User login
Due to the COVID-19 pandemic, the AAN urges feds to further expand telehealth benefits
On March 17, the Trump administration announced an expansion of telehealth benefits to help stop the spread of COVID-19 and allow more Medicare patients to receive virtual care without having to visit a healthcare center or physician office.
Under the expansion, Medicare will pay for office, hospital, and other visits furnished via telehealth across the country and including in the patient’s home, delivered by a range of providers, such as physicians, nurse practitioners, clinical psychologists, and licensed clinical social workers.
Prior to this waiver, Medicare would only pay for telehealth on a limited basis, such as when the patient receiving the service was in a designated rural area.
However, in a letter to Alex Azar, secretary of the U.S. Department of Health & Human Services (HHS), the AAN says the easing of restrictions on telehealth should be extended beyond Medicare fee-for-service to both Medicare Advantage and Medicaid patients.
Practice changing?
“It is very heartening that the government is stepping up to the plate” and lifting many telemedicine restrictions, Neil Busis, MD, member of the AAN Health Policy Subcommittee, said in an interview.
Dr. Busis, who leads the telemedicine program for the department of neurology at NYU Langone Health in New York, said the global pandemic has “heightened, focused, and sharpened” attention to the need for telehealth services, particularly for neurology.
“By definition, a lot of neurology patients have mobility problems, traveling is a burden, making it difficult to see a neurologist,” he said.
Dr. Busis hopes these waivers in telehealth, made on a temporary and emergency basis, will become permanent once the COVID-19 pandemic has passed.
“What we hope is that the usefulness of various virtual technologies tested in the crucible of this pandemic will stimulate people to think about it once the pandemic is over and not rescind these loosening of restrictions, and that this will be the beginning of a new era for telemedicine,” he said.
The COVID-19 pandemic may be a “catalyst to accelerate the incorporation of non-face-to-face care into our armamentarium,” he added.
“What we have discovered in recent years is non-face-to-face care with enabling communication technologies is as effective in many clinical situations as face-to-face care. Now is the time to really focus on making the virtual experience as good as possible and to make it as available to as many people as possible,” said Dr. Busis.
Reduce regulatory burdens
The AAN also calls on the federal government to urge states to take action to ensure access to telehealth services and allow telehealth companies to provide telehealth technology and education free of charge to providers who don’t currently use telehealth in their practices.
“The AAN notes that doing so may implicate provisions of the Anti-Kickback Statute. We believe during the current emergency that HHS should issue guidance making it clear to providers that accepting free access to telehealth platforms and education does not put them at risk of violating fraud and abuse laws,” the letter, signed by AAN President James Stevens, MD, stated.
The AAN also wants the government to reduce regulatory burdens during this public health emergency to allow physicians more time to focus on patient care. “This is especially true for providers that are self-quarantining or are in a practice that is experiencing staffing shortages due to self-quarantines,” he wrote.
Specifically, the AAN asked the Centers for Medicare & Medicaid Services to extend the March 31 deadline for physicians to submit their data for the Merit-based Incentive Payment System program for calendar year 2019 (and other compliance deadlines) by at least 30 days.
The AAN also calls on the CMS to delay implementation of the Appropriate Use Criteria program by 1 year, saying that many providers will not have the capacity to “meaningfully” participate in the current testing year for the program.
A version of this article originally appeared on Medscape.com.
On March 17, the Trump administration announced an expansion of telehealth benefits to help stop the spread of COVID-19 and allow more Medicare patients to receive virtual care without having to visit a healthcare center or physician office.
Under the expansion, Medicare will pay for office, hospital, and other visits furnished via telehealth across the country and including in the patient’s home, delivered by a range of providers, such as physicians, nurse practitioners, clinical psychologists, and licensed clinical social workers.
Prior to this waiver, Medicare would only pay for telehealth on a limited basis, such as when the patient receiving the service was in a designated rural area.
However, in a letter to Alex Azar, secretary of the U.S. Department of Health & Human Services (HHS), the AAN says the easing of restrictions on telehealth should be extended beyond Medicare fee-for-service to both Medicare Advantage and Medicaid patients.
Practice changing?
“It is very heartening that the government is stepping up to the plate” and lifting many telemedicine restrictions, Neil Busis, MD, member of the AAN Health Policy Subcommittee, said in an interview.
Dr. Busis, who leads the telemedicine program for the department of neurology at NYU Langone Health in New York, said the global pandemic has “heightened, focused, and sharpened” attention to the need for telehealth services, particularly for neurology.
“By definition, a lot of neurology patients have mobility problems, traveling is a burden, making it difficult to see a neurologist,” he said.
Dr. Busis hopes these waivers in telehealth, made on a temporary and emergency basis, will become permanent once the COVID-19 pandemic has passed.
“What we hope is that the usefulness of various virtual technologies tested in the crucible of this pandemic will stimulate people to think about it once the pandemic is over and not rescind these loosening of restrictions, and that this will be the beginning of a new era for telemedicine,” he said.
The COVID-19 pandemic may be a “catalyst to accelerate the incorporation of non-face-to-face care into our armamentarium,” he added.
“What we have discovered in recent years is non-face-to-face care with enabling communication technologies is as effective in many clinical situations as face-to-face care. Now is the time to really focus on making the virtual experience as good as possible and to make it as available to as many people as possible,” said Dr. Busis.
Reduce regulatory burdens
The AAN also calls on the federal government to urge states to take action to ensure access to telehealth services and allow telehealth companies to provide telehealth technology and education free of charge to providers who don’t currently use telehealth in their practices.
“The AAN notes that doing so may implicate provisions of the Anti-Kickback Statute. We believe during the current emergency that HHS should issue guidance making it clear to providers that accepting free access to telehealth platforms and education does not put them at risk of violating fraud and abuse laws,” the letter, signed by AAN President James Stevens, MD, stated.
The AAN also wants the government to reduce regulatory burdens during this public health emergency to allow physicians more time to focus on patient care. “This is especially true for providers that are self-quarantining or are in a practice that is experiencing staffing shortages due to self-quarantines,” he wrote.
Specifically, the AAN asked the Centers for Medicare & Medicaid Services to extend the March 31 deadline for physicians to submit their data for the Merit-based Incentive Payment System program for calendar year 2019 (and other compliance deadlines) by at least 30 days.
The AAN also calls on the CMS to delay implementation of the Appropriate Use Criteria program by 1 year, saying that many providers will not have the capacity to “meaningfully” participate in the current testing year for the program.
A version of this article originally appeared on Medscape.com.
On March 17, the Trump administration announced an expansion of telehealth benefits to help stop the spread of COVID-19 and allow more Medicare patients to receive virtual care without having to visit a healthcare center or physician office.
Under the expansion, Medicare will pay for office, hospital, and other visits furnished via telehealth across the country and including in the patient’s home, delivered by a range of providers, such as physicians, nurse practitioners, clinical psychologists, and licensed clinical social workers.
Prior to this waiver, Medicare would only pay for telehealth on a limited basis, such as when the patient receiving the service was in a designated rural area.
However, in a letter to Alex Azar, secretary of the U.S. Department of Health & Human Services (HHS), the AAN says the easing of restrictions on telehealth should be extended beyond Medicare fee-for-service to both Medicare Advantage and Medicaid patients.
Practice changing?
“It is very heartening that the government is stepping up to the plate” and lifting many telemedicine restrictions, Neil Busis, MD, member of the AAN Health Policy Subcommittee, said in an interview.
Dr. Busis, who leads the telemedicine program for the department of neurology at NYU Langone Health in New York, said the global pandemic has “heightened, focused, and sharpened” attention to the need for telehealth services, particularly for neurology.
“By definition, a lot of neurology patients have mobility problems, traveling is a burden, making it difficult to see a neurologist,” he said.
Dr. Busis hopes these waivers in telehealth, made on a temporary and emergency basis, will become permanent once the COVID-19 pandemic has passed.
“What we hope is that the usefulness of various virtual technologies tested in the crucible of this pandemic will stimulate people to think about it once the pandemic is over and not rescind these loosening of restrictions, and that this will be the beginning of a new era for telemedicine,” he said.
The COVID-19 pandemic may be a “catalyst to accelerate the incorporation of non-face-to-face care into our armamentarium,” he added.
“What we have discovered in recent years is non-face-to-face care with enabling communication technologies is as effective in many clinical situations as face-to-face care. Now is the time to really focus on making the virtual experience as good as possible and to make it as available to as many people as possible,” said Dr. Busis.
Reduce regulatory burdens
The AAN also calls on the federal government to urge states to take action to ensure access to telehealth services and allow telehealth companies to provide telehealth technology and education free of charge to providers who don’t currently use telehealth in their practices.
“The AAN notes that doing so may implicate provisions of the Anti-Kickback Statute. We believe during the current emergency that HHS should issue guidance making it clear to providers that accepting free access to telehealth platforms and education does not put them at risk of violating fraud and abuse laws,” the letter, signed by AAN President James Stevens, MD, stated.
The AAN also wants the government to reduce regulatory burdens during this public health emergency to allow physicians more time to focus on patient care. “This is especially true for providers that are self-quarantining or are in a practice that is experiencing staffing shortages due to self-quarantines,” he wrote.
Specifically, the AAN asked the Centers for Medicare & Medicaid Services to extend the March 31 deadline for physicians to submit their data for the Merit-based Incentive Payment System program for calendar year 2019 (and other compliance deadlines) by at least 30 days.
The AAN also calls on the CMS to delay implementation of the Appropriate Use Criteria program by 1 year, saying that many providers will not have the capacity to “meaningfully” participate in the current testing year for the program.
A version of this article originally appeared on Medscape.com.
‘Larger-than-life’ physician Stephen Schwartz dies of COVID-19 at 78
Stephen M. Schwartz, MD, PhD, a pioneer in the field of vascular biology and a longtime professor of pathology at the University of Washington, Seattle, died March 17, 2020, after being hospitalized with COVID-19. He was 78.
“This has become all too real,” UW president Ana Mari Cauce said on Facebook, where she described Dr. Schwartz as “larger than life,” and superimposed a photo of him in front of Mount Rainier, according to a report in the Seattle Times.
Dr. Schwartz is “rightfully considered a giant among investigators of the biology of smooth muscle cells and the structure of blood vessels,” Paul Ramsey, MD, CEO of UW Medicine, said in a statement. He will be remembered for his “vigorous advocacy for research and for the field of vascular biology as well as for his many trainees who have gone on to great success as independent investigators in the field of vascular pathobiology,” Dr. Ramsey said.
Dr. Schwartz received a BA in biology from Harvard University in 1963 and an MD from Boston University in 1967. Dr. Schwartz started a residency in the UW department of pathology in 1967 and received his PhD from the institution in 1973. From 1974 to 1979, he was an assistant professor of pathology and became a full professor in 1984.
Dr. Schwartz was also an adjunct professor in the UW departments of bioengineering and medicine, “reflective of his many collaborative relationships with faculty in other departments in our medical school and in the world,” Dr. Ramsey said.
“Dr. Schwartz left a lasting imprint on the UW School of Medicine and the broader scientific community. He will be greatly missed,” he added.
‘A great loss’
Dr. Schwartz chaired numerous national and international meetings in the field of vascular biology. He was the founding chair of the Gordon Research Conference on Vascular Biology and a cofounder and second president of the North American Vascular Biology Organization (NAVBO). He created NAVBO’s flagship summer course, Vasculata.
“The NAVBO community has suffered a great loss,” Bernadette Englert, executive officer for the organization, said in a statement. He will be “sorely missed by generations of vascular biologists and pathologists.”
News of Dr. Schwartz’s passing lit up Twitter. Here are just a few comments:
UW lost to COVID-19 “beloved professor Stephen Schwartz, a pioneer in vascular biology and a larger-than-life scientist. Steve, you will be missed!” Rong Tian, MD, PhD, with the bioengineering department, wrote in a tweet.
“Stephen Schwartz was a giant in vascular biology and a mentor to countless faculty and trainees, including myself. He will be deeply missed,” said Kelly Stevens, PhD, also from the bioengineering department.
A version of this article originally appeared on Medscape.com.
Stephen M. Schwartz, MD, PhD, a pioneer in the field of vascular biology and a longtime professor of pathology at the University of Washington, Seattle, died March 17, 2020, after being hospitalized with COVID-19. He was 78.
“This has become all too real,” UW president Ana Mari Cauce said on Facebook, where she described Dr. Schwartz as “larger than life,” and superimposed a photo of him in front of Mount Rainier, according to a report in the Seattle Times.
Dr. Schwartz is “rightfully considered a giant among investigators of the biology of smooth muscle cells and the structure of blood vessels,” Paul Ramsey, MD, CEO of UW Medicine, said in a statement. He will be remembered for his “vigorous advocacy for research and for the field of vascular biology as well as for his many trainees who have gone on to great success as independent investigators in the field of vascular pathobiology,” Dr. Ramsey said.
