggest an underlying malignancy. Hence, Netter reminds us that we should never convince ourselves that we see something simply because we know it should be there. Instead, we should always rely on what we see. This is, after all, how Vesalius debunked Galen’s anatomic errors: by seeing for himself. Tom McCrae, Osler’s right-hand man at Johns Hopkins, used to warn his students that one misses more by not seeing than by not knowing. Leonardo put it simply: “Wisdom is the daughter of [visual] experience.”20 In the end, Netter’s drawing reminds us that a picture is truly worth a thousand words.
TEACHING STUDENTS TO OBSERVE
Unfortunately, detecting detail is difficult. It is also very difficult to teach. For the past few months I’ve been asking astute clinicians how they observe, and most of them seem befuddled, as if I had asked which muscles they contract in order to walk. They just walk. And they just observe.
So, how can we rekindle this important but underappreciated component of the physician’s skill set? First of all, by becoming cognizant of its fundamental role in medicine. Second, by accepting that this is something that cannot be easily tested by single-best- answer, black-and-white, multiple-choice exams. Recognizing the complexity of clinical skills reminds us that not all that counts in medicine can be counted, and not all that can be counted counts. Yet it also provides a hurdle, since testing typically drives curriculum. If we cannot assess observation, how can we reincorporate it in the curriculum? Lastly, we need to regain ownership of the teaching of this skill. No art instructor can properly identify and interpret clinical findings. Hence, physicians ought to teach it. In the end, learning how to properly observe is a personal and lifelong effort. As Osler put it, “There is no more difficult art to acquire than the art of observation.”21
Leonardo used to quip that “There are three classes of people: those who see, those who see when they are shown, and those who do not see.”22 Yet this time Leonardo might have been wrong. There are really only two kinds of people: those who have been taught how to observe and those who have not. Leonardo was lucky enough to have been apprenticed to an artist whose nickname was Verrocchio, which resembles the Italian words vero occhio, a “fine eye.” Without Verrocchio, even Leonardo might not have become such a skilled observer. How many Verrocchios are around today?
- Doyle AC. A case of identity. In: The Adventures of Sherlock Holmes. London, UK: George Newnes; 1892.
- Grandjean R, Huber LC. Thinker’s sign. Cleve Clin J Med 2019; 86(7):439. doi:10.3949/ccjm.86a.19036
- Osler W. The natural method of teaching the subject of medicine. JAMA 1901; 36(24):1673–1679. doi:10.1001/jama.1901.52470240001001
- Mangione S, Del Maestro R. Was Leonardo da Vinci dyslexic? Am J Med 2019 Mar 7; pii:S0002-9343(19)30214-1. Epub ahead of print. doi:10.1016/j.amjmed.2019.02.019
- Leonardo Da Vinci. Studies of the Heart of an Ox, Great Vessels and Bronchial Tree (c. 1513); pen and ink on blue paper, Windsor, London, UK Royal Library (19071r).
- Holmes OW Sr. Gray’s Anatomy. The Boston Medical and Surgical Journal 1859; 60(25):489–496.
- O’Neill S, McCarthy DS. Postural relief of dyspnoea in severe chronic airflow limitation: relationship to respiratory muscle strength. Thorax 1983; 38(8):595–600. pmid:6612651
- Banzett RB, Topulos GP, Leith DE, Nations CS. Bracing arms increases the capacity for sustained hyperpnea. Am Rev Respir Dis 1988; 138(1):106–109. doi:10.1164/ajrccm/138.1.106
- Mueller RE, Petty TL, Filley GF. Ventilation and arterial blood gas changes induced by pursed lips breathing. J Appl Physiol 1970; 28(6):784–789. doi:10.1152/jappl.1970.28.6.784
- Thoman RL, Stoker GL, Ross JC. The efficacy of pursed-lips breathing in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1966; 93(1):100–106.
- Breslin EH. The pattern of respiratory muscle recruitment during pursed-lip breathing. Chest 1992; 101(1):75–78. pmid:1729114
- Jones AY, Dean E, Chow CC. Comparison of the oxygen cost of breathing exercises and spontaneous breathing in patients with stable chronic obstructive pulmonary disease. Phys Ther 2003; 83(5):424–431. pmid:12718708
- el-Manshawi A, Killian KJ, Summers E, Jones NL. Breathlessness during exercise with and without resistive loading. J Appl Physiol (1985) 1986; 61(3):896–905. doi:10.1152/jappl.1986.61.3.896
- Nield MA, Soo Hoo GW, Roper JM, Santiago S. Efficacy of pursed-lips breathing: a breathing pattern retraining strategy for dyspnea reduction. J Cardiopulm Rehabil Prev 2007; 27(4):237–244. doi:10.1097/01.HCR.0000281770.82652.cb
- Mattos WL, Signori LG, Borges FK, Bergamin JA, Machado V. Accuracy of clinical examination findings in the diagnosis of COPD. J Bras Pneumol 2009; 35(5):404–408. pmid:19547847
- Stubbing DG. Physical signs in the evaluation of patients with chronic obstructive pulmonary disease. Pract Cardiol 1984;10:114–120.
- Godfrey S, Edwards RH, Campbell EJ, Newton-Howes J. Clinical and physiological associations of some physical signs observed in patients with chronic airways obstruction. Thorax 1970; 25(3):285–287. pmid:5452279
- Anderson CL, Shankar PS, Scott JH. Physiological significance of sternomastoid muscle contraction in chronic obstructive pulmonary disease. Respir Care 1980; 25(9):937–939.
- Myers KA, Farquhar DR. The rational clinical examination. Does this patient have clubbing? JAMA 2001; 286(3):341–347. pmid:11466101
- Richter JP. The Notebooks of Leonardo Da Vinci. New York: Dover Books; 1970.
- Osler W. On the educational value of the medical society. Yale Medical Journal 1903; 9(10):325.
- Goodreads. Leonardo da Vinci Quotable Quote. http://www.goodreads.com/quotes/243423-there-are-three-classes-of-people-those-whosee-those. Accessed April 15, 2019.
"I have trained myself to see what others overlook."
—Sherlock Holmes1
The article by Grandjean and Huber in this issue2 is a timely reminder of the importance of skilled observation in medical care. Osler3 considered observation to represent “the whole art of medicine,” but warned that “for some men it is quite as difficult to record an observation in brief and plain language.” This insight captures not only the never-ending feud between written and visual communication, but also the higher efficiency of images. Leonardo da Vinci, a visual thinker with a touch of dyslexia,4 often boasted in colorful terms about the superiority of the visual. Next to his amazing rendition of a bovine heart he scribbled, “[Writer] how could you describe this heart in words without filling a whole book? So, don’t bother with words unless you are speaking to the blind…you will always be overruled by the painter.”5
See related article and editorial
Ironically, physicians have often preferred the written over the visual. Oliver Wendell Holmes Sr., professor of anatomy at Harvard Medical School and renowned essayist, once wrote a scathing review of a new anatomy textbook that, according to him, had just too many pictures. “Let a student have illustrations,” he thundered “and just so surely will he use them at the expense of the text.”6 The book was Gray’s Anatomy, but Holmes’ tirade exemplifies the conundrum of our profession: to become physicians we must read (and memorize) lots of written text, with little emphasis on how much more efficiently information might be conveyed through a single picture.
