User login
Genomic prostate score does not improve risk assessment
A genomic prostate score (GPS) has little value in predicting adverse outcomes in men who have undergone a period of active surveillance before having a radical prostatectomy, according to a study published in the Journal of Clinical of Oncology.
The hazard ratio for adverse pathology using the 17-gene Oncotype DX Genomic Prostate Score did not reach statistical significance in a multivariate model (HR, 1.17; P = .066). This model took into account factors such as the prostate-specific antigen density (PSAD) and the Gleason grade group at diagnosis.
“In our study, the independent association of GPS with adverse pathology after initial active surveillance was not statistically significant,” Daniel W. Lin, MD, of the Fred Hutchinson Cancer Research Center in Seattle, and colleagues wrote.
There was also no association between the GPS and having upgraded biopsy findings during active surveillance.
Active surveillance is the “preferred management strategy” for men with low-risk prostate cancer, observed Dr. Lin and colleagues, but its use is often tempered by the worry that there may be underlying pathology that is not detected using routine clinical measures such as prostate-specific antigen testing. In their study, the investigators looked to see if using the GPS could help risk-stratify men undergoing active surveillance.
They noted that the biopsy-based genomic test had been shown to predict adverse surgical pathology and recurrence in men with low- and intermediate-risk prostate cancer who had undergone immediate radical prostatectomy. The team therefore wanted to clarify the test’s role in men who had been initially managed with a period of active surveillance.
To calculate the GPS, the investigators retrospectively analyzed diagnostic biopsy samples that had been prospectively collected from 432 men in the Canary Prostate Active Surveillance Study. The primary endpoint was adverse pathology in men who underwent radical prostatectomy after initial surveillance. Adverse pathology was defined as a Gleason grade of 3 or greater, a staging of pT3a or higher (with or without N1), or both.
After a median follow-up of 4.6 years, 167 (39%) men experienced upgrading of their prostate cancer at a surveillance biopsy, with 51 (12%) being upgraded to a Gleason grade group of 3 or higher. A total of 101 (23%) men had radical prostatectomy at a median of 2.1 years after their diagnostic biopsy, and just over half (n = 52; 51%) had adverse pathology at this time point.
GPS was associated with adverse pathology when the diagnostic Gleason grade group was taken into account (HR, 1.18; P = .030) but not when the investigators adjusted for both PSAD and diagnostic Gleason grade group. By contrast, PSAD (HR, 1.75; P = .025) was significantly associated with adverse pathology.
“Adding GPS to a model containing PSAD and diagnostic [Gleason grade group] did not significantly improve stratification of risk for [adverse pathology] over the clinical variables alone,” Dr. Lin and colleagues concluded.
This work was supported by the Canary Foundation, the Department of Defense, the National Institutes of Health, and Genomic Health. The authors disclosed relationships with Genomic Health and other companies.
SOURCE: Lin DW et al. J Clin Oncol. 2020 Mar 4. doi: 10.1200/JCO.19.02267.
A genomic prostate score (GPS) has little value in predicting adverse outcomes in men who have undergone a period of active surveillance before having a radical prostatectomy, according to a study published in the Journal of Clinical of Oncology.
The hazard ratio for adverse pathology using the 17-gene Oncotype DX Genomic Prostate Score did not reach statistical significance in a multivariate model (HR, 1.17; P = .066). This model took into account factors such as the prostate-specific antigen density (PSAD) and the Gleason grade group at diagnosis.
“In our study, the independent association of GPS with adverse pathology after initial active surveillance was not statistically significant,” Daniel W. Lin, MD, of the Fred Hutchinson Cancer Research Center in Seattle, and colleagues wrote.
There was also no association between the GPS and having upgraded biopsy findings during active surveillance.
Active surveillance is the “preferred management strategy” for men with low-risk prostate cancer, observed Dr. Lin and colleagues, but its use is often tempered by the worry that there may be underlying pathology that is not detected using routine clinical measures such as prostate-specific antigen testing. In their study, the investigators looked to see if using the GPS could help risk-stratify men undergoing active surveillance.
They noted that the biopsy-based genomic test had been shown to predict adverse surgical pathology and recurrence in men with low- and intermediate-risk prostate cancer who had undergone immediate radical prostatectomy. The team therefore wanted to clarify the test’s role in men who had been initially managed with a period of active surveillance.
To calculate the GPS, the investigators retrospectively analyzed diagnostic biopsy samples that had been prospectively collected from 432 men in the Canary Prostate Active Surveillance Study. The primary endpoint was adverse pathology in men who underwent radical prostatectomy after initial surveillance. Adverse pathology was defined as a Gleason grade of 3 or greater, a staging of pT3a or higher (with or without N1), or both.
After a median follow-up of 4.6 years, 167 (39%) men experienced upgrading of their prostate cancer at a surveillance biopsy, with 51 (12%) being upgraded to a Gleason grade group of 3 or higher. A total of 101 (23%) men had radical prostatectomy at a median of 2.1 years after their diagnostic biopsy, and just over half (n = 52; 51%) had adverse pathology at this time point.
GPS was associated with adverse pathology when the diagnostic Gleason grade group was taken into account (HR, 1.18; P = .030) but not when the investigators adjusted for both PSAD and diagnostic Gleason grade group. By contrast, PSAD (HR, 1.75; P = .025) was significantly associated with adverse pathology.
“Adding GPS to a model containing PSAD and diagnostic [Gleason grade group] did not significantly improve stratification of risk for [adverse pathology] over the clinical variables alone,” Dr. Lin and colleagues concluded.
This work was supported by the Canary Foundation, the Department of Defense, the National Institutes of Health, and Genomic Health. The authors disclosed relationships with Genomic Health and other companies.
SOURCE: Lin DW et al. J Clin Oncol. 2020 Mar 4. doi: 10.1200/JCO.19.02267.
A genomic prostate score (GPS) has little value in predicting adverse outcomes in men who have undergone a period of active surveillance before having a radical prostatectomy, according to a study published in the Journal of Clinical of Oncology.
The hazard ratio for adverse pathology using the 17-gene Oncotype DX Genomic Prostate Score did not reach statistical significance in a multivariate model (HR, 1.17; P = .066). This model took into account factors such as the prostate-specific antigen density (PSAD) and the Gleason grade group at diagnosis.
“In our study, the independent association of GPS with adverse pathology after initial active surveillance was not statistically significant,” Daniel W. Lin, MD, of the Fred Hutchinson Cancer Research Center in Seattle, and colleagues wrote.
There was also no association between the GPS and having upgraded biopsy findings during active surveillance.
Active surveillance is the “preferred management strategy” for men with low-risk prostate cancer, observed Dr. Lin and colleagues, but its use is often tempered by the worry that there may be underlying pathology that is not detected using routine clinical measures such as prostate-specific antigen testing. In their study, the investigators looked to see if using the GPS could help risk-stratify men undergoing active surveillance.
They noted that the biopsy-based genomic test had been shown to predict adverse surgical pathology and recurrence in men with low- and intermediate-risk prostate cancer who had undergone immediate radical prostatectomy. The team therefore wanted to clarify the test’s role in men who had been initially managed with a period of active surveillance.
To calculate the GPS, the investigators retrospectively analyzed diagnostic biopsy samples that had been prospectively collected from 432 men in the Canary Prostate Active Surveillance Study. The primary endpoint was adverse pathology in men who underwent radical prostatectomy after initial surveillance. Adverse pathology was defined as a Gleason grade of 3 or greater, a staging of pT3a or higher (with or without N1), or both.
After a median follow-up of 4.6 years, 167 (39%) men experienced upgrading of their prostate cancer at a surveillance biopsy, with 51 (12%) being upgraded to a Gleason grade group of 3 or higher. A total of 101 (23%) men had radical prostatectomy at a median of 2.1 years after their diagnostic biopsy, and just over half (n = 52; 51%) had adverse pathology at this time point.
GPS was associated with adverse pathology when the diagnostic Gleason grade group was taken into account (HR, 1.18; P = .030) but not when the investigators adjusted for both PSAD and diagnostic Gleason grade group. By contrast, PSAD (HR, 1.75; P = .025) was significantly associated with adverse pathology.
“Adding GPS to a model containing PSAD and diagnostic [Gleason grade group] did not significantly improve stratification of risk for [adverse pathology] over the clinical variables alone,” Dr. Lin and colleagues concluded.
This work was supported by the Canary Foundation, the Department of Defense, the National Institutes of Health, and Genomic Health. The authors disclosed relationships with Genomic Health and other companies.
SOURCE: Lin DW et al. J Clin Oncol. 2020 Mar 4. doi: 10.1200/JCO.19.02267.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Study: Delays filling biologic prescriptions have consequences
Insurance and specialty pharmacy delays in authorizing new biologic prescriptions for severe allergies leave waiting patients at risk of asthma attacks, hospitalizations, emergency department visits and prednisone shots and their known side effects, according to a single-center study that was to have been presented at the annual meeting of the American Academy of Allergy, Asthma and Immunology.
The AAAAI canceled their annual meeting and provided abstracts and access to presenters for press coverage.
The study of 80 patients in State College, Pa., found that they waited an average of 44 days from when their doctor submitted the preauthorization request to the insurance company until the practice received the shipment for dispensing to the patient, investigator Faoud Ishmael, MD, PhD, of Mount Nittany Medical Group said in an interview. “The implication here is that these are really the most severe patients who, you would argue, need their medications the quickest, and it’s taking longer to get them than it would an inhaler,” Dr. Ishmael said.
The study focused on patients with severe asthma (n = 60) or urticarial (n = 20) who received a new prescription of monoclonal antibody therapy from March 2014 to August 2019. For asthma treatments, the average time was 45.8 days; for urticaria, 40.6 days (P = .573), Dr. Ishmael said. The researchers divided the total amount of time into two components: insurance plan review and approval (P = .654, and specialty pharmacy review and dispensing of the medicine, each of which averaged 22.8 days (P = .384), he said.
He also noted wide disparity in the range of approval times. “The shortest approval time was 1 day, and the longest 97 days,” Dr. Ishmael said. “It’s interesting that we had this really broad spread.”
What’s more, the study found no trend for the delays among insurers and specialty pharmacies, Dr. Ishmael added. “When these prescriptions get submitted, it’s like a black box,” he said. “It really seems arbitrary why some of them take so long and some of them don’t.” The findings were independent of type of coverage, whether commercial or government, or even specific insurance plans. “It’s more the process that is flawed rather than one insurance company being the bad guy,” he said.
The study also looked at what happened to patients while they were waiting for their prescriptions to be delivered. “What we found is that over half of asthmatics had an exacerbation – 51% had at least one asthma attack where they needed prednisone,” Dr. Ishmael said (P = .0015), “and we had three patients admitted to the hospital over that time frame when they were waiting for the drugs.” One of those patients had been admitted twice, making four total hospitalizations. Preliminary data analysis showed that about 40% of the patients who had attacks went to the emergency department.
For asthmatics who needed prednisone, the average dose was 480 mg (P = .284) – “a pretty substantial number,” in Dr. Ishmael’s words. He noted that a large portion of the study patients were obese, with a mean body mass index of 33 kg/m2. Other comorbidities prevalent in the study population were hypertension and type 2 diabetes. “Prednisone is something that could worsen all of those conditions, so it’s not a trivial issue,” he said.
The study, however, didn’t evaluate costs of the interventions during the delay period vs. the costs of the medications themselves. Of the 80 prescriptions Dr. Ishmael and coauthors submitted, only one was rejected, that person being a smoker, he said. “I understand these are expensive medicines, but it’s counterproductive to delay them because in the long run the insurance company ends up paying for the hospitalization and the drug rather than just the drug,” he said.
Timothy Craig, DO, of Penn State Health Allergy, Asthma, and Immunology and professor of medicine and pediatrics at Penn State College of Medicine, both in Hershey, said he was surprised at the brevity of the delays reported in Dr. Ishmael’s study. “They do much better than we do with preauthorization,” he said, noting that, in his experience, these approvals take much longer. He added that his own research has found faulty insurance plan algorithms are at the heart of these delays. “We need more studies to clarify how much this is interfering with patient care and how much risk they’re putting patients in,” he said.
The COVID-19 pandemic poses a double-edged sword for physicians managing patients with severe asthma, Dr. Craig noted. “Their asthma care is important, especially if they do test for COVID-19,” he said. On the other hand, doctors and nurses attending to COVID-19 patients will have less time to haggle with payers to expedite coverage for biologics for their severe asthma patients, he said. “I hope the flexibility is there, especially at this time to allow people to get on the biologics and stay on them,” he said.
Dr. Ishmael said these findings have serious implications because biologics are getting prescribed ever more frequently for asthma and hives. Steps his practice has taken to streamline the process include following the payer’s approval guidelines as closely as possible. This sometimes can mean making sure a patient with severe asthma has been maximized on controller medications before submitting the biologic prescription, he said. Another step is to use drug company programs to remove barriers to coverage.
Nonetheless, the approval process can be daunting even when taking those steps, he said. “Those guidelines that constitute approval may vary a lot from one insurer to another; and sometimes those guidelines are different from the criteria that studies may have used when these drugs were being evaluated in clinical trials,” he said. It would be helpful, he said, if payers used the National Heart, Lung and Blood institute and the Global Initiative for Asthma guidelines for biologics.
One of the goals of the researchers is to present their findings to payers, “to let them know, here are some of the hang-ups and the real risks associated with delaying these medications,” Dr. Ishmael said.
“When specialists especially prescribe these therapies, there’s usually a valid reason,” he said. “We really need to do something about the current process – if there are ways to make it more transparent, faster.”
Dr. Ishmael has no relevant financial relationships to disclose.
SOURCE: Ishmael F et al. AAAAI 2020. Session 3609, Presentation 558.
Insurance and specialty pharmacy delays in authorizing new biologic prescriptions for severe allergies leave waiting patients at risk of asthma attacks, hospitalizations, emergency department visits and prednisone shots and their known side effects, according to a single-center study that was to have been presented at the annual meeting of the American Academy of Allergy, Asthma and Immunology.
The AAAAI canceled their annual meeting and provided abstracts and access to presenters for press coverage.
The study of 80 patients in State College, Pa., found that they waited an average of 44 days from when their doctor submitted the preauthorization request to the insurance company until the practice received the shipment for dispensing to the patient, investigator Faoud Ishmael, MD, PhD, of Mount Nittany Medical Group said in an interview. “The implication here is that these are really the most severe patients who, you would argue, need their medications the quickest, and it’s taking longer to get them than it would an inhaler,” Dr. Ishmael said.
The study focused on patients with severe asthma (n = 60) or urticarial (n = 20) who received a new prescription of monoclonal antibody therapy from March 2014 to August 2019. For asthma treatments, the average time was 45.8 days; for urticaria, 40.6 days (P = .573), Dr. Ishmael said. The researchers divided the total amount of time into two components: insurance plan review and approval (P = .654, and specialty pharmacy review and dispensing of the medicine, each of which averaged 22.8 days (P = .384), he said.
He also noted wide disparity in the range of approval times. “The shortest approval time was 1 day, and the longest 97 days,” Dr. Ishmael said. “It’s interesting that we had this really broad spread.”
What’s more, the study found no trend for the delays among insurers and specialty pharmacies, Dr. Ishmael added. “When these prescriptions get submitted, it’s like a black box,” he said. “It really seems arbitrary why some of them take so long and some of them don’t.” The findings were independent of type of coverage, whether commercial or government, or even specific insurance plans. “It’s more the process that is flawed rather than one insurance company being the bad guy,” he said.
The study also looked at what happened to patients while they were waiting for their prescriptions to be delivered. “What we found is that over half of asthmatics had an exacerbation – 51% had at least one asthma attack where they needed prednisone,” Dr. Ishmael said (P = .0015), “and we had three patients admitted to the hospital over that time frame when they were waiting for the drugs.” One of those patients had been admitted twice, making four total hospitalizations. Preliminary data analysis showed that about 40% of the patients who had attacks went to the emergency department.
For asthmatics who needed prednisone, the average dose was 480 mg (P = .284) – “a pretty substantial number,” in Dr. Ishmael’s words. He noted that a large portion of the study patients were obese, with a mean body mass index of 33 kg/m2. Other comorbidities prevalent in the study population were hypertension and type 2 diabetes. “Prednisone is something that could worsen all of those conditions, so it’s not a trivial issue,” he said.
The study, however, didn’t evaluate costs of the interventions during the delay period vs. the costs of the medications themselves. Of the 80 prescriptions Dr. Ishmael and coauthors submitted, only one was rejected, that person being a smoker, he said. “I understand these are expensive medicines, but it’s counterproductive to delay them because in the long run the insurance company ends up paying for the hospitalization and the drug rather than just the drug,” he said.
Timothy Craig, DO, of Penn State Health Allergy, Asthma, and Immunology and professor of medicine and pediatrics at Penn State College of Medicine, both in Hershey, said he was surprised at the brevity of the delays reported in Dr. Ishmael’s study. “They do much better than we do with preauthorization,” he said, noting that, in his experience, these approvals take much longer. He added that his own research has found faulty insurance plan algorithms are at the heart of these delays. “We need more studies to clarify how much this is interfering with patient care and how much risk they’re putting patients in,” he said.
The COVID-19 pandemic poses a double-edged sword for physicians managing patients with severe asthma, Dr. Craig noted. “Their asthma care is important, especially if they do test for COVID-19,” he said. On the other hand, doctors and nurses attending to COVID-19 patients will have less time to haggle with payers to expedite coverage for biologics for their severe asthma patients, he said. “I hope the flexibility is there, especially at this time to allow people to get on the biologics and stay on them,” he said.
Dr. Ishmael said these findings have serious implications because biologics are getting prescribed ever more frequently for asthma and hives. Steps his practice has taken to streamline the process include following the payer’s approval guidelines as closely as possible. This sometimes can mean making sure a patient with severe asthma has been maximized on controller medications before submitting the biologic prescription, he said. Another step is to use drug company programs to remove barriers to coverage.
Nonetheless, the approval process can be daunting even when taking those steps, he said. “Those guidelines that constitute approval may vary a lot from one insurer to another; and sometimes those guidelines are different from the criteria that studies may have used when these drugs were being evaluated in clinical trials,” he said. It would be helpful, he said, if payers used the National Heart, Lung and Blood institute and the Global Initiative for Asthma guidelines for biologics.
One of the goals of the researchers is to present their findings to payers, “to let them know, here are some of the hang-ups and the real risks associated with delaying these medications,” Dr. Ishmael said.
“When specialists especially prescribe these therapies, there’s usually a valid reason,” he said. “We really need to do something about the current process – if there are ways to make it more transparent, faster.”
Dr. Ishmael has no relevant financial relationships to disclose.
SOURCE: Ishmael F et al. AAAAI 2020. Session 3609, Presentation 558.
Insurance and specialty pharmacy delays in authorizing new biologic prescriptions for severe allergies leave waiting patients at risk of asthma attacks, hospitalizations, emergency department visits and prednisone shots and their known side effects, according to a single-center study that was to have been presented at the annual meeting of the American Academy of Allergy, Asthma and Immunology.
The AAAAI canceled their annual meeting and provided abstracts and access to presenters for press coverage.
The study of 80 patients in State College, Pa., found that they waited an average of 44 days from when their doctor submitted the preauthorization request to the insurance company until the practice received the shipment for dispensing to the patient, investigator Faoud Ishmael, MD, PhD, of Mount Nittany Medical Group said in an interview. “The implication here is that these are really the most severe patients who, you would argue, need their medications the quickest, and it’s taking longer to get them than it would an inhaler,” Dr. Ishmael said.
The study focused on patients with severe asthma (n = 60) or urticarial (n = 20) who received a new prescription of monoclonal antibody therapy from March 2014 to August 2019. For asthma treatments, the average time was 45.8 days; for urticaria, 40.6 days (P = .573), Dr. Ishmael said. The researchers divided the total amount of time into two components: insurance plan review and approval (P = .654, and specialty pharmacy review and dispensing of the medicine, each of which averaged 22.8 days (P = .384), he said.
