User login
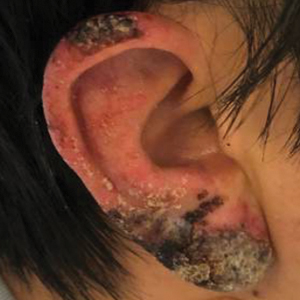
Crusted Papules on the Bilateral Helices and Lobules
The Diagnosis: Kikuchi-Fujimoto Disease
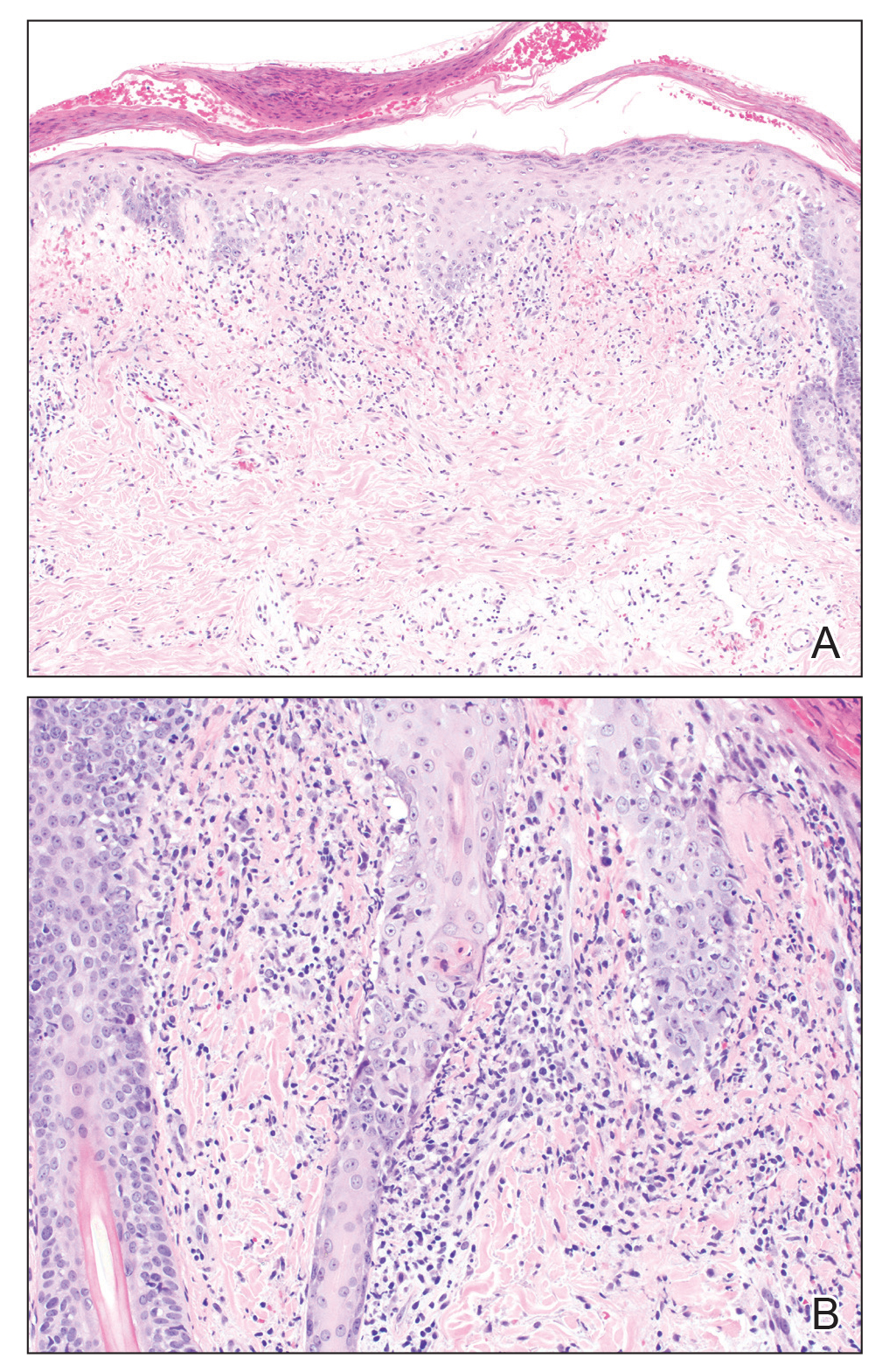
A skin biopsy from the left helix was obtained. Histopathologic examination revealed a vacuolar interface reaction with marked papillary dermal edema and a patchy perijunctional lymphocytic infiltrate. The dermis was free of increased mucin (Figure 1). Immunohistochemical staining for CD56 and Epstein-Barr virus (EBV)–encoded small nuclear RNA chromogenic in situ hybridization were negative. Laboratory workup was remarkable for elevated transaminases and inflammatory markers (eg, C-reactive protein, erythrocyte sedimentation rate) but negative for rheumatologic markers (eg, antinuclear antibodies, antineutrophil cytoplasmic antibodies, myeloperoxidase antibodies, serine protease IgG). An extensive infectious workup was unrevealing. Computed tomography highlighted prominent lymphadenopathy throughout the cervical and supraclavicular chains and a large necrotic lymph node in the porta hepatis (Figure 2). Right neck lymph node aspiration revealed necrotizing lymphadenitis in a background of histiocytes and mixed lymphocytes. Coupling the clinical presentation and histomorphology with imaging, a diagnosis of Kikuchi-Fujimoto disease (KD) was rendered.

Kikuchi-Fujimoto disease is a rare illness of unknown etiology characterized by cervical lymphadenopathy and fever. Originally described in Japan, KD affects all racial and ethnic groups1,2 but more commonly is seen in women and patients younger than 40 years.3 It can be associated with systemic lupus erythematosus (SLE) and other autoimmune diseases (eg, relapsing polychondritis, adult-onset Still disease),3 and lymphoma.4 Multiple infections have been implicated in the pathogenesis of KD, including EBV and other human herpesviruses; HIV; human T-cell leukemia virus type 1; dengue virus; parvovirus B19; and Yersinia enterocolitica, Bartonella, Brucella, and Toxoplasma infections.3,5,6
Kikuchi-Fujimoto disease classically presents with fever and cervical lymphadenopathy. In a retrospective review of 244 patients with KD, the 3 most common manifestations included lymphadenopathy, fever, and rash.7 A diagnosis of KD is rendered based on clinical presentation and lymph node histopathologic findings of paracortical necrosis and florid histiocytic infiltrate.1
The cutaneous manifestations of KD are heterogeneous yet mostly transient. Cutaneous involvement is reported in 16.6% to 40% of patients.3,5,6 Common cutaneous manifestations include erythematous macules, papules, patches, and plaques; erosions, nodules, and bullae less commonly can occur.6 A variety of cutaneous manifestations have been reported in KD, including lesions mimicking pigmented purpuric dermatoses, vasculitis, Sweet syndrome, drug eruptions, and viral exanthems.6 Signs and symptoms of KD usually resolve within 1 to 4 months. Although there are no established treatments for this disease, patients with severe or persistent symptoms can be treated with steroids or hydroxychloroquine. Recurrences after treatment have been reported.8
Systemic lupus erythematosus is a multiorgan disease with protean manifestations. Cutaneous manifestations of SLE include malar erythema and discoid, annular, and papulosquamous lesions. Histopathologic patterns frequently observed in cutaneous lesions associated with SLE include interface dermatitis with perivascular infiltrates, dermal mucin, and plasmacytoid dendritic cells (marked by CD123 staining); these findings were notably absent in our case.6
Lupus vulgaris is a form of cutaneous tuberculosis that results from reactivation of Mycobacterium tuberculosis in tubercles formed during preceding hematogenous dissemination. The head and neck region is the most common location, particularly the nose, cheeks, and earlobes. Small, brown-red, soft papules coalesce into gelatinous plaques, demonstrating a characteristic apple jelly appearance on diascopy. Other clinical manifestations include the plaque/plane, hypertrophic/tumorlike, and ulcerative/scarring forms.9 Delayed-type hypersensitivity testing by tuberculin skin test, interferon-gamma release assay, or polymerase chain reaction–based assays can detect Mycobacterium tuberculosis. Histopathology shows well-formed granulomas surrounded by chronic inflammatory cells and central necrosis.
Hydroa vacciniforme–like (HV-like) eruption is a rare photosensitive disorder characterized by vesiculopapules on sun-exposed areas. Hydroa vacciniforme–like eruptions rarely have been reported to progress to EBVassociated malignant lymphoma.10 Unlike typical hydroa vacciniforme, which resolves by early adulthood, HV-like eruptions can become more severe with age and are associated with systemic manifestations, including fevers, lymphadenopathy, and liver damage. Histopathologic examination reveals a dense infiltrate of atypical T lymphocytes or natural killer cells (CD56+), which stain positive for EBV-encoded small nuclear RNA,10 in contrast to the patchy perijunctional lymphocytic infiltrate seen in KD.
This case highlights the protean cutaneous manifestations of a rare rheumatologic entity. It demonstrates the importance of a full systemic workup when considering an enigmatic disease. Our patient was started on prednisone 20 mg and hydroxychloroquine 200 mg daily. Within 24 hours, the fevers and rash both improved.
- Turner RR, Martin J, Dorfman RF. Necrotizing lymphadenitis. a study of 30 cases. Am J Surg Pathol. 1983;7:115-123.
- Dorfman RF, Berry GJ. Kikuchi’s histiocytic necrotizing lymphadenitis: an analysis of 108 cases with emphasis on differential diagnosis. Semin Diagn Pathol. 1988;5:329-345.
- Atwater AR, Longley BJ, Aughenbaugh WD. Kikuchi’s disease: case report and systematic review of cutaneous and histopathologic presentations. J Am Acad Dermatol. 2008;59:130-136.
- Yoshino T, Mannami T, Ichimura K, et al. Two cases of histiocytic necrotizing lymphadenitis (Kikuchi-Fujimoto’s disease) following diffuse large B-cell lymphoma. Hum Pathol. 2000;31:1328-1331.
- Yen A, Fearneyhough P, Raimer SS, et al. EBV-associated Kikuchi’s histiocytic necrotizing lymphadenitis with cutaneous manifestations. J Am Acad Dermatol. 1997;36:342-346.
- Kim JH, Kim YB, In SI, et al. The cutaneous lesions of Kikuchi’s disease: a comprehensive analysis of 16 cases based on the clinicopathologic, immunohistochemical, and immunofluorescence studies with an emphasis on the differential diagnosis. Hum Pathol. 2010;41:1245-1254.
- Kucukardali Y, Solmazgul E, Kunter E, et al. Kikuchi-Fujimoto Disease: analysis of 244 cases. Clin Rheumatol. 2007;26:50-54.
- Smith KG, Becker GJ, Busmanis I. Recurrent Kikuchi’s disease. Lancet. 1992;340:124.
- Macgregor R. Cutaneous tuberculosis. Clin Dermatol. 1995;13:245-255.
- Iwatsuki K, Ohtsuka M, Harada H, et al. Clinicopathologic manifestations of Epstein-Barr virus–associated cutaneous lymphoproliferative disorders. Arch Dermatol. 1997;133:1081-1086.
The Diagnosis: Kikuchi-Fujimoto Disease
A skin biopsy from the left helix was obtained. Histopathologic examination revealed a vacuolar interface reaction with marked papillary dermal edema and a patchy perijunctional lymphocytic infiltrate. The dermis was free of increased mucin (Figure 1). Immunohistochemical staining for CD56 and Epstein-Barr virus (EBV)–encoded small nuclear RNA chromogenic in situ hybridization were negative. Laboratory workup was remarkable for elevated transaminases and inflammatory markers (eg, C-reactive protein, erythrocyte sedimentation rate) but negative for rheumatologic markers (eg, antinuclear antibodies, antineutrophil cytoplasmic antibodies, myeloperoxidase antibodies, serine protease IgG). An extensive infectious workup was unrevealing. Computed tomography highlighted prominent lymphadenopathy throughout the cervical and supraclavicular chains and a large necrotic lymph node in the porta hepatis (Figure 2). Right neck lymph node aspiration revealed necrotizing lymphadenitis in a background of histiocytes and mixed lymphocytes. Coupling the clinical presentation and histomorphology with imaging, a diagnosis of Kikuchi-Fujimoto disease (KD) was rendered.


Kikuchi-Fujimoto disease is a rare illness of unknown etiology characterized by cervical lymphadenopathy and fever. Originally described in Japan, KD affects all racial and ethnic groups1,2 but more commonly is seen in women and patients younger than 40 years.3 It can be associated with systemic lupus erythematosus (SLE) and other autoimmune diseases (eg, relapsing polychondritis, adult-onset Still disease),3 and lymphoma.4 Multiple infections have been implicated in the pathogenesis of KD, including EBV and other human herpesviruses; HIV; human T-cell leukemia virus type 1; dengue virus; parvovirus B19; and Yersinia enterocolitica, Bartonella, Brucella, and Toxoplasma infections.3,5,6
Kikuchi-Fujimoto disease classically presents with fever and cervical lymphadenopathy. In a retrospective review of 244 patients with KD, the 3 most common manifestations included lymphadenopathy, fever, and rash.7 A diagnosis of KD is rendered based on clinical presentation and lymph node histopathologic findings of paracortical necrosis and florid histiocytic infiltrate.1
The cutaneous manifestations of KD are heterogeneous yet mostly transient. Cutaneous involvement is reported in 16.6% to 40% of patients.3,5,6 Common cutaneous manifestations include erythematous macules, papules, patches, and plaques; erosions, nodules, and bullae less commonly can occur.6 A variety of cutaneous manifestations have been reported in KD, including lesions mimicking pigmented purpuric dermatoses, vasculitis, Sweet syndrome, drug eruptions, and viral exanthems.6 Signs and symptoms of KD usually resolve within 1 to 4 months. Although there are no established treatments for this disease, patients with severe or persistent symptoms can be treated with steroids or hydroxychloroquine. Recurrences after treatment have been reported.8
Systemic lupus erythematosus is a multiorgan disease with protean manifestations. Cutaneous manifestations of SLE include malar erythema and discoid, annular, and papulosquamous lesions. Histopathologic patterns frequently observed in cutaneous lesions associated with SLE include interface dermatitis with perivascular infiltrates, dermal mucin, and plasmacytoid dendritic cells (marked by CD123 staining); these findings were notably absent in our case.6
Lupus vulgaris is a form of cutaneous tuberculosis that results from reactivation of Mycobacterium tuberculosis in tubercles formed during preceding hematogenous dissemination. The head and neck region is the most common location, particularly the nose, cheeks, and earlobes. Small, brown-red, soft papules coalesce into gelatinous plaques, demonstrating a characteristic apple jelly appearance on diascopy. Other clinical manifestations include the plaque/plane, hypertrophic/tumorlike, and ulcerative/scarring forms.9 Delayed-type hypersensitivity testing by tuberculin skin test, interferon-gamma release assay, or polymerase chain reaction–based assays can detect Mycobacterium tuberculosis. Histopathology shows well-formed granulomas surrounded by chronic inflammatory cells and central necrosis.
Hydroa vacciniforme–like (HV-like) eruption is a rare photosensitive disorder characterized by vesiculopapules on sun-exposed areas. Hydroa vacciniforme–like eruptions rarely have been reported to progress to EBVassociated malignant lymphoma.10 Unlike typical hydroa vacciniforme, which resolves by early adulthood, HV-like eruptions can become more severe with age and are associated with systemic manifestations, including fevers, lymphadenopathy, and liver damage. Histopathologic examination reveals a dense infiltrate of atypical T lymphocytes or natural killer cells (CD56+), which stain positive for EBV-encoded small nuclear RNA,10 in contrast to the patchy perijunctional lymphocytic infiltrate seen in KD.
This case highlights the protean cutaneous manifestations of a rare rheumatologic entity. It demonstrates the importance of a full systemic workup when considering an enigmatic disease. Our patient was started on prednisone 20 mg and hydroxychloroquine 200 mg daily. Within 24 hours, the fevers and rash both improved.
The Diagnosis: Kikuchi-Fujimoto Disease
A skin biopsy from the left helix was obtained. Histopathologic examination revealed a vacuolar interface reaction with marked papillary dermal edema and a patchy perijunctional lymphocytic infiltrate. The dermis was free of increased mucin (Figure 1). Immunohistochemical staining for CD56 and Epstein-Barr virus (EBV)–encoded small nuclear RNA chromogenic in situ hybridization were negative. Laboratory workup was remarkable for elevated transaminases and inflammatory markers (eg, C-reactive protein, erythrocyte sedimentation rate) but negative for rheumatologic markers (eg, antinuclear antibodies, antineutrophil cytoplasmic antibodies, myeloperoxidase antibodies, serine protease IgG). An extensive infectious workup was unrevealing. Computed tomography highlighted prominent lymphadenopathy throughout the cervical and supraclavicular chains and a large necrotic lymph node in the porta hepatis (Figure 2). Right neck lymph node aspiration revealed necrotizing lymphadenitis in a background of histiocytes and mixed lymphocytes. Coupling the clinical presentation and histomorphology with imaging, a diagnosis of Kikuchi-Fujimoto disease (KD) was rendered.


Kikuchi-Fujimoto disease is a rare illness of unknown etiology characterized by cervical lymphadenopathy and fever. Originally described in Japan, KD affects all racial and ethnic groups1,2 but more commonly is seen in women and patients younger than 40 years.3 It can be associated with systemic lupus erythematosus (SLE) and other autoimmune diseases (eg, relapsing polychondritis, adult-onset Still disease),3 and lymphoma.4 Multiple infections have been implicated in the pathogenesis of KD, including EBV and other human herpesviruses; HIV; human T-cell leukemia virus type 1; dengue virus; parvovirus B19; and Yersinia enterocolitica, Bartonella, Brucella, and Toxoplasma infections.3,5,6
Kikuchi-Fujimoto disease classically presents with fever and cervical lymphadenopathy. In a retrospective review of 244 patients with KD, the 3 most common manifestations included lymphadenopathy, fever, and rash.7 A diagnosis of KD is rendered based on clinical presentation and lymph node histopathologic findings of paracortical necrosis and florid histiocytic infiltrate.1
The cutaneous manifestations of KD are heterogeneous yet mostly transient. Cutaneous involvement is reported in 16.6% to 40% of patients.3,5,6 Common cutaneous manifestations include erythematous macules, papules, patches, and plaques; erosions, nodules, and bullae less commonly can occur.6 A variety of cutaneous manifestations have been reported in KD, including lesions mimicking pigmented purpuric dermatoses, vasculitis, Sweet syndrome, drug eruptions, and viral exanthems.6 Signs and symptoms of KD usually resolve within 1 to 4 months. Although there are no established treatments for this disease, patients with severe or persistent symptoms can be treated with steroids or hydroxychloroquine. Recurrences after treatment have been reported.8
Systemic lupus erythematosus is a multiorgan disease with protean manifestations. Cutaneous manifestations of SLE include malar erythema and discoid, annular, and papulosquamous lesions. Histopathologic patterns frequently observed in cutaneous lesions associated with SLE include interface dermatitis with perivascular infiltrates, dermal mucin, and plasmacytoid dendritic cells (marked by CD123 staining); these findings were notably absent in our case.6
Lupus vulgaris is a form of cutaneous tuberculosis that results from reactivation of Mycobacterium tuberculosis in tubercles formed during preceding hematogenous dissemination. The head and neck region is the most common location, particularly the nose, cheeks, and earlobes. Small, brown-red, soft papules coalesce into gelatinous plaques, demonstrating a characteristic apple jelly appearance on diascopy. Other clinical manifestations include the plaque/plane, hypertrophic/tumorlike, and ulcerative/scarring forms.9 Delayed-type hypersensitivity testing by tuberculin skin test, interferon-gamma release assay, or polymerase chain reaction–based assays can detect Mycobacterium tuberculosis. Histopathology shows well-formed granulomas surrounded by chronic inflammatory cells and central necrosis.
Hydroa vacciniforme–like (HV-like) eruption is a rare photosensitive disorder characterized by vesiculopapules on sun-exposed areas. Hydroa vacciniforme–like eruptions rarely have been reported to progress to EBVassociated malignant lymphoma.10 Unlike typical hydroa vacciniforme, which resolves by early adulthood, HV-like eruptions can become more severe with age and are associated with systemic manifestations, including fevers, lymphadenopathy, and liver damage. Histopathologic examination reveals a dense infiltrate of atypical T lymphocytes or natural killer cells (CD56+), which stain positive for EBV-encoded small nuclear RNA,10 in contrast to the patchy perijunctional lymphocytic infiltrate seen in KD.
This case highlights the protean cutaneous manifestations of a rare rheumatologic entity. It demonstrates the importance of a full systemic workup when considering an enigmatic disease. Our patient was started on prednisone 20 mg and hydroxychloroquine 200 mg daily. Within 24 hours, the fevers and rash both improved.
- Turner RR, Martin J, Dorfman RF. Necrotizing lymphadenitis. a study of 30 cases. Am J Surg Pathol. 1983;7:115-123.
- Dorfman RF, Berry GJ. Kikuchi’s histiocytic necrotizing lymphadenitis: an analysis of 108 cases with emphasis on differential diagnosis. Semin Diagn Pathol. 1988;5:329-345.
- Atwater AR, Longley BJ, Aughenbaugh WD. Kikuchi’s disease: case report and systematic review of cutaneous and histopathologic presentations. J Am Acad Dermatol. 2008;59:130-136.
- Yoshino T, Mannami T, Ichimura K, et al. Two cases of histiocytic necrotizing lymphadenitis (Kikuchi-Fujimoto’s disease) following diffuse large B-cell lymphoma. Hum Pathol. 2000;31:1328-1331.
- Yen A, Fearneyhough P, Raimer SS, et al. EBV-associated Kikuchi’s histiocytic necrotizing lymphadenitis with cutaneous manifestations. J Am Acad Dermatol. 1997;36:342-346.
- Kim JH, Kim YB, In SI, et al. The cutaneous lesions of Kikuchi’s disease: a comprehensive analysis of 16 cases based on the clinicopathologic, immunohistochemical, and immunofluorescence studies with an emphasis on the differential diagnosis. Hum Pathol. 2010;41:1245-1254.
- Kucukardali Y, Solmazgul E, Kunter E, et al. Kikuchi-Fujimoto Disease: analysis of 244 cases. Clin Rheumatol. 2007;26:50-54.
- Smith KG, Becker GJ, Busmanis I. Recurrent Kikuchi’s disease. Lancet. 1992;340:124.
- Macgregor R. Cutaneous tuberculosis. Clin Dermatol. 1995;13:245-255.
- Iwatsuki K, Ohtsuka M, Harada H, et al. Clinicopathologic manifestations of Epstein-Barr virus–associated cutaneous lymphoproliferative disorders. Arch Dermatol. 1997;133:1081-1086.
- Turner RR, Martin J, Dorfman RF. Necrotizing lymphadenitis. a study of 30 cases. Am J Surg Pathol. 1983;7:115-123.
- Dorfman RF, Berry GJ. Kikuchi’s histiocytic necrotizing lymphadenitis: an analysis of 108 cases with emphasis on differential diagnosis. Semin Diagn Pathol. 1988;5:329-345.
- Atwater AR, Longley BJ, Aughenbaugh WD. Kikuchi’s disease: case report and systematic review of cutaneous and histopathologic presentations. J Am Acad Dermatol. 2008;59:130-136.
- Yoshino T, Mannami T, Ichimura K, et al. Two cases of histiocytic necrotizing lymphadenitis (Kikuchi-Fujimoto’s disease) following diffuse large B-cell lymphoma. Hum Pathol. 2000;31:1328-1331.
- Yen A, Fearneyhough P, Raimer SS, et al. EBV-associated Kikuchi’s histiocytic necrotizing lymphadenitis with cutaneous manifestations. J Am Acad Dermatol. 1997;36:342-346.
- Kim JH, Kim YB, In SI, et al. The cutaneous lesions of Kikuchi’s disease: a comprehensive analysis of 16 cases based on the clinicopathologic, immunohistochemical, and immunofluorescence studies with an emphasis on the differential diagnosis. Hum Pathol. 2010;41:1245-1254.
- Kucukardali Y, Solmazgul E, Kunter E, et al. Kikuchi-Fujimoto Disease: analysis of 244 cases. Clin Rheumatol. 2007;26:50-54.
- Smith KG, Becker GJ, Busmanis I. Recurrent Kikuchi’s disease. Lancet. 1992;340:124.
- Macgregor R. Cutaneous tuberculosis. Clin Dermatol. 1995;13:245-255.
- Iwatsuki K, Ohtsuka M, Harada H, et al. Clinicopathologic manifestations of Epstein-Barr virus–associated cutaneous lymphoproliferative disorders. Arch Dermatol. 1997;133:1081-1086.
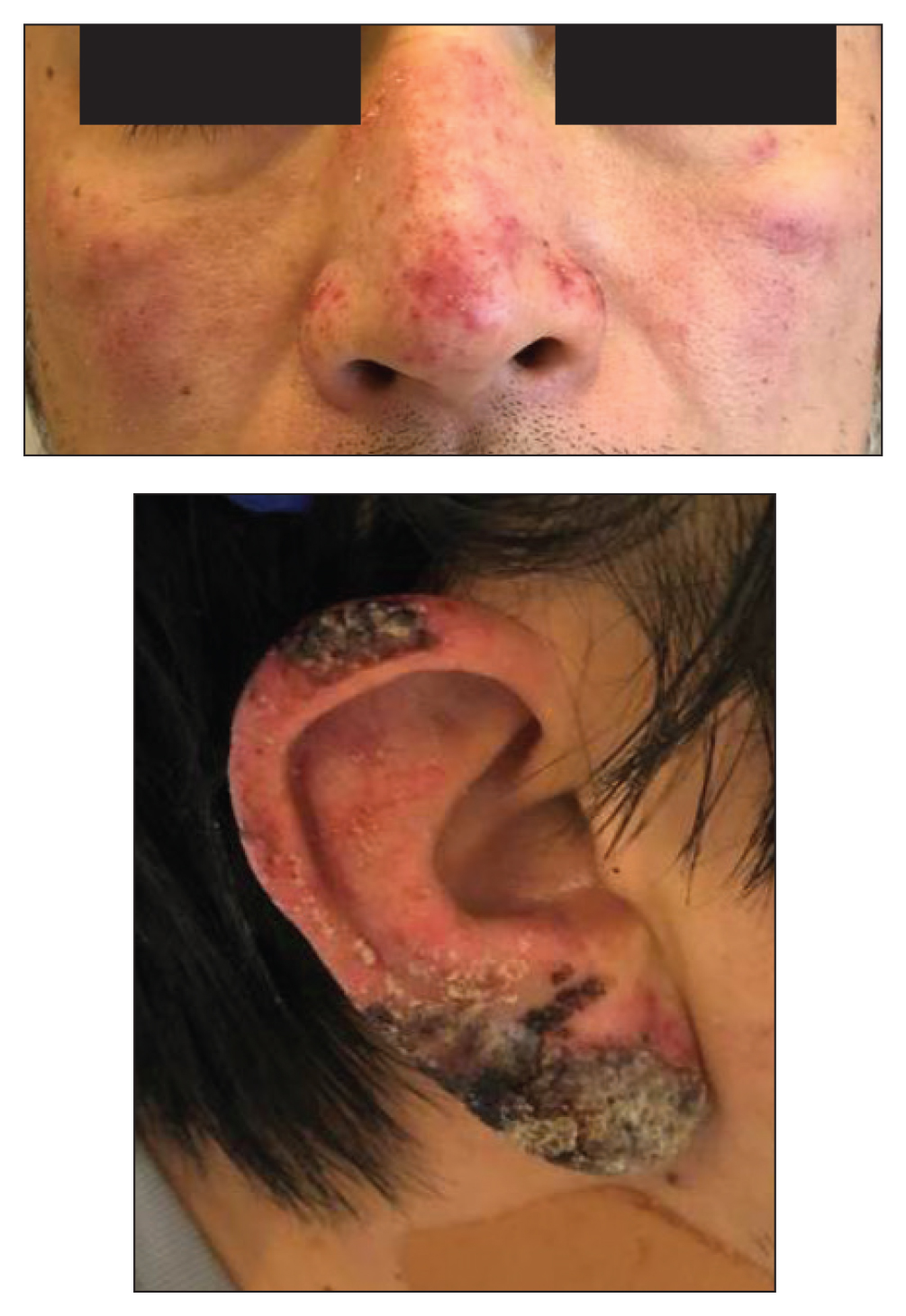
A healthy 42-year-old Japanese man presented with painful lymphadenopathy and fevers of 1 month’s duration as well as a pruritic rash and bilateral ear redness and crusting of 1 week’s duration. He initially was seen at an outside facility and was treated with antibiotics and supportive care for cervical adenitis. During clinical evaluation, he denied joint pain, photosensitivity, and oral lesions. His medical and family history were noncontributory. Although he reported recent travel to multiple countries, he denied exposure to animals, ticks, or sick individuals. Physical examination revealed erythematous blanching papules on the nose and cheeks (top) as well as crusted papules coalescing into plaques on the bilateral helices and lobules (bottom).
Depression screening after ACS does not change outcomes
Background: Depression after ACS is common and is associated with increased mortality. Professional societies have recommended routine depression screening in these patients; however, this has not been consistently implemented because there is a lack of data to support routine screening.
Study design: Multicenter randomized clinical trial.
Setting: Four geographically diverse health systems in the United States.
Synopsis: In the CODIACS-QoL trial, 1,500 patients were randomized to three groups within 12 months of documented ACS: depression screening with notification to primary care and treatment, screening and notification to primary care, and no screening. Only 7.7% of the patients in the screen, notify, and treat group and 6.6% of screen and notify group screened positive for depression. There were no differences for the primary outcome of quality-adjusted life-years or the secondary outcome of depression-free days between groups. Additionally, there was no difference in mortality or patient-reported harms of screening between groups. The study excluded patients who already had a history of depression, psychiatric history, or other severe life-threatening medical conditions, which may have affected the outcomes.
Depression remains a substantial factor in coronary disease and quality of life; however, systematic depression screening appears to have limited population-level benefits.
Bottom line: Systematic depression screening with or without treatment offerings did not alter quality of life, depression-free days, or mortality in patients with ACS.
Citation: Kronish IM et al. Effect of depression screening after acute coronary syndrome on quality of life. JAMA Intern Med. 2020;180(1):45-53.
Dr. Ciarkowski is a hospitalist and clinical instructor of medicine at the University of Utah, Salt Lake City.
Background: Depression after ACS is common and is associated with increased mortality. Professional societies have recommended routine depression screening in these patients; however, this has not been consistently implemented because there is a lack of data to support routine screening.
Study design: Multicenter randomized clinical trial.
Setting: Four geographically diverse health systems in the United States.
Synopsis: In the CODIACS-QoL trial, 1,500 patients were randomized to three groups within 12 months of documented ACS: depression screening with notification to primary care and treatment, screening and notification to primary care, and no screening. Only 7.7% of the patients in the screen, notify, and treat group and 6.6% of screen and notify group screened positive for depression. There were no differences for the primary outcome of quality-adjusted life-years or the secondary outcome of depression-free days between groups. Additionally, there was no difference in mortality or patient-reported harms of screening between groups. The study excluded patients who already had a history of depression, psychiatric history, or other severe life-threatening medical conditions, which may have affected the outcomes.
Depression remains a substantial factor in coronary disease and quality of life; however, systematic depression screening appears to have limited population-level benefits.
Bottom line: Systematic depression screening with or without treatment offerings did not alter quality of life, depression-free days, or mortality in patients with ACS.
Citation: Kronish IM et al. Effect of depression screening after acute coronary syndrome on quality of life. JAMA Intern Med. 2020;180(1):45-53.
Dr. Ciarkowski is a hospitalist and clinical instructor of medicine at the University of Utah, Salt Lake City.
Background: Depression after ACS is common and is associated with increased mortality. Professional societies have recommended routine depression screening in these patients; however, this has not been consistently implemented because there is a lack of data to support routine screening.
Study design: Multicenter randomized clinical trial.
Setting: Four geographically diverse health systems in the United States.
Synopsis: In the CODIACS-QoL trial, 1,500 patients were randomized to three groups within 12 months of documented ACS: depression screening with notification to primary care and treatment, screening and notification to primary care, and no screening. Only 7.7% of the patients in the screen, notify, and treat group and 6.6% of screen and notify group screened positive for depression. There were no differences for the primary outcome of quality-adjusted life-years or the secondary outcome of depression-free days between groups. Additionally, there was no difference in mortality or patient-reported harms of screening between groups. The study excluded patients who already had a history of depression, psychiatric history, or other severe life-threatening medical conditions, which may have affected the outcomes.
Depression remains a substantial factor in coronary disease and quality of life; however, systematic depression screening appears to have limited population-level benefits.
Bottom line: Systematic depression screening with or without treatment offerings did not alter quality of life, depression-free days, or mortality in patients with ACS.
Citation: Kronish IM et al. Effect of depression screening after acute coronary syndrome on quality of life. JAMA Intern Med. 2020;180(1):45-53.
Dr. Ciarkowski is a hospitalist and clinical instructor of medicine at the University of Utah, Salt Lake City.
High-intensity interval training cuts cardiometabolic risks in women with PCOS
High-intensity interval training (HIIT) was better than moderate-intensity continuous training (MICT) for improving several measures of cardiometabolic health in women with polycystic ovary syndrome (PCOS) in a prospective, randomized, single-center study with 27 women.
After 12 weeks on a supervised exercise regimen, the women with PCOS who followed the HIIT program had significantly better improvements in aerobic capacity, insulin sensitivity, and level of sex hormone–binding globulin, Rhiannon K. Patten, MSc, said at the annual meeting of the Endocrine Society.
“HIIT can offer superior improvements in health outcomes, and should be considered as an effective tool to reduce cardiometabolic risk in women with PCOS,” concluded Ms. Patten, a researcher in the Institute for Health and Sport at Victoria University in Melbourne in her presentation (Abstract OR10-1).
“The changes we see [after 12 weeks on the HIIT regimen] seem to occur despite no change in body mass index, so rather than focus on weight loss we encourage participants to focus on the health improvements that seem to be greater with HIIT. We actively encourage the HIIT protocol right now,” she said.
Both regimens use a stationary cycle ergometer. In the HIIT protocol patients twice weekly pedal through 12 1-minute intervals at a heart rate of 90%-100% maximum, interspersed with 1 minute rest intervals. On a third day per week, patients pedal to a heart rate of 90%-95% maximum for 6-8 intervals maintained for 2 minutes and interspersed with rest intervals of 2 minutes. The MICT regimen used as a comparator has participants pedal to 60%-70% of their maximum heart rate continuously for 50 minutes 3 days weekly.
HIIT saves time
“These findings are relevant to clinical practice, because they demonstrate that HIIT is effective in women with PCOS. Reducing the time devoted to exercise to achieve fitness goals is attractive to patients. The reduced time to achieve training benefits with HIIT should improve patient compliance,” commented Andrea Dunaif, MD, professor and chief of the division of endocrinology, diabetes, and bone disease of the Mount Sinai Health System in New York, who was not involved with the study.
The overall weekly exercise time on the MICT regimen, 150 minutes, halves down to 75 minutes a week in the HIIT program. Guideline recommendations released in 2018 by the International PCOS Network recommended these as acceptable alternative exercise strategies. Ms. Patten and her associates sought to determine whether one strategy surpassed the other, the first time this has been examined in women with PCOS, she said.
They randomized 27 sedentary women 18-45 years old with a body mass index (BMI) above 25 kg/m2 and diagnosed with PCOS by the Rotterdam criteria to a 12-week supervised exercise program on either the HIIT or MICT protocol. Their average BMI at entry was 36-37 kg/m2. The study excluded women who smoked, were pregnant, had an illness or injury that would prevent exercise, or were on an oral contraceptive or insulin-sensitizing medication.
At the end of 12 weeks, neither group had a significant change in average weight or BMI, and waist circumference dropped by an average of just over 2 cm in both treatment groups. Lean mass increased by a mean 1 kg in the HIIT group, a significant change, compared with a nonsignificant 0.3 kg average increase in the MICT group.
Increased aerobic capacity ‘partially explains’ improved insulin sensitivity
Aerobic capacity, measured as peak oxygen consumption (VO2peak), increased by an average 5.7 mL/kg per min among the HIIT patients, significantly more than the mean 3.2 mL/kg per min increase among those in the MICT program.
The insulin sensitivity index rose by a significant, relative 35% among the HIIT patients, but barely budged in the MICT group. Fasting glucose fell significantly and the glucose infusion rate increased significantly among the women who performed HIIT, but again showed little change among those doing MICT.
Analysis showed a significant link between the increase in VO2peak and the increase in insulin sensitivity among the women engaged in HIIT, Ms. Patten reported. The improvement in the insulin sensitivity index was “partially explained” by the increase in VO2peak, she said.
Assessment of hormone levels showed a significant increase in sex hormone–binding globulin in the HIIT patients while those in the MICT group showed a small decline in this level. The free androgen index fell by a relative 39% on average in the HIIT group, a significant drop, but decreased by a much smaller and not significant amount among the women who did MICT. The women who performed HIIT also showed a significant drop in their free testosterone level, a change not seen with MICT.
Women who performed the HIIT protocol also had a significant improvement in their menstrual cyclicity, and significant improvements in depression, stress, and anxiety, Ms Patten reported. She next plans to do longer follow-up on study participants, out to 6 and 12 months after the end of the exercise protocol.
“Overall, the findings suggest that HIIT is superior to MICT for improving fitness and insulin sensitivity in the short term. Results from a number of studies in individuals without PCOS suggest that HIIT is superior to MICT for improving fitness short term,” commented Dr. Dunaif. “This study makes an important contribution by directly investigating the impact of training intensity in women with PCOS. Larger studies will be needed before the superiority of HIIT is established for women with PCOS, and study durations of at least several months will be needed to assess the impact on reproductive outcomes such as ovulation,” she said in an interview. She also called for assessing the effects of HIIT in more diverse populations of women with PCOS.
Ms. Patten had no disclosures. Dr. Dunaif has been a consultant to Equator Therapeutics, Fractyl Laboratories, and Globe Life Sciences.
High-intensity interval training (HIIT) was better than moderate-intensity continuous training (MICT) for improving several measures of cardiometabolic health in women with polycystic ovary syndrome (PCOS) in a prospective, randomized, single-center study with 27 women.
After 12 weeks on a supervised exercise regimen, the women with PCOS who followed the HIIT program had significantly better improvements in aerobic capacity, insulin sensitivity, and level of sex hormone–binding globulin, Rhiannon K. Patten, MSc, said at the annual meeting of the Endocrine Society.
“HIIT can offer superior improvements in health outcomes, and should be considered as an effective tool to reduce cardiometabolic risk in women with PCOS,” concluded Ms. Patten, a researcher in the Institute for Health and Sport at Victoria University in Melbourne in her presentation (Abstract OR10-1).
“The changes we see [after 12 weeks on the HIIT regimen] seem to occur despite no change in body mass index, so rather than focus on weight loss we encourage participants to focus on the health improvements that seem to be greater with HIIT. We actively encourage the HIIT protocol right now,” she said.
Both regimens use a stationary cycle ergometer. In the HIIT protocol patients twice weekly pedal through 12 1-minute intervals at a heart rate of 90%-100% maximum, interspersed with 1 minute rest intervals. On a third day per week, patients pedal to a heart rate of 90%-95% maximum for 6-8 intervals maintained for 2 minutes and interspersed with rest intervals of 2 minutes. The MICT regimen used as a comparator has participants pedal to 60%-70% of their maximum heart rate continuously for 50 minutes 3 days weekly.
HIIT saves time
“These findings are relevant to clinical practice, because they demonstrate that HIIT is effective in women with PCOS. Reducing the time devoted to exercise to achieve fitness goals is attractive to patients. The reduced time to achieve training benefits with HIIT should improve patient compliance,” commented Andrea Dunaif, MD, professor and chief of the division of endocrinology, diabetes, and bone disease of the Mount Sinai Health System in New York, who was not involved with the study.
The overall weekly exercise time on the MICT regimen, 150 minutes, halves down to 75 minutes a week in the HIIT program. Guideline recommendations released in 2018 by the International PCOS Network recommended these as acceptable alternative exercise strategies. Ms. Patten and her associates sought to determine whether one strategy surpassed the other, the first time this has been examined in women with PCOS, she said.
They randomized 27 sedentary women 18-45 years old with a body mass index (BMI) above 25 kg/m2 and diagnosed with PCOS by the Rotterdam criteria to a 12-week supervised exercise program on either the HIIT or MICT protocol. Their average BMI at entry was 36-37 kg/m2. The study excluded women who smoked, were pregnant, had an illness or injury that would prevent exercise, or were on an oral contraceptive or insulin-sensitizing medication.
At the end of 12 weeks, neither group had a significant change in average weight or BMI, and waist circumference dropped by an average of just over 2 cm in both treatment groups. Lean mass increased by a mean 1 kg in the HIIT group, a significant change, compared with a nonsignificant 0.3 kg average increase in the MICT group.
Increased aerobic capacity ‘partially explains’ improved insulin sensitivity
Aerobic capacity, measured as peak oxygen consumption (VO2peak), increased by an average 5.7 mL/kg per min among the HIIT patients, significantly more than the mean 3.2 mL/kg per min increase among those in the MICT program.
The insulin sensitivity index rose by a significant, relative 35% among the HIIT patients, but barely budged in the MICT group. Fasting glucose fell significantly and the glucose infusion rate increased significantly among the women who performed HIIT, but again showed little change among those doing MICT.
Analysis showed a significant link between the increase in VO2peak and the increase in insulin sensitivity among the women engaged in HIIT, Ms. Patten reported. The improvement in the insulin sensitivity index was “partially explained” by the increase in VO2peak, she said.
Assessment of hormone levels showed a significant increase in sex hormone–binding globulin in the HIIT patients while those in the MICT group showed a small decline in this level. The free androgen index fell by a relative 39% on average in the HIIT group, a significant drop, but decreased by a much smaller and not significant amount among the women who did MICT. The women who performed HIIT also showed a significant drop in their free testosterone level, a change not seen with MICT.
Women who performed the HIIT protocol also had a significant improvement in their menstrual cyclicity, and significant improvements in depression, stress, and anxiety, Ms Patten reported. She next plans to do longer follow-up on study participants, out to 6 and 12 months after the end of the exercise protocol.
“Overall, the findings suggest that HIIT is superior to MICT for improving fitness and insulin sensitivity in the short term. Results from a number of studies in individuals without PCOS suggest that HIIT is superior to MICT for improving fitness short term,” commented Dr. Dunaif. “This study makes an important contribution by directly investigating the impact of training intensity in women with PCOS. Larger studies will be needed before the superiority of HIIT is established for women with PCOS, and study durations of at least several months will be needed to assess the impact on reproductive outcomes such as ovulation,” she said in an interview. She also called for assessing the effects of HIIT in more diverse populations of women with PCOS.
Ms. Patten had no disclosures. Dr. Dunaif has been a consultant to Equator Therapeutics, Fractyl Laboratories, and Globe Life Sciences.
High-intensity interval training (HIIT) was better than moderate-intensity continuous training (MICT) for improving several measures of cardiometabolic health in women with polycystic ovary syndrome (PCOS) in a prospective, randomized, single-center study with 27 women.
After 12 weeks on a supervised exercise regimen, the women with PCOS who followed the HIIT program had significantly better improvements in aerobic capacity, insulin sensitivity, and level of sex hormone–binding globulin, Rhiannon K. Patten, MSc, said at the annual meeting of the Endocrine Society.
“HIIT can offer superior improvements in health outcomes, and should be considered as an effective tool to reduce cardiometabolic risk in women with PCOS,” concluded Ms. Patten, a researcher in the Institute for Health and Sport at Victoria University in Melbourne in her presentation (Abstract OR10-1).
“The changes we see [after 12 weeks on the HIIT regimen] seem to occur despite no change in body mass index, so rather than focus on weight loss we encourage participants to focus on the health improvements that seem to be greater with HIIT. We actively encourage the HIIT protocol right now,” she said.
Both regimens use a stationary cycle ergometer. In the HIIT protocol patients twice weekly pedal through 12 1-minute intervals at a heart rate of 90%-100% maximum, interspersed with 1 minute rest intervals. On a third day per week, patients pedal to a heart rate of 90%-95% maximum for 6-8 intervals maintained for 2 minutes and interspersed with rest intervals of 2 minutes. The MICT regimen used as a comparator has participants pedal to 60%-70% of their maximum heart rate continuously for 50 minutes 3 days weekly.
HIIT saves time
“These findings are relevant to clinical practice, because they demonstrate that HIIT is effective in women with PCOS. Reducing the time devoted to exercise to achieve fitness goals is attractive to patients. The reduced time to achieve training benefits with HIIT should improve patient compliance,” commented Andrea Dunaif, MD, professor and chief of the division of endocrinology, diabetes, and bone disease of the Mount Sinai Health System in New York, who was not involved with the study.
The overall weekly exercise time on the MICT regimen, 150 minutes, halves down to 75 minutes a week in the HIIT program. Guideline recommendations released in 2018 by the International PCOS Network recommended these as acceptable alternative exercise strategies. Ms. Patten and her associates sought to determine whether one strategy surpassed the other, the first time this has been examined in women with PCOS, she said.
They randomized 27 sedentary women 18-45 years old with a body mass index (BMI) above 25 kg/m2 and diagnosed with PCOS by the Rotterdam criteria to a 12-week supervised exercise program on either the HIIT or MICT protocol. Their average BMI at entry was 36-37 kg/m2. The study excluded women who smoked, were pregnant, had an illness or injury that would prevent exercise, or were on an oral contraceptive or insulin-sensitizing medication.
At the end of 12 weeks, neither group had a significant change in average weight or BMI, and waist circumference dropped by an average of just over 2 cm in both treatment groups. Lean mass increased by a mean 1 kg in the HIIT group, a significant change, compared with a nonsignificant 0.3 kg average increase in the MICT group.
Increased aerobic capacity ‘partially explains’ improved insulin sensitivity
Aerobic capacity, measured as peak oxygen consumption (VO2peak), increased by an average 5.7 mL/kg per min among the HIIT patients, significantly more than the mean 3.2 mL/kg per min increase among those in the MICT program.
The insulin sensitivity index rose by a significant, relative 35% among the HIIT patients, but barely budged in the MICT group. Fasting glucose fell significantly and the glucose infusion rate increased significantly among the women who performed HIIT, but again showed little change among those doing MICT.
Analysis showed a significant link between the increase in VO2peak and the increase in insulin sensitivity among the women engaged in HIIT, Ms. Patten reported. The improvement in the insulin sensitivity index was “partially explained” by the increase in VO2peak, she said.
Assessment of hormone levels showed a significant increase in sex hormone–binding globulin in the HIIT patients while those in the MICT group showed a small decline in this level. The free androgen index fell by a relative 39% on average in the HIIT group, a significant drop, but decreased by a much smaller and not significant amount among the women who did MICT. The women who performed HIIT also showed a significant drop in their free testosterone level, a change not seen with MICT.
Women who performed the HIIT protocol also had a significant improvement in their menstrual cyclicity, and significant improvements in depression, stress, and anxiety, Ms Patten reported. She next plans to do longer follow-up on study participants, out to 6 and 12 months after the end of the exercise protocol.
“Overall, the findings suggest that HIIT is superior to MICT for improving fitness and insulin sensitivity in the short term. Results from a number of studies in individuals without PCOS suggest that HIIT is superior to MICT for improving fitness short term,” commented Dr. Dunaif. “This study makes an important contribution by directly investigating the impact of training intensity in women with PCOS. Larger studies will be needed before the superiority of HIIT is established for women with PCOS, and study durations of at least several months will be needed to assess the impact on reproductive outcomes such as ovulation,” she said in an interview. She also called for assessing the effects of HIIT in more diverse populations of women with PCOS.
Ms. Patten had no disclosures. Dr. Dunaif has been a consultant to Equator Therapeutics, Fractyl Laboratories, and Globe Life Sciences.
FROM ENDO 2021
Match Day 2021: Dermatology holds steady
Despite the pandemic, , according to the National Resident Matching Program (NRMP).
Available dermatology PGY-2 slots fell by 0.2% from 478 in 2020 to 477 in 2021, but 471 slots were filled in 2021, 2 more than last year, for an increase of 0.4%. The overall fill rate was 98.7% in 2021, with 86.6% being filled by U.S. graduates. Just under 88% of filled positions went to MD and DO seniors.
While Match Day 2021 set a record for positions offered and filled at 38,106 slots and 36,179, respectively, an overall total of 2,699 PGY-2 slots were offered in 2021, which is a decrease of 1.6% from last year’s total of 2,742 slots. Of the available PGY-2 slots, 97.6% were filled in 2021, compared with 96.6% in 2020.
“The NRMP is honored to have delivered a strong Match to the many applicants pursuing their dreams of medicine. We admire all the Match participants for their hard work and their commitment to train and serve alongside their peers. The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP President and CEO, said in a press release.
Despite the pandemic, , according to the National Resident Matching Program (NRMP).

Available dermatology PGY-2 slots fell by 0.2% from 478 in 2020 to 477 in 2021, but 471 slots were filled in 2021, 2 more than last year, for an increase of 0.4%. The overall fill rate was 98.7% in 2021, with 86.6% being filled by U.S. graduates. Just under 88% of filled positions went to MD and DO seniors.
While Match Day 2021 set a record for positions offered and filled at 38,106 slots and 36,179, respectively, an overall total of 2,699 PGY-2 slots were offered in 2021, which is a decrease of 1.6% from last year’s total of 2,742 slots. Of the available PGY-2 slots, 97.6% were filled in 2021, compared with 96.6% in 2020.
“The NRMP is honored to have delivered a strong Match to the many applicants pursuing their dreams of medicine. We admire all the Match participants for their hard work and their commitment to train and serve alongside their peers. The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP President and CEO, said in a press release.
Despite the pandemic, , according to the National Resident Matching Program (NRMP).

Available dermatology PGY-2 slots fell by 0.2% from 478 in 2020 to 477 in 2021, but 471 slots were filled in 2021, 2 more than last year, for an increase of 0.4%. The overall fill rate was 98.7% in 2021, with 86.6% being filled by U.S. graduates. Just under 88% of filled positions went to MD and DO seniors.
While Match Day 2021 set a record for positions offered and filled at 38,106 slots and 36,179, respectively, an overall total of 2,699 PGY-2 slots were offered in 2021, which is a decrease of 1.6% from last year’s total of 2,742 slots. Of the available PGY-2 slots, 97.6% were filled in 2021, compared with 96.6% in 2020.
“The NRMP is honored to have delivered a strong Match to the many applicants pursuing their dreams of medicine. We admire all the Match participants for their hard work and their commitment to train and serve alongside their peers. The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP President and CEO, said in a press release.
New analysis eyes the surgical landscape for hidradenitis suppurativa
yet these options should be balanced against potentially higher morbidity of extensive procedures.
Those are among the key findings of a systematic review and meta-analysis published online in Dermatologic Surgery.
“There is a major need to better understand the best surgical approaches to HS,” one of the study authors, Christopher Sayed, MD, associate professor of dermatology at the University of North Carolina at Chapel Hill, said in an interview. Previous studies have mostly reviewed outcomes for procedure types in individual cohorts, “but no recent reports have combined and analyzed data from recent studies.”
When Dr. Sayed and colleagues set out to summarize the literature on HS surgery regarding patient characteristics, surgical approaches, and study quality, as well as compare postsurgical recurrence rates, the most recent meta-analysis on postoperative recurrence rates of HS included studies published between 1990 and 2015. “In the past few years, surgical management of HS has become an increasingly popular area of study,” corresponding author Ashley Riddle, MD, MPH, who is currently an internal medicine resident at the Carolinas Medical Center, Charlotte, said in an interview. “We sought to provide an updated picture of the HS surgical landscape by analyzing studies published between 2004 to 2019. We also limited our analysis to studies with follow-up periods of greater than 1 year and included information on disease severity, adverse events, and patient satisfaction when available.”
Of 715 relevant studies identified in the medical literature, the researchers included 59 in the review and 33 in the meta-analysis. Of these 59 studies, 56 were case series, 2 were randomized, controlled trials, and one was a retrospective cohort study.
Of the 50 studies reporting gender and age at time of surgery, 61% of patients were female and their average age was 37 years. Of the 25 studies that reported Hurley scores, 73% had Hurley stage 3 HS. Of the 38 studies reporting the number of procedures per anatomic region, the most commonly operated on regions were the axilla (59%) and the inguinal region (20%).
The researchers found that 22 studies of wide excision had the lowest pooled recurrence rate at 8%, while local excision had the highest pooled recurrence rate at 34%. Meanwhile, among studies of wide/radical excision, flap repair had a pooled recurrence rate of 0%, while delayed primary closure had the highest pooled recurrence rate at 38%.
“Extensive excisions of HS seem to portend a lower risk of postoperative recurrence, but there are many approaches available that may be more appropriate for certain patients,” Dr. Riddle said. “The influence of patient factors such as comorbidities and disease severity on surgical outcomes is unclear and is a potential area of future study.”
Dr. Sayed, an author of the 2019 North American guidelines for the clinical management of HS, pointed out that most studies in the review and meta-analysis included patients who had diabetes, were on biologics or other therapy, were actively smoking, or had other comorbidities that sometimes influence surgeons to delay surgical treatment because they consider it elective. “Most studies indicated minimal or no risk of significant complications relating to these factors, so they should ideally not become obstacles for patients interested in surgical care,” he said.
Dr. Riddle said that she was surprised by how relatively few studies had been published on more conservative surgical approaches such as skin tissue–sparing excision with electrosurgical peeling, deroofing, local excision, and CO2 laser–based evaporation.
The researchers acknowledged certain limitations of their work, including the high risk of bias for most included studies. “Almost all studies were retrospective with substantial methodological limitations, and there were no head-to-head comparisons of different surgical approaches,” Dr. Riddle said. “Patient comorbidities and postoperative complications were variably reported.”
Dr. Sayed disclosed that he is a speaker for AbbVie and Novartis; an investigator for AbbVie, Novartis, InflaRx, and UCB; and on the advisory board of AbbVie and InflaRx. The remaining authors reported having no financial disclosures.
yet these options should be balanced against potentially higher morbidity of extensive procedures.
Those are among the key findings of a systematic review and meta-analysis published online in Dermatologic Surgery.
“There is a major need to better understand the best surgical approaches to HS,” one of the study authors, Christopher Sayed, MD, associate professor of dermatology at the University of North Carolina at Chapel Hill, said in an interview. Previous studies have mostly reviewed outcomes for procedure types in individual cohorts, “but no recent reports have combined and analyzed data from recent studies.”
When Dr. Sayed and colleagues set out to summarize the literature on HS surgery regarding patient characteristics, surgical approaches, and study quality, as well as compare postsurgical recurrence rates, the most recent meta-analysis on postoperative recurrence rates of HS included studies published between 1990 and 2015. “In the past few years, surgical management of HS has become an increasingly popular area of study,” corresponding author Ashley Riddle, MD, MPH, who is currently an internal medicine resident at the Carolinas Medical Center, Charlotte, said in an interview. “We sought to provide an updated picture of the HS surgical landscape by analyzing studies published between 2004 to 2019. We also limited our analysis to studies with follow-up periods of greater than 1 year and included information on disease severity, adverse events, and patient satisfaction when available.”
Of 715 relevant studies identified in the medical literature, the researchers included 59 in the review and 33 in the meta-analysis. Of these 59 studies, 56 were case series, 2 were randomized, controlled trials, and one was a retrospective cohort study.
Of the 50 studies reporting gender and age at time of surgery, 61% of patients were female and their average age was 37 years. Of the 25 studies that reported Hurley scores, 73% had Hurley stage 3 HS. Of the 38 studies reporting the number of procedures per anatomic region, the most commonly operated on regions were the axilla (59%) and the inguinal region (20%).
The researchers found that 22 studies of wide excision had the lowest pooled recurrence rate at 8%, while local excision had the highest pooled recurrence rate at 34%. Meanwhile, among studies of wide/radical excision, flap repair had a pooled recurrence rate of 0%, while delayed primary closure had the highest pooled recurrence rate at 38%.
“Extensive excisions of HS seem to portend a lower risk of postoperative recurrence, but there are many approaches available that may be more appropriate for certain patients,” Dr. Riddle said. “The influence of patient factors such as comorbidities and disease severity on surgical outcomes is unclear and is a potential area of future study.”
Dr. Sayed, an author of the 2019 North American guidelines for the clinical management of HS, pointed out that most studies in the review and meta-analysis included patients who had diabetes, were on biologics or other therapy, were actively smoking, or had other comorbidities that sometimes influence surgeons to delay surgical treatment because they consider it elective. “Most studies indicated minimal or no risk of significant complications relating to these factors, so they should ideally not become obstacles for patients interested in surgical care,” he said.
Dr. Riddle said that she was surprised by how relatively few studies had been published on more conservative surgical approaches such as skin tissue–sparing excision with electrosurgical peeling, deroofing, local excision, and CO2 laser–based evaporation.
The researchers acknowledged certain limitations of their work, including the high risk of bias for most included studies. “Almost all studies were retrospective with substantial methodological limitations, and there were no head-to-head comparisons of different surgical approaches,” Dr. Riddle said. “Patient comorbidities and postoperative complications were variably reported.”
Dr. Sayed disclosed that he is a speaker for AbbVie and Novartis; an investigator for AbbVie, Novartis, InflaRx, and UCB; and on the advisory board of AbbVie and InflaRx. The remaining authors reported having no financial disclosures.
yet these options should be balanced against potentially higher morbidity of extensive procedures.
Those are among the key findings of a systematic review and meta-analysis published online in Dermatologic Surgery.
“There is a major need to better understand the best surgical approaches to HS,” one of the study authors, Christopher Sayed, MD, associate professor of dermatology at the University of North Carolina at Chapel Hill, said in an interview. Previous studies have mostly reviewed outcomes for procedure types in individual cohorts, “but no recent reports have combined and analyzed data from recent studies.”
When Dr. Sayed and colleagues set out to summarize the literature on HS surgery regarding patient characteristics, surgical approaches, and study quality, as well as compare postsurgical recurrence rates, the most recent meta-analysis on postoperative recurrence rates of HS included studies published between 1990 and 2015. “In the past few years, surgical management of HS has become an increasingly popular area of study,” corresponding author Ashley Riddle, MD, MPH, who is currently an internal medicine resident at the Carolinas Medical Center, Charlotte, said in an interview. “We sought to provide an updated picture of the HS surgical landscape by analyzing studies published between 2004 to 2019. We also limited our analysis to studies with follow-up periods of greater than 1 year and included information on disease severity, adverse events, and patient satisfaction when available.”
Of 715 relevant studies identified in the medical literature, the researchers included 59 in the review and 33 in the meta-analysis. Of these 59 studies, 56 were case series, 2 were randomized, controlled trials, and one was a retrospective cohort study.
Of the 50 studies reporting gender and age at time of surgery, 61% of patients were female and their average age was 37 years. Of the 25 studies that reported Hurley scores, 73% had Hurley stage 3 HS. Of the 38 studies reporting the number of procedures per anatomic region, the most commonly operated on regions were the axilla (59%) and the inguinal region (20%).
The researchers found that 22 studies of wide excision had the lowest pooled recurrence rate at 8%, while local excision had the highest pooled recurrence rate at 34%. Meanwhile, among studies of wide/radical excision, flap repair had a pooled recurrence rate of 0%, while delayed primary closure had the highest pooled recurrence rate at 38%.
“Extensive excisions of HS seem to portend a lower risk of postoperative recurrence, but there are many approaches available that may be more appropriate for certain patients,” Dr. Riddle said. “The influence of patient factors such as comorbidities and disease severity on surgical outcomes is unclear and is a potential area of future study.”
Dr. Sayed, an author of the 2019 North American guidelines for the clinical management of HS, pointed out that most studies in the review and meta-analysis included patients who had diabetes, were on biologics or other therapy, were actively smoking, or had other comorbidities that sometimes influence surgeons to delay surgical treatment because they consider it elective. “Most studies indicated minimal or no risk of significant complications relating to these factors, so they should ideally not become obstacles for patients interested in surgical care,” he said.
Dr. Riddle said that she was surprised by how relatively few studies had been published on more conservative surgical approaches such as skin tissue–sparing excision with electrosurgical peeling, deroofing, local excision, and CO2 laser–based evaporation.
The researchers acknowledged certain limitations of their work, including the high risk of bias for most included studies. “Almost all studies were retrospective with substantial methodological limitations, and there were no head-to-head comparisons of different surgical approaches,” Dr. Riddle said. “Patient comorbidities and postoperative complications were variably reported.”
Dr. Sayed disclosed that he is a speaker for AbbVie and Novartis; an investigator for AbbVie, Novartis, InflaRx, and UCB; and on the advisory board of AbbVie and InflaRx. The remaining authors reported having no financial disclosures.
FROM DERMATOLOGIC SURGERY
Prenatal dietary folate not enough to offset AEDs’ effect on kids’ cognition
New research underscores the importance of folic acid supplementation for pregnant women with epilepsy who are taking antiepileptic drugs (AEDs).
Dietary folate alone, even in the United States, where food is fortified with folic acid, is “not sufficient” to improve cognitive outcomes for children of women who take AEDs during pregnancy, the researchers report.
“We found that dietary folate was not related to outcomes,” study investigator Kimford Meador, MD, professor of neurology and neurologic sciences, Stanford (Calif.) University, told this news organization.
“Only when the mother was taking extra folate did we see an improvement in child outcomes,” he added.
The findings were published online Feb. 23 in Epilepsy and Behavior.
Cognitive boost
“Daily folate is recommended to women in the general populations to reduce congenital malformations,” Dr. Meador said. In addition, periconceptional use of folate has been shown in previous research to improve neurodevelopmental outcomes for children of mothers with epilepsy who are taking AEDs.
Whether folate-fortified food alone, without supplements, has any effect on cognitive outcomes in this population of children has not been examined previously.
To investigate, the researchers assessed 117 children from the Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study, a prospective, observational study of women with epilepsy who were taking one of four AEDs: carbamazepine, lamotrigine, phenytoin, or valproate.
Results showed that dietary folate from fortified food alone, without supplements, had no significant impact on IQ at age 6 years among children with prenatal exposure to AEDs.
In contrast, use of periconceptual folate supplements was significantly associated with a 10-point higher IQ at age 6 in the adjusted analyses (95% confidence interval, 5.2-15.0; P < .001).
These six other nutrients from food and supplements had no significant association with IQ at age 6 years: vitamins C, D, and E, omega-3, gamma tocopherol, and vitamin B12.
Optimal dose unclear
The findings indicate that folates, including natural folate and folic acid, in food do not have positive cognitive effects for children of women with epilepsy who take AEDs, the researchers write.
Dr. Meador noted that the optimal dose of folic acid supplementation to provide a cognitive benefit remains unclear.
The U.S. Centers for Disease Control recommends 0.4 mg/d for the general population of women of childbearing age. In Europe, the recommendation is 1 mg/d.
“Higher doses are recommended if there is a personal or family history of spina bifida in prior pregnancies, but there is some concern that very high doses of folate may be detrimental,” Dr. Meador said.
For women with epilepsy, he would recommend “at least 1 mg/d and not more than 4 mg/d.”
Proves a point?
Commenting on the study for this news organization, Derek Chong, MD, vice chair of neurology and director of epilepsy at Lenox Hill Hospital, New York, said the finding that folate fortification of food alone is not adequate for women with epilepsy is “not groundbreaking” but does prove something previously thought.
“Folic acid is important for all women, but it does seem like folic acid may be even more important in the epilepsy population,” said Dr. Chong, who was not involved with the research.
He cautioned that the current analysis included only four medications, three of which are not used very often anymore.
“Lamotrigine is probably the most commonly used one now. It’s unfortunate that this study did not include Keppra [levetiracetam], which probably is the number one medication that we use now,” Dr. Chong said.
The research was supported by the National Institutes of Health. Dr. Meador and Dr. Chong have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New research underscores the importance of folic acid supplementation for pregnant women with epilepsy who are taking antiepileptic drugs (AEDs).
Dietary folate alone, even in the United States, where food is fortified with folic acid, is “not sufficient” to improve cognitive outcomes for children of women who take AEDs during pregnancy, the researchers report.
“We found that dietary folate was not related to outcomes,” study investigator Kimford Meador, MD, professor of neurology and neurologic sciences, Stanford (Calif.) University, told this news organization.
“Only when the mother was taking extra folate did we see an improvement in child outcomes,” he added.
The findings were published online Feb. 23 in Epilepsy and Behavior.
Cognitive boost
“Daily folate is recommended to women in the general populations to reduce congenital malformations,” Dr. Meador said. In addition, periconceptional use of folate has been shown in previous research to improve neurodevelopmental outcomes for children of mothers with epilepsy who are taking AEDs.
Whether folate-fortified food alone, without supplements, has any effect on cognitive outcomes in this population of children has not been examined previously.
To investigate, the researchers assessed 117 children from the Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study, a prospective, observational study of women with epilepsy who were taking one of four AEDs: carbamazepine, lamotrigine, phenytoin, or valproate.
Results showed that dietary folate from fortified food alone, without supplements, had no significant impact on IQ at age 6 years among children with prenatal exposure to AEDs.
In contrast, use of periconceptual folate supplements was significantly associated with a 10-point higher IQ at age 6 in the adjusted analyses (95% confidence interval, 5.2-15.0; P < .001).
These six other nutrients from food and supplements had no significant association with IQ at age 6 years: vitamins C, D, and E, omega-3, gamma tocopherol, and vitamin B12.
Optimal dose unclear
The findings indicate that folates, including natural folate and folic acid, in food do not have positive cognitive effects for children of women with epilepsy who take AEDs, the researchers write.
Dr. Meador noted that the optimal dose of folic acid supplementation to provide a cognitive benefit remains unclear.
The U.S. Centers for Disease Control recommends 0.4 mg/d for the general population of women of childbearing age. In Europe, the recommendation is 1 mg/d.
“Higher doses are recommended if there is a personal or family history of spina bifida in prior pregnancies, but there is some concern that very high doses of folate may be detrimental,” Dr. Meador said.
For women with epilepsy, he would recommend “at least 1 mg/d and not more than 4 mg/d.”
Proves a point?
Commenting on the study for this news organization, Derek Chong, MD, vice chair of neurology and director of epilepsy at Lenox Hill Hospital, New York, said the finding that folate fortification of food alone is not adequate for women with epilepsy is “not groundbreaking” but does prove something previously thought.
“Folic acid is important for all women, but it does seem like folic acid may be even more important in the epilepsy population,” said Dr. Chong, who was not involved with the research.
He cautioned that the current analysis included only four medications, three of which are not used very often anymore.
“Lamotrigine is probably the most commonly used one now. It’s unfortunate that this study did not include Keppra [levetiracetam], which probably is the number one medication that we use now,” Dr. Chong said.
The research was supported by the National Institutes of Health. Dr. Meador and Dr. Chong have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New research underscores the importance of folic acid supplementation for pregnant women with epilepsy who are taking antiepileptic drugs (AEDs).
Dietary folate alone, even in the United States, where food is fortified with folic acid, is “not sufficient” to improve cognitive outcomes for children of women who take AEDs during pregnancy, the researchers report.
“We found that dietary folate was not related to outcomes,” study investigator Kimford Meador, MD, professor of neurology and neurologic sciences, Stanford (Calif.) University, told this news organization.
“Only when the mother was taking extra folate did we see an improvement in child outcomes,” he added.
The findings were published online Feb. 23 in Epilepsy and Behavior.
Cognitive boost
“Daily folate is recommended to women in the general populations to reduce congenital malformations,” Dr. Meador said. In addition, periconceptional use of folate has been shown in previous research to improve neurodevelopmental outcomes for children of mothers with epilepsy who are taking AEDs.
Whether folate-fortified food alone, without supplements, has any effect on cognitive outcomes in this population of children has not been examined previously.
To investigate, the researchers assessed 117 children from the Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study, a prospective, observational study of women with epilepsy who were taking one of four AEDs: carbamazepine, lamotrigine, phenytoin, or valproate.
Results showed that dietary folate from fortified food alone, without supplements, had no significant impact on IQ at age 6 years among children with prenatal exposure to AEDs.
In contrast, use of periconceptual folate supplements was significantly associated with a 10-point higher IQ at age 6 in the adjusted analyses (95% confidence interval, 5.2-15.0; P < .001).
These six other nutrients from food and supplements had no significant association with IQ at age 6 years: vitamins C, D, and E, omega-3, gamma tocopherol, and vitamin B12.
Optimal dose unclear
The findings indicate that folates, including natural folate and folic acid, in food do not have positive cognitive effects for children of women with epilepsy who take AEDs, the researchers write.
Dr. Meador noted that the optimal dose of folic acid supplementation to provide a cognitive benefit remains unclear.
The U.S. Centers for Disease Control recommends 0.4 mg/d for the general population of women of childbearing age. In Europe, the recommendation is 1 mg/d.
“Higher doses are recommended if there is a personal or family history of spina bifida in prior pregnancies, but there is some concern that very high doses of folate may be detrimental,” Dr. Meador said.
For women with epilepsy, he would recommend “at least 1 mg/d and not more than 4 mg/d.”
Proves a point?
Commenting on the study for this news organization, Derek Chong, MD, vice chair of neurology and director of epilepsy at Lenox Hill Hospital, New York, said the finding that folate fortification of food alone is not adequate for women with epilepsy is “not groundbreaking” but does prove something previously thought.
“Folic acid is important for all women, but it does seem like folic acid may be even more important in the epilepsy population,” said Dr. Chong, who was not involved with the research.
He cautioned that the current analysis included only four medications, three of which are not used very often anymore.
“Lamotrigine is probably the most commonly used one now. It’s unfortunate that this study did not include Keppra [levetiracetam], which probably is the number one medication that we use now,” Dr. Chong said.
The research was supported by the National Institutes of Health. Dr. Meador and Dr. Chong have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cannabinoids promising for improving appetite, behavior in dementia
For patients with dementia, cannabinoids may be a promising intervention for treating neuropsychiatric symptoms (NPS) and the refusing of food, new research suggests.
Results of a systematic literature review, presented at the 2021 meeting of the American Association for Geriatric Psychiatry, showed that cannabinoids were associated with reduced agitation, longer sleep, and lower NPS. They were also linked to increased meal consumption and weight gain.
Refusing food is a common problem for patients with dementia, often resulting in worsening sleep, agitation, and mood, study investigator Niraj Asthana, MD, a second-year resident in the department of psychiatry, University of California, San Diego, said in an interview. Dr. Asthana noted that certain cannabinoid analogues are now used to stimulate appetite for patients undergoing chemotherapy.
Filling a treatment gap
After years of legal and other problems affecting cannabinoid research, there is renewed interest in investigating its use for patients with dementia. Early evidence suggests that cannabinoids may also be beneficial for pain, sleep, and aggression.
The researchers noted that cannabinoids may be especially valuable in areas where there are currently limited therapies, including food refusal and NPS.
“Unfortunately, there are limited treatments available for food refusal, so we’re left with appetite stimulants and electroconvulsive therapy, and although atypical antipsychotics are commonly used to treat NPS, they’re associated with an increased risk of serious adverse events and mortality in older patients,” said Dr. Asthana.
Dr. Asthana and colleague Dan Sewell, MD, carried out a systematic literature review of relevant studies of the use of cannabinoids for dementia patients.
“We found there are lot of studies, but they’re small scale; I’d say the largest was probably about 50 patients, with most studies having 10-50 patients,” said Dr. Asthana. In part, this may be because, until very recently, research on cannabinoids was controversial.
To review the current literature on the potential applications of cannabinoids in the treatment of food refusal and NPS in dementia patients, the researchers conducted a literature review.
They identified 23 relevant studies of the use of synthetic cannabinoids, including dronabinol and nabilone, for dementia patients. These products contain tetrahydrocannabinol (THC), the main psychoactive compound in cannabis.
More research coming
Several studies showed that cannabinoid use was associated with reduced nighttime motor activity, improved sleep duration, reduced agitation, and lower Neuropsychiatric Inventory scores.
One crossover placebo-controlled trial showed an overall increase in body weight among dementia patients who took dronabinol.
This suggests there might be something to the “colloquial cultural association between cannabinoids and the munchies,” said Dr. Asthana.
Possible mechanisms for the effects on appetite may be that cannabinoids increase levels of the hormone ghrelin, which is also known as the “hunger hormone,” and decrease leptin levels, a hormone that inhibits hunger. Dr. Asthana noted that, in these studies, the dose of THC was low and that overall, cannabinoids appeared to be safe.
“We found that, at least in these small-scale studies, cannabinoid analogues are well tolerated,” possibly because of the relatively low doses of THC, said Dr. Asthana. “They generally don’t seem to have a ton of side effects; they may make people a little sleepy, which is actually good, because these patents also have a lot of trouble sleeping.”
He noted that more recent research suggests cannabidiol oil may reduce agitation by up to 40%.
“Now that cannabis is losing a lot of its stigma, both culturally and in the scientific community, you’re seeing a lot of grant applications for clinical trials,” said Dr. Asthana. “I’m excited to see what we find in the next 5-10 years.”
In a comment, Kirsten Wilkins, MD, associate professor of psychiatry, Yale University, New Haven, Conn., who is also a geriatric psychiatrist at the Veterans Affairs Connecticut Health Care System, welcomed the new research in this area.
“With limited safe and effective treatments for food refusal and neuropsychiatric symptoms of dementia, Dr. Asthana and Dr. Sewell highlight the growing body of literature suggesting cannabinoids may be a novel treatment option,” she said.
A version of this article first appeared on Medscape.com.
For patients with dementia, cannabinoids may be a promising intervention for treating neuropsychiatric symptoms (NPS) and the refusing of food, new research suggests.
Results of a systematic literature review, presented at the 2021 meeting of the American Association for Geriatric Psychiatry, showed that cannabinoids were associated with reduced agitation, longer sleep, and lower NPS. They were also linked to increased meal consumption and weight gain.
Refusing food is a common problem for patients with dementia, often resulting in worsening sleep, agitation, and mood, study investigator Niraj Asthana, MD, a second-year resident in the department of psychiatry, University of California, San Diego, said in an interview. Dr. Asthana noted that certain cannabinoid analogues are now used to stimulate appetite for patients undergoing chemotherapy.
Filling a treatment gap
After years of legal and other problems affecting cannabinoid research, there is renewed interest in investigating its use for patients with dementia. Early evidence suggests that cannabinoids may also be beneficial for pain, sleep, and aggression.
The researchers noted that cannabinoids may be especially valuable in areas where there are currently limited therapies, including food refusal and NPS.
“Unfortunately, there are limited treatments available for food refusal, so we’re left with appetite stimulants and electroconvulsive therapy, and although atypical antipsychotics are commonly used to treat NPS, they’re associated with an increased risk of serious adverse events and mortality in older patients,” said Dr. Asthana.
Dr. Asthana and colleague Dan Sewell, MD, carried out a systematic literature review of relevant studies of the use of cannabinoids for dementia patients.
“We found there are lot of studies, but they’re small scale; I’d say the largest was probably about 50 patients, with most studies having 10-50 patients,” said Dr. Asthana. In part, this may be because, until very recently, research on cannabinoids was controversial.
To review the current literature on the potential applications of cannabinoids in the treatment of food refusal and NPS in dementia patients, the researchers conducted a literature review.
They identified 23 relevant studies of the use of synthetic cannabinoids, including dronabinol and nabilone, for dementia patients. These products contain tetrahydrocannabinol (THC), the main psychoactive compound in cannabis.
More research coming
Several studies showed that cannabinoid use was associated with reduced nighttime motor activity, improved sleep duration, reduced agitation, and lower Neuropsychiatric Inventory scores.
One crossover placebo-controlled trial showed an overall increase in body weight among dementia patients who took dronabinol.
This suggests there might be something to the “colloquial cultural association between cannabinoids and the munchies,” said Dr. Asthana.
Possible mechanisms for the effects on appetite may be that cannabinoids increase levels of the hormone ghrelin, which is also known as the “hunger hormone,” and decrease leptin levels, a hormone that inhibits hunger. Dr. Asthana noted that, in these studies, the dose of THC was low and that overall, cannabinoids appeared to be safe.
“We found that, at least in these small-scale studies, cannabinoid analogues are well tolerated,” possibly because of the relatively low doses of THC, said Dr. Asthana. “They generally don’t seem to have a ton of side effects; they may make people a little sleepy, which is actually good, because these patents also have a lot of trouble sleeping.”
He noted that more recent research suggests cannabidiol oil may reduce agitation by up to 40%.
“Now that cannabis is losing a lot of its stigma, both culturally and in the scientific community, you’re seeing a lot of grant applications for clinical trials,” said Dr. Asthana. “I’m excited to see what we find in the next 5-10 years.”
In a comment, Kirsten Wilkins, MD, associate professor of psychiatry, Yale University, New Haven, Conn., who is also a geriatric psychiatrist at the Veterans Affairs Connecticut Health Care System, welcomed the new research in this area.
“With limited safe and effective treatments for food refusal and neuropsychiatric symptoms of dementia, Dr. Asthana and Dr. Sewell highlight the growing body of literature suggesting cannabinoids may be a novel treatment option,” she said.
A version of this article first appeared on Medscape.com.
For patients with dementia, cannabinoids may be a promising intervention for treating neuropsychiatric symptoms (NPS) and the refusing of food, new research suggests.
Results of a systematic literature review, presented at the 2021 meeting of the American Association for Geriatric Psychiatry, showed that cannabinoids were associated with reduced agitation, longer sleep, and lower NPS. They were also linked to increased meal consumption and weight gain.
Refusing food is a common problem for patients with dementia, often resulting in worsening sleep, agitation, and mood, study investigator Niraj Asthana, MD, a second-year resident in the department of psychiatry, University of California, San Diego, said in an interview. Dr. Asthana noted that certain cannabinoid analogues are now used to stimulate appetite for patients undergoing chemotherapy.
Filling a treatment gap
After years of legal and other problems affecting cannabinoid research, there is renewed interest in investigating its use for patients with dementia. Early evidence suggests that cannabinoids may also be beneficial for pain, sleep, and aggression.
The researchers noted that cannabinoids may be especially valuable in areas where there are currently limited therapies, including food refusal and NPS.
“Unfortunately, there are limited treatments available for food refusal, so we’re left with appetite stimulants and electroconvulsive therapy, and although atypical antipsychotics are commonly used to treat NPS, they’re associated with an increased risk of serious adverse events and mortality in older patients,” said Dr. Asthana.
Dr. Asthana and colleague Dan Sewell, MD, carried out a systematic literature review of relevant studies of the use of cannabinoids for dementia patients.
“We found there are lot of studies, but they’re small scale; I’d say the largest was probably about 50 patients, with most studies having 10-50 patients,” said Dr. Asthana. In part, this may be because, until very recently, research on cannabinoids was controversial.
To review the current literature on the potential applications of cannabinoids in the treatment of food refusal and NPS in dementia patients, the researchers conducted a literature review.
They identified 23 relevant studies of the use of synthetic cannabinoids, including dronabinol and nabilone, for dementia patients. These products contain tetrahydrocannabinol (THC), the main psychoactive compound in cannabis.
More research coming
Several studies showed that cannabinoid use was associated with reduced nighttime motor activity, improved sleep duration, reduced agitation, and lower Neuropsychiatric Inventory scores.
One crossover placebo-controlled trial showed an overall increase in body weight among dementia patients who took dronabinol.
This suggests there might be something to the “colloquial cultural association between cannabinoids and the munchies,” said Dr. Asthana.
Possible mechanisms for the effects on appetite may be that cannabinoids increase levels of the hormone ghrelin, which is also known as the “hunger hormone,” and decrease leptin levels, a hormone that inhibits hunger. Dr. Asthana noted that, in these studies, the dose of THC was low and that overall, cannabinoids appeared to be safe.
“We found that, at least in these small-scale studies, cannabinoid analogues are well tolerated,” possibly because of the relatively low doses of THC, said Dr. Asthana. “They generally don’t seem to have a ton of side effects; they may make people a little sleepy, which is actually good, because these patents also have a lot of trouble sleeping.”
He noted that more recent research suggests cannabidiol oil may reduce agitation by up to 40%.
“Now that cannabis is losing a lot of its stigma, both culturally and in the scientific community, you’re seeing a lot of grant applications for clinical trials,” said Dr. Asthana. “I’m excited to see what we find in the next 5-10 years.”
In a comment, Kirsten Wilkins, MD, associate professor of psychiatry, Yale University, New Haven, Conn., who is also a geriatric psychiatrist at the Veterans Affairs Connecticut Health Care System, welcomed the new research in this area.
“With limited safe and effective treatments for food refusal and neuropsychiatric symptoms of dementia, Dr. Asthana and Dr. Sewell highlight the growing body of literature suggesting cannabinoids may be a novel treatment option,” she said.
A version of this article first appeared on Medscape.com.
Ruxolitinib cream for atopic dermatitis is in regulatory home stretch
, demonstrated a dual mechanism of action in two pivotal phase 3 trials: antipruritic and anti-inflammatory, Kim A. Papp, MD, PhD, said at Innovations in Dermatology: Virtual Spring Conference 2021. He presented a pooled analysis of the TRuE-AD1 and TRuE-AD2 trials, in which 1,249 patients with AD affecting 3%-20% of the body surface area were randomized 2:2:1 double-blind to ruxolitinib cream 0.75%, 1.5%, or vehicle twice daily for 8 weeks.
Striking evidence of the drug’s antipruritic effect comes from the finding that patient-reported itch scores separated significantly from the vehicle controls within just 12 hours after the first application. The margin of difference grew over time such that at 4 weeks, 48.5% of patients on ruxolitinib 1.5% experienced a clinically meaningful reduction in itch – defined by at least a 4-point improvement on the itch numeric rating scale – as did 30.1% of those on ruxolitinib 0.75% and 6.1% of controls. By week 8, these figures had further improved to 51.5%, 41.5%, and 15.8%, respectively, noted Dr. Papp, a dermatologist and president of Probity Medical Research in Waterloo, Ont.
Ruxolitinib’s anti-inflammatory mechanism of action was on display in the primary study endpoint, which was the proportion of patients achieving an Investigator Global Assessment score of 0 or 1 with at least a 2-grade improvement from baseline at week 8. The rates were 52.6% with ruxolitinib 1.5% and 44.7% at the lower dose, both significantly better than the 11.5% rate with vehicle.
For the secondary endpoint of at least a 75% improvement in Eczema Area and Severity Index score at week 8, the rates were 62% with ruxolitinib 1.5% and 53.8% at the 0.75% concentration, compared with 19.7% with vehicle.
The topical JAK inhibitor also showed superior efficacy in terms of improvement on the Patient-Reported Outcomes Measurement Information System Sleep Disturbance Score, with a clinically meaningful 6-point or greater improvement in 23.9% and 20.9% of patients in the high- and low-dose ruxolitinib groups, versus 14.2% in controls.
Plasma drug levels remained consistently low and near-flat throughout the study.
Session comoderator Lawrence F. Eichenfield, MD, was struck by what he termed the “incredibly low” rates of irritancy, burning, and stinging in the ruxolitinib-treated patients: 7 cases of application-site burning in 999 treated patients, compared with 11 cases in 250 vehicle-treated patients, and 4 cases of application-site pruritus in nearly 1,000 patients on ruxolitinib, versus 6 cases in one-fourth as many controls.
“If that’s really true in clinical practice, it would be tremendous to have a nonsteroid that doesn’t have stinging and burning and may have that efficacy,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice-chair of dermatology at the University of California, San Diego.
“I think the fast action is an exciting aspect of this,” said comoderator Jonathan I. Silverberg, MD, PhD, MBA, director of clinical research and contact dermatitis in the department of dermatology at George Washington University in Washington.
He noted that in an earlier phase 2 study, ruxolitinib cream was at least as efficacious as 0.1% triamcinolone cream, providing dermatologists with a rough yardstick as to where the topical JAK inhibitor lies on the potency spectrum for AD treatment.
The FDA is expected to issue a decision on the application for approval of ruxolitinib cream in June. Dr. Eichenfield expects the drug to easily win approval. The big unanswered question is whether the regulatory agency will require boxed safety warnings, as it does for the oral JAK inhibitors approved for various indications, even though safety issues haven’t arisen with the topical agent in the clinical trials.
Dr. Papp reported receiving research grants from and serving as a consultant to Incyte Corp., which funded the ruxolitinib studies, as well as numerous other pharmaceutical companies. MedscapeLive and this news organization are owned by the same parent company.
, demonstrated a dual mechanism of action in two pivotal phase 3 trials: antipruritic and anti-inflammatory, Kim A. Papp, MD, PhD, said at Innovations in Dermatology: Virtual Spring Conference 2021. He presented a pooled analysis of the TRuE-AD1 and TRuE-AD2 trials, in which 1,249 patients with AD affecting 3%-20% of the body surface area were randomized 2:2:1 double-blind to ruxolitinib cream 0.75%, 1.5%, or vehicle twice daily for 8 weeks.
Striking evidence of the drug’s antipruritic effect comes from the finding that patient-reported itch scores separated significantly from the vehicle controls within just 12 hours after the first application. The margin of difference grew over time such that at 4 weeks, 48.5% of patients on ruxolitinib 1.5% experienced a clinically meaningful reduction in itch – defined by at least a 4-point improvement on the itch numeric rating scale – as did 30.1% of those on ruxolitinib 0.75% and 6.1% of controls. By week 8, these figures had further improved to 51.5%, 41.5%, and 15.8%, respectively, noted Dr. Papp, a dermatologist and president of Probity Medical Research in Waterloo, Ont.
Ruxolitinib’s anti-inflammatory mechanism of action was on display in the primary study endpoint, which was the proportion of patients achieving an Investigator Global Assessment score of 0 or 1 with at least a 2-grade improvement from baseline at week 8. The rates were 52.6% with ruxolitinib 1.5% and 44.7% at the lower dose, both significantly better than the 11.5% rate with vehicle.
For the secondary endpoint of at least a 75% improvement in Eczema Area and Severity Index score at week 8, the rates were 62% with ruxolitinib 1.5% and 53.8% at the 0.75% concentration, compared with 19.7% with vehicle.
The topical JAK inhibitor also showed superior efficacy in terms of improvement on the Patient-Reported Outcomes Measurement Information System Sleep Disturbance Score, with a clinically meaningful 6-point or greater improvement in 23.9% and 20.9% of patients in the high- and low-dose ruxolitinib groups, versus 14.2% in controls.
Plasma drug levels remained consistently low and near-flat throughout the study.
Session comoderator Lawrence F. Eichenfield, MD, was struck by what he termed the “incredibly low” rates of irritancy, burning, and stinging in the ruxolitinib-treated patients: 7 cases of application-site burning in 999 treated patients, compared with 11 cases in 250 vehicle-treated patients, and 4 cases of application-site pruritus in nearly 1,000 patients on ruxolitinib, versus 6 cases in one-fourth as many controls.
“If that’s really true in clinical practice, it would be tremendous to have a nonsteroid that doesn’t have stinging and burning and may have that efficacy,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice-chair of dermatology at the University of California, San Diego.
“I think the fast action is an exciting aspect of this,” said comoderator Jonathan I. Silverberg, MD, PhD, MBA, director of clinical research and contact dermatitis in the department of dermatology at George Washington University in Washington.
He noted that in an earlier phase 2 study, ruxolitinib cream was at least as efficacious as 0.1% triamcinolone cream, providing dermatologists with a rough yardstick as to where the topical JAK inhibitor lies on the potency spectrum for AD treatment.
The FDA is expected to issue a decision on the application for approval of ruxolitinib cream in June. Dr. Eichenfield expects the drug to easily win approval. The big unanswered question is whether the regulatory agency will require boxed safety warnings, as it does for the oral JAK inhibitors approved for various indications, even though safety issues haven’t arisen with the topical agent in the clinical trials.
Dr. Papp reported receiving research grants from and serving as a consultant to Incyte Corp., which funded the ruxolitinib studies, as well as numerous other pharmaceutical companies. MedscapeLive and this news organization are owned by the same parent company.
, demonstrated a dual mechanism of action in two pivotal phase 3 trials: antipruritic and anti-inflammatory, Kim A. Papp, MD, PhD, said at Innovations in Dermatology: Virtual Spring Conference 2021. He presented a pooled analysis of the TRuE-AD1 and TRuE-AD2 trials, in which 1,249 patients with AD affecting 3%-20% of the body surface area were randomized 2:2:1 double-blind to ruxolitinib cream 0.75%, 1.5%, or vehicle twice daily for 8 weeks.
Striking evidence of the drug’s antipruritic effect comes from the finding that patient-reported itch scores separated significantly from the vehicle controls within just 12 hours after the first application. The margin of difference grew over time such that at 4 weeks, 48.5% of patients on ruxolitinib 1.5% experienced a clinically meaningful reduction in itch – defined by at least a 4-point improvement on the itch numeric rating scale – as did 30.1% of those on ruxolitinib 0.75% and 6.1% of controls. By week 8, these figures had further improved to 51.5%, 41.5%, and 15.8%, respectively, noted Dr. Papp, a dermatologist and president of Probity Medical Research in Waterloo, Ont.
Ruxolitinib’s anti-inflammatory mechanism of action was on display in the primary study endpoint, which was the proportion of patients achieving an Investigator Global Assessment score of 0 or 1 with at least a 2-grade improvement from baseline at week 8. The rates were 52.6% with ruxolitinib 1.5% and 44.7% at the lower dose, both significantly better than the 11.5% rate with vehicle.
For the secondary endpoint of at least a 75% improvement in Eczema Area and Severity Index score at week 8, the rates were 62% with ruxolitinib 1.5% and 53.8% at the 0.75% concentration, compared with 19.7% with vehicle.
The topical JAK inhibitor also showed superior efficacy in terms of improvement on the Patient-Reported Outcomes Measurement Information System Sleep Disturbance Score, with a clinically meaningful 6-point or greater improvement in 23.9% and 20.9% of patients in the high- and low-dose ruxolitinib groups, versus 14.2% in controls.
Plasma drug levels remained consistently low and near-flat throughout the study.
Session comoderator Lawrence F. Eichenfield, MD, was struck by what he termed the “incredibly low” rates of irritancy, burning, and stinging in the ruxolitinib-treated patients: 7 cases of application-site burning in 999 treated patients, compared with 11 cases in 250 vehicle-treated patients, and 4 cases of application-site pruritus in nearly 1,000 patients on ruxolitinib, versus 6 cases in one-fourth as many controls.
“If that’s really true in clinical practice, it would be tremendous to have a nonsteroid that doesn’t have stinging and burning and may have that efficacy,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice-chair of dermatology at the University of California, San Diego.
“I think the fast action is an exciting aspect of this,” said comoderator Jonathan I. Silverberg, MD, PhD, MBA, director of clinical research and contact dermatitis in the department of dermatology at George Washington University in Washington.
He noted that in an earlier phase 2 study, ruxolitinib cream was at least as efficacious as 0.1% triamcinolone cream, providing dermatologists with a rough yardstick as to where the topical JAK inhibitor lies on the potency spectrum for AD treatment.
The FDA is expected to issue a decision on the application for approval of ruxolitinib cream in June. Dr. Eichenfield expects the drug to easily win approval. The big unanswered question is whether the regulatory agency will require boxed safety warnings, as it does for the oral JAK inhibitors approved for various indications, even though safety issues haven’t arisen with the topical agent in the clinical trials.
Dr. Papp reported receiving research grants from and serving as a consultant to Incyte Corp., which funded the ruxolitinib studies, as well as numerous other pharmaceutical companies. MedscapeLive and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
THC persists in breast milk 6 weeks after quitting cannabis
Delta-9-Tetrahydrocannabinol (THC), the main psychoactive component of cannabis, remains detectable in breast milk even after weeks of abstinence, new data show. The estimated half-life of THC in breast milk is 17 days, according to the study results, with a projected time to elimination of more than 6 weeks. The clinical importance of the remaining THC is up for debate, according to some experts.
“To limit THC effects on fetal brain development and promote safe breastfeeding, it is critical to emphasize marijuana abstention both early in pregnancy and post partum,” Erica M. Wymore, MD, MPH, an assistant professor of pediatrics and neonatology at the University of Colorado at Denver, Aurora, and colleagues wrote. The group published their results online March 8, 2021, in JAMA Pediatrics.
And while the study was a pharmacokinetic analysis rather than a safety investigation, Dr. Wymore said in an interview that the detectable levels of THC suggest any use is of concern and no safety thresholds have been established. “We wish we had more data on the potential effects on the neurocognitive development of children, but for now we must discourage any use in prepregnancy, pregnancy, and breastfeeding, as our national guidelines recommend.”
Therefore, the findings support current guidelines discouraging any cannabis use in mothers-to-be and breast-feeding mothers issued by national organizations, including those from the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, and the Academy of Breastfeeding Medicine.
Furthermore, the difficulties many mothers face in abstaining from marijuana, a commonly used drug in pregnancy, and the persistence of THC in maternal milk led the authors to question the feasibility of having women who use marijuana simply discard their breast milk until THC is cleared.
“We report challenges in abstention and prolonged excretion of THC in breast milk greater than 6 weeks among women with prenatal marijuana use,” they wrote. “These findings make the recommendations for mothers to discard breast milk until THC is undetectable unrealistic for mothers committed to breastfeeding.”
However, not all experts are equally concerned about low THC concentrations in breast milk. Neonatal pharmacologist Thomas R. Hale, PhD, a professor of pediatrics at Texas Tech University, Lubbock, said a previous study by his group showed that THC levels in maternal milk peaked within 60 minutes of a moderate dose of inhaled marijuana and fell to quite low levels over the next 4 hours. The highest concentration in maternal milk occurred shortly after the peak in plasma.
“So you can see that, just because a mom is drug screen positive, the clinical dose transferred to the infant is probably exceedingly low,” he said in an interview.
Dr. Hale also stressed that judgments about drugs in this context should weigh the risk of the drug against the risk of not breastfeeding. “All of us caution women not to use cannabis when pregnant or breastfeeding,” Dr. Hale said. “But when the decision has to be made as to whether a mom breastfeeds or not if she is drug screen positive, a lot of other factors must be analyzed to make such a decision.”
Study cohort
For the study, Dr. Wymore and colleagues screened 394 women who gave birth between Nov. 1, 2016, and June 30, 2019. Of those, 25 women, with a median age of 26 years, were eligible and enrolled. Inclusion criteria included known prenatal marijuana use, intention to breastfeed, and self-reported abstinence. Prenatal use primarily involved inhaling cannabis more than twice a week.
Of the 25 enrolled mothers, 12 who self-reported marijuana abstinence were in fact found to be abstinent according to the results of plasma analysis. Those who continued to use the substance were younger than the overall sample, with a median age of 21, and were less likely to have attended college (23%) than abstainers (58%).
The researchers prospectively collected data on self-reported marijuana usage and paired maternal plasma and breast milk samples several times a week. All participants had detectable THC in breast milk throughout the study. Initial median THC concentrations were 3.2 ng/mL (interquartile range, 1.2-6.8) within the first week after delivery. These increased to 5.5 ng/mL (IQR, 4.4-16.0) at 2 weeks and declined to 1.9 ng/mL (IQR, 1.1-4.3) at 6 weeks. In terms of ratio, the milk:plasma partition coefficient for THC was approximately 6:1 (IQR, 3.8:1-8.1:1).
Dr. Hale noted that, although THC was detectable in milk, the levels were exceedingly low. “This is where the risk assessment comes in. There’s a lot of hysteria in the cannabis field right now, and we’re going to need time and a lot more studies to really be able to predict any untoward complications.”
Dr. Wymore, however, countered that THC levels were low only in those who abstained and that her concerns relate not just to postpartum breast milk levels but the health effects on children of mothers’ cannabis use over the course of prepregnancy, pregnancy, and lactation. “[Dr. Hale’s] message makes it difficult for clinicians to counsel mothers since it goes against national guidelines,” she said. “We need to be consistent.”
But Dr. Wymore and other experts acknowledge the dilemma faced in that breast milk clearly offers substantial benefits for infant and child health. “The risks of an infant’s exposure to marijuana versus the benefit of breast milk must be considered,” said Amy B. Hair, MD, assistant professor of pediatrics and neonatal medicine at Baylor College of Medicine, Houston, who was not involved in the Colorado study. “And it’s unrealistic, as the study suggests, for mothers to discard breast milk for 6 weeks.”
Nevertheless, calling the findings of THC persistence after abstinence “troublesome,” Dr. Hair said the legalization of marijuana in some states gives the public the impression it’s safe to use marijuana even during pregnancy and lactation. “Research studies, however, are concerning for potential detrimental effects on brain growth and development in infants whose mothers use marijuana during pregnancy and breastfeeding,” she added.
Dr. Wymore stressed that more U.S. cannabis dispensaries must engage in rigorous point-of-sale counseling to women on the potential harms during pregnancy. This is the case in Canada, she noted, where recreational and medicinal cannabis has been legal since 2018 and more than 90% of outlets (vs. two thirds of their U.S. counterparts) advise women not to use cannabis during pregnancy or lactation, even for nausea.
“This is where many women are getting their information on cannabis,” she said. “We learned the hard way with alcohol and we don’t want to make the same mistake with marijuana.”
The study was funded by the Colorado Department of Public Health and Environment, the Children’s Hospital Colorado Research Institute, the Colorado Fetal Care Center, the Colorado Perinatal Clinical and Translational Research Center, and the Children’s Colorado Research Institute. Two study coauthors disclosed relationships with the private sector outside the submitted work. Dr. Hale and Dr. Hair have disclosed no competing interests with regard to their comments.
A version of this article first appeared on Medscape.com.
Delta-9-Tetrahydrocannabinol (THC), the main psychoactive component of cannabis, remains detectable in breast milk even after weeks of abstinence, new data show. The estimated half-life of THC in breast milk is 17 days, according to the study results, with a projected time to elimination of more than 6 weeks. The clinical importance of the remaining THC is up for debate, according to some experts.
“To limit THC effects on fetal brain development and promote safe breastfeeding, it is critical to emphasize marijuana abstention both early in pregnancy and post partum,” Erica M. Wymore, MD, MPH, an assistant professor of pediatrics and neonatology at the University of Colorado at Denver, Aurora, and colleagues wrote. The group published their results online March 8, 2021, in JAMA Pediatrics.
And while the study was a pharmacokinetic analysis rather than a safety investigation, Dr. Wymore said in an interview that the detectable levels of THC suggest any use is of concern and no safety thresholds have been established. “We wish we had more data on the potential effects on the neurocognitive development of children, but for now we must discourage any use in prepregnancy, pregnancy, and breastfeeding, as our national guidelines recommend.”
Therefore, the findings support current guidelines discouraging any cannabis use in mothers-to-be and breast-feeding mothers issued by national organizations, including those from the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, and the Academy of Breastfeeding Medicine.
Furthermore, the difficulties many mothers face in abstaining from marijuana, a commonly used drug in pregnancy, and the persistence of THC in maternal milk led the authors to question the feasibility of having women who use marijuana simply discard their breast milk until THC is cleared.
“We report challenges in abstention and prolonged excretion of THC in breast milk greater than 6 weeks among women with prenatal marijuana use,” they wrote. “These findings make the recommendations for mothers to discard breast milk until THC is undetectable unrealistic for mothers committed to breastfeeding.”
However, not all experts are equally concerned about low THC concentrations in breast milk. Neonatal pharmacologist Thomas R. Hale, PhD, a professor of pediatrics at Texas Tech University, Lubbock, said a previous study by his group showed that THC levels in maternal milk peaked within 60 minutes of a moderate dose of inhaled marijuana and fell to quite low levels over the next 4 hours. The highest concentration in maternal milk occurred shortly after the peak in plasma.
“So you can see that, just because a mom is drug screen positive, the clinical dose transferred to the infant is probably exceedingly low,” he said in an interview.
Dr. Hale also stressed that judgments about drugs in this context should weigh the risk of the drug against the risk of not breastfeeding. “All of us caution women not to use cannabis when pregnant or breastfeeding,” Dr. Hale said. “But when the decision has to be made as to whether a mom breastfeeds or not if she is drug screen positive, a lot of other factors must be analyzed to make such a decision.”
Study cohort
For the study, Dr. Wymore and colleagues screened 394 women who gave birth between Nov. 1, 2016, and June 30, 2019. Of those, 25 women, with a median age of 26 years, were eligible and enrolled. Inclusion criteria included known prenatal marijuana use, intention to breastfeed, and self-reported abstinence. Prenatal use primarily involved inhaling cannabis more than twice a week.
Of the 25 enrolled mothers, 12 who self-reported marijuana abstinence were in fact found to be abstinent according to the results of plasma analysis. Those who continued to use the substance were younger than the overall sample, with a median age of 21, and were less likely to have attended college (23%) than abstainers (58%).
The researchers prospectively collected data on self-reported marijuana usage and paired maternal plasma and breast milk samples several times a week. All participants had detectable THC in breast milk throughout the study. Initial median THC concentrations were 3.2 ng/mL (interquartile range, 1.2-6.8) within the first week after delivery. These increased to 5.5 ng/mL (IQR, 4.4-16.0) at 2 weeks and declined to 1.9 ng/mL (IQR, 1.1-4.3) at 6 weeks. In terms of ratio, the milk:plasma partition coefficient for THC was approximately 6:1 (IQR, 3.8:1-8.1:1).
Dr. Hale noted that, although THC was detectable in milk, the levels were exceedingly low. “This is where the risk assessment comes in. There’s a lot of hysteria in the cannabis field right now, and we’re going to need time and a lot more studies to really be able to predict any untoward complications.”
Dr. Wymore, however, countered that THC levels were low only in those who abstained and that her concerns relate not just to postpartum breast milk levels but the health effects on children of mothers’ cannabis use over the course of prepregnancy, pregnancy, and lactation. “[Dr. Hale’s] message makes it difficult for clinicians to counsel mothers since it goes against national guidelines,” she said. “We need to be consistent.”
But Dr. Wymore and other experts acknowledge the dilemma faced in that breast milk clearly offers substantial benefits for infant and child health. “The risks of an infant’s exposure to marijuana versus the benefit of breast milk must be considered,” said Amy B. Hair, MD, assistant professor of pediatrics and neonatal medicine at Baylor College of Medicine, Houston, who was not involved in the Colorado study. “And it’s unrealistic, as the study suggests, for mothers to discard breast milk for 6 weeks.”
Nevertheless, calling the findings of THC persistence after abstinence “troublesome,” Dr. Hair said the legalization of marijuana in some states gives the public the impression it’s safe to use marijuana even during pregnancy and lactation. “Research studies, however, are concerning for potential detrimental effects on brain growth and development in infants whose mothers use marijuana during pregnancy and breastfeeding,” she added.
Dr. Wymore stressed that more U.S. cannabis dispensaries must engage in rigorous point-of-sale counseling to women on the potential harms during pregnancy. This is the case in Canada, she noted, where recreational and medicinal cannabis has been legal since 2018 and more than 90% of outlets (vs. two thirds of their U.S. counterparts) advise women not to use cannabis during pregnancy or lactation, even for nausea.
“This is where many women are getting their information on cannabis,” she said. “We learned the hard way with alcohol and we don’t want to make the same mistake with marijuana.”
The study was funded by the Colorado Department of Public Health and Environment, the Children’s Hospital Colorado Research Institute, the Colorado Fetal Care Center, the Colorado Perinatal Clinical and Translational Research Center, and the Children’s Colorado Research Institute. Two study coauthors disclosed relationships with the private sector outside the submitted work. Dr. Hale and Dr. Hair have disclosed no competing interests with regard to their comments.
A version of this article first appeared on Medscape.com.
Delta-9-Tetrahydrocannabinol (THC), the main psychoactive component of cannabis, remains detectable in breast milk even after weeks of abstinence, new data show. The estimated half-life of THC in breast milk is 17 days, according to the study results, with a projected time to elimination of more than 6 weeks. The clinical importance of the remaining THC is up for debate, according to some experts.
“To limit THC effects on fetal brain development and promote safe breastfeeding, it is critical to emphasize marijuana abstention both early in pregnancy and post partum,” Erica M. Wymore, MD, MPH, an assistant professor of pediatrics and neonatology at the University of Colorado at Denver, Aurora, and colleagues wrote. The group published their results online March 8, 2021, in JAMA Pediatrics.
And while the study was a pharmacokinetic analysis rather than a safety investigation, Dr. Wymore said in an interview that the detectable levels of THC suggest any use is of concern and no safety thresholds have been established. “We wish we had more data on the potential effects on the neurocognitive development of children, but for now we must discourage any use in prepregnancy, pregnancy, and breastfeeding, as our national guidelines recommend.”
Therefore, the findings support current guidelines discouraging any cannabis use in mothers-to-be and breast-feeding mothers issued by national organizations, including those from the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, and the Academy of Breastfeeding Medicine.
Furthermore, the difficulties many mothers face in abstaining from marijuana, a commonly used drug in pregnancy, and the persistence of THC in maternal milk led the authors to question the feasibility of having women who use marijuana simply discard their breast milk until THC is cleared.
“We report challenges in abstention and prolonged excretion of THC in breast milk greater than 6 weeks among women with prenatal marijuana use,” they wrote. “These findings make the recommendations for mothers to discard breast milk until THC is undetectable unrealistic for mothers committed to breastfeeding.”
However, not all experts are equally concerned about low THC concentrations in breast milk. Neonatal pharmacologist Thomas R. Hale, PhD, a professor of pediatrics at Texas Tech University, Lubbock, said a previous study by his group showed that THC levels in maternal milk peaked within 60 minutes of a moderate dose of inhaled marijuana and fell to quite low levels over the next 4 hours. The highest concentration in maternal milk occurred shortly after the peak in plasma.
“So you can see that, just because a mom is drug screen positive, the clinical dose transferred to the infant is probably exceedingly low,” he said in an interview.
Dr. Hale also stressed that judgments about drugs in this context should weigh the risk of the drug against the risk of not breastfeeding. “All of us caution women not to use cannabis when pregnant or breastfeeding,” Dr. Hale said. “But when the decision has to be made as to whether a mom breastfeeds or not if she is drug screen positive, a lot of other factors must be analyzed to make such a decision.”
Study cohort
For the study, Dr. Wymore and colleagues screened 394 women who gave birth between Nov. 1, 2016, and June 30, 2019. Of those, 25 women, with a median age of 26 years, were eligible and enrolled. Inclusion criteria included known prenatal marijuana use, intention to breastfeed, and self-reported abstinence. Prenatal use primarily involved inhaling cannabis more than twice a week.
Of the 25 enrolled mothers, 12 who self-reported marijuana abstinence were in fact found to be abstinent according to the results of plasma analysis. Those who continued to use the substance were younger than the overall sample, with a median age of 21, and were less likely to have attended college (23%) than abstainers (58%).
The researchers prospectively collected data on self-reported marijuana usage and paired maternal plasma and breast milk samples several times a week. All participants had detectable THC in breast milk throughout the study. Initial median THC concentrations were 3.2 ng/mL (interquartile range, 1.2-6.8) within the first week after delivery. These increased to 5.5 ng/mL (IQR, 4.4-16.0) at 2 weeks and declined to 1.9 ng/mL (IQR, 1.1-4.3) at 6 weeks. In terms of ratio, the milk:plasma partition coefficient for THC was approximately 6:1 (IQR, 3.8:1-8.1:1).
Dr. Hale noted that, although THC was detectable in milk, the levels were exceedingly low. “This is where the risk assessment comes in. There’s a lot of hysteria in the cannabis field right now, and we’re going to need time and a lot more studies to really be able to predict any untoward complications.”
Dr. Wymore, however, countered that THC levels were low only in those who abstained and that her concerns relate not just to postpartum breast milk levels but the health effects on children of mothers’ cannabis use over the course of prepregnancy, pregnancy, and lactation. “[Dr. Hale’s] message makes it difficult for clinicians to counsel mothers since it goes against national guidelines,” she said. “We need to be consistent.”
But Dr. Wymore and other experts acknowledge the dilemma faced in that breast milk clearly offers substantial benefits for infant and child health. “The risks of an infant’s exposure to marijuana versus the benefit of breast milk must be considered,” said Amy B. Hair, MD, assistant professor of pediatrics and neonatal medicine at Baylor College of Medicine, Houston, who was not involved in the Colorado study. “And it’s unrealistic, as the study suggests, for mothers to discard breast milk for 6 weeks.”
Nevertheless, calling the findings of THC persistence after abstinence “troublesome,” Dr. Hair said the legalization of marijuana in some states gives the public the impression it’s safe to use marijuana even during pregnancy and lactation. “Research studies, however, are concerning for potential detrimental effects on brain growth and development in infants whose mothers use marijuana during pregnancy and breastfeeding,” she added.
Dr. Wymore stressed that more U.S. cannabis dispensaries must engage in rigorous point-of-sale counseling to women on the potential harms during pregnancy. This is the case in Canada, she noted, where recreational and medicinal cannabis has been legal since 2018 and more than 90% of outlets (vs. two thirds of their U.S. counterparts) advise women not to use cannabis during pregnancy or lactation, even for nausea.
“This is where many women are getting their information on cannabis,” she said. “We learned the hard way with alcohol and we don’t want to make the same mistake with marijuana.”
The study was funded by the Colorado Department of Public Health and Environment, the Children’s Hospital Colorado Research Institute, the Colorado Fetal Care Center, the Colorado Perinatal Clinical and Translational Research Center, and the Children’s Colorado Research Institute. Two study coauthors disclosed relationships with the private sector outside the submitted work. Dr. Hale and Dr. Hair have disclosed no competing interests with regard to their comments.
A version of this article first appeared on Medscape.com.
Direct transfer to angiography improves outcome in large-vessel stroke
in a new study.
Results of the ANGIO-CAT trial were presented at the International Stroke Conference sponsored by the American Heart Association.
The study involved patients suspected of having a large-vessel occlusion, as assessed in the prehospital setting by paramedics using the Rapid Arterial Occlusion Evaluation (RACE) score.
In his presentation, Manuel Requena, PhD, a neurologist and neurointerventionalist fellow at Vall d’Hebron Hospital, Barcelona, explained that, if patients were within 6 hours of symptom onset with a RACE scale score greater than 4, paramedics called ahead to a stroke neurologist, who met the patient directly at the hospital.
If on clinical examination the National Institutes of Health Stroke Scale (NIHSS) score was greater than 10, patients could be enrolled into the study. Upon enrollment, they were randomly assigned either to be taken directly to the angiography suite or to receive standard care.
Bypassing the emergency department
Dr. Requena noted that, at his center, patients who receive standard care are transferred to the CT imaging suite, where they are evaluated with noncontrast CT and CT angiography. CT perfusion is also performed if the treating physician deems it necessary.
If a large-vessel occlusion is confirmed, patients are then transferred to the angiography suite for endovascular treatment. He added that in many centers, patients are evaluated in the ED before undergoing CT scanning.
Patients in the direct angiography group received a “flat-panel” noncontrast CT in the angiography suite to rule out intracranial hemorrhage or a large, established infarct. The large-vessel occlusion would be confirmed by arteriography before the endovascular procedure was performed.
After CT scanning, patients received thrombolysis as recommended in the guidelines.
The current interim analysis includes the 174 patients who have been enrolled so far in the study. The median RACE score for these patients was 7, and the median NIHSS score was 17. Large-vessel occlusion was confirmed in 84% of patients, and 8% had an intracerebral hemorrhage.
Results showed that of the 147 patients who received endovascular therapy, puncture time was shorter for those who were taken directly to angiography (median, 18 min vs. 42 min), as was time to reperfusion (median, 57 min vs. 84 min).
The primary outcome was a shift analysis of the Modified Rankin Scale functional outcome scale at 90 days (odds of 1-point improvement or more). In the direct angiography group, the adjusted odds ratio for an improved functional outcome was 2.2 (95% confidence interval, 1.2-.1).
There were no significant differences in safety endpoints. There was a trend toward more procedural complications in those receiving endovascular therapy in the direct angiography group (8.1% vs. 2.7%; P = .6), but there was also a trend toward lower 90-day mortality in this group (20.2% vs. 32.9%; P = .07)
Dr. Requena reported no significant difference in safety outcomes among those with a hemorrhagic stroke.
“Our study is the first clinical trial that shows the superiority of direct transfer to an angiography suite,” said Dr. Requena. “Our findings were close to what we expected, and we were surprised that they occurred so early in the study. We trust that they will be confirmed in ongoing, multicenter, international trials.”
Stroke patients who were transferred directly to an angiography suite were also less likely to be dependent on assistance with daily activities than were those who received the current standard of care, Dr. Requena said. “More frequent and more rapid treatment can help improve outcomes for our stroke patients.”
A limitation of this study is that the hospital had extensive experience with immediate angiography, so findings may differ at hospitals or care centers with less angiography expertise or experience, Dr. Requena said.
He added that retrospective studies conducted in hospitals in the United States, Germany, and Switzerland show that this kind of protocol can be developed in any high-volume stroke center, although multicenter, international trials are needed.
The cost of speed
Commenting on the ANGIO-CAT study, Michael Hill, MD, a professor at the University of Calgary (Alta.), said the 27-minute improvement in door-to-reperfusion time achieved in the study was meaningful and correlates with the degree of improved outcomes observed. “So, the improvement in speed of treatment resulting in better outcomes makes sense,” he added.
He cautioned that this strategy would only be feasible in certain centers with selected patients and that cost will be a fundamental issue.
“If you identify patients at angiography, you risk having some patients with no target large-vessel occlusion,” Dr. Hill added. “The real question is, how many of these patients without a large-vessel occlusion can the system tolerate before it becomes uneconomical and not fruitful or harmful, given that groin puncture is not totally harmless?”
The moderator of the ISC news conference on the study, Mitchell Elkind, MD, professor of neurology at Columbia University, New York, who is also president of the American Stroke Association, said the study reflects the growing recognition of the importance of speed when treating stroke. “If we can shorten time to treatment using rapid evaluation and imaging protocols, this will help save brain,” he said.
Also commenting on the study, Louisa McCullough, MD, PhD, chief of neurology at Memorial Hermann Hospital–Texas Medical Center, Houston, who is the ISC meeting chair, said she thought the study would be relevant to the United States. “Speed is really of the essence. Whenever we can reduce delays, that will make a big difference to patients.”
Referring to this study on improving hospital systems, as well as a second study that was presented at the meeting that showed benefits from delivery of prehospital thrombolysis via a mobile stroke unit, Dr. McCullough added that “we need to set up models so we can get the best of both these worlds. These studies are really leading the way on how we can change the stroke systems of care.”
The study was funded by Vall d’Hebron Research Institute. Dr. Requena disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in a new study.
Results of the ANGIO-CAT trial were presented at the International Stroke Conference sponsored by the American Heart Association.
The study involved patients suspected of having a large-vessel occlusion, as assessed in the prehospital setting by paramedics using the Rapid Arterial Occlusion Evaluation (RACE) score.
In his presentation, Manuel Requena, PhD, a neurologist and neurointerventionalist fellow at Vall d’Hebron Hospital, Barcelona, explained that, if patients were within 6 hours of symptom onset with a RACE scale score greater than 4, paramedics called ahead to a stroke neurologist, who met the patient directly at the hospital.
If on clinical examination the National Institutes of Health Stroke Scale (NIHSS) score was greater than 10, patients could be enrolled into the study. Upon enrollment, they were randomly assigned either to be taken directly to the angiography suite or to receive standard care.
Bypassing the emergency department
Dr. Requena noted that, at his center, patients who receive standard care are transferred to the CT imaging suite, where they are evaluated with noncontrast CT and CT angiography. CT perfusion is also performed if the treating physician deems it necessary.
If a large-vessel occlusion is confirmed, patients are then transferred to the angiography suite for endovascular treatment. He added that in many centers, patients are evaluated in the ED before undergoing CT scanning.
Patients in the direct angiography group received a “flat-panel” noncontrast CT in the angiography suite to rule out intracranial hemorrhage or a large, established infarct. The large-vessel occlusion would be confirmed by arteriography before the endovascular procedure was performed.
After CT scanning, patients received thrombolysis as recommended in the guidelines.
The current interim analysis includes the 174 patients who have been enrolled so far in the study. The median RACE score for these patients was 7, and the median NIHSS score was 17. Large-vessel occlusion was confirmed in 84% of patients, and 8% had an intracerebral hemorrhage.
Results showed that of the 147 patients who received endovascular therapy, puncture time was shorter for those who were taken directly to angiography (median, 18 min vs. 42 min), as was time to reperfusion (median, 57 min vs. 84 min).
The primary outcome was a shift analysis of the Modified Rankin Scale functional outcome scale at 90 days (odds of 1-point improvement or more). In the direct angiography group, the adjusted odds ratio for an improved functional outcome was 2.2 (95% confidence interval, 1.2-.1).
There were no significant differences in safety endpoints. There was a trend toward more procedural complications in those receiving endovascular therapy in the direct angiography group (8.1% vs. 2.7%; P = .6), but there was also a trend toward lower 90-day mortality in this group (20.2% vs. 32.9%; P = .07)
Dr. Requena reported no significant difference in safety outcomes among those with a hemorrhagic stroke.
“Our study is the first clinical trial that shows the superiority of direct transfer to an angiography suite,” said Dr. Requena. “Our findings were close to what we expected, and we were surprised that they occurred so early in the study. We trust that they will be confirmed in ongoing, multicenter, international trials.”
Stroke patients who were transferred directly to an angiography suite were also less likely to be dependent on assistance with daily activities than were those who received the current standard of care, Dr. Requena said. “More frequent and more rapid treatment can help improve outcomes for our stroke patients.”
A limitation of this study is that the hospital had extensive experience with immediate angiography, so findings may differ at hospitals or care centers with less angiography expertise or experience, Dr. Requena said.
He added that retrospective studies conducted in hospitals in the United States, Germany, and Switzerland show that this kind of protocol can be developed in any high-volume stroke center, although multicenter, international trials are needed.
The cost of speed
Commenting on the ANGIO-CAT study, Michael Hill, MD, a professor at the University of Calgary (Alta.), said the 27-minute improvement in door-to-reperfusion time achieved in the study was meaningful and correlates with the degree of improved outcomes observed. “So, the improvement in speed of treatment resulting in better outcomes makes sense,” he added.
He cautioned that this strategy would only be feasible in certain centers with selected patients and that cost will be a fundamental issue.
“If you identify patients at angiography, you risk having some patients with no target large-vessel occlusion,” Dr. Hill added. “The real question is, how many of these patients without a large-vessel occlusion can the system tolerate before it becomes uneconomical and not fruitful or harmful, given that groin puncture is not totally harmless?”
The moderator of the ISC news conference on the study, Mitchell Elkind, MD, professor of neurology at Columbia University, New York, who is also president of the American Stroke Association, said the study reflects the growing recognition of the importance of speed when treating stroke. “If we can shorten time to treatment using rapid evaluation and imaging protocols, this will help save brain,” he said.
Also commenting on the study, Louisa McCullough, MD, PhD, chief of neurology at Memorial Hermann Hospital–Texas Medical Center, Houston, who is the ISC meeting chair, said she thought the study would be relevant to the United States. “Speed is really of the essence. Whenever we can reduce delays, that will make a big difference to patients.”
Referring to this study on improving hospital systems, as well as a second study that was presented at the meeting that showed benefits from delivery of prehospital thrombolysis via a mobile stroke unit, Dr. McCullough added that “we need to set up models so we can get the best of both these worlds. These studies are really leading the way on how we can change the stroke systems of care.”
The study was funded by Vall d’Hebron Research Institute. Dr. Requena disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in a new study.
Results of the ANGIO-CAT trial were presented at the International Stroke Conference sponsored by the American Heart Association.
The study involved patients suspected of having a large-vessel occlusion, as assessed in the prehospital setting by paramedics using the Rapid Arterial Occlusion Evaluation (RACE) score.
In his presentation, Manuel Requena, PhD, a neurologist and neurointerventionalist fellow at Vall d’Hebron Hospital, Barcelona, explained that, if patients were within 6 hours of symptom onset with a RACE scale score greater than 4, paramedics called ahead to a stroke neurologist, who met the patient directly at the hospital.
If on clinical examination the National Institutes of Health Stroke Scale (NIHSS) score was greater than 10, patients could be enrolled into the study. Upon enrollment, they were randomly assigned either to be taken directly to the angiography suite or to receive standard care.
Bypassing the emergency department
Dr. Requena noted that, at his center, patients who receive standard care are transferred to the CT imaging suite, where they are evaluated with noncontrast CT and CT angiography. CT perfusion is also performed if the treating physician deems it necessary.
If a large-vessel occlusion is confirmed, patients are then transferred to the angiography suite for endovascular treatment. He added that in many centers, patients are evaluated in the ED before undergoing CT scanning.
Patients in the direct angiography group received a “flat-panel” noncontrast CT in the angiography suite to rule out intracranial hemorrhage or a large, established infarct. The large-vessel occlusion would be confirmed by arteriography before the endovascular procedure was performed.
After CT scanning, patients received thrombolysis as recommended in the guidelines.
The current interim analysis includes the 174 patients who have been enrolled so far in the study. The median RACE score for these patients was 7, and the median NIHSS score was 17. Large-vessel occlusion was confirmed in 84% of patients, and 8% had an intracerebral hemorrhage.
Results showed that of the 147 patients who received endovascular therapy, puncture time was shorter for those who were taken directly to angiography (median, 18 min vs. 42 min), as was time to reperfusion (median, 57 min vs. 84 min).
The primary outcome was a shift analysis of the Modified Rankin Scale functional outcome scale at 90 days (odds of 1-point improvement or more). In the direct angiography group, the adjusted odds ratio for an improved functional outcome was 2.2 (95% confidence interval, 1.2-.1).
There were no significant differences in safety endpoints. There was a trend toward more procedural complications in those receiving endovascular therapy in the direct angiography group (8.1% vs. 2.7%; P = .6), but there was also a trend toward lower 90-day mortality in this group (20.2% vs. 32.9%; P = .07)
Dr. Requena reported no significant difference in safety outcomes among those with a hemorrhagic stroke.
“Our study is the first clinical trial that shows the superiority of direct transfer to an angiography suite,” said Dr. Requena. “Our findings were close to what we expected, and we were surprised that they occurred so early in the study. We trust that they will be confirmed in ongoing, multicenter, international trials.”
Stroke patients who were transferred directly to an angiography suite were also less likely to be dependent on assistance with daily activities than were those who received the current standard of care, Dr. Requena said. “More frequent and more rapid treatment can help improve outcomes for our stroke patients.”
A limitation of this study is that the hospital had extensive experience with immediate angiography, so findings may differ at hospitals or care centers with less angiography expertise or experience, Dr. Requena said.
He added that retrospective studies conducted in hospitals in the United States, Germany, and Switzerland show that this kind of protocol can be developed in any high-volume stroke center, although multicenter, international trials are needed.
The cost of speed
Commenting on the ANGIO-CAT study, Michael Hill, MD, a professor at the University of Calgary (Alta.), said the 27-minute improvement in door-to-reperfusion time achieved in the study was meaningful and correlates with the degree of improved outcomes observed. “So, the improvement in speed of treatment resulting in better outcomes makes sense,” he added.
He cautioned that this strategy would only be feasible in certain centers with selected patients and that cost will be a fundamental issue.
“If you identify patients at angiography, you risk having some patients with no target large-vessel occlusion,” Dr. Hill added. “The real question is, how many of these patients without a large-vessel occlusion can the system tolerate before it becomes uneconomical and not fruitful or harmful, given that groin puncture is not totally harmless?”
The moderator of the ISC news conference on the study, Mitchell Elkind, MD, professor of neurology at Columbia University, New York, who is also president of the American Stroke Association, said the study reflects the growing recognition of the importance of speed when treating stroke. “If we can shorten time to treatment using rapid evaluation and imaging protocols, this will help save brain,” he said.
Also commenting on the study, Louisa McCullough, MD, PhD, chief of neurology at Memorial Hermann Hospital–Texas Medical Center, Houston, who is the ISC meeting chair, said she thought the study would be relevant to the United States. “Speed is really of the essence. Whenever we can reduce delays, that will make a big difference to patients.”
Referring to this study on improving hospital systems, as well as a second study that was presented at the meeting that showed benefits from delivery of prehospital thrombolysis via a mobile stroke unit, Dr. McCullough added that “we need to set up models so we can get the best of both these worlds. These studies are really leading the way on how we can change the stroke systems of care.”
The study was funded by Vall d’Hebron Research Institute. Dr. Requena disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ISC 2021