User login
FDA clears nonstimulant for ADHD in children aged 6 years and up
The Food and Drug Administration has approved the nonstimulant medication viloxazine extended-release capsules (Qelbree, Supernus Pharmaceuticals) for the treatment of attention deficit hyperactivity disorder (ADHD) in children aged 6-17 years, the company has announced.
Viloxazine (formerly SPN-812) is a selective norepinephrine reuptake inhibitor. Capsules may be swallowed whole or opened and the entire contents sprinkled onto applesauce, as needed.
The approval of viloxazine is supported by data from four phase 3 clinical trials involving more than 1,000 pediatric patients aged 6-17 years, the company said.
In one randomized, placebo-controlled phase 3 study that included more than 400 children, viloxazine reduced symptoms of ADHD as soon as 1 week after dosing and was well tolerated.
As reported by this news organization, the study was published last July in Clinical Therapeutics.
In addition to its fast onset of action, the fact that it was effective for both inattentive and hyperactive/impulsive clusters of symptoms is “impressive,” study investigator Andrew Cutler, MD, clinical associate professor of psychiatry, SUNY Upstate Medical University, Syracuse, N.Y., said in an interview.
Also noteworthy was the improvement in measures of quality of life and function, “especially function in the areas of school, home life, family relations, and peer relationships, which can be really disrupted with ADHD,” Dr. Cutler said.
The prescribing label for viloxazine includes a boxed warning regarding the potential for suicidal thoughts and behaviors in some children with ADHD treated with the drug, especially within the first few months of treatment or when the dose is changed.
In clinical trials, higher rates of suicidal thoughts and behavior were reported in pediatric patients treated with viloxazine than in patients treated with placebo. Patients taking viloxazine should be closely monitored for any new or sudden changes in mood, behavior, thoughts, and feelings.
Viloxazine has shown promise in a phase 3 trial involving adults with ADHD.
The company plans to submit a supplemental new drug application to the FDA for viloxazine in adults later this year.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the nonstimulant medication viloxazine extended-release capsules (Qelbree, Supernus Pharmaceuticals) for the treatment of attention deficit hyperactivity disorder (ADHD) in children aged 6-17 years, the company has announced.
Viloxazine (formerly SPN-812) is a selective norepinephrine reuptake inhibitor. Capsules may be swallowed whole or opened and the entire contents sprinkled onto applesauce, as needed.
The approval of viloxazine is supported by data from four phase 3 clinical trials involving more than 1,000 pediatric patients aged 6-17 years, the company said.
In one randomized, placebo-controlled phase 3 study that included more than 400 children, viloxazine reduced symptoms of ADHD as soon as 1 week after dosing and was well tolerated.
As reported by this news organization, the study was published last July in Clinical Therapeutics.
In addition to its fast onset of action, the fact that it was effective for both inattentive and hyperactive/impulsive clusters of symptoms is “impressive,” study investigator Andrew Cutler, MD, clinical associate professor of psychiatry, SUNY Upstate Medical University, Syracuse, N.Y., said in an interview.
Also noteworthy was the improvement in measures of quality of life and function, “especially function in the areas of school, home life, family relations, and peer relationships, which can be really disrupted with ADHD,” Dr. Cutler said.
The prescribing label for viloxazine includes a boxed warning regarding the potential for suicidal thoughts and behaviors in some children with ADHD treated with the drug, especially within the first few months of treatment or when the dose is changed.
In clinical trials, higher rates of suicidal thoughts and behavior were reported in pediatric patients treated with viloxazine than in patients treated with placebo. Patients taking viloxazine should be closely monitored for any new or sudden changes in mood, behavior, thoughts, and feelings.
Viloxazine has shown promise in a phase 3 trial involving adults with ADHD.
The company plans to submit a supplemental new drug application to the FDA for viloxazine in adults later this year.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the nonstimulant medication viloxazine extended-release capsules (Qelbree, Supernus Pharmaceuticals) for the treatment of attention deficit hyperactivity disorder (ADHD) in children aged 6-17 years, the company has announced.
Viloxazine (formerly SPN-812) is a selective norepinephrine reuptake inhibitor. Capsules may be swallowed whole or opened and the entire contents sprinkled onto applesauce, as needed.
The approval of viloxazine is supported by data from four phase 3 clinical trials involving more than 1,000 pediatric patients aged 6-17 years, the company said.
In one randomized, placebo-controlled phase 3 study that included more than 400 children, viloxazine reduced symptoms of ADHD as soon as 1 week after dosing and was well tolerated.
As reported by this news organization, the study was published last July in Clinical Therapeutics.
In addition to its fast onset of action, the fact that it was effective for both inattentive and hyperactive/impulsive clusters of symptoms is “impressive,” study investigator Andrew Cutler, MD, clinical associate professor of psychiatry, SUNY Upstate Medical University, Syracuse, N.Y., said in an interview.
Also noteworthy was the improvement in measures of quality of life and function, “especially function in the areas of school, home life, family relations, and peer relationships, which can be really disrupted with ADHD,” Dr. Cutler said.
The prescribing label for viloxazine includes a boxed warning regarding the potential for suicidal thoughts and behaviors in some children with ADHD treated with the drug, especially within the first few months of treatment or when the dose is changed.
In clinical trials, higher rates of suicidal thoughts and behavior were reported in pediatric patients treated with viloxazine than in patients treated with placebo. Patients taking viloxazine should be closely monitored for any new or sudden changes in mood, behavior, thoughts, and feelings.
Viloxazine has shown promise in a phase 3 trial involving adults with ADHD.
The company plans to submit a supplemental new drug application to the FDA for viloxazine in adults later this year.
A version of this article first appeared on Medscape.com.
The pandemic is making periods unbearable for some women
Stories of how the pandemic has disrupted women’s periods reverberated across the Internet. Here’s what docs can do to help.
Following a recent article in the Guardian, the Internet has erupted with tales of periods gone awry. The stress and loss of normalcy over the last year appears to have altered cycles and amplified the premenstrual syndrome (PMS) symptoms many women experience. And after the piece published, many responded on social media with the same sentiment: “So, it’s not just me?”
Women have experienced the loss of their period, excessive and prolonged bleeding, severe mood swings, and irritability, according to the Guardian article. London-based gynecologist Anita Mitra, MBChB, PhD, took an informal survey and found that 65% of 5,677 respondents had noticed a change in their menstrual cycle, the Guardian reported. Another survey, which was posted on medRxiv but hasn’t been peer reviewed yet, found 53% of the 749 respondents had noticed a change in their menstrual cycle, including increased cycle length.
“The pandemic in itself has made more stress for women,” said Karen Carlson, MD, obstetrician and gynecologist at Nebraska Medicine. There’s preliminary evidence that the cycling progesterone and estrogen experienced by reproductive age women actually offers a protective effect against COVID-19, which is good news. But Dr. Carlson said that because they are less likely than men and the elderly to become seriously ill, many women have taken on a lot of the additional responsibilities brought on by the pandemic. They often juggle homeschooling and elder care in addition to the ubiquitous stressors of isolation and concerns around personal health.
“Abnormal bleeding is the most common reason people present to the gynecologist,” Dr. Carlson said in an interview. But in recent months, Dr. Carlson said she’s seen a slight uptick in these issues, and there might have been even more women presenting to their physicians if the pandemic hadn’t also suppressed access to care.
Stress, or rather the cortisol it causes the body to produce, is the culprit for disrupted cycles. It can suppress pituitary hormones that stimulate ovulation. “Some women don’t feel right because they are stuck in the one phase of the cycle,” Dr. Carlson said. They may go months without a period and when they do eventually shed their uterine lining the bleeding goes on for a while.
Some irregularity in a person’s cycle is a normal response to stress and even likely, given the last year. However, bleeding for more than 2 weeks or irregularity for more than 3 months could point to something more serious like an infection or cancer, Dr. Carlson said. Getting a clear history so you know when you need to do blood and hormone workups is critical.
Anxiety and depression amplified
For some women it’s not bleeding that’s a problem, rather their PMS has become crippling. And some of their significant others have noticed drastic changes in their mood. In the Guardian article, one woman said she’d gone from feeling withdrawn during her period to being totally unreachable and experiencing intense anxiety.
Maureen Whelihan, MD, a gynecologist in Palm Beach, Fla., said that, for the majority of her patients under 39 years of age, these feelings aren’t a hormone issue, but a stress and neuroreceptor issue. She says she’s seen approximately a 30% increase in mood disorders since the start of the pandemic. Even though many of her patients are cycling relatively normally, their anxiety and depression have been amplified.
Caroline Gurvich, PhD, a neuroscientist at Monash University in Melbourne, attributes this to the loss of typical coping mechanisms. “Having changes to the support system and routine and things that would keep them mentally healthy can exacerbate PMS,” she said in an interview. Dr. Gurvich’s advice is to build routines into the pandemic lifestyle. Normal wake and sleep times, healthy eating, and practices that bring happiness can be “crucial to keeping those PMS systems as controlled as possible.”
Telehealth has made it much easier to access some patients struggling with PMS and offer them the medication or counseling they need, Dr. Carlson said. But that approach doesn’t work for everyone. “I feel like there are a lot of silent sufferers,” she said.
This is where screening practices like the Patient Health Questionnaire-9 are so critical, according to Dr. Whelihan, who screens every patient as part of their routine iPad check-in process. Even in a normal year, “I think one-third of gynecology is psychiatry,” she said in an interview. She finds many of the patients struggling with excessive PMS symptoms, both during the pandemic and before, benefit from a child-sized dose of antidepressant. This may allow them to get to a place where they can make impactful routine decisions about exercise or sleep, and then taper off the antidepressant.
It may also be important for clinicians to help patients make the initial connection between their worsening mood or cognitive function and their period. Knowing their feelings of stress, irritability, fogginess, or being withdrawn are linked to their hormone cycle and possibly worsened by the stress of the pandemic can be helpful, Dr. Gurvich said. “If they become conscious of how they are feeling it can be helpful for management of these stressful symptoms,” she said.
Stories of how the pandemic has disrupted women’s periods reverberated across the Internet. Here’s what docs can do to help.
Stories of how the pandemic has disrupted women’s periods reverberated across the Internet. Here’s what docs can do to help.
Following a recent article in the Guardian, the Internet has erupted with tales of periods gone awry. The stress and loss of normalcy over the last year appears to have altered cycles and amplified the premenstrual syndrome (PMS) symptoms many women experience. And after the piece published, many responded on social media with the same sentiment: “So, it’s not just me?”
Women have experienced the loss of their period, excessive and prolonged bleeding, severe mood swings, and irritability, according to the Guardian article. London-based gynecologist Anita Mitra, MBChB, PhD, took an informal survey and found that 65% of 5,677 respondents had noticed a change in their menstrual cycle, the Guardian reported. Another survey, which was posted on medRxiv but hasn’t been peer reviewed yet, found 53% of the 749 respondents had noticed a change in their menstrual cycle, including increased cycle length.
“The pandemic in itself has made more stress for women,” said Karen Carlson, MD, obstetrician and gynecologist at Nebraska Medicine. There’s preliminary evidence that the cycling progesterone and estrogen experienced by reproductive age women actually offers a protective effect against COVID-19, which is good news. But Dr. Carlson said that because they are less likely than men and the elderly to become seriously ill, many women have taken on a lot of the additional responsibilities brought on by the pandemic. They often juggle homeschooling and elder care in addition to the ubiquitous stressors of isolation and concerns around personal health.
“Abnormal bleeding is the most common reason people present to the gynecologist,” Dr. Carlson said in an interview. But in recent months, Dr. Carlson said she’s seen a slight uptick in these issues, and there might have been even more women presenting to their physicians if the pandemic hadn’t also suppressed access to care.
Stress, or rather the cortisol it causes the body to produce, is the culprit for disrupted cycles. It can suppress pituitary hormones that stimulate ovulation. “Some women don’t feel right because they are stuck in the one phase of the cycle,” Dr. Carlson said. They may go months without a period and when they do eventually shed their uterine lining the bleeding goes on for a while.
Some irregularity in a person’s cycle is a normal response to stress and even likely, given the last year. However, bleeding for more than 2 weeks or irregularity for more than 3 months could point to something more serious like an infection or cancer, Dr. Carlson said. Getting a clear history so you know when you need to do blood and hormone workups is critical.
Anxiety and depression amplified
For some women it’s not bleeding that’s a problem, rather their PMS has become crippling. And some of their significant others have noticed drastic changes in their mood. In the Guardian article, one woman said she’d gone from feeling withdrawn during her period to being totally unreachable and experiencing intense anxiety.
Maureen Whelihan, MD, a gynecologist in Palm Beach, Fla., said that, for the majority of her patients under 39 years of age, these feelings aren’t a hormone issue, but a stress and neuroreceptor issue. She says she’s seen approximately a 30% increase in mood disorders since the start of the pandemic. Even though many of her patients are cycling relatively normally, their anxiety and depression have been amplified.
Caroline Gurvich, PhD, a neuroscientist at Monash University in Melbourne, attributes this to the loss of typical coping mechanisms. “Having changes to the support system and routine and things that would keep them mentally healthy can exacerbate PMS,” she said in an interview. Dr. Gurvich’s advice is to build routines into the pandemic lifestyle. Normal wake and sleep times, healthy eating, and practices that bring happiness can be “crucial to keeping those PMS systems as controlled as possible.”
Telehealth has made it much easier to access some patients struggling with PMS and offer them the medication or counseling they need, Dr. Carlson said. But that approach doesn’t work for everyone. “I feel like there are a lot of silent sufferers,” she said.
This is where screening practices like the Patient Health Questionnaire-9 are so critical, according to Dr. Whelihan, who screens every patient as part of their routine iPad check-in process. Even in a normal year, “I think one-third of gynecology is psychiatry,” she said in an interview. She finds many of the patients struggling with excessive PMS symptoms, both during the pandemic and before, benefit from a child-sized dose of antidepressant. This may allow them to get to a place where they can make impactful routine decisions about exercise or sleep, and then taper off the antidepressant.
It may also be important for clinicians to help patients make the initial connection between their worsening mood or cognitive function and their period. Knowing their feelings of stress, irritability, fogginess, or being withdrawn are linked to their hormone cycle and possibly worsened by the stress of the pandemic can be helpful, Dr. Gurvich said. “If they become conscious of how they are feeling it can be helpful for management of these stressful symptoms,” she said.
Following a recent article in the Guardian, the Internet has erupted with tales of periods gone awry. The stress and loss of normalcy over the last year appears to have altered cycles and amplified the premenstrual syndrome (PMS) symptoms many women experience. And after the piece published, many responded on social media with the same sentiment: “So, it’s not just me?”
Women have experienced the loss of their period, excessive and prolonged bleeding, severe mood swings, and irritability, according to the Guardian article. London-based gynecologist Anita Mitra, MBChB, PhD, took an informal survey and found that 65% of 5,677 respondents had noticed a change in their menstrual cycle, the Guardian reported. Another survey, which was posted on medRxiv but hasn’t been peer reviewed yet, found 53% of the 749 respondents had noticed a change in their menstrual cycle, including increased cycle length.
“The pandemic in itself has made more stress for women,” said Karen Carlson, MD, obstetrician and gynecologist at Nebraska Medicine. There’s preliminary evidence that the cycling progesterone and estrogen experienced by reproductive age women actually offers a protective effect against COVID-19, which is good news. But Dr. Carlson said that because they are less likely than men and the elderly to become seriously ill, many women have taken on a lot of the additional responsibilities brought on by the pandemic. They often juggle homeschooling and elder care in addition to the ubiquitous stressors of isolation and concerns around personal health.
“Abnormal bleeding is the most common reason people present to the gynecologist,” Dr. Carlson said in an interview. But in recent months, Dr. Carlson said she’s seen a slight uptick in these issues, and there might have been even more women presenting to their physicians if the pandemic hadn’t also suppressed access to care.
Stress, or rather the cortisol it causes the body to produce, is the culprit for disrupted cycles. It can suppress pituitary hormones that stimulate ovulation. “Some women don’t feel right because they are stuck in the one phase of the cycle,” Dr. Carlson said. They may go months without a period and when they do eventually shed their uterine lining the bleeding goes on for a while.
Some irregularity in a person’s cycle is a normal response to stress and even likely, given the last year. However, bleeding for more than 2 weeks or irregularity for more than 3 months could point to something more serious like an infection or cancer, Dr. Carlson said. Getting a clear history so you know when you need to do blood and hormone workups is critical.
Anxiety and depression amplified
For some women it’s not bleeding that’s a problem, rather their PMS has become crippling. And some of their significant others have noticed drastic changes in their mood. In the Guardian article, one woman said she’d gone from feeling withdrawn during her period to being totally unreachable and experiencing intense anxiety.
Maureen Whelihan, MD, a gynecologist in Palm Beach, Fla., said that, for the majority of her patients under 39 years of age, these feelings aren’t a hormone issue, but a stress and neuroreceptor issue. She says she’s seen approximately a 30% increase in mood disorders since the start of the pandemic. Even though many of her patients are cycling relatively normally, their anxiety and depression have been amplified.
Caroline Gurvich, PhD, a neuroscientist at Monash University in Melbourne, attributes this to the loss of typical coping mechanisms. “Having changes to the support system and routine and things that would keep them mentally healthy can exacerbate PMS,” she said in an interview. Dr. Gurvich’s advice is to build routines into the pandemic lifestyle. Normal wake and sleep times, healthy eating, and practices that bring happiness can be “crucial to keeping those PMS systems as controlled as possible.”
Telehealth has made it much easier to access some patients struggling with PMS and offer them the medication or counseling they need, Dr. Carlson said. But that approach doesn’t work for everyone. “I feel like there are a lot of silent sufferers,” she said.
This is where screening practices like the Patient Health Questionnaire-9 are so critical, according to Dr. Whelihan, who screens every patient as part of their routine iPad check-in process. Even in a normal year, “I think one-third of gynecology is psychiatry,” she said in an interview. She finds many of the patients struggling with excessive PMS symptoms, both during the pandemic and before, benefit from a child-sized dose of antidepressant. This may allow them to get to a place where they can make impactful routine decisions about exercise or sleep, and then taper off the antidepressant.
It may also be important for clinicians to help patients make the initial connection between their worsening mood or cognitive function and their period. Knowing their feelings of stress, irritability, fogginess, or being withdrawn are linked to their hormone cycle and possibly worsened by the stress of the pandemic can be helpful, Dr. Gurvich said. “If they become conscious of how they are feeling it can be helpful for management of these stressful symptoms,” she said.
Researchers stress importance of second COVID-19 vaccine dose for infliximab users
Patients being treated with infliximab had weakened immune responses to the first dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, compared with patients on vedolizumab (Entyvio), although a very significant number of patients from both groups seroconverted after their second dose, according to a new U.K. study of patients with inflammatory bowel disease (IBD).
“Antibody testing and adapted vaccine schedules should be considered to protect these at-risk patients,” Nicholas A. Kennedy, PhD, MBBS, of the University of Exeter (England) and colleagues wrote in a preprint published March 29 on MedRxiv.
Infliximab is an anti–tumor necrosis factor (anti-TNF) monoclonal antibody that’s approved to treat adult and pediatric Crohn’s disease and ulcerative colitis, as well as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, whereas vedolizumab, a gut selective anti-integrin alpha4beta7 monoclonal antibody that is not associated with impaired systemic immune responses, is approved to treat Crohn’s disease and ulcerative colitis in adults.
A previous study from Kennedy and colleagues revealed that IBD patients on infliximab showed a weakened COVID-19 antibody response compared with patients on vedolizumab. To determine if treatment with anti-TNF drugs impacted the efficacy of the first shot of these two-dose COVID-19 vaccines, the researchers used data from the CLARITY IBD study to assess 865 infliximab- and 428 vedolizumab-treated participants without evidence of prior SARS-CoV-2 infection who had received uninterrupted biologic therapy since being recruited between Sept. 22 and Dec. 23, 2020.
In the 3-10 weeks after initial vaccination, geometric mean concentrations for SARS-CoV-2 anti-spike protein receptor-binding protein antibodies were lower in patients on infliximab, compared with patients on vedolizumab for both the Pfizer (6.0 U/mL [5.9] versus 28.8 U/mL [5.4], P < .0001) and AstraZeneca (4.7 U/mL [4.9] versus 13.8 U/mL [5.9]; P < .0001) vaccines. The researchers’ multivariable models reinforced those findings, with antibody concentrations lower in infliximab-treated patients for both the Pfizer (fold change, 0.29; 95% confidence interval, 0.21-0.40; P < .0001) and AstraZeneca (FC, 0.39; 95% CI, 0.30-0.51; P < .0001) vaccines.
After second doses of the two-dose Pfizer vaccine, 85% of patients on infliximab and 86% of patients on vedolizumab seroconverted (P = .68); similarly high seroconversion rates were seen in patients who had been infected with SARS-CoV-2 prior to receiving either vaccine. Several patient characteristics were associated with lower antibody concentrations regardless of vaccine type: being 60 years or older, use of immunomodulators, having Crohn’s disease, and being a smoker. Alternatively, non-White ethnicity was associated with higher antibody concentrations.
Evidence has ‘unclear clinical significance’
“These data, which require peer review, do not change my opinion on the safety and efficacy of COVID-19 vaccines in patients taking TNF inhibitors such as infliximab as monotherapy for the treatment of psoriatic disease,” Joel M. Gelfand MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, said in an interview.
“First, two peer-reviewed studies found good antibody response in patients on TNF inhibitors receiving COVID-19 vaccines (doi: 10.1136/annrheumdis-2021-220289; 10.1136/annrheumdis-2021-220272). Second, antibody responses were robust in the small cohort that received the second dose of a COVID-19 vaccine. We already know that, for the two messenger RNA-based vaccines available under emergency use authorization in the U.S., a second dose is required for optimal efficacy. Thus, evidence of a reduced antibody response after just one dose is of unclear clinical significance. Third, antibody responses are only a surrogate marker, and a low antibody response doesn’t necessarily mean the patient will not be protected by the vaccine.”
Focus on the second dose of a two-dose regimen
“Tell me about the response in people who got both doses of a vaccine that you’re supposed to get both doses of,” Jeffrey Curtis, MD, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham, said in an interview. “The number of patients in that subset was small [n = 27] but in my opinion that’s the most clinically relevant analysis and the one that patients and clinicians want answered.”
He also emphasized the uncertainty around what ‘protection’ means in these early days of studying COVID-19 vaccine responses. “You can define seroprotection or seroconversion as some absolute level of an antibody response, but if you want to say ‘Mrs. Smith, your antibody level was X,’ on whatever arbitrary scale with whoever’s arbitrary lab test, nobody actually knows that Mrs. Smith is now protected from SARS-CoV-2, or how protected,” he said.
“What is not terribly controversial is: If you can’t detect antibodies, the vaccine didn’t ‘take,’ if you will. But if I tell you that the mean antibody level was X with one drug and then 2X with another drug, does that mean that you’re twice as protected? We don’t know that. I’m fearful that people are looking at these studies and thinking that more is better. It might be, but we don’t know that to be true.”
Debating the cause of weakened immune responses
“The biological plausibility of being on an anti-TNF affecting your immune reaction to a messenger RNA or even a replication-deficient viral vector vaccine doesn’t make sense,” David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the National Scientific Advisory Committee of the Crohn’s and Colitis Foundation, said in an interview.
“I’m sure immunologists may differ with me on this, but given what we have come to appreciate about these vaccine mechanisms, this finding doesn’t make intuitive sense. So we need to make sure that, when this happens, we look to the next studies and try to understand, was there any other confounder that may have resulted in these findings that was not adequately adjusted for or addressed in some other way?
“When you have a study of this size, you argue, ‘Because it’s so large, any effect that was seen must be real,’ ” he added. “Alternatively, to have a study of this size, by its very nature you are limited in being able to control for certain other factors or differences between the groups.”
That said, he commended the authors for their study and acknowledged the potential questions it raises about the single-shot Johnson & Johnson vaccine. “If you only get one and you’re on infliximab, this study implies that maybe that’s not enough,” he said. “Despite the fact that Johnson & Johnson was approved as a single dose, it may be necessary to think about it as the first of two, or maybe it’s not the preferred vaccine in this group of patients.”
The study was supported by the Royal Devon and Exeter and Hull University Hospital Foundation NHS Trusts and unrestricted educational grants from Biogen (Switzerland), Celltrion Healthcare (South Korea), Galapagos NV (Belgium), and F. Hoffmann-La Roche (Switzerland). The authors acknowledged numerous potential conflicts of interest, including receiving grants, personal fees, and nonfinancial support from various pharmaceutical companies.
Patients being treated with infliximab had weakened immune responses to the first dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, compared with patients on vedolizumab (Entyvio), although a very significant number of patients from both groups seroconverted after their second dose, according to a new U.K. study of patients with inflammatory bowel disease (IBD).
“Antibody testing and adapted vaccine schedules should be considered to protect these at-risk patients,” Nicholas A. Kennedy, PhD, MBBS, of the University of Exeter (England) and colleagues wrote in a preprint published March 29 on MedRxiv.
Infliximab is an anti–tumor necrosis factor (anti-TNF) monoclonal antibody that’s approved to treat adult and pediatric Crohn’s disease and ulcerative colitis, as well as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, whereas vedolizumab, a gut selective anti-integrin alpha4beta7 monoclonal antibody that is not associated with impaired systemic immune responses, is approved to treat Crohn’s disease and ulcerative colitis in adults.
A previous study from Kennedy and colleagues revealed that IBD patients on infliximab showed a weakened COVID-19 antibody response compared with patients on vedolizumab. To determine if treatment with anti-TNF drugs impacted the efficacy of the first shot of these two-dose COVID-19 vaccines, the researchers used data from the CLARITY IBD study to assess 865 infliximab- and 428 vedolizumab-treated participants without evidence of prior SARS-CoV-2 infection who had received uninterrupted biologic therapy since being recruited between Sept. 22 and Dec. 23, 2020.
In the 3-10 weeks after initial vaccination, geometric mean concentrations for SARS-CoV-2 anti-spike protein receptor-binding protein antibodies were lower in patients on infliximab, compared with patients on vedolizumab for both the Pfizer (6.0 U/mL [5.9] versus 28.8 U/mL [5.4], P < .0001) and AstraZeneca (4.7 U/mL [4.9] versus 13.8 U/mL [5.9]; P < .0001) vaccines. The researchers’ multivariable models reinforced those findings, with antibody concentrations lower in infliximab-treated patients for both the Pfizer (fold change, 0.29; 95% confidence interval, 0.21-0.40; P < .0001) and AstraZeneca (FC, 0.39; 95% CI, 0.30-0.51; P < .0001) vaccines.
After second doses of the two-dose Pfizer vaccine, 85% of patients on infliximab and 86% of patients on vedolizumab seroconverted (P = .68); similarly high seroconversion rates were seen in patients who had been infected with SARS-CoV-2 prior to receiving either vaccine. Several patient characteristics were associated with lower antibody concentrations regardless of vaccine type: being 60 years or older, use of immunomodulators, having Crohn’s disease, and being a smoker. Alternatively, non-White ethnicity was associated with higher antibody concentrations.
Evidence has ‘unclear clinical significance’
“These data, which require peer review, do not change my opinion on the safety and efficacy of COVID-19 vaccines in patients taking TNF inhibitors such as infliximab as monotherapy for the treatment of psoriatic disease,” Joel M. Gelfand MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, said in an interview.
“First, two peer-reviewed studies found good antibody response in patients on TNF inhibitors receiving COVID-19 vaccines (doi: 10.1136/annrheumdis-2021-220289; 10.1136/annrheumdis-2021-220272). Second, antibody responses were robust in the small cohort that received the second dose of a COVID-19 vaccine. We already know that, for the two messenger RNA-based vaccines available under emergency use authorization in the U.S., a second dose is required for optimal efficacy. Thus, evidence of a reduced antibody response after just one dose is of unclear clinical significance. Third, antibody responses are only a surrogate marker, and a low antibody response doesn’t necessarily mean the patient will not be protected by the vaccine.”
Focus on the second dose of a two-dose regimen
“Tell me about the response in people who got both doses of a vaccine that you’re supposed to get both doses of,” Jeffrey Curtis, MD, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham, said in an interview. “The number of patients in that subset was small [n = 27] but in my opinion that’s the most clinically relevant analysis and the one that patients and clinicians want answered.”
He also emphasized the uncertainty around what ‘protection’ means in these early days of studying COVID-19 vaccine responses. “You can define seroprotection or seroconversion as some absolute level of an antibody response, but if you want to say ‘Mrs. Smith, your antibody level was X,’ on whatever arbitrary scale with whoever’s arbitrary lab test, nobody actually knows that Mrs. Smith is now protected from SARS-CoV-2, or how protected,” he said.
“What is not terribly controversial is: If you can’t detect antibodies, the vaccine didn’t ‘take,’ if you will. But if I tell you that the mean antibody level was X with one drug and then 2X with another drug, does that mean that you’re twice as protected? We don’t know that. I’m fearful that people are looking at these studies and thinking that more is better. It might be, but we don’t know that to be true.”
Debating the cause of weakened immune responses
“The biological plausibility of being on an anti-TNF affecting your immune reaction to a messenger RNA or even a replication-deficient viral vector vaccine doesn’t make sense,” David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the National Scientific Advisory Committee of the Crohn’s and Colitis Foundation, said in an interview.
“I’m sure immunologists may differ with me on this, but given what we have come to appreciate about these vaccine mechanisms, this finding doesn’t make intuitive sense. So we need to make sure that, when this happens, we look to the next studies and try to understand, was there any other confounder that may have resulted in these findings that was not adequately adjusted for or addressed in some other way?
“When you have a study of this size, you argue, ‘Because it’s so large, any effect that was seen must be real,’ ” he added. “Alternatively, to have a study of this size, by its very nature you are limited in being able to control for certain other factors or differences between the groups.”
That said, he commended the authors for their study and acknowledged the potential questions it raises about the single-shot Johnson & Johnson vaccine. “If you only get one and you’re on infliximab, this study implies that maybe that’s not enough,” he said. “Despite the fact that Johnson & Johnson was approved as a single dose, it may be necessary to think about it as the first of two, or maybe it’s not the preferred vaccine in this group of patients.”
The study was supported by the Royal Devon and Exeter and Hull University Hospital Foundation NHS Trusts and unrestricted educational grants from Biogen (Switzerland), Celltrion Healthcare (South Korea), Galapagos NV (Belgium), and F. Hoffmann-La Roche (Switzerland). The authors acknowledged numerous potential conflicts of interest, including receiving grants, personal fees, and nonfinancial support from various pharmaceutical companies.
Patients being treated with infliximab had weakened immune responses to the first dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, compared with patients on vedolizumab (Entyvio), although a very significant number of patients from both groups seroconverted after their second dose, according to a new U.K. study of patients with inflammatory bowel disease (IBD).
“Antibody testing and adapted vaccine schedules should be considered to protect these at-risk patients,” Nicholas A. Kennedy, PhD, MBBS, of the University of Exeter (England) and colleagues wrote in a preprint published March 29 on MedRxiv.
Infliximab is an anti–tumor necrosis factor (anti-TNF) monoclonal antibody that’s approved to treat adult and pediatric Crohn’s disease and ulcerative colitis, as well as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, whereas vedolizumab, a gut selective anti-integrin alpha4beta7 monoclonal antibody that is not associated with impaired systemic immune responses, is approved to treat Crohn’s disease and ulcerative colitis in adults.
A previous study from Kennedy and colleagues revealed that IBD patients on infliximab showed a weakened COVID-19 antibody response compared with patients on vedolizumab. To determine if treatment with anti-TNF drugs impacted the efficacy of the first shot of these two-dose COVID-19 vaccines, the researchers used data from the CLARITY IBD study to assess 865 infliximab- and 428 vedolizumab-treated participants without evidence of prior SARS-CoV-2 infection who had received uninterrupted biologic therapy since being recruited between Sept. 22 and Dec. 23, 2020.
In the 3-10 weeks after initial vaccination, geometric mean concentrations for SARS-CoV-2 anti-spike protein receptor-binding protein antibodies were lower in patients on infliximab, compared with patients on vedolizumab for both the Pfizer (6.0 U/mL [5.9] versus 28.8 U/mL [5.4], P < .0001) and AstraZeneca (4.7 U/mL [4.9] versus 13.8 U/mL [5.9]; P < .0001) vaccines. The researchers’ multivariable models reinforced those findings, with antibody concentrations lower in infliximab-treated patients for both the Pfizer (fold change, 0.29; 95% confidence interval, 0.21-0.40; P < .0001) and AstraZeneca (FC, 0.39; 95% CI, 0.30-0.51; P < .0001) vaccines.
After second doses of the two-dose Pfizer vaccine, 85% of patients on infliximab and 86% of patients on vedolizumab seroconverted (P = .68); similarly high seroconversion rates were seen in patients who had been infected with SARS-CoV-2 prior to receiving either vaccine. Several patient characteristics were associated with lower antibody concentrations regardless of vaccine type: being 60 years or older, use of immunomodulators, having Crohn’s disease, and being a smoker. Alternatively, non-White ethnicity was associated with higher antibody concentrations.
Evidence has ‘unclear clinical significance’
“These data, which require peer review, do not change my opinion on the safety and efficacy of COVID-19 vaccines in patients taking TNF inhibitors such as infliximab as monotherapy for the treatment of psoriatic disease,” Joel M. Gelfand MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, said in an interview.
“First, two peer-reviewed studies found good antibody response in patients on TNF inhibitors receiving COVID-19 vaccines (doi: 10.1136/annrheumdis-2021-220289; 10.1136/annrheumdis-2021-220272). Second, antibody responses were robust in the small cohort that received the second dose of a COVID-19 vaccine. We already know that, for the two messenger RNA-based vaccines available under emergency use authorization in the U.S., a second dose is required for optimal efficacy. Thus, evidence of a reduced antibody response after just one dose is of unclear clinical significance. Third, antibody responses are only a surrogate marker, and a low antibody response doesn’t necessarily mean the patient will not be protected by the vaccine.”
Focus on the second dose of a two-dose regimen
“Tell me about the response in people who got both doses of a vaccine that you’re supposed to get both doses of,” Jeffrey Curtis, MD, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham, said in an interview. “The number of patients in that subset was small [n = 27] but in my opinion that’s the most clinically relevant analysis and the one that patients and clinicians want answered.”
He also emphasized the uncertainty around what ‘protection’ means in these early days of studying COVID-19 vaccine responses. “You can define seroprotection or seroconversion as some absolute level of an antibody response, but if you want to say ‘Mrs. Smith, your antibody level was X,’ on whatever arbitrary scale with whoever’s arbitrary lab test, nobody actually knows that Mrs. Smith is now protected from SARS-CoV-2, or how protected,” he said.
“What is not terribly controversial is: If you can’t detect antibodies, the vaccine didn’t ‘take,’ if you will. But if I tell you that the mean antibody level was X with one drug and then 2X with another drug, does that mean that you’re twice as protected? We don’t know that. I’m fearful that people are looking at these studies and thinking that more is better. It might be, but we don’t know that to be true.”
Debating the cause of weakened immune responses
“The biological plausibility of being on an anti-TNF affecting your immune reaction to a messenger RNA or even a replication-deficient viral vector vaccine doesn’t make sense,” David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the National Scientific Advisory Committee of the Crohn’s and Colitis Foundation, said in an interview.
“I’m sure immunologists may differ with me on this, but given what we have come to appreciate about these vaccine mechanisms, this finding doesn’t make intuitive sense. So we need to make sure that, when this happens, we look to the next studies and try to understand, was there any other confounder that may have resulted in these findings that was not adequately adjusted for or addressed in some other way?
“When you have a study of this size, you argue, ‘Because it’s so large, any effect that was seen must be real,’ ” he added. “Alternatively, to have a study of this size, by its very nature you are limited in being able to control for certain other factors or differences between the groups.”
That said, he commended the authors for their study and acknowledged the potential questions it raises about the single-shot Johnson & Johnson vaccine. “If you only get one and you’re on infliximab, this study implies that maybe that’s not enough,” he said. “Despite the fact that Johnson & Johnson was approved as a single dose, it may be necessary to think about it as the first of two, or maybe it’s not the preferred vaccine in this group of patients.”
The study was supported by the Royal Devon and Exeter and Hull University Hospital Foundation NHS Trusts and unrestricted educational grants from Biogen (Switzerland), Celltrion Healthcare (South Korea), Galapagos NV (Belgium), and F. Hoffmann-La Roche (Switzerland). The authors acknowledged numerous potential conflicts of interest, including receiving grants, personal fees, and nonfinancial support from various pharmaceutical companies.
FROM MEDRXIV
Hyperphagia, anxiety eased with carbetocin in patients with Prader-Willi syndrome
Children and adolescents with Prader-Willi syndrome (PWS) who received three daily, intranasal doses of carbetocin, an investigational, long-acting oxytocin analogue, had significant improvement in hyperphagia and anxiety during 8 weeks on treatment, compared with placebo in a multicenter, phase 3 trial with 119 patients.
The treatment also appeared safe during up to 56 additional weeks on active treatment, with no serious adverse effects nor “unexpected” events, and once completing the study about 95% of enrolled patients opted to remain on active treatment, Cheri L. Deal, MD, PhD, said at the annual meeting of the Endocrine Society.
Based on “the significant results for the placebo-controlled period, as well as for those finishing the 56-week extension, we may well have a new armament for helping these kids and their families deal with the unrelenting hunger of patients with PWS as well as some of the behavioral symptoms,” Dr. Deal, chief of endocrinology and diabetes at the Sainte-Justine Mother-Child University of Montreal Hospital, said in an interview. No treatment currently has labeling for addressing the hyperphagia or anxiety that is characteristic and often problematic for children and adolescents with PWS, an autosomal dominant genetic disease with an incidence of about 1 in 15,000 births and an estimated U.S. prevalence of about 9,000 cases, or about 1 case for every 37,000 people.
‘Gorgeous’ safety
“The results looked pretty positive, and we’re encouraged by what appears to be a good safety profile, so overall I think the PWS community is very excited by the results and is very interested in getting access to this drug,” commented Theresa V. Strong, PhD, director of research programs for the Foundation for Prader-Willi Research in Walnut, Calif., a group not involved with the study. Currently, “we have no effective treatments for these difficult behaviors” of hyperphagia and anxiety. Surveys and studies run by the foundation have documented that hyperphagia and anxiety “were the two most important symptoms that families would like to see treated,” Dr. Strong added in an interview.
PWS “is complex and affects almost every aspect of the lives of affected people and their families. Any treatment that can chip away at some of the problems these patients have can be a huge benefit to the patients and their families,” said Jennifer L. Miller, MD, a professor of pediatric endocrinology at the University of Florida, Gainesville, and a coinvestigator on the study.
But the finding that carbetocin appeared to address, at least in part, this unmet need while compiling a safety record that Dr. Miller called “gorgeous” and “remarkable,” also came with a few limitations.
Fewer patients than planned, and muddled outcomes
The CARE-PWS trial aimed to enroll 175 patients, but fell short once the COVID-19 pandemic hit. Plus the trial had two prespecified primary endpoints – improvements in a measure of hyperphagia, and in a measure of obsessive and compulsive behaviors – specifically in the 40 patients who received the higher of the two dosages studied, 9.6 mg t.i.d. intranasally. Neither endpoint showed significant improvement among the patients on this dosage, compared with the 40 patients who received placebo, although both outcomes trended in the right direction in the actively treated patients.
The study’s positive results came in a secondary treatment group, 39 patients who received 3.2 mg t.i.d., also intranasally. This subgroup had significant benefit, compared with placebo, for reducing hyperphagia symptoms as measured on the Hyperphagia Questionnaire for Clinical Trials (HQ-CT) Total Score. After the first 8 weeks on treatment, patients on the lower carbetocin dosage had an average reduction in their HQ-CT score of greater than 5 points, more than double the reduction seen among control patients who received placebo.
Those on the 3.2-mg t.i.d. dosage also showed significant improvements, compared with placebo, for anxiety, measured by the PWS Anxiety and Distress Questionnaire Total Score, as well as on measures of clinical global impression of severity, and of clinical global impression of change. Like the higher-dosage patients the lower-dosage subgroup did not show a significant difference compared with placebo for the other primary endpoint, change in obsessive and compulsive behaviors as measured by the Children’s Yale-Brown Obsessive-Compulsive Scale Total Score, although also like the higher dosage the effect from the lower dosage trended toward benefit.
A further limitation was that, at the time of her report, presented in abstract OR16-3 at the meeting, Dr. Deal could only present complete 64-week follow-up for 72 patients, although this reassuringly showed that, as time on the 9.6-mg t.i.d. dosage continued beyond 8 weeks, patients gradually improved their HQ-CT response so that by 64 weeks on treatment their hyperphagia score had improved as much as in the patients who received the lower dosage.
In short, documented benefits occurred on the lower dosage, especially for clinically meaningful symptoms like hyperphagia and anxiety, but the study’s overall results were not fully consistent by statistical criteria.
Benefiting an unmet need?
“While it is regrettable that we did not get to 175 patients because of COVID-19, the dataset is significant enough for me to feel that the FDA [Food and Drug Administration] needs to take a very serious look and consider approval,” Dr. Deal said in an interview. “Once safety is assured, which I think it is, I can only hope that regulatory officials understand their unmet needs of this rare disease community and will allow the drug to move to the next stage.”
“This is a very rare disease, and having families participate in trials is really challenging,” especially while the COVID-19 pandemic continues, Dr. Strong said. For the pediatric and adolescent patients targeted in this study “it will take a while for COVID to go away and for families to feel safe again being in a trial, so a real concern is that a need for more clinical trials is not terribly feasible now. Given that the safety profile looked good and one dose seemed to have good efficacy, as long as the long-term data continue to look good we’d love for the FDA to look at the existing data and see whether there is a path forward.”
Dr. Miller highlighted the limitations of what the CARE-PWS findings show.
“Given that it was only an 8-week trial of drug against placebo, and the fact that the primary outcomes weren’t met for the higher dose, my thought is that potentially we need to study more patients for a longer period at the 3.2-mg dose,” she said. She acknowledged that the metric used in the study to assess obsessive and compulsive behaviors is “very difficult” to apply to patients with PWS because of uncertainties in scoring obsessions in patients “who are not very good at telling you what they’re thinking.” Plus, “it’s absolutely not a problem that we did not see an effect on obsession and compulsions if the treatment potentially improves anxiety and hyperphagia, which are very common.” A treatment that reliably reduces these symptoms “would be amazing,” Dr. Miller added.
“PWS is very rare, so it’s very hard to do trials. Maybe the FDA will approve carbetocin because it was safe and gave a signal of efficacy at the lower dose. But my thought is that additional treatment trials are needed with only the lower dose and with longer duration,” she said.
CARE-PWS enrolled patients with nutritional phase 3 PWS who were aged 7-18 years at any of 24 sites in the United States, Canada, or Australia during 2018-2020. They averaged about 12 years of age, and 56% were girls.
The most common adverse effect from carbetocin was flushing, occurring in 14% of those on the lower dose and 21% on the higher dose, but not in any placebo patient. Other adverse effects more common on the lower dose than in the placebo group included headache in 16%, and diarrhea in 9%.
Carbetocin is not only long-lasting in circulation, it also has better affinity for oxytocin receptors than for vasopressin receptors, reducing the potential for causing hyponatremia. The idea to use it in patients with PWS followed prior studies with oxytocin, which had shown dopamine interactions that reduced anxiety and influenced food ingestion behavior. Brain autopsy studies had shown that patients with Prader-Willi syndrome have substantially fewer neurons than usual producing oxytocin. Treatment with intranasal carbetocin had shown efficacy for improving hyperphagia and behavior in a controlled phase 2 study with 37 patients.
Carbetocin is approved for use in reducing excessive bleeding after childbirth, particularly cesarean, in more than 20 countries outside the United States.
CARE-PWS was sponsored by Levo Therapeutics, the company developing carbetocin. Dr. Deal has been an adviser to Levo Therapeutics. Dr. Strong is an employee of the Foundation for Prader-Willi Research, which has received support from Levo Therapeutics as well as from other drug companies, but which receives most of its funding from individuals. Dr. Miller has received research funding from Levo Therapeutics and also from Harmony Biosciences, Rhythm Pharmaceuticals, and Soleno Therapeutics.
Children and adolescents with Prader-Willi syndrome (PWS) who received three daily, intranasal doses of carbetocin, an investigational, long-acting oxytocin analogue, had significant improvement in hyperphagia and anxiety during 8 weeks on treatment, compared with placebo in a multicenter, phase 3 trial with 119 patients.
The treatment also appeared safe during up to 56 additional weeks on active treatment, with no serious adverse effects nor “unexpected” events, and once completing the study about 95% of enrolled patients opted to remain on active treatment, Cheri L. Deal, MD, PhD, said at the annual meeting of the Endocrine Society.
Based on “the significant results for the placebo-controlled period, as well as for those finishing the 56-week extension, we may well have a new armament for helping these kids and their families deal with the unrelenting hunger of patients with PWS as well as some of the behavioral symptoms,” Dr. Deal, chief of endocrinology and diabetes at the Sainte-Justine Mother-Child University of Montreal Hospital, said in an interview. No treatment currently has labeling for addressing the hyperphagia or anxiety that is characteristic and often problematic for children and adolescents with PWS, an autosomal dominant genetic disease with an incidence of about 1 in 15,000 births and an estimated U.S. prevalence of about 9,000 cases, or about 1 case for every 37,000 people.
‘Gorgeous’ safety
“The results looked pretty positive, and we’re encouraged by what appears to be a good safety profile, so overall I think the PWS community is very excited by the results and is very interested in getting access to this drug,” commented Theresa V. Strong, PhD, director of research programs for the Foundation for Prader-Willi Research in Walnut, Calif., a group not involved with the study. Currently, “we have no effective treatments for these difficult behaviors” of hyperphagia and anxiety. Surveys and studies run by the foundation have documented that hyperphagia and anxiety “were the two most important symptoms that families would like to see treated,” Dr. Strong added in an interview.
PWS “is complex and affects almost every aspect of the lives of affected people and their families. Any treatment that can chip away at some of the problems these patients have can be a huge benefit to the patients and their families,” said Jennifer L. Miller, MD, a professor of pediatric endocrinology at the University of Florida, Gainesville, and a coinvestigator on the study.
But the finding that carbetocin appeared to address, at least in part, this unmet need while compiling a safety record that Dr. Miller called “gorgeous” and “remarkable,” also came with a few limitations.
Fewer patients than planned, and muddled outcomes
The CARE-PWS trial aimed to enroll 175 patients, but fell short once the COVID-19 pandemic hit. Plus the trial had two prespecified primary endpoints – improvements in a measure of hyperphagia, and in a measure of obsessive and compulsive behaviors – specifically in the 40 patients who received the higher of the two dosages studied, 9.6 mg t.i.d. intranasally. Neither endpoint showed significant improvement among the patients on this dosage, compared with the 40 patients who received placebo, although both outcomes trended in the right direction in the actively treated patients.
The study’s positive results came in a secondary treatment group, 39 patients who received 3.2 mg t.i.d., also intranasally. This subgroup had significant benefit, compared with placebo, for reducing hyperphagia symptoms as measured on the Hyperphagia Questionnaire for Clinical Trials (HQ-CT) Total Score. After the first 8 weeks on treatment, patients on the lower carbetocin dosage had an average reduction in their HQ-CT score of greater than 5 points, more than double the reduction seen among control patients who received placebo.
Those on the 3.2-mg t.i.d. dosage also showed significant improvements, compared with placebo, for anxiety, measured by the PWS Anxiety and Distress Questionnaire Total Score, as well as on measures of clinical global impression of severity, and of clinical global impression of change. Like the higher-dosage patients the lower-dosage subgroup did not show a significant difference compared with placebo for the other primary endpoint, change in obsessive and compulsive behaviors as measured by the Children’s Yale-Brown Obsessive-Compulsive Scale Total Score, although also like the higher dosage the effect from the lower dosage trended toward benefit.
A further limitation was that, at the time of her report, presented in abstract OR16-3 at the meeting, Dr. Deal could only present complete 64-week follow-up for 72 patients, although this reassuringly showed that, as time on the 9.6-mg t.i.d. dosage continued beyond 8 weeks, patients gradually improved their HQ-CT response so that by 64 weeks on treatment their hyperphagia score had improved as much as in the patients who received the lower dosage.
In short, documented benefits occurred on the lower dosage, especially for clinically meaningful symptoms like hyperphagia and anxiety, but the study’s overall results were not fully consistent by statistical criteria.
Benefiting an unmet need?
“While it is regrettable that we did not get to 175 patients because of COVID-19, the dataset is significant enough for me to feel that the FDA [Food and Drug Administration] needs to take a very serious look and consider approval,” Dr. Deal said in an interview. “Once safety is assured, which I think it is, I can only hope that regulatory officials understand their unmet needs of this rare disease community and will allow the drug to move to the next stage.”
“This is a very rare disease, and having families participate in trials is really challenging,” especially while the COVID-19 pandemic continues, Dr. Strong said. For the pediatric and adolescent patients targeted in this study “it will take a while for COVID to go away and for families to feel safe again being in a trial, so a real concern is that a need for more clinical trials is not terribly feasible now. Given that the safety profile looked good and one dose seemed to have good efficacy, as long as the long-term data continue to look good we’d love for the FDA to look at the existing data and see whether there is a path forward.”
Dr. Miller highlighted the limitations of what the CARE-PWS findings show.
“Given that it was only an 8-week trial of drug against placebo, and the fact that the primary outcomes weren’t met for the higher dose, my thought is that potentially we need to study more patients for a longer period at the 3.2-mg dose,” she said. She acknowledged that the metric used in the study to assess obsessive and compulsive behaviors is “very difficult” to apply to patients with PWS because of uncertainties in scoring obsessions in patients “who are not very good at telling you what they’re thinking.” Plus, “it’s absolutely not a problem that we did not see an effect on obsession and compulsions if the treatment potentially improves anxiety and hyperphagia, which are very common.” A treatment that reliably reduces these symptoms “would be amazing,” Dr. Miller added.
“PWS is very rare, so it’s very hard to do trials. Maybe the FDA will approve carbetocin because it was safe and gave a signal of efficacy at the lower dose. But my thought is that additional treatment trials are needed with only the lower dose and with longer duration,” she said.
CARE-PWS enrolled patients with nutritional phase 3 PWS who were aged 7-18 years at any of 24 sites in the United States, Canada, or Australia during 2018-2020. They averaged about 12 years of age, and 56% were girls.
The most common adverse effect from carbetocin was flushing, occurring in 14% of those on the lower dose and 21% on the higher dose, but not in any placebo patient. Other adverse effects more common on the lower dose than in the placebo group included headache in 16%, and diarrhea in 9%.
Carbetocin is not only long-lasting in circulation, it also has better affinity for oxytocin receptors than for vasopressin receptors, reducing the potential for causing hyponatremia. The idea to use it in patients with PWS followed prior studies with oxytocin, which had shown dopamine interactions that reduced anxiety and influenced food ingestion behavior. Brain autopsy studies had shown that patients with Prader-Willi syndrome have substantially fewer neurons than usual producing oxytocin. Treatment with intranasal carbetocin had shown efficacy for improving hyperphagia and behavior in a controlled phase 2 study with 37 patients.
Carbetocin is approved for use in reducing excessive bleeding after childbirth, particularly cesarean, in more than 20 countries outside the United States.
CARE-PWS was sponsored by Levo Therapeutics, the company developing carbetocin. Dr. Deal has been an adviser to Levo Therapeutics. Dr. Strong is an employee of the Foundation for Prader-Willi Research, which has received support from Levo Therapeutics as well as from other drug companies, but which receives most of its funding from individuals. Dr. Miller has received research funding from Levo Therapeutics and also from Harmony Biosciences, Rhythm Pharmaceuticals, and Soleno Therapeutics.
Children and adolescents with Prader-Willi syndrome (PWS) who received three daily, intranasal doses of carbetocin, an investigational, long-acting oxytocin analogue, had significant improvement in hyperphagia and anxiety during 8 weeks on treatment, compared with placebo in a multicenter, phase 3 trial with 119 patients.
The treatment also appeared safe during up to 56 additional weeks on active treatment, with no serious adverse effects nor “unexpected” events, and once completing the study about 95% of enrolled patients opted to remain on active treatment, Cheri L. Deal, MD, PhD, said at the annual meeting of the Endocrine Society.
Based on “the significant results for the placebo-controlled period, as well as for those finishing the 56-week extension, we may well have a new armament for helping these kids and their families deal with the unrelenting hunger of patients with PWS as well as some of the behavioral symptoms,” Dr. Deal, chief of endocrinology and diabetes at the Sainte-Justine Mother-Child University of Montreal Hospital, said in an interview. No treatment currently has labeling for addressing the hyperphagia or anxiety that is characteristic and often problematic for children and adolescents with PWS, an autosomal dominant genetic disease with an incidence of about 1 in 15,000 births and an estimated U.S. prevalence of about 9,000 cases, or about 1 case for every 37,000 people.
‘Gorgeous’ safety
“The results looked pretty positive, and we’re encouraged by what appears to be a good safety profile, so overall I think the PWS community is very excited by the results and is very interested in getting access to this drug,” commented Theresa V. Strong, PhD, director of research programs for the Foundation for Prader-Willi Research in Walnut, Calif., a group not involved with the study. Currently, “we have no effective treatments for these difficult behaviors” of hyperphagia and anxiety. Surveys and studies run by the foundation have documented that hyperphagia and anxiety “were the two most important symptoms that families would like to see treated,” Dr. Strong added in an interview.
PWS “is complex and affects almost every aspect of the lives of affected people and their families. Any treatment that can chip away at some of the problems these patients have can be a huge benefit to the patients and their families,” said Jennifer L. Miller, MD, a professor of pediatric endocrinology at the University of Florida, Gainesville, and a coinvestigator on the study.
But the finding that carbetocin appeared to address, at least in part, this unmet need while compiling a safety record that Dr. Miller called “gorgeous” and “remarkable,” also came with a few limitations.
Fewer patients than planned, and muddled outcomes
The CARE-PWS trial aimed to enroll 175 patients, but fell short once the COVID-19 pandemic hit. Plus the trial had two prespecified primary endpoints – improvements in a measure of hyperphagia, and in a measure of obsessive and compulsive behaviors – specifically in the 40 patients who received the higher of the two dosages studied, 9.6 mg t.i.d. intranasally. Neither endpoint showed significant improvement among the patients on this dosage, compared with the 40 patients who received placebo, although both outcomes trended in the right direction in the actively treated patients.
The study’s positive results came in a secondary treatment group, 39 patients who received 3.2 mg t.i.d., also intranasally. This subgroup had significant benefit, compared with placebo, for reducing hyperphagia symptoms as measured on the Hyperphagia Questionnaire for Clinical Trials (HQ-CT) Total Score. After the first 8 weeks on treatment, patients on the lower carbetocin dosage had an average reduction in their HQ-CT score of greater than 5 points, more than double the reduction seen among control patients who received placebo.
Those on the 3.2-mg t.i.d. dosage also showed significant improvements, compared with placebo, for anxiety, measured by the PWS Anxiety and Distress Questionnaire Total Score, as well as on measures of clinical global impression of severity, and of clinical global impression of change. Like the higher-dosage patients the lower-dosage subgroup did not show a significant difference compared with placebo for the other primary endpoint, change in obsessive and compulsive behaviors as measured by the Children’s Yale-Brown Obsessive-Compulsive Scale Total Score, although also like the higher dosage the effect from the lower dosage trended toward benefit.
A further limitation was that, at the time of her report, presented in abstract OR16-3 at the meeting, Dr. Deal could only present complete 64-week follow-up for 72 patients, although this reassuringly showed that, as time on the 9.6-mg t.i.d. dosage continued beyond 8 weeks, patients gradually improved their HQ-CT response so that by 64 weeks on treatment their hyperphagia score had improved as much as in the patients who received the lower dosage.
In short, documented benefits occurred on the lower dosage, especially for clinically meaningful symptoms like hyperphagia and anxiety, but the study’s overall results were not fully consistent by statistical criteria.
Benefiting an unmet need?
“While it is regrettable that we did not get to 175 patients because of COVID-19, the dataset is significant enough for me to feel that the FDA [Food and Drug Administration] needs to take a very serious look and consider approval,” Dr. Deal said in an interview. “Once safety is assured, which I think it is, I can only hope that regulatory officials understand their unmet needs of this rare disease community and will allow the drug to move to the next stage.”
“This is a very rare disease, and having families participate in trials is really challenging,” especially while the COVID-19 pandemic continues, Dr. Strong said. For the pediatric and adolescent patients targeted in this study “it will take a while for COVID to go away and for families to feel safe again being in a trial, so a real concern is that a need for more clinical trials is not terribly feasible now. Given that the safety profile looked good and one dose seemed to have good efficacy, as long as the long-term data continue to look good we’d love for the FDA to look at the existing data and see whether there is a path forward.”
Dr. Miller highlighted the limitations of what the CARE-PWS findings show.
“Given that it was only an 8-week trial of drug against placebo, and the fact that the primary outcomes weren’t met for the higher dose, my thought is that potentially we need to study more patients for a longer period at the 3.2-mg dose,” she said. She acknowledged that the metric used in the study to assess obsessive and compulsive behaviors is “very difficult” to apply to patients with PWS because of uncertainties in scoring obsessions in patients “who are not very good at telling you what they’re thinking.” Plus, “it’s absolutely not a problem that we did not see an effect on obsession and compulsions if the treatment potentially improves anxiety and hyperphagia, which are very common.” A treatment that reliably reduces these symptoms “would be amazing,” Dr. Miller added.
“PWS is very rare, so it’s very hard to do trials. Maybe the FDA will approve carbetocin because it was safe and gave a signal of efficacy at the lower dose. But my thought is that additional treatment trials are needed with only the lower dose and with longer duration,” she said.
CARE-PWS enrolled patients with nutritional phase 3 PWS who were aged 7-18 years at any of 24 sites in the United States, Canada, or Australia during 2018-2020. They averaged about 12 years of age, and 56% were girls.
The most common adverse effect from carbetocin was flushing, occurring in 14% of those on the lower dose and 21% on the higher dose, but not in any placebo patient. Other adverse effects more common on the lower dose than in the placebo group included headache in 16%, and diarrhea in 9%.
Carbetocin is not only long-lasting in circulation, it also has better affinity for oxytocin receptors than for vasopressin receptors, reducing the potential for causing hyponatremia. The idea to use it in patients with PWS followed prior studies with oxytocin, which had shown dopamine interactions that reduced anxiety and influenced food ingestion behavior. Brain autopsy studies had shown that patients with Prader-Willi syndrome have substantially fewer neurons than usual producing oxytocin. Treatment with intranasal carbetocin had shown efficacy for improving hyperphagia and behavior in a controlled phase 2 study with 37 patients.
Carbetocin is approved for use in reducing excessive bleeding after childbirth, particularly cesarean, in more than 20 countries outside the United States.
CARE-PWS was sponsored by Levo Therapeutics, the company developing carbetocin. Dr. Deal has been an adviser to Levo Therapeutics. Dr. Strong is an employee of the Foundation for Prader-Willi Research, which has received support from Levo Therapeutics as well as from other drug companies, but which receives most of its funding from individuals. Dr. Miller has received research funding from Levo Therapeutics and also from Harmony Biosciences, Rhythm Pharmaceuticals, and Soleno Therapeutics.
FROM ENDO 2021
Study suggests no added risk of blood clots in COVID-19 outpatients
The incidence of venous thromboembolism (VTE) in nonhospitalized patients with COVID-19 was not significantly different from patients without the infectious disease, according to a new study published in JAMA Internal Medicine.
National Institutes of Health guidelines recommend blood thinners to prevent blood clots in patients hospitalized with COVID-19. However, the new study provides more insight on the best treatment approach for COVID-19 outpatients.
“[COVID-19’s] rapid global progression and impact has caused us to make and modify treatment decisions at a pace that we never have in modern medicine,” study author Nareg Roubinian, MD, an investigator at Kaiser Permanente, Oakland, Calif., said in an interview.
“As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes,” Dr. Roubinian added.
The increased risk of blood clots in patients hospitalized with COVID-19 has been a major issue throughout the pandemic. In fact, one study published in November 2020 found that more than half of patients hospitalized with the illness have prothrombotic antiphospholipid (aPL) autoantibodies in their blood, which could contribute to venous and arterial thromboembolism.
Although it was clear many hospitalized patients diagnosed with COVID-19 were developing more clots, researchers of the current study were not sure if this trend would also be seen in outpatients.
“Most people with COVID-19 do not need to be hospitalized, and we needed to know how often patients outside the hospital were having blood clots,” said Dr. Roubinian.
For the study, Dr. Roubinian and colleagues examined data on 220,588 patients who were members of Kaiser Permanente Northern California health plan and were tested for COVID-19 between Feb. 25 and Aug. 31, 2020. They then reported on the 30-day incidence of outpatient and hospital-associated blood clots following the COVID-19 diagnosis. Patients who were asymptomatic at the time of testing or had received anticoagulants within the last year were excluded.
“We knew from other studies that patients with COVID-19 often get sicker in the first few weeks after infection. What we didn’t know was whether COVID-19 patients were developing blood clots but not pneumonia or were developing blood clots at the same time as they developed pneumonia,” said Dr. Roubinian, an intensive care doctor with the Permanente Medical Group in Oakland, Calif. “Following the patients for 30 days allowed us to focus on the time period from infection to when blood clots were most likely to develop.”
Researchers found that of the cohort who took the COVID-19 test, 11.8% had a positive result. Within 30 days of the COVID-19 test, 0.8% of patients with a positive result were diagnosed with VTE compared to 0.5% of those who received a negative test result. They also found that viral testing took place in an outpatient setting for 59.1% of the patients with a positive viral test who later developed VTE. Of those patients, 76.1% had to be hospitalized.
Dr. Roubinian said he was surprised to see that the blood clotting in outpatients with COVID-19 was similar in frequency to what he saw in patients without the infection.
“Our findings suggest that blood clots do occur in COVID-19 patients but not on a scale where we need to put all or many COVID outpatients on blood thinners,” he said. “As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes.”
In December 2020, three trials investigating the risk and benefits of increased levels of anticoagulation in hospitalized COVID-19 patients were paused because of safety issues. The trials would have enrolled critically ill COVID-19 patients for whom therapeutic doses of anticoagulation drugs showed no benefit.
Anticoagulants are associated with bleeding risks, including prolonged nosebleeds and vomiting or coughing up blood.
Instead of prescribing the routine use of thromboprophylactic drugs to COVID-19 outpatients, Dr. Roubinian believes it would be helpful to learn how to determine whether a patient at risk of becoming sick or being hospitalized would benefit from being treated with such drugs.
Dr. Roubinian reported receiving grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute during the conduct of the study.
The incidence of venous thromboembolism (VTE) in nonhospitalized patients with COVID-19 was not significantly different from patients without the infectious disease, according to a new study published in JAMA Internal Medicine.
National Institutes of Health guidelines recommend blood thinners to prevent blood clots in patients hospitalized with COVID-19. However, the new study provides more insight on the best treatment approach for COVID-19 outpatients.
“[COVID-19’s] rapid global progression and impact has caused us to make and modify treatment decisions at a pace that we never have in modern medicine,” study author Nareg Roubinian, MD, an investigator at Kaiser Permanente, Oakland, Calif., said in an interview.
“As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes,” Dr. Roubinian added.
The increased risk of blood clots in patients hospitalized with COVID-19 has been a major issue throughout the pandemic. In fact, one study published in November 2020 found that more than half of patients hospitalized with the illness have prothrombotic antiphospholipid (aPL) autoantibodies in their blood, which could contribute to venous and arterial thromboembolism.
Although it was clear many hospitalized patients diagnosed with COVID-19 were developing more clots, researchers of the current study were not sure if this trend would also be seen in outpatients.
“Most people with COVID-19 do not need to be hospitalized, and we needed to know how often patients outside the hospital were having blood clots,” said Dr. Roubinian.
For the study, Dr. Roubinian and colleagues examined data on 220,588 patients who were members of Kaiser Permanente Northern California health plan and were tested for COVID-19 between Feb. 25 and Aug. 31, 2020. They then reported on the 30-day incidence of outpatient and hospital-associated blood clots following the COVID-19 diagnosis. Patients who were asymptomatic at the time of testing or had received anticoagulants within the last year were excluded.
“We knew from other studies that patients with COVID-19 often get sicker in the first few weeks after infection. What we didn’t know was whether COVID-19 patients were developing blood clots but not pneumonia or were developing blood clots at the same time as they developed pneumonia,” said Dr. Roubinian, an intensive care doctor with the Permanente Medical Group in Oakland, Calif. “Following the patients for 30 days allowed us to focus on the time period from infection to when blood clots were most likely to develop.”
Researchers found that of the cohort who took the COVID-19 test, 11.8% had a positive result. Within 30 days of the COVID-19 test, 0.8% of patients with a positive result were diagnosed with VTE compared to 0.5% of those who received a negative test result. They also found that viral testing took place in an outpatient setting for 59.1% of the patients with a positive viral test who later developed VTE. Of those patients, 76.1% had to be hospitalized.
Dr. Roubinian said he was surprised to see that the blood clotting in outpatients with COVID-19 was similar in frequency to what he saw in patients without the infection.
“Our findings suggest that blood clots do occur in COVID-19 patients but not on a scale where we need to put all or many COVID outpatients on blood thinners,” he said. “As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes.”
In December 2020, three trials investigating the risk and benefits of increased levels of anticoagulation in hospitalized COVID-19 patients were paused because of safety issues. The trials would have enrolled critically ill COVID-19 patients for whom therapeutic doses of anticoagulation drugs showed no benefit.
Anticoagulants are associated with bleeding risks, including prolonged nosebleeds and vomiting or coughing up blood.
Instead of prescribing the routine use of thromboprophylactic drugs to COVID-19 outpatients, Dr. Roubinian believes it would be helpful to learn how to determine whether a patient at risk of becoming sick or being hospitalized would benefit from being treated with such drugs.
Dr. Roubinian reported receiving grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute during the conduct of the study.
The incidence of venous thromboembolism (VTE) in nonhospitalized patients with COVID-19 was not significantly different from patients without the infectious disease, according to a new study published in JAMA Internal Medicine.
National Institutes of Health guidelines recommend blood thinners to prevent blood clots in patients hospitalized with COVID-19. However, the new study provides more insight on the best treatment approach for COVID-19 outpatients.
“[COVID-19’s] rapid global progression and impact has caused us to make and modify treatment decisions at a pace that we never have in modern medicine,” study author Nareg Roubinian, MD, an investigator at Kaiser Permanente, Oakland, Calif., said in an interview.
“As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes,” Dr. Roubinian added.
The increased risk of blood clots in patients hospitalized with COVID-19 has been a major issue throughout the pandemic. In fact, one study published in November 2020 found that more than half of patients hospitalized with the illness have prothrombotic antiphospholipid (aPL) autoantibodies in their blood, which could contribute to venous and arterial thromboembolism.
Although it was clear many hospitalized patients diagnosed with COVID-19 were developing more clots, researchers of the current study were not sure if this trend would also be seen in outpatients.
“Most people with COVID-19 do not need to be hospitalized, and we needed to know how often patients outside the hospital were having blood clots,” said Dr. Roubinian.
For the study, Dr. Roubinian and colleagues examined data on 220,588 patients who were members of Kaiser Permanente Northern California health plan and were tested for COVID-19 between Feb. 25 and Aug. 31, 2020. They then reported on the 30-day incidence of outpatient and hospital-associated blood clots following the COVID-19 diagnosis. Patients who were asymptomatic at the time of testing or had received anticoagulants within the last year were excluded.
“We knew from other studies that patients with COVID-19 often get sicker in the first few weeks after infection. What we didn’t know was whether COVID-19 patients were developing blood clots but not pneumonia or were developing blood clots at the same time as they developed pneumonia,” said Dr. Roubinian, an intensive care doctor with the Permanente Medical Group in Oakland, Calif. “Following the patients for 30 days allowed us to focus on the time period from infection to when blood clots were most likely to develop.”
Researchers found that of the cohort who took the COVID-19 test, 11.8% had a positive result. Within 30 days of the COVID-19 test, 0.8% of patients with a positive result were diagnosed with VTE compared to 0.5% of those who received a negative test result. They also found that viral testing took place in an outpatient setting for 59.1% of the patients with a positive viral test who later developed VTE. Of those patients, 76.1% had to be hospitalized.
Dr. Roubinian said he was surprised to see that the blood clotting in outpatients with COVID-19 was similar in frequency to what he saw in patients without the infection.
“Our findings suggest that blood clots do occur in COVID-19 patients but not on a scale where we need to put all or many COVID outpatients on blood thinners,” he said. “As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes.”
In December 2020, three trials investigating the risk and benefits of increased levels of anticoagulation in hospitalized COVID-19 patients were paused because of safety issues. The trials would have enrolled critically ill COVID-19 patients for whom therapeutic doses of anticoagulation drugs showed no benefit.
Anticoagulants are associated with bleeding risks, including prolonged nosebleeds and vomiting or coughing up blood.
Instead of prescribing the routine use of thromboprophylactic drugs to COVID-19 outpatients, Dr. Roubinian believes it would be helpful to learn how to determine whether a patient at risk of becoming sick or being hospitalized would benefit from being treated with such drugs.
Dr. Roubinian reported receiving grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute during the conduct of the study.
COVID-19 in children: New cases back on the decline
New cases of COVID-19 in children in the United States fell slightly, but even that small dip was enough to reverse 2 straight weeks of increases, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
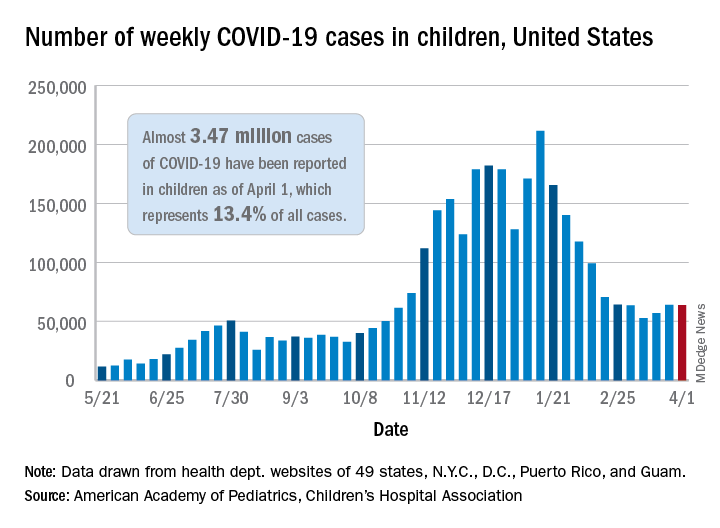
the AAP and the CHA said in their weekly COVID-19 report. For the week ending April 1, children represented 18.1% of all new cases reported in the United States, down from a pandemic-high 19.1% the week before.
COVID-19 cases in children now total just under 3.47 million, which works out to 13.4% of reported cases for all ages and 4,610 cases per 100,000 children since the beginning of the pandemic, the AAP and the CHA said based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Among those jurisdictions, Vermont has the highest proportion of its cases occurring in children at 21.0%, and North Dakota has the highest cumulative rate at 8,958 cases per 100,000 children. Looking at those states from the bottoms of their respective lists are Florida, where children aged 0-14 years represent 8.4% of all cases, and Hawaii, with 1,133 cases per 100,000 children aged 0-17 years, the AAP/CHA report shows.
The data on more serious illness show that Minnesota has the highest proportion of hospitalizations occurring in children at 3.1%, while New York City has the highest hospitalization rate among infected children, 2.0%. Among the other 23 states reporting on such admissions, children make up only 1.3% of hospitalizations in Florida and in New Hampshire, which also has the lowest hospitalization rate at 0.1%, the AAP and CHA said.
Five more deaths were reported in children during the week ending April 1, bringing the total to 284 in the 43 states, along with New York City, Puerto Rico, and Guam, that are sharing age-distribution data on mortality.
New cases of COVID-19 in children in the United States fell slightly, but even that small dip was enough to reverse 2 straight weeks of increases, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and the CHA said in their weekly COVID-19 report. For the week ending April 1, children represented 18.1% of all new cases reported in the United States, down from a pandemic-high 19.1% the week before.
COVID-19 cases in children now total just under 3.47 million, which works out to 13.4% of reported cases for all ages and 4,610 cases per 100,000 children since the beginning of the pandemic, the AAP and the CHA said based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Among those jurisdictions, Vermont has the highest proportion of its cases occurring in children at 21.0%, and North Dakota has the highest cumulative rate at 8,958 cases per 100,000 children. Looking at those states from the bottoms of their respective lists are Florida, where children aged 0-14 years represent 8.4% of all cases, and Hawaii, with 1,133 cases per 100,000 children aged 0-17 years, the AAP/CHA report shows.
The data on more serious illness show that Minnesota has the highest proportion of hospitalizations occurring in children at 3.1%, while New York City has the highest hospitalization rate among infected children, 2.0%. Among the other 23 states reporting on such admissions, children make up only 1.3% of hospitalizations in Florida and in New Hampshire, which also has the lowest hospitalization rate at 0.1%, the AAP and CHA said.
Five more deaths were reported in children during the week ending April 1, bringing the total to 284 in the 43 states, along with New York City, Puerto Rico, and Guam, that are sharing age-distribution data on mortality.
New cases of COVID-19 in children in the United States fell slightly, but even that small dip was enough to reverse 2 straight weeks of increases, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and the CHA said in their weekly COVID-19 report. For the week ending April 1, children represented 18.1% of all new cases reported in the United States, down from a pandemic-high 19.1% the week before.
COVID-19 cases in children now total just under 3.47 million, which works out to 13.4% of reported cases for all ages and 4,610 cases per 100,000 children since the beginning of the pandemic, the AAP and the CHA said based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Among those jurisdictions, Vermont has the highest proportion of its cases occurring in children at 21.0%, and North Dakota has the highest cumulative rate at 8,958 cases per 100,000 children. Looking at those states from the bottoms of their respective lists are Florida, where children aged 0-14 years represent 8.4% of all cases, and Hawaii, with 1,133 cases per 100,000 children aged 0-17 years, the AAP/CHA report shows.
The data on more serious illness show that Minnesota has the highest proportion of hospitalizations occurring in children at 3.1%, while New York City has the highest hospitalization rate among infected children, 2.0%. Among the other 23 states reporting on such admissions, children make up only 1.3% of hospitalizations in Florida and in New Hampshire, which also has the lowest hospitalization rate at 0.1%, the AAP and CHA said.
Five more deaths were reported in children during the week ending April 1, bringing the total to 284 in the 43 states, along with New York City, Puerto Rico, and Guam, that are sharing age-distribution data on mortality.
Clinical Edge Commentary: RA April 2021
Iguratimod (IGU) is a novel small-molecule synthetic DMARD being studied for RA; its exact mechanism is unknown, but it inhibits NF-kB activation and seems to reduce bone resorption via RANKL and cartilage erosion via matrix metalloproteinases. Currently, it is approved in Japan and China for treatment of RA; initial studies from 2008-2010 showed improved ACR20 rates compared to placebo and comparable rates with methotrexate monotherapy; combination therapy with methotrexate appears to be more effective than either methotrexate or IGU monotherapy. Yoshikawa et al present a small study of 47 patients receiving methotrexate or methotrexate with IGU and show promising results regarding tapering of methotrexate with sustained remission/low disease activity and decrease in ultrasound evidence of synovitis in patients receiving IGU, suggesting that it is a reasonable longer-term option for RA patients. However, the medication is not approved in the US, nor have studies in patients in the US been published, so generalizability in patients outside of Japan and China is uncertain, in addition to concerns related to the small sample size.
In contrast, tocilizumab is an established therapy for RA; Pappas et al present real-world clinical practice data from the Corrona RA registry. Among nearly 1800 RA patients who initiated tocilizumab therapy, the mean durability of response was over 3 years in terms of maintaining minimum clinically important difference (MCID) over baseline CDAI and over 1 year in terms of maintaining low disease activity. Most patients in the study had previously received biologic therapy. Though the observational nature of the study limits its generalizability, the addition of efficacy data to persistence on therapy lend weight to this evidence regarding durability of response to tocilizumab, as the proportion of patients maintaining MCID was >50% at 3 years.
Treatment options are limited for RA patients who have ILD or other pulmonary complications. The fear of causing or exacerbating pulmonary toxicity limits the use of methotrexate, leflunomide, and anti-TNF biologics. Some data support the safety of rituximab and abatacept. A retrospective observational study looked at outcomes in RA patients on rituximab and JAK inhibitors. Reassuringly, respiratory event rates were no different between the two groups though number of patients and trial design prevents wider generalization; it is also unknown whether RA-ILD improves with ritixumab or JAK-inhibition. However, given these results, studying JAK inhibitors in larger prospective studies would be reasonable.
Iguratimod (IGU) is a novel small-molecule synthetic DMARD being studied for RA; its exact mechanism is unknown, but it inhibits NF-kB activation and seems to reduce bone resorption via RANKL and cartilage erosion via matrix metalloproteinases. Currently, it is approved in Japan and China for treatment of RA; initial studies from 2008-2010 showed improved ACR20 rates compared to placebo and comparable rates with methotrexate monotherapy; combination therapy with methotrexate appears to be more effective than either methotrexate or IGU monotherapy. Yoshikawa et al present a small study of 47 patients receiving methotrexate or methotrexate with IGU and show promising results regarding tapering of methotrexate with sustained remission/low disease activity and decrease in ultrasound evidence of synovitis in patients receiving IGU, suggesting that it is a reasonable longer-term option for RA patients. However, the medication is not approved in the US, nor have studies in patients in the US been published, so generalizability in patients outside of Japan and China is uncertain, in addition to concerns related to the small sample size.
In contrast, tocilizumab is an established therapy for RA; Pappas et al present real-world clinical practice data from the Corrona RA registry. Among nearly 1800 RA patients who initiated tocilizumab therapy, the mean durability of response was over 3 years in terms of maintaining minimum clinically important difference (MCID) over baseline CDAI and over 1 year in terms of maintaining low disease activity. Most patients in the study had previously received biologic therapy. Though the observational nature of the study limits its generalizability, the addition of efficacy data to persistence on therapy lend weight to this evidence regarding durability of response to tocilizumab, as the proportion of patients maintaining MCID was >50% at 3 years.
Treatment options are limited for RA patients who have ILD or other pulmonary complications. The fear of causing or exacerbating pulmonary toxicity limits the use of methotrexate, leflunomide, and anti-TNF biologics. Some data support the safety of rituximab and abatacept. A retrospective observational study looked at outcomes in RA patients on rituximab and JAK inhibitors. Reassuringly, respiratory event rates were no different between the two groups though number of patients and trial design prevents wider generalization; it is also unknown whether RA-ILD improves with ritixumab or JAK-inhibition. However, given these results, studying JAK inhibitors in larger prospective studies would be reasonable.
Iguratimod (IGU) is a novel small-molecule synthetic DMARD being studied for RA; its exact mechanism is unknown, but it inhibits NF-kB activation and seems to reduce bone resorption via RANKL and cartilage erosion via matrix metalloproteinases. Currently, it is approved in Japan and China for treatment of RA; initial studies from 2008-2010 showed improved ACR20 rates compared to placebo and comparable rates with methotrexate monotherapy; combination therapy with methotrexate appears to be more effective than either methotrexate or IGU monotherapy. Yoshikawa et al present a small study of 47 patients receiving methotrexate or methotrexate with IGU and show promising results regarding tapering of methotrexate with sustained remission/low disease activity and decrease in ultrasound evidence of synovitis in patients receiving IGU, suggesting that it is a reasonable longer-term option for RA patients. However, the medication is not approved in the US, nor have studies in patients in the US been published, so generalizability in patients outside of Japan and China is uncertain, in addition to concerns related to the small sample size.
In contrast, tocilizumab is an established therapy for RA; Pappas et al present real-world clinical practice data from the Corrona RA registry. Among nearly 1800 RA patients who initiated tocilizumab therapy, the mean durability of response was over 3 years in terms of maintaining minimum clinically important difference (MCID) over baseline CDAI and over 1 year in terms of maintaining low disease activity. Most patients in the study had previously received biologic therapy. Though the observational nature of the study limits its generalizability, the addition of efficacy data to persistence on therapy lend weight to this evidence regarding durability of response to tocilizumab, as the proportion of patients maintaining MCID was >50% at 3 years.
Treatment options are limited for RA patients who have ILD or other pulmonary complications. The fear of causing or exacerbating pulmonary toxicity limits the use of methotrexate, leflunomide, and anti-TNF biologics. Some data support the safety of rituximab and abatacept. A retrospective observational study looked at outcomes in RA patients on rituximab and JAK inhibitors. Reassuringly, respiratory event rates were no different between the two groups though number of patients and trial design prevents wider generalization; it is also unknown whether RA-ILD improves with ritixumab or JAK-inhibition. However, given these results, studying JAK inhibitors in larger prospective studies would be reasonable.
RA: Difficult-to-treat cases persist in real world despite intensive treatment
Key clinical point: Considerable proportions of cases with rheumatoid arthritis (RA) are still difficult to treat in real world despite intensive treatment.
Major finding: Among patients with RA, 10.1% were categorized as having difficult-to-treat RA despite being treated intensively. Main reasons for difficulty in treating RA were multidrug resistance (34.1%), comorbidities (9.8%), and socioeconomic reasons (56.1%).
Study details: Data come from an analysis of 1,709 patients with RA who visited Keio University Hospital between 2016 and 2017.
Disclosures: The study did not receive any funding. S Takanashi reported no conflicts of interest. Y Kaneko and T Takeuchi reported receiving grants or speaker fees from various sources.
Source: Takanashi S et al. Rheumatology (Oxford). 2021 Mar 2. doi: 10.1093/rheumatology/keab209.
Key clinical point: Considerable proportions of cases with rheumatoid arthritis (RA) are still difficult to treat in real world despite intensive treatment.
Major finding: Among patients with RA, 10.1% were categorized as having difficult-to-treat RA despite being treated intensively. Main reasons for difficulty in treating RA were multidrug resistance (34.1%), comorbidities (9.8%), and socioeconomic reasons (56.1%).
Study details: Data come from an analysis of 1,709 patients with RA who visited Keio University Hospital between 2016 and 2017.
Disclosures: The study did not receive any funding. S Takanashi reported no conflicts of interest. Y Kaneko and T Takeuchi reported receiving grants or speaker fees from various sources.
Source: Takanashi S et al. Rheumatology (Oxford). 2021 Mar 2. doi: 10.1093/rheumatology/keab209.
Key clinical point: Considerable proportions of cases with rheumatoid arthritis (RA) are still difficult to treat in real world despite intensive treatment.
Major finding: Among patients with RA, 10.1% were categorized as having difficult-to-treat RA despite being treated intensively. Main reasons for difficulty in treating RA were multidrug resistance (34.1%), comorbidities (9.8%), and socioeconomic reasons (56.1%).
Study details: Data come from an analysis of 1,709 patients with RA who visited Keio University Hospital between 2016 and 2017.
Disclosures: The study did not receive any funding. S Takanashi reported no conflicts of interest. Y Kaneko and T Takeuchi reported receiving grants or speaker fees from various sources.
Source: Takanashi S et al. Rheumatology (Oxford). 2021 Mar 2. doi: 10.1093/rheumatology/keab209.
Low cardiorespiratory fitness contributes to excess all-cause mortality in RA
Key clinical point: Low cardiorespiratory fitness (CRF) is an important mediator of increased long-term all-cause mortality among patients with rheumatoid arthritis (RA).
Major finding: Patients with RA having CRF below sex-specific and age-specific median had a 28% excess relative risk of mortality (P = .035), of which 5% was associated with the disease itself and 23% was mediated by direct and indirect effects of low CRF.
Study details: Data come from an analysis of patients with RA (n=348) and controls (n=60,938) who participated in the second and third waves of the longitudinal population-based Trøndelag Health Study.
Disclosures: This project was funded by a grant to MH Liff from the Central Norway Regional Health Authority, allocated via the Liaison Committee for Education, Research and Innovation in Central Norway. All the authors declared no conflicts of interest.
Source: Liff MH et al. RMD Open. 2021 March 8. doi: 10.1136/rmdopen-2020-001545.
Key clinical point: Low cardiorespiratory fitness (CRF) is an important mediator of increased long-term all-cause mortality among patients with rheumatoid arthritis (RA).
Major finding: Patients with RA having CRF below sex-specific and age-specific median had a 28% excess relative risk of mortality (P = .035), of which 5% was associated with the disease itself and 23% was mediated by direct and indirect effects of low CRF.
Study details: Data come from an analysis of patients with RA (n=348) and controls (n=60,938) who participated in the second and third waves of the longitudinal population-based Trøndelag Health Study.
Disclosures: This project was funded by a grant to MH Liff from the Central Norway Regional Health Authority, allocated via the Liaison Committee for Education, Research and Innovation in Central Norway. All the authors declared no conflicts of interest.
Source: Liff MH et al. RMD Open. 2021 March 8. doi: 10.1136/rmdopen-2020-001545.
Key clinical point: Low cardiorespiratory fitness (CRF) is an important mediator of increased long-term all-cause mortality among patients with rheumatoid arthritis (RA).
Major finding: Patients with RA having CRF below sex-specific and age-specific median had a 28% excess relative risk of mortality (P = .035), of which 5% was associated with the disease itself and 23% was mediated by direct and indirect effects of low CRF.
Study details: Data come from an analysis of patients with RA (n=348) and controls (n=60,938) who participated in the second and third waves of the longitudinal population-based Trøndelag Health Study.
Disclosures: This project was funded by a grant to MH Liff from the Central Norway Regional Health Authority, allocated via the Liaison Committee for Education, Research and Innovation in Central Norway. All the authors declared no conflicts of interest.
Source: Liff MH et al. RMD Open. 2021 March 8. doi: 10.1136/rmdopen-2020-001545.
Simple blood test plus AI may flag early-stage Alzheimer’s disease
, raising the prospect of early intervention when effective treatments become available.
In a study, investigators used six AI methodologies, including Deep Learning, to assess blood leukocyte epigenomic biomarkers. They found more than 150 genetic differences among study participants with Alzheimer’s disease in comparison with participants who did not have Alzheimer’s disease.
All of the AI platforms were effective in predicting Alzheimer’s disease. Deep Learning’s assessment of intragenic cytosine-phosphate-guanines (CpGs) had sensitivity and specificity rates of 97%.
“It’s almost as if the leukocytes have become a newspaper to tell us, ‘This is what is going on in the brain,’ “ lead author Ray Bahado-Singh, MD, chair of the department of obstetrics and gynecology, Oakland University, Auburn Hills, Mich., said in a news release.
The researchers noted that the findings, if replicated in future studies, may help in providing Alzheimer’s disease diagnoses “much earlier” in the disease process. “The holy grail is to identify patients in the preclinical stage so effective early interventions, including new medications, can be studied and ultimately used,” Dr. Bahado-Singh said.
“This certainly isn’t the final step in Alzheimer’s research, but I think this represents a significant change in direction,” he told attendees at a press briefing.
The findings were published online March 31 in PLOS ONE.
Silver tsunami
The investigators noted that Alzheimer’s disease is often diagnosed when the disease is in its later stages, after irreversible brain damage has occurred. “There is currently no cure for the disease, and the treatment is limited to drugs that attempt to treat symptoms and have little effect on the disease’s progression,” they noted.
Coinvestigator Khaled Imam, MD, director of geriatric medicine for Beaumont Health in Michigan, pointed out that although MRI and lumbar puncture can identify Alzheimer’s disease early on, the processes are expensive and/or invasive.
“Having biomarkers in the blood ... and being able to identify [Alzheimer’s disease] years before symptoms start, hopefully we’d be able to intervene early on in the process of the disease,” Dr. Imam said.
It is estimated that the number of Americans aged 85 and older will triple by 2050. This impending “silver tsunami,” which will come with a commensurate increase in Alzheimer’s disease cases, makes it even more important to be able to diagnose the disease early on, he noted.
The study included 24 individuals with late-onset Alzheimer’s disease (70.8% women; mean age, 83 years); 24 were deemed to be “cognitively healthy” (66.7% women; mean age, 80 years). About 500 ng of genomic DNA was extracted from whole-blood samples from each participant.
The researchers used the Infinium MethylationEPIC BeadChip array, and the samples were then examined for markers of methylation that would “indicate the disease process has started,” they noted.
In addition to Deep Learning, the five other AI platforms were the Support Vector Machine, Generalized Linear Model, Prediction Analysis for Microarrays, Random Forest, and Linear Discriminant Analysis.
These platforms were used to assess leukocyte genome changes. To predict Alzheimer’s disease, the researchers also used Ingenuity Pathway Analysis.
Significant “chemical changes”
Results showed that the Alzheimer’s disease group had 152 significantly differentially methylated CpGs in 171 genes in comparison with the non-Alzheimer’s disease group (false discovery rate P value < .05).
As a whole, using intragenic and intergenic/extragenic CpGs, the AI platforms were effective in predicting who had Alzheimer’s disease (area under the curve [AUC], ≥ 0.93). Using intragenic markers, the AUC for Deep Learning was 0.99.
“We looked at close to a million different sites, and we saw some chemical changes that we know are associated with alteration or change in gene function,” Dr. Bahado-Singh said.
Altered genes that were found in the Alzheimer’s disease group included CR1L, CTSV, S1PR1, and LTB4R – all of which “have been previously linked with Alzheimer’s disease and dementia,” the researchers noted. They also found the methylated genes CTSV and PRMT5, both of which have been previously associated with cardiovascular disease.
“A significant strength of our study is the novelty, i.e. the use of blood leukocytes to accurately detect Alzheimer’s disease and also for interrogating the pathogenesis of Alzheimer’s disease,” the investigators wrote.
Dr. Bahado-Singh said that the test let them identify changes in cells in the blood, “giving us a comprehensive account not only of the fact that the brain is being affected by Alzheimer’s disease but it’s telling us what kinds of processes are going on in the brain.
“Normally you don’t have access to the brain. This gives us a simple blood test to get an ongoing reading of the course of events in the brain – and potentially tell us very early on before the onset of symptoms,” he added.
Cautiously optimistic
During the question-and-answer session following his presentation at the briefing, Dr. Bahado-Singh reiterated that they are at a very early stage in the research and were not able to make clinical recommendations at this point. However, he added, “There was evidence that DNA methylation change could likely precede the onset of abnormalities in the cells that give rise to the disease.”
Coinvestigator Stewart Graham, PhD, director of Alzheimer’s research at Beaumont Health, added that although the initial study findings led to some excitement for the team, “we have to be very conservative with what we say.”
He noted that the findings need to be replicated in a more diverse population. Still, “we’re excited at the moment and looking forward to seeing what the future results hold,” Dr. Graham said.
Dr. Bahado-Singh said that if larger studies confirm the findings and the test is viable, it would make sense to use it as a screen for individuals older than 65. He noted that because of the aging of the population, “this subset of individuals will constitute a larger and larger fraction of the population globally.”
