User login
Hospitalists and medical malpractice
A look at some sobering trends
Among the pressures felt by hospitalists are concerns about being subject to a malpractice claim. Anxiety about malpractice influences the way hospitalists practice, giving rise to defensive medicine.
One survey, which asked hospitalists to retrospectively rate which of their orders represented defensive medicine, found that 28% of orders were deemed defensive.1 Defensive medicine can lead to low-value medical care, drive up health care costs, and potentially subject patients to unnecessary testing.2,3
Encouragingly, medical malpractice claims rates have, overall, been downtrending. An analysis of data from the National Practitioner Data Bank, which is a repository of all paid malpractice claims against individual physicians, found that malpractice claims rates decreased by 55.7% from 1992 to 2014 among all specialties, and by 46.1% for internal medicine physicians.4 The data used in this analysis did not separate hospitalists from other internal medicine physicians. An older study of malpractice claims against hospitalists found that hospitalists had significantly lower claims rates than non-hospitalist internal medicine physicians.5
Current malpractice environment for hospitalists
Seeking to shed light on the current malpractice environment faced by hospitalists, a recent study examined claims against hospitalists using the Comparative Benchmarking System (CBS), a national database of malpractice claims containing approximately 30% of all U.S. malpractice claims, which is maintained by CRICO, the malpractice insurer for the Harvard-affiliated medical institutions.6
Claims in the CBS database are examined by trained nurse coders who review the claims, along with the associated medical and legal records, to understand the contributing factors behind the adverse event leading to the claim.
Contrary to the trends for nearly all other physician specialties, the malpractice claims rates of hospitalists were not downtrending, going from 1.77 claims per 100 physician-years from 2009-2013 to 2.08 claims per 100 physician-years from 2014-2018. The overall claims rate for hospitalists was significantly higher than that for internal medicine subspecialists (though roughly the same as the claims rate for non-hospitalist general internal medicine physicians). These sobering findings raise the important question of why hospitalists claims rates are heading in the wrong direction.
One possible answer relates the ever-broadening scope of hospitalist practice. Hospitalists are being asked to care for surgical patients and other patient populations that they may have less familiarity with, increasing the risk of medical errors. Among the other specialties most commonly also named in hospitalist claims, general surgery and orthopedic surgery are in the top five. The extraordinary growth in the field of hospital medicine has meant a need to hire an increasing number of hospitalists, leading to less-experienced physicians entering the field.
Making hospital medicine safer
A more urgent question than what is driving the trends in hospitalist claims rates is what can be done to avoid adverse events and make hospital medicine safer. One potential answer is thoughtful collaboration arrangements with the surgical and other specialties with whom hospitalists may be co-managing patients.
Questions about who responds to what types of clinical issues that might arise and specific domains of responsibility should be defined in advance, so that a lack of role clarity does not negatively impact patient care. Given that hospitalists will be less comfortable addressing more technical surgical issues, expectations about surgeons’ availability should be established. Nocturnists may be tasked with overnight cross-coverage of patients on services, such as oncology and cardiology, that subspecialty physicians have responsibility for during the day. Agreeing upon triggers for when the nocturnist should contact the daytime subspecialty attending (for example, if a rapid response is called on their patient) should be considered, so that nocturnists are not left deciding, in the moment, whether to call the daytime attending. Measures such as this ensure that everyone’s expectations are aligned. In addition, new hospitalists need to be offered support, in the form of training and mentorship.
CBS malpractice data, which includes the contributing factors underlying what went wrong, illuminates potential targets for programs designed to enhance patient safety. In the recent hospitalist malpractice study, the two contributing factors that were the best predictors of a hospitalist malpractice claim closing with payment to the claimant were clinical judgment errors and communication breakdowns. Identifying measures that are effective in promoting patient safety by refining the clinical judgement of clinicians is a challenge, and there are limited data demonstrating what programs are effective in this area.
Clinical decision support (CDS) systems have shown promise in promoting guideline-concordant care.7 However, the role of CDS in aiding the higher-stakes clinical decisions that may be called into question after an adverse outcome is not well defined. Alerts that a patient may be developing sepsis is one type of CDS that has been extensively studied and has been shown to be of some benefit.8 The importance of clinical judgment to whether payment is made on a malpractice claim can inform risk management strategies. Hospitalists should document the thought process behind their decision making in the chart, especially for important clinical decisions. A note showing that the clinician was thoughtfully weighing the risks and benefits using the data available at the time will help make a case defensible if an adverse outcome occurs.
The effect of communication breakdowns on hospitalist case outcomes highlights the importance of measures to improve and systematize communication among clinicians, particularly at vulnerable junctures – such as handoffs from the day team to the night team, and transitions from one care setting to another. An example of an intervention to improve handoffs with cogent evidence to support it is I-PASS, which is an approach to handoffs between teams in which information about the patient’s illness severity, clinical background, and contingencies is conveyed and synthesized in a structured manner. A study of the effect of implementation of I-PASS among nine pediatric residency programs demonstrated a 30% reduction in preventable adverse events.9
Applying insights from malpractice claims analysis to clinical practice
The systematic review of malpractice cases to determine the contributing factors and other case attributes is an important source of patient safety insights. The process breakdowns described by the contributing factors can inform the design of patient safety initiatives. In addition, malpractice data provides information on which specialties and what types of clinicians are being named together in malpractice claims.
In the hospitalist malpractice study, in addition to general surgery and orthopedic surgery, the other clinical services most commonly subject to claims along with hospitalists were nursing, emergency medicine, and cardiology. Another observation was that physician assistants and nurse practitioners are increasingly being named in hospitalist claims. This information is crucial to guiding who needs to be in the room with hospitalists when efforts are undertaken to enhance patient safety within hospital medicine.
An understandable response to the finding that hospitalist claims rates are not decreasing is for hospitalists to seek ways to lower their risk of being named in a malpractice claim. Of course, avoiding adverse events by providing the safest possible care is paramount. Even when patients do suffer adverse events due to a physician negligence, only rarely, less than 5% of the time, does this result in a malpractice claim.10 Important lessons in risk management can be learned from examining why patients decide to sue when mistakes lead to bad outcomes.
An analysis of plaintiffs’ depositions found that the key reasons that patients decided to file a malpractice claim include a poor relationship with the physician – specifically, a lack of empathy from the physician, feeling deserted by the physician, and feeling devalued by the physician.11 These findings support the use of programs that assist physicians in compassionately disclosing adverse events to patients. Among inpatient physicians, patient satisfaction survey questions about the time the physician spent with the patient and the physician’s concern for the patient are better predictors of the physicians’ risk management performance than is the question about the skill of the physician.12 In the aftermath of an adverse event, focusing on maintaining a strong patient-physician relationship is not only the right the thing to do, the data tell us that it is also a sensible approach to reducing medicolegal risk.
Dr. Schaffer practices as a member of the Hospital Medicine Unit at Brigham and Women’s Hospital, Boston, where he serves as an attending physician on the inpatient general medicine services. An instructor at Harvard Medical School, his academic interests include research using large medical malpractice databases to examine temporal trends in medical malpractice.
References
1. Rothberg MB, et al. The cost of defensive medicine on 3 hospital medicine services. JAMA Intern Med. 2014;174(11):1867-1868. doi:10.1001/jamainternmed.2014.4649.
2. Kachalia A, et al. Overuse of testing in preoperative evaluation and syncope: A survey of hospitalists. Ann Intern Med. 2015;162(2):100-108. doi: 10.7326/M14-0694.
3. Mello MM, et al. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577. doi: 10.1377/hlthaff.2009.0807.
4. Schaffer AC, et al. Rates and characteristics of paid malpractice claims among U.S. physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177(5):710-718. doi:10.1001/jamainternmed.2017.0311.
5. Schaffer AC, et al. Liability impact of the hospitalist model of care. J Hosp Med. 2014;9(12):750-755. doi: 10.1002/jhm.2244.
6. Schaffer AC, et al. Rates and characteristics of medical malpractice claims against hospitalists. J Hosp Med. 2021 Jul;16(7):390-396. doi: 10.12788/jhm.3557.
7. Poon EG. Clinical decision support: a tool of the hospital trade. J Hosp Med. 2015;10(1):60-61. doi: 10.1002/jhm.2295.
8. Makam AN, Nguyen OK, Auerbach AD. Diagnostic accuracy and effectiveness of automated electronic sepsis alert systems: A systematic review. J Hosp Med. 2015;10(6):396-402. doi: 10.1002/jhm.2347.
9. Starmer AJ, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. doi: 10.1056/NEJMsa1405556.
10. Localio AR, et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. doi: 10.1056/NEJM199107253250405.
11. Beckman HB, et al. The doctor-patient relationship and malpractice. Lessons from plaintiff depositions. Arch Intern Med. 1994;154(12):1365-1370. doi:10.1001/archinte.1994.00420120093010.
12. Stelfox HT, et al. The relation of patient satisfaction with complaints against physicians and malpractice lawsuits. Am J Med. 2005;118(10):1126-1133. doi: 10.1016/j.amjmed.2005.01.060.
A look at some sobering trends
A look at some sobering trends
Among the pressures felt by hospitalists are concerns about being subject to a malpractice claim. Anxiety about malpractice influences the way hospitalists practice, giving rise to defensive medicine.
One survey, which asked hospitalists to retrospectively rate which of their orders represented defensive medicine, found that 28% of orders were deemed defensive.1 Defensive medicine can lead to low-value medical care, drive up health care costs, and potentially subject patients to unnecessary testing.2,3
Encouragingly, medical malpractice claims rates have, overall, been downtrending. An analysis of data from the National Practitioner Data Bank, which is a repository of all paid malpractice claims against individual physicians, found that malpractice claims rates decreased by 55.7% from 1992 to 2014 among all specialties, and by 46.1% for internal medicine physicians.4 The data used in this analysis did not separate hospitalists from other internal medicine physicians. An older study of malpractice claims against hospitalists found that hospitalists had significantly lower claims rates than non-hospitalist internal medicine physicians.5
Current malpractice environment for hospitalists
Seeking to shed light on the current malpractice environment faced by hospitalists, a recent study examined claims against hospitalists using the Comparative Benchmarking System (CBS), a national database of malpractice claims containing approximately 30% of all U.S. malpractice claims, which is maintained by CRICO, the malpractice insurer for the Harvard-affiliated medical institutions.6
Claims in the CBS database are examined by trained nurse coders who review the claims, along with the associated medical and legal records, to understand the contributing factors behind the adverse event leading to the claim.
