User login
Dupilumab for the Treatment of Lichen Planus
To the Editor:
Lichen planus (LP) is an inflammatory mucocutaneous disorder that primarily affects adults aged 30 to 60 years.1 It can present across various regions such as the skin, scalp, oral cavity, genitalia, nails, and hair. It classically presents with pruritic, purple, polygonal papules or plaques. The proposed pathogenesis of this condition involves autoimmune destruction of epidermal basal keratinocytes.2 Management involves a stepwise approach, beginning with topical therapies such as corticosteroids and phototherapy and proceeding to systemic therapy including oral corticosteroids and retinoids. Additional medications with reported positive results include immunomodulators such as cyclosporine, tacrolimus, and mycophenolate mofetil.2-4 Dupilumab is a biologic immunomodulator and antagonist to the IL-4Rα on helper T cells (TH1). Although indicated for the treatment of moderate to severe atopic dermatitis, this medication’s immunomodulatory properties have been shown to aid various inflammatory cutaneous conditions, including prurigo nodularis.5-9 We present a case of dupilumab therapy for treatment-refractory LP.
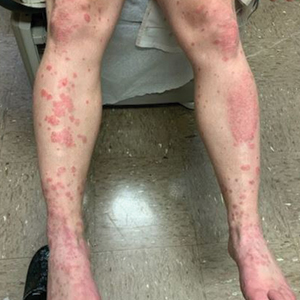
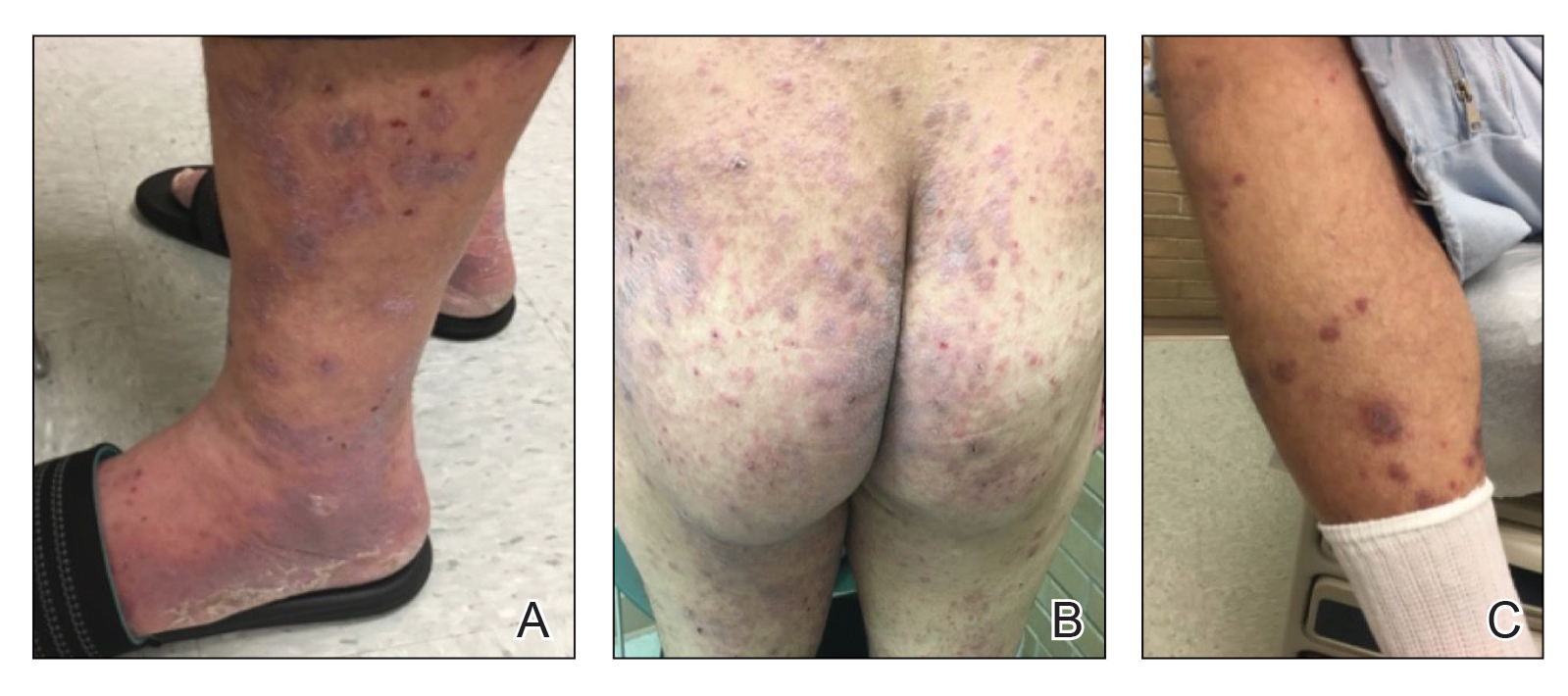
A 52-year-old man presented with a new-onset progressive rash over the prior 6 months. He reported no history of atopic dermatitis. The patient described the rash as “severely pruritic” with a numeric rating scale itch intensity of 9/10 (0 being no itch; 10 being the worst itch imaginable). Physical examination revealed purple polygonal scaly papules on the arms, hands, legs, feet, chest, and back (Figure 1).
Three biopsies were taken, all indicative of lichenoid dermatitis consistent with LP. Rapid plasma reagin as well as HIV and hepatitis C virus serology tests were negative. Halobetasol ointment, tacrolimus ointment, and oral prednisone (28-day taper starting at 40 mg) all failed. Acitretin subsequently was initiated and failed to provide any benefit. The patient was unable to come to clinic 3 times a week for phototherapy due to his work schedule.
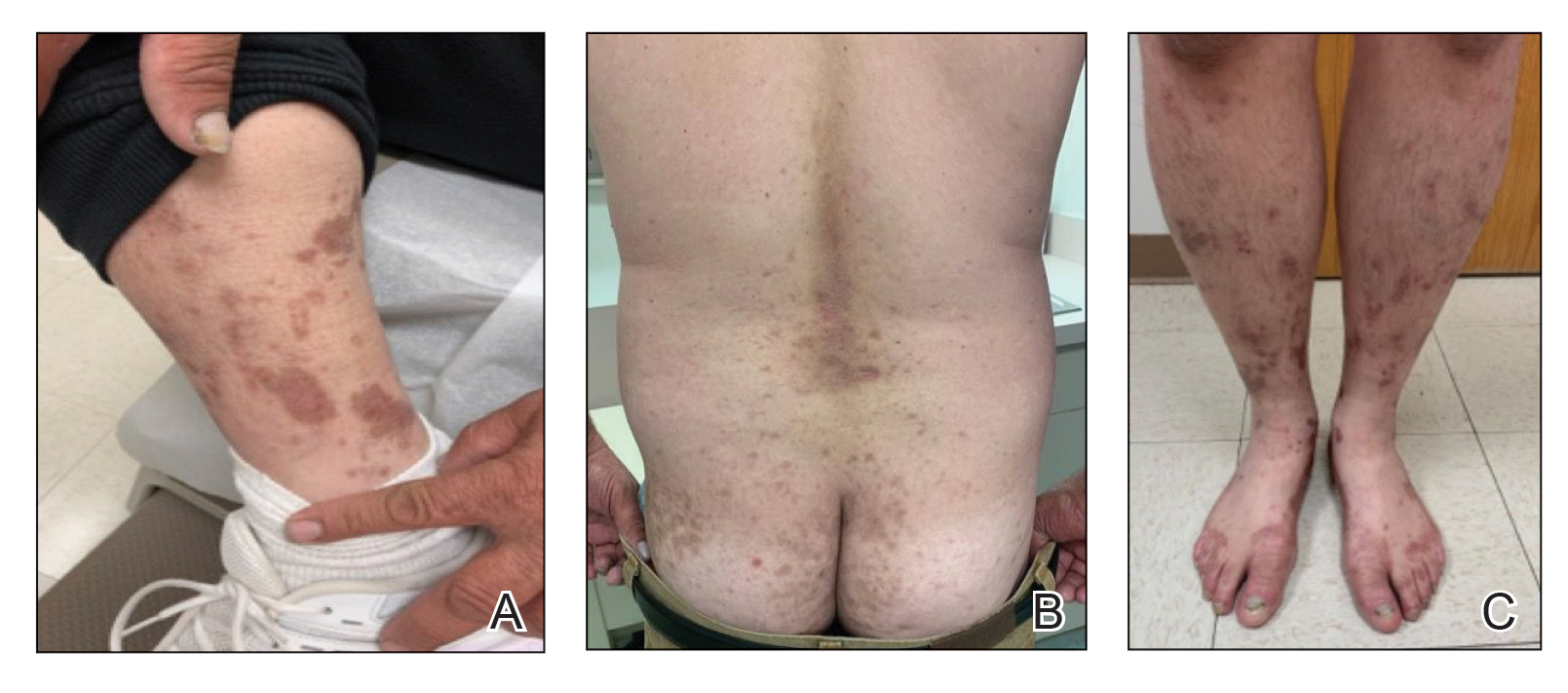
Due to the chronic, severe, and recalcitrant nature of his condition, as well as the lack of US Food and Drug Administration–approved treatments, the patient agreed to begin off-label treatment with dupilumab. Upon documentation, the patient’s primary diagnosis was listed as LP, clearly stating all commonly accepted treatments were attempted, except off-label therapy, and failed, and the plan was to treat him with dupilumab as if he had a severe form of atopic dermatitis. Dupilumab was approved with this documentation with a minimal co-pay, as the patient was on Medicaid. At 3-month follow-up (after 4 administrations of the medication), the patient showed remarkable improvement in appearance, and his numeric rating scale itch intensity score improved to 1/10.
Lichen planus is an immune-mediated, inflammatory condition that can affect the skin, hair, nails, and oral cavity. Although its etiology is not fully understood, research supports a primarily TH1 immunologic reaction.10 These T cells promote cytotoxic CD8 T-cell differentiation and migration, leading to subsequent destruction of epidermal basal keratinocytes. An important cytokine in this pathway—tumor necrosis factor α—stimulates a series of proinflammatory factors, including IL-1α, IL-8, and IL-6. IL-6 is of particular interest, as its elevation has been identified in the serum of patients with LP, with levels correlating to disease severity.11 This increase is thought to be multifactorial and a reliable predictor of disease activity.12,13 In addition to its proinflammatory role, IL-6 promotes the activity of IL-4, an essential cytokine in TH2 T-cell differentiation.
The TH2 pathway, enhanced by IL-6, increases the activity of downstream cytokines IL-4, IL-5, and IL-13. This pathway promotes IgE class switching and eosinophil maturation, pivotal factors in the development of atopic conditions such as allergic rhinitis, asthma, and atopic dermatitis. The role of IL-4 and TH2 cells in the pathogenesis of LP remains poorly understood.14 In prior basic laboratory studies, utilizing tissue sampling, RNA extraction, and real-time polymerase chain reaction assays, Yamauchi et al15 proposed that TH2-related chemokines played a pathogenic role in oral LP. Additional reports propose the pathogenic involvement of TH17, TH0, and TH2 T cells.16 These findings suggest that elevated IL-6 in those with LP may stimulate an increase in IL-4 and subsequent TH2 response. Dupilumab, a monoclonal antibody that targets IL-4Rα found on T cells, inhibits both IL-4 and IL-13 signaling, decreasing subsequent effector cell function.17,18 Several case reports have described dupilumab successfully treating various additional dermatoses, including prurigo nodularis, chronic pruritus, and bullous pemphigoid.5-9 Our case demonstrates an example of LP responsive to dupilumab. Our findings suggest that dupilumab interacts with the pathogenic cascade of LP, potentially implicating the role of TH2 in the pathophysiology of LP.
Treatment-refractory LP remains difficult to manage for both the patient and provider. Treatment regimens remain limited to small uncontrolled studies and case reports. Although primarily considered a TH1-mediated disease, the interplay of various alternative signaling pathways has been suggested. Our case of dupilumab-responsive LP suggests an underlying pathologic role of TH2-mediated activity. Dupilumab shows promise as an effective therapy for refractory LP, as evidenced by our patient’s remarkable response. Larger studies are warranted regarding the role of TH2-mediated inflammation and the use of dupilumab in LP.
- Cleach LL, Chosidow O. Clinical practice. lichen planus. N Engl J Med. 2012;266:723-732.
- Lehman, JS, Tollefson MM, Gibson LE. Lichen planus. Int J Dermatol. 2009;48:682-694.
- Frieling U, Bonsmann G, Schwarz T, et al. Treatment of severe lichen planus with mycophenolate mofetil. J Am Acad Dermatol. 2003;49:1063-1066.
- Cribier B, Frances C, Chosidow O. Treatment of lichen planus. an evidence-based medicine analysis of efficacy. Arch Dermatol. 1998;134:1521-1530.
- Calugareanu A, Jachiet C, Lepelletier C, et al. Dramatic improvement of generalized prurigo nodularis with dupilumab. J Eur Acad Dermatol Venereol. 2019;33:E303-E304.
- Kaye A, Gordon SC, Deverapalli SC, et al. Dupilumab for the treatment of recalcitrant bullous pemphigoid. JAMA Dermatol. 2018;154:1225-1226.
- Mollanazar NK, Qiu CC, Aldrich JL, et al. Use of dupilumab in HIV-positive patients: report of four cases. Br J Dermatol. 2019;181:1311-1312.
- Zhai LL, Savage KT, Qiu CC, et al. Chronic pruritus responding to dupilumab—a case series. Medicines (Basel). 2019;6:72.
- Mollanazar NK, Elgash M, Weaver L, et al. Reduced itch associated with dupilumab treatment in 4 patients with prurigo nodularis. JAMA Dermatol. 2019;155:121-122.
- Lodi G, Scully C, Carrozzo M, et al. Current controversies in oral lichen planus: report of an international consensus meeting. part 1. viral infections and etiopathogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:40-51.
- Yin M, Li G, Song H, et al. Identifying the association between interleukin-6 and lichen planus: a meta-analysis. Biomed Rep. 2017;6:571-575.
- Sun A, Chia JS, Chang YF, et al. Serum interleukin-6 level is a useful marker in evaluating therapeutic effects of levamisole and Chinese medicinal herbs on patients with oral lichen planus. J Oral Pathol Med. 2002;31:196-203.
- Rhodus NL, Cheng B, Bowles W, et al. Proinflammatory cytokine levels in saliva before and after treatment of (erosive) oral lichen planus with dexamethasone. Oral Dis. 2006;12:112-116.
- Carrozzo M. Understanding the pathobiology of oral lichen planus. Curr Oral Health Rep. 2014;1:173-179.
- Yamauchi M, Moriyama M, Hayashida JN, et al. Myeloid dendritic cells stimulated by thymic stromal lymphopoietin promote Th2 immune responses and the pathogenesis of oral lichen planus. Plos One. 2017:12:e0173017.
- Piccinni M-P, Lombardell L, Logidice F, et al. Potential pathogenetic role of Th17, Th0, and Th2 cells in erosive and reticular oral lichen planus. Oral Dis. 2013:20:212-218.
- Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223-246.
- Noda S, Kruefer JG, Guttum-Yassky E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J Allergy Clin Immunol. 2015;135:324-336.
To the Editor:
Lichen planus (LP) is an inflammatory mucocutaneous disorder that primarily affects adults aged 30 to 60 years.1 It can present across various regions such as the skin, scalp, oral cavity, genitalia, nails, and hair. It classically presents with pruritic, purple, polygonal papules or plaques. The proposed pathogenesis of this condition involves autoimmune destruction of epidermal basal keratinocytes.2 Management involves a stepwise approach, beginning with topical therapies such as corticosteroids and phototherapy and proceeding to systemic therapy including oral corticosteroids and retinoids. Additional medications with reported positive results include immunomodulators such as cyclosporine, tacrolimus, and mycophenolate mofetil.2-4 Dupilumab is a biologic immunomodulator and antagonist to the IL-4Rα on helper T cells (TH1). Although indicated for the treatment of moderate to severe atopic dermatitis, this medication’s immunomodulatory properties have been shown to aid various inflammatory cutaneous conditions, including prurigo nodularis.5-9 We present a case of dupilumab therapy for treatment-refractory LP.
A 52-year-old man presented with a new-onset progressive rash over the prior 6 months. He reported no history of atopic dermatitis. The patient described the rash as “severely pruritic” with a numeric rating scale itch intensity of 9/10 (0 being no itch; 10 being the worst itch imaginable). Physical examination revealed purple polygonal scaly papules on the arms, hands, legs, feet, chest, and back (Figure 1).


Three biopsies were taken, all indicative of lichenoid dermatitis consistent with LP. Rapid plasma reagin as well as HIV and hepatitis C virus serology tests were negative. Halobetasol ointment, tacrolimus ointment, and oral prednisone (28-day taper starting at 40 mg) all failed. Acitretin subsequently was initiated and failed to provide any benefit. The patient was unable to come to clinic 3 times a week for phototherapy due to his work schedule.
Due to the chronic, severe, and recalcitrant nature of his condition, as well as the lack of US Food and Drug Administration–approved treatments, the patient agreed to begin off-label treatment with dupilumab. Upon documentation, the patient’s primary diagnosis was listed as LP, clearly stating all commonly accepted treatments were attempted, except off-label therapy, and failed, and the plan was to treat him with dupilumab as if he had a severe form of atopic dermatitis. Dupilumab was approved with this documentation with a minimal co-pay, as the patient was on Medicaid. At 3-month follow-up (after 4 administrations of the medication), the patient showed remarkable improvement in appearance, and his numeric rating scale itch intensity score improved to 1/10.
Lichen planus is an immune-mediated, inflammatory condition that can affect the skin, hair, nails, and oral cavity. Although its etiology is not fully understood, research supports a primarily TH1 immunologic reaction.10 These T cells promote cytotoxic CD8 T-cell differentiation and migration, leading to subsequent destruction of epidermal basal keratinocytes. An important cytokine in this pathway—tumor necrosis factor α—stimulates a series of proinflammatory factors, including IL-1α, IL-8, and IL-6. IL-6 is of particular interest, as its elevation has been identified in the serum of patients with LP, with levels correlating to disease severity.11 This increase is thought to be multifactorial and a reliable predictor of disease activity.12,13 In addition to its proinflammatory role, IL-6 promotes the activity of IL-4, an essential cytokine in TH2 T-cell differentiation.
The TH2 pathway, enhanced by IL-6, increases the activity of downstream cytokines IL-4, IL-5, and IL-13. This pathway promotes IgE class switching and eosinophil maturation, pivotal factors in the development of atopic conditions such as allergic rhinitis, asthma, and atopic dermatitis. The role of IL-4 and TH2 cells in the pathogenesis of LP remains poorly understood.14 In prior basic laboratory studies, utilizing tissue sampling, RNA extraction, and real-time polymerase chain reaction assays, Yamauchi et al15 proposed that TH2-related chemokines played a pathogenic role in oral LP. Additional reports propose the pathogenic involvement of TH17, TH0, and TH2 T cells.16 These findings suggest that elevated IL-6 in those with LP may stimulate an increase in IL-4 and subsequent TH2 response. Dupilumab, a monoclonal antibody that targets IL-4Rα found on T cells, inhibits both IL-4 and IL-13 signaling, decreasing subsequent effector cell function.17,18 Several case reports have described dupilumab successfully treating various additional dermatoses, including prurigo nodularis, chronic pruritus, and bullous pemphigoid.5-9 Our case demonstrates an example of LP responsive to dupilumab. Our findings suggest that dupilumab interacts with the pathogenic cascade of LP, potentially implicating the role of TH2 in the pathophysiology of LP.
Treatment-refractory LP remains difficult to manage for both the patient and provider. Treatment regimens remain limited to small uncontrolled studies and case reports. Although primarily considered a TH1-mediated disease, the interplay of various alternative signaling pathways has been suggested. Our case of dupilumab-responsive LP suggests an underlying pathologic role of TH2-mediated activity. Dupilumab shows promise as an effective therapy for refractory LP, as evidenced by our patient’s remarkable response. Larger studies are warranted regarding the role of TH2-mediated inflammation and the use of dupilumab in LP.
To the Editor:
Lichen planus (LP) is an inflammatory mucocutaneous disorder that primarily affects adults aged 30 to 60 years.1 It can present across various regions such as the skin, scalp, oral cavity, genitalia, nails, and hair. It classically presents with pruritic, purple, polygonal papules or plaques. The proposed pathogenesis of this condition involves autoimmune destruction of epidermal basal keratinocytes.2 Management involves a stepwise approach, beginning with topical therapies such as corticosteroids and phototherapy and proceeding to systemic therapy including oral corticosteroids and retinoids. Additional medications with reported positive results include immunomodulators such as cyclosporine, tacrolimus, and mycophenolate mofetil.2-4 Dupilumab is a biologic immunomodulator and antagonist to the IL-4Rα on helper T cells (TH1). Although indicated for the treatment of moderate to severe atopic dermatitis, this medication’s immunomodulatory properties have been shown to aid various inflammatory cutaneous conditions, including prurigo nodularis.5-9 We present a case of dupilumab therapy for treatment-refractory LP.
A 52-year-old man presented with a new-onset progressive rash over the prior 6 months. He reported no history of atopic dermatitis. The patient described the rash as “severely pruritic” with a numeric rating scale itch intensity of 9/10 (0 being no itch; 10 being the worst itch imaginable). Physical examination revealed purple polygonal scaly papules on the arms, hands, legs, feet, chest, and back (Figure 1).


Three biopsies were taken, all indicative of lichenoid dermatitis consistent with LP. Rapid plasma reagin as well as HIV and hepatitis C virus serology tests were negative. Halobetasol ointment, tacrolimus ointment, and oral prednisone (28-day taper starting at 40 mg) all failed. Acitretin subsequently was initiated and failed to provide any benefit. The patient was unable to come to clinic 3 times a week for phototherapy due to his work schedule.
Due to the chronic, severe, and recalcitrant nature of his condition, as well as the lack of US Food and Drug Administration–approved treatments, the patient agreed to begin off-label treatment with dupilumab. Upon documentation, the patient’s primary diagnosis was listed as LP, clearly stating all commonly accepted treatments were attempted, except off-label therapy, and failed, and the plan was to treat him with dupilumab as if he had a severe form of atopic dermatitis. Dupilumab was approved with this documentation with a minimal co-pay, as the patient was on Medicaid. At 3-month follow-up (after 4 administrations of the medication), the patient showed remarkable improvement in appearance, and his numeric rating scale itch intensity score improved to 1/10.
Lichen planus is an immune-mediated, inflammatory condition that can affect the skin, hair, nails, and oral cavity. Although its etiology is not fully understood, research supports a primarily TH1 immunologic reaction.10 These T cells promote cytotoxic CD8 T-cell differentiation and migration, leading to subsequent destruction of epidermal basal keratinocytes. An important cytokine in this pathway—tumor necrosis factor α—stimulates a series of proinflammatory factors, including IL-1α, IL-8, and IL-6. IL-6 is of particular interest, as its elevation has been identified in the serum of patients with LP, with levels correlating to disease severity.11 This increase is thought to be multifactorial and a reliable predictor of disease activity.12,13 In addition to its proinflammatory role, IL-6 promotes the activity of IL-4, an essential cytokine in TH2 T-cell differentiation.
The TH2 pathway, enhanced by IL-6, increases the activity of downstream cytokines IL-4, IL-5, and IL-13. This pathway promotes IgE class switching and eosinophil maturation, pivotal factors in the development of atopic conditions such as allergic rhinitis, asthma, and atopic dermatitis. The role of IL-4 and TH2 cells in the pathogenesis of LP remains poorly understood.14 In prior basic laboratory studies, utilizing tissue sampling, RNA extraction, and real-time polymerase chain reaction assays, Yamauchi et al15 proposed that TH2-related chemokines played a pathogenic role in oral LP. Additional reports propose the pathogenic involvement of TH17, TH0, and TH2 T cells.16 These findings suggest that elevated IL-6 in those with LP may stimulate an increase in IL-4 and subsequent TH2 response. Dupilumab, a monoclonal antibody that targets IL-4Rα found on T cells, inhibits both IL-4 and IL-13 signaling, decreasing subsequent effector cell function.17,18 Several case reports have described dupilumab successfully treating various additional dermatoses, including prurigo nodularis, chronic pruritus, and bullous pemphigoid.5-9 Our case demonstrates an example of LP responsive to dupilumab. Our findings suggest that dupilumab interacts with the pathogenic cascade of LP, potentially implicating the role of TH2 in the pathophysiology of LP.
Treatment-refractory LP remains difficult to manage for both the patient and provider. Treatment regimens remain limited to small uncontrolled studies and case reports. Although primarily considered a TH1-mediated disease, the interplay of various alternative signaling pathways has been suggested. Our case of dupilumab-responsive LP suggests an underlying pathologic role of TH2-mediated activity. Dupilumab shows promise as an effective therapy for refractory LP, as evidenced by our patient’s remarkable response. Larger studies are warranted regarding the role of TH2-mediated inflammation and the use of dupilumab in LP.
- Cleach LL, Chosidow O. Clinical practice. lichen planus. N Engl J Med. 2012;266:723-732.
- Lehman, JS, Tollefson MM, Gibson LE. Lichen planus. Int J Dermatol. 2009;48:682-694.
- Frieling U, Bonsmann G, Schwarz T, et al. Treatment of severe lichen planus with mycophenolate mofetil. J Am Acad Dermatol. 2003;49:1063-1066.
- Cribier B, Frances C, Chosidow O. Treatment of lichen planus. an evidence-based medicine analysis of efficacy. Arch Dermatol. 1998;134:1521-1530.
- Calugareanu A, Jachiet C, Lepelletier C, et al. Dramatic improvement of generalized prurigo nodularis with dupilumab. J Eur Acad Dermatol Venereol. 2019;33:E303-E304.
- Kaye A, Gordon SC, Deverapalli SC, et al. Dupilumab for the treatment of recalcitrant bullous pemphigoid. JAMA Dermatol. 2018;154:1225-1226.
- Mollanazar NK, Qiu CC, Aldrich JL, et al. Use of dupilumab in HIV-positive patients: report of four cases. Br J Dermatol. 2019;181:1311-1312.
- Zhai LL, Savage KT, Qiu CC, et al. Chronic pruritus responding to dupilumab—a case series. Medicines (Basel). 2019;6:72.
- Mollanazar NK, Elgash M, Weaver L, et al. Reduced itch associated with dupilumab treatment in 4 patients with prurigo nodularis. JAMA Dermatol. 2019;155:121-122.
- Lodi G, Scully C, Carrozzo M, et al. Current controversies in oral lichen planus: report of an international consensus meeting. part 1. viral infections and etiopathogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:40-51.
- Yin M, Li G, Song H, et al. Identifying the association between interleukin-6 and lichen planus: a meta-analysis. Biomed Rep. 2017;6:571-575.
- Sun A, Chia JS, Chang YF, et al. Serum interleukin-6 level is a useful marker in evaluating therapeutic effects of levamisole and Chinese medicinal herbs on patients with oral lichen planus. J Oral Pathol Med. 2002;31:196-203.
- Rhodus NL, Cheng B, Bowles W, et al. Proinflammatory cytokine levels in saliva before and after treatment of (erosive) oral lichen planus with dexamethasone. Oral Dis. 2006;12:112-116.
- Carrozzo M. Understanding the pathobiology of oral lichen planus. Curr Oral Health Rep. 2014;1:173-179.
- Yamauchi M, Moriyama M, Hayashida JN, et al. Myeloid dendritic cells stimulated by thymic stromal lymphopoietin promote Th2 immune responses and the pathogenesis of oral lichen planus. Plos One. 2017:12:e0173017.
- Piccinni M-P, Lombardell L, Logidice F, et al. Potential pathogenetic role of Th17, Th0, and Th2 cells in erosive and reticular oral lichen planus. Oral Dis. 2013:20:212-218.
- Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223-246.
- Noda S, Kruefer JG, Guttum-Yassky E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J Allergy Clin Immunol. 2015;135:324-336.
- Cleach LL, Chosidow O. Clinical practice. lichen planus. N Engl J Med. 2012;266:723-732.
- Lehman, JS, Tollefson MM, Gibson LE. Lichen planus. Int J Dermatol. 2009;48:682-694.
- Frieling U, Bonsmann G, Schwarz T, et al. Treatment of severe lichen planus with mycophenolate mofetil. J Am Acad Dermatol. 2003;49:1063-1066.
- Cribier B, Frances C, Chosidow O. Treatment of lichen planus. an evidence-based medicine analysis of efficacy. Arch Dermatol. 1998;134:1521-1530.
- Calugareanu A, Jachiet C, Lepelletier C, et al. Dramatic improvement of generalized prurigo nodularis with dupilumab. J Eur Acad Dermatol Venereol. 2019;33:E303-E304.
- Kaye A, Gordon SC, Deverapalli SC, et al. Dupilumab for the treatment of recalcitrant bullous pemphigoid. JAMA Dermatol. 2018;154:1225-1226.
- Mollanazar NK, Qiu CC, Aldrich JL, et al. Use of dupilumab in HIV-positive patients: report of four cases. Br J Dermatol. 2019;181:1311-1312.
- Zhai LL, Savage KT, Qiu CC, et al. Chronic pruritus responding to dupilumab—a case series. Medicines (Basel). 2019;6:72.
- Mollanazar NK, Elgash M, Weaver L, et al. Reduced itch associated with dupilumab treatment in 4 patients with prurigo nodularis. JAMA Dermatol. 2019;155:121-122.
- Lodi G, Scully C, Carrozzo M, et al. Current controversies in oral lichen planus: report of an international consensus meeting. part 1. viral infections and etiopathogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:40-51.
- Yin M, Li G, Song H, et al. Identifying the association between interleukin-6 and lichen planus: a meta-analysis. Biomed Rep. 2017;6:571-575.
- Sun A, Chia JS, Chang YF, et al. Serum interleukin-6 level is a useful marker in evaluating therapeutic effects of levamisole and Chinese medicinal herbs on patients with oral lichen planus. J Oral Pathol Med. 2002;31:196-203.
- Rhodus NL, Cheng B, Bowles W, et al. Proinflammatory cytokine levels in saliva before and after treatment of (erosive) oral lichen planus with dexamethasone. Oral Dis. 2006;12:112-116.
- Carrozzo M. Understanding the pathobiology of oral lichen planus. Curr Oral Health Rep. 2014;1:173-179.
- Yamauchi M, Moriyama M, Hayashida JN, et al. Myeloid dendritic cells stimulated by thymic stromal lymphopoietin promote Th2 immune responses and the pathogenesis of oral lichen planus. Plos One. 2017:12:e0173017.
- Piccinni M-P, Lombardell L, Logidice F, et al. Potential pathogenetic role of Th17, Th0, and Th2 cells in erosive and reticular oral lichen planus. Oral Dis. 2013:20:212-218.
- Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223-246.
- Noda S, Kruefer JG, Guttum-Yassky E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J Allergy Clin Immunol. 2015;135:324-336.
Practice Points
- Lichen planus (LP) is an inflammatory mucocutaneous disorder that can present across various regions of the body with pruritic, purple, polygonal papules or plaques.
- The proposed pathogenesis of LP involves autoimmune destruction of epidermal basal keratinocytes.
- The immunomodulatory properties of dupilumab have been shown to aid various inflammatory cutaneous conditions.
Deaths tied to reprocessed urologic endoscopes, FDA warns
The U.S. Food and Drug Administration is warning health care providers about the risk for potentially life-threatening infections associated with reprocessed endoscopes used for viewing the urinary tract, including cystoscopes, cystouerthroscopes, and ureteroscopes.
The federal agency is investigating more than 450 medical device reports, including three reports of deaths, received between Jan. 1, 2017, and Feb. 20, 2021, that describe post-procedure infections and other possible contamination problems associated with the reprocessing or cleaning and sterilization of the devices.
Although it’s early in the investigation, on the basis of available data, the FDA believes the risk for infection is low.
“We are very concerned about the three reported deaths – outside of the United States – associated with these infections, and we’re acting fast to communicate with health care providers and the public about what we know and what is still an emerging issue,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement released on April 1.
Manufacturer Olympus Corporation submitted three reports of deaths attributed to a bacterial infection. In two of those reports, the infection was linked to a forceps/irrigation plug, an accessory component used to control water flow and enable access to the working channel of the endoscope. Lab tests confirmed that the bacteria that caused the infection was present in the forceps/irrigation plug.
The FDA said the third victim’s death involved a cystoscope that did not pass a leak test. It is possible that the damaged device was a factor in the patient’s becoming infected.
It’s not known to what degree the reported infections or patient comorbidities played a part in the patient deaths. The FDA also hasn’t concluded that any specific manufacturer or brand of these devices is associated with higher risks than others.
The FDA released recommendations for processing and using these devices and emphasized the importance of following manufacturers’ labeling and reprocessing instructions to minimize the risk for infection.
In addition to following reprocessing instructions, the recommendations include not using a device that has failed a leak test, developing schedules for routine device inspection and maintenance, and discussing the potential benefits and risks associated with procedures involving reprocessed urologic endoscopes with patients.
The newly reported concerns with urologic endoscopes are similar to problems associated with reprocessed duodenoscopes. In 2018, the FDA warned about higher-than-expected contamination rates for reprocessed duodenoscopes. The FDA has taken action on infections related to the reprocessing of duodenoscopes. In 2015, it required postmarket safety studies and the updating of sampling and culturing protocols. In 2019, the FDA approved single-use duodenoscopes in an effort to curb infections.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration is warning health care providers about the risk for potentially life-threatening infections associated with reprocessed endoscopes used for viewing the urinary tract, including cystoscopes, cystouerthroscopes, and ureteroscopes.
The federal agency is investigating more than 450 medical device reports, including three reports of deaths, received between Jan. 1, 2017, and Feb. 20, 2021, that describe post-procedure infections and other possible contamination problems associated with the reprocessing or cleaning and sterilization of the devices.
Although it’s early in the investigation, on the basis of available data, the FDA believes the risk for infection is low.
“We are very concerned about the three reported deaths – outside of the United States – associated with these infections, and we’re acting fast to communicate with health care providers and the public about what we know and what is still an emerging issue,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement released on April 1.
Manufacturer Olympus Corporation submitted three reports of deaths attributed to a bacterial infection. In two of those reports, the infection was linked to a forceps/irrigation plug, an accessory component used to control water flow and enable access to the working channel of the endoscope. Lab tests confirmed that the bacteria that caused the infection was present in the forceps/irrigation plug.
The FDA said the third victim’s death involved a cystoscope that did not pass a leak test. It is possible that the damaged device was a factor in the patient’s becoming infected.
It’s not known to what degree the reported infections or patient comorbidities played a part in the patient deaths. The FDA also hasn’t concluded that any specific manufacturer or brand of these devices is associated with higher risks than others.
The FDA released recommendations for processing and using these devices and emphasized the importance of following manufacturers’ labeling and reprocessing instructions to minimize the risk for infection.
In addition to following reprocessing instructions, the recommendations include not using a device that has failed a leak test, developing schedules for routine device inspection and maintenance, and discussing the potential benefits and risks associated with procedures involving reprocessed urologic endoscopes with patients.
The newly reported concerns with urologic endoscopes are similar to problems associated with reprocessed duodenoscopes. In 2018, the FDA warned about higher-than-expected contamination rates for reprocessed duodenoscopes. The FDA has taken action on infections related to the reprocessing of duodenoscopes. In 2015, it required postmarket safety studies and the updating of sampling and culturing protocols. In 2019, the FDA approved single-use duodenoscopes in an effort to curb infections.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration is warning health care providers about the risk for potentially life-threatening infections associated with reprocessed endoscopes used for viewing the urinary tract, including cystoscopes, cystouerthroscopes, and ureteroscopes.
The federal agency is investigating more than 450 medical device reports, including three reports of deaths, received between Jan. 1, 2017, and Feb. 20, 2021, that describe post-procedure infections and other possible contamination problems associated with the reprocessing or cleaning and sterilization of the devices.
Although it’s early in the investigation, on the basis of available data, the FDA believes the risk for infection is low.
“We are very concerned about the three reported deaths – outside of the United States – associated with these infections, and we’re acting fast to communicate with health care providers and the public about what we know and what is still an emerging issue,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement released on April 1.
Manufacturer Olympus Corporation submitted three reports of deaths attributed to a bacterial infection. In two of those reports, the infection was linked to a forceps/irrigation plug, an accessory component used to control water flow and enable access to the working channel of the endoscope. Lab tests confirmed that the bacteria that caused the infection was present in the forceps/irrigation plug.
The FDA said the third victim’s death involved a cystoscope that did not pass a leak test. It is possible that the damaged device was a factor in the patient’s becoming infected.
It’s not known to what degree the reported infections or patient comorbidities played a part in the patient deaths. The FDA also hasn’t concluded that any specific manufacturer or brand of these devices is associated with higher risks than others.
The FDA released recommendations for processing and using these devices and emphasized the importance of following manufacturers’ labeling and reprocessing instructions to minimize the risk for infection.
In addition to following reprocessing instructions, the recommendations include not using a device that has failed a leak test, developing schedules for routine device inspection and maintenance, and discussing the potential benefits and risks associated with procedures involving reprocessed urologic endoscopes with patients.
The newly reported concerns with urologic endoscopes are similar to problems associated with reprocessed duodenoscopes. In 2018, the FDA warned about higher-than-expected contamination rates for reprocessed duodenoscopes. The FDA has taken action on infections related to the reprocessing of duodenoscopes. In 2015, it required postmarket safety studies and the updating of sampling and culturing protocols. In 2019, the FDA approved single-use duodenoscopes in an effort to curb infections.
A version of this article first appeared on Medscape.com.
Erethism Mercurialis and Reactions to Elemental Mercury
Evidence of human exposure to mercury dates as far back as the Egyptians in 1500
Mercury release in the environment primarily is a function of human activity, including coal-fired power plants, residential heating, and mining.9,10 Mercury from these sources is commonly found in the sediment of lakes and bays, where it is enzymatically converted to methylmercury by aquatic microorganisms; subsequent food chain biomagnification results in elevated mercury levels in apex predators. Substantial release of mercury into the environment also can be attributed to health care facilities from their use of thermometers containing 0.5 to 3 g of elemental mercury,11 blood pressure monitors, and medical waste incinerators.5
Mercury has been reported as the second most common cause of heavy metal poisoning after lead.12 Standards from the US Food and Drug Administration dictate that methylmercury levels in fish and wheat products must not exceed 1 ppm.13 Most plant and animal food sources contain methylmercury at levels between 0.0001 and 0.01 ppm; mercury concentrations are especially high in tuna, averaging 0.4 ppm, while larger predatory fish contain levels in excess of 1 ppm.14 The use of mercury-containing cosmetic products also presents a substantial exposure risk to consumers.5,10 In one study, 3.3% of skin-lightening creams and soaps purchased within the United States contained concentrations of mercury exceeding 1000 ppm.15
We describe a case of mercury toxicity resulting from intentional injection of liquid mercury into the right antecubital fossa in a suicide attempt.
Case Report
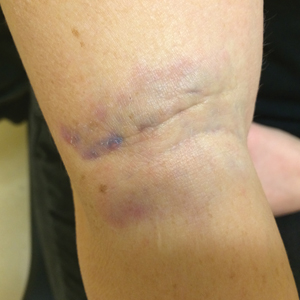
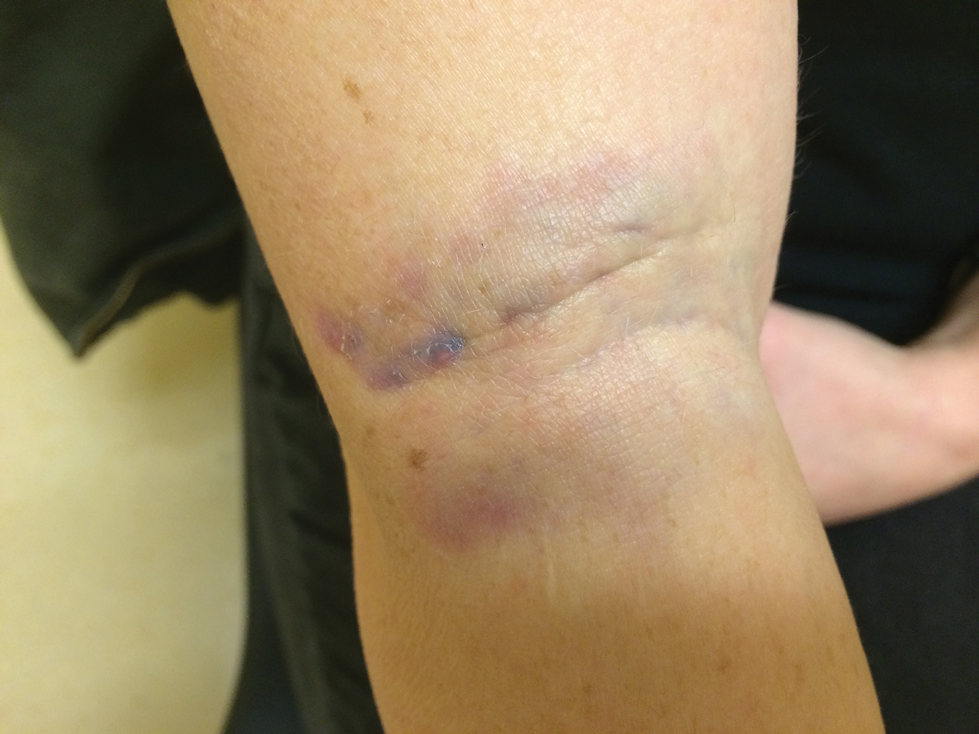
A 31-year-old woman presented to the family practice center for evaluation of a firm stained area on the skin of the right arm. She reported increasing anxiety, depression, tremors, irritability, and difficulty concentrating over the last 6 months. She denied headache and joint or muscle pain. Four years earlier, she had broken apart a thermometer and injected approximately 0.7 mL of its contents into the right arm in a suicide attempt. She intended to inject the thermometer’s contents directly into a vein, but the material instead entered the surrounding tissue. She denied notable pain or itching overlying the injection site. Her medications included aripiprazole and buspirone. She noted that she smoked half a pack of cigarettes per day and had a history of methamphetamine abuse. She was homeless and unemployed. Physical examination revealed an anxious tremulous woman with an erythematous to bluish gray, firm plaque on the right antecubital fossa (Figure 1). There were no notable tremors and no gait disturbance.
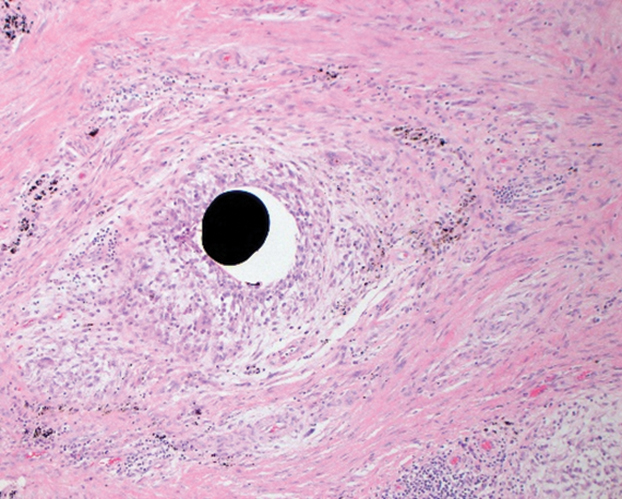
Her blood mercury level was greater than 100 µg/L and urine mercury was 477 µg/g (reference ranges, 1–8 μg/L and 4–5 μg/L, respectively). A radiograph of the right elbow area revealed scattered punctate foci of increased density within or overlying the anterolateral elbow soft tissues. She was diagnosed with mercury granuloma causing chronic mercury elevation. She underwent excision of the granuloma (Figure 2) with endovascular surgery via an elliptical incision. The patient was subsequently lost to follow-up.
Comment
Elemental mercury is a silver liquid at room temperature that spontaneously evaporates to form mercury vapor, an invisible, odorless, toxic gas. Accidental cutaneous exposure typically is safely managed by washing exposed skin with soap and water,16 though there is a potential risk for systemic absorption, especially when the skin is inflamed. When metallic mercury is subcutaneously injected, it is advised to promptly excise all subcutaneous areas containing mercury, regardless of any symptoms of systemic toxicity. Patients should subsequently be monitored for signs of both central nervous system (CNS) and renal deficits, undergo chelation therapy when systemic effects are apparent, and finally receive psychiatric consultation and treatment when necessary.17
Inorganic mercury compounds are formed when elemental mercury combines with sulfur or oxygen and often take the form of mercury salts, which appear as white crystals.16 These salts occur naturally in the environment and are used in pesticides, antiseptics, and skin-lightening creams and soaps.18
Methylmercury is a highly toxic, organic compound that is capable of crossing the placental and blood-brain barriers. It is the most common organic mercury compound found in the environment.16 Most humans have trace amounts of methylmercury in their bodies, typically as a result of consuming seafood.5
Exposure to mercury most commonly occurs through chronic consumption of methylmercury in seafood or acute inhalation of elemental mercury vapors.9 Iatrogenic cases of mercury exposure via injection also have been reported in the literature, including a case resulting in acute poisoning due to peritoneal lavage with mercury bichloride.19 Acute mercury-induced pulmonary damage typically resolves completely. However, there have been reported cases of exposure progressing to interstitial emphysema, pneumatocele, pneumothorax, pneumomediastinum, interstitial fibrosis, and chronic respiratory insufficiency, with examples of fatal acute respiratory distress syndrome being reported.5,16,20 Although individuals who inhale mercury vapors initially may be unaware of exposure due to little upper airway irritation, symptoms following an initial acute exposure may include ptyalism, a metallic taste, dysphagia, enteritis, diarrhea, nausea, renal damage, and CNS effects.16 Additionally, exposure may lead to confusion with signs and symptoms of metal fume fever, including shortness of breath, pleuritic chest pain, stomatitis, lethargy, and vomiting.20
Chronic exposure to mercury vapor can result in accumulation of mercury in the body, leading to neuropsychiatric, dermatologic, oropharyngeal, and renal manifestations. Sore throat, fever, headache, fatigue, dyspnea, chest pain, and pneumonitis are common.16 Typically, low-level exposure to elemental mercury does not lead to long-lasting health effects. However, individuals exposed to high-level elemental mercury vapors may require hospitalization. Treatment of acute mercury poisoning consists of removing the source of exposure, followed by cardiopulmonary support.16
Specific assays for mercury levels in blood and urine are useful to assess the level of exposure and risk to the patient. Blood mercury concentrations of 20 µg/L or below are considered within reference range; however, once blood and urine concentrations of mercury exceed 100 µg/L, clinical signs of acute mercury poisoning typically manifest.21 Chest radiographs can reveal pulmonary damage, while complete blood cell count, metabolic panel, and urinalysis can assess damage to other organs. Neuropsychiatric testing and nerve conduction studies may provide objective evidence of CNS toxicity. Assays for N-acetyl-β-D-glucosaminidase can provide an indication of early renal tubular dysfunction.16
Elemental mercury is not absorbed from the gastrointestinal tract, posing minimal risk for acute toxicity from ingestion. Generally, less than 10% of ingested inorganic mercury is absorbed from the gut, while elemental mercury is nonabsorbable.10 If an individual ingests a large amount of mercury, it may persist in the gastrointestinal tract for an extended period. Mercury is radiopaque, and abdominal radiographs should be obtained in all cases of ingestion.16
Mercury is toxic to the CNS and peripheral nervous system, resulting in erethism mercurialis, a constellation of neuropsychologic signs and symptoms including restlessness, irritability, insomnia, emotional lability, difficulty concentrating, and impaired memory. In severe cases, delirium and psychosis may develop. Other CNS effects include tremors, paresthesia, dysarthria, neuromuscular changes, headaches, polyneuropathy, and cerebellar ataxia, as well as ophthalmologic and audiologic impairment.5,16
Upon inhalation exposure, patients with respiratory concerns should be given oxygen. Bronchospasms are treated with bronchodilators; however, if multiple chemical exposures are suspected, bronchial-sensitizing agents may pose additional risks. Corticosteroids and antibiotics have been recommended for treatment of chemical pneumonitis, but their efficacy has not been substantiated.16
Skin reactions associated with skin contact to elemental mercury are rare. However, hives and dermatitis have been observed following accidental contact with inorganic mercury compounds.5 Manifestation in children chronically exposed to mercury includes a nonallergic hypersensitivity (acrodynia),5,17 which is characterized by pain and dusky pink discoloration in the hands and feet, most often seen in children chronically exposed to mercury absorbed from vapor inhalation or cutaneous exposure.16
Renal conditions associated with acute inhalation of elemental mercury vapor include proteinuria, nephrotic syndrome, temporary tubular dysfunction, acute tubular necrosis, and oliguric renal failure.16 Chronic exposure to inorganic mercury compounds also has been reported to cause renal damage.5 Chelation therapy should be performed for any symptomatic patient with a clear history of acute elemental mercury exposure.16 The most frequently used chelation agent in cases of acute inorganic mercury exposures is dimercaprol. In rare cases of mercury intoxication, hemodialysis is required in the treatment of renal failure and to expedite removal of dimercaprol-mercury complexes.16
Cardiovascular symptoms associated with acute inhalation of high levels of elemental mercury include tachycardia and hypertension.16 Increases in blood pressure, palpitations, and heart rate also have been observed in instances of acute elemental mercury exposure. Studies show that exposure to mercury increases both the risk for acute myocardial infarction as well as death from coronary heart and cardiovascular diseases.5
Conclusion
Mercury poisoning presents with varied neuropsychologic signs and symptoms. Our case provides insight into a unique route of exposure for mercury toxicity. In addition to the unusual presentation of a mercury granuloma, our case illustrates how surgical techniques can aid in removal of cutaneous reservoirs in the setting of percutaneous exposure.
- History of mercury. Government of Canada website. Modified April 26, 2010. Accessed March 11, 2021. https://www.canada.ca/en/environment-climate-change/services/pollutants/mercury-environment/about/history.html
- Dartmouth Toxic Metals Superfund Research Program website. Accessed March 11, 2021. https://sites.dartmouth.edu/toxmetal/
- Norn S, Permin H, Kruse E, et al. Mercury—a major agent in the history of medicine and alchemy [in Danish]. Dan Medicinhist Arbog. 2008;36:21-40.
- Waldron HA. Did the Mad Hatter have mercury poisoning? Br Med J (Clin Res Ed). 1983;287:1961.
- Poulin J, Gibb H. Mercury: assessing the environmental burden of disease at national and local levels. WHO Environmental Burden of Disease Series No. 16. World Health Organization; 2008.
- Charcot JM. Clinical lectures of the diseases of the nervous system. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:186.
- Kinnier Wilson SA. Neurology. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:739-740.
- Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1-24.
- Mercury and health. World Health Organization website. Updated March 31, 2017. Accessed March 12, 2021. http://www.whoint/mediacentre/factsheets/fs361/en/
- Olson DA. Mercury toxicity. Updated November 5, 2018. Accessed March 12, 2021.http://emedicine.medscape.com/article/1175560-overview
- Mercury thermometers. Environmental Protection Agency website. Updated June 26, 2018. https://www.epa.gov/mercury/mercury-thermometers
- Jao-Tan C, Pope E. Cutaneous poisoning syndromes in children: a review. Curr Opin Pediatr. 2006;18:410-416.
- US Department of Health and Human Services: Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological profile for mercury: regulations and advisories. Published March 1999. Accessed March 23, 2021. https://www.atsdr.cdc.gov/toxprofiles/tp46.pdf
- US Food and Drug Administration. Mercury levels in commercial fish and shellfish (1990-2012). Updated October 25, 2017. Accessed March 16, 2021. https://www.fda.gov/food/metals-and-your-food/mercury-levels-commercial-fish-and-shellfish-1990-2012
- Hamann CR, Boonchai W, Wen L, et al. Spectrometric analysis of mercury content in 549 skin-lightening products: is mercury toxicity a hidden global health hazard? J Am Acad Dermatol. 2014;70:281-287.e3.
- Mercury. Managing Hazardous Materials Incidents. Agency for Toxic Substances and Disease Registry website. Accessed March 16, 2021. https://www.atsdr.cdc.gov/MHMI/mmg46.pdf
- Krohn IT, Solof A, Mobini J, et al. Subcutaneous injection of metallic mercury. JAMA. 1980;243:548-549.
- Lai O, Parsi KK, Wu D, et al. Mercury toxicity presenting acrodynia and a papulovesicular eruption in a 5-year-old girl. Dermatol Online J. 2016;16;22:13030/qt6444r7nc.
- Dolianiti M, Tasiopoulou K, Kalostou A, et al. Mercury bichloride iatrogenic poisoning: a case report. J Clin Toxicol. 2016;6:2. doi:10.4172/2161-0495.1000290
- Broussard LA, Hammett-Stabler CA, Winecker RE, et al. The toxicology of mercury. Lab Med. 2002;33:614-625. doi:10.1309/5HY1-V3NE-2LFL-P9MT
- Byeong-Jin Y, Byoung-Gwon K, Man-Joong J, et al. Evaluation of mercury exposure levels, clinical diagnosis and treatment for mercury intoxication. Ann Occup Environ Med. 2016;28:5.
Evidence of human exposure to mercury dates as far back as the Egyptians in 1500
Mercury release in the environment primarily is a function of human activity, including coal-fired power plants, residential heating, and mining.9,10 Mercury from these sources is commonly found in the sediment of lakes and bays, where it is enzymatically converted to methylmercury by aquatic microorganisms; subsequent food chain biomagnification results in elevated mercury levels in apex predators. Substantial release of mercury into the environment also can be attributed to health care facilities from their use of thermometers containing 0.5 to 3 g of elemental mercury,11 blood pressure monitors, and medical waste incinerators.5
Mercury has been reported as the second most common cause of heavy metal poisoning after lead.12 Standards from the US Food and Drug Administration dictate that methylmercury levels in fish and wheat products must not exceed 1 ppm.13 Most plant and animal food sources contain methylmercury at levels between 0.0001 and 0.01 ppm; mercury concentrations are especially high in tuna, averaging 0.4 ppm, while larger predatory fish contain levels in excess of 1 ppm.14 The use of mercury-containing cosmetic products also presents a substantial exposure risk to consumers.5,10 In one study, 3.3% of skin-lightening creams and soaps purchased within the United States contained concentrations of mercury exceeding 1000 ppm.15
We describe a case of mercury toxicity resulting from intentional injection of liquid mercury into the right antecubital fossa in a suicide attempt.
Case Report
A 31-year-old woman presented to the family practice center for evaluation of a firm stained area on the skin of the right arm. She reported increasing anxiety, depression, tremors, irritability, and difficulty concentrating over the last 6 months. She denied headache and joint or muscle pain. Four years earlier, she had broken apart a thermometer and injected approximately 0.7 mL of its contents into the right arm in a suicide attempt. She intended to inject the thermometer’s contents directly into a vein, but the material instead entered the surrounding tissue. She denied notable pain or itching overlying the injection site. Her medications included aripiprazole and buspirone. She noted that she smoked half a pack of cigarettes per day and had a history of methamphetamine abuse. She was homeless and unemployed. Physical examination revealed an anxious tremulous woman with an erythematous to bluish gray, firm plaque on the right antecubital fossa (Figure 1). There were no notable tremors and no gait disturbance.

