User login
Pediatric NAFLD almost always stems from excess body weight, not other etiologies
Nonalcoholic fatty liver disease (NAFLD) in children is almost always caused by excess body weight, not other etiologies, based on a retrospective analysis of 900 patients.
Just 2% of children with overweight or obesity and suspected NAFLD had other causes of liver disease, and none tested positive for autoimmune hepatitis (AIH), reported lead author Toshifumi Yodoshi, MD, PhD, of Cincinnati Children’s Hospital Medical Center, and colleagues.
“Currently, recommended testing of patients with suspected NAFLD includes ruling out the following conditions: AIH, Wilson disease, hemochromatosis, alpha-1 antitrypsin [A1AT] deficiency, viral hepatitis, celiac disease, and thyroid dysfunction,” the investigators wrote in Pediatrics.
Yet evidence supporting this particular battery of tests is scant; just one previous pediatric study has estimated the prevalence of other liver diseases among children with suspected NAFLD. The study showed that the second-most common etiology, after NAFLD, was AIH, at a rate of 4%.
But “the generalizability of these findings is uncertain,” noted Dr. Yodoshi and colleagues, as the study was conducted at one tertiary center in the western United States, among a population that was predominantly Hispanic.
This uncertainty spurred the present study, which was conducted at two pediatric centers: Cincinnati Children’s Hospital Medical Center (2009-2017) and Yale New Haven (Conn.) Children’s Hospital (2012-2017).
The final analysis involved 900 patients aged 18 years or younger with suspected NAFLD based on hepatic steatosis detected via imaging and/or elevated serum aminotransferases. Demographically, a slight majority of the patients were boys (63%), and approximately one-quarter (26%) were Hispanic. Median BMI z score was 2.45, with three out of four patients (76%) exhibiting severe obesity. Out of 900 patients, 358 (40%) underwent liver biopsy, among whom 46% had confirmed nonalcoholic steatohepatitis.
All patients underwent testing to exclude the aforementioned conditions using various diagnostics, revealing that just 2% of the population had etiologies other than NAFLD. Specifically, 11 children had thyroid dysfunction (1.2%), 3 had celiac disease (0.4%), 3 had A1AT deficiency (0.4%), 1 had hemophagocytic lymphohistiocytosis, and 1 had Hodgkin’s lymphoma. None of the children had Wilson disease, hepatitis B or C, or AIH.
Dr. Yodoshi and colleagues highlighted the latter finding, noting that 13% of the patients had autoantibodies for AIH, but “none met composite criteria.” This contrasts with the previous study from 2013, which found an AIH rate of 4%.
“Nonetheless,” the investigators went on, “NAFLD remains a diagnosis of exclusion, and key conditions that require specific treatments must be ruled out in the workup of patients with suspected NAFLD. In the future, the cost-effectiveness of this approach will need to be investigated.”
Interpreting the findings, Francis E. Rushton, MD, of Beaufort (S.C.) Memorial Hospital emphasized the implications for preventive and interventional health care.
“This study showing an absence of etiologies other than obesity in overweight children with NAFLD provides further impetus for pediatricians to work on both preventive and treatment regimens for weight issues,” Dr. Rushton said. “Linking community-based initiatives focused on adequate nutritional support with pediatric clinical support services is critical in solving issues related to overweight in children. Tracking BMI over time and developing healthy habit goals for patients are key parts of clinical interventions.”
The study was funded by the National Institutes of Health. The investigators reported no conflicts of interest.
Nonalcoholic fatty liver disease (NAFLD) in children is almost always caused by excess body weight, not other etiologies, based on a retrospective analysis of 900 patients.
Just 2% of children with overweight or obesity and suspected NAFLD had other causes of liver disease, and none tested positive for autoimmune hepatitis (AIH), reported lead author Toshifumi Yodoshi, MD, PhD, of Cincinnati Children’s Hospital Medical Center, and colleagues.
“Currently, recommended testing of patients with suspected NAFLD includes ruling out the following conditions: AIH, Wilson disease, hemochromatosis, alpha-1 antitrypsin [A1AT] deficiency, viral hepatitis, celiac disease, and thyroid dysfunction,” the investigators wrote in Pediatrics.
Yet evidence supporting this particular battery of tests is scant; just one previous pediatric study has estimated the prevalence of other liver diseases among children with suspected NAFLD. The study showed that the second-most common etiology, after NAFLD, was AIH, at a rate of 4%.
But “the generalizability of these findings is uncertain,” noted Dr. Yodoshi and colleagues, as the study was conducted at one tertiary center in the western United States, among a population that was predominantly Hispanic.
This uncertainty spurred the present study, which was conducted at two pediatric centers: Cincinnati Children’s Hospital Medical Center (2009-2017) and Yale New Haven (Conn.) Children’s Hospital (2012-2017).
The final analysis involved 900 patients aged 18 years or younger with suspected NAFLD based on hepatic steatosis detected via imaging and/or elevated serum aminotransferases. Demographically, a slight majority of the patients were boys (63%), and approximately one-quarter (26%) were Hispanic. Median BMI z score was 2.45, with three out of four patients (76%) exhibiting severe obesity. Out of 900 patients, 358 (40%) underwent liver biopsy, among whom 46% had confirmed nonalcoholic steatohepatitis.
All patients underwent testing to exclude the aforementioned conditions using various diagnostics, revealing that just 2% of the population had etiologies other than NAFLD. Specifically, 11 children had thyroid dysfunction (1.2%), 3 had celiac disease (0.4%), 3 had A1AT deficiency (0.4%), 1 had hemophagocytic lymphohistiocytosis, and 1 had Hodgkin’s lymphoma. None of the children had Wilson disease, hepatitis B or C, or AIH.
Dr. Yodoshi and colleagues highlighted the latter finding, noting that 13% of the patients had autoantibodies for AIH, but “none met composite criteria.” This contrasts with the previous study from 2013, which found an AIH rate of 4%.
“Nonetheless,” the investigators went on, “NAFLD remains a diagnosis of exclusion, and key conditions that require specific treatments must be ruled out in the workup of patients with suspected NAFLD. In the future, the cost-effectiveness of this approach will need to be investigated.”
Interpreting the findings, Francis E. Rushton, MD, of Beaufort (S.C.) Memorial Hospital emphasized the implications for preventive and interventional health care.
“This study showing an absence of etiologies other than obesity in overweight children with NAFLD provides further impetus for pediatricians to work on both preventive and treatment regimens for weight issues,” Dr. Rushton said. “Linking community-based initiatives focused on adequate nutritional support with pediatric clinical support services is critical in solving issues related to overweight in children. Tracking BMI over time and developing healthy habit goals for patients are key parts of clinical interventions.”
The study was funded by the National Institutes of Health. The investigators reported no conflicts of interest.
Nonalcoholic fatty liver disease (NAFLD) in children is almost always caused by excess body weight, not other etiologies, based on a retrospective analysis of 900 patients.
Just 2% of children with overweight or obesity and suspected NAFLD had other causes of liver disease, and none tested positive for autoimmune hepatitis (AIH), reported lead author Toshifumi Yodoshi, MD, PhD, of Cincinnati Children’s Hospital Medical Center, and colleagues.
“Currently, recommended testing of patients with suspected NAFLD includes ruling out the following conditions: AIH, Wilson disease, hemochromatosis, alpha-1 antitrypsin [A1AT] deficiency, viral hepatitis, celiac disease, and thyroid dysfunction,” the investigators wrote in Pediatrics.
Yet evidence supporting this particular battery of tests is scant; just one previous pediatric study has estimated the prevalence of other liver diseases among children with suspected NAFLD. The study showed that the second-most common etiology, after NAFLD, was AIH, at a rate of 4%.
But “the generalizability of these findings is uncertain,” noted Dr. Yodoshi and colleagues, as the study was conducted at one tertiary center in the western United States, among a population that was predominantly Hispanic.
This uncertainty spurred the present study, which was conducted at two pediatric centers: Cincinnati Children’s Hospital Medical Center (2009-2017) and Yale New Haven (Conn.) Children’s Hospital (2012-2017).
The final analysis involved 900 patients aged 18 years or younger with suspected NAFLD based on hepatic steatosis detected via imaging and/or elevated serum aminotransferases. Demographically, a slight majority of the patients were boys (63%), and approximately one-quarter (26%) were Hispanic. Median BMI z score was 2.45, with three out of four patients (76%) exhibiting severe obesity. Out of 900 patients, 358 (40%) underwent liver biopsy, among whom 46% had confirmed nonalcoholic steatohepatitis.
All patients underwent testing to exclude the aforementioned conditions using various diagnostics, revealing that just 2% of the population had etiologies other than NAFLD. Specifically, 11 children had thyroid dysfunction (1.2%), 3 had celiac disease (0.4%), 3 had A1AT deficiency (0.4%), 1 had hemophagocytic lymphohistiocytosis, and 1 had Hodgkin’s lymphoma. None of the children had Wilson disease, hepatitis B or C, or AIH.
Dr. Yodoshi and colleagues highlighted the latter finding, noting that 13% of the patients had autoantibodies for AIH, but “none met composite criteria.” This contrasts with the previous study from 2013, which found an AIH rate of 4%.
“Nonetheless,” the investigators went on, “NAFLD remains a diagnosis of exclusion, and key conditions that require specific treatments must be ruled out in the workup of patients with suspected NAFLD. In the future, the cost-effectiveness of this approach will need to be investigated.”
Interpreting the findings, Francis E. Rushton, MD, of Beaufort (S.C.) Memorial Hospital emphasized the implications for preventive and interventional health care.
“This study showing an absence of etiologies other than obesity in overweight children with NAFLD provides further impetus for pediatricians to work on both preventive and treatment regimens for weight issues,” Dr. Rushton said. “Linking community-based initiatives focused on adequate nutritional support with pediatric clinical support services is critical in solving issues related to overweight in children. Tracking BMI over time and developing healthy habit goals for patients are key parts of clinical interventions.”
The study was funded by the National Institutes of Health. The investigators reported no conflicts of interest.
FROM PEDIATRICS
Real-world outcomes of caplacizumab for iTTP comparable to clinical trial results
Real-world data for caplacizumab outcomes matched those seen in randomized controlled trials (RCTs) for the treatment of immune-mediated thrombotic thrombocytopenic purpura (iTTP), according to the results of a retrospective study.
Data collected from 2018 to 2020 were assessed for 85 patients (4 of them children) receiving caplacizumab at 22 United Kingdom hospitals, according to a report published online in Blood.
Researchers Tina Dutt, PhD, from the Liverpool (England) University Hospitals NHS Foundation Trust, and her colleagues compared patient characteristics and outcomes in these real-world clinical settings to those of caplacizumab trial endpoint results and to historical outcomes in the precaplacizumab era.
Acquired thrombotic thrombocytopenic purpura is an immune-mediated deficiency of the von Willebrand factor–cleaving protease (ADAMTS13), which allows unrestrained adhesion of von Willebrand factor multimers to platelets, leading to thrombocytopenia, hemolytic anemia, and tissue ischemia.
Standard management of iTTP has focused on the replacement of ADAMTS13 and the removal of autoantibodies using plasma exchange and immunosuppression, an approach which has reduced the mortality of acute TTP from greater than 90% to between 10% and 20%, according to the report.
Caplacizumab is a novel anti–von Willebrand factor immunoglobulin fragment that inhibits this interaction between von Willebrand factor multimers and platelets and is now added to the standard treatment regimen. The drug has been assessed in two pivotal multicenter RCTs that led to European Union and U.S. Food and Drug Administration approval.
Benefits and risk
Eighty-four of 85 patients received steroid and rituximab as well as plasma exchange along with caplacizumab treatment. All patients had ADAMTS13 activity at presentation less than 20 IU/dL, with 99% of patients (84/85) having ADAMTS13 activity less than 10 IU/dL, confirming a clinical diagnosis of acute TTP, according to the researchers.
The median time to platelet count normalization (3 days), the median duration of plasma exchange (7 days), and the median hospital stay (12 days) were all comparable with the RCT data, according to the researchers. In addition, the median duration of plasma exchange and time from beginning plasma exchange to platelet count normalization were favorable, compared with historical outcomes (P < .05).
TTP recurred in 5 of the 85 patients, all of whom had persistent ADAMTS13 activity less than 5 IU/dL.
There were 31 adverse events reported in 26 patients, 17 of these (55%) were bleeding episodes, and 5 of 31 (16%) were thrombotic events (2 unrelated to caplacizumab). The overall mortality was 6% (five patients), with no deaths attributed to caplacizumab. In four of the five deaths, caplacizumab was introduced more than 48 hours after plasma exchange initiation (range 3-21 days).
“This real-world evidence from the largest series of TTP patients receiving caplacizumab, outside of the pivotal studies, provides confirmation of the therapeutic benefits of caplacizumab and its inherent bleeding risk,” the researchers concluded.
Dr. Dutt and several of her colleagues reported receiving honoraria from Sanofi for serving on advisory boards, as well as speaker fees from Sanofi and Alexion.
Real-world data for caplacizumab outcomes matched those seen in randomized controlled trials (RCTs) for the treatment of immune-mediated thrombotic thrombocytopenic purpura (iTTP), according to the results of a retrospective study.
Data collected from 2018 to 2020 were assessed for 85 patients (4 of them children) receiving caplacizumab at 22 United Kingdom hospitals, according to a report published online in Blood.
Researchers Tina Dutt, PhD, from the Liverpool (England) University Hospitals NHS Foundation Trust, and her colleagues compared patient characteristics and outcomes in these real-world clinical settings to those of caplacizumab trial endpoint results and to historical outcomes in the precaplacizumab era.
Acquired thrombotic thrombocytopenic purpura is an immune-mediated deficiency of the von Willebrand factor–cleaving protease (ADAMTS13), which allows unrestrained adhesion of von Willebrand factor multimers to platelets, leading to thrombocytopenia, hemolytic anemia, and tissue ischemia.
Standard management of iTTP has focused on the replacement of ADAMTS13 and the removal of autoantibodies using plasma exchange and immunosuppression, an approach which has reduced the mortality of acute TTP from greater than 90% to between 10% and 20%, according to the report.
Caplacizumab is a novel anti–von Willebrand factor immunoglobulin fragment that inhibits this interaction between von Willebrand factor multimers and platelets and is now added to the standard treatment regimen. The drug has been assessed in two pivotal multicenter RCTs that led to European Union and U.S. Food and Drug Administration approval.
Benefits and risk
Eighty-four of 85 patients received steroid and rituximab as well as plasma exchange along with caplacizumab treatment. All patients had ADAMTS13 activity at presentation less than 20 IU/dL, with 99% of patients (84/85) having ADAMTS13 activity less than 10 IU/dL, confirming a clinical diagnosis of acute TTP, according to the researchers.
The median time to platelet count normalization (3 days), the median duration of plasma exchange (7 days), and the median hospital stay (12 days) were all comparable with the RCT data, according to the researchers. In addition, the median duration of plasma exchange and time from beginning plasma exchange to platelet count normalization were favorable, compared with historical outcomes (P < .05).
TTP recurred in 5 of the 85 patients, all of whom had persistent ADAMTS13 activity less than 5 IU/dL.
There were 31 adverse events reported in 26 patients, 17 of these (55%) were bleeding episodes, and 5 of 31 (16%) were thrombotic events (2 unrelated to caplacizumab). The overall mortality was 6% (five patients), with no deaths attributed to caplacizumab. In four of the five deaths, caplacizumab was introduced more than 48 hours after plasma exchange initiation (range 3-21 days).
“This real-world evidence from the largest series of TTP patients receiving caplacizumab, outside of the pivotal studies, provides confirmation of the therapeutic benefits of caplacizumab and its inherent bleeding risk,” the researchers concluded.
Dr. Dutt and several of her colleagues reported receiving honoraria from Sanofi for serving on advisory boards, as well as speaker fees from Sanofi and Alexion.
Real-world data for caplacizumab outcomes matched those seen in randomized controlled trials (RCTs) for the treatment of immune-mediated thrombotic thrombocytopenic purpura (iTTP), according to the results of a retrospective study.
Data collected from 2018 to 2020 were assessed for 85 patients (4 of them children) receiving caplacizumab at 22 United Kingdom hospitals, according to a report published online in Blood.
Researchers Tina Dutt, PhD, from the Liverpool (England) University Hospitals NHS Foundation Trust, and her colleagues compared patient characteristics and outcomes in these real-world clinical settings to those of caplacizumab trial endpoint results and to historical outcomes in the precaplacizumab era.
Acquired thrombotic thrombocytopenic purpura is an immune-mediated deficiency of the von Willebrand factor–cleaving protease (ADAMTS13), which allows unrestrained adhesion of von Willebrand factor multimers to platelets, leading to thrombocytopenia, hemolytic anemia, and tissue ischemia.
Standard management of iTTP has focused on the replacement of ADAMTS13 and the removal of autoantibodies using plasma exchange and immunosuppression, an approach which has reduced the mortality of acute TTP from greater than 90% to between 10% and 20%, according to the report.
Caplacizumab is a novel anti–von Willebrand factor immunoglobulin fragment that inhibits this interaction between von Willebrand factor multimers and platelets and is now added to the standard treatment regimen. The drug has been assessed in two pivotal multicenter RCTs that led to European Union and U.S. Food and Drug Administration approval.
Benefits and risk
Eighty-four of 85 patients received steroid and rituximab as well as plasma exchange along with caplacizumab treatment. All patients had ADAMTS13 activity at presentation less than 20 IU/dL, with 99% of patients (84/85) having ADAMTS13 activity less than 10 IU/dL, confirming a clinical diagnosis of acute TTP, according to the researchers.
The median time to platelet count normalization (3 days), the median duration of plasma exchange (7 days), and the median hospital stay (12 days) were all comparable with the RCT data, according to the researchers. In addition, the median duration of plasma exchange and time from beginning plasma exchange to platelet count normalization were favorable, compared with historical outcomes (P < .05).