Dr. Schwartz received a BA in biology from Harvard University in 1963 and an MD from Boston University in 1967. Dr. Schwartz started a residency in the UW department of pathology in 1967 and received his PhD from the institution in 1973. From 1974 to 1979, he was an assistant professor of pathology and became a full professor in 1984.
Dr. Schwartz was also an adjunct professor in the UW departments of bioengineering and medicine, “reflective of his many collaborative relationships with faculty in other departments in our medical school and in the world,” Dr. Ramsey said.
“Dr. Schwartz left a lasting imprint on the UW School of Medicine and the broader scientific community. He will be greatly missed,” he added.
‘A great loss’
Dr. Schwartz chaired numerous national and international meetings in the field of vascular biology. He was the founding chair of the Gordon Research Conference on Vascular Biology and a cofounder and second president of the North American Vascular Biology Organization (NAVBO). He created NAVBO’s flagship summer course, Vasculata.
“The NAVBO community has suffered a great loss,” Bernadette Englert, executive officer for the organization, said in a statement. He will be “sorely missed by generations of vascular biologists and pathologists.”
News of Dr. Schwartz’s passing lit up Twitter. Here are just a few comments:
UW lost to COVID-19 “beloved professor Stephen Schwartz, a pioneer in vascular biology and a larger-than-life scientist. Steve, you will be missed!” Rong Tian, MD, PhD, with the bioengineering department, wrote in a tweet.
“Stephen Schwartz was a giant in vascular biology and a mentor to countless faculty and trainees, including myself. He will be deeply missed,” said Kelly Stevens, PhD, also from the bioengineering department.
A version of this article originally appeared on Medscape.com.
Stephen M. Schwartz, MD, PhD, a pioneer in the field of vascular biology and a longtime professor of pathology at the University of Washington, Seattle, died March 17, 2020, after being hospitalized with COVID-19. He was 78.
“This has become all too real,” UW president Ana Mari Cauce said on Facebook, where she described Dr. Schwartz as “larger than life,” and superimposed a photo of him in front of Mount Rainier, according to a report in the Seattle Times.
Dr. Schwartz is “rightfully considered a giant among investigators of the biology of smooth muscle cells and the structure of blood vessels,” Paul Ramsey, MD, CEO of UW Medicine, said in a statement. He will be remembered for his “vigorous advocacy for research and for the field of vascular biology as well as for his many trainees who have gone on to great success as independent investigators in the field of vascular pathobiology,” Dr. Ramsey said.
Dr. Schwartz received a BA in biology from Harvard University in 1963 and an MD from Boston University in 1967. Dr. Schwartz started a residency in the UW department of pathology in 1967 and received his PhD from the institution in 1973. From 1974 to 1979, he was an assistant professor of pathology and became a full professor in 1984.
Dr. Schwartz was also an adjunct professor in the UW departments of bioengineering and medicine, “reflective of his many collaborative relationships with faculty in other departments in our medical school and in the world,” Dr. Ramsey said.
“Dr. Schwartz left a lasting imprint on the UW School of Medicine and the broader scientific community. He will be greatly missed,” he added.
‘A great loss’
Dr. Schwartz chaired numerous national and international meetings in the field of vascular biology. He was the founding chair of the Gordon Research Conference on Vascular Biology and a cofounder and second president of the North American Vascular Biology Organization (NAVBO). He created NAVBO’s flagship summer course, Vasculata.
“The NAVBO community has suffered a great loss,” Bernadette Englert, executive officer for the organization, said in a statement. He will be “sorely missed by generations of vascular biologists and pathologists.”
News of Dr. Schwartz’s passing lit up Twitter. Here are just a few comments:
UW lost to COVID-19 “beloved professor Stephen Schwartz, a pioneer in vascular biology and a larger-than-life scientist. Steve, you will be missed!” Rong Tian, MD, PhD, with the bioengineering department, wrote in a tweet.
“Stephen Schwartz was a giant in vascular biology and a mentor to countless faculty and trainees, including myself. He will be deeply missed,” said Kelly Stevens, PhD, also from the bioengineering department.
A version of this article originally appeared on Medscape.com.
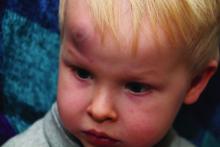
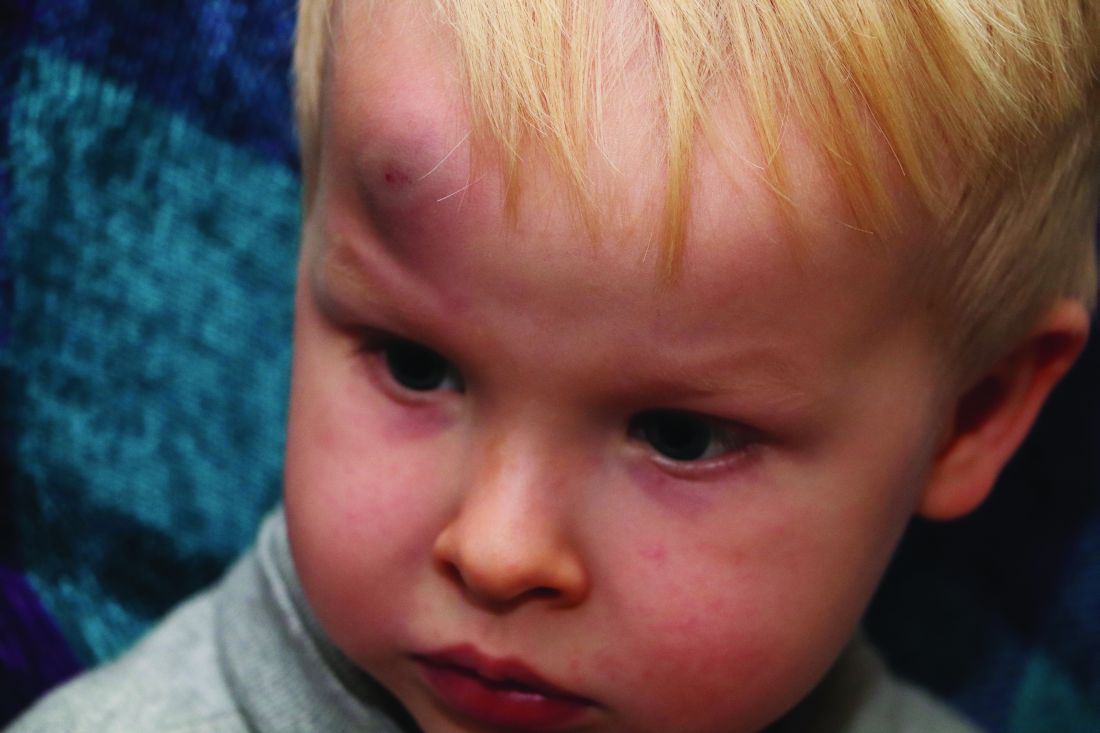
AAP adds specifics to policy on abusive head trauma
the American Academy of Pediatrics said in an updated policy statement.
Abusive head trauma (AHT) is fatal in approximately one-quarter of cases in infants during the first year of life, and less-obvious clinical signs such as vomiting and fussiness often are missed, wrote Sandeep K. Narang, MD, JD, of Northwestern University, Chicago, and colleagues on the AAP Council on Child Abuse and Neglect.
In a policy statement published in Pediatrics, the AAP cautioned physicians to remain vigilant for signs that are common in AHT cases. In particular, bruising on the torso, ears, and neck in children aged younger than 4 years, or any bruising in infants younger than 4 months should be a red flag. In addition, the most recent data indicate that apnea and retinal hemorrhages are more common in cases of abuse than in accidental injuries. The AAP also recommends a skeletal survey in suspected AHT for children younger than 2 years to identify occult fractures.
“Oral injuries in infants, such as frenulum tears, may also accompany or precede AHT,” Dr. Narang and associates said.
In addition, secondary brain injury as a result of AHT can lead to poor outcomes that may be observed. “Almost 70% of survivors of AHT have some degree of lasting neurologic impairment, including static encephalopathy, intellectual disability, cerebral palsy, cortical blindness, seizure disorders, behavior problems, and learning disabilities,” according to the statement.
Endocrine dysfunction also is common in children with a history of AHT, but might not present until years later, the authors noted.
When AHT is suspected in a patient, the policy statement recommends that a subspecialist in child abuse pediatrics or in related areas including radiology, ophthalmology, neurosurgery, neurology, and general pediatric surgery “should also be consulted when necessary to ensure a complete and accurate evaluation.”
Although falls from a height of 1.5 m or 5 feet often are used as an explanation for AHT injuries, “numerous lines of clinical research have clarified the extreme rarity of short falls as a cause of severe neurologic injury or death in young infants,” Dr. Narang and associates wrote.
Other recommendations in the updated policy encourage use of the term “abusive head trauma” in medical communications, as well as encourage caregivers to serve as a medical home for survivors of AHT or refer them to medical homes for rehabilitation and monitoring. Parents and caregivers may need to be educated about the dangers of shaking or striking an infant, shown safe ways to manage a crying baby, and given tools to manage their own stress and frustration.
Physicians are legally required to report suspected cases of child abuse or neglect, and should be prepared to educate stakeholders if you are called on to work with legal and child protective services about the science behind AHT.
“The role of the pediatric practitioner is not to apportion blame or investigate potential criminal activity but to identify the medical problem, evaluate and treat the child’s injuries, and offer honest medical information to parents, families, investigators, and attorneys and/or judges,” Dr. Narang and associates wrote.
This policy statement updates the previous policy statement issued in 2009 and affirmed in 2013. The policy had no external funding, and the authors had no financial conflicts to disclose. Dr. Narang, Amanda Fingarson, DO, and James Lukefahr, MD, have served as paid expert witnesses/consultants in cases of abusive head trauma in infants and children.
SOURCE: Narang SK et al. Pediatrics. 2020 Mar 23. doi: 10.1542/peds.2020-0203.
the American Academy of Pediatrics said in an updated policy statement.
Abusive head trauma (AHT) is fatal in approximately one-quarter of cases in infants during the first year of life, and less-obvious clinical signs such as vomiting and fussiness often are missed, wrote Sandeep K. Narang, MD, JD, of Northwestern University, Chicago, and colleagues on the AAP Council on Child Abuse and Neglect.
In a policy statement published in Pediatrics, the AAP cautioned physicians to remain vigilant for signs that are common in AHT cases. In particular, bruising on the torso, ears, and neck in children aged younger than 4 years, or any bruising in infants younger than 4 months should be a red flag. In addition, the most recent data indicate that apnea and retinal hemorrhages are more common in cases of abuse than in accidental injuries. The AAP also recommends a skeletal survey in suspected AHT for children younger than 2 years to identify occult fractures.
“Oral injuries in infants, such as frenulum tears, may also accompany or precede AHT,” Dr. Narang and associates said.
In addition, secondary brain injury as a result of AHT can lead to poor outcomes that may be observed. “Almost 70% of survivors of AHT have some degree of lasting neurologic impairment, including static encephalopathy, intellectual disability, cerebral palsy, cortical blindness, seizure disorders, behavior problems, and learning disabilities,” according to the statement.
Endocrine dysfunction also is common in children with a history of AHT, but might not present until years later, the authors noted.
When AHT is suspected in a patient, the policy statement recommends that a subspecialist in child abuse pediatrics or in related areas including radiology, ophthalmology, neurosurgery, neurology, and general pediatric surgery “should also be consulted when necessary to ensure a complete and accurate evaluation.”
Although falls from a height of 1.5 m or 5 feet often are used as an explanation for AHT injuries, “numerous lines of clinical research have clarified the extreme rarity of short falls as a cause of severe neurologic injury or death in young infants,” Dr. Narang and associates wrote.
Other recommendations in the updated policy encourage use of the term “abusive head trauma” in medical communications, as well as encourage caregivers to serve as a medical home for survivors of AHT or refer them to medical homes for rehabilitation and monitoring. Parents and caregivers may need to be educated about the dangers of shaking or striking an infant, shown safe ways to manage a crying baby, and given tools to manage their own stress and frustration.
Physicians are legally required to report suspected cases of child abuse or neglect, and should be prepared to educate stakeholders if you are called on to work with legal and child protective services about the science behind AHT.
“The role of the pediatric practitioner is not to apportion blame or investigate potential criminal activity but to identify the medical problem, evaluate and treat the child’s injuries, and offer honest medical information to parents, families, investigators, and attorneys and/or judges,” Dr. Narang and associates wrote.
This policy statement updates the previous policy statement issued in 2009 and affirmed in 2013. The policy had no external funding, and the authors had no financial conflicts to disclose. Dr. Narang, Amanda Fingarson, DO, and James Lukefahr, MD, have served as paid expert witnesses/consultants in cases of abusive head trauma in infants and children.
SOURCE: Narang SK et al. Pediatrics. 2020 Mar 23. doi: 10.1542/peds.2020-0203.
the American Academy of Pediatrics said in an updated policy statement.
