User login
CDC emphasizes pandemic not over, need to avoid large gatherings
Robert Redfield, MD, Director, CDC, and Jay C. Butler, MD, Deputy Director of Infectious Diseases and COVID-19 Response Incident Manager, CDC, discussed two new sets of CDC guidance on deciding to go out and attending group gatherings.
“We recognize that we’re all getting tired of staying at home; people long for the life that they had back in December, and as we head into the summer months, we know that Americans will be looking forward to reconnecting with family and friends and being able to attend events, and we want that to occur as safely as possible,” Butler said.
“Our recommendations evolved based on new information that becomes available, but it continues to be extremely important that we embrace the recommendations of social distancing, handwashing, and wearing a face covering when we’re in public as some of the key defenses that we have against this virus,” Redfield explained.
“The pandemic is not over and it’s important to recognize that. While COVID-19 is still making headlines everywhere, we know the pandemic hasn’t affected everyone everywhere in the same way,” Butler said.
He noted that it is important to prepare for next fall and winter, when we can expect influenza season to complicate matters. “If anything, we must be overly-prepared for what we might face later this year,” he continued, adding that it is important to get vaccinated against influenza. “[F]lu and COVID-19 could be circulating together as we move into the fall and winter months,” he concluded.
Americans Mostly Following Guidelines
The agency also presented data from an article published online June 12 in Morbidity and Mortality Weekly Report that “underscores the fact that American people have taken mitigation efforts seriously…and it demonstrates our collective spirit in responding to the pandemic,” Butler said.
In it, the researchers describe representative panel surveys conducted among 4042 adults aged 18 years or older in New York City and Los Angeles — the two most populous cities in the United States — and “broadly across the United States” during May 5 to May 12, 2020.
Most respondents supported stay-at-home orders and nonessential business closures (United States, 79.5%; New York City, 86.7%; Los Angeles, 81.5%) and always or often wore cloth face coverings in public (United States, 74.1%; New York City, 89.6%; Los Angeles, 89.8%). Respondents also agreed that nonessential workers should remain at home (United States, 67.3%; New York City, 76.6%; Los Angeles, 69.1%), report Mark É. Czeisler, from Monash University and Austin Health, both in Melbourne, Australia, and colleagues.
There was wide support with public health guidelines: more than 87% of individuals in each area agreed that individuals should keep six feet of distance between themselves and others, and more than 82% in each area said that people should limit gatherings to fewer than 10 individuals.
At the time the survey was conducted, most were against indoor dining at restaurants (United States, 66.6%; New York City, 81.5%; Los Angeles, 71.8%).
Adherence “Widespread,” Survey Finds
Most respondents said they were adhering to COVID-19 mitigation guidance, including self-isolating (United States, 77.3%; New York City, 84.6%; Los Angeles, 83.0%) and “always or often” kept at least six feet between themselves and others (New York City, 85.7%; Los Angeles, 82.6%).
More than 85% of respondents in each of the three cohorts said they always or often avoided groups of 10 or more individuals.
About 90% of respondents said they had been in a public area during the last week, with 74.1% of those saying they always or often covered their face in public; respondents in New York City (89.6%) and Los Angeles (89.8%) had higher percentages of this behavior compared with respondents from the United States overall.
Most respondents felt that restrictions in their state were balanced or too lax (United States, 84.3%; New York City, 89.7%; Los Angeles, 79.7%) and said they would feel unsafe if restrictions were eased nationwide at that time (United States, 74.3%; New York City, 81.5%; Los Angeles, 73.4%). However, some individuals who said they would feel unsafe still wanted community mitigation strategies eased and were willing to accept risks resulting from lifting restrictions (United States, 17.1%; New York City, 12.6%; Los Angeles, 12.7%).
“Reported prevalence of self-isolation and feeling safe if community mitigation strategies were lifted differed significantly by age, employment status, and essential worker status among adults in the U.S. survey cohort,” the authors write.
Reports of self-isolation were highest among persons aged 18 to 24 years (92.3%) and lowest among those aged 45 to 54 years (71.5%). Yet, young adults aged 18 to 24 years (43.1%) were more than twice as likely to say they would feel safe if community mitigation strategies were eased, compared with adults aged 65 years or older (19.2%).
Almost half (47.2%) of employed respondents in the US cohort were essential workers; essential workers were “significantly less likely” to report self-isolating when compared with nonessential workers (63.1% vs 80.6%). Some 37.7% of essential workers said they would feel safe if community mitigation strategies were eased, compared with 23.7% of nonessential workers.
“Respondents who were male, employed, or essential workers were significantly more likely to report having been in public areas in the past week. Among respondents who had been in public areas during the preceding week, significantly higher percentages of women, adults aged ≥ 65 years, retired persons, and those living in urban areas reported wearing cloth face coverings,” the authors explain.
The findings are subject to several limitations, including self-reporting and the fact that some respondents may have known someone who tested positive for COVID-19 or died from it, the authors note. Respondents were not representative of the US population and the findings may not be generalizable.
This article first appeared on Medscape.com.
Robert Redfield, MD, Director, CDC, and Jay C. Butler, MD, Deputy Director of Infectious Diseases and COVID-19 Response Incident Manager, CDC, discussed two new sets of CDC guidance on deciding to go out and attending group gatherings.
“We recognize that we’re all getting tired of staying at home; people long for the life that they had back in December, and as we head into the summer months, we know that Americans will be looking forward to reconnecting with family and friends and being able to attend events, and we want that to occur as safely as possible,” Butler said.
“Our recommendations evolved based on new information that becomes available, but it continues to be extremely important that we embrace the recommendations of social distancing, handwashing, and wearing a face covering when we’re in public as some of the key defenses that we have against this virus,” Redfield explained.
“The pandemic is not over and it’s important to recognize that. While COVID-19 is still making headlines everywhere, we know the pandemic hasn’t affected everyone everywhere in the same way,” Butler said.
He noted that it is important to prepare for next fall and winter, when we can expect influenza season to complicate matters. “If anything, we must be overly-prepared for what we might face later this year,” he continued, adding that it is important to get vaccinated against influenza. “[F]lu and COVID-19 could be circulating together as we move into the fall and winter months,” he concluded.
Americans Mostly Following Guidelines
The agency also presented data from an article published online June 12 in Morbidity and Mortality Weekly Report that “underscores the fact that American people have taken mitigation efforts seriously…and it demonstrates our collective spirit in responding to the pandemic,” Butler said.
In it, the researchers describe representative panel surveys conducted among 4042 adults aged 18 years or older in New York City and Los Angeles — the two most populous cities in the United States — and “broadly across the United States” during May 5 to May 12, 2020.
Most respondents supported stay-at-home orders and nonessential business closures (United States, 79.5%; New York City, 86.7%; Los Angeles, 81.5%) and always or often wore cloth face coverings in public (United States, 74.1%; New York City, 89.6%; Los Angeles, 89.8%). Respondents also agreed that nonessential workers should remain at home (United States, 67.3%; New York City, 76.6%; Los Angeles, 69.1%), report Mark É. Czeisler, from Monash University and Austin Health, both in Melbourne, Australia, and colleagues.
There was wide support with public health guidelines: more than 87% of individuals in each area agreed that individuals should keep six feet of distance between themselves and others, and more than 82% in each area said that people should limit gatherings to fewer than 10 individuals.
At the time the survey was conducted, most were against indoor dining at restaurants (United States, 66.6%; New York City, 81.5%; Los Angeles, 71.8%).
Adherence “Widespread,” Survey Finds
Most respondents said they were adhering to COVID-19 mitigation guidance, including self-isolating (United States, 77.3%; New York City, 84.6%; Los Angeles, 83.0%) and “always or often” kept at least six feet between themselves and others (New York City, 85.7%; Los Angeles, 82.6%).
More than 85% of respondents in each of the three cohorts said they always or often avoided groups of 10 or more individuals.
About 90% of respondents said they had been in a public area during the last week, with 74.1% of those saying they always or often covered their face in public; respondents in New York City (89.6%) and Los Angeles (89.8%) had higher percentages of this behavior compared with respondents from the United States overall.
Most respondents felt that restrictions in their state were balanced or too lax (United States, 84.3%; New York City, 89.7%; Los Angeles, 79.7%) and said they would feel unsafe if restrictions were eased nationwide at that time (United States, 74.3%; New York City, 81.5%; Los Angeles, 73.4%). However, some individuals who said they would feel unsafe still wanted community mitigation strategies eased and were willing to accept risks resulting from lifting restrictions (United States, 17.1%; New York City, 12.6%; Los Angeles, 12.7%).
“Reported prevalence of self-isolation and feeling safe if community mitigation strategies were lifted differed significantly by age, employment status, and essential worker status among adults in the U.S. survey cohort,” the authors write.
Reports of self-isolation were highest among persons aged 18 to 24 years (92.3%) and lowest among those aged 45 to 54 years (71.5%). Yet, young adults aged 18 to 24 years (43.1%) were more than twice as likely to say they would feel safe if community mitigation strategies were eased, compared with adults aged 65 years or older (19.2%).
Almost half (47.2%) of employed respondents in the US cohort were essential workers; essential workers were “significantly less likely” to report self-isolating when compared with nonessential workers (63.1% vs 80.6%). Some 37.7% of essential workers said they would feel safe if community mitigation strategies were eased, compared with 23.7% of nonessential workers.
“Respondents who were male, employed, or essential workers were significantly more likely to report having been in public areas in the past week. Among respondents who had been in public areas during the preceding week, significantly higher percentages of women, adults aged ≥ 65 years, retired persons, and those living in urban areas reported wearing cloth face coverings,” the authors explain.
The findings are subject to several limitations, including self-reporting and the fact that some respondents may have known someone who tested positive for COVID-19 or died from it, the authors note. Respondents were not representative of the US population and the findings may not be generalizable.
This article first appeared on Medscape.com.
Robert Redfield, MD, Director, CDC, and Jay C. Butler, MD, Deputy Director of Infectious Diseases and COVID-19 Response Incident Manager, CDC, discussed two new sets of CDC guidance on deciding to go out and attending group gatherings.
“We recognize that we’re all getting tired of staying at home; people long for the life that they had back in December, and as we head into the summer months, we know that Americans will be looking forward to reconnecting with family and friends and being able to attend events, and we want that to occur as safely as possible,” Butler said.
“Our recommendations evolved based on new information that becomes available, but it continues to be extremely important that we embrace the recommendations of social distancing, handwashing, and wearing a face covering when we’re in public as some of the key defenses that we have against this virus,” Redfield explained.
“The pandemic is not over and it’s important to recognize that. While COVID-19 is still making headlines everywhere, we know the pandemic hasn’t affected everyone everywhere in the same way,” Butler said.
He noted that it is important to prepare for next fall and winter, when we can expect influenza season to complicate matters. “If anything, we must be overly-prepared for what we might face later this year,” he continued, adding that it is important to get vaccinated against influenza. “[F]lu and COVID-19 could be circulating together as we move into the fall and winter months,” he concluded.
Americans Mostly Following Guidelines
The agency also presented data from an article published online June 12 in Morbidity and Mortality Weekly Report that “underscores the fact that American people have taken mitigation efforts seriously…and it demonstrates our collective spirit in responding to the pandemic,” Butler said.
In it, the researchers describe representative panel surveys conducted among 4042 adults aged 18 years or older in New York City and Los Angeles — the two most populous cities in the United States — and “broadly across the United States” during May 5 to May 12, 2020.
Most respondents supported stay-at-home orders and nonessential business closures (United States, 79.5%; New York City, 86.7%; Los Angeles, 81.5%) and always or often wore cloth face coverings in public (United States, 74.1%; New York City, 89.6%; Los Angeles, 89.8%). Respondents also agreed that nonessential workers should remain at home (United States, 67.3%; New York City, 76.6%; Los Angeles, 69.1%), report Mark É. Czeisler, from Monash University and Austin Health, both in Melbourne, Australia, and colleagues.
There was wide support with public health guidelines: more than 87% of individuals in each area agreed that individuals should keep six feet of distance between themselves and others, and more than 82% in each area said that people should limit gatherings to fewer than 10 individuals.
At the time the survey was conducted, most were against indoor dining at restaurants (United States, 66.6%; New York City, 81.5%; Los Angeles, 71.8%).
Adherence “Widespread,” Survey Finds
Most respondents said they were adhering to COVID-19 mitigation guidance, including self-isolating (United States, 77.3%; New York City, 84.6%; Los Angeles, 83.0%) and “always or often” kept at least six feet between themselves and others (New York City, 85.7%; Los Angeles, 82.6%).
More than 85% of respondents in each of the three cohorts said they always or often avoided groups of 10 or more individuals.
About 90% of respondents said they had been in a public area during the last week, with 74.1% of those saying they always or often covered their face in public; respondents in New York City (89.6%) and Los Angeles (89.8%) had higher percentages of this behavior compared with respondents from the United States overall.
Most respondents felt that restrictions in their state were balanced or too lax (United States, 84.3%; New York City, 89.7%; Los Angeles, 79.7%) and said they would feel unsafe if restrictions were eased nationwide at that time (United States, 74.3%; New York City, 81.5%; Los Angeles, 73.4%). However, some individuals who said they would feel unsafe still wanted community mitigation strategies eased and were willing to accept risks resulting from lifting restrictions (United States, 17.1%; New York City, 12.6%; Los Angeles, 12.7%).
“Reported prevalence of self-isolation and feeling safe if community mitigation strategies were lifted differed significantly by age, employment status, and essential worker status among adults in the U.S. survey cohort,” the authors write.
Reports of self-isolation were highest among persons aged 18 to 24 years (92.3%) and lowest among those aged 45 to 54 years (71.5%). Yet, young adults aged 18 to 24 years (43.1%) were more than twice as likely to say they would feel safe if community mitigation strategies were eased, compared with adults aged 65 years or older (19.2%).
Almost half (47.2%) of employed respondents in the US cohort were essential workers; essential workers were “significantly less likely” to report self-isolating when compared with nonessential workers (63.1% vs 80.6%). Some 37.7% of essential workers said they would feel safe if community mitigation strategies were eased, compared with 23.7% of nonessential workers.
“Respondents who were male, employed, or essential workers were significantly more likely to report having been in public areas in the past week. Among respondents who had been in public areas during the preceding week, significantly higher percentages of women, adults aged ≥ 65 years, retired persons, and those living in urban areas reported wearing cloth face coverings,” the authors explain.
The findings are subject to several limitations, including self-reporting and the fact that some respondents may have known someone who tested positive for COVID-19 or died from it, the authors note. Respondents were not representative of the US population and the findings may not be generalizable.
This article first appeared on Medscape.com.
Automated insulin delivery system ‘getting better and better’
Medtronic’s next-generation automated insulin delivery system offers significant improvements over the currently available 670G hybrid closed-loop, particularly in young people with type 1 diabetes, new data suggest.
Automated insulin delivery systems are comprised of an insulin pump, continuous glucose monitor (CGM), and an automated insulin dosing algorithm.
Data from three trials of such systems using Medtronic’s advanced hybrid closed-loop (AHCL) algorithm (trade name SmartGuard) were presented June 12 during the virtual American Diabetes Association (ADA) 80th Scientific Sessions. The AHCL is the algorithm used in Medtronic’s new MiniMed 780G system, which received a CE Mark on June 11 for the treatment of type 1 diabetes in people aged 7 to 80 years.
One trial, presented by Bruce W. Bode, MD, of Atlanta Diabetes Associates, Georgia, was the US pivotal safety study that will be submitted to the US Food and Drug Administration for approval of the Medtronic 780G.
Another trial, presented by Richard M. Bergenstal, MD, executive director of the International Diabetes Center at Park Nicollet, Minneapolis, Minnesota, was a separate comparison of the AHCL with the 670G. (The AHCL-based system used in the three trials was identical to the 780G except it didn’t include Bluetooth, which will be a feature of the final product.)
A third trial, presented by Martin de Bock, PhD, of the University of Otago, New Zealand, included the CE Mark dataset for the 780G.
In contrast to the 670G, the 780G adds automated correction boluses for high blood glucose levels (rather than simply adjusting the basal infusion) and allows for adjustment of target glucose levels down to 100 mg/dL rather than a minimum of 120 mg/dL.
Taken together, the data from the three trials showed that the AHCL-based system improved glycemic time-in-range with no increased risk for hypoglycemia, including in children and teenagers, with high patient-reported satisfaction. And specifically compared to the 670G, the AHCL-based system reverts to open-loop far less often because it only exits closed-loop mode when the sensor stops working or during sensor changes, but not during hyperglycemia even above 300 mg/dL.
Asked to comment, session moderator Timothy S. Bailey, MD, president and CEO of the AMCR Institute, Escondido, California, told Medscape Medical News: “Automated insulin delivery systems are getting better and better.”
“None of these devices is perfect, but they are a substantial improvement over what we’ve had ... They all take people from where they are now to better time-in-range, less time with hypoglycemia, and most important, they might make the quality of their lives better. That’s really underappreciated.”
One factor that has allowed for the improvements, Bailey said, is the recognition by regulatory bodies that the hybrid closed-loop devices are generally safer than current open-loop type 1 diabetes management so that fewer “safety” device features that interfere with tight glycemic control are necessary.
With first-generation closed-loop systems, “If a wide variety of conditions occur, users get kicked off [hybrid closed-loop mode]. Originally it was perceived by the regulatory agencies as a safety feature because they perceived the standard of care as safe. The new system was allowed to have fewer rules.”
Pivotal trial: Time-in-range improved, 96% say system easy to use
The goal of the AHCL system is to maximize the time-in-range of blood glucose between 70-180 mg/dL. Automated basal delivery of insulin is programmed to a set-point of 100 or 120 mg/dL, with dosing every 5 minutes.
The US pivotal trial was a single-arm, 16-center, in-home trial of 157 people with type 1 diabetes, including 39 adolescents aged 14-21 years and 118 adults aged 22-75 years. All had type 1 diabetes for at least 2 years, A1c levels below 10%, and had been using insulin pumps for at least 6 months, with or without CGMs.
After a 14-day run-in, they wore the systems with a 100 or 120 mg/dL set-point for 45 days, then switched to the other setpoint for another 45 days. Average A1c dropped from 7.5% to 7.0%, with the proportions having an A1c ≤ 7.0% increasing from 34% to 61%.
Overall time-in-range was 75% compared to 69% at baseline, with time below range (< 70 mg/dL) of 1.8%. Overnight time-in-range was 82%, with 1.5% below range. Time-in-range increased from 62% to 73% in the adolescents and from 71% to 75% in the adults.
There were no incidences of severe hypoglycemia or diabetic ketoacidosis, and no device-related serious adverse events.
Participants reported being in hybrid closed-loop, or auto-mode, 95% of the time, compared with 33% for those who had been previously using the 670G.
The number of AHCL exits was 1.3 per week, significantly less than with the 670G. Of those, 29% were user-initiated while the rest were implemented by the device, most often when the sensor wasn’t working.
In a study questionnaire, 96% reported that the system was easy to use.
AHCL vs 670G: Major improvements seen
Bergenstal presented data from the Fuzzy Logic Automated Insulin Regulation (FLAIR) study, funded by the National Institute of Diabetes and Digestive and Kidney Disease, comparing Medtronic’s AHCL-based system with the currently marketed 670G hybrid closed-loop, in 113 individuals with type 1 diabetes aged 14-29 years.
“This age group has traditionally been the most difficult group in which to optimize glucose management,” Bergenstal said.
FLAIR is believed to be the first-ever study comparing an investigational automated insulin delivery system with a commercially approved system, he noted. All participants used each automated insulin delivery system for 3 months in the randomized crossover trial.
The primary outcome, time spent above 180 mg/dL during the day combined with time below 54 mg/dL over 24 hours at baseline with the 670G and AHCL went from 42% to 37% to 34%, respectively, for the former and from 0.46% to 0.50% to 0.45%, respectively, for the latter.
The percentage time-in-range over 24 hours went from 57% at baseline to 67% with the AHCL versus 63% with the 670G. A1c levels dropped from 7.9% at baseline to 7.6% with the 670G and 7.4% with AHCL.
“Remember, these are the adolescents who are the toughest of the tough, yet there was a 10% increase in time-in-range ... this is very clinically significant,” Bergenstal said.
Even among 14 patients who had been using multiple daily injections without CGM prior to the study, a group often excluded from closed-loop studies, time-in-range improved from 45% at baseline to 63% with the 670G to 65% with AHCL.