TTP recurred in 5 of the 85 patients, all of whom had persistent ADAMTS13 activity less than 5 IU/dL.
There were 31 adverse events reported in 26 patients, 17 of these (55%) were bleeding episodes, and 5 of 31 (16%) were thrombotic events (2 unrelated to caplacizumab). The overall mortality was 6% (five patients), with no deaths attributed to caplacizumab. In four of the five deaths, caplacizumab was introduced more than 48 hours after plasma exchange initiation (range 3-21 days).
“This real-world evidence from the largest series of TTP patients receiving caplacizumab, outside of the pivotal studies, provides confirmation of the therapeutic benefits of caplacizumab and its inherent bleeding risk,” the researchers concluded.
Dr. Dutt and several of her colleagues reported receiving honoraria from Sanofi for serving on advisory boards, as well as speaker fees from Sanofi and Alexion.
FROM BLOOD
Is ketamine effective and safe for treatment-resistant depression?
Evidence Summary
Single-dose IV ketamine elicits a short-term response
A meta-analysis of RCTs evaluating a single dose of IV ketamine vs placebo for severe depression found that it increased the chance of a treatment response for up to 1 week afterward. Studies included patients with severe (N = 30), treatment-resistant (N = 40), and psychotic depression (N = 10), based on Diagnostic and Statistical Manual of Mental Disorders–Fifth Edition criteria.1
The primary outcome was treatment response: either an improvement of > 50% on a standardized depression scale or a Clinical Global Impression–Improvement scale score of 1 or 2 (“very much” and “much” improved, respectively, as assessed by a clinician). Ketamine increased the likelihood of short-term response or improvement at 24 hours (3 RCTs; N = 56; odds ratio [OR] = 11; 95% CI, 2-58); at 72 hours (3 RCTs; N = 56; OR = 13; 95% CI, 2-66); and at 7 days (4 RCTs; N = 88; OR = 2.6; 95% CI, 1.1-6.2).1 Response rates equaled placebo at 2 weeks. The authors rated the RCTs as low quality.
Another systematic review of single-dose IV ketamine vs placebo for major depression and bipolar disorder included 3 additional small, low-quality RCTs, 2 of which showed short-term response to ketamine. The authors used Hedge’s g statistic to standardize effect size (a score of magnitude 0.2 indicates a small effect; 0.6, moderate; 1.2, large; and 2, very large). One RCT (n = 26) found a very large 1-day response (effect size: –2; 95% CI, –2.8 to –1.3), and 2 RCTs found conflicting responses at 12 days (RCT with N = 18: effect size: –0.2; 95% CI, –0.4 to 0.02 [no significant response] vs RCT with N = 8: effect size: –1.5; 95% CI, –2.5 to –0.5).2
More frequent dosing of IV ketamine improves symptoms
An RCT (N = 67) evaluating twice- or thrice-weekly IV ketamine vs placebo in patients with recurrent depression (with at least 1 treatment failure) found that ketamine significantly improved standardized depression scores and response rates at 15 days. Patients with clinically significant suicidality were excluded.3
Researchers randomized patients to IV ketamine (0.05 mg/kg) twice or thrice weekly or to saline control and used the 60-point Montgomery-Asberg Depression Rating Scale (MADRS). A response was defined as a reduction of the MADRS score by 50%.
Both ketamine arms produced greater symptom improvement at 15 days, compared to placebo (twice weekly: −18.4 vs −5.7; P < 0.001; thrice weekly: −17.7 vs −3.1; P < 0.001) in addition to higher response rates (twice weekly: 69% vs 15%; P = .005; number needed to treat [NNT] = 2; and thrice-weekly: 54% vs 6%; P = .004; NNT = 2).3 There was no significant difference between twice- or thrice-weekly dosing. The study was flawed by dropouts (N = 57 at 15 days and N = 25 at 28 days), primarily attributed to ketamine adverse effects, that prevented assessment beyond 2 weeks.
Oral ketamine has a moderate effecton depression
A systematic review included 2 low-quality RCTs evaluating oral ketamine vs placebo as adjunctive treatment with sertraline, and oral ketamine vs diclofenac, and found improvement in patients with moderate depression.4 In the first RCT (n = 45), researchers found that oral ketamine (25 mg bid) plus sertraline (25 mg titrated up to 150 mg/d) produced more treatment responses (> 50% reduction on a standardized depression rating scale) than placebo plus sertraline (2 weeks: 85.4% vs 42.5%; P < .001; 6 weeks: 85.4% vs 57.5%; P = .005).4
In the second RCT (n = 23), researchers randomized patients with mild-to-moderate depression and comorbid chronic headaches to take oral ketamine (50 mg tid) or oral diclofenac (50 mg tid) and measured effect size on standardized depression scores at 3 weeks (no difference) and 6 weeks (Cohen d effect size = 0.79 [rated as a moderate effect]; P = .017).4
Nasal esketamine + oral antidepressants boosts treatment response rates
A meta-analysis with 4 RCTs (N = 708) evaluating intranasal esketamine vs placebo as an adjunct to oral antidepressants for patients with predominantly treatment-resistant major depression found that it boosted response rates by about 40%. Researchers randomized patients to intranasal esketamine (mostly 28-84 mg twice weekly for 28 days) or placebo spray as an adjunct to oral antidepressants (duloxetine, escitalopram, sertraline, venlafaxine).
The primary outcomes were treatment response (≥ 50% reduction in depression scores) or remission (a MADRS score < 12). Adjunctive intranasal esketamine produced greater rates of treatment response compared to placebo at 24 hours (21% vs 7%; relative risk [RR] = 8.4; 95% CI, 1.4 to 21.2; P < .02; NNT = 7) and at 28 days (59% vs 43%; RR = 1.4; 95% CI, 1.2 to 1.60; P < .0001; NNT = 6).5 Adjunctive intranasal esketamine also produced greater rates of remission at the end of the study (mostly at 28 days), compared with placebo (41% vs 25%; RR = 1.4; 95% CI, 1.2 to 1.7; P = .0004; NNT = 7).5 The authors rated study quality as moderate to high.
Adverse effects are common, may cause Tx discontinuation
Ketamine-produced adverse effects (AEs) included confusion (2 trials; N = 76; OR = 3.7; 95% CI, 1.1-12) and emotional blunting (1 trial; N = 30; OR = 23; 95% CI, 1.1-489).1
A 2018 systematic review assessed the safety of ketamine in depression after single and repeated dose in 60 studies (N = 899; 20 RCTs, 17 open-label-trials, 20 case series, and 3 retrospective studies). The most common AEs reported were headache (35% of studies), dizziness (33%), dissociation (28%), elevated blood pressure (28%), and blurred vision (23%), with the majority reported to resolve shortly after administration. The most common psychiatric AE was anxiety (15%).6 Included studies varied greatly in design and dosage form, and no meta-analysis could be performed.
Nasal esketamine produced more AEs causing discontinuation than did placebo (5.8% vs 1.5%; RR = 3.5; 95% CI, 1.34-8.9; number needed to harm [NNH] = 23), including blurred vision, dizziness, sedation, nausea, and dysphoria.5A review (5 RCTs and 1 open-label trial; N = 1708) analyzing the cardiac safety profile of intranasal esketamine adjuvant therapy found that it produced transient and asymptomatic blood pressure elevations (OR = 3.2; 95% CI, 1.9-5.8; NNH = 13).7
Recommendations from others
A clinical practice guideline from the US Veterans Administration lists IV ketamine as 1 of the therapeutic options for patients with treatment-resistant depression and suicidal ideation.8 However, a Department of Veterans Affairs Panel restricted its use to a pre-approved case-by-case basis.8
Editor’s takeaway
Physicians with patients facing the all-too-common problem of treatment-resistant major depression will be wondering if ketamine, either by itself or as an augmentation therapy, can help. Unfortunately, the outcomes we report here are too short term to answer that question, and we must await the results of further studies. Augmentation with intranasal esketamine, at a cost of $370/month, might offer some promise.
1. Caddy C, Amit BH, McCloud TL, et al. Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev. 2015;(9):CD011612.
2. Coyle CM, Laws KR. The use of ketamine as an antidepressant: a systematic review and meta-analysis. Hum Psychopharmacol. 2015;30:152‐163.
3. Singh JB, Fedgchin M, Daly EJ, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173:816‐826.
4. Rosenblat JD, Carvalho AF, Li M, et al. Oral ketamine for depression: a systematic review. J Clin Psychiatry. 2019;80:18r12475.
5. Zheng W, Cai DB, Xiang YQ, et al. Adjunctive intranasal esketamine for major depressive disorder: a systematic review of randomized double-blind controlled-placebo studies. J Affect Disord. 2020;265:63‐70.
6. Short B, Fong J, Galvez V, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5:65‐78.
7. Doherty T, Wajs E, Melkote R, et al. Cardiac safety of esketamine nasal spray in treatment-resistant depression: results from the Clinical Development Program. CNS Drugs. 2020;34:299‐310.
8. Sall J, Brenner L, Millikan Bell AM, et al. Assessment and management of patients at risk for suicide: synopsis of the 2019 US Department of Veterans Affairs and US Department of Defense Clinical Practice Guidelines. Ann Intern Med. 2019;171:343-353.
Evidence Summary
Single-dose IV ketamine elicits a short-term response
A meta-analysis of RCTs evaluating a single dose of IV ketamine vs placebo for severe depression found that it increased the chance of a treatment response for up to 1 week afterward. Studies included patients with severe (N = 30), treatment-resistant (N = 40), and psychotic depression (N = 10), based on Diagnostic and Statistical Manual of Mental Disorders–Fifth Edition criteria.1
The primary outcome was treatment response: either an improvement of > 50% on a standardized depression scale or a Clinical Global Impression–Improvement scale score of 1 or 2 (“very much” and “much” improved, respectively, as assessed by a clinician). Ketamine increased the likelihood of short-term response or improvement at 24 hours (3 RCTs; N = 56; odds ratio [OR] = 11; 95% CI, 2-58); at 72 hours (3 RCTs; N = 56; OR = 13; 95% CI, 2-66); and at 7 days (4 RCTs; N = 88; OR = 2.6; 95% CI, 1.1-6.2).1 Response rates equaled placebo at 2 weeks. The authors rated the RCTs as low quality.
Another systematic review of single-dose IV ketamine vs placebo for major depression and bipolar disorder included 3 additional small, low-quality RCTs, 2 of which showed short-term response to ketamine. The authors used Hedge’s g statistic to standardize effect size (a score of magnitude 0.2 indicates a small effect; 0.6, moderate; 1.2, large; and 2, very large). One RCT (n = 26) found a very large 1-day response (effect size: –2; 95% CI, –2.8 to –1.3), and 2 RCTs found conflicting responses at 12 days (RCT with N = 18: effect size: –0.2; 95% CI, –0.4 to 0.02 [no significant response] vs RCT with N = 8: effect size: –1.5; 95% CI, –2.5 to –0.5).2
More frequent dosing of IV ketamine improves symptoms
An RCT (N = 67) evaluating twice- or thrice-weekly IV ketamine vs placebo in patients with recurrent depression (with at least 1 treatment failure) found that ketamine significantly improved standardized depression scores and response rates at 15 days. Patients with clinically significant suicidality were excluded.3
Researchers randomized patients to IV ketamine (0.05 mg/kg) twice or thrice weekly or to saline control and used the 60-point Montgomery-Asberg Depression Rating Scale (MADRS). A response was defined as a reduction of the MADRS score by 50%.
Both ketamine arms produced greater symptom improvement at 15 days, compared to placebo (twice weekly: −18.4 vs −5.7; P < 0.001; thrice weekly: −17.7 vs −3.1; P < 0.001) in addition to higher response rates (twice weekly: 69% vs 15%; P = .005; number needed to treat [NNT] = 2; and thrice-weekly: 54% vs 6%; P = .004; NNT = 2).3 There was no significant difference between twice- or thrice-weekly dosing. The study was flawed by dropouts (N = 57 at 15 days and N = 25 at 28 days), primarily attributed to ketamine adverse effects, that prevented assessment beyond 2 weeks.
Oral ketamine has a moderate effecton depression
A systematic review included 2 low-quality RCTs evaluating oral ketamine vs placebo as adjunctive treatment with sertraline, and oral ketamine vs diclofenac, and found improvement in patients with moderate depression.4 In the first RCT (n = 45), researchers found that oral ketamine (25 mg bid) plus sertraline (25 mg titrated up to 150 mg/d) produced more treatment responses (> 50% reduction on a standardized depression rating scale) than placebo plus sertraline (2 weeks: 85.4% vs 42.5%; P < .001; 6 weeks: 85.4% vs 57.5%; P = .005).4
In the second RCT (n = 23), researchers randomized patients with mild-to-moderate depression and comorbid chronic headaches to take oral ketamine (50 mg tid) or oral diclofenac (50 mg tid) and measured effect size on standardized depression scores at 3 weeks (no difference) and 6 weeks (Cohen d effect size = 0.79 [rated as a moderate effect]; P = .017).4
Nasal esketamine + oral antidepressants boosts treatment response rates
A meta-analysis with 4 RCTs (N = 708) evaluating intranasal esketamine vs placebo as an adjunct to oral antidepressants for patients with predominantly treatment-resistant major depression found that it boosted response rates by about 40%. Researchers randomized patients to intranasal esketamine (mostly 28-84 mg twice weekly for 28 days) or placebo spray as an adjunct to oral antidepressants (duloxetine, escitalopram, sertraline, venlafaxine).
The primary outcomes were treatment response (≥ 50% reduction in depression scores) or remission (a MADRS score < 12). Adjunctive intranasal esketamine produced greater rates of treatment response compared to placebo at 24 hours (21% vs 7%; relative risk [RR] = 8.4; 95% CI, 1.4 to 21.2; P < .02; NNT = 7) and at 28 days (59% vs 43%; RR = 1.4; 95% CI, 1.2 to 1.60; P < .0001; NNT = 6).5 Adjunctive intranasal esketamine also produced greater rates of remission at the end of the study (mostly at 28 days), compared with placebo (41% vs 25%; RR = 1.4; 95% CI, 1.2 to 1.7; P = .0004; NNT = 7).5 The authors rated study quality as moderate to high.
Adverse effects are common, may cause Tx discontinuation
Ketamine-produced adverse effects (AEs) included confusion (2 trials; N = 76; OR = 3.7; 95% CI, 1.1-12) and emotional blunting (1 trial; N = 30; OR = 23; 95% CI, 1.1-489).1
A 2018 systematic review assessed the safety of ketamine in depression after single and repeated dose in 60 studies (N = 899; 20 RCTs, 17 open-label-trials, 20 case series, and 3 retrospective studies). The most common AEs reported were headache (35% of studies), dizziness (33%), dissociation (28%), elevated blood pressure (28%), and blurred vision (23%), with the majority reported to resolve shortly after administration. The most common psychiatric AE was anxiety (15%).6 Included studies varied greatly in design and dosage form, and no meta-analysis could be performed.
Nasal esketamine produced more AEs causing discontinuation than did placebo (5.8% vs 1.5%; RR = 3.5; 95% CI, 1.34-8.9; number needed to harm [NNH] = 23), including blurred vision, dizziness, sedation, nausea, and dysphoria.5A review (5 RCTs and 1 open-label trial; N = 1708) analyzing the cardiac safety profile of intranasal esketamine adjuvant therapy found that it produced transient and asymptomatic blood pressure elevations (OR = 3.2; 95% CI, 1.9-5.8; NNH = 13).7
Recommendations from others
A clinical practice guideline from the US Veterans Administration lists IV ketamine as 1 of the therapeutic options for patients with treatment-resistant depression and suicidal ideation.8 However, a Department of Veterans Affairs Panel restricted its use to a pre-approved case-by-case basis.8
Editor’s takeaway
Physicians with patients facing the all-too-common problem of treatment-resistant major depression will be wondering if ketamine, either by itself or as an augmentation therapy, can help. Unfortunately, the outcomes we report here are too short term to answer that question, and we must await the results of further studies. Augmentation with intranasal esketamine, at a cost of $370/month, might offer some promise.
Evidence Summary
Single-dose IV ketamine elicits a short-term response
A meta-analysis of RCTs evaluating a single dose of IV ketamine vs placebo for severe depression found that it increased the chance of a treatment response for up to 1 week afterward. Studies included patients with severe (N = 30), treatment-resistant (N = 40), and psychotic depression (N = 10), based on Diagnostic and Statistical Manual of Mental Disorders–Fifth Edition criteria.1
The primary outcome was treatment response: either an improvement of > 50% on a standardized depression scale or a Clinical Global Impression–Improvement scale score of 1 or 2 (“very much” and “much” improved, respectively, as assessed by a clinician). Ketamine increased the likelihood of short-term response or improvement at 24 hours (3 RCTs; N = 56; odds ratio [OR] = 11; 95% CI, 2-58); at 72 hours (3 RCTs; N = 56; OR = 13; 95% CI, 2-66); and at 7 days (4 RCTs; N = 88; OR = 2.6; 95% CI, 1.1-6.2).1 Response rates equaled placebo at 2 weeks. The authors rated the RCTs as low quality.
Another systematic review of single-dose IV ketamine vs placebo for major depression and bipolar disorder included 3 additional small, low-quality RCTs, 2 of which showed short-term response to ketamine. The authors used Hedge’s g statistic to standardize effect size (a score of magnitude 0.2 indicates a small effect; 0.6, moderate; 1.2, large; and 2, very large). One RCT (n = 26) found a very large 1-day response (effect size: –2; 95% CI, –2.8 to –1.3), and 2 RCTs found conflicting responses at 12 days (RCT with N = 18: effect size: –0.2; 95% CI, –0.4 to 0.02 [no significant response] vs RCT with N = 8: effect size: –1.5; 95% CI, –2.5 to –0.5).2
More frequent dosing of IV ketamine improves symptoms
An RCT (N = 67) evaluating twice- or thrice-weekly IV ketamine vs placebo in patients with recurrent depression (with at least 1 treatment failure) found that ketamine significantly improved standardized depression scores and response rates at 15 days. Patients with clinically significant suicidality were excluded.3
Researchers randomized patients to IV ketamine (0.05 mg/kg) twice or thrice weekly or to saline control and used the 60-point Montgomery-Asberg Depression Rating Scale (MADRS). A response was defined as a reduction of the MADRS score by 50%.