Abusive head trauma (AHT) is fatal in approximately one-quarter of cases in infants during the first year of life, and less-obvious clinical signs such as vomiting and fussiness often are missed, wrote Sandeep K. Narang, MD, JD, of Northwestern University, Chicago, and colleagues on the AAP Council on Child Abuse and Neglect.
In a policy statement published in Pediatrics, the AAP cautioned physicians to remain vigilant for signs that are common in AHT cases. In particular, bruising on the torso, ears, and neck in children aged younger than 4 years, or any bruising in infants younger than 4 months should be a red flag. In addition, the most recent data indicate that apnea and retinal hemorrhages are more common in cases of abuse than in accidental injuries. The AAP also recommends a skeletal survey in suspected AHT for children younger than 2 years to identify occult fractures.
“Oral injuries in infants, such as frenulum tears, may also accompany or precede AHT,” Dr. Narang and associates said.
In addition, secondary brain injury as a result of AHT can lead to poor outcomes that may be observed. “Almost 70% of survivors of AHT have some degree of lasting neurologic impairment, including static encephalopathy, intellectual disability, cerebral palsy, cortical blindness, seizure disorders, behavior problems, and learning disabilities,” according to the statement.
Endocrine dysfunction also is common in children with a history of AHT, but might not present until years later, the authors noted.
When AHT is suspected in a patient, the policy statement recommends that a subspecialist in child abuse pediatrics or in related areas including radiology, ophthalmology, neurosurgery, neurology, and general pediatric surgery “should also be consulted when necessary to ensure a complete and accurate evaluation.”
Although falls from a height of 1.5 m or 5 feet often are used as an explanation for AHT injuries, “numerous lines of clinical research have clarified the extreme rarity of short falls as a cause of severe neurologic injury or death in young infants,” Dr. Narang and associates wrote.
Other recommendations in the updated policy encourage use of the term “abusive head trauma” in medical communications, as well as encourage caregivers to serve as a medical home for survivors of AHT or refer them to medical homes for rehabilitation and monitoring. Parents and caregivers may need to be educated about the dangers of shaking or striking an infant, shown safe ways to manage a crying baby, and given tools to manage their own stress and frustration.
Physicians are legally required to report suspected cases of child abuse or neglect, and should be prepared to educate stakeholders if you are called on to work with legal and child protective services about the science behind AHT.
“The role of the pediatric practitioner is not to apportion blame or investigate potential criminal activity but to identify the medical problem, evaluate and treat the child’s injuries, and offer honest medical information to parents, families, investigators, and attorneys and/or judges,” Dr. Narang and associates wrote.
This policy statement updates the previous policy statement issued in 2009 and affirmed in 2013. The policy had no external funding, and the authors had no financial conflicts to disclose. Dr. Narang, Amanda Fingarson, DO, and James Lukefahr, MD, have served as paid expert witnesses/consultants in cases of abusive head trauma in infants and children.
SOURCE: Narang SK et al. Pediatrics. 2020 Mar 23. doi: 10.1542/peds.2020-0203.
FROM PEDIATRICS
Treatment options for COVID-19: Dr. Annie Luetkemeyer
Annie Luetkemeyer, MD, professor of infectious diseases at UCSF, is an expert on the treatment of viral infections. Robert Wachter, MD, MHM, chair of the UCSF Department of Medicine, interviewed her about the evidence behind potential treatments for COVID-19 (including chloroquine/hydroxychloroquine, remdesivir, and others), as well as how to assess new and existing drugs in a pandemic.
Annie Luetkemeyer, MD, professor of infectious diseases at UCSF, is an expert on the treatment of viral infections. Robert Wachter, MD, MHM, chair of the UCSF Department of Medicine, interviewed her about the evidence behind potential treatments for COVID-19 (including chloroquine/hydroxychloroquine, remdesivir, and others), as well as how to assess new and existing drugs in a pandemic.
Annie Luetkemeyer, MD, professor of infectious diseases at UCSF, is an expert on the treatment of viral infections. Robert Wachter, MD, MHM, chair of the UCSF Department of Medicine, interviewed her about the evidence behind potential treatments for COVID-19 (including chloroquine/hydroxychloroquine, remdesivir, and others), as well as how to assess new and existing drugs in a pandemic.
Week-old COVID-19 urology guidelines already outdated
Recommendations to help clinicians triage surgical procedures during the COVID-19 pandemic, developed quickly by a team of urology experts from around the world and shared last week, are already out of date.
“I would change some things we said a week ago,” said David Canes, MD, from Lahey Hospital and Medical Center in Burlington, Massachusetts, and Derry, New Hampshire, who was one of those experts.
“We now know it’s not possible to create a cookbook in the face of a rapidly evolving pandemic,” he told Medscape Medical News.
“It’s heartening that we could do it so fast, but now it’s a snapshot in time, a starting point. People have to have conversations locally, in their community, taking into account where they are in relation to a surge of COVID patients, to make good decisions,” Canes said.
Long-thought-out guidance can no longer come from societies. “As the pace of information changes so rapidly,” Canes said he has changed the way he disseminates information and searches for guidance. “I’m even looking to nontraditional channels, like Twitter.”
As the COVID-19 pandemic evolves, informal discussions on social media are helping specialists make decisions. “Threads about various cancers and how people are handling them are helpful,” he said.
He described, for example, a thoughtful discussion on the use of androgen-deprivation therapy, a hormone therapy that can block the effects of androgens and can slow the growth of prostate cancer. “This is not a standard-of-care treatment,” he said, but now it’s being discussed very seriously to treat patients whose care might get delayed.
A multiple-choice survey was posted on Twitter by Ashish Kamat, MD, MBBS, from the MD Anderson Cancer Center in Houston, asking respondents what they would do for a patient with stage T2 high-grade muscle invasive bladder cancer and normal glomerular filtration during the pandemic.
In less than 20 hours, his post received 290 votes in response.
And when Badar Mian, MD, from the Albany Medical Center in New York, asked 23 urologists whether they would recommend radiotherapy (20 fractions) without any chemotherapy, he quickly got two responses: one yes and one no, with explanations.
People are responding to posts quickly. “With the COVID pandemic, we can’t wait for consensus guidelines from the American Urology Association or European Association of Urology,” Canes said.
One Week Changed Everything
When Canes and his coauthors said last week that prostatectomies should be delayed, they didn’t know the extent to which surgery was going to be halted. “When we wrote this statement, most facilities were still allowing elective surgeries or were just on the cusp of shutting down.”
Today, if you’re in an area where elective surgeries are still allowed or it is early in the crisis, “you might still take a patient with a Gleason 9 and a PSA of 25 and judiciously get the surgery done.”
As of March 23, however, surgery in New York City is entirely off the table. “No cancer surgery is happening anymore,” Canes reported.
The recommendations suggested using “shared decision-making” to guide radiation therapy choices. “But now, bringing a patient in for daily radiation treatment may not even be feasible, with the effort it takes to clean, the consumption of PPEs, etc,” he added.
When the dust settles, there will be a lot of assessment of current decision-making. “We’ll see if there are blips in mortality according to decisions being made,” Canes said.
The bottom line is that “we’re running on a 24-hour news cycle,” he pointed out. “It’s humbling to see how quickly decision-making changes and how nimble we have to be in making these very difficult decisions that we’ve never had to make before.”
For his own patients, Canes said he is doing consultations by phone or video at this point. “My patients have been very gracious; everyone has a general feeling we’re all in this together.”
And so far, “I haven’t had a situation where I thought the patient wasn’t going to survive,” he added.
This article first appeared on Medscape.com.
Recommendations to help clinicians triage surgical procedures during the COVID-19 pandemic, developed quickly by a team of urology experts from around the world and shared last week, are already out of date.
“I would change some things we said a week ago,” said David Canes, MD, from Lahey Hospital and Medical Center in Burlington, Massachusetts, and Derry, New Hampshire, who was one of those experts.
“We now know it’s not possible to create a cookbook in the face of a rapidly evolving pandemic,” he told Medscape Medical News.
“It’s heartening that we could do it so fast, but now it’s a snapshot in time, a starting point. People have to have conversations locally, in their community, taking into account where they are in relation to a surge of COVID patients, to make good decisions,” Canes said.
Long-thought-out guidance can no longer come from societies. “As the pace of information changes so rapidly,” Canes said he has changed the way he disseminates information and searches for guidance. “I’m even looking to nontraditional channels, like Twitter.”
As the COVID-19 pandemic evolves, informal discussions on social media are helping specialists make decisions. “Threads about various cancers and how people are handling them are helpful,” he said.
He described, for example, a thoughtful discussion on the use of androgen-deprivation therapy, a hormone therapy that can block the effects of androgens and can slow the growth of prostate cancer. “This is not a standard-of-care treatment,” he said, but now it’s being discussed very seriously to treat patients whose care might get delayed.
A multiple-choice survey was posted on Twitter by Ashish Kamat, MD, MBBS, from the MD Anderson Cancer Center in Houston, asking respondents what they would do for a patient with stage T2 high-grade muscle invasive bladder cancer and normal glomerular filtration during the pandemic.
In less than 20 hours, his post received 290 votes in response.
And when Badar Mian, MD, from the Albany Medical Center in New York, asked 23 urologists whether they would recommend radiotherapy (20 fractions) without any chemotherapy, he quickly got two responses: one yes and one no, with explanations.
People are responding to posts quickly. “With the COVID pandemic, we can’t wait for consensus guidelines from the American Urology Association or European Association of Urology,” Canes said.
One Week Changed Everything
When Canes and his coauthors said last week that prostatectomies should be delayed, they didn’t know the extent to which surgery was going to be halted. “When we wrote this statement, most facilities were still allowing elective surgeries or were just on the cusp of shutting down.”
Today, if you’re in an area where elective surgeries are still allowed or it is early in the crisis, “you might still take a patient with a Gleason 9 and a PSA of 25 and judiciously get the surgery done.”
As of March 23, however, surgery in New York City is entirely off the table. “No cancer surgery is happening anymore,” Canes reported.
The recommendations suggested using “shared decision-making” to guide radiation therapy choices. “But now, bringing a patient in for daily radiation treatment may not even be feasible, with the effort it takes to clean, the consumption of PPEs, etc,” he added.
When the dust settles, there will be a lot of assessment of current decision-making. “We’ll see if there are blips in mortality according to decisions being made,” Canes said.
The bottom line is that “we’re running on a 24-hour news cycle,” he pointed out. “It’s humbling to see how quickly decision-making changes and how nimble we have to be in making these very difficult decisions that we’ve never had to make before.”
For his own patients, Canes said he is doing consultations by phone or video at this point. “My patients have been very gracious; everyone has a general feeling we’re all in this together.”
And so far, “I haven’t had a situation where I thought the patient wasn’t going to survive,” he added.
This article first appeared on Medscape.com.
Recommendations to help clinicians triage surgical procedures during the COVID-19 pandemic, developed quickly by a team of urology experts from around the world and shared last week, are already out of date.
“I would change some things we said a week ago,” said David Canes, MD, from Lahey Hospital and Medical Center in Burlington, Massachusetts, and Derry, New Hampshire, who was one of those experts.
“We now know it’s not possible to create a cookbook in the face of a rapidly evolving pandemic,” he told Medscape Medical News.
“It’s heartening that we could do it so fast, but now it’s a snapshot in time, a starting point. People have to have conversations locally, in their community, taking into account where they are in relation to a surge of COVID patients, to make good decisions,” Canes said.
Long-thought-out guidance can no longer come from societies. “As the pace of information changes so rapidly,” Canes said he has changed the way he disseminates information and searches for guidance. “I’m even looking to nontraditional channels, like Twitter.”
As the COVID-19 pandemic evolves, informal discussions on social media are helping specialists make decisions. “Threads about various cancers and how people are handling them are helpful,” he said.
He described, for example, a thoughtful discussion on the use of androgen-deprivation therapy, a hormone therapy that can block the effects of androgens and can slow the growth of prostate cancer. “This is not a standard-of-care treatment,” he said, but now it’s being discussed very seriously to treat patients whose care might get delayed.
A multiple-choice survey was posted on Twitter by Ashish Kamat, MD, MBBS, from the MD Anderson Cancer Center in Houston, asking respondents what they would do for a patient with stage T2 high-grade muscle invasive bladder cancer and normal glomerular filtration during the pandemic.
In less than 20 hours, his post received 290 votes in response.
And when Badar Mian, MD, from the Albany Medical Center in New York, asked 23 urologists whether they would recommend radiotherapy (20 fractions) without any chemotherapy, he quickly got two responses: one yes and one no, with explanations.
People are responding to posts quickly. “With the COVID pandemic, we can’t wait for consensus guidelines from the American Urology Association or European Association of Urology,” Canes said.
One Week Changed Everything
When Canes and his coauthors said last week that prostatectomies should be delayed, they didn’t know the extent to which surgery was going to be halted. “When we wrote this statement, most facilities were still allowing elective surgeries or were just on the cusp of shutting down.”
Today, if you’re in an area where elective surgeries are still allowed or it is early in the crisis, “you might still take a patient with a Gleason 9 and a PSA of 25 and judiciously get the surgery done.”
As of March 23, however, surgery in New York City is entirely off the table. “No cancer surgery is happening anymore,” Canes reported.
The recommendations suggested using “shared decision-making” to guide radiation therapy choices. “But now, bringing a patient in for daily radiation treatment may not even be feasible, with the effort it takes to clean, the consumption of PPEs, etc,” he added.
When the dust settles, there will be a lot of assessment of current decision-making. “We’ll see if there are blips in mortality according to decisions being made,” Canes said.
The bottom line is that “we’re running on a 24-hour news cycle,” he pointed out. “It’s humbling to see how quickly decision-making changes and how nimble we have to be in making these very difficult decisions that we’ve never had to make before.”
For his own patients, Canes said he is doing consultations by phone or video at this point. “My patients have been very gracious; everyone has a general feeling we’re all in this together.”
And so far, “I haven’t had a situation where I thought the patient wasn’t going to survive,” he added.
This article first appeared on Medscape.com.
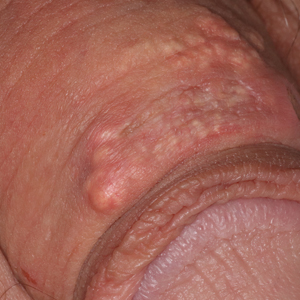
Multinodular Plaque on the Penis
The Diagnosis: Tophaceous Gout
Biopsy revealed amorphous pink material within the center of palisading granulomas lined by histiocytes and giant cells. Scattered crystal remnants also were identified within the center of the granulomas; however, the majority of the crystals were dissolved during the formalin processing of the tissue to become the amorphous material. A perivascular mixed inflammatory infiltrate composed of lymphocytes, histiocytes, and plasma cells surrounded the tophi nodules. A biopsy confirmed the diagnosis of tophaceous gout (Figure).
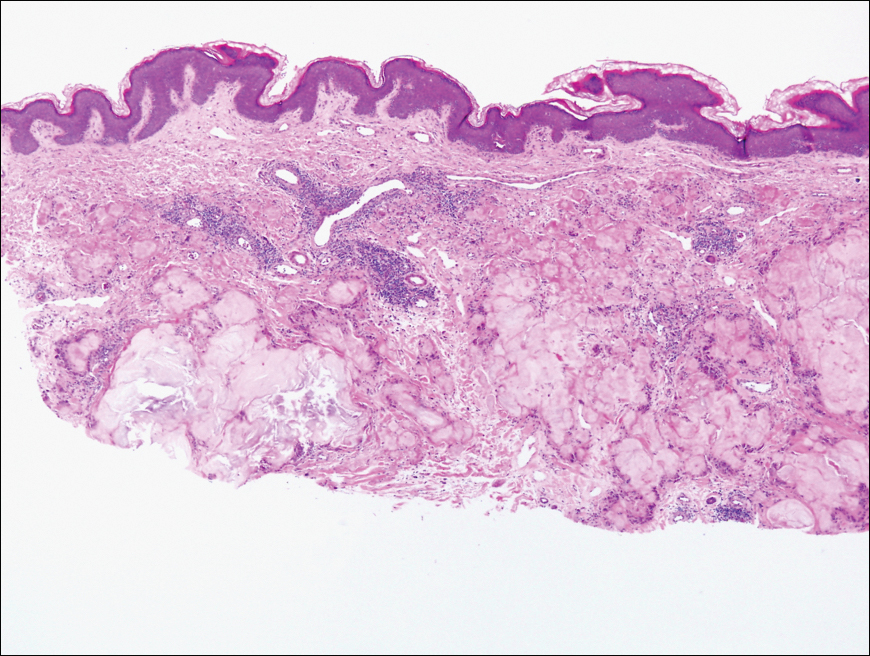
Gout is a systemic metabolic disease characterized by the supersaturation of monosodium urate (MSU) crystals in joints and bursae. Peripheral joints most commonly are affected due to the poor solubility of MSU crystals at low temperatures.1 It is one of the most common forms of inflammatory arthritis, with an estimated prevalence of 4% of adults in the United States.2 An estimated $1 billion is spent each year on ambulatory care for gout.3 Gout occurs most commonly in men and usually manifests in the fifth or sixth decades of life.4 Risk factors for the development of gout include obesity, hypertension, poor dietary habits and kidney function, excessive alcohol intake, and diuretic use.3
Disease manifestations range from asymptomatic hyperuricemia to acute gouty arthritis and chronic tophaceous gout. Patients may present with chronic tophaceous gout without a prior clinically apparent acute gout episode.5,6 Uncontrolled gout may result in large accumulations of MSU crystals, leading to well-circumscribed masses (known as tophi), as demonstrated in our patient.1 Tophi are pathognomonic features of gout and are the sine qua non of advanced gout (also known as chronic tophaceous gout).2 Clinically, these tophi appear as subcutaneous, yellowish white, firm and smooth nodules that are highlighted on the skin.4 Tophi most commonly are found on the helix, articular and periarticular tissue, and the tissue of the hands and feet. They usually are visible on physical examination but also may be detected on imaging studies.2,4
Gouty tophi have been reported in extraordinary locations, such as in sclerae; vocal cords; heart valves; abdominal striae; nerves; axial skeleton4,7; and the penis, as in our patient and one other case.2 These gouty deposits can appear similarly to lipomas, rheumatoid and osteoarthritic nodules, and infectious and malignant processes.1,5 When tophi present in unusual locations, tissue biopsy often is necessary to confirm the diagnosis. Tissue preservation in alcohol is required to preserve the urate crystals. Microscopically, urate crystals appear as tightly packed, brown, needle-shaped crystals surrounded by granulomatous inflammation with foreign body giant cells, macrophages, and possibly some fibrosis. When examined under polarized light, the MSU crystals are negatively birefringent. However, when clinical suspicion for gout is low and the tissue is instead formalin fixed, as was performed in our case, the crystals dissolve into fibrillary amorphous deposits within the center of the granulomatous inflammation, which is another characteristic histologic finding in tophaceous gout.8
Management of gout focuses on urate-lowering therapy including lifestyle changes. Lower serum urate levels are associated with a decreased incidence of acute gout attacks and chronic tophaceous gout.2 Urate-lowering drugs often are combined with anti-inflammatory drugs during acute attacks. Lifestyle changes, such as weight loss, exercise, reduced alcohol consumption, high fluid intake, and a low-purine diet also are beneficial.3,4 Although gout cannot be cured, it can be effectively managed, and appropriate treatment can improve quality of life and reduce the risk for permanent joint damage and structural deformities. If medical treatment and lifestyle changes fail to adequately control tophaceous gout or if tophi become symptomatic, surgical removal of tophi is appropriate.4
At follow-up, our patient opted for surgical removal of the penile tophi. Using local anesthesia, surgical debulking via curettage was performed. Open defects were closed with fine absorbable sutures, and prophylactic antibiotics were given. Allopurinol also was started. Six weeks following extraction, the patient reported no complications and the area was continuing to heal.
Tophaceous gout would be distinguished from conditions in the differential diagnosis based on histologic findings from hematoxylin and eosin (H&E)-stained sections. Actinomycotic mycetoma is rare in the United States and is characterized by a seropurulent or stringy exudate with grains, ulcerations, melicerous scabs, and retractable scarring.9 On H&E-stained sections, actinomyces appear filamentous with deeply basophilic staining and radially oriented acidophilic projections.10 Calcinosis cutis of the penis has been reported to appear as asymptomatic papules; however, microscopic sections reveal deeply basophilic calcium deposits within the tissue.11 Multinodular syphilis shows characteristic histology with lichenoid or vacuolar interface dermatitis, slender acanthosis, plasma cells, and endothelial swelling of the small vessels. A Treponema pallidum immunoperoxidase stain shows numerous organisms. Planar xanthoma shows xanthomatous or foamy histiocytes throughout the dermis on H&E-stained sections.12
- Ragab G, Elshahaly M, Bardin T. Gout: an old disease in new perspective--a review. J Adv Res. 2007;8:495-511.
- Flores Martín JF, Vázquez Alonso F, Puche Sanz I, et al. Gouty tophi in the penis: a case report and review of the literature. Case Rep Urol. 2012;2012:594905.
- Qaseem A, Harris RP, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Management of acute and recurrent gout: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:58-68.
- Forbess LJ, Fields TR. The broad spectrum of urate crystal deposition: unusual presentations of gouty tophi. Semin Arthritis Rheum. 2012;42:146-154.
- Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64:1431-1446.
- Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. part 2: therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res. 2012;64:1447-1461.
- Gaviria JL, Ortega VG, Gaona J. Unusual dermatological manifestations of gout: review of literature and a case report. Plast Reconstr Surg Glob Open. 2015;3:E445.
- Patterson JW, Hosler GA, Weedon D. Weedon's Skin Pathology. Edinburgh, Scotland: Churchill Livingstone/Elsevier; 2016.
- Guerra-Leal JD, Medrano-Danés LA, Montemayor-Martinez A, et al. The importance of diagnostic imaging of mycetoma in the foot [published online December 18, 2018]. Int J Dermatol. 2019;58:600-604.
- Fazeli MS, Bateni H. Actinomycosis: a rare soft tissue infection. Dermatol Online J. 2005;11:18.
- Cohen PR, Tschen JA. Idiopathic calcinosis cutis of the penis. J Clin Aesthet Dermatol. 2012;5:23-30.
- Ko C, Elston DM, Ferringer T. Dermatopathology. 3rd ed. Philadelphia, PA: Elsevier; 2019.
The Diagnosis: Tophaceous Gout
Biopsy revealed amorphous pink material within the center of palisading granulomas lined by histiocytes and giant cells. Scattered crystal remnants also were identified within the center of the granulomas; however, the majority of the crystals were dissolved during the formalin processing of the tissue to become the amorphous material. A perivascular mixed inflammatory infiltrate composed of lymphocytes, histiocytes, and plasma cells surrounded the tophi nodules. A biopsy confirmed the diagnosis of tophaceous gout (Figure).

Gout is a systemic metabolic disease characterized by the supersaturation of monosodium urate (MSU) crystals in joints and bursae. Peripheral joints most commonly are affected due to the poor solubility of MSU crystals at low temperatures.1 It is one of the most common forms of inflammatory arthritis, with an estimated prevalence of 4% of adults in the United States.2 An estimated $1 billion is spent each year on ambulatory care for gout.3 Gout occurs most commonly in men and usually manifests in the fifth or sixth decades of life.4 Risk factors for the development of gout include obesity, hypertension, poor dietary habits and kidney function, excessive alcohol intake, and diuretic use.3
Disease manifestations range from asymptomatic hyperuricemia to acute gouty arthritis and chronic tophaceous gout. Patients may present with chronic tophaceous gout without a prior clinically apparent acute gout episode.5,6 Uncontrolled gout may result in large accumulations of MSU crystals, leading to well-circumscribed masses (known as tophi), as demonstrated in our patient.1 Tophi are pathognomonic features of gout and are the sine qua non of advanced gout (also known as chronic tophaceous gout).2 Clinically, these tophi appear as subcutaneous, yellowish white, firm and smooth nodules that are highlighted on the skin.4 Tophi most commonly are found on the helix, articular and periarticular tissue, and the tissue of the hands and feet. They usually are visible on physical examination but also may be detected on imaging studies.2,4
Gouty tophi have been reported in extraordinary locations, such as in sclerae; vocal cords; heart valves; abdominal striae; nerves; axial skeleton4,7; and the penis, as in our patient and one other case.2 These gouty deposits can appear similarly to lipomas, rheumatoid and osteoarthritic nodules, and infectious and malignant processes.1,5 When tophi present in unusual locations, tissue biopsy often is necessary to confirm the diagnosis. Tissue preservation in alcohol is required to preserve the urate crystals. Microscopically, urate crystals appear as tightly packed, brown, needle-shaped crystals surrounded by granulomatous inflammation with foreign body giant cells, macrophages, and possibly some fibrosis. When examined under polarized light, the MSU crystals are negatively birefringent. However, when clinical suspicion for gout is low and the tissue is instead formalin fixed, as was performed in our case, the crystals dissolve into fibrillary amorphous deposits within the center of the granulomatous inflammation, which is another characteristic histologic finding in tophaceous gout.8
Management of gout focuses on urate-lowering therapy including lifestyle changes. Lower serum urate levels are associated with a decreased incidence of acute gout attacks and chronic tophaceous gout.2 Urate-lowering drugs often are combined with anti-inflammatory drugs during acute attacks. Lifestyle changes, such as weight loss, exercise, reduced alcohol consumption, high fluid intake, and a low-purine diet also are beneficial.3,4 Although gout cannot be cured, it can be effectively managed, and appropriate treatment can improve quality of life and reduce the risk for permanent joint damage and structural deformities. If medical treatment and lifestyle changes fail to adequately control tophaceous gout or if tophi become symptomatic, surgical removal of tophi is appropriate.4
At follow-up, our patient opted for surgical removal of the penile tophi. Using local anesthesia, surgical debulking via curettage was performed. Open defects were closed with fine absorbable sutures, and prophylactic antibiotics were given. Allopurinol also was started. Six weeks following extraction, the patient reported no complications and the area was continuing to heal.
Tophaceous gout would be distinguished from conditions in the differential diagnosis based on histologic findings from hematoxylin and eosin (H&E)-stained sections. Actinomycotic mycetoma is rare in the United States and is characterized by a seropurulent or stringy exudate with grains, ulcerations, melicerous scabs, and retractable scarring.9 On H&E-stained sections, actinomyces appear filamentous with deeply basophilic staining and radially oriented acidophilic projections.10 Calcinosis cutis of the penis has been reported to appear as asymptomatic papules; however, microscopic sections reveal deeply basophilic calcium deposits within the tissue.11 Multinodular syphilis shows characteristic histology with lichenoid or vacuolar interface dermatitis, slender acanthosis, plasma cells, and endothelial swelling of the small vessels. A Treponema pallidum immunoperoxidase stain shows numerous organisms. Planar xanthoma shows xanthomatous or foamy histiocytes throughout the dermis on H&E-stained sections.12
The Diagnosis: Tophaceous Gout
Biopsy revealed amorphous pink material within the center of palisading granulomas lined by histiocytes and giant cells. Scattered crystal remnants also were identified within the center of the granulomas; however, the majority of the crystals were dissolved during the formalin processing of the tissue to become the amorphous material. A perivascular mixed inflammatory infiltrate composed of lymphocytes, histiocytes, and plasma cells surrounded the tophi nodules. A biopsy confirmed the diagnosis of tophaceous gout (Figure).

Gout is a systemic metabolic disease characterized by the supersaturation of monosodium urate (MSU) crystals in joints and bursae. Peripheral joints most commonly are affected due to the poor solubility of MSU crystals at low temperatures.1 It is one of the most common forms of inflammatory arthritis, with an estimated prevalence of 4% of adults in the United States.2 An estimated $1 billion is spent each year on ambulatory care for gout.3 Gout occurs most commonly in men and usually manifests in the fifth or sixth decades of life.4 Risk factors for the development of gout include obesity, hypertension, poor dietary habits and kidney function, excessive alcohol intake, and diuretic use.3
Disease manifestations range from asymptomatic hyperuricemia to acute gouty arthritis and chronic tophaceous gout. Patients may present with chronic tophaceous gout without a prior clinically apparent acute gout episode.5,6 Uncontrolled gout may result in large accumulations of MSU crystals, leading to well-circumscribed masses (known as tophi), as demonstrated in our patient.1 Tophi are pathognomonic features of gout and are the sine qua non of advanced gout (also known as chronic tophaceous gout).2 Clinically, these tophi appear as subcutaneous, yellowish white, firm and smooth nodules that are highlighted on the skin.4 Tophi most commonly are found on the helix, articular and periarticular tissue, and the tissue of the hands and feet. They usually are visible on physical examination but also may be detected on imaging studies.2,4
Gouty tophi have been reported in extraordinary locations, such as in sclerae; vocal cords; heart valves; abdominal striae; nerves; axial skeleton4,7; and the penis, as in our patient and one other case.2 These gouty deposits can appear similarly to lipomas, rheumatoid and osteoarthritic nodules, and infectious and malignant processes.1,5 When tophi present in unusual locations, tissue biopsy often is necessary to confirm the diagnosis. Tissue preservation in alcohol is required to preserve the urate crystals. Microscopically, urate crystals appear as tightly packed, brown, needle-shaped crystals surrounded by granulomatous inflammation with foreign body giant cells, macrophages, and possibly some fibrosis. When examined under polarized light, the MSU crystals are negatively birefringent. However, when clinical suspicion for gout is low and the tissue is instead formalin fixed, as was performed in our case, the crystals dissolve into fibrillary amorphous deposits within the center of the granulomatous inflammation, which is another characteristic histologic finding in tophaceous gout.8
Management of gout focuses on urate-lowering therapy including lifestyle changes. Lower serum urate levels are associated with a decreased incidence of acute gout attacks and chronic tophaceous gout.2 Urate-lowering drugs often are combined with anti-inflammatory drugs during acute attacks. Lifestyle changes, such as weight loss, exercise, reduced alcohol consumption, high fluid intake, and a low-purine diet also are beneficial.3,4 Although gout cannot be cured, it can be effectively managed, and appropriate treatment can improve quality of life and reduce the risk for permanent joint damage and structural deformities. If medical treatment and lifestyle changes fail to adequately control tophaceous gout or if tophi become symptomatic, surgical removal of tophi is appropriate.4
At follow-up, our patient opted for surgical removal of the penile tophi. Using local anesthesia, surgical debulking via curettage was performed. Open defects were closed with fine absorbable sutures, and prophylactic antibiotics were given. Allopurinol also was started. Six weeks following extraction, the patient reported no complications and the area was continuing to heal.
Tophaceous gout would be distinguished from conditions in the differential diagnosis based on histologic findings from hematoxylin and eosin (H&E)-stained sections. Actinomycotic mycetoma is rare in the United States and is characterized by a seropurulent or stringy exudate with grains, ulcerations, melicerous scabs, and retractable scarring.9 On H&E-stained sections, actinomyces appear filamentous with deeply basophilic staining and radially oriented acidophilic projections.10 Calcinosis cutis of the penis has been reported to appear as asymptomatic papules; however, microscopic sections reveal deeply basophilic calcium deposits within the tissue.11 Multinodular syphilis shows characteristic histology with lichenoid or vacuolar interface dermatitis, slender acanthosis, plasma cells, and endothelial swelling of the small vessels. A Treponema pallidum immunoperoxidase stain shows numerous organisms. Planar xanthoma shows xanthomatous or foamy histiocytes throughout the dermis on H&E-stained sections.12
- Ragab G, Elshahaly M, Bardin T. Gout: an old disease in new perspective--a review. J Adv Res. 2007;8:495-511.
- Flores Martín JF, Vázquez Alonso F, Puche Sanz I, et al. Gouty tophi in the penis: a case report and review of the literature. Case Rep Urol. 2012;2012:594905.
- Qaseem A, Harris RP, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Management of acute and recurrent gout: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:58-68.
- Forbess LJ, Fields TR. The broad spectrum of urate crystal deposition: unusual presentations of gouty tophi. Semin Arthritis Rheum. 2012;42:146-154.
- Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64:1431-1446.
- Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. part 2: therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res. 2012;64:1447-1461.
- Gaviria JL, Ortega VG, Gaona J. Unusual dermatological manifestations of gout: review of literature and a case report. Plast Reconstr Surg Glob Open. 2015;3:E445.
- Patterson JW, Hosler GA, Weedon D. Weedon's Skin Pathology. Edinburgh, Scotland: Churchill Livingstone/Elsevier; 2016.
- Guerra-Leal JD, Medrano-Danés LA, Montemayor-Martinez A, et al. The importance of diagnostic imaging of mycetoma in the foot [published online December 18, 2018]. Int J Dermatol. 2019;58:600-604.
- Fazeli MS, Bateni H. Actinomycosis: a rare soft tissue infection. Dermatol Online J. 2005;11:18.
- Cohen PR, Tschen JA. Idiopathic calcinosis cutis of the penis. J Clin Aesthet Dermatol. 2012;5:23-30.
- Ko C, Elston DM, Ferringer T. Dermatopathology. 3rd ed. Philadelphia, PA: Elsevier; 2019.
- Ragab G, Elshahaly M, Bardin T. Gout: an old disease in new perspective--a review. J Adv Res. 2007;8:495-511.
- Flores Martín JF, Vázquez Alonso F, Puche Sanz I, et al. Gouty tophi in the penis: a case report and review of the literature. Case Rep Urol. 2012;2012:594905.
- Qaseem A, Harris RP, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Management of acute and recurrent gout: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:58-68.
- Forbess LJ, Fields TR. The broad spectrum of urate crystal deposition: unusual presentations of gouty tophi. Semin Arthritis Rheum. 2012;42:146-154.
- Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64:1431-1446.
- Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. part 2: therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res. 2012;64:1447-1461.
- Gaviria JL, Ortega VG, Gaona J. Unusual dermatological manifestations of gout: review of literature and a case report. Plast Reconstr Surg Glob Open. 2015;3:E445.
- Patterson JW, Hosler GA, Weedon D. Weedon's Skin Pathology. Edinburgh, Scotland: Churchill Livingstone/Elsevier; 2016.
- Guerra-Leal JD, Medrano-Danés LA, Montemayor-Martinez A, et al. The importance of diagnostic imaging of mycetoma in the foot [published online December 18, 2018]. Int J Dermatol. 2019;58:600-604.
- Fazeli MS, Bateni H. Actinomycosis: a rare soft tissue infection. Dermatol Online J. 2005;11:18.
- Cohen PR, Tschen JA. Idiopathic calcinosis cutis of the penis. J Clin Aesthet Dermatol. 2012;5:23-30.
- Ko C, Elston DM, Ferringer T. Dermatopathology. 3rd ed. Philadelphia, PA: Elsevier; 2019.
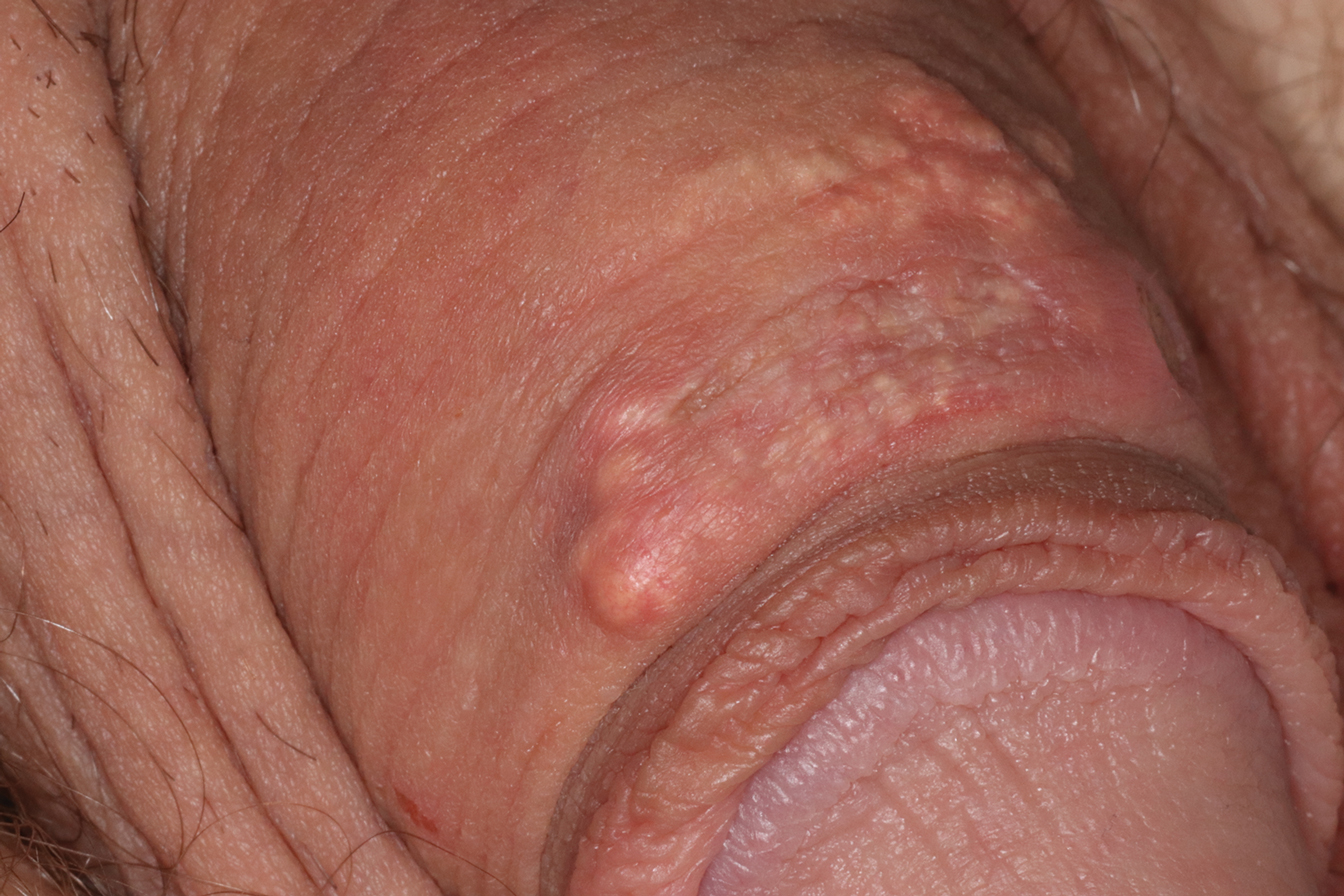
A 34-year-old man presented for evaluation of a slowly growing group of firm white bumps on the penis. The lesions were nontender and asymptomatic. Medical and family history was notable for gout, though he was not being treated. Physical examination revealed a 3-cm, firm, multinodular, chalky white plaque on the dorsal aspect of the penile shaft. A tangential biopsy was performed and sent for hematoxylin and eosin staining.