“I’m making a plea not to exclude people just because they haven’t previously used technology,” Bergenstal said.
One patient who had dosed with extra insulin manually had a severe hypoglycemia event with AHCL. No patient had diabetic ketoacidosis.
The proportion of insulin given as auto-correction boluses was 36%, which is important as it means that the system was compensating for missed meal doses, a common phenomenon among teenagers, Bergenstal noted.
“There is still room for further improvement in glycemic control in this population of patients with type 1 diabetes, but AHCL represents a significant step forward,” he concluded.
New Zealand study: More data in youth show AHCL benefits
Unlike the US study populations of just teens aged 14 and older, and adults, the study data used for approval in the EU — from New Zealand — included a total of 60 patients with 20 children aged 7-15 years. It, too, was a 10-week randomized crossover clinical trial comparing the AHCL to a sensor-augmented pump system with an algorithm only for predictive low-glucose management (PLGM) and no adjustments for high blood glucose.
Time-in-range was 59% at baseline and 58% with PLGM, compared to 70.4% with AHCL, and most of the time-in-range improvement occurred at night. Time below 70 mg/dL dropped from 3.1% to 2.5% to 2.1%, respectively.
Similar to the US studies, participants spent 96% of the time in closed-loop mode with only 1.2 exits per week. On a questionnaire, 95% of patients agreed that the system was easy to use and 85% that the system improved their quality of life.
De Bock showed a slide with some quotes, including one from a parent saying, “We didn’t have to be fearful at night or have that thought when we opened her bedroom door in the morning that she might not be conscious,” and from a patient, “I forgot I had diabetes today.”
Bailey commented: “Of course these devices are not free. So, the challenge is how do we make them available, less expensive, and easy to use? We have our work cut out for us, but this is heartening data. Everything has gotten better but we’re not out of a job yet.”
Bailey has reported receiving research support from Abbott, Capillary Biomedical, Dexcom, Diasome, Eli Lilly, Kowa, Lexicon, Medtronic, Medtrum, Novo Nordisk, REMD, Sanofi, Senseonics, ViaCyte, vTv Therapeutics, Zealand Pharma, and consulting or speaking honoraria from Abbott, LifeScan, Novo Nordisk, Sanofi, and Medtronic. Bode has reported receiving consulting and speaker fees from Medtronic. Bergenstal has reported participating in clinical research, being an advisory board member, and/or serving as a consultant for Abbott Diabetes Care, Ascensia, CeQure, Dexcom, Eli Lilly, Hygieia, Senseonics, and United Healthcare. De Bock has reported receiving honoraria or expenses from Novo Nordisk, Sanofi, Pfizer, Medtronic, and Lilly, and research funds from Novo Nordisk and Medtronic.
This article first appeared on Medscape.com.
Medtronic’s next-generation automated insulin delivery system offers significant improvements over the currently available 670G hybrid closed-loop, particularly in young people with type 1 diabetes, new data suggest.
Automated insulin delivery systems are comprised of an insulin pump, continuous glucose monitor (CGM), and an automated insulin dosing algorithm.
Data from three trials of such systems using Medtronic’s advanced hybrid closed-loop (AHCL) algorithm (trade name SmartGuard) were presented June 12 during the virtual American Diabetes Association (ADA) 80th Scientific Sessions. The AHCL is the algorithm used in Medtronic’s new MiniMed 780G system, which received a CE Mark on June 11 for the treatment of type 1 diabetes in people aged 7 to 80 years.
One trial, presented by Bruce W. Bode, MD, of Atlanta Diabetes Associates, Georgia, was the US pivotal safety study that will be submitted to the US Food and Drug Administration for approval of the Medtronic 780G.
Another trial, presented by Richard M. Bergenstal, MD, executive director of the International Diabetes Center at Park Nicollet, Minneapolis, Minnesota, was a separate comparison of the AHCL with the 670G. (The AHCL-based system used in the three trials was identical to the 780G except it didn’t include Bluetooth, which will be a feature of the final product.)
A third trial, presented by Martin de Bock, PhD, of the University of Otago, New Zealand, included the CE Mark dataset for the 780G.
In contrast to the 670G, the 780G adds automated correction boluses for high blood glucose levels (rather than simply adjusting the basal infusion) and allows for adjustment of target glucose levels down to 100 mg/dL rather than a minimum of 120 mg/dL.
Taken together, the data from the three trials showed that the AHCL-based system improved glycemic time-in-range with no increased risk for hypoglycemia, including in children and teenagers, with high patient-reported satisfaction. And specifically compared to the 670G, the AHCL-based system reverts to open-loop far less often because it only exits closed-loop mode when the sensor stops working or during sensor changes, but not during hyperglycemia even above 300 mg/dL.
Asked to comment, session moderator Timothy S. Bailey, MD, president and CEO of the AMCR Institute, Escondido, California, told Medscape Medical News: “Automated insulin delivery systems are getting better and better.”
“None of these devices is perfect, but they are a substantial improvement over what we’ve had ... They all take people from where they are now to better time-in-range, less time with hypoglycemia, and most important, they might make the quality of their lives better. That’s really underappreciated.”
One factor that has allowed for the improvements, Bailey said, is the recognition by regulatory bodies that the hybrid closed-loop devices are generally safer than current open-loop type 1 diabetes management so that fewer “safety” device features that interfere with tight glycemic control are necessary.
With first-generation closed-loop systems, “If a wide variety of conditions occur, users get kicked off [hybrid closed-loop mode]. Originally it was perceived by the regulatory agencies as a safety feature because they perceived the standard of care as safe. The new system was allowed to have fewer rules.”
Pivotal trial: Time-in-range improved, 96% say system easy to use
The goal of the AHCL system is to maximize the time-in-range of blood glucose between 70-180 mg/dL. Automated basal delivery of insulin is programmed to a set-point of 100 or 120 mg/dL, with dosing every 5 minutes.
The US pivotal trial was a single-arm, 16-center, in-home trial of 157 people with type 1 diabetes, including 39 adolescents aged 14-21 years and 118 adults aged 22-75 years. All had type 1 diabetes for at least 2 years, A1c levels below 10%, and had been using insulin pumps for at least 6 months, with or without CGMs.
After a 14-day run-in, they wore the systems with a 100 or 120 mg/dL set-point for 45 days, then switched to the other setpoint for another 45 days. Average A1c dropped from 7.5% to 7.0%, with the proportions having an A1c ≤ 7.0% increasing from 34% to 61%.
Overall time-in-range was 75% compared to 69% at baseline, with time below range (< 70 mg/dL) of 1.8%. Overnight time-in-range was 82%, with 1.5% below range. Time-in-range increased from 62% to 73% in the adolescents and from 71% to 75% in the adults.
There were no incidences of severe hypoglycemia or diabetic ketoacidosis, and no device-related serious adverse events.
Participants reported being in hybrid closed-loop, or auto-mode, 95% of the time, compared with 33% for those who had been previously using the 670G.
The number of AHCL exits was 1.3 per week, significantly less than with the 670G. Of those, 29% were user-initiated while the rest were implemented by the device, most often when the sensor wasn’t working.
In a study questionnaire, 96% reported that the system was easy to use.
AHCL vs 670G: Major improvements seen
Bergenstal presented data from the Fuzzy Logic Automated Insulin Regulation (FLAIR) study, funded by the National Institute of Diabetes and Digestive and Kidney Disease, comparing Medtronic’s AHCL-based system with the currently marketed 670G hybrid closed-loop, in 113 individuals with type 1 diabetes aged 14-29 years.
“This age group has traditionally been the most difficult group in which to optimize glucose management,” Bergenstal said.
FLAIR is believed to be the first-ever study comparing an investigational automated insulin delivery system with a commercially approved system, he noted. All participants used each automated insulin delivery system for 3 months in the randomized crossover trial.
The primary outcome, time spent above 180 mg/dL during the day combined with time below 54 mg/dL over 24 hours at baseline with the 670G and AHCL went from 42% to 37% to 34%, respectively, for the former and from 0.46% to 0.50% to 0.45%, respectively, for the latter.
The percentage time-in-range over 24 hours went from 57% at baseline to 67% with the AHCL versus 63% with the 670G. A1c levels dropped from 7.9% at baseline to 7.6% with the 670G and 7.4% with AHCL.
“Remember, these are the adolescents who are the toughest of the tough, yet there was a 10% increase in time-in-range ... this is very clinically significant,” Bergenstal said.
Even among 14 patients who had been using multiple daily injections without CGM prior to the study, a group often excluded from closed-loop studies, time-in-range improved from 45% at baseline to 63% with the 670G to 65% with AHCL.
“I’m making a plea not to exclude people just because they haven’t previously used technology,” Bergenstal said.
One patient who had dosed with extra insulin manually had a severe hypoglycemia event with AHCL. No patient had diabetic ketoacidosis.
The proportion of insulin given as auto-correction boluses was 36%, which is important as it means that the system was compensating for missed meal doses, a common phenomenon among teenagers, Bergenstal noted.
“There is still room for further improvement in glycemic control in this population of patients with type 1 diabetes, but AHCL represents a significant step forward,” he concluded.
New Zealand study: More data in youth show AHCL benefits
Unlike the US study populations of just teens aged 14 and older, and adults, the study data used for approval in the EU — from New Zealand — included a total of 60 patients with 20 children aged 7-15 years. It, too, was a 10-week randomized crossover clinical trial comparing the AHCL to a sensor-augmented pump system with an algorithm only for predictive low-glucose management (PLGM) and no adjustments for high blood glucose.
Time-in-range was 59% at baseline and 58% with PLGM, compared to 70.4% with AHCL, and most of the time-in-range improvement occurred at night. Time below 70 mg/dL dropped from 3.1% to 2.5% to 2.1%, respectively.
Similar to the US studies, participants spent 96% of the time in closed-loop mode with only 1.2 exits per week. On a questionnaire, 95% of patients agreed that the system was easy to use and 85% that the system improved their quality of life.
De Bock showed a slide with some quotes, including one from a parent saying, “We didn’t have to be fearful at night or have that thought when we opened her bedroom door in the morning that she might not be conscious,” and from a patient, “I forgot I had diabetes today.”
Bailey commented: “Of course these devices are not free. So, the challenge is how do we make them available, less expensive, and easy to use? We have our work cut out for us, but this is heartening data. Everything has gotten better but we’re not out of a job yet.”
Bailey has reported receiving research support from Abbott, Capillary Biomedical, Dexcom, Diasome, Eli Lilly, Kowa, Lexicon, Medtronic, Medtrum, Novo Nordisk, REMD, Sanofi, Senseonics, ViaCyte, vTv Therapeutics, Zealand Pharma, and consulting or speaking honoraria from Abbott, LifeScan, Novo Nordisk, Sanofi, and Medtronic. Bode has reported receiving consulting and speaker fees from Medtronic. Bergenstal has reported participating in clinical research, being an advisory board member, and/or serving as a consultant for Abbott Diabetes Care, Ascensia, CeQure, Dexcom, Eli Lilly, Hygieia, Senseonics, and United Healthcare. De Bock has reported receiving honoraria or expenses from Novo Nordisk, Sanofi, Pfizer, Medtronic, and Lilly, and research funds from Novo Nordisk and Medtronic.
This article first appeared on Medscape.com.
Medtronic’s next-generation automated insulin delivery system offers significant improvements over the currently available 670G hybrid closed-loop, particularly in young people with type 1 diabetes, new data suggest.
Automated insulin delivery systems are comprised of an insulin pump, continuous glucose monitor (CGM), and an automated insulin dosing algorithm.
Data from three trials of such systems using Medtronic’s advanced hybrid closed-loop (AHCL) algorithm (trade name SmartGuard) were presented June 12 during the virtual American Diabetes Association (ADA) 80th Scientific Sessions. The AHCL is the algorithm used in Medtronic’s new MiniMed 780G system, which received a CE Mark on June 11 for the treatment of type 1 diabetes in people aged 7 to 80 years.
One trial, presented by Bruce W. Bode, MD, of Atlanta Diabetes Associates, Georgia, was the US pivotal safety study that will be submitted to the US Food and Drug Administration for approval of the Medtronic 780G.
Another trial, presented by Richard M. Bergenstal, MD, executive director of the International Diabetes Center at Park Nicollet, Minneapolis, Minnesota, was a separate comparison of the AHCL with the 670G. (The AHCL-based system used in the three trials was identical to the 780G except it didn’t include Bluetooth, which will be a feature of the final product.)
A third trial, presented by Martin de Bock, PhD, of the University of Otago, New Zealand, included the CE Mark dataset for the 780G.
In contrast to the 670G, the 780G adds automated correction boluses for high blood glucose levels (rather than simply adjusting the basal infusion) and allows for adjustment of target glucose levels down to 100 mg/dL rather than a minimum of 120 mg/dL.
Taken together, the data from the three trials showed that the AHCL-based system improved glycemic time-in-range with no increased risk for hypoglycemia, including in children and teenagers, with high patient-reported satisfaction. And specifically compared to the 670G, the AHCL-based system reverts to open-loop far less often because it only exits closed-loop mode when the sensor stops working or during sensor changes, but not during hyperglycemia even above 300 mg/dL.
Asked to comment, session moderator Timothy S. Bailey, MD, president and CEO of the AMCR Institute, Escondido, California, told Medscape Medical News: “Automated insulin delivery systems are getting better and better.”
“None of these devices is perfect, but they are a substantial improvement over what we’ve had ... They all take people from where they are now to better time-in-range, less time with hypoglycemia, and most important, they might make the quality of their lives better. That’s really underappreciated.”
One factor that has allowed for the improvements, Bailey said, is the recognition by regulatory bodies that the hybrid closed-loop devices are generally safer than current open-loop type 1 diabetes management so that fewer “safety” device features that interfere with tight glycemic control are necessary.
With first-generation closed-loop systems, “If a wide variety of conditions occur, users get kicked off [hybrid closed-loop mode]. Originally it was perceived by the regulatory agencies as a safety feature because they perceived the standard of care as safe. The new system was allowed to have fewer rules.”
Pivotal trial: Time-in-range improved, 96% say system easy to use
The goal of the AHCL system is to maximize the time-in-range of blood glucose between 70-180 mg/dL. Automated basal delivery of insulin is programmed to a set-point of 100 or 120 mg/dL, with dosing every 5 minutes.
The US pivotal trial was a single-arm, 16-center, in-home trial of 157 people with type 1 diabetes, including 39 adolescents aged 14-21 years and 118 adults aged 22-75 years. All had type 1 diabetes for at least 2 years, A1c levels below 10%, and had been using insulin pumps for at least 6 months, with or without CGMs.
After a 14-day run-in, they wore the systems with a 100 or 120 mg/dL set-point for 45 days, then switched to the other setpoint for another 45 days. Average A1c dropped from 7.5% to 7.0%, with the proportions having an A1c ≤ 7.0% increasing from 34% to 61%.
Overall time-in-range was 75% compared to 69% at baseline, with time below range (< 70 mg/dL) of 1.8%. Overnight time-in-range was 82%, with 1.5% below range. Time-in-range increased from 62% to 73% in the adolescents and from 71% to 75% in the adults.
There were no incidences of severe hypoglycemia or diabetic ketoacidosis, and no device-related serious adverse events.
Participants reported being in hybrid closed-loop, or auto-mode, 95% of the time, compared with 33% for those who had been previously using the 670G.
The number of AHCL exits was 1.3 per week, significantly less than with the 670G. Of those, 29% were user-initiated while the rest were implemented by the device, most often when the sensor wasn’t working.
In a study questionnaire, 96% reported that the system was easy to use.
AHCL vs 670G: Major improvements seen
Bergenstal presented data from the Fuzzy Logic Automated Insulin Regulation (FLAIR) study, funded by the National Institute of Diabetes and Digestive and Kidney Disease, comparing Medtronic’s AHCL-based system with the currently marketed 670G hybrid closed-loop, in 113 individuals with type 1 diabetes aged 14-29 years.
“This age group has traditionally been the most difficult group in which to optimize glucose management,” Bergenstal said.
FLAIR is believed to be the first-ever study comparing an investigational automated insulin delivery system with a commercially approved system, he noted. All participants used each automated insulin delivery system for 3 months in the randomized crossover trial.
The primary outcome, time spent above 180 mg/dL during the day combined with time below 54 mg/dL over 24 hours at baseline with the 670G and AHCL went from 42% to 37% to 34%, respectively, for the former and from 0.46% to 0.50% to 0.45%, respectively, for the latter.
The percentage time-in-range over 24 hours went from 57% at baseline to 67% with the AHCL versus 63% with the 670G. A1c levels dropped from 7.9% at baseline to 7.6% with the 670G and 7.4% with AHCL.
“Remember, these are the adolescents who are the toughest of the tough, yet there was a 10% increase in time-in-range ... this is very clinically significant,” Bergenstal said.
Even among 14 patients who had been using multiple daily injections without CGM prior to the study, a group often excluded from closed-loop studies, time-in-range improved from 45% at baseline to 63% with the 670G to 65% with AHCL.
“I’m making a plea not to exclude people just because they haven’t previously used technology,” Bergenstal said.
One patient who had dosed with extra insulin manually had a severe hypoglycemia event with AHCL. No patient had diabetic ketoacidosis.
The proportion of insulin given as auto-correction boluses was 36%, which is important as it means that the system was compensating for missed meal doses, a common phenomenon among teenagers, Bergenstal noted.
“There is still room for further improvement in glycemic control in this population of patients with type 1 diabetes, but AHCL represents a significant step forward,” he concluded.
New Zealand study: More data in youth show AHCL benefits
Unlike the US study populations of just teens aged 14 and older, and adults, the study data used for approval in the EU — from New Zealand — included a total of 60 patients with 20 children aged 7-15 years. It, too, was a 10-week randomized crossover clinical trial comparing the AHCL to a sensor-augmented pump system with an algorithm only for predictive low-glucose management (PLGM) and no adjustments for high blood glucose.
Time-in-range was 59% at baseline and 58% with PLGM, compared to 70.4% with AHCL, and most of the time-in-range improvement occurred at night. Time below 70 mg/dL dropped from 3.1% to 2.5% to 2.1%, respectively.
Similar to the US studies, participants spent 96% of the time in closed-loop mode with only 1.2 exits per week. On a questionnaire, 95% of patients agreed that the system was easy to use and 85% that the system improved their quality of life.
De Bock showed a slide with some quotes, including one from a parent saying, “We didn’t have to be fearful at night or have that thought when we opened her bedroom door in the morning that she might not be conscious,” and from a patient, “I forgot I had diabetes today.”
Bailey commented: “Of course these devices are not free. So, the challenge is how do we make them available, less expensive, and easy to use? We have our work cut out for us, but this is heartening data. Everything has gotten better but we’re not out of a job yet.”
Bailey has reported receiving research support from Abbott, Capillary Biomedical, Dexcom, Diasome, Eli Lilly, Kowa, Lexicon, Medtronic, Medtrum, Novo Nordisk, REMD, Sanofi, Senseonics, ViaCyte, vTv Therapeutics, Zealand Pharma, and consulting or speaking honoraria from Abbott, LifeScan, Novo Nordisk, Sanofi, and Medtronic. Bode has reported receiving consulting and speaker fees from Medtronic. Bergenstal has reported participating in clinical research, being an advisory board member, and/or serving as a consultant for Abbott Diabetes Care, Ascensia, CeQure, Dexcom, Eli Lilly, Hygieia, Senseonics, and United Healthcare. De Bock has reported receiving honoraria or expenses from Novo Nordisk, Sanofi, Pfizer, Medtronic, and Lilly, and research funds from Novo Nordisk and Medtronic.
This article first appeared on Medscape.com.
FROM ADA 2020
Ventricular tachycardia storm responds to magnetic stimulation
In a pilot study of five patients with ventricular tachycardia (VT) storm that was refractory to antiarrhythmic drug therapy, treatment with noninvasive transcutaneous magnetic stimulation (TCMS) was associated with a lower arrhythmia burden.
The five patients were men aged 40 to 68 years with VT storm, defined as at least three episodes of sustained VT in the preceding 24 hours. The patients experienced a drop in both sustained and nonsustained VT with TCMS.
The study “aimed at developing a novel system for noninvasively and nondestructively interrupting the sympathetic tone,” corresponding author Timothy M. Markman, MD, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania, told theheart.org | Medscape Cardiology. “We demonstrated that the technique was safe and that there was a strong signal of efficacy,” he added.
“We know that interrupting the sympathetic tone in these patients is beneficial,” said Markman, “but our strategies for doing so are mostly invasive and associated with a significant risk profile.”