Her blood mercury level was greater than 100 µg/L and urine mercury was 477 µg/g (reference ranges, 1–8 μg/L and 4–5 μg/L, respectively). A radiograph of the right elbow area revealed scattered punctate foci of increased density within or overlying the anterolateral elbow soft tissues. She was diagnosed with mercury granuloma causing chronic mercury elevation. She underwent excision of the granuloma (Figure 2) with endovascular surgery via an elliptical incision. The patient was subsequently lost to follow-up.

Comment
Elemental mercury is a silver liquid at room temperature that spontaneously evaporates to form mercury vapor, an invisible, odorless, toxic gas. Accidental cutaneous exposure typically is safely managed by washing exposed skin with soap and water,16 though there is a potential risk for systemic absorption, especially when the skin is inflamed. When metallic mercury is subcutaneously injected, it is advised to promptly excise all subcutaneous areas containing mercury, regardless of any symptoms of systemic toxicity. Patients should subsequently be monitored for signs of both central nervous system (CNS) and renal deficits, undergo chelation therapy when systemic effects are apparent, and finally receive psychiatric consultation and treatment when necessary.17
Inorganic mercury compounds are formed when elemental mercury combines with sulfur or oxygen and often take the form of mercury salts, which appear as white crystals.16 These salts occur naturally in the environment and are used in pesticides, antiseptics, and skin-lightening creams and soaps.18
Methylmercury is a highly toxic, organic compound that is capable of crossing the placental and blood-brain barriers. It is the most common organic mercury compound found in the environment.16 Most humans have trace amounts of methylmercury in their bodies, typically as a result of consuming seafood.5
Exposure to mercury most commonly occurs through chronic consumption of methylmercury in seafood or acute inhalation of elemental mercury vapors.9 Iatrogenic cases of mercury exposure via injection also have been reported in the literature, including a case resulting in acute poisoning due to peritoneal lavage with mercury bichloride.19 Acute mercury-induced pulmonary damage typically resolves completely. However, there have been reported cases of exposure progressing to interstitial emphysema, pneumatocele, pneumothorax, pneumomediastinum, interstitial fibrosis, and chronic respiratory insufficiency, with examples of fatal acute respiratory distress syndrome being reported.5,16,20 Although individuals who inhale mercury vapors initially may be unaware of exposure due to little upper airway irritation, symptoms following an initial acute exposure may include ptyalism, a metallic taste, dysphagia, enteritis, diarrhea, nausea, renal damage, and CNS effects.16 Additionally, exposure may lead to confusion with signs and symptoms of metal fume fever, including shortness of breath, pleuritic chest pain, stomatitis, lethargy, and vomiting.20
Chronic exposure to mercury vapor can result in accumulation of mercury in the body, leading to neuropsychiatric, dermatologic, oropharyngeal, and renal manifestations. Sore throat, fever, headache, fatigue, dyspnea, chest pain, and pneumonitis are common.16 Typically, low-level exposure to elemental mercury does not lead to long-lasting health effects. However, individuals exposed to high-level elemental mercury vapors may require hospitalization. Treatment of acute mercury poisoning consists of removing the source of exposure, followed by cardiopulmonary support.16
Specific assays for mercury levels in blood and urine are useful to assess the level of exposure and risk to the patient. Blood mercury concentrations of 20 µg/L or below are considered within reference range; however, once blood and urine concentrations of mercury exceed 100 µg/L, clinical signs of acute mercury poisoning typically manifest.21 Chest radiographs can reveal pulmonary damage, while complete blood cell count, metabolic panel, and urinalysis can assess damage to other organs. Neuropsychiatric testing and nerve conduction studies may provide objective evidence of CNS toxicity. Assays for N-acetyl-β-D-glucosaminidase can provide an indication of early renal tubular dysfunction.16
Elemental mercury is not absorbed from the gastrointestinal tract, posing minimal risk for acute toxicity from ingestion. Generally, less than 10% of ingested inorganic mercury is absorbed from the gut, while elemental mercury is nonabsorbable.10 If an individual ingests a large amount of mercury, it may persist in the gastrointestinal tract for an extended period. Mercury is radiopaque, and abdominal radiographs should be obtained in all cases of ingestion.16
Mercury is toxic to the CNS and peripheral nervous system, resulting in erethism mercurialis, a constellation of neuropsychologic signs and symptoms including restlessness, irritability, insomnia, emotional lability, difficulty concentrating, and impaired memory. In severe cases, delirium and psychosis may develop. Other CNS effects include tremors, paresthesia, dysarthria, neuromuscular changes, headaches, polyneuropathy, and cerebellar ataxia, as well as ophthalmologic and audiologic impairment.5,16
Upon inhalation exposure, patients with respiratory concerns should be given oxygen. Bronchospasms are treated with bronchodilators; however, if multiple chemical exposures are suspected, bronchial-sensitizing agents may pose additional risks. Corticosteroids and antibiotics have been recommended for treatment of chemical pneumonitis, but their efficacy has not been substantiated.16
Skin reactions associated with skin contact to elemental mercury are rare. However, hives and dermatitis have been observed following accidental contact with inorganic mercury compounds.5 Manifestation in children chronically exposed to mercury includes a nonallergic hypersensitivity (acrodynia),5,17 which is characterized by pain and dusky pink discoloration in the hands and feet, most often seen in children chronically exposed to mercury absorbed from vapor inhalation or cutaneous exposure.16
Renal conditions associated with acute inhalation of elemental mercury vapor include proteinuria, nephrotic syndrome, temporary tubular dysfunction, acute tubular necrosis, and oliguric renal failure.16 Chronic exposure to inorganic mercury compounds also has been reported to cause renal damage.5 Chelation therapy should be performed for any symptomatic patient with a clear history of acute elemental mercury exposure.16 The most frequently used chelation agent in cases of acute inorganic mercury exposures is dimercaprol. In rare cases of mercury intoxication, hemodialysis is required in the treatment of renal failure and to expedite removal of dimercaprol-mercury complexes.16
Cardiovascular symptoms associated with acute inhalation of high levels of elemental mercury include tachycardia and hypertension.16 Increases in blood pressure, palpitations, and heart rate also have been observed in instances of acute elemental mercury exposure. Studies show that exposure to mercury increases both the risk for acute myocardial infarction as well as death from coronary heart and cardiovascular diseases.5
Conclusion
Mercury poisoning presents with varied neuropsychologic signs and symptoms. Our case provides insight into a unique route of exposure for mercury toxicity. In addition to the unusual presentation of a mercury granuloma, our case illustrates how surgical techniques can aid in removal of cutaneous reservoirs in the setting of percutaneous exposure.
Evidence of human exposure to mercury dates as far back as the Egyptians in 1500
Mercury release in the environment primarily is a function of human activity, including coal-fired power plants, residential heating, and mining.9,10 Mercury from these sources is commonly found in the sediment of lakes and bays, where it is enzymatically converted to methylmercury by aquatic microorganisms; subsequent food chain biomagnification results in elevated mercury levels in apex predators. Substantial release of mercury into the environment also can be attributed to health care facilities from their use of thermometers containing 0.5 to 3 g of elemental mercury,11 blood pressure monitors, and medical waste incinerators.5
Mercury has been reported as the second most common cause of heavy metal poisoning after lead.12 Standards from the US Food and Drug Administration dictate that methylmercury levels in fish and wheat products must not exceed 1 ppm.13 Most plant and animal food sources contain methylmercury at levels between 0.0001 and 0.01 ppm; mercury concentrations are especially high in tuna, averaging 0.4 ppm, while larger predatory fish contain levels in excess of 1 ppm.14 The use of mercury-containing cosmetic products also presents a substantial exposure risk to consumers.5,10 In one study, 3.3% of skin-lightening creams and soaps purchased within the United States contained concentrations of mercury exceeding 1000 ppm.15
We describe a case of mercury toxicity resulting from intentional injection of liquid mercury into the right antecubital fossa in a suicide attempt.
Case Report
A 31-year-old woman presented to the family practice center for evaluation of a firm stained area on the skin of the right arm. She reported increasing anxiety, depression, tremors, irritability, and difficulty concentrating over the last 6 months. She denied headache and joint or muscle pain. Four years earlier, she had broken apart a thermometer and injected approximately 0.7 mL of its contents into the right arm in a suicide attempt. She intended to inject the thermometer’s contents directly into a vein, but the material instead entered the surrounding tissue. She denied notable pain or itching overlying the injection site. Her medications included aripiprazole and buspirone. She noted that she smoked half a pack of cigarettes per day and had a history of methamphetamine abuse. She was homeless and unemployed. Physical examination revealed an anxious tremulous woman with an erythematous to bluish gray, firm plaque on the right antecubital fossa (Figure 1). There were no notable tremors and no gait disturbance.

Her blood mercury level was greater than 100 µg/L and urine mercury was 477 µg/g (reference ranges, 1–8 μg/L and 4–5 μg/L, respectively). A radiograph of the right elbow area revealed scattered punctate foci of increased density within or overlying the anterolateral elbow soft tissues. She was diagnosed with mercury granuloma causing chronic mercury elevation. She underwent excision of the granuloma (Figure 2) with endovascular surgery via an elliptical incision. The patient was subsequently lost to follow-up.

Comment
Elemental mercury is a silver liquid at room temperature that spontaneously evaporates to form mercury vapor, an invisible, odorless, toxic gas. Accidental cutaneous exposure typically is safely managed by washing exposed skin with soap and water,16 though there is a potential risk for systemic absorption, especially when the skin is inflamed. When metallic mercury is subcutaneously injected, it is advised to promptly excise all subcutaneous areas containing mercury, regardless of any symptoms of systemic toxicity. Patients should subsequently be monitored for signs of both central nervous system (CNS) and renal deficits, undergo chelation therapy when systemic effects are apparent, and finally receive psychiatric consultation and treatment when necessary.17
Inorganic mercury compounds are formed when elemental mercury combines with sulfur or oxygen and often take the form of mercury salts, which appear as white crystals.16 These salts occur naturally in the environment and are used in pesticides, antiseptics, and skin-lightening creams and soaps.18
Methylmercury is a highly toxic, organic compound that is capable of crossing the placental and blood-brain barriers. It is the most common organic mercury compound found in the environment.16 Most humans have trace amounts of methylmercury in their bodies, typically as a result of consuming seafood.5
Exposure to mercury most commonly occurs through chronic consumption of methylmercury in seafood or acute inhalation of elemental mercury vapors.9 Iatrogenic cases of mercury exposure via injection also have been reported in the literature, including a case resulting in acute poisoning due to peritoneal lavage with mercury bichloride.19 Acute mercury-induced pulmonary damage typically resolves completely. However, there have been reported cases of exposure progressing to interstitial emphysema, pneumatocele, pneumothorax, pneumomediastinum, interstitial fibrosis, and chronic respiratory insufficiency, with examples of fatal acute respiratory distress syndrome being reported.5,16,20 Although individuals who inhale mercury vapors initially may be unaware of exposure due to little upper airway irritation, symptoms following an initial acute exposure may include ptyalism, a metallic taste, dysphagia, enteritis, diarrhea, nausea, renal damage, and CNS effects.16 Additionally, exposure may lead to confusion with signs and symptoms of metal fume fever, including shortness of breath, pleuritic chest pain, stomatitis, lethargy, and vomiting.20
Chronic exposure to mercury vapor can result in accumulation of mercury in the body, leading to neuropsychiatric, dermatologic, oropharyngeal, and renal manifestations. Sore throat, fever, headache, fatigue, dyspnea, chest pain, and pneumonitis are common.16 Typically, low-level exposure to elemental mercury does not lead to long-lasting health effects. However, individuals exposed to high-level elemental mercury vapors may require hospitalization. Treatment of acute mercury poisoning consists of removing the source of exposure, followed by cardiopulmonary support.16
Specific assays for mercury levels in blood and urine are useful to assess the level of exposure and risk to the patient. Blood mercury concentrations of 20 µg/L or below are considered within reference range; however, once blood and urine concentrations of mercury exceed 100 µg/L, clinical signs of acute mercury poisoning typically manifest.21 Chest radiographs can reveal pulmonary damage, while complete blood cell count, metabolic panel, and urinalysis can assess damage to other organs. Neuropsychiatric testing and nerve conduction studies may provide objective evidence of CNS toxicity. Assays for N-acetyl-β-D-glucosaminidase can provide an indication of early renal tubular dysfunction.16
Elemental mercury is not absorbed from the gastrointestinal tract, posing minimal risk for acute toxicity from ingestion. Generally, less than 10% of ingested inorganic mercury is absorbed from the gut, while elemental mercury is nonabsorbable.10 If an individual ingests a large amount of mercury, it may persist in the gastrointestinal tract for an extended period. Mercury is radiopaque, and abdominal radiographs should be obtained in all cases of ingestion.16
Mercury is toxic to the CNS and peripheral nervous system, resulting in erethism mercurialis, a constellation of neuropsychologic signs and symptoms including restlessness, irritability, insomnia, emotional lability, difficulty concentrating, and impaired memory. In severe cases, delirium and psychosis may develop. Other CNS effects include tremors, paresthesia, dysarthria, neuromuscular changes, headaches, polyneuropathy, and cerebellar ataxia, as well as ophthalmologic and audiologic impairment.5,16
Upon inhalation exposure, patients with respiratory concerns should be given oxygen. Bronchospasms are treated with bronchodilators; however, if multiple chemical exposures are suspected, bronchial-sensitizing agents may pose additional risks. Corticosteroids and antibiotics have been recommended for treatment of chemical pneumonitis, but their efficacy has not been substantiated.16
Skin reactions associated with skin contact to elemental mercury are rare. However, hives and dermatitis have been observed following accidental contact with inorganic mercury compounds.5 Manifestation in children chronically exposed to mercury includes a nonallergic hypersensitivity (acrodynia),5,17 which is characterized by pain and dusky pink discoloration in the hands and feet, most often seen in children chronically exposed to mercury absorbed from vapor inhalation or cutaneous exposure.16
Renal conditions associated with acute inhalation of elemental mercury vapor include proteinuria, nephrotic syndrome, temporary tubular dysfunction, acute tubular necrosis, and oliguric renal failure.16 Chronic exposure to inorganic mercury compounds also has been reported to cause renal damage.5 Chelation therapy should be performed for any symptomatic patient with a clear history of acute elemental mercury exposure.16 The most frequently used chelation agent in cases of acute inorganic mercury exposures is dimercaprol. In rare cases of mercury intoxication, hemodialysis is required in the treatment of renal failure and to expedite removal of dimercaprol-mercury complexes.16
Cardiovascular symptoms associated with acute inhalation of high levels of elemental mercury include tachycardia and hypertension.16 Increases in blood pressure, palpitations, and heart rate also have been observed in instances of acute elemental mercury exposure. Studies show that exposure to mercury increases both the risk for acute myocardial infarction as well as death from coronary heart and cardiovascular diseases.5
Conclusion
Mercury poisoning presents with varied neuropsychologic signs and symptoms. Our case provides insight into a unique route of exposure for mercury toxicity. In addition to the unusual presentation of a mercury granuloma, our case illustrates how surgical techniques can aid in removal of cutaneous reservoirs in the setting of percutaneous exposure.
- History of mercury. Government of Canada website. Modified April 26, 2010. Accessed March 11, 2021. https://www.canada.ca/en/environment-climate-change/services/pollutants/mercury-environment/about/history.html
- Dartmouth Toxic Metals Superfund Research Program website. Accessed March 11, 2021. https://sites.dartmouth.edu/toxmetal/
- Norn S, Permin H, Kruse E, et al. Mercury—a major agent in the history of medicine and alchemy [in Danish]. Dan Medicinhist Arbog. 2008;36:21-40.
- Waldron HA. Did the Mad Hatter have mercury poisoning? Br Med J (Clin Res Ed). 1983;287:1961.
- Poulin J, Gibb H. Mercury: assessing the environmental burden of disease at national and local levels. WHO Environmental Burden of Disease Series No. 16. World Health Organization; 2008.
- Charcot JM. Clinical lectures of the diseases of the nervous system. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:186.
- Kinnier Wilson SA. Neurology. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:739-740.
- Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1-24.
- Mercury and health. World Health Organization website. Updated March 31, 2017. Accessed March 12, 2021. http://www.whoint/mediacentre/factsheets/fs361/en/
- Olson DA. Mercury toxicity. Updated November 5, 2018. Accessed March 12, 2021.http://emedicine.medscape.com/article/1175560-overview
- Mercury thermometers. Environmental Protection Agency website. Updated June 26, 2018. https://www.epa.gov/mercury/mercury-thermometers
- Jao-Tan C, Pope E. Cutaneous poisoning syndromes in children: a review. Curr Opin Pediatr. 2006;18:410-416.
- US Department of Health and Human Services: Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological profile for mercury: regulations and advisories. Published March 1999. Accessed March 23, 2021. https://www.atsdr.cdc.gov/toxprofiles/tp46.pdf
- US Food and Drug Administration. Mercury levels in commercial fish and shellfish (1990-2012). Updated October 25, 2017. Accessed March 16, 2021. https://www.fda.gov/food/metals-and-your-food/mercury-levels-commercial-fish-and-shellfish-1990-2012
- Hamann CR, Boonchai W, Wen L, et al. Spectrometric analysis of mercury content in 549 skin-lightening products: is mercury toxicity a hidden global health hazard? J Am Acad Dermatol. 2014;70:281-287.e3.
- Mercury. Managing Hazardous Materials Incidents. Agency for Toxic Substances and Disease Registry website. Accessed March 16, 2021. https://www.atsdr.cdc.gov/MHMI/mmg46.pdf
- Krohn IT, Solof A, Mobini J, et al. Subcutaneous injection of metallic mercury. JAMA. 1980;243:548-549.
- Lai O, Parsi KK, Wu D, et al. Mercury toxicity presenting acrodynia and a papulovesicular eruption in a 5-year-old girl. Dermatol Online J. 2016;16;22:13030/qt6444r7nc.
- Dolianiti M, Tasiopoulou K, Kalostou A, et al. Mercury bichloride iatrogenic poisoning: a case report. J Clin Toxicol. 2016;6:2. doi:10.4172/2161-0495.1000290
- Broussard LA, Hammett-Stabler CA, Winecker RE, et al. The toxicology of mercury. Lab Med. 2002;33:614-625. doi:10.1309/5HY1-V3NE-2LFL-P9MT
- Byeong-Jin Y, Byoung-Gwon K, Man-Joong J, et al. Evaluation of mercury exposure levels, clinical diagnosis and treatment for mercury intoxication. Ann Occup Environ Med. 2016;28:5.
- History of mercury. Government of Canada website. Modified April 26, 2010. Accessed March 11, 2021. https://www.canada.ca/en/environment-climate-change/services/pollutants/mercury-environment/about/history.html
- Dartmouth Toxic Metals Superfund Research Program website. Accessed March 11, 2021. https://sites.dartmouth.edu/toxmetal/
- Norn S, Permin H, Kruse E, et al. Mercury—a major agent in the history of medicine and alchemy [in Danish]. Dan Medicinhist Arbog. 2008;36:21-40.
- Waldron HA. Did the Mad Hatter have mercury poisoning? Br Med J (Clin Res Ed). 1983;287:1961.
- Poulin J, Gibb H. Mercury: assessing the environmental burden of disease at national and local levels. WHO Environmental Burden of Disease Series No. 16. World Health Organization; 2008.
- Charcot JM. Clinical lectures of the diseases of the nervous system. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:186.
- Kinnier Wilson SA. Neurology. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:739-740.
- Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1-24.
- Mercury and health. World Health Organization website. Updated March 31, 2017. Accessed March 12, 2021. http://www.whoint/mediacentre/factsheets/fs361/en/
- Olson DA. Mercury toxicity. Updated November 5, 2018. Accessed March 12, 2021.http://emedicine.medscape.com/article/1175560-overview
- Mercury thermometers. Environmental Protection Agency website. Updated June 26, 2018. https://www.epa.gov/mercury/mercury-thermometers
- Jao-Tan C, Pope E. Cutaneous poisoning syndromes in children: a review. Curr Opin Pediatr. 2006;18:410-416.
- US Department of Health and Human Services: Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological profile for mercury: regulations and advisories. Published March 1999. Accessed March 23, 2021. https://www.atsdr.cdc.gov/toxprofiles/tp46.pdf
- US Food and Drug Administration. Mercury levels in commercial fish and shellfish (1990-2012). Updated October 25, 2017. Accessed March 16, 2021. https://www.fda.gov/food/metals-and-your-food/mercury-levels-commercial-fish-and-shellfish-1990-2012
- Hamann CR, Boonchai W, Wen L, et al. Spectrometric analysis of mercury content in 549 skin-lightening products: is mercury toxicity a hidden global health hazard? J Am Acad Dermatol. 2014;70:281-287.e3.
- Mercury. Managing Hazardous Materials Incidents. Agency for Toxic Substances and Disease Registry website. Accessed March 16, 2021. https://www.atsdr.cdc.gov/MHMI/mmg46.pdf
- Krohn IT, Solof A, Mobini J, et al. Subcutaneous injection of metallic mercury. JAMA. 1980;243:548-549.
- Lai O, Parsi KK, Wu D, et al. Mercury toxicity presenting acrodynia and a papulovesicular eruption in a 5-year-old girl. Dermatol Online J. 2016;16;22:13030/qt6444r7nc.
- Dolianiti M, Tasiopoulou K, Kalostou A, et al. Mercury bichloride iatrogenic poisoning: a case report. J Clin Toxicol. 2016;6:2. doi:10.4172/2161-0495.1000290
- Broussard LA, Hammett-Stabler CA, Winecker RE, et al. The toxicology of mercury. Lab Med. 2002;33:614-625. doi:10.1309/5HY1-V3NE-2LFL-P9MT
- Byeong-Jin Y, Byoung-Gwon K, Man-Joong J, et al. Evaluation of mercury exposure levels, clinical diagnosis and treatment for mercury intoxication. Ann Occup Environ Med. 2016;28:5.
Practice Points
- Chronic mercury granulomas can present as firm, erythematous to bluish gray plaques.
- Accidental skin contact to elemental mercury may cause urticaria and dermatitis.
- Blood mercury concentrations below 20 11µg/L are considered within reference range; once blood and urine concentrations exceed 100 11µg/L, clinical signs of acute mercury poisoning typically manifest.
- Mercury is toxic to the central and peripheral nervous systems, resulting in erethism mercurialis, a constellation of neuropsychologic signs and symptoms including restlessness, irritability, insomnia, emotional lability, difficulty concentrating, and impaired memory.
Comparison of Dermatologist Ratings on Health Care–Specific and General Consumer Websites
Health care–specific (eg, Healthgrades, Zocdoc, Vitals, WebMD) and general consumer websites (eg, Google, Yelp) are popular platforms for patients to find physicians, schedule appointments, and review physician experiences. Patients find ratings on these websites more trustworthy than standardized surveys distributed by hospitals, but many physicians do not trust the reviews on these sites. For example, in a survey of both physicians (n=828) and patients (n=494), 36% of physicians trusted online reviews compared to 57% of patients.1 The objective of this study was to determine if health care–specific or general consumer websites more accurately reflect overall patient sentiment. This knowledge can help physicians who are seeking to improve the patient experience understand which websites have more accurate and trustworthy reviews.
Methods
A list of dermatologists from the top 10 most and least dermatologist–dense areas in the United States was compiled to examine different physician populations.2 Equal numbers of male and female dermatologists were randomly selected from the most dense areas. All physicians were included from the least dense areas because of limited sample size. Ratings were collected from websites most likely to appear on the first page of a Google search for a physician name, as these are most likely to be seen by patients. Descriptive statistics were generated to describe the study population; mean and median physician rating (using a scale of 1–5); SD; and minimum, maximum, and interquartile ranges. Spearman correlation coefficients were generated to examine the strength of association between ratings from website pairs. P<.05 was considered statistically significant, with analyses performed in R (3.6.2) for Windows (the R Foundation).
Results
A total of 167 representative physicians were included in this analysis; 141 from the most dense areas, and 26 from the least dense areas. The lowest average ratings for the entire sample and most dermatologist–dense areas were found on Yelp (3.61 and 3.60, respectively), and the lowest ratings in the least dermatologist–dense areas were found on Google (3.45)(Table 1). Correlation coefficient values were lowest for Zocdoc and Healthgrades (0.263) and highest for Vitals and WebMD (0.963)(Table 2). The health care–specific sites were closer to the overall average (4.06) than the general consumer sites (eFigure).
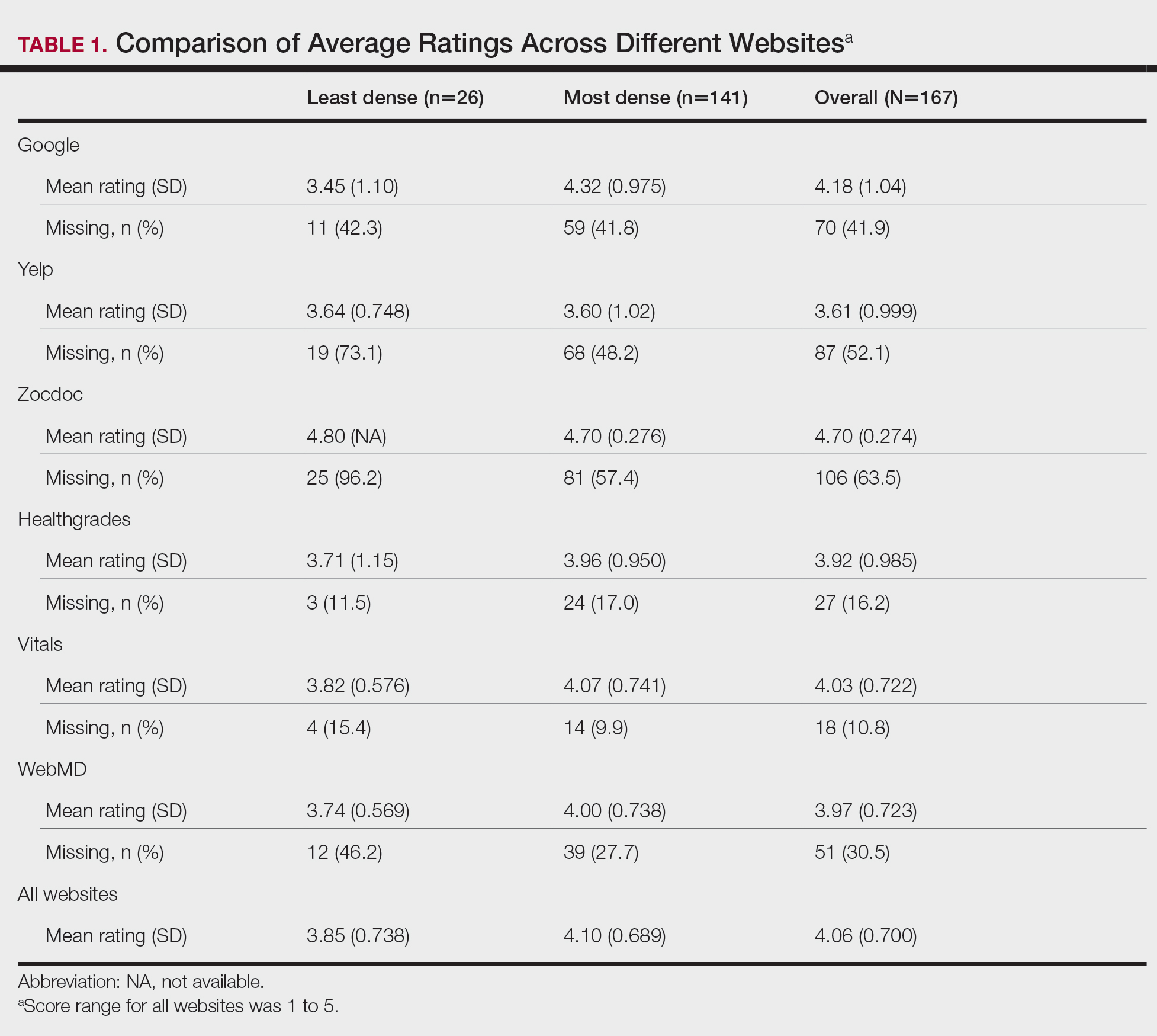
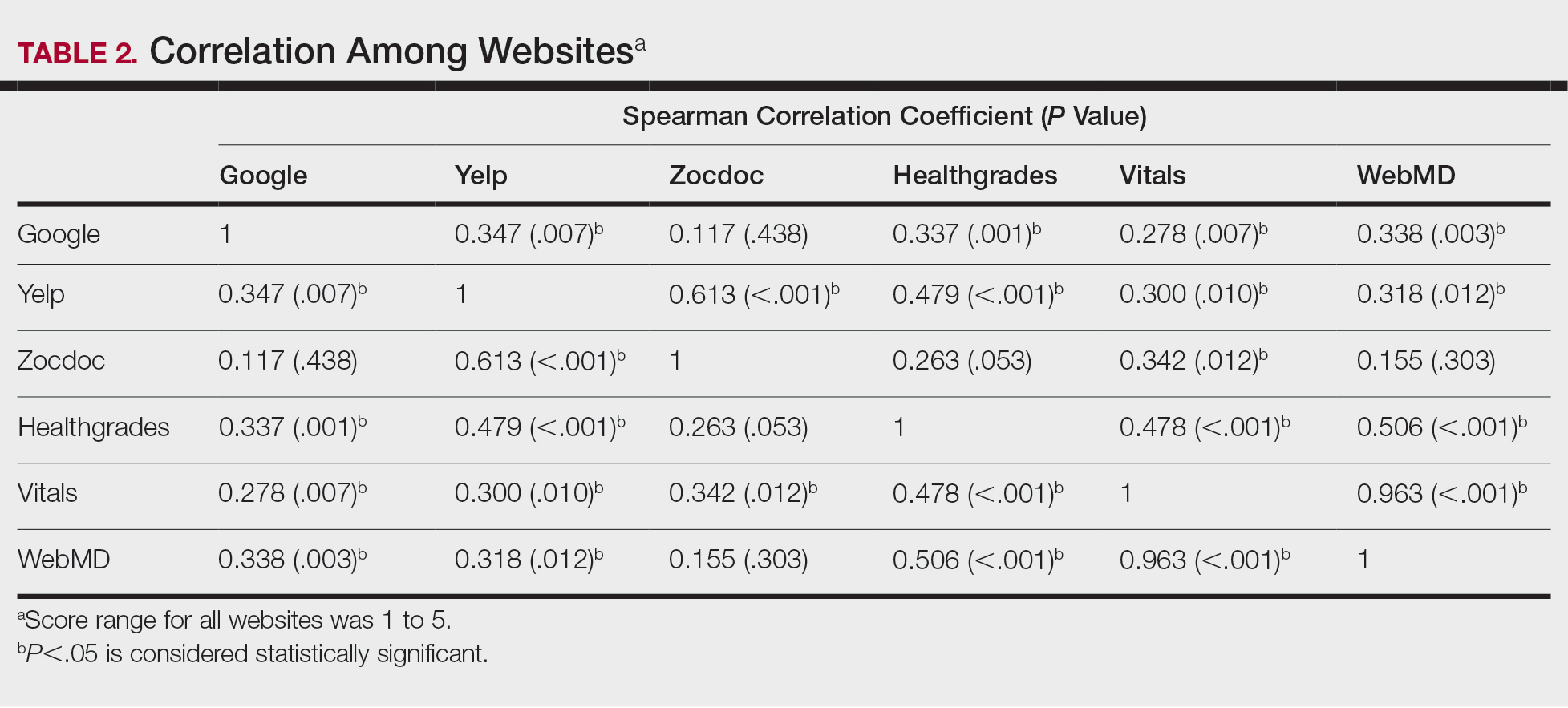
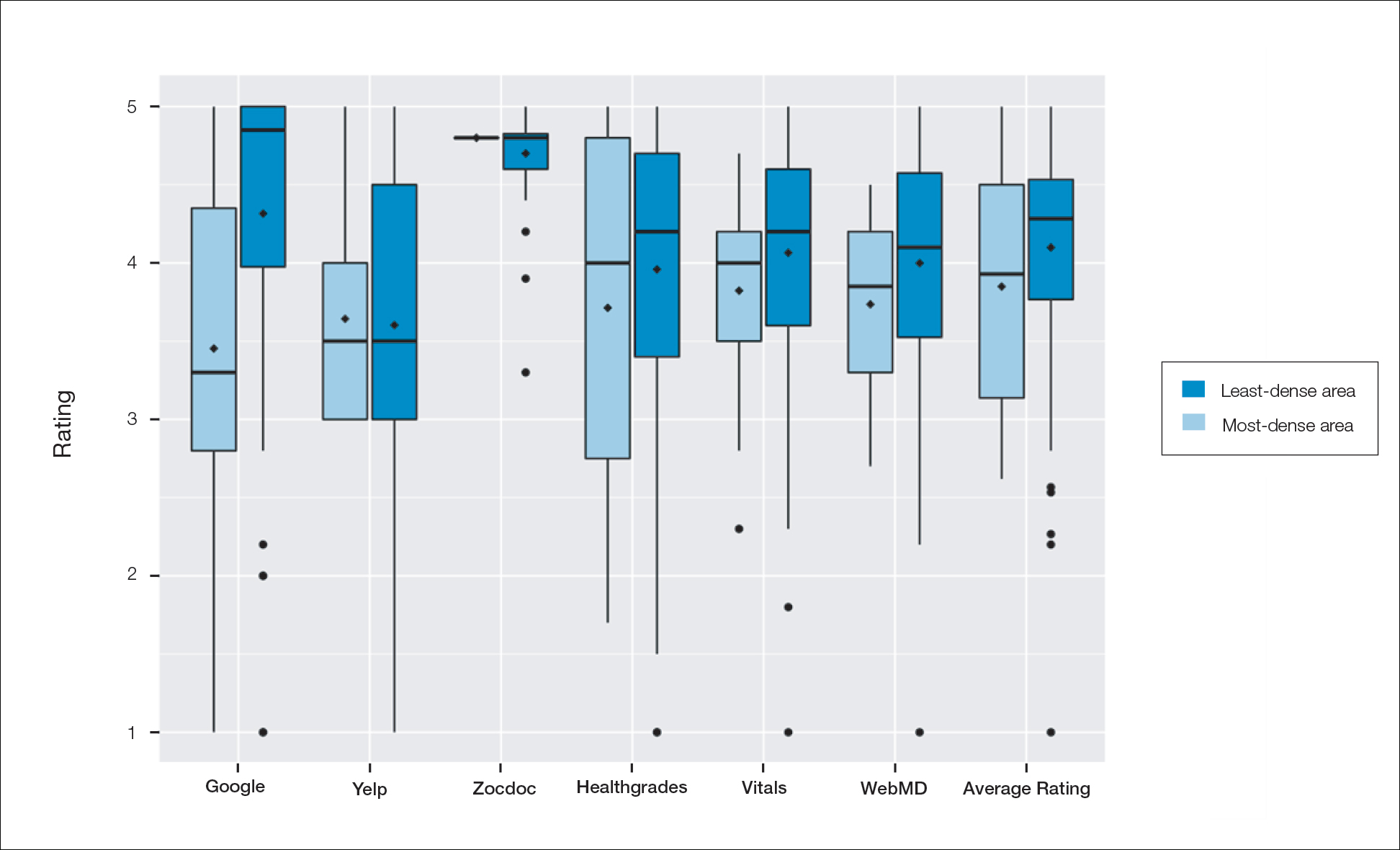
Comment
Although dermatologist ratings on each site had a broad range, we found that patients typically expressed negative interactions on general consumer websites rather than health care–specific websites. When comparing the ratings of the same group of dermatologists across different sites, ratings on health care–specific sites had a higher degree of correlation, with physician ratings more similar between 2 health care–specific sites and less similar between a health care–specific and a general consumer website. This pattern was consistent in both dermatologist-dense and dermatologist-poor areas, despite patients having varying levels of access to dermatologic care and medical resources and potentially different regional preferences of consumer websites. Taken together, these findings imply that health care–specific websites more consistently reflect overall patient sentiment.
Although one 2016 study comparing reviews of dermatology practices on Zocdoc and Yelp also demonstrated lower average ratings on Yelp,3 our study suggests that this trend is not isolated to these 2 sites but can be seen when comparing many health care–specific sites vs general consumer sites.
Our study compared ratings of dermatologists among popular websites to understand those that are most representative of patient attitudes toward physicians. These findings are important because online reviews reflect the entire patient experience, not just the patient-physician interaction, which may explain why physician scores on standardized questionnaires, such as Press Ganey surveys, do not correlate well with their online reviews.4 In a study comparing 98 physicians with negative online ratings to 82 physicians in similar departments with positive ratings, there was no significant difference in scores on patient-physician interaction questions on the Press Ganey survey.5 However, physicians who received negative online reviews scored lower on Press Ganey questions related to nonphysician interactions (eg, office cleanliness, interactions with staff).
The current study was subject to several limitations. Our analysis included all physicians in our random selection without accounting for those physicians with a greater online presence who might be more cognizant of these ratings and try to manipulate them through a reputation-management company or public relations consultant.
Conclusion
Our study suggests that consumer websites are not primarily used by disgruntled patients wishing to express grievances; instead, on average, most physicians received positive reviews. Furthermore, health care–specific websites show a higher degree of concordance than and may more accurately reflect overall patient attitudes toward their physicians than general consumer sites. Reviews from these health care–specific sites may be more helpful than general consumer websites in allowing physicians to understand patient sentiment and improve patient experiences.
- Frost C, Mesfin A. Online reviews of orthopedic surgeons: an emerging trend. Orthopedics. 2015;38:e257-e262. doi:10.3928/01477447-20150402-52
- Waqas B, Cooley V, Lipner SR. Association of sex, location, and experience with online patient ratings of dermatologists. J Am Acad Dermatol. 2020;83:954-955.
- Smith RJ, Lipoff JB. Evaluation of dermatology practice online reviews: lessons from qualitative analysis. JAMA Dermatol. 2016;152:153-157. doi:10.1001/jamadermatol.2015.3950
- Chen J, Presson A, Zhang C, et al. Online physician review websites poorly correlate to a validated metric of patient satisfaction. J Surg Res. 2018;227:1-6.
- Widmer RJ, Maurer MJ, Nayar VR, et al. Online physician reviews do not reflect patient satisfaction survey responses. Mayo Clinic Proc. 2018;93:453-457.
Health care–specific (eg, Healthgrades, Zocdoc, Vitals, WebMD) and general consumer websites (eg, Google, Yelp) are popular platforms for patients to find physicians, schedule appointments, and review physician experiences. Patients find ratings on these websites more trustworthy than standardized surveys distributed by hospitals, but many physicians do not trust the reviews on these sites. For example, in a survey of both physicians (n=828) and patients (n=494), 36% of physicians trusted online reviews compared to 57% of patients.1 The objective of this study was to determine if health care–specific or general consumer websites more accurately reflect overall patient sentiment. This knowledge can help physicians who are seeking to improve the patient experience understand which websites have more accurate and trustworthy reviews.
Methods
A list of dermatologists from the top 10 most and least dermatologist–dense areas in the United States was compiled to examine different physician populations.2 Equal numbers of male and female dermatologists were randomly selected from the most dense areas. All physicians were included from the least dense areas because of limited sample size. Ratings were collected from websites most likely to appear on the first page of a Google search for a physician name, as these are most likely to be seen by patients. Descriptive statistics were generated to describe the study population; mean and median physician rating (using a scale of 1–5); SD; and minimum, maximum, and interquartile ranges. Spearman correlation coefficients were generated to examine the strength of association between ratings from website pairs. P<.05 was considered statistically significant, with analyses performed in R (3.6.2) for Windows (the R Foundation).
Results
A total of 167 representative physicians were included in this analysis; 141 from the most dense areas, and 26 from the least dense areas. The lowest average ratings for the entire sample and most dermatologist–dense areas were found on Yelp (3.61 and 3.60, respectively), and the lowest ratings in the least dermatologist–dense areas were found on Google (3.45)(Table 1). Correlation coefficient values were lowest for Zocdoc and Healthgrades (0.263) and highest for Vitals and WebMD (0.963)(Table 2). The health care–specific sites were closer to the overall average (4.06) than the general consumer sites (eFigure).



Comment
Although dermatologist ratings on each site had a broad range, we found that patients typically expressed negative interactions on general consumer websites rather than health care–specific websites. When comparing the ratings of the same group of dermatologists across different sites, ratings on health care–specific sites had a higher degree of correlation, with physician ratings more similar between 2 health care–specific sites and less similar between a health care–specific and a general consumer website. This pattern was consistent in both dermatologist-dense and dermatologist-poor areas, despite patients having varying levels of access to dermatologic care and medical resources and potentially different regional preferences of consumer websites. Taken together, these findings imply that health care–specific websites more consistently reflect overall patient sentiment.
Although one 2016 study comparing reviews of dermatology practices on Zocdoc and Yelp also demonstrated lower average ratings on Yelp,3 our study suggests that this trend is not isolated to these 2 sites but can be seen when comparing many health care–specific sites vs general consumer sites.
Our study compared ratings of dermatologists among popular websites to understand those that are most representative of patient attitudes toward physicians. These findings are important because online reviews reflect the entire patient experience, not just the patient-physician interaction, which may explain why physician scores on standardized questionnaires, such as Press Ganey surveys, do not correlate well with their online reviews.4 In a study comparing 98 physicians with negative online ratings to 82 physicians in similar departments with positive ratings, there was no significant difference in scores on patient-physician interaction questions on the Press Ganey survey.5 However, physicians who received negative online reviews scored lower on Press Ganey questions related to nonphysician interactions (eg, office cleanliness, interactions with staff).
The current study was subject to several limitations. Our analysis included all physicians in our random selection without accounting for those physicians with a greater online presence who might be more cognizant of these ratings and try to manipulate them through a reputation-management company or public relations consultant.
Conclusion
Our study suggests that consumer websites are not primarily used by disgruntled patients wishing to express grievances; instead, on average, most physicians received positive reviews. Furthermore, health care–specific websites show a higher degree of concordance than and may more accurately reflect overall patient attitudes toward their physicians than general consumer sites. Reviews from these health care–specific sites may be more helpful than general consumer websites in allowing physicians to understand patient sentiment and improve patient experiences.
Health care–specific (eg, Healthgrades, Zocdoc, Vitals, WebMD) and general consumer websites (eg, Google, Yelp) are popular platforms for patients to find physicians, schedule appointments, and review physician experiences. Patients find ratings on these websites more trustworthy than standardized surveys distributed by hospitals, but many physicians do not trust the reviews on these sites. For example, in a survey of both physicians (n=828) and patients (n=494), 36% of physicians trusted online reviews compared to 57% of patients.1 The objective of this study was to determine if health care–specific or general consumer websites more accurately reflect overall patient sentiment. This knowledge can help physicians who are seeking to improve the patient experience understand which websites have more accurate and trustworthy reviews.
Methods
A list of dermatologists from the top 10 most and least dermatologist–dense areas in the United States was compiled to examine different physician populations.2 Equal numbers of male and female dermatologists were randomly selected from the most dense areas. All physicians were included from the least dense areas because of limited sample size. Ratings were collected from websites most likely to appear on the first page of a Google search for a physician name, as these are most likely to be seen by patients. Descriptive statistics were generated to describe the study population; mean and median physician rating (using a scale of 1–5); SD; and minimum, maximum, and interquartile ranges. Spearman correlation coefficients were generated to examine the strength of association between ratings from website pairs. P<.05 was considered statistically significant, with analyses performed in R (3.6.2) for Windows (the R Foundation).
Results
A total of 167 representative physicians were included in this analysis; 141 from the most dense areas, and 26 from the least dense areas. The lowest average ratings for the entire sample and most dermatologist–dense areas were found on Yelp (3.61 and 3.60, respectively), and the lowest ratings in the least dermatologist–dense areas were found on Google (3.45)(Table 1). Correlation coefficient values were lowest for Zocdoc and Healthgrades (0.263) and highest for Vitals and WebMD (0.963)(Table 2). The health care–specific sites were closer to the overall average (4.06) than the general consumer sites (eFigure).



Comment
Although dermatologist ratings on each site had a broad range, we found that patients typically expressed negative interactions on general consumer websites rather than health care–specific websites. When comparing the ratings of the same group of dermatologists across different sites, ratings on health care–specific sites had a higher degree of correlation, with physician ratings more similar between 2 health care–specific sites and less similar between a health care–specific and a general consumer website. This pattern was consistent in both dermatologist-dense and dermatologist-poor areas, despite patients having varying levels of access to dermatologic care and medical resources and potentially different regional preferences of consumer websites. Taken together, these findings imply that health care–specific websites more consistently reflect overall patient sentiment.
Although one 2016 study comparing reviews of dermatology practices on Zocdoc and Yelp also demonstrated lower average ratings on Yelp,3 our study suggests that this trend is not isolated to these 2 sites but can be seen when comparing many health care–specific sites vs general consumer sites.
Our study compared ratings of dermatologists among popular websites to understand those that are most representative of patient attitudes toward physicians. These findings are important because online reviews reflect the entire patient experience, not just the patient-physician interaction, which may explain why physician scores on standardized questionnaires, such as Press Ganey surveys, do not correlate well with their online reviews.4 In a study comparing 98 physicians with negative online ratings to 82 physicians in similar departments with positive ratings, there was no significant difference in scores on patient-physician interaction questions on the Press Ganey survey.5 However, physicians who received negative online reviews scored lower on Press Ganey questions related to nonphysician interactions (eg, office cleanliness, interactions with staff).
The current study was subject to several limitations. Our analysis included all physicians in our random selection without accounting for those physicians with a greater online presence who might be more cognizant of these ratings and try to manipulate them through a reputation-management company or public relations consultant.
Conclusion
Our study suggests that consumer websites are not primarily used by disgruntled patients wishing to express grievances; instead, on average, most physicians received positive reviews. Furthermore, health care–specific websites show a higher degree of concordance than and may more accurately reflect overall patient attitudes toward their physicians than general consumer sites. Reviews from these health care–specific sites may be more helpful than general consumer websites in allowing physicians to understand patient sentiment and improve patient experiences.
- Frost C, Mesfin A. Online reviews of orthopedic surgeons: an emerging trend. Orthopedics. 2015;38:e257-e262. doi:10.3928/01477447-20150402-52
- Waqas B, Cooley V, Lipner SR. Association of sex, location, and experience with online patient ratings of dermatologists. J Am Acad Dermatol. 2020;83:954-955.
- Smith RJ, Lipoff JB. Evaluation of dermatology practice online reviews: lessons from qualitative analysis. JAMA Dermatol. 2016;152:153-157. doi:10.1001/jamadermatol.2015.3950
- Chen J, Presson A, Zhang C, et al. Online physician review websites poorly correlate to a validated metric of patient satisfaction. J Surg Res. 2018;227:1-6.
- Widmer RJ, Maurer MJ, Nayar VR, et al. Online physician reviews do not reflect patient satisfaction survey responses. Mayo Clinic Proc. 2018;93:453-457.
- Frost C, Mesfin A. Online reviews of orthopedic surgeons: an emerging trend. Orthopedics. 2015;38:e257-e262. doi:10.3928/01477447-20150402-52
- Waqas B, Cooley V, Lipner SR. Association of sex, location, and experience with online patient ratings of dermatologists. J Am Acad Dermatol. 2020;83:954-955.
- Smith RJ, Lipoff JB. Evaluation of dermatology practice online reviews: lessons from qualitative analysis. JAMA Dermatol. 2016;152:153-157. doi:10.1001/jamadermatol.2015.3950
- Chen J, Presson A, Zhang C, et al. Online physician review websites poorly correlate to a validated metric of patient satisfaction. J Surg Res. 2018;227:1-6.
- Widmer RJ, Maurer MJ, Nayar VR, et al. Online physician reviews do not reflect patient satisfaction survey responses. Mayo Clinic Proc. 2018;93:453-457.
Practice Points
- Online physician-rating websites are commonly used by patients to find physicians and review experiences.
- Health care–specific sites may more accurately reflect patient sentiment than general consumer sites.
- Dermatologists can use health care–specific sites to understand patient sentiment and learn how to improve patient experiences.
Apremilast Uses and Relevance to the Military
Apremilast is a small-molecule biologic approved by the US Food and Drug Administration (FDA) for use in plaque psoriasis, psoriatic arthritis, and Behçet disease.1-6 Although apremilast is seemingly a less favorable choice for treating psoriasis in the era of injectable biologics, the drug is an important option for patients in the military. In recent months, apremilast also emerged as one of a few systemic medications recommended for the treatment of psoriasis and other dermatologic conditions during the COVID-19 pandemic.7
In this article, we review on-label indications and off-label uses for apremilast; highlight the importance of apremilast for managing psoriasis in the military population; and propose other patient populations in whom the use of apremilast is favorable. We also present a case report that highlights and embodies the benefit of apremilast for military service members.
CASE REPORT
A 28-year-old active-duty male US Navy service member developed extensive guttate psoriasis in a distribution too wide to manage with topical medication (Figure, A–C). His condition did not improve with a trial of oral antibiotics, and he reported itch that affected his sleep. He denied new joint pain, swelling, or deformity.
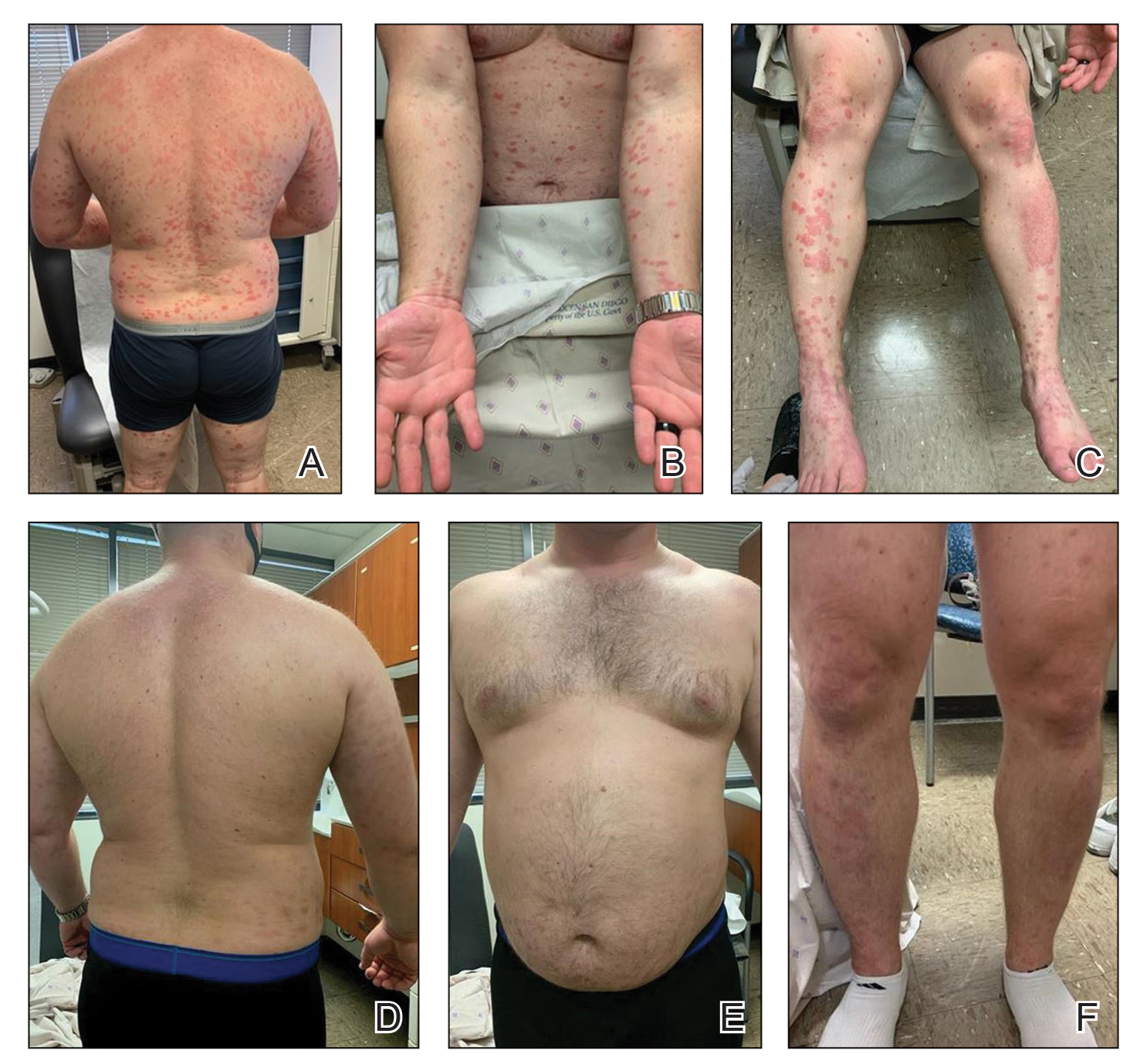
A review of the patient’s service history revealed that he was serving aboard a guided-missile cruiser ship for a tour extending an additional 2 years. Limited medical resources and lack of refrigeration made the use of injectable biologics, such as adalimumab, infeasible. Furthermore, the patient was too critical to the mission to be transported frequently off the ship to a higher level of care for injection of medication. He also had trouble returning for appointments and refills because of the high operational tempo of his command.
After discussion with the patient, oral apremilast was started at 30 mg/d and titrated up to the standard dosing of 30 mg twice daily, with excellent results by 3 months after he started therapy (Figure, D–F).
COMMENT
We reviewed the research on apremilast for its approved indications, including psoriasis; its off-label uses; and strategies for using the drug to treat psoriasis and other dermatologic conditions in military populations. The most recent evidence regarding the use of apremilast in dermatology, rheumatology, and other medical specialties was assessed using published English-language research data and review articles. We conducted a PubMed search of articles indexed for MEDLINE using the following terms: apremilast, Otezla, psoriasis, psoriatic arthritis, arthritis, off-label, Behçet’s, hidradenitis suppurativa, military, and armed forces. We also reviewed citations within relevant articles to identify additional relevant sources.
Off-label uses reviewed here are based on data from randomized controlled trials, large open-label trials, and large prospective case series. Articles with less evidence are not included in this review.
On-Label Usage Profile
Apremilast is an orally administered, small-molecule inhibitor of phosphodiesterase 4. Small-molecule inhibitors are a class of medications with low molecular weight, high stability, and short half-life. They act intracellularly to modulate proinflammatory states through regulation of the proinflammatory cytokine milieu.
Apremilast has been approved by the FDA for use in adult psoriasis and psoriatic arthritis since 2014 and for use in treating oral ulcers of Behçet disease since 2019.1-3,5,6 Recently, a phase 2, multicenter, open-label study on the use of apremilast in pediatric psoriasis patients (aged 12–17 years) demonstrated a similar safety profile with weight-based dosing8; phase 3 trials in this population are in the recruitment phase (ClinicalTrials.gov Identifier NCT03701763).
Because information regarding its use in pregnancy is limited, apremilast is not recommended in this population. It is unknown whether apremilast is present in breast milk; although the manufacturer does not make explicit recommendations regarding use during breastfeeding, an expert panel reviewing management of psoriasis in pregnant and breastfeeding women recommended avoiding its use while breastfeeding.9
Common Adverse Effects
Common adverse effects (AEs) include weight loss (>5% total body weight in 5% of patients; 5%–10% of total body weight in 10%–12% of patients; and ≥10% total body weight in 2% of patients), diarrhea and nausea, headache, and upper respiratory tract infection.10,11 Gastrointestinal AEs tend to be self-limited and improve or resolve after the first few weeks of therapy. Caution is advised in patients older than 65 years and in those at risk for hypotension or volume depletion. Although depressed mood is a rare AE (<1%), apremilast should be used cautiously in patients with a history of depression or suicidal ideation. Weight loss generally is self-limited; routine monitoring of weight is recommended.11
Apremilast in Psoriasis and Psoriatic Arthritis
Psoriasis
The ESTEEM trials established the safety and efficacy of apremilast for use in psoriasis.2,3 In a phase 3, multicenter, double-blind, placebo-controlled trial of 844 patients, apremilast demonstrated a statistically significant 75% or greater reduction from the baseline psoriasis area and severity index score (PASI-75) in 33.1% of patients receiving the medication compared to 5.3% of those receiving placebo.2 Data from real-world practice (outside constraints of clinical trials) suggest slightly greater efficacy than was demonstrated in the ESTEEM trials.
A recently published retrospective, cross-sectional study of 480 patients with psoriasis treated with apremilast reported that 48.6% of patients continuing therapy for a mean (SD) of 6 (1) months achieved PASI-75. Furthermore, the mean dermatology life quality index (DLQI) score of the surveyed population decreased from 13.4 at initiation of treatment to 5.7 at 6 (1) months of treatment—a marked improvement in quality of life.12 Other single-center and smaller study populations also have suggested increased real-world benefit.13,14
Nonetheless, the rate and degree of clearance of plaques with apremilast seem to lag behind what is observed with many of the biologics and traditional medications employed to treat psoriasis.15-19 Furthermore, indirect cost analysis comparisons suggest a much higher cost per level of PASI for apremilast compared to several biologics and to methotrexate.20,21 A study that used indirect methods of comparison to analyze the comparative cost and efficacy of apremilast and methotrexate found no evidence of greater efficacy for apremilast and that the incremental cost to achieve 1 additional PASI-75 responder by using apremilast is $187,888 annually.21
Psoriatic Arthritis
The PALACE clinical trials 1, 2, and 3 assessed the efficacy of apremilast in patients who had prior treatment with conventional disease-modifying antirheumatic drugs or biologics, or both. PALACE 4 evaluated efficacy in treatment-naïve patients; standard dosing of apremilast was found to produce improvement in psoriatic arthritis in treatment-naïve and non–treatment-naïve patients.4-6,22 In the 24-week placebo-controlled phase of the PALACE 1 trial, the American College of Rheumatology (ACR) baseline composite measurement of 20% disease improvement, or ACR20, was achieved in 40% of patients randomized to the standard dosing regimen compared to 19% of patients receiving placebo, a statistically significant result (P<.001).22
Evaluation of long-term study data is beyond the scope of this review, but those data suggest that disease outcomes continue to improve the longer therapy is utilized, with a greater percentage of patients achieving ACR20 as well as ACR50 (50% improvement) and ACR70 (70% improvement) responses. Indirect comparisons analyzing the cost and effectiveness for adalimumab, apremilast, and methotrexate in patients with psoriatic arthritis found that apremilast was less effective than adalimumab and as efficacious as methotrexate, though apremilast carries the highest price tag of these drugs.23
Off-Label Uses
Ease of oral administration and a favorable safety profile have prompted off-label study of apremilast in other inflammatory skin diseases, including atopic dermatitis, hidradenitis suppurativa, lichen planus, rosacea, alopecia areata, and cutaneous sarcoidosis. Publications with a minimum case series of 10 patients are included in the Table.24-32
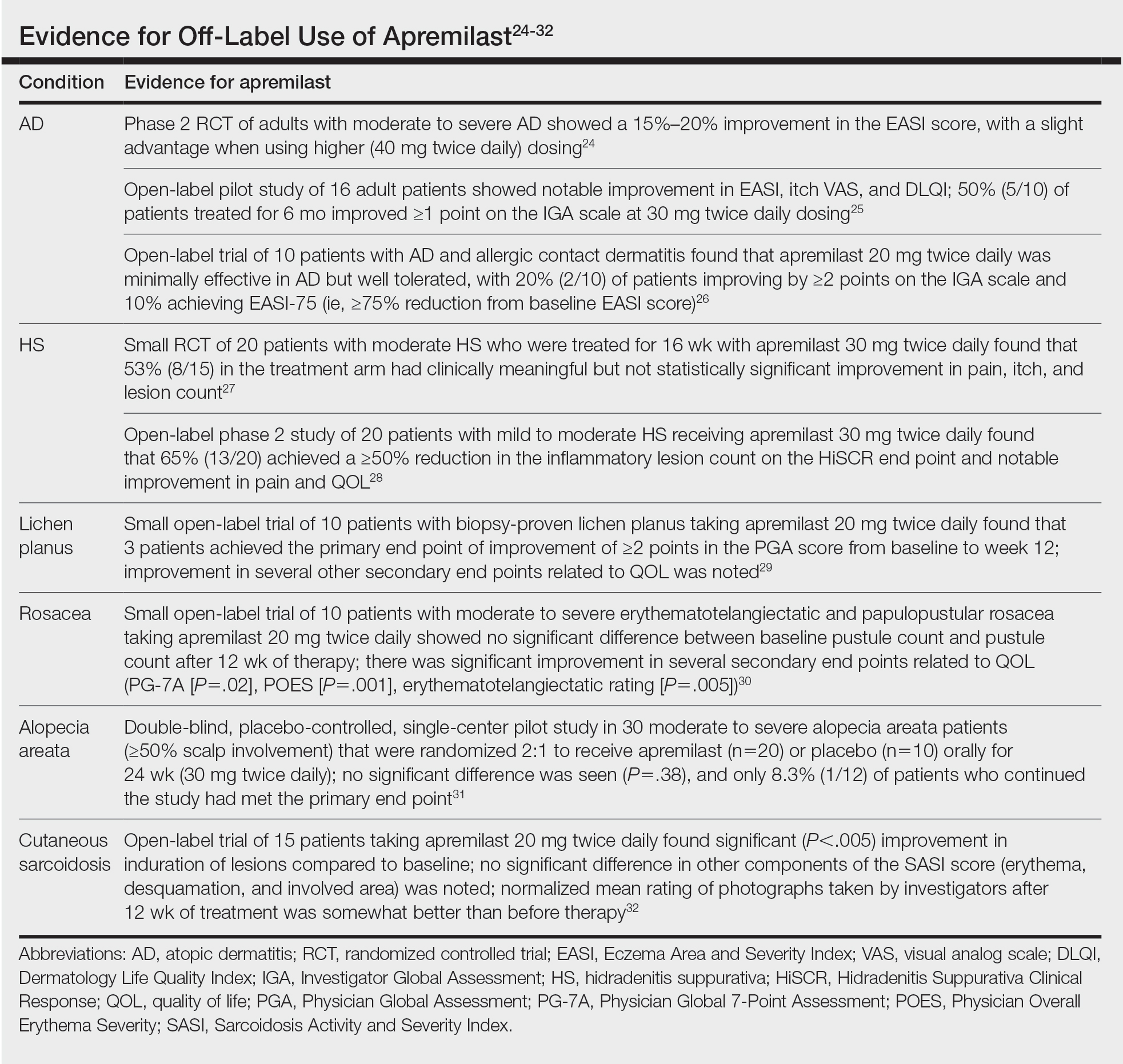
Use in the Military and Beyond
Psoriasis and other inflammatory skin conditions are common in the military and can greatly hinder a service member’s ability to perform their duties and remain ready to deploy. A history of psoriasis is disqualifying for military recruits, but early entry into service, misdiagnosis, and low or no burden of disease at time of entry into the service all contribute to a substantial population of active-duty service members who require treatment of psoriasis.33 Necessity dictates that treatment of this condition extend to theater operations; from 2008 to 2015, more than 3600 soldiers sought care for psoriasis while deployed to a combat theater.34
In some cases, poorly controlled inflammatory skin conditions lead to medical separation.33 Although there are limited data on the use of apremilast in the military, its use during deployment for the treatment of psoriasis and psoriatic arthritis has been reported, with the great majority of service members retaining their deployable status even 1 year after the study period.35
The ideal medication for deployable military personnel should have low toxicity, simple storage, and minimal monitoring requirements, and it should not expose a service member to increased risk while in a combat theater. Worldwide deployability is a requirement for most military occupations. The risk for immunosuppression with targeted immune therapy must be fully weighed, as certain duty stations and deployments might increase the risk for exposure to Mycobacterium tuberculosis, endemic mycopathogens, hepatitis C virus, HIV, Leishmania, and Strongyloides.34
Furthermore, the tumor necrosis factor α inhibitors and IL-17 and IL-23 blockers used to treat psoriasis all require refrigeration; often, this requirement cannot be met in austere overseas settings. Additional requirements for laboratory monitoring, titration of medications, and frequent office visits might prohibit a service member from performing their duties, which, in turn, is detrimental to military readiness and the career of that service member.
Last, the Centers for Disease Control and Prevention recommend avoiding live virus vaccination while taking targeted immune therapy because of safety and effectiveness concerns during immunosuppression.36 This recommendation might disqualify military personnel from deployment to certain locations that require the protection that such vaccines afford. Therefore, apremilast is an ideal option for the military patient population, with many military-specific advantages.
Of course, the military is not the only population in whom ease of use and storage and simplified monitoring parameters are essential. Benefits of apremilast also may translate to patients who are placed in austere conditions or who participate in extended worldwide travel for work or leisure, such as government contractors who deploy in support of military operations, firefighters or national park employees who spend extended periods in resource-limited settings, and foreign-aid workers and diplomats who are engaged in frequent travel around the world. Furthermore, travel to certain regions might increase the risk for exposure to atypical pathogens as well as the desire for a therapeutic option that does not have potential to suppress the immune system. This subset of psoriasis patients might be better treated with novel agents such as apremilast than other drugs that would be the presumed standard of care in a domestic setting.
Final Thoughts
The benefits of apremilast translate to all patients in austere environments with limited resources and during times when immune function is of utmost concern. For military service members and many civilians in austere environments worldwide, apremilast could be considered a first-line systemic agent for psoriasis and psoriatic arthritis. In patients unable to use or tolerate other treatments, apremilast can be considered for off-label therapy (Table24-32). There are times when the approach to prescribing must look beyond primary efficacy, AE profile, and cost—to include occupation, environment, or duties—to select the optimal medication for a patient.
- Hatemi G, Melikoglu M, Tunc R, et al. Apremilast for Behçet’s syndrome—a phase 2, placebo-controlled study. N Engl J Med. 2015;372:1510-1518. doi:10.1056/NEJMoa1408684
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j.jaad.2015.03.049
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate‐to‐severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399. doi:10.1111/bjd.14164
- Cutolo M, Myerson GE, Fleischmann RM, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol. 2016;43:1724-1734. doi:10.3899/jrheum.151376
- Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis. 2016;75:1065-1073. doi:10.1136/annrheumdis-2015-207963
- Wells AF, Edwards CJ, Kivitz AJ, et al. Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: results of the randomized, placebo-controlled PALACE 4 trial. Rheumatology (Oxford). 2018;57:1253-1263. doi:10.1093/rheumatology/key032
- Niaki OZ, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Paller AS, Hong Y, Becker EM, et al. Pharmacokinetics and safety of apremilast in pediatric patients with moderate to severe plaque psoriasis: results from a phase 2 open-label study. J Am Acad Dermatol. 2020;82:389-397. doi:10.1016/j.jaad.2019.08.019
- Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. The Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100. doi:10.1111/ajd.12641
- Otezla. Prescribing information. Amgen Inc; June 2020. Accessed March 13, 2021. www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/otezla/otezla_pi_english.ashx
- Otezla. Product monograph. Amgen Canada Inc; Revised August 2020. Accessed March 13, 2021. www.amgen.ca/products/~/media/FB841218E06B4508B0E7213BC578E641.ashx
- Augustin M, Kleyn CE, Conrad C, et al. Characteristics and outcomes of patients treated with apremilast in the real world: Results from the APPRECIATE study. J Eur Acad Dermatol Venereol. 2020;35:123-134. doi:10.1111/jdv.16431
- Papadavid E, Rompoti N, Theodoropoulos K, et al. Real‐world data on the efficacy and safety of apremilast in patients with moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2018;32:1173-1179. doi:10.1111/jdv.14832
- Wong TH, Sinclair S, Smith B, et al. Real‐world, single‐centre experience of apremilast for the treatment of moderate to severe psoriasis. Clin Exp Dermatol. 2017;42:675-676. doi:10.1111/ced.13150
- Saurat, J‐H, Stingl G, Dubertret L, et al; . Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158:558-566. doi:10.1111/j.1365-2133.2007.08315.x
- Kimball AB, Papp KA, Wasfi Y, et al; Long‐term efficacy of ustekinumab in patients with moderate‐to‐severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535-1545. doi:10.1111/jdv.12046
- Langley, RG, Elewski BE, Lebwohl M, et al; ; Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338. doi:10.1056/NEJMoa1314258
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328. doi:10.1056/NEJMoa1503824
- Papp KA, Leonaridi CL, Blauvelt A, et al. Ixekizumab treatment for psoriasis: integrated efficacy analysis of three double‐blinded, controlled studies (UNCOVER‐1, UNCOVER‐2, UNCOVER‐3). Br J Dermatol. 2018;178:674-681. doi:10.1111/bjd.16050
- Kromer C, Celis D, Sonntag D, et al. Biologicals and small molecules in psoriasis: a systematic review of economic evaluations. PloS One. 2018;13:e0189765. doi:10.1371/journal.pone.0189765
- Armstrong AW, Betts KA, Sundaram M, et al. Comparative efficacy and incremental cost per responder of methotrexate versus apremilast for methotrexate-naïve patients with psoriasis. J Am Acad Dermatol. 2016;75:740-746. doi:10.1016/j.jaad.2016.05.040
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026. doi:10.1136/annrheumdis-2013-205056
- Betts KA, Griffith J, Friedman A, et al. An indirect comparison and cost per responder analysis of adalimumab, methotrexate and apremilast in the treatment of methotrexate-naïve patients with psoriatic arthritis. Curr Med Res Opin. 2016;32:721-729. doi:10.1185/03007995.2016.114002624. Simpson EL, Imafuku S, Poulin Y, et al. A phase 2 randomized trial of apremilast in patients with atopic dermatitis. J Invest Dermatol. 2019;139:1063-1072. doi:10.1016/j.jid.2018.10.043
- Samrao A, Berry TM, Goreshi R, et al. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol. 2012;148:890-897. doi:10.1001/archdermatol.2012.812
- Volf EM, Au S-C, Dumont N, et al. A phase 2, open-label, investigator-initiated study to evaluate the safety and efficacy of apremilast in subjects with recalcitrant allergic contact or atopic dermatitis. J Drugs Dermatol. 2012;11:341-346.
- Vossen ARJV, van Doorn MBA, van der Zee HH, et al. Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial. J Am Acad Dermatol. 2019;80:80-88. doi:10.1016/j.jaad.2018.06.046
- Kerdel FR, Azevedo FA, Don CK, et al. Apremilast for the treatment of mild-to-moderate hidradenitis suppurativa in a prospective, open-label, phase 2 study. J Drugs Dermatol. 2019;18:170-176.
- Paul J, Foss CE, Hirano SA, et al. An open-label pilot study of apremilast for the treatment of moderate to severe lichen planus: a case series. J Am Acad Dermatol. 2013;68:255-261. doi:10.1016/j.jaad.2012.07.014
- Thompson BJ, Furniss M, Zhao W, et al. An oral phosphodiesterase inhibitor (apremilast) for inflammatory rosacea in adults: a pilot study. JAMA Dermatol. 2014;150:1013-1014. doi:10.1001/jamadermatol.2013.10526
- Mikhaylov D, Pavel A, Yao C, et al. A randomized placebo-controlled single-center pilot study of the safety and efficacy of apremilast in subjects with moderate-to-severe alopecia areata. Arch Dermatol Res. 2019;311(1):29-36. doi:10.1007/s00403-018-1876-y
- Baughman RP, Judson MA, Ingledue R, et al. Efficacy and safety of apremilast in chronic cutaneous sarcoidosis. Arch Dermatol. 2012;148:262-264. doi:10.1001/archdermatol.2011.301
- Navy Medicine, US Navy. Manual of the Medical Department (MANMED), NAVMED P-117. Chapter 15. Updated October 20, 2020. Accessed March 13, 2021. https://www.med.navy.mil/directives/Pages/NAVMEDP-MANMED.aspx
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631. doi:10.7205/MILMED-D-17-00047
- Price AD, Wagler VD, Donaldson C, et al. The effects of apremilast therapy on deployability in active duty US Army soldiers with plaque psoriasis and psoriatic arthritis [published online October 30, 2020]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001601
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 13th ed. Washington D.C. Public Health Foundation, 2015. Accessed March 25,2021; https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/table-of-contents.pdf
Apremilast is a small-molecule biologic approved by the US Food and Drug Administration (FDA) for use in plaque psoriasis, psoriatic arthritis, and Behçet disease.1-6 Although apremilast is seemingly a less favorable choice for treating psoriasis in the era of injectable biologics, the drug is an important option for patients in the military. In recent months, apremilast also emerged as one of a few systemic medications recommended for the treatment of psoriasis and other dermatologic conditions during the COVID-19 pandemic.7
In this article, we review on-label indications and off-label uses for apremilast; highlight the importance of apremilast for managing psoriasis in the military population; and propose other patient populations in whom the use of apremilast is favorable. We also present a case report that highlights and embodies the benefit of apremilast for military service members.
CASE REPORT
A 28-year-old active-duty male US Navy service member developed extensive guttate psoriasis in a distribution too wide to manage with topical medication (Figure, A–C). His condition did not improve with a trial of oral antibiotics, and he reported itch that affected his sleep. He denied new joint pain, swelling, or deformity.