This trend is probably worsening. When I first came to the United States 43 years ago, I was amazed at how many of my professors immediately grabbed a sheet of paper and started drawing their explanations to my questions. But I have not seen much of this lately, and that is a pity, since pictures are undoubtedly a better way of communicating.
OBSERVING A PATIENT WITH COPD
Netter’s patient is also exhaling through pursed lips. This reduces the respiratory rate and carbon dioxide level, while improving distribution of ventilation,9,10 oxygen saturation, tidal volume, inspiratory muscle strength, and diaphragmatic efficiency.11,12 Since less inspiratory force is required for each breath, dyspnea is also improved.13,14 Diagnostically, pursed‑lip breathing increases the probability of chronic obstructive pulmonary disease (COPD), with a likelihood ratio of 5.05.15
The man in The Pink Puffer is using accessory respiratory muscles, which not only represents one of the earliest signs of airway obstruction, but also reflects severe disease. In fact, use of accessory respiratory muscles occurs in more than 90% of COPD patients admitted for acute exacerbations.7
Lastly, Netter’s patient exhibits inspiratory retraction of supraclavicular fossae and interspaces (tirage), which indicates increased airway resistance and reduced forced expiratory volume in 1 second (FEV1).16,17 A clavicular “lift” of more than 5 mm correlates with an FEV1 of 0.6 L.18
But what is odd about this patient is what Netter did not portray: clubbing. This goes against the conventional wisdom of the time but is actually correct, since we now know that clubbing is more a feature of chronic bronchitis than emphysema.19 In fact, if present in a “pink puffer,” it should suggest an underlying malignancy. Hence, Netter reminds us that we should never convince ourselves that we see something simply because we know it should be there. Instead, we should always rely on what we see. This is, after all, how Vesalius debunked Galen’s anatomic errors: by seeing for himself. Tom McCrae, Osler’s right-hand man at Johns Hopkins, used to warn his students that one misses more by not seeing than by not knowing. Leonardo put it simply: “Wisdom is the daughter of [visual] experience.”20 In the end, Netter’s drawing reminds us that a picture is truly worth a thousand words.
TEACHING STUDENTS TO OBSERVE
Unfortunately, detecting detail is difficult. It is also very difficult to teach. For the past few months I’ve been asking astute clinicians how they observe, and most of them seem befuddled, as if I had asked which muscles they contract in order to walk. They just walk. And they just observe.
So, how can we rekindle this important but underappreciated component of the physician’s skill set? First of all, by becoming cognizant of its fundamental role in medicine. Second, by accepting that this is something that cannot be easily tested by single-best- answer, black-and-white, multiple-choice exams. Recognizing the complexity of clinical skills reminds us that not all that counts in medicine can be counted, and not all that can be counted counts. Yet it also provides a hurdle, since testing typically drives curriculum. If we cannot assess observation, how can we reincorporate it in the curriculum? Lastly, we need to regain ownership of the teaching of this skill. No art instructor can properly identify and interpret clinical findings. Hence, physicians ought to teach it. In the end, learning how to properly observe is a personal and lifelong effort. As Osler put it, “There is no more difficult art to acquire than the art of observation.”21
Leonardo used to quip that “There are three classes of people: those who see, those who see when they are shown, and those who do not see.”22 Yet this time Leonardo might have been wrong. There are really only two kinds of people: those who have been taught how to observe and those who have not. Leonardo was lucky enough to have been apprenticed to an artist whose nickname was Verrocchio, which resembles the Italian words vero occhio, a “fine eye.” Without Verrocchio, even Leonardo might not have become such a skilled observer. How many Verrocchios are around today?
"I have trained myself to see what others overlook."
—Sherlock Holmes1
The article by Grandjean and Huber in this issue2 is a timely reminder of the importance of skilled observation in medical care. Osler3 considered observation to represent “the whole art of medicine,” but warned that “for some men it is quite as difficult to record an observation in brief and plain language.” This insight captures not only the never-ending feud between written and visual communication, but also the higher efficiency of images. Leonardo da Vinci, a visual thinker with a touch of dyslexia,4 often boasted in colorful terms about the superiority of the visual. Next to his amazing rendition of a bovine heart he scribbled, “[Writer] how could you describe this heart in words without filling a whole book? So, don’t bother with words unless you are speaking to the blind…you will always be overruled by the painter.”5
See related article and editorial
Ironically, physicians have often preferred the written over the visual. Oliver Wendell Holmes Sr., professor of anatomy at Harvard Medical School and renowned essayist, once wrote a scathing review of a new anatomy textbook that, according to him, had just too many pictures. “Let a student have illustrations,” he thundered “and just so surely will he use them at the expense of the text.”6 The book was Gray’s Anatomy, but Holmes’ tirade exemplifies the conundrum of our profession: to become physicians we must read (and memorize) lots of written text, with little emphasis on how much more efficiently information might be conveyed through a single picture.
This trend is probably worsening. When I first came to the United States 43 years ago, I was amazed at how many of my professors immediately grabbed a sheet of paper and started drawing their explanations to my questions. But I have not seen much of this lately, and that is a pity, since pictures are undoubtedly a better way of communicating.
OBSERVING A PATIENT WITH COPD
Netter’s patient is also exhaling through pursed lips. This reduces the respiratory rate and carbon dioxide level, while improving distribution of ventilation,9,10 oxygen saturation, tidal volume, inspiratory muscle strength, and diaphragmatic efficiency.11,12 Since less inspiratory force is required for each breath, dyspnea is also improved.13,14 Diagnostically, pursed‑lip breathing increases the probability of chronic obstructive pulmonary disease (COPD), with a likelihood ratio of 5.05.15
The man in The Pink Puffer is using accessory respiratory muscles, which not only represents one of the earliest signs of airway obstruction, but also reflects severe disease. In fact, use of accessory respiratory muscles occurs in more than 90% of COPD patients admitted for acute exacerbations.7
Lastly, Netter’s patient exhibits inspiratory retraction of supraclavicular fossae and interspaces (tirage), which indicates increased airway resistance and reduced forced expiratory volume in 1 second (FEV1).16,17 A clavicular “lift” of more than 5 mm correlates with an FEV1 of 0.6 L.18
But what is odd about this patient is what Netter did not portray: clubbing. This goes against the conventional wisdom of the time but is actually correct, since we now know that clubbing is more a feature of chronic bronchitis than emphysema.19 In fact, if present in a “pink puffer,” it should suggest an underlying malignancy. Hence, Netter reminds us that we should never convince ourselves that we see something simply because we know it should be there. Instead, we should always rely on what we see. This is, after all, how Vesalius debunked Galen’s anatomic errors: by seeing for himself. Tom McCrae, Osler’s right-hand man at Johns Hopkins, used to warn his students that one misses more by not seeing than by not knowing. Leonardo put it simply: “Wisdom is the daughter of [visual] experience.”20 In the end, Netter’s drawing reminds us that a picture is truly worth a thousand words.