Contrary to the trends for nearly all other physician specialties, the malpractice claims rates of hospitalists were not downtrending, going from 1.77 claims per 100 physician-years from 2009-2013 to 2.08 claims per 100 physician-years from 2014-2018. The overall claims rate for hospitalists was significantly higher than that for internal medicine subspecialists (though roughly the same as the claims rate for non-hospitalist general internal medicine physicians). These sobering findings raise the important question of why hospitalists claims rates are heading in the wrong direction.
One possible answer relates the ever-broadening scope of hospitalist practice. Hospitalists are being asked to care for surgical patients and other patient populations that they may have less familiarity with, increasing the risk of medical errors. Among the other specialties most commonly also named in hospitalist claims, general surgery and orthopedic surgery are in the top five. The extraordinary growth in the field of hospital medicine has meant a need to hire an increasing number of hospitalists, leading to less-experienced physicians entering the field.
Making hospital medicine safer
A more urgent question than what is driving the trends in hospitalist claims rates is what can be done to avoid adverse events and make hospital medicine safer. One potential answer is thoughtful collaboration arrangements with the surgical and other specialties with whom hospitalists may be co-managing patients.
Questions about who responds to what types of clinical issues that might arise and specific domains of responsibility should be defined in advance, so that a lack of role clarity does not negatively impact patient care. Given that hospitalists will be less comfortable addressing more technical surgical issues, expectations about surgeons’ availability should be established. Nocturnists may be tasked with overnight cross-coverage of patients on services, such as oncology and cardiology, that subspecialty physicians have responsibility for during the day. Agreeing upon triggers for when the nocturnist should contact the daytime subspecialty attending (for example, if a rapid response is called on their patient) should be considered, so that nocturnists are not left deciding, in the moment, whether to call the daytime attending. Measures such as this ensure that everyone’s expectations are aligned. In addition, new hospitalists need to be offered support, in the form of training and mentorship.
CBS malpractice data, which includes the contributing factors underlying what went wrong, illuminates potential targets for programs designed to enhance patient safety. In the recent hospitalist malpractice study, the two contributing factors that were the best predictors of a hospitalist malpractice claim closing with payment to the claimant were clinical judgment errors and communication breakdowns. Identifying measures that are effective in promoting patient safety by refining the clinical judgement of clinicians is a challenge, and there are limited data demonstrating what programs are effective in this area.
Clinical decision support (CDS) systems have shown promise in promoting guideline-concordant care.7 However, the role of CDS in aiding the higher-stakes clinical decisions that may be called into question after an adverse outcome is not well defined. Alerts that a patient may be developing sepsis is one type of CDS that has been extensively studied and has been shown to be of some benefit.8 The importance of clinical judgment to whether payment is made on a malpractice claim can inform risk management strategies. Hospitalists should document the thought process behind their decision making in the chart, especially for important clinical decisions. A note showing that the clinician was thoughtfully weighing the risks and benefits using the data available at the time will help make a case defensible if an adverse outcome occurs.
The effect of communication breakdowns on hospitalist case outcomes highlights the importance of measures to improve and systematize communication among clinicians, particularly at vulnerable junctures – such as handoffs from the day team to the night team, and transitions from one care setting to another. An example of an intervention to improve handoffs with cogent evidence to support it is I-PASS, which is an approach to handoffs between teams in which information about the patient’s illness severity, clinical background, and contingencies is conveyed and synthesized in a structured manner. A study of the effect of implementation of I-PASS among nine pediatric residency programs demonstrated a 30% reduction in preventable adverse events.9
Applying insights from malpractice claims analysis to clinical practice
The systematic review of malpractice cases to determine the contributing factors and other case attributes is an important source of patient safety insights. The process breakdowns described by the contributing factors can inform the design of patient safety initiatives. In addition, malpractice data provides information on which specialties and what types of clinicians are being named together in malpractice claims.
In the hospitalist malpractice study, in addition to general surgery and orthopedic surgery, the other clinical services most commonly subject to claims along with hospitalists were nursing, emergency medicine, and cardiology. Another observation was that physician assistants and nurse practitioners are increasingly being named in hospitalist claims. This information is crucial to guiding who needs to be in the room with hospitalists when efforts are undertaken to enhance patient safety within hospital medicine.
An understandable response to the finding that hospitalist claims rates are not decreasing is for hospitalists to seek ways to lower their risk of being named in a malpractice claim. Of course, avoiding adverse events by providing the safest possible care is paramount. Even when patients do suffer adverse events due to a physician negligence, only rarely, less than 5% of the time, does this result in a malpractice claim.10 Important lessons in risk management can be learned from examining why patients decide to sue when mistakes lead to bad outcomes.
An analysis of plaintiffs’ depositions found that the key reasons that patients decided to file a malpractice claim include a poor relationship with the physician – specifically, a lack of empathy from the physician, feeling deserted by the physician, and feeling devalued by the physician.11 These findings support the use of programs that assist physicians in compassionately disclosing adverse events to patients. Among inpatient physicians, patient satisfaction survey questions about the time the physician spent with the patient and the physician’s concern for the patient are better predictors of the physicians’ risk management performance than is the question about the skill of the physician.12 In the aftermath of an adverse event, focusing on maintaining a strong patient-physician relationship is not only the right the thing to do, the data tell us that it is also a sensible approach to reducing medicolegal risk.
Dr. Schaffer practices as a member of the Hospital Medicine Unit at Brigham and Women’s Hospital, Boston, where he serves as an attending physician on the inpatient general medicine services. An instructor at Harvard Medical School, his academic interests include research using large medical malpractice databases to examine temporal trends in medical malpractice.
References
1. Rothberg MB, et al. The cost of defensive medicine on 3 hospital medicine services. JAMA Intern Med. 2014;174(11):1867-1868. doi:10.1001/jamainternmed.2014.4649.
2. Kachalia A, et al. Overuse of testing in preoperative evaluation and syncope: A survey of hospitalists. Ann Intern Med. 2015;162(2):100-108. doi: 10.7326/M14-0694.
3. Mello MM, et al. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577. doi: 10.1377/hlthaff.2009.0807.
4. Schaffer AC, et al. Rates and characteristics of paid malpractice claims among U.S. physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177(5):710-718. doi:10.1001/jamainternmed.2017.0311.
5. Schaffer AC, et al. Liability impact of the hospitalist model of care. J Hosp Med. 2014;9(12):750-755. doi: 10.1002/jhm.2244.
6. Schaffer AC, et al. Rates and characteristics of medical malpractice claims against hospitalists. J Hosp Med. 2021 Jul;16(7):390-396. doi: 10.12788/jhm.3557.
7. Poon EG. Clinical decision support: a tool of the hospital trade. J Hosp Med. 2015;10(1):60-61. doi: 10.1002/jhm.2295.
8. Makam AN, Nguyen OK, Auerbach AD. Diagnostic accuracy and effectiveness of automated electronic sepsis alert systems: A systematic review. J Hosp Med. 2015;10(6):396-402. doi: 10.1002/jhm.2347.
9. Starmer AJ, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. doi: 10.1056/NEJMsa1405556.
10. Localio AR, et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. doi: 10.1056/NEJM199107253250405.
11. Beckman HB, et al. The doctor-patient relationship and malpractice. Lessons from plaintiff depositions. Arch Intern Med. 1994;154(12):1365-1370. doi:10.1001/archinte.1994.00420120093010.
12. Stelfox HT, et al. The relation of patient satisfaction with complaints against physicians and malpractice lawsuits. Am J Med. 2005;118(10):1126-1133. doi: 10.1016/j.amjmed.2005.01.060.
Among the pressures felt by hospitalists are concerns about being subject to a malpractice claim. Anxiety about malpractice influences the way hospitalists practice, giving rise to defensive medicine.
One survey, which asked hospitalists to retrospectively rate which of their orders represented defensive medicine, found that 28% of orders were deemed defensive.1 Defensive medicine can lead to low-value medical care, drive up health care costs, and potentially subject patients to unnecessary testing.2,3
Encouragingly, medical malpractice claims rates have, overall, been downtrending. An analysis of data from the National Practitioner Data Bank, which is a repository of all paid malpractice claims against individual physicians, found that malpractice claims rates decreased by 55.7% from 1992 to 2014 among all specialties, and by 46.1% for internal medicine physicians.4 The data used in this analysis did not separate hospitalists from other internal medicine physicians. An older study of malpractice claims against hospitalists found that hospitalists had significantly lower claims rates than non-hospitalist internal medicine physicians.5
Current malpractice environment for hospitalists
Seeking to shed light on the current malpractice environment faced by hospitalists, a recent study examined claims against hospitalists using the Comparative Benchmarking System (CBS), a national database of malpractice claims containing approximately 30% of all U.S. malpractice claims, which is maintained by CRICO, the malpractice insurer for the Harvard-affiliated medical institutions.6
Claims in the CBS database are examined by trained nurse coders who review the claims, along with the associated medical and legal records, to understand the contributing factors behind the adverse event leading to the claim.
Contrary to the trends for nearly all other physician specialties, the malpractice claims rates of hospitalists were not downtrending, going from 1.77 claims per 100 physician-years from 2009-2013 to 2.08 claims per 100 physician-years from 2014-2018. The overall claims rate for hospitalists was significantly higher than that for internal medicine subspecialists (though roughly the same as the claims rate for non-hospitalist general internal medicine physicians). These sobering findings raise the important question of why hospitalists claims rates are heading in the wrong direction.