The research letter was published online May 5 in the Journal of the American Medical Association. It was also presented during the virtual Heart Rhythm Society 2020 conference.
Growing body of evidence
Numerous studies have linked autonomic neuromodulation, including local blockade of the left stellate ganglion, with a reduction of cardiac sympathetic input in patients with VT storm, the authors write.
“This adds to a growing body of literature that autonomic neuromodulation is a valuable tool in the management of arrhythmias,” said Markman.
The use of magnetic stimulation to treat arrhythmias by targeting cardiac sympathetic innervation has been demonstrated in animal studies. The authors note that, to their knowledge, this is the first study involving humans.
Evidence suggests that TCMS may serve as a bridge for patients with difficult-to- treat VT to reduce VT and eliminate antiarrhythmic drug therapies and the associated risks, the authors say.
A lower VT burden
Five participants were included in the study. The patients were followed from March 2019 to June 2019. All had experienced at least three episodes of sustained VT (>30 sec) in the 24 hours preceding treatment. Patients with implantable cardiac devices were excluded.
The investigators used a figure 8 TCMS coil that was attached to a magnetic stimulation system positioned lateral to the C7 spinous process in approximation of the left stellate ganglion. TCMS was delivered at 80% of the left trapezius motor threshold at a frequency of 0.9 Hz for 60 minutes, the authors write. For one patient (patient no. 4), TCMS was shut off after 17 minutes, owing to the coil’s overheating. That resulted in the patient’s not being able to complete the protocol, they note.
Patients were monitored during and immediately after treatment for adverse events, including hemodynamic compromise, local discomfort, and skin irritation.
Results showed that compared to the 24-hour baseline period, sustained VT was reduced from 99 to five episodes, and nonsustained VT was reduced from 150 to 58 episodes in the 48 hours following TCMS.
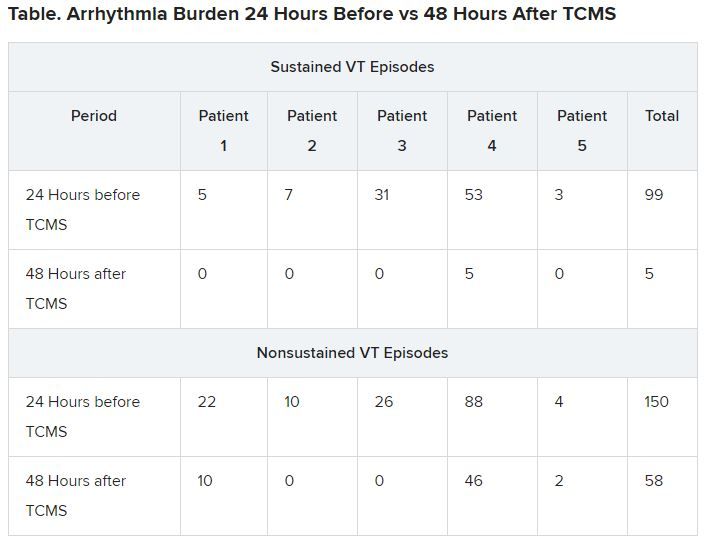
In addition, 41 total external shocks were performed at the 24-hour baseline before TCMS. No external shocks were performed 48 hours after TCMS treatment.
Of the three patients who were not under sedation, none reported discomfort from TCMS.
Before TCMS treatment began, VT was refractory to a mean (SD) of 2.5 (2.1) antiarrhythmic drugs per patient. Within the 48-hour follow-up, patients received a mean of 1.2 (0.7) antiarrhythmic drugs. No additional antiarrhythmic drug was added, the authors note. Only patient no. 4, who did not complete the protocol, underwent ablation 36 hours post enrollment, they add.
The authors note some limitations, such as small case number. Markman told theheart.org | Medscape Cardiology that enrollment of patients in a randomized, sham-controlled trial to demonstrate efficacy is underway.
Physiology studies to evaluate the effects of this therapy while optimizing the technical aspects of the delivery of transcutaneous magnetic stimulation are also being conducted, he adds. Other limitations include the absence of control measures and exclusion of patients with implantable cardiac devices.
A potential addition to treatment
Gordon F. Tomaselli, MD, past president of the American Heart Association and current dean of the Albert Einstein College of Medicine, New York City, who was not involved in the research, told theheart.org | Medscape Cardiology that “the results are kind of interesting; it actually changes the function in the ganglion in the neck that actually innervates the heart, excites the heart, if you will.
“Clearly it wasn’t something that was just happening while this therapy was applied, but instead there’s some changes made when the sympathetic ganglion is targeted,” Tomaselli said. “They’re changing it functionally somehow, reducing the stimulating input to the heart, and in doing so, reducing the frequency of arrhythmias.”
Tomaselli suggested TCMS might be helpful in choosing among alternative treatments, such as sympathetic denervation. “It might also be a way to decide whether or not somebody might benefit, for example, from permanent dissection,” he said. “If you do this therapy, if it quiets things down but then it comes back after a while, you may consider denervation of that ganglion.”
Tomaselli adds that this treatment might be applied in different ways. “In some future iteration, it could even be implantable, could be patient activated or automatically activated ― for example, if a rapid heart rate is detected, that kind of thing.”
He noted that “there may be applications of this ultra-low frequency to other arrythmias, more common arrythmias, less life-threatening arrythmias, like atrial fibrillation; so there are a number of ways you might consider using this to treat cardiac rhythm disturbances by targeting the nervous system.”
Nazarian has consulted for Siemens, CardioSolv, and Circle Software and is a principle investigator for research funding to the University of Pennsylvania from Biosense-Webster, Siemens, ImriCor, and the National Institutes of Health. No other relevant financial relationships have been disclosed.
This story first appeared on Medscape.com.
In a pilot study of five patients with ventricular tachycardia (VT) storm that was refractory to antiarrhythmic drug therapy, treatment with noninvasive transcutaneous magnetic stimulation (TCMS) was associated with a lower arrhythmia burden.
The five patients were men aged 40 to 68 years with VT storm, defined as at least three episodes of sustained VT in the preceding 24 hours. The patients experienced a drop in both sustained and nonsustained VT with TCMS.
The study “aimed at developing a novel system for noninvasively and nondestructively interrupting the sympathetic tone,” corresponding author Timothy M. Markman, MD, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania, told theheart.org | Medscape Cardiology. “We demonstrated that the technique was safe and that there was a strong signal of efficacy,” he added.
“We know that interrupting the sympathetic tone in these patients is beneficial,” said Markman, “but our strategies for doing so are mostly invasive and associated with a significant risk profile.”
The research letter was published online May 5 in the Journal of the American Medical Association. It was also presented during the virtual Heart Rhythm Society 2020 conference.
Growing body of evidence
Numerous studies have linked autonomic neuromodulation, including local blockade of the left stellate ganglion, with a reduction of cardiac sympathetic input in patients with VT storm, the authors write.
“This adds to a growing body of literature that autonomic neuromodulation is a valuable tool in the management of arrhythmias,” said Markman.
The use of magnetic stimulation to treat arrhythmias by targeting cardiac sympathetic innervation has been demonstrated in animal studies. The authors note that, to their knowledge, this is the first study involving humans.
Evidence suggests that TCMS may serve as a bridge for patients with difficult-to- treat VT to reduce VT and eliminate antiarrhythmic drug therapies and the associated risks, the authors say.
A lower VT burden
Five participants were included in the study. The patients were followed from March 2019 to June 2019. All had experienced at least three episodes of sustained VT (>30 sec) in the 24 hours preceding treatment. Patients with implantable cardiac devices were excluded.
The investigators used a figure 8 TCMS coil that was attached to a magnetic stimulation system positioned lateral to the C7 spinous process in approximation of the left stellate ganglion. TCMS was delivered at 80% of the left trapezius motor threshold at a frequency of 0.9 Hz for 60 minutes, the authors write. For one patient (patient no. 4), TCMS was shut off after 17 minutes, owing to the coil’s overheating. That resulted in the patient’s not being able to complete the protocol, they note.
Patients were monitored during and immediately after treatment for adverse events, including hemodynamic compromise, local discomfort, and skin irritation.
Results showed that compared to the 24-hour baseline period, sustained VT was reduced from 99 to five episodes, and nonsustained VT was reduced from 150 to 58 episodes in the 48 hours following TCMS.

In addition, 41 total external shocks were performed at the 24-hour baseline before TCMS. No external shocks were performed 48 hours after TCMS treatment.
Of the three patients who were not under sedation, none reported discomfort from TCMS.
Before TCMS treatment began, VT was refractory to a mean (SD) of 2.5 (2.1) antiarrhythmic drugs per patient. Within the 48-hour follow-up, patients received a mean of 1.2 (0.7) antiarrhythmic drugs. No additional antiarrhythmic drug was added, the authors note. Only patient no. 4, who did not complete the protocol, underwent ablation 36 hours post enrollment, they add.
The authors note some limitations, such as small case number. Markman told theheart.org | Medscape Cardiology that enrollment of patients in a randomized, sham-controlled trial to demonstrate efficacy is underway.
Physiology studies to evaluate the effects of this therapy while optimizing the technical aspects of the delivery of transcutaneous magnetic stimulation are also being conducted, he adds. Other limitations include the absence of control measures and exclusion of patients with implantable cardiac devices.
A potential addition to treatment
Gordon F. Tomaselli, MD, past president of the American Heart Association and current dean of the Albert Einstein College of Medicine, New York City, who was not involved in the research, told theheart.org | Medscape Cardiology that “the results are kind of interesting; it actually changes the function in the ganglion in the neck that actually innervates the heart, excites the heart, if you will.
“Clearly it wasn’t something that was just happening while this therapy was applied, but instead there’s some changes made when the sympathetic ganglion is targeted,” Tomaselli said. “They’re changing it functionally somehow, reducing the stimulating input to the heart, and in doing so, reducing the frequency of arrhythmias.”
Tomaselli suggested TCMS might be helpful in choosing among alternative treatments, such as sympathetic denervation. “It might also be a way to decide whether or not somebody might benefit, for example, from permanent dissection,” he said. “If you do this therapy, if it quiets things down but then it comes back after a while, you may consider denervation of that ganglion.”
Tomaselli adds that this treatment might be applied in different ways. “In some future iteration, it could even be implantable, could be patient activated or automatically activated ― for example, if a rapid heart rate is detected, that kind of thing.”
He noted that “there may be applications of this ultra-low frequency to other arrythmias, more common arrythmias, less life-threatening arrythmias, like atrial fibrillation; so there are a number of ways you might consider using this to treat cardiac rhythm disturbances by targeting the nervous system.”
Nazarian has consulted for Siemens, CardioSolv, and Circle Software and is a principle investigator for research funding to the University of Pennsylvania from Biosense-Webster, Siemens, ImriCor, and the National Institutes of Health. No other relevant financial relationships have been disclosed.
This story first appeared on Medscape.com.
In a pilot study of five patients with ventricular tachycardia (VT) storm that was refractory to antiarrhythmic drug therapy, treatment with noninvasive transcutaneous magnetic stimulation (TCMS) was associated with a lower arrhythmia burden.
The five patients were men aged 40 to 68 years with VT storm, defined as at least three episodes of sustained VT in the preceding 24 hours. The patients experienced a drop in both sustained and nonsustained VT with TCMS.
The study “aimed at developing a novel system for noninvasively and nondestructively interrupting the sympathetic tone,” corresponding author Timothy M. Markman, MD, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania, told theheart.org | Medscape Cardiology. “We demonstrated that the technique was safe and that there was a strong signal of efficacy,” he added.
“We know that interrupting the sympathetic tone in these patients is beneficial,” said Markman, “but our strategies for doing so are mostly invasive and associated with a significant risk profile.”
The research letter was published online May 5 in the Journal of the American Medical Association. It was also presented during the virtual Heart Rhythm Society 2020 conference.
Growing body of evidence
Numerous studies have linked autonomic neuromodulation, including local blockade of the left stellate ganglion, with a reduction of cardiac sympathetic input in patients with VT storm, the authors write.
“This adds to a growing body of literature that autonomic neuromodulation is a valuable tool in the management of arrhythmias,” said Markman.
The use of magnetic stimulation to treat arrhythmias by targeting cardiac sympathetic innervation has been demonstrated in animal studies. The authors note that, to their knowledge, this is the first study involving humans.
Evidence suggests that TCMS may serve as a bridge for patients with difficult-to- treat VT to reduce VT and eliminate antiarrhythmic drug therapies and the associated risks, the authors say.
A lower VT burden
Five participants were included in the study. The patients were followed from March 2019 to June 2019. All had experienced at least three episodes of sustained VT (>30 sec) in the 24 hours preceding treatment. Patients with implantable cardiac devices were excluded.
The investigators used a figure 8 TCMS coil that was attached to a magnetic stimulation system positioned lateral to the C7 spinous process in approximation of the left stellate ganglion. TCMS was delivered at 80% of the left trapezius motor threshold at a frequency of 0.9 Hz for 60 minutes, the authors write. For one patient (patient no. 4), TCMS was shut off after 17 minutes, owing to the coil’s overheating. That resulted in the patient’s not being able to complete the protocol, they note.
Patients were monitored during and immediately after treatment for adverse events, including hemodynamic compromise, local discomfort, and skin irritation.
Results showed that compared to the 24-hour baseline period, sustained VT was reduced from 99 to five episodes, and nonsustained VT was reduced from 150 to 58 episodes in the 48 hours following TCMS.

In addition, 41 total external shocks were performed at the 24-hour baseline before TCMS. No external shocks were performed 48 hours after TCMS treatment.
Of the three patients who were not under sedation, none reported discomfort from TCMS.
Before TCMS treatment began, VT was refractory to a mean (SD) of 2.5 (2.1) antiarrhythmic drugs per patient. Within the 48-hour follow-up, patients received a mean of 1.2 (0.7) antiarrhythmic drugs. No additional antiarrhythmic drug was added, the authors note. Only patient no. 4, who did not complete the protocol, underwent ablation 36 hours post enrollment, they add.
The authors note some limitations, such as small case number. Markman told theheart.org | Medscape Cardiology that enrollment of patients in a randomized, sham-controlled trial to demonstrate efficacy is underway.
Physiology studies to evaluate the effects of this therapy while optimizing the technical aspects of the delivery of transcutaneous magnetic stimulation are also being conducted, he adds. Other limitations include the absence of control measures and exclusion of patients with implantable cardiac devices.
A potential addition to treatment
Gordon F. Tomaselli, MD, past president of the American Heart Association and current dean of the Albert Einstein College of Medicine, New York City, who was not involved in the research, told theheart.org | Medscape Cardiology that “the results are kind of interesting; it actually changes the function in the ganglion in the neck that actually innervates the heart, excites the heart, if you will.
“Clearly it wasn’t something that was just happening while this therapy was applied, but instead there’s some changes made when the sympathetic ganglion is targeted,” Tomaselli said. “They’re changing it functionally somehow, reducing the stimulating input to the heart, and in doing so, reducing the frequency of arrhythmias.”
Tomaselli suggested TCMS might be helpful in choosing among alternative treatments, such as sympathetic denervation. “It might also be a way to decide whether or not somebody might benefit, for example, from permanent dissection,” he said. “If you do this therapy, if it quiets things down but then it comes back after a while, you may consider denervation of that ganglion.”
Tomaselli adds that this treatment might be applied in different ways. “In some future iteration, it could even be implantable, could be patient activated or automatically activated ― for example, if a rapid heart rate is detected, that kind of thing.”
He noted that “there may be applications of this ultra-low frequency to other arrythmias, more common arrythmias, less life-threatening arrythmias, like atrial fibrillation; so there are a number of ways you might consider using this to treat cardiac rhythm disturbances by targeting the nervous system.”
Nazarian has consulted for Siemens, CardioSolv, and Circle Software and is a principle investigator for research funding to the University of Pennsylvania from Biosense-Webster, Siemens, ImriCor, and the National Institutes of Health. No other relevant financial relationships have been disclosed.
This story first appeared on Medscape.com.
Daily Recap: Stressed out primary care docs, ‘hospital at home’ for cancer patients
Here are the stories our MDedge editors across specialties think you need to know about today:
Racism, COVID-19 lead to sky-high stress levels
Primary care clinicians, already experiencing all-time high stress levels related to COVID-19, are now struggling to cope with the fallout from racism and the death of George Floyd, according to a survey conducted June 5-8.
When asked how the situation has affected their practices, 12% of the survey’s 586 respondents “drew clear connections between the current racial unrest and the health of their patients,” the Larry A. Green Center said in a recent statement. One-third of the clinicians also said that recent racism-related events have had a negative effect on their own well-being.
In a related survey of 1,111 patients conducted June 8 about 65% of patients said that racism affected emotional, psychological, and behavioral health.
“The fact that so many patients and clinicians agree that racism is a driver of health points to the incredible role primary care plays in creating safe spaces to process deep societal and personal issues,” said Christine Bechtel, cofounder of 3rd Conversation, a community of patients and providers. Read more.
Medical teams take to the streets
They stanched bleeding wounds and plucked disoriented teenagers from clouds of gas, entering dangerous corners where on-duty emergency health responders may fear to go. Many are medical professionals who see parallels between the front lines of COVID-19, where they confront stark racial imbalances among those stricken by the coronavirus, and what they see as racialized police brutality.
Iris Butler, a 21-year-old certified nursing assistant who works in a nursing home, decided to offer her skills after seeing a man injured by a rubber bullet on her first night at the Denver protests. She showed up as a medic every night thereafter. “I am working full time and basically being at the protest after getting straight off of work,” said Ms. Butler, who is black. That’s tiring, she added, but so is being a black woman in America. Read more.
At-home management of type 1 diabetes, COVID-19
Although hyperglycemia and diabetic ketoacidosis are common in people with type 1 diabetes who develop COVID-19, many are still able to manage the illness at home and overall mortality is relatively low.
These new findings are still preliminary and were published online June 5 in Diabetes Care by Osagie A. Ebekozien, MD, vice president, quality improvement and population health at the T1D Exchange, and colleagues.
The published study includes data as of May 5 on 64 individuals from a total of 64 US sites. Since the paper was submitted, there are now 220 patients from 68 sites. There were two deaths in the preliminary report, Dr. Ebekozien said. There have been a few more deaths in the larger dataset, but the mortality rate remains relatively low.
Overall, 34.9% of patients were able to manage COVID-19 entirely at home. At the other extreme, 22.2% of patients overall were admitted to the intensive care unit. Including the small proportion of patients sent home after being seen in emergency or urgent care, overall roughly half were not admitted to the hospital. “Even in this preliminary study, half were managed at home via telemedicine with an endocrinologist and infectious disease specialist ... I think it continues to be a case-by-case clinical decision between the patient and their provider,” Dr. Ebekozien said. Read more.
‘Hospital at home’ for cancer patients
Visits to the emergency department (ED) and hospitalizations are often frequent occurrences for cancer patients, but what if the “hospital” could be brought into the home instead?
A new American cohort study provides evidence that this can be a workable option for cancer patients. The authors report improved patient outcomes, with 56% lower odds of unplanned hospitalizations, 45% lower odds of ED visits, and 50% lower cumulative charges, as compared with patients who received usual care.
“The oncology hospital-at-home model of care that extends acute-level care to the patient at home offers promise in addressing a long-term gap in cancer care service delivery,” said lead author Kathi Mooney, PhD, RN, distinguished professor of nursing at the University of Utah, Salt Lake City. “In light of the current global pandemic, we are compelled to consider new ways to provide cancer care, and the oncology hospital-at-home model is on point to address critical elements of an improved cancer care delivery system.”
Dr. Mooney presented the findings during the virtual scientific program of the American Society of Clinical Oncology 2020 annual meeting (abstract 7000). Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Racism, COVID-19 lead to sky-high stress levels
Primary care clinicians, already experiencing all-time high stress levels related to COVID-19, are now struggling to cope with the fallout from racism and the death of George Floyd, according to a survey conducted June 5-8.
When asked how the situation has affected their practices, 12% of the survey’s 586 respondents “drew clear connections between the current racial unrest and the health of their patients,” the Larry A. Green Center said in a recent statement. One-third of the clinicians also said that recent racism-related events have had a negative effect on their own well-being.
In a related survey of 1,111 patients conducted June 8 about 65% of patients said that racism affected emotional, psychological, and behavioral health.