He also noted wide disparity in the range of approval times. “The shortest approval time was 1 day, and the longest 97 days,” Dr. Ishmael said. “It’s interesting that we had this really broad spread.”
What’s more, the study found no trend for the delays among insurers and specialty pharmacies, Dr. Ishmael added. “When these prescriptions get submitted, it’s like a black box,” he said. “It really seems arbitrary why some of them take so long and some of them don’t.” The findings were independent of type of coverage, whether commercial or government, or even specific insurance plans. “It’s more the process that is flawed rather than one insurance company being the bad guy,” he said.
The study also looked at what happened to patients while they were waiting for their prescriptions to be delivered. “What we found is that over half of asthmatics had an exacerbation – 51% had at least one asthma attack where they needed prednisone,” Dr. Ishmael said (P = .0015), “and we had three patients admitted to the hospital over that time frame when they were waiting for the drugs.” One of those patients had been admitted twice, making four total hospitalizations. Preliminary data analysis showed that about 40% of the patients who had attacks went to the emergency department.
For asthmatics who needed prednisone, the average dose was 480 mg (P = .284) – “a pretty substantial number,” in Dr. Ishmael’s words. He noted that a large portion of the study patients were obese, with a mean body mass index of 33 kg/m2. Other comorbidities prevalent in the study population were hypertension and type 2 diabetes. “Prednisone is something that could worsen all of those conditions, so it’s not a trivial issue,” he said.
The study, however, didn’t evaluate costs of the interventions during the delay period vs. the costs of the medications themselves. Of the 80 prescriptions Dr. Ishmael and coauthors submitted, only one was rejected, that person being a smoker, he said. “I understand these are expensive medicines, but it’s counterproductive to delay them because in the long run the insurance company ends up paying for the hospitalization and the drug rather than just the drug,” he said.
Timothy Craig, DO, of Penn State Health Allergy, Asthma, and Immunology and professor of medicine and pediatrics at Penn State College of Medicine, both in Hershey, said he was surprised at the brevity of the delays reported in Dr. Ishmael’s study. “They do much better than we do with preauthorization,” he said, noting that, in his experience, these approvals take much longer. He added that his own research has found faulty insurance plan algorithms are at the heart of these delays. “We need more studies to clarify how much this is interfering with patient care and how much risk they’re putting patients in,” he said.
The COVID-19 pandemic poses a double-edged sword for physicians managing patients with severe asthma, Dr. Craig noted. “Their asthma care is important, especially if they do test for COVID-19,” he said. On the other hand, doctors and nurses attending to COVID-19 patients will have less time to haggle with payers to expedite coverage for biologics for their severe asthma patients, he said. “I hope the flexibility is there, especially at this time to allow people to get on the biologics and stay on them,” he said.
Dr. Ishmael said these findings have serious implications because biologics are getting prescribed ever more frequently for asthma and hives. Steps his practice has taken to streamline the process include following the payer’s approval guidelines as closely as possible. This sometimes can mean making sure a patient with severe asthma has been maximized on controller medications before submitting the biologic prescription, he said. Another step is to use drug company programs to remove barriers to coverage.
Nonetheless, the approval process can be daunting even when taking those steps, he said. “Those guidelines that constitute approval may vary a lot from one insurer to another; and sometimes those guidelines are different from the criteria that studies may have used when these drugs were being evaluated in clinical trials,” he said. It would be helpful, he said, if payers used the National Heart, Lung and Blood institute and the Global Initiative for Asthma guidelines for biologics.
One of the goals of the researchers is to present their findings to payers, “to let them know, here are some of the hang-ups and the real risks associated with delaying these medications,” Dr. Ishmael said.
“When specialists especially prescribe these therapies, there’s usually a valid reason,” he said. “We really need to do something about the current process – if there are ways to make it more transparent, faster.”
Dr. Ishmael has no relevant financial relationships to disclose.
SOURCE: Ishmael F et al. AAAAI 2020. Session 3609, Presentation 558.
REPORTING FROM AAAAI
How long is it safe to delay gynecologic cancer surgery?
As I write this column, there are more than 25,000 current cases of COVID-19 in the United States with an expected exponential rise in these numbers. Hospitals are issuing directives to cancel or postpone “elective” surgery to preserve the finite essential personal protective equipment (PPE), encourage social distancing, prevent exposure of at-risk patients within the hospital, and ensure bed and ventilator capacity for the impending surge in COVID-19 patients.
Many health systems have defined which surgeries they consider permissible, typically by using time parameters such as would not cause patient harm if not performed within 4 weeks, or 7 days, or 24 hours. This leaves surgeons in the unfamiliar position of rationing health care, a role with which, over the coming months, we may have to become increasingly comfortable. This is an enormous responsibility, the shift of resources between one population in need and another, and decisions should be based on data, not bias or hunch. We know that untreated cancer is life threatening, but there is a difference between untreated and delayed. What is a safe time to wait for gynecologic cancer surgery after diagnosis without negatively affecting survival from that cancer?
As I looked through my own upcoming surgical schedule, I sought guidance from the American College of Surgeons’ website, updated on March 17, 2020. In this site they tabulate an “Elective Surgery Acuity Scale” in which “most cancers” fit into tier 3a, which corresponds to high acuity surgery – “do not postpone.” This definition is fairly generalized and blunt; it does not account for the differences in cancers and occasional voluntary needs to postpone a patient’s cancer surgery for health optimization. There are limited data that measure the impact of surgical wait times on survival from gynecologic cancer. Most of this research is observational, and therefore, is influenced by confounders causing delay in surgery (e.g., comorbid conditions or socioeconomic factors that limit access to care). However, the current enforced delays are involuntary; driven by the system, not the patient; and access is universally restricted.
Endometrial cancer
Most data regarding outcomes and gynecologic cancer delay come from endometrial cancer. In 2016, Shalowitz et al. evaluated 182,000 endometrial cancer cases documented within the National Cancer Database (NCDB), which captures approximately 70% of cancer surgeries in the United States.1 They separated these patients into groups of low-grade (grade 1 and 2 endometrioid) and high-grade (grade 3 endometrioid and nonendometrioid) cancers, and evaluated the groups for their overall survival, stratified by the time period between diagnosis and surgery. Interestingly, those whose surgery was performed under 2 weeks from diagnosis had worse perioperative mortality and long-term survival. This seems to be a function of lack of medical optimization; low-volume, nonspecialized centers having less wait time; and the presentation of more advanced and symptomatic disease demanding a more urgent surgery. After those initial 2 weeks of worse outcomes, there was a period of stable outcomes and safety in waiting that extended up to 8 weeks for patients with low-grade cancers and up to 18 weeks for patients with high-grade cancers.
It may be counterintuitive to think that surgical delay affects patients with high-grade endometrial cancers less. These are more aggressive cancers, and there is patient and provider concern for metastatic spread with time elapsed. But an expedited surgery does not appear to be necessary for this group. The Shalowitz study demonstrated no risk for upstaging with surgical delay, meaning that advanced stage was not more likely to be identified in patients whose surgery was delayed, compared with those performed earlier. This observation suggests that the survival from high-grade endometrial cancers is largely determined by factors that cannot be controlled by the surgeon such as the stage at diagnosis, occult spread, and decreased responsiveness of the tumor to adjuvant therapy. In other words, fast-tracking these patients to surgery has limited influence on the outcomes for high-grade endometrial cancers.
For low-grade cancers, adverse outcomes were seen with a surgical delay of more than 8 weeks. But this may not have been caused by progression of disease (low-grade cancers also were not upstaged with delays), but rather may reflect that, in normal times, elective delays of more than 8 weeks are a function of necessary complex medical optimization of comorbidities (such as obesity-related disease). The survival that is measured by NCDB is not disease specific, and patients with comorbidities will be more likely to have impaired overall survival.
A systematic review of all papers that looked at endometrial cancer outcomes associated with surgical delay determined that it is reasonable to delay surgery for up to 8 weeks.2
Ovarian cancer
The data for ovarian cancer surgery is more limited. Most literature discusses the impact of delay in the time between surgery and the receipt of adjuvant chemotherapy, but there are limited data exploring how a delay in primary debulking negatively affects patients. This is perhaps because advanced ovarian cancer surgery rarely is delayed because of symptoms and apparent advanced stage at diagnosis. When a patient’s surgery does need to be voluntarily delayed, for example for medical optimization, there is the option of neoadjuvant chemotherapy (NACT) in which surgery is performed after three or more cycles of chemotherapy. NACT has been shown in multiple studies to have noninferior cancer outcomes, compared with primary debulking surgery.3,4
Perhaps in this current environment in which access to operating rooms and supplies is rationed, we should consider offering more, or all, patients NACT? Hospital stays after primary cytoreductive surgeries are typically 3-7 days in length, and these patients are at a higher risk, compared with other gynecologic cancer surgeries, of ICU admission and blood transfusions, both limited resources in this current environment. The disadvantage of this approach is that, while chemotherapy can keep patients out of the hospital so that they can practice social distancing, this particular therapy adds to the immunocompromised population. However, even patients who undergo primary surgical cytoreductive surgery will need to rapidly transition to immunosuppressive cytotoxic therapy; therefore it is unlikely that this can be avoided entirely during this time.
Lower genital tract cancers
Surgery for patients with lower genital tract cancers – such as cervical and vulvar cancer – also can probably be safely delayed for a 4-week period, and possibly longer. A Canadian retrospective study looked collectively at cervical, vaginal, and vulvar cancers evaluating for disease progression associated with delay to surgery, using 28 days as a benchmark for delayed surgery.5 They found no significant increased progression associated with surgical delay greater than 28 days. This study evaluated progression of cancer and did not measure cancer survival, although it is unlikely we would see impaired survival without a significant increase in disease progression.
We also can look to outcomes from delayed radical hysterectomy for stage I cervical cancer in pregnancy to provided us with some data. A retrospective cohort study observed no difference in survival when 28 women with early-stage cervical cancer who were diagnosed in pregnancy (average wait time 20 weeks from diagnosis to treatment) were compared with the outcomes of 52 matched nonpregnant control patients (average wait time 8 weeks). Their survival was 89% versus 94% respectively (P = .08).6
Summary
Synthesizing this data, it appears that, in an environment of competing needs and resources, it is reasonable and safe to delay surgery for patients with gynecologic cancers for 4-6 weeks and potentially longer. This includes patients with high-grade endometrial cancers. Clearly, these decisions should be individualized to patients and different health systems. For example, a patient who presents with a cancer-associated life-threatening bowel obstruction or hemorrhage may need an immediate intervention, and communities minimally affected by the coronavirus pandemic may have more allowances for surgery. With respect to patient anxiety, most patients with cancer are keen to have surgery promptly, and breaking the news to them that their surgery may be delayed because of institutional and public health needs will be difficult. However, the data support that this is likely safe.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email Dr. Rossi at [email protected].
References
1. Am J Obstet Gynecol 2017;216(3):268 e1-68 e18.
2. Eur J Obstet Gynecol Reprod Biol 2020;246:1-6. doi: 10.1016/j.ejogrb.2020.01.004.
3. N Engl J Med 2010;363(10):943-53.
4. Lancet 2015;386(9990):249-57.
5. J Obstet Gynaecol Can 2015;37(4):338-44.
6. Am J Obstet Gynecol 2017;216(3):276 e1-76 e6. doi: 10.1016/j.ajog.2016.10.034.
As I write this column, there are more than 25,000 current cases of COVID-19 in the United States with an expected exponential rise in these numbers. Hospitals are issuing directives to cancel or postpone “elective” surgery to preserve the finite essential personal protective equipment (PPE), encourage social distancing, prevent exposure of at-risk patients within the hospital, and ensure bed and ventilator capacity for the impending surge in COVID-19 patients.
Many health systems have defined which surgeries they consider permissible, typically by using time parameters such as would not cause patient harm if not performed within 4 weeks, or 7 days, or 24 hours. This leaves surgeons in the unfamiliar position of rationing health care, a role with which, over the coming months, we may have to become increasingly comfortable. This is an enormous responsibility, the shift of resources between one population in need and another, and decisions should be based on data, not bias or hunch. We know that untreated cancer is life threatening, but there is a difference between untreated and delayed. What is a safe time to wait for gynecologic cancer surgery after diagnosis without negatively affecting survival from that cancer?
As I looked through my own upcoming surgical schedule, I sought guidance from the American College of Surgeons’ website, updated on March 17, 2020. In this site they tabulate an “Elective Surgery Acuity Scale” in which “most cancers” fit into tier 3a, which corresponds to high acuity surgery – “do not postpone.” This definition is fairly generalized and blunt; it does not account for the differences in cancers and occasional voluntary needs to postpone a patient’s cancer surgery for health optimization. There are limited data that measure the impact of surgical wait times on survival from gynecologic cancer. Most of this research is observational, and therefore, is influenced by confounders causing delay in surgery (e.g., comorbid conditions or socioeconomic factors that limit access to care). However, the current enforced delays are involuntary; driven by the system, not the patient; and access is universally restricted.
Endometrial cancer
Most data regarding outcomes and gynecologic cancer delay come from endometrial cancer. In 2016, Shalowitz et al. evaluated 182,000 endometrial cancer cases documented within the National Cancer Database (NCDB), which captures approximately 70% of cancer surgeries in the United States.1 They separated these patients into groups of low-grade (grade 1 and 2 endometrioid) and high-grade (grade 3 endometrioid and nonendometrioid) cancers, and evaluated the groups for their overall survival, stratified by the time period between diagnosis and surgery. Interestingly, those whose surgery was performed under 2 weeks from diagnosis had worse perioperative mortality and long-term survival. This seems to be a function of lack of medical optimization; low-volume, nonspecialized centers having less wait time; and the presentation of more advanced and symptomatic disease demanding a more urgent surgery. After those initial 2 weeks of worse outcomes, there was a period of stable outcomes and safety in waiting that extended up to 8 weeks for patients with low-grade cancers and up to 18 weeks for patients with high-grade cancers.
It may be counterintuitive to think that surgical delay affects patients with high-grade endometrial cancers less. These are more aggressive cancers, and there is patient and provider concern for metastatic spread with time elapsed. But an expedited surgery does not appear to be necessary for this group. The Shalowitz study demonstrated no risk for upstaging with surgical delay, meaning that advanced stage was not more likely to be identified in patients whose surgery was delayed, compared with those performed earlier. This observation suggests that the survival from high-grade endometrial cancers is largely determined by factors that cannot be controlled by the surgeon such as the stage at diagnosis, occult spread, and decreased responsiveness of the tumor to adjuvant therapy. In other words, fast-tracking these patients to surgery has limited influence on the outcomes for high-grade endometrial cancers.
For low-grade cancers, adverse outcomes were seen with a surgical delay of more than 8 weeks. But this may not have been caused by progression of disease (low-grade cancers also were not upstaged with delays), but rather may reflect that, in normal times, elective delays of more than 8 weeks are a function of necessary complex medical optimization of comorbidities (such as obesity-related disease). The survival that is measured by NCDB is not disease specific, and patients with comorbidities will be more likely to have impaired overall survival.
A systematic review of all papers that looked at endometrial cancer outcomes associated with surgical delay determined that it is reasonable to delay surgery for up to 8 weeks.2
Ovarian cancer
The data for ovarian cancer surgery is more limited. Most literature discusses the impact of delay in the time between surgery and the receipt of adjuvant chemotherapy, but there are limited data exploring how a delay in primary debulking negatively affects patients. This is perhaps because advanced ovarian cancer surgery rarely is delayed because of symptoms and apparent advanced stage at diagnosis. When a patient’s surgery does need to be voluntarily delayed, for example for medical optimization, there is the option of neoadjuvant chemotherapy (NACT) in which surgery is performed after three or more cycles of chemotherapy. NACT has been shown in multiple studies to have noninferior cancer outcomes, compared with primary debulking surgery.3,4
Perhaps in this current environment in which access to operating rooms and supplies is rationed, we should consider offering more, or all, patients NACT? Hospital stays after primary cytoreductive surgeries are typically 3-7 days in length, and these patients are at a higher risk, compared with other gynecologic cancer surgeries, of ICU admission and blood transfusions, both limited resources in this current environment. The disadvantage of this approach is that, while chemotherapy can keep patients out of the hospital so that they can practice social distancing, this particular therapy adds to the immunocompromised population. However, even patients who undergo primary surgical cytoreductive surgery will need to rapidly transition to immunosuppressive cytotoxic therapy; therefore it is unlikely that this can be avoided entirely during this time.
Lower genital tract cancers
Surgery for patients with lower genital tract cancers – such as cervical and vulvar cancer – also can probably be safely delayed for a 4-week period, and possibly longer. A Canadian retrospective study looked collectively at cervical, vaginal, and vulvar cancers evaluating for disease progression associated with delay to surgery, using 28 days as a benchmark for delayed surgery.5 They found no significant increased progression associated with surgical delay greater than 28 days. This study evaluated progression of cancer and did not measure cancer survival, although it is unlikely we would see impaired survival without a significant increase in disease progression.
We also can look to outcomes from delayed radical hysterectomy for stage I cervical cancer in pregnancy to provided us with some data. A retrospective cohort study observed no difference in survival when 28 women with early-stage cervical cancer who were diagnosed in pregnancy (average wait time 20 weeks from diagnosis to treatment) were compared with the outcomes of 52 matched nonpregnant control patients (average wait time 8 weeks). Their survival was 89% versus 94% respectively (P = .08).6
Summary
Synthesizing this data, it appears that, in an environment of competing needs and resources, it is reasonable and safe to delay surgery for patients with gynecologic cancers for 4-6 weeks and potentially longer. This includes patients with high-grade endometrial cancers. Clearly, these decisions should be individualized to patients and different health systems. For example, a patient who presents with a cancer-associated life-threatening bowel obstruction or hemorrhage may need an immediate intervention, and communities minimally affected by the coronavirus pandemic may have more allowances for surgery. With respect to patient anxiety, most patients with cancer are keen to have surgery promptly, and breaking the news to them that their surgery may be delayed because of institutional and public health needs will be difficult. However, the data support that this is likely safe.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email Dr. Rossi at [email protected].
References
1. Am J Obstet Gynecol 2017;216(3):268 e1-68 e18.
2. Eur J Obstet Gynecol Reprod Biol 2020;246:1-6. doi: 10.1016/j.ejogrb.2020.01.004.
3. N Engl J Med 2010;363(10):943-53.
4. Lancet 2015;386(9990):249-57.
5. J Obstet Gynaecol Can 2015;37(4):338-44.
6. Am J Obstet Gynecol 2017;216(3):276 e1-76 e6. doi: 10.1016/j.ajog.2016.10.034.
As I write this column, there are more than 25,000 current cases of COVID-19 in the United States with an expected exponential rise in these numbers. Hospitals are issuing directives to cancel or postpone “elective” surgery to preserve the finite essential personal protective equipment (PPE), encourage social distancing, prevent exposure of at-risk patients within the hospital, and ensure bed and ventilator capacity for the impending surge in COVID-19 patients.
Many health systems have defined which surgeries they consider permissible, typically by using time parameters such as would not cause patient harm if not performed within 4 weeks, or 7 days, or 24 hours. This leaves surgeons in the unfamiliar position of rationing health care, a role with which, over the coming months, we may have to become increasingly comfortable. This is an enormous responsibility, the shift of resources between one population in need and another, and decisions should be based on data, not bias or hunch. We know that untreated cancer is life threatening, but there is a difference between untreated and delayed. What is a safe time to wait for gynecologic cancer surgery after diagnosis without negatively affecting survival from that cancer?