One possible answer relates the ever-broadening scope of hospitalist practice. Hospitalists are being asked to care for surgical patients and other patient populations that they may have less familiarity with, increasing the risk of medical errors. Among the other specialties most commonly also named in hospitalist claims, general surgery and orthopedic surgery are in the top five. The extraordinary growth in the field of hospital medicine has meant a need to hire an increasing number of hospitalists, leading to less-experienced physicians entering the field.
Making hospital medicine safer
A more urgent question than what is driving the trends in hospitalist claims rates is what can be done to avoid adverse events and make hospital medicine safer. One potential answer is thoughtful collaboration arrangements with the surgical and other specialties with whom hospitalists may be co-managing patients.
Questions about who responds to what types of clinical issues that might arise and specific domains of responsibility should be defined in advance, so that a lack of role clarity does not negatively impact patient care. Given that hospitalists will be less comfortable addressing more technical surgical issues, expectations about surgeons’ availability should be established. Nocturnists may be tasked with overnight cross-coverage of patients on services, such as oncology and cardiology, that subspecialty physicians have responsibility for during the day. Agreeing upon triggers for when the nocturnist should contact the daytime subspecialty attending (for example, if a rapid response is called on their patient) should be considered, so that nocturnists are not left deciding, in the moment, whether to call the daytime attending. Measures such as this ensure that everyone’s expectations are aligned. In addition, new hospitalists need to be offered support, in the form of training and mentorship.
CBS malpractice data, which includes the contributing factors underlying what went wrong, illuminates potential targets for programs designed to enhance patient safety. In the recent hospitalist malpractice study, the two contributing factors that were the best predictors of a hospitalist malpractice claim closing with payment to the claimant were clinical judgment errors and communication breakdowns. Identifying measures that are effective in promoting patient safety by refining the clinical judgement of clinicians is a challenge, and there are limited data demonstrating what programs are effective in this area.
Clinical decision support (CDS) systems have shown promise in promoting guideline-concordant care.7 However, the role of CDS in aiding the higher-stakes clinical decisions that may be called into question after an adverse outcome is not well defined. Alerts that a patient may be developing sepsis is one type of CDS that has been extensively studied and has been shown to be of some benefit.8 The importance of clinical judgment to whether payment is made on a malpractice claim can inform risk management strategies. Hospitalists should document the thought process behind their decision making in the chart, especially for important clinical decisions. A note showing that the clinician was thoughtfully weighing the risks and benefits using the data available at the time will help make a case defensible if an adverse outcome occurs.
The effect of communication breakdowns on hospitalist case outcomes highlights the importance of measures to improve and systematize communication among clinicians, particularly at vulnerable junctures – such as handoffs from the day team to the night team, and transitions from one care setting to another. An example of an intervention to improve handoffs with cogent evidence to support it is I-PASS, which is an approach to handoffs between teams in which information about the patient’s illness severity, clinical background, and contingencies is conveyed and synthesized in a structured manner. A study of the effect of implementation of I-PASS among nine pediatric residency programs demonstrated a 30% reduction in preventable adverse events.9
Applying insights from malpractice claims analysis to clinical practice
The systematic review of malpractice cases to determine the contributing factors and other case attributes is an important source of patient safety insights. The process breakdowns described by the contributing factors can inform the design of patient safety initiatives. In addition, malpractice data provides information on which specialties and what types of clinicians are being named together in malpractice claims.
In the hospitalist malpractice study, in addition to general surgery and orthopedic surgery, the other clinical services most commonly subject to claims along with hospitalists were nursing, emergency medicine, and cardiology. Another observation was that physician assistants and nurse practitioners are increasingly being named in hospitalist claims. This information is crucial to guiding who needs to be in the room with hospitalists when efforts are undertaken to enhance patient safety within hospital medicine.
An understandable response to the finding that hospitalist claims rates are not decreasing is for hospitalists to seek ways to lower their risk of being named in a malpractice claim. Of course, avoiding adverse events by providing the safest possible care is paramount. Even when patients do suffer adverse events due to a physician negligence, only rarely, less than 5% of the time, does this result in a malpractice claim.10 Important lessons in risk management can be learned from examining why patients decide to sue when mistakes lead to bad outcomes.
An analysis of plaintiffs’ depositions found that the key reasons that patients decided to file a malpractice claim include a poor relationship with the physician – specifically, a lack of empathy from the physician, feeling deserted by the physician, and feeling devalued by the physician.11 These findings support the use of programs that assist physicians in compassionately disclosing adverse events to patients. Among inpatient physicians, patient satisfaction survey questions about the time the physician spent with the patient and the physician’s concern for the patient are better predictors of the physicians’ risk management performance than is the question about the skill of the physician.12 In the aftermath of an adverse event, focusing on maintaining a strong patient-physician relationship is not only the right the thing to do, the data tell us that it is also a sensible approach to reducing medicolegal risk.
Dr. Schaffer practices as a member of the Hospital Medicine Unit at Brigham and Women’s Hospital, Boston, where he serves as an attending physician on the inpatient general medicine services. An instructor at Harvard Medical School, his academic interests include research using large medical malpractice databases to examine temporal trends in medical malpractice.
References
1. Rothberg MB, et al. The cost of defensive medicine on 3 hospital medicine services. JAMA Intern Med. 2014;174(11):1867-1868. doi:10.1001/jamainternmed.2014.4649.
2. Kachalia A, et al. Overuse of testing in preoperative evaluation and syncope: A survey of hospitalists. Ann Intern Med. 2015;162(2):100-108. doi: 10.7326/M14-0694.
3. Mello MM, et al. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577. doi: 10.1377/hlthaff.2009.0807.
4. Schaffer AC, et al. Rates and characteristics of paid malpractice claims among U.S. physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177(5):710-718. doi:10.1001/jamainternmed.2017.0311.
5. Schaffer AC, et al. Liability impact of the hospitalist model of care. J Hosp Med. 2014;9(12):750-755. doi: 10.1002/jhm.2244.
6. Schaffer AC, et al. Rates and characteristics of medical malpractice claims against hospitalists. J Hosp Med. 2021 Jul;16(7):390-396. doi: 10.12788/jhm.3557.
7. Poon EG. Clinical decision support: a tool of the hospital trade. J Hosp Med. 2015;10(1):60-61. doi: 10.1002/jhm.2295.
8. Makam AN, Nguyen OK, Auerbach AD. Diagnostic accuracy and effectiveness of automated electronic sepsis alert systems: A systematic review. J Hosp Med. 2015;10(6):396-402. doi: 10.1002/jhm.2347.
9. Starmer AJ, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. doi: 10.1056/NEJMsa1405556.
10. Localio AR, et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. doi: 10.1056/NEJM199107253250405.
11. Beckman HB, et al. The doctor-patient relationship and malpractice. Lessons from plaintiff depositions. Arch Intern Med. 1994;154(12):1365-1370. doi:10.1001/archinte.1994.00420120093010.
12. Stelfox HT, et al. The relation of patient satisfaction with complaints against physicians and malpractice lawsuits. Am J Med. 2005;118(10):1126-1133. doi: 10.1016/j.amjmed.2005.01.060.
One in three cancer articles on social media has wrong info
Of the 200 most popular articles (50 each for prostate, lung, breast, and colorectal cancer), about a third (32.5%, n = 65) contained misinformation.
Among these articles containing misinformation, 76.9% (50/65) contained harmful information.
“The Internet is a leading source of health misinformation,” the study authors wrote. This is “particularly true for social media, where false information spreads faster and more broadly than fact-checked information,” they said, citing other research.
“We need to address these issues head on,” said lead author Skyler Johnson, MD, of the University of Utah’s Huntsman Cancer Institute in Salt Lake City.
“As a medical community, we can’t ignore the problem of cancer misinformation on social media or ask our patients to ignore it. We must empathize with our patients and help them when they encounter this type of information,” he said in a statement. “My goal is to help answer their questions, and provide cancer patients with accurate information that will give them the best chance for the best outcome.”
The study was published online July 22 in the Journal of the National Cancer Institute.
The study period ran from 2018 to 2019, and looked at articles posted on social media platforms Facebook, Reddit, Twitter, or Pinterest. Popularity was measured by engagement with readers, such as upvotes, comments, reactions, and shares.
Some of the articles came from long-established news entities such as CBS News, The New York Times, and medical journals, while others came from fleeting crowdfunding web pages and fledging nontraditional news sites.
One example of popular and harmful misinformation highlighted by Dr. Johnson in an interview was titled, “44-Year-Old Mother Claims CBD Oil Cured Her of Breast Cancer within 5 Months.” Posted on truththeory.com in February 2018, the article is tagged as “opinion” by the publisher and in turn links to another news story about the same woman in the UK’s Daily Mail newspaper.
The ideas and claims in such articles can be very influential, Jennifer L. Lycette, MD, suggested in a recent blog post.
“After 18 years as a cancer doctor, it sadly doesn’t come as a surprise anymore when a patient declines treatment recommendations and instead opts for ‘alternative’ treatment,” she wrote.
Sometimes, misinformation is not sensational but is still effective via clever wording and presentation, observed Brian G. Southwell, PhD, of Duke University, Durham, N.C., who has studied patients and misinformation.
“It isn’t the falsehood that is somehow magically attractive, per se, but the way that misinformation is often framed that can make it attractive,” he said in an interview.
Dr. Southwell recommends that clinicians be proactive about medical misinformation.
“Rather than expect patients to raise concerns without prompting, health care providers should invite conversations about potential misinformation with their patients,” he wrote in a recent essay in the American Journal of Public Health.
In short, ask patients what they know about the treatment of their cancer, he suggests.
“Patients don’t typically know that the misinformation they are encountering is misinformation,” said Dr. Southwell. “Approaching patients with compassion and empathy is a good first step.”
Study details
For the study, reported by Johnson et al., two National Comprehensive Cancer Network panel members were selected as content experts for each of the four cancers and were tasked with reviewing the primary medical claims in each article. The experts then completed a set of ratings to arrive at the proportion of misinformation and potential for harm in each article.