Both ketamine arms produced greater symptom improvement at 15 days, compared to placebo (twice weekly: −18.4 vs −5.7; P < 0.001; thrice weekly: −17.7 vs −3.1; P < 0.001) in addition to higher response rates (twice weekly: 69% vs 15%; P = .005; number needed to treat [NNT] = 2; and thrice-weekly: 54% vs 6%; P = .004; NNT = 2).3 There was no significant difference between twice- or thrice-weekly dosing. The study was flawed by dropouts (N = 57 at 15 days and N = 25 at 28 days), primarily attributed to ketamine adverse effects, that prevented assessment beyond 2 weeks.
Oral ketamine has a moderate effecton depression
A systematic review included 2 low-quality RCTs evaluating oral ketamine vs placebo as adjunctive treatment with sertraline, and oral ketamine vs diclofenac, and found improvement in patients with moderate depression.4 In the first RCT (n = 45), researchers found that oral ketamine (25 mg bid) plus sertraline (25 mg titrated up to 150 mg/d) produced more treatment responses (> 50% reduction on a standardized depression rating scale) than placebo plus sertraline (2 weeks: 85.4% vs 42.5%; P < .001; 6 weeks: 85.4% vs 57.5%; P = .005).4
In the second RCT (n = 23), researchers randomized patients with mild-to-moderate depression and comorbid chronic headaches to take oral ketamine (50 mg tid) or oral diclofenac (50 mg tid) and measured effect size on standardized depression scores at 3 weeks (no difference) and 6 weeks (Cohen d effect size = 0.79 [rated as a moderate effect]; P = .017).4
Nasal esketamine + oral antidepressants boosts treatment response rates
A meta-analysis with 4 RCTs (N = 708) evaluating intranasal esketamine vs placebo as an adjunct to oral antidepressants for patients with predominantly treatment-resistant major depression found that it boosted response rates by about 40%. Researchers randomized patients to intranasal esketamine (mostly 28-84 mg twice weekly for 28 days) or placebo spray as an adjunct to oral antidepressants (duloxetine, escitalopram, sertraline, venlafaxine).
The primary outcomes were treatment response (≥ 50% reduction in depression scores) or remission (a MADRS score < 12). Adjunctive intranasal esketamine produced greater rates of treatment response compared to placebo at 24 hours (21% vs 7%; relative risk [RR] = 8.4; 95% CI, 1.4 to 21.2; P < .02; NNT = 7) and at 28 days (59% vs 43%; RR = 1.4; 95% CI, 1.2 to 1.60; P < .0001; NNT = 6).5 Adjunctive intranasal esketamine also produced greater rates of remission at the end of the study (mostly at 28 days), compared with placebo (41% vs 25%; RR = 1.4; 95% CI, 1.2 to 1.7; P = .0004; NNT = 7).5 The authors rated study quality as moderate to high.
Adverse effects are common, may cause Tx discontinuation
Ketamine-produced adverse effects (AEs) included confusion (2 trials; N = 76; OR = 3.7; 95% CI, 1.1-12) and emotional blunting (1 trial; N = 30; OR = 23; 95% CI, 1.1-489).1
A 2018 systematic review assessed the safety of ketamine in depression after single and repeated dose in 60 studies (N = 899; 20 RCTs, 17 open-label-trials, 20 case series, and 3 retrospective studies). The most common AEs reported were headache (35% of studies), dizziness (33%), dissociation (28%), elevated blood pressure (28%), and blurred vision (23%), with the majority reported to resolve shortly after administration. The most common psychiatric AE was anxiety (15%).6 Included studies varied greatly in design and dosage form, and no meta-analysis could be performed.
Nasal esketamine produced more AEs causing discontinuation than did placebo (5.8% vs 1.5%; RR = 3.5; 95% CI, 1.34-8.9; number needed to harm [NNH] = 23), including blurred vision, dizziness, sedation, nausea, and dysphoria.5A review (5 RCTs and 1 open-label trial; N = 1708) analyzing the cardiac safety profile of intranasal esketamine adjuvant therapy found that it produced transient and asymptomatic blood pressure elevations (OR = 3.2; 95% CI, 1.9-5.8; NNH = 13).7
Recommendations from others
A clinical practice guideline from the US Veterans Administration lists IV ketamine as 1 of the therapeutic options for patients with treatment-resistant depression and suicidal ideation.8 However, a Department of Veterans Affairs Panel restricted its use to a pre-approved case-by-case basis.8
Editor’s takeaway
Physicians with patients facing the all-too-common problem of treatment-resistant major depression will be wondering if ketamine, either by itself or as an augmentation therapy, can help. Unfortunately, the outcomes we report here are too short term to answer that question, and we must await the results of further studies. Augmentation with intranasal esketamine, at a cost of $370/month, might offer some promise.
1. Caddy C, Amit BH, McCloud TL, et al. Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev. 2015;(9):CD011612.
2. Coyle CM, Laws KR. The use of ketamine as an antidepressant: a systematic review and meta-analysis. Hum Psychopharmacol. 2015;30:152‐163.
3. Singh JB, Fedgchin M, Daly EJ, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173:816‐826.
4. Rosenblat JD, Carvalho AF, Li M, et al. Oral ketamine for depression: a systematic review. J Clin Psychiatry. 2019;80:18r12475.
5. Zheng W, Cai DB, Xiang YQ, et al. Adjunctive intranasal esketamine for major depressive disorder: a systematic review of randomized double-blind controlled-placebo studies. J Affect Disord. 2020;265:63‐70.
6. Short B, Fong J, Galvez V, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5:65‐78.
7. Doherty T, Wajs E, Melkote R, et al. Cardiac safety of esketamine nasal spray in treatment-resistant depression: results from the Clinical Development Program. CNS Drugs. 2020;34:299‐310.
8. Sall J, Brenner L, Millikan Bell AM, et al. Assessment and management of patients at risk for suicide: synopsis of the 2019 US Department of Veterans Affairs and US Department of Defense Clinical Practice Guidelines. Ann Intern Med. 2019;171:343-353.
1. Caddy C, Amit BH, McCloud TL, et al. Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev. 2015;(9):CD011612.
2. Coyle CM, Laws KR. The use of ketamine as an antidepressant: a systematic review and meta-analysis. Hum Psychopharmacol. 2015;30:152‐163.
3. Singh JB, Fedgchin M, Daly EJ, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173:816‐826.
4. Rosenblat JD, Carvalho AF, Li M, et al. Oral ketamine for depression: a systematic review. J Clin Psychiatry. 2019;80:18r12475.
5. Zheng W, Cai DB, Xiang YQ, et al. Adjunctive intranasal esketamine for major depressive disorder: a systematic review of randomized double-blind controlled-placebo studies. J Affect Disord. 2020;265:63‐70.
6. Short B, Fong J, Galvez V, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5:65‐78.
7. Doherty T, Wajs E, Melkote R, et al. Cardiac safety of esketamine nasal spray in treatment-resistant depression: results from the Clinical Development Program. CNS Drugs. 2020;34:299‐310.
8. Sall J, Brenner L, Millikan Bell AM, et al. Assessment and management of patients at risk for suicide: synopsis of the 2019 US Department of Veterans Affairs and US Department of Defense Clinical Practice Guidelines. Ann Intern Med. 2019;171:343-353.
EVIDENCE-BASED ANSWER:
MAYBE, but it’s too soon to tell. There is limited evidence that ketamine by itself is effective in the very short term. Single-dose intravenous (IV) ketamine is more likely than placebo (odds ratio = 11-13) to produce improvement (> 50%) in standardized depression scores in 1 to 3 days, lasting up to a week. Twice- or thrice-weekly IV ketamine improves symptom scores by 20%-25% over 2 weeks (strength of recommendation [SOR]: B, meta-analysis of small, low-quality, randomized controlled trials [RCTs] and a single small RCT).
Augmentation of sertraline with daily oral ketamine moderately improves symptom scores for 6 weeks in patients with moderate depression (SOR: B, small, low-quality RCTs).
Augmentation of oral antidepressants (duloxetine, escitalopram, sertraline, venlafaxine) with intranasal esketamine spray improves response and remission rates at 4 weeks (16% for both outcomes) in patients with predominantly treatment-resistant major depression (SOR: A, meta-analysis of RCTs).
Ketamine therapy is associated with confusion, emotional blunting, headache, dizziness, and blurred vision (SOR: A, meta-analyses).
Nasal esketamine spray produces the adverse effects of dizziness, vertigo, and blurred vision severe enough to cause discontinuation in 4% of patients; it also can produce transient elevation of blood pressure (SOR: A, meta-analyses).
List of COVID-19 high-risk comorbidities expanded
The list of medical according to the Centers for Disease Control and Prevention.
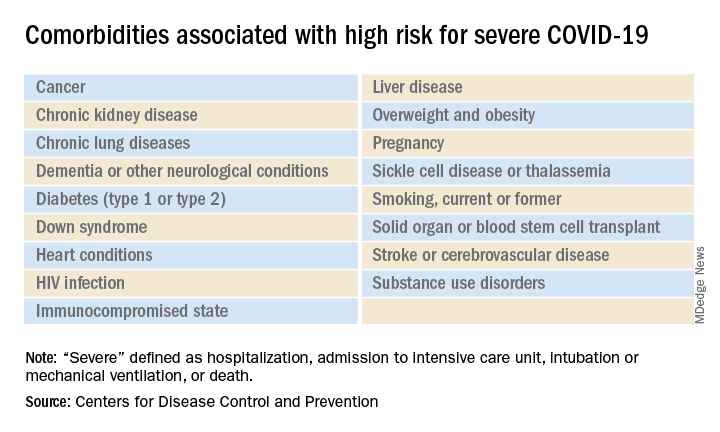
The CDC’s latest list consists of 17 conditions or groups of related conditions that may increase patients’ risk of developing severe outcomes of COVID-19, the CDC said on a web page intended for the general public.
On a separate page, the CDC defines severe outcomes “as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
Asthma is included in the newly expanded list with other chronic lung diseases such as chronic obstructive pulmonary disease and cystic fibrosis; the list’s heart disease entry covers coronary artery disease, heart failure, cardiomyopathies, and hypertension, the CDC said.
The list of medical according to the Centers for Disease Control and Prevention.

The CDC’s latest list consists of 17 conditions or groups of related conditions that may increase patients’ risk of developing severe outcomes of COVID-19, the CDC said on a web page intended for the general public.
On a separate page, the CDC defines severe outcomes “as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
Asthma is included in the newly expanded list with other chronic lung diseases such as chronic obstructive pulmonary disease and cystic fibrosis; the list’s heart disease entry covers coronary artery disease, heart failure, cardiomyopathies, and hypertension, the CDC said.
The list of medical according to the Centers for Disease Control and Prevention.

The CDC’s latest list consists of 17 conditions or groups of related conditions that may increase patients’ risk of developing severe outcomes of COVID-19, the CDC said on a web page intended for the general public.
On a separate page, the CDC defines severe outcomes “as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
Asthma is included in the newly expanded list with other chronic lung diseases such as chronic obstructive pulmonary disease and cystic fibrosis; the list’s heart disease entry covers coronary artery disease, heart failure, cardiomyopathies, and hypertension, the CDC said.
Endocrinologist charged after bomb-making supplies found
An endocrinologist in Naples, Fla., faces multiple federal charges after police found homemade explosives and bomb-making supplies, as well as numerous illegal drugs, in his home.
Police were executing a search warrant at the home of Christy Daniel Cugini, MD, 63, on March 30 when they found the items, according to Collier County Sheriff’s Office (CCSO).
“An investigation continues and more charges could be brought,” the sheriff’s office said in a statement. As of April 1, Dr. Cugini was out on bond. His next court appearance is on April 26.
A search of his bedroom turned up marijuana, tramadol, oxycodone, and hydrocodone, the sheriff’s office said. According to nbcmiami.com, police also found 560 grams of marijuana and $20,000 in cash and jewelry in a safe.
“Some of the narcotics were in pill bottles with other people’s names on them. Many of the substances were of trafficking quantities. The search also turned up numerous items of narcotic paraphernalia, including heat seal bags, a vacuum sealer, and a scale,” the CCSO report said.
Charges against Dr. Cugini include narcotics trafficking; possession of marijuana with intent to sell/manufacture/deliver; possession of more than 20 grams of marijuana; possession of a controlled substance; and possession of narcotic paraphernalia, according to the report.
He was also charged with nine counts of making/possessing a destructive device.
The CCSO bomb squad was brought in to investigate the homemade explosive devices and supplies, including potassium nitrate and ammonium nitrate – which can be used as oxidizers – PVC pipe, and flash powders used in fireworks in Dr. Cugini’s house and garage.
Newsweek reported that the bomb squad found six red cylindrical devices about 4 inches long, according to information reported in an affidavit from Collier County Officer Jeffrey Tayar. They may have been intended to be a hand-tossed improvised explosive device, Mr. Tayar wrote.
An officer also found three other devices made up of PVC pipe attached to a small wood square. A rifle round was inserted into the PVC pipe, Mr. Tayar’s report said.
“The device could be placed on the ground in such a manner as to leave the rifle round facing up,” Mr. Tayar reportedly wrote. “If downward pressure were applied on the tip of the round ... the rifle round [would] discharge, launching the projectile portion of the round upward, presumably into the foot of the subject stepping on it.”
NBC News reported that deputies said Dr. Cugini appeared to live only with his young daughter.
He initially agreed to speak with deputies but then invoked his Miranda rights and stopped answering questions, NBC said.
Dr. Cugini’s profile has been removed from the Millennium Physician Group website.
His employer offered this statement via spokesperson Liza Fernandez: “We are shocked at the allegations regarding Dr. Christy Cugini. He has been placed on administrative leave until further notice. Millennium is committed to cooperating with law enforcement and is conducting an internal investigation.”
According to U.S. News & World Report, Dr. Cugini is affiliated with NCH Baker Hospital. He received his medical degree from Ross University School of Medicine, now located in Barbados, and has been practicing for more than 20 years.
Attempts to contact Dr. Cugini were unsuccessful.
A version of this article first appeared on Medscape.com.
An endocrinologist in Naples, Fla., faces multiple federal charges after police found homemade explosives and bomb-making supplies, as well as numerous illegal drugs, in his home.
Police were executing a search warrant at the home of Christy Daniel Cugini, MD, 63, on March 30 when they found the items, according to Collier County Sheriff’s Office (CCSO).
“An investigation continues and more charges could be brought,” the sheriff’s office said in a statement. As of April 1, Dr. Cugini was out on bond. His next court appearance is on April 26.
A search of his bedroom turned up marijuana, tramadol, oxycodone, and hydrocodone, the sheriff’s office said. According to nbcmiami.com, police also found 560 grams of marijuana and $20,000 in cash and jewelry in a safe.
“Some of the narcotics were in pill bottles with other people’s names on them. Many of the substances were of trafficking quantities. The search also turned up numerous items of narcotic paraphernalia, including heat seal bags, a vacuum sealer, and a scale,” the CCSO report said.
Charges against Dr. Cugini include narcotics trafficking; possession of marijuana with intent to sell/manufacture/deliver; possession of more than 20 grams of marijuana; possession of a controlled substance; and possession of narcotic paraphernalia, according to the report.
He was also charged with nine counts of making/possessing a destructive device.
The CCSO bomb squad was brought in to investigate the homemade explosive devices and supplies, including potassium nitrate and ammonium nitrate – which can be used as oxidizers – PVC pipe, and flash powders used in fireworks in Dr. Cugini’s house and garage.
Newsweek reported that the bomb squad found six red cylindrical devices about 4 inches long, according to information reported in an affidavit from Collier County Officer Jeffrey Tayar. They may have been intended to be a hand-tossed improvised explosive device, Mr. Tayar wrote.
An officer also found three other devices made up of PVC pipe attached to a small wood square. A rifle round was inserted into the PVC pipe, Mr. Tayar’s report said.
“The device could be placed on the ground in such a manner as to leave the rifle round facing up,” Mr. Tayar reportedly wrote. “If downward pressure were applied on the tip of the round ... the rifle round [would] discharge, launching the projectile portion of the round upward, presumably into the foot of the subject stepping on it.”
NBC News reported that deputies said Dr. Cugini appeared to live only with his young daughter.
He initially agreed to speak with deputies but then invoked his Miranda rights and stopped answering questions, NBC said.
Dr. Cugini’s profile has been removed from the Millennium Physician Group website.
His employer offered this statement via spokesperson Liza Fernandez: “We are shocked at the allegations regarding Dr. Christy Cugini. He has been placed on administrative leave until further notice. Millennium is committed to cooperating with law enforcement and is conducting an internal investigation.”
According to U.S. News & World Report, Dr. Cugini is affiliated with NCH Baker Hospital. He received his medical degree from Ross University School of Medicine, now located in Barbados, and has been practicing for more than 20 years.
Attempts to contact Dr. Cugini were unsuccessful.
A version of this article first appeared on Medscape.com.
An endocrinologist in Naples, Fla., faces multiple federal charges after police found homemade explosives and bomb-making supplies, as well as numerous illegal drugs, in his home.
Police were executing a search warrant at the home of Christy Daniel Cugini, MD, 63, on March 30 when they found the items, according to Collier County Sheriff’s Office (CCSO).
“An investigation continues and more charges could be brought,” the sheriff’s office said in a statement. As of April 1, Dr. Cugini was out on bond. His next court appearance is on April 26.
A search of his bedroom turned up marijuana, tramadol, oxycodone, and hydrocodone, the sheriff’s office said. According to nbcmiami.com, police also found 560 grams of marijuana and $20,000 in cash and jewelry in a safe.
“Some of the narcotics were in pill bottles with other people’s names on them. Many of the substances were of trafficking quantities. The search also turned up numerous items of narcotic paraphernalia, including heat seal bags, a vacuum sealer, and a scale,” the CCSO report said.
Charges against Dr. Cugini include narcotics trafficking; possession of marijuana with intent to sell/manufacture/deliver; possession of more than 20 grams of marijuana; possession of a controlled substance; and possession of narcotic paraphernalia, according to the report.
He was also charged with nine counts of making/possessing a destructive device.
The CCSO bomb squad was brought in to investigate the homemade explosive devices and supplies, including potassium nitrate and ammonium nitrate – which can be used as oxidizers – PVC pipe, and flash powders used in fireworks in Dr. Cugini’s house and garage.
Newsweek reported that the bomb squad found six red cylindrical devices about 4 inches long, according to information reported in an affidavit from Collier County Officer Jeffrey Tayar. They may have been intended to be a hand-tossed improvised explosive device, Mr. Tayar wrote.
An officer also found three other devices made up of PVC pipe attached to a small wood square. A rifle round was inserted into the PVC pipe, Mr. Tayar’s report said.
“The device could be placed on the ground in such a manner as to leave the rifle round facing up,” Mr. Tayar reportedly wrote. “If downward pressure were applied on the tip of the round ... the rifle round [would] discharge, launching the projectile portion of the round upward, presumably into the foot of the subject stepping on it.”
NBC News reported that deputies said Dr. Cugini appeared to live only with his young daughter.
He initially agreed to speak with deputies but then invoked his Miranda rights and stopped answering questions, NBC said.
Dr. Cugini’s profile has been removed from the Millennium Physician Group website.
His employer offered this statement via spokesperson Liza Fernandez: “We are shocked at the allegations regarding Dr. Christy Cugini. He has been placed on administrative leave until further notice. Millennium is committed to cooperating with law enforcement and is conducting an internal investigation.”
According to U.S. News & World Report, Dr. Cugini is affiliated with NCH Baker Hospital. He received his medical degree from Ross University School of Medicine, now located in Barbados, and has been practicing for more than 20 years.
Attempts to contact Dr. Cugini were unsuccessful.
A version of this article first appeared on Medscape.com.
Green light puts the stop on migraine
, according to results of a small study from the University of Arizona, Tucson.
“This is the first clinical study to evaluate green light exposure as a potential preventive therapy for patients with migraine, “ senior author Mohab M. Ibrahim, MD, PhD, said in a press release. “Now I have another tool in my toolbox to treat one of the most difficult neurologic conditions – migraine.”
“Given the safety, affordability, and efficacy of green light exposure, there is merit to conduct a larger study,” he and coauthors from the university wrote in their paper.
The study included 29 adult patients (average age 52.2 years), 22 with chronic migraine and the rest with episodic migraine who were recruited from the University of Arizona/Banner Medical Center chronic pain clinic. To be included, patients had to meet the International Headache Society diagnostic criteria for chronic or episodic migraine, have an average headache pain intensity of 5 out of 10 or greater on the numeric pain scale (NPS) over the 10 weeks prior to enrolling in the study, and be dissatisfied with their current migraine therapy.
The patients were free to start, continue, or discontinue any other migraine treatments as recommended by their physicians as long as this was reported to the study team.
White versus green
The one-way crossover design involved exposure to 10 weeks of white light emitting diodes, for 1-2 hours per day, followed by a 2-week washout period and then 10 weeks’ exposure to green light emitting diodes (GLED) for the same daily duration. The protocol involved use of a light strip emitting an intensity of between 4 and 100 lux measured at approximately 2 m and 1 m from a lux meter.
Patients were instructed to use the light in a dark room, without falling asleep, and to participate in activities that did not require external light sources, such as listening to music, reading books, doing exercises, or engaging in similar activities. The daily minimum exposure of 1 hour, up to a maximum of 2 hours, was to be completed in one sitting.
The primary outcome measure was the number of headache days per month, defined as days with moderate to severe headache pain for at least 4 hours. Secondary outcomes included perceived reduction in duration and intensity of the headache phase of the migraine episodes assessed every 2 weeks with the NPS, improved ability to fall and stay asleep, improved ability to perform work and daily activity, improved quality of life, and reduction of pain medications.
The researchers found that when the patients with chronic migraine and episodic migraine were examined as separate groups, white light exposure did not significantly reduce the number of headache days per month, but when the chronic migraine and episodic migraine groups were combined there was a significant reduction from 18.2 to 16.5 headache days per month.
On the other hand, green light did result in significantly reduced headache days both in the separate (from 7.9 to 2.4 days in the episodic migraine group and 22.3 to 9.4 days in the chronic migraine group) and combined groups (from 18.4 to 7.4 days).
“While some improvement in secondary outcomes was observed with white light emitting diodes, more secondary outcomes with significantly greater magnitude including assessments of quality of life, Short-Form McGill Pain Questionnaire, Headache Impact Test-6, and Five-level version of the EuroQol five-dimensional survey without reported side effects were observed with green light emitting diodes,” the authors reported.
“The use of a nonpharmacological therapy such as green light can be of tremendous help to a variety of patients that either do not want to be on medications or do not respond to them,” coauthor Amol M. Patwardhan, MD, PhD, said in the press release. “The beauty of this approach is the lack of associated side effects. If at all, it appears to improve sleep and other quality of life measures,” said Dr. Patwardhan, associate professor and vice chair of research in the University of Arizona’s department of anesthesiology.
Better than white light
Asked to comment on the findings, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, said research has shown for some time that exposure to green light has beneficial effects in migraine patients. This study, although small, does indicate that green light is more beneficial than is white light and reduces headache days and intensity. “I believe patients would be willing to spend 1-2 hours a day in green light to reduce and improve their migraine with few side effects. A larger randomized trial should be done,” he said.
The study was funded by support from the National Center for Complementary and Integrative Health (to Dr. Ibrahim), the Comprehensive Chronic Pain and Addiction Center–University of Arizona, and the University of Arizona CHiLLI initiative. Dr. Ibrahim and one coauthor have a patent pending through the University of Arizona for use of green light therapy for the management of chronic pain. Dr. Rapoport is a former president of the International Headache Society. He is an editor of Headache and CNS Drugs, and Editor-in-Chief of Neurology Reviews. He reviews for many peer-reviewed journals such as Cephalalgia, Neurology, New England Journal of Medicine, and Headache.
, according to results of a small study from the University of Arizona, Tucson.
“This is the first clinical study to evaluate green light exposure as a potential preventive therapy for patients with migraine, “ senior author Mohab M. Ibrahim, MD, PhD, said in a press release. “Now I have another tool in my toolbox to treat one of the most difficult neurologic conditions – migraine.”
“Given the safety, affordability, and efficacy of green light exposure, there is merit to conduct a larger study,” he and coauthors from the university wrote in their paper.
The study included 29 adult patients (average age 52.2 years), 22 with chronic migraine and the rest with episodic migraine who were recruited from the University of Arizona/Banner Medical Center chronic pain clinic. To be included, patients had to meet the International Headache Society diagnostic criteria for chronic or episodic migraine, have an average headache pain intensity of 5 out of 10 or greater on the numeric pain scale (NPS) over the 10 weeks prior to enrolling in the study, and be dissatisfied with their current migraine therapy.
The patients were free to start, continue, or discontinue any other migraine treatments as recommended by their physicians as long as this was reported to the study team.
White versus green
The one-way crossover design involved exposure to 10 weeks of white light emitting diodes, for 1-2 hours per day, followed by a 2-week washout period and then 10 weeks’ exposure to green light emitting diodes (GLED) for the same daily duration. The protocol involved use of a light strip emitting an intensity of between 4 and 100 lux measured at approximately 2 m and 1 m from a lux meter.
Patients were instructed to use the light in a dark room, without falling asleep, and to participate in activities that did not require external light sources, such as listening to music, reading books, doing exercises, or engaging in similar activities. The daily minimum exposure of 1 hour, up to a maximum of 2 hours, was to be completed in one sitting.
The primary outcome measure was the number of headache days per month, defined as days with moderate to severe headache pain for at least 4 hours. Secondary outcomes included perceived reduction in duration and intensity of the headache phase of the migraine episodes assessed every 2 weeks with the NPS, improved ability to fall and stay asleep, improved ability to perform work and daily activity, improved quality of life, and reduction of pain medications.
The researchers found that when the patients with chronic migraine and episodic migraine were examined as separate groups, white light exposure did not significantly reduce the number of headache days per month, but when the chronic migraine and episodic migraine groups were combined there was a significant reduction from 18.2 to 16.5 headache days per month.
On the other hand, green light did result in significantly reduced headache days both in the separate (from 7.9 to 2.4 days in the episodic migraine group and 22.3 to 9.4 days in the chronic migraine group) and combined groups (from 18.4 to 7.4 days).
“While some improvement in secondary outcomes was observed with white light emitting diodes, more secondary outcomes with significantly greater magnitude including assessments of quality of life, Short-Form McGill Pain Questionnaire, Headache Impact Test-6, and Five-level version of the EuroQol five-dimensional survey without reported side effects were observed with green light emitting diodes,” the authors reported.
“The use of a nonpharmacological therapy such as green light can be of tremendous help to a variety of patients that either do not want to be on medications or do not respond to them,” coauthor Amol M. Patwardhan, MD, PhD, said in the press release. “The beauty of this approach is the lack of associated side effects. If at all, it appears to improve sleep and other quality of life measures,” said Dr. Patwardhan, associate professor and vice chair of research in the University of Arizona’s department of anesthesiology.
Better than white light
Asked to comment on the findings, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, said research has shown for some time that exposure to green light has beneficial effects in migraine patients. This study, although small, does indicate that green light is more beneficial than is white light and reduces headache days and intensity. “I believe patients would be willing to spend 1-2 hours a day in green light to reduce and improve their migraine with few side effects. A larger randomized trial should be done,” he said.
The study was funded by support from the National Center for Complementary and Integrative Health (to Dr. Ibrahim), the Comprehensive Chronic Pain and Addiction Center–University of Arizona, and the University of Arizona CHiLLI initiative. Dr. Ibrahim and one coauthor have a patent pending through the University of Arizona for use of green light therapy for the management of chronic pain. Dr. Rapoport is a former president of the International Headache Society. He is an editor of Headache and CNS Drugs, and Editor-in-Chief of Neurology Reviews. He reviews for many peer-reviewed journals such as Cephalalgia, Neurology, New England Journal of Medicine, and Headache.
, according to results of a small study from the University of Arizona, Tucson.
“This is the first clinical study to evaluate green light exposure as a potential preventive therapy for patients with migraine, “ senior author Mohab M. Ibrahim, MD, PhD, said in a press release. “Now I have another tool in my toolbox to treat one of the most difficult neurologic conditions – migraine.”
“Given the safety, affordability, and efficacy of green light exposure, there is merit to conduct a larger study,” he and coauthors from the university wrote in their paper.
The study included 29 adult patients (average age 52.2 years), 22 with chronic migraine and the rest with episodic migraine who were recruited from the University of Arizona/Banner Medical Center chronic pain clinic. To be included, patients had to meet the International Headache Society diagnostic criteria for chronic or episodic migraine, have an average headache pain intensity of 5 out of 10 or greater on the numeric pain scale (NPS) over the 10 weeks prior to enrolling in the study, and be dissatisfied with their current migraine therapy.
The patients were free to start, continue, or discontinue any other migraine treatments as recommended by their physicians as long as this was reported to the study team.
White versus green
The one-way crossover design involved exposure to 10 weeks of white light emitting diodes, for 1-2 hours per day, followed by a 2-week washout period and then 10 weeks’ exposure to green light emitting diodes (GLED) for the same daily duration. The protocol involved use of a light strip emitting an intensity of between 4 and 100 lux measured at approximately 2 m and 1 m from a lux meter.
Patients were instructed to use the light in a dark room, without falling asleep, and to participate in activities that did not require external light sources, such as listening to music, reading books, doing exercises, or engaging in similar activities. The daily minimum exposure of 1 hour, up to a maximum of 2 hours, was to be completed in one sitting.
The primary outcome measure was the number of headache days per month, defined as days with moderate to severe headache pain for at least 4 hours. Secondary outcomes included perceived reduction in duration and intensity of the headache phase of the migraine episodes assessed every 2 weeks with the NPS, improved ability to fall and stay asleep, improved ability to perform work and daily activity, improved quality of life, and reduction of pain medications.
The researchers found that when the patients with chronic migraine and episodic migraine were examined as separate groups, white light exposure did not significantly reduce the number of headache days per month, but when the chronic migraine and episodic migraine groups were combined there was a significant reduction from 18.2 to 16.5 headache days per month.
On the other hand, green light did result in significantly reduced headache days both in the separate (from 7.9 to 2.4 days in the episodic migraine group and 22.3 to 9.4 days in the chronic migraine group) and combined groups (from 18.4 to 7.4 days).
“While some improvement in secondary outcomes was observed with white light emitting diodes, more secondary outcomes with significantly greater magnitude including assessments of quality of life, Short-Form McGill Pain Questionnaire, Headache Impact Test-6, and Five-level version of the EuroQol five-dimensional survey without reported side effects were observed with green light emitting diodes,” the authors reported.
“The use of a nonpharmacological therapy such as green light can be of tremendous help to a variety of patients that either do not want to be on medications or do not respond to them,” coauthor Amol M. Patwardhan, MD, PhD, said in the press release. “The beauty of this approach is the lack of associated side effects. If at all, it appears to improve sleep and other quality of life measures,” said Dr. Patwardhan, associate professor and vice chair of research in the University of Arizona’s department of anesthesiology.
Better than white light
Asked to comment on the findings, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, said research has shown for some time that exposure to green light has beneficial effects in migraine patients. This study, although small, does indicate that green light is more beneficial than is white light and reduces headache days and intensity. “I believe patients would be willing to spend 1-2 hours a day in green light to reduce and improve their migraine with few side effects. A larger randomized trial should be done,” he said.
The study was funded by support from the National Center for Complementary and Integrative Health (to Dr. Ibrahim), the Comprehensive Chronic Pain and Addiction Center–University of Arizona, and the University of Arizona CHiLLI initiative. Dr. Ibrahim and one coauthor have a patent pending through the University of Arizona for use of green light therapy for the management of chronic pain. Dr. Rapoport is a former president of the International Headache Society. He is an editor of Headache and CNS Drugs, and Editor-in-Chief of Neurology Reviews. He reviews for many peer-reviewed journals such as Cephalalgia, Neurology, New England Journal of Medicine, and Headache.
FROM CEPHALALGIA
The COVID-19 push to evolve
Has anyone else noticed how slow it has been on your pediatric floors? Well, you are not alone.
The COVID pandemic has had a significant impact on health care volumes, with pediatric volumes decreasing across the nation. A Children’s Hospital Association CEO survey, currently unpublished, noted a 10%-20% decline in inpatient admissions and a 30%-50% decline in pediatric ED visits this past year. Even our usual respiratory surge has been disrupted. The rate of influenza tracked by the CDC is around 1%, compared with the usual seasonal flu baseline national rate of 2.6%. These COVID-related declines have occurred amidst the backdrop of already-decreasing inpatient admissions because of the great work of the pediatric hospital medicine (PHM) community in reducing unnecessary admissions and lengths of stay.
For many hospitals, several factors related to the pandemic have raised significant financial concerns. According to Becker Hospital Review, as of August 2020 over 500 hospitals had furloughed workers. While 26 of those hospitals had brought back workers by December 2020, many did not. Similar financial concerns were noted in a Kaufmann Hall report from January 2021, which showed a median drop of 55% in operating margins. The CARES Act helped reduce some of the detrimental impact on operating margins, but it did not diminish the added burden of personal protective equipment expenses, longer length of stay for COVID patients, and a reimbursement shift to more government payors and uninsured caused by pandemic-forced job losses.
COVID’s impact specific to pediatric hospital medicine has been substantial. A recent unpublished survey by the PHM Economics Research Collaborative (PERC) demonstrated how COVID has affected pediatric hospital medicine programs. Forty-five unique PHM programs from over 21 states responded, with 98% reporting a decrease in pediatric inpatient admissions as well as ED visits. About 11% reported temporary unit closures, while 51% of all programs reported staffing restrictions ranging from hiring freezes to downsizing the number of hospitalists in the group. Salaries decreased in 26% of reporting programs, and 20%-56% described reduced benefits, ranging from less CME/vacation time and stipends to retirement benefits. The three most frequent benefit losses included annual salary increases, educational stipends, and bonuses.
Community hospitals felt the palpable, financial strain of decreasing pediatric admissions well before the pandemic. Hospitals like MedStar Franklin Square Hospital in Baltimore and Harrington Hospital in Southbridge, Mass., had decided to close their pediatrics units before COVID hit. In a 2014 unpublished survey of 349 community PHM (CPHM) programs, 57% of respondents felt that finances and justification for a pediatric program were primary concerns.
Responding to financial stressors is not a novel challenge for CPHM programs. To keep these vital pediatric programs in place despite lower inpatient volumes, those of us in CPHM have learned many lessons over the years on how to adapt. Such adaptations have included diversification in procedures and multifloor coverage in the hospital. Voiding cystourethrogram catheterizations and circumcisions are now more commonly performed by CPHM providers, who may also cover multiple areas of the hospital, including the ED, NICU, and well-newborn nursery. Comanagement of subspecialty or surgical patients is yet another example of such diversification.
Furthermore, the PERC survey showed that some PHM programs temporarily covered pediatric ICUs and step-down units and began doing ED and urgent care coverage as primary providers Most programs reported no change in newborn visits while 16% reported an increase in newborn volume and 14% reported a decrease in newborn volume. My own health system was one of the groups that had an increase in newborn volume. This was caused by community pediatricians who had stopped coming in to see their own newborns. This coverage adjustment has yet to return to baseline and will likely become permanent.
There was a 11% increase from prepandemic baselines (from 9% to 20%) in programs doing telemedicine. Most respondents stated that they will continue to offer telemedicine with an additional 25% of programs considering starting. There was also a slight increase during the pandemic of coverage of mental health units (from 11% to 13%), which may have led 11% of respondents to consider the addition of this service. The survey also noted that about 28% of PHM programs performed circumcisions, frenectomies, and sedation prepandemic, and 14%-18% are considering adding these services.
Overall, the financial stressors are improving, but our need to adapt in PHM is more pressing than ever. The pandemic has given us the push for evolution and some opportunities that did not exist before. One is the use of telemedicine to expand our subspecialty support to community hospitals, as well as to children’s hospitals in areas where subspecialists are in short supply. These telemedicine consults are being reimbursed for the first time, which allows more access to these services.
With the pandemic, many hospitals are moving to single room occupancy models. Construction to add more beds is costly, and unnecessary if we can utilize community hospitals to keep appropriate patients in their home communities. The opportunity to partner with community hospital programs to provide telemedicine support should not be overlooked. This is also an opportunity for academic referral centers to have more open beds for critical care and highly specialized patients.
Another opportunity is to expand scope by changing age limits, as 18% of respondents to the PERC survey reported that they had started to care for adults since the pandemic. The Pediatric Overflow Planning Contingency Response Network (POPCoRN) has been a valuable resource for education on caring for adults, guidance on which patient populations are appropriate, and the resources needed to do this. While caring for older adults, even in their 90s, was a pandemic-related phenomenon, there is an opportunity to see if the age limit we care for should be raised to 21, or even 25, as some CPHM programs had been doing prepandemic.
Along with the expansion of age limits, there are many other areas of opportunity highlighted within the PERC survey. These include expanding coverage within pediatric ICUs, EDs, and urgent care areas, along with coverage of well newborns that were previously covered by community pediatricians. Also, the increase of mental health admissions is another area where PHM programs might expand their services.
While I hope the financial stressors improve, hope is not a plan and therefore we need to think and prepare for what the post-COVID future may look like. Some have predicted a rebound pediatric respiratory surge next year as the masks come off and children return to in-person learning and daycare. This may be true, but we would be foolish not to use lessons from the pandemic as well as the past to consider options in our toolkit to become more financially stable. POPCoRN, as well as the American Academy of Pediatrics’ listserv and subcommittees, have been a source of collaboration and shared knowledge during a time when we have needed to quickly respond to ever-changing information. These networks and information sharing should be leveraged once the dust settles for us to prepare for future challenges.
New innovations may arise as we look at how we address the growing need for mental health services and incorporate new procedures, like point of care ultrasound. As Charles Darwin said: “It is not the strongest of the species that survives nor the most intelligent that survives. It is the one that is most adaptable to change.” It is time for us to evolve.
Dr. Dias is a clinical associate professor of pediatrics at Yale University, New Haven, Conn., in the division of pediatric hospital medicine. She has practiced community pediatric hospital medicine for over 21 years in New Jersey, Pennsylvania, and Connecticut. She is the chair of the Education Working Group for the AAP’s section on hospital medicine’s subcommittee on community hospitalists as well as the cochair of the Community Hospital Operations Group of the POPCoRN network.
Has anyone else noticed how slow it has been on your pediatric floors? Well, you are not alone.
The COVID pandemic has had a significant impact on health care volumes, with pediatric volumes decreasing across the nation. A Children’s Hospital Association CEO survey, currently unpublished, noted a 10%-20% decline in inpatient admissions and a 30%-50% decline in pediatric ED visits this past year. Even our usual respiratory surge has been disrupted. The rate of influenza tracked by the CDC is around 1%, compared with the usual seasonal flu baseline national rate of 2.6%. These COVID-related declines have occurred amidst the backdrop of already-decreasing inpatient admissions because of the great work of the pediatric hospital medicine (PHM) community in reducing unnecessary admissions and lengths of stay.
For many hospitals, several factors related to the pandemic have raised significant financial concerns. According to Becker Hospital Review, as of August 2020 over 500 hospitals had furloughed workers. While 26 of those hospitals had brought back workers by December 2020, many did not. Similar financial concerns were noted in a Kaufmann Hall report from January 2021, which showed a median drop of 55% in operating margins. The CARES Act helped reduce some of the detrimental impact on operating margins, but it did not diminish the added burden of personal protective equipment expenses, longer length of stay for COVID patients, and a reimbursement shift to more government payors and uninsured caused by pandemic-forced job losses.
COVID’s impact specific to pediatric hospital medicine has been substantial. A recent unpublished survey by the PHM Economics Research Collaborative (PERC) demonstrated how COVID has affected pediatric hospital medicine programs. Forty-five unique PHM programs from over 21 states responded, with 98% reporting a decrease in pediatric inpatient admissions as well as ED visits. About 11% reported temporary unit closures, while 51% of all programs reported staffing restrictions ranging from hiring freezes to downsizing the number of hospitalists in the group. Salaries decreased in 26% of reporting programs, and 20%-56% described reduced benefits, ranging from less CME/vacation time and stipends to retirement benefits. The three most frequent benefit losses included annual salary increases, educational stipends, and bonuses.
Community hospitals felt the palpable, financial strain of decreasing pediatric admissions well before the pandemic. Hospitals like MedStar Franklin Square Hospital in Baltimore and Harrington Hospital in Southbridge, Mass., had decided to close their pediatrics units before COVID hit. In a 2014 unpublished survey of 349 community PHM (CPHM) programs, 57% of respondents felt that finances and justification for a pediatric program were primary concerns.
Responding to financial stressors is not a novel challenge for CPHM programs. To keep these vital pediatric programs in place despite lower inpatient volumes, those of us in CPHM have learned many lessons over the years on how to adapt. Such adaptations have included diversification in procedures and multifloor coverage in the hospital. Voiding cystourethrogram catheterizations and circumcisions are now more commonly performed by CPHM providers, who may also cover multiple areas of the hospital, including the ED, NICU, and well-newborn nursery. Comanagement of subspecialty or surgical patients is yet another example of such diversification.
Furthermore, the PERC survey showed that some PHM programs temporarily covered pediatric ICUs and step-down units and began doing ED and urgent care coverage as primary providers Most programs reported no change in newborn visits while 16% reported an increase in newborn volume and 14% reported a decrease in newborn volume. My own health system was one of the groups that had an increase in newborn volume. This was caused by community pediatricians who had stopped coming in to see their own newborns. This coverage adjustment has yet to return to baseline and will likely become permanent.
There was a 11% increase from prepandemic baselines (from 9% to 20%) in programs doing telemedicine. Most respondents stated that they will continue to offer telemedicine with an additional 25% of programs considering starting. There was also a slight increase during the pandemic of coverage of mental health units (from 11% to 13%), which may have led 11% of respondents to consider the addition of this service. The survey also noted that about 28% of PHM programs performed circumcisions, frenectomies, and sedation prepandemic, and 14%-18% are considering adding these services.
Overall, the financial stressors are improving, but our need to adapt in PHM is more pressing than ever. The pandemic has given us the push for evolution and some opportunities that did not exist before. One is the use of telemedicine to expand our subspecialty support to community hospitals, as well as to children’s hospitals in areas where subspecialists are in short supply. These telemedicine consults are being reimbursed for the first time, which allows more access to these services.
With the pandemic, many hospitals are moving to single room occupancy models. Construction to add more beds is costly, and unnecessary if we can utilize community hospitals to keep appropriate patients in their home communities. The opportunity to partner with community hospital programs to provide telemedicine support should not be overlooked. This is also an opportunity for academic referral centers to have more open beds for critical care and highly specialized patients.
Another opportunity is to expand scope by changing age limits, as 18% of respondents to the PERC survey reported that they had started to care for adults since the pandemic. The Pediatric Overflow Planning Contingency Response Network (POPCoRN) has been a valuable resource for education on caring for adults, guidance on which patient populations are appropriate, and the resources needed to do this. While caring for older adults, even in their 90s, was a pandemic-related phenomenon, there is an opportunity to see if the age limit we care for should be raised to 21, or even 25, as some CPHM programs had been doing prepandemic.
Along with the expansion of age limits, there are many other areas of opportunity highlighted within the PERC survey. These include expanding coverage within pediatric ICUs, EDs, and urgent care areas, along with coverage of well newborns that were previously covered by community pediatricians. Also, the increase of mental health admissions is another area where PHM programs might expand their services.
While I hope the financial stressors improve, hope is not a plan and therefore we need to think and prepare for what the post-COVID future may look like. Some have predicted a rebound pediatric respiratory surge next year as the masks come off and children return to in-person learning and daycare. This may be true, but we would be foolish not to use lessons from the pandemic as well as the past to consider options in our toolkit to become more financially stable. POPCoRN, as well as the American Academy of Pediatrics’ listserv and subcommittees, have been a source of collaboration and shared knowledge during a time when we have needed to quickly respond to ever-changing information. These networks and information sharing should be leveraged once the dust settles for us to prepare for future challenges.
New innovations may arise as we look at how we address the growing need for mental health services and incorporate new procedures, like point of care ultrasound. As Charles Darwin said: “It is not the strongest of the species that survives nor the most intelligent that survives. It is the one that is most adaptable to change.” It is time for us to evolve.
Dr. Dias is a clinical associate professor of pediatrics at Yale University, New Haven, Conn., in the division of pediatric hospital medicine. She has practiced community pediatric hospital medicine for over 21 years in New Jersey, Pennsylvania, and Connecticut. She is the chair of the Education Working Group for the AAP’s section on hospital medicine’s subcommittee on community hospitalists as well as the cochair of the Community Hospital Operations Group of the POPCoRN network.
Has anyone else noticed how slow it has been on your pediatric floors? Well, you are not alone.
The COVID pandemic has had a significant impact on health care volumes, with pediatric volumes decreasing across the nation. A Children’s Hospital Association CEO survey, currently unpublished, noted a 10%-20% decline in inpatient admissions and a 30%-50% decline in pediatric ED visits this past year. Even our usual respiratory surge has been disrupted. The rate of influenza tracked by the CDC is around 1%, compared with the usual seasonal flu baseline national rate of 2.6%. These COVID-related declines have occurred amidst the backdrop of already-decreasing inpatient admissions because of the great work of the pediatric hospital medicine (PHM) community in reducing unnecessary admissions and lengths of stay.
For many hospitals, several factors related to the pandemic have raised significant financial concerns. According to Becker Hospital Review, as of August 2020 over 500 hospitals had furloughed workers. While 26 of those hospitals had brought back workers by December 2020, many did not. Similar financial concerns were noted in a Kaufmann Hall report from January 2021, which showed a median drop of 55% in operating margins. The CARES Act helped reduce some of the detrimental impact on operating margins, but it did not diminish the added burden of personal protective equipment expenses, longer length of stay for COVID patients, and a reimbursement shift to more government payors and uninsured caused by pandemic-forced job losses.
COVID’s impact specific to pediatric hospital medicine has been substantial. A recent unpublished survey by the PHM Economics Research Collaborative (PERC) demonstrated how COVID has affected pediatric hospital medicine programs. Forty-five unique PHM programs from over 21 states responded, with 98% reporting a decrease in pediatric inpatient admissions as well as ED visits. About 11% reported temporary unit closures, while 51% of all programs reported staffing restrictions ranging from hiring freezes to downsizing the number of hospitalists in the group. Salaries decreased in 26% of reporting programs, and 20%-56% described reduced benefits, ranging from less CME/vacation time and stipends to retirement benefits. The three most frequent benefit losses included annual salary increases, educational stipends, and bonuses.
Community hospitals felt the palpable, financial strain of decreasing pediatric admissions well before the pandemic. Hospitals like MedStar Franklin Square Hospital in Baltimore and Harrington Hospital in Southbridge, Mass., had decided to close their pediatrics units before COVID hit. In a 2014 unpublished survey of 349 community PHM (CPHM) programs, 57% of respondents felt that finances and justification for a pediatric program were primary concerns.
Responding to financial stressors is not a novel challenge for CPHM programs. To keep these vital pediatric programs in place despite lower inpatient volumes, those of us in CPHM have learned many lessons over the years on how to adapt. Such adaptations have included diversification in procedures and multifloor coverage in the hospital. Voiding cystourethrogram catheterizations and circumcisions are now more commonly performed by CPHM providers, who may also cover multiple areas of the hospital, including the ED, NICU, and well-newborn nursery. Comanagement of subspecialty or surgical patients is yet another example of such diversification.
Furthermore, the PERC survey showed that some PHM programs temporarily covered pediatric ICUs and step-down units and began doing ED and urgent care coverage as primary providers Most programs reported no change in newborn visits while 16% reported an increase in newborn volume and 14% reported a decrease in newborn volume. My own health system was one of the groups that had an increase in newborn volume. This was caused by community pediatricians who had stopped coming in to see their own newborns. This coverage adjustment has yet to return to baseline and will likely become permanent.
There was a 11% increase from prepandemic baselines (from 9% to 20%) in programs doing telemedicine. Most respondents stated that they will continue to offer telemedicine with an additional 25% of programs considering starting. There was also a slight increase during the pandemic of coverage of mental health units (from 11% to 13%), which may have led 11% of respondents to consider the addition of this service. The survey also noted that about 28% of PHM programs performed circumcisions, frenectomies, and sedation prepandemic, and 14%-18% are considering adding these services.
Overall, the financial stressors are improving, but our need to adapt in PHM is more pressing than ever. The pandemic has given us the push for evolution and some opportunities that did not exist before. One is the use of telemedicine to expand our subspecialty support to community hospitals, as well as to children’s hospitals in areas where subspecialists are in short supply. These telemedicine consults are being reimbursed for the first time, which allows more access to these services.
With the pandemic, many hospitals are moving to single room occupancy models. Construction to add more beds is costly, and unnecessary if we can utilize community hospitals to keep appropriate patients in their home communities. The opportunity to partner with community hospital programs to provide telemedicine support should not be overlooked. This is also an opportunity for academic referral centers to have more open beds for critical care and highly specialized patients.
Another opportunity is to expand scope by changing age limits, as 18% of respondents to the PERC survey reported that they had started to care for adults since the pandemic. The Pediatric Overflow Planning Contingency Response Network (POPCoRN) has been a valuable resource for education on caring for adults, guidance on which patient populations are appropriate, and the resources needed to do this. While caring for older adults, even in their 90s, was a pandemic-related phenomenon, there is an opportunity to see if the age limit we care for should be raised to 21, or even 25, as some CPHM programs had been doing prepandemic.
Along with the expansion of age limits, there are many other areas of opportunity highlighted within the PERC survey. These include expanding coverage within pediatric ICUs, EDs, and urgent care areas, along with coverage of well newborns that were previously covered by community pediatricians. Also, the increase of mental health admissions is another area where PHM programs might expand their services.
While I hope the financial stressors improve, hope is not a plan and therefore we need to think and prepare for what the post-COVID future may look like. Some have predicted a rebound pediatric respiratory surge next year as the masks come off and children return to in-person learning and daycare. This may be true, but we would be foolish not to use lessons from the pandemic as well as the past to consider options in our toolkit to become more financially stable. POPCoRN, as well as the American Academy of Pediatrics’ listserv and subcommittees, have been a source of collaboration and shared knowledge during a time when we have needed to quickly respond to ever-changing information. These networks and information sharing should be leveraged once the dust settles for us to prepare for future challenges.
New innovations may arise as we look at how we address the growing need for mental health services and incorporate new procedures, like point of care ultrasound. As Charles Darwin said: “It is not the strongest of the species that survives nor the most intelligent that survives. It is the one that is most adaptable to change.” It is time for us to evolve.
Dr. Dias is a clinical associate professor of pediatrics at Yale University, New Haven, Conn., in the division of pediatric hospital medicine. She has practiced community pediatric hospital medicine for over 21 years in New Jersey, Pennsylvania, and Connecticut. She is the chair of the Education Working Group for the AAP’s section on hospital medicine’s subcommittee on community hospitalists as well as the cochair of the Community Hospital Operations Group of the POPCoRN network.
Risk for erectile dysfunction sixfold higher in men with COVID-19
COVID-19 increases the risk of developing erectile dysfunction (ED) by nearly sixfold, according to data from the first study to investigate the association between ED and COVID-19 in young men in a real-life setting.
Men with ED are more than five times more likely to have COVID-19 (odds ratio, 5.27).
For men with a history of COVID-19, the odds ratio of developing ED was 5.66. The strength of the association remained after adjusting for factors considered to affect ED.
The study, which was led by Emmanuele A. Jannini, MD, professor of endocrinology and medical sexology, University of Rome Tor Vergata, was published on March 20 in Andrology.
‘Mask up to keep it up’
ED can be both a short-term and a long-term complication of COVID-19, Dr. Jannini suggests.
“When offered, men should have the COVID vaccination. It also gives a whole new meaning to wearing the mask – mask up to keep it up,” he said. “It could possibly have the added benefit of preventing sexual dysfunction.”
He points out that older age, diabetes, high body mass index, and smoking increase the risk of contracting COVID-19.
“These are the same as risk factors for ED. Results of our study agree with the pathophysiological mechanisms linking ED, endothelial dysfunction, and COVID-19. Basically, endothelial dysfunction is common in both conditions [COVID-10 and ED].
“We would like to find some sort of biomarker of endothelial dysfunction post COVID, because it seems that there are many sequelae that coexist for a long time after infection,” added Dr. Jannini. “Asking a patient if they have ED after COVID might provide a measure of systemic wellness.”