“The fact that so many patients and clinicians agree that racism is a driver of health points to the incredible role primary care plays in creating safe spaces to process deep societal and personal issues,” said Christine Bechtel, cofounder of 3rd Conversation, a community of patients and providers. Read more.
Medical teams take to the streets
They stanched bleeding wounds and plucked disoriented teenagers from clouds of gas, entering dangerous corners where on-duty emergency health responders may fear to go. Many are medical professionals who see parallels between the front lines of COVID-19, where they confront stark racial imbalances among those stricken by the coronavirus, and what they see as racialized police brutality.
Iris Butler, a 21-year-old certified nursing assistant who works in a nursing home, decided to offer her skills after seeing a man injured by a rubber bullet on her first night at the Denver protests. She showed up as a medic every night thereafter. “I am working full time and basically being at the protest after getting straight off of work,” said Ms. Butler, who is black. That’s tiring, she added, but so is being a black woman in America. Read more.
At-home management of type 1 diabetes, COVID-19
Although hyperglycemia and diabetic ketoacidosis are common in people with type 1 diabetes who develop COVID-19, many are still able to manage the illness at home and overall mortality is relatively low.
These new findings are still preliminary and were published online June 5 in Diabetes Care by Osagie A. Ebekozien, MD, vice president, quality improvement and population health at the T1D Exchange, and colleagues.
The published study includes data as of May 5 on 64 individuals from a total of 64 US sites. Since the paper was submitted, there are now 220 patients from 68 sites. There were two deaths in the preliminary report, Dr. Ebekozien said. There have been a few more deaths in the larger dataset, but the mortality rate remains relatively low.
Overall, 34.9% of patients were able to manage COVID-19 entirely at home. At the other extreme, 22.2% of patients overall were admitted to the intensive care unit. Including the small proportion of patients sent home after being seen in emergency or urgent care, overall roughly half were not admitted to the hospital. “Even in this preliminary study, half were managed at home via telemedicine with an endocrinologist and infectious disease specialist ... I think it continues to be a case-by-case clinical decision between the patient and their provider,” Dr. Ebekozien said. Read more.
‘Hospital at home’ for cancer patients
Visits to the emergency department (ED) and hospitalizations are often frequent occurrences for cancer patients, but what if the “hospital” could be brought into the home instead?
A new American cohort study provides evidence that this can be a workable option for cancer patients. The authors report improved patient outcomes, with 56% lower odds of unplanned hospitalizations, 45% lower odds of ED visits, and 50% lower cumulative charges, as compared with patients who received usual care.
“The oncology hospital-at-home model of care that extends acute-level care to the patient at home offers promise in addressing a long-term gap in cancer care service delivery,” said lead author Kathi Mooney, PhD, RN, distinguished professor of nursing at the University of Utah, Salt Lake City. “In light of the current global pandemic, we are compelled to consider new ways to provide cancer care, and the oncology hospital-at-home model is on point to address critical elements of an improved cancer care delivery system.”
Dr. Mooney presented the findings during the virtual scientific program of the American Society of Clinical Oncology 2020 annual meeting (abstract 7000). Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Racism, COVID-19 lead to sky-high stress levels
Primary care clinicians, already experiencing all-time high stress levels related to COVID-19, are now struggling to cope with the fallout from racism and the death of George Floyd, according to a survey conducted June 5-8.
When asked how the situation has affected their practices, 12% of the survey’s 586 respondents “drew clear connections between the current racial unrest and the health of their patients,” the Larry A. Green Center said in a recent statement. One-third of the clinicians also said that recent racism-related events have had a negative effect on their own well-being.
In a related survey of 1,111 patients conducted June 8 about 65% of patients said that racism affected emotional, psychological, and behavioral health.
“The fact that so many patients and clinicians agree that racism is a driver of health points to the incredible role primary care plays in creating safe spaces to process deep societal and personal issues,” said Christine Bechtel, cofounder of 3rd Conversation, a community of patients and providers. Read more.
Medical teams take to the streets
They stanched bleeding wounds and plucked disoriented teenagers from clouds of gas, entering dangerous corners where on-duty emergency health responders may fear to go. Many are medical professionals who see parallels between the front lines of COVID-19, where they confront stark racial imbalances among those stricken by the coronavirus, and what they see as racialized police brutality.
Iris Butler, a 21-year-old certified nursing assistant who works in a nursing home, decided to offer her skills after seeing a man injured by a rubber bullet on her first night at the Denver protests. She showed up as a medic every night thereafter. “I am working full time and basically being at the protest after getting straight off of work,” said Ms. Butler, who is black. That’s tiring, she added, but so is being a black woman in America. Read more.
At-home management of type 1 diabetes, COVID-19
Although hyperglycemia and diabetic ketoacidosis are common in people with type 1 diabetes who develop COVID-19, many are still able to manage the illness at home and overall mortality is relatively low.
These new findings are still preliminary and were published online June 5 in Diabetes Care by Osagie A. Ebekozien, MD, vice president, quality improvement and population health at the T1D Exchange, and colleagues.
The published study includes data as of May 5 on 64 individuals from a total of 64 US sites. Since the paper was submitted, there are now 220 patients from 68 sites. There were two deaths in the preliminary report, Dr. Ebekozien said. There have been a few more deaths in the larger dataset, but the mortality rate remains relatively low.
Overall, 34.9% of patients were able to manage COVID-19 entirely at home. At the other extreme, 22.2% of patients overall were admitted to the intensive care unit. Including the small proportion of patients sent home after being seen in emergency or urgent care, overall roughly half were not admitted to the hospital. “Even in this preliminary study, half were managed at home via telemedicine with an endocrinologist and infectious disease specialist ... I think it continues to be a case-by-case clinical decision between the patient and their provider,” Dr. Ebekozien said. Read more.
‘Hospital at home’ for cancer patients
Visits to the emergency department (ED) and hospitalizations are often frequent occurrences for cancer patients, but what if the “hospital” could be brought into the home instead?
A new American cohort study provides evidence that this can be a workable option for cancer patients. The authors report improved patient outcomes, with 56% lower odds of unplanned hospitalizations, 45% lower odds of ED visits, and 50% lower cumulative charges, as compared with patients who received usual care.
“The oncology hospital-at-home model of care that extends acute-level care to the patient at home offers promise in addressing a long-term gap in cancer care service delivery,” said lead author Kathi Mooney, PhD, RN, distinguished professor of nursing at the University of Utah, Salt Lake City. “In light of the current global pandemic, we are compelled to consider new ways to provide cancer care, and the oncology hospital-at-home model is on point to address critical elements of an improved cancer care delivery system.”
Dr. Mooney presented the findings during the virtual scientific program of the American Society of Clinical Oncology 2020 annual meeting (abstract 7000). Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Many COVID patients shed virus in feces, even without GI symptoms
Even without GI symptoms, many patients with COVID-19 shed viral RNA in feces, suggesting that stool testing and prevention of fecal-oral transmission may be needed to combat the ongoing pandemic, according to investigators.
A meta-analysis of 29 studies showed that 12% of patients with COVID-19 developed nausea, diarrhea, or vomiting, while 41% shed viral RNA in feces, reported lead author Sravanthi Parasa, MD, of Swedish Medical Center, Seattle.Writing in JAMA Network Open, Dr. Parasa and colleagues emphasized that respiratory symptoms remain the predominant form of disease; however, GI symptoms can occur.
“In fact, the first reported patient with COVID-19 in the U.S. reported GI symptoms of loose bowel movements and abdominal discomfort,” the investigators wrote, noting that the patient went on to test positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in both respiratory and stool specimens.
“This raises the question of inadvertent human-to-human transmission via the fecal route despite public health emphasis on droplet transmission and precautions for contact with respiratory secretions,” the investigators wrote.
To address this question, the investigators conducted a systematic review and meta-analysis involving 23 published and 6 preprint studies involving a total of 4,805 patients, all of whom tested positive for SARS-CoV-2 based on PCR results from nasopharyngeal swabs. Dr. Parasa and colleagues noted that most of the studies “scored between 8 and 10 on the MINORS quality assessment,” suggesting moderate quality.
Pooled data from these studies showed that 4.6% of patients reported nausea or vomiting, while 7.4% reported diarrhea. Such symptoms may serve as an early warning flag for clinicians, the investigators noted.
“[T]he presence of GI symptoms may portend a worse outcome for patients infected with SARS-CoV-2,” they wrote, citing a study by Pan and colleagues, which found that GI symptoms were associated with lower rates of recovery and hospital discharge.
Regardless of GI symptoms, 40.5% of patients in the meta-analysis tested positive for viral RNA in feces (95% confidence interval, 27.4%-55.1%). Duration of viral shedding in feces lasted up to 11 days after symptom onset, or in a single-patient case study, 18 days after hospitalization.
The investigators called these duration figures “particularly concerning,” especially in light of a study published by Xiao and colleagues, which showed that 23.3% of patients with negative respiratory tests were still shedding live virus in feces.
“[T]he fecal-oral route of transmission could be an additional potential source of infection spread,” wrote Dr. Parasa and colleagues. “Our results also suggest that testing of the virus in feces ... could be helpful in disease monitoring and surveillance.”
David A. Johnson, MD, professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, said that the findings confirm what has been suspected for some time: GI disease is relatively common with COVID-19.
“The evidence is clear now that a sizable percentage of patients have GI symptoms,” Dr. Johnson said in an interview.
GI issues may precede respiratory signs, he added, so clinicians should be aware that nausea, vomiting, or diarrhea could be early indicators of COVID-19, and possibly, a worse outcome.
“The other highlight of this study is that stool shedding may be extended beyond respiratory shedding,” Dr. Johnson said.
He suggested that this finding could influence current CDC criteria, which define absence of infectious risk by two consecutive, negative nasopharyngeal swabs. Instead, fecal testing may be needed, he said, along with measures to prevent fecal-oral transmission.
Dr. Johnson expressed particular concern for risk of infection via toilet plume, in which toilet flushing aerosolizes viral particles.
“As much as people try to social distance by 6 feet – you can do that when you walk into a store, or a building, but you can’t necessarily do that when you walk into a public toilet, where the plume may have been expansive for a period of time,” he said. “That toilet may never really get cleaned to a high level of disinfection, and those droplets set up potential for fecal-oral spread.”
Dr. Sharma disclosed relationships with Medtronic, Fujifilm, Boston Scientific, and others. Dr. Johnson disclosed no relevant conflicts of interest.
SOURCE: Parasa S et al. JAMA Network Open. 2020 Jun 11. doi: 10.1001/jamanetworkopen.2020.11335.
Even without GI symptoms, many patients with COVID-19 shed viral RNA in feces, suggesting that stool testing and prevention of fecal-oral transmission may be needed to combat the ongoing pandemic, according to investigators.
A meta-analysis of 29 studies showed that 12% of patients with COVID-19 developed nausea, diarrhea, or vomiting, while 41% shed viral RNA in feces, reported lead author Sravanthi Parasa, MD, of Swedish Medical Center, Seattle.Writing in JAMA Network Open, Dr. Parasa and colleagues emphasized that respiratory symptoms remain the predominant form of disease; however, GI symptoms can occur.
“In fact, the first reported patient with COVID-19 in the U.S. reported GI symptoms of loose bowel movements and abdominal discomfort,” the investigators wrote, noting that the patient went on to test positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in both respiratory and stool specimens.
“This raises the question of inadvertent human-to-human transmission via the fecal route despite public health emphasis on droplet transmission and precautions for contact with respiratory secretions,” the investigators wrote.
To address this question, the investigators conducted a systematic review and meta-analysis involving 23 published and 6 preprint studies involving a total of 4,805 patients, all of whom tested positive for SARS-CoV-2 based on PCR results from nasopharyngeal swabs. Dr. Parasa and colleagues noted that most of the studies “scored between 8 and 10 on the MINORS quality assessment,” suggesting moderate quality.
Pooled data from these studies showed that 4.6% of patients reported nausea or vomiting, while 7.4% reported diarrhea. Such symptoms may serve as an early warning flag for clinicians, the investigators noted.
“[T]he presence of GI symptoms may portend a worse outcome for patients infected with SARS-CoV-2,” they wrote, citing a study by Pan and colleagues, which found that GI symptoms were associated with lower rates of recovery and hospital discharge.
Regardless of GI symptoms, 40.5% of patients in the meta-analysis tested positive for viral RNA in feces (95% confidence interval, 27.4%-55.1%). Duration of viral shedding in feces lasted up to 11 days after symptom onset, or in a single-patient case study, 18 days after hospitalization.
The investigators called these duration figures “particularly concerning,” especially in light of a study published by Xiao and colleagues, which showed that 23.3% of patients with negative respiratory tests were still shedding live virus in feces.
“[T]he fecal-oral route of transmission could be an additional potential source of infection spread,” wrote Dr. Parasa and colleagues. “Our results also suggest that testing of the virus in feces ... could be helpful in disease monitoring and surveillance.”
David A. Johnson, MD, professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, said that the findings confirm what has been suspected for some time: GI disease is relatively common with COVID-19.
“The evidence is clear now that a sizable percentage of patients have GI symptoms,” Dr. Johnson said in an interview.
GI issues may precede respiratory signs, he added, so clinicians should be aware that nausea, vomiting, or diarrhea could be early indicators of COVID-19, and possibly, a worse outcome.
“The other highlight of this study is that stool shedding may be extended beyond respiratory shedding,” Dr. Johnson said.
He suggested that this finding could influence current CDC criteria, which define absence of infectious risk by two consecutive, negative nasopharyngeal swabs. Instead, fecal testing may be needed, he said, along with measures to prevent fecal-oral transmission.
Dr. Johnson expressed particular concern for risk of infection via toilet plume, in which toilet flushing aerosolizes viral particles.
“As much as people try to social distance by 6 feet – you can do that when you walk into a store, or a building, but you can’t necessarily do that when you walk into a public toilet, where the plume may have been expansive for a period of time,” he said. “That toilet may never really get cleaned to a high level of disinfection, and those droplets set up potential for fecal-oral spread.”
Dr. Sharma disclosed relationships with Medtronic, Fujifilm, Boston Scientific, and others. Dr. Johnson disclosed no relevant conflicts of interest.
SOURCE: Parasa S et al. JAMA Network Open. 2020 Jun 11. doi: 10.1001/jamanetworkopen.2020.11335.
Even without GI symptoms, many patients with COVID-19 shed viral RNA in feces, suggesting that stool testing and prevention of fecal-oral transmission may be needed to combat the ongoing pandemic, according to investigators.
A meta-analysis of 29 studies showed that 12% of patients with COVID-19 developed nausea, diarrhea, or vomiting, while 41% shed viral RNA in feces, reported lead author Sravanthi Parasa, MD, of Swedish Medical Center, Seattle.Writing in JAMA Network Open, Dr. Parasa and colleagues emphasized that respiratory symptoms remain the predominant form of disease; however, GI symptoms can occur.
“In fact, the first reported patient with COVID-19 in the U.S. reported GI symptoms of loose bowel movements and abdominal discomfort,” the investigators wrote, noting that the patient went on to test positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in both respiratory and stool specimens.
“This raises the question of inadvertent human-to-human transmission via the fecal route despite public health emphasis on droplet transmission and precautions for contact with respiratory secretions,” the investigators wrote.
To address this question, the investigators conducted a systematic review and meta-analysis involving 23 published and 6 preprint studies involving a total of 4,805 patients, all of whom tested positive for SARS-CoV-2 based on PCR results from nasopharyngeal swabs. Dr. Parasa and colleagues noted that most of the studies “scored between 8 and 10 on the MINORS quality assessment,” suggesting moderate quality.
Pooled data from these studies showed that 4.6% of patients reported nausea or vomiting, while 7.4% reported diarrhea. Such symptoms may serve as an early warning flag for clinicians, the investigators noted.
“[T]he presence of GI symptoms may portend a worse outcome for patients infected with SARS-CoV-2,” they wrote, citing a study by Pan and colleagues, which found that GI symptoms were associated with lower rates of recovery and hospital discharge.
Regardless of GI symptoms, 40.5% of patients in the meta-analysis tested positive for viral RNA in feces (95% confidence interval, 27.4%-55.1%). Duration of viral shedding in feces lasted up to 11 days after symptom onset, or in a single-patient case study, 18 days after hospitalization.
The investigators called these duration figures “particularly concerning,” especially in light of a study published by Xiao and colleagues, which showed that 23.3% of patients with negative respiratory tests were still shedding live virus in feces.
“[T]he fecal-oral route of transmission could be an additional potential source of infection spread,” wrote Dr. Parasa and colleagues. “Our results also suggest that testing of the virus in feces ... could be helpful in disease monitoring and surveillance.”
David A. Johnson, MD, professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, said that the findings confirm what has been suspected for some time: GI disease is relatively common with COVID-19.
“The evidence is clear now that a sizable percentage of patients have GI symptoms,” Dr. Johnson said in an interview.
GI issues may precede respiratory signs, he added, so clinicians should be aware that nausea, vomiting, or diarrhea could be early indicators of COVID-19, and possibly, a worse outcome.
“The other highlight of this study is that stool shedding may be extended beyond respiratory shedding,” Dr. Johnson said.
He suggested that this finding could influence current CDC criteria, which define absence of infectious risk by two consecutive, negative nasopharyngeal swabs. Instead, fecal testing may be needed, he said, along with measures to prevent fecal-oral transmission.
Dr. Johnson expressed particular concern for risk of infection via toilet plume, in which toilet flushing aerosolizes viral particles.
“As much as people try to social distance by 6 feet – you can do that when you walk into a store, or a building, but you can’t necessarily do that when you walk into a public toilet, where the plume may have been expansive for a period of time,” he said. “That toilet may never really get cleaned to a high level of disinfection, and those droplets set up potential for fecal-oral spread.”
Dr. Sharma disclosed relationships with Medtronic, Fujifilm, Boston Scientific, and others. Dr. Johnson disclosed no relevant conflicts of interest.
SOURCE: Parasa S et al. JAMA Network Open. 2020 Jun 11. doi: 10.1001/jamanetworkopen.2020.11335.
FROM JAMA NETWORK OPEN
Half of type 1 diabetes patients with COVID-19 manage at home
New preliminary data from the T1D Exchange suggest that, although hyperglycemia and diabetic ketoacidosis (DKA) are common in people with type 1 diabetes who develop COVID-19, many are still able to manage the illness at home and overall mortality is relatively low.
The new findings – the first US data on individuals with type 1 diabetes and COVID-19 – were published online June 5 in Diabetes Care by Osagie A. Ebekozien, MD, vice president, quality improvement and population health at the T1D Exchange, and colleagues.
Two UK studies are the only prior ones to previously examine the topic.
The newly published study includes data as of May 5 on 64 individuals from a total of 64 US sites, including 15 T1D Exchange member clinics and an additional 49 endocrinology clinics from around the country. Since the paper was submitted, there are now 220 patients from 68 sites. Another publication with a more detailed analysis of risk factors and adjustment for confounders is planned for later this year.
Some of the findings from the preliminary data have shifted, but many aspects remain consistent, Ebekozien told Medscape Medical News.
“One thing still very true, even with the unpublished findings, is the influence of A1c and glycemic management. ...With higher A1c levels, we’re seeing more COVID-19 hospitalizations and worse outcomes,” he said.
And as has been generally reported for COVID-19, high body mass index was a major risk factor in the preliminary dataset – and remains so.
There were two deaths in the preliminary report, both individuals with comorbidities in addition to type 1 diabetes, Ebekozien said. There have been a few more deaths in the larger dataset, but the mortality rate remains relatively low.
Interestingly, females predominate in both cohorts. That may be a reporting phenomenon, another factor that is being analyzed.
Hyperglycemia Remains a Major Risk Factor
The study is specifically being conducted by the T1D Exchange’s Quality Improvement Collaborative, which Ebekozien heads.
Data were obtained for 33 patients with type 1 diabetes who tested positive for COVID-19, and another 31 who were classified as “COVID-19–like” because they had symptoms consistent with COVID-19, as identified by the Centers for Disease Control and Prevention, but hadn’t been tested for the virus.
For all 64 patients, the mean age was 20.9 years and two thirds (65.6%) were aged 18 or younger. A higher proportion of the COVID-19–like patients were pediatric than the confirmed cases. The larger dataset includes more adult patients, Ebekozien told Medscape Medical News.
Overall, 60.9% of patients were female. Nearly half were white, a quarter Hispanic, and 18.8% black. More confirmed COVID-19 cases were black compared with suspected cases (30.3% vs 6.5%).