Of the 200 articles, 41.5% were from nontraditional news (digital only), 37.5% were from traditional news sources (online versions of print and/or broadcast media), 17% were from medical journals, 3% were from a crowdfunding site, and 1% were from personal blogs.
This expert review concluded that nearly one-third of the articles contained misinformation, as noted above. The misinformation was described as misleading (title not supported by text or statistics/data do not support conclusion, 28.8%), strength of the evidence mischaracterized (weak evidence portrayed as strong or vice versa, 27.7%) and unproven therapies (not studied or insufficient evidence, 26.7%).
Notably, the median number of engagements, such as likes on Twitter, for articles with misinformation was greater than that of factual articles (median, 2,300 vs. 1,600; P = .05).
In total, 30.5% of all 200 articles contained harmful information. This was described as harmful inaction (could lead to delay or not seeking medical attention for treatable/curable condition, 31.0%), economic harm (out-of-pocket financial costs associated with treatment/travel, 27.7%), harmful action (potentially toxic effects of the suggested test/treatment, 17.0%), and harmful interactions (known/unknown medical interactions with curative therapies, 16.2%).
The median number of engagements for articles with harmful information was statistically significantly greater than that of articles with correct information (median, 2,300 vs. 1,500; P = .007).
A limitation of the study is that it included only the most popular English language cancer articles.
This study was funded in part by the Huntsman Cancer Institute. Dr. Johnson, Dr. Lycette, and Dr. Southwell have disclosed no relevant financial relationships. Some study authors have ties to the pharmaceutical industry.
A version of this article first appeared on Medscape.com.
Of the 200 most popular articles (50 each for prostate, lung, breast, and colorectal cancer), about a third (32.5%, n = 65) contained misinformation.
Among these articles containing misinformation, 76.9% (50/65) contained harmful information.
“The Internet is a leading source of health misinformation,” the study authors wrote. This is “particularly true for social media, where false information spreads faster and more broadly than fact-checked information,” they said, citing other research.
“We need to address these issues head on,” said lead author Skyler Johnson, MD, of the University of Utah’s Huntsman Cancer Institute in Salt Lake City.
“As a medical community, we can’t ignore the problem of cancer misinformation on social media or ask our patients to ignore it. We must empathize with our patients and help them when they encounter this type of information,” he said in a statement. “My goal is to help answer their questions, and provide cancer patients with accurate information that will give them the best chance for the best outcome.”
The study was published online July 22 in the Journal of the National Cancer Institute.
The study period ran from 2018 to 2019, and looked at articles posted on social media platforms Facebook, Reddit, Twitter, or Pinterest. Popularity was measured by engagement with readers, such as upvotes, comments, reactions, and shares.
Some of the articles came from long-established news entities such as CBS News, The New York Times, and medical journals, while others came from fleeting crowdfunding web pages and fledging nontraditional news sites.
One example of popular and harmful misinformation highlighted by Dr. Johnson in an interview was titled, “44-Year-Old Mother Claims CBD Oil Cured Her of Breast Cancer within 5 Months.” Posted on truththeory.com in February 2018, the article is tagged as “opinion” by the publisher and in turn links to another news story about the same woman in the UK’s Daily Mail newspaper.
The ideas and claims in such articles can be very influential, Jennifer L. Lycette, MD, suggested in a recent blog post.
“After 18 years as a cancer doctor, it sadly doesn’t come as a surprise anymore when a patient declines treatment recommendations and instead opts for ‘alternative’ treatment,” she wrote.
Sometimes, misinformation is not sensational but is still effective via clever wording and presentation, observed Brian G. Southwell, PhD, of Duke University, Durham, N.C., who has studied patients and misinformation.
“It isn’t the falsehood that is somehow magically attractive, per se, but the way that misinformation is often framed that can make it attractive,” he said in an interview.
Dr. Southwell recommends that clinicians be proactive about medical misinformation.
“Rather than expect patients to raise concerns without prompting, health care providers should invite conversations about potential misinformation with their patients,” he wrote in a recent essay in the American Journal of Public Health.
In short, ask patients what they know about the treatment of their cancer, he suggests.
“Patients don’t typically know that the misinformation they are encountering is misinformation,” said Dr. Southwell. “Approaching patients with compassion and empathy is a good first step.”
Study details
For the study, reported by Johnson et al., two National Comprehensive Cancer Network panel members were selected as content experts for each of the four cancers and were tasked with reviewing the primary medical claims in each article. The experts then completed a set of ratings to arrive at the proportion of misinformation and potential for harm in each article.
Of the 200 articles, 41.5% were from nontraditional news (digital only), 37.5% were from traditional news sources (online versions of print and/or broadcast media), 17% were from medical journals, 3% were from a crowdfunding site, and 1% were from personal blogs.
This expert review concluded that nearly one-third of the articles contained misinformation, as noted above. The misinformation was described as misleading (title not supported by text or statistics/data do not support conclusion, 28.8%), strength of the evidence mischaracterized (weak evidence portrayed as strong or vice versa, 27.7%) and unproven therapies (not studied or insufficient evidence, 26.7%).
Notably, the median number of engagements, such as likes on Twitter, for articles with misinformation was greater than that of factual articles (median, 2,300 vs. 1,600; P = .05).
In total, 30.5% of all 200 articles contained harmful information. This was described as harmful inaction (could lead to delay or not seeking medical attention for treatable/curable condition, 31.0%), economic harm (out-of-pocket financial costs associated with treatment/travel, 27.7%), harmful action (potentially toxic effects of the suggested test/treatment, 17.0%), and harmful interactions (known/unknown medical interactions with curative therapies, 16.2%).
The median number of engagements for articles with harmful information was statistically significantly greater than that of articles with correct information (median, 2,300 vs. 1,500; P = .007).
A limitation of the study is that it included only the most popular English language cancer articles.
This study was funded in part by the Huntsman Cancer Institute. Dr. Johnson, Dr. Lycette, and Dr. Southwell have disclosed no relevant financial relationships. Some study authors have ties to the pharmaceutical industry.
A version of this article first appeared on Medscape.com.
Of the 200 most popular articles (50 each for prostate, lung, breast, and colorectal cancer), about a third (32.5%, n = 65) contained misinformation.
Among these articles containing misinformation, 76.9% (50/65) contained harmful information.
“The Internet is a leading source of health misinformation,” the study authors wrote. This is “particularly true for social media, where false information spreads faster and more broadly than fact-checked information,” they said, citing other research.
“We need to address these issues head on,” said lead author Skyler Johnson, MD, of the University of Utah’s Huntsman Cancer Institute in Salt Lake City.
“As a medical community, we can’t ignore the problem of cancer misinformation on social media or ask our patients to ignore it. We must empathize with our patients and help them when they encounter this type of information,” he said in a statement. “My goal is to help answer their questions, and provide cancer patients with accurate information that will give them the best chance for the best outcome.”
The study was published online July 22 in the Journal of the National Cancer Institute.
The study period ran from 2018 to 2019, and looked at articles posted on social media platforms Facebook, Reddit, Twitter, or Pinterest. Popularity was measured by engagement with readers, such as upvotes, comments, reactions, and shares.
Some of the articles came from long-established news entities such as CBS News, The New York Times, and medical journals, while others came from fleeting crowdfunding web pages and fledging nontraditional news sites.
One example of popular and harmful misinformation highlighted by Dr. Johnson in an interview was titled, “44-Year-Old Mother Claims CBD Oil Cured Her of Breast Cancer within 5 Months.” Posted on truththeory.com in February 2018, the article is tagged as “opinion” by the publisher and in turn links to another news story about the same woman in the UK’s Daily Mail newspaper.
The ideas and claims in such articles can be very influential, Jennifer L. Lycette, MD, suggested in a recent blog post.
“After 18 years as a cancer doctor, it sadly doesn’t come as a surprise anymore when a patient declines treatment recommendations and instead opts for ‘alternative’ treatment,” she wrote.
Sometimes, misinformation is not sensational but is still effective via clever wording and presentation, observed Brian G. Southwell, PhD, of Duke University, Durham, N.C., who has studied patients and misinformation.
“It isn’t the falsehood that is somehow magically attractive, per se, but the way that misinformation is often framed that can make it attractive,” he said in an interview.
Dr. Southwell recommends that clinicians be proactive about medical misinformation.
“Rather than expect patients to raise concerns without prompting, health care providers should invite conversations about potential misinformation with their patients,” he wrote in a recent essay in the American Journal of Public Health.
In short, ask patients what they know about the treatment of their cancer, he suggests.
“Patients don’t typically know that the misinformation they are encountering is misinformation,” said Dr. Southwell. “Approaching patients with compassion and empathy is a good first step.”
Study details
For the study, reported by Johnson et al., two National Comprehensive Cancer Network panel members were selected as content experts for each of the four cancers and were tasked with reviewing the primary medical claims in each article. The experts then completed a set of ratings to arrive at the proportion of misinformation and potential for harm in each article.
Of the 200 articles, 41.5% were from nontraditional news (digital only), 37.5% were from traditional news sources (online versions of print and/or broadcast media), 17% were from medical journals, 3% were from a crowdfunding site, and 1% were from personal blogs.
This expert review concluded that nearly one-third of the articles contained misinformation, as noted above. The misinformation was described as misleading (title not supported by text or statistics/data do not support conclusion, 28.8%), strength of the evidence mischaracterized (weak evidence portrayed as strong or vice versa, 27.7%) and unproven therapies (not studied or insufficient evidence, 26.7%).
Notably, the median number of engagements, such as likes on Twitter, for articles with misinformation was greater than that of factual articles (median, 2,300 vs. 1,600; P = .05).
In total, 30.5% of all 200 articles contained harmful information. This was described as harmful inaction (could lead to delay or not seeking medical attention for treatable/curable condition, 31.0%), economic harm (out-of-pocket financial costs associated with treatment/travel, 27.7%), harmful action (potentially toxic effects of the suggested test/treatment, 17.0%), and harmful interactions (known/unknown medical interactions with curative therapies, 16.2%).