FDA to allow alternative respiratory devices to treat COVID-19
“Whenever possible, health care facilities should use FDA-cleared conventional/standard full-featured ventilators when necessary to support patients with respiratory failure, or a device subject to an Emergency Use Authorization (EUA), if any,” FDA stated in a guidance document issued March 22.
“However, to help ensure the availability of the greatest possible number of devices for this purpose, ... FDA does not intend to object to limited modifications to indications, claims, functionality, or to the hardware, software, or materials of FDA-cleared devices used to support patients with respiratory failure or respiratory insufficiency, without prior submission of a premarket notification” for the duration of the declared national emergency related to the COVID-19 pandemic.
FDA Commissioner Stephen Hahn, MD, said in a statement that the agency is doing everything it can to support patients, health care professionals, and others during this pandemic.
“One of the most impactful steps we can take is to help with access and availability to life-saving medical treatments,” he said. “Our policy issued today demonstrates our ability to react and adapt quickly during this pandemic and help very ill patients access the lifesaving ventilator support they need. To do that, we are providing maximum regulatory flexibility to facilitate an increase in ventilator inventory, while still providing crucial FDA oversight. We believe this action will immediately increase ventilator availability.”
The document identified examples of where modifications would not create undue risk, including the use of powered emergency ventilators and anesthesia gas machines for patients needing mechanical ventilation; the use of ventilators outside of their cleared environment; the use of devices used to treat patients with sleep apnea, such as CPAPs and BiPAPs, to treat respiratory insufficiency when appropriate design mitigations are in place to minimize aerosolization; and the use of oxygen concentrators for primary supply when medically necessary and clinically appropriate.
The agency also is allowing for changes to the hardware, software, and materials to FDA-cleared ventilators and anesthesia gas machines, such as modifications to motors, batteries, or other electrical components; material changes to components in the gas pathways or with other patient tissue contact; the introduction of filtration to minimize aerosolization; and other hardware and software modifications.
FDA is also allowing for products to be used past their indicated shelf life.
“Whenever possible, health care facilities should use FDA-cleared conventional/standard full-featured ventilators when necessary to support patients with respiratory failure, or a device subject to an Emergency Use Authorization (EUA), if any,” FDA stated in a guidance document issued March 22.
“However, to help ensure the availability of the greatest possible number of devices for this purpose, ... FDA does not intend to object to limited modifications to indications, claims, functionality, or to the hardware, software, or materials of FDA-cleared devices used to support patients with respiratory failure or respiratory insufficiency, without prior submission of a premarket notification” for the duration of the declared national emergency related to the COVID-19 pandemic.
FDA Commissioner Stephen Hahn, MD, said in a statement that the agency is doing everything it can to support patients, health care professionals, and others during this pandemic.
“One of the most impactful steps we can take is to help with access and availability to life-saving medical treatments,” he said. “Our policy issued today demonstrates our ability to react and adapt quickly during this pandemic and help very ill patients access the lifesaving ventilator support they need. To do that, we are providing maximum regulatory flexibility to facilitate an increase in ventilator inventory, while still providing crucial FDA oversight. We believe this action will immediately increase ventilator availability.”
The document identified examples of where modifications would not create undue risk, including the use of powered emergency ventilators and anesthesia gas machines for patients needing mechanical ventilation; the use of ventilators outside of their cleared environment; the use of devices used to treat patients with sleep apnea, such as CPAPs and BiPAPs, to treat respiratory insufficiency when appropriate design mitigations are in place to minimize aerosolization; and the use of oxygen concentrators for primary supply when medically necessary and clinically appropriate.
The agency also is allowing for changes to the hardware, software, and materials to FDA-cleared ventilators and anesthesia gas machines, such as modifications to motors, batteries, or other electrical components; material changes to components in the gas pathways or with other patient tissue contact; the introduction of filtration to minimize aerosolization; and other hardware and software modifications.
FDA is also allowing for products to be used past their indicated shelf life.
“Whenever possible, health care facilities should use FDA-cleared conventional/standard full-featured ventilators when necessary to support patients with respiratory failure, or a device subject to an Emergency Use Authorization (EUA), if any,” FDA stated in a guidance document issued March 22.
“However, to help ensure the availability of the greatest possible number of devices for this purpose, ... FDA does not intend to object to limited modifications to indications, claims, functionality, or to the hardware, software, or materials of FDA-cleared devices used to support patients with respiratory failure or respiratory insufficiency, without prior submission of a premarket notification” for the duration of the declared national emergency related to the COVID-19 pandemic.
FDA Commissioner Stephen Hahn, MD, said in a statement that the agency is doing everything it can to support patients, health care professionals, and others during this pandemic.
“One of the most impactful steps we can take is to help with access and availability to life-saving medical treatments,” he said. “Our policy issued today demonstrates our ability to react and adapt quickly during this pandemic and help very ill patients access the lifesaving ventilator support they need. To do that, we are providing maximum regulatory flexibility to facilitate an increase in ventilator inventory, while still providing crucial FDA oversight. We believe this action will immediately increase ventilator availability.”
The document identified examples of where modifications would not create undue risk, including the use of powered emergency ventilators and anesthesia gas machines for patients needing mechanical ventilation; the use of ventilators outside of their cleared environment; the use of devices used to treat patients with sleep apnea, such as CPAPs and BiPAPs, to treat respiratory insufficiency when appropriate design mitigations are in place to minimize aerosolization; and the use of oxygen concentrators for primary supply when medically necessary and clinically appropriate.
The agency also is allowing for changes to the hardware, software, and materials to FDA-cleared ventilators and anesthesia gas machines, such as modifications to motors, batteries, or other electrical components; material changes to components in the gas pathways or with other patient tissue contact; the introduction of filtration to minimize aerosolization; and other hardware and software modifications.
FDA is also allowing for products to be used past their indicated shelf life.
Live zoster vaccine confers limited protection during tofacitinib therapy
The live attenuated zoster vaccine (Zostavax) does not provide adequate long-term protection in patients with rheumatoid arthritis (RA) starting tofacitinib, suggests the ORAL Sequel extension study.
The incidence of herpes zoster in patients with RA taking tofacitinib (Xeljanz), an oral Janus kinase inhibitor, is about double the rate seen with biologic disease-modifying antirheumatic drugs, noted the investigators, who were led by Kevin L. Winthrop, MD, professor of infectious diseases, ophthalmology, public health, and preventive medicine at Oregon Health & Science University, Portland. The American College of Rheumatology’s guideline for the treatment of RA recommends herpes zoster vaccination before patients aged 50 years or older initiate any of these agents.
The investigators studied 100 patients with RA from an index randomized, placebo-controlled trial of tofacitinib who started the long-term extension study 14 weeks after receiving the live attenuated zoster vaccine. All were given open-label tofacitinib, at 5 or 10 mg two times per day, along with background RA therapy as needed.
With a follow-up of 27 months, five patients (5%) developed herpes zoster, including two treated with the 5-mg dose and three treated with the 10-mg dose, according to results reported in Annals of the Rheumatic Diseases. Cases occurred between 218 and 741 days after vaccination.
Four of the patients had herpes zoster involving a single dermatome, while one had involvement of five dermatomes. All episodes were mild or moderate, and resolved with antiviral therapy.
Humoral and cell-mediated immunity to the varicella zoster virus were assessed with immunoglobulin G titer and an interferon-gamma enzyme-linked immunosorbent spot assay, respectively. Results showed that three of the patients developing herpes zoster had undetectable cell-mediated immunity to the virus at baseline and week 6 after vaccination. The other two patients had an adequate humoral and cell-mediated immune response to the vaccine, as assessed from changes from baseline, but had below-average immunoglobulin G titer at baseline and week 6.
“These results suggest that [live attenuated zoster vaccine] may not provide adequate long-term protection, as previously demonstrated in healthy individuals aged ≥60 years 3 years post-vaccination, in which [herpes zoster] risk was reduced by 51%,” Dr. Winthrop and colleagues wrote.
“While it is possible that [live attenuated zoster vaccine] booster vaccinations may improve vaccine efficacy, to date there is a lack of data on the use and timing of booster vaccinations, and no recommendations on the use of [live attenuated zoster vaccine] booster vaccinations currently exist,” they concluded. “This highlights the importance of evaluating the newly approved subunit non-live vaccine (Shingrix) in patients with RA receiving tofacitinib.”
The study was sponsored by Pfizer. Dr. Winthrop disclosed consulting for AbbVie, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, Pfizer, and UCB and receiving grant/research support from Bristol-Myers Squibb. Two coauthors disclosed financial relationships with Pfizer and other pharmaceutical companies, and the other seven coauthors were employees and shareholders of Pfizer.
SOURCE: Winthrop KL et al. Ann Rheum Dis. 2020 Mar 11. doi: 10.1136/annrheumdis-2019-216566.
The live attenuated zoster vaccine (Zostavax) does not provide adequate long-term protection in patients with rheumatoid arthritis (RA) starting tofacitinib, suggests the ORAL Sequel extension study.
The incidence of herpes zoster in patients with RA taking tofacitinib (Xeljanz), an oral Janus kinase inhibitor, is about double the rate seen with biologic disease-modifying antirheumatic drugs, noted the investigators, who were led by Kevin L. Winthrop, MD, professor of infectious diseases, ophthalmology, public health, and preventive medicine at Oregon Health & Science University, Portland. The American College of Rheumatology’s guideline for the treatment of RA recommends herpes zoster vaccination before patients aged 50 years or older initiate any of these agents.
The investigators studied 100 patients with RA from an index randomized, placebo-controlled trial of tofacitinib who started the long-term extension study 14 weeks after receiving the live attenuated zoster vaccine. All were given open-label tofacitinib, at 5 or 10 mg two times per day, along with background RA therapy as needed.
With a follow-up of 27 months, five patients (5%) developed herpes zoster, including two treated with the 5-mg dose and three treated with the 10-mg dose, according to results reported in Annals of the Rheumatic Diseases. Cases occurred between 218 and 741 days after vaccination.
Four of the patients had herpes zoster involving a single dermatome, while one had involvement of five dermatomes. All episodes were mild or moderate, and resolved with antiviral therapy.
Humoral and cell-mediated immunity to the varicella zoster virus were assessed with immunoglobulin G titer and an interferon-gamma enzyme-linked immunosorbent spot assay, respectively. Results showed that three of the patients developing herpes zoster had undetectable cell-mediated immunity to the virus at baseline and week 6 after vaccination. The other two patients had an adequate humoral and cell-mediated immune response to the vaccine, as assessed from changes from baseline, but had below-average immunoglobulin G titer at baseline and week 6.
“These results suggest that [live attenuated zoster vaccine] may not provide adequate long-term protection, as previously demonstrated in healthy individuals aged ≥60 years 3 years post-vaccination, in which [herpes zoster] risk was reduced by 51%,” Dr. Winthrop and colleagues wrote.
“While it is possible that [live attenuated zoster vaccine] booster vaccinations may improve vaccine efficacy, to date there is a lack of data on the use and timing of booster vaccinations, and no recommendations on the use of [live attenuated zoster vaccine] booster vaccinations currently exist,” they concluded. “This highlights the importance of evaluating the newly approved subunit non-live vaccine (Shingrix) in patients with RA receiving tofacitinib.”
The study was sponsored by Pfizer. Dr. Winthrop disclosed consulting for AbbVie, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, Pfizer, and UCB and receiving grant/research support from Bristol-Myers Squibb. Two coauthors disclosed financial relationships with Pfizer and other pharmaceutical companies, and the other seven coauthors were employees and shareholders of Pfizer.
SOURCE: Winthrop KL et al. Ann Rheum Dis. 2020 Mar 11. doi: 10.1136/annrheumdis-2019-216566.
The live attenuated zoster vaccine (Zostavax) does not provide adequate long-term protection in patients with rheumatoid arthritis (RA) starting tofacitinib, suggests the ORAL Sequel extension study.
The incidence of herpes zoster in patients with RA taking tofacitinib (Xeljanz), an oral Janus kinase inhibitor, is about double the rate seen with biologic disease-modifying antirheumatic drugs, noted the investigators, who were led by Kevin L. Winthrop, MD, professor of infectious diseases, ophthalmology, public health, and preventive medicine at Oregon Health & Science University, Portland. The American College of Rheumatology’s guideline for the treatment of RA recommends herpes zoster vaccination before patients aged 50 years or older initiate any of these agents.
