User login
New sickle cell drugs give hope, but access remains a barrier
Sickle cell disease (SCD) is an incurable genetic blood disorder that reduces patients’ lifespan and quality of life. Many patients live into their 40s or 50s. Yet, throughout their lives, patients are plagued by lethargy, unpredictable painful crises, and frequent hospitalizations. For nearly 20 years, clinicians only had one drug to treat SCD. Since 2017, three new drugs have been approved, but their costs and lack of long-term data have spawned questions regarding access and benefit.
“SCD reduces lifespan by 30 years, and that’s very hard to quantify,” says Ifeyinwa Osunkwo, MD, professor of medicine and pediatrics and director of Sickle Cell Disease Enterprise at the Levine Cancer Institute at Atrium Health in Charlotte, N.C. “If you put a dollar amount on what someone would make working for 30 adult years, that would be more reflective of the true cost of treating the disease.”
In 1984, hydroxyurea (Hydrea, Droxia) became the first drug to treat SCD in adults.
Originally developed as a myelosuppressive antineoplastic, hydroxyurea is used to treat resistant chronic myeloid leukemia and certain head and neck cancers. In SCD, it increases levels of hemoglobin and fetal hemoglobin.
Despite its benefit, hydroxyurea has two major drawbacks: It is only effective in two genotypes – HbSS or HbS/Beta0thal.
HbSS or HbS/Beta0thal genotypes account for 60% of the SCD population, but further studies are required to elucidate hydroxyurea’s effect in other forms of SCD, according to Dr. Osunkwo.
Secondly, hydroxyurea only reduces the frequency of painful episodes by 50% – not enough to ameliorate the pain, said John J. Strouse, MD, associate professor of medicine at Duke University School of Medicine.
Newer therapies offer the potential to enhance the effects of hydroxyurea when used concomitantly. They also give clinicians additional options for patients who either fail hydroxyurea therapy or for whom it is inappropriate.
The amino acid L-glutamine (Endari) became the second drug approved for sickle cell in 2017. Indicated for patients 5 years of age and older, Dr. Osunkwo says many patients were thrilled to have a nonchemotherapeutic option available. However, the medical community received the drug with some skepticism. “The data show Endari is moderately effective at best,” said Dr. Strouse. “Also, the mechanism of action is unclear.”
Additionally, the drug’s powder form and twice-daily dosing regimen make adherence more challenging than swallowing a few hydroxyurea tablets or capsules once a day. In Dr. Osunkwo’s experience, patients who respond best to Endari tend to be those who are naturally motivated individuals who are intentional in their efforts at optimizing their nutrition and self-care.
“It takes a lot to be adherent to Endari,” she said. “You have to work at it.”
On Nov. 15, 2019, the FDA approved crizanlizumab-tmca (Adakveo) for patients 16 years of age and older to decrease the occurrence of vasoocclusive crises.
The drug works by blocking selectin – a protein involved in the painful vascular pathophysiology. Patients receive a loading dose of 5 mg/kg administered via intravenous infusion over 30 minutes at the initiation of therapy, as well as weeks 2 and 4. After that, patients undergo treatment once a month. Nausea, back pain, pyrexia, and arthralgia are the most frequently reported adverse reactions. Clinicians must monitor patients for signs and symptoms of infusion-related reactions.
Ten days later, the FDA approved voxelotor (Oxbryta) for patients ages 12 years and up. The drug inhibits hemoglobin S polymerization and increases hemoglobin levels. Like hydroxyurea, the drug offers the convenience of once-daily dosing, and the tablet can be taken without regard to food. The drug dose requires adjustment for patients with severe hepatic impairment. Headache, fatigue, rash, and gastrointestinal disturbances such as diarrhea, nausea, and abdominal pain fall among the most commonly reported side effects.
Endari, Adakveo, and Oxbryta can all be used as monotherapy. They also provide additional benefits in reducing pain and hospitalizations and improving anemia when used concomitantly with hydroxyurea.
Like so many drugs, these novel therapies are expensive. The cost of these novel treatments has raised some eyebrows.
Annual costs of generic hydroxyurea range in the neighborhood of $1,200. In a 2017 CNBC interview, Endari manufacturer Emmaus stated that it aimed to keep drug costs under $20,000 a year. Annual costs for Adakveo and Oxbryta costs are in the neighborhood of $100,000. Adakveo manufacturer Novartis reportedly priced vials at $2,347. Most patients will require at least three of the maximum four vials per treatment. In a press release, Global Therapeutics stated that Oxbyta would cost $10,417 a month.
However, Dr. Osunkwo says the benefits of these new drugs far exceed the costs from both monetary and quality of life standpoints.
“Sickle cell disease is costly to manage,” she said in an interview. “One hospitalization can cost $10,000.”
Additionally, many SCD patients are publicly insured because of the profound disability and loss of productive work they encounter as a direct consequence of their disease and its complications. Those too sick to complete their high school and postsecondary education find limited employment opportunities.
Those fortunate enough to secure employment face significantly fewer years they can work because their pain, fatigue, frequent hospitalizations, and cumulative organ damage result in permanent disability. Only a smaller number of patients with less severe disease manifestations can secure steady employment and pursue careers that allow them to obtain private insurance.
Even if the newer therapies can help cut some costs, clinicians should be aware that prior authorizations can delay patient access to Adakveo and Oxbyta.
“We wrote the first prescription for Oxbryta in November of 2019, but the prior authorization wasn’t approved until February of 2020,” she said. Adding Adakveo to her institution’s formulary required several months of navigation. Given the arduous process, Dr. Osunkwo anticipates it will take at least year after approval before Adakveo is available for all eligible patients.
The long-term impact of these drugs also remains to be seen, so hydroxyurea will likely remain the drug of choice for many patients, according to Dr. Strouse.
Dr. Osunkwo believes SCD needs more drugs in order to truly optimize outcomes, contain costs, and enhance the patient experience.
Dr. Osunkwo reports consultancy and being on the speaker’s bureau and participating in the advisory board for Novartis, which markets Adakveo, and relationships with a variety of other pharmaceutical companies. She is the editor in chief for Hematology News. Dr. Strouse reports consultancy for Global Therapeutics, which markets Oxbryta.
Sickle cell disease (SCD) is an incurable genetic blood disorder that reduces patients’ lifespan and quality of life. Many patients live into their 40s or 50s. Yet, throughout their lives, patients are plagued by lethargy, unpredictable painful crises, and frequent hospitalizations. For nearly 20 years, clinicians only had one drug to treat SCD. Since 2017, three new drugs have been approved, but their costs and lack of long-term data have spawned questions regarding access and benefit.
“SCD reduces lifespan by 30 years, and that’s very hard to quantify,” says Ifeyinwa Osunkwo, MD, professor of medicine and pediatrics and director of Sickle Cell Disease Enterprise at the Levine Cancer Institute at Atrium Health in Charlotte, N.C. “If you put a dollar amount on what someone would make working for 30 adult years, that would be more reflective of the true cost of treating the disease.”
In 1984, hydroxyurea (Hydrea, Droxia) became the first drug to treat SCD in adults.
Originally developed as a myelosuppressive antineoplastic, hydroxyurea is used to treat resistant chronic myeloid leukemia and certain head and neck cancers. In SCD, it increases levels of hemoglobin and fetal hemoglobin.
Despite its benefit, hydroxyurea has two major drawbacks: It is only effective in two genotypes – HbSS or HbS/Beta0thal.
HbSS or HbS/Beta0thal genotypes account for 60% of the SCD population, but further studies are required to elucidate hydroxyurea’s effect in other forms of SCD, according to Dr. Osunkwo.
Secondly, hydroxyurea only reduces the frequency of painful episodes by 50% – not enough to ameliorate the pain, said John J. Strouse, MD, associate professor of medicine at Duke University School of Medicine.
Newer therapies offer the potential to enhance the effects of hydroxyurea when used concomitantly. They also give clinicians additional options for patients who either fail hydroxyurea therapy or for whom it is inappropriate.
The amino acid L-glutamine (Endari) became the second drug approved for sickle cell in 2017. Indicated for patients 5 years of age and older, Dr. Osunkwo says many patients were thrilled to have a nonchemotherapeutic option available. However, the medical community received the drug with some skepticism. “The data show Endari is moderately effective at best,” said Dr. Strouse. “Also, the mechanism of action is unclear.”
Additionally, the drug’s powder form and twice-daily dosing regimen make adherence more challenging than swallowing a few hydroxyurea tablets or capsules once a day. In Dr. Osunkwo’s experience, patients who respond best to Endari tend to be those who are naturally motivated individuals who are intentional in their efforts at optimizing their nutrition and self-care.
“It takes a lot to be adherent to Endari,” she said. “You have to work at it.”