The median number of engagements for articles with harmful information was statistically significantly greater than that of articles with correct information (median, 2,300 vs. 1,500; P = .007).
A limitation of the study is that it included only the most popular English language cancer articles.
This study was funded in part by the Huntsman Cancer Institute. Dr. Johnson, Dr. Lycette, and Dr. Southwell have disclosed no relevant financial relationships. Some study authors have ties to the pharmaceutical industry.
A version of this article first appeared on Medscape.com.
Bone drugs for prostate cancer may result in survival benefit
Results from a retrospective study show that the addition of bone resorption inhibitors (BRIs), including zoledronic acid and denosumab (Xgeva), to abiraterone plus prednisolone was associated with significantly longer overall survival (OS). The median OS was increased by nearly 9 months among recipients, compared with men who didn’t receive these drugs in this setting.
The findings were published online July 22, 2021, in JAMA Network Open.
All men with prostate cancer should receive BRIs “as the disease reaches the castration resistance with bone metastases stage, as recommended by the international guidelines,” lead author Edoardo Francini, MD, PhD, of the University of Florence (Italy) said in a comment.
While there is no evidence that BRIs – when used alone – may improve survival in metastatic castration-resistant prostate cancer (mCRPC) with bone involvement, there has been a “suggestion” of a survival benefit with BRIs when combined with other anticancer therapies in this setting, say the authors.
So Dr. Francini and a team of international coinvestigators looked at the medical records of men with mCRPC and bone metastases treated at eight institutions in Canada, Europe, and the United States and focused on patients who received abiraterone acetate (with prednisone) because it is the most common first-line therapy in this setting.
Patients were classified by receipt versus nonreceipt of concomitant BRIs and subclassified by volume of disease (high or low volume).
There were two cohorts in the study population: 529 men (71.0%) who received abiraterone alone and 216 men (29.0%) who received abiraterone plus BRIs. The median follow-up was 23.5 months.
Patients in the BRI cohort experienced significantly longer OS compared with those in the abiraterone alone cohort (31.8 vs. 23.0 months; hazard ratio, 0.65; P < .001).
Notably, the OS benefit in the BRI cohort was greater for patients with high-volume versus low-volume disease (33.6 vs. 19.7 months; HR, 0.51; P < .001).
Dr. Francini hopes the new study results can effect change. “Hopefully, clinicians will be more inclined to use bone resorption inhibitors in combination with abiraterone acetate plus prednisone as soon as the disease reaches the castration-resistance with bone metastases stage, as recommended by the international guidelines.”
Importance of bone-targeted drugs
“This study highlights the importance of bone-targeted therapy in current practice for men with mCRPC and bone metastases,” Samuel Takvorian, MD, and Naomi Haas, MD, of the University of Pennsylvania, Philadelphia, wrote in an accompanying editorial.
But the study also reveals that work needs to be done to get clinicians to prescribe BRIs, they said, and that clinical pathways and behavioral “nudges” could help promote adoption.
Most (71%) of the men in this study did not get bone protective drug therapy, they pointed out, even though they were being treated at major hospital systems.
So, why aren’t more men receiving BRIs?
“I think this is less likely due to poor communication from professional societies (the guidelines are clear) and more likely due to bone health being low on the list of priorities for these patients and clinician uncertainty and/or lack of appreciation of the clinical benefit of these agents,” Dr. Takvorian said in an interview.
“When prostate cancer progresses to the castration-resistant phase, clinicians (and patients) rightfully are focused on the next cancer-directed therapy. However, this may be at the expense of supportive care, like bone agents, which often gets short shrift,” he added.
As would be expected, the men who were taking BRIs had a significantly shorter time to first skeletal-related events (SREs), compared with those who were not (32.4 vs. 42.7 months; HR, 1.27; P = .04), and the risk of a first SRE was more than double in the subgroup with low-volume disease (HR, 2.29; P < .001).
“These SREs collectively represent a clinically meaningful outcome that is often measured in clinical trials,” the editorialists observed. In the current study, SREs were comprised of pathological fractures, spinal cord compression, or the need for surgery or radiotherapy to bone.
“Up to one-half of men with mCRPC, the advanced and often fatal stage of disease, experience SREs, which are associated with considerable morbidity, decreased survival, and increased health care utilization and costs,” they wrote.
Costly vs. inexpensive BRI
The study found no difference in the OS benefit between the different BRIs used, that is, between that seen with zoledronic acid versus denosumab.
The editorialists suggested that this finding is important, even though it “must be considered preliminary given the limitations of a retrospective study.” These results add “to data suggesting that these agents are comparably beneficial; thus, decisions between them should focus on clinical factors, such as kidney function, patient preference, and cost.”
The two agents differ mechanistically, they added, with zoledronic acid preferentially inhibiting osteoclast proliferation and denosumab inhibiting an important factor in osteoclast maturation.
In terms of having differentiating characteristics, the editorialists say that zoledronic acid is “more often associated with acute phase reactions and required monitoring of kidney function” while “denosumab conferred a higher risk of hypocalcemia.” Rates of osteonecrosis of the jaw are comparable.
International guidelines endorse the use of either agent for the treatment of men with mCRPC. But “some argue that the marginal benefit of denosumab must be weighed against its dramatically higher cost (the annual cost of zoledronic acid is approximately $140 vs. $29,000 for denosumab),” the editorialists said.
The dramatically higher cost of denosumab versus zoledronic acid has also been noted by other oncologists treating patients with other cancers, including multiple myeloma.
In addition to drug costs, there is another issue at stake: the prescribing oncologist is reimbursed by Medicare Part D at 6% for whichever drug is chosen, which represents a “financial conflict” for oncologists, said Vincent Rajkumar, MD, professor of medicine and a hematologist/oncologist at the Mayo Clinic, Rochester, Minn.
There is also a difference in how the drugs are administered, which may influence patient preference, the myeloma experts noted. Zoledronic acid is given intravenously every 3 months and requires a 15-minute infusion at a center, while denosumab needs to be given more frequently (every month) but is administered by subcutaneous injection.
Dr. Francini reported receiving grants from Roche Italia and personal fees for research travel from Janssen-Cilag outside the submitted work. A number of other authors disclosed financial ties to Janssen or Amgen, makers of abiraterone and denosumab, respectively. The editorialists reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from a retrospective study show that the addition of bone resorption inhibitors (BRIs), including zoledronic acid and denosumab (Xgeva), to abiraterone plus prednisolone was associated with significantly longer overall survival (OS). The median OS was increased by nearly 9 months among recipients, compared with men who didn’t receive these drugs in this setting.
The findings were published online July 22, 2021, in JAMA Network Open.
All men with prostate cancer should receive BRIs “as the disease reaches the castration resistance with bone metastases stage, as recommended by the international guidelines,” lead author Edoardo Francini, MD, PhD, of the University of Florence (Italy) said in a comment.
While there is no evidence that BRIs – when used alone – may improve survival in metastatic castration-resistant prostate cancer (mCRPC) with bone involvement, there has been a “suggestion” of a survival benefit with BRIs when combined with other anticancer therapies in this setting, say the authors.
So Dr. Francini and a team of international coinvestigators looked at the medical records of men with mCRPC and bone metastases treated at eight institutions in Canada, Europe, and the United States and focused on patients who received abiraterone acetate (with prednisone) because it is the most common first-line therapy in this setting.
Patients were classified by receipt versus nonreceipt of concomitant BRIs and subclassified by volume of disease (high or low volume).
There were two cohorts in the study population: 529 men (71.0%) who received abiraterone alone and 216 men (29.0%) who received abiraterone plus BRIs. The median follow-up was 23.5 months.
Patients in the BRI cohort experienced significantly longer OS compared with those in the abiraterone alone cohort (31.8 vs. 23.0 months; hazard ratio, 0.65; P < .001).
Notably, the OS benefit in the BRI cohort was greater for patients with high-volume versus low-volume disease (33.6 vs. 19.7 months; HR, 0.51; P < .001).
Dr. Francini hopes the new study results can effect change. “Hopefully, clinicians will be more inclined to use bone resorption inhibitors in combination with abiraterone acetate plus prednisone as soon as the disease reaches the castration-resistance with bone metastases stage, as recommended by the international guidelines.”
Importance of bone-targeted drugs
“This study highlights the importance of bone-targeted therapy in current practice for men with mCRPC and bone metastases,” Samuel Takvorian, MD, and Naomi Haas, MD, of the University of Pennsylvania, Philadelphia, wrote in an accompanying editorial.
But the study also reveals that work needs to be done to get clinicians to prescribe BRIs, they said, and that clinical pathways and behavioral “nudges” could help promote adoption.
Most (71%) of the men in this study did not get bone protective drug therapy, they pointed out, even though they were being treated at major hospital systems.
So, why aren’t more men receiving BRIs?
“I think this is less likely due to poor communication from professional societies (the guidelines are clear) and more likely due to bone health being low on the list of priorities for these patients and clinician uncertainty and/or lack of appreciation of the clinical benefit of these agents,” Dr. Takvorian said in an interview.
“When prostate cancer progresses to the castration-resistant phase, clinicians (and patients) rightfully are focused on the next cancer-directed therapy. However, this may be at the expense of supportive care, like bone agents, which often gets short shrift,” he added.
As would be expected, the men who were taking BRIs had a significantly shorter time to first skeletal-related events (SREs), compared with those who were not (32.4 vs. 42.7 months; HR, 1.27; P = .04), and the risk of a first SRE was more than double in the subgroup with low-volume disease (HR, 2.29; P < .001).
“These SREs collectively represent a clinically meaningful outcome that is often measured in clinical trials,” the editorialists observed. In the current study, SREs were comprised of pathological fractures, spinal cord compression, or the need for surgery or radiotherapy to bone.
“Up to one-half of men with mCRPC, the advanced and often fatal stage of disease, experience SREs, which are associated with considerable morbidity, decreased survival, and increased health care utilization and costs,” they wrote.