The investigators studied 100 patients with RA from an index randomized, placebo-controlled trial of tofacitinib who started the long-term extension study 14 weeks after receiving the live attenuated zoster vaccine. All were given open-label tofacitinib, at 5 or 10 mg two times per day, along with background RA therapy as needed.
With a follow-up of 27 months, five patients (5%) developed herpes zoster, including two treated with the 5-mg dose and three treated with the 10-mg dose, according to results reported in Annals of the Rheumatic Diseases. Cases occurred between 218 and 741 days after vaccination.
Four of the patients had herpes zoster involving a single dermatome, while one had involvement of five dermatomes. All episodes were mild or moderate, and resolved with antiviral therapy.
Humoral and cell-mediated immunity to the varicella zoster virus were assessed with immunoglobulin G titer and an interferon-gamma enzyme-linked immunosorbent spot assay, respectively. Results showed that three of the patients developing herpes zoster had undetectable cell-mediated immunity to the virus at baseline and week 6 after vaccination. The other two patients had an adequate humoral and cell-mediated immune response to the vaccine, as assessed from changes from baseline, but had below-average immunoglobulin G titer at baseline and week 6.
“These results suggest that [live attenuated zoster vaccine] may not provide adequate long-term protection, as previously demonstrated in healthy individuals aged ≥60 years 3 years post-vaccination, in which [herpes zoster] risk was reduced by 51%,” Dr. Winthrop and colleagues wrote.
“While it is possible that [live attenuated zoster vaccine] booster vaccinations may improve vaccine efficacy, to date there is a lack of data on the use and timing of booster vaccinations, and no recommendations on the use of [live attenuated zoster vaccine] booster vaccinations currently exist,” they concluded. “This highlights the importance of evaluating the newly approved subunit non-live vaccine (Shingrix) in patients with RA receiving tofacitinib.”
The study was sponsored by Pfizer. Dr. Winthrop disclosed consulting for AbbVie, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, Pfizer, and UCB and receiving grant/research support from Bristol-Myers Squibb. Two coauthors disclosed financial relationships with Pfizer and other pharmaceutical companies, and the other seven coauthors were employees and shareholders of Pfizer.
SOURCE: Winthrop KL et al. Ann Rheum Dis. 2020 Mar 11. doi: 10.1136/annrheumdis-2019-216566.
FROM ANNALS OF THE RHEUMATIC DISEASES
Survey explores the role of social media in choosing a dermatologist
Fewer than one-quarter of consumers rely heavily on social media for choosing a dermatologist, results from an online survey suggest.
In a video presentation during a virtual meeting held by the George Washington University department of dermatology, Kamaria Nelson, MD, said that, as of 2019, 79% of Americans have a social media account, with the majority using platforms such as Facebook, Instagram, Twitter, and YouTube. “There’s also a high predominance of social media use in the dermatology field, with many dermatologists assuming that it will improve their personal brands,” said Dr. Nelson, a research fellow in the department of dermatology at George Washington University, Washington. “Some even hire social media managers to monitor online reviews and mitigate any damage. So, although social media is commonly used, it’s unknown if it impacts patient knowledge, access to health care, or provider choice.”
To evaluate how social media influences patients when choosing a dermatologist, she and her colleagues used Survey Monkey to create a 10-item questionnaire that they distributed to 1,481 individuals in the general U.S. population in May 2019. Individuals qualified for the study if they used social media and if they had ever been to a dermatologist. Dr. Nelson reported that 726 individuals (58%) qualified for the survey and 715 completed it, for a response rate of 98%. The researchers used Chi-square tests to compare frequency and importance of social media by visit type, age, gender, and educational level.
When the respondents were stratified by visit type, 43% who saw a dermatologist for cosmetic reasons were more likely to view social media as “extremely important” or “very important,” compared with 15% of patients who saw a dermatologist for medical reasons (P less than .0001).
When stratified by age, about 12% of respondents between the ages of 18 and 44 years considered social media as extremely important when choosing a dermatologist, compared with only 9% of those aged 45-60 years and about 2% of those older than age 60 (P less than .0001).
When stratified by educational level, 30% of respondents with a high school degree or less were more likely to view social media as extremely important or very important when choosing a dermatologist, while 62% of those with more than a high school degree were more likely to view social media as “not at all important” or only “slightly important” (P = .0006).
One of the survey questions was, “When choosing a dermatologist to see, how important is his or her social media site?” Only 9% of respondents said extremely important, 13% said very important, 21% said “moderately important,” 24% said slightly important, and 33% said not at all important. “This left about 22% of respondents who viewed the social media site as extremely important or very important when choosing a dermatologist,” Dr. Nelson said.
Factors deemed important on a dermatologist’s social media profile were patient reviews (68%), years of experience (61%), and the amount of medical information written by the dermatologist (59%).
“There seems to be a low reliance on social media when selecting a dermatologist,” Dr. Nelson concluded. “We also found that cosmetic patients, patients with lower levels of education, and younger patients were more likely to value social media. Therefore, social media may only be useful for targeting specific patient populations. When doing so, medical information written by a provider is most often desired.”
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic. Dr. Nelson reported having no disclosures.
Fewer than one-quarter of consumers rely heavily on social media for choosing a dermatologist, results from an online survey suggest.
In a video presentation during a virtual meeting held by the George Washington University department of dermatology, Kamaria Nelson, MD, said that, as of 2019, 79% of Americans have a social media account, with the majority using platforms such as Facebook, Instagram, Twitter, and YouTube. “There’s also a high predominance of social media use in the dermatology field, with many dermatologists assuming that it will improve their personal brands,” said Dr. Nelson, a research fellow in the department of dermatology at George Washington University, Washington. “Some even hire social media managers to monitor online reviews and mitigate any damage. So, although social media is commonly used, it’s unknown if it impacts patient knowledge, access to health care, or provider choice.”
To evaluate how social media influences patients when choosing a dermatologist, she and her colleagues used Survey Monkey to create a 10-item questionnaire that they distributed to 1,481 individuals in the general U.S. population in May 2019. Individuals qualified for the study if they used social media and if they had ever been to a dermatologist. Dr. Nelson reported that 726 individuals (58%) qualified for the survey and 715 completed it, for a response rate of 98%. The researchers used Chi-square tests to compare frequency and importance of social media by visit type, age, gender, and educational level.
When the respondents were stratified by visit type, 43% who saw a dermatologist for cosmetic reasons were more likely to view social media as “extremely important” or “very important,” compared with 15% of patients who saw a dermatologist for medical reasons (P less than .0001).
When stratified by age, about 12% of respondents between the ages of 18 and 44 years considered social media as extremely important when choosing a dermatologist, compared with only 9% of those aged 45-60 years and about 2% of those older than age 60 (P less than .0001).
When stratified by educational level, 30% of respondents with a high school degree or less were more likely to view social media as extremely important or very important when choosing a dermatologist, while 62% of those with more than a high school degree were more likely to view social media as “not at all important” or only “slightly important” (P = .0006).
One of the survey questions was, “When choosing a dermatologist to see, how important is his or her social media site?” Only 9% of respondents said extremely important, 13% said very important, 21% said “moderately important,” 24% said slightly important, and 33% said not at all important. “This left about 22% of respondents who viewed the social media site as extremely important or very important when choosing a dermatologist,” Dr. Nelson said.
Factors deemed important on a dermatologist’s social media profile were patient reviews (68%), years of experience (61%), and the amount of medical information written by the dermatologist (59%).
“There seems to be a low reliance on social media when selecting a dermatologist,” Dr. Nelson concluded. “We also found that cosmetic patients, patients with lower levels of education, and younger patients were more likely to value social media. Therefore, social media may only be useful for targeting specific patient populations. When doing so, medical information written by a provider is most often desired.”
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic. Dr. Nelson reported having no disclosures.
Fewer than one-quarter of consumers rely heavily on social media for choosing a dermatologist, results from an online survey suggest.
In a video presentation during a virtual meeting held by the George Washington University department of dermatology, Kamaria Nelson, MD, said that, as of 2019, 79% of Americans have a social media account, with the majority using platforms such as Facebook, Instagram, Twitter, and YouTube. “There’s also a high predominance of social media use in the dermatology field, with many dermatologists assuming that it will improve their personal brands,” said Dr. Nelson, a research fellow in the department of dermatology at George Washington University, Washington. “Some even hire social media managers to monitor online reviews and mitigate any damage. So, although social media is commonly used, it’s unknown if it impacts patient knowledge, access to health care, or provider choice.”
To evaluate how social media influences patients when choosing a dermatologist, she and her colleagues used Survey Monkey to create a 10-item questionnaire that they distributed to 1,481 individuals in the general U.S. population in May 2019. Individuals qualified for the study if they used social media and if they had ever been to a dermatologist. Dr. Nelson reported that 726 individuals (58%) qualified for the survey and 715 completed it, for a response rate of 98%. The researchers used Chi-square tests to compare frequency and importance of social media by visit type, age, gender, and educational level.
When the respondents were stratified by visit type, 43% who saw a dermatologist for cosmetic reasons were more likely to view social media as “extremely important” or “very important,” compared with 15% of patients who saw a dermatologist for medical reasons (P less than .0001).
When stratified by age, about 12% of respondents between the ages of 18 and 44 years considered social media as extremely important when choosing a dermatologist, compared with only 9% of those aged 45-60 years and about 2% of those older than age 60 (P less than .0001).
When stratified by educational level, 30% of respondents with a high school degree or less were more likely to view social media as extremely important or very important when choosing a dermatologist, while 62% of those with more than a high school degree were more likely to view social media as “not at all important” or only “slightly important” (P = .0006).
One of the survey questions was, “When choosing a dermatologist to see, how important is his or her social media site?” Only 9% of respondents said extremely important, 13% said very important, 21% said “moderately important,” 24% said slightly important, and 33% said not at all important. “This left about 22% of respondents who viewed the social media site as extremely important or very important when choosing a dermatologist,” Dr. Nelson said.
Factors deemed important on a dermatologist’s social media profile were patient reviews (68%), years of experience (61%), and the amount of medical information written by the dermatologist (59%).
“There seems to be a low reliance on social media when selecting a dermatologist,” Dr. Nelson concluded. “We also found that cosmetic patients, patients with lower levels of education, and younger patients were more likely to value social media. Therefore, social media may only be useful for targeting specific patient populations. When doing so, medical information written by a provider is most often desired.”
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic. Dr. Nelson reported having no disclosures.
Stronger links forged between RA and asthma, COPD
Asthma and chronic obstructive pulmonary disease were both linked to an increased risk of rheumatoid arthritis in a recent large, prospective cohort study, researchers have reported, which adds to a growing body of evidence that airway inflammation is implicated in the development of this disease.
RA risk was increased by about 50% among asthma patients, even when excluding those who had ever smoked, according to the study’s results, which were based on more than 200,000 women in the Nurses’ Health Study I and II.
Risk of RA nearly doubled among those with chronic obstructive pulmonary disease (COPD), with an even stronger association seen in older ever-smokers, according to authors of the study.
The findings not only strengthen the case for the potential role of obstructive lung diseases in RA development, according to the study’s authors, but also suggest that health care providers need to lower the bar for evaluation of patients with lung diseases and inflammatory joint symptoms.
“If these patients develop arthralgias, then the clinicians taking care of them should have a low threshold to consider RA, and perhaps refer, or check these patients with a diagnostic test for RA,” said researcher Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital and Harvard Medical School in Boston.
What’s perhaps not as clear now is whether screening obstructive lung disease patients in the absence of early RA signs would be warranted: “I don’t know if we’re quite at the point where we would need to screen these patients if they’re not symptomatic,” Dr. Sparks said in an interview.
The study by Dr. Sparks and colleagues is, by far, not the first study to implicate asthma or other lung conditions in RA development. However, most previous studies are retrospective, and interpretation of the findings has been subject to limitations such as inadequate power to detect an increased risk or lack of adjustment for important confounding factors, such as smoking.
As such, the study by Dr. Sparks and colleagues is believed to be the first-ever prospective study to evaluate asthma and COPD as risk factors for RA, study authors reported in Arthritis & Rheumatology.
Researchers were able to identify 1,060 incident RA cases that developed in 15,148 women with asthma and 3,573 with COPD in the study with more than 4 million person-years of follow-up.
The association between asthma and increased RA risk was seen not only for the asthma population as a whole (hazard ratio, 1.53; 95% confidence interval, 1.24-1.88), but also for the subset of women who had never smoked, to a similar degree (HR, 1.53; 95% CI, 1.14-2.05), the report shows.
COPD’s association with RA risk was apparent overall (HR, 1.89; 95% CI, 1.31-2.75) and even more so in the subgroup of ever-smokers 55 years of age and older (HR, 2.20; 95% CI, 1.38-3.51), the data further show.
Findings of studies looking at the inflammation of airways and other mucosal sites are “critically important to understand” when it comes to trying to prevent RA, said Kevin Deane, MD, of the University of Colorado at Denver at Aurora.
