User login
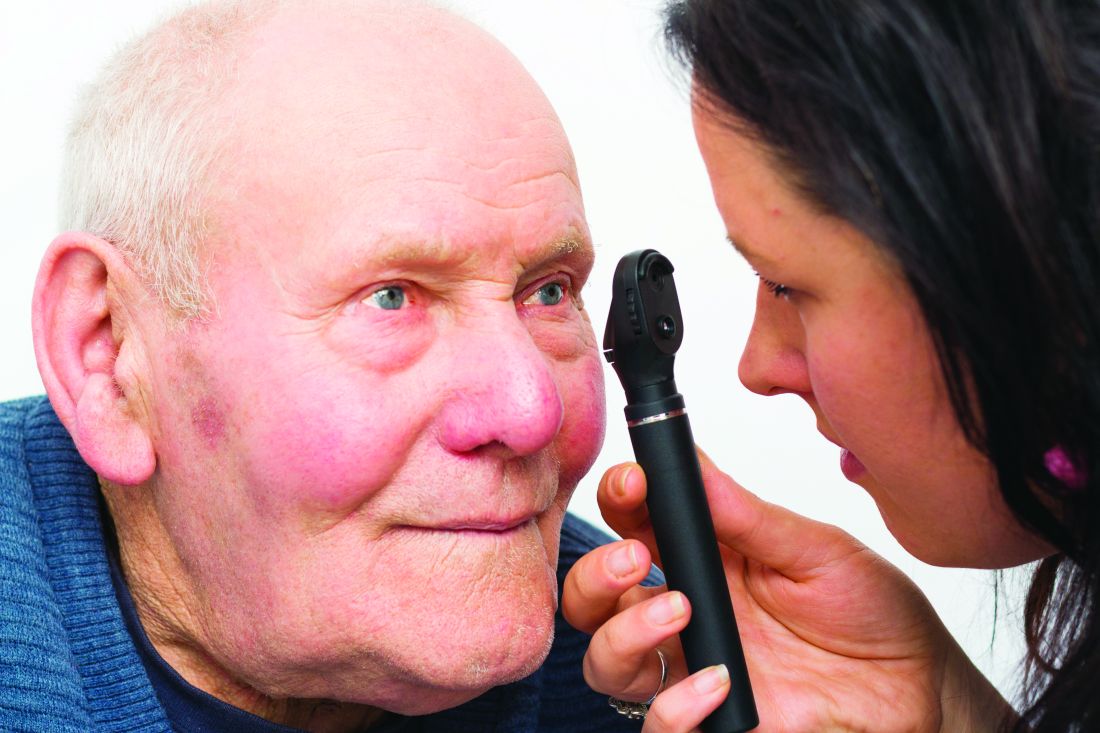
Diabetes screening program in optometry offices to expand
The program is sponsored by VSP Vision Care, a vision benefits company with over 40,000 network optometrists and nearly 90 million consumer members worldwide. “Optometrists are often the first to detect signs of diabetes by looking at the blood vessels in the eye during a comprehensive eye exam,” the company said in a statement.
In the pilot program, conducted from May 2019 to February 2020* in 12 VSP practices in five states, 818 patients who had come in for their annual vision exam were given the American Diabetes Association Risk Test for type 2 diabetes, and 287 identified at risk were offered an in-office fingerstick hemoglobin A1c test.
Materials were provided free to the optometrists, who were paid a professional fee to perform the HbA1c screenings.
Of the 287 eligible for the HbA1c test, 85% took it. Of those 244, 31% and 5% had levels in the prediabetes and diabetes range, respectively. None had been aware of their status previously, and 92% rated the screening as an extremely or very positive experience.
Now, VSP is expanding the pilot program for another year with two large clients in Ohio covering about 90,000 members.
“Coupled with the fact that VSP members are more likely to get their annual eye exam over their annual physical exam with their primary care physician, HbA1c screenings provided by eye doctors offer another critical way to detect the chronic condition earlier and help prevent eye disease and even vision loss caused by diabetes,” according to the statement.
In an interview, a VSP spokesperson explained that if the patient provides their primary care provider information to the optometrist, the optometrist will send a referral with exam information to that provider and also instruct the patient to make an appointment with the provider for follow-up testing and care.
The optometrist also educates the patient about the connection between eye health and overall health and provides them with a flier that gives tips on lifestyle changes they can make to help slow or prevent the progression to type 2 diabetes, the spokesperson said.
Thirty states, including Ohio, allow optometrists to perform in-office blood testing, including HbA1c screening, provided they obtain a Clinical Laboratory Improvement Amendments Certificate of Waiver. VSP is providing online training for participating optometrists on administering the HbA1c screening.
The pilot program is part of an alliance between VSP and the American Diabetes Association formed in November 2019 to raise awareness of eye health in people with diabetes and those at risk for it.
*Correction: The original article included the wrong end date for the pilot program.
The program is sponsored by VSP Vision Care, a vision benefits company with over 40,000 network optometrists and nearly 90 million consumer members worldwide. “Optometrists are often the first to detect signs of diabetes by looking at the blood vessels in the eye during a comprehensive eye exam,” the company said in a statement.
In the pilot program, conducted from May 2019 to February 2020* in 12 VSP practices in five states, 818 patients who had come in for their annual vision exam were given the American Diabetes Association Risk Test for type 2 diabetes, and 287 identified at risk were offered an in-office fingerstick hemoglobin A1c test.
Materials were provided free to the optometrists, who were paid a professional fee to perform the HbA1c screenings.
Of the 287 eligible for the HbA1c test, 85% took it. Of those 244, 31% and 5% had levels in the prediabetes and diabetes range, respectively. None had been aware of their status previously, and 92% rated the screening as an extremely or very positive experience.
Now, VSP is expanding the pilot program for another year with two large clients in Ohio covering about 90,000 members.
“Coupled with the fact that VSP members are more likely to get their annual eye exam over their annual physical exam with their primary care physician, HbA1c screenings provided by eye doctors offer another critical way to detect the chronic condition earlier and help prevent eye disease and even vision loss caused by diabetes,” according to the statement.
In an interview, a VSP spokesperson explained that if the patient provides their primary care provider information to the optometrist, the optometrist will send a referral with exam information to that provider and also instruct the patient to make an appointment with the provider for follow-up testing and care.
The optometrist also educates the patient about the connection between eye health and overall health and provides them with a flier that gives tips on lifestyle changes they can make to help slow or prevent the progression to type 2 diabetes, the spokesperson said.
Thirty states, including Ohio, allow optometrists to perform in-office blood testing, including HbA1c screening, provided they obtain a Clinical Laboratory Improvement Amendments Certificate of Waiver. VSP is providing online training for participating optometrists on administering the HbA1c screening.
The pilot program is part of an alliance between VSP and the American Diabetes Association formed in November 2019 to raise awareness of eye health in people with diabetes and those at risk for it.
*Correction: The original article included the wrong end date for the pilot program.
The program is sponsored by VSP Vision Care, a vision benefits company with over 40,000 network optometrists and nearly 90 million consumer members worldwide. “Optometrists are often the first to detect signs of diabetes by looking at the blood vessels in the eye during a comprehensive eye exam,” the company said in a statement.
In the pilot program, conducted from May 2019 to February 2020* in 12 VSP practices in five states, 818 patients who had come in for their annual vision exam were given the American Diabetes Association Risk Test for type 2 diabetes, and 287 identified at risk were offered an in-office fingerstick hemoglobin A1c test.
Materials were provided free to the optometrists, who were paid a professional fee to perform the HbA1c screenings.
Of the 287 eligible for the HbA1c test, 85% took it. Of those 244, 31% and 5% had levels in the prediabetes and diabetes range, respectively. None had been aware of their status previously, and 92% rated the screening as an extremely or very positive experience.
Now, VSP is expanding the pilot program for another year with two large clients in Ohio covering about 90,000 members.
“Coupled with the fact that VSP members are more likely to get their annual eye exam over their annual physical exam with their primary care physician, HbA1c screenings provided by eye doctors offer another critical way to detect the chronic condition earlier and help prevent eye disease and even vision loss caused by diabetes,” according to the statement.
In an interview, a VSP spokesperson explained that if the patient provides their primary care provider information to the optometrist, the optometrist will send a referral with exam information to that provider and also instruct the patient to make an appointment with the provider for follow-up testing and care.
The optometrist also educates the patient about the connection between eye health and overall health and provides them with a flier that gives tips on lifestyle changes they can make to help slow or prevent the progression to type 2 diabetes, the spokesperson said.
Thirty states, including Ohio, allow optometrists to perform in-office blood testing, including HbA1c screening, provided they obtain a Clinical Laboratory Improvement Amendments Certificate of Waiver. VSP is providing online training for participating optometrists on administering the HbA1c screening.
The pilot program is part of an alliance between VSP and the American Diabetes Association formed in November 2019 to raise awareness of eye health in people with diabetes and those at risk for it.
*Correction: The original article included the wrong end date for the pilot program.
AMA reports a crash in physician revenues, visits over summer
according to a new American Medical Association survey of 3,500 physicians, conducted from mid-July to August. That period coincided with the second wave of the coronavirus pandemic in the United States.
A third of practices reported a revenue drop of 25%-49%; 15% said their volume had fallen by 50%-74%, and 4% saw a decrease of 75% or more.
Because of the pandemic, 81% of physicians were providing fewer in-person visits than in February. In-person visits dropped by 50% or more for more than one-third of physicians. The average number of in-person visits fell from 95 to 57 per week.
Physicians who responded to the survey held an average of six weekly telehealth visits before the pandemic, 29 at the height of the pandemic in the spring, and 16 the week they were surveyed. About 20% of respondents with any telehealth visits had conducted them before the pandemic, 77% at the height of the crisis, and 68% in the survey week.
Among the doctors who weren’t involved in telehealth visits before the pandemic, only 23% conducted them at the pandemic’s peak; 12% conducted them in the survey week.
Despite the telehealth increase, almost 70% of physicians were providing fewer total visits, including in-person and virtual encounters, than before the pandemic, the survey showed. About 21% saw a decrease of 25%-49%; 11%, a drop of 50%-74%; and 10%, a falloff of at least 75%. On average, total visits fell from 101 to 72 per week.
Other surveys more upbeat
A larger survey by Harvard University, the Commonwealth Fund, and the technology company Phreesia found that total outpatient visits in early October had rebounded to the level of March 1. This was a major turnaround from late March, when visits had plunged by nearly 60%.
According to the Harvard/Commonwealth Fund’s ongoing survey, visits started recovering in late June, although they were still off by 10%. They began rising further around Labor Day. The AMA researchers began conducting their survey in mid-June. The summertime surge in COVID-19 likely accounted for their finding that practice revenues were off by a third from the February baseline.
If so, the return to normalcy early this month may not represent the current situation as the virus sweeps across the country for a third time. In any case, even if patient visits and revenues have recovered more than the AMA data indicate, most practices will not have recovered from their losses earlier in the year.
A third survey more closely mirrors the AMA results. At the end of June, according to data from the Medical Group Management Association, revenues for the association’s members were 76% of what they had been in June 2019, and patient volume was 78% of that in the previous year.
Practice expenses rise
The AMA survey also found that, since February, practice spending on personal protective equipment (PPE) had increased by 57% or more, on average. About 64% of practice owners said their PPE expenditures were up from what they had been before the pandemic. For nearly 40% of practice owners, this expense had increased by 50% or more.
About 36% of the respondents said that acquiring PPE was very or extremely difficult. This was an especially big challenge for smaller practices, which do not have the purchasing power to compete with big health care systems for masks, gowns, and gloves, the AMA noted.
About 41% of doctors in practices with one to five physicians said they had difficulty getting PPE, compared with 30% of those in practices of 50 or more doctors. Only 25% of respondents in practices owned by hospitals and health systems said this was a problem.
Acquiring sufficient PPE is just one factor in the increase in practice expenses attributable to COVID-19. Still, it is indicative of the financial woes affecting physicians during the pandemic.
Nearly all respondents agreed that federal financial relief early in the pandemic was helpful and was appreciated. Among these programs was the CARES Act, which authorized the Provider Relief Fund, which accepted applications through Aug.28; the Medicare Accelerated and Advance Payment Program, which was suspended in April; and the SBA Paycheck Protection Program, which ended on Aug. 8.
To date, Congress had not approved the renewal of any these programs.
“Physician practices continue to be under significant financial stress due to reductions in patient volume and revenue, in addition to higher expenses for supplies that are scarce for some physicians,” said AMA President Susan R. Bailey, MD, in a news release on the survey’s findings. “More economic relief is needed now from Congress as some medical practices contemplate the brink of viability, particularly smaller practices that are facing a difficult road to recovery.”
A version of this article originally appeared on Medscape.com.
according to a new American Medical Association survey of 3,500 physicians, conducted from mid-July to August. That period coincided with the second wave of the coronavirus pandemic in the United States.
A third of practices reported a revenue drop of 25%-49%; 15% said their volume had fallen by 50%-74%, and 4% saw a decrease of 75% or more.
Because of the pandemic, 81% of physicians were providing fewer in-person visits than in February. In-person visits dropped by 50% or more for more than one-third of physicians. The average number of in-person visits fell from 95 to 57 per week.
Physicians who responded to the survey held an average of six weekly telehealth visits before the pandemic, 29 at the height of the pandemic in the spring, and 16 the week they were surveyed. About 20% of respondents with any telehealth visits had conducted them before the pandemic, 77% at the height of the crisis, and 68% in the survey week.
Among the doctors who weren’t involved in telehealth visits before the pandemic, only 23% conducted them at the pandemic’s peak; 12% conducted them in the survey week.
Despite the telehealth increase, almost 70% of physicians were providing fewer total visits, including in-person and virtual encounters, than before the pandemic, the survey showed. About 21% saw a decrease of 25%-49%; 11%, a drop of 50%-74%; and 10%, a falloff of at least 75%. On average, total visits fell from 101 to 72 per week.
Other surveys more upbeat
A larger survey by Harvard University, the Commonwealth Fund, and the technology company Phreesia found that total outpatient visits in early October had rebounded to the level of March 1. This was a major turnaround from late March, when visits had plunged by nearly 60%.
According to the Harvard/Commonwealth Fund’s ongoing survey, visits started recovering in late June, although they were still off by 10%. They began rising further around Labor Day. The AMA researchers began conducting their survey in mid-June. The summertime surge in COVID-19 likely accounted for their finding that practice revenues were off by a third from the February baseline.
If so, the return to normalcy early this month may not represent the current situation as the virus sweeps across the country for a third time. In any case, even if patient visits and revenues have recovered more than the AMA data indicate, most practices will not have recovered from their losses earlier in the year.
A third survey more closely mirrors the AMA results. At the end of June, according to data from the Medical Group Management Association, revenues for the association’s members were 76% of what they had been in June 2019, and patient volume was 78% of that in the previous year.
Practice expenses rise
The AMA survey also found that, since February, practice spending on personal protective equipment (PPE) had increased by 57% or more, on average. About 64% of practice owners said their PPE expenditures were up from what they had been before the pandemic. For nearly 40% of practice owners, this expense had increased by 50% or more.
About 36% of the respondents said that acquiring PPE was very or extremely difficult. This was an especially big challenge for smaller practices, which do not have the purchasing power to compete with big health care systems for masks, gowns, and gloves, the AMA noted.
About 41% of doctors in practices with one to five physicians said they had difficulty getting PPE, compared with 30% of those in practices of 50 or more doctors. Only 25% of respondents in practices owned by hospitals and health systems said this was a problem.