Costly vs. inexpensive BRI
The study found no difference in the OS benefit between the different BRIs used, that is, between that seen with zoledronic acid versus denosumab.
The editorialists suggested that this finding is important, even though it “must be considered preliminary given the limitations of a retrospective study.” These results add “to data suggesting that these agents are comparably beneficial; thus, decisions between them should focus on clinical factors, such as kidney function, patient preference, and cost.”
The two agents differ mechanistically, they added, with zoledronic acid preferentially inhibiting osteoclast proliferation and denosumab inhibiting an important factor in osteoclast maturation.
In terms of having differentiating characteristics, the editorialists say that zoledronic acid is “more often associated with acute phase reactions and required monitoring of kidney function” while “denosumab conferred a higher risk of hypocalcemia.” Rates of osteonecrosis of the jaw are comparable.
International guidelines endorse the use of either agent for the treatment of men with mCRPC. But “some argue that the marginal benefit of denosumab must be weighed against its dramatically higher cost (the annual cost of zoledronic acid is approximately $140 vs. $29,000 for denosumab),” the editorialists said.
The dramatically higher cost of denosumab versus zoledronic acid has also been noted by other oncologists treating patients with other cancers, including multiple myeloma.
In addition to drug costs, there is another issue at stake: the prescribing oncologist is reimbursed by Medicare Part D at 6% for whichever drug is chosen, which represents a “financial conflict” for oncologists, said Vincent Rajkumar, MD, professor of medicine and a hematologist/oncologist at the Mayo Clinic, Rochester, Minn.
There is also a difference in how the drugs are administered, which may influence patient preference, the myeloma experts noted. Zoledronic acid is given intravenously every 3 months and requires a 15-minute infusion at a center, while denosumab needs to be given more frequently (every month) but is administered by subcutaneous injection.
Dr. Francini reported receiving grants from Roche Italia and personal fees for research travel from Janssen-Cilag outside the submitted work. A number of other authors disclosed financial ties to Janssen or Amgen, makers of abiraterone and denosumab, respectively. The editorialists reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from a retrospective study show that the addition of bone resorption inhibitors (BRIs), including zoledronic acid and denosumab (Xgeva), to abiraterone plus prednisolone was associated with significantly longer overall survival (OS). The median OS was increased by nearly 9 months among recipients, compared with men who didn’t receive these drugs in this setting.
The findings were published online July 22, 2021, in JAMA Network Open.
All men with prostate cancer should receive BRIs “as the disease reaches the castration resistance with bone metastases stage, as recommended by the international guidelines,” lead author Edoardo Francini, MD, PhD, of the University of Florence (Italy) said in a comment.
While there is no evidence that BRIs – when used alone – may improve survival in metastatic castration-resistant prostate cancer (mCRPC) with bone involvement, there has been a “suggestion” of a survival benefit with BRIs when combined with other anticancer therapies in this setting, say the authors.
So Dr. Francini and a team of international coinvestigators looked at the medical records of men with mCRPC and bone metastases treated at eight institutions in Canada, Europe, and the United States and focused on patients who received abiraterone acetate (with prednisone) because it is the most common first-line therapy in this setting.
Patients were classified by receipt versus nonreceipt of concomitant BRIs and subclassified by volume of disease (high or low volume).
There were two cohorts in the study population: 529 men (71.0%) who received abiraterone alone and 216 men (29.0%) who received abiraterone plus BRIs. The median follow-up was 23.5 months.
Patients in the BRI cohort experienced significantly longer OS compared with those in the abiraterone alone cohort (31.8 vs. 23.0 months; hazard ratio, 0.65; P < .001).
Notably, the OS benefit in the BRI cohort was greater for patients with high-volume versus low-volume disease (33.6 vs. 19.7 months; HR, 0.51; P < .001).
Dr. Francini hopes the new study results can effect change. “Hopefully, clinicians will be more inclined to use bone resorption inhibitors in combination with abiraterone acetate plus prednisone as soon as the disease reaches the castration-resistance with bone metastases stage, as recommended by the international guidelines.”
Importance of bone-targeted drugs
“This study highlights the importance of bone-targeted therapy in current practice for men with mCRPC and bone metastases,” Samuel Takvorian, MD, and Naomi Haas, MD, of the University of Pennsylvania, Philadelphia, wrote in an accompanying editorial.
But the study also reveals that work needs to be done to get clinicians to prescribe BRIs, they said, and that clinical pathways and behavioral “nudges” could help promote adoption.
Most (71%) of the men in this study did not get bone protective drug therapy, they pointed out, even though they were being treated at major hospital systems.
So, why aren’t more men receiving BRIs?
“I think this is less likely due to poor communication from professional societies (the guidelines are clear) and more likely due to bone health being low on the list of priorities for these patients and clinician uncertainty and/or lack of appreciation of the clinical benefit of these agents,” Dr. Takvorian said in an interview.
“When prostate cancer progresses to the castration-resistant phase, clinicians (and patients) rightfully are focused on the next cancer-directed therapy. However, this may be at the expense of supportive care, like bone agents, which often gets short shrift,” he added.
As would be expected, the men who were taking BRIs had a significantly shorter time to first skeletal-related events (SREs), compared with those who were not (32.4 vs. 42.7 months; HR, 1.27; P = .04), and the risk of a first SRE was more than double in the subgroup with low-volume disease (HR, 2.29; P < .001).
“These SREs collectively represent a clinically meaningful outcome that is often measured in clinical trials,” the editorialists observed. In the current study, SREs were comprised of pathological fractures, spinal cord compression, or the need for surgery or radiotherapy to bone.
“Up to one-half of men with mCRPC, the advanced and often fatal stage of disease, experience SREs, which are associated with considerable morbidity, decreased survival, and increased health care utilization and costs,” they wrote.
Costly vs. inexpensive BRI
The study found no difference in the OS benefit between the different BRIs used, that is, between that seen with zoledronic acid versus denosumab.
The editorialists suggested that this finding is important, even though it “must be considered preliminary given the limitations of a retrospective study.” These results add “to data suggesting that these agents are comparably beneficial; thus, decisions between them should focus on clinical factors, such as kidney function, patient preference, and cost.”
The two agents differ mechanistically, they added, with zoledronic acid preferentially inhibiting osteoclast proliferation and denosumab inhibiting an important factor in osteoclast maturation.
In terms of having differentiating characteristics, the editorialists say that zoledronic acid is “more often associated with acute phase reactions and required monitoring of kidney function” while “denosumab conferred a higher risk of hypocalcemia.” Rates of osteonecrosis of the jaw are comparable.
International guidelines endorse the use of either agent for the treatment of men with mCRPC. But “some argue that the marginal benefit of denosumab must be weighed against its dramatically higher cost (the annual cost of zoledronic acid is approximately $140 vs. $29,000 for denosumab),” the editorialists said.
The dramatically higher cost of denosumab versus zoledronic acid has also been noted by other oncologists treating patients with other cancers, including multiple myeloma.
In addition to drug costs, there is another issue at stake: the prescribing oncologist is reimbursed by Medicare Part D at 6% for whichever drug is chosen, which represents a “financial conflict” for oncologists, said Vincent Rajkumar, MD, professor of medicine and a hematologist/oncologist at the Mayo Clinic, Rochester, Minn.
There is also a difference in how the drugs are administered, which may influence patient preference, the myeloma experts noted. Zoledronic acid is given intravenously every 3 months and requires a 15-minute infusion at a center, while denosumab needs to be given more frequently (every month) but is administered by subcutaneous injection.
Dr. Francini reported receiving grants from Roche Italia and personal fees for research travel from Janssen-Cilag outside the submitted work. A number of other authors disclosed financial ties to Janssen or Amgen, makers of abiraterone and denosumab, respectively. The editorialists reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Shorter antibiotic course okay for UTIs in men with no fever
A week of antibiotics appears just as effective as 2 weeks in treating afebrile men with urinary tract infections (UTIs), researchers say.
Shortening the course of treatment could spare patients side effects from the medications and reduce the risk that bacteria will develop resistance to the drugs, said Dimitri Drekonja, MD, chief of infectious diseases at the Minneapolis VA Medical Center.
“You’d like to be on these drugs for as short [an] amount of time as gets the job done,” he told this news organization. The study was published online July 28 in JAMA.
Researchers have recently found that shorter courses of antimicrobials are effective in the treatment of other types of infection and for UTIs in women. However, UTIs in men are thought to be more complicated because the male urethra is longer.
To see whether reducing length of treatment could be effective in men as well, Dr. Drekonja and colleagues compared 7-day and 14-day regimens in men treated at U.S. Veterans Affairs medical centers in Minnesota and Texas.
They recruited 272 men who had symptoms of UTI and were willing to participate. All the men received trimethoprim/sulfamethoxazole or ciprofloxacin for 7 days. Half the men were randomly assigned to continue this treatment for an additional 7 days; the other half received placebo pills for an additional 7 days.
The average age of the men was 69 years. Urine samples were cultured from 87.9% of the men. In 60.7% of these samples, the researchers found more than 100,000 CFU/mL; in 16.3%, they found lower colony counts; and in 23.0%, they found no growth of bacteria. The most common organism they isolated was Escherchia coli.
Results for the two groups were similar. Symptoms resolved 14 days after completion of the course of treatment in 90.4% of those who received 14 days of antibiotics, versus 91.9% of those who received 7 days of antibiotics plus 7 days of placebo pills. At 1.5%, the difference between the two arms was within the predetermined boundary for noninferiority.
The percentage of those who experienced recurrence of symptoms within 28 days of stopping medication was also similar between the two groups. Among those who received 7 days of antibiotics, 10.3% experienced recurrence of symptoms, compared to 16.9% of those assigned to 14 days of antibiotics.
There was no significant difference in the resolution of UTI symptoms between the two groups by type of antibiotic, pretreatment bacteriuria count, or study site.