TEACHING STUDENTS TO OBSERVE
Unfortunately, detecting detail is difficult. It is also very difficult to teach. For the past few months I’ve been asking astute clinicians how they observe, and most of them seem befuddled, as if I had asked which muscles they contract in order to walk. They just walk. And they just observe.
So, how can we rekindle this important but underappreciated component of the physician’s skill set? First of all, by becoming cognizant of its fundamental role in medicine. Second, by accepting that this is something that cannot be easily tested by single-best- answer, black-and-white, multiple-choice exams. Recognizing the complexity of clinical skills reminds us that not all that counts in medicine can be counted, and not all that can be counted counts. Yet it also provides a hurdle, since testing typically drives curriculum. If we cannot assess observation, how can we reincorporate it in the curriculum? Lastly, we need to regain ownership of the teaching of this skill. No art instructor can properly identify and interpret clinical findings. Hence, physicians ought to teach it. In the end, learning how to properly observe is a personal and lifelong effort. As Osler put it, “There is no more difficult art to acquire than the art of observation.”21
Leonardo used to quip that “There are three classes of people: those who see, those who see when they are shown, and those who do not see.”22 Yet this time Leonardo might have been wrong. There are really only two kinds of people: those who have been taught how to observe and those who have not. Leonardo was lucky enough to have been apprenticed to an artist whose nickname was Verrocchio, which resembles the Italian words vero occhio, a “fine eye.” Without Verrocchio, even Leonardo might not have become such a skilled observer. How many Verrocchios are around today?
- Doyle AC. A case of identity. In: The Adventures of Sherlock Holmes. London, UK: George Newnes; 1892.
- Grandjean R, Huber LC. Thinker’s sign. Cleve Clin J Med 2019; 86(7):439. doi:10.3949/ccjm.86a.19036
- Osler W. The natural method of teaching the subject of medicine. JAMA 1901; 36(24):1673–1679. doi:10.1001/jama.1901.52470240001001
- Mangione S, Del Maestro R. Was Leonardo da Vinci dyslexic? Am J Med 2019 Mar 7; pii:S0002-9343(19)30214-1. Epub ahead of print. doi:10.1016/j.amjmed.2019.02.019
- Leonardo Da Vinci. Studies of the Heart of an Ox, Great Vessels and Bronchial Tree (c. 1513); pen and ink on blue paper, Windsor, London, UK Royal Library (19071r).
- Holmes OW Sr. Gray’s Anatomy. The Boston Medical and Surgical Journal 1859; 60(25):489–496.
- O’Neill S, McCarthy DS. Postural relief of dyspnoea in severe chronic airflow limitation: relationship to respiratory muscle strength. Thorax 1983; 38(8):595–600. pmid:6612651
- Banzett RB, Topulos GP, Leith DE, Nations CS. Bracing arms increases the capacity for sustained hyperpnea. Am Rev Respir Dis 1988; 138(1):106–109. doi:10.1164/ajrccm/138.1.106
- Mueller RE, Petty TL, Filley GF. Ventilation and arterial blood gas changes induced by pursed lips breathing. J Appl Physiol 1970; 28(6):784–789. doi:10.1152/jappl.1970.28.6.784
- Thoman RL, Stoker GL, Ross JC. The efficacy of pursed-lips breathing in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1966; 93(1):100–106.
- Breslin EH. The pattern of respiratory muscle recruitment during pursed-lip breathing. Chest 1992; 101(1):75–78. pmid:1729114
- Jones AY, Dean E, Chow CC. Comparison of the oxygen cost of breathing exercises and spontaneous breathing in patients with stable chronic obstructive pulmonary disease. Phys Ther 2003; 83(5):424–431. pmid:12718708
- el-Manshawi A, Killian KJ, Summers E, Jones NL. Breathlessness during exercise with and without resistive loading. J Appl Physiol (1985) 1986; 61(3):896–905. doi:10.1152/jappl.1986.61.3.896
- Nield MA, Soo Hoo GW, Roper JM, Santiago S. Efficacy of pursed-lips breathing: a breathing pattern retraining strategy for dyspnea reduction. J Cardiopulm Rehabil Prev 2007; 27(4):237–244. doi:10.1097/01.HCR.0000281770.82652.cb
- Mattos WL, Signori LG, Borges FK, Bergamin JA, Machado V. Accuracy of clinical examination findings in the diagnosis of COPD. J Bras Pneumol 2009; 35(5):404–408. pmid:19547847
- Stubbing DG. Physical signs in the evaluation of patients with chronic obstructive pulmonary disease. Pract Cardiol 1984;10:114–120.
- Godfrey S, Edwards RH, Campbell EJ, Newton-Howes J. Clinical and physiological associations of some physical signs observed in patients with chronic airways obstruction. Thorax 1970; 25(3):285–287. pmid:5452279
- Anderson CL, Shankar PS, Scott JH. Physiological significance of sternomastoid muscle contraction in chronic obstructive pulmonary disease. Respir Care 1980; 25(9):937–939.
- Myers KA, Farquhar DR. The rational clinical examination. Does this patient have clubbing? JAMA 2001; 286(3):341–347. pmid:11466101
- Richter JP. The Notebooks of Leonardo Da Vinci. New York: Dover Books; 1970.
- Osler W. On the educational value of the medical society. Yale Medical Journal 1903; 9(10):325.
- Goodreads. Leonardo da Vinci Quotable Quote. http://www.goodreads.com/quotes/243423-there-are-three-classes-of-people-those-whosee-those. Accessed April 15, 2019.
- Doyle AC. A case of identity. In: The Adventures of Sherlock Holmes. London, UK: George Newnes; 1892.
- Grandjean R, Huber LC. Thinker’s sign. Cleve Clin J Med 2019; 86(7):439. doi:10.3949/ccjm.86a.19036
- Osler W. The natural method of teaching the subject of medicine. JAMA 1901; 36(24):1673–1679. doi:10.1001/jama.1901.52470240001001
- Mangione S, Del Maestro R. Was Leonardo da Vinci dyslexic? Am J Med 2019 Mar 7; pii:S0002-9343(19)30214-1. Epub ahead of print. doi:10.1016/j.amjmed.2019.02.019
- Leonardo Da Vinci. Studies of the Heart of an Ox, Great Vessels and Bronchial Tree (c. 1513); pen and ink on blue paper, Windsor, London, UK Royal Library (19071r).
- Holmes OW Sr. Gray’s Anatomy. The Boston Medical and Surgical Journal 1859; 60(25):489–496.
- O’Neill S, McCarthy DS. Postural relief of dyspnoea in severe chronic airflow limitation: relationship to respiratory muscle strength. Thorax 1983; 38(8):595–600. pmid:6612651
- Banzett RB, Topulos GP, Leith DE, Nations CS. Bracing arms increases the capacity for sustained hyperpnea. Am Rev Respir Dis 1988; 138(1):106–109. doi:10.1164/ajrccm/138.1.106
- Mueller RE, Petty TL, Filley GF. Ventilation and arterial blood gas changes induced by pursed lips breathing. J Appl Physiol 1970; 28(6):784–789. doi:10.1152/jappl.1970.28.6.784
- Thoman RL, Stoker GL, Ross JC. The efficacy of pursed-lips breathing in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1966; 93(1):100–106.