On Nov. 15, 2019, the FDA approved crizanlizumab-tmca (Adakveo) for patients 16 years of age and older to decrease the occurrence of vasoocclusive crises.
The drug works by blocking selectin – a protein involved in the painful vascular pathophysiology. Patients receive a loading dose of 5 mg/kg administered via intravenous infusion over 30 minutes at the initiation of therapy, as well as weeks 2 and 4. After that, patients undergo treatment once a month. Nausea, back pain, pyrexia, and arthralgia are the most frequently reported adverse reactions. Clinicians must monitor patients for signs and symptoms of infusion-related reactions.
Ten days later, the FDA approved voxelotor (Oxbryta) for patients ages 12 years and up. The drug inhibits hemoglobin S polymerization and increases hemoglobin levels. Like hydroxyurea, the drug offers the convenience of once-daily dosing, and the tablet can be taken without regard to food. The drug dose requires adjustment for patients with severe hepatic impairment. Headache, fatigue, rash, and gastrointestinal disturbances such as diarrhea, nausea, and abdominal pain fall among the most commonly reported side effects.
Endari, Adakveo, and Oxbryta can all be used as monotherapy. They also provide additional benefits in reducing pain and hospitalizations and improving anemia when used concomitantly with hydroxyurea.
Like so many drugs, these novel therapies are expensive. The cost of these novel treatments has raised some eyebrows.
Annual costs of generic hydroxyurea range in the neighborhood of $1,200. In a 2017 CNBC interview, Endari manufacturer Emmaus stated that it aimed to keep drug costs under $20,000 a year. Annual costs for Adakveo and Oxbryta costs are in the neighborhood of $100,000. Adakveo manufacturer Novartis reportedly priced vials at $2,347. Most patients will require at least three of the maximum four vials per treatment. In a press release, Global Therapeutics stated that Oxbyta would cost $10,417 a month.
However, Dr. Osunkwo says the benefits of these new drugs far exceed the costs from both monetary and quality of life standpoints.
“Sickle cell disease is costly to manage,” she said in an interview. “One hospitalization can cost $10,000.”
Additionally, many SCD patients are publicly insured because of the profound disability and loss of productive work they encounter as a direct consequence of their disease and its complications. Those too sick to complete their high school and postsecondary education find limited employment opportunities.
Those fortunate enough to secure employment face significantly fewer years they can work because their pain, fatigue, frequent hospitalizations, and cumulative organ damage result in permanent disability. Only a smaller number of patients with less severe disease manifestations can secure steady employment and pursue careers that allow them to obtain private insurance.
Even if the newer therapies can help cut some costs, clinicians should be aware that prior authorizations can delay patient access to Adakveo and Oxbyta.
“We wrote the first prescription for Oxbryta in November of 2019, but the prior authorization wasn’t approved until February of 2020,” she said. Adding Adakveo to her institution’s formulary required several months of navigation. Given the arduous process, Dr. Osunkwo anticipates it will take at least year after approval before Adakveo is available for all eligible patients.
The long-term impact of these drugs also remains to be seen, so hydroxyurea will likely remain the drug of choice for many patients, according to Dr. Strouse.
Dr. Osunkwo believes SCD needs more drugs in order to truly optimize outcomes, contain costs, and enhance the patient experience.
Dr. Osunkwo reports consultancy and being on the speaker’s bureau and participating in the advisory board for Novartis, which markets Adakveo, and relationships with a variety of other pharmaceutical companies. She is the editor in chief for Hematology News. Dr. Strouse reports consultancy for Global Therapeutics, which markets Oxbryta.
Sickle cell disease (SCD) is an incurable genetic blood disorder that reduces patients’ lifespan and quality of life. Many patients live into their 40s or 50s. Yet, throughout their lives, patients are plagued by lethargy, unpredictable painful crises, and frequent hospitalizations. For nearly 20 years, clinicians only had one drug to treat SCD. Since 2017, three new drugs have been approved, but their costs and lack of long-term data have spawned questions regarding access and benefit.
“SCD reduces lifespan by 30 years, and that’s very hard to quantify,” says Ifeyinwa Osunkwo, MD, professor of medicine and pediatrics and director of Sickle Cell Disease Enterprise at the Levine Cancer Institute at Atrium Health in Charlotte, N.C. “If you put a dollar amount on what someone would make working for 30 adult years, that would be more reflective of the true cost of treating the disease.”
In 1984, hydroxyurea (Hydrea, Droxia) became the first drug to treat SCD in adults.
Originally developed as a myelosuppressive antineoplastic, hydroxyurea is used to treat resistant chronic myeloid leukemia and certain head and neck cancers. In SCD, it increases levels of hemoglobin and fetal hemoglobin.
Despite its benefit, hydroxyurea has two major drawbacks: It is only effective in two genotypes – HbSS or HbS/Beta0thal.
HbSS or HbS/Beta0thal genotypes account for 60% of the SCD population, but further studies are required to elucidate hydroxyurea’s effect in other forms of SCD, according to Dr. Osunkwo.
Secondly, hydroxyurea only reduces the frequency of painful episodes by 50% – not enough to ameliorate the pain, said John J. Strouse, MD, associate professor of medicine at Duke University School of Medicine.
Newer therapies offer the potential to enhance the effects of hydroxyurea when used concomitantly. They also give clinicians additional options for patients who either fail hydroxyurea therapy or for whom it is inappropriate.
The amino acid L-glutamine (Endari) became the second drug approved for sickle cell in 2017. Indicated for patients 5 years of age and older, Dr. Osunkwo says many patients were thrilled to have a nonchemotherapeutic option available. However, the medical community received the drug with some skepticism. “The data show Endari is moderately effective at best,” said Dr. Strouse. “Also, the mechanism of action is unclear.”
Additionally, the drug’s powder form and twice-daily dosing regimen make adherence more challenging than swallowing a few hydroxyurea tablets or capsules once a day. In Dr. Osunkwo’s experience, patients who respond best to Endari tend to be those who are naturally motivated individuals who are intentional in their efforts at optimizing their nutrition and self-care.
“It takes a lot to be adherent to Endari,” she said. “You have to work at it.”
On Nov. 15, 2019, the FDA approved crizanlizumab-tmca (Adakveo) for patients 16 years of age and older to decrease the occurrence of vasoocclusive crises.
The drug works by blocking selectin – a protein involved in the painful vascular pathophysiology. Patients receive a loading dose of 5 mg/kg administered via intravenous infusion over 30 minutes at the initiation of therapy, as well as weeks 2 and 4. After that, patients undergo treatment once a month. Nausea, back pain, pyrexia, and arthralgia are the most frequently reported adverse reactions. Clinicians must monitor patients for signs and symptoms of infusion-related reactions.
Ten days later, the FDA approved voxelotor (Oxbryta) for patients ages 12 years and up. The drug inhibits hemoglobin S polymerization and increases hemoglobin levels. Like hydroxyurea, the drug offers the convenience of once-daily dosing, and the tablet can be taken without regard to food. The drug dose requires adjustment for patients with severe hepatic impairment. Headache, fatigue, rash, and gastrointestinal disturbances such as diarrhea, nausea, and abdominal pain fall among the most commonly reported side effects.
Endari, Adakveo, and Oxbryta can all be used as monotherapy. They also provide additional benefits in reducing pain and hospitalizations and improving anemia when used concomitantly with hydroxyurea.
Like so many drugs, these novel therapies are expensive. The cost of these novel treatments has raised some eyebrows.
Annual costs of generic hydroxyurea range in the neighborhood of $1,200. In a 2017 CNBC interview, Endari manufacturer Emmaus stated that it aimed to keep drug costs under $20,000 a year. Annual costs for Adakveo and Oxbryta costs are in the neighborhood of $100,000. Adakveo manufacturer Novartis reportedly priced vials at $2,347. Most patients will require at least three of the maximum four vials per treatment. In a press release, Global Therapeutics stated that Oxbyta would cost $10,417 a month.
However, Dr. Osunkwo says the benefits of these new drugs far exceed the costs from both monetary and quality of life standpoints.
“Sickle cell disease is costly to manage,” she said in an interview. “One hospitalization can cost $10,000.”
Additionally, many SCD patients are publicly insured because of the profound disability and loss of productive work they encounter as a direct consequence of their disease and its complications. Those too sick to complete their high school and postsecondary education find limited employment opportunities.
Those fortunate enough to secure employment face significantly fewer years they can work because their pain, fatigue, frequent hospitalizations, and cumulative organ damage result in permanent disability. Only a smaller number of patients with less severe disease manifestations can secure steady employment and pursue careers that allow them to obtain private insurance.
Even if the newer therapies can help cut some costs, clinicians should be aware that prior authorizations can delay patient access to Adakveo and Oxbyta.