Allan Pacey, MD, professor of andrology at the University of Sheffield (England), welcomed the research, noting, “This seems to be a well-conducted study. However, at the moment, the relationship is just a correlation, and it might be that some of the comorbidities that increased the men’s chances of getting a significant COVID-19 infection may have also independently increased their chances of erectile dysfunction.
“But the authors offer a plausible mechanism by which COVID-19 may impact directly on erectile function,” agrees Dr. Pacey. However, “There’s more work to be done,” he said. “I’d also argue it’s a good reason for men to wear a mask, practice social distancing, and take the vaccine when it’s offered to them.”
Urologist John Mulhall, MD, from Memorial Sloan Kettering Cancer Center, New York, remarked, “It was a highly preliminary study, but the data are suggestive of a potential link between COVID-19 infection and ED.
“However, it raises enough questions such that further large, more long-term analyses are required to define causation. Future studies assessing testosterone levels and erectile hemodynamics will be needed to provide definite evidence of a causative link,» he stressed.
Erectile problems a ‘hallmark’ of systemic endothelial dysfunction
Prior research has suggested that asymptomatic COVID-19 could be associated with subclinical microvascular involvement with long-term cardiovascular effects.
“Indeed, COVID-19 is by all means an endothelial disease, in which systemic manifestations ... can potentially be due to alterations in the endothelial thrombotic/fibrinolytic balance,” emphasized Dr. Jannini. “In addition, endothelial cells express many of the cofactors used by SARS-CoV-2 to invade host cells.
“Erectile dysfunction has often been considered a hallmark of endothelial dysfunction, and as such, a potential association between ED and COVID-19 has also been postulated and underpinned the investigation in this study,” he explained.
The study was predicated on the fact that ED is often considered a clinical marker of impaired overall health status, which often features cardiovascular events at an early age. It aimed to investigate the bidirectional relationship between COVID-19 and ED. It asked whether ED could be a risk factor for contracting COVID-19 and whether having COVID-19 predisposes to developing ED.
“This would possibly suggest that men with ED, due to the underlying conditions which impair erectile response, could also be more susceptible to contracting COVID-19,” said Dr. Jannini.
Data were drawn from the Sex@COVID online survey, which was conducted from April 7 to May 4, 2020, in Italy. The survey included 6,821 participants aged 18 years or older (4,177 women; 2,644 men; mean age, 32.83 ± 11.24 years). Participants were stratified on the basis of marital status and sexual activity during lockdown. From these participants, 985 sexually active men were identified, among whom 25 (2.54%) reported having tested positive for COVID-19. These persons were matched with 75 COVID-19–negative men using propensity score matching in a 1:3 ratio.
The researchers used standardized psychometric tools to measure the effects of lockdown and social distancing on the intrapsychic, relational, and sexual health of the participants.
Erectile function was measured with the International Index of Erectile Function or the Sexual Health Inventory for Men, which are often used in clinical settings. In light of the two-way interaction between sexual activity and psychological well-being, results were adjusted for any influence of anxiety and depression, which were measured with recognized scales for use in patients with a history of COVID-19.
Results showed that the prevalence of ED was significantly higher among men who self-reported a history of COVID-19, compared with a matching COVID-negative population (28% vs. 9.33%; P = .027).
After adjusting for variables that are considered to have a bearing on the development of ED, such as psychological status, age, and BMI, the odds ratio for developing ED after having had COVID-19 was 5.66 (95% confidence interval, 1.50-24.01).
Similarly, after adjusting for age and BMI, men with ED were more likely to have COVID‐19 (OR, 5.27; 95% CI, 1.49-20.09).
The authors note that persons who experience “a sudden onset or worsening of ED might also consider precautionary quarantine or nasopharyngeal swab, as COVID‐19 might act as a potential initiating trigger for the onset of erectile impairment, or an aggravating factor for its progression to more severe forms.”
Similarly, patients who have ED “should consider their erectile impairment as a sign of possible underlying conditions that could increase the likelihood of suffering from COVID‐19,” they write.
Dr. Mulhall highlighted several limitations of the study, including its retrospective nature, recall bias associated with the use of online questionnaires, and the inclusion of COVID‐19 diagnoses that were based on the response to the survey rather than on testing with nasopharyngeal swabs. In addition, comorbidity data were incomplete, and there was no indication of duration after COVID-19 infection, the severity of COVID-19, or the severity of ED.
The authors have disclosed no relevant financial relationships. Dr. Pacey is chairman of the advisory committee of the U.K. National External Quality Assurance Schemes in Andrology, editor-in-chief of Human Fertility, trustee of the Progress Educational Trust, and trustee of the British Fertility Society (all unpaid). Dr. Mulhall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 increases the risk of developing erectile dysfunction (ED) by nearly sixfold, according to data from the first study to investigate the association between ED and COVID-19 in young men in a real-life setting.
Men with ED are more than five times more likely to have COVID-19 (odds ratio, 5.27).
For men with a history of COVID-19, the odds ratio of developing ED was 5.66. The strength of the association remained after adjusting for factors considered to affect ED.
The study, which was led by Emmanuele A. Jannini, MD, professor of endocrinology and medical sexology, University of Rome Tor Vergata, was published on March 20 in Andrology.
‘Mask up to keep it up’
ED can be both a short-term and a long-term complication of COVID-19, Dr. Jannini suggests.
“When offered, men should have the COVID vaccination. It also gives a whole new meaning to wearing the mask – mask up to keep it up,” he said. “It could possibly have the added benefit of preventing sexual dysfunction.”
He points out that older age, diabetes, high body mass index, and smoking increase the risk of contracting COVID-19.
“These are the same as risk factors for ED. Results of our study agree with the pathophysiological mechanisms linking ED, endothelial dysfunction, and COVID-19. Basically, endothelial dysfunction is common in both conditions [COVID-10 and ED].
“We would like to find some sort of biomarker of endothelial dysfunction post COVID, because it seems that there are many sequelae that coexist for a long time after infection,” added Dr. Jannini. “Asking a patient if they have ED after COVID might provide a measure of systemic wellness.”
Allan Pacey, MD, professor of andrology at the University of Sheffield (England), welcomed the research, noting, “This seems to be a well-conducted study. However, at the moment, the relationship is just a correlation, and it might be that some of the comorbidities that increased the men’s chances of getting a significant COVID-19 infection may have also independently increased their chances of erectile dysfunction.
“But the authors offer a plausible mechanism by which COVID-19 may impact directly on erectile function,” agrees Dr. Pacey. However, “There’s more work to be done,” he said. “I’d also argue it’s a good reason for men to wear a mask, practice social distancing, and take the vaccine when it’s offered to them.”
Urologist John Mulhall, MD, from Memorial Sloan Kettering Cancer Center, New York, remarked, “It was a highly preliminary study, but the data are suggestive of a potential link between COVID-19 infection and ED.
“However, it raises enough questions such that further large, more long-term analyses are required to define causation. Future studies assessing testosterone levels and erectile hemodynamics will be needed to provide definite evidence of a causative link,» he stressed.
Erectile problems a ‘hallmark’ of systemic endothelial dysfunction
Prior research has suggested that asymptomatic COVID-19 could be associated with subclinical microvascular involvement with long-term cardiovascular effects.
“Indeed, COVID-19 is by all means an endothelial disease, in which systemic manifestations ... can potentially be due to alterations in the endothelial thrombotic/fibrinolytic balance,” emphasized Dr. Jannini. “In addition, endothelial cells express many of the cofactors used by SARS-CoV-2 to invade host cells.
“Erectile dysfunction has often been considered a hallmark of endothelial dysfunction, and as such, a potential association between ED and COVID-19 has also been postulated and underpinned the investigation in this study,” he explained.
The study was predicated on the fact that ED is often considered a clinical marker of impaired overall health status, which often features cardiovascular events at an early age. It aimed to investigate the bidirectional relationship between COVID-19 and ED. It asked whether ED could be a risk factor for contracting COVID-19 and whether having COVID-19 predisposes to developing ED.
“This would possibly suggest that men with ED, due to the underlying conditions which impair erectile response, could also be more susceptible to contracting COVID-19,” said Dr. Jannini.
Data were drawn from the Sex@COVID online survey, which was conducted from April 7 to May 4, 2020, in Italy. The survey included 6,821 participants aged 18 years or older (4,177 women; 2,644 men; mean age, 32.83 ± 11.24 years). Participants were stratified on the basis of marital status and sexual activity during lockdown. From these participants, 985 sexually active men were identified, among whom 25 (2.54%) reported having tested positive for COVID-19. These persons were matched with 75 COVID-19–negative men using propensity score matching in a 1:3 ratio.
The researchers used standardized psychometric tools to measure the effects of lockdown and social distancing on the intrapsychic, relational, and sexual health of the participants.
Erectile function was measured with the International Index of Erectile Function or the Sexual Health Inventory for Men, which are often used in clinical settings. In light of the two-way interaction between sexual activity and psychological well-being, results were adjusted for any influence of anxiety and depression, which were measured with recognized scales for use in patients with a history of COVID-19.
Results showed that the prevalence of ED was significantly higher among men who self-reported a history of COVID-19, compared with a matching COVID-negative population (28% vs. 9.33%; P = .027).
After adjusting for variables that are considered to have a bearing on the development of ED, such as psychological status, age, and BMI, the odds ratio for developing ED after having had COVID-19 was 5.66 (95% confidence interval, 1.50-24.01).
Similarly, after adjusting for age and BMI, men with ED were more likely to have COVID‐19 (OR, 5.27; 95% CI, 1.49-20.09).
The authors note that persons who experience “a sudden onset or worsening of ED might also consider precautionary quarantine or nasopharyngeal swab, as COVID‐19 might act as a potential initiating trigger for the onset of erectile impairment, or an aggravating factor for its progression to more severe forms.”
Similarly, patients who have ED “should consider their erectile impairment as a sign of possible underlying conditions that could increase the likelihood of suffering from COVID‐19,” they write.
Dr. Mulhall highlighted several limitations of the study, including its retrospective nature, recall bias associated with the use of online questionnaires, and the inclusion of COVID‐19 diagnoses that were based on the response to the survey rather than on testing with nasopharyngeal swabs. In addition, comorbidity data were incomplete, and there was no indication of duration after COVID-19 infection, the severity of COVID-19, or the severity of ED.
The authors have disclosed no relevant financial relationships. Dr. Pacey is chairman of the advisory committee of the U.K. National External Quality Assurance Schemes in Andrology, editor-in-chief of Human Fertility, trustee of the Progress Educational Trust, and trustee of the British Fertility Society (all unpaid). Dr. Mulhall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 increases the risk of developing erectile dysfunction (ED) by nearly sixfold, according to data from the first study to investigate the association between ED and COVID-19 in young men in a real-life setting.
Men with ED are more than five times more likely to have COVID-19 (odds ratio, 5.27).
For men with a history of COVID-19, the odds ratio of developing ED was 5.66. The strength of the association remained after adjusting for factors considered to affect ED.
The study, which was led by Emmanuele A. Jannini, MD, professor of endocrinology and medical sexology, University of Rome Tor Vergata, was published on March 20 in Andrology.
‘Mask up to keep it up’
ED can be both a short-term and a long-term complication of COVID-19, Dr. Jannini suggests.
“When offered, men should have the COVID vaccination. It also gives a whole new meaning to wearing the mask – mask up to keep it up,” he said. “It could possibly have the added benefit of preventing sexual dysfunction.”
He points out that older age, diabetes, high body mass index, and smoking increase the risk of contracting COVID-19.
“These are the same as risk factors for ED. Results of our study agree with the pathophysiological mechanisms linking ED, endothelial dysfunction, and COVID-19. Basically, endothelial dysfunction is common in both conditions [COVID-10 and ED].
“We would like to find some sort of biomarker of endothelial dysfunction post COVID, because it seems that there are many sequelae that coexist for a long time after infection,” added Dr. Jannini. “Asking a patient if they have ED after COVID might provide a measure of systemic wellness.”
Allan Pacey, MD, professor of andrology at the University of Sheffield (England), welcomed the research, noting, “This seems to be a well-conducted study. However, at the moment, the relationship is just a correlation, and it might be that some of the comorbidities that increased the men’s chances of getting a significant COVID-19 infection may have also independently increased their chances of erectile dysfunction.
“But the authors offer a plausible mechanism by which COVID-19 may impact directly on erectile function,” agrees Dr. Pacey. However, “There’s more work to be done,” he said. “I’d also argue it’s a good reason for men to wear a mask, practice social distancing, and take the vaccine when it’s offered to them.”
Urologist John Mulhall, MD, from Memorial Sloan Kettering Cancer Center, New York, remarked, “It was a highly preliminary study, but the data are suggestive of a potential link between COVID-19 infection and ED.
“However, it raises enough questions such that further large, more long-term analyses are required to define causation. Future studies assessing testosterone levels and erectile hemodynamics will be needed to provide definite evidence of a causative link,» he stressed.
Erectile problems a ‘hallmark’ of systemic endothelial dysfunction
Prior research has suggested that asymptomatic COVID-19 could be associated with subclinical microvascular involvement with long-term cardiovascular effects.
“Indeed, COVID-19 is by all means an endothelial disease, in which systemic manifestations ... can potentially be due to alterations in the endothelial thrombotic/fibrinolytic balance,” emphasized Dr. Jannini. “In addition, endothelial cells express many of the cofactors used by SARS-CoV-2 to invade host cells.
“Erectile dysfunction has often been considered a hallmark of endothelial dysfunction, and as such, a potential association between ED and COVID-19 has also been postulated and underpinned the investigation in this study,” he explained.
The study was predicated on the fact that ED is often considered a clinical marker of impaired overall health status, which often features cardiovascular events at an early age. It aimed to investigate the bidirectional relationship between COVID-19 and ED. It asked whether ED could be a risk factor for contracting COVID-19 and whether having COVID-19 predisposes to developing ED.
“This would possibly suggest that men with ED, due to the underlying conditions which impair erectile response, could also be more susceptible to contracting COVID-19,” said Dr. Jannini.
Data were drawn from the Sex@COVID online survey, which was conducted from April 7 to May 4, 2020, in Italy. The survey included 6,821 participants aged 18 years or older (4,177 women; 2,644 men; mean age, 32.83 ± 11.24 years). Participants were stratified on the basis of marital status and sexual activity during lockdown. From these participants, 985 sexually active men were identified, among whom 25 (2.54%) reported having tested positive for COVID-19. These persons were matched with 75 COVID-19–negative men using propensity score matching in a 1:3 ratio.
The researchers used standardized psychometric tools to measure the effects of lockdown and social distancing on the intrapsychic, relational, and sexual health of the participants.
Erectile function was measured with the International Index of Erectile Function or the Sexual Health Inventory for Men, which are often used in clinical settings. In light of the two-way interaction between sexual activity and psychological well-being, results were adjusted for any influence of anxiety and depression, which were measured with recognized scales for use in patients with a history of COVID-19.
Results showed that the prevalence of ED was significantly higher among men who self-reported a history of COVID-19, compared with a matching COVID-negative population (28% vs. 9.33%; P = .027).
After adjusting for variables that are considered to have a bearing on the development of ED, such as psychological status, age, and BMI, the odds ratio for developing ED after having had COVID-19 was 5.66 (95% confidence interval, 1.50-24.01).
Similarly, after adjusting for age and BMI, men with ED were more likely to have COVID‐19 (OR, 5.27; 95% CI, 1.49-20.09).
The authors note that persons who experience “a sudden onset or worsening of ED might also consider precautionary quarantine or nasopharyngeal swab, as COVID‐19 might act as a potential initiating trigger for the onset of erectile impairment, or an aggravating factor for its progression to more severe forms.”
Similarly, patients who have ED “should consider their erectile impairment as a sign of possible underlying conditions that could increase the likelihood of suffering from COVID‐19,” they write.
Dr. Mulhall highlighted several limitations of the study, including its retrospective nature, recall bias associated with the use of online questionnaires, and the inclusion of COVID‐19 diagnoses that were based on the response to the survey rather than on testing with nasopharyngeal swabs. In addition, comorbidity data were incomplete, and there was no indication of duration after COVID-19 infection, the severity of COVID-19, or the severity of ED.
The authors have disclosed no relevant financial relationships. Dr. Pacey is chairman of the advisory committee of the U.K. National External Quality Assurance Schemes in Andrology, editor-in-chief of Human Fertility, trustee of the Progress Educational Trust, and trustee of the British Fertility Society (all unpaid). Dr. Mulhall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiovascular risks elevated in transgender youth
Cardiovascular and metabolic risk factors are increased among transgender youths, compared with youths who are not transgender. Elevations in lipid levels and body mass index (BMI) also occur in adult transgender patients, new research shows.
“This is the first study of its size in the United States of which we are aware that looks at the odds of youth with a diagnosis of gender dysphoria having medical diagnoses that relate to overall metabolic and cardiovascular health,” first author Anna Valentine, MD, of Children’s Hospital Colorado, Aurora, said in a press statement.
Although previous studies have shown that among transgender adults, BMI is higher and there is an increased risk for cardiovascular events, such as stroke or heart attack, compared with nontransgender people, research on adolescent transgender patients has been lacking.
With a recent survey showing that nearly 2% of adolescents identify as transgender, interest in health outcomes among younger patients is high.
To investigate, Dr. Valentine, and colleagues evaluated data from the PEDSnet pediatric database on 4,177 youths who had received a diagnosis of gender dysphoria. The participants had been enrolled at six sites from 2009 to 2019. The researchers compared these patients in a ratio of 1:4 with 16,664 control persons who had not been diagnosed with gender dysphoria. They reported their findings as a poster at the annual meeting of the Endocrine Society.
For the propensity-score analysis, participants were matched according to year of birth, age at last visit, site, race, ethnicity, insurance status, and duration in the database.
In both the transgender and control groups, about 66% were female at birth, 73% were White, and 9% Hispanic. For both groups, the average age was 16.2 years at the last visit. The average duration in the database was 7 years.
Study didn’t distinguish between those receiving and those not receiving gender-affirming hormones
In the retrospective study, among those who identified as transgender, the rates of diagnoses of dyslipidemia (odds ratio, 1.6; P < .0001) and metabolic syndrome (OR, 1.9; P = .0086) were significantly higher, compared with those without gender dysphoria.
Among the transgender male patients (born female) but not transgender female patients (born male), rates of diagnoses of overweight/obesity (OR, 1.7; P < .0001) and polycystic ovary syndrome were higher (OR, 1.9, P = .0006), compared with controls.
Gender-affirming hormone therapy, such as with testosterone or estradiol, is among the suspected culprits for the cardiovascular effects. However, importantly, this study did not differentiate between patients who had received estradiol or testosterone for gender affirmation and those who had not, Dr. Valentine said.
“We don’t know [whether gender-affirming hormone therapy is a cause], as we have not looked at this yet,” she said in an interview. “We are looking at that in our next analyses and will be including that in our future publication.
“We’ll also be looking at the relationship between having overweight/obesity and the other diagnoses that influence cardiovascular health (high blood pressure, liver dysfunction, and abnormal cholesterol), as that could certainly be playing a role as well,” she said.
For many transgender patients, gender-affirming hormone therapy is lifelong. One question that needs to be evaluated concerns whether the dose of such therapy has a role on cardiovascular effects and if so, whether adjustments could be made without compromising the therapeutic effect, Dr. Valentine noted.
“This is an important question, and future research is needed to evaluate whether doses [of gender-affirming hormones] are related to cardiometabolic outcomes,” she said.
Potential confounders in the study include the fact that rates of overweight and obesity are higher among youths with gender dysphoria. This can in itself can increase the risk for other disorders, Dr. Valentine noted.
Furthermore, rates of mental health comorbidities are higher among youths with gender dysphoria. One consequence of this may be that they engage in less physical activity, she said.
Hormone therapy, health care disparities, or both could explain risk
In commenting on the study, Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery, the Mount Sinai Health System, New York, said that although similar cardiovascular effects are known to occur in transgender adults as well, they may or may not be hormone related. Other factors can increase the risk.
“With transgender adults, any differences in lipids or cardiac risk factors relative to cisgender people might be attributable either to hormone therapy or to health care disparities,” he said in an interview.
“The data are mixed. It may be that most differences relate to lack of access to care and to mistreatment by society,” he said. “Even studies that focus on hormones see a worsened situation for trans women versus trans men.”
Other recent research that shows potential cardiovascular effects among adult transgender men includes a study of more than 1,000 transgender men (born female) who received testosterone. That study, which was also presented at the ENDO meeting and was reported by this news organization, found an increased risk for high hematocrit levels, which could lead to a thrombotic event.
However, a study published in Pediatrics, which was also reported by this news organization, that included 611 transgender youths who had taken gender-affirming hormone therapy for more than a year found no increased risk for thrombosis, even in the presence of thrombosis risk factors, including obesity, tobacco use, and family history of thrombosis. However, the senior author of that study pointed out that the duration of follow-up in that study was relatively short, which may have been why they did not find an increased risk for thrombosis.
Dr. Safer noted that transgender youths and adults alike face a host of cultural factors that could play a role in increased cardiovascular risks.
“For adults, the major candidate explanations for worse BMI and cardiac risk factors are societal mistreatment, and for trans women specifically, progestins. For youth, the major candidate explanations are societal mistreatment and lack of access to athletics,” he said.
The authors and Dr. Safer disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiovascular and metabolic risk factors are increased among transgender youths, compared with youths who are not transgender. Elevations in lipid levels and body mass index (BMI) also occur in adult transgender patients, new research shows.
“This is the first study of its size in the United States of which we are aware that looks at the odds of youth with a diagnosis of gender dysphoria having medical diagnoses that relate to overall metabolic and cardiovascular health,” first author Anna Valentine, MD, of Children’s Hospital Colorado, Aurora, said in a press statement.
Although previous studies have shown that among transgender adults, BMI is higher and there is an increased risk for cardiovascular events, such as stroke or heart attack, compared with nontransgender people, research on adolescent transgender patients has been lacking.
With a recent survey showing that nearly 2% of adolescents identify as transgender, interest in health outcomes among younger patients is high.
To investigate, Dr. Valentine, and colleagues evaluated data from the PEDSnet pediatric database on 4,177 youths who had received a diagnosis of gender dysphoria. The participants had been enrolled at six sites from 2009 to 2019. The researchers compared these patients in a ratio of 1:4 with 16,664 control persons who had not been diagnosed with gender dysphoria. They reported their findings as a poster at the annual meeting of the Endocrine Society.
For the propensity-score analysis, participants were matched according to year of birth, age at last visit, site, race, ethnicity, insurance status, and duration in the database.
In both the transgender and control groups, about 66% were female at birth, 73% were White, and 9% Hispanic. For both groups, the average age was 16.2 years at the last visit. The average duration in the database was 7 years.
Study didn’t distinguish between those receiving and those not receiving gender-affirming hormones
In the retrospective study, among those who identified as transgender, the rates of diagnoses of dyslipidemia (odds ratio, 1.6; P < .0001) and metabolic syndrome (OR, 1.9; P = .0086) were significantly higher, compared with those without gender dysphoria.
Among the transgender male patients (born female) but not transgender female patients (born male), rates of diagnoses of overweight/obesity (OR, 1.7; P < .0001) and polycystic ovary syndrome were higher (OR, 1.9, P = .0006), compared with controls.
Gender-affirming hormone therapy, such as with testosterone or estradiol, is among the suspected culprits for the cardiovascular effects. However, importantly, this study did not differentiate between patients who had received estradiol or testosterone for gender affirmation and those who had not, Dr. Valentine said.
“We don’t know [whether gender-affirming hormone therapy is a cause], as we have not looked at this yet,” she said in an interview. “We are looking at that in our next analyses and will be including that in our future publication.
“We’ll also be looking at the relationship between having overweight/obesity and the other diagnoses that influence cardiovascular health (high blood pressure, liver dysfunction, and abnormal cholesterol), as that could certainly be playing a role as well,” she said.
For many transgender patients, gender-affirming hormone therapy is lifelong. One question that needs to be evaluated concerns whether the dose of such therapy has a role on cardiovascular effects and if so, whether adjustments could be made without compromising the therapeutic effect, Dr. Valentine noted.
“This is an important question, and future research is needed to evaluate whether doses [of gender-affirming hormones] are related to cardiometabolic outcomes,” she said.
Potential confounders in the study include the fact that rates of overweight and obesity are higher among youths with gender dysphoria. This can in itself can increase the risk for other disorders, Dr. Valentine noted.
Furthermore, rates of mental health comorbidities are higher among youths with gender dysphoria. One consequence of this may be that they engage in less physical activity, she said.
Hormone therapy, health care disparities, or both could explain risk
In commenting on the study, Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery, the Mount Sinai Health System, New York, said that although similar cardiovascular effects are known to occur in transgender adults as well, they may or may not be hormone related. Other factors can increase the risk.
“With transgender adults, any differences in lipids or cardiac risk factors relative to cisgender people might be attributable either to hormone therapy or to health care disparities,” he said in an interview.
“The data are mixed. It may be that most differences relate to lack of access to care and to mistreatment by society,” he said. “Even studies that focus on hormones see a worsened situation for trans women versus trans men.”
Other recent research that shows potential cardiovascular effects among adult transgender men includes a study of more than 1,000 transgender men (born female) who received testosterone. That study, which was also presented at the ENDO meeting and was reported by this news organization, found an increased risk for high hematocrit levels, which could lead to a thrombotic event.
However, a study published in Pediatrics, which was also reported by this news organization, that included 611 transgender youths who had taken gender-affirming hormone therapy for more than a year found no increased risk for thrombosis, even in the presence of thrombosis risk factors, including obesity, tobacco use, and family history of thrombosis. However, the senior author of that study pointed out that the duration of follow-up in that study was relatively short, which may have been why they did not find an increased risk for thrombosis.
Dr. Safer noted that transgender youths and adults alike face a host of cultural factors that could play a role in increased cardiovascular risks.
“For adults, the major candidate explanations for worse BMI and cardiac risk factors are societal mistreatment, and for trans women specifically, progestins. For youth, the major candidate explanations are societal mistreatment and lack of access to athletics,” he said.
The authors and Dr. Safer disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiovascular and metabolic risk factors are increased among transgender youths, compared with youths who are not transgender. Elevations in lipid levels and body mass index (BMI) also occur in adult transgender patients, new research shows.
“This is the first study of its size in the United States of which we are aware that looks at the odds of youth with a diagnosis of gender dysphoria having medical diagnoses that relate to overall metabolic and cardiovascular health,” first author Anna Valentine, MD, of Children’s Hospital Colorado, Aurora, said in a press statement.
Although previous studies have shown that among transgender adults, BMI is higher and there is an increased risk for cardiovascular events, such as stroke or heart attack, compared with nontransgender people, research on adolescent transgender patients has been lacking.
With a recent survey showing that nearly 2% of adolescents identify as transgender, interest in health outcomes among younger patients is high.
To investigate, Dr. Valentine, and colleagues evaluated data from the PEDSnet pediatric database on 4,177 youths who had received a diagnosis of gender dysphoria. The participants had been enrolled at six sites from 2009 to 2019. The researchers compared these patients in a ratio of 1:4 with 16,664 control persons who had not been diagnosed with gender dysphoria. They reported their findings as a poster at the annual meeting of the Endocrine Society.
For the propensity-score analysis, participants were matched according to year of birth, age at last visit, site, race, ethnicity, insurance status, and duration in the database.
In both the transgender and control groups, about 66% were female at birth, 73% were White, and 9% Hispanic. For both groups, the average age was 16.2 years at the last visit. The average duration in the database was 7 years.
Study didn’t distinguish between those receiving and those not receiving gender-affirming hormones
In the retrospective study, among those who identified as transgender, the rates of diagnoses of dyslipidemia (odds ratio, 1.6; P < .0001) and metabolic syndrome (OR, 1.9; P = .0086) were significantly higher, compared with those without gender dysphoria.
Among the transgender male patients (born female) but not transgender female patients (born male), rates of diagnoses of overweight/obesity (OR, 1.7; P < .0001) and polycystic ovary syndrome were higher (OR, 1.9, P = .0006), compared with controls.
Gender-affirming hormone therapy, such as with testosterone or estradiol, is among the suspected culprits for the cardiovascular effects. However, importantly, this study did not differentiate between patients who had received estradiol or testosterone for gender affirmation and those who had not, Dr. Valentine said.
“We don’t know [whether gender-affirming hormone therapy is a cause], as we have not looked at this yet,” she said in an interview. “We are looking at that in our next analyses and will be including that in our future publication.
“We’ll also be looking at the relationship between having overweight/obesity and the other diagnoses that influence cardiovascular health (high blood pressure, liver dysfunction, and abnormal cholesterol), as that could certainly be playing a role as well,” she said.
For many transgender patients, gender-affirming hormone therapy is lifelong. One question that needs to be evaluated concerns whether the dose of such therapy has a role on cardiovascular effects and if so, whether adjustments could be made without compromising the therapeutic effect, Dr. Valentine noted.
“This is an important question, and future research is needed to evaluate whether doses [of gender-affirming hormones] are related to cardiometabolic outcomes,” she said.
Potential confounders in the study include the fact that rates of overweight and obesity are higher among youths with gender dysphoria. This can in itself can increase the risk for other disorders, Dr. Valentine noted.
Furthermore, rates of mental health comorbidities are higher among youths with gender dysphoria. One consequence of this may be that they engage in less physical activity, she said.
Hormone therapy, health care disparities, or both could explain risk
In commenting on the study, Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery, the Mount Sinai Health System, New York, said that although similar cardiovascular effects are known to occur in transgender adults as well, they may or may not be hormone related. Other factors can increase the risk.
“With transgender adults, any differences in lipids or cardiac risk factors relative to cisgender people might be attributable either to hormone therapy or to health care disparities,” he said in an interview.
“The data are mixed. It may be that most differences relate to lack of access to care and to mistreatment by society,” he said. “Even studies that focus on hormones see a worsened situation for trans women versus trans men.”
Other recent research that shows potential cardiovascular effects among adult transgender men includes a study of more than 1,000 transgender men (born female) who received testosterone. That study, which was also presented at the ENDO meeting and was reported by this news organization, found an increased risk for high hematocrit levels, which could lead to a thrombotic event.
However, a study published in Pediatrics, which was also reported by this news organization, that included 611 transgender youths who had taken gender-affirming hormone therapy for more than a year found no increased risk for thrombosis, even in the presence of thrombosis risk factors, including obesity, tobacco use, and family history of thrombosis. However, the senior author of that study pointed out that the duration of follow-up in that study was relatively short, which may have been why they did not find an increased risk for thrombosis.
Dr. Safer noted that transgender youths and adults alike face a host of cultural factors that could play a role in increased cardiovascular risks.
“For adults, the major candidate explanations for worse BMI and cardiac risk factors are societal mistreatment, and for trans women specifically, progestins. For youth, the major candidate explanations are societal mistreatment and lack of access to athletics,” he said.
The authors and Dr. Safer disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New guidelines on antibiotic prescribing focus on shorter courses
An antibiotic course of 5 days is usually just as effective as longer courses but with fewer side effects and decreased overall antibiotic exposure for a number of common bacterial conditions, according to new clinical guidelines published by the American College of Physicians.
The guidelines focus on treatment of uncomplicated cases involving pneumonia, urinary tract infections (UTIs), cellulitis, chronic obstructive pulmonary disease (COPD) exacerbations, and acute bronchitis. The goal of the guidelines is to continue improving antibiotic stewardship given the increasing threat of antibiotic resistance and the adverse effects of antibiotics.
“Any use of antibiotics (including necessary use) has downstream effects outside of treating infection,” Dawn Nolt, MD, MPH, a professor of pediatric infection disease at Oregon Health & Science University, Portland, said in an interview. Dr. Nolt was not involved in developing these guidelines. “Undesirable outcomes include allergic reactions, diarrhea, and antibiotic-resistant bacteria. When we reduce unnecessary antibiotic, we reduce undesirable outcomes,” she said.
According to background information in the paper, 1 in 10 patients receives an antibiotic prescription during visits, yet nearly a third of these (30%) are unnecessary and last too long, especially for sinusitis and bronchitis. Meanwhile, overuse of antibiotics, particularly broad-spectrum ones, leads to resistance and adverse effects in up to 20% of patients.
“Prescribing practices can vary based on the type of provider, the setting where the antibiotic is being prescribed, what geographic area you are looking at, the medical reason for which the antibiotic is being prescribed, the actual germ being targeted, and the type of patient,” Dr. Nolt said. “But this variability can be reduced when prescribing providers are aware and follow best practice standards as through this article.”
The new ACP guidelines are a distillation of recommendations from preexisting infectious disease organizations, Dr. Nolt said, but aimed specifically at those practicing internal medicine.
“We define appropriate antibiotic use as prescribing the right antibiotic at the right dose for the right duration for a specific condition,” Rachael A. Lee, MD, MSPH, of the University of Alabama at Birmingham, and colleagues wrote in the article detailing the new guidelines. “Despite evidence and guidelines supporting shorter durations of antibiotic use, many physicians do not prescribe short-course therapy, frequently defaulting to 10-day courses regardless of the condition.”
The reasons for this default response vary. Though some clinicians prescribe longer courses specifically to prevent antibiotic resistance, no evidence shows that continuing to take antibiotics after symptoms have resolved actually reduces likelihood of resistance, the authors noted.
“In fact, resistance is a documented side effect of prolonged antibiotic use due to natural selection pressure,” they wrote.
Another common reason is habit.
“This was the ‘conventional wisdom’ for so long, just trying to make sure all bacteria causing the infection were completely eradicated, with no stragglers that had been exposed to the antibiotic but were not gone and now could evolve into resistant organisms,” Jacqueline W. Fincher, MD, a primary care physician and president of the ACP, said in an interview. “While antibiotic stewardship has been very important for over a decade, we now have more recent head-to-head studies/data showing that, in these four conditions, shorter courses of treatment are just as efficacious with less side effects and adverse events.”
The researchers reviewed all existing clinical guidelines related to bronchitis with COPD exacerbations, community-acquired pneumonia, UTIs, and cellulitis, as well as any other relevant studies in the literature. Although they did not conduct a formal systematic review, they compiled the guidelines specifically for all internists, family physicians and other clinicians caring for patients with these conditions.
“Although most patients with these infections will be seen in the outpatient setting, these best-practice advice statements also apply to patients who present in the inpatient setting,” the authors wrote. They also note the importance of ensuring the patient has the correct diagnosis and appropriate corresponding antibiotic prescription. “If a patient is not improving with appropriate antibiotics, it is important for the clinician to reassess for other causes of symptoms rather than defaulting to a longer duration of antibiotic therapy,” they wrote, calling a longer course “the exception and not the rule.”
Acute bronchitis with COPD exacerbations
Antibiotic treatment for COPD exacerbations and acute uncomplicated bronchitis with signs of a bacterial infection should last no longer than 5 days. The authors define this condition as an acute respiratory infection with a normal chest x-ray, most often caused by a virus. Although patients with bronchitis do not automatically need antibiotics if there’s no evidence of pneumonia, the authors did advise antibiotics in cases involving COPD and a high likelihood of bacterial infection. Clinicians should base their choice of antibiotics on the most common bacterial etiology: Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis. Ideal candidates for therapy may include aminopenicillin with clavulanic acid, a macrolide, or a tetracycline.