- Breslin EH. The pattern of respiratory muscle recruitment during pursed-lip breathing. Chest 1992; 101(1):75–78. pmid:1729114
- Jones AY, Dean E, Chow CC. Comparison of the oxygen cost of breathing exercises and spontaneous breathing in patients with stable chronic obstructive pulmonary disease. Phys Ther 2003; 83(5):424–431. pmid:12718708
- el-Manshawi A, Killian KJ, Summers E, Jones NL. Breathlessness during exercise with and without resistive loading. J Appl Physiol (1985) 1986; 61(3):896–905. doi:10.1152/jappl.1986.61.3.896
- Nield MA, Soo Hoo GW, Roper JM, Santiago S. Efficacy of pursed-lips breathing: a breathing pattern retraining strategy for dyspnea reduction. J Cardiopulm Rehabil Prev 2007; 27(4):237–244. doi:10.1097/01.HCR.0000281770.82652.cb
- Mattos WL, Signori LG, Borges FK, Bergamin JA, Machado V. Accuracy of clinical examination findings in the diagnosis of COPD. J Bras Pneumol 2009; 35(5):404–408. pmid:19547847
- Stubbing DG. Physical signs in the evaluation of patients with chronic obstructive pulmonary disease. Pract Cardiol 1984;10:114–120.
- Godfrey S, Edwards RH, Campbell EJ, Newton-Howes J. Clinical and physiological associations of some physical signs observed in patients with chronic airways obstruction. Thorax 1970; 25(3):285–287. pmid:5452279
- Anderson CL, Shankar PS, Scott JH. Physiological significance of sternomastoid muscle contraction in chronic obstructive pulmonary disease. Respir Care 1980; 25(9):937–939.
- Myers KA, Farquhar DR. The rational clinical examination. Does this patient have clubbing? JAMA 2001; 286(3):341–347. pmid:11466101
- Richter JP. The Notebooks of Leonardo Da Vinci. New York: Dover Books; 1970.
- Osler W. On the educational value of the medical society. Yale Medical Journal 1903; 9(10):325.
- Goodreads. Leonardo da Vinci Quotable Quote. http://www.goodreads.com/quotes/243423-there-are-three-classes-of-people-those-whosee-those. Accessed April 15, 2019.
If a picture is worth a thousand words, a patient is worth ten thousand
Today’s most prominent medical journals have a “clinical images” section. High- quality, readily accessible digital photography can transport a patient to the journal’s pages, as demonstrated by Grandjean and Huber’s “Thinker sign” images in this issue of the Journal.1 Images challenge healthcare practitioners to recall diseases via pattern recognition, or to deduce them by higher-order cognition. Images can reinforce prior learning, change perspective, and challenge preconceived notions.
See related article and editorial
I have used clinical images—physical examination findings, skin rashes, blood smears, radiography—for more than 20 years as a medical educator. I have dimmed the lights in conference rooms and lecture halls from Maine to Northern California, challenging students, residents, and faculty to contemplate a snippet of history and describe what they see to arrive at a diagnosis. Images are compelling teaching tools for first-year medical students beginning to make clinical observations, and for seasoned clinicians who have seen thousands of patients.
In my experience, clinical image presentations are consistently engaging. Introducing an audience to 8 to 10 patients in an hour loosely mimics the experience of seeing patients over the course of morning hospital rounds or clinic. The images I use are assembled from a collection of images of patients I have seen during my career in medical education. Showing images of patients I’ve personally cared for consistently prompts people to engage. “Here is a patient I saw last week on the medicine wards” reignites the sagging eyes and fading attention of the audience. In retelling a patient encounter, I create a human connection between a picture on the screen—my patient—and the listener. My patient becomes a patient of anyone in the room, a patient someone might see tomorrow on hospital rounds or in clinic.
Sometimes, instead of presenting a brief clinical history or select physical findings, I tell a story about the patient in the image. Whether sad or funny, these stories often bring learners together, prompting them to wonder how there could ever be a better job than the one they have. A prominent educator once approached me after a clinical images presentation to opine, “What you did with us today is the cure for physician burnout.” Hyperbole, perhaps, but I understood what he meant. Over the course of an hour, the audience had been transported to numerous bedsides and examination rooms, witnessing the interesting and delightfully mundane jewels our patients often bring—true pearls, indeed.
However, as educational, fun, and intellectually challenging as clinical images can be, they can never replace the experience of being at the bedside. There is nothing as engaging as the stories the patients themselves tell us. Unfiltered musings come to life, physical findings are indelibly seared into memory.
But unfortunately, even as trainees spend less time than ever before with their patients,2,3 bedside rounding has dramatically faded, replaced by rounds in conference rooms and hospital hallways.4 The underlying cause is multifactorial—declining physical examination skills, increasing use of radiography and other advanced imaging, the electronic health record, and the overwhelming volume of clinical tasks carried out at a distance from the patient.
But this is not the whole story. I also believe that teachers and leaders fear the “thin ice” of rounding at the patient’s bedside. One never knows what will happen there—what will be said, what will be asked, what will be uncovered. What if, while talking to and examining the patient with the Dahl sign shown in Grandjean and Huber,1 the patient’s condition would suddenly deteriorate, urgently requiring nebulized beta-2 agonists and transfer to the medical intensive care unit? What if the patient rambles for 5 minutes about extraneous details not relevant to his or her disease? What if the nurse needs to dispense scheduled medications or hang the next dose of antibiotics? What if the patient asks to use the bedpan at the moment digital clubbing was to be pointed out and discussed?
Of course, the patient may have lots to say, or nothing at all. But in those moments when the ice does not break, when the patient is not suddenly wheeled away to radiology, key clinical findings are seen and remembered, often for an entire career. If the ice does not break, the patient, the story, and the clinical finding—otherwise seen on a large screen in a dark room or on a page in a textbook or journal—come together in that moment, in a way nothing else ever quite can.
In this golden age of technology, we must remember that these images portray real patients with stories to tell, sometimes mundane and sometimes profound, but always worth hearing.
Acknowledgment: The author wishes to thank Mark C. Henderson, MD, for his helpful comments on this manuscript.
- Grandjean R, Huber LC. Thinker’s sign. Cleve Clin J Med 2019; 86(7):439. doi:10.3949/ccjm.86a.19036
- Chaiyachati KH, Shea JA, Asch DA, et al. Assessment of inpatient time allocation among first-year internal medicine residents using time-motion observations. JAMA Intern Med 2019. Epub ahead of print. doi:10.1001/jamainternmed.2019.0095
- Block L, Habicht R, Wu AW, et al. In the wake of the 2003 and 2011 duty hours regulations, how do internal medicine interns spend their time? J Gen Intern Med 2013; 28(8):1042–1047. doi:10.1007/s11606-013-2376-6
- Crumlish CM, Yialamas MA, McMahon GT. Quantification of bedside teaching by an academic hospitalist group. J Hosp Med 2009; 4(5):304–307. doi:10.1002/jhm.540
Today’s most prominent medical journals have a “clinical images” section. High- quality, readily accessible digital photography can transport a patient to the journal’s pages, as demonstrated by Grandjean and Huber’s “Thinker sign” images in this issue of the Journal.1 Images challenge healthcare practitioners to recall diseases via pattern recognition, or to deduce them by higher-order cognition. Images can reinforce prior learning, change perspective, and challenge preconceived notions.
See related article and editorial
I have used clinical images—physical examination findings, skin rashes, blood smears, radiography—for more than 20 years as a medical educator. I have dimmed the lights in conference rooms and lecture halls from Maine to Northern California, challenging students, residents, and faculty to contemplate a snippet of history and describe what they see to arrive at a diagnosis. Images are compelling teaching tools for first-year medical students beginning to make clinical observations, and for seasoned clinicians who have seen thousands of patients.
In my experience, clinical image presentations are consistently engaging. Introducing an audience to 8 to 10 patients in an hour loosely mimics the experience of seeing patients over the course of morning hospital rounds or clinic. The images I use are assembled from a collection of images of patients I have seen during my career in medical education. Showing images of patients I’ve personally cared for consistently prompts people to engage. “Here is a patient I saw last week on the medicine wards” reignites the sagging eyes and fading attention of the audience. In retelling a patient encounter, I create a human connection between a picture on the screen—my patient—and the listener. My patient becomes a patient of anyone in the room, a patient someone might see tomorrow on hospital rounds or in clinic.
Sometimes, instead of presenting a brief clinical history or select physical findings, I tell a story about the patient in the image. Whether sad or funny, these stories often bring learners together, prompting them to wonder how there could ever be a better job than the one they have. A prominent educator once approached me after a clinical images presentation to opine, “What you did with us today is the cure for physician burnout.” Hyperbole, perhaps, but I understood what he meant. Over the course of an hour, the audience had been transported to numerous bedsides and examination rooms, witnessing the interesting and delightfully mundane jewels our patients often bring—true pearls, indeed.
However, as educational, fun, and intellectually challenging as clinical images can be, they can never replace the experience of being at the bedside. There is nothing as engaging as the stories the patients themselves tell us. Unfiltered musings come to life, physical findings are indelibly seared into memory.
But unfortunately, even as trainees spend less time than ever before with their patients,2,3 bedside rounding has dramatically faded, replaced by rounds in conference rooms and hospital hallways.4 The underlying cause is multifactorial—declining physical examination skills, increasing use of radiography and other advanced imaging, the electronic health record, and the overwhelming volume of clinical tasks carried out at a distance from the patient.
But this is not the whole story. I also believe that teachers and leaders fear the “thin ice” of rounding at the patient’s bedside. One never knows what will happen there—what will be said, what will be asked, what will be uncovered. What if, while talking to and examining the patient with the Dahl sign shown in Grandjean and Huber,1 the patient’s condition would suddenly deteriorate, urgently requiring nebulized beta-2 agonists and transfer to the medical intensive care unit? What if the patient rambles for 5 minutes about extraneous details not relevant to his or her disease? What if the nurse needs to dispense scheduled medications or hang the next dose of antibiotics? What if the patient asks to use the bedpan at the moment digital clubbing was to be pointed out and discussed?
Of course, the patient may have lots to say, or nothing at all. But in those moments when the ice does not break, when the patient is not suddenly wheeled away to radiology, key clinical findings are seen and remembered, often for an entire career. If the ice does not break, the patient, the story, and the clinical finding—otherwise seen on a large screen in a dark room or on a page in a textbook or journal—come together in that moment, in a way nothing else ever quite can.
In this golden age of technology, we must remember that these images portray real patients with stories to tell, sometimes mundane and sometimes profound, but always worth hearing.
Acknowledgment: The author wishes to thank Mark C. Henderson, MD, for his helpful comments on this manuscript.
Today’s most prominent medical journals have a “clinical images” section. High- quality, readily accessible digital photography can transport a patient to the journal’s pages, as demonstrated by Grandjean and Huber’s “Thinker sign” images in this issue of the Journal.1 Images challenge healthcare practitioners to recall diseases via pattern recognition, or to deduce them by higher-order cognition. Images can reinforce prior learning, change perspective, and challenge preconceived notions.
See related article and editorial
I have used clinical images—physical examination findings, skin rashes, blood smears, radiography—for more than 20 years as a medical educator. I have dimmed the lights in conference rooms and lecture halls from Maine to Northern California, challenging students, residents, and faculty to contemplate a snippet of history and describe what they see to arrive at a diagnosis. Images are compelling teaching tools for first-year medical students beginning to make clinical observations, and for seasoned clinicians who have seen thousands of patients.
In my experience, clinical image presentations are consistently engaging. Introducing an audience to 8 to 10 patients in an hour loosely mimics the experience of seeing patients over the course of morning hospital rounds or clinic. The images I use are assembled from a collection of images of patients I have seen during my career in medical education. Showing images of patients I’ve personally cared for consistently prompts people to engage. “Here is a patient I saw last week on the medicine wards” reignites the sagging eyes and fading attention of the audience. In retelling a patient encounter, I create a human connection between a picture on the screen—my patient—and the listener. My patient becomes a patient of anyone in the room, a patient someone might see tomorrow on hospital rounds or in clinic.
Sometimes, instead of presenting a brief clinical history or select physical findings, I tell a story about the patient in the image. Whether sad or funny, these stories often bring learners together, prompting them to wonder how there could ever be a better job than the one they have. A prominent educator once approached me after a clinical images presentation to opine, “What you did with us today is the cure for physician burnout.” Hyperbole, perhaps, but I understood what he meant. Over the course of an hour, the audience had been transported to numerous bedsides and examination rooms, witnessing the interesting and delightfully mundane jewels our patients often bring—true pearls, indeed.
However, as educational, fun, and intellectually challenging as clinical images can be, they can never replace the experience of being at the bedside. There is nothing as engaging as the stories the patients themselves tell us. Unfiltered musings come to life, physical findings are indelibly seared into memory.
But unfortunately, even as trainees spend less time than ever before with their patients,2,3 bedside rounding has dramatically faded, replaced by rounds in conference rooms and hospital hallways.4 The underlying cause is multifactorial—declining physical examination skills, increasing use of radiography and other advanced imaging, the electronic health record, and the overwhelming volume of clinical tasks carried out at a distance from the patient.
But this is not the whole story. I also believe that teachers and leaders fear the “thin ice” of rounding at the patient’s bedside. One never knows what will happen there—what will be said, what will be asked, what will be uncovered. What if, while talking to and examining the patient with the Dahl sign shown in Grandjean and Huber,1 the patient’s condition would suddenly deteriorate, urgently requiring nebulized beta-2 agonists and transfer to the medical intensive care unit? What if the patient rambles for 5 minutes about extraneous details not relevant to his or her disease? What if the nurse needs to dispense scheduled medications or hang the next dose of antibiotics? What if the patient asks to use the bedpan at the moment digital clubbing was to be pointed out and discussed?