Acquiring sufficient PPE is just one factor in the increase in practice expenses attributable to COVID-19. Still, it is indicative of the financial woes affecting physicians during the pandemic.
Nearly all respondents agreed that federal financial relief early in the pandemic was helpful and was appreciated. Among these programs was the CARES Act, which authorized the Provider Relief Fund, which accepted applications through Aug.28; the Medicare Accelerated and Advance Payment Program, which was suspended in April; and the SBA Paycheck Protection Program, which ended on Aug. 8.
To date, Congress had not approved the renewal of any these programs.
“Physician practices continue to be under significant financial stress due to reductions in patient volume and revenue, in addition to higher expenses for supplies that are scarce for some physicians,” said AMA President Susan R. Bailey, MD, in a news release on the survey’s findings. “More economic relief is needed now from Congress as some medical practices contemplate the brink of viability, particularly smaller practices that are facing a difficult road to recovery.”
A version of this article originally appeared on Medscape.com.
according to a new American Medical Association survey of 3,500 physicians, conducted from mid-July to August. That period coincided with the second wave of the coronavirus pandemic in the United States.
A third of practices reported a revenue drop of 25%-49%; 15% said their volume had fallen by 50%-74%, and 4% saw a decrease of 75% or more.
Because of the pandemic, 81% of physicians were providing fewer in-person visits than in February. In-person visits dropped by 50% or more for more than one-third of physicians. The average number of in-person visits fell from 95 to 57 per week.
Physicians who responded to the survey held an average of six weekly telehealth visits before the pandemic, 29 at the height of the pandemic in the spring, and 16 the week they were surveyed. About 20% of respondents with any telehealth visits had conducted them before the pandemic, 77% at the height of the crisis, and 68% in the survey week.
Among the doctors who weren’t involved in telehealth visits before the pandemic, only 23% conducted them at the pandemic’s peak; 12% conducted them in the survey week.
Despite the telehealth increase, almost 70% of physicians were providing fewer total visits, including in-person and virtual encounters, than before the pandemic, the survey showed. About 21% saw a decrease of 25%-49%; 11%, a drop of 50%-74%; and 10%, a falloff of at least 75%. On average, total visits fell from 101 to 72 per week.
Other surveys more upbeat
A larger survey by Harvard University, the Commonwealth Fund, and the technology company Phreesia found that total outpatient visits in early October had rebounded to the level of March 1. This was a major turnaround from late March, when visits had plunged by nearly 60%.
According to the Harvard/Commonwealth Fund’s ongoing survey, visits started recovering in late June, although they were still off by 10%. They began rising further around Labor Day. The AMA researchers began conducting their survey in mid-June. The summertime surge in COVID-19 likely accounted for their finding that practice revenues were off by a third from the February baseline.
If so, the return to normalcy early this month may not represent the current situation as the virus sweeps across the country for a third time. In any case, even if patient visits and revenues have recovered more than the AMA data indicate, most practices will not have recovered from their losses earlier in the year.
A third survey more closely mirrors the AMA results. At the end of June, according to data from the Medical Group Management Association, revenues for the association’s members were 76% of what they had been in June 2019, and patient volume was 78% of that in the previous year.
Practice expenses rise
The AMA survey also found that, since February, practice spending on personal protective equipment (PPE) had increased by 57% or more, on average. About 64% of practice owners said their PPE expenditures were up from what they had been before the pandemic. For nearly 40% of practice owners, this expense had increased by 50% or more.
About 36% of the respondents said that acquiring PPE was very or extremely difficult. This was an especially big challenge for smaller practices, which do not have the purchasing power to compete with big health care systems for masks, gowns, and gloves, the AMA noted.
About 41% of doctors in practices with one to five physicians said they had difficulty getting PPE, compared with 30% of those in practices of 50 or more doctors. Only 25% of respondents in practices owned by hospitals and health systems said this was a problem.
Acquiring sufficient PPE is just one factor in the increase in practice expenses attributable to COVID-19. Still, it is indicative of the financial woes affecting physicians during the pandemic.
Nearly all respondents agreed that federal financial relief early in the pandemic was helpful and was appreciated. Among these programs was the CARES Act, which authorized the Provider Relief Fund, which accepted applications through Aug.28; the Medicare Accelerated and Advance Payment Program, which was suspended in April; and the SBA Paycheck Protection Program, which ended on Aug. 8.
To date, Congress had not approved the renewal of any these programs.
“Physician practices continue to be under significant financial stress due to reductions in patient volume and revenue, in addition to higher expenses for supplies that are scarce for some physicians,” said AMA President Susan R. Bailey, MD, in a news release on the survey’s findings. “More economic relief is needed now from Congress as some medical practices contemplate the brink of viability, particularly smaller practices that are facing a difficult road to recovery.”
A version of this article originally appeared on Medscape.com.
Subscription services a consideration for aesthetic patients
According to W. Grant Stevens, MD, an estimated 73% of aesthetic patients fall short when it comes to compliance with recommended treatment intervals for toxins, fillers, and other procedures.
“When we talk about how often the average patient should be treated with Botox, for instance, we say every 3-4 months,” Dr. Stevens, founder and CEO of Marina Plastic Surgery in Marina Del Rey, Calif., said during the virtual annual Masters of Aesthetics Symposium. But in reality, he added, “it’s more like every 7 months.” A 2015 survey of 23 Bay Area aesthetic practices conducted by HintMD found that 73% of patients were noncompliant and that they came in fewer than 3-4 times per year for treatments. “Not only did they come in infrequently, but they oftentimes were undercorrected and the revenue was being left on the table because of discounting and undercorrection,” said Dr. Stevens, who is also a professor of surgery in the division of plastic surgery at the University of Southern California, Los Angeles.
On average, each patient from the 23 practices surveyed spent $601.88 on treatments 1.44 times per year, yet the industry standard for neuromodulators is 3-4 times per year and every 2 months for HydraFacials and med spa facials. “What’s the problem?” he asked “Why are we falling off? For our practices, noncompliance leads to unhappy, undertreated patients, so they may write negative reviews. In addition to that, we lose revenue.” He cited results from a 2016 focus group of aesthetic patients who were asked about the perceived barriers to treatment compliance. More than two-thirds (68%) said cost was the issue, followed by the number of treatments required (43%) and effectiveness (16%).
Three years ago, Dr. Stevens used the HintMD platform to implement a treatment plan subscription service to 472 active members of his practice. Prior to implementation, patients were coming in for treatment with toxins an average of 1.8 times per year. After implementation, that rose to an average of 3.1 times per year. “That was almost an $800 incremental average increase spent on toxins alone,” Dr. Stevens said. “More importantly, the patients were therapeutic all year long.” With toxin and filler services combined, the average increased income grew to more than $1,100 per patient, which translated into increased annual revenue of $519,200.
Dr. Stevens said that many of his patients favor subscription services because most use them in other aspects of their lives, such as with Amazon Prime, Blue Apron, and Netflix. “They like it because it is personalized and customized,” he said. “If we want to adjust the amount of toxin or filler, we can do it that very day, and it’s customized for them. It’s not a one-size-fits-all program. It also allows them to have convenient, smaller monthly payments. That’s the key. That way, they budget. So, if they’re spending $200 a month or $500 a month or $1,000 a month, it’s a convenient monthly payment.”
Dr. Stevens disclosed that he is an adviser to Viveve, Venus, Aesthetics Biomedical, Alastin, Cypris Medical, Allergan, CoolSculpting, HydraFacial, Revance, Ampersand, and HintMD.
According to W. Grant Stevens, MD, an estimated 73% of aesthetic patients fall short when it comes to compliance with recommended treatment intervals for toxins, fillers, and other procedures.
“When we talk about how often the average patient should be treated with Botox, for instance, we say every 3-4 months,” Dr. Stevens, founder and CEO of Marina Plastic Surgery in Marina Del Rey, Calif., said during the virtual annual Masters of Aesthetics Symposium. But in reality, he added, “it’s more like every 7 months.” A 2015 survey of 23 Bay Area aesthetic practices conducted by HintMD found that 73% of patients were noncompliant and that they came in fewer than 3-4 times per year for treatments. “Not only did they come in infrequently, but they oftentimes were undercorrected and the revenue was being left on the table because of discounting and undercorrection,” said Dr. Stevens, who is also a professor of surgery in the division of plastic surgery at the University of Southern California, Los Angeles.
On average, each patient from the 23 practices surveyed spent $601.88 on treatments 1.44 times per year, yet the industry standard for neuromodulators is 3-4 times per year and every 2 months for HydraFacials and med spa facials. “What’s the problem?” he asked “Why are we falling off? For our practices, noncompliance leads to unhappy, undertreated patients, so they may write negative reviews. In addition to that, we lose revenue.” He cited results from a 2016 focus group of aesthetic patients who were asked about the perceived barriers to treatment compliance. More than two-thirds (68%) said cost was the issue, followed by the number of treatments required (43%) and effectiveness (16%).
Three years ago, Dr. Stevens used the HintMD platform to implement a treatment plan subscription service to 472 active members of his practice. Prior to implementation, patients were coming in for treatment with toxins an average of 1.8 times per year. After implementation, that rose to an average of 3.1 times per year. “That was almost an $800 incremental average increase spent on toxins alone,” Dr. Stevens said. “More importantly, the patients were therapeutic all year long.” With toxin and filler services combined, the average increased income grew to more than $1,100 per patient, which translated into increased annual revenue of $519,200.
Dr. Stevens said that many of his patients favor subscription services because most use them in other aspects of their lives, such as with Amazon Prime, Blue Apron, and Netflix. “They like it because it is personalized and customized,” he said. “If we want to adjust the amount of toxin or filler, we can do it that very day, and it’s customized for them. It’s not a one-size-fits-all program. It also allows them to have convenient, smaller monthly payments. That’s the key. That way, they budget. So, if they’re spending $200 a month or $500 a month or $1,000 a month, it’s a convenient monthly payment.”
Dr. Stevens disclosed that he is an adviser to Viveve, Venus, Aesthetics Biomedical, Alastin, Cypris Medical, Allergan, CoolSculpting, HydraFacial, Revance, Ampersand, and HintMD.
According to W. Grant Stevens, MD, an estimated 73% of aesthetic patients fall short when it comes to compliance with recommended treatment intervals for toxins, fillers, and other procedures.
“When we talk about how often the average patient should be treated with Botox, for instance, we say every 3-4 months,” Dr. Stevens, founder and CEO of Marina Plastic Surgery in Marina Del Rey, Calif., said during the virtual annual Masters of Aesthetics Symposium. But in reality, he added, “it’s more like every 7 months.” A 2015 survey of 23 Bay Area aesthetic practices conducted by HintMD found that 73% of patients were noncompliant and that they came in fewer than 3-4 times per year for treatments. “Not only did they come in infrequently, but they oftentimes were undercorrected and the revenue was being left on the table because of discounting and undercorrection,” said Dr. Stevens, who is also a professor of surgery in the division of plastic surgery at the University of Southern California, Los Angeles.
On average, each patient from the 23 practices surveyed spent $601.88 on treatments 1.44 times per year, yet the industry standard for neuromodulators is 3-4 times per year and every 2 months for HydraFacials and med spa facials. “What’s the problem?” he asked “Why are we falling off? For our practices, noncompliance leads to unhappy, undertreated patients, so they may write negative reviews. In addition to that, we lose revenue.” He cited results from a 2016 focus group of aesthetic patients who were asked about the perceived barriers to treatment compliance. More than two-thirds (68%) said cost was the issue, followed by the number of treatments required (43%) and effectiveness (16%).
Three years ago, Dr. Stevens used the HintMD platform to implement a treatment plan subscription service to 472 active members of his practice. Prior to implementation, patients were coming in for treatment with toxins an average of 1.8 times per year. After implementation, that rose to an average of 3.1 times per year. “That was almost an $800 incremental average increase spent on toxins alone,” Dr. Stevens said. “More importantly, the patients were therapeutic all year long.” With toxin and filler services combined, the average increased income grew to more than $1,100 per patient, which translated into increased annual revenue of $519,200.
Dr. Stevens said that many of his patients favor subscription services because most use them in other aspects of their lives, such as with Amazon Prime, Blue Apron, and Netflix. “They like it because it is personalized and customized,” he said. “If we want to adjust the amount of toxin or filler, we can do it that very day, and it’s customized for them. It’s not a one-size-fits-all program. It also allows them to have convenient, smaller monthly payments. That’s the key. That way, they budget. So, if they’re spending $200 a month or $500 a month or $1,000 a month, it’s a convenient monthly payment.”
Dr. Stevens disclosed that he is an adviser to Viveve, Venus, Aesthetics Biomedical, Alastin, Cypris Medical, Allergan, CoolSculpting, HydraFacial, Revance, Ampersand, and HintMD.
FROM MOA 2020
HHS extends deadline for patient access to your clinical notes
The Department of Health & Human Services on Oct. 29 extended the deadline for health care groups to provide patients with immediate electronic access to their doctors’ clinical notes as well as test results and reports from pathology and imaging.
The mandate, called “open notes” by many, is part of the 21st Century Cures Act, and will now go into effect April 5.
The announcement comes just 4 days before the previously established Nov. 2 deadline and gives the pandemic as the reason for the delay.
“We are hearing that, while there is strong support for advancing patient access … stakeholders also must manage the needs being experienced during the current pandemic,” Don Rucker, MD, national coordinator for health information technology at HHS, said in a press statement.
“To be clear, the Office of the National Coordinator is not removing the requirements advancing patient access to their health information,” he added.
‘What you make of it’
Scott MacDonald, MD, electronic health record medical director at the University of California, Davis, said his organization is proceeding anyway. “UC Davis is going to start releasing notes and test results on Nov. 12,” he said in an interview.
Other organizations and practices now have more time, he said, but the law stays the same. “There’s no change to the what or why – only to the when,” Dr. MacDonald pointed out.
Vanderbilt University Medical Center in Nashville, Tenn., will take advantage of the extra time, Trent Rosenbloom, MD, MPH, director of patient portals, said in an interview.
“Given the super-short time frame we had to work under as this emerged out from dealing with COVID, we feel that we have not addressed all the potential legal-edge cases such as dealing with adolescent medicine and child abuse,” he said.
On Oct. 21, this news organization reported on the then-imminent start of the new law, which irked many readers. They cited, among other things, the likelihood of patient confusion with fast patient access to all clinical notes.
“To me, the biggest issue is that we speak a foreign language that most outside of medicine don’t speak. Our job is to explain it to the patient at a level they can understand. What will 100% happen now is that a patient will not be able to reconcile what is in the note to what they’ve been told,” Andrew White, MD, wrote in a reader comment.
But benefits of open notes outweigh the risks, say proponents, who claim that doctor-patient communication and trust actually improve with information access and that research indicates other benefits such as improved medication adherence.
Open notes are “what you make of it,” said Marlene Millen, MD, an internist at UC San Diego Health, which has had a pilot open-notes program for 3 years.
“I actually end all of my appointments with: ‘Don’t forget to read your note later,’ ” she said in an interview.
Dr. Millen feared open notes initially but, within the first 3 months of usage, about 15 patients gave her direct feedback on how much they appreciated her notes. “It seemed to really reassure them that they were getting good care.”
Dr. MacDonald and Dr. Millen disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The Department of Health & Human Services on Oct. 29 extended the deadline for health care groups to provide patients with immediate electronic access to their doctors’ clinical notes as well as test results and reports from pathology and imaging.
The mandate, called “open notes” by many, is part of the 21st Century Cures Act, and will now go into effect April 5.
The announcement comes just 4 days before the previously established Nov. 2 deadline and gives the pandemic as the reason for the delay.