Community-acquired pneumonia
The initial course of antibiotics should be at least 5 days for pneumonia and only extended after considering validated evidence of the patient’s clinical stability, such as resuming normal vital signs, mental activity, and the ability to eat. Multiple randomized, controlled trials have shown no improved benefit from longer courses, though longer courses are linked to increased adverse events and mortality.
Again, antibiotics used should “cover common pathogens, such as S. pneumoniae, H. influenzae, Mycoplasma pneumoniae, and Staphylococcus aureus, and atypical pathogens, such as Legionella species,” the authors wrote. Options include “amoxicillin, doxycycline, or a macrolide for healthy adults or a beta-lactam with a macrolide or a respiratory fluoroquinolone in patients with comorbidities.”
UTIs: Uncomplicated cystitis and pyelonephritis
For women’s bacterial cystitis – 75% of which is caused by Escherichia coli – the guidelines recommend nitrofurantoin for 5 days, trimethoprim-sulfamethoxazole for 3 days, or fosfomycin as a single dose. For uncomplicated pyelonephritis in both men and women, clinicians can consider fluoroquinolones for 5-7 days or trimethoprim-sulfamethoxazole for 14 days, depending on antibiotic susceptibility.
This recommendation does not include UTIs in women who are pregnant or UTIs with other functional abnormalities present, such as obstruction. The authors also intentionally left out acute bacterial prostatitis because of its complexity and how long it can take to treat.
Cellulitis
MRSA, which has been increasing in prevalence, is a leading cause of skin and soft-tissue infections, such as necrotizing infections, cellulitis, and erysipelas. Unless the patient has penetrating trauma, evidence of MRSA infection elsewhere, injection drug use, nasal colonization of MRSA, or systemic inflammatory response syndrome, the guidelines recommend a 5- to 6-day course of cephalosporin, penicillin, or clindamycin, extended only if the infection has not improved in 5 days. Further research can narrow down the most appropriate treatment course.
This guidance does not apply to purulent cellulitis, such as conditions with abscesses, furuncles, or carbuncles that typically require incision and drainage.
Continuing to get the message out
Dr. Fincher emphasized the importance of continuing to disseminate messaging for clinicians about reducing unnecessary antibiotic use.
“In medicine we are constantly bombarded with new information. It is those patients and disease states that we see and treat every day that are especially important for us as physicians and other clinicians to keep our skills and knowledge base up to date when it comes to use of antibiotics,” Dr. Fincher said in an interview. “We just need to continue to educate and push out the data, guidelines, and recommendations.”
Dr. Nolt added that it’s important to emphasize how to translate these national recommendations into local practices since local guidance can also raise awareness and encourage local compliance.
Other strategies for reducing overuse of antibiotics “include restriction on antibiotics available at health care systems (formulary restriction), not allowing use of antibiotics unless there is discussion about the patient’s case (preauthorization), and reviewing cases of patients on antibiotics and advising on next steps (prospective audit and feedback),” she said.
The research was funded by the ACP. Dr. Lee has received personal fees from this news organization and Prime Education. Dr. Fincher owns stock in Johnson & Johnson and Procter and Gamble. Dr. Nolt and the article’s coauthors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An antibiotic course of 5 days is usually just as effective as longer courses but with fewer side effects and decreased overall antibiotic exposure for a number of common bacterial conditions, according to new clinical guidelines published by the American College of Physicians.
The guidelines focus on treatment of uncomplicated cases involving pneumonia, urinary tract infections (UTIs), cellulitis, chronic obstructive pulmonary disease (COPD) exacerbations, and acute bronchitis. The goal of the guidelines is to continue improving antibiotic stewardship given the increasing threat of antibiotic resistance and the adverse effects of antibiotics.
“Any use of antibiotics (including necessary use) has downstream effects outside of treating infection,” Dawn Nolt, MD, MPH, a professor of pediatric infection disease at Oregon Health & Science University, Portland, said in an interview. Dr. Nolt was not involved in developing these guidelines. “Undesirable outcomes include allergic reactions, diarrhea, and antibiotic-resistant bacteria. When we reduce unnecessary antibiotic, we reduce undesirable outcomes,” she said.
According to background information in the paper, 1 in 10 patients receives an antibiotic prescription during visits, yet nearly a third of these (30%) are unnecessary and last too long, especially for sinusitis and bronchitis. Meanwhile, overuse of antibiotics, particularly broad-spectrum ones, leads to resistance and adverse effects in up to 20% of patients.
“Prescribing practices can vary based on the type of provider, the setting where the antibiotic is being prescribed, what geographic area you are looking at, the medical reason for which the antibiotic is being prescribed, the actual germ being targeted, and the type of patient,” Dr. Nolt said. “But this variability can be reduced when prescribing providers are aware and follow best practice standards as through this article.”
The new ACP guidelines are a distillation of recommendations from preexisting infectious disease organizations, Dr. Nolt said, but aimed specifically at those practicing internal medicine.
“We define appropriate antibiotic use as prescribing the right antibiotic at the right dose for the right duration for a specific condition,” Rachael A. Lee, MD, MSPH, of the University of Alabama at Birmingham, and colleagues wrote in the article detailing the new guidelines. “Despite evidence and guidelines supporting shorter durations of antibiotic use, many physicians do not prescribe short-course therapy, frequently defaulting to 10-day courses regardless of the condition.”
The reasons for this default response vary. Though some clinicians prescribe longer courses specifically to prevent antibiotic resistance, no evidence shows that continuing to take antibiotics after symptoms have resolved actually reduces likelihood of resistance, the authors noted.
“In fact, resistance is a documented side effect of prolonged antibiotic use due to natural selection pressure,” they wrote.
Another common reason is habit.
“This was the ‘conventional wisdom’ for so long, just trying to make sure all bacteria causing the infection were completely eradicated, with no stragglers that had been exposed to the antibiotic but were not gone and now could evolve into resistant organisms,” Jacqueline W. Fincher, MD, a primary care physician and president of the ACP, said in an interview. “While antibiotic stewardship has been very important for over a decade, we now have more recent head-to-head studies/data showing that, in these four conditions, shorter courses of treatment are just as efficacious with less side effects and adverse events.”
The researchers reviewed all existing clinical guidelines related to bronchitis with COPD exacerbations, community-acquired pneumonia, UTIs, and cellulitis, as well as any other relevant studies in the literature. Although they did not conduct a formal systematic review, they compiled the guidelines specifically for all internists, family physicians and other clinicians caring for patients with these conditions.
“Although most patients with these infections will be seen in the outpatient setting, these best-practice advice statements also apply to patients who present in the inpatient setting,” the authors wrote. They also note the importance of ensuring the patient has the correct diagnosis and appropriate corresponding antibiotic prescription. “If a patient is not improving with appropriate antibiotics, it is important for the clinician to reassess for other causes of symptoms rather than defaulting to a longer duration of antibiotic therapy,” they wrote, calling a longer course “the exception and not the rule.”
Acute bronchitis with COPD exacerbations
Antibiotic treatment for COPD exacerbations and acute uncomplicated bronchitis with signs of a bacterial infection should last no longer than 5 days. The authors define this condition as an acute respiratory infection with a normal chest x-ray, most often caused by a virus. Although patients with bronchitis do not automatically need antibiotics if there’s no evidence of pneumonia, the authors did advise antibiotics in cases involving COPD and a high likelihood of bacterial infection. Clinicians should base their choice of antibiotics on the most common bacterial etiology: Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis. Ideal candidates for therapy may include aminopenicillin with clavulanic acid, a macrolide, or a tetracycline.
Community-acquired pneumonia
The initial course of antibiotics should be at least 5 days for pneumonia and only extended after considering validated evidence of the patient’s clinical stability, such as resuming normal vital signs, mental activity, and the ability to eat. Multiple randomized, controlled trials have shown no improved benefit from longer courses, though longer courses are linked to increased adverse events and mortality.
Again, antibiotics used should “cover common pathogens, such as S. pneumoniae, H. influenzae, Mycoplasma pneumoniae, and Staphylococcus aureus, and atypical pathogens, such as Legionella species,” the authors wrote. Options include “amoxicillin, doxycycline, or a macrolide for healthy adults or a beta-lactam with a macrolide or a respiratory fluoroquinolone in patients with comorbidities.”
UTIs: Uncomplicated cystitis and pyelonephritis
For women’s bacterial cystitis – 75% of which is caused by Escherichia coli – the guidelines recommend nitrofurantoin for 5 days, trimethoprim-sulfamethoxazole for 3 days, or fosfomycin as a single dose. For uncomplicated pyelonephritis in both men and women, clinicians can consider fluoroquinolones for 5-7 days or trimethoprim-sulfamethoxazole for 14 days, depending on antibiotic susceptibility.
This recommendation does not include UTIs in women who are pregnant or UTIs with other functional abnormalities present, such as obstruction. The authors also intentionally left out acute bacterial prostatitis because of its complexity and how long it can take to treat.
Cellulitis
MRSA, which has been increasing in prevalence, is a leading cause of skin and soft-tissue infections, such as necrotizing infections, cellulitis, and erysipelas. Unless the patient has penetrating trauma, evidence of MRSA infection elsewhere, injection drug use, nasal colonization of MRSA, or systemic inflammatory response syndrome, the guidelines recommend a 5- to 6-day course of cephalosporin, penicillin, or clindamycin, extended only if the infection has not improved in 5 days. Further research can narrow down the most appropriate treatment course.
This guidance does not apply to purulent cellulitis, such as conditions with abscesses, furuncles, or carbuncles that typically require incision and drainage.
Continuing to get the message out
Dr. Fincher emphasized the importance of continuing to disseminate messaging for clinicians about reducing unnecessary antibiotic use.
“In medicine we are constantly bombarded with new information. It is those patients and disease states that we see and treat every day that are especially important for us as physicians and other clinicians to keep our skills and knowledge base up to date when it comes to use of antibiotics,” Dr. Fincher said in an interview. “We just need to continue to educate and push out the data, guidelines, and recommendations.”
Dr. Nolt added that it’s important to emphasize how to translate these national recommendations into local practices since local guidance can also raise awareness and encourage local compliance.
Other strategies for reducing overuse of antibiotics “include restriction on antibiotics available at health care systems (formulary restriction), not allowing use of antibiotics unless there is discussion about the patient’s case (preauthorization), and reviewing cases of patients on antibiotics and advising on next steps (prospective audit and feedback),” she said.
The research was funded by the ACP. Dr. Lee has received personal fees from this news organization and Prime Education. Dr. Fincher owns stock in Johnson & Johnson and Procter and Gamble. Dr. Nolt and the article’s coauthors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An antibiotic course of 5 days is usually just as effective as longer courses but with fewer side effects and decreased overall antibiotic exposure for a number of common bacterial conditions, according to new clinical guidelines published by the American College of Physicians.
The guidelines focus on treatment of uncomplicated cases involving pneumonia, urinary tract infections (UTIs), cellulitis, chronic obstructive pulmonary disease (COPD) exacerbations, and acute bronchitis. The goal of the guidelines is to continue improving antibiotic stewardship given the increasing threat of antibiotic resistance and the adverse effects of antibiotics.
“Any use of antibiotics (including necessary use) has downstream effects outside of treating infection,” Dawn Nolt, MD, MPH, a professor of pediatric infection disease at Oregon Health & Science University, Portland, said in an interview. Dr. Nolt was not involved in developing these guidelines. “Undesirable outcomes include allergic reactions, diarrhea, and antibiotic-resistant bacteria. When we reduce unnecessary antibiotic, we reduce undesirable outcomes,” she said.
According to background information in the paper, 1 in 10 patients receives an antibiotic prescription during visits, yet nearly a third of these (30%) are unnecessary and last too long, especially for sinusitis and bronchitis. Meanwhile, overuse of antibiotics, particularly broad-spectrum ones, leads to resistance and adverse effects in up to 20% of patients.
“Prescribing practices can vary based on the type of provider, the setting where the antibiotic is being prescribed, what geographic area you are looking at, the medical reason for which the antibiotic is being prescribed, the actual germ being targeted, and the type of patient,” Dr. Nolt said. “But this variability can be reduced when prescribing providers are aware and follow best practice standards as through this article.”
The new ACP guidelines are a distillation of recommendations from preexisting infectious disease organizations, Dr. Nolt said, but aimed specifically at those practicing internal medicine.
“We define appropriate antibiotic use as prescribing the right antibiotic at the right dose for the right duration for a specific condition,” Rachael A. Lee, MD, MSPH, of the University of Alabama at Birmingham, and colleagues wrote in the article detailing the new guidelines. “Despite evidence and guidelines supporting shorter durations of antibiotic use, many physicians do not prescribe short-course therapy, frequently defaulting to 10-day courses regardless of the condition.”
The reasons for this default response vary. Though some clinicians prescribe longer courses specifically to prevent antibiotic resistance, no evidence shows that continuing to take antibiotics after symptoms have resolved actually reduces likelihood of resistance, the authors noted.
“In fact, resistance is a documented side effect of prolonged antibiotic use due to natural selection pressure,” they wrote.
Another common reason is habit.
“This was the ‘conventional wisdom’ for so long, just trying to make sure all bacteria causing the infection were completely eradicated, with no stragglers that had been exposed to the antibiotic but were not gone and now could evolve into resistant organisms,” Jacqueline W. Fincher, MD, a primary care physician and president of the ACP, said in an interview. “While antibiotic stewardship has been very important for over a decade, we now have more recent head-to-head studies/data showing that, in these four conditions, shorter courses of treatment are just as efficacious with less side effects and adverse events.”
The researchers reviewed all existing clinical guidelines related to bronchitis with COPD exacerbations, community-acquired pneumonia, UTIs, and cellulitis, as well as any other relevant studies in the literature. Although they did not conduct a formal systematic review, they compiled the guidelines specifically for all internists, family physicians and other clinicians caring for patients with these conditions.
“Although most patients with these infections will be seen in the outpatient setting, these best-practice advice statements also apply to patients who present in the inpatient setting,” the authors wrote. They also note the importance of ensuring the patient has the correct diagnosis and appropriate corresponding antibiotic prescription. “If a patient is not improving with appropriate antibiotics, it is important for the clinician to reassess for other causes of symptoms rather than defaulting to a longer duration of antibiotic therapy,” they wrote, calling a longer course “the exception and not the rule.”
Acute bronchitis with COPD exacerbations
Antibiotic treatment for COPD exacerbations and acute uncomplicated bronchitis with signs of a bacterial infection should last no longer than 5 days. The authors define this condition as an acute respiratory infection with a normal chest x-ray, most often caused by a virus. Although patients with bronchitis do not automatically need antibiotics if there’s no evidence of pneumonia, the authors did advise antibiotics in cases involving COPD and a high likelihood of bacterial infection. Clinicians should base their choice of antibiotics on the most common bacterial etiology: Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis. Ideal candidates for therapy may include aminopenicillin with clavulanic acid, a macrolide, or a tetracycline.
Community-acquired pneumonia
The initial course of antibiotics should be at least 5 days for pneumonia and only extended after considering validated evidence of the patient’s clinical stability, such as resuming normal vital signs, mental activity, and the ability to eat. Multiple randomized, controlled trials have shown no improved benefit from longer courses, though longer courses are linked to increased adverse events and mortality.
Again, antibiotics used should “cover common pathogens, such as S. pneumoniae, H. influenzae, Mycoplasma pneumoniae, and Staphylococcus aureus, and atypical pathogens, such as Legionella species,” the authors wrote. Options include “amoxicillin, doxycycline, or a macrolide for healthy adults or a beta-lactam with a macrolide or a respiratory fluoroquinolone in patients with comorbidities.”
UTIs: Uncomplicated cystitis and pyelonephritis
For women’s bacterial cystitis – 75% of which is caused by Escherichia coli – the guidelines recommend nitrofurantoin for 5 days, trimethoprim-sulfamethoxazole for 3 days, or fosfomycin as a single dose. For uncomplicated pyelonephritis in both men and women, clinicians can consider fluoroquinolones for 5-7 days or trimethoprim-sulfamethoxazole for 14 days, depending on antibiotic susceptibility.
This recommendation does not include UTIs in women who are pregnant or UTIs with other functional abnormalities present, such as obstruction. The authors also intentionally left out acute bacterial prostatitis because of its complexity and how long it can take to treat.
Cellulitis
MRSA, which has been increasing in prevalence, is a leading cause of skin and soft-tissue infections, such as necrotizing infections, cellulitis, and erysipelas. Unless the patient has penetrating trauma, evidence of MRSA infection elsewhere, injection drug use, nasal colonization of MRSA, or systemic inflammatory response syndrome, the guidelines recommend a 5- to 6-day course of cephalosporin, penicillin, or clindamycin, extended only if the infection has not improved in 5 days. Further research can narrow down the most appropriate treatment course.
This guidance does not apply to purulent cellulitis, such as conditions with abscesses, furuncles, or carbuncles that typically require incision and drainage.
Continuing to get the message out
Dr. Fincher emphasized the importance of continuing to disseminate messaging for clinicians about reducing unnecessary antibiotic use.
“In medicine we are constantly bombarded with new information. It is those patients and disease states that we see and treat every day that are especially important for us as physicians and other clinicians to keep our skills and knowledge base up to date when it comes to use of antibiotics,” Dr. Fincher said in an interview. “We just need to continue to educate and push out the data, guidelines, and recommendations.”
Dr. Nolt added that it’s important to emphasize how to translate these national recommendations into local practices since local guidance can also raise awareness and encourage local compliance.
Other strategies for reducing overuse of antibiotics “include restriction on antibiotics available at health care systems (formulary restriction), not allowing use of antibiotics unless there is discussion about the patient’s case (preauthorization), and reviewing cases of patients on antibiotics and advising on next steps (prospective audit and feedback),” she said.
The research was funded by the ACP. Dr. Lee has received personal fees from this news organization and Prime Education. Dr. Fincher owns stock in Johnson & Johnson and Procter and Gamble. Dr. Nolt and the article’s coauthors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.




