User login
Even a few days of steroids may be risky, new study suggests
Extended use of corticosteroids for chronic inflammatory conditions puts patients at risk for serious adverse events (AEs), including cardiovascular disease, osteoporosis, cataracts, and diabetes. Now, a growing body of evidence suggests that even short bursts of these drugs are associated with serious risks.
Most recently, a population-based study of more than 2.6 million people found that taking corticosteroids for 14 days or less was associated with a substantially greater risk for gastrointestinal (GI) bleeding, sepsis, and heart failure, particularly within the first 30 days after therapy.
In the study, Tsung-Chieh Yao, MD, PhD, a professor in the division of allergy, asthma, and rheumatology in the department of pediatrics at Chang Gung Memorial Hospital in Taoyuan, Taiwan, and colleagues used a self-controlled case series to analyze data from Taiwan’s National Health Insurance Research Database of medical claims. They compared patients’ conditions in the period from 5 to 90 days before treatment to conditions from the periods from 5 to 30 days and from 31 to 90 days after therapy.
With a median duration of 3 days of treatment, the incidence rate ratios (IRRs) were 1.80 (95% confidence interval, 1.75-1.84) for GI bleeding, 1.99 (95% CI, 1.70-2.32) for sepsis, and 2.37 (95% CI, 2.13-2.63) for heart failure.
Given the findings, physicians should weigh the benefits against the risks of rare but potentially serious consequences of these anti-inflammatory drugs, according to the authors.
“After initiating patients on oral steroid bursts, physicians should be on the lookout for these severe adverse events, particularly within the first month after initiation of steroid therapy,” Dr. Yao said in an interview.
The findings were published online July 6 in Annals of Internal Medicine.
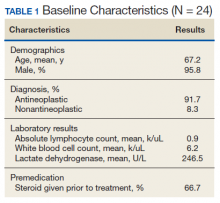
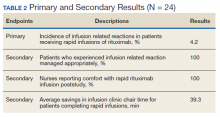
Of the 15,859,129 adult Asians in the Taiwanese database, the study included 2,623,327 adults aged 20-64 years who received single steroid bursts (14 days or less) between Jan. 1, 2013, and Dec. 31, 2015.
Almost 60% of the indications were for skin disorders, such as eczema and urticaria, and for respiratory tract infections, such as sinusitis and acute pharyngitis. Among specialties, dermatology, otolaryngology, family practice, internal medicine, and pediatrics accounted for 88% of prescriptions.
“Our findings are important for physicians and guideline developers because short-term use of oral corticosteroids is common and the real-world safety of this approach remains unclear,” the authors wrote. They acknowledged that the database did not provide information on such potential confounders as disease severity and lifestyle factors, nor did it include children and vulnerable individuals, which may limit the generalizability of the results.
The findings echo those of a 2017 cohort study conducted by researchers at the University of Michigan in Ann Arbor. That study, by Akbar K. Waljee, MD, assistant professor of gastroenterology, University of Michigan, Ann Arbor, and colleagues, included data on more than 1.5 million privately insured U.S. adults. The researchers included somewhat longer steroid bursts of up to 30 days’ duration and found that use of the drugs was associated with a greater than fivefold increased risk for sepsis, a more than threefold increased risk for venous thromboembolism, and a nearly twofold increased risk for fracture within 30 days of starting treatment.
Furthermore, the elevated risk persisted at prednisone-equivalent doses of less than 20 mg/d (IRR, 4.02 for sepsis, 3.61 for venous thromboembolism, and 1.83 for fracture; all P < .001).
The U.S. study also found that during the 3-year period from 2012 to 2014, more than 20% of patients were prescribed short-term oral corticosteroids.
“Both studies indicate that these short-term regimens are more common in the real world than was previously thought and are not risk free,” Dr. Yao said.
Recognition that corticosteroids are associated with adverse events has been building for decades, according to the authors of an editorial that accompanies the new study.
“However, we commonly use short corticosteroid ‘bursts’ for minor ailments despite a lack of evidence for meaningful benefit. We are now learning that bursts as short as 3 days may increase risk for serious AEs, even in young and healthy people,” wrote editorialists Beth I. Wallace, MD, of the Center for Clinical Management Research at the VA Ann Arbor Healthcare System and the Institute for Healthcare Policy and Innovation at Michigan Medicine, Ann Arbor, and Dr. Waljee, who led the 2017 study.
Dr. Wallace and Dr. Waljee drew parallels between corticosteroid bursts and other short-term regimens, such as of antibiotics and opiates, in which prescriber preference and sometimes patient pressure play a role. “All of these treatments have well-defined indications but can cause net harm when used. We can thus conceive of a corticosteroid stewardship model of targeted interventions that aims to reduce inappropriate prescribing,” they wrote.
In an interview, Dr. Wallace, a rheumatologist who prescribes oral steroids fairly frequently, noted that the Taiwan study is the first to investigate steroid bursts. “Up till now, these very short courses have flown under the radar. Clinicians very commonly prescribe short courses to help relieve symptoms of self-limited conditions like bronchitis, and we assume that because the exposure duration is short, the risks are low, especially for patients who are otherwise healthy.”
She warned that the data in the current study indicate that these short bursts – even at the lower end of the 1- to 2-week courses American physicians prescribe most often – carry small but real increases in risk for serious AEs. “And these increases were seen in young, healthy people, not just in people with preexisting conditions,” she said. “So, we might need to start thinking harder about how we are prescribing even these very short courses of steroids and try to use steroids only when their meaningful benefits really outweigh the risk.”
She noted that a patient with a chronic inflammatory condition such as rheumatoid arthritis may benefit substantially from short-term steroids to treat a disease flare. In that specific case, the benefits of short-term steroids may outweigh the risks, Dr. Wallace said.
But not everyone thinks a new strategy is needed. For Whitney A. High, MD, associate professor of dermatology and pathology at the University of Colorado at Denver, Aurora, the overprescribing of short-term corticosteroids is not a problem, and dermatologists are already exercising caution.
“I only prescribe these drugs short term to, at a guess, about 1 in 40 patients and only when a patient is miserable and quality of life is being seriously affected,” he said in an interview. “And that’s something that can’t be measured in a database study like the one from Taiwan but only in a risk-benefit analysis,” he said.
Furthermore, dermatologists have other drugs and technologies in their armamentarium, including topical steroids with occlusion or with wet wraps, phototherapy, phosphodiesterase inhibitors, calcipotriene, methotrexate and other immunosuppressive agents, and biologics. “In fact, many of these agents are specifically referred to as steroid-sparing,” Dr. High said.
Nor does he experience much pressure from patients to prescribe these drugs. “While occasionally I may encounter a patient who places pressure on me for oral steroids, it’s probably not nearly as frequently as providers in other fields are pressured to prescribe antibiotics or narcotics,” he said.
According to the Taiwanese researchers, the next step is to conduct more studies, including clinical trials, to determine optimal use of corticosteroids by monitoring adverse events. In the meantime, for practitioners such as Dr. Wallace and Dr. High, there is ample evidence from several recent studies of the harms of short-term corticosteroids, whereas the benefits for patients with self-limiting conditions remain uncertain. “This and other studies like it quite appropriately remind providers to avoid oral steroids when they’re not necessary and to seek alternatives where possible,” Dr. High said.
The study was supported by the National Health Research Institutes of Taiwan, the Ministry of Science and Technology of Taiwan, the Chang Gung Medical Foundation, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH). Dr. Yao has disclosed no relevant financial relationships. Dr. Wu has received grants from GlaxoSmithKline outside the submitted work. The editorialists and Dr. High have disclosed no relevant financial relationships. Dr. Wallace received an NIH grant during the writing of the editorial.
A version of this article originally appeared on Medscape.com.
Extended use of corticosteroids for chronic inflammatory conditions puts patients at risk for serious adverse events (AEs), including cardiovascular disease, osteoporosis, cataracts, and diabetes. Now, a growing body of evidence suggests that even short bursts of these drugs are associated with serious risks.
Most recently, a population-based study of more than 2.6 million people found that taking corticosteroids for 14 days or less was associated with a substantially greater risk for gastrointestinal (GI) bleeding, sepsis, and heart failure, particularly within the first 30 days after therapy.
In the study, Tsung-Chieh Yao, MD, PhD, a professor in the division of allergy, asthma, and rheumatology in the department of pediatrics at Chang Gung Memorial Hospital in Taoyuan, Taiwan, and colleagues used a self-controlled case series to analyze data from Taiwan’s National Health Insurance Research Database of medical claims. They compared patients’ conditions in the period from 5 to 90 days before treatment to conditions from the periods from 5 to 30 days and from 31 to 90 days after therapy.
With a median duration of 3 days of treatment, the incidence rate ratios (IRRs) were 1.80 (95% confidence interval, 1.75-1.84) for GI bleeding, 1.99 (95% CI, 1.70-2.32) for sepsis, and 2.37 (95% CI, 2.13-2.63) for heart failure.
Given the findings, physicians should weigh the benefits against the risks of rare but potentially serious consequences of these anti-inflammatory drugs, according to the authors.
“After initiating patients on oral steroid bursts, physicians should be on the lookout for these severe adverse events, particularly within the first month after initiation of steroid therapy,” Dr. Yao said in an interview.
The findings were published online July 6 in Annals of Internal Medicine.
Of the 15,859,129 adult Asians in the Taiwanese database, the study included 2,623,327 adults aged 20-64 years who received single steroid bursts (14 days or less) between Jan. 1, 2013, and Dec. 31, 2015.
Almost 60% of the indications were for skin disorders, such as eczema and urticaria, and for respiratory tract infections, such as sinusitis and acute pharyngitis. Among specialties, dermatology, otolaryngology, family practice, internal medicine, and pediatrics accounted for 88% of prescriptions.
“Our findings are important for physicians and guideline developers because short-term use of oral corticosteroids is common and the real-world safety of this approach remains unclear,” the authors wrote. They acknowledged that the database did not provide information on such potential confounders as disease severity and lifestyle factors, nor did it include children and vulnerable individuals, which may limit the generalizability of the results.
The findings echo those of a 2017 cohort study conducted by researchers at the University of Michigan in Ann Arbor. That study, by Akbar K. Waljee, MD, assistant professor of gastroenterology, University of Michigan, Ann Arbor, and colleagues, included data on more than 1.5 million privately insured U.S. adults. The researchers included somewhat longer steroid bursts of up to 30 days’ duration and found that use of the drugs was associated with a greater than fivefold increased risk for sepsis, a more than threefold increased risk for venous thromboembolism, and a nearly twofold increased risk for fracture within 30 days of starting treatment.
Furthermore, the elevated risk persisted at prednisone-equivalent doses of less than 20 mg/d (IRR, 4.02 for sepsis, 3.61 for venous thromboembolism, and 1.83 for fracture; all P < .001).
The U.S. study also found that during the 3-year period from 2012 to 2014, more than 20% of patients were prescribed short-term oral corticosteroids.
“Both studies indicate that these short-term regimens are more common in the real world than was previously thought and are not risk free,” Dr. Yao said.
Recognition that corticosteroids are associated with adverse events has been building for decades, according to the authors of an editorial that accompanies the new study.
“However, we commonly use short corticosteroid ‘bursts’ for minor ailments despite a lack of evidence for meaningful benefit. We are now learning that bursts as short as 3 days may increase risk for serious AEs, even in young and healthy people,” wrote editorialists Beth I. Wallace, MD, of the Center for Clinical Management Research at the VA Ann Arbor Healthcare System and the Institute for Healthcare Policy and Innovation at Michigan Medicine, Ann Arbor, and Dr. Waljee, who led the 2017 study.
Dr. Wallace and Dr. Waljee drew parallels between corticosteroid bursts and other short-term regimens, such as of antibiotics and opiates, in which prescriber preference and sometimes patient pressure play a role. “All of these treatments have well-defined indications but can cause net harm when used. We can thus conceive of a corticosteroid stewardship model of targeted interventions that aims to reduce inappropriate prescribing,” they wrote.
In an interview, Dr. Wallace, a rheumatologist who prescribes oral steroids fairly frequently, noted that the Taiwan study is the first to investigate steroid bursts. “Up till now, these very short courses have flown under the radar. Clinicians very commonly prescribe short courses to help relieve symptoms of self-limited conditions like bronchitis, and we assume that because the exposure duration is short, the risks are low, especially for patients who are otherwise healthy.”
She warned that the data in the current study indicate that these short bursts – even at the lower end of the 1- to 2-week courses American physicians prescribe most often – carry small but real increases in risk for serious AEs. “And these increases were seen in young, healthy people, not just in people with preexisting conditions,” she said. “So, we might need to start thinking harder about how we are prescribing even these very short courses of steroids and try to use steroids only when their meaningful benefits really outweigh the risk.”
She noted that a patient with a chronic inflammatory condition such as rheumatoid arthritis may benefit substantially from short-term steroids to treat a disease flare. In that specific case, the benefits of short-term steroids may outweigh the risks, Dr. Wallace said.
But not everyone thinks a new strategy is needed. For Whitney A. High, MD, associate professor of dermatology and pathology at the University of Colorado at Denver, Aurora, the overprescribing of short-term corticosteroids is not a problem, and dermatologists are already exercising caution.
“I only prescribe these drugs short term to, at a guess, about 1 in 40 patients and only when a patient is miserable and quality of life is being seriously affected,” he said in an interview. “And that’s something that can’t be measured in a database study like the one from Taiwan but only in a risk-benefit analysis,” he said.
Furthermore, dermatologists have other drugs and technologies in their armamentarium, including topical steroids with occlusion or with wet wraps, phototherapy, phosphodiesterase inhibitors, calcipotriene, methotrexate and other immunosuppressive agents, and biologics. “In fact, many of these agents are specifically referred to as steroid-sparing,” Dr. High said.
Nor does he experience much pressure from patients to prescribe these drugs. “While occasionally I may encounter a patient who places pressure on me for oral steroids, it’s probably not nearly as frequently as providers in other fields are pressured to prescribe antibiotics or narcotics,” he said.
According to the Taiwanese researchers, the next step is to conduct more studies, including clinical trials, to determine optimal use of corticosteroids by monitoring adverse events. In the meantime, for practitioners such as Dr. Wallace and Dr. High, there is ample evidence from several recent studies of the harms of short-term corticosteroids, whereas the benefits for patients with self-limiting conditions remain uncertain. “This and other studies like it quite appropriately remind providers to avoid oral steroids when they’re not necessary and to seek alternatives where possible,” Dr. High said.
The study was supported by the National Health Research Institutes of Taiwan, the Ministry of Science and Technology of Taiwan, the Chang Gung Medical Foundation, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH). Dr. Yao has disclosed no relevant financial relationships. Dr. Wu has received grants from GlaxoSmithKline outside the submitted work. The editorialists and Dr. High have disclosed no relevant financial relationships. Dr. Wallace received an NIH grant during the writing of the editorial.
A version of this article originally appeared on Medscape.com.
Extended use of corticosteroids for chronic inflammatory conditions puts patients at risk for serious adverse events (AEs), including cardiovascular disease, osteoporosis, cataracts, and diabetes. Now, a growing body of evidence suggests that even short bursts of these drugs are associated with serious risks.
Most recently, a population-based study of more than 2.6 million people found that taking corticosteroids for 14 days or less was associated with a substantially greater risk for gastrointestinal (GI) bleeding, sepsis, and heart failure, particularly within the first 30 days after therapy.
In the study, Tsung-Chieh Yao, MD, PhD, a professor in the division of allergy, asthma, and rheumatology in the department of pediatrics at Chang Gung Memorial Hospital in Taoyuan, Taiwan, and colleagues used a self-controlled case series to analyze data from Taiwan’s National Health Insurance Research Database of medical claims. They compared patients’ conditions in the period from 5 to 90 days before treatment to conditions from the periods from 5 to 30 days and from 31 to 90 days after therapy.
With a median duration of 3 days of treatment, the incidence rate ratios (IRRs) were 1.80 (95% confidence interval, 1.75-1.84) for GI bleeding, 1.99 (95% CI, 1.70-2.32) for sepsis, and 2.37 (95% CI, 2.13-2.63) for heart failure.
Given the findings, physicians should weigh the benefits against the risks of rare but potentially serious consequences of these anti-inflammatory drugs, according to the authors.
“After initiating patients on oral steroid bursts, physicians should be on the lookout for these severe adverse events, particularly within the first month after initiation of steroid therapy,” Dr. Yao said in an interview.
The findings were published online July 6 in Annals of Internal Medicine.
Of the 15,859,129 adult Asians in the Taiwanese database, the study included 2,623,327 adults aged 20-64 years who received single steroid bursts (14 days or less) between Jan. 1, 2013, and Dec. 31, 2015.
Almost 60% of the indications were for skin disorders, such as eczema and urticaria, and for respiratory tract infections, such as sinusitis and acute pharyngitis. Among specialties, dermatology, otolaryngology, family practice, internal medicine, and pediatrics accounted for 88% of prescriptions.
“Our findings are important for physicians and guideline developers because short-term use of oral corticosteroids is common and the real-world safety of this approach remains unclear,” the authors wrote. They acknowledged that the database did not provide information on such potential confounders as disease severity and lifestyle factors, nor did it include children and vulnerable individuals, which may limit the generalizability of the results.
The findings echo those of a 2017 cohort study conducted by researchers at the University of Michigan in Ann Arbor. That study, by Akbar K. Waljee, MD, assistant professor of gastroenterology, University of Michigan, Ann Arbor, and colleagues, included data on more than 1.5 million privately insured U.S. adults. The researchers included somewhat longer steroid bursts of up to 30 days’ duration and found that use of the drugs was associated with a greater than fivefold increased risk for sepsis, a more than threefold increased risk for venous thromboembolism, and a nearly twofold increased risk for fracture within 30 days of starting treatment.
Furthermore, the elevated risk persisted at prednisone-equivalent doses of less than 20 mg/d (IRR, 4.02 for sepsis, 3.61 for venous thromboembolism, and 1.83 for fracture; all P < .001).
The U.S. study also found that during the 3-year period from 2012 to 2014, more than 20% of patients were prescribed short-term oral corticosteroids.
“Both studies indicate that these short-term regimens are more common in the real world than was previously thought and are not risk free,” Dr. Yao said.
Recognition that corticosteroids are associated with adverse events has been building for decades, according to the authors of an editorial that accompanies the new study.
“However, we commonly use short corticosteroid ‘bursts’ for minor ailments despite a lack of evidence for meaningful benefit. We are now learning that bursts as short as 3 days may increase risk for serious AEs, even in young and healthy people,” wrote editorialists Beth I. Wallace, MD, of the Center for Clinical Management Research at the VA Ann Arbor Healthcare System and the Institute for Healthcare Policy and Innovation at Michigan Medicine, Ann Arbor, and Dr. Waljee, who led the 2017 study.
Dr. Wallace and Dr. Waljee drew parallels between corticosteroid bursts and other short-term regimens, such as of antibiotics and opiates, in which prescriber preference and sometimes patient pressure play a role. “All of these treatments have well-defined indications but can cause net harm when used. We can thus conceive of a corticosteroid stewardship model of targeted interventions that aims to reduce inappropriate prescribing,” they wrote.
In an interview, Dr. Wallace, a rheumatologist who prescribes oral steroids fairly frequently, noted that the Taiwan study is the first to investigate steroid bursts. “Up till now, these very short courses have flown under the radar. Clinicians very commonly prescribe short courses to help relieve symptoms of self-limited conditions like bronchitis, and we assume that because the exposure duration is short, the risks are low, especially for patients who are otherwise healthy.”
She warned that the data in the current study indicate that these short bursts – even at the lower end of the 1- to 2-week courses American physicians prescribe most often – carry small but real increases in risk for serious AEs. “And these increases were seen in young, healthy people, not just in people with preexisting conditions,” she said. “So, we might need to start thinking harder about how we are prescribing even these very short courses of steroids and try to use steroids only when their meaningful benefits really outweigh the risk.”
She noted that a patient with a chronic inflammatory condition such as rheumatoid arthritis may benefit substantially from short-term steroids to treat a disease flare. In that specific case, the benefits of short-term steroids may outweigh the risks, Dr. Wallace said.
But not everyone thinks a new strategy is needed. For Whitney A. High, MD, associate professor of dermatology and pathology at the University of Colorado at Denver, Aurora, the overprescribing of short-term corticosteroids is not a problem, and dermatologists are already exercising caution.
“I only prescribe these drugs short term to, at a guess, about 1 in 40 patients and only when a patient is miserable and quality of life is being seriously affected,” he said in an interview. “And that’s something that can’t be measured in a database study like the one from Taiwan but only in a risk-benefit analysis,” he said.
Furthermore, dermatologists have other drugs and technologies in their armamentarium, including topical steroids with occlusion or with wet wraps, phototherapy, phosphodiesterase inhibitors, calcipotriene, methotrexate and other immunosuppressive agents, and biologics. “In fact, many of these agents are specifically referred to as steroid-sparing,” Dr. High said.
Nor does he experience much pressure from patients to prescribe these drugs. “While occasionally I may encounter a patient who places pressure on me for oral steroids, it’s probably not nearly as frequently as providers in other fields are pressured to prescribe antibiotics or narcotics,” he said.
According to the Taiwanese researchers, the next step is to conduct more studies, including clinical trials, to determine optimal use of corticosteroids by monitoring adverse events. In the meantime, for practitioners such as Dr. Wallace and Dr. High, there is ample evidence from several recent studies of the harms of short-term corticosteroids, whereas the benefits for patients with self-limiting conditions remain uncertain. “This and other studies like it quite appropriately remind providers to avoid oral steroids when they’re not necessary and to seek alternatives where possible,” Dr. High said.
The study was supported by the National Health Research Institutes of Taiwan, the Ministry of Science and Technology of Taiwan, the Chang Gung Medical Foundation, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH). Dr. Yao has disclosed no relevant financial relationships. Dr. Wu has received grants from GlaxoSmithKline outside the submitted work. The editorialists and Dr. High have disclosed no relevant financial relationships. Dr. Wallace received an NIH grant during the writing of the editorial.
A version of this article originally appeared on Medscape.com.
Access to Pain Care From Compensation Clinics: A Relational Coordination Perspective
Chronic pain is common in veterans, and early engagement in pain treatment is recommended to forestall consequences of untreated pain, including depression, disability, and substance use disorders. The Veterans Health Administration (VHA) employs a stepped care model of pain treatment, with the majority of pain care based in primary care (step 1), and an array of specialty/multimodal treatment options made available at each step in the model for patients with more complex problems, or those who do not respond to more conservative interventions.1
Recognizing the need for comprehensive pain care, the US Congress passed the Comprehensive Addiction and Recovery Act, 21 USC §1521 (2016), which included provisions for VHA facilities to offer multimodal pain treatment and to report the availability of pain care options at each step in the stepped care model.2, With the passage of the Veterans Access, Choice, and Accountability Act of 2014, 38 USC §101 (2014) and now the MISSION Act of 2018, 38 USC §703 (2018) veterans whose VHA facilities are too distant, who require care unavailable at that facility, or who have to wait too long to receive care are eligible for treatment at either VHA or non-VHA facilities.3 These laws allocate the same pool of funds to both VHA and community care and thus create an incentive to engage veterans in care within the VHA network so the funds are not spent out of network.4
An opportunity to connect veterans with VHA care arises at specialized VHA Compensation and Pension (C&P) clinics during examinations that determine whether a veteran’s health conditions were caused or exacerbated by their military service. Veterans file claims with the US Department of Veterans Affairs (VA) Veterans Benefits Administration (VBA), which sends the patient to either a VHA facility or private practitioners for these examinations. Although the number of examinations conducted each year is not available, there were 274,528 veterans newly awarded compensation in fiscal year 2018, and a substantial number of the total of 4,743,108 veterans with C&P awards had reevaluation examinations for at least 1 of their conditions during that year.5 Based largely on the compensation examination results, military service records, and medical records, veterans are granted a service-connected rating for conditions deemed related to military service. A service-connection rating between 0% and 100% is assigned by the VBA, with higher ratings indicating more impairment and, consequently, more financial compensation. Service-connection ratings also are used to decide which veterans are in the highest priority groups for receipt of VHA health care services and are exempt from copayments.
Although traditionally thought of as a forensic evaluation with no clinical purpose, the C&P examination process affords many opportunities to explain VHA care to veterans in distress who file claims.6 A randomized clinical trials (RCT) involving veterans with mental health claims and a second RCT including veterans with musculoskeletal claims each found that veterans use more VHA services if offered outreach at the time of the C&P examination.7,8 In addition to clinical benefits, outreach around the time of C&P examinations also might mitigate the well documented adversarial aspects of the service-connection claims process.6,9,10 Currently, such outreach is not part of routine VHA procedures. Ironically, it is the VBA and not VHA that contacts veterans who are awarded service-connection with information about their eligibility for VHA care based on their award.
Connecting veterans to pain treatment can involve clarifying eligibility for VHA care for veterans in whom eligibility is unknown, involving primary care providers (PCPs) who are the fulcrum of VHA pain care referrals, and motivating veterans to seek specific pain treatment modalities. Connecting veterans to treatment at the time of their compensation examinations also likely involves bidirectional cooperation between the specialized C&P clinics where veterans are examined and the clinics that provide treatment.
Relational coordination is a theoretical framework that can describe the horizontal relationships between different teams within the same medical facility. Relational coordination theorizes that communication between workgroups is related synergistically to the quality of relationships between workgroups. Relational coordination is better between workgroups that share goals and often have high levels of relational coordination, which is thought to be especially important when activities are ambiguous, require cooperation, and are conducted under time pressure.11 High relational coordination also has been associated with high staff job satisfaction, high satisfaction with delivered services, and adherence to treatment guidelines.12-14 An observational cohort study suggested that relational coordination can be improved by targeted interventions that bring workgroups together and facilitate intercommunication.15
To better understand referral and engagement for pain treatment at compensation examinations, VA staff from primary care, mental health, pain management, and C&P teams at the 8 VHA medical centers in New England were invited to complete a validated relational coordination survey.11,16 A subset of invited staff participated in a semistructured interview about pain treatment referral practices within their medical centers.
Assessments were conducted as part of a mixed methods formative evaluation involving quantitative and qualitative methods for a clinical trial at the 8 VHA medical centers in New England. The trial is testing an intervention in which veterans presenting for service-connection examinations for musculoskeletal conditions receive brief counseling to engage them in nonopioid pain treatments. The VHA Central Institutional Review Board approved this formative evaluation and the clinical trial has begun (ClinicalTrials.gov NCT04062214).
Potential interviewees were involved in referrals to and provision of nonpharmacologic pain treatment and were identified by site investigators in the randomized trial. Identified interviewees were clinical and administrative staff belonging to VHA Primary Care, Pain Management, and Compensation and Pension clinics. A total of 83 staff were identified.
Semistructured Interviews
A subset of the 83 staff were invited to participate in a semistructured interview because their position impacted coordination of pain care at their facilities or they worked in C&P. Staff at a site were interviewed until no new themes emerged from additional interviews, and each of the 8 sites was represented. Interviews were conducted between June and August 2018. Standardized scripts describing the study and inviting participation in a semistructured interview were e-mailed to VA staff. At the time of the interview the study purpose was restated and consent for audiotaping was obtained. The interviews followed a guide designed to assess a relational coordination framework among various workgroups. The data in this manuscript were elicited by specific prompts concerning: (1) How veterans learn about pain care when they come through C&P; and (2) How staff in C&P communicate with treatment providers about veterans who have chronic pain. Each interview lasted about 30 minutes.
Relational Coordination Survey
All identified staff were invited to participate in a relational coordination survey. The survey was administered through VA REDCap. Survey invitations were e-mailed from REDCap to VA staff and included a description of the study and assurances of the confidentiality of data collected. Surveys took < 10 minutes to complete. To begin, respondents identified their primary workgroup (C&P, primary care, pain management, or administrative leadership or staff), secondary workgroup (if they were in > 1), and site. Respondents provided no other identifying information and were assured their responses would be confidential.
The survey consisted of 7 questions regarding beliefs about the quality of communication and interactions among workgroup members in obtaining a shared goal.11 The shared goal in the survey used in this study was providing pain care services for veterans with musculoskeletal conditions. Using a 5-point Likert scale, the 7 questions concerned frequency, timeliness, and accuracy of communication; response to problems providing pain services; sharing goals; and knowledge and respect for respondent’s job function. Higher scores indicated better relational coordination among members of a workgroup. Using the survey’s 7 items, composite mean relational coordination scores were calculated for each of the 4 primary workgroups. To account for the possibility that a member rated their own workgroups, 2 scores were created for each workgroup; one included members of the workgroup and another excluded them.
Data Analysis
The audio-recorded semistructured interviews were transcribed and entered into Atlas.ti qualitative data analysis software. To identify cross-cutting themes, a semistructured telephone interview guide was developed by the qualitative study team that emphasized interrelationships between different clinical teams. The transcripts were then analyzed using the grounded theory approach, a systematic methodology to reduce themes from collected qualitative data. Two research staff read each transcript twice; first to familiarize themselves with the text and then, using open coding, to identify important concepts that emerged from the language and assign codes to segments of text. To ensure accuracy, researchers included suitable contextual information in the coding. Using the constant comparative method, research staff then met to examine the themes that emerged in the interviews, discuss and coalesce coding discrepancies, and compare perspectives.17
The composite score (mean of the 7 items and 95% CI) of the survey responses was analyzed to identify significant differences in coordination across the 4 workgroups. Analysis of variance (ANOVA) was used to examine each relational coordination score by respondents’ workgroup. Post hoc analyses examined relational coordination survey differences among the 4 respondent groups.
Results
Thirty-nine survey respondents participated in the semistructured interviews. C&P examiners expressed varying degrees of comfort with their role in extending access to pain care for veterans. Some of the examiners strongly believed that their role was purely forensic, and going beyond this forensic role to refer or recommend treatment to veterans would be a violation of their role to conduct a forensic examination. “We don’t have an ongoing therapeutic relationship with any of the patients,” a C&P examiner explained: “We see them once; they’re out the door. It’s forensic. We’re investigating the person as a claimant, we’re investigating it and using our tools to go and review information from 30, 40 years ago.”
Other examiners had a less strict approach for working with veterans in C&P, even though examiners are asked not to provide advice or therapy. One C&P examiner noted that because he “can’t watch people in pain,” during the examination this doctor recommends that patients go to the office that determines whether they are eligible for benefits and choose a PCP. Another C&P examiner concurred with this approach. “I certainly spend a little time with the veteran talking to them about their personal life, who they are, what they do, what they’ve done, what they’re going to do to kind of break the ice between us,” the second examiner explained. “At the end, I will make some suggestions to them. I’m comfortable doing that. I don’t know that everybody is.”
Many of the VHA providers we interviewed had little knowledge of the C&P process or whether C&P examiners had any role or responsibilities in referring veterans for pain care. Most VHA providers could not name any C&P examiners at their facility and were generally unfamiliar with the content of C&P examinations. One provider bluntly said, “I’ve never communicated with anyone in comp and pen [C&P].”
Another PCP also expressed concerns with referrals, suggesting that C&P and primary care “are totally separate and should remain separate,” the PCP explained. “My concern with getting referral from comp and pen is that is it then they’re seeking all sorts of treatment that they wouldn’t necessarily need or ask for otherwise.”
Conversely a different PCP had a positive outlook on how C&P examiners might help ease the transition into the VHA for veterans with pain, especially for newly discharged veterans. “Having comp and pen address these issues is really going to be helpful. I think it could be significant that the topic is introduced early on.”
Relational Coordination Survey
Relational coordination surveys were sent to 83 participants of whom 66 responded. Respondents were from
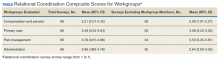
The relational coordination composite scores were lowest for C&P. This finding remained whether C&P staff surveys were included or removed from the C&P responses. As demonstrated by the 95% CI, when team members’ surveys were included, C&P scores (95% CI, 2.01-2.42) were significantly lower than the primary care (95% CI, 3.34-3.64) and pain management (95% CI, 3.61-3.96) groups. All the relational coordination composite scores were slightly lower when staff who described their own workgroup were removed (ie, respondents rated their own workgroups as having higher relational coordination than others did). Using the composite scores excluding same workgroup members, the composite scores of the C&P remained significantly lower than all 3 other workgroups (Table). Means values for each individual item in the C&P group were significantly less than all other group means for each item except for the question on responses to problems providing pain services (data not shown). On this item only, the mean C&P rating was > 3 (3.19), but this was still lower than the means of the primary care and pain management workgroups.
Further analyses were undertaken to understand the importance of stakeholders’ ratings of their own workgroup compared with ratings by others of that workgroup. A 1-way ANOVA of workgroup was conducted and displayed significant workgroup differences between member and nonmember relational coordination ratings on 3 of the 4 workgroup’s scores C&P (F = 5.75, 3, 62 df; P < .01) primary care (F = 4.30, 3, 62 df; P < .008) and pain management (F = 8.22, 3, 62 df; P < .001). Post hoc contrasts between the different workgroups doing the rating revealed: (1) significant differences in the assessment of the C&P workgroup between the C&P workgroup and both the primary care (P < .01) and pain management groups (P < .001) with C&P rating their own workgroup significantly higher; (2) a significant difference in the scoring of the primary care workgroup with the primary care group rating themselves significantly higher than the C&P group; and (3) significant differences in the scoring of the pain management workgroup with both pain management and primary care groups rating the pain management group significantly higher than the C&P group. The results were not substantially changed by removing the 18 respondents who identified themselves as being part of > 1 workgroup .
Discussion
Mixed methods revealed disparate viewpoints about the role of C&P in referring veterans to pain care services. Overall, C&P teams coordinated less with other workgroups than the other groups coordinated with each other, and the C&P clinics took only limited steps to engage veterans in VHA treatment. The relational coordination results appeared to be valid. The mean scores were near the middle of the relational coordination rating scale, with standard deviations indicating a range of responses. The lower relational coordination scores of the C&P group remained after removing stakeholders who were rating their own workgroup. Further support for the validity of the relational coordination survey results is that they were consistent with the reports of C&P clinic isolation in the semistructured interviews.
The interview data suggest that one reason the C&P teams had low relational coordination scores is that VA staff interpret the emphasis on evaluative rather than therapeutic examinations to preclude other attempts to engage veterans into VHA treatment, even though such treatment engagement is permitted within existing guidelines. VBA referrals for examinations say nothing, either way, about engaging veterans in VHA care. The relational coordination results suggest that an intervention that might increase treatment referrals from the C&P clinics would be to explain the (existing) policy allowing for outreach around the time of compensation examinations to VHA staff so this goal is clearly agreed-upon. Another approach to facilitating treatment engagement at the C&P examination is to use other interventions that have been associated with better relational coordination such as intergroup meetings, horizontal integration more generally, and an atmosphere is which people from different backgrounds feel empowered to speak frankly to each other.15,18,19 An important linkage to forge is between C&P teams and the administrative workgroups responsible for verifying a veteran’s eligibility for VHA care and enrolling eligible veterans in VHA treatment. Having C&P clinicians who are familiar with the eligibility and treatment engagement processes would facilitate providing that information to veterans, without compromising the evaluative format of the compensation examination.
An interesting ancillary finding is that relational coordination ratings by members of 3 of the 4 workgroups were higher than ratings by other staff of that workgroup. A possible explanation for this finding is that workgroup members are more aware of the relational coordination efforts made by their own workgroup than those by other workgroups, and therefore rate their own workgroup higher. This also might be part of a broader self-aggrandizement heuristic that has been described in multiple domains.20 Staff may apply this heuristic in reporting that their staff engage in more relational coordination, reflecting the social desirability of being cooperative.
There are simple facility-level interventions that would facilitate veterans access to care such as conducting C&P examinations for potentially treatment-eligible veterans at VHA facilities (vs conducted outside VHA) and having access to materials that explain the treatment options to veterans when they check in for their compensation examinations. The approach to C&P-based treatment engagement that was successfully employed in 2 clinical trials involved having counselors not connected with the C&P clinic contact veterans around the time of their compensation examination to explain VA treatment options and motivate veterans to pursue treatment.8,9 This independent counselor approach is being evaluated in a larger study.
Limitations
These data are from a small number of VA staff evaluating veterans in a single region of the US. They do not show causation, and it is possible that relational coordination is not necessary for referrals from C&P clinics. Relational coordination might not be necessary when referral processes can be simply routinized with little need for communication.11 However, other analyses in these clinics have found that pain treatment referrals in fact are not routinized, with substantial variability within and across institutions. Another possibility is that features that have been associated with less relational coordination, such as male gender and medical specialist guild, were disproportionately present in C&P clinics compared to the other clinics.21
Conclusions
There have been public calls to improve the evaluation of service-connection claims such that this process includes approaches to engage veterans in treatment.22 Referring veterans to treatment when they come for C&P examinations will likely involve improving relational coordination between the C&P service and other parts of VHA. Nationwide, sites that integrate C&P more fully may have valuable lessons to impart about the benefits of such integration. An important step towards better relational coordination will be clarifying that engaging veterans in VHA care around the time of their C&P examinations is a facility-wide goal.
Acknowledgments
The authors thank Brian Linde and Efia James for their perspectives on C&P procedures. This work was supported by the Veterans Integrated Service Network 1 Mental Illness Research Education and Clinical Center (MIRECC) and National Institute of Health, National Center for Complementary and Integrative Health Project # 5UG3AT009758-02. (MIR, SM mPIs).
1. US Department Veterans Affairs, Veterans Health Administration. VHA Directive 2009-053: pain management. https://www.va.gov/painmanagement/docs/vha09paindirective.pdf. Published October 28, 2019. Accessed June 18, 2020.
2. Rosenberger PH, Phillip EJ, Lee A, Kerns RD. The VHA’s national pain management strategy: implementing the stepped care model. Fed Pract. 2011;28(8):39-42.
3. Mattocks KM, Mengeling M, Sadler A, Baldor R, Bastian L. The Veterans Choice Act: A Qualitative Examination of Rapid Policy Implementation in the Department of Veterans Affairs. Med Care. 2017;55 Suppl 7 Suppl 1:S71-S75. doi:10.1097/MLR.0000000000000667
4. Rieselbach RE, Epperly T, Nycz G, Shin P. Community health centers could provide better outsourced primary care for veterans. J Gen Intern Med. 2019;34(1):150-153. doi:10.1007/s11606-018-4691-4
5. US Department of Veterans Affairs, Veterans Benefit Administration. VBA annual benefits report fiscal year 2018. https://www.benefits.va.gov/REPORTS/abr/docs/2018-abr.pdf. Updated March 29, 2019. Accessed June 17, 2020.
6. Rosen MI. Compensation examinations for PTSD-an opportunity for treatment? J Rehabil Res Dev. 2010;47(5):xv-xxii. doi:10.1682/jrrd.2010.04.0075
7. Rosen MI, Ablondi K, Black AC, et al. Work outcomes after benefits counseling among veterans applying for service connection for a psychiatric condition. Psychiatr Serv. 2014;65(12):1426-1432. doi:10.1176/appi.ps.201300478
8. Rosen MI, Becker WC, Black AC, Martino S, Edens EL, Kerns RD. Brief counseling for veterans with musculoskeletal disorder, risky substance use, and service connection claims. Pain Med. 2019;20(3):528-542. doi:10.1093/pm/pny071
9. Meshberg-Cohen S, DeViva JC, Rosen MI. Counseling veterans applying for service connection status for mental health conditions. Psychiatr Serv. 2017;68(4):396-399. doi:10.1176/appi.ps.201500533
10. Sayer NA, Spoont M, Nelson DB. Post-traumatic stress disorder claims from the viewpoint of veterans service officers. Mil Med. 2005;170(10):867-870. doi:10.7205/milmed.170.10.867
11. Gittell JH. Coordinating mechanisms in care provider groups: relational coordination as a mediator and input uncertainty as a moderator of performance effects. Manage Sci. 2002;48(11):1408-1426. doi: 10.1287/mnsc.48.11.1408.268
12. Havens DS, Gittell JH, Vasey J. Impact of relational coordination on nurse job satisfaction, work engagement and burnout: achieving the quadruple aim. J Nurs Adm. 2018;48(3):132-140. doi:10.1097/NNA.0000000000000587
13. Gittell JH, Logan C, Cronenwett J, et al. Impact of relational coordination on staff and patient outcomes in outpatient surgical clinics. Health Care Manage Rev. 2020;45(1):12-20. doi:10.1097/HMR.0000000000000192
14. Cramm JM, Nieboer AP. Relational coordination promotes quality of chronic care delivery in Dutch disease-management programs. Health Care Manage Rev. 2012;37(4):301-309. doi:10.1097/HMR.0b013e3182355ea4
15. Abu-Rish Blakeney E, Lavallee DC, Baik D, Pambianco S, O’Brien KD, Zierler BK. Purposeful interprofessional team intervention improves relational coordination among advanced heart failure care teams. J Interprof Care. 2019;33(5):481-489. doi:10.1080/13561820.2018.1560248
16. Valentine MA, Nembhard IM, Edmondson AC. Measuring teamwork in health care settings: a review of survey instruments. Med Care. 2015;53(4):e16-e30. doi:10.1097/MLR.0b013e31827feef6
17. Glaser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL. Transaction Publishers; 2009.
18. Gittell JH. How interdependent parties build relational coordination to achieve their desired outcomes. Negot J. 2015;31(4):387-391. doi: 10.1111/nejo.12114
19. Solberg MT, Hansen TW, Bjørk IT. The need for predictability in coordination of ventilator treatment of newborn infants--a qualitative study. Intensive Crit Care Nurs. 2015;31(4):205-212. doi:10.1016/j.iccn.2014.12.003
20. Taylor SE, Brown JD. Illusion and well-being: a social psychological perspective on mental health. Psychol Bull. 1988;103(2):193-210.
21. Hartgerink JM, Cramm JM, Bakker TJ, van Eijsden AM, Mackenbach JP, Nieboer AP. The importance of multidisciplinary teamwork and team climate for relational coordination among teams delivering care to older patients. J Adv Nurs. 2014;70(4):791-799. doi:10.1111/jan.12233
22. Bilmes L. soldiers returning from iraq and afghanistan: the long-term costs of providing veterans medical care and disability benefits RWP07-001. https://research.hks.harvard.edu/publications/getFile.aspx?Id=237. Published January 2007. Accessed June 18, 2020.
Chronic pain is common in veterans, and early engagement in pain treatment is recommended to forestall consequences of untreated pain, including depression, disability, and substance use disorders. The Veterans Health Administration (VHA) employs a stepped care model of pain treatment, with the majority of pain care based in primary care (step 1), and an array of specialty/multimodal treatment options made available at each step in the model for patients with more complex problems, or those who do not respond to more conservative interventions.1
Recognizing the need for comprehensive pain care, the US Congress passed the Comprehensive Addiction and Recovery Act, 21 USC §1521 (2016), which included provisions for VHA facilities to offer multimodal pain treatment and to report the availability of pain care options at each step in the stepped care model.2, With the passage of the Veterans Access, Choice, and Accountability Act of 2014, 38 USC §101 (2014) and now the MISSION Act of 2018, 38 USC §703 (2018) veterans whose VHA facilities are too distant, who require care unavailable at that facility, or who have to wait too long to receive care are eligible for treatment at either VHA or non-VHA facilities.3 These laws allocate the same pool of funds to both VHA and community care and thus create an incentive to engage veterans in care within the VHA network so the funds are not spent out of network.4
An opportunity to connect veterans with VHA care arises at specialized VHA Compensation and Pension (C&P) clinics during examinations that determine whether a veteran’s health conditions were caused or exacerbated by their military service. Veterans file claims with the US Department of Veterans Affairs (VA) Veterans Benefits Administration (VBA), which sends the patient to either a VHA facility or private practitioners for these examinations. Although the number of examinations conducted each year is not available, there were 274,528 veterans newly awarded compensation in fiscal year 2018, and a substantial number of the total of 4,743,108 veterans with C&P awards had reevaluation examinations for at least 1 of their conditions during that year.5 Based largely on the compensation examination results, military service records, and medical records, veterans are granted a service-connected rating for conditions deemed related to military service. A service-connection rating between 0% and 100% is assigned by the VBA, with higher ratings indicating more impairment and, consequently, more financial compensation. Service-connection ratings also are used to decide which veterans are in the highest priority groups for receipt of VHA health care services and are exempt from copayments.
Although traditionally thought of as a forensic evaluation with no clinical purpose, the C&P examination process affords many opportunities to explain VHA care to veterans in distress who file claims.6 A randomized clinical trials (RCT) involving veterans with mental health claims and a second RCT including veterans with musculoskeletal claims each found that veterans use more VHA services if offered outreach at the time of the C&P examination.7,8 In addition to clinical benefits, outreach around the time of C&P examinations also might mitigate the well documented adversarial aspects of the service-connection claims process.6,9,10 Currently, such outreach is not part of routine VHA procedures. Ironically, it is the VBA and not VHA that contacts veterans who are awarded service-connection with information about their eligibility for VHA care based on their award.
Connecting veterans to pain treatment can involve clarifying eligibility for VHA care for veterans in whom eligibility is unknown, involving primary care providers (PCPs) who are the fulcrum of VHA pain care referrals, and motivating veterans to seek specific pain treatment modalities. Connecting veterans to treatment at the time of their compensation examinations also likely involves bidirectional cooperation between the specialized C&P clinics where veterans are examined and the clinics that provide treatment.
Relational coordination is a theoretical framework that can describe the horizontal relationships between different teams within the same medical facility. Relational coordination theorizes that communication between workgroups is related synergistically to the quality of relationships between workgroups. Relational coordination is better between workgroups that share goals and often have high levels of relational coordination, which is thought to be especially important when activities are ambiguous, require cooperation, and are conducted under time pressure.11 High relational coordination also has been associated with high staff job satisfaction, high satisfaction with delivered services, and adherence to treatment guidelines.12-14 An observational cohort study suggested that relational coordination can be improved by targeted interventions that bring workgroups together and facilitate intercommunication.15
To better understand referral and engagement for pain treatment at compensation examinations, VA staff from primary care, mental health, pain management, and C&P teams at the 8 VHA medical centers in New England were invited to complete a validated relational coordination survey.11,16 A subset of invited staff participated in a semistructured interview about pain treatment referral practices within their medical centers.
Assessments were conducted as part of a mixed methods formative evaluation involving quantitative and qualitative methods for a clinical trial at the 8 VHA medical centers in New England. The trial is testing an intervention in which veterans presenting for service-connection examinations for musculoskeletal conditions receive brief counseling to engage them in nonopioid pain treatments. The VHA Central Institutional Review Board approved this formative evaluation and the clinical trial has begun (ClinicalTrials.gov NCT04062214).
Potential interviewees were involved in referrals to and provision of nonpharmacologic pain treatment and were identified by site investigators in the randomized trial. Identified interviewees were clinical and administrative staff belonging to VHA Primary Care, Pain Management, and Compensation and Pension clinics. A total of 83 staff were identified.
Semistructured Interviews
A subset of the 83 staff were invited to participate in a semistructured interview because their position impacted coordination of pain care at their facilities or they worked in C&P. Staff at a site were interviewed until no new themes emerged from additional interviews, and each of the 8 sites was represented. Interviews were conducted between June and August 2018. Standardized scripts describing the study and inviting participation in a semistructured interview were e-mailed to VA staff. At the time of the interview the study purpose was restated and consent for audiotaping was obtained. The interviews followed a guide designed to assess a relational coordination framework among various workgroups. The data in this manuscript were elicited by specific prompts concerning: (1) How veterans learn about pain care when they come through C&P; and (2) How staff in C&P communicate with treatment providers about veterans who have chronic pain. Each interview lasted about 30 minutes.
Relational Coordination Survey
All identified staff were invited to participate in a relational coordination survey. The survey was administered through VA REDCap. Survey invitations were e-mailed from REDCap to VA staff and included a description of the study and assurances of the confidentiality of data collected. Surveys took < 10 minutes to complete. To begin, respondents identified their primary workgroup (C&P, primary care, pain management, or administrative leadership or staff), secondary workgroup (if they were in > 1), and site. Respondents provided no other identifying information and were assured their responses would be confidential.
The survey consisted of 7 questions regarding beliefs about the quality of communication and interactions among workgroup members in obtaining a shared goal.11 The shared goal in the survey used in this study was providing pain care services for veterans with musculoskeletal conditions. Using a 5-point Likert scale, the 7 questions concerned frequency, timeliness, and accuracy of communication; response to problems providing pain services; sharing goals; and knowledge and respect for respondent’s job function. Higher scores indicated better relational coordination among members of a workgroup. Using the survey’s 7 items, composite mean relational coordination scores were calculated for each of the 4 primary workgroups. To account for the possibility that a member rated their own workgroups, 2 scores were created for each workgroup; one included members of the workgroup and another excluded them.
Data Analysis
The audio-recorded semistructured interviews were transcribed and entered into Atlas.ti qualitative data analysis software. To identify cross-cutting themes, a semistructured telephone interview guide was developed by the qualitative study team that emphasized interrelationships between different clinical teams. The transcripts were then analyzed using the grounded theory approach, a systematic methodology to reduce themes from collected qualitative data. Two research staff read each transcript twice; first to familiarize themselves with the text and then, using open coding, to identify important concepts that emerged from the language and assign codes to segments of text. To ensure accuracy, researchers included suitable contextual information in the coding. Using the constant comparative method, research staff then met to examine the themes that emerged in the interviews, discuss and coalesce coding discrepancies, and compare perspectives.17
The composite score (mean of the 7 items and 95% CI) of the survey responses was analyzed to identify significant differences in coordination across the 4 workgroups. Analysis of variance (ANOVA) was used to examine each relational coordination score by respondents’ workgroup. Post hoc analyses examined relational coordination survey differences among the 4 respondent groups.
Results
Thirty-nine survey respondents participated in the semistructured interviews. C&P examiners expressed varying degrees of comfort with their role in extending access to pain care for veterans. Some of the examiners strongly believed that their role was purely forensic, and going beyond this forensic role to refer or recommend treatment to veterans would be a violation of their role to conduct a forensic examination. “We don’t have an ongoing therapeutic relationship with any of the patients,” a C&P examiner explained: “We see them once; they’re out the door. It’s forensic. We’re investigating the person as a claimant, we’re investigating it and using our tools to go and review information from 30, 40 years ago.”
Other examiners had a less strict approach for working with veterans in C&P, even though examiners are asked not to provide advice or therapy. One C&P examiner noted that because he “can’t watch people in pain,” during the examination this doctor recommends that patients go to the office that determines whether they are eligible for benefits and choose a PCP. Another C&P examiner concurred with this approach. “I certainly spend a little time with the veteran talking to them about their personal life, who they are, what they do, what they’ve done, what they’re going to do to kind of break the ice between us,” the second examiner explained. “At the end, I will make some suggestions to them. I’m comfortable doing that. I don’t know that everybody is.”
Many of the VHA providers we interviewed had little knowledge of the C&P process or whether C&P examiners had any role or responsibilities in referring veterans for pain care. Most VHA providers could not name any C&P examiners at their facility and were generally unfamiliar with the content of C&P examinations. One provider bluntly said, “I’ve never communicated with anyone in comp and pen [C&P].”
Another PCP also expressed concerns with referrals, suggesting that C&P and primary care “are totally separate and should remain separate,” the PCP explained. “My concern with getting referral from comp and pen is that is it then they’re seeking all sorts of treatment that they wouldn’t necessarily need or ask for otherwise.”
Conversely a different PCP had a positive outlook on how C&P examiners might help ease the transition into the VHA for veterans with pain, especially for newly discharged veterans. “Having comp and pen address these issues is really going to be helpful. I think it could be significant that the topic is introduced early on.”
Relational Coordination Survey
Relational coordination surveys were sent to 83 participants of whom 66 responded. Respondents were from
The relational coordination composite scores were lowest for C&P. This finding remained whether C&P staff surveys were included or removed from the C&P responses. As demonstrated by the 95% CI, when team members’ surveys were included, C&P scores (95% CI, 2.01-2.42) were significantly lower than the primary care (95% CI, 3.34-3.64) and pain management (95% CI, 3.61-3.96) groups. All the relational coordination composite scores were slightly lower when staff who described their own workgroup were removed (ie, respondents rated their own workgroups as having higher relational coordination than others did). Using the composite scores excluding same workgroup members, the composite scores of the C&P remained significantly lower than all 3 other workgroups (Table). Means values for each individual item in the C&P group were significantly less than all other group means for each item except for the question on responses to problems providing pain services (data not shown). On this item only, the mean C&P rating was > 3 (3.19), but this was still lower than the means of the primary care and pain management workgroups.
Further analyses were undertaken to understand the importance of stakeholders’ ratings of their own workgroup compared with ratings by others of that workgroup. A 1-way ANOVA of workgroup was conducted and displayed significant workgroup differences between member and nonmember relational coordination ratings on 3 of the 4 workgroup’s scores C&P (F = 5.75, 3, 62 df; P < .01) primary care (F = 4.30, 3, 62 df; P < .008) and pain management (F = 8.22, 3, 62 df; P < .001). Post hoc contrasts between the different workgroups doing the rating revealed: (1) significant differences in the assessment of the C&P workgroup between the C&P workgroup and both the primary care (P < .01) and pain management groups (P < .001) with C&P rating their own workgroup significantly higher; (2) a significant difference in the scoring of the primary care workgroup with the primary care group rating themselves significantly higher than the C&P group; and (3) significant differences in the scoring of the pain management workgroup with both pain management and primary care groups rating the pain management group significantly higher than the C&P group. The results were not substantially changed by removing the 18 respondents who identified themselves as being part of > 1 workgroup .
Discussion
Mixed methods revealed disparate viewpoints about the role of C&P in referring veterans to pain care services. Overall, C&P teams coordinated less with other workgroups than the other groups coordinated with each other, and the C&P clinics took only limited steps to engage veterans in VHA treatment. The relational coordination results appeared to be valid. The mean scores were near the middle of the relational coordination rating scale, with standard deviations indicating a range of responses. The lower relational coordination scores of the C&P group remained after removing stakeholders who were rating their own workgroup. Further support for the validity of the relational coordination survey results is that they were consistent with the reports of C&P clinic isolation in the semistructured interviews.
The interview data suggest that one reason the C&P teams had low relational coordination scores is that VA staff interpret the emphasis on evaluative rather than therapeutic examinations to preclude other attempts to engage veterans into VHA treatment, even though such treatment engagement is permitted within existing guidelines. VBA referrals for examinations say nothing, either way, about engaging veterans in VHA care. The relational coordination results suggest that an intervention that might increase treatment referrals from the C&P clinics would be to explain the (existing) policy allowing for outreach around the time of compensation examinations to VHA staff so this goal is clearly agreed-upon. Another approach to facilitating treatment engagement at the C&P examination is to use other interventions that have been associated with better relational coordination such as intergroup meetings, horizontal integration more generally, and an atmosphere is which people from different backgrounds feel empowered to speak frankly to each other.15,18,19 An important linkage to forge is between C&P teams and the administrative workgroups responsible for verifying a veteran’s eligibility for VHA care and enrolling eligible veterans in VHA treatment. Having C&P clinicians who are familiar with the eligibility and treatment engagement processes would facilitate providing that information to veterans, without compromising the evaluative format of the compensation examination.
An interesting ancillary finding is that relational coordination ratings by members of 3 of the 4 workgroups were higher than ratings by other staff of that workgroup. A possible explanation for this finding is that workgroup members are more aware of the relational coordination efforts made by their own workgroup than those by other workgroups, and therefore rate their own workgroup higher. This also might be part of a broader self-aggrandizement heuristic that has been described in multiple domains.20 Staff may apply this heuristic in reporting that their staff engage in more relational coordination, reflecting the social desirability of being cooperative.
There are simple facility-level interventions that would facilitate veterans access to care such as conducting C&P examinations for potentially treatment-eligible veterans at VHA facilities (vs conducted outside VHA) and having access to materials that explain the treatment options to veterans when they check in for their compensation examinations. The approach to C&P-based treatment engagement that was successfully employed in 2 clinical trials involved having counselors not connected with the C&P clinic contact veterans around the time of their compensation examination to explain VA treatment options and motivate veterans to pursue treatment.8,9 This independent counselor approach is being evaluated in a larger study.
Limitations
These data are from a small number of VA staff evaluating veterans in a single region of the US. They do not show causation, and it is possible that relational coordination is not necessary for referrals from C&P clinics. Relational coordination might not be necessary when referral processes can be simply routinized with little need for communication.11 However, other analyses in these clinics have found that pain treatment referrals in fact are not routinized, with substantial variability within and across institutions. Another possibility is that features that have been associated with less relational coordination, such as male gender and medical specialist guild, were disproportionately present in C&P clinics compared to the other clinics.21
Conclusions
There have been public calls to improve the evaluation of service-connection claims such that this process includes approaches to engage veterans in treatment.22 Referring veterans to treatment when they come for C&P examinations will likely involve improving relational coordination between the C&P service and other parts of VHA. Nationwide, sites that integrate C&P more fully may have valuable lessons to impart about the benefits of such integration. An important step towards better relational coordination will be clarifying that engaging veterans in VHA care around the time of their C&P examinations is a facility-wide goal.
Acknowledgments
The authors thank Brian Linde and Efia James for their perspectives on C&P procedures. This work was supported by the Veterans Integrated Service Network 1 Mental Illness Research Education and Clinical Center (MIRECC) and National Institute of Health, National Center for Complementary and Integrative Health Project # 5UG3AT009758-02. (MIR, SM mPIs).
Chronic pain is common in veterans, and early engagement in pain treatment is recommended to forestall consequences of untreated pain, including depression, disability, and substance use disorders. The Veterans Health Administration (VHA) employs a stepped care model of pain treatment, with the majority of pain care based in primary care (step 1), and an array of specialty/multimodal treatment options made available at each step in the model for patients with more complex problems, or those who do not respond to more conservative interventions.1
Recognizing the need for comprehensive pain care, the US Congress passed the Comprehensive Addiction and Recovery Act, 21 USC §1521 (2016), which included provisions for VHA facilities to offer multimodal pain treatment and to report the availability of pain care options at each step in the stepped care model.2, With the passage of the Veterans Access, Choice, and Accountability Act of 2014, 38 USC §101 (2014) and now the MISSION Act of 2018, 38 USC §703 (2018) veterans whose VHA facilities are too distant, who require care unavailable at that facility, or who have to wait too long to receive care are eligible for treatment at either VHA or non-VHA facilities.3 These laws allocate the same pool of funds to both VHA and community care and thus create an incentive to engage veterans in care within the VHA network so the funds are not spent out of network.4
An opportunity to connect veterans with VHA care arises at specialized VHA Compensation and Pension (C&P) clinics during examinations that determine whether a veteran’s health conditions were caused or exacerbated by their military service. Veterans file claims with the US Department of Veterans Affairs (VA) Veterans Benefits Administration (VBA), which sends the patient to either a VHA facility or private practitioners for these examinations. Although the number of examinations conducted each year is not available, there were 274,528 veterans newly awarded compensation in fiscal year 2018, and a substantial number of the total of 4,743,108 veterans with C&P awards had reevaluation examinations for at least 1 of their conditions during that year.5 Based largely on the compensation examination results, military service records, and medical records, veterans are granted a service-connected rating for conditions deemed related to military service. A service-connection rating between 0% and 100% is assigned by the VBA, with higher ratings indicating more impairment and, consequently, more financial compensation. Service-connection ratings also are used to decide which veterans are in the highest priority groups for receipt of VHA health care services and are exempt from copayments.
Although traditionally thought of as a forensic evaluation with no clinical purpose, the C&P examination process affords many opportunities to explain VHA care to veterans in distress who file claims.6 A randomized clinical trials (RCT) involving veterans with mental health claims and a second RCT including veterans with musculoskeletal claims each found that veterans use more VHA services if offered outreach at the time of the C&P examination.7,8 In addition to clinical benefits, outreach around the time of C&P examinations also might mitigate the well documented adversarial aspects of the service-connection claims process.6,9,10 Currently, such outreach is not part of routine VHA procedures. Ironically, it is the VBA and not VHA that contacts veterans who are awarded service-connection with information about their eligibility for VHA care based on their award.
Connecting veterans to pain treatment can involve clarifying eligibility for VHA care for veterans in whom eligibility is unknown, involving primary care providers (PCPs) who are the fulcrum of VHA pain care referrals, and motivating veterans to seek specific pain treatment modalities. Connecting veterans to treatment at the time of their compensation examinations also likely involves bidirectional cooperation between the specialized C&P clinics where veterans are examined and the clinics that provide treatment.
Relational coordination is a theoretical framework that can describe the horizontal relationships between different teams within the same medical facility. Relational coordination theorizes that communication between workgroups is related synergistically to the quality of relationships between workgroups. Relational coordination is better between workgroups that share goals and often have high levels of relational coordination, which is thought to be especially important when activities are ambiguous, require cooperation, and are conducted under time pressure.11 High relational coordination also has been associated with high staff job satisfaction, high satisfaction with delivered services, and adherence to treatment guidelines.12-14 An observational cohort study suggested that relational coordination can be improved by targeted interventions that bring workgroups together and facilitate intercommunication.15
To better understand referral and engagement for pain treatment at compensation examinations, VA staff from primary care, mental health, pain management, and C&P teams at the 8 VHA medical centers in New England were invited to complete a validated relational coordination survey.11,16 A subset of invited staff participated in a semistructured interview about pain treatment referral practices within their medical centers.
Assessments were conducted as part of a mixed methods formative evaluation involving quantitative and qualitative methods for a clinical trial at the 8 VHA medical centers in New England. The trial is testing an intervention in which veterans presenting for service-connection examinations for musculoskeletal conditions receive brief counseling to engage them in nonopioid pain treatments. The VHA Central Institutional Review Board approved this formative evaluation and the clinical trial has begun (ClinicalTrials.gov NCT04062214).
Potential interviewees were involved in referrals to and provision of nonpharmacologic pain treatment and were identified by site investigators in the randomized trial. Identified interviewees were clinical and administrative staff belonging to VHA Primary Care, Pain Management, and Compensation and Pension clinics. A total of 83 staff were identified.
Semistructured Interviews
A subset of the 83 staff were invited to participate in a semistructured interview because their position impacted coordination of pain care at their facilities or they worked in C&P. Staff at a site were interviewed until no new themes emerged from additional interviews, and each of the 8 sites was represented. Interviews were conducted between June and August 2018. Standardized scripts describing the study and inviting participation in a semistructured interview were e-mailed to VA staff. At the time of the interview the study purpose was restated and consent for audiotaping was obtained. The interviews followed a guide designed to assess a relational coordination framework among various workgroups. The data in this manuscript were elicited by specific prompts concerning: (1) How veterans learn about pain care when they come through C&P; and (2) How staff in C&P communicate with treatment providers about veterans who have chronic pain. Each interview lasted about 30 minutes.
Relational Coordination Survey
All identified staff were invited to participate in a relational coordination survey. The survey was administered through VA REDCap. Survey invitations were e-mailed from REDCap to VA staff and included a description of the study and assurances of the confidentiality of data collected. Surveys took < 10 minutes to complete. To begin, respondents identified their primary workgroup (C&P, primary care, pain management, or administrative leadership or staff), secondary workgroup (if they were in > 1), and site. Respondents provided no other identifying information and were assured their responses would be confidential.
The survey consisted of 7 questions regarding beliefs about the quality of communication and interactions among workgroup members in obtaining a shared goal.11 The shared goal in the survey used in this study was providing pain care services for veterans with musculoskeletal conditions. Using a 5-point Likert scale, the 7 questions concerned frequency, timeliness, and accuracy of communication; response to problems providing pain services; sharing goals; and knowledge and respect for respondent’s job function. Higher scores indicated better relational coordination among members of a workgroup. Using the survey’s 7 items, composite mean relational coordination scores were calculated for each of the 4 primary workgroups. To account for the possibility that a member rated their own workgroups, 2 scores were created for each workgroup; one included members of the workgroup and another excluded them.
Data Analysis
The audio-recorded semistructured interviews were transcribed and entered into Atlas.ti qualitative data analysis software. To identify cross-cutting themes, a semistructured telephone interview guide was developed by the qualitative study team that emphasized interrelationships between different clinical teams. The transcripts were then analyzed using the grounded theory approach, a systematic methodology to reduce themes from collected qualitative data. Two research staff read each transcript twice; first to familiarize themselves with the text and then, using open coding, to identify important concepts that emerged from the language and assign codes to segments of text. To ensure accuracy, researchers included suitable contextual information in the coding. Using the constant comparative method, research staff then met to examine the themes that emerged in the interviews, discuss and coalesce coding discrepancies, and compare perspectives.17
The composite score (mean of the 7 items and 95% CI) of the survey responses was analyzed to identify significant differences in coordination across the 4 workgroups. Analysis of variance (ANOVA) was used to examine each relational coordination score by respondents’ workgroup. Post hoc analyses examined relational coordination survey differences among the 4 respondent groups.
Results
Thirty-nine survey respondents participated in the semistructured interviews. C&P examiners expressed varying degrees of comfort with their role in extending access to pain care for veterans. Some of the examiners strongly believed that their role was purely forensic, and going beyond this forensic role to refer or recommend treatment to veterans would be a violation of their role to conduct a forensic examination. “We don’t have an ongoing therapeutic relationship with any of the patients,” a C&P examiner explained: “We see them once; they’re out the door. It’s forensic. We’re investigating the person as a claimant, we’re investigating it and using our tools to go and review information from 30, 40 years ago.”
Other examiners had a less strict approach for working with veterans in C&P, even though examiners are asked not to provide advice or therapy. One C&P examiner noted that because he “can’t watch people in pain,” during the examination this doctor recommends that patients go to the office that determines whether they are eligible for benefits and choose a PCP. Another C&P examiner concurred with this approach. “I certainly spend a little time with the veteran talking to them about their personal life, who they are, what they do, what they’ve done, what they’re going to do to kind of break the ice between us,” the second examiner explained. “At the end, I will make some suggestions to them. I’m comfortable doing that. I don’t know that everybody is.”
Many of the VHA providers we interviewed had little knowledge of the C&P process or whether C&P examiners had any role or responsibilities in referring veterans for pain care. Most VHA providers could not name any C&P examiners at their facility and were generally unfamiliar with the content of C&P examinations. One provider bluntly said, “I’ve never communicated with anyone in comp and pen [C&P].”
Another PCP also expressed concerns with referrals, suggesting that C&P and primary care “are totally separate and should remain separate,” the PCP explained. “My concern with getting referral from comp and pen is that is it then they’re seeking all sorts of treatment that they wouldn’t necessarily need or ask for otherwise.”
Conversely a different PCP had a positive outlook on how C&P examiners might help ease the transition into the VHA for veterans with pain, especially for newly discharged veterans. “Having comp and pen address these issues is really going to be helpful. I think it could be significant that the topic is introduced early on.”
Relational Coordination Survey
Relational coordination surveys were sent to 83 participants of whom 66 responded. Respondents were from
The relational coordination composite scores were lowest for C&P. This finding remained whether C&P staff surveys were included or removed from the C&P responses. As demonstrated by the 95% CI, when team members’ surveys were included, C&P scores (95% CI, 2.01-2.42) were significantly lower than the primary care (95% CI, 3.34-3.64) and pain management (95% CI, 3.61-3.96) groups. All the relational coordination composite scores were slightly lower when staff who described their own workgroup were removed (ie, respondents rated their own workgroups as having higher relational coordination than others did). Using the composite scores excluding same workgroup members, the composite scores of the C&P remained significantly lower than all 3 other workgroups (Table). Means values for each individual item in the C&P group were significantly less than all other group means for each item except for the question on responses to problems providing pain services (data not shown). On this item only, the mean C&P rating was > 3 (3.19), but this was still lower than the means of the primary care and pain management workgroups.
Further analyses were undertaken to understand the importance of stakeholders’ ratings of their own workgroup compared with ratings by others of that workgroup. A 1-way ANOVA of workgroup was conducted and displayed significant workgroup differences between member and nonmember relational coordination ratings on 3 of the 4 workgroup’s scores C&P (F = 5.75, 3, 62 df; P < .01) primary care (F = 4.30, 3, 62 df; P < .008) and pain management (F = 8.22, 3, 62 df; P < .001). Post hoc contrasts between the different workgroups doing the rating revealed: (1) significant differences in the assessment of the C&P workgroup between the C&P workgroup and both the primary care (P < .01) and pain management groups (P < .001) with C&P rating their own workgroup significantly higher; (2) a significant difference in the scoring of the primary care workgroup with the primary care group rating themselves significantly higher than the C&P group; and (3) significant differences in the scoring of the pain management workgroup with both pain management and primary care groups rating the pain management group significantly higher than the C&P group. The results were not substantially changed by removing the 18 respondents who identified themselves as being part of > 1 workgroup .
Discussion
Mixed methods revealed disparate viewpoints about the role of C&P in referring veterans to pain care services. Overall, C&P teams coordinated less with other workgroups than the other groups coordinated with each other, and the C&P clinics took only limited steps to engage veterans in VHA treatment. The relational coordination results appeared to be valid. The mean scores were near the middle of the relational coordination rating scale, with standard deviations indicating a range of responses. The lower relational coordination scores of the C&P group remained after removing stakeholders who were rating their own workgroup. Further support for the validity of the relational coordination survey results is that they were consistent with the reports of C&P clinic isolation in the semistructured interviews.
The interview data suggest that one reason the C&P teams had low relational coordination scores is that VA staff interpret the emphasis on evaluative rather than therapeutic examinations to preclude other attempts to engage veterans into VHA treatment, even though such treatment engagement is permitted within existing guidelines. VBA referrals for examinations say nothing, either way, about engaging veterans in VHA care. The relational coordination results suggest that an intervention that might increase treatment referrals from the C&P clinics would be to explain the (existing) policy allowing for outreach around the time of compensation examinations to VHA staff so this goal is clearly agreed-upon. Another approach to facilitating treatment engagement at the C&P examination is to use other interventions that have been associated with better relational coordination such as intergroup meetings, horizontal integration more generally, and an atmosphere is which people from different backgrounds feel empowered to speak frankly to each other.15,18,19 An important linkage to forge is between C&P teams and the administrative workgroups responsible for verifying a veteran’s eligibility for VHA care and enrolling eligible veterans in VHA treatment. Having C&P clinicians who are familiar with the eligibility and treatment engagement processes would facilitate providing that information to veterans, without compromising the evaluative format of the compensation examination.
An interesting ancillary finding is that relational coordination ratings by members of 3 of the 4 workgroups were higher than ratings by other staff of that workgroup. A possible explanation for this finding is that workgroup members are more aware of the relational coordination efforts made by their own workgroup than those by other workgroups, and therefore rate their own workgroup higher. This also might be part of a broader self-aggrandizement heuristic that has been described in multiple domains.20 Staff may apply this heuristic in reporting that their staff engage in more relational coordination, reflecting the social desirability of being cooperative.
There are simple facility-level interventions that would facilitate veterans access to care such as conducting C&P examinations for potentially treatment-eligible veterans at VHA facilities (vs conducted outside VHA) and having access to materials that explain the treatment options to veterans when they check in for their compensation examinations. The approach to C&P-based treatment engagement that was successfully employed in 2 clinical trials involved having counselors not connected with the C&P clinic contact veterans around the time of their compensation examination to explain VA treatment options and motivate veterans to pursue treatment.8,9 This independent counselor approach is being evaluated in a larger study.
Limitations
These data are from a small number of VA staff evaluating veterans in a single region of the US. They do not show causation, and it is possible that relational coordination is not necessary for referrals from C&P clinics. Relational coordination might not be necessary when referral processes can be simply routinized with little need for communication.11 However, other analyses in these clinics have found that pain treatment referrals in fact are not routinized, with substantial variability within and across institutions. Another possibility is that features that have been associated with less relational coordination, such as male gender and medical specialist guild, were disproportionately present in C&P clinics compared to the other clinics.21
Conclusions
There have been public calls to improve the evaluation of service-connection claims such that this process includes approaches to engage veterans in treatment.22 Referring veterans to treatment when they come for C&P examinations will likely involve improving relational coordination between the C&P service and other parts of VHA. Nationwide, sites that integrate C&P more fully may have valuable lessons to impart about the benefits of such integration. An important step towards better relational coordination will be clarifying that engaging veterans in VHA care around the time of their C&P examinations is a facility-wide goal.
Acknowledgments
The authors thank Brian Linde and Efia James for their perspectives on C&P procedures. This work was supported by the Veterans Integrated Service Network 1 Mental Illness Research Education and Clinical Center (MIRECC) and National Institute of Health, National Center for Complementary and Integrative Health Project # 5UG3AT009758-02. (MIR, SM mPIs).
1. US Department Veterans Affairs, Veterans Health Administration. VHA Directive 2009-053: pain management. https://www.va.gov/painmanagement/docs/vha09paindirective.pdf. Published October 28, 2019. Accessed June 18, 2020.
2. Rosenberger PH, Phillip EJ, Lee A, Kerns RD. The VHA’s national pain management strategy: implementing the stepped care model. Fed Pract. 2011;28(8):39-42.
3. Mattocks KM, Mengeling M, Sadler A, Baldor R, Bastian L. The Veterans Choice Act: A Qualitative Examination of Rapid Policy Implementation in the Department of Veterans Affairs. Med Care. 2017;55 Suppl 7 Suppl 1:S71-S75. doi:10.1097/MLR.0000000000000667
4. Rieselbach RE, Epperly T, Nycz G, Shin P. Community health centers could provide better outsourced primary care for veterans. J Gen Intern Med. 2019;34(1):150-153. doi:10.1007/s11606-018-4691-4
5. US Department of Veterans Affairs, Veterans Benefit Administration. VBA annual benefits report fiscal year 2018. https://www.benefits.va.gov/REPORTS/abr/docs/2018-abr.pdf. Updated March 29, 2019. Accessed June 17, 2020.
6. Rosen MI. Compensation examinations for PTSD-an opportunity for treatment? J Rehabil Res Dev. 2010;47(5):xv-xxii. doi:10.1682/jrrd.2010.04.0075
7. Rosen MI, Ablondi K, Black AC, et al. Work outcomes after benefits counseling among veterans applying for service connection for a psychiatric condition. Psychiatr Serv. 2014;65(12):1426-1432. doi:10.1176/appi.ps.201300478
8. Rosen MI, Becker WC, Black AC, Martino S, Edens EL, Kerns RD. Brief counseling for veterans with musculoskeletal disorder, risky substance use, and service connection claims. Pain Med. 2019;20(3):528-542. doi:10.1093/pm/pny071
9. Meshberg-Cohen S, DeViva JC, Rosen MI. Counseling veterans applying for service connection status for mental health conditions. Psychiatr Serv. 2017;68(4):396-399. doi:10.1176/appi.ps.201500533
10. Sayer NA, Spoont M, Nelson DB. Post-traumatic stress disorder claims from the viewpoint of veterans service officers. Mil Med. 2005;170(10):867-870. doi:10.7205/milmed.170.10.867
11. Gittell JH. Coordinating mechanisms in care provider groups: relational coordination as a mediator and input uncertainty as a moderator of performance effects. Manage Sci. 2002;48(11):1408-1426. doi: 10.1287/mnsc.48.11.1408.268
12. Havens DS, Gittell JH, Vasey J. Impact of relational coordination on nurse job satisfaction, work engagement and burnout: achieving the quadruple aim. J Nurs Adm. 2018;48(3):132-140. doi:10.1097/NNA.0000000000000587
13. Gittell JH, Logan C, Cronenwett J, et al. Impact of relational coordination on staff and patient outcomes in outpatient surgical clinics. Health Care Manage Rev. 2020;45(1):12-20. doi:10.1097/HMR.0000000000000192
14. Cramm JM, Nieboer AP. Relational coordination promotes quality of chronic care delivery in Dutch disease-management programs. Health Care Manage Rev. 2012;37(4):301-309. doi:10.1097/HMR.0b013e3182355ea4
15. Abu-Rish Blakeney E, Lavallee DC, Baik D, Pambianco S, O’Brien KD, Zierler BK. Purposeful interprofessional team intervention improves relational coordination among advanced heart failure care teams. J Interprof Care. 2019;33(5):481-489. doi:10.1080/13561820.2018.1560248
16. Valentine MA, Nembhard IM, Edmondson AC. Measuring teamwork in health care settings: a review of survey instruments. Med Care. 2015;53(4):e16-e30. doi:10.1097/MLR.0b013e31827feef6
17. Glaser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL. Transaction Publishers; 2009.
18. Gittell JH. How interdependent parties build relational coordination to achieve their desired outcomes. Negot J. 2015;31(4):387-391. doi: 10.1111/nejo.12114
19. Solberg MT, Hansen TW, Bjørk IT. The need for predictability in coordination of ventilator treatment of newborn infants--a qualitative study. Intensive Crit Care Nurs. 2015;31(4):205-212. doi:10.1016/j.iccn.2014.12.003
20. Taylor SE, Brown JD. Illusion and well-being: a social psychological perspective on mental health. Psychol Bull. 1988;103(2):193-210.
21. Hartgerink JM, Cramm JM, Bakker TJ, van Eijsden AM, Mackenbach JP, Nieboer AP. The importance of multidisciplinary teamwork and team climate for relational coordination among teams delivering care to older patients. J Adv Nurs. 2014;70(4):791-799. doi:10.1111/jan.12233
22. Bilmes L. soldiers returning from iraq and afghanistan: the long-term costs of providing veterans medical care and disability benefits RWP07-001. https://research.hks.harvard.edu/publications/getFile.aspx?Id=237. Published January 2007. Accessed June 18, 2020.
1. US Department Veterans Affairs, Veterans Health Administration. VHA Directive 2009-053: pain management. https://www.va.gov/painmanagement/docs/vha09paindirective.pdf. Published October 28, 2019. Accessed June 18, 2020.
2. Rosenberger PH, Phillip EJ, Lee A, Kerns RD. The VHA’s national pain management strategy: implementing the stepped care model. Fed Pract. 2011;28(8):39-42.
3. Mattocks KM, Mengeling M, Sadler A, Baldor R, Bastian L. The Veterans Choice Act: A Qualitative Examination of Rapid Policy Implementation in the Department of Veterans Affairs. Med Care. 2017;55 Suppl 7 Suppl 1:S71-S75. doi:10.1097/MLR.0000000000000667
4. Rieselbach RE, Epperly T, Nycz G, Shin P. Community health centers could provide better outsourced primary care for veterans. J Gen Intern Med. 2019;34(1):150-153. doi:10.1007/s11606-018-4691-4
5. US Department of Veterans Affairs, Veterans Benefit Administration. VBA annual benefits report fiscal year 2018. https://www.benefits.va.gov/REPORTS/abr/docs/2018-abr.pdf. Updated March 29, 2019. Accessed June 17, 2020.
6. Rosen MI. Compensation examinations for PTSD-an opportunity for treatment? J Rehabil Res Dev. 2010;47(5):xv-xxii. doi:10.1682/jrrd.2010.04.0075
7. Rosen MI, Ablondi K, Black AC, et al. Work outcomes after benefits counseling among veterans applying for service connection for a psychiatric condition. Psychiatr Serv. 2014;65(12):1426-1432. doi:10.1176/appi.ps.201300478
8. Rosen MI, Becker WC, Black AC, Martino S, Edens EL, Kerns RD. Brief counseling for veterans with musculoskeletal disorder, risky substance use, and service connection claims. Pain Med. 2019;20(3):528-542. doi:10.1093/pm/pny071
9. Meshberg-Cohen S, DeViva JC, Rosen MI. Counseling veterans applying for service connection status for mental health conditions. Psychiatr Serv. 2017;68(4):396-399. doi:10.1176/appi.ps.201500533
10. Sayer NA, Spoont M, Nelson DB. Post-traumatic stress disorder claims from the viewpoint of veterans service officers. Mil Med. 2005;170(10):867-870. doi:10.7205/milmed.170.10.867
11. Gittell JH. Coordinating mechanisms in care provider groups: relational coordination as a mediator and input uncertainty as a moderator of performance effects. Manage Sci. 2002;48(11):1408-1426. doi: 10.1287/mnsc.48.11.1408.268
12. Havens DS, Gittell JH, Vasey J. Impact of relational coordination on nurse job satisfaction, work engagement and burnout: achieving the quadruple aim. J Nurs Adm. 2018;48(3):132-140. doi:10.1097/NNA.0000000000000587
13. Gittell JH, Logan C, Cronenwett J, et al. Impact of relational coordination on staff and patient outcomes in outpatient surgical clinics. Health Care Manage Rev. 2020;45(1):12-20. doi:10.1097/HMR.0000000000000192
14. Cramm JM, Nieboer AP. Relational coordination promotes quality of chronic care delivery in Dutch disease-management programs. Health Care Manage Rev. 2012;37(4):301-309. doi:10.1097/HMR.0b013e3182355ea4
15. Abu-Rish Blakeney E, Lavallee DC, Baik D, Pambianco S, O’Brien KD, Zierler BK. Purposeful interprofessional team intervention improves relational coordination among advanced heart failure care teams. J Interprof Care. 2019;33(5):481-489. doi:10.1080/13561820.2018.1560248
16. Valentine MA, Nembhard IM, Edmondson AC. Measuring teamwork in health care settings: a review of survey instruments. Med Care. 2015;53(4):e16-e30. doi:10.1097/MLR.0b013e31827feef6
17. Glaser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL. Transaction Publishers; 2009.
18. Gittell JH. How interdependent parties build relational coordination to achieve their desired outcomes. Negot J. 2015;31(4):387-391. doi: 10.1111/nejo.12114
19. Solberg MT, Hansen TW, Bjørk IT. The need for predictability in coordination of ventilator treatment of newborn infants--a qualitative study. Intensive Crit Care Nurs. 2015;31(4):205-212. doi:10.1016/j.iccn.2014.12.003
20. Taylor SE, Brown JD. Illusion and well-being: a social psychological perspective on mental health. Psychol Bull. 1988;103(2):193-210.
21. Hartgerink JM, Cramm JM, Bakker TJ, van Eijsden AM, Mackenbach JP, Nieboer AP. The importance of multidisciplinary teamwork and team climate for relational coordination among teams delivering care to older patients. J Adv Nurs. 2014;70(4):791-799. doi:10.1111/jan.12233
22. Bilmes L. soldiers returning from iraq and afghanistan: the long-term costs of providing veterans medical care and disability benefits RWP07-001. https://research.hks.harvard.edu/publications/getFile.aspx?Id=237. Published January 2007. Accessed June 18, 2020.
Implementation and Evaluation of a 90-Minute Rituximab Infusion Protocol at the Richard L. Roudebush VA Medical Center
Rituximab is a genetically engineered chimeric immunoglobulin G1 monoclonal antibody. It functions by binding to the CD20 antigen on the surface of B-cell lymphocytes, leading to complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity.1 The US Food and Drug Administration approved this therapy to treat patients with B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia, along with other nonmalignant indications, including pemphigus vulgaris and rheumatoid arthritis (RA). Historically, a significant amount of time and labor on behalf of medical personnel has been required to administer rituximab according to the original manufacturer’s labeling due to the boxed warning associated with infusion-related reactions (IRRs).2
Originally, the elongated infusion times that were recommended for rituximab were largely due to the perceived risk of serious infusion-related adverse drug reactions. Slower infusion times should reduce the risk of a reaction and are considered to be a good option for those patients who are at a high risk of having a severe IRRs to rituximab. Examples of high-risk patients from previous studies include those with significant cardiovascular disease, a circulating lymphocyte count ≤ 5,000/µL at the start of infusion, and those who have previously had a reaction to rituximab.3-5 In appropriate patients, research has shown a decreasing incidence of all-grade IRRs for patients who are prescribed rituximab as they receive more doses of the drug.2,6 The ability to identify suitable patients for 90-minute infusions of rituximab and the prospect of better health system resource utilization has led investigators to study the effects of shortened infusion times.
The RATE trial addressed this subject with a phase 3 safety study on the effects of a 90-minute rituximab infusion for patients with previously untreated diffuse large B-cell and follicular lymphoma.3 The patients in this study received their first dose of rituximab using the traditional infusion approach. If it was well-tolerated, they received subsequent rituximab infusions using a 90-minute protocol. Only 1.1% of patients who had previously received a rituximab infusion developed a grade 3 or 4 IRR when receiving a faster infusion of the drug for the first time.3 This result led to the addition of instructions for a 90-minute infusion to the package insert.2
In contrast to the RATE trial, the RATE-RA trial evaluated the incidence of IRRs in patients who received rituximab for nonmalignant indications. This study assessed patients with RA receiving rituximab for > 120 minutes. The authors reported 0.6% of the patients in the study developed a grade 3 or 4 IRR associated with the first 120-minute infusion of the medication.5 The researchers concluded that rituximab can be administered at a faster rate during second and subsequent infusions in patients who have been shown to tolerate traditional infusions without increasing the risk or severity of IRRs.5
The US Department of Veterans Affairs (VA) Richard L. Roudebush VA Medical Center (RLRVAMC) in Indianapolis, Indiana, uses traditional directions for the infusion of rituximab due to perceived tolerability and safety concerns specifically in a veteran population—even while other VA medical centers have implemented shortened infusion protocols. This also is despite the fact that available research shows rapid infusions of the drug are well tolerated in a variety of community settings.7,8 Anticipated benefits of implementing a protocol include savings in chair time at the institution’s infusion clinic along with increased nursing and patient satisfaction. This project was conducted to prepare, implement, and assess the safety of a 90-minute rituximab protocol at the RLRVAMC.
Methods
Proactive measures were required before and during the implementation of the 90-minute protocol to ensure patient safety and staff satisfaction. Updates to the RLRVAMC policy for the management of medical emergencies within the infusion center were reviewed and approved by the acute care committee and nursing leadership. A protocol was developed to identify eligible patients, outline the hypersensitivity protocol, instruct pharmacy personnel on admixture preparation, and provide a titration schedule based on dose. Order sets also were created to assist health care providers (HCPs) with the prescribing of rituximab for nonantineoplastic indications. Educational materials were crafted to assist with order verification, product preparation, labeling, and programming of infusion pumps. Live education was provided for physicians, pharmacists, and nurses to ensure smooth implementation of the protocol and appropriate management of medical emergencies based on the updated policy.
Study Design
Nursing staff in the infusion clinic were surveyed once before a live education session and again after the conclusion of the study. The purpose of the survey was to assess the prior experience and current comfort level of the nursing staff with administering rituximab over 90 minutes. Nurses were asked the following questions: (1) Do you have prior experience administering rituximab via 90-minute infusion; and (2) do you feel comfortable administering rituximab via 90-minute infusion?
A weekly report of patients who received rituximab between November 1, 2018 through April 1, 2019 at the RLRVAMC was generated. HCPs were alerted to eligible patients based on protocol requirements. The HCPs then made the final determination and entered orders accordingly.
This study was a retrospective chart review of all who patients received a rapid infusion of rituximab. Patients who were included if they were aged ≥ 18 years, received rituximab infusions in the RLRVAMC infusion clinic, had an absolute lymphocyte count ≤ 5,000/mm3 at the time of their rapid infusions, had no significant baseline cardiovascular disease or respiratory compromise, and had no prior grade 3 or 4 rituximab IRRs as defined by Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.9 This study was a quality improvement initiative and considered exempt by the institutional review board. All data were deidentified and secured to ensure patient privacy.
The primary endpoint for this study was the incidence of grade 3 or 4 IRRs associated with the rapid infusion of rituximab. Secondary endpoints included the proportion of patients who experienced a grade 3 or 4 infusion reaction, who received proper treatment according to the institution’s hypersensitivity protocol, savings in infusion clinic chair time, and nursing satisfaction with education and implementation of the rapid infusion rituximab protocol.
The following data were collected for all included patients: demographics, lactic acid dehydrogenase level, white blood cell count, and absolute lymphocyte count prior to rituximab infusion, indication for treatment, dose of rituximab for 90-minute infusion, date of infusion, starting time, ending time, number of previous rituximab infusions within the past 3 months, symptoms of infusion reactions during rituximab infusion, and grade of any infusion reactions that occurred.
Estimated savings in infusion clinic chair time was calculated by taking the difference in time between each completed rapid infusion and the estimated amount of time it would have taken for each patient to receive a traditional infusion. The estimated amount of time for traditional infusion was determined by following the institution’s protocol for administering rituximab to patients who previously tolerated their first dose of the drug (eg, 100 mg/h starting rate and increasing by 100 mg/h every 30 minutes to a maximum infusion rate of 400 mg/h). All endpoints were analyzed using descriptive statistics.
Results
Between November 1, 2018 and April 1, 2019, 11 patients received a total of 24 rapid infusions of rituximab. The majority of patients included in the study were older males, and the most common indication for rapid infusion was follicular lymphoma (Table 1).
Primary Endpoint
All patients who received a rapid infusion of rituximab were reviewed in the analysis of the primary and secondary endpoints. Among the 24 rapid infusions of rituximab, 1 infusion was stopped due to the patient experiencing a grade 3 IRR according to criteria from CTCAE Version 5.0. The patient was found to have dysphagia at baseline and experienced severe symptoms in the days following the first infusion that put the patient at high risk for subsequent infusion related concerns. Eligibility criteria for the 90-minute protocol were updated based on these findings. No patient experienced a grade 4 or 5 IRR. The remaining 23 infusions were well tolerated by the patients with no clinically significant events.
Secondary Endpoints
The patient who experienced a grade 3 IRR to rituximab received proper treatment by infusion clinic nurses according to the RLRVAMC hypersensitivity protocol. Patients who received rapid infusions of rituximab had a mean length of infusion of 95.0 minutes. This was in contrast to the mean time of each patient’s previous nonrapid infusion of 134.3 minutes. The difference between the 2 values equated to a savings in infusion clinic chair mean time of 39.3 minutes per patient.
Nurses were asked whether they had prior experience administering rituximab via 90-minute infusion and whether they felt comfortable administering a 90-minute rituximab infusion. Before the live education session, none of the nurses surveyed had prior experience or felt comfortable administering rituximab over 90 minutes. When the nurses were surveyed poststudy, all reported that they were experienced administering rituximab and felt comfortable with the process (Table 2).
Discussion
The infusion of rituximab has been associated with significant challenges related to the time and labor required. Although a vast number of institutions across the country now infuse the medication over an abbreviated time, HCP concerns for patient safety and appropriate use of hypersensitivity protocol in a veteran population delayed implementation at RLRVAMC. The results from this quality improvement initiative highlight the positive impact of the proactive measures that were used to implement the rapid infusion protocol for rituximab on improving HCP prescribing rates, nursing satisfaction, and appropriate management of IRRs.
Rapid infusion saved on average 39.3 minutes per patient in infusion clinic chair time. Each successful rapid infusion of rituximab potentially opened additional time in clinic for ≥ 1 patients to receive an infusion therapy. The RLRVAMC usually operated at maximum capacity, so the ability to accommodate more patients helped decrease hospital admittances for time-sensitive infusions.
The initial criteria used to screen patients to determine whether a rapid infusion of rituximab would be appropriate was based on inclusion and exclusion criteria for past studies on the same subject.3-5 The incidence of hypersensitivity reactions associated with study participants who received rapid rituximab infusions also resembles past research done on the subject, which is important to note due to prior misconceptions of staff at the institution of a higher risk of reaction in this specific veteran population. One patient with RA experienced a grade 3 IRR in this study. Although this patient met the original inclusion criteria, the patient had baseline dysphagia, and following the first infusion, reported to the emergency department (ED) with symptoms of delayed anaphylaxis. In this case, the order for rapid infusion was placed in advance and the prescriber was unaware of the ED visit. Based on this event, eligibility criteria for 90-minute rituximab infusions were updated to include additional information specifying that candidates for a rapid infusion also may have no baseline airway compromise. This hypersensitivity reaction also highlighted the need for decision support technology to assist HCPs in patient selection as well as empowering nursing and pharmacy staff to identify concerns once they place orders.
Over the course of the study, investigators assisted the HCPs with preparation of orders for the rapid infusion of rituximab for antineoplastic indications. Due to feasibility issues with this practice moving forward, order sets containing rituximab were updated to include a 90-minute option. This created a more standardized process that allowed HCPs to screen potential patients on their own. The expectation is that HCPs will be more likely to order 90-minute infusions for eligible patients in the future with this efficient and safer process.
Limitations
The small sample size in this study was a limitation. Retrospective data related to the management of infusion reactions and length of infusions were collected from nursing notes. The prospective use of a standardized evaluation tool for adverse drug reactions as well as bar code medication administration technology would improve the data available for this study. Additional studies also would be useful to validate the results.
Conclusions
The proactive measures that were used to implement the rapid infusion rituximab protocol improved HCP prescribing rates, nursing satisfaction, and the management of IRRs. Potential time savings with each infusion was significant. This study confirmed appropriateness of rapid administration of rituximab in this veteran population and has increased interest in implementing other rapid infusion protocols. Protocols, education, and order sets are being developed for daratumumab and infliximab.
1. Feugier P. A review of rituximab, the first anti-CD20 monoclonal antibody used in the treatment of B non-Hodgkin’s lymphomas. Future Oncol. 2015;11(9):1327-1342. doi:10.2217/fon.15.57
2. Rituxan [package insert]. South San Francisco, CA: Genentech; 2016.
3. Dakhil S, Hermann R, Schreeder MT, et al. Phase III safety study of rituximab administered as a 90-minute infusion in patients with previously untreated diffuse large B-cell and follicular lymphoma. Leuk Lymphoma. 2014;55(10):2335-2340. doi:10.3109/10428194.2013.877135
4. Dotson E, Crawford B, Phillips G, Jones J. Sixty-minute infusion rituximab protocol allows for safe and efficient workflow. Support Care Cancer. 2016;24(3):1125-1129. doi:10.1007/s00520-015-2869-4
5. Pritchard CH, Greenwald MW, Kremer JM, et al. Safety of infusing rituximab at a more rapid rate in patients with rheumatoid arthritis: results from the RATE-RA study. BMC Musculoskelet Disord. 2014;15:177. doi:10.1186/1471-2474-15-177
6. Hainsworth JD, Litchy S, Barton JH, et al. Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2003;21(9):1746-1751. doi:10.1200/JCO.2003.09.027
7. Can M, Alibaz-Öner F, Yılmaz-Öner S, Atagündüz P, Înanç N, Direskeneli H. Accelerated infusion rates of rituximab are well tolerated and safe in rheumatology practice: a single-centre experience. Clin Rheumatol. 2013;32(1):87-90. doi:10.1007/s10067-012-2094-1
8. Sehn LH, Donaldson J, Filewich A, et al. Rapid infusion rituximab in combination with corticosteroid-containing chemotherapy or as maintenance therapy is well tolerated and can safely be delivered in the community setting. Blood. 2007;109(10):4171-4173. doi:10.1182/blood-2006-11-059469
9. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Updated March 27 2020. Accessed June 15, 2020.
Rituximab is a genetically engineered chimeric immunoglobulin G1 monoclonal antibody. It functions by binding to the CD20 antigen on the surface of B-cell lymphocytes, leading to complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity.1 The US Food and Drug Administration approved this therapy to treat patients with B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia, along with other nonmalignant indications, including pemphigus vulgaris and rheumatoid arthritis (RA). Historically, a significant amount of time and labor on behalf of medical personnel has been required to administer rituximab according to the original manufacturer’s labeling due to the boxed warning associated with infusion-related reactions (IRRs).2
Originally, the elongated infusion times that were recommended for rituximab were largely due to the perceived risk of serious infusion-related adverse drug reactions. Slower infusion times should reduce the risk of a reaction and are considered to be a good option for those patients who are at a high risk of having a severe IRRs to rituximab. Examples of high-risk patients from previous studies include those with significant cardiovascular disease, a circulating lymphocyte count ≤ 5,000/µL at the start of infusion, and those who have previously had a reaction to rituximab.3-5 In appropriate patients, research has shown a decreasing incidence of all-grade IRRs for patients who are prescribed rituximab as they receive more doses of the drug.2,6 The ability to identify suitable patients for 90-minute infusions of rituximab and the prospect of better health system resource utilization has led investigators to study the effects of shortened infusion times.
The RATE trial addressed this subject with a phase 3 safety study on the effects of a 90-minute rituximab infusion for patients with previously untreated diffuse large B-cell and follicular lymphoma.3 The patients in this study received their first dose of rituximab using the traditional infusion approach. If it was well-tolerated, they received subsequent rituximab infusions using a 90-minute protocol. Only 1.1% of patients who had previously received a rituximab infusion developed a grade 3 or 4 IRR when receiving a faster infusion of the drug for the first time.3 This result led to the addition of instructions for a 90-minute infusion to the package insert.2
In contrast to the RATE trial, the RATE-RA trial evaluated the incidence of IRRs in patients who received rituximab for nonmalignant indications. This study assessed patients with RA receiving rituximab for > 120 minutes. The authors reported 0.6% of the patients in the study developed a grade 3 or 4 IRR associated with the first 120-minute infusion of the medication.5 The researchers concluded that rituximab can be administered at a faster rate during second and subsequent infusions in patients who have been shown to tolerate traditional infusions without increasing the risk or severity of IRRs.5
The US Department of Veterans Affairs (VA) Richard L. Roudebush VA Medical Center (RLRVAMC) in Indianapolis, Indiana, uses traditional directions for the infusion of rituximab due to perceived tolerability and safety concerns specifically in a veteran population—even while other VA medical centers have implemented shortened infusion protocols. This also is despite the fact that available research shows rapid infusions of the drug are well tolerated in a variety of community settings.7,8 Anticipated benefits of implementing a protocol include savings in chair time at the institution’s infusion clinic along with increased nursing and patient satisfaction. This project was conducted to prepare, implement, and assess the safety of a 90-minute rituximab protocol at the RLRVAMC.
Methods
Proactive measures were required before and during the implementation of the 90-minute protocol to ensure patient safety and staff satisfaction. Updates to the RLRVAMC policy for the management of medical emergencies within the infusion center were reviewed and approved by the acute care committee and nursing leadership. A protocol was developed to identify eligible patients, outline the hypersensitivity protocol, instruct pharmacy personnel on admixture preparation, and provide a titration schedule based on dose. Order sets also were created to assist health care providers (HCPs) with the prescribing of rituximab for nonantineoplastic indications. Educational materials were crafted to assist with order verification, product preparation, labeling, and programming of infusion pumps. Live education was provided for physicians, pharmacists, and nurses to ensure smooth implementation of the protocol and appropriate management of medical emergencies based on the updated policy.
Study Design
Nursing staff in the infusion clinic were surveyed once before a live education session and again after the conclusion of the study. The purpose of the survey was to assess the prior experience and current comfort level of the nursing staff with administering rituximab over 90 minutes. Nurses were asked the following questions: (1) Do you have prior experience administering rituximab via 90-minute infusion; and (2) do you feel comfortable administering rituximab via 90-minute infusion?
A weekly report of patients who received rituximab between November 1, 2018 through April 1, 2019 at the RLRVAMC was generated. HCPs were alerted to eligible patients based on protocol requirements. The HCPs then made the final determination and entered orders accordingly.
This study was a retrospective chart review of all who patients received a rapid infusion of rituximab. Patients who were included if they were aged ≥ 18 years, received rituximab infusions in the RLRVAMC infusion clinic, had an absolute lymphocyte count ≤ 5,000/mm3 at the time of their rapid infusions, had no significant baseline cardiovascular disease or respiratory compromise, and had no prior grade 3 or 4 rituximab IRRs as defined by Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.9 This study was a quality improvement initiative and considered exempt by the institutional review board. All data were deidentified and secured to ensure patient privacy.
The primary endpoint for this study was the incidence of grade 3 or 4 IRRs associated with the rapid infusion of rituximab. Secondary endpoints included the proportion of patients who experienced a grade 3 or 4 infusion reaction, who received proper treatment according to the institution’s hypersensitivity protocol, savings in infusion clinic chair time, and nursing satisfaction with education and implementation of the rapid infusion rituximab protocol.
The following data were collected for all included patients: demographics, lactic acid dehydrogenase level, white blood cell count, and absolute lymphocyte count prior to rituximab infusion, indication for treatment, dose of rituximab for 90-minute infusion, date of infusion, starting time, ending time, number of previous rituximab infusions within the past 3 months, symptoms of infusion reactions during rituximab infusion, and grade of any infusion reactions that occurred.
Estimated savings in infusion clinic chair time was calculated by taking the difference in time between each completed rapid infusion and the estimated amount of time it would have taken for each patient to receive a traditional infusion. The estimated amount of time for traditional infusion was determined by following the institution’s protocol for administering rituximab to patients who previously tolerated their first dose of the drug (eg, 100 mg/h starting rate and increasing by 100 mg/h every 30 minutes to a maximum infusion rate of 400 mg/h). All endpoints were analyzed using descriptive statistics.
Results
Between November 1, 2018 and April 1, 2019, 11 patients received a total of 24 rapid infusions of rituximab. The majority of patients included in the study were older males, and the most common indication for rapid infusion was follicular lymphoma (Table 1).
Primary Endpoint
All patients who received a rapid infusion of rituximab were reviewed in the analysis of the primary and secondary endpoints. Among the 24 rapid infusions of rituximab, 1 infusion was stopped due to the patient experiencing a grade 3 IRR according to criteria from CTCAE Version 5.0. The patient was found to have dysphagia at baseline and experienced severe symptoms in the days following the first infusion that put the patient at high risk for subsequent infusion related concerns. Eligibility criteria for the 90-minute protocol were updated based on these findings. No patient experienced a grade 4 or 5 IRR. The remaining 23 infusions were well tolerated by the patients with no clinically significant events.
Secondary Endpoints
The patient who experienced a grade 3 IRR to rituximab received proper treatment by infusion clinic nurses according to the RLRVAMC hypersensitivity protocol. Patients who received rapid infusions of rituximab had a mean length of infusion of 95.0 minutes. This was in contrast to the mean time of each patient’s previous nonrapid infusion of 134.3 minutes. The difference between the 2 values equated to a savings in infusion clinic chair mean time of 39.3 minutes per patient.
Nurses were asked whether they had prior experience administering rituximab via 90-minute infusion and whether they felt comfortable administering a 90-minute rituximab infusion. Before the live education session, none of the nurses surveyed had prior experience or felt comfortable administering rituximab over 90 minutes. When the nurses were surveyed poststudy, all reported that they were experienced administering rituximab and felt comfortable with the process (Table 2).
Discussion
The infusion of rituximab has been associated with significant challenges related to the time and labor required. Although a vast number of institutions across the country now infuse the medication over an abbreviated time, HCP concerns for patient safety and appropriate use of hypersensitivity protocol in a veteran population delayed implementation at RLRVAMC. The results from this quality improvement initiative highlight the positive impact of the proactive measures that were used to implement the rapid infusion protocol for rituximab on improving HCP prescribing rates, nursing satisfaction, and appropriate management of IRRs.
Rapid infusion saved on average 39.3 minutes per patient in infusion clinic chair time. Each successful rapid infusion of rituximab potentially opened additional time in clinic for ≥ 1 patients to receive an infusion therapy. The RLRVAMC usually operated at maximum capacity, so the ability to accommodate more patients helped decrease hospital admittances for time-sensitive infusions.
The initial criteria used to screen patients to determine whether a rapid infusion of rituximab would be appropriate was based on inclusion and exclusion criteria for past studies on the same subject.3-5 The incidence of hypersensitivity reactions associated with study participants who received rapid rituximab infusions also resembles past research done on the subject, which is important to note due to prior misconceptions of staff at the institution of a higher risk of reaction in this specific veteran population. One patient with RA experienced a grade 3 IRR in this study. Although this patient met the original inclusion criteria, the patient had baseline dysphagia, and following the first infusion, reported to the emergency department (ED) with symptoms of delayed anaphylaxis. In this case, the order for rapid infusion was placed in advance and the prescriber was unaware of the ED visit. Based on this event, eligibility criteria for 90-minute rituximab infusions were updated to include additional information specifying that candidates for a rapid infusion also may have no baseline airway compromise. This hypersensitivity reaction also highlighted the need for decision support technology to assist HCPs in patient selection as well as empowering nursing and pharmacy staff to identify concerns once they place orders.
Over the course of the study, investigators assisted the HCPs with preparation of orders for the rapid infusion of rituximab for antineoplastic indications. Due to feasibility issues with this practice moving forward, order sets containing rituximab were updated to include a 90-minute option. This created a more standardized process that allowed HCPs to screen potential patients on their own. The expectation is that HCPs will be more likely to order 90-minute infusions for eligible patients in the future with this efficient and safer process.
Limitations
The small sample size in this study was a limitation. Retrospective data related to the management of infusion reactions and length of infusions were collected from nursing notes. The prospective use of a standardized evaluation tool for adverse drug reactions as well as bar code medication administration technology would improve the data available for this study. Additional studies also would be useful to validate the results.
Conclusions
The proactive measures that were used to implement the rapid infusion rituximab protocol improved HCP prescribing rates, nursing satisfaction, and the management of IRRs. Potential time savings with each infusion was significant. This study confirmed appropriateness of rapid administration of rituximab in this veteran population and has increased interest in implementing other rapid infusion protocols. Protocols, education, and order sets are being developed for daratumumab and infliximab.
Rituximab is a genetically engineered chimeric immunoglobulin G1 monoclonal antibody. It functions by binding to the CD20 antigen on the surface of B-cell lymphocytes, leading to complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity.1 The US Food and Drug Administration approved this therapy to treat patients with B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia, along with other nonmalignant indications, including pemphigus vulgaris and rheumatoid arthritis (RA). Historically, a significant amount of time and labor on behalf of medical personnel has been required to administer rituximab according to the original manufacturer’s labeling due to the boxed warning associated with infusion-related reactions (IRRs).2
Originally, the elongated infusion times that were recommended for rituximab were largely due to the perceived risk of serious infusion-related adverse drug reactions. Slower infusion times should reduce the risk of a reaction and are considered to be a good option for those patients who are at a high risk of having a severe IRRs to rituximab. Examples of high-risk patients from previous studies include those with significant cardiovascular disease, a circulating lymphocyte count ≤ 5,000/µL at the start of infusion, and those who have previously had a reaction to rituximab.3-5 In appropriate patients, research has shown a decreasing incidence of all-grade IRRs for patients who are prescribed rituximab as they receive more doses of the drug.2,6 The ability to identify suitable patients for 90-minute infusions of rituximab and the prospect of better health system resource utilization has led investigators to study the effects of shortened infusion times.
The RATE trial addressed this subject with a phase 3 safety study on the effects of a 90-minute rituximab infusion for patients with previously untreated diffuse large B-cell and follicular lymphoma.3 The patients in this study received their first dose of rituximab using the traditional infusion approach. If it was well-tolerated, they received subsequent rituximab infusions using a 90-minute protocol. Only 1.1% of patients who had previously received a rituximab infusion developed a grade 3 or 4 IRR when receiving a faster infusion of the drug for the first time.3 This result led to the addition of instructions for a 90-minute infusion to the package insert.2
In contrast to the RATE trial, the RATE-RA trial evaluated the incidence of IRRs in patients who received rituximab for nonmalignant indications. This study assessed patients with RA receiving rituximab for > 120 minutes. The authors reported 0.6% of the patients in the study developed a grade 3 or 4 IRR associated with the first 120-minute infusion of the medication.5 The researchers concluded that rituximab can be administered at a faster rate during second and subsequent infusions in patients who have been shown to tolerate traditional infusions without increasing the risk or severity of IRRs.5
The US Department of Veterans Affairs (VA) Richard L. Roudebush VA Medical Center (RLRVAMC) in Indianapolis, Indiana, uses traditional directions for the infusion of rituximab due to perceived tolerability and safety concerns specifically in a veteran population—even while other VA medical centers have implemented shortened infusion protocols. This also is despite the fact that available research shows rapid infusions of the drug are well tolerated in a variety of community settings.7,8 Anticipated benefits of implementing a protocol include savings in chair time at the institution’s infusion clinic along with increased nursing and patient satisfaction. This project was conducted to prepare, implement, and assess the safety of a 90-minute rituximab protocol at the RLRVAMC.
Methods
Proactive measures were required before and during the implementation of the 90-minute protocol to ensure patient safety and staff satisfaction. Updates to the RLRVAMC policy for the management of medical emergencies within the infusion center were reviewed and approved by the acute care committee and nursing leadership. A protocol was developed to identify eligible patients, outline the hypersensitivity protocol, instruct pharmacy personnel on admixture preparation, and provide a titration schedule based on dose. Order sets also were created to assist health care providers (HCPs) with the prescribing of rituximab for nonantineoplastic indications. Educational materials were crafted to assist with order verification, product preparation, labeling, and programming of infusion pumps. Live education was provided for physicians, pharmacists, and nurses to ensure smooth implementation of the protocol and appropriate management of medical emergencies based on the updated policy.
Study Design
Nursing staff in the infusion clinic were surveyed once before a live education session and again after the conclusion of the study. The purpose of the survey was to assess the prior experience and current comfort level of the nursing staff with administering rituximab over 90 minutes. Nurses were asked the following questions: (1) Do you have prior experience administering rituximab via 90-minute infusion; and (2) do you feel comfortable administering rituximab via 90-minute infusion?
A weekly report of patients who received rituximab between November 1, 2018 through April 1, 2019 at the RLRVAMC was generated. HCPs were alerted to eligible patients based on protocol requirements. The HCPs then made the final determination and entered orders accordingly.
This study was a retrospective chart review of all who patients received a rapid infusion of rituximab. Patients who were included if they were aged ≥ 18 years, received rituximab infusions in the RLRVAMC infusion clinic, had an absolute lymphocyte count ≤ 5,000/mm3 at the time of their rapid infusions, had no significant baseline cardiovascular disease or respiratory compromise, and had no prior grade 3 or 4 rituximab IRRs as defined by Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.9 This study was a quality improvement initiative and considered exempt by the institutional review board. All data were deidentified and secured to ensure patient privacy.
The primary endpoint for this study was the incidence of grade 3 or 4 IRRs associated with the rapid infusion of rituximab. Secondary endpoints included the proportion of patients who experienced a grade 3 or 4 infusion reaction, who received proper treatment according to the institution’s hypersensitivity protocol, savings in infusion clinic chair time, and nursing satisfaction with education and implementation of the rapid infusion rituximab protocol.
The following data were collected for all included patients: demographics, lactic acid dehydrogenase level, white blood cell count, and absolute lymphocyte count prior to rituximab infusion, indication for treatment, dose of rituximab for 90-minute infusion, date of infusion, starting time, ending time, number of previous rituximab infusions within the past 3 months, symptoms of infusion reactions during rituximab infusion, and grade of any infusion reactions that occurred.
Estimated savings in infusion clinic chair time was calculated by taking the difference in time between each completed rapid infusion and the estimated amount of time it would have taken for each patient to receive a traditional infusion. The estimated amount of time for traditional infusion was determined by following the institution’s protocol for administering rituximab to patients who previously tolerated their first dose of the drug (eg, 100 mg/h starting rate and increasing by 100 mg/h every 30 minutes to a maximum infusion rate of 400 mg/h). All endpoints were analyzed using descriptive statistics.
Results
Between November 1, 2018 and April 1, 2019, 11 patients received a total of 24 rapid infusions of rituximab. The majority of patients included in the study were older males, and the most common indication for rapid infusion was follicular lymphoma (Table 1).
Primary Endpoint
All patients who received a rapid infusion of rituximab were reviewed in the analysis of the primary and secondary endpoints. Among the 24 rapid infusions of rituximab, 1 infusion was stopped due to the patient experiencing a grade 3 IRR according to criteria from CTCAE Version 5.0. The patient was found to have dysphagia at baseline and experienced severe symptoms in the days following the first infusion that put the patient at high risk for subsequent infusion related concerns. Eligibility criteria for the 90-minute protocol were updated based on these findings. No patient experienced a grade 4 or 5 IRR. The remaining 23 infusions were well tolerated by the patients with no clinically significant events.
Secondary Endpoints
The patient who experienced a grade 3 IRR to rituximab received proper treatment by infusion clinic nurses according to the RLRVAMC hypersensitivity protocol. Patients who received rapid infusions of rituximab had a mean length of infusion of 95.0 minutes. This was in contrast to the mean time of each patient’s previous nonrapid infusion of 134.3 minutes. The difference between the 2 values equated to a savings in infusion clinic chair mean time of 39.3 minutes per patient.
Nurses were asked whether they had prior experience administering rituximab via 90-minute infusion and whether they felt comfortable administering a 90-minute rituximab infusion. Before the live education session, none of the nurses surveyed had prior experience or felt comfortable administering rituximab over 90 minutes. When the nurses were surveyed poststudy, all reported that they were experienced administering rituximab and felt comfortable with the process (Table 2).
Discussion
The infusion of rituximab has been associated with significant challenges related to the time and labor required. Although a vast number of institutions across the country now infuse the medication over an abbreviated time, HCP concerns for patient safety and appropriate use of hypersensitivity protocol in a veteran population delayed implementation at RLRVAMC. The results from this quality improvement initiative highlight the positive impact of the proactive measures that were used to implement the rapid infusion protocol for rituximab on improving HCP prescribing rates, nursing satisfaction, and appropriate management of IRRs.
Rapid infusion saved on average 39.3 minutes per patient in infusion clinic chair time. Each successful rapid infusion of rituximab potentially opened additional time in clinic for ≥ 1 patients to receive an infusion therapy. The RLRVAMC usually operated at maximum capacity, so the ability to accommodate more patients helped decrease hospital admittances for time-sensitive infusions.
The initial criteria used to screen patients to determine whether a rapid infusion of rituximab would be appropriate was based on inclusion and exclusion criteria for past studies on the same subject.3-5 The incidence of hypersensitivity reactions associated with study participants who received rapid rituximab infusions also resembles past research done on the subject, which is important to note due to prior misconceptions of staff at the institution of a higher risk of reaction in this specific veteran population. One patient with RA experienced a grade 3 IRR in this study. Although this patient met the original inclusion criteria, the patient had baseline dysphagia, and following the first infusion, reported to the emergency department (ED) with symptoms of delayed anaphylaxis. In this case, the order for rapid infusion was placed in advance and the prescriber was unaware of the ED visit. Based on this event, eligibility criteria for 90-minute rituximab infusions were updated to include additional information specifying that candidates for a rapid infusion also may have no baseline airway compromise. This hypersensitivity reaction also highlighted the need for decision support technology to assist HCPs in patient selection as well as empowering nursing and pharmacy staff to identify concerns once they place orders.
Over the course of the study, investigators assisted the HCPs with preparation of orders for the rapid infusion of rituximab for antineoplastic indications. Due to feasibility issues with this practice moving forward, order sets containing rituximab were updated to include a 90-minute option. This created a more standardized process that allowed HCPs to screen potential patients on their own. The expectation is that HCPs will be more likely to order 90-minute infusions for eligible patients in the future with this efficient and safer process.
Limitations
The small sample size in this study was a limitation. Retrospective data related to the management of infusion reactions and length of infusions were collected from nursing notes. The prospective use of a standardized evaluation tool for adverse drug reactions as well as bar code medication administration technology would improve the data available for this study. Additional studies also would be useful to validate the results.
Conclusions
The proactive measures that were used to implement the rapid infusion rituximab protocol improved HCP prescribing rates, nursing satisfaction, and the management of IRRs. Potential time savings with each infusion was significant. This study confirmed appropriateness of rapid administration of rituximab in this veteran population and has increased interest in implementing other rapid infusion protocols. Protocols, education, and order sets are being developed for daratumumab and infliximab.
1. Feugier P. A review of rituximab, the first anti-CD20 monoclonal antibody used in the treatment of B non-Hodgkin’s lymphomas. Future Oncol. 2015;11(9):1327-1342. doi:10.2217/fon.15.57
2. Rituxan [package insert]. South San Francisco, CA: Genentech; 2016.
3. Dakhil S, Hermann R, Schreeder MT, et al. Phase III safety study of rituximab administered as a 90-minute infusion in patients with previously untreated diffuse large B-cell and follicular lymphoma. Leuk Lymphoma. 2014;55(10):2335-2340. doi:10.3109/10428194.2013.877135
4. Dotson E, Crawford B, Phillips G, Jones J. Sixty-minute infusion rituximab protocol allows for safe and efficient workflow. Support Care Cancer. 2016;24(3):1125-1129. doi:10.1007/s00520-015-2869-4
5. Pritchard CH, Greenwald MW, Kremer JM, et al. Safety of infusing rituximab at a more rapid rate in patients with rheumatoid arthritis: results from the RATE-RA study. BMC Musculoskelet Disord. 2014;15:177. doi:10.1186/1471-2474-15-177
6. Hainsworth JD, Litchy S, Barton JH, et al. Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2003;21(9):1746-1751. doi:10.1200/JCO.2003.09.027
7. Can M, Alibaz-Öner F, Yılmaz-Öner S, Atagündüz P, Înanç N, Direskeneli H. Accelerated infusion rates of rituximab are well tolerated and safe in rheumatology practice: a single-centre experience. Clin Rheumatol. 2013;32(1):87-90. doi:10.1007/s10067-012-2094-1
8. Sehn LH, Donaldson J, Filewich A, et al. Rapid infusion rituximab in combination with corticosteroid-containing chemotherapy or as maintenance therapy is well tolerated and can safely be delivered in the community setting. Blood. 2007;109(10):4171-4173. doi:10.1182/blood-2006-11-059469
9. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Updated March 27 2020. Accessed June 15, 2020.
1. Feugier P. A review of rituximab, the first anti-CD20 monoclonal antibody used in the treatment of B non-Hodgkin’s lymphomas. Future Oncol. 2015;11(9):1327-1342. doi:10.2217/fon.15.57
2. Rituxan [package insert]. South San Francisco, CA: Genentech; 2016.
3. Dakhil S, Hermann R, Schreeder MT, et al. Phase III safety study of rituximab administered as a 90-minute infusion in patients with previously untreated diffuse large B-cell and follicular lymphoma. Leuk Lymphoma. 2014;55(10):2335-2340. doi:10.3109/10428194.2013.877135
4. Dotson E, Crawford B, Phillips G, Jones J. Sixty-minute infusion rituximab protocol allows for safe and efficient workflow. Support Care Cancer. 2016;24(3):1125-1129. doi:10.1007/s00520-015-2869-4
5. Pritchard CH, Greenwald MW, Kremer JM, et al. Safety of infusing rituximab at a more rapid rate in patients with rheumatoid arthritis: results from the RATE-RA study. BMC Musculoskelet Disord. 2014;15:177. doi:10.1186/1471-2474-15-177
6. Hainsworth JD, Litchy S, Barton JH, et al. Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2003;21(9):1746-1751. doi:10.1200/JCO.2003.09.027
7. Can M, Alibaz-Öner F, Yılmaz-Öner S, Atagündüz P, Înanç N, Direskeneli H. Accelerated infusion rates of rituximab are well tolerated and safe in rheumatology practice: a single-centre experience. Clin Rheumatol. 2013;32(1):87-90. doi:10.1007/s10067-012-2094-1
8. Sehn LH, Donaldson J, Filewich A, et al. Rapid infusion rituximab in combination with corticosteroid-containing chemotherapy or as maintenance therapy is well tolerated and can safely be delivered in the community setting. Blood. 2007;109(10):4171-4173. doi:10.1182/blood-2006-11-059469
9. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Updated March 27 2020. Accessed June 15, 2020.
Assessment of Consolidated Mail Outpatient Pharmacy Utilization in the Indian Health Service
Consolidated mail outpatient pharmacy (CMOP) is an automated prescription order processing and delivery system developed by the US Department of Veterans Affairs (VA) in 1994 to provide medications to VA patients.1 In fiscal year (FY) 2016, CMOP filled about 80% of VA outpatient prescriptions.2
Formalized by the 2010 Memorandum of Understanding between Indian Health Service (IHS) and VA, CMOP is a partnership undertaken to improve the delivery of care to patients by both agencies.3 The number of prescriptions filled by CMOP for IHS patients increased from 1,972 in FY 2010 to 840,109 in FY 2018.4 In the fourth quarter of FY 2018, there were 94 CMOP-enrolled IHS federal and tribal sites.5 It is only appropriate that a growing number of IHS sites are adopting CMOP considering the evidence for mail-order pharmacy on better patient adherence, improved health outcomes, and potential cost savings.6-9 Furthermore, using a centralized pharmacy operation, such as CMOP, can lead to better quality services.10
Crownpoint Health Care Facility (CHCF) serves > 30,000 American Indians and is in Crownpoint, New Mexico, a small community of about 3,000 people.11 Most of the patients served by the facility live in distant places. Many of these underserved patients do not have a stable means of transportation.12 Therefore, these patients may have difficulty traveling to the facility for their health care needs, including medication pickups. More than 2.5 million American Indians and Alaska Natives IHS beneficiaries face similar challenges due to the rurality of their communities.13 CMOP can be a method to increase access to care for this vulnerable population. However, the utilization of CMOP varies significantly among IHS facilities. While some IHS facilities process large numbers of prescriptions through CMOP, other facilities process few, if any. There also are IHS facilities, such as CHCF, which are at the initial stage of implementing CMOP or trying to increase the volume of prescriptions processed through CMOP. Although the utilization of CMOP has grown exponentially among IHS facilities, there is currently no available resource that summarizes the relative advantages and disadvantages, the challenges and opportunities, and the strengths and weaknesses of implementing CMOP for IHS facilities
Methods
A questionnaire encompassing various aspects of CMOP prescription processing was developed and distributed to the primary CMOP contacts for IHS facilities. The questionnaire was first distributed by e-mail on December 19, 2018. It was e-mailed for a second time on January 16, 2019, and the questionnaire was open for responses until the end of January 2019 (Table).
Results
Forty-four of 94 CMOP-enrolled IHS sites responded to the questionnaire. Most sites train the majority of their pharmacists in CMOP prescription processing. Overall, 310 of 347 pharmacists (89%) in these 44 IHS sites can process prescriptions through CMOP. Thirty-one sites have all their pharmacists trained in CMOP prescription processing. Only 1 facility had less than half (2 of 17 pharmacists) of its pharmacists trained in CMOP prescription processing. More than half the total number of pharmacists, 185 out of 347 (53%), check electronic messages via Resource and Patient Management System (RPMS) MailMan to get information about prescriptions rejected by CMOP. Twenty sites have all their pharmacists check messages about CMOP rejections. However, 2 facilities reported that they do not check the rejection messages at all. Twenty-six of the 44 responding sites (59%) transmit prescriptions to CMOP manually in the electronic system. The rest (18 of 44) rely on the auto-transmission (AT) setup to transmit the CMOP-suspended prescriptions at specified times of the day.
Half the sites (8 of 16) that rely on patients asking for prescriptions to be mailed at the time of refill request do not use any method to designate a CMOP patient. Twenty-four sites use the narrative field on the patient’s profile in RPMS, the health information system used by most IHS facilities, to designate CMOP patients. Eighteen sites use pop-up messages on ScriptPro, a pharmacy automation system, as a designation method. Most of the sites (12 of 15) that use both RPMS and ScriptPro designation methods do not require patients to ask for prescriptions to be mailed at the time of refill request; prescriptions for these patients are routed through CMOP unless patients request otherwise. Only 3 of 44 sites use both methods and rely on patients asking for prescriptions to be mailed at the time of refill request. Some other reported designation methods were using the electronic health record (EHR) posting box, keeping a manual list of CMOP patients, and solely utilizing the Prescription Mail Delivery field in RPMS. Three sites also noted that they keep manual lists to auto-refill prescriptions through CMOP.
Thirty sites (68%) reported that they process every prescription through CMOP even if the patient had prescriptions with specified CMOP quantities. Only 8 sites (18%) said that they used the local mail-out program to keep the same days’ supply for all medication orders. For patients with CMOP-ineligible prescriptions, 34 of the 44 sites (77%) process the eligible prescriptions through CMOP and refill the rest of the prescriptions locally. Six sites (14%) process all medication orders locally for patients with any CMOP-ineligible prescriptions.
Only 12 of 44 sites (27%) involve pharmacy technicians in CMOP prescription processing. Five sites have technicians process prescription refills through CMOP. Two of these sites mentioned the strategy of technicians suspending the prescriptions to be sent to CMOP on the refill due date. Other technician roles included tracking CMOP packages, checking electronic messages for CMOP rejections, and signing up patients for CMOP.
Only 3 of the 44 sites (7%) have measured patient satisfaction with the CMOP program. One of these 3 sites reported that the overall satisfaction was high with CMOP. This site administered the survey to patients who came to the clinic for appointments. The second facility called patients and asked for their feedback. The third site conducted the survey by using student pharmacists. Two sites reported that they use the survey results from the CMOP-conducted patient satisfaction surveys, although they have not measured patient satisfaction at their specific facilities.
Most sites have not assessed CMOP’s impact on their insurance (point of sale) collections. However, 13 sites (30%) reported that they believe they are losing on collections by utilizing CMOP. The use of repackaged products by CMOP, which are usually nonreimbursable, is an issue that was mentioned multiple times. In contrast, 2 sites mentioned that CMOP has led to increased insurance collections for their facilities.
Discussion
The utility of CMOP among the responding IHS sites varies quite significantly. Some sites appreciate the convenience of CMOP while acknowledging its limitations, such as the possible decrease in insurance collections, lengthy prescription processing time, or medication backorders. However, some sites have reserved CMOP for special circumstances (eg, mailing refrigerated items to the patient’s street address) due to various complexities that may come with CMOP. One site reported that it compares IHS contract drug prices with VA contract drug prices quarterly to determine which prescriptions should be sent through CMOP.
Most of the IHS pharmacists (89%) are trained in CMOP prescription processing. If an IHS site wants to increase its volume of CMOP prescriptions, it is sensible to train as many pharmacists as possible so that the responsibility does not fall on a few pharmacists. Newly hired pharmacists can receive guidance from trained pharmacists. Designation methods for CMOP patients can be beneficial for these pharmacists to identify CMOP-enrolled patients, especially if the site does not require patients to ask for prescriptions to be mailed at the time of refill request. Only 3 sites (7%) use multiple designation methods in addition to relying on patients to ask for prescriptions to be mailed. Proper implementation of designation methods can remove this extra burden on patients. Conversely, requiring patients to ask for prescriptions to be sent through CMOP can prevent spontaneous mail-outs if a CMOP-designated patient wants to pick up prescriptions locally. Overall, 16 sites (36%) rely on patients asking for prescriptions to be mailed.
One of the main benefits of CMOP is the ability to mail refrigerated items. Local pharmacy mail-out programs may not have this ability. Patients at rural locations often use post office (PO) boxes because they are unable to receive postal services at their physical addresses; however, they may receive packages through United Parcel Service (UPS) at their physical addresses. CMOP uses UPS to send refrigerated items, but UPS does not deliver to PO boxes. Therefore, remotely located sites like CHCF have difficulty in fully optimizing this benefit. One solution is documenting both the physical and mailing addresses on the patient’s EHR, which enables CMOP to send refrigerated items to the patient’s home address via UPS and mail the rest of the prescriptions to the patient’s PO box address with the US Postal Service. The physical address must be listed above the PO box address to ensure that refrigerated items are not rejected by CMOP. Furthermore, both the physical address and the PO box address must be in the same city for this method to work. Two sites noted mailing refrigerated items as one of the major challenges in CMOP prescription processing.
CMOP-enrolled patients must be educated about requesting medications 7 to 10 days before they run out. There is no standard time line for prescriptions filled by CMOP. However, 1 site reported that it may take up to “10 days from time requested to mailbox.” This delay leads to pharmacies facing a dilemma as processing prescriptions too early can lead to insurance rejections, but processing them too late can lead to the patient not receiving the medication by the time they run out of their current supply. However, CMOP provides the ability to track prescriptions sent through CMOP. Pharmacists and technicians need to have access to BestWay Parcel Services Client Portal (genco-mms.bestwayparcel.com) to track CMOP packages. Tracking CMOP prescriptions is a way pharmacy technicians can be involved in CMOP prescription processing. Technicians seem to be underutilized, as only 27% of the responding sites utilize them to some degree in the CMOP process. One site delegated the responsibility of checking CMOP rejection messages to pharmacy technicians. Since 2 of the responding sites do not check CMOP rejection messages at all, this is an excellent opportunity to get pharmacy technicians involved.
A CMOP auto-refill program can potentially be utilized to avoid missed or late medications. In an auto-refill program, a pharmacist can refill prescriptions through CMOP on the due date without a patient request. They may get rejected by insurance the first time they are processed through CMOP for refilling too early if the processing time is taken into account. However, the subsequent refills do not have to consider the CMOP processing time as they would already be synchronized based on the last refill date. Though, if CMOP is out of stock on a medication and it is expected to be available soon, CMOP may take a few extra days to either fill the prescription or reject it if the drug stays unavailable. One of the sites reported “the amount of time [CMOP] holds medications if they are out of stock” as “the hardest thing to work around.” A couple of sites also mentioned the longer than usual delay in processing prescriptions by CMOP during the holidays as one of the major challenges.
CMOP use of repackaged products also may lead insurance companies to deny reimbursement. Repackaged products are usually cheaper to buy.14 However, most insurances do not reimburse for prescriptions filled with these products.15 The local drug file on RPMS may have a national drug code (NDC) that is reimbursable by insurance, but CMOP will change it to the repackaged NDC if they are filling the prescription with a repackaged product. One potential solution to this problem would be filling these prescriptions locally. Furthermore, insurance claims are processed when the prescriptions are filled by CMOP. Sites cannot return/cancel the prescription anymore at that point. Therefore, the inability to see real-time rejections as the medication orders are processed on-site makes it challenging to prevent avoidable insurance rejections, such as a refill too soon. One site calculated that it lost $26,386.45 by utilizing CMOP from January 9, 2018 to December 12, 2018. However, it is unclear whether this loss was representative of other sites. It is also worth noting that IHS sites can save a substantial amount of money on certain products by utilizing CMOP because VA buys these products at a reduced price.16
CMOP-transmitted prescriptions can be rejected for various reasons, such as CMOP manufacturer’s backorder, a different quantity from CMOP stock size, etc. Information about these rejected prescriptions is accessed through electronic messages on RPMS. CMOP does not dispense less than a full, unopened package for most over-the-counter (OTC) medications. The quantity on these prescriptions must be equal to or multiples of the package size for them to be filled by CMOP. This can lead to a patient having prescriptions with different days’ supplies, which results in various refill due dates. If a site has a local mail-out program available, it can potentially keep the same days’ supply for all prescriptions by mailing these OTC medications locally rather than utilizing CMOP. However, this can partially negate CMOP’s benefit of reduced workload.
CMOP also has specified quantities on some prescription medications. One survey respondent viewed “the quantity and day supply required by CMOP” as a negative influence on the site’s insurance collection. It is possible that CMOP does not carry all the medications that a CMOP-enrolled patient is prescribed. Most sites (77%) still send eligible prescriptions through CMOP for the patients who also have CMOP-ineligible prescriptions. There are a small number of sites (14%) that utilize local mail-out program for the patients with any CMOP-ineligible prescriptions, possibly to simplify the process. Schedule II controlled substances cannot be processed through CMOP either; however, facilities may have local policies that prohibit mailing any controlled substances.
Prescriptions can be manually transmitted to CMOP, or they can be automatically transmitted based on the run time and frequency of the auto-transmission setup. The prescriptions that are waiting to be transmitted to CMOP must be in the “suspended” status. The apparent advantage of relying on auto-transmission is that you do not have to complete the steps manually to transmit suspended CMOP prescriptions, thereby making the process more convenient. However, the manual transmission can be utilized as a checkpoint to verify that prescriptions were properly suspended for CMOP, as the prescription status changes from “S” (suspended) to “AT” once the transmission is completed. If a prescription is not properly suspended for CMOP, the status will remain as S even after manual transmission. More than half (59%) of the responding sites must find the manual transmission feature useful as they use it either over or in addition to the auto-transmission setup.
Despite the challenges, many IHS sites process thousands of monthly prescriptions through CMOP. Of the 94 CMOP-enrolled IHS sites, 17 processed > 1,000 prescriptions from March 27, 2019 to April 25, 2019.17 Five sites processed > 5,000 prescriptions.17 At the rate of > 5,000 prescriptions per month, the yearly CMOP prescription count will be > 60,000. That is more than one-third of the prescriptions processed by CHCF in 2018. By handling these prescriptions through CMOP, it can decrease pharmacy filling and dispensing workload, thereby freeing pharmacists to participate in other services.18 Furthermore, implementing CMOP does not incur any cost for the IHS site. There is a nondrug cost for each prescription that is filled through CMOP. This cost was $2.67 during FY 2016.19 The fee covers prescription vial, label, packaging for mail, postage, personnel, building overhead, and equipment capitalization.19 The nondrug cost of filling a prescription locally at the site can potentially exceed the cost charged by CMOP.19
A lack of objective data exists to assess the net impact of CMOP on patients. Different theoretical assumptions can be made, such as CMOP resulting in better patient adherence. However, there is no objective information about how much CMOP improves patient adherence if it does at all. Though J.D. Power US Pharmacy Study ranks CMOP as “among the best” mail-order pharmacies in customer satisfaction, only 3 of the 44 responding sites have measured patient satisfaction locally.20 Only 1 site had objective data about CMOP’s impact on the point of sale. Therefore, it is currently difficult to perform a cost-benefit analysis of the CMOP program. There are opportunities for further studies on these topics.
Limitations
One limitation of this study is that < 50% of the CMOP-enrolled sites (44 of 94) responded to the questionnaire. It is possible that the facilities that had a significantly positive or negative experience with CMOP were more inclined to share their views. Therefore, it is difficult to conclude whether the responding sites are an accurate representative sample. Another limitation of the study was the questionnaire design and the reliance on free-text responses as opposed to structured data. The free-text responses had to be analyzed manually to determine whether they fall in the same category, thereby increasing the risk of interpretation error.
Conclusion
CMOP has its unique challenges but provides many benefits that local pharmacy mail-out programs may not possess, such as the abilities to mail refrigerated items and track packages. One must be familiar with CMOP’s various idiosyncrasies to make the best use of the program. Extensive staff education and orientation for new staff members must be done to familiarize them with the program. Nevertheless, the successful implementation of CMOP can lead to reduced pharmacy workload while increasing access to care for patients with transportation issues.
Acknowledgments
The authors thank LCDR Karsten Smith, PharmD, BCGP, the IHS CMOP Coordinator for providing the list of primary CMOP contacts and CDR Kendall Van Tyle, PharmD, BCPS, for proofreading the article.
1. US Department of Veterans Affairs, Office of Inspector General. Audit of Consolidated Mail Outpatient Pharmacy contract management. https://www.va.gov/oig/52/reports/2009/VAOIG-09-00026-143.pdf. Published June 10, 2009. Accessed June 11, 2020.
2. US Department of Veterans Affairs. Pharmacy Benefits Management Services. VA mail order pharmacy. https://www.pbm.va.gov/PBM/CMOP/VA_Mail_Order_Pharmacy.asp. Updated July 18, 2018. Accessed July 16, 2019.
3. US Department of Veterans Affairs. Memorandum of understanding between the Department of Veterans Affairs (VA) and Indian Health Service (IHS). https://www.va.gov/TRIBALGOVERNMENT/docs/Signed2010VA-IHSMOU.pdf. Published October 1, 2010. Accessed June 11, 2020.
4. US Department of Veterans Affairs, Office of Tribal Government Relations, Office of Rural Health, US Department of Health and Human Services, Indian Health Service. U.S. Department of Veterans Affairs and Indian Health Service memorandum of understanding annual report fiscal year 2018. https://www.ruralhealth.va.gov/docs/VA-IHS_MOU_AnnualReport_FY2018_FINAL.pdf. Published December 2018. Accessed June 11, 2020.
5. Karsten S. CMOP items of interest. Published October 12, 2018. [Nonpublic document]
6. Fernandez EV, McDaniel JA, Carroll NV. Examination of the link between medication adherence and use of mail-order pharmacies in chronic disease states. J Manag Care Spec Pharm. 2016;22(11):1247‐1259. doi:10.18553/jmcp.2016.22.11.1247
7. Schwab P, Racsa P, Rascati K, Mourer M, Meah Y, Worley K. A retrospective database study comparing diabetes-related medication adherence and health outcomes for mail-order versus community pharmacy. J Manag Care Spec Pharm. 2019;25(3):332‐340. doi:10.18553/jmcp.2019.25.3.332
8. Schmittdiel JA, Karter AJ, Dyer W, et al. The comparative effectiveness of mail order pharmacy use vs. local pharmacy use on LDL-C control in new statin users. J Gen Intern Med. 2011;26(12):1396‐1402. doi:10.1007/s11606-011-1805-7
9. Devine S, Vlahiotis A, Sundar H. A comparison of diabetes medication adherence and healthcare costs in patients using mail order pharmacy and retail pharmacy. J Med Econ. 2010;13(2):203‐211. doi:10.3111/13696991003741801
10. Kappenman AM, Ragsdale R, Rim MH, Tyler LS, Nickman NA. Implementation of a centralized mail-order pharmacy service. Am J Health Syst Pharm. 2019;76(suppl 3):S74‐S78. doi:10.1093/ajhp/zxz138
11. US Department of Health and Human Services, Indian Health Service. Crownpoint service unit. www.ihs.gov/crownpoint. Accessed June 11, 2020.
12. Chaco P. Roads and transportation on the Navajo Nation. https://obamawhitehouse.archives.gov/microsite/blog/31387?page=135. Published February 15, 2012. Accessed June 11, 2020.
13. US Department of Health and Human Services, Indian Health Service. Disparities. www.ihs.gov/newsroom/factsheets/disparities. Updated October 2019. Accessed June 11, 2020.
14. Golden State Medical Supply. National contracts. www.gsms.us/wp-content/uploads/2018/10/National-Contracts-Flyer.pdf. Updated October 4, 2018. Accessed June 11, 2020.
15. Arizona Health Care Cost Containment System. IHS/Tribal provider billing manual chapter 9, hospital and clinic services. www.azahcccs.gov/PlansProviders/Downloads/IHS-TribalManual/IHS-Chap09HospClinic.pdf. Updated February 28, 2019. Accessed June 11, 2020.
16. US Department of Veterans Affairs, Office of Inspector General. The impact of VA allowing government agencies to be excluded from temporary price reductions on federal supply schedule pharmaceutical contracts. www.va.gov/oig/pubs/VAOIG-18-04451-06.pdf. Published October 30, 2019. Accessed June 11, 2020.
17. Karsten S. IHS Billing Report-Apr. Indian Health Service SharePoint. Published May 3, 2019. [Nonpublic document]
18. Aragon BR, Pierce RA 2nd, Jones WN. VA CMOPs: producing a pattern of quality and efficiency in government. J Am Pharm Assoc (2003). 2012;52(6):810‐815. doi:10.1331/JAPhA.2012.11075
19. Todd W. VA-IHS Consolidated Mail Outpatient Pharmacy program (CMOP). www.npaihb.org/wp-content/uploads/2017/01/CMOP-Slides-for-Portland-Area-Tribal-Sites.pdf. Published 2017. Accessed June 11, 2020.
20. J.D. Power. Pharmacy customers slow to adopt digital offerings but satisfaction increases when they do, J.D. Power finds. www.jdpower.com/business/press-releases/2019-us-pharmacy-study. Published August 20, 2019. Accessed June 11, 2020.
Consolidated mail outpatient pharmacy (CMOP) is an automated prescription order processing and delivery system developed by the US Department of Veterans Affairs (VA) in 1994 to provide medications to VA patients.1 In fiscal year (FY) 2016, CMOP filled about 80% of VA outpatient prescriptions.2
Formalized by the 2010 Memorandum of Understanding between Indian Health Service (IHS) and VA, CMOP is a partnership undertaken to improve the delivery of care to patients by both agencies.3 The number of prescriptions filled by CMOP for IHS patients increased from 1,972 in FY 2010 to 840,109 in FY 2018.4 In the fourth quarter of FY 2018, there were 94 CMOP-enrolled IHS federal and tribal sites.5 It is only appropriate that a growing number of IHS sites are adopting CMOP considering the evidence for mail-order pharmacy on better patient adherence, improved health outcomes, and potential cost savings.6-9 Furthermore, using a centralized pharmacy operation, such as CMOP, can lead to better quality services.10
Crownpoint Health Care Facility (CHCF) serves > 30,000 American Indians and is in Crownpoint, New Mexico, a small community of about 3,000 people.11 Most of the patients served by the facility live in distant places. Many of these underserved patients do not have a stable means of transportation.12 Therefore, these patients may have difficulty traveling to the facility for their health care needs, including medication pickups. More than 2.5 million American Indians and Alaska Natives IHS beneficiaries face similar challenges due to the rurality of their communities.13 CMOP can be a method to increase access to care for this vulnerable population. However, the utilization of CMOP varies significantly among IHS facilities. While some IHS facilities process large numbers of prescriptions through CMOP, other facilities process few, if any. There also are IHS facilities, such as CHCF, which are at the initial stage of implementing CMOP or trying to increase the volume of prescriptions processed through CMOP. Although the utilization of CMOP has grown exponentially among IHS facilities, there is currently no available resource that summarizes the relative advantages and disadvantages, the challenges and opportunities, and the strengths and weaknesses of implementing CMOP for IHS facilities
Methods
A questionnaire encompassing various aspects of CMOP prescription processing was developed and distributed to the primary CMOP contacts for IHS facilities. The questionnaire was first distributed by e-mail on December 19, 2018. It was e-mailed for a second time on January 16, 2019, and the questionnaire was open for responses until the end of January 2019 (Table).
Results
Forty-four of 94 CMOP-enrolled IHS sites responded to the questionnaire. Most sites train the majority of their pharmacists in CMOP prescription processing. Overall, 310 of 347 pharmacists (89%) in these 44 IHS sites can process prescriptions through CMOP. Thirty-one sites have all their pharmacists trained in CMOP prescription processing. Only 1 facility had less than half (2 of 17 pharmacists) of its pharmacists trained in CMOP prescription processing. More than half the total number of pharmacists, 185 out of 347 (53%), check electronic messages via Resource and Patient Management System (RPMS) MailMan to get information about prescriptions rejected by CMOP. Twenty sites have all their pharmacists check messages about CMOP rejections. However, 2 facilities reported that they do not check the rejection messages at all. Twenty-six of the 44 responding sites (59%) transmit prescriptions to CMOP manually in the electronic system. The rest (18 of 44) rely on the auto-transmission (AT) setup to transmit the CMOP-suspended prescriptions at specified times of the day.
Half the sites (8 of 16) that rely on patients asking for prescriptions to be mailed at the time of refill request do not use any method to designate a CMOP patient. Twenty-four sites use the narrative field on the patient’s profile in RPMS, the health information system used by most IHS facilities, to designate CMOP patients. Eighteen sites use pop-up messages on ScriptPro, a pharmacy automation system, as a designation method. Most of the sites (12 of 15) that use both RPMS and ScriptPro designation methods do not require patients to ask for prescriptions to be mailed at the time of refill request; prescriptions for these patients are routed through CMOP unless patients request otherwise. Only 3 of 44 sites use both methods and rely on patients asking for prescriptions to be mailed at the time of refill request. Some other reported designation methods were using the electronic health record (EHR) posting box, keeping a manual list of CMOP patients, and solely utilizing the Prescription Mail Delivery field in RPMS. Three sites also noted that they keep manual lists to auto-refill prescriptions through CMOP.
Thirty sites (68%) reported that they process every prescription through CMOP even if the patient had prescriptions with specified CMOP quantities. Only 8 sites (18%) said that they used the local mail-out program to keep the same days’ supply for all medication orders. For patients with CMOP-ineligible prescriptions, 34 of the 44 sites (77%) process the eligible prescriptions through CMOP and refill the rest of the prescriptions locally. Six sites (14%) process all medication orders locally for patients with any CMOP-ineligible prescriptions.
Only 12 of 44 sites (27%) involve pharmacy technicians in CMOP prescription processing. Five sites have technicians process prescription refills through CMOP. Two of these sites mentioned the strategy of technicians suspending the prescriptions to be sent to CMOP on the refill due date. Other technician roles included tracking CMOP packages, checking electronic messages for CMOP rejections, and signing up patients for CMOP.
Only 3 of the 44 sites (7%) have measured patient satisfaction with the CMOP program. One of these 3 sites reported that the overall satisfaction was high with CMOP. This site administered the survey to patients who came to the clinic for appointments. The second facility called patients and asked for their feedback. The third site conducted the survey by using student pharmacists. Two sites reported that they use the survey results from the CMOP-conducted patient satisfaction surveys, although they have not measured patient satisfaction at their specific facilities.
Most sites have not assessed CMOP’s impact on their insurance (point of sale) collections. However, 13 sites (30%) reported that they believe they are losing on collections by utilizing CMOP. The use of repackaged products by CMOP, which are usually nonreimbursable, is an issue that was mentioned multiple times. In contrast, 2 sites mentioned that CMOP has led to increased insurance collections for their facilities.
Discussion
The utility of CMOP among the responding IHS sites varies quite significantly. Some sites appreciate the convenience of CMOP while acknowledging its limitations, such as the possible decrease in insurance collections, lengthy prescription processing time, or medication backorders. However, some sites have reserved CMOP for special circumstances (eg, mailing refrigerated items to the patient’s street address) due to various complexities that may come with CMOP. One site reported that it compares IHS contract drug prices with VA contract drug prices quarterly to determine which prescriptions should be sent through CMOP.
Most of the IHS pharmacists (89%) are trained in CMOP prescription processing. If an IHS site wants to increase its volume of CMOP prescriptions, it is sensible to train as many pharmacists as possible so that the responsibility does not fall on a few pharmacists. Newly hired pharmacists can receive guidance from trained pharmacists. Designation methods for CMOP patients can be beneficial for these pharmacists to identify CMOP-enrolled patients, especially if the site does not require patients to ask for prescriptions to be mailed at the time of refill request. Only 3 sites (7%) use multiple designation methods in addition to relying on patients to ask for prescriptions to be mailed. Proper implementation of designation methods can remove this extra burden on patients. Conversely, requiring patients to ask for prescriptions to be sent through CMOP can prevent spontaneous mail-outs if a CMOP-designated patient wants to pick up prescriptions locally. Overall, 16 sites (36%) rely on patients asking for prescriptions to be mailed.
One of the main benefits of CMOP is the ability to mail refrigerated items. Local pharmacy mail-out programs may not have this ability. Patients at rural locations often use post office (PO) boxes because they are unable to receive postal services at their physical addresses; however, they may receive packages through United Parcel Service (UPS) at their physical addresses. CMOP uses UPS to send refrigerated items, but UPS does not deliver to PO boxes. Therefore, remotely located sites like CHCF have difficulty in fully optimizing this benefit. One solution is documenting both the physical and mailing addresses on the patient’s EHR, which enables CMOP to send refrigerated items to the patient’s home address via UPS and mail the rest of the prescriptions to the patient’s PO box address with the US Postal Service. The physical address must be listed above the PO box address to ensure that refrigerated items are not rejected by CMOP. Furthermore, both the physical address and the PO box address must be in the same city for this method to work. Two sites noted mailing refrigerated items as one of the major challenges in CMOP prescription processing.
CMOP-enrolled patients must be educated about requesting medications 7 to 10 days before they run out. There is no standard time line for prescriptions filled by CMOP. However, 1 site reported that it may take up to “10 days from time requested to mailbox.” This delay leads to pharmacies facing a dilemma as processing prescriptions too early can lead to insurance rejections, but processing them too late can lead to the patient not receiving the medication by the time they run out of their current supply. However, CMOP provides the ability to track prescriptions sent through CMOP. Pharmacists and technicians need to have access to BestWay Parcel Services Client Portal (genco-mms.bestwayparcel.com) to track CMOP packages. Tracking CMOP prescriptions is a way pharmacy technicians can be involved in CMOP prescription processing. Technicians seem to be underutilized, as only 27% of the responding sites utilize them to some degree in the CMOP process. One site delegated the responsibility of checking CMOP rejection messages to pharmacy technicians. Since 2 of the responding sites do not check CMOP rejection messages at all, this is an excellent opportunity to get pharmacy technicians involved.
A CMOP auto-refill program can potentially be utilized to avoid missed or late medications. In an auto-refill program, a pharmacist can refill prescriptions through CMOP on the due date without a patient request. They may get rejected by insurance the first time they are processed through CMOP for refilling too early if the processing time is taken into account. However, the subsequent refills do not have to consider the CMOP processing time as they would already be synchronized based on the last refill date. Though, if CMOP is out of stock on a medication and it is expected to be available soon, CMOP may take a few extra days to either fill the prescription or reject it if the drug stays unavailable. One of the sites reported “the amount of time [CMOP] holds medications if they are out of stock” as “the hardest thing to work around.” A couple of sites also mentioned the longer than usual delay in processing prescriptions by CMOP during the holidays as one of the major challenges.
CMOP use of repackaged products also may lead insurance companies to deny reimbursement. Repackaged products are usually cheaper to buy.14 However, most insurances do not reimburse for prescriptions filled with these products.15 The local drug file on RPMS may have a national drug code (NDC) that is reimbursable by insurance, but CMOP will change it to the repackaged NDC if they are filling the prescription with a repackaged product. One potential solution to this problem would be filling these prescriptions locally. Furthermore, insurance claims are processed when the prescriptions are filled by CMOP. Sites cannot return/cancel the prescription anymore at that point. Therefore, the inability to see real-time rejections as the medication orders are processed on-site makes it challenging to prevent avoidable insurance rejections, such as a refill too soon. One site calculated that it lost $26,386.45 by utilizing CMOP from January 9, 2018 to December 12, 2018. However, it is unclear whether this loss was representative of other sites. It is also worth noting that IHS sites can save a substantial amount of money on certain products by utilizing CMOP because VA buys these products at a reduced price.16
CMOP-transmitted prescriptions can be rejected for various reasons, such as CMOP manufacturer’s backorder, a different quantity from CMOP stock size, etc. Information about these rejected prescriptions is accessed through electronic messages on RPMS. CMOP does not dispense less than a full, unopened package for most over-the-counter (OTC) medications. The quantity on these prescriptions must be equal to or multiples of the package size for them to be filled by CMOP. This can lead to a patient having prescriptions with different days’ supplies, which results in various refill due dates. If a site has a local mail-out program available, it can potentially keep the same days’ supply for all prescriptions by mailing these OTC medications locally rather than utilizing CMOP. However, this can partially negate CMOP’s benefit of reduced workload.
CMOP also has specified quantities on some prescription medications. One survey respondent viewed “the quantity and day supply required by CMOP” as a negative influence on the site’s insurance collection. It is possible that CMOP does not carry all the medications that a CMOP-enrolled patient is prescribed. Most sites (77%) still send eligible prescriptions through CMOP for the patients who also have CMOP-ineligible prescriptions. There are a small number of sites (14%) that utilize local mail-out program for the patients with any CMOP-ineligible prescriptions, possibly to simplify the process. Schedule II controlled substances cannot be processed through CMOP either; however, facilities may have local policies that prohibit mailing any controlled substances.
Prescriptions can be manually transmitted to CMOP, or they can be automatically transmitted based on the run time and frequency of the auto-transmission setup. The prescriptions that are waiting to be transmitted to CMOP must be in the “suspended” status. The apparent advantage of relying on auto-transmission is that you do not have to complete the steps manually to transmit suspended CMOP prescriptions, thereby making the process more convenient. However, the manual transmission can be utilized as a checkpoint to verify that prescriptions were properly suspended for CMOP, as the prescription status changes from “S” (suspended) to “AT” once the transmission is completed. If a prescription is not properly suspended for CMOP, the status will remain as S even after manual transmission. More than half (59%) of the responding sites must find the manual transmission feature useful as they use it either over or in addition to the auto-transmission setup.
Despite the challenges, many IHS sites process thousands of monthly prescriptions through CMOP. Of the 94 CMOP-enrolled IHS sites, 17 processed > 1,000 prescriptions from March 27, 2019 to April 25, 2019.17 Five sites processed > 5,000 prescriptions.17 At the rate of > 5,000 prescriptions per month, the yearly CMOP prescription count will be > 60,000. That is more than one-third of the prescriptions processed by CHCF in 2018. By handling these prescriptions through CMOP, it can decrease pharmacy filling and dispensing workload, thereby freeing pharmacists to participate in other services.18 Furthermore, implementing CMOP does not incur any cost for the IHS site. There is a nondrug cost for each prescription that is filled through CMOP. This cost was $2.67 during FY 2016.19 The fee covers prescription vial, label, packaging for mail, postage, personnel, building overhead, and equipment capitalization.19 The nondrug cost of filling a prescription locally at the site can potentially exceed the cost charged by CMOP.19
A lack of objective data exists to assess the net impact of CMOP on patients. Different theoretical assumptions can be made, such as CMOP resulting in better patient adherence. However, there is no objective information about how much CMOP improves patient adherence if it does at all. Though J.D. Power US Pharmacy Study ranks CMOP as “among the best” mail-order pharmacies in customer satisfaction, only 3 of the 44 responding sites have measured patient satisfaction locally.20 Only 1 site had objective data about CMOP’s impact on the point of sale. Therefore, it is currently difficult to perform a cost-benefit analysis of the CMOP program. There are opportunities for further studies on these topics.
Limitations
One limitation of this study is that < 50% of the CMOP-enrolled sites (44 of 94) responded to the questionnaire. It is possible that the facilities that had a significantly positive or negative experience with CMOP were more inclined to share their views. Therefore, it is difficult to conclude whether the responding sites are an accurate representative sample. Another limitation of the study was the questionnaire design and the reliance on free-text responses as opposed to structured data. The free-text responses had to be analyzed manually to determine whether they fall in the same category, thereby increasing the risk of interpretation error.
Conclusion
CMOP has its unique challenges but provides many benefits that local pharmacy mail-out programs may not possess, such as the abilities to mail refrigerated items and track packages. One must be familiar with CMOP’s various idiosyncrasies to make the best use of the program. Extensive staff education and orientation for new staff members must be done to familiarize them with the program. Nevertheless, the successful implementation of CMOP can lead to reduced pharmacy workload while increasing access to care for patients with transportation issues.
Acknowledgments
The authors thank LCDR Karsten Smith, PharmD, BCGP, the IHS CMOP Coordinator for providing the list of primary CMOP contacts and CDR Kendall Van Tyle, PharmD, BCPS, for proofreading the article.
Consolidated mail outpatient pharmacy (CMOP) is an automated prescription order processing and delivery system developed by the US Department of Veterans Affairs (VA) in 1994 to provide medications to VA patients.1 In fiscal year (FY) 2016, CMOP filled about 80% of VA outpatient prescriptions.2
Formalized by the 2010 Memorandum of Understanding between Indian Health Service (IHS) and VA, CMOP is a partnership undertaken to improve the delivery of care to patients by both agencies.3 The number of prescriptions filled by CMOP for IHS patients increased from 1,972 in FY 2010 to 840,109 in FY 2018.4 In the fourth quarter of FY 2018, there were 94 CMOP-enrolled IHS federal and tribal sites.5 It is only appropriate that a growing number of IHS sites are adopting CMOP considering the evidence for mail-order pharmacy on better patient adherence, improved health outcomes, and potential cost savings.6-9 Furthermore, using a centralized pharmacy operation, such as CMOP, can lead to better quality services.10
Crownpoint Health Care Facility (CHCF) serves > 30,000 American Indians and is in Crownpoint, New Mexico, a small community of about 3,000 people.11 Most of the patients served by the facility live in distant places. Many of these underserved patients do not have a stable means of transportation.12 Therefore, these patients may have difficulty traveling to the facility for their health care needs, including medication pickups. More than 2.5 million American Indians and Alaska Natives IHS beneficiaries face similar challenges due to the rurality of their communities.13 CMOP can be a method to increase access to care for this vulnerable population. However, the utilization of CMOP varies significantly among IHS facilities. While some IHS facilities process large numbers of prescriptions through CMOP, other facilities process few, if any. There also are IHS facilities, such as CHCF, which are at the initial stage of implementing CMOP or trying to increase the volume of prescriptions processed through CMOP. Although the utilization of CMOP has grown exponentially among IHS facilities, there is currently no available resource that summarizes the relative advantages and disadvantages, the challenges and opportunities, and the strengths and weaknesses of implementing CMOP for IHS facilities
Methods
A questionnaire encompassing various aspects of CMOP prescription processing was developed and distributed to the primary CMOP contacts for IHS facilities. The questionnaire was first distributed by e-mail on December 19, 2018. It was e-mailed for a second time on January 16, 2019, and the questionnaire was open for responses until the end of January 2019 (Table).
Results
Forty-four of 94 CMOP-enrolled IHS sites responded to the questionnaire. Most sites train the majority of their pharmacists in CMOP prescription processing. Overall, 310 of 347 pharmacists (89%) in these 44 IHS sites can process prescriptions through CMOP. Thirty-one sites have all their pharmacists trained in CMOP prescription processing. Only 1 facility had less than half (2 of 17 pharmacists) of its pharmacists trained in CMOP prescription processing. More than half the total number of pharmacists, 185 out of 347 (53%), check electronic messages via Resource and Patient Management System (RPMS) MailMan to get information about prescriptions rejected by CMOP. Twenty sites have all their pharmacists check messages about CMOP rejections. However, 2 facilities reported that they do not check the rejection messages at all. Twenty-six of the 44 responding sites (59%) transmit prescriptions to CMOP manually in the electronic system. The rest (18 of 44) rely on the auto-transmission (AT) setup to transmit the CMOP-suspended prescriptions at specified times of the day.
Half the sites (8 of 16) that rely on patients asking for prescriptions to be mailed at the time of refill request do not use any method to designate a CMOP patient. Twenty-four sites use the narrative field on the patient’s profile in RPMS, the health information system used by most IHS facilities, to designate CMOP patients. Eighteen sites use pop-up messages on ScriptPro, a pharmacy automation system, as a designation method. Most of the sites (12 of 15) that use both RPMS and ScriptPro designation methods do not require patients to ask for prescriptions to be mailed at the time of refill request; prescriptions for these patients are routed through CMOP unless patients request otherwise. Only 3 of 44 sites use both methods and rely on patients asking for prescriptions to be mailed at the time of refill request. Some other reported designation methods were using the electronic health record (EHR) posting box, keeping a manual list of CMOP patients, and solely utilizing the Prescription Mail Delivery field in RPMS. Three sites also noted that they keep manual lists to auto-refill prescriptions through CMOP.
Thirty sites (68%) reported that they process every prescription through CMOP even if the patient had prescriptions with specified CMOP quantities. Only 8 sites (18%) said that they used the local mail-out program to keep the same days’ supply for all medication orders. For patients with CMOP-ineligible prescriptions, 34 of the 44 sites (77%) process the eligible prescriptions through CMOP and refill the rest of the prescriptions locally. Six sites (14%) process all medication orders locally for patients with any CMOP-ineligible prescriptions.
Only 12 of 44 sites (27%) involve pharmacy technicians in CMOP prescription processing. Five sites have technicians process prescription refills through CMOP. Two of these sites mentioned the strategy of technicians suspending the prescriptions to be sent to CMOP on the refill due date. Other technician roles included tracking CMOP packages, checking electronic messages for CMOP rejections, and signing up patients for CMOP.
Only 3 of the 44 sites (7%) have measured patient satisfaction with the CMOP program. One of these 3 sites reported that the overall satisfaction was high with CMOP. This site administered the survey to patients who came to the clinic for appointments. The second facility called patients and asked for their feedback. The third site conducted the survey by using student pharmacists. Two sites reported that they use the survey results from the CMOP-conducted patient satisfaction surveys, although they have not measured patient satisfaction at their specific facilities.
Most sites have not assessed CMOP’s impact on their insurance (point of sale) collections. However, 13 sites (30%) reported that they believe they are losing on collections by utilizing CMOP. The use of repackaged products by CMOP, which are usually nonreimbursable, is an issue that was mentioned multiple times. In contrast, 2 sites mentioned that CMOP has led to increased insurance collections for their facilities.
Discussion
The utility of CMOP among the responding IHS sites varies quite significantly. Some sites appreciate the convenience of CMOP while acknowledging its limitations, such as the possible decrease in insurance collections, lengthy prescription processing time, or medication backorders. However, some sites have reserved CMOP for special circumstances (eg, mailing refrigerated items to the patient’s street address) due to various complexities that may come with CMOP. One site reported that it compares IHS contract drug prices with VA contract drug prices quarterly to determine which prescriptions should be sent through CMOP.
Most of the IHS pharmacists (89%) are trained in CMOP prescription processing. If an IHS site wants to increase its volume of CMOP prescriptions, it is sensible to train as many pharmacists as possible so that the responsibility does not fall on a few pharmacists. Newly hired pharmacists can receive guidance from trained pharmacists. Designation methods for CMOP patients can be beneficial for these pharmacists to identify CMOP-enrolled patients, especially if the site does not require patients to ask for prescriptions to be mailed at the time of refill request. Only 3 sites (7%) use multiple designation methods in addition to relying on patients to ask for prescriptions to be mailed. Proper implementation of designation methods can remove this extra burden on patients. Conversely, requiring patients to ask for prescriptions to be sent through CMOP can prevent spontaneous mail-outs if a CMOP-designated patient wants to pick up prescriptions locally. Overall, 16 sites (36%) rely on patients asking for prescriptions to be mailed.
One of the main benefits of CMOP is the ability to mail refrigerated items. Local pharmacy mail-out programs may not have this ability. Patients at rural locations often use post office (PO) boxes because they are unable to receive postal services at their physical addresses; however, they may receive packages through United Parcel Service (UPS) at their physical addresses. CMOP uses UPS to send refrigerated items, but UPS does not deliver to PO boxes. Therefore, remotely located sites like CHCF have difficulty in fully optimizing this benefit. One solution is documenting both the physical and mailing addresses on the patient’s EHR, which enables CMOP to send refrigerated items to the patient’s home address via UPS and mail the rest of the prescriptions to the patient’s PO box address with the US Postal Service. The physical address must be listed above the PO box address to ensure that refrigerated items are not rejected by CMOP. Furthermore, both the physical address and the PO box address must be in the same city for this method to work. Two sites noted mailing refrigerated items as one of the major challenges in CMOP prescription processing.
CMOP-enrolled patients must be educated about requesting medications 7 to 10 days before they run out. There is no standard time line for prescriptions filled by CMOP. However, 1 site reported that it may take up to “10 days from time requested to mailbox.” This delay leads to pharmacies facing a dilemma as processing prescriptions too early can lead to insurance rejections, but processing them too late can lead to the patient not receiving the medication by the time they run out of their current supply. However, CMOP provides the ability to track prescriptions sent through CMOP. Pharmacists and technicians need to have access to BestWay Parcel Services Client Portal (genco-mms.bestwayparcel.com) to track CMOP packages. Tracking CMOP prescriptions is a way pharmacy technicians can be involved in CMOP prescription processing. Technicians seem to be underutilized, as only 27% of the responding sites utilize them to some degree in the CMOP process. One site delegated the responsibility of checking CMOP rejection messages to pharmacy technicians. Since 2 of the responding sites do not check CMOP rejection messages at all, this is an excellent opportunity to get pharmacy technicians involved.
A CMOP auto-refill program can potentially be utilized to avoid missed or late medications. In an auto-refill program, a pharmacist can refill prescriptions through CMOP on the due date without a patient request. They may get rejected by insurance the first time they are processed through CMOP for refilling too early if the processing time is taken into account. However, the subsequent refills do not have to consider the CMOP processing time as they would already be synchronized based on the last refill date. Though, if CMOP is out of stock on a medication and it is expected to be available soon, CMOP may take a few extra days to either fill the prescription or reject it if the drug stays unavailable. One of the sites reported “the amount of time [CMOP] holds medications if they are out of stock” as “the hardest thing to work around.” A couple of sites also mentioned the longer than usual delay in processing prescriptions by CMOP during the holidays as one of the major challenges.
CMOP use of repackaged products also may lead insurance companies to deny reimbursement. Repackaged products are usually cheaper to buy.14 However, most insurances do not reimburse for prescriptions filled with these products.15 The local drug file on RPMS may have a national drug code (NDC) that is reimbursable by insurance, but CMOP will change it to the repackaged NDC if they are filling the prescription with a repackaged product. One potential solution to this problem would be filling these prescriptions locally. Furthermore, insurance claims are processed when the prescriptions are filled by CMOP. Sites cannot return/cancel the prescription anymore at that point. Therefore, the inability to see real-time rejections as the medication orders are processed on-site makes it challenging to prevent avoidable insurance rejections, such as a refill too soon. One site calculated that it lost $26,386.45 by utilizing CMOP from January 9, 2018 to December 12, 2018. However, it is unclear whether this loss was representative of other sites. It is also worth noting that IHS sites can save a substantial amount of money on certain products by utilizing CMOP because VA buys these products at a reduced price.16
CMOP-transmitted prescriptions can be rejected for various reasons, such as CMOP manufacturer’s backorder, a different quantity from CMOP stock size, etc. Information about these rejected prescriptions is accessed through electronic messages on RPMS. CMOP does not dispense less than a full, unopened package for most over-the-counter (OTC) medications. The quantity on these prescriptions must be equal to or multiples of the package size for them to be filled by CMOP. This can lead to a patient having prescriptions with different days’ supplies, which results in various refill due dates. If a site has a local mail-out program available, it can potentially keep the same days’ supply for all prescriptions by mailing these OTC medications locally rather than utilizing CMOP. However, this can partially negate CMOP’s benefit of reduced workload.
CMOP also has specified quantities on some prescription medications. One survey respondent viewed “the quantity and day supply required by CMOP” as a negative influence on the site’s insurance collection. It is possible that CMOP does not carry all the medications that a CMOP-enrolled patient is prescribed. Most sites (77%) still send eligible prescriptions through CMOP for the patients who also have CMOP-ineligible prescriptions. There are a small number of sites (14%) that utilize local mail-out program for the patients with any CMOP-ineligible prescriptions, possibly to simplify the process. Schedule II controlled substances cannot be processed through CMOP either; however, facilities may have local policies that prohibit mailing any controlled substances.
Prescriptions can be manually transmitted to CMOP, or they can be automatically transmitted based on the run time and frequency of the auto-transmission setup. The prescriptions that are waiting to be transmitted to CMOP must be in the “suspended” status. The apparent advantage of relying on auto-transmission is that you do not have to complete the steps manually to transmit suspended CMOP prescriptions, thereby making the process more convenient. However, the manual transmission can be utilized as a checkpoint to verify that prescriptions were properly suspended for CMOP, as the prescription status changes from “S” (suspended) to “AT” once the transmission is completed. If a prescription is not properly suspended for CMOP, the status will remain as S even after manual transmission. More than half (59%) of the responding sites must find the manual transmission feature useful as they use it either over or in addition to the auto-transmission setup.
Despite the challenges, many IHS sites process thousands of monthly prescriptions through CMOP. Of the 94 CMOP-enrolled IHS sites, 17 processed > 1,000 prescriptions from March 27, 2019 to April 25, 2019.17 Five sites processed > 5,000 prescriptions.17 At the rate of > 5,000 prescriptions per month, the yearly CMOP prescription count will be > 60,000. That is more than one-third of the prescriptions processed by CHCF in 2018. By handling these prescriptions through CMOP, it can decrease pharmacy filling and dispensing workload, thereby freeing pharmacists to participate in other services.18 Furthermore, implementing CMOP does not incur any cost for the IHS site. There is a nondrug cost for each prescription that is filled through CMOP. This cost was $2.67 during FY 2016.19 The fee covers prescription vial, label, packaging for mail, postage, personnel, building overhead, and equipment capitalization.19 The nondrug cost of filling a prescription locally at the site can potentially exceed the cost charged by CMOP.19
A lack of objective data exists to assess the net impact of CMOP on patients. Different theoretical assumptions can be made, such as CMOP resulting in better patient adherence. However, there is no objective information about how much CMOP improves patient adherence if it does at all. Though J.D. Power US Pharmacy Study ranks CMOP as “among the best” mail-order pharmacies in customer satisfaction, only 3 of the 44 responding sites have measured patient satisfaction locally.20 Only 1 site had objective data about CMOP’s impact on the point of sale. Therefore, it is currently difficult to perform a cost-benefit analysis of the CMOP program. There are opportunities for further studies on these topics.
Limitations
One limitation of this study is that < 50% of the CMOP-enrolled sites (44 of 94) responded to the questionnaire. It is possible that the facilities that had a significantly positive or negative experience with CMOP were more inclined to share their views. Therefore, it is difficult to conclude whether the responding sites are an accurate representative sample. Another limitation of the study was the questionnaire design and the reliance on free-text responses as opposed to structured data. The free-text responses had to be analyzed manually to determine whether they fall in the same category, thereby increasing the risk of interpretation error.
Conclusion
CMOP has its unique challenges but provides many benefits that local pharmacy mail-out programs may not possess, such as the abilities to mail refrigerated items and track packages. One must be familiar with CMOP’s various idiosyncrasies to make the best use of the program. Extensive staff education and orientation for new staff members must be done to familiarize them with the program. Nevertheless, the successful implementation of CMOP can lead to reduced pharmacy workload while increasing access to care for patients with transportation issues.
Acknowledgments
The authors thank LCDR Karsten Smith, PharmD, BCGP, the IHS CMOP Coordinator for providing the list of primary CMOP contacts and CDR Kendall Van Tyle, PharmD, BCPS, for proofreading the article.
1. US Department of Veterans Affairs, Office of Inspector General. Audit of Consolidated Mail Outpatient Pharmacy contract management. https://www.va.gov/oig/52/reports/2009/VAOIG-09-00026-143.pdf. Published June 10, 2009. Accessed June 11, 2020.
2. US Department of Veterans Affairs. Pharmacy Benefits Management Services. VA mail order pharmacy. https://www.pbm.va.gov/PBM/CMOP/VA_Mail_Order_Pharmacy.asp. Updated July 18, 2018. Accessed July 16, 2019.
3. US Department of Veterans Affairs. Memorandum of understanding between the Department of Veterans Affairs (VA) and Indian Health Service (IHS). https://www.va.gov/TRIBALGOVERNMENT/docs/Signed2010VA-IHSMOU.pdf. Published October 1, 2010. Accessed June 11, 2020.
4. US Department of Veterans Affairs, Office of Tribal Government Relations, Office of Rural Health, US Department of Health and Human Services, Indian Health Service. U.S. Department of Veterans Affairs and Indian Health Service memorandum of understanding annual report fiscal year 2018. https://www.ruralhealth.va.gov/docs/VA-IHS_MOU_AnnualReport_FY2018_FINAL.pdf. Published December 2018. Accessed June 11, 2020.
5. Karsten S. CMOP items of interest. Published October 12, 2018. [Nonpublic document]
6. Fernandez EV, McDaniel JA, Carroll NV. Examination of the link between medication adherence and use of mail-order pharmacies in chronic disease states. J Manag Care Spec Pharm. 2016;22(11):1247‐1259. doi:10.18553/jmcp.2016.22.11.1247
7. Schwab P, Racsa P, Rascati K, Mourer M, Meah Y, Worley K. A retrospective database study comparing diabetes-related medication adherence and health outcomes for mail-order versus community pharmacy. J Manag Care Spec Pharm. 2019;25(3):332‐340. doi:10.18553/jmcp.2019.25.3.332
8. Schmittdiel JA, Karter AJ, Dyer W, et al. The comparative effectiveness of mail order pharmacy use vs. local pharmacy use on LDL-C control in new statin users. J Gen Intern Med. 2011;26(12):1396‐1402. doi:10.1007/s11606-011-1805-7
9. Devine S, Vlahiotis A, Sundar H. A comparison of diabetes medication adherence and healthcare costs in patients using mail order pharmacy and retail pharmacy. J Med Econ. 2010;13(2):203‐211. doi:10.3111/13696991003741801
10. Kappenman AM, Ragsdale R, Rim MH, Tyler LS, Nickman NA. Implementation of a centralized mail-order pharmacy service. Am J Health Syst Pharm. 2019;76(suppl 3):S74‐S78. doi:10.1093/ajhp/zxz138
11. US Department of Health and Human Services, Indian Health Service. Crownpoint service unit. www.ihs.gov/crownpoint. Accessed June 11, 2020.
12. Chaco P. Roads and transportation on the Navajo Nation. https://obamawhitehouse.archives.gov/microsite/blog/31387?page=135. Published February 15, 2012. Accessed June 11, 2020.
13. US Department of Health and Human Services, Indian Health Service. Disparities. www.ihs.gov/newsroom/factsheets/disparities. Updated October 2019. Accessed June 11, 2020.
14. Golden State Medical Supply. National contracts. www.gsms.us/wp-content/uploads/2018/10/National-Contracts-Flyer.pdf. Updated October 4, 2018. Accessed June 11, 2020.
15. Arizona Health Care Cost Containment System. IHS/Tribal provider billing manual chapter 9, hospital and clinic services. www.azahcccs.gov/PlansProviders/Downloads/IHS-TribalManual/IHS-Chap09HospClinic.pdf. Updated February 28, 2019. Accessed June 11, 2020.
16. US Department of Veterans Affairs, Office of Inspector General. The impact of VA allowing government agencies to be excluded from temporary price reductions on federal supply schedule pharmaceutical contracts. www.va.gov/oig/pubs/VAOIG-18-04451-06.pdf. Published October 30, 2019. Accessed June 11, 2020.
17. Karsten S. IHS Billing Report-Apr. Indian Health Service SharePoint. Published May 3, 2019. [Nonpublic document]
18. Aragon BR, Pierce RA 2nd, Jones WN. VA CMOPs: producing a pattern of quality and efficiency in government. J Am Pharm Assoc (2003). 2012;52(6):810‐815. doi:10.1331/JAPhA.2012.11075
19. Todd W. VA-IHS Consolidated Mail Outpatient Pharmacy program (CMOP). www.npaihb.org/wp-content/uploads/2017/01/CMOP-Slides-for-Portland-Area-Tribal-Sites.pdf. Published 2017. Accessed June 11, 2020.
20. J.D. Power. Pharmacy customers slow to adopt digital offerings but satisfaction increases when they do, J.D. Power finds. www.jdpower.com/business/press-releases/2019-us-pharmacy-study. Published August 20, 2019. Accessed June 11, 2020.
1. US Department of Veterans Affairs, Office of Inspector General. Audit of Consolidated Mail Outpatient Pharmacy contract management. https://www.va.gov/oig/52/reports/2009/VAOIG-09-00026-143.pdf. Published June 10, 2009. Accessed June 11, 2020.
2. US Department of Veterans Affairs. Pharmacy Benefits Management Services. VA mail order pharmacy. https://www.pbm.va.gov/PBM/CMOP/VA_Mail_Order_Pharmacy.asp. Updated July 18, 2018. Accessed July 16, 2019.
3. US Department of Veterans Affairs. Memorandum of understanding between the Department of Veterans Affairs (VA) and Indian Health Service (IHS). https://www.va.gov/TRIBALGOVERNMENT/docs/Signed2010VA-IHSMOU.pdf. Published October 1, 2010. Accessed June 11, 2020.
4. US Department of Veterans Affairs, Office of Tribal Government Relations, Office of Rural Health, US Department of Health and Human Services, Indian Health Service. U.S. Department of Veterans Affairs and Indian Health Service memorandum of understanding annual report fiscal year 2018. https://www.ruralhealth.va.gov/docs/VA-IHS_MOU_AnnualReport_FY2018_FINAL.pdf. Published December 2018. Accessed June 11, 2020.
5. Karsten S. CMOP items of interest. Published October 12, 2018. [Nonpublic document]
6. Fernandez EV, McDaniel JA, Carroll NV. Examination of the link between medication adherence and use of mail-order pharmacies in chronic disease states. J Manag Care Spec Pharm. 2016;22(11):1247‐1259. doi:10.18553/jmcp.2016.22.11.1247
7. Schwab P, Racsa P, Rascati K, Mourer M, Meah Y, Worley K. A retrospective database study comparing diabetes-related medication adherence and health outcomes for mail-order versus community pharmacy. J Manag Care Spec Pharm. 2019;25(3):332‐340. doi:10.18553/jmcp.2019.25.3.332
8. Schmittdiel JA, Karter AJ, Dyer W, et al. The comparative effectiveness of mail order pharmacy use vs. local pharmacy use on LDL-C control in new statin users. J Gen Intern Med. 2011;26(12):1396‐1402. doi:10.1007/s11606-011-1805-7
9. Devine S, Vlahiotis A, Sundar H. A comparison of diabetes medication adherence and healthcare costs in patients using mail order pharmacy and retail pharmacy. J Med Econ. 2010;13(2):203‐211. doi:10.3111/13696991003741801
10. Kappenman AM, Ragsdale R, Rim MH, Tyler LS, Nickman NA. Implementation of a centralized mail-order pharmacy service. Am J Health Syst Pharm. 2019;76(suppl 3):S74‐S78. doi:10.1093/ajhp/zxz138
11. US Department of Health and Human Services, Indian Health Service. Crownpoint service unit. www.ihs.gov/crownpoint. Accessed June 11, 2020.
12. Chaco P. Roads and transportation on the Navajo Nation. https://obamawhitehouse.archives.gov/microsite/blog/31387?page=135. Published February 15, 2012. Accessed June 11, 2020.
13. US Department of Health and Human Services, Indian Health Service. Disparities. www.ihs.gov/newsroom/factsheets/disparities. Updated October 2019. Accessed June 11, 2020.
14. Golden State Medical Supply. National contracts. www.gsms.us/wp-content/uploads/2018/10/National-Contracts-Flyer.pdf. Updated October 4, 2018. Accessed June 11, 2020.
15. Arizona Health Care Cost Containment System. IHS/Tribal provider billing manual chapter 9, hospital and clinic services. www.azahcccs.gov/PlansProviders/Downloads/IHS-TribalManual/IHS-Chap09HospClinic.pdf. Updated February 28, 2019. Accessed June 11, 2020.
16. US Department of Veterans Affairs, Office of Inspector General. The impact of VA allowing government agencies to be excluded from temporary price reductions on federal supply schedule pharmaceutical contracts. www.va.gov/oig/pubs/VAOIG-18-04451-06.pdf. Published October 30, 2019. Accessed June 11, 2020.
17. Karsten S. IHS Billing Report-Apr. Indian Health Service SharePoint. Published May 3, 2019. [Nonpublic document]
18. Aragon BR, Pierce RA 2nd, Jones WN. VA CMOPs: producing a pattern of quality and efficiency in government. J Am Pharm Assoc (2003). 2012;52(6):810‐815. doi:10.1331/JAPhA.2012.11075
19. Todd W. VA-IHS Consolidated Mail Outpatient Pharmacy program (CMOP). www.npaihb.org/wp-content/uploads/2017/01/CMOP-Slides-for-Portland-Area-Tribal-Sites.pdf. Published 2017. Accessed June 11, 2020.
20. J.D. Power. Pharmacy customers slow to adopt digital offerings but satisfaction increases when they do, J.D. Power finds. www.jdpower.com/business/press-releases/2019-us-pharmacy-study. Published August 20, 2019. Accessed June 11, 2020.
Retreatment of Hepatitis C Infection With Direct-Acting Antivirals
An estimated 3.5 million people in the US have chronic hepatitis C virus (HCV) infection, and between 10% and 20% of those developed cirrhosis over 20 to 30 years.1 There are at least 6 genotypes (GTs) of HCV, with GT1 being the most common in the US and previously one of the most difficult to treat.2,3 The goal of treatment is to achieve viral cure, called sustained virologic response (SVR) when HCV viral load remains undetectable several weeks after therapy completion. In the 2000s, pegylated interferon (pegIFN) and ribavirin (RBV) were the standard of care.2 For patients with GT1 infections, an SVR of 40 to 50% was commonly seen after 48 weeks of pegIFN/RBV regimens compared with 70 to 80% SVR for GT2 or GT3 after 24 weeks of pegIFN/RBV therapy.2 However, treatment has evolved rapidly (Table 1).2-17
In 2011, the US Food and Drug Administration (FDA) approved the protease inhibitors (PIs) boceprevir and telaprevir, which added a new class of agents with increased SVR for patients with GT1 infection; however, pegIFN and RBV were still needed for treatment.4 In addition, both PIs required multiple doses per day and strict adherence to an 8-hour schedule.4 Boceprevir required treatment with RBV and pegIFN for 48 weeks unless futility rule was met at 24 weeks of treatment (ie, viral load still detectable).4 The SVR in patients with GT1 infection improved to > 65% for patients in clinical trials.2 FDA approval of the direct-acting antivirals (DAAs) sofosbuvir and simeprevir in late 2013 decreased the usual duration of therapy to only 12 weeks with improved SVR rates 12 weeks posttherapy (SVR12) to 90% or higher.2,6,10
FDA approval of ledipasvir (LDV)/sofosbuvir (SOF) in October 2014 resulted in the first interferon-free all-oral regimen indicated for HCV GT1 infection.11 In December 2014, FDA approved a combination of paritaprevir, ritonavir, ombitasvir, and dasabuvir (PrOD).12 In 2015 GT-specific approvals were issued for daclastavir to be used with SOV for GT1 and GT3 and a combination similar to PrOD without dasabuvir (PrO) for GT4.13 In 2016, a combination of elbasvir (ERB) and grazoprevir (GZP) was approved for GT1 and GT4.14
In 2016, a pangenotypic DAA of SOF and velpatasvir (VEL) was approved.15 Most recently, combinations of SOF, VEL, and voxilaprevir (VOX), and glecaprevir (GLE) and pibrentasvir (PIB) were approved for patients with previous DAA treatment failures.7, 8,16,17 These oral regimens avoided the significant adverse events (AEs) associated with pegIFN and RBV (eg, thrombocytopenia, depression), were expected to improve treatment adherence and shorten duration of therapy.
The West Palm Beach Veterans Affairs has had a nurse practitioner (NP)-based HCV treatment clinic since the late 1990s. When PIs became available, a CPS started reviewing patient electronic health records (EHRs) and monitored response to therapy along with the NP to ensure discontinuation of therapy if futility criteria were met.7 Our unpublished experience showed SVR > 60% with both boceprevir and SOF regimens and > 90% with oral DAA regimens.
This review will provide the SVR rates for patients that needed retreatment for HCV infection since 2015 until December 2019. We treated all willing patients, beginning with the patients who had experienced failures with previous regimens. Patients first received education on HCV infection and treatment options in a group class then they were seen by the NP individually for specific education on treatment. The CPS reviewed the patient’s medical record to assess for appropriate therapy, possible drug-drug interactions and contraindications to therapy. In addition, patient outcomes (eg, viral load, AEs) were documented by the CPS in collaboration with the NP throughout treatment until viral load for SVR evaluation was obtained.
Methods
A retrospective EHR review of patients retreated from January 2015 to December 2019 was conducted. Data collected included age, sex, HCV GT, previous therapy, new medications prescribed, creatinine clearance, and achievement of SVR12. This retrospective review was approved by the facility’s scientific advisory committee as part of performance improvement efforts. Descriptive statistics are provided.
Results
Boceprevir
We treated 31 patients with boceprevir of which 3 met futility rule and 28 completed therapy. Eighteen of 28 responded (64%) to the treatment. The 10 patients who failed treatment were retreated with LDV/SOF, and all achieved SVR.
Sofosbuvir
A total of 53 patients were treated with SOF, RBV, and pegIFN for 12 weeks. Forty-one achieved SVR (77%). Of the 12 who failed therapy, all have been retreated and achieved SVR (Table 2).
Interferon-Free DAA Oral Regimens
More than 900 patients have been treated with interferon-free regimens since 2015 and outcomes were documented for > 800 patients. The SVR rates by GT were as follows: GT1 639 of 676 (95%); GT2 76 of 79 (96%); GT3 40 of 48 (83%); and GT4 6 of 6 (100%). Eighty-four percent of patients had GT1 infection. The median age of patient was 62 years, 72% were treatment naïve, and 35% having cirrhosis (based on liver biopsy or FIB4 score).18
Of 48 treatment failures, 30 patients were retreated; the rest of the patients were lost to follow-up (n = 9) or unable to receive retreatment (n = 9) mainly due to decompensated cirrhosis or liver cancer and short life expectancy. The median age of patient in this retreatment group was 62 years, 62% had cirrhosis, and most were infected with GT1. The average creatinine clearance was 73 mL/min. Twenty-two patients who failed therapy with ledipasvir/SOF were retreated (Table 3). A total of 13 patients out of the 19 tested eventually achieved SVR (68%). Four of the patients who had treatment failure again had GT1 infection and the other 2 GT3. All had cirrhosis.
Thirty-five patients were treated with PrOD, and 32 achieved SVR (91%). All 3 patients were retreated. One patient each achieved SVR with ERB/GZP, SOF/VEL and SOF/VEL/VOX. Fifty patients were treated with ERB/GZP and 45 achieved SVR (90%). All 5 treatment failures were retreated. Four achieved SVR and 1 was lost to follow-up (Table 4). Overall, of 30 patients who were retreated after failure with an all-oral DAA regimen, 27 patients had SVR values available and 21 achieved it (78%).
Discussion
Overall SVR was very high for patients who received oral treatment for HCV infection. A low number of patients failed therapy and were retreated. Patients who failed therapy again were similar in age but were more likely to have cirrhosis when compared with the overall interferon-free treated group. Thus, prompt treatment after HCV detection and before disease progression may improve treatment outcomes. Achieving SVR has been shown to improve fibrosis, portal hypertension, splenomegaly and cirrhosis, and reduce the risk of hepatocellular carcinoma by 70% and liver-related mortality by 90%.19-21/
Patients who failed therapy primarily had GT1—the most prevalent GT treated. A higher prevalence of GT1 is expected since it is the most common GT in the US.6 However, disease progression occurs more rapidly in those with GT3 and is more difficult to treat.22 The overall response rate was lower with this GT (83%) in this report, with only 1 of 3 patients retreated achieving an SVR.
Similar results are documented in retreatment trials.23 In the POLARIS-1 trial, treatment with SOF/VEL/VOX resulted in an overall response rate of 96% but only 91% for patients with GT3, compared with 95 to 100% for GTs 1, 2, or 4.23 In the current report, only 1 patient (GT1) failed retreatment with SOF/VEL/VOX. At this time, there are no clear treatment options for this patient. However, patients who fail GLE/PIB (none so far in the current report) may be able to receive SOF/VEL/VOX.24 In a small study, 29 of 31 patients achieved SVR with SOF/VEL/VOX after GLE/PIB failure (12 of 13 GT1 and 17 of 18 GT3).24
Limitations
This review was an observational, nonrandomized design, and only 1 medical center was involved. These results may not be applicable to other patient populations without a clinic set up with routine follow-ups to encourage adherence and completion of therapy.
Conclusions
Treatment of HCV infection has improved significantly over the past 10 years. Use of DAAs results in SVR for > 90% of patients, especially if the disease had not progressed to cirrhosis. Failure after retreatment for HCV infection was rare as well. Given that cirrhosis seems to increase the chance of treatment failure, it is imperative to identify candidates for treatment before the infection has progressed to cirrhosis. Patients infected with GT3 in particular should be more aggressively identified and treated.
Acknowledgments
The authors thank Nick P. Becky, PharmD, for his contributions to the identification of patients needing treatment for their HCV infection and review of initial manuscript information.
1. Centers for Disease Control and Prevention. Viral hepatitis: hepatitis C information. https://www.cdc.gov/hepatitis/hcv/index.htm. Reviewed April 14, 2020. Accessed June 16, 2020.
2. American Association for the Study of Liver Disease, Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. https://www.hcvguidelines.org. Accessed June 16, 2020.
3. Lingala S, Ghany MG. Natural history of hepatitis C. Gastroenterol Clin N Am. 2015;44(4):717-734. doi:10.1016/j.gtc.2015.07.003
4. Foote BS, Spooner LM, Belliveau PP. Boceprevir: a protease inhibitor for the treatment of chronic hepatitis C. Ann Pharmacother. 2011;45(9):1085-1093. doi:10.1345/aph.1P744
5. Kayali Z, Schmidt WN. Finally sofosbuvir: an oral anti-HCV drug with wide performance capability. Pharmgenomics Pers Med. 2014:7:387-398. doi:10.2147/PGPM.S52629
6. Falade-Nwulis O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. 2017;166(9):637-648. doi:10.7326/M16-2575
7. Carrion AF, Martin P. Glecaprevir + pibrentasvir for treatment of hepatitis C. Expert Opinion Pharmacother. 2018;19(4):413-419. doi:10.1080/14656566.2018.1444030
8. Chahine EB, Kelley D, Childs-Kean LM. Sofosbuvir/velpatasvir/voxilaprevir: a pan-genotypic direct-acting antiviral combination for hepatitis C. Ann Pharmacother. 2018;52(4):352-363. doi:10.1177/1060028017741508
9. Lagasca AM, Kan VL. Hepatitis C treatment at a Veteran Affairs medical center after the availability of direct-acting agents: things are looking up. Clin Infect Dis. 2015:61(8):1347-1349. doi:10.1093/cid/civ573
10. Sovaldi (sofosbuvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
11. Harvoni (ledipasvir and sofosbuvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
12. Viekira Pak (ombitasvir, paritaprevir and ritonavir; dasabuvir) [package insert]. North Chicago, IL: AbbVie Inc; 2018.
13. Technivie (ombitasvir, paritaprevir and ritonavir) [package insert]. North Chicago, IL: AbbVie Inc; 2018.
14. Zepatier (elbasvir and grazoprevir) [package insert]. Whitehouse Station, NJ: Merck & Co Inc; 2018.
15. Epclusa (sofosbuvir and velpatasvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
16. Mavyret (glecaprevir and pibrentasvir) [package insert]. North Chicago, IL: AbbVie Inc; 2019.
17. Vosevi (sofosbuvir, velpatasvir and voxilaprevir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
18. Vallet-Pichard A, Mallet V, Nalpas V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and FibroTest. Hepatology. 2017;46(1):32-36. doi:10.1002/hep.21669
19. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5, pt 1):329-337. doi:10.7326/0003-4819-158-5-201303050-00005
20. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584-2593. doi:10.1001/jama.2012.144878
21. Veldt BJ, Heathcote EJ, Wedemeyer H, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147(10):677-684. doi:10.7326/0003-4819-147-10-200711200-00003
22. Chen A, Patel K, Naggie S. Genotype 3 infection: the last stand of hepatitis C virus. Drugs. 2017;77(2):131-144. doi:10.1007/s40265-016-0685-x
23. Bourlière M, Gordon SC, Flamm SL, et al; POLARIS-1 and POLARIS-4 Investigators. Sofosbuvir, velpatasvir and voxilaprevir for previously treated HCV infection. N Engl J Med. 2017;376(22):2136-2146. doi:10.1056/NEJMoa1613512
24. Pearlman B, Perrys M, Hinds A. Sofosbuvir/velpatasvir/voxilaprevir for previous treatment failures with glecaprevir/pibrentasvir in chronic hepatitis C infection. Am J Gastroenterol. 2019;114(9):1550-1552. doi:10.14309/ajg.0000000000000248
An estimated 3.5 million people in the US have chronic hepatitis C virus (HCV) infection, and between 10% and 20% of those developed cirrhosis over 20 to 30 years.1 There are at least 6 genotypes (GTs) of HCV, with GT1 being the most common in the US and previously one of the most difficult to treat.2,3 The goal of treatment is to achieve viral cure, called sustained virologic response (SVR) when HCV viral load remains undetectable several weeks after therapy completion. In the 2000s, pegylated interferon (pegIFN) and ribavirin (RBV) were the standard of care.2 For patients with GT1 infections, an SVR of 40 to 50% was commonly seen after 48 weeks of pegIFN/RBV regimens compared with 70 to 80% SVR for GT2 or GT3 after 24 weeks of pegIFN/RBV therapy.2 However, treatment has evolved rapidly (Table 1).2-17
In 2011, the US Food and Drug Administration (FDA) approved the protease inhibitors (PIs) boceprevir and telaprevir, which added a new class of agents with increased SVR for patients with GT1 infection; however, pegIFN and RBV were still needed for treatment.4 In addition, both PIs required multiple doses per day and strict adherence to an 8-hour schedule.4 Boceprevir required treatment with RBV and pegIFN for 48 weeks unless futility rule was met at 24 weeks of treatment (ie, viral load still detectable).4 The SVR in patients with GT1 infection improved to > 65% for patients in clinical trials.2 FDA approval of the direct-acting antivirals (DAAs) sofosbuvir and simeprevir in late 2013 decreased the usual duration of therapy to only 12 weeks with improved SVR rates 12 weeks posttherapy (SVR12) to 90% or higher.2,6,10
FDA approval of ledipasvir (LDV)/sofosbuvir (SOF) in October 2014 resulted in the first interferon-free all-oral regimen indicated for HCV GT1 infection.11 In December 2014, FDA approved a combination of paritaprevir, ritonavir, ombitasvir, and dasabuvir (PrOD).12 In 2015 GT-specific approvals were issued for daclastavir to be used with SOV for GT1 and GT3 and a combination similar to PrOD without dasabuvir (PrO) for GT4.13 In 2016, a combination of elbasvir (ERB) and grazoprevir (GZP) was approved for GT1 and GT4.14
In 2016, a pangenotypic DAA of SOF and velpatasvir (VEL) was approved.15 Most recently, combinations of SOF, VEL, and voxilaprevir (VOX), and glecaprevir (GLE) and pibrentasvir (PIB) were approved for patients with previous DAA treatment failures.7, 8,16,17 These oral regimens avoided the significant adverse events (AEs) associated with pegIFN and RBV (eg, thrombocytopenia, depression), were expected to improve treatment adherence and shorten duration of therapy.
The West Palm Beach Veterans Affairs has had a nurse practitioner (NP)-based HCV treatment clinic since the late 1990s. When PIs became available, a CPS started reviewing patient electronic health records (EHRs) and monitored response to therapy along with the NP to ensure discontinuation of therapy if futility criteria were met.7 Our unpublished experience showed SVR > 60% with both boceprevir and SOF regimens and > 90% with oral DAA regimens.
This review will provide the SVR rates for patients that needed retreatment for HCV infection since 2015 until December 2019. We treated all willing patients, beginning with the patients who had experienced failures with previous regimens. Patients first received education on HCV infection and treatment options in a group class then they were seen by the NP individually for specific education on treatment. The CPS reviewed the patient’s medical record to assess for appropriate therapy, possible drug-drug interactions and contraindications to therapy. In addition, patient outcomes (eg, viral load, AEs) were documented by the CPS in collaboration with the NP throughout treatment until viral load for SVR evaluation was obtained.
Methods
A retrospective EHR review of patients retreated from January 2015 to December 2019 was conducted. Data collected included age, sex, HCV GT, previous therapy, new medications prescribed, creatinine clearance, and achievement of SVR12. This retrospective review was approved by the facility’s scientific advisory committee as part of performance improvement efforts. Descriptive statistics are provided.
Results
Boceprevir
We treated 31 patients with boceprevir of which 3 met futility rule and 28 completed therapy. Eighteen of 28 responded (64%) to the treatment. The 10 patients who failed treatment were retreated with LDV/SOF, and all achieved SVR.
Sofosbuvir
A total of 53 patients were treated with SOF, RBV, and pegIFN for 12 weeks. Forty-one achieved SVR (77%). Of the 12 who failed therapy, all have been retreated and achieved SVR (Table 2).
Interferon-Free DAA Oral Regimens
More than 900 patients have been treated with interferon-free regimens since 2015 and outcomes were documented for > 800 patients. The SVR rates by GT were as follows: GT1 639 of 676 (95%); GT2 76 of 79 (96%); GT3 40 of 48 (83%); and GT4 6 of 6 (100%). Eighty-four percent of patients had GT1 infection. The median age of patient was 62 years, 72% were treatment naïve, and 35% having cirrhosis (based on liver biopsy or FIB4 score).18
Of 48 treatment failures, 30 patients were retreated; the rest of the patients were lost to follow-up (n = 9) or unable to receive retreatment (n = 9) mainly due to decompensated cirrhosis or liver cancer and short life expectancy. The median age of patient in this retreatment group was 62 years, 62% had cirrhosis, and most were infected with GT1. The average creatinine clearance was 73 mL/min. Twenty-two patients who failed therapy with ledipasvir/SOF were retreated (Table 3). A total of 13 patients out of the 19 tested eventually achieved SVR (68%). Four of the patients who had treatment failure again had GT1 infection and the other 2 GT3. All had cirrhosis.
Thirty-five patients were treated with PrOD, and 32 achieved SVR (91%). All 3 patients were retreated. One patient each achieved SVR with ERB/GZP, SOF/VEL and SOF/VEL/VOX. Fifty patients were treated with ERB/GZP and 45 achieved SVR (90%). All 5 treatment failures were retreated. Four achieved SVR and 1 was lost to follow-up (Table 4). Overall, of 30 patients who were retreated after failure with an all-oral DAA regimen, 27 patients had SVR values available and 21 achieved it (78%).
Discussion
Overall SVR was very high for patients who received oral treatment for HCV infection. A low number of patients failed therapy and were retreated. Patients who failed therapy again were similar in age but were more likely to have cirrhosis when compared with the overall interferon-free treated group. Thus, prompt treatment after HCV detection and before disease progression may improve treatment outcomes. Achieving SVR has been shown to improve fibrosis, portal hypertension, splenomegaly and cirrhosis, and reduce the risk of hepatocellular carcinoma by 70% and liver-related mortality by 90%.19-21/
Patients who failed therapy primarily had GT1—the most prevalent GT treated. A higher prevalence of GT1 is expected since it is the most common GT in the US.6 However, disease progression occurs more rapidly in those with GT3 and is more difficult to treat.22 The overall response rate was lower with this GT (83%) in this report, with only 1 of 3 patients retreated achieving an SVR.
Similar results are documented in retreatment trials.23 In the POLARIS-1 trial, treatment with SOF/VEL/VOX resulted in an overall response rate of 96% but only 91% for patients with GT3, compared with 95 to 100% for GTs 1, 2, or 4.23 In the current report, only 1 patient (GT1) failed retreatment with SOF/VEL/VOX. At this time, there are no clear treatment options for this patient. However, patients who fail GLE/PIB (none so far in the current report) may be able to receive SOF/VEL/VOX.24 In a small study, 29 of 31 patients achieved SVR with SOF/VEL/VOX after GLE/PIB failure (12 of 13 GT1 and 17 of 18 GT3).24
Limitations
This review was an observational, nonrandomized design, and only 1 medical center was involved. These results may not be applicable to other patient populations without a clinic set up with routine follow-ups to encourage adherence and completion of therapy.
Conclusions
Treatment of HCV infection has improved significantly over the past 10 years. Use of DAAs results in SVR for > 90% of patients, especially if the disease had not progressed to cirrhosis. Failure after retreatment for HCV infection was rare as well. Given that cirrhosis seems to increase the chance of treatment failure, it is imperative to identify candidates for treatment before the infection has progressed to cirrhosis. Patients infected with GT3 in particular should be more aggressively identified and treated.
Acknowledgments
The authors thank Nick P. Becky, PharmD, for his contributions to the identification of patients needing treatment for their HCV infection and review of initial manuscript information.
An estimated 3.5 million people in the US have chronic hepatitis C virus (HCV) infection, and between 10% and 20% of those developed cirrhosis over 20 to 30 years.1 There are at least 6 genotypes (GTs) of HCV, with GT1 being the most common in the US and previously one of the most difficult to treat.2,3 The goal of treatment is to achieve viral cure, called sustained virologic response (SVR) when HCV viral load remains undetectable several weeks after therapy completion. In the 2000s, pegylated interferon (pegIFN) and ribavirin (RBV) were the standard of care.2 For patients with GT1 infections, an SVR of 40 to 50% was commonly seen after 48 weeks of pegIFN/RBV regimens compared with 70 to 80% SVR for GT2 or GT3 after 24 weeks of pegIFN/RBV therapy.2 However, treatment has evolved rapidly (Table 1).2-17
In 2011, the US Food and Drug Administration (FDA) approved the protease inhibitors (PIs) boceprevir and telaprevir, which added a new class of agents with increased SVR for patients with GT1 infection; however, pegIFN and RBV were still needed for treatment.4 In addition, both PIs required multiple doses per day and strict adherence to an 8-hour schedule.4 Boceprevir required treatment with RBV and pegIFN for 48 weeks unless futility rule was met at 24 weeks of treatment (ie, viral load still detectable).4 The SVR in patients with GT1 infection improved to > 65% for patients in clinical trials.2 FDA approval of the direct-acting antivirals (DAAs) sofosbuvir and simeprevir in late 2013 decreased the usual duration of therapy to only 12 weeks with improved SVR rates 12 weeks posttherapy (SVR12) to 90% or higher.2,6,10
FDA approval of ledipasvir (LDV)/sofosbuvir (SOF) in October 2014 resulted in the first interferon-free all-oral regimen indicated for HCV GT1 infection.11 In December 2014, FDA approved a combination of paritaprevir, ritonavir, ombitasvir, and dasabuvir (PrOD).12 In 2015 GT-specific approvals were issued for daclastavir to be used with SOV for GT1 and GT3 and a combination similar to PrOD without dasabuvir (PrO) for GT4.13 In 2016, a combination of elbasvir (ERB) and grazoprevir (GZP) was approved for GT1 and GT4.14
In 2016, a pangenotypic DAA of SOF and velpatasvir (VEL) was approved.15 Most recently, combinations of SOF, VEL, and voxilaprevir (VOX), and glecaprevir (GLE) and pibrentasvir (PIB) were approved for patients with previous DAA treatment failures.7, 8,16,17 These oral regimens avoided the significant adverse events (AEs) associated with pegIFN and RBV (eg, thrombocytopenia, depression), were expected to improve treatment adherence and shorten duration of therapy.
The West Palm Beach Veterans Affairs has had a nurse practitioner (NP)-based HCV treatment clinic since the late 1990s. When PIs became available, a CPS started reviewing patient electronic health records (EHRs) and monitored response to therapy along with the NP to ensure discontinuation of therapy if futility criteria were met.7 Our unpublished experience showed SVR > 60% with both boceprevir and SOF regimens and > 90% with oral DAA regimens.
This review will provide the SVR rates for patients that needed retreatment for HCV infection since 2015 until December 2019. We treated all willing patients, beginning with the patients who had experienced failures with previous regimens. Patients first received education on HCV infection and treatment options in a group class then they were seen by the NP individually for specific education on treatment. The CPS reviewed the patient’s medical record to assess for appropriate therapy, possible drug-drug interactions and contraindications to therapy. In addition, patient outcomes (eg, viral load, AEs) were documented by the CPS in collaboration with the NP throughout treatment until viral load for SVR evaluation was obtained.
Methods
A retrospective EHR review of patients retreated from January 2015 to December 2019 was conducted. Data collected included age, sex, HCV GT, previous therapy, new medications prescribed, creatinine clearance, and achievement of SVR12. This retrospective review was approved by the facility’s scientific advisory committee as part of performance improvement efforts. Descriptive statistics are provided.
Results
Boceprevir
We treated 31 patients with boceprevir of which 3 met futility rule and 28 completed therapy. Eighteen of 28 responded (64%) to the treatment. The 10 patients who failed treatment were retreated with LDV/SOF, and all achieved SVR.
Sofosbuvir
A total of 53 patients were treated with SOF, RBV, and pegIFN for 12 weeks. Forty-one achieved SVR (77%). Of the 12 who failed therapy, all have been retreated and achieved SVR (Table 2).
Interferon-Free DAA Oral Regimens
More than 900 patients have been treated with interferon-free regimens since 2015 and outcomes were documented for > 800 patients. The SVR rates by GT were as follows: GT1 639 of 676 (95%); GT2 76 of 79 (96%); GT3 40 of 48 (83%); and GT4 6 of 6 (100%). Eighty-four percent of patients had GT1 infection. The median age of patient was 62 years, 72% were treatment naïve, and 35% having cirrhosis (based on liver biopsy or FIB4 score).18
Of 48 treatment failures, 30 patients were retreated; the rest of the patients were lost to follow-up (n = 9) or unable to receive retreatment (n = 9) mainly due to decompensated cirrhosis or liver cancer and short life expectancy. The median age of patient in this retreatment group was 62 years, 62% had cirrhosis, and most were infected with GT1. The average creatinine clearance was 73 mL/min. Twenty-two patients who failed therapy with ledipasvir/SOF were retreated (Table 3). A total of 13 patients out of the 19 tested eventually achieved SVR (68%). Four of the patients who had treatment failure again had GT1 infection and the other 2 GT3. All had cirrhosis.
Thirty-five patients were treated with PrOD, and 32 achieved SVR (91%). All 3 patients were retreated. One patient each achieved SVR with ERB/GZP, SOF/VEL and SOF/VEL/VOX. Fifty patients were treated with ERB/GZP and 45 achieved SVR (90%). All 5 treatment failures were retreated. Four achieved SVR and 1 was lost to follow-up (Table 4). Overall, of 30 patients who were retreated after failure with an all-oral DAA regimen, 27 patients had SVR values available and 21 achieved it (78%).
Discussion
Overall SVR was very high for patients who received oral treatment for HCV infection. A low number of patients failed therapy and were retreated. Patients who failed therapy again were similar in age but were more likely to have cirrhosis when compared with the overall interferon-free treated group. Thus, prompt treatment after HCV detection and before disease progression may improve treatment outcomes. Achieving SVR has been shown to improve fibrosis, portal hypertension, splenomegaly and cirrhosis, and reduce the risk of hepatocellular carcinoma by 70% and liver-related mortality by 90%.19-21/
Patients who failed therapy primarily had GT1—the most prevalent GT treated. A higher prevalence of GT1 is expected since it is the most common GT in the US.6 However, disease progression occurs more rapidly in those with GT3 and is more difficult to treat.22 The overall response rate was lower with this GT (83%) in this report, with only 1 of 3 patients retreated achieving an SVR.
Similar results are documented in retreatment trials.23 In the POLARIS-1 trial, treatment with SOF/VEL/VOX resulted in an overall response rate of 96% but only 91% for patients with GT3, compared with 95 to 100% for GTs 1, 2, or 4.23 In the current report, only 1 patient (GT1) failed retreatment with SOF/VEL/VOX. At this time, there are no clear treatment options for this patient. However, patients who fail GLE/PIB (none so far in the current report) may be able to receive SOF/VEL/VOX.24 In a small study, 29 of 31 patients achieved SVR with SOF/VEL/VOX after GLE/PIB failure (12 of 13 GT1 and 17 of 18 GT3).24
Limitations
This review was an observational, nonrandomized design, and only 1 medical center was involved. These results may not be applicable to other patient populations without a clinic set up with routine follow-ups to encourage adherence and completion of therapy.
Conclusions
Treatment of HCV infection has improved significantly over the past 10 years. Use of DAAs results in SVR for > 90% of patients, especially if the disease had not progressed to cirrhosis. Failure after retreatment for HCV infection was rare as well. Given that cirrhosis seems to increase the chance of treatment failure, it is imperative to identify candidates for treatment before the infection has progressed to cirrhosis. Patients infected with GT3 in particular should be more aggressively identified and treated.
Acknowledgments
The authors thank Nick P. Becky, PharmD, for his contributions to the identification of patients needing treatment for their HCV infection and review of initial manuscript information.
1. Centers for Disease Control and Prevention. Viral hepatitis: hepatitis C information. https://www.cdc.gov/hepatitis/hcv/index.htm. Reviewed April 14, 2020. Accessed June 16, 2020.
2. American Association for the Study of Liver Disease, Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. https://www.hcvguidelines.org. Accessed June 16, 2020.
3. Lingala S, Ghany MG. Natural history of hepatitis C. Gastroenterol Clin N Am. 2015;44(4):717-734. doi:10.1016/j.gtc.2015.07.003
4. Foote BS, Spooner LM, Belliveau PP. Boceprevir: a protease inhibitor for the treatment of chronic hepatitis C. Ann Pharmacother. 2011;45(9):1085-1093. doi:10.1345/aph.1P744
5. Kayali Z, Schmidt WN. Finally sofosbuvir: an oral anti-HCV drug with wide performance capability. Pharmgenomics Pers Med. 2014:7:387-398. doi:10.2147/PGPM.S52629
6. Falade-Nwulis O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. 2017;166(9):637-648. doi:10.7326/M16-2575
7. Carrion AF, Martin P. Glecaprevir + pibrentasvir for treatment of hepatitis C. Expert Opinion Pharmacother. 2018;19(4):413-419. doi:10.1080/14656566.2018.1444030
8. Chahine EB, Kelley D, Childs-Kean LM. Sofosbuvir/velpatasvir/voxilaprevir: a pan-genotypic direct-acting antiviral combination for hepatitis C. Ann Pharmacother. 2018;52(4):352-363. doi:10.1177/1060028017741508
9. Lagasca AM, Kan VL. Hepatitis C treatment at a Veteran Affairs medical center after the availability of direct-acting agents: things are looking up. Clin Infect Dis. 2015:61(8):1347-1349. doi:10.1093/cid/civ573
10. Sovaldi (sofosbuvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
11. Harvoni (ledipasvir and sofosbuvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
12. Viekira Pak (ombitasvir, paritaprevir and ritonavir; dasabuvir) [package insert]. North Chicago, IL: AbbVie Inc; 2018.
13. Technivie (ombitasvir, paritaprevir and ritonavir) [package insert]. North Chicago, IL: AbbVie Inc; 2018.
14. Zepatier (elbasvir and grazoprevir) [package insert]. Whitehouse Station, NJ: Merck & Co Inc; 2018.
15. Epclusa (sofosbuvir and velpatasvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
16. Mavyret (glecaprevir and pibrentasvir) [package insert]. North Chicago, IL: AbbVie Inc; 2019.
17. Vosevi (sofosbuvir, velpatasvir and voxilaprevir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
18. Vallet-Pichard A, Mallet V, Nalpas V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and FibroTest. Hepatology. 2017;46(1):32-36. doi:10.1002/hep.21669
19. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5, pt 1):329-337. doi:10.7326/0003-4819-158-5-201303050-00005
20. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584-2593. doi:10.1001/jama.2012.144878
21. Veldt BJ, Heathcote EJ, Wedemeyer H, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147(10):677-684. doi:10.7326/0003-4819-147-10-200711200-00003
22. Chen A, Patel K, Naggie S. Genotype 3 infection: the last stand of hepatitis C virus. Drugs. 2017;77(2):131-144. doi:10.1007/s40265-016-0685-x
23. Bourlière M, Gordon SC, Flamm SL, et al; POLARIS-1 and POLARIS-4 Investigators. Sofosbuvir, velpatasvir and voxilaprevir for previously treated HCV infection. N Engl J Med. 2017;376(22):2136-2146. doi:10.1056/NEJMoa1613512
24. Pearlman B, Perrys M, Hinds A. Sofosbuvir/velpatasvir/voxilaprevir for previous treatment failures with glecaprevir/pibrentasvir in chronic hepatitis C infection. Am J Gastroenterol. 2019;114(9):1550-1552. doi:10.14309/ajg.0000000000000248
1. Centers for Disease Control and Prevention. Viral hepatitis: hepatitis C information. https://www.cdc.gov/hepatitis/hcv/index.htm. Reviewed April 14, 2020. Accessed June 16, 2020.
2. American Association for the Study of Liver Disease, Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. https://www.hcvguidelines.org. Accessed June 16, 2020.
3. Lingala S, Ghany MG. Natural history of hepatitis C. Gastroenterol Clin N Am. 2015;44(4):717-734. doi:10.1016/j.gtc.2015.07.003
4. Foote BS, Spooner LM, Belliveau PP. Boceprevir: a protease inhibitor for the treatment of chronic hepatitis C. Ann Pharmacother. 2011;45(9):1085-1093. doi:10.1345/aph.1P744
5. Kayali Z, Schmidt WN. Finally sofosbuvir: an oral anti-HCV drug with wide performance capability. Pharmgenomics Pers Med. 2014:7:387-398. doi:10.2147/PGPM.S52629
6. Falade-Nwulis O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. 2017;166(9):637-648. doi:10.7326/M16-2575
7. Carrion AF, Martin P. Glecaprevir + pibrentasvir for treatment of hepatitis C. Expert Opinion Pharmacother. 2018;19(4):413-419. doi:10.1080/14656566.2018.1444030
8. Chahine EB, Kelley D, Childs-Kean LM. Sofosbuvir/velpatasvir/voxilaprevir: a pan-genotypic direct-acting antiviral combination for hepatitis C. Ann Pharmacother. 2018;52(4):352-363. doi:10.1177/1060028017741508
9. Lagasca AM, Kan VL. Hepatitis C treatment at a Veteran Affairs medical center after the availability of direct-acting agents: things are looking up. Clin Infect Dis. 2015:61(8):1347-1349. doi:10.1093/cid/civ573
10. Sovaldi (sofosbuvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
11. Harvoni (ledipasvir and sofosbuvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
12. Viekira Pak (ombitasvir, paritaprevir and ritonavir; dasabuvir) [package insert]. North Chicago, IL: AbbVie Inc; 2018.
13. Technivie (ombitasvir, paritaprevir and ritonavir) [package insert]. North Chicago, IL: AbbVie Inc; 2018.
14. Zepatier (elbasvir and grazoprevir) [package insert]. Whitehouse Station, NJ: Merck & Co Inc; 2018.
15. Epclusa (sofosbuvir and velpatasvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
16. Mavyret (glecaprevir and pibrentasvir) [package insert]. North Chicago, IL: AbbVie Inc; 2019.
17. Vosevi (sofosbuvir, velpatasvir and voxilaprevir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
18. Vallet-Pichard A, Mallet V, Nalpas V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and FibroTest. Hepatology. 2017;46(1):32-36. doi:10.1002/hep.21669
19. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5, pt 1):329-337. doi:10.7326/0003-4819-158-5-201303050-00005
20. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584-2593. doi:10.1001/jama.2012.144878
21. Veldt BJ, Heathcote EJ, Wedemeyer H, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147(10):677-684. doi:10.7326/0003-4819-147-10-200711200-00003
22. Chen A, Patel K, Naggie S. Genotype 3 infection: the last stand of hepatitis C virus. Drugs. 2017;77(2):131-144. doi:10.1007/s40265-016-0685-x
23. Bourlière M, Gordon SC, Flamm SL, et al; POLARIS-1 and POLARIS-4 Investigators. Sofosbuvir, velpatasvir and voxilaprevir for previously treated HCV infection. N Engl J Med. 2017;376(22):2136-2146. doi:10.1056/NEJMoa1613512
24. Pearlman B, Perrys M, Hinds A. Sofosbuvir/velpatasvir/voxilaprevir for previous treatment failures with glecaprevir/pibrentasvir in chronic hepatitis C infection. Am J Gastroenterol. 2019;114(9):1550-1552. doi:10.14309/ajg.0000000000000248
Healthy Aging Project-Brain: A Psychoeducational and Motivational Group for Older Veterans
With a rapidly growing older adult population, increased attention has been given to cognitive changes that occur with age, with a focus on optimizing the cognitive health of aging individuals.1 Given the absence of pharmaceutical treatments to prevent cognitive decline, there is an increased need for health care systems to offer alternative or behavioral interventions that can mitigate the effects of cognitive decline in aging.
Notably, many individuals are able to maintain or even improve cognitive functioning throughout their lifespan, with some research implicating health behaviors as an important factor for promoting brain health with age. Specifically, sleep, exercise, eating habits, social engagement, and cognitive stimulation have been linked to improved cognitive functioning.2-8 In addition to the potential benefits for brain health, there is evidence that greater investment in attaining health goals is associated with subjective reports of higher well-being, fewer mental health symptoms, lower physical health stresses, decreased caregiver burden, and increased functional independence linked with longer independent living.9 The latter has a substantial financial impact, such that the positive consequence of increased independence is likely staving off the need for admission to assisted living and adult family homes, which can be costly.
Despite the role of health behaviors in brain aging and overall health and functioning, research indicates that only a small number of older adults (12.8%) follow recommended guidelines for healthy lifestyle factors.10 Education has been identified as one factor associated with the likelihood of engaging in positive health behaviors, prompting the delivery of health-education interventions. Most psychoeducational interventions have traditionally focused on one aspect of behavior change at a time (eg, sleep); however, Gross and colleaguesconducted a meta-analysis of cognitive interventions and in addition to the overall positive benefits (effect size 0.38), they also found suggestive evidence that interventions that combined multiple training strategies were associated with larger training gains (P = .04) after adjusting for multiple comparisons.11 For example, Miller and colleagues found a significant improvement on both subjective and objective measures of memory following a multicomponent approach that combined training in memory skills, stress reduction, nutrition, and physical activity.12
In addition to the potential positive impacts of health behaviors on brain health, findings suggest that targeted emphasis on health behavior change may have the potential to stave off mild cognitiveimpairment (MCI) or dementia even if for a short time. Given the increasing prevalence rates of MCI with age (6.7% in adults aged 60-64 years, reaching 25.2% in adults aged 80-84 years13) and dementia (prevalence of MCI converting to dementia is 18-40%14), as well as the corresponding emotional, financial, and family-oriented consequences (eg, impact on the well-being of family caregivers), the need for behavioral interventions that seek to optimize brain health is becoming increasingly apparent.
More than 9 million veterans are now aged ≥ 65 years.15 In addition to representing nearly half of all veterans and a sizable portion of aging adults in the US, older veterans are at increased risk of frailty, mortality, and high rates of chronic medical/mental health conditions that can lead to accelerated cognitive aging.6-17 Together, these conditions highlight the importance of developing comprehensive psychoeducational and behavioral interventions in this population. To address this need, we developed a novel psychoeducation and behavior change group called the Healthy Aging Project-Brain (HAP-B, pronounced “happy”). The HAP-B intervention was designed to promote healthy brain aging by using empirically supported health behavior change strategies, including education, personalized goal setting, and community support. The primary aim of this project was to develop and implement an intervention that was feasible and acceptable (eg, could be implemented in our setting, was appropriate for a veteran population) and to determine any positive outcomes/preliminary effects on overall health and well-being.
Methods
We recruited veterans aged ≥ 50 years through primary care clinics and self-referrals via flyers in the US Department of Veterans Affairs (VA) Puget Sound Health Care System (VAPSHCS), Seattle Division hospital. We targeted the “worried well” and welcomed veterans with MCI and mental health diagnoses. Notably, if there were significant mental health and/or substance use concerns, we encouraged veterans to seek focused care and stabilization prior to or concurrent with group participation. Exclusion criteria included presence of suicidality/homicidality, untreated or unstable substance use disorder, or a diagnosis of dementia. Exclusion criteria were assessed by the referring health care providers (HCPs), when appropriate, and through a health record review. Group facilitators used their clinical judgment to monitor participants if they began experiencing more severe cognitive impairment or acute mental health concerns. Although we did not encounter any of these instances, facilitators were prepared to discuss any concerns with the veteran and their referring HCP. Participants sampled were from 1 of 5 groups offered between January 2018 and March 2019. A waiver from the institutional review board was obtained after meeting criteria for quality improvement/quality assurance (QI/QA) for this study.
Procedures
At the initial stages of development, our team conducted a needs assessment to identify health-related areas where HCPs felt veterans would benefit from additional education and support. The needs assessment was conducted across primary care, geriatric extended care, and the Geriatric Research, Education, and Clinical Center (GRECC) at VAPSHCS. Combining the needs assessment results with the available research base, we identified sleep, physical activity, social engagement, and cognitive stimulation as areas for focus. Notably, although nutrition has been identified as an important factor in cognitive aging, a diet and nutrition class was already available to older veterans at the Seattle VA; hence, we chose to limit overlap by not covering this topic in our group.
The group was offered on a quarterly basis as six 90-minute psychoeducational classes to allow time for didactics, discussion, and practice without overloading participants with information. Each group consisted of 4 to 9 veterans led by 2 cofacilitators. Group structure allowed for feedback and ideas from group members as well as accountability for engaging in behavior change. Cognitive functioning was not formally evaluated. Attendees were asked but not required to complete questionnaires before the classes began and again at completion. In addition at the completion of each group, feedback was collected from veterans and used to modify group content (Figure).
Two pilot groups were implemented in early and mid-2018 with iterative changes after each group. Then we revised the assessment battery and implemented the current version (v1.0), which was first offered in the fall of 2018 and was used with the final 3 groups. Noteworthy changes included weekly check-ins to assess use of health behavior logs and progress toward individual goals, additional pre-and postgroup measures, and in vivo skills practice relevant to the topic being discussed that day.
Each session began with a check-in, which included a review of daily logs and SMART (specific, measurable, attainable, relevant/realistic, and timebound) goals from the previous week.18 This allowed for praise/reinforcement of health behaviors as well as discussion of potential barriers. Second, an overview of research focusing on the relationship between aging, brain health, and the topic of the day was presented. As an example, in the discussion of social engagement, research was presented about the link between social isolation and cognitive decline; the indirect benefits of social support (eg, social support is linked to improved physical and mental health, which, in turn, is associated with less cognitive decline); and the direct benefits of social support (eg, high levels of emotional support are associated with better cognitive function) (Table 1).6
Next, facilitators reviewed skills and strategies to improve functioning in the topic of discussion. During the social engagement group, for example, facilitators discussed tips to improve social skills (eg, asking open-ended questions) and how to build social support into a daily routine (eg, scheduling weekly phone calls with family and friends). Following this discussion of skills, an activity was practiced, reinforcing learned material. During the social engagement group, veterans were invited to use small talk strategies with fellow group members. Finally, group sessions ended with each participant identifying a SMART goal for the coming week and troubleshooting potential barriers to success. SMART goals were kept broad, so veterans could choose a goal related to the topic discussed at the group that day (eg, scheduling a phone call with a friend twice in the coming week during the social engagement-focused group) or choose any other goal to focus on (eg, a sleep-related goal). Similarly, goals could change week to week, or could remain the same throughout the 6-week classes.
Measures
The questionnaires used for QI/QA analyses included the Satisfaction with Life Scale (SWLS); Geriatric Depression Scale-Short Form (GDS-S); Social Support Survey Instrument (SSSI); Pittsburg Sleep Quality Index (PSQI); Medical Outcomes Survey-Short Form (MOS-36 SF); and a self-efficacy scale (adapted from Huckans and colleagues for traumatic brain injury).19-24 Written feedback was collected at the end of the last group to assess perception of progress, self-perceived behavior change, what was helpful or unhelpful, and how likely the participants were to recommend the group to other veterans (0 to 3, very unlikely to very likely).
To promote consistency with other health and behavior change interventions at the VA, HAP-B used resources from the Whole Health model SMART goals. Research supports the use of self-monitoring techniques like SMART goals for behavior change.25
To facilitate skills practice and self-monitoring between classes, veterans were asked to complete 2 homework assignments. First, at the end of each group, each veteran identified a specific SMART goal to focus on and track in the coming week. Goals were unique to each veteran and allowed to change from week to week. Group discussion around SMART goals involved plans for how to address potential barriers; progress toward goals was discussed at the beginning of the following group. Second, veterans were asked to complete a worksheet used to track progress toward the weekly SMART goal and the specific health behaviors related to the 4 domains targeted by HAP-B. For example, when tracking sleep behaviors, veterans noted bedtime, waketime, number of times they woke up during the night, and length of daytime naps if applicable. Tracking logs were provided at the end of each class for personal purposes only. We asked veterans to rate themselves each week on whether they used the tracking sheet to monitor health behaviors; and how successful they were at accomplishing their previously identified SMART goal. We recorded responses on a 0 to 2 scale (0, not good; 1, fair; 2, good). This rating system was developed and implemented in later groups to promote self-monitoring, accountability, and discussion of potential barriers. However, due to the small sample that completed these ratings and the absence of objective corroborating data, these ratings were not included in the current analyses.
Every participant received a manual in binder format, which provided the didactic information for each group session, skills and strategies discussed in each session, and relevant resources in both the VA and community. For example, social engagement resources included information about volunteer opportunities, VA groups that focus on developing interpersonal skills, and recommendations from past group members on social events (eg, dance lessons at a senior center). We also developed a facilitator version of the manual in which we added comments and guidance on topics for discussion. Materials were developed with the goal of optimizing the ease of dissemination to other sites.
Results
Across the 5 groups, 31 veterans enrolled as participants and completed the initial intake measures, with an average of 6 participants per group (range 4-9). The majority (80%) attended at least 5 of the 6 classes. The mean
At the start of the class, the mean (SD) reports of participants were mild depressive symptoms 5.96 (3.8) on the GDS scale, moderate levels of self-efficacy 3.69 (0.5) on the self-efficacy scale, and moderate levels of satisfaction with life 18.08 (6.8) on the SWLS scale (Table 2). Data from 25 of 31 veterans who completed both pregroup and postgroup surveys were analyzed and paired samples t tests without corrections indicated a reduction in depressive symptoms (P = .01), improved self-efficacy (P = .08), and improved satisfaction with life (P = .03). There were no significant differences in self-reported sleep quality or perceived social support from pregroup to postgroup evaluations. Because the sample size was smaller for the MOS-36, which was not used until group 3, and the subscales are composed of few items each, we conducted exploratory analyses of the 8 MOS-36 subscales and found that well-being, physical functioning, role limitations due to physical and emotional functioning, and energy/fatigue significantly improved over time (Ps < .04).
Twenty-eight veterans provided written feedback following the final session. Qualitative feedback received at the completion of the group focused on participants’ desire for increased number of classes, longer sessions (eg, 2 participants recommended lengthening the group to 2 hours), and integrating mindfulness-based activities into each class. Participants rated themselves somewhat likely to very likely to recommend this group to other veterans (mean, 2.9 [SD, 0.4]).
Discussion
The ability and need to promote brain health with age is an emerging priority as our aging population grows. A growing body of evidence supports the role of health behaviors in healthy brain aging. Education and skills training in a group setting provides a supportive, cost-effective approach for increasing overall health in aging adults. Yet older adults are statistically less likely to engage in these behaviors on a regular basis. The current investigation provides preliminary support for a model of care that uses a comprehensive, experiential psychoeducational approach to facilitate behavior change in older adults. Our aim was to develop and implement an intervention that was feasible and acceptable to our older veterans and to determine any positive outcomes/preliminary effects on overall health and well-being.
Participants indicated that they enjoyed the group, learned new skills (per participant feedback and facilitator observation), and experienced improvements in mood, self-efficacy, and life satisfaction. Given the participants’ positive response to the group and its content, as well as continued referrals by HCPs to this group and low difficulty with ongoing recruitment, this program was deemed both feasible and acceptable in our veteran health care setting. Questions remain about the extent to which participants modified their health behaviors given that we did not collect objective measurements of behaviors (eg, time spent exercising), the duration of behavior change (ie, how long during and after the group were behaviors maintained), and the role of premorbid or concurrent characteristics that may moderate the effect of the intervention on health-related outcomes (eg, sleep quality, perceived social support, overall functioning, concurrent interventions, medications).
Strengths and Limitations
This study had a limited sample size and no control group. However, evidence of significant improvements in depressive symptoms, self-efficacy, and life satisfaction in the development groups without a control group is encouraging. This is particularly noteworthy given that older veterans as a group have higher rates of frailty and mortality than do other similarly aged counterparts.17An additional weakness is the absence of a brief cognitive assessment or other formal assessment as part of the inclusion/exclusion criteria. However, this program development project provides data from a realistic condition (recruited broadly and with few exclusions, offered in similar format as other VA classes), thus adding strength to the interpretation and possibly the generalizability of these findings.
Conclusions
Future directions include disseminating HAP-B materials and procedures across a variety of sites, both VA and non-VA. In line with this goal, we hope to increase sample size and sample diversity while optimizing protocol integrity during the exportation phase. With a greater sample size and power, we aim to examine the role of self-efficacy and other premorbid factors (eg, cognitive functioning at baseline) as mediators for observed changes in pre-/postmeasures and outcomes. We also hope to incorporate objective measures of behavior change, such as fitness trackers, heart rate/pulse monitors, and actigraphy for monitoring sleep. Finally, we are interested in conducting follow-up with past and future participants to detect changes that may occur with learning new skills following the completion of the group (eg, changes in sleep behavior that take time to take effect) and the extent to which participants continue to use the health behavior skills and strategies to maintain or enhance progress in behavioral goals. Finally, although this intervention was initially designed for use with older veterans receiving health care through the VA, we believe the concepts and work products described here can be used with older adults across a wide range of health care settings. Providers interested in trialing HAP-B at their local site are encouraged to contact the authors.
1. Jacobsen LA, Kent M, Lee M, Mather M. America’s aging population. Popul Bull. 2011;66(1):1-20.
2. Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):85-592. doi:10.1093/sleep/33.5.585
3. Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev. 2014;16:12-31. doi:10.1016/j.arr.2014.05.002
4. Middleton LE, Manini TM, Simonsick EM, et al. Activity energy expenditure and incident cognitive impairment in older adults. Arch Intern Med. 2011;171(14):1251-1257. doi:10.1001/archinternmed.2011.277
5. World Health Organization. Interventions on diet and physical activity: what works. https://www.who.int/dietphysicalactivity/whatworks/en/. Published 2009. Accessed June 19, 2020.
6. Seeman TE, Lusignolo TM, Albert M, Berkman L. Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychol. 2001;20(4):243-255. doi:10.1037//0278-6133.20.4.243
7. La Rue A. Healthy brain aging: role of cognitive reserve, cognitive stimulation and cognitive exercises. Clin Geriatr Med. 2010;26(1):99-111. doi:10.1016/j.cger.2009.11.003
8. Salthouse TA, Berish DE, Miles JD. The role of cognitive stimulation on the relations between age and cognitive functioning. Psychol Aging. 2002;17(4):548-557. doi:10.1037//0882-7974.17.4.548
9. Wrosch C, Schulz R, Heckhausen J. Health stresses and depressive symptomatology in the elderly: the importance of health engagement control strategies. Health Psychol. 2002;21(4):340-348. doi:10.1037//0278-6133.21.4.340
10. Pronk NP, Anderson LH, Crain AL, et al. Meeting recommendations for multiple healthy lifestyle factors: prevalence, clustering, and predictors among adolescent, adult, and senior health plan members. Am J Prev Med. 2004;27(suppl 2):25-33. doi:10.1016/j.amepre.2004.04.022
11. Gross AL, Parisi JM, Spira AP, et al. Memory training interventions for older adults: a meta-analysis. Aging Ment Health. 2012;16(6):722-734. doi:10.1080/13607863.2012.667783
12. Miller KJ, Siddarth P, Gaines JM, et al. The memory fitness program: cognitive effects of a healthy aging intervention. Am J Geriat Psychiatry. 2012;20(6):514-523. doi:10.1097/JGP.0b013e318227f821
13. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126-135. doi:10.1212/WNL.0000000000004826
14. Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006;367(9518):1262-1270. doi:10.1016/S0140-6736(06)68542-5
15. US Department of Veteran Affairs, National Center for Veteran Analysis and Statistics.Veteran population. 2020. https://www.va.gov/vetdata/Veteran_Population.asp. Updated May 21, 2020 . Accessed June 17, 2020.
16. Eibner C, Krull H, Brown K, et al. Current and projected characteristics and unique healthcare needs of the patient population served by the Department of Veterans Affairs. RAND Health Q. 2016;5(4):13.
17. Orkaby AR, Nussbaum L, Ho Y, et al. The burden of frailty among U.S. Veterans and its association with mortality, 2002-2012. J Gerontol A Biol Med Sci. 2019;74(8):1257-1264. doi:10.1093/gerona/gly232
18. Doran GT. There’s a S.M.A.R.T. way to write management’s goals and objectives. Manag Rev. 1981;70(11):35-36.
19. Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. J Pers Assess. 1985;49(1):71-75. doi:10.1207/s15327752jpa4901-13
20. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5(1-2):165-173. doi:10.1300/J018v05n01_09
21. Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705-714. doi:10.1016/0277-9536(91)90150-b
22. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193-213. doi:10.1016/0165-1781(89)90047-4
23. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
24. Huckans M, Pavawalla S, Demadura T, et al. A pilot study examining effects of group-based cognitive strategy training treatment on self-reported cognitive problems, psychiatric symptoms, functioning, and compensatory strategy use in OIF/OEF combat veterans with persistent mild cognitive disorder and history of traumatic brain injury. J Rehabil Res Dev. 2010;47(1):43-60. doi:10.1682/jrrd.2009.02.0019
25. Pearson ES. Goal setting as a health behavior change strategy in overweight and obese adults: a systematic literature review examining intervention components. Patient Educ Couns. 2012;87(1):32-42. doi:10.1016/j.pec.2011.07.018
With a rapidly growing older adult population, increased attention has been given to cognitive changes that occur with age, with a focus on optimizing the cognitive health of aging individuals.1 Given the absence of pharmaceutical treatments to prevent cognitive decline, there is an increased need for health care systems to offer alternative or behavioral interventions that can mitigate the effects of cognitive decline in aging.
Notably, many individuals are able to maintain or even improve cognitive functioning throughout their lifespan, with some research implicating health behaviors as an important factor for promoting brain health with age. Specifically, sleep, exercise, eating habits, social engagement, and cognitive stimulation have been linked to improved cognitive functioning.2-8 In addition to the potential benefits for brain health, there is evidence that greater investment in attaining health goals is associated with subjective reports of higher well-being, fewer mental health symptoms, lower physical health stresses, decreased caregiver burden, and increased functional independence linked with longer independent living.9 The latter has a substantial financial impact, such that the positive consequence of increased independence is likely staving off the need for admission to assisted living and adult family homes, which can be costly.
Despite the role of health behaviors in brain aging and overall health and functioning, research indicates that only a small number of older adults (12.8%) follow recommended guidelines for healthy lifestyle factors.10 Education has been identified as one factor associated with the likelihood of engaging in positive health behaviors, prompting the delivery of health-education interventions. Most psychoeducational interventions have traditionally focused on one aspect of behavior change at a time (eg, sleep); however, Gross and colleaguesconducted a meta-analysis of cognitive interventions and in addition to the overall positive benefits (effect size 0.38), they also found suggestive evidence that interventions that combined multiple training strategies were associated with larger training gains (P = .04) after adjusting for multiple comparisons.11 For example, Miller and colleagues found a significant improvement on both subjective and objective measures of memory following a multicomponent approach that combined training in memory skills, stress reduction, nutrition, and physical activity.12
In addition to the potential positive impacts of health behaviors on brain health, findings suggest that targeted emphasis on health behavior change may have the potential to stave off mild cognitiveimpairment (MCI) or dementia even if for a short time. Given the increasing prevalence rates of MCI with age (6.7% in adults aged 60-64 years, reaching 25.2% in adults aged 80-84 years13) and dementia (prevalence of MCI converting to dementia is 18-40%14), as well as the corresponding emotional, financial, and family-oriented consequences (eg, impact on the well-being of family caregivers), the need for behavioral interventions that seek to optimize brain health is becoming increasingly apparent.
More than 9 million veterans are now aged ≥ 65 years.15 In addition to representing nearly half of all veterans and a sizable portion of aging adults in the US, older veterans are at increased risk of frailty, mortality, and high rates of chronic medical/mental health conditions that can lead to accelerated cognitive aging.6-17 Together, these conditions highlight the importance of developing comprehensive psychoeducational and behavioral interventions in this population. To address this need, we developed a novel psychoeducation and behavior change group called the Healthy Aging Project-Brain (HAP-B, pronounced “happy”). The HAP-B intervention was designed to promote healthy brain aging by using empirically supported health behavior change strategies, including education, personalized goal setting, and community support. The primary aim of this project was to develop and implement an intervention that was feasible and acceptable (eg, could be implemented in our setting, was appropriate for a veteran population) and to determine any positive outcomes/preliminary effects on overall health and well-being.
Methods
We recruited veterans aged ≥ 50 years through primary care clinics and self-referrals via flyers in the US Department of Veterans Affairs (VA) Puget Sound Health Care System (VAPSHCS), Seattle Division hospital. We targeted the “worried well” and welcomed veterans with MCI and mental health diagnoses. Notably, if there were significant mental health and/or substance use concerns, we encouraged veterans to seek focused care and stabilization prior to or concurrent with group participation. Exclusion criteria included presence of suicidality/homicidality, untreated or unstable substance use disorder, or a diagnosis of dementia. Exclusion criteria were assessed by the referring health care providers (HCPs), when appropriate, and through a health record review. Group facilitators used their clinical judgment to monitor participants if they began experiencing more severe cognitive impairment or acute mental health concerns. Although we did not encounter any of these instances, facilitators were prepared to discuss any concerns with the veteran and their referring HCP. Participants sampled were from 1 of 5 groups offered between January 2018 and March 2019. A waiver from the institutional review board was obtained after meeting criteria for quality improvement/quality assurance (QI/QA) for this study.
Procedures
At the initial stages of development, our team conducted a needs assessment to identify health-related areas where HCPs felt veterans would benefit from additional education and support. The needs assessment was conducted across primary care, geriatric extended care, and the Geriatric Research, Education, and Clinical Center (GRECC) at VAPSHCS. Combining the needs assessment results with the available research base, we identified sleep, physical activity, social engagement, and cognitive stimulation as areas for focus. Notably, although nutrition has been identified as an important factor in cognitive aging, a diet and nutrition class was already available to older veterans at the Seattle VA; hence, we chose to limit overlap by not covering this topic in our group.
The group was offered on a quarterly basis as six 90-minute psychoeducational classes to allow time for didactics, discussion, and practice without overloading participants with information. Each group consisted of 4 to 9 veterans led by 2 cofacilitators. Group structure allowed for feedback and ideas from group members as well as accountability for engaging in behavior change. Cognitive functioning was not formally evaluated. Attendees were asked but not required to complete questionnaires before the classes began and again at completion. In addition at the completion of each group, feedback was collected from veterans and used to modify group content (Figure).
Two pilot groups were implemented in early and mid-2018 with iterative changes after each group. Then we revised the assessment battery and implemented the current version (v1.0), which was first offered in the fall of 2018 and was used with the final 3 groups. Noteworthy changes included weekly check-ins to assess use of health behavior logs and progress toward individual goals, additional pre-and postgroup measures, and in vivo skills practice relevant to the topic being discussed that day.
Each session began with a check-in, which included a review of daily logs and SMART (specific, measurable, attainable, relevant/realistic, and timebound) goals from the previous week.18 This allowed for praise/reinforcement of health behaviors as well as discussion of potential barriers. Second, an overview of research focusing on the relationship between aging, brain health, and the topic of the day was presented. As an example, in the discussion of social engagement, research was presented about the link between social isolation and cognitive decline; the indirect benefits of social support (eg, social support is linked to improved physical and mental health, which, in turn, is associated with less cognitive decline); and the direct benefits of social support (eg, high levels of emotional support are associated with better cognitive function) (Table 1).6
Next, facilitators reviewed skills and strategies to improve functioning in the topic of discussion. During the social engagement group, for example, facilitators discussed tips to improve social skills (eg, asking open-ended questions) and how to build social support into a daily routine (eg, scheduling weekly phone calls with family and friends). Following this discussion of skills, an activity was practiced, reinforcing learned material. During the social engagement group, veterans were invited to use small talk strategies with fellow group members. Finally, group sessions ended with each participant identifying a SMART goal for the coming week and troubleshooting potential barriers to success. SMART goals were kept broad, so veterans could choose a goal related to the topic discussed at the group that day (eg, scheduling a phone call with a friend twice in the coming week during the social engagement-focused group) or choose any other goal to focus on (eg, a sleep-related goal). Similarly, goals could change week to week, or could remain the same throughout the 6-week classes.
Measures
The questionnaires used for QI/QA analyses included the Satisfaction with Life Scale (SWLS); Geriatric Depression Scale-Short Form (GDS-S); Social Support Survey Instrument (SSSI); Pittsburg Sleep Quality Index (PSQI); Medical Outcomes Survey-Short Form (MOS-36 SF); and a self-efficacy scale (adapted from Huckans and colleagues for traumatic brain injury).19-24 Written feedback was collected at the end of the last group to assess perception of progress, self-perceived behavior change, what was helpful or unhelpful, and how likely the participants were to recommend the group to other veterans (0 to 3, very unlikely to very likely).
To promote consistency with other health and behavior change interventions at the VA, HAP-B used resources from the Whole Health model SMART goals. Research supports the use of self-monitoring techniques like SMART goals for behavior change.25
To facilitate skills practice and self-monitoring between classes, veterans were asked to complete 2 homework assignments. First, at the end of each group, each veteran identified a specific SMART goal to focus on and track in the coming week. Goals were unique to each veteran and allowed to change from week to week. Group discussion around SMART goals involved plans for how to address potential barriers; progress toward goals was discussed at the beginning of the following group. Second, veterans were asked to complete a worksheet used to track progress toward the weekly SMART goal and the specific health behaviors related to the 4 domains targeted by HAP-B. For example, when tracking sleep behaviors, veterans noted bedtime, waketime, number of times they woke up during the night, and length of daytime naps if applicable. Tracking logs were provided at the end of each class for personal purposes only. We asked veterans to rate themselves each week on whether they used the tracking sheet to monitor health behaviors; and how successful they were at accomplishing their previously identified SMART goal. We recorded responses on a 0 to 2 scale (0, not good; 1, fair; 2, good). This rating system was developed and implemented in later groups to promote self-monitoring, accountability, and discussion of potential barriers. However, due to the small sample that completed these ratings and the absence of objective corroborating data, these ratings were not included in the current analyses.
Every participant received a manual in binder format, which provided the didactic information for each group session, skills and strategies discussed in each session, and relevant resources in both the VA and community. For example, social engagement resources included information about volunteer opportunities, VA groups that focus on developing interpersonal skills, and recommendations from past group members on social events (eg, dance lessons at a senior center). We also developed a facilitator version of the manual in which we added comments and guidance on topics for discussion. Materials were developed with the goal of optimizing the ease of dissemination to other sites.
Results
Across the 5 groups, 31 veterans enrolled as participants and completed the initial intake measures, with an average of 6 participants per group (range 4-9). The majority (80%) attended at least 5 of the 6 classes. The mean
At the start of the class, the mean (SD) reports of participants were mild depressive symptoms 5.96 (3.8) on the GDS scale, moderate levels of self-efficacy 3.69 (0.5) on the self-efficacy scale, and moderate levels of satisfaction with life 18.08 (6.8) on the SWLS scale (Table 2). Data from 25 of 31 veterans who completed both pregroup and postgroup surveys were analyzed and paired samples t tests without corrections indicated a reduction in depressive symptoms (P = .01), improved self-efficacy (P = .08), and improved satisfaction with life (P = .03). There were no significant differences in self-reported sleep quality or perceived social support from pregroup to postgroup evaluations. Because the sample size was smaller for the MOS-36, which was not used until group 3, and the subscales are composed of few items each, we conducted exploratory analyses of the 8 MOS-36 subscales and found that well-being, physical functioning, role limitations due to physical and emotional functioning, and energy/fatigue significantly improved over time (Ps < .04).
Twenty-eight veterans provided written feedback following the final session. Qualitative feedback received at the completion of the group focused on participants’ desire for increased number of classes, longer sessions (eg, 2 participants recommended lengthening the group to 2 hours), and integrating mindfulness-based activities into each class. Participants rated themselves somewhat likely to very likely to recommend this group to other veterans (mean, 2.9 [SD, 0.4]).
Discussion
The ability and need to promote brain health with age is an emerging priority as our aging population grows. A growing body of evidence supports the role of health behaviors in healthy brain aging. Education and skills training in a group setting provides a supportive, cost-effective approach for increasing overall health in aging adults. Yet older adults are statistically less likely to engage in these behaviors on a regular basis. The current investigation provides preliminary support for a model of care that uses a comprehensive, experiential psychoeducational approach to facilitate behavior change in older adults. Our aim was to develop and implement an intervention that was feasible and acceptable to our older veterans and to determine any positive outcomes/preliminary effects on overall health and well-being.
Participants indicated that they enjoyed the group, learned new skills (per participant feedback and facilitator observation), and experienced improvements in mood, self-efficacy, and life satisfaction. Given the participants’ positive response to the group and its content, as well as continued referrals by HCPs to this group and low difficulty with ongoing recruitment, this program was deemed both feasible and acceptable in our veteran health care setting. Questions remain about the extent to which participants modified their health behaviors given that we did not collect objective measurements of behaviors (eg, time spent exercising), the duration of behavior change (ie, how long during and after the group were behaviors maintained), and the role of premorbid or concurrent characteristics that may moderate the effect of the intervention on health-related outcomes (eg, sleep quality, perceived social support, overall functioning, concurrent interventions, medications).
Strengths and Limitations
This study had a limited sample size and no control group. However, evidence of significant improvements in depressive symptoms, self-efficacy, and life satisfaction in the development groups without a control group is encouraging. This is particularly noteworthy given that older veterans as a group have higher rates of frailty and mortality than do other similarly aged counterparts.17An additional weakness is the absence of a brief cognitive assessment or other formal assessment as part of the inclusion/exclusion criteria. However, this program development project provides data from a realistic condition (recruited broadly and with few exclusions, offered in similar format as other VA classes), thus adding strength to the interpretation and possibly the generalizability of these findings.
Conclusions
Future directions include disseminating HAP-B materials and procedures across a variety of sites, both VA and non-VA. In line with this goal, we hope to increase sample size and sample diversity while optimizing protocol integrity during the exportation phase. With a greater sample size and power, we aim to examine the role of self-efficacy and other premorbid factors (eg, cognitive functioning at baseline) as mediators for observed changes in pre-/postmeasures and outcomes. We also hope to incorporate objective measures of behavior change, such as fitness trackers, heart rate/pulse monitors, and actigraphy for monitoring sleep. Finally, we are interested in conducting follow-up with past and future participants to detect changes that may occur with learning new skills following the completion of the group (eg, changes in sleep behavior that take time to take effect) and the extent to which participants continue to use the health behavior skills and strategies to maintain or enhance progress in behavioral goals. Finally, although this intervention was initially designed for use with older veterans receiving health care through the VA, we believe the concepts and work products described here can be used with older adults across a wide range of health care settings. Providers interested in trialing HAP-B at their local site are encouraged to contact the authors.
With a rapidly growing older adult population, increased attention has been given to cognitive changes that occur with age, with a focus on optimizing the cognitive health of aging individuals.1 Given the absence of pharmaceutical treatments to prevent cognitive decline, there is an increased need for health care systems to offer alternative or behavioral interventions that can mitigate the effects of cognitive decline in aging.
Notably, many individuals are able to maintain or even improve cognitive functioning throughout their lifespan, with some research implicating health behaviors as an important factor for promoting brain health with age. Specifically, sleep, exercise, eating habits, social engagement, and cognitive stimulation have been linked to improved cognitive functioning.2-8 In addition to the potential benefits for brain health, there is evidence that greater investment in attaining health goals is associated with subjective reports of higher well-being, fewer mental health symptoms, lower physical health stresses, decreased caregiver burden, and increased functional independence linked with longer independent living.9 The latter has a substantial financial impact, such that the positive consequence of increased independence is likely staving off the need for admission to assisted living and adult family homes, which can be costly.
Despite the role of health behaviors in brain aging and overall health and functioning, research indicates that only a small number of older adults (12.8%) follow recommended guidelines for healthy lifestyle factors.10 Education has been identified as one factor associated with the likelihood of engaging in positive health behaviors, prompting the delivery of health-education interventions. Most psychoeducational interventions have traditionally focused on one aspect of behavior change at a time (eg, sleep); however, Gross and colleaguesconducted a meta-analysis of cognitive interventions and in addition to the overall positive benefits (effect size 0.38), they also found suggestive evidence that interventions that combined multiple training strategies were associated with larger training gains (P = .04) after adjusting for multiple comparisons.11 For example, Miller and colleagues found a significant improvement on both subjective and objective measures of memory following a multicomponent approach that combined training in memory skills, stress reduction, nutrition, and physical activity.12
In addition to the potential positive impacts of health behaviors on brain health, findings suggest that targeted emphasis on health behavior change may have the potential to stave off mild cognitiveimpairment (MCI) or dementia even if for a short time. Given the increasing prevalence rates of MCI with age (6.7% in adults aged 60-64 years, reaching 25.2% in adults aged 80-84 years13) and dementia (prevalence of MCI converting to dementia is 18-40%14), as well as the corresponding emotional, financial, and family-oriented consequences (eg, impact on the well-being of family caregivers), the need for behavioral interventions that seek to optimize brain health is becoming increasingly apparent.
More than 9 million veterans are now aged ≥ 65 years.15 In addition to representing nearly half of all veterans and a sizable portion of aging adults in the US, older veterans are at increased risk of frailty, mortality, and high rates of chronic medical/mental health conditions that can lead to accelerated cognitive aging.6-17 Together, these conditions highlight the importance of developing comprehensive psychoeducational and behavioral interventions in this population. To address this need, we developed a novel psychoeducation and behavior change group called the Healthy Aging Project-Brain (HAP-B, pronounced “happy”). The HAP-B intervention was designed to promote healthy brain aging by using empirically supported health behavior change strategies, including education, personalized goal setting, and community support. The primary aim of this project was to develop and implement an intervention that was feasible and acceptable (eg, could be implemented in our setting, was appropriate for a veteran population) and to determine any positive outcomes/preliminary effects on overall health and well-being.
Methods
We recruited veterans aged ≥ 50 years through primary care clinics and self-referrals via flyers in the US Department of Veterans Affairs (VA) Puget Sound Health Care System (VAPSHCS), Seattle Division hospital. We targeted the “worried well” and welcomed veterans with MCI and mental health diagnoses. Notably, if there were significant mental health and/or substance use concerns, we encouraged veterans to seek focused care and stabilization prior to or concurrent with group participation. Exclusion criteria included presence of suicidality/homicidality, untreated or unstable substance use disorder, or a diagnosis of dementia. Exclusion criteria were assessed by the referring health care providers (HCPs), when appropriate, and through a health record review. Group facilitators used their clinical judgment to monitor participants if they began experiencing more severe cognitive impairment or acute mental health concerns. Although we did not encounter any of these instances, facilitators were prepared to discuss any concerns with the veteran and their referring HCP. Participants sampled were from 1 of 5 groups offered between January 2018 and March 2019. A waiver from the institutional review board was obtained after meeting criteria for quality improvement/quality assurance (QI/QA) for this study.
Procedures
At the initial stages of development, our team conducted a needs assessment to identify health-related areas where HCPs felt veterans would benefit from additional education and support. The needs assessment was conducted across primary care, geriatric extended care, and the Geriatric Research, Education, and Clinical Center (GRECC) at VAPSHCS. Combining the needs assessment results with the available research base, we identified sleep, physical activity, social engagement, and cognitive stimulation as areas for focus. Notably, although nutrition has been identified as an important factor in cognitive aging, a diet and nutrition class was already available to older veterans at the Seattle VA; hence, we chose to limit overlap by not covering this topic in our group.
The group was offered on a quarterly basis as six 90-minute psychoeducational classes to allow time for didactics, discussion, and practice without overloading participants with information. Each group consisted of 4 to 9 veterans led by 2 cofacilitators. Group structure allowed for feedback and ideas from group members as well as accountability for engaging in behavior change. Cognitive functioning was not formally evaluated. Attendees were asked but not required to complete questionnaires before the classes began and again at completion. In addition at the completion of each group, feedback was collected from veterans and used to modify group content (Figure).
Two pilot groups were implemented in early and mid-2018 with iterative changes after each group. Then we revised the assessment battery and implemented the current version (v1.0), which was first offered in the fall of 2018 and was used with the final 3 groups. Noteworthy changes included weekly check-ins to assess use of health behavior logs and progress toward individual goals, additional pre-and postgroup measures, and in vivo skills practice relevant to the topic being discussed that day.
Each session began with a check-in, which included a review of daily logs and SMART (specific, measurable, attainable, relevant/realistic, and timebound) goals from the previous week.18 This allowed for praise/reinforcement of health behaviors as well as discussion of potential barriers. Second, an overview of research focusing on the relationship between aging, brain health, and the topic of the day was presented. As an example, in the discussion of social engagement, research was presented about the link between social isolation and cognitive decline; the indirect benefits of social support (eg, social support is linked to improved physical and mental health, which, in turn, is associated with less cognitive decline); and the direct benefits of social support (eg, high levels of emotional support are associated with better cognitive function) (Table 1).6
Next, facilitators reviewed skills and strategies to improve functioning in the topic of discussion. During the social engagement group, for example, facilitators discussed tips to improve social skills (eg, asking open-ended questions) and how to build social support into a daily routine (eg, scheduling weekly phone calls with family and friends). Following this discussion of skills, an activity was practiced, reinforcing learned material. During the social engagement group, veterans were invited to use small talk strategies with fellow group members. Finally, group sessions ended with each participant identifying a SMART goal for the coming week and troubleshooting potential barriers to success. SMART goals were kept broad, so veterans could choose a goal related to the topic discussed at the group that day (eg, scheduling a phone call with a friend twice in the coming week during the social engagement-focused group) or choose any other goal to focus on (eg, a sleep-related goal). Similarly, goals could change week to week, or could remain the same throughout the 6-week classes.
Measures
The questionnaires used for QI/QA analyses included the Satisfaction with Life Scale (SWLS); Geriatric Depression Scale-Short Form (GDS-S); Social Support Survey Instrument (SSSI); Pittsburg Sleep Quality Index (PSQI); Medical Outcomes Survey-Short Form (MOS-36 SF); and a self-efficacy scale (adapted from Huckans and colleagues for traumatic brain injury).19-24 Written feedback was collected at the end of the last group to assess perception of progress, self-perceived behavior change, what was helpful or unhelpful, and how likely the participants were to recommend the group to other veterans (0 to 3, very unlikely to very likely).
To promote consistency with other health and behavior change interventions at the VA, HAP-B used resources from the Whole Health model SMART goals. Research supports the use of self-monitoring techniques like SMART goals for behavior change.25
To facilitate skills practice and self-monitoring between classes, veterans were asked to complete 2 homework assignments. First, at the end of each group, each veteran identified a specific SMART goal to focus on and track in the coming week. Goals were unique to each veteran and allowed to change from week to week. Group discussion around SMART goals involved plans for how to address potential barriers; progress toward goals was discussed at the beginning of the following group. Second, veterans were asked to complete a worksheet used to track progress toward the weekly SMART goal and the specific health behaviors related to the 4 domains targeted by HAP-B. For example, when tracking sleep behaviors, veterans noted bedtime, waketime, number of times they woke up during the night, and length of daytime naps if applicable. Tracking logs were provided at the end of each class for personal purposes only. We asked veterans to rate themselves each week on whether they used the tracking sheet to monitor health behaviors; and how successful they were at accomplishing their previously identified SMART goal. We recorded responses on a 0 to 2 scale (0, not good; 1, fair; 2, good). This rating system was developed and implemented in later groups to promote self-monitoring, accountability, and discussion of potential barriers. However, due to the small sample that completed these ratings and the absence of objective corroborating data, these ratings were not included in the current analyses.
Every participant received a manual in binder format, which provided the didactic information for each group session, skills and strategies discussed in each session, and relevant resources in both the VA and community. For example, social engagement resources included information about volunteer opportunities, VA groups that focus on developing interpersonal skills, and recommendations from past group members on social events (eg, dance lessons at a senior center). We also developed a facilitator version of the manual in which we added comments and guidance on topics for discussion. Materials were developed with the goal of optimizing the ease of dissemination to other sites.
Results
Across the 5 groups, 31 veterans enrolled as participants and completed the initial intake measures, with an average of 6 participants per group (range 4-9). The majority (80%) attended at least 5 of the 6 classes. The mean
At the start of the class, the mean (SD) reports of participants were mild depressive symptoms 5.96 (3.8) on the GDS scale, moderate levels of self-efficacy 3.69 (0.5) on the self-efficacy scale, and moderate levels of satisfaction with life 18.08 (6.8) on the SWLS scale (Table 2). Data from 25 of 31 veterans who completed both pregroup and postgroup surveys were analyzed and paired samples t tests without corrections indicated a reduction in depressive symptoms (P = .01), improved self-efficacy (P = .08), and improved satisfaction with life (P = .03). There were no significant differences in self-reported sleep quality or perceived social support from pregroup to postgroup evaluations. Because the sample size was smaller for the MOS-36, which was not used until group 3, and the subscales are composed of few items each, we conducted exploratory analyses of the 8 MOS-36 subscales and found that well-being, physical functioning, role limitations due to physical and emotional functioning, and energy/fatigue significantly improved over time (Ps < .04).
Twenty-eight veterans provided written feedback following the final session. Qualitative feedback received at the completion of the group focused on participants’ desire for increased number of classes, longer sessions (eg, 2 participants recommended lengthening the group to 2 hours), and integrating mindfulness-based activities into each class. Participants rated themselves somewhat likely to very likely to recommend this group to other veterans (mean, 2.9 [SD, 0.4]).
Discussion
The ability and need to promote brain health with age is an emerging priority as our aging population grows. A growing body of evidence supports the role of health behaviors in healthy brain aging. Education and skills training in a group setting provides a supportive, cost-effective approach for increasing overall health in aging adults. Yet older adults are statistically less likely to engage in these behaviors on a regular basis. The current investigation provides preliminary support for a model of care that uses a comprehensive, experiential psychoeducational approach to facilitate behavior change in older adults. Our aim was to develop and implement an intervention that was feasible and acceptable to our older veterans and to determine any positive outcomes/preliminary effects on overall health and well-being.
Participants indicated that they enjoyed the group, learned new skills (per participant feedback and facilitator observation), and experienced improvements in mood, self-efficacy, and life satisfaction. Given the participants’ positive response to the group and its content, as well as continued referrals by HCPs to this group and low difficulty with ongoing recruitment, this program was deemed both feasible and acceptable in our veteran health care setting. Questions remain about the extent to which participants modified their health behaviors given that we did not collect objective measurements of behaviors (eg, time spent exercising), the duration of behavior change (ie, how long during and after the group were behaviors maintained), and the role of premorbid or concurrent characteristics that may moderate the effect of the intervention on health-related outcomes (eg, sleep quality, perceived social support, overall functioning, concurrent interventions, medications).
Strengths and Limitations
This study had a limited sample size and no control group. However, evidence of significant improvements in depressive symptoms, self-efficacy, and life satisfaction in the development groups without a control group is encouraging. This is particularly noteworthy given that older veterans as a group have higher rates of frailty and mortality than do other similarly aged counterparts.17An additional weakness is the absence of a brief cognitive assessment or other formal assessment as part of the inclusion/exclusion criteria. However, this program development project provides data from a realistic condition (recruited broadly and with few exclusions, offered in similar format as other VA classes), thus adding strength to the interpretation and possibly the generalizability of these findings.
Conclusions
Future directions include disseminating HAP-B materials and procedures across a variety of sites, both VA and non-VA. In line with this goal, we hope to increase sample size and sample diversity while optimizing protocol integrity during the exportation phase. With a greater sample size and power, we aim to examine the role of self-efficacy and other premorbid factors (eg, cognitive functioning at baseline) as mediators for observed changes in pre-/postmeasures and outcomes. We also hope to incorporate objective measures of behavior change, such as fitness trackers, heart rate/pulse monitors, and actigraphy for monitoring sleep. Finally, we are interested in conducting follow-up with past and future participants to detect changes that may occur with learning new skills following the completion of the group (eg, changes in sleep behavior that take time to take effect) and the extent to which participants continue to use the health behavior skills and strategies to maintain or enhance progress in behavioral goals. Finally, although this intervention was initially designed for use with older veterans receiving health care through the VA, we believe the concepts and work products described here can be used with older adults across a wide range of health care settings. Providers interested in trialing HAP-B at their local site are encouraged to contact the authors.
1. Jacobsen LA, Kent M, Lee M, Mather M. America’s aging population. Popul Bull. 2011;66(1):1-20.
2. Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):85-592. doi:10.1093/sleep/33.5.585
3. Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev. 2014;16:12-31. doi:10.1016/j.arr.2014.05.002
4. Middleton LE, Manini TM, Simonsick EM, et al. Activity energy expenditure and incident cognitive impairment in older adults. Arch Intern Med. 2011;171(14):1251-1257. doi:10.1001/archinternmed.2011.277
5. World Health Organization. Interventions on diet and physical activity: what works. https://www.who.int/dietphysicalactivity/whatworks/en/. Published 2009. Accessed June 19, 2020.
6. Seeman TE, Lusignolo TM, Albert M, Berkman L. Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychol. 2001;20(4):243-255. doi:10.1037//0278-6133.20.4.243
7. La Rue A. Healthy brain aging: role of cognitive reserve, cognitive stimulation and cognitive exercises. Clin Geriatr Med. 2010;26(1):99-111. doi:10.1016/j.cger.2009.11.003
8. Salthouse TA, Berish DE, Miles JD. The role of cognitive stimulation on the relations between age and cognitive functioning. Psychol Aging. 2002;17(4):548-557. doi:10.1037//0882-7974.17.4.548
9. Wrosch C, Schulz R, Heckhausen J. Health stresses and depressive symptomatology in the elderly: the importance of health engagement control strategies. Health Psychol. 2002;21(4):340-348. doi:10.1037//0278-6133.21.4.340
10. Pronk NP, Anderson LH, Crain AL, et al. Meeting recommendations for multiple healthy lifestyle factors: prevalence, clustering, and predictors among adolescent, adult, and senior health plan members. Am J Prev Med. 2004;27(suppl 2):25-33. doi:10.1016/j.amepre.2004.04.022
11. Gross AL, Parisi JM, Spira AP, et al. Memory training interventions for older adults: a meta-analysis. Aging Ment Health. 2012;16(6):722-734. doi:10.1080/13607863.2012.667783
12. Miller KJ, Siddarth P, Gaines JM, et al. The memory fitness program: cognitive effects of a healthy aging intervention. Am J Geriat Psychiatry. 2012;20(6):514-523. doi:10.1097/JGP.0b013e318227f821
13. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126-135. doi:10.1212/WNL.0000000000004826
14. Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006;367(9518):1262-1270. doi:10.1016/S0140-6736(06)68542-5
15. US Department of Veteran Affairs, National Center for Veteran Analysis and Statistics.Veteran population. 2020. https://www.va.gov/vetdata/Veteran_Population.asp. Updated May 21, 2020 . Accessed June 17, 2020.
16. Eibner C, Krull H, Brown K, et al. Current and projected characteristics and unique healthcare needs of the patient population served by the Department of Veterans Affairs. RAND Health Q. 2016;5(4):13.
17. Orkaby AR, Nussbaum L, Ho Y, et al. The burden of frailty among U.S. Veterans and its association with mortality, 2002-2012. J Gerontol A Biol Med Sci. 2019;74(8):1257-1264. doi:10.1093/gerona/gly232
18. Doran GT. There’s a S.M.A.R.T. way to write management’s goals and objectives. Manag Rev. 1981;70(11):35-36.
19. Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. J Pers Assess. 1985;49(1):71-75. doi:10.1207/s15327752jpa4901-13
20. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5(1-2):165-173. doi:10.1300/J018v05n01_09
21. Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705-714. doi:10.1016/0277-9536(91)90150-b
22. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193-213. doi:10.1016/0165-1781(89)90047-4
23. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
24. Huckans M, Pavawalla S, Demadura T, et al. A pilot study examining effects of group-based cognitive strategy training treatment on self-reported cognitive problems, psychiatric symptoms, functioning, and compensatory strategy use in OIF/OEF combat veterans with persistent mild cognitive disorder and history of traumatic brain injury. J Rehabil Res Dev. 2010;47(1):43-60. doi:10.1682/jrrd.2009.02.0019
25. Pearson ES. Goal setting as a health behavior change strategy in overweight and obese adults: a systematic literature review examining intervention components. Patient Educ Couns. 2012;87(1):32-42. doi:10.1016/j.pec.2011.07.018
1. Jacobsen LA, Kent M, Lee M, Mather M. America’s aging population. Popul Bull. 2011;66(1):1-20.
2. Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):85-592. doi:10.1093/sleep/33.5.585
3. Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev. 2014;16:12-31. doi:10.1016/j.arr.2014.05.002
4. Middleton LE, Manini TM, Simonsick EM, et al. Activity energy expenditure and incident cognitive impairment in older adults. Arch Intern Med. 2011;171(14):1251-1257. doi:10.1001/archinternmed.2011.277
5. World Health Organization. Interventions on diet and physical activity: what works. https://www.who.int/dietphysicalactivity/whatworks/en/. Published 2009. Accessed June 19, 2020.
6. Seeman TE, Lusignolo TM, Albert M, Berkman L. Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychol. 2001;20(4):243-255. doi:10.1037//0278-6133.20.4.243
7. La Rue A. Healthy brain aging: role of cognitive reserve, cognitive stimulation and cognitive exercises. Clin Geriatr Med. 2010;26(1):99-111. doi:10.1016/j.cger.2009.11.003
8. Salthouse TA, Berish DE, Miles JD. The role of cognitive stimulation on the relations between age and cognitive functioning. Psychol Aging. 2002;17(4):548-557. doi:10.1037//0882-7974.17.4.548
9. Wrosch C, Schulz R, Heckhausen J. Health stresses and depressive symptomatology in the elderly: the importance of health engagement control strategies. Health Psychol. 2002;21(4):340-348. doi:10.1037//0278-6133.21.4.340
10. Pronk NP, Anderson LH, Crain AL, et al. Meeting recommendations for multiple healthy lifestyle factors: prevalence, clustering, and predictors among adolescent, adult, and senior health plan members. Am J Prev Med. 2004;27(suppl 2):25-33. doi:10.1016/j.amepre.2004.04.022
11. Gross AL, Parisi JM, Spira AP, et al. Memory training interventions for older adults: a meta-analysis. Aging Ment Health. 2012;16(6):722-734. doi:10.1080/13607863.2012.667783
12. Miller KJ, Siddarth P, Gaines JM, et al. The memory fitness program: cognitive effects of a healthy aging intervention. Am J Geriat Psychiatry. 2012;20(6):514-523. doi:10.1097/JGP.0b013e318227f821
13. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126-135. doi:10.1212/WNL.0000000000004826
14. Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006;367(9518):1262-1270. doi:10.1016/S0140-6736(06)68542-5
15. US Department of Veteran Affairs, National Center for Veteran Analysis and Statistics.Veteran population. 2020. https://www.va.gov/vetdata/Veteran_Population.asp. Updated May 21, 2020 . Accessed June 17, 2020.
16. Eibner C, Krull H, Brown K, et al. Current and projected characteristics and unique healthcare needs of the patient population served by the Department of Veterans Affairs. RAND Health Q. 2016;5(4):13.
17. Orkaby AR, Nussbaum L, Ho Y, et al. The burden of frailty among U.S. Veterans and its association with mortality, 2002-2012. J Gerontol A Biol Med Sci. 2019;74(8):1257-1264. doi:10.1093/gerona/gly232
18. Doran GT. There’s a S.M.A.R.T. way to write management’s goals and objectives. Manag Rev. 1981;70(11):35-36.
19. Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. J Pers Assess. 1985;49(1):71-75. doi:10.1207/s15327752jpa4901-13
20. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5(1-2):165-173. doi:10.1300/J018v05n01_09
21. Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705-714. doi:10.1016/0277-9536(91)90150-b
22. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193-213. doi:10.1016/0165-1781(89)90047-4
23. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
24. Huckans M, Pavawalla S, Demadura T, et al. A pilot study examining effects of group-based cognitive strategy training treatment on self-reported cognitive problems, psychiatric symptoms, functioning, and compensatory strategy use in OIF/OEF combat veterans with persistent mild cognitive disorder and history of traumatic brain injury. J Rehabil Res Dev. 2010;47(1):43-60. doi:10.1682/jrrd.2009.02.0019
25. Pearson ES. Goal setting as a health behavior change strategy in overweight and obese adults: a systematic literature review examining intervention components. Patient Educ Couns. 2012;87(1):32-42. doi:10.1016/j.pec.2011.07.018
Trauma-Informed Telehealth in the COVID-19 Era and Beyond
COVID-19 has created stressors that are unprecedented in our modern era, prompting health care systems to adapt rapidly. Demand for telehealth has skyrocketed, and clinicians, many of whom had planned to adopt virtual practices in the future, have been pressured to do so immediately.1 In March 2020, the Centers for Medicare and Medicaid Services (CMS) expanded telehealth services, removing many barriers to virtual care.2 Similar remedy was not necessary for the Veterans Health Administration (VHA) which reported more than 2.6 million episodes of telehealth care in 2019.3 By the time the pandemic was underway in the US, use of telehealth was widespread across the agency. In late March 2020, VHA released a COVID-19 Response Plan, in which telehealth played a critical role in safe, uninterrupted delivery of services.4 While telehealth has been widely used in VHA, the call for replacement of most in-person outpatient visits with telehealth visits was a fundamental paradigm shift for many patients and clinicians.4
The Coronavirus Aid, Relief, and Economic Security (CARES) Act (HR 748) gave the US Department of Veterans Affairs (VA) funding to expand coronavirus-related telehealth services, including the purchase of mobile devices and broadband expansion. CARES authorized the agency to expand telemental health services, enter into short-term agreements with telecommunications companies to provide temporary broadband services to veterans, temporarily waived an in-person home visit requirement (accepting video and phone calls as an alternative), and provided means to make telehealth available for homeless veterans and case managers through the HUD-VASH (US Department of Housing and Urban Development-VA Supportive Housing) program.
VHA is a national telehealth exemplar, initiating telehealth by use of closed-circuit televisions as early as 1968, and continuing to expand through 2017 with the implementation of the Veterans Video Connect (VVC) platform.5 VVC has enabled veterans to participate in virtual visits from distant locations, including their homes. VVC was used successfully during hurricanes Sandy, Harvey, Irma, and Maria and is being widely deployed in the current crisis.6-8
While telehealth can take many forms, the current discussion will focus on live (synchronous) videoconferencing: a 2-way audiovisual link between a patient and clinician, such as VVC, which enables patients to maintain a safe and social distance from others while connecting with the health care team and receiving urgent as well as ongoing medical care for both new and established conditions.9 VHA has developed multiple training resources for use of VVC across many settings, including primary care, mental health, and specialties. In this review, we will make the novel case for applying a trauma-informed lens to telehealth care across VHA and beyond to other health care systems.
Trauma-Informed Care
Although our current focus is rightly on mitigating the health effects of a pandemic, we must recognize that stressful phenomena like COVID-19 occur against a backdrop of widespread physical, sexual, psychological, and racial trauma in our communities. The Substance Abuse and Mental Health Services Administration (SAMHSA) describes trauma as resulting from “an event, series of events, or set of circumstances that is experienced by an individual as physically or emotionally harmful or life threatening and that has lasting adverse effects on the individual’s functioning and mental, physical, social, emotional, or spiritual well-being.”10 Trauma exposure is both ubiquitous worldwide and inequitably distributed, with vulnerable populations disproportionately impacted.11,12
Veterans as a population are often highly trauma exposed, and while VHA routinely screens for experiences of trauma, such as military sexual trauma (MST) and intimate partner violence (IPV), and potential mental health sequelae of trauma, including posttraumatic stress disorder (PTSD) and suicidality, veterans may experience other forms of trauma or be unwilling or unable to talk about past exposures.13 One common example is that of adverse childhood experiences (ACEs), which include household dysfunction, neglect, and physical and sexual abuse before the age of 18 years.14 ACEs have been associated with a wide range of risk behaviors and poor health outcomes in adulthood.14 In population-based data, both male and female veterans have reported higher ACE scores.15 In addition, ACE scores are higher overall for those serving in the all-volunteer era (after July 1, 1973).16 Because trauma may be unseen, unmeasured, and unnamed, it is important to deliver all medical care with sensitivity to its potential presence.
It is important to distinguish the concept of trauma-informed care (TIC) from trauma-focused services. Trauma-focused or trauma-specific treatment refers to evidence-based and best practice treatment models that have been proven to facilitate recovery from problems resulting from the experience of trauma, such as PTSD.17 These treatments directly address the emotional, behavioral, and physiologic impact of trauma on an individual’s life and facilitate improvement in related symptoms and functioning: They are designed to treat the consequences of trauma. VHA offers a wide range of trauma-specific treatments, and considerable experience in delivering evidence-based trauma-focused treatment through telehealth exists.18,19 Given the range of possible responses to the experience of trauma, not all veterans with trauma histories need to, chose to, or feel ready to access trauma-specific treatments.20
In contrast, TIC is a global, universal precautions approach to providing quality care that can be applied to all aspects of health care and to all patients.21 TIC is a strengths-based service delivery framework that is grounded in an understanding of, and responsiveness to, the disempowering impact of experiencing trauma. It seeks to maximize physical, psychological, and emotional safety in all health care encounters, not just those that are specifically trauma-focused, and creates opportunities to rebuild a sense of control and empowerment while fostering healing through safe and collaborative patient-clinician relationships.22 TIC is not accomplished through any single technique or checklist but through continuous appraisal of approaches to care delivery. SAMHSA has elucidated 6 fundamental principles of TIC: safety; trustworthiness and transparency; peer support; collaboration and mutuality; empowerment; voice and choice; and sensitivity to cultural, historical, and gender issues.10
TIC is based on the understanding that often traditional service delivery models of care may trigger, silence, or disempower survivors of trauma, exacerbating physical and mental health symptoms and potentially increasing disengagement from care and poorer outcomes.23 Currier and colleagues aptly noted, “TIC assumes that trustworthiness is not something that an organization creates in a veteran client, but something that he or she will freely grant to an organization.”24 Given the global prevalence of trauma, its well-established and deleterious impact on lifelong health, and the potential for health care itself to be traumatizing, TIC is a fundamental construct to apply universally with any patient at any time, especially in the context of a large-scale community trauma, such as a pandemic.12
Trauma-Informed COVID-19 Care
Catastrophic events, such as natural disasters and pandemics, may serve as both newly traumatic and as potential triggers for survivors who have endured prior trauma.25,26 Increases in depression, PTSD, and substance use disorder (SUD) are common sequalae, occurring during the event, the immediate aftermath, and beyond.25,27 In 2003, quarantine contained the spread of Severe acute respiratory syndrome (SARS) but resulted in a high prevalence of psychological distress, including PTSD and depression.27 Many veterans may have deployed in support of humanitarian assistance/disaster relief missions, which typically do not involve armed combat but may expose service members to warlike situations, including social insecurity and suffering populations.28 COVID-19 may be reminiscent of some of these deployments as well.
The impact of the current COVID-19 pandemic on patients is pervasive. Those with preexisting financial insecurity now face additional economic hardship and health challenges, which are amplified by loneliness and loss of social support networks.26 Widespread unemployment and closures of many businesses add to stress and may exacerbate preexisting mental and physical health concerns for many; some veterans also may be at increased risk.29 While previous postdisaster research suggests that psychopathology in the general population will significantly remit over time, high-risk groups remain vulnerable to PTSD and bear the brunt of social and economic consequences associated with the crisis.25 Veterans with preexisting trauma histories and mental health conditions are at increased risk for being retraumatized by the current pandemic and impacted by isolation and unplanned job or wage loss from it.29 Compounding this, social distancing serves to protect communities but may amplify isolation and danger in abusive relationships or exacerbate underlying mental illness.26,30
Thus, as we expand our use of telehealth, replacing our face-to-face visits with virtual encounters, it is critical for clinicians to be mindful that the pandemic and public health responses to it may result in trauma and retraumatization for veterans and other vulnerable patients, which in turn can impact both access and response to care. The application of trauma-informed principles to our virtual encounters has the potential to mitigate some of these health impacts, increase engagement in care, and provide opportunities for protective, healing connections.
In the setting of the continued fear and uncertainty of the COVID-19 pandemic, we believe that application of a trauma-informed lens to telehealth efforts is timely. While virtual visits may seem to lack the warmth and immediacy of traditional medical encounters, accumulated experience suggests otherwise.19 Telehealth is fundamentally more patient-focused than traditional encounters, overcomes service delivery barriers, offers a greater range of options for treatment engagement, and can enhance clinician-patient partnerships.6,31,32 Although the rapid transition to telehealth may be challenging for those new to it, experienced clinicians and patients express high degrees of satisfaction with virtual care because direct communication is unhampered by in-office challenges and travel logistics.33
While it may feel daunting to integrate principles of TIC into telehealth during a crisis-driven scale-up, a growing practice and body of research can inform these efforts. To help better understand how trauma-exposed patients respond to telehealth, we reviewed findings from trauma-focused telemental health (TMH) treatment. This research demonstrates that telehealth promotes safety and collaboration—fundamental principles of TIC—that can, in turn, be applied to telehealth visits in primary care and other medical and surgical specialties. When compared with traditional in-person treatment, studies of both individual and group formats of TMH found no significant differences in satisfaction, acceptability, or outcomes (such as reduction in PTSD symptom severity scores34), and TMH did not impede development of rapport.19,35
Although counterintuitive, the virtual space created by the combined physical and psychological distance of videoconferencing has been shown to promote safety and transparency. In TMH, patients have reported greater honesty due to the protection afforded by this virtual space.31 Engaging in telehealth visits from the comfort of one’s home can feel emotionally safer than having to travel to a medical office, resulting in feeling more at ease during encounters.31 In one TMH study, veterans with PTSD described high comfort levels and ability to let their guard down during virtual treatment.19 Similarly, in palliative telehealth care, patients reported that clinicians successfully nurtured an experience of intimacy, expressed empathy verbally and nonverbally, and responded to the patient’s unique situation and emotions.33
Trauma-Informed Telehealth
We have discussed how telehealth’s greater flexibility may create an ideal environment in which to implement principles of TIC. It may allow increased collaboration and closeness between patients and clinicians, empowering patients to codesign their care.31,33 The Table reviews 6 core SAMHSA principles of TIC and offers examples of their application to telehealth visits. The following case illustrates the application of trauma-informed telehealth care.
Case Presentation
S is a 45-year-old male veteran of Operation Enduring Freedom (OEF) who served as a combat medic. He has a history of osteoarthritis and PTSD related to combat experiences like caring for traumatic amputees. Before the pandemic began, he was employed as a server at a local restaurant but was laid off as the business transitioned to takeout orders only. The patient worked near a VA primary care clinic and frequently dropped by to see the staff and to pick up prescriptions. He had never agreed to video visits despite receiving encouragement from his medical team. He was reluctant to try telehealth, but he had developed a painful, itchy rash on his lower leg and was concerned about getting care.
For patients like S who may be reluctant to try telehealth, it is important to understand the cause. Potential barriers to telehealth may include lack of Internet access or familiarity with technology, discomfort with being on video, shame about the appearance of one’s home, or a strong cultural preference for face-to-face medical visits. Some may miss the social support benefit of coming into a clinic, particularly in VHA, which is designed specifically for veteran patients. For these reasons it is important to offer the patient a choice and to begin with a supportive phone call that explores and strives to address the patient’s concerns about videoconferencing.
The clinic nurse called S who agreed to try a VVC visit with gentle encouragement. He shared that he was embarrassed about the appearance of his apartment and fearful about pictures being recorded of his body due to “a bad experience in my past.” The patient was reassured that visits are private and will not be recorded. The nurse also reminded him that he can choose the location in which the visit will take place and can turn his camera off at any time. Importantly, the nurse did not ask him to recount additional details of what happened in his past. Next, the nurse verified his location and contact information and explained why obtaining this information was necessary. Next, she asked his consent to proceed with the visit, reminding him that the visit can end at any point if he feels uncomfortable. After finishing this initial discussion, the nurse told him that his primary care physician (PCP) would join the visit and address his concerns with his leg.
S was happy to see his PCP despite his hesitations about video care. The PCP noticed that he seemed anxious and was avoiding talking about the rash. Knowing that he was anxious about this VVC visit, the PCP was careful to look directly at the camera to make eye contact and to be sure her face was well lit and not in shadows. She gave him some time to acclimate to the virtual environment and thanked him for joining the visit. Knowing that he was a combat veteran, she warned him that there have been sudden, loud construction noises outside her window. Although the PCP was pressed for time, she was aware that S may have had a previous difficult experience around images of his body or even combat-related trauma. She gently brought up the rash and asked for permission to examine it, avoiding commands or personalizing language such as “show me your leg” or “take off your pants for me.”36After some hesitation, the patient revealed his leg that appeared to have multiple excoriations and old scars from picking. After the examination, the PCP waited until the patient’s leg was fully covered before beginning a discussion of the care plan. Together they collaboratively reviewed treatments that would soothe the skin. They decided to virtually consult a social worker to obtain emergency economic assistance and to speak with the patient’s care team psychologist to reduce some of the anxiety that may be leading to his leg scratching.
