User login
GI cancer death disproportional to incidence rates
Cancers of the gastrointestinal tract represented about one in four cancer cases in 2018 but more than one in three cancer-related deaths, underscoring the importance of preventive measures, according to an analysis of global cancer datasets that identified trends in five major GI cancer types – esophageal, stomach, colorectal, liver, and pancreatic – published in Gastroenterology.
“Although the incidence of some GI cancer types has decreased, this group of malignancies continues to pose major challenges to public health,” wrote Melina Arnold, PhD, of the International for Research on Cancer in Lyon, France, and colleagues. “Primary and secondary prevention measures are important for controlling these malignancies – most importantly, reducing consumption of tobacco and alcohol, obesity control, immunizing populations against hepatitis B virus infection, and screening for colorectal cancer.”
For example, the study found that the proportion of GI cancer deaths in Asia exceeds that of new cases, with the greatest disparity in China, while the opposite can be said of GI cancer trends in Europe and North America.
The study reported 4.8 million new cases of GI cancer and 3.4 million deaths worldwide in 2018. GI cancers accounted for 26% of the global cancer burden but 35% of cancer-related deaths. Incidence-to-death disparities were greatest for Africa, accounting for 4% of new cases and 5% of deaths; and Asia, with about 63% of new cases but 65% of deaths. The disparity was even wider in China, which accounted for 38% of worldwide cases but 41% of the deaths. Europe and North America, on the other hand, accounted for 26% of global cases but 23% of deaths. High death rates of these cancers are associated with late detection, Dr. Arnold and colleagues noted.
Regarding the five different types of GI cancer, the study reported the following:
- Esophageal cancer accounted for 572,000 new cases and 508,000 deaths in 2018, making it the sixth-most-deadly cancer worldwide. Rates in men are two to three times higher than in women. Eastern Asia has the highest rates, 12.2 per 100,000 person-years, followed by eastern Africa (8.3) and southern Africa (7.4). China alone accounts for 54% of the global burden. A large percentage of these cancers in developing countries are squamous cell carcinoma, the most common form of esophageal cancer globally, which has been linked to tobacco use, heavy alcohol consumption, opium intake, air pollution, and diet.
- Gastric cancer accounted for more than 1 million new cases and nearly 800,000 deaths in 2018. Again, the incidence is twice as high in men as in women, and eastern Asia has the highest rates of 22 per 100,000 vs. < 5 in Africa, North America, and northern Europe. Cardia gastric cancer (CGC), associated with obesity and gastroesophageal reflux disease, is more prevalent in Western countries, while noncardia gastric cancer (CG) is more prevalent in countries with higher rates of Helicobacter pylori infection. In the United States specifically, CGC is more common in non-Hispanic whites than other ethnic groups. Gastric cancer rates have been declining in recent years, although trends of CGC and cancer of the gastric corpus, which the study terms “a noncardia subsite,” among younger people “may lead to a deceleration or a reversal” of declining gastric cancer rates, stated Dr. Arnold and colleagues.
- Colorectal cancer remained the most commonly diagnosed GI cancer in 2018, accounting for 1.8 million cases and 881,000 deaths, which represents 1 in 10 cancer deaths. The highest incidence was found in Australia/New Zealand, the lowest in south and central Asia. Colorectal cancers are in “transition” from infection-related cancers to those related to “rapid societal and economic change.” Dr. Arnold and colleagues attributed these changes to higher dietary intake of fats, sugar, and animal-source foods and increases in sedentary behavior and obesity. Despite advances in cures for colorectal cancer, disparities continue, even in high-income countries, and screening programs have been limited, according to the study. Colorectal cancer will be “one of the main contributors” to the doubling of cancer rates in older adults by 2035.
- Liver cancer comprised 841,000 cases and 782,000 deaths in 2018, making it the sixth-most-diagnosed cancer but the fourth most deadly. “Transitioning countries” in eastern Asia, Micronesia, and northern Africa have the highest rates. In eastern Asia, hepatitis B infections and aflatoxins are the primary risk factors for hepatocellular carcinoma, while in Japan and Europe hepatitis C is the main cause for hepatocellular carcinoma. Decreases in both infections and aflatoxins may explain declines in liver cancer rates in those regions. Whereas in lower-risk areas, increasing liver cancer rates caused by more widespread obesity and diabetes may be offsetting declines in HBV and HCV rates.
- Pancreatic cancer was the 12th-most-common cancer but the seventh leading cause of cancer death, with 432,000 cases and 459,000 deaths in 2018. Wealthy countries have incidence and death rates three to four times higher than do less-developed countries, with rates highest in Europe, North America, and Australia/New Zealand. Because pancreatic cancer isn’t typically diagnosed until it is in the metastatic or locally advanced state, curative surgery isn’t feasible, Dr. Arnold and colleagues stated. Population aging and growth, along with advances in treating other types of cancers, mean that pancreatic cancer “has become or is set out to become one of the leading causes of cancer-related death in many countries,” Dr. Arnold and colleagues stated. They added that, in the European Union, pancreatic cancer is already the third-leading cause of cancer death after lung and colorectal cancer.
The findings, Dr. Arnold and colleagues wrote, underscore the shift of the cancer burden toward transitioning countries, “which are less equipped to manage this increasing burden.” In the United States, the rates of all five GI cancers in young adults (aged 25-49 years) have increased.
GI cancers, with the exception of colorectal cancers, also contribute disproportionately to cancer-related death rates, mostly because all but colorectal cancers are difficult to diagnose.
However, early detection and screening programs for gastric cancer in Japan and Korea, and esophageal cancer in China “have shown promising results,” Dr. Arnold and colleagues said. “Pancreatic cancer, on the other hand, is becoming a more important contributor to cancer-related mortality as a consequence of improved diagnosis and management of the historically most common forms of cancer death,” they wrote.
Prevention remains key, and lifestyle choices like smoking, alcohol intake, and physical activity are all drivers of GI cancer burden. “Primary and secondary prevention measures remain the most important tools to control this group of malignancies, particularly in light of their preventability and often dreadful prognosis,” Dr. Arnold and colleagues wrote.
Dr. Arnold and colleagues have no financial relationships to disclose.
SOURCE: Arnold M et al. Gastroenterology. 2020 Apr 2;S0016-5085(20)30452-2. doi: 10.1053/j.gastro.2020.02.068.
Cancers of the gastrointestinal tract represented about one in four cancer cases in 2018 but more than one in three cancer-related deaths, underscoring the importance of preventive measures, according to an analysis of global cancer datasets that identified trends in five major GI cancer types – esophageal, stomach, colorectal, liver, and pancreatic – published in Gastroenterology.
“Although the incidence of some GI cancer types has decreased, this group of malignancies continues to pose major challenges to public health,” wrote Melina Arnold, PhD, of the International for Research on Cancer in Lyon, France, and colleagues. “Primary and secondary prevention measures are important for controlling these malignancies – most importantly, reducing consumption of tobacco and alcohol, obesity control, immunizing populations against hepatitis B virus infection, and screening for colorectal cancer.”
For example, the study found that the proportion of GI cancer deaths in Asia exceeds that of new cases, with the greatest disparity in China, while the opposite can be said of GI cancer trends in Europe and North America.
The study reported 4.8 million new cases of GI cancer and 3.4 million deaths worldwide in 2018. GI cancers accounted for 26% of the global cancer burden but 35% of cancer-related deaths. Incidence-to-death disparities were greatest for Africa, accounting for 4% of new cases and 5% of deaths; and Asia, with about 63% of new cases but 65% of deaths. The disparity was even wider in China, which accounted for 38% of worldwide cases but 41% of the deaths. Europe and North America, on the other hand, accounted for 26% of global cases but 23% of deaths. High death rates of these cancers are associated with late detection, Dr. Arnold and colleagues noted.
Regarding the five different types of GI cancer, the study reported the following:
- Esophageal cancer accounted for 572,000 new cases and 508,000 deaths in 2018, making it the sixth-most-deadly cancer worldwide. Rates in men are two to three times higher than in women. Eastern Asia has the highest rates, 12.2 per 100,000 person-years, followed by eastern Africa (8.3) and southern Africa (7.4). China alone accounts for 54% of the global burden. A large percentage of these cancers in developing countries are squamous cell carcinoma, the most common form of esophageal cancer globally, which has been linked to tobacco use, heavy alcohol consumption, opium intake, air pollution, and diet.
- Gastric cancer accounted for more than 1 million new cases and nearly 800,000 deaths in 2018. Again, the incidence is twice as high in men as in women, and eastern Asia has the highest rates of 22 per 100,000 vs. < 5 in Africa, North America, and northern Europe. Cardia gastric cancer (CGC), associated with obesity and gastroesophageal reflux disease, is more prevalent in Western countries, while noncardia gastric cancer (CG) is more prevalent in countries with higher rates of Helicobacter pylori infection. In the United States specifically, CGC is more common in non-Hispanic whites than other ethnic groups. Gastric cancer rates have been declining in recent years, although trends of CGC and cancer of the gastric corpus, which the study terms “a noncardia subsite,” among younger people “may lead to a deceleration or a reversal” of declining gastric cancer rates, stated Dr. Arnold and colleagues.
- Colorectal cancer remained the most commonly diagnosed GI cancer in 2018, accounting for 1.8 million cases and 881,000 deaths, which represents 1 in 10 cancer deaths. The highest incidence was found in Australia/New Zealand, the lowest in south and central Asia. Colorectal cancers are in “transition” from infection-related cancers to those related to “rapid societal and economic change.” Dr. Arnold and colleagues attributed these changes to higher dietary intake of fats, sugar, and animal-source foods and increases in sedentary behavior and obesity. Despite advances in cures for colorectal cancer, disparities continue, even in high-income countries, and screening programs have been limited, according to the study. Colorectal cancer will be “one of the main contributors” to the doubling of cancer rates in older adults by 2035.
- Liver cancer comprised 841,000 cases and 782,000 deaths in 2018, making it the sixth-most-diagnosed cancer but the fourth most deadly. “Transitioning countries” in eastern Asia, Micronesia, and northern Africa have the highest rates. In eastern Asia, hepatitis B infections and aflatoxins are the primary risk factors for hepatocellular carcinoma, while in Japan and Europe hepatitis C is the main cause for hepatocellular carcinoma. Decreases in both infections and aflatoxins may explain declines in liver cancer rates in those regions. Whereas in lower-risk areas, increasing liver cancer rates caused by more widespread obesity and diabetes may be offsetting declines in HBV and HCV rates.
- Pancreatic cancer was the 12th-most-common cancer but the seventh leading cause of cancer death, with 432,000 cases and 459,000 deaths in 2018. Wealthy countries have incidence and death rates three to four times higher than do less-developed countries, with rates highest in Europe, North America, and Australia/New Zealand. Because pancreatic cancer isn’t typically diagnosed until it is in the metastatic or locally advanced state, curative surgery isn’t feasible, Dr. Arnold and colleagues stated. Population aging and growth, along with advances in treating other types of cancers, mean that pancreatic cancer “has become or is set out to become one of the leading causes of cancer-related death in many countries,” Dr. Arnold and colleagues stated. They added that, in the European Union, pancreatic cancer is already the third-leading cause of cancer death after lung and colorectal cancer.
The findings, Dr. Arnold and colleagues wrote, underscore the shift of the cancer burden toward transitioning countries, “which are less equipped to manage this increasing burden.” In the United States, the rates of all five GI cancers in young adults (aged 25-49 years) have increased.
GI cancers, with the exception of colorectal cancers, also contribute disproportionately to cancer-related death rates, mostly because all but colorectal cancers are difficult to diagnose.
However, early detection and screening programs for gastric cancer in Japan and Korea, and esophageal cancer in China “have shown promising results,” Dr. Arnold and colleagues said. “Pancreatic cancer, on the other hand, is becoming a more important contributor to cancer-related mortality as a consequence of improved diagnosis and management of the historically most common forms of cancer death,” they wrote.
Prevention remains key, and lifestyle choices like smoking, alcohol intake, and physical activity are all drivers of GI cancer burden. “Primary and secondary prevention measures remain the most important tools to control this group of malignancies, particularly in light of their preventability and often dreadful prognosis,” Dr. Arnold and colleagues wrote.
Dr. Arnold and colleagues have no financial relationships to disclose.
SOURCE: Arnold M et al. Gastroenterology. 2020 Apr 2;S0016-5085(20)30452-2. doi: 10.1053/j.gastro.2020.02.068.
Cancers of the gastrointestinal tract represented about one in four cancer cases in 2018 but more than one in three cancer-related deaths, underscoring the importance of preventive measures, according to an analysis of global cancer datasets that identified trends in five major GI cancer types – esophageal, stomach, colorectal, liver, and pancreatic – published in Gastroenterology.
“Although the incidence of some GI cancer types has decreased, this group of malignancies continues to pose major challenges to public health,” wrote Melina Arnold, PhD, of the International for Research on Cancer in Lyon, France, and colleagues. “Primary and secondary prevention measures are important for controlling these malignancies – most importantly, reducing consumption of tobacco and alcohol, obesity control, immunizing populations against hepatitis B virus infection, and screening for colorectal cancer.”
For example, the study found that the proportion of GI cancer deaths in Asia exceeds that of new cases, with the greatest disparity in China, while the opposite can be said of GI cancer trends in Europe and North America.
The study reported 4.8 million new cases of GI cancer and 3.4 million deaths worldwide in 2018. GI cancers accounted for 26% of the global cancer burden but 35% of cancer-related deaths. Incidence-to-death disparities were greatest for Africa, accounting for 4% of new cases and 5% of deaths; and Asia, with about 63% of new cases but 65% of deaths. The disparity was even wider in China, which accounted for 38% of worldwide cases but 41% of the deaths. Europe and North America, on the other hand, accounted for 26% of global cases but 23% of deaths. High death rates of these cancers are associated with late detection, Dr. Arnold and colleagues noted.
Regarding the five different types of GI cancer, the study reported the following:
- Esophageal cancer accounted for 572,000 new cases and 508,000 deaths in 2018, making it the sixth-most-deadly cancer worldwide. Rates in men are two to three times higher than in women. Eastern Asia has the highest rates, 12.2 per 100,000 person-years, followed by eastern Africa (8.3) and southern Africa (7.4). China alone accounts for 54% of the global burden. A large percentage of these cancers in developing countries are squamous cell carcinoma, the most common form of esophageal cancer globally, which has been linked to tobacco use, heavy alcohol consumption, opium intake, air pollution, and diet.
- Gastric cancer accounted for more than 1 million new cases and nearly 800,000 deaths in 2018. Again, the incidence is twice as high in men as in women, and eastern Asia has the highest rates of 22 per 100,000 vs. < 5 in Africa, North America, and northern Europe. Cardia gastric cancer (CGC), associated with obesity and gastroesophageal reflux disease, is more prevalent in Western countries, while noncardia gastric cancer (CG) is more prevalent in countries with higher rates of Helicobacter pylori infection. In the United States specifically, CGC is more common in non-Hispanic whites than other ethnic groups. Gastric cancer rates have been declining in recent years, although trends of CGC and cancer of the gastric corpus, which the study terms “a noncardia subsite,” among younger people “may lead to a deceleration or a reversal” of declining gastric cancer rates, stated Dr. Arnold and colleagues.
- Colorectal cancer remained the most commonly diagnosed GI cancer in 2018, accounting for 1.8 million cases and 881,000 deaths, which represents 1 in 10 cancer deaths. The highest incidence was found in Australia/New Zealand, the lowest in south and central Asia. Colorectal cancers are in “transition” from infection-related cancers to those related to “rapid societal and economic change.” Dr. Arnold and colleagues attributed these changes to higher dietary intake of fats, sugar, and animal-source foods and increases in sedentary behavior and obesity. Despite advances in cures for colorectal cancer, disparities continue, even in high-income countries, and screening programs have been limited, according to the study. Colorectal cancer will be “one of the main contributors” to the doubling of cancer rates in older adults by 2035.
- Liver cancer comprised 841,000 cases and 782,000 deaths in 2018, making it the sixth-most-diagnosed cancer but the fourth most deadly. “Transitioning countries” in eastern Asia, Micronesia, and northern Africa have the highest rates. In eastern Asia, hepatitis B infections and aflatoxins are the primary risk factors for hepatocellular carcinoma, while in Japan and Europe hepatitis C is the main cause for hepatocellular carcinoma. Decreases in both infections and aflatoxins may explain declines in liver cancer rates in those regions. Whereas in lower-risk areas, increasing liver cancer rates caused by more widespread obesity and diabetes may be offsetting declines in HBV and HCV rates.
- Pancreatic cancer was the 12th-most-common cancer but the seventh leading cause of cancer death, with 432,000 cases and 459,000 deaths in 2018. Wealthy countries have incidence and death rates three to four times higher than do less-developed countries, with rates highest in Europe, North America, and Australia/New Zealand. Because pancreatic cancer isn’t typically diagnosed until it is in the metastatic or locally advanced state, curative surgery isn’t feasible, Dr. Arnold and colleagues stated. Population aging and growth, along with advances in treating other types of cancers, mean that pancreatic cancer “has become or is set out to become one of the leading causes of cancer-related death in many countries,” Dr. Arnold and colleagues stated. They added that, in the European Union, pancreatic cancer is already the third-leading cause of cancer death after lung and colorectal cancer.
The findings, Dr. Arnold and colleagues wrote, underscore the shift of the cancer burden toward transitioning countries, “which are less equipped to manage this increasing burden.” In the United States, the rates of all five GI cancers in young adults (aged 25-49 years) have increased.
GI cancers, with the exception of colorectal cancers, also contribute disproportionately to cancer-related death rates, mostly because all but colorectal cancers are difficult to diagnose.
However, early detection and screening programs for gastric cancer in Japan and Korea, and esophageal cancer in China “have shown promising results,” Dr. Arnold and colleagues said. “Pancreatic cancer, on the other hand, is becoming a more important contributor to cancer-related mortality as a consequence of improved diagnosis and management of the historically most common forms of cancer death,” they wrote.
Prevention remains key, and lifestyle choices like smoking, alcohol intake, and physical activity are all drivers of GI cancer burden. “Primary and secondary prevention measures remain the most important tools to control this group of malignancies, particularly in light of their preventability and often dreadful prognosis,” Dr. Arnold and colleagues wrote.
Dr. Arnold and colleagues have no financial relationships to disclose.
SOURCE: Arnold M et al. Gastroenterology. 2020 Apr 2;S0016-5085(20)30452-2. doi: 10.1053/j.gastro.2020.02.068.
FROM GASTROENTEROLOGY
AGA clinical practice update: Pancreatic cancer screening
Individuals at high risk for pancreatic cancer should at least be considered for screening for the disease, states a new clinical practice update from the American Gastroenterological Association that further defines what constitutes high risk for pancreatic cancer, when and how screenings should occur, and the role of genetic testing and counseling (Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.03.088).
Individuals who have a first-degree relative with two or more genetically related relatives with pancreatic cancer should be considered for screening, as should people with Peutz-Jeghers syndrome, a CDKN2A gene mutation, one or more first-degree relatives with pancreatic cancer with Lynch syndrome and mutations in the BRCA1, BRCA2, PALB2, and ATM genes, the clinical update stated. Screening in high-risk individuals should begin at age 50, but some groups should start having screening earlier: age 40 in carriers of the CKDN2A and PRSS1 mutations with hereditary pancreatitis; and age 35 in those with Peutz-Jeghers syndrome.
“Studies to date have demonstrated variability regarding definitions of high-risk groups and the age at which screening should be initiated,” wrote Harry R. Aslanian, MD, AGAF, of Yale New Haven (Conn.) Hospital, and coauthors. “The genetic basis of much of the inherited susceptibility to pancreas cancer remains unexplained (in approximately 90% of cases) and familial history is important in risk stratification.”
Genetic testing and counseling should be considered for any familial pancreas cancer relative – that is, a person with two or more first-degree relatives with pancreas cancer that’s outside the definition of other hereditary cancers. “A positive germline mutation is associated with an increased risk of neoplastic progression and may also lead to screening for other relevant associated cancers,” wrote Dr. Aslanian and coauthors.
The screening itself should consist of MRI and endoscopic ultrasonography (EUS) in combination, the clinical practice update states. It defines detectable targets as stage 1 pancreatic ductal adenocarcinoma and high-risk neoplasms such as intraductal papillary mucinous neoplasms with high-grade dysplasia and some enlarged pancreatic intraepithelial neoplasms.
High-risk patients having screening should also be enrolled in a registry and referred to a pancreas center of excellence.
The update suggests screening every 12 months when the baseline screening is negative for any suspect lesions, with shorter intervals for EUS when suspected lesions are found: 6-12 months for low-risk lesions; 3-6 months for intermediate lesions; and 3 months for high-risk lesions if the patient hasn’t had surgery to remove the lesions.
Regarding management of positive screening results, a multidisciplinary team should confer with the individual and family to determine therapy. If surgery is indicated, it should be done at a high-volume center.
The update also provides guidance for two scenarios when patients shouldn’t undergo screening: those at average risk; and those at high-risk more likely to die from another cause. Of course, the physician should review the limitations and risk of screening with patients beforehand.
Dr. Aslanian and coauthors noted that a number of areas require further study, including defining the highest-risk groups and refining screening tests with high sensitivity and specificity to detect high-grade precursors, along with more data on risks of precursor lesions themselves.
They also acknowledged the need for more study into the effectiveness of pancreatic cancer screening, although a randomized clinical trial comparing screening vs. no screening “might be challenging to conduct given implementation of clinical screening as standard of care in some practices.” Blood tests for pancreatic cancer screening in high-risk patients also need more study, they stated.
The authors did not report any funding sources or conflicts of interest.
SOURCE: Aslanian HR et al. Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.03.088.
Individuals at high risk for pancreatic cancer should at least be considered for screening for the disease, states a new clinical practice update from the American Gastroenterological Association that further defines what constitutes high risk for pancreatic cancer, when and how screenings should occur, and the role of genetic testing and counseling (Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.03.088).
Individuals who have a first-degree relative with two or more genetically related relatives with pancreatic cancer should be considered for screening, as should people with Peutz-Jeghers syndrome, a CDKN2A gene mutation, one or more first-degree relatives with pancreatic cancer with Lynch syndrome and mutations in the BRCA1, BRCA2, PALB2, and ATM genes, the clinical update stated. Screening in high-risk individuals should begin at age 50, but some groups should start having screening earlier: age 40 in carriers of the CKDN2A and PRSS1 mutations with hereditary pancreatitis; and age 35 in those with Peutz-Jeghers syndrome.
“Studies to date have demonstrated variability regarding definitions of high-risk groups and the age at which screening should be initiated,” wrote Harry R. Aslanian, MD, AGAF, of Yale New Haven (Conn.) Hospital, and coauthors. “The genetic basis of much of the inherited susceptibility to pancreas cancer remains unexplained (in approximately 90% of cases) and familial history is important in risk stratification.”
Genetic testing and counseling should be considered for any familial pancreas cancer relative – that is, a person with two or more first-degree relatives with pancreas cancer that’s outside the definition of other hereditary cancers. “A positive germline mutation is associated with an increased risk of neoplastic progression and may also lead to screening for other relevant associated cancers,” wrote Dr. Aslanian and coauthors.
The screening itself should consist of MRI and endoscopic ultrasonography (EUS) in combination, the clinical practice update states. It defines detectable targets as stage 1 pancreatic ductal adenocarcinoma and high-risk neoplasms such as intraductal papillary mucinous neoplasms with high-grade dysplasia and some enlarged pancreatic intraepithelial neoplasms.
High-risk patients having screening should also be enrolled in a registry and referred to a pancreas center of excellence.
The update suggests screening every 12 months when the baseline screening is negative for any suspect lesions, with shorter intervals for EUS when suspected lesions are found: 6-12 months for low-risk lesions; 3-6 months for intermediate lesions; and 3 months for high-risk lesions if the patient hasn’t had surgery to remove the lesions.
Regarding management of positive screening results, a multidisciplinary team should confer with the individual and family to determine therapy. If surgery is indicated, it should be done at a high-volume center.
The update also provides guidance for two scenarios when patients shouldn’t undergo screening: those at average risk; and those at high-risk more likely to die from another cause. Of course, the physician should review the limitations and risk of screening with patients beforehand.
Dr. Aslanian and coauthors noted that a number of areas require further study, including defining the highest-risk groups and refining screening tests with high sensitivity and specificity to detect high-grade precursors, along with more data on risks of precursor lesions themselves.
They also acknowledged the need for more study into the effectiveness of pancreatic cancer screening, although a randomized clinical trial comparing screening vs. no screening “might be challenging to conduct given implementation of clinical screening as standard of care in some practices.” Blood tests for pancreatic cancer screening in high-risk patients also need more study, they stated.
The authors did not report any funding sources or conflicts of interest.
SOURCE: Aslanian HR et al. Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.03.088.
Individuals at high risk for pancreatic cancer should at least be considered for screening for the disease, states a new clinical practice update from the American Gastroenterological Association that further defines what constitutes high risk for pancreatic cancer, when and how screenings should occur, and the role of genetic testing and counseling (Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.03.088).
Individuals who have a first-degree relative with two or more genetically related relatives with pancreatic cancer should be considered for screening, as should people with Peutz-Jeghers syndrome, a CDKN2A gene mutation, one or more first-degree relatives with pancreatic cancer with Lynch syndrome and mutations in the BRCA1, BRCA2, PALB2, and ATM genes, the clinical update stated. Screening in high-risk individuals should begin at age 50, but some groups should start having screening earlier: age 40 in carriers of the CKDN2A and PRSS1 mutations with hereditary pancreatitis; and age 35 in those with Peutz-Jeghers syndrome.
“Studies to date have demonstrated variability regarding definitions of high-risk groups and the age at which screening should be initiated,” wrote Harry R. Aslanian, MD, AGAF, of Yale New Haven (Conn.) Hospital, and coauthors. “The genetic basis of much of the inherited susceptibility to pancreas cancer remains unexplained (in approximately 90% of cases) and familial history is important in risk stratification.”
Genetic testing and counseling should be considered for any familial pancreas cancer relative – that is, a person with two or more first-degree relatives with pancreas cancer that’s outside the definition of other hereditary cancers. “A positive germline mutation is associated with an increased risk of neoplastic progression and may also lead to screening for other relevant associated cancers,” wrote Dr. Aslanian and coauthors.
The screening itself should consist of MRI and endoscopic ultrasonography (EUS) in combination, the clinical practice update states. It defines detectable targets as stage 1 pancreatic ductal adenocarcinoma and high-risk neoplasms such as intraductal papillary mucinous neoplasms with high-grade dysplasia and some enlarged pancreatic intraepithelial neoplasms.
High-risk patients having screening should also be enrolled in a registry and referred to a pancreas center of excellence.
The update suggests screening every 12 months when the baseline screening is negative for any suspect lesions, with shorter intervals for EUS when suspected lesions are found: 6-12 months for low-risk lesions; 3-6 months for intermediate lesions; and 3 months for high-risk lesions if the patient hasn’t had surgery to remove the lesions.
Regarding management of positive screening results, a multidisciplinary team should confer with the individual and family to determine therapy. If surgery is indicated, it should be done at a high-volume center.
The update also provides guidance for two scenarios when patients shouldn’t undergo screening: those at average risk; and those at high-risk more likely to die from another cause. Of course, the physician should review the limitations and risk of screening with patients beforehand.
Dr. Aslanian and coauthors noted that a number of areas require further study, including defining the highest-risk groups and refining screening tests with high sensitivity and specificity to detect high-grade precursors, along with more data on risks of precursor lesions themselves.
They also acknowledged the need for more study into the effectiveness of pancreatic cancer screening, although a randomized clinical trial comparing screening vs. no screening “might be challenging to conduct given implementation of clinical screening as standard of care in some practices.” Blood tests for pancreatic cancer screening in high-risk patients also need more study, they stated.
The authors did not report any funding sources or conflicts of interest.
SOURCE: Aslanian HR et al. Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.03.088.
Large study finds no link between gluten, IBD risk
Among women without celiac disease, dietary gluten intake was not associated with the risk of developing either Crohn’s disease or ulcerative colitis, investigators reported.
The findings spanned subgroups stratified by age, body mass index, smoking status, and whether individuals primarily consumed refined or whole grains, said Emily Walsh Lopes, MD, gastroenterology clinical and research fellow at Massachusetts General Hospital in Boston. She and associates reported the combined analysis of the prospective Nurses’ Health Study and Nurses’ Health Study II in an abstract released as part of the annual Digestive Disease Week.®
“Avoidance of dietary gluten is common, and many patients attribute gastrointestinal symptoms to gluten intake,” Dr. Lopes said in an interview. “Though our findings warrant further study, the results suggest to patients and providers that eating gluten does not increase a person’s chance of getting diagnosed with inflammatory bowel disease.”
Prior studies have found that many individuals with inflammatory bowel disease avoid gluten and report subsequent improvements in gastrointestinal symptoms, even if they do not have celiac disease. However, it remains unclear whether dietary gluten is a risk factor for new-onset inflammatory bowel disease.
To address this question, Dr. Lopes and associates analyzed data collected from 165,327 women who took part in the Nurses’ Health Study (1986 to 2016) or the Nurses’ Health Study II (1991 through 2017). None of the women had a preexisting diagnosis of celiac disease or inflammatory bowel disease. Dietary gluten intake was estimated based on food frequency questionnaires completed by the women at baseline and every 4 years. The researchers also reviewed medical records to confirm self-reported cases of new-onset ulcerative colitis and Crohn’s disease.
Over 4.02 million person-years of follow-up, 277 women developed Crohn’s disease and 359 developed ulcerative colitis. Gluten intake was not associated with the risk of either type of inflammatory bowel disease, even after the researchers controlled for multiple demographic and clinical risk factors.
After submitting their abstract, Dr. Lopes and coinvestigators expanded the dataset to include a large cohort of men from the prospective Health Professionals Follow-up Study. The final pooled cohort included more than 208,000 women and men followed for more than 20 years. Through the end of follow-up, the researchers documented 337 cases of Crohn’s disease and 446 cases of ulcerative colitis. “Inclusion of the male cohort in the pooled analysis did not materially change our estimates,” Dr. Lopes told MDedge. “That is, no association was seen between gluten intake and risk of either Crohn’s disease or ulcerative colitis in the final cohort.”
She noted that the findings cannot be extrapolated to individuals who are already diagnosed with inflammatory bowel disease. “It is possible that different mechanisms exist to explain how gluten intake impacts those already diagnosed with IBD, and this topic warrants further study,” she said. Also, because the three cohort studies were observational, they are subject to bias. “While we tried to account for this in our analyses, residual bias may still exist.”
Dr. Lopes reported having no conflicts of interest.
SOURCE: Walsh Lopes E et al. DDW 2020, abstract 847.
Among women without celiac disease, dietary gluten intake was not associated with the risk of developing either Crohn’s disease or ulcerative colitis, investigators reported.
The findings spanned subgroups stratified by age, body mass index, smoking status, and whether individuals primarily consumed refined or whole grains, said Emily Walsh Lopes, MD, gastroenterology clinical and research fellow at Massachusetts General Hospital in Boston. She and associates reported the combined analysis of the prospective Nurses’ Health Study and Nurses’ Health Study II in an abstract released as part of the annual Digestive Disease Week.®
“Avoidance of dietary gluten is common, and many patients attribute gastrointestinal symptoms to gluten intake,” Dr. Lopes said in an interview. “Though our findings warrant further study, the results suggest to patients and providers that eating gluten does not increase a person’s chance of getting diagnosed with inflammatory bowel disease.”
Prior studies have found that many individuals with inflammatory bowel disease avoid gluten and report subsequent improvements in gastrointestinal symptoms, even if they do not have celiac disease. However, it remains unclear whether dietary gluten is a risk factor for new-onset inflammatory bowel disease.
To address this question, Dr. Lopes and associates analyzed data collected from 165,327 women who took part in the Nurses’ Health Study (1986 to 2016) or the Nurses’ Health Study II (1991 through 2017). None of the women had a preexisting diagnosis of celiac disease or inflammatory bowel disease. Dietary gluten intake was estimated based on food frequency questionnaires completed by the women at baseline and every 4 years. The researchers also reviewed medical records to confirm self-reported cases of new-onset ulcerative colitis and Crohn’s disease.
Over 4.02 million person-years of follow-up, 277 women developed Crohn’s disease and 359 developed ulcerative colitis. Gluten intake was not associated with the risk of either type of inflammatory bowel disease, even after the researchers controlled for multiple demographic and clinical risk factors.
After submitting their abstract, Dr. Lopes and coinvestigators expanded the dataset to include a large cohort of men from the prospective Health Professionals Follow-up Study. The final pooled cohort included more than 208,000 women and men followed for more than 20 years. Through the end of follow-up, the researchers documented 337 cases of Crohn’s disease and 446 cases of ulcerative colitis. “Inclusion of the male cohort in the pooled analysis did not materially change our estimates,” Dr. Lopes told MDedge. “That is, no association was seen between gluten intake and risk of either Crohn’s disease or ulcerative colitis in the final cohort.”
She noted that the findings cannot be extrapolated to individuals who are already diagnosed with inflammatory bowel disease. “It is possible that different mechanisms exist to explain how gluten intake impacts those already diagnosed with IBD, and this topic warrants further study,” she said. Also, because the three cohort studies were observational, they are subject to bias. “While we tried to account for this in our analyses, residual bias may still exist.”
Dr. Lopes reported having no conflicts of interest.
SOURCE: Walsh Lopes E et al. DDW 2020, abstract 847.
Among women without celiac disease, dietary gluten intake was not associated with the risk of developing either Crohn’s disease or ulcerative colitis, investigators reported.
The findings spanned subgroups stratified by age, body mass index, smoking status, and whether individuals primarily consumed refined or whole grains, said Emily Walsh Lopes, MD, gastroenterology clinical and research fellow at Massachusetts General Hospital in Boston. She and associates reported the combined analysis of the prospective Nurses’ Health Study and Nurses’ Health Study II in an abstract released as part of the annual Digestive Disease Week.®
“Avoidance of dietary gluten is common, and many patients attribute gastrointestinal symptoms to gluten intake,” Dr. Lopes said in an interview. “Though our findings warrant further study, the results suggest to patients and providers that eating gluten does not increase a person’s chance of getting diagnosed with inflammatory bowel disease.”
Prior studies have found that many individuals with inflammatory bowel disease avoid gluten and report subsequent improvements in gastrointestinal symptoms, even if they do not have celiac disease. However, it remains unclear whether dietary gluten is a risk factor for new-onset inflammatory bowel disease.
To address this question, Dr. Lopes and associates analyzed data collected from 165,327 women who took part in the Nurses’ Health Study (1986 to 2016) or the Nurses’ Health Study II (1991 through 2017). None of the women had a preexisting diagnosis of celiac disease or inflammatory bowel disease. Dietary gluten intake was estimated based on food frequency questionnaires completed by the women at baseline and every 4 years. The researchers also reviewed medical records to confirm self-reported cases of new-onset ulcerative colitis and Crohn’s disease.
Over 4.02 million person-years of follow-up, 277 women developed Crohn’s disease and 359 developed ulcerative colitis. Gluten intake was not associated with the risk of either type of inflammatory bowel disease, even after the researchers controlled for multiple demographic and clinical risk factors.
After submitting their abstract, Dr. Lopes and coinvestigators expanded the dataset to include a large cohort of men from the prospective Health Professionals Follow-up Study. The final pooled cohort included more than 208,000 women and men followed for more than 20 years. Through the end of follow-up, the researchers documented 337 cases of Crohn’s disease and 446 cases of ulcerative colitis. “Inclusion of the male cohort in the pooled analysis did not materially change our estimates,” Dr. Lopes told MDedge. “That is, no association was seen between gluten intake and risk of either Crohn’s disease or ulcerative colitis in the final cohort.”
She noted that the findings cannot be extrapolated to individuals who are already diagnosed with inflammatory bowel disease. “It is possible that different mechanisms exist to explain how gluten intake impacts those already diagnosed with IBD, and this topic warrants further study,” she said. Also, because the three cohort studies were observational, they are subject to bias. “While we tried to account for this in our analyses, residual bias may still exist.”
Dr. Lopes reported having no conflicts of interest.
SOURCE: Walsh Lopes E et al. DDW 2020, abstract 847.
FROM DDW 2020
Topical Clobetasol Propionate Treatment and Cutaneous Adverse Effects in Patients With Early-Stage Mycosis Fungoides: An Observational Study
Mycosis fungoides (MF), the most common variant of cutaneous T-cell lymphoma, is a non-Hodgkin lymphoma of T-cell origin that primarily develops in the skin and has a chronic relapsing course. Early-stage MF (stages IA–IIA) is defined as papules, patches, or plaques with limited (if any) lymph node and blood involvement and no visceral involvement.1 Early-stage MF has a favorable prognosis, and first-line treatments are skin-directed therapies including topical corticosteroids (CSs), topical chemotherapy (nitrogen mustard or carmustine), topical retinoids, topical imiquimod, local radiation, or phototherapy.2 Topical CSs are effective in treating early-stage MF and have been widely used for this indication for several decades; however, there are very little data in the literature on topical CS use in MF.3 Superpotent topical CSs have been shown to have a high overall response rate in early-stage MF3; however, cutaneous side effects associated with long-term topical use include cutaneous atrophy, striae formation, skin fragility, and irritation.
The US Food and Drug Administration (FDA) approved bexarotene gel and mechlorethamine gel for topical treatment of cutaneous lesions in patients with stage IA and IB MF in 2000 and 2013, respectively. Although each may be effective in achieving complete or partial response in MF, both agents are associated with cutaneous side effects, mainly irritation and frequent contact hypersensitivity reactions, respectively.4,5 Additionally, their high prices and limited availability are other major drawbacks of treatment.
At our institution, high-potency topical CSs, specifically once or twice daily clobetasol propionate cream 0.05% prescribed as monotherapy for at least several months, remain the mainstay of treatment in patients with limited patches, papules, and plaques covering less than 10% of the skin surface (stage IA). In this study, we aimed to assess the risk of cutaneous side effects in patients with early-stage MF who were treated with long-term, high-potency topical CSs.
Methods
This prospective observational cohort study included patients with early-stage MF who were seen at the Cutaneous Lymphoma Clinic at Memorial Sloan Kettering Cancer Center (MSKCC) in New York, New York, and were started on a superpotent (class I) topical CS (clobetasol propionate cream 0.05%) as monotherapy for MF from July 2016 to July 2017. The diagnosis of MF had to be supported by clinical findings and histopathologic features. All patients were Fitzpatrick skin types I, II, or III. Eligible patients were evaluated for development of CS-induced cutaneous AEs by physical examination and clinical photography of the treated lesions performed at baseline and as part of routine follow-up visits (usually scheduled every 2 to 6 months) at the MSKCC Cutaneous Lymphoma Clinic. Patients’ skin was evaluated clinically for MF activity, atrophy, telangiectasia, purpura, hypopigmentation, and stretch marks (striae). Use of the topical CS was self-reported and also was documented at follow-up visits. Treatment response was defined as follows: complete clinical response (CCR) if the treated lesions resolved completely compared to initial photography; minimal active disease (MAD) if resolution of the vast majority (≥75%) of lesions was seen; and partial response (PR) if some of the lesions resolved (<75%). We analyzed the treatment response rates and adverse effects (AEs). Results were summarized using descriptive statistics.
Results
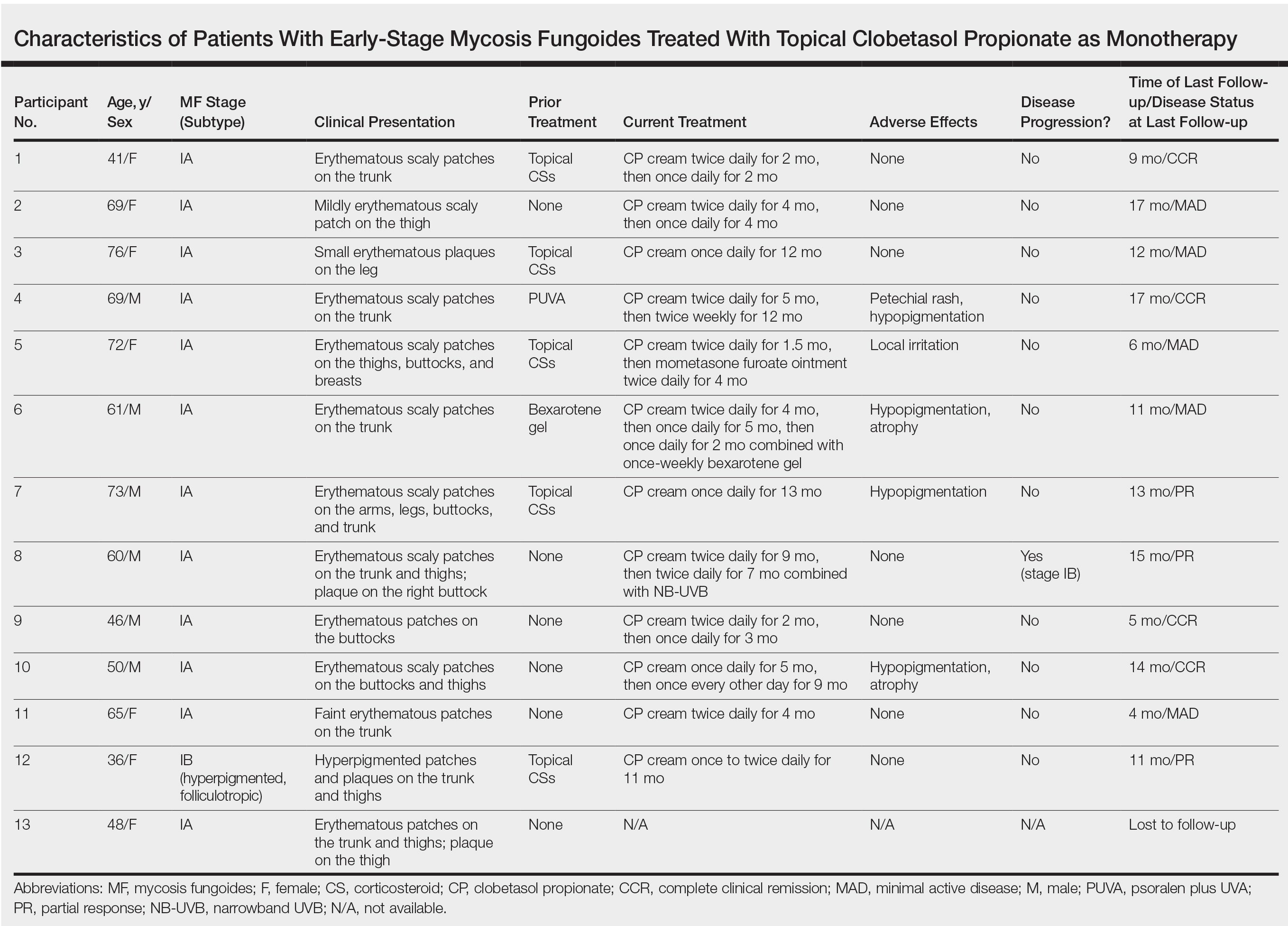
We identified 13 patients who were started on topical clobetasol propionate as monotherapy for early-stage MF during the study period. Our cohort included 6 males and 7 females aged 36 to 76 years (median age, 61 years). All but 1 participant were diagnosed with stage IA MF (12/13 [92.3%]); of those, 9 (75.0%) had patch-stage disease and 3 (25.0%) presented with plaques. One (7.7%) participant presented with hyperpigmented patches and plaques that involved a little more than 10% of the skin surface (stage IB), and involvement of the hair follicles was noted on histology (folliculotropic MF). All prior treatments were stopped when participants started the superpotent topical CS: 6 (46.2%) participants had been treated with lower-potency topical agents and 1 (7.7%) participant was getting psoralen plus UVA therapy, while the other 6 (46.2%) participants were receiving no therapy for MF prior to starting the study. All participants were prescribed clobetasol propionate cream 0.05% once or twice daily as monotherapy and were instructed to apply it to the MF lesions only, avoiding skin folds and the face. One participant was lost to follow-up, and another stopped using the clobetasol propionate cream after 1.5 months due to local irritation associated with treatment. At their follow-up visits, the other 11 participants were advised to continue with once-daily treatment with clobetasol propionate or were tapered to once every other day, twice weekly, or once weekly depending on their response to treatment and AEs (Table). Participants were advised not to use more than 50 g of clobetasol propionate cream weekly.
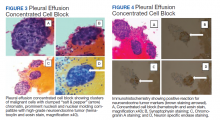
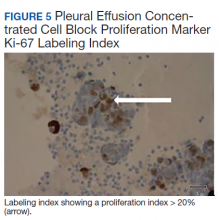
All participants responded to the clobetasol propionate cream, and improvement was noted in the treated lesions; however, progression of disease (from stage IA to stage IB) occurred in 1 (8.3%) participant, and phototherapy was added with good response. The participants in our cohort were followed for 4 to 17 months (median, 11.5 months). At the last follow-up visit, all 12 participants showed treatment response: 4 (33.3%) had CCR, 5 (41.7%) had MAD; and 3 (25.0%) had PR. In one participant with a history of partial response to bexarotene gel 1%, daily clobetasol propionate cream 0.05% initially was used alone for 9 months and was later combined with bexarotene gel once weekly, resulting in MAD.
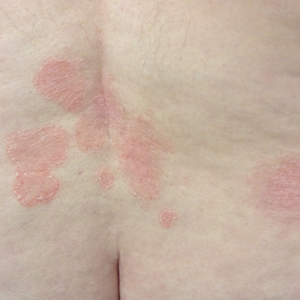
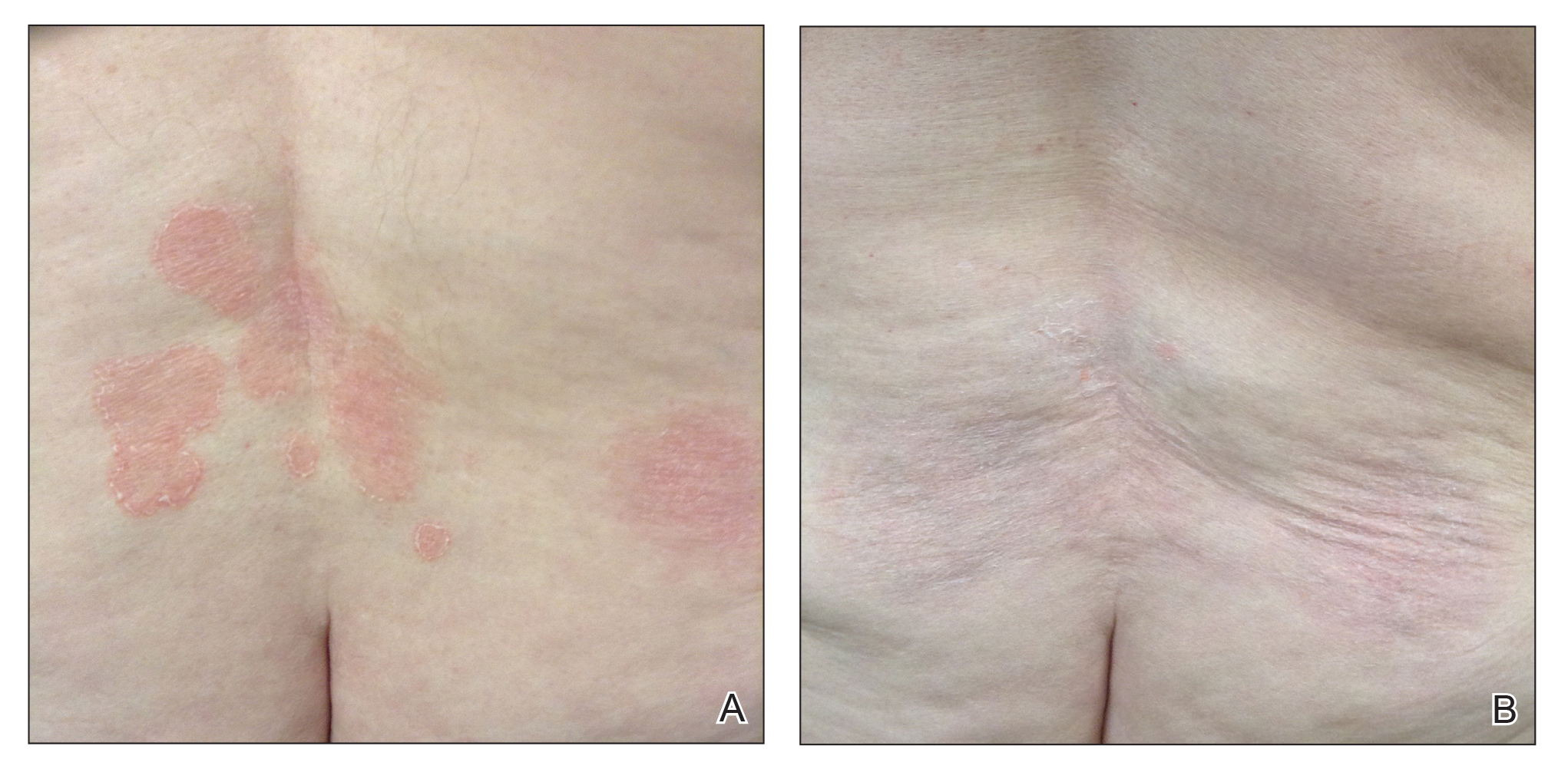
In 7 (58.3%) participants, no AEs to topical clobetasol propionate were recorded. Four (33.3%) participants developed local hypopigmentation at the application site, and 2 (16.7%) developed cutaneous atrophy with local fine wrinkling of the skin (Figure 1); none of the participants developed stretch marks (striae), telangiectases, or skin fragility. One (8.3%) participant developed a petechial rash at the clobetasol propionate application site that resolved once treatment was discontinued and did not recur after restarting clobetasol propionate twice weekly.
Comment
Topical CSs are the most commonly prescribed agents, either as monotherapy or in combination with other agents, in the treatment of numerous dermatologic conditions, including cutaneous T-cell lymphoma and MF. Cutaneous and systemic AEs have been associated with topical CS use. Local AEs are encountered more frequently and include cutaneous atrophy, striae, telangiectasia, purpura, skin fragility, hypopigmentation, hyperpigmentation, acneform eruptions, and hypertrichosis.6 Factors other than potency of the topical CS agent may affect the development of skin atrophy, including anatomic location, duration of therapy, vehicle, and method and frequency of application.7 The potential for systemic AEs due to percutaneous absorption of high-potency CSs, specifically Cushing syndrome and pathologic adrenal suppression, has been a long-standing concern and led the FDA to recommend limiting the use of superpotent CSs to 50 g weekly for 2 or 4 consecutive weeks.8 However, if using an excess of 50 g weekly is avoided, superpotent topical CSs may be safe to use consecutively for months, perhaps even years, without causing systemic effects.9
The effects of topical CSs in MF include induction of apoptosis; inhibition of lymphocyte binding to the endothelium; and downregulation of transcription factors with decreased cytokines, adhesion molecules, and production of growth factors.2 For patients with limited early-stage MF patches and thin plaques, topical CSs often control the disease for many years and frequently are the only form of therapy required. Intralesional steroids can be effective in treating thicker lesions, such as plaques or tumors.10 In an uncontrolled study, Zackheim et al11 prospectively evaluated the effectiveness and safety of twice-daily use of mainly high-potency topical CSs in 79 patients with MF stages IA to IB and observed an overall response rate of 94%. None of the patients were using systemic agents while being treated with topical CSs. Adverse effects were rare: 2 (2.5%) patients experienced temporary minor irritation from the topical CS, 1 (1.3%) patient developed localized skin atrophy under the breast that resolved several months after she stopped treatment, and 1 (1.3%) patient developed stretch marks on the thighs.11 Zackheim12 later reported treatment of approximately 200 patients with class I topical CSs, and overall response rates were over 90% in stage T1 and over 80% in stage T2 patients. Response to topical CS was reported to be evident within 3 months and often much sooner. Side effects were most likely related to the more prolonged treatment periods. Irritant dermatitis or purpura developed in approximately 10% to 20% of patients, and purpura was seen at the sites of treatment as well as at distant sites. Only a small number of patients developed cutaneous atrophy and striae, which were reversible.12 Successful use of intralesional steroids for treatment-resistant MF was reported in 4 patients who tolerated treatment well without any side effects other than local hypopigmentation in a single patient.13
At MSKCC, the first line of treatment in localized (stage IA) MF in light-skinned individuals most frequently is class I topical CSs, usually clobetasol propionate cream 0.05%. Patients are instructed to apply the cream twice daily on active MF lesions uninterruptedly until completely clear and to avoid using it on the face and in skin folds (axillary, inguinal, and abdominal). Patients are instructed to observe themselves for possible cutaneous AEs related to treatment and to stop or taper treatment if any AEs are noticed. In patients with darker skin, we may recommend other modalities such as narrowband UVB phototherapy for even limited MF disease because of the risk for uneven/hypopigmentation with superpotent CSs.
The current study offers a real-life observation of topical high-potency CSs for treatment of early-stage MF and the associated cutaneous AEs. Local hypopigmentation was identified in 4 participants (33.3%), local skin atrophy was seen in 2 participants (16.7%), and local purpura and irritation were seen in 1 participant each (8.3%). All patients responded to therapy and 75.0% (9/12) achieved CCR or showed only MAD at their last follow-up visit. The limitations of our study were the small number of patients included and the relatively short follow-up period.
In MF patients, patches can present as fine wrinkling of the skin resembling atrophy, which can make it difficult to differentiate active MF from CS-induced atrophy in patients treated with topical CSs (Figure 1) and may have caused us to overestimate the occurrence of this AE. Corticosteroid-induced skin atrophy has been studied mainly in normal skin and to a lesser extent in pathological skin in psoriasis and atopic dermatitis. Some of these studies reported that CS-induced atrophy is reversible, and skin thickness can return to normal after topical application of CS is stopped.7
When hypopigmentation is seen around MF lesions, it is a confirmation that the patient is compliant with the therapy. From our experience, local hypopigmentation due to topical CSs is reversible (Figure 2). In some cases, MF patients have applied topical clobetasol propionate to lesional and surrounding skin, and hypopigmentation can be lessened with more careful limited application. In most cases, after discontinuation or tapering of the therapy, the skin returns to its normal color.
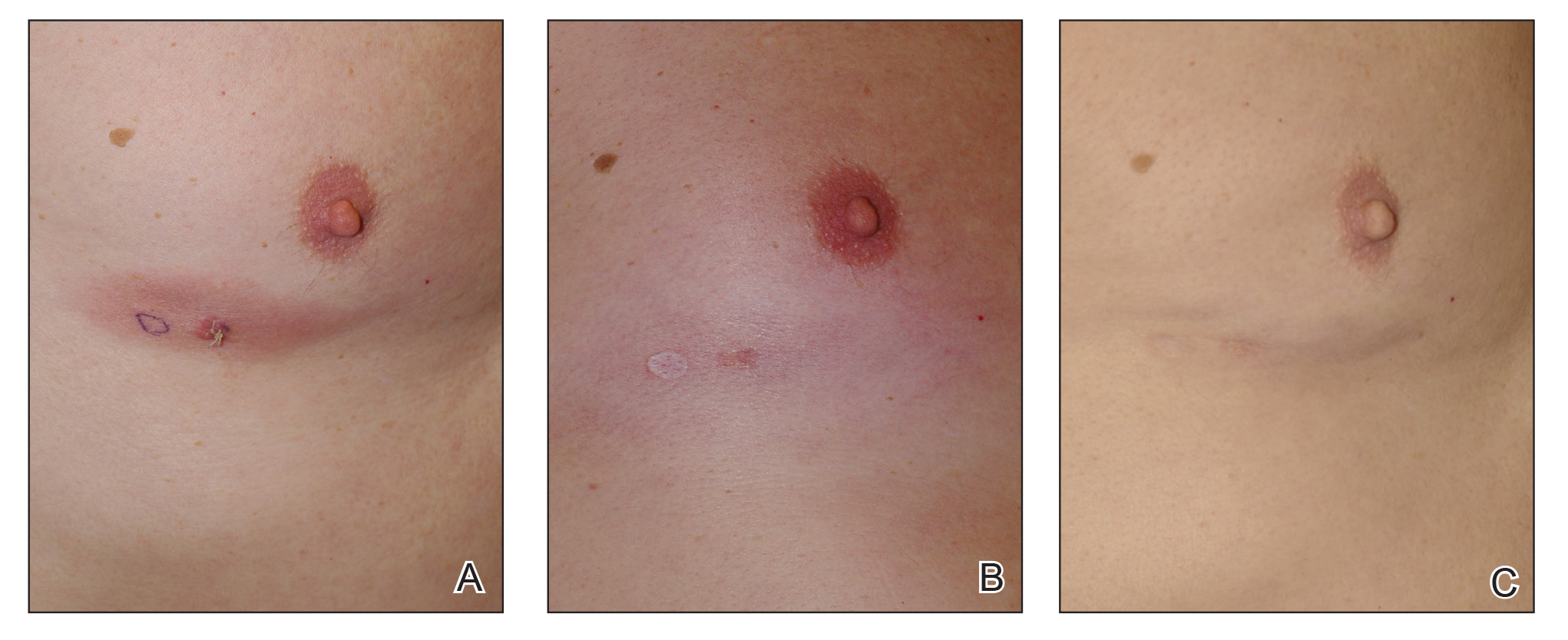
Based on our experience and the results of the current study, we conclude that topical superpotent CSs should remain the first-choice treatment for patients with early-stage MF (stage IA). Although bexarotene gel and mechlorethamine gel are FDA approved for early-stage MF, they are not widely available outside of the United States and are associated with AEs, mainly local skin irritation, rash, and pruritus.4,5 In contrast to bexarotene gel and mechlorethamine gel, topical clobetasol propionate can be used in young children (>12 years) and is classified as pregnancy category C.8
Conclusion
Patients with early-stage MF should be treated with skin-directed therapies, and the choice between different therapeutic options is made based on the physician’s experience with the treatment, patient characteristics, location and morphology of the MF lesions, and the AE profile of the treatment. Based on our experience, superpotent topical CSs are readily available and easily applied, have minor side effects, and remain the mainstay of therapy in patients with stage IA disease. Patients with MF on superpotent topical CS therapy should be monitored periodically and instructed how to identify cutaneous AEs related to treatment.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sezary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e221-217; quiz 240-222.
- Weberschock T, Strametz R, Lorenz M, et al. Interventions for mycosis fungoides [published online September 12, 2012]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD008946.pub2.
- Heald P, Mehlmauer M, Martin AG, et al. Topical bexarotene therapy for patients with refractory or persistent early-stage cutaneous T-cell lymphoma: results of the phase III clinical trial. J Am Acad Dermatol. 2003;49:801-815.
- Lessin SR, Duvic M, Guitart J, et al. Topical chemotherapy in cutaneous T-cell lymphoma: positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. 2013;149:25-32.
- Tadicherla S, Ross K, Shenefelt PD, et al. Topical corticosteroids in dermatology. J Drugs Dermatol. 2009;8:1093-1105.
- Barnes L, Kaya G, Rollason V. Topical corticosteroid-induced skin atrophy: a comprehensive review. Drug Saf. 2015;38:493-509.
- Temovate E (Clobetasol Propionate) Cream, 0.05% [package insert]. Melville, NY: PharmaDerm, a division of Fougera Pharmaceuticals Inc; 2012.
- Nakamura M, Abrouk M, Zhu H, et al. Update on the systemic risks of superpotent topical steroids. J Drugs Dermatol. 2017;16:643-648.
- Prince HM, Whittaker S, Hoppe RT. How I treat mycosis fungoides and Sezary syndrome. Blood. 2009;114:4337-4353.
- Zackheim HS, Kashani-Sabet M, Amin S. Topical corticosteroids for mycosis fungoides. experience in 79 patients. Arch Dermatol. 1998;134:949-954.
- Zackheim HS. Treatment of patch-stage mycosis fungoides with topical corticosteroids. Dermatol Ther. 2003;16:283-287.
- Liu DY, Shaath T, Rajpara AN, et al. Safe and efficacious use of intralesional steroids for the treatment of focally resistant mycosis fungoides. J Drugs Dermatol. 2015;14:466-471.
Mycosis fungoides (MF), the most common variant of cutaneous T-cell lymphoma, is a non-Hodgkin lymphoma of T-cell origin that primarily develops in the skin and has a chronic relapsing course. Early-stage MF (stages IA–IIA) is defined as papules, patches, or plaques with limited (if any) lymph node and blood involvement and no visceral involvement.1 Early-stage MF has a favorable prognosis, and first-line treatments are skin-directed therapies including topical corticosteroids (CSs), topical chemotherapy (nitrogen mustard or carmustine), topical retinoids, topical imiquimod, local radiation, or phototherapy.2 Topical CSs are effective in treating early-stage MF and have been widely used for this indication for several decades; however, there are very little data in the literature on topical CS use in MF.3 Superpotent topical CSs have been shown to have a high overall response rate in early-stage MF3; however, cutaneous side effects associated with long-term topical use include cutaneous atrophy, striae formation, skin fragility, and irritation.
The US Food and Drug Administration (FDA) approved bexarotene gel and mechlorethamine gel for topical treatment of cutaneous lesions in patients with stage IA and IB MF in 2000 and 2013, respectively. Although each may be effective in achieving complete or partial response in MF, both agents are associated with cutaneous side effects, mainly irritation and frequent contact hypersensitivity reactions, respectively.4,5 Additionally, their high prices and limited availability are other major drawbacks of treatment.
At our institution, high-potency topical CSs, specifically once or twice daily clobetasol propionate cream 0.05% prescribed as monotherapy for at least several months, remain the mainstay of treatment in patients with limited patches, papules, and plaques covering less than 10% of the skin surface (stage IA). In this study, we aimed to assess the risk of cutaneous side effects in patients with early-stage MF who were treated with long-term, high-potency topical CSs.
Methods
This prospective observational cohort study included patients with early-stage MF who were seen at the Cutaneous Lymphoma Clinic at Memorial Sloan Kettering Cancer Center (MSKCC) in New York, New York, and were started on a superpotent (class I) topical CS (clobetasol propionate cream 0.05%) as monotherapy for MF from July 2016 to July 2017. The diagnosis of MF had to be supported by clinical findings and histopathologic features. All patients were Fitzpatrick skin types I, II, or III. Eligible patients were evaluated for development of CS-induced cutaneous AEs by physical examination and clinical photography of the treated lesions performed at baseline and as part of routine follow-up visits (usually scheduled every 2 to 6 months) at the MSKCC Cutaneous Lymphoma Clinic. Patients’ skin was evaluated clinically for MF activity, atrophy, telangiectasia, purpura, hypopigmentation, and stretch marks (striae). Use of the topical CS was self-reported and also was documented at follow-up visits. Treatment response was defined as follows: complete clinical response (CCR) if the treated lesions resolved completely compared to initial photography; minimal active disease (MAD) if resolution of the vast majority (≥75%) of lesions was seen; and partial response (PR) if some of the lesions resolved (<75%). We analyzed the treatment response rates and adverse effects (AEs). Results were summarized using descriptive statistics.
Results
We identified 13 patients who were started on topical clobetasol propionate as monotherapy for early-stage MF during the study period. Our cohort included 6 males and 7 females aged 36 to 76 years (median age, 61 years). All but 1 participant were diagnosed with stage IA MF (12/13 [92.3%]); of those, 9 (75.0%) had patch-stage disease and 3 (25.0%) presented with plaques. One (7.7%) participant presented with hyperpigmented patches and plaques that involved a little more than 10% of the skin surface (stage IB), and involvement of the hair follicles was noted on histology (folliculotropic MF). All prior treatments were stopped when participants started the superpotent topical CS: 6 (46.2%) participants had been treated with lower-potency topical agents and 1 (7.7%) participant was getting psoralen plus UVA therapy, while the other 6 (46.2%) participants were receiving no therapy for MF prior to starting the study. All participants were prescribed clobetasol propionate cream 0.05% once or twice daily as monotherapy and were instructed to apply it to the MF lesions only, avoiding skin folds and the face. One participant was lost to follow-up, and another stopped using the clobetasol propionate cream after 1.5 months due to local irritation associated with treatment. At their follow-up visits, the other 11 participants were advised to continue with once-daily treatment with clobetasol propionate or were tapered to once every other day, twice weekly, or once weekly depending on their response to treatment and AEs (Table). Participants were advised not to use more than 50 g of clobetasol propionate cream weekly.

All participants responded to the clobetasol propionate cream, and improvement was noted in the treated lesions; however, progression of disease (from stage IA to stage IB) occurred in 1 (8.3%) participant, and phototherapy was added with good response. The participants in our cohort were followed for 4 to 17 months (median, 11.5 months). At the last follow-up visit, all 12 participants showed treatment response: 4 (33.3%) had CCR, 5 (41.7%) had MAD; and 3 (25.0%) had PR. In one participant with a history of partial response to bexarotene gel 1%, daily clobetasol propionate cream 0.05% initially was used alone for 9 months and was later combined with bexarotene gel once weekly, resulting in MAD.
In 7 (58.3%) participants, no AEs to topical clobetasol propionate were recorded. Four (33.3%) participants developed local hypopigmentation at the application site, and 2 (16.7%) developed cutaneous atrophy with local fine wrinkling of the skin (Figure 1); none of the participants developed stretch marks (striae), telangiectases, or skin fragility. One (8.3%) participant developed a petechial rash at the clobetasol propionate application site that resolved once treatment was discontinued and did not recur after restarting clobetasol propionate twice weekly.

Comment
Topical CSs are the most commonly prescribed agents, either as monotherapy or in combination with other agents, in the treatment of numerous dermatologic conditions, including cutaneous T-cell lymphoma and MF. Cutaneous and systemic AEs have been associated with topical CS use. Local AEs are encountered more frequently and include cutaneous atrophy, striae, telangiectasia, purpura, skin fragility, hypopigmentation, hyperpigmentation, acneform eruptions, and hypertrichosis.6 Factors other than potency of the topical CS agent may affect the development of skin atrophy, including anatomic location, duration of therapy, vehicle, and method and frequency of application.7 The potential for systemic AEs due to percutaneous absorption of high-potency CSs, specifically Cushing syndrome and pathologic adrenal suppression, has been a long-standing concern and led the FDA to recommend limiting the use of superpotent CSs to 50 g weekly for 2 or 4 consecutive weeks.8 However, if using an excess of 50 g weekly is avoided, superpotent topical CSs may be safe to use consecutively for months, perhaps even years, without causing systemic effects.9
The effects of topical CSs in MF include induction of apoptosis; inhibition of lymphocyte binding to the endothelium; and downregulation of transcription factors with decreased cytokines, adhesion molecules, and production of growth factors.2 For patients with limited early-stage MF patches and thin plaques, topical CSs often control the disease for many years and frequently are the only form of therapy required. Intralesional steroids can be effective in treating thicker lesions, such as plaques or tumors.10 In an uncontrolled study, Zackheim et al11 prospectively evaluated the effectiveness and safety of twice-daily use of mainly high-potency topical CSs in 79 patients with MF stages IA to IB and observed an overall response rate of 94%. None of the patients were using systemic agents while being treated with topical CSs. Adverse effects were rare: 2 (2.5%) patients experienced temporary minor irritation from the topical CS, 1 (1.3%) patient developed localized skin atrophy under the breast that resolved several months after she stopped treatment, and 1 (1.3%) patient developed stretch marks on the thighs.11 Zackheim12 later reported treatment of approximately 200 patients with class I topical CSs, and overall response rates were over 90% in stage T1 and over 80% in stage T2 patients. Response to topical CS was reported to be evident within 3 months and often much sooner. Side effects were most likely related to the more prolonged treatment periods. Irritant dermatitis or purpura developed in approximately 10% to 20% of patients, and purpura was seen at the sites of treatment as well as at distant sites. Only a small number of patients developed cutaneous atrophy and striae, which were reversible.12 Successful use of intralesional steroids for treatment-resistant MF was reported in 4 patients who tolerated treatment well without any side effects other than local hypopigmentation in a single patient.13
At MSKCC, the first line of treatment in localized (stage IA) MF in light-skinned individuals most frequently is class I topical CSs, usually clobetasol propionate cream 0.05%. Patients are instructed to apply the cream twice daily on active MF lesions uninterruptedly until completely clear and to avoid using it on the face and in skin folds (axillary, inguinal, and abdominal). Patients are instructed to observe themselves for possible cutaneous AEs related to treatment and to stop or taper treatment if any AEs are noticed. In patients with darker skin, we may recommend other modalities such as narrowband UVB phototherapy for even limited MF disease because of the risk for uneven/hypopigmentation with superpotent CSs.
The current study offers a real-life observation of topical high-potency CSs for treatment of early-stage MF and the associated cutaneous AEs. Local hypopigmentation was identified in 4 participants (33.3%), local skin atrophy was seen in 2 participants (16.7%), and local purpura and irritation were seen in 1 participant each (8.3%). All patients responded to therapy and 75.0% (9/12) achieved CCR or showed only MAD at their last follow-up visit. The limitations of our study were the small number of patients included and the relatively short follow-up period.
In MF patients, patches can present as fine wrinkling of the skin resembling atrophy, which can make it difficult to differentiate active MF from CS-induced atrophy in patients treated with topical CSs (Figure 1) and may have caused us to overestimate the occurrence of this AE. Corticosteroid-induced skin atrophy has been studied mainly in normal skin and to a lesser extent in pathological skin in psoriasis and atopic dermatitis. Some of these studies reported that CS-induced atrophy is reversible, and skin thickness can return to normal after topical application of CS is stopped.7
When hypopigmentation is seen around MF lesions, it is a confirmation that the patient is compliant with the therapy. From our experience, local hypopigmentation due to topical CSs is reversible (Figure 2). In some cases, MF patients have applied topical clobetasol propionate to lesional and surrounding skin, and hypopigmentation can be lessened with more careful limited application. In most cases, after discontinuation or tapering of the therapy, the skin returns to its normal color.

Based on our experience and the results of the current study, we conclude that topical superpotent CSs should remain the first-choice treatment for patients with early-stage MF (stage IA). Although bexarotene gel and mechlorethamine gel are FDA approved for early-stage MF, they are not widely available outside of the United States and are associated with AEs, mainly local skin irritation, rash, and pruritus.4,5 In contrast to bexarotene gel and mechlorethamine gel, topical clobetasol propionate can be used in young children (>12 years) and is classified as pregnancy category C.8
Conclusion
Patients with early-stage MF should be treated with skin-directed therapies, and the choice between different therapeutic options is made based on the physician’s experience with the treatment, patient characteristics, location and morphology of the MF lesions, and the AE profile of the treatment. Based on our experience, superpotent topical CSs are readily available and easily applied, have minor side effects, and remain the mainstay of therapy in patients with stage IA disease. Patients with MF on superpotent topical CS therapy should be monitored periodically and instructed how to identify cutaneous AEs related to treatment.
Mycosis fungoides (MF), the most common variant of cutaneous T-cell lymphoma, is a non-Hodgkin lymphoma of T-cell origin that primarily develops in the skin and has a chronic relapsing course. Early-stage MF (stages IA–IIA) is defined as papules, patches, or plaques with limited (if any) lymph node and blood involvement and no visceral involvement.1 Early-stage MF has a favorable prognosis, and first-line treatments are skin-directed therapies including topical corticosteroids (CSs), topical chemotherapy (nitrogen mustard or carmustine), topical retinoids, topical imiquimod, local radiation, or phototherapy.2 Topical CSs are effective in treating early-stage MF and have been widely used for this indication for several decades; however, there are very little data in the literature on topical CS use in MF.3 Superpotent topical CSs have been shown to have a high overall response rate in early-stage MF3; however, cutaneous side effects associated with long-term topical use include cutaneous atrophy, striae formation, skin fragility, and irritation.
The US Food and Drug Administration (FDA) approved bexarotene gel and mechlorethamine gel for topical treatment of cutaneous lesions in patients with stage IA and IB MF in 2000 and 2013, respectively. Although each may be effective in achieving complete or partial response in MF, both agents are associated with cutaneous side effects, mainly irritation and frequent contact hypersensitivity reactions, respectively.4,5 Additionally, their high prices and limited availability are other major drawbacks of treatment.
At our institution, high-potency topical CSs, specifically once or twice daily clobetasol propionate cream 0.05% prescribed as monotherapy for at least several months, remain the mainstay of treatment in patients with limited patches, papules, and plaques covering less than 10% of the skin surface (stage IA). In this study, we aimed to assess the risk of cutaneous side effects in patients with early-stage MF who were treated with long-term, high-potency topical CSs.
Methods
This prospective observational cohort study included patients with early-stage MF who were seen at the Cutaneous Lymphoma Clinic at Memorial Sloan Kettering Cancer Center (MSKCC) in New York, New York, and were started on a superpotent (class I) topical CS (clobetasol propionate cream 0.05%) as monotherapy for MF from July 2016 to July 2017. The diagnosis of MF had to be supported by clinical findings and histopathologic features. All patients were Fitzpatrick skin types I, II, or III. Eligible patients were evaluated for development of CS-induced cutaneous AEs by physical examination and clinical photography of the treated lesions performed at baseline and as part of routine follow-up visits (usually scheduled every 2 to 6 months) at the MSKCC Cutaneous Lymphoma Clinic. Patients’ skin was evaluated clinically for MF activity, atrophy, telangiectasia, purpura, hypopigmentation, and stretch marks (striae). Use of the topical CS was self-reported and also was documented at follow-up visits. Treatment response was defined as follows: complete clinical response (CCR) if the treated lesions resolved completely compared to initial photography; minimal active disease (MAD) if resolution of the vast majority (≥75%) of lesions was seen; and partial response (PR) if some of the lesions resolved (<75%). We analyzed the treatment response rates and adverse effects (AEs). Results were summarized using descriptive statistics.
Results
We identified 13 patients who were started on topical clobetasol propionate as monotherapy for early-stage MF during the study period. Our cohort included 6 males and 7 females aged 36 to 76 years (median age, 61 years). All but 1 participant were diagnosed with stage IA MF (12/13 [92.3%]); of those, 9 (75.0%) had patch-stage disease and 3 (25.0%) presented with plaques. One (7.7%) participant presented with hyperpigmented patches and plaques that involved a little more than 10% of the skin surface (stage IB), and involvement of the hair follicles was noted on histology (folliculotropic MF). All prior treatments were stopped when participants started the superpotent topical CS: 6 (46.2%) participants had been treated with lower-potency topical agents and 1 (7.7%) participant was getting psoralen plus UVA therapy, while the other 6 (46.2%) participants were receiving no therapy for MF prior to starting the study. All participants were prescribed clobetasol propionate cream 0.05% once or twice daily as monotherapy and were instructed to apply it to the MF lesions only, avoiding skin folds and the face. One participant was lost to follow-up, and another stopped using the clobetasol propionate cream after 1.5 months due to local irritation associated with treatment. At their follow-up visits, the other 11 participants were advised to continue with once-daily treatment with clobetasol propionate or were tapered to once every other day, twice weekly, or once weekly depending on their response to treatment and AEs (Table). Participants were advised not to use more than 50 g of clobetasol propionate cream weekly.

All participants responded to the clobetasol propionate cream, and improvement was noted in the treated lesions; however, progression of disease (from stage IA to stage IB) occurred in 1 (8.3%) participant, and phototherapy was added with good response. The participants in our cohort were followed for 4 to 17 months (median, 11.5 months). At the last follow-up visit, all 12 participants showed treatment response: 4 (33.3%) had CCR, 5 (41.7%) had MAD; and 3 (25.0%) had PR. In one participant with a history of partial response to bexarotene gel 1%, daily clobetasol propionate cream 0.05% initially was used alone for 9 months and was later combined with bexarotene gel once weekly, resulting in MAD.
In 7 (58.3%) participants, no AEs to topical clobetasol propionate were recorded. Four (33.3%) participants developed local hypopigmentation at the application site, and 2 (16.7%) developed cutaneous atrophy with local fine wrinkling of the skin (Figure 1); none of the participants developed stretch marks (striae), telangiectases, or skin fragility. One (8.3%) participant developed a petechial rash at the clobetasol propionate application site that resolved once treatment was discontinued and did not recur after restarting clobetasol propionate twice weekly.

Comment
Topical CSs are the most commonly prescribed agents, either as monotherapy or in combination with other agents, in the treatment of numerous dermatologic conditions, including cutaneous T-cell lymphoma and MF. Cutaneous and systemic AEs have been associated with topical CS use. Local AEs are encountered more frequently and include cutaneous atrophy, striae, telangiectasia, purpura, skin fragility, hypopigmentation, hyperpigmentation, acneform eruptions, and hypertrichosis.6 Factors other than potency of the topical CS agent may affect the development of skin atrophy, including anatomic location, duration of therapy, vehicle, and method and frequency of application.7 The potential for systemic AEs due to percutaneous absorption of high-potency CSs, specifically Cushing syndrome and pathologic adrenal suppression, has been a long-standing concern and led the FDA to recommend limiting the use of superpotent CSs to 50 g weekly for 2 or 4 consecutive weeks.8 However, if using an excess of 50 g weekly is avoided, superpotent topical CSs may be safe to use consecutively for months, perhaps even years, without causing systemic effects.9
The effects of topical CSs in MF include induction of apoptosis; inhibition of lymphocyte binding to the endothelium; and downregulation of transcription factors with decreased cytokines, adhesion molecules, and production of growth factors.2 For patients with limited early-stage MF patches and thin plaques, topical CSs often control the disease for many years and frequently are the only form of therapy required. Intralesional steroids can be effective in treating thicker lesions, such as plaques or tumors.10 In an uncontrolled study, Zackheim et al11 prospectively evaluated the effectiveness and safety of twice-daily use of mainly high-potency topical CSs in 79 patients with MF stages IA to IB and observed an overall response rate of 94%. None of the patients were using systemic agents while being treated with topical CSs. Adverse effects were rare: 2 (2.5%) patients experienced temporary minor irritation from the topical CS, 1 (1.3%) patient developed localized skin atrophy under the breast that resolved several months after she stopped treatment, and 1 (1.3%) patient developed stretch marks on the thighs.11 Zackheim12 later reported treatment of approximately 200 patients with class I topical CSs, and overall response rates were over 90% in stage T1 and over 80% in stage T2 patients. Response to topical CS was reported to be evident within 3 months and often much sooner. Side effects were most likely related to the more prolonged treatment periods. Irritant dermatitis or purpura developed in approximately 10% to 20% of patients, and purpura was seen at the sites of treatment as well as at distant sites. Only a small number of patients developed cutaneous atrophy and striae, which were reversible.12 Successful use of intralesional steroids for treatment-resistant MF was reported in 4 patients who tolerated treatment well without any side effects other than local hypopigmentation in a single patient.13
At MSKCC, the first line of treatment in localized (stage IA) MF in light-skinned individuals most frequently is class I topical CSs, usually clobetasol propionate cream 0.05%. Patients are instructed to apply the cream twice daily on active MF lesions uninterruptedly until completely clear and to avoid using it on the face and in skin folds (axillary, inguinal, and abdominal). Patients are instructed to observe themselves for possible cutaneous AEs related to treatment and to stop or taper treatment if any AEs are noticed. In patients with darker skin, we may recommend other modalities such as narrowband UVB phototherapy for even limited MF disease because of the risk for uneven/hypopigmentation with superpotent CSs.
The current study offers a real-life observation of topical high-potency CSs for treatment of early-stage MF and the associated cutaneous AEs. Local hypopigmentation was identified in 4 participants (33.3%), local skin atrophy was seen in 2 participants (16.7%), and local purpura and irritation were seen in 1 participant each (8.3%). All patients responded to therapy and 75.0% (9/12) achieved CCR or showed only MAD at their last follow-up visit. The limitations of our study were the small number of patients included and the relatively short follow-up period.
In MF patients, patches can present as fine wrinkling of the skin resembling atrophy, which can make it difficult to differentiate active MF from CS-induced atrophy in patients treated with topical CSs (Figure 1) and may have caused us to overestimate the occurrence of this AE. Corticosteroid-induced skin atrophy has been studied mainly in normal skin and to a lesser extent in pathological skin in psoriasis and atopic dermatitis. Some of these studies reported that CS-induced atrophy is reversible, and skin thickness can return to normal after topical application of CS is stopped.7
When hypopigmentation is seen around MF lesions, it is a confirmation that the patient is compliant with the therapy. From our experience, local hypopigmentation due to topical CSs is reversible (Figure 2). In some cases, MF patients have applied topical clobetasol propionate to lesional and surrounding skin, and hypopigmentation can be lessened with more careful limited application. In most cases, after discontinuation or tapering of the therapy, the skin returns to its normal color.

Based on our experience and the results of the current study, we conclude that topical superpotent CSs should remain the first-choice treatment for patients with early-stage MF (stage IA). Although bexarotene gel and mechlorethamine gel are FDA approved for early-stage MF, they are not widely available outside of the United States and are associated with AEs, mainly local skin irritation, rash, and pruritus.4,5 In contrast to bexarotene gel and mechlorethamine gel, topical clobetasol propionate can be used in young children (>12 years) and is classified as pregnancy category C.8
Conclusion
Patients with early-stage MF should be treated with skin-directed therapies, and the choice between different therapeutic options is made based on the physician’s experience with the treatment, patient characteristics, location and morphology of the MF lesions, and the AE profile of the treatment. Based on our experience, superpotent topical CSs are readily available and easily applied, have minor side effects, and remain the mainstay of therapy in patients with stage IA disease. Patients with MF on superpotent topical CS therapy should be monitored periodically and instructed how to identify cutaneous AEs related to treatment.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sezary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e221-217; quiz 240-222.
- Weberschock T, Strametz R, Lorenz M, et al. Interventions for mycosis fungoides [published online September 12, 2012]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD008946.pub2.
- Heald P, Mehlmauer M, Martin AG, et al. Topical bexarotene therapy for patients with refractory or persistent early-stage cutaneous T-cell lymphoma: results of the phase III clinical trial. J Am Acad Dermatol. 2003;49:801-815.
- Lessin SR, Duvic M, Guitart J, et al. Topical chemotherapy in cutaneous T-cell lymphoma: positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. 2013;149:25-32.
- Tadicherla S, Ross K, Shenefelt PD, et al. Topical corticosteroids in dermatology. J Drugs Dermatol. 2009;8:1093-1105.
- Barnes L, Kaya G, Rollason V. Topical corticosteroid-induced skin atrophy: a comprehensive review. Drug Saf. 2015;38:493-509.
- Temovate E (Clobetasol Propionate) Cream, 0.05% [package insert]. Melville, NY: PharmaDerm, a division of Fougera Pharmaceuticals Inc; 2012.
- Nakamura M, Abrouk M, Zhu H, et al. Update on the systemic risks of superpotent topical steroids. J Drugs Dermatol. 2017;16:643-648.
- Prince HM, Whittaker S, Hoppe RT. How I treat mycosis fungoides and Sezary syndrome. Blood. 2009;114:4337-4353.
- Zackheim HS, Kashani-Sabet M, Amin S. Topical corticosteroids for mycosis fungoides. experience in 79 patients. Arch Dermatol. 1998;134:949-954.
- Zackheim HS. Treatment of patch-stage mycosis fungoides with topical corticosteroids. Dermatol Ther. 2003;16:283-287.
- Liu DY, Shaath T, Rajpara AN, et al. Safe and efficacious use of intralesional steroids for the treatment of focally resistant mycosis fungoides. J Drugs Dermatol. 2015;14:466-471.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sezary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e221-217; quiz 240-222.
- Weberschock T, Strametz R, Lorenz M, et al. Interventions for mycosis fungoides [published online September 12, 2012]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD008946.pub2.
- Heald P, Mehlmauer M, Martin AG, et al. Topical bexarotene therapy for patients with refractory or persistent early-stage cutaneous T-cell lymphoma: results of the phase III clinical trial. J Am Acad Dermatol. 2003;49:801-815.
- Lessin SR, Duvic M, Guitart J, et al. Topical chemotherapy in cutaneous T-cell lymphoma: positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. 2013;149:25-32.
- Tadicherla S, Ross K, Shenefelt PD, et al. Topical corticosteroids in dermatology. J Drugs Dermatol. 2009;8:1093-1105.
- Barnes L, Kaya G, Rollason V. Topical corticosteroid-induced skin atrophy: a comprehensive review. Drug Saf. 2015;38:493-509.
- Temovate E (Clobetasol Propionate) Cream, 0.05% [package insert]. Melville, NY: PharmaDerm, a division of Fougera Pharmaceuticals Inc; 2012.
- Nakamura M, Abrouk M, Zhu H, et al. Update on the systemic risks of superpotent topical steroids. J Drugs Dermatol. 2017;16:643-648.
- Prince HM, Whittaker S, Hoppe RT. How I treat mycosis fungoides and Sezary syndrome. Blood. 2009;114:4337-4353.
- Zackheim HS, Kashani-Sabet M, Amin S. Topical corticosteroids for mycosis fungoides. experience in 79 patients. Arch Dermatol. 1998;134:949-954.
- Zackheim HS. Treatment of patch-stage mycosis fungoides with topical corticosteroids. Dermatol Ther. 2003;16:283-287.
- Liu DY, Shaath T, Rajpara AN, et al. Safe and efficacious use of intralesional steroids for the treatment of focally resistant mycosis fungoides. J Drugs Dermatol. 2015;14:466-471.
Practice Points
- Topical corticosteroid (CS) treatment is a safe skin-directed therapy that can effectively obtain complete and long-term response in patients with early-stage mycosis fungoides (MF).
- Despite the availability of optional topical treatments in MF, topical superpotent class I CSs are still considered the first-line treatment in patients with limited disease (stage IA).
- Patients using prolonged topical superpotent CSs should be monitored periodically and instructed on how to identify cutaneous adverse effects related to treatment, mainly local hypopigmentation and skin atrophy.
No link seen between methotrexate, interstitial lung disease in RA
Patients with rheumatoid arthritis (RA) have an elevated risk of interstitial lung disease (ILD), but methotrexate does not accentuate that risk and may in fact be protective, new data show. These were among key findings of a pair of studies reported at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Although a guideline-recommended cornerstone in the management of RA, methotrexate has been associated with both hypersensitivity pneumonitis and diffuse lung disease. However, its involvement in the development of ILD among patients with RA is unclear.
A Danish study of more than 30,000 RA patients reported at the congress found that their risk of ILD was about three to five times that of the general population. However, risk did not differ significantly whether they had filled a methotrexate prescription or not.
In addition, a multinational case-control study of more than 1,000 RA patients also reported at the congress found that, compared with never-users of methotrexate, ever-users actually had a 59% lower likelihood of developing ILD.
However, both studies were limited by their retrospective design, Elizabeth R. Volkmann, MD, codirector of the connective tissue disease–related interstitial lung disease program at the University of California, Los Angeles, cautioned in an interview. Hence, there was likely systematic bias and confounding.
“I would interpret the conclusions of both studies with caution,” she maintained. “To understand how a particular intervention, such as methotrexate use, affects the outcome of ILD development, a prospective design is needed, which adequately adjusts for known ILD risk factors, such as male sex and smoking.”
As to whether the new findings are practice changing and how they might affect patient counseling, “the answers to these questions are not straightforward and depend on other patient-related factors,” according to Dr. Volkmann.
Danish nationwide study
René Cordtz, MD, a clinical assistant at the Center for Rheumatology and Spine Diseases, Rigshospitalet‐Gentofte, Copenhagen, and colleagues conducted a nationwide population-based cohort study using registry data from 1997 to 2015 to assess lung disease among patients with RA by prescriptions filled.
Results based on 30,512 RA patients showed that, compared with peers filling no methotrexate prescriptions, patients filling at least one did not have a significantly elevated risk of ILD at either 1 year of follow-up (hazard ratio, 1.03) or 5 years of follow-up (HR, 1.00). (Findings were similar for sulfasalazine, with respective nonsignificant HRs of 0.88 and 1.14.)
In addition, patients with RA had a similarly sharply elevated 5-year risk of ILD relative to the general population regardless of whether they had filled neither methotrexate nor sulfasalazine prescriptions (standardized incidence ratio, 3.38) or had filled prescriptions for methotrexate only (SIR, 3.63), sulfasalazine only (SIR, 4.12), or both (SIR, 5.45).
“RA patients have an increased risk of ILD, compared to the general population, which was not surprising, but very importantly, that risk was not further exacerbated in those treated with methotrexate,” Dr. Cordtz concluded. “We do acknowledge that purchasing your medicine is different from taking your medicine, which is why we found it extra reassuring that when requiring at least two methotrexate prescriptions to be considered exposed, it did not change our results.”
Multinational study
Pierre-Antoine Juge, MD, a rheumatologist at Bichat-Claude Bernard Hospital, Paris, and colleagues performed a case-control study among 482 RA patients with ILD and 741 RA patients without ILD in three cohorts: a French discovery cohort, a multinational (Brazilian, Italian, Mexican, United Kingdom, and United States) replication cohort, and a combined cohort. Those with methotrexate hypersensitivity pneumonitis were excluded.
Results showed that relative to peers without ILD, patients with ILD had a lower prevalence of ever having used methotrexate and had received a lower cumulative methotrexate dose, findings that were consistent across all three cohorts.
Methotrexate ever-use was associated with a significantly lower adjusted likelihood of ILD in the discovery cohort (odds ratio, 0.46), the replication cohort (OR, 0.38), and the combined cohort (OR, 0.41). Furthermore, ever-users were less commonly represented among patients with ILD regardless of chest high-resolution CT pattern (usual interstitial pneumonia pattern vs. not).
Finally, methotrexate use appeared to delay the adjusted time to onset of ILD by 3.5 years in the discovery cohort (P = .001), by 3.2 years in the replication cohort (P < .0001), and by 3.5 years in the combined cohort (P < .0001).
“Outside of methotrexate hypersensitivity pneumonitis, methotrexate was not a risk factor for RA-associated ILD in our study. We observed an inverse relationship that was similar whatever the high-resolution CT pattern,” Dr. Juge commented. “But this possible protective effect should be confirmed through a dedicated prospective, randomized, controlled trial.”
“Methotrexate should not be considered as a causal factor for RA-associated ILD, and its [discontinuation] should be discussed through a multidisciplinary discussion,” he recommended. In addition, “this study does not investigate the impact of methotrexate use on RA-associated ILD prognosis.”
The Danish study did not receive any specific funding, and none of its authors reported having any financial disclosures. The multinational study did not receive any specific funding. Dr. Juge disclosed that he had no relevant conflicts of interest, but many of his coauthors reported financial relationships with industry. Dr. Volkmann disclosed consulting for Boehringer Ingelheim and Forbius, and receiving grant support from Forbius and Corbus.
SOURCES: Cordtz R et al. Ann Rheum Dis. 2020;79[suppl 1]:147-8, Abstract OP0232; Juge P-A et al. Ann Rheum Dis. 2020;79[suppl 1]:25, Abstract OP0236.
Patients with rheumatoid arthritis (RA) have an elevated risk of interstitial lung disease (ILD), but methotrexate does not accentuate that risk and may in fact be protective, new data show. These were among key findings of a pair of studies reported at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Although a guideline-recommended cornerstone in the management of RA, methotrexate has been associated with both hypersensitivity pneumonitis and diffuse lung disease. However, its involvement in the development of ILD among patients with RA is unclear.
A Danish study of more than 30,000 RA patients reported at the congress found that their risk of ILD was about three to five times that of the general population. However, risk did not differ significantly whether they had filled a methotrexate prescription or not.
In addition, a multinational case-control study of more than 1,000 RA patients also reported at the congress found that, compared with never-users of methotrexate, ever-users actually had a 59% lower likelihood of developing ILD.
However, both studies were limited by their retrospective design, Elizabeth R. Volkmann, MD, codirector of the connective tissue disease–related interstitial lung disease program at the University of California, Los Angeles, cautioned in an interview. Hence, there was likely systematic bias and confounding.
“I would interpret the conclusions of both studies with caution,” she maintained. “To understand how a particular intervention, such as methotrexate use, affects the outcome of ILD development, a prospective design is needed, which adequately adjusts for known ILD risk factors, such as male sex and smoking.”
As to whether the new findings are practice changing and how they might affect patient counseling, “the answers to these questions are not straightforward and depend on other patient-related factors,” according to Dr. Volkmann.
Danish nationwide study
René Cordtz, MD, a clinical assistant at the Center for Rheumatology and Spine Diseases, Rigshospitalet‐Gentofte, Copenhagen, and colleagues conducted a nationwide population-based cohort study using registry data from 1997 to 2015 to assess lung disease among patients with RA by prescriptions filled.
Results based on 30,512 RA patients showed that, compared with peers filling no methotrexate prescriptions, patients filling at least one did not have a significantly elevated risk of ILD at either 1 year of follow-up (hazard ratio, 1.03) or 5 years of follow-up (HR, 1.00). (Findings were similar for sulfasalazine, with respective nonsignificant HRs of 0.88 and 1.14.)
In addition, patients with RA had a similarly sharply elevated 5-year risk of ILD relative to the general population regardless of whether they had filled neither methotrexate nor sulfasalazine prescriptions (standardized incidence ratio, 3.38) or had filled prescriptions for methotrexate only (SIR, 3.63), sulfasalazine only (SIR, 4.12), or both (SIR, 5.45).
“RA patients have an increased risk of ILD, compared to the general population, which was not surprising, but very importantly, that risk was not further exacerbated in those treated with methotrexate,” Dr. Cordtz concluded. “We do acknowledge that purchasing your medicine is different from taking your medicine, which is why we found it extra reassuring that when requiring at least two methotrexate prescriptions to be considered exposed, it did not change our results.”
Multinational study
Pierre-Antoine Juge, MD, a rheumatologist at Bichat-Claude Bernard Hospital, Paris, and colleagues performed a case-control study among 482 RA patients with ILD and 741 RA patients without ILD in three cohorts: a French discovery cohort, a multinational (Brazilian, Italian, Mexican, United Kingdom, and United States) replication cohort, and a combined cohort. Those with methotrexate hypersensitivity pneumonitis were excluded.
Results showed that relative to peers without ILD, patients with ILD had a lower prevalence of ever having used methotrexate and had received a lower cumulative methotrexate dose, findings that were consistent across all three cohorts.
Methotrexate ever-use was associated with a significantly lower adjusted likelihood of ILD in the discovery cohort (odds ratio, 0.46), the replication cohort (OR, 0.38), and the combined cohort (OR, 0.41). Furthermore, ever-users were less commonly represented among patients with ILD regardless of chest high-resolution CT pattern (usual interstitial pneumonia pattern vs. not).
Finally, methotrexate use appeared to delay the adjusted time to onset of ILD by 3.5 years in the discovery cohort (P = .001), by 3.2 years in the replication cohort (P < .0001), and by 3.5 years in the combined cohort (P < .0001).
“Outside of methotrexate hypersensitivity pneumonitis, methotrexate was not a risk factor for RA-associated ILD in our study. We observed an inverse relationship that was similar whatever the high-resolution CT pattern,” Dr. Juge commented. “But this possible protective effect should be confirmed through a dedicated prospective, randomized, controlled trial.”
“Methotrexate should not be considered as a causal factor for RA-associated ILD, and its [discontinuation] should be discussed through a multidisciplinary discussion,” he recommended. In addition, “this study does not investigate the impact of methotrexate use on RA-associated ILD prognosis.”
The Danish study did not receive any specific funding, and none of its authors reported having any financial disclosures. The multinational study did not receive any specific funding. Dr. Juge disclosed that he had no relevant conflicts of interest, but many of his coauthors reported financial relationships with industry. Dr. Volkmann disclosed consulting for Boehringer Ingelheim and Forbius, and receiving grant support from Forbius and Corbus.
SOURCES: Cordtz R et al. Ann Rheum Dis. 2020;79[suppl 1]:147-8, Abstract OP0232; Juge P-A et al. Ann Rheum Dis. 2020;79[suppl 1]:25, Abstract OP0236.
Patients with rheumatoid arthritis (RA) have an elevated risk of interstitial lung disease (ILD), but methotrexate does not accentuate that risk and may in fact be protective, new data show. These were among key findings of a pair of studies reported at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Although a guideline-recommended cornerstone in the management of RA, methotrexate has been associated with both hypersensitivity pneumonitis and diffuse lung disease. However, its involvement in the development of ILD among patients with RA is unclear.
A Danish study of more than 30,000 RA patients reported at the congress found that their risk of ILD was about three to five times that of the general population. However, risk did not differ significantly whether they had filled a methotrexate prescription or not.
In addition, a multinational case-control study of more than 1,000 RA patients also reported at the congress found that, compared with never-users of methotrexate, ever-users actually had a 59% lower likelihood of developing ILD.
However, both studies were limited by their retrospective design, Elizabeth R. Volkmann, MD, codirector of the connective tissue disease–related interstitial lung disease program at the University of California, Los Angeles, cautioned in an interview. Hence, there was likely systematic bias and confounding.
“I would interpret the conclusions of both studies with caution,” she maintained. “To understand how a particular intervention, such as methotrexate use, affects the outcome of ILD development, a prospective design is needed, which adequately adjusts for known ILD risk factors, such as male sex and smoking.”
As to whether the new findings are practice changing and how they might affect patient counseling, “the answers to these questions are not straightforward and depend on other patient-related factors,” according to Dr. Volkmann.
Danish nationwide study
René Cordtz, MD, a clinical assistant at the Center for Rheumatology and Spine Diseases, Rigshospitalet‐Gentofte, Copenhagen, and colleagues conducted a nationwide population-based cohort study using registry data from 1997 to 2015 to assess lung disease among patients with RA by prescriptions filled.
Results based on 30,512 RA patients showed that, compared with peers filling no methotrexate prescriptions, patients filling at least one did not have a significantly elevated risk of ILD at either 1 year of follow-up (hazard ratio, 1.03) or 5 years of follow-up (HR, 1.00). (Findings were similar for sulfasalazine, with respective nonsignificant HRs of 0.88 and 1.14.)
In addition, patients with RA had a similarly sharply elevated 5-year risk of ILD relative to the general population regardless of whether they had filled neither methotrexate nor sulfasalazine prescriptions (standardized incidence ratio, 3.38) or had filled prescriptions for methotrexate only (SIR, 3.63), sulfasalazine only (SIR, 4.12), or both (SIR, 5.45).
“RA patients have an increased risk of ILD, compared to the general population, which was not surprising, but very importantly, that risk was not further exacerbated in those treated with methotrexate,” Dr. Cordtz concluded. “We do acknowledge that purchasing your medicine is different from taking your medicine, which is why we found it extra reassuring that when requiring at least two methotrexate prescriptions to be considered exposed, it did not change our results.”
Multinational study
Pierre-Antoine Juge, MD, a rheumatologist at Bichat-Claude Bernard Hospital, Paris, and colleagues performed a case-control study among 482 RA patients with ILD and 741 RA patients without ILD in three cohorts: a French discovery cohort, a multinational (Brazilian, Italian, Mexican, United Kingdom, and United States) replication cohort, and a combined cohort. Those with methotrexate hypersensitivity pneumonitis were excluded.
Results showed that relative to peers without ILD, patients with ILD had a lower prevalence of ever having used methotrexate and had received a lower cumulative methotrexate dose, findings that were consistent across all three cohorts.
Methotrexate ever-use was associated with a significantly lower adjusted likelihood of ILD in the discovery cohort (odds ratio, 0.46), the replication cohort (OR, 0.38), and the combined cohort (OR, 0.41). Furthermore, ever-users were less commonly represented among patients with ILD regardless of chest high-resolution CT pattern (usual interstitial pneumonia pattern vs. not).
Finally, methotrexate use appeared to delay the adjusted time to onset of ILD by 3.5 years in the discovery cohort (P = .001), by 3.2 years in the replication cohort (P < .0001), and by 3.5 years in the combined cohort (P < .0001).
“Outside of methotrexate hypersensitivity pneumonitis, methotrexate was not a risk factor for RA-associated ILD in our study. We observed an inverse relationship that was similar whatever the high-resolution CT pattern,” Dr. Juge commented. “But this possible protective effect should be confirmed through a dedicated prospective, randomized, controlled trial.”
“Methotrexate should not be considered as a causal factor for RA-associated ILD, and its [discontinuation] should be discussed through a multidisciplinary discussion,” he recommended. In addition, “this study does not investigate the impact of methotrexate use on RA-associated ILD prognosis.”
The Danish study did not receive any specific funding, and none of its authors reported having any financial disclosures. The multinational study did not receive any specific funding. Dr. Juge disclosed that he had no relevant conflicts of interest, but many of his coauthors reported financial relationships with industry. Dr. Volkmann disclosed consulting for Boehringer Ingelheim and Forbius, and receiving grant support from Forbius and Corbus.
SOURCES: Cordtz R et al. Ann Rheum Dis. 2020;79[suppl 1]:147-8, Abstract OP0232; Juge P-A et al. Ann Rheum Dis. 2020;79[suppl 1]:25, Abstract OP0236.
FROM THE EULAR 2020 E-CONGRESS
ACR reacts to study disclosing industry donations
Institutions receive most research funding
Second only to the American Society of Clinical Oncology, the volunteer leaders of the American College of Rheumatology with ties to industry received the highest median payment amounts, a new cross-sectional study reveals.
Total research payments exceeded $54 million to ASCO leaders and $20 million to ACR leaders, for example. The investigators identified the 10 most common and costly conditions in the United States – including heart disease, trauma-related disorders, mental disorders, and others. They then used a new national database to explore the financial relationships between pharmaceutical and device manufacturers and the leaders of “influential U.S. professional medical associations active across these disease areas.” Steven Echard, executive vice president of the American College of Rheumatology, responded to the study findings, published in the BMJ. “We require our leaders to tell us about any payments they receive as part of their professional and personal activities that may pose actual or potential conflicts, post this information transparently to our website, and adjust what projects and initiatives they can participate in to avoid any undue influence,” he said in an interview.
“Many of the disclosures included in the study were due to individuals participating in research projects to move the needle forward in the care of rheumatology patients,” he added.
There remains an ongoing debate about how close the relationships should be between medical associations and industry, wrote lead author Ray Moynihan, PhD, assistant professor in the Institute for Evidence-Based Healthcare at Bond University, Gold Coast, Australia, and colleagues.
Majority report relationship with industry
Dr. Moynihan and associates conducted the first study to evaluate these relationships in such detail. Using the U.S. government’s Open Payments database, established in 2013, they assessed research payments and general payments for consultancy, royalties, and hospitality. The study included the current year of board membership, as well as the 4 years prior and 1 year after membership.
Overall, out of 293 physician association leaders, 235 or 80% had a financial relationship. None of the associations in the study featured a leadership free of financial ties.
Payments totaled almost $130 million, including nearly $25 million in general payments, almost $105 million for research, and about half a million in other payments. The research payments went primarily to institutions with leaders named as principal investigators, they noted.
“The most common misperception [in the BMJ study] is that all the funding identified represents personal payments to the individuals,” Mr. Echard said. “Because research dollars are included, almost 80% of the payments referenced in the article – sometimes more – go to individuals’ academic institutions.” These funds cover overhead, lab materials, protected time for said research, research assistant salaries, and other expenses.
“We have other board of director members who have no industry relationships at all,” he added.
Part of business model
“Faculty members are expected to bring in funding to support research, so most academic institutions receive industry funding, and it’s considered part of their business model,” Mr. Echard said. “For this reason, we generally do not consider these payments the same as being on advisory boards, speakers bureaus, or going to dinners, etc.”
The median total amounts linked to individual leaders varied between associations. For example, the median amount was $518,000 for ASCO leaders, as previously reported by Medscape Medical News.
Volunteer leaders for the ACR received a median $251,000. Not all ACR board of director members receive funding in this range, Mr. Echard said. “We have board of director members who work at academic institutions that can receive funding that high due to their participation in research studies, particularly when looking over a 4-year period prior to their leadership role, as the study did.”
At the lower end, median total payments were $404 for leaders of the American College of Physicians and $212 for those of the American Psychiatric Association.
In an accompanying editorial, Jake Checketts, DO, and Matt Vassar, PhD, of the Oklahoma State University Center for Health Sciences, Tulsa, proposed five actions that “could mitigate or even eliminate the overwhelming presence of financial conflicts of interest among medical societies and associations. This would protect these groups from producing biased documents or policies, which in turn would protect all physicians and the patients they treat.”
They made five proposals:
- Each association must take the initiative to evaluate its present conflicts using open payments.
- Associations should alter their recruitment processes to yield balanced and diverse groups of physician leaders largely free from financial conflicts of interest.
- The creation of standards for promoting medical associations that are free from financial conflicts of interest, similar to the framework within the Institute of Medicine’s standards for producing clinical practice guidelines we can trust.
- Greater reliance on the Sunshine Act and open payments in the United States (and elsewhere for countries with similar data) could eliminate the need for the traditional “honor system” of financial self-disclosure, which is ineffective and inaccurate at best.
- All medical associations, guidelines groups, and policy makers provide links from their documents and websites to open payments data for each U.S.-based physician contributor. Such links would make it easier for anyone, including patients, to evaluate any risk of bias.
On a final note, the ACR prohibits key college leaders, including the ACR president, ACR president-elect, foundation president, and others from having direct financial ties to commercial entities in their conflict of interest guidelines.
“We agree that actively managing conflict of interest is important to maintaining the integrity and reputation of an association with the medical community and with the public,” Mr. Echard said. “And all actual, potential, and perceived conflicts of interest should be addressed and managed through a disclosure process.”
Several authors of the study reported receiving grants from the Australian National Health and Medical Research Council. Mr. Echard, Dr. Checketts, and Dr. Vassar had no relevant disclosures.
SOURCE: Moynihan R et al. BMJ. 2020;369:m1505.
Institutions receive most research funding
Institutions receive most research funding
Second only to the American Society of Clinical Oncology, the volunteer leaders of the American College of Rheumatology with ties to industry received the highest median payment amounts, a new cross-sectional study reveals.
Total research payments exceeded $54 million to ASCO leaders and $20 million to ACR leaders, for example. The investigators identified the 10 most common and costly conditions in the United States – including heart disease, trauma-related disorders, mental disorders, and others. They then used a new national database to explore the financial relationships between pharmaceutical and device manufacturers and the leaders of “influential U.S. professional medical associations active across these disease areas.” Steven Echard, executive vice president of the American College of Rheumatology, responded to the study findings, published in the BMJ. “We require our leaders to tell us about any payments they receive as part of their professional and personal activities that may pose actual or potential conflicts, post this information transparently to our website, and adjust what projects and initiatives they can participate in to avoid any undue influence,” he said in an interview.
“Many of the disclosures included in the study were due to individuals participating in research projects to move the needle forward in the care of rheumatology patients,” he added.
There remains an ongoing debate about how close the relationships should be between medical associations and industry, wrote lead author Ray Moynihan, PhD, assistant professor in the Institute for Evidence-Based Healthcare at Bond University, Gold Coast, Australia, and colleagues.
Majority report relationship with industry
Dr. Moynihan and associates conducted the first study to evaluate these relationships in such detail. Using the U.S. government’s Open Payments database, established in 2013, they assessed research payments and general payments for consultancy, royalties, and hospitality. The study included the current year of board membership, as well as the 4 years prior and 1 year after membership.
Overall, out of 293 physician association leaders, 235 or 80% had a financial relationship. None of the associations in the study featured a leadership free of financial ties.
Payments totaled almost $130 million, including nearly $25 million in general payments, almost $105 million for research, and about half a million in other payments. The research payments went primarily to institutions with leaders named as principal investigators, they noted.
“The most common misperception [in the BMJ study] is that all the funding identified represents personal payments to the individuals,” Mr. Echard said. “Because research dollars are included, almost 80% of the payments referenced in the article – sometimes more – go to individuals’ academic institutions.” These funds cover overhead, lab materials, protected time for said research, research assistant salaries, and other expenses.
“We have other board of director members who have no industry relationships at all,” he added.
Part of business model
“Faculty members are expected to bring in funding to support research, so most academic institutions receive industry funding, and it’s considered part of their business model,” Mr. Echard said. “For this reason, we generally do not consider these payments the same as being on advisory boards, speakers bureaus, or going to dinners, etc.”
The median total amounts linked to individual leaders varied between associations. For example, the median amount was $518,000 for ASCO leaders, as previously reported by Medscape Medical News.
Volunteer leaders for the ACR received a median $251,000. Not all ACR board of director members receive funding in this range, Mr. Echard said. “We have board of director members who work at academic institutions that can receive funding that high due to their participation in research studies, particularly when looking over a 4-year period prior to their leadership role, as the study did.”
At the lower end, median total payments were $404 for leaders of the American College of Physicians and $212 for those of the American Psychiatric Association.
In an accompanying editorial, Jake Checketts, DO, and Matt Vassar, PhD, of the Oklahoma State University Center for Health Sciences, Tulsa, proposed five actions that “could mitigate or even eliminate the overwhelming presence of financial conflicts of interest among medical societies and associations. This would protect these groups from producing biased documents or policies, which in turn would protect all physicians and the patients they treat.”
They made five proposals:
- Each association must take the initiative to evaluate its present conflicts using open payments.
- Associations should alter their recruitment processes to yield balanced and diverse groups of physician leaders largely free from financial conflicts of interest.
- The creation of standards for promoting medical associations that are free from financial conflicts of interest, similar to the framework within the Institute of Medicine’s standards for producing clinical practice guidelines we can trust.
- Greater reliance on the Sunshine Act and open payments in the United States (and elsewhere for countries with similar data) could eliminate the need for the traditional “honor system” of financial self-disclosure, which is ineffective and inaccurate at best.
- All medical associations, guidelines groups, and policy makers provide links from their documents and websites to open payments data for each U.S.-based physician contributor. Such links would make it easier for anyone, including patients, to evaluate any risk of bias.
On a final note, the ACR prohibits key college leaders, including the ACR president, ACR president-elect, foundation president, and others from having direct financial ties to commercial entities in their conflict of interest guidelines.
“We agree that actively managing conflict of interest is important to maintaining the integrity and reputation of an association with the medical community and with the public,” Mr. Echard said. “And all actual, potential, and perceived conflicts of interest should be addressed and managed through a disclosure process.”
Several authors of the study reported receiving grants from the Australian National Health and Medical Research Council. Mr. Echard, Dr. Checketts, and Dr. Vassar had no relevant disclosures.
SOURCE: Moynihan R et al. BMJ. 2020;369:m1505.
Second only to the American Society of Clinical Oncology, the volunteer leaders of the American College of Rheumatology with ties to industry received the highest median payment amounts, a new cross-sectional study reveals.
Total research payments exceeded $54 million to ASCO leaders and $20 million to ACR leaders, for example. The investigators identified the 10 most common and costly conditions in the United States – including heart disease, trauma-related disorders, mental disorders, and others. They then used a new national database to explore the financial relationships between pharmaceutical and device manufacturers and the leaders of “influential U.S. professional medical associations active across these disease areas.” Steven Echard, executive vice president of the American College of Rheumatology, responded to the study findings, published in the BMJ. “We require our leaders to tell us about any payments they receive as part of their professional and personal activities that may pose actual or potential conflicts, post this information transparently to our website, and adjust what projects and initiatives they can participate in to avoid any undue influence,” he said in an interview.
“Many of the disclosures included in the study were due to individuals participating in research projects to move the needle forward in the care of rheumatology patients,” he added.
There remains an ongoing debate about how close the relationships should be between medical associations and industry, wrote lead author Ray Moynihan, PhD, assistant professor in the Institute for Evidence-Based Healthcare at Bond University, Gold Coast, Australia, and colleagues.
Majority report relationship with industry
Dr. Moynihan and associates conducted the first study to evaluate these relationships in such detail. Using the U.S. government’s Open Payments database, established in 2013, they assessed research payments and general payments for consultancy, royalties, and hospitality. The study included the current year of board membership, as well as the 4 years prior and 1 year after membership.
Overall, out of 293 physician association leaders, 235 or 80% had a financial relationship. None of the associations in the study featured a leadership free of financial ties.
Payments totaled almost $130 million, including nearly $25 million in general payments, almost $105 million for research, and about half a million in other payments. The research payments went primarily to institutions with leaders named as principal investigators, they noted.
“The most common misperception [in the BMJ study] is that all the funding identified represents personal payments to the individuals,” Mr. Echard said. “Because research dollars are included, almost 80% of the payments referenced in the article – sometimes more – go to individuals’ academic institutions.” These funds cover overhead, lab materials, protected time for said research, research assistant salaries, and other expenses.
“We have other board of director members who have no industry relationships at all,” he added.
Part of business model
“Faculty members are expected to bring in funding to support research, so most academic institutions receive industry funding, and it’s considered part of their business model,” Mr. Echard said. “For this reason, we generally do not consider these payments the same as being on advisory boards, speakers bureaus, or going to dinners, etc.”
The median total amounts linked to individual leaders varied between associations. For example, the median amount was $518,000 for ASCO leaders, as previously reported by Medscape Medical News.
Volunteer leaders for the ACR received a median $251,000. Not all ACR board of director members receive funding in this range, Mr. Echard said. “We have board of director members who work at academic institutions that can receive funding that high due to their participation in research studies, particularly when looking over a 4-year period prior to their leadership role, as the study did.”
At the lower end, median total payments were $404 for leaders of the American College of Physicians and $212 for those of the American Psychiatric Association.
In an accompanying editorial, Jake Checketts, DO, and Matt Vassar, PhD, of the Oklahoma State University Center for Health Sciences, Tulsa, proposed five actions that “could mitigate or even eliminate the overwhelming presence of financial conflicts of interest among medical societies and associations. This would protect these groups from producing biased documents or policies, which in turn would protect all physicians and the patients they treat.”
They made five proposals:
- Each association must take the initiative to evaluate its present conflicts using open payments.
- Associations should alter their recruitment processes to yield balanced and diverse groups of physician leaders largely free from financial conflicts of interest.
- The creation of standards for promoting medical associations that are free from financial conflicts of interest, similar to the framework within the Institute of Medicine’s standards for producing clinical practice guidelines we can trust.
- Greater reliance on the Sunshine Act and open payments in the United States (and elsewhere for countries with similar data) could eliminate the need for the traditional “honor system” of financial self-disclosure, which is ineffective and inaccurate at best.
- All medical associations, guidelines groups, and policy makers provide links from their documents and websites to open payments data for each U.S.-based physician contributor. Such links would make it easier for anyone, including patients, to evaluate any risk of bias.
On a final note, the ACR prohibits key college leaders, including the ACR president, ACR president-elect, foundation president, and others from having direct financial ties to commercial entities in their conflict of interest guidelines.
“We agree that actively managing conflict of interest is important to maintaining the integrity and reputation of an association with the medical community and with the public,” Mr. Echard said. “And all actual, potential, and perceived conflicts of interest should be addressed and managed through a disclosure process.”
Several authors of the study reported receiving grants from the Australian National Health and Medical Research Council. Mr. Echard, Dr. Checketts, and Dr. Vassar had no relevant disclosures.
SOURCE: Moynihan R et al. BMJ. 2020;369:m1505.
FROM THE BMJ
The CDI APP adviser
A novel approach to APP documentation engagement
As hospitals and clinicians, we are facing increased scrutiny of the care we provide to our patients. There is increased demand for more transparency of our outcomes and a need for increased efficiency of the care we provide in the setting of already significant documentation burden and its known impact on provider burnout.
Clinical documentation integrity (CDI) is an instrumental department which supports complete and accurate documentation, serves as a bridge between physicians and hospital coders such that hospital reimbursement is appropriate and quality metrics are attributed appropriately to the hospital, service lines, and individual providers. Complete and accurate documentation also leads to the submission of coded/claims-based data reflecting provider true intent and to clinically valid data for research and patient centric purposes. For this reason, the physician adviser role as a liaison between physicians and CDI and coding, in addition to utilization management and case management, has become more commonplace. The physician adviser role has been a mainstay of CDI programs across the United States since as early as 2012.
At the University of Colorado Health (UCHealth), the physician adviser role first began in 2015 at our major academic medical center, the University of Colorado Hospital (UCH). That physician adviser, after the additional physician adviser FTE at UCH, having established relationships with physicians across service lines, began to focus on CDI-related education and communication as it pertained to inpatient documentation.
At our institution we have approximately 500 advance practice providers (APPs). Approximately two-thirds of the APPs care for inpatients on a myriad of different service lines and, along with physician learners from interns to fellows, complete the bulk of the documentation in the electronic health record.
In early 2018, the UCHealth office of advanced practice collaborated with CDI in its mission to optimize documentation with the aim to have a positive impact on reimbursement and quality metrics while highlighting APP value. In the relatively early stages of the collaboration it became evident that an APP adviser could be an innovative and effective approach in engaging our many APPs with CDI as faculty members who are generally service line based and, as such, invested in hospital and service line outcomes.
A business case for a new position of APP adviser for CDI was formulated based on not only the number of APP faculty and learners at our institution, but also on the premise that the level of consistency APPs provide would increase reliability in the adoption and adaptation of documentation practices as medicine and coding rules evolve. In addition, APP documentation can stand alone without physician attestation or signature, unlike physicians in training, further making them ideally suited collaborators. The position was approved by hospital leadership and the first APP adviser for CDI in the country (of whom we are aware) was hired at UCH in July 2019.
A dedicated APP CDI adviser facilitates the success of a CDI/APP collaboration through a better understanding of APP engagement needs largely by creating new and/or fostering existing relationships between the APP adviser and the APPs for each service line. The APP CDI adviser identifies the needs of the team in order to maximally enhance their documentation while illustrating how the work/collaboration can positively contribute to APP clinical and/or academic goals. The APP CDI adviser possesses a deeper knowledge of APP clinical work flow and how that work flow might be impacting the documentation. He or she utilizes information gathered from the APP team to create more efficient note templates, provide lunch and learns with different service line APPs, and offering 1:1 drop-in documentation support, allowing for more feedback flexibility in context of their clinical work flow.
This real time input may be received more positively and be perceived as less intimidating in the peer-to-peer context. The APP adviser also attends various educational forums to which the physician advisers may not have access. For example, the APP adviser attends monthly APP orientation to meet new APPs for the institution, attends APP council, is a member of the APP steering committee, and provides documentation tips for the APP monthly newsletter.
At this point we are in the process of collecting pre- and post data to illustrate the benefit of a CDI APP adviser (and the CDI APP collaboration as a whole) through metrics such as CC/MCC capture rate, case mix index, and mortality and length of stay as influenced by the level of complexity in documentation. We hope to add APPs as advisers across the UCHealth system over time and to continue to highlight and publish the experience and outcomes related to this innovative role as it evolves such that other institutions across the country will consider this type of collaboration.
Dr. Anoff is associate professor of clinical practice in the division of hospital medicine and medical director of clinical documentation integrity at University of Colorado Health, Denver. Ms. Brill is senior instructor in the department of neurosurgery and APP adviser of clinical documentation integrity at UCHealth Denver Metro.
A novel approach to APP documentation engagement
A novel approach to APP documentation engagement
As hospitals and clinicians, we are facing increased scrutiny of the care we provide to our patients. There is increased demand for more transparency of our outcomes and a need for increased efficiency of the care we provide in the setting of already significant documentation burden and its known impact on provider burnout.
Clinical documentation integrity (CDI) is an instrumental department which supports complete and accurate documentation, serves as a bridge between physicians and hospital coders such that hospital reimbursement is appropriate and quality metrics are attributed appropriately to the hospital, service lines, and individual providers. Complete and accurate documentation also leads to the submission of coded/claims-based data reflecting provider true intent and to clinically valid data for research and patient centric purposes. For this reason, the physician adviser role as a liaison between physicians and CDI and coding, in addition to utilization management and case management, has become more commonplace. The physician adviser role has been a mainstay of CDI programs across the United States since as early as 2012.
At the University of Colorado Health (UCHealth), the physician adviser role first began in 2015 at our major academic medical center, the University of Colorado Hospital (UCH). That physician adviser, after the additional physician adviser FTE at UCH, having established relationships with physicians across service lines, began to focus on CDI-related education and communication as it pertained to inpatient documentation.
At our institution we have approximately 500 advance practice providers (APPs). Approximately two-thirds of the APPs care for inpatients on a myriad of different service lines and, along with physician learners from interns to fellows, complete the bulk of the documentation in the electronic health record.
In early 2018, the UCHealth office of advanced practice collaborated with CDI in its mission to optimize documentation with the aim to have a positive impact on reimbursement and quality metrics while highlighting APP value. In the relatively early stages of the collaboration it became evident that an APP adviser could be an innovative and effective approach in engaging our many APPs with CDI as faculty members who are generally service line based and, as such, invested in hospital and service line outcomes.
A business case for a new position of APP adviser for CDI was formulated based on not only the number of APP faculty and learners at our institution, but also on the premise that the level of consistency APPs provide would increase reliability in the adoption and adaptation of documentation practices as medicine and coding rules evolve. In addition, APP documentation can stand alone without physician attestation or signature, unlike physicians in training, further making them ideally suited collaborators. The position was approved by hospital leadership and the first APP adviser for CDI in the country (of whom we are aware) was hired at UCH in July 2019.
A dedicated APP CDI adviser facilitates the success of a CDI/APP collaboration through a better understanding of APP engagement needs largely by creating new and/or fostering existing relationships between the APP adviser and the APPs for each service line. The APP CDI adviser identifies the needs of the team in order to maximally enhance their documentation while illustrating how the work/collaboration can positively contribute to APP clinical and/or academic goals. The APP CDI adviser possesses a deeper knowledge of APP clinical work flow and how that work flow might be impacting the documentation. He or she utilizes information gathered from the APP team to create more efficient note templates, provide lunch and learns with different service line APPs, and offering 1:1 drop-in documentation support, allowing for more feedback flexibility in context of their clinical work flow.
This real time input may be received more positively and be perceived as less intimidating in the peer-to-peer context. The APP adviser also attends various educational forums to which the physician advisers may not have access. For example, the APP adviser attends monthly APP orientation to meet new APPs for the institution, attends APP council, is a member of the APP steering committee, and provides documentation tips for the APP monthly newsletter.
At this point we are in the process of collecting pre- and post data to illustrate the benefit of a CDI APP adviser (and the CDI APP collaboration as a whole) through metrics such as CC/MCC capture rate, case mix index, and mortality and length of stay as influenced by the level of complexity in documentation. We hope to add APPs as advisers across the UCHealth system over time and to continue to highlight and publish the experience and outcomes related to this innovative role as it evolves such that other institutions across the country will consider this type of collaboration.
Dr. Anoff is associate professor of clinical practice in the division of hospital medicine and medical director of clinical documentation integrity at University of Colorado Health, Denver. Ms. Brill is senior instructor in the department of neurosurgery and APP adviser of clinical documentation integrity at UCHealth Denver Metro.
As hospitals and clinicians, we are facing increased scrutiny of the care we provide to our patients. There is increased demand for more transparency of our outcomes and a need for increased efficiency of the care we provide in the setting of already significant documentation burden and its known impact on provider burnout.
Clinical documentation integrity (CDI) is an instrumental department which supports complete and accurate documentation, serves as a bridge between physicians and hospital coders such that hospital reimbursement is appropriate and quality metrics are attributed appropriately to the hospital, service lines, and individual providers. Complete and accurate documentation also leads to the submission of coded/claims-based data reflecting provider true intent and to clinically valid data for research and patient centric purposes. For this reason, the physician adviser role as a liaison between physicians and CDI and coding, in addition to utilization management and case management, has become more commonplace. The physician adviser role has been a mainstay of CDI programs across the United States since as early as 2012.
At the University of Colorado Health (UCHealth), the physician adviser role first began in 2015 at our major academic medical center, the University of Colorado Hospital (UCH). That physician adviser, after the additional physician adviser FTE at UCH, having established relationships with physicians across service lines, began to focus on CDI-related education and communication as it pertained to inpatient documentation.
At our institution we have approximately 500 advance practice providers (APPs). Approximately two-thirds of the APPs care for inpatients on a myriad of different service lines and, along with physician learners from interns to fellows, complete the bulk of the documentation in the electronic health record.
In early 2018, the UCHealth office of advanced practice collaborated with CDI in its mission to optimize documentation with the aim to have a positive impact on reimbursement and quality metrics while highlighting APP value. In the relatively early stages of the collaboration it became evident that an APP adviser could be an innovative and effective approach in engaging our many APPs with CDI as faculty members who are generally service line based and, as such, invested in hospital and service line outcomes.
A business case for a new position of APP adviser for CDI was formulated based on not only the number of APP faculty and learners at our institution, but also on the premise that the level of consistency APPs provide would increase reliability in the adoption and adaptation of documentation practices as medicine and coding rules evolve. In addition, APP documentation can stand alone without physician attestation or signature, unlike physicians in training, further making them ideally suited collaborators. The position was approved by hospital leadership and the first APP adviser for CDI in the country (of whom we are aware) was hired at UCH in July 2019.
A dedicated APP CDI adviser facilitates the success of a CDI/APP collaboration through a better understanding of APP engagement needs largely by creating new and/or fostering existing relationships between the APP adviser and the APPs for each service line. The APP CDI adviser identifies the needs of the team in order to maximally enhance their documentation while illustrating how the work/collaboration can positively contribute to APP clinical and/or academic goals. The APP CDI adviser possesses a deeper knowledge of APP clinical work flow and how that work flow might be impacting the documentation. He or she utilizes information gathered from the APP team to create more efficient note templates, provide lunch and learns with different service line APPs, and offering 1:1 drop-in documentation support, allowing for more feedback flexibility in context of their clinical work flow.
This real time input may be received more positively and be perceived as less intimidating in the peer-to-peer context. The APP adviser also attends various educational forums to which the physician advisers may not have access. For example, the APP adviser attends monthly APP orientation to meet new APPs for the institution, attends APP council, is a member of the APP steering committee, and provides documentation tips for the APP monthly newsletter.
At this point we are in the process of collecting pre- and post data to illustrate the benefit of a CDI APP adviser (and the CDI APP collaboration as a whole) through metrics such as CC/MCC capture rate, case mix index, and mortality and length of stay as influenced by the level of complexity in documentation. We hope to add APPs as advisers across the UCHealth system over time and to continue to highlight and publish the experience and outcomes related to this innovative role as it evolves such that other institutions across the country will consider this type of collaboration.
Dr. Anoff is associate professor of clinical practice in the division of hospital medicine and medical director of clinical documentation integrity at University of Colorado Health, Denver. Ms. Brill is senior instructor in the department of neurosurgery and APP adviser of clinical documentation integrity at UCHealth Denver Metro.
Former smokers using e-cigarettes at risk for cigarette smoking relapse
The use of , results from a large longitudinal cohort study demonstrated.
“For the many clinicians treating former smokers who have successfully quit all nicotine products, the implications are that use of [electronic nicotine delivery systems] should be discouraged, just as use of all other tobacco products is discouraged,” researchers led by Colm D. Everard, PhD, reported in a study published in JAMA Network Open (2020 Jun 5. doi: 10.1001/jamanetworkopen.2020.4813).
Dr. Everard, of the National Institute on Drug Abuse, and colleagues based their comments on results from a survey of adult former smokers who participated in Waves 1-4 of the Population Assessment of Tobacco and Health (PATH) Study (2013-2018). They limited the analysis to 2,273 former cigarette smokers who self-reported reported no tobacco product use at Wave 1, and categorized them as recent former smokers (defined as having last smoked within the past 12 previous months) or as long-term former smokers (defined as having last smoked for longer ago than in the previous 12 months). The main outcome of interest was the self-reported current use of cigarettes at follow-up interviews, which was defined as every day or some days. Electronic nicotine delivery systems (ENDS) comprised e-cigarettes, e-cigars, e-pipes, and e-hookahs. Other tobacco products included cigars, pipe tobacco, hookahs, snus tobacco, other smokeless tobacco, and dissolvable tobacco.
Of the 2,273 adult former smokers, 52% were women, 60% were older than age 50, and 80% were non-Hispanic white. Adjusted hazard ratio (AHR) analysis revealed that the use of ENDS was associated with significant risk of cigarette smoking relapse among recent former smokers (AHR 1.63) and among long-term former smokers (AHR 3.79). The use of other tobacco products was associated with significant risk for cigarette smoking relapse among recent former smokers (AHR 1.97) and among long-term former smokers (AHR 3.82).
The authors acknowledged certain limitations of the study, including the fact that it did not assess different ENDS devices, different e-liquid nicotine levels, or frequency of ENDS use and their associations with cigarette smoking relapse. It also did not explore the mechanism by which ENDS use may lead to reestablishing or reinforcing nicotine-seeking behavior among former cigarette users. “Determining pharmacologic, behavioral, or some other explanation for these findings may require laboratory-based research,” they wrote.
The PATH Study is supported with federal funds from the National Institute on Drug Abuse, the National Institutes of Health, and the Food and Drug Administration and Department of Health and Human Services under a contract to Westat. One of the study authors, Wilson M. Compton, MD, reported having long-term stock holdings in General Electric, 3M, and Pfizer. The other authors reported having no financial disclosures.
The use of , results from a large longitudinal cohort study demonstrated.
“For the many clinicians treating former smokers who have successfully quit all nicotine products, the implications are that use of [electronic nicotine delivery systems] should be discouraged, just as use of all other tobacco products is discouraged,” researchers led by Colm D. Everard, PhD, reported in a study published in JAMA Network Open (2020 Jun 5. doi: 10.1001/jamanetworkopen.2020.4813).
Dr. Everard, of the National Institute on Drug Abuse, and colleagues based their comments on results from a survey of adult former smokers who participated in Waves 1-4 of the Population Assessment of Tobacco and Health (PATH) Study (2013-2018). They limited the analysis to 2,273 former cigarette smokers who self-reported reported no tobacco product use at Wave 1, and categorized them as recent former smokers (defined as having last smoked within the past 12 previous months) or as long-term former smokers (defined as having last smoked for longer ago than in the previous 12 months). The main outcome of interest was the self-reported current use of cigarettes at follow-up interviews, which was defined as every day or some days. Electronic nicotine delivery systems (ENDS) comprised e-cigarettes, e-cigars, e-pipes, and e-hookahs. Other tobacco products included cigars, pipe tobacco, hookahs, snus tobacco, other smokeless tobacco, and dissolvable tobacco.
Of the 2,273 adult former smokers, 52% were women, 60% were older than age 50, and 80% were non-Hispanic white. Adjusted hazard ratio (AHR) analysis revealed that the use of ENDS was associated with significant risk of cigarette smoking relapse among recent former smokers (AHR 1.63) and among long-term former smokers (AHR 3.79). The use of other tobacco products was associated with significant risk for cigarette smoking relapse among recent former smokers (AHR 1.97) and among long-term former smokers (AHR 3.82).
The authors acknowledged certain limitations of the study, including the fact that it did not assess different ENDS devices, different e-liquid nicotine levels, or frequency of ENDS use and their associations with cigarette smoking relapse. It also did not explore the mechanism by which ENDS use may lead to reestablishing or reinforcing nicotine-seeking behavior among former cigarette users. “Determining pharmacologic, behavioral, or some other explanation for these findings may require laboratory-based research,” they wrote.
The PATH Study is supported with federal funds from the National Institute on Drug Abuse, the National Institutes of Health, and the Food and Drug Administration and Department of Health and Human Services under a contract to Westat. One of the study authors, Wilson M. Compton, MD, reported having long-term stock holdings in General Electric, 3M, and Pfizer. The other authors reported having no financial disclosures.
The use of , results from a large longitudinal cohort study demonstrated.
“For the many clinicians treating former smokers who have successfully quit all nicotine products, the implications are that use of [electronic nicotine delivery systems] should be discouraged, just as use of all other tobacco products is discouraged,” researchers led by Colm D. Everard, PhD, reported in a study published in JAMA Network Open (2020 Jun 5. doi: 10.1001/jamanetworkopen.2020.4813).
Dr. Everard, of the National Institute on Drug Abuse, and colleagues based their comments on results from a survey of adult former smokers who participated in Waves 1-4 of the Population Assessment of Tobacco and Health (PATH) Study (2013-2018). They limited the analysis to 2,273 former cigarette smokers who self-reported reported no tobacco product use at Wave 1, and categorized them as recent former smokers (defined as having last smoked within the past 12 previous months) or as long-term former smokers (defined as having last smoked for longer ago than in the previous 12 months). The main outcome of interest was the self-reported current use of cigarettes at follow-up interviews, which was defined as every day or some days. Electronic nicotine delivery systems (ENDS) comprised e-cigarettes, e-cigars, e-pipes, and e-hookahs. Other tobacco products included cigars, pipe tobacco, hookahs, snus tobacco, other smokeless tobacco, and dissolvable tobacco.
Of the 2,273 adult former smokers, 52% were women, 60% were older than age 50, and 80% were non-Hispanic white. Adjusted hazard ratio (AHR) analysis revealed that the use of ENDS was associated with significant risk of cigarette smoking relapse among recent former smokers (AHR 1.63) and among long-term former smokers (AHR 3.79). The use of other tobacco products was associated with significant risk for cigarette smoking relapse among recent former smokers (AHR 1.97) and among long-term former smokers (AHR 3.82).
The authors acknowledged certain limitations of the study, including the fact that it did not assess different ENDS devices, different e-liquid nicotine levels, or frequency of ENDS use and their associations with cigarette smoking relapse. It also did not explore the mechanism by which ENDS use may lead to reestablishing or reinforcing nicotine-seeking behavior among former cigarette users. “Determining pharmacologic, behavioral, or some other explanation for these findings may require laboratory-based research,” they wrote.
The PATH Study is supported with federal funds from the National Institute on Drug Abuse, the National Institutes of Health, and the Food and Drug Administration and Department of Health and Human Services under a contract to Westat. One of the study authors, Wilson M. Compton, MD, reported having long-term stock holdings in General Electric, 3M, and Pfizer. The other authors reported having no financial disclosures.
FROM JAMA NETWORK OPEN
Outcomes Comparison of the Veterans’ Choice Program With the Veterans Affairs Health Care System for Hepatitis C Treatment
Population studies show high prevalence of chronic hepatitis C virus (HCV) infection among veterans, especially Vietnam War era veterans.1,2 The development of safe and efficacious direct-acting antiviral (DAA) medications to treat HCV infection made the majority of those infected eligible for treatment. However, the large number of veterans needing DAA treatment stressed the resources of the US Department of Veterans Affairs (VA) health care system. This occurred while Congress was focused on reducing wait times for veterans receiving care at the VA.
Congress passed the Veterans Access, Choice, and Accountability Act on August 7, 2014, leading to the creation of the Veterans Choice Program. Legislators felt there were inappropriate delays in care at the VA, and the Choice program was meant to address this problem and provide an “apples-to-apples comparison [of the VA] with non-VA hospitals.”3
Congress acknowledged the importance of curing HCV in the veteran population and allocated $1.5 billion for fiscal year (FY) 2016 for DAAs. The VA Central Office (VACO) carefully monitored these resources. The first policy memorandum from VACO for HCV care, issued on May 21, 2015, recommended that the sickest patients who will benefit from the treatment “receive priority over those who are less ill.”4,5 Those who met criteria for advanced liver disease were prioritized for treatment at the VA, while those who did not meet criteria were given the option of receiving treatment through Choice, or waiting for a change in policy.6 Over time, revisions to the guidelines relaxed the criteria for VA treatment eligibility, and on February 24, 2016, all restrictions on HCV treatment at the VA were lifted.7,8
The aim of this study was to provide a comparison of VA and non-VA care, specifically to determine whether care provided through Choice was timelier and more cost effective than care provided by the VA, and whether there was a quality difference. The high prevalence among veterans, wellestablished standards of care, and finite treatment course with clear indicators of success and failure makes HCV treatment an ideal disease with which to make this comparison.
Methods
We retrospectively analyzed the VA electronic health records of all veterans seen in the VA Loma Linda Healthcare System (VALLHCS) Hepatology clinic for chronic HCV infection during FY 2016 who were referred to Choice for HCV treatment. One hundred veterans met these criteria, encompassing the Choice population; 71 were seen at least once by a non-VA (Choice) health care provider (HCP) and 61 completed a treatment regimen through Choice. Treatment completion was defined as cessation of medication after the planned duration of therapy, or early termination of medication without resumption by that HCP. The Choice population was matched to an equal number of veterans who received HCV treatment from VALLHCS HCPs.
Data collected included age, gender, HCV genotype, determinants of liver fibrosis, and treatment success (defined as sustained virologic response at 12 weeks after the last dose of medication [SVR12]). Determinants of liver fibrosis included documented cirrhosis or complications of cirrhosis, Fibrosis-4 score (Fib-4), and platelet count.
Treatment failures were categorized as nonresponse (defined as detectable HCV viral load at the end of treatment), relapse (defined as an undetectable HCV viral load at the end of treatment with a subsequent positive test), and early termination (defined as a failure to complete the planned treatment regimen). Documented patient nonadherence, medical comorbidities that affected the treatment protocol, mental health diagnoses, and active social issues (defined as active or history of heavy alcohol use, active or history of illicit drug use, lack of social support, and homelessness) were noted.
Timeliness of delivery of care was measured in days. For the VA group, the wait time was defined as the date the consult for HCV treatment was placed to the date of the initial appointment with the HCV treatment provider. For the Choice group, the wait time was defined as the date the referral to the Choice program was made to the date of the initial appointment with the Choice HCP. Treatment regimens were evaluated for appropriateness based on guidelines from VACO and the American Association for the Study of Liver Diseases.9-11
Tests performed by Choice providers were evaluated for whether they were relevant to HCV care and whether similar data already were available from VALLHCS. Tests that were not indicated were identified as unnecessary costs incurred by the Choice program, as were tests that had to be repeated at the VA because of a lack of documentation from the Choice provider. All medications given inappropriately were considered added costs. Medicare reimbursement rates for the most applicable Current Procedural Terminology (CPT) code and VA national contract pricing for medications were used for calculations. This study was approved by the VALLHCS institutional review board.
Statistical Analysis
IBM (Armonk, NY) Statistical Package for Social Sciences software was used to evaluate for differences in Fib-4, platelet count, prevalence of cirrhosis, prevalence of medical comorbidities, prevalence of mental health comorbidities, prevalence of the social issues defined in the Methods section, time from referral to time of appointment date, and SVR12 rate between the VA and Choice groups.
Exclusions
There were 15 veterans in the VA group who had a wait time of > 100 days. Of these, 5 (33%) were initially Choice referrals, but due to negative interactions with the Choice provider, the veterans returned to VALLHCS for care. Two of the 15 (13%) did not keep appointments and were lost to follow up. Six of the 15 (40%) had medical comorbidities that required more immediate attention, so HCV treatment initiation was deliberately moved back. The final 2 veterans scheduled their appointments unusually far apart, artificially increasing their wait time. Given that these were unique situations and some of the veterans received care from both Choice and VA providers, a decision was made to exclude these individuals from the study.
It has been shown that platelet count correlates with degree of liver fibrosis, a concept that is the basis for the Fib-4 scoring system.12 Studies have shown that platelet count is a survival predictor in those with cirrhosis, and thrombocytopenia is a negative predictor of HCV treatment success using peginterferon and ribavirin13,14 Therefore, the VA memorandum automatically assigned the sickest individuals to the VA for HCV treatment. The goal of this study was to compare the impact of factors other than stage of fibrosis on HCV treatment success, which is why the 12 veterans with platelet count < 100,000 in the VA group were excluded. There were no veterans with platelet count < 100,000 in the Choice group.
When comparing SVR12 rates between the VA and Choice groups, every veteran treated at VALLHCS in FY 2016 was included, increasing the number in the VA group from 100 to 320 and therefore the power of this comparison.
Results
A summary of the statistical analysis can be found in Table 1. The genotype distribution was consistent with epidemiological studies, including those specific to veterans.15,16 There were statistically significant differences (P < .001) in mean Fib-4 and mean platelet count. The VA group had a higher Fib-4 and lower platelet count. Seventy-four percent of the VA population was defined as cirrhotic, while only 3% of the Choice population met similar criteria (P < .001). The VA and Choice groups were similar in terms of age, gender, and genotype distribution (Table 2).
The VA group was found to have a higher prevalence of comorbidities that affected HCV treatment. These conditions included but were not limited to: chronic kidney disease that precluded the use of certain medications, any condition that required medication with a known interaction with DAAs (ie, proton pump inhibitors, statins, and amiodarone), and cirrhosis if it impacted the treatment regimen. The difference in the prevalence of mental health comorbidities was not significant (P = .39), but there was a higher prevalence of social issues among the VA group (P = .002).
The mean wait time from referral to appointment was 28.6 days for the VA group and 42.3 days for the Choice group (P < .001), indicating that a Choice referral took longer to complete than a referral within the VA for HCV treatment. Thirty of the 71 (42%) veterans seen by a Choice provider accrued extraneous cost, with a mean additional cost of $8,561.40 per veteran. In the Choice group, 61 veterans completed a treatment regimen with the Choice HCP. Fifty-five veterans completed treatment and had available SVR12 data (6 were lost to follow up without SVR12 testing) and 50 (91%) had confirmed SVR12. The charts of the 5 treatment failures were reviewed to discern the cause for failure. Two cases involved early termination of therapy, 3 involved relapse and 2 failed to comply with medication instructions. There was 1 case of the Choice HCP not addressing simultaneous use of ledipasvir and a proton pump inhibitor, potentially causing an interaction, and 1 case where both the VA and Choice providers failed to recognize indicators of cirrhosis, which impacted the regimen used.
In the VALLHCS group, records of 320 veterans who completed treatment and had SVR12 testing were reviewed. While the Choice memorandum was active, veterans selected to be treated at VALLHCS had advanced liver fibrosis or cirrhosis, medical and mental health comorbidities that increased the risk of treatment complications or were considered to have difficulty adhering to the medication regimen. For this group, 296 (93%) had confirmed SVR12. Eighteen of the 24 (75%) treatment failures were complicated by nonadherence, including all 13 cases of early termination. One patient died from complications of decompensated cirrhosis before completing treatment, and 1 did not receive HCV medications during a hospital admission due to poor coordination of care between the VA inpatient and outpatient pharmacy services, leading to multiple missed doses.
The difference in SVR12 rates (ie, treatment failure rates), between the VA and Choice groups was not statistically significant (P = .61). None of the specific reasons for treatment failure had a statistically significant difference between groups. A treatment failure analysis is shown in Table 3, and Table 4 indicates the breakdown of treatment regimens.
Discussion
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, consisting of 152 medical centers and > 1,700 sites of care. The VA has the potential to meet the health care needs of 21.6 million veterans. About 9 million veterans are enrolled in the VA system and 5.9 million received health care through VHA.17 However, every medical service cannot realistically be made available at every facility, and some veterans have difficulty gaining access to VHA care; distance and wait times have been well-publicized issues that need further exploration.18,19 The Choice program is an attempt to meet gaps in VA coverage using non-VA HCPs.
HCV infection is a specific diagnosis with national treatment guidelines and wellstudied treatments; it can be cured, with an evidence- based definition of cure. The VACO policy memorandum to refer less sick veterans to Choice while treating sicker veterans at the VA provided the opportunity to directly compare the quality of the 2 programs. The SVR12 rates of VALLHCS and Choice providers were comparable to the national average at the time, and while the difference in SVR12 rate was not significant, VALLHCS treated a significantly higher number of patients with cirrhosis because of the referral criteria.20
The significant difference in medical comorbidities between the VA and Choice groups was not surprising, partly because of the referral criteria. Cirrhosis can impact the treatment regimen, especially in regard to use of ribavirin. Since the presence of mental health comorbidities did not affect selection into the Choice group, it makes sense that there was no significant difference in prevalence between the groups.
VACO allowed veterans with HCV treatment plans that VA HCPs felt were too complicated for the Choice program to be treated by VHA HCPs.9 VALLHCS exercised this right for veterans at risk for nonadherence, because in HCV treatment, nonadherence leads to treatment failure and development of drug resistant virus strains. Therefore, veterans who would have difficulty traveling to VALLHCS to pick up medications, those who lacked means of communication (such as those who were homeless), and those who had active substance abuse were treated at the VA, where closer monitoring and immediate access to a wide range of services was possible. Studies have confirmed the impact of these types of issues on HCV treatment adherence and success. 21 This explains the higher prevalence of social issues in the VA group.
For an internal referral for HCV treatment at VALLHCS, the hepatology provider submits a consult request to the HCV treatment provider, who works in the same office, making direct communication simple. The main administrative limiting factor to minimizing wait times is the number of HCPs, which is dependent on hiring allowances.
When a veteran is referred to Choice, the VA provider places a consult for non-VA care, which the VA Office of Community Care processes by compiling relevant documents and sending the package to Triwest Healthcare Alliance, a private insurance processor contracted with the VA. Triwest selects the Choice provider, often without any input from the VA, and arranges the veteran’s initial appointment.22 Geographic distance to the veteran’s address is the main selection criteria for Triwest. Documents sent between the Choice and VA HCPs go through the Office of Community Care and Triwest. This significantly increases the potential for delays and failed communication. Triwest had a comprehensive list of providers deemed to be qualified to treat HCV within the geographic catchment of VALLHCS. This list was reviewed, and all veterans referred to Choice had HCPs near their home address; therefore, availability of Choice HCPs was not an issue.
The VA can provide guidance on management of the veteran in the form of bundle packages containing a list of services for which the Choice provider is authorized to provide, and ones the Choice provider is not authorized to provide. Some Choice HCPs ordered tests that were not authorized for HCV treatment such as esophagogastroduodenoscopy, colonoscopy, and liver biopsy. In all, 17 of 71 (24%) veterans seen by Choice HCPs had tests performed or ordered that VA HCPs would not have obtained for the purpose of HCV treatment (Table 5).
In order to prevent veterans from receiving unnecessary tests, a VALLHCS hepatologist asked to be notified by VA administrators overseeing Choice referrals whenever a secondary authorization request (SAR) was submitted by a Choice HCP. This strategy is not standard VA practice, therefore at many VA sites these requested tests would have been performed by the Choice HCP, which is why SARs were factored into cost analysis.
SVR12 test results that were drawn too early and had to be repeated at VALLHCS were a cost unique to the Choice program. Duplicate tests, particularly imaging studies and blood work, were extraneous costs. The largest extraneous costs were treatment regimens prescribed by Choice HCPs that did not follow standard of care and required VA provider intervention. Thirty of the 71 (42%) veterans seen by a Choice provider accrued a mean $8,561.40 in extra costs. As a result, the Choice program cost VHA $250,000 more to provide care for 30 veterans (enough to pay for a physician’s annual salary).
Some inappropriate treatment regimens were the result of Choice HCP error, such as 1 case in which a veteran was inadvertently switched from ledipasvir/sofosbuvir to ombitasvir/ paritaprevir/ritonavir/dasabuvir after week 4. The veteran had to start therapy over but still achieved SVR12. Other cases saw veterans receive regimens for which they had clear contraindications, such as creatinine clearance < 30 mL/min/1.73m2 for sofosbuvir or a positive resistance panel for specific medications. Eleven of 62 (18%) veterans who were started on HCV treatment by a Choice HCP received a regimen not consistent with VA guidelines—an alarming result.
Follow up for veterans referred to Choice was extremely labor intensive, and assessment of personnel requirements in a Choice-based VA system must take this into consideration. The Choice HCP has no obligation to communicate with the VA HCP. At the time of chart review, 57 of 71 (80%) Choice veterans had inadequate documentation to make a confident assessment of the treatment outcome. Multiple calls to the offices of the Choice HCP were needed to acquire records, and veterans had to be tracked down for additional tests. Veterans often would complete treatment and stop following up with the Choice provider before SVR12 confirmation. The VA hepatology provider reviewing Choice referrals served as clinician, case manager, and clerk in order to get veterans to an appropriate end point in their hepatitis C treatment, with mostly unmeasured hours of work.
Limitations
The study population size was limited by the number of veterans able to complete treatment through Choice. The parameters in the VACO policy memos automatically selected the VA and Choice groups but made them clinically distinct populations. New treatment medications were released during the study period, which impacted management strategy. Occasionally, VA and non- VA HCPs preferred different treatment regimens, leading to variation in the distribution of regimens used despite similar genotype distribution (Tables 2 and 4). In addition, a retrospective study is at risk for recall bias. A prospective study randomizing veterans to the Choice and VA groups is an important future endeavor. Comparing veteran satisfaction for Choice and VA services is also crucial.
Conclusions
This study demonstrates that the VA was able to provide more cost-effective and more timely care for HCV treatment to a relatively sicker population with no reduction in treatment success when compared with non-VA HCPs through the Choice program. While the Choice program can help veterans receive services they may otherwise not have access to and reduce travel time, the current system introduces inefficiencies that delay care and decrease cost-effectiveness. The Choice HCP selection process is based on proximity rather than quality, which may place the veteran at risk for receiving substandard care. Large-scale quality of care studies that compare efficiency measures, clinical outcomes, patient demographics, travel distance, cost efficacy and patient satisfaction for veterans receiving similar services at a VA facility and through Choice should be performed to ensure that veterans receive the best care available.
1. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300. doi:10.7326/M13-1133
2. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88-96. doi:10.1002/hep.20502
3. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
4. Tuchschmidt J. Attachment C: Provision of hepatitis C treatment. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/education /choice-provision-hcv-treatment.asp. Published May 21, 2015. [Nonpublic site.]
5. Tuchschmidt J. Attachment A: Provision of hepatitis C (HCV) treatment through the Choice program. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http:// vaww.hepatitis.va.gov/pdf/choice-attachment-a-FY16 .pdf. Published May 21, 2015. [Nonpublic site.]
6. Tuchschmidt J. Attachment B: Initiation of hepatitis C virus (HCV) treatment: protocol for prioritization. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww .hepatitis.va.gov/pdf/provision-HCV-treatment-attach ment-b.pdf. Published May 21, 2015. [Nonpublic site.]
7. Murphy, JP. Hepatitis C virus funding and prioritization status. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations. http://vaww.hepatitis .va.gov/education/choice-memo-hcv-funding-and -prioritization-status-01272016.asp. Published January 27, 2016. [Nonpublic site.]
8. Lynch TJ, McCarthy MF. Hepatitis C virus funding and prioritization status update. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations and Acting Assistant Deputy Under Secretary for Health for Patient Care Services. http://vaww.hepatitis.va.gov /education/choice-funding-update-feb-2016.asp. Published February 24, 2016. [Nonpublic site.]
9. Morgan TR, Yee H; US Department of Veterans Affairs National Hepatitis C Resource Center Program and the National Viral Hepatitis Program in the Office of Patient Care Services. Chronic hepatitis C virus (HCV) infection: treatment considerations. http://vaww.hepatitis.va.gov /pdf/treatment-considerations-2016-03-28.pdf. Published March 28, 2016. [Nonpublic site.]
10. American Association for the Study of Liver Diseases; Infectious Diseases Society of America. Initial Treatment Box. http://hcvguidelines.org/full-report/initial-treatment -box-summary-recommendations-patients-who-are -initiating-therapy-hcv. Updated November 6, 2019. Accessed May 11, 2020.
11. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3): 932-954. doi:10.1002/hep.27950
12. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006; 43(6):1317-1325. doi:10.1002/hep.21178
13. Realdi G, Fattovich G, Hadziyannis S, et al. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. The Investigators of the European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol. 1994;21(4):656-666. doi:10.1016/s0168 -8278(94)80115-0
14. Kanda T, Kato K, Tsubota A, et al. Platelet count and sustained virological response in hepatitis C treatment. World J Hepatol. 2013;5(4):182-188. doi:10.4254/wjh.v5.i4.182
15. Manos MM, Shvachko VA, Murphy RC, Arduino JM, Shire NJ. Distribution of hepatitis C virus genotypes in a diverse US integrated health care population. J Med Virol. 2012;84(11):1744-1750. doi:10.1002/jmv.23399
16. Cheung RC. Epidemiology of hepatitis C virus infection in American veterans. Am J Gastroenterol. 2000;95(3): 740-747. doi:10.1111/j.1572-0241.2000.01854.x
17. Bagalman E. The number of Veterans that use VA health care services: a fact sheet. Congressional Research Service Report R43579. https://fas.org/sgp/crs/misc/R43579.pdf. Published June 3, 2014. Accessed May 11, 2020.
18. US General Accounting Office. Report to the Ranking Minority Member, Subcommittee on Compensation, Pension, Insurance, and Memorial Affairs, Committee on Veterans’ Affairs, US House of Representatives. How distance from VA facilities affects veterans’ use of VA services. https:// www.gao.gov/assets/230/221992.pdf. Published December 1995. Accessed May 11, 2020.
19. Bronstein S, Griffin D. A fatal wait: Veterans languish and die on a VA hospital’s secret list. http://www.cnn .com/2014/04/23/health/veterans-dying-health-care -delays/index.html. Published April 23, 2014. Accessed May 11, 2020.
20. Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs national health care system. Gastroenterology. 2016;151(3):457- 471. doi:10.1053/j.gastro.2016.05.049
21. Malespin MH, Harris C, Kanar O, et al. Barriers to treatment of chronic hepatitis C with direct acting antivirals in an urban clinic. Ann Hepatol. 2019;18(2):304-309. doi:10.1016/j.aohep.2018.06.001
22. Tuchschmidt J. Attachment D: Hepatitis C virus (HCV) fact sheet for Veterans Choice Program for both VA and Choice providers. US Department of Veterans Affairs Central Office Memorandum from the Deputy Under Secretary for Health for Policy and Services and the Acting Deputy Undersecretary for Health for Operations and Management. http://vaww .hepatitis.va.gov/educatiochoice-provision-HCV-treatment -additional.asp. [Nonpublic site.]
Population studies show high prevalence of chronic hepatitis C virus (HCV) infection among veterans, especially Vietnam War era veterans.1,2 The development of safe and efficacious direct-acting antiviral (DAA) medications to treat HCV infection made the majority of those infected eligible for treatment. However, the large number of veterans needing DAA treatment stressed the resources of the US Department of Veterans Affairs (VA) health care system. This occurred while Congress was focused on reducing wait times for veterans receiving care at the VA.
Congress passed the Veterans Access, Choice, and Accountability Act on August 7, 2014, leading to the creation of the Veterans Choice Program. Legislators felt there were inappropriate delays in care at the VA, and the Choice program was meant to address this problem and provide an “apples-to-apples comparison [of the VA] with non-VA hospitals.”3
Congress acknowledged the importance of curing HCV in the veteran population and allocated $1.5 billion for fiscal year (FY) 2016 for DAAs. The VA Central Office (VACO) carefully monitored these resources. The first policy memorandum from VACO for HCV care, issued on May 21, 2015, recommended that the sickest patients who will benefit from the treatment “receive priority over those who are less ill.”4,5 Those who met criteria for advanced liver disease were prioritized for treatment at the VA, while those who did not meet criteria were given the option of receiving treatment through Choice, or waiting for a change in policy.6 Over time, revisions to the guidelines relaxed the criteria for VA treatment eligibility, and on February 24, 2016, all restrictions on HCV treatment at the VA were lifted.7,8
The aim of this study was to provide a comparison of VA and non-VA care, specifically to determine whether care provided through Choice was timelier and more cost effective than care provided by the VA, and whether there was a quality difference. The high prevalence among veterans, wellestablished standards of care, and finite treatment course with clear indicators of success and failure makes HCV treatment an ideal disease with which to make this comparison.
Methods
We retrospectively analyzed the VA electronic health records of all veterans seen in the VA Loma Linda Healthcare System (VALLHCS) Hepatology clinic for chronic HCV infection during FY 2016 who were referred to Choice for HCV treatment. One hundred veterans met these criteria, encompassing the Choice population; 71 were seen at least once by a non-VA (Choice) health care provider (HCP) and 61 completed a treatment regimen through Choice. Treatment completion was defined as cessation of medication after the planned duration of therapy, or early termination of medication without resumption by that HCP. The Choice population was matched to an equal number of veterans who received HCV treatment from VALLHCS HCPs.
Data collected included age, gender, HCV genotype, determinants of liver fibrosis, and treatment success (defined as sustained virologic response at 12 weeks after the last dose of medication [SVR12]). Determinants of liver fibrosis included documented cirrhosis or complications of cirrhosis, Fibrosis-4 score (Fib-4), and platelet count.
Treatment failures were categorized as nonresponse (defined as detectable HCV viral load at the end of treatment), relapse (defined as an undetectable HCV viral load at the end of treatment with a subsequent positive test), and early termination (defined as a failure to complete the planned treatment regimen). Documented patient nonadherence, medical comorbidities that affected the treatment protocol, mental health diagnoses, and active social issues (defined as active or history of heavy alcohol use, active or history of illicit drug use, lack of social support, and homelessness) were noted.
Timeliness of delivery of care was measured in days. For the VA group, the wait time was defined as the date the consult for HCV treatment was placed to the date of the initial appointment with the HCV treatment provider. For the Choice group, the wait time was defined as the date the referral to the Choice program was made to the date of the initial appointment with the Choice HCP. Treatment regimens were evaluated for appropriateness based on guidelines from VACO and the American Association for the Study of Liver Diseases.9-11
Tests performed by Choice providers were evaluated for whether they were relevant to HCV care and whether similar data already were available from VALLHCS. Tests that were not indicated were identified as unnecessary costs incurred by the Choice program, as were tests that had to be repeated at the VA because of a lack of documentation from the Choice provider. All medications given inappropriately were considered added costs. Medicare reimbursement rates for the most applicable Current Procedural Terminology (CPT) code and VA national contract pricing for medications were used for calculations. This study was approved by the VALLHCS institutional review board.
Statistical Analysis
IBM (Armonk, NY) Statistical Package for Social Sciences software was used to evaluate for differences in Fib-4, platelet count, prevalence of cirrhosis, prevalence of medical comorbidities, prevalence of mental health comorbidities, prevalence of the social issues defined in the Methods section, time from referral to time of appointment date, and SVR12 rate between the VA and Choice groups.
Exclusions
There were 15 veterans in the VA group who had a wait time of > 100 days. Of these, 5 (33%) were initially Choice referrals, but due to negative interactions with the Choice provider, the veterans returned to VALLHCS for care. Two of the 15 (13%) did not keep appointments and were lost to follow up. Six of the 15 (40%) had medical comorbidities that required more immediate attention, so HCV treatment initiation was deliberately moved back. The final 2 veterans scheduled their appointments unusually far apart, artificially increasing their wait time. Given that these were unique situations and some of the veterans received care from both Choice and VA providers, a decision was made to exclude these individuals from the study.
It has been shown that platelet count correlates with degree of liver fibrosis, a concept that is the basis for the Fib-4 scoring system.12 Studies have shown that platelet count is a survival predictor in those with cirrhosis, and thrombocytopenia is a negative predictor of HCV treatment success using peginterferon and ribavirin13,14 Therefore, the VA memorandum automatically assigned the sickest individuals to the VA for HCV treatment. The goal of this study was to compare the impact of factors other than stage of fibrosis on HCV treatment success, which is why the 12 veterans with platelet count < 100,000 in the VA group were excluded. There were no veterans with platelet count < 100,000 in the Choice group.
When comparing SVR12 rates between the VA and Choice groups, every veteran treated at VALLHCS in FY 2016 was included, increasing the number in the VA group from 100 to 320 and therefore the power of this comparison.
Results
A summary of the statistical analysis can be found in Table 1. The genotype distribution was consistent with epidemiological studies, including those specific to veterans.15,16 There were statistically significant differences (P < .001) in mean Fib-4 and mean platelet count. The VA group had a higher Fib-4 and lower platelet count. Seventy-four percent of the VA population was defined as cirrhotic, while only 3% of the Choice population met similar criteria (P < .001). The VA and Choice groups were similar in terms of age, gender, and genotype distribution (Table 2).
The VA group was found to have a higher prevalence of comorbidities that affected HCV treatment. These conditions included but were not limited to: chronic kidney disease that precluded the use of certain medications, any condition that required medication with a known interaction with DAAs (ie, proton pump inhibitors, statins, and amiodarone), and cirrhosis if it impacted the treatment regimen. The difference in the prevalence of mental health comorbidities was not significant (P = .39), but there was a higher prevalence of social issues among the VA group (P = .002).
The mean wait time from referral to appointment was 28.6 days for the VA group and 42.3 days for the Choice group (P < .001), indicating that a Choice referral took longer to complete than a referral within the VA for HCV treatment. Thirty of the 71 (42%) veterans seen by a Choice provider accrued extraneous cost, with a mean additional cost of $8,561.40 per veteran. In the Choice group, 61 veterans completed a treatment regimen with the Choice HCP. Fifty-five veterans completed treatment and had available SVR12 data (6 were lost to follow up without SVR12 testing) and 50 (91%) had confirmed SVR12. The charts of the 5 treatment failures were reviewed to discern the cause for failure. Two cases involved early termination of therapy, 3 involved relapse and 2 failed to comply with medication instructions. There was 1 case of the Choice HCP not addressing simultaneous use of ledipasvir and a proton pump inhibitor, potentially causing an interaction, and 1 case where both the VA and Choice providers failed to recognize indicators of cirrhosis, which impacted the regimen used.
In the VALLHCS group, records of 320 veterans who completed treatment and had SVR12 testing were reviewed. While the Choice memorandum was active, veterans selected to be treated at VALLHCS had advanced liver fibrosis or cirrhosis, medical and mental health comorbidities that increased the risk of treatment complications or were considered to have difficulty adhering to the medication regimen. For this group, 296 (93%) had confirmed SVR12. Eighteen of the 24 (75%) treatment failures were complicated by nonadherence, including all 13 cases of early termination. One patient died from complications of decompensated cirrhosis before completing treatment, and 1 did not receive HCV medications during a hospital admission due to poor coordination of care between the VA inpatient and outpatient pharmacy services, leading to multiple missed doses.
The difference in SVR12 rates (ie, treatment failure rates), between the VA and Choice groups was not statistically significant (P = .61). None of the specific reasons for treatment failure had a statistically significant difference between groups. A treatment failure analysis is shown in Table 3, and Table 4 indicates the breakdown of treatment regimens.
Discussion
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, consisting of 152 medical centers and > 1,700 sites of care. The VA has the potential to meet the health care needs of 21.6 million veterans. About 9 million veterans are enrolled in the VA system and 5.9 million received health care through VHA.17 However, every medical service cannot realistically be made available at every facility, and some veterans have difficulty gaining access to VHA care; distance and wait times have been well-publicized issues that need further exploration.18,19 The Choice program is an attempt to meet gaps in VA coverage using non-VA HCPs.
HCV infection is a specific diagnosis with national treatment guidelines and wellstudied treatments; it can be cured, with an evidence- based definition of cure. The VACO policy memorandum to refer less sick veterans to Choice while treating sicker veterans at the VA provided the opportunity to directly compare the quality of the 2 programs. The SVR12 rates of VALLHCS and Choice providers were comparable to the national average at the time, and while the difference in SVR12 rate was not significant, VALLHCS treated a significantly higher number of patients with cirrhosis because of the referral criteria.20
The significant difference in medical comorbidities between the VA and Choice groups was not surprising, partly because of the referral criteria. Cirrhosis can impact the treatment regimen, especially in regard to use of ribavirin. Since the presence of mental health comorbidities did not affect selection into the Choice group, it makes sense that there was no significant difference in prevalence between the groups.
VACO allowed veterans with HCV treatment plans that VA HCPs felt were too complicated for the Choice program to be treated by VHA HCPs.9 VALLHCS exercised this right for veterans at risk for nonadherence, because in HCV treatment, nonadherence leads to treatment failure and development of drug resistant virus strains. Therefore, veterans who would have difficulty traveling to VALLHCS to pick up medications, those who lacked means of communication (such as those who were homeless), and those who had active substance abuse were treated at the VA, where closer monitoring and immediate access to a wide range of services was possible. Studies have confirmed the impact of these types of issues on HCV treatment adherence and success. 21 This explains the higher prevalence of social issues in the VA group.
For an internal referral for HCV treatment at VALLHCS, the hepatology provider submits a consult request to the HCV treatment provider, who works in the same office, making direct communication simple. The main administrative limiting factor to minimizing wait times is the number of HCPs, which is dependent on hiring allowances.
When a veteran is referred to Choice, the VA provider places a consult for non-VA care, which the VA Office of Community Care processes by compiling relevant documents and sending the package to Triwest Healthcare Alliance, a private insurance processor contracted with the VA. Triwest selects the Choice provider, often without any input from the VA, and arranges the veteran’s initial appointment.22 Geographic distance to the veteran’s address is the main selection criteria for Triwest. Documents sent between the Choice and VA HCPs go through the Office of Community Care and Triwest. This significantly increases the potential for delays and failed communication. Triwest had a comprehensive list of providers deemed to be qualified to treat HCV within the geographic catchment of VALLHCS. This list was reviewed, and all veterans referred to Choice had HCPs near their home address; therefore, availability of Choice HCPs was not an issue.
The VA can provide guidance on management of the veteran in the form of bundle packages containing a list of services for which the Choice provider is authorized to provide, and ones the Choice provider is not authorized to provide. Some Choice HCPs ordered tests that were not authorized for HCV treatment such as esophagogastroduodenoscopy, colonoscopy, and liver biopsy. In all, 17 of 71 (24%) veterans seen by Choice HCPs had tests performed or ordered that VA HCPs would not have obtained for the purpose of HCV treatment (Table 5).
In order to prevent veterans from receiving unnecessary tests, a VALLHCS hepatologist asked to be notified by VA administrators overseeing Choice referrals whenever a secondary authorization request (SAR) was submitted by a Choice HCP. This strategy is not standard VA practice, therefore at many VA sites these requested tests would have been performed by the Choice HCP, which is why SARs were factored into cost analysis.
SVR12 test results that were drawn too early and had to be repeated at VALLHCS were a cost unique to the Choice program. Duplicate tests, particularly imaging studies and blood work, were extraneous costs. The largest extraneous costs were treatment regimens prescribed by Choice HCPs that did not follow standard of care and required VA provider intervention. Thirty of the 71 (42%) veterans seen by a Choice provider accrued a mean $8,561.40 in extra costs. As a result, the Choice program cost VHA $250,000 more to provide care for 30 veterans (enough to pay for a physician’s annual salary).
Some inappropriate treatment regimens were the result of Choice HCP error, such as 1 case in which a veteran was inadvertently switched from ledipasvir/sofosbuvir to ombitasvir/ paritaprevir/ritonavir/dasabuvir after week 4. The veteran had to start therapy over but still achieved SVR12. Other cases saw veterans receive regimens for which they had clear contraindications, such as creatinine clearance < 30 mL/min/1.73m2 for sofosbuvir or a positive resistance panel for specific medications. Eleven of 62 (18%) veterans who were started on HCV treatment by a Choice HCP received a regimen not consistent with VA guidelines—an alarming result.
Follow up for veterans referred to Choice was extremely labor intensive, and assessment of personnel requirements in a Choice-based VA system must take this into consideration. The Choice HCP has no obligation to communicate with the VA HCP. At the time of chart review, 57 of 71 (80%) Choice veterans had inadequate documentation to make a confident assessment of the treatment outcome. Multiple calls to the offices of the Choice HCP were needed to acquire records, and veterans had to be tracked down for additional tests. Veterans often would complete treatment and stop following up with the Choice provider before SVR12 confirmation. The VA hepatology provider reviewing Choice referrals served as clinician, case manager, and clerk in order to get veterans to an appropriate end point in their hepatitis C treatment, with mostly unmeasured hours of work.
Limitations
The study population size was limited by the number of veterans able to complete treatment through Choice. The parameters in the VACO policy memos automatically selected the VA and Choice groups but made them clinically distinct populations. New treatment medications were released during the study period, which impacted management strategy. Occasionally, VA and non- VA HCPs preferred different treatment regimens, leading to variation in the distribution of regimens used despite similar genotype distribution (Tables 2 and 4). In addition, a retrospective study is at risk for recall bias. A prospective study randomizing veterans to the Choice and VA groups is an important future endeavor. Comparing veteran satisfaction for Choice and VA services is also crucial.
Conclusions
This study demonstrates that the VA was able to provide more cost-effective and more timely care for HCV treatment to a relatively sicker population with no reduction in treatment success when compared with non-VA HCPs through the Choice program. While the Choice program can help veterans receive services they may otherwise not have access to and reduce travel time, the current system introduces inefficiencies that delay care and decrease cost-effectiveness. The Choice HCP selection process is based on proximity rather than quality, which may place the veteran at risk for receiving substandard care. Large-scale quality of care studies that compare efficiency measures, clinical outcomes, patient demographics, travel distance, cost efficacy and patient satisfaction for veterans receiving similar services at a VA facility and through Choice should be performed to ensure that veterans receive the best care available.
Population studies show high prevalence of chronic hepatitis C virus (HCV) infection among veterans, especially Vietnam War era veterans.1,2 The development of safe and efficacious direct-acting antiviral (DAA) medications to treat HCV infection made the majority of those infected eligible for treatment. However, the large number of veterans needing DAA treatment stressed the resources of the US Department of Veterans Affairs (VA) health care system. This occurred while Congress was focused on reducing wait times for veterans receiving care at the VA.
Congress passed the Veterans Access, Choice, and Accountability Act on August 7, 2014, leading to the creation of the Veterans Choice Program. Legislators felt there were inappropriate delays in care at the VA, and the Choice program was meant to address this problem and provide an “apples-to-apples comparison [of the VA] with non-VA hospitals.”3
Congress acknowledged the importance of curing HCV in the veteran population and allocated $1.5 billion for fiscal year (FY) 2016 for DAAs. The VA Central Office (VACO) carefully monitored these resources. The first policy memorandum from VACO for HCV care, issued on May 21, 2015, recommended that the sickest patients who will benefit from the treatment “receive priority over those who are less ill.”4,5 Those who met criteria for advanced liver disease were prioritized for treatment at the VA, while those who did not meet criteria were given the option of receiving treatment through Choice, or waiting for a change in policy.6 Over time, revisions to the guidelines relaxed the criteria for VA treatment eligibility, and on February 24, 2016, all restrictions on HCV treatment at the VA were lifted.7,8
The aim of this study was to provide a comparison of VA and non-VA care, specifically to determine whether care provided through Choice was timelier and more cost effective than care provided by the VA, and whether there was a quality difference. The high prevalence among veterans, wellestablished standards of care, and finite treatment course with clear indicators of success and failure makes HCV treatment an ideal disease with which to make this comparison.
Methods
We retrospectively analyzed the VA electronic health records of all veterans seen in the VA Loma Linda Healthcare System (VALLHCS) Hepatology clinic for chronic HCV infection during FY 2016 who were referred to Choice for HCV treatment. One hundred veterans met these criteria, encompassing the Choice population; 71 were seen at least once by a non-VA (Choice) health care provider (HCP) and 61 completed a treatment regimen through Choice. Treatment completion was defined as cessation of medication after the planned duration of therapy, or early termination of medication without resumption by that HCP. The Choice population was matched to an equal number of veterans who received HCV treatment from VALLHCS HCPs.
Data collected included age, gender, HCV genotype, determinants of liver fibrosis, and treatment success (defined as sustained virologic response at 12 weeks after the last dose of medication [SVR12]). Determinants of liver fibrosis included documented cirrhosis or complications of cirrhosis, Fibrosis-4 score (Fib-4), and platelet count.
Treatment failures were categorized as nonresponse (defined as detectable HCV viral load at the end of treatment), relapse (defined as an undetectable HCV viral load at the end of treatment with a subsequent positive test), and early termination (defined as a failure to complete the planned treatment regimen). Documented patient nonadherence, medical comorbidities that affected the treatment protocol, mental health diagnoses, and active social issues (defined as active or history of heavy alcohol use, active or history of illicit drug use, lack of social support, and homelessness) were noted.
Timeliness of delivery of care was measured in days. For the VA group, the wait time was defined as the date the consult for HCV treatment was placed to the date of the initial appointment with the HCV treatment provider. For the Choice group, the wait time was defined as the date the referral to the Choice program was made to the date of the initial appointment with the Choice HCP. Treatment regimens were evaluated for appropriateness based on guidelines from VACO and the American Association for the Study of Liver Diseases.9-11
Tests performed by Choice providers were evaluated for whether they were relevant to HCV care and whether similar data already were available from VALLHCS. Tests that were not indicated were identified as unnecessary costs incurred by the Choice program, as were tests that had to be repeated at the VA because of a lack of documentation from the Choice provider. All medications given inappropriately were considered added costs. Medicare reimbursement rates for the most applicable Current Procedural Terminology (CPT) code and VA national contract pricing for medications were used for calculations. This study was approved by the VALLHCS institutional review board.
Statistical Analysis
IBM (Armonk, NY) Statistical Package for Social Sciences software was used to evaluate for differences in Fib-4, platelet count, prevalence of cirrhosis, prevalence of medical comorbidities, prevalence of mental health comorbidities, prevalence of the social issues defined in the Methods section, time from referral to time of appointment date, and SVR12 rate between the VA and Choice groups.
Exclusions
There were 15 veterans in the VA group who had a wait time of > 100 days. Of these, 5 (33%) were initially Choice referrals, but due to negative interactions with the Choice provider, the veterans returned to VALLHCS for care. Two of the 15 (13%) did not keep appointments and were lost to follow up. Six of the 15 (40%) had medical comorbidities that required more immediate attention, so HCV treatment initiation was deliberately moved back. The final 2 veterans scheduled their appointments unusually far apart, artificially increasing their wait time. Given that these were unique situations and some of the veterans received care from both Choice and VA providers, a decision was made to exclude these individuals from the study.
It has been shown that platelet count correlates with degree of liver fibrosis, a concept that is the basis for the Fib-4 scoring system.12 Studies have shown that platelet count is a survival predictor in those with cirrhosis, and thrombocytopenia is a negative predictor of HCV treatment success using peginterferon and ribavirin13,14 Therefore, the VA memorandum automatically assigned the sickest individuals to the VA for HCV treatment. The goal of this study was to compare the impact of factors other than stage of fibrosis on HCV treatment success, which is why the 12 veterans with platelet count < 100,000 in the VA group were excluded. There were no veterans with platelet count < 100,000 in the Choice group.
When comparing SVR12 rates between the VA and Choice groups, every veteran treated at VALLHCS in FY 2016 was included, increasing the number in the VA group from 100 to 320 and therefore the power of this comparison.
Results
A summary of the statistical analysis can be found in Table 1. The genotype distribution was consistent with epidemiological studies, including those specific to veterans.15,16 There were statistically significant differences (P < .001) in mean Fib-4 and mean platelet count. The VA group had a higher Fib-4 and lower platelet count. Seventy-four percent of the VA population was defined as cirrhotic, while only 3% of the Choice population met similar criteria (P < .001). The VA and Choice groups were similar in terms of age, gender, and genotype distribution (Table 2).
The VA group was found to have a higher prevalence of comorbidities that affected HCV treatment. These conditions included but were not limited to: chronic kidney disease that precluded the use of certain medications, any condition that required medication with a known interaction with DAAs (ie, proton pump inhibitors, statins, and amiodarone), and cirrhosis if it impacted the treatment regimen. The difference in the prevalence of mental health comorbidities was not significant (P = .39), but there was a higher prevalence of social issues among the VA group (P = .002).
The mean wait time from referral to appointment was 28.6 days for the VA group and 42.3 days for the Choice group (P < .001), indicating that a Choice referral took longer to complete than a referral within the VA for HCV treatment. Thirty of the 71 (42%) veterans seen by a Choice provider accrued extraneous cost, with a mean additional cost of $8,561.40 per veteran. In the Choice group, 61 veterans completed a treatment regimen with the Choice HCP. Fifty-five veterans completed treatment and had available SVR12 data (6 were lost to follow up without SVR12 testing) and 50 (91%) had confirmed SVR12. The charts of the 5 treatment failures were reviewed to discern the cause for failure. Two cases involved early termination of therapy, 3 involved relapse and 2 failed to comply with medication instructions. There was 1 case of the Choice HCP not addressing simultaneous use of ledipasvir and a proton pump inhibitor, potentially causing an interaction, and 1 case where both the VA and Choice providers failed to recognize indicators of cirrhosis, which impacted the regimen used.
In the VALLHCS group, records of 320 veterans who completed treatment and had SVR12 testing were reviewed. While the Choice memorandum was active, veterans selected to be treated at VALLHCS had advanced liver fibrosis or cirrhosis, medical and mental health comorbidities that increased the risk of treatment complications or were considered to have difficulty adhering to the medication regimen. For this group, 296 (93%) had confirmed SVR12. Eighteen of the 24 (75%) treatment failures were complicated by nonadherence, including all 13 cases of early termination. One patient died from complications of decompensated cirrhosis before completing treatment, and 1 did not receive HCV medications during a hospital admission due to poor coordination of care between the VA inpatient and outpatient pharmacy services, leading to multiple missed doses.
The difference in SVR12 rates (ie, treatment failure rates), between the VA and Choice groups was not statistically significant (P = .61). None of the specific reasons for treatment failure had a statistically significant difference between groups. A treatment failure analysis is shown in Table 3, and Table 4 indicates the breakdown of treatment regimens.
Discussion
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, consisting of 152 medical centers and > 1,700 sites of care. The VA has the potential to meet the health care needs of 21.6 million veterans. About 9 million veterans are enrolled in the VA system and 5.9 million received health care through VHA.17 However, every medical service cannot realistically be made available at every facility, and some veterans have difficulty gaining access to VHA care; distance and wait times have been well-publicized issues that need further exploration.18,19 The Choice program is an attempt to meet gaps in VA coverage using non-VA HCPs.
HCV infection is a specific diagnosis with national treatment guidelines and wellstudied treatments; it can be cured, with an evidence- based definition of cure. The VACO policy memorandum to refer less sick veterans to Choice while treating sicker veterans at the VA provided the opportunity to directly compare the quality of the 2 programs. The SVR12 rates of VALLHCS and Choice providers were comparable to the national average at the time, and while the difference in SVR12 rate was not significant, VALLHCS treated a significantly higher number of patients with cirrhosis because of the referral criteria.20
The significant difference in medical comorbidities between the VA and Choice groups was not surprising, partly because of the referral criteria. Cirrhosis can impact the treatment regimen, especially in regard to use of ribavirin. Since the presence of mental health comorbidities did not affect selection into the Choice group, it makes sense that there was no significant difference in prevalence between the groups.
VACO allowed veterans with HCV treatment plans that VA HCPs felt were too complicated for the Choice program to be treated by VHA HCPs.9 VALLHCS exercised this right for veterans at risk for nonadherence, because in HCV treatment, nonadherence leads to treatment failure and development of drug resistant virus strains. Therefore, veterans who would have difficulty traveling to VALLHCS to pick up medications, those who lacked means of communication (such as those who were homeless), and those who had active substance abuse were treated at the VA, where closer monitoring and immediate access to a wide range of services was possible. Studies have confirmed the impact of these types of issues on HCV treatment adherence and success. 21 This explains the higher prevalence of social issues in the VA group.
For an internal referral for HCV treatment at VALLHCS, the hepatology provider submits a consult request to the HCV treatment provider, who works in the same office, making direct communication simple. The main administrative limiting factor to minimizing wait times is the number of HCPs, which is dependent on hiring allowances.
When a veteran is referred to Choice, the VA provider places a consult for non-VA care, which the VA Office of Community Care processes by compiling relevant documents and sending the package to Triwest Healthcare Alliance, a private insurance processor contracted with the VA. Triwest selects the Choice provider, often without any input from the VA, and arranges the veteran’s initial appointment.22 Geographic distance to the veteran’s address is the main selection criteria for Triwest. Documents sent between the Choice and VA HCPs go through the Office of Community Care and Triwest. This significantly increases the potential for delays and failed communication. Triwest had a comprehensive list of providers deemed to be qualified to treat HCV within the geographic catchment of VALLHCS. This list was reviewed, and all veterans referred to Choice had HCPs near their home address; therefore, availability of Choice HCPs was not an issue.
The VA can provide guidance on management of the veteran in the form of bundle packages containing a list of services for which the Choice provider is authorized to provide, and ones the Choice provider is not authorized to provide. Some Choice HCPs ordered tests that were not authorized for HCV treatment such as esophagogastroduodenoscopy, colonoscopy, and liver biopsy. In all, 17 of 71 (24%) veterans seen by Choice HCPs had tests performed or ordered that VA HCPs would not have obtained for the purpose of HCV treatment (Table 5).
In order to prevent veterans from receiving unnecessary tests, a VALLHCS hepatologist asked to be notified by VA administrators overseeing Choice referrals whenever a secondary authorization request (SAR) was submitted by a Choice HCP. This strategy is not standard VA practice, therefore at many VA sites these requested tests would have been performed by the Choice HCP, which is why SARs were factored into cost analysis.
SVR12 test results that were drawn too early and had to be repeated at VALLHCS were a cost unique to the Choice program. Duplicate tests, particularly imaging studies and blood work, were extraneous costs. The largest extraneous costs were treatment regimens prescribed by Choice HCPs that did not follow standard of care and required VA provider intervention. Thirty of the 71 (42%) veterans seen by a Choice provider accrued a mean $8,561.40 in extra costs. As a result, the Choice program cost VHA $250,000 more to provide care for 30 veterans (enough to pay for a physician’s annual salary).
Some inappropriate treatment regimens were the result of Choice HCP error, such as 1 case in which a veteran was inadvertently switched from ledipasvir/sofosbuvir to ombitasvir/ paritaprevir/ritonavir/dasabuvir after week 4. The veteran had to start therapy over but still achieved SVR12. Other cases saw veterans receive regimens for which they had clear contraindications, such as creatinine clearance < 30 mL/min/1.73m2 for sofosbuvir or a positive resistance panel for specific medications. Eleven of 62 (18%) veterans who were started on HCV treatment by a Choice HCP received a regimen not consistent with VA guidelines—an alarming result.
Follow up for veterans referred to Choice was extremely labor intensive, and assessment of personnel requirements in a Choice-based VA system must take this into consideration. The Choice HCP has no obligation to communicate with the VA HCP. At the time of chart review, 57 of 71 (80%) Choice veterans had inadequate documentation to make a confident assessment of the treatment outcome. Multiple calls to the offices of the Choice HCP were needed to acquire records, and veterans had to be tracked down for additional tests. Veterans often would complete treatment and stop following up with the Choice provider before SVR12 confirmation. The VA hepatology provider reviewing Choice referrals served as clinician, case manager, and clerk in order to get veterans to an appropriate end point in their hepatitis C treatment, with mostly unmeasured hours of work.
Limitations
The study population size was limited by the number of veterans able to complete treatment through Choice. The parameters in the VACO policy memos automatically selected the VA and Choice groups but made them clinically distinct populations. New treatment medications were released during the study period, which impacted management strategy. Occasionally, VA and non- VA HCPs preferred different treatment regimens, leading to variation in the distribution of regimens used despite similar genotype distribution (Tables 2 and 4). In addition, a retrospective study is at risk for recall bias. A prospective study randomizing veterans to the Choice and VA groups is an important future endeavor. Comparing veteran satisfaction for Choice and VA services is also crucial.
Conclusions
This study demonstrates that the VA was able to provide more cost-effective and more timely care for HCV treatment to a relatively sicker population with no reduction in treatment success when compared with non-VA HCPs through the Choice program. While the Choice program can help veterans receive services they may otherwise not have access to and reduce travel time, the current system introduces inefficiencies that delay care and decrease cost-effectiveness. The Choice HCP selection process is based on proximity rather than quality, which may place the veteran at risk for receiving substandard care. Large-scale quality of care studies that compare efficiency measures, clinical outcomes, patient demographics, travel distance, cost efficacy and patient satisfaction for veterans receiving similar services at a VA facility and through Choice should be performed to ensure that veterans receive the best care available.
1. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300. doi:10.7326/M13-1133
2. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88-96. doi:10.1002/hep.20502
3. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
4. Tuchschmidt J. Attachment C: Provision of hepatitis C treatment. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/education /choice-provision-hcv-treatment.asp. Published May 21, 2015. [Nonpublic site.]
5. Tuchschmidt J. Attachment A: Provision of hepatitis C (HCV) treatment through the Choice program. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http:// vaww.hepatitis.va.gov/pdf/choice-attachment-a-FY16 .pdf. Published May 21, 2015. [Nonpublic site.]
6. Tuchschmidt J. Attachment B: Initiation of hepatitis C virus (HCV) treatment: protocol for prioritization. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww .hepatitis.va.gov/pdf/provision-HCV-treatment-attach ment-b.pdf. Published May 21, 2015. [Nonpublic site.]
7. Murphy, JP. Hepatitis C virus funding and prioritization status. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations. http://vaww.hepatitis .va.gov/education/choice-memo-hcv-funding-and -prioritization-status-01272016.asp. Published January 27, 2016. [Nonpublic site.]
8. Lynch TJ, McCarthy MF. Hepatitis C virus funding and prioritization status update. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations and Acting Assistant Deputy Under Secretary for Health for Patient Care Services. http://vaww.hepatitis.va.gov /education/choice-funding-update-feb-2016.asp. Published February 24, 2016. [Nonpublic site.]
9. Morgan TR, Yee H; US Department of Veterans Affairs National Hepatitis C Resource Center Program and the National Viral Hepatitis Program in the Office of Patient Care Services. Chronic hepatitis C virus (HCV) infection: treatment considerations. http://vaww.hepatitis.va.gov /pdf/treatment-considerations-2016-03-28.pdf. Published March 28, 2016. [Nonpublic site.]
10. American Association for the Study of Liver Diseases; Infectious Diseases Society of America. Initial Treatment Box. http://hcvguidelines.org/full-report/initial-treatment -box-summary-recommendations-patients-who-are -initiating-therapy-hcv. Updated November 6, 2019. Accessed May 11, 2020.
11. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3): 932-954. doi:10.1002/hep.27950
12. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006; 43(6):1317-1325. doi:10.1002/hep.21178
13. Realdi G, Fattovich G, Hadziyannis S, et al. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. The Investigators of the European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol. 1994;21(4):656-666. doi:10.1016/s0168 -8278(94)80115-0
14. Kanda T, Kato K, Tsubota A, et al. Platelet count and sustained virological response in hepatitis C treatment. World J Hepatol. 2013;5(4):182-188. doi:10.4254/wjh.v5.i4.182
15. Manos MM, Shvachko VA, Murphy RC, Arduino JM, Shire NJ. Distribution of hepatitis C virus genotypes in a diverse US integrated health care population. J Med Virol. 2012;84(11):1744-1750. doi:10.1002/jmv.23399
16. Cheung RC. Epidemiology of hepatitis C virus infection in American veterans. Am J Gastroenterol. 2000;95(3): 740-747. doi:10.1111/j.1572-0241.2000.01854.x
17. Bagalman E. The number of Veterans that use VA health care services: a fact sheet. Congressional Research Service Report R43579. https://fas.org/sgp/crs/misc/R43579.pdf. Published June 3, 2014. Accessed May 11, 2020.
18. US General Accounting Office. Report to the Ranking Minority Member, Subcommittee on Compensation, Pension, Insurance, and Memorial Affairs, Committee on Veterans’ Affairs, US House of Representatives. How distance from VA facilities affects veterans’ use of VA services. https:// www.gao.gov/assets/230/221992.pdf. Published December 1995. Accessed May 11, 2020.
19. Bronstein S, Griffin D. A fatal wait: Veterans languish and die on a VA hospital’s secret list. http://www.cnn .com/2014/04/23/health/veterans-dying-health-care -delays/index.html. Published April 23, 2014. Accessed May 11, 2020.
20. Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs national health care system. Gastroenterology. 2016;151(3):457- 471. doi:10.1053/j.gastro.2016.05.049
21. Malespin MH, Harris C, Kanar O, et al. Barriers to treatment of chronic hepatitis C with direct acting antivirals in an urban clinic. Ann Hepatol. 2019;18(2):304-309. doi:10.1016/j.aohep.2018.06.001
22. Tuchschmidt J. Attachment D: Hepatitis C virus (HCV) fact sheet for Veterans Choice Program for both VA and Choice providers. US Department of Veterans Affairs Central Office Memorandum from the Deputy Under Secretary for Health for Policy and Services and the Acting Deputy Undersecretary for Health for Operations and Management. http://vaww .hepatitis.va.gov/educatiochoice-provision-HCV-treatment -additional.asp. [Nonpublic site.]
1. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300. doi:10.7326/M13-1133
2. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88-96. doi:10.1002/hep.20502
3. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
4. Tuchschmidt J. Attachment C: Provision of hepatitis C treatment. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/education /choice-provision-hcv-treatment.asp. Published May 21, 2015. [Nonpublic site.]
5. Tuchschmidt J. Attachment A: Provision of hepatitis C (HCV) treatment through the Choice program. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http:// vaww.hepatitis.va.gov/pdf/choice-attachment-a-FY16 .pdf. Published May 21, 2015. [Nonpublic site.]
6. Tuchschmidt J. Attachment B: Initiation of hepatitis C virus (HCV) treatment: protocol for prioritization. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww .hepatitis.va.gov/pdf/provision-HCV-treatment-attach ment-b.pdf. Published May 21, 2015. [Nonpublic site.]
7. Murphy, JP. Hepatitis C virus funding and prioritization status. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations. http://vaww.hepatitis .va.gov/education/choice-memo-hcv-funding-and -prioritization-status-01272016.asp. Published January 27, 2016. [Nonpublic site.]
8. Lynch TJ, McCarthy MF. Hepatitis C virus funding and prioritization status update. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations and Acting Assistant Deputy Under Secretary for Health for Patient Care Services. http://vaww.hepatitis.va.gov /education/choice-funding-update-feb-2016.asp. Published February 24, 2016. [Nonpublic site.]
9. Morgan TR, Yee H; US Department of Veterans Affairs National Hepatitis C Resource Center Program and the National Viral Hepatitis Program in the Office of Patient Care Services. Chronic hepatitis C virus (HCV) infection: treatment considerations. http://vaww.hepatitis.va.gov /pdf/treatment-considerations-2016-03-28.pdf. Published March 28, 2016. [Nonpublic site.]
10. American Association for the Study of Liver Diseases; Infectious Diseases Society of America. Initial Treatment Box. http://hcvguidelines.org/full-report/initial-treatment -box-summary-recommendations-patients-who-are -initiating-therapy-hcv. Updated November 6, 2019. Accessed May 11, 2020.
11. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3): 932-954. doi:10.1002/hep.27950
12. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006; 43(6):1317-1325. doi:10.1002/hep.21178
13. Realdi G, Fattovich G, Hadziyannis S, et al. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. The Investigators of the European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol. 1994;21(4):656-666. doi:10.1016/s0168 -8278(94)80115-0
14. Kanda T, Kato K, Tsubota A, et al. Platelet count and sustained virological response in hepatitis C treatment. World J Hepatol. 2013;5(4):182-188. doi:10.4254/wjh.v5.i4.182
15. Manos MM, Shvachko VA, Murphy RC, Arduino JM, Shire NJ. Distribution of hepatitis C virus genotypes in a diverse US integrated health care population. J Med Virol. 2012;84(11):1744-1750. doi:10.1002/jmv.23399
16. Cheung RC. Epidemiology of hepatitis C virus infection in American veterans. Am J Gastroenterol. 2000;95(3): 740-747. doi:10.1111/j.1572-0241.2000.01854.x
17. Bagalman E. The number of Veterans that use VA health care services: a fact sheet. Congressional Research Service Report R43579. https://fas.org/sgp/crs/misc/R43579.pdf. Published June 3, 2014. Accessed May 11, 2020.
18. US General Accounting Office. Report to the Ranking Minority Member, Subcommittee on Compensation, Pension, Insurance, and Memorial Affairs, Committee on Veterans’ Affairs, US House of Representatives. How distance from VA facilities affects veterans’ use of VA services. https:// www.gao.gov/assets/230/221992.pdf. Published December 1995. Accessed May 11, 2020.
19. Bronstein S, Griffin D. A fatal wait: Veterans languish and die on a VA hospital’s secret list. http://www.cnn .com/2014/04/23/health/veterans-dying-health-care -delays/index.html. Published April 23, 2014. Accessed May 11, 2020.
20. Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs national health care system. Gastroenterology. 2016;151(3):457- 471. doi:10.1053/j.gastro.2016.05.049
21. Malespin MH, Harris C, Kanar O, et al. Barriers to treatment of chronic hepatitis C with direct acting antivirals in an urban clinic. Ann Hepatol. 2019;18(2):304-309. doi:10.1016/j.aohep.2018.06.001
22. Tuchschmidt J. Attachment D: Hepatitis C virus (HCV) fact sheet for Veterans Choice Program for both VA and Choice providers. US Department of Veterans Affairs Central Office Memorandum from the Deputy Under Secretary for Health for Policy and Services and the Acting Deputy Undersecretary for Health for Operations and Management. http://vaww .hepatitis.va.gov/educatiochoice-provision-HCV-treatment -additional.asp. [Nonpublic site.]
Pulmonary Neuroendocrine Tumor Presenting as a Left Pleural Effusion
Neuroendocrine tumors (NETs) account for about 0.5% of all newly diagnosed malignancies.1 Pulmonary NETs are rare, accounting for 1 to 2% of all invasive lung malignancies and involve about 20 to 25% of primary lung malignancies. 2,3 Their prevalence has increased by an estimated 6% per year over the past 30 years.2 Nonetheless, the time of diagnosis is frequently delayed because of nonspecific symptoms that may imitate other pulmonary conditions.
In the normal pleural space, there is a steady state in which there is a roughly equal rate of fluid formation and absorption. Any disequilibrium may produce a pleural effusion. Pleural fluids can be transudates or exudates. Transudates result from imbalances in hydrostatic and oncotic pressures in the pleural space. Exudates result primarily from pleural and/or lung inflammation or from impaired lymphatic drainage of the pleural space. Clinical manifestations include cough, wheezing, recurrent pneumonia, hemoptysis and pleural effusions. We present a case of a man who developed a large left pleural effusion with a pathology report suggesting a pulmonary NET as the etiology. Being aware of this rare entity may help improve prognosis by making an earlier diagnosis and starting treatment sooner.
Case Presentation
A 90-year-old man with a medical history of arterial hypertension, hyperlipidemia, type 2 diabetes mellitus, coronary artery disease, and vascular dementia presented to the emergency department with hypoactivity, poor appetite, productive cough, and shortness of breath. The patient was a former smoker (unknown pack-years) who quit smoking cigarettes 7 years prior. Vital signs showed sinus tachycardia and peripheral oxygen saturation of 90% at room air. The initial physical examination was remarkable for decreased breath sounds and crackles at the left lung base. Laboratory findings showed leukocytosis with neutrophilia and chronic normocytic anemia. Chest computed tomography (CT) showed a large left-sided pleural effusion occupying most of the left hemithorax with adjacent atelectatic lung, enlarged pretracheal, subcarinal, and left perihilar lymph nodes (Figure 1).
The patient was admitted to the internal medicine ward with the diagnosis of left pneumonic process and started on IV levofloxacin. However, despite 7 days of antibiotic therapy, the patient’s respiratory symptoms worsened. This clinical deterioration prompted pulmonary service consultation. Chest radiography demonstrated an enlarging left pleural effusion (Figure 2). A thoracentesis drained 1.2 L of serosanguineous pleural fluid. Pleural fluid analysis showed a cell count of 947/cm3 with 79% of lymphocytes, total protein 3.8 g/dL, lactic dehydrogenase (LDH) level 607 U/L, and glucose level 109 mg/dL. Serum total protein was 6.62 g/dL, LDH 666 U/L and glucose 92 mg/dL (Tables 1 and 2). Alanine transaminase (ALT) and aspartate aminotransferase (AST) were 11 U/L and 21 U/L, respectively. Using Light criteria, the pleural:serum protein ratio was 0.57, the pleural:serum LDH ratio was 0.91, and the pleural LDH was more than two-thirds of the serum LDH. These calculations were consistent with an exudative effusion. An infectious disease workup, including blood and pleural fluid cultures, was negative.
The pleural fluid concentrated cell block hematoxylin and eosin (H&E) staining showed chromatin, prominent nucleoli, and nuclear molding, which was compatible with high-grade lung NET (Figure 3). The cell block immunohistochemistry (IHC) was positive for synaptophysin, chromogranin A, and neuron specific enolase (NSE) also consistent with a high-grade pulmonary NET (Figure 4). The proliferation marker protein Ki-67 labeling index (LI) showed a proliferation index > 20% (Figure 5). The patient did not have decision-making capacity given vascular dementia. Multiple attempts to contact the next of kin or family members were unsuccessful. Risks vs benefits were evaluated, and given the patient’s advanced age and multiple comorbidities, a conservative management approach under palliative care was chosen. For this reason, further genomic studies were not done.
Discussion
NETs are a group of neoplasms that differ in site, amount of cell propagation, and clinical manifestations.4 These tumors are rare with an estimated incidence of 25 to 50 per 100,000.4 The most commonly affected organ systems are the gastroenteropancreatic and the bronchopulmonary tracts, accounting for 60% and 25% of the tumors, respectively.4 The incidence is increasing over the past years in part because of novel diagnostic techniques.
The average age of diagnosis is between the fourth and sixth decades, affecting more women than men.5 Smoking has been identified as a possible culprit for the development of these neoplasms; nonetheless, the association is still not clear.4 For example, poorly differentiated pulmonary NETs have a strong association with smoking but not well-differentiated pulmonary NETs.2
Patients typically present with cough, wheezing, hemoptysis, and recurrent pneumonias, which are in part a consequence of obstruction caused by the mass.2 Sometimes, obstruction may yield persistent pleural effusions. Hemoptysis may be seen secondary to the vascularity of pulmonary NETs.
The diagnosis is often delayed because patients are frequently treated for infection before being diagnosed with the malignancy, such as in our case. Radiologic image findings include round opacities, central masses, and atelectasis. Pulmonary NETs are frequently found incidentally as solitary lung nodules. The CT scan is the most common diagnostic modality and can provide information about the borders of the tumor, the location and surrounding structures, including the presence of atelectasis.5 Pulmonary NETs are usually centrally located in an accessible region for lung biopsy. In cases where the mass is not easily reachable, thoracentesis may provide the only available specimen.
The 2015 World Health Organization classification has identified 4 histologic types of pulmonary NETs, namely, typical carcinoid (TC), atypical carcinoid (AC), large cell neuroendocrine carcinoma (LCNEC) and small cell lung carcinoma (SCLC).6 The low-grade pulmonary NET, the typical carcinoid, is slow growing and has lower rates of metastasis. The intermediate-grade NET, the atypical carcinoid, is more aggressive. The highgrade NETs, the LCNEC and the SCLC, are aggressive and spread quickly to other places.6 Consequently, LCNEC and SCLC have higher mortalities with a 5-year survival, ranging from 13 to 57% and 5%, respectively.7
Tumors may be histomorphologically classified by H&E staining. The main characteristics that differentiate the low- and high-grade NETs are the presence of necrosis and the mitotic rate. Both categories form neuropeptides and have dense granular cores when seen with an electron microscopy.6 The TC and AC have welldefined, organized histologic patterns, no necrosis, and scarce mitosis. On the other hand, the LCNEC and SCLC are poorly differentiated tumors with necrosis, atypia, and mitosis.6 LCNEC can be separated from SCLC and other tumors by IHC staining, whereas SCLC is primarily distinguished by morphology.
If the biopsy sample size is small, then IHC morphology and markers are helpful for subclassification.8 IHC is used to discern between neuroendocrine (NE) vs non-NE. The evaluation of pleural fluid includes preparation of cell blocks. Cell block staining is deemed better for IHC because it mimics a small biopsy that enables superior stains.9 The need for a pleural biopsy in cases where the cytology is negative depends on treatment aims, the kind of tumor, and the presence of metastasis.10 In almost 80% of cases, pleural biopsy and cytology are the only specimens obtained for analysis.Therefore, identification of these markers is practical for diagnosis.10 For this reason, pleural effusion samples are appropriate options to lung biopsy for molecular studies.10
Ki-67 LI in samples has the highest specificity and sensitivity for low-tointermediate- grade vs high-grade tumors. It is being used for guiding clinical and treatment decisions.6 In SCLC, the Ki-67 LI is not necessary for diagnosis but will be about 80%.11 The tumor cells will show epithelial characteristics with positive cytokeratin AE1/AE3 and monoclonal antibody CAM5.2 and neuroendocrine markers, including NCAM/CD56, chromogranin A, and synaptophysin.11
Thyroid transcription factor-1 (TTF- 1) is positive in most cases. In LCNEC, the Ki-67 LI is between 40% and 80%. NCAM/ CD56, chromogranin A, and synaptophysin are present in 92 to 100%, 80 to 85%, and 50 to 60%, respectively.11 TTF-1 is identified in half of the tumors. All these tumors express pancytokeratin (AE1/AE3), cytokeratin 7 or low-molecular-weight cytokeratin. Likewise, the carcinoids will show markers, such as chromogranin A, synaptophysin, CD56, and epithelial markers like pancytokeratin.11 However, the high-molecular-weight cytokeratin and TTF-1 are negative. Furthermore, NSE is considered a good tumor marker in the diagnosis and prognosis of SCLC. NSE also has been reported in NSCLC. The level of NSE correlates with tumor burden, number of metastatic sites, and response to treatment. 12 A potentially useful marker is the insulinoma-associated protein 1, which is a nuclear determinant of NE differentiation that stains all types of pulmonary NETs irrespective of the histology but does not stain adenocarcinoma or squamous cell carcinoma (SCC).6
Recently, genomic studies have identified gene alterations that have become standard of care for diagnosis and targeted therapies.8 For example, epidermal growth factor receptor (EGFR) and echinoderm microtubule- associated proteinlike 4, and anaplastic lymphoma kinase (EML4-ALK) mutations have been found in about 25% of lung adenocarcinomas. 8 Other abnormalities in LKB1/STK11, NF1, CDKN2A, SMARCA4 and KEAP1, KRAS, MET, ROS1, and RET have also been identified.8 On the other hand, SCC rarely have derangements in EGFR and EML4-ALK, but do show changes in RTKs, DDR2M, FGGRs, among others.8 In TC and AC, observed molecular alterations include MEN1 mutations, mTOR, and SSTRs pathway activation, and GC/ CEACAM1 and CD44/OTP expression.13 LCNEC and SCLC have shown TP53 and RB1 mutations and CDX2/VIL1/BAI3 expression. DLL3 expression and MET mutations may be present in SCLC.13 Last, chromatin remodeling gene mutations have been identified in all these lung NET types.13
Furthermore, neuropeptides and neuroamines may be measured in the blood and urine.14 Pulmonary NETs may be functional and secrete these substances, leading to systemic symptoms based on the released molecules.15 However, pulmonary NETs produce less serotonin than gastrointestinal NETs; therefore, carcinoid syndrome is less frequent in pulmonary NETs.16 Liver metastasis is often present when it occurs.5 Other possible clinical features include Cushing syndrome and acromegaly depending on the secreted hormones.5
In a recent metanalysis, serum LDH has been found to have a prognostic role in Ewing sarcoma, urologic cancers, malignant mesothelioma, among others.17 It demonstrated that a higher LDH concentration is associated with worse survival in patients with lung cancer.17 Serum LDH is an enzyme that catalyzes the reaction between lactic acid and pyruvic acid that typically takes place in anaerobic conditions.17 LDH levels are elevated in malignancies because tumors have an anaerobic environment. Elevated LDH levels correlate with the anaerobic metabolism in the tumor. Other studies also have noted that patients with high metastatic score have higher LDH levels.17 Therefore, LDH may reflect tumor extension.
In addition, other techniques, such as somatostatin- receptor imaging are specifically beneficial in tumors that express the somatostatin receptor.16 For this reason, this type of study is typically indicated in patients with known metastasis, not in patients with low-grade tumors. Abdominal CT scans are done because the liver is a common site for metastasis.
Our case report demonstrates how biomarkers help diagnose these potentially aggressive and life-threatening tumors that may present as a common condition such as a pleural effusion. Using a less invasive and quicker approach with thoracentesis rather than with lung biopsies is a diagnostic tool in this entity. IHC in cell blocks is a reasonable diagnostic method especially in patients in whom performing a lung biopsy is difficult.
Conclusions
The presence of a symptomatic and recurrent unilateral pleural effusion must urge physicians to consider thoracentesis with mindful use of biomarkers not only for therapeutic purposes, but also for diagnosis of a variety of etiologies, both benign and malignant.
1. Oronsky B, Ma PC, Morgensztern D, Carter CA. Nothing but NET: a review of neuroendocrine tumors and carcinomas. Neoplasia. 2017;19(12):991-1002. doi: 10.1016/j.neo.2017.09.002
2. Hendifar AE, Marchevsky AM, Tuli R. Neuroendocrine tumors of the lung: current challenges and advances in the diagnosis and management of well-differentiated disease. J Thorac Oncol. 2017;12(3):425-436. doi: 10.1016/j.jtho.2016.11.2222
3. Fisseler-Eckhoff A, Demes M. Neuroendocrine tumors of the lung. Cancers (Basel). 2012;4(3):777-798. doi: 10.3390/cancers4030777
4. Mandegaran R, David S, Screaton N. Cardiothoracic manifestations of neuroendocrine tumours. Br J Radiol. 2016;89(1060). doi: 10.1259/bjr.20150787
5. Caplin ME, Baudin E, Ferolla P, et al; ENETS consensus conference participants. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol. 2015;26(8):1604-1620. doi: 10.1093/annonc/mdv041
6. Pelosi G, Sonzogni A, Harari S, et al. Classification of pulmonary neuroendocrine tumors: new insights. Transl Lung Cancer Res. 2017;6(5):513-529. doi: 10.21037/tlcr.2017.09.04
7. Rossi G, Bertero L, Marchiò C, Papotti M. Molecular alterations of neuroendocrine tumours of the lung. Histopathology. 2018;72(1):142-152. doi: 10.1111/his.13394.
8. Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): moving from targeted therapy to immunotherapy. Semin Cancer Biol. 2018;52(pt 1):103-109. doi: 10.1016/j.semcancer.2017.11.019
9. Kaur G, Nijhawan R, Gupta N, Singh N, Rajwanshi A. Pleural fluid cytology samples in cases of suspected lung cancer: an experience from a tertiary care centre. Diagn Cytopathol. 2017;45(3):195-201.
10. Porcel JM. Biomarkers in the diagnosis of pleural diseases: a 2018 update. Ther Adv Respir Dis. 2018;12. doi: 10.1177/1753466618808660
11. Kim JY, Hong SM, Ro JY. Recent updates on grading and classification of neuroendocrine tumors. Ann Diagn Pathol. 2017;29:11-16. doi: 10.1016/j.anndiagpath.2017.04.005
12. Isgrò MA, Bottoni P, Scatena R. Neuron-specific enolase as a biomarker: biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:125-143. doi: 10.1007/978-94-017-7215-0_9
13. Rossi G, Bertero L, Marchiò C, Papotti M. Molecular alterations of neuroendocrine tumours of the lung. Histopathology. 2018;72(1):142-152. doi: 10.1111/his.13394
14. Eriksson B, Oberg K, Stridsberg M. Tumor markers in neuroendocrine tumors. Digestion. 2000;62(suppl 1):33-38.
15. Melosky B. Low grade neuroendocrine tumors of the lung. Front Oncol. 2017;7:119. doi: 10.3389/fonc.2017.00119
16. Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2001;113(1):5-21. https://doi.org/10.1002/cncr.23542
17. Deng T, Zhang J, Meng Y, Zhou Y, Li W. Higher pretreatment lactate dehydrogenase concentration predicts worse overall survival in patients with lung cancer. Medicine (Baltimore). 2018;97(38):e12524
Neuroendocrine tumors (NETs) account for about 0.5% of all newly diagnosed malignancies.1 Pulmonary NETs are rare, accounting for 1 to 2% of all invasive lung malignancies and involve about 20 to 25% of primary lung malignancies. 2,3 Their prevalence has increased by an estimated 6% per year over the past 30 years.2 Nonetheless, the time of diagnosis is frequently delayed because of nonspecific symptoms that may imitate other pulmonary conditions.
In the normal pleural space, there is a steady state in which there is a roughly equal rate of fluid formation and absorption. Any disequilibrium may produce a pleural effusion. Pleural fluids can be transudates or exudates. Transudates result from imbalances in hydrostatic and oncotic pressures in the pleural space. Exudates result primarily from pleural and/or lung inflammation or from impaired lymphatic drainage of the pleural space. Clinical manifestations include cough, wheezing, recurrent pneumonia, hemoptysis and pleural effusions. We present a case of a man who developed a large left pleural effusion with a pathology report suggesting a pulmonary NET as the etiology. Being aware of this rare entity may help improve prognosis by making an earlier diagnosis and starting treatment sooner.
Case Presentation
A 90-year-old man with a medical history of arterial hypertension, hyperlipidemia, type 2 diabetes mellitus, coronary artery disease, and vascular dementia presented to the emergency department with hypoactivity, poor appetite, productive cough, and shortness of breath. The patient was a former smoker (unknown pack-years) who quit smoking cigarettes 7 years prior. Vital signs showed sinus tachycardia and peripheral oxygen saturation of 90% at room air. The initial physical examination was remarkable for decreased breath sounds and crackles at the left lung base. Laboratory findings showed leukocytosis with neutrophilia and chronic normocytic anemia. Chest computed tomography (CT) showed a large left-sided pleural effusion occupying most of the left hemithorax with adjacent atelectatic lung, enlarged pretracheal, subcarinal, and left perihilar lymph nodes (Figure 1).
The patient was admitted to the internal medicine ward with the diagnosis of left pneumonic process and started on IV levofloxacin. However, despite 7 days of antibiotic therapy, the patient’s respiratory symptoms worsened. This clinical deterioration prompted pulmonary service consultation. Chest radiography demonstrated an enlarging left pleural effusion (Figure 2). A thoracentesis drained 1.2 L of serosanguineous pleural fluid. Pleural fluid analysis showed a cell count of 947/cm3 with 79% of lymphocytes, total protein 3.8 g/dL, lactic dehydrogenase (LDH) level 607 U/L, and glucose level 109 mg/dL. Serum total protein was 6.62 g/dL, LDH 666 U/L and glucose 92 mg/dL (Tables 1 and 2). Alanine transaminase (ALT) and aspartate aminotransferase (AST) were 11 U/L and 21 U/L, respectively. Using Light criteria, the pleural:serum protein ratio was 0.57, the pleural:serum LDH ratio was 0.91, and the pleural LDH was more than two-thirds of the serum LDH. These calculations were consistent with an exudative effusion. An infectious disease workup, including blood and pleural fluid cultures, was negative.
The pleural fluid concentrated cell block hematoxylin and eosin (H&E) staining showed chromatin, prominent nucleoli, and nuclear molding, which was compatible with high-grade lung NET (Figure 3). The cell block immunohistochemistry (IHC) was positive for synaptophysin, chromogranin A, and neuron specific enolase (NSE) also consistent with a high-grade pulmonary NET (Figure 4). The proliferation marker protein Ki-67 labeling index (LI) showed a proliferation index > 20% (Figure 5). The patient did not have decision-making capacity given vascular dementia. Multiple attempts to contact the next of kin or family members were unsuccessful. Risks vs benefits were evaluated, and given the patient’s advanced age and multiple comorbidities, a conservative management approach under palliative care was chosen. For this reason, further genomic studies were not done.
Discussion
NETs are a group of neoplasms that differ in site, amount of cell propagation, and clinical manifestations.4 These tumors are rare with an estimated incidence of 25 to 50 per 100,000.4 The most commonly affected organ systems are the gastroenteropancreatic and the bronchopulmonary tracts, accounting for 60% and 25% of the tumors, respectively.4 The incidence is increasing over the past years in part because of novel diagnostic techniques.
The average age of diagnosis is between the fourth and sixth decades, affecting more women than men.5 Smoking has been identified as a possible culprit for the development of these neoplasms; nonetheless, the association is still not clear.4 For example, poorly differentiated pulmonary NETs have a strong association with smoking but not well-differentiated pulmonary NETs.2
Patients typically present with cough, wheezing, hemoptysis, and recurrent pneumonias, which are in part a consequence of obstruction caused by the mass.2 Sometimes, obstruction may yield persistent pleural effusions. Hemoptysis may be seen secondary to the vascularity of pulmonary NETs.
The diagnosis is often delayed because patients are frequently treated for infection before being diagnosed with the malignancy, such as in our case. Radiologic image findings include round opacities, central masses, and atelectasis. Pulmonary NETs are frequently found incidentally as solitary lung nodules. The CT scan is the most common diagnostic modality and can provide information about the borders of the tumor, the location and surrounding structures, including the presence of atelectasis.5 Pulmonary NETs are usually centrally located in an accessible region for lung biopsy. In cases where the mass is not easily reachable, thoracentesis may provide the only available specimen.
The 2015 World Health Organization classification has identified 4 histologic types of pulmonary NETs, namely, typical carcinoid (TC), atypical carcinoid (AC), large cell neuroendocrine carcinoma (LCNEC) and small cell lung carcinoma (SCLC).6 The low-grade pulmonary NET, the typical carcinoid, is slow growing and has lower rates of metastasis. The intermediate-grade NET, the atypical carcinoid, is more aggressive. The highgrade NETs, the LCNEC and the SCLC, are aggressive and spread quickly to other places.6 Consequently, LCNEC and SCLC have higher mortalities with a 5-year survival, ranging from 13 to 57% and 5%, respectively.7
Tumors may be histomorphologically classified by H&E staining. The main characteristics that differentiate the low- and high-grade NETs are the presence of necrosis and the mitotic rate. Both categories form neuropeptides and have dense granular cores when seen with an electron microscopy.6 The TC and AC have welldefined, organized histologic patterns, no necrosis, and scarce mitosis. On the other hand, the LCNEC and SCLC are poorly differentiated tumors with necrosis, atypia, and mitosis.6 LCNEC can be separated from SCLC and other tumors by IHC staining, whereas SCLC is primarily distinguished by morphology.
If the biopsy sample size is small, then IHC morphology and markers are helpful for subclassification.8 IHC is used to discern between neuroendocrine (NE) vs non-NE. The evaluation of pleural fluid includes preparation of cell blocks. Cell block staining is deemed better for IHC because it mimics a small biopsy that enables superior stains.9 The need for a pleural biopsy in cases where the cytology is negative depends on treatment aims, the kind of tumor, and the presence of metastasis.10 In almost 80% of cases, pleural biopsy and cytology are the only specimens obtained for analysis.Therefore, identification of these markers is practical for diagnosis.10 For this reason, pleural effusion samples are appropriate options to lung biopsy for molecular studies.10
Ki-67 LI in samples has the highest specificity and sensitivity for low-tointermediate- grade vs high-grade tumors. It is being used for guiding clinical and treatment decisions.6 In SCLC, the Ki-67 LI is not necessary for diagnosis but will be about 80%.11 The tumor cells will show epithelial characteristics with positive cytokeratin AE1/AE3 and monoclonal antibody CAM5.2 and neuroendocrine markers, including NCAM/CD56, chromogranin A, and synaptophysin.11
Thyroid transcription factor-1 (TTF- 1) is positive in most cases. In LCNEC, the Ki-67 LI is between 40% and 80%. NCAM/ CD56, chromogranin A, and synaptophysin are present in 92 to 100%, 80 to 85%, and 50 to 60%, respectively.11 TTF-1 is identified in half of the tumors. All these tumors express pancytokeratin (AE1/AE3), cytokeratin 7 or low-molecular-weight cytokeratin. Likewise, the carcinoids will show markers, such as chromogranin A, synaptophysin, CD56, and epithelial markers like pancytokeratin.11 However, the high-molecular-weight cytokeratin and TTF-1 are negative. Furthermore, NSE is considered a good tumor marker in the diagnosis and prognosis of SCLC. NSE also has been reported in NSCLC. The level of NSE correlates with tumor burden, number of metastatic sites, and response to treatment. 12 A potentially useful marker is the insulinoma-associated protein 1, which is a nuclear determinant of NE differentiation that stains all types of pulmonary NETs irrespective of the histology but does not stain adenocarcinoma or squamous cell carcinoma (SCC).6
Recently, genomic studies have identified gene alterations that have become standard of care for diagnosis and targeted therapies.8 For example, epidermal growth factor receptor (EGFR) and echinoderm microtubule- associated proteinlike 4, and anaplastic lymphoma kinase (EML4-ALK) mutations have been found in about 25% of lung adenocarcinomas. 8 Other abnormalities in LKB1/STK11, NF1, CDKN2A, SMARCA4 and KEAP1, KRAS, MET, ROS1, and RET have also been identified.8 On the other hand, SCC rarely have derangements in EGFR and EML4-ALK, but do show changes in RTKs, DDR2M, FGGRs, among others.8 In TC and AC, observed molecular alterations include MEN1 mutations, mTOR, and SSTRs pathway activation, and GC/ CEACAM1 and CD44/OTP expression.13 LCNEC and SCLC have shown TP53 and RB1 mutations and CDX2/VIL1/BAI3 expression. DLL3 expression and MET mutations may be present in SCLC.13 Last, chromatin remodeling gene mutations have been identified in all these lung NET types.13
Furthermore, neuropeptides and neuroamines may be measured in the blood and urine.14 Pulmonary NETs may be functional and secrete these substances, leading to systemic symptoms based on the released molecules.15 However, pulmonary NETs produce less serotonin than gastrointestinal NETs; therefore, carcinoid syndrome is less frequent in pulmonary NETs.16 Liver metastasis is often present when it occurs.5 Other possible clinical features include Cushing syndrome and acromegaly depending on the secreted hormones.5
In a recent metanalysis, serum LDH has been found to have a prognostic role in Ewing sarcoma, urologic cancers, malignant mesothelioma, among others.17 It demonstrated that a higher LDH concentration is associated with worse survival in patients with lung cancer.17 Serum LDH is an enzyme that catalyzes the reaction between lactic acid and pyruvic acid that typically takes place in anaerobic conditions.17 LDH levels are elevated in malignancies because tumors have an anaerobic environment. Elevated LDH levels correlate with the anaerobic metabolism in the tumor. Other studies also have noted that patients with high metastatic score have higher LDH levels.17 Therefore, LDH may reflect tumor extension.
In addition, other techniques, such as somatostatin- receptor imaging are specifically beneficial in tumors that express the somatostatin receptor.16 For this reason, this type of study is typically indicated in patients with known metastasis, not in patients with low-grade tumors. Abdominal CT scans are done because the liver is a common site for metastasis.
Our case report demonstrates how biomarkers help diagnose these potentially aggressive and life-threatening tumors that may present as a common condition such as a pleural effusion. Using a less invasive and quicker approach with thoracentesis rather than with lung biopsies is a diagnostic tool in this entity. IHC in cell blocks is a reasonable diagnostic method especially in patients in whom performing a lung biopsy is difficult.
Conclusions
The presence of a symptomatic and recurrent unilateral pleural effusion must urge physicians to consider thoracentesis with mindful use of biomarkers not only for therapeutic purposes, but also for diagnosis of a variety of etiologies, both benign and malignant.
Neuroendocrine tumors (NETs) account for about 0.5% of all newly diagnosed malignancies.1 Pulmonary NETs are rare, accounting for 1 to 2% of all invasive lung malignancies and involve about 20 to 25% of primary lung malignancies. 2,3 Their prevalence has increased by an estimated 6% per year over the past 30 years.2 Nonetheless, the time of diagnosis is frequently delayed because of nonspecific symptoms that may imitate other pulmonary conditions.
In the normal pleural space, there is a steady state in which there is a roughly equal rate of fluid formation and absorption. Any disequilibrium may produce a pleural effusion. Pleural fluids can be transudates or exudates. Transudates result from imbalances in hydrostatic and oncotic pressures in the pleural space. Exudates result primarily from pleural and/or lung inflammation or from impaired lymphatic drainage of the pleural space. Clinical manifestations include cough, wheezing, recurrent pneumonia, hemoptysis and pleural effusions. We present a case of a man who developed a large left pleural effusion with a pathology report suggesting a pulmonary NET as the etiology. Being aware of this rare entity may help improve prognosis by making an earlier diagnosis and starting treatment sooner.
Case Presentation
A 90-year-old man with a medical history of arterial hypertension, hyperlipidemia, type 2 diabetes mellitus, coronary artery disease, and vascular dementia presented to the emergency department with hypoactivity, poor appetite, productive cough, and shortness of breath. The patient was a former smoker (unknown pack-years) who quit smoking cigarettes 7 years prior. Vital signs showed sinus tachycardia and peripheral oxygen saturation of 90% at room air. The initial physical examination was remarkable for decreased breath sounds and crackles at the left lung base. Laboratory findings showed leukocytosis with neutrophilia and chronic normocytic anemia. Chest computed tomography (CT) showed a large left-sided pleural effusion occupying most of the left hemithorax with adjacent atelectatic lung, enlarged pretracheal, subcarinal, and left perihilar lymph nodes (Figure 1).
The patient was admitted to the internal medicine ward with the diagnosis of left pneumonic process and started on IV levofloxacin. However, despite 7 days of antibiotic therapy, the patient’s respiratory symptoms worsened. This clinical deterioration prompted pulmonary service consultation. Chest radiography demonstrated an enlarging left pleural effusion (Figure 2). A thoracentesis drained 1.2 L of serosanguineous pleural fluid. Pleural fluid analysis showed a cell count of 947/cm3 with 79% of lymphocytes, total protein 3.8 g/dL, lactic dehydrogenase (LDH) level 607 U/L, and glucose level 109 mg/dL. Serum total protein was 6.62 g/dL, LDH 666 U/L and glucose 92 mg/dL (Tables 1 and 2). Alanine transaminase (ALT) and aspartate aminotransferase (AST) were 11 U/L and 21 U/L, respectively. Using Light criteria, the pleural:serum protein ratio was 0.57, the pleural:serum LDH ratio was 0.91, and the pleural LDH was more than two-thirds of the serum LDH. These calculations were consistent with an exudative effusion. An infectious disease workup, including blood and pleural fluid cultures, was negative.
The pleural fluid concentrated cell block hematoxylin and eosin (H&E) staining showed chromatin, prominent nucleoli, and nuclear molding, which was compatible with high-grade lung NET (Figure 3). The cell block immunohistochemistry (IHC) was positive for synaptophysin, chromogranin A, and neuron specific enolase (NSE) also consistent with a high-grade pulmonary NET (Figure 4). The proliferation marker protein Ki-67 labeling index (LI) showed a proliferation index > 20% (Figure 5). The patient did not have decision-making capacity given vascular dementia. Multiple attempts to contact the next of kin or family members were unsuccessful. Risks vs benefits were evaluated, and given the patient’s advanced age and multiple comorbidities, a conservative management approach under palliative care was chosen. For this reason, further genomic studies were not done.
Discussion
NETs are a group of neoplasms that differ in site, amount of cell propagation, and clinical manifestations.4 These tumors are rare with an estimated incidence of 25 to 50 per 100,000.4 The most commonly affected organ systems are the gastroenteropancreatic and the bronchopulmonary tracts, accounting for 60% and 25% of the tumors, respectively.4 The incidence is increasing over the past years in part because of novel diagnostic techniques.
The average age of diagnosis is between the fourth and sixth decades, affecting more women than men.5 Smoking has been identified as a possible culprit for the development of these neoplasms; nonetheless, the association is still not clear.4 For example, poorly differentiated pulmonary NETs have a strong association with smoking but not well-differentiated pulmonary NETs.2
Patients typically present with cough, wheezing, hemoptysis, and recurrent pneumonias, which are in part a consequence of obstruction caused by the mass.2 Sometimes, obstruction may yield persistent pleural effusions. Hemoptysis may be seen secondary to the vascularity of pulmonary NETs.
The diagnosis is often delayed because patients are frequently treated for infection before being diagnosed with the malignancy, such as in our case. Radiologic image findings include round opacities, central masses, and atelectasis. Pulmonary NETs are frequently found incidentally as solitary lung nodules. The CT scan is the most common diagnostic modality and can provide information about the borders of the tumor, the location and surrounding structures, including the presence of atelectasis.5 Pulmonary NETs are usually centrally located in an accessible region for lung biopsy. In cases where the mass is not easily reachable, thoracentesis may provide the only available specimen.
The 2015 World Health Organization classification has identified 4 histologic types of pulmonary NETs, namely, typical carcinoid (TC), atypical carcinoid (AC), large cell neuroendocrine carcinoma (LCNEC) and small cell lung carcinoma (SCLC).6 The low-grade pulmonary NET, the typical carcinoid, is slow growing and has lower rates of metastasis. The intermediate-grade NET, the atypical carcinoid, is more aggressive. The highgrade NETs, the LCNEC and the SCLC, are aggressive and spread quickly to other places.6 Consequently, LCNEC and SCLC have higher mortalities with a 5-year survival, ranging from 13 to 57% and 5%, respectively.7
Tumors may be histomorphologically classified by H&E staining. The main characteristics that differentiate the low- and high-grade NETs are the presence of necrosis and the mitotic rate. Both categories form neuropeptides and have dense granular cores when seen with an electron microscopy.6 The TC and AC have welldefined, organized histologic patterns, no necrosis, and scarce mitosis. On the other hand, the LCNEC and SCLC are poorly differentiated tumors with necrosis, atypia, and mitosis.6 LCNEC can be separated from SCLC and other tumors by IHC staining, whereas SCLC is primarily distinguished by morphology.
If the biopsy sample size is small, then IHC morphology and markers are helpful for subclassification.8 IHC is used to discern between neuroendocrine (NE) vs non-NE. The evaluation of pleural fluid includes preparation of cell blocks. Cell block staining is deemed better for IHC because it mimics a small biopsy that enables superior stains.9 The need for a pleural biopsy in cases where the cytology is negative depends on treatment aims, the kind of tumor, and the presence of metastasis.10 In almost 80% of cases, pleural biopsy and cytology are the only specimens obtained for analysis.Therefore, identification of these markers is practical for diagnosis.10 For this reason, pleural effusion samples are appropriate options to lung biopsy for molecular studies.10
Ki-67 LI in samples has the highest specificity and sensitivity for low-tointermediate- grade vs high-grade tumors. It is being used for guiding clinical and treatment decisions.6 In SCLC, the Ki-67 LI is not necessary for diagnosis but will be about 80%.11 The tumor cells will show epithelial characteristics with positive cytokeratin AE1/AE3 and monoclonal antibody CAM5.2 and neuroendocrine markers, including NCAM/CD56, chromogranin A, and synaptophysin.11
Thyroid transcription factor-1 (TTF- 1) is positive in most cases. In LCNEC, the Ki-67 LI is between 40% and 80%. NCAM/ CD56, chromogranin A, and synaptophysin are present in 92 to 100%, 80 to 85%, and 50 to 60%, respectively.11 TTF-1 is identified in half of the tumors. All these tumors express pancytokeratin (AE1/AE3), cytokeratin 7 or low-molecular-weight cytokeratin. Likewise, the carcinoids will show markers, such as chromogranin A, synaptophysin, CD56, and epithelial markers like pancytokeratin.11 However, the high-molecular-weight cytokeratin and TTF-1 are negative. Furthermore, NSE is considered a good tumor marker in the diagnosis and prognosis of SCLC. NSE also has been reported in NSCLC. The level of NSE correlates with tumor burden, number of metastatic sites, and response to treatment. 12 A potentially useful marker is the insulinoma-associated protein 1, which is a nuclear determinant of NE differentiation that stains all types of pulmonary NETs irrespective of the histology but does not stain adenocarcinoma or squamous cell carcinoma (SCC).6
Recently, genomic studies have identified gene alterations that have become standard of care for diagnosis and targeted therapies.8 For example, epidermal growth factor receptor (EGFR) and echinoderm microtubule- associated proteinlike 4, and anaplastic lymphoma kinase (EML4-ALK) mutations have been found in about 25% of lung adenocarcinomas. 8 Other abnormalities in LKB1/STK11, NF1, CDKN2A, SMARCA4 and KEAP1, KRAS, MET, ROS1, and RET have also been identified.8 On the other hand, SCC rarely have derangements in EGFR and EML4-ALK, but do show changes in RTKs, DDR2M, FGGRs, among others.8 In TC and AC, observed molecular alterations include MEN1 mutations, mTOR, and SSTRs pathway activation, and GC/ CEACAM1 and CD44/OTP expression.13 LCNEC and SCLC have shown TP53 and RB1 mutations and CDX2/VIL1/BAI3 expression. DLL3 expression and MET mutations may be present in SCLC.13 Last, chromatin remodeling gene mutations have been identified in all these lung NET types.13
Furthermore, neuropeptides and neuroamines may be measured in the blood and urine.14 Pulmonary NETs may be functional and secrete these substances, leading to systemic symptoms based on the released molecules.15 However, pulmonary NETs produce less serotonin than gastrointestinal NETs; therefore, carcinoid syndrome is less frequent in pulmonary NETs.16 Liver metastasis is often present when it occurs.5 Other possible clinical features include Cushing syndrome and acromegaly depending on the secreted hormones.5
In a recent metanalysis, serum LDH has been found to have a prognostic role in Ewing sarcoma, urologic cancers, malignant mesothelioma, among others.17 It demonstrated that a higher LDH concentration is associated with worse survival in patients with lung cancer.17 Serum LDH is an enzyme that catalyzes the reaction between lactic acid and pyruvic acid that typically takes place in anaerobic conditions.17 LDH levels are elevated in malignancies because tumors have an anaerobic environment. Elevated LDH levels correlate with the anaerobic metabolism in the tumor. Other studies also have noted that patients with high metastatic score have higher LDH levels.17 Therefore, LDH may reflect tumor extension.
In addition, other techniques, such as somatostatin- receptor imaging are specifically beneficial in tumors that express the somatostatin receptor.16 For this reason, this type of study is typically indicated in patients with known metastasis, not in patients with low-grade tumors. Abdominal CT scans are done because the liver is a common site for metastasis.
Our case report demonstrates how biomarkers help diagnose these potentially aggressive and life-threatening tumors that may present as a common condition such as a pleural effusion. Using a less invasive and quicker approach with thoracentesis rather than with lung biopsies is a diagnostic tool in this entity. IHC in cell blocks is a reasonable diagnostic method especially in patients in whom performing a lung biopsy is difficult.
Conclusions
The presence of a symptomatic and recurrent unilateral pleural effusion must urge physicians to consider thoracentesis with mindful use of biomarkers not only for therapeutic purposes, but also for diagnosis of a variety of etiologies, both benign and malignant.
1. Oronsky B, Ma PC, Morgensztern D, Carter CA. Nothing but NET: a review of neuroendocrine tumors and carcinomas. Neoplasia. 2017;19(12):991-1002. doi: 10.1016/j.neo.2017.09.002
2. Hendifar AE, Marchevsky AM, Tuli R. Neuroendocrine tumors of the lung: current challenges and advances in the diagnosis and management of well-differentiated disease. J Thorac Oncol. 2017;12(3):425-436. doi: 10.1016/j.jtho.2016.11.2222
3. Fisseler-Eckhoff A, Demes M. Neuroendocrine tumors of the lung. Cancers (Basel). 2012;4(3):777-798. doi: 10.3390/cancers4030777
4. Mandegaran R, David S, Screaton N. Cardiothoracic manifestations of neuroendocrine tumours. Br J Radiol. 2016;89(1060). doi: 10.1259/bjr.20150787
5. Caplin ME, Baudin E, Ferolla P, et al; ENETS consensus conference participants. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol. 2015;26(8):1604-1620. doi: 10.1093/annonc/mdv041
6. Pelosi G, Sonzogni A, Harari S, et al. Classification of pulmonary neuroendocrine tumors: new insights. Transl Lung Cancer Res. 2017;6(5):513-529. doi: 10.21037/tlcr.2017.09.04
7. Rossi G, Bertero L, Marchiò C, Papotti M. Molecular alterations of neuroendocrine tumours of the lung. Histopathology. 2018;72(1):142-152. doi: 10.1111/his.13394.
8. Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): moving from targeted therapy to immunotherapy. Semin Cancer Biol. 2018;52(pt 1):103-109. doi: 10.1016/j.semcancer.2017.11.019
9. Kaur G, Nijhawan R, Gupta N, Singh N, Rajwanshi A. Pleural fluid cytology samples in cases of suspected lung cancer: an experience from a tertiary care centre. Diagn Cytopathol. 2017;45(3):195-201.
10. Porcel JM. Biomarkers in the diagnosis of pleural diseases: a 2018 update. Ther Adv Respir Dis. 2018;12. doi: 10.1177/1753466618808660
11. Kim JY, Hong SM, Ro JY. Recent updates on grading and classification of neuroendocrine tumors. Ann Diagn Pathol. 2017;29:11-16. doi: 10.1016/j.anndiagpath.2017.04.005
12. Isgrò MA, Bottoni P, Scatena R. Neuron-specific enolase as a biomarker: biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:125-143. doi: 10.1007/978-94-017-7215-0_9
13. Rossi G, Bertero L, Marchiò C, Papotti M. Molecular alterations of neuroendocrine tumours of the lung. Histopathology. 2018;72(1):142-152. doi: 10.1111/his.13394
14. Eriksson B, Oberg K, Stridsberg M. Tumor markers in neuroendocrine tumors. Digestion. 2000;62(suppl 1):33-38.
15. Melosky B. Low grade neuroendocrine tumors of the lung. Front Oncol. 2017;7:119. doi: 10.3389/fonc.2017.00119
16. Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2001;113(1):5-21. https://doi.org/10.1002/cncr.23542
17. Deng T, Zhang J, Meng Y, Zhou Y, Li W. Higher pretreatment lactate dehydrogenase concentration predicts worse overall survival in patients with lung cancer. Medicine (Baltimore). 2018;97(38):e12524
1. Oronsky B, Ma PC, Morgensztern D, Carter CA. Nothing but NET: a review of neuroendocrine tumors and carcinomas. Neoplasia. 2017;19(12):991-1002. doi: 10.1016/j.neo.2017.09.002
2. Hendifar AE, Marchevsky AM, Tuli R. Neuroendocrine tumors of the lung: current challenges and advances in the diagnosis and management of well-differentiated disease. J Thorac Oncol. 2017;12(3):425-436. doi: 10.1016/j.jtho.2016.11.2222
3. Fisseler-Eckhoff A, Demes M. Neuroendocrine tumors of the lung. Cancers (Basel). 2012;4(3):777-798. doi: 10.3390/cancers4030777
4. Mandegaran R, David S, Screaton N. Cardiothoracic manifestations of neuroendocrine tumours. Br J Radiol. 2016;89(1060). doi: 10.1259/bjr.20150787
5. Caplin ME, Baudin E, Ferolla P, et al; ENETS consensus conference participants. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol. 2015;26(8):1604-1620. doi: 10.1093/annonc/mdv041
6. Pelosi G, Sonzogni A, Harari S, et al. Classification of pulmonary neuroendocrine tumors: new insights. Transl Lung Cancer Res. 2017;6(5):513-529. doi: 10.21037/tlcr.2017.09.04
7. Rossi G, Bertero L, Marchiò C, Papotti M. Molecular alterations of neuroendocrine tumours of the lung. Histopathology. 2018;72(1):142-152. doi: 10.1111/his.13394.
8. Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): moving from targeted therapy to immunotherapy. Semin Cancer Biol. 2018;52(pt 1):103-109. doi: 10.1016/j.semcancer.2017.11.019
9. Kaur G, Nijhawan R, Gupta N, Singh N, Rajwanshi A. Pleural fluid cytology samples in cases of suspected lung cancer: an experience from a tertiary care centre. Diagn Cytopathol. 2017;45(3):195-201.
10. Porcel JM. Biomarkers in the diagnosis of pleural diseases: a 2018 update. Ther Adv Respir Dis. 2018;12. doi: 10.1177/1753466618808660
11. Kim JY, Hong SM, Ro JY. Recent updates on grading and classification of neuroendocrine tumors. Ann Diagn Pathol. 2017;29:11-16. doi: 10.1016/j.anndiagpath.2017.04.005
12. Isgrò MA, Bottoni P, Scatena R. Neuron-specific enolase as a biomarker: biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:125-143. doi: 10.1007/978-94-017-7215-0_9
13. Rossi G, Bertero L, Marchiò C, Papotti M. Molecular alterations of neuroendocrine tumours of the lung. Histopathology. 2018;72(1):142-152. doi: 10.1111/his.13394
14. Eriksson B, Oberg K, Stridsberg M. Tumor markers in neuroendocrine tumors. Digestion. 2000;62(suppl 1):33-38.
15. Melosky B. Low grade neuroendocrine tumors of the lung. Front Oncol. 2017;7:119. doi: 10.3389/fonc.2017.00119
16. Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2001;113(1):5-21. https://doi.org/10.1002/cncr.23542
17. Deng T, Zhang J, Meng Y, Zhou Y, Li W. Higher pretreatment lactate dehydrogenase concentration predicts worse overall survival in patients with lung cancer. Medicine (Baltimore). 2018;97(38):e12524