User login
Risk factors predict graft failure in pediatric acute leukemia patients
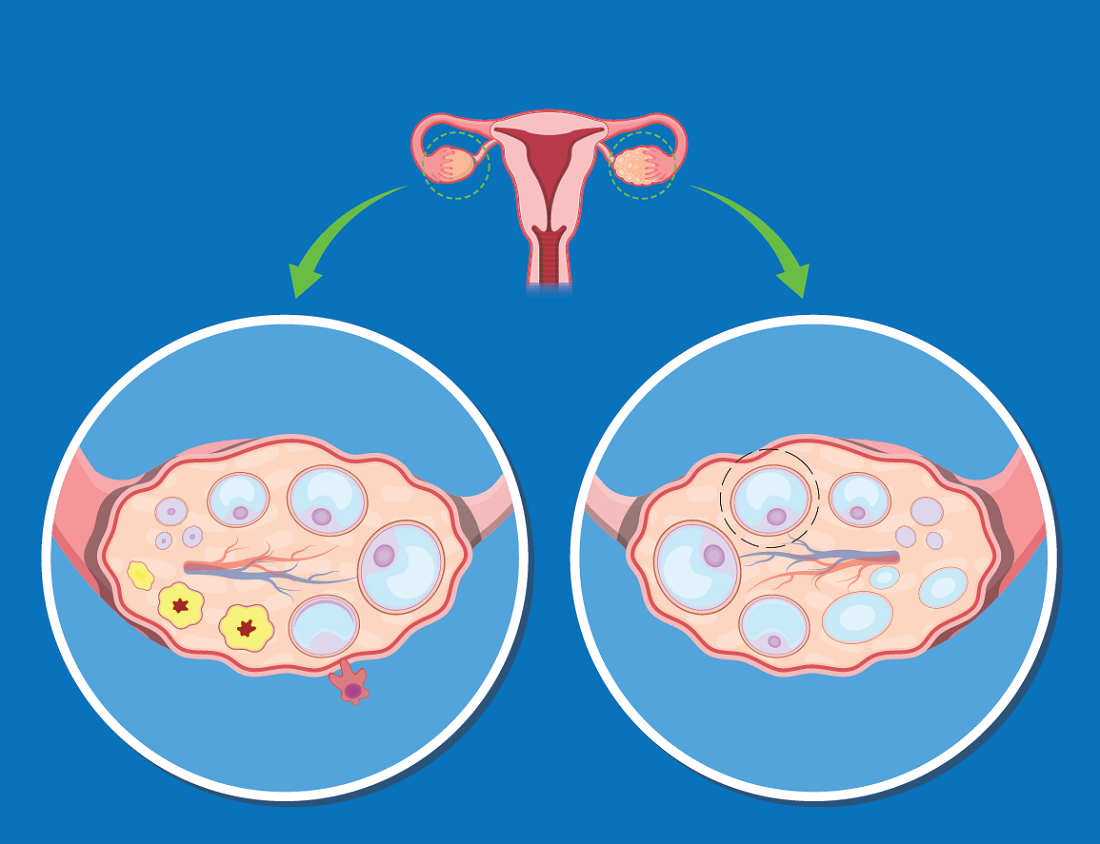
Researchers developed a predictive score for the risk of graft failure in patients with acute leukemia who underwent allogeneic hematopoietic stem cell transplantation (aHSCT) with ex vivo T-cell depletion. T-cell depletion is performed in an effort to prevent subsequent graft-versus-host disease (GVHD) after transplant.
The risk score was based on patient age and the T-lymphocyte population pre-aHSCT with 1 point of risk possible in each category. Patients with 1 point had a graft failure risk of 5% and 13% if they had 2 points, according to the results of the study presented at the virtual meeting of the European Society for Blood and Marrow Transplantation.
Graft failure is a potentially severe complication in patients treated with aHSCT, but there are few studies analyzing risk factors when ex vivo T-cell depletion is used, Ivan López Torija of the Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues noted in their presentation, which won the Best Young Poster Abstract Award at the meeting.
The researchers assessed 148 pediatric patients (64% boys) with acute leukemia who underwent allogeneic HSCT from haploidentical donors using ex vivo T-cell depletion between 2005 and 2020. About 53% of the patients were diagnosed with acute lymphoblastic leukemia, the rest with acute myeloid leukemia. The donor mean age was 40 years, and all transplant patients received toxicity reduction conditioning based on fludarabine busulfan and thiotepa.
Predictive results
Multivariate analysis showed that T-cell count (CD3+/CD8+ ≥ 350/mL: hazard ratio, 2,6; P = .01) and patient age (less than 9 years: HR; 5.0; P = .04) were associated with graft failure. A risk score was established using these results and based on patient age and T lymphocyte pre-aHSCT with 1 point each for each increased risk category. Patients with 1 point had a graft failure risk of 5% and a risk of 13% if they had 2 points.
However, in this particular population, with a mean follow up of 4 years, the overall survival rate was 60%, with no significant differences seen between patients that presented graft failure and those without graft failure.
“Patient age and pretransplant number of CD3+/CD8+ are associated with [graft failure] in pediatric patients with acute leukemia undergoing ex vivo T-cell–depleted haploidentical transplantation. These findings highlight the importance of preexisting cellular immunity in the transplant recipient and support T-cell population analysis as part of a pretransplant working program,” the researchers concluded.
The authors reported that they had no disclosures.
Researchers developed a predictive score for the risk of graft failure in patients with acute leukemia who underwent allogeneic hematopoietic stem cell transplantation (aHSCT) with ex vivo T-cell depletion. T-cell depletion is performed in an effort to prevent subsequent graft-versus-host disease (GVHD) after transplant.
The risk score was based on patient age and the T-lymphocyte population pre-aHSCT with 1 point of risk possible in each category. Patients with 1 point had a graft failure risk of 5% and 13% if they had 2 points, according to the results of the study presented at the virtual meeting of the European Society for Blood and Marrow Transplantation.
Graft failure is a potentially severe complication in patients treated with aHSCT, but there are few studies analyzing risk factors when ex vivo T-cell depletion is used, Ivan López Torija of the Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues noted in their presentation, which won the Best Young Poster Abstract Award at the meeting.
The researchers assessed 148 pediatric patients (64% boys) with acute leukemia who underwent allogeneic HSCT from haploidentical donors using ex vivo T-cell depletion between 2005 and 2020. About 53% of the patients were diagnosed with acute lymphoblastic leukemia, the rest with acute myeloid leukemia. The donor mean age was 40 years, and all transplant patients received toxicity reduction conditioning based on fludarabine busulfan and thiotepa.
Predictive results
Multivariate analysis showed that T-cell count (CD3+/CD8+ ≥ 350/mL: hazard ratio, 2,6; P = .01) and patient age (less than 9 years: HR; 5.0; P = .04) were associated with graft failure. A risk score was established using these results and based on patient age and T lymphocyte pre-aHSCT with 1 point each for each increased risk category. Patients with 1 point had a graft failure risk of 5% and a risk of 13% if they had 2 points.
However, in this particular population, with a mean follow up of 4 years, the overall survival rate was 60%, with no significant differences seen between patients that presented graft failure and those without graft failure.
“Patient age and pretransplant number of CD3+/CD8+ are associated with [graft failure] in pediatric patients with acute leukemia undergoing ex vivo T-cell–depleted haploidentical transplantation. These findings highlight the importance of preexisting cellular immunity in the transplant recipient and support T-cell population analysis as part of a pretransplant working program,” the researchers concluded.
The authors reported that they had no disclosures.
Researchers developed a predictive score for the risk of graft failure in patients with acute leukemia who underwent allogeneic hematopoietic stem cell transplantation (aHSCT) with ex vivo T-cell depletion. T-cell depletion is performed in an effort to prevent subsequent graft-versus-host disease (GVHD) after transplant.
The risk score was based on patient age and the T-lymphocyte population pre-aHSCT with 1 point of risk possible in each category. Patients with 1 point had a graft failure risk of 5% and 13% if they had 2 points, according to the results of the study presented at the virtual meeting of the European Society for Blood and Marrow Transplantation.
Graft failure is a potentially severe complication in patients treated with aHSCT, but there are few studies analyzing risk factors when ex vivo T-cell depletion is used, Ivan López Torija of the Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues noted in their presentation, which won the Best Young Poster Abstract Award at the meeting.
The researchers assessed 148 pediatric patients (64% boys) with acute leukemia who underwent allogeneic HSCT from haploidentical donors using ex vivo T-cell depletion between 2005 and 2020. About 53% of the patients were diagnosed with acute lymphoblastic leukemia, the rest with acute myeloid leukemia. The donor mean age was 40 years, and all transplant patients received toxicity reduction conditioning based on fludarabine busulfan and thiotepa.
Predictive results
Multivariate analysis showed that T-cell count (CD3+/CD8+ ≥ 350/mL: hazard ratio, 2,6; P = .01) and patient age (less than 9 years: HR; 5.0; P = .04) were associated with graft failure. A risk score was established using these results and based on patient age and T lymphocyte pre-aHSCT with 1 point each for each increased risk category. Patients with 1 point had a graft failure risk of 5% and a risk of 13% if they had 2 points.
However, in this particular population, with a mean follow up of 4 years, the overall survival rate was 60%, with no significant differences seen between patients that presented graft failure and those without graft failure.
“Patient age and pretransplant number of CD3+/CD8+ are associated with [graft failure] in pediatric patients with acute leukemia undergoing ex vivo T-cell–depleted haploidentical transplantation. These findings highlight the importance of preexisting cellular immunity in the transplant recipient and support T-cell population analysis as part of a pretransplant working program,” the researchers concluded.
The authors reported that they had no disclosures.
FROM EBMT 2021
Obesity pegged as source of marked increased risk of diabetes in PCOS
The increased risk of type 2 diabetes in women with polycystic ovary syndrome is well established, but a new analysis has shown that obesity is the major mediator and a target for preventing or reversing this comorbidity.
“Most women with PCOS are obese, complicating the effort to understand whether high rates of diabetes in this population are due to PCOS or excess weight, but our study now suggest that obesity isa targetable risk factor,” reported Panagiotis Anagnostis, MD, PhD, a reproductive endocrinologist at the Medical School of Aristotle University, Thessaloniki, Greece.
Obesity is also a known risk factor for type 2 diabetes (T2D), but there is reason to suspect that PCOS, which is associated with abnormal carbohydrate metabolism, has a direct impact on the risk of developing T2D, according to Dr. Anagnostis. It is also reasonable to expect “a synergistic deleterious effect” from PCOS and obesity on adverse changes in glucose metabolism that lead to T2D.
Even though rates of obesity among women with PCOS reach 80% in some studies, Dr. Anagnostis attempted to disentangle the relationship between obesity, PCOS, and risk of T2D using a large set of data drawn from a comprehensive search of published studies.
After screening with predefined criteria, 12 studies provided data on 224,284 women, of whom 45,361 had PCOS and 5,717 had T2D. Not least of the criteria for inclusion in this analysis, all studies stratified women as obese, defined as a body mass index (BMI) greater than 30 kg/m2, or nonobese, he reported at the annual meeting of the Endocrine Society.
Diabetes risk tripled in PCOS
When compared without regard to BMI, the relative risk of having T2D among those with PCOS relative to those without this condition was more than three times greater (RR 3.13; P < .001). When women with PCOS were stratified for BMI, obesity was associated with a more than fourfold increased risk relative to controls without PCOS (RR, 4.06; P < .001).
In women who were nonobese, the risk of T2D was numerically higher for those with PCOS than those without (RR, 2.68), but it was only a trend with a large confidence interval (95% confidence interval, 0.97-7.49).
Among women with PCOS, those who were obese also had a more than fourfold and highly significant increased risk of T2D relative to those who were not obese (RR, 4.20; P < .001).
The message from these data is that obesity is a major and potentially modifiable risk factor for diabetes in women with PCOS, according to Dr. Anagnostis.
He said these data provide the basis for recommending weight loss specifically for managing this common PCOS comorbidity.
Almost the same relative risk of diabetes was derived from an analysis of a women’s health database published 2 years ago in Diabetes Care. In that study with 1,916 person-years of follow-up, the hazard ratio for T2D was also more than three times greater (HR, 3.23; P < .001) for those with PCOS relative to those without the syndrome.
However, normal BMI did not eliminate risk of developing diabetes in this study. Rather, the relative risk of T2D in women with PCOS was higher in those of normal weight, compared with those who were obese (HR, 4.68 vs. 2.36; P < .005). The investigators recommend screening all women with PCOS at least every 3 years with more frequent screening in those with risk factors.
PCOS complexity challenges simple conclusions
The complexity of disturbed metabolic pathways in patients with PCOS and obesity might explain some of the difficulty in unraveling the relationship between these two disease states and diabetes risk. In one recent review, it was suggested that obesity and PCOS share interrelated adverse effects on glucose metabolism. As a result, these associations are “more complex than a simple cause-and-effect process.” the authors of that article concluded.
Furthermore, in their examination of metabolic pathways, genetic susceptibility, and behavioral factors that might link PCOS, weight gain, and T2D, the authors did not ignore the psychological impact of PCOS in causing obesity and, as a byproduct, diabetes. These psychological factors might be relevant to treatment.
For example, depression and stress “might hamper ongoing attempts at lifestyle change and therefore effective weight loss” in at least some women, they cautioned.
However, in encouraging weight loss in overweight women with PCOS, the debate about cause of T2D might be moot in practical terms, according to Michael Dansinger, MD, founding director of the diabetes reversal program at Tufts Medical Center, Boston.
“Reducing excess body fat reduces the risk of type 2 diabetes,” Dr. Dansinger said in an interview. “Since women with obesity and PCOS are clearly at risk for future type 2 diabetes, that’s another reason to lose excess body fat through healthy eating and exercise.”
Dr. Anagnostis and Dr. Dansinger reported no relevant conflicts of interest.
The increased risk of type 2 diabetes in women with polycystic ovary syndrome is well established, but a new analysis has shown that obesity is the major mediator and a target for preventing or reversing this comorbidity.
“Most women with PCOS are obese, complicating the effort to understand whether high rates of diabetes in this population are due to PCOS or excess weight, but our study now suggest that obesity isa targetable risk factor,” reported Panagiotis Anagnostis, MD, PhD, a reproductive endocrinologist at the Medical School of Aristotle University, Thessaloniki, Greece.
Obesity is also a known risk factor for type 2 diabetes (T2D), but there is reason to suspect that PCOS, which is associated with abnormal carbohydrate metabolism, has a direct impact on the risk of developing T2D, according to Dr. Anagnostis. It is also reasonable to expect “a synergistic deleterious effect” from PCOS and obesity on adverse changes in glucose metabolism that lead to T2D.
Even though rates of obesity among women with PCOS reach 80% in some studies, Dr. Anagnostis attempted to disentangle the relationship between obesity, PCOS, and risk of T2D using a large set of data drawn from a comprehensive search of published studies.
After screening with predefined criteria, 12 studies provided data on 224,284 women, of whom 45,361 had PCOS and 5,717 had T2D. Not least of the criteria for inclusion in this analysis, all studies stratified women as obese, defined as a body mass index (BMI) greater than 30 kg/m2, or nonobese, he reported at the annual meeting of the Endocrine Society.
Diabetes risk tripled in PCOS
When compared without regard to BMI, the relative risk of having T2D among those with PCOS relative to those without this condition was more than three times greater (RR 3.13; P < .001). When women with PCOS were stratified for BMI, obesity was associated with a more than fourfold increased risk relative to controls without PCOS (RR, 4.06; P < .001).
In women who were nonobese, the risk of T2D was numerically higher for those with PCOS than those without (RR, 2.68), but it was only a trend with a large confidence interval (95% confidence interval, 0.97-7.49).
Among women with PCOS, those who were obese also had a more than fourfold and highly significant increased risk of T2D relative to those who were not obese (RR, 4.20; P < .001).
The message from these data is that obesity is a major and potentially modifiable risk factor for diabetes in women with PCOS, according to Dr. Anagnostis.
He said these data provide the basis for recommending weight loss specifically for managing this common PCOS comorbidity.
Almost the same relative risk of diabetes was derived from an analysis of a women’s health database published 2 years ago in Diabetes Care. In that study with 1,916 person-years of follow-up, the hazard ratio for T2D was also more than three times greater (HR, 3.23; P < .001) for those with PCOS relative to those without the syndrome.
However, normal BMI did not eliminate risk of developing diabetes in this study. Rather, the relative risk of T2D in women with PCOS was higher in those of normal weight, compared with those who were obese (HR, 4.68 vs. 2.36; P < .005). The investigators recommend screening all women with PCOS at least every 3 years with more frequent screening in those with risk factors.
PCOS complexity challenges simple conclusions
The complexity of disturbed metabolic pathways in patients with PCOS and obesity might explain some of the difficulty in unraveling the relationship between these two disease states and diabetes risk. In one recent review, it was suggested that obesity and PCOS share interrelated adverse effects on glucose metabolism. As a result, these associations are “more complex than a simple cause-and-effect process.” the authors of that article concluded.
Furthermore, in their examination of metabolic pathways, genetic susceptibility, and behavioral factors that might link PCOS, weight gain, and T2D, the authors did not ignore the psychological impact of PCOS in causing obesity and, as a byproduct, diabetes. These psychological factors might be relevant to treatment.
For example, depression and stress “might hamper ongoing attempts at lifestyle change and therefore effective weight loss” in at least some women, they cautioned.
However, in encouraging weight loss in overweight women with PCOS, the debate about cause of T2D might be moot in practical terms, according to Michael Dansinger, MD, founding director of the diabetes reversal program at Tufts Medical Center, Boston.
“Reducing excess body fat reduces the risk of type 2 diabetes,” Dr. Dansinger said in an interview. “Since women with obesity and PCOS are clearly at risk for future type 2 diabetes, that’s another reason to lose excess body fat through healthy eating and exercise.”
Dr. Anagnostis and Dr. Dansinger reported no relevant conflicts of interest.
The increased risk of type 2 diabetes in women with polycystic ovary syndrome is well established, but a new analysis has shown that obesity is the major mediator and a target for preventing or reversing this comorbidity.
“Most women with PCOS are obese, complicating the effort to understand whether high rates of diabetes in this population are due to PCOS or excess weight, but our study now suggest that obesity isa targetable risk factor,” reported Panagiotis Anagnostis, MD, PhD, a reproductive endocrinologist at the Medical School of Aristotle University, Thessaloniki, Greece.
Obesity is also a known risk factor for type 2 diabetes (T2D), but there is reason to suspect that PCOS, which is associated with abnormal carbohydrate metabolism, has a direct impact on the risk of developing T2D, according to Dr. Anagnostis. It is also reasonable to expect “a synergistic deleterious effect” from PCOS and obesity on adverse changes in glucose metabolism that lead to T2D.
Even though rates of obesity among women with PCOS reach 80% in some studies, Dr. Anagnostis attempted to disentangle the relationship between obesity, PCOS, and risk of T2D using a large set of data drawn from a comprehensive search of published studies.
After screening with predefined criteria, 12 studies provided data on 224,284 women, of whom 45,361 had PCOS and 5,717 had T2D. Not least of the criteria for inclusion in this analysis, all studies stratified women as obese, defined as a body mass index (BMI) greater than 30 kg/m2, or nonobese, he reported at the annual meeting of the Endocrine Society.
Diabetes risk tripled in PCOS
When compared without regard to BMI, the relative risk of having T2D among those with PCOS relative to those without this condition was more than three times greater (RR 3.13; P < .001). When women with PCOS were stratified for BMI, obesity was associated with a more than fourfold increased risk relative to controls without PCOS (RR, 4.06; P < .001).
In women who were nonobese, the risk of T2D was numerically higher for those with PCOS than those without (RR, 2.68), but it was only a trend with a large confidence interval (95% confidence interval, 0.97-7.49).
Among women with PCOS, those who were obese also had a more than fourfold and highly significant increased risk of T2D relative to those who were not obese (RR, 4.20; P < .001).
The message from these data is that obesity is a major and potentially modifiable risk factor for diabetes in women with PCOS, according to Dr. Anagnostis.
He said these data provide the basis for recommending weight loss specifically for managing this common PCOS comorbidity.
Almost the same relative risk of diabetes was derived from an analysis of a women’s health database published 2 years ago in Diabetes Care. In that study with 1,916 person-years of follow-up, the hazard ratio for T2D was also more than three times greater (HR, 3.23; P < .001) for those with PCOS relative to those without the syndrome.
However, normal BMI did not eliminate risk of developing diabetes in this study. Rather, the relative risk of T2D in women with PCOS was higher in those of normal weight, compared with those who were obese (HR, 4.68 vs. 2.36; P < .005). The investigators recommend screening all women with PCOS at least every 3 years with more frequent screening in those with risk factors.
PCOS complexity challenges simple conclusions
The complexity of disturbed metabolic pathways in patients with PCOS and obesity might explain some of the difficulty in unraveling the relationship between these two disease states and diabetes risk. In one recent review, it was suggested that obesity and PCOS share interrelated adverse effects on glucose metabolism. As a result, these associations are “more complex than a simple cause-and-effect process.” the authors of that article concluded.
Furthermore, in their examination of metabolic pathways, genetic susceptibility, and behavioral factors that might link PCOS, weight gain, and T2D, the authors did not ignore the psychological impact of PCOS in causing obesity and, as a byproduct, diabetes. These psychological factors might be relevant to treatment.
For example, depression and stress “might hamper ongoing attempts at lifestyle change and therefore effective weight loss” in at least some women, they cautioned.
However, in encouraging weight loss in overweight women with PCOS, the debate about cause of T2D might be moot in practical terms, according to Michael Dansinger, MD, founding director of the diabetes reversal program at Tufts Medical Center, Boston.
“Reducing excess body fat reduces the risk of type 2 diabetes,” Dr. Dansinger said in an interview. “Since women with obesity and PCOS are clearly at risk for future type 2 diabetes, that’s another reason to lose excess body fat through healthy eating and exercise.”
Dr. Anagnostis and Dr. Dansinger reported no relevant conflicts of interest.
FROM ENDO 2021
Match Day 2021: Interest in family medicine remains strong
which were up 3.5% over last year, according to the National Resident Matching Program.
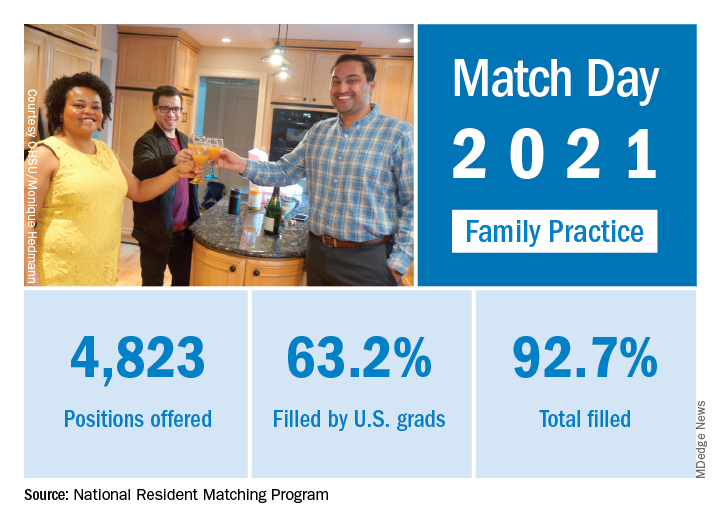
“Rather than faltering in these uncertain times, program fill rates increased across the board,” the NRMP said in a written statement. Overall, the 2021 Main Residency Match offered (35,194) and filled (33,353) more first-year (PGY-1) slots than ever before, for a fill rate of 94.8%, compared with 94.6% the year before.
Family medicine offered 4,823 positions in this year’s Match, up by 3.5% over 2020, and filled 4,472, for a 1-year increase of 3.7% and a fill rate of 92.7%. Just over 63% (3,046) of the available slots were given to U.S. seniors (MDs and DOs), while 25.4% went to international medical graduates. The corresponding PGY-1 numbers for the Match as a whole were 70.4% U.S. and 21.1% international medical graduates, based on NRMP data.
“In the last five years, the Main Residency Match has seen sizable increases in the number of positions offered” in family medicine – up by 1,467 (43.7%) since 2017 – and such growth over time may “be a predictor of future physician workforce supply,” the NRMP said. Family medicine also increased its share of all available residency positions from 11.6% in 2017 to 13.7% this year.
“Concerns about the impact of virtual recruitment on applicants’ matching into PGY-1 positions were not realized,” the NRMP noted, as “growth in registration was seen in every applicant group.” Compared with 2020, submissions of rank-order lists of programs were up by 2.8% for U.S. MD seniors, 7.9% for U.S. DO seniors, 2.5% for U.S.-citizen IMGs, and 15.0% for non–U.S.-citizen IMGs.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” said Donna L. Lamb, DHSc, MBA, who is president and CEO of the NRMP.
which were up 3.5% over last year, according to the National Resident Matching Program.

“Rather than faltering in these uncertain times, program fill rates increased across the board,” the NRMP said in a written statement. Overall, the 2021 Main Residency Match offered (35,194) and filled (33,353) more first-year (PGY-1) slots than ever before, for a fill rate of 94.8%, compared with 94.6% the year before.
Family medicine offered 4,823 positions in this year’s Match, up by 3.5% over 2020, and filled 4,472, for a 1-year increase of 3.7% and a fill rate of 92.7%. Just over 63% (3,046) of the available slots were given to U.S. seniors (MDs and DOs), while 25.4% went to international medical graduates. The corresponding PGY-1 numbers for the Match as a whole were 70.4% U.S. and 21.1% international medical graduates, based on NRMP data.
“In the last five years, the Main Residency Match has seen sizable increases in the number of positions offered” in family medicine – up by 1,467 (43.7%) since 2017 – and such growth over time may “be a predictor of future physician workforce supply,” the NRMP said. Family medicine also increased its share of all available residency positions from 11.6% in 2017 to 13.7% this year.
“Concerns about the impact of virtual recruitment on applicants’ matching into PGY-1 positions were not realized,” the NRMP noted, as “growth in registration was seen in every applicant group.” Compared with 2020, submissions of rank-order lists of programs were up by 2.8% for U.S. MD seniors, 7.9% for U.S. DO seniors, 2.5% for U.S.-citizen IMGs, and 15.0% for non–U.S.-citizen IMGs.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” said Donna L. Lamb, DHSc, MBA, who is president and CEO of the NRMP.
which were up 3.5% over last year, according to the National Resident Matching Program.

“Rather than faltering in these uncertain times, program fill rates increased across the board,” the NRMP said in a written statement. Overall, the 2021 Main Residency Match offered (35,194) and filled (33,353) more first-year (PGY-1) slots than ever before, for a fill rate of 94.8%, compared with 94.6% the year before.
Family medicine offered 4,823 positions in this year’s Match, up by 3.5% over 2020, and filled 4,472, for a 1-year increase of 3.7% and a fill rate of 92.7%. Just over 63% (3,046) of the available slots were given to U.S. seniors (MDs and DOs), while 25.4% went to international medical graduates. The corresponding PGY-1 numbers for the Match as a whole were 70.4% U.S. and 21.1% international medical graduates, based on NRMP data.
“In the last five years, the Main Residency Match has seen sizable increases in the number of positions offered” in family medicine – up by 1,467 (43.7%) since 2017 – and such growth over time may “be a predictor of future physician workforce supply,” the NRMP said. Family medicine also increased its share of all available residency positions from 11.6% in 2017 to 13.7% this year.
“Concerns about the impact of virtual recruitment on applicants’ matching into PGY-1 positions were not realized,” the NRMP noted, as “growth in registration was seen in every applicant group.” Compared with 2020, submissions of rank-order lists of programs were up by 2.8% for U.S. MD seniors, 7.9% for U.S. DO seniors, 2.5% for U.S.-citizen IMGs, and 15.0% for non–U.S.-citizen IMGs.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” said Donna L. Lamb, DHSc, MBA, who is president and CEO of the NRMP.
Pink plaque on the ear
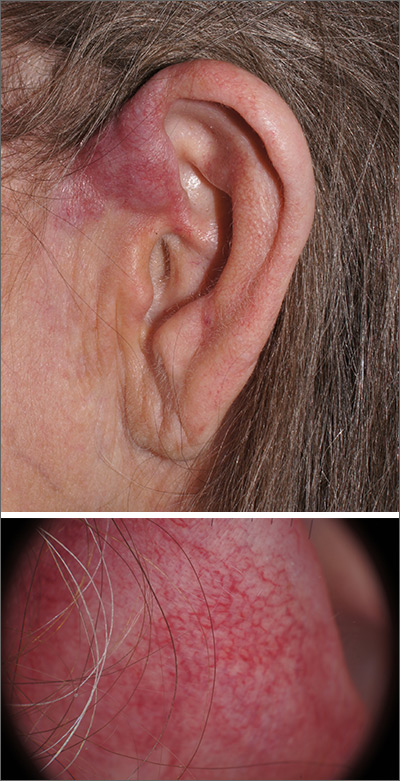
A 4-mm punch biopsy was performed and revealed B-cell lymphoma, consistent with extranodal marginal zone lymphoma. The plaque was palpated carefully and the location of branches of the superficial temporal artery, which usually course anterior to the helix, were mapped, and avoided as a biopsy site.
Marginal zone lymphoma is a relatively indolent B-cell lymphoma that occurs in adults in mucosal associated lymphoid tissue, most often in the gastrointestinal (GI) tract. Neoplasms also occur in the lungs, eyes, and skin. Initial symptoms vary according to the site of manifestation. Patients with GI tumors may present with GI bleeding, abdominal pain, and weight loss. Pulmonary lesions are often asymptomatic and picked up on chest imaging for other indications. Chronic gastritis associated with Helicobacter pylori contributes to cases and occasionally eradication of H. pylori may clear patients of disease. Autoimmune diseases, particularly Sjögren disease and Hashimoto thyroiditis, also have a causative association. Rarely, transformation into high-grade disease occurs.
On the skin, marginal zone lymphoma may be exhibited as soft salmon-colored patches with serpentine vascular markings, as seen here on dermoscopy.1 The differential diagnosis includes keloid scar, basal cell carcinoma, Merkel cell carcinoma, arthropod bites, and amelanotic melanoma.
This patient had been under the care of Medical Oncology for surveillance and was offered observation as a potential treatment strategy because of the relatively indolent nature of the tumor. However, because the tumor was painful and growing, she opted for focal palliative radiation therapy.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Geller S, Marghoob AA, Scope A, et al. Dermoscopy and the diagnosis of primary cutaneous B-cell lymphoma. J Eur Acad Dermatol Venereol. 2018;32:53-56. doi:10.1111/jdv.14549

A 4-mm punch biopsy was performed and revealed B-cell lymphoma, consistent with extranodal marginal zone lymphoma. The plaque was palpated carefully and the location of branches of the superficial temporal artery, which usually course anterior to the helix, were mapped, and avoided as a biopsy site.
Marginal zone lymphoma is a relatively indolent B-cell lymphoma that occurs in adults in mucosal associated lymphoid tissue, most often in the gastrointestinal (GI) tract. Neoplasms also occur in the lungs, eyes, and skin. Initial symptoms vary according to the site of manifestation. Patients with GI tumors may present with GI bleeding, abdominal pain, and weight loss. Pulmonary lesions are often asymptomatic and picked up on chest imaging for other indications. Chronic gastritis associated with Helicobacter pylori contributes to cases and occasionally eradication of H. pylori may clear patients of disease. Autoimmune diseases, particularly Sjögren disease and Hashimoto thyroiditis, also have a causative association. Rarely, transformation into high-grade disease occurs.
On the skin, marginal zone lymphoma may be exhibited as soft salmon-colored patches with serpentine vascular markings, as seen here on dermoscopy.1 The differential diagnosis includes keloid scar, basal cell carcinoma, Merkel cell carcinoma, arthropod bites, and amelanotic melanoma.
This patient had been under the care of Medical Oncology for surveillance and was offered observation as a potential treatment strategy because of the relatively indolent nature of the tumor. However, because the tumor was painful and growing, she opted for focal palliative radiation therapy.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

A 4-mm punch biopsy was performed and revealed B-cell lymphoma, consistent with extranodal marginal zone lymphoma. The plaque was palpated carefully and the location of branches of the superficial temporal artery, which usually course anterior to the helix, were mapped, and avoided as a biopsy site.
Marginal zone lymphoma is a relatively indolent B-cell lymphoma that occurs in adults in mucosal associated lymphoid tissue, most often in the gastrointestinal (GI) tract. Neoplasms also occur in the lungs, eyes, and skin. Initial symptoms vary according to the site of manifestation. Patients with GI tumors may present with GI bleeding, abdominal pain, and weight loss. Pulmonary lesions are often asymptomatic and picked up on chest imaging for other indications. Chronic gastritis associated with Helicobacter pylori contributes to cases and occasionally eradication of H. pylori may clear patients of disease. Autoimmune diseases, particularly Sjögren disease and Hashimoto thyroiditis, also have a causative association. Rarely, transformation into high-grade disease occurs.
On the skin, marginal zone lymphoma may be exhibited as soft salmon-colored patches with serpentine vascular markings, as seen here on dermoscopy.1 The differential diagnosis includes keloid scar, basal cell carcinoma, Merkel cell carcinoma, arthropod bites, and amelanotic melanoma.
This patient had been under the care of Medical Oncology for surveillance and was offered observation as a potential treatment strategy because of the relatively indolent nature of the tumor. However, because the tumor was painful and growing, she opted for focal palliative radiation therapy.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Geller S, Marghoob AA, Scope A, et al. Dermoscopy and the diagnosis of primary cutaneous B-cell lymphoma. J Eur Acad Dermatol Venereol. 2018;32:53-56. doi:10.1111/jdv.14549
1. Geller S, Marghoob AA, Scope A, et al. Dermoscopy and the diagnosis of primary cutaneous B-cell lymphoma. J Eur Acad Dermatol Venereol. 2018;32:53-56. doi:10.1111/jdv.14549
Infliximab weakens COVID-19 antibody response for IBD patients
Patients treated with infliximab for inflammatory bowel disease (IBD) showed significantly reduced response to COVID-19 antibodies, compared with those treated with vedolizumab, according to data from nearly 7,000 patients.
Although anti–tumor necrosis factor (anti-TNF) drugs are routinely used for patients with IBD, the impact of their immune-suppressing properties on protective immunity to COVID-19 is unknown, wrote Nicholas A. Kennedy, MD, of the University of Exeter (England) and colleagues. These drugs have been reported to impair protective immunity following vaccines for other diseases, such as those for influenza and viral hepatitis.
“By suppressing immune responses, biological and immunosuppression therapies may lead to chronic SARS-CoV-2 infection and have recently been implicated in the evolution and emergence of novel variants,” they noted, citing a study published in Cell.
In the current study, published in Gut, the researchers used data from the CLARITY IBD study to identify 6,935 patients with IBD aged 5 years and older seen at 92 hospitals in the United Kingdom between Sept. 22, 2020, and Dec. 23, 2020. Of these, 4,685 were treated with infliximab, and 2,250 received vedolizumab. The proportion of study participants with a positive anti–SARS-CoV-2 antibody test was the primary outcome, with secondary outcomes including proportion with positive antibodies following positive polymerase chain reaction test for SARS-CoV-2 and the magnitude of antibody reactivity.
Substantial seroprevalence differences seen
Overall, rates of symptomatic and proven SARS-CoV-2 infection and hospitalization were similar between infliximab-treated and vedolizumab-treated patients with IBD. However, seroprevalence was significantly lower in the infliximab group, compared with the vedolizumab group (3.4% vs. 6.0%; P < .0001). In addition, infliximab and immunomodulator use were each independently associated with lower seropositivity, compared with vedolizumab (odds ratio, 0.66 for infliximab and OR, 0.70 for immunomodulators) in a multivariate analysis.
In a sensitivity analysis, 39 of 81 infliximab-treated patients with polymerase chain reaction–confirmed COVID-19 infection seroconverted (48%), compared with 30 of 36 vedolizumab-treated patients (83%) (P < .00044). Infliximab-treated patients with confirmed infections also showed a lower magnitude of anti–SARS-CoV-2 reactivity, compared with vedolizumab-treated patients (P < .0001).
From a clinical perspective, the lower seroconversion rates and reduced levels of anti–SARS-CoV-2 antibody reactivity might increase susceptibility to recurrent COVID-19 infections in infliximab-treated IBD patients, the researchers noted. In addition, the impaired serological responses might promote chronic nasopharyngeal colonization and consequently promote the development of COVID-19 variants and drive persistent transmission, the researchers said.
The study findings were limited by several factors including lack of knowledge on the impact of attenuated immune response on infection risk, the potential for recall bias associated with patient reports, and the focus on infliximab only, the researchers pointed out. However, the key findings are likely apply to other anti-TNF monoclonal antibodies including adalimumab, certolizumab and golimumab, they suggested.
The study was strengthened by the recruitment of a large number of patients in a narrow time frame and comprehensive collection of data on patient-reported outcomes, COVID-19 testing, and serological assay results, the researchers said. Overall, the findings support the public health value of serological testing and virus surveillance to identify suboptimal vaccine response and to consider implications for practice, they added. “If attenuated serological responses following vaccination are also observed, then modified immunization strategies will need to be designed for millions of patients worldwide,” they emphasized.
Findings inform clinical practice and public health
The study is very important for many reasons, said Kim L. Isaacs, MD, PhD, AGAF, of the University of North Carolina at Chapel Hill in an interview. “It is known that there is decreased responsiveness to a number of routine vaccinations in IBD patients on immune active therapy. In terms of SARS-CoV-2, development of an immune response with infection is important in terms of severity of infection, reinfection, and possibly limiting spread of infection in this patient population,” she said. “Looking at both serum seroconversion and reactivity of immune response in patients with known SARS-CoV-2 infection will help to define clinical and public health guidance, and also may be predictive as to what might happen with SARS-CoV-2 immunization based on background biologic or immunosuppressant therapy,” she noted.
Dr. Isaacs said that she was not surprised by the study findings. “Anti-TNF, thiopurine, and methotrexate therapy are all thought to be systemically active and likely to suppress the immune response to infection and vaccination,” she said. Vedolizumab, on the other hand, is thought to be less systemically active and clinically is associated with fewer serious infections.
Data will drive patient counseling
“These results affect counseling of IBD patients on immune active therapy who have had a SARS-CoV-2 infection,” said Dr. Isaacs. “They should be made aware that infection does not indicate protection for further infection. Although the issues that are raised in this study are of concern, patients should not have clinically beneficial therapy discontinued or switched based on these results,” she said.
“Additional research is needed to determine what the seroconversion rate is with the currently available immunizations for SARS-CoV-2,” said Dr. Isaacs. More questions to address include whether there are differences in the different products available, whether immunization after SARS-CoV-2 infection improves both seroconversion and immune reactivity, and whether there is any benefit to transiently stopping dual immune active therapy during the time of immunization, she said.
Further studies can fill knowledge gaps
“There is a knowledge gap in our understanding of susceptibility to SARS-CoV-2 infections among patients with IBD who have previously been infected,” Shirley Cohen-Mekelburg, MD, MS, staff physician and research scientist in the inflammatory bowel disease program at the Veterans Affairs Ann Arbor (Mich.) Healthcare System, said in an interview. ”This is a first step in beginning to narrow this gap – to provide patients and providers with data to drive recommendations during this COVID-19 pandemic.”
She added that, while further work needs to be done, the study findings do support potential benefit for ongoing vigilance among patients receiving infliximab for IBD. “The study findings also drive us to seek answers to more questions: For example, should we consider serological testing for patients on infliximab? How does the presence or absence of anti–SARS-CoV-2 antibodies associate with susceptibility to infection for patients with infliximab?
“Further studies examining anti–SARS-CoV-2 reactivity are necessary to better understand antibody responses between patients with IBD to the general population, or between patients on immunosuppressive therapy and the general population,” she said. “Observational studies are also not designed to examine the causal relationship between infections, medications, and antibody responses. There may be some inherent differences to patients who receive infliximab as compared to vedolizumab for IBD.”
The study was supported by Biogen (Switzerland), Celltrion Healthcare, Galapagos, F. Hoffmann-La Roche, Hull University Teaching Hospital NHS Trust, and the Royal Devon and Exeter NHS Foundation Trust. The study authors disclosed financial and nonfinancial relationships with numerous companies, including AbbVie, Biogen, Celltrion Healthcare, Galapagos, F. Hoffmann-La Roche, and Immundiagnostik, as well as Janssen, who markets infliximab, and Takeda, who markets vedolizumab. Dr. Isaacs and Dr. Cohen-Mekelburg had no relevant financial conflicts to disclose.
This article was updated 3/31/21.
Patients treated with infliximab for inflammatory bowel disease (IBD) showed significantly reduced response to COVID-19 antibodies, compared with those treated with vedolizumab, according to data from nearly 7,000 patients.
Although anti–tumor necrosis factor (anti-TNF) drugs are routinely used for patients with IBD, the impact of their immune-suppressing properties on protective immunity to COVID-19 is unknown, wrote Nicholas A. Kennedy, MD, of the University of Exeter (England) and colleagues. These drugs have been reported to impair protective immunity following vaccines for other diseases, such as those for influenza and viral hepatitis.
“By suppressing immune responses, biological and immunosuppression therapies may lead to chronic SARS-CoV-2 infection and have recently been implicated in the evolution and emergence of novel variants,” they noted, citing a study published in Cell.
In the current study, published in Gut, the researchers used data from the CLARITY IBD study to identify 6,935 patients with IBD aged 5 years and older seen at 92 hospitals in the United Kingdom between Sept. 22, 2020, and Dec. 23, 2020. Of these, 4,685 were treated with infliximab, and 2,250 received vedolizumab. The proportion of study participants with a positive anti–SARS-CoV-2 antibody test was the primary outcome, with secondary outcomes including proportion with positive antibodies following positive polymerase chain reaction test for SARS-CoV-2 and the magnitude of antibody reactivity.
Substantial seroprevalence differences seen
Overall, rates of symptomatic and proven SARS-CoV-2 infection and hospitalization were similar between infliximab-treated and vedolizumab-treated patients with IBD. However, seroprevalence was significantly lower in the infliximab group, compared with the vedolizumab group (3.4% vs. 6.0%; P < .0001). In addition, infliximab and immunomodulator use were each independently associated with lower seropositivity, compared with vedolizumab (odds ratio, 0.66 for infliximab and OR, 0.70 for immunomodulators) in a multivariate analysis.
In a sensitivity analysis, 39 of 81 infliximab-treated patients with polymerase chain reaction–confirmed COVID-19 infection seroconverted (48%), compared with 30 of 36 vedolizumab-treated patients (83%) (P < .00044). Infliximab-treated patients with confirmed infections also showed a lower magnitude of anti–SARS-CoV-2 reactivity, compared with vedolizumab-treated patients (P < .0001).
From a clinical perspective, the lower seroconversion rates and reduced levels of anti–SARS-CoV-2 antibody reactivity might increase susceptibility to recurrent COVID-19 infections in infliximab-treated IBD patients, the researchers noted. In addition, the impaired serological responses might promote chronic nasopharyngeal colonization and consequently promote the development of COVID-19 variants and drive persistent transmission, the researchers said.
The study findings were limited by several factors including lack of knowledge on the impact of attenuated immune response on infection risk, the potential for recall bias associated with patient reports, and the focus on infliximab only, the researchers pointed out. However, the key findings are likely apply to other anti-TNF monoclonal antibodies including adalimumab, certolizumab and golimumab, they suggested.
The study was strengthened by the recruitment of a large number of patients in a narrow time frame and comprehensive collection of data on patient-reported outcomes, COVID-19 testing, and serological assay results, the researchers said. Overall, the findings support the public health value of serological testing and virus surveillance to identify suboptimal vaccine response and to consider implications for practice, they added. “If attenuated serological responses following vaccination are also observed, then modified immunization strategies will need to be designed for millions of patients worldwide,” they emphasized.
Findings inform clinical practice and public health
The study is very important for many reasons, said Kim L. Isaacs, MD, PhD, AGAF, of the University of North Carolina at Chapel Hill in an interview. “It is known that there is decreased responsiveness to a number of routine vaccinations in IBD patients on immune active therapy. In terms of SARS-CoV-2, development of an immune response with infection is important in terms of severity of infection, reinfection, and possibly limiting spread of infection in this patient population,” she said. “Looking at both serum seroconversion and reactivity of immune response in patients with known SARS-CoV-2 infection will help to define clinical and public health guidance, and also may be predictive as to what might happen with SARS-CoV-2 immunization based on background biologic or immunosuppressant therapy,” she noted.
Dr. Isaacs said that she was not surprised by the study findings. “Anti-TNF, thiopurine, and methotrexate therapy are all thought to be systemically active and likely to suppress the immune response to infection and vaccination,” she said. Vedolizumab, on the other hand, is thought to be less systemically active and clinically is associated with fewer serious infections.
Data will drive patient counseling
“These results affect counseling of IBD patients on immune active therapy who have had a SARS-CoV-2 infection,” said Dr. Isaacs. “They should be made aware that infection does not indicate protection for further infection. Although the issues that are raised in this study are of concern, patients should not have clinically beneficial therapy discontinued or switched based on these results,” she said.
“Additional research is needed to determine what the seroconversion rate is with the currently available immunizations for SARS-CoV-2,” said Dr. Isaacs. More questions to address include whether there are differences in the different products available, whether immunization after SARS-CoV-2 infection improves both seroconversion and immune reactivity, and whether there is any benefit to transiently stopping dual immune active therapy during the time of immunization, she said.
Further studies can fill knowledge gaps
“There is a knowledge gap in our understanding of susceptibility to SARS-CoV-2 infections among patients with IBD who have previously been infected,” Shirley Cohen-Mekelburg, MD, MS, staff physician and research scientist in the inflammatory bowel disease program at the Veterans Affairs Ann Arbor (Mich.) Healthcare System, said in an interview. ”This is a first step in beginning to narrow this gap – to provide patients and providers with data to drive recommendations during this COVID-19 pandemic.”
She added that, while further work needs to be done, the study findings do support potential benefit for ongoing vigilance among patients receiving infliximab for IBD. “The study findings also drive us to seek answers to more questions: For example, should we consider serological testing for patients on infliximab? How does the presence or absence of anti–SARS-CoV-2 antibodies associate with susceptibility to infection for patients with infliximab?
“Further studies examining anti–SARS-CoV-2 reactivity are necessary to better understand antibody responses between patients with IBD to the general population, or between patients on immunosuppressive therapy and the general population,” she said. “Observational studies are also not designed to examine the causal relationship between infections, medications, and antibody responses. There may be some inherent differences to patients who receive infliximab as compared to vedolizumab for IBD.”
The study was supported by Biogen (Switzerland), Celltrion Healthcare, Galapagos, F. Hoffmann-La Roche, Hull University Teaching Hospital NHS Trust, and the Royal Devon and Exeter NHS Foundation Trust. The study authors disclosed financial and nonfinancial relationships with numerous companies, including AbbVie, Biogen, Celltrion Healthcare, Galapagos, F. Hoffmann-La Roche, and Immundiagnostik, as well as Janssen, who markets infliximab, and Takeda, who markets vedolizumab. Dr. Isaacs and Dr. Cohen-Mekelburg had no relevant financial conflicts to disclose.
This article was updated 3/31/21.
Patients treated with infliximab for inflammatory bowel disease (IBD) showed significantly reduced response to COVID-19 antibodies, compared with those treated with vedolizumab, according to data from nearly 7,000 patients.
Although anti–tumor necrosis factor (anti-TNF) drugs are routinely used for patients with IBD, the impact of their immune-suppressing properties on protective immunity to COVID-19 is unknown, wrote Nicholas A. Kennedy, MD, of the University of Exeter (England) and colleagues. These drugs have been reported to impair protective immunity following vaccines for other diseases, such as those for influenza and viral hepatitis.
“By suppressing immune responses, biological and immunosuppression therapies may lead to chronic SARS-CoV-2 infection and have recently been implicated in the evolution and emergence of novel variants,” they noted, citing a study published in Cell.
In the current study, published in Gut, the researchers used data from the CLARITY IBD study to identify 6,935 patients with IBD aged 5 years and older seen at 92 hospitals in the United Kingdom between Sept. 22, 2020, and Dec. 23, 2020. Of these, 4,685 were treated with infliximab, and 2,250 received vedolizumab. The proportion of study participants with a positive anti–SARS-CoV-2 antibody test was the primary outcome, with secondary outcomes including proportion with positive antibodies following positive polymerase chain reaction test for SARS-CoV-2 and the magnitude of antibody reactivity.
Substantial seroprevalence differences seen
Overall, rates of symptomatic and proven SARS-CoV-2 infection and hospitalization were similar between infliximab-treated and vedolizumab-treated patients with IBD. However, seroprevalence was significantly lower in the infliximab group, compared with the vedolizumab group (3.4% vs. 6.0%; P < .0001). In addition, infliximab and immunomodulator use were each independently associated with lower seropositivity, compared with vedolizumab (odds ratio, 0.66 for infliximab and OR, 0.70 for immunomodulators) in a multivariate analysis.
In a sensitivity analysis, 39 of 81 infliximab-treated patients with polymerase chain reaction–confirmed COVID-19 infection seroconverted (48%), compared with 30 of 36 vedolizumab-treated patients (83%) (P < .00044). Infliximab-treated patients with confirmed infections also showed a lower magnitude of anti–SARS-CoV-2 reactivity, compared with vedolizumab-treated patients (P < .0001).
From a clinical perspective, the lower seroconversion rates and reduced levels of anti–SARS-CoV-2 antibody reactivity might increase susceptibility to recurrent COVID-19 infections in infliximab-treated IBD patients, the researchers noted. In addition, the impaired serological responses might promote chronic nasopharyngeal colonization and consequently promote the development of COVID-19 variants and drive persistent transmission, the researchers said.
The study findings were limited by several factors including lack of knowledge on the impact of attenuated immune response on infection risk, the potential for recall bias associated with patient reports, and the focus on infliximab only, the researchers pointed out. However, the key findings are likely apply to other anti-TNF monoclonal antibodies including adalimumab, certolizumab and golimumab, they suggested.
The study was strengthened by the recruitment of a large number of patients in a narrow time frame and comprehensive collection of data on patient-reported outcomes, COVID-19 testing, and serological assay results, the researchers said. Overall, the findings support the public health value of serological testing and virus surveillance to identify suboptimal vaccine response and to consider implications for practice, they added. “If attenuated serological responses following vaccination are also observed, then modified immunization strategies will need to be designed for millions of patients worldwide,” they emphasized.
Findings inform clinical practice and public health
The study is very important for many reasons, said Kim L. Isaacs, MD, PhD, AGAF, of the University of North Carolina at Chapel Hill in an interview. “It is known that there is decreased responsiveness to a number of routine vaccinations in IBD patients on immune active therapy. In terms of SARS-CoV-2, development of an immune response with infection is important in terms of severity of infection, reinfection, and possibly limiting spread of infection in this patient population,” she said. “Looking at both serum seroconversion and reactivity of immune response in patients with known SARS-CoV-2 infection will help to define clinical and public health guidance, and also may be predictive as to what might happen with SARS-CoV-2 immunization based on background biologic or immunosuppressant therapy,” she noted.
Dr. Isaacs said that she was not surprised by the study findings. “Anti-TNF, thiopurine, and methotrexate therapy are all thought to be systemically active and likely to suppress the immune response to infection and vaccination,” she said. Vedolizumab, on the other hand, is thought to be less systemically active and clinically is associated with fewer serious infections.
Data will drive patient counseling
“These results affect counseling of IBD patients on immune active therapy who have had a SARS-CoV-2 infection,” said Dr. Isaacs. “They should be made aware that infection does not indicate protection for further infection. Although the issues that are raised in this study are of concern, patients should not have clinically beneficial therapy discontinued or switched based on these results,” she said.
“Additional research is needed to determine what the seroconversion rate is with the currently available immunizations for SARS-CoV-2,” said Dr. Isaacs. More questions to address include whether there are differences in the different products available, whether immunization after SARS-CoV-2 infection improves both seroconversion and immune reactivity, and whether there is any benefit to transiently stopping dual immune active therapy during the time of immunization, she said.
Further studies can fill knowledge gaps
“There is a knowledge gap in our understanding of susceptibility to SARS-CoV-2 infections among patients with IBD who have previously been infected,” Shirley Cohen-Mekelburg, MD, MS, staff physician and research scientist in the inflammatory bowel disease program at the Veterans Affairs Ann Arbor (Mich.) Healthcare System, said in an interview. ”This is a first step in beginning to narrow this gap – to provide patients and providers with data to drive recommendations during this COVID-19 pandemic.”
She added that, while further work needs to be done, the study findings do support potential benefit for ongoing vigilance among patients receiving infliximab for IBD. “The study findings also drive us to seek answers to more questions: For example, should we consider serological testing for patients on infliximab? How does the presence or absence of anti–SARS-CoV-2 antibodies associate with susceptibility to infection for patients with infliximab?
“Further studies examining anti–SARS-CoV-2 reactivity are necessary to better understand antibody responses between patients with IBD to the general population, or between patients on immunosuppressive therapy and the general population,” she said. “Observational studies are also not designed to examine the causal relationship between infections, medications, and antibody responses. There may be some inherent differences to patients who receive infliximab as compared to vedolizumab for IBD.”
The study was supported by Biogen (Switzerland), Celltrion Healthcare, Galapagos, F. Hoffmann-La Roche, Hull University Teaching Hospital NHS Trust, and the Royal Devon and Exeter NHS Foundation Trust. The study authors disclosed financial and nonfinancial relationships with numerous companies, including AbbVie, Biogen, Celltrion Healthcare, Galapagos, F. Hoffmann-La Roche, and Immundiagnostik, as well as Janssen, who markets infliximab, and Takeda, who markets vedolizumab. Dr. Isaacs and Dr. Cohen-Mekelburg had no relevant financial conflicts to disclose.
This article was updated 3/31/21.
FROM GUT
Itch response faster with abrocitinib in trial comparing JAK inhibitor to dupilumab
, in a multicenter, randomized trial.
In addition, in the study, those on 200-mg and 100-mg daily doses of abrocitinib experienced significantly greater reductions in signs and symptoms of AD at 12 and 16 weeks, than those on placebo, the authors reported.
The findings from the JADE COMPARE trial, published on March 25 in the New England Journal of Medicine, suggest abrocitinib will provide clinicians with another treatment option for patients who don’t get adequate relief from either topical medications or dupilumab. Abrocitinib is associated with a different set of adverse reactions than dupilumab, according to investigators.
In 2017, dupilumab (Dupixent) became the first systemic drug approved by the Food and Drug Administration specifically for AD, though systemic steroids and other immunosuppressant drugs are sometimes prescribed. A monoclonal antibody delivered by subcutaneous injection, dupilumab binds to interleukin-4 receptors to block signaling pathways involved in AD; it is now approved for treatment of patients with moderate to severe AD down to age 6 years.
“It is sort of the bar for efficacy and for safety in those patients, because that’s what we have right now,” said one of the JADE Compare investigators and study author, Jonathan I. Silverberg, MD, PhD, MPH, associate professor of dermatology and director of clinical research and contact dermatitis at George Washington University, Washington, said in an interview. “For any new therapy coming to market, we really do want to understand how it compares to what’s out there.”
Abrocitinib is a small molecule that inhibits JAK1, which is thought to modulate multiple cytokines involved in AD, including interleukin (IL)–4, IL-13, IL-31, IL-22, and thymic stromal lymphopoietin. Two other JAK1 inhibitors, baricitinib and upadacitinib, are also being investigated as systemic treatments for AD.
In JADE COMPARE, people with moderate to severe AD from 18 countries on four continents, entered a 28-day screening period during which they discontinued treatments. They began using emollients twice a day at least 7 days before being randomly assigned to a treatment group, and continued on topical medication once daily. Topical treatments included low- or medium-potency topical glucocorticoids, calcineurin inhibitors, and phosphodiesterase-4 inhibitors.
The researchers randomly assigned 838 to trial groups: 226 received 200 mg of abrocitinib orally once a day, 238 received 100 mg of abrocitinib once a day, 243 received a 300-mg dupilumab injection every other week, and 131 received placebo versions of both medications, for 16 weeks. The mean age of the patients overall was about 38 years; about two-thirds were White.
At 2 weeks, half of the patients on 200 mg of abrocitinib and 31.8% of those on the 100-mg dose had an itch response, defined as at least a 4-point improvement from baseline in the 0-10 Peak Pruritus Numerical Rating Scale. This was compared with 26.4% of those on dupilumab and 13.8% of those on placebo.
And at 12 weeks, more of the patients in the 200-mg abrocitinib group than in the other groups had an Investigator’s Global Assessment (IGA) response (defined as clear or almost clear) and more had an Eczema Area and Severity Index (EASI-75) response (defined as an improvement of at least 75%). (See Table) EASI-75 and IGA responses at week 12 were the primary outcomes of the study.
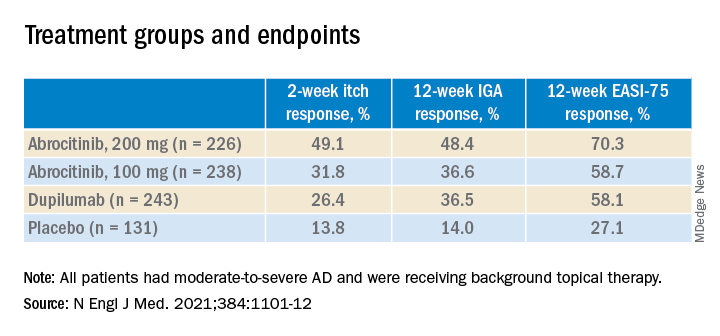
The differences between both abrocitinib groups and the placebo group were statistically significant by all these measures (P < .001). The difference between the 200-mg abrocitinib and the dupilumab group was only significant for itch at 2 weeks, and the difference in itch response between the 100-mg group and the dupilumab group at 2 weeks was not significant (P < .20).
At 16 weeks, the EASI-75 response (a secondary endpoint) among those on either dose of abrocitinib was not significantly different than among those on dupilumab (71% and 60.3% among those on 200 mg and 100 mg, respectively; and 65.5% among those on dupilumab, compared with 30.6% of those on placebo).
“The patients I have on this medicine [abrocitinib] are very happy,” said one of the study authors, Melinda Gooderham, MsC, MD, an assistant professor at Queen’s University, Kingston, Ont., an investigator in the trial. “It works very quickly for itch,” she said in an interview.
The study didn’t have sufficient statistical power to fully explore the comparison to dupilumab, and future trials will go deeper into the comparison, she added.
Still, in this trial, abrocitinib demonstrated a clear advantage in the speed and depth of efficacy, Dr. Silverberg noted. “The 100-mg dose of abrocitinib was about as effective as, or maybe slightly less effective than, dupilumab, and the 200-mg dose was more effective than dupilumab.”
The overall incidence of adverse events was higher in the 200-mg abrocitinib arm than in the other groups, but the incidence of serious or severe adverse events, and the incidence of adverse events that resulted in discontinuing the medication, were similar across the trial groups.
However, nausea affected 11.1% of the patients in the 200-mg abrocitinib group and 4.2% of those in the 100-mg abrocitinib group. Acne was also reported in these groups (6.6% and 2.9%, among those on 200 mg and 100 mg, respectively, compared with 1.2% of those on dupilumab and none of those on placebo). In a few of those on abrocitinib, herpes zoster flared up. And median platelet counts decreased among the patients taking abrocitinib, although none dropped below 75,000/mm3. Serious infections were reported in two patients on abrocitinib, but resolved.
By contrast, only 2.9% of the patients on dupilumab had nausea. But 6.2% in the dupilumab group had conjunctivitis, compared with 1.3% of patients in the 200-mg abrocitinib group and 0.8 in the 100-mg abrocitinib group.
As an oral medication, abrocitinib will appeal to patients who want to avoid injections, and dosing will be easier to adjust, Dr. Silverberg said. On the other hand, he added, dupilumab will have an advantage for patients who don’t want to take a daily medication, or who are concerned about the adverse events associated with abrocitinib, particularly those with blood-clotting disorders.
On the basis of two previous JADE phase 3 trials, Pfizer has submitted a new drug application for abrocitinib for treating moderate to severe AD in patients aged 12 and older to the FDA; a decision is expected in April, according to the company. The company has also applied to market the drug in Europe and the United Kingdom.
The study was funded by Pfizer. Dr. Silverberg’s disclosures included serving as a consultant to companies including AbbVie, Pfizer, and Regeneron. Several authors are Pfizer employees; other authors had disclosures related to Pfizer and other pharmaceutical companies.
, in a multicenter, randomized trial.
In addition, in the study, those on 200-mg and 100-mg daily doses of abrocitinib experienced significantly greater reductions in signs and symptoms of AD at 12 and 16 weeks, than those on placebo, the authors reported.
The findings from the JADE COMPARE trial, published on March 25 in the New England Journal of Medicine, suggest abrocitinib will provide clinicians with another treatment option for patients who don’t get adequate relief from either topical medications or dupilumab. Abrocitinib is associated with a different set of adverse reactions than dupilumab, according to investigators.
In 2017, dupilumab (Dupixent) became the first systemic drug approved by the Food and Drug Administration specifically for AD, though systemic steroids and other immunosuppressant drugs are sometimes prescribed. A monoclonal antibody delivered by subcutaneous injection, dupilumab binds to interleukin-4 receptors to block signaling pathways involved in AD; it is now approved for treatment of patients with moderate to severe AD down to age 6 years.
“It is sort of the bar for efficacy and for safety in those patients, because that’s what we have right now,” said one of the JADE Compare investigators and study author, Jonathan I. Silverberg, MD, PhD, MPH, associate professor of dermatology and director of clinical research and contact dermatitis at George Washington University, Washington, said in an interview. “For any new therapy coming to market, we really do want to understand how it compares to what’s out there.”
Abrocitinib is a small molecule that inhibits JAK1, which is thought to modulate multiple cytokines involved in AD, including interleukin (IL)–4, IL-13, IL-31, IL-22, and thymic stromal lymphopoietin. Two other JAK1 inhibitors, baricitinib and upadacitinib, are also being investigated as systemic treatments for AD.
In JADE COMPARE, people with moderate to severe AD from 18 countries on four continents, entered a 28-day screening period during which they discontinued treatments. They began using emollients twice a day at least 7 days before being randomly assigned to a treatment group, and continued on topical medication once daily. Topical treatments included low- or medium-potency topical glucocorticoids, calcineurin inhibitors, and phosphodiesterase-4 inhibitors.
The researchers randomly assigned 838 to trial groups: 226 received 200 mg of abrocitinib orally once a day, 238 received 100 mg of abrocitinib once a day, 243 received a 300-mg dupilumab injection every other week, and 131 received placebo versions of both medications, for 16 weeks. The mean age of the patients overall was about 38 years; about two-thirds were White.
At 2 weeks, half of the patients on 200 mg of abrocitinib and 31.8% of those on the 100-mg dose had an itch response, defined as at least a 4-point improvement from baseline in the 0-10 Peak Pruritus Numerical Rating Scale. This was compared with 26.4% of those on dupilumab and 13.8% of those on placebo.
And at 12 weeks, more of the patients in the 200-mg abrocitinib group than in the other groups had an Investigator’s Global Assessment (IGA) response (defined as clear or almost clear) and more had an Eczema Area and Severity Index (EASI-75) response (defined as an improvement of at least 75%). (See Table) EASI-75 and IGA responses at week 12 were the primary outcomes of the study.

The differences between both abrocitinib groups and the placebo group were statistically significant by all these measures (P < .001). The difference between the 200-mg abrocitinib and the dupilumab group was only significant for itch at 2 weeks, and the difference in itch response between the 100-mg group and the dupilumab group at 2 weeks was not significant (P < .20).
At 16 weeks, the EASI-75 response (a secondary endpoint) among those on either dose of abrocitinib was not significantly different than among those on dupilumab (71% and 60.3% among those on 200 mg and 100 mg, respectively; and 65.5% among those on dupilumab, compared with 30.6% of those on placebo).
“The patients I have on this medicine [abrocitinib] are very happy,” said one of the study authors, Melinda Gooderham, MsC, MD, an assistant professor at Queen’s University, Kingston, Ont., an investigator in the trial. “It works very quickly for itch,” she said in an interview.
The study didn’t have sufficient statistical power to fully explore the comparison to dupilumab, and future trials will go deeper into the comparison, she added.
Still, in this trial, abrocitinib demonstrated a clear advantage in the speed and depth of efficacy, Dr. Silverberg noted. “The 100-mg dose of abrocitinib was about as effective as, or maybe slightly less effective than, dupilumab, and the 200-mg dose was more effective than dupilumab.”
The overall incidence of adverse events was higher in the 200-mg abrocitinib arm than in the other groups, but the incidence of serious or severe adverse events, and the incidence of adverse events that resulted in discontinuing the medication, were similar across the trial groups.
However, nausea affected 11.1% of the patients in the 200-mg abrocitinib group and 4.2% of those in the 100-mg abrocitinib group. Acne was also reported in these groups (6.6% and 2.9%, among those on 200 mg and 100 mg, respectively, compared with 1.2% of those on dupilumab and none of those on placebo). In a few of those on abrocitinib, herpes zoster flared up. And median platelet counts decreased among the patients taking abrocitinib, although none dropped below 75,000/mm3. Serious infections were reported in two patients on abrocitinib, but resolved.
By contrast, only 2.9% of the patients on dupilumab had nausea. But 6.2% in the dupilumab group had conjunctivitis, compared with 1.3% of patients in the 200-mg abrocitinib group and 0.8 in the 100-mg abrocitinib group.
As an oral medication, abrocitinib will appeal to patients who want to avoid injections, and dosing will be easier to adjust, Dr. Silverberg said. On the other hand, he added, dupilumab will have an advantage for patients who don’t want to take a daily medication, or who are concerned about the adverse events associated with abrocitinib, particularly those with blood-clotting disorders.
On the basis of two previous JADE phase 3 trials, Pfizer has submitted a new drug application for abrocitinib for treating moderate to severe AD in patients aged 12 and older to the FDA; a decision is expected in April, according to the company. The company has also applied to market the drug in Europe and the United Kingdom.
The study was funded by Pfizer. Dr. Silverberg’s disclosures included serving as a consultant to companies including AbbVie, Pfizer, and Regeneron. Several authors are Pfizer employees; other authors had disclosures related to Pfizer and other pharmaceutical companies.
, in a multicenter, randomized trial.
In addition, in the study, those on 200-mg and 100-mg daily doses of abrocitinib experienced significantly greater reductions in signs and symptoms of AD at 12 and 16 weeks, than those on placebo, the authors reported.
The findings from the JADE COMPARE trial, published on March 25 in the New England Journal of Medicine, suggest abrocitinib will provide clinicians with another treatment option for patients who don’t get adequate relief from either topical medications or dupilumab. Abrocitinib is associated with a different set of adverse reactions than dupilumab, according to investigators.
In 2017, dupilumab (Dupixent) became the first systemic drug approved by the Food and Drug Administration specifically for AD, though systemic steroids and other immunosuppressant drugs are sometimes prescribed. A monoclonal antibody delivered by subcutaneous injection, dupilumab binds to interleukin-4 receptors to block signaling pathways involved in AD; it is now approved for treatment of patients with moderate to severe AD down to age 6 years.
“It is sort of the bar for efficacy and for safety in those patients, because that’s what we have right now,” said one of the JADE Compare investigators and study author, Jonathan I. Silverberg, MD, PhD, MPH, associate professor of dermatology and director of clinical research and contact dermatitis at George Washington University, Washington, said in an interview. “For any new therapy coming to market, we really do want to understand how it compares to what’s out there.”
Abrocitinib is a small molecule that inhibits JAK1, which is thought to modulate multiple cytokines involved in AD, including interleukin (IL)–4, IL-13, IL-31, IL-22, and thymic stromal lymphopoietin. Two other JAK1 inhibitors, baricitinib and upadacitinib, are also being investigated as systemic treatments for AD.
In JADE COMPARE, people with moderate to severe AD from 18 countries on four continents, entered a 28-day screening period during which they discontinued treatments. They began using emollients twice a day at least 7 days before being randomly assigned to a treatment group, and continued on topical medication once daily. Topical treatments included low- or medium-potency topical glucocorticoids, calcineurin inhibitors, and phosphodiesterase-4 inhibitors.
The researchers randomly assigned 838 to trial groups: 226 received 200 mg of abrocitinib orally once a day, 238 received 100 mg of abrocitinib once a day, 243 received a 300-mg dupilumab injection every other week, and 131 received placebo versions of both medications, for 16 weeks. The mean age of the patients overall was about 38 years; about two-thirds were White.
At 2 weeks, half of the patients on 200 mg of abrocitinib and 31.8% of those on the 100-mg dose had an itch response, defined as at least a 4-point improvement from baseline in the 0-10 Peak Pruritus Numerical Rating Scale. This was compared with 26.4% of those on dupilumab and 13.8% of those on placebo.
And at 12 weeks, more of the patients in the 200-mg abrocitinib group than in the other groups had an Investigator’s Global Assessment (IGA) response (defined as clear or almost clear) and more had an Eczema Area and Severity Index (EASI-75) response (defined as an improvement of at least 75%). (See Table) EASI-75 and IGA responses at week 12 were the primary outcomes of the study.

The differences between both abrocitinib groups and the placebo group were statistically significant by all these measures (P < .001). The difference between the 200-mg abrocitinib and the dupilumab group was only significant for itch at 2 weeks, and the difference in itch response between the 100-mg group and the dupilumab group at 2 weeks was not significant (P < .20).
At 16 weeks, the EASI-75 response (a secondary endpoint) among those on either dose of abrocitinib was not significantly different than among those on dupilumab (71% and 60.3% among those on 200 mg and 100 mg, respectively; and 65.5% among those on dupilumab, compared with 30.6% of those on placebo).
“The patients I have on this medicine [abrocitinib] are very happy,” said one of the study authors, Melinda Gooderham, MsC, MD, an assistant professor at Queen’s University, Kingston, Ont., an investigator in the trial. “It works very quickly for itch,” she said in an interview.
The study didn’t have sufficient statistical power to fully explore the comparison to dupilumab, and future trials will go deeper into the comparison, she added.
Still, in this trial, abrocitinib demonstrated a clear advantage in the speed and depth of efficacy, Dr. Silverberg noted. “The 100-mg dose of abrocitinib was about as effective as, or maybe slightly less effective than, dupilumab, and the 200-mg dose was more effective than dupilumab.”
The overall incidence of adverse events was higher in the 200-mg abrocitinib arm than in the other groups, but the incidence of serious or severe adverse events, and the incidence of adverse events that resulted in discontinuing the medication, were similar across the trial groups.
However, nausea affected 11.1% of the patients in the 200-mg abrocitinib group and 4.2% of those in the 100-mg abrocitinib group. Acne was also reported in these groups (6.6% and 2.9%, among those on 200 mg and 100 mg, respectively, compared with 1.2% of those on dupilumab and none of those on placebo). In a few of those on abrocitinib, herpes zoster flared up. And median platelet counts decreased among the patients taking abrocitinib, although none dropped below 75,000/mm3. Serious infections were reported in two patients on abrocitinib, but resolved.
By contrast, only 2.9% of the patients on dupilumab had nausea. But 6.2% in the dupilumab group had conjunctivitis, compared with 1.3% of patients in the 200-mg abrocitinib group and 0.8 in the 100-mg abrocitinib group.
As an oral medication, abrocitinib will appeal to patients who want to avoid injections, and dosing will be easier to adjust, Dr. Silverberg said. On the other hand, he added, dupilumab will have an advantage for patients who don’t want to take a daily medication, or who are concerned about the adverse events associated with abrocitinib, particularly those with blood-clotting disorders.
On the basis of two previous JADE phase 3 trials, Pfizer has submitted a new drug application for abrocitinib for treating moderate to severe AD in patients aged 12 and older to the FDA; a decision is expected in April, according to the company. The company has also applied to market the drug in Europe and the United Kingdom.
The study was funded by Pfizer. Dr. Silverberg’s disclosures included serving as a consultant to companies including AbbVie, Pfizer, and Regeneron. Several authors are Pfizer employees; other authors had disclosures related to Pfizer and other pharmaceutical companies.
Match Day 2021: Internal medicine keeps growing
according to the National Resident Matching Program.
“Rather than faltering in these uncertain times, program fill rates increased across the board,” the NRMP said in a written statement. Overall, the 2021 Main Residency Match offered (35,194) and filled (33,353) record numbers of first-year (PGY-1) slots. That fill rate of 94.8% was up from 94.6% the year before.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” said Donna L. Lamb, DHSc, MBA, president and CEO of the NRMP.
Internal medicine offered 9,024 positions in this year’s Match, up by 3.8% over 2020, and filled 8,632, for a 1-year increase of 3.7% and a fill rate of 95.7%. Over 55% (5,005) of the available slots were given to U.S. seniors (MDs and DOs), while 37.9% went to international medical graduates. The corresponding PGY-1 numbers for the Match as a whole were 70.4% U.S. and 21.1% international medical graduates, based on NRMP data.
The number of positions offered in internal medicine residencies has increased by 1,791 (24.8%) since 2017, and such growth over time may “be a predictor of future physician workforce supply,” the NRMP suggested. Internal medicine also increased its share of all available residency positions from 24.9% in 2018 to 25.6% in 2021.
“Concerns about the impact of virtual recruitment on applicants’ matching into PGY-1 positions were not realized,” the NRMP noted, as “growth in registration was seen in every applicant group.” Compared with 2020, submissions of rank-order lists of programs were up by 2.8% for U.S. MD seniors, 7.9% for U.S. DO seniors, 2.5% among U.S.-citizen IMGs, and 15.0% for non–U.S.-citizen IMGs.
“The internal medicine workforce remains the backbone of our health care system, and expansion of this workforce is imperative to provide access to specialty and subspecialty medical care for future patients,” Philip A. Masters, MD, vice president of membership and global engagement at the American College of Physicians, said in a separate statement.
according to the National Resident Matching Program.

“Rather than faltering in these uncertain times, program fill rates increased across the board,” the NRMP said in a written statement. Overall, the 2021 Main Residency Match offered (35,194) and filled (33,353) record numbers of first-year (PGY-1) slots. That fill rate of 94.8% was up from 94.6% the year before.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” said Donna L. Lamb, DHSc, MBA, president and CEO of the NRMP.
Internal medicine offered 9,024 positions in this year’s Match, up by 3.8% over 2020, and filled 8,632, for a 1-year increase of 3.7% and a fill rate of 95.7%. Over 55% (5,005) of the available slots were given to U.S. seniors (MDs and DOs), while 37.9% went to international medical graduates. The corresponding PGY-1 numbers for the Match as a whole were 70.4% U.S. and 21.1% international medical graduates, based on NRMP data.
The number of positions offered in internal medicine residencies has increased by 1,791 (24.8%) since 2017, and such growth over time may “be a predictor of future physician workforce supply,” the NRMP suggested. Internal medicine also increased its share of all available residency positions from 24.9% in 2018 to 25.6% in 2021.
“Concerns about the impact of virtual recruitment on applicants’ matching into PGY-1 positions were not realized,” the NRMP noted, as “growth in registration was seen in every applicant group.” Compared with 2020, submissions of rank-order lists of programs were up by 2.8% for U.S. MD seniors, 7.9% for U.S. DO seniors, 2.5% among U.S.-citizen IMGs, and 15.0% for non–U.S.-citizen IMGs.
“The internal medicine workforce remains the backbone of our health care system, and expansion of this workforce is imperative to provide access to specialty and subspecialty medical care for future patients,” Philip A. Masters, MD, vice president of membership and global engagement at the American College of Physicians, said in a separate statement.
according to the National Resident Matching Program.

“Rather than faltering in these uncertain times, program fill rates increased across the board,” the NRMP said in a written statement. Overall, the 2021 Main Residency Match offered (35,194) and filled (33,353) record numbers of first-year (PGY-1) slots. That fill rate of 94.8% was up from 94.6% the year before.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” said Donna L. Lamb, DHSc, MBA, president and CEO of the NRMP.
Internal medicine offered 9,024 positions in this year’s Match, up by 3.8% over 2020, and filled 8,632, for a 1-year increase of 3.7% and a fill rate of 95.7%. Over 55% (5,005) of the available slots were given to U.S. seniors (MDs and DOs), while 37.9% went to international medical graduates. The corresponding PGY-1 numbers for the Match as a whole were 70.4% U.S. and 21.1% international medical graduates, based on NRMP data.
The number of positions offered in internal medicine residencies has increased by 1,791 (24.8%) since 2017, and such growth over time may “be a predictor of future physician workforce supply,” the NRMP suggested. Internal medicine also increased its share of all available residency positions from 24.9% in 2018 to 25.6% in 2021.
“Concerns about the impact of virtual recruitment on applicants’ matching into PGY-1 positions were not realized,” the NRMP noted, as “growth in registration was seen in every applicant group.” Compared with 2020, submissions of rank-order lists of programs were up by 2.8% for U.S. MD seniors, 7.9% for U.S. DO seniors, 2.5% among U.S.-citizen IMGs, and 15.0% for non–U.S.-citizen IMGs.
“The internal medicine workforce remains the backbone of our health care system, and expansion of this workforce is imperative to provide access to specialty and subspecialty medical care for future patients,” Philip A. Masters, MD, vice president of membership and global engagement at the American College of Physicians, said in a separate statement.
Postintubation tracheal injury in the COVID-19 era
Postintubation laryngeal and tracheal injuries may be yet another part of recovery from severe COVID-19 infection for some patients.
Evidence has been accumulating on the link between prolonged intubation and lingering breathing and speaking difficulties, a concern that has become more germane in the wake of the COVID-19 pandemic. Now, researchers in Italy led by Giacomo Fiacchini, MD, and Luca Bruschini, MD, of the University of Pisa have published new research suggesting tracheal complications were particularly common in COVID-19 patients intubated for prolonged periods during the pandemic.
The study may be revealing effects of the pandemic itself, as resources and staff were at times overwhelmed by critical care patients. Of the 98 patients admitted from March 1 to May 31, 47% intubated for longer than 14 days developed full-thickness tracheal lesions, compared with 2.2% of a control group treated during the same time frame in 2019. The difference is eye-popping, but may not be generalizable. “I have not observed an increased rate of tracheal injury, but we haven’t carefully studied that outcome as far as I know,” said Daniel Ouellette, MD, FCCP, who is a senior staff physician and director of the pulmonary inpatient unit at Henry Ford Health System, and an associate professor at Wayne State University, Detroit.
He expressed concern about the retrospective nature of the study, and wondered if the different outcomes might be because of disruptions caused by the pandemic. “It’s not hard to imagine that these patients were seen [during] a great rush of patients, whereas the control group was looked at during a period where that kind of volume didn’t exist. There might have been a tendency for more inexperienced practitioners to be intubating patients because they were in the middle of the epidemic. There might have been less supervision of trainees. Individuals, physicians, teams may have been more rushed. Protocols may not have been followed as closely. It may all be an effect of the epidemic itself,” said Dr. Ouellette.
The investigators suggested that implementation of pronation maneuvers may have increased cuff pressure on the tracheal walls leading to some injuries. In addition, the prothrombotic and antifibrinolytic state of patients with COVID-19 may have contributed, along with the impact of systemic steroids that may have altered normal healing of tracheal wall microwounds caused by intubation, cuff pressure, or tracheostomy.
Other research has suggested increased complications from intubation among COVID-19 patients, including a case series that found heightened frequency of pneumomediastinum. The authors of that study suggested that aggressive disease pathophysiology and accompanying risk of alveolar damage and tracheobronchial injury may be to blame, along with larger-bore tracheal tubes and higher ventilation pressures. That study may also be reflecting the conditions of intubation during the pandemic.
Not all institutions saw an uptick in tracheal injury or pneumomediastinum. Mary Jo S. Farmer, MD, PhD, FCCP, of the department of medicine at University of Massachusetts, Springfield, asked one of the institute’s statisticians to examine pneumothorax frequency from March 15, 2020, to March 1, 2021, comparing the rates between patients who tested positive for SARS-CoV-2 within 14 days of admission, and those who tested negative. The rate was 0.5% in patients who tested positive versus 0.4% in those who tested negative. “My division chief’s gut sense is it’s just the same. The prevalence [of pneumomediastinum] is what we were seeing before,” said Dr. Farmer.
Shortly before the COVID-19 pandemic, researchers at Vanderbilt University Medical Center found that more than half of patients undergoing prolonged intubation experience breathing and speaking difficulties at 10 weeks post intubation. The group has followed up that study with another study looking at treatment timing and outcomes.
The researchers reviewed the experiences of 29 patients with laryngeal injury from endotracheal intubation between May 1, 2014,- and June 1, 2018. Ten patients with posterior glottis injury received early treatment, at a median of 34.7 days to presentation (interquartile range, 1.5-44.8 days). Nineteen patients with posterior glottis stenosis received treatment at a median of 341.9 days (absolute difference, 307.2 days; 95% confidence interval, 124.4-523.3 days). Demographic characteristics and comorbidities were similar between the two groups. At last follow-up, 90% of the early-treatment group were decannulated, compared with 58% of the late group (absolute difference, 32%; 95% CI, –3% to 68%). The early group required a mean of 2.2 interventions, compared with 11.5 in the late group (absolute difference, 9.3; 95% CI, 6.4-12.1). No patients in the early group required an open procedure, compared with 90% of the late-treatment group.
Although early treatment seems promising, the timing of laryngeal injury repair would be a key consideration. “You would worry about patient stability, [making] sure they’re clinically stable and didn’t have any acute ill effects from the injury itself or the underlying illness that led to intubation,” said Dr. Ouellette. For COVID-19 patients, that would mean recovery from pneumonia or any other lung problems, he added.
Together, the studies raise concerns and questions over tracheal and laryngeal injury in the context of COVID-19, but fall short of providing clinical guidance. “It raises the awareness in the mind of the critical care physician about these potential injuries to the larynx surrounding intubation,” said Dr. Farmer.
The studies received no funding. Dr. Ouellette and Dr. Farmer reported no relevant financial disclosures.
Postintubation laryngeal and tracheal injuries may be yet another part of recovery from severe COVID-19 infection for some patients.
Evidence has been accumulating on the link between prolonged intubation and lingering breathing and speaking difficulties, a concern that has become more germane in the wake of the COVID-19 pandemic. Now, researchers in Italy led by Giacomo Fiacchini, MD, and Luca Bruschini, MD, of the University of Pisa have published new research suggesting tracheal complications were particularly common in COVID-19 patients intubated for prolonged periods during the pandemic.
The study may be revealing effects of the pandemic itself, as resources and staff were at times overwhelmed by critical care patients. Of the 98 patients admitted from March 1 to May 31, 47% intubated for longer than 14 days developed full-thickness tracheal lesions, compared with 2.2% of a control group treated during the same time frame in 2019. The difference is eye-popping, but may not be generalizable. “I have not observed an increased rate of tracheal injury, but we haven’t carefully studied that outcome as far as I know,” said Daniel Ouellette, MD, FCCP, who is a senior staff physician and director of the pulmonary inpatient unit at Henry Ford Health System, and an associate professor at Wayne State University, Detroit.
He expressed concern about the retrospective nature of the study, and wondered if the different outcomes might be because of disruptions caused by the pandemic. “It’s not hard to imagine that these patients were seen [during] a great rush of patients, whereas the control group was looked at during a period where that kind of volume didn’t exist. There might have been a tendency for more inexperienced practitioners to be intubating patients because they were in the middle of the epidemic. There might have been less supervision of trainees. Individuals, physicians, teams may have been more rushed. Protocols may not have been followed as closely. It may all be an effect of the epidemic itself,” said Dr. Ouellette.
The investigators suggested that implementation of pronation maneuvers may have increased cuff pressure on the tracheal walls leading to some injuries. In addition, the prothrombotic and antifibrinolytic state of patients with COVID-19 may have contributed, along with the impact of systemic steroids that may have altered normal healing of tracheal wall microwounds caused by intubation, cuff pressure, or tracheostomy.
Other research has suggested increased complications from intubation among COVID-19 patients, including a case series that found heightened frequency of pneumomediastinum. The authors of that study suggested that aggressive disease pathophysiology and accompanying risk of alveolar damage and tracheobronchial injury may be to blame, along with larger-bore tracheal tubes and higher ventilation pressures. That study may also be reflecting the conditions of intubation during the pandemic.
Not all institutions saw an uptick in tracheal injury or pneumomediastinum. Mary Jo S. Farmer, MD, PhD, FCCP, of the department of medicine at University of Massachusetts, Springfield, asked one of the institute’s statisticians to examine pneumothorax frequency from March 15, 2020, to March 1, 2021, comparing the rates between patients who tested positive for SARS-CoV-2 within 14 days of admission, and those who tested negative. The rate was 0.5% in patients who tested positive versus 0.4% in those who tested negative. “My division chief’s gut sense is it’s just the same. The prevalence [of pneumomediastinum] is what we were seeing before,” said Dr. Farmer.
Shortly before the COVID-19 pandemic, researchers at Vanderbilt University Medical Center found that more than half of patients undergoing prolonged intubation experience breathing and speaking difficulties at 10 weeks post intubation. The group has followed up that study with another study looking at treatment timing and outcomes.
The researchers reviewed the experiences of 29 patients with laryngeal injury from endotracheal intubation between May 1, 2014,- and June 1, 2018. Ten patients with posterior glottis injury received early treatment, at a median of 34.7 days to presentation (interquartile range, 1.5-44.8 days). Nineteen patients with posterior glottis stenosis received treatment at a median of 341.9 days (absolute difference, 307.2 days; 95% confidence interval, 124.4-523.3 days). Demographic characteristics and comorbidities were similar between the two groups. At last follow-up, 90% of the early-treatment group were decannulated, compared with 58% of the late group (absolute difference, 32%; 95% CI, –3% to 68%). The early group required a mean of 2.2 interventions, compared with 11.5 in the late group (absolute difference, 9.3; 95% CI, 6.4-12.1). No patients in the early group required an open procedure, compared with 90% of the late-treatment group.
Although early treatment seems promising, the timing of laryngeal injury repair would be a key consideration. “You would worry about patient stability, [making] sure they’re clinically stable and didn’t have any acute ill effects from the injury itself or the underlying illness that led to intubation,” said Dr. Ouellette. For COVID-19 patients, that would mean recovery from pneumonia or any other lung problems, he added.
Together, the studies raise concerns and questions over tracheal and laryngeal injury in the context of COVID-19, but fall short of providing clinical guidance. “It raises the awareness in the mind of the critical care physician about these potential injuries to the larynx surrounding intubation,” said Dr. Farmer.
The studies received no funding. Dr. Ouellette and Dr. Farmer reported no relevant financial disclosures.
Postintubation laryngeal and tracheal injuries may be yet another part of recovery from severe COVID-19 infection for some patients.
Evidence has been accumulating on the link between prolonged intubation and lingering breathing and speaking difficulties, a concern that has become more germane in the wake of the COVID-19 pandemic. Now, researchers in Italy led by Giacomo Fiacchini, MD, and Luca Bruschini, MD, of the University of Pisa have published new research suggesting tracheal complications were particularly common in COVID-19 patients intubated for prolonged periods during the pandemic.
The study may be revealing effects of the pandemic itself, as resources and staff were at times overwhelmed by critical care patients. Of the 98 patients admitted from March 1 to May 31, 47% intubated for longer than 14 days developed full-thickness tracheal lesions, compared with 2.2% of a control group treated during the same time frame in 2019. The difference is eye-popping, but may not be generalizable. “I have not observed an increased rate of tracheal injury, but we haven’t carefully studied that outcome as far as I know,” said Daniel Ouellette, MD, FCCP, who is a senior staff physician and director of the pulmonary inpatient unit at Henry Ford Health System, and an associate professor at Wayne State University, Detroit.
He expressed concern about the retrospective nature of the study, and wondered if the different outcomes might be because of disruptions caused by the pandemic. “It’s not hard to imagine that these patients were seen [during] a great rush of patients, whereas the control group was looked at during a period where that kind of volume didn’t exist. There might have been a tendency for more inexperienced practitioners to be intubating patients because they were in the middle of the epidemic. There might have been less supervision of trainees. Individuals, physicians, teams may have been more rushed. Protocols may not have been followed as closely. It may all be an effect of the epidemic itself,” said Dr. Ouellette.
The investigators suggested that implementation of pronation maneuvers may have increased cuff pressure on the tracheal walls leading to some injuries. In addition, the prothrombotic and antifibrinolytic state of patients with COVID-19 may have contributed, along with the impact of systemic steroids that may have altered normal healing of tracheal wall microwounds caused by intubation, cuff pressure, or tracheostomy.
Other research has suggested increased complications from intubation among COVID-19 patients, including a case series that found heightened frequency of pneumomediastinum. The authors of that study suggested that aggressive disease pathophysiology and accompanying risk of alveolar damage and tracheobronchial injury may be to blame, along with larger-bore tracheal tubes and higher ventilation pressures. That study may also be reflecting the conditions of intubation during the pandemic.
Not all institutions saw an uptick in tracheal injury or pneumomediastinum. Mary Jo S. Farmer, MD, PhD, FCCP, of the department of medicine at University of Massachusetts, Springfield, asked one of the institute’s statisticians to examine pneumothorax frequency from March 15, 2020, to March 1, 2021, comparing the rates between patients who tested positive for SARS-CoV-2 within 14 days of admission, and those who tested negative. The rate was 0.5% in patients who tested positive versus 0.4% in those who tested negative. “My division chief’s gut sense is it’s just the same. The prevalence [of pneumomediastinum] is what we were seeing before,” said Dr. Farmer.
Shortly before the COVID-19 pandemic, researchers at Vanderbilt University Medical Center found that more than half of patients undergoing prolonged intubation experience breathing and speaking difficulties at 10 weeks post intubation. The group has followed up that study with another study looking at treatment timing and outcomes.
The researchers reviewed the experiences of 29 patients with laryngeal injury from endotracheal intubation between May 1, 2014,- and June 1, 2018. Ten patients with posterior glottis injury received early treatment, at a median of 34.7 days to presentation (interquartile range, 1.5-44.8 days). Nineteen patients with posterior glottis stenosis received treatment at a median of 341.9 days (absolute difference, 307.2 days; 95% confidence interval, 124.4-523.3 days). Demographic characteristics and comorbidities were similar between the two groups. At last follow-up, 90% of the early-treatment group were decannulated, compared with 58% of the late group (absolute difference, 32%; 95% CI, –3% to 68%). The early group required a mean of 2.2 interventions, compared with 11.5 in the late group (absolute difference, 9.3; 95% CI, 6.4-12.1). No patients in the early group required an open procedure, compared with 90% of the late-treatment group.
Although early treatment seems promising, the timing of laryngeal injury repair would be a key consideration. “You would worry about patient stability, [making] sure they’re clinically stable and didn’t have any acute ill effects from the injury itself or the underlying illness that led to intubation,” said Dr. Ouellette. For COVID-19 patients, that would mean recovery from pneumonia or any other lung problems, he added.
Together, the studies raise concerns and questions over tracheal and laryngeal injury in the context of COVID-19, but fall short of providing clinical guidance. “It raises the awareness in the mind of the critical care physician about these potential injuries to the larynx surrounding intubation,” said Dr. Farmer.
The studies received no funding. Dr. Ouellette and Dr. Farmer reported no relevant financial disclosures.
FROM JAMA OTOLARYNGOLOGY–HEAD & NECK SURGERY
Allo-HSCT plus monoclonal antibody treatment can improve survival in patients with r/r B-ALL
The use of allogeneic hematopoietic stem cell transplantation (allo-HSCT) can improve survival in minimal residual disease (MRD)–negative remission patients with relapsed/refractory (r/r) B-cell acute lymphoblastic leukemia (B-ALL) after the start of monoclonal antibody treatment, according to the results of a landmark analysis presented at the virtual meeting of the European Society for Blood and Marrow Transplantation.
Previous studies have indicated that allo-HSCT improves the results of treatment in r/r B-ALL patients, compared with chemotherapy alone. In addition, it has been found that the monoclonal antibodies (Mab), anti-CD19-blinatumomab and anti-CD22-inotuzumab ozogamicin, induced remission in a significant proportion of such patients.
To determine if the use of allo-HSCT improves the outcome of patients in MRD-negative remission with or without Mab treatment, researchers performed a landmark analysis of 110 patients who achieved MRD-negative status after Mab treatment. The analysis examined results at 2, 4, and 6 months subsequent to the initiation of Mab treatment, according to poster presentation by Inna V. Markova, MD, and colleagues at Pavlov University, Saint Petersburg, Russian Federation.
Study details
The researchers included 110 patients who achieved MRD-negative status outside of clinical trials at a single institution in the analysis. Forty of the patients (36%) were children and 70 (64%) were adults. The median age for all patients was 23 years and the median follow up was 24 months. Fifty-seven (52%) and 53 (48%) patients received Mab for hematological relapse and persistent measurable residual disease or for molecular relapse, respectively. Therapy with Mab alone without subsequent allo-HSCT was used in 36 (31%) patients (30 received blinatumomab and 6 received inotuzumab ozogamicin). A total of 74 (69%) patients received allo-HSCT from a matched related or unrelated donor (MD-HSCT, n = 38) or haploidentical donor (Haplo-HSCT, n = 36). All patients received posttransplantation cyclophosphamide (PTCY)–based graft-versus host disease (GVHD) prophylaxis. Landmark analysis was performed at 2, 4, and 6 months after Mab therapy initiation to determine the effect of allo-HSCT on the outcome and the optimal timing of HSCT. Overall survival and disease-free survival (DFS) were used as outcomes.
Promising results
No significant differences between the MD-HSCT, Mab alone, and Haplo-HSCT groups were observed in 2-month landmark analysis (P = .4 for OS and P =.65 for DFS). However, the 4-month landmark analysis demonstrated superior overall survival and DFS in patients after MD-HSCT, but not Haplo-HSCT, compared with Mab alone: 2-year OS was 75%, 50%, and 27,7% (P = .032) and DFS was 53.5%, 51.3%, and 16.6% (P = .02) for MD-HSCT, Mab alone and Haplo-HSCT groups, respectively. In addition, 6-month analysis showed that there was no benefit from subsequent transplantation, according to the authors, with regard to overall survival (P = .11).
“Our study demonstrated that at least MD-HSCT with PTCY platform improves survival in MRD-negative remission if performed during the first 4 months after Mab initiation. Haplo-HSCT or MD-HSCT beyond 4 months are not associated with improved outcomes in this groups of patients,” the researchers concluded.
The researchers reported they had no conflicts of interest to declare.
The use of allogeneic hematopoietic stem cell transplantation (allo-HSCT) can improve survival in minimal residual disease (MRD)–negative remission patients with relapsed/refractory (r/r) B-cell acute lymphoblastic leukemia (B-ALL) after the start of monoclonal antibody treatment, according to the results of a landmark analysis presented at the virtual meeting of the European Society for Blood and Marrow Transplantation.
Previous studies have indicated that allo-HSCT improves the results of treatment in r/r B-ALL patients, compared with chemotherapy alone. In addition, it has been found that the monoclonal antibodies (Mab), anti-CD19-blinatumomab and anti-CD22-inotuzumab ozogamicin, induced remission in a significant proportion of such patients.
To determine if the use of allo-HSCT improves the outcome of patients in MRD-negative remission with or without Mab treatment, researchers performed a landmark analysis of 110 patients who achieved MRD-negative status after Mab treatment. The analysis examined results at 2, 4, and 6 months subsequent to the initiation of Mab treatment, according to poster presentation by Inna V. Markova, MD, and colleagues at Pavlov University, Saint Petersburg, Russian Federation.
Study details
The researchers included 110 patients who achieved MRD-negative status outside of clinical trials at a single institution in the analysis. Forty of the patients (36%) were children and 70 (64%) were adults. The median age for all patients was 23 years and the median follow up was 24 months. Fifty-seven (52%) and 53 (48%) patients received Mab for hematological relapse and persistent measurable residual disease or for molecular relapse, respectively. Therapy with Mab alone without subsequent allo-HSCT was used in 36 (31%) patients (30 received blinatumomab and 6 received inotuzumab ozogamicin). A total of 74 (69%) patients received allo-HSCT from a matched related or unrelated donor (MD-HSCT, n = 38) or haploidentical donor (Haplo-HSCT, n = 36). All patients received posttransplantation cyclophosphamide (PTCY)–based graft-versus host disease (GVHD) prophylaxis. Landmark analysis was performed at 2, 4, and 6 months after Mab therapy initiation to determine the effect of allo-HSCT on the outcome and the optimal timing of HSCT. Overall survival and disease-free survival (DFS) were used as outcomes.
Promising results
No significant differences between the MD-HSCT, Mab alone, and Haplo-HSCT groups were observed in 2-month landmark analysis (P = .4 for OS and P =.65 for DFS). However, the 4-month landmark analysis demonstrated superior overall survival and DFS in patients after MD-HSCT, but not Haplo-HSCT, compared with Mab alone: 2-year OS was 75%, 50%, and 27,7% (P = .032) and DFS was 53.5%, 51.3%, and 16.6% (P = .02) for MD-HSCT, Mab alone and Haplo-HSCT groups, respectively. In addition, 6-month analysis showed that there was no benefit from subsequent transplantation, according to the authors, with regard to overall survival (P = .11).
“Our study demonstrated that at least MD-HSCT with PTCY platform improves survival in MRD-negative remission if performed during the first 4 months after Mab initiation. Haplo-HSCT or MD-HSCT beyond 4 months are not associated with improved outcomes in this groups of patients,” the researchers concluded.
The researchers reported they had no conflicts of interest to declare.
The use of allogeneic hematopoietic stem cell transplantation (allo-HSCT) can improve survival in minimal residual disease (MRD)–negative remission patients with relapsed/refractory (r/r) B-cell acute lymphoblastic leukemia (B-ALL) after the start of monoclonal antibody treatment, according to the results of a landmark analysis presented at the virtual meeting of the European Society for Blood and Marrow Transplantation.
Previous studies have indicated that allo-HSCT improves the results of treatment in r/r B-ALL patients, compared with chemotherapy alone. In addition, it has been found that the monoclonal antibodies (Mab), anti-CD19-blinatumomab and anti-CD22-inotuzumab ozogamicin, induced remission in a significant proportion of such patients.
To determine if the use of allo-HSCT improves the outcome of patients in MRD-negative remission with or without Mab treatment, researchers performed a landmark analysis of 110 patients who achieved MRD-negative status after Mab treatment. The analysis examined results at 2, 4, and 6 months subsequent to the initiation of Mab treatment, according to poster presentation by Inna V. Markova, MD, and colleagues at Pavlov University, Saint Petersburg, Russian Federation.
Study details
The researchers included 110 patients who achieved MRD-negative status outside of clinical trials at a single institution in the analysis. Forty of the patients (36%) were children and 70 (64%) were adults. The median age for all patients was 23 years and the median follow up was 24 months. Fifty-seven (52%) and 53 (48%) patients received Mab for hematological relapse and persistent measurable residual disease or for molecular relapse, respectively. Therapy with Mab alone without subsequent allo-HSCT was used in 36 (31%) patients (30 received blinatumomab and 6 received inotuzumab ozogamicin). A total of 74 (69%) patients received allo-HSCT from a matched related or unrelated donor (MD-HSCT, n = 38) or haploidentical donor (Haplo-HSCT, n = 36). All patients received posttransplantation cyclophosphamide (PTCY)–based graft-versus host disease (GVHD) prophylaxis. Landmark analysis was performed at 2, 4, and 6 months after Mab therapy initiation to determine the effect of allo-HSCT on the outcome and the optimal timing of HSCT. Overall survival and disease-free survival (DFS) were used as outcomes.
Promising results
No significant differences between the MD-HSCT, Mab alone, and Haplo-HSCT groups were observed in 2-month landmark analysis (P = .4 for OS and P =.65 for DFS). However, the 4-month landmark analysis demonstrated superior overall survival and DFS in patients after MD-HSCT, but not Haplo-HSCT, compared with Mab alone: 2-year OS was 75%, 50%, and 27,7% (P = .032) and DFS was 53.5%, 51.3%, and 16.6% (P = .02) for MD-HSCT, Mab alone and Haplo-HSCT groups, respectively. In addition, 6-month analysis showed that there was no benefit from subsequent transplantation, according to the authors, with regard to overall survival (P = .11).
“Our study demonstrated that at least MD-HSCT with PTCY platform improves survival in MRD-negative remission if performed during the first 4 months after Mab initiation. Haplo-HSCT or MD-HSCT beyond 4 months are not associated with improved outcomes in this groups of patients,” the researchers concluded.
The researchers reported they had no conflicts of interest to declare.
FROM EBMT 2021
Frail status may be better than age for predicting ovarian cancer outcomes
Baseball great Satchel Paige’s famous adage, “Age is a case of mind over matter. If you don’t mind, it don’t matter,” may also apply to candidates for ovarian cancer surgery. That’s because investigators have found that physical frailty is a better determinant of fitness for surgery than is calendar age.
Investigators conducted a retrospective analysis of 591 patients considered for primary resection of stage II to IV high-grade ovarian, fallopian tube, or peritoneal cancer. Results showed that a 10-item modified frailty index was better than patient age for predicting survival outcomes.
“Frailty does seem to correlate with age and increase with age, but it is not synonymous with age,” said investigator Katelyn F. Handley, MD, of the University of Texas MD Anderson Cancer Center in Houston.
“Frailty is a spectrum, and we can see patients of the same chronological age, but one may be a 76-year-old, ultra-distance triathlete, while another is in failing health and diminishing function,” Dr. Handley said at the Society of Gynecologic Oncology’s Virtual Annual Meeting on Women’s Cancer (Abstract 10463).
Dr. Handley cited a consensus definition of frailty, published in 2013, as “a medical syndrome with multiple causes and contributors that is characterized by diminished strength, endurance, and reduced physiologic function that increases an individual’s vulnerability for developing increased dependency and/or death.”
Ten-item score
To assess the effect of frailty in ovarian cancer patients on surgical procedures and outcomes, the investigators retrospectively applied a modified frailty index (mFI) to patients who were treated at MD Anderson Cancer Center from April 2013 through September 2017.
The index is a sum of 10 items: Chronic obstructive pulmonary disease or recent pneumonia, heart failure, myocardial infarction, coronary artery disease, diabetes, hypertension, peripheral vascular disease, cerebrovascular disease, cerebrovascular accident with neurologic deficit, and poor Eastern Cooperative Oncology Group performance status (3 or 4).
Of the 591 patients who met inclusion criteria, 57% had mFI scores of 0, indicating no frailty, 29% had one frailty factor, and 14% had two or more factors.
Patient age did significantly correlate with mFI scores. Patients with an mFI score of 0 had a median age of 62 years, the median age in those with a score of 1 was 69 years, and the median age for those with scores of 2 or higher was 70.5 years (P <. 001).
Charlson comorbidity index scores also significantly increased with age, with mean scores of 3.00, 3.83, and 5.14 in patients with mFI scores of 0, 1, or 2, respectively (P <. 001).
“But if you look at the age ranges in each category, you’ll notice that there are patients as young as 47 with an mFI of greater than or equal to 2, and as old as 89 with an mFI of 0,” Dr. Handley pointed out.
Higher scores, fewer assessments
The investigators found that patients with suspected ovarian cancer with frailty scores of 2 or higher were less likely to be offered laparoscopic assessment to determine primary resectability than were those with scores of 1 or 0 (28% vs. 43% and 49%, respectively, P = .004).
Among all patients who underwent laparoscopic assessment, the predictive index score (modified Fagotti score) was more likely to be 8 or higher in patients with high frailty scores (58%, 48%, and 34% for scores of 2 or greater, 1, and 0, respectively; P = .038).
Only 17% of the most frail patients went on to primary debulking surgery, compared with 26% of patients with a single frailty factor and 34% of those with none (P = .015).
Patients with higher frailty scores were less likely to undergo primary or interval tumor reductive surgery (59% vs. 74% for those with mFI scores of 1 and 85% for those with scores of 0; P <. 001). The frailest patients were significantly more likely to undergo splenectomy (20%, 3%, and 6%, respectively; P = .001) and small bowel resection (14%, 8%, and 3%, respectively; P = .006).
Two-thirds of the most frail patients (64%) had postoperative complications, primarily gastrointestinal and renal complications, compared with 56% and 44% of patients with mFI scores of 1 or 0, respectively (P = .014).
Frailty was predictive of 30-day postoperative mortality (P = .005) but not postoperative length of stay.
Frailer patients were more likely to receive neoadjuvant chemotherapy (P = .033) but less likely to receive adjuvant chemotherapy (P <. 001).
mFI scores of 2 or greater and 1 were both associated with significantly worse progression-free survival (P < .001 and P = .022, respectively), but only an mFI of 2 or greater was associated with significantly worse overall survival (P <. 001).
On multivariate analysis controlling for frailty, age, stage, BRCA status, and tumor reductive surgery status, high frailty was associated with worse progression-free survival (P = .009) and a trend toward worse overall survival (P = .079).