Still early days
Commenting on the findings, Heather Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, noted that the investigators used an “interesting” diagnostic process.
“It was a unique approach to looking at and trying to understand what might be some of the biological underpinnings and using these tools and technologies to determine if they’re able to differentiate individuals with Alzheimer’s disease” from those without Alzheimer’s disease, said Dr. Snyder, who was not involved with the research.
“Ultimately, we want to know who is at greater risk, who may have some of the changing biology at the earliest time point so that we can intervene to stop the progression of the disease,” she said.
She pointed out that a number of types of biomarker tests are currently under investigation, many of which are measuring different outcomes. “And that’s what we want to see going forward. We want to have as many tools in our toolbox that allow us to accurately diagnose at that earliest time point,” Dr. Snyder said.
“At this point, [the current study] is still pretty early, so it needs to be replicated and then expanded to larger groups to really understand what they may be seeing,” she added.
Dr. Bahado-Singh, Dr. Imam, Dr. Graham, and Dr. Snyder have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, raising the prospect of early intervention when effective treatments become available.
In a study, investigators used six AI methodologies, including Deep Learning, to assess blood leukocyte epigenomic biomarkers. They found more than 150 genetic differences among study participants with Alzheimer’s disease in comparison with participants who did not have Alzheimer’s disease.
All of the AI platforms were effective in predicting Alzheimer’s disease. Deep Learning’s assessment of intragenic cytosine-phosphate-guanines (CpGs) had sensitivity and specificity rates of 97%.
“It’s almost as if the leukocytes have become a newspaper to tell us, ‘This is what is going on in the brain,’ “ lead author Ray Bahado-Singh, MD, chair of the department of obstetrics and gynecology, Oakland University, Auburn Hills, Mich., said in a news release.
The researchers noted that the findings, if replicated in future studies, may help in providing Alzheimer’s disease diagnoses “much earlier” in the disease process. “The holy grail is to identify patients in the preclinical stage so effective early interventions, including new medications, can be studied and ultimately used,” Dr. Bahado-Singh said.
“This certainly isn’t the final step in Alzheimer’s research, but I think this represents a significant change in direction,” he told attendees at a press briefing.
The findings were published online March 31 in PLOS ONE.
Silver tsunami
The investigators noted that Alzheimer’s disease is often diagnosed when the disease is in its later stages, after irreversible brain damage has occurred. “There is currently no cure for the disease, and the treatment is limited to drugs that attempt to treat symptoms and have little effect on the disease’s progression,” they noted.
Coinvestigator Khaled Imam, MD, director of geriatric medicine for Beaumont Health in Michigan, pointed out that although MRI and lumbar puncture can identify Alzheimer’s disease early on, the processes are expensive and/or invasive.
“Having biomarkers in the blood ... and being able to identify [Alzheimer’s disease] years before symptoms start, hopefully we’d be able to intervene early on in the process of the disease,” Dr. Imam said.
It is estimated that the number of Americans aged 85 and older will triple by 2050. This impending “silver tsunami,” which will come with a commensurate increase in Alzheimer’s disease cases, makes it even more important to be able to diagnose the disease early on, he noted.
The study included 24 individuals with late-onset Alzheimer’s disease (70.8% women; mean age, 83 years); 24 were deemed to be “cognitively healthy” (66.7% women; mean age, 80 years). About 500 ng of genomic DNA was extracted from whole-blood samples from each participant.
The researchers used the Infinium MethylationEPIC BeadChip array, and the samples were then examined for markers of methylation that would “indicate the disease process has started,” they noted.
In addition to Deep Learning, the five other AI platforms were the Support Vector Machine, Generalized Linear Model, Prediction Analysis for Microarrays, Random Forest, and Linear Discriminant Analysis.
These platforms were used to assess leukocyte genome changes. To predict Alzheimer’s disease, the researchers also used Ingenuity Pathway Analysis.
Significant “chemical changes”
Results showed that the Alzheimer’s disease group had 152 significantly differentially methylated CpGs in 171 genes in comparison with the non-Alzheimer’s disease group (false discovery rate P value < .05).
As a whole, using intragenic and intergenic/extragenic CpGs, the AI platforms were effective in predicting who had Alzheimer’s disease (area under the curve [AUC], ≥ 0.93). Using intragenic markers, the AUC for Deep Learning was 0.99.
“We looked at close to a million different sites, and we saw some chemical changes that we know are associated with alteration or change in gene function,” Dr. Bahado-Singh said.
Altered genes that were found in the Alzheimer’s disease group included CR1L, CTSV, S1PR1, and LTB4R – all of which “have been previously linked with Alzheimer’s disease and dementia,” the researchers noted. They also found the methylated genes CTSV and PRMT5, both of which have been previously associated with cardiovascular disease.
“A significant strength of our study is the novelty, i.e. the use of blood leukocytes to accurately detect Alzheimer’s disease and also for interrogating the pathogenesis of Alzheimer’s disease,” the investigators wrote.
Dr. Bahado-Singh said that the test let them identify changes in cells in the blood, “giving us a comprehensive account not only of the fact that the brain is being affected by Alzheimer’s disease but it’s telling us what kinds of processes are going on in the brain.
“Normally you don’t have access to the brain. This gives us a simple blood test to get an ongoing reading of the course of events in the brain – and potentially tell us very early on before the onset of symptoms,” he added.
Cautiously optimistic
During the question-and-answer session following his presentation at the briefing, Dr. Bahado-Singh reiterated that they are at a very early stage in the research and were not able to make clinical recommendations at this point. However, he added, “There was evidence that DNA methylation change could likely precede the onset of abnormalities in the cells that give rise to the disease.”
Coinvestigator Stewart Graham, PhD, director of Alzheimer’s research at Beaumont Health, added that although the initial study findings led to some excitement for the team, “we have to be very conservative with what we say.”
He noted that the findings need to be replicated in a more diverse population. Still, “we’re excited at the moment and looking forward to seeing what the future results hold,” Dr. Graham said.
Dr. Bahado-Singh said that if larger studies confirm the findings and the test is viable, it would make sense to use it as a screen for individuals older than 65. He noted that because of the aging of the population, “this subset of individuals will constitute a larger and larger fraction of the population globally.”
Still early days
Commenting on the findings, Heather Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, noted that the investigators used an “interesting” diagnostic process.
“It was a unique approach to looking at and trying to understand what might be some of the biological underpinnings and using these tools and technologies to determine if they’re able to differentiate individuals with Alzheimer’s disease” from those without Alzheimer’s disease, said Dr. Snyder, who was not involved with the research.
“Ultimately, we want to know who is at greater risk, who may have some of the changing biology at the earliest time point so that we can intervene to stop the progression of the disease,” she said.
She pointed out that a number of types of biomarker tests are currently under investigation, many of which are measuring different outcomes. “And that’s what we want to see going forward. We want to have as many tools in our toolbox that allow us to accurately diagnose at that earliest time point,” Dr. Snyder said.
“At this point, [the current study] is still pretty early, so it needs to be replicated and then expanded to larger groups to really understand what they may be seeing,” she added.
Dr. Bahado-Singh, Dr. Imam, Dr. Graham, and Dr. Snyder have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, raising the prospect of early intervention when effective treatments become available.
In a study, investigators used six AI methodologies, including Deep Learning, to assess blood leukocyte epigenomic biomarkers. They found more than 150 genetic differences among study participants with Alzheimer’s disease in comparison with participants who did not have Alzheimer’s disease.
All of the AI platforms were effective in predicting Alzheimer’s disease. Deep Learning’s assessment of intragenic cytosine-phosphate-guanines (CpGs) had sensitivity and specificity rates of 97%.
“It’s almost as if the leukocytes have become a newspaper to tell us, ‘This is what is going on in the brain,’ “ lead author Ray Bahado-Singh, MD, chair of the department of obstetrics and gynecology, Oakland University, Auburn Hills, Mich., said in a news release.
The researchers noted that the findings, if replicated in future studies, may help in providing Alzheimer’s disease diagnoses “much earlier” in the disease process. “The holy grail is to identify patients in the preclinical stage so effective early interventions, including new medications, can be studied and ultimately used,” Dr. Bahado-Singh said.
“This certainly isn’t the final step in Alzheimer’s research, but I think this represents a significant change in direction,” he told attendees at a press briefing.
The findings were published online March 31 in PLOS ONE.
Silver tsunami
The investigators noted that Alzheimer’s disease is often diagnosed when the disease is in its later stages, after irreversible brain damage has occurred. “There is currently no cure for the disease, and the treatment is limited to drugs that attempt to treat symptoms and have little effect on the disease’s progression,” they noted.
Coinvestigator Khaled Imam, MD, director of geriatric medicine for Beaumont Health in Michigan, pointed out that although MRI and lumbar puncture can identify Alzheimer’s disease early on, the processes are expensive and/or invasive.
“Having biomarkers in the blood ... and being able to identify [Alzheimer’s disease] years before symptoms start, hopefully we’d be able to intervene early on in the process of the disease,” Dr. Imam said.
It is estimated that the number of Americans aged 85 and older will triple by 2050. This impending “silver tsunami,” which will come with a commensurate increase in Alzheimer’s disease cases, makes it even more important to be able to diagnose the disease early on, he noted.
The study included 24 individuals with late-onset Alzheimer’s disease (70.8% women; mean age, 83 years); 24 were deemed to be “cognitively healthy” (66.7% women; mean age, 80 years). About 500 ng of genomic DNA was extracted from whole-blood samples from each participant.
The researchers used the Infinium MethylationEPIC BeadChip array, and the samples were then examined for markers of methylation that would “indicate the disease process has started,” they noted.
In addition to Deep Learning, the five other AI platforms were the Support Vector Machine, Generalized Linear Model, Prediction Analysis for Microarrays, Random Forest, and Linear Discriminant Analysis.
These platforms were used to assess leukocyte genome changes. To predict Alzheimer’s disease, the researchers also used Ingenuity Pathway Analysis.
Significant “chemical changes”
Results showed that the Alzheimer’s disease group had 152 significantly differentially methylated CpGs in 171 genes in comparison with the non-Alzheimer’s disease group (false discovery rate P value < .05).
As a whole, using intragenic and intergenic/extragenic CpGs, the AI platforms were effective in predicting who had Alzheimer’s disease (area under the curve [AUC], ≥ 0.93). Using intragenic markers, the AUC for Deep Learning was 0.99.
“We looked at close to a million different sites, and we saw some chemical changes that we know are associated with alteration or change in gene function,” Dr. Bahado-Singh said.
Altered genes that were found in the Alzheimer’s disease group included CR1L, CTSV, S1PR1, and LTB4R – all of which “have been previously linked with Alzheimer’s disease and dementia,” the researchers noted. They also found the methylated genes CTSV and PRMT5, both of which have been previously associated with cardiovascular disease.
“A significant strength of our study is the novelty, i.e. the use of blood leukocytes to accurately detect Alzheimer’s disease and also for interrogating the pathogenesis of Alzheimer’s disease,” the investigators wrote.
Dr. Bahado-Singh said that the test let them identify changes in cells in the blood, “giving us a comprehensive account not only of the fact that the brain is being affected by Alzheimer’s disease but it’s telling us what kinds of processes are going on in the brain.
“Normally you don’t have access to the brain. This gives us a simple blood test to get an ongoing reading of the course of events in the brain – and potentially tell us very early on before the onset of symptoms,” he added.
Cautiously optimistic
During the question-and-answer session following his presentation at the briefing, Dr. Bahado-Singh reiterated that they are at a very early stage in the research and were not able to make clinical recommendations at this point. However, he added, “There was evidence that DNA methylation change could likely precede the onset of abnormalities in the cells that give rise to the disease.”
Coinvestigator Stewart Graham, PhD, director of Alzheimer’s research at Beaumont Health, added that although the initial study findings led to some excitement for the team, “we have to be very conservative with what we say.”
He noted that the findings need to be replicated in a more diverse population. Still, “we’re excited at the moment and looking forward to seeing what the future results hold,” Dr. Graham said.
Dr. Bahado-Singh said that if larger studies confirm the findings and the test is viable, it would make sense to use it as a screen for individuals older than 65. He noted that because of the aging of the population, “this subset of individuals will constitute a larger and larger fraction of the population globally.”
Still early days
Commenting on the findings, Heather Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, noted that the investigators used an “interesting” diagnostic process.
“It was a unique approach to looking at and trying to understand what might be some of the biological underpinnings and using these tools and technologies to determine if they’re able to differentiate individuals with Alzheimer’s disease” from those without Alzheimer’s disease, said Dr. Snyder, who was not involved with the research.
“Ultimately, we want to know who is at greater risk, who may have some of the changing biology at the earliest time point so that we can intervene to stop the progression of the disease,” she said.
She pointed out that a number of types of biomarker tests are currently under investigation, many of which are measuring different outcomes. “And that’s what we want to see going forward. We want to have as many tools in our toolbox that allow us to accurately diagnose at that earliest time point,” Dr. Snyder said.
“At this point, [the current study] is still pretty early, so it needs to be replicated and then expanded to larger groups to really understand what they may be seeing,” she added.
Dr. Bahado-Singh, Dr. Imam, Dr. Graham, and Dr. Snyder have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PLOS ONE