Median A1c for the overall group (including suspected COVID-19 cases) was 8.0%, but it was 8.5% among confirmed cases. Overall, six patients (9.8%) presented with new-onset type 1 diabetes after they developed COVID-19.
Hyperglycemia was present in half (32) of patients overall. DKA occurred in 19 people (30.2%): 15 of the confirmed COVID-19 cases (45.5%) versus just 4 (13.3%) of the COVID-19–like cases. Nausea was reported in 30.2% of patients overall.
Other symptoms were typical of COVID-19, including fever (41.3%), dry cough (38.1%), and shortness of breath (27.0%). Loss of taste and smell was less common, at just 9.5% overall.
Obesity was present in 39.7% of patients overall, with similar proportions in the confirmed and suspected COVID-19 groups. Hypertension and/or cardiovascular disease were present in 14.3% of patients overall, and the rate was similar between the two subgroups.
One of the two patients who died was a 79-year-old man who had hypertension and a prior stroke in addition to type 1 diabetes. The other was a 19-year-old woman with a history of asthma who developed a pulmonary embolism during the onset of COVID-19. Neither had DKA.
Even in Type 1 Diabetes, COVID-19 Can Be Managed at Home
Overall, 34.9% of patients were able to manage COVID-19 entirely at home, with 27.3% of the confirmed and 43.3% of the suspected cases able to do so.
At the other extreme, 22.2% of patients overall were admitted to the intensive care unit; 30.3% of the confirmed versus 13.3% of suspected cases.
Including the small proportion of patients sent home after being seen in emergency or urgent care, overall roughly half were not admitted to hospital.
“Interestingly, even in this preliminary study, half were managed at home via telemedicine with an endocrinologist and infectious disease specialist. ... I think it continues to be a case-by-case clinical decision between the patient and their provider,” Ebekozien said.
“But, we’re seeing a good number of patients who are managed at home and the symptoms resolve in a week or two, and the illness runs its course, and they don’t have to even be seen,” he added.
The research team is also collecting data on barriers to remote care, including challenges with telemedicine and how frontline providers are navigating them.
“Those are all things that our future paper will be able to shed more light on,” he explained.
Endocrinologists around the country are invited to report cases of COVID-19 in patients with type 1 diabetes to the T1D Exchange by emailing [email protected].
And in fact, Ebekozien also requested that clinicians with a large type 1 diabetes population also report if they’ve had no COVID-19 cases.
“Even if they haven’t had a case, that’s very useful information for us to know. One of the things we want to calculate down the line is the incidence ratio. Not all participating sites have had a case.”
Endocrinologists from all the participating sites have formed a dedicated community that meets regularly via webinars to share information, he noted. “It’s been a very selfless effort to work collaboratively as a community to quickly answer critical questions.”
The Helmsley Charitable Trust funds the T1D Exchange Quality Improvement Collaborative. The T1D Exchange received financial support for this study from Abbott Diabetes, Dexcom, JDRF, Insulet Corporation, Lilly, Medtronic, and Tandem Diabetes Care. No other relevant financial relationships were reported.
This article first appeared on Medscape.com.
New preliminary data from the T1D Exchange suggest that, although hyperglycemia and diabetic ketoacidosis (DKA) are common in people with type 1 diabetes who develop COVID-19, many are still able to manage the illness at home and overall mortality is relatively low.
The new findings – the first US data on individuals with type 1 diabetes and COVID-19 – were published online June 5 in Diabetes Care by Osagie A. Ebekozien, MD, vice president, quality improvement and population health at the T1D Exchange, and colleagues.
Two UK studies are the only prior ones to previously examine the topic.
The newly published study includes data as of May 5 on 64 individuals from a total of 64 US sites, including 15 T1D Exchange member clinics and an additional 49 endocrinology clinics from around the country. Since the paper was submitted, there are now 220 patients from 68 sites. Another publication with a more detailed analysis of risk factors and adjustment for confounders is planned for later this year.
Some of the findings from the preliminary data have shifted, but many aspects remain consistent, Ebekozien told Medscape Medical News.
“One thing still very true, even with the unpublished findings, is the influence of A1c and glycemic management. ...With higher A1c levels, we’re seeing more COVID-19 hospitalizations and worse outcomes,” he said.
And as has been generally reported for COVID-19, high body mass index was a major risk factor in the preliminary dataset – and remains so.
There were two deaths in the preliminary report, both individuals with comorbidities in addition to type 1 diabetes, Ebekozien said. There have been a few more deaths in the larger dataset, but the mortality rate remains relatively low.
Interestingly, females predominate in both cohorts. That may be a reporting phenomenon, another factor that is being analyzed.
Hyperglycemia Remains a Major Risk Factor
The study is specifically being conducted by the T1D Exchange’s Quality Improvement Collaborative, which Ebekozien heads.
Data were obtained for 33 patients with type 1 diabetes who tested positive for COVID-19, and another 31 who were classified as “COVID-19–like” because they had symptoms consistent with COVID-19, as identified by the Centers for Disease Control and Prevention, but hadn’t been tested for the virus.
For all 64 patients, the mean age was 20.9 years and two thirds (65.6%) were aged 18 or younger. A higher proportion of the COVID-19–like patients were pediatric than the confirmed cases. The larger dataset includes more adult patients, Ebekozien told Medscape Medical News.
Overall, 60.9% of patients were female. Nearly half were white, a quarter Hispanic, and 18.8% black. More confirmed COVID-19 cases were black compared with suspected cases (30.3% vs 6.5%).
Median A1c for the overall group (including suspected COVID-19 cases) was 8.0%, but it was 8.5% among confirmed cases. Overall, six patients (9.8%) presented with new-onset type 1 diabetes after they developed COVID-19.
Hyperglycemia was present in half (32) of patients overall. DKA occurred in 19 people (30.2%): 15 of the confirmed COVID-19 cases (45.5%) versus just 4 (13.3%) of the COVID-19–like cases. Nausea was reported in 30.2% of patients overall.
Other symptoms were typical of COVID-19, including fever (41.3%), dry cough (38.1%), and shortness of breath (27.0%). Loss of taste and smell was less common, at just 9.5% overall.
Obesity was present in 39.7% of patients overall, with similar proportions in the confirmed and suspected COVID-19 groups. Hypertension and/or cardiovascular disease were present in 14.3% of patients overall, and the rate was similar between the two subgroups.
One of the two patients who died was a 79-year-old man who had hypertension and a prior stroke in addition to type 1 diabetes. The other was a 19-year-old woman with a history of asthma who developed a pulmonary embolism during the onset of COVID-19. Neither had DKA.
Even in Type 1 Diabetes, COVID-19 Can Be Managed at Home
Overall, 34.9% of patients were able to manage COVID-19 entirely at home, with 27.3% of the confirmed and 43.3% of the suspected cases able to do so.
At the other extreme, 22.2% of patients overall were admitted to the intensive care unit; 30.3% of the confirmed versus 13.3% of suspected cases.
Including the small proportion of patients sent home after being seen in emergency or urgent care, overall roughly half were not admitted to hospital.
“Interestingly, even in this preliminary study, half were managed at home via telemedicine with an endocrinologist and infectious disease specialist. ... I think it continues to be a case-by-case clinical decision between the patient and their provider,” Ebekozien said.
“But, we’re seeing a good number of patients who are managed at home and the symptoms resolve in a week or two, and the illness runs its course, and they don’t have to even be seen,” he added.
The research team is also collecting data on barriers to remote care, including challenges with telemedicine and how frontline providers are navigating them.
“Those are all things that our future paper will be able to shed more light on,” he explained.
Endocrinologists around the country are invited to report cases of COVID-19 in patients with type 1 diabetes to the T1D Exchange by emailing [email protected].
And in fact, Ebekozien also requested that clinicians with a large type 1 diabetes population also report if they’ve had no COVID-19 cases.
“Even if they haven’t had a case, that’s very useful information for us to know. One of the things we want to calculate down the line is the incidence ratio. Not all participating sites have had a case.”
Endocrinologists from all the participating sites have formed a dedicated community that meets regularly via webinars to share information, he noted. “It’s been a very selfless effort to work collaboratively as a community to quickly answer critical questions.”
The Helmsley Charitable Trust funds the T1D Exchange Quality Improvement Collaborative. The T1D Exchange received financial support for this study from Abbott Diabetes, Dexcom, JDRF, Insulet Corporation, Lilly, Medtronic, and Tandem Diabetes Care. No other relevant financial relationships were reported.
This article first appeared on Medscape.com.
New preliminary data from the T1D Exchange suggest that, although hyperglycemia and diabetic ketoacidosis (DKA) are common in people with type 1 diabetes who develop COVID-19, many are still able to manage the illness at home and overall mortality is relatively low.
The new findings – the first US data on individuals with type 1 diabetes and COVID-19 – were published online June 5 in Diabetes Care by Osagie A. Ebekozien, MD, vice president, quality improvement and population health at the T1D Exchange, and colleagues.
Two UK studies are the only prior ones to previously examine the topic.
The newly published study includes data as of May 5 on 64 individuals from a total of 64 US sites, including 15 T1D Exchange member clinics and an additional 49 endocrinology clinics from around the country. Since the paper was submitted, there are now 220 patients from 68 sites. Another publication with a more detailed analysis of risk factors and adjustment for confounders is planned for later this year.
Some of the findings from the preliminary data have shifted, but many aspects remain consistent, Ebekozien told Medscape Medical News.
“One thing still very true, even with the unpublished findings, is the influence of A1c and glycemic management. ...With higher A1c levels, we’re seeing more COVID-19 hospitalizations and worse outcomes,” he said.
And as has been generally reported for COVID-19, high body mass index was a major risk factor in the preliminary dataset – and remains so.
There were two deaths in the preliminary report, both individuals with comorbidities in addition to type 1 diabetes, Ebekozien said. There have been a few more deaths in the larger dataset, but the mortality rate remains relatively low.
Interestingly, females predominate in both cohorts. That may be a reporting phenomenon, another factor that is being analyzed.
Hyperglycemia Remains a Major Risk Factor
The study is specifically being conducted by the T1D Exchange’s Quality Improvement Collaborative, which Ebekozien heads.
Data were obtained for 33 patients with type 1 diabetes who tested positive for COVID-19, and another 31 who were classified as “COVID-19–like” because they had symptoms consistent with COVID-19, as identified by the Centers for Disease Control and Prevention, but hadn’t been tested for the virus.
For all 64 patients, the mean age was 20.9 years and two thirds (65.6%) were aged 18 or younger. A higher proportion of the COVID-19–like patients were pediatric than the confirmed cases. The larger dataset includes more adult patients, Ebekozien told Medscape Medical News.
Overall, 60.9% of patients were female. Nearly half were white, a quarter Hispanic, and 18.8% black. More confirmed COVID-19 cases were black compared with suspected cases (30.3% vs 6.5%).
Median A1c for the overall group (including suspected COVID-19 cases) was 8.0%, but it was 8.5% among confirmed cases. Overall, six patients (9.8%) presented with new-onset type 1 diabetes after they developed COVID-19.
Hyperglycemia was present in half (32) of patients overall. DKA occurred in 19 people (30.2%): 15 of the confirmed COVID-19 cases (45.5%) versus just 4 (13.3%) of the COVID-19–like cases. Nausea was reported in 30.2% of patients overall.
Other symptoms were typical of COVID-19, including fever (41.3%), dry cough (38.1%), and shortness of breath (27.0%). Loss of taste and smell was less common, at just 9.5% overall.
Obesity was present in 39.7% of patients overall, with similar proportions in the confirmed and suspected COVID-19 groups. Hypertension and/or cardiovascular disease were present in 14.3% of patients overall, and the rate was similar between the two subgroups.
One of the two patients who died was a 79-year-old man who had hypertension and a prior stroke in addition to type 1 diabetes. The other was a 19-year-old woman with a history of asthma who developed a pulmonary embolism during the onset of COVID-19. Neither had DKA.
Even in Type 1 Diabetes, COVID-19 Can Be Managed at Home
Overall, 34.9% of patients were able to manage COVID-19 entirely at home, with 27.3% of the confirmed and 43.3% of the suspected cases able to do so.
At the other extreme, 22.2% of patients overall were admitted to the intensive care unit; 30.3% of the confirmed versus 13.3% of suspected cases.
Including the small proportion of patients sent home after being seen in emergency or urgent care, overall roughly half were not admitted to hospital.
“Interestingly, even in this preliminary study, half were managed at home via telemedicine with an endocrinologist and infectious disease specialist. ... I think it continues to be a case-by-case clinical decision between the patient and their provider,” Ebekozien said.
“But, we’re seeing a good number of patients who are managed at home and the symptoms resolve in a week or two, and the illness runs its course, and they don’t have to even be seen,” he added.
The research team is also collecting data on barriers to remote care, including challenges with telemedicine and how frontline providers are navigating them.
“Those are all things that our future paper will be able to shed more light on,” he explained.
Endocrinologists around the country are invited to report cases of COVID-19 in patients with type 1 diabetes to the T1D Exchange by emailing [email protected].
And in fact, Ebekozien also requested that clinicians with a large type 1 diabetes population also report if they’ve had no COVID-19 cases.
“Even if they haven’t had a case, that’s very useful information for us to know. One of the things we want to calculate down the line is the incidence ratio. Not all participating sites have had a case.”
Endocrinologists from all the participating sites have formed a dedicated community that meets regularly via webinars to share information, he noted. “It’s been a very selfless effort to work collaboratively as a community to quickly answer critical questions.”
The Helmsley Charitable Trust funds the T1D Exchange Quality Improvement Collaborative. The T1D Exchange received financial support for this study from Abbott Diabetes, Dexcom, JDRF, Insulet Corporation, Lilly, Medtronic, and Tandem Diabetes Care. No other relevant financial relationships were reported.
This article first appeared on Medscape.com.
Electrosurgical choices lead to similar results
For endoscopists performing electrosurgical snare resection of large colorectal polyps, choosing between the blue foot pedal and the yellow foot pedal may be the least important step of the day, according to data from almost 1,000 patients.
Risks of severe adverse events and polyp recurrence were similar between cases in which blended current (yellow pedal) was used and those in which coagulation current (blue pedal) was used, reported lead author Heiko Pohl, MD, of Geisel School of Medicine at Dartmouth, Hanover, N.H., and colleagues.
“Although electrosurgical application is a fundamental aspect of polypectomy, various currents and settings are clinically used, and there are no accepted standards of practice,” the investigators wrote in Gastroenterology.
According to Dr. Pohl and colleagues, a 2004 study showed that the split between endoscopists using coagulation current and those using blended current was about 50-50 (46% vs. 46%), but no studies to date have tested the relative safety or efficacy of these approaches.
The investigators aimed to address this knowledge gap with a single-blinded study involving 928 patients who underwent endoscopic mucosal resection of nonpedunculated, large (20 mm or larger) colorectal polyps with an Erbe Vio® 300D electrosurgical unit (Erbe USA Inc., Marietta, Ga.) at 18 medical centers.
Patients were randomized in 2x2 factorial design involving clip closure versus no clip closure, and blended current (Endocut Q) versus pure coagulation current (Forced Coagulation). Although electrosurgical setting was initially a secondary intervention in the trial, post hoc analysis showed that interaction between the interventions was not significant (P = .957), allowing for the present, independent analysis of current type.
For this analysis, the primary outcome was severe adverse event rate, both during the procedure, and after the procedure for up to 30 days. Secondary outcomes included proportion of polyps completely excised and recurrence rate at time of first surveillance endoscopy.
Out of 928 patients randomized, 919 completed 30-day follow-up, and 675 underwent first surveillance colonoscopy. Baseline characteristics were similar between groups, apart from the proportion of individuals with more than one large polyp, which was significantly greater in the Endocut Q group (8.6% vs. 4.5%; P = .012), although the investigators noted that this imbalance did not affect main outcomes.
Rates of severe adverse events were similar between groups: 7.2% for the Endocut Q group and 7.9% for the Forced Coagulation group (P = .762). Groups also had similar rates of intra- and postprocedure adverse events, and types of adverse events.
Efficacy measures also revealed high similarity between cutting techniques. Endoscopists using Endocut achieved complete polyp removal 96% of the time, compared with 95% of the time when using Forced Coagulation (P = .267). Piecemeal resection rates were similar, at 90% and 87% for Endocut Q and Forced Coagulation, respectively (P = .270).
Although Endocut Q less frequently resulted in small residual tissue islands after initial snare resection (35% vs. 41%; P = .041), it more often caused intraprocedural bleeding that required treatment (17% vs. 11%; P = .006).
According to Dr. Pohl and colleagues, previous discussions have included concerns that such bleeding may impair visualization and therefore lead to higher rates of polyp recurrence; but surveillance colonoscopy, which was performed in 79% of patients, revealed a polyp recurrence rate of 17% for each group.
“Although we did not find a difference in recurrence between the two groups, our study cannot completely exclude this possibility,” the investigators added.
They also noted that six perforations occurred in the Endocut Q group, compared with three in the Forced Coagulation group, and suggested that this risk may be real, yet statistically unsupported by the present analysis because of sample size.
“Endoscopists using Endocut should therefore be aware of this potential risk and [ensure] that no muscularis propria is entrapped in the snare before electrosurgery is applied,” the investigators wrote.
Still, the investigators’ final conclusion supported the existing method of decision-making: personal choice.
“Overall, polyp resection with Endocut or Forced Coagulation did not differ with respect to severe adverse events, complete resection rate, or polyp recurrence,” they wrote. “This study therefore supports an individual approach based on endoscopist preference.”
The study was funded by Boston Scientific and the American College of Gastroenterology. The investigators disclosed additional relationships with Medtronic, Olympus, Cook Endoscopy, and others.
SOURCE: Pohl H et al. Gastroenterology. 2020 Mar 12. doi: 10.1053/j.gastro.2020.03.014.
There has long been a debate over which type of electrosurgical setting is best for colon polyp resection. Endoscopists can use either a blended current (yellow pedal) or a coagulation current (blue pedal). The choice is based on the endoscopists’ preference. However, few data have been available to support one setting versus the other. This study by Pohl et al. pursued the burning question of yellow or blue pedal? This single-blind randomized multicenter trial compared the two commonly used electrosurgical settings (Blended Current/Endocut Q vs. Forced Coagulation) for the resection of large colorectal polyps and found no difference in the risk of serious adverse events, complete resection rate, or polyp recurrence, thus supporting the current practice that electrosurgical settings can be selected based on endoscopist expertise and preference.
A few important highlights from this well designed study are worth mentioning. Although there was no significant difference in perforation, it should be noted that fewer patients had a perforation event in the Forced Coagulation group than in the Endocut Q group (3 vs. 6 patients; P = .320). In addition, the study demonstrated that the rate of polyp recurrence did not differ significantly between the two groups (17.4% vs. 16.5%; P = .762). while the rate of macroscopically visible recurrence was not statistically different, a histologic recurrence without visible polyp tissue was found slightly less frequent in the Forced Coagulation group than in the Endocut Q group (3.1% vs. 6.0%; P = .07). Finally, another important observation, intraprocedural bleeding requiring treatment, occurred less frequently during resection with Forced Coagulation than with Endocut Q (11% vs. 17%, P = .006); however, this difference did not affect overall safety and efficacy. This is an important finding since bleeding can affect the field of view during polypectomy, which that can potentially increase the risk of other serious adverse events such as perforation or increase the risk of recurrence because the endoscopist may not completely resect the polyp.
This study provides important insights into the potential risks associated with blended vs. coagulation currents. It further shows there is no difference in safety and efficacy of polypectomy using either a blended current or coagulation current, thus supporting current practice. However, the authors make it clear that a larger study will be needed to better answer such questions as polyp recurrence and perforation more definitively.
Frank G. Gress, MD, MBA, is senior faculty at the Icahn School of Medicine at Mount Sinai, New York; chief, division of gastroenterology and hepatology, and director of the Center for Interventional Endoscopy at Mount Sinai Hospital South Nassau. He has no conflicts.
There has long been a debate over which type of electrosurgical setting is best for colon polyp resection. Endoscopists can use either a blended current (yellow pedal) or a coagulation current (blue pedal). The choice is based on the endoscopists’ preference. However, few data have been available to support one setting versus the other. This study by Pohl et al. pursued the burning question of yellow or blue pedal? This single-blind randomized multicenter trial compared the two commonly used electrosurgical settings (Blended Current/Endocut Q vs. Forced Coagulation) for the resection of large colorectal polyps and found no difference in the risk of serious adverse events, complete resection rate, or polyp recurrence, thus supporting the current practice that electrosurgical settings can be selected based on endoscopist expertise and preference.
A few important highlights from this well designed study are worth mentioning. Although there was no significant difference in perforation, it should be noted that fewer patients had a perforation event in the Forced Coagulation group than in the Endocut Q group (3 vs. 6 patients; P = .320). In addition, the study demonstrated that the rate of polyp recurrence did not differ significantly between the two groups (17.4% vs. 16.5%; P = .762). while the rate of macroscopically visible recurrence was not statistically different, a histologic recurrence without visible polyp tissue was found slightly less frequent in the Forced Coagulation group than in the Endocut Q group (3.1% vs. 6.0%; P = .07). Finally, another important observation, intraprocedural bleeding requiring treatment, occurred less frequently during resection with Forced Coagulation than with Endocut Q (11% vs. 17%, P = .006); however, this difference did not affect overall safety and efficacy. This is an important finding since bleeding can affect the field of view during polypectomy, which that can potentially increase the risk of other serious adverse events such as perforation or increase the risk of recurrence because the endoscopist may not completely resect the polyp.
This study provides important insights into the potential risks associated with blended vs. coagulation currents. It further shows there is no difference in safety and efficacy of polypectomy using either a blended current or coagulation current, thus supporting current practice. However, the authors make it clear that a larger study will be needed to better answer such questions as polyp recurrence and perforation more definitively.
Frank G. Gress, MD, MBA, is senior faculty at the Icahn School of Medicine at Mount Sinai, New York; chief, division of gastroenterology and hepatology, and director of the Center for Interventional Endoscopy at Mount Sinai Hospital South Nassau. He has no conflicts.
There has long been a debate over which type of electrosurgical setting is best for colon polyp resection. Endoscopists can use either a blended current (yellow pedal) or a coagulation current (blue pedal). The choice is based on the endoscopists’ preference. However, few data have been available to support one setting versus the other. This study by Pohl et al. pursued the burning question of yellow or blue pedal? This single-blind randomized multicenter trial compared the two commonly used electrosurgical settings (Blended Current/Endocut Q vs. Forced Coagulation) for the resection of large colorectal polyps and found no difference in the risk of serious adverse events, complete resection rate, or polyp recurrence, thus supporting the current practice that electrosurgical settings can be selected based on endoscopist expertise and preference.
A few important highlights from this well designed study are worth mentioning. Although there was no significant difference in perforation, it should be noted that fewer patients had a perforation event in the Forced Coagulation group than in the Endocut Q group (3 vs. 6 patients; P = .320). In addition, the study demonstrated that the rate of polyp recurrence did not differ significantly between the two groups (17.4% vs. 16.5%; P = .762). while the rate of macroscopically visible recurrence was not statistically different, a histologic recurrence without visible polyp tissue was found slightly less frequent in the Forced Coagulation group than in the Endocut Q group (3.1% vs. 6.0%; P = .07). Finally, another important observation, intraprocedural bleeding requiring treatment, occurred less frequently during resection with Forced Coagulation than with Endocut Q (11% vs. 17%, P = .006); however, this difference did not affect overall safety and efficacy. This is an important finding since bleeding can affect the field of view during polypectomy, which that can potentially increase the risk of other serious adverse events such as perforation or increase the risk of recurrence because the endoscopist may not completely resect the polyp.
This study provides important insights into the potential risks associated with blended vs. coagulation currents. It further shows there is no difference in safety and efficacy of polypectomy using either a blended current or coagulation current, thus supporting current practice. However, the authors make it clear that a larger study will be needed to better answer such questions as polyp recurrence and perforation more definitively.
Frank G. Gress, MD, MBA, is senior faculty at the Icahn School of Medicine at Mount Sinai, New York; chief, division of gastroenterology and hepatology, and director of the Center for Interventional Endoscopy at Mount Sinai Hospital South Nassau. He has no conflicts.
For endoscopists performing electrosurgical snare resection of large colorectal polyps, choosing between the blue foot pedal and the yellow foot pedal may be the least important step of the day, according to data from almost 1,000 patients.
Risks of severe adverse events and polyp recurrence were similar between cases in which blended current (yellow pedal) was used and those in which coagulation current (blue pedal) was used, reported lead author Heiko Pohl, MD, of Geisel School of Medicine at Dartmouth, Hanover, N.H., and colleagues.
“Although electrosurgical application is a fundamental aspect of polypectomy, various currents and settings are clinically used, and there are no accepted standards of practice,” the investigators wrote in Gastroenterology.
According to Dr. Pohl and colleagues, a 2004 study showed that the split between endoscopists using coagulation current and those using blended current was about 50-50 (46% vs. 46%), but no studies to date have tested the relative safety or efficacy of these approaches.
The investigators aimed to address this knowledge gap with a single-blinded study involving 928 patients who underwent endoscopic mucosal resection of nonpedunculated, large (20 mm or larger) colorectal polyps with an Erbe Vio® 300D electrosurgical unit (Erbe USA Inc., Marietta, Ga.) at 18 medical centers.
Patients were randomized in 2x2 factorial design involving clip closure versus no clip closure, and blended current (Endocut Q) versus pure coagulation current (Forced Coagulation). Although electrosurgical setting was initially a secondary intervention in the trial, post hoc analysis showed that interaction between the interventions was not significant (P = .957), allowing for the present, independent analysis of current type.
For this analysis, the primary outcome was severe adverse event rate, both during the procedure, and after the procedure for up to 30 days. Secondary outcomes included proportion of polyps completely excised and recurrence rate at time of first surveillance endoscopy.
Out of 928 patients randomized, 919 completed 30-day follow-up, and 675 underwent first surveillance colonoscopy. Baseline characteristics were similar between groups, apart from the proportion of individuals with more than one large polyp, which was significantly greater in the Endocut Q group (8.6% vs. 4.5%; P = .012), although the investigators noted that this imbalance did not affect main outcomes.
Rates of severe adverse events were similar between groups: 7.2% for the Endocut Q group and 7.9% for the Forced Coagulation group (P = .762). Groups also had similar rates of intra- and postprocedure adverse events, and types of adverse events.
Efficacy measures also revealed high similarity between cutting techniques. Endoscopists using Endocut achieved complete polyp removal 96% of the time, compared with 95% of the time when using Forced Coagulation (P = .267). Piecemeal resection rates were similar, at 90% and 87% for Endocut Q and Forced Coagulation, respectively (P = .270).
Although Endocut Q less frequently resulted in small residual tissue islands after initial snare resection (35% vs. 41%; P = .041), it more often caused intraprocedural bleeding that required treatment (17% vs. 11%; P = .006).
According to Dr. Pohl and colleagues, previous discussions have included concerns that such bleeding may impair visualization and therefore lead to higher rates of polyp recurrence; but surveillance colonoscopy, which was performed in 79% of patients, revealed a polyp recurrence rate of 17% for each group.
“Although we did not find a difference in recurrence between the two groups, our study cannot completely exclude this possibility,” the investigators added.
They also noted that six perforations occurred in the Endocut Q group, compared with three in the Forced Coagulation group, and suggested that this risk may be real, yet statistically unsupported by the present analysis because of sample size.
“Endoscopists using Endocut should therefore be aware of this potential risk and [ensure] that no muscularis propria is entrapped in the snare before electrosurgery is applied,” the investigators wrote.
Still, the investigators’ final conclusion supported the existing method of decision-making: personal choice.
“Overall, polyp resection with Endocut or Forced Coagulation did not differ with respect to severe adverse events, complete resection rate, or polyp recurrence,” they wrote. “This study therefore supports an individual approach based on endoscopist preference.”
The study was funded by Boston Scientific and the American College of Gastroenterology. The investigators disclosed additional relationships with Medtronic, Olympus, Cook Endoscopy, and others.
SOURCE: Pohl H et al. Gastroenterology. 2020 Mar 12. doi: 10.1053/j.gastro.2020.03.014.
For endoscopists performing electrosurgical snare resection of large colorectal polyps, choosing between the blue foot pedal and the yellow foot pedal may be the least important step of the day, according to data from almost 1,000 patients.
Risks of severe adverse events and polyp recurrence were similar between cases in which blended current (yellow pedal) was used and those in which coagulation current (blue pedal) was used, reported lead author Heiko Pohl, MD, of Geisel School of Medicine at Dartmouth, Hanover, N.H., and colleagues.
“Although electrosurgical application is a fundamental aspect of polypectomy, various currents and settings are clinically used, and there are no accepted standards of practice,” the investigators wrote in Gastroenterology.
According to Dr. Pohl and colleagues, a 2004 study showed that the split between endoscopists using coagulation current and those using blended current was about 50-50 (46% vs. 46%), but no studies to date have tested the relative safety or efficacy of these approaches.
The investigators aimed to address this knowledge gap with a single-blinded study involving 928 patients who underwent endoscopic mucosal resection of nonpedunculated, large (20 mm or larger) colorectal polyps with an Erbe Vio® 300D electrosurgical unit (Erbe USA Inc., Marietta, Ga.) at 18 medical centers.
Patients were randomized in 2x2 factorial design involving clip closure versus no clip closure, and blended current (Endocut Q) versus pure coagulation current (Forced Coagulation). Although electrosurgical setting was initially a secondary intervention in the trial, post hoc analysis showed that interaction between the interventions was not significant (P = .957), allowing for the present, independent analysis of current type.
For this analysis, the primary outcome was severe adverse event rate, both during the procedure, and after the procedure for up to 30 days. Secondary outcomes included proportion of polyps completely excised and recurrence rate at time of first surveillance endoscopy.
Out of 928 patients randomized, 919 completed 30-day follow-up, and 675 underwent first surveillance colonoscopy. Baseline characteristics were similar between groups, apart from the proportion of individuals with more than one large polyp, which was significantly greater in the Endocut Q group (8.6% vs. 4.5%; P = .012), although the investigators noted that this imbalance did not affect main outcomes.
Rates of severe adverse events were similar between groups: 7.2% for the Endocut Q group and 7.9% for the Forced Coagulation group (P = .762). Groups also had similar rates of intra- and postprocedure adverse events, and types of adverse events.
Efficacy measures also revealed high similarity between cutting techniques. Endoscopists using Endocut achieved complete polyp removal 96% of the time, compared with 95% of the time when using Forced Coagulation (P = .267). Piecemeal resection rates were similar, at 90% and 87% for Endocut Q and Forced Coagulation, respectively (P = .270).
Although Endocut Q less frequently resulted in small residual tissue islands after initial snare resection (35% vs. 41%; P = .041), it more often caused intraprocedural bleeding that required treatment (17% vs. 11%; P = .006).
According to Dr. Pohl and colleagues, previous discussions have included concerns that such bleeding may impair visualization and therefore lead to higher rates of polyp recurrence; but surveillance colonoscopy, which was performed in 79% of patients, revealed a polyp recurrence rate of 17% for each group.
“Although we did not find a difference in recurrence between the two groups, our study cannot completely exclude this possibility,” the investigators added.
They also noted that six perforations occurred in the Endocut Q group, compared with three in the Forced Coagulation group, and suggested that this risk may be real, yet statistically unsupported by the present analysis because of sample size.
“Endoscopists using Endocut should therefore be aware of this potential risk and [ensure] that no muscularis propria is entrapped in the snare before electrosurgery is applied,” the investigators wrote.
Still, the investigators’ final conclusion supported the existing method of decision-making: personal choice.
“Overall, polyp resection with Endocut or Forced Coagulation did not differ with respect to severe adverse events, complete resection rate, or polyp recurrence,” they wrote. “This study therefore supports an individual approach based on endoscopist preference.”
The study was funded by Boston Scientific and the American College of Gastroenterology. The investigators disclosed additional relationships with Medtronic, Olympus, Cook Endoscopy, and others.
SOURCE: Pohl H et al. Gastroenterology. 2020 Mar 12. doi: 10.1053/j.gastro.2020.03.014.
FROM GASTROENTEROLOGY
FibroScan: M probe underestimates hepatic fat content
When performing transient elastography (FibroScan) to evaluate patients for hepatic steatosis, using an M probe instead of an XL probe may significantly underestimate hepatic fat content, according to investigators.
The findings, which were independent of body weight, suggest that probe-specific controlled attenuation parameter (CAP) thresholds are needed to accurately interpret FibroScan results, reported lead author Cyrielle Caussy, MD, PhD, of the University of California, San Diego, and colleagues.
“We have previously determined the optimal threshold of CAP using either [an] M or XL probe for the detection of ... nonalcoholic fatty liver disease (NAFLD),” the investigators wrote in Clinical Gastroenterology and Hepatology. “However, head-to-head comparison of consecutive measurements of CAP with both the M and XL probes versus MRI-PDFF [proton density fat fraction] ... has not been reported yet.”
Dr. Caussy and colleagues set out to do just that. They enrolled 105 individuals with and without NAFLD who had a mean body mass index (BMI) of 30.6 kg/m2, as this represented a typical population screened for NAFLD. After evaluation for other causes of hepatic steatosis and liver disease, participants underwent MRI-PDFF, which served as a gold standard, followed by FibroScan using both M and XL probes on the same day.
The primary outcome was hepatic steatosis (MRI-PDFF of at least 5%), while the secondary outcome was MRI-PDFF–detected hepatic fat content of at least 10%, the latter of which has been “used in several therapeutic trials as inclusion criteria,” the investigators noted.
A total of 100 participants were included in the final analysis, of whom two-thirds (66%) underwent MRI and FibroScan on the same day, with a mean interval between test types of 11 days. Most participants (68%) had an MRI-PDFF of at least 5%, while almost half (48%) exceeded an MRI-PDFF of 10%.
The mean CAP measurement with the M probe was 310 dB/m, which was significantly lower than the mean value detected by the XL probe, which was 317 dB/m (P = .007). In participants with hepatic steatosis, when the M probe was used for those with a BMI of less than 30, and the XL probe was used for those with a BMI of 30 or more, the M probe still provided a significantly lower measure of hepatic fat content (312 vs. 345 dB/m; P = .0035).
“[T]hese results have direct application in routine clinical practice,” the investigators wrote, “as [they] will help clinicians interpreting CAP measurements depending on the type of probe used.”
Dr. Caussy and colleagues went on to offer a diagnostic algorithm involving optimal probe-specific thresholds for CAP based on hepatic fat content. Individuals screened with an M probe who have a CAP of 294 dB/m or more should be considered positive for NAFLD, while patients screened with an XL probe need to have a CAP of at least 307 dB/m to be NAFLD positive.
For the XL probe, but not the M probe, diagnostic accuracy depended upon an interquartile range of less than 30 dB/m. The investigators noted that this finding should alter the interpretation of a 2019 study by Eddowes and colleagues, which concluded that interquartile range was unrelated to diagnostic accuracy.
“As Eddowes et al. did not perform head-to-head comparison of CAP measurement with both the M and XL probes, this important difference could not have been observed,” the investigators wrote, noting that “an interquartile range of CAP below 30 dB/m should be considered as a quality indicator that significantly improves the diagnostic performance of CAP using the XL probe for the detection of hepatic steatosis in NAFLD.”
The investigators concluded by suggesting that their findings will drive research forward.
“The use of these new thresholds will help to further assess the clinical utility of CAP for the detection of hepatic steatosis and its cost-effectiveness, compared with other modalities, to develop optimal strategies for the screening of NAFLD,” they wrote.
The study was funded by Atlantic Philanthropies, the John A. Hartford Foundation, the American Gastroenterological Association, and others. The investigators disclosed no conflicts of interest.
SOURCE: Caussy C et al. Clin Gastro Hepatol. 2019 Dec 13. doi: 10.1016/j.cgh.2019.11.060.
When performing transient elastography (FibroScan) to evaluate patients for hepatic steatosis, using an M probe instead of an XL probe may significantly underestimate hepatic fat content, according to investigators.
The findings, which were independent of body weight, suggest that probe-specific controlled attenuation parameter (CAP) thresholds are needed to accurately interpret FibroScan results, reported lead author Cyrielle Caussy, MD, PhD, of the University of California, San Diego, and colleagues.
“We have previously determined the optimal threshold of CAP using either [an] M or XL probe for the detection of ... nonalcoholic fatty liver disease (NAFLD),” the investigators wrote in Clinical Gastroenterology and Hepatology. “However, head-to-head comparison of consecutive measurements of CAP with both the M and XL probes versus MRI-PDFF [proton density fat fraction] ... has not been reported yet.”
Dr. Caussy and colleagues set out to do just that. They enrolled 105 individuals with and without NAFLD who had a mean body mass index (BMI) of 30.6 kg/m2, as this represented a typical population screened for NAFLD. After evaluation for other causes of hepatic steatosis and liver disease, participants underwent MRI-PDFF, which served as a gold standard, followed by FibroScan using both M and XL probes on the same day.
The primary outcome was hepatic steatosis (MRI-PDFF of at least 5%), while the secondary outcome was MRI-PDFF–detected hepatic fat content of at least 10%, the latter of which has been “used in several therapeutic trials as inclusion criteria,” the investigators noted.
A total of 100 participants were included in the final analysis, of whom two-thirds (66%) underwent MRI and FibroScan on the same day, with a mean interval between test types of 11 days. Most participants (68%) had an MRI-PDFF of at least 5%, while almost half (48%) exceeded an MRI-PDFF of 10%.
The mean CAP measurement with the M probe was 310 dB/m, which was significantly lower than the mean value detected by the XL probe, which was 317 dB/m (P = .007). In participants with hepatic steatosis, when the M probe was used for those with a BMI of less than 30, and the XL probe was used for those with a BMI of 30 or more, the M probe still provided a significantly lower measure of hepatic fat content (312 vs. 345 dB/m; P = .0035).
“[T]hese results have direct application in routine clinical practice,” the investigators wrote, “as [they] will help clinicians interpreting CAP measurements depending on the type of probe used.”
Dr. Caussy and colleagues went on to offer a diagnostic algorithm involving optimal probe-specific thresholds for CAP based on hepatic fat content. Individuals screened with an M probe who have a CAP of 294 dB/m or more should be considered positive for NAFLD, while patients screened with an XL probe need to have a CAP of at least 307 dB/m to be NAFLD positive.
For the XL probe, but not the M probe, diagnostic accuracy depended upon an interquartile range of less than 30 dB/m. The investigators noted that this finding should alter the interpretation of a 2019 study by Eddowes and colleagues, which concluded that interquartile range was unrelated to diagnostic accuracy.
“As Eddowes et al. did not perform head-to-head comparison of CAP measurement with both the M and XL probes, this important difference could not have been observed,” the investigators wrote, noting that “an interquartile range of CAP below 30 dB/m should be considered as a quality indicator that significantly improves the diagnostic performance of CAP using the XL probe for the detection of hepatic steatosis in NAFLD.”
The investigators concluded by suggesting that their findings will drive research forward.
“The use of these new thresholds will help to further assess the clinical utility of CAP for the detection of hepatic steatosis and its cost-effectiveness, compared with other modalities, to develop optimal strategies for the screening of NAFLD,” they wrote.
The study was funded by Atlantic Philanthropies, the John A. Hartford Foundation, the American Gastroenterological Association, and others. The investigators disclosed no conflicts of interest.
SOURCE: Caussy C et al. Clin Gastro Hepatol. 2019 Dec 13. doi: 10.1016/j.cgh.2019.11.060.
When performing transient elastography (FibroScan) to evaluate patients for hepatic steatosis, using an M probe instead of an XL probe may significantly underestimate hepatic fat content, according to investigators.
The findings, which were independent of body weight, suggest that probe-specific controlled attenuation parameter (CAP) thresholds are needed to accurately interpret FibroScan results, reported lead author Cyrielle Caussy, MD, PhD, of the University of California, San Diego, and colleagues.
“We have previously determined the optimal threshold of CAP using either [an] M or XL probe for the detection of ... nonalcoholic fatty liver disease (NAFLD),” the investigators wrote in Clinical Gastroenterology and Hepatology. “However, head-to-head comparison of consecutive measurements of CAP with both the M and XL probes versus MRI-PDFF [proton density fat fraction] ... has not been reported yet.”
Dr. Caussy and colleagues set out to do just that. They enrolled 105 individuals with and without NAFLD who had a mean body mass index (BMI) of 30.6 kg/m2, as this represented a typical population screened for NAFLD. After evaluation for other causes of hepatic steatosis and liver disease, participants underwent MRI-PDFF, which served as a gold standard, followed by FibroScan using both M and XL probes on the same day.
The primary outcome was hepatic steatosis (MRI-PDFF of at least 5%), while the secondary outcome was MRI-PDFF–detected hepatic fat content of at least 10%, the latter of which has been “used in several therapeutic trials as inclusion criteria,” the investigators noted.
A total of 100 participants were included in the final analysis, of whom two-thirds (66%) underwent MRI and FibroScan on the same day, with a mean interval between test types of 11 days. Most participants (68%) had an MRI-PDFF of at least 5%, while almost half (48%) exceeded an MRI-PDFF of 10%.
The mean CAP measurement with the M probe was 310 dB/m, which was significantly lower than the mean value detected by the XL probe, which was 317 dB/m (P = .007). In participants with hepatic steatosis, when the M probe was used for those with a BMI of less than 30, and the XL probe was used for those with a BMI of 30 or more, the M probe still provided a significantly lower measure of hepatic fat content (312 vs. 345 dB/m; P = .0035).
“[T]hese results have direct application in routine clinical practice,” the investigators wrote, “as [they] will help clinicians interpreting CAP measurements depending on the type of probe used.”
Dr. Caussy and colleagues went on to offer a diagnostic algorithm involving optimal probe-specific thresholds for CAP based on hepatic fat content. Individuals screened with an M probe who have a CAP of 294 dB/m or more should be considered positive for NAFLD, while patients screened with an XL probe need to have a CAP of at least 307 dB/m to be NAFLD positive.
For the XL probe, but not the M probe, diagnostic accuracy depended upon an interquartile range of less than 30 dB/m. The investigators noted that this finding should alter the interpretation of a 2019 study by Eddowes and colleagues, which concluded that interquartile range was unrelated to diagnostic accuracy.
“As Eddowes et al. did not perform head-to-head comparison of CAP measurement with both the M and XL probes, this important difference could not have been observed,” the investigators wrote, noting that “an interquartile range of CAP below 30 dB/m should be considered as a quality indicator that significantly improves the diagnostic performance of CAP using the XL probe for the detection of hepatic steatosis in NAFLD.”
The investigators concluded by suggesting that their findings will drive research forward.
“The use of these new thresholds will help to further assess the clinical utility of CAP for the detection of hepatic steatosis and its cost-effectiveness, compared with other modalities, to develop optimal strategies for the screening of NAFLD,” they wrote.
The study was funded by Atlantic Philanthropies, the John A. Hartford Foundation, the American Gastroenterological Association, and others. The investigators disclosed no conflicts of interest.
SOURCE: Caussy C et al. Clin Gastro Hepatol. 2019 Dec 13. doi: 10.1016/j.cgh.2019.11.060.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
‘Hospital at home’ cuts ED visits and costs for cancer patients
Visits to the emergency department (ED) and hospitalizations are often frequent occurrences for cancer patients, but what if the “hospital” could be brought into the home instead?
A new American cohort study provides evidence that this can be a workable option for cancer patients. The authors report improved patient outcomes, with 56% lower odds of unplanned hospitalizations (P = .001), 45% lower odds of ED visits (P = .037), and 50% lower cumulative charges (P = .001), as compared with patients who received usual care.
“The oncology hospital-at-home model of care that extends acute-level care to the patient at home offers promise in addressing a long-term gap in cancer care service delivery,” said lead author Kathi Mooney, PhD, RN, interim senior director of population sciences at the Huntsman Cancer Institute and distinguished professor of nursing at the University of Utah, Salt Lake City. “In light of the current global pandemic, we are compelled to consider new ways to provide cancer care, and the oncology hospital-at-home model is on point to address critical elements of an improved cancer care delivery system.”
Mooney presented the findings during the virtual scientific program of the American Society of Clinical Oncology 2020 annual meeting (abstract 7000).
The hospital-at-home model of care provides hospital-level care in the comfort of the patient’s home and is a component of many healthcare systems worldwide. Although it was introduced in the United States more than 2 decades ago, it has not been widely adopted or studied specifically in oncology.
Most cancer treatment is provided on an outpatient basis, which means that patients experience significant adverse events, toxicities, and disease progression while they are at home. Thus, Mooney noted, patients tend to rely heavily on the ED and sometimes experience unplanned hospitalizations and 30-day readmissions.
“These care patterns are distressing to the patients and their families and tax healthcare resources,” she said. “They are even more concerning and salient as we endeavor to protect cancer patients and provide cancer care during a pandemic.”
Currently, strategies to evaluate and support cancer patients and caregivers at home are limited. In 2018, the Huntsman Cancer Institute implemented Huntsman at Home, a demonstration project to evaluate the utility of an oncology hospital-at-home model.
Significantly Fewer Unplanned Hospitalizations
Huntsman at Home is run by nurse practitioner and registered nurse teams who deliver acute-level care at home. Physicians provide backup support for both medical oncology and palliative care. Nurse practitioners also work directly with the patient’s oncology team to coordinate care needs, including services such as social work and physical therapy.
To evaluate the hospital-at-home model, Mooney and colleagues compared patients who were enrolled in the program with those who received usual care. The usual-care comparison group was drawn from patients who lived in the Salt Lake City area. These patients would have qualified for enrollment in the Huntsman at Home program, but they lived outside the 20-mile service area.
The cohort included 367 patients (169 Huntsman at Home patients and 198 usual-care patients). Of those patients, 77% had stage IV cancer. A range of cancer types was represented; the most common were colon, gynecologic, prostate, and lung cancers. As compared to the usual-care group, those in the home model were more likely to be women (61% vs 43%).
During the first 30 days after admission, Huntsman at Home patients had significantly fewer unplanned hospitalizations (19.5% vs 35.4%) and a shorter length of stay (1.4 vs 2.6 days). Their care was also less expensive. The estimated charges for the hospital-at-home patients was $10,238, compared with $21,363 for the usual-care patients. There was no real difference in stays in the intensive care unit between the two groups.
Mooney noted that since there have been few studies of the hospital-at-home model for oncology patients, the investigators’ initial focus was on patients at hospital discharge who needed continued acute-level care and those who had acute problems identified through their oncology care clinic. Therefore, patients were not admitted to the program directly from emergency services, and chemotherapy infusions were not provided, although these are “other areas to consider in an oncology hospital-at-home model.”
Other limitations of the study were that it was not a randomized trial, and the evaluation was from a single program located at one comprehensive cancer center.
“These findings provide the oncology community with an opportunity to rethink cancer care as solely hospital- and clinic-based and instead reimagine care delivery that moves with the patient with key components provided at home,” said Mooney. “We plan to continue the development and evaluation of Huntsman at Home and extend care to admission from the emergency department.”
She added that, together with Flatiron Health, they are validating a tool to prospectively predict, on the basis of the likelihood of ED use, which patients may benefit from Huntsman at Home support. They also plan to extend care to patients who live at a distance from the cancer center and in rural communities, and may include chemotherapy infusion services.
Palliative Care Patients Prefer Home-Based Treatment
In a discussion of the paper, Lynne Wagner, PhD, a professor in the Department of Social Sciences and Health Policy with the Wake Forest School of Medicine, Winston-Salem, North Carolina, and a member of the Wake Forest Baptist Comprehensive Cancer Center, explained that some “aspects of healthcare are more translatable to a virtual or alternative delivery model than others. An area of cancer care greatly in need of innovation and quality improvement pertains to the management of oncologic emergencies.”
She pointed out that optimal care for oncologic emergencies requires the “intersection of oncology and emergency medicine specialists,” but there are often no well-defined processes for care coordination in place.
“Emergency department utilization could be reduced through greater precision with regard to risk stratification and early intervention and improved outpatient management, including improved symptom management,” said Wagner.
Wagner suggested that research should incorporate patient-reported outcomes so as to measure patient-centered benefits of home-based care. “Patients who are receiving palliative care services prefer home-based care, and it’s reasonable to anticipate this finding would extrapolate to the investigator’s target population,” she said. “However, there may also be unanticipated consequences, potentially including increased anxiety or increased burden on caretakers.”
In addition, the tangible and intangible costs associated with traveling to receive healthcare services and time away from work can be reduced with home-based care, and this should also be quantified. “The costs associated with COVID infection should be estimated to realize the full economic value of this care model, given significant reductions in cohort exposure afforded by home-based visits,” Wagner added.
The Huntsman at Home program is funded by the Huntsman Cancer Institute. The evaluation was funded by the Cambia Health Foundation. Mooney has a consulting or advisory role with Cognitive Medical System, Inc, and has patents, royalties, and other intellectual property for the development of Symptom Care at Home, a remote symptom-monitoring platform developed through research grants funded by the National Cancer Institute. No royalties have been received to date. Wagner has relationships with Celgene, Eli Lilly, Gilead Sciences, and Johnson & Johnson.
This article first appeared on Medscape.com.
Visits to the emergency department (ED) and hospitalizations are often frequent occurrences for cancer patients, but what if the “hospital” could be brought into the home instead?
A new American cohort study provides evidence that this can be a workable option for cancer patients. The authors report improved patient outcomes, with 56% lower odds of unplanned hospitalizations (P = .001), 45% lower odds of ED visits (P = .037), and 50% lower cumulative charges (P = .001), as compared with patients who received usual care.
“The oncology hospital-at-home model of care that extends acute-level care to the patient at home offers promise in addressing a long-term gap in cancer care service delivery,” said lead author Kathi Mooney, PhD, RN, interim senior director of population sciences at the Huntsman Cancer Institute and distinguished professor of nursing at the University of Utah, Salt Lake City. “In light of the current global pandemic, we are compelled to consider new ways to provide cancer care, and the oncology hospital-at-home model is on point to address critical elements of an improved cancer care delivery system.”
Mooney presented the findings during the virtual scientific program of the American Society of Clinical Oncology 2020 annual meeting (abstract 7000).
The hospital-at-home model of care provides hospital-level care in the comfort of the patient’s home and is a component of many healthcare systems worldwide. Although it was introduced in the United States more than 2 decades ago, it has not been widely adopted or studied specifically in oncology.
Most cancer treatment is provided on an outpatient basis, which means that patients experience significant adverse events, toxicities, and disease progression while they are at home. Thus, Mooney noted, patients tend to rely heavily on the ED and sometimes experience unplanned hospitalizations and 30-day readmissions.
“These care patterns are distressing to the patients and their families and tax healthcare resources,” she said. “They are even more concerning and salient as we endeavor to protect cancer patients and provide cancer care during a pandemic.”
Currently, strategies to evaluate and support cancer patients and caregivers at home are limited. In 2018, the Huntsman Cancer Institute implemented Huntsman at Home, a demonstration project to evaluate the utility of an oncology hospital-at-home model.
Significantly Fewer Unplanned Hospitalizations
Huntsman at Home is run by nurse practitioner and registered nurse teams who deliver acute-level care at home. Physicians provide backup support for both medical oncology and palliative care. Nurse practitioners also work directly with the patient’s oncology team to coordinate care needs, including services such as social work and physical therapy.
To evaluate the hospital-at-home model, Mooney and colleagues compared patients who were enrolled in the program with those who received usual care. The usual-care comparison group was drawn from patients who lived in the Salt Lake City area. These patients would have qualified for enrollment in the Huntsman at Home program, but they lived outside the 20-mile service area.
The cohort included 367 patients (169 Huntsman at Home patients and 198 usual-care patients). Of those patients, 77% had stage IV cancer. A range of cancer types was represented; the most common were colon, gynecologic, prostate, and lung cancers. As compared to the usual-care group, those in the home model were more likely to be women (61% vs 43%).
During the first 30 days after admission, Huntsman at Home patients had significantly fewer unplanned hospitalizations (19.5% vs 35.4%) and a shorter length of stay (1.4 vs 2.6 days). Their care was also less expensive. The estimated charges for the hospital-at-home patients was $10,238, compared with $21,363 for the usual-care patients. There was no real difference in stays in the intensive care unit between the two groups.
Mooney noted that since there have been few studies of the hospital-at-home model for oncology patients, the investigators’ initial focus was on patients at hospital discharge who needed continued acute-level care and those who had acute problems identified through their oncology care clinic. Therefore, patients were not admitted to the program directly from emergency services, and chemotherapy infusions were not provided, although these are “other areas to consider in an oncology hospital-at-home model.”
Other limitations of the study were that it was not a randomized trial, and the evaluation was from a single program located at one comprehensive cancer center.
“These findings provide the oncology community with an opportunity to rethink cancer care as solely hospital- and clinic-based and instead reimagine care delivery that moves with the patient with key components provided at home,” said Mooney. “We plan to continue the development and evaluation of Huntsman at Home and extend care to admission from the emergency department.”
She added that, together with Flatiron Health, they are validating a tool to prospectively predict, on the basis of the likelihood of ED use, which patients may benefit from Huntsman at Home support. They also plan to extend care to patients who live at a distance from the cancer center and in rural communities, and may include chemotherapy infusion services.
Palliative Care Patients Prefer Home-Based Treatment
In a discussion of the paper, Lynne Wagner, PhD, a professor in the Department of Social Sciences and Health Policy with the Wake Forest School of Medicine, Winston-Salem, North Carolina, and a member of the Wake Forest Baptist Comprehensive Cancer Center, explained that some “aspects of healthcare are more translatable to a virtual or alternative delivery model than others. An area of cancer care greatly in need of innovation and quality improvement pertains to the management of oncologic emergencies.”
She pointed out that optimal care for oncologic emergencies requires the “intersection of oncology and emergency medicine specialists,” but there are often no well-defined processes for care coordination in place.
“Emergency department utilization could be reduced through greater precision with regard to risk stratification and early intervention and improved outpatient management, including improved symptom management,” said Wagner.
Wagner suggested that research should incorporate patient-reported outcomes so as to measure patient-centered benefits of home-based care. “Patients who are receiving palliative care services prefer home-based care, and it’s reasonable to anticipate this finding would extrapolate to the investigator’s target population,” she said. “However, there may also be unanticipated consequences, potentially including increased anxiety or increased burden on caretakers.”
In addition, the tangible and intangible costs associated with traveling to receive healthcare services and time away from work can be reduced with home-based care, and this should also be quantified. “The costs associated with COVID infection should be estimated to realize the full economic value of this care model, given significant reductions in cohort exposure afforded by home-based visits,” Wagner added.
The Huntsman at Home program is funded by the Huntsman Cancer Institute. The evaluation was funded by the Cambia Health Foundation. Mooney has a consulting or advisory role with Cognitive Medical System, Inc, and has patents, royalties, and other intellectual property for the development of Symptom Care at Home, a remote symptom-monitoring platform developed through research grants funded by the National Cancer Institute. No royalties have been received to date. Wagner has relationships with Celgene, Eli Lilly, Gilead Sciences, and Johnson & Johnson.
This article first appeared on Medscape.com.
Visits to the emergency department (ED) and hospitalizations are often frequent occurrences for cancer patients, but what if the “hospital” could be brought into the home instead?
A new American cohort study provides evidence that this can be a workable option for cancer patients. The authors report improved patient outcomes, with 56% lower odds of unplanned hospitalizations (P = .001), 45% lower odds of ED visits (P = .037), and 50% lower cumulative charges (P = .001), as compared with patients who received usual care.
“The oncology hospital-at-home model of care that extends acute-level care to the patient at home offers promise in addressing a long-term gap in cancer care service delivery,” said lead author Kathi Mooney, PhD, RN, interim senior director of population sciences at the Huntsman Cancer Institute and distinguished professor of nursing at the University of Utah, Salt Lake City. “In light of the current global pandemic, we are compelled to consider new ways to provide cancer care, and the oncology hospital-at-home model is on point to address critical elements of an improved cancer care delivery system.”
Mooney presented the findings during the virtual scientific program of the American Society of Clinical Oncology 2020 annual meeting (abstract 7000).
The hospital-at-home model of care provides hospital-level care in the comfort of the patient’s home and is a component of many healthcare systems worldwide. Although it was introduced in the United States more than 2 decades ago, it has not been widely adopted or studied specifically in oncology.
Most cancer treatment is provided on an outpatient basis, which means that patients experience significant adverse events, toxicities, and disease progression while they are at home. Thus, Mooney noted, patients tend to rely heavily on the ED and sometimes experience unplanned hospitalizations and 30-day readmissions.
“These care patterns are distressing to the patients and their families and tax healthcare resources,” she said. “They are even more concerning and salient as we endeavor to protect cancer patients and provide cancer care during a pandemic.”
Currently, strategies to evaluate and support cancer patients and caregivers at home are limited. In 2018, the Huntsman Cancer Institute implemented Huntsman at Home, a demonstration project to evaluate the utility of an oncology hospital-at-home model.
Significantly Fewer Unplanned Hospitalizations
Huntsman at Home is run by nurse practitioner and registered nurse teams who deliver acute-level care at home. Physicians provide backup support for both medical oncology and palliative care. Nurse practitioners also work directly with the patient’s oncology team to coordinate care needs, including services such as social work and physical therapy.
To evaluate the hospital-at-home model, Mooney and colleagues compared patients who were enrolled in the program with those who received usual care. The usual-care comparison group was drawn from patients who lived in the Salt Lake City area. These patients would have qualified for enrollment in the Huntsman at Home program, but they lived outside the 20-mile service area.
The cohort included 367 patients (169 Huntsman at Home patients and 198 usual-care patients). Of those patients, 77% had stage IV cancer. A range of cancer types was represented; the most common were colon, gynecologic, prostate, and lung cancers. As compared to the usual-care group, those in the home model were more likely to be women (61% vs 43%).
During the first 30 days after admission, Huntsman at Home patients had significantly fewer unplanned hospitalizations (19.5% vs 35.4%) and a shorter length of stay (1.4 vs 2.6 days). Their care was also less expensive. The estimated charges for the hospital-at-home patients was $10,238, compared with $21,363 for the usual-care patients. There was no real difference in stays in the intensive care unit between the two groups.
Mooney noted that since there have been few studies of the hospital-at-home model for oncology patients, the investigators’ initial focus was on patients at hospital discharge who needed continued acute-level care and those who had acute problems identified through their oncology care clinic. Therefore, patients were not admitted to the program directly from emergency services, and chemotherapy infusions were not provided, although these are “other areas to consider in an oncology hospital-at-home model.”
Other limitations of the study were that it was not a randomized trial, and the evaluation was from a single program located at one comprehensive cancer center.
“These findings provide the oncology community with an opportunity to rethink cancer care as solely hospital- and clinic-based and instead reimagine care delivery that moves with the patient with key components provided at home,” said Mooney. “We plan to continue the development and evaluation of Huntsman at Home and extend care to admission from the emergency department.”
She added that, together with Flatiron Health, they are validating a tool to prospectively predict, on the basis of the likelihood of ED use, which patients may benefit from Huntsman at Home support. They also plan to extend care to patients who live at a distance from the cancer center and in rural communities, and may include chemotherapy infusion services.
Palliative Care Patients Prefer Home-Based Treatment
In a discussion of the paper, Lynne Wagner, PhD, a professor in the Department of Social Sciences and Health Policy with the Wake Forest School of Medicine, Winston-Salem, North Carolina, and a member of the Wake Forest Baptist Comprehensive Cancer Center, explained that some “aspects of healthcare are more translatable to a virtual or alternative delivery model than others. An area of cancer care greatly in need of innovation and quality improvement pertains to the management of oncologic emergencies.”
She pointed out that optimal care for oncologic emergencies requires the “intersection of oncology and emergency medicine specialists,” but there are often no well-defined processes for care coordination in place.
“Emergency department utilization could be reduced through greater precision with regard to risk stratification and early intervention and improved outpatient management, including improved symptom management,” said Wagner.
Wagner suggested that research should incorporate patient-reported outcomes so as to measure patient-centered benefits of home-based care. “Patients who are receiving palliative care services prefer home-based care, and it’s reasonable to anticipate this finding would extrapolate to the investigator’s target population,” she said. “However, there may also be unanticipated consequences, potentially including increased anxiety or increased burden on caretakers.”
In addition, the tangible and intangible costs associated with traveling to receive healthcare services and time away from work can be reduced with home-based care, and this should also be quantified. “The costs associated with COVID infection should be estimated to realize the full economic value of this care model, given significant reductions in cohort exposure afforded by home-based visits,” Wagner added.
The Huntsman at Home program is funded by the Huntsman Cancer Institute. The evaluation was funded by the Cambia Health Foundation. Mooney has a consulting or advisory role with Cognitive Medical System, Inc, and has patents, royalties, and other intellectual property for the development of Symptom Care at Home, a remote symptom-monitoring platform developed through research grants funded by the National Cancer Institute. No royalties have been received to date. Wagner has relationships with Celgene, Eli Lilly, Gilead Sciences, and Johnson & Johnson.