“We wrote the first prescription for Oxbryta in November of 2019, but the prior authorization wasn’t approved until February of 2020,” she said. Adding Adakveo to her institution’s formulary required several months of navigation. Given the arduous process, Dr. Osunkwo anticipates it will take at least year after approval before Adakveo is available for all eligible patients.
The long-term impact of these drugs also remains to be seen, so hydroxyurea will likely remain the drug of choice for many patients, according to Dr. Strouse.
Dr. Osunkwo believes SCD needs more drugs in order to truly optimize outcomes, contain costs, and enhance the patient experience.
Dr. Osunkwo reports consultancy and being on the speaker’s bureau and participating in the advisory board for Novartis, which markets Adakveo, and relationships with a variety of other pharmaceutical companies. She is the editor in chief for Hematology News. Dr. Strouse reports consultancy for Global Therapeutics, which markets Oxbryta.
New melanoma treatments linked to mortality decline
Recent advances in treatment appear to have reversed the course of melanoma mortality since 2013, according to data published in the American Journal of Public Health.
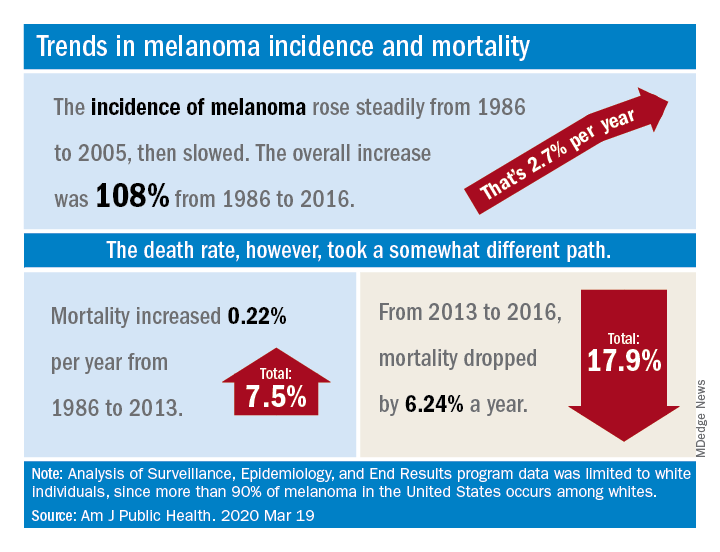
The U.S. death rate for melanoma, which had been rising at a rate of 0.22% a year for more than 2 decades, dropped by 17.9%, or 6.24% per year, during 2013-2016. That decline “coincides with the introduction of multiple new and efficacious treatments for metastatic melanoma,” such as BRAF inhibitors and immune checkpoint inhibitors, study author Juliana Berk-Krauss, MD, of the State University of New York Downstate Medical Center in Brooklyn and colleagues wrote.
The other possible explanation for the decline in deaths, “education and early detection resulting in migration toward earlier stage melanomas with a greater chance of surgical cure,” is unlikely, according to the investigators. That’s because the small decrease in median tumor thickness that occurred during 1989-2009 “is not associated with changes in prognosis.”
The investigators’ analysis encompassed data from the Surveillance, Epidemiology, and End Results registry recorded during 1986-2016. Nine registry areas were included (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah), which covered about 9.4% of the U.S. population. The analysis was limited to the white population, which accounts for more than 90% of melanoma cases in the United States.
The data showed a slight decline in annual percent change in melanoma incidence, from 3.24% for 1986-2005 to 1.72% for 2006-2016. However, over the whole period studied (1986-2016), melanoma incidence increased by 108%, or about 2.7% per year.
“Given the increased incidence of melanoma throughout this period and the lack of stage migration, these data strongly suggest that the mortality decline is due to the extended survival associated with these [newer] treatments,” the investigators wrote.
This study was funded by NYU Langone. Two investigators disclosed potential conflicts of interest, including relationships with Bio-Rad Laboratories, Novartis, Merck, and several other companies.
SOURCE: Berk-Krauss J et al. Am J Public Health. 2020 Mar 19. doi: 10.2105/AJPH.2020.305567.
Recent advances in treatment appear to have reversed the course of melanoma mortality since 2013, according to data published in the American Journal of Public Health.

The U.S. death rate for melanoma, which had been rising at a rate of 0.22% a year for more than 2 decades, dropped by 17.9%, or 6.24% per year, during 2013-2016. That decline “coincides with the introduction of multiple new and efficacious treatments for metastatic melanoma,” such as BRAF inhibitors and immune checkpoint inhibitors, study author Juliana Berk-Krauss, MD, of the State University of New York Downstate Medical Center in Brooklyn and colleagues wrote.
The other possible explanation for the decline in deaths, “education and early detection resulting in migration toward earlier stage melanomas with a greater chance of surgical cure,” is unlikely, according to the investigators. That’s because the small decrease in median tumor thickness that occurred during 1989-2009 “is not associated with changes in prognosis.”
The investigators’ analysis encompassed data from the Surveillance, Epidemiology, and End Results registry recorded during 1986-2016. Nine registry areas were included (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah), which covered about 9.4% of the U.S. population. The analysis was limited to the white population, which accounts for more than 90% of melanoma cases in the United States.
The data showed a slight decline in annual percent change in melanoma incidence, from 3.24% for 1986-2005 to 1.72% for 2006-2016. However, over the whole period studied (1986-2016), melanoma incidence increased by 108%, or about 2.7% per year.
“Given the increased incidence of melanoma throughout this period and the lack of stage migration, these data strongly suggest that the mortality decline is due to the extended survival associated with these [newer] treatments,” the investigators wrote.
This study was funded by NYU Langone. Two investigators disclosed potential conflicts of interest, including relationships with Bio-Rad Laboratories, Novartis, Merck, and several other companies.
SOURCE: Berk-Krauss J et al. Am J Public Health. 2020 Mar 19. doi: 10.2105/AJPH.2020.305567.
Recent advances in treatment appear to have reversed the course of melanoma mortality since 2013, according to data published in the American Journal of Public Health.

The U.S. death rate for melanoma, which had been rising at a rate of 0.22% a year for more than 2 decades, dropped by 17.9%, or 6.24% per year, during 2013-2016. That decline “coincides with the introduction of multiple new and efficacious treatments for metastatic melanoma,” such as BRAF inhibitors and immune checkpoint inhibitors, study author Juliana Berk-Krauss, MD, of the State University of New York Downstate Medical Center in Brooklyn and colleagues wrote.
The other possible explanation for the decline in deaths, “education and early detection resulting in migration toward earlier stage melanomas with a greater chance of surgical cure,” is unlikely, according to the investigators. That’s because the small decrease in median tumor thickness that occurred during 1989-2009 “is not associated with changes in prognosis.”
The investigators’ analysis encompassed data from the Surveillance, Epidemiology, and End Results registry recorded during 1986-2016. Nine registry areas were included (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah), which covered about 9.4% of the U.S. population. The analysis was limited to the white population, which accounts for more than 90% of melanoma cases in the United States.
The data showed a slight decline in annual percent change in melanoma incidence, from 3.24% for 1986-2005 to 1.72% for 2006-2016. However, over the whole period studied (1986-2016), melanoma incidence increased by 108%, or about 2.7% per year.
“Given the increased incidence of melanoma throughout this period and the lack of stage migration, these data strongly suggest that the mortality decline is due to the extended survival associated with these [newer] treatments,” the investigators wrote.
This study was funded by NYU Langone. Two investigators disclosed potential conflicts of interest, including relationships with Bio-Rad Laboratories, Novartis, Merck, and several other companies.
SOURCE: Berk-Krauss J et al. Am J Public Health. 2020 Mar 19. doi: 10.2105/AJPH.2020.305567.
FROM THE AMERICAN JOURNAL OF PUBLIC HEALTH
April 2020
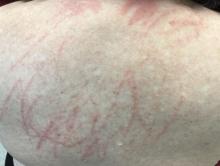
Shiitake mushroom flagellate dermatitis
that resemble whiplash marks. The lesions may be extremely pruritic, and petechiae may be present in the streaks. The trunk is most commonly affected, although lesions can occur on the limbs. Mucosa is not affected. Sun exposure may exacerbate the condition. The dermatitis has been described in all ages and races, and males seem to be more affected than females.
Shiitake mushroom flagellate dermatitis typically occurs following the ingestion of raw or undercooked shiitake mushrooms (Lentinula edodes). The mushrooms contain a polysaccharide called lentinan. Ingestion of lentinan activates interleukin-1 (IL-1), resulting in vasodilation and the subsequent dermatitis that can occur within a few hours and up to 5 days post ingestion. Associated gastrointestinal symptoms, fever, and localized swelling have been reported. The rash will resolve spontaneously over a few days to weeks.
Flagellate erythema has been described with bleomycin treatment. Other reported associations include peplomycin (a bleomycin derivative) and docetaxel. The rash may appear following administration of bleomycin by any route and has been shown to be dose independent. Onset occurs anywhere from 1 day to several months after exposure. Over time, the erythema will develop into postinflammatory hyperpigmentation.
Dermatomyositis may present with flagellate erythema. Other symptoms include muscle weakness and an inflammatory myopathy. A heliotrope rash on the eyelids, Gottron’s papules on the hands, ragged cuticles with prominent vessels on nail folds may be seen. Blood work may reveal elevated antinuclear antibodies (ANA), anti–Mi-2 and anti–Jo-1. Adult-onset Still disease is characterized by fever, arthritis, and salmon-colored patches.
Our patient’s dermatitis resolved spontaneously without treatment.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Shiitake mushroom flagellate dermatitis
that resemble whiplash marks. The lesions may be extremely pruritic, and petechiae may be present in the streaks. The trunk is most commonly affected, although lesions can occur on the limbs. Mucosa is not affected. Sun exposure may exacerbate the condition. The dermatitis has been described in all ages and races, and males seem to be more affected than females.
Shiitake mushroom flagellate dermatitis typically occurs following the ingestion of raw or undercooked shiitake mushrooms (Lentinula edodes). The mushrooms contain a polysaccharide called lentinan. Ingestion of lentinan activates interleukin-1 (IL-1), resulting in vasodilation and the subsequent dermatitis that can occur within a few hours and up to 5 days post ingestion. Associated gastrointestinal symptoms, fever, and localized swelling have been reported. The rash will resolve spontaneously over a few days to weeks.
Flagellate erythema has been described with bleomycin treatment. Other reported associations include peplomycin (a bleomycin derivative) and docetaxel. The rash may appear following administration of bleomycin by any route and has been shown to be dose independent. Onset occurs anywhere from 1 day to several months after exposure. Over time, the erythema will develop into postinflammatory hyperpigmentation.
Dermatomyositis may present with flagellate erythema. Other symptoms include muscle weakness and an inflammatory myopathy. A heliotrope rash on the eyelids, Gottron’s papules on the hands, ragged cuticles with prominent vessels on nail folds may be seen. Blood work may reveal elevated antinuclear antibodies (ANA), anti–Mi-2 and anti–Jo-1. Adult-onset Still disease is characterized by fever, arthritis, and salmon-colored patches.
Our patient’s dermatitis resolved spontaneously without treatment.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Shiitake mushroom flagellate dermatitis
that resemble whiplash marks. The lesions may be extremely pruritic, and petechiae may be present in the streaks. The trunk is most commonly affected, although lesions can occur on the limbs. Mucosa is not affected. Sun exposure may exacerbate the condition. The dermatitis has been described in all ages and races, and males seem to be more affected than females.
Shiitake mushroom flagellate dermatitis typically occurs following the ingestion of raw or undercooked shiitake mushrooms (Lentinula edodes). The mushrooms contain a polysaccharide called lentinan. Ingestion of lentinan activates interleukin-1 (IL-1), resulting in vasodilation and the subsequent dermatitis that can occur within a few hours and up to 5 days post ingestion. Associated gastrointestinal symptoms, fever, and localized swelling have been reported. The rash will resolve spontaneously over a few days to weeks.
Flagellate erythema has been described with bleomycin treatment. Other reported associations include peplomycin (a bleomycin derivative) and docetaxel. The rash may appear following administration of bleomycin by any route and has been shown to be dose independent. Onset occurs anywhere from 1 day to several months after exposure. Over time, the erythema will develop into postinflammatory hyperpigmentation.
Dermatomyositis may present with flagellate erythema. Other symptoms include muscle weakness and an inflammatory myopathy. A heliotrope rash on the eyelids, Gottron’s papules on the hands, ragged cuticles with prominent vessels on nail folds may be seen. Blood work may reveal elevated antinuclear antibodies (ANA), anti–Mi-2 and anti–Jo-1. Adult-onset Still disease is characterized by fever, arthritis, and salmon-colored patches.
Our patient’s dermatitis resolved spontaneously without treatment.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Carboplatin plus pemetrexed should be ‘a standard option’ in elderly patients with NSCLC
For patients age 75 and older with nonsquamous non–small cell lung cancer (NSCLC) not previously treated with chemotherapy, combination carboplatin and pemetrexed followed by pemetrexed maintenance is both effective and tolerable, suggest the results of a phase 3 trial.
The median overall survival was 18.7 months for patients randomized to carboplatin/pemetrexed and 15.5 months for patients randomized to docetaxel monotherapy.
The combination met the prespecified endpoint for noninferiority to docetaxel but was not shown to be superior in terms of overall survival, investigator Isamu Okamoto, MD, of Kyushu University in Fukuoka, Japan, and colleagues reported in JAMA Oncology.
Still, progression-free survival was significantly longer in the carboplatin/pemetrexed arm, and dose reductions were less frequent with the combination than with docetaxel.
“The combination of carboplatin and pemetrexed followed by pemetrexed maintenance ... provides a clinically significant benefit with regard to its effectiveness and tolerability,” the investigators wrote. “This combination should therefore be considered as a standard option for treatment in this setting.”
Dr. Okamoto and colleagues noted that the lung cancer incidence in elderly patients – 75 years and older – is increasing, and cytotoxic chemotherapy remains the standard treatment for patients whose tumors do not carry targetable mutations or are resistant to immunotherapy.
“In anticipation of a further increase in the number of elderly individuals with advanced NSCLC, it will be important to develop more optimal chemotherapeutic regimens for this patient group,” the investigators wrote.
To that end, they conducted a phase 3 trial of carboplatin/pemetrexed in patients aged 75 and older with NSCLC who had not been exposed to cytotoxic chemotherapy.
Patients and treatment
There were 433 patients enrolled in the trial. Their median age was 78 years (range, 75-88 years), and 57.7% were men. All patients had Eastern Cooperative Oncology Group performance status of 0 or 1.
Patients were stratified by clinical stage (III, IV, or recurrence), sex, epidermal growth factor receptor variant status (wild-type, exon 19 deletion, L858R variant of exon 21, or unknown), and treatment center.
The patients were then randomly assigned on a 1:1 basis to receive either intravenous docetaxel at 60 mg/m2 for 60 minutes on day 1 every 3 weeks or pemetrexed at 500 mg/m2 for 10 minutes followed by an infusion of carboplatin at an area under the curve of 5 for 30 minutes on day 1 every 3 weeks. The combination therapy was repeated for up to four courses and followed by 3-week courses of maintenance therapy with the same dose of pemetrexed.
Both regimens were continued until disease progression or the development of unacceptable toxicities.
Efficacy and safety
All 433 randomized patients were included in the efficacy analysis, but the safety analysis included 428 patients. Three patients assigned to docetaxel and two assigned to carboplatin/pemetrexed did not receive protocol treatment.
The respective median overall survival for the docetaxel and carboplatin/pemetrexed arms was 15.5 months and 18.7 months, which translated to a stratified hazard ratio for death of 0.85, meeting the prespecified noninferiority endpoint (P = .003).
However, the upper limit of the 95% confidence interval was 1.056, exceeding the prespecified superiority margin of 1.000. Therefore, the combination could not be proven superior to docetaxel with regard to overall survival.
On the other hand, progression-free survival was significantly longer with carboplatin/pemetrexed. The median progression-free survival was 6.4 months in the combination arm and 4.3 months in the docetaxel arm (unstratified HR, 0.739; P < .001).
The overall response rate with carboplatin/pemetrexed was 36.8%, compared with 28.2% for docetaxel, but this difference was not statistically significant.
Adverse events that were more common in the docetaxel arm than in the combination arm included grade 3/4 decreases in white blood cell count (68.7% and 28%, respectively), grade 3/4 decreases in neutrophil count (86% and 46.3%, respectively), and febrile neutropenia (17.8% and 4.2%, respectively).
Adverse events more frequently seen with the combination than with docetaxel included anemia (29.4% and 1.9%, respectively) and decreased platelet counts (25.7% and 1.4%, respectively).
Two patients in each arm died from treatment-related causes. In the docetaxel arm, the deaths were caused by acute respiratory distress syndrome and pneumonitis. In the combination arm, the deaths were caused by dyspnea and pneumonitis.
Approximately 29% of patients in each arm reported improvement in quality of life at 18 weeks, compared with baseline.
Performance status is key
A lung cancer specialist who was not involved in the study agreed with the authors that age should not be the primary determinant for choice of a treatment regimen.
“There’s a convergence of data over the last decade or so that has really clearly shown that our treatment decisions should be based on performance status much more than chronologic age, certainly for our patients who are in their 70s, and even potentially into their early 80s,” Howard (Jack) West, MD, of City of Hope Comprehensive Cancer Center in Duarte, Calif., said in an interview.
“The available data really say that patients with a good performance status who are in their 70s should be treated just like patients in their 60s and 50s,” he said.
He added, however, that for patients such as those in the study without targetable driver mutations, the best treatment would likely be immunotherapy or immunotherapy combined with chemotherapy.
“If there were a patient with a nonsquamous non–small cell lung cancer where we would be thinking about carboplatin and pemetrexed, I would go further than just carbo and pemetrexed; I would give carbo and pemetrexed with pembrolizumab for most of these patients,” he said.
Dr. West said the study primarily offers reassurances about the efficacy and tolerability of the carboplatin/pemetrexed combination in patients aged 75 years and older.
The study was funded by agencies of the Japanese government. The investigators disclosed relationships with Boehringer Ingelheim, AstraZeneca, Eli Lilly Japan KK, and many other companies. Dr. West disclosed consulting for Merck.
SOURCE: Okamoto I et al. JAMA Oncol. 2020 Mar 12. doi: 10.1001/jamaoncol.2019.6828.
For patients age 75 and older with nonsquamous non–small cell lung cancer (NSCLC) not previously treated with chemotherapy, combination carboplatin and pemetrexed followed by pemetrexed maintenance is both effective and tolerable, suggest the results of a phase 3 trial.
The median overall survival was 18.7 months for patients randomized to carboplatin/pemetrexed and 15.5 months for patients randomized to docetaxel monotherapy.
The combination met the prespecified endpoint for noninferiority to docetaxel but was not shown to be superior in terms of overall survival, investigator Isamu Okamoto, MD, of Kyushu University in Fukuoka, Japan, and colleagues reported in JAMA Oncology.
Still, progression-free survival was significantly longer in the carboplatin/pemetrexed arm, and dose reductions were less frequent with the combination than with docetaxel.
“The combination of carboplatin and pemetrexed followed by pemetrexed maintenance ... provides a clinically significant benefit with regard to its effectiveness and tolerability,” the investigators wrote. “This combination should therefore be considered as a standard option for treatment in this setting.”
Dr. Okamoto and colleagues noted that the lung cancer incidence in elderly patients – 75 years and older – is increasing, and cytotoxic chemotherapy remains the standard treatment for patients whose tumors do not carry targetable mutations or are resistant to immunotherapy.
“In anticipation of a further increase in the number of elderly individuals with advanced NSCLC, it will be important to develop more optimal chemotherapeutic regimens for this patient group,” the investigators wrote.
To that end, they conducted a phase 3 trial of carboplatin/pemetrexed in patients aged 75 and older with NSCLC who had not been exposed to cytotoxic chemotherapy.
Patients and treatment
There were 433 patients enrolled in the trial. Their median age was 78 years (range, 75-88 years), and 57.7% were men. All patients had Eastern Cooperative Oncology Group performance status of 0 or 1.
Patients were stratified by clinical stage (III, IV, or recurrence), sex, epidermal growth factor receptor variant status (wild-type, exon 19 deletion, L858R variant of exon 21, or unknown), and treatment center.
The patients were then randomly assigned on a 1:1 basis to receive either intravenous docetaxel at 60 mg/m2 for 60 minutes on day 1 every 3 weeks or pemetrexed at 500 mg/m2 for 10 minutes followed by an infusion of carboplatin at an area under the curve of 5 for 30 minutes on day 1 every 3 weeks. The combination therapy was repeated for up to four courses and followed by 3-week courses of maintenance therapy with the same dose of pemetrexed.
Both regimens were continued until disease progression or the development of unacceptable toxicities.
Efficacy and safety
All 433 randomized patients were included in the efficacy analysis, but the safety analysis included 428 patients. Three patients assigned to docetaxel and two assigned to carboplatin/pemetrexed did not receive protocol treatment.
The respective median overall survival for the docetaxel and carboplatin/pemetrexed arms was 15.5 months and 18.7 months, which translated to a stratified hazard ratio for death of 0.85, meeting the prespecified noninferiority endpoint (P = .003).
However, the upper limit of the 95% confidence interval was 1.056, exceeding the prespecified superiority margin of 1.000. Therefore, the combination could not be proven superior to docetaxel with regard to overall survival.
On the other hand, progression-free survival was significantly longer with carboplatin/pemetrexed. The median progression-free survival was 6.4 months in the combination arm and 4.3 months in the docetaxel arm (unstratified HR, 0.739; P < .001).
The overall response rate with carboplatin/pemetrexed was 36.8%, compared with 28.2% for docetaxel, but this difference was not statistically significant.
Adverse events that were more common in the docetaxel arm than in the combination arm included grade 3/4 decreases in white blood cell count (68.7% and 28%, respectively), grade 3/4 decreases in neutrophil count (86% and 46.3%, respectively), and febrile neutropenia (17.8% and 4.2%, respectively).
Adverse events more frequently seen with the combination than with docetaxel included anemia (29.4% and 1.9%, respectively) and decreased platelet counts (25.7% and 1.4%, respectively).
Two patients in each arm died from treatment-related causes. In the docetaxel arm, the deaths were caused by acute respiratory distress syndrome and pneumonitis. In the combination arm, the deaths were caused by dyspnea and pneumonitis.
Approximately 29% of patients in each arm reported improvement in quality of life at 18 weeks, compared with baseline.
Performance status is key
A lung cancer specialist who was not involved in the study agreed with the authors that age should not be the primary determinant for choice of a treatment regimen.
“There’s a convergence of data over the last decade or so that has really clearly shown that our treatment decisions should be based on performance status much more than chronologic age, certainly for our patients who are in their 70s, and even potentially into their early 80s,” Howard (Jack) West, MD, of City of Hope Comprehensive Cancer Center in Duarte, Calif., said in an interview.
“The available data really say that patients with a good performance status who are in their 70s should be treated just like patients in their 60s and 50s,” he said.
He added, however, that for patients such as those in the study without targetable driver mutations, the best treatment would likely be immunotherapy or immunotherapy combined with chemotherapy.
“If there were a patient with a nonsquamous non–small cell lung cancer where we would be thinking about carboplatin and pemetrexed, I would go further than just carbo and pemetrexed; I would give carbo and pemetrexed with pembrolizumab for most of these patients,” he said.
Dr. West said the study primarily offers reassurances about the efficacy and tolerability of the carboplatin/pemetrexed combination in patients aged 75 years and older.
The study was funded by agencies of the Japanese government. The investigators disclosed relationships with Boehringer Ingelheim, AstraZeneca, Eli Lilly Japan KK, and many other companies. Dr. West disclosed consulting for Merck.
SOURCE: Okamoto I et al. JAMA Oncol. 2020 Mar 12. doi: 10.1001/jamaoncol.2019.6828.
For patients age 75 and older with nonsquamous non–small cell lung cancer (NSCLC) not previously treated with chemotherapy, combination carboplatin and pemetrexed followed by pemetrexed maintenance is both effective and tolerable, suggest the results of a phase 3 trial.
The median overall survival was 18.7 months for patients randomized to carboplatin/pemetrexed and 15.5 months for patients randomized to docetaxel monotherapy.
The combination met the prespecified endpoint for noninferiority to docetaxel but was not shown to be superior in terms of overall survival, investigator Isamu Okamoto, MD, of Kyushu University in Fukuoka, Japan, and colleagues reported in JAMA Oncology.
Still, progression-free survival was significantly longer in the carboplatin/pemetrexed arm, and dose reductions were less frequent with the combination than with docetaxel.
“The combination of carboplatin and pemetrexed followed by pemetrexed maintenance ... provides a clinically significant benefit with regard to its effectiveness and tolerability,” the investigators wrote. “This combination should therefore be considered as a standard option for treatment in this setting.”
Dr. Okamoto and colleagues noted that the lung cancer incidence in elderly patients – 75 years and older – is increasing, and cytotoxic chemotherapy remains the standard treatment for patients whose tumors do not carry targetable mutations or are resistant to immunotherapy.
“In anticipation of a further increase in the number of elderly individuals with advanced NSCLC, it will be important to develop more optimal chemotherapeutic regimens for this patient group,” the investigators wrote.
To that end, they conducted a phase 3 trial of carboplatin/pemetrexed in patients aged 75 and older with NSCLC who had not been exposed to cytotoxic chemotherapy.
Patients and treatment
There were 433 patients enrolled in the trial. Their median age was 78 years (range, 75-88 years), and 57.7% were men. All patients had Eastern Cooperative Oncology Group performance status of 0 or 1.
Patients were stratified by clinical stage (III, IV, or recurrence), sex, epidermal growth factor receptor variant status (wild-type, exon 19 deletion, L858R variant of exon 21, or unknown), and treatment center.
The patients were then randomly assigned on a 1:1 basis to receive either intravenous docetaxel at 60 mg/m2 for 60 minutes on day 1 every 3 weeks or pemetrexed at 500 mg/m2 for 10 minutes followed by an infusion of carboplatin at an area under the curve of 5 for 30 minutes on day 1 every 3 weeks. The combination therapy was repeated for up to four courses and followed by 3-week courses of maintenance therapy with the same dose of pemetrexed.
Both regimens were continued until disease progression or the development of unacceptable toxicities.
Efficacy and safety
All 433 randomized patients were included in the efficacy analysis, but the safety analysis included 428 patients. Three patients assigned to docetaxel and two assigned to carboplatin/pemetrexed did not receive protocol treatment.
The respective median overall survival for the docetaxel and carboplatin/pemetrexed arms was 15.5 months and 18.7 months, which translated to a stratified hazard ratio for death of 0.85, meeting the prespecified noninferiority endpoint (P = .003).
However, the upper limit of the 95% confidence interval was 1.056, exceeding the prespecified superiority margin of 1.000. Therefore, the combination could not be proven superior to docetaxel with regard to overall survival.
On the other hand, progression-free survival was significantly longer with carboplatin/pemetrexed. The median progression-free survival was 6.4 months in the combination arm and 4.3 months in the docetaxel arm (unstratified HR, 0.739; P < .001).
The overall response rate with carboplatin/pemetrexed was 36.8%, compared with 28.2% for docetaxel, but this difference was not statistically significant.
Adverse events that were more common in the docetaxel arm than in the combination arm included grade 3/4 decreases in white blood cell count (68.7% and 28%, respectively), grade 3/4 decreases in neutrophil count (86% and 46.3%, respectively), and febrile neutropenia (17.8% and 4.2%, respectively).
Adverse events more frequently seen with the combination than with docetaxel included anemia (29.4% and 1.9%, respectively) and decreased platelet counts (25.7% and 1.4%, respectively).
Two patients in each arm died from treatment-related causes. In the docetaxel arm, the deaths were caused by acute respiratory distress syndrome and pneumonitis. In the combination arm, the deaths were caused by dyspnea and pneumonitis.
Approximately 29% of patients in each arm reported improvement in quality of life at 18 weeks, compared with baseline.
Performance status is key
A lung cancer specialist who was not involved in the study agreed with the authors that age should not be the primary determinant for choice of a treatment regimen.
“There’s a convergence of data over the last decade or so that has really clearly shown that our treatment decisions should be based on performance status much more than chronologic age, certainly for our patients who are in their 70s, and even potentially into their early 80s,” Howard (Jack) West, MD, of City of Hope Comprehensive Cancer Center in Duarte, Calif., said in an interview.
“The available data really say that patients with a good performance status who are in their 70s should be treated just like patients in their 60s and 50s,” he said.
He added, however, that for patients such as those in the study without targetable driver mutations, the best treatment would likely be immunotherapy or immunotherapy combined with chemotherapy.
“If there were a patient with a nonsquamous non–small cell lung cancer where we would be thinking about carboplatin and pemetrexed, I would go further than just carbo and pemetrexed; I would give carbo and pemetrexed with pembrolizumab for most of these patients,” he said.
Dr. West said the study primarily offers reassurances about the efficacy and tolerability of the carboplatin/pemetrexed combination in patients aged 75 years and older.
The study was funded by agencies of the Japanese government. The investigators disclosed relationships with Boehringer Ingelheim, AstraZeneca, Eli Lilly Japan KK, and many other companies. Dr. West disclosed consulting for Merck.
SOURCE: Okamoto I et al. JAMA Oncol. 2020 Mar 12. doi: 10.1001/jamaoncol.2019.6828.
FROM JAMA ONCOLOGY
N-acetyl cysteine may positively affect cerebral glucose metabolism in MS
Key clinical point: In patients with multiple sclerosis (MS), N-acetyl cysteine (NAC) positively affects cerebral glucose metabolism, and this appears to be associated with improved symptoms related to cognition and attention.
Major finding: Based on fluorodeoxyglucose (FDG) positron emission tomography (PET) data, NAC group vs. the control group demonstrated significant increases in cerebral glucose metabolism after therapy in several brain regions including the lateral and middle temporal lobes, inferior frontal lobe, and caudate (P less than .05). NAC group had significant improvements in self-reported scores related to cognition and attention.
Study details: Twenty-four patients with a diagnosis of MS were randomly assigned to NAC plus standard of care or standard of care only (waitlist control group). FDG PET was used to assess cerebral glucose metabolism at baseline and after 2 months in all patients.
Disclosures: This study was supported by a grant from the Coors Foundation. The authors declared no potential conflicts of interest.
Citation: Monti DA et al. Front Neurol. 2020 Feb 14. doi: 10.3389/fneur.2020.00088.
Key clinical point: In patients with multiple sclerosis (MS), N-acetyl cysteine (NAC) positively affects cerebral glucose metabolism, and this appears to be associated with improved symptoms related to cognition and attention.
Major finding: Based on fluorodeoxyglucose (FDG) positron emission tomography (PET) data, NAC group vs. the control group demonstrated significant increases in cerebral glucose metabolism after therapy in several brain regions including the lateral and middle temporal lobes, inferior frontal lobe, and caudate (P less than .05). NAC group had significant improvements in self-reported scores related to cognition and attention.
Study details: Twenty-four patients with a diagnosis of MS were randomly assigned to NAC plus standard of care or standard of care only (waitlist control group). FDG PET was used to assess cerebral glucose metabolism at baseline and after 2 months in all patients.
Disclosures: This study was supported by a grant from the Coors Foundation. The authors declared no potential conflicts of interest.
Citation: Monti DA et al. Front Neurol. 2020 Feb 14. doi: 10.3389/fneur.2020.00088.
Key clinical point: In patients with multiple sclerosis (MS), N-acetyl cysteine (NAC) positively affects cerebral glucose metabolism, and this appears to be associated with improved symptoms related to cognition and attention.
Major finding: Based on fluorodeoxyglucose (FDG) positron emission tomography (PET) data, NAC group vs. the control group demonstrated significant increases in cerebral glucose metabolism after therapy in several brain regions including the lateral and middle temporal lobes, inferior frontal lobe, and caudate (P less than .05). NAC group had significant improvements in self-reported scores related to cognition and attention.
Study details: Twenty-four patients with a diagnosis of MS were randomly assigned to NAC plus standard of care or standard of care only (waitlist control group). FDG PET was used to assess cerebral glucose metabolism at baseline and after 2 months in all patients.
Disclosures: This study was supported by a grant from the Coors Foundation. The authors declared no potential conflicts of interest.
Citation: Monti DA et al. Front Neurol. 2020 Feb 14. doi: 10.3389/fneur.2020.00088.
Does diet quality influence late-onset MS risk?
Key clinical point: Diet quality is not associated with the risk for late-onset multiple sclerosis (MS) in middle-aged individuals.
Major finding: Diet quality, assessed by means of the Alternative Healthy Eating Index-2010, was not statistically significantly associated with late-onset MS diagnosis risk (hazard ratio highest vs. lowest tertile, 0.79; test for trend: P = .22). Smoking did not modify the association.
Study details: Prospective cohort study based on the Danish cohort “Diet, Cancer and Health” evaluated the association between diet quality and the hazards of late-onset MS diagnosis in 56,867 individuals (age, 50-64 years) who were followed for a median of 20.4 years; 124 patients with MS were identified.
Disclosures: This study did not receive any specific funding. The data collection for the Diet, Cancer and Health cohort was funded by the Danish Cancer Society. Ulrik Dalgas has received research support, travel grants, and/or teaching honorary from Biogen Idec, Merck Serono, Novartis, Bayer Schering, and Sanofi Aventis as well as honoraria from serving on scientific advisory boards of Biogen Idec and Genzyme. The other authors reported no conflicts of interest.
Citation: Pommerich UM et al. Mult Scler Relat Disord. 2020 Jan 26. doi: 10.1016/j.msard.2020.101968.
Key clinical point: Diet quality is not associated with the risk for late-onset multiple sclerosis (MS) in middle-aged individuals.
Major finding: Diet quality, assessed by means of the Alternative Healthy Eating Index-2010, was not statistically significantly associated with late-onset MS diagnosis risk (hazard ratio highest vs. lowest tertile, 0.79; test for trend: P = .22). Smoking did not modify the association.
Study details: Prospective cohort study based on the Danish cohort “Diet, Cancer and Health” evaluated the association between diet quality and the hazards of late-onset MS diagnosis in 56,867 individuals (age, 50-64 years) who were followed for a median of 20.4 years; 124 patients with MS were identified.
Disclosures: This study did not receive any specific funding. The data collection for the Diet, Cancer and Health cohort was funded by the Danish Cancer Society. Ulrik Dalgas has received research support, travel grants, and/or teaching honorary from Biogen Idec, Merck Serono, Novartis, Bayer Schering, and Sanofi Aventis as well as honoraria from serving on scientific advisory boards of Biogen Idec and Genzyme. The other authors reported no conflicts of interest.
Citation: Pommerich UM et al. Mult Scler Relat Disord. 2020 Jan 26. doi: 10.1016/j.msard.2020.101968.
Key clinical point: Diet quality is not associated with the risk for late-onset multiple sclerosis (MS) in middle-aged individuals.
Major finding: Diet quality, assessed by means of the Alternative Healthy Eating Index-2010, was not statistically significantly associated with late-onset MS diagnosis risk (hazard ratio highest vs. lowest tertile, 0.79; test for trend: P = .22). Smoking did not modify the association.
Study details: Prospective cohort study based on the Danish cohort “Diet, Cancer and Health” evaluated the association between diet quality and the hazards of late-onset MS diagnosis in 56,867 individuals (age, 50-64 years) who were followed for a median of 20.4 years; 124 patients with MS were identified.
Disclosures: This study did not receive any specific funding. The data collection for the Diet, Cancer and Health cohort was funded by the Danish Cancer Society. Ulrik Dalgas has received research support, travel grants, and/or teaching honorary from Biogen Idec, Merck Serono, Novartis, Bayer Schering, and Sanofi Aventis as well as honoraria from serving on scientific advisory boards of Biogen Idec and Genzyme. The other authors reported no conflicts of interest.
Citation: Pommerich UM et al. Mult Scler Relat Disord. 2020 Jan 26. doi: 10.1016/j.msard.2020.101968.
Elevated leptin levels linked with MS risk
Key clinical point: Increase in leptin concentration is associated with an elevated multiple sclerosis (MS) risk among young individuals.
Major finding: A 1-unit increase in leptin z-score was associated with higher MS risk in individuals younger than 20 years (odds ratio [OR], 1.4; 95% confidence interval, 1.1-1.9) and in all men (OR, 1.4; 95% confidence interval, 1.0-2.0). In contrast, MS risk reduced with increased leptin levels in women aged 30-39 years after adjustment for insulin levels (OR, 0.74; 95% confidence interval, 0.54-1.0).
Study details: This nested case-control study used blood samples from Swedish biobanks and compared leptin and insulin concentrations in 649 individuals who later developed relapsing-remitting MS and 649 matched controls.
Disclosures: This study was supported by the Swedish Research Council. Lucia Alonso-Magdalena has received speaking fees from Merck Serono and served on advisory board for Merck Serono and Biogen. Magnus Vrethem has received honoraria for lectures from Genzyme and for advisory boards from Roche and Novartis.
Citation: Biström M et al. Mult Scler. 2020 Feb 7. doi: 10.1177/1352458520905033.
Key clinical point: Increase in leptin concentration is associated with an elevated multiple sclerosis (MS) risk among young individuals.
Major finding: A 1-unit increase in leptin z-score was associated with higher MS risk in individuals younger than 20 years (odds ratio [OR], 1.4; 95% confidence interval, 1.1-1.9) and in all men (OR, 1.4; 95% confidence interval, 1.0-2.0). In contrast, MS risk reduced with increased leptin levels in women aged 30-39 years after adjustment for insulin levels (OR, 0.74; 95% confidence interval, 0.54-1.0).
Study details: This nested case-control study used blood samples from Swedish biobanks and compared leptin and insulin concentrations in 649 individuals who later developed relapsing-remitting MS and 649 matched controls.
Disclosures: This study was supported by the Swedish Research Council. Lucia Alonso-Magdalena has received speaking fees from Merck Serono and served on advisory board for Merck Serono and Biogen. Magnus Vrethem has received honoraria for lectures from Genzyme and for advisory boards from Roche and Novartis.
Citation: Biström M et al. Mult Scler. 2020 Feb 7. doi: 10.1177/1352458520905033.
Key clinical point: Increase in leptin concentration is associated with an elevated multiple sclerosis (MS) risk among young individuals.
Major finding: A 1-unit increase in leptin z-score was associated with higher MS risk in individuals younger than 20 years (odds ratio [OR], 1.4; 95% confidence interval, 1.1-1.9) and in all men (OR, 1.4; 95% confidence interval, 1.0-2.0). In contrast, MS risk reduced with increased leptin levels in women aged 30-39 years after adjustment for insulin levels (OR, 0.74; 95% confidence interval, 0.54-1.0).
Study details: This nested case-control study used blood samples from Swedish biobanks and compared leptin and insulin concentrations in 649 individuals who later developed relapsing-remitting MS and 649 matched controls.
Disclosures: This study was supported by the Swedish Research Council. Lucia Alonso-Magdalena has received speaking fees from Merck Serono and served on advisory board for Merck Serono and Biogen. Magnus Vrethem has received honoraria for lectures from Genzyme and for advisory boards from Roche and Novartis.
Citation: Biström M et al. Mult Scler. 2020 Feb 7. doi: 10.1177/1352458520905033.
Concussion in adolescents tied to MS risk
Key clinical point: Concussions in adolescents correlate to an elevated risk for multiple sclerosis (MS).
Major finding: The risk for MS was higher among patients exposed to a concussion in adolescence (hazard ratio [HR], 1.29; P = .03). Sex-specific analysis revealed a higher risk for MS only in males who sustained a concussion in adolescence (HR, 1.41; P = 0.04).
Study details: Retrospective study included 97,965 patients (age, 11-18 years) exposed to a concussion who were matched to 293,895 unexposed patients; primary outcome was MS diagnosis.
Disclosures: This study was funded by an unrestricted investigator-initiated trial grant from Roche Canada and supported by ICES. The corresponding author has served on advisory boards for Biogen Idec, EMD Serono, Genzyme Canada, Novartis, and Roche; has received Investigator Initiated Grant Funds from Biogen Idec, Novartis, and Roche; and has acted as site PI for multicenter trials funded by Novartis, Genzyme, Roche, and AbbVie. All other authors declared no conflicts of interest.
Citation: Povolo CA et al. Mult Scler. 2020 Feb 24. doi: 10.1177/1352458520908037.
Key clinical point: Concussions in adolescents correlate to an elevated risk for multiple sclerosis (MS).
Major finding: The risk for MS was higher among patients exposed to a concussion in adolescence (hazard ratio [HR], 1.29; P = .03). Sex-specific analysis revealed a higher risk for MS only in males who sustained a concussion in adolescence (HR, 1.41; P = 0.04).
Study details: Retrospective study included 97,965 patients (age, 11-18 years) exposed to a concussion who were matched to 293,895 unexposed patients; primary outcome was MS diagnosis.
Disclosures: This study was funded by an unrestricted investigator-initiated trial grant from Roche Canada and supported by ICES. The corresponding author has served on advisory boards for Biogen Idec, EMD Serono, Genzyme Canada, Novartis, and Roche; has received Investigator Initiated Grant Funds from Biogen Idec, Novartis, and Roche; and has acted as site PI for multicenter trials funded by Novartis, Genzyme, Roche, and AbbVie. All other authors declared no conflicts of interest.
Citation: Povolo CA et al. Mult Scler. 2020 Feb 24. doi: 10.1177/1352458520908037.
Key clinical point: Concussions in adolescents correlate to an elevated risk for multiple sclerosis (MS).
Major finding: The risk for MS was higher among patients exposed to a concussion in adolescence (hazard ratio [HR], 1.29; P = .03). Sex-specific analysis revealed a higher risk for MS only in males who sustained a concussion in adolescence (HR, 1.41; P = 0.04).
Study details: Retrospective study included 97,965 patients (age, 11-18 years) exposed to a concussion who were matched to 293,895 unexposed patients; primary outcome was MS diagnosis.
Disclosures: This study was funded by an unrestricted investigator-initiated trial grant from Roche Canada and supported by ICES. The corresponding author has served on advisory boards for Biogen Idec, EMD Serono, Genzyme Canada, Novartis, and Roche; has received Investigator Initiated Grant Funds from Biogen Idec, Novartis, and Roche; and has acted as site PI for multicenter trials funded by Novartis, Genzyme, Roche, and AbbVie. All other authors declared no conflicts of interest.
Citation: Povolo CA et al. Mult Scler. 2020 Feb 24. doi: 10.1177/1352458520908037.
Rheumatologists to share knowledge in COVID-19 patient-centered registry
Rheumatologists the world over are joining forces to create a COVID-19 rheumatology registry designed to help both patients and providers learn from each other regarding management of rheumatologic diseases and risk of infection among patients who are commonly on chronic immunosuppressive medications.
The COVID-19 Global Rheumatology Alliance, a consortium supported by more than 50 major clinical societies and foundations, quickly grew from messages on social media platforms to a multinational group focused on the common goal of helping to “guide rheumatology clinicians in assessing and treating patients with rheumatologic disease and in evaluating the risk of infection in patients on immunosuppression.”
As of this writing, the rheumatology registry is still being assembled, and organizers are currently seeking approvals from various authorities. As of March 17, 2020, the Institutional Review Board (IRB) at the University of California, San Francisco, has determined that the registry is exempt from IRB approval requirements, a finding that should apply elsewhere in the United States, according to the registry website.
When it is fully up and running, clinicians will be able to report to the secure website on any and all cases of patients with rheumatologic disorders who present with COVID-19 of any severity, including patients with mild disease or asymptomatic patients who test positive.
“We are aiming for 5 to 10 minutes to input the data. We don’t want to drag them away from their clinical duties too much, but if clinicians are able to spare a few minutes to put in details about a patient, then that’s going to help build our knowledge and it’s going to help them with other patients,” said Philip Robinson, MBChB, associate professor of medicine at the University of Queensland in Brisbane, Australia, and the chief architect of the registry.
The data will be deindentified, with no protected health care information required or included, and made available to the global rheumatology community, but the registry will not offer clinical advice, Dr. Robinson said in an interview.
“This is observational data, it’s not randomized, but our approach is that some data is better than no data,” he said.
He also cautioned that the data will need careful interpretation, because information about patients with mild symptoms may offer false reassurances about the severity or extent of infection.
“For example, the patients with severe cases may be in the ICU, and can’t tell their doctors that they’re on methotrexate, so you can see how we need to be really careful about the messages from that data and not misinterpret it,” he said.
The COVID-19 rheumatology registry was inspired by a similar effort in the gastroenterology community, the Surveillance Epidemiology of Coronavirus Under Research Exclusion (SECURE-IBD) registry. Patients with inflammatory bowel disease are often treated with immunosuppressive biologic agents familiar to the rheumatology community, such as infliximab (Remicade and biosimilars) and adalimumab (Humira and biosimilars), and methotrexate.
Rheumatologists the world over are joining forces to create a COVID-19 rheumatology registry designed to help both patients and providers learn from each other regarding management of rheumatologic diseases and risk of infection among patients who are commonly on chronic immunosuppressive medications.
The COVID-19 Global Rheumatology Alliance, a consortium supported by more than 50 major clinical societies and foundations, quickly grew from messages on social media platforms to a multinational group focused on the common goal of helping to “guide rheumatology clinicians in assessing and treating patients with rheumatologic disease and in evaluating the risk of infection in patients on immunosuppression.”
As of this writing, the rheumatology registry is still being assembled, and organizers are currently seeking approvals from various authorities. As of March 17, 2020, the Institutional Review Board (IRB) at the University of California, San Francisco, has determined that the registry is exempt from IRB approval requirements, a finding that should apply elsewhere in the United States, according to the registry website.
When it is fully up and running, clinicians will be able to report to the secure website on any and all cases of patients with rheumatologic disorders who present with COVID-19 of any severity, including patients with mild disease or asymptomatic patients who test positive.
“We are aiming for 5 to 10 minutes to input the data. We don’t want to drag them away from their clinical duties too much, but if clinicians are able to spare a few minutes to put in details about a patient, then that’s going to help build our knowledge and it’s going to help them with other patients,” said Philip Robinson, MBChB, associate professor of medicine at the University of Queensland in Brisbane, Australia, and the chief architect of the registry.
The data will be deindentified, with no protected health care information required or included, and made available to the global rheumatology community, but the registry will not offer clinical advice, Dr. Robinson said in an interview.
“This is observational data, it’s not randomized, but our approach is that some data is better than no data,” he said.
He also cautioned that the data will need careful interpretation, because information about patients with mild symptoms may offer false reassurances about the severity or extent of infection.
“For example, the patients with severe cases may be in the ICU, and can’t tell their doctors that they’re on methotrexate, so you can see how we need to be really careful about the messages from that data and not misinterpret it,” he said.
The COVID-19 rheumatology registry was inspired by a similar effort in the gastroenterology community, the Surveillance Epidemiology of Coronavirus Under Research Exclusion (SECURE-IBD) registry. Patients with inflammatory bowel disease are often treated with immunosuppressive biologic agents familiar to the rheumatology community, such as infliximab (Remicade and biosimilars) and adalimumab (Humira and biosimilars), and methotrexate.
Rheumatologists the world over are joining forces to create a COVID-19 rheumatology registry designed to help both patients and providers learn from each other regarding management of rheumatologic diseases and risk of infection among patients who are commonly on chronic immunosuppressive medications.
The COVID-19 Global Rheumatology Alliance, a consortium supported by more than 50 major clinical societies and foundations, quickly grew from messages on social media platforms to a multinational group focused on the common goal of helping to “guide rheumatology clinicians in assessing and treating patients with rheumatologic disease and in evaluating the risk of infection in patients on immunosuppression.”
As of this writing, the rheumatology registry is still being assembled, and organizers are currently seeking approvals from various authorities. As of March 17, 2020, the Institutional Review Board (IRB) at the University of California, San Francisco, has determined that the registry is exempt from IRB approval requirements, a finding that should apply elsewhere in the United States, according to the registry website.
When it is fully up and running, clinicians will be able to report to the secure website on any and all cases of patients with rheumatologic disorders who present with COVID-19 of any severity, including patients with mild disease or asymptomatic patients who test positive.
“We are aiming for 5 to 10 minutes to input the data. We don’t want to drag them away from their clinical duties too much, but if clinicians are able to spare a few minutes to put in details about a patient, then that’s going to help build our knowledge and it’s going to help them with other patients,” said Philip Robinson, MBChB, associate professor of medicine at the University of Queensland in Brisbane, Australia, and the chief architect of the registry.
The data will be deindentified, with no protected health care information required or included, and made available to the global rheumatology community, but the registry will not offer clinical advice, Dr. Robinson said in an interview.
“This is observational data, it’s not randomized, but our approach is that some data is better than no data,” he said.
He also cautioned that the data will need careful interpretation, because information about patients with mild symptoms may offer false reassurances about the severity or extent of infection.
“For example, the patients with severe cases may be in the ICU, and can’t tell their doctors that they’re on methotrexate, so you can see how we need to be really careful about the messages from that data and not misinterpret it,” he said.
The COVID-19 rheumatology registry was inspired by a similar effort in the gastroenterology community, the Surveillance Epidemiology of Coronavirus Under Research Exclusion (SECURE-IBD) registry. Patients with inflammatory bowel disease are often treated with immunosuppressive biologic agents familiar to the rheumatology community, such as infliximab (Remicade and biosimilars) and adalimumab (Humira and biosimilars), and methotrexate.
Vitamin D levels tied to clinical and radiological outcomes in early relapsing MS
Key clinical point: Serum 25(OH)D levels are associated with a modest decrease in relapse rate and radiological inflammatory activities in patients with early relapsing multiple sclerosis (MS).
Major finding: Each 25 nmol/L increase in serum 25(OH)D levels is associated with a decrease in clinical relapse rate (risk ratio [RR], 0.90), gadolinium-enhancing lesions (RR, 0.69), new/enlarging T2 lesions (RR, 0.86), and new active lesions (RR, 0.81) in the magnetic resonance imaging.
Study details: Meta-analysis of 13 studies including 3,498 patients.
Disclosures: No study sponsor was identified.
Citation: Martínez-Lapiscina EH et al. J Neurol Sci. 2020 Jan 25. doi: 10.1016/j.jns.2020.116668.
Key clinical point: Serum 25(OH)D levels are associated with a modest decrease in relapse rate and radiological inflammatory activities in patients with early relapsing multiple sclerosis (MS).
Major finding: Each 25 nmol/L increase in serum 25(OH)D levels is associated with a decrease in clinical relapse rate (risk ratio [RR], 0.90), gadolinium-enhancing lesions (RR, 0.69), new/enlarging T2 lesions (RR, 0.86), and new active lesions (RR, 0.81) in the magnetic resonance imaging.
Study details: Meta-analysis of 13 studies including 3,498 patients.
Disclosures: No study sponsor was identified.
Citation: Martínez-Lapiscina EH et al. J Neurol Sci. 2020 Jan 25. doi: 10.1016/j.jns.2020.116668.
Key clinical point: Serum 25(OH)D levels are associated with a modest decrease in relapse rate and radiological inflammatory activities in patients with early relapsing multiple sclerosis (MS).
Major finding: Each 25 nmol/L increase in serum 25(OH)D levels is associated with a decrease in clinical relapse rate (risk ratio [RR], 0.90), gadolinium-enhancing lesions (RR, 0.69), new/enlarging T2 lesions (RR, 0.86), and new active lesions (RR, 0.81) in the magnetic resonance imaging.
Study details: Meta-analysis of 13 studies including 3,498 patients.
Disclosures: No study sponsor was identified.
Citation: Martínez-Lapiscina EH et al. J Neurol Sci. 2020 Jan 25. doi: 10.1016/j.jns.2020.116668.