User login
Flu now riding on COVID-19’s coattails
The viral tsunami that is COVID-19 has hit the United States, and influenza appears to be riding the crest of the wave.

according to the Centers for Disease Control. Flu-related visits went from 5.2% of all outpatient visits the week before to 5.8% during the week ending March 14.
“The COVID-19 outbreak unfolding in the United States may affect healthcare seeking behavior which in turn would impact data from” the U.S. Outpatient Influenza-like Illness Surveillance Network, the CDC explained.
Data from clinical laboratories show that, despite the increased activity, fewer respiratory specimens tested positive for influenza: 15.3% for the week of March 8-14, compared with 21.1% the week before, the CDC’s influenza division said in its latest FluView report.
Influenza activity also increased slightly among the states, with 35 states and Puerto Rico at the highest level on the CDC’s 1-10 scale, versus 34 states and Puerto Rico the previous week. The count was down to 33 for the last week of February, CDC data show.
Severity measures remain mixed as overall hospitalization continues to be moderate but rates for children aged 0-4 years and adults aged 18-49 years are the highest on record and rates for children aged 5-17 years are the highest since the 2009 pandemic, the influenza division said.
Mortality data present a similar picture: The overall death rate is low, but the 149 flu-related deaths reported among children is the most for this point of the season since 2009, the CDC said.
The viral tsunami that is COVID-19 has hit the United States, and influenza appears to be riding the crest of the wave.

according to the Centers for Disease Control. Flu-related visits went from 5.2% of all outpatient visits the week before to 5.8% during the week ending March 14.
“The COVID-19 outbreak unfolding in the United States may affect healthcare seeking behavior which in turn would impact data from” the U.S. Outpatient Influenza-like Illness Surveillance Network, the CDC explained.
Data from clinical laboratories show that, despite the increased activity, fewer respiratory specimens tested positive for influenza: 15.3% for the week of March 8-14, compared with 21.1% the week before, the CDC’s influenza division said in its latest FluView report.
Influenza activity also increased slightly among the states, with 35 states and Puerto Rico at the highest level on the CDC’s 1-10 scale, versus 34 states and Puerto Rico the previous week. The count was down to 33 for the last week of February, CDC data show.
Severity measures remain mixed as overall hospitalization continues to be moderate but rates for children aged 0-4 years and adults aged 18-49 years are the highest on record and rates for children aged 5-17 years are the highest since the 2009 pandemic, the influenza division said.
Mortality data present a similar picture: The overall death rate is low, but the 149 flu-related deaths reported among children is the most for this point of the season since 2009, the CDC said.
The viral tsunami that is COVID-19 has hit the United States, and influenza appears to be riding the crest of the wave.

according to the Centers for Disease Control. Flu-related visits went from 5.2% of all outpatient visits the week before to 5.8% during the week ending March 14.
“The COVID-19 outbreak unfolding in the United States may affect healthcare seeking behavior which in turn would impact data from” the U.S. Outpatient Influenza-like Illness Surveillance Network, the CDC explained.
Data from clinical laboratories show that, despite the increased activity, fewer respiratory specimens tested positive for influenza: 15.3% for the week of March 8-14, compared with 21.1% the week before, the CDC’s influenza division said in its latest FluView report.
Influenza activity also increased slightly among the states, with 35 states and Puerto Rico at the highest level on the CDC’s 1-10 scale, versus 34 states and Puerto Rico the previous week. The count was down to 33 for the last week of February, CDC data show.
Severity measures remain mixed as overall hospitalization continues to be moderate but rates for children aged 0-4 years and adults aged 18-49 years are the highest on record and rates for children aged 5-17 years are the highest since the 2009 pandemic, the influenza division said.
Mortality data present a similar picture: The overall death rate is low, but the 149 flu-related deaths reported among children is the most for this point of the season since 2009, the CDC said.
Inflammatory Changes in Actinic Keratoses Associated With Afatinib Therapy
To the Editor:
Afatinib is a small molecule covalently binding and inhibiting the epidermal growth factor receptor (EGFR) as well as HER2 and HER4 receptor tyrosine kinases.1 The EGFR family is part of a complex signal transduction network that is central to several critical cellular processes.2 The human EGFR family is dysregulated in many solid tumors, making it an attractive target for anticancer therapy.2 In 2013, the US Food and Drug Administration approved afatinib as a first-line treatment of patients with metastatic non–small cell lung cancer whose tumors have EGFR exon 19 deletions or exon 21 (L858R) substitution mutations.3
Treatment with afatinib and other EGFR inhibitors is frequently associated with cutaneous adverse effects that occur in up to 90% of patients. These cutaneous reactions are typical for this drug family and distinct from the skin adverse effects related to other types of anticancer chemotherapy.4 The most frequent skin manifestations following afatinib treatment consist of an acneform pustular eruption in up to 90% of patients.5,6 Other dermatologic reactions include nonspecific maculopapular rashes (90%), stomatitis (71%), paronychia with some nail changes (58%), xerosis (31%), pruritus (21%), and hand-foot syndrome (7%)5,6; however, grade 3 dermatologic reactions occurred in only 0.15% of patients.
Inflammatory changes in both preexisting and undetected actinic keratoses (AKs) and even progression to squamous cell carcinoma (SCC) have been previously described as uncommon dermatologic adverse effects of 2 EGFR inhibitors, sorafenib and erlotinib.7-9 Seven of 131 patients with metastatic renal cell carcinoma treated with single-agent sorafenib developed cutaneous SCC and 3 more had AKs.9 One patient demonstrated self-limited inflammatory flare-up of AKs during erlotinib treatment.8 We report acute inflammation of AKs from afatinib treatment.
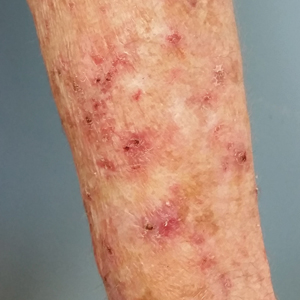
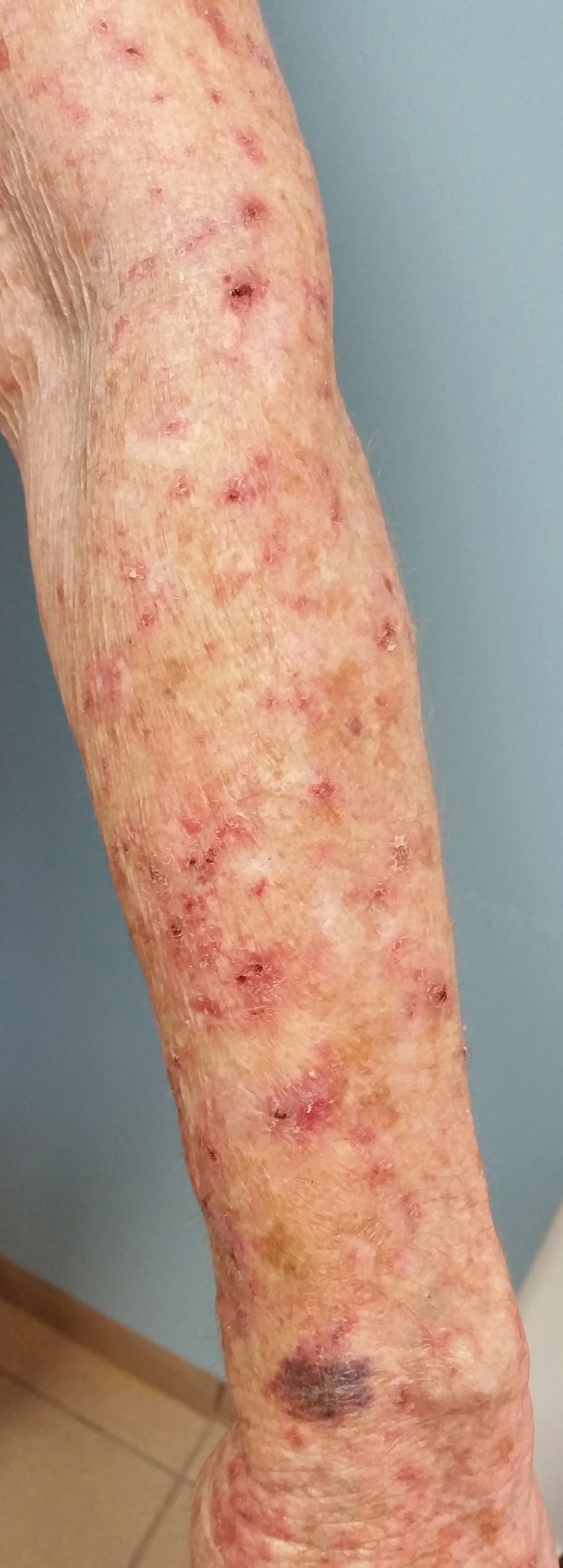
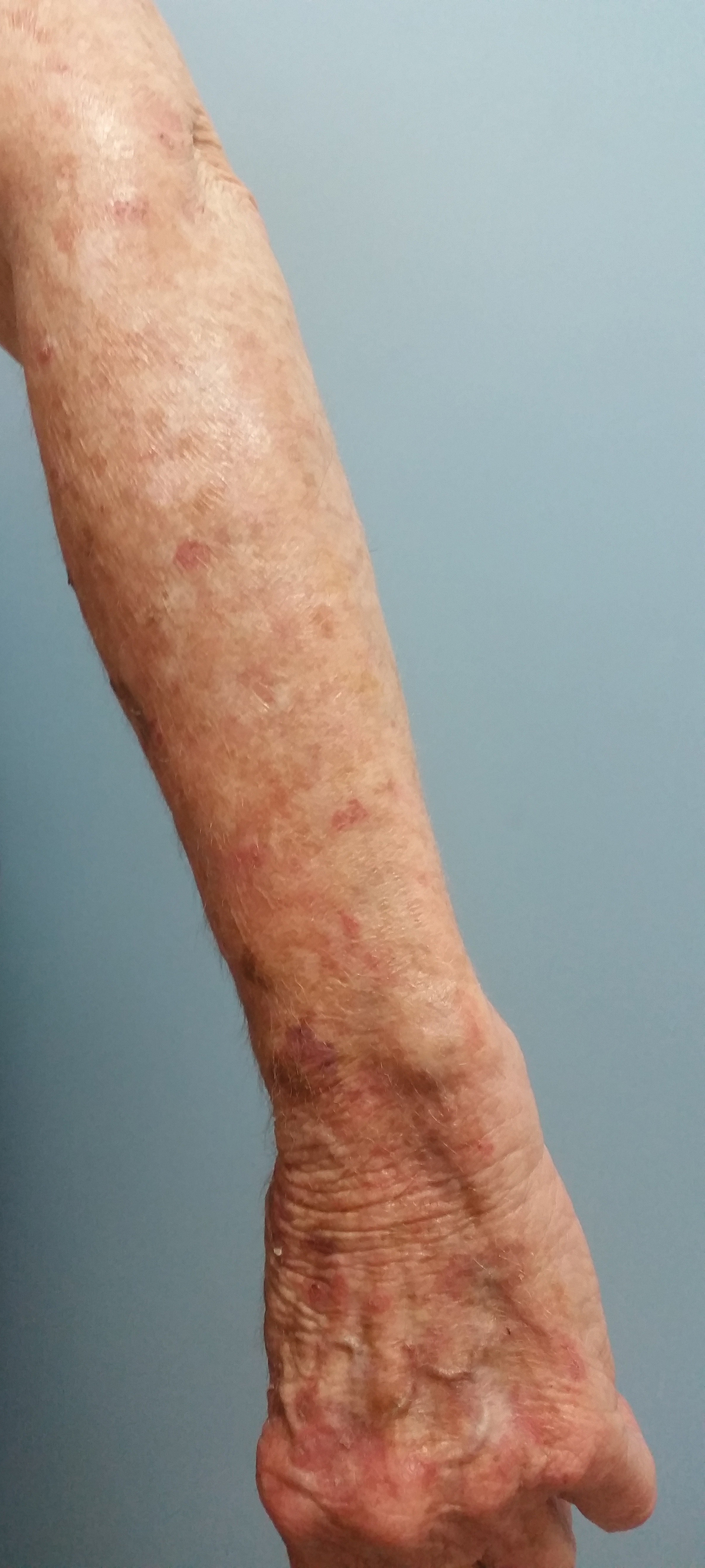
A 78-year-old woman with fair skin who was previously treated for several AKs in sun-exposed areas presented with inflammatory changes that appeared at the site of AKs on photoexposed areas 110 days after initiating afatinib therapy (40 mg/d). Physical examination revealed multiple erythematous scaly plaques on the face, neck, chest, and forearms (Figure 1).
In the previous 2 decades, lesions that were surgically removed and histopathologically examined included Bowen disease (2 lesions), 2 basal cell carcinomas, 2 blue nevi, and a seborrheic keratosis. Several AKs also were surgically removed and confirmed histopathologically.
Eighteen months prior to the current presentation, the patient was diagnosed with locally advanced, inoperable, stage IIIA adenocarcinoma of the lung with deletion in exon 19 of the EGFR gene. She received definitive concomitant chemoradiation with the carboplatin-vinorelbine regimen and 60-Gy radiation. Four months later, a positron emission tomography (PET)–fludeoxyglucose scan revealed a single bone lesion in the L5 vertebra leading to irradiation to the lumbar spine. Subsequently, new metastases to the neck, right lung, T5 vertebra, and left acetabulum were detected by PET–computed tomography. One year later, afatinib 40 mg/d was initiated. A PET scan after 2 months of treatment showed excellent response.
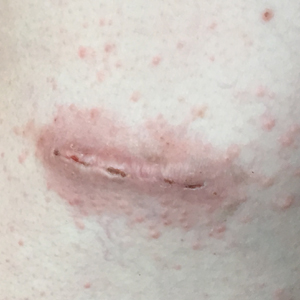
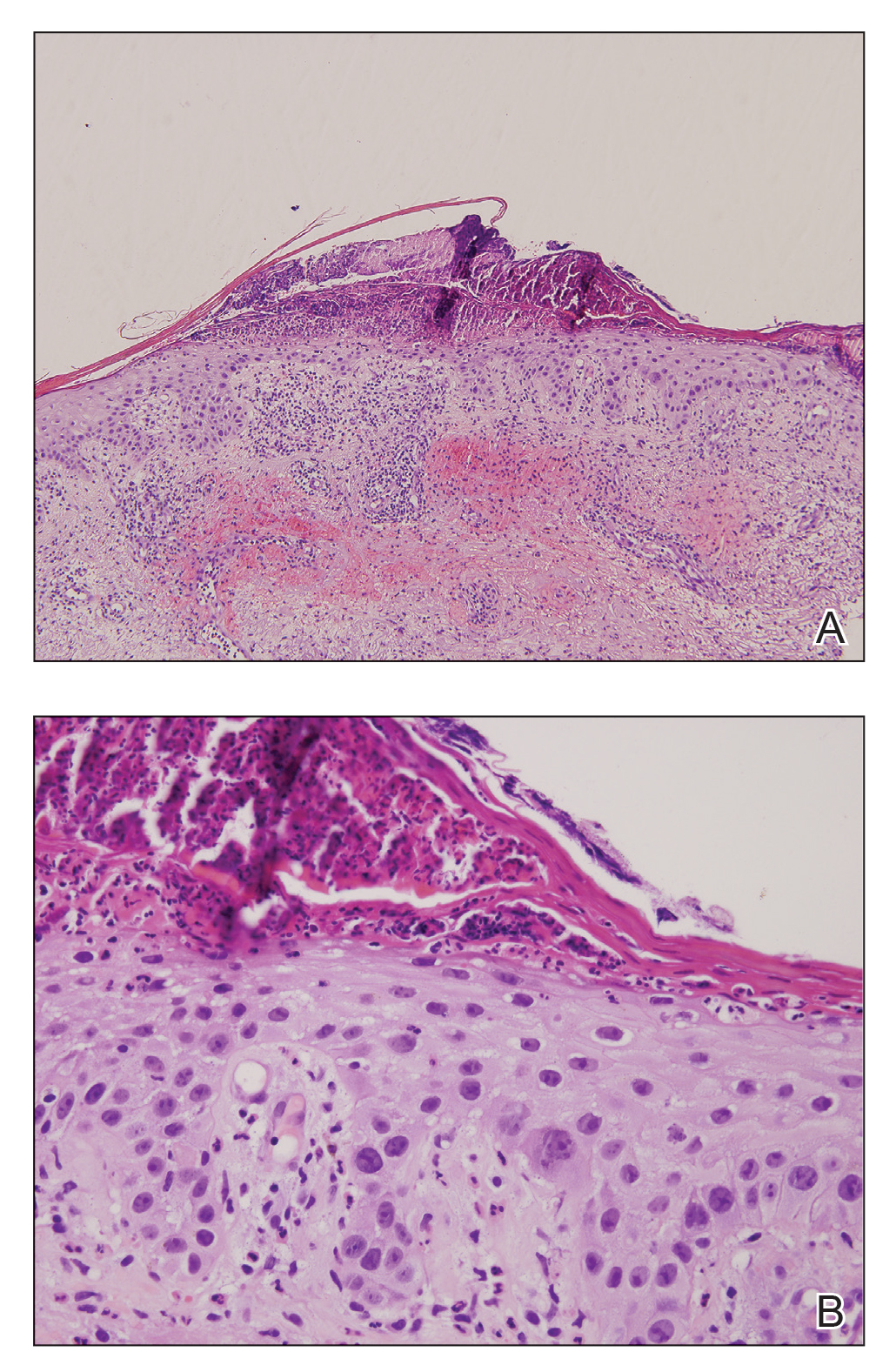
At the current presentation, a punch biopsy obtained from an inflammatory lesion on the left dorsal forearm revealed findings consistent with an eroded and inflamed AK; the biopsy showed marked dysplasia of the keratinocytes that was predominately located in the basal layer of the epidermis. The lesion was accompanied by a dense mixed inflammatory cell infiltrate that was centered in the papillary dermis and extended to the epidermis (Figure 2). Because of this grade 3 skin toxicity, the afatinib dosage was reduced to 20 mg/d, and betamethasone cream 0.1% and emollients were applied locally for 2 weeks. A reduction in the number of AKs and clinical regression of the inflammatory changes was observed 2 weeks later (Figure 3).
Chronically sun-exposed skin is prone to develop AKs that are at risk to progress to SCC.10-12 These lesions are increasingly diagnosed in older patients when internal cancers also are prevalent.13 Inflammatory flare-up of AKs is typically present during the regression phase14,15 but also during progression to SCC.16
There are many strategies for treating AKs. Physical procedures for destroying the lesions are commonly used. Some topical drugs, including imiquimod, 5-fluorouracil, and diclofenac sodium, also have proven efficacy.17
Conventional chemotherapeutic agents that have been described to be associated with the inflammation of AKs include docetaxel; doxorubicin; capecitabine; pentostatin; and the combination of dactinomycin, vincristine, dacarbazine and doxorubicin, cytarabine, and 6-thioguanine.7,18 The mechanism leading to this effect is unknown, though abnormal DNA synthesis and a type of radiation recall phenomenon have been postulated.7
We described inflammatory changes in AKs associated with afatinib treatment. The precise mechanism by which afatinib induces inflammation in AK has not been elucidated; however, it is known that EGFR normally downregulates chemokine expression in keratinocytes. Conversely, EGFR signaling blockade produces opposite effects, with increased CCL2, CCL5, and CXCL10, as well as reduced CXCL8 expression, leading to enhanced skin inflammation.19 Afatinib is a targeted agent that modulates the Ras/Raf/MEK/ERK signaling circuit, which is a key intracellular signal transduction pathway.20 This pathway and its downstream effectors have been implicated in cutaneous squamous cell carcinogenesis that might be accompanied by inflammatory changes.21,22 The remarkable clinical improvement of the AKs in our patient following the inflammatory flare-up supports the notion that the anticancer effect on intraepidermal neoplasms might be mediated by inflammation.23
- Katakami N, Atagi S, Goto K, et al. LUX-lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol. 2013;31:3335-3342.
- Liao BC, Lin CC, Yang JCH. First-line management of EGFR-mutated advanced lung adenocarcinoma: recent developments. Drugs. 2013;73:357-369.
- Jain P, Khanal R, Sharma A, et al. Afatinib and lung cancer. Expert Rev Anticancer Ther. 2014;14:1391-1406.
- Wyatt AJ, Leonard GD, Sachs DL. Cutaneous reactions to chemotherapy and their management. Am J Clin Dermatol. 2006;7:45-63.
- Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16:1425-1433.
- Agero ALC, Dusza SW, Benvenuto-Andrade C, et al. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55:657-670.
- Lacouture ME, Desai A, Soltani K, et al. Inflammation of actinic keratoses subsequent to therapy with sorafenib, a multitargeted tyrosine-kinase inhibitor. Clin Exp Dermatol. 2006;31:783-785.
- Hermanns JF, Piérard GE, Quatresooz P. Erlotinib-responsive actinic keratoses. Oncol Rep. 2007;18:581-584.
- Dubauskas Z, Kunishige J, Prieto VG, et al. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23.
- Czarnecki D, Meehan CJ, Bruce F, et al. The majority of cutaneous squamous cell carcinomas arise in actinic keratoses. J Cutan Med Surg. 2002;6:207-209.
- Ehrig T, Cockerell C, Piacquadio D, et al. Actinic keratoses and the incidence of occult squamous cell carcinoma: a clinical-histopathologic correlation. Dermatolog Surg. 2006;32:1261-1265.
- Quaedvlieg PJF, Tirsi E, Thissen MRTM, et al. Actinic keratosis: how to differentiate the good from the bad ones? Eur J Dermatol. 2006;16:335-339.
- Atkins D, Bang RH, Sternberg MR, et al. Reliable methods to evaluate the burden of actinic keratoses. J Invest Dermatol. 2006;126:591-594.
- Ooi T, Barnetson RS, Zhuang L, et al. Imiquimod-induced regression of actinic keratosis is associated with infiltration by T lymphocytes and dendritic cells: a randomized controlled trial. Br J Dermatol. 2006;154:72-78.
- Quatresooz P, Piérard GE. Imiquimod-responsive basal cell carcinomas and factor XIIIa enriched dendrocytes. Clin Exp Dermatol. 2003;28(suppl 1):27-29.
- Berhane T, Halliday GM, Cooke B, et al. Inflammation is associated with progression of actinic keratoses to squamous cell carcinomas in humans. Br J Dermatol. 2002;146:810-815.
- Ceilley RI, Jorizzo JL. Current issues in the management of actinic keratosis. J Am Acad Dermatol. 2013;68(1 suppl 1):S28-S38.
- Susser WS, Whitaker-Worth DL, Grant-Kels JM. Mucocutaneous reactions to chemotherapy. J Am Acad Dermatol. 1999;40:367-398.