A review of the patient’s service history revealed that he was serving aboard a guided-missile cruiser ship for a tour extending an additional 2 years. Limited medical resources and lack of refrigeration made the use of injectable biologics, such as adalimumab, infeasible. Furthermore, the patient was too critical to the mission to be transported frequently off the ship to a higher level of care for injection of medication. He also had trouble returning for appointments and refills because of the high operational tempo of his command.
After discussion with the patient, oral apremilast was started at 30 mg/d and titrated up to the standard dosing of 30 mg twice daily, with excellent results by 3 months after he started therapy (Figure, D–F).
COMMENT
We reviewed the research on apremilast for its approved indications, including psoriasis; its off-label uses; and strategies for using the drug to treat psoriasis and other dermatologic conditions in military populations. The most recent evidence regarding the use of apremilast in dermatology, rheumatology, and other medical specialties was assessed using published English-language research data and review articles. We conducted a PubMed search of articles indexed for MEDLINE using the following terms: apremilast, Otezla, psoriasis, psoriatic arthritis, arthritis, off-label, Behçet’s, hidradenitis suppurativa, military, and armed forces. We also reviewed citations within relevant articles to identify additional relevant sources.
Off-label uses reviewed here are based on data from randomized controlled trials, large open-label trials, and large prospective case series. Articles with less evidence are not included in this review.
On-Label Usage Profile
Apremilast is an orally administered, small-molecule inhibitor of phosphodiesterase 4. Small-molecule inhibitors are a class of medications with low molecular weight, high stability, and short half-life. They act intracellularly to modulate proinflammatory states through regulation of the proinflammatory cytokine milieu.
Apremilast has been approved by the FDA for use in adult psoriasis and psoriatic arthritis since 2014 and for use in treating oral ulcers of Behçet disease since 2019.1-3,5,6 Recently, a phase 2, multicenter, open-label study on the use of apremilast in pediatric psoriasis patients (aged 12–17 years) demonstrated a similar safety profile with weight-based dosing8; phase 3 trials in this population are in the recruitment phase (ClinicalTrials.gov Identifier NCT03701763).
Because information regarding its use in pregnancy is limited, apremilast is not recommended in this population. It is unknown whether apremilast is present in breast milk; although the manufacturer does not make explicit recommendations regarding use during breastfeeding, an expert panel reviewing management of psoriasis in pregnant and breastfeeding women recommended avoiding its use while breastfeeding.9
Common Adverse Effects
Common adverse effects (AEs) include weight loss (>5% total body weight in 5% of patients; 5%–10% of total body weight in 10%–12% of patients; and ≥10% total body weight in 2% of patients), diarrhea and nausea, headache, and upper respiratory tract infection.10,11 Gastrointestinal AEs tend to be self-limited and improve or resolve after the first few weeks of therapy. Caution is advised in patients older than 65 years and in those at risk for hypotension or volume depletion. Although depressed mood is a rare AE (<1%), apremilast should be used cautiously in patients with a history of depression or suicidal ideation. Weight loss generally is self-limited; routine monitoring of weight is recommended.11
Apremilast in Psoriasis and Psoriatic Arthritis
Psoriasis
The ESTEEM trials established the safety and efficacy of apremilast for use in psoriasis.2,3 In a phase 3, multicenter, double-blind, placebo-controlled trial of 844 patients, apremilast demonstrated a statistically significant 75% or greater reduction from the baseline psoriasis area and severity index score (PASI-75) in 33.1% of patients receiving the medication compared to 5.3% of those receiving placebo.2 Data from real-world practice (outside constraints of clinical trials) suggest slightly greater efficacy than was demonstrated in the ESTEEM trials.
A recently published retrospective, cross-sectional study of 480 patients with psoriasis treated with apremilast reported that 48.6% of patients continuing therapy for a mean (SD) of 6 (1) months achieved PASI-75. Furthermore, the mean dermatology life quality index (DLQI) score of the surveyed population decreased from 13.4 at initiation of treatment to 5.7 at 6 (1) months of treatment—a marked improvement in quality of life.12 Other single-center and smaller study populations also have suggested increased real-world benefit.13,14
Nonetheless, the rate and degree of clearance of plaques with apremilast seem to lag behind what is observed with many of the biologics and traditional medications employed to treat psoriasis.15-19 Furthermore, indirect cost analysis comparisons suggest a much higher cost per level of PASI for apremilast compared to several biologics and to methotrexate.20,21 A study that used indirect methods of comparison to analyze the comparative cost and efficacy of apremilast and methotrexate found no evidence of greater efficacy for apremilast and that the incremental cost to achieve 1 additional PASI-75 responder by using apremilast is $187,888 annually.21
Psoriatic Arthritis
The PALACE clinical trials 1, 2, and 3 assessed the efficacy of apremilast in patients who had prior treatment with conventional disease-modifying antirheumatic drugs or biologics, or both. PALACE 4 evaluated efficacy in treatment-naïve patients; standard dosing of apremilast was found to produce improvement in psoriatic arthritis in treatment-naïve and non–treatment-naïve patients.4-6,22 In the 24-week placebo-controlled phase of the PALACE 1 trial, the American College of Rheumatology (ACR) baseline composite measurement of 20% disease improvement, or ACR20, was achieved in 40% of patients randomized to the standard dosing regimen compared to 19% of patients receiving placebo, a statistically significant result (P<.001).22
Evaluation of long-term study data is beyond the scope of this review, but those data suggest that disease outcomes continue to improve the longer therapy is utilized, with a greater percentage of patients achieving ACR20 as well as ACR50 (50% improvement) and ACR70 (70% improvement) responses. Indirect comparisons analyzing the cost and effectiveness for adalimumab, apremilast, and methotrexate in patients with psoriatic arthritis found that apremilast was less effective than adalimumab and as efficacious as methotrexate, though apremilast carries the highest price tag of these drugs.23
Off-Label Uses
Ease of oral administration and a favorable safety profile have prompted off-label study of apremilast in other inflammatory skin diseases, including atopic dermatitis, hidradenitis suppurativa, lichen planus, rosacea, alopecia areata, and cutaneous sarcoidosis. Publications with a minimum case series of 10 patients are included in the Table.24-32

Use in the Military and Beyond
Psoriasis and other inflammatory skin conditions are common in the military and can greatly hinder a service member’s ability to perform their duties and remain ready to deploy. A history of psoriasis is disqualifying for military recruits, but early entry into service, misdiagnosis, and low or no burden of disease at time of entry into the service all contribute to a substantial population of active-duty service members who require treatment of psoriasis.33 Necessity dictates that treatment of this condition extend to theater operations; from 2008 to 2015, more than 3600 soldiers sought care for psoriasis while deployed to a combat theater.34
In some cases, poorly controlled inflammatory skin conditions lead to medical separation.33 Although there are limited data on the use of apremilast in the military, its use during deployment for the treatment of psoriasis and psoriatic arthritis has been reported, with the great majority of service members retaining their deployable status even 1 year after the study period.35
The ideal medication for deployable military personnel should have low toxicity, simple storage, and minimal monitoring requirements, and it should not expose a service member to increased risk while in a combat theater. Worldwide deployability is a requirement for most military occupations. The risk for immunosuppression with targeted immune therapy must be fully weighed, as certain duty stations and deployments might increase the risk for exposure to Mycobacterium tuberculosis, endemic mycopathogens, hepatitis C virus, HIV, Leishmania, and Strongyloides.34
Furthermore, the tumor necrosis factor α inhibitors and IL-17 and IL-23 blockers used to treat psoriasis all require refrigeration; often, this requirement cannot be met in austere overseas settings. Additional requirements for laboratory monitoring, titration of medications, and frequent office visits might prohibit a service member from performing their duties, which, in turn, is detrimental to military readiness and the career of that service member.
Last, the Centers for Disease Control and Prevention recommend avoiding live virus vaccination while taking targeted immune therapy because of safety and effectiveness concerns during immunosuppression.36 This recommendation might disqualify military personnel from deployment to certain locations that require the protection that such vaccines afford. Therefore, apremilast is an ideal option for the military patient population, with many military-specific advantages.
Of course, the military is not the only population in whom ease of use and storage and simplified monitoring parameters are essential. Benefits of apremilast also may translate to patients who are placed in austere conditions or who participate in extended worldwide travel for work or leisure, such as government contractors who deploy in support of military operations, firefighters or national park employees who spend extended periods in resource-limited settings, and foreign-aid workers and diplomats who are engaged in frequent travel around the world. Furthermore, travel to certain regions might increase the risk for exposure to atypical pathogens as well as the desire for a therapeutic option that does not have potential to suppress the immune system. This subset of psoriasis patients might be better treated with novel agents such as apremilast than other drugs that would be the presumed standard of care in a domestic setting.
Final Thoughts
The benefits of apremilast translate to all patients in austere environments with limited resources and during times when immune function is of utmost concern. For military service members and many civilians in austere environments worldwide, apremilast could be considered a first-line systemic agent for psoriasis and psoriatic arthritis. In patients unable to use or tolerate other treatments, apremilast can be considered for off-label therapy (Table24-32). There are times when the approach to prescribing must look beyond primary efficacy, AE profile, and cost—to include occupation, environment, or duties—to select the optimal medication for a patient.
Apremilast is a small-molecule biologic approved by the US Food and Drug Administration (FDA) for use in plaque psoriasis, psoriatic arthritis, and Behçet disease.1-6 Although apremilast is seemingly a less favorable choice for treating psoriasis in the era of injectable biologics, the drug is an important option for patients in the military. In recent months, apremilast also emerged as one of a few systemic medications recommended for the treatment of psoriasis and other dermatologic conditions during the COVID-19 pandemic.7
In this article, we review on-label indications and off-label uses for apremilast; highlight the importance of apremilast for managing psoriasis in the military population; and propose other patient populations in whom the use of apremilast is favorable. We also present a case report that highlights and embodies the benefit of apremilast for military service members.
CASE REPORT
A 28-year-old active-duty male US Navy service member developed extensive guttate psoriasis in a distribution too wide to manage with topical medication (Figure, A–C). His condition did not improve with a trial of oral antibiotics, and he reported itch that affected his sleep. He denied new joint pain, swelling, or deformity.

A review of the patient’s service history revealed that he was serving aboard a guided-missile cruiser ship for a tour extending an additional 2 years. Limited medical resources and lack of refrigeration made the use of injectable biologics, such as adalimumab, infeasible. Furthermore, the patient was too critical to the mission to be transported frequently off the ship to a higher level of care for injection of medication. He also had trouble returning for appointments and refills because of the high operational tempo of his command.
After discussion with the patient, oral apremilast was started at 30 mg/d and titrated up to the standard dosing of 30 mg twice daily, with excellent results by 3 months after he started therapy (Figure, D–F).
COMMENT
We reviewed the research on apremilast for its approved indications, including psoriasis; its off-label uses; and strategies for using the drug to treat psoriasis and other dermatologic conditions in military populations. The most recent evidence regarding the use of apremilast in dermatology, rheumatology, and other medical specialties was assessed using published English-language research data and review articles. We conducted a PubMed search of articles indexed for MEDLINE using the following terms: apremilast, Otezla, psoriasis, psoriatic arthritis, arthritis, off-label, Behçet’s, hidradenitis suppurativa, military, and armed forces. We also reviewed citations within relevant articles to identify additional relevant sources.
Off-label uses reviewed here are based on data from randomized controlled trials, large open-label trials, and large prospective case series. Articles with less evidence are not included in this review.
On-Label Usage Profile
Apremilast is an orally administered, small-molecule inhibitor of phosphodiesterase 4. Small-molecule inhibitors are a class of medications with low molecular weight, high stability, and short half-life. They act intracellularly to modulate proinflammatory states through regulation of the proinflammatory cytokine milieu.
Apremilast has been approved by the FDA for use in adult psoriasis and psoriatic arthritis since 2014 and for use in treating oral ulcers of Behçet disease since 2019.1-3,5,6 Recently, a phase 2, multicenter, open-label study on the use of apremilast in pediatric psoriasis patients (aged 12–17 years) demonstrated a similar safety profile with weight-based dosing8; phase 3 trials in this population are in the recruitment phase (ClinicalTrials.gov Identifier NCT03701763).
Because information regarding its use in pregnancy is limited, apremilast is not recommended in this population. It is unknown whether apremilast is present in breast milk; although the manufacturer does not make explicit recommendations regarding use during breastfeeding, an expert panel reviewing management of psoriasis in pregnant and breastfeeding women recommended avoiding its use while breastfeeding.9
Common Adverse Effects
Common adverse effects (AEs) include weight loss (>5% total body weight in 5% of patients; 5%–10% of total body weight in 10%–12% of patients; and ≥10% total body weight in 2% of patients), diarrhea and nausea, headache, and upper respiratory tract infection.10,11 Gastrointestinal AEs tend to be self-limited and improve or resolve after the first few weeks of therapy. Caution is advised in patients older than 65 years and in those at risk for hypotension or volume depletion. Although depressed mood is a rare AE (<1%), apremilast should be used cautiously in patients with a history of depression or suicidal ideation. Weight loss generally is self-limited; routine monitoring of weight is recommended.11
Apremilast in Psoriasis and Psoriatic Arthritis
Psoriasis
The ESTEEM trials established the safety and efficacy of apremilast for use in psoriasis.2,3 In a phase 3, multicenter, double-blind, placebo-controlled trial of 844 patients, apremilast demonstrated a statistically significant 75% or greater reduction from the baseline psoriasis area and severity index score (PASI-75) in 33.1% of patients receiving the medication compared to 5.3% of those receiving placebo.2 Data from real-world practice (outside constraints of clinical trials) suggest slightly greater efficacy than was demonstrated in the ESTEEM trials.
A recently published retrospective, cross-sectional study of 480 patients with psoriasis treated with apremilast reported that 48.6% of patients continuing therapy for a mean (SD) of 6 (1) months achieved PASI-75. Furthermore, the mean dermatology life quality index (DLQI) score of the surveyed population decreased from 13.4 at initiation of treatment to 5.7 at 6 (1) months of treatment—a marked improvement in quality of life.12 Other single-center and smaller study populations also have suggested increased real-world benefit.13,14
Nonetheless, the rate and degree of clearance of plaques with apremilast seem to lag behind what is observed with many of the biologics and traditional medications employed to treat psoriasis.15-19 Furthermore, indirect cost analysis comparisons suggest a much higher cost per level of PASI for apremilast compared to several biologics and to methotrexate.20,21 A study that used indirect methods of comparison to analyze the comparative cost and efficacy of apremilast and methotrexate found no evidence of greater efficacy for apremilast and that the incremental cost to achieve 1 additional PASI-75 responder by using apremilast is $187,888 annually.21
Psoriatic Arthritis
The PALACE clinical trials 1, 2, and 3 assessed the efficacy of apremilast in patients who had prior treatment with conventional disease-modifying antirheumatic drugs or biologics, or both. PALACE 4 evaluated efficacy in treatment-naïve patients; standard dosing of apremilast was found to produce improvement in psoriatic arthritis in treatment-naïve and non–treatment-naïve patients.4-6,22 In the 24-week placebo-controlled phase of the PALACE 1 trial, the American College of Rheumatology (ACR) baseline composite measurement of 20% disease improvement, or ACR20, was achieved in 40% of patients randomized to the standard dosing regimen compared to 19% of patients receiving placebo, a statistically significant result (P<.001).22
Evaluation of long-term study data is beyond the scope of this review, but those data suggest that disease outcomes continue to improve the longer therapy is utilized, with a greater percentage of patients achieving ACR20 as well as ACR50 (50% improvement) and ACR70 (70% improvement) responses. Indirect comparisons analyzing the cost and effectiveness for adalimumab, apremilast, and methotrexate in patients with psoriatic arthritis found that apremilast was less effective than adalimumab and as efficacious as methotrexate, though apremilast carries the highest price tag of these drugs.23
Off-Label Uses
Ease of oral administration and a favorable safety profile have prompted off-label study of apremilast in other inflammatory skin diseases, including atopic dermatitis, hidradenitis suppurativa, lichen planus, rosacea, alopecia areata, and cutaneous sarcoidosis. Publications with a minimum case series of 10 patients are included in the Table.24-32

Use in the Military and Beyond
Psoriasis and other inflammatory skin conditions are common in the military and can greatly hinder a service member’s ability to perform their duties and remain ready to deploy. A history of psoriasis is disqualifying for military recruits, but early entry into service, misdiagnosis, and low or no burden of disease at time of entry into the service all contribute to a substantial population of active-duty service members who require treatment of psoriasis.33 Necessity dictates that treatment of this condition extend to theater operations; from 2008 to 2015, more than 3600 soldiers sought care for psoriasis while deployed to a combat theater.34
In some cases, poorly controlled inflammatory skin conditions lead to medical separation.33 Although there are limited data on the use of apremilast in the military, its use during deployment for the treatment of psoriasis and psoriatic arthritis has been reported, with the great majority of service members retaining their deployable status even 1 year after the study period.35
The ideal medication for deployable military personnel should have low toxicity, simple storage, and minimal monitoring requirements, and it should not expose a service member to increased risk while in a combat theater. Worldwide deployability is a requirement for most military occupations. The risk for immunosuppression with targeted immune therapy must be fully weighed, as certain duty stations and deployments might increase the risk for exposure to Mycobacterium tuberculosis, endemic mycopathogens, hepatitis C virus, HIV, Leishmania, and Strongyloides.34
Furthermore, the tumor necrosis factor α inhibitors and IL-17 and IL-23 blockers used to treat psoriasis all require refrigeration; often, this requirement cannot be met in austere overseas settings. Additional requirements for laboratory monitoring, titration of medications, and frequent office visits might prohibit a service member from performing their duties, which, in turn, is detrimental to military readiness and the career of that service member.
Last, the Centers for Disease Control and Prevention recommend avoiding live virus vaccination while taking targeted immune therapy because of safety and effectiveness concerns during immunosuppression.36 This recommendation might disqualify military personnel from deployment to certain locations that require the protection that such vaccines afford. Therefore, apremilast is an ideal option for the military patient population, with many military-specific advantages.
Of course, the military is not the only population in whom ease of use and storage and simplified monitoring parameters are essential. Benefits of apremilast also may translate to patients who are placed in austere conditions or who participate in extended worldwide travel for work or leisure, such as government contractors who deploy in support of military operations, firefighters or national park employees who spend extended periods in resource-limited settings, and foreign-aid workers and diplomats who are engaged in frequent travel around the world. Furthermore, travel to certain regions might increase the risk for exposure to atypical pathogens as well as the desire for a therapeutic option that does not have potential to suppress the immune system. This subset of psoriasis patients might be better treated with novel agents such as apremilast than other drugs that would be the presumed standard of care in a domestic setting.
Final Thoughts
The benefits of apremilast translate to all patients in austere environments with limited resources and during times when immune function is of utmost concern. For military service members and many civilians in austere environments worldwide, apremilast could be considered a first-line systemic agent for psoriasis and psoriatic arthritis. In patients unable to use or tolerate other treatments, apremilast can be considered for off-label therapy (Table24-32). There are times when the approach to prescribing must look beyond primary efficacy, AE profile, and cost—to include occupation, environment, or duties—to select the optimal medication for a patient.
- Hatemi G, Melikoglu M, Tunc R, et al. Apremilast for Behçet’s syndrome—a phase 2, placebo-controlled study. N Engl J Med. 2015;372:1510-1518. doi:10.1056/NEJMoa1408684
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j.jaad.2015.03.049
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate‐to‐severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399. doi:10.1111/bjd.14164
- Cutolo M, Myerson GE, Fleischmann RM, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol. 2016;43:1724-1734. doi:10.3899/jrheum.151376
- Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis. 2016;75:1065-1073. doi:10.1136/annrheumdis-2015-207963
- Wells AF, Edwards CJ, Kivitz AJ, et al. Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: results of the randomized, placebo-controlled PALACE 4 trial. Rheumatology (Oxford). 2018;57:1253-1263. doi:10.1093/rheumatology/key032
- Niaki OZ, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Paller AS, Hong Y, Becker EM, et al. Pharmacokinetics and safety of apremilast in pediatric patients with moderate to severe plaque psoriasis: results from a phase 2 open-label study. J Am Acad Dermatol. 2020;82:389-397. doi:10.1016/j.jaad.2019.08.019
- Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. The Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100. doi:10.1111/ajd.12641
- Otezla. Prescribing information. Amgen Inc; June 2020. Accessed March 13, 2021. www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/otezla/otezla_pi_english.ashx
- Otezla. Product monograph. Amgen Canada Inc; Revised August 2020. Accessed March 13, 2021. www.amgen.ca/products/~/media/FB841218E06B4508B0E7213BC578E641.ashx
- Augustin M, Kleyn CE, Conrad C, et al. Characteristics and outcomes of patients treated with apremilast in the real world: Results from the APPRECIATE study. J Eur Acad Dermatol Venereol. 2020;35:123-134. doi:10.1111/jdv.16431
- Papadavid E, Rompoti N, Theodoropoulos K, et al. Real‐world data on the efficacy and safety of apremilast in patients with moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2018;32:1173-1179. doi:10.1111/jdv.14832
- Wong TH, Sinclair S, Smith B, et al. Real‐world, single‐centre experience of apremilast for the treatment of moderate to severe psoriasis. Clin Exp Dermatol. 2017;42:675-676. doi:10.1111/ced.13150
- Saurat, J‐H, Stingl G, Dubertret L, et al; . Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158:558-566. doi:10.1111/j.1365-2133.2007.08315.x
- Kimball AB, Papp KA, Wasfi Y, et al; Long‐term efficacy of ustekinumab in patients with moderate‐to‐severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535-1545. doi:10.1111/jdv.12046
- Langley, RG, Elewski BE, Lebwohl M, et al; ; Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338. doi:10.1056/NEJMoa1314258
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328. doi:10.1056/NEJMoa1503824
- Papp KA, Leonaridi CL, Blauvelt A, et al. Ixekizumab treatment for psoriasis: integrated efficacy analysis of three double‐blinded, controlled studies (UNCOVER‐1, UNCOVER‐2, UNCOVER‐3). Br J Dermatol. 2018;178:674-681. doi:10.1111/bjd.16050
- Kromer C, Celis D, Sonntag D, et al. Biologicals and small molecules in psoriasis: a systematic review of economic evaluations. PloS One. 2018;13:e0189765. doi:10.1371/journal.pone.0189765
- Armstrong AW, Betts KA, Sundaram M, et al. Comparative efficacy and incremental cost per responder of methotrexate versus apremilast for methotrexate-naïve patients with psoriasis. J Am Acad Dermatol. 2016;75:740-746. doi:10.1016/j.jaad.2016.05.040
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026. doi:10.1136/annrheumdis-2013-205056
- Betts KA, Griffith J, Friedman A, et al. An indirect comparison and cost per responder analysis of adalimumab, methotrexate and apremilast in the treatment of methotrexate-naïve patients with psoriatic arthritis. Curr Med Res Opin. 2016;32:721-729. doi:10.1185/03007995.2016.114002624. Simpson EL, Imafuku S, Poulin Y, et al. A phase 2 randomized trial of apremilast in patients with atopic dermatitis. J Invest Dermatol. 2019;139:1063-1072. doi:10.1016/j.jid.2018.10.043
- Samrao A, Berry TM, Goreshi R, et al. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol. 2012;148:890-897. doi:10.1001/archdermatol.2012.812
- Volf EM, Au S-C, Dumont N, et al. A phase 2, open-label, investigator-initiated study to evaluate the safety and efficacy of apremilast in subjects with recalcitrant allergic contact or atopic dermatitis. J Drugs Dermatol. 2012;11:341-346.
- Vossen ARJV, van Doorn MBA, van der Zee HH, et al. Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial. J Am Acad Dermatol. 2019;80:80-88. doi:10.1016/j.jaad.2018.06.046
- Kerdel FR, Azevedo FA, Don CK, et al. Apremilast for the treatment of mild-to-moderate hidradenitis suppurativa in a prospective, open-label, phase 2 study. J Drugs Dermatol. 2019;18:170-176.
- Paul J, Foss CE, Hirano SA, et al. An open-label pilot study of apremilast for the treatment of moderate to severe lichen planus: a case series. J Am Acad Dermatol. 2013;68:255-261. doi:10.1016/j.jaad.2012.07.014
- Thompson BJ, Furniss M, Zhao W, et al. An oral phosphodiesterase inhibitor (apremilast) for inflammatory rosacea in adults: a pilot study. JAMA Dermatol. 2014;150:1013-1014. doi:10.1001/jamadermatol.2013.10526
- Mikhaylov D, Pavel A, Yao C, et al. A randomized placebo-controlled single-center pilot study of the safety and efficacy of apremilast in subjects with moderate-to-severe alopecia areata. Arch Dermatol Res. 2019;311(1):29-36. doi:10.1007/s00403-018-1876-y
- Baughman RP, Judson MA, Ingledue R, et al. Efficacy and safety of apremilast in chronic cutaneous sarcoidosis. Arch Dermatol. 2012;148:262-264. doi:10.1001/archdermatol.2011.301
- Navy Medicine, US Navy. Manual of the Medical Department (MANMED), NAVMED P-117. Chapter 15. Updated October 20, 2020. Accessed March 13, 2021. https://www.med.navy.mil/directives/Pages/NAVMEDP-MANMED.aspx
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631. doi:10.7205/MILMED-D-17-00047
- Price AD, Wagler VD, Donaldson C, et al. The effects of apremilast therapy on deployability in active duty US Army soldiers with plaque psoriasis and psoriatic arthritis [published online October 30, 2020]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001601
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 13th ed. Washington D.C. Public Health Foundation, 2015. Accessed March 25,2021; https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/table-of-contents.pdf
- Hatemi G, Melikoglu M, Tunc R, et al. Apremilast for Behçet’s syndrome—a phase 2, placebo-controlled study. N Engl J Med. 2015;372:1510-1518. doi:10.1056/NEJMoa1408684
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j.jaad.2015.03.049
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate‐to‐severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399. doi:10.1111/bjd.14164
- Cutolo M, Myerson GE, Fleischmann RM, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol. 2016;43:1724-1734. doi:10.3899/jrheum.151376
- Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis. 2016;75:1065-1073. doi:10.1136/annrheumdis-2015-207963
- Wells AF, Edwards CJ, Kivitz AJ, et al. Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: results of the randomized, placebo-controlled PALACE 4 trial. Rheumatology (Oxford). 2018;57:1253-1263. doi:10.1093/rheumatology/key032
- Niaki OZ, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Paller AS, Hong Y, Becker EM, et al. Pharmacokinetics and safety of apremilast in pediatric patients with moderate to severe plaque psoriasis: results from a phase 2 open-label study. J Am Acad Dermatol. 2020;82:389-397. doi:10.1016/j.jaad.2019.08.019
- Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. The Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100. doi:10.1111/ajd.12641
- Otezla. Prescribing information. Amgen Inc; June 2020. Accessed March 13, 2021. www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/otezla/otezla_pi_english.ashx
- Otezla. Product monograph. Amgen Canada Inc; Revised August 2020. Accessed March 13, 2021. www.amgen.ca/products/~/media/FB841218E06B4508B0E7213BC578E641.ashx
- Augustin M, Kleyn CE, Conrad C, et al. Characteristics and outcomes of patients treated with apremilast in the real world: Results from the APPRECIATE study. J Eur Acad Dermatol Venereol. 2020;35:123-134. doi:10.1111/jdv.16431
- Papadavid E, Rompoti N, Theodoropoulos K, et al. Real‐world data on the efficacy and safety of apremilast in patients with moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2018;32:1173-1179. doi:10.1111/jdv.14832
- Wong TH, Sinclair S, Smith B, et al. Real‐world, single‐centre experience of apremilast for the treatment of moderate to severe psoriasis. Clin Exp Dermatol. 2017;42:675-676. doi:10.1111/ced.13150
- Saurat, J‐H, Stingl G, Dubertret L, et al; . Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158:558-566. doi:10.1111/j.1365-2133.2007.08315.x
- Kimball AB, Papp KA, Wasfi Y, et al; Long‐term efficacy of ustekinumab in patients with moderate‐to‐severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535-1545. doi:10.1111/jdv.12046
- Langley, RG, Elewski BE, Lebwohl M, et al; ; Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338. doi:10.1056/NEJMoa1314258
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328. doi:10.1056/NEJMoa1503824
- Papp KA, Leonaridi CL, Blauvelt A, et al. Ixekizumab treatment for psoriasis: integrated efficacy analysis of three double‐blinded, controlled studies (UNCOVER‐1, UNCOVER‐2, UNCOVER‐3). Br J Dermatol. 2018;178:674-681. doi:10.1111/bjd.16050
- Kromer C, Celis D, Sonntag D, et al. Biologicals and small molecules in psoriasis: a systematic review of economic evaluations. PloS One. 2018;13:e0189765. doi:10.1371/journal.pone.0189765
- Armstrong AW, Betts KA, Sundaram M, et al. Comparative efficacy and incremental cost per responder of methotrexate versus apremilast for methotrexate-naïve patients with psoriasis. J Am Acad Dermatol. 2016;75:740-746. doi:10.1016/j.jaad.2016.05.040
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026. doi:10.1136/annrheumdis-2013-205056
- Betts KA, Griffith J, Friedman A, et al. An indirect comparison and cost per responder analysis of adalimumab, methotrexate and apremilast in the treatment of methotrexate-naïve patients with psoriatic arthritis. Curr Med Res Opin. 2016;32:721-729. doi:10.1185/03007995.2016.114002624. Simpson EL, Imafuku S, Poulin Y, et al. A phase 2 randomized trial of apremilast in patients with atopic dermatitis. J Invest Dermatol. 2019;139:1063-1072. doi:10.1016/j.jid.2018.10.043
- Samrao A, Berry TM, Goreshi R, et al. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol. 2012;148:890-897. doi:10.1001/archdermatol.2012.812
- Volf EM, Au S-C, Dumont N, et al. A phase 2, open-label, investigator-initiated study to evaluate the safety and efficacy of apremilast in subjects with recalcitrant allergic contact or atopic dermatitis. J Drugs Dermatol. 2012;11:341-346.
- Vossen ARJV, van Doorn MBA, van der Zee HH, et al. Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial. J Am Acad Dermatol. 2019;80:80-88. doi:10.1016/j.jaad.2018.06.046
- Kerdel FR, Azevedo FA, Don CK, et al. Apremilast for the treatment of mild-to-moderate hidradenitis suppurativa in a prospective, open-label, phase 2 study. J Drugs Dermatol. 2019;18:170-176.
- Paul J, Foss CE, Hirano SA, et al. An open-label pilot study of apremilast for the treatment of moderate to severe lichen planus: a case series. J Am Acad Dermatol. 2013;68:255-261. doi:10.1016/j.jaad.2012.07.014
- Thompson BJ, Furniss M, Zhao W, et al. An oral phosphodiesterase inhibitor (apremilast) for inflammatory rosacea in adults: a pilot study. JAMA Dermatol. 2014;150:1013-1014. doi:10.1001/jamadermatol.2013.10526
- Mikhaylov D, Pavel A, Yao C, et al. A randomized placebo-controlled single-center pilot study of the safety and efficacy of apremilast in subjects with moderate-to-severe alopecia areata. Arch Dermatol Res. 2019;311(1):29-36. doi:10.1007/s00403-018-1876-y
- Baughman RP, Judson MA, Ingledue R, et al. Efficacy and safety of apremilast in chronic cutaneous sarcoidosis. Arch Dermatol. 2012;148:262-264. doi:10.1001/archdermatol.2011.301
- Navy Medicine, US Navy. Manual of the Medical Department (MANMED), NAVMED P-117. Chapter 15. Updated October 20, 2020. Accessed March 13, 2021. https://www.med.navy.mil/directives/Pages/NAVMEDP-MANMED.aspx
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631. doi:10.7205/MILMED-D-17-00047
- Price AD, Wagler VD, Donaldson C, et al. The effects of apremilast therapy on deployability in active duty US Army soldiers with plaque psoriasis and psoriatic arthritis [published online October 30, 2020]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001601
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 13th ed. Washington D.C. Public Health Foundation, 2015. Accessed March 25,2021; https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/table-of-contents.pdf
Practice Points
- Apremilast is a versatile and easy-to-use therapeutic option for treatment of psoriasis and psoriatic arthritis.
- Ease of transport and storage as well as lack of necessary laboratory monitoring have made apremilast a compelling treatment option for psoriasis and psoriatic arthritis in military populations with high operational tempos.
- Dermatologists should consider apremilast for treatment in populations that work for prolonged periods in austere or resource-limited environments.
Cutaneous Manifestations of COVID-19: Characteristics, Pathogenesis, and the Role of Dermatology in the Pandemic
The virus that causes COVID-19—SARS-CoV-2—has infected more than 128 million individuals, resulting in more than 2.8 million deaths worldwide between December 2019 and April 2021. Disease mortality primarily is driven by hypoxemic respiratory failure and systemic hypercoagulability, resulting in multisystem organ failure.1 With more than 17 million Americans infected, the virus is estimated to have impacted someone within the social circle of nearly every American.2
The COVID-19 pandemic has highlighted resource limitations, delayed elective and preventive care, and rapidly increased the adoption of telemedicine, presenting a host of new challenges to providers in every medical specialty, including dermatology. Although COVID-19 primarily is a respiratory disease, clinical manifestations have been observed in nearly every organ, including the skin. The cutaneous manifestations of COVID-19 provide insight into disease diagnosis, prognosis, and pathophysiology. In this article, we review the cutaneous manifestations of COVID-19 and explore the state of knowledge regarding their pathophysiology and clinical significance. Finally, we discuss the role of dermatology consultants in the care of patients with COVID-19, and the impact of the pandemic on the field of dermatology.
Prevalence of Cutaneous Findings in COVID-19
Early reports characterizing the clinical presentation of patients hospitalized with COVID-19 suggested skin findings associated with the disease were rare. Cohort studies from Europe, China, and New York City in January through March 2020 reported a low prevalence or made no mention of rash.3-7 However, reports from dermatologists in Italy that emerged in May 2020 indicated a substantially higher proportion of cutaneous disease: 18 of 88 (20.4%) hospitalized patients were found to have cutaneous involvement, primarily consisting of erythematous rash, along with some cases of urticarial and vesicular lesions.8 In October 2020, a retrospective cohort study from Spain examining 2761 patients presenting to the emergency department or admitted to the hospital for COVID-19 found that 58 (2.1%) patients had skin lesions attributed to COVID-19.9
The wide range in reported prevalence of skin lesions may be due to variable involvement of dermatologic specialists in patient care, particularly in China.10 Some variation also may be due to variability in the timing of clinical examination, as well as demographic and clinical differences in patient populations. Of note, a multisystem inflammatory disease seen in US children subsequent to infection with COVID-19 has been associated with rash in as many as 74% of cases.11 Although COVID-19 disproportionately impacts people with skin of color, there are few reports of cutaneous manifestations in that population,12 highlighting the challenges of the dermatologic examination in individuals with darker skin and suggesting the prevalence of dermatologic disease in COVID-19 may be greater than reported.
Morphologic Patterns of Cutaneous Involvement in COVID-19
Researchers in Europe and the United States have attempted to classify the cutaneous manifestations of COVID-19. A registry established through the American Academy of Dermatology published a compilation of reports from 31 countries, totaling 716 patient profiles.13 A prospective Spanish study detailed the cutaneous involvement of 375 patients with suspected or confirmed COVID-19.14 Together, these efforts have revealed several distinct patterns of cutaneous involvement associated with COVID-19 (Table).9,15-18
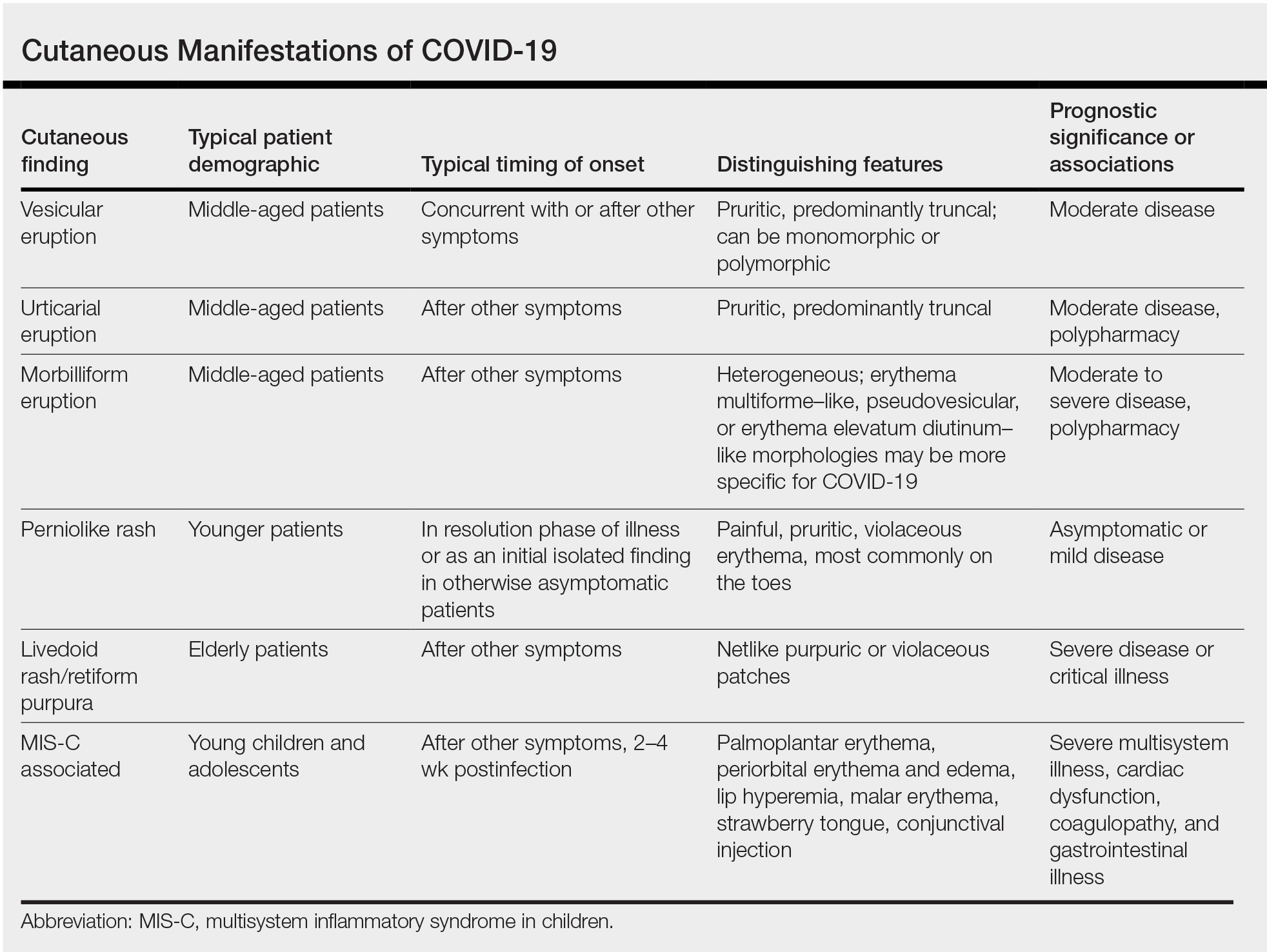
Vesicular Rash
Vesicular rash associated with COVID-19 has been described in several studies and case series8,13,14 and is considered, along with the pseudopernio (or pseudochilblains) morphology, to be one of the more disease-specific patterns in COVID-19.14,18 Vesicular rash appears to comprise roughly one-tenth of all COVID-19–associated rashes.13,14 It usually is described as pruritic, with 72% to 83% of patients reporting itch.13,16
Small monomorphic or polymorphic vesicles predominantly on the trunk and to a lesser extent the extremities and head have been described by multiple authors.14,16 Vesicular rash is most common among middle-aged individuals, with studies reporting median and mean ages ranging from 40.5 to 55 years.9,13,14,16
Vesicular rash develops concurrent with or after other presenting symptoms of COVID-19; in 2 studies, vesicular rash preceded development of other symptoms in only 15% and 5.6% of cases, respectively.13,14 Prognostically, vesicular rash is associated with moderate disease severity.14,16 It may persist for an average of 8 to 10 days.14,16,18
Histopathologic examination reveals basal layer vacuolar degeneration, hyperchromatic keratinocytes, acantholysis, and dyskeratosis.9,16,18
Urticarial Rash
Urticarial lesions represent approximately 7% to 19% of reported COVID-19–associated rashes.9,13,14 Urticarial rashes in patients testing positive for SARS-CoV-2 primarily occur on the trunk.14 The urticaria, which typically last about 1 week,14 are seen most frequently in middle-aged patients (mean/median age, 42–48 years)13,14 and are associated with pruritus, which has been reported in 74% to 92% of patients.13,14 Urticarial lesions typically do not precede other symptoms of COVID-19 and are nonspecific, making them less useful diagnostically.14
Urticaria appears to be associated with more severe COVID-19 illness in several studies, but this finding may be confounded by several factors, including older age, increased tobacco use, and polypharmacy. Of 104 patients with reported urticarial rash and suspected or confirmed COVID-19 across 3 studies, only 1 death was reported.9,13,14
The histopathologic appearance is that of typical hives, demonstrating a perivascular infiltrate of lymphocytes and eosinophils with edema of the upper dermis.9,19
Morbilliform Eruption
Morbilliform eruption is a commonly reported morphology associated with COVID-19, accounting for 20% to 47% of rashes.9,13,14 This categorization may have limited utility from a diagnostic and prognostic perspective, given that morbilliform eruptions are common, nonspecific, and heterogenous and can arise from many causes.9,13,14 Onset of morbilliform eruption appears to coincide with14 or follow13,20,21 the development of other COVID-19–related symptoms, with 5% of patients reporting morbilliform rash as the initial manifestation of infection.13,14 Morbilliform eruptions have been observed to occur in patients with more severe disease.9,13,14
Certain morphologic subtypes, such as erythema multiforme–like, erythema elevatum diutinum–like, or pseudovesicular, may be more specific to COVID-19 infection.14 A small case series highlighted 4 patients with erythema multiforme–like eruptions, 3 of whom also were found to have petechial enanthem occurring after COVID-19 diagnosis; however, the investigators were unable to exclude drug reaction as a potential cause of rash in these patients.22 Another case series of 21 patients with COVID-19 and skin rash described a (primarily) petechial enanthem on the palate in 6 (28.5%) patients.23 It is unclear to what extent oral enanthem may be underrecognized given that some physicians may be disinclined to remove the masks of known COVID-19–positive patients to examine the oral cavity.
The histologic appearance of morbilliform rash seen in association with COVID-19 has been described as spongiotic with interface dermatitis with perivascular lymphocytic inflammation.9,21
COVID Toes, Pseudochilblains Rash, Perniolike Rash, and Acral Erythema/Edema
Of all the rashes associated with COVID-19, COVID toes, or pseudochilblains rash, has perhaps attracted the most attention. The characteristic violaceous erythema on the fingers and/or toes may be itchy or painful, presenting similar to idiopathic cases of pernio (Figure 1).14 The entity has been controversial because of an absence of a clear correlation with a positive SARS-CoV-2 polymerase chain reaction test or antibodies to the virus in a subset of reported cases.24,25 Onset of the rash late in the disease course, generally after symptom resolution in mild or asymptomatic cases, may explain the absence of viral DNA in the nasopharynx by the time of lesion appearance.14,26 Seronegative patients may have cleared SARS-CoV-2 infection before humoral immunity could occur via a strong type 1 interferon response.25
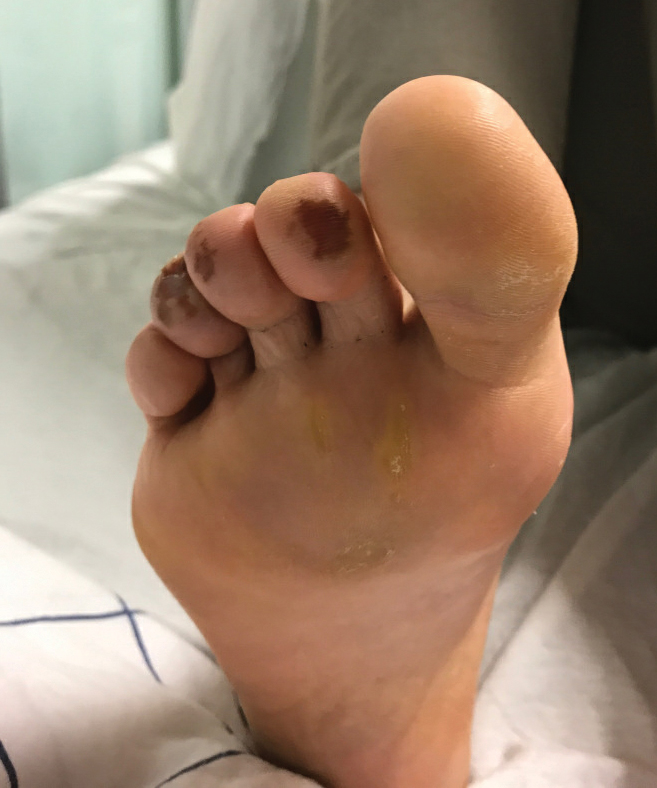
Across 3 studies, perniolike skin lesions constituted 18% to 29% of COVID-19–associated skin findings9,13,14 and persisted for an average of 12 to 14 days.13,14 Perniolike lesions portend a favorable outcome; patients with COVID toes rarely present with systemic symptoms or laboratory or imaging abnormalities9 and less commonly require hospitalization for severe illness. Perniolike lesions have been reported most frequently in younger patients, with a median or mean age of 32 to 35 years.13,14
Histology demonstrates lichenoid dermatitis with perivascular and periadnexal lymphocytic infiltrates.9 Notably, one study observed interface dermatitis of the intraepidermal portion of the acrosyringium, a rare finding in chilblain lupus, in 83% of patients (N=40).25 Direct immunofluorescence demonstrates a vasculopathic pattern, with some patients showing deposition of IgM or IgG, C3, and fibrinogen in dermal blood vessels. Vascular C9 deposits also have been demonstrated on immunohistochemistry.9 Biopsies of perniolike lesions in COVID-19 patients have demonstrated the presence of SARS-CoV-2 RNA,27 have identified SARS-CoV-2 spike protein in endothelial cells on immunohistochemistry, and have visualized intracytoplasmic viral particles in vascular endothelium on electron microscopy.28
Livedoid Rash/Retiform Purpura
Netlike purpuric or violaceous patches signifying vessel damage or occlusion have been seen in association with COVID-19, constituting approximately 6% of COVID-19–associated skin findings in 2 studies.13,14 Livedoid rash (Figure 2) and retiform purpura (Figure 3) are associated with older age and occur primarily in severely ill patients, including those requiring intensive care. In a registry of 716 patients with COVID-19, 100% of patients with retiform purpura were hospitalized, and 82% had acute respiratory distress syndrome.13 In another study, 33% (7/21) of patients with livedoid and necrotic lesions required intensive care, and 10% (2/21) died.14
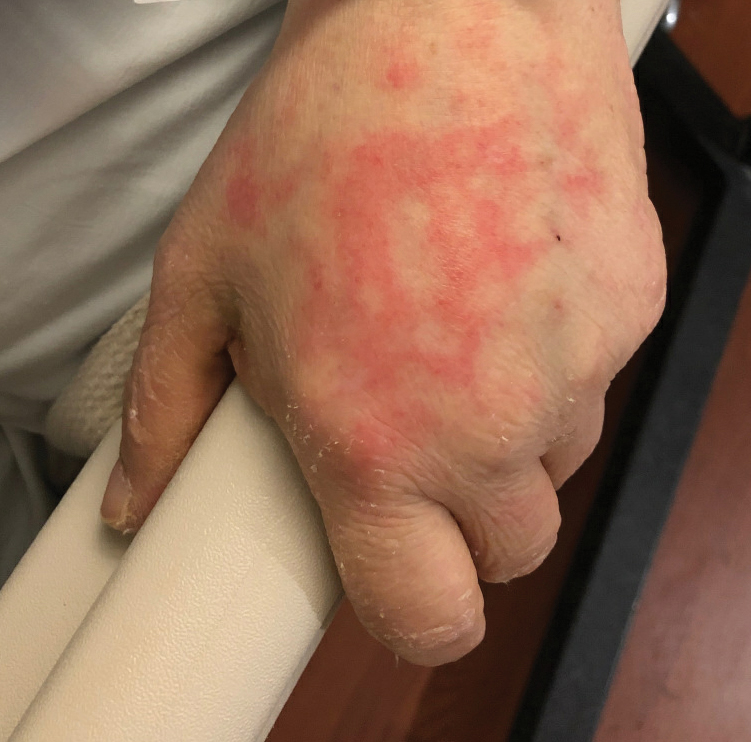
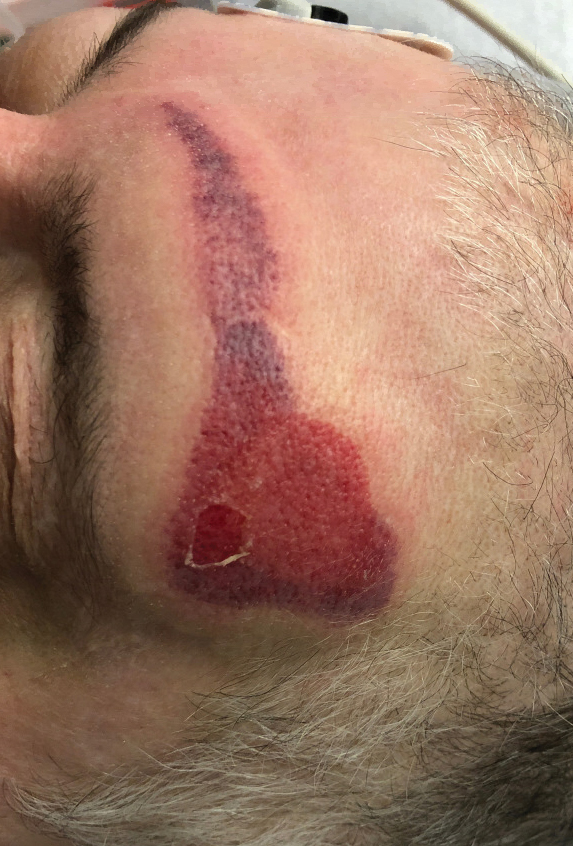
Livedoid lesions and retiform purpura represent thrombotic disease in the skin due to vasculopathy/coagulopathy. Dermatopathology available through the American Academy of Dermatology registry revealed thrombotic vasculopathy.13 A case series of 4 patients with livedo racemosa and retiform purpura demonstrated pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, and arterioles with complement deposition.29 Livedoid and retiform lesions in the skin may be associated with a COVID-19–induced coagulopathy, a propensity for systemic clotting including pulmonary embolism, which mostly occurs in hospitalized patients with severe illness.30
Multisystem Inflammatory Disease in Children
A hyperinflammatory syndrome similar to Kawasaki disease and toxic shock syndrome associated with mucocutaneous, cardiac, and gastrointestinal manifestations has been reported following COVID-19 infection.31 This syndrome, known as multisystem inflammatory syndrome in children (MIS-C), predominantly affects adolescents and children older than 5 years,11 typically occurs 2 to 4 weeks after infection, and appears to be at least 100-times less common than COVID-19 infection among the same age group.31 Sixty percent31 to 74%11 of affected patients have mucocutaneous involvement, with the most common clinical findings being conjunctival injection, palmoplantar erythema, lip hyperemia, periorbital erythema and edema, strawberry tongue, and malar erythema, respectively.32
Because this condition appears to reflect an immune response to the virus, the majority of cases demonstrate negative SARS-CoV-2 polymerase chain reaction and positive antibody testing.33 Although cutaneous findings are similar to those seen in Kawasaki disease, certain findings have been noted in MIS-C that are not typical of Kawasaki disease, including heliotrope rash–like periorbital edema and erythema as well as erythema infectiosum–like malar erythema and reticulated erythematous eruptions.32
The course of MIS-C can be severe; in one case series of patients presenting with MIS-C, 80% (79/99) required intensive care unit admission, with 10% requiring mechanical ventilation and 2% of patients dying during admission.31 Cardiac dysfunction, coagulopathy, and gastrointestinal symptoms are common.11,31 It has been postulated that a superantigenlike region of the SARS-CoV-2 spike protein, similar to that of staphylococcal enterotoxin B, may underlie MIS-C and account for its similarities to toxic shock syndrome.34 Of note, a similar multisystem inflammatory syndrome associated with COVID-19 also has been described in adults, and it too may present with rash as a cardinal feature.35
Pathophysiology of COVID-19: What the Skin May Reveal About the Disease
The diverse range of cutaneous manifestations in COVID-19 reflects a spectrum of host immunologicresponses to SARS-CoV-2 and may inform the pathophysiology of the disease as well as potential treatment modalities.
Host Response to SARS-CoV-2
The body’s response to viral infection is 2-pronged, involving activation of cellular antiviral defenses mediated by type I and III interferons, as well as recruitment of leukocytes, mobilized by cytokines and chemokines.36,37 Infection with SARS-CoV-2 results in a unique inflammatory response characterized by suppression of interferons, juxtaposed with a rampant proinflammatory cytokine and chemokine response, reminiscent of a cytokine storm. Reflective of this imbalance, a study of 50 COVID-19 patients and 20 healthy controls found decreased natural killer cells and CD3+ T cells in COVID-19 patients, particularly severely or critically ill patients, with an increase in B cells and monocytes.38 This distinctive immune imbalance positions SARS-CoV-2 to thrive in the absence of inhibitory interferon activity while submitting the host to the deleterious effects of a cytokine surge.36
Type I Interferons
The perniolike lesions associated with mild COVID-19 disease14 may represent a robust immune response via effective stimulation of type I interferons (IFN-1). Similar perniolike lesions are observed in Aicardi-Goutières syndrome37 and familial chilblain lupus, hereditary interferonopathies associated with mutations in the TREX1 (three prime repair exonuclease 1) gene and characterized by inappropriate upregulation of IFN-1,39 resulting in chilblains. It has been suggested that perniolike lesions in COVID-19 result from IFN-1 activation—a robust effective immunologic response to the virus.14,26,40
On the other end of the spectrum, patients with severe COVID-19 may have a blunted IFN-1 response and reduced IFN-1–stimulated gene expression.36,38 Notably, low IFN-1 response preceded clinical deterioration and was associated with increased risk for evolution to critical illness.38 Severe disease from COVID-19 also is more commonly observed in older patients and those with comorbidities,1 both of which are known factors associated with depressed IFN-1 function.38,41 Reflective of this disparate IFN-1 response, biopsies of COVID-19 perniosis have demonstrated striking expression of myxovirus resistance protein A (MXA), a marker for IFN-1 signaling in tissue, whereas its expression is absent in COVID-19 livedo/retiform purpura.27
Familial chilblain lupus may be effectively treated by the Janus kinase inhibitor baricitinib,39 which inhibits IFN-1 signaling. Baricitinib recently received emergency use authorization by the US Food and Drug Administration for treatment of severe COVID-19 pneumonia,42,43 hinting to disordered IFN-1 signaling in the COVID-19 pathophysiology.
The impaired IFN-1 response in COVID-19 patients may be due to a unique characteristic of SARS-CoV-2: its ORF3b gene is a potent IFN-1 antagonist. In a series of experiments comparing SARS-CoV-2 to the related virus severe acute respiratory disease coronavirus (which was responsible for an epidemic in 2002), Konno et al44 found that SARS-CoV-2 is more effectively able to downregulate host IFN-1, likely due to premature stop codons on ORF3b that produce a truncated version of the gene with amplified anti–IFN-1 activity.
Cytokine Storm and Coagulation Cascade
This dulled interferon response is juxtaposed with a surge of inflammatory chemokines and cytokines, including IL-6, IL-8, IL-10, and tumor necrosis factor α, impairing innate immunity and leading to end-organ damage. This inflammatory response is associated with the influx of innate immune cells, specifically neutrophils and monocytes, which likely contribute to lung injury in COVID-19 acute respiratory distress syndrome.38 It also is thought to lead to downstream activation of coagulation, with a high incidence of thrombotic events observed in patients with severe COVID-19.1 In a retrospective study of 184 intensive care patients with COVID-19 receiving at least standard doses of thromboprophylaxis, venous thromboembolism occurred in 27% and arterial thrombotic events occurred in 3.7%.45
Livedo racemosa and retiform purpura are cutaneous markers of hypercoagulability, which indicate an increased risk for systemic clotting in COVID-19. A positive feedback loop between the complement and coagulation cascades appears to be important.13,14,29,46-48 In addition, a few studies have reported antiphospholipid antibody positivity in hospitalized COVID-19 patients.49,50
The high incidence of coagulopathy in severe COVID-19 has prompted many institutions to develop aggressive prophylactic anticoagulation protocols. Elevation of proinflammatory cytokines and observation of terminal complement activation in the skin and other organs has led to therapeutic trials of IL-6 inhibitors such as tocilizumab,51 complement inhibitors such as eculizumab, and Janus kinase inhibitors such as ruxolitinib and baricitinib.42,48
COVID Long-Haulers
The long-term effects of immune dysregulation in COVID-19 patients remain to be seen. Viral triggering of autoimmune disease is a well-established phenomenon, seen in DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome and other dermatologic diseases, raising the possibility that dermatologists will see a rising incidence of cutaneous autoimmune disease in the aftermath of the pandemic. Disordered interferon stimulation could lead to increased incidence of interferon-mediated disorders, such as sarcoidosis and other granulomatous diseases. Vasculitislike skin lesions could persist beyond the acute infectious period. Recent data from a registry of 990 COVID-19 cases from 39 countries suggest that COVID-19 perniolike lesions may persist as long as 150 days.52 In a time of many unknowns, these questions serve as a call to action for rigorous data collection, contribution to existing registries for dermatologic manifestations of COVID-19, and long-term follow-up of COVID-19 patients by the dermatology community.
Pandemic Dermatology
The pandemic has posed unprecedented challenges for patient care. The use of hydroxychloroquine as a popular but unproven treatment for COVID-19, 53 particularly early in the pandemic, has resulted in drug shortages for patients with lupus and other autoimmune skin diseases. Meanwhile, the need for patients with complex dermatologic conditions to receive systemic immunosuppression has had to be balanced against the associated risks during a global pandemic. To help dermatologists navigate this dilemma, various subspecialty groups have issued guidelines, including the COVID-19 Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists, which recommends a stepwise approach to shared decision-making with the goal of minimizing both the risk for disease flare and that of infection. The use of systemic steroids and rituximab, as well as the dose of immunosuppression—particularly broad-acting immunosuppression—should be limited where permitted. 54
Rapid adoption of telemedicine and remote monitoring strategies has enabled dermatologists to provide safe and timely care when in-person visits have not been possible, including for patients with confirmed or suspected COVID-19, as well as for hospitalized patients. 55-57 Use of telemedicine has facilitated preservation of personal protective equipment at a time when these important resources have been scarce. For patients with transportation or scheduling barriers, telemedicine has even expanded access to care.
However, this strategy cannot completely replace comprehensive in-person evaluation. Variability in video and photographic quality limits evaluation, while in-person physical examination can reveal subtle morphologic clues necessary for diagnosis. 5 8 Additionally, unequal access to technology may disadvantage some patients. For dermatologists to provide optimal care and continue to contribute accurate and insightful observations into COVID-19, it is essential to be physically present in the clinic and in the hospital when necessary, caring for patients in need of dermatologic expertise. Creative management strategies developed during this time will benefit patients and expand the reach of the specialty . 5 8
Final Thoughts
The COVID-19 pandemic has profoundly challenged the medical community and dermatology is no exception. By documenting and characterizing the diverse cutaneous manifestations of this novel disease, dermatologists have furthered understanding of its pathophysiology and management. By adapting quickly and developing creative ways to deliver care, dermatologists have found ways to contribute, both large and small. As we take stock at this juncture of the pandemic, it is clear there remains much to learn. We hope dermatologists will continue to take an active role in meeting the challenges of this time.
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA . 2020;324:782-793. doi:10.1001/jama.2020.12839
- New York Times . Updated December 23, 2020. Accessed March 22, 2021. https://www.nytimes.com/2020/11/15/us/coronavirus-us-cases-deaths.html
- Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med . 2020;382:1708-1720. doi:10.1056/NEJMoa2002032
- Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med . 2020;288:335-344. doi:https://doi.org/10.1111/joim.13089
- Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu province: a multicenter descriptive study. Clin Infect Dis . 2020;71:706-712. doi:10.1093/cid/ciaa199
- Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med . 2020;382:2372-2374. doi:10.1056/NEJMc2010419
- Sun L, Shen L, Fan J, et al. Clinical features of patients with coronavirus disease 2019 from a designated hospital in Beijing, China. J Med Virol . 2020;92:2055-2066. https://doi.org/10.1002/jmv.25966
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatology Venereol . 2020;34:E212-E213. https://doi.org/10.1111/jdv.16387
- Giavedoni P, Podlipnik S, Pericàs JM, et al. Skin manifestations in COVID-19: prevalence and relationship with disease severity. J Clin Med . 2020;9:3261. doi:10.3390/jcm9103261
- Jimenez-Cauhe J, Ortega-Quijano D, Prieto-Barrios M, et al. Reply to “COVID-19 can present with a rash and be mistaken for dengue”: petechial rash in a patient with COVID-19 infection. J Am Acad Dermatol . 2020;83:E141-E142. doi:10.1016/j.jaad.2020.04.016
- Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med . 2020;383:334-346. doi:10.1056/NEJMoa2021680
- Shinkai K, Bruckner AL. Dermatology and COVID-19. JAMA . 2020;324:1133-1134. doi:10.1001/jama.2020.15276
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol . 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol . 2020;183:71-77. https://doi.org/10.1111/bjd.19163
- Bouaziz JD, Duong TA, Jachiet M, et al. Vascular skin symptoms in COVID-19: a French observational study. J Eur Acad Dermatology Venereol . 2020;34:E451-E452. https://doi.org/10.1111/jdv.16544
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol . 2020;45:872-875. https://doi.org/10.1111/ced.14277
- Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol . 2020;83:E61-E63. doi:10.1016/j.jaad.2020.04.093
- Marzano AV, Genovese G, Fabbrocini G, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: Multicenter case series of 22 patients. J Am Acad Dermatol . 2020;83:280-285. doi:10.1016/j.jaad.2020.04.044
- Fernandez-Nieto D, Ortega-Quijano D, Segurado-Miravalles G, et al. Comment on: cutaneous manifestations in COVID-19: a first perspective. safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol . 2020;34:E252-E254. https://doi.org/10.1111/jdv.16470
- Herrero-Moyano M, Capusan TM, Andreu-Barasoain M, et al. A clinicopathological study of eight patients with COVID-19 pneumonia and a late-onset exanthema. J Eur Acad Dermatol Venereol . 2020;34:E460-E464. https://doi.org/10.1111/jdv.16631
- Rubio-Muniz CA, Puerta-Peñ a M, Falkenhain-L ópez D, et al. The broad spectrum of dermatological manifestations in COVID-19: clinical and histopathological features learned from a series of 34 cases. J Eur Acad Dermatol Venereol . 2020;34:E574-E576. https://doi.org/10.1111/jdv.16734
- Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings. Clin Exp Dermatol . 2020;45:892-895. https://doi.org/10.1111/ced.14281
- Jimenez-Cauhe J, Ortega-Quijano D, de Perosanz-Lobo D, et al. Enanthem in patients with COVID-19 and skin rash. JAMA Dermatol . 2020;156:1134-1136. doi:10.1001/jamadermatol.2020.2550
- Le Cleach L, Dousset L, Assier H, et al. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol . 2020;183:866-874. https://doi.org/10.1111/bjd.19377
- Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic [published online November 25, 2020]. JAMA Dermatol . doi:10.1001/jamadermatol.2020.4324
- Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol . 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Magro CM, Mulvey JJ, Laurence J, et al. The differing pathophysiologies that underlie COVID-19-associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol . 2021;184:141-150. https://doi.org/10.1111/bjd.19415
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol . 2020;183:729-737. doi:10.1111/bjd.19327
- Droesch C, Do MH, DeSancho M, et al. Livedoid and purpuric skin eruptions associated with coagulopathy in severe COVID-19. JAMA Dermatol . 2020;156:1-3. doi:10.1001/jamadermatol.2020.2800
- Asakura H, Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol . 2021;113:45-57. doi:10.1007/s12185-020-03029-y
- Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med . 2020;383:347-358. doi:10.1056/NEJMoa2021756
- Young TK, Shaw KS, Shah JK, et al. Mucocutaneous manifestations of multisystem inflammatory syndrome in children during the COVID-19 pandemic. JAMA Dermatol . 2021;157:207-212. doi:10.1001/jamadermatol.2020.4779
- Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259-269. doi:10.1001/jama.2020.10369
- Cheng MH, Zhang S, Porritt RA, et al. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation.
- Morris SB, Schwartz NG, Patel P, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 Infection—United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1450-1456. doi:10.15585/mmwr.mm6940e1
- Blanco-Melo D, Nilsson-Payant BE, Liu W-C, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036.e9-1045.e9. doi:10.1016/j.cell.2020.04.026
- Crow YJ, Manel N. Aicardi–Goutières syndrome and the type I interferonopathies. Nat Rev Immunol. 2015;15:429-440. doi:10.1038/nri3850
- Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718-724. doi:10.1126/science.abc6027
- Zimmermann N, Wolf C, Schwenke R, et al. Assessment of clinical response to janus kinase inhibition in patients with familial chilblain lupus and TREX1 mutation. JAMA Dermatol. 2019;155:342-346. doi:10.1001/jamadermatol.2018.5077
- Hubiche T, Le Duff F, Chiaverini C, et al. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions. Lancet Infect Dis. 2021;21:315-316. doi:10.1016/S1473-3099(20)30518-1
- Agrawal A. Mechanisms and implications of age-associated impaired innate interferon secretion by dendritic cells: a mini-review. Gerontology. 2013;59:421-426. doi:10.1159/000350536
- Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795-807. doi:10.1056/NEJMoa2031994
- US Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization (EUA) of baricitinib. Accessed March 29, 2021. https://www.fda.gov/media/143823/download
- Konno Y, Kimura I, Uriu K, et al. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep. 2020;32:108185. doi:10.1016/j.celrep.2020.108185
- Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke: from the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO). J Vasc Interv Radiol. 2018;29:441-453. doi:10.1016/j.jvir.2017.11.026
- Lo MW, Kemper C, Woodruff TM. COVID-19: complement, coagulation, and collateral damage. J Immunol. 2020;205:1488-1495. doi:10.4049/jimmunol.2000644
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Yan B, Freiwald T, Chauss D, et al. SARS-CoV2 drives JAK1/2-dependent local and systemic complement hyper-activation [published online June 9, 2020]. Res Sq. doi:10.21203/rs.3.rs-33390/v1
- Marietta M, Coluccio V, Luppi M. COVID-19, coagulopathy and venous thromboembolism: more questions than answers. Intern Emerg Med. 2020;15:1375-1387. doi:10.1007/s11739-020-02432-x
- Zuo Y, Estes SK, Ali RA, et al. Prothrombotic antiphospholipid antibodies in COVID-19 [published online June 17, 2020]. medRxiv. doi:10.1101/2020.06.15.20131607
- Lan S-H, Lai C-C, Huang H-T, et al. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56:106103. doi:10.1016/j.ijantimicag.2020.106103
- McMahon D, Gallman A, Hruza G, et al. COVID-19 “long-haulers” in dermatology? duration of dermatologic symptoms in an international registry from 39 countries. Abstract presented at: 29th EADV Congress; October 29, 2020. Accessed March 29, 2020. https://eadvdistribute.m-anage.com/from.storage?image=PXQEdDtICIihN3sM_8nAmh7p_y9AFijhQlf2-_KjrtYgOsOXNVwGxDdti95GZ2Yh0
- Saag MS. Misguided use of hydroxychloroquine for COVID-19: the infusion of politics into science. JAMA. 2020;324:2161-2162. doi:10.1001/jama.2020.22389
- Zahedi Niaki O, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Hammond MI, Sharma TR, Cooper KD, et al. Conducting inpatient dermatology consultations and maintaining resident education in the COVID-19 telemedicine era. J Am Acad Dermatol. 2020;83:E317-E318. doi:10.1016/j.jaad.2020.07.008
- Brunasso AMG, Massone C. Teledermatologic monitoring for chronic cutaneous autoimmune diseases with smartworking during COVID-19 emergency in a tertiary center in Italy. Dermatol Ther. 2020;33:E13495-E13495. doi:10.1111/dth.13695
- Trinidad J, Kroshinsky D, Kaffenberger BH, et al. Telemedicine for inpatient dermatology consultations in response to the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E69-E71. doi:10.1016/j.jaad.2020.04.096
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatology. 2020;156:733-734. doi:10.1001/jamadermatol.2020.1438
The virus that causes COVID-19—SARS-CoV-2—has infected more than 128 million individuals, resulting in more than 2.8 million deaths worldwide between December 2019 and April 2021. Disease mortality primarily is driven by hypoxemic respiratory failure and systemic hypercoagulability, resulting in multisystem organ failure.1 With more than 17 million Americans infected, the virus is estimated to have impacted someone within the social circle of nearly every American.2
The COVID-19 pandemic has highlighted resource limitations, delayed elective and preventive care, and rapidly increased the adoption of telemedicine, presenting a host of new challenges to providers in every medical specialty, including dermatology. Although COVID-19 primarily is a respiratory disease, clinical manifestations have been observed in nearly every organ, including the skin. The cutaneous manifestations of COVID-19 provide insight into disease diagnosis, prognosis, and pathophysiology. In this article, we review the cutaneous manifestations of COVID-19 and explore the state of knowledge regarding their pathophysiology and clinical significance. Finally, we discuss the role of dermatology consultants in the care of patients with COVID-19, and the impact of the pandemic on the field of dermatology.
Prevalence of Cutaneous Findings in COVID-19
Early reports characterizing the clinical presentation of patients hospitalized with COVID-19 suggested skin findings associated with the disease were rare. Cohort studies from Europe, China, and New York City in January through March 2020 reported a low prevalence or made no mention of rash.3-7 However, reports from dermatologists in Italy that emerged in May 2020 indicated a substantially higher proportion of cutaneous disease: 18 of 88 (20.4%) hospitalized patients were found to have cutaneous involvement, primarily consisting of erythematous rash, along with some cases of urticarial and vesicular lesions.8 In October 2020, a retrospective cohort study from Spain examining 2761 patients presenting to the emergency department or admitted to the hospital for COVID-19 found that 58 (2.1%) patients had skin lesions attributed to COVID-19.9
The wide range in reported prevalence of skin lesions may be due to variable involvement of dermatologic specialists in patient care, particularly in China.10 Some variation also may be due to variability in the timing of clinical examination, as well as demographic and clinical differences in patient populations. Of note, a multisystem inflammatory disease seen in US children subsequent to infection with COVID-19 has been associated with rash in as many as 74% of cases.11 Although COVID-19 disproportionately impacts people with skin of color, there are few reports of cutaneous manifestations in that population,12 highlighting the challenges of the dermatologic examination in individuals with darker skin and suggesting the prevalence of dermatologic disease in COVID-19 may be greater than reported.
Morphologic Patterns of Cutaneous Involvement in COVID-19
Researchers in Europe and the United States have attempted to classify the cutaneous manifestations of COVID-19. A registry established through the American Academy of Dermatology published a compilation of reports from 31 countries, totaling 716 patient profiles.13 A prospective Spanish study detailed the cutaneous involvement of 375 patients with suspected or confirmed COVID-19.14 Together, these efforts have revealed several distinct patterns of cutaneous involvement associated with COVID-19 (Table).9,15-18

Vesicular Rash
Vesicular rash associated with COVID-19 has been described in several studies and case series8,13,14 and is considered, along with the pseudopernio (or pseudochilblains) morphology, to be one of the more disease-specific patterns in COVID-19.14,18 Vesicular rash appears to comprise roughly one-tenth of all COVID-19–associated rashes.13,14 It usually is described as pruritic, with 72% to 83% of patients reporting itch.13,16
Small monomorphic or polymorphic vesicles predominantly on the trunk and to a lesser extent the extremities and head have been described by multiple authors.14,16 Vesicular rash is most common among middle-aged individuals, with studies reporting median and mean ages ranging from 40.5 to 55 years.9,13,14,16
Vesicular rash develops concurrent with or after other presenting symptoms of COVID-19; in 2 studies, vesicular rash preceded development of other symptoms in only 15% and 5.6% of cases, respectively.13,14 Prognostically, vesicular rash is associated with moderate disease severity.14,16 It may persist for an average of 8 to 10 days.14,16,18
Histopathologic examination reveals basal layer vacuolar degeneration, hyperchromatic keratinocytes, acantholysis, and dyskeratosis.9,16,18
Urticarial Rash
Urticarial lesions represent approximately 7% to 19% of reported COVID-19–associated rashes.9,13,14 Urticarial rashes in patients testing positive for SARS-CoV-2 primarily occur on the trunk.14 The urticaria, which typically last about 1 week,14 are seen most frequently in middle-aged patients (mean/median age, 42–48 years)13,14 and are associated with pruritus, which has been reported in 74% to 92% of patients.13,14 Urticarial lesions typically do not precede other symptoms of COVID-19 and are nonspecific, making them less useful diagnostically.14
Urticaria appears to be associated with more severe COVID-19 illness in several studies, but this finding may be confounded by several factors, including older age, increased tobacco use, and polypharmacy. Of 104 patients with reported urticarial rash and suspected or confirmed COVID-19 across 3 studies, only 1 death was reported.9,13,14
The histopathologic appearance is that of typical hives, demonstrating a perivascular infiltrate of lymphocytes and eosinophils with edema of the upper dermis.9,19
Morbilliform Eruption
Morbilliform eruption is a commonly reported morphology associated with COVID-19, accounting for 20% to 47% of rashes.9,13,14 This categorization may have limited utility from a diagnostic and prognostic perspective, given that morbilliform eruptions are common, nonspecific, and heterogenous and can arise from many causes.9,13,14 Onset of morbilliform eruption appears to coincide with14 or follow13,20,21 the development of other COVID-19–related symptoms, with 5% of patients reporting morbilliform rash as the initial manifestation of infection.13,14 Morbilliform eruptions have been observed to occur in patients with more severe disease.9,13,14
Certain morphologic subtypes, such as erythema multiforme–like, erythema elevatum diutinum–like, or pseudovesicular, may be more specific to COVID-19 infection.14 A small case series highlighted 4 patients with erythema multiforme–like eruptions, 3 of whom also were found to have petechial enanthem occurring after COVID-19 diagnosis; however, the investigators were unable to exclude drug reaction as a potential cause of rash in these patients.22 Another case series of 21 patients with COVID-19 and skin rash described a (primarily) petechial enanthem on the palate in 6 (28.5%) patients.23 It is unclear to what extent oral enanthem may be underrecognized given that some physicians may be disinclined to remove the masks of known COVID-19–positive patients to examine the oral cavity.
The histologic appearance of morbilliform rash seen in association with COVID-19 has been described as spongiotic with interface dermatitis with perivascular lymphocytic inflammation.9,21
COVID Toes, Pseudochilblains Rash, Perniolike Rash, and Acral Erythema/Edema
Of all the rashes associated with COVID-19, COVID toes, or pseudochilblains rash, has perhaps attracted the most attention. The characteristic violaceous erythema on the fingers and/or toes may be itchy or painful, presenting similar to idiopathic cases of pernio (Figure 1).14 The entity has been controversial because of an absence of a clear correlation with a positive SARS-CoV-2 polymerase chain reaction test or antibodies to the virus in a subset of reported cases.24,25 Onset of the rash late in the disease course, generally after symptom resolution in mild or asymptomatic cases, may explain the absence of viral DNA in the nasopharynx by the time of lesion appearance.14,26 Seronegative patients may have cleared SARS-CoV-2 infection before humoral immunity could occur via a strong type 1 interferon response.25

Across 3 studies, perniolike skin lesions constituted 18% to 29% of COVID-19–associated skin findings9,13,14 and persisted for an average of 12 to 14 days.13,14 Perniolike lesions portend a favorable outcome; patients with COVID toes rarely present with systemic symptoms or laboratory or imaging abnormalities9 and less commonly require hospitalization for severe illness. Perniolike lesions have been reported most frequently in younger patients, with a median or mean age of 32 to 35 years.13,14
Histology demonstrates lichenoid dermatitis with perivascular and periadnexal lymphocytic infiltrates.9 Notably, one study observed interface dermatitis of the intraepidermal portion of the acrosyringium, a rare finding in chilblain lupus, in 83% of patients (N=40).25 Direct immunofluorescence demonstrates a vasculopathic pattern, with some patients showing deposition of IgM or IgG, C3, and fibrinogen in dermal blood vessels. Vascular C9 deposits also have been demonstrated on immunohistochemistry.9 Biopsies of perniolike lesions in COVID-19 patients have demonstrated the presence of SARS-CoV-2 RNA,27 have identified SARS-CoV-2 spike protein in endothelial cells on immunohistochemistry, and have visualized intracytoplasmic viral particles in vascular endothelium on electron microscopy.28
Livedoid Rash/Retiform Purpura
Netlike purpuric or violaceous patches signifying vessel damage or occlusion have been seen in association with COVID-19, constituting approximately 6% of COVID-19–associated skin findings in 2 studies.13,14 Livedoid rash (Figure 2) and retiform purpura (Figure 3) are associated with older age and occur primarily in severely ill patients, including those requiring intensive care. In a registry of 716 patients with COVID-19, 100% of patients with retiform purpura were hospitalized, and 82% had acute respiratory distress syndrome.13 In another study, 33% (7/21) of patients with livedoid and necrotic lesions required intensive care, and 10% (2/21) died.14


Livedoid lesions and retiform purpura represent thrombotic disease in the skin due to vasculopathy/coagulopathy. Dermatopathology available through the American Academy of Dermatology registry revealed thrombotic vasculopathy.13 A case series of 4 patients with livedo racemosa and retiform purpura demonstrated pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, and arterioles with complement deposition.29 Livedoid and retiform lesions in the skin may be associated with a COVID-19–induced coagulopathy, a propensity for systemic clotting including pulmonary embolism, which mostly occurs in hospitalized patients with severe illness.30
Multisystem Inflammatory Disease in Children
A hyperinflammatory syndrome similar to Kawasaki disease and toxic shock syndrome associated with mucocutaneous, cardiac, and gastrointestinal manifestations has been reported following COVID-19 infection.31 This syndrome, known as multisystem inflammatory syndrome in children (MIS-C), predominantly affects adolescents and children older than 5 years,11 typically occurs 2 to 4 weeks after infection, and appears to be at least 100-times less common than COVID-19 infection among the same age group.31 Sixty percent31 to 74%11 of affected patients have mucocutaneous involvement, with the most common clinical findings being conjunctival injection, palmoplantar erythema, lip hyperemia, periorbital erythema and edema, strawberry tongue, and malar erythema, respectively.32
Because this condition appears to reflect an immune response to the virus, the majority of cases demonstrate negative SARS-CoV-2 polymerase chain reaction and positive antibody testing.33 Although cutaneous findings are similar to those seen in Kawasaki disease, certain findings have been noted in MIS-C that are not typical of Kawasaki disease, including heliotrope rash–like periorbital edema and erythema as well as erythema infectiosum–like malar erythema and reticulated erythematous eruptions.32
The course of MIS-C can be severe; in one case series of patients presenting with MIS-C, 80% (79/99) required intensive care unit admission, with 10% requiring mechanical ventilation and 2% of patients dying during admission.31 Cardiac dysfunction, coagulopathy, and gastrointestinal symptoms are common.11,31 It has been postulated that a superantigenlike region of the SARS-CoV-2 spike protein, similar to that of staphylococcal enterotoxin B, may underlie MIS-C and account for its similarities to toxic shock syndrome.34 Of note, a similar multisystem inflammatory syndrome associated with COVID-19 also has been described in adults, and it too may present with rash as a cardinal feature.35
Pathophysiology of COVID-19: What the Skin May Reveal About the Disease
The diverse range of cutaneous manifestations in COVID-19 reflects a spectrum of host immunologicresponses to SARS-CoV-2 and may inform the pathophysiology of the disease as well as potential treatment modalities.
Host Response to SARS-CoV-2
The body’s response to viral infection is 2-pronged, involving activation of cellular antiviral defenses mediated by type I and III interferons, as well as recruitment of leukocytes, mobilized by cytokines and chemokines.36,37 Infection with SARS-CoV-2 results in a unique inflammatory response characterized by suppression of interferons, juxtaposed with a rampant proinflammatory cytokine and chemokine response, reminiscent of a cytokine storm. Reflective of this imbalance, a study of 50 COVID-19 patients and 20 healthy controls found decreased natural killer cells and CD3+ T cells in COVID-19 patients, particularly severely or critically ill patients, with an increase in B cells and monocytes.38 This distinctive immune imbalance positions SARS-CoV-2 to thrive in the absence of inhibitory interferon activity while submitting the host to the deleterious effects of a cytokine surge.36
Type I Interferons
The perniolike lesions associated with mild COVID-19 disease14 may represent a robust immune response via effective stimulation of type I interferons (IFN-1). Similar perniolike lesions are observed in Aicardi-Goutières syndrome37 and familial chilblain lupus, hereditary interferonopathies associated with mutations in the TREX1 (three prime repair exonuclease 1) gene and characterized by inappropriate upregulation of IFN-1,39 resulting in chilblains. It has been suggested that perniolike lesions in COVID-19 result from IFN-1 activation—a robust effective immunologic response to the virus.14,26,40
On the other end of the spectrum, patients with severe COVID-19 may have a blunted IFN-1 response and reduced IFN-1–stimulated gene expression.36,38 Notably, low IFN-1 response preceded clinical deterioration and was associated with increased risk for evolution to critical illness.38 Severe disease from COVID-19 also is more commonly observed in older patients and those with comorbidities,1 both of which are known factors associated with depressed IFN-1 function.38,41 Reflective of this disparate IFN-1 response, biopsies of COVID-19 perniosis have demonstrated striking expression of myxovirus resistance protein A (MXA), a marker for IFN-1 signaling in tissue, whereas its expression is absent in COVID-19 livedo/retiform purpura.27
Familial chilblain lupus may be effectively treated by the Janus kinase inhibitor baricitinib,39 which inhibits IFN-1 signaling. Baricitinib recently received emergency use authorization by the US Food and Drug Administration for treatment of severe COVID-19 pneumonia,42,43 hinting to disordered IFN-1 signaling in the COVID-19 pathophysiology.
The impaired IFN-1 response in COVID-19 patients may be due to a unique characteristic of SARS-CoV-2: its ORF3b gene is a potent IFN-1 antagonist. In a series of experiments comparing SARS-CoV-2 to the related virus severe acute respiratory disease coronavirus (which was responsible for an epidemic in 2002), Konno et al44 found that SARS-CoV-2 is more effectively able to downregulate host IFN-1, likely due to premature stop codons on ORF3b that produce a truncated version of the gene with amplified anti–IFN-1 activity.
Cytokine Storm and Coagulation Cascade
This dulled interferon response is juxtaposed with a surge of inflammatory chemokines and cytokines, including IL-6, IL-8, IL-10, and tumor necrosis factor α, impairing innate immunity and leading to end-organ damage. This inflammatory response is associated with the influx of innate immune cells, specifically neutrophils and monocytes, which likely contribute to lung injury in COVID-19 acute respiratory distress syndrome.38 It also is thought to lead to downstream activation of coagulation, with a high incidence of thrombotic events observed in patients with severe COVID-19.1 In a retrospective study of 184 intensive care patients with COVID-19 receiving at least standard doses of thromboprophylaxis, venous thromboembolism occurred in 27% and arterial thrombotic events occurred in 3.7%.45
Livedo racemosa and retiform purpura are cutaneous markers of hypercoagulability, which indicate an increased risk for systemic clotting in COVID-19. A positive feedback loop between the complement and coagulation cascades appears to be important.13,14,29,46-48 In addition, a few studies have reported antiphospholipid antibody positivity in hospitalized COVID-19 patients.49,50
The high incidence of coagulopathy in severe COVID-19 has prompted many institutions to develop aggressive prophylactic anticoagulation protocols. Elevation of proinflammatory cytokines and observation of terminal complement activation in the skin and other organs has led to therapeutic trials of IL-6 inhibitors such as tocilizumab,51 complement inhibitors such as eculizumab, and Janus kinase inhibitors such as ruxolitinib and baricitinib.42,48
COVID Long-Haulers
The long-term effects of immune dysregulation in COVID-19 patients remain to be seen. Viral triggering of autoimmune disease is a well-established phenomenon, seen in DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome and other dermatologic diseases, raising the possibility that dermatologists will see a rising incidence of cutaneous autoimmune disease in the aftermath of the pandemic. Disordered interferon stimulation could lead to increased incidence of interferon-mediated disorders, such as sarcoidosis and other granulomatous diseases. Vasculitislike skin lesions could persist beyond the acute infectious period. Recent data from a registry of 990 COVID-19 cases from 39 countries suggest that COVID-19 perniolike lesions may persist as long as 150 days.52 In a time of many unknowns, these questions serve as a call to action for rigorous data collection, contribution to existing registries for dermatologic manifestations of COVID-19, and long-term follow-up of COVID-19 patients by the dermatology community.
Pandemic Dermatology
The pandemic has posed unprecedented challenges for patient care. The use of hydroxychloroquine as a popular but unproven treatment for COVID-19, 53 particularly early in the pandemic, has resulted in drug shortages for patients with lupus and other autoimmune skin diseases. Meanwhile, the need for patients with complex dermatologic conditions to receive systemic immunosuppression has had to be balanced against the associated risks during a global pandemic. To help dermatologists navigate this dilemma, various subspecialty groups have issued guidelines, including the COVID-19 Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists, which recommends a stepwise approach to shared decision-making with the goal of minimizing both the risk for disease flare and that of infection. The use of systemic steroids and rituximab, as well as the dose of immunosuppression—particularly broad-acting immunosuppression—should be limited where permitted. 54
Rapid adoption of telemedicine and remote monitoring strategies has enabled dermatologists to provide safe and timely care when in-person visits have not been possible, including for patients with confirmed or suspected COVID-19, as well as for hospitalized patients. 55-57 Use of telemedicine has facilitated preservation of personal protective equipment at a time when these important resources have been scarce. For patients with transportation or scheduling barriers, telemedicine has even expanded access to care.
However, this strategy cannot completely replace comprehensive in-person evaluation. Variability in video and photographic quality limits evaluation, while in-person physical examination can reveal subtle morphologic clues necessary for diagnosis. 5 8 Additionally, unequal access to technology may disadvantage some patients. For dermatologists to provide optimal care and continue to contribute accurate and insightful observations into COVID-19, it is essential to be physically present in the clinic and in the hospital when necessary, caring for patients in need of dermatologic expertise. Creative management strategies developed during this time will benefit patients and expand the reach of the specialty . 5 8
Final Thoughts
The COVID-19 pandemic has profoundly challenged the medical community and dermatology is no exception. By documenting and characterizing the diverse cutaneous manifestations of this novel disease, dermatologists have furthered understanding of its pathophysiology and management. By adapting quickly and developing creative ways to deliver care, dermatologists have found ways to contribute, both large and small. As we take stock at this juncture of the pandemic, it is clear there remains much to learn. We hope dermatologists will continue to take an active role in meeting the challenges of this time.
The virus that causes COVID-19—SARS-CoV-2—has infected more than 128 million individuals, resulting in more than 2.8 million deaths worldwide between December 2019 and April 2021. Disease mortality primarily is driven by hypoxemic respiratory failure and systemic hypercoagulability, resulting in multisystem organ failure.1 With more than 17 million Americans infected, the virus is estimated to have impacted someone within the social circle of nearly every American.2
The COVID-19 pandemic has highlighted resource limitations, delayed elective and preventive care, and rapidly increased the adoption of telemedicine, presenting a host of new challenges to providers in every medical specialty, including dermatology. Although COVID-19 primarily is a respiratory disease, clinical manifestations have been observed in nearly every organ, including the skin. The cutaneous manifestations of COVID-19 provide insight into disease diagnosis, prognosis, and pathophysiology. In this article, we review the cutaneous manifestations of COVID-19 and explore the state of knowledge regarding their pathophysiology and clinical significance. Finally, we discuss the role of dermatology consultants in the care of patients with COVID-19, and the impact of the pandemic on the field of dermatology.
Prevalence of Cutaneous Findings in COVID-19
Early reports characterizing the clinical presentation of patients hospitalized with COVID-19 suggested skin findings associated with the disease were rare. Cohort studies from Europe, China, and New York City in January through March 2020 reported a low prevalence or made no mention of rash.3-7 However, reports from dermatologists in Italy that emerged in May 2020 indicated a substantially higher proportion of cutaneous disease: 18 of 88 (20.4%) hospitalized patients were found to have cutaneous involvement, primarily consisting of erythematous rash, along with some cases of urticarial and vesicular lesions.8 In October 2020, a retrospective cohort study from Spain examining 2761 patients presenting to the emergency department or admitted to the hospital for COVID-19 found that 58 (2.1%) patients had skin lesions attributed to COVID-19.9
The wide range in reported prevalence of skin lesions may be due to variable involvement of dermatologic specialists in patient care, particularly in China.10 Some variation also may be due to variability in the timing of clinical examination, as well as demographic and clinical differences in patient populations. Of note, a multisystem inflammatory disease seen in US children subsequent to infection with COVID-19 has been associated with rash in as many as 74% of cases.11 Although COVID-19 disproportionately impacts people with skin of color, there are few reports of cutaneous manifestations in that population,12 highlighting the challenges of the dermatologic examination in individuals with darker skin and suggesting the prevalence of dermatologic disease in COVID-19 may be greater than reported.
Morphologic Patterns of Cutaneous Involvement in COVID-19
Researchers in Europe and the United States have attempted to classify the cutaneous manifestations of COVID-19. A registry established through the American Academy of Dermatology published a compilation of reports from 31 countries, totaling 716 patient profiles.13 A prospective Spanish study detailed the cutaneous involvement of 375 patients with suspected or confirmed COVID-19.14 Together, these efforts have revealed several distinct patterns of cutaneous involvement associated with COVID-19 (Table).9,15-18

Vesicular Rash
Vesicular rash associated with COVID-19 has been described in several studies and case series8,13,14 and is considered, along with the pseudopernio (or pseudochilblains) morphology, to be one of the more disease-specific patterns in COVID-19.14,18 Vesicular rash appears to comprise roughly one-tenth of all COVID-19–associated rashes.13,14 It usually is described as pruritic, with 72% to 83% of patients reporting itch.13,16
Small monomorphic or polymorphic vesicles predominantly on the trunk and to a lesser extent the extremities and head have been described by multiple authors.14,16 Vesicular rash is most common among middle-aged individuals, with studies reporting median and mean ages ranging from 40.5 to 55 years.9,13,14,16
Vesicular rash develops concurrent with or after other presenting symptoms of COVID-19; in 2 studies, vesicular rash preceded development of other symptoms in only 15% and 5.6% of cases, respectively.13,14 Prognostically, vesicular rash is associated with moderate disease severity.14,16 It may persist for an average of 8 to 10 days.14,16,18
Histopathologic examination reveals basal layer vacuolar degeneration, hyperchromatic keratinocytes, acantholysis, and dyskeratosis.9,16,18
Urticarial Rash
Urticarial lesions represent approximately 7% to 19% of reported COVID-19–associated rashes.9,13,14 Urticarial rashes in patients testing positive for SARS-CoV-2 primarily occur on the trunk.14 The urticaria, which typically last about 1 week,14 are seen most frequently in middle-aged patients (mean/median age, 42–48 years)13,14 and are associated with pruritus, which has been reported in 74% to 92% of patients.13,14 Urticarial lesions typically do not precede other symptoms of COVID-19 and are nonspecific, making them less useful diagnostically.14
Urticaria appears to be associated with more severe COVID-19 illness in several studies, but this finding may be confounded by several factors, including older age, increased tobacco use, and polypharmacy. Of 104 patients with reported urticarial rash and suspected or confirmed COVID-19 across 3 studies, only 1 death was reported.9,13,14
The histopathologic appearance is that of typical hives, demonstrating a perivascular infiltrate of lymphocytes and eosinophils with edema of the upper dermis.9,19
Morbilliform Eruption
Morbilliform eruption is a commonly reported morphology associated with COVID-19, accounting for 20% to 47% of rashes.9,13,14 This categorization may have limited utility from a diagnostic and prognostic perspective, given that morbilliform eruptions are common, nonspecific, and heterogenous and can arise from many causes.9,13,14 Onset of morbilliform eruption appears to coincide with14 or follow13,20,21 the development of other COVID-19–related symptoms, with 5% of patients reporting morbilliform rash as the initial manifestation of infection.13,14 Morbilliform eruptions have been observed to occur in patients with more severe disease.9,13,14
Certain morphologic subtypes, such as erythema multiforme–like, erythema elevatum diutinum–like, or pseudovesicular, may be more specific to COVID-19 infection.14 A small case series highlighted 4 patients with erythema multiforme–like eruptions, 3 of whom also were found to have petechial enanthem occurring after COVID-19 diagnosis; however, the investigators were unable to exclude drug reaction as a potential cause of rash in these patients.22 Another case series of 21 patients with COVID-19 and skin rash described a (primarily) petechial enanthem on the palate in 6 (28.5%) patients.23 It is unclear to what extent oral enanthem may be underrecognized given that some physicians may be disinclined to remove the masks of known COVID-19–positive patients to examine the oral cavity.
The histologic appearance of morbilliform rash seen in association with COVID-19 has been described as spongiotic with interface dermatitis with perivascular lymphocytic inflammation.9,21
COVID Toes, Pseudochilblains Rash, Perniolike Rash, and Acral Erythema/Edema
Of all the rashes associated with COVID-19, COVID toes, or pseudochilblains rash, has perhaps attracted the most attention. The characteristic violaceous erythema on the fingers and/or toes may be itchy or painful, presenting similar to idiopathic cases of pernio (Figure 1).14 The entity has been controversial because of an absence of a clear correlation with a positive SARS-CoV-2 polymerase chain reaction test or antibodies to the virus in a subset of reported cases.24,25 Onset of the rash late in the disease course, generally after symptom resolution in mild or asymptomatic cases, may explain the absence of viral DNA in the nasopharynx by the time of lesion appearance.14,26 Seronegative patients may have cleared SARS-CoV-2 infection before humoral immunity could occur via a strong type 1 interferon response.25

Across 3 studies, perniolike skin lesions constituted 18% to 29% of COVID-19–associated skin findings9,13,14 and persisted for an average of 12 to 14 days.13,14 Perniolike lesions portend a favorable outcome; patients with COVID toes rarely present with systemic symptoms or laboratory or imaging abnormalities9 and less commonly require hospitalization for severe illness. Perniolike lesions have been reported most frequently in younger patients, with a median or mean age of 32 to 35 years.13,14
Histology demonstrates lichenoid dermatitis with perivascular and periadnexal lymphocytic infiltrates.9 Notably, one study observed interface dermatitis of the intraepidermal portion of the acrosyringium, a rare finding in chilblain lupus, in 83% of patients (N=40).25 Direct immunofluorescence demonstrates a vasculopathic pattern, with some patients showing deposition of IgM or IgG, C3, and fibrinogen in dermal blood vessels. Vascular C9 deposits also have been demonstrated on immunohistochemistry.9 Biopsies of perniolike lesions in COVID-19 patients have demonstrated the presence of SARS-CoV-2 RNA,27 have identified SARS-CoV-2 spike protein in endothelial cells on immunohistochemistry, and have visualized intracytoplasmic viral particles in vascular endothelium on electron microscopy.28
Livedoid Rash/Retiform Purpura
Netlike purpuric or violaceous patches signifying vessel damage or occlusion have been seen in association with COVID-19, constituting approximately 6% of COVID-19–associated skin findings in 2 studies.13,14 Livedoid rash (Figure 2) and retiform purpura (Figure 3) are associated with older age and occur primarily in severely ill patients, including those requiring intensive care. In a registry of 716 patients with COVID-19, 100% of patients with retiform purpura were hospitalized, and 82% had acute respiratory distress syndrome.13 In another study, 33% (7/21) of patients with livedoid and necrotic lesions required intensive care, and 10% (2/21) died.14


Livedoid lesions and retiform purpura represent thrombotic disease in the skin due to vasculopathy/coagulopathy. Dermatopathology available through the American Academy of Dermatology registry revealed thrombotic vasculopathy.13 A case series of 4 patients with livedo racemosa and retiform purpura demonstrated pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, and arterioles with complement deposition.29 Livedoid and retiform lesions in the skin may be associated with a COVID-19–induced coagulopathy, a propensity for systemic clotting including pulmonary embolism, which mostly occurs in hospitalized patients with severe illness.30
Multisystem Inflammatory Disease in Children
A hyperinflammatory syndrome similar to Kawasaki disease and toxic shock syndrome associated with mucocutaneous, cardiac, and gastrointestinal manifestations has been reported following COVID-19 infection.31 This syndrome, known as multisystem inflammatory syndrome in children (MIS-C), predominantly affects adolescents and children older than 5 years,11 typically occurs 2 to 4 weeks after infection, and appears to be at least 100-times less common than COVID-19 infection among the same age group.31 Sixty percent31 to 74%11 of affected patients have mucocutaneous involvement, with the most common clinical findings being conjunctival injection, palmoplantar erythema, lip hyperemia, periorbital erythema and edema, strawberry tongue, and malar erythema, respectively.32
Because this condition appears to reflect an immune response to the virus, the majority of cases demonstrate negative SARS-CoV-2 polymerase chain reaction and positive antibody testing.33 Although cutaneous findings are similar to those seen in Kawasaki disease, certain findings have been noted in MIS-C that are not typical of Kawasaki disease, including heliotrope rash–like periorbital edema and erythema as well as erythema infectiosum–like malar erythema and reticulated erythematous eruptions.32
The course of MIS-C can be severe; in one case series of patients presenting with MIS-C, 80% (79/99) required intensive care unit admission, with 10% requiring mechanical ventilation and 2% of patients dying during admission.31 Cardiac dysfunction, coagulopathy, and gastrointestinal symptoms are common.11,31 It has been postulated that a superantigenlike region of the SARS-CoV-2 spike protein, similar to that of staphylococcal enterotoxin B, may underlie MIS-C and account for its similarities to toxic shock syndrome.34 Of note, a similar multisystem inflammatory syndrome associated with COVID-19 also has been described in adults, and it too may present with rash as a cardinal feature.35
Pathophysiology of COVID-19: What the Skin May Reveal About the Disease
The diverse range of cutaneous manifestations in COVID-19 reflects a spectrum of host immunologicresponses to SARS-CoV-2 and may inform the pathophysiology of the disease as well as potential treatment modalities.
Host Response to SARS-CoV-2
The body’s response to viral infection is 2-pronged, involving activation of cellular antiviral defenses mediated by type I and III interferons, as well as recruitment of leukocytes, mobilized by cytokines and chemokines.36,37 Infection with SARS-CoV-2 results in a unique inflammatory response characterized by suppression of interferons, juxtaposed with a rampant proinflammatory cytokine and chemokine response, reminiscent of a cytokine storm. Reflective of this imbalance, a study of 50 COVID-19 patients and 20 healthy controls found decreased natural killer cells and CD3+ T cells in COVID-19 patients, particularly severely or critically ill patients, with an increase in B cells and monocytes.38 This distinctive immune imbalance positions SARS-CoV-2 to thrive in the absence of inhibitory interferon activity while submitting the host to the deleterious effects of a cytokine surge.36
Type I Interferons
The perniolike lesions associated with mild COVID-19 disease14 may represent a robust immune response via effective stimulation of type I interferons (IFN-1). Similar perniolike lesions are observed in Aicardi-Goutières syndrome37 and familial chilblain lupus, hereditary interferonopathies associated with mutations in the TREX1 (three prime repair exonuclease 1) gene and characterized by inappropriate upregulation of IFN-1,39 resulting in chilblains. It has been suggested that perniolike lesions in COVID-19 result from IFN-1 activation—a robust effective immunologic response to the virus.14,26,40
On the other end of the spectrum, patients with severe COVID-19 may have a blunted IFN-1 response and reduced IFN-1–stimulated gene expression.36,38 Notably, low IFN-1 response preceded clinical deterioration and was associated with increased risk for evolution to critical illness.38 Severe disease from COVID-19 also is more commonly observed in older patients and those with comorbidities,1 both of which are known factors associated with depressed IFN-1 function.38,41 Reflective of this disparate IFN-1 response, biopsies of COVID-19 perniosis have demonstrated striking expression of myxovirus resistance protein A (MXA), a marker for IFN-1 signaling in tissue, whereas its expression is absent in COVID-19 livedo/retiform purpura.27
Familial chilblain lupus may be effectively treated by the Janus kinase inhibitor baricitinib,39 which inhibits IFN-1 signaling. Baricitinib recently received emergency use authorization by the US Food and Drug Administration for treatment of severe COVID-19 pneumonia,42,43 hinting to disordered IFN-1 signaling in the COVID-19 pathophysiology.
The impaired IFN-1 response in COVID-19 patients may be due to a unique characteristic of SARS-CoV-2: its ORF3b gene is a potent IFN-1 antagonist. In a series of experiments comparing SARS-CoV-2 to the related virus severe acute respiratory disease coronavirus (which was responsible for an epidemic in 2002), Konno et al44 found that SARS-CoV-2 is more effectively able to downregulate host IFN-1, likely due to premature stop codons on ORF3b that produce a truncated version of the gene with amplified anti–IFN-1 activity.
Cytokine Storm and Coagulation Cascade
This dulled interferon response is juxtaposed with a surge of inflammatory chemokines and cytokines, including IL-6, IL-8, IL-10, and tumor necrosis factor α, impairing innate immunity and leading to end-organ damage. This inflammatory response is associated with the influx of innate immune cells, specifically neutrophils and monocytes, which likely contribute to lung injury in COVID-19 acute respiratory distress syndrome.38 It also is thought to lead to downstream activation of coagulation, with a high incidence of thrombotic events observed in patients with severe COVID-19.1 In a retrospective study of 184 intensive care patients with COVID-19 receiving at least standard doses of thromboprophylaxis, venous thromboembolism occurred in 27% and arterial thrombotic events occurred in 3.7%.45
Livedo racemosa and retiform purpura are cutaneous markers of hypercoagulability, which indicate an increased risk for systemic clotting in COVID-19. A positive feedback loop between the complement and coagulation cascades appears to be important.13,14,29,46-48 In addition, a few studies have reported antiphospholipid antibody positivity in hospitalized COVID-19 patients.49,50
The high incidence of coagulopathy in severe COVID-19 has prompted many institutions to develop aggressive prophylactic anticoagulation protocols. Elevation of proinflammatory cytokines and observation of terminal complement activation in the skin and other organs has led to therapeutic trials of IL-6 inhibitors such as tocilizumab,51 complement inhibitors such as eculizumab, and Janus kinase inhibitors such as ruxolitinib and baricitinib.42,48
COVID Long-Haulers
The long-term effects of immune dysregulation in COVID-19 patients remain to be seen. Viral triggering of autoimmune disease is a well-established phenomenon, seen in DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome and other dermatologic diseases, raising the possibility that dermatologists will see a rising incidence of cutaneous autoimmune disease in the aftermath of the pandemic. Disordered interferon stimulation could lead to increased incidence of interferon-mediated disorders, such as sarcoidosis and other granulomatous diseases. Vasculitislike skin lesions could persist beyond the acute infectious period. Recent data from a registry of 990 COVID-19 cases from 39 countries suggest that COVID-19 perniolike lesions may persist as long as 150 days.52 In a time of many unknowns, these questions serve as a call to action for rigorous data collection, contribution to existing registries for dermatologic manifestations of COVID-19, and long-term follow-up of COVID-19 patients by the dermatology community.
Pandemic Dermatology
The pandemic has posed unprecedented challenges for patient care. The use of hydroxychloroquine as a popular but unproven treatment for COVID-19, 53 particularly early in the pandemic, has resulted in drug shortages for patients with lupus and other autoimmune skin diseases. Meanwhile, the need for patients with complex dermatologic conditions to receive systemic immunosuppression has had to be balanced against the associated risks during a global pandemic. To help dermatologists navigate this dilemma, various subspecialty groups have issued guidelines, including the COVID-19 Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists, which recommends a stepwise approach to shared decision-making with the goal of minimizing both the risk for disease flare and that of infection. The use of systemic steroids and rituximab, as well as the dose of immunosuppression—particularly broad-acting immunosuppression—should be limited where permitted. 54
Rapid adoption of telemedicine and remote monitoring strategies has enabled dermatologists to provide safe and timely care when in-person visits have not been possible, including for patients with confirmed or suspected COVID-19, as well as for hospitalized patients. 55-57 Use of telemedicine has facilitated preservation of personal protective equipment at a time when these important resources have been scarce. For patients with transportation or scheduling barriers, telemedicine has even expanded access to care.
However, this strategy cannot completely replace comprehensive in-person evaluation. Variability in video and photographic quality limits evaluation, while in-person physical examination can reveal subtle morphologic clues necessary for diagnosis. 5 8 Additionally, unequal access to technology may disadvantage some patients. For dermatologists to provide optimal care and continue to contribute accurate and insightful observations into COVID-19, it is essential to be physically present in the clinic and in the hospital when necessary, caring for patients in need of dermatologic expertise. Creative management strategies developed during this time will benefit patients and expand the reach of the specialty . 5 8
Final Thoughts
The COVID-19 pandemic has profoundly challenged the medical community and dermatology is no exception. By documenting and characterizing the diverse cutaneous manifestations of this novel disease, dermatologists have furthered understanding of its pathophysiology and management. By adapting quickly and developing creative ways to deliver care, dermatologists have found ways to contribute, both large and small. As we take stock at this juncture of the pandemic, it is clear there remains much to learn. We hope dermatologists will continue to take an active role in meeting the challenges of this time.
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA . 2020;324:782-793. doi:10.1001/jama.2020.12839
- New York Times . Updated December 23, 2020. Accessed March 22, 2021. https://www.nytimes.com/2020/11/15/us/coronavirus-us-cases-deaths.html
- Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med . 2020;382:1708-1720. doi:10.1056/NEJMoa2002032
- Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med . 2020;288:335-344. doi:https://doi.org/10.1111/joim.13089
- Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu province: a multicenter descriptive study. Clin Infect Dis . 2020;71:706-712. doi:10.1093/cid/ciaa199
- Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med . 2020;382:2372-2374. doi:10.1056/NEJMc2010419
- Sun L, Shen L, Fan J, et al. Clinical features of patients with coronavirus disease 2019 from a designated hospital in Beijing, China. J Med Virol . 2020;92:2055-2066. https://doi.org/10.1002/jmv.25966
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatology Venereol . 2020;34:E212-E213. https://doi.org/10.1111/jdv.16387
- Giavedoni P, Podlipnik S, Pericàs JM, et al. Skin manifestations in COVID-19: prevalence and relationship with disease severity. J Clin Med . 2020;9:3261. doi:10.3390/jcm9103261
- Jimenez-Cauhe J, Ortega-Quijano D, Prieto-Barrios M, et al. Reply to “COVID-19 can present with a rash and be mistaken for dengue”: petechial rash in a patient with COVID-19 infection. J Am Acad Dermatol . 2020;83:E141-E142. doi:10.1016/j.jaad.2020.04.016
- Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med . 2020;383:334-346. doi:10.1056/NEJMoa2021680
- Shinkai K, Bruckner AL. Dermatology and COVID-19. JAMA . 2020;324:1133-1134. doi:10.1001/jama.2020.15276
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol . 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol . 2020;183:71-77. https://doi.org/10.1111/bjd.19163
- Bouaziz JD, Duong TA, Jachiet M, et al. Vascular skin symptoms in COVID-19: a French observational study. J Eur Acad Dermatology Venereol . 2020;34:E451-E452. https://doi.org/10.1111/jdv.16544
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol . 2020;45:872-875. https://doi.org/10.1111/ced.14277
- Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol . 2020;83:E61-E63. doi:10.1016/j.jaad.2020.04.093
- Marzano AV, Genovese G, Fabbrocini G, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: Multicenter case series of 22 patients. J Am Acad Dermatol . 2020;83:280-285. doi:10.1016/j.jaad.2020.04.044
- Fernandez-Nieto D, Ortega-Quijano D, Segurado-Miravalles G, et al. Comment on: cutaneous manifestations in COVID-19: a first perspective. safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol . 2020;34:E252-E254. https://doi.org/10.1111/jdv.16470
- Herrero-Moyano M, Capusan TM, Andreu-Barasoain M, et al. A clinicopathological study of eight patients with COVID-19 pneumonia and a late-onset exanthema. J Eur Acad Dermatol Venereol . 2020;34:E460-E464. https://doi.org/10.1111/jdv.16631
- Rubio-Muniz CA, Puerta-Peñ a M, Falkenhain-L ópez D, et al. The broad spectrum of dermatological manifestations in COVID-19: clinical and histopathological features learned from a series of 34 cases. J Eur Acad Dermatol Venereol . 2020;34:E574-E576. https://doi.org/10.1111/jdv.16734
- Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings. Clin Exp Dermatol . 2020;45:892-895. https://doi.org/10.1111/ced.14281
- Jimenez-Cauhe J, Ortega-Quijano D, de Perosanz-Lobo D, et al. Enanthem in patients with COVID-19 and skin rash. JAMA Dermatol . 2020;156:1134-1136. doi:10.1001/jamadermatol.2020.2550
- Le Cleach L, Dousset L, Assier H, et al. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol . 2020;183:866-874. https://doi.org/10.1111/bjd.19377
- Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic [published online November 25, 2020]. JAMA Dermatol . doi:10.1001/jamadermatol.2020.4324
- Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol . 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Magro CM, Mulvey JJ, Laurence J, et al. The differing pathophysiologies that underlie COVID-19-associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol . 2021;184:141-150. https://doi.org/10.1111/bjd.19415
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol . 2020;183:729-737. doi:10.1111/bjd.19327
- Droesch C, Do MH, DeSancho M, et al. Livedoid and purpuric skin eruptions associated with coagulopathy in severe COVID-19. JAMA Dermatol . 2020;156:1-3. doi:10.1001/jamadermatol.2020.2800
- Asakura H, Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol . 2021;113:45-57. doi:10.1007/s12185-020-03029-y
- Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med . 2020;383:347-358. doi:10.1056/NEJMoa2021756
- Young TK, Shaw KS, Shah JK, et al. Mucocutaneous manifestations of multisystem inflammatory syndrome in children during the COVID-19 pandemic. JAMA Dermatol . 2021;157:207-212. doi:10.1001/jamadermatol.2020.4779
- Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259-269. doi:10.1001/jama.2020.10369
- Cheng MH, Zhang S, Porritt RA, et al. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation.
- Morris SB, Schwartz NG, Patel P, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 Infection—United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1450-1456. doi:10.15585/mmwr.mm6940e1
- Blanco-Melo D, Nilsson-Payant BE, Liu W-C, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036.e9-1045.e9. doi:10.1016/j.cell.2020.04.026
- Crow YJ, Manel N. Aicardi–Goutières syndrome and the type I interferonopathies. Nat Rev Immunol. 2015;15:429-440. doi:10.1038/nri3850
- Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718-724. doi:10.1126/science.abc6027
- Zimmermann N, Wolf C, Schwenke R, et al. Assessment of clinical response to janus kinase inhibition in patients with familial chilblain lupus and TREX1 mutation. JAMA Dermatol. 2019;155:342-346. doi:10.1001/jamadermatol.2018.5077
- Hubiche T, Le Duff F, Chiaverini C, et al. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions. Lancet Infect Dis. 2021;21:315-316. doi:10.1016/S1473-3099(20)30518-1
- Agrawal A. Mechanisms and implications of age-associated impaired innate interferon secretion by dendritic cells: a mini-review. Gerontology. 2013;59:421-426. doi:10.1159/000350536
- Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795-807. doi:10.1056/NEJMoa2031994
- US Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization (EUA) of baricitinib. Accessed March 29, 2021. https://www.fda.gov/media/143823/download
- Konno Y, Kimura I, Uriu K, et al. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep. 2020;32:108185. doi:10.1016/j.celrep.2020.108185
- Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke: from the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO). J Vasc Interv Radiol. 2018;29:441-453. doi:10.1016/j.jvir.2017.11.026
- Lo MW, Kemper C, Woodruff TM. COVID-19: complement, coagulation, and collateral damage. J Immunol. 2020;205:1488-1495. doi:10.4049/jimmunol.2000644
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Yan B, Freiwald T, Chauss D, et al. SARS-CoV2 drives JAK1/2-dependent local and systemic complement hyper-activation [published online June 9, 2020]. Res Sq. doi:10.21203/rs.3.rs-33390/v1
- Marietta M, Coluccio V, Luppi M. COVID-19, coagulopathy and venous thromboembolism: more questions than answers. Intern Emerg Med. 2020;15:1375-1387. doi:10.1007/s11739-020-02432-x
- Zuo Y, Estes SK, Ali RA, et al. Prothrombotic antiphospholipid antibodies in COVID-19 [published online June 17, 2020]. medRxiv. doi:10.1101/2020.06.15.20131607
- Lan S-H, Lai C-C, Huang H-T, et al. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56:106103. doi:10.1016/j.ijantimicag.2020.106103
- McMahon D, Gallman A, Hruza G, et al. COVID-19 “long-haulers” in dermatology? duration of dermatologic symptoms in an international registry from 39 countries. Abstract presented at: 29th EADV Congress; October 29, 2020. Accessed March 29, 2020. https://eadvdistribute.m-anage.com/from.storage?image=PXQEdDtICIihN3sM_8nAmh7p_y9AFijhQlf2-_KjrtYgOsOXNVwGxDdti95GZ2Yh0
- Saag MS. Misguided use of hydroxychloroquine for COVID-19: the infusion of politics into science. JAMA. 2020;324:2161-2162. doi:10.1001/jama.2020.22389
- Zahedi Niaki O, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Hammond MI, Sharma TR, Cooper KD, et al. Conducting inpatient dermatology consultations and maintaining resident education in the COVID-19 telemedicine era. J Am Acad Dermatol. 2020;83:E317-E318. doi:10.1016/j.jaad.2020.07.008
- Brunasso AMG, Massone C. Teledermatologic monitoring for chronic cutaneous autoimmune diseases with smartworking during COVID-19 emergency in a tertiary center in Italy. Dermatol Ther. 2020;33:E13495-E13495. doi:10.1111/dth.13695
- Trinidad J, Kroshinsky D, Kaffenberger BH, et al. Telemedicine for inpatient dermatology consultations in response to the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E69-E71. doi:10.1016/j.jaad.2020.04.096
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatology. 2020;156:733-734. doi:10.1001/jamadermatol.2020.1438
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA . 2020;324:782-793. doi:10.1001/jama.2020.12839
- New York Times . Updated December 23, 2020. Accessed March 22, 2021. https://www.nytimes.com/2020/11/15/us/coronavirus-us-cases-deaths.html
- Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med . 2020;382:1708-1720. doi:10.1056/NEJMoa2002032
- Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med . 2020;288:335-344. doi:https://doi.org/10.1111/joim.13089
- Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu province: a multicenter descriptive study. Clin Infect Dis . 2020;71:706-712. doi:10.1093/cid/ciaa199
- Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med . 2020;382:2372-2374. doi:10.1056/NEJMc2010419
- Sun L, Shen L, Fan J, et al. Clinical features of patients with coronavirus disease 2019 from a designated hospital in Beijing, China. J Med Virol . 2020;92:2055-2066. https://doi.org/10.1002/jmv.25966
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatology Venereol . 2020;34:E212-E213. https://doi.org/10.1111/jdv.16387
- Giavedoni P, Podlipnik S, Pericàs JM, et al. Skin manifestations in COVID-19: prevalence and relationship with disease severity. J Clin Med . 2020;9:3261. doi:10.3390/jcm9103261
- Jimenez-Cauhe J, Ortega-Quijano D, Prieto-Barrios M, et al. Reply to “COVID-19 can present with a rash and be mistaken for dengue”: petechial rash in a patient with COVID-19 infection. J Am Acad Dermatol . 2020;83:E141-E142. doi:10.1016/j.jaad.2020.04.016
- Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med . 2020;383:334-346. doi:10.1056/NEJMoa2021680
- Shinkai K, Bruckner AL. Dermatology and COVID-19. JAMA . 2020;324:1133-1134. doi:10.1001/jama.2020.15276
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol . 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol . 2020;183:71-77. https://doi.org/10.1111/bjd.19163
- Bouaziz JD, Duong TA, Jachiet M, et al. Vascular skin symptoms in COVID-19: a French observational study. J Eur Acad Dermatology Venereol . 2020;34:E451-E452. https://doi.org/10.1111/jdv.16544
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol . 2020;45:872-875. https://doi.org/10.1111/ced.14277
- Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol . 2020;83:E61-E63. doi:10.1016/j.jaad.2020.04.093
- Marzano AV, Genovese G, Fabbrocini G, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: Multicenter case series of 22 patients. J Am Acad Dermatol . 2020;83:280-285. doi:10.1016/j.jaad.2020.04.044
- Fernandez-Nieto D, Ortega-Quijano D, Segurado-Miravalles G, et al. Comment on: cutaneous manifestations in COVID-19: a first perspective. safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol . 2020;34:E252-E254. https://doi.org/10.1111/jdv.16470
- Herrero-Moyano M, Capusan TM, Andreu-Barasoain M, et al. A clinicopathological study of eight patients with COVID-19 pneumonia and a late-onset exanthema. J Eur Acad Dermatol Venereol . 2020;34:E460-E464. https://doi.org/10.1111/jdv.16631
- Rubio-Muniz CA, Puerta-Peñ a M, Falkenhain-L ópez D, et al. The broad spectrum of dermatological manifestations in COVID-19: clinical and histopathological features learned from a series of 34 cases. J Eur Acad Dermatol Venereol . 2020;34:E574-E576. https://doi.org/10.1111/jdv.16734
- Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings. Clin Exp Dermatol . 2020;45:892-895. https://doi.org/10.1111/ced.14281
- Jimenez-Cauhe J, Ortega-Quijano D, de Perosanz-Lobo D, et al. Enanthem in patients with COVID-19 and skin rash. JAMA Dermatol . 2020;156:1134-1136. doi:10.1001/jamadermatol.2020.2550
- Le Cleach L, Dousset L, Assier H, et al. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol . 2020;183:866-874. https://doi.org/10.1111/bjd.19377
- Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic [published online November 25, 2020]. JAMA Dermatol . doi:10.1001/jamadermatol.2020.4324
- Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol . 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Magro CM, Mulvey JJ, Laurence J, et al. The differing pathophysiologies that underlie COVID-19-associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol . 2021;184:141-150. https://doi.org/10.1111/bjd.19415
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol . 2020;183:729-737. doi:10.1111/bjd.19327
- Droesch C, Do MH, DeSancho M, et al. Livedoid and purpuric skin eruptions associated with coagulopathy in severe COVID-19. JAMA Dermatol . 2020;156:1-3. doi:10.1001/jamadermatol.2020.2800
- Asakura H, Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol . 2021;113:45-57. doi:10.1007/s12185-020-03029-y
- Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med . 2020;383:347-358. doi:10.1056/NEJMoa2021756
- Young TK, Shaw KS, Shah JK, et al. Mucocutaneous manifestations of multisystem inflammatory syndrome in children during the COVID-19 pandemic. JAMA Dermatol . 2021;157:207-212. doi:10.1001/jamadermatol.2020.4779
- Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259-269. doi:10.1001/jama.2020.10369
- Cheng MH, Zhang S, Porritt RA, et al. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation.
- Morris SB, Schwartz NG, Patel P, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 Infection—United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1450-1456. doi:10.15585/mmwr.mm6940e1
- Blanco-Melo D, Nilsson-Payant BE, Liu W-C, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036.e9-1045.e9. doi:10.1016/j.cell.2020.04.026
- Crow YJ, Manel N. Aicardi–Goutières syndrome and the type I interferonopathies. Nat Rev Immunol. 2015;15:429-440. doi:10.1038/nri3850
- Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718-724. doi:10.1126/science.abc6027
- Zimmermann N, Wolf C, Schwenke R, et al. Assessment of clinical response to janus kinase inhibition in patients with familial chilblain lupus and TREX1 mutation. JAMA Dermatol. 2019;155:342-346. doi:10.1001/jamadermatol.2018.5077
- Hubiche T, Le Duff F, Chiaverini C, et al. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions. Lancet Infect Dis. 2021;21:315-316. doi:10.1016/S1473-3099(20)30518-1
- Agrawal A. Mechanisms and implications of age-associated impaired innate interferon secretion by dendritic cells: a mini-review. Gerontology. 2013;59:421-426. doi:10.1159/000350536
- Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795-807. doi:10.1056/NEJMoa2031994
- US Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization (EUA) of baricitinib. Accessed March 29, 2021. https://www.fda.gov/media/143823/download
- Konno Y, Kimura I, Uriu K, et al. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep. 2020;32:108185. doi:10.1016/j.celrep.2020.108185
- Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke: from the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO). J Vasc Interv Radiol. 2018;29:441-453. doi:10.1016/j.jvir.2017.11.026
- Lo MW, Kemper C, Woodruff TM. COVID-19: complement, coagulation, and collateral damage. J Immunol. 2020;205:1488-1495. doi:10.4049/jimmunol.2000644
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Yan B, Freiwald T, Chauss D, et al. SARS-CoV2 drives JAK1/2-dependent local and systemic complement hyper-activation [published online June 9, 2020]. Res Sq. doi:10.21203/rs.3.rs-33390/v1
- Marietta M, Coluccio V, Luppi M. COVID-19, coagulopathy and venous thromboembolism: more questions than answers. Intern Emerg Med. 2020;15:1375-1387. doi:10.1007/s11739-020-02432-x
- Zuo Y, Estes SK, Ali RA, et al. Prothrombotic antiphospholipid antibodies in COVID-19 [published online June 17, 2020]. medRxiv. doi:10.1101/2020.06.15.20131607
- Lan S-H, Lai C-C, Huang H-T, et al. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56:106103. doi:10.1016/j.ijantimicag.2020.106103
- McMahon D, Gallman A, Hruza G, et al. COVID-19 “long-haulers” in dermatology? duration of dermatologic symptoms in an international registry from 39 countries. Abstract presented at: 29th EADV Congress; October 29, 2020. Accessed March 29, 2020. https://eadvdistribute.m-anage.com/from.storage?image=PXQEdDtICIihN3sM_8nAmh7p_y9AFijhQlf2-_KjrtYgOsOXNVwGxDdti95GZ2Yh0
- Saag MS. Misguided use of hydroxychloroquine for COVID-19: the infusion of politics into science. JAMA. 2020;324:2161-2162. doi:10.1001/jama.2020.22389
- Zahedi Niaki O, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Hammond MI, Sharma TR, Cooper KD, et al. Conducting inpatient dermatology consultations and maintaining resident education in the COVID-19 telemedicine era. J Am Acad Dermatol. 2020;83:E317-E318. doi:10.1016/j.jaad.2020.07.008
- Brunasso AMG, Massone C. Teledermatologic monitoring for chronic cutaneous autoimmune diseases with smartworking during COVID-19 emergency in a tertiary center in Italy. Dermatol Ther. 2020;33:E13495-E13495. doi:10.1111/dth.13695
- Trinidad J, Kroshinsky D, Kaffenberger BH, et al. Telemedicine for inpatient dermatology consultations in response to the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E69-E71. doi:10.1016/j.jaad.2020.04.096
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatology. 2020;156:733-734. doi:10.1001/jamadermatol.2020.1438
Practice Points
- Cutaneous manifestations of COVID-19 may reflect the range of host immunologic responses to SARS-CoV-2.
- Perniosis appears to be a late manifestation of COVID-19 associated with a comparatively benign disease course, whereas livedoid or other vasculopathic lesions portend poorer outcomes and may warrant further workup for occult thrombotic disease.
- Maculopapular, vesicular, and urticarial eruptions may be seen in association with COVID-19 but are nonspecific and necessitate a broad differential and workup.
- Challenges posed by the COVID-19 pandemic necessitate creative management strategies for immunosuppression and clinical assessment.
Quantifying Itch: Measurement on the Way to Management
Itch is one of the most protean manifestations of skin disease and can take a substantial physical and emotional toll on patients. For physicians, it is a frequent—if often dreaded—patient concern with a rising incidence. Lack of specific itch therapies as well as associations with multiple dermatologic conditions, including xerosis, psoriasis, atopic dermatitis, cutaneous lymphoma, contact dermatitis, and internal malignancies, make management of these itchy patients challenging and deserving of our attention. Studies evaluating patients with chronic pruritus identified a considerable impact on health-related quality of life, including development of depression, inability to perform activities of daily living, and sleep difficulties. 1
How to Classify Itching
Itch, or pruritus, originally was defined as an unpleasant sensation that provokes the desire to scratch,2 but this definition likely limits our ability to assess itch.
Pain is another complex subjective symptom but is one that has been better studied. A previous intensity theory postulated that itch is a form of pain: low-intensity noxious stimuli are perceived as itch, while high-intensity stimuli are perceived as pain. Over time, our understanding of itch evolved, and it became clear that a specific neuronal pathway for itch also exists.3 However, the pathophysiology of itch and pain remain intertwined. Scratching may elicit pain, providing a change in sensation that replaces the itch, whereas opioid analgesics suppress pain but may worsen the itch.
We are gaining a better understanding of the biology and classification of itch, which will hopefully enable the development of new measures to accurately assess itch. Four main categories of itch currently exist: neurogenic, psychogenic, neuropathic, and pruritoceptive.4 Patients may have one or multiple types of itch, which can be differentiated clinically and biochemically. Neurogenic (also known as systemic) itch is transmitted via the central nervous system with possible involvement of itch-specific neurons in the spinal cord and encompasses itch associated with pruritus from other organ systems. As the term implies, psychogenic itch is associated with psychiatric disorders. Neuropathic itch is generated from the inappropriate firing of peripheral or central sensory neurons in the absence of pruritogenic stimuli, which can be seen in notalgia paresthetica, brachioradial pruritus, and postherpetic neuralgia. Pruritoceptive itch most commonly is encountered in dermatology and is associated with skin inflammation or other dermatoses.4
How to Assess Itch Quantitatively
There currently are 2 major questions about quantitative assessments of itch. First, how do we measure itch in studies that are designed to relieve a different skin disease that is associated with itch? Most clinical trials investigating therapeutic options for atopic dermatitis and psoriasis now include itch assessment and improvement as a secondary outcome. Second, how do we measure itch in studies that are designed with relief of itch as the primary end point? Both of these scenarios require a fundamental set of decisions. Itch clearly is a subjective experience, but it also is one that can be local, regional, generalized, or transitory. Just as with pain, an individual can be distracted from their itch to some extent and consequently experience it more acutely when there are fewer stimuli in their environment. Classically, patients will report that itching is worse at night, preventing them from sleeping. Sleep disruption previously has been demonstrated.5 Of course, the environment also can exacerbate itch, as dry air and in some cases humidity can flare the sensation.
Fundamentally, therefore, the questions that are asked to assess itch are incredibly relevant, and there is a matrix of possible avenues of inquiry. Should you measure the peak itch in one area or the peak itch overall? Is the duration, the frequency, or the persistence of the itching most relevant? What is the correct time frame in which to do an assessment: the last 24 hours, the last 48 hours, or the last week? Because these parameters have been so challenging, most investigators have used a visual analog scale, similar to what is used to assess pain, at a 24-hour interval to decrease recall bias. The most commonly employed tool is the itch numeric rating scale (NRS), which asks patients to rate their symptoms on a scale of 0 (no itch) to 10 (worst imaginable itch). Although the psychometric properties of the itch NRS have been validated, debate still exists as to whether the itch NRS is best administered at a specific time of day or if it should be updated to evaluate peak pruritus scores explicitly. Regardless, implementing these scales often is time consuming and burdensome in the clinical trial setting, as participants are asked to complete daily diaries at the same time each day using either paper forms or electronic tablets.
Once scores are collected, we then need to quantitate a meaningful difference in itch. For pain, there has been some acceptance of a 30% difference, or a 2-point reduction, as being clinically meaningful; however, there was substantial debate at the time of the approval of ixekizumab as to whether that was a similarly appropriate threshold for itch. Using data from ixekizumab phase 2 and phase 3 trials, a 4-point reduction in itch NRS was found to be optimal for evaluating clinically significant changes in moderate to severe psoriasis.6 A more recent study of the validity of the itch NRS in prurigo nodularis suggested a 1-point change was correlated with minimal clinical improvement.7 Thus, the interesting question of how assessment of itch varies across clinical trials and disease states needs to be raised. Psoriasis classically has been thought of as not particularly itchy, and atopic dermatitis and prurigo nodularis have been regarded as extraordinarily itchy, yet one study comparing baseline itch scores in psoriasis and atopic dermatitis suggested that the experience actually is somewhat similar.8
Final Thoughts
The subjective nature of itch makes NRSs our best option at this time, but the best disease severity assessment tools are objective, sensitive, and generalizable. Unfortunately, we do not have such tools available to us yet, but technology—smart devices to monitor nocturnal scratching and machine learning algorithms that use electromagnetic impact to capture motion associated with itching and scratching9—may offer new objective measures for itch that can be used to further validate the current itch NRS. Even if these technology-based approaches become the standard of measurement, they will certainly help us understand what we are measuring. And even better, the focus on how to develop meaningful end points around the improvement of itch will likely lead us to measure it more and drive the development of therapeutics that address the effect and consequences of this pernicious problem.
- Kini SP, DeLong LK, Veledar E, et al. The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol. 2011;147:1153-1156. doi:10.1001/archdermatol.2011.178
- Savin JA. How should we define itching? J Am Acad Dermatol. 1998;39(2 pt 1):268-269. doi:10.1016/s0190-9622(98)70087-8
- Ikoma A, Rukwied R, Ständer S, et al. Neurophysiology of pruritus: interaction of itch and pain. Arch Dermatol. 2003;139:1475-1478. doi:10.1001/archderm.139.11.1475
- Garibyan L, Rheingold CG, Lerner EA. Understanding the pathophysiology of itch. Dermatol Ther. 2013;26:84-91. doi:10.1111/dth.12025
- Kimball AB, Luger T, Gottlieb A, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156-1161. doi:10.1016/j.jaad.2016.07.034
- Kimball AB, Naegeli AN, Edson-Heredia E, et al. Psychometric properties of the Itch Numeric Rating Scale in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:157-162. doi:10.1111/bjd.14464
- Kimel M, Zeidler C, Kwon P, et al. validation of psychometric properties of the itch numeric rating scale for pruritus associated with prurigo nodularis: a secondary analysis of a randomized clinical trial. JAMA Dermatol. 2020;156:1354-1358. doi:10.1001/jamadermatol.2020.3071
- Shahwan KT, Kimball AB. Itch intensity in moderate-to-severe plaque psoriasis versus atopic dermatitis: a meta-analysis. J Am Acad Dermatol. 2017;76:1198.el-1200.e1. doi:10.1016/j.jaad.2017.02.002
- Smith MP, Ly K, Thibodeaux Q, et al. Emerging methods to objectively assess pruritus in atopic dermatitis. Dermatol Ther (Heidelb). 2019;9:407-420. doi:10.1007/s13555-019-0312-3
Itch is one of the most protean manifestations of skin disease and can take a substantial physical and emotional toll on patients. For physicians, it is a frequent—if often dreaded—patient concern with a rising incidence. Lack of specific itch therapies as well as associations with multiple dermatologic conditions, including xerosis, psoriasis, atopic dermatitis, cutaneous lymphoma, contact dermatitis, and internal malignancies, make management of these itchy patients challenging and deserving of our attention. Studies evaluating patients with chronic pruritus identified a considerable impact on health-related quality of life, including development of depression, inability to perform activities of daily living, and sleep difficulties. 1
How to Classify Itching
Itch, or pruritus, originally was defined as an unpleasant sensation that provokes the desire to scratch,2 but this definition likely limits our ability to assess itch.
Pain is another complex subjective symptom but is one that has been better studied. A previous intensity theory postulated that itch is a form of pain: low-intensity noxious stimuli are perceived as itch, while high-intensity stimuli are perceived as pain. Over time, our understanding of itch evolved, and it became clear that a specific neuronal pathway for itch also exists.3 However, the pathophysiology of itch and pain remain intertwined. Scratching may elicit pain, providing a change in sensation that replaces the itch, whereas opioid analgesics suppress pain but may worsen the itch.
We are gaining a better understanding of the biology and classification of itch, which will hopefully enable the development of new measures to accurately assess itch. Four main categories of itch currently exist: neurogenic, psychogenic, neuropathic, and pruritoceptive.4 Patients may have one or multiple types of itch, which can be differentiated clinically and biochemically. Neurogenic (also known as systemic) itch is transmitted via the central nervous system with possible involvement of itch-specific neurons in the spinal cord and encompasses itch associated with pruritus from other organ systems. As the term implies, psychogenic itch is associated with psychiatric disorders. Neuropathic itch is generated from the inappropriate firing of peripheral or central sensory neurons in the absence of pruritogenic stimuli, which can be seen in notalgia paresthetica, brachioradial pruritus, and postherpetic neuralgia. Pruritoceptive itch most commonly is encountered in dermatology and is associated with skin inflammation or other dermatoses.4
How to Assess Itch Quantitatively
There currently are 2 major questions about quantitative assessments of itch. First, how do we measure itch in studies that are designed to relieve a different skin disease that is associated with itch? Most clinical trials investigating therapeutic options for atopic dermatitis and psoriasis now include itch assessment and improvement as a secondary outcome. Second, how do we measure itch in studies that are designed with relief of itch as the primary end point? Both of these scenarios require a fundamental set of decisions. Itch clearly is a subjective experience, but it also is one that can be local, regional, generalized, or transitory. Just as with pain, an individual can be distracted from their itch to some extent and consequently experience it more acutely when there are fewer stimuli in their environment. Classically, patients will report that itching is worse at night, preventing them from sleeping. Sleep disruption previously has been demonstrated.5 Of course, the environment also can exacerbate itch, as dry air and in some cases humidity can flare the sensation.
Fundamentally, therefore, the questions that are asked to assess itch are incredibly relevant, and there is a matrix of possible avenues of inquiry. Should you measure the peak itch in one area or the peak itch overall? Is the duration, the frequency, or the persistence of the itching most relevant? What is the correct time frame in which to do an assessment: the last 24 hours, the last 48 hours, or the last week? Because these parameters have been so challenging, most investigators have used a visual analog scale, similar to what is used to assess pain, at a 24-hour interval to decrease recall bias. The most commonly employed tool is the itch numeric rating scale (NRS), which asks patients to rate their symptoms on a scale of 0 (no itch) to 10 (worst imaginable itch). Although the psychometric properties of the itch NRS have been validated, debate still exists as to whether the itch NRS is best administered at a specific time of day or if it should be updated to evaluate peak pruritus scores explicitly. Regardless, implementing these scales often is time consuming and burdensome in the clinical trial setting, as participants are asked to complete daily diaries at the same time each day using either paper forms or electronic tablets.
Once scores are collected, we then need to quantitate a meaningful difference in itch. For pain, there has been some acceptance of a 30% difference, or a 2-point reduction, as being clinically meaningful; however, there was substantial debate at the time of the approval of ixekizumab as to whether that was a similarly appropriate threshold for itch. Using data from ixekizumab phase 2 and phase 3 trials, a 4-point reduction in itch NRS was found to be optimal for evaluating clinically significant changes in moderate to severe psoriasis.6 A more recent study of the validity of the itch NRS in prurigo nodularis suggested a 1-point change was correlated with minimal clinical improvement.7 Thus, the interesting question of how assessment of itch varies across clinical trials and disease states needs to be raised. Psoriasis classically has been thought of as not particularly itchy, and atopic dermatitis and prurigo nodularis have been regarded as extraordinarily itchy, yet one study comparing baseline itch scores in psoriasis and atopic dermatitis suggested that the experience actually is somewhat similar.8
Final Thoughts
The subjective nature of itch makes NRSs our best option at this time, but the best disease severity assessment tools are objective, sensitive, and generalizable. Unfortunately, we do not have such tools available to us yet, but technology—smart devices to monitor nocturnal scratching and machine learning algorithms that use electromagnetic impact to capture motion associated with itching and scratching9—may offer new objective measures for itch that can be used to further validate the current itch NRS. Even if these technology-based approaches become the standard of measurement, they will certainly help us understand what we are measuring. And even better, the focus on how to develop meaningful end points around the improvement of itch will likely lead us to measure it more and drive the development of therapeutics that address the effect and consequences of this pernicious problem.
Itch is one of the most protean manifestations of skin disease and can take a substantial physical and emotional toll on patients. For physicians, it is a frequent—if often dreaded—patient concern with a rising incidence. Lack of specific itch therapies as well as associations with multiple dermatologic conditions, including xerosis, psoriasis, atopic dermatitis, cutaneous lymphoma, contact dermatitis, and internal malignancies, make management of these itchy patients challenging and deserving of our attention. Studies evaluating patients with chronic pruritus identified a considerable impact on health-related quality of life, including development of depression, inability to perform activities of daily living, and sleep difficulties. 1
How to Classify Itching
Itch, or pruritus, originally was defined as an unpleasant sensation that provokes the desire to scratch,2 but this definition likely limits our ability to assess itch.
Pain is another complex subjective symptom but is one that has been better studied. A previous intensity theory postulated that itch is a form of pain: low-intensity noxious stimuli are perceived as itch, while high-intensity stimuli are perceived as pain. Over time, our understanding of itch evolved, and it became clear that a specific neuronal pathway for itch also exists.3 However, the pathophysiology of itch and pain remain intertwined. Scratching may elicit pain, providing a change in sensation that replaces the itch, whereas opioid analgesics suppress pain but may worsen the itch.
We are gaining a better understanding of the biology and classification of itch, which will hopefully enable the development of new measures to accurately assess itch. Four main categories of itch currently exist: neurogenic, psychogenic, neuropathic, and pruritoceptive.4 Patients may have one or multiple types of itch, which can be differentiated clinically and biochemically. Neurogenic (also known as systemic) itch is transmitted via the central nervous system with possible involvement of itch-specific neurons in the spinal cord and encompasses itch associated with pruritus from other organ systems. As the term implies, psychogenic itch is associated with psychiatric disorders. Neuropathic itch is generated from the inappropriate firing of peripheral or central sensory neurons in the absence of pruritogenic stimuli, which can be seen in notalgia paresthetica, brachioradial pruritus, and postherpetic neuralgia. Pruritoceptive itch most commonly is encountered in dermatology and is associated with skin inflammation or other dermatoses.4
How to Assess Itch Quantitatively
There currently are 2 major questions about quantitative assessments of itch. First, how do we measure itch in studies that are designed to relieve a different skin disease that is associated with itch? Most clinical trials investigating therapeutic options for atopic dermatitis and psoriasis now include itch assessment and improvement as a secondary outcome. Second, how do we measure itch in studies that are designed with relief of itch as the primary end point? Both of these scenarios require a fundamental set of decisions. Itch clearly is a subjective experience, but it also is one that can be local, regional, generalized, or transitory. Just as with pain, an individual can be distracted from their itch to some extent and consequently experience it more acutely when there are fewer stimuli in their environment. Classically, patients will report that itching is worse at night, preventing them from sleeping. Sleep disruption previously has been demonstrated.5 Of course, the environment also can exacerbate itch, as dry air and in some cases humidity can flare the sensation.
Fundamentally, therefore, the questions that are asked to assess itch are incredibly relevant, and there is a matrix of possible avenues of inquiry. Should you measure the peak itch in one area or the peak itch overall? Is the duration, the frequency, or the persistence of the itching most relevant? What is the correct time frame in which to do an assessment: the last 24 hours, the last 48 hours, or the last week? Because these parameters have been so challenging, most investigators have used a visual analog scale, similar to what is used to assess pain, at a 24-hour interval to decrease recall bias. The most commonly employed tool is the itch numeric rating scale (NRS), which asks patients to rate their symptoms on a scale of 0 (no itch) to 10 (worst imaginable itch). Although the psychometric properties of the itch NRS have been validated, debate still exists as to whether the itch NRS is best administered at a specific time of day or if it should be updated to evaluate peak pruritus scores explicitly. Regardless, implementing these scales often is time consuming and burdensome in the clinical trial setting, as participants are asked to complete daily diaries at the same time each day using either paper forms or electronic tablets.
Once scores are collected, we then need to quantitate a meaningful difference in itch. For pain, there has been some acceptance of a 30% difference, or a 2-point reduction, as being clinically meaningful; however, there was substantial debate at the time of the approval of ixekizumab as to whether that was a similarly appropriate threshold for itch. Using data from ixekizumab phase 2 and phase 3 trials, a 4-point reduction in itch NRS was found to be optimal for evaluating clinically significant changes in moderate to severe psoriasis.6 A more recent study of the validity of the itch NRS in prurigo nodularis suggested a 1-point change was correlated with minimal clinical improvement.7 Thus, the interesting question of how assessment of itch varies across clinical trials and disease states needs to be raised. Psoriasis classically has been thought of as not particularly itchy, and atopic dermatitis and prurigo nodularis have been regarded as extraordinarily itchy, yet one study comparing baseline itch scores in psoriasis and atopic dermatitis suggested that the experience actually is somewhat similar.8
Final Thoughts
The subjective nature of itch makes NRSs our best option at this time, but the best disease severity assessment tools are objective, sensitive, and generalizable. Unfortunately, we do not have such tools available to us yet, but technology—smart devices to monitor nocturnal scratching and machine learning algorithms that use electromagnetic impact to capture motion associated with itching and scratching9—may offer new objective measures for itch that can be used to further validate the current itch NRS. Even if these technology-based approaches become the standard of measurement, they will certainly help us understand what we are measuring. And even better, the focus on how to develop meaningful end points around the improvement of itch will likely lead us to measure it more and drive the development of therapeutics that address the effect and consequences of this pernicious problem.
- Kini SP, DeLong LK, Veledar E, et al. The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol. 2011;147:1153-1156. doi:10.1001/archdermatol.2011.178
- Savin JA. How should we define itching? J Am Acad Dermatol. 1998;39(2 pt 1):268-269. doi:10.1016/s0190-9622(98)70087-8
- Ikoma A, Rukwied R, Ständer S, et al. Neurophysiology of pruritus: interaction of itch and pain. Arch Dermatol. 2003;139:1475-1478. doi:10.1001/archderm.139.11.1475
- Garibyan L, Rheingold CG, Lerner EA. Understanding the pathophysiology of itch. Dermatol Ther. 2013;26:84-91. doi:10.1111/dth.12025
- Kimball AB, Luger T, Gottlieb A, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156-1161. doi:10.1016/j.jaad.2016.07.034
- Kimball AB, Naegeli AN, Edson-Heredia E, et al. Psychometric properties of the Itch Numeric Rating Scale in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:157-162. doi:10.1111/bjd.14464
- Kimel M, Zeidler C, Kwon P, et al. validation of psychometric properties of the itch numeric rating scale for pruritus associated with prurigo nodularis: a secondary analysis of a randomized clinical trial. JAMA Dermatol. 2020;156:1354-1358. doi:10.1001/jamadermatol.2020.3071
- Shahwan KT, Kimball AB. Itch intensity in moderate-to-severe plaque psoriasis versus atopic dermatitis: a meta-analysis. J Am Acad Dermatol. 2017;76:1198.el-1200.e1. doi:10.1016/j.jaad.2017.02.002
- Smith MP, Ly K, Thibodeaux Q, et al. Emerging methods to objectively assess pruritus in atopic dermatitis. Dermatol Ther (Heidelb). 2019;9:407-420. doi:10.1007/s13555-019-0312-3
- Kini SP, DeLong LK, Veledar E, et al. The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol. 2011;147:1153-1156. doi:10.1001/archdermatol.2011.178
- Savin JA. How should we define itching? J Am Acad Dermatol. 1998;39(2 pt 1):268-269. doi:10.1016/s0190-9622(98)70087-8
- Ikoma A, Rukwied R, Ständer S, et al. Neurophysiology of pruritus: interaction of itch and pain. Arch Dermatol. 2003;139:1475-1478. doi:10.1001/archderm.139.11.1475
- Garibyan L, Rheingold CG, Lerner EA. Understanding the pathophysiology of itch. Dermatol Ther. 2013;26:84-91. doi:10.1111/dth.12025
- Kimball AB, Luger T, Gottlieb A, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156-1161. doi:10.1016/j.jaad.2016.07.034
- Kimball AB, Naegeli AN, Edson-Heredia E, et al. Psychometric properties of the Itch Numeric Rating Scale in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:157-162. doi:10.1111/bjd.14464
- Kimel M, Zeidler C, Kwon P, et al. validation of psychometric properties of the itch numeric rating scale for pruritus associated with prurigo nodularis: a secondary analysis of a randomized clinical trial. JAMA Dermatol. 2020;156:1354-1358. doi:10.1001/jamadermatol.2020.3071
- Shahwan KT, Kimball AB. Itch intensity in moderate-to-severe plaque psoriasis versus atopic dermatitis: a meta-analysis. J Am Acad Dermatol. 2017;76:1198.el-1200.e1. doi:10.1016/j.jaad.2017.02.002
- Smith MP, Ly K, Thibodeaux Q, et al. Emerging methods to objectively assess pruritus in atopic dermatitis. Dermatol Ther (Heidelb). 2019;9:407-420. doi:10.1007/s13555-019-0312-3
Isolated Perianal Erosive Lichen Planus: A Diagnostic Challenge
To the Editor:
Erosive lichen planus (LP) often is painful, debilitating, and resistant to topical therapy making it both a diagnostic and therapeutic challenge. We report the case of an elderly woman with isolated perianal erosive LP, a rare clinical manifestation. We also review cases of erosive perianal LP reported in the literature.
A 72-year-old woman was referred to our dermatology clinic for evaluation of multiple pruritic and painful perianal lesions of 1 year’s duration. The lesions had remained stable since onset, with no other reported lesions elsewhere on body, including the mucosae. Her medical history was notable for rheumatoid arthritis, osteoporosis, hypercholesterolemia, and hypertension. She was taking methotrexate, folic acid, abatacept, alendronate, atorvastatin, and lisinopril. The patient reported she had been using abatacept for 3 years and lisinopril for 2 years. Her primary care physician initially treated the lesions as hemorrhoids but referred her to a gastroenterologist when they failed to improve. Gastroenterology evaluated the patient, and a colonoscopy was performed with unremarkable results. Thus, she was referred to dermatology for further evaluation.
Physical examination revealed 2 tender, sharply defined, angulated erosions with irregular violaceous borders involving the perianal skin (Figure 1). A biopsy of one of the lesions was taken. Histopathologic examination revealed acanthosis of the epidermis with slight compact hyperkeratosis, scattered dyskeratotic keratinocytes, and a dense bandlike lymphohistiocytic infiltrate that obliterated the dermoepidermal junction (Figure 2). A diagnosis of perianal erosive LP was made. The patient was prescribed mometasone ointment 0.1% daily with notable improvement after 2 months.
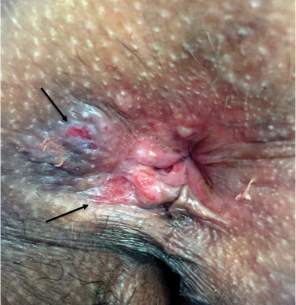
Erosive LP is an extremely rare variant of LP.1 It typically manifests as chronic painful erosions that often can progress to scarring, ulceration, and tissue destruction. Although erosive LP most commonly involves the mucosal surfaces of the genitalia and oral mucosa, it also has been reported in the palmoplantar skin, lacrimal duct, external auditory meatus, and esophagus.2-7 However, isolated perianal involvement is extremely rare. A PubMed search of articles indexed for MEDLINE using the terms erosive or ulcerative and lichen planus and perianal revealed 10 cases of perianal erosive LP, and weak data exist regarding therapy (Table).8-12 Of these cases, only 3 reported isolated perianal involvement.8-10 In most reported cases, perianal involvement manifested as extremely painful and occasionally pruritic, sharply angulated erosions and ulcers arising 0.5 to 3 cm from the anus with macerated, whitish, and violaceous borders. Most of the lesions occurred unilaterally, with only 1 case of bilateral perianal involvement.10
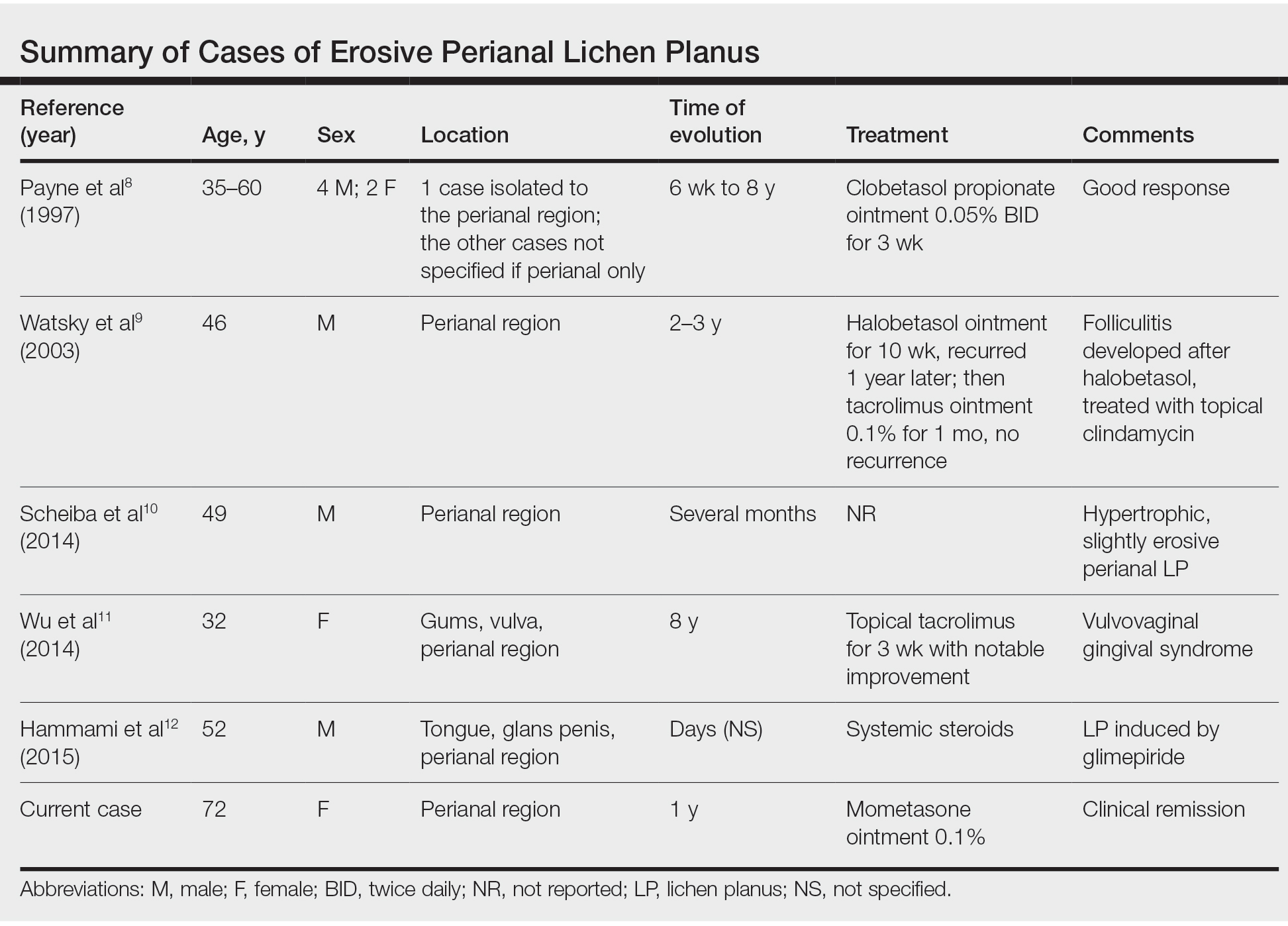
The differential diagnosis of perianal erosions is extensive and includes cutaneous Crohn disease, extramammary Paget disease, cutaneous malignancy, herpes simplex virus, cytomegalovirus, external hemorrhoids, lichen sclerosus, Behçet disease, lichen simplex chronicus, and drug-induced lichenoid reaction, among others. It is worth emphasizing infectious processes and cutaneous malignancies in light of our patient’s immunosuppression. Perianal cytomegalovirus has been reported in the literature in association with HIV, and it is a clinically challenging diagnosis.13 Cutaneous malignancy associated with the use of methotrexate also was considered in the differential diagnosis for our patient, given the increased risk for nonmelanoma skin cancer with the use of immunosuppresants.14
Along with a thorough patient history and physical examination, skin biopsy and clinicopathologic correlation are key to determine the exact etiology. Histologically, LP is characterized by a lichenoid interface dermatitis with a dense bandlike lymphohistiocytic infiltrate at the dermoepidermal junction. Other distinguishing factors include irregular acanthosis, hyperkeratosis, basal cell vacuolar degeneration, and Civatte bodies. Drug-induced LP is a possibility, but it is unclear if abatacept or lisinopril may have played a role in our patient. However, absence of eosinophils and parakeratosis suggested an idiopathic rather than drug-induced etiology. In 2016, Day et al2 published a clinicopathologic review of 60 cases of perianal lichenoid dermatoses in which only 17% of lesions were LP. Of note, 90% of perianal LP lesions were of the hypertrophic variant, and none were of the erosive variant, further supporting that our case represents a rare clinical manifestation of perianal LP.
Treatment of LP varies depending on the location and subtype of the lesions and is primarily aimed at improving symptoms. Topical corticosteroids are the standard treatment of LP; however, there is limited evidence regarding their efficacy for mucosal LP. Although randomized controlled trials assessing the efficacy of different interventions on oral erosive LP are available in the literature,15 there is a paucity of studies addressing this topic for genital or perianal LP. A review of the literature regarding perianal erosive LP suggests good response to high-potency topical steroids and calcineurin inhibitors with resolution of lesions within 3 to 4 weeks.11,15-18
Erosive LP is a painful variant that can cause erosions, ulcerations, and scarring. It rarely is seen in the perianal region alone and presents a diagnostic challenge. Treatment with high-potency topical steroid therapy seems to be effective in the few cases that have been reported as well as in our case. More comprehensive data from randomized controlled trials would be needed to evaluate their efficacy compared to other therapies.
- Rebora A. Erosive lichen planus: what is this? Dermatology. 2002;205:226-228; discussion 227.
- Day T, Bohl TG, Scurry J. Perianal lichen dermatoses: a review of 60 cases. Australas J Dermatol. 2016;57:210-215.
- Fox LP, Lightdale CJ, Grossman ME. Lichen planus of the esophagus: what dermatologists need to know. J Am Acad Dermatol. 2011;65:175-883.
- Holmstrup P, Thorn JJ, Rindum J, et al. Malignant development of lichen planus-affected oral mucosa. J Oral Pathol. 1988;17:219-225.
- Lewi, FM, Bogliatto F. Erosive vulval lichen planus—a diagnosis not to be missed: a clinical review. Eur J Obstet Gynecol Reprod Biol. 2013;171:214-219.
- Webber NK, Setterfield JF, Lewis FM, et al. Lacrimal canalicular duct scarring in patients with lichen planus. Arch Dermatol. 2012;148:224-227.
- Martin L, Moriniere S, Machet MC, et al. Bilateral conductive deafness related to erosive lichen planus. J Laryngol Otol. 1998;112:365-366.
- Payne CM, McPartlin JF, Hawley PR. Ulcerative perianal lichen planus. Br J Dermatol. 1997;136:479.
- Watsky KL. Erosive perianal lichen planus responsive to tacrolimus. Int J Dermatol. 2003;42:217-218.
- Scheiba N, Toberer F, Lenhard BH, et al. Erythema and erosions of the perianal region in a 49-year-old man. J Dtsch Dermatol Ges. 2014;12:162-165.
- Wu Y, Qiao J, Fang H. Syndrome in question. An Bras Dermatol. 2014;89:843-844.
- Hammami S, Ksouda K, Affes H, et al. Mucosal lichenoid drug reaction associated with glimepiride: a case report. Eur Rev Med Pharmacol Sci. 2015;19:2301-2302.
- Meyerle JH, Turiansky GW. Perianal ulcer in a patient with AIDS. Arch Dermatol. 2004;140:877-882.
- Scott FI, Mamtani R, Brensinger CM, et al. Risk of nonmelanoma skin cancer associated with the use of immunosuppressant and biologic agents in patients with a history of autoimmune disease and nonmelanoma skin cancer. JAMA Dermatol. 2016;152:164-172.
- Cheng S, Kirtschig G, Cooper S, et al. Interventions for erosive lichen planus affecting mucosal sites. Cochrane Database Syst Rev. 2012:Cd008092.
- Gunther S. Effect of retinoic acid in lichen planus of the genitalia and perianal region. Br J Vener Dis. 1973;49:553-554.
- Vente C, Reich K, Neumann C. Erosive mucosal lichen planus: response to topical treatment with tacrolimus. Br J Dermatol. 1999;140:338-342.
- Lonsdale-Eccles AA, Velangi S. Topical pimecrolimus in the treatment of genital lichen planus: a prospective case series. Br J Dermatol. 2005;153:390-394.
To the Editor:
Erosive lichen planus (LP) often is painful, debilitating, and resistant to topical therapy making it both a diagnostic and therapeutic challenge. We report the case of an elderly woman with isolated perianal erosive LP, a rare clinical manifestation. We also review cases of erosive perianal LP reported in the literature.
A 72-year-old woman was referred to our dermatology clinic for evaluation of multiple pruritic and painful perianal lesions of 1 year’s duration. The lesions had remained stable since onset, with no other reported lesions elsewhere on body, including the mucosae. Her medical history was notable for rheumatoid arthritis, osteoporosis, hypercholesterolemia, and hypertension. She was taking methotrexate, folic acid, abatacept, alendronate, atorvastatin, and lisinopril. The patient reported she had been using abatacept for 3 years and lisinopril for 2 years. Her primary care physician initially treated the lesions as hemorrhoids but referred her to a gastroenterologist when they failed to improve. Gastroenterology evaluated the patient, and a colonoscopy was performed with unremarkable results. Thus, she was referred to dermatology for further evaluation.
Physical examination revealed 2 tender, sharply defined, angulated erosions with irregular violaceous borders involving the perianal skin (Figure 1). A biopsy of one of the lesions was taken. Histopathologic examination revealed acanthosis of the epidermis with slight compact hyperkeratosis, scattered dyskeratotic keratinocytes, and a dense bandlike lymphohistiocytic infiltrate that obliterated the dermoepidermal junction (Figure 2). A diagnosis of perianal erosive LP was made. The patient was prescribed mometasone ointment 0.1% daily with notable improvement after 2 months.


Erosive LP is an extremely rare variant of LP.1 It typically manifests as chronic painful erosions that often can progress to scarring, ulceration, and tissue destruction. Although erosive LP most commonly involves the mucosal surfaces of the genitalia and oral mucosa, it also has been reported in the palmoplantar skin, lacrimal duct, external auditory meatus, and esophagus.2-7 However, isolated perianal involvement is extremely rare. A PubMed search of articles indexed for MEDLINE using the terms erosive or ulcerative and lichen planus and perianal revealed 10 cases of perianal erosive LP, and weak data exist regarding therapy (Table).8-12 Of these cases, only 3 reported isolated perianal involvement.8-10 In most reported cases, perianal involvement manifested as extremely painful and occasionally pruritic, sharply angulated erosions and ulcers arising 0.5 to 3 cm from the anus with macerated, whitish, and violaceous borders. Most of the lesions occurred unilaterally, with only 1 case of bilateral perianal involvement.10

The differential diagnosis of perianal erosions is extensive and includes cutaneous Crohn disease, extramammary Paget disease, cutaneous malignancy, herpes simplex virus, cytomegalovirus, external hemorrhoids, lichen sclerosus, Behçet disease, lichen simplex chronicus, and drug-induced lichenoid reaction, among others. It is worth emphasizing infectious processes and cutaneous malignancies in light of our patient’s immunosuppression. Perianal cytomegalovirus has been reported in the literature in association with HIV, and it is a clinically challenging diagnosis.13 Cutaneous malignancy associated with the use of methotrexate also was considered in the differential diagnosis for our patient, given the increased risk for nonmelanoma skin cancer with the use of immunosuppresants.14
Along with a thorough patient history and physical examination, skin biopsy and clinicopathologic correlation are key to determine the exact etiology. Histologically, LP is characterized by a lichenoid interface dermatitis with a dense bandlike lymphohistiocytic infiltrate at the dermoepidermal junction. Other distinguishing factors include irregular acanthosis, hyperkeratosis, basal cell vacuolar degeneration, and Civatte bodies. Drug-induced LP is a possibility, but it is unclear if abatacept or lisinopril may have played a role in our patient. However, absence of eosinophils and parakeratosis suggested an idiopathic rather than drug-induced etiology. In 2016, Day et al2 published a clinicopathologic review of 60 cases of perianal lichenoid dermatoses in which only 17% of lesions were LP. Of note, 90% of perianal LP lesions were of the hypertrophic variant, and none were of the erosive variant, further supporting that our case represents a rare clinical manifestation of perianal LP.
Treatment of LP varies depending on the location and subtype of the lesions and is primarily aimed at improving symptoms. Topical corticosteroids are the standard treatment of LP; however, there is limited evidence regarding their efficacy for mucosal LP. Although randomized controlled trials assessing the efficacy of different interventions on oral erosive LP are available in the literature,15 there is a paucity of studies addressing this topic for genital or perianal LP. A review of the literature regarding perianal erosive LP suggests good response to high-potency topical steroids and calcineurin inhibitors with resolution of lesions within 3 to 4 weeks.11,15-18
Erosive LP is a painful variant that can cause erosions, ulcerations, and scarring. It rarely is seen in the perianal region alone and presents a diagnostic challenge. Treatment with high-potency topical steroid therapy seems to be effective in the few cases that have been reported as well as in our case. More comprehensive data from randomized controlled trials would be needed to evaluate their efficacy compared to other therapies.
To the Editor:
Erosive lichen planus (LP) often is painful, debilitating, and resistant to topical therapy making it both a diagnostic and therapeutic challenge. We report the case of an elderly woman with isolated perianal erosive LP, a rare clinical manifestation. We also review cases of erosive perianal LP reported in the literature.
A 72-year-old woman was referred to our dermatology clinic for evaluation of multiple pruritic and painful perianal lesions of 1 year’s duration. The lesions had remained stable since onset, with no other reported lesions elsewhere on body, including the mucosae. Her medical history was notable for rheumatoid arthritis, osteoporosis, hypercholesterolemia, and hypertension. She was taking methotrexate, folic acid, abatacept, alendronate, atorvastatin, and lisinopril. The patient reported she had been using abatacept for 3 years and lisinopril for 2 years. Her primary care physician initially treated the lesions as hemorrhoids but referred her to a gastroenterologist when they failed to improve. Gastroenterology evaluated the patient, and a colonoscopy was performed with unremarkable results. Thus, she was referred to dermatology for further evaluation.
Physical examination revealed 2 tender, sharply defined, angulated erosions with irregular violaceous borders involving the perianal skin (Figure 1). A biopsy of one of the lesions was taken. Histopathologic examination revealed acanthosis of the epidermis with slight compact hyperkeratosis, scattered dyskeratotic keratinocytes, and a dense bandlike lymphohistiocytic infiltrate that obliterated the dermoepidermal junction (Figure 2). A diagnosis of perianal erosive LP was made. The patient was prescribed mometasone ointment 0.1% daily with notable improvement after 2 months.