Adverse events were also similar in the two groups, occurring in 20.6% of the men who received 7 days of antibiotics, versus 24.3% of the men who received 14 days of treatment. In both groups, 8.8% of patients had diarrhea, which was the most common adverse event.
Clinicians should not worry that antibiotic resistance is more likely to develop or that symptoms will recur when patients don’t finish a prescribed course of treatment, Dr. Drekonja said. “That is an old piece of guidance that has persisted for such a long time,” he said. “And it makes all of us in the infectious disease field cringe.”
Rather, the current thinking is that the more antibiotics patients take, the more resistance bacteria will develop, he said.
The success of the 7-day regimen raises the question of whether an even shorter course would work equally well. It’s not clear how short a course of antibiotics will do the trick. Research in certain populations, such as patients with spinal cord injuries, has suggested that recurrences are more frequent with 3 days of antibiotics than with 14, “so there could be a floor that you do need to go beyond,” Dr. Drekonja said.
“We’re not really sure how much people need,” agreed Daniel Morgan, MD, a professor of epidemiology and public health and medicine at the University of Maryland, Baltimore, which is why this study is important. “It really defined that 1 week is better than 2 weeks,” he said in an interview.
Another way that clinicians can reduce the use of antibiotics by men with UTIs is to consider alternative diagnoses and to culture urine samples when UTI seems like the most likely cause of their symptoms, said Dr. Morgan, who co-authored an accompanying editorial.
He pointed out that the U.S. Food and Drug Administration has issued a black box warning on fluoroquinolones, including ciprofloxacin, because they increase the risk for tendinitis and tendon rupture. Nitrofurantoin and amoxicillin-clavulanate are better alternatives for UTIs, he said.
Even some men with fevers and UTIs may need no more than 7 days of antibiotics, said Dr. Morgan. Dr. Drekonja said he generally prescribes at least 10 days antibiotics for these men.
The study was funded by the VA Merit Review Program. Dr. Drekonja and Dr. Morgan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A week of antibiotics appears just as effective as 2 weeks in treating afebrile men with urinary tract infections (UTIs), researchers say.
Shortening the course of treatment could spare patients side effects from the medications and reduce the risk that bacteria will develop resistance to the drugs, said Dimitri Drekonja, MD, chief of infectious diseases at the Minneapolis VA Medical Center.
“You’d like to be on these drugs for as short [an] amount of time as gets the job done,” he told this news organization. The study was published online July 28 in JAMA.
Researchers have recently found that shorter courses of antimicrobials are effective in the treatment of other types of infection and for UTIs in women. However, UTIs in men are thought to be more complicated because the male urethra is longer.
To see whether reducing length of treatment could be effective in men as well, Dr. Drekonja and colleagues compared 7-day and 14-day regimens in men treated at U.S. Veterans Affairs medical centers in Minnesota and Texas.
They recruited 272 men who had symptoms of UTI and were willing to participate. All the men received trimethoprim/sulfamethoxazole or ciprofloxacin for 7 days. Half the men were randomly assigned to continue this treatment for an additional 7 days; the other half received placebo pills for an additional 7 days.
The average age of the men was 69 years. Urine samples were cultured from 87.9% of the men. In 60.7% of these samples, the researchers found more than 100,000 CFU/mL; in 16.3%, they found lower colony counts; and in 23.0%, they found no growth of bacteria. The most common organism they isolated was Escherchia coli.
Results for the two groups were similar. Symptoms resolved 14 days after completion of the course of treatment in 90.4% of those who received 14 days of antibiotics, versus 91.9% of those who received 7 days of antibiotics plus 7 days of placebo pills. At 1.5%, the difference between the two arms was within the predetermined boundary for noninferiority.
The percentage of those who experienced recurrence of symptoms within 28 days of stopping medication was also similar between the two groups. Among those who received 7 days of antibiotics, 10.3% experienced recurrence of symptoms, compared to 16.9% of those assigned to 14 days of antibiotics.
There was no significant difference in the resolution of UTI symptoms between the two groups by type of antibiotic, pretreatment bacteriuria count, or study site.
Adverse events were also similar in the two groups, occurring in 20.6% of the men who received 7 days of antibiotics, versus 24.3% of the men who received 14 days of treatment. In both groups, 8.8% of patients had diarrhea, which was the most common adverse event.
Clinicians should not worry that antibiotic resistance is more likely to develop or that symptoms will recur when patients don’t finish a prescribed course of treatment, Dr. Drekonja said. “That is an old piece of guidance that has persisted for such a long time,” he said. “And it makes all of us in the infectious disease field cringe.”
Rather, the current thinking is that the more antibiotics patients take, the more resistance bacteria will develop, he said.
The success of the 7-day regimen raises the question of whether an even shorter course would work equally well. It’s not clear how short a course of antibiotics will do the trick. Research in certain populations, such as patients with spinal cord injuries, has suggested that recurrences are more frequent with 3 days of antibiotics than with 14, “so there could be a floor that you do need to go beyond,” Dr. Drekonja said.
“We’re not really sure how much people need,” agreed Daniel Morgan, MD, a professor of epidemiology and public health and medicine at the University of Maryland, Baltimore, which is why this study is important. “It really defined that 1 week is better than 2 weeks,” he said in an interview.
Another way that clinicians can reduce the use of antibiotics by men with UTIs is to consider alternative diagnoses and to culture urine samples when UTI seems like the most likely cause of their symptoms, said Dr. Morgan, who co-authored an accompanying editorial.
He pointed out that the U.S. Food and Drug Administration has issued a black box warning on fluoroquinolones, including ciprofloxacin, because they increase the risk for tendinitis and tendon rupture. Nitrofurantoin and amoxicillin-clavulanate are better alternatives for UTIs, he said.
Even some men with fevers and UTIs may need no more than 7 days of antibiotics, said Dr. Morgan. Dr. Drekonja said he generally prescribes at least 10 days antibiotics for these men.
The study was funded by the VA Merit Review Program. Dr. Drekonja and Dr. Morgan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A week of antibiotics appears just as effective as 2 weeks in treating afebrile men with urinary tract infections (UTIs), researchers say.
Shortening the course of treatment could spare patients side effects from the medications and reduce the risk that bacteria will develop resistance to the drugs, said Dimitri Drekonja, MD, chief of infectious diseases at the Minneapolis VA Medical Center.
“You’d like to be on these drugs for as short [an] amount of time as gets the job done,” he told this news organization. The study was published online July 28 in JAMA.
Researchers have recently found that shorter courses of antimicrobials are effective in the treatment of other types of infection and for UTIs in women. However, UTIs in men are thought to be more complicated because the male urethra is longer.
To see whether reducing length of treatment could be effective in men as well, Dr. Drekonja and colleagues compared 7-day and 14-day regimens in men treated at U.S. Veterans Affairs medical centers in Minnesota and Texas.
They recruited 272 men who had symptoms of UTI and were willing to participate. All the men received trimethoprim/sulfamethoxazole or ciprofloxacin for 7 days. Half the men were randomly assigned to continue this treatment for an additional 7 days; the other half received placebo pills for an additional 7 days.
The average age of the men was 69 years. Urine samples were cultured from 87.9% of the men. In 60.7% of these samples, the researchers found more than 100,000 CFU/mL; in 16.3%, they found lower colony counts; and in 23.0%, they found no growth of bacteria. The most common organism they isolated was Escherchia coli.
Results for the two groups were similar. Symptoms resolved 14 days after completion of the course of treatment in 90.4% of those who received 14 days of antibiotics, versus 91.9% of those who received 7 days of antibiotics plus 7 days of placebo pills. At 1.5%, the difference between the two arms was within the predetermined boundary for noninferiority.
The percentage of those who experienced recurrence of symptoms within 28 days of stopping medication was also similar between the two groups. Among those who received 7 days of antibiotics, 10.3% experienced recurrence of symptoms, compared to 16.9% of those assigned to 14 days of antibiotics.
There was no significant difference in the resolution of UTI symptoms between the two groups by type of antibiotic, pretreatment bacteriuria count, or study site.
Adverse events were also similar in the two groups, occurring in 20.6% of the men who received 7 days of antibiotics, versus 24.3% of the men who received 14 days of treatment. In both groups, 8.8% of patients had diarrhea, which was the most common adverse event.
Clinicians should not worry that antibiotic resistance is more likely to develop or that symptoms will recur when patients don’t finish a prescribed course of treatment, Dr. Drekonja said. “That is an old piece of guidance that has persisted for such a long time,” he said. “And it makes all of us in the infectious disease field cringe.”
Rather, the current thinking is that the more antibiotics patients take, the more resistance bacteria will develop, he said.
The success of the 7-day regimen raises the question of whether an even shorter course would work equally well. It’s not clear how short a course of antibiotics will do the trick. Research in certain populations, such as patients with spinal cord injuries, has suggested that recurrences are more frequent with 3 days of antibiotics than with 14, “so there could be a floor that you do need to go beyond,” Dr. Drekonja said.
“We’re not really sure how much people need,” agreed Daniel Morgan, MD, a professor of epidemiology and public health and medicine at the University of Maryland, Baltimore, which is why this study is important. “It really defined that 1 week is better than 2 weeks,” he said in an interview.
Another way that clinicians can reduce the use of antibiotics by men with UTIs is to consider alternative diagnoses and to culture urine samples when UTI seems like the most likely cause of their symptoms, said Dr. Morgan, who co-authored an accompanying editorial.
He pointed out that the U.S. Food and Drug Administration has issued a black box warning on fluoroquinolones, including ciprofloxacin, because they increase the risk for tendinitis and tendon rupture. Nitrofurantoin and amoxicillin-clavulanate are better alternatives for UTIs, he said.
Even some men with fevers and UTIs may need no more than 7 days of antibiotics, said Dr. Morgan. Dr. Drekonja said he generally prescribes at least 10 days antibiotics for these men.
The study was funded by the VA Merit Review Program. Dr. Drekonja and Dr. Morgan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Recombinant factor IX fusion protein benefited untreated patients with hemophilia B
Recombinant factor IX Fc fusion protein was effective, both as prophylaxis and in the treatment of bleeding episodes in previously untreated boys (< 18 years of age) with hemophilia B, according to Beatrice Nolan, MD, of Children’s Health Ireland at Crumlin, Dublin, and colleagues.
PUPs B-LONG is an open-label, single-arm, multicenter, phase 3 trial (Clinicaltrials.gov: NCT02234310) of rFIXFc in 33 previously untreated patients (PUPs) with hemophilia B; 79% of the patients were younger than 1 year of age at study entry. The primary endpoint was occurrence of inhibitor development, with a secondary endpoint of annualized bleed rate, according to the results of the study, published online in Blood Advances.
At the onset of the study, 22 patients (67%) received on-demand treatment and 11 (33%) started with a prophylactic regimen. Seventeen (77%) of 22 patients who initially received on-demand treatment subsequently switched to prophylaxis, for a total of 28 patients receiving prophylaxis during the study, according to the researchers. Twenty-seven patients (82%) completed the study, and six (18%) discontinued early.
Promising results
The median patient annualized bleeding rate was 1.24 for patients receiving prophylaxis. Most (85%) bleeding episodes required only one infusion for bleed resolution, according to the researchers.
One patient developed a low-titer inhibitor after 11 exposure days to rFIXFc; no high-titer inhibitors were detected. However, 23 patients (70%) had 58 treatment-emergent serious adverse events, of which 2 were assessed as related to treatment (FIX inhibition and hypersensitivity in 1 patient, resulting in withdrawal).
“rFIXFc was generally well tolerated. The incidence of inhibitor development was consistent with other FIX products, with 1 low-titer inhibitor and no high-titer inhibitors detected. In addition, rFIXFc was effective, both as prophylaxis and in the treatment of bleeding episodes,” the researchers concluded.
The PUPs B-LONG study was sponsored by Sanofi and Sobi. Dr. Nolan reported personal fees from Sobi and sponsorship from Sanofi and several other pharmaceutical companies. The other authors reported financial support from a variety of pharmaceutical companies.
Recombinant factor IX Fc fusion protein was effective, both as prophylaxis and in the treatment of bleeding episodes in previously untreated boys (< 18 years of age) with hemophilia B, according to Beatrice Nolan, MD, of Children’s Health Ireland at Crumlin, Dublin, and colleagues.
PUPs B-LONG is an open-label, single-arm, multicenter, phase 3 trial (Clinicaltrials.gov: NCT02234310) of rFIXFc in 33 previously untreated patients (PUPs) with hemophilia B; 79% of the patients were younger than 1 year of age at study entry. The primary endpoint was occurrence of inhibitor development, with a secondary endpoint of annualized bleed rate, according to the results of the study, published online in Blood Advances.
At the onset of the study, 22 patients (67%) received on-demand treatment and 11 (33%) started with a prophylactic regimen. Seventeen (77%) of 22 patients who initially received on-demand treatment subsequently switched to prophylaxis, for a total of 28 patients receiving prophylaxis during the study, according to the researchers. Twenty-seven patients (82%) completed the study, and six (18%) discontinued early.
Promising results
The median patient annualized bleeding rate was 1.24 for patients receiving prophylaxis. Most (85%) bleeding episodes required only one infusion for bleed resolution, according to the researchers.
One patient developed a low-titer inhibitor after 11 exposure days to rFIXFc; no high-titer inhibitors were detected. However, 23 patients (70%) had 58 treatment-emergent serious adverse events, of which 2 were assessed as related to treatment (FIX inhibition and hypersensitivity in 1 patient, resulting in withdrawal).
“rFIXFc was generally well tolerated. The incidence of inhibitor development was consistent with other FIX products, with 1 low-titer inhibitor and no high-titer inhibitors detected. In addition, rFIXFc was effective, both as prophylaxis and in the treatment of bleeding episodes,” the researchers concluded.
The PUPs B-LONG study was sponsored by Sanofi and Sobi. Dr. Nolan reported personal fees from Sobi and sponsorship from Sanofi and several other pharmaceutical companies. The other authors reported financial support from a variety of pharmaceutical companies.
Recombinant factor IX Fc fusion protein was effective, both as prophylaxis and in the treatment of bleeding episodes in previously untreated boys (< 18 years of age) with hemophilia B, according to Beatrice Nolan, MD, of Children’s Health Ireland at Crumlin, Dublin, and colleagues.
PUPs B-LONG is an open-label, single-arm, multicenter, phase 3 trial (Clinicaltrials.gov: NCT02234310) of rFIXFc in 33 previously untreated patients (PUPs) with hemophilia B; 79% of the patients were younger than 1 year of age at study entry. The primary endpoint was occurrence of inhibitor development, with a secondary endpoint of annualized bleed rate, according to the results of the study, published online in Blood Advances.
At the onset of the study, 22 patients (67%) received on-demand treatment and 11 (33%) started with a prophylactic regimen. Seventeen (77%) of 22 patients who initially received on-demand treatment subsequently switched to prophylaxis, for a total of 28 patients receiving prophylaxis during the study, according to the researchers. Twenty-seven patients (82%) completed the study, and six (18%) discontinued early.
Promising results
The median patient annualized bleeding rate was 1.24 for patients receiving prophylaxis. Most (85%) bleeding episodes required only one infusion for bleed resolution, according to the researchers.
One patient developed a low-titer inhibitor after 11 exposure days to rFIXFc; no high-titer inhibitors were detected. However, 23 patients (70%) had 58 treatment-emergent serious adverse events, of which 2 were assessed as related to treatment (FIX inhibition and hypersensitivity in 1 patient, resulting in withdrawal).
“rFIXFc was generally well tolerated. The incidence of inhibitor development was consistent with other FIX products, with 1 low-titer inhibitor and no high-titer inhibitors detected. In addition, rFIXFc was effective, both as prophylaxis and in the treatment of bleeding episodes,” the researchers concluded.
The PUPs B-LONG study was sponsored by Sanofi and Sobi. Dr. Nolan reported personal fees from Sobi and sponsorship from Sanofi and several other pharmaceutical companies. The other authors reported financial support from a variety of pharmaceutical companies.
FROM BLOOD ADVANCES
Pink, Scaly, Annular Plaques in Concentric Rings Localized to Vitiliginous Patches
The Diagnosis: Tinea Pseudoimbricata
Tinea pseudoimbricata and tinea indecisiva are synonyms describing cases of tinea corporis that manifest in scaly plaques in concentric rings evocative of those present in tinea imbricata. However, in contrast to tinea imbricata, cases of tinea pseudoimbricata are caused by dermatophytes other than Trichophyton concentricum. 1 Tinea pseudoimbricata usually presents in association with immunosuppression, either systemic or local, and can be produced by application of topical medications such as corticosteroids.2 Mask-Bull et al3 reported the case of a 21-year-old man in the United States with no history of immunosuppressive conditions who presented with scaly erythematous annular plaques on the lateral neck that resolved with 2 pulsed doses of terbinafine. Potassium hydroxide preparation and fungal culture were both consistent with Trichophyton tonsurans.3
Trichophyton concentricum is an anthropophilic species of dermatophyte endemic to areas within the South Pacific, Southeast Asia, and Central and South America. Infection with T concentricum produces tinea imbricata, which presents with concentric, scaly, annular rings. Cutaneous lesions of tinea imbricata caused by T concentricum have a more generalized distribution and more densely grouped, concentric circles than the cutaneous findings seen in patients with tinea pseudoimbricata.4 Affected patients typically demonstrate negative delayed-type hypersensitivity to T concentricum cytoplasmic antigen and T-lymphocyte hyporeactivity, which may contribute to the development of sequential waves of scaling observed in tinea imbricata.5
Trichophyton rubrum, the most common cause of tinea corporis, has been reported to cause some cases of tinea pseudoimbricata (indecisiva).1,2 It utilizes keratinases such as subtilisins (Sub3 and Sub4), leucine aminopeptidases (Lap1 and Lap2), and dipeptidyl peptidases (DppIV and DppV) to invade the skin. Once inside, mannans, glycoprotein constituents of the cell wall, are released and bind to the cell surface of mononuclear phagocytes, subsequently moving into the cell by phagocytosis, thereafter interfering with RNA synthesis that is necessary for presentation of antigens to appropriate T cells and allowing for initiation of chronic infection.6,7 The cytotoxic response to superficial dermatophyte infection is triggered by major histocompatibility complex class I molecule activation of CD8+ cells.6,8
Our case is of interest given the localization of the superficial dermatophyte infection to only vitiliginous skin. This distribution and appearance while undergoing narrowband UVB (NB-UVB) treatment is rare. We postulate that our patient likely represents a case of locus minoris resistentiae, a phenomenon in which an area of skin exhibits a compromised immune microenvironment that predisposes it to disease.9
In vitiligo, NB-UVB modulates the immune response by increasing IL-10, thereby promoting regulatory T-cell differentiation with suppression of autoreactive T cells and induction of direct T-lymphocyte apoptosis.10,11 Although the mechanism accounting for our patient’s presentation is unknown, we suspect NB-UVB–induced immunosuppression enabled persistence of the dermatophyte infection. The localization of the infection to the vitiliginous patches may result from the greater penetration of the UV light relative to the surrounding, normally pigmented skin. This relative difference in UV penetration would be expected to result in increased immunosuppression in the vitiliginous lesions and enhanced susceptibility to the fungal organisms.
Erythema annulare centrifugum is characterized by annular lesions with a trailing scale instead of the concentric rings seen in tinea pseudoimbricata. Erythema marginatum is seen in acute rheumatic fever and presents with a transient nonpruritic rash, usually on the trunk or extremities. Erythema migrans presents with fewer lesions that are less circinate in shape, and the patient often has a history of a tick bite. Tinea imbricata is caused by T concentricum, while tinea pseudoimbricata is caused by T tonsurans and other dermatophytes.
With the increasing use of immunosuppressant drugs, the prevalence of tinea pseudoimbricata is hypