User login
New Insights Into the Dermatology Residency Application Process Amid the COVID-19 Pandemic
Residency application is an arduous experience for many medical students. The National Resident Matching Program reported that US medical school seniors who matched into dermatology applied to a median of 90 programs and attended 9 interviews in 2019.1 High application and interview travel costs are a disadvantage for applicants from lower socioeconomic backgrounds. We propose that the coronavirus disease 2019 (COVID-19) pandemic should serve as a call to action for dermatology to update and promote a more equitable, time-effective, and cost-efficient residency interview process.
In light of COVID-19, dermatology residency program directors have recommended a holistic application review process, taking into consideration “disparities in strength of applications due to lack of opportunity for students with smaller home programs or in areas more affected by this crisis.”2 However, in a 2018 survey of 180 dermatology faculty members, 80% stated that time spent reviewing residency applications was already excessive.3 The Association of American Medical Colleges reported that for medical student applicants with US Medical Licensing Examination Step 1 scores lower than 237 or higher than 251, the value added by submitting one additional application beyond means of 43 (95% confidence interval [CI], 34-53) and 34 (95% CI, 28-41), respectively, is reduced relative to the value added by each application before reaching the point of diminishing returns.4 Therefore, we suggest limiting the number of applications per applicant to the upper bounds of the CI for the lower US Medical Licensing Examination Step 1 score (53), facilitating a more detailed review of fewer applications by each program and limiting student expenses.
On May 7, 2020, the Association of American Medical Colleges made a statement strongly encouraging medical school and teaching hospital faculty to conduct interviews through videoconferencing.5 Videoconferencing interviews (VCIs) minimize travel-associated health risks, providing a more equitable structure for applicants and programs in areas disproportionately impacted by the pandemic. In the 2018 survey of dermatology faculty members, only 11% believed that applicants interviewing virtually received equal consideration to those interviewing in person; a solution to this problem would be to mandate that all applicants use VCIs during the COVID-19 pandemic.3 This coming year, residency programs may elect to replace in-person interviews with VCIs, or they may utilize VCIs as screening tools to narrow down the applicant pool and for students to rank their preferred programs prior to an in-person interview. By inviting fewer applicants for in-person interviews, travel-associated health risks, financial costs, and missed educational activities would be minimized. Given that many medical students have had academic activities cancelled or postponed due to COVID-19, student opportunities for live clinical experiences should be maximized.
As programs plan for future application cycles beyond COVID-19, they must work to balance competing interests. Videoconferencing interviews allow for improved access to interviewing for applicants of lower socioeconomic classes, improved geographic mobility of applicants, and increased flexibility in accommodating faculty schedules with reduced time away from patient care and research; however, with VCIs one may lose the personal element that comes from the in-person interview, including interactions among applicants, faculty, current residents, and staff on the day of interview, as well as the departmental tour. Additionally, the quality of VCIs may be diminished by technical difficulties and the possibility of distractions, making standardization of the interview experience for applicants challenging.
The COVID-19 pandemic will surely leave its mark on the residency application cycle. We must take time now to collaborate and brainstorm creative solutions to maximize the equity and efficiency of the application process for both residency programs and students. We welcome reader feedback on these ideas and other possible solutions in the form of Letters to the Editor.
- National Resident Matching Program. Results of the 2019 NRMP Applicant Survey by Preferred Specialty and Applicant Type. Washington, DC: National Resident Matching Program; 2019. https://mk0nrmp3oyqui6wqfm.kinstacdn.com/wp-content/uploads/2019/06/Applicant-Survey-Report-2019.pdf. Accessed June 22, 2020.
- Association of American Medical Colleges. Specialty response to COVID-19: dermatology residency program director consensus statement on 2020-21 application cycle. https://aamc-orange.global.ssl.fastly.net/production/media/filer_
public/0f/7b/0f7b547e-65b5-4d93-8247-951206e7f726/updated_dermatology_program_director_
statement_on_2020-21_application_cycle_.pdf. Updated June 1, 2020. Accessed June 24, 2020. - Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159.
- Association of American Medical Colleges. Apply smart: data to consider when applying to residency. https://www.students-residents.aamc.org/applying-residency/filteredresult/apply-smart-data-consider-when-applying-residency/. Accessed June 22, 2020.
- Association of American Medical Colleges. Conducting interviews during the coronavirus pandemic. https://www.aamc.org/what-we-do/mission-areas/medical-education/conducting-interviews-during-coronavirus-pandemic/. Published May 7, 2020. Accessed June 22, 2020.
Residency application is an arduous experience for many medical students. The National Resident Matching Program reported that US medical school seniors who matched into dermatology applied to a median of 90 programs and attended 9 interviews in 2019.1 High application and interview travel costs are a disadvantage for applicants from lower socioeconomic backgrounds. We propose that the coronavirus disease 2019 (COVID-19) pandemic should serve as a call to action for dermatology to update and promote a more equitable, time-effective, and cost-efficient residency interview process.
In light of COVID-19, dermatology residency program directors have recommended a holistic application review process, taking into consideration “disparities in strength of applications due to lack of opportunity for students with smaller home programs or in areas more affected by this crisis.”2 However, in a 2018 survey of 180 dermatology faculty members, 80% stated that time spent reviewing residency applications was already excessive.3 The Association of American Medical Colleges reported that for medical student applicants with US Medical Licensing Examination Step 1 scores lower than 237 or higher than 251, the value added by submitting one additional application beyond means of 43 (95% confidence interval [CI], 34-53) and 34 (95% CI, 28-41), respectively, is reduced relative to the value added by each application before reaching the point of diminishing returns.4 Therefore, we suggest limiting the number of applications per applicant to the upper bounds of the CI for the lower US Medical Licensing Examination Step 1 score (53), facilitating a more detailed review of fewer applications by each program and limiting student expenses.
On May 7, 2020, the Association of American Medical Colleges made a statement strongly encouraging medical school and teaching hospital faculty to conduct interviews through videoconferencing.5 Videoconferencing interviews (VCIs) minimize travel-associated health risks, providing a more equitable structure for applicants and programs in areas disproportionately impacted by the pandemic. In the 2018 survey of dermatology faculty members, only 11% believed that applicants interviewing virtually received equal consideration to those interviewing in person; a solution to this problem would be to mandate that all applicants use VCIs during the COVID-19 pandemic.3 This coming year, residency programs may elect to replace in-person interviews with VCIs, or they may utilize VCIs as screening tools to narrow down the applicant pool and for students to rank their preferred programs prior to an in-person interview. By inviting fewer applicants for in-person interviews, travel-associated health risks, financial costs, and missed educational activities would be minimized. Given that many medical students have had academic activities cancelled or postponed due to COVID-19, student opportunities for live clinical experiences should be maximized.
As programs plan for future application cycles beyond COVID-19, they must work to balance competing interests. Videoconferencing interviews allow for improved access to interviewing for applicants of lower socioeconomic classes, improved geographic mobility of applicants, and increased flexibility in accommodating faculty schedules with reduced time away from patient care and research; however, with VCIs one may lose the personal element that comes from the in-person interview, including interactions among applicants, faculty, current residents, and staff on the day of interview, as well as the departmental tour. Additionally, the quality of VCIs may be diminished by technical difficulties and the possibility of distractions, making standardization of the interview experience for applicants challenging.
The COVID-19 pandemic will surely leave its mark on the residency application cycle. We must take time now to collaborate and brainstorm creative solutions to maximize the equity and efficiency of the application process for both residency programs and students. We welcome reader feedback on these ideas and other possible solutions in the form of Letters to the Editor.
Residency application is an arduous experience for many medical students. The National Resident Matching Program reported that US medical school seniors who matched into dermatology applied to a median of 90 programs and attended 9 interviews in 2019.1 High application and interview travel costs are a disadvantage for applicants from lower socioeconomic backgrounds. We propose that the coronavirus disease 2019 (COVID-19) pandemic should serve as a call to action for dermatology to update and promote a more equitable, time-effective, and cost-efficient residency interview process.
In light of COVID-19, dermatology residency program directors have recommended a holistic application review process, taking into consideration “disparities in strength of applications due to lack of opportunity for students with smaller home programs or in areas more affected by this crisis.”2 However, in a 2018 survey of 180 dermatology faculty members, 80% stated that time spent reviewing residency applications was already excessive.3 The Association of American Medical Colleges reported that for medical student applicants with US Medical Licensing Examination Step 1 scores lower than 237 or higher than 251, the value added by submitting one additional application beyond means of 43 (95% confidence interval [CI], 34-53) and 34 (95% CI, 28-41), respectively, is reduced relative to the value added by each application before reaching the point of diminishing returns.4 Therefore, we suggest limiting the number of applications per applicant to the upper bounds of the CI for the lower US Medical Licensing Examination Step 1 score (53), facilitating a more detailed review of fewer applications by each program and limiting student expenses.
On May 7, 2020, the Association of American Medical Colleges made a statement strongly encouraging medical school and teaching hospital faculty to conduct interviews through videoconferencing.5 Videoconferencing interviews (VCIs) minimize travel-associated health risks, providing a more equitable structure for applicants and programs in areas disproportionately impacted by the pandemic. In the 2018 survey of dermatology faculty members, only 11% believed that applicants interviewing virtually received equal consideration to those interviewing in person; a solution to this problem would be to mandate that all applicants use VCIs during the COVID-19 pandemic.3 This coming year, residency programs may elect to replace in-person interviews with VCIs, or they may utilize VCIs as screening tools to narrow down the applicant pool and for students to rank their preferred programs prior to an in-person interview. By inviting fewer applicants for in-person interviews, travel-associated health risks, financial costs, and missed educational activities would be minimized. Given that many medical students have had academic activities cancelled or postponed due to COVID-19, student opportunities for live clinical experiences should be maximized.
As programs plan for future application cycles beyond COVID-19, they must work to balance competing interests. Videoconferencing interviews allow for improved access to interviewing for applicants of lower socioeconomic classes, improved geographic mobility of applicants, and increased flexibility in accommodating faculty schedules with reduced time away from patient care and research; however, with VCIs one may lose the personal element that comes from the in-person interview, including interactions among applicants, faculty, current residents, and staff on the day of interview, as well as the departmental tour. Additionally, the quality of VCIs may be diminished by technical difficulties and the possibility of distractions, making standardization of the interview experience for applicants challenging.
The COVID-19 pandemic will surely leave its mark on the residency application cycle. We must take time now to collaborate and brainstorm creative solutions to maximize the equity and efficiency of the application process for both residency programs and students. We welcome reader feedback on these ideas and other possible solutions in the form of Letters to the Editor.
- National Resident Matching Program. Results of the 2019 NRMP Applicant Survey by Preferred Specialty and Applicant Type. Washington, DC: National Resident Matching Program; 2019. https://mk0nrmp3oyqui6wqfm.kinstacdn.com/wp-content/uploads/2019/06/Applicant-Survey-Report-2019.pdf. Accessed June 22, 2020.
- Association of American Medical Colleges. Specialty response to COVID-19: dermatology residency program director consensus statement on 2020-21 application cycle. https://aamc-orange.global.ssl.fastly.net/production/media/filer_
public/0f/7b/0f7b547e-65b5-4d93-8247-951206e7f726/updated_dermatology_program_director_
statement_on_2020-21_application_cycle_.pdf. Updated June 1, 2020. Accessed June 24, 2020. - Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159.
- Association of American Medical Colleges. Apply smart: data to consider when applying to residency. https://www.students-residents.aamc.org/applying-residency/filteredresult/apply-smart-data-consider-when-applying-residency/. Accessed June 22, 2020.
- Association of American Medical Colleges. Conducting interviews during the coronavirus pandemic. https://www.aamc.org/what-we-do/mission-areas/medical-education/conducting-interviews-during-coronavirus-pandemic/. Published May 7, 2020. Accessed June 22, 2020.
- National Resident Matching Program. Results of the 2019 NRMP Applicant Survey by Preferred Specialty and Applicant Type. Washington, DC: National Resident Matching Program; 2019. https://mk0nrmp3oyqui6wqfm.kinstacdn.com/wp-content/uploads/2019/06/Applicant-Survey-Report-2019.pdf. Accessed June 22, 2020.
- Association of American Medical Colleges. Specialty response to COVID-19: dermatology residency program director consensus statement on 2020-21 application cycle. https://aamc-orange.global.ssl.fastly.net/production/media/filer_
public/0f/7b/0f7b547e-65b5-4d93-8247-951206e7f726/updated_dermatology_program_director_
statement_on_2020-21_application_cycle_.pdf. Updated June 1, 2020. Accessed June 24, 2020. - Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159.
- Association of American Medical Colleges. Apply smart: data to consider when applying to residency. https://www.students-residents.aamc.org/applying-residency/filteredresult/apply-smart-data-consider-when-applying-residency/. Accessed June 22, 2020.
- Association of American Medical Colleges. Conducting interviews during the coronavirus pandemic. https://www.aamc.org/what-we-do/mission-areas/medical-education/conducting-interviews-during-coronavirus-pandemic/. Published May 7, 2020. Accessed June 22, 2020.
Practice Points
- We propose that the coronavirus disease 2019 pandemic should serve as a call to action for dermatology to update and promote a more equitable, time-effective, and cost-efficient residency interview process.
- A limitation on the number of applications per candidate may lower expenses and allow for a more holistic review process by residency programs.
- The benefits and challenges of videoconferencing interviews must be weighed as residency programs decide on their continued use beyond this application cycle.
What’s Eating You? Megalopyge opercularis
Lepidoptera is the second largest order of the class Insecta and comprises approximately 160,000 species of butterflies and moths classified among approximately 124 families and subfamilies. Venomous properties have been identified in 12 of these families, posing a serious threat to human health. 1
The clinical manifestations from Lepidoptera envenomation can range from general systemic symptoms such as fever and abdominal distress; to more complex focal affections including hemorrhage, ophthalmologic lesions, and irritation of the respiratory tracts; to less severe reactions of the skin, which are the most common presentation.1
Terminology
Lepidopterism is the term used to address a clinical spectrum of systemic manifestations from direct contact with venomous butterflies or moths and/or their products.2 Conversely, erucism is a term used to describe localized cutaneous reactions after direct contact with toxins from caterpillars.
Lepidopterism is derived from the Greek roots lepis, meaning scale, and pteron, meaning wing. The term erucism stems from the Latin word eruca, which means larva.2
Ideally, lepidopterism should refer solely to reactions from butterflies and moths—adult forms of insects with scaly wings—while erucism should refer to reactions from contact with caterpillars—the larval form of butterflies and moths.
In common use, lepidopterism can describe any reaction from caterpillars, moths, or adult butterflies, as well as any case of Lepidoptera exposure with only systemic manifestations, regardless of cutaneous findings. Concurrently, erucism has been defined as either any reaction from caterpillars or any skin reaction from contact with caterpillars or moths.2
Because caterpillars are the larval form of butterflies and moths, caterpillar-associated skin reactions also have been conveniently denominated caterpillar dermatitis.1 Henceforth in this article, both terms erucism and caterpillar dermatitis are used interchangeably.
Caterpillar Envenomation
Caterpillars cause the vast majority of adverse events from lepidopteran exposures.2 Envenomation by caterpillars might stand as the world’s most common envenomation given the larvae proximity to humans.3 Although involvement of internal organs (eg, renal failure), cerebral hemorrhage, and joint lesions can occur, skin manifestations are more predominant with the majority of species. Initial localized pain, edema, and erythema usually are present at the site of direct contact and subsequently progress toward maculopapular to bullous lesions, erosions, petechiae, necrosis, and ulceration depending on the offending species.1,4
Megalopyge opercularis
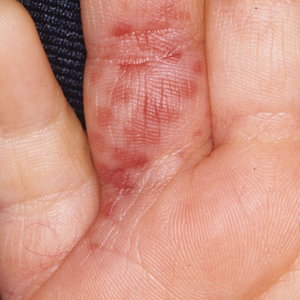
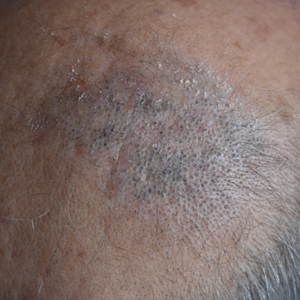
In the United States, more than 50 species of caterpillars have been identified as poisonous or venomous.5 Megalopyge opercularis (Figure 1), the larval form of the flannel moth, is an important cause of caterpillar-associated dermatitis in the southern United States.6,7 Megalopyge opercularis also is commonly known as the puss caterpillar, opossum bug, wooly slug, el perrito, tree asp, or Italian asp.6 This lepidopteran insect is mainly found in the southeastern and southcentral United States, with noted particular abundance in Texas, Louisiana, and Florida.6,8 The puss caterpillar has 2 generations per year; the first develops during the months of June to July, and the second develops from September to October, carrying seasonal health hazards.6,8

Megalopyge opercularis is tapered at the ends and can measure 2.5 to 3.5×1 cm at maturity. It is covered by silky, long-streaked, wavy hairs that may appear single colored or as a mix of colors—from white to gray to brown—forming a mid-dorsal crest.6 Beneath this furry coat, rows of short sharp spines are hidden. Upon contact with the human skin, these spines will break and discharge venom.1,6,8 Toxins contained within the hollow spines are thought to be produced by specialized basal cells, but there still is little knowledge about the dynamics and composition of the venom.1
Clinical Manifestations
The severity of the reaction depends on the caterpillar’s size and the extent of contact.1,4 Contact with M opercularis instantly presents with a throbbing or burning pain that may be followed by localized erythema and rash.1,6 A characteristic gridlike pattern of erythematous macules develops, reflecting each site of puncture from the insect’s spines (Figure 2).8,9 Skin lesions can progress from erythematous macules to hemorrhagic vesicles or pustules, usually self-resolving after a few days. The reaction also can present with radiating pain to regional lymph nodes and numbness of the affected area.1,6,8 Moreover, some patients may report urticaria and pruritus.9
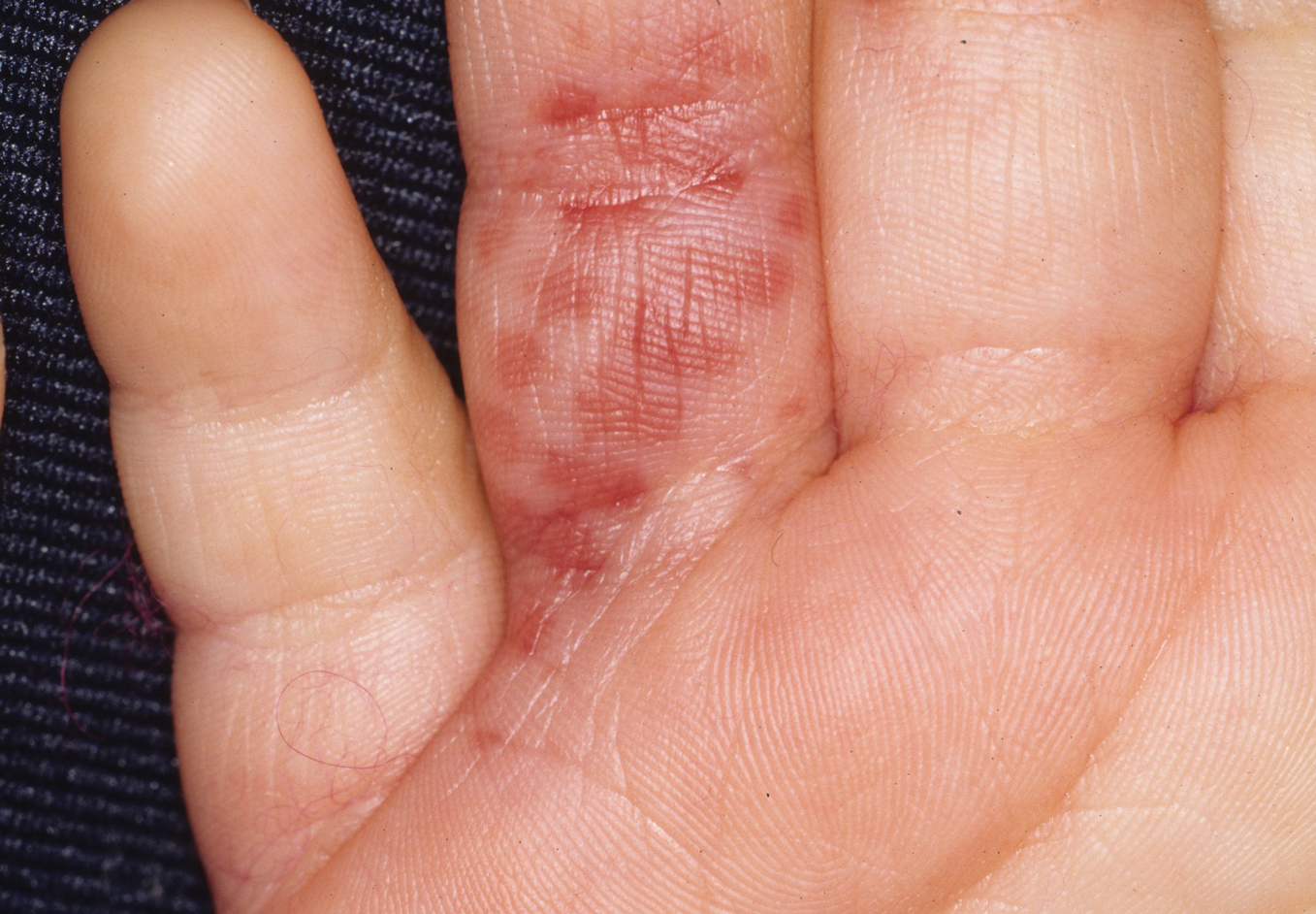
Envenomation by a puss caterpillar also can present with systemic manifestations including fever, headache, nausea, vomiting, shocklike symptoms, and seizures.1,6,7 Anaphylactic reaction is rare but also can present.7 Uncommon cases have been reported with severe abdominal pain and muscle spasm mimicking acute appendicitis and latrodectism, respectively.7,9
Diagnosis
The diagnosis of M opercularis envenomation is made clinically based on the morphology of the skin lesions and a history of probable exposure. Coexistent leukocytosis is likely, but laboratory testing is not warranted, as it is both nonspecific and insensitive.9
Management/Treatment
The most commonly reported immediate approaches to treatment involve attempts to remove the spines from the skin with tape (stripping), application of ice packs over the affected area, oral antihistamines, topical and intralesional anesthetics, regional nerve block, and oral analgesics.6,9 There have been several cases detailing the successful use of parenteral calcium gluconate,5,7 and diazepam has been used to treat severe muscle spasms. Anaphylactic reactions should be managed in a controlled monitored setting with subcutaneous epinephrine.7 Despite their common use, some data suggest that ice packs and mid- to high-potency topical steroids are ineffective.9
Incidence
From 2001 to 2005, a mean average of 94,552 annual cases of animal bites and stings were reported to poison control centers in the United States, of which 2094 were linked to caterpillars in this 5-year period.10 There were 3484 M opercularis caterpillar stings reported to the Texas Poison Center Network from 2000 to 2016.5,6 Given their ability to sting throughout their life cycle, thousands of M opercularis caterpillar stings can occur each year.1,6 Existing literature on M opercularis caterpillar stings mainly involves case reports with affections of the skin and oral mucosa, self-reported envenomation, and case studies.5,6,8
Although multiple health concerns associated with caterpillar envenomation have been reported worldwide, the lack of official epidemiologic reports highly suggests that this problem remains underestimated. There also may be many unreported cases because certain reactions are mild or self-limited and can even go unnoticed.11 Nonetheless, there is an evident rise of cases reported in the United States. According to the 2018 annual report of the American Association of Poison Control Centers, there were 2815 case mentions from caterpillar envenomation.12
In 1921 and 1952, some public schools in Texas were temporarily closed due to outbreaks of puss caterpillar–associated dermatitis.8 Similar outbreaks also have been reported in South Carolina, Virginia, and Oklahoma.9 Emerging data suggest that plant oil products and the pesticide cypermethrin may be helpful in controlling local infestations of the puss caterpillar.8
- Villas-Boas IM, Bonfa G, Tambourgi DV. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon. 2018;153:39-52.
- Hossler EW. Caterpillars and moths: part I. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:1-10; quiz 11-12.
- Haddad Junior V, Amorim PC, Haddad Junior WT, et al. Venomous and poisonous arthropods: identification, clinical manifestations of envenomation, and treatments used in human injuries. Rev Soc Bras Med Trop. 2015;48:650-657.
- Haddad V Jr, Cardoso JL, Lupi O, et al. Tropical dermatology: venomous arthropods and human skin: part I. Insecta. J Am Acad Dermatol. 2012;67:331.e1-331.e14; quiz 345.
- Pappano DA, Trout Fryxell R, Warren M. Oral mucosal envenomation of an infant by a puss caterpillar. Pediatr Emerg Care. 2017;33:424-426.
- Forrester MB. Megalopyge opercularis caterpillar stings reported to Texas poison centers. Wilderness Environ Med. 2018;29:215-220.
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:13-28; quiz 29-30.
- Eagleman DM. Envenomation by the asp caterpillar (Megalopyge opercularis). Clin Toxicol (Phila). 2008;46:201-205.
- Greene SC, Carey JM. Puss caterpillar envenomation: erucism mimicking appendicitis in a young child [published online May 23, 2018]. Pediatr Emerg Care. doi:10.1097/PEC.0000000000001514.
- Langley RL. Animal bites and stings reported by United States Poison Control Centers, 2001-2005. Wilderness Environ Med. 2008;19:7-14.
- Seldeslachts A, Peigneur S, Tytgat J. Caterpillar venom: a health hazard of the 21st century [published online May 30, 2020]. Biomedicines. doi:10.3390/biomedicines8060143.
- Gummin DD, Mowry JB, Spyker DA, et al. 2018 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th annual report. Clin Toxicol (Phila). 2019;57:1220-1413.
Lepidoptera is the second largest order of the class Insecta and comprises approximately 160,000 species of butterflies and moths classified among approximately 124 families and subfamilies. Venomous properties have been identified in 12 of these families, posing a serious threat to human health. 1
The clinical manifestations from Lepidoptera envenomation can range from general systemic symptoms such as fever and abdominal distress; to more complex focal affections including hemorrhage, ophthalmologic lesions, and irritation of the respiratory tracts; to less severe reactions of the skin, which are the most common presentation.1
Terminology
Lepidopterism is the term used to address a clinical spectrum of systemic manifestations from direct contact with venomous butterflies or moths and/or their products.2 Conversely, erucism is a term used to describe localized cutaneous reactions after direct contact with toxins from caterpillars.
Lepidopterism is derived from the Greek roots lepis, meaning scale, and pteron, meaning wing. The term erucism stems from the Latin word eruca, which means larva.2
Ideally, lepidopterism should refer solely to reactions from butterflies and moths—adult forms of insects with scaly wings—while erucism should refer to reactions from contact with caterpillars—the larval form of butterflies and moths.
In common use, lepidopterism can describe any reaction from caterpillars, moths, or adult butterflies, as well as any case of Lepidoptera exposure with only systemic manifestations, regardless of cutaneous findings. Concurrently, erucism has been defined as either any reaction from caterpillars or any skin reaction from contact with caterpillars or moths.2
Because caterpillars are the larval form of butterflies and moths, caterpillar-associated skin reactions also have been conveniently denominated caterpillar dermatitis.1 Henceforth in this article, both terms erucism and caterpillar dermatitis are used interchangeably.
Caterpillar Envenomation
Caterpillars cause the vast majority of adverse events from lepidopteran exposures.2 Envenomation by caterpillars might stand as the world’s most common envenomation given the larvae proximity to humans.3 Although involvement of internal organs (eg, renal failure), cerebral hemorrhage, and joint lesions can occur, skin manifestations are more predominant with the majority of species. Initial localized pain, edema, and erythema usually are present at the site of direct contact and subsequently progress toward maculopapular to bullous lesions, erosions, petechiae, necrosis, and ulceration depending on the offending species.1,4
Megalopyge opercularis
In the United States, more than 50 species of caterpillars have been identified as poisonous or venomous.5 Megalopyge opercularis (Figure 1), the larval form of the flannel moth, is an important cause of caterpillar-associated dermatitis in the southern United States.6,7 Megalopyge opercularis also is commonly known as the puss caterpillar, opossum bug, wooly slug, el perrito, tree asp, or Italian asp.6 This lepidopteran insect is mainly found in the southeastern and southcentral United States, with noted particular abundance in Texas, Louisiana, and Florida.6,8 The puss caterpillar has 2 generations per year; the first develops during the months of June to July, and the second develops from September to October, carrying seasonal health hazards.6,8

Megalopyge opercularis is tapered at the ends and can measure 2.5 to 3.5×1 cm at maturity. It is covered by silky, long-streaked, wavy hairs that may appear single colored or as a mix of colors—from white to gray to brown—forming a mid-dorsal crest.6 Beneath this furry coat, rows of short sharp spines are hidden. Upon contact with the human skin, these spines will break and discharge venom.1,6,8 Toxins contained within the hollow spines are thought to be produced by specialized basal cells, but there still is little knowledge about the dynamics and composition of the venom.1
Clinical Manifestations
The severity of the reaction depends on the caterpillar’s size and the extent of contact.1,4 Contact with M opercularis instantly presents with a throbbing or burning pain that may be followed by localized erythema and rash.1,6 A characteristic gridlike pattern of erythematous macules develops, reflecting each site of puncture from the insect’s spines (Figure 2).8,9 Skin lesions can progress from erythematous macules to hemorrhagic vesicles or pustules, usually self-resolving after a few days. The reaction also can present with radiating pain to regional lymph nodes and numbness of the affected area.1,6,8 Moreover, some patients may report urticaria and pruritus.9

Envenomation by a puss caterpillar also can present with systemic manifestations including fever, headache, nausea, vomiting, shocklike symptoms, and seizures.1,6,7 Anaphylactic reaction is rare but also can present.7 Uncommon cases have been reported with severe abdominal pain and muscle spasm mimicking acute appendicitis and latrodectism, respectively.7,9
Diagnosis
The diagnosis of M opercularis envenomation is made clinically based on the morphology of the skin lesions and a history of probable exposure. Coexistent leukocytosis is likely, but laboratory testing is not warranted, as it is both nonspecific and insensitive.9
Management/Treatment
The most commonly reported immediate approaches to treatment involve attempts to remove the spines from the skin with tape (stripping), application of ice packs over the affected area, oral antihistamines, topical and intralesional anesthetics, regional nerve block, and oral analgesics.6,9 There have been several cases detailing the successful use of parenteral calcium gluconate,5,7 and diazepam has been used to treat severe muscle spasms. Anaphylactic reactions should be managed in a controlled monitored setting with subcutaneous epinephrine.7 Despite their common use, some data suggest that ice packs and mid- to high-potency topical steroids are ineffective.9
Incidence
From 2001 to 2005, a mean average of 94,552 annual cases of animal bites and stings were reported to poison control centers in the United States, of which 2094 were linked to caterpillars in this 5-year period.10 There were 3484 M opercularis caterpillar stings reported to the Texas Poison Center Network from 2000 to 2016.5,6 Given their ability to sting throughout their life cycle, thousands of M opercularis caterpillar stings can occur each year.1,6 Existing literature on M opercularis caterpillar stings mainly involves case reports with affections of the skin and oral mucosa, self-reported envenomation, and case studies.5,6,8
Although multiple health concerns associated with caterpillar envenomation have been reported worldwide, the lack of official epidemiologic reports highly suggests that this problem remains underestimated. There also may be many unreported cases because certain reactions are mild or self-limited and can even go unnoticed.11 Nonetheless, there is an evident rise of cases reported in the United States. According to the 2018 annual report of the American Association of Poison Control Centers, there were 2815 case mentions from caterpillar envenomation.12
In 1921 and 1952, some public schools in Texas were temporarily closed due to outbreaks of puss caterpillar–associated dermatitis.8 Similar outbreaks also have been reported in South Carolina, Virginia, and Oklahoma.9 Emerging data suggest that plant oil products and the pesticide cypermethrin may be helpful in controlling local infestations of the puss caterpillar.8
Lepidoptera is the second largest order of the class Insecta and comprises approximately 160,000 species of butterflies and moths classified among approximately 124 families and subfamilies. Venomous properties have been identified in 12 of these families, posing a serious threat to human health. 1
The clinical manifestations from Lepidoptera envenomation can range from general systemic symptoms such as fever and abdominal distress; to more complex focal affections including hemorrhage, ophthalmologic lesions, and irritation of the respiratory tracts; to less severe reactions of the skin, which are the most common presentation.1
Terminology
Lepidopterism is the term used to address a clinical spectrum of systemic manifestations from direct contact with venomous butterflies or moths and/or their products.2 Conversely, erucism is a term used to describe localized cutaneous reactions after direct contact with toxins from caterpillars.
Lepidopterism is derived from the Greek roots lepis, meaning scale, and pteron, meaning wing. The term erucism stems from the Latin word eruca, which means larva.2
Ideally, lepidopterism should refer solely to reactions from butterflies and moths—adult forms of insects with scaly wings—while erucism should refer to reactions from contact with caterpillars—the larval form of butterflies and moths.
In common use, lepidopterism can describe any reaction from caterpillars, moths, or adult butterflies, as well as any case of Lepidoptera exposure with only systemic manifestations, regardless of cutaneous findings. Concurrently, erucism has been defined as either any reaction from caterpillars or any skin reaction from contact with caterpillars or moths.2
Because caterpillars are the larval form of butterflies and moths, caterpillar-associated skin reactions also have been conveniently denominated caterpillar dermatitis.1 Henceforth in this article, both terms erucism and caterpillar dermatitis are used interchangeably.
Caterpillar Envenomation
Caterpillars cause the vast majority of adverse events from lepidopteran exposures.2 Envenomation by caterpillars might stand as the world’s most common envenomation given the larvae proximity to humans.3 Although involvement of internal organs (eg, renal failure), cerebral hemorrhage, and joint lesions can occur, skin manifestations are more predominant with the majority of species. Initial localized pain, edema, and erythema usually are present at the site of direct contact and subsequently progress toward maculopapular to bullous lesions, erosions, petechiae, necrosis, and ulceration depending on the offending species.1,4
Megalopyge opercularis
In the United States, more than 50 species of caterpillars have been identified as poisonous or venomous.5 Megalopyge opercularis (Figure 1), the larval form of the flannel moth, is an important cause of caterpillar-associated dermatitis in the southern United States.6,7 Megalopyge opercularis also is commonly known as the puss caterpillar, opossum bug, wooly slug, el perrito, tree asp, or Italian asp.6 This lepidopteran insect is mainly found in the southeastern and southcentral United States, with noted particular abundance in Texas, Louisiana, and Florida.6,8 The puss caterpillar has 2 generations per year; the first develops during the months of June to July, and the second develops from September to October, carrying seasonal health hazards.6,8

Megalopyge opercularis is tapered at the ends and can measure 2.5 to 3.5×1 cm at maturity. It is covered by silky, long-streaked, wavy hairs that may appear single colored or as a mix of colors—from white to gray to brown—forming a mid-dorsal crest.6 Beneath this furry coat, rows of short sharp spines are hidden. Upon contact with the human skin, these spines will break and discharge venom.1,6,8 Toxins contained within the hollow spines are thought to be produced by specialized basal cells, but there still is little knowledge about the dynamics and composition of the venom.1
Clinical Manifestations
The severity of the reaction depends on the caterpillar’s size and the extent of contact.1,4 Contact with M opercularis instantly presents with a throbbing or burning pain that may be followed by localized erythema and rash.1,6 A characteristic gridlike pattern of erythematous macules develops, reflecting each site of puncture from the insect’s spines (Figure 2).8,9 Skin lesions can progress from erythematous macules to hemorrhagic vesicles or pustules, usually self-resolving after a few days. The reaction also can present with radiating pain to regional lymph nodes and numbness of the affected area.1,6,8 Moreover, some patients may report urticaria and pruritus.9

Envenomation by a puss caterpillar also can present with systemic manifestations including fever, headache, nausea, vomiting, shocklike symptoms, and seizures.1,6,7 Anaphylactic reaction is rare but also can present.7 Uncommon cases have been reported with severe abdominal pain and muscle spasm mimicking acute appendicitis and latrodectism, respectively.7,9
Diagnosis
The diagnosis of M opercularis envenomation is made clinically based on the morphology of the skin lesions and a history of probable exposure. Coexistent leukocytosis is likely, but laboratory testing is not warranted, as it is both nonspecific and insensitive.9
Management/Treatment
The most commonly reported immediate approaches to treatment involve attempts to remove the spines from the skin with tape (stripping), application of ice packs over the affected area, oral antihistamines, topical and intralesional anesthetics, regional nerve block, and oral analgesics.6,9 There have been several cases detailing the successful use of parenteral calcium gluconate,5,7 and diazepam has been used to treat severe muscle spasms. Anaphylactic reactions should be managed in a controlled monitored setting with subcutaneous epinephrine.7 Despite their common use, some data suggest that ice packs and mid- to high-potency topical steroids are ineffective.9
Incidence
From 2001 to 2005, a mean average of 94,552 annual cases of animal bites and stings were reported to poison control centers in the United States, of which 2094 were linked to caterpillars in this 5-year period.10 There were 3484 M opercularis caterpillar stings reported to the Texas Poison Center Network from 2000 to 2016.5,6 Given their ability to sting throughout their life cycle, thousands of M opercularis caterpillar stings can occur each year.1,6 Existing literature on M opercularis caterpillar stings mainly involves case reports with affections of the skin and oral mucosa, self-reported envenomation, and case studies.5,6,8
Although multiple health concerns associated with caterpillar envenomation have been reported worldwide, the lack of official epidemiologic reports highly suggests that this problem remains underestimated. There also may be many unreported cases because certain reactions are mild or self-limited and can even go unnoticed.11 Nonetheless, there is an evident rise of cases reported in the United States. According to the 2018 annual report of the American Association of Poison Control Centers, there were 2815 case mentions from caterpillar envenomation.12
In 1921 and 1952, some public schools in Texas were temporarily closed due to outbreaks of puss caterpillar–associated dermatitis.8 Similar outbreaks also have been reported in South Carolina, Virginia, and Oklahoma.9 Emerging data suggest that plant oil products and the pesticide cypermethrin may be helpful in controlling local infestations of the puss caterpillar.8
- Villas-Boas IM, Bonfa G, Tambourgi DV. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon. 2018;153:39-52.
- Hossler EW. Caterpillars and moths: part I. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:1-10; quiz 11-12.
- Haddad Junior V, Amorim PC, Haddad Junior WT, et al. Venomous and poisonous arthropods: identification, clinical manifestations of envenomation, and treatments used in human injuries. Rev Soc Bras Med Trop. 2015;48:650-657.
- Haddad V Jr, Cardoso JL, Lupi O, et al. Tropical dermatology: venomous arthropods and human skin: part I. Insecta. J Am Acad Dermatol. 2012;67:331.e1-331.e14; quiz 345.
- Pappano DA, Trout Fryxell R, Warren M. Oral mucosal envenomation of an infant by a puss caterpillar. Pediatr Emerg Care. 2017;33:424-426.
- Forrester MB. Megalopyge opercularis caterpillar stings reported to Texas poison centers. Wilderness Environ Med. 2018;29:215-220.
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:13-28; quiz 29-30.
- Eagleman DM. Envenomation by the asp caterpillar (Megalopyge opercularis). Clin Toxicol (Phila). 2008;46:201-205.
- Greene SC, Carey JM. Puss caterpillar envenomation: erucism mimicking appendicitis in a young child [published online May 23, 2018]. Pediatr Emerg Care. doi:10.1097/PEC.0000000000001514.
- Langley RL. Animal bites and stings reported by United States Poison Control Centers, 2001-2005. Wilderness Environ Med. 2008;19:7-14.
- Seldeslachts A, Peigneur S, Tytgat J. Caterpillar venom: a health hazard of the 21st century [published online May 30, 2020]. Biomedicines. doi:10.3390/biomedicines8060143.
- Gummin DD, Mowry JB, Spyker DA, et al. 2018 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th annual report. Clin Toxicol (Phila). 2019;57:1220-1413.
- Villas-Boas IM, Bonfa G, Tambourgi DV. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon. 2018;153:39-52.
- Hossler EW. Caterpillars and moths: part I. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:1-10; quiz 11-12.
- Haddad Junior V, Amorim PC, Haddad Junior WT, et al. Venomous and poisonous arthropods: identification, clinical manifestations of envenomation, and treatments used in human injuries. Rev Soc Bras Med Trop. 2015;48:650-657.
- Haddad V Jr, Cardoso JL, Lupi O, et al. Tropical dermatology: venomous arthropods and human skin: part I. Insecta. J Am Acad Dermatol. 2012;67:331.e1-331.e14; quiz 345.
- Pappano DA, Trout Fryxell R, Warren M. Oral mucosal envenomation of an infant by a puss caterpillar. Pediatr Emerg Care. 2017;33:424-426.
- Forrester MB. Megalopyge opercularis caterpillar stings reported to Texas poison centers. Wilderness Environ Med. 2018;29:215-220.
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:13-28; quiz 29-30.
- Eagleman DM. Envenomation by the asp caterpillar (Megalopyge opercularis). Clin Toxicol (Phila). 2008;46:201-205.
- Greene SC, Carey JM. Puss caterpillar envenomation: erucism mimicking appendicitis in a young child [published online May 23, 2018]. Pediatr Emerg Care. doi:10.1097/PEC.0000000000001514.
- Langley RL. Animal bites and stings reported by United States Poison Control Centers, 2001-2005. Wilderness Environ Med. 2008;19:7-14.
- Seldeslachts A, Peigneur S, Tytgat J. Caterpillar venom: a health hazard of the 21st century [published online May 30, 2020]. Biomedicines. doi:10.3390/biomedicines8060143.
- Gummin DD, Mowry JB, Spyker DA, et al. 2018 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th annual report. Clin Toxicol (Phila). 2019;57:1220-1413.
Practice Points
- Megalopyge opercularis is the most widely distributed caterpillar species in the Americas, and envenomation by it can occur year-round.
- Skin reactions to M opercularis stings can present as maculopapular dermatitis, eczematous eruptions, or urticarial reactions.
- During the initial presentation, patients experience intense throbbing pain, yet the severity of symptoms depends on the caterpillar’s size and the extent of contact.
- A history of caterpillar exposure helps with diagnosis, and treatment remains empiric.
Fighting Acne for the Fighting Forces
Acne treatment presents unique challenges in the active-duty military population. Lesions on the face may interfere with proper fit and seal of protective masks and helmets, while those involving the shoulders or back may cause considerable discomfort beneath safety restraints, parachute harnesses, or flak jackets. Therefore, untreated acne may limit servicemembers from performing their assigned duties. Treatments themselves also may be limiting; for instance, aircrew members who are taking oral doxycycline, tetracycline, or erythromycin may be grounded (ie, temporarily removed from duty) during and after therapy to monitor for side effects. Minocycline is considered unacceptable for aviators and is completely restricted for use due to risk for central nervous system side effects. Isotretinoin is restricted in aircrew members, submariners, and divers. If initiated, isotretinoin requires grounding for the entire duration of therapy and up to 3 months following treatment. Normalization of triglyceride levels and slit-lamp ocular examination also must take place prior to return to full duty, which may lead to additional grounding time. Well-established topical and oral treatments not impacting military duty are omitted from this review.
Antibiotics
Minocycline
Minocycline carries a small risk for development of systemic lupus erythematosus and other autoimmune treatment-emergent adverse effects. It has known gastrointestinal tract side effects, and long-term use also can lead to bluish discoloration of the skin.1 Systemic minocycline is restricted in aircrew members due to its risk for central nervous system side effects, including light-headedness, dizziness, and vertigo.2-5
A topical formulation of minocycline recently was developed and approved by the US Food and Drug Administration as a means to reduce systemic adverse effects. This 4% minocycline foam has thus far been safe and well tolerated, with adverse events reported in less than 1% of study participants.1,6 In addition, topical minocycline was shown in a recent phase 3 study to notably reduce inflammatory lesion counts when compared to control vehicles at as early as 3 weeks.7 Topical minocycline may emerge as a viable treatment option for active-duty servicemembers in the future.
Doxycycline
Doxycycline is not medically disqualifying. Even so, it may still necessitate grounding for a period of time while monitoring for side effects.4 Doxycycline can lead to photosensitivity, which could be difficult to tolerate for active-duty personnel training in sunny climates. Fortunately, uniform regulations and personal protective equipment requirements provide cover for most of the body surfaces aside from the face, which is protected by various forms of covers. If the patient tolerates the medication well without considerable side effects, he/she may be returned to full duty, making doxycycline an acceptable alternative to minocycline in the military population.
Sarecycline
This novel compound is a tetracycline-class antibiotic with a narrower spectrum of activity, with reduced activity against enteric gram-negative bacteria. It has shown efficacy in reducing inflammatory and noninflammatory acne lesions, including lesions on the face, back, and chest. Common adverse side effects are nausea, headache, nasopharyngitis, and vomiting. Vestibular and phototoxic adverse effects were reported in less than 1% of patients.1,8 The US Food and Drug Administration approved sarecycline as a once-daily oral formulation for moderate to severe acne vulgaris, the first new antibiotic to be approved for the disease in the last 40 years. Sarecycline is not mentioned in any US military guidelines with regard to medical readiness and duty status; however, given its lack of vestibular side effects and narrower activity spectrum, it may become another acceptable treatment option in the military population.
Isotretinoin
Isotretinoin is well established as an excellent treatment of acne and stands alone as the only currently available medication that alters the disease course and prevents relapse in many patients. Nearly all patients on isotretinoin experience considerable mucocutaneous dryness, and up to 25% of patients on high-dose isotretinoin develop myalgia.9 Isotretinoin causes serious retinoid embryopathy, requiring all patients to be enrolled in the iPLEDGE program (https://www.ipledgeprogram.com/iPledgeUI/home.u) and to use 2 methods of contraception during treatment. Although it is uncommon to have notable elevations in lipids and transaminases during treatment with isotretinoin, routine laboratory monitoring generally is performed until the patient reaches steady dosing.
Isotretinoin is not permitted for use in active aircrew members, submariners, or divers. Servicemembers pursuing isotretinoin therapy are removed from their duty and are nondeployable for the entirety of their treatment course and several months after completion.4,5
Photodynamic Therapy
Aminolevulinic acid and photodynamic therapy (ALA-PDT) has been successfully used in the management of acne.10 In addition to inducing selective damage to sebaceous glands, it has been proposed that PDT also destroys Propionibacterium acnes and reduces keratinocyte shedding and immunologic changes that play key roles in the development of acne.10
A recent randomized controlled trial comparing the efficacy of ALA-PDT vs adapalene gel plus oral doxycycline for treatment of moderate acne included 46 patients with moderate inflammatory acne.10 Twenty-three participants received 2 sessions (spaced 2 weeks apart) of 20% ALA incubated for 90 minutes before red light irradiation with a fluence of 37 J/cm2, and the other 23 received 100 mg/d of oral doxycycline plus adapalene gel 0.1%. By 6-week follow-up, there was a significantly higher reduction in total lesions within the PDT group (P=.038), which was sustained at the secondary 12-week follow-up (P=.026). There was a 79% total reduction of lesions in the ALA-PDT group vs 67% in the doxycycline plus adapalene group.10
Although some studies have shown promise for PDT as an emerging treatment option for acne, further research is needed. A 2016 systematic review of the related literature determined that although 20% ALA-PDT with red light was more effective than lower concentrations of ALA and also more effective than ALA-PDT with blue light—which offered no additional benefit when compared with blue light alone—high-quality evidence on the use of PDT for acne is lacking overall.11 At the time of the review, there was little certainty as to the usefulness of ALA-PDT with red or blue light as a standard treatment for individuals with moderate to severe acne. A 2019 review by Marson and Baldwin12 echoed this sentiment, recommending more stringently designed studies to elucidate the true role of PDT as a monotherapy or adjunctive treatment of acne.
Pulsed Dye Laser
Pulsed dye laser (PDL) was first shown to be a potential therapy for acne by Seaton et al,13 who conducted a small-scale, randomized, controlled trial with 41 patients, each assigned to either a single PDL treatment or a sham treatment. Patients were re-evaluated at 12 weeks, measuring acne severity by the Leeds revised acne grading system and taking total lesion counts. Acne severity (P=.007) and total lesion counts (P=.023) were significantly improved in the treatment group, with a 53% reduction in total lesion count following a single PDL treatment.13
In 2007, a Spanish study described use of PDL every 4 weeks for a total of 12 weeks in 36 patients with mild to moderate acne. Using lesion counts as their primary outcome measure, the investigators found results similar to those from Seaton et al,13 with a 57% decrease in active lesions.14 Others still have found similar outcomes. A 2009 study of 45 patients with mild to moderate acne compared patients treated with PDL every 2 weeks for 12 weeks to patients receiving either topical therapy or chemical peels with 25% trichloroacetic acid. At 12 weeks, they noted the best results were in the PDL group.15
Karsai et al16 compared PDL as an adjuvant treatment of acne to proven treatment with clindamycin plus benzoyl peroxide gel. Eighty patients were randomized to topical therapy plus PDL or topical therapy alone and were followed at 2 and 4 weeks after the initial treatment. Although both groups showed improvement as measured by inflammatory lesion count and dermatology life quality index, there was no statistically significant difference noted between groups.16
Case Report
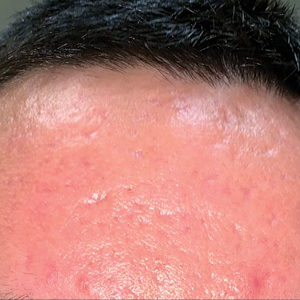
A 24-year-old active-duty male servicemember was referred to the dermatology department for evaluation of treatment-resistant nodulocystic scarring acne. Prior to his arrival to dermatology, he had completed 2 weeks of isotretinoin before discontinuation due to notable mood alteration. Following the isotretinoin, he was then switched to doxycycline 100 mg twice daily, which he trialed for 3 months. Even on the antibiotic, the patient continued to develop new pustules and cysts, prompting referral to dermatology for additional treatment options (Figure, A). All of the previous topical and oral medications had been discontinued at the current presentation.
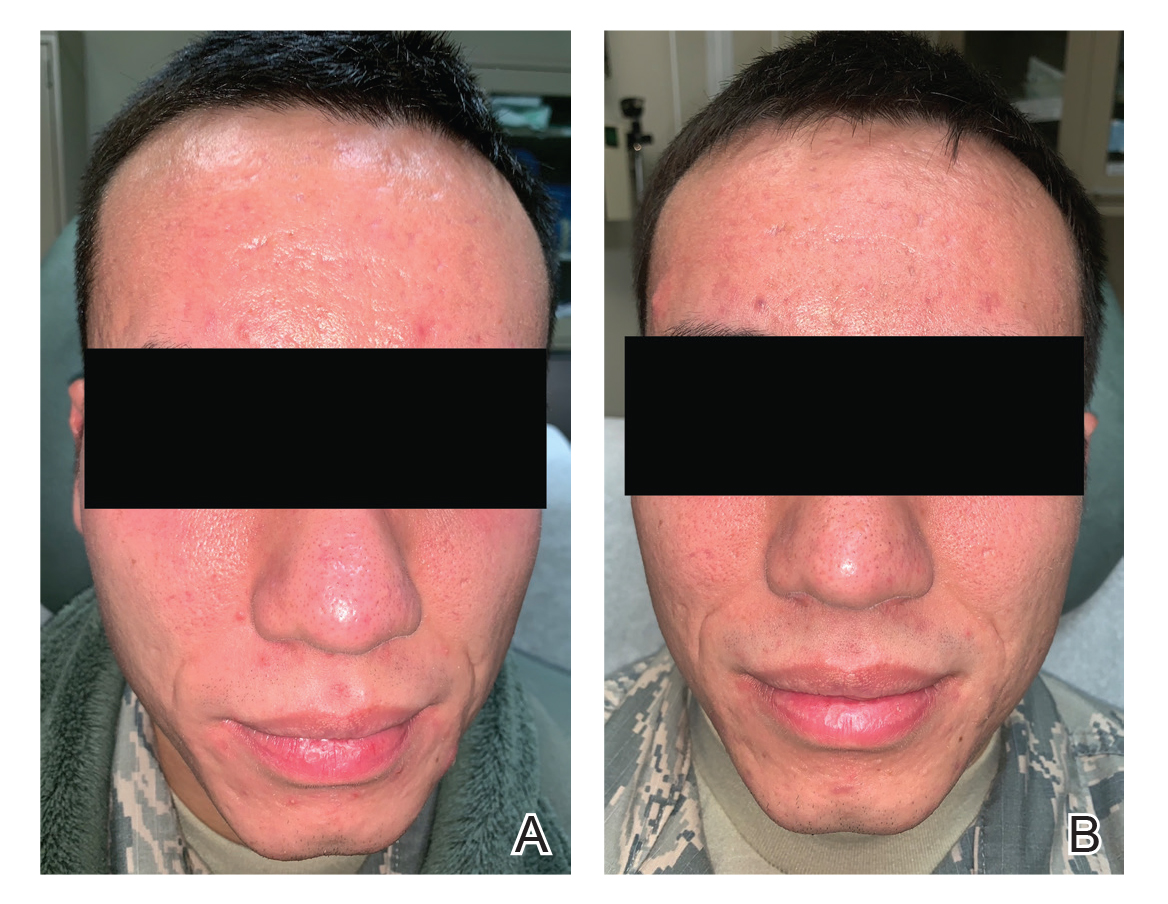
The patient received 3 treatments with the 595-nm PDL (spot size, 10 mm; fluence, 7 J/cm2; pulse width, 6 milliseconds) spaced 4 weeks apart. At each treatment, fewer than 10 total inflammatory lesions were treated, including inflammatory papules, pustules, and nodules. Nodular lesions were treated with 2 pulses. After each treatment, the patient reported that all treated lesions resolved within 2 days (Figure, B). Subsequent treated lesions all occurred at previously uninvolved sites.
Final Thoughts
Antibiotic resistance is a known and growing problem throughout the medical community. In 2013, the US Centers for Disease Control and Prevention reported that dermatologists prescribe more antibiotics than any other specialty.17 Aside from antibiotic stewardship, systemic antibiotics come with various considerations when selecting ideal acne treatment regimens in military populations, as they are either medically disqualifying or lead to temporary grounding status. Numerous guidelines on acne have recommended limiting the use of antibiotics, instead pursuing alternative therapies such as spironolactone, oral contraceptives, or isotretinoin.9,18 Both spironolactone and oral contraceptives work well via antiandrogenic and antisebogenic properties; however, these therapies are limited to female patients only, who make up a minority of patients in the active-duty military setting. Isotretinoin is highly effective in the treatment of acne, but it requires grounding for the entirety of treatment and for months afterward, which comes at great personal and financial costs to servicemembers and their commanders due to limited-duty status and inability to deploy.
Given the operational constraints with isotretinoin and the continual rise of antibiotic resistance, PDL appears to be a safe and effective alternative therapy for acne. In our case, the patient had complete resolution of active inflammatory lesions after each of his treatments. He had no adverse effects and tolerated the treatments well. We report this case here to highlight the use of PDL as an effective therapy for spot treatment in patients limited by personal or operational constraints and as a means to reduce antibiotic use in the face of a growing tide of antibiotic resistance.
- Kircik LH. What’s new in the management of acne vulgaris. Cutis. 2019;104:48-52.
- US Department of the Army. Standards of medical fitness. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN8673_AR40_501_FINAL_WEB.pdf. Published June 27, 2019. Accessed June 23, 2020.
- US Department of the Air Force. Medical examinations and standards. http://aangfs.com/wp-content/uploads/2012/10/AFI-48-123-Medical-Examination-Standards.pdf. Published January 29, 2013. Accessed June 23, 2020.
- US Navy Aeromedical Reference and Waiver Guide. Navy Medicine website. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf. Published September 4, 2019. Accessed June 17, 2020.
- Burke KR, Larrymore DC, Cho S. Treatment considerations for US military members with skin disease. Cutis. 2019:103:329-332.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;30:168-177.
- Raoof J, Hooper D, Moore A, et al. FMX101 4% topical minocycline foam for the treatment of moderate to severe acne vulgaris: efficacy and safety from a phase 3 randomized, double-blind, vehicle-controlled study. Poster presented at: 2018 Fall Clinical Dermatology Conference; October 18-21, 2018; Las Vegas, NV.
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris; results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments.J Am Acad Dermatol. 2019;80:538-549.
- Nicklas C, Rubio R, Cardenas C, et al. Comparison of efficacy of aminolaevulinic acid photodynamic therapy vs. adapalene gel plus oral doxycycline for treatment of moderate acne vulgaris—a simple, blind, randomized, and controlled trial. Photodermatol Photoimmunol Photomed. 2019;35:3-10.
- Barbaric J, Abbott R, Posadzki P, et al. Light therapies for acne [published online September 27, 2016]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD007917.pub2.
- Marson JW, Baldwin HE. New concepts, concerns, and creations in acne. Dermatol Clin. 2019;37:1-9.
- Seaton ED, Charakida A, Mouser PE, et al. Pulsed-dye laser treatment for inflammatory acne vulgaris: randomised controlled trial. Lancet Lond Engl. 2003;362:1347-1352.
- Harto A, Garcia-Morales I, Belmar P, et al. Pulsed dye laser treatment of acne. study of clinical efficacy and mechanism of action. Actas Dermosifiliogr. 2007;98:415-419.
- Leheta TM. Role of the 585-nm pulsed dye laser in the treatment of acne in comparison with other topical therapeutic modalities. J Cosmet Laser Ther Off Publ Eur Soc Laser Dermatol. 2009;11:118-124.
- Karsai S, Schmitt L, Raulin C. The pulsed-dye laser as an adjuvant treatment modality in acne vulgaris: a randomized controlled single-blinded trial. Br J Dermatol. 2010;163:395-401.
- Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions—United States. annual report 2013.https://www.cdc.gov/antibiotic-use/community/pdfs/Annual-ReportSummary_2013.pdf. Accessed June 23, 2020.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33.
Acne treatment presents unique challenges in the active-duty military population. Lesions on the face may interfere with proper fit and seal of protective masks and helmets, while those involving the shoulders or back may cause considerable discomfort beneath safety restraints, parachute harnesses, or flak jackets. Therefore, untreated acne may limit servicemembers from performing their assigned duties. Treatments themselves also may be limiting; for instance, aircrew members who are taking oral doxycycline, tetracycline, or erythromycin may be grounded (ie, temporarily removed from duty) during and after therapy to monitor for side effects. Minocycline is considered unacceptable for aviators and is completely restricted for use due to risk for central nervous system side effects. Isotretinoin is restricted in aircrew members, submariners, and divers. If initiated, isotretinoin requires grounding for the entire duration of therapy and up to 3 months following treatment. Normalization of triglyceride levels and slit-lamp ocular examination also must take place prior to return to full duty, which may lead to additional grounding time. Well-established topical and oral treatments not impacting military duty are omitted from this review.
Antibiotics
Minocycline
Minocycline carries a small risk for development of systemic lupus erythematosus and other autoimmune treatment-emergent adverse effects. It has known gastrointestinal tract side effects, and long-term use also can lead to bluish discoloration of the skin.1 Systemic minocycline is restricted in aircrew members due to its risk for central nervous system side effects, including light-headedness, dizziness, and vertigo.2-5
A topical formulation of minocycline recently was developed and approved by the US Food and Drug Administration as a means to reduce systemic adverse effects. This 4% minocycline foam has thus far been safe and well tolerated, with adverse events reported in less than 1% of study participants.1,6 In addition, topical minocycline was shown in a recent phase 3 study to notably reduce inflammatory lesion counts when compared to control vehicles at as early as 3 weeks.7 Topical minocycline may emerge as a viable treatment option for active-duty servicemembers in the future.
Doxycycline
Doxycycline is not medically disqualifying. Even so, it may still necessitate grounding for a period of time while monitoring for side effects.4 Doxycycline can lead to photosensitivity, which could be difficult to tolerate for active-duty personnel training in sunny climates. Fortunately, uniform regulations and personal protective equipment requirements provide cover for most of the body surfaces aside from the face, which is protected by various forms of covers. If the patient tolerates the medication well without considerable side effects, he/she may be returned to full duty, making doxycycline an acceptable alternative to minocycline in the military population.
Sarecycline
This novel compound is a tetracycline-class antibiotic with a narrower spectrum of activity, with reduced activity against enteric gram-negative bacteria. It has shown efficacy in reducing inflammatory and noninflammatory acne lesions, including lesions on the face, back, and chest. Common adverse side effects are nausea, headache, nasopharyngitis, and vomiting. Vestibular and phototoxic adverse effects were reported in less than 1% of patients.1,8 The US Food and Drug Administration approved sarecycline as a once-daily oral formulation for moderate to severe acne vulgaris, the first new antibiotic to be approved for the disease in the last 40 years. Sarecycline is not mentioned in any US military guidelines with regard to medical readiness and duty status; however, given its lack of vestibular side effects and narrower activity spectrum, it may become another acceptable treatment option in the military population.
Isotretinoin
Isotretinoin is well established as an excellent treatment of acne and stands alone as the only currently available medication that alters the disease course and prevents relapse in many patients. Nearly all patients on isotretinoin experience considerable mucocutaneous dryness, and up to 25% of patients on high-dose isotretinoin develop myalgia.9 Isotretinoin causes serious retinoid embryopathy, requiring all patients to be enrolled in the iPLEDGE program (https://www.ipledgeprogram.com/iPledgeUI/home.u) and to use 2 methods of contraception during treatment. Although it is uncommon to have notable elevations in lipids and transaminases during treatment with isotretinoin, routine laboratory monitoring generally is performed until the patient reaches steady dosing.
Isotretinoin is not permitted for use in active aircrew members, submariners, or divers. Servicemembers pursuing isotretinoin therapy are removed from their duty and are nondeployable for the entirety of their treatment course and several months after completion.4,5
Photodynamic Therapy
Aminolevulinic acid and photodynamic therapy (ALA-PDT) has been successfully used in the management of acne.10 In addition to inducing selective damage to sebaceous glands, it has been proposed that PDT also destroys Propionibacterium acnes and reduces keratinocyte shedding and immunologic changes that play key roles in the development of acne.10
A recent randomized controlled trial comparing the efficacy of ALA-PDT vs adapalene gel plus oral doxycycline for treatment of moderate acne included 46 patients with moderate inflammatory acne.10 Twenty-three participants received 2 sessions (spaced 2 weeks apart) of 20% ALA incubated for 90 minutes before red light irradiation with a fluence of 37 J/cm2, and the other 23 received 100 mg/d of oral doxycycline plus adapalene gel 0.1%. By 6-week follow-up, there was a significantly higher reduction in total lesions within the PDT group (P=.038), which was sustained at the secondary 12-week follow-up (P=.026). There was a 79% total reduction of lesions in the ALA-PDT group vs 67% in the doxycycline plus adapalene group.10
Although some studies have shown promise for PDT as an emerging treatment option for acne, further research is needed. A 2016 systematic review of the related literature determined that although 20% ALA-PDT with red light was more effective than lower concentrations of ALA and also more effective than ALA-PDT with blue light—which offered no additional benefit when compared with blue light alone—high-quality evidence on the use of PDT for acne is lacking overall.11 At the time of the review, there was little certainty as to the usefulness of ALA-PDT with red or blue light as a standard treatment for individuals with moderate to severe acne. A 2019 review by Marson and Baldwin12 echoed this sentiment, recommending more stringently designed studies to elucidate the true role of PDT as a monotherapy or adjunctive treatment of acne.
Pulsed Dye Laser
Pulsed dye laser (PDL) was first shown to be a potential therapy for acne by Seaton et al,13 who conducted a small-scale, randomized, controlled trial with 41 patients, each assigned to either a single PDL treatment or a sham treatment. Patients were re-evaluated at 12 weeks, measuring acne severity by the Leeds revised acne grading system and taking total lesion counts. Acne severity (P=.007) and total lesion counts (P=.023) were significantly improved in the treatment group, with a 53% reduction in total lesion count following a single PDL treatment.13
In 2007, a Spanish study described use of PDL every 4 weeks for a total of 12 weeks in 36 patients with mild to moderate acne. Using lesion counts as their primary outcome measure, the investigators found results similar to those from Seaton et al,13 with a 57% decrease in active lesions.14 Others still have found similar outcomes. A 2009 study of 45 patients with mild to moderate acne compared patients treated with PDL every 2 weeks for 12 weeks to patients receiving either topical therapy or chemical peels with 25% trichloroacetic acid. At 12 weeks, they noted the best results were in the PDL group.15
Karsai et al16 compared PDL as an adjuvant treatment of acne to proven treatment with clindamycin plus benzoyl peroxide gel. Eighty patients were randomized to topical therapy plus PDL or topical therapy alone and were followed at 2 and 4 weeks after the initial treatment. Although both groups showed improvement as measured by inflammatory lesion count and dermatology life quality index, there was no statistically significant difference noted between groups.16
Case Report
A 24-year-old active-duty male servicemember was referred to the dermatology department for evaluation of treatment-resistant nodulocystic scarring acne. Prior to his arrival to dermatology, he had completed 2 weeks of isotretinoin before discontinuation due to notable mood alteration. Following the isotretinoin, he was then switched to doxycycline 100 mg twice daily, which he trialed for 3 months. Even on the antibiotic, the patient continued to develop new pustules and cysts, prompting referral to dermatology for additional treatment options (Figure, A). All of the previous topical and oral medications had been discontinued at the current presentation.

The patient received 3 treatments with the 595-nm PDL (spot size, 10 mm; fluence, 7 J/cm2; pulse width, 6 milliseconds) spaced 4 weeks apart. At each treatment, fewer than 10 total inflammatory lesions were treated, including inflammatory papules, pustules, and nodules. Nodular lesions were treated with 2 pulses. After each treatment, the patient reported that all treated lesions resolved within 2 days (Figure, B). Subsequent treated lesions all occurred at previously uninvolved sites.
Final Thoughts
Antibiotic resistance is a known and growing problem throughout the medical community. In 2013, the US Centers for Disease Control and Prevention reported that dermatologists prescribe more antibiotics than any other specialty.17 Aside from antibiotic stewardship, systemic antibiotics come with various considerations when selecting ideal acne treatment regimens in military populations, as they are either medically disqualifying or lead to temporary grounding status. Numerous guidelines on acne have recommended limiting the use of antibiotics, instead pursuing alternative therapies such as spironolactone, oral contraceptives, or isotretinoin.9,18 Both spironolactone and oral contraceptives work well via antiandrogenic and antisebogenic properties; however, these therapies are limited to female patients only, who make up a minority of patients in the active-duty military setting. Isotretinoin is highly effective in the treatment of acne, but it requires grounding for the entirety of treatment and for months afterward, which comes at great personal and financial costs to servicemembers and their commanders due to limited-duty status and inability to deploy.
Given the operational constraints with isotretinoin and the continual rise of antibiotic resistance, PDL appears to be a safe and effective alternative therapy for acne. In our case, the patient had complete resolution of active inflammatory lesions after each of his treatments. He had no adverse effects and tolerated the treatments well. We report this case here to highlight the use of PDL as an effective therapy for spot treatment in patients limited by personal or operational constraints and as a means to reduce antibiotic use in the face of a growing tide of antibiotic resistance.
Acne treatment presents unique challenges in the active-duty military population. Lesions on the face may interfere with proper fit and seal of protective masks and helmets, while those involving the shoulders or back may cause considerable discomfort beneath safety restraints, parachute harnesses, or flak jackets. Therefore, untreated acne may limit servicemembers from performing their assigned duties. Treatments themselves also may be limiting; for instance, aircrew members who are taking oral doxycycline, tetracycline, or erythromycin may be grounded (ie, temporarily removed from duty) during and after therapy to monitor for side effects. Minocycline is considered unacceptable for aviators and is completely restricted for use due to risk for central nervous system side effects. Isotretinoin is restricted in aircrew members, submariners, and divers. If initiated, isotretinoin requires grounding for the entire duration of therapy and up to 3 months following treatment. Normalization of triglyceride levels and slit-lamp ocular examination also must take place prior to return to full duty, which may lead to additional grounding time. Well-established topical and oral treatments not impacting military duty are omitted from this review.
Antibiotics
Minocycline
Minocycline carries a small risk for development of systemic lupus erythematosus and other autoimmune treatment-emergent adverse effects. It has known gastrointestinal tract side effects, and long-term use also can lead to bluish discoloration of the skin.1 Systemic minocycline is restricted in aircrew members due to its risk for central nervous system side effects, including light-headedness, dizziness, and vertigo.2-5
A topical formulation of minocycline recently was developed and approved by the US Food and Drug Administration as a means to reduce systemic adverse effects. This 4% minocycline foam has thus far been safe and well tolerated, with adverse events reported in less than 1% of study participants.1,6 In addition, topical minocycline was shown in a recent phase 3 study to notably reduce inflammatory lesion counts when compared to control vehicles at as early as 3 weeks.7 Topical minocycline may emerge as a viable treatment option for active-duty servicemembers in the future.
Doxycycline
Doxycycline is not medically disqualifying. Even so, it may still necessitate grounding for a period of time while monitoring for side effects.4 Doxycycline can lead to photosensitivity, which could be difficult to tolerate for active-duty personnel training in sunny climates. Fortunately, uniform regulations and personal protective equipment requirements provide cover for most of the body surfaces aside from the face, which is protected by various forms of covers. If the patient tolerates the medication well without considerable side effects, he/she may be returned to full duty, making doxycycline an acceptable alternative to minocycline in the military population.
Sarecycline
This novel compound is a tetracycline-class antibiotic with a narrower spectrum of activity, with reduced activity against enteric gram-negative bacteria. It has shown efficacy in reducing inflammatory and noninflammatory acne lesions, including lesions on the face, back, and chest. Common adverse side effects are nausea, headache, nasopharyngitis, and vomiting. Vestibular and phototoxic adverse effects were reported in less than 1% of patients.1,8 The US Food and Drug Administration approved sarecycline as a once-daily oral formulation for moderate to severe acne vulgaris, the first new antibiotic to be approved for the disease in the last 40 years. Sarecycline is not mentioned in any US military guidelines with regard to medical readiness and duty status; however, given its lack of vestibular side effects and narrower activity spectrum, it may become another acceptable treatment option in the military population.
Isotretinoin
Isotretinoin is well established as an excellent treatment of acne and stands alone as the only currently available medication that alters the disease course and prevents relapse in many patients. Nearly all patients on isotretinoin experience considerable mucocutaneous dryness, and up to 25% of patients on high-dose isotretinoin develop myalgia.9 Isotretinoin causes serious retinoid embryopathy, requiring all patients to be enrolled in the iPLEDGE program (https://www.ipledgeprogram.com/iPledgeUI/home.u) and to use 2 methods of contraception during treatment. Although it is uncommon to have notable elevations in lipids and transaminases during treatment with isotretinoin, routine laboratory monitoring generally is performed until the patient reaches steady dosing.
Isotretinoin is not permitted for use in active aircrew members, submariners, or divers. Servicemembers pursuing isotretinoin therapy are removed from their duty and are nondeployable for the entirety of their treatment course and several months after completion.4,5
Photodynamic Therapy
Aminolevulinic acid and photodynamic therapy (ALA-PDT) has been successfully used in the management of acne.10 In addition to inducing selective damage to sebaceous glands, it has been proposed that PDT also destroys Propionibacterium acnes and reduces keratinocyte shedding and immunologic changes that play key roles in the development of acne.10
A recent randomized controlled trial comparing the efficacy of ALA-PDT vs adapalene gel plus oral doxycycline for treatment of moderate acne included 46 patients with moderate inflammatory acne.10 Twenty-three participants received 2 sessions (spaced 2 weeks apart) of 20% ALA incubated for 90 minutes before red light irradiation with a fluence of 37 J/cm2, and the other 23 received 100 mg/d of oral doxycycline plus adapalene gel 0.1%. By 6-week follow-up, there was a significantly higher reduction in total lesions within the PDT group (P=.038), which was sustained at the secondary 12-week follow-up (P=.026). There was a 79% total reduction of lesions in the ALA-PDT group vs 67% in the doxycycline plus adapalene group.10
Although some studies have shown promise for PDT as an emerging treatment option for acne, further research is needed. A 2016 systematic review of the related literature determined that although 20% ALA-PDT with red light was more effective than lower concentrations of ALA and also more effective than ALA-PDT with blue light—which offered no additional benefit when compared with blue light alone—high-quality evidence on the use of PDT for acne is lacking overall.11 At the time of the review, there was little certainty as to the usefulness of ALA-PDT with red or blue light as a standard treatment for individuals with moderate to severe acne. A 2019 review by Marson and Baldwin12 echoed this sentiment, recommending more stringently designed studies to elucidate the true role of PDT as a monotherapy or adjunctive treatment of acne.
Pulsed Dye Laser
Pulsed dye laser (PDL) was first shown to be a potential therapy for acne by Seaton et al,13 who conducted a small-scale, randomized, controlled trial with 41 patients, each assigned to either a single PDL treatment or a sham treatment. Patients were re-evaluated at 12 weeks, measuring acne severity by the Leeds revised acne grading system and taking total lesion counts. Acne severity (P=.007) and total lesion counts (P=.023) were significantly improved in the treatment group, with a 53% reduction in total lesion count following a single PDL treatment.13
In 2007, a Spanish study described use of PDL every 4 weeks for a total of 12 weeks in 36 patients with mild to moderate acne. Using lesion counts as their primary outcome measure, the investigators found results similar to those from Seaton et al,13 with a 57% decrease in active lesions.14 Others still have found similar outcomes. A 2009 study of 45 patients with mild to moderate acne compared patients treated with PDL every 2 weeks for 12 weeks to patients receiving either topical therapy or chemical peels with 25% trichloroacetic acid. At 12 weeks, they noted the best results were in the PDL group.15
Karsai et al16 compared PDL as an adjuvant treatment of acne to proven treatment with clindamycin plus benzoyl peroxide gel. Eighty patients were randomized to topical therapy plus PDL or topical therapy alone and were followed at 2 and 4 weeks after the initial treatment. Although both groups showed improvement as measured by inflammatory lesion count and dermatology life quality index, there was no statistically significant difference noted between groups.16
Case Report
A 24-year-old active-duty male servicemember was referred to the dermatology department for evaluation of treatment-resistant nodulocystic scarring acne. Prior to his arrival to dermatology, he had completed 2 weeks of isotretinoin before discontinuation due to notable mood alteration. Following the isotretinoin, he was then switched to doxycycline 100 mg twice daily, which he trialed for 3 months. Even on the antibiotic, the patient continued to develop new pustules and cysts, prompting referral to dermatology for additional treatment options (Figure, A). All of the previous topical and oral medications had been discontinued at the current presentation.

The patient received 3 treatments with the 595-nm PDL (spot size, 10 mm; fluence, 7 J/cm2; pulse width, 6 milliseconds) spaced 4 weeks apart. At each treatment, fewer than 10 total inflammatory lesions were treated, including inflammatory papules, pustules, and nodules. Nodular lesions were treated with 2 pulses. After each treatment, the patient reported that all treated lesions resolved within 2 days (Figure, B). Subsequent treated lesions all occurred at previously uninvolved sites.
Final Thoughts
Antibiotic resistance is a known and growing problem throughout the medical community. In 2013, the US Centers for Disease Control and Prevention reported that dermatologists prescribe more antibiotics than any other specialty.17 Aside from antibiotic stewardship, systemic antibiotics come with various considerations when selecting ideal acne treatment regimens in military populations, as they are either medically disqualifying or lead to temporary grounding status. Numerous guidelines on acne have recommended limiting the use of antibiotics, instead pursuing alternative therapies such as spironolactone, oral contraceptives, or isotretinoin.9,18 Both spironolactone and oral contraceptives work well via antiandrogenic and antisebogenic properties; however, these therapies are limited to female patients only, who make up a minority of patients in the active-duty military setting. Isotretinoin is highly effective in the treatment of acne, but it requires grounding for the entirety of treatment and for months afterward, which comes at great personal and financial costs to servicemembers and their commanders due to limited-duty status and inability to deploy.
Given the operational constraints with isotretinoin and the continual rise of antibiotic resistance, PDL appears to be a safe and effective alternative therapy for acne. In our case, the patient had complete resolution of active inflammatory lesions after each of his treatments. He had no adverse effects and tolerated the treatments well. We report this case here to highlight the use of PDL as an effective therapy for spot treatment in patients limited by personal or operational constraints and as a means to reduce antibiotic use in the face of a growing tide of antibiotic resistance.
- Kircik LH. What’s new in the management of acne vulgaris. Cutis. 2019;104:48-52.
- US Department of the Army. Standards of medical fitness. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN8673_AR40_501_FINAL_WEB.pdf. Published June 27, 2019. Accessed June 23, 2020.
- US Department of the Air Force. Medical examinations and standards. http://aangfs.com/wp-content/uploads/2012/10/AFI-48-123-Medical-Examination-Standards.pdf. Published January 29, 2013. Accessed June 23, 2020.
- US Navy Aeromedical Reference and Waiver Guide. Navy Medicine website. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf. Published September 4, 2019. Accessed June 17, 2020.
- Burke KR, Larrymore DC, Cho S. Treatment considerations for US military members with skin disease. Cutis. 2019:103:329-332.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;30:168-177.
- Raoof J, Hooper D, Moore A, et al. FMX101 4% topical minocycline foam for the treatment of moderate to severe acne vulgaris: efficacy and safety from a phase 3 randomized, double-blind, vehicle-controlled study. Poster presented at: 2018 Fall Clinical Dermatology Conference; October 18-21, 2018; Las Vegas, NV.
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris; results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments.J Am Acad Dermatol. 2019;80:538-549.
- Nicklas C, Rubio R, Cardenas C, et al. Comparison of efficacy of aminolaevulinic acid photodynamic therapy vs. adapalene gel plus oral doxycycline for treatment of moderate acne vulgaris—a simple, blind, randomized, and controlled trial. Photodermatol Photoimmunol Photomed. 2019;35:3-10.
- Barbaric J, Abbott R, Posadzki P, et al. Light therapies for acne [published online September 27, 2016]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD007917.pub2.
- Marson JW, Baldwin HE. New concepts, concerns, and creations in acne. Dermatol Clin. 2019;37:1-9.
- Seaton ED, Charakida A, Mouser PE, et al. Pulsed-dye laser treatment for inflammatory acne vulgaris: randomised controlled trial. Lancet Lond Engl. 2003;362:1347-1352.
- Harto A, Garcia-Morales I, Belmar P, et al. Pulsed dye laser treatment of acne. study of clinical efficacy and mechanism of action. Actas Dermosifiliogr. 2007;98:415-419.
- Leheta TM. Role of the 585-nm pulsed dye laser in the treatment of acne in comparison with other topical therapeutic modalities. J Cosmet Laser Ther Off Publ Eur Soc Laser Dermatol. 2009;11:118-124.
- Karsai S, Schmitt L, Raulin C. The pulsed-dye laser as an adjuvant treatment modality in acne vulgaris: a randomized controlled single-blinded trial. Br J Dermatol. 2010;163:395-401.
- Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions—United States. annual report 2013.https://www.cdc.gov/antibiotic-use/community/pdfs/Annual-ReportSummary_2013.pdf. Accessed June 23, 2020.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33.
- Kircik LH. What’s new in the management of acne vulgaris. Cutis. 2019;104:48-52.
- US Department of the Army. Standards of medical fitness. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN8673_AR40_501_FINAL_WEB.pdf. Published June 27, 2019. Accessed June 23, 2020.
- US Department of the Air Force. Medical examinations and standards. http://aangfs.com/wp-content/uploads/2012/10/AFI-48-123-Medical-Examination-Standards.pdf. Published January 29, 2013. Accessed June 23, 2020.
- US Navy Aeromedical Reference and Waiver Guide. Navy Medicine website. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf. Published September 4, 2019. Accessed June 17, 2020.
- Burke KR, Larrymore DC, Cho S. Treatment considerations for US military members with skin disease. Cutis. 2019:103:329-332.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;30:168-177.
- Raoof J, Hooper D, Moore A, et al. FMX101 4% topical minocycline foam for the treatment of moderate to severe acne vulgaris: efficacy and safety from a phase 3 randomized, double-blind, vehicle-controlled study. Poster presented at: 2018 Fall Clinical Dermatology Conference; October 18-21, 2018; Las Vegas, NV.
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris; results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments.J Am Acad Dermatol. 2019;80:538-549.
- Nicklas C, Rubio R, Cardenas C, et al. Comparison of efficacy of aminolaevulinic acid photodynamic therapy vs. adapalene gel plus oral doxycycline for treatment of moderate acne vulgaris—a simple, blind, randomized, and controlled trial. Photodermatol Photoimmunol Photomed. 2019;35:3-10.
- Barbaric J, Abbott R, Posadzki P, et al. Light therapies for acne [published online September 27, 2016]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD007917.pub2.
- Marson JW, Baldwin HE. New concepts, concerns, and creations in acne. Dermatol Clin. 2019;37:1-9.
- Seaton ED, Charakida A, Mouser PE, et al. Pulsed-dye laser treatment for inflammatory acne vulgaris: randomised controlled trial. Lancet Lond Engl. 2003;362:1347-1352.
- Harto A, Garcia-Morales I, Belmar P, et al. Pulsed dye laser treatment of acne. study of clinical efficacy and mechanism of action. Actas Dermosifiliogr. 2007;98:415-419.
- Leheta TM. Role of the 585-nm pulsed dye laser in the treatment of acne in comparison with other topical therapeutic modalities. J Cosmet Laser Ther Off Publ Eur Soc Laser Dermatol. 2009;11:118-124.
- Karsai S, Schmitt L, Raulin C. The pulsed-dye laser as an adjuvant treatment modality in acne vulgaris: a randomized controlled single-blinded trial. Br J Dermatol. 2010;163:395-401.
- Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions—United States. annual report 2013.https://www.cdc.gov/antibiotic-use/community/pdfs/Annual-ReportSummary_2013.pdf. Accessed June 23, 2020.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33.
Practice Points
- Acne is a common disease that may cause considerable physical and psychological morbidity. Numerous therapies are available, each with their respective risks and benefits.
- Military servicemembers face unique challenges in the management of acne due to operational and medical readiness considerations.
- Less conventional treatments such as photodynamic therapy and pulsed dye laser may be available to military servicemembers.
- Pulsed dye laser is an effective alternative treatment of acne, especially in an age of growing antibiotic resistance.
Why Is It That the Biggest Resistance With Fighting the Battle Against Bacterial Resistance Seems to Fall on Dermatology Clinicians?
This discussion focuses on antibiotic resistance in acne therapy but also includes general principles related to this subject. “Seeing is believing” is a concept we have all heard many times, and we generally can all agree with and relate to what this is saying to us. However, it is harder to get a consensus of agreement on concepts that are happening beneath the surface but are not visibly apparent. Antibiotic resistance is a concept that falls into this latter category, especially in acne treatment. Many clinicians are not convinced antibiotic resistance is clinically relevant, exclaiming “I just do not see it in my practice.” The problem is—especially in the case of acne where oral tetracycline agents commonly are prescribed—how does the clinician “see” antibiotic resistance? Clinicians do not obtain bacterial cultures or perform sensitivity testing as they might do when evaluating a suspected cutaneous infection such as folliculitis, an inflamed postsurgical wound, a purulent leg ulcer, or an abscess. Additionally, if the selected therapy is not as effective as anticipated, it may be attributed to the patient needing another type of treatment or something “stronger,” or maybe they are not fully compliant. In fact, a very possible reason for inadequate therapeutic response may be that the predominant Cutibacterium acnes strains in a particular case are proinflammatory, and many of the strains are not highly sensitive to the chosen antibiotic.1
In the United States, antibiotic resistance in C acnes is most prevalent with erythromycin, followed by clindamycin, tetracycline, doxycycline, and minocycline, respectively.2 The relative patterns of antibiotic resistance in specific geographic regions correlate with the magnitude of specific antibiotic use, and that consistent reduction in use of a given antibiotic in a community can reverse the prevalence of resistance to that antibiotic progressively over time.3 Combination therapy approaches to mitigate emergence of resistant bacteria during acne treatment with an exit plan explained up-front with the patient are important to reduce prolonged use or repeated cycles of antibiotic use and in some cases to circumvent antibiotic use and incorporate a different therapeutic approach.1-3 Interestingly, in a retrospective chart review of acne patients who were eventually treated with oral isotretinoin at dermatology practices within a major university health system, approximately two-thirds received oral antibiotics for 6 months or longer and one-third for 1 year or longer.4 It is easy for all of us to have good intentions; however, in reality it is not always easy, practical, or in the best interest of the patient to stringently enforce recommendations that are determined not to be the best option at that time. Patients get a vote, too, as long as they are fully informed of benefits vs risks.
The concern about emergence of less-sensitive bacteria during acne antibiotic treatment is not limited to discussion of C acnes resistance. Use of both oral and topical antibiotics creates “ecologic mischief,” which is the emergence of less-sensitive strains of other bacteria exposed to the antibiotic—both commensal and opportunistic—especially at anatomic sites such as the skin, nasopharyngeal region, and gastrointestinal and genitourinary tracts.5-7 Application of topical erythromycin to the face can induce erythromycin-resistant bacteria such as staphylococci on the face as well as at remote sites such as the nares (nasal vestibule) and the back.6 Antibiotics used to treat acne, predominantly oral tetracyclines, showed positive oropharyngeal cultures for Streptococcus pyogenes in 33% (13/39) of treated patients; among these positive cultures, 85% (11/13) were resistant to at least one tetracycline antibiotic.7 Importantly, the streptococcal colonization of the oropharynx in individuals taking an oral antibiotic for acne may not induce a clinically apparent pharyngitis in that individual, but that person can carry and spread that streptococcal pathogen to others. In either case, the dermatology clinician, even if he/she suspects the connection related to antibiotic selection pressure and resistance, would not “see” the antibiotic resistance, as the individuals who develop a “sore throat” or strep throat do not seek care for this problem through a dermatology office.
The first formally organized and independent group in dermatology to address antibiotic use and resistance issues was the Scientific Panel on Antibiotic Use in Dermatology, which I put together in 2004 with James J. Leyden, MD (Philadelphia, Pennsylvania) and Guy F. Webster, MD (Hockessin, Delaware), and was comprised mostly of interested dermatologists with contributions from microbiologists and infectious disease specialists. A series of meetings, publications, and presentations have emerged from this group, which now falls under the auspices of the American Acne & Rosacea Society. Through the efforts of these organizations and other groups and companies with a strong interest in combating antibiotic resistance, we continue to see slow but steady progress in enlightening dermatology clinicians to think about if and when antibiotic therapy is needed and for how long. The subject of when antibiotics are not necessary also has been addressed, including both oral and topical antibiotics in many common scenarios encountered in dermatology practice.8 Examples include incision and drainage of an inflamed epidermal cyst without antibiotic therapy and use of white petrolatum instead of a topical antibiotic after most dermatologic procedures such as biopsies, tangential procedures, and closures after excisional procedures. Overall, the potential for topical antibiotics containing bacitracin and/or neomycin to induce allergic contact dermatitis is higher than the risk for postoperative wound infection. A major reason to avoid facilitating the emergence of antibiotic-resistant bacteria is that these organisms are efficient in packaging their resistance genes along with those from other bacteria, thus creating multidrug-resistant bacterial strains. This situation creates bigger challenges with trying to select effective therapies.
A cross-sectional analysis of antibiotics prescribed by dermatologists from January 1, 2008, to December 31, 2016, performed via a large commercial prescription claims database showed that among almost 1 million courses of oral antibiotics prescribed by approximately 12,000 unique dermatology prescribers, overall antibiotic prescribing decreased 36.6%, reflecting a drop of 1.23 courses per 100 visits, with much of the reduction occurring among extended antibiotic courses for acne and rosacea.9 Dermatology clinicians appear to be increasing their consideration of treatment alternatives such as oral spironolactone in adult female patients or earlier transition to oral isotretinoin therapy before starting another cycle with the same or a different oral antibiotic. Some have increased the use of physical device therapies. Importantly, we do not want to throw out the baby with the bathwater. Oral antibiotics remain important agents for treatment of moderate to severe inflammatory acne and in rosacea when subantibiotic-dose doxycycline is not accessible or is not effective after an adequate trial of therapy. Last but not least, a full-court press with an optimal topical regimen is the foundation of acne therapy, as monotherapy with an oral antibiotic for acne is considered dermatologic heresy and for good reason.
- Del Rosso JQ, Leyden JJ. Status report on antibiotic resistance: implications for the dermatologist. Dermatol Clin. 2007;25:127-132.
- Leyden JJ, Del Rosso JQ, Webster GF. Clinical considerations in the treatment of acne vulgaris and other inflammatory skin disorders: a status report. Dermatol Clin. 2009;27:1-15.
- Del Rosso JQ, Zeichner JA. The clinical relevance of antibiotic resistance: thirteen principles that every dermatologist needs to consider when prescribing antibiotic therapy. Dermatol Clin. 2016;34:167-173.
- Nagler AR, Milam EC, Orlow SJ. The use of oral antibiotics before isotretinoin therapy in patients with acne. J Am Acad Dermatol. 2016;74:273-279.
- Del Rosso JQ, Kim GK. Topical antibiotics: therapeutic value or ecologic mischief? Dermatol Ther. 2009;22:398-406.
- Bowe WP, Leyden JJ. Clinical implications of antibiotic resistance: risk of systemic infection from Staphylococcus and Streptococcus. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, England: Informa Healthcare; 2011:125-133.
- Levy RM, Huang EY, Roling D, et al. Effect of antibiotics on the oropharyngeal flora in patients with acne. Arch Dermatol. 2003;139:467-471.
- Hirschmann JV. When antibiotics are unnecessary. Dermatol Clin. 2009;27:75-83.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297.
This discussion focuses on antibiotic resistance in acne therapy but also includes general principles related to this subject. “Seeing is believing” is a concept we have all heard many times, and we generally can all agree with and relate to what this is saying to us. However, it is harder to get a consensus of agreement on concepts that are happening beneath the surface but are not visibly apparent. Antibiotic resistance is a concept that falls into this latter category, especially in acne treatment. Many clinicians are not convinced antibiotic resistance is clinically relevant, exclaiming “I just do not see it in my practice.” The problem is—especially in the case of acne where oral tetracycline agents commonly are prescribed—how does the clinician “see” antibiotic resistance? Clinicians do not obtain bacterial cultures or perform sensitivity testing as they might do when evaluating a suspected cutaneous infection such as folliculitis, an inflamed postsurgical wound, a purulent leg ulcer, or an abscess. Additionally, if the selected therapy is not as effective as anticipated, it may be attributed to the patient needing another type of treatment or something “stronger,” or maybe they are not fully compliant. In fact, a very possible reason for inadequate therapeutic response may be that the predominant Cutibacterium acnes strains in a particular case are proinflammatory, and many of the strains are not highly sensitive to the chosen antibiotic.1
In the United States, antibiotic resistance in C acnes is most prevalent with erythromycin, followed by clindamycin, tetracycline, doxycycline, and minocycline, respectively.2 The relative patterns of antibiotic resistance in specific geographic regions correlate with the magnitude of specific antibiotic use, and that consistent reduction in use of a given antibiotic in a community can reverse the prevalence of resistance to that antibiotic progressively over time.3 Combination therapy approaches to mitigate emergence of resistant bacteria during acne treatment with an exit plan explained up-front with the patient are important to reduce prolonged use or repeated cycles of antibiotic use and in some cases to circumvent antibiotic use and incorporate a different therapeutic approach.1-3 Interestingly, in a retrospective chart review of acne patients who were eventually treated with oral isotretinoin at dermatology practices within a major university health system, approximately two-thirds received oral antibiotics for 6 months or longer and one-third for 1 year or longer.4 It is easy for all of us to have good intentions; however, in reality it is not always easy, practical, or in the best interest of the patient to stringently enforce recommendations that are determined not to be the best option at that time. Patients get a vote, too, as long as they are fully informed of benefits vs risks.
The concern about emergence of less-sensitive bacteria during acne antibiotic treatment is not limited to discussion of C acnes resistance. Use of both oral and topical antibiotics creates “ecologic mischief,” which is the emergence of less-sensitive strains of other bacteria exposed to the antibiotic—both commensal and opportunistic—especially at anatomic sites such as the skin, nasopharyngeal region, and gastrointestinal and genitourinary tracts.5-7 Application of topical erythromycin to the face can induce erythromycin-resistant bacteria such as staphylococci on the face as well as at remote sites such as the nares (nasal vestibule) and the back.6 Antibiotics used to treat acne, predominantly oral tetracyclines, showed positive oropharyngeal cultures for Streptococcus pyogenes in 33% (13/39) of treated patients; among these positive cultures, 85% (11/13) were resistant to at least one tetracycline antibiotic.7 Importantly, the streptococcal colonization of the oropharynx in individuals taking an oral antibiotic for acne may not induce a clinically apparent pharyngitis in that individual, but that person can carry and spread that streptococcal pathogen to others. In either case, the dermatology clinician, even if he/she suspects the connection related to antibiotic selection pressure and resistance, would not “see” the antibiotic resistance, as the individuals who develop a “sore throat” or strep throat do not seek care for this problem through a dermatology office.
The first formally organized and independent group in dermatology to address antibiotic use and resistance issues was the Scientific Panel on Antibiotic Use in Dermatology, which I put together in 2004 with James J. Leyden, MD (Philadelphia, Pennsylvania) and Guy F. Webster, MD (Hockessin, Delaware), and was comprised mostly of interested dermatologists with contributions from microbiologists and infectious disease specialists. A series of meetings, publications, and presentations have emerged from this group, which now falls under the auspices of the American Acne & Rosacea Society. Through the efforts of these organizations and other groups and companies with a strong interest in combating antibiotic resistance, we continue to see slow but steady progress in enlightening dermatology clinicians to think about if and when antibiotic therapy is needed and for how long. The subject of when antibiotics are not necessary also has been addressed, including both oral and topical antibiotics in many common scenarios encountered in dermatology practice.8 Examples include incision and drainage of an inflamed epidermal cyst without antibiotic therapy and use of white petrolatum instead of a topical antibiotic after most dermatologic procedures such as biopsies, tangential procedures, and closures after excisional procedures. Overall, the potential for topical antibiotics containing bacitracin and/or neomycin to induce allergic contact dermatitis is higher than the risk for postoperative wound infection. A major reason to avoid facilitating the emergence of antibiotic-resistant bacteria is that these organisms are efficient in packaging their resistance genes along with those from other bacteria, thus creating multidrug-resistant bacterial strains. This situation creates bigger challenges with trying to select effective therapies.
A cross-sectional analysis of antibiotics prescribed by dermatologists from January 1, 2008, to December 31, 2016, performed via a large commercial prescription claims database showed that among almost 1 million courses of oral antibiotics prescribed by approximately 12,000 unique dermatology prescribers, overall antibiotic prescribing decreased 36.6%, reflecting a drop of 1.23 courses per 100 visits, with much of the reduction occurring among extended antibiotic courses for acne and rosacea.9 Dermatology clinicians appear to be increasing their consideration of treatment alternatives such as oral spironolactone in adult female patients or earlier transition to oral isotretinoin therapy before starting another cycle with the same or a different oral antibiotic. Some have increased the use of physical device therapies. Importantly, we do not want to throw out the baby with the bathwater. Oral antibiotics remain important agents for treatment of moderate to severe inflammatory acne and in rosacea when subantibiotic-dose doxycycline is not accessible or is not effective after an adequate trial of therapy. Last but not least, a full-court press with an optimal topical regimen is the foundation of acne therapy, as monotherapy with an oral antibiotic for acne is considered dermatologic heresy and for good reason.
This discussion focuses on antibiotic resistance in acne therapy but also includes general principles related to this subject. “Seeing is believing” is a concept we have all heard many times, and we generally can all agree with and relate to what this is saying to us. However, it is harder to get a consensus of agreement on concepts that are happening beneath the surface but are not visibly apparent. Antibiotic resistance is a concept that falls into this latter category, especially in acne treatment. Many clinicians are not convinced antibiotic resistance is clinically relevant, exclaiming “I just do not see it in my practice.” The problem is—especially in the case of acne where oral tetracycline agents commonly are prescribed—how does the clinician “see” antibiotic resistance? Clinicians do not obtain bacterial cultures or perform sensitivity testing as they might do when evaluating a suspected cutaneous infection such as folliculitis, an inflamed postsurgical wound, a purulent leg ulcer, or an abscess. Additionally, if the selected therapy is not as effective as anticipated, it may be attributed to the patient needing another type of treatment or something “stronger,” or maybe they are not fully compliant. In fact, a very possible reason for inadequate therapeutic response may be that the predominant Cutibacterium acnes strains in a particular case are proinflammatory, and many of the strains are not highly sensitive to the chosen antibiotic.1
In the United States, antibiotic resistance in C acnes is most prevalent with erythromycin, followed by clindamycin, tetracycline, doxycycline, and minocycline, respectively.2 The relative patterns of antibiotic resistance in specific geographic regions correlate with the magnitude of specific antibiotic use, and that consistent reduction in use of a given antibiotic in a community can reverse the prevalence of resistance to that antibiotic progressively over time.3 Combination therapy approaches to mitigate emergence of resistant bacteria during acne treatment with an exit plan explained up-front with the patient are important to reduce prolonged use or repeated cycles of antibiotic use and in some cases to circumvent antibiotic use and incorporate a different therapeutic approach.1-3 Interestingly, in a retrospective chart review of acne patients who were eventually treated with oral isotretinoin at dermatology practices within a major university health system, approximately two-thirds received oral antibiotics for 6 months or longer and one-third for 1 year or longer.4 It is easy for all of us to have good intentions; however, in reality it is not always easy, practical, or in the best interest of the patient to stringently enforce recommendations that are determined not to be the best option at that time. Patients get a vote, too, as long as they are fully informed of benefits vs risks.
The concern about emergence of less-sensitive bacteria during acne antibiotic treatment is not limited to discussion of C acnes resistance. Use of both oral and topical antibiotics creates “ecologic mischief,” which is the emergence of less-sensitive strains of other bacteria exposed to the antibiotic—both commensal and opportunistic—especially at anatomic sites such as the skin, nasopharyngeal region, and gastrointestinal and genitourinary tracts.5-7 Application of topical erythromycin to the face can induce erythromycin-resistant bacteria such as staphylococci on the face as well as at remote sites such as the nares (nasal vestibule) and the back.6 Antibiotics used to treat acne, predominantly oral tetracyclines, showed positive oropharyngeal cultures for Streptococcus pyogenes in 33% (13/39) of treated patients; among these positive cultures, 85% (11/13) were resistant to at least one tetracycline antibiotic.7 Importantly, the streptococcal colonization of the oropharynx in individuals taking an oral antibiotic for acne may not induce a clinically apparent pharyngitis in that individual, but that person can carry and spread that streptococcal pathogen to others. In either case, the dermatology clinician, even if he/she suspects the connection related to antibiotic selection pressure and resistance, would not “see” the antibiotic resistance, as the individuals who develop a “sore throat” or strep throat do not seek care for this problem through a dermatology office.
The first formally organized and independent group in dermatology to address antibiotic use and resistance issues was the Scientific Panel on Antibiotic Use in Dermatology, which I put together in 2004 with James J. Leyden, MD (Philadelphia, Pennsylvania) and Guy F. Webster, MD (Hockessin, Delaware), and was comprised mostly of interested dermatologists with contributions from microbiologists and infectious disease specialists. A series of meetings, publications, and presentations have emerged from this group, which now falls under the auspices of the American Acne & Rosacea Society. Through the efforts of these organizations and other groups and companies with a strong interest in combating antibiotic resistance, we continue to see slow but steady progress in enlightening dermatology clinicians to think about if and when antibiotic therapy is needed and for how long. The subject of when antibiotics are not necessary also has been addressed, including both oral and topical antibiotics in many common scenarios encountered in dermatology practice.8 Examples include incision and drainage of an inflamed epidermal cyst without antibiotic therapy and use of white petrolatum instead of a topical antibiotic after most dermatologic procedures such as biopsies, tangential procedures, and closures after excisional procedures. Overall, the potential for topical antibiotics containing bacitracin and/or neomycin to induce allergic contact dermatitis is higher than the risk for postoperative wound infection. A major reason to avoid facilitating the emergence of antibiotic-resistant bacteria is that these organisms are efficient in packaging their resistance genes along with those from other bacteria, thus creating multidrug-resistant bacterial strains. This situation creates bigger challenges with trying to select effective therapies.
A cross-sectional analysis of antibiotics prescribed by dermatologists from January 1, 2008, to December 31, 2016, performed via a large commercial prescription claims database showed that among almost 1 million courses of oral antibiotics prescribed by approximately 12,000 unique dermatology prescribers, overall antibiotic prescribing decreased 36.6%, reflecting a drop of 1.23 courses per 100 visits, with much of the reduction occurring among extended antibiotic courses for acne and rosacea.9 Dermatology clinicians appear to be increasing their consideration of treatment alternatives such as oral spironolactone in adult female patients or earlier transition to oral isotretinoin therapy before starting another cycle with the same or a different oral antibiotic. Some have increased the use of physical device therapies. Importantly, we do not want to throw out the baby with the bathwater. Oral antibiotics remain important agents for treatment of moderate to severe inflammatory acne and in rosacea when subantibiotic-dose doxycycline is not accessible or is not effective after an adequate trial of therapy. Last but not least, a full-court press with an optimal topical regimen is the foundation of acne therapy, as monotherapy with an oral antibiotic for acne is considered dermatologic heresy and for good reason.
- Del Rosso JQ, Leyden JJ. Status report on antibiotic resistance: implications for the dermatologist. Dermatol Clin. 2007;25:127-132.
- Leyden JJ, Del Rosso JQ, Webster GF. Clinical considerations in the treatment of acne vulgaris and other inflammatory skin disorders: a status report. Dermatol Clin. 2009;27:1-15.
- Del Rosso JQ, Zeichner JA. The clinical relevance of antibiotic resistance: thirteen principles that every dermatologist needs to consider when prescribing antibiotic therapy. Dermatol Clin. 2016;34:167-173.
- Nagler AR, Milam EC, Orlow SJ. The use of oral antibiotics before isotretinoin therapy in patients with acne. J Am Acad Dermatol. 2016;74:273-279.
- Del Rosso JQ, Kim GK. Topical antibiotics: therapeutic value or ecologic mischief? Dermatol Ther. 2009;22:398-406.
- Bowe WP, Leyden JJ. Clinical implications of antibiotic resistance: risk of systemic infection from Staphylococcus and Streptococcus. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, England: Informa Healthcare; 2011:125-133.
- Levy RM, Huang EY, Roling D, et al. Effect of antibiotics on the oropharyngeal flora in patients with acne. Arch Dermatol. 2003;139:467-471.
- Hirschmann JV. When antibiotics are unnecessary. Dermatol Clin. 2009;27:75-83.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297.
- Del Rosso JQ, Leyden JJ. Status report on antibiotic resistance: implications for the dermatologist. Dermatol Clin. 2007;25:127-132.
- Leyden JJ, Del Rosso JQ, Webster GF. Clinical considerations in the treatment of acne vulgaris and other inflammatory skin disorders: a status report. Dermatol Clin. 2009;27:1-15.
- Del Rosso JQ, Zeichner JA. The clinical relevance of antibiotic resistance: thirteen principles that every dermatologist needs to consider when prescribing antibiotic therapy. Dermatol Clin. 2016;34:167-173.
- Nagler AR, Milam EC, Orlow SJ. The use of oral antibiotics before isotretinoin therapy in patients with acne. J Am Acad Dermatol. 2016;74:273-279.
- Del Rosso JQ, Kim GK. Topical antibiotics: therapeutic value or ecologic mischief? Dermatol Ther. 2009;22:398-406.
- Bowe WP, Leyden JJ. Clinical implications of antibiotic resistance: risk of systemic infection from Staphylococcus and Streptococcus. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, England: Informa Healthcare; 2011:125-133.
- Levy RM, Huang EY, Roling D, et al. Effect of antibiotics on the oropharyngeal flora in patients with acne. Arch Dermatol. 2003;139:467-471.
- Hirschmann JV. When antibiotics are unnecessary. Dermatol Clin. 2009;27:75-83.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297.
Severe Phymatous Rosacea of the Nose, Cheeks, and Chin Treated With Hydrosurgery
Phymatous rosacea is a rare and severe form of rosacea that manifests as disfiguring soft-tissue hypertrophy and hyperplasia as well as fibrosis of the sebaceous glands. 1 Treatments for phymatous rosacea include pharmacotherapeutic and surgical modalities; most cases are treated surgically. Surgical modalities vary, ranging from cryosurgery to conventional excision, and consensus guidelines for surgical management do not exist because data are largely limited to case reports and small case series. 2 The Versajet II Hydrosurgery System (Smith-Nephew) is a high-pressure, pulsatile lavage system that has been used for phymatous rosacea and then only for rosacea of the nose (rhinophyma). We present the case of a patient with phymatous rosacea of the nose, cheeks, and chin who was successfully treated with the Versajet II Hydrosurgery System beyond just the nose region.
Case Report
A 75-year-old man presented to the dermatology clinic for evaluation of severe phymatous rosacea of the nose, cheeks, and chin that had been present for several years. Examination revealed verruciform, thickened, erythematous skin of the nose, cheeks, and chin; marked blue-gray hyperpigmentation on the neck and hands; generalized facial redness; and cystic and depressed scars (Figure 1). The patient had been treated with topical metronidazole without response, and isotretinoin worsened the symptoms. He also was taking minocycline but stopped it at our request because of concern that the drug was causing the blue-gray hyperpigmentation. The patient was referred to plastic surgery and tangential excision was recommended. Fractional ablative laser therapy was considered but deferred because the patient wanted quicker results.

The patient received tangential excision of the phymatous areas of the chin, bilateral cheeks, and nose with the Versajet II Hydrosurgery System until a pleasing contour was noted. At 1-month follow-up, the patient had an excellent contour of the nose, cheeks, and chin (Figure 2).
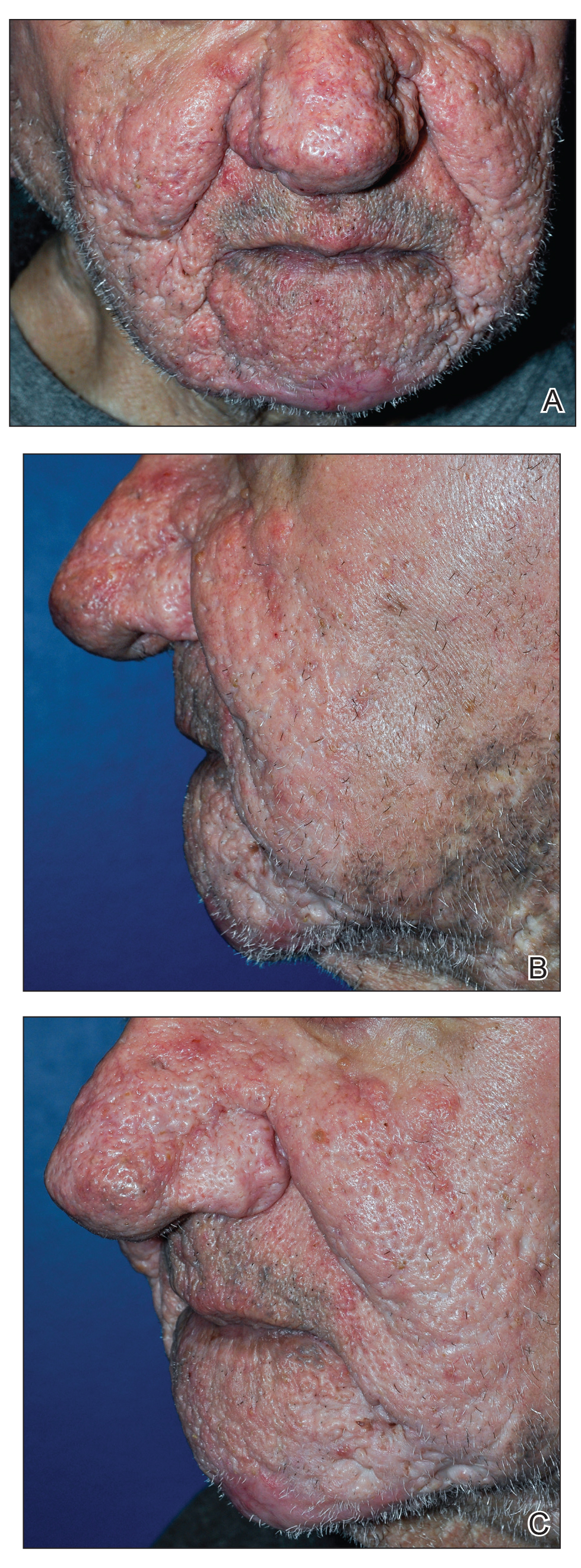
Comment
Phymatous rosacea is a rare disfiguring disease that most commonly presents on the nose but also can affect the chin, cheeks, eyelids, ears, and forehead. Incidence is greater in individuals of Scottish descent and in men due to the influence of androgens. The etiology of the condition is unknown.1
Aside from clinical findings of hyperplastic and fibrotic sebaceous glands in conjunction with enlargement of the affected facial areas, histopathologic findings of phymatous rosacea vary but typically include hypertrophy of subcutaneous tissue, enlarged sebaceous ducts filled with keratin and sebum, atrophy of the dermis, and abnormal vascular development in the form of telangiectases.
Phymatous rosacea adversely affects patients’ physical, mental, and social well-being. Left untreated, it can cause nasal obstruction and recurrent bacterial infections. Furthermore, because of the potential extent of facial deformity, phymatous rosacea can be highly stigmatizing.3 Nonmelanoma skin cancers have been reported within phymatous skin, but evidence of an association between the 2 diseases remains inconclusive.4 Excised tissue from our patient was not submitted to pathology for analysis.
Given the far-reaching physical and psychological consequences of phymatous rosacea, treatment is critical but, regrettably, challenging. Although medical and surgical interventions exist, surgery is the most common practice. Oral isotretinoin may help, but many cases are recalcitrant, as was the disease in our patient. Therefore, procedural remedies often are sought, including scalpel excision, cryosurgery, argon laser, CO2 laser, dermabrasion, and electrocautery.2
Our patient underwent Versajet II Hydrosurgery System treatment of the phymatous rosacea on the nose, cheeks, and chin. Versajet is not yet commonly used to treat phymatous rosacea, likely due to the upfront cost of obtaining a new device, lack of physician familiarity, and few reports of its use for phymatous skin. A search of PubMed, EMBASE, and the Web of Science using the terms Rosacea AND (Versajet OR Hydrosurgery) yielded only 6 cases of rosacea treated by hydrosurgery; all were limited to rhinophyma and reported excellent cosmetic and functional results.5-10 Our case was unique in that hydrosurgery was used to treat phymatous rosacea beyond the nose.
Hydrosurgery has many advantages in the treatment of phymatous rosacea and other conditions in which surgical debridement is necessary, such as burns and wounds. A randomized clinical trial demonstrated that hydrosurgery is more cost-effective than conventional excision because of decreased operative time and intraoperative blood loss, fewer debridement procedures, and fewer postoperative complications.11
Rennekampff et al12 showed that Versajet debridement is superior to conventional surgery in contouring facial and acral sites and has a lower probability of infection. They proposed that by running a highly pressurized constant stream of saline across the device, Versajet clears blood and debris from the surgical site during excision.12 Hydrosurgical debridement also has been shown to reduce Staphylococcus aureus inoculate levels from in vitro–contaminated equine models significantly more than conventional debridement methods (P<.05).13
Versajet surgery appears to be well tolerated, with side effects comparable to those of classic surgical excision. A randomized controlled trial in burn patients in which treatment with Versajet was compared to traditional debridement found no significant difference in postoperative pain, healing time, and contracture rate.13
Overall, tangential excision of our patient’s phymatous rosacea using the Versajet II Hydrosurgery System yielded excellent contouring. However, due to the paucity of literature on the subject, it is difficult to discern the optimal treatment modality. Therefore, more research—ideally randomized trials—should be pursued to examine the comparative effectiveness of different interventions for phymatous rosacea.
- Curnier A, Choudhary S. Rhinophyma: dispelling the myths. Plast Reconstr Surg. 2004;114:351-354.
- Sadick H, Goepel B, Bersch C, et al. Rhinophyma: diagnosis and treatment options for a disfiguring tumor of the nose. Ann Plast Surg. 2008;61:114-120.
- Dirschka T, Micali G, Papadopoulos L, et al. Perceptions on the psychological impact of facial erythema associated with rosacea: results of international survey. Dermatol Ther (Heidelb). 2015;5:117-127.
- Lazzeri D, Colizzi L, Licata G, et al. Malignancies within rhinophyma: report of three new cases and review of the literature. Aesthetic Plast Surg. 2012;36:396-405.
- Dunne JA, Saleh DB, Rawlins JM. Management of rhinophyma with Versajet™ and ReCell®. Br J Oral Maxillofac Surg. 2013;51:e282-e284.
- Yildiz K, Kayan BR, Dulgeroglu T, et al. Treatment of rhinophyma with the Versajet™ Hydrosurgery System and autologous cell suspension (ReCELL®): a case report. J Cosmet Laser Ther. 2018;20:114-116.
- Nicolas J, Garmi R, Labbé D, et al. The role of Versajet in the surgical treatment of rhinophyma. case report. Ann Chir Plast Esthet. 2009;54:78-81.
- Novati FC, Franchi A, Roggio T, et al. Treatment of a double-giant rhinophyma with electrocautery and Versajet Hydrosurgery System. Ann Ital Chir. 2015;86. pii: S2239253X15023269.
- Taghizadeh R, Mackay SP, Gilbert PM. Treatment of rhinophyma with the Versajet Hydrosurgery System. J Plast Reconstr Aesthet Surg. 2008;61:330-333.
- Wong WL, Wong She R, Mathy JA. Rhinophyma treatment using Versajet Hydrosurgery. ANZ J Surg. 2017;87:E331-E332.
- Liu J, Ko JH, Secretov E, et al. Comparing the hydrosurgery system to conventional debridement techniques for the treatment of delayed healing wounds: a prospective, randomised clinical trial to investigate clinical efficacy and cost-effectiveness. Int Wound J. 2015;12:456-461.
- Rennekampff H-O, Schaller H-E, Wisser D, et al. Debridement of burn wounds with a water jet surgical tool. Burns. 2006;32:64-69.
- Skarlina EM, Wilmink JM, Fall N, et al. Effectiveness of conventional and hydrosurgical debridement methods in reducing Staphylococcus aureus inoculation of equine muscle in vitro. Equine Vet J. 2015;47:218-222.
Phymatous rosacea is a rare and severe form of rosacea that manifests as disfiguring soft-tissue hypertrophy and hyperplasia as well as fibrosis of the sebaceous glands. 1 Treatments for phymatous rosacea include pharmacotherapeutic and surgical modalities; most cases are treated surgically. Surgical modalities vary, ranging from cryosurgery to conventional excision, and consensus guidelines for surgical management do not exist because data are largely limited to case reports and small case series. 2 The Versajet II Hydrosurgery System (Smith-Nephew) is a high-pressure, pulsatile lavage system that has been used for phymatous rosacea and then only for rosacea of the nose (rhinophyma). We present the case of a patient with phymatous rosacea of the nose, cheeks, and chin who was successfully treated with the Versajet II Hydrosurgery System beyond just the nose region.
Case Report
A 75-year-old man presented to the dermatology clinic for evaluation of severe phymatous rosacea of the nose, cheeks, and chin that had been present for several years. Examination revealed verruciform, thickened, erythematous skin of the nose, cheeks, and chin; marked blue-gray hyperpigmentation on the neck and hands; generalized facial redness; and cystic and depressed scars (Figure 1). The patient had been treated with topical metronidazole without response, and isotretinoin worsened the symptoms. He also was taking minocycline but stopped it at our request because of concern that the drug was causing the blue-gray hyperpigmentation. The patient was referred to plastic surgery and tangential excision was recommended. Fractional ablative laser therapy was considered but deferred because the patient wanted quicker results.

The patient received tangential excision of the phymatous areas of the chin, bilateral cheeks, and nose with the Versajet II Hydrosurgery System until a pleasing contour was noted. At 1-month follow-up, the patient had an excellent contour of the nose, cheeks, and chin (Figure 2).

Comment
Phymatous rosacea is a rare disfiguring disease that most commonly presents on the nose but also can affect the chin, cheeks, eyelids, ears, and forehead. Incidence is greater in individuals of Scottish descent and in men due to the influence of androgens. The etiology of the condition is unknown.1
Aside from clinical findings of hyperplastic and fibrotic sebaceous glands in conjunction with enlargement of the affected facial areas, histopathologic findings of phymatous rosacea vary but typically include hypertrophy of subcutaneous tissue, enlarged sebaceous ducts filled with keratin and sebum, atrophy of the dermis, and abnormal vascular development in the form of telangiectases.
Phymatous rosacea adversely affects patients’ physical, mental, and social well-being. Left untreated, it can cause nasal obstruction and recurrent bacterial infections. Furthermore, because of the potential extent of facial deformity, phymatous rosacea can be highly stigmatizing.3 Nonmelanoma skin cancers have been reported within phymatous skin, but evidence of an association between the 2 diseases remains inconclusive.4 Excised tissue from our patient was not submitted to pathology for analysis.
Given the far-reaching physical and psychological consequences of phymatous rosacea, treatment is critical but, regrettably, challenging. Although medical and surgical interventions exist, surgery is the most common practice. Oral isotretinoin may help, but many cases are recalcitrant, as was the disease in our patient. Therefore, procedural remedies often are sought, including scalpel excision, cryosurgery, argon laser, CO2 laser, dermabrasion, and electrocautery.2
Our patient underwent Versajet II Hydrosurgery System treatment of the phymatous rosacea on the nose, cheeks, and chin. Versajet is not yet commonly used to treat phymatous rosacea, likely due to the upfront cost of obtaining a new device, lack of physician familiarity, and few reports of its use for phymatous skin. A search of PubMed, EMBASE, and the Web of Science using the terms Rosacea AND (Versajet OR Hydrosurgery) yielded only 6 cases of rosacea treated by hydrosurgery; all were limited to rhinophyma and reported excellent cosmetic and functional results.5-10 Our case was unique in that hydrosurgery was used to treat phymatous rosacea beyond the nose.
Hydrosurgery has many advantages in the treatment of phymatous rosacea and other conditions in which surgical debridement is necessary, such as burns and wounds. A randomized clinical trial demonstrated that hydrosurgery is more cost-effective than conventional excision because of decreased operative time and intraoperative blood loss, fewer debridement procedures, and fewer postoperative complications.11
Rennekampff et al12 showed that Versajet debridement is superior to conventional surgery in contouring facial and acral sites and has a lower probability of infection. They proposed that by running a highly pressurized constant stream of saline across the device, Versajet clears blood and debris from the surgical site during excision.12 Hydrosurgical debridement also has been shown to reduce Staphylococcus aureus inoculate levels from in vitro–contaminated equine models significantly more than conventional debridement methods (P<.05).13
Versajet surgery appears to be well tolerated, with side effects comparable to those of classic surgical excision. A randomized controlled trial in burn patients in which treatment with Versajet was compared to traditional debridement found no significant difference in postoperative pain, healing time, and contracture rate.13
Overall, tangential excision of our patient’s phymatous rosacea using the Versajet II Hydrosurgery System yielded excellent contouring. However, due to the paucity of literature on the subject, it is difficult to discern the optimal treatment modality. Therefore, more research—ideally randomized trials—should be pursued to examine the comparative effectiveness of different interventions for phymatous rosacea.
Phymatous rosacea is a rare and severe form of rosacea that manifests as disfiguring soft-tissue hypertrophy and hyperplasia as well as fibrosis of the sebaceous glands. 1 Treatments for phymatous rosacea include pharmacotherapeutic and surgical modalities; most cases are treated surgically. Surgical modalities vary, ranging from cryosurgery to conventional excision, and consensus guidelines for surgical management do not exist because data are largely limited to case reports and small case series. 2 The Versajet II Hydrosurgery System (Smith-Nephew) is a high-pressure, pulsatile lavage system that has been used for phymatous rosacea and then only for rosacea of the nose (rhinophyma). We present the case of a patient with phymatous rosacea of the nose, cheeks, and chin who was successfully treated with the Versajet II Hydrosurgery System beyond just the nose region.
Case Report
A 75-year-old man presented to the dermatology clinic for evaluation of severe phymatous rosacea of the nose, cheeks, and chin that had been present for several years. Examination revealed verruciform, thickened, erythematous skin of the nose, cheeks, and chin; marked blue-gray hyperpigmentation on the neck and hands; generalized facial redness; and cystic and depressed scars (Figure 1). The patient had been treated with topical metronidazole without response, and isotretinoin worsened the symptoms. He also was taking minocycline but stopped it at our request because of concern that the drug was causing the blue-gray hyperpigmentation. The patient was referred to plastic surgery and tangential excision was recommended. Fractional ablative laser therapy was considered but deferred because the patient wanted quicker results.

The patient received tangential excision of the phymatous areas of the chin, bilateral cheeks, and nose with the Versajet II Hydrosurgery System until a pleasing contour was noted. At 1-month follow-up, the patient had an excellent contour of the nose, cheeks, and chin (Figure 2).

Comment
Phymatous rosacea is a rare disfiguring disease that most commonly presents on the nose but also can affect the chin, cheeks, eyelids, ears, and forehead. Incidence is greater in individuals of Scottish descent and in men due to the influence of androgens. The etiology of the condition is unknown.1
Aside from clinical findings of hyperplastic and fibrotic sebaceous glands in conjunction with enlargement of the affected facial areas, histopathologic findings of phymatous rosacea vary but typically include hypertrophy of subcutaneous tissue, enlarged sebaceous ducts filled with keratin and sebum, atrophy of the dermis, and abnormal vascular development in the form of telangiectases.
Phymatous rosacea adversely affects patients’ physical, mental, and social well-being. Left untreated, it can cause nasal obstruction and recurrent bacterial infections. Furthermore, because of the potential extent of facial deformity, phymatous rosacea can be highly stigmatizing.3 Nonmelanoma skin cancers have been reported within phymatous skin, but evidence of an association between the 2 diseases remains inconclusive.4 Excised tissue from our patient was not submitted to pathology for analysis.
Given the far-reaching physical and psychological consequences of phymatous rosacea, treatment is critical but, regrettably, challenging. Although medical and surgical interventions exist, surgery is the most common practice. Oral isotretinoin may help, but many cases are recalcitrant, as was the disease in our patient. Therefore, procedural remedies often are sought, including scalpel excision, cryosurgery, argon laser, CO2 laser, dermabrasion, and electrocautery.2
Our patient underwent Versajet II Hydrosurgery System treatment of the phymatous rosacea on the nose, cheeks, and chin. Versajet is not yet commonly used to treat phymatous rosacea, likely due to the upfront cost of obtaining a new device, lack of physician familiarity, and few reports of its use for phymatous skin. A search of PubMed, EMBASE, and the Web of Science using the terms Rosacea AND (Versajet OR Hydrosurgery) yielded only 6 cases of rosacea treated by hydrosurgery; all were limited to rhinophyma and reported excellent cosmetic and functional results.5-10 Our case was unique in that hydrosurgery was used to treat phymatous rosacea beyond the nose.
Hydrosurgery has many advantages in the treatment of phymatous rosacea and other conditions in which surgical debridement is necessary, such as burns and wounds. A randomized clinical trial demonstrated that hydrosurgery is more cost-effective than conventional excision because of decreased operative time and intraoperative blood loss, fewer debridement procedures, and fewer postoperative complications.11
Rennekampff et al12 showed that Versajet debridement is superior to conventional surgery in contouring facial and acral sites and has a lower probability of infection. They proposed that by running a highly pressurized constant stream of saline across the device, Versajet clears blood and debris from the surgical site during excision.12 Hydrosurgical debridement also has been shown to reduce Staphylococcus aureus inoculate levels from in vitro–contaminated equine models significantly more than conventional debridement methods (P<.05).13
Versajet surgery appears to be well tolerated, with side effects comparable to those of classic surgical excision. A randomized controlled trial in burn patients in which treatment with Versajet was compared to traditional debridement found no significant difference in postoperative pain, healing time, and contracture rate.13
Overall, tangential excision of our patient’s phymatous rosacea using the Versajet II Hydrosurgery System yielded excellent contouring. However, due to the paucity of literature on the subject, it is difficult to discern the optimal treatment modality. Therefore, more research—ideally randomized trials—should be pursued to examine the comparative effectiveness of different interventions for phymatous rosacea.
- Curnier A, Choudhary S. Rhinophyma: dispelling the myths. Plast Reconstr Surg. 2004;114:351-354.
- Sadick H, Goepel B, Bersch C, et al. Rhinophyma: diagnosis and treatment options for a disfiguring tumor of the nose. Ann Plast Surg. 2008;61:114-120.
- Dirschka T, Micali G, Papadopoulos L, et al. Perceptions on the psychological impact of facial erythema associated with rosacea: results of international survey. Dermatol Ther (Heidelb). 2015;5:117-127.
- Lazzeri D, Colizzi L, Licata G, et al. Malignancies within rhinophyma: report of three new cases and review of the literature. Aesthetic Plast Surg. 2012;36:396-405.
- Dunne JA, Saleh DB, Rawlins JM. Management of rhinophyma with Versajet™ and ReCell®. Br J Oral Maxillofac Surg. 2013;51:e282-e284.
- Yildiz K, Kayan BR, Dulgeroglu T, et al. Treatment of rhinophyma with the Versajet™ Hydrosurgery System and autologous cell suspension (ReCELL®): a case report. J Cosmet Laser Ther. 2018;20:114-116.
- Nicolas J, Garmi R, Labbé D, et al. The role of Versajet in the surgical treatment of rhinophyma. case report. Ann Chir Plast Esthet. 2009;54:78-81.
- Novati FC, Franchi A, Roggio T, et al. Treatment of a double-giant rhinophyma with electrocautery and Versajet Hydrosurgery System. Ann Ital Chir. 2015;86. pii: S2239253X15023269.
- Taghizadeh R, Mackay SP, Gilbert PM. Treatment of rhinophyma with the Versajet Hydrosurgery System. J Plast Reconstr Aesthet Surg. 2008;61:330-333.
- Wong WL, Wong She R, Mathy JA. Rhinophyma treatment using Versajet Hydrosurgery. ANZ J Surg. 2017;87:E331-E332.
- Liu J, Ko JH, Secretov E, et al. Comparing the hydrosurgery system to conventional debridement techniques for the treatment of delayed healing wounds: a prospective, randomised clinical trial to investigate clinical efficacy and cost-effectiveness. Int Wound J. 2015;12:456-461.
- Rennekampff H-O, Schaller H-E, Wisser D, et al. Debridement of burn wounds with a water jet surgical tool. Burns. 2006;32:64-69.
- Skarlina EM, Wilmink JM, Fall N, et al. Effectiveness of conventional and hydrosurgical debridement methods in reducing Staphylococcus aureus inoculation of equine muscle in vitro. Equine Vet J. 2015;47:218-222.
- Curnier A, Choudhary S. Rhinophyma: dispelling the myths. Plast Reconstr Surg. 2004;114:351-354.
- Sadick H, Goepel B, Bersch C, et al. Rhinophyma: diagnosis and treatment options for a disfiguring tumor of the nose. Ann Plast Surg. 2008;61:114-120.
- Dirschka T, Micali G, Papadopoulos L, et al. Perceptions on the psychological impact of facial erythema associated with rosacea: results of international survey. Dermatol Ther (Heidelb). 2015;5:117-127.
- Lazzeri D, Colizzi L, Licata G, et al. Malignancies within rhinophyma: report of three new cases and review of the literature. Aesthetic Plast Surg. 2012;36:396-405.
- Dunne JA, Saleh DB, Rawlins JM. Management of rhinophyma with Versajet™ and ReCell®. Br J Oral Maxillofac Surg. 2013;51:e282-e284.
- Yildiz K, Kayan BR, Dulgeroglu T, et al. Treatment of rhinophyma with the Versajet™ Hydrosurgery System and autologous cell suspension (ReCELL®): a case report. J Cosmet Laser Ther. 2018;20:114-116.
- Nicolas J, Garmi R, Labbé D, et al. The role of Versajet in the surgical treatment of rhinophyma. case report. Ann Chir Plast Esthet. 2009;54:78-81.
- Novati FC, Franchi A, Roggio T, et al. Treatment of a double-giant rhinophyma with electrocautery and Versajet Hydrosurgery System. Ann Ital Chir. 2015;86. pii: S2239253X15023269.
- Taghizadeh R, Mackay SP, Gilbert PM. Treatment of rhinophyma with the Versajet Hydrosurgery System. J Plast Reconstr Aesthet Surg. 2008;61:330-333.
- Wong WL, Wong She R, Mathy JA. Rhinophyma treatment using Versajet Hydrosurgery. ANZ J Surg. 2017;87:E331-E332.
- Liu J, Ko JH, Secretov E, et al. Comparing the hydrosurgery system to conventional debridement techniques for the treatment of delayed healing wounds: a prospective, randomised clinical trial to investigate clinical efficacy and cost-effectiveness. Int Wound J. 2015;12:456-461.
- Rennekampff H-O, Schaller H-E, Wisser D, et al. Debridement of burn wounds with a water jet surgical tool. Burns. 2006;32:64-69.
- Skarlina EM, Wilmink JM, Fall N, et al. Effectiveness of conventional and hydrosurgical debridement methods in reducing Staphylococcus aureus inoculation of equine muscle in vitro. Equine Vet J. 2015;47:218-222.
Practice Points
- Phymatous rosacea is a rare disfiguring disease that most commonly affects men and can have considerable effects on a patient’s physical, mental, and social well-being.
- Treatment of phymatous rosacea usually is surgical; however, no consensus guidelines exist for best surgical management.
- The Versajet II Hydrosurgery System can be useful and effective for the treatment of phymatous rosacea, not only on the nose but elsewhere on the face.
Rapid Screening of Invasive Fungal Infections in the Hospital Setting Using the (1,3)-β-D-glucan Assay
Practice Gap
Invasive fungal infections are a leading cause of morbidity and mortality among neutropenic, immunocompromised, and critically ill patients. Candida species are the most common cause of fungemia, with portals of entry into the bloodstream including the gastrointestinal tract, contaminated intravascular catheters, and localized foci of infection.1 Diagnosis of invasive candidiasis remains challenging due to an absence of specific clinical signs and symptoms, varying from a mild fever that is unresponsive to antibiotics to florid sepsis. When present, clinical clues may include chorioretinitis; muscle abscesses; and skin eruptions, characteristically with Candida tropicalis. Cutaneous manifestations of disseminated Candida infections appear in only 13% of affected patients.1 The lesions typically present as 5- to 10-mm pink dermal papules or painless pustules on an erythematous base and may be singular, localized, or diffuse in distribution. Body regions normally involved are the trunk, arms, and legs, rarely the head and neck.1 Cutaneous lesions often develop at a time when patients are febrile, are not responding to antibiotics, and are clinically deteriorating.
A 15-year-old adolescent boy with pre–B-cell acute lymphoblastic leukemia was admitted with febrile neutropenia for presumed septic shock secondary to an unknown infectious etiology. The patient was started on broad-spectrum intravenous antibiotics, and blood cultures were obtained. On the second day of hospitalization, he developed approximately 10 to 15 discrete, 3- to 6-mm, pink to violaceous papules scattered on the chest and arms (Figure 1). Over several hours, the number of lesions increased to more than 50 with involvement of the legs (Figure 2). A punch biopsy of lesional skin from the left dorsal wrist demonstrated a circumscribed abscess of yeast in the papillary dermis, which was highlighted by periodic acid–Schiff staining with minimal associated inflammation (Figure 3). Blood and tissue cultures persistently grew C tropicalis. The patient was started on intravenous liposomal amphotericin B but died on day 5 of hospitalization after developing endocarditis.
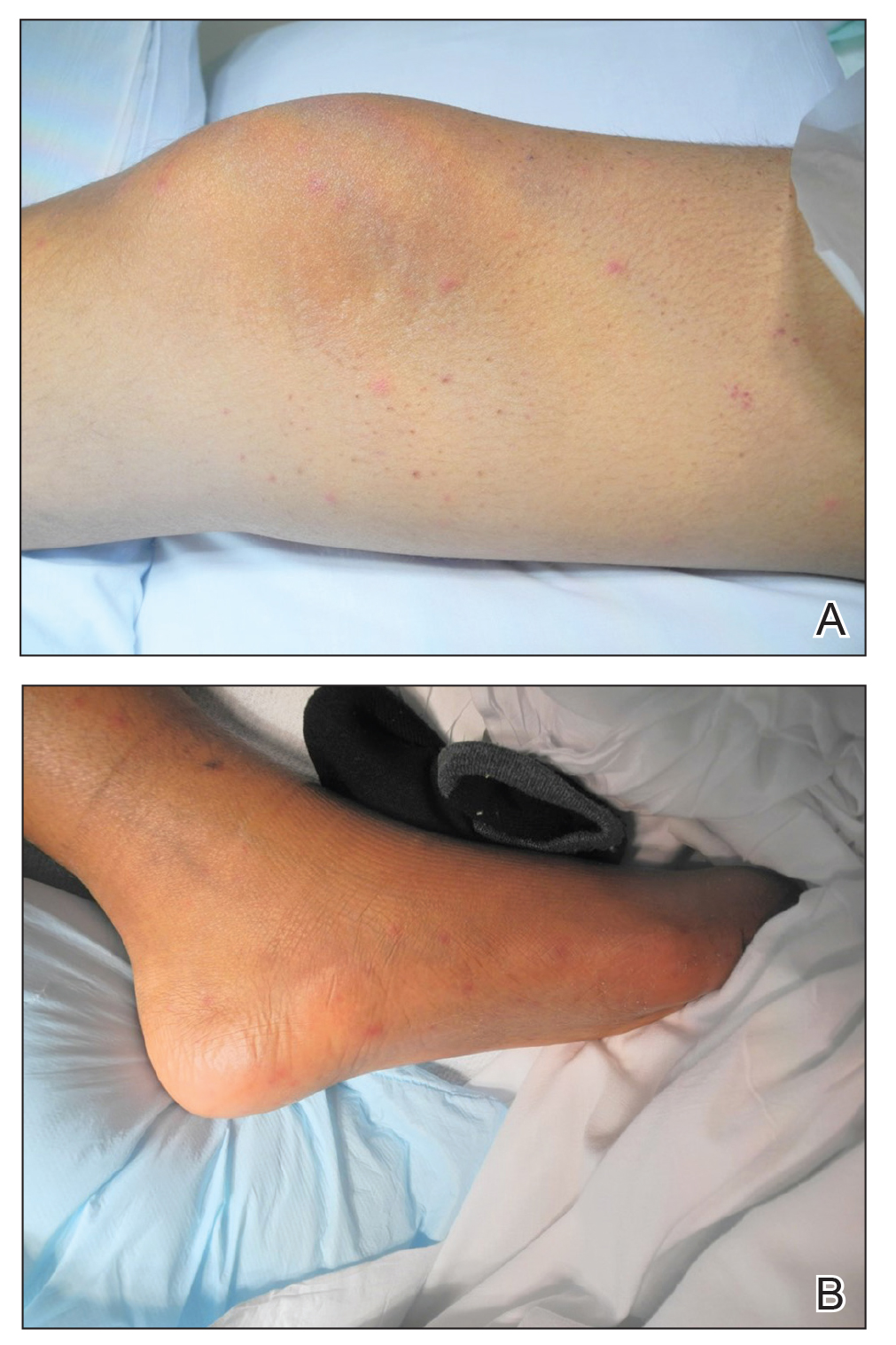
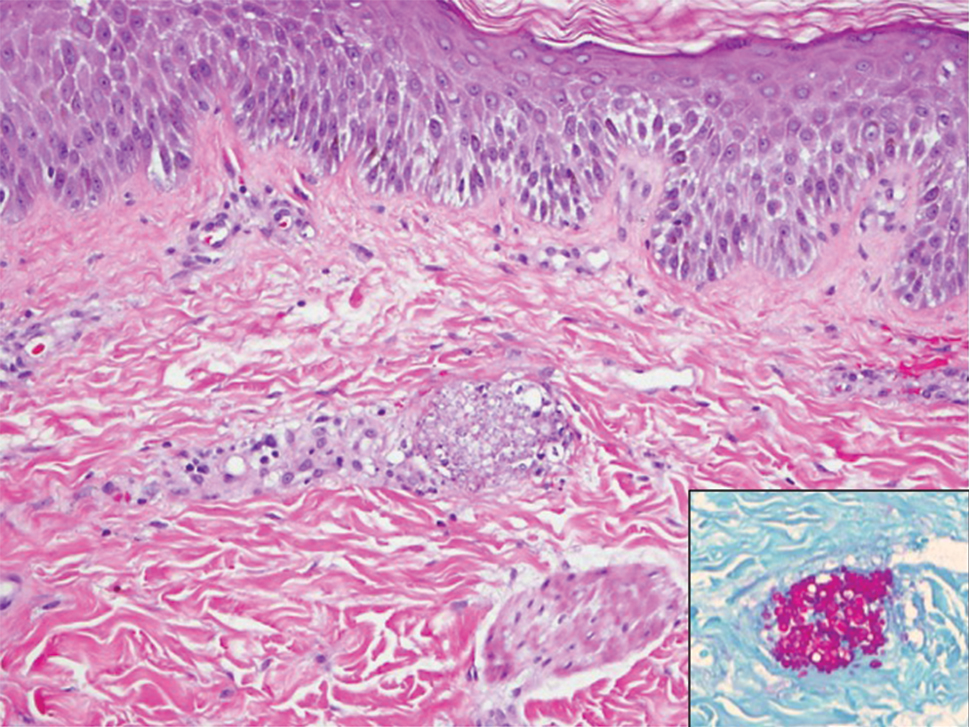
Early and reliable diagnosis of Candida species fungemia is of critical importance to successful treatment, particularly with the emergence of multidrug-resistant strains such as Candida auris.2 In patients with apparent cutaneous manifestations, a lesional punch biopsy for culture and histopathologic evaluation is recommended in addition to blood culture; however, organisms may or may not be present in large numbers, and they may be difficult to identify on routine hematoxylin and eosin–stained tissue sections. To enhance the likelihood of highlighting the fungus within the sample, the pathologist must be made aware of the presumptive diagnosis of disseminated candidiasis so that special techniques can be utilized, such as periodic acid–Schiff stain.
Although positive blood culture is the gold standard for candidemia diagnosis, only 30% to 50% of patients with disseminated candidiasis had positive blood cultures at autopsy.1 Another study showed the sensitivity of blood culture for the detection of invasive fungal infection to be as low as 8.3%.3 In cases with positive blood cultures, the median time to positivity is 2 to 3 days, but it can take as long as 8 days, thus limiting its clinical utility in acutely ill patients.4 Given the low sensitivity and prolonged time required for culture growth of most fungal organisms, novel assays for rapid, non–culture-based diagnosis of systemic fungal infections hold substantial clinical promise moving forward.
The Technique
One of the more promising non–culture-based fungal diagnostic methodologies is an antigen assay based on the detection of serum (1,3)-β-D-glucan (BDG), a major cell wall constituent of most pathogenic fungi. This assay is not specific for Candida species and can be positive for Aspergillosis species, Fusarium species, Coccidioides immitis, Histoplasma capsulatum, and Pneumocystis jirovecii pneumonia, among others; therefore, it functions as a general biomarker for fungi in the bloodstream.4,5 (1,3)-β-D-glucan assay can be useful as an adjunct for blood cultures and punch biopsy, especially when cultures are negative or the results remain outstanding. The results of the BDG assay are available in less than 24 hours at minimal cost, and the test is approved by the US Food and Drug Administration for use as an aid in invasive fungal disease diagnosis. In a meta-analysis of 11 studies, BDG sensitivity was 75%.4 In a study based on autopsy cases from 6 years, BDG specificity was 98.4% with positive and negative predictive values of 86.7% and 97.1%, respectively.3 Optimal results were achieved when 2 consecutive tests were positive.4 The serum assay output is based on spectrophotometer readings, which are converted to BDG concentrations (negative, <60 pg/mL; indeterminate, 60–79 pg/mL; positive ≥80 pg/mL).5 Although we cannot be certain, utilizing the BDG assay in our patient may have led to earlier treatment and a better outcome.
A disadvantage of the BDG assay is the potential for false-positive results, which have been reported in lung transplant recipients with respiratory mold colonization and patients with other systemic bacterial infections.4 False-positive results also have been associated with use of ampicillin-clavulanate and piperacillin-tazobactam antibiotics and human blood products, hemodialysis, and severe mucositis, thus reaffirming the importance of judicious interpretation of BDG assay results by the clinician.4,6 There also is a potential for false-negative results, as the BDG assay does not detect certain fungal species such as Cryptococcus species and Blastomyces dermatitidis, which produce very low levels of BDG, or zygomycetes (Absidia, Mucor, and Rizopus species), which are not known to produce BDG.6
Practice Implications
In the setting of invasive fungal infections, a high degree of clinical suspicion is paramount due to the often subtle nature of cutaneous manifestations. A positive BDG assay can be used to identify high-risk patients for empiric antifungal therapy, prompting early intervention and improved outcomes in these acutely ill patients. The BDG assay’s excellent negative predictive value is useful in ruling out invasive Candida infections and may justify stopping unnecessary empiric antifungal therapy.4 For the dermatology hospitalist, incorporation of the BDG assay as a noninvasive screening tool may allow for more rapid initiation of appropriate antifungal therapy while awaiting confirmatory skin biopsy or culture results in disseminated candidemia and other invasive fungal infections.
- Mays SR, Bogle MA, Bodey GP. Cutaneous fungal infections in the oncology patient: recognition and management. Am J Clin Dermatol. 2006;7:31-43.
- Candida auris. Centers for Disease Control and Prevention website. https://www.cdc.gov/fungal/candida-auris/. Updated May 15, 2020. Accessed July 10, 2020.
- Obayashi T, Negishi K, Suzuki T, et al. Reappraisal of the serum (1,3)-β-D-glucan assay for the diagnosis of invasive fungal infections—a study based on autopsy cases from 6 years. Clin Infect Dis. 2008;46:1864-1870.
- Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56:1284-1292.
- McCarthy MW, Petraitiene R, Walsh TJ. Translational development and application of (1→3)-β-d-glucan for diagnosis and therapeutic monitoring of invasive mycoses [published online May 24, 2017]. Int J Mol Sci. doi:10.3390/ijms18061124.
- Beta-D glucan assay. MiraVista Diagnostics website. https://miravistalabs.com/medical-fungal-infection-testing/antigen-detection/beta-d-glucan-test/. Accessed June 5, 2020.
Practice Gap
Invasive fungal infections are a leading cause of morbidity and mortality among neutropenic, immunocompromised, and critically ill patients. Candida species are the most common cause of fungemia, with portals of entry into the bloodstream including the gastrointestinal tract, contaminated intravascular catheters, and localized foci of infection.1 Diagnosis of invasive candidiasis remains challenging due to an absence of specific clinical signs and symptoms, varying from a mild fever that is unresponsive to antibiotics to florid sepsis. When present, clinical clues may include chorioretinitis; muscle abscesses; and skin eruptions, characteristically with Candida tropicalis. Cutaneous manifestations of disseminated Candida infections appear in only 13% of affected patients.1 The lesions typically present as 5- to 10-mm pink dermal papules or painless pustules on an erythematous base and may be singular, localized, or diffuse in distribution. Body regions normally involved are the trunk, arms, and legs, rarely the head and neck.1 Cutaneous lesions often develop at a time when patients are febrile, are not responding to antibiotics, and are clinically deteriorating.
A 15-year-old adolescent boy with pre–B-cell acute lymphoblastic leukemia was admitted with febrile neutropenia for presumed septic shock secondary to an unknown infectious etiology. The patient was started on broad-spectrum intravenous antibiotics, and blood cultures were obtained. On the second day of hospitalization, he developed approximately 10 to 15 discrete, 3- to 6-mm, pink to violaceous papules scattered on the chest and arms (Figure 1). Over several hours, the number of lesions increased to more than 50 with involvement of the legs (Figure 2). A punch biopsy of lesional skin from the left dorsal wrist demonstrated a circumscribed abscess of yeast in the papillary dermis, which was highlighted by periodic acid–Schiff staining with minimal associated inflammation (Figure 3). Blood and tissue cultures persistently grew C tropicalis. The patient was started on intravenous liposomal amphotericin B but died on day 5 of hospitalization after developing endocarditis.



Early and reliable diagnosis of Candida species fungemia is of critical importance to successful treatment, particularly with the emergence of multidrug-resistant strains such as Candida auris.2 In patients with apparent cutaneous manifestations, a lesional punch biopsy for culture and histopathologic evaluation is recommended in addition to blood culture; however, organisms may or may not be present in large numbers, and they may be difficult to identify on routine hematoxylin and eosin–stained tissue sections. To enhance the likelihood of highlighting the fungus within the sample, the pathologist must be made aware of the presumptive diagnosis of disseminated candidiasis so that special techniques can be utilized, such as periodic acid–Schiff stain.
Although positive blood culture is the gold standard for candidemia diagnosis, only 30% to 50% of patients with disseminated candidiasis had positive blood cultures at autopsy.1 Another study showed the sensitivity of blood culture for the detection of invasive fungal infection to be as low as 8.3%.3 In cases with positive blood cultures, the median time to positivity is 2 to 3 days, but it can take as long as 8 days, thus limiting its clinical utility in acutely ill patients.4 Given the low sensitivity and prolonged time required for culture growth of most fungal organisms, novel assays for rapid, non–culture-based diagnosis of systemic fungal infections hold substantial clinical promise moving forward.
The Technique
One of the more promising non–culture-based fungal diagnostic methodologies is an antigen assay based on the detection of serum (1,3)-β-D-glucan (BDG), a major cell wall constituent of most pathogenic fungi. This assay is not specific for Candida species and can be positive for Aspergillosis species, Fusarium species, Coccidioides immitis, Histoplasma capsulatum, and Pneumocystis jirovecii pneumonia, among others; therefore, it functions as a general biomarker for fungi in the bloodstream.4,5 (1,3)-β-D-glucan assay can be useful as an adjunct for blood cultures and punch biopsy, especially when cultures are negative or the results remain outstanding. The results of the BDG assay are available in less than 24 hours at minimal cost, and the test is approved by the US Food and Drug Administration for use as an aid in invasive fungal disease diagnosis. In a meta-analysis of 11 studies, BDG sensitivity was 75%.4 In a study based on autopsy cases from 6 years, BDG specificity was 98.4% with positive and negative predictive values of 86.7% and 97.1%, respectively.3 Optimal results were achieved when 2 consecutive tests were positive.4 The serum assay output is based on spectrophotometer readings, which are converted to BDG concentrations (negative, <60 pg/mL; indeterminate, 60–79 pg/mL; positive ≥80 pg/mL).5 Although we cannot be certain, utilizing the BDG assay in our patient may have led to earlier treatment and a better outcome.
A disadvantage of the BDG assay is the potential for false-positive results, which have been reported in lung transplant recipients with respiratory mold colonization and patients with other systemic bacterial infections.4 False-positive results also have been associated with use of ampicillin-clavulanate and piperacillin-tazobactam antibiotics and human blood products, hemodialysis, and severe mucositis, thus reaffirming the importance of judicious interpretation of BDG assay results by the clinician.4,6 There also is a potential for false-negative results, as the BDG assay does not detect certain fungal species such as Cryptococcus species and Blastomyces dermatitidis, which produce very low levels of BDG, or zygomycetes (Absidia, Mucor, and Rizopus species), which are not known to produce BDG.6
Practice Implications
In the setting of invasive fungal infections, a high degree of clinical suspicion is paramount due to the often subtle nature of cutaneous manifestations. A positive BDG assay can be used to identify high-risk patients for empiric antifungal therapy, prompting early intervention and improved outcomes in these acutely ill patients. The BDG assay’s excellent negative predictive value is useful in ruling out invasive Candida infections and may justify stopping unnecessary empiric antifungal therapy.4 For the dermatology hospitalist, incorporation of the BDG assay as a noninvasive screening tool may allow for more rapid initiation of appropriate antifungal therapy while awaiting confirmatory skin biopsy or culture results in disseminated candidemia and other invasive fungal infections.
Practice Gap
Invasive fungal infections are a leading cause of morbidity and mortality among neutropenic, immunocompromised, and critically ill patients. Candida species are the most common cause of fungemia, with portals of entry into the bloodstream including the gastrointestinal tract, contaminated intravascular catheters, and localized foci of infection.1 Diagnosis of invasive candidiasis remains challenging due to an absence of specific clinical signs and symptoms, varying from a mild fever that is unresponsive to antibiotics to florid sepsis. When present, clinical clues may include chorioretinitis; muscle abscesses; and skin eruptions, characteristically with Candida tropicalis. Cutaneous manifestations of disseminated Candida infections appear in only 13% of affected patients.1 The lesions typically present as 5- to 10-mm pink dermal papules or painless pustules on an erythematous base and may be singular, localized, or diffuse in distribution. Body regions normally involved are the trunk, arms, and legs, rarely the head and neck.1 Cutaneous lesions often develop at a time when patients are febrile, are not responding to antibiotics, and are clinically deteriorating.
A 15-year-old adolescent boy with pre–B-cell acute lymphoblastic leukemia was admitted with febrile neutropenia for presumed septic shock secondary to an unknown infectious etiology. The patient was started on broad-spectrum intravenous antibiotics, and blood cultures were obtained. On the second day of hospitalization, he developed approximately 10 to 15 discrete, 3- to 6-mm, pink to violaceous papules scattered on the chest and arms (Figure 1). Over several hours, the number of lesions increased to more than 50 with involvement of the legs (Figure 2). A punch biopsy of lesional skin from the left dorsal wrist demonstrated a circumscribed abscess of yeast in the papillary dermis, which was highlighted by periodic acid–Schiff staining with minimal associated inflammation (Figure 3). Blood and tissue cultures persistently grew C tropicalis. The patient was started on intravenous liposomal amphotericin B but died on day 5 of hospitalization after developing endocarditis.



Early and reliable diagnosis of Candida species fungemia is of critical importance to successful treatment, particularly with the emergence of multidrug-resistant strains such as Candida auris.2 In patients with apparent cutaneous manifestations, a lesional punch biopsy for culture and histopathologic evaluation is recommended in addition to blood culture; however, organisms may or may not be present in large numbers, and they may be difficult to identify on routine hematoxylin and eosin–stained tissue sections. To enhance the likelihood of highlighting the fungus within the sample, the pathologist must be made aware of the presumptive diagnosis of disseminated candidiasis so that special techniques can be utilized, such as periodic acid–Schiff stain.
Although positive blood culture is the gold standard for candidemia diagnosis, only 30% to 50% of patients with disseminated candidiasis had positive blood cultures at autopsy.1 Another study showed the sensitivity of blood culture for the detection of invasive fungal infection to be as low as 8.3%.3 In cases with positive blood cultures, the median time to positivity is 2 to 3 days, but it can take as long as 8 days, thus limiting its clinical utility in acutely ill patients.4 Given the low sensitivity and prolonged time required for culture growth of most fungal organisms, novel assays for rapid, non–culture-based diagnosis of systemic fungal infections hold substantial clinical promise moving forward.
The Technique
One of the more promising non–culture-based fungal diagnostic methodologies is an antigen assay based on the detection of serum (1,3)-β-D-glucan (BDG), a major cell wall constituent of most pathogenic fungi. This assay is not specific for Candida species and can be positive for Aspergillosis species, Fusarium species, Coccidioides immitis, Histoplasma capsulatum, and Pneumocystis jirovecii pneumonia, among others; therefore, it functions as a general biomarker for fungi in the bloodstream.4,5 (1,3)-β-D-glucan assay can be useful as an adjunct for blood cultures and punch biopsy, especially when cultures are negative or the results remain outstanding. The results of the BDG assay are available in less than 24 hours at minimal cost, and the test is approved by the US Food and Drug Administration for use as an aid in invasive fungal disease diagnosis. In a meta-analysis of 11 studies, BDG sensitivity was 75%.4 In a study based on autopsy cases from 6 years, BDG specificity was 98.4% with positive and negative predictive values of 86.7% and 97.1%, respectively.3 Optimal results were achieved when 2 consecutive tests were positive.4 The serum assay output is based on spectrophotometer readings, which are converted to BDG concentrations (negative, <60 pg/mL; indeterminate, 60–79 pg/mL; positive ≥80 pg/mL).5 Although we cannot be certain, utilizing the BDG assay in our patient may have led to earlier treatment and a better outcome.
A disadvantage of the BDG assay is the potential for false-positive results, which have been reported in lung transplant recipients with respiratory mold colonization and patients with other systemic bacterial infections.4 False-positive results also have been associated with use of ampicillin-clavulanate and piperacillin-tazobactam antibiotics and human blood products, hemodialysis, and severe mucositis, thus reaffirming the importance of judicious interpretation of BDG assay results by the clinician.4,6 There also is a potential for false-negative results, as the BDG assay does not detect certain fungal species such as Cryptococcus species and Blastomyces dermatitidis, which produce very low levels of BDG, or zygomycetes (Absidia, Mucor, and Rizopus species), which are not known to produce BDG.6
Practice Implications
In the setting of invasive fungal infections, a high degree of clinical suspicion is paramount due to the often subtle nature of cutaneous manifestations. A positive BDG assay can be used to identify high-risk patients for empiric antifungal therapy, prompting early intervention and improved outcomes in these acutely ill patients. The BDG assay’s excellent negative predictive value is useful in ruling out invasive Candida infections and may justify stopping unnecessary empiric antifungal therapy.4 For the dermatology hospitalist, incorporation of the BDG assay as a noninvasive screening tool may allow for more rapid initiation of appropriate antifungal therapy while awaiting confirmatory skin biopsy or culture results in disseminated candidemia and other invasive fungal infections.
- Mays SR, Bogle MA, Bodey GP. Cutaneous fungal infections in the oncology patient: recognition and management. Am J Clin Dermatol. 2006;7:31-43.
- Candida auris. Centers for Disease Control and Prevention website. https://www.cdc.gov/fungal/candida-auris/. Updated May 15, 2020. Accessed July 10, 2020.
- Obayashi T, Negishi K, Suzuki T, et al. Reappraisal of the serum (1,3)-β-D-glucan assay for the diagnosis of invasive fungal infections—a study based on autopsy cases from 6 years. Clin Infect Dis. 2008;46:1864-1870.
- Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56:1284-1292.
- McCarthy MW, Petraitiene R, Walsh TJ. Translational development and application of (1→3)-β-d-glucan for diagnosis and therapeutic monitoring of invasive mycoses [published online May 24, 2017]. Int J Mol Sci. doi:10.3390/ijms18061124.
- Beta-D glucan assay. MiraVista Diagnostics website. https://miravistalabs.com/medical-fungal-infection-testing/antigen-detection/beta-d-glucan-test/. Accessed June 5, 2020.
- Mays SR, Bogle MA, Bodey GP. Cutaneous fungal infections in the oncology patient: recognition and management. Am J Clin Dermatol. 2006;7:31-43.
- Candida auris. Centers for Disease Control and Prevention website. https://www.cdc.gov/fungal/candida-auris/. Updated May 15, 2020. Accessed July 10, 2020.
- Obayashi T, Negishi K, Suzuki T, et al. Reappraisal of the serum (1,3)-β-D-glucan assay for the diagnosis of invasive fungal infections—a study based on autopsy cases from 6 years. Clin Infect Dis. 2008;46:1864-1870.
- Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56:1284-1292.
- McCarthy MW, Petraitiene R, Walsh TJ. Translational development and application of (1→3)-β-d-glucan for diagnosis and therapeutic monitoring of invasive mycoses [published online May 24, 2017]. Int J Mol Sci. doi:10.3390/ijms18061124.
- Beta-D glucan assay. MiraVista Diagnostics website. https://miravistalabs.com/medical-fungal-infection-testing/antigen-detection/beta-d-glucan-test/. Accessed June 5, 2020.
Brensocatib reduced bronchiectasis exacerbations
Brensocatib, an experimental small-molecule inhibitor targeted to inflammation-regulating neutrophil serine proteases, may be a novel, nonantibiotic option for reducing exacerbations in patients with bronchiectasis, investigators in the phase 2 WILLOW study said.
Among 256 adults with a recent history of bronchiectasis exacerbations, oral brensocatib at doses of both 10 mg and 25 mg daily for 24 weeks was associated with significantly longer time to first exacerbation than placebo, and the 10-mg dose was associated with a significant reduction in the annualized rate of exacerbations, reported James Chalmers, MB, ChB, PhD of Ninewells Hospital and Medical School in Dundee (England).
“We also observed a dose-dependent reduction in neutrophil elastase levels in sputum, which supports the mechanism of action of this drug, and importantly showed a link between reducing neutrophil serine protease activity and clinical benefits in people with bronchiectasis,” he said in at the American Thoracic Society’s virtual clinical trial session.
“This is a very important trial, a landmark trial for people with bronchiectasis, because this is a drug that for the first time appears to be able to target directly neutrophilic inflammation, resulting in clinical benefit,” he said.
Pulmonologist Jennifer L. Taylor-Cousar, MD, MSCS, of National Jewish Health in Denver, who was facilitator for the online presentation but was not involved in the study, said that it offered welcome news.
“For those of us who treat bronchiectasis, a safe and effective anti-inflammatory has really been the Holy Grail, so this is really exciting,” she said.
Novel mechanism of action
Frequent exacerbations in bronchiectasis are related to uncontrolled neutrophilic inflammation, and proinflammatory neutrophil serine proteases (NSPs), including neutrophil elastase, are seen at increased levels in sputum of patients with bronchiectasis. In addition, the presence in sputum of elevated NSPs are associated with exacerbations and poor quality of life, Dr. Chalmers said.
Brensocatib is an inhibitor of dipeptidyl peptidase 1 (DPP1), a lysosomal cysteine protease that is responsible for NSP activation in bone marrow during the neutrophil maturation cycle.
In phase 1 trials, brensocatib was associated with a dose-dependent reduction in neutrophil elastase in healthy volunteers.
Three WILLOW branches
In the phase 2 WILLOW trial, patients with bronchiectasis not related to cystic fibrosis were screened and stratified by Pseudomonas aeruginosa on sputum culture and use of macrolide antibiotics and then randomized in equal proportions to receive either brensocatib at daily oral doses of 25 mg or 10 mg, or placebo for 24 weeks, followed by a 4-week off-treatment period.
Both doses of brensocatib met the primary endpoint of time to first exacerbation, compared with placebo. The hazard ratio (HR) for the 10-mg brensocatib dose, compared with placebo was 0.58 (P = .029), and the HR for the 25-mg dose was 0.62 (P = .046).
The exacerbation rate over 24 weeks among patients on placebo was 48.3%, compared with 31.7% of patients on 10 mg brensocatib (P = .033) and 33.3% of patients on the 25 mg dose (P = .038).
The annualized exacerbation rate was 1.37 for patients on placebo, compared with 0.88 with 10 mg brensocatib (P = .041) and 1.03 with 25 mg brensocatib (nonsignificant).
In both brensocatib groups there were significant reductions from baseline neutrophil elastase concentrations in sputum, compared with placebo (P = .034 for 10 mg and .021 for 25 mg). During the 4-week period following treatment neutrophil elastase levels in both active drug arms rose rapidly and returned to baseline.
The importance of these reductions was reflected in pooled data from the two brensocatib cohorts, which showed that patients who achieved neutrophil elastase levels below the limit of quantification had a significantly lower incidence of bronchiectasis exacerbations (HR 0.28, P < .0001).
Although the study was not powered to compare changes in postbronchodilator forced expiratory volume in 1 second (FEV1) levels, placebo-treated patients had a numerically larger decline in lung function from baseline, compared with brensocatib-treated patients.
Safety
Expected adverse events with brensocatib included those associated with Papillon-Lefèvre syndrome, a rare congenital condition caused by the absence of the gene coding for DPP1, resulting in keratinization leading to redness, thickening of soles and palms, and severe, destructive periodontal disease, as well as reduced immune response to bacterial infection.
Treatment-emergent adverse events (TEAEs) resulting in study discontinuation occurred in only three patients on placebo and 10 mg brensocatib, and four on the 25-mg dose. TEAEs resulting in treatment discontinuation were more common in the placebo arm, occurring in nine patients compared with six each in the brensocatib arms.
Serious TEAEs occurring in more than 3% of patients in any group included infective exacerbations in three patients on placebo, none on the 10-mg dose, and four on the 25-mg dose of brensocatib. Respective numbers of patients with treatment-emergent pneumonia were three, zero, and four.
Other TEAEs included cough, headache, sputum increase, dyspnea, and diarrhea.
Adverse events of special interest included skin events in 10 patients on placebo, 12 on the 10-mg dose, and 21 on the 25-mg brensocatib dose. Dental changes occurred in 3, 13, and 9 patients, and infections in 9, 12, and 14 patients, respectively.
A phase 3 study to confirm efficacy and establish the optimal dose of brensocatib is planned for the end of 2020, “COVID willing,” Dr. Chalmers said.
Dr. Chalmers disclosed consultancy with and research funding from Insmed, which funded the study. Dr. Taylor-Cousar has disclosed grants and/or personal fees from various companies.
Brensocatib, an experimental small-molecule inhibitor targeted to inflammation-regulating neutrophil serine proteases, may be a novel, nonantibiotic option for reducing exacerbations in patients with bronchiectasis, investigators in the phase 2 WILLOW study said.
Among 256 adults with a recent history of bronchiectasis exacerbations, oral brensocatib at doses of both 10 mg and 25 mg daily for 24 weeks was associated with significantly longer time to first exacerbation than placebo, and the 10-mg dose was associated with a significant reduction in the annualized rate of exacerbations, reported James Chalmers, MB, ChB, PhD of Ninewells Hospital and Medical School in Dundee (England).
“We also observed a dose-dependent reduction in neutrophil elastase levels in sputum, which supports the mechanism of action of this drug, and importantly showed a link between reducing neutrophil serine protease activity and clinical benefits in people with bronchiectasis,” he said in at the American Thoracic Society’s virtual clinical trial session.
“This is a very important trial, a landmark trial for people with bronchiectasis, because this is a drug that for the first time appears to be able to target directly neutrophilic inflammation, resulting in clinical benefit,” he said.
Pulmonologist Jennifer L. Taylor-Cousar, MD, MSCS, of National Jewish Health in Denver, who was facilitator for the online presentation but was not involved in the study, said that it offered welcome news.
“For those of us who treat bronchiectasis, a safe and effective anti-inflammatory has really been the Holy Grail, so this is really exciting,” she said.
Novel mechanism of action
Frequent exacerbations in bronchiectasis are related to uncontrolled neutrophilic inflammation, and proinflammatory neutrophil serine proteases (NSPs), including neutrophil elastase, are seen at increased levels in sputum of patients with bronchiectasis. In addition, the presence in sputum of elevated NSPs are associated with exacerbations and poor quality of life, Dr. Chalmers said.
Brensocatib is an inhibitor of dipeptidyl peptidase 1 (DPP1), a lysosomal cysteine protease that is responsible for NSP activation in bone marrow during the neutrophil maturation cycle.
In phase 1 trials, brensocatib was associated with a dose-dependent reduction in neutrophil elastase in healthy volunteers.
Three WILLOW branches
In the phase 2 WILLOW trial, patients with bronchiectasis not related to cystic fibrosis were screened and stratified by Pseudomonas aeruginosa on sputum culture and use of macrolide antibiotics and then randomized in equal proportions to receive either brensocatib at daily oral doses of 25 mg or 10 mg, or placebo for 24 weeks, followed by a 4-week off-treatment period.
Both doses of brensocatib met the primary endpoint of time to first exacerbation, compared with placebo. The hazard ratio (HR) for the 10-mg brensocatib dose, compared with placebo was 0.58 (P = .029), and the HR for the 25-mg dose was 0.62 (P = .046).
The exacerbation rate over 24 weeks among patients on placebo was 48.3%, compared with 31.7% of patients on 10 mg brensocatib (P = .033) and 33.3% of patients on the 25 mg dose (P = .038).
The annualized exacerbation rate was 1.37 for patients on placebo, compared with 0.88 with 10 mg brensocatib (P = .041) and 1.03 with 25 mg brensocatib (nonsignificant).
In both brensocatib groups there were significant reductions from baseline neutrophil elastase concentrations in sputum, compared with placebo (P = .034 for 10 mg and .021 for 25 mg). During the 4-week period following treatment neutrophil elastase levels in both active drug arms rose rapidly and returned to baseline.
The importance of these reductions was reflected in pooled data from the two brensocatib cohorts, which showed that patients who achieved neutrophil elastase levels below the limit of quantification had a significantly lower incidence of bronchiectasis exacerbations (HR 0.28, P < .0001).
Although the study was not powered to compare changes in postbronchodilator forced expiratory volume in 1 second (FEV1) levels, placebo-treated patients had a numerically larger decline in lung function from baseline, compared with brensocatib-treated patients.
Safety
Expected adverse events with brensocatib included those associated with Papillon-Lefèvre syndrome, a rare congenital condition caused by the absence of the gene coding for DPP1, resulting in keratinization leading to redness, thickening of soles and palms, and severe, destructive periodontal disease, as well as reduced immune response to bacterial infection.
Treatment-emergent adverse events (TEAEs) resulting in study discontinuation occurred in only three patients on placebo and 10 mg brensocatib, and four on the 25-mg dose. TEAEs resulting in treatment discontinuation were more common in the placebo arm, occurring in nine patients compared with six each in the brensocatib arms.
Serious TEAEs occurring in more than 3% of patients in any group included infective exacerbations in three patients on placebo, none on the 10-mg dose, and four on the 25-mg dose of brensocatib. Respective numbers of patients with treatment-emergent pneumonia were three, zero, and four.
Other TEAEs included cough, headache, sputum increase, dyspnea, and diarrhea.
Adverse events of special interest included skin events in 10 patients on placebo, 12 on the 10-mg dose, and 21 on the 25-mg brensocatib dose. Dental changes occurred in 3, 13, and 9 patients, and infections in 9, 12, and 14 patients, respectively.
A phase 3 study to confirm efficacy and establish the optimal dose of brensocatib is planned for the end of 2020, “COVID willing,” Dr. Chalmers said.
Dr. Chalmers disclosed consultancy with and research funding from Insmed, which funded the study. Dr. Taylor-Cousar has disclosed grants and/or personal fees from various companies.
Brensocatib, an experimental small-molecule inhibitor targeted to inflammation-regulating neutrophil serine proteases, may be a novel, nonantibiotic option for reducing exacerbations in patients with bronchiectasis, investigators in the phase 2 WILLOW study said.
Among 256 adults with a recent history of bronchiectasis exacerbations, oral brensocatib at doses of both 10 mg and 25 mg daily for 24 weeks was associated with significantly longer time to first exacerbation than placebo, and the 10-mg dose was associated with a significant reduction in the annualized rate of exacerbations, reported James Chalmers, MB, ChB, PhD of Ninewells Hospital and Medical School in Dundee (England).
“We also observed a dose-dependent reduction in neutrophil elastase levels in sputum, which supports the mechanism of action of this drug, and importantly showed a link between reducing neutrophil serine protease activity and clinical benefits in people with bronchiectasis,” he said in at the American Thoracic Society’s virtual clinical trial session.
“This is a very important trial, a landmark trial for people with bronchiectasis, because this is a drug that for the first time appears to be able to target directly neutrophilic inflammation, resulting in clinical benefit,” he said.
Pulmonologist Jennifer L. Taylor-Cousar, MD, MSCS, of National Jewish Health in Denver, who was facilitator for the online presentation but was not involved in the study, said that it offered welcome news.
“For those of us who treat bronchiectasis, a safe and effective anti-inflammatory has really been the Holy Grail, so this is really exciting,” she said.
Novel mechanism of action
Frequent exacerbations in bronchiectasis are related to uncontrolled neutrophilic inflammation, and proinflammatory neutrophil serine proteases (NSPs), including neutrophil elastase, are seen at increased levels in sputum of patients with bronchiectasis. In addition, the presence in sputum of elevated NSPs are associated with exacerbations and poor quality of life, Dr. Chalmers said.
Brensocatib is an inhibitor of dipeptidyl peptidase 1 (DPP1), a lysosomal cysteine protease that is responsible for NSP activation in bone marrow during the neutrophil maturation cycle.
In phase 1 trials, brensocatib was associated with a dose-dependent reduction in neutrophil elastase in healthy volunteers.
Three WILLOW branches
In the phase 2 WILLOW trial, patients with bronchiectasis not related to cystic fibrosis were screened and stratified by Pseudomonas aeruginosa on sputum culture and use of macrolide antibiotics and then randomized in equal proportions to receive either brensocatib at daily oral doses of 25 mg or 10 mg, or placebo for 24 weeks, followed by a 4-week off-treatment period.
Both doses of brensocatib met the primary endpoint of time to first exacerbation, compared with placebo. The hazard ratio (HR) for the 10-mg brensocatib dose, compared with placebo was 0.58 (P = .029), and the HR for the 25-mg dose was 0.62 (P = .046).
The exacerbation rate over 24 weeks among patients on placebo was 48.3%, compared with 31.7% of patients on 10 mg brensocatib (P = .033) and 33.3% of patients on the 25 mg dose (P = .038).
The annualized exacerbation rate was 1.37 for patients on placebo, compared with 0.88 with 10 mg brensocatib (P = .041) and 1.03 with 25 mg brensocatib (nonsignificant).
In both brensocatib groups there were significant reductions from baseline neutrophil elastase concentrations in sputum, compared with placebo (P = .034 for 10 mg and .021 for 25 mg). During the 4-week period following treatment neutrophil elastase levels in both active drug arms rose rapidly and returned to baseline.
The importance of these reductions was reflected in pooled data from the two brensocatib cohorts, which showed that patients who achieved neutrophil elastase levels below the limit of quantification had a significantly lower incidence of bronchiectasis exacerbations (HR 0.28, P < .0001).
Although the study was not powered to compare changes in postbronchodilator forced expiratory volume in 1 second (FEV1) levels, placebo-treated patients had a numerically larger decline in lung function from baseline, compared with brensocatib-treated patients.
Safety
Expected adverse events with brensocatib included those associated with Papillon-Lefèvre syndrome, a rare congenital condition caused by the absence of the gene coding for DPP1, resulting in keratinization leading to redness, thickening of soles and palms, and severe, destructive periodontal disease, as well as reduced immune response to bacterial infection.
Treatment-emergent adverse events (TEAEs) resulting in study discontinuation occurred in only three patients on placebo and 10 mg brensocatib, and four on the 25-mg dose. TEAEs resulting in treatment discontinuation were more common in the placebo arm, occurring in nine patients compared with six each in the brensocatib arms.
Serious TEAEs occurring in more than 3% of patients in any group included infective exacerbations in three patients on placebo, none on the 10-mg dose, and four on the 25-mg dose of brensocatib. Respective numbers of patients with treatment-emergent pneumonia were three, zero, and four.
Other TEAEs included cough, headache, sputum increase, dyspnea, and diarrhea.
Adverse events of special interest included skin events in 10 patients on placebo, 12 on the 10-mg dose, and 21 on the 25-mg brensocatib dose. Dental changes occurred in 3, 13, and 9 patients, and infections in 9, 12, and 14 patients, respectively.
A phase 3 study to confirm efficacy and establish the optimal dose of brensocatib is planned for the end of 2020, “COVID willing,” Dr. Chalmers said.
Dr. Chalmers disclosed consultancy with and research funding from Insmed, which funded the study. Dr. Taylor-Cousar has disclosed grants and/or personal fees from various companies.
FROM ATS 2020
Breast density asymmetry might increase breast cancer risk
The 854 women in the study had been referred for biopsy after an abnormal mammogram.
Researchers used the mammograms to assess global bilateral asymmetry, which was the overall absolute difference in percent fibroglandular tissue volume (%FGV) between the ipsilateral (biopsied) breast and the contralateral (unaffected) breast.
The researchers also assessed local bilateral asymmetry, which was the perilesional %FGV difference in an area twice the size of, but excluding, the biopsy target, and the corresponding area in the unaffected breast.
The women were then divided into quartiles based on breast density asymmetry.
Most of the women had benign breast disease, including proliferative (43%) and nonproliferative (33%) disease, but 23% had carcinoma in situ or invasive breast cancer.
The trend for higher risk of in situ or invasive cancer with increasing breast density asymmetry was observed only in the local analysis. The odds ratio was 1.59 (95% confidence interval, 0.94-2.69) for women in the highest quartile of breast density asymmetry (absolute difference, > 8.23) versus those in the lowest quartile (absolute difference, ≤ –5.55; P = .067).
When compared with women who had proliferative benign disease, women with carcinoma in situ or invasive breast cancer “were more likely to be in the higher than lower quartiles,” said lead investigator Maeve Mullooly, PhD, a research fellow at the Royal College of Surgeons in Dublin.
There was no association between breast density asymmetry and traditional breast cancer risk factors such as age, body mass index, race, and hormone therapy. However, among women diagnosed with benign nonproliferative disease, women with a breast cancer family history were more likely to have higher overall breast density asymmetry.
Study rationale and details
Higher breast density is a known risk factor for breast cancer. Breast asymmetry also has been reported as a possible risk factor (Breast Cancer Res. 2006;8[2]:R14), and incorporation of breast density asymmetry into traditional risk factors in one study improved risk prediction (Breast Cancer Res. 2017 Mar 14;19[1]:29).
Building on that work, the goal of Dr. Mullooly’s study was to “learn how to better use breast density to inform breast cancer risk prediction,” she said.
To that end, her team turned to 854 women enrolled from 2007-2010 in the National Cancer Institute’s Breast Radiology Evaluation and Study of Tissues Project, a cross-sectional molecular epidemiologic study designed to understand how breast density measures are related to breast cancer etiology.
Most of the women were non-Hispanic white. The mean age was 51 years (range, 40-65), and the median body mass index was 25 kg/m2.
About three-quarters of the women (76%) had a breast density asymmetry of at least 2% on the global analysis, with 43% having higher %FGV in the biopsied breast and 33% having higher %FGV in the unaffected breast. In all, 89% of women had local breast density asymmetry, with higher density in the biopsied breast in 61% of women and higher density in the contralateral breast in 28%.
Next steps
This research is ongoing, and additional follow-up is planned, according to Dr. Mullooly. She said the researchers hope to apply more recent analytical techniques to the mammograms and to study the histologic differences in their breast biopsy specimens, among other steps, to see if stronger relationships with greater clinical utility emerge.
It was a “very well done study” with “very provocative data,” said presentation moderator Jennifer Wargo, MD, professor of genomic medicine and surgical oncology at the University of Texas MD Anderson Cancer Center in Houston.
She was interested in the planned next steps, particularly the histologic analysis of dense versus less dense breast tissue. There could be “differences in stroma or hormonal levels even at the microenvironmental level” that “represent a potential field defect, which later puts someone at risk,” she said, adding that it’s “great” that the work is continuing.
The National Cancer Institute funded the research. Dr. Mullooly reported no relevant disclosures. Dr. Wargo disclosed relationships with Bristol-Myers Squibb, Roche/Genentech, Novartis, GlaxoSmithKline, AstraZeneca, Imedex, Dava Oncology, Omniprex, Illumina, Gilead, PeerView, Physician Education Resource, MedImmune, Merck, Biothera Pharmaceuticals, and Microbiome DX.
SOURCE: Mullooly M et al. AACR 2020, Abstract NG15.
The 854 women in the study had been referred for biopsy after an abnormal mammogram.
Researchers used the mammograms to assess global bilateral asymmetry, which was the overall absolute difference in percent fibroglandular tissue volume (%FGV) between the ipsilateral (biopsied) breast and the contralateral (unaffected) breast.
The researchers also assessed local bilateral asymmetry, which was the perilesional %FGV difference in an area twice the size of, but excluding, the biopsy target, and the corresponding area in the unaffected breast.
The women were then divided into quartiles based on breast density asymmetry.
Most of the women had benign breast disease, including proliferative (43%) and nonproliferative (33%) disease, but 23% had carcinoma in situ or invasive breast cancer.
The trend for higher risk of in situ or invasive cancer with increasing breast density asymmetry was observed only in the local analysis. The odds ratio was 1.59 (95% confidence interval, 0.94-2.69) for women in the highest quartile of breast density asymmetry (absolute difference, > 8.23) versus those in the lowest quartile (absolute difference, ≤ –5.55; P = .067).
When compared with women who had proliferative benign disease, women with carcinoma in situ or invasive breast cancer “were more likely to be in the higher than lower quartiles,” said lead investigator Maeve Mullooly, PhD, a research fellow at the Royal College of Surgeons in Dublin.
There was no association between breast density asymmetry and traditional breast cancer risk factors such as age, body mass index, race, and hormone therapy. However, among women diagnosed with benign nonproliferative disease, women with a breast cancer family history were more likely to have higher overall breast density asymmetry.
Study rationale and details
Higher breast density is a known risk factor for breast cancer. Breast asymmetry also has been reported as a possible risk factor (Breast Cancer Res. 2006;8[2]:R14), and incorporation of breast density asymmetry into traditional risk factors in one study improved risk prediction (Breast Cancer Res. 2017 Mar 14;19[1]:29).
Building on that work, the goal of Dr. Mullooly’s study was to “learn how to better use breast density to inform breast cancer risk prediction,” she said.
To that end, her team turned to 854 women enrolled from 2007-2010 in the National Cancer Institute’s Breast Radiology Evaluation and Study of Tissues Project, a cross-sectional molecular epidemiologic study designed to understand how breast density measures are related to breast cancer etiology.
Most of the women were non-Hispanic white. The mean age was 51 years (range, 40-65), and the median body mass index was 25 kg/m2.
About three-quarters of the women (76%) had a breast density asymmetry of at least 2% on the global analysis, with 43% having higher %FGV in the biopsied breast and 33% having higher %FGV in the unaffected breast. In all, 89% of women had local breast density asymmetry, with higher density in the biopsied breast in 61% of women and higher density in the contralateral breast in 28%.
Next steps
This research is ongoing, and additional follow-up is planned, according to Dr. Mullooly. She said the researchers hope to apply more recent analytical techniques to the mammograms and to study the histologic differences in their breast biopsy specimens, among other steps, to see if stronger relationships with greater clinical utility emerge.
It was a “very well done study” with “very provocative data,” said presentation moderator Jennifer Wargo, MD, professor of genomic medicine and surgical oncology at the University of Texas MD Anderson Cancer Center in Houston.
She was interested in the planned next steps, particularly the histologic analysis of dense versus less dense breast tissue. There could be “differences in stroma or hormonal levels even at the microenvironmental level” that “represent a potential field defect, which later puts someone at risk,” she said, adding that it’s “great” that the work is continuing.
The National Cancer Institute funded the research. Dr. Mullooly reported no relevant disclosures. Dr. Wargo disclosed relationships with Bristol-Myers Squibb, Roche/Genentech, Novartis, GlaxoSmithKline, AstraZeneca, Imedex, Dava Oncology, Omniprex, Illumina, Gilead, PeerView, Physician Education Resource, MedImmune, Merck, Biothera Pharmaceuticals, and Microbiome DX.
SOURCE: Mullooly M et al. AACR 2020, Abstract NG15.
The 854 women in the study had been referred for biopsy after an abnormal mammogram.
Researchers used the mammograms to assess global bilateral asymmetry, which was the overall absolute difference in percent fibroglandular tissue volume (%FGV) between the ipsilateral (biopsied) breast and the contralateral (unaffected) breast.
The researchers also assessed local bilateral asymmetry, which was the perilesional %FGV difference in an area twice the size of, but excluding, the biopsy target, and the corresponding area in the unaffected breast.
The women were then divided into quartiles based on breast density asymmetry.
Most of the women had benign breast disease, including proliferative (43%) and nonproliferative (33%) disease, but 23% had carcinoma in situ or invasive breast cancer.
The trend for higher risk of in situ or invasive cancer with increasing breast density asymmetry was observed only in the local analysis. The odds ratio was 1.59 (95% confidence interval, 0.94-2.69) for women in the highest quartile of breast density asymmetry (absolute difference, > 8.23) versus those in the lowest quartile (absolute difference, ≤ –5.55; P = .067).
When compared with women who had proliferative benign disease, women with carcinoma in situ or invasive breast cancer “were more likely to be in the higher than lower quartiles,” said lead investigator Maeve Mullooly, PhD, a research fellow at the Royal College of Surgeons in Dublin.
There was no association between breast density asymmetry and traditional breast cancer risk factors such as age, body mass index, race, and hormone therapy. However, among women diagnosed with benign nonproliferative disease, women with a breast cancer family history were more likely to have higher overall breast density asymmetry.
Study rationale and details
Higher breast density is a known risk factor for breast cancer. Breast asymmetry also has been reported as a possible risk factor (Breast Cancer Res. 2006;8[2]:R14), and incorporation of breast density asymmetry into traditional risk factors in one study improved risk prediction (Breast Cancer Res. 2017 Mar 14;19[1]:29).
Building on that work, the goal of Dr. Mullooly’s study was to “learn how to better use breast density to inform breast cancer risk prediction,” she said.
To that end, her team turned to 854 women enrolled from 2007-2010 in the National Cancer Institute’s Breast Radiology Evaluation and Study of Tissues Project, a cross-sectional molecular epidemiologic study designed to understand how breast density measures are related to breast cancer etiology.
Most of the women were non-Hispanic white. The mean age was 51 years (range, 40-65), and the median body mass index was 25 kg/m2.
About three-quarters of the women (76%) had a breast density asymmetry of at least 2% on the global analysis, with 43% having higher %FGV in the biopsied breast and 33% having higher %FGV in the unaffected breast. In all, 89% of women had local breast density asymmetry, with higher density in the biopsied breast in 61% of women and higher density in the contralateral breast in 28%.
Next steps
This research is ongoing, and additional follow-up is planned, according to Dr. Mullooly. She said the researchers hope to apply more recent analytical techniques to the mammograms and to study the histologic differences in their breast biopsy specimens, among other steps, to see if stronger relationships with greater clinical utility emerge.
It was a “very well done study” with “very provocative data,” said presentation moderator Jennifer Wargo, MD, professor of genomic medicine and surgical oncology at the University of Texas MD Anderson Cancer Center in Houston.
She was interested in the planned next steps, particularly the histologic analysis of dense versus less dense breast tissue. There could be “differences in stroma or hormonal levels even at the microenvironmental level” that “represent a potential field defect, which later puts someone at risk,” she said, adding that it’s “great” that the work is continuing.
The National Cancer Institute funded the research. Dr. Mullooly reported no relevant disclosures. Dr. Wargo disclosed relationships with Bristol-Myers Squibb, Roche/Genentech, Novartis, GlaxoSmithKline, AstraZeneca, Imedex, Dava Oncology, Omniprex, Illumina, Gilead, PeerView, Physician Education Resource, MedImmune, Merck, Biothera Pharmaceuticals, and Microbiome DX.
SOURCE: Mullooly M et al. AACR 2020, Abstract NG15.
FROM AACR 2020
Asymptomatic Plaque on the Scalp
The Diagnosis: Nevus Comedonicus
Dermoscopy showed multiple dilated follicular openings plugged with keratinous material (Figure 1). Histopathology revealed dilated follicular infundibula with dilation and orthokeratotic plugging (Figure 2). Routine laboratory tests including complete blood cell count and blood chemistry were within reference range. Thus, on the basis of clinical, dermoscopy, and histopathological findings, a diagnosis of nevus comedonicus (NC) was made. The patient refused treatment for cosmetic reasons.
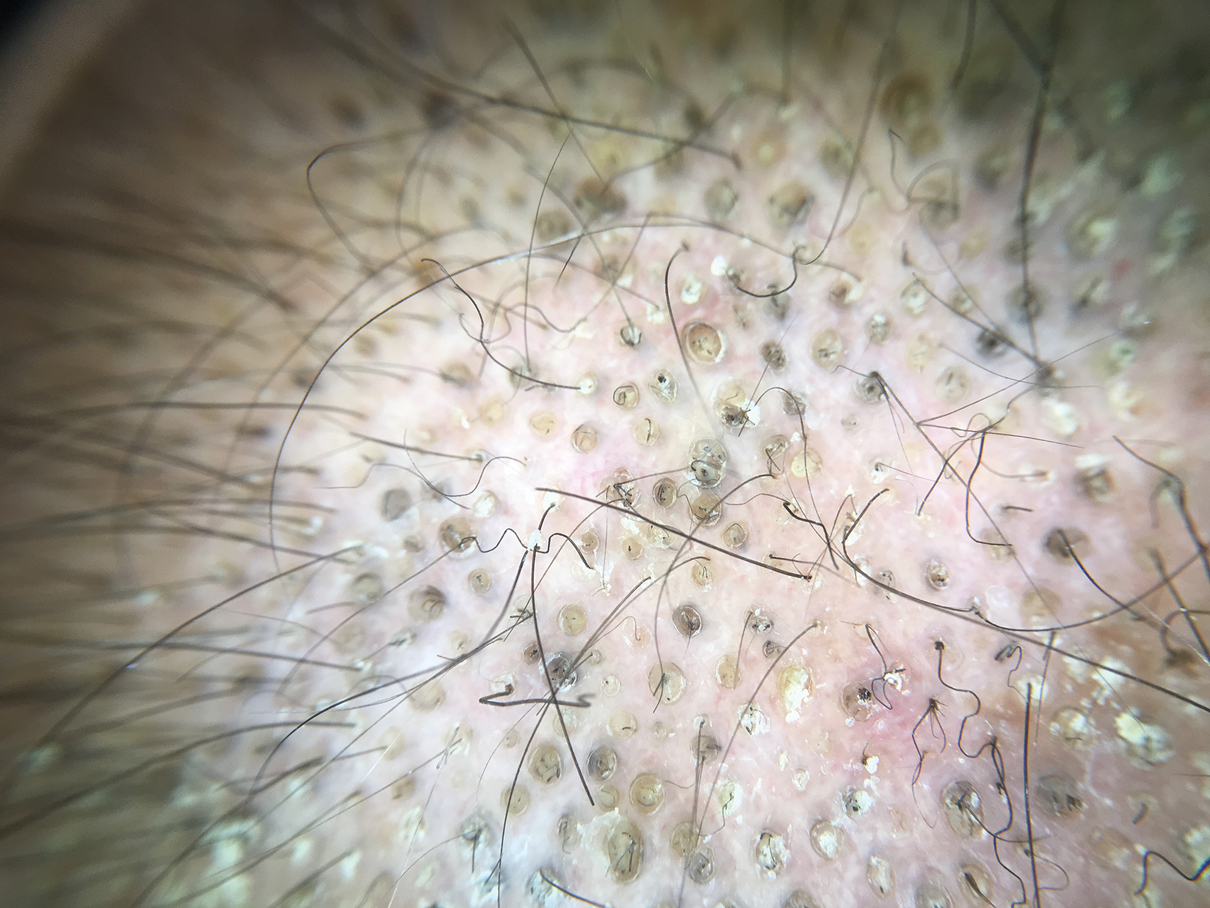
Nevus comedonicus is a rare hamartoma first described by Kofmann1 in 1895. It is thought to be a developmental defect of the pilosebaceous unit; the resulting structure is unable to produce mature hairs, matrix cells, or sebaceous glands and is capable only of forming soft keratin.2 Clinically, it is characterized by closely grouped papules with hyperkeratotic plugs that mimic comedones. It has a predilection for the face, neck, and trunk area. Nevertheless, scalp involvement rarely has been reported in the literature.2-4 Nevus comedonicus usually appears at birth or during childhood and generally is asymptomatic; however, an inflammatory variant of NC with cyst formation and recurrent infections also has been described.5 Moreover, a syndromic variant was reported and characterized by a combination of NC with ocular, skeletal, or neurological defects.5 Most lesions grow proportionately with age and usually stabilize by late adolescence.2 Our patient's plaque increased in size with age. No triggering factors were found. Although NC usually has a benign course, squamous cell carcinoma arising in NC has been reported.6 Consequently, routine surveillance is necessary.
Diagnosis often is easily made by considering the characteristic morphology of the lesions and the early age of its appearance. However, in atypical NC presentations, acne, seborrheic keratosis, porokeratotic eccrine ostial and dermal duct nevus, folliculotropic mycosis fungoides, Favre-Racouchot syndrome, or familial dyskeratotic comedones should be considered. Dermoscopy has been reported to be useful in the diagnosis of NC. Typical dermoscopy findings are numerous circular and barrel-shaped homogenous areas in light and dark brown shades with remarkable keratin plugs.7,8
Folliculotropic mycosis fungoides is a variant of mycosis fungoides characterized by hair follicle invasion of mature, CD4+, small, lymphoid cells with cerebriform nuclei.9 Patients may present with grouped follicular papules that preferentially involve the head and neck area. It typically occurs in adults but occasionally may affect children. Histopathology is characterized by the presence of folliculotropic infiltrates with variable infiltration of the follicular epithelium, often with sparing of the epidermis. Familial dyskeratotic comedones, rare autosomal-dominant genodermatoses, clinically are characterized by symmetrically scattered comedonelike hyperkeratotic papules. These lesions appear around puberty and show a predilection for the trunk, arms, and face. Histopathology reveals craterlike invaginations filled with keratinous material and evidence of dyskeratosis. Porokeratotic eccrine ostial and dermal duct nevus is a rare adnexal hamartoma with eccrine differentiation. It is characterized by asymptomatic grouped keratotic papules and plaques. The lesions usually present at birth or in childhood and favor the palms and soles. Widespread involvement along Blaschko lines also can occur. Cornoid lamella involving an eccrine duct is the characteristic histopathologic feature of this condition.9
Treatment of NC is essentially reserved for cosmetic reasons or when there are complications such as discomfort or infection. Treatment options include topical corticosteroids, topical retinoids, and keratolytic agents such as ammonium lactate or salicylic acid.10 The use of oral isotretinoin is controversial.2 Surgical excision is useful for localized lesions. Nevus comedonicus, especially occurring at unusual sites such as the scalp, is uncommon. Therefore, a high index of suspicion is required to reach a diagnosis.
- Kofmann S. Ein fall von seltener localisation und verbreitiing von comedonen. Arch Derm Syph. 1895;32:177-178.
- Sikorski D, Parker J, Shwayder T. A boy with an unusual scalp birthmark. Int J Dermatol. 2011;50:670-672.
- Ghaninezhad H, Ehsani AH, Mansoori P, et al. Naevus comedonicus of the scalp. J Eur Acad Dermatol Venereol. 2006;20:184-185.
- Kikkeri N, Priyanka R, Parshawanath H. Nevus comedonicus on scalp: a rare site. Indian J Dermatol. 2015;60:105.
- Happle R. The group of epidermal nevus syndromes. J Am Acad Dermatol. 2010;63:1-22.
- Walling HW, Swick BL. Squamous cell carcinoma arising in nevus comedonicus. Dermatol Surg. 2009;35:144-146.
- Kamin´ska-Winciorek G, S´piewak R. Dermoscopy on nevus comedonicus: a case report and review of the literature. Postepy Dermatol Alergol. 2013;30:252-254.
- Vora R, Kota R, Sheth N. Dermoscopy of nevus comedonicus. Indian Dermatol Online J. 2017;8:388.
- Wang NS, Meola T, Orlow SJ, et al. Porokeratotic eccrine ostial and dermal duct nevus: a report of 2 cases and review of the literature. Am J Dermatopathol. 2009;31:582-586.
- Ferrari B, Taliercio V, Restrepo P, et al. Nevus comedonicus: a case series. Pediatr Dermatol. 2015;32:216-219
The Diagnosis: Nevus Comedonicus
Dermoscopy showed multiple dilated follicular openings plugged with keratinous material (Figure 1). Histopathology revealed dilated follicular infundibula with dilation and orthokeratotic plugging (Figure 2). Routine laboratory tests including complete blood cell count and blood chemistry were within reference range. Thus, on the basis of clinical, dermoscopy, and histopathological findings, a diagnosis of nevus comedonicus (NC) was made. The patient refused treatment for cosmetic reasons.


Nevus comedonicus is a rare hamartoma first described by Kofmann1 in 1895. It is thought to be a developmental defect of the pilosebaceous unit; the resulting structure is unable to produce mature hairs, matrix cells, or sebaceous glands and is capable only of forming soft keratin.2 Clinically, it is characterized by closely grouped papules with hyperkeratotic plugs that mimic comedones. It has a predilection for the face, neck, and trunk area. Nevertheless, scalp involvement rarely has been reported in the literature.2-4 Nevus comedonicus usually appears at birth or during childhood and generally is asymptomatic; however, an inflammatory variant of NC with cyst formation and recurrent infections also has been described.5 Moreover, a syndromic variant was reported and characterized by a combination of NC with ocular, skeletal, or neurological defects.5 Most lesions grow proportionately with age and usually stabilize by late adolescence.2 Our patient's plaque increased in size with age. No triggering factors were found. Although NC usually has a benign course, squamous cell carcinoma arising in NC has been reported.6 Consequently, routine surveillance is necessary.
Diagnosis often is easily made by considering the characteristic morphology of the lesions and the early age of its appearance. However, in atypical NC presentations, acne, seborrheic keratosis, porokeratotic eccrine ostial and dermal duct nevus, folliculotropic mycosis fungoides, Favre-Racouchot syndrome, or familial dyskeratotic comedones should be considered. Dermoscopy has been reported to be useful in the diagnosis of NC. Typical dermoscopy findings are numerous circular and barrel-shaped homogenous areas in light and dark brown shades with remarkable keratin plugs.7,8
Folliculotropic mycosis fungoides is a variant of mycosis fungoides characterized by hair follicle invasion of mature, CD4+, small, lymphoid cells with cerebriform nuclei.9 Patients may present with grouped follicular papules that preferentially involve the head and neck area. It typically occurs in adults but occasionally may affect children. Histopathology is characterized by the presence of folliculotropic infiltrates with variable infiltration of the follicular epithelium, often with sparing of the epidermis. Familial dyskeratotic comedones, rare autosomal-dominant genodermatoses, clinically are characterized by symmetrically scattered comedonelike hyperkeratotic papules. These lesions appear around puberty and show a predilection for the trunk, arms, and face. Histopathology reveals craterlike invaginations filled with keratinous material and evidence of dyskeratosis. Porokeratotic eccrine ostial and dermal duct nevus is a rare adnexal hamartoma with eccrine differentiation. It is characterized by asymptomatic grouped keratotic papules and plaques. The lesions usually present at birth or in childhood and favor the palms and soles. Widespread involvement along Blaschko lines also can occur. Cornoid lamella involving an eccrine duct is the characteristic histopathologic feature of this condition.9
Treatment of NC is essentially reserved for cosmetic reasons or when there are complications such as discomfort or infection. Treatment options include topical corticosteroids, topical retinoids, and keratolytic agents such as ammonium lactate or salicylic acid.10 The use of oral isotretinoin is controversial.2 Surgical excision is useful for localized lesions. Nevus comedonicus, especially occurring at unusual sites such as the scalp, is uncommon. Therefore, a high index of suspicion is required to reach a diagnosis.
The Diagnosis: Nevus Comedonicus
Dermoscopy showed multiple dilated follicular openings plugged with keratinous material (Figure 1). Histopathology revealed dilated follicular infundibula with dilation and orthokeratotic plugging (Figure 2). Routine laboratory tests including complete blood cell count and blood chemistry were within reference range. Thus, on the basis of clinical, dermoscopy, and histopathological findings, a diagnosis of nevus comedonicus (NC) was made. The patient refused treatment for cosmetic reasons.


Nevus comedonicus is a rare hamartoma first described by Kofmann1 in 1895. It is thought to be a developmental defect of the pilosebaceous unit; the resulting structure is unable to produce mature hairs, matrix cells, or sebaceous glands and is capable only of forming soft keratin.2 Clinically, it is characterized by closely grouped papules with hyperkeratotic plugs that mimic comedones. It has a predilection for the face, neck, and trunk area. Nevertheless, scalp involvement rarely has been reported in the literature.2-4 Nevus comedonicus usually appears at birth or during childhood and generally is asymptomatic; however, an inflammatory variant of NC with cyst formation and recurrent infections also has been described.5 Moreover, a syndromic variant was reported and characterized by a combination of NC with ocular, skeletal, or neurological defects.5 Most lesions grow proportionately with age and usually stabilize by late adolescence.2 Our patient's plaque increased in size with age. No triggering factors were found. Although NC usually has a benign course, squamous cell carcinoma arising in NC has been reported.6 Consequently, routine surveillance is necessary.
Diagnosis often is easily made by considering the characteristic morphology of the lesions and the early age of its appearance. However, in atypical NC presentations, acne, seborrheic keratosis, porokeratotic eccrine ostial and dermal duct nevus, folliculotropic mycosis fungoides, Favre-Racouchot syndrome, or familial dyskeratotic comedones should be considered. Dermoscopy has been reported to be useful in the diagnosis of NC. Typical dermoscopy findings are numerous circular and barrel-shaped homogenous areas in light and dark brown shades with remarkable keratin plugs.7,8
Folliculotropic mycosis fungoides is a variant of mycosis fungoides characterized by hair follicle invasion of mature, CD4+, small, lymphoid cells with cerebriform nuclei.9 Patients may present with grouped follicular papules that preferentially involve the head and neck area. It typically occurs in adults but occasionally may affect children. Histopathology is characterized by the presence of folliculotropic infiltrates with variable infiltration of the follicular epithelium, often with sparing of the epidermis. Familial dyskeratotic comedones, rare autosomal-dominant genodermatoses, clinically are characterized by symmetrically scattered comedonelike hyperkeratotic papules. These lesions appear around puberty and show a predilection for the trunk, arms, and face. Histopathology reveals craterlike invaginations filled with keratinous material and evidence of dyskeratosis. Porokeratotic eccrine ostial and dermal duct nevus is a rare adnexal hamartoma with eccrine differentiation. It is characterized by asymptomatic grouped keratotic papules and plaques. The lesions usually present at birth or in childhood and favor the palms and soles. Widespread involvement along Blaschko lines also can occur. Cornoid lamella involving an eccrine duct is the characteristic histopathologic feature of this condition.9
Treatment of NC is essentially reserved for cosmetic reasons or when there are complications such as discomfort or infection. Treatment options include topical corticosteroids, topical retinoids, and keratolytic agents such as ammonium lactate or salicylic acid.10 The use of oral isotretinoin is controversial.2 Surgical excision is useful for localized lesions. Nevus comedonicus, especially occurring at unusual sites such as the scalp, is uncommon. Therefore, a high index of suspicion is required to reach a diagnosis.
- Kofmann S. Ein fall von seltener localisation und verbreitiing von comedonen. Arch Derm Syph. 1895;32:177-178.
- Sikorski D, Parker J, Shwayder T. A boy with an unusual scalp birthmark. Int J Dermatol. 2011;50:670-672.
- Ghaninezhad H, Ehsani AH, Mansoori P, et al. Naevus comedonicus of the scalp. J Eur Acad Dermatol Venereol. 2006;20:184-185.
- Kikkeri N, Priyanka R, Parshawanath H. Nevus comedonicus on scalp: a rare site. Indian J Dermatol. 2015;60:105.
- Happle R. The group of epidermal nevus syndromes. J Am Acad Dermatol. 2010;63:1-22.
- Walling HW, Swick BL. Squamous cell carcinoma arising in nevus comedonicus. Dermatol Surg. 2009;35:144-146.
- Kamin´ska-Winciorek G, S´piewak R. Dermoscopy on nevus comedonicus: a case report and review of the literature. Postepy Dermatol Alergol. 2013;30:252-254.
- Vora R, Kota R, Sheth N. Dermoscopy of nevus comedonicus. Indian Dermatol Online J. 2017;8:388.
- Wang NS, Meola T, Orlow SJ, et al. Porokeratotic eccrine ostial and dermal duct nevus: a report of 2 cases and review of the literature. Am J Dermatopathol. 2009;31:582-586.
- Ferrari B, Taliercio V, Restrepo P, et al. Nevus comedonicus: a case series. Pediatr Dermatol. 2015;32:216-219
- Kofmann S. Ein fall von seltener localisation und verbreitiing von comedonen. Arch Derm Syph. 1895;32:177-178.
- Sikorski D, Parker J, Shwayder T. A boy with an unusual scalp birthmark. Int J Dermatol. 2011;50:670-672.
- Ghaninezhad H, Ehsani AH, Mansoori P, et al. Naevus comedonicus of the scalp. J Eur Acad Dermatol Venereol. 2006;20:184-185.
- Kikkeri N, Priyanka R, Parshawanath H. Nevus comedonicus on scalp: a rare site. Indian J Dermatol. 2015;60:105.
- Happle R. The group of epidermal nevus syndromes. J Am Acad Dermatol. 2010;63:1-22.
- Walling HW, Swick BL. Squamous cell carcinoma arising in nevus comedonicus. Dermatol Surg. 2009;35:144-146.
- Kamin´ska-Winciorek G, S´piewak R. Dermoscopy on nevus comedonicus: a case report and review of the literature. Postepy Dermatol Alergol. 2013;30:252-254.
- Vora R, Kota R, Sheth N. Dermoscopy of nevus comedonicus. Indian Dermatol Online J. 2017;8:388.
- Wang NS, Meola T, Orlow SJ, et al. Porokeratotic eccrine ostial and dermal duct nevus: a report of 2 cases and review of the literature. Am J Dermatopathol. 2009;31:582-586.
- Ferrari B, Taliercio V, Restrepo P, et al. Nevus comedonicus: a case series. Pediatr Dermatol. 2015;32:216-219
A 50-year-old man presented to the dermatology department with an asymptomatic plaque on the scalp that had been present since childhood. The size of the plaque gradually progressed initially but had notably increased in size in the last 6 months. There was no association with trauma or irritation. There was no family history of similar lesions. Physical examination revealed a 3.0×2.5-cm plaque on the vertex of the scalp consisting of aggregated pits plugged with keratinous material resembling comedones. There were no lesions elsewhere on the body. Dermoscopy and a 4-mm punch biopsy were performed.
Neural tube defect risk from dolutegravir drops as clinical experience grows
The newest data, based on 3,591 deliveries among women in Botswana infected by HIV and treated with dolutegravir at the time of conception during a little more than 5.5 years through April 2020, showed that dolutegravir use at conception linked with 7 cases of neonatal neural tube defects (NTDs), a 0.19% rate that exceeded comparator rates by about 1 in every 1,000 deliveries, far below the 0.94% rate initially found and that raised a red flag 2 years ago (New Engl J Med. 2018 Sep 6;379[10]:979-81). “The prevalence of NTDs among infants born to women on dolutegravir at conception may be stabilizing at approximately 2 per 1,000,” said Rebecca Zash, MD, during the virtual meeting of the International AIDS conference.
“This small absolute risk for neural tube defects is far outweighed by the potential benefits from dolutegravir” for better tolerability than alternative drugs and fewer drug-drug interactions. “This should allow for broader use of dolutegravir in women,” added Dr. Zash, an HIV specialist at Beth Israel Deaconess Medical Center and codirector of the Placental Scientific Working Group of the Harvard University Center for AIDS Research, both in Boston.
“What this has taught us is that women are not a niche population” of people infected with HIV, but rather constitute about half of HIV patients worldwide. “Maintaining gender equity in HIV treatment requires safety data for treatments during pregnancy,” she said during a press briefing.
The new findings mean that it’s “time to lay to rest” concerns about neural tube defects (NTDs) in infants born to women treated with dolutegravir, “given the incredible benefits of dolutegravir,” commented Monica Gandhi, MD, professor of medicine and associate chief of the division of HIV, infectious disease, and global medicine at the University of California, San Francisco. Another benefit from removing any caveats about use of dolutegravir in women who could become pregnant is that it would simplify treatment recommendations and make dolutegravir the unqualified first-line agent for treating HIV infection, Dr. Gandhi said during the briefing. “It’s super reassuring to have these data, as the incidence of NTDs goes down and down,” she added.
Following the alarm raised by initial findings from the Tsepamo study in 2018, Dr. Zash and associates first updated their data through March 2019, when they reported a revised cumulative NTD incidence rate of 0.3% (New Engl J Med. 2019 Aug 29;381[9]:827-40). The Tsepamo study began by following the pregnancy outcomes of women at eight Botswana sites during August 2014–July 2018, representing 45% of the country’s deliveries. This expanded to 18 sites and 72% of deliveries during July-September 2018, and then starting in September 2019 the scope slightly reduced to 16 Botswana sites with 70% of the nation’s deliveries.
Folate supplementation to women who might conceive is vital, but remains spotty in Botswana. “Folate supplementation is a no-brainer, but has had really slow adoption in many countries,” Dr. Zash said. “Folate supplementation, especially in food so that everyone gets it, will reduce NTDs by half.” The two most recent cases of infants born with a NTD to mothers who had been on dolutegravir at conception occurred in mothers who had received no folate supplementation, Dr. Zash reported.
The most recent HIV treatment guidelines for adults from the Department of Health & Human Services, which date from late 2019, designated dolutegravir plus lamivudine as a first-line regimen for most, but flagged it as an “alternative” antiretroviral drug when treating women who have childbearing potential and are either trying to conceive or are sexually active but not using contraception.
The study had no commercial funding. Dr. Zash has been a researcher in studies funded by CytoDyn, Fulcrum, and Gilead. Dr. Gandhi had no commercial disclosures.
The newest data, based on 3,591 deliveries among women in Botswana infected by HIV and treated with dolutegravir at the time of conception during a little more than 5.5 years through April 2020, showed that dolutegravir use at conception linked with 7 cases of neonatal neural tube defects (NTDs), a 0.19% rate that exceeded comparator rates by about 1 in every 1,000 deliveries, far below the 0.94% rate initially found and that raised a red flag 2 years ago (New Engl J Med. 2018 Sep 6;379[10]:979-81). “The prevalence of NTDs among infants born to women on dolutegravir at conception may be stabilizing at approximately 2 per 1,000,” said Rebecca Zash, MD, during the virtual meeting of the International AIDS conference.
“This small absolute risk for neural tube defects is far outweighed by the potential benefits from dolutegravir” for better tolerability than alternative drugs and fewer drug-drug interactions. “This should allow for broader use of dolutegravir in women,” added Dr. Zash, an HIV specialist at Beth Israel Deaconess Medical Center and codirector of the Placental Scientific Working Group of the Harvard University Center for AIDS Research, both in Boston.
“What this has taught us is that women are not a niche population” of people infected with HIV, but rather constitute about half of HIV patients worldwide. “Maintaining gender equity in HIV treatment requires safety data for treatments during pregnancy,” she said during a press briefing.
The new findings mean that it’s “time to lay to rest” concerns about neural tube defects (NTDs) in infants born to women treated with dolutegravir, “given the incredible benefits of dolutegravir,” commented Monica Gandhi, MD, professor of medicine and associate chief of the division of HIV, infectious disease, and global medicine at the University of California, San Francisco. Another benefit from removing any caveats about use of dolutegravir in women who could become pregnant is that it would simplify treatment recommendations and make dolutegravir the unqualified first-line agent for treating HIV infection, Dr. Gandhi said during the briefing. “It’s super reassuring to have these data, as the incidence of NTDs goes down and down,” she added.
Following the alarm raised by initial findings from the Tsepamo study in 2018, Dr. Zash and associates first updated their data through March 2019, when they reported a revised cumulative NTD incidence rate of 0.3% (New Engl J Med. 2019 Aug 29;381[9]:827-40). The Tsepamo study began by following the pregnancy outcomes of women at eight Botswana sites during August 2014–July 2018, representing 45% of the country’s deliveries. This expanded to 18 sites and 72% of deliveries during July-September 2018, and then starting in September 2019 the scope slightly reduced to 16 Botswana sites with 70% of the nation’s deliveries.
Folate supplementation to women who might conceive is vital, but remains spotty in Botswana. “Folate supplementation is a no-brainer, but has had really slow adoption in many countries,” Dr. Zash said. “Folate supplementation, especially in food so that everyone gets it, will reduce NTDs by half.” The two most recent cases of infants born with a NTD to mothers who had been on dolutegravir at conception occurred in mothers who had received no folate supplementation, Dr. Zash reported.
The most recent HIV treatment guidelines for adults from the Department of Health & Human Services, which date from late 2019, designated dolutegravir plus lamivudine as a first-line regimen for most, but flagged it as an “alternative” antiretroviral drug when treating women who have childbearing potential and are either trying to conceive or are sexually active but not using contraception.
The study had no commercial funding. Dr. Zash has been a researcher in studies funded by CytoDyn, Fulcrum, and Gilead. Dr. Gandhi had no commercial disclosures.
The newest data, based on 3,591 deliveries among women in Botswana infected by HIV and treated with dolutegravir at the time of conception during a little more than 5.5 years through April 2020, showed that dolutegravir use at conception linked with 7 cases of neonatal neural tube defects (NTDs), a 0.19% rate that exceeded comparator rates by about 1 in every 1,000 deliveries, far below the 0.94% rate initially found and that raised a red flag 2 years ago (New Engl J Med. 2018 Sep 6;379[10]:979-81). “The prevalence of NTDs among infants born to women on dolutegravir at conception may be stabilizing at approximately 2 per 1,000,” said Rebecca Zash, MD, during the virtual meeting of the International AIDS conference.
“This small absolute risk for neural tube defects is far outweighed by the potential benefits from dolutegravir” for better tolerability than alternative drugs and fewer drug-drug interactions. “This should allow for broader use of dolutegravir in women,” added Dr. Zash, an HIV specialist at Beth Israel Deaconess Medical Center and codirector of the Placental Scientific Working Group of the Harvard University Center for AIDS Research, both in Boston.
“What this has taught us is that women are not a niche population” of people infected with HIV, but rather constitute about half of HIV patients worldwide. “Maintaining gender equity in HIV treatment requires safety data for treatments during pregnancy,” she said during a press briefing.
The new findings mean that it’s “time to lay to rest” concerns about neural tube defects (NTDs) in infants born to women treated with dolutegravir, “given the incredible benefits of dolutegravir,” commented Monica Gandhi, MD, professor of medicine and associate chief of the division of HIV, infectious disease, and global medicine at the University of California, San Francisco. Another benefit from removing any caveats about use of dolutegravir in women who could become pregnant is that it would simplify treatment recommendations and make dolutegravir the unqualified first-line agent for treating HIV infection, Dr. Gandhi said during the briefing. “It’s super reassuring to have these data, as the incidence of NTDs goes down and down,” she added.
Following the alarm raised by initial findings from the Tsepamo study in 2018, Dr. Zash and associates first updated their data through March 2019, when they reported a revised cumulative NTD incidence rate of 0.3% (New Engl J Med. 2019 Aug 29;381[9]:827-40). The Tsepamo study began by following the pregnancy outcomes of women at eight Botswana sites during August 2014–July 2018, representing 45% of the country’s deliveries. This expanded to 18 sites and 72% of deliveries during July-September 2018, and then starting in September 2019 the scope slightly reduced to 16 Botswana sites with 70% of the nation’s deliveries.
Folate supplementation to women who might conceive is vital, but remains spotty in Botswana. “Folate supplementation is a no-brainer, but has had really slow adoption in many countries,” Dr. Zash said. “Folate supplementation, especially in food so that everyone gets it, will reduce NTDs by half.” The two most recent cases of infants born with a NTD to mothers who had been on dolutegravir at conception occurred in mothers who had received no folate supplementation, Dr. Zash reported.
The most recent HIV treatment guidelines for adults from the Department of Health & Human Services, which date from late 2019, designated dolutegravir plus lamivudine as a first-line regimen for most, but flagged it as an “alternative” antiretroviral drug when treating women who have childbearing potential and are either trying to conceive or are sexually active but not using contraception.
The study had no commercial funding. Dr. Zash has been a researcher in studies funded by CytoDyn, Fulcrum, and Gilead. Dr. Gandhi had no commercial disclosures.
FROM AIDS 2020