“If we indeed are trying to prevent rheumatoid arthritis in terms of the joint disease, we may need to look at these mucosal sites in individuals who don’t yet have joint disease as potential sites to target for prevention, or at least areas to study to understand how prevention may work,” said Dr. Deane, principal investigator on the National Institutes of Health–funded Strategy for the Prevention of RA (StopRA) trial.
With that in mind, it’s conceivable targeting a lung process might prevent joint disease in a patient with asthma or airway inflammation and blood markers that indicate a risk of RA, Dr. Deane said in an interview.
Blood markers of RA have been evaluated in some recent studies, with findings that provide further evidence of a link between lung diseases and RA, and vice versa.
In particular, anti–citrullinated protein antibodies (ACPA) are clearly central to RA pathogenesis. And while asthma is increasingly linked to RA risk, there have been relatively little data on any potential links between ACPA and asthma.
That research gap led to a case-control study of the Nurses’ Health Study I and II (on which Dr. Sparks was senior author) showing that asthma was strongly linked to elevated ACPA in blood drawn from patients prior to a diagnosis of RA.
Results, published last year in Arthritis Research & Therapy, showed a significant association between asthma and pre-RA ACPA elevation (odds ratio, 3.57; 95% CI, 1.58-8.04), after adjustment for smoking and other potentially confounding factors. Investigators said the findings provided evidence that chronic mucosal airway inflammation is a factor in the development of ACPA and in the pathogenesis of RA.
In a follow-up study published more recently in Arthritis Care & Research, investigators showed that, among women in the Nurses Health Study I and II, pre-RA ACPA elevation was linked to increased risk of COPD, compared with controls (HR, 3.04; 95% CI, 1.33-7.00), while the risk for development of asthma was similar in women with or without elevated pre-RA ACPA.
That study was in part an attempt to establish a “timeline” related to antibodies, lung diseases, and RA onset, Dr. Sparks said in the interview.
“We think that probably the asthma is more important in developing the antibody, but that once you have the antibody, if you didn’t have asthma by then, you’re unlikely to develop it,” he said. “So asthma seems to be something that could happen before the antibody production, whereas COPD seems to happen after – but ACPA seems to be the common link in both of these obstructive lung diseases.”
The study in Arthritis & Rheumatology linking asthma and COPD to risk of incident RA was supported by the National Institutes of Health. Dr. Sparks reported grant support from Amgen and Bristol Myers Squibb and consulting fees from Inova and Optum. Coauthors provided disclosures related to GlaxoSmithKline, AstraZeneca, Merck, Neutrolis, and Genentech.
SOURCE: Ford JA et al. Arthritis Rheumatol. 2020 Mar 4. doi: 10.1002/art.41194.
Asthma and chronic obstructive pulmonary disease were both linked to an increased risk of rheumatoid arthritis in a recent large, prospective cohort study, researchers have reported, which adds to a growing body of evidence that airway inflammation is implicated in the development of this disease.
RA risk was increased by about 50% among asthma patients, even when excluding those who had ever smoked, according to the study’s results, which were based on more than 200,000 women in the Nurses’ Health Study I and II.
Risk of RA nearly doubled among those with chronic obstructive pulmonary disease (COPD), with an even stronger association seen in older ever-smokers, according to authors of the study.
The findings not only strengthen the case for the potential role of obstructive lung diseases in RA development, according to the study’s authors, but also suggest that health care providers need to lower the bar for evaluation of patients with lung diseases and inflammatory joint symptoms.
“If these patients develop arthralgias, then the clinicians taking care of them should have a low threshold to consider RA, and perhaps refer, or check these patients with a diagnostic test for RA,” said researcher Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital and Harvard Medical School in Boston.
What’s perhaps not as clear now is whether screening obstructive lung disease patients in the absence of early RA signs would be warranted: “I don’t know if we’re quite at the point where we would need to screen these patients if they’re not symptomatic,” Dr. Sparks said in an interview.
The study by Dr. Sparks and colleagues is, by far, not the first study to implicate asthma or other lung conditions in RA development. However, most previous studies are retrospective, and interpretation of the findings has been subject to limitations such as inadequate power to detect an increased risk or lack of adjustment for important confounding factors, such as smoking.
As such, the study by Dr. Sparks and colleagues is believed to be the first-ever prospective study to evaluate asthma and COPD as risk factors for RA, study authors reported in Arthritis & Rheumatology.
Researchers were able to identify 1,060 incident RA cases that developed in 15,148 women with asthma and 3,573 with COPD in the study with more than 4 million person-years of follow-up.
The association between asthma and increased RA risk was seen not only for the asthma population as a whole (hazard ratio, 1.53; 95% confidence interval, 1.24-1.88), but also for the subset of women who had never smoked, to a similar degree (HR, 1.53; 95% CI, 1.14-2.05), the report shows.
COPD’s association with RA risk was apparent overall (HR, 1.89; 95% CI, 1.31-2.75) and even more so in the subgroup of ever-smokers 55 years of age and older (HR, 2.20; 95% CI, 1.38-3.51), the data further show.
Findings of studies looking at the inflammation of airways and other mucosal sites are “critically important to understand” when it comes to trying to prevent RA, said Kevin Deane, MD, of the University of Colorado at Denver at Aurora.
“If we indeed are trying to prevent rheumatoid arthritis in terms of the joint disease, we may need to look at these mucosal sites in individuals who don’t yet have joint disease as potential sites to target for prevention, or at least areas to study to understand how prevention may work,” said Dr. Deane, principal investigator on the National Institutes of Health–funded Strategy for the Prevention of RA (StopRA) trial.
With that in mind, it’s conceivable targeting a lung process might prevent joint disease in a patient with asthma or airway inflammation and blood markers that indicate a risk of RA, Dr. Deane said in an interview.
Blood markers of RA have been evaluated in some recent studies, with findings that provide further evidence of a link between lung diseases and RA, and vice versa.
In particular, anti–citrullinated protein antibodies (ACPA) are clearly central to RA pathogenesis. And while asthma is increasingly linked to RA risk, there have been relatively little data on any potential links between ACPA and asthma.
That research gap led to a case-control study of the Nurses’ Health Study I and II (on which Dr. Sparks was senior author) showing that asthma was strongly linked to elevated ACPA in blood drawn from patients prior to a diagnosis of RA.
Results, published last year in Arthritis Research & Therapy, showed a significant association between asthma and pre-RA ACPA elevation (odds ratio, 3.57; 95% CI, 1.58-8.04), after adjustment for smoking and other potentially confounding factors. Investigators said the findings provided evidence that chronic mucosal airway inflammation is a factor in the development of ACPA and in the pathogenesis of RA.
In a follow-up study published more recently in Arthritis Care & Research, investigators showed that, among women in the Nurses Health Study I and II, pre-RA ACPA elevation was linked to increased risk of COPD, compared with controls (HR, 3.04; 95% CI, 1.33-7.00), while the risk for development of asthma was similar in women with or without elevated pre-RA ACPA.
That study was in part an attempt to establish a “timeline” related to antibodies, lung diseases, and RA onset, Dr. Sparks said in the interview.
“We think that probably the asthma is more important in developing the antibody, but that once you have the antibody, if you didn’t have asthma by then, you’re unlikely to develop it,” he said. “So asthma seems to be something that could happen before the antibody production, whereas COPD seems to happen after – but ACPA seems to be the common link in both of these obstructive lung diseases.”
The study in Arthritis & Rheumatology linking asthma and COPD to risk of incident RA was supported by the National Institutes of Health. Dr. Sparks reported grant support from Amgen and Bristol Myers Squibb and consulting fees from Inova and Optum. Coauthors provided disclosures related to GlaxoSmithKline, AstraZeneca, Merck, Neutrolis, and Genentech.
SOURCE: Ford JA et al. Arthritis Rheumatol. 2020 Mar 4. doi: 10.1002/art.41194.
Asthma and chronic obstructive pulmonary disease were both linked to an increased risk of rheumatoid arthritis in a recent large, prospective cohort study, researchers have reported, which adds to a growing body of evidence that airway inflammation is implicated in the development of this disease.
RA risk was increased by about 50% among asthma patients, even when excluding those who had ever smoked, according to the study’s results, which were based on more than 200,000 women in the Nurses’ Health Study I and II.
Risk of RA nearly doubled among those with chronic obstructive pulmonary disease (COPD), with an even stronger association seen in older ever-smokers, according to authors of the study.
The findings not only strengthen the case for the potential role of obstructive lung diseases in RA development, according to the study’s authors, but also suggest that health care providers need to lower the bar for evaluation of patients with lung diseases and inflammatory joint symptoms.
“If these patients develop arthralgias, then the clinicians taking care of them should have a low threshold to consider RA, and perhaps refer, or check these patients with a diagnostic test for RA,” said researcher Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital and Harvard Medical School in Boston.
What’s perhaps not as clear now is whether screening obstructive lung disease patients in the absence of early RA signs would be warranted: “I don’t know if we’re quite at the point where we would need to screen these patients if they’re not symptomatic,” Dr. Sparks said in an interview.
The study by Dr. Sparks and colleagues is, by far, not the first study to implicate asthma or other lung conditions in RA development. However, most previous studies are retrospective, and interpretation of the findings has been subject to limitations such as inadequate power to detect an increased risk or lack of adjustment for important confounding factors, such as smoking.
As such, the study by Dr. Sparks and colleagues is believed to be the first-ever prospective study to evaluate asthma and COPD as risk factors for RA, study authors reported in Arthritis & Rheumatology.
Researchers were able to identify 1,060 incident RA cases that developed in 15,148 women with asthma and 3,573 with COPD in the study with more than 4 million person-years of follow-up.
The association between asthma and increased RA risk was seen not only for the asthma population as a whole (hazard ratio, 1.53; 95% confidence interval, 1.24-1.88), but also for the subset of women who had never smoked, to a similar degree (HR, 1.53; 95% CI, 1.14-2.05), the report shows.
COPD’s association with RA risk was apparent overall (HR, 1.89; 95% CI, 1.31-2.75) and even more so in the subgroup of ever-smokers 55 years of age and older (HR, 2.20; 95% CI, 1.38-3.51), the data further show.
Findings of studies looking at the inflammation of airways and other mucosal sites are “critically important to understand” when it comes to trying to prevent RA, said Kevin Deane, MD, of the University of Colorado at Denver at Aurora.
“If we indeed are trying to prevent rheumatoid arthritis in terms of the joint disease, we may need to look at these mucosal sites in individuals who don’t yet have joint disease as potential sites to target for prevention, or at least areas to study to understand how prevention may work,” said Dr. Deane, principal investigator on the National Institutes of Health–funded Strategy for the Prevention of RA (StopRA) trial.
With that in mind, it’s conceivable targeting a lung process might prevent joint disease in a patient with asthma or airway inflammation and blood markers that indicate a risk of RA, Dr. Deane said in an interview.
Blood markers of RA have been evaluated in some recent studies, with findings that provide further evidence of a link between lung diseases and RA, and vice versa.
In particular, anti–citrullinated protein antibodies (ACPA) are clearly central to RA pathogenesis. And while asthma is increasingly linked to RA risk, there have been relatively little data on any potential links between ACPA and asthma.
That research gap led to a case-control study of the Nurses’ Health Study I and II (on which Dr. Sparks was senior author) showing that asthma was strongly linked to elevated ACPA in blood drawn from patients prior to a diagnosis of RA.
Results, published last year in Arthritis Research & Therapy, showed a significant association between asthma and pre-RA ACPA elevation (odds ratio, 3.57; 95% CI, 1.58-8.04), after adjustment for smoking and other potentially confounding factors. Investigators said the findings provided evidence that chronic mucosal airway inflammation is a factor in the development of ACPA and in the pathogenesis of RA.
In a follow-up study published more recently in Arthritis Care & Research, investigators showed that, among women in the Nurses Health Study I and II, pre-RA ACPA elevation was linked to increased risk of COPD, compared with controls (HR, 3.04; 95% CI, 1.33-7.00), while the risk for development of asthma was similar in women with or without elevated pre-RA ACPA.
That study was in part an attempt to establish a “timeline” related to antibodies, lung diseases, and RA onset, Dr. Sparks said in the interview.
“We think that probably the asthma is more important in developing the antibody, but that once you have the antibody, if you didn’t have asthma by then, you’re unlikely to develop it,” he said. “So asthma seems to be something that could happen before the antibody production, whereas COPD seems to happen after – but ACPA seems to be the common link in both of these obstructive lung diseases.”
The study in Arthritis & Rheumatology linking asthma and COPD to risk of incident RA was supported by the National Institutes of Health. Dr. Sparks reported grant support from Amgen and Bristol Myers Squibb and consulting fees from Inova and Optum. Coauthors provided disclosures related to GlaxoSmithKline, AstraZeneca, Merck, Neutrolis, and Genentech.
SOURCE: Ford JA et al. Arthritis Rheumatol. 2020 Mar 4. doi: 10.1002/art.41194.
FROM ARTHRITIS & RHEUMATOLOGY