“We are hearing that, while there is strong support for advancing patient access … stakeholders also must manage the needs being experienced during the current pandemic,” Don Rucker, MD, national coordinator for health information technology at HHS, said in a press statement.
“To be clear, the Office of the National Coordinator is not removing the requirements advancing patient access to their health information,” he added.
‘What you make of it’
Scott MacDonald, MD, electronic health record medical director at the University of California, Davis, said his organization is proceeding anyway. “UC Davis is going to start releasing notes and test results on Nov. 12,” he said in an interview.
Other organizations and practices now have more time, he said, but the law stays the same. “There’s no change to the what or why – only to the when,” Dr. MacDonald pointed out.
Vanderbilt University Medical Center in Nashville, Tenn., will take advantage of the extra time, Trent Rosenbloom, MD, MPH, director of patient portals, said in an interview.
“Given the super-short time frame we had to work under as this emerged out from dealing with COVID, we feel that we have not addressed all the potential legal-edge cases such as dealing with adolescent medicine and child abuse,” he said.
On Oct. 21, this news organization reported on the then-imminent start of the new law, which irked many readers. They cited, among other things, the likelihood of patient confusion with fast patient access to all clinical notes.
“To me, the biggest issue is that we speak a foreign language that most outside of medicine don’t speak. Our job is to explain it to the patient at a level they can understand. What will 100% happen now is that a patient will not be able to reconcile what is in the note to what they’ve been told,” Andrew White, MD, wrote in a reader comment.
But benefits of open notes outweigh the risks, say proponents, who claim that doctor-patient communication and trust actually improve with information access and that research indicates other benefits such as improved medication adherence.
Open notes are “what you make of it,” said Marlene Millen, MD, an internist at UC San Diego Health, which has had a pilot open-notes program for 3 years.
“I actually end all of my appointments with: ‘Don’t forget to read your note later,’ ” she said in an interview.
Dr. Millen feared open notes initially but, within the first 3 months of usage, about 15 patients gave her direct feedback on how much they appreciated her notes. “It seemed to really reassure them that they were getting good care.”
Dr. MacDonald and Dr. Millen disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The Department of Health & Human Services on Oct. 29 extended the deadline for health care groups to provide patients with immediate electronic access to their doctors’ clinical notes as well as test results and reports from pathology and imaging.
The mandate, called “open notes” by many, is part of the 21st Century Cures Act, and will now go into effect April 5.
The announcement comes just 4 days before the previously established Nov. 2 deadline and gives the pandemic as the reason for the delay.
“We are hearing that, while there is strong support for advancing patient access … stakeholders also must manage the needs being experienced during the current pandemic,” Don Rucker, MD, national coordinator for health information technology at HHS, said in a press statement.
“To be clear, the Office of the National Coordinator is not removing the requirements advancing patient access to their health information,” he added.
‘What you make of it’
Scott MacDonald, MD, electronic health record medical director at the University of California, Davis, said his organization is proceeding anyway. “UC Davis is going to start releasing notes and test results on Nov. 12,” he said in an interview.
Other organizations and practices now have more time, he said, but the law stays the same. “There’s no change to the what or why – only to the when,” Dr. MacDonald pointed out.
Vanderbilt University Medical Center in Nashville, Tenn., will take advantage of the extra time, Trent Rosenbloom, MD, MPH, director of patient portals, said in an interview.
“Given the super-short time frame we had to work under as this emerged out from dealing with COVID, we feel that we have not addressed all the potential legal-edge cases such as dealing with adolescent medicine and child abuse,” he said.
On Oct. 21, this news organization reported on the then-imminent start of the new law, which irked many readers. They cited, among other things, the likelihood of patient confusion with fast patient access to all clinical notes.
“To me, the biggest issue is that we speak a foreign language that most outside of medicine don’t speak. Our job is to explain it to the patient at a level they can understand. What will 100% happen now is that a patient will not be able to reconcile what is in the note to what they’ve been told,” Andrew White, MD, wrote in a reader comment.
But benefits of open notes outweigh the risks, say proponents, who claim that doctor-patient communication and trust actually improve with information access and that research indicates other benefits such as improved medication adherence.
Open notes are “what you make of it,” said Marlene Millen, MD, an internist at UC San Diego Health, which has had a pilot open-notes program for 3 years.
“I actually end all of my appointments with: ‘Don’t forget to read your note later,’ ” she said in an interview.
Dr. Millen feared open notes initially but, within the first 3 months of usage, about 15 patients gave her direct feedback on how much they appreciated her notes. “It seemed to really reassure them that they were getting good care.”
Dr. MacDonald and Dr. Millen disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
HIV drugs prevent type 2 diabetes, may be path to new therapy
A class of drugs long used to treat HIV and hepatitis B viral infections appears to prevent the development of diabetes in a substantial proportion of patients who take these agents, an analysis of multiple databases has shown.
“Nucleoside reverse transcriptase inhibitors [NRTIs], drugs approved to treat HIV-1 and hepatitis B infections, also block inflammasome activation,” Jayakrishna Ambati, MD, University of Virginia, Charlottesville, and colleagues wrote in Nature Communications.
“[We showed that] the adjusted risk of incident diabetes is 33% lower in patients with NRTI exposure. ... These data suggest the possibility of repurposing an approved class of drugs for prevention of diabetes,” they wrote.
The researchers made a small chemical modification to NRTIs that led to their developing a new class of drugs, which they have termed “kamuvudines.” Kamuvudines are nontoxic derivatives of NRTIs, Dr. Ambati said in an interview.
“People take NRTIs because they need to live with HIV, but giving them to the general population is not a great idea because of the toxicities associated with long-term NRTI use. So our focus is not to go forward specifically with NRTIs but rather with these new molecules that are far less toxic, and that is how we envision a clinical trial going forward,” Dr. Ambati noted.
Researchers screened five databases of >100,000 patients
Dr. Ambati and colleagues analyzed information from five databases in which patients who had been exposed to an NRTI but who had not previously been diagnosed with type 2 diabetes were assessed for the subsequent development of diabetes over varying time intervals. In one, the Veterans Health Administration database – from the largest integrated health care system in the United States – the analysis spanned a period of 17 years.
Of 79,744 patients with a confirmed diagnosis of HIV or hepatitis B in the Veterans Health Administration database, the risk for type 2 diabetes was reduced by 34% among NRTI users, compared with nonusers after adjusting for potential confounders (P < .0001).
The reduction in diabetes risk was similar among HIV-positive and hepatitis B–positive patients.
These results were reaffirmed by further analyses of four other databases, the investigators reported. One of these, the employer-based health insurance Truven database, had data on 23,634 patients who had been diagnosed with HIV or hepatitis B. After adjusting for potential confounders, NRTI users had a 39% lower risk of developing type 2 diabetes, compared with nonusers (P < .0001).
The risk of developing type 2 diabetes was somewhat lower among NRTI users in the Pearl Diver database, which includes predominantly private health insurance claims. Of 16,045 patients diagnosed with HIV or hepatitis B included in this database, the risk for type 2 diabetes was 26% lower among NRTI users, compared with nonusers (P = .004).
A similar magnitude of risk reduction was seen in the analysis of the Clinformatics dataset. Among 6,341 users of NRTIs, the risk for type 2 diabetes was 27% lower than it was for nonusers (P = .009).
The least reduction in diabetes risk was in the Medicare database, in which only 3,097 patients had been diagnosed with either HIV or hepatitis B. Among these patients, the risk for diabetes was 17% lower among NRTI users than it was for nonusers (P = .137).
One-third reduction across multiple databases enhances confidence
“Collectively, among 128,861 patients with HIV-1 or hepatitis B, users of NRTIs had a 33% reduced hazard of developing type 2 diabetes,” Dr. Ambati and colleagues emphasize.
“The fact that the protective effect against the development of diabetes was replicated in multiple databases in studies from multiple institutions enhances confidence in the results,” Dr. Ambati noted in a statement from the University of Virginia.
Dr. Ambati and colleagues also showed that the NRTI lamivudine restores insulin sensitivity in human cells from type 2 diabetes patients.
That drug prevented induction of insulin resistance in human cells from people who did not have diabetes. It also prevented inflammasome activation in mice fed a high-fat diet.
“These investigations of human cell, mouse and population database systems collectively suggest a potential beneficial effect of NRTIs in forestalling diabetes onset,” they stressed.
Trial assessing kamuvudines slated to begin next year
In the interview, Dr. Ambati explained that inflammasomes are protein complexes that form a large superstructure within the cell. “When activated, they lead to the production of some very powerful inflammatory cytokines, including interleukin-1 beta and IL-18.”
Although there are many different types of inflammasomes, the one implicated in type 2 diabetes, as well as many other chronic diseases, including macular degeneration, is the NLRP3 inflammasome.
Activation of this molecule promotes insulin resistance, a key driver of type 2 diabetes, he explained.
Importantly, previous research showed that the way the NRTIs block this inflammasome has nothing to do with their anti-HIV activity.
After making a small chemical modification in the NRTIs, Dr. Ambati and colleagues were able to show that the resulting agents, which they have dubbed “kamuvudines,” are able to block inflammasome activation independently of their antiviral effects.
They hope that this modification will reduce the toxicities associated with the agents. This would be necessary if kamuvudines were to be more widely used in a noninfected, healthier population, Ambati stressed.
Dr. Ambati and his colleague, Paul Ashton, PhD, cofounder of Inflammasone Therapeutics, plan a clinical trial with one of these kamuvudines in macular degeneration, which they hope will begin early next year.
“We are trying to pick a disease where we can show efficacy fairly quickly in a small number of people,” Dr. Ashton explained in an interview. “We’re very enthusiastic about this as it looks really, really promising.”
Dr. Ambati and Dr. Ashton cofounded Inflammasone Therapeutics, located in Boston. Dr. Ashton is the CEO of the company.
A version of this article originally appeared on Medscape.com.
A class of drugs long used to treat HIV and hepatitis B viral infections appears to prevent the development of diabetes in a substantial proportion of patients who take these agents, an analysis of multiple databases has shown.
“Nucleoside reverse transcriptase inhibitors [NRTIs], drugs approved to treat HIV-1 and hepatitis B infections, also block inflammasome activation,” Jayakrishna Ambati, MD, University of Virginia, Charlottesville, and colleagues wrote in Nature Communications.
“[We showed that] the adjusted risk of incident diabetes is 33% lower in patients with NRTI exposure. ... These data suggest the possibility of repurposing an approved class of drugs for prevention of diabetes,” they wrote.
The researchers made a small chemical modification to NRTIs that led to their developing a new class of drugs, which they have termed “kamuvudines.” Kamuvudines are nontoxic derivatives of NRTIs, Dr. Ambati said in an interview.
“People take NRTIs because they need to live with HIV, but giving them to the general population is not a great idea because of the toxicities associated with long-term NRTI use. So our focus is not to go forward specifically with NRTIs but rather with these new molecules that are far less toxic, and that is how we envision a clinical trial going forward,” Dr. Ambati noted.
Researchers screened five databases of >100,000 patients
Dr. Ambati and colleagues analyzed information from five databases in which patients who had been exposed to an NRTI but who had not previously been diagnosed with type 2 diabetes were assessed for the subsequent development of diabetes over varying time intervals. In one, the Veterans Health Administration database – from the largest integrated health care system in the United States – the analysis spanned a period of 17 years.
Of 79,744 patients with a confirmed diagnosis of HIV or hepatitis B in the Veterans Health Administration database, the risk for type 2 diabetes was reduced by 34% among NRTI users, compared with nonusers after adjusting for potential confounders (P < .0001).
The reduction in diabetes risk was similar among HIV-positive and hepatitis B–positive patients.
These results were reaffirmed by further analyses of four other databases, the investigators reported. One of these, the employer-based health insurance Truven database, had data on 23,634 patients who had been diagnosed with HIV or hepatitis B. After adjusting for potential confounders, NRTI users had a 39% lower risk of developing type 2 diabetes, compared with nonusers (P < .0001).
The risk of developing type 2 diabetes was somewhat lower among NRTI users in the Pearl Diver database, which includes predominantly private health insurance claims. Of 16,045 patients diagnosed with HIV or hepatitis B included in this database, the risk for type 2 diabetes was 26% lower among NRTI users, compared with nonusers (P = .004).
A similar magnitude of risk reduction was seen in the analysis of the Clinformatics dataset. Among 6,341 users of NRTIs, the risk for type 2 diabetes was 27% lower than it was for nonusers (P = .009).
The least reduction in diabetes risk was in the Medicare database, in which only 3,097 patients had been diagnosed with either HIV or hepatitis B. Among these patients, the risk for diabetes was 17% lower among NRTI users than it was for nonusers (P = .137).
One-third reduction across multiple databases enhances confidence
“Collectively, among 128,861 patients with HIV-1 or hepatitis B, users of NRTIs had a 33% reduced hazard of developing type 2 diabetes,” Dr. Ambati and colleagues emphasize.
“The fact that the protective effect against the development of diabetes was replicated in multiple databases in studies from multiple institutions enhances confidence in the results,” Dr. Ambati noted in a statement from the University of Virginia.
Dr. Ambati and colleagues also showed that the NRTI lamivudine restores insulin sensitivity in human cells from type 2 diabetes patients.
That drug prevented induction of insulin resistance in human cells from people who did not have diabetes. It also prevented inflammasome activation in mice fed a high-fat diet.
“These investigations of human cell, mouse and population database systems collectively suggest a potential beneficial effect of NRTIs in forestalling diabetes onset,” they stressed.
Trial assessing kamuvudines slated to begin next year
In the interview, Dr. Ambati explained that inflammasomes are protein complexes that form a large superstructure within the cell. “When activated, they lead to the production of some very powerful inflammatory cytokines, including interleukin-1 beta and IL-18.”
Although there are many different types of inflammasomes, the one implicated in type 2 diabetes, as well as many other chronic diseases, including macular degeneration, is the NLRP3 inflammasome.
Activation of this molecule promotes insulin resistance, a key driver of type 2 diabetes, he explained.
Importantly, previous research showed that the way the NRTIs block this inflammasome has nothing to do with their anti-HIV activity.
After making a small chemical modification in the NRTIs, Dr. Ambati and colleagues were able to show that the resulting agents, which they have dubbed “kamuvudines,” are able to block inflammasome activation independently of their antiviral effects.
They hope that this modification will reduce the toxicities associated with the agents. This would be necessary if kamuvudines were to be more widely used in a noninfected, healthier population, Ambati stressed.
Dr. Ambati and his colleague, Paul Ashton, PhD, cofounder of Inflammasone Therapeutics, plan a clinical trial with one of these kamuvudines in macular degeneration, which they hope will begin early next year.
“We are trying to pick a disease where we can show efficacy fairly quickly in a small number of people,” Dr. Ashton explained in an interview. “We’re very enthusiastic about this as it looks really, really promising.”
Dr. Ambati and Dr. Ashton cofounded Inflammasone Therapeutics, located in Boston. Dr. Ashton is the CEO of the company.
A version of this article originally appeared on Medscape.com.
A class of drugs long used to treat HIV and hepatitis B viral infections appears to prevent the development of diabetes in a substantial proportion of patients who take these agents, an analysis of multiple databases has shown.
“Nucleoside reverse transcriptase inhibitors [NRTIs], drugs approved to treat HIV-1 and hepatitis B infections, also block inflammasome activation,” Jayakrishna Ambati, MD, University of Virginia, Charlottesville, and colleagues wrote in Nature Communications.
“[We showed that] the adjusted risk of incident diabetes is 33% lower in patients with NRTI exposure. ... These data suggest the possibility of repurposing an approved class of drugs for prevention of diabetes,” they wrote.
The researchers made a small chemical modification to NRTIs that led to their developing a new class of drugs, which they have termed “kamuvudines.” Kamuvudines are nontoxic derivatives of NRTIs, Dr. Ambati said in an interview.
“People take NRTIs because they need to live with HIV, but giving them to the general population is not a great idea because of the toxicities associated with long-term NRTI use. So our focus is not to go forward specifically with NRTIs but rather with these new molecules that are far less toxic, and that is how we envision a clinical trial going forward,” Dr. Ambati noted.
Researchers screened five databases of >100,000 patients
Dr. Ambati and colleagues analyzed information from five databases in which patients who had been exposed to an NRTI but who had not previously been diagnosed with type 2 diabetes were assessed for the subsequent development of diabetes over varying time intervals. In one, the Veterans Health Administration database – from the largest integrated health care system in the United States – the analysis spanned a period of 17 years.
Of 79,744 patients with a confirmed diagnosis of HIV or hepatitis B in the Veterans Health Administration database, the risk for type 2 diabetes was reduced by 34% among NRTI users, compared with nonusers after adjusting for potential confounders (P < .0001).
The reduction in diabetes risk was similar among HIV-positive and hepatitis B–positive patients.
These results were reaffirmed by further analyses of four other databases, the investigators reported. One of these, the employer-based health insurance Truven database, had data on 23,634 patients who had been diagnosed with HIV or hepatitis B. After adjusting for potential confounders, NRTI users had a 39% lower risk of developing type 2 diabetes, compared with nonusers (P < .0001).
The risk of developing type 2 diabetes was somewhat lower among NRTI users in the Pearl Diver database, which includes predominantly private health insurance claims. Of 16,045 patients diagnosed with HIV or hepatitis B included in this database, the risk for type 2 diabetes was 26% lower among NRTI users, compared with nonusers (P = .004).
A similar magnitude of risk reduction was seen in the analysis of the Clinformatics dataset. Among 6,341 users of NRTIs, the risk for type 2 diabetes was 27% lower than it was for nonusers (P = .009).
The least reduction in diabetes risk was in the Medicare database, in which only 3,097 patients had been diagnosed with either HIV or hepatitis B. Among these patients, the risk for diabetes was 17% lower among NRTI users than it was for nonusers (P = .137).
One-third reduction across multiple databases enhances confidence
“Collectively, among 128,861 patients with HIV-1 or hepatitis B, users of NRTIs had a 33% reduced hazard of developing type 2 diabetes,” Dr. Ambati and colleagues emphasize.
“The fact that the protective effect against the development of diabetes was replicated in multiple databases in studies from multiple institutions enhances confidence in the results,” Dr. Ambati noted in a statement from the University of Virginia.
Dr. Ambati and colleagues also showed that the NRTI lamivudine restores insulin sensitivity in human cells from type 2 diabetes patients.
That drug prevented induction of insulin resistance in human cells from people who did not have diabetes. It also prevented inflammasome activation in mice fed a high-fat diet.
“These investigations of human cell, mouse and population database systems collectively suggest a potential beneficial effect of NRTIs in forestalling diabetes onset,” they stressed.
Trial assessing kamuvudines slated to begin next year
In the interview, Dr. Ambati explained that inflammasomes are protein complexes that form a large superstructure within the cell. “When activated, they lead to the production of some very powerful inflammatory cytokines, including interleukin-1 beta and IL-18.”
Although there are many different types of inflammasomes, the one implicated in type 2 diabetes, as well as many other chronic diseases, including macular degeneration, is the NLRP3 inflammasome.
Activation of this molecule promotes insulin resistance, a key driver of type 2 diabetes, he explained.
Importantly, previous research showed that the way the NRTIs block this inflammasome has nothing to do with their anti-HIV activity.
After making a small chemical modification in the NRTIs, Dr. Ambati and colleagues were able to show that the resulting agents, which they have dubbed “kamuvudines,” are able to block inflammasome activation independently of their antiviral effects.
They hope that this modification will reduce the toxicities associated with the agents. This would be necessary if kamuvudines were to be more widely used in a noninfected, healthier population, Ambati stressed.
Dr. Ambati and his colleague, Paul Ashton, PhD, cofounder of Inflammasone Therapeutics, plan a clinical trial with one of these kamuvudines in macular degeneration, which they hope will begin early next year.
“We are trying to pick a disease where we can show efficacy fairly quickly in a small number of people,” Dr. Ashton explained in an interview. “We’re very enthusiastic about this as it looks really, really promising.”
Dr. Ambati and Dr. Ashton cofounded Inflammasone Therapeutics, located in Boston. Dr. Ashton is the CEO of the company.
A version of this article originally appeared on Medscape.com.
‘Landmark’ study pushed detection of covert consciousness in TBI
Compelling advances in the ability to detect signs of consciousness in unconscious patients who have experienced traumatic brain injury (TBI) are leading to unprecedented changes in the field. There is now hope of improving outcomes and even sparing lives of patients who may otherwise have been mistakenly assessed as having no chance of recovery.
That research, published in the New England Journal of Medicine in June 2019, linked the promising signals of consciousness in comatose patients, detected only on imaging, with remarkable outcomes a year later.
“This was a landmark study,” said Brian L. Edlow, MD, in a presentation on the issue of covert consciousness at the virtual annual meeting of the American Neurological Association.
“Importantly, it is the first compelling evidence that early detection of covert consciousness also predicts 1-year outcomes in the Glasgow Outcome Scale Extended (GOSE), showing that covert consciousness in the ICU appears to be relevant for predicting long-term outcomes,” said Dr. Edlow, who is associate director of the Center for Neurotechnology and Neurorecovery, Massachusetts General Hospital, in Boston.
The researchers showed that 15% of unconscious patients with acute brain injury in the study exhibited significant brain activity on EEG in response to stimuli that included verbal commands such as envisioning that they are playing tennis.
Although other studies have shown similar effects with task-based stimuli, the New England Journal of Medicine study further showed that a year later, the patients who had shown signs of covert consciousness, also called “cognitive motor dissociation” (CMD), were significantly more likely to have a good functional outcome, said the study’s senior author, Jan Claassen, MD, director of critical care neurology at Columbia University, New York, who also presented at the ANA session.
“Importantly, a year later after injury, we found that 44% of patients with CMD and only 14% of non-CMD patients had a good functional outcome, defined as a GOSE score indicating a state where they can at least take care of themselves for 8 hours in a day,” he said.
“[Whether] these patients in a CMD state represent a parallel state or a transitory state on the road to recovery remains to be shown,” he said.
Jennifer Frontera, MD, a professor in the department of neurology at NYU Langone Health in New York and comoderator of the session, agreed that the research is “remarkable.”
“Also,” she said, “it is practical, since many could potentially apply and validate his algorithms, since EEG technology is portable and widely available.”
Research has ushered in a ‘sea change’ in neurocritical care
The research has helped push forward recommendations on the treatment of unconscious patients, Dr. Edlow said. “This has led to a sea change in our field just over the last 2 years, with multiple guidelines published suggesting that it may be time for us to consider incorporating task-based fMRI and EEG techniques into our clinical assessment of patients with disorders of consciousness,” Dr. Edlow said.
Among those updating their recommendations was the American Academy of Neurology, which revised guidelines on practice parameters for patients in a persistent vegetative state. Those guidelines had not been updated since 1995.
Although concluding that “no diagnostic assessment procedure had moderate or strong evidence for use,” the guidelines acknowledge that “it is possible that a positive electromyographic (EMG) response to command, EEG reactivity to sensory stimuli, laser-evoked potentials, and the Perturbational Complexity Index can distinguish a minimally conscious state from vegetative state/unresponsive wakefulness syndrome (VS/UWS).”
Earlier this year, the European Academy of Neurology followed suit with updated guidelines of its own. In the EAN guideline, the academy’s Panel on Coma, Disorders of Consciousness recommends that task-based fMRI, EEG, and other advanced assessments be performed as part of a composite assessment of consciousness and that a patient’s best performance or highest level of consciousness on any of those tests should be a reflection of their diagnosis, Dr. Edlow explained.
“What this means is that our field is moving toward a multimodal assessment of consciousness in the ICU as well as beyond, in the subacute to chronic setting, whereby the behavioral exam, advanced DG, and advanced MRI methods all also contribute to the diagnosis of consciousness,” he said.
The standard for assessment of disorders of consciousness is the Coma Recovery Scale–Revised, with a 25-item scale for diagnosis, prediction of outcome, and assessment of potential treatment efficacy.
But much uncertainty can remain despite the assessment, Dr. Claassen said. “Behavioral assessments of patients with acute brain injury are challenging because examinations fluctuate, and there’s variability between assessors,” he said. “Nevertheless, patients and their families demand guidance from us.”
Dr. Edlow pointed out that the largest study to date of the causes of death among patients with TBI in the ICU underscores the need for better assessments.
The study of more than 600 patients at six level l trauma centers in Canada showed that 70% of patients who died in the ICU from TBI did so as the result of the withdrawal of life-sustaining therapy. However, only about a half (57%) had an unreactive pupil, and only about a quarter (23.7%) had evidence of herniation on CT, findings that are commonly associated with a poor prognosis.
“What emerges from this is that the manner in which the clinicians communicated the prognosis to families was a primary determinant of decisions to withdraw life-sustaining therapy,” Dr. Edlow said.
Negative response not necessarily conclusive
Dr. Edlow added a word of caution that the science is still far from perfect. He noted that, for 25% of healthy patients who are given a motor imagery task, neuroimaging might not show a response, implying that the lack of a signal may not be conclusive.
He described the case of a patient who was comatose at the time she was scanned on day 3 after injury and who showed no responses to language, music, or motor imagery during the MRI, yet a year later, she was functionally independent, back in the workforce, and had very few residual symptoms from her trauma.
“So if a patient does not show a response, that does not prove the patient is not conscious, and it does not prove that the patient is likely to have a poor outcome,” Dr. Edlow said. Such cases underscore the need for more advances in understanding the inner workings of brain injury.
Dr. Edlow and his colleagues are embarking on a trial of the effects of intravenous methylphenidate in targeting the stimulation of dopaminergic circuits within the subcortical ascending arousal network in patients with severe brain injuries.
“The scientific premise of the trial is that personalized brain network mapping in the ICU can identify patients whose connectomes are amenable to neuromodulation,” Dr. Edlow and his colleague report in an article in Neurocritical Care.
The trial, called STIMPACT (Stimulant Therapy Targeted to Individualized Connectivity Maps to Promote ReACTivation of Consciousness), is part of the newly launched Connectome-based Clinical Trial Platform, which the authors describe as “a new paradigm for developing and testing targeted therapies that promote early recovery of consciousness in the ICU.”
Such efforts are essential, given the high stakes of TBI outcomes, Dr. Edlow said.
“Let’s be clear about the stakes of an incorrect prognosis,” he said. “If we’re overly pessimistic, then a patient who could have potential for meaningful recovery will likely die in our ICU. On the other hand, if we are overly optimistic, then a patient could end up in a vegetative or minimally conscious state that he or she may never have found to be acceptable,” he said.
Access to technologies a ‘civil right?’
Some ethicists in the field are recommending that patients be given access to the advanced techniques as a civil right, similar to the rights described in the Convention on the Rights of Persons With Disabilities, which was adopted by the United Nations in 2008, Dr. Edlow noted.
“So the question that we as clinicians are going to face moving forward from an ethical standpoint is, if we have access to these techniques, is it an ethical obligation to offer them now?” he said.
Dr. Edlow underscored the need to consider the reality that “there are profound issues relating to resource allocation and access to these advanced techniques, but we’re going to have to consider this together as we move forward.”
Dr. Edlow has received funding from the National Institutes of Health. Dr. Claassen is a minority shareholder with ICE Neurosystems. Dr. Frontera has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Compelling advances in the ability to detect signs of consciousness in unconscious patients who have experienced traumatic brain injury (TBI) are leading to unprecedented changes in the field. There is now hope of improving outcomes and even sparing lives of patients who may otherwise have been mistakenly assessed as having no chance of recovery.
That research, published in the New England Journal of Medicine in June 2019, linked the promising signals of consciousness in comatose patients, detected only on imaging, with remarkable outcomes a year later.
“This was a landmark study,” said Brian L. Edlow, MD, in a presentation on the issue of covert consciousness at the virtual annual meeting of the American Neurological Association.
“Importantly, it is the first compelling evidence that early detection of covert consciousness also predicts 1-year outcomes in the Glasgow Outcome Scale Extended (GOSE), showing that covert consciousness in the ICU appears to be relevant for predicting long-term outcomes,” said Dr. Edlow, who is associate director of the Center for Neurotechnology and Neurorecovery, Massachusetts General Hospital, in Boston.
The researchers showed that 15% of unconscious patients with acute brain injury in the study exhibited significant brain activity on EEG in response to stimuli that included verbal commands such as envisioning that they are playing tennis.
Although other studies have shown similar effects with task-based stimuli, the New England Journal of Medicine study further showed that a year later, the patients who had shown signs of covert consciousness, also called “cognitive motor dissociation” (CMD), were significantly more likely to have a good functional outcome, said the study’s senior author, Jan Claassen, MD, director of critical care neurology at Columbia University, New York, who also presented at the ANA session.
“Importantly, a year later after injury, we found that 44% of patients with CMD and only 14% of non-CMD patients had a good functional outcome, defined as a GOSE score indicating a state where they can at least take care of themselves for 8 hours in a day,” he said.
“[Whether] these patients in a CMD state represent a parallel state or a transitory state on the road to recovery remains to be shown,” he said.
Jennifer Frontera, MD, a professor in the department of neurology at NYU Langone Health in New York and comoderator of the session, agreed that the research is “remarkable.”
“Also,” she said, “it is practical, since many could potentially apply and validate his algorithms, since EEG technology is portable and widely available.”
Research has ushered in a ‘sea change’ in neurocritical care
The research has helped push forward recommendations on the treatment of unconscious patients, Dr. Edlow said. “This has led to a sea change in our field just over the last 2 years, with multiple guidelines published suggesting that it may be time for us to consider incorporating task-based fMRI and EEG techniques into our clinical assessment of patients with disorders of consciousness,” Dr. Edlow said.
Among those updating their recommendations was the American Academy of Neurology, which revised guidelines on practice parameters for patients in a persistent vegetative state. Those guidelines had not been updated since 1995.
Although concluding that “no diagnostic assessment procedure had moderate or strong evidence for use,” the guidelines acknowledge that “it is possible that a positive electromyographic (EMG) response to command, EEG reactivity to sensory stimuli, laser-evoked potentials, and the Perturbational Complexity Index can distinguish a minimally conscious state from vegetative state/unresponsive wakefulness syndrome (VS/UWS).”
Earlier this year, the European Academy of Neurology followed suit with updated guidelines of its own. In the EAN guideline, the academy’s Panel on Coma, Disorders of Consciousness recommends that task-based fMRI, EEG, and other advanced assessments be performed as part of a composite assessment of consciousness and that a patient’s best performance or highest level of consciousness on any of those tests should be a reflection of their diagnosis, Dr. Edlow explained.
“What this means is that our field is moving toward a multimodal assessment of consciousness in the ICU as well as beyond, in the subacute to chronic setting, whereby the behavioral exam, advanced DG, and advanced MRI methods all also contribute to the diagnosis of consciousness,” he said.
The standard for assessment of disorders of consciousness is the Coma Recovery Scale–Revised, with a 25-item scale for diagnosis, prediction of outcome, and assessment of potential treatment efficacy.
But much uncertainty can remain despite the assessment, Dr. Claassen said. “Behavioral assessments of patients with acute brain injury are challenging because examinations fluctuate, and there’s variability between assessors,” he said. “Nevertheless, patients and their families demand guidance from us.”
Dr. Edlow pointed out that the largest study to date of the causes of death among patients with TBI in the ICU underscores the need for better assessments.
The study of more than 600 patients at six level l trauma centers in Canada showed that 70% of patients who died in the ICU from TBI did so as the result of the withdrawal of life-sustaining therapy. However, only about a half (57%) had an unreactive pupil, and only about a quarter (23.7%) had evidence of herniation on CT, findings that are commonly associated with a poor prognosis.
“What emerges from this is that the manner in which the clinicians communicated the prognosis to families was a primary determinant of decisions to withdraw life-sustaining therapy,” Dr. Edlow said.
Negative response not necessarily conclusive
Dr. Edlow added a word of caution that the science is still far from perfect. He noted that, for 25% of healthy patients who are given a motor imagery task, neuroimaging might not show a response, implying that the lack of a signal may not be conclusive.
He described the case of a patient who was comatose at the time she was scanned on day 3 after injury and who showed no responses to language, music, or motor imagery during the MRI, yet a year later, she was functionally independent, back in the workforce, and had very few residual symptoms from her trauma.
“So if a patient does not show a response, that does not prove the patient is not conscious, and it does not prove that the patient is likely to have a poor outcome,” Dr. Edlow said. Such cases underscore the need for more advances in understanding the inner workings of brain injury.
Dr. Edlow and his colleagues are embarking on a trial of the effects of intravenous methylphenidate in targeting the stimulation of dopaminergic circuits within the subcortical ascending arousal network in patients with severe brain injuries.
“The scientific premise of the trial is that personalized brain network mapping in the ICU can identify patients whose connectomes are amenable to neuromodulation,” Dr. Edlow and his colleague report in an article in Neurocritical Care.
The trial, called STIMPACT (Stimulant Therapy Targeted to Individualized Connectivity Maps to Promote ReACTivation of Consciousness), is part of the newly launched Connectome-based Clinical Trial Platform, which the authors describe as “a new paradigm for developing and testing targeted therapies that promote early recovery of consciousness in the ICU.”
Such efforts are essential, given the high stakes of TBI outcomes, Dr. Edlow said.
“Let’s be clear about the stakes of an incorrect prognosis,” he said. “If we’re overly pessimistic, then a patient who could have potential for meaningful recovery will likely die in our ICU. On the other hand, if we are overly optimistic, then a patient could end up in a vegetative or minimally conscious state that he or she may never have found to be acceptable,” he said.
Access to technologies a ‘civil right?’
Some ethicists in the field are recommending that patients be given access to the advanced techniques as a civil right, similar to the rights described in the Convention on the Rights of Persons With Disabilities, which was adopted by the United Nations in 2008, Dr. Edlow noted.
“So the question that we as clinicians are going to face moving forward from an ethical standpoint is, if we have access to these techniques, is it an ethical obligation to offer them now?” he said.
Dr. Edlow underscored the need to consider the reality that “there are profound issues relating to resource allocation and access to these advanced techniques, but we’re going to have to consider this together as we move forward.”
Dr. Edlow has received funding from the National Institutes of Health. Dr. Claassen is a minority shareholder with ICE Neurosystems. Dr. Frontera has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Compelling advances in the ability to detect signs of consciousness in unconscious patients who have experienced traumatic brain injury (TBI) are leading to unprecedented changes in the field. There is now hope of improving outcomes and even sparing lives of patients who may otherwise have been mistakenly assessed as having no chance of recovery.
That research, published in the New England Journal of Medicine in June 2019, linked the promising signals of consciousness in comatose patients, detected only on imaging, with remarkable outcomes a year later.
“This was a landmark study,” said Brian L. Edlow, MD, in a presentation on the issue of covert consciousness at the virtual annual meeting of the American Neurological Association.
“Importantly, it is the first compelling evidence that early detection of covert consciousness also predicts 1-year outcomes in the Glasgow Outcome Scale Extended (GOSE), showing that covert consciousness in the ICU appears to be relevant for predicting long-term outcomes,” said Dr. Edlow, who is associate director of the Center for Neurotechnology and Neurorecovery, Massachusetts General Hospital, in Boston.
The researchers showed that 15% of unconscious patients with acute brain injury in the study exhibited significant brain activity on EEG in response to stimuli that included verbal commands such as envisioning that they are playing tennis.
Although other studies have shown similar effects with task-based stimuli, the New England Journal of Medicine study further showed that a year later, the patients who had shown signs of covert consciousness, also called “cognitive motor dissociation” (CMD), were significantly more likely to have a good functional outcome, said the study’s senior author, Jan Claassen, MD, director of critical care neurology at Columbia University, New York, who also presented at the ANA session.
“Importantly, a year later after injury, we found that 44% of patients with CMD and only 14% of non-CMD patients had a good functional outcome, defined as a GOSE score indicating a state where they can at least take care of themselves for 8 hours in a day,” he said.
“[Whether] these patients in a CMD state represent a parallel state or a transitory state on the road to recovery remains to be shown,” he said.
Jennifer Frontera, MD, a professor in the department of neurology at NYU Langone Health in New York and comoderator of the session, agreed that the research is “remarkable.”
“Also,” she said, “it is practical, since many could potentially apply and validate his algorithms, since EEG technology is portable and widely available.”
Research has ushered in a ‘sea change’ in neurocritical care
The research has helped push forward recommendations on the treatment of unconscious patients, Dr. Edlow said. “This has led to a sea change in our field just over the last 2 years, with multiple guidelines published suggesting that it may be time for us to consider incorporating task-based fMRI and EEG techniques into our clinical assessment of patients with disorders of consciousness,” Dr. Edlow said.
Among those updating their recommendations was the American Academy of Neurology, which revised guidelines on practice parameters for patients in a persistent vegetative state. Those guidelines had not been updated since 1995.
Although concluding that “no diagnostic assessment procedure had moderate or strong evidence for use,” the guidelines acknowledge that “it is possible that a positive electromyographic (EMG) response to command, EEG reactivity to sensory stimuli, laser-evoked potentials, and the Perturbational Complexity Index can distinguish a minimally conscious state from vegetative state/unresponsive wakefulness syndrome (VS/UWS).”
Earlier this year, the European Academy of Neurology followed suit with updated guidelines of its own. In the EAN guideline, the academy’s Panel on Coma, Disorders of Consciousness recommends that task-based fMRI, EEG, and other advanced assessments be performed as part of a composite assessment of consciousness and that a patient’s best performance or highest level of consciousness on any of those tests should be a reflection of their diagnosis, Dr. Edlow explained.
“What this means is that our field is moving toward a multimodal assessment of consciousness in the ICU as well as beyond, in the subacute to chronic setting, whereby the behavioral exam, advanced DG, and advanced MRI methods all also contribute to the diagnosis of consciousness,” he said.
The standard for assessment of disorders of consciousness is the Coma Recovery Scale–Revised, with a 25-item scale for diagnosis, prediction of outcome, and assessment of potential treatment efficacy.
But much uncertainty can remain despite the assessment, Dr. Claassen said. “Behavioral assessments of patients with acute brain injury are challenging because examinations fluctuate, and there’s variability between assessors,” he said. “Nevertheless, patients and their families demand guidance from us.”
Dr. Edlow pointed out that the largest study to date of the causes of death among patients with TBI in the ICU underscores the need for better assessments.
The study of more than 600 patients at six level l trauma centers in Canada showed that 70% of patients who died in the ICU from TBI did so as the result of the withdrawal of life-sustaining therapy. However, only about a half (57%) had an unreactive pupil, and only about a quarter (23.7%) had evidence of herniation on CT, findings that are commonly associated with a poor prognosis.
“What emerges from this is that the manner in which the clinicians communicated the prognosis to families was a primary determinant of decisions to withdraw life-sustaining therapy,” Dr. Edlow said.
Negative response not necessarily conclusive
Dr. Edlow added a word of caution that the science is still far from perfect. He noted that, for 25% of healthy patients who are given a motor imagery task, neuroimaging might not show a response, implying that the lack of a signal may not be conclusive.
He described the case of a patient who was comatose at the time she was scanned on day 3 after injury and who showed no responses to language, music, or motor imagery during the MRI, yet a year later, she was functionally independent, back in the workforce, and had very few residual symptoms from her trauma.
“So if a patient does not show a response, that does not prove the patient is not conscious, and it does not prove that the patient is likely to have a poor outcome,” Dr. Edlow said. Such cases underscore the need for more advances in understanding the inner workings of brain injury.
Dr. Edlow and his colleagues are embarking on a trial of the effects of intravenous methylphenidate in targeting the stimulation of dopaminergic circuits within the subcortical ascending arousal network in patients with severe brain injuries.
“The scientific premise of the trial is that personalized brain network mapping in the ICU can identify patients whose connectomes are amenable to neuromodulation,” Dr. Edlow and his colleague report in an article in Neurocritical Care.
The trial, called STIMPACT (Stimulant Therapy Targeted to Individualized Connectivity Maps to Promote ReACTivation of Consciousness), is part of the newly launched Connectome-based Clinical Trial Platform, which the authors describe as “a new paradigm for developing and testing targeted therapies that promote early recovery of consciousness in the ICU.”
Such efforts are essential, given the high stakes of TBI outcomes, Dr. Edlow said.
“Let’s be clear about the stakes of an incorrect prognosis,” he said. “If we’re overly pessimistic, then a patient who could have potential for meaningful recovery will likely die in our ICU. On the other hand, if we are overly optimistic, then a patient could end up in a vegetative or minimally conscious state that he or she may never have found to be acceptable,” he said.
Access to technologies a ‘civil right?’
Some ethicists in the field are recommending that patients be given access to the advanced techniques as a civil right, similar to the rights described in the Convention on the Rights of Persons With Disabilities, which was adopted by the United Nations in 2008, Dr. Edlow noted.
“So the question that we as clinicians are going to face moving forward from an ethical standpoint is, if we have access to these techniques, is it an ethical obligation to offer them now?” he said.
Dr. Edlow underscored the need to consider the reality that “there are profound issues relating to resource allocation and access to these advanced techniques, but we’re going to have to consider this together as we move forward.”
Dr. Edlow has received funding from the National Institutes of Health. Dr. Claassen is a minority shareholder with ICE Neurosystems. Dr. Frontera has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ANA 2020
Online tool offers diabetes device information free of industry funding
A new online tool aims to help patients with insulin-treated diabetes and their health care providers to identify the best diabetes technology based on individual needs and preferences.
The “Device Finder” tool is a new feature of the DiabetesWise website, www.diabeteswise.org, which is funded by the Leona M. and Harry B. Helmsley Charitable Trust with no industry contributions. It is intended for use by patients with either type 1 diabetes or insulin-treated type 2 diabetes and by endocrinologists and primary care clinicians in their discussions with patients.
The main DiabetesWise site was launched in June 2019 by a team led by Stanford (Calif.) University psychologist Korey K. Hood, PhD; this team included endocrinologists, psychologists, diabetes care and education specialists, nurses, and patients. The information provided in it was based on work from the past several years in examining human variables that influence diabetes technology uptake, Dr. Hood said in an interview.
“We realized there wasn’t really a great resource for people to actually compare different devices and understand what might fit their lifestyle and priorities. You had to go to a device manufacturer to get that information, and ... that’s probably a little bit biased,” said Dr. Hood, who is professor of pediatrics and psychiatry & behavioral sciences at Stanford.
The site offers a quick “Check Up” that asks patients about what devices they’re currently using, how they feel they’re handling their diabetes management, and about their priorities regarding devices. The new “Device Finder” tool provides information about different combinations of insulin pumps, continuous glucose monitors (CGMs), injections, and fingerstick glucose meters. The site also features resources for patients on speaking with their doctors, costs and health insurance, coping with COVID-19, and “wisdom” with patient narratives. Patients can download reports to share with their clinicians.
Asked to comment, diabetes technology expert David Ahn, MD, program director of the Allen Diabetes Center, Hoag Health, Newport Beach, Calif., said, “I love that DiabetesWise.org offers patients a way to compare and contrast different products all in one place that is not directly influenced or funded by a specific manufacturer or industry in general. I especially appreciate the patient stories and how they each arrived at their current devices.”
However, Dr. Ahn also noted, “when talking to my patients, I feel like having a personal discussion can lead to a better sense of their desires and preferences than a website that is just following an algorithm. ... The challenge with any resource like this is fully appreciating the nuances of each individual and device since choosing a device or combination of devices can be more of an art than a science.”
Nonetheless, he said that the site may be “a good starting place to learn key concepts and product details” for newly diagnosed patients and nonspecialist clinicians.
Indeed, Dr. Hood said, “It’s not perfect. We will revise it as we get more data.” The team is currently following about 500 patients with type 1 and type 2 diabetes, most of them not in specialty care and not initially using advanced devices (pumps/CGMs) to see how they’re engaging with the site and whether they adopt new technologies. “We were pretty encouraged that, in the first month, people were reaching out to their providers to get a prescription. I think we’re generating the awareness that we thought we would.”
Unfortunately, the COVID-19 pandemic has had a negative impact. “We queried people, [and] about half had lost some portion of employment and with that was tied their access to benefits and health insurance. We saw a dip in how much people could actually access. We’ll report that when we have all the data.”
Pending funding, Dr. Hood said the team also hopes to create a clinician-facing versions of the site. “We won’t forget about endocrinologists, but really we’re interested in making it a tool that primary care clinicians and even pharmacists can use to help with the engagement and uptake of diabetes devices, because the rate of use of these diabetes devices in adults with type 1 who aren’t in specialty care is pretty low. So we’re trying to reach the groups that will have a bigger impact.”
In addition to his work on DiabetesWise, Dr. Hood is a consultant for Cecelia Health. Dr. Ahn is a consultant for Senseonics and Eli Lilly and on the speaker’s bureau for Lilly.
A new online tool aims to help patients with insulin-treated diabetes and their health care providers to identify the best diabetes technology based on individual needs and preferences.
The “Device Finder” tool is a new feature of the DiabetesWise website, www.diabeteswise.org, which is funded by the Leona M. and Harry B. Helmsley Charitable Trust with no industry contributions. It is intended for use by patients with either type 1 diabetes or insulin-treated type 2 diabetes and by endocrinologists and primary care clinicians in their discussions with patients.
The main DiabetesWise site was launched in June 2019 by a team led by Stanford (Calif.) University psychologist Korey K. Hood, PhD; this team included endocrinologists, psychologists, diabetes care and education specialists, nurses, and patients. The information provided in it was based on work from the past several years in examining human variables that influence diabetes technology uptake, Dr. Hood said in an interview.
“We realized there wasn’t really a great resource for people to actually compare different devices and understand what might fit their lifestyle and priorities. You had to go to a device manufacturer to get that information, and ... that’s probably a little bit biased,” said Dr. Hood, who is professor of pediatrics and psychiatry & behavioral sciences at Stanford.
The site offers a quick “Check Up” that asks patients about what devices they’re currently using, how they feel they’re handling their diabetes management, and about their priorities regarding devices. The new “Device Finder” tool provides information about different combinations of insulin pumps, continuous glucose monitors (CGMs), injections, and fingerstick glucose meters. The site also features resources for patients on speaking with their doctors, costs and health insurance, coping with COVID-19, and “wisdom” with patient narratives. Patients can download reports to share with their clinicians.
Asked to comment, diabetes technology expert David Ahn, MD, program director of the Allen Diabetes Center, Hoag Health, Newport Beach, Calif., said, “I love that DiabetesWise.org offers patients a way to compare and contrast different products all in one place that is not directly influenced or funded by a specific manufacturer or industry in general. I especially appreciate the patient stories and how they each arrived at their current devices.”
However, Dr. Ahn also noted, “when talking to my patients, I feel like having a personal discussion can lead to a better sense of their desires and preferences than a website that is just following an algorithm. ... The challenge with any resource like this is fully appreciating the nuances of each individual and device since choosing a device or combination of devices can be more of an art than a science.”
Nonetheless, he said that the site may be “a good starting place to learn key concepts and product details” for newly diagnosed patients and nonspecialist clinicians.
Indeed, Dr. Hood said, “It’s not perfect. We will revise it as we get more data.” The team is currently following about 500 patients with type 1 and type 2 diabetes, most of them not in specialty care and not initially using advanced devices (pumps/CGMs) to see how they’re engaging with the site and whether they adopt new technologies. “We were pretty encouraged that, in the first month, people were reaching out to their providers to get a prescription. I think we’re generating the awareness that we thought we would.”
Unfortunately, the COVID-19 pandemic has had a negative impact. “We queried people, [and] about half had lost some portion of employment and with that was tied their access to benefits and health insurance. We saw a dip in how much people could actually access. We’ll report that when we have all the data.”
Pending funding, Dr. Hood said the team also hopes to create a clinician-facing versions of the site. “We won’t forget about endocrinologists, but really we’re interested in making it a tool that primary care clinicians and even pharmacists can use to help with the engagement and uptake of diabetes devices, because the rate of use of these diabetes devices in adults with type 1 who aren’t in specialty care is pretty low. So we’re trying to reach the groups that will have a bigger impact.”
In addition to his work on DiabetesWise, Dr. Hood is a consultant for Cecelia Health. Dr. Ahn is a consultant for Senseonics and Eli Lilly and on the speaker’s bureau for Lilly.
A new online tool aims to help patients with insulin-treated diabetes and their health care providers to identify the best diabetes technology based on individual needs and preferences.
The “Device Finder” tool is a new feature of the DiabetesWise website, www.diabeteswise.org, which is funded by the Leona M. and Harry B. Helmsley Charitable Trust with no industry contributions. It is intended for use by patients with either type 1 diabetes or insulin-treated type 2 diabetes and by endocrinologists and primary care clinicians in their discussions with patients.
The main DiabetesWise site was launched in June 2019 by a team led by Stanford (Calif.) University psychologist Korey K. Hood, PhD; this team included endocrinologists, psychologists, diabetes care and education specialists, nurses, and patients. The information provided in it was based on work from the past several years in examining human variables that influence diabetes technology uptake, Dr. Hood said in an interview.
“We realized there wasn’t really a great resource for people to actually compare different devices and understand what might fit their lifestyle and priorities. You had to go to a device manufacturer to get that information, and ... that’s probably a little bit biased,” said Dr. Hood, who is professor of pediatrics and psychiatry & behavioral sciences at Stanford.
The site offers a quick “Check Up” that asks patients about what devices they’re currently using, how they feel they’re handling their diabetes management, and about their priorities regarding devices. The new “Device Finder” tool provides information about different combinations of insulin pumps, continuous glucose monitors (CGMs), injections, and fingerstick glucose meters. The site also features resources for patients on speaking with their doctors, costs and health insurance, coping with COVID-19, and “wisdom” with patient narratives. Patients can download reports to share with their clinicians.
Asked to comment, diabetes technology expert David Ahn, MD, program director of the Allen Diabetes Center, Hoag Health, Newport Beach, Calif., said, “I love that DiabetesWise.org offers patients a way to compare and contrast different products all in one place that is not directly influenced or funded by a specific manufacturer or industry in general. I especially appreciate the patient stories and how they each arrived at their current devices.”
However, Dr. Ahn also noted, “when talking to my patients, I feel like having a personal discussion can lead to a better sense of their desires and preferences than a website that is just following an algorithm. ... The challenge with any resource like this is fully appreciating the nuances of each individual and device since choosing a device or combination of devices can be more of an art than a science.”
Nonetheless, he said that the site may be “a good starting place to learn key concepts and product details” for newly diagnosed patients and nonspecialist clinicians.
Indeed, Dr. Hood said, “It’s not perfect. We will revise it as we get more data.” The team is currently following about 500 patients with type 1 and type 2 diabetes, most of them not in specialty care and not initially using advanced devices (pumps/CGMs) to see how they’re engaging with the site and whether they adopt new technologies. “We were pretty encouraged that, in the first month, people were reaching out to their providers to get a prescription. I think we’re generating the awareness that we thought we would.”
Unfortunately, the COVID-19 pandemic has had a negative impact. “We queried people, [and] about half had lost some portion of employment and with that was tied their access to benefits and health insurance. We saw a dip in how much people could actually access. We’ll report that when we have all the data.”
Pending funding, Dr. Hood said the team also hopes to create a clinician-facing versions of the site. “We won’t forget about endocrinologists, but really we’re interested in making it a tool that primary care clinicians and even pharmacists can use to help with the engagement and uptake of diabetes devices, because the rate of use of these diabetes devices in adults with type 1 who aren’t in specialty care is pretty low. So we’re trying to reach the groups that will have a bigger impact.”
In addition to his work on DiabetesWise, Dr. Hood is a consultant for Cecelia Health. Dr. Ahn is a consultant for Senseonics and Eli Lilly and on the speaker’s bureau for Lilly.
New estimates for breast cancer risk with HRT
The study was published online on October 28 in The BMJ.
“The study confirms increased risk of breast cancer in patients taking HRT but shows that the magnitude of risk depends on a number of factors,” first author Yana Vinogradova, PhD, said in an interview. Dr. Vinogradova is a medical statistician at the University of Nottingham (England).
The study also suggests the risk may be lower than was estimated in a large meta-analysis of 24 trials that was published in 2019 in The Lancet. In that study, researchers suggested the risk for breast cancer with HRT was higher and persisted longer than had been thought.
This conclusion from the meta-analysis was widely reported in the lay press and led to the UK Medicine and Healthcare Products Regulatory Agency issuing a safety alert for HRT regarding breast cancer. Experts in the field questioned the alert and said it caused undue anxiety. The European Medicines Agency also issued a safety alert because of the study.
This new study was begun before publication of the meta-analysis. Although the results are broadly similar in suggesting increased risk for breast cancer with HRT use, findings from the new study suggest the risk is lower than had been estimated in the meta-analysis and that the risk diminishes more rapidly after stopping HRT than was suggested by the meta-analysis.
“The publicity surrounding publication of the meta-analysis highlighted unexpectedly high risks and led to a heightened level of concern in some quarters,” Dr. Vinogradova commented. “Our study, based on general population data, has not confirmed any such findings. In general, it showed lower levels of risk and clarified the variability of magnitude within them.”
Dr. Vinogradova said the discrepancy could be related to the fact that the studies were designed differently. The meta-analysis relied on results from 24 studies that were conducted around the world at different periods and included women of different ages and backgrounds. The studies in the meta-analysis used different methods, including questionnaires that relied on women’s memories and therefore could have been biased, she said.
In contrast, the new study analyzed EMR data collected prospectively by general practices in the United Kingdom. The data came from the QResearch and from the Clinical Practice Research Datalink (CPRD) databases, the two largest primary care databases in the United Kingdom, which were linked to hospital, mortality, and cancer registries.
Because this study used a “consistent design” and “consistent data sources,” these new results “are likely to be more accurate and reliable for assessing risks among HRT users,” Dr. Vinogradova commented.
This study used an observational design, so it cannot prove that HRT causes breast cancer. These results may better represent women in the general U.K. population, compared with the earlier meta-analysis, she added.
Commenting on the new study, Michael Jones, PhD, senior staff scientist in genetics and epidemiology at the Institute of Cancer Research, London, also emphasized that it was large and its data came from general practitioner medical records, “so the strong statistical associations are unlikely to be due to chance.
“The results of this study generally confirm what has been seen before and is well established – that the use of combined estrogen plus progestogen HRT is associated with increased risk of breast cancer, and this risk increases with duration of use. But reassuringly, after stopping HRT, the raised risk of breast cancer mostly returns to that seen in nonusers of HRT,” he said.
“It’s important to note that no one study should be considered in isolation,” he added. “Even though some risks were found to be slightly smaller than those reported in another meta-analysis of the worldwide epidemiological evidence recently published in 2019, women considering use of HRT should still follow advice given to them by their [general practitioners].”
Study details
In the study, researchers evaluated all types of HRT commonly prescribed in the United Kingdom over the past 20 years, including topical estrogen, vaginal pessaries, and creams. They grouped HRT use by recent (within the past 5 years) and past (5 or more years ago) and HRT duration as short term (less than 5 years) and long term (5 years or longer). Results were adjusted for a range of factors that could affect breast cancer risk, including lifestyle, smoking, alcohol consumption, other medical conditions, family history, and use of other prescribed drugs.
The analysis included 98,611 women aged 50-79 years who were first diagnosed with breast cancer between 1998 and 2019. These women were matched by age and general practice to 457,498 women who were not diagnosed with breast cancer over these years. HRT use was reported in 34% (33,703) of women with breast cancer and in 31% (134,391) of women without breast cancer.
Overall, the risk for breast cancer was increased with use of most HRT drugs (adjusted odds ratio, 1.21; 95% confidence, 1.19-1.23), compared with not using HRT drugs. The highest risk was tied to combined estrogen/progestogen HRT (adjusted OR, 1.26; 95% CI, 1.24-1.29). The lowest risk was tied to estrogen-only HRT (adjusted OR, 1.06; 95% CI, 1.03-1.10). Estrogen cream and vaginal estrogen were not associated with increased breast cancer risk.
In general, breast cancer risk was higher among recent HRT users and those receiving long-term therapy. HRT-associated breast cancer risk increased with age and declined after discontinuing treatment. Therapy of less than 1 year was not associated with increased breast cancer risk.
Women who had recently been receiving long-term combined estrogen/progestogen HRT had a 79% increased risk for breast cancer (adjusted OR, 1.79; 95% CI, 1.73-1.85), compared with never-users. Among recent long-term users of combined HRT, breast cancer risk was highest for norethisterone (adjusted OR, 1.88; 95% CI, 1.79-1.99) and lowest for dydrogesterone (adjusted OR, 1.24; 95% CI, 1.03-1.48). Women who had recently been receiving long-term estrogen-only HRT had a 15% increased risk for breast cancer compared to never-users (adjusted OR, 1.15; 95% CI, 1.09-1.21).
Among women who discontinued HRT 5 or more years ago, risk for breast cancer was no longer increased for long-term estrogen-only therapy and short-term estrogen/progestogen therapy. However, breast cancer risk remained elevated 5 years after discontinuing long-term estrogen/progestogen (adjusted OR, 1.16; 95% CI, 1.11-1.21).
HRT-associated risk for breast cancer increased with age across all durations of therapy.
Compared with never-use, recent long-term estrogen-only therapy was associated with zero extra breast cancer cases per 10,000 women-years among women aged 50-59 years and eight extra cases per 10,000 women-years among women aged 70-79.
Recent long-term estrogen/progestogen use was associated with 15 extra breast cancer cases among women aged 50-59 and 36 extra cases among women aged 70-79 per 10,000 women-years.
Past long-term estrogen/progestogen use was associated with zero extra breast cancer cases among women aged 50-59 and eight extra cases among women aged 70-79 per 10,000 women-years.
Summarizing, Dr. Vinogradova said the increased risk for breast cancer with HRT appears to be “relatively small, particularly for younger women and for any women who use HRT only for a restricted period.”
Decisions about whether to use HRT and which type to use should depend on symptom severity, patient factors, and suitability of other treatment options, she commented.
“Particularly for those women who our study has shown to be most at risk, these decisions should be made through discussions between the patient and her doctor,” she concluded. “We hope that the new and more detailed information provided by our study will facilitate such prescribing decisions.”
The study was partially funded by the School for Primary Care Research of the National Institute for Health Research, by Cancer Research UK, and by the Cancer Research UK Oxford Center. Dr. Vinogradova has disclosed no relevant financial relationships. Senior author Julia Hippisley-Cox is an unpaid director of QResearch and was a paid director of ClinRisk until 2019. The other authors have disclosed no relevant financial relationships.
A version of this story originally appeared on Medscape.com.
The study was published online on October 28 in The BMJ.
“The study confirms increased risk of breast cancer in patients taking HRT but shows that the magnitude of risk depends on a number of factors,” first author Yana Vinogradova, PhD, said in an interview. Dr. Vinogradova is a medical statistician at the University of Nottingham (England).
The study also suggests the risk may be lower than was estimated in a large meta-analysis of 24 trials that was published in 2019 in The Lancet. In that study, researchers suggested the risk for breast cancer with HRT was higher and persisted longer than had been thought.
This conclusion from the meta-analysis was widely reported in the lay press and led to the UK Medicine and Healthcare Products Regulatory Agency issuing a safety alert for HRT regarding breast cancer. Experts in the field questioned the alert and said it caused undue anxiety. The European Medicines Agency also issued a safety alert because of the study.
This new study was begun before publication of the meta-analysis. Although the results are broadly similar in suggesting increased risk for breast cancer with HRT use, findings from the new study suggest the risk is lower than had been estimated in the meta-analysis and that the risk diminishes more rapidly after stopping HRT than was suggested by the meta-analysis.
“The publicity surrounding publication of the meta-analysis highlighted unexpectedly high risks and led to a heightened level of concern in some quarters,” Dr. Vinogradova commented. “Our study, based on general population data, has not confirmed any such findings. In general, it showed lower levels of risk and clarified the variability of magnitude within them.”
Dr. Vinogradova said the discrepancy could be related to the fact that the studies were designed differently. The meta-analysis relied on results from 24 studies that were conducted around the world at different periods and included women of different ages and backgrounds. The studies in the meta-analysis used different methods, including questionnaires that relied on women’s memories and therefore could have been biased, she said.
In contrast, the new study analyzed EMR data collected prospectively by general practices in the United Kingdom. The data came from the QResearch and from the Clinical Practice Research Datalink (CPRD) databases, the two largest primary care databases in the United Kingdom, which were linked to hospital, mortality, and cancer registries.
Because this study used a “consistent design” and “consistent data sources,” these new results “are likely to be more accurate and reliable for assessing risks among HRT users,” Dr. Vinogradova commented.
This study used an observational design, so it cannot prove that HRT causes breast cancer. These results may better represent women in the general U.K. population, compared with the earlier meta-analysis, she added.
Commenting on the new study, Michael Jones, PhD, senior staff scientist in genetics and epidemiology at the Institute of Cancer Research, London, also emphasized that it was large and its data came from general practitioner medical records, “so the strong statistical associations are unlikely to be due to chance.
“The results of this study generally confirm what has been seen before and is well established – that the use of combined estrogen plus progestogen HRT is associated with increased risk of breast cancer, and this risk increases with duration of use. But reassuringly, after stopping HRT, the raised risk of breast cancer mostly returns to that seen in nonusers of HRT,” he said.
“It’s important to note that no one study should be considered in isolation,” he added. “Even though some risks were found to be slightly smaller than those reported in another meta-analysis of the worldwide epidemiological evidence recently published in 2019, women considering use of HRT should still follow advice given to them by their [general practitioners].”
Study details
In the study, researchers evaluated all types of HRT commonly prescribed in the United Kingdom over the past 20 years, including topical estrogen, vaginal pessaries, and creams. They grouped HRT use by recent (within the past 5 years) and past (5 or more years ago) and HRT duration as short term (less than 5 years) and long term (5 years or longer). Results were adjusted for a range of factors that could affect breast cancer risk, including lifestyle, smoking, alcohol consumption, other medical conditions, family history, and use of other prescribed drugs.
The analysis included 98,611 women aged 50-79 years who were first diagnosed with breast cancer between 1998 and 2019. These women were matched by age and general practice to 457,498 women who were not diagnosed with breast cancer over these years. HRT use was reported in 34% (33,703) of women with breast cancer and in 31% (134,391) of women without breast cancer.
Overall, the risk for breast cancer was increased with use of most HRT drugs (adjusted odds ratio, 1.21; 95% confidence, 1.19-1.23), compared with not using HRT drugs. The highest risk was tied to combined estrogen/progestogen HRT (adjusted OR, 1.26; 95% CI, 1.24-1.29). The lowest risk was tied to estrogen-only HRT (adjusted OR, 1.06; 95% CI, 1.03-1.10). Estrogen cream and vaginal estrogen were not associated with increased breast cancer risk.
In general, breast cancer risk was higher among recent HRT users and those receiving long-term therapy. HRT-associated breast cancer risk increased with age and declined after discontinuing treatment. Therapy of less than 1 year was not associated with increased breast cancer risk.
Women who had recently been receiving long-term combined estrogen/progestogen HRT had a 79% increased risk for breast cancer (adjusted OR, 1.79; 95% CI, 1.73-1.85), compared with never-users. Among recent long-term users of combined HRT, breast cancer risk was highest for norethisterone (adjusted OR, 1.88; 95% CI, 1.79-1.99) and lowest for dydrogesterone (adjusted OR, 1.24; 95% CI, 1.03-1.48). Women who had recently been receiving long-term estrogen-only HRT had a 15% increased risk for breast cancer compared to never-users (adjusted OR, 1.15; 95% CI, 1.09-1.21).
Among women who discontinued HRT 5 or more years ago, risk for breast cancer was no longer increased for long-term estrogen-only therapy and short-term estrogen/progestogen therapy. However, breast cancer risk remained elevated 5 years after discontinuing long-term estrogen/progestogen (adjusted OR, 1.16; 95% CI, 1.11-1.21).
HRT-associated risk for breast cancer increased with age across all durations of therapy.
Compared with never-use, recent long-term estrogen-only therapy was associated with zero extra breast cancer cases per 10,000 women-years among women aged 50-59 years and eight extra cases per 10,000 women-years among women aged 70-79.
Recent long-term estrogen/progestogen use was associated with 15 extra breast cancer cases among women aged 50-59 and 36 extra cases among women aged 70-79 per 10,000 women-years.
Past long-term estrogen/progestogen use was associated with zero extra breast cancer cases among women aged 50-59 and eight extra cases among women aged 70-79 per 10,000 women-years.
Summarizing, Dr. Vinogradova said the increased risk for breast cancer with HRT appears to be “relatively small, particularly for younger women and for any women who use HRT only for a restricted period.”
Decisions about whether to use HRT and which type to use should depend on symptom severity, patient factors, and suitability of other treatment options, she commented.
“Particularly for those women who our study has shown to be most at risk, these decisions should be made through discussions between the patient and her doctor,” she concluded. “We hope that the new and more detailed information provided by our study will facilitate such prescribing decisions.”
The study was partially funded by the School for Primary Care Research of the National Institute for Health Research, by Cancer Research UK, and by the Cancer Research UK Oxford Center. Dr. Vinogradova has disclosed no relevant financial relationships. Senior author Julia Hippisley-Cox is an unpaid director of QResearch and was a paid director of ClinRisk until 2019. The other authors have disclosed no relevant financial relationships.
A version of this story originally appeared on Medscape.com.
The study was published online on October 28 in The BMJ.
“The study confirms increased risk of breast cancer in patients taking HRT but shows that the magnitude of risk depends on a number of factors,” first author Yana Vinogradova, PhD, said in an interview. Dr. Vinogradova is a medical statistician at the University of Nottingham (England).
The study also suggests the risk may be lower than was estimated in a large meta-analysis of 24 trials that was published in 2019 in The Lancet. In that study, researchers suggested the risk for breast cancer with HRT was higher and persisted longer than had been thought.
This conclusion from the meta-analysis was widely reported in the lay press and led to the UK Medicine and Healthcare Products Regulatory Agency issuing a safety alert for HRT regarding breast cancer. Experts in the field questioned the alert and said it caused undue anxiety. The European Medicines Agency also issued a safety alert because of the study.
This new study was begun before publication of the meta-analysis. Although the results are broadly similar in suggesting increased risk for breast cancer with HRT use, findings from the new study suggest the risk is lower than had been estimated in the meta-analysis and that the risk diminishes more rapidly after stopping HRT than was suggested by the meta-analysis.
“The publicity surrounding publication of the meta-analysis highlighted unexpectedly high risks and led to a heightened level of concern in some quarters,” Dr. Vinogradova commented. “Our study, based on general population data, has not confirmed any such findings. In general, it showed lower levels of risk and clarified the variability of magnitude within them.”
Dr. Vinogradova said the discrepancy could be related to the fact that the studies were designed differently. The meta-analysis relied on results from 24 studies that were conducted around the world at different periods and included women of different ages and backgrounds. The studies in the meta-analysis used different methods, including questionnaires that relied on women’s memories and therefore could have been biased, she said.
In contrast, the new study analyzed EMR data collected prospectively by general practices in the United Kingdom. The data came from the QResearch and from the Clinical Practice Research Datalink (CPRD) databases, the two largest primary care databases in the United Kingdom, which were linked to hospital, mortality, and cancer registries.
Because this study used a “consistent design” and “consistent data sources,” these new results “are likely to be more accurate and reliable for assessing risks among HRT users,” Dr. Vinogradova commented.
This study used an observational design, so it cannot prove that HRT causes breast cancer. These results may better represent women in the general U.K. population, compared with the earlier meta-analysis, she added.
Commenting on the new study, Michael Jones, PhD, senior staff scientist in genetics and epidemiology at the Institute of Cancer Research, London, also emphasized that it was large and its data came from general practitioner medical records, “so the strong statistical associations are unlikely to be due to chance.
“The results of this study generally confirm what has been seen before and is well established – that the use of combined estrogen plus progestogen HRT is associated with increased risk of breast cancer, and this risk increases with duration of use. But reassuringly, after stopping HRT, the raised risk of breast cancer mostly returns to that seen in nonusers of HRT,” he said.
“It’s important to note that no one study should be considered in isolation,” he added. “Even though some risks were found to be slightly smaller than those reported in another meta-analysis of the worldwide epidemiological evidence recently published in 2019, women considering use of HRT should still follow advice given to them by their [general practitioners].”
Study details
In the study, researchers evaluated all types of HRT commonly prescribed in the United Kingdom over the past 20 years, including topical estrogen, vaginal pessaries, and creams. They grouped HRT use by recent (within the past 5 years) and past (5 or more years ago) and HRT duration as short term (less than 5 years) and long term (5 years or longer). Results were adjusted for a range of factors that could affect breast cancer risk, including lifestyle, smoking, alcohol consumption, other medical conditions, family history, and use of other prescribed drugs.
The analysis included 98,611 women aged 50-79 years who were first diagnosed with breast cancer between 1998 and 2019. These women were matched by age and general practice to 457,498 women who were not diagnosed with breast cancer over these years. HRT use was reported in 34% (33,703) of women with breast cancer and in 31% (134,391) of women without breast cancer.
Overall, the risk for breast cancer was increased with use of most HRT drugs (adjusted odds ratio, 1.21; 95% confidence, 1.19-1.23), compared with not using HRT drugs. The highest risk was tied to combined estrogen/progestogen HRT (adjusted OR, 1.26; 95% CI, 1.24-1.29). The lowest risk was tied to estrogen-only HRT (adjusted OR, 1.06; 95% CI, 1.03-1.10). Estrogen cream and vaginal estrogen were not associated with increased breast cancer risk.
In general, breast cancer risk was higher among recent HRT users and those receiving long-term therapy. HRT-associated breast cancer risk increased with age and declined after discontinuing treatment. Therapy of less than 1 year was not associated with increased breast cancer risk.
Women who had recently been receiving long-term combined estrogen/progestogen HRT had a 79% increased risk for breast cancer (adjusted OR, 1.79; 95% CI, 1.73-1.85), compared with never-users. Among recent long-term users of combined HRT, breast cancer risk was highest for norethisterone (adjusted OR, 1.88; 95% CI, 1.79-1.99) and lowest for dydrogesterone (adjusted OR, 1.24; 95% CI, 1.03-1.48). Women who had recently been receiving long-term estrogen-only HRT had a 15% increased risk for breast cancer compared to never-users (adjusted OR, 1.15; 95% CI, 1.09-1.21).
Among women who discontinued HRT 5 or more years ago, risk for breast cancer was no longer increased for long-term estrogen-only therapy and short-term estrogen/progestogen therapy. However, breast cancer risk remained elevated 5 years after discontinuing long-term estrogen/progestogen (adjusted OR, 1.16; 95% CI, 1.11-1.21).
HRT-associated risk for breast cancer increased with age across all durations of therapy.
Compared with never-use, recent long-term estrogen-only therapy was associated with zero extra breast cancer cases per 10,000 women-years among women aged 50-59 years and eight extra cases per 10,000 women-years among women aged 70-79.
Recent long-term estrogen/progestogen use was associated with 15 extra breast cancer cases among women aged 50-59 and 36 extra cases among women aged 70-79 per 10,000 women-years.
Past long-term estrogen/progestogen use was associated with zero extra breast cancer cases among women aged 50-59 and eight extra cases among women aged 70-79 per 10,000 women-years.
Summarizing, Dr. Vinogradova said the increased risk for breast cancer with HRT appears to be “relatively small, particularly for younger women and for any women who use HRT only for a restricted period.”
Decisions about whether to use HRT and which type to use should depend on symptom severity, patient factors, and suitability of other treatment options, she commented.
“Particularly for those women who our study has shown to be most at risk, these decisions should be made through discussions between the patient and her doctor,” she concluded. “We hope that the new and more detailed information provided by our study will facilitate such prescribing decisions.”
The study was partially funded by the School for Primary Care Research of the National Institute for Health Research, by Cancer Research UK, and by the Cancer Research UK Oxford Center. Dr. Vinogradova has disclosed no relevant financial relationships. Senior author Julia Hippisley-Cox is an unpaid director of QResearch and was a paid director of ClinRisk until 2019. The other authors have disclosed no relevant financial relationships.
A version of this story originally appeared on Medscape.com.
Surgery for adhesive small-bowel obstruction linked with lower risk of recurrence
Background: Guidelines recommend nonoperative monitoring for aSBO; however, long-term association of operative versus nonoperative management and aSBO recurrence is poorly understood.
Study design: Longitudinal, retrospective cohort.
Setting: Hospitals in Ontario.
Synopsis: Administrative data for 2005-2014 was used to identify 27,904 adults hospitalized for an initial episode of aSBO who did not have known inflammatory bowel disease, history of radiotherapy, malignancy, ileus, impaction, or anatomical obstruction. Approximately 22% of patients were managed surgically during the index admission. Patients were followed for a median of 3.6 years. Overall, 19.6% of patients experienced at least one admission for recurrence of aSBO during the study period. With each recurrence, the probability of subsequent recurrence increased, the time between episodes decreased, and the probability of being treated surgically decreased.
Patients were then matched into operative (n = 6,160) and nonoperative (n = 6,160) cohorts based on a propensity score which incorporated comorbidity burden, age, gender, and socioeconomic status. Patients who underwent operative management during their index admission for aSBO had a lower overall risk of recurrence compared to patients managed nonoperatively (13.0% vs. 21.3%; P less than .001). Operative intervention was associated with lower hazards of recurrence even when accounting for death. Additionally, surgical intervention after any episode was associated with a significantly lower risk of recurrence, compared with nonoperative management.
Bottom line: Contrary to surgical dogma, surgical intervention is associated with reduced risk of recurrent aSBO in patients without complicating factors. Hospitalists should consider recurrence risk when managing these patients nonoperatively.
Citation: Behman R et al. Association of surgical intervention for adhesive small-bowel obstruction with the risk of recurrence. JAMA Surg. 2019 May 1;154(5):413-20.
Dr. Liu is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
Background: Guidelines recommend nonoperative monitoring for aSBO; however, long-term association of operative versus nonoperative management and aSBO recurrence is poorly understood.
Study design: Longitudinal, retrospective cohort.
Setting: Hospitals in Ontario.
Synopsis: Administrative data for 2005-2014 was used to identify 27,904 adults hospitalized for an initial episode of aSBO who did not have known inflammatory bowel disease, history of radiotherapy, malignancy, ileus, impaction, or anatomical obstruction. Approximately 22% of patients were managed surgically during the index admission. Patients were followed for a median of 3.6 years. Overall, 19.6% of patients experienced at least one admission for recurrence of aSBO during the study period. With each recurrence, the probability of subsequent recurrence increased, the time between episodes decreased, and the probability of being treated surgically decreased.
Patients were then matched into operative (n = 6,160) and nonoperative (n = 6,160) cohorts based on a propensity score which incorporated comorbidity burden, age, gender, and socioeconomic status. Patients who underwent operative management during their index admission for aSBO had a lower overall risk of recurrence compared to patients managed nonoperatively (13.0% vs. 21.3%; P less than .001). Operative intervention was associated with lower hazards of recurrence even when accounting for death. Additionally, surgical intervention after any episode was associated with a significantly lower risk of recurrence, compared with nonoperative management.
Bottom line: Contrary to surgical dogma, surgical intervention is associated with reduced risk of recurrent aSBO in patients without complicating factors. Hospitalists should consider recurrence risk when managing these patients nonoperatively.
Citation: Behman R et al. Association of surgical intervention for adhesive small-bowel obstruction with the risk of recurrence. JAMA Surg. 2019 May 1;154(5):413-20.
Dr. Liu is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
Background: Guidelines recommend nonoperative monitoring for aSBO; however, long-term association of operative versus nonoperative management and aSBO recurrence is poorly understood.
Study design: Longitudinal, retrospective cohort.
Setting: Hospitals in Ontario.
Synopsis: Administrative data for 2005-2014 was used to identify 27,904 adults hospitalized for an initial episode of aSBO who did not have known inflammatory bowel disease, history of radiotherapy, malignancy, ileus, impaction, or anatomical obstruction. Approximately 22% of patients were managed surgically during the index admission. Patients were followed for a median of 3.6 years. Overall, 19.6% of patients experienced at least one admission for recurrence of aSBO during the study period. With each recurrence, the probability of subsequent recurrence increased, the time between episodes decreased, and the probability of being treated surgically decreased.
Patients were then matched into operative (n = 6,160) and nonoperative (n = 6,160) cohorts based on a propensity score which incorporated comorbidity burden, age, gender, and socioeconomic status. Patients who underwent operative management during their index admission for aSBO had a lower overall risk of recurrence compared to patients managed nonoperatively (13.0% vs. 21.3%; P less than .001). Operative intervention was associated with lower hazards of recurrence even when accounting for death. Additionally, surgical intervention after any episode was associated with a significantly lower risk of recurrence, compared with nonoperative management.
Bottom line: Contrary to surgical dogma, surgical intervention is associated with reduced risk of recurrent aSBO in patients without complicating factors. Hospitalists should consider recurrence risk when managing these patients nonoperatively.
Citation: Behman R et al. Association of surgical intervention for adhesive small-bowel obstruction with the risk of recurrence. JAMA Surg. 2019 May 1;154(5):413-20.
Dr. Liu is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
Facebook $52M settlement flags need to screen for vicarious trauma
The images are graphic, disturbing, and endless, said a former Facebook employee. Her job as a content moderator required that she review and remove disturbing posts. That work, she claimed in a lawsuit, caused her to suffer serious psychological trauma.
In September 2018, she filed a complaint with the Superior Court of California.
“Every day, Facebook users post millions of videos, images, and livestream broadcasts of child sexual abuse, rape, torture, bestiality, beheadings, suicide, and murder,” reads the complaint. “By requiring its content moderators to work in dangerous conditions that cause debilitating physical and psychological harm, Facebook violates California law.”
In May, Facebook settled the case, agreeing to pay $52 million to content moderators to compensate them for the consequences their work had on their mental health. The settlement was the first to officially recognize the psychological toll of exposure to disturbing material resulting from online moderator jobs. It also highlights an emerging understanding of vicarious trauma.
Also known as secondary trauma, vicarious trauma can result from exposure to images, stories, or accounts that someone does not directly experience, said Françoise Mathieu, MEd, CCC, RP, a compassion fatigue specialist and executive director of TEND, a company in Kingston, Ont., that offers resources and training for people who work in high-stress, trauma-exposed workplaces.
Secondary trauma can affect people much as any other kind of intensely stressful experience. “What I can tell you as a specialist is that trauma is trauma,” Mathieu said. “Our brain doesn’t necessarily know the difference.”
The potential for vicarious trauma has long been recognized as a risk for journalists, health care providers, and anyone who watches television coverage of a disaster. Only recently, Mathieu said, have researchers started to investigate the psychological impact of jobs that require people to look at extreme, graphic, or disturbing images.
Physical fallout
In a 2017 study of digital forensic examiners, researchers found that examiners who worked on cases involving sexual crimes against children were at elevated risk of developing secondary trauma.
However, the exploratory study did not quantify the risks, and the study investigators concluded that more research is needed to understand how best to help people deal with PTSD resulting from working in the criminal justice system.
Content moderation requires sifting through upsetting images, and people can react in different ways to the task, says Anthony Ng, MD, a psychiatrist at Hartford (Conn.) Healthcare in Mansfield Center.
Dr. Ng says some individuals may become emotionally numb in order to protect themselves. Others might relate to what they are seeing, either because of their own life circumstances or because of experiences they have had in the past. For example, individuals might think: “I could see that kid being my son, I could see that woman who was assaulted as my wife who got beaten up”
that ramps up activity in an area of the brain called the locus coeruleus, Dr. Ng said.
Heart rate rises. Breathing rate goes up. Muscles become tense. If a threat occurs once and then dissipates, the body can often recover a state of calm. However, when that threat is part of the daily workday, it can cause chronic harm to mental and physical health. Unlike with direct, or primary, trauma, he adds, secondary trauma can take a while to become symptomatic.
“Your heart is not designed to be constantly pumping at a high rate,” Dr. Ng says. “We just can’t sustain that for long periods of time without starting to develop stress reactions.”
Under the radar
Some types of work appear to confer greater risk for trauma than others. Overall, estimates show that up to 8% of the U.S. population will develop PTSD at some point in their lives, Ms. Mathieu said.
For police officers, the rate is 15%. According to reporting by The Verge, lawyers in the Facebook lawsuit cited vicarious trauma rates of up to 50% among content moderators.
There are multiple reasons why content moderators suffer such high rates of mental health problems, Ms. Mathieu said. Content moderation is a low-paying, thankless, and solo job that can seem never-ending, she said.
Furthermore, content moderators are generally uninformed about the psychological risks associated with their occupation. They aren’t given the time to process what they are exposed to and generally don’t feel recognized or appreciated for the work they do.
That makes their jobs different from those of people such as law enforcement officers who investigate Internet crimes. For people pursuing justice, a sense of unity can counterbalance the exposure to tough imagery and information.
Going forward, Ms. Mathieu said, the only way to make content moderation safer is to institute changes such as better pay, more flexible schedules to allow breaks from exposure, and access to mental health professionals who can help employees process what they have seen.
Climate of fear
“This can’t be a climate of fear where people are afraid to ask for help,” Ms. Mathieu said. “They are really important jobs, but people need to feel that they are safe in expressing when it’s impacting them so that they’re not worried that they’re actually going to lose their work.”
It would help if content moderators received evidence-based guidance to help process their experiences, Ms. Mathieu added. However, to avoid doing more harm than good, debriefing has to be administered correctly.
For example, a method called “critical incident stress debriefing,” a longstanding approach that research has shown can do more harm than good, is still widely used in law enforcement agencies. The technique requires individuals to talk about their traumatic experience immediately after it happens, which can cause retraumatization.
Instead, Dr. Ng recommended a more self-aware approach called low-impact debriefing. The method involves strategies such as giving fair warning, asking for consent from listeners, and being selective about the details shared.
Employees should also be taught to recognize and report early signs and symptoms so that they can seek help before psychological distress becomes overwhelming, Dr. Ng says.
Plenty of moderators do not develop PTSD, he said, despite their exposure to upsetting imagery. This suggests an important avenue for research – understanding what makes some people resilient, even in the face of graphic and disturbing stressors.
A version of this story originally appeared on Medscape.com.
The images are graphic, disturbing, and endless, said a former Facebook employee. Her job as a content moderator required that she review and remove disturbing posts. That work, she claimed in a lawsuit, caused her to suffer serious psychological trauma.
In September 2018, she filed a complaint with the Superior Court of California.
“Every day, Facebook users post millions of videos, images, and livestream broadcasts of child sexual abuse, rape, torture, bestiality, beheadings, suicide, and murder,” reads the complaint. “By requiring its content moderators to work in dangerous conditions that cause debilitating physical and psychological harm, Facebook violates California law.”
In May, Facebook settled the case, agreeing to pay $52 million to content moderators to compensate them for the consequences their work had on their mental health. The settlement was the first to officially recognize the psychological toll of exposure to disturbing material resulting from online moderator jobs. It also highlights an emerging understanding of vicarious trauma.
Also known as secondary trauma, vicarious trauma can result from exposure to images, stories, or accounts that someone does not directly experience, said Françoise Mathieu, MEd, CCC, RP, a compassion fatigue specialist and executive director of TEND, a company in Kingston, Ont., that offers resources and training for people who work in high-stress, trauma-exposed workplaces.
Secondary trauma can affect people much as any other kind of intensely stressful experience. “What I can tell you as a specialist is that trauma is trauma,” Mathieu said. “Our brain doesn’t necessarily know the difference.”
The potential for vicarious trauma has long been recognized as a risk for journalists, health care providers, and anyone who watches television coverage of a disaster. Only recently, Mathieu said, have researchers started to investigate the psychological impact of jobs that require people to look at extreme, graphic, or disturbing images.
Physical fallout
In a 2017 study of digital forensic examiners, researchers found that examiners who worked on cases involving sexual crimes against children were at elevated risk of developing secondary trauma.
However, the exploratory study did not quantify the risks, and the study investigators concluded that more research is needed to understand how best to help people deal with PTSD resulting from working in the criminal justice system.
Content moderation requires sifting through upsetting images, and people can react in different ways to the task, says Anthony Ng, MD, a psychiatrist at Hartford (Conn.) Healthcare in Mansfield Center.
Dr. Ng says some individuals may become emotionally numb in order to protect themselves. Others might relate to what they are seeing, either because of their own life circumstances or because of experiences they have had in the past. For example, individuals might think: “I could see that kid being my son, I could see that woman who was assaulted as my wife who got beaten up”
that ramps up activity in an area of the brain called the locus coeruleus, Dr. Ng said.
Heart rate rises. Breathing rate goes up. Muscles become tense. If a threat occurs once and then dissipates, the body can often recover a state of calm. However, when that threat is part of the daily workday, it can cause chronic harm to mental and physical health. Unlike with direct, or primary, trauma, he adds, secondary trauma can take a while to become symptomatic.
“Your heart is not designed to be constantly pumping at a high rate,” Dr. Ng says. “We just can’t sustain that for long periods of time without starting to develop stress reactions.”
Under the radar
Some types of work appear to confer greater risk for trauma than others. Overall, estimates show that up to 8% of the U.S. population will develop PTSD at some point in their lives, Ms. Mathieu said.
For police officers, the rate is 15%. According to reporting by The Verge, lawyers in the Facebook lawsuit cited vicarious trauma rates of up to 50% among content moderators.
There are multiple reasons why content moderators suffer such high rates of mental health problems, Ms. Mathieu said. Content moderation is a low-paying, thankless, and solo job that can seem never-ending, she said.
Furthermore, content moderators are generally uninformed about the psychological risks associated with their occupation. They aren’t given the time to process what they are exposed to and generally don’t feel recognized or appreciated for the work they do.
That makes their jobs different from those of people such as law enforcement officers who investigate Internet crimes. For people pursuing justice, a sense of unity can counterbalance the exposure to tough imagery and information.
Going forward, Ms. Mathieu said, the only way to make content moderation safer is to institute changes such as better pay, more flexible schedules to allow breaks from exposure, and access to mental health professionals who can help employees process what they have seen.
Climate of fear
“This can’t be a climate of fear where people are afraid to ask for help,” Ms. Mathieu said. “They are really important jobs, but people need to feel that they are safe in expressing when it’s impacting them so that they’re not worried that they’re actually going to lose their work.”
It would help if content moderators received evidence-based guidance to help process their experiences, Ms. Mathieu added. However, to avoid doing more harm than good, debriefing has to be administered correctly.
For example, a method called “critical incident stress debriefing,” a longstanding approach that research has shown can do more harm than good, is still widely used in law enforcement agencies. The technique requires individuals to talk about their traumatic experience immediately after it happens, which can cause retraumatization.
Instead, Dr. Ng recommended a more self-aware approach called low-impact debriefing. The method involves strategies such as giving fair warning, asking for consent from listeners, and being selective about the details shared.
Employees should also be taught to recognize and report early signs and symptoms so that they can seek help before psychological distress becomes overwhelming, Dr. Ng says.
Plenty of moderators do not develop PTSD, he said, despite their exposure to upsetting imagery. This suggests an important avenue for research – understanding what makes some people resilient, even in the face of graphic and disturbing stressors.
A version of this story originally appeared on Medscape.com.
The images are graphic, disturbing, and endless, said a former Facebook employee. Her job as a content moderator required that she review and remove disturbing posts. That work, she claimed in a lawsuit, caused her to suffer serious psychological trauma.
In September 2018, she filed a complaint with the Superior Court of California.
“Every day, Facebook users post millions of videos, images, and livestream broadcasts of child sexual abuse, rape, torture, bestiality, beheadings, suicide, and murder,” reads the complaint. “By requiring its content moderators to work in dangerous conditions that cause debilitating physical and psychological harm, Facebook violates California law.”
In May, Facebook settled the case, agreeing to pay $52 million to content moderators to compensate them for the consequences their work had on their mental health. The settlement was the first to officially recognize the psychological toll of exposure to disturbing material resulting from online moderator jobs. It also highlights an emerging understanding of vicarious trauma.
Also known as secondary trauma, vicarious trauma can result from exposure to images, stories, or accounts that someone does not directly experience, said Françoise Mathieu, MEd, CCC, RP, a compassion fatigue specialist and executive director of TEND, a company in Kingston, Ont., that offers resources and training for people who work in high-stress, trauma-exposed workplaces.
Secondary trauma can affect people much as any other kind of intensely stressful experience. “What I can tell you as a specialist is that trauma is trauma,” Mathieu said. “Our brain doesn’t necessarily know the difference.”
The potential for vicarious trauma has long been recognized as a risk for journalists, health care providers, and anyone who watches television coverage of a disaster. Only recently, Mathieu said, have researchers started to investigate the psychological impact of jobs that require people to look at extreme, graphic, or disturbing images.
Physical fallout
In a 2017 study of digital forensic examiners, researchers found that examiners who worked on cases involving sexual crimes against children were at elevated risk of developing secondary trauma.
However, the exploratory study did not quantify the risks, and the study investigators concluded that more research is needed to understand how best to help people deal with PTSD resulting from working in the criminal justice system.
Content moderation requires sifting through upsetting images, and people can react in different ways to the task, says Anthony Ng, MD, a psychiatrist at Hartford (Conn.) Healthcare in Mansfield Center.
Dr. Ng says some individuals may become emotionally numb in order to protect themselves. Others might relate to what they are seeing, either because of their own life circumstances or because of experiences they have had in the past. For example, individuals might think: “I could see that kid being my son, I could see that woman who was assaulted as my wife who got beaten up”
that ramps up activity in an area of the brain called the locus coeruleus, Dr. Ng said.
Heart rate rises. Breathing rate goes up. Muscles become tense. If a threat occurs once and then dissipates, the body can often recover a state of calm. However, when that threat is part of the daily workday, it can cause chronic harm to mental and physical health. Unlike with direct, or primary, trauma, he adds, secondary trauma can take a while to become symptomatic.
“Your heart is not designed to be constantly pumping at a high rate,” Dr. Ng says. “We just can’t sustain that for long periods of time without starting to develop stress reactions.”
Under the radar
Some types of work appear to confer greater risk for trauma than others. Overall, estimates show that up to 8% of the U.S. population will develop PTSD at some point in their lives, Ms. Mathieu said.
For police officers, the rate is 15%. According to reporting by The Verge, lawyers in the Facebook lawsuit cited vicarious trauma rates of up to 50% among content moderators.
There are multiple reasons why content moderators suffer such high rates of mental health problems, Ms. Mathieu said. Content moderation is a low-paying, thankless, and solo job that can seem never-ending, she said.
Furthermore, content moderators are generally uninformed about the psychological risks associated with their occupation. They aren’t given the time to process what they are exposed to and generally don’t feel recognized or appreciated for the work they do.
That makes their jobs different from those of people such as law enforcement officers who investigate Internet crimes. For people pursuing justice, a sense of unity can counterbalance the exposure to tough imagery and information.
Going forward, Ms. Mathieu said, the only way to make content moderation safer is to institute changes such as better pay, more flexible schedules to allow breaks from exposure, and access to mental health professionals who can help employees process what they have seen.
Climate of fear
“This can’t be a climate of fear where people are afraid to ask for help,” Ms. Mathieu said. “They are really important jobs, but people need to feel that they are safe in expressing when it’s impacting them so that they’re not worried that they’re actually going to lose their work.”
It would help if content moderators received evidence-based guidance to help process their experiences, Ms. Mathieu added. However, to avoid doing more harm than good, debriefing has to be administered correctly.
For example, a method called “critical incident stress debriefing,” a longstanding approach that research has shown can do more harm than good, is still widely used in law enforcement agencies. The technique requires individuals to talk about their traumatic experience immediately after it happens, which can cause retraumatization.
Instead, Dr. Ng recommended a more self-aware approach called low-impact debriefing. The method involves strategies such as giving fair warning, asking for consent from listeners, and being selective about the details shared.
Employees should also be taught to recognize and report early signs and symptoms so that they can seek help before psychological distress becomes overwhelming, Dr. Ng says.
Plenty of moderators do not develop PTSD, he said, despite their exposure to upsetting imagery. This suggests an important avenue for research – understanding what makes some people resilient, even in the face of graphic and disturbing stressors.
A version of this story originally appeared on Medscape.com.