As I looked through my own upcoming surgical schedule, I sought guidance from the American College of Surgeons’ website, updated on March 17, 2020. In this site they tabulate an “Elective Surgery Acuity Scale” in which “most cancers” fit into tier 3a, which corresponds to high acuity surgery – “do not postpone.” This definition is fairly generalized and blunt; it does not account for the differences in cancers and occasional voluntary needs to postpone a patient’s cancer surgery for health optimization. There are limited data that measure the impact of surgical wait times on survival from gynecologic cancer. Most of this research is observational, and therefore, is influenced by confounders causing delay in surgery (e.g., comorbid conditions or socioeconomic factors that limit access to care). However, the current enforced delays are involuntary; driven by the system, not the patient; and access is universally restricted.
Endometrial cancer
Most data regarding outcomes and gynecologic cancer delay come from endometrial cancer. In 2016, Shalowitz et al. evaluated 182,000 endometrial cancer cases documented within the National Cancer Database (NCDB), which captures approximately 70% of cancer surgeries in the United States.1 They separated these patients into groups of low-grade (grade 1 and 2 endometrioid) and high-grade (grade 3 endometrioid and nonendometrioid) cancers, and evaluated the groups for their overall survival, stratified by the time period between diagnosis and surgery. Interestingly, those whose surgery was performed under 2 weeks from diagnosis had worse perioperative mortality and long-term survival. This seems to be a function of lack of medical optimization; low-volume, nonspecialized centers having less wait time; and the presentation of more advanced and symptomatic disease demanding a more urgent surgery. After those initial 2 weeks of worse outcomes, there was a period of stable outcomes and safety in waiting that extended up to 8 weeks for patients with low-grade cancers and up to 18 weeks for patients with high-grade cancers.
It may be counterintuitive to think that surgical delay affects patients with high-grade endometrial cancers less. These are more aggressive cancers, and there is patient and provider concern for metastatic spread with time elapsed. But an expedited surgery does not appear to be necessary for this group. The Shalowitz study demonstrated no risk for upstaging with surgical delay, meaning that advanced stage was not more likely to be identified in patients whose surgery was delayed, compared with those performed earlier. This observation suggests that the survival from high-grade endometrial cancers is largely determined by factors that cannot be controlled by the surgeon such as the stage at diagnosis, occult spread, and decreased responsiveness of the tumor to adjuvant therapy. In other words, fast-tracking these patients to surgery has limited influence on the outcomes for high-grade endometrial cancers.
For low-grade cancers, adverse outcomes were seen with a surgical delay of more than 8 weeks. But this may not have been caused by progression of disease (low-grade cancers also were not upstaged with delays), but rather may reflect that, in normal times, elective delays of more than 8 weeks are a function of necessary complex medical optimization of comorbidities (such as obesity-related disease). The survival that is measured by NCDB is not disease specific, and patients with comorbidities will be more likely to have impaired overall survival.
A systematic review of all papers that looked at endometrial cancer outcomes associated with surgical delay determined that it is reasonable to delay surgery for up to 8 weeks.2
Ovarian cancer
The data for ovarian cancer surgery is more limited. Most literature discusses the impact of delay in the time between surgery and the receipt of adjuvant chemotherapy, but there are limited data exploring how a delay in primary debulking negatively affects patients. This is perhaps because advanced ovarian cancer surgery rarely is delayed because of symptoms and apparent advanced stage at diagnosis. When a patient’s surgery does need to be voluntarily delayed, for example for medical optimization, there is the option of neoadjuvant chemotherapy (NACT) in which surgery is performed after three or more cycles of chemotherapy. NACT has been shown in multiple studies to have noninferior cancer outcomes, compared with primary debulking surgery.3,4
Perhaps in this current environment in which access to operating rooms and supplies is rationed, we should consider offering more, or all, patients NACT? Hospital stays after primary cytoreductive surgeries are typically 3-7 days in length, and these patients are at a higher risk, compared with other gynecologic cancer surgeries, of ICU admission and blood transfusions, both limited resources in this current environment. The disadvantage of this approach is that, while chemotherapy can keep patients out of the hospital so that they can practice social distancing, this particular therapy adds to the immunocompromised population. However, even patients who undergo primary surgical cytoreductive surgery will need to rapidly transition to immunosuppressive cytotoxic therapy; therefore it is unlikely that this can be avoided entirely during this time.
Lower genital tract cancers
Surgery for patients with lower genital tract cancers – such as cervical and vulvar cancer – also can probably be safely delayed for a 4-week period, and possibly longer. A Canadian retrospective study looked collectively at cervical, vaginal, and vulvar cancers evaluating for disease progression associated with delay to surgery, using 28 days as a benchmark for delayed surgery.5 They found no significant increased progression associated with surgical delay greater than 28 days. This study evaluated progression of cancer and did not measure cancer survival, although it is unlikely we would see impaired survival without a significant increase in disease progression.
We also can look to outcomes from delayed radical hysterectomy for stage I cervical cancer in pregnancy to provided us with some data. A retrospective cohort study observed no difference in survival when 28 women with early-stage cervical cancer who were diagnosed in pregnancy (average wait time 20 weeks from diagnosis to treatment) were compared with the outcomes of 52 matched nonpregnant control patients (average wait time 8 weeks). Their survival was 89% versus 94% respectively (P = .08).6
Summary
Synthesizing this data, it appears that, in an environment of competing needs and resources, it is reasonable and safe to delay surgery for patients with gynecologic cancers for 4-6 weeks and potentially longer. This includes patients with high-grade endometrial cancers. Clearly, these decisions should be individualized to patients and different health systems. For example, a patient who presents with a cancer-associated life-threatening bowel obstruction or hemorrhage may need an immediate intervention, and communities minimally affected by the coronavirus pandemic may have more allowances for surgery. With respect to patient anxiety, most patients with cancer are keen to have surgery promptly, and breaking the news to them that their surgery may be delayed because of institutional and public health needs will be difficult. However, the data support that this is likely safe.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email Dr. Rossi at [email protected].
References
1. Am J Obstet Gynecol 2017;216(3):268 e1-68 e18.
2. Eur J Obstet Gynecol Reprod Biol 2020;246:1-6. doi: 10.1016/j.ejogrb.2020.01.004.
3. N Engl J Med 2010;363(10):943-53.
4. Lancet 2015;386(9990):249-57.
5. J Obstet Gynaecol Can 2015;37(4):338-44.
6. Am J Obstet Gynecol 2017;216(3):276 e1-76 e6. doi: 10.1016/j.ajog.2016.10.034.
Liver cancer increase driven mainly by NASH in men over 60
Liver cancer rates have been increasing, but a new analysis finds that the increase has occurred primarily in men older than 60 years in developed countries.
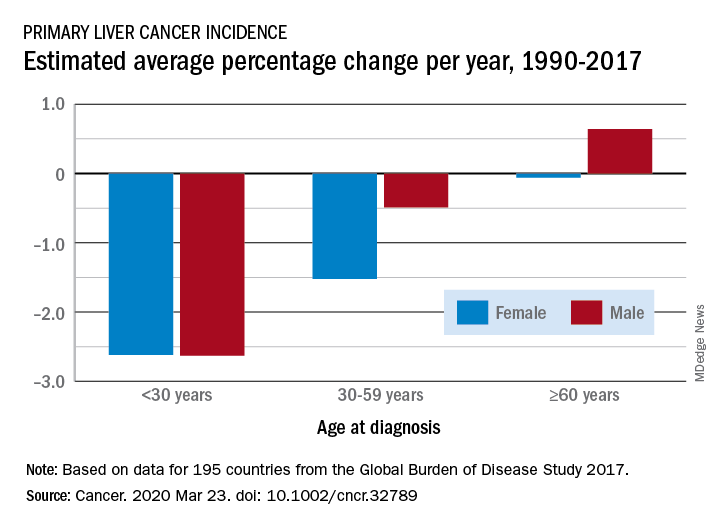
The findings come from an analysis of data from the Global Burden of Disease (GBD) Study 2017, published online March 23 in Cancer.
From 1990 to 2017, the number of cancer cases increased nearly threefold in older men and more than twofold in older women (aged 60 years or more). This increase was driven mainly by an increase in liver cancer caused by nonalcoholic steatohepatitis (NASH), also termed fatty liver disease, note the authors.
In contrast, the incidence of liver cancer among men and women who are younger than 30 years and those aged 30 to 59 years declined during this period.
The decreases seen in younger adults were largely ascribed to hepatitis B virus (HBV) vaccination and were consistent in most regions except in developed countries, where liver cancer rates increased irrespective of sex and age.
“Our findings suggest a lack of attention for older people in current liver cancer prevention efforts and highlight the emerging concern of obesity as a risk factor for liver cancer,” lead author Xingdong Chen, MD, PhD, Fudan University, China, said in a statement.
“Liver cancer prevention strategies in both developing and developed countries should be tailored and updated,” he added.
The authors point out that liver cancer was previously considered to be rare in the Western hemisphere.
“However, we found a significant increase in primary liver cancer incidence – regardless of etiology, sex, or age – in most of these countries over the last few decades,” they observe.
The fact that the most pronounced increase in liver cancer was caused by NASH suggests that more attention should be paid to weight management and obesity control as primary prevention strategies in these regions, they suggest.
Study design
Annual incidence data were collected from 1990 to 2017 and were categorized by sex, region, country, age group, and etiology.
“Data from a total of 195 countries and territories were available,” the investigators note, “and these countries and territories were categorized into 5 regions in terms of sociodemographic index (SDI),” they add.
Data were also retrieved regarding five etiologies of liver cancer: HBV infection, hepatitis C virus (HCV) infection, alcohol use, NASH, and others.
The authors note that age-standardized incidence rates of primary liver cancer caused by those five etiologies increased significantly in Australasia, Western Europe, and high-income regions of North America. The most significant increase was found in liver cancer caused by NASH in the Netherlands (in men) and in Finland (in women).
An increasing trend was observed in most countries for primary liver cancer among people aged 60 years or older, the authors note. They suggest that population expansion, aging, and increasing prevalence of obesity and diabetes might partly explain the marked increase, especially the dramatic increase in the number of cases among older people. Additionally, the “lag effect” of the large HBV infection reservoir in several countries might also contribute to the increase, the authors state. They explain that people infected with HBV early in life may experience progression to liver cancer as they age.
Primary prevention
Prevention of HBV infection – the primary cause of liver cancer – has been possible since the introduction of the HBV vaccine in 1982.
“By the end of 2017, 187 countries had introduced the HBV vaccine into their national immunization schedules, with global coverage with 3 doses of the hepatitis B vaccine ... estimated at 84%,” the authors point out.
This has “dramatically” reduced both the prevalence of HBV infection and the incidence of liver cancer caused by it among younger people in high-risk countries, they comment.
The investigators also observed a significant decrease in the incidence of liver cancer caused by HBV infection in people aged 30 to 59 years, although the decline was smaller than it was for those younger than 30.
Moreover, HCV infection has emerged as a concerning cause of liver cancer among those who used to be at low risk for HCV infection.
Although there is optimism that global control of HCV infection can be achieved through direct-acting antiviral agents, “the high cost, drug resistance, and reinfection rates are still major obstacles to fulfilling this ambitious goal,” Chen and colleagues point out.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Liver cancer rates have been increasing, but a new analysis finds that the increase has occurred primarily in men older than 60 years in developed countries.

The findings come from an analysis of data from the Global Burden of Disease (GBD) Study 2017, published online March 23 in Cancer.
From 1990 to 2017, the number of cancer cases increased nearly threefold in older men and more than twofold in older women (aged 60 years or more). This increase was driven mainly by an increase in liver cancer caused by nonalcoholic steatohepatitis (NASH), also termed fatty liver disease, note the authors.
In contrast, the incidence of liver cancer among men and women who are younger than 30 years and those aged 30 to 59 years declined during this period.
The decreases seen in younger adults were largely ascribed to hepatitis B virus (HBV) vaccination and were consistent in most regions except in developed countries, where liver cancer rates increased irrespective of sex and age.
“Our findings suggest a lack of attention for older people in current liver cancer prevention efforts and highlight the emerging concern of obesity as a risk factor for liver cancer,” lead author Xingdong Chen, MD, PhD, Fudan University, China, said in a statement.
“Liver cancer prevention strategies in both developing and developed countries should be tailored and updated,” he added.
The authors point out that liver cancer was previously considered to be rare in the Western hemisphere.
“However, we found a significant increase in primary liver cancer incidence – regardless of etiology, sex, or age – in most of these countries over the last few decades,” they observe.
The fact that the most pronounced increase in liver cancer was caused by NASH suggests that more attention should be paid to weight management and obesity control as primary prevention strategies in these regions, they suggest.
Study design
Annual incidence data were collected from 1990 to 2017 and were categorized by sex, region, country, age group, and etiology.
“Data from a total of 195 countries and territories were available,” the investigators note, “and these countries and territories were categorized into 5 regions in terms of sociodemographic index (SDI),” they add.
Data were also retrieved regarding five etiologies of liver cancer: HBV infection, hepatitis C virus (HCV) infection, alcohol use, NASH, and others.
The authors note that age-standardized incidence rates of primary liver cancer caused by those five etiologies increased significantly in Australasia, Western Europe, and high-income regions of North America. The most significant increase was found in liver cancer caused by NASH in the Netherlands (in men) and in Finland (in women).
An increasing trend was observed in most countries for primary liver cancer among people aged 60 years or older, the authors note. They suggest that population expansion, aging, and increasing prevalence of obesity and diabetes might partly explain the marked increase, especially the dramatic increase in the number of cases among older people. Additionally, the “lag effect” of the large HBV infection reservoir in several countries might also contribute to the increase, the authors state. They explain that people infected with HBV early in life may experience progression to liver cancer as they age.
Primary prevention
Prevention of HBV infection – the primary cause of liver cancer – has been possible since the introduction of the HBV vaccine in 1982.
“By the end of 2017, 187 countries had introduced the HBV vaccine into their national immunization schedules, with global coverage with 3 doses of the hepatitis B vaccine ... estimated at 84%,” the authors point out.
This has “dramatically” reduced both the prevalence of HBV infection and the incidence of liver cancer caused by it among younger people in high-risk countries, they comment.
The investigators also observed a significant decrease in the incidence of liver cancer caused by HBV infection in people aged 30 to 59 years, although the decline was smaller than it was for those younger than 30.
Moreover, HCV infection has emerged as a concerning cause of liver cancer among those who used to be at low risk for HCV infection.
Although there is optimism that global control of HCV infection can be achieved through direct-acting antiviral agents, “the high cost, drug resistance, and reinfection rates are still major obstacles to fulfilling this ambitious goal,” Chen and colleagues point out.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Liver cancer rates have been increasing, but a new analysis finds that the increase has occurred primarily in men older than 60 years in developed countries.

The findings come from an analysis of data from the Global Burden of Disease (GBD) Study 2017, published online March 23 in Cancer.
From 1990 to 2017, the number of cancer cases increased nearly threefold in older men and more than twofold in older women (aged 60 years or more). This increase was driven mainly by an increase in liver cancer caused by nonalcoholic steatohepatitis (NASH), also termed fatty liver disease, note the authors.
In contrast, the incidence of liver cancer among men and women who are younger than 30 years and those aged 30 to 59 years declined during this period.
The decreases seen in younger adults were largely ascribed to hepatitis B virus (HBV) vaccination and were consistent in most regions except in developed countries, where liver cancer rates increased irrespective of sex and age.
“Our findings suggest a lack of attention for older people in current liver cancer prevention efforts and highlight the emerging concern of obesity as a risk factor for liver cancer,” lead author Xingdong Chen, MD, PhD, Fudan University, China, said in a statement.
“Liver cancer prevention strategies in both developing and developed countries should be tailored and updated,” he added.
The authors point out that liver cancer was previously considered to be rare in the Western hemisphere.
“However, we found a significant increase in primary liver cancer incidence – regardless of etiology, sex, or age – in most of these countries over the last few decades,” they observe.
The fact that the most pronounced increase in liver cancer was caused by NASH suggests that more attention should be paid to weight management and obesity control as primary prevention strategies in these regions, they suggest.
Study design
Annual incidence data were collected from 1990 to 2017 and were categorized by sex, region, country, age group, and etiology.
“Data from a total of 195 countries and territories were available,” the investigators note, “and these countries and territories were categorized into 5 regions in terms of sociodemographic index (SDI),” they add.
Data were also retrieved regarding five etiologies of liver cancer: HBV infection, hepatitis C virus (HCV) infection, alcohol use, NASH, and others.
The authors note that age-standardized incidence rates of primary liver cancer caused by those five etiologies increased significantly in Australasia, Western Europe, and high-income regions of North America. The most significant increase was found in liver cancer caused by NASH in the Netherlands (in men) and in Finland (in women).
An increasing trend was observed in most countries for primary liver cancer among people aged 60 years or older, the authors note. They suggest that population expansion, aging, and increasing prevalence of obesity and diabetes might partly explain the marked increase, especially the dramatic increase in the number of cases among older people. Additionally, the “lag effect” of the large HBV infection reservoir in several countries might also contribute to the increase, the authors state. They explain that people infected with HBV early in life may experience progression to liver cancer as they age.
Primary prevention
Prevention of HBV infection – the primary cause of liver cancer – has been possible since the introduction of the HBV vaccine in 1982.
“By the end of 2017, 187 countries had introduced the HBV vaccine into their national immunization schedules, with global coverage with 3 doses of the hepatitis B vaccine ... estimated at 84%,” the authors point out.
This has “dramatically” reduced both the prevalence of HBV infection and the incidence of liver cancer caused by it among younger people in high-risk countries, they comment.
The investigators also observed a significant decrease in the incidence of liver cancer caused by HBV infection in people aged 30 to 59 years, although the decline was smaller than it was for those younger than 30.
Moreover, HCV infection has emerged as a concerning cause of liver cancer among those who used to be at low risk for HCV infection.
Although there is optimism that global control of HCV infection can be achieved through direct-acting antiviral agents, “the high cost, drug resistance, and reinfection rates are still major obstacles to fulfilling this ambitious goal,” Chen and colleagues point out.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Sleep-disordered breathing linked with Alzheimer’s disease biomarkers in cognitively normal older adults
investigators have found.
Among 127 adults enrolled in a randomized clinical trial of interventions to promote mental well-being in older adults, those with sleep-disordered breathing had significantly greater amyloid burden and gray-matter volume, as well as increased perfusion and metabolism in parietal-occipital regions, reported Claire André, PhD, from the French Institute of Health and Medical Research (INSERM) unit in Caen, and colleagues.
“Our findings highlight the need to treat sleep disorders in the older population, even in the absence of cognitive or behavioral manifestations,” they wrote in a study published in JAMA Neurology.
Previous studies of the possible association between sleep-disordered breathing and dementia risk have shown conflicting or inconsistent results, the authors noted.
“These discrepancies may be explained by the characteristics of patients with sleep-disordered breathing (e.g., recruited from sleep clinics versus from the community, differences in age and disease duration), the scoring criteria of respiratory events, sample sizes, or the lack of controls for possibly biasing covariates,” they wrote.
To see whether they could clear up the confusion, the investigators conducted a retrospective analysis of 127 patients who were enrolled in the Age-Well randomized, controlled trial of the Medit-Ageing European project. The participants were community-dwelling adults (mean age, 69.1 years; 63% women), who were enrolled in the trial and underwent evaluation from 2016 to 2018 at the Cyceron Cancer Center in Caen.
The participants, all of whom were cognitively unimpaired at baseline, underwent neuropsychological assessment, polysomnography, MRI, plus florbetapir- and fluorodeoxyglucose-labeled PET.
The investigators defined sleep-disordered breathing as 15 apnea-hypopnea index events per hour or higher, and compared results between those with sleep-disordered breathing and those without for each imaging modality.
Participants with sleep-disordered breathing has significantly greater amyloid burden (P = .04), gray-matter volume (P = .04), perfusion (P = .04), and metabolism (P = .001), primarily overlapping the posterior cingulate cortex and precuneus, areas known to be significantly involved in Alzheimer’s disease.