Erosive LP is an extremely rare variant of LP.1 It typically manifests as chronic painful erosions that often can progress to scarring, ulceration, and tissue destruction. Although erosive LP most commonly involves the mucosal surfaces of the genitalia and oral mucosa, it also has been reported in the palmoplantar skin, lacrimal duct, external auditory meatus, and esophagus.2-7 However, isolated perianal involvement is extremely rare. A PubMed search of articles indexed for MEDLINE using the terms erosive or ulcerative and lichen planus and perianal revealed 10 cases of perianal erosive LP, and weak data exist regarding therapy (Table).8-12 Of these cases, only 3 reported isolated perianal involvement.8-10 In most reported cases, perianal involvement manifested as extremely painful and occasionally pruritic, sharply angulated erosions and ulcers arising 0.5 to 3 cm from the anus with macerated, whitish, and violaceous borders. Most of the lesions occurred unilaterally, with only 1 case of bilateral perianal involvement.10

The differential diagnosis of perianal erosions is extensive and includes cutaneous Crohn disease, extramammary Paget disease, cutaneous malignancy, herpes simplex virus, cytomegalovirus, external hemorrhoids, lichen sclerosus, Behçet disease, lichen simplex chronicus, and drug-induced lichenoid reaction, among others. It is worth emphasizing infectious processes and cutaneous malignancies in light of our patient’s immunosuppression. Perianal cytomegalovirus has been reported in the literature in association with HIV, and it is a clinically challenging diagnosis.13 Cutaneous malignancy associated with the use of methotrexate also was considered in the differential diagnosis for our patient, given the increased risk for nonmelanoma skin cancer with the use of immunosuppresants.14
Along with a thorough patient history and physical examination, skin biopsy and clinicopathologic correlation are key to determine the exact etiology. Histologically, LP is characterized by a lichenoid interface dermatitis with a dense bandlike lymphohistiocytic infiltrate at the dermoepidermal junction. Other distinguishing factors include irregular acanthosis, hyperkeratosis, basal cell vacuolar degeneration, and Civatte bodies. Drug-induced LP is a possibility, but it is unclear if abatacept or lisinopril may have played a role in our patient. However, absence of eosinophils and parakeratosis suggested an idiopathic rather than drug-induced etiology. In 2016, Day et al2 published a clinicopathologic review of 60 cases of perianal lichenoid dermatoses in which only 17% of lesions were LP. Of note, 90% of perianal LP lesions were of the hypertrophic variant, and none were of the erosive variant, further supporting that our case represents a rare clinical manifestation of perianal LP.
Treatment of LP varies depending on the location and subtype of the lesions and is primarily aimed at improving symptoms. Topical corticosteroids are the standard treatment of LP; however, there is limited evidence regarding their efficacy for mucosal LP. Although randomized controlled trials assessing the efficacy of different interventions on oral erosive LP are available in the literature,15 there is a paucity of studies addressing this topic for genital or perianal LP. A review of the literature regarding perianal erosive LP suggests good response to high-potency topical steroids and calcineurin inhibitors with resolution of lesions within 3 to 4 weeks.11,15-18
Erosive LP is a painful variant that can cause erosions, ulcerations, and scarring. It rarely is seen in the perianal region alone and presents a diagnostic challenge. Treatment with high-potency topical steroid therapy seems to be effective in the few cases that have been reported as well as in our case. More comprehensive data from randomized controlled trials would be needed to evaluate their efficacy compared to other therapies.
- Rebora A. Erosive lichen planus: what is this? Dermatology. 2002;205:226-228; discussion 227.
- Day T, Bohl TG, Scurry J. Perianal lichen dermatoses: a review of 60 cases. Australas J Dermatol. 2016;57:210-215.
- Fox LP, Lightdale CJ, Grossman ME. Lichen planus of the esophagus: what dermatologists need to know. J Am Acad Dermatol. 2011;65:175-883.
- Holmstrup P, Thorn JJ, Rindum J, et al. Malignant development of lichen planus-affected oral mucosa. J Oral Pathol. 1988;17:219-225.
- Lewi, FM, Bogliatto F. Erosive vulval lichen planus—a diagnosis not to be missed: a clinical review. Eur J Obstet Gynecol Reprod Biol. 2013;171:214-219.
- Webber NK, Setterfield JF, Lewis FM, et al. Lacrimal canalicular duct scarring in patients with lichen planus. Arch Dermatol. 2012;148:224-227.
- Martin L, Moriniere S, Machet MC, et al. Bilateral conductive deafness related to erosive lichen planus. J Laryngol Otol. 1998;112:365-366.
- Payne CM, McPartlin JF, Hawley PR. Ulcerative perianal lichen planus. Br J Dermatol. 1997;136:479.
- Watsky KL. Erosive perianal lichen planus responsive to tacrolimus. Int J Dermatol. 2003;42:217-218.
- Scheiba N, Toberer F, Lenhard BH, et al. Erythema and erosions of the perianal region in a 49-year-old man. J Dtsch Dermatol Ges. 2014;12:162-165.
- Wu Y, Qiao J, Fang H. Syndrome in question. An Bras Dermatol. 2014;89:843-844.
- Hammami S, Ksouda K, Affes H, et al. Mucosal lichenoid drug reaction associated with glimepiride: a case report. Eur Rev Med Pharmacol Sci. 2015;19:2301-2302.
- Meyerle JH, Turiansky GW. Perianal ulcer in a patient with AIDS. Arch Dermatol. 2004;140:877-882.
- Scott FI, Mamtani R, Brensinger CM, et al. Risk of nonmelanoma skin cancer associated with the use of immunosuppressant and biologic agents in patients with a history of autoimmune disease and nonmelanoma skin cancer. JAMA Dermatol. 2016;152:164-172.
- Cheng S, Kirtschig G, Cooper S, et al. Interventions for erosive lichen planus affecting mucosal sites. Cochrane Database Syst Rev. 2012:Cd008092.
- Gunther S. Effect of retinoic acid in lichen planus of the genitalia and perianal region. Br J Vener Dis. 1973;49:553-554.
- Vente C, Reich K, Neumann C. Erosive mucosal lichen planus: response to topical treatment with tacrolimus. Br J Dermatol. 1999;140:338-342.
- Lonsdale-Eccles AA, Velangi S. Topical pimecrolimus in the treatment of genital lichen planus: a prospective case series. Br J Dermatol. 2005;153:390-394.
- Rebora A. Erosive lichen planus: what is this? Dermatology. 2002;205:226-228; discussion 227.
- Day T, Bohl TG, Scurry J. Perianal lichen dermatoses: a review of 60 cases. Australas J Dermatol. 2016;57:210-215.
- Fox LP, Lightdale CJ, Grossman ME. Lichen planus of the esophagus: what dermatologists need to know. J Am Acad Dermatol. 2011;65:175-883.
- Holmstrup P, Thorn JJ, Rindum J, et al. Malignant development of lichen planus-affected oral mucosa. J Oral Pathol. 1988;17:219-225.
- Lewi, FM, Bogliatto F. Erosive vulval lichen planus—a diagnosis not to be missed: a clinical review. Eur J Obstet Gynecol Reprod Biol. 2013;171:214-219.
- Webber NK, Setterfield JF, Lewis FM, et al. Lacrimal canalicular duct scarring in patients with lichen planus. Arch Dermatol. 2012;148:224-227.
- Martin L, Moriniere S, Machet MC, et al. Bilateral conductive deafness related to erosive lichen planus. J Laryngol Otol. 1998;112:365-366.
- Payne CM, McPartlin JF, Hawley PR. Ulcerative perianal lichen planus. Br J Dermatol. 1997;136:479.
- Watsky KL. Erosive perianal lichen planus responsive to tacrolimus. Int J Dermatol. 2003;42:217-218.
- Scheiba N, Toberer F, Lenhard BH, et al. Erythema and erosions of the perianal region in a 49-year-old man. J Dtsch Dermatol Ges. 2014;12:162-165.
- Wu Y, Qiao J, Fang H. Syndrome in question. An Bras Dermatol. 2014;89:843-844.
- Hammami S, Ksouda K, Affes H, et al. Mucosal lichenoid drug reaction associated with glimepiride: a case report. Eur Rev Med Pharmacol Sci. 2015;19:2301-2302.
- Meyerle JH, Turiansky GW. Perianal ulcer in a patient with AIDS. Arch Dermatol. 2004;140:877-882.
- Scott FI, Mamtani R, Brensinger CM, et al. Risk of nonmelanoma skin cancer associated with the use of immunosuppressant and biologic agents in patients with a history of autoimmune disease and nonmelanoma skin cancer. JAMA Dermatol. 2016;152:164-172.
- Cheng S, Kirtschig G, Cooper S, et al. Interventions for erosive lichen planus affecting mucosal sites. Cochrane Database Syst Rev. 2012:Cd008092.
- Gunther S. Effect of retinoic acid in lichen planus of the genitalia and perianal region. Br J Vener Dis. 1973;49:553-554.
- Vente C, Reich K, Neumann C. Erosive mucosal lichen planus: response to topical treatment with tacrolimus. Br J Dermatol. 1999;140:338-342.
- Lonsdale-Eccles AA, Velangi S. Topical pimecrolimus in the treatment of genital lichen planus: a prospective case series. Br J Dermatol. 2005;153:390-394.
Practice Points
- Erosive lichen planus (LP) is an underrecognized variant of LP presenting with painful erosions, ulcerations, and scarring.
- Although rare, perianal erosive LP should be included in the differential diagnosis of perianal erosions.
- Treatment with high-potency steroids is an effective therapeutic option resulting in notable improvement.
Hypofractionated radiotherapy: New normal for lung cancer?
The U.K.-based study showed that patients with stage I-III lung cancer who were set to undergo radiotherapy with curative intent were more likely to receive fewer fractions at higher doses when treated between April and October 2020. During that period, 19% of patients had their radiotherapy dose or fractionation schedule changed to deviate from standard care.
In addition, 8% of patients who were set to undergo surgery ultimately received radiotherapy instead, presumably to ease pressures on already struggling intensive care services, said Kathryn Banfill, MBChB, of Christie NHS Foundation Trust in Manchester, England.
Dr. Banfill presented results from the COVID-RT Lung study at the European Lung Cancer Virtual Congress 2021 (Abstract 203MO).
New guidelines prompt study
When the COVID-19 pandemic began, European and joint European and North American guidelines were issued to try to ensure that lung cancer patients would continue to receive the best possible treatment under the circumstances. This included guidance on how and when to use treatments such as radiotherapy.
One U.K. guideline included recommendations on the use of hypofractionation in the COVID-19 era. The recommendations focused on altering the dosage or length of radiotherapy treatments to try to reduce the number of hospital visits, thereby reducing the risk of exposing patients to SARS-CoV-2.
“The aim of these guidelines is very much to reduce the risk to patients,” Dr. Banfill said. “These patients are often at higher risk of serious COVID-19, both as a result of their cancer and also as a result of many of the coexisting medical conditions that they have, such as COPD [chronic obstructive pulmonary disease],” she explained.
The COVID-RT Lung study was essentially born out of these guidelines. The goals of the study were to see what changes to radiotherapy practice occurred as a result of the guidelines and to assess how the changes have affected patient outcomes.
Changes to diagnosis and treatment
COVID-RT Lung is an ongoing, prospective study of patients with biopsy- or imaging-proven stage I–III lung cancer who were referred for, or treated with, radical radiotherapy at one of 26 oncology centers in the United Kingdom between April and October 2020.
Records on 1,117 patients were available for the initial analysis. The patients’ median age was 72 years (range, 38-93 years), and half were women.
The records showed changes to diagnostic investigations in 14% of patients (n = 160). Changes included not obtaining histology (4.6%, n = 51), not conducting nodal sampling (3.1%, n = 35), not performing pulmonary function tests (1.8%, n = 20), not conducting brain imaging (2.9%, n = 32), not performing PET/CT scans or having out-of-date scans (4.2%, n = 47), and delays in diagnosis (0.6%, n = 7).
Changes to treatment – deviations from standard care – occurred in 37% of patients (n = 415). This included 19% of patients (n = 210) having changes to radiotherapy dose or fractionation schedule, 8% (n = 86) undergoing radiotherapy instead of surgery, and 13% (n = 143) having their chemotherapy omitted or reduced.
The median number of radiotherapy fractions was 15 for patients who had their radiotherapy adjusted and 20 for those who had no treatment amendments.
“Those who had their treatment changed were more likely to have hypofractionated or ultra-hypofractionated radiotherapy,” Dr. Banfill said.
This was particularly true for patients with early-stage disease, she noted, where there was an increase in the percentage of patients getting more than 15 Gy per fraction. Even in stage III disease, there was an increased use of 3–5 Gy per fraction, although “virtually nobody” who had a change in treatment received less than 2 Gy per fraction, Dr. Banfill said.
“The changes are in line with what was reported in international recommendations,” observed Yolande Lievens, MD, PhD, of Ghent University Hospital in Belgium, who discussed the findings at the meeting.
Few patients had COVID-19
“It was striking to me to see that so few patients developed COVID-19 prior to radiotherapy or during radiotherapy,” Dr. Lievens noted. “This is actually something that we’ve also experienced in our setting.”
Indeed, just 15 patients (1%) were diagnosed with COVID-19, 10 of whom were diagnosed before receiving radiotherapy.
Dr. Banfill observed that the COVID-19 diagnosis had been “a reasonable time” before the patients started radiotherapy, and some had been diagnosed with lung cancer as a result of having a chest x-ray for suspected COVID-19.
Of the four patients who were diagnosed during treatment, two had their radiotherapy interrupted as a result.
The low COVID-19 rate is perhaps a result of the protective measures recommended in the United Kingdom, such as advising patients to shield from others, Dr. Banfill said.
Are changes to practice likely to hold?
“Part of the reason we actually stopped the data collection in October was that people were starting to go, ‘Well, is this actually a change?’ because they’d been doing it for 6 months,” Dr. Banfill observed during the discussion session.
“It was becoming almost normal for some of these hypofractionated changes. I think there is potential for these to become more embedded going forward,” she said. Data on how these changes might affect patients in the long term is going to be the focus of a future analysis.
“There is ongoing data collection on recurrence and survival and toxicity, which will hopefully provide more information on the outcomes of this patient group,” Dr. Banfill said.
The COVID-RT Lung project is supported by the NIHR Manchester Biomedical Research Centre. Dr. Banfill and Dr. Lievens reported no relevant conflicts of interest.
The U.K.-based study showed that patients with stage I-III lung cancer who were set to undergo radiotherapy with curative intent were more likely to receive fewer fractions at higher doses when treated between April and October 2020. During that period, 19% of patients had their radiotherapy dose or fractionation schedule changed to deviate from standard care.
In addition, 8% of patients who were set to undergo surgery ultimately received radiotherapy instead, presumably to ease pressures on already struggling intensive care services, said Kathryn Banfill, MBChB, of Christie NHS Foundation Trust in Manchester, England.
Dr. Banfill presented results from the COVID-RT Lung study at the European Lung Cancer Virtual Congress 2021 (Abstract 203MO).
New guidelines prompt study
When the COVID-19 pandemic began, European and joint European and North American guidelines were issued to try to ensure that lung cancer patients would continue to receive the best possible treatment under the circumstances. This included guidance on how and when to use treatments such as radiotherapy.
One U.K. guideline included recommendations on the use of hypofractionation in the COVID-19 era. The recommendations focused on altering the dosage or length of radiotherapy treatments to try to reduce the number of hospital visits, thereby reducing the risk of exposing patients to SARS-CoV-2.
“The aim of these guidelines is very much to reduce the risk to patients,” Dr. Banfill said. “These patients are often at higher risk of serious COVID-19, both as a result of their cancer and also as a result of many of the coexisting medical conditions that they have, such as COPD [chronic obstructive pulmonary disease],” she explained.
The COVID-RT Lung study was essentially born out of these guidelines. The goals of the study were to see what changes to radiotherapy practice occurred as a result of the guidelines and to assess how the changes have affected patient outcomes.
Changes to diagnosis and treatment
COVID-RT Lung is an ongoing, prospective study of patients with biopsy- or imaging-proven stage I–III lung cancer who were referred for, or treated with, radical radiotherapy at one of 26 oncology centers in the United Kingdom between April and October 2020.
Records on 1,117 patients were available for the initial analysis. The patients’ median age was 72 years (range, 38-93 years), and half were women.
The records showed changes to diagnostic investigations in 14% of patients (n = 160). Changes included not obtaining histology (4.6%, n = 51), not conducting nodal sampling (3.1%, n = 35), not performing pulmonary function tests (1.8%, n = 20), not conducting brain imaging (2.9%, n = 32), not performing PET/CT scans or having out-of-date scans (4.2%, n = 47), and delays in diagnosis (0.6%, n = 7).
Changes to treatment – deviations from standard care – occurred in 37% of patients (n = 415). This included 19% of patients (n = 210) having changes to radiotherapy dose or fractionation schedule, 8% (n = 86) undergoing radiotherapy instead of surgery, and 13% (n = 143) having their chemotherapy omitted or reduced.
The median number of radiotherapy fractions was 15 for patients who had their radiotherapy adjusted and 20 for those who had no treatment amendments.
“Those who had their treatment changed were more likely to have hypofractionated or ultra-hypofractionated radiotherapy,” Dr. Banfill said.
This was particularly true for patients with early-stage disease, she noted, where there was an increase in the percentage of patients getting more than 15 Gy per fraction. Even in stage III disease, there was an increased use of 3–5 Gy per fraction, although “virtually nobody” who had a change in treatment received less than 2 Gy per fraction, Dr. Banfill said.
“The changes are in line with what was reported in international recommendations,” observed Yolande Lievens, MD, PhD, of Ghent University Hospital in Belgium, who discussed the findings at the meeting.
Few patients had COVID-19
“It was striking to me to see that so few patients developed COVID-19 prior to radiotherapy or during radiotherapy,” Dr. Lievens noted. “This is actually something that we’ve also experienced in our setting.”
Indeed, just 15 patients (1%) were diagnosed with COVID-19, 10 of whom were diagnosed before receiving radiotherapy.
Dr. Banfill observed that the COVID-19 diagnosis had been “a reasonable time” before the patients started radiotherapy, and some had been diagnosed with lung cancer as a result of having a chest x-ray for suspected COVID-19.
Of the four patients who were diagnosed during treatment, two had their radiotherapy interrupted as a result.
The low COVID-19 rate is perhaps a result of the protective measures recommended in the United Kingdom, such as advising patients to shield from others, Dr. Banfill said.
Are changes to practice likely to hold?
“Part of the reason we actually stopped the data collection in October was that people were starting to go, ‘Well, is this actually a change?’ because they’d been doing it for 6 months,” Dr. Banfill observed during the discussion session.
“It was becoming almost normal for some of these hypofractionated changes. I think there is potential for these to become more embedded going forward,” she said. Data on how these changes might affect patients in the long term is going to be the focus of a future analysis.
“There is ongoing data collection on recurrence and survival and toxicity, which will hopefully provide more information on the outcomes of this patient group,” Dr. Banfill said.
The COVID-RT Lung project is supported by the NIHR Manchester Biomedical Research Centre. Dr. Banfill and Dr. Lievens reported no relevant conflicts of interest.
The U.K.-based study showed that patients with stage I-III lung cancer who were set to undergo radiotherapy with curative intent were more likely to receive fewer fractions at higher doses when treated between April and October 2020. During that period, 19% of patients had their radiotherapy dose or fractionation schedule changed to deviate from standard care.
In addition, 8% of patients who were set to undergo surgery ultimately received radiotherapy instead, presumably to ease pressures on already struggling intensive care services, said Kathryn Banfill, MBChB, of Christie NHS Foundation Trust in Manchester, England.
Dr. Banfill presented results from the COVID-RT Lung study at the European Lung Cancer Virtual Congress 2021 (Abstract 203MO).
New guidelines prompt study
When the COVID-19 pandemic began, European and joint European and North American guidelines were issued to try to ensure that lung cancer patients would continue to receive the best possible treatment under the circumstances. This included guidance on how and when to use treatments such as radiotherapy.
One U.K. guideline included recommendations on the use of hypofractionation in the COVID-19 era. The recommendations focused on altering the dosage or length of radiotherapy treatments to try to reduce the number of hospital visits, thereby reducing the risk of exposing patients to SARS-CoV-2.
“The aim of these guidelines is very much to reduce the risk to patients,” Dr. Banfill said. “These patients are often at higher risk of serious COVID-19, both as a result of their cancer and also as a result of many of the coexisting medical conditions that they have, such as COPD [chronic obstructive pulmonary disease],” she explained.
The COVID-RT Lung study was essentially born out of these guidelines. The goals of the study were to see what changes to radiotherapy practice occurred as a result of the guidelines and to assess how the changes have affected patient outcomes.
Changes to diagnosis and treatment
COVID-RT Lung is an ongoing, prospective study of patients with biopsy- or imaging-proven stage I–III lung cancer who were referred for, or treated with, radical radiotherapy at one of 26 oncology centers in the United Kingdom between April and October 2020.
Records on 1,117 patients were available for the initial analysis. The patients’ median age was 72 years (range, 38-93 years), and half were women.
The records showed changes to diagnostic investigations in 14% of patients (n = 160). Changes included not obtaining histology (4.6%, n = 51), not conducting nodal sampling (3.1%, n = 35), not performing pulmonary function tests (1.8%, n = 20), not conducting brain imaging (2.9%, n = 32), not performing PET/CT scans or having out-of-date scans (4.2%, n = 47), and delays in diagnosis (0.6%, n = 7).
Changes to treatment – deviations from standard care – occurred in 37% of patients (n = 415). This included 19% of patients (n = 210) having changes to radiotherapy dose or fractionation schedule, 8% (n = 86) undergoing radiotherapy instead of surgery, and 13% (n = 143) having their chemotherapy omitted or reduced.
The median number of radiotherapy fractions was 15 for patients who had their radiotherapy adjusted and 20 for those who had no treatment amendments.
“Those who had their treatment changed were more likely to have hypofractionated or ultra-hypofractionated radiotherapy,” Dr. Banfill said.
This was particularly true for patients with early-stage disease, she noted, where there was an increase in the percentage of patients getting more than 15 Gy per fraction. Even in stage III disease, there was an increased use of 3–5 Gy per fraction, although “virtually nobody” who had a change in treatment received less than 2 Gy per fraction, Dr. Banfill said.
“The changes are in line with what was reported in international recommendations,” observed Yolande Lievens, MD, PhD, of Ghent University Hospital in Belgium, who discussed the findings at the meeting.
Few patients had COVID-19
“It was striking to me to see that so few patients developed COVID-19 prior to radiotherapy or during radiotherapy,” Dr. Lievens noted. “This is actually something that we’ve also experienced in our setting.”
Indeed, just 15 patients (1%) were diagnosed with COVID-19, 10 of whom were diagnosed before receiving radiotherapy.
Dr. Banfill observed that the COVID-19 diagnosis had been “a reasonable time” before the patients started radiotherapy, and some had been diagnosed with lung cancer as a result of having a chest x-ray for suspected COVID-19.
Of the four patients who were diagnosed during treatment, two had their radiotherapy interrupted as a result.
The low COVID-19 rate is perhaps a result of the protective measures recommended in the United Kingdom, such as advising patients to shield from others, Dr. Banfill said.
Are changes to practice likely to hold?
“Part of the reason we actually stopped the data collection in October was that people were starting to go, ‘Well, is this actually a change?’ because they’d been doing it for 6 months,” Dr. Banfill observed during the discussion session.
“It was becoming almost normal for some of these hypofractionated changes. I think there is potential for these to become more embedded going forward,” she said. Data on how these changes might affect patients in the long term is going to be the focus of a future analysis.
“There is ongoing data collection on recurrence and survival and toxicity, which will hopefully provide more information on the outcomes of this patient group,” Dr. Banfill said.
The COVID-RT Lung project is supported by the NIHR Manchester Biomedical Research Centre. Dr. Banfill and Dr. Lievens reported no relevant conflicts of interest.
FROM ELCC 2021
Age-related cognitive decline not inevitable?
Investigators found that despite the presence of neuropathologies associated with Alzheimer’s disease (AD), many centenarians maintained high levels of cognitive performance.
“Cognitive decline is not inevitable,” senior author Henne Holstege, PhD, assistant professor, Amsterdam Alzheimer Center and Clinical Genetics, Amsterdam University Medical Center, said in an interview.
“At 100 years or older, high levels of cognitive performance can be maintained for several years, even when individuals are exposed to risk factors associated with cognitive decline,” she said.
The study was published online Jan. 15 in JAMA Network Open.
Escaping cognitive decline
Dr. Holstege said her interest in researching aging and cognitive health was inspired by the “fascinating” story of Hendrikje van Andel-Schipper, who died at age 115 in 2015 “completely cognitively healthy.” Her mother, who died at age 100, also was cognitively intact at the end of her life.
“I wanted to know how it is possible that some people can completely escape all aspects of cognitive decline while reaching extreme ages,” Dr. Holstege said.
To discover the secret to cognitive health in the oldest old, Dr. Holstege initiated the 100-Plus Study, which involved a cohort of healthy centenarians.
The investigators conducted extensive neuropsychological testing and collected blood and fecal samples to examine “the myriad factors that influence physical health, including genetics, neuropathology, blood markers, and the gut microbiome, to explore the molecular and neuropsychologic constellations associated with the escape from cognitive decline.”
The goal of the research was to investigate “to what extent centenarians were able to maintain their cognitive health after study inclusion, and to what extent this was associated with genetic, physical, or neuropathological features,” she said.
The study included 330 centenarians who completed one or more neuropsychological assessments. Neuropathologic studies were available for 44 participants.
To assess baseline cognitive performance, the researchers administered a wide array of neurocognitive tests, as well as the Mini–Mental State Examination, from which mean z scores for cognitive domains were calculated.
Additional factors in the analysis included sex, age, APOE status, cognitive reserve, physical health, and whether participants lived independently.
At autopsy, amyloid-beta (A-beta) level, the level of intracellular accumulation of phosphorylated tau protein in neurofibrillary tangles (NFTs), and the neuritic plaque (NP) load were assessed.
Resilience and cognitive reserve
At baseline, the median age of the centenarians (n = 330, 72.4% women) was 100.5 years (interquartile range, 100.2-101.7). A little over half (56.7%) lived independently, and the majority had good vision (65%) and hearing (56.4%). Most (78.8%) were able to walk independently, and 37.9% had achieved the highest International Standard Classification of Education level of postsecondary education.
The researchers found “varying degrees of neuropathology” in the brains of the 44 donors, including A-beta, NFT, and NPs.
The duration of follow-up in analyzing cognitive trajectories ranged from 0 to 4 years (median, 1.6 years).
Assessments of all cognitive domains showed no decline, with the exception of a “slight” decrement in memory function (beta −.10 SD per year; 95% confidence interval, –.14 to –.05 SD; P < .001).
Cognitive performance was associated with factors of physical health or cognitive reserve, for example, greater independence in performing activities of daily living, as assessed by the Barthel index (beta .37 SD per year; 95% CI, .24-.49; P < .001), or higher educational level (beta .41 SD per year; 95% CI, .29-.53; P < .001).
Despite findings of neuropathologic “hallmarks” of AD post mortem in the brains of the centenarians, these were not associated with cognitive performance or rate of decline.
APOE epsilon-4 or an APOE epsilon-3 alleles also were not significantly associated with cognitive performance or decline, suggesting that the “effects of APOE alleles are exerted before the age of 100 years,” the authors noted.
“Our findings suggest that after reaching age 100 years, cognitive performance remains relatively stable during ensuing years. Therefore, these centenarians might be resilient or resistant against different risk factors of cognitive decline,” the authors wrote. They also speculate that resilience may be attributable to greater cognitive reserve.
“Our preliminary data indicate that approximately 60% of the chance to reach 100 years old is heritable. Therefore, to get a better understanding of which genetic factors associate with the prolonged maintenance of cognitive health, we are looking into which genetic variants occur more commonly in centenarians compared to younger individuals,” said Dr. Holstege.
“Of course, more research needs to be performed to get a better understanding of how such genetic elements might sustain brain health,” she added.
A ‘landmark study’
Commenting on the study in an interview, Thomas Perls, MD, MPH, professor of medicine, Boston University, called it a “landmark” study in research on exceptional longevity in humans.
Dr. Perls, the author of an accompanying editorial, noted that “one cannot absolutely assume a certain level or disability or risk for disease just because a person has achieved extreme age – in fact, if anything, their ability to achieve much older ages likely indicates that they have resistance or resilience to aging-related problems.”
Understanding the mechanism of the resilience could lead to treatment or prevention of AD, said Dr. Perls, who was not involved in the research.
“People have to be careful about ageist myths and attitudes and not have the ageist idea that the older you get, the sicker you get, because many individuals disprove that,” he cautioned.
The study was supported by Stichting Alzheimer Nederland and Stichting Vumc Fonds. Research from the Alzheimer Center Amsterdam is part of the neurodegeneration research program of Amsterdam Neuroscience. Dr. Holstege and Dr. Perls reported having no relevant financial relationships. The other authors’ disclosures are listed on the original article.
A version of this article first appeared on Medscape.com.
Investigators found that despite the presence of neuropathologies associated with Alzheimer’s disease (AD), many centenarians maintained high levels of cognitive performance.
“Cognitive decline is not inevitable,” senior author Henne Holstege, PhD, assistant professor, Amsterdam Alzheimer Center and Clinical Genetics, Amsterdam University Medical Center, said in an interview.
“At 100 years or older, high levels of cognitive performance can be maintained for several years, even when individuals are exposed to risk factors associated with cognitive decline,” she said.
The study was published online Jan. 15 in JAMA Network Open.
Escaping cognitive decline
Dr. Holstege said her interest in researching aging and cognitive health was inspired by the “fascinating” story of Hendrikje van Andel-Schipper, who died at age 115 in 2015 “completely cognitively healthy.” Her mother, who died at age 100, also was cognitively intact at the end of her life.
“I wanted to know how it is possible that some people can completely escape all aspects of cognitive decline while reaching extreme ages,” Dr. Holstege said.
To discover the secret to cognitive health in the oldest old, Dr. Holstege initiated the 100-Plus Study, which involved a cohort of healthy centenarians.
The investigators conducted extensive neuropsychological testing and collected blood and fecal samples to examine “the myriad factors that influence physical health, including genetics, neuropathology, blood markers, and the gut microbiome, to explore the molecular and neuropsychologic constellations associated with the escape from cognitive decline.”
The goal of the research was to investigate “to what extent centenarians were able to maintain their cognitive health after study inclusion, and to what extent this was associated with genetic, physical, or neuropathological features,” she said.
The study included 330 centenarians who completed one or more neuropsychological assessments. Neuropathologic studies were available for 44 participants.
To assess baseline cognitive performance, the researchers administered a wide array of neurocognitive tests, as well as the Mini–Mental State Examination, from which mean z scores for cognitive domains were calculated.
Additional factors in the analysis included sex, age, APOE status, cognitive reserve, physical health, and whether participants lived independently.
At autopsy, amyloid-beta (A-beta) level, the level of intracellular accumulation of phosphorylated tau protein in neurofibrillary tangles (NFTs), and the neuritic plaque (NP) load were assessed.
Resilience and cognitive reserve
At baseline, the median age of the centenarians (n = 330, 72.4% women) was 100.5 years (interquartile range, 100.2-101.7). A little over half (56.7%) lived independently, and the majority had good vision (65%) and hearing (56.4%). Most (78.8%) were able to walk independently, and 37.9% had achieved the highest International Standard Classification of Education level of postsecondary education.
The researchers found “varying degrees of neuropathology” in the brains of the 44 donors, including A-beta, NFT, and NPs.
The duration of follow-up in analyzing cognitive trajectories ranged from 0 to 4 years (median, 1.6 years).
Assessments of all cognitive domains showed no decline, with the exception of a “slight” decrement in memory function (beta −.10 SD per year; 95% confidence interval, –.14 to –.05 SD; P < .001).
Cognitive performance was associated with factors of physical health or cognitive reserve, for example, greater independence in performing activities of daily living, as assessed by the Barthel index (beta .37 SD per year; 95% CI, .24-.49; P < .001), or higher educational level (beta .41 SD per year; 95% CI, .29-.53; P < .001).
Despite findings of neuropathologic “hallmarks” of AD post mortem in the brains of the centenarians, these were not associated with cognitive performance or rate of decline.
APOE epsilon-4 or an APOE epsilon-3 alleles also were not significantly associated with cognitive performance or decline, suggesting that the “effects of APOE alleles are exerted before the age of 100 years,” the authors noted.
“Our findings suggest that after reaching age 100 years, cognitive performance remains relatively stable during ensuing years. Therefore, these centenarians might be resilient or resistant against different risk factors of cognitive decline,” the authors wrote. They also speculate that resilience may be attributable to greater cognitive reserve.
“Our preliminary data indicate that approximately 60% of the chance to reach 100 years old is heritable. Therefore, to get a better understanding of which genetic factors associate with the prolonged maintenance of cognitive health, we are looking into which genetic variants occur more commonly in centenarians compared to younger individuals,” said Dr. Holstege.
“Of course, more research needs to be performed to get a better understanding of how such genetic elements might sustain brain health,” she added.
A ‘landmark study’
Commenting on the study in an interview, Thomas Perls, MD, MPH, professor of medicine, Boston University, called it a “landmark” study in research on exceptional longevity in humans.
Dr. Perls, the author of an accompanying editorial, noted that “one cannot absolutely assume a certain level or disability or risk for disease just because a person has achieved extreme age – in fact, if anything, their ability to achieve much older ages likely indicates that they have resistance or resilience to aging-related problems.”
Understanding the mechanism of the resilience could lead to treatment or prevention of AD, said Dr. Perls, who was not involved in the research.
“People have to be careful about ageist myths and attitudes and not have the ageist idea that the older you get, the sicker you get, because many individuals disprove that,” he cautioned.
The study was supported by Stichting Alzheimer Nederland and Stichting Vumc Fonds. Research from the Alzheimer Center Amsterdam is part of the neurodegeneration research program of Amsterdam Neuroscience. Dr. Holstege and Dr. Perls reported having no relevant financial relationships. The other authors’ disclosures are listed on the original article.
A version of this article first appeared on Medscape.com.
Investigators found that despite the presence of neuropathologies associated with Alzheimer’s disease (AD), many centenarians maintained high levels of cognitive performance.
“Cognitive decline is not inevitable,” senior author Henne Holstege, PhD, assistant professor, Amsterdam Alzheimer Center and Clinical Genetics, Amsterdam University Medical Center, said in an interview.
“At 100 years or older, high levels of cognitive performance can be maintained for several years, even when individuals are exposed to risk factors associated with cognitive decline,” she said.
The study was published online Jan. 15 in JAMA Network Open.
Escaping cognitive decline
Dr. Holstege said her interest in researching aging and cognitive health was inspired by the “fascinating” story of Hendrikje van Andel-Schipper, who died at age 115 in 2015 “completely cognitively healthy.” Her mother, who died at age 100, also was cognitively intact at the end of her life.
“I wanted to know how it is possible that some people can completely escape all aspects of cognitive decline while reaching extreme ages,” Dr. Holstege said.
To discover the secret to cognitive health in the oldest old, Dr. Holstege initiated the 100-Plus Study, which involved a cohort of healthy centenarians.
The investigators conducted extensive neuropsychological testing and collected blood and fecal samples to examine “the myriad factors that influence physical health, including genetics, neuropathology, blood markers, and the gut microbiome, to explore the molecular and neuropsychologic constellations associated with the escape from cognitive decline.”
The goal of the research was to investigate “to what extent centenarians were able to maintain their cognitive health after study inclusion, and to what extent this was associated with genetic, physical, or neuropathological features,” she said.
The study included 330 centenarians who completed one or more neuropsychological assessments. Neuropathologic studies were available for 44 participants.
To assess baseline cognitive performance, the researchers administered a wide array of neurocognitive tests, as well as the Mini–Mental State Examination, from which mean z scores for cognitive domains were calculated.
Additional factors in the analysis included sex, age, APOE status, cognitive reserve, physical health, and whether participants lived independently.
At autopsy, amyloid-beta (A-beta) level, the level of intracellular accumulation of phosphorylated tau protein in neurofibrillary tangles (NFTs), and the neuritic plaque (NP) load were assessed.
Resilience and cognitive reserve
At baseline, the median age of the centenarians (n = 330, 72.4% women) was 100.5 years (interquartile range, 100.2-101.7). A little over half (56.7%) lived independently, and the majority had good vision (65%) and hearing (56.4%). Most (78.8%) were able to walk independently, and 37.9% had achieved the highest International Standard Classification of Education level of postsecondary education.
The researchers found “varying degrees of neuropathology” in the brains of the 44 donors, including A-beta, NFT, and NPs.
The duration of follow-up in analyzing cognitive trajectories ranged from 0 to 4 years (median, 1.6 years).
Assessments of all cognitive domains showed no decline, with the exception of a “slight” decrement in memory function (beta −.10 SD per year; 95% confidence interval, –.14 to –.05 SD; P < .001).
Cognitive performance was associated with factors of physical health or cognitive reserve, for example, greater independence in performing activities of daily living, as assessed by the Barthel index (beta .37 SD per year; 95% CI, .24-.49; P < .001), or higher educational level (beta .41 SD per year; 95% CI, .29-.53; P < .001).
Despite findings of neuropathologic “hallmarks” of AD post mortem in the brains of the centenarians, these were not associated with cognitive performance or rate of decline.
APOE epsilon-4 or an APOE epsilon-3 alleles also were not significantly associated with cognitive performance or decline, suggesting that the “effects of APOE alleles are exerted before the age of 100 years,” the authors noted.
“Our findings suggest that after reaching age 100 years, cognitive performance remains relatively stable during ensuing years. Therefore, these centenarians might be resilient or resistant against different risk factors of cognitive decline,” the authors wrote. They also speculate that resilience may be attributable to greater cognitive reserve.
“Our preliminary data indicate that approximately 60% of the chance to reach 100 years old is heritable. Therefore, to get a better understanding of which genetic factors associate with the prolonged maintenance of cognitive health, we are looking into which genetic variants occur more commonly in centenarians compared to younger individuals,” said Dr. Holstege.
“Of course, more research needs to be performed to get a better understanding of how such genetic elements might sustain brain health,” she added.
A ‘landmark study’
Commenting on the study in an interview, Thomas Perls, MD, MPH, professor of medicine, Boston University, called it a “landmark” study in research on exceptional longevity in humans.
Dr. Perls, the author of an accompanying editorial, noted that “one cannot absolutely assume a certain level or disability or risk for disease just because a person has achieved extreme age – in fact, if anything, their ability to achieve much older ages likely indicates that they have resistance or resilience to aging-related problems.”
Understanding the mechanism of the resilience could lead to treatment or prevention of AD, said Dr. Perls, who was not involved in the research.
“People have to be careful about ageist myths and attitudes and not have the ageist idea that the older you get, the sicker you get, because many individuals disprove that,” he cautioned.
The study was supported by Stichting Alzheimer Nederland and Stichting Vumc Fonds. Research from the Alzheimer Center Amsterdam is part of the neurodegeneration research program of Amsterdam Neuroscience. Dr. Holstege and Dr. Perls reported having no relevant financial relationships. The other authors’ disclosures are listed on the original article.
A version of this article first appeared on Medscape.com.