Of course, the patient may have lots to say, or nothing at all. But in those moments when the ice does not break, when the patient is not suddenly wheeled away to radiology, key clinical findings are seen and remembered, often for an entire career. If the ice does not break, the patient, the story, and the clinical finding—otherwise seen on a large screen in a dark room or on a page in a textbook or journal—come together in that moment, in a way nothing else ever quite can.
In this golden age of technology, we must remember that these images portray real patients with stories to tell, sometimes mundane and sometimes profound, but always worth hearing.
Acknowledgment: The author wishes to thank Mark C. Henderson, MD, for his helpful comments on this manuscript.
- Grandjean R, Huber LC. Thinker’s sign. Cleve Clin J Med 2019; 86(7):439. doi:10.3949/ccjm.86a.19036
- Chaiyachati KH, Shea JA, Asch DA, et al. Assessment of inpatient time allocation among first-year internal medicine residents using time-motion observations. JAMA Intern Med 2019. Epub ahead of print. doi:10.1001/jamainternmed.2019.0095
- Block L, Habicht R, Wu AW, et al. In the wake of the 2003 and 2011 duty hours regulations, how do internal medicine interns spend their time? J Gen Intern Med 2013; 28(8):1042–1047. doi:10.1007/s11606-013-2376-6
- Crumlish CM, Yialamas MA, McMahon GT. Quantification of bedside teaching by an academic hospitalist group. J Hosp Med 2009; 4(5):304–307. doi:10.1002/jhm.540
- Grandjean R, Huber LC. Thinker’s sign. Cleve Clin J Med 2019; 86(7):439. doi:10.3949/ccjm.86a.19036
- Chaiyachati KH, Shea JA, Asch DA, et al. Assessment of inpatient time allocation among first-year internal medicine residents using time-motion observations. JAMA Intern Med 2019. Epub ahead of print. doi:10.1001/jamainternmed.2019.0095
- Block L, Habicht R, Wu AW, et al. In the wake of the 2003 and 2011 duty hours regulations, how do internal medicine interns spend their time? J Gen Intern Med 2013; 28(8):1042–1047. doi:10.1007/s11606-013-2376-6
- Crumlish CM, Yialamas MA, McMahon GT. Quantification of bedside teaching by an academic hospitalist group. J Hosp Med 2009; 4(5):304–307. doi:10.1002/jhm.540
Apply for the Research Career Development Travel Award
The SVS Foundation developed the Research Career Development Travel Awards program to develop strong leaders in vascular surgery research. Recipients of the award will be assigned SVS research mentors who will provide guidance and discuss academic career advancement. They’ll also receive financial support to be used for travel, hotel accommodations and registration expenses for a research course. Applicants must be an SVS Candidate or Active Member who’s completed postgraduate clinical training in vascular surgery and has been in practice no more than seven years. Apply before August 15 to be considered.
The SVS Foundation developed the Research Career Development Travel Awards program to develop strong leaders in vascular surgery research. Recipients of the award will be assigned SVS research mentors who will provide guidance and discuss academic career advancement. They’ll also receive financial support to be used for travel, hotel accommodations and registration expenses for a research course. Applicants must be an SVS Candidate or Active Member who’s completed postgraduate clinical training in vascular surgery and has been in practice no more than seven years. Apply before August 15 to be considered.
The SVS Foundation developed the Research Career Development Travel Awards program to develop strong leaders in vascular surgery research. Recipients of the award will be assigned SVS research mentors who will provide guidance and discuss academic career advancement. They’ll also receive financial support to be used for travel, hotel accommodations and registration expenses for a research course. Applicants must be an SVS Candidate or Active Member who’s completed postgraduate clinical training in vascular surgery and has been in practice no more than seven years. Apply before August 15 to be considered.
Whose needs come first – the patient’s or the trial’s?
Debra Banks (not her real name) had hope. There was a clinical trial open at an academic hospital 200 miles from where she lived. She would commute or find local housing. It would cost her, but this is what her savings were for, she reasoned. What expense could be more important than her life?
Next came the tests. Blood tests, an ultrasound of her heart, breathing tests. She gave vials of blood, lay in scanners, and eagerly jumped through every hoop placed before her. Then came the call from the trial coordinator. Her heart ultrasound showed a mild dysfunction in how it pumped. It excluded her from the trial.
“Not eligible.” The two words that took away everything reverberated in her mind. Her heart had never caused her any problems before. So after the shock wore off, she tried to bargain with the trial coordinator: Had the study drug been shown to cause or worsen heart damage? Could they repeat the ultrasound? Did this blip in her heart function really matter?
When the trial coordinator couldn’t answer all these questions, she encouraged Debra to come into the clinic and talk to the doctors directly. That’s where I met her.
Debra found herself in the middle of a painful crossroads she had no interest being in. What happens when the needs of an individual patient and the needs of medical research are at odds? From Debra’s perspective, she had one goal. She wanted the therapy that would give her the best chance of living.
But the aim of the trial was not to help Debra – not directly, at least. Clinical trials help patient populations. The goal is to add to a body of knowledge: To study new therapies, demonstrate safety and efficacy, and ultimately find better treatments. The bulk of benefit goes to future patients, not individual participants. If an individual participant does benefit, all the better. But this is a bonus, not a requirement.
In order to meet these goals, trials come with inclusion and exclusion criteria. These are often strict. Individuals with certain other medical conditions are frequently excluded, as the person needs to be able to tolerate the toxicities of the drug being tested.
This, of course, is very different from our usual approach to patient care. Outside of trials, the needs of the individual patient are our North Star. Instead of inclusion and exclusion criteria, we have guidelines: general goalposts that hint at the right answer, but are able to be bent based on individual circumstances. It’s something I love about medicine. Part science, part art. Part algorithmic, part creative.
I can give chemotherapy to a patient with a low platelet count, if I think it’s best. I can override an elevated bilirubin. I can simply not check a heart ultrasound in the first place, if I don’t believe it will change my management.
I understand why trial criteria exist. I fully support investing in novel therapies that will help future patients on a large scale. There will invariably be individuals for whom a clinical trial is unsafe or inappropriate for a multitude of reasons, and our job as oncologists is to make that call and convey that news.
Still, that can be hard to square with the human being sitting in front of you. Debra was only in her mid-50s. She was an artist, an educator, a parent. She was a person who was so, so not ready to die. That she would because of a glitch in her heart function – the significance of which nobody knew – was excruciating.
While we can’t enroll every patient in every trial, the least we can do is comb through trial criteria thoughtfully. With the role of clinical investigator comes great responsibility. Are we choosing a cutoff because it makes clinical sense – or because that’s how it was done before? Is there a medical justification behind each and every exclusion criterion? A careless cutoff is not just a line on a protocol. It can be the difference between someone’s last hope – and no more options.
Every time I saw Debra in clinic, she asked about the trial. Then one day she stopped asking. She was distracted by more pressing problems. Her breathing had worsened and her energy levels were so low she could hardly get out of bed. Debra became sicker and sicker until she could no longer request the last hope that might make her better.