Case Discussion
This case illustrates the ways in which TIC can be applied to telehealth for a veteran with combat-related PTSD who may have experienced additional interpersonal trauma. It was not necessary to know more detail about the veteran’s trauma history to conduct the visit in a trauma-informed manner. Connecting to patients at home while considering these principles may thus foster mutuality, mitigate retraumatization, and cultivate enhanced collaboration with health care teams in this era of social distancing.
While a virtual physical examination creates both limitations and opportunity in telehealth, patients may find the greater degree of choice over their clothing and surroundings to be empowering. Telehealth also can allow for a greater portion of time to be dedicated to quality discussion and collaborative planning, with the clinician hearing and responding to the patient’s needs with reduced distraction. This may include opportunities to discuss mental health concerns openly, normalize emotional reactions, and offer connection to mental health and support services available through telehealth, including for patients who have not previously engaged in such care.
Conclusions
1. Wosik J, Fudim M, Cameron B, et al. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc. 2020;27(6):957-962. doi:10.1093/jamia/ocaa067
2. Centers for Medicare and Medicaid Services. Medicare and Medicaid programs; policy and regulatory revisions in response to the COVID-19 public health emergency. CMS-1744-IFC. https://www.cms.gov/files/document/covid-final-ifc.pdf. Published March 24, 2020. Accessed April 8, 2020.
3. Eddy N. VA sees a surge in veterans’ use of telehealth services. https://www.healthcareitnews.com/news/va-sees-surge-veterans-use-telehealth-services. Published November 25, 2019. Accessed June 17, 2020.
4. Veterans Health Administration, Office of Emergency Management. COVID-19 response plan. Version 1.6. Published March 23, 2020. Accessed June 17, 2020.
5. Caudill RL, Sager Z. Institutionally based videoconferencing. Int Rev Psychiatry. 2015;27(6):496-503. doi:10.3109/09540261.2015.1085369
6. Heyworth L. Sharing Connections [published correction appears in JAMA. 2018 May 8;319(18):1939]. JAMA. 2018;319(13):1323-1324. doi:10.1001/jama.2018.2717
7. Dobalian A. U.S. Department of Veterans Affairs’ (VA’s) response to the 2017 hurricanes. Presented at: American Public Health Association 2019 Annual Meeting and Exposition; November 2-6, 2019; Philadelphia, PA. https://apha.confex.com/apha/2019/meetingapp.cgi/Session/58543. Accessed June 16, 2020.
8. Der-Martirosian C, Griffin AR, Chu K, Dobalian A. Telehealth at the US Department of Veterans Affairs after Hurricane Sandy. J Telemed Telecare. 2019;25(5):310-317. doi:10.1177/1357633X17751005
9. The Office of the National Coordinator for Health Information Technology. Telemedicine and telehealth. https://www.healthit.gov/topic/health-it-initiatives/telemedicine-and-telehealth. Updated September 28, 2017. Accessed June 16, 2020.
10. Substance Abuse and Mental Health Services Administration, Trauma and Justice Strategic Initiative. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. https://ncsacw.samhsa.gov/userfiles/files/SAMHSA_Trauma.pdf. Published July 2014. Accessed June 16, 2020.
11. Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26(5):537-547. doi:10.1002/jts.21848
12. Kimberg L, Wheeler M. Trauma and Trauma-informed Care. In: Gerber MR, ed. Trauma-informed Healthcare Approaches: A Guide for Primary Care. Cham, Switzerland: Springer Nature; 2019:25-56.
13. Gerber MR. Trauma-informed care of veterans. In: Gerber MR, ed. Trauma-informed Healthcare Approaches: A Guide for Primary Care. Cham, Switzerland: Springer Nature; 2019:25-56.
14. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245-258. doi:10.1016/s0749-3797(98)00017-8
15. Katon JG, Lehavot K, Simpson TL, et al. Adverse childhood experiences, Military service, and adult health. Am J Prev Med. 2015;49(4):573-582. doi:10.1016/j.amepre.2015.03.020
16. Blosnich JR, Dichter ME, Cerulli C, Batten SV, Bossarte RM. Disparities in adverse childhood experiences among individuals with a history of military service. JAMA Psychiatry. 2014;71(9):1041-1048. doi:10.1001/jamapsychiatry.2014.724
17. Center for Substance Abuse Treatment. Treatment improvement protocol (TIP). Series, No. 57. In: SAMHSA, ed. Trauma-Informed Care in Behavioral Health Services. SAMHSA: Rockville, MD; 2014:137-155.
18. US Department of Veterans Affairs, Veterans Health Administration, National Center for PTSD. Trauma, PTSD and treatment. https://www.ptsd.va.gov/PTSD/professional/treat/index.asp. Updated July 5, 2019. Accessed June 17, 2020.
19. Turgoose D, Ashwick R, Murphy D. Systematic review of lessons learned from delivering tele-therapy to veterans with post-traumatic stress disorder. J Telemed Telecare. 2018;24(9):575-585. doi:10.1177/1357633X17730443
20. Cook JM, Simiola V, Hamblen JL, Bernardy N, Schnurr PP. The influence of patient readiness on implementation of evidence-based PTSD treatments in Veterans Affairs residential programs. Psychol Trauma. 2017;9(suppl 1):51-58. doi:10.1037/tra0000162
21. Raja S, Hasnain M, Hoersch M, Gove-Yin S, Rajagopalan C. Trauma informed care in medicine: current knowledge and future research directions. Fam Community Health. 2015;38(3):216-226. doi:10.1097/FCH.0000000000000071
22. Hopper EK, Bassuk EL, Olivet J. Shelter from the storm: trauma-informed care in homeless service settings. Open Health Serv Policy J. 2009;2:131-151.
23. Kelly U, Boyd MA, Valente SM, Czekanski E. Trauma-informed care: keeping mental health settings safe for veterans [published correction appears in Issues Ment Health Nurs. 2015 Jun;36(6):482]. Issues Ment Health Nurs. 2014;35(6):413-419. doi:10.3109/01612840.2014.881941
24. Currier JM, Stefurak T, Carroll TD, Shatto EH. Applying trauma-informed care to community-based mental health services for military veterans. Best Pract Ment Health. 2017;13(1):47-64.
25. Neria Y, Nandi A, Galea S. Post-traumatic stress disorder following disasters: a systematic review. Psychol Med. 2008;38(4):467-480. doi:10.1017/S0033291707001353
26. Galea S, Merchant RM, Lurie N. the mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention [published online ahead of print, 2020 Apr 10]. JAMA Intern Med. 2020;10.1001/jamainternmed.2020.1562. doi:10.1001/jamainternmed.2020.1562
27. Hawryluck L, Gold WL, Robinson S, Pogorski S, Galea S, Styra R. SARS control and psychological effects of quarantine, Toronto, Canada. Emerg Infect Dis. 2004;10(7):1206-1212. doi:10.3201/eid1007.030703
28. Cunha JM, Shen YC, Burke ZR. Contrasting the impacts of combat and humanitarian assistance/disaster relief missions on the mental health of military service members. Def Peace Economics. 2018;29(1):62-77. doi: 10.1080/10242694.2017.1349365
29. Ramchand R, Harrell MC, Berglass N, Lauck M. Veterans and COVID-19: Projecting the Economic, Social and Mental Health Needs of America’s Veterans. New York, NY: The Bob Woodruff Foundation; 2020.
30. van Gelder N, Peterman A, Potts A, et al. COVID-19: reducing the risk of infection might increase the risk of intimate partner violence [published online ahead of print, 2020 Apr 11]. EClinicalMedicine. 2020;21:100348. doi:10.1016/j.eclinm.2020.100348
31. Azarang A, Pakyurek M, Giroux C, Nordahl TE, Yellowlees P. Information technologies: an augmentation to post-traumatic stress disorder treatment among trauma survivors. Telemed J E Health. 2019;25(4):263-271. doi:10.1089/tmj.2018.0068.
32. Gilmore AK, Davis MT, Grubaugh A, et al. “Do you expect me to receive PTSD care in a setting where most of the other patients remind me of the perpetrator?”: Home-based telemedicine to address barriers to care unique to military sexual trauma and veterans affairs hospitals. Contemp Clin Trials. 2016;48:59-64. doi:10.1016/j.cct.2016.03.004.
33. van Gurp J, van Selm M, Vissers K, van Leeuwen E, Hasselaar J. How outpatient palliative care teleconsultation facilitates empathic patient-professional relationships: a qualitative study. PLoS One. 2015;10(4):e0124387. Published 2015 Apr 22. doi:10.1371/journal.pone.0124387
34. Morland LA, Mackintosh MA, Glassman LH, et al. Home-based delivery of variable length prolonged exposure therapy: a comparison of clinical efficacy between service modalities. Depress Anxiety. 2020;37(4):346-355. doi:10.1002/da.22979
35. Morland LA, Hynes AK, Mackintosh MA, Resick PA, Chard KM. Group cognitive processing therapy delivered to veterans via telehealth: a pilot cohort. J Trauma Stress. 2011;24(4):465-469. doi:10.1002/jts.20661
36. Elisseou S, Puranam S, Nandi M. A novel, trauma-informed physical examination curriculum. Med Educ. 2018;52(5):555-556. doi:10.1111/medu.13569
COVID-19 has created stressors that are unprecedented in our modern era, prompting health care systems to adapt rapidly. Demand for telehealth has skyrocketed, and clinicians, many of whom had planned to adopt virtual practices in the future, have been pressured to do so immediately.1 In March 2020, the Centers for Medicare and Medicaid Services (CMS) expanded telehealth services, removing many barriers to virtual care.2 Similar remedy was not necessary for the Veterans Health Administration (VHA) which reported more than 2.6 million episodes of telehealth care in 2019.3 By the time the pandemic was underway in the US, use of telehealth was widespread across the agency. In late March 2020, VHA released a COVID-19 Response Plan, in which telehealth played a critical role in safe, uninterrupted delivery of services.4 While telehealth has been widely used in VHA, the call for replacement of most in-person outpatient visits with telehealth visits was a fundamental paradigm shift for many patients and clinicians.4
The Coronavirus Aid, Relief, and Economic Security (CARES) Act (HR 748) gave the US Department of Veterans Affairs (VA) funding to expand coronavirus-related telehealth services, including the purchase of mobile devices and broadband expansion. CARES authorized the agency to expand telemental health services, enter into short-term agreements with telecommunications companies to provide temporary broadband services to veterans, temporarily waived an in-person home visit requirement (accepting video and phone calls as an alternative), and provided means to make telehealth available for homeless veterans and case managers through the HUD-VASH (US Department of Housing and Urban Development-VA Supportive Housing) program.
VHA is a national telehealth exemplar, initiating telehealth by use of closed-circuit televisions as early as 1968, and continuing to expand through 2017 with the implementation of the Veterans Video Connect (VVC) platform.5 VVC has enabled veterans to participate in virtual visits from distant locations, including their homes. VVC was used successfully during hurricanes Sandy, Harvey, Irma, and Maria and is being widely deployed in the current crisis.6-8
While telehealth can take many forms, the current discussion will focus on live (synchronous) videoconferencing: a 2-way audiovisual link between a patient and clinician, such as VVC, which enables patients to maintain a safe and social distance from others while connecting with the health care team and receiving urgent as well as ongoing medical care for both new and established conditions.9 VHA has developed multiple training resources for use of VVC across many settings, including primary care, mental health, and specialties. In this review, we will make the novel case for applying a trauma-informed lens to telehealth care across VHA and beyond to other health care systems.
Trauma-Informed Care
Although our current focus is rightly on mitigating the health effects of a pandemic, we must recognize that stressful phenomena like COVID-19 occur against a backdrop of widespread physical, sexual, psychological, and racial trauma in our communities. The Substance Abuse and Mental Health Services Administration (SAMHSA) describes trauma as resulting from “an event, series of events, or set of circumstances that is experienced by an individual as physically or emotionally harmful or life threatening and that has lasting adverse effects on the individual’s functioning and mental, physical, social, emotional, or spiritual well-being.”10 Trauma exposure is both ubiquitous worldwide and inequitably distributed, with vulnerable populations disproportionately impacted.11,12
Veterans as a population are often highly trauma exposed, and while VHA routinely screens for experiences of trauma, such as military sexual trauma (MST) and intimate partner violence (IPV), and potential mental health sequelae of trauma, including posttraumatic stress disorder (PTSD) and suicidality, veterans may experience other forms of trauma or be unwilling or unable to talk about past exposures.13 One common example is that of adverse childhood experiences (ACEs), which include household dysfunction, neglect, and physical and sexual abuse before the age of 18 years.14 ACEs have been associated with a wide range of risk behaviors and poor health outcomes in adulthood.14 In population-based data, both male and female veterans have reported higher ACE scores.15 In addition, ACE scores are higher overall for those serving in the all-volunteer era (after July 1, 1973).16 Because trauma may be unseen, unmeasured, and unnamed, it is important to deliver all medical care with sensitivity to its potential presence.
It is important to distinguish the concept of trauma-informed care (TIC) from trauma-focused services. Trauma-focused or trauma-specific treatment refers to evidence-based and best practice treatment models that have been proven to facilitate recovery from problems resulting from the experience of trauma, such as PTSD.17 These treatments directly address the emotional, behavioral, and physiologic impact of trauma on an individual’s life and facilitate improvement in related symptoms and functioning: They are designed to treat the consequences of trauma. VHA offers a wide range of trauma-specific treatments, and considerable experience in delivering evidence-based trauma-focused treatment through telehealth exists.18,19 Given the range of possible responses to the experience of trauma, not all veterans with trauma histories need to, chose to, or feel ready to access trauma-specific treatments.20
In contrast, TIC is a global, universal precautions approach to providing quality care that can be applied to all aspects of health care and to all patients.21 TIC is a strengths-based service delivery framework that is grounded in an understanding of, and responsiveness to, the disempowering impact of experiencing trauma. It seeks to maximize physical, psychological, and emotional safety in all health care encounters, not just those that are specifically trauma-focused, and creates opportunities to rebuild a sense of control and empowerment while fostering healing through safe and collaborative patient-clinician relationships.22 TIC is not accomplished through any single technique or checklist but through continuous appraisal of approaches to care delivery. SAMHSA has elucidated 6 fundamental principles of TIC: safety; trustworthiness and transparency; peer support; collaboration and mutuality; empowerment; voice and choice; and sensitivity to cultural, historical, and gender issues.10
TIC is based on the understanding that often traditional service delivery models of care may trigger, silence, or disempower survivors of trauma, exacerbating physical and mental health symptoms and potentially increasing disengagement from care and poorer outcomes.23 Currier and colleagues aptly noted, “TIC assumes that trustworthiness is not something that an organization creates in a veteran client, but something that he or she will freely grant to an organization.”24 Given the global prevalence of trauma, its well-established and deleterious impact on lifelong health, and the potential for health care itself to be traumatizing, TIC is a fundamental construct to apply universally with any patient at any time, especially in the context of a large-scale community trauma, such as a pandemic.12
Trauma-Informed COVID-19 Care
Catastrophic events, such as natural disasters and pandemics, may serve as both newly traumatic and as potential triggers for survivors who have endured prior trauma.25,26 Increases in depression, PTSD, and substance use disorder (SUD) are common sequalae, occurring during the event, the immediate aftermath, and beyond.25,27 In 2003, quarantine contained the spread of Severe acute respiratory syndrome (SARS) but resulted in a high prevalence of psychological distress, including PTSD and depression.27 Many veterans may have deployed in support of humanitarian assistance/disaster relief missions, which typically do not involve armed combat but may expose service members to warlike situations, including social insecurity and suffering populations.28 COVID-19 may be reminiscent of some of these deployments as well.
The impact of the current COVID-19 pandemic on patients is pervasive. Those with preexisting financial insecurity now face additional economic hardship and health challenges, which are amplified by loneliness and loss of social support networks.26 Widespread unemployment and closures of many businesses add to stress and may exacerbate preexisting mental and physical health concerns for many; some veterans also may be at increased risk.29 While previous postdisaster research suggests that psychopathology in the general population will significantly remit over time, high-risk groups remain vulnerable to PTSD and bear the brunt of social and economic consequences associated with the crisis.25 Veterans with preexisting trauma histories and mental health conditions are at increased risk for being retraumatized by the current pandemic and impacted by isolation and unplanned job or wage loss from it.29 Compounding this, social distancing serves to protect communities but may amplify isolation and danger in abusive relationships or exacerbate underlying mental illness.26,30
Thus, as we expand our use of telehealth, replacing our face-to-face visits with virtual encounters, it is critical for clinicians to be mindful that the pandemic and public health responses to it may result in trauma and retraumatization for veterans and other vulnerable patients, which in turn can impact both access and response to care. The application of trauma-informed principles to our virtual encounters has the potential to mitigate some of these health impacts, increase engagement in care, and provide opportunities for protective, healing connections.
In the setting of the continued fear and uncertainty of the COVID-19 pandemic, we believe that application of a trauma-informed lens to telehealth efforts is timely. While virtual visits may seem to lack the warmth and immediacy of traditional medical encounters, accumulated experience suggests otherwise.19 Telehealth is fundamentally more patient-focused than traditional encounters, overcomes service delivery barriers, offers a greater range of options for treatment engagement, and can enhance clinician-patient partnerships.6,31,32 Although the rapid transition to telehealth may be challenging for those new to it, experienced clinicians and patients express high degrees of satisfaction with virtual care because direct communication is unhampered by in-office challenges and travel logistics.33
While it may feel daunting to integrate principles of TIC into telehealth during a crisis-driven scale-up, a growing practice and body of research can inform these efforts. To help better understand how trauma-exposed patients respond to telehealth, we reviewed findings from trauma-focused telemental health (TMH) treatment. This research demonstrates that telehealth promotes safety and collaboration—fundamental principles of TIC—that can, in turn, be applied to telehealth visits in primary care and other medical and surgical specialties. When compared with traditional in-person treatment, studies of both individual and group formats of TMH found no significant differences in satisfaction, acceptability, or outcomes (such as reduction in PTSD symptom severity scores34), and TMH did not impede development of rapport.19,35
Although counterintuitive, the virtual space created by the combined physical and psychological distance of videoconferencing has been shown to promote safety and transparency. In TMH, patients have reported greater honesty due to the protection afforded by this virtual space.31 Engaging in telehealth visits from the comfort of one’s home can feel emotionally safer than having to travel to a medical office, resulting in feeling more at ease during encounters.31 In one TMH study, veterans with PTSD described high comfort levels and ability to let their guard down during virtual treatment.19 Similarly, in palliative telehealth care, patients reported that clinicians successfully nurtured an experience of intimacy, expressed empathy verbally and nonverbally, and responded to the patient’s unique situation and emotions.33
Trauma-Informed Telehealth
We have discussed how telehealth’s greater flexibility may create an ideal environment in which to implement principles of TIC. It may allow increased collaboration and closeness between patients and clinicians, empowering patients to codesign their care.31,33 The Table reviews 6 core SAMHSA principles of TIC and offers examples of their application to telehealth visits. The following case illustrates the application of trauma-informed telehealth care.
Case Presentation
S is a 45-year-old male veteran of Operation Enduring Freedom (OEF) who served as a combat medic. He has a history of osteoarthritis and PTSD related to combat experiences like caring for traumatic amputees. Before the pandemic began, he was employed as a server at a local restaurant but was laid off as the business transitioned to takeout orders only. The patient worked near a VA primary care clinic and frequently dropped by to see the staff and to pick up prescriptions. He had never agreed to video visits despite receiving encouragement from his medical team. He was reluctant to try telehealth, but he had developed a painful, itchy rash on his lower leg and was concerned about getting care.
For patients like S who may be reluctant to try telehealth, it is important to understand the cause. Potential barriers to telehealth may include lack of Internet access or familiarity with technology, discomfort with being on video, shame about the appearance of one’s home, or a strong cultural preference for face-to-face medical visits. Some may miss the social support benefit of coming into a clinic, particularly in VHA, which is designed specifically for veteran patients. For these reasons it is important to offer the patient a choice and to begin with a supportive phone call that explores and strives to address the patient’s concerns about videoconferencing.
The clinic nurse called S who agreed to try a VVC visit with gentle encouragement. He shared that he was embarrassed about the appearance of his apartment and fearful about pictures being recorded of his body due to “a bad experience in my past.” The patient was reassured that visits are private and will not be recorded. The nurse also reminded him that he can choose the location in which the visit will take place and can turn his camera off at any time. Importantly, the nurse did not ask him to recount additional details of what happened in his past. Next, the nurse verified his location and contact information and explained why obtaining this information was necessary. Next, she asked his consent to proceed with the visit, reminding him that the visit can end at any point if he feels uncomfortable. After finishing this initial discussion, the nurse told him that his primary care physician (PCP) would join the visit and address his concerns with his leg.
S was happy to see his PCP despite his hesitations about video care. The PCP noticed that he seemed anxious and was avoiding talking about the rash. Knowing that he was anxious about this VVC visit, the PCP was careful to look directly at the camera to make eye contact and to be sure her face was well lit and not in shadows. She gave him some time to acclimate to the virtual environment and thanked him for joining the visit. Knowing that he was a combat veteran, she warned him that there have been sudden, loud construction noises outside her window. Although the PCP was pressed for time, she was aware that S may have had a previous difficult experience around images of his body or even combat-related trauma. She gently brought up the rash and asked for permission to examine it, avoiding commands or personalizing language such as “show me your leg” or “take off your pants for me.”36After some hesitation, the patient revealed his leg that appeared to have multiple excoriations and old scars from picking. After the examination, the PCP waited until the patient’s leg was fully covered before beginning a discussion of the care plan. Together they collaboratively reviewed treatments that would soothe the skin. They decided to virtually consult a social worker to obtain emergency economic assistance and to speak with the patient’s care team psychologist to reduce some of the anxiety that may be leading to his leg scratching.
Case Discussion
This case illustrates the ways in which TIC can be applied to telehealth for a veteran with combat-related PTSD who may have experienced additional interpersonal trauma. It was not necessary to know more detail about the veteran’s trauma history to conduct the visit in a trauma-informed manner. Connecting to patients at home while considering these principles may thus foster mutuality, mitigate retraumatization, and cultivate enhanced collaboration with health care teams in this era of social distancing.
While a virtual physical examination creates both limitations and opportunity in telehealth, patients may find the greater degree of choice over their clothing and surroundings to be empowering. Telehealth also can allow for a greater portion of time to be dedicated to quality discussion and collaborative planning, with the clinician hearing and responding to the patient’s needs with reduced distraction. This may include opportunities to discuss mental health concerns openly, normalize emotional reactions, and offer connection to mental health and support services available through telehealth, including for patients who have not previously engaged in such care.
Conclusions
COVID-19 has created stressors that are unprecedented in our modern era, prompting health care systems to adapt rapidly. Demand for telehealth has skyrocketed, and clinicians, many of whom had planned to adopt virtual practices in the future, have been pressured to do so immediately.1 In March 2020, the Centers for Medicare and Medicaid Services (CMS) expanded telehealth services, removing many barriers to virtual care.2 Similar remedy was not necessary for the Veterans Health Administration (VHA) which reported more than 2.6 million episodes of telehealth care in 2019.3 By the time the pandemic was underway in the US, use of telehealth was widespread across the agency. In late March 2020, VHA released a COVID-19 Response Plan, in which telehealth played a critical role in safe, uninterrupted delivery of services.4 While telehealth has been widely used in VHA, the call for replacement of most in-person outpatient visits with telehealth visits was a fundamental paradigm shift for many patients and clinicians.4
The Coronavirus Aid, Relief, and Economic Security (CARES) Act (HR 748) gave the US Department of Veterans Affairs (VA) funding to expand coronavirus-related telehealth services, including the purchase of mobile devices and broadband expansion. CARES authorized the agency to expand telemental health services, enter into short-term agreements with telecommunications companies to provide temporary broadband services to veterans, temporarily waived an in-person home visit requirement (accepting video and phone calls as an alternative), and provided means to make telehealth available for homeless veterans and case managers through the HUD-VASH (US Department of Housing and Urban Development-VA Supportive Housing) program.
VHA is a national telehealth exemplar, initiating telehealth by use of closed-circuit televisions as early as 1968, and continuing to expand through 2017 with the implementation of the Veterans Video Connect (VVC) platform.5 VVC has enabled veterans to participate in virtual visits from distant locations, including their homes. VVC was used successfully during hurricanes Sandy, Harvey, Irma, and Maria and is being widely deployed in the current crisis.6-8
While telehealth can take many forms, the current discussion will focus on live (synchronous) videoconferencing: a 2-way audiovisual link between a patient and clinician, such as VVC, which enables patients to maintain a safe and social distance from others while connecting with the health care team and receiving urgent as well as ongoing medical care for both new and established conditions.9 VHA has developed multiple training resources for use of VVC across many settings, including primary care, mental health, and specialties. In this review, we will make the novel case for applying a trauma-informed lens to telehealth care across VHA and beyond to other health care systems.
Trauma-Informed Care
Although our current focus is rightly on mitigating the health effects of a pandemic, we must recognize that stressful phenomena like COVID-19 occur against a backdrop of widespread physical, sexual, psychological, and racial trauma in our communities. The Substance Abuse and Mental Health Services Administration (SAMHSA) describes trauma as resulting from “an event, series of events, or set of circumstances that is experienced by an individual as physically or emotionally harmful or life threatening and that has lasting adverse effects on the individual’s functioning and mental, physical, social, emotional, or spiritual well-being.”10 Trauma exposure is both ubiquitous worldwide and inequitably distributed, with vulnerable populations disproportionately impacted.11,12
Veterans as a population are often highly trauma exposed, and while VHA routinely screens for experiences of trauma, such as military sexual trauma (MST) and intimate partner violence (IPV), and potential mental health sequelae of trauma, including posttraumatic stress disorder (PTSD) and suicidality, veterans may experience other forms of trauma or be unwilling or unable to talk about past exposures.13 One common example is that of adverse childhood experiences (ACEs), which include household dysfunction, neglect, and physical and sexual abuse before the age of 18 years.14 ACEs have been associated with a wide range of risk behaviors and poor health outcomes in adulthood.14 In population-based data, both male and female veterans have reported higher ACE scores.15 In addition, ACE scores are higher overall for those serving in the all-volunteer era (after July 1, 1973).16 Because trauma may be unseen, unmeasured, and unnamed, it is important to deliver all medical care with sensitivity to its potential presence.
It is important to distinguish the concept of trauma-informed care (TIC) from trauma-focused services. Trauma-focused or trauma-specific treatment refers to evidence-based and best practice treatment models that have been proven to facilitate recovery from problems resulting from the experience of trauma, such as PTSD.17 These treatments directly address the emotional, behavioral, and physiologic impact of trauma on an individual’s life and facilitate improvement in related symptoms and functioning: They are designed to treat the consequences of trauma. VHA offers a wide range of trauma-specific treatments, and considerable experience in delivering evidence-based trauma-focused treatment through telehealth exists.18,19 Given the range of possible responses to the experience of trauma, not all veterans with trauma histories need to, chose to, or feel ready to access trauma-specific treatments.20
In contrast, TIC is a global, universal precautions approach to providing quality care that can be applied to all aspects of health care and to all patients.21 TIC is a strengths-based service delivery framework that is grounded in an understanding of, and responsiveness to, the disempowering impact of experiencing trauma. It seeks to maximize physical, psychological, and emotional safety in all health care encounters, not just those that are specifically trauma-focused, and creates opportunities to rebuild a sense of control and empowerment while fostering healing through safe and collaborative patient-clinician relationships.22 TIC is not accomplished through any single technique or checklist but through continuous appraisal of approaches to care delivery. SAMHSA has elucidated 6 fundamental principles of TIC: safety; trustworthiness and transparency; peer support; collaboration and mutuality; empowerment; voice and choice; and sensitivity to cultural, historical, and gender issues.10
TIC is based on the understanding that often traditional service delivery models of care may trigger, silence, or disempower survivors of trauma, exacerbating physical and mental health symptoms and potentially increasing disengagement from care and poorer outcomes.23 Currier and colleagues aptly noted, “TIC assumes that trustworthiness is not something that an organization creates in a veteran client, but something that he or she will freely grant to an organization.”24 Given the global prevalence of trauma, its well-established and deleterious impact on lifelong health, and the potential for health care itself to be traumatizing, TIC is a fundamental construct to apply universally with any patient at any time, especially in the context of a large-scale community trauma, such as a pandemic.12
Trauma-Informed COVID-19 Care
Catastrophic events, such as natural disasters and pandemics, may serve as both newly traumatic and as potential triggers for survivors who have endured prior trauma.25,26 Increases in depression, PTSD, and substance use disorder (SUD) are common sequalae, occurring during the event, the immediate aftermath, and beyond.25,27 In 2003, quarantine contained the spread of Severe acute respiratory syndrome (SARS) but resulted in a high prevalence of psychological distress, including PTSD and depression.27 Many veterans may have deployed in support of humanitarian assistance/disaster relief missions, which typically do not involve armed combat but may expose service members to warlike situations, including social insecurity and suffering populations.28 COVID-19 may be reminiscent of some of these deployments as well.
The impact of the current COVID-19 pandemic on patients is pervasive. Those with preexisting financial insecurity now face additional economic hardship and health challenges, which are amplified by loneliness and loss of social support networks.26 Widespread unemployment and closures of many businesses add to stress and may exacerbate preexisting mental and physical health concerns for many; some veterans also may be at increased risk.29 While previous postdisaster research suggests that psychopathology in the general population will significantly remit over time, high-risk groups remain vulnerable to PTSD and bear the brunt of social and economic consequences associated with the crisis.25 Veterans with preexisting trauma histories and mental health conditions are at increased risk for being retraumatized by the current pandemic and impacted by isolation and unplanned job or wage loss from it.29 Compounding this, social distancing serves to protect communities but may amplify isolation and danger in abusive relationships or exacerbate underlying mental illness.26,30
Thus, as we expand our use of telehealth, replacing our face-to-face visits with virtual encounters, it is critical for clinicians to be mindful that the pandemic and public health responses to it may result in trauma and retraumatization for veterans and other vulnerable patients, which in turn can impact both access and response to care. The application of trauma-informed principles to our virtual encounters has the potential to mitigate some of these health impacts, increase engagement in care, and provide opportunities for protective, healing connections.
In the setting of the continued fear and uncertainty of the COVID-19 pandemic, we believe that application of a trauma-informed lens to telehealth efforts is timely. While virtual visits may seem to lack the warmth and immediacy of traditional medical encounters, accumulated experience suggests otherwise.19 Telehealth is fundamentally more patient-focused than traditional encounters, overcomes service delivery barriers, offers a greater range of options for treatment engagement, and can enhance clinician-patient partnerships.6,31,32 Although the rapid transition to telehealth may be challenging for those new to it, experienced clinicians and patients express high degrees of satisfaction with virtual care because direct communication is unhampered by in-office challenges and travel logistics.33
While it may feel daunting to integrate principles of TIC into telehealth during a crisis-driven scale-up, a growing practice and body of research can inform these efforts. To help better understand how trauma-exposed patients respond to telehealth, we reviewed findings from trauma-focused telemental health (TMH) treatment. This research demonstrates that telehealth promotes safety and collaboration—fundamental principles of TIC—that can, in turn, be applied to telehealth visits in primary care and other medical and surgical specialties. When compared with traditional in-person treatment, studies of both individual and group formats of TMH found no significant differences in satisfaction, acceptability, or outcomes (such as reduction in PTSD symptom severity scores34), and TMH did not impede development of rapport.19,35
Although counterintuitive, the virtual space created by the combined physical and psychological distance of videoconferencing has been shown to promote safety and transparency. In TMH, patients have reported greater honesty due to the protection afforded by this virtual space.31 Engaging in telehealth visits from the comfort of one’s home can feel emotionally safer than having to travel to a medical office, resulting in feeling more at ease during encounters.31 In one TMH study, veterans with PTSD described high comfort levels and ability to let their guard down during virtual treatment.19 Similarly, in palliative telehealth care, patients reported that clinicians successfully nurtured an experience of intimacy, expressed empathy verbally and nonverbally, and responded to the patient’s unique situation and emotions.33
Trauma-Informed Telehealth
We have discussed how telehealth’s greater flexibility may create an ideal environment in which to implement principles of TIC. It may allow increased collaboration and closeness between patients and clinicians, empowering patients to codesign their care.31,33 The Table reviews 6 core SAMHSA principles of TIC and offers examples of their application to telehealth visits. The following case illustrates the application of trauma-informed telehealth care.
Case Presentation
S is a 45-year-old male veteran of Operation Enduring Freedom (OEF) who served as a combat medic. He has a history of osteoarthritis and PTSD related to combat experiences like caring for traumatic amputees. Before the pandemic began, he was employed as a server at a local restaurant but was laid off as the business transitioned to takeout orders only. The patient worked near a VA primary care clinic and frequently dropped by to see the staff and to pick up prescriptions. He had never agreed to video visits despite receiving encouragement from his medical team. He was reluctant to try telehealth, but he had developed a painful, itchy rash on his lower leg and was concerned about getting care.
For patients like S who may be reluctant to try telehealth, it is important to understand the cause. Potential barriers to telehealth may include lack of Internet access or familiarity with technology, discomfort with being on video, shame about the appearance of one’s home, or a strong cultural preference for face-to-face medical visits. Some may miss the social support benefit of coming into a clinic, particularly in VHA, which is designed specifically for veteran patients. For these reasons it is important to offer the patient a choice and to begin with a supportive phone call that explores and strives to address the patient’s concerns about videoconferencing.
The clinic nurse called S who agreed to try a VVC visit with gentle encouragement. He shared that he was embarrassed about the appearance of his apartment and fearful about pictures being recorded of his body due to “a bad experience in my past.” The patient was reassured that visits are private and will not be recorded. The nurse also reminded him that he can choose the location in which the visit will take place and can turn his camera off at any time. Importantly, the nurse did not ask him to recount additional details of what happened in his past. Next, the nurse verified his location and contact information and explained why obtaining this information was necessary. Next, she asked his consent to proceed with the visit, reminding him that the visit can end at any point if he feels uncomfortable. After finishing this initial discussion, the nurse told him that his primary care physician (PCP) would join the visit and address his concerns with his leg.
S was happy to see his PCP despite his hesitations about video care. The PCP noticed that he seemed anxious and was avoiding talking about the rash. Knowing that he was anxious about this VVC visit, the PCP was careful to look directly at the camera to make eye contact and to be sure her face was well lit and not in shadows. She gave him some time to acclimate to the virtual environment and thanked him for joining the visit. Knowing that he was a combat veteran, she warned him that there have been sudden, loud construction noises outside her window. Although the PCP was pressed for time, she was aware that S may have had a previous difficult experience around images of his body or even combat-related trauma. She gently brought up the rash and asked for permission to examine it, avoiding commands or personalizing language such as “show me your leg” or “take off your pants for me.”36After some hesitation, the patient revealed his leg that appeared to have multiple excoriations and old scars from picking. After the examination, the PCP waited until the patient’s leg was fully covered before beginning a discussion of the care plan. Together they collaboratively reviewed treatments that would soothe the skin. They decided to virtually consult a social worker to obtain emergency economic assistance and to speak with the patient’s care team psychologist to reduce some of the anxiety that may be leading to his leg scratching.
Case Discussion
This case illustrates the ways in which TIC can be applied to telehealth for a veteran with combat-related PTSD who may have experienced additional interpersonal trauma. It was not necessary to know more detail about the veteran’s trauma history to conduct the visit in a trauma-informed manner. Connecting to patients at home while considering these principles may thus foster mutuality, mitigate retraumatization, and cultivate enhanced collaboration with health care teams in this era of social distancing.
While a virtual physical examination creates both limitations and opportunity in telehealth, patients may find the greater degree of choice over their clothing and surroundings to be empowering. Telehealth also can allow for a greater portion of time to be dedicated to quality discussion and collaborative planning, with the clinician hearing and responding to the patient’s needs with reduced distraction. This may include opportunities to discuss mental health concerns openly, normalize emotional reactions, and offer connection to mental health and support services available through telehealth, including for patients who have not previously engaged in such care.
Conclusions
1. Wosik J, Fudim M, Cameron B, et al. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc. 2020;27(6):957-962. doi:10.1093/jamia/ocaa067
2. Centers for Medicare and Medicaid Services. Medicare and Medicaid programs; policy and regulatory revisions in response to the COVID-19 public health emergency. CMS-1744-IFC. https://www.cms.gov/files/document/covid-final-ifc.pdf. Published March 24, 2020. Accessed April 8, 2020.
3. Eddy N. VA sees a surge in veterans’ use of telehealth services. https://www.healthcareitnews.com/news/va-sees-surge-veterans-use-telehealth-services. Published November 25, 2019. Accessed June 17, 2020.
4. Veterans Health Administration, Office of Emergency Management. COVID-19 response plan. Version 1.6. Published March 23, 2020. Accessed June 17, 2020.
5. Caudill RL, Sager Z. Institutionally based videoconferencing. Int Rev Psychiatry. 2015;27(6):496-503. doi:10.3109/09540261.2015.1085369
6. Heyworth L. Sharing Connections [published correction appears in JAMA. 2018 May 8;319(18):1939]. JAMA. 2018;319(13):1323-1324. doi:10.1001/jama.2018.2717
7. Dobalian A. U.S. Department of Veterans Affairs’ (VA’s) response to the 2017 hurricanes. Presented at: American Public Health Association 2019 Annual Meeting and Exposition; November 2-6, 2019; Philadelphia, PA. https://apha.confex.com/apha/2019/meetingapp.cgi/Session/58543. Accessed June 16, 2020.
8. Der-Martirosian C, Griffin AR, Chu K, Dobalian A. Telehealth at the US Department of Veterans Affairs after Hurricane Sandy. J Telemed Telecare. 2019;25(5):310-317. doi:10.1177/1357633X17751005
9. The Office of the National Coordinator for Health Information Technology. Telemedicine and telehealth. https://www.healthit.gov/topic/health-it-initiatives/telemedicine-and-telehealth. Updated September 28, 2017. Accessed June 16, 2020.
10. Substance Abuse and Mental Health Services Administration, Trauma and Justice Strategic Initiative. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. https://ncsacw.samhsa.gov/userfiles/files/SAMHSA_Trauma.pdf. Published July 2014. Accessed June 16, 2020.
11. Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26(5):537-547. doi:10.1002/jts.21848
12. Kimberg L, Wheeler M. Trauma and Trauma-informed Care. In: Gerber MR, ed. Trauma-informed Healthcare Approaches: A Guide for Primary Care. Cham, Switzerland: Springer Nature; 2019:25-56.
13. Gerber MR. Trauma-informed care of veterans. In: Gerber MR, ed. Trauma-informed Healthcare Approaches: A Guide for Primary Care. Cham, Switzerland: Springer Nature; 2019:25-56.
14. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245-258. doi:10.1016/s0749-3797(98)00017-8
15. Katon JG, Lehavot K, Simpson TL, et al. Adverse childhood experiences, Military service, and adult health. Am J Prev Med. 2015;49(4):573-582. doi:10.1016/j.amepre.2015.03.020
16. Blosnich JR, Dichter ME, Cerulli C, Batten SV, Bossarte RM. Disparities in adverse childhood experiences among individuals with a history of military service. JAMA Psychiatry. 2014;71(9):1041-1048. doi:10.1001/jamapsychiatry.2014.724
17. Center for Substance Abuse Treatment. Treatment improvement protocol (TIP). Series, No. 57. In: SAMHSA, ed. Trauma-Informed Care in Behavioral Health Services. SAMHSA: Rockville, MD; 2014:137-155.
18. US Department of Veterans Affairs, Veterans Health Administration, National Center for PTSD. Trauma, PTSD and treatment. https://www.ptsd.va.gov/PTSD/professional/treat/index.asp. Updated July 5, 2019. Accessed June 17, 2020.
19. Turgoose D, Ashwick R, Murphy D. Systematic review of lessons learned from delivering tele-therapy to veterans with post-traumatic stress disorder. J Telemed Telecare. 2018;24(9):575-585. doi:10.1177/1357633X17730443
20. Cook JM, Simiola V, Hamblen JL, Bernardy N, Schnurr PP. The influence of patient readiness on implementation of evidence-based PTSD treatments in Veterans Affairs residential programs. Psychol Trauma. 2017;9(suppl 1):51-58. doi:10.1037/tra0000162
21. Raja S, Hasnain M, Hoersch M, Gove-Yin S, Rajagopalan C. Trauma informed care in medicine: current knowledge and future research directions. Fam Community Health. 2015;38(3):216-226. doi:10.1097/FCH.0000000000000071
22. Hopper EK, Bassuk EL, Olivet J. Shelter from the storm: trauma-informed care in homeless service settings. Open Health Serv Policy J. 2009;2:131-151.
23. Kelly U, Boyd MA, Valente SM, Czekanski E. Trauma-informed care: keeping mental health settings safe for veterans [published correction appears in Issues Ment Health Nurs. 2015 Jun;36(6):482]. Issues Ment Health Nurs. 2014;35(6):413-419. doi:10.3109/01612840.2014.881941
24. Currier JM, Stefurak T, Carroll TD, Shatto EH. Applying trauma-informed care to community-based mental health services for military veterans. Best Pract Ment Health. 2017;13(1):47-64.
25. Neria Y, Nandi A, Galea S. Post-traumatic stress disorder following disasters: a systematic review. Psychol Med. 2008;38(4):467-480. doi:10.1017/S0033291707001353
26. Galea S, Merchant RM, Lurie N. the mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention [published online ahead of print, 2020 Apr 10]. JAMA Intern Med. 2020;10.1001/jamainternmed.2020.1562. doi:10.1001/jamainternmed.2020.1562
27. Hawryluck L, Gold WL, Robinson S, Pogorski S, Galea S, Styra R. SARS control and psychological effects of quarantine, Toronto, Canada. Emerg Infect Dis. 2004;10(7):1206-1212. doi:10.3201/eid1007.030703
28. Cunha JM, Shen YC, Burke ZR. Contrasting the impacts of combat and humanitarian assistance/disaster relief missions on the mental health of military service members. Def Peace Economics. 2018;29(1):62-77. doi: 10.1080/10242694.2017.1349365
29. Ramchand R, Harrell MC, Berglass N, Lauck M. Veterans and COVID-19: Projecting the Economic, Social and Mental Health Needs of America’s Veterans. New York, NY: The Bob Woodruff Foundation; 2020.
30. van Gelder N, Peterman A, Potts A, et al. COVID-19: reducing the risk of infection might increase the risk of intimate partner violence [published online ahead of print, 2020 Apr 11]. EClinicalMedicine. 2020;21:100348. doi:10.1016/j.eclinm.2020.100348
31. Azarang A, Pakyurek M, Giroux C, Nordahl TE, Yellowlees P. Information technologies: an augmentation to post-traumatic stress disorder treatment among trauma survivors. Telemed J E Health. 2019;25(4):263-271. doi:10.1089/tmj.2018.0068.
32. Gilmore AK, Davis MT, Grubaugh A, et al. “Do you expect me to receive PTSD care in a setting where most of the other patients remind me of the perpetrator?”: Home-based telemedicine to address barriers to care unique to military sexual trauma and veterans affairs hospitals. Contemp Clin Trials. 2016;48:59-64. doi:10.1016/j.cct.2016.03.004.
33. van Gurp J, van Selm M, Vissers K, van Leeuwen E, Hasselaar J. How outpatient palliative care teleconsultation facilitates empathic patient-professional relationships: a qualitative study. PLoS One. 2015;10(4):e0124387. Published 2015 Apr 22. doi:10.1371/journal.pone.0124387
34. Morland LA, Mackintosh MA, Glassman LH, et al. Home-based delivery of variable length prolonged exposure therapy: a comparison of clinical efficacy between service modalities. Depress Anxiety. 2020;37(4):346-355. doi:10.1002/da.22979
35. Morland LA, Hynes AK, Mackintosh MA, Resick PA, Chard KM. Group cognitive processing therapy delivered to veterans via telehealth: a pilot cohort. J Trauma Stress. 2011;24(4):465-469. doi:10.1002/jts.20661
36. Elisseou S, Puranam S, Nandi M. A novel, trauma-informed physical examination curriculum. Med Educ. 2018;52(5):555-556. doi:10.1111/medu.13569
1. Wosik J, Fudim M, Cameron B, et al. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc. 2020;27(6):957-962. doi:10.1093/jamia/ocaa067
2. Centers for Medicare and Medicaid Services. Medicare and Medicaid programs; policy and regulatory revisions in response to the COVID-19 public health emergency. CMS-1744-IFC. https://www.cms.gov/files/document/covid-final-ifc.pdf. Published March 24, 2020. Accessed April 8, 2020.
3. Eddy N. VA sees a surge in veterans’ use of telehealth services. https://www.healthcareitnews.com/news/va-sees-surge-veterans-use-telehealth-services. Published November 25, 2019. Accessed June 17, 2020.
4. Veterans Health Administration, Office of Emergency Management. COVID-19 response plan. Version 1.6. Published March 23, 2020. Accessed June 17, 2020.
5. Caudill RL, Sager Z. Institutionally based videoconferencing. Int Rev Psychiatry. 2015;27(6):496-503. doi:10.3109/09540261.2015.1085369
6. Heyworth L. Sharing Connections [published correction appears in JAMA. 2018 May 8;319(18):1939]. JAMA. 2018;319(13):1323-1324. doi:10.1001/jama.2018.2717
7. Dobalian A. U.S. Department of Veterans Affairs’ (VA’s) response to the 2017 hurricanes. Presented at: American Public Health Association 2019 Annual Meeting and Exposition; November 2-6, 2019; Philadelphia, PA. https://apha.confex.com/apha/2019/meetingapp.cgi/Session/58543. Accessed June 16, 2020.
8. Der-Martirosian C, Griffin AR, Chu K, Dobalian A. Telehealth at the US Department of Veterans Affairs after Hurricane Sandy. J Telemed Telecare. 2019;25(5):310-317. doi:10.1177/1357633X17751005
9. The Office of the National Coordinator for Health Information Technology. Telemedicine and telehealth. https://www.healthit.gov/topic/health-it-initiatives/telemedicine-and-telehealth. Updated September 28, 2017. Accessed June 16, 2020.
10. Substance Abuse and Mental Health Services Administration, Trauma and Justice Strategic Initiative. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. https://ncsacw.samhsa.gov/userfiles/files/SAMHSA_Trauma.pdf. Published July 2014. Accessed June 16, 2020.
11. Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26(5):537-547. doi:10.1002/jts.21848
12. Kimberg L, Wheeler M. Trauma and Trauma-informed Care. In: Gerber MR, ed. Trauma-informed Healthcare Approaches: A Guide for Primary Care. Cham, Switzerland: Springer Nature; 2019:25-56.
13. Gerber MR. Trauma-informed care of veterans. In: Gerber MR, ed. Trauma-informed Healthcare Approaches: A Guide for Primary Care. Cham, Switzerland: Springer Nature; 2019:25-56.
14. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245-258. doi:10.1016/s0749-3797(98)00017-8
15. Katon JG, Lehavot K, Simpson TL, et al. Adverse childhood experiences, Military service, and adult health. Am J Prev Med. 2015;49(4):573-582. doi:10.1016/j.amepre.2015.03.020
16. Blosnich JR, Dichter ME, Cerulli C, Batten SV, Bossarte RM. Disparities in adverse childhood experiences among individuals with a history of military service. JAMA Psychiatry. 2014;71(9):1041-1048. doi:10.1001/jamapsychiatry.2014.724
17. Center for Substance Abuse Treatment. Treatment improvement protocol (TIP). Series, No. 57. In: SAMHSA, ed. Trauma-Informed Care in Behavioral Health Services. SAMHSA: Rockville, MD; 2014:137-155.
18. US Department of Veterans Affairs, Veterans Health Administration, National Center for PTSD. Trauma, PTSD and treatment. https://www.ptsd.va.gov/PTSD/professional/treat/index.asp. Updated July 5, 2019. Accessed June 17, 2020.
19. Turgoose D, Ashwick R, Murphy D. Systematic review of lessons learned from delivering tele-therapy to veterans with post-traumatic stress disorder. J Telemed Telecare. 2018;24(9):575-585. doi:10.1177/1357633X17730443
20. Cook JM, Simiola V, Hamblen JL, Bernardy N, Schnurr PP. The influence of patient readiness on implementation of evidence-based PTSD treatments in Veterans Affairs residential programs. Psychol Trauma. 2017;9(suppl 1):51-58. doi:10.1037/tra0000162
21. Raja S, Hasnain M, Hoersch M, Gove-Yin S, Rajagopalan C. Trauma informed care in medicine: current knowledge and future research directions. Fam Community Health. 2015;38(3):216-226. doi:10.1097/FCH.0000000000000071
22. Hopper EK, Bassuk EL, Olivet J. Shelter from the storm: trauma-informed care in homeless service settings. Open Health Serv Policy J. 2009;2:131-151.
23. Kelly U, Boyd MA, Valente SM, Czekanski E. Trauma-informed care: keeping mental health settings safe for veterans [published correction appears in Issues Ment Health Nurs. 2015 Jun;36(6):482]. Issues Ment Health Nurs. 2014;35(6):413-419. doi:10.3109/01612840.2014.881941
24. Currier JM, Stefurak T, Carroll TD, Shatto EH. Applying trauma-informed care to community-based mental health services for military veterans. Best Pract Ment Health. 2017;13(1):47-64.
25. Neria Y, Nandi A, Galea S. Post-traumatic stress disorder following disasters: a systematic review. Psychol Med. 2008;38(4):467-480. doi:10.1017/S0033291707001353
26. Galea S, Merchant RM, Lurie N. the mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention [published online ahead of print, 2020 Apr 10]. JAMA Intern Med. 2020;10.1001/jamainternmed.2020.1562. doi:10.1001/jamainternmed.2020.1562
27. Hawryluck L, Gold WL, Robinson S, Pogorski S, Galea S, Styra R. SARS control and psychological effects of quarantine, Toronto, Canada. Emerg Infect Dis. 2004;10(7):1206-1212. doi:10.3201/eid1007.030703
28. Cunha JM, Shen YC, Burke ZR. Contrasting the impacts of combat and humanitarian assistance/disaster relief missions on the mental health of military service members. Def Peace Economics. 2018;29(1):62-77. doi: 10.1080/10242694.2017.1349365
29. Ramchand R, Harrell MC, Berglass N, Lauck M. Veterans and COVID-19: Projecting the Economic, Social and Mental Health Needs of America’s Veterans. New York, NY: The Bob Woodruff Foundation; 2020.
30. van Gelder N, Peterman A, Potts A, et al. COVID-19: reducing the risk of infection might increase the risk of intimate partner violence [published online ahead of print, 2020 Apr 11]. EClinicalMedicine. 2020;21:100348. doi:10.1016/j.eclinm.2020.100348
31. Azarang A, Pakyurek M, Giroux C, Nordahl TE, Yellowlees P. Information technologies: an augmentation to post-traumatic stress disorder treatment among trauma survivors. Telemed J E Health. 2019;25(4):263-271. doi:10.1089/tmj.2018.0068.
32. Gilmore AK, Davis MT, Grubaugh A, et al. “Do you expect me to receive PTSD care in a setting where most of the other patients remind me of the perpetrator?”: Home-based telemedicine to address barriers to care unique to military sexual trauma and veterans affairs hospitals. Contemp Clin Trials. 2016;48:59-64. doi:10.1016/j.cct.2016.03.004.
33. van Gurp J, van Selm M, Vissers K, van Leeuwen E, Hasselaar J. How outpatient palliative care teleconsultation facilitates empathic patient-professional relationships: a qualitative study. PLoS One. 2015;10(4):e0124387. Published 2015 Apr 22. doi:10.1371/journal.pone.0124387
34. Morland LA, Mackintosh MA, Glassman LH, et al. Home-based delivery of variable length prolonged exposure therapy: a comparison of clinical efficacy between service modalities. Depress Anxiety. 2020;37(4):346-355. doi:10.1002/da.22979
35. Morland LA, Hynes AK, Mackintosh MA, Resick PA, Chard KM. Group cognitive processing therapy delivered to veterans via telehealth: a pilot cohort. J Trauma Stress. 2011;24(4):465-469. doi:10.1002/jts.20661
36. Elisseou S, Puranam S, Nandi M. A novel, trauma-informed physical examination curriculum. Med Educ. 2018;52(5):555-556. doi:10.1111/medu.13569
The Dog Days of COVID-19
The editorials I have written so far in this series on COVID-19 have been on weighty topics as befits the serious situation of the pandemic, which as of June 30, 2020 had taken more than 500,000 lives across the globe and caused anguish and sorrow such as the world has not known since the 1918 influenza pandemic.2
The human spirit can bear only so much distress and tragedy before it is bowed and unable to stand. Stand though we must; not just against the inanimate invasion of viruses from the outside, but also our own endemic national tensions and conflicts. A periodic lifting of our burdens and a recharging of our psychological and spiritual energies are crucial to the resilience and flexibility that are necessary to walk the long difficult road ahead of us as a nation and as public servants in health care. This column takes a lighter look at COVID-19 and considers the restorative role companion animals, especially, for me, my beloved canines, have played in caring for and about us humans during the pandemic.
You will likely read this editorial during the official dog days of summer, which run from July 22 to August 22. We all may imagine a big dog laying on a porch in the American South while his owners drink lemonade and quietly rock in chairs watching the long lazy days pass in a simpler time.
However pleasant this bucolic picture, it has little to do with the origin of the expression, which dates back to ancient Greece. The dog refers not to our literal furry friends but, according to National Geographic (and who should know better), to the position of the “dog star” in a constellation in the night sky.3 Unfortunately, we cannot completely get away from the sobering theme of the pandemic: The rise of the star to prominence during the peak of the Mediterranean summer’s heat was a period associated with disaster and illness.
Real dogs, cats, and assorted other so-called pandemic pets, though, have been another type of star in this difficult period. Early in the shelter-in-place, pet adoptions from city and county animal shelters and rescue organizations skyrocketed.4 Although animal welfare experts have legitimate concerns that some of these adoptees will be surrendered if there is ever a return to normal. For now many people feel it is the perfect time to adopt, precisely because they now have space to bring a new member into the family. Before adopting, as a recent National Public Radio report emphasized, individuals should consider whether they truly have the resources both material and emotional to care for a pet.5 For those who take stock honestly and believe they have the room in their heart and budget, rescuing a companion is good psychological news, arguably even more for the human than for the animal.
Sheltering-in-place has reduced the transmission of the virus, which scientists estimate has saved thousands of lives.6 But it also has triggered a second health crisis, this time of mental health with an unprecedented increase in rates of depression, anxiety, suicide attempts, and substance use that is expected to worsen over the coming months and years.7 Companion animals certainly cannot solve this complex and mammoth public health problem; however, they can contribute in simple and small yet very significant ways to the mental health of individuals.8
Caring for a pet who shows unconditional love and loyalty to you can reduce isolation; foster hope; provide meaning, comfort, and cheer to you when you are down or afraid; and offer a routine and reason to get out of bed every day and take a walk outside. Research shows that those positive effects can decrease the risk of the very mental health conditions that are now plaguing us in such alarming numbers.9,10
“How many more lives are we willing to sacrifice in the name of containing the virus?” Elinore McCance-Katz, MD, PhD, the nation’s top mental health official ominously asked about the potential effects of another shutdown during a cabinet meeting.11 For some of us, a companion animal who does not require physical distancing (at least when you are healthy) may permit us to prevent the spread of the virus while protecting our mental health.
Nor is emotional support the only clinical way in which animals are helping pandemic- beleaguered humans. There is a low risk we can infect household pets, and dogs are not likely to transmit the virus. In fact, they even can be trained to serve as highly efficient virus testers who don’t need scarce reagents or carry high price tags—just a pat on the head and an occasional treat.12 Medscape reported that clinical trials starting in the United Kingdom are set to evaluate the accuracy of these “bio-detection” dogs. The story quotes a leading British public health official as saying, “Properly trained sniffer dogs could revolutionise our approach to this whole pandemic, screening 250 people an hour for the virus.”13
Canines are not only healers who can ease our troubles through the pandemic but also peacemakers. As injustice and violence rock the country, we would do well to imitate their attitudes of nonjudgmental acceptance. “Dogs are our link to paradise. They do not know evil or jealousy or discontent,” wrote novelist Milan Kundera. “To sit with a dog on a hillside on a glorious afternoon is to be back in Eden, where doing nothing was not boring—it was peace.”14 Those indeed would be dog days as when better nature we sometimes share with animals prevailed.
1. Buber M. I and Thou . Kaufmann W, trans. New York: Charles Scribner’s Sons: 1970:144.
2. World Health Organization. Coronavirus disease (COVID-19). Situation report-153. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200621-covid-19-sitrep-153.pdf?sfvrsn=c896464d_2. Published June 21, 2020. Accessed June 22, 2020.
3. Little B. Why do we call them the ‘dog days’ of summer. National Geographic . July 10, 2015. https://www.nationalgeographic.com/news/2015/07/150710-dog-days-summer-sirius-star-astronomy-weather-language. Accessed June 21, 2020.
4. Ellis EG. Thanks to sheltering in place, animal shelters are empty. https://www.wired.com/story/coronavirus-pet-adoption-boom. Published April 10, 2020. Accessed June 21, 2020.
5. Balaban S. Should I adopt a dog during the coronavirus crisis? Read this first. https://www.npr.org/2020/05/08/853088872/should-i-adopt-a-dog-during-the-coronavirus-crisis-read-this-first. Published May 11, 2020. Accessed June 21, 2020
6. Hsiang S, Allen D, Annan-Phan S, et al. The effect of large-scale anti-contagion policies on the COVID-19 pandemic [published online ahead of print, 2020 Jun 8]. Nature. 2020;10.1038/s41586-020-2404-8. doi:10.1038/s41586-020-2404-8
7. Galea S, Merchant RM, Lurie N. The Mental Health Consequences of COVID-19 and Physical Distancing: The Need for Prevention and Early Intervention [published online ahead of print, 2020 Apr 10]. JAMA Intern Med. 2020;10.1001/jamainternmed.2020.1562. doi:10.1001/jamainternmed.2020.1562
8. Rajewski G. How animals help us during the COVID-19 pandemic. https://now.tufts.edu/articles/how-animals-help-us-during-covid-19-pandemic. Published Mach 30, 2020. Accessed June 21, 2020
9. Fitzpatrick KM, Harris C, Drawve G. Fear of COVID-19 and the mental health consequences in America [published online ahead of print, 2020 Jun 4]. Psychol Trauma . 2020;10.1037/tra0000924. doi:10.1037/tra0000924
10. Rajkumar RP. COVID-19 and mental health: A review of the existing literature [published online ahead of print, 2020 Apr 10]. Asian J Psychiatr. 2020;52:102066. doi:10.1016/j.ajp.2020.102066
11. The White House. Remarks by President Trump in cabinet meeting. https://www.whitehouse.gov/briefings-statements/remarks-president-trump-cabinet-meeting-17. Published May 19, 2020. Accessed June 21, 2020
12. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). COVID -19 and animals. https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/animals.html. Updated June 22, 2020. Accessed June 24, 2020.
13. Russell P. Could bio-detection dogs sniff out COVID-19 infection. https://www.medscape.com/viewarticle/930652. Published May 17, 2020. Accessed June 21, 2020.
14. Kundera M. The Unbearable Lightness of Being . New York: Harper & Row; 1984.
The editorials I have written so far in this series on COVID-19 have been on weighty topics as befits the serious situation of the pandemic, which as of June 30, 2020 had taken more than 500,000 lives across the globe and caused anguish and sorrow such as the world has not known since the 1918 influenza pandemic.2
The human spirit can bear only so much distress and tragedy before it is bowed and unable to stand. Stand though we must; not just against the inanimate invasion of viruses from the outside, but also our own endemic national tensions and conflicts. A periodic lifting of our burdens and a recharging of our psychological and spiritual energies are crucial to the resilience and flexibility that are necessary to walk the long difficult road ahead of us as a nation and as public servants in health care. This column takes a lighter look at COVID-19 and considers the restorative role companion animals, especially, for me, my beloved canines, have played in caring for and about us humans during the pandemic.
You will likely read this editorial during the official dog days of summer, which run from July 22 to August 22. We all may imagine a big dog laying on a porch in the American South while his owners drink lemonade and quietly rock in chairs watching the long lazy days pass in a simpler time.
However pleasant this bucolic picture, it has little to do with the origin of the expression, which dates back to ancient Greece. The dog refers not to our literal furry friends but, according to National Geographic (and who should know better), to the position of the “dog star” in a constellation in the night sky.3 Unfortunately, we cannot completely get away from the sobering theme of the pandemic: The rise of the star to prominence during the peak of the Mediterranean summer’s heat was a period associated with disaster and illness.
Real dogs, cats, and assorted other so-called pandemic pets, though, have been another type of star in this difficult period. Early in the shelter-in-place, pet adoptions from city and county animal shelters and rescue organizations skyrocketed.4 Although animal welfare experts have legitimate concerns that some of these adoptees will be surrendered if there is ever a return to normal. For now many people feel it is the perfect time to adopt, precisely because they now have space to bring a new member into the family. Before adopting, as a recent National Public Radio report emphasized, individuals should consider whether they truly have the resources both material and emotional to care for a pet.5 For those who take stock honestly and believe they have the room in their heart and budget, rescuing a companion is good psychological news, arguably even more for the human than for the animal.
Sheltering-in-place has reduced the transmission of the virus, which scientists estimate has saved thousands of lives.6 But it also has triggered a second health crisis, this time of mental health with an unprecedented increase in rates of depression, anxiety, suicide attempts, and substance use that is expected to worsen over the coming months and years.7 Companion animals certainly cannot solve this complex and mammoth public health problem; however, they can contribute in simple and small yet very significant ways to the mental health of individuals.8
Caring for a pet who shows unconditional love and loyalty to you can reduce isolation; foster hope; provide meaning, comfort, and cheer to you when you are down or afraid; and offer a routine and reason to get out of bed every day and take a walk outside. Research shows that those positive effects can decrease the risk of the very mental health conditions that are now plaguing us in such alarming numbers.9,10
“How many more lives are we willing to sacrifice in the name of containing the virus?” Elinore McCance-Katz, MD, PhD, the nation’s top mental health official ominously asked about the potential effects of another shutdown during a cabinet meeting.11 For some of us, a companion animal who does not require physical distancing (at least when you are healthy) may permit us to prevent the spread of the virus while protecting our mental health.
Nor is emotional support the only clinical way in which animals are helping pandemic- beleaguered humans. There is a low risk we can infect household pets, and dogs are not likely to transmit the virus. In fact, they even can be trained to serve as highly efficient virus testers who don’t need scarce reagents or carry high price tags—just a pat on the head and an occasional treat.12 Medscape reported that clinical trials starting in the United Kingdom are set to evaluate the accuracy of these “bio-detection” dogs. The story quotes a leading British public health official as saying, “Properly trained sniffer dogs could revolutionise our approach to this whole pandemic, screening 250 people an hour for the virus.”13
Canines are not only healers who can ease our troubles through the pandemic but also peacemakers. As injustice and violence rock the country, we would do well to imitate their attitudes of nonjudgmental acceptance. “Dogs are our link to paradise. They do not know evil or jealousy or discontent,” wrote novelist Milan Kundera. “To sit with a dog on a hillside on a glorious afternoon is to be back in Eden, where doing nothing was not boring—it was peace.”14 Those indeed would be dog days as when better nature we sometimes share with animals prevailed.
The editorials I have written so far in this series on COVID-19 have been on weighty topics as befits the serious situation of the pandemic, which as of June 30, 2020 had taken more than 500,000 lives across the globe and caused anguish and sorrow such as the world has not known since the 1918 influenza pandemic.2
The human spirit can bear only so much distress and tragedy before it is bowed and unable to stand. Stand though we must; not just against the inanimate invasion of viruses from the outside, but also our own endemic national tensions and conflicts. A periodic lifting of our burdens and a recharging of our psychological and spiritual energies are crucial to the resilience and flexibility that are necessary to walk the long difficult road ahead of us as a nation and as public servants in health care. This column takes a lighter look at COVID-19 and considers the restorative role companion animals, especially, for me, my beloved canines, have played in caring for and about us humans during the pandemic.
You will likely read this editorial during the official dog days of summer, which run from July 22 to August 22. We all may imagine a big dog laying on a porch in the American South while his owners drink lemonade and quietly rock in chairs watching the long lazy days pass in a simpler time.
However pleasant this bucolic picture, it has little to do with the origin of the expression, which dates back to ancient Greece. The dog refers not to our literal furry friends but, according to National Geographic (and who should know better), to the position of the “dog star” in a constellation in the night sky.3 Unfortunately, we cannot completely get away from the sobering theme of the pandemic: The rise of the star to prominence during the peak of the Mediterranean summer’s heat was a period associated with disaster and illness.
Real dogs, cats, and assorted other so-called pandemic pets, though, have been another type of star in this difficult period. Early in the shelter-in-place, pet adoptions from city and county animal shelters and rescue organizations skyrocketed.4 Although animal welfare experts have legitimate concerns that some of these adoptees will be surrendered if there is ever a return to normal. For now many people feel it is the perfect time to adopt, precisely because they now have space to bring a new member into the family. Before adopting, as a recent National Public Radio report emphasized, individuals should consider whether they truly have the resources both material and emotional to care for a pet.5 For those who take stock honestly and believe they have the room in their heart and budget, rescuing a companion is good psychological news, arguably even more for the human than for the animal.
Sheltering-in-place has reduced the transmission of the virus, which scientists estimate has saved thousands of lives.6 But it also has triggered a second health crisis, this time of mental health with an unprecedented increase in rates of depression, anxiety, suicide attempts, and substance use that is expected to worsen over the coming months and years.7 Companion animals certainly cannot solve this complex and mammoth public health problem; however, they can contribute in simple and small yet very significant ways to the mental health of individuals.8
Caring for a pet who shows unconditional love and loyalty to you can reduce isolation; foster hope; provide meaning, comfort, and cheer to you when you are down or afraid; and offer a routine and reason to get out of bed every day and take a walk outside. Research shows that those positive effects can decrease the risk of the very mental health conditions that are now plaguing us in such alarming numbers.9,10
“How many more lives are we willing to sacrifice in the name of containing the virus?” Elinore McCance-Katz, MD, PhD, the nation’s top mental health official ominously asked about the potential effects of another shutdown during a cabinet meeting.11 For some of us, a companion animal who does not require physical distancing (at least when you are healthy) may permit us to prevent the spread of the virus while protecting our mental health.
Nor is emotional support the only clinical way in which animals are helping pandemic- beleaguered humans. There is a low risk we can infect household pets, and dogs are not likely to transmit the virus. In fact, they even can be trained to serve as highly efficient virus testers who don’t need scarce reagents or carry high price tags—just a pat on the head and an occasional treat.12 Medscape reported that clinical trials starting in the United Kingdom are set to evaluate the accuracy of these “bio-detection” dogs. The story quotes a leading British public health official as saying, “Properly trained sniffer dogs could revolutionise our approach to this whole pandemic, screening 250 people an hour for the virus.”13
Canines are not only healers who can ease our troubles through the pandemic but also peacemakers. As injustice and violence rock the country, we would do well to imitate their attitudes of nonjudgmental acceptance. “Dogs are our link to paradise. They do not know evil or jealousy or discontent,” wrote novelist Milan Kundera. “To sit with a dog on a hillside on a glorious afternoon is to be back in Eden, where doing nothing was not boring—it was peace.”14 Those indeed would be dog days as when better nature we sometimes share with animals prevailed.
1. Buber M. I and Thou . Kaufmann W, trans. New York: Charles Scribner’s Sons: 1970:144.
2. World Health Organization. Coronavirus disease (COVID-19). Situation report-153. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200621-covid-19-sitrep-153.pdf?sfvrsn=c896464d_2. Published June 21, 2020. Accessed June 22, 2020.
3. Little B. Why do we call them the ‘dog days’ of summer. National Geographic . July 10, 2015. https://www.nationalgeographic.com/news/2015/07/150710-dog-days-summer-sirius-star-astronomy-weather-language. Accessed June 21, 2020.
4. Ellis EG. Thanks to sheltering in place, animal shelters are empty. https://www.wired.com/story/coronavirus-pet-adoption-boom. Published April 10, 2020. Accessed June 21, 2020.
5. Balaban S. Should I adopt a dog during the coronavirus crisis? Read this first. https://www.npr.org/2020/05/08/853088872/should-i-adopt-a-dog-during-the-coronavirus-crisis-read-this-first. Published May 11, 2020. Accessed June 21, 2020
6. Hsiang S, Allen D, Annan-Phan S, et al. The effect of large-scale anti-contagion policies on the COVID-19 pandemic [published online ahead of print, 2020 Jun 8]. Nature. 2020;10.1038/s41586-020-2404-8. doi:10.1038/s41586-020-2404-8
7. Galea S, Merchant RM, Lurie N. The Mental Health Consequences of COVID-19 and Physical Distancing: The Need for Prevention and Early Intervention [published online ahead of print, 2020 Apr 10]. JAMA Intern Med. 2020;10.1001/jamainternmed.2020.1562. doi:10.1001/jamainternmed.2020.1562
8. Rajewski G. How animals help us during the COVID-19 pandemic. https://now.tufts.edu/articles/how-animals-help-us-during-covid-19-pandemic. Published Mach 30, 2020. Accessed June 21, 2020
9. Fitzpatrick KM, Harris C, Drawve G. Fear of COVID-19 and the mental health consequences in America [published online ahead of print, 2020 Jun 4]. Psychol Trauma . 2020;10.1037/tra0000924. doi:10.1037/tra0000924
10. Rajkumar RP. COVID-19 and mental health: A review of the existing literature [published online ahead of print, 2020 Apr 10]. Asian J Psychiatr. 2020;52:102066. doi:10.1016/j.ajp.2020.102066
11. The White House. Remarks by President Trump in cabinet meeting. https://www.whitehouse.gov/briefings-statements/remarks-president-trump-cabinet-meeting-17. Published May 19, 2020. Accessed June 21, 2020
12. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). COVID -19 and animals. https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/animals.html. Updated June 22, 2020. Accessed June 24, 2020.
13. Russell P. Could bio-detection dogs sniff out COVID-19 infection. https://www.medscape.com/viewarticle/930652. Published May 17, 2020. Accessed June 21, 2020.
14. Kundera M. The Unbearable Lightness of Being . New York: Harper & Row; 1984.
1. Buber M. I and Thou . Kaufmann W, trans. New York: Charles Scribner’s Sons: 1970:144.
2. World Health Organization. Coronavirus disease (COVID-19). Situation report-153. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200621-covid-19-sitrep-153.pdf?sfvrsn=c896464d_2. Published June 21, 2020. Accessed June 22, 2020.
3. Little B. Why do we call them the ‘dog days’ of summer. National Geographic . July 10, 2015. https://www.nationalgeographic.com/news/2015/07/150710-dog-days-summer-sirius-star-astronomy-weather-language. Accessed June 21, 2020.
4. Ellis EG. Thanks to sheltering in place, animal shelters are empty. https://www.wired.com/story/coronavirus-pet-adoption-boom. Published April 10, 2020. Accessed June 21, 2020.
5. Balaban S. Should I adopt a dog during the coronavirus crisis? Read this first. https://www.npr.org/2020/05/08/853088872/should-i-adopt-a-dog-during-the-coronavirus-crisis-read-this-first. Published May 11, 2020. Accessed June 21, 2020
6. Hsiang S, Allen D, Annan-Phan S, et al. The effect of large-scale anti-contagion policies on the COVID-19 pandemic [published online ahead of print, 2020 Jun 8]. Nature. 2020;10.1038/s41586-020-2404-8. doi:10.1038/s41586-020-2404-8
7. Galea S, Merchant RM, Lurie N. The Mental Health Consequences of COVID-19 and Physical Distancing: The Need for Prevention and Early Intervention [published online ahead of print, 2020 Apr 10]. JAMA Intern Med. 2020;10.1001/jamainternmed.2020.1562. doi:10.1001/jamainternmed.2020.1562
8. Rajewski G. How animals help us during the COVID-19 pandemic. https://now.tufts.edu/articles/how-animals-help-us-during-covid-19-pandemic. Published Mach 30, 2020. Accessed June 21, 2020
9. Fitzpatrick KM, Harris C, Drawve G. Fear of COVID-19 and the mental health consequences in America [published online ahead of print, 2020 Jun 4]. Psychol Trauma . 2020;10.1037/tra0000924. doi:10.1037/tra0000924
10. Rajkumar RP. COVID-19 and mental health: A review of the existing literature [published online ahead of print, 2020 Apr 10]. Asian J Psychiatr. 2020;52:102066. doi:10.1016/j.ajp.2020.102066
11. The White House. Remarks by President Trump in cabinet meeting. https://www.whitehouse.gov/briefings-statements/remarks-president-trump-cabinet-meeting-17. Published May 19, 2020. Accessed June 21, 2020
12. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). COVID -19 and animals. https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/animals.html. Updated June 22, 2020. Accessed June 24, 2020.
13. Russell P. Could bio-detection dogs sniff out COVID-19 infection. https://www.medscape.com/viewarticle/930652. Published May 17, 2020. Accessed June 21, 2020.
14. Kundera M. The Unbearable Lightness of Being . New York: Harper & Row; 1984.
Wear N95 masks during endoscopy
COVID-19 guideline update
Nausea, vomiting, and diarrhea are gastrointestinal symptoms that COVID-19 patients have had, and up to 30% have been reported to have liver symptoms. Because patients with these symptoms may require endoscopy, the American Gastroenterological Association has issued a rapid recommendation document that advises physicians and health care workers to use N95 masks, double gloves, and negative-pressure rooms when performing GI procedures during the COVID-19 pandemic.
The recommendations, published in Gastroenterology (2020. doi: 10.1053/j.gastro.2020.03.072), also cover non–COVID-19 patients and situations where N95 masks should be used, offer guidelines for triaging patients for endoscopy and timing of nonurgent procedures, and evaluate the latest evidence in the incidence of GI and liver manifestations of COVID-19. The guideline panel met in March.
The document includes seven recommendations for use of personal protective equipment by physicians and nurses performing GI procedures. The recommendations and the level of evidence supporting them fall under four categories:
1. Masks, comprising four recommendations: use of N95 masks for upper and lower GI procedures regardless of a patient’s COVID-19 status; no use of only surgical masks in confirmed COVID-19 patients or suspected cases; and use of reused N95 masks when fresh ones aren’t available instead of using a surgical mask only (very low to moderate level of evidence depending on the recommendation).
2. Double-gloving when performing any GI procedure regardless of the patient’s COVID-19 status (moderate quality evidence).
3. When available, a negative-pressure room for any COVID-19 patient or suspect rather than a regular endoscopy room (very low certainty of evidence).
4. Standard cleaning, endoscopic disinfection, and reprocessing protocols regardless of a patient’s COVID-19 status (good practice statement).
For decontamination, the panel noted that commonly used biocidal agents, such as hydrogen peroxide, alcohols, sodium hypochlorite, or benzalkonium chloride have proved effective for decontaminating of coronavirus.
For implementing the PPE recommendations, the panel stated that personnel still need to practice don and doff standard protocols, and that N95 masks should be fitted for each individual.
Other steps include banning personal belongings in the procedure area; minimizing the number of personnel in the room; avoiding change of personnel and keeping nonprocedural personnel out during the procedure; considering use of nursing teams that follow the patient through preprocedure, procedure, and recovery, and considering having endoscopy teams remain together during the day to minimize exposure.
The triage recommendations stated that “trained medical personnel” should review all procedures and categorize them as time-sensitive or not time-sensitive, based on a framework the recommendation includes. In “an open-access endoscopy system” when there isn’t enough information to determine timing for the procedure, the recommendation provides a three-step approach: a phone consult with the referring physician, a telehealth visit with the patient, or a multidisciplinary team approach or virtual disease/tumor board.
“The proposed framework of separating procedures into time-sensitive and non–time-sensitive cases may be useful in determining which procedures if delayed may negatively impact on patient-important outcomes,” wrote Shahnaz Sultan, MD, AGAF, of the University of Minnesota, Minneapolis, and colleagues. The panel noted decision-making should focus on “patient-important outcomes.”
For nonurgent procedures, the panel arrived at a consensus that 8 weeks was an appropriate window for reassessment of deferred procedures, depending on the availability of resources and if the time-sensitivity of the procedure changes.
The panel also attempted to determine the likelihood of GI and liver manifestations of COVID-19 by evaluating published cohort studies. They found that 2%-13.8% of patients had diarrhea, 1%-10.1% had nausea or vomiting, and one study reported 2% had abdominal pain (Am J Gastroenterol. 2020 May;115[5]766-73). What’s more, some studies have shown stool samples positive for SARS-CoV-2 RNA even after respiratory samples were negative.
The evidence on liver manifestations isn’t as robust, but one study reported that 20%-30% of patients had liver injury upon diagnosis of COVID-19 (Gastroenterology. 2020;158:1518-9), and that severe hepatitis has been reported but liver failure seems rare (Lancet. 2020 Feb 15;395[10223]:507-13). “The pattern of liver injury appears to be predominantly hepatocellular, and the etiology remains uncertain but may represent a secondary effect of the systemic inflammatory response observed with COVID-19 disease, although direct viral infection and drug-induced liver injury cannot be excluded,” Dr. Sultan and colleagues noted.
There were no relevant author conflicts of interest. The American Gastroenterological Association (AGA) Institute funded the study.
SOURCE: Sultan S et al. Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.03.072.
Check out the AGA COVID-19 Resource Library for new clinical guidance, education, research and physician resources, including recent guidance on how to treat patients with IBD during the pandemic, at www.gastro.org/covid.
COVID-19 guideline update
COVID-19 guideline update
Nausea, vomiting, and diarrhea are gastrointestinal symptoms that COVID-19 patients have had, and up to 30% have been reported to have liver symptoms. Because patients with these symptoms may require endoscopy, the American Gastroenterological Association has issued a rapid recommendation document that advises physicians and health care workers to use N95 masks, double gloves, and negative-pressure rooms when performing GI procedures during the COVID-19 pandemic.
The recommendations, published in Gastroenterology (2020. doi: 10.1053/j.gastro.2020.03.072), also cover non–COVID-19 patients and situations where N95 masks should be used, offer guidelines for triaging patients for endoscopy and timing of nonurgent procedures, and evaluate the latest evidence in the incidence of GI and liver manifestations of COVID-19. The guideline panel met in March.
The document includes seven recommendations for use of personal protective equipment by physicians and nurses performing GI procedures. The recommendations and the level of evidence supporting them fall under four categories:
1. Masks, comprising four recommendations: use of N95 masks for upper and lower GI procedures regardless of a patient’s COVID-19 status; no use of only surgical masks in confirmed COVID-19 patients or suspected cases; and use of reused N95 masks when fresh ones aren’t available instead of using a surgical mask only (very low to moderate level of evidence depending on the recommendation).
2. Double-gloving when performing any GI procedure regardless of the patient’s COVID-19 status (moderate quality evidence).
3. When available, a negative-pressure room for any COVID-19 patient or suspect rather than a regular endoscopy room (very low certainty of evidence).
4. Standard cleaning, endoscopic disinfection, and reprocessing protocols regardless of a patient’s COVID-19 status (good practice statement).
For decontamination, the panel noted that commonly used biocidal agents, such as hydrogen peroxide, alcohols, sodium hypochlorite, or benzalkonium chloride have proved effective for decontaminating of coronavirus.
For implementing the PPE recommendations, the panel stated that personnel still need to practice don and doff standard protocols, and that N95 masks should be fitted for each individual.
Other steps include banning personal belongings in the procedure area; minimizing the number of personnel in the room; avoiding change of personnel and keeping nonprocedural personnel out during the procedure; considering use of nursing teams that follow the patient through preprocedure, procedure, and recovery, and considering having endoscopy teams remain together during the day to minimize exposure.
The triage recommendations stated that “trained medical personnel” should review all procedures and categorize them as time-sensitive or not time-sensitive, based on a framework the recommendation includes. In “an open-access endoscopy system” when there isn’t enough information to determine timing for the procedure, the recommendation provides a three-step approach: a phone consult with the referring physician, a telehealth visit with the patient, or a multidisciplinary team approach or virtual disease/tumor board.
“The proposed framework of separating procedures into time-sensitive and non–time-sensitive cases may be useful in determining which procedures if delayed may negatively impact on patient-important outcomes,” wrote Shahnaz Sultan, MD, AGAF, of the University of Minnesota, Minneapolis, and colleagues. The panel noted decision-making should focus on “patient-important outcomes.”
For nonurgent procedures, the panel arrived at a consensus that 8 weeks was an appropriate window for reassessment of deferred procedures, depending on the availability of resources and if the time-sensitivity of the procedure changes.
The panel also attempted to determine the likelihood of GI and liver manifestations of COVID-19 by evaluating published cohort studies. They found that 2%-13.8% of patients had diarrhea, 1%-10.1% had nausea or vomiting, and one study reported 2% had abdominal pain (Am J Gastroenterol. 2020 May;115[5]766-73). What’s more, some studies have shown stool samples positive for SARS-CoV-2 RNA even after respiratory samples were negative.
The evidence on liver manifestations isn’t as robust, but one study reported that 20%-30% of patients had liver injury upon diagnosis of COVID-19 (Gastroenterology. 2020;158:1518-9), and that severe hepatitis has been reported but liver failure seems rare (Lancet. 2020 Feb 15;395[10223]:507-13). “The pattern of liver injury appears to be predominantly hepatocellular, and the etiology remains uncertain but may represent a secondary effect of the systemic inflammatory response observed with COVID-19 disease, although direct viral infection and drug-induced liver injury cannot be excluded,” Dr. Sultan and colleagues noted.
There were no relevant author conflicts of interest. The American Gastroenterological Association (AGA) Institute funded the study.
SOURCE: Sultan S et al. Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.03.072.
Check out the AGA COVID-19 Resource Library for new clinical guidance, education, research and physician resources, including recent guidance on how to treat patients with IBD during the pandemic, at www.gastro.org/covid.
Nausea, vomiting, and diarrhea are gastrointestinal symptoms that COVID-19 patients have had, and up to 30% have been reported to have liver symptoms. Because patients with these symptoms may require endoscopy, the American Gastroenterological Association has issued a rapid recommendation document that advises physicians and health care workers to use N95 masks, double gloves, and negative-pressure rooms when performing GI procedures during the COVID-19 pandemic.
The recommendations, published in Gastroenterology (2020. doi: 10.1053/j.gastro.2020.03.072), also cover non–COVID-19 patients and situations where N95 masks should be used, offer guidelines for triaging patients for endoscopy and timing of nonurgent procedures, and evaluate the latest evidence in the incidence of GI and liver manifestations of COVID-19. The guideline panel met in March.
The document includes seven recommendations for use of personal protective equipment by physicians and nurses performing GI procedures. The recommendations and the level of evidence supporting them fall under four categories:
1. Masks, comprising four recommendations: use of N95 masks for upper and lower GI procedures regardless of a patient’s COVID-19 status; no use of only surgical masks in confirmed COVID-19 patients or suspected cases; and use of reused N95 masks when fresh ones aren’t available instead of using a surgical mask only (very low to moderate level of evidence depending on the recommendation).
2. Double-gloving when performing any GI procedure regardless of the patient’s COVID-19 status (moderate quality evidence).
3. When available, a negative-pressure room for any COVID-19 patient or suspect rather than a regular endoscopy room (very low certainty of evidence).
4. Standard cleaning, endoscopic disinfection, and reprocessing protocols regardless of a patient’s COVID-19 status (good practice statement).
For decontamination, the panel noted that commonly used biocidal agents, such as hydrogen peroxide, alcohols, sodium hypochlorite, or benzalkonium chloride have proved effective for decontaminating of coronavirus.
For implementing the PPE recommendations, the panel stated that personnel still need to practice don and doff standard protocols, and that N95 masks should be fitted for each individual.
Other steps include banning personal belongings in the procedure area; minimizing the number of personnel in the room; avoiding change of personnel and keeping nonprocedural personnel out during the procedure; considering use of nursing teams that follow the patient through preprocedure, procedure, and recovery, and considering having endoscopy teams remain together during the day to minimize exposure.
The triage recommendations stated that “trained medical personnel” should review all procedures and categorize them as time-sensitive or not time-sensitive, based on a framework the recommendation includes. In “an open-access endoscopy system” when there isn’t enough information to determine timing for the procedure, the recommendation provides a three-step approach: a phone consult with the referring physician, a telehealth visit with the patient, or a multidisciplinary team approach or virtual disease/tumor board.
“The proposed framework of separating procedures into time-sensitive and non–time-sensitive cases may be useful in determining which procedures if delayed may negatively impact on patient-important outcomes,” wrote Shahnaz Sultan, MD, AGAF, of the University of Minnesota, Minneapolis, and colleagues. The panel noted decision-making should focus on “patient-important outcomes.”
For nonurgent procedures, the panel arrived at a consensus that 8 weeks was an appropriate window for reassessment of deferred procedures, depending on the availability of resources and if the time-sensitivity of the procedure changes.
The panel also attempted to determine the likelihood of GI and liver manifestations of COVID-19 by evaluating published cohort studies. They found that 2%-13.8% of patients had diarrhea, 1%-10.1% had nausea or vomiting, and one study reported 2% had abdominal pain (Am J Gastroenterol. 2020 May;115[5]766-73). What’s more, some studies have shown stool samples positive for SARS-CoV-2 RNA even after respiratory samples were negative.
The evidence on liver manifestations isn’t as robust, but one study reported that 20%-30% of patients had liver injury upon diagnosis of COVID-19 (Gastroenterology. 2020;158:1518-9), and that severe hepatitis has been reported but liver failure seems rare (Lancet. 2020 Feb 15;395[10223]:507-13). “The pattern of liver injury appears to be predominantly hepatocellular, and the etiology remains uncertain but may represent a secondary effect of the systemic inflammatory response observed with COVID-19 disease, although direct viral infection and drug-induced liver injury cannot be excluded,” Dr. Sultan and colleagues noted.
There were no relevant author conflicts of interest. The American Gastroenterological Association (AGA) Institute funded the study.
SOURCE: Sultan S et al. Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.03.072.
Check out the AGA COVID-19 Resource Library for new clinical guidance, education, research and physician resources, including recent guidance on how to treat patients with IBD during the pandemic, at www.gastro.org/covid.
A 72-year-old with an acute, pruritic, bullous eruption involving his right pretibial extremity
Localized bullous pemphigoid
with a predilection in the elderly population.1
Localized variants of bullous pemphigoid (BP) are rare and have been reported to arise at sites of mechanical trauma, prior radiation, lymphedema, surgical scars, burns, fistulas, and ostomies.1-3 Although the mechanism remains unclear, the Koebner phenomenon is thought to induce dysregulation of immunologic and vascular factors in sites of mechanical shear and trauma in susceptible individuals.3
Localized BP is an important entity for the dermatologist to be familiar with, as the diagnosis is often delayed. The localized, well-defined skin lesions frequently mimic contact dermatitis. In fact, previous reports have shown the most likely misdiagnosis of localized BP is acute allergic contact dermatitis, stasis dermatitis, and eczematous dermatitis.4,5
In this patient, histopathologic examination of a biopsy revealed a subepidermal blister with numerous eosinophils. Direct immunofluorescence study of perilesional skin showed strong linear IgG and C3 deposits at the basal membrane level. Serum level of autoantibody to BP180 antigen was elevated. Bacterial culture was positive for Staphylococcus aureus. These findings were suggestive of unilateral, localized BP with superimposed bacterial infection. Initial treatment with an extended course of doxycycline 200 mg twice daily, topical triamcinolone 0.1% ointment twice daily with compression therapy, and leg elevation led to clinical improvement with healing of previous lesions on the leg. At follow-up 3 weeks later, the patient had continued to develop new bullous lesions on the trunk and upper thighs. He was subsequently started on systemic immunosuppressive therapy for generalized bullous pemphigoid.
Importantly, localized BP generally follows a more benign disease course, although long-term follow-up is recommended for monitoring given the potential risk of developing the generalized form of BP of approximately 15%.3 Topical corticosteroids and oral antibiotics are recommended as the first-line therapy in these patients, with an escalated systemic therapy if needed for disease progression.3,5
Our case represents an important differential diagnosis to consider when evaluating an acute localized bullous eruption in an elderly patient.
Dr. Cusick and Dr. Dolohanty are with the department of dermatology, University of Rochester (N.Y.), and provided the case and photo. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kohroh K et al. J Dermatol. 2007 Jul;34(7):482-5.
2. Nguyen T et al. Dermatology 2014;229(2):88-96.
3. Sen BB et al. Indian J Dermatol Venereol Leprol. 2013;79(4):554.
4. Salomon RJ et al. Arch Dermatol. 1987 Mar;123(3):389-92.
5. Tran JT, Mutasim DF. Int J Dermatol. 2005 Nov;44(11):942-5.
Localized bullous pemphigoid
with a predilection in the elderly population.1
Localized variants of bullous pemphigoid (BP) are rare and have been reported to arise at sites of mechanical trauma, prior radiation, lymphedema, surgical scars, burns, fistulas, and ostomies.1-3 Although the mechanism remains unclear, the Koebner phenomenon is thought to induce dysregulation of immunologic and vascular factors in sites of mechanical shear and trauma in susceptible individuals.3
Localized BP is an important entity for the dermatologist to be familiar with, as the diagnosis is often delayed. The localized, well-defined skin lesions frequently mimic contact dermatitis. In fact, previous reports have shown the most likely misdiagnosis of localized BP is acute allergic contact dermatitis, stasis dermatitis, and eczematous dermatitis.4,5
In this patient, histopathologic examination of a biopsy revealed a subepidermal blister with numerous eosinophils. Direct immunofluorescence study of perilesional skin showed strong linear IgG and C3 deposits at the basal membrane level. Serum level of autoantibody to BP180 antigen was elevated. Bacterial culture was positive for Staphylococcus aureus. These findings were suggestive of unilateral, localized BP with superimposed bacterial infection. Initial treatment with an extended course of doxycycline 200 mg twice daily, topical triamcinolone 0.1% ointment twice daily with compression therapy, and leg elevation led to clinical improvement with healing of previous lesions on the leg. At follow-up 3 weeks later, the patient had continued to develop new bullous lesions on the trunk and upper thighs. He was subsequently started on systemic immunosuppressive therapy for generalized bullous pemphigoid.
Importantly, localized BP generally follows a more benign disease course, although long-term follow-up is recommended for monitoring given the potential risk of developing the generalized form of BP of approximately 15%.3 Topical corticosteroids and oral antibiotics are recommended as the first-line therapy in these patients, with an escalated systemic therapy if needed for disease progression.3,5
Our case represents an important differential diagnosis to consider when evaluating an acute localized bullous eruption in an elderly patient.
Dr. Cusick and Dr. Dolohanty are with the department of dermatology, University of Rochester (N.Y.), and provided the case and photo. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kohroh K et al. J Dermatol. 2007 Jul;34(7):482-5.
2. Nguyen T et al. Dermatology 2014;229(2):88-96.
3. Sen BB et al. Indian J Dermatol Venereol Leprol. 2013;79(4):554.
4. Salomon RJ et al. Arch Dermatol. 1987 Mar;123(3):389-92.
5. Tran JT, Mutasim DF. Int J Dermatol. 2005 Nov;44(11):942-5.
Localized bullous pemphigoid
with a predilection in the elderly population.1
Localized variants of bullous pemphigoid (BP) are rare and have been reported to arise at sites of mechanical trauma, prior radiation, lymphedema, surgical scars, burns, fistulas, and ostomies.1-3 Although the mechanism remains unclear, the Koebner phenomenon is thought to induce dysregulation of immunologic and vascular factors in sites of mechanical shear and trauma in susceptible individuals.3
Localized BP is an important entity for the dermatologist to be familiar with, as the diagnosis is often delayed. The localized, well-defined skin lesions frequently mimic contact dermatitis. In fact, previous reports have shown the most likely misdiagnosis of localized BP is acute allergic contact dermatitis, stasis dermatitis, and eczematous dermatitis.4,5
In this patient, histopathologic examination of a biopsy revealed a subepidermal blister with numerous eosinophils. Direct immunofluorescence study of perilesional skin showed strong linear IgG and C3 deposits at the basal membrane level. Serum level of autoantibody to BP180 antigen was elevated. Bacterial culture was positive for Staphylococcus aureus. These findings were suggestive of unilateral, localized BP with superimposed bacterial infection. Initial treatment with an extended course of doxycycline 200 mg twice daily, topical triamcinolone 0.1% ointment twice daily with compression therapy, and leg elevation led to clinical improvement with healing of previous lesions on the leg. At follow-up 3 weeks later, the patient had continued to develop new bullous lesions on the trunk and upper thighs. He was subsequently started on systemic immunosuppressive therapy for generalized bullous pemphigoid.
Importantly, localized BP generally follows a more benign disease course, although long-term follow-up is recommended for monitoring given the potential risk of developing the generalized form of BP of approximately 15%.3 Topical corticosteroids and oral antibiotics are recommended as the first-line therapy in these patients, with an escalated systemic therapy if needed for disease progression.3,5
Our case represents an important differential diagnosis to consider when evaluating an acute localized bullous eruption in an elderly patient.
Dr. Cusick and Dr. Dolohanty are with the department of dermatology, University of Rochester (N.Y.), and provided the case and photo. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kohroh K et al. J Dermatol. 2007 Jul;34(7):482-5.
2. Nguyen T et al. Dermatology 2014;229(2):88-96.
3. Sen BB et al. Indian J Dermatol Venereol Leprol. 2013;79(4):554.
4. Salomon RJ et al. Arch Dermatol. 1987 Mar;123(3):389-92.
5. Tran JT, Mutasim DF. Int J Dermatol. 2005 Nov;44(11):942-5.