This article first appeared on Medscape.com.
FROM ASCO 2020
Addressing suicide prevention among South Asian Americans
Multifaceted strategies are needed to address unique cultural factors
On first glance, the age-adjusted rate of suicide for Asian and Pacific Islander populations living in the United States looks comparatively low.
Over the past 2 decades in the United States, for example, the overall rate increased by 35%, from, 10.5 to 14.2 per 100,000 individuals. That compares with a rate of 7.0 per 100,000 among Asian and Pacific Islander communities.1
However, because of the aggregate nature (national suicide mortality data combine people of Asian, Native Hawaiian, and other Pacific Islander descent into a single group) in which these data are reported, a significant amount of salient information on subgroups of Asian Americans is lost.2 There is a growing body of research on the mental health of Asian Americans, but the dearth of information and research on suicide in South Asians is striking.3 In fact, a review of literature finds fewer than 10 articles on the topic that have been published in peer-reviewed journals in the last decade. to provide effective, culturally sensitive care.
Diverse group
There are 3.4 million individuals of South Asian descent in the United States. Geographically, South Asians may have familial and cultural/historical roots in Bangladesh, Bhutan, India, Maldives, Nepal, and Pakistan.4 They enjoy a rich diversity in terms of cultural and religious beliefs, language, socioeconomic status, modes of acculturation, and immigration patterns. Asian Indians are the largest group of South Asians in the United States. They are highly educated, with a larger proportion of them pursuing an undergraduate and/or graduate level education than the general population. The median household income of Asian Indians is also higher than the national average.5
In general, suicide, like all mental health issues, is a stigmatized and taboo topic in the South Asian community.6 Also, South Asian Americans are hesitant to seek mental health care because of a perceived inability of Western health care professionals to understand their cultural views. Extrapolation from data on South Asians in the United Kingdom, aggregate statistics for Asian Americans and Pacific Islanders, and studies on South Asians in the United States highlight two South Asian subgroups that are particularly vulnerable to suicide. These are young adults (aged 18-24 years) and women.7
Suicide is the second-leading cause of death for young Asian American men in the United States. Rates of lifetime suicidal ideation and attempts are higher among younger Asian Americans (aged 18-24 years) than among older Asian American adults. Young Asian American adults have been found to have higher levels of suicidal ideation than their white counterparts.8,9 Acculturation or assimilating into a different culture, familial violence as a child, hopelessness or a thought pattern with a pessimistic outlook, depression, and childhood sexual abuse have all been found to be positively correlated with suicidal ideation and attempted suicide in South Asian Americans. One study that conducted0 in-group analysis on undergraduate university students of South Asian descent living in New York found higher levels of hopelessness and depression in Asian Indians relative to Bangladeshi or Pakistani Americans.10
In addition, higher levels of suicidal ideation are reported in Asian Indians relative to Bangladeshi or Pakistani Americans. These results resemble findings from similar studies in the United Kingdom. A posited reason for these findings is a difference in religious beliefs. Pakistani and Bangladeshi Americans are predominantly Muslim, have stronger moral beliefs against suicide, and consider it a sin as defined by Islamic beliefs. Asian Indians, in contrast, are majority Hindu and believe in reincarnation – a context that might make suicide seem more permissible.11
South Asian women are particularly vulnerable to domestic violence, childhood sexual abuse, intimate partner violence, and/or familial violence. Cultural gender norms, traditional norms, and patriarchal ideology in the South Asian community make quantifying the level of childhood sexual abuse and familial violence a challenge. Furthermore, culturally, South Asian women are often considered subordinate relative to men, and discussion around family violence and childhood sexual abuse is avoided. Studies from the United Kingdom find a lack of knowledge around, disclosure of, and fear of reporting childhood sexual abuse in South Asian women. A study of a sample of representative South Asian American women found that 25.2% had experienced some form of childhood sexual abuse.12
Research also suggests that South Asians in the United States have some of the highest rates of intimate partner violence. Another study in the United States found that two out of five South Asian women have experienced physical and/or sexual intimate partner violence. This is much higher than the rate found in representative general U.S. population samples.
Literature suggests that exposure to these factors increases womens’ risk for suicidal ideation and attempted suicide. In the United Kingdom, research on South Asian women (aged 18-24 years) has found rates of attempted suicide to be three times higher than those of their white counterparts. Research from the United Kingdom and the United States suggests that younger married South Asian women are exposed to emotional and/or physical abuse from their spouse or in-laws, which is often a mediating factor in their increased risk for suicide.
Attempts to address suicide in the South Asian American community have to be multifaceted. An ideal approach would consist of educating, and connecting with, the community through ethnic media and trusted community sources, such as primary care doctors, caregivers, and social workers. In line with established American Psychological Association guidelines on caring for individuals of immigrant origin, health care professionals should document the patient’s number of generations in the country, number of years in the country, language fluency, family and community support, educational level, social status changes related to immigration, intimate relationships with people of different backgrounds, and stress related to acculturation. Special attention should be paid to South Asian women. Health care professionals should screen South Asian women for past and current intimate partner violence, provide culturally appropriate intimate partner violence resources, and be prepared to refer them to legal counseling services. Also, South Asian women should be screened for a history of exposure to familial violence and childhood sexual abuse.1
To adequately serve this population, there is a need to build capacity in the provision of culturally appropriate mental health services. Access to mental health care professionals through settings such as shelters for abused women, South Asian community–based organizations, youth centers, college counseling, and senior centers would encourage individuals to seek care without the threat of being stigmatized.
References
1. Hedegaard H et al. Suicide mortality in the United States, 1999–2017. NCHS Data Brief, No. 330. 2018 Nov.
2. Ahmad-Stout DJ and Nath SR. J College Stud Psychother. 2013 Jan 10;27(1):43-61.
3. Li H and Keshavan M. Asian J Psychiatry. 2011;4(1):1.
4. Nagaraj NC et al. J Immigr Minor Health. 2019 Oct;21(5):978-1003.
5. Nagaraj NC et al. J Comm Health. 2018;43(3):543-51.
6. Cao KO. Generations. 2014;30(4):82-5.
7. Hurwitz EJ et al. J Immigr Minor Health. 2006;8(3):251-61.
8. Polanco-Roman L et al. Cultur Divers Ethnic Minor Psychol. 2019 Dec 23. doi: 10.1037/cpd0000313.
9. Erausquin JT et al. J Youth Adolesc. 2019 Sep;48(9):1796-1805.
10. Lane R et al. Asian Am J Psychol. 2016;7(2):120-8.
11. Nath SR et al. Asian Am J Psychol. 2018;9(4):334-343.
12. Robertson HA et al. J Immigr Minor Health. 2016 Jul 31;18(4):921-7.
Mr. Kaleka is a medical student in the class of 2021 at Central Michigan University (CMU) College of Medicine, Mt. Pleasant. He has no disclosures. Mr. Kaleka would like to thank his mentor, Furhut Janssen, DO, for her continued guidance and support in research on mental health in immigrant populations.
Multifaceted strategies are needed to address unique cultural factors
Multifaceted strategies are needed to address unique cultural factors
On first glance, the age-adjusted rate of suicide for Asian and Pacific Islander populations living in the United States looks comparatively low.
Over the past 2 decades in the United States, for example, the overall rate increased by 35%, from, 10.5 to 14.2 per 100,000 individuals. That compares with a rate of 7.0 per 100,000 among Asian and Pacific Islander communities.1
However, because of the aggregate nature (national suicide mortality data combine people of Asian, Native Hawaiian, and other Pacific Islander descent into a single group) in which these data are reported, a significant amount of salient information on subgroups of Asian Americans is lost.2 There is a growing body of research on the mental health of Asian Americans, but the dearth of information and research on suicide in South Asians is striking.3 In fact, a review of literature finds fewer than 10 articles on the topic that have been published in peer-reviewed journals in the last decade. to provide effective, culturally sensitive care.
Diverse group
There are 3.4 million individuals of South Asian descent in the United States. Geographically, South Asians may have familial and cultural/historical roots in Bangladesh, Bhutan, India, Maldives, Nepal, and Pakistan.4 They enjoy a rich diversity in terms of cultural and religious beliefs, language, socioeconomic status, modes of acculturation, and immigration patterns. Asian Indians are the largest group of South Asians in the United States. They are highly educated, with a larger proportion of them pursuing an undergraduate and/or graduate level education than the general population. The median household income of Asian Indians is also higher than the national average.5
In general, suicide, like all mental health issues, is a stigmatized and taboo topic in the South Asian community.6 Also, South Asian Americans are hesitant to seek mental health care because of a perceived inability of Western health care professionals to understand their cultural views. Extrapolation from data on South Asians in the United Kingdom, aggregate statistics for Asian Americans and Pacific Islanders, and studies on South Asians in the United States highlight two South Asian subgroups that are particularly vulnerable to suicide. These are young adults (aged 18-24 years) and women.7
Suicide is the second-leading cause of death for young Asian American men in the United States. Rates of lifetime suicidal ideation and attempts are higher among younger Asian Americans (aged 18-24 years) than among older Asian American adults. Young Asian American adults have been found to have higher levels of suicidal ideation than their white counterparts.8,9 Acculturation or assimilating into a different culture, familial violence as a child, hopelessness or a thought pattern with a pessimistic outlook, depression, and childhood sexual abuse have all been found to be positively correlated with suicidal ideation and attempted suicide in South Asian Americans. One study that conducted0 in-group analysis on undergraduate university students of South Asian descent living in New York found higher levels of hopelessness and depression in Asian Indians relative to Bangladeshi or Pakistani Americans.10
In addition, higher levels of suicidal ideation are reported in Asian Indians relative to Bangladeshi or Pakistani Americans. These results resemble findings from similar studies in the United Kingdom. A posited reason for these findings is a difference in religious beliefs. Pakistani and Bangladeshi Americans are predominantly Muslim, have stronger moral beliefs against suicide, and consider it a sin as defined by Islamic beliefs. Asian Indians, in contrast, are majority Hindu and believe in reincarnation – a context that might make suicide seem more permissible.11
South Asian women are particularly vulnerable to domestic violence, childhood sexual abuse, intimate partner violence, and/or familial violence. Cultural gender norms, traditional norms, and patriarchal ideology in the South Asian community make quantifying the level of childhood sexual abuse and familial violence a challenge. Furthermore, culturally, South Asian women are often considered subordinate relative to men, and discussion around family violence and childhood sexual abuse is avoided. Studies from the United Kingdom find a lack of knowledge around, disclosure of, and fear of reporting childhood sexual abuse in South Asian women. A study of a sample of representative South Asian American women found that 25.2% had experienced some form of childhood sexual abuse.12
Research also suggests that South Asians in the United States have some of the highest rates of intimate partner violence. Another study in the United States found that two out of five South Asian women have experienced physical and/or sexual intimate partner violence. This is much higher than the rate found in representative general U.S. population samples.
Literature suggests that exposure to these factors increases womens’ risk for suicidal ideation and attempted suicide. In the United Kingdom, research on South Asian women (aged 18-24 years) has found rates of attempted suicide to be three times higher than those of their white counterparts. Research from the United Kingdom and the United States suggests that younger married South Asian women are exposed to emotional and/or physical abuse from their spouse or in-laws, which is often a mediating factor in their increased risk for suicide.
Attempts to address suicide in the South Asian American community have to be multifaceted. An ideal approach would consist of educating, and connecting with, the community through ethnic media and trusted community sources, such as primary care doctors, caregivers, and social workers. In line with established American Psychological Association guidelines on caring for individuals of immigrant origin, health care professionals should document the patient’s number of generations in the country, number of years in the country, language fluency, family and community support, educational level, social status changes related to immigration, intimate relationships with people of different backgrounds, and stress related to acculturation. Special attention should be paid to South Asian women. Health care professionals should screen South Asian women for past and current intimate partner violence, provide culturally appropriate intimate partner violence resources, and be prepared to refer them to legal counseling services. Also, South Asian women should be screened for a history of exposure to familial violence and childhood sexual abuse.1
To adequately serve this population, there is a need to build capacity in the provision of culturally appropriate mental health services. Access to mental health care professionals through settings such as shelters for abused women, South Asian community–based organizations, youth centers, college counseling, and senior centers would encourage individuals to seek care without the threat of being stigmatized.
References
1. Hedegaard H et al. Suicide mortality in the United States, 1999–2017. NCHS Data Brief, No. 330. 2018 Nov.
2. Ahmad-Stout DJ and Nath SR. J College Stud Psychother. 2013 Jan 10;27(1):43-61.
3. Li H and Keshavan M. Asian J Psychiatry. 2011;4(1):1.
4. Nagaraj NC et al. J Immigr Minor Health. 2019 Oct;21(5):978-1003.
5. Nagaraj NC et al. J Comm Health. 2018;43(3):543-51.
6. Cao KO. Generations. 2014;30(4):82-5.
7. Hurwitz EJ et al. J Immigr Minor Health. 2006;8(3):251-61.
8. Polanco-Roman L et al. Cultur Divers Ethnic Minor Psychol. 2019 Dec 23. doi: 10.1037/cpd0000313.
9. Erausquin JT et al. J Youth Adolesc. 2019 Sep;48(9):1796-1805.
10. Lane R et al. Asian Am J Psychol. 2016;7(2):120-8.
11. Nath SR et al. Asian Am J Psychol. 2018;9(4):334-343.
12. Robertson HA et al. J Immigr Minor Health. 2016 Jul 31;18(4):921-7.
Mr. Kaleka is a medical student in the class of 2021 at Central Michigan University (CMU) College of Medicine, Mt. Pleasant. He has no disclosures. Mr. Kaleka would like to thank his mentor, Furhut Janssen, DO, for her continued guidance and support in research on mental health in immigrant populations.
On first glance, the age-adjusted rate of suicide for Asian and Pacific Islander populations living in the United States looks comparatively low.
Over the past 2 decades in the United States, for example, the overall rate increased by 35%, from, 10.5 to 14.2 per 100,000 individuals. That compares with a rate of 7.0 per 100,000 among Asian and Pacific Islander communities.1
However, because of the aggregate nature (national suicide mortality data combine people of Asian, Native Hawaiian, and other Pacific Islander descent into a single group) in which these data are reported, a significant amount of salient information on subgroups of Asian Americans is lost.2 There is a growing body of research on the mental health of Asian Americans, but the dearth of information and research on suicide in South Asians is striking.3 In fact, a review of literature finds fewer than 10 articles on the topic that have been published in peer-reviewed journals in the last decade. to provide effective, culturally sensitive care.
Diverse group
There are 3.4 million individuals of South Asian descent in the United States. Geographically, South Asians may have familial and cultural/historical roots in Bangladesh, Bhutan, India, Maldives, Nepal, and Pakistan.4 They enjoy a rich diversity in terms of cultural and religious beliefs, language, socioeconomic status, modes of acculturation, and immigration patterns. Asian Indians are the largest group of South Asians in the United States. They are highly educated, with a larger proportion of them pursuing an undergraduate and/or graduate level education than the general population. The median household income of Asian Indians is also higher than the national average.5
In general, suicide, like all mental health issues, is a stigmatized and taboo topic in the South Asian community.6 Also, South Asian Americans are hesitant to seek mental health care because of a perceived inability of Western health care professionals to understand their cultural views. Extrapolation from data on South Asians in the United Kingdom, aggregate statistics for Asian Americans and Pacific Islanders, and studies on South Asians in the United States highlight two South Asian subgroups that are particularly vulnerable to suicide. These are young adults (aged 18-24 years) and women.7
Suicide is the second-leading cause of death for young Asian American men in the United States. Rates of lifetime suicidal ideation and attempts are higher among younger Asian Americans (aged 18-24 years) than among older Asian American adults. Young Asian American adults have been found to have higher levels of suicidal ideation than their white counterparts.8,9 Acculturation or assimilating into a different culture, familial violence as a child, hopelessness or a thought pattern with a pessimistic outlook, depression, and childhood sexual abuse have all been found to be positively correlated with suicidal ideation and attempted suicide in South Asian Americans. One study that conducted0 in-group analysis on undergraduate university students of South Asian descent living in New York found higher levels of hopelessness and depression in Asian Indians relative to Bangladeshi or Pakistani Americans.10
In addition, higher levels of suicidal ideation are reported in Asian Indians relative to Bangladeshi or Pakistani Americans. These results resemble findings from similar studies in the United Kingdom. A posited reason for these findings is a difference in religious beliefs. Pakistani and Bangladeshi Americans are predominantly Muslim, have stronger moral beliefs against suicide, and consider it a sin as defined by Islamic beliefs. Asian Indians, in contrast, are majority Hindu and believe in reincarnation – a context that might make suicide seem more permissible.11
South Asian women are particularly vulnerable to domestic violence, childhood sexual abuse, intimate partner violence, and/or familial violence. Cultural gender norms, traditional norms, and patriarchal ideology in the South Asian community make quantifying the level of childhood sexual abuse and familial violence a challenge. Furthermore, culturally, South Asian women are often considered subordinate relative to men, and discussion around family violence and childhood sexual abuse is avoided. Studies from the United Kingdom find a lack of knowledge around, disclosure of, and fear of reporting childhood sexual abuse in South Asian women. A study of a sample of representative South Asian American women found that 25.2% had experienced some form of childhood sexual abuse.12
Research also suggests that South Asians in the United States have some of the highest rates of intimate partner violence. Another study in the United States found that two out of five South Asian women have experienced physical and/or sexual intimate partner violence. This is much higher than the rate found in representative general U.S. population samples.
Literature suggests that exposure to these factors increases womens’ risk for suicidal ideation and attempted suicide. In the United Kingdom, research on South Asian women (aged 18-24 years) has found rates of attempted suicide to be three times higher than those of their white counterparts. Research from the United Kingdom and the United States suggests that younger married South Asian women are exposed to emotional and/or physical abuse from their spouse or in-laws, which is often a mediating factor in their increased risk for suicide.
Attempts to address suicide in the South Asian American community have to be multifaceted. An ideal approach would consist of educating, and connecting with, the community through ethnic media and trusted community sources, such as primary care doctors, caregivers, and social workers. In line with established American Psychological Association guidelines on caring for individuals of immigrant origin, health care professionals should document the patient’s number of generations in the country, number of years in the country, language fluency, family and community support, educational level, social status changes related to immigration, intimate relationships with people of different backgrounds, and stress related to acculturation. Special attention should be paid to South Asian women. Health care professionals should screen South Asian women for past and current intimate partner violence, provide culturally appropriate intimate partner violence resources, and be prepared to refer them to legal counseling services. Also, South Asian women should be screened for a history of exposure to familial violence and childhood sexual abuse.1
To adequately serve this population, there is a need to build capacity in the provision of culturally appropriate mental health services. Access to mental health care professionals through settings such as shelters for abused women, South Asian community–based organizations, youth centers, college counseling, and senior centers would encourage individuals to seek care without the threat of being stigmatized.
References
1. Hedegaard H et al. Suicide mortality in the United States, 1999–2017. NCHS Data Brief, No. 330. 2018 Nov.
2. Ahmad-Stout DJ and Nath SR. J College Stud Psychother. 2013 Jan 10;27(1):43-61.
3. Li H and Keshavan M. Asian J Psychiatry. 2011;4(1):1.
4. Nagaraj NC et al. J Immigr Minor Health. 2019 Oct;21(5):978-1003.
5. Nagaraj NC et al. J Comm Health. 2018;43(3):543-51.
6. Cao KO. Generations. 2014;30(4):82-5.
7. Hurwitz EJ et al. J Immigr Minor Health. 2006;8(3):251-61.
8. Polanco-Roman L et al. Cultur Divers Ethnic Minor Psychol. 2019 Dec 23. doi: 10.1037/cpd0000313.
9. Erausquin JT et al. J Youth Adolesc. 2019 Sep;48(9):1796-1805.
10. Lane R et al. Asian Am J Psychol. 2016;7(2):120-8.
11. Nath SR et al. Asian Am J Psychol. 2018;9(4):334-343.
12. Robertson HA et al. J Immigr Minor Health. 2016 Jul 31;18(4):921-7.
Mr. Kaleka is a medical student in the class of 2021 at Central Michigan University (CMU) College of Medicine, Mt. Pleasant. He has no disclosures. Mr. Kaleka would like to thank his mentor, Furhut Janssen, DO, for her continued guidance and support in research on mental health in immigrant populations.