A wonderful physician-scientist I worked with once said she split her time between patient care and medical research because they complement each other. Whenever she lost a patient, she turned that pain into motivation to delve deeper into her research. She coped with individual loss by helping to make small, incremental improvements for the needs of many.
I think about this, months later, as I look around the empty exam room where I first met Debra. I imagine a roomful of patients, alive and healthy, for whom the research she was excluded from has benefited.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
Debra Banks (not her real name) had hope. There was a clinical trial open at an academic hospital 200 miles from where she lived. She would commute or find local housing. It would cost her, but this is what her savings were for, she reasoned. What expense could be more important than her life?
Next came the tests. Blood tests, an ultrasound of her heart, breathing tests. She gave vials of blood, lay in scanners, and eagerly jumped through every hoop placed before her. Then came the call from the trial coordinator. Her heart ultrasound showed a mild dysfunction in how it pumped. It excluded her from the trial.
“Not eligible.” The two words that took away everything reverberated in her mind. Her heart had never caused her any problems before. So after the shock wore off, she tried to bargain with the trial coordinator: Had the study drug been shown to cause or worsen heart damage? Could they repeat the ultrasound? Did this blip in her heart function really matter?
When the trial coordinator couldn’t answer all these questions, she encouraged Debra to come into the clinic and talk to the doctors directly. That’s where I met her.
Debra found herself in the middle of a painful crossroads she had no interest being in. What happens when the needs of an individual patient and the needs of medical research are at odds? From Debra’s perspective, she had one goal. She wanted the therapy that would give her the best chance of living.
But the aim of the trial was not to help Debra – not directly, at least. Clinical trials help patient populations. The goal is to add to a body of knowledge: To study new therapies, demonstrate safety and efficacy, and ultimately find better treatments. The bulk of benefit goes to future patients, not individual participants. If an individual participant does benefit, all the better. But this is a bonus, not a requirement.
In order to meet these goals, trials come with inclusion and exclusion criteria. These are often strict. Individuals with certain other medical conditions are frequently excluded, as the person needs to be able to tolerate the toxicities of the drug being tested.
This, of course, is very different from our usual approach to patient care. Outside of trials, the needs of the individual patient are our North Star. Instead of inclusion and exclusion criteria, we have guidelines: general goalposts that hint at the right answer, but are able to be bent based on individual circumstances. It’s something I love about medicine. Part science, part art. Part algorithmic, part creative.
I can give chemotherapy to a patient with a low platelet count, if I think it’s best. I can override an elevated bilirubin. I can simply not check a heart ultrasound in the first place, if I don’t believe it will change my management.
I understand why trial criteria exist. I fully support investing in novel therapies that will help future patients on a large scale. There will invariably be individuals for whom a clinical trial is unsafe or inappropriate for a multitude of reasons, and our job as oncologists is to make that call and convey that news.
Still, that can be hard to square with the human being sitting in front of you. Debra was only in her mid-50s. She was an artist, an educator, a parent. She was a person who was so, so not ready to die. That she would because of a glitch in her heart function – the significance of which nobody knew – was excruciating.
While we can’t enroll every patient in every trial, the least we can do is comb through trial criteria thoughtfully. With the role of clinical investigator comes great responsibility. Are we choosing a cutoff because it makes clinical sense – or because that’s how it was done before? Is there a medical justification behind each and every exclusion criterion? A careless cutoff is not just a line on a protocol. It can be the difference between someone’s last hope – and no more options.
Every time I saw Debra in clinic, she asked about the trial. Then one day she stopped asking. She was distracted by more pressing problems. Her breathing had worsened and her energy levels were so low she could hardly get out of bed. Debra became sicker and sicker until she could no longer request the last hope that might make her better.
A wonderful physician-scientist I worked with once said she split her time between patient care and medical research because they complement each other. Whenever she lost a patient, she turned that pain into motivation to delve deeper into her research. She coped with individual loss by helping to make small, incremental improvements for the needs of many.
I think about this, months later, as I look around the empty exam room where I first met Debra. I imagine a roomful of patients, alive and healthy, for whom the research she was excluded from has benefited.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
Debra Banks (not her real name) had hope. There was a clinical trial open at an academic hospital 200 miles from where she lived. She would commute or find local housing. It would cost her, but this is what her savings were for, she reasoned. What expense could be more important than her life?
Next came the tests. Blood tests, an ultrasound of her heart, breathing tests. She gave vials of blood, lay in scanners, and eagerly jumped through every hoop placed before her. Then came the call from the trial coordinator. Her heart ultrasound showed a mild dysfunction in how it pumped. It excluded her from the trial.
“Not eligible.” The two words that took away everything reverberated in her mind. Her heart had never caused her any problems before. So after the shock wore off, she tried to bargain with the trial coordinator: Had the study drug been shown to cause or worsen heart damage? Could they repeat the ultrasound? Did this blip in her heart function really matter?
When the trial coordinator couldn’t answer all these questions, she encouraged Debra to come into the clinic and talk to the doctors directly. That’s where I met her.
Debra found herself in the middle of a painful crossroads she had no interest being in. What happens when the needs of an individual patient and the needs of medical research are at odds? From Debra’s perspective, she had one goal. She wanted the therapy that would give her the best chance of living.
But the aim of the trial was not to help Debra – not directly, at least. Clinical trials help patient populations. The goal is to add to a body of knowledge: To study new therapies, demonstrate safety and efficacy, and ultimately find better treatments. The bulk of benefit goes to future patients, not individual participants. If an individual participant does benefit, all the better. But this is a bonus, not a requirement.
In order to meet these goals, trials come with inclusion and exclusion criteria. These are often strict. Individuals with certain other medical conditions are frequently excluded, as the person needs to be able to tolerate the toxicities of the drug being tested.
This, of course, is very different from our usual approach to patient care. Outside of trials, the needs of the individual patient are our North Star. Instead of inclusion and exclusion criteria, we have guidelines: general goalposts that hint at the right answer, but are able to be bent based on individual circumstances. It’s something I love about medicine. Part science, part art. Part algorithmic, part creative.
I can give chemotherapy to a patient with a low platelet count, if I think it’s best. I can override an elevated bilirubin. I can simply not check a heart ultrasound in the first place, if I don’t believe it will change my management.
I understand why trial criteria exist. I fully support investing in novel therapies that will help future patients on a large scale. There will invariably be individuals for whom a clinical trial is unsafe or inappropriate for a multitude of reasons, and our job as oncologists is to make that call and convey that news.
Still, that can be hard to square with the human being sitting in front of you. Debra was only in her mid-50s. She was an artist, an educator, a parent. She was a person who was so, so not ready to die. That she would because of a glitch in her heart function – the significance of which nobody knew – was excruciating.
While we can’t enroll every patient in every trial, the least we can do is comb through trial criteria thoughtfully. With the role of clinical investigator comes great responsibility. Are we choosing a cutoff because it makes clinical sense – or because that’s how it was done before? Is there a medical justification behind each and every exclusion criterion? A careless cutoff is not just a line on a protocol. It can be the difference between someone’s last hope – and no more options.