When the investigators looked for behavioral and cognitive correlates of sleep-disordered breathing severity with associated brain changes, however, they found no associations with either cognitive performance, self-reported cognitive or sleep difficulties, or symptoms of daytime sleepiness.
“Importantly, to the best of our knowledge, our results show in vivo for the first time that greater amyloid burden colocalizes with greater gray-matter volume, perfusion, and metabolism in older participants with sleep-disordered breathing who are cognitively unimpaired. We believe that these overlapping patterns reinforce the likelihood of common underlying mechanisms,” they wrote.
The Age-Well randomized clinical trial is part of the Medit-Ageing project and is funded through the European Union’s Horizon 2020 Research and Innovation Program, INSERM, and Fondation d’ Entreprise MMA des Entrepreneurs du Futur. Dr. André reported no conflicts of interest to disclose.
SOURCE: André C et al. JAMA Neurol. 2020 Mar 23. doi: 10.1001/jamaneurol.2020.0311.
investigators have found.
Among 127 adults enrolled in a randomized clinical trial of interventions to promote mental well-being in older adults, those with sleep-disordered breathing had significantly greater amyloid burden and gray-matter volume, as well as increased perfusion and metabolism in parietal-occipital regions, reported Claire André, PhD, from the French Institute of Health and Medical Research (INSERM) unit in Caen, and colleagues.
“Our findings highlight the need to treat sleep disorders in the older population, even in the absence of cognitive or behavioral manifestations,” they wrote in a study published in JAMA Neurology.
Previous studies of the possible association between sleep-disordered breathing and dementia risk have shown conflicting or inconsistent results, the authors noted.
“These discrepancies may be explained by the characteristics of patients with sleep-disordered breathing (e.g., recruited from sleep clinics versus from the community, differences in age and disease duration), the scoring criteria of respiratory events, sample sizes, or the lack of controls for possibly biasing covariates,” they wrote.
To see whether they could clear up the confusion, the investigators conducted a retrospective analysis of 127 patients who were enrolled in the Age-Well randomized, controlled trial of the Medit-Ageing European project. The participants were community-dwelling adults (mean age, 69.1 years; 63% women), who were enrolled in the trial and underwent evaluation from 2016 to 2018 at the Cyceron Cancer Center in Caen.
The participants, all of whom were cognitively unimpaired at baseline, underwent neuropsychological assessment, polysomnography, MRI, plus florbetapir- and fluorodeoxyglucose-labeled PET.
The investigators defined sleep-disordered breathing as 15 apnea-hypopnea index events per hour or higher, and compared results between those with sleep-disordered breathing and those without for each imaging modality.
Participants with sleep-disordered breathing has significantly greater amyloid burden (P = .04), gray-matter volume (P = .04), perfusion (P = .04), and metabolism (P = .001), primarily overlapping the posterior cingulate cortex and precuneus, areas known to be significantly involved in Alzheimer’s disease.
When the investigators looked for behavioral and cognitive correlates of sleep-disordered breathing severity with associated brain changes, however, they found no associations with either cognitive performance, self-reported cognitive or sleep difficulties, or symptoms of daytime sleepiness.
“Importantly, to the best of our knowledge, our results show in vivo for the first time that greater amyloid burden colocalizes with greater gray-matter volume, perfusion, and metabolism in older participants with sleep-disordered breathing who are cognitively unimpaired. We believe that these overlapping patterns reinforce the likelihood of common underlying mechanisms,” they wrote.
The Age-Well randomized clinical trial is part of the Medit-Ageing project and is funded through the European Union’s Horizon 2020 Research and Innovation Program, INSERM, and Fondation d’ Entreprise MMA des Entrepreneurs du Futur. Dr. André reported no conflicts of interest to disclose.
SOURCE: André C et al. JAMA Neurol. 2020 Mar 23. doi: 10.1001/jamaneurol.2020.0311.
investigators have found.
Among 127 adults enrolled in a randomized clinical trial of interventions to promote mental well-being in older adults, those with sleep-disordered breathing had significantly greater amyloid burden and gray-matter volume, as well as increased perfusion and metabolism in parietal-occipital regions, reported Claire André, PhD, from the French Institute of Health and Medical Research (INSERM) unit in Caen, and colleagues.
“Our findings highlight the need to treat sleep disorders in the older population, even in the absence of cognitive or behavioral manifestations,” they wrote in a study published in JAMA Neurology.
Previous studies of the possible association between sleep-disordered breathing and dementia risk have shown conflicting or inconsistent results, the authors noted.
“These discrepancies may be explained by the characteristics of patients with sleep-disordered breathing (e.g., recruited from sleep clinics versus from the community, differences in age and disease duration), the scoring criteria of respiratory events, sample sizes, or the lack of controls for possibly biasing covariates,” they wrote.
To see whether they could clear up the confusion, the investigators conducted a retrospective analysis of 127 patients who were enrolled in the Age-Well randomized, controlled trial of the Medit-Ageing European project. The participants were community-dwelling adults (mean age, 69.1 years; 63% women), who were enrolled in the trial and underwent evaluation from 2016 to 2018 at the Cyceron Cancer Center in Caen.
The participants, all of whom were cognitively unimpaired at baseline, underwent neuropsychological assessment, polysomnography, MRI, plus florbetapir- and fluorodeoxyglucose-labeled PET.
The investigators defined sleep-disordered breathing as 15 apnea-hypopnea index events per hour or higher, and compared results between those with sleep-disordered breathing and those without for each imaging modality.
Participants with sleep-disordered breathing has significantly greater amyloid burden (P = .04), gray-matter volume (P = .04), perfusion (P = .04), and metabolism (P = .001), primarily overlapping the posterior cingulate cortex and precuneus, areas known to be significantly involved in Alzheimer’s disease.
When the investigators looked for behavioral and cognitive correlates of sleep-disordered breathing severity with associated brain changes, however, they found no associations with either cognitive performance, self-reported cognitive or sleep difficulties, or symptoms of daytime sleepiness.
“Importantly, to the best of our knowledge, our results show in vivo for the first time that greater amyloid burden colocalizes with greater gray-matter volume, perfusion, and metabolism in older participants with sleep-disordered breathing who are cognitively unimpaired. We believe that these overlapping patterns reinforce the likelihood of common underlying mechanisms,” they wrote.
The Age-Well randomized clinical trial is part of the Medit-Ageing project and is funded through the European Union’s Horizon 2020 Research and Innovation Program, INSERM, and Fondation d’ Entreprise MMA des Entrepreneurs du Futur. Dr. André reported no conflicts of interest to disclose.
SOURCE: André C et al. JAMA Neurol. 2020 Mar 23. doi: 10.1001/jamaneurol.2020.0311.
FROM JAMA NEUROLOGY
Intracranial artery stenting shows promising 1-year results
LOS ANGELES –
One-year after 129 patients received an intracranial stent that’s already on the U.S. market for treating severe atherosclerotic disease following an ischemic stroke, their incidence of death or repeat stroke was 9%, low enough to suggest benefit compared with historic control patients who were medically managed and had a 12% 1-year rate of death or stroke. This signal of incremental benefit from intracranial stenting may help spark renewed interest in an intervention that was largely forsaken in recent years because of safety concerns.
Assessing restenosis frequency
“Stenting seemed to confer some protection against severe or fatal strokes” in a study that provided “the largest 1-year follow-up of stenting” for intracranial atherosclerotic disease, the trigger for roughly 10% of all U.S. stroke cases, Michael J. Alexander, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
What 1-year follow-up highlighted was the restenosis frequency, based on imaging follow-up for 107 of these 129 patients who had received a Wingspan nitinol, self-expanding stent. Seven patients developed symptomatic restenosis in the region of stent placement, and another 11 patients had asymptomatic restenosis that occluded at least 70% of the stented artery, a total 1-year restenosis rate of 18/107 (17%), reported Dr. Alexander, professor of neurosurgery and director of the Neurovascular Center at Cedars-Sinai Medical Center in Los Angeles. The mean time to detection of restenosis was 5 months, with a range of 1-11 months.
Intracranial stenting falls out of favor
The tested Wingspan stent first received Food and Drug Administration approval for intracranial artery placement in 2005, and then in August 2012 the agency tightened the labeled indication to a much smaller, more specifically defined group of patients: those 22-80 years old, with 70%-99% stenosis in a cerebral artery, with a history of at least two strokes, with stent placement timed more than 7 days following the most recent stroke, and refractory to medical therapy. This 2012 label change came in response to a concerning rate of periprocedural complications in patients who received intracrania artery stents as part of a study reported in 2011 that included many patients with clinical characteristics that fell outside the limits the agency later set in 2012. Results from the SAMMPRIS (Stenting vs. Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis) trial showed that among 451 randomized patients the 224 assigned to stenting had a 15% 30-day rate of death or stroke, compared with a 6% rate among control patients who did not receive a stent, a statistically significant difference that led to early stopping of the trial (New Engl J Med. 2011 Sep 15;365[11]:993-1003). At least half of the patients who received a stent in SAMMPRIS were 7 or fewer days out from their index event, a majority had either a single prior stroke or transient ischemic attack as their index event, and many had not been established as refractory to medical management.
The SAMMPRIS results and the subsequent relabeling of the Wingspan stent by the FDA had two consequences. First came a steep drop in the use of intracranial stenting. After SAMMPRIS, vascular neurologists “abandoned the stent; no one does intracranial stenting” today, commented Louise D. McCullough, MD, professor and chair of neurology at the University of Texas Health Science Center at Houston. The second consequence was an FDA mandate to run a new randomized study to reassess the periprocedural complications when clinicians placed the Wingspan intracranial stent in patients who fully matched the revised 2012 labeling.
That mandated study was the WEAVE (Post Market Surveillance Study of the Wingspan Stent System) trial, which enrolled 152 patients at 24 U.S. sites in a single-arm study, and found a 2.6% rate of death or stroke during the 30 days following intervention that beat the 4% benchmark rate prespecified in the trial’s design (Stroke. 2019 Apr;50[4]:889-94). The WEAVE findings provided even more evidence of the need for the tight labeling the device received in 2012. A safety communication from the FDA in April 2019 noted that an additional 46 patients received an intracranial artery stent during WEAVE despite falling outside the 2012 labeled indications, and this off-label group had a 24% incidence of periprocedural complications, compared with the 2.6% rate in the on-label group. The FDA’s statement reaffirmed the labeling restrictions and highlighted additional cautions and recommendations for using the device.
The WOVEN study
The 1-year follow-up of the WEAVE patients, an extension called the WOVEN (Wingspan One Year Vascular Imaging Events and Neurologic Outcomes) study, was investigator initiated with no commercial funding and included 129 of the original on-label patients (85%) at 15 of the original 24 participating centers.
In addition to the data collected in WOVEN on restenosis rates, follow-up tallied seven patients with a stroke in the vascular territory of the stent during the period that began 30 days after the procedure (when the WEAVE follow-up finished) and continued through 12 months, with no neurologic deaths. When combined with the 4 periprocedural events that occurred during WEAVE, the final WOVEN tally was 11 total events in 129 patients followed for 1 year (9%). Because WEAVE and WOVEN included no control patients, Dr. Alexander compared this 1-year incidence rate with the 12% rate among medically managed control patients in SAMMPRIS.
According to Dr. Alexander, the next step in the path to rehabilitating a clinical role for intracranial stenting is a new randomized study that compares stenting used exclusively to the 2012 labeling with medical management in high-risk patients, those with hemodynamic compromise.
Encouraging data, but is it compelling?
“There may be a benefit” from intracranial stenting, but “we need a larger trial to convince people” said Dr. McCullough. The WEAVE and new WOVEN findings provide a “signal that stenting may be better than medical therapy, but this was only in just over 100 patients. We’ll need a larger study,” she said in an interview. The findings also reinforced that restenosis remains a challenge for intracranial artery stenting.
“Intracranial atherosclerosis is very difficult to treat, and we need new strategies for these patients.” The WEAVE and WOVEN results “suggest that while the restenosis rate may be high, it may also be manageable.” Delaying stent placement to no sooner than 8 days after a stroke may be a key step for improving safety, but new approaches are also need to minimize the restenosis risk, Dr. McCullough noted.
WEAVE was sponsored by Stryker Neurovascular, the company that markets the Wingspan intracranial artery stent. WOVEN received no commercial funding. Dr. Alexander has been a consultant to Stryker Neurovascular. Dr. McCullough had no disclosures.
SOURCE: Alexander MJ et al. International Stroke Conference, Abstract LB4.
LOS ANGELES –
One-year after 129 patients received an intracranial stent that’s already on the U.S. market for treating severe atherosclerotic disease following an ischemic stroke, their incidence of death or repeat stroke was 9%, low enough to suggest benefit compared with historic control patients who were medically managed and had a 12% 1-year rate of death or stroke. This signal of incremental benefit from intracranial stenting may help spark renewed interest in an intervention that was largely forsaken in recent years because of safety concerns.
Assessing restenosis frequency
“Stenting seemed to confer some protection against severe or fatal strokes” in a study that provided “the largest 1-year follow-up of stenting” for intracranial atherosclerotic disease, the trigger for roughly 10% of all U.S. stroke cases, Michael J. Alexander, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
What 1-year follow-up highlighted was the restenosis frequency, based on imaging follow-up for 107 of these 129 patients who had received a Wingspan nitinol, self-expanding stent. Seven patients developed symptomatic restenosis in the region of stent placement, and another 11 patients had asymptomatic restenosis that occluded at least 70% of the stented artery, a total 1-year restenosis rate of 18/107 (17%), reported Dr. Alexander, professor of neurosurgery and director of the Neurovascular Center at Cedars-Sinai Medical Center in Los Angeles. The mean time to detection of restenosis was 5 months, with a range of 1-11 months.
Intracranial stenting falls out of favor
The tested Wingspan stent first received Food and Drug Administration approval for intracranial artery placement in 2005, and then in August 2012 the agency tightened the labeled indication to a much smaller, more specifically defined group of patients: those 22-80 years old, with 70%-99% stenosis in a cerebral artery, with a history of at least two strokes, with stent placement timed more than 7 days following the most recent stroke, and refractory to medical therapy. This 2012 label change came in response to a concerning rate of periprocedural complications in patients who received intracrania artery stents as part of a study reported in 2011 that included many patients with clinical characteristics that fell outside the limits the agency later set in 2012. Results from the SAMMPRIS (Stenting vs. Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis) trial showed that among 451 randomized patients the 224 assigned to stenting had a 15% 30-day rate of death or stroke, compared with a 6% rate among control patients who did not receive a stent, a statistically significant difference that led to early stopping of the trial (New Engl J Med. 2011 Sep 15;365[11]:993-1003). At least half of the patients who received a stent in SAMMPRIS were 7 or fewer days out from their index event, a majority had either a single prior stroke or transient ischemic attack as their index event, and many had not been established as refractory to medical management.
The SAMMPRIS results and the subsequent relabeling of the Wingspan stent by the FDA had two consequences. First came a steep drop in the use of intracranial stenting. After SAMMPRIS, vascular neurologists “abandoned the stent; no one does intracranial stenting” today, commented Louise D. McCullough, MD, professor and chair of neurology at the University of Texas Health Science Center at Houston. The second consequence was an FDA mandate to run a new randomized study to reassess the periprocedural complications when clinicians placed the Wingspan intracranial stent in patients who fully matched the revised 2012 labeling.
That mandated study was the WEAVE (Post Market Surveillance Study of the Wingspan Stent System) trial, which enrolled 152 patients at 24 U.S. sites in a single-arm study, and found a 2.6% rate of death or stroke during the 30 days following intervention that beat the 4% benchmark rate prespecified in the trial’s design (Stroke. 2019 Apr;50[4]:889-94). The WEAVE findings provided even more evidence of the need for the tight labeling the device received in 2012. A safety communication from the FDA in April 2019 noted that an additional 46 patients received an intracranial artery stent during WEAVE despite falling outside the 2012 labeled indications, and this off-label group had a 24% incidence of periprocedural complications, compared with the 2.6% rate in the on-label group. The FDA’s statement reaffirmed the labeling restrictions and highlighted additional cautions and recommendations for using the device.
The WOVEN study
The 1-year follow-up of the WEAVE patients, an extension called the WOVEN (Wingspan One Year Vascular Imaging Events and Neurologic Outcomes) study, was investigator initiated with no commercial funding and included 129 of the original on-label patients (85%) at 15 of the original 24 participating centers.
In addition to the data collected in WOVEN on restenosis rates, follow-up tallied seven patients with a stroke in the vascular territory of the stent during the period that began 30 days after the procedure (when the WEAVE follow-up finished) and continued through 12 months, with no neurologic deaths. When combined with the 4 periprocedural events that occurred during WEAVE, the final WOVEN tally was 11 total events in 129 patients followed for 1 year (9%). Because WEAVE and WOVEN included no control patients, Dr. Alexander compared this 1-year incidence rate with the 12% rate among medically managed control patients in SAMMPRIS.
According to Dr. Alexander, the next step in the path to rehabilitating a clinical role for intracranial stenting is a new randomized study that compares stenting used exclusively to the 2012 labeling with medical management in high-risk patients, those with hemodynamic compromise.
Encouraging data, but is it compelling?
“There may be a benefit” from intracranial stenting, but “we need a larger trial to convince people” said Dr. McCullough. The WEAVE and new WOVEN findings provide a “signal that stenting may be better than medical therapy, but this was only in just over 100 patients. We’ll need a larger study,” she said in an interview. The findings also reinforced that restenosis remains a challenge for intracranial artery stenting.
“Intracranial atherosclerosis is very difficult to treat, and we need new strategies for these patients.” The WEAVE and WOVEN results “suggest that while the restenosis rate may be high, it may also be manageable.” Delaying stent placement to no sooner than 8 days after a stroke may be a key step for improving safety, but new approaches are also need to minimize the restenosis risk, Dr. McCullough noted.
WEAVE was sponsored by Stryker Neurovascular, the company that markets the Wingspan intracranial artery stent. WOVEN received no commercial funding. Dr. Alexander has been a consultant to Stryker Neurovascular. Dr. McCullough had no disclosures.
SOURCE: Alexander MJ et al. International Stroke Conference, Abstract LB4.
LOS ANGELES –
One-year after 129 patients received an intracranial stent that’s already on the U.S. market for treating severe atherosclerotic disease following an ischemic stroke, their incidence of death or repeat stroke was 9%, low enough to suggest benefit compared with historic control patients who were medically managed and had a 12% 1-year rate of death or stroke. This signal of incremental benefit from intracranial stenting may help spark renewed interest in an intervention that was largely forsaken in recent years because of safety concerns.
Assessing restenosis frequency
“Stenting seemed to confer some protection against severe or fatal strokes” in a study that provided “the largest 1-year follow-up of stenting” for intracranial atherosclerotic disease, the trigger for roughly 10% of all U.S. stroke cases, Michael J. Alexander, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
What 1-year follow-up highlighted was the restenosis frequency, based on imaging follow-up for 107 of these 129 patients who had received a Wingspan nitinol, self-expanding stent. Seven patients developed symptomatic restenosis in the region of stent placement, and another 11 patients had asymptomatic restenosis that occluded at least 70% of the stented artery, a total 1-year restenosis rate of 18/107 (17%), reported Dr. Alexander, professor of neurosurgery and director of the Neurovascular Center at Cedars-Sinai Medical Center in Los Angeles. The mean time to detection of restenosis was 5 months, with a range of 1-11 months.
Intracranial stenting falls out of favor
The tested Wingspan stent first received Food and Drug Administration approval for intracranial artery placement in 2005, and then in August 2012 the agency tightened the labeled indication to a much smaller, more specifically defined group of patients: those 22-80 years old, with 70%-99% stenosis in a cerebral artery, with a history of at least two strokes, with stent placement timed more than 7 days following the most recent stroke, and refractory to medical therapy. This 2012 label change came in response to a concerning rate of periprocedural complications in patients who received intracrania artery stents as part of a study reported in 2011 that included many patients with clinical characteristics that fell outside the limits the agency later set in 2012. Results from the SAMMPRIS (Stenting vs. Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis) trial showed that among 451 randomized patients the 224 assigned to stenting had a 15% 30-day rate of death or stroke, compared with a 6% rate among control patients who did not receive a stent, a statistically significant difference that led to early stopping of the trial (New Engl J Med. 2011 Sep 15;365[11]:993-1003). At least half of the patients who received a stent in SAMMPRIS were 7 or fewer days out from their index event, a majority had either a single prior stroke or transient ischemic attack as their index event, and many had not been established as refractory to medical management.
The SAMMPRIS results and the subsequent relabeling of the Wingspan stent by the FDA had two consequences. First came a steep drop in the use of intracranial stenting. After SAMMPRIS, vascular neurologists “abandoned the stent; no one does intracranial stenting” today, commented Louise D. McCullough, MD, professor and chair of neurology at the University of Texas Health Science Center at Houston. The second consequence was an FDA mandate to run a new randomized study to reassess the periprocedural complications when clinicians placed the Wingspan intracranial stent in patients who fully matched the revised 2012 labeling.
That mandated study was the WEAVE (Post Market Surveillance Study of the Wingspan Stent System) trial, which enrolled 152 patients at 24 U.S. sites in a single-arm study, and found a 2.6% rate of death or stroke during the 30 days following intervention that beat the 4% benchmark rate prespecified in the trial’s design (Stroke. 2019 Apr;50[4]:889-94). The WEAVE findings provided even more evidence of the need for the tight labeling the device received in 2012. A safety communication from the FDA in April 2019 noted that an additional 46 patients received an intracranial artery stent during WEAVE despite falling outside the 2012 labeled indications, and this off-label group had a 24% incidence of periprocedural complications, compared with the 2.6% rate in the on-label group. The FDA’s statement reaffirmed the labeling restrictions and highlighted additional cautions and recommendations for using the device.
The WOVEN study
The 1-year follow-up of the WEAVE patients, an extension called the WOVEN (Wingspan One Year Vascular Imaging Events and Neurologic Outcomes) study, was investigator initiated with no commercial funding and included 129 of the original on-label patients (85%) at 15 of the original 24 participating centers.
In addition to the data collected in WOVEN on restenosis rates, follow-up tallied seven patients with a stroke in the vascular territory of the stent during the period that began 30 days after the procedure (when the WEAVE follow-up finished) and continued through 12 months, with no neurologic deaths. When combined with the 4 periprocedural events that occurred during WEAVE, the final WOVEN tally was 11 total events in 129 patients followed for 1 year (9%). Because WEAVE and WOVEN included no control patients, Dr. Alexander compared this 1-year incidence rate with the 12% rate among medically managed control patients in SAMMPRIS.
According to Dr. Alexander, the next step in the path to rehabilitating a clinical role for intracranial stenting is a new randomized study that compares stenting used exclusively to the 2012 labeling with medical management in high-risk patients, those with hemodynamic compromise.
Encouraging data, but is it compelling?
“There may be a benefit” from intracranial stenting, but “we need a larger trial to convince people” said Dr. McCullough. The WEAVE and new WOVEN findings provide a “signal that stenting may be better than medical therapy, but this was only in just over 100 patients. We’ll need a larger study,” she said in an interview. The findings also reinforced that restenosis remains a challenge for intracranial artery stenting.
“Intracranial atherosclerosis is very difficult to treat, and we need new strategies for these patients.” The WEAVE and WOVEN results “suggest that while the restenosis rate may be high, it may also be manageable.” Delaying stent placement to no sooner than 8 days after a stroke may be a key step for improving safety, but new approaches are also need to minimize the restenosis risk, Dr. McCullough noted.
WEAVE was sponsored by Stryker Neurovascular, the company that markets the Wingspan intracranial artery stent. WOVEN received no commercial funding. Dr. Alexander has been a consultant to Stryker Neurovascular. Dr. McCullough had no disclosures.
SOURCE: Alexander MJ et al. International Stroke Conference, Abstract LB4.
REPORTING FROM ISC 2020
AMA offers resources for front-line physicians
.
The literature include news, advocacy, and other information to help front-line physicians provide care to patients and keep themselves safe “in a rapidly changing environment,” the organization said in a statement.
“The AMA continues to forcefully advocate for [personal protective equipment] and critical policy and regulatory changes needed to address our public health and health system needs. Because so many of the challenges of the pandemic are felt at a practice level, we are also providing new tools and information to help physicians respond,” AMA President Patrice A. Harris, MD, said in the statement.
The COVID-19 physician and practice resources released by the AMA include:
- A Physicians Guide to COVID-19 .
- An AMA COVID-19 online resource center and a COVID-19 FAQ.
- A Quick Guide to Telemedicine in Practice.
- Ethical guidance for physicians .
- Evidence-based resources from the The JAMA Network COVID-19 Resource Center.
- CME for physicians through the JAMA Network’s JN Learning website.
.
The literature include news, advocacy, and other information to help front-line physicians provide care to patients and keep themselves safe “in a rapidly changing environment,” the organization said in a statement.
“The AMA continues to forcefully advocate for [personal protective equipment] and critical policy and regulatory changes needed to address our public health and health system needs. Because so many of the challenges of the pandemic are felt at a practice level, we are also providing new tools and information to help physicians respond,” AMA President Patrice A. Harris, MD, said in the statement.
The COVID-19 physician and practice resources released by the AMA include:
- A Physicians Guide to COVID-19 .
- An AMA COVID-19 online resource center and a COVID-19 FAQ.
- A Quick Guide to Telemedicine in Practice.
- Ethical guidance for physicians .
- Evidence-based resources from the The JAMA Network COVID-19 Resource Center.
- CME for physicians through the JAMA Network’s JN Learning website.
.
The literature include news, advocacy, and other information to help front-line physicians provide care to patients and keep themselves safe “in a rapidly changing environment,” the organization said in a statement.
“The AMA continues to forcefully advocate for [personal protective equipment] and critical policy and regulatory changes needed to address our public health and health system needs. Because so many of the challenges of the pandemic are felt at a practice level, we are also providing new tools and information to help physicians respond,” AMA President Patrice A. Harris, MD, said in the statement.
The COVID-19 physician and practice resources released by the AMA include:
- A Physicians Guide to COVID-19 .
- An AMA COVID-19 online resource center and a COVID-19 FAQ.
- A Quick Guide to Telemedicine in Practice.
- Ethical guidance for physicians .
- Evidence-based resources from the The JAMA Network COVID-19 Resource Center.
- CME for physicians through the JAMA Network’s JN Learning website.
Hand washing and hand sanitizer on the skin and COVID-19 infection risk
As we deal with the effects of the COVID-19 pandemic, hand washing and the use of hand sanitizers have been key for infection prevention. With drier, colder weather in many of the communities initially affected by COVID-19, skin was already prone to dryness and a skin barrier compromised, and hand eczema was more prevalent because of these factors alone. This article explores the while maintaining the maximum possible degree of infection prevention.
With many viruses, including coronavirus, the virus is a self-assembled nanoparticle in which the most vulnerable structure is the outer lipid bilayer. Soaps dissolve the lipid membrane and the virus breaks apart, inactivating it; they are also alkaline surfactants that pick up particles – including dirt, bacteria, and viruses – which are removed from the surface of the skin when the soaps are rinsed off. In the process of washing, the alkalinity of the soap (pH approximately 9-10), compared with the normal outer skin pH of approximately 5.5 or lower, also can affect the skin barrier as well as the resident skin microflora. In a study by Lambers et al., it was found that an acid skin pH (4-4.5) keeps the resident bacterial flora attached to the skin, whereas an alkaline pH (8-9) promotes the dispersal from the skin in assessments of the volar forearm.
With regard to the effectiveness of hand washing against viruses, the length of time spent hand washing has been shown to have an impact on influenza-like illness. In a recent study of 2,082 participants by Bin Abdulrahman et al., those who spent only 5-10 seconds hand washing with soap and hand rubbing were at a higher risk of more frequent influenza-like illness (odds ratio, 1.37; 95% confidence interval, 1.08-1.75), compared with those who washed their hands for 15 seconds or longer. Moreover, hand washing with soap and rubbing after shaking hands was found to be an independent protective factor against frequent influenza-like illness (adjusted OR, 0.59; 95% confidence interval, 0.37-0.94). Previous studies on the impact of hand washing on bacterial and parasitic illnesses also found similar results: Hand washing for 15-20 seconds or longer reduces infection.
Alcohol, long known as a disinfectant, has been recommended for disinfecting the hands since the late 1800s. Most alcohol-based hand antiseptics contain isopropanol, ethanol, N-propanol, or a combination of two of these products. The antimicrobial activity of alcohols can be attributed to their ability to denature and coagulate proteins, thereby lysing microorganisms’ cells, and disrupting their cellular metabolism. Alcohol solutions containing 60%-95% alcohol are the most effective. Notably, very high concentrations of alcohol are less potent because less water is found in higher concentrations of alcohol and proteins are not denatured easily in the absence of water. Alcohol-based hand sanitizers also often contain humectants, such as glycerin and/or aloe vera, to help prevent skin dryness and replace water content that is stripped by the use of alcohol on the skin surface.
Other topical disinfectants can also be used to inactivate coronaviruses from surfaces, including the skin. A recently published analysis of 22 studies found that human coronaviruses – such as severe acute respiratory syndrome (SARS) coronavirus, Middle East respiratory syndrome (MERS) coronavirus, or endemic human coronaviruses (HCoV) – can persist on inanimate surfaces such as metal, glass, or plastic for up to 9 days (COVID-19 was found in a study to persist on metal for up to 2-3 days), but can be efficiently inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate, are less effective.
In the case of SARS, treatment of SARS-CoV with povidone-iodine products for 2 minutes reduced virus infectivity to below the detectable level, equivalent to the effect of ethanol, in one study. Formalin fixation of the infected cells and heating the virus to 56° C, as used in routine tissue processing, were found to inactivate several coronaviruses as well. Based on this information, ethanol-based hand sanitizers, typically containing ethanol content of 60% or higher, can be used to inactivate coronaviruses on the skin, including COVID-19.
In patients with influenza-virus infections, whether pathogens were in wet or dried mucus played a role in whether hand washing or rubbing with hand sanitizer was more effective. In a study that examined the effects of hand washing versus antiseptic hand rubbing with an ethanol-based hand disinfectant on inactivation of influenza A virus adhered to the hands, the investigators showed that the effectiveness of the ethanol-based disinfectant against influenza A virus in mucus was reduced, compared with influenza A virus in saline. Influenza A in mucus remained active, despite 120 seconds of hand rubbing with hand sanitizer; however, influenza A in saline was completely inactivated within 30 seconds. Interestingly, rubbing hands with an ethanol-based disinfectant inactivated influenza A virus in mucus within 30 seconds with mucus that had dried completely because the hydrogel characteristics had been eliminated. Hand washing rapidly inactivated influenza A virus whether in mucus form, saline, or dried mucous.
It is important to note that in COVID-19 infections, a productive cough or rhinorrhea are not as common compared with dry cough. Regardless, the findings of the study described above should be considered if mucous symptoms develop during a COVID-19 infection when determining infection control. Luckily, with COVID-19, both hand washing and use of an ethanol-based hand sanitizer are seemingly effective in inactivating the virus or removing it from the skin surface.
After frequent hand washing, we all can experience dryness and potentially cracked skin as well. With hand sanitizer, the alcohol content can also cause burning of skin, especially compromised skin.
Vanilloid receptor-1 (VR1), a heat-gated ion channel, is responsible for the burning sensation caused by capsaicin. Ethanol lowers the amount of heat needed to turn on VR1 nocioceptive pain receptors by almost ten degrees, resulting in a potential burning sensation when applied.
Nails are affected as well with frequent hand washing and/or application of hand sanitizer and can become cracked or brittle. Contact dermatitis, both irritant and allergic, can occur with increased use of disinfectants, particularly household cleaners without proper barrier protection.
We’ve previously mentioned the effect of hand washing disrupting the resident skin microflora. Maintaining the skin microflora and barrier is an important component of skin health for preventing both dermatitis and infection. Hand washing or use of hand sanitizer is of paramount importance and effective in infection control for COVID-19. To maintain skin health and the skin barrier, applying lotion or cream after hand washing is recommended. It is recommended to avoid scrubbing hands while washing, since this causes breaks in the skin. Using water that is too hot is not recommended as it can inflame the skin further and disrupt the skin barrier.
Wearing gloves, if possible, is recommended when using household disinfectant products to further decrease skin irritation, barrier disruption, and risk of contact dermatitis. I have found hand emollients that contain ceramides or ingredients higher in omega 6 fatty acids, such as borage seed oil or other oils high in linoleic acid content, to be helpful. In addition to improving the skin barrier, emollients and perhaps those with topical pre- or probiotics, may help restore the skin microflora, potentially improving infection control further. Application of hand moisturizer each time after hand washing to maintain better infection control and barrier protection was also recommended by the recent consensus statement of Chinese experts on protection of skin and mucous membrane barrier for health care workers fighting against COVID-19.
We and our patients have remarked how it seems like our hands have aged 20-50 years in the previous 2 weeks. No one is complaining, everyone understands that protecting themselves and others against a potentially lethal virus is paramount. Maintaining skin health is of secondary concern, but maintaining healthy skin may also protect the skin barrier, another important component of potential infection control.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. They had no relevant disclosures. Write to them at [email protected].
Resources
Lambers H et al. Int J Cosmet Sci. 2006 Oct;28(5):359-70.
Bin Abdulrahman AK et al. BMC Public Health. 2019 Oct 22;19(1):1324. doi: 10.1186/s12889-019-77.
Kariwa H et al. Dermatology. 2006;212 Suppl 1:119-23.
HIrose R et al. mSphere. 2019 Sep 18;4(5). pii: e00474-19. doi: 10.1128/mSphere.00474-19.
Trevisani M et al. Nat Neurosci. 2002 Jun;5(6):546-51.
Yan Y et al. Dermatol Ther. 2020 Mar 13:e13310. doi: 10.1111/dth.13310.
As we deal with the effects of the COVID-19 pandemic, hand washing and the use of hand sanitizers have been key for infection prevention. With drier, colder weather in many of the communities initially affected by COVID-19, skin was already prone to dryness and a skin barrier compromised, and hand eczema was more prevalent because of these factors alone. This article explores the while maintaining the maximum possible degree of infection prevention.
With many viruses, including coronavirus, the virus is a self-assembled nanoparticle in which the most vulnerable structure is the outer lipid bilayer. Soaps dissolve the lipid membrane and the virus breaks apart, inactivating it; they are also alkaline surfactants that pick up particles – including dirt, bacteria, and viruses – which are removed from the surface of the skin when the soaps are rinsed off. In the process of washing, the alkalinity of the soap (pH approximately 9-10), compared with the normal outer skin pH of approximately 5.5 or lower, also can affect the skin barrier as well as the resident skin microflora. In a study by Lambers et al., it was found that an acid skin pH (4-4.5) keeps the resident bacterial flora attached to the skin, whereas an alkaline pH (8-9) promotes the dispersal from the skin in assessments of the volar forearm.
With regard to the effectiveness of hand washing against viruses, the length of time spent hand washing has been shown to have an impact on influenza-like illness. In a recent study of 2,082 participants by Bin Abdulrahman et al., those who spent only 5-10 seconds hand washing with soap and hand rubbing were at a higher risk of more frequent influenza-like illness (odds ratio, 1.37; 95% confidence interval, 1.08-1.75), compared with those who washed their hands for 15 seconds or longer. Moreover, hand washing with soap and rubbing after shaking hands was found to be an independent protective factor against frequent influenza-like illness (adjusted OR, 0.59; 95% confidence interval, 0.37-0.94). Previous studies on the impact of hand washing on bacterial and parasitic illnesses also found similar results: Hand washing for 15-20 seconds or longer reduces infection.
Alcohol, long known as a disinfectant, has been recommended for disinfecting the hands since the late 1800s. Most alcohol-based hand antiseptics contain isopropanol, ethanol, N-propanol, or a combination of two of these products. The antimicrobial activity of alcohols can be attributed to their ability to denature and coagulate proteins, thereby lysing microorganisms’ cells, and disrupting their cellular metabolism. Alcohol solutions containing 60%-95% alcohol are the most effective. Notably, very high concentrations of alcohol are less potent because less water is found in higher concentrations of alcohol and proteins are not denatured easily in the absence of water. Alcohol-based hand sanitizers also often contain humectants, such as glycerin and/or aloe vera, to help prevent skin dryness and replace water content that is stripped by the use of alcohol on the skin surface.
Other topical disinfectants can also be used to inactivate coronaviruses from surfaces, including the skin. A recently published analysis of 22 studies found that human coronaviruses – such as severe acute respiratory syndrome (SARS) coronavirus, Middle East respiratory syndrome (MERS) coronavirus, or endemic human coronaviruses (HCoV) – can persist on inanimate surfaces such as metal, glass, or plastic for up to 9 days (COVID-19 was found in a study to persist on metal for up to 2-3 days), but can be efficiently inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate, are less effective.
In the case of SARS, treatment of SARS-CoV with povidone-iodine products for 2 minutes reduced virus infectivity to below the detectable level, equivalent to the effect of ethanol, in one study. Formalin fixation of the infected cells and heating the virus to 56° C, as used in routine tissue processing, were found to inactivate several coronaviruses as well. Based on this information, ethanol-based hand sanitizers, typically containing ethanol content of 60% or higher, can be used to inactivate coronaviruses on the skin, including COVID-19.
In patients with influenza-virus infections, whether pathogens were in wet or dried mucus played a role in whether hand washing or rubbing with hand sanitizer was more effective. In a study that examined the effects of hand washing versus antiseptic hand rubbing with an ethanol-based hand disinfectant on inactivation of influenza A virus adhered to the hands, the investigators showed that the effectiveness of the ethanol-based disinfectant against influenza A virus in mucus was reduced, compared with influenza A virus in saline. Influenza A in mucus remained active, despite 120 seconds of hand rubbing with hand sanitizer; however, influenza A in saline was completely inactivated within 30 seconds. Interestingly, rubbing hands with an ethanol-based disinfectant inactivated influenza A virus in mucus within 30 seconds with mucus that had dried completely because the hydrogel characteristics had been eliminated. Hand washing rapidly inactivated influenza A virus whether in mucus form, saline, or dried mucous.
It is important to note that in COVID-19 infections, a productive cough or rhinorrhea are not as common compared with dry cough. Regardless, the findings of the study described above should be considered if mucous symptoms develop during a COVID-19 infection when determining infection control. Luckily, with COVID-19, both hand washing and use of an ethanol-based hand sanitizer are seemingly effective in inactivating the virus or removing it from the skin surface.
After frequent hand washing, we all can experience dryness and potentially cracked skin as well. With hand sanitizer, the alcohol content can also cause burning of skin, especially compromised skin.
Vanilloid receptor-1 (VR1), a heat-gated ion channel, is responsible for the burning sensation caused by capsaicin. Ethanol lowers the amount of heat needed to turn on VR1 nocioceptive pain receptors by almost ten degrees, resulting in a potential burning sensation when applied.
Nails are affected as well with frequent hand washing and/or application of hand sanitizer and can become cracked or brittle. Contact dermatitis, both irritant and allergic, can occur with increased use of disinfectants, particularly household cleaners without proper barrier protection.
We’ve previously mentioned the effect of hand washing disrupting the resident skin microflora. Maintaining the skin microflora and barrier is an important component of skin health for preventing both dermatitis and infection. Hand washing or use of hand sanitizer is of paramount importance and effective in infection control for COVID-19. To maintain skin health and the skin barrier, applying lotion or cream after hand washing is recommended. It is recommended to avoid scrubbing hands while washing, since this causes breaks in the skin. Using water that is too hot is not recommended as it can inflame the skin further and disrupt the skin barrier.
Wearing gloves, if possible, is recommended when using household disinfectant products to further decrease skin irritation, barrier disruption, and risk of contact dermatitis. I have found hand emollients that contain ceramides or ingredients higher in omega 6 fatty acids, such as borage seed oil or other oils high in linoleic acid content, to be helpful. In addition to improving the skin barrier, emollients and perhaps those with topical pre- or probiotics, may help restore the skin microflora, potentially improving infection control further. Application of hand moisturizer each time after hand washing to maintain better infection control and barrier protection was also recommended by the recent consensus statement of Chinese experts on protection of skin and mucous membrane barrier for health care workers fighting against COVID-19.
We and our patients have remarked how it seems like our hands have aged 20-50 years in the previous 2 weeks. No one is complaining, everyone understands that protecting themselves and others against a potentially lethal virus is paramount. Maintaining skin health is of secondary concern, but maintaining healthy skin may also protect the skin barrier, another important component of potential infection control.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. They had no relevant disclosures. Write to them at [email protected].
Resources
Lambers H et al. Int J Cosmet Sci. 2006 Oct;28(5):359-70.
Bin Abdulrahman AK et al. BMC Public Health. 2019 Oct 22;19(1):1324. doi: 10.1186/s12889-019-77.
Kariwa H et al. Dermatology. 2006;212 Suppl 1:119-23.
HIrose R et al. mSphere. 2019 Sep 18;4(5). pii: e00474-19. doi: 10.1128/mSphere.00474-19.
Trevisani M et al. Nat Neurosci. 2002 Jun;5(6):546-51.
Yan Y et al. Dermatol Ther. 2020 Mar 13:e13310. doi: 10.1111/dth.13310.
As we deal with the effects of the COVID-19 pandemic, hand washing and the use of hand sanitizers have been key for infection prevention. With drier, colder weather in many of the communities initially affected by COVID-19, skin was already prone to dryness and a skin barrier compromised, and hand eczema was more prevalent because of these factors alone. This article explores the while maintaining the maximum possible degree of infection prevention.
With many viruses, including coronavirus, the virus is a self-assembled nanoparticle in which the most vulnerable structure is the outer lipid bilayer. Soaps dissolve the lipid membrane and the virus breaks apart, inactivating it; they are also alkaline surfactants that pick up particles – including dirt, bacteria, and viruses – which are removed from the surface of the skin when the soaps are rinsed off. In the process of washing, the alkalinity of the soap (pH approximately 9-10), compared with the normal outer skin pH of approximately 5.5 or lower, also can affect the skin barrier as well as the resident skin microflora. In a study by Lambers et al., it was found that an acid skin pH (4-4.5) keeps the resident bacterial flora attached to the skin, whereas an alkaline pH (8-9) promotes the dispersal from the skin in assessments of the volar forearm.
With regard to the effectiveness of hand washing against viruses, the length of time spent hand washing has been shown to have an impact on influenza-like illness. In a recent study of 2,082 participants by Bin Abdulrahman et al., those who spent only 5-10 seconds hand washing with soap and hand rubbing were at a higher risk of more frequent influenza-like illness (odds ratio, 1.37; 95% confidence interval, 1.08-1.75), compared with those who washed their hands for 15 seconds or longer. Moreover, hand washing with soap and rubbing after shaking hands was found to be an independent protective factor against frequent influenza-like illness (adjusted OR, 0.59; 95% confidence interval, 0.37-0.94). Previous studies on the impact of hand washing on bacterial and parasitic illnesses also found similar results: Hand washing for 15-20 seconds or longer reduces infection.
Alcohol, long known as a disinfectant, has been recommended for disinfecting the hands since the late 1800s. Most alcohol-based hand antiseptics contain isopropanol, ethanol, N-propanol, or a combination of two of these products. The antimicrobial activity of alcohols can be attributed to their ability to denature and coagulate proteins, thereby lysing microorganisms’ cells, and disrupting their cellular metabolism. Alcohol solutions containing 60%-95% alcohol are the most effective. Notably, very high concentrations of alcohol are less potent because less water is found in higher concentrations of alcohol and proteins are not denatured easily in the absence of water. Alcohol-based hand sanitizers also often contain humectants, such as glycerin and/or aloe vera, to help prevent skin dryness and replace water content that is stripped by the use of alcohol on the skin surface.
Other topical disinfectants can also be used to inactivate coronaviruses from surfaces, including the skin. A recently published analysis of 22 studies found that human coronaviruses – such as severe acute respiratory syndrome (SARS) coronavirus, Middle East respiratory syndrome (MERS) coronavirus, or endemic human coronaviruses (HCoV) – can persist on inanimate surfaces such as metal, glass, or plastic for up to 9 days (COVID-19 was found in a study to persist on metal for up to 2-3 days), but can be efficiently inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate, are less effective.
In the case of SARS, treatment of SARS-CoV with povidone-iodine products for 2 minutes reduced virus infectivity to below the detectable level, equivalent to the effect of ethanol, in one study. Formalin fixation of the infected cells and heating the virus to 56° C, as used in routine tissue processing, were found to inactivate several coronaviruses as well. Based on this information, ethanol-based hand sanitizers, typically containing ethanol content of 60% or higher, can be used to inactivate coronaviruses on the skin, including COVID-19.
In patients with influenza-virus infections, whether pathogens were in wet or dried mucus played a role in whether hand washing or rubbing with hand sanitizer was more effective. In a study that examined the effects of hand washing versus antiseptic hand rubbing with an ethanol-based hand disinfectant on inactivation of influenza A virus adhered to the hands, the investigators showed that the effectiveness of the ethanol-based disinfectant against influenza A virus in mucus was reduced, compared with influenza A virus in saline. Influenza A in mucus remained active, despite 120 seconds of hand rubbing with hand sanitizer; however, influenza A in saline was completely inactivated within 30 seconds. Interestingly, rubbing hands with an ethanol-based disinfectant inactivated influenza A virus in mucus within 30 seconds with mucus that had dried completely because the hydrogel characteristics had been eliminated. Hand washing rapidly inactivated influenza A virus whether in mucus form, saline, or dried mucous.
It is important to note that in COVID-19 infections, a productive cough or rhinorrhea are not as common compared with dry cough. Regardless, the findings of the study described above should be considered if mucous symptoms develop during a COVID-19 infection when determining infection control. Luckily, with COVID-19, both hand washing and use of an ethanol-based hand sanitizer are seemingly effective in inactivating the virus or removing it from the skin surface.
After frequent hand washing, we all can experience dryness and potentially cracked skin as well. With hand sanitizer, the alcohol content can also cause burning of skin, especially compromised skin.
Vanilloid receptor-1 (VR1), a heat-gated ion channel, is responsible for the burning sensation caused by capsaicin. Ethanol lowers the amount of heat needed to turn on VR1 nocioceptive pain receptors by almost ten degrees, resulting in a potential burning sensation when applied.
Nails are affected as well with frequent hand washing and/or application of hand sanitizer and can become cracked or brittle. Contact dermatitis, both irritant and allergic, can occur with increased use of disinfectants, particularly household cleaners without proper barrier protection.
We’ve previously mentioned the effect of hand washing disrupting the resident skin microflora. Maintaining the skin microflora and barrier is an important component of skin health for preventing both dermatitis and infection. Hand washing or use of hand sanitizer is of paramount importance and effective in infection control for COVID-19. To maintain skin health and the skin barrier, applying lotion or cream after hand washing is recommended. It is recommended to avoid scrubbing hands while washing, since this causes breaks in the skin. Using water that is too hot is not recommended as it can inflame the skin further and disrupt the skin barrier.
Wearing gloves, if possible, is recommended when using household disinfectant products to further decrease skin irritation, barrier disruption, and risk of contact dermatitis. I have found hand emollients that contain ceramides or ingredients higher in omega 6 fatty acids, such as borage seed oil or other oils high in linoleic acid content, to be helpful. In addition to improving the skin barrier, emollients and perhaps those with topical pre- or probiotics, may help restore the skin microflora, potentially improving infection control further. Application of hand moisturizer each time after hand washing to maintain better infection control and barrier protection was also recommended by the recent consensus statement of Chinese experts on protection of skin and mucous membrane barrier for health care workers fighting against COVID-19.
We and our patients have remarked how it seems like our hands have aged 20-50 years in the previous 2 weeks. No one is complaining, everyone understands that protecting themselves and others against a potentially lethal virus is paramount. Maintaining skin health is of secondary concern, but maintaining healthy skin may also protect the skin barrier, another important component of potential infection control.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. They had no relevant disclosures. Write to them at [email protected].
Resources
Lambers H et al. Int J Cosmet Sci. 2006 Oct;28(5):359-70.
Bin Abdulrahman AK et al. BMC Public Health. 2019 Oct 22;19(1):1324. doi: 10.1186/s12889-019-77.
Kariwa H et al. Dermatology. 2006;212 Suppl 1:119-23.
HIrose R et al. mSphere. 2019 Sep 18;4(5). pii: e00474-19. doi: 10.1128/mSphere.00474-19.
Trevisani M et al. Nat Neurosci. 2002 Jun;5(6):546-51.
Yan Y et al. Dermatol Ther. 2020 Mar 13:e13310. doi: 10.1111/dth.13310.
Cancer care and COVID-19 in Seattle, the first U.S. epicenter
Two months after the first patient with COVID-19 was identified in China, the first case was reported in the United States in the Seattle, Washington, metropolitan area.
Seattle rapidly became the first US epicenter for COVID-19, and local experts are now offering their expertise and advice on how to provide optimal cancer care during the pandemic in a special feature published online March 20 in the Journal of the National Comprehensive Cancer Network.
“We began implementing measures in early March, including infection control and screening of visitors, staff, and patients at the door,” said lead author Masumi Ueda, MD, who holds positions at the Seattle Cancer Care Alliance, the University of Washington, and the Fred Hutchinson Research Center.
“A lot of changes have been implemented, and it changes on a daily basis. We are responding to the growing rate of COVID-19 infection in the community,” she told Medscape Medical News.
Ueda notes that as a result of the quick implementation of new procedures, so far, very few cancer patients at their facilities have been infected by the virus. “It has not hit our cancer population hard, which is a good thing,” she said.
Create “Incident Command Structure”
In sharing their experience, the authors emphasize the importance of keeping channels of communication open between all stakeholders ― administrators and staff, patients, caregivers, and the general public. They also recommend that each facility create an “incident command structure” that can provide early coordination of institution-wide efforts and that can rapidly respond to changing information.
Ueda noted that their command structure was set up very early on, “so we could get communication set up and start building an infrastructure for response.”
Several areas of care that required new strategies were addressed, both to protect patients and to work around staff shortages caused by possible exposure and/or school closings, as well as projected shortages of supplies and hospital resources.
First and foremost was to identify patients and visitors who had respiratory symptoms and to provide them with masks. Although this is always routine practice during the respiratory virus season, screening has now been initiated at entry points throughout the system.
“We were lucky in Seattle and Washington state in that the University of Washington virology lab developed PCR [polymerase chain reaction] testing early on for COVID-19, which subsequently got FDA approval,” said Ueda. “So we were able to have local testing and didn’t have to rely on the state lab. Testing has also been rapidly scaled up.”
Initiating a comprehensive policy for testing staff, tracking results and exposures for persons under investigation, and defining when it is possible to return to work are essential elements for maintaining a stable workforce. In addition, reinforcing a strict “stay at home when ill” policy and providing access to testing for symptomatic staff have been key to limiting exposures.
“What is unique to our region is that we had testing early on, and we are turning it around in 24 hours,” she pointed out. “This is important for staff to be able to return to work.” Currently, staff, patients, and visitors are being tested only if they show the cardinal symptoms associated with COVID-19: fever, shortness of breath, and cough, although muscle aches have recently been added to their testing protocol.
“I think if we had unlimited capacity, we might consider testing people who are asymptomatic,” Ueda noted, “although if you don’t have symptoms, you may not have the viral load needed for an accurate test.”
Educational materials explaining infection control were also needed for patients and families, along with signs and a website to provide COVID-19 education. These were quickly developed.
In addition, a telephone triage line was established for patients with mild symptoms in order to minimize exposures in clinics and to lessen the number of patients presenting at emergency departments.
Outpatient Care
Because theirs is a referral center, many cancer patients come from out of town, and so there is concern about exposing nonlocal patients to COVID-19 as the virus spreads in the Seattle area. In addition, staffing shortages due to factors such as illness, exposure, and school closures are anticipated.
To address these problems, an initial priority was to establish a “multilayer” coverage system for the clinics in the event that practitioners had to be quarantined on short notice, the authors explain.
One decision was to reschedule all wellness visits for current patients or to use telemedicine. Capacity for that option expanded quickly, which was greatly helped by the recent decision by the Centers for Medicare & Medicaid Services to lift Medicare restrictions on the use of certain telemedicine services.
Another approach is to defer all consultations for second opinions for patients who were already undergoing treatment and to increase clinic hours of operations and capabilities for acute evaluations. This helps reserve emergency departments and hospital resources for patients who require higher-level care, the authors comment.
Treatment Decisions
Treatment decisions were more challenging to make, the authors note. One decision was that, despite the risk for COVID-19 for patients with solid tumors, adjuvant therapy with curative intent should proceed, they note. Similarly, patients with metastatic disease might lose the window of opportunity for treatment if it is delayed.
Treatment for aggressive hematologic malignancies is usually urgent, and stem cell transplant and cellular immunotherapies that provide curative treatments cannot be delayed in many cases.
Enrollment in clinical trials will most likely be limited to those trials that are most likely to benefit the patient.
Ueda noted that, because their patients come from all over the country, they are now conducting consultations for stem cell transplant by telephone so that nonlocal patients do not have to travel to Seattle. “If there is some way we can delay the treatment, we have taken that approach,” Ueda told Medscape Medical News. “If we can divert a patient to an area that is not as heavily affected, that’s another option we are taking.”
Although cancer surgery is not considered elective, surgical intervention needs to be prioritized, the authors comment. In the Seattle system, there is currently a 2-week ban on elective surgery in the healthcare system, owing to limited availability of personal protective equipment (PPE), staffing, and beds.
The oncology teams are currently reviewing treatment regimens to determine which treatments might lessen immunosuppression and which treatment options can be moved from the inpatient to the outpatient setting or can be delayed.
Inpatient Care
For hospitalized patients, several issues are being addressed. The priority is to prepare for an upcoming shortage of beds and resources because of the surge of patients with COVID-19 that is predicted. For both clinic and hospitalized patients, shortages of blood products have necessitated stricter adherence to thresholds for transfusion, and consideration is being given to lowering those thresholds.
Another important problem is the need to conserve PPE, which includes masks, gowns, gloves, and other products. The Seattle teams have implemented solutions such as favoring handwashing with soap and water over the use of hand gel for standard-precaution rooms, limiting the number of personnel entering patient rooms (so as to use less PPE), and reducing nursing procedures that require PPE, such as measuring urine output, unless they are necessary.
In addition, a no-visitor policy has been adopted in inpatient units to conserve PPE, with the exception of end-of-life situations.
The Future
The future trajectory of the COVID-19 pandemic is uncertain, Ueda commented. She emphasized that “we must continue to prepare for its widespread impact. The unknown is what we are looking at. We are expecting it to evolve, and the number of infections cannot go down.”
Ueda and coauthors end their article on a positive note. “To many of us, this has become the health care challenge of our generation, one that modern cancer therapy has never had to face. We will prevail, and when the pandemic ends, we will all be proud of what we did for our patients and each other in this critical moment for humanity.”
Two months after the first patient with COVID-19 was identified in China, the first case was reported in the United States in the Seattle, Washington, metropolitan area.
Seattle rapidly became the first US epicenter for COVID-19, and local experts are now offering their expertise and advice on how to provide optimal cancer care during the pandemic in a special feature published online March 20 in the Journal of the National Comprehensive Cancer Network.
“We began implementing measures in early March, including infection control and screening of visitors, staff, and patients at the door,” said lead author Masumi Ueda, MD, who holds positions at the Seattle Cancer Care Alliance, the University of Washington, and the Fred Hutchinson Research Center.
“A lot of changes have been implemented, and it changes on a daily basis. We are responding to the growing rate of COVID-19 infection in the community,” she told Medscape Medical News.
Ueda notes that as a result of the quick implementation of new procedures, so far, very few cancer patients at their facilities have been infected by the virus. “It has not hit our cancer population hard, which is a good thing,” she said.
Create “Incident Command Structure”
In sharing their experience, the authors emphasize the importance of keeping channels of communication open between all stakeholders ― administrators and staff, patients, caregivers, and the general public. They also recommend that each facility create an “incident command structure” that can provide early coordination of institution-wide efforts and that can rapidly respond to changing information.
Ueda noted that their command structure was set up very early on, “so we could get communication set up and start building an infrastructure for response.”
Several areas of care that required new strategies were addressed, both to protect patients and to work around staff shortages caused by possible exposure and/or school closings, as well as projected shortages of supplies and hospital resources.
First and foremost was to identify patients and visitors who had respiratory symptoms and to provide them with masks. Although this is always routine practice during the respiratory virus season, screening has now been initiated at entry points throughout the system.
“We were lucky in Seattle and Washington state in that the University of Washington virology lab developed PCR [polymerase chain reaction] testing early on for COVID-19, which subsequently got FDA approval,” said Ueda. “So we were able to have local testing and didn’t have to rely on the state lab. Testing has also been rapidly scaled up.”
Initiating a comprehensive policy for testing staff, tracking results and exposures for persons under investigation, and defining when it is possible to return to work are essential elements for maintaining a stable workforce. In addition, reinforcing a strict “stay at home when ill” policy and providing access to testing for symptomatic staff have been key to limiting exposures.
“What is unique to our region is that we had testing early on, and we are turning it around in 24 hours,” she pointed out. “This is important for staff to be able to return to work.” Currently, staff, patients, and visitors are being tested only if they show the cardinal symptoms associated with COVID-19: fever, shortness of breath, and cough, although muscle aches have recently been added to their testing protocol.
“I think if we had unlimited capacity, we might consider testing people who are asymptomatic,” Ueda noted, “although if you don’t have symptoms, you may not have the viral load needed for an accurate test.”
Educational materials explaining infection control were also needed for patients and families, along with signs and a website to provide COVID-19 education. These were quickly developed.
In addition, a telephone triage line was established for patients with mild symptoms in order to minimize exposures in clinics and to lessen the number of patients presenting at emergency departments.
Outpatient Care
Because theirs is a referral center, many cancer patients come from out of town, and so there is concern about exposing nonlocal patients to COVID-19 as the virus spreads in the Seattle area. In addition, staffing shortages due to factors such as illness, exposure, and school closures are anticipated.
To address these problems, an initial priority was to establish a “multilayer” coverage system for the clinics in the event that practitioners had to be quarantined on short notice, the authors explain.
One decision was to reschedule all wellness visits for current patients or to use telemedicine. Capacity for that option expanded quickly, which was greatly helped by the recent decision by the Centers for Medicare & Medicaid Services to lift Medicare restrictions on the use of certain telemedicine services.
Another approach is to defer all consultations for second opinions for patients who were already undergoing treatment and to increase clinic hours of operations and capabilities for acute evaluations. This helps reserve emergency departments and hospital resources for patients who require higher-level care, the authors comment.
Treatment Decisions
Treatment decisions were more challenging to make, the authors note. One decision was that, despite the risk for COVID-19 for patients with solid tumors, adjuvant therapy with curative intent should proceed, they note. Similarly, patients with metastatic disease might lose the window of opportunity for treatment if it is delayed.
Treatment for aggressive hematologic malignancies is usually urgent, and stem cell transplant and cellular immunotherapies that provide curative treatments cannot be delayed in many cases.
Enrollment in clinical trials will most likely be limited to those trials that are most likely to benefit the patient.
Ueda noted that, because their patients come from all over the country, they are now conducting consultations for stem cell transplant by telephone so that nonlocal patients do not have to travel to Seattle. “If there is some way we can delay the treatment, we have taken that approach,” Ueda told Medscape Medical News. “If we can divert a patient to an area that is not as heavily affected, that’s another option we are taking.”
Although cancer surgery is not considered elective, surgical intervention needs to be prioritized, the authors comment. In the Seattle system, there is currently a 2-week ban on elective surgery in the healthcare system, owing to limited availability of personal protective equipment (PPE), staffing, and beds.
The oncology teams are currently reviewing treatment regimens to determine which treatments might lessen immunosuppression and which treatment options can be moved from the inpatient to the outpatient setting or can be delayed.
Inpatient Care
For hospitalized patients, several issues are being addressed. The priority is to prepare for an upcoming shortage of beds and resources because of the surge of patients with COVID-19 that is predicted. For both clinic and hospitalized patients, shortages of blood products have necessitated stricter adherence to thresholds for transfusion, and consideration is being given to lowering those thresholds.
Another important problem is the need to conserve PPE, which includes masks, gowns, gloves, and other products. The Seattle teams have implemented solutions such as favoring handwashing with soap and water over the use of hand gel for standard-precaution rooms, limiting the number of personnel entering patient rooms (so as to use less PPE), and reducing nursing procedures that require PPE, such as measuring urine output, unless they are necessary.
In addition, a no-visitor policy has been adopted in inpatient units to conserve PPE, with the exception of end-of-life situations.
The Future
The future trajectory of the COVID-19 pandemic is uncertain, Ueda commented. She emphasized that “we must continue to prepare for its widespread impact. The unknown is what we are looking at. We are expecting it to evolve, and the number of infections cannot go down.”
Ueda and coauthors end their article on a positive note. “To many of us, this has become the health care challenge of our generation, one that modern cancer therapy has never had to face. We will prevail, and when the pandemic ends, we will all be proud of what we did for our patients and each other in this critical moment for humanity.”
Two months after the first patient with COVID-19 was identified in China, the first case was reported in the United States in the Seattle, Washington, metropolitan area.
Seattle rapidly became the first US epicenter for COVID-19, and local experts are now offering their expertise and advice on how to provide optimal cancer care during the pandemic in a special feature published online March 20 in the Journal of the National Comprehensive Cancer Network.
“We began implementing measures in early March, including infection control and screening of visitors, staff, and patients at the door,” said lead author Masumi Ueda, MD, who holds positions at the Seattle Cancer Care Alliance, the University of Washington, and the Fred Hutchinson Research Center.
“A lot of changes have been implemented, and it changes on a daily basis. We are responding to the growing rate of COVID-19 infection in the community,” she told Medscape Medical News.
Ueda notes that as a result of the quick implementation of new procedures, so far, very few cancer patients at their facilities have been infected by the virus. “It has not hit our cancer population hard, which is a good thing,” she said.
Create “Incident Command Structure”
In sharing their experience, the authors emphasize the importance of keeping channels of communication open between all stakeholders ― administrators and staff, patients, caregivers, and the general public. They also recommend that each facility create an “incident command structure” that can provide early coordination of institution-wide efforts and that can rapidly respond to changing information.
Ueda noted that their command structure was set up very early on, “so we could get communication set up and start building an infrastructure for response.”
Several areas of care that required new strategies were addressed, both to protect patients and to work around staff shortages caused by possible exposure and/or school closings, as well as projected shortages of supplies and hospital resources.
First and foremost was to identify patients and visitors who had respiratory symptoms and to provide them with masks. Although this is always routine practice during the respiratory virus season, screening has now been initiated at entry points throughout the system.
“We were lucky in Seattle and Washington state in that the University of Washington virology lab developed PCR [polymerase chain reaction] testing early on for COVID-19, which subsequently got FDA approval,” said Ueda. “So we were able to have local testing and didn’t have to rely on the state lab. Testing has also been rapidly scaled up.”
Initiating a comprehensive policy for testing staff, tracking results and exposures for persons under investigation, and defining when it is possible to return to work are essential elements for maintaining a stable workforce. In addition, reinforcing a strict “stay at home when ill” policy and providing access to testing for symptomatic staff have been key to limiting exposures.
“What is unique to our region is that we had testing early on, and we are turning it around in 24 hours,” she pointed out. “This is important for staff to be able to return to work.” Currently, staff, patients, and visitors are being tested only if they show the cardinal symptoms associated with COVID-19: fever, shortness of breath, and cough, although muscle aches have recently been added to their testing protocol.
“I think if we had unlimited capacity, we might consider testing people who are asymptomatic,” Ueda noted, “although if you don’t have symptoms, you may not have the viral load needed for an accurate test.”
Educational materials explaining infection control were also needed for patients and families, along with signs and a website to provide COVID-19 education. These were quickly developed.
In addition, a telephone triage line was established for patients with mild symptoms in order to minimize exposures in clinics and to lessen the number of patients presenting at emergency departments.
Outpatient Care
Because theirs is a referral center, many cancer patients come from out of town, and so there is concern about exposing nonlocal patients to COVID-19 as the virus spreads in the Seattle area. In addition, staffing shortages due to factors such as illness, exposure, and school closures are anticipated.
To address these problems, an initial priority was to establish a “multilayer” coverage system for the clinics in the event that practitioners had to be quarantined on short notice, the authors explain.
One decision was to reschedule all wellness visits for current patients or to use telemedicine. Capacity for that option expanded quickly, which was greatly helped by the recent decision by the Centers for Medicare & Medicaid Services to lift Medicare restrictions on the use of certain telemedicine services.
Another approach is to defer all consultations for second opinions for patients who were already undergoing treatment and to increase clinic hours of operations and capabilities for acute evaluations. This helps reserve emergency departments and hospital resources for patients who require higher-level care, the authors comment.
Treatment Decisions
Treatment decisions were more challenging to make, the authors note. One decision was that, despite the risk for COVID-19 for patients with solid tumors, adjuvant therapy with curative intent should proceed, they note. Similarly, patients with metastatic disease might lose the window of opportunity for treatment if it is delayed.
Treatment for aggressive hematologic malignancies is usually urgent, and stem cell transplant and cellular immunotherapies that provide curative treatments cannot be delayed in many cases.
Enrollment in clinical trials will most likely be limited to those trials that are most likely to benefit the patient.
Ueda noted that, because their patients come from all over the country, they are now conducting consultations for stem cell transplant by telephone so that nonlocal patients do not have to travel to Seattle. “If there is some way we can delay the treatment, we have taken that approach,” Ueda told Medscape Medical News. “If we can divert a patient to an area that is not as heavily affected, that’s another option we are taking.”
Although cancer surgery is not considered elective, surgical intervention needs to be prioritized, the authors comment. In the Seattle system, there is currently a 2-week ban on elective surgery in the healthcare system, owing to limited availability of personal protective equipment (PPE), staffing, and beds.
The oncology teams are currently reviewing treatment regimens to determine which treatments might lessen immunosuppression and which treatment options can be moved from the inpatient to the outpatient setting or can be delayed.
Inpatient Care
For hospitalized patients, several issues are being addressed. The priority is to prepare for an upcoming shortage of beds and resources because of the surge of patients with COVID-19 that is predicted. For both clinic and hospitalized patients, shortages of blood products have necessitated stricter adherence to thresholds for transfusion, and consideration is being given to lowering those thresholds.
Another important problem is the need to conserve PPE, which includes masks, gowns, gloves, and other products. The Seattle teams have implemented solutions such as favoring handwashing with soap and water over the use of hand gel for standard-precaution rooms, limiting the number of personnel entering patient rooms (so as to use less PPE), and reducing nursing procedures that require PPE, such as measuring urine output, unless they are necessary.
In addition, a no-visitor policy has been adopted in inpatient units to conserve PPE, with the exception of end-of-life situations.
The Future
The future trajectory of the COVID-19 pandemic is uncertain, Ueda commented. She emphasized that “we must continue to prepare for its widespread impact. The unknown is what we are looking at. We are expecting it to evolve, and the number of infections cannot go down.”
Ueda and coauthors end their article on a positive note. “To many of us, this has become the health care challenge of our generation, one that modern cancer therapy has never had to face. We will prevail, and when the pandemic ends, we will all be proud of what we did for our patients and each other in this critical moment for humanity.”
7 tips for running your practice in the coronavirus crisis
At one large practice in Bergen County, New Jersey, the waiting room is empty — but its patients are still receiving care. As of mid-March, the practice is still operating, thanks to the group’s willingness to adapt its work flow, sometimes radically, to mitigate the threat of the COVID-19 pandemic.
For example, patients now call the receptionist from their vehicles when they arrive, and wait there until receiving a call back telling them the clinician is ready. The practice has also started using telemedicine for the first time, to the extent it can be adopted in a hurry, and some clinicians are working from home on tasks such as medication refills.
Still, the rapidly increasing numbers of COVID-19 cases in the United States raises the possibility that some physician offices will decide or be forced to close temporarily, as occurred in London last month.
Many practices across the country are having to adjust the way they operate, amid daily changes in the pandemic. What should you do to adapt to this new way of operating your practice?
1. Create a task force to manage change
The readiness of medical practices to address the myriad challenges posed by this crisis has so far been a mixed bag, said Owen Dahl, MBA, a Texas-based medical practice management consultant. “Leadership is going to have to assess what’s happening in the community, what’s happening with staff members who may or may not have the disease and may or may not have to self-quarantine,” Dahl said.
The physicians, the administrator, CEO, or managing partner should be involved in decision making as the global crisis unfolds, added Laurie Morgan, MBA, a California-based practice management consultant. And depending on the size of the practice, it may be useful to delegate specific components of this work to various department managers or other individuals in the group.
The team should assess:
- Recommendations and/or mandates from local, state, and federal governments
- Guidance from specialty and state medical societies
- How to triage patients over the phone, virtual visits, or referral to an alternate site of care
- Where to send patients for testing
- The practice’s inventory of personal protective equipment (PPE)
- Review of and possible revision of current infection control policies
- Possible collaborations within the community
- Reimbursement policies for suspected COVID-19 triage, testing, and follow-up treatment — in office or virtually
- Whether some employees’ work (eg, billing, coding) can be done remotely
- Options for paying personnel in the case of a temporary shutdown
- What’s covered and excluded by the group’s business interruption insurance
2. Consider postponing nonessential appointments
What’s more, it’s crucial for practices to form a strategy that does not involve bringing patients into the office, said Javeed Siddiqui, MD, MPH, an infectious disease physician, epidemiologist, and chief medical officer of TeleMed2U. “One thing we really have to recognize in this pandemic is that we don’t want people going and sitting in our waiting room. We don’t want people coming, and not only exposing other patients, but also further exposing staff. Forward triaging is going to be essential in this type of pandemic.”
Reliant Medical Group, with multiple locations in Massachusetts, for example, announced to patients recently that it will postpone appointments for some routine and elective procedures, as determined by the group’s physicians and clinical staff.
“Taking this step will help limit the number of people passing through our facilities, which will help slow the spread of illness [as recommended by the CDC],” noted an email blast to patients.
3. Overcommunicate to patients
With a situation as dynamic and unprecedented as this, constant and clear communication with patients is crucial. “In general, in my experience, practices don’t realize how much communication is necessary,” said Morgan. “In order to be effective and get the word out, you have to be overcommunicating.”
Today’s practices have multiple ways to communicate to keep people informed, including email, text messaging, social media, patient portals, and even local television and radio.
One email or text message to the patient population can help direct them to the appropriate streams of information. Helping direct patients to updated information is critical.
In contrast, having the front desk field multitudes of calls from concerned patients ties up precious resources, according Siddiqui. “Right now, practices are absolutely inundated, patients are waiting on hold, and that creates a great deal of frustration,” he said.
“We really need to take a page from every other industry in the United States, and that is using secure SMS, email communication, and telehealth,” Siddiqui said. “Healthcare generally tends to be a laggard in this because so many people think, ‘Well, you can’t do that in healthcare,’ as opposed to thinking, ‘How can we do that in healthcare?’”
4. Take advantage of telemedicine
Fortunately, technology to interact with patients remotely is almost ubiquitous. Even for practices with little experience in this arena, various vendors exist that can get secure, HIPAA-compliant technologies up and running quickly.
Various payers have issued guidance regarding reimbursement for telemedicine specific to COVID-19, and on March 6, Congress passed a law regarding Medicare coverage and payment for virtual services during a government-declared state of emergency. Some of the rules about HIPAA compliance in telemedicine have been eased for this emergency.
But even with well-established telemedicine modalities in place, it’s crunch time for applying it to COVID-19. “You need to find a way to have telemedicine available and use it, because depending on how this goes, that’s going to be clearly the safest, best way to care for a huge number of people,” said Darryl Elmouchi, MD, MBA, chief medical officer of Spectrum Health System and president of Spectrum Health Medical Group in Michigan.
“What we recognize now, both with our past experience with telehealth for many years and specifically with this coronavirus testing we’ve done, is that it’s incredibly useful both for the clinicians and the patients,” Elmouchi said.
One possibility to consider is the tactic used by Spectrum, a large integrated healthcare system. The company mobilized its existing telemedicine program to offer free virtual screenings for anyone in Michigan showing possible symptoms of COVID-19. “We wanted to keep people out of our clinics, emergency rooms, and urgent care centers if they didn’t need to be there, and help allay fears,” he said.
Elmouchi said his company faced the problems that other physicians would also have to deal with. “It was a ton of work with a dedicated team that was focused on this. The hardest part was probably trying to determine how we can staff it,” he said.
With their dedicated virtual team still seeing regularly scheduled virtual patients, the system had to reassign its traditional teams, such as urgent care and primary care clinicians, to the virtual screening effort. “Then we had to figure out how we could operationalize it. It was a lot of work,” Elmouchi said.
Telemedicine capabilities are not limited to screening patients, but can also be used to stay in touch with patients who may be quarantined and provide follow-up care, he noted.
5. Identify COVID-19 testing sites
Access to tests remains a problem in the US, but is improving by the week. For practices that can attain the tests themselves, it will still require some creativity to administer them with as little risk as possible. In South Korea, for example, and increasingly in the United States, healthcare organizations are instructing patients waiting to be tested to stay in their cars and have a practitioner wearing the proper PPE go out to patients to test them there.
Alternatively, some practices may opt to have PPE-wearing staff members bring PPE to patients in their cars and then escort them to a designated testing area in the building —through the back door if noninfected patients are still being seen.
“Once in the office, you still need to isolate virus patients in any way you can,” Dahl said. “In fact, you want a negative-pressure environment if possible, with the air being sucked out rather than circulating,” he said, adding that a large restroom with a ventilation system could be repurposed as a makeshift exam room.
Community testing sites are another possibility, given proper coordination with other healthcare organizations and community officials. Siddiqui has been working with several communities in which individual clinics and hospitals are unable to handle testing on their own, and have instead collaborated to create community testing sites in tents on local athletic fields.
“One of our communities is looking at using the local college parking lot to do drive-through testing there,” he said. “We really need to embrace collaboration much more than we’ve ever done.”
Collaboration also requires sharing supplies and PPE, noted Dahl. “Don’t hoard them because of the shortage. Look at your inventory and make sure you can help out whomever you may be sending patients to.” And if your office is falling short, Dahl advises checking with offices in your community that may be closing — such as dentists or plastic surgeons — for supplies you can purchase or simply have.
The US Food and Drug Administration has issued some guidance to healthcare providers about shortages of surgical masks and gowns, including advice about reusable cloth alternatives to gowns.
In addition, some hospitals have asked clinicians to keep their masks and provided guidance on how to conserve supplies.
6. Preparing to potentially shut down
A temporary closure may be inevitable for some practices. “Maybe the physician owners will not feel like they have a choice,” said Morgan. “They might feel like they want to stay open for as long as they can; but if it’s not safe for patients or not safe for employees, maybe they’ll feel it’s better if they check out for a bit.”
Should practices make the decision to close or reduce hours, multimodal communication with patients and the public is paramount. Patients will want to know whom to call if they are feeling ill for any reason, where to seek care, and when the practice expects to reopen. Again, proactive outreach will be more efficient and comforting to patients.
Handling financial ramifications of closure is a top priority as well, and will require a full understanding of what is and isn’t covered by the practice’s business interruption insurance. Practices that don’t have a line of credit should reach out to banks and the Small Business Administration immediately, according to Dahl. Practices that have lines of credit already may want to ask for an increase, added Morgan.
Protecting employees’ income is challenging as well. For employees who are furloughed, consider allowing them to use their sick and vacation time during the shutdown — and possibly let staff ‘borrow’ not-yet accrued paid time off.
“However, there’s a risk with certain jobs in a medical practice that tend to have extremely high turnover, so physicians and administrators may be reluctant to pay people too much because they don’t know for sure those employees will come back to those jobs,” Morgan said. “On the other hand, if you have had a stable team for a very long time and feel confident that those employees are going to stay, then you may make a different decision.”
7. Seize work-from-home opportunities
Even if the practice isn’t seeing patients, there may be opportunities for some employees, such as billers and schedulers, to continue to work from home,” Morgan noted. Particularly if a practice is behind on its billing, a closure or slowdown is an ideal time to catch up. This measure will keep at least some people working — perhaps including some individuals who can be cross-trained to do other tasks — and maintain some cashflow when the practice needs it most.
Other remote-friendly jobs that often fall by the wayside when practices are busy include marketing tasks such as setting up or updating Google business pages, Healthgrades profiles, and so on, noted Morgan.
“Another thing that can be even more important, and is often overlooked, is making sure health plan directories have correct information about your practice,” she added. “These are pesky, often tedious tasks that may require repeated contact with health plans to fix things — perfect things to do when the office is not busy or closed.”
For administrators and billers, if the practice is able to keep paying these employees while partially or fully closed, it can also be an excellent time to do the sort of analysis that takes a lot of focused attention and is hard to do when busy. Some examples: a detailed comparison of payer performance, analysis of referral patterns, or a review of coding accuracy, Morgan suggested.
Although practices have varying levels of comfort in letting employees work from home, it’s not much different from working with external billing or scheduling services that have grown more popular in recent years, Morgan said.
As with many technologies, HIPAA is a leading concern, though it needn’t be, according to Morgan. “If you are on a cloud-based electronic medical record and practice management system, there’s a good chance that it’s very straightforward to set someone up to work from elsewhere and have that data be secure,” she said.
Finally, as the crisis begins to abate, practices must keep working in teams to evaluate and structure an orderly return to business as usual, gleaning best practices from colleagues whenever possible.
“I would tell practices this is not a time when anyone is competing with anyone,” said Elmouchi. “The more collaboration between practices and health systems that have larger resources, the better.”
This article was originally published on Medscape.com.
At one large practice in Bergen County, New Jersey, the waiting room is empty — but its patients are still receiving care. As of mid-March, the practice is still operating, thanks to the group’s willingness to adapt its work flow, sometimes radically, to mitigate the threat of the COVID-19 pandemic.
For example, patients now call the receptionist from their vehicles when they arrive, and wait there until receiving a call back telling them the clinician is ready. The practice has also started using telemedicine for the first time, to the extent it can be adopted in a hurry, and some clinicians are working from home on tasks such as medication refills.
Still, the rapidly increasing numbers of COVID-19 cases in the United States raises the possibility that some physician offices will decide or be forced to close temporarily, as occurred in London last month.
Many practices across the country are having to adjust the way they operate, amid daily changes in the pandemic. What should you do to adapt to this new way of operating your practice?
1. Create a task force to manage change
The readiness of medical practices to address the myriad challenges posed by this crisis has so far been a mixed bag, said Owen Dahl, MBA, a Texas-based medical practice management consultant. “Leadership is going to have to assess what’s happening in the community, what’s happening with staff members who may or may not have the disease and may or may not have to self-quarantine,” Dahl said.
The physicians, the administrator, CEO, or managing partner should be involved in decision making as the global crisis unfolds, added Laurie Morgan, MBA, a California-based practice management consultant. And depending on the size of the practice, it may be useful to delegate specific components of this work to various department managers or other individuals in the group.
The team should assess:
- Recommendations and/or mandates from local, state, and federal governments
- Guidance from specialty and state medical societies
- How to triage patients over the phone, virtual visits, or referral to an alternate site of care
- Where to send patients for testing
- The practice’s inventory of personal protective equipment (PPE)
- Review of and possible revision of current infection control policies
- Possible collaborations within the community
- Reimbursement policies for suspected COVID-19 triage, testing, and follow-up treatment — in office or virtually
- Whether some employees’ work (eg, billing, coding) can be done remotely
- Options for paying personnel in the case of a temporary shutdown
- What’s covered and excluded by the group’s business interruption insurance
2. Consider postponing nonessential appointments
What’s more, it’s crucial for practices to form a strategy that does not involve bringing patients into the office, said Javeed Siddiqui, MD, MPH, an infectious disease physician, epidemiologist, and chief medical officer of TeleMed2U. “One thing we really have to recognize in this pandemic is that we don’t want people going and sitting in our waiting room. We don’t want people coming, and not only exposing other patients, but also further exposing staff. Forward triaging is going to be essential in this type of pandemic.”
Reliant Medical Group, with multiple locations in Massachusetts, for example, announced to patients recently that it will postpone appointments for some routine and elective procedures, as determined by the group’s physicians and clinical staff.
“Taking this step will help limit the number of people passing through our facilities, which will help slow the spread of illness [as recommended by the CDC],” noted an email blast to patients.
3. Overcommunicate to patients
With a situation as dynamic and unprecedented as this, constant and clear communication with patients is crucial. “In general, in my experience, practices don’t realize how much communication is necessary,” said Morgan. “In order to be effective and get the word out, you have to be overcommunicating.”
Today’s practices have multiple ways to communicate to keep people informed, including email, text messaging, social media, patient portals, and even local television and radio.
One email or text message to the patient population can help direct them to the appropriate streams of information. Helping direct patients to updated information is critical.
In contrast, having the front desk field multitudes of calls from concerned patients ties up precious resources, according Siddiqui. “Right now, practices are absolutely inundated, patients are waiting on hold, and that creates a great deal of frustration,” he said.
“We really need to take a page from every other industry in the United States, and that is using secure SMS, email communication, and telehealth,” Siddiqui said. “Healthcare generally tends to be a laggard in this because so many people think, ‘Well, you can’t do that in healthcare,’ as opposed to thinking, ‘How can we do that in healthcare?’”
4. Take advantage of telemedicine
Fortunately, technology to interact with patients remotely is almost ubiquitous. Even for practices with little experience in this arena, various vendors exist that can get secure, HIPAA-compliant technologies up and running quickly.
Various payers have issued guidance regarding reimbursement for telemedicine specific to COVID-19, and on March 6, Congress passed a law regarding Medicare coverage and payment for virtual services during a government-declared state of emergency. Some of the rules about HIPAA compliance in telemedicine have been eased for this emergency.
But even with well-established telemedicine modalities in place, it’s crunch time for applying it to COVID-19. “You need to find a way to have telemedicine available and use it, because depending on how this goes, that’s going to be clearly the safest, best way to care for a huge number of people,” said Darryl Elmouchi, MD, MBA, chief medical officer of Spectrum Health System and president of Spectrum Health Medical Group in Michigan.
“What we recognize now, both with our past experience with telehealth for many years and specifically with this coronavirus testing we’ve done, is that it’s incredibly useful both for the clinicians and the patients,” Elmouchi said.
One possibility to consider is the tactic used by Spectrum, a large integrated healthcare system. The company mobilized its existing telemedicine program to offer free virtual screenings for anyone in Michigan showing possible symptoms of COVID-19. “We wanted to keep people out of our clinics, emergency rooms, and urgent care centers if they didn’t need to be there, and help allay fears,” he said.
Elmouchi said his company faced the problems that other physicians would also have to deal with. “It was a ton of work with a dedicated team that was focused on this. The hardest part was probably trying to determine how we can staff it,” he said.
With their dedicated virtual team still seeing regularly scheduled virtual patients, the system had to reassign its traditional teams, such as urgent care and primary care clinicians, to the virtual screening effort. “Then we had to figure out how we could operationalize it. It was a lot of work,” Elmouchi said.
Telemedicine capabilities are not limited to screening patients, but can also be used to stay in touch with patients who may be quarantined and provide follow-up care, he noted.
5. Identify COVID-19 testing sites
Access to tests remains a problem in the US, but is improving by the week. For practices that can attain the tests themselves, it will still require some creativity to administer them with as little risk as possible. In South Korea, for example, and increasingly in the United States, healthcare organizations are instructing patients waiting to be tested to stay in their cars and have a practitioner wearing the proper PPE go out to patients to test them there.
Alternatively, some practices may opt to have PPE-wearing staff members bring PPE to patients in their cars and then escort them to a designated testing area in the building —through the back door if noninfected patients are still being seen.
“Once in the office, you still need to isolate virus patients in any way you can,” Dahl said. “In fact, you want a negative-pressure environment if possible, with the air being sucked out rather than circulating,” he said, adding that a large restroom with a ventilation system could be repurposed as a makeshift exam room.
Community testing sites are another possibility, given proper coordination with other healthcare organizations and community officials. Siddiqui has been working with several communities in which individual clinics and hospitals are unable to handle testing on their own, and have instead collaborated to create community testing sites in tents on local athletic fields.
“One of our communities is looking at using the local college parking lot to do drive-through testing there,” he said. “We really need to embrace collaboration much more than we’ve ever done.”
Collaboration also requires sharing supplies and PPE, noted Dahl. “Don’t hoard them because of the shortage. Look at your inventory and make sure you can help out whomever you may be sending patients to.” And if your office is falling short, Dahl advises checking with offices in your community that may be closing — such as dentists or plastic surgeons — for supplies you can purchase or simply have.
The US Food and Drug Administration has issued some guidance to healthcare providers about shortages of surgical masks and gowns, including advice about reusable cloth alternatives to gowns.
In addition, some hospitals have asked clinicians to keep their masks and provided guidance on how to conserve supplies.
6. Preparing to potentially shut down
A temporary closure may be inevitable for some practices. “Maybe the physician owners will not feel like they have a choice,” said Morgan. “They might feel like they want to stay open for as long as they can; but if it’s not safe for patients or not safe for employees, maybe they’ll feel it’s better if they check out for a bit.”
Should practices make the decision to close or reduce hours, multimodal communication with patients and the public is paramount. Patients will want to know whom to call if they are feeling ill for any reason, where to seek care, and when the practice expects to reopen. Again, proactive outreach will be more efficient and comforting to patients.
Handling financial ramifications of closure is a top priority as well, and will require a full understanding of what is and isn’t covered by the practice’s business interruption insurance. Practices that don’t have a line of credit should reach out to banks and the Small Business Administration immediately, according to Dahl. Practices that have lines of credit already may want to ask for an increase, added Morgan.
Protecting employees’ income is challenging as well. For employees who are furloughed, consider allowing them to use their sick and vacation time during the shutdown — and possibly let staff ‘borrow’ not-yet accrued paid time off.
“However, there’s a risk with certain jobs in a medical practice that tend to have extremely high turnover, so physicians and administrators may be reluctant to pay people too much because they don’t know for sure those employees will come back to those jobs,” Morgan said. “On the other hand, if you have had a stable team for a very long time and feel confident that those employees are going to stay, then you may make a different decision.”
7. Seize work-from-home opportunities
Even if the practice isn’t seeing patients, there may be opportunities for some employees, such as billers and schedulers, to continue to work from home,” Morgan noted. Particularly if a practice is behind on its billing, a closure or slowdown is an ideal time to catch up. This measure will keep at least some people working — perhaps including some individuals who can be cross-trained to do other tasks — and maintain some cashflow when the practice needs it most.
Other remote-friendly jobs that often fall by the wayside when practices are busy include marketing tasks such as setting up or updating Google business pages, Healthgrades profiles, and so on, noted Morgan.
“Another thing that can be even more important, and is often overlooked, is making sure health plan directories have correct information about your practice,” she added. “These are pesky, often tedious tasks that may require repeated contact with health plans to fix things — perfect things to do when the office is not busy or closed.”
For administrators and billers, if the practice is able to keep paying these employees while partially or fully closed, it can also be an excellent time to do the sort of analysis that takes a lot of focused attention and is hard to do when busy. Some examples: a detailed comparison of payer performance, analysis of referral patterns, or a review of coding accuracy, Morgan suggested.
Although practices have varying levels of comfort in letting employees work from home, it’s not much different from working with external billing or scheduling services that have grown more popular in recent years, Morgan said.
As with many technologies, HIPAA is a leading concern, though it needn’t be, according to Morgan. “If you are on a cloud-based electronic medical record and practice management system, there’s a good chance that it’s very straightforward to set someone up to work from elsewhere and have that data be secure,” she said.
Finally, as the crisis begins to abate, practices must keep working in teams to evaluate and structure an orderly return to business as usual, gleaning best practices from colleagues whenever possible.
“I would tell practices this is not a time when anyone is competing with anyone,” said Elmouchi. “The more collaboration between practices and health systems that have larger resources, the better.”
This article was originally published on Medscape.com.
At one large practice in Bergen County, New Jersey, the waiting room is empty — but its patients are still receiving care. As of mid-March, the practice is still operating, thanks to the group’s willingness to adapt its work flow, sometimes radically, to mitigate the threat of the COVID-19 pandemic.
For example, patients now call the receptionist from their vehicles when they arrive, and wait there until receiving a call back telling them the clinician is ready. The practice has also started using telemedicine for the first time, to the extent it can be adopted in a hurry, and some clinicians are working from home on tasks such as medication refills.
Still, the rapidly increasing numbers of COVID-19 cases in the United States raises the possibility that some physician offices will decide or be forced to close temporarily, as occurred in London last month.
Many practices across the country are having to adjust the way they operate, amid daily changes in the pandemic. What should you do to adapt to this new way of operating your practice?
1. Create a task force to manage change
The readiness of medical practices to address the myriad challenges posed by this crisis has so far been a mixed bag, said Owen Dahl, MBA, a Texas-based medical practice management consultant. “Leadership is going to have to assess what’s happening in the community, what’s happening with staff members who may or may not have the disease and may or may not have to self-quarantine,” Dahl said.
The physicians, the administrator, CEO, or managing partner should be involved in decision making as the global crisis unfolds, added Laurie Morgan, MBA, a California-based practice management consultant. And depending on the size of the practice, it may be useful to delegate specific components of this work to various department managers or other individuals in the group.
The team should assess:
- Recommendations and/or mandates from local, state, and federal governments
- Guidance from specialty and state medical societies
- How to triage patients over the phone, virtual visits, or referral to an alternate site of care
- Where to send patients for testing
- The practice’s inventory of personal protective equipment (PPE)
- Review of and possible revision of current infection control policies
- Possible collaborations within the community
- Reimbursement policies for suspected COVID-19 triage, testing, and follow-up treatment — in office or virtually
- Whether some employees’ work (eg, billing, coding) can be done remotely
- Options for paying personnel in the case of a temporary shutdown
- What’s covered and excluded by the group’s business interruption insurance
2. Consider postponing nonessential appointments
What’s more, it’s crucial for practices to form a strategy that does not involve bringing patients into the office, said Javeed Siddiqui, MD, MPH, an infectious disease physician, epidemiologist, and chief medical officer of TeleMed2U. “One thing we really have to recognize in this pandemic is that we don’t want people going and sitting in our waiting room. We don’t want people coming, and not only exposing other patients, but also further exposing staff. Forward triaging is going to be essential in this type of pandemic.”
Reliant Medical Group, with multiple locations in Massachusetts, for example, announced to patients recently that it will postpone appointments for some routine and elective procedures, as determined by the group’s physicians and clinical staff.
“Taking this step will help limit the number of people passing through our facilities, which will help slow the spread of illness [as recommended by the CDC],” noted an email blast to patients.
3. Overcommunicate to patients
With a situation as dynamic and unprecedented as this, constant and clear communication with patients is crucial. “In general, in my experience, practices don’t realize how much communication is necessary,” said Morgan. “In order to be effective and get the word out, you have to be overcommunicating.”
Today’s practices have multiple ways to communicate to keep people informed, including email, text messaging, social media, patient portals, and even local television and radio.
One email or text message to the patient population can help direct them to the appropriate streams of information. Helping direct patients to updated information is critical.
In contrast, having the front desk field multitudes of calls from concerned patients ties up precious resources, according Siddiqui. “Right now, practices are absolutely inundated, patients are waiting on hold, and that creates a great deal of frustration,” he said.
“We really need to take a page from every other industry in the United States, and that is using secure SMS, email communication, and telehealth,” Siddiqui said. “Healthcare generally tends to be a laggard in this because so many people think, ‘Well, you can’t do that in healthcare,’ as opposed to thinking, ‘How can we do that in healthcare?’”
4. Take advantage of telemedicine
Fortunately, technology to interact with patients remotely is almost ubiquitous. Even for practices with little experience in this arena, various vendors exist that can get secure, HIPAA-compliant technologies up and running quickly.
Various payers have issued guidance regarding reimbursement for telemedicine specific to COVID-19, and on March 6, Congress passed a law regarding Medicare coverage and payment for virtual services during a government-declared state of emergency. Some of the rules about HIPAA compliance in telemedicine have been eased for this emergency.
But even with well-established telemedicine modalities in place, it’s crunch time for applying it to COVID-19. “You need to find a way to have telemedicine available and use it, because depending on how this goes, that’s going to be clearly the safest, best way to care for a huge number of people,” said Darryl Elmouchi, MD, MBA, chief medical officer of Spectrum Health System and president of Spectrum Health Medical Group in Michigan.
“What we recognize now, both with our past experience with telehealth for many years and specifically with this coronavirus testing we’ve done, is that it’s incredibly useful both for the clinicians and the patients,” Elmouchi said.
One possibility to consider is the tactic used by Spectrum, a large integrated healthcare system. The company mobilized its existing telemedicine program to offer free virtual screenings for anyone in Michigan showing possible symptoms of COVID-19. “We wanted to keep people out of our clinics, emergency rooms, and urgent care centers if they didn’t need to be there, and help allay fears,” he said.
Elmouchi said his company faced the problems that other physicians would also have to deal with. “It was a ton of work with a dedicated team that was focused on this. The hardest part was probably trying to determine how we can staff it,” he said.
With their dedicated virtual team still seeing regularly scheduled virtual patients, the system had to reassign its traditional teams, such as urgent care and primary care clinicians, to the virtual screening effort. “Then we had to figure out how we could operationalize it. It was a lot of work,” Elmouchi said.
Telemedicine capabilities are not limited to screening patients, but can also be used to stay in touch with patients who may be quarantined and provide follow-up care, he noted.
5. Identify COVID-19 testing sites
Access to tests remains a problem in the US, but is improving by the week. For practices that can attain the tests themselves, it will still require some creativity to administer them with as little risk as possible. In South Korea, for example, and increasingly in the United States, healthcare organizations are instructing patients waiting to be tested to stay in their cars and have a practitioner wearing the proper PPE go out to patients to test them there.
Alternatively, some practices may opt to have PPE-wearing staff members bring PPE to patients in their cars and then escort them to a designated testing area in the building —through the back door if noninfected patients are still being seen.
“Once in the office, you still need to isolate virus patients in any way you can,” Dahl said. “In fact, you want a negative-pressure environment if possible, with the air being sucked out rather than circulating,” he said, adding that a large restroom with a ventilation system could be repurposed as a makeshift exam room.
Community testing sites are another possibility, given proper coordination with other healthcare organizations and community officials. Siddiqui has been working with several communities in which individual clinics and hospitals are unable to handle testing on their own, and have instead collaborated to create community testing sites in tents on local athletic fields.
“One of our communities is looking at using the local college parking lot to do drive-through testing there,” he said. “We really need to embrace collaboration much more than we’ve ever done.”
Collaboration also requires sharing supplies and PPE, noted Dahl. “Don’t hoard them because of the shortage. Look at your inventory and make sure you can help out whomever you may be sending patients to.” And if your office is falling short, Dahl advises checking with offices in your community that may be closing — such as dentists or plastic surgeons — for supplies you can purchase or simply have.
The US Food and Drug Administration has issued some guidance to healthcare providers about shortages of surgical masks and gowns, including advice about reusable cloth alternatives to gowns.
In addition, some hospitals have asked clinicians to keep their masks and provided guidance on how to conserve supplies.
6. Preparing to potentially shut down
A temporary closure may be inevitable for some practices. “Maybe the physician owners will not feel like they have a choice,” said Morgan. “They might feel like they want to stay open for as long as they can; but if it’s not safe for patients or not safe for employees, maybe they’ll feel it’s better if they check out for a bit.”
Should practices make the decision to close or reduce hours, multimodal communication with patients and the public is paramount. Patients will want to know whom to call if they are feeling ill for any reason, where to seek care, and when the practice expects to reopen. Again, proactive outreach will be more efficient and comforting to patients.
Handling financial ramifications of closure is a top priority as well, and will require a full understanding of what is and isn’t covered by the practice’s business interruption insurance. Practices that don’t have a line of credit should reach out to banks and the Small Business Administration immediately, according to Dahl. Practices that have lines of credit already may want to ask for an increase, added Morgan.
Protecting employees’ income is challenging as well. For employees who are furloughed, consider allowing them to use their sick and vacation time during the shutdown — and possibly let staff ‘borrow’ not-yet accrued paid time off.
“However, there’s a risk with certain jobs in a medical practice that tend to have extremely high turnover, so physicians and administrators may be reluctant to pay people too much because they don’t know for sure those employees will come back to those jobs,” Morgan said. “On the other hand, if you have had a stable team for a very long time and feel confident that those employees are going to stay, then you may make a different decision.”
7. Seize work-from-home opportunities
Even if the practice isn’t seeing patients, there may be opportunities for some employees, such as billers and schedulers, to continue to work from home,” Morgan noted. Particularly if a practice is behind on its billing, a closure or slowdown is an ideal time to catch up. This measure will keep at least some people working — perhaps including some individuals who can be cross-trained to do other tasks — and maintain some cashflow when the practice needs it most.
Other remote-friendly jobs that often fall by the wayside when practices are busy include marketing tasks such as setting up or updating Google business pages, Healthgrades profiles, and so on, noted Morgan.
“Another thing that can be even more important, and is often overlooked, is making sure health plan directories have correct information about your practice,” she added. “These are pesky, often tedious tasks that may require repeated contact with health plans to fix things — perfect things to do when the office is not busy or closed.”
For administrators and billers, if the practice is able to keep paying these employees while partially or fully closed, it can also be an excellent time to do the sort of analysis that takes a lot of focused attention and is hard to do when busy. Some examples: a detailed comparison of payer performance, analysis of referral patterns, or a review of coding accuracy, Morgan suggested.
Although practices have varying levels of comfort in letting employees work from home, it’s not much different from working with external billing or scheduling services that have grown more popular in recent years, Morgan said.
As with many technologies, HIPAA is a leading concern, though it needn’t be, according to Morgan. “If you are on a cloud-based electronic medical record and practice management system, there’s a good chance that it’s very straightforward to set someone up to work from elsewhere and have that data be secure,” she said.
Finally, as the crisis begins to abate, practices must keep working in teams to evaluate and structure an orderly return to business as usual, gleaning best practices from colleagues whenever possible.
“I would tell practices this is not a time when anyone is competing with anyone,” said Elmouchi. “The more collaboration between practices and health systems that have larger resources, the better.”
This article was originally published on Medscape.com.