High frailty was better than age for predicting worse progression-free survival (hazard ratio, 1.50; P = .017) and overall survival (HR, 1.57; P = .047)
Volume counts
In a separate presentation during the same session, Morcos Nakhla, of the University of California, Los Angeles, reported finding similar associations between frailty and worse surgical outcomes for ovarian cancer patients (Abstract 11016).
Mr. Nakhla and colleagues found that frail patients had a twofold increase in the risk of postoperative complications, a threefold increase in the risk for non-home dismissal, and a threefold increased risk of death (P <. 001 for all).
The team also found, however, that mortality improved from 2005 through 2017, despite an increase in frail patients over that time period.
In addition, higher surgical volumes were associated with decreased mortality among frail patients undergoing ovarian cancer surgery.
Navigating treatment
“Frailty syndrome is a medical syndrome. It’s not a disability,” said Jamie N. Bakkum-Gamez, MD, of the Mayo Clinic in Rochester, Minn., the invited discussant. “No patient or human wants to be frail, but at some point, we may all be at risk for frailty syndrome, and as we navigate much-needed novel ways to treat this medical syndrome, it’s imperative that we listen to the voice of the customer and that our communication and technology doesn’t add unanticipated stress.”
Dr. Bakkum-Gamez emphasized the importance of shared decision-making, screening for frailty syndrome, referral to higher volume surgical centers when practical, and surgical alternatives such as neoadjuvant chemotherapy with or without interval debulking surgery and palliative care.
Interventions for ameliorating frailty may include exercise, high-protein calorie supplementation, reduction of polypharmacy, and vitamin D supplementation.
“Sometimes, shared decision-making means deciding not to operate,” Dr. Bakkum-Gamez said. “This is sometimes amongst the hardest decisions for a surgeon. We know when we make the wrong decision in operating if our patient experiences a major, life-shortening complication, but it’s less clear to know if we make the wrong decision to not operate.”
The study by Dr. Handley and colleagues was funded by the Gulf Coast Consortia, MD Anderson, National Institutes of Health, American Cancer Society, and GOG Foundation. Mr. Nakhla and colleagues did not disclose a funding source. Dr. Handley, Mr. Nakhla, and Dr. Bakkum-Gamez all reported no relevant conflicts of interest.
Baseball great Satchel Paige’s famous adage, “Age is a case of mind over matter. If you don’t mind, it don’t matter,” may also apply to candidates for ovarian cancer surgery. That’s because investigators have found that physical frailty is a better determinant of fitness for surgery than is calendar age.
Investigators conducted a retrospective analysis of 591 patients considered for primary resection of stage II to IV high-grade ovarian, fallopian tube, or peritoneal cancer. Results showed that a 10-item modified frailty index was better than patient age for predicting survival outcomes.
“Frailty does seem to correlate with age and increase with age, but it is not synonymous with age,” said investigator Katelyn F. Handley, MD, of the University of Texas MD Anderson Cancer Center in Houston.
“Frailty is a spectrum, and we can see patients of the same chronological age, but one may be a 76-year-old, ultra-distance triathlete, while another is in failing health and diminishing function,” Dr. Handley said at the Society of Gynecologic Oncology’s Virtual Annual Meeting on Women’s Cancer (Abstract 10463).
Dr. Handley cited a consensus definition of frailty, published in 2013, as “a medical syndrome with multiple causes and contributors that is characterized by diminished strength, endurance, and reduced physiologic function that increases an individual’s vulnerability for developing increased dependency and/or death.”
Ten-item score
To assess the effect of frailty in ovarian cancer patients on surgical procedures and outcomes, the investigators retrospectively applied a modified frailty index (mFI) to patients who were treated at MD Anderson Cancer Center from April 2013 through September 2017.
The index is a sum of 10 items: Chronic obstructive pulmonary disease or recent pneumonia, heart failure, myocardial infarction, coronary artery disease, diabetes, hypertension, peripheral vascular disease, cerebrovascular disease, cerebrovascular accident with neurologic deficit, and poor Eastern Cooperative Oncology Group performance status (3 or 4).
Of the 591 patients who met inclusion criteria, 57% had mFI scores of 0, indicating no frailty, 29% had one frailty factor, and 14% had two or more factors.
Patient age did significantly correlate with mFI scores. Patients with an mFI score of 0 had a median age of 62 years, the median age in those with a score of 1 was 69 years, and the median age for those with scores of 2 or higher was 70.5 years (P <. 001).
Charlson comorbidity index scores also significantly increased with age, with mean scores of 3.00, 3.83, and 5.14 in patients with mFI scores of 0, 1, or 2, respectively (P <. 001).
“But if you look at the age ranges in each category, you’ll notice that there are patients as young as 47 with an mFI of greater than or equal to 2, and as old as 89 with an mFI of 0,” Dr. Handley pointed out.
Higher scores, fewer assessments
The investigators found that patients with suspected ovarian cancer with frailty scores of 2 or higher were less likely to be offered laparoscopic assessment to determine primary resectability than were those with scores of 1 or 0 (28% vs. 43% and 49%, respectively, P = .004).
Among all patients who underwent laparoscopic assessment, the predictive index score (modified Fagotti score) was more likely to be 8 or higher in patients with high frailty scores (58%, 48%, and 34% for scores of 2 or greater, 1, and 0, respectively; P = .038).
Only 17% of the most frail patients went on to primary debulking surgery, compared with 26% of patients with a single frailty factor and 34% of those with none (P = .015).
Patients with higher frailty scores were less likely to undergo primary or interval tumor reductive surgery (59% vs. 74% for those with mFI scores of 1 and 85% for those with scores of 0; P <. 001). The frailest patients were significantly more likely to undergo splenectomy (20%, 3%, and 6%, respectively; P = .001) and small bowel resection (14%, 8%, and 3%, respectively; P = .006).
Two-thirds of the most frail patients (64%) had postoperative complications, primarily gastrointestinal and renal complications, compared with 56% and 44% of patients with mFI scores of 1 or 0, respectively (P = .014).
Frailty was predictive of 30-day postoperative mortality (P = .005) but not postoperative length of stay.
Frailer patients were more likely to receive neoadjuvant chemotherapy (P = .033) but less likely to receive adjuvant chemotherapy (P <. 001).
mFI scores of 2 or greater and 1 were both associated with significantly worse progression-free survival (P < .001 and P = .022, respectively), but only an mFI of 2 or greater was associated with significantly worse overall survival (P <. 001).
On multivariate analysis controlling for frailty, age, stage, BRCA status, and tumor reductive surgery status, high frailty was associated with worse progression-free survival (P = .009) and a trend toward worse overall survival (P = .079).
High frailty was better than age for predicting worse progression-free survival (hazard ratio, 1.50; P = .017) and overall survival (HR, 1.57; P = .047)
Volume counts
In a separate presentation during the same session, Morcos Nakhla, of the University of California, Los Angeles, reported finding similar associations between frailty and worse surgical outcomes for ovarian cancer patients (Abstract 11016).
Mr. Nakhla and colleagues found that frail patients had a twofold increase in the risk of postoperative complications, a threefold increase in the risk for non-home dismissal, and a threefold increased risk of death (P <. 001 for all).
The team also found, however, that mortality improved from 2005 through 2017, despite an increase in frail patients over that time period.
In addition, higher surgical volumes were associated with decreased mortality among frail patients undergoing ovarian cancer surgery.
Navigating treatment
“Frailty syndrome is a medical syndrome. It’s not a disability,” said Jamie N. Bakkum-Gamez, MD, of the Mayo Clinic in Rochester, Minn., the invited discussant. “No patient or human wants to be frail, but at some point, we may all be at risk for frailty syndrome, and as we navigate much-needed novel ways to treat this medical syndrome, it’s imperative that we listen to the voice of the customer and that our communication and technology doesn’t add unanticipated stress.”
Dr. Bakkum-Gamez emphasized the importance of shared decision-making, screening for frailty syndrome, referral to higher volume surgical centers when practical, and surgical alternatives such as neoadjuvant chemotherapy with or without interval debulking surgery and palliative care.
Interventions for ameliorating frailty may include exercise, high-protein calorie supplementation, reduction of polypharmacy, and vitamin D supplementation.
“Sometimes, shared decision-making means deciding not to operate,” Dr. Bakkum-Gamez said. “This is sometimes amongst the hardest decisions for a surgeon. We know when we make the wrong decision in operating if our patient experiences a major, life-shortening complication, but it’s less clear to know if we make the wrong decision to not operate.”
The study by Dr. Handley and colleagues was funded by the Gulf Coast Consortia, MD Anderson, National Institutes of Health, American Cancer Society, and GOG Foundation. Mr. Nakhla and colleagues did not disclose a funding source. Dr. Handley, Mr. Nakhla, and Dr. Bakkum-Gamez all reported no relevant conflicts of interest.
Baseball great Satchel Paige’s famous adage, “Age is a case of mind over matter. If you don’t mind, it don’t matter,” may also apply to candidates for ovarian cancer surgery. That’s because investigators have found that physical frailty is a better determinant of fitness for surgery than is calendar age.
Investigators conducted a retrospective analysis of 591 patients considered for primary resection of stage II to IV high-grade ovarian, fallopian tube, or peritoneal cancer. Results showed that a 10-item modified frailty index was better than patient age for predicting survival outcomes.
“Frailty does seem to correlate with age and increase with age, but it is not synonymous with age,” said investigator Katelyn F. Handley, MD, of the University of Texas MD Anderson Cancer Center in Houston.
“Frailty is a spectrum, and we can see patients of the same chronological age, but one may be a 76-year-old, ultra-distance triathlete, while another is in failing health and diminishing function,” Dr. Handley said at the Society of Gynecologic Oncology’s Virtual Annual Meeting on Women’s Cancer (Abstract 10463).
Dr. Handley cited a consensus definition of frailty, published in 2013, as “a medical syndrome with multiple causes and contributors that is characterized by diminished strength, endurance, and reduced physiologic function that increases an individual’s vulnerability for developing increased dependency and/or death.”
Ten-item score
To assess the effect of frailty in ovarian cancer patients on surgical procedures and outcomes, the investigators retrospectively applied a modified frailty index (mFI) to patients who were treated at MD Anderson Cancer Center from April 2013 through September 2017.
The index is a sum of 10 items: Chronic obstructive pulmonary disease or recent pneumonia, heart failure, myocardial infarction, coronary artery disease, diabetes, hypertension, peripheral vascular disease, cerebrovascular disease, cerebrovascular accident with neurologic deficit, and poor Eastern Cooperative Oncology Group performance status (3 or 4).
Of the 591 patients who met inclusion criteria, 57% had mFI scores of 0, indicating no frailty, 29% had one frailty factor, and 14% had two or more factors.
Patient age did significantly correlate with mFI scores. Patients with an mFI score of 0 had a median age of 62 years, the median age in those with a score of 1 was 69 years, and the median age for those with scores of 2 or higher was 70.5 years (P <. 001).
Charlson comorbidity index scores also significantly increased with age, with mean scores of 3.00, 3.83, and 5.14 in patients with mFI scores of 0, 1, or 2, respectively (P <. 001).
“But if you look at the age ranges in each category, you’ll notice that there are patients as young as 47 with an mFI of greater than or equal to 2, and as old as 89 with an mFI of 0,” Dr. Handley pointed out.
Higher scores, fewer assessments
The investigators found that patients with suspected ovarian cancer with frailty scores of 2 or higher were less likely to be offered laparoscopic assessment to determine primary resectability than were those with scores of 1 or 0 (28% vs. 43% and 49%, respectively, P = .004).
Among all patients who underwent laparoscopic assessment, the predictive index score (modified Fagotti score) was more likely to be 8 or higher in patients with high frailty scores (58%, 48%, and 34% for scores of 2 or greater, 1, and 0, respectively; P = .038).
Only 17% of the most frail patients went on to primary debulking surgery, compared with 26% of patients with a single frailty factor and 34% of those with none (P = .015).
Patients with higher frailty scores were less likely to undergo primary or interval tumor reductive surgery (59% vs. 74% for those with mFI scores of 1 and 85% for those with scores of 0; P <. 001). The frailest patients were significantly more likely to undergo splenectomy (20%, 3%, and 6%, respectively; P = .001) and small bowel resection (14%, 8%, and 3%, respectively; P = .006).
Two-thirds of the most frail patients (64%) had postoperative complications, primarily gastrointestinal and renal complications, compared with 56% and 44% of patients with mFI scores of 1 or 0, respectively (P = .014).
Frailty was predictive of 30-day postoperative mortality (P = .005) but not postoperative length of stay.
Frailer patients were more likely to receive neoadjuvant chemotherapy (P = .033) but less likely to receive adjuvant chemotherapy (P <. 001).
mFI scores of 2 or greater and 1 were both associated with significantly worse progression-free survival (P < .001 and P = .022, respectively), but only an mFI of 2 or greater was associated with significantly worse overall survival (P <. 001).
On multivariate analysis controlling for frailty, age, stage, BRCA status, and tumor reductive surgery status, high frailty was associated with worse progression-free survival (P = .009) and a trend toward worse overall survival (P = .079).
High frailty was better than age for predicting worse progression-free survival (hazard ratio, 1.50; P = .017) and overall survival (HR, 1.57; P = .047)
Volume counts
In a separate presentation during the same session, Morcos Nakhla, of the University of California, Los Angeles, reported finding similar associations between frailty and worse surgical outcomes for ovarian cancer patients (Abstract 11016).
Mr. Nakhla and colleagues found that frail patients had a twofold increase in the risk of postoperative complications, a threefold increase in the risk for non-home dismissal, and a threefold increased risk of death (P <. 001 for all).
The team also found, however, that mortality improved from 2005 through 2017, despite an increase in frail patients over that time period.
In addition, higher surgical volumes were associated with decreased mortality among frail patients undergoing ovarian cancer surgery.
Navigating treatment
“Frailty syndrome is a medical syndrome. It’s not a disability,” said Jamie N. Bakkum-Gamez, MD, of the Mayo Clinic in Rochester, Minn., the invited discussant. “No patient or human wants to be frail, but at some point, we may all be at risk for frailty syndrome, and as we navigate much-needed novel ways to treat this medical syndrome, it’s imperative that we listen to the voice of the customer and that our communication and technology doesn’t add unanticipated stress.”
Dr. Bakkum-Gamez emphasized the importance of shared decision-making, screening for frailty syndrome, referral to higher volume surgical centers when practical, and surgical alternatives such as neoadjuvant chemotherapy with or without interval debulking surgery and palliative care.
Interventions for ameliorating frailty may include exercise, high-protein calorie supplementation, reduction of polypharmacy, and vitamin D supplementation.
“Sometimes, shared decision-making means deciding not to operate,” Dr. Bakkum-Gamez said. “This is sometimes amongst the hardest decisions for a surgeon. We know when we make the wrong decision in operating if our patient experiences a major, life-shortening complication, but it’s less clear to know if we make the wrong decision to not operate.”
The study by Dr. Handley and colleagues was funded by the Gulf Coast Consortia, MD Anderson, National Institutes of Health, American Cancer Society, and GOG Foundation. Mr. Nakhla and colleagues did not disclose a funding source. Dr. Handley, Mr. Nakhla, and Dr. Bakkum-Gamez all reported no relevant conflicts of interest.
FROM SGO 2021