Every time I saw Debra in clinic, she asked about the trial. Then one day she stopped asking. She was distracted by more pressing problems. Her breathing had worsened and her energy levels were so low she could hardly get out of bed. Debra became sicker and sicker until she could no longer request the last hope that might make her better.
A wonderful physician-scientist I worked with once said she split her time between patient care and medical research because they complement each other. Whenever she lost a patient, she turned that pain into motivation to delve deeper into her research. She coped with individual loss by helping to make small, incremental improvements for the needs of many.
I think about this, months later, as I look around the empty exam room where I first met Debra. I imagine a roomful of patients, alive and healthy, for whom the research she was excluded from has benefited.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
NIH Study Will Test New Preventive Drug for Multidrug-Resistant TB
Tuberculosis (TB) kills more people each year than any other infectious disease. Not only the patients, but their nearest and dearest are at risk, as well. They are more likely to acquire latent TB infection and many will progress to active TB.
NIH is launching a study to compare delamanid, a new drug for multidrug-resistant TB (MDR-TB) with isoniazid, the long-time standard. The study hypothesis is that prophylactic delamanid will better protect family and other household members of patients with MDR-TB. Existing treatments for MDR-TB are often highly toxic and poorly tolerated, putting patients at risk while curing them only about half the time. Delamanid is one of the first drugs available specifically to treat people with MDR-TB and the first formulation suitable for children.
“A highly effective preventive TB therapy for vulnerable household members of people with active MDR-TB disease would be a game-changer in TB care,” says Dr. Anneke Hesseling, MD, PhD, one of the study leaders.
The phase 3 trial, Protecting Households on Exposure to Newly Diagnosed Index Multidrug-Resistant Tuberculosis Patients (PHOENIx MDR-TB), will take place at > 27 sites in at ≥ 12 countries. The researchers plan to enroll 2,158 adults being treated for confirmed active MDR-TB and 3,452 members of their households who are at high risk for developing active TB. The household members will be assigned randomly to receive oral delamanid daily for 26 weeks or oral isoniazid plus vitamin B6 daily for 26 weeks. All at-risk members of the same household will receive the same drug regimen.
Every 2 to 12 weeks, participating household contacts will have physical exams and other health assessments. The researchers will follow them for 96 weeks. Final results are expected in 2024.
TB is the leading cause of death among people with HIV. Both delamanid and isoniazid have minimal potential for interacting with antiretroviral drugs. Study participants with HIV who have not yet begun treatment will be referred to local health care providers for antiretroviral treatment.
Tuberculosis (TB) kills more people each year than any other infectious disease. Not only the patients, but their nearest and dearest are at risk, as well. They are more likely to acquire latent TB infection and many will progress to active TB.
NIH is launching a study to compare delamanid, a new drug for multidrug-resistant TB (MDR-TB) with isoniazid, the long-time standard. The study hypothesis is that prophylactic delamanid will better protect family and other household members of patients with MDR-TB. Existing treatments for MDR-TB are often highly toxic and poorly tolerated, putting patients at risk while curing them only about half the time. Delamanid is one of the first drugs available specifically to treat people with MDR-TB and the first formulation suitable for children.
“A highly effective preventive TB therapy for vulnerable household members of people with active MDR-TB disease would be a game-changer in TB care,” says Dr. Anneke Hesseling, MD, PhD, one of the study leaders.
The phase 3 trial, Protecting Households on Exposure to Newly Diagnosed Index Multidrug-Resistant Tuberculosis Patients (PHOENIx MDR-TB), will take place at > 27 sites in at ≥ 12 countries. The researchers plan to enroll 2,158 adults being treated for confirmed active MDR-TB and 3,452 members of their households who are at high risk for developing active TB. The household members will be assigned randomly to receive oral delamanid daily for 26 weeks or oral isoniazid plus vitamin B6 daily for 26 weeks. All at-risk members of the same household will receive the same drug regimen.
Every 2 to 12 weeks, participating household contacts will have physical exams and other health assessments. The researchers will follow them for 96 weeks. Final results are expected in 2024.
TB is the leading cause of death among people with HIV. Both delamanid and isoniazid have minimal potential for interacting with antiretroviral drugs. Study participants with HIV who have not yet begun treatment will be referred to local health care providers for antiretroviral treatment.
Tuberculosis (TB) kills more people each year than any other infectious disease. Not only the patients, but their nearest and dearest are at risk, as well. They are more likely to acquire latent TB infection and many will progress to active TB.
NIH is launching a study to compare delamanid, a new drug for multidrug-resistant TB (MDR-TB) with isoniazid, the long-time standard. The study hypothesis is that prophylactic delamanid will better protect family and other household members of patients with MDR-TB. Existing treatments for MDR-TB are often highly toxic and poorly tolerated, putting patients at risk while curing them only about half the time. Delamanid is one of the first drugs available specifically to treat people with MDR-TB and the first formulation suitable for children.
“A highly effective preventive TB therapy for vulnerable household members of people with active MDR-TB disease would be a game-changer in TB care,” says Dr. Anneke Hesseling, MD, PhD, one of the study leaders.
The phase 3 trial, Protecting Households on Exposure to Newly Diagnosed Index Multidrug-Resistant Tuberculosis Patients (PHOENIx MDR-TB), will take place at > 27 sites in at ≥ 12 countries. The researchers plan to enroll 2,158 adults being treated for confirmed active MDR-TB and 3,452 members of their households who are at high risk for developing active TB. The household members will be assigned randomly to receive oral delamanid daily for 26 weeks or oral isoniazid plus vitamin B6 daily for 26 weeks. All at-risk members of the same household will receive the same drug regimen.
Every 2 to 12 weeks, participating household contacts will have physical exams and other health assessments. The researchers will follow them for 96 weeks. Final results are expected in 2024.
TB is the leading cause of death among people with HIV. Both delamanid and isoniazid have minimal potential for interacting with antiretroviral drugs. Study participants with HIV who have not yet begun treatment will be referred to local health care providers for antiretroviral treatment.
Federal Health Care Data Trends 2019
Click here to access Federal Health Care Data Trends 2019
Table of Contents
- American Indian/Alaska Native
- Women's Health
- Department of Veterans Affairs
- Neurologic Disorders
- Diabetes Mellitus
- Infectious Disease
- Hematology/Oncology
- Mental Health
- Respiratory Disorders
- Gastrointestinal Disorders
- Department of Defense
- References

Click here to access Federal Health Care Data Trends 2019
Table of Contents
- American Indian/Alaska Native
- Women's Health
- Department of Veterans Affairs
- Neurologic Disorders
- Diabetes Mellitus
- Infectious Disease
- Hematology/Oncology
- Mental Health
- Respiratory Disorders
- Gastrointestinal Disorders
- Department of Defense
- References

Click here to access Federal Health Care Data Trends 2019
Table of Contents
- American Indian/Alaska Native
- Women's Health
- Department of Veterans Affairs
- Neurologic Disorders
- Diabetes Mellitus
- Infectious Disease
- Hematology/Oncology
- Mental Health
- Respiratory Disorders
- Gastrointestinal Disorders
- Department of Defense
- References