- Mascia F, Mariani V, Girolomoni G, et al. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303-312.
- Zebisch A, Czernilofsky AP, Keri G, et al. Signaling through RAS-RAF-MEK ERK: from basics to bedside. Curr Med Chem. 2007;14:601-623.
- Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis. 2005;26:1657-1667.
- Malliri A, Collard JG. Role of Rho-family proteins in cell adhesion and cancer. Curr Opin Cell Biol. 2003;15:583-589.
- Kumar S, Kumar R, Medhi B, et al. Novel strategies for effective actinic keratosis treatment: a review. Curr Cancer Ther Rev. 2015;11:119-1132.
To the Editor:
Afatinib is a small molecule covalently binding and inhibiting the epidermal growth factor receptor (EGFR) as well as HER2 and HER4 receptor tyrosine kinases.1 The EGFR family is part of a complex signal transduction network that is central to several critical cellular processes.2 The human EGFR family is dysregulated in many solid tumors, making it an attractive target for anticancer therapy.2 In 2013, the US Food and Drug Administration approved afatinib as a first-line treatment of patients with metastatic non–small cell lung cancer whose tumors have EGFR exon 19 deletions or exon 21 (L858R) substitution mutations.3
Treatment with afatinib and other EGFR inhibitors is frequently associated with cutaneous adverse effects that occur in up to 90% of patients. These cutaneous reactions are typical for this drug family and distinct from the skin adverse effects related to other types of anticancer chemotherapy.4 The most frequent skin manifestations following afatinib treatment consist of an acneform pustular eruption in up to 90% of patients.5,6 Other dermatologic reactions include nonspecific maculopapular rashes (90%), stomatitis (71%), paronychia with some nail changes (58%), xerosis (31%), pruritus (21%), and hand-foot syndrome (7%)5,6; however, grade 3 dermatologic reactions occurred in only 0.15% of patients.
Inflammatory changes in both preexisting and undetected actinic keratoses (AKs) and even progression to squamous cell carcinoma (SCC) have been previously described as uncommon dermatologic adverse effects of 2 EGFR inhibitors, sorafenib and erlotinib.7-9 Seven of 131 patients with metastatic renal cell carcinoma treated with single-agent sorafenib developed cutaneous SCC and 3 more had AKs.9 One patient demonstrated self-limited inflammatory flare-up of AKs during erlotinib treatment.8 We report acute inflammation of AKs from afatinib treatment.
A 78-year-old woman with fair skin who was previously treated for several AKs in sun-exposed areas presented with inflammatory changes that appeared at the site of AKs on photoexposed areas 110 days after initiating afatinib therapy (40 mg/d). Physical examination revealed multiple erythematous scaly plaques on the face, neck, chest, and forearms (Figure 1).

In the previous 2 decades, lesions that were surgically removed and histopathologically examined included Bowen disease (2 lesions), 2 basal cell carcinomas, 2 blue nevi, and a seborrheic keratosis. Several AKs also were surgically removed and confirmed histopathologically.
Eighteen months prior to the current presentation, the patient was diagnosed with locally advanced, inoperable, stage IIIA adenocarcinoma of the lung with deletion in exon 19 of the EGFR gene. She received definitive concomitant chemoradiation with the carboplatin-vinorelbine regimen and 60-Gy radiation. Four months later, a positron emission tomography (PET)–fludeoxyglucose scan revealed a single bone lesion in the L5 vertebra leading to irradiation to the lumbar spine. Subsequently, new metastases to the neck, right lung, T5 vertebra, and left acetabulum were detected by PET–computed tomography. One year later, afatinib 40 mg/d was initiated. A PET scan after 2 months of treatment showed excellent response.
At the current presentation, a punch biopsy obtained from an inflammatory lesion on the left dorsal forearm revealed findings consistent with an eroded and inflamed AK; the biopsy showed marked dysplasia of the keratinocytes that was predominately located in the basal layer of the epidermis. The lesion was accompanied by a dense mixed inflammatory cell infiltrate that was centered in the papillary dermis and extended to the epidermis (Figure 2). Because of this grade 3 skin toxicity, the afatinib dosage was reduced to 20 mg/d, and betamethasone cream 0.1% and emollients were applied locally for 2 weeks. A reduction in the number of AKs and clinical regression of the inflammatory changes was observed 2 weeks later (Figure 3).


Chronically sun-exposed skin is prone to develop AKs that are at risk to progress to SCC.10-12 These lesions are increasingly diagnosed in older patients when internal cancers also are prevalent.13 Inflammatory flare-up of AKs is typically present during the regression phase14,15 but also during progression to SCC.16
There are many strategies for treating AKs. Physical procedures for destroying the lesions are commonly used. Some topical drugs, including imiquimod, 5-fluorouracil, and diclofenac sodium, also have proven efficacy.17
Conventional chemotherapeutic agents that have been described to be associated with the inflammation of AKs include docetaxel; doxorubicin; capecitabine; pentostatin; and the combination of dactinomycin, vincristine, dacarbazine and doxorubicin, cytarabine, and 6-thioguanine.7,18 The mechanism leading to this effect is unknown, though abnormal DNA synthesis and a type of radiation recall phenomenon have been postulated.7
We described inflammatory changes in AKs associated with afatinib treatment. The precise mechanism by which afatinib induces inflammation in AK has not been elucidated; however, it is known that EGFR normally downregulates chemokine expression in keratinocytes. Conversely, EGFR signaling blockade produces opposite effects, with increased CCL2, CCL5, and CXCL10, as well as reduced CXCL8 expression, leading to enhanced skin inflammation.19 Afatinib is a targeted agent that modulates the Ras/Raf/MEK/ERK signaling circuit, which is a key intracellular signal transduction pathway.20 This pathway and its downstream effectors have been implicated in cutaneous squamous cell carcinogenesis that might be accompanied by inflammatory changes.21,22 The remarkable clinical improvement of the AKs in our patient following the inflammatory flare-up supports the notion that the anticancer effect on intraepidermal neoplasms might be mediated by inflammation.23
To the Editor:
Afatinib is a small molecule covalently binding and inhibiting the epidermal growth factor receptor (EGFR) as well as HER2 and HER4 receptor tyrosine kinases.1 The EGFR family is part of a complex signal transduction network that is central to several critical cellular processes.2 The human EGFR family is dysregulated in many solid tumors, making it an attractive target for anticancer therapy.2 In 2013, the US Food and Drug Administration approved afatinib as a first-line treatment of patients with metastatic non–small cell lung cancer whose tumors have EGFR exon 19 deletions or exon 21 (L858R) substitution mutations.3
Treatment with afatinib and other EGFR inhibitors is frequently associated with cutaneous adverse effects that occur in up to 90% of patients. These cutaneous reactions are typical for this drug family and distinct from the skin adverse effects related to other types of anticancer chemotherapy.4 The most frequent skin manifestations following afatinib treatment consist of an acneform pustular eruption in up to 90% of patients.5,6 Other dermatologic reactions include nonspecific maculopapular rashes (90%), stomatitis (71%), paronychia with some nail changes (58%), xerosis (31%), pruritus (21%), and hand-foot syndrome (7%)5,6; however, grade 3 dermatologic reactions occurred in only 0.15% of patients.
Inflammatory changes in both preexisting and undetected actinic keratoses (AKs) and even progression to squamous cell carcinoma (SCC) have been previously described as uncommon dermatologic adverse effects of 2 EGFR inhibitors, sorafenib and erlotinib.7-9 Seven of 131 patients with metastatic renal cell carcinoma treated with single-agent sorafenib developed cutaneous SCC and 3 more had AKs.9 One patient demonstrated self-limited inflammatory flare-up of AKs during erlotinib treatment.8 We report acute inflammation of AKs from afatinib treatment.
A 78-year-old woman with fair skin who was previously treated for several AKs in sun-exposed areas presented with inflammatory changes that appeared at the site of AKs on photoexposed areas 110 days after initiating afatinib therapy (40 mg/d). Physical examination revealed multiple erythematous scaly plaques on the face, neck, chest, and forearms (Figure 1).

In the previous 2 decades, lesions that were surgically removed and histopathologically examined included Bowen disease (2 lesions), 2 basal cell carcinomas, 2 blue nevi, and a seborrheic keratosis. Several AKs also were surgically removed and confirmed histopathologically.
Eighteen months prior to the current presentation, the patient was diagnosed with locally advanced, inoperable, stage IIIA adenocarcinoma of the lung with deletion in exon 19 of the EGFR gene. She received definitive concomitant chemoradiation with the carboplatin-vinorelbine regimen and 60-Gy radiation. Four months later, a positron emission tomography (PET)–fludeoxyglucose scan revealed a single bone lesion in the L5 vertebra leading to irradiation to the lumbar spine. Subsequently, new metastases to the neck, right lung, T5 vertebra, and left acetabulum were detected by PET–computed tomography. One year later, afatinib 40 mg/d was initiated. A PET scan after 2 months of treatment showed excellent response.
At the current presentation, a punch biopsy obtained from an inflammatory lesion on the left dorsal forearm revealed findings consistent with an eroded and inflamed AK; the biopsy showed marked dysplasia of the keratinocytes that was predominately located in the basal layer of the epidermis. The lesion was accompanied by a dense mixed inflammatory cell infiltrate that was centered in the papillary dermis and extended to the epidermis (Figure 2). Because of this grade 3 skin toxicity, the afatinib dosage was reduced to 20 mg/d, and betamethasone cream 0.1% and emollients were applied locally for 2 weeks. A reduction in the number of AKs and clinical regression of the inflammatory changes was observed 2 weeks later (Figure 3).


Chronically sun-exposed skin is prone to develop AKs that are at risk to progress to SCC.10-12 These lesions are increasingly diagnosed in older patients when internal cancers also are prevalent.13 Inflammatory flare-up of AKs is typically present during the regression phase14,15 but also during progression to SCC.16
There are many strategies for treating AKs. Physical procedures for destroying the lesions are commonly used. Some topical drugs, including imiquimod, 5-fluorouracil, and diclofenac sodium, also have proven efficacy.17
Conventional chemotherapeutic agents that have been described to be associated with the inflammation of AKs include docetaxel; doxorubicin; capecitabine; pentostatin; and the combination of dactinomycin, vincristine, dacarbazine and doxorubicin, cytarabine, and 6-thioguanine.7,18 The mechanism leading to this effect is unknown, though abnormal DNA synthesis and a type of radiation recall phenomenon have been postulated.7
We described inflammatory changes in AKs associated with afatinib treatment. The precise mechanism by which afatinib induces inflammation in AK has not been elucidated; however, it is known that EGFR normally downregulates chemokine expression in keratinocytes. Conversely, EGFR signaling blockade produces opposite effects, with increased CCL2, CCL5, and CXCL10, as well as reduced CXCL8 expression, leading to enhanced skin inflammation.19 Afatinib is a targeted agent that modulates the Ras/Raf/MEK/ERK signaling circuit, which is a key intracellular signal transduction pathway.20 This pathway and its downstream effectors have been implicated in cutaneous squamous cell carcinogenesis that might be accompanied by inflammatory changes.21,22 The remarkable clinical improvement of the AKs in our patient following the inflammatory flare-up supports the notion that the anticancer effect on intraepidermal neoplasms might be mediated by inflammation.23
- Katakami N, Atagi S, Goto K, et al. LUX-lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol. 2013;31:3335-3342.
- Liao BC, Lin CC, Yang JCH. First-line management of EGFR-mutated advanced lung adenocarcinoma: recent developments. Drugs. 2013;73:357-369.
- Jain P, Khanal R, Sharma A, et al. Afatinib and lung cancer. Expert Rev Anticancer Ther. 2014;14:1391-1406.
- Wyatt AJ, Leonard GD, Sachs DL. Cutaneous reactions to chemotherapy and their management. Am J Clin Dermatol. 2006;7:45-63.
- Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16:1425-1433.
- Agero ALC, Dusza SW, Benvenuto-Andrade C, et al. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55:657-670.
- Lacouture ME, Desai A, Soltani K, et al. Inflammation of actinic keratoses subsequent to therapy with sorafenib, a multitargeted tyrosine-kinase inhibitor. Clin Exp Dermatol. 2006;31:783-785.
- Hermanns JF, Piérard GE, Quatresooz P. Erlotinib-responsive actinic keratoses. Oncol Rep. 2007;18:581-584.
- Dubauskas Z, Kunishige J, Prieto VG, et al. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23.
- Czarnecki D, Meehan CJ, Bruce F, et al. The majority of cutaneous squamous cell carcinomas arise in actinic keratoses. J Cutan Med Surg. 2002;6:207-209.
- Ehrig T, Cockerell C, Piacquadio D, et al. Actinic keratoses and the incidence of occult squamous cell carcinoma: a clinical-histopathologic correlation. Dermatolog Surg. 2006;32:1261-1265.
- Quaedvlieg PJF, Tirsi E, Thissen MRTM, et al. Actinic keratosis: how to differentiate the good from the bad ones? Eur J Dermatol. 2006;16:335-339.
- Atkins D, Bang RH, Sternberg MR, et al. Reliable methods to evaluate the burden of actinic keratoses. J Invest Dermatol. 2006;126:591-594.
- Ooi T, Barnetson RS, Zhuang L, et al. Imiquimod-induced regression of actinic keratosis is associated with infiltration by T lymphocytes and dendritic cells: a randomized controlled trial. Br J Dermatol. 2006;154:72-78.
- Quatresooz P, Piérard GE. Imiquimod-responsive basal cell carcinomas and factor XIIIa enriched dendrocytes. Clin Exp Dermatol. 2003;28(suppl 1):27-29.
- Berhane T, Halliday GM, Cooke B, et al. Inflammation is associated with progression of actinic keratoses to squamous cell carcinomas in humans. Br J Dermatol. 2002;146:810-815.
- Ceilley RI, Jorizzo JL. Current issues in the management of actinic keratosis. J Am Acad Dermatol. 2013;68(1 suppl 1):S28-S38.
- Susser WS, Whitaker-Worth DL, Grant-Kels JM. Mucocutaneous reactions to chemotherapy. J Am Acad Dermatol. 1999;40:367-398.
- Mascia F, Mariani V, Girolomoni G, et al. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303-312.
- Zebisch A, Czernilofsky AP, Keri G, et al. Signaling through RAS-RAF-MEK ERK: from basics to bedside. Curr Med Chem. 2007;14:601-623.
- Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis. 2005;26:1657-1667.
- Malliri A, Collard JG. Role of Rho-family proteins in cell adhesion and cancer. Curr Opin Cell Biol. 2003;15:583-589.
- Kumar S, Kumar R, Medhi B, et al. Novel strategies for effective actinic keratosis treatment: a review. Curr Cancer Ther Rev. 2015;11:119-1132.
- Katakami N, Atagi S, Goto K, et al. LUX-lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol. 2013;31:3335-3342.
- Liao BC, Lin CC, Yang JCH. First-line management of EGFR-mutated advanced lung adenocarcinoma: recent developments. Drugs. 2013;73:357-369.
- Jain P, Khanal R, Sharma A, et al. Afatinib and lung cancer. Expert Rev Anticancer Ther. 2014;14:1391-1406.
- Wyatt AJ, Leonard GD, Sachs DL. Cutaneous reactions to chemotherapy and their management. Am J Clin Dermatol. 2006;7:45-63.
- Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16:1425-1433.
- Agero ALC, Dusza SW, Benvenuto-Andrade C, et al. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55:657-670.
- Lacouture ME, Desai A, Soltani K, et al. Inflammation of actinic keratoses subsequent to therapy with sorafenib, a multitargeted tyrosine-kinase inhibitor. Clin Exp Dermatol. 2006;31:783-785.
- Hermanns JF, Piérard GE, Quatresooz P. Erlotinib-responsive actinic keratoses. Oncol Rep. 2007;18:581-584.
- Dubauskas Z, Kunishige J, Prieto VG, et al. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23.
- Czarnecki D, Meehan CJ, Bruce F, et al. The majority of cutaneous squamous cell carcinomas arise in actinic keratoses. J Cutan Med Surg. 2002;6:207-209.
- Ehrig T, Cockerell C, Piacquadio D, et al. Actinic keratoses and the incidence of occult squamous cell carcinoma: a clinical-histopathologic correlation. Dermatolog Surg. 2006;32:1261-1265.
- Quaedvlieg PJF, Tirsi E, Thissen MRTM, et al. Actinic keratosis: how to differentiate the good from the bad ones? Eur J Dermatol. 2006;16:335-339.
- Atkins D, Bang RH, Sternberg MR, et al. Reliable methods to evaluate the burden of actinic keratoses. J Invest Dermatol. 2006;126:591-594.
- Ooi T, Barnetson RS, Zhuang L, et al. Imiquimod-induced regression of actinic keratosis is associated with infiltration by T lymphocytes and dendritic cells: a randomized controlled trial. Br J Dermatol. 2006;154:72-78.
- Quatresooz P, Piérard GE. Imiquimod-responsive basal cell carcinomas and factor XIIIa enriched dendrocytes. Clin Exp Dermatol. 2003;28(suppl 1):27-29.
- Berhane T, Halliday GM, Cooke B, et al. Inflammation is associated with progression of actinic keratoses to squamous cell carcinomas in humans. Br J Dermatol. 2002;146:810-815.
- Ceilley RI, Jorizzo JL. Current issues in the management of actinic keratosis. J Am Acad Dermatol. 2013;68(1 suppl 1):S28-S38.
- Susser WS, Whitaker-Worth DL, Grant-Kels JM. Mucocutaneous reactions to chemotherapy. J Am Acad Dermatol. 1999;40:367-398.
- Mascia F, Mariani V, Girolomoni G, et al. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303-312.
- Zebisch A, Czernilofsky AP, Keri G, et al. Signaling through RAS-RAF-MEK ERK: from basics to bedside. Curr Med Chem. 2007;14:601-623.
- Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis. 2005;26:1657-1667.
- Malliri A, Collard JG. Role of Rho-family proteins in cell adhesion and cancer. Curr Opin Cell Biol. 2003;15:583-589.
- Kumar S, Kumar R, Medhi B, et al. Novel strategies for effective actinic keratosis treatment: a review. Curr Cancer Ther Rev. 2015;11:119-1132.
Practice Points
- One of the underreported adverse events of afatinibis is the induction of inflammatory changes in actinic keratoses (AKs).
- Our cases showed that inflammatory changes eventually led to shrinkage and resolution of the underlying AK.
Hyperoxia in the ICU: Is less more?
“All things are poison and nothing is without poison, only the dose permits something not to be poisonous.” Paracelsus once said.
A bit of history
Oxygen was discovered in 1775 and was since noted to be both vital and poisonous. It was much later in 1899 that it was demonstrated that partial pressures of oxygen up to 75% led to both severe lung injury and death as compared with levels of 40% to 50%. While the administration of oxygen in hypoxic patients is beneficial, this intervention in healthy subjects leads to a reduction in heart rate, cardiac index, and an increase in mean arterial pressure, systemic vascular resistance, and large artery stiffness.
While oxygen itself is not toxic, the reactive oxygen species that form as a result of oxygen metabolism are. A study showed that supplementation of oxygen in patients with COPD, or in women undergoing C-section with the use of spinal anesthesia, leads to an increase in reactive oxygen species (Winslow RM. Transfusion. 2013;53[2]:424).
Hyperoxia has multiple clinical effects on lung physiology and gas exchange that include worsening hypoxemia secondary to absorptive atelectasis and damage to the airways and lung parenchyma (Sackner MA, et al. Ann Intern Med. 1975;82[1]:40).
High levels of inspired oxygen could also lead to accentuation of hypercapnia as explained by the Haldane effect; a reduction of the affinity for carbon dioxide leading to an increase in PaC02. High oxygen levels can also decrease the hypoxic drive for ventilation leading to worsening hypercapnia.
Hyperoxia is a situation routinely encountered in clinical practice, as well, often resulting from an overzealous attempt to prevent or reverse hypoxia. ICU physicians, though aware of potential threats of hyperoxia, often fail to translate such concerns in their clinical practice (Helmerhorst HJ, et al. Ann Intensive Care. 2014;4:23).
Effects of hyperoxia in CNS and cardiovascular disease
The last 2 decades have seen several studies looking into the effects of hyperoxia in specific clinical scenarios. Arterial hyperoxia was found to be independently associated with in-hospital death in ventilated stroke patients in the ICU, as compared with either arterial normoxia or hypoxia (Rincon F, et al. Crit Care Med. 2014;42[2]:387). The AVOID trial showed that supplemental oxygen therapy in patients with ST-elevation myocardial infarction, but without hypoxia, increased early myocardial injury with risk of larger myocardial infarct size at 6 months. (Stub D, et al. Circulation. 2015;131[24]:2143).
Hyperoxia in the ICU
Although the potential risks of hyperoxia in conditions such as stroke and cardiac arrest had been observed, the jury was still out on its effects on a critically ill, mixed population, as routinely encountered in the ICU. Oxygen-ICU, a single center trial published in 2016, was one of the first looking at a mixed ICU population, while assessing the effects of a conservative oxygen delivery strategy against a conventional one (Girardis M, et al. JAMA. 2016;316[15]:1583). The researchers noted a significant mortality difference favoring conservative oxygen therapy, particularly in intubated patients. The IOTA group’s systematic review and meta-analysis of 16,000 patients, showed an increased relative risk of death in-hospital with hyperoxia, that persisted over a prolonged period while conferring no obvious advantages (Chu DK, et al. Lancet. 2018;391[10131]:1693).
With the growing body of evidence, the need of the hour was an ICU-based randomized trial that may settle the debate. The 21 center, 1,000 patient ICU-ROX trial promised to deliver on that (Mackle D, et al. N Engl J Med. 2019 Oct 14. doi: 10.1056/NEJMoa1903297). The study design was more reflective of real-life clinical scenarios than some of its predecessors, with the control group exposed to usual-oxygen therapy instead of liberal hyperoxia. Both groups had a lower saturation threshold of 91% while the conservative-oxygen group had an upper limit of 97% along with a conscious effort made to drop the FIO2 to 21%. Though both groups had similar median PaO2 levels, the conservative group spent much greater time (median 29 hours) at 21% FIO2 than the usual group (median 1 hour). SpO2 targets also allowed frequent changes to oxygen delivery without the need for blood gases.
Presuming the primary effect of oxygen toxicity would be on the lungs, the study was powered for a primary outcome of ventilator-free-days, which showed no significant difference among the groups. No significant differences in mortality or other secondary outcomes were observed.
The ICU-ROX trial leaves us with a few questions, the most important are:
Are the detrimental effects of hyperoxia limited to certain disease-specific groups or generally applicable?
The evidence is substantial inpatients with cardiac arrest/myocardial injury. A prespecified subgroup analysis in ICU-ROX indicated a higher number of ventilator-free days with conservative oxygen therapy in patients with hypoxic ischemic encephalopathy. When asked, Dr. Paul Young, one of the investigators of the ICU-ROX group, states, “These are actually pretty small subgroups, and the number of mortality events is quite small. My belief is that these data are best viewed as hypothesis generating rather than practice changing”
Where do we stand?
While we look for further answers regarding the consequences of hyperoxia, it is established that conservative oxygen therapy aimed at reducing delivered FIO2 is a safe practice without any adverse outcomes. The conservative oxygen group in ICU-ROX allowed SpO2 levels as low as 91% with no serious hypoxic events. On the other hand, the IOTA group in their data analysis suggested a possible increase in mortality risk, which was dose-dependent on the magnitude of increase in SpO2, in the range of 94% to 96%. Based on the available evidence, it is reasonable to encourage targeting lowest FIO2 values needed to maintain SpO2 between 91% and 96% in our ICU patients. There would always be a small fraction of patients, such as those with ARDS or severe hypoxic respiratory failure, in whom this may not be achievable given fluctuating and unreliable SpO2 levels in the setting of profound hypoxia.
What lies ahead?
As the debate rages on, in an effort to answer this question for once and for all, the researchers of ICU-ROX are planning to conduct a multinational, multicenter RCT, the MEGA-ROX. An ICU trial of this size has not been attempted before and, given the sample size, Dr. Young feels the MEGA-ROX will be powered to detect an absolute mortality difference as low as 1.5%, if it does exist. There is a distinct possibility that conservative oxygen therapy will be best for patients with some diagnoses while liberal oxygen will be best for patients with other diagnoses. “We are conducting a number of parallel nested trials within the overall 40,000 participant trial sample. Each of these nested trials will evaluate a prespecified hypothesis in a specific cohort of critically ill patients and is accompanied by an appropriate power calculation. This will be able to address any heterogeneity of treatment effect among the different subgroups,” he concluded. As we eagerly await the results of MEGA-ROX, there may be a growing belief among intensivists that when it comes to oxygen in the ICU, less may be truly more.
Dr. Chaaban and Dr. Sen are with the University of Kentucky College of Medicine, Lexington, Kentucky.
Correction, 4/10/20: An earlier version of this article misstated Dr. Sen's name
“All things are poison and nothing is without poison, only the dose permits something not to be poisonous.” Paracelsus once said.
A bit of history
Oxygen was discovered in 1775 and was since noted to be both vital and poisonous. It was much later in 1899 that it was demonstrated that partial pressures of oxygen up to 75% led to both severe lung injury and death as compared with levels of 40% to 50%. While the administration of oxygen in hypoxic patients is beneficial, this intervention in healthy subjects leads to a reduction in heart rate, cardiac index, and an increase in mean arterial pressure, systemic vascular resistance, and large artery stiffness.
While oxygen itself is not toxic, the reactive oxygen species that form as a result of oxygen metabolism are. A study showed that supplementation of oxygen in patients with COPD, or in women undergoing C-section with the use of spinal anesthesia, leads to an increase in reactive oxygen species (Winslow RM. Transfusion. 2013;53[2]:424).
Hyperoxia has multiple clinical effects on lung physiology and gas exchange that include worsening hypoxemia secondary to absorptive atelectasis and damage to the airways and lung parenchyma (Sackner MA, et al. Ann Intern Med. 1975;82[1]:40).
High levels of inspired oxygen could also lead to accentuation of hypercapnia as explained by the Haldane effect; a reduction of the affinity for carbon dioxide leading to an increase in PaC02. High oxygen levels can also decrease the hypoxic drive for ventilation leading to worsening hypercapnia.
Hyperoxia is a situation routinely encountered in clinical practice, as well, often resulting from an overzealous attempt to prevent or reverse hypoxia. ICU physicians, though aware of potential threats of hyperoxia, often fail to translate such concerns in their clinical practice (Helmerhorst HJ, et al. Ann Intensive Care. 2014;4:23).
Effects of hyperoxia in CNS and cardiovascular disease
The last 2 decades have seen several studies looking into the effects of hyperoxia in specific clinical scenarios. Arterial hyperoxia was found to be independently associated with in-hospital death in ventilated stroke patients in the ICU, as compared with either arterial normoxia or hypoxia (Rincon F, et al. Crit Care Med. 2014;42[2]:387). The AVOID trial showed that supplemental oxygen therapy in patients with ST-elevation myocardial infarction, but without hypoxia, increased early myocardial injury with risk of larger myocardial infarct size at 6 months. (Stub D, et al. Circulation. 2015;131[24]:2143).
Hyperoxia in the ICU
Although the potential risks of hyperoxia in conditions such as stroke and cardiac arrest had been observed, the jury was still out on its effects on a critically ill, mixed population, as routinely encountered in the ICU. Oxygen-ICU, a single center trial published in 2016, was one of the first looking at a mixed ICU population, while assessing the effects of a conservative oxygen delivery strategy against a conventional one (Girardis M, et al. JAMA. 2016;316[15]:1583). The researchers noted a significant mortality difference favoring conservative oxygen therapy, particularly in intubated patients. The IOTA group’s systematic review and meta-analysis of 16,000 patients, showed an increased relative risk of death in-hospital with hyperoxia, that persisted over a prolonged period while conferring no obvious advantages (Chu DK, et al. Lancet. 2018;391[10131]:1693).
With the growing body of evidence, the need of the hour was an ICU-based randomized trial that may settle the debate. The 21 center, 1,000 patient ICU-ROX trial promised to deliver on that (Mackle D, et al. N Engl J Med. 2019 Oct 14. doi: 10.1056/NEJMoa1903297). The study design was more reflective of real-life clinical scenarios than some of its predecessors, with the control group exposed to usual-oxygen therapy instead of liberal hyperoxia. Both groups had a lower saturation threshold of 91% while the conservative-oxygen group had an upper limit of 97% along with a conscious effort made to drop the FIO2 to 21%. Though both groups had similar median PaO2 levels, the conservative group spent much greater time (median 29 hours) at 21% FIO2 than the usual group (median 1 hour). SpO2 targets also allowed frequent changes to oxygen delivery without the need for blood gases.
Presuming the primary effect of oxygen toxicity would be on the lungs, the study was powered for a primary outcome of ventilator-free-days, which showed no significant difference among the groups. No significant differences in mortality or other secondary outcomes were observed.
The ICU-ROX trial leaves us with a few questions, the most important are:
Are the detrimental effects of hyperoxia limited to certain disease-specific groups or generally applicable?
The evidence is substantial inpatients with cardiac arrest/myocardial injury. A prespecified subgroup analysis in ICU-ROX indicated a higher number of ventilator-free days with conservative oxygen therapy in patients with hypoxic ischemic encephalopathy. When asked, Dr. Paul Young, one of the investigators of the ICU-ROX group, states, “These are actually pretty small subgroups, and the number of mortality events is quite small. My belief is that these data are best viewed as hypothesis generating rather than practice changing”
Where do we stand?
While we look for further answers regarding the consequences of hyperoxia, it is established that conservative oxygen therapy aimed at reducing delivered FIO2 is a safe practice without any adverse outcomes. The conservative oxygen group in ICU-ROX allowed SpO2 levels as low as 91% with no serious hypoxic events. On the other hand, the IOTA group in their data analysis suggested a possible increase in mortality risk, which was dose-dependent on the magnitude of increase in SpO2, in the range of 94% to 96%. Based on the available evidence, it is reasonable to encourage targeting lowest FIO2 values needed to maintain SpO2 between 91% and 96% in our ICU patients. There would always be a small fraction of patients, such as those with ARDS or severe hypoxic respiratory failure, in whom this may not be achievable given fluctuating and unreliable SpO2 levels in the setting of profound hypoxia.
What lies ahead?
As the debate rages on, in an effort to answer this question for once and for all, the researchers of ICU-ROX are planning to conduct a multinational, multicenter RCT, the MEGA-ROX. An ICU trial of this size has not been attempted before and, given the sample size, Dr. Young feels the MEGA-ROX will be powered to detect an absolute mortality difference as low as 1.5%, if it does exist. There is a distinct possibility that conservative oxygen therapy will be best for patients with some diagnoses while liberal oxygen will be best for patients with other diagnoses. “We are conducting a number of parallel nested trials within the overall 40,000 participant trial sample. Each of these nested trials will evaluate a prespecified hypothesis in a specific cohort of critically ill patients and is accompanied by an appropriate power calculation. This will be able to address any heterogeneity of treatment effect among the different subgroups,” he concluded. As we eagerly await the results of MEGA-ROX, there may be a growing belief among intensivists that when it comes to oxygen in the ICU, less may be truly more.
Dr. Chaaban and Dr. Sen are with the University of Kentucky College of Medicine, Lexington, Kentucky.
Correction, 4/10/20: An earlier version of this article misstated Dr. Sen's name
“All things are poison and nothing is without poison, only the dose permits something not to be poisonous.” Paracelsus once said.
A bit of history
Oxygen was discovered in 1775 and was since noted to be both vital and poisonous. It was much later in 1899 that it was demonstrated that partial pressures of oxygen up to 75% led to both severe lung injury and death as compared with levels of 40% to 50%. While the administration of oxygen in hypoxic patients is beneficial, this intervention in healthy subjects leads to a reduction in heart rate, cardiac index, and an increase in mean arterial pressure, systemic vascular resistance, and large artery stiffness.
While oxygen itself is not toxic, the reactive oxygen species that form as a result of oxygen metabolism are. A study showed that supplementation of oxygen in patients with COPD, or in women undergoing C-section with the use of spinal anesthesia, leads to an increase in reactive oxygen species (Winslow RM. Transfusion. 2013;53[2]:424).
Hyperoxia has multiple clinical effects on lung physiology and gas exchange that include worsening hypoxemia secondary to absorptive atelectasis and damage to the airways and lung parenchyma (Sackner MA, et al. Ann Intern Med. 1975;82[1]:40).
High levels of inspired oxygen could also lead to accentuation of hypercapnia as explained by the Haldane effect; a reduction of the affinity for carbon dioxide leading to an increase in PaC02. High oxygen levels can also decrease the hypoxic drive for ventilation leading to worsening hypercapnia.
Hyperoxia is a situation routinely encountered in clinical practice, as well, often resulting from an overzealous attempt to prevent or reverse hypoxia. ICU physicians, though aware of potential threats of hyperoxia, often fail to translate such concerns in their clinical practice (Helmerhorst HJ, et al. Ann Intensive Care. 2014;4:23).
Effects of hyperoxia in CNS and cardiovascular disease
The last 2 decades have seen several studies looking into the effects of hyperoxia in specific clinical scenarios. Arterial hyperoxia was found to be independently associated with in-hospital death in ventilated stroke patients in the ICU, as compared with either arterial normoxia or hypoxia (Rincon F, et al. Crit Care Med. 2014;42[2]:387). The AVOID trial showed that supplemental oxygen therapy in patients with ST-elevation myocardial infarction, but without hypoxia, increased early myocardial injury with risk of larger myocardial infarct size at 6 months. (Stub D, et al. Circulation. 2015;131[24]:2143).
Hyperoxia in the ICU
Although the potential risks of hyperoxia in conditions such as stroke and cardiac arrest had been observed, the jury was still out on its effects on a critically ill, mixed population, as routinely encountered in the ICU. Oxygen-ICU, a single center trial published in 2016, was one of the first looking at a mixed ICU population, while assessing the effects of a conservative oxygen delivery strategy against a conventional one (Girardis M, et al. JAMA. 2016;316[15]:1583). The researchers noted a significant mortality difference favoring conservative oxygen therapy, particularly in intubated patients. The IOTA group’s systematic review and meta-analysis of 16,000 patients, showed an increased relative risk of death in-hospital with hyperoxia, that persisted over a prolonged period while conferring no obvious advantages (Chu DK, et al. Lancet. 2018;391[10131]:1693).
With the growing body of evidence, the need of the hour was an ICU-based randomized trial that may settle the debate. The 21 center, 1,000 patient ICU-ROX trial promised to deliver on that (Mackle D, et al. N Engl J Med. 2019 Oct 14. doi: 10.1056/NEJMoa1903297). The study design was more reflective of real-life clinical scenarios than some of its predecessors, with the control group exposed to usual-oxygen therapy instead of liberal hyperoxia. Both groups had a lower saturation threshold of 91% while the conservative-oxygen group had an upper limit of 97% along with a conscious effort made to drop the FIO2 to 21%. Though both groups had similar median PaO2 levels, the conservative group spent much greater time (median 29 hours) at 21% FIO2 than the usual group (median 1 hour). SpO2 targets also allowed frequent changes to oxygen delivery without the need for blood gases.
Presuming the primary effect of oxygen toxicity would be on the lungs, the study was powered for a primary outcome of ventilator-free-days, which showed no significant difference among the groups. No significant differences in mortality or other secondary outcomes were observed.
The ICU-ROX trial leaves us with a few questions, the most important are:
Are the detrimental effects of hyperoxia limited to certain disease-specific groups or generally applicable?
The evidence is substantial inpatients with cardiac arrest/myocardial injury. A prespecified subgroup analysis in ICU-ROX indicated a higher number of ventilator-free days with conservative oxygen therapy in patients with hypoxic ischemic encephalopathy. When asked, Dr. Paul Young, one of the investigators of the ICU-ROX group, states, “These are actually pretty small subgroups, and the number of mortality events is quite small. My belief is that these data are best viewed as hypothesis generating rather than practice changing”
Where do we stand?
While we look for further answers regarding the consequences of hyperoxia, it is established that conservative oxygen therapy aimed at reducing delivered FIO2 is a safe practice without any adverse outcomes. The conservative oxygen group in ICU-ROX allowed SpO2 levels as low as 91% with no serious hypoxic events. On the other hand, the IOTA group in their data analysis suggested a possible increase in mortality risk, which was dose-dependent on the magnitude of increase in SpO2, in the range of 94% to 96%. Based on the available evidence, it is reasonable to encourage targeting lowest FIO2 values needed to maintain SpO2 between 91% and 96% in our ICU patients. There would always be a small fraction of patients, such as those with ARDS or severe hypoxic respiratory failure, in whom this may not be achievable given fluctuating and unreliable SpO2 levels in the setting of profound hypoxia.
What lies ahead?
As the debate rages on, in an effort to answer this question for once and for all, the researchers of ICU-ROX are planning to conduct a multinational, multicenter RCT, the MEGA-ROX. An ICU trial of this size has not been attempted before and, given the sample size, Dr. Young feels the MEGA-ROX will be powered to detect an absolute mortality difference as low as 1.5%, if it does exist. There is a distinct possibility that conservative oxygen therapy will be best for patients with some diagnoses while liberal oxygen will be best for patients with other diagnoses. “We are conducting a number of parallel nested trials within the overall 40,000 participant trial sample. Each of these nested trials will evaluate a prespecified hypothesis in a specific cohort of critically ill patients and is accompanied by an appropriate power calculation. This will be able to address any heterogeneity of treatment effect among the different subgroups,” he concluded. As we eagerly await the results of MEGA-ROX, there may be a growing belief among intensivists that when it comes to oxygen in the ICU, less may be truly more.
Dr. Chaaban and Dr. Sen are with the University of Kentucky College of Medicine, Lexington, Kentucky.
Correction, 4/10/20: An earlier version of this article misstated Dr. Sen's name
Expansion of the donor pool in lung transplantation
Lung transplants are increasing, with 2,562 performed in the United States in 2018 – a 31% increase over the preceding 5 years. With this increased demand for donor lungs, waitlist mortality in the United States is 9.4 deaths per 100 waitlist-years for obstructive lung diseases and as high as 29.7 deaths per 100 waitlist-years for restrictive lung diseases (Valapour M, et al. Lung. Am J Transplant. 2020;20[suppl s1]:427). Conversely, lungs are utilized from eligible multiorgan donors only 15% to 20% of the time, usually due to concerns over donor history or organ quality (Young KA, et al. Chest. 2019;155[3]:465). In light of this imbalance of supply and demand, lung transplant specialists are making significant efforts to expand the donor pool of available organs. Three of these strategies include: (1) applications of ex-vivo lung perfusion (EVLP) technology; (2) use of lungs from hepatitis C-positive donors for hep-C negative recipients; and (3) increasing utilization of donation after cardiac death.
Normothermic ex-vivo lung perfusion is a technology which allows donor lungs to be perfused and ventilated after removal from the donor but before transplant into the recipient. This is in contrast to the traditional method of cold static preservation. The proposed advantage of using this technology is to allow time for a more thorough assessment of graft quality and to improve function of grafts not meeting established criteria for transplant, all-the-while decreasing organ ischemia despite an increased cross-clamp time. There are currently four commercial systems available capable of EVLP. Broadly speaking, three EVLP management protocols exist (Toronto, Lund, and OCS), which differ in perfusate composition, target flow, pulmonary arterial pressure, left atrial pressure, and ventilatory settings. Notably, the Toronto protocol uses a closed left atrium, whereas the Lund and OCS protocol use an open left atrium. There are excellent published reviews of the different systems (Possoz J, et al. J Thorac Dis. 2019;11[4]:1635). EVLP has now been studied for two different goals: (1) to allow an extended evaluation of lungs of questionable quality before transplant; or (2) for routine use in all lung transplantations in place of cold static preservation.
In most studies concerning the use of EVLP for reconditioning of donor lungs, “high risk” or “extended criteria” refers to one or more of the following: P/F ratios < 300 on arterial blood gas, macroscopic abnormalities (eg, pulmonary edema, poor lung compliance), donation after circulatory death, or high-risk history (eg, aspiration). The largest cohort with the longest follow-up addressing the role of EVLP for donation of lungs with extended criteria was published from the Toronto Lung Transplant Group. Their results have demonstrated equivalent graft survival and rates of chronic lung allograft dysfunction (CLAD) up to 9 years posttransplant compared with standard criteria donor lungs, despite utilizing lower quality lungs and having a longer median preservation (Divithotawela C, et al. JAMA Surg. 2019;154[12]:1143). The group’s subsequent lung transplant rates have increased over the past decade.
A separate study addressed the same question but differed in that it was a single-arm, multicenter, international trial that tracked the outcomes of 93 extended criteria lungs placed on EVLP (including a large proportion acquired via donation after circulatory death) (Loor G, et al. Lancet Respir Med. 2019;7[11]:975). Among these, 87% of eligible lungs were transplanted, and outcomes were excellent, albeit shorter in follow-up compared with the Toronto cohort (eg, primary graft dysfunction grade 3 (PGD3) within 72 hours was 44% and 1-year survival was 91%). Based on these trials and many other retrospective reports, it has been concluded by many experts in the field that EVLP-treated extended criteria donor lungs perform equally well to standard criteria donor lungs.
Two RCTs have been conducted to evaluate whether EVLP is noninferior to static cold storage with donor lungs meeting “standard criteria” for transplant. The first was a single center study at the Medical University of Vienna, that looked at 80 recipient/donor pairs. Lungs in the EVLP arm underwent 4 hours of perfusion with frequent reassessment of quality before transplant, whereas the lungs in the control arm went directly to transplant. This study met noninferiority criteria looking at primary outcomes of PGD grade >1 and 30-day survival (Slama A, et al. J Heart Lung Transplant. 2017;36[7]:744). The second study was a phase 3, multicenter, international trial that included 320 recipient/donor pairs randomized to either EVLP (without a prespecified time on the EVLP system) or static cold storage. This trial met noninferiority for safety endpoints (lung graft-related adverse events within 30 days) and a composite primary outcome of PGD grade 3 incidence within 72 hours and 30-day survival (Warnecke G, et al. Lancet Respir Med. 2018;6[5]:357). The authors also tested and found superiority of EVLP in lower PGD grade 3 frequency compared with control. While these RCTs may suggest a role for EVLP in the procurement process of standard criteria organs in addition to extended criteria organs in the future, major criticisms for these trials include the lack of a demonstrable clinical benefit over cold storage beyond the lower PGD3 rates.
In the era of direct-acting antiviral agents available to treat HCV infection, there has been efforts to study the early use of anti-HCV medications to prevent infection as a result of heart or lung transplant from HCV viremic donors to HCV-negative recipients. In one major trial on efficacy, it was found that 4 weeks of sofosbuvir and velpatasvir, when started within a few hours of transplant, was sufficient to achieve a sustained (undetectable) virologic response at 12 weeks after completion of the antiviral regimen (Woolley AE, et al. N Engl J Med. 2019;380[17]:1606). Therefore, many transplant centers have adopted protocols to increase the donor pool (by CDC estimates about 4% of solid organ donors are HCV-positive) by accepting HCV nucleic acid amplification test (NAT)-positive donors for HCV-negative recipients, after appropriate informed consent.
Donation after cardiac death (DCD), which is alternatively known as donation after circulatory determination of death (DCDD), generally refers to organ procurement taking place after cessation of circulation, often after inpatient withdrawal of support. This is in contrast to the much more common practice of donation after brain death (DBD). Addressing concerns over the quality of lungs donated in the context of DCD compared with DBD, analyses of ISHLT registry data have demonstrated no differences in hospital length of stay or survival at 1 or 5 years (Van Raemdonck D, et al. J Heart Lung Transplant. 2019;38[12]:1235). Outcomes comparing specific mechanisms of donor death in DCD remain relatively unknown, such as outcomes from donors withdrawn from life support vs donors who had an uncontrolled cardiac death.
These methods for expanding the donor pool are not mutually exclusive, and, in fact, application of EVLP for lungs obtained in the context of DCD seems to be increasingly common. Optimization of protocols with collaboration between lung transplant centers will be paramount as we move forward in advancing this field. As we do so, efforts to successfully increase the donor pool will serve to provide a life-saving therapy to an ever-growing number of patients with end-stage lung disease.
Dr. Sala and Dr. Tomic are with the Division of Pulmonary and Critical Care Medicine, Northwestern University, Chicago, Illinois.
Lung transplants are increasing, with 2,562 performed in the United States in 2018 – a 31% increase over the preceding 5 years. With this increased demand for donor lungs, waitlist mortality in the United States is 9.4 deaths per 100 waitlist-years for obstructive lung diseases and as high as 29.7 deaths per 100 waitlist-years for restrictive lung diseases (Valapour M, et al. Lung. Am J Transplant. 2020;20[suppl s1]:427). Conversely, lungs are utilized from eligible multiorgan donors only 15% to 20% of the time, usually due to concerns over donor history or organ quality (Young KA, et al. Chest. 2019;155[3]:465). In light of this imbalance of supply and demand, lung transplant specialists are making significant efforts to expand the donor pool of available organs. Three of these strategies include: (1) applications of ex-vivo lung perfusion (EVLP) technology; (2) use of lungs from hepatitis C-positive donors for hep-C negative recipients; and (3) increasing utilization of donation after cardiac death.
Normothermic ex-vivo lung perfusion is a technology which allows donor lungs to be perfused and ventilated after removal from the donor but before transplant into the recipient. This is in contrast to the traditional method of cold static preservation. The proposed advantage of using this technology is to allow time for a more thorough assessment of graft quality and to improve function of grafts not meeting established criteria for transplant, all-the-while decreasing organ ischemia despite an increased cross-clamp time. There are currently four commercial systems available capable of EVLP. Broadly speaking, three EVLP management protocols exist (Toronto, Lund, and OCS), which differ in perfusate composition, target flow, pulmonary arterial pressure, left atrial pressure, and ventilatory settings. Notably, the Toronto protocol uses a closed left atrium, whereas the Lund and OCS protocol use an open left atrium. There are excellent published reviews of the different systems (Possoz J, et al. J Thorac Dis. 2019;11[4]:1635). EVLP has now been studied for two different goals: (1) to allow an extended evaluation of lungs of questionable quality before transplant; or (2) for routine use in all lung transplantations in place of cold static preservation.
In most studies concerning the use of EVLP for reconditioning of donor lungs, “high risk” or “extended criteria” refers to one or more of the following: P/F ratios < 300 on arterial blood gas, macroscopic abnormalities (eg, pulmonary edema, poor lung compliance), donation after circulatory death, or high-risk history (eg, aspiration). The largest cohort with the longest follow-up addressing the role of EVLP for donation of lungs with extended criteria was published from the Toronto Lung Transplant Group. Their results have demonstrated equivalent graft survival and rates of chronic lung allograft dysfunction (CLAD) up to 9 years posttransplant compared with standard criteria donor lungs, despite utilizing lower quality lungs and having a longer median preservation (Divithotawela C, et al. JAMA Surg. 2019;154[12]:1143). The group’s subsequent lung transplant rates have increased over the past decade.
A separate study addressed the same question but differed in that it was a single-arm, multicenter, international trial that tracked the outcomes of 93 extended criteria lungs placed on EVLP (including a large proportion acquired via donation after circulatory death) (Loor G, et al. Lancet Respir Med. 2019;7[11]:975). Among these, 87% of eligible lungs were transplanted, and outcomes were excellent, albeit shorter in follow-up compared with the Toronto cohort (eg, primary graft dysfunction grade 3 (PGD3) within 72 hours was 44% and 1-year survival was 91%). Based on these trials and many other retrospective reports, it has been concluded by many experts in the field that EVLP-treated extended criteria donor lungs perform equally well to standard criteria donor lungs.
Two RCTs have been conducted to evaluate whether EVLP is noninferior to static cold storage with donor lungs meeting “standard criteria” for transplant. The first was a single center study at the Medical University of Vienna, that looked at 80 recipient/donor pairs. Lungs in the EVLP arm underwent 4 hours of perfusion with frequent reassessment of quality before transplant, whereas the lungs in the control arm went directly to transplant. This study met noninferiority criteria looking at primary outcomes of PGD grade >1 and 30-day survival (Slama A, et al. J Heart Lung Transplant. 2017;36[7]:744). The second study was a phase 3, multicenter, international trial that included 320 recipient/donor pairs randomized to either EVLP (without a prespecified time on the EVLP system) or static cold storage. This trial met noninferiority for safety endpoints (lung graft-related adverse events within 30 days) and a composite primary outcome of PGD grade 3 incidence within 72 hours and 30-day survival (Warnecke G, et al. Lancet Respir Med. 2018;6[5]:357). The authors also tested and found superiority of EVLP in lower PGD grade 3 frequency compared with control. While these RCTs may suggest a role for EVLP in the procurement process of standard criteria organs in addition to extended criteria organs in the future, major criticisms for these trials include the lack of a demonstrable clinical benefit over cold storage beyond the lower PGD3 rates.
In the era of direct-acting antiviral agents available to treat HCV infection, there has been efforts to study the early use of anti-HCV medications to prevent infection as a result of heart or lung transplant from HCV viremic donors to HCV-negative recipients. In one major trial on efficacy, it was found that 4 weeks of sofosbuvir and velpatasvir, when started within a few hours of transplant, was sufficient to achieve a sustained (undetectable) virologic response at 12 weeks after completion of the antiviral regimen (Woolley AE, et al. N Engl J Med. 2019;380[17]:1606). Therefore, many transplant centers have adopted protocols to increase the donor pool (by CDC estimates about 4% of solid organ donors are HCV-positive) by accepting HCV nucleic acid amplification test (NAT)-positive donors for HCV-negative recipients, after appropriate informed consent.
Donation after cardiac death (DCD), which is alternatively known as donation after circulatory determination of death (DCDD), generally refers to organ procurement taking place after cessation of circulation, often after inpatient withdrawal of support. This is in contrast to the much more common practice of donation after brain death (DBD). Addressing concerns over the quality of lungs donated in the context of DCD compared with DBD, analyses of ISHLT registry data have demonstrated no differences in hospital length of stay or survival at 1 or 5 years (Van Raemdonck D, et al. J Heart Lung Transplant. 2019;38[12]:1235). Outcomes comparing specific mechanisms of donor death in DCD remain relatively unknown, such as outcomes from donors withdrawn from life support vs donors who had an uncontrolled cardiac death.
These methods for expanding the donor pool are not mutually exclusive, and, in fact, application of EVLP for lungs obtained in the context of DCD seems to be increasingly common. Optimization of protocols with collaboration between lung transplant centers will be paramount as we move forward in advancing this field. As we do so, efforts to successfully increase the donor pool will serve to provide a life-saving therapy to an ever-growing number of patients with end-stage lung disease.
Dr. Sala and Dr. Tomic are with the Division of Pulmonary and Critical Care Medicine, Northwestern University, Chicago, Illinois.
Lung transplants are increasing, with 2,562 performed in the United States in 2018 – a 31% increase over the preceding 5 years. With this increased demand for donor lungs, waitlist mortality in the United States is 9.4 deaths per 100 waitlist-years for obstructive lung diseases and as high as 29.7 deaths per 100 waitlist-years for restrictive lung diseases (Valapour M, et al. Lung. Am J Transplant. 2020;20[suppl s1]:427). Conversely, lungs are utilized from eligible multiorgan donors only 15% to 20% of the time, usually due to concerns over donor history or organ quality (Young KA, et al. Chest. 2019;155[3]:465). In light of this imbalance of supply and demand, lung transplant specialists are making significant efforts to expand the donor pool of available organs. Three of these strategies include: (1) applications of ex-vivo lung perfusion (EVLP) technology; (2) use of lungs from hepatitis C-positive donors for hep-C negative recipients; and (3) increasing utilization of donation after cardiac death.
Normothermic ex-vivo lung perfusion is a technology which allows donor lungs to be perfused and ventilated after removal from the donor but before transplant into the recipient. This is in contrast to the traditional method of cold static preservation. The proposed advantage of using this technology is to allow time for a more thorough assessment of graft quality and to improve function of grafts not meeting established criteria for transplant, all-the-while decreasing organ ischemia despite an increased cross-clamp time. There are currently four commercial systems available capable of EVLP. Broadly speaking, three EVLP management protocols exist (Toronto, Lund, and OCS), which differ in perfusate composition, target flow, pulmonary arterial pressure, left atrial pressure, and ventilatory settings. Notably, the Toronto protocol uses a closed left atrium, whereas the Lund and OCS protocol use an open left atrium. There are excellent published reviews of the different systems (Possoz J, et al. J Thorac Dis. 2019;11[4]:1635). EVLP has now been studied for two different goals: (1) to allow an extended evaluation of lungs of questionable quality before transplant; or (2) for routine use in all lung transplantations in place of cold static preservation.
In most studies concerning the use of EVLP for reconditioning of donor lungs, “high risk” or “extended criteria” refers to one or more of the following: P/F ratios < 300 on arterial blood gas, macroscopic abnormalities (eg, pulmonary edema, poor lung compliance), donation after circulatory death, or high-risk history (eg, aspiration). The largest cohort with the longest follow-up addressing the role of EVLP for donation of lungs with extended criteria was published from the Toronto Lung Transplant Group. Their results have demonstrated equivalent graft survival and rates of chronic lung allograft dysfunction (CLAD) up to 9 years posttransplant compared with standard criteria donor lungs, despite utilizing lower quality lungs and having a longer median preservation (Divithotawela C, et al. JAMA Surg. 2019;154[12]:1143). The group’s subsequent lung transplant rates have increased over the past decade.
A separate study addressed the same question but differed in that it was a single-arm, multicenter, international trial that tracked the outcomes of 93 extended criteria lungs placed on EVLP (including a large proportion acquired via donation after circulatory death) (Loor G, et al. Lancet Respir Med. 2019;7[11]:975). Among these, 87% of eligible lungs were transplanted, and outcomes were excellent, albeit shorter in follow-up compared with the Toronto cohort (eg, primary graft dysfunction grade 3 (PGD3) within 72 hours was 44% and 1-year survival was 91%). Based on these trials and many other retrospective reports, it has been concluded by many experts in the field that EVLP-treated extended criteria donor lungs perform equally well to standard criteria donor lungs.
Two RCTs have been conducted to evaluate whether EVLP is noninferior to static cold storage with donor lungs meeting “standard criteria” for transplant. The first was a single center study at the Medical University of Vienna, that looked at 80 recipient/donor pairs. Lungs in the EVLP arm underwent 4 hours of perfusion with frequent reassessment of quality before transplant, whereas the lungs in the control arm went directly to transplant. This study met noninferiority criteria looking at primary outcomes of PGD grade >1 and 30-day survival (Slama A, et al. J Heart Lung Transplant. 2017;36[7]:744). The second study was a phase 3, multicenter, international trial that included 320 recipient/donor pairs randomized to either EVLP (without a prespecified time on the EVLP system) or static cold storage. This trial met noninferiority for safety endpoints (lung graft-related adverse events within 30 days) and a composite primary outcome of PGD grade 3 incidence within 72 hours and 30-day survival (Warnecke G, et al. Lancet Respir Med. 2018;6[5]:357). The authors also tested and found superiority of EVLP in lower PGD grade 3 frequency compared with control. While these RCTs may suggest a role for EVLP in the procurement process of standard criteria organs in addition to extended criteria organs in the future, major criticisms for these trials include the lack of a demonstrable clinical benefit over cold storage beyond the lower PGD3 rates.
In the era of direct-acting antiviral agents available to treat HCV infection, there has been efforts to study the early use of anti-HCV medications to prevent infection as a result of heart or lung transplant from HCV viremic donors to HCV-negative recipients. In one major trial on efficacy, it was found that 4 weeks of sofosbuvir and velpatasvir, when started within a few hours of transplant, was sufficient to achieve a sustained (undetectable) virologic response at 12 weeks after completion of the antiviral regimen (Woolley AE, et al. N Engl J Med. 2019;380[17]:1606). Therefore, many transplant centers have adopted protocols to increase the donor pool (by CDC estimates about 4% of solid organ donors are HCV-positive) by accepting HCV nucleic acid amplification test (NAT)-positive donors for HCV-negative recipients, after appropriate informed consent.
Donation after cardiac death (DCD), which is alternatively known as donation after circulatory determination of death (DCDD), generally refers to organ procurement taking place after cessation of circulation, often after inpatient withdrawal of support. This is in contrast to the much more common practice of donation after brain death (DBD). Addressing concerns over the quality of lungs donated in the context of DCD compared with DBD, analyses of ISHLT registry data have demonstrated no differences in hospital length of stay or survival at 1 or 5 years (Van Raemdonck D, et al. J Heart Lung Transplant. 2019;38[12]:1235). Outcomes comparing specific mechanisms of donor death in DCD remain relatively unknown, such as outcomes from donors withdrawn from life support vs donors who had an uncontrolled cardiac death.
These methods for expanding the donor pool are not mutually exclusive, and, in fact, application of EVLP for lungs obtained in the context of DCD seems to be increasingly common. Optimization of protocols with collaboration between lung transplant centers will be paramount as we move forward in advancing this field. As we do so, efforts to successfully increase the donor pool will serve to provide a life-saving therapy to an ever-growing number of patients with end-stage lung disease.
Dr. Sala and Dr. Tomic are with the Division of Pulmonary and Critical Care Medicine, Northwestern University, Chicago, Illinois.
Cutaneous Id Reaction After Using Cyanoacrylate for Wound Closure
To the Editor:
In 1998, 2-octyl-cyanoacrylate (2-CA) tissue adhesive gained US Food and Drug Administration approval for topical application to easily hold closed approximated skin edges from surgical excisions and simple trauma-induced lacerations.1 It has since been employed for a number of off-label indications, including sutureless circumcision,2 skin graft fixation,3 pericatheter leakage,4 and intracorporeal use to control air leaks during lung resection.5 Animal investigations additionally have attempted to elucidate potential future uses of 2-CA for procedures such as inguinal hernia repair,6 bowel anastomosis,7 incisional hernia repair with mesh,8 and microvascular anastomosis.9 Compared to sutures, 2-CA offers ease and rapidity of application, a water-resistant barrier, and equivalent cosmetic results, as well as eliminates the need for suture removal.10 As 2-CA is used with increasing frequency across a variety of settings, there arises a greater need to be mindful of the potential complications of its use, such as irritant contact dermatitis (ICD), allergic contact dermatitis (ACD), and cutaneous id reaction.
A 14-year-old adolescent boy with no notable medical history and no known allergies underwent a minimally invasive Nuss procedure11 (performed by P.L.G.) for the repair of severe pectus excavatum. Two 4-cm incisions were made—one in each lateral chest wall at the approximately eighth intercostal space—to facilitate the introduction of the Nuss bar. The surgical wounds were closed with 2 layers of running polyglactin 910 suture before 2-CA was applied topically to the incision sites. The surgery was well tolerated, and the patient’s wounds healed without incident. When the patient was evaluated for Nuss bar removal 3 years later, incision sites were noted to be well healed, and he exhibited no other skin lesions. The original incision sites (bilateral chest walls) were utilized to facilitate surgical Nuss bar removal. The wounds were closed in 4 layers and 2-CA was again applied topically to the incision sites. There were no intraoperative complications; no devices, drains, or tissue implants were left in the patient at the conclusion of the procedure.
One week later, via text message and digital photographs, the patient reported intense pruritus at the bilateral chest wall incision sites, which were now surrounded by symmetric 1-cm erythematous plaques and associated sparse erythematous satellite papules (Figure 1). The patient denied any fevers, pain, swelling, or purulent discharge from the wounds. He was started on hydrocortisone cream 1% twice daily as well as oral diphenhydramine 25 mg at bedtime with initial good effect.
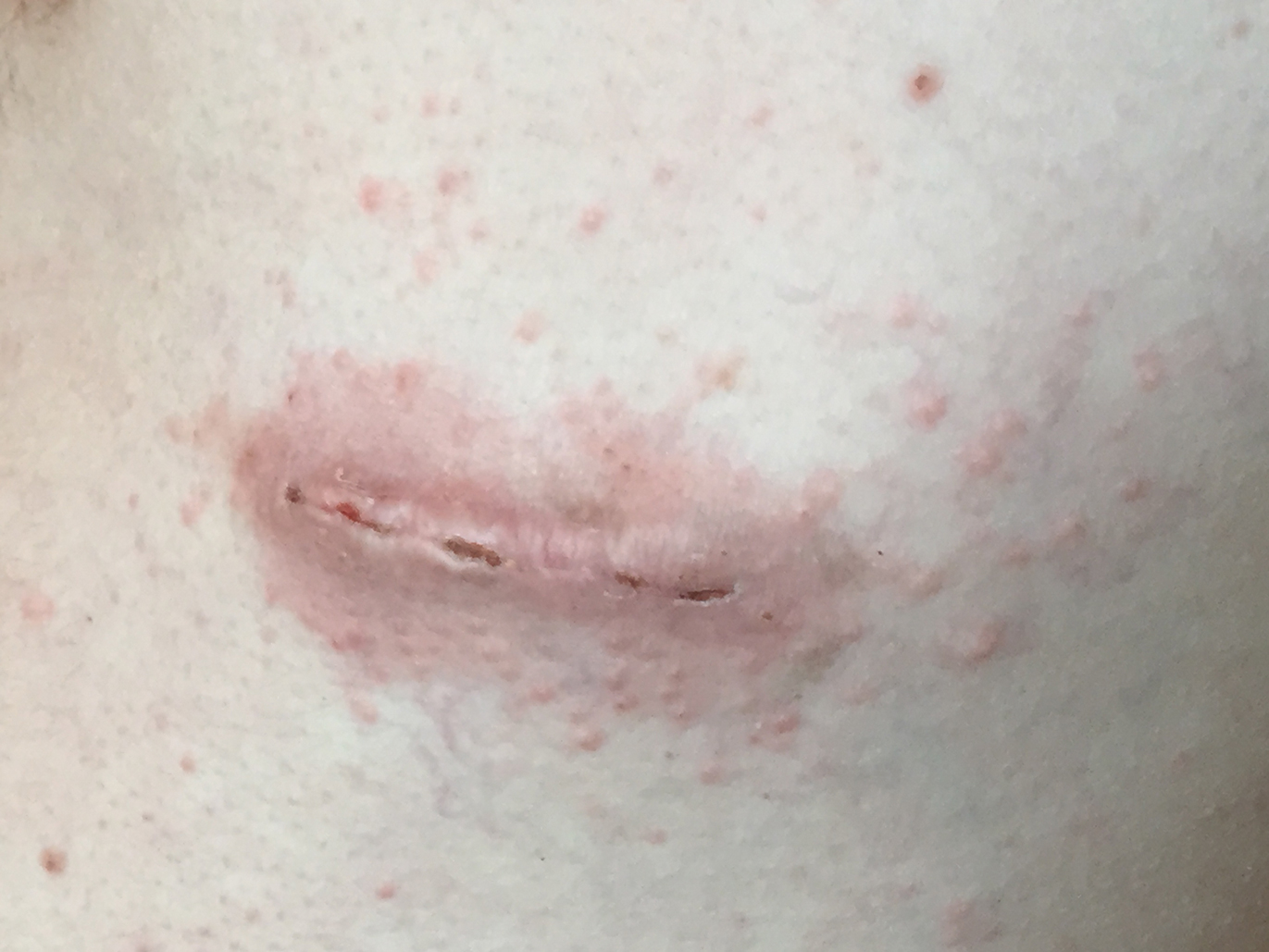
Three days later, the patient sent digital photographs of a morphologically similar–appearing rash that had progressed beyond the lateral chest walls to include the central chest and bilateral upper and lower extremities (Figure 2). He continued to deny any local or systemic signs of infection. Dermatology was consulted, and a diagnosis of ACD with cutaneous id reaction was made. The patient’s medication regimen was modified to include triamcinolone acetonide cream 0.1% applied twice daily to the rash away from the wounds, clobetasol propionate ointment 0.05% applied twice daily to the rash at the wound sites, oral levocetirizine 5 mg once daily, and oral hydroxyzine 25 to 50 mg every 6 hours as needed for pruritus. Additional recommendations included the use of a fragrance-free soap and application of an over-the-counter anti-itch lotion containing menthol and camphor applied as needed. Within 24 hours of starting this modified treatment regimen, the patient began to notice an improvement in symptoms, with full resolution over the course of the ensuing 2 weeks. The patient was counseled to inform his physicians—present and future—of his allergy to 2-CA.

Contact dermatitis associated with the use of 2-CA has been described in the literature.12-15 We report progression to an id reaction, which is characterized by the diffuse symmetric spread of a cutaneous eruption at a site distant from the primary localized dermatitis that develops within a few days of the primary lesion and exhibits the same morphologic and histopathologic findings.16,17 In our patient, pruritic erythematous papules and plaques symmetrically distributed on the arms, legs, and chest appeared 3 days after he first reported a similar eruption at the 2-CA application sites. It is theorized that id reactions develop when the sensitization phase of a type IV hypersensitivity reaction generates a population of T cells that not only recognizes a hapten but also recognizes keratinocyte-derived epitopes.16 A hapten is a small molecule (<500 Da) that is capable of penetrating the stratum corneum and binding skin components. A contact allergen is a hapten that has bound epidermal proteins to create a new antigenic determinant.18 The secondary dermatitis that characterizes id reactions results from an abnormal autoimmune response. Id reactions associated with exposure to adhesive material are rare.19
Allergic contact dermatitis is a type IV hypersensitivity reaction that appears after initial sensitization to an allergen followed by re-exposure. Our patient presented with symmetric erythematous plaques at the surgical incision sites 1 week after 2-CA had been applied. During this interval, sensitization to the inciting allergen occurred. The allergen is taken up by antigen-presenting cells, which then migrate to lymph nodes where they encounter naïve T lymphocytes that subsequently undergo clonal expansion to produce a cohort of T cells that are capable of recognizing the allergen. If subsequent exposure to the specific allergen takes place, an elicitation phase occurs in which primed T cells are incited to release mediators of inflammation that engender the manifestations of ACD within 24 to 72 hours.18,20 Sensitization may be promoted by skin barrier impairments such as dermatitis or a frank wound.12,20 In most cases, the patient is unaware that sensitization has occurred, though a primary ACD within 5 to 15 days after initial exposure to the inciting allergen rarely may be observed.18 Although our patient had 2-CA applied to his surgical wounds at 14 years of age, it was unlikely that sensitization took place at that time, as it was 1 week rather than 1 to 3 days before he experienced the cutaneous eruption associated with his second 2-CA exposure at 17 years of age.
Cyanoacrylate tissue adhesive also may cause ICD resulting from histotoxic degradation products such as formaldehyde and cyanoacetate that are capable of compromising cutaneous barrier function. Keratinocytes that have had their membranes disturbed release proinflammatory cytokines, which recruit cells of the innate immune system as well as T lymphocytes to the site of insult to facilitate the inflammatory response. The manifestations of ICD include erythema, edema, and local necrosis that can compromise wound healing.20 The speed at which a given cyanoacrylate adhesive degrades is proportional to the length of its carbon side chain. Those with shorter side chains—ethyl and methyl cyanoacrylate—degrade more rapidly into formaldehyde and cyanoacetate; 2-CA possesses a longer side chain and therefore degrades more slowly, which should, in theory, lessen its potential to cause ICD.20 Because it may take 7 to 14 days before 2-CA will spontaneously peel from the application site, however, its potential to evoke ICD nevertheless exists.
Treatment of ICD entails removing the irritant while concurrently working to restore the skin’s barrier with emollients. Although topical corticosteroids often are reflexively prescribed to treat rashes, some believe that their use should be avoided in cases of ICD, as their inhibitory effects on epidermal lipid synthesis may further impair the skin’s barrier.21 For cases of ACD, with or without an accompanying id reaction, topical corticosteroids are the mainstay of therapy. It is customary to start with a higher-potency topical steroid such as clobetasol and taper to lower-potency steroids as the patient’s condition improves. Steroid ointments are petroleum based and are capable of causing 2-CA to separate from the skin.10 As a result, they should be used with care when being applied to an area where 2-CA is maintaining dermal closure. Systemic corticosteroids may be warranted in cases with involvement of more than 20% of the body surface area and should start to provide relief within 12 to 24 hours.22 Oral antihistamines and cold water compresses can be added to help address pruritus and discomfort in both ACD and ICD.
Instances of contact dermatitis caused by 2-CA are rare, and progression to an id reaction is rarer still. Physicians should be aware of the possibility of encountering a patient that manifests one or both of these complications whenever 2-CA is employed for skin closure. Physicians who employ 2-CA for skin closure should first ask patients about prior cutaneous reactions to cyanoacrylates including 2-CA and other commonly encountered acrylate-containing products including adhesive wound dressings, dental cements and prostheses, superglue, artificial nails, and adhesives for wigs and false eyelashes. Still, many patients who exhibit acrylate-induced contact dermatitis, with or without an associated id reaction, will not attest to a history of adverse reactions; they simply may not recognize acrylate as the inciting agent. Practitioners across a range of specialties outside of dermatology—surgeons, emergency physicians, and primary care providers—should be prepared to both recognize contact dermatitis and id reaction arising from the use of 2-CA and implement a basic treatment plan that will bring the patient relief without compromising wound closure.
- US Food and Drug Administration. Premarket approval (PMA). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=p960052. Accessed March 4, 2020.
- Elmore JM, Smith EA, Kirsch AJ. Sutureless circumcision using 2-octyl cyanoacrylate (Dermabond): appraisal after 18-month experience. Urology. 2007;70:803-806.
- Kilic A, Ozdengil E. Skin graft fixation by applying cyanoacrylate without any complication. Plast Reconstr Surg. 2002;110:370-371.
- Gurnaney H, Kraemer FW, Ganesh A. Dermabond decreases pericatheter local anesthetic leakage after continuous perineural infusions. Anesth Analg. 2011;113:206.
- Carr JA. The intracorporeal use of 2-octyl cyanoacrylate resin to control air leaks after lung resection. Eur J Cardiothorac Surg. 2011;39:579-583.
- Miyano G, Yamataka A, Kato Y, et al. Laparoscopic injection of Dermabond tissue adhesive for the repair of inguinal hernia: short- and long-term follow-up. J Pediatr Surg. 2004;39:1867-1870.
- Paral J, Subrt Z, Lochman P, et al. Suture-free anastomosis of the colon. experimental comparison of two cyanoacrylate adhesives. J Gastrointest Surg. 2011;15:451-459.
- Birch DW, Park A. Octylcyanoacrylate tissue adhesive as an alternative to mechanical fixation of expanded polytetrafluoroethylene prosthesis. Am Surg. 2001;67:974-978.
- Ang ES, Tan KC, Tan LH, et al. 2-octylcyanoacrylate-assisted microvascular anastomosis: comparison with a conventional suture technique in rat femoral arteries. J Reconstr Microsurg. 2001;17:193-201.
- Bruns TB, Worthington JM. Using tissue adhesive for wound repair: a practical guide to Dermabond. Am Fam Physician. 2000;61:1383-1388.
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998;33:545-552.
- Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octylcyanoacrylate. Arch Dermatol. 2008;144:814-815.
- Howard BK, Downey SE. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
- Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
- Sachse MM, Junghans T, Rose C, et al. Allergic contact dermatitis caused by topical 2-octyl-cyanoacrylate. Contact Dermatitis. 2013;68:317-319.
- Fehr BS, Takashima A, Bergstresser PR, et al. T cells reactive to keratinocyte antigens are generated during induction of contact hypersensitivity in mice. a model for autoeczematization in humans? Am J Contact Dermat. 2000;11:145-154.
- Gonzalez-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Vocanson M, Hennino A, Rozières A, et al. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy. 2009;64:1699-1714.
- Sommer LL, Hejazi EZ, Heymann WR. An acute linear pruritic eruption following allergic contact dermatitis. J Clin Aesthet Dermatol. 2014;7:42-44.
- Rietschel RL, Fowler JF. Plastics, adhesives, and synthetic resins. In: Rietschek RL, Fowler JF, eds. Fisher’s Contact Dermatitis. Hamilton, BC: Decker Inc; 2008:542-560.
- Kao JS, Fluhr JW, Man M, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003;120:456-464.
- American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Contact dermatitis: a practice parameter. Ann Allergy Asthma Immunol. 2006;97(3 suppl 2):S1-S38.
To the Editor:
In 1998, 2-octyl-cyanoacrylate (2-CA) tissue adhesive gained US Food and Drug Administration approval for topical application to easily hold closed approximated skin edges from surgical excisions and simple trauma-induced lacerations.1 It has since been employed for a number of off-label indications, including sutureless circumcision,2 skin graft fixation,3 pericatheter leakage,4 and intracorporeal use to control air leaks during lung resection.5 Animal investigations additionally have attempted to elucidate potential future uses of 2-CA for procedures such as inguinal hernia repair,6 bowel anastomosis,7 incisional hernia repair with mesh,8 and microvascular anastomosis.9 Compared to sutures, 2-CA offers ease and rapidity of application, a water-resistant barrier, and equivalent cosmetic results, as well as eliminates the need for suture removal.10 As 2-CA is used with increasing frequency across a variety of settings, there arises a greater need to be mindful of the potential complications of its use, such as irritant contact dermatitis (ICD), allergic contact dermatitis (ACD), and cutaneous id reaction.
A 14-year-old adolescent boy with no notable medical history and no known allergies underwent a minimally invasive Nuss procedure11 (performed by P.L.G.) for the repair of severe pectus excavatum. Two 4-cm incisions were made—one in each lateral chest wall at the approximately eighth intercostal space—to facilitate the introduction of the Nuss bar. The surgical wounds were closed with 2 layers of running polyglactin 910 suture before 2-CA was applied topically to the incision sites. The surgery was well tolerated, and the patient’s wounds healed without incident. When the patient was evaluated for Nuss bar removal 3 years later, incision sites were noted to be well healed, and he exhibited no other skin lesions. The original incision sites (bilateral chest walls) were utilized to facilitate surgical Nuss bar removal. The wounds were closed in 4 layers and 2-CA was again applied topically to the incision sites. There were no intraoperative complications; no devices, drains, or tissue implants were left in the patient at the conclusion of the procedure.
One week later, via text message and digital photographs, the patient reported intense pruritus at the bilateral chest wall incision sites, which were now surrounded by symmetric 1-cm erythematous plaques and associated sparse erythematous satellite papules (Figure 1). The patient denied any fevers, pain, swelling, or purulent discharge from the wounds. He was started on hydrocortisone cream 1% twice daily as well as oral diphenhydramine 25 mg at bedtime with initial good effect.

Three days later, the patient sent digital photographs of a morphologically similar–appearing rash that had progressed beyond the lateral chest walls to include the central chest and bilateral upper and lower extremities (Figure 2). He continued to deny any local or systemic signs of infection. Dermatology was consulted, and a diagnosis of ACD with cutaneous id reaction was made. The patient’s medication regimen was modified to include triamcinolone acetonide cream 0.1% applied twice daily to the rash away from the wounds, clobetasol propionate ointment 0.05% applied twice daily to the rash at the wound sites, oral levocetirizine 5 mg once daily, and oral hydroxyzine 25 to 50 mg every 6 hours as needed for pruritus. Additional recommendations included the use of a fragrance-free soap and application of an over-the-counter anti-itch lotion containing menthol and camphor applied as needed. Within 24 hours of starting this modified treatment regimen, the patient began to notice an improvement in symptoms, with full resolution over the course of the ensuing 2 weeks. The patient was counseled to inform his physicians—present and future—of his allergy to 2-CA.

Contact dermatitis associated with the use of 2-CA has been described in the literature.12-15 We report progression to an id reaction, which is characterized by the diffuse symmetric spread of a cutaneous eruption at a site distant from the primary localized dermatitis that develops within a few days of the primary lesion and exhibits the same morphologic and histopathologic findings.16,17 In our patient, pruritic erythematous papules and plaques symmetrically distributed on the arms, legs, and chest appeared 3 days after he first reported a similar eruption at the 2-CA application sites. It is theorized that id reactions develop when the sensitization phase of a type IV hypersensitivity reaction generates a population of T cells that not only recognizes a hapten but also recognizes keratinocyte-derived epitopes.16 A hapten is a small molecule (<500 Da) that is capable of penetrating the stratum corneum and binding skin components. A contact allergen is a hapten that has bound epidermal proteins to create a new antigenic determinant.18 The secondary dermatitis that characterizes id reactions results from an abnormal autoimmune response. Id reactions associated with exposure to adhesive material are rare.19
Allergic contact dermatitis is a type IV hypersensitivity reaction that appears after initial sensitization to an allergen followed by re-exposure. Our patient presented with symmetric erythematous plaques at the surgical incision sites 1 week after 2-CA had been applied. During this interval, sensitization to the inciting allergen occurred. The allergen is taken up by antigen-presenting cells, which then migrate to lymph nodes where they encounter naïve T lymphocytes that subsequently undergo clonal expansion to produce a cohort of T cells that are capable of recognizing the allergen. If subsequent exposure to the specific allergen takes place, an elicitation phase occurs in which primed T cells are incited to release mediators of inflammation that engender the manifestations of ACD within 24 to 72 hours.18,20 Sensitization may be promoted by skin barrier impairments such as dermatitis or a frank wound.12,20 In most cases, the patient is unaware that sensitization has occurred, though a primary ACD within 5 to 15 days after initial exposure to the inciting allergen rarely may be observed.18 Although our patient had 2-CA applied to his surgical wounds at 14 years of age, it was unlikely that sensitization took place at that time, as it was 1 week rather than 1 to 3 days before he experienced the cutaneous eruption associated with his second 2-CA exposure at 17 years of age.
Cyanoacrylate tissue adhesive also may cause ICD resulting from histotoxic degradation products such as formaldehyde and cyanoacetate that are capable of compromising cutaneous barrier function. Keratinocytes that have had their membranes disturbed release proinflammatory cytokines, which recruit cells of the innate immune system as well as T lymphocytes to the site of insult to facilitate the inflammatory response. The manifestations of ICD include erythema, edema, and local necrosis that can compromise wound healing.20 The speed at which a given cyanoacrylate adhesive degrades is proportional to the length of its carbon side chain. Those with shorter side chains—ethyl and methyl cyanoacrylate—degrade more rapidly into formaldehyde and cyanoacetate; 2-CA possesses a longer side chain and therefore degrades more slowly, which should, in theory, lessen its potential to cause ICD.20 Because it may take 7 to 14 days before 2-CA will spontaneously peel from the application site, however, its potential to evoke ICD nevertheless exists.
Treatment of ICD entails removing the irritant while concurrently working to restore the skin’s barrier with emollients. Although topical corticosteroids often are reflexively prescribed to treat rashes, some believe that their use should be avoided in cases of ICD, as their inhibitory effects on epidermal lipid synthesis may further impair the skin’s barrier.21 For cases of ACD, with or without an accompanying id reaction, topical corticosteroids are the mainstay of therapy. It is customary to start with a higher-potency topical steroid such as clobetasol and taper to lower-potency steroids as the patient’s condition improves. Steroid ointments are petroleum based and are capable of causing 2-CA to separate from the skin.10 As a result, they should be used with care when being applied to an area where 2-CA is maintaining dermal closure. Systemic corticosteroids may be warranted in cases with involvement of more than 20% of the body surface area and should start to provide relief within 12 to 24 hours.22 Oral antihistamines and cold water compresses can be added to help address pruritus and discomfort in both ACD and ICD.
Instances of contact dermatitis caused by 2-CA are rare, and progression to an id reaction is rarer still. Physicians should be aware of the possibility of encountering a patient that manifests one or both of these complications whenever 2-CA is employed for skin closure. Physicians who employ 2-CA for skin closure should first ask patients about prior cutaneous reactions to cyanoacrylates including 2-CA and other commonly encountered acrylate-containing products including adhesive wound dressings, dental cements and prostheses, superglue, artificial nails, and adhesives for wigs and false eyelashes. Still, many patients who exhibit acrylate-induced contact dermatitis, with or without an associated id reaction, will not attest to a history of adverse reactions; they simply may not recognize acrylate as the inciting agent. Practitioners across a range of specialties outside of dermatology—surgeons, emergency physicians, and primary care providers—should be prepared to both recognize contact dermatitis and id reaction arising from the use of 2-CA and implement a basic treatment plan that will bring the patient relief without compromising wound closure.
To the Editor:
In 1998, 2-octyl-cyanoacrylate (2-CA) tissue adhesive gained US Food and Drug Administration approval for topical application to easily hold closed approximated skin edges from surgical excisions and simple trauma-induced lacerations.1 It has since been employed for a number of off-label indications, including sutureless circumcision,2 skin graft fixation,3 pericatheter leakage,4 and intracorporeal use to control air leaks during lung resection.5 Animal investigations additionally have attempted to elucidate potential future uses of 2-CA for procedures such as inguinal hernia repair,6 bowel anastomosis,7 incisional hernia repair with mesh,8 and microvascular anastomosis.9 Compared to sutures, 2-CA offers ease and rapidity of application, a water-resistant barrier, and equivalent cosmetic results, as well as eliminates the need for suture removal.10 As 2-CA is used with increasing frequency across a variety of settings, there arises a greater need to be mindful of the potential complications of its use, such as irritant contact dermatitis (ICD), allergic contact dermatitis (ACD), and cutaneous id reaction.
A 14-year-old adolescent boy with no notable medical history and no known allergies underwent a minimally invasive Nuss procedure11 (performed by P.L.G.) for the repair of severe pectus excavatum. Two 4-cm incisions were made—one in each lateral chest wall at the approximately eighth intercostal space—to facilitate the introduction of the Nuss bar. The surgical wounds were closed with 2 layers of running polyglactin 910 suture before 2-CA was applied topically to the incision sites. The surgery was well tolerated, and the patient’s wounds healed without incident. When the patient was evaluated for Nuss bar removal 3 years later, incision sites were noted to be well healed, and he exhibited no other skin lesions. The original incision sites (bilateral chest walls) were utilized to facilitate surgical Nuss bar removal. The wounds were closed in 4 layers and 2-CA was again applied topically to the incision sites. There were no intraoperative complications; no devices, drains, or tissue implants were left in the patient at the conclusion of the procedure.
One week later, via text message and digital photographs, the patient reported intense pruritus at the bilateral chest wall incision sites, which were now surrounded by symmetric 1-cm erythematous plaques and associated sparse erythematous satellite papules (Figure 1). The patient denied any fevers, pain, swelling, or purulent discharge from the wounds. He was started on hydrocortisone cream 1% twice daily as well as oral diphenhydramine 25 mg at bedtime with initial good effect.

Three days later, the patient sent digital photographs of a morphologically similar–appearing rash that had progressed beyond the lateral chest walls to include the central chest and bilateral upper and lower extremities (Figure 2). He continued to deny any local or systemic signs of infection. Dermatology was consulted, and a diagnosis of ACD with cutaneous id reaction was made. The patient’s medication regimen was modified to include triamcinolone acetonide cream 0.1% applied twice daily to the rash away from the wounds, clobetasol propionate ointment 0.05% applied twice daily to the rash at the wound sites, oral levocetirizine 5 mg once daily, and oral hydroxyzine 25 to 50 mg every 6 hours as needed for pruritus. Additional recommendations included the use of a fragrance-free soap and application of an over-the-counter anti-itch lotion containing menthol and camphor applied as needed. Within 24 hours of starting this modified treatment regimen, the patient began to notice an improvement in symptoms, with full resolution over the course of the ensuing 2 weeks. The patient was counseled to inform his physicians—present and future—of his allergy to 2-CA.

Contact dermatitis associated with the use of 2-CA has been described in the literature.12-15 We report progression to an id reaction, which is characterized by the diffuse symmetric spread of a cutaneous eruption at a site distant from the primary localized dermatitis that develops within a few days of the primary lesion and exhibits the same morphologic and histopathologic findings.16,17 In our patient, pruritic erythematous papules and plaques symmetrically distributed on the arms, legs, and chest appeared 3 days after he first reported a similar eruption at the 2-CA application sites. It is theorized that id reactions develop when the sensitization phase of a type IV hypersensitivity reaction generates a population of T cells that not only recognizes a hapten but also recognizes keratinocyte-derived epitopes.16 A hapten is a small molecule (<500 Da) that is capable of penetrating the stratum corneum and binding skin components. A contact allergen is a hapten that has bound epidermal proteins to create a new antigenic determinant.18 The secondary dermatitis that characterizes id reactions results from an abnormal autoimmune response. Id reactions associated with exposure to adhesive material are rare.19
Allergic contact dermatitis is a type IV hypersensitivity reaction that appears after initial sensitization to an allergen followed by re-exposure. Our patient presented with symmetric erythematous plaques at the surgical incision sites 1 week after 2-CA had been applied. During this interval, sensitization to the inciting allergen occurred. The allergen is taken up by antigen-presenting cells, which then migrate to lymph nodes where they encounter naïve T lymphocytes that subsequently undergo clonal expansion to produce a cohort of T cells that are capable of recognizing the allergen. If subsequent exposure to the specific allergen takes place, an elicitation phase occurs in which primed T cells are incited to release mediators of inflammation that engender the manifestations of ACD within 24 to 72 hours.18,20 Sensitization may be promoted by skin barrier impairments such as dermatitis or a frank wound.12,20 In most cases, the patient is unaware that sensitization has occurred, though a primary ACD within 5 to 15 days after initial exposure to the inciting allergen rarely may be observed.18 Although our patient had 2-CA applied to his surgical wounds at 14 years of age, it was unlikely that sensitization took place at that time, as it was 1 week rather than 1 to 3 days before he experienced the cutaneous eruption associated with his second 2-CA exposure at 17 years of age.
Cyanoacrylate tissue adhesive also may cause ICD resulting from histotoxic degradation products such as formaldehyde and cyanoacetate that are capable of compromising cutaneous barrier function. Keratinocytes that have had their membranes disturbed release proinflammatory cytokines, which recruit cells of the innate immune system as well as T lymphocytes to the site of insult to facilitate the inflammatory response. The manifestations of ICD include erythema, edema, and local necrosis that can compromise wound healing.20 The speed at which a given cyanoacrylate adhesive degrades is proportional to the length of its carbon side chain. Those with shorter side chains—ethyl and methyl cyanoacrylate—degrade more rapidly into formaldehyde and cyanoacetate; 2-CA possesses a longer side chain and therefore degrades more slowly, which should, in theory, lessen its potential to cause ICD.20 Because it may take 7 to 14 days before 2-CA will spontaneously peel from the application site, however, its potential to evoke ICD nevertheless exists.
Treatment of ICD entails removing the irritant while concurrently working to restore the skin’s barrier with emollients. Although topical corticosteroids often are reflexively prescribed to treat rashes, some believe that their use should be avoided in cases of ICD, as their inhibitory effects on epidermal lipid synthesis may further impair the skin’s barrier.21 For cases of ACD, with or without an accompanying id reaction, topical corticosteroids are the mainstay of therapy. It is customary to start with a higher-potency topical steroid such as clobetasol and taper to lower-potency steroids as the patient’s condition improves. Steroid ointments are petroleum based and are capable of causing 2-CA to separate from the skin.10 As a result, they should be used with care when being applied to an area where 2-CA is maintaining dermal closure. Systemic corticosteroids may be warranted in cases with involvement of more than 20% of the body surface area and should start to provide relief within 12 to 24 hours.22 Oral antihistamines and cold water compresses can be added to help address pruritus and discomfort in both ACD and ICD.
Instances of contact dermatitis caused by 2-CA are rare, and progression to an id reaction is rarer still. Physicians should be aware of the possibility of encountering a patient that manifests one or both of these complications whenever 2-CA is employed for skin closure. Physicians who employ 2-CA for skin closure should first ask patients about prior cutaneous reactions to cyanoacrylates including 2-CA and other commonly encountered acrylate-containing products including adhesive wound dressings, dental cements and prostheses, superglue, artificial nails, and adhesives for wigs and false eyelashes. Still, many patients who exhibit acrylate-induced contact dermatitis, with or without an associated id reaction, will not attest to a history of adverse reactions; they simply may not recognize acrylate as the inciting agent. Practitioners across a range of specialties outside of dermatology—surgeons, emergency physicians, and primary care providers—should be prepared to both recognize contact dermatitis and id reaction arising from the use of 2-CA and implement a basic treatment plan that will bring the patient relief without compromising wound closure.
- US Food and Drug Administration. Premarket approval (PMA). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=p960052. Accessed March 4, 2020.
- Elmore JM, Smith EA, Kirsch AJ. Sutureless circumcision using 2-octyl cyanoacrylate (Dermabond): appraisal after 18-month experience. Urology. 2007;70:803-806.
- Kilic A, Ozdengil E. Skin graft fixation by applying cyanoacrylate without any complication. Plast Reconstr Surg. 2002;110:370-371.
- Gurnaney H, Kraemer FW, Ganesh A. Dermabond decreases pericatheter local anesthetic leakage after continuous perineural infusions. Anesth Analg. 2011;113:206.
- Carr JA. The intracorporeal use of 2-octyl cyanoacrylate resin to control air leaks after lung resection. Eur J Cardiothorac Surg. 2011;39:579-583.
- Miyano G, Yamataka A, Kato Y, et al. Laparoscopic injection of Dermabond tissue adhesive for the repair of inguinal hernia: short- and long-term follow-up. J Pediatr Surg. 2004;39:1867-1870.
- Paral J, Subrt Z, Lochman P, et al. Suture-free anastomosis of the colon. experimental comparison of two cyanoacrylate adhesives. J Gastrointest Surg. 2011;15:451-459.
- Birch DW, Park A. Octylcyanoacrylate tissue adhesive as an alternative to mechanical fixation of expanded polytetrafluoroethylene prosthesis. Am Surg. 2001;67:974-978.
- Ang ES, Tan KC, Tan LH, et al. 2-octylcyanoacrylate-assisted microvascular anastomosis: comparison with a conventional suture technique in rat femoral arteries. J Reconstr Microsurg. 2001;17:193-201.
- Bruns TB, Worthington JM. Using tissue adhesive for wound repair: a practical guide to Dermabond. Am Fam Physician. 2000;61:1383-1388.
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998;33:545-552.
- Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octylcyanoacrylate. Arch Dermatol. 2008;144:814-815.
- Howard BK, Downey SE. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
- Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
- Sachse MM, Junghans T, Rose C, et al. Allergic contact dermatitis caused by topical 2-octyl-cyanoacrylate. Contact Dermatitis. 2013;68:317-319.
- Fehr BS, Takashima A, Bergstresser PR, et al. T cells reactive to keratinocyte antigens are generated during induction of contact hypersensitivity in mice. a model for autoeczematization in humans? Am J Contact Dermat. 2000;11:145-154.
- Gonzalez-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Vocanson M, Hennino A, Rozières A, et al. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy. 2009;64:1699-1714.
- Sommer LL, Hejazi EZ, Heymann WR. An acute linear pruritic eruption following allergic contact dermatitis. J Clin Aesthet Dermatol. 2014;7:42-44.
- Rietschel RL, Fowler JF. Plastics, adhesives, and synthetic resins. In: Rietschek RL, Fowler JF, eds. Fisher’s Contact Dermatitis. Hamilton, BC: Decker Inc; 2008:542-560.
- Kao JS, Fluhr JW, Man M, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003;120:456-464.
- American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Contact dermatitis: a practice parameter. Ann Allergy Asthma Immunol. 2006;97(3 suppl 2):S1-S38.
- US Food and Drug Administration. Premarket approval (PMA). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=p960052. Accessed March 4, 2020.
- Elmore JM, Smith EA, Kirsch AJ. Sutureless circumcision using 2-octyl cyanoacrylate (Dermabond): appraisal after 18-month experience. Urology. 2007;70:803-806.
- Kilic A, Ozdengil E. Skin graft fixation by applying cyanoacrylate without any complication. Plast Reconstr Surg. 2002;110:370-371.
- Gurnaney H, Kraemer FW, Ganesh A. Dermabond decreases pericatheter local anesthetic leakage after continuous perineural infusions. Anesth Analg. 2011;113:206.
- Carr JA. The intracorporeal use of 2-octyl cyanoacrylate resin to control air leaks after lung resection. Eur J Cardiothorac Surg. 2011;39:579-583.
- Miyano G, Yamataka A, Kato Y, et al. Laparoscopic injection of Dermabond tissue adhesive for the repair of inguinal hernia: short- and long-term follow-up. J Pediatr Surg. 2004;39:1867-1870.
- Paral J, Subrt Z, Lochman P, et al. Suture-free anastomosis of the colon. experimental comparison of two cyanoacrylate adhesives. J Gastrointest Surg. 2011;15:451-459.
- Birch DW, Park A. Octylcyanoacrylate tissue adhesive as an alternative to mechanical fixation of expanded polytetrafluoroethylene prosthesis. Am Surg. 2001;67:974-978.
- Ang ES, Tan KC, Tan LH, et al. 2-octylcyanoacrylate-assisted microvascular anastomosis: comparison with a conventional suture technique in rat femoral arteries. J Reconstr Microsurg. 2001;17:193-201.
- Bruns TB, Worthington JM. Using tissue adhesive for wound repair: a practical guide to Dermabond. Am Fam Physician. 2000;61:1383-1388.
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998;33:545-552.
- Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octylcyanoacrylate. Arch Dermatol. 2008;144:814-815.
- Howard BK, Downey SE. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
- Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
- Sachse MM, Junghans T, Rose C, et al. Allergic contact dermatitis caused by topical 2-octyl-cyanoacrylate. Contact Dermatitis. 2013;68:317-319.
- Fehr BS, Takashima A, Bergstresser PR, et al. T cells reactive to keratinocyte antigens are generated during induction of contact hypersensitivity in mice. a model for autoeczematization in humans? Am J Contact Dermat. 2000;11:145-154.
- Gonzalez-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Vocanson M, Hennino A, Rozières A, et al. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy. 2009;64:1699-1714.
- Sommer LL, Hejazi EZ, Heymann WR. An acute linear pruritic eruption following allergic contact dermatitis. J Clin Aesthet Dermatol. 2014;7:42-44.
- Rietschel RL, Fowler JF. Plastics, adhesives, and synthetic resins. In: Rietschek RL, Fowler JF, eds. Fisher’s Contact Dermatitis. Hamilton, BC: Decker Inc; 2008:542-560.
- Kao JS, Fluhr JW, Man M, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003;120:456-464.
- American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Contact dermatitis: a practice parameter. Ann Allergy Asthma Immunol. 2006;97(3 suppl 2):S1-S38.
Practice Points
- 2-Octyl-cyanoacrylate (2-CA) tissue adhesive has been reported to cause contact dermatitis when applied topically for surgical site closure.
- Id reactions resulting from the use of 2-CA tissue adhesive are possible, though less commonly observed.
- Id reactions caused by 2-CA tissue adhesive respond well to treatment with a combination of topical steroids and oral antihistamines. Systemic corticosteroids may be warranted in cases involving greater than 20% body surface area.
Give me an occupation, Miss Dashwood
“I’ve been watching YouTube videos on how to set a ventilator,” said one of our dermatologists. The absurdity, levity, and gravity of that statement captures in a single sentence where we are today.
None of us alive have experience with such a crisis. It is as if our planet passed through a wormhole and we’ve been transported to the late medieval period: We doctors fighting the Black Death donned in beaked masks filled with juniper berries, mint, and clove to protect us from the miasma. Now, though, we spray store-bought lavender disinfectant on surgical masks.
“A crisis shows you a person’s soul,” said New York Governor Andrew Cuomo, adding: “It shows you what they’re made of, the weaknesses explode and the strengths ... emboldened.” Most of us have traveled through life with no experience of peril. Such mortal danger explodes and emboldens us, dividing us in two, the fearful or the phlegmatic.
When President Trump proclaimed that plaquenil was a promising treatment for the virus, prescriptions for the drug soared so quickly that four of eight manufacturers reported being in shortage by the end of the day. Many of those prescriptions were written by physicians for themselves and their families. Private Facebook physician groups shared insider tips for how to get around constraints and find the drug – as hoardable as toilet paper. As a department chief and fellow human being, I understand why some of us might behave this way. We didn’t sign up to be dermatologists or nephrologists or surgeons or pulmonologists agreeing that, to do so, we might die. We are all afraid.
The track of this epic storm became clear last week and now, terrifyingly, it appears it will be a direct hit. I braced for an onslaught of anxiety from our doctors and staff. But as the forecast became more grim, the courage began to well up and creativity climbed. Doctors went to local stores and bought all the masks and shields on their own. Rolls of toilet paper and diapers began magically appearing in our mom-doctors’ offices, delivered by angels in scrubs. I’ve practically had to install a velvet rope at my door to organize the queue of people wanting to talk to me about their ideas to help – keep 6 feet apart please! Stories like this abound. Even at the EvergreenHealth hospital in Washington they’ve not had shortages of staff. Rather than calling out sick, they called in: “If you need me, I’m available.”
Doctors are afraid and frustrated. Some of the things we will do in the coming weeks will first do no good, perhaps even harm. But I believe it’s because we’ve yet to embolden our strengths. It’s our job as leaders, attendings, administrators to inform and enable them.
When Marianne fell deathly ill in “Sense and Sensibility,” Colonel Branden wrung his hands and paced the floor. “Give me an occupation, Miss Dashwood, or I shall run mad.” Doctors are running, mad. And, just in case, some dermatologists are relearning how to intubate, waiting for that occupation to be given.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. He has no relevant conflicts of interest related to this column. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“I’ve been watching YouTube videos on how to set a ventilator,” said one of our dermatologists. The absurdity, levity, and gravity of that statement captures in a single sentence where we are today.
None of us alive have experience with such a crisis. It is as if our planet passed through a wormhole and we’ve been transported to the late medieval period: We doctors fighting the Black Death donned in beaked masks filled with juniper berries, mint, and clove to protect us from the miasma. Now, though, we spray store-bought lavender disinfectant on surgical masks.
“A crisis shows you a person’s soul,” said New York Governor Andrew Cuomo, adding: “It shows you what they’re made of, the weaknesses explode and the strengths ... emboldened.” Most of us have traveled through life with no experience of peril. Such mortal danger explodes and emboldens us, dividing us in two, the fearful or the phlegmatic.
When President Trump proclaimed that plaquenil was a promising treatment for the virus, prescriptions for the drug soared so quickly that four of eight manufacturers reported being in shortage by the end of the day. Many of those prescriptions were written by physicians for themselves and their families. Private Facebook physician groups shared insider tips for how to get around constraints and find the drug – as hoardable as toilet paper. As a department chief and fellow human being, I understand why some of us might behave this way. We didn’t sign up to be dermatologists or nephrologists or surgeons or pulmonologists agreeing that, to do so, we might die. We are all afraid.
The track of this epic storm became clear last week and now, terrifyingly, it appears it will be a direct hit. I braced for an onslaught of anxiety from our doctors and staff. But as the forecast became more grim, the courage began to well up and creativity climbed. Doctors went to local stores and bought all the masks and shields on their own. Rolls of toilet paper and diapers began magically appearing in our mom-doctors’ offices, delivered by angels in scrubs. I’ve practically had to install a velvet rope at my door to organize the queue of people wanting to talk to me about their ideas to help – keep 6 feet apart please! Stories like this abound. Even at the EvergreenHealth hospital in Washington they’ve not had shortages of staff. Rather than calling out sick, they called in: “If you need me, I’m available.”
Doctors are afraid and frustrated. Some of the things we will do in the coming weeks will first do no good, perhaps even harm. But I believe it’s because we’ve yet to embolden our strengths. It’s our job as leaders, attendings, administrators to inform and enable them.
When Marianne fell deathly ill in “Sense and Sensibility,” Colonel Branden wrung his hands and paced the floor. “Give me an occupation, Miss Dashwood, or I shall run mad.” Doctors are running, mad. And, just in case, some dermatologists are relearning how to intubate, waiting for that occupation to be given.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. He has no relevant conflicts of interest related to this column. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“I’ve been watching YouTube videos on how to set a ventilator,” said one of our dermatologists. The absurdity, levity, and gravity of that statement captures in a single sentence where we are today.
None of us alive have experience with such a crisis. It is as if our planet passed through a wormhole and we’ve been transported to the late medieval period: We doctors fighting the Black Death donned in beaked masks filled with juniper berries, mint, and clove to protect us from the miasma. Now, though, we spray store-bought lavender disinfectant on surgical masks.
“A crisis shows you a person’s soul,” said New York Governor Andrew Cuomo, adding: “It shows you what they’re made of, the weaknesses explode and the strengths ... emboldened.” Most of us have traveled through life with no experience of peril. Such mortal danger explodes and emboldens us, dividing us in two, the fearful or the phlegmatic.
When President Trump proclaimed that plaquenil was a promising treatment for the virus, prescriptions for the drug soared so quickly that four of eight manufacturers reported being in shortage by the end of the day. Many of those prescriptions were written by physicians for themselves and their families. Private Facebook physician groups shared insider tips for how to get around constraints and find the drug – as hoardable as toilet paper. As a department chief and fellow human being, I understand why some of us might behave this way. We didn’t sign up to be dermatologists or nephrologists or surgeons or pulmonologists agreeing that, to do so, we might die. We are all afraid.
The track of this epic storm became clear last week and now, terrifyingly, it appears it will be a direct hit. I braced for an onslaught of anxiety from our doctors and staff. But as the forecast became more grim, the courage began to well up and creativity climbed. Doctors went to local stores and bought all the masks and shields on their own. Rolls of toilet paper and diapers began magically appearing in our mom-doctors’ offices, delivered by angels in scrubs. I’ve practically had to install a velvet rope at my door to organize the queue of people wanting to talk to me about their ideas to help – keep 6 feet apart please! Stories like this abound. Even at the EvergreenHealth hospital in Washington they’ve not had shortages of staff. Rather than calling out sick, they called in: “If you need me, I’m available.”
Doctors are afraid and frustrated. Some of the things we will do in the coming weeks will first do no good, perhaps even harm. But I believe it’s because we’ve yet to embolden our strengths. It’s our job as leaders, attendings, administrators to inform and enable them.
When Marianne fell deathly ill in “Sense and Sensibility,” Colonel Branden wrung his hands and paced the floor. “Give me an occupation, Miss Dashwood, or I shall run mad.” Doctors are running, mad. And, just in case, some dermatologists are relearning how to intubate, waiting for that occupation to be given.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. He has no relevant conflicts of interest related to this column. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Are CRMO and SAPHO syndrome one and the same?
MAUI, HAWAII – Chronic recurrent multifocal osteomyelitis (CRMO) in children and SAPHO syndrome in adults may well be a single clinical syndrome.
That contention, recently put forth by Austrian investigators, resonates with Anne M. Stevens, MD, PhD, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
“Is CRMO just for kids? No,” she asserted at the 2020 Rheumatology Winter Clinical Symposium.
First off, she noted that the nomenclature is shifting: The more familiar acronym CRMO is giving way to CNO (chronic nonbacterial osteomyelitis) in light of evidence that roughly 30% of patients with CRMO start out with a single characteristic bone lesion, with the disease turning multifocal in the subsequent 4 years in the great majority of cases.
SAPHO syndrome – an acronym for synovitis, acne, pustulosis, hyperostosis, and osteitis – a formerly obscure disease entity first described in 1987 in France, has suddenly become a trendy research topic, with three small studies presented at the 2019 annual meeting of the American College of Rheumatology.
CNO is a pediatric autoinflammatory bone disease characterized by sterile bone lesions, most often on the clavicle, spine, mandible, and lower extremities. It is marked by prominent focal bone and/or joint pain, worse at night, with or without swelling. With no agreed-upon diagnostic criteria or biomarkers, CNO is a diagnosis of exclusion. Two-thirds of the time the condition is initially misdiagnosed as bacterial osteomyelitis or a malignant tumor.
Austrian investigators at the University of Graz recently conducted a retrospective comparison of 24 pediatric patients diagnosed with CNO and 10 adults with SAPHO syndrome. The median age at diagnosis of CNO was 12.3 years versus 32.5 years for SAPHO syndrome. The two groups shared compelling similarities in mean number of bone lesions, prevalence of skin involvement, and other aspects of initial clinical presentation, as well as laboratory and histopathologic findings on bone biopsy.
There were, however, several notable clinical differences in this small dataset: CNO bone lesions affected mainly the lower extremities, clavicle, spine, and mandible, while SAPHO syndrome more commonly involved the sternum (50% vs. 8%) and vertebrae (50% vs. 21%). Also, the most frequent cutaneous manifestation was palmoplantar pustulosis in adults with SAPHO syndrome, while severe acne predominated in children with CNO. In both children and adults, the skin lesions most often arose after the bone symptoms, making early diagnosis a challenge.
Another similarity: Although there have been no randomized treatment trials in either CNO or SAPHO syndrome, case series suggest the same treatments are effective for both, with NSAIDs as first line, followed by nonbiologic disease-modifying antirheumatic drugs, tumor necrosis factor (TNF) inhibitors, or bisphosphonates.
CNO diagnosis, treatment, and follow-up
Various investigators have pegged the sensitivity of physical examination for diagnosis of CNO at 31%, radiographs at a lowly 13%, and bone scintigraphy at 74%, all in comparison with MRI.
“Our go-to now is MRI with STIR [short tau inversion recovery],” according to Dr. Stevens. “There’s no contrast – so no IV – no radiation, and it’s fast, 20 minutes for a whole body MRI in a little kid, 45 minutes in a big one.”
Insurers are reluctant to pay for serial whole-body MRIs for patient follow-up, so it’s often necessary to order a series of images covering different body parts.
Her University of Washington colleague Dan Zhao, MD, PhD, is developing infrared thermal imaging as an inexpensive, convenient alternative to MRI which could theoretically be done at home. In a pilot study in 30 children with CNO and 31 controls, inflamed leg segments showed significantly higher temperatures. Larger studies are planned.
Dr. Stevens advised leaning towards a diagnosis of CNO with avoidance of bone biopsy in a patient with multifocal osteomyelitis at the typical sites, a normal CBC, the typical extraosseous manifestations, and normal or only mildly elevated erythrocyte sedimentation rate and C-reactive protein in an otherwise well-appearing child. In contrast, strongly consider a bone biopsy to rule out malignancy or infection if the child has unexplained highly elevated C-reactive protein and erythrocyte sedimentation rate, cytopenia, high fever, excessive pain, lymphadenopathy, hepatosplenomegaly, or suspicious imaging findings.
German rheumatologists have developed a clinical score for diagnosis of CNO. A normal blood cell count gets 13 points; symmetric bone lesions 10; lesions with marginal sclerosis 10; a normal body temperature 9; two or more radiologically proven lesions 7; a C-reactive protein of 1 mg/dL or greater 6; and vertebral, clavicular, or sternal lesions 8. A score of 39 points or more out of a possible 63 had a 97% positive predictive value for CNO in a retrospective study of 224 children with CNO, proven bacterial osteomyelitis, or malignant bone tumors. A score of 28 points or less had a 97% negative predictive value for CNO. An indeterminate score of 29-38 warrants close monitoring.
The scoring system hasn’t been validated, but most pediatric rheumatologists agree that it’s useful, according to Dr. Stevens.
The Childhood Arthritis and Rheumatology Research Alliance (CARRA) is in the process of developing standardized diagnostic and classification criteria and treatment plans for CNO. Dr. Zhao was first author of a CARRA consensus treatment plan for CNO refractory to NSAID monotherapy. The plan for the first 12 months includes three options: methotrexate or sulfasalazine, TNF inhibitors with or without methotrexate, and bisphosphonates.
“The main point of this is you try a medicine and then wait 3 months. If they’re not responding then, switch medicines or add another drug. Monitor every 3 months based upon pain,” she said.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
MAUI, HAWAII – Chronic recurrent multifocal osteomyelitis (CRMO) in children and SAPHO syndrome in adults may well be a single clinical syndrome.
That contention, recently put forth by Austrian investigators, resonates with Anne M. Stevens, MD, PhD, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
“Is CRMO just for kids? No,” she asserted at the 2020 Rheumatology Winter Clinical Symposium.
First off, she noted that the nomenclature is shifting: The more familiar acronym CRMO is giving way to CNO (chronic nonbacterial osteomyelitis) in light of evidence that roughly 30% of patients with CRMO start out with a single characteristic bone lesion, with the disease turning multifocal in the subsequent 4 years in the great majority of cases.
SAPHO syndrome – an acronym for synovitis, acne, pustulosis, hyperostosis, and osteitis – a formerly obscure disease entity first described in 1987 in France, has suddenly become a trendy research topic, with three small studies presented at the 2019 annual meeting of the American College of Rheumatology.
CNO is a pediatric autoinflammatory bone disease characterized by sterile bone lesions, most often on the clavicle, spine, mandible, and lower extremities. It is marked by prominent focal bone and/or joint pain, worse at night, with or without swelling. With no agreed-upon diagnostic criteria or biomarkers, CNO is a diagnosis of exclusion. Two-thirds of the time the condition is initially misdiagnosed as bacterial osteomyelitis or a malignant tumor.
Austrian investigators at the University of Graz recently conducted a retrospective comparison of 24 pediatric patients diagnosed with CNO and 10 adults with SAPHO syndrome. The median age at diagnosis of CNO was 12.3 years versus 32.5 years for SAPHO syndrome. The two groups shared compelling similarities in mean number of bone lesions, prevalence of skin involvement, and other aspects of initial clinical presentation, as well as laboratory and histopathologic findings on bone biopsy.
There were, however, several notable clinical differences in this small dataset: CNO bone lesions affected mainly the lower extremities, clavicle, spine, and mandible, while SAPHO syndrome more commonly involved the sternum (50% vs. 8%) and vertebrae (50% vs. 21%). Also, the most frequent cutaneous manifestation was palmoplantar pustulosis in adults with SAPHO syndrome, while severe acne predominated in children with CNO. In both children and adults, the skin lesions most often arose after the bone symptoms, making early diagnosis a challenge.
Another similarity: Although there have been no randomized treatment trials in either CNO or SAPHO syndrome, case series suggest the same treatments are effective for both, with NSAIDs as first line, followed by nonbiologic disease-modifying antirheumatic drugs, tumor necrosis factor (TNF) inhibitors, or bisphosphonates.
CNO diagnosis, treatment, and follow-up
Various investigators have pegged the sensitivity of physical examination for diagnosis of CNO at 31%, radiographs at a lowly 13%, and bone scintigraphy at 74%, all in comparison with MRI.
“Our go-to now is MRI with STIR [short tau inversion recovery],” according to Dr. Stevens. “There’s no contrast – so no IV – no radiation, and it’s fast, 20 minutes for a whole body MRI in a little kid, 45 minutes in a big one.”
Insurers are reluctant to pay for serial whole-body MRIs for patient follow-up, so it’s often necessary to order a series of images covering different body parts.
Her University of Washington colleague Dan Zhao, MD, PhD, is developing infrared thermal imaging as an inexpensive, convenient alternative to MRI which could theoretically be done at home. In a pilot study in 30 children with CNO and 31 controls, inflamed leg segments showed significantly higher temperatures. Larger studies are planned.
Dr. Stevens advised leaning towards a diagnosis of CNO with avoidance of bone biopsy in a patient with multifocal osteomyelitis at the typical sites, a normal CBC, the typical extraosseous manifestations, and normal or only mildly elevated erythrocyte sedimentation rate and C-reactive protein in an otherwise well-appearing child. In contrast, strongly consider a bone biopsy to rule out malignancy or infection if the child has unexplained highly elevated C-reactive protein and erythrocyte sedimentation rate, cytopenia, high fever, excessive pain, lymphadenopathy, hepatosplenomegaly, or suspicious imaging findings.
German rheumatologists have developed a clinical score for diagnosis of CNO. A normal blood cell count gets 13 points; symmetric bone lesions 10; lesions with marginal sclerosis 10; a normal body temperature 9; two or more radiologically proven lesions 7; a C-reactive protein of 1 mg/dL or greater 6; and vertebral, clavicular, or sternal lesions 8. A score of 39 points or more out of a possible 63 had a 97% positive predictive value for CNO in a retrospective study of 224 children with CNO, proven bacterial osteomyelitis, or malignant bone tumors. A score of 28 points or less had a 97% negative predictive value for CNO. An indeterminate score of 29-38 warrants close monitoring.
The scoring system hasn’t been validated, but most pediatric rheumatologists agree that it’s useful, according to Dr. Stevens.
The Childhood Arthritis and Rheumatology Research Alliance (CARRA) is in the process of developing standardized diagnostic and classification criteria and treatment plans for CNO. Dr. Zhao was first author of a CARRA consensus treatment plan for CNO refractory to NSAID monotherapy. The plan for the first 12 months includes three options: methotrexate or sulfasalazine, TNF inhibitors with or without methotrexate, and bisphosphonates.
“The main point of this is you try a medicine and then wait 3 months. If they’re not responding then, switch medicines or add another drug. Monitor every 3 months based upon pain,” she said.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
MAUI, HAWAII – Chronic recurrent multifocal osteomyelitis (CRMO) in children and SAPHO syndrome in adults may well be a single clinical syndrome.
That contention, recently put forth by Austrian investigators, resonates with Anne M. Stevens, MD, PhD, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
“Is CRMO just for kids? No,” she asserted at the 2020 Rheumatology Winter Clinical Symposium.
First off, she noted that the nomenclature is shifting: The more familiar acronym CRMO is giving way to CNO (chronic nonbacterial osteomyelitis) in light of evidence that roughly 30% of patients with CRMO start out with a single characteristic bone lesion, with the disease turning multifocal in the subsequent 4 years in the great majority of cases.
SAPHO syndrome – an acronym for synovitis, acne, pustulosis, hyperostosis, and osteitis – a formerly obscure disease entity first described in 1987 in France, has suddenly become a trendy research topic, with three small studies presented at the 2019 annual meeting of the American College of Rheumatology.
CNO is a pediatric autoinflammatory bone disease characterized by sterile bone lesions, most often on the clavicle, spine, mandible, and lower extremities. It is marked by prominent focal bone and/or joint pain, worse at night, with or without swelling. With no agreed-upon diagnostic criteria or biomarkers, CNO is a diagnosis of exclusion. Two-thirds of the time the condition is initially misdiagnosed as bacterial osteomyelitis or a malignant tumor.
Austrian investigators at the University of Graz recently conducted a retrospective comparison of 24 pediatric patients diagnosed with CNO and 10 adults with SAPHO syndrome. The median age at diagnosis of CNO was 12.3 years versus 32.5 years for SAPHO syndrome. The two groups shared compelling similarities in mean number of bone lesions, prevalence of skin involvement, and other aspects of initial clinical presentation, as well as laboratory and histopathologic findings on bone biopsy.
There were, however, several notable clinical differences in this small dataset: CNO bone lesions affected mainly the lower extremities, clavicle, spine, and mandible, while SAPHO syndrome more commonly involved the sternum (50% vs. 8%) and vertebrae (50% vs. 21%). Also, the most frequent cutaneous manifestation was palmoplantar pustulosis in adults with SAPHO syndrome, while severe acne predominated in children with CNO. In both children and adults, the skin lesions most often arose after the bone symptoms, making early diagnosis a challenge.
Another similarity: Although there have been no randomized treatment trials in either CNO or SAPHO syndrome, case series suggest the same treatments are effective for both, with NSAIDs as first line, followed by nonbiologic disease-modifying antirheumatic drugs, tumor necrosis factor (TNF) inhibitors, or bisphosphonates.
CNO diagnosis, treatment, and follow-up
Various investigators have pegged the sensitivity of physical examination for diagnosis of CNO at 31%, radiographs at a lowly 13%, and bone scintigraphy at 74%, all in comparison with MRI.
“Our go-to now is MRI with STIR [short tau inversion recovery],” according to Dr. Stevens. “There’s no contrast – so no IV – no radiation, and it’s fast, 20 minutes for a whole body MRI in a little kid, 45 minutes in a big one.”
Insurers are reluctant to pay for serial whole-body MRIs for patient follow-up, so it’s often necessary to order a series of images covering different body parts.
Her University of Washington colleague Dan Zhao, MD, PhD, is developing infrared thermal imaging as an inexpensive, convenient alternative to MRI which could theoretically be done at home. In a pilot study in 30 children with CNO and 31 controls, inflamed leg segments showed significantly higher temperatures. Larger studies are planned.
Dr. Stevens advised leaning towards a diagnosis of CNO with avoidance of bone biopsy in a patient with multifocal osteomyelitis at the typical sites, a normal CBC, the typical extraosseous manifestations, and normal or only mildly elevated erythrocyte sedimentation rate and C-reactive protein in an otherwise well-appearing child. In contrast, strongly consider a bone biopsy to rule out malignancy or infection if the child has unexplained highly elevated C-reactive protein and erythrocyte sedimentation rate, cytopenia, high fever, excessive pain, lymphadenopathy, hepatosplenomegaly, or suspicious imaging findings.
German rheumatologists have developed a clinical score for diagnosis of CNO. A normal blood cell count gets 13 points; symmetric bone lesions 10; lesions with marginal sclerosis 10; a normal body temperature 9; two or more radiologically proven lesions 7; a C-reactive protein of 1 mg/dL or greater 6; and vertebral, clavicular, or sternal lesions 8. A score of 39 points or more out of a possible 63 had a 97% positive predictive value for CNO in a retrospective study of 224 children with CNO, proven bacterial osteomyelitis, or malignant bone tumors. A score of 28 points or less had a 97% negative predictive value for CNO. An indeterminate score of 29-38 warrants close monitoring.
The scoring system hasn’t been validated, but most pediatric rheumatologists agree that it’s useful, according to Dr. Stevens.
The Childhood Arthritis and Rheumatology Research Alliance (CARRA) is in the process of developing standardized diagnostic and classification criteria and treatment plans for CNO. Dr. Zhao was first author of a CARRA consensus treatment plan for CNO refractory to NSAID monotherapy. The plan for the first 12 months includes three options: methotrexate or sulfasalazine, TNF inhibitors with or without methotrexate, and bisphosphonates.
“The main point of this is you try a medicine and then wait 3 months. If they’re not responding then, switch medicines or add another drug. Monitor every 3 months based upon pain,” she said.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
EXPERT ANALYSIS FROM RWCS 2020
Preventable diseases could gain a foothold because of COVID-19
There is a highly infectious virus spreading around the world and it is targeting the most vulnerable among us. It is among the most contagious of human diseases, spreading through the air unseen. No, it isn’t the novel coronavirus, COVID-19. It’s measles.
Remember measles? Outbreaks in recent years have brought the disease, which once was declared eliminated in the United States, back into the news and public awareness, but measles never has really gone away. Every year there are millions of cases worldwide – in 2018 alone there were nearly 10 million estimated cases and 142,300 deaths, according to the World Health Organization. The good news is that measles vaccination is highly effective, at about 97% after the recommended two doses. According to the Centers for Disease Control and Prevention, “because of vaccination, more than 21 million lives have been saved and measles deaths have been reduced by 80% since 2000.” This is a tremendous public health success and a cause for celebration. But our work is not done. The recent increases in vaccine hesitancy and refusal in many countries has contributed to the resurgence of measles worldwide.
Influenza still is in full swing with the CDC reporting high activity in 1 states for the week ending April 4th. Seasonal influenza, according to currently available data, has a lower fatality rate than COVID-19, but that doesn’t mean it is harmless. Thus far in the 2019-2020 flu season, there have been at least 24,000 deaths because of influenza in the United States alone, 166 of which were among pediatric patients.*
Like many pediatricians, I have seen firsthand the impact of vaccine-preventable illnesses like influenza, pertussis, and varicella. I have personally cared for an infant with pertussis who had to be intubated and on a ventilator for nearly a week. I have told the family of a child with cancer that they would have to be admitted to the hospital yet again for intravenous antiviral medication because that little rash turned out to be varicella. I have performed CPR on a previously healthy teenager with the flu whose heart was failing despite maximum ventilator support. All these illnesses might have been prevented had these patients or those around them been appropriately vaccinated.
Right now, the United States and governments around the world are taking unprecedented public health measures to prevent the spread of COVID-19, directing the public to stay home, avoid unnecessary contact with other people, practice good hand-washing and infection-control techniques. In order to promote social distancing, many primary care clinics are canceling nonurgent appointments or converting them to virtual visits, including some visits for routine vaccinations for older children, teens, and adults. This is a responsible choice to keep potentially asymptomatic people from spreading COVID-19, but once restrictions begin to lift, we all will need to act to help our patients catch up on these missing vaccinations.
This pandemic has made it more apparent than ever that we all rely upon each other to stay healthy. While this pandemic has disrupted nearly every aspect of daily life, we can’t let it disrupt one of the great successes in health care today: the prevention of serious illnesses. As soon as it is safe to do so, we must help and encourage patients to catch up on missing vaccinations. It’s rare that preventative public health measures and vaccine developments are in the nightly news, so we should use this increased public awareness to ensure patients are well educated and protected from every disease. As part of this, we must continue our efforts to share accurate information on the safety and efficacy of routine vaccination. And when there is a vaccine for COVID-19? Let’s make sure everyone gets that too.
Dr. Leighton is a pediatrician in the ED at Children’s National Hospital and currently is completing her MPH in health policy at George Washington University, both in Washington. She had no relevant financial disclosures.*
* This article was updated 4/10/2020.
There is a highly infectious virus spreading around the world and it is targeting the most vulnerable among us. It is among the most contagious of human diseases, spreading through the air unseen. No, it isn’t the novel coronavirus, COVID-19. It’s measles.
Remember measles? Outbreaks in recent years have brought the disease, which once was declared eliminated in the United States, back into the news and public awareness, but measles never has really gone away. Every year there are millions of cases worldwide – in 2018 alone there were nearly 10 million estimated cases and 142,300 deaths, according to the World Health Organization. The good news is that measles vaccination is highly effective, at about 97% after the recommended two doses. According to the Centers for Disease Control and Prevention, “because of vaccination, more than 21 million lives have been saved and measles deaths have been reduced by 80% since 2000.” This is a tremendous public health success and a cause for celebration. But our work is not done. The recent increases in vaccine hesitancy and refusal in many countries has contributed to the resurgence of measles worldwide.
Influenza still is in full swing with the CDC reporting high activity in 1 states for the week ending April 4th. Seasonal influenza, according to currently available data, has a lower fatality rate than COVID-19, but that doesn’t mean it is harmless. Thus far in the 2019-2020 flu season, there have been at least 24,000 deaths because of influenza in the United States alone, 166 of which were among pediatric patients.*
Like many pediatricians, I have seen firsthand the impact of vaccine-preventable illnesses like influenza, pertussis, and varicella. I have personally cared for an infant with pertussis who had to be intubated and on a ventilator for nearly a week. I have told the family of a child with cancer that they would have to be admitted to the hospital yet again for intravenous antiviral medication because that little rash turned out to be varicella. I have performed CPR on a previously healthy teenager with the flu whose heart was failing despite maximum ventilator support. All these illnesses might have been prevented had these patients or those around them been appropriately vaccinated.
Right now, the United States and governments around the world are taking unprecedented public health measures to prevent the spread of COVID-19, directing the public to stay home, avoid unnecessary contact with other people, practice good hand-washing and infection-control techniques. In order to promote social distancing, many primary care clinics are canceling nonurgent appointments or converting them to virtual visits, including some visits for routine vaccinations for older children, teens, and adults. This is a responsible choice to keep potentially asymptomatic people from spreading COVID-19, but once restrictions begin to lift, we all will need to act to help our patients catch up on these missing vaccinations.
This pandemic has made it more apparent than ever that we all rely upon each other to stay healthy. While this pandemic has disrupted nearly every aspect of daily life, we can’t let it disrupt one of the great successes in health care today: the prevention of serious illnesses. As soon as it is safe to do so, we must help and encourage patients to catch up on missing vaccinations. It’s rare that preventative public health measures and vaccine developments are in the nightly news, so we should use this increased public awareness to ensure patients are well educated and protected from every disease. As part of this, we must continue our efforts to share accurate information on the safety and efficacy of routine vaccination. And when there is a vaccine for COVID-19? Let’s make sure everyone gets that too.
Dr. Leighton is a pediatrician in the ED at Children’s National Hospital and currently is completing her MPH in health policy at George Washington University, both in Washington. She had no relevant financial disclosures.*
* This article was updated 4/10/2020.
There is a highly infectious virus spreading around the world and it is targeting the most vulnerable among us. It is among the most contagious of human diseases, spreading through the air unseen. No, it isn’t the novel coronavirus, COVID-19. It’s measles.
Remember measles? Outbreaks in recent years have brought the disease, which once was declared eliminated in the United States, back into the news and public awareness, but measles never has really gone away. Every year there are millions of cases worldwide – in 2018 alone there were nearly 10 million estimated cases and 142,300 deaths, according to the World Health Organization. The good news is that measles vaccination is highly effective, at about 97% after the recommended two doses. According to the Centers for Disease Control and Prevention, “because of vaccination, more than 21 million lives have been saved and measles deaths have been reduced by 80% since 2000.” This is a tremendous public health success and a cause for celebration. But our work is not done. The recent increases in vaccine hesitancy and refusal in many countries has contributed to the resurgence of measles worldwide.
Influenza still is in full swing with the CDC reporting high activity in 1 states for the week ending April 4th. Seasonal influenza, according to currently available data, has a lower fatality rate than COVID-19, but that doesn’t mean it is harmless. Thus far in the 2019-2020 flu season, there have been at least 24,000 deaths because of influenza in the United States alone, 166 of which were among pediatric patients.*
Like many pediatricians, I have seen firsthand the impact of vaccine-preventable illnesses like influenza, pertussis, and varicella. I have personally cared for an infant with pertussis who had to be intubated and on a ventilator for nearly a week. I have told the family of a child with cancer that they would have to be admitted to the hospital yet again for intravenous antiviral medication because that little rash turned out to be varicella. I have performed CPR on a previously healthy teenager with the flu whose heart was failing despite maximum ventilator support. All these illnesses might have been prevented had these patients or those around them been appropriately vaccinated.
Right now, the United States and governments around the world are taking unprecedented public health measures to prevent the spread of COVID-19, directing the public to stay home, avoid unnecessary contact with other people, practice good hand-washing and infection-control techniques. In order to promote social distancing, many primary care clinics are canceling nonurgent appointments or converting them to virtual visits, including some visits for routine vaccinations for older children, teens, and adults. This is a responsible choice to keep potentially asymptomatic people from spreading COVID-19, but once restrictions begin to lift, we all will need to act to help our patients catch up on these missing vaccinations.
This pandemic has made it more apparent than ever that we all rely upon each other to stay healthy. While this pandemic has disrupted nearly every aspect of daily life, we can’t let it disrupt one of the great successes in health care today: the prevention of serious illnesses. As soon as it is safe to do so, we must help and encourage patients to catch up on missing vaccinations. It’s rare that preventative public health measures and vaccine developments are in the nightly news, so we should use this increased public awareness to ensure patients are well educated and protected from every disease. As part of this, we must continue our efforts to share accurate information on the safety and efficacy of routine vaccination. And when there is a vaccine for COVID-19? Let’s make sure everyone gets that too.
Dr. Leighton is a pediatrician in the ED at Children’s National Hospital and currently is completing her MPH in health policy at George Washington University, both in Washington. She had no relevant financial disclosures.*
* This article was updated 4/10/2020.
Milestone Match Day sees record highs; soar in DO applicants
Unifying allopathic (MD) and osteopathic (DO) applicants for the first time in a single matching program, 2020’s Match Day results underscored the continuing growth of DOs in the field, boosting numbers in primary care medicine and the Match as a whole.
The 2020 Main Residency Match bested 2019’s record as the largest in the history of the National Resident Matching Program (NRMP), with 40,084 applicants submitting program choices for 37,256 positions. This compares with 38,376 applicants vying for 35,185 positions last year.
It’s the seventh consecutive year in which overall match numbers are up, according to the NRMP. Although the number of applicants increased, so did the number of positions, resulting in a slight drop in the percent of positions filled during 2019-2020.
Available first-year (PGY-1) positions rose to 34,266, an increase of 2,072 (6.4%) over 2019. “This was, in part, due to the last migration of osteopathic program positions into the Main Residency Match,” Donna L. Lamb, DHSc, NRMP president and CEO, said in an interview. An agreement the Accreditation Council for Graduate Medical Education, American Osteopathic Association and American Association of Colleges of Osteopathic Medicine reached in 2014 recognized ACGME as the primary accrediting body for graduate medical education programs by 2020.
This led to the first single match for U.S. MD and DO senior students and graduates and the inclusion of DO senior students as sponsored applicants in 2020, Dr. Lamb noted.
Gains, trends in 2020 match
Growth in U.S. DO senior participation also pushed this year’s Match to record highs. There were 6,581 U.S. DO medical school seniors who submitted rank order lists, 1,103 more than in 2019. Among those seniors, 90.7% matched to PGY-1 positions, driving the match rate for U.S. DO seniors up 2.6 percentage points from 2019.
Since 2016, the number of U.S. DO seniors seeking positions has risen by 3,599 or 120%. “Of course, the number of U.S. MD seniors who submitted program choices was also record-high: 19,326, an increase of 401 over 2019. The 93.7% match rate to first-year positions for this group has remained very consistent for many years,” Dr. Lamb said.
Among individual specialties, the NRMP reported extremely high fill rates for dermatology, medicine-emergency medicine, neurological surgery, physical medicine and rehabilitation (categorical), integrated plastic surgery, and thoracic surgery. Other competitive specialties included medicine-pediatrics, orthopedic surgery, otolaryngology, and vascular surgery.
Participation of international medical school students and graduates (IMGs) went up in 2020, breaking a 3-year cycle of decline. More than 61% matched to first-year positions, 2.5 percentage points higher than 2019 – and the highest match rate since 1990. “IMGs generally are having the most success matching to primary care specialties, including internal medicine, family medicine, and pediatrics,” Dr. Lamb said.
Primary care benefits from DO growth
DO candidates also helped drive up the numbers in primary care.
Internal medicine offered 8,697 categorical positions, 581 more than in 2019, reflecting a fill rate of 95.7%. More than 40% of these slots were filled by U.S. MD seniors, a category that’s seen decreases over the last 5 years, due in part to administrative and financial burdens associated with primary care internal medicine.
“In addition, the steady growth of internal medicine has increased the overall number of training positions available, and with the growth of other specialties in parallel, it has also likely had some effect on decreasing the percentage of U.S. graduates entering the field,” Phil Masters, MD, vice president of membership and global engagement at the American College of Physicians, said in an interview.
However, fill rates for U.S. DO seniors reached 16% in 2020, a notable rise from 6.9% in 2016. “As the number of osteopathic trainees increases, we are happy that more are choosing internal medicine as a career path,” Dr. Masters said, adding that the slightly different training and practice orientation of osteopathic physicians “complements that of their allopathic colleagues, and add richness to the many different practice settings that internal medicine encompasses.”
A record number of DO seniors also matched in family medicine (1,392), accounting for nearly 30% of all applicants. The single match led to an important net increase in filled family medicine residency positions, Clif Knight, MD, senior vice president for education at the American Academy of Family Physicians, said in an interview.
Overall, family medicine filled 92.5% of its 4,662 positions, 555 more than in 2019. The results show that family medicine and primary care are on solid footing, Dr. Knight said. “We are excited that the number of filled family medicine residency positions increased from last year. This is important as we work to meet the significant primary care workforce shortage,” he added.
In other specialties:
- Pediatrics filled more than 98% of its 2,864 categorical positions, 17 more than in 2019. U.S. MD seniors filled 1,731 (60.4%) of those slots. “We’re very excited about our newly matched pediatricians,” Sara “Sally” H. Goza, MD, president of the American Academy of Pediatrics, said in an interview. “The coronavirus outbreak has shown us how valuable the pediatric workforce is and how much we’re needed.’’
- Dermatology offered 478 positions, achieving a fill rate of 98.1%. “Looking at our own program’s Match results, I feel very satisfied that we are accomplishing our specific aim to serve rural populations and to create a diverse workforce in dermatology,” Erik Stratman, MD, an expert on dermatologic education in U.S. medical schools/residency programs, and a member of the American Academy of Dermatology, said in an interview. “It’s nice to see the fruits of the specialty’s expanding efforts to get the right people in the specialty who reflect those populations we serve.”
- Obstetrics-gynecology offered 1,433 first-year positions – 48 more than in 2019 – achieving a fill rate of 99.8%, with U.S. MD seniors filling more than 75% of those slots.
- Neurology filled more than 97.5% of 682 offered positions in 2020. However, U.S. MD seniors represented just under half of those filled positions (46.5%).
- Psychiatry offered 1,858 positions in 2020, achieving an overall fill rate of 98.9%, 61.2% for U.S. MD seniors.
- Emergency Medicine filled 99.5% of the 2,665 positions offered this year. In this profession, the U.S. MD fill rate was 64.3%. These new interns are sorely needed at a time when EM physicians are on the front lines of a pandemic, Hannah R. Hughes, MD, president of the Emergency Medicine Residents’ Association, said in an interview.
Unifying allopathic (MD) and osteopathic (DO) applicants for the first time in a single matching program, 2020’s Match Day results underscored the continuing growth of DOs in the field, boosting numbers in primary care medicine and the Match as a whole.

The 2020 Main Residency Match bested 2019’s record as the largest in the history of the National Resident Matching Program (NRMP), with 40,084 applicants submitting program choices for 37,256 positions. This compares with 38,376 applicants vying for 35,185 positions last year.
It’s the seventh consecutive year in which overall match numbers are up, according to the NRMP. Although the number of applicants increased, so did the number of positions, resulting in a slight drop in the percent of positions filled during 2019-2020.
Available first-year (PGY-1) positions rose to 34,266, an increase of 2,072 (6.4%) over 2019. “This was, in part, due to the last migration of osteopathic program positions into the Main Residency Match,” Donna L. Lamb, DHSc, NRMP president and CEO, said in an interview. An agreement the Accreditation Council for Graduate Medical Education, American Osteopathic Association and American Association of Colleges of Osteopathic Medicine reached in 2014 recognized ACGME as the primary accrediting body for graduate medical education programs by 2020.
This led to the first single match for U.S. MD and DO senior students and graduates and the inclusion of DO senior students as sponsored applicants in 2020, Dr. Lamb noted.
Gains, trends in 2020 match
Growth in U.S. DO senior participation also pushed this year’s Match to record highs. There were 6,581 U.S. DO medical school seniors who submitted rank order lists, 1,103 more than in 2019. Among those seniors, 90.7% matched to PGY-1 positions, driving the match rate for U.S. DO seniors up 2.6 percentage points from 2019.
Since 2016, the number of U.S. DO seniors seeking positions has risen by 3,599 or 120%. “Of course, the number of U.S. MD seniors who submitted program choices was also record-high: 19,326, an increase of 401 over 2019. The 93.7% match rate to first-year positions for this group has remained very consistent for many years,” Dr. Lamb said.
Among individual specialties, the NRMP reported extremely high fill rates for dermatology, medicine-emergency medicine, neurological surgery, physical medicine and rehabilitation (categorical), integrated plastic surgery, and thoracic surgery. Other competitive specialties included medicine-pediatrics, orthopedic surgery, otolaryngology, and vascular surgery.
Participation of international medical school students and graduates (IMGs) went up in 2020, breaking a 3-year cycle of decline. More than 61% matched to first-year positions, 2.5 percentage points higher than 2019 – and the highest match rate since 1990. “IMGs generally are having the most success matching to primary care specialties, including internal medicine, family medicine, and pediatrics,” Dr. Lamb said.
Primary care benefits from DO growth
DO candidates also helped drive up the numbers in primary care.
Internal medicine offered 8,697 categorical positions, 581 more than in 2019, reflecting a fill rate of 95.7%. More than 40% of these slots were filled by U.S. MD seniors, a category that’s seen decreases over the last 5 years, due in part to administrative and financial burdens associated with primary care internal medicine.
“In addition, the steady growth of internal medicine has increased the overall number of training positions available, and with the growth of other specialties in parallel, it has also likely had some effect on decreasing the percentage of U.S. graduates entering the field,” Phil Masters, MD, vice president of membership and global engagement at the American College of Physicians, said in an interview.
However, fill rates for U.S. DO seniors reached 16% in 2020, a notable rise from 6.9% in 2016. “As the number of osteopathic trainees increases, we are happy that more are choosing internal medicine as a career path,” Dr. Masters said, adding that the slightly different training and practice orientation of osteopathic physicians “complements that of their allopathic colleagues, and add richness to the many different practice settings that internal medicine encompasses.”
A record number of DO seniors also matched in family medicine (1,392), accounting for nearly 30% of all applicants. The single match led to an important net increase in filled family medicine residency positions, Clif Knight, MD, senior vice president for education at the American Academy of Family Physicians, said in an interview.
Overall, family medicine filled 92.5% of its 4,662 positions, 555 more than in 2019. The results show that family medicine and primary care are on solid footing, Dr. Knight said. “We are excited that the number of filled family medicine residency positions increased from last year. This is important as we work to meet the significant primary care workforce shortage,” he added.
In other specialties:
- Pediatrics filled more than 98% of its 2,864 categorical positions, 17 more than in 2019. U.S. MD seniors filled 1,731 (60.4%) of those slots. “We’re very excited about our newly matched pediatricians,” Sara “Sally” H. Goza, MD, president of the American Academy of Pediatrics, said in an interview. “The coronavirus outbreak has shown us how valuable the pediatric workforce is and how much we’re needed.’’
- Dermatology offered 478 positions, achieving a fill rate of 98.1%. “Looking at our own program’s Match results, I feel very satisfied that we are accomplishing our specific aim to serve rural populations and to create a diverse workforce in dermatology,” Erik Stratman, MD, an expert on dermatologic education in U.S. medical schools/residency programs, and a member of the American Academy of Dermatology, said in an interview. “It’s nice to see the fruits of the specialty’s expanding efforts to get the right people in the specialty who reflect those populations we serve.”
- Obstetrics-gynecology offered 1,433 first-year positions – 48 more than in 2019 – achieving a fill rate of 99.8%, with U.S. MD seniors filling more than 75% of those slots.
- Neurology filled more than 97.5% of 682 offered positions in 2020. However, U.S. MD seniors represented just under half of those filled positions (46.5%).
- Psychiatry offered 1,858 positions in 2020, achieving an overall fill rate of 98.9%, 61.2% for U.S. MD seniors.
- Emergency Medicine filled 99.5% of the 2,665 positions offered this year. In this profession, the U.S. MD fill rate was 64.3%. These new interns are sorely needed at a time when EM physicians are on the front lines of a pandemic, Hannah R. Hughes, MD, president of the Emergency Medicine Residents’ Association, said in an interview.
Unifying allopathic (MD) and osteopathic (DO) applicants for the first time in a single matching program, 2020’s Match Day results underscored the continuing growth of DOs in the field, boosting numbers in primary care medicine and the Match as a whole.

The 2020 Main Residency Match bested 2019’s record as the largest in the history of the National Resident Matching Program (NRMP), with 40,084 applicants submitting program choices for 37,256 positions. This compares with 38,376 applicants vying for 35,185 positions last year.
It’s the seventh consecutive year in which overall match numbers are up, according to the NRMP. Although the number of applicants increased, so did the number of positions, resulting in a slight drop in the percent of positions filled during 2019-2020.
Available first-year (PGY-1) positions rose to 34,266, an increase of 2,072 (6.4%) over 2019. “This was, in part, due to the last migration of osteopathic program positions into the Main Residency Match,” Donna L. Lamb, DHSc, NRMP president and CEO, said in an interview. An agreement the Accreditation Council for Graduate Medical Education, American Osteopathic Association and American Association of Colleges of Osteopathic Medicine reached in 2014 recognized ACGME as the primary accrediting body for graduate medical education programs by 2020.
This led to the first single match for U.S. MD and DO senior students and graduates and the inclusion of DO senior students as sponsored applicants in 2020, Dr. Lamb noted.
Gains, trends in 2020 match
Growth in U.S. DO senior participation also pushed this year’s Match to record highs. There were 6,581 U.S. DO medical school seniors who submitted rank order lists, 1,103 more than in 2019. Among those seniors, 90.7% matched to PGY-1 positions, driving the match rate for U.S. DO seniors up 2.6 percentage points from 2019.
Since 2016, the number of U.S. DO seniors seeking positions has risen by 3,599 or 120%. “Of course, the number of U.S. MD seniors who submitted program choices was also record-high: 19,326, an increase of 401 over 2019. The 93.7% match rate to first-year positions for this group has remained very consistent for many years,” Dr. Lamb said.
Among individual specialties, the NRMP reported extremely high fill rates for dermatology, medicine-emergency medicine, neurological surgery, physical medicine and rehabilitation (categorical), integrated plastic surgery, and thoracic surgery. Other competitive specialties included medicine-pediatrics, orthopedic surgery, otolaryngology, and vascular surgery.
Participation of international medical school students and graduates (IMGs) went up in 2020, breaking a 3-year cycle of decline. More than 61% matched to first-year positions, 2.5 percentage points higher than 2019 – and the highest match rate since 1990. “IMGs generally are having the most success matching to primary care specialties, including internal medicine, family medicine, and pediatrics,” Dr. Lamb said.
Primary care benefits from DO growth
DO candidates also helped drive up the numbers in primary care.
Internal medicine offered 8,697 categorical positions, 581 more than in 2019, reflecting a fill rate of 95.7%. More than 40% of these slots were filled by U.S. MD seniors, a category that’s seen decreases over the last 5 years, due in part to administrative and financial burdens associated with primary care internal medicine.
“In addition, the steady growth of internal medicine has increased the overall number of training positions available, and with the growth of other specialties in parallel, it has also likely had some effect on decreasing the percentage of U.S. graduates entering the field,” Phil Masters, MD, vice president of membership and global engagement at the American College of Physicians, said in an interview.
However, fill rates for U.S. DO seniors reached 16% in 2020, a notable rise from 6.9% in 2016. “As the number of osteopathic trainees increases, we are happy that more are choosing internal medicine as a career path,” Dr. Masters said, adding that the slightly different training and practice orientation of osteopathic physicians “complements that of their allopathic colleagues, and add richness to the many different practice settings that internal medicine encompasses.”
A record number of DO seniors also matched in family medicine (1,392), accounting for nearly 30% of all applicants. The single match led to an important net increase in filled family medicine residency positions, Clif Knight, MD, senior vice president for education at the American Academy of Family Physicians, said in an interview.
Overall, family medicine filled 92.5% of its 4,662 positions, 555 more than in 2019. The results show that family medicine and primary care are on solid footing, Dr. Knight said. “We are excited that the number of filled family medicine residency positions increased from last year. This is important as we work to meet the significant primary care workforce shortage,” he added.
In other specialties:
- Pediatrics filled more than 98% of its 2,864 categorical positions, 17 more than in 2019. U.S. MD seniors filled 1,731 (60.4%) of those slots. “We’re very excited about our newly matched pediatricians,” Sara “Sally” H. Goza, MD, president of the American Academy of Pediatrics, said in an interview. “The coronavirus outbreak has shown us how valuable the pediatric workforce is and how much we’re needed.’’
- Dermatology offered 478 positions, achieving a fill rate of 98.1%. “Looking at our own program’s Match results, I feel very satisfied that we are accomplishing our specific aim to serve rural populations and to create a diverse workforce in dermatology,” Erik Stratman, MD, an expert on dermatologic education in U.S. medical schools/residency programs, and a member of the American Academy of Dermatology, said in an interview. “It’s nice to see the fruits of the specialty’s expanding efforts to get the right people in the specialty who reflect those populations we serve.”
- Obstetrics-gynecology offered 1,433 first-year positions – 48 more than in 2019 – achieving a fill rate of 99.8%, with U.S. MD seniors filling more than 75% of those slots.
- Neurology filled more than 97.5% of 682 offered positions in 2020. However, U.S. MD seniors represented just under half of those filled positions (46.5%).
- Psychiatry offered 1,858 positions in 2020, achieving an overall fill rate of 98.9%, 61.2% for U.S. MD seniors.
- Emergency Medicine filled 99.5% of the 2,665 positions offered this year. In this profession, the U.S. MD fill rate was 64.3%. These new interns are sorely needed at a time when EM physicians are on the front lines of a pandemic, Hannah R. Hughes, MD, president of the Emergency Medicine Residents’ Association, said in an interview.
Emergency Rule: Docs can bill for telehealth and COVID-19 tests. Here’s how
Many medical practices have long wanted to use telehealth to perform office visits and other evaluation and management (E/M) services. The technology readily exists and many electronic health records are set up to do telehealth visits. The problem has been getting paid for those visits. Medicare limited telehealth services to patients in underserved areas, and commercial insurances wouldn’t pay. But amid the COVID-19 crisis, things have changed.
On March 17, Congress passed a law allowing Medicare to waive some telehealth restrictions during a government state of emergency only, which we are in now. Specifically, the patient no longer needs to be in a medically underserved area and no longer needs to go to an originating site, such as a hospital. The patient can be located anywhere in the country and be in their own home.
Further, the Centers for Medicare & Medicaid is waiving the requirement that the practitioner use a HIPAA-compliant platform for the telehealth service. The service must still be provided using a real-time audiovisual platform, but that could be via FaceTime or Skype, both of which are readily available via a patient’s smartphone or home computer. Audio alone – that is, phone calls between physician and patient – is still insufficient.
Billing for telemedicine
There are two lists of services that you can bill for telehealth. One of the lists is in Medicare’s telehealth fact sheet and includes both CPT and HCPCS codes. The second is in your CPT book, Appendix P, and lists only CPT codes.
Practices may bill all of the Medicare-covered telehealth services using these new rules. This includes new and established patient visits 99201–99215. It includes inpatient and skilled nursing services, for which CMS uses HCPCS codes in place of CPT codes.
Some notable additional services that you may bill via telehealth are: smoking cessation, transitional care management, advanced care planning, psychiatric diagnostic interviews and psychotherapy, and initial and subsequent Medicare wellness visits. The Welcome to Medicare visit is not on the list.
Report these services to Medicare with the correct CPT code and use place of service 02 (telehealth) on the claim. There is a CPT modifier for telehealth (Modifier -95 Synchronous Telemedicine Service Rendered Via a Real-Time Interactive Audio and Video Telecommunications System) but Medicare does not require it.
If you perform an office visit and also do smoking cessation, document those just as you would if you saw the patient in person. Document the history; observational exam, if relevant; and the assessment and plan. Note the additional time spent in smoking cessation counseling. If it was a level three established patient, code 99213-25 and 99406 (smoking and tobacco use cessation counseling visit, intermediate, 3-10 minutes).
The Office of Inspector General is allowing practices to reduce or waive copays and patient due amounts. However, a practice is not required to waive the copay or patient due amount for a telehealth service.
Medicare Advantage plans are required to cover all services that original Medicare covers. State Medicaid plans and Medicaid managed care organizations can set their own rules.
What about commercial payers?
While CMS has issued its Medicare guidelines, commercial insurance companies can also set their own rules about covering telehealth services. Many of them have rushed to update their policies to allow office visits to be billed via telehealth.
Unfortunately, each payer can set its own rules about whether to cover telehealth and if the place of service 02 and/or modifier -95 is needed. UnitedHealthcare is covering telehealth visits for all of its Medicare Advantage, Medicaid, and commercial accounts.
Humana also is covering telemedicine for urgent care needs. Some private insurers are continuing to offer virtual visits with their contracted telehealth provider, not with the patient’s own physician. It is likely that this will change in the days ahead, but it means practices must check their payer policies and pay attention to the emails they receive from the payers. If patient foot traffic is slow, this may be a good time to call each payer to not only find out their telehealth rules, but to also learn what else is being suspended during the COVID-19 pandemic.
This would also be a good job for an employee to do from home versus coming into the practice.
None of the payers are limiting the diagnosis code for telemedicine services. The patient does not need to have a cough or fever to have telemedicine covered. Any diagnosis or condition is eligible to be billed via telehealth.
The waived restrictions by Medicare are in place only as long as the government state of emergency. Commercial payers are also describing these as temporary. However, it may be hard to put the genie back in the bottle. Medical practices and patients may find that these visits are just what the doctor ordered.
COVID-19 testing
Although testing is still not widely available, the American Medical Association has developed a CPT code for the test:
- 87635: Infectious agent detection by nucleic acid (DNA or RNA); severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Coronavirus disease [COVID-19]), amplified probe technique
CMS has also developed codes for testing for this new coronavirus. One (U0001) is specifically for tests done in the CDC lab. The second (U0002) was for other labs, but it seems likely that the CPT code will replace it.
In February, the U.S. Food and Drug Administration issued a new policy for certain labs to develop their own validated COVID-19 diagnostics. This second HCPCS code could be used for such tests when submitting claims to Medicare or other insurers.
The hope by CMS is that having these specific codes will encourage further testing and improve tracking of the virus.
This article first appeared on Medscape.com.
Many medical practices have long wanted to use telehealth to perform office visits and other evaluation and management (E/M) services. The technology readily exists and many electronic health records are set up to do telehealth visits. The problem has been getting paid for those visits. Medicare limited telehealth services to patients in underserved areas, and commercial insurances wouldn’t pay. But amid the COVID-19 crisis, things have changed.
On March 17, Congress passed a law allowing Medicare to waive some telehealth restrictions during a government state of emergency only, which we are in now. Specifically, the patient no longer needs to be in a medically underserved area and no longer needs to go to an originating site, such as a hospital. The patient can be located anywhere in the country and be in their own home.
Further, the Centers for Medicare & Medicaid is waiving the requirement that the practitioner use a HIPAA-compliant platform for the telehealth service. The service must still be provided using a real-time audiovisual platform, but that could be via FaceTime or Skype, both of which are readily available via a patient’s smartphone or home computer. Audio alone – that is, phone calls between physician and patient – is still insufficient.
Billing for telemedicine
There are two lists of services that you can bill for telehealth. One of the lists is in Medicare’s telehealth fact sheet and includes both CPT and HCPCS codes. The second is in your CPT book, Appendix P, and lists only CPT codes.
Practices may bill all of the Medicare-covered telehealth services using these new rules. This includes new and established patient visits 99201–99215. It includes inpatient and skilled nursing services, for which CMS uses HCPCS codes in place of CPT codes.
Some notable additional services that you may bill via telehealth are: smoking cessation, transitional care management, advanced care planning, psychiatric diagnostic interviews and psychotherapy, and initial and subsequent Medicare wellness visits. The Welcome to Medicare visit is not on the list.
Report these services to Medicare with the correct CPT code and use place of service 02 (telehealth) on the claim. There is a CPT modifier for telehealth (Modifier -95 Synchronous Telemedicine Service Rendered Via a Real-Time Interactive Audio and Video Telecommunications System) but Medicare does not require it.
If you perform an office visit and also do smoking cessation, document those just as you would if you saw the patient in person. Document the history; observational exam, if relevant; and the assessment and plan. Note the additional time spent in smoking cessation counseling. If it was a level three established patient, code 99213-25 and 99406 (smoking and tobacco use cessation counseling visit, intermediate, 3-10 minutes).
The Office of Inspector General is allowing practices to reduce or waive copays and patient due amounts. However, a practice is not required to waive the copay or patient due amount for a telehealth service.
Medicare Advantage plans are required to cover all services that original Medicare covers. State Medicaid plans and Medicaid managed care organizations can set their own rules.
What about commercial payers?
While CMS has issued its Medicare guidelines, commercial insurance companies can also set their own rules about covering telehealth services. Many of them have rushed to update their policies to allow office visits to be billed via telehealth.
Unfortunately, each payer can set its own rules about whether to cover telehealth and if the place of service 02 and/or modifier -95 is needed. UnitedHealthcare is covering telehealth visits for all of its Medicare Advantage, Medicaid, and commercial accounts.
Humana also is covering telemedicine for urgent care needs. Some private insurers are continuing to offer virtual visits with their contracted telehealth provider, not with the patient’s own physician. It is likely that this will change in the days ahead, but it means practices must check their payer policies and pay attention to the emails they receive from the payers. If patient foot traffic is slow, this may be a good time to call each payer to not only find out their telehealth rules, but to also learn what else is being suspended during the COVID-19 pandemic.
This would also be a good job for an employee to do from home versus coming into the practice.
None of the payers are limiting the diagnosis code for telemedicine services. The patient does not need to have a cough or fever to have telemedicine covered. Any diagnosis or condition is eligible to be billed via telehealth.
The waived restrictions by Medicare are in place only as long as the government state of emergency. Commercial payers are also describing these as temporary. However, it may be hard to put the genie back in the bottle. Medical practices and patients may find that these visits are just what the doctor ordered.
COVID-19 testing
Although testing is still not widely available, the American Medical Association has developed a CPT code for the test:
- 87635: Infectious agent detection by nucleic acid (DNA or RNA); severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Coronavirus disease [COVID-19]), amplified probe technique
CMS has also developed codes for testing for this new coronavirus. One (U0001) is specifically for tests done in the CDC lab. The second (U0002) was for other labs, but it seems likely that the CPT code will replace it.
In February, the U.S. Food and Drug Administration issued a new policy for certain labs to develop their own validated COVID-19 diagnostics. This second HCPCS code could be used for such tests when submitting claims to Medicare or other insurers.
The hope by CMS is that having these specific codes will encourage further testing and improve tracking of the virus.
This article first appeared on Medscape.com.
Many medical practices have long wanted to use telehealth to perform office visits and other evaluation and management (E/M) services. The technology readily exists and many electronic health records are set up to do telehealth visits. The problem has been getting paid for those visits. Medicare limited telehealth services to patients in underserved areas, and commercial insurances wouldn’t pay. But amid the COVID-19 crisis, things have changed.
On March 17, Congress passed a law allowing Medicare to waive some telehealth restrictions during a government state of emergency only, which we are in now. Specifically, the patient no longer needs to be in a medically underserved area and no longer needs to go to an originating site, such as a hospital. The patient can be located anywhere in the country and be in their own home.
Further, the Centers for Medicare & Medicaid is waiving the requirement that the practitioner use a HIPAA-compliant platform for the telehealth service. The service must still be provided using a real-time audiovisual platform, but that could be via FaceTime or Skype, both of which are readily available via a patient’s smartphone or home computer. Audio alone – that is, phone calls between physician and patient – is still insufficient.
Billing for telemedicine
There are two lists of services that you can bill for telehealth. One of the lists is in Medicare’s telehealth fact sheet and includes both CPT and HCPCS codes. The second is in your CPT book, Appendix P, and lists only CPT codes.
Practices may bill all of the Medicare-covered telehealth services using these new rules. This includes new and established patient visits 99201–99215. It includes inpatient and skilled nursing services, for which CMS uses HCPCS codes in place of CPT codes.
Some notable additional services that you may bill via telehealth are: smoking cessation, transitional care management, advanced care planning, psychiatric diagnostic interviews and psychotherapy, and initial and subsequent Medicare wellness visits. The Welcome to Medicare visit is not on the list.
Report these services to Medicare with the correct CPT code and use place of service 02 (telehealth) on the claim. There is a CPT modifier for telehealth (Modifier -95 Synchronous Telemedicine Service Rendered Via a Real-Time Interactive Audio and Video Telecommunications System) but Medicare does not require it.
If you perform an office visit and also do smoking cessation, document those just as you would if you saw the patient in person. Document the history; observational exam, if relevant; and the assessment and plan. Note the additional time spent in smoking cessation counseling. If it was a level three established patient, code 99213-25 and 99406 (smoking and tobacco use cessation counseling visit, intermediate, 3-10 minutes).
The Office of Inspector General is allowing practices to reduce or waive copays and patient due amounts. However, a practice is not required to waive the copay or patient due amount for a telehealth service.
Medicare Advantage plans are required to cover all services that original Medicare covers. State Medicaid plans and Medicaid managed care organizations can set their own rules.
What about commercial payers?
While CMS has issued its Medicare guidelines, commercial insurance companies can also set their own rules about covering telehealth services. Many of them have rushed to update their policies to allow office visits to be billed via telehealth.
Unfortunately, each payer can set its own rules about whether to cover telehealth and if the place of service 02 and/or modifier -95 is needed. UnitedHealthcare is covering telehealth visits for all of its Medicare Advantage, Medicaid, and commercial accounts.
Humana also is covering telemedicine for urgent care needs. Some private insurers are continuing to offer virtual visits with their contracted telehealth provider, not with the patient’s own physician. It is likely that this will change in the days ahead, but it means practices must check their payer policies and pay attention to the emails they receive from the payers. If patient foot traffic is slow, this may be a good time to call each payer to not only find out their telehealth rules, but to also learn what else is being suspended during the COVID-19 pandemic.
This would also be a good job for an employee to do from home versus coming into the practice.
None of the payers are limiting the diagnosis code for telemedicine services. The patient does not need to have a cough or fever to have telemedicine covered. Any diagnosis or condition is eligible to be billed via telehealth.
The waived restrictions by Medicare are in place only as long as the government state of emergency. Commercial payers are also describing these as temporary. However, it may be hard to put the genie back in the bottle. Medical practices and patients may find that these visits are just what the doctor ordered.
COVID-19 testing
Although testing is still not widely available, the American Medical Association has developed a CPT code for the test:
- 87635: Infectious agent detection by nucleic acid (DNA or RNA); severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Coronavirus disease [COVID-19]), amplified probe technique
CMS has also developed codes for testing for this new coronavirus. One (U0001) is specifically for tests done in the CDC lab. The second (U0002) was for other labs, but it seems likely that the CPT code will replace it.
In February, the U.S. Food and Drug Administration issued a new policy for certain labs to develop their own validated COVID-19 diagnostics. This second HCPCS code could be used for such tests when submitting claims to Medicare or other insurers.
The hope by CMS is that having these specific codes will encourage further testing and improve tracking of the virus.
This article first appeared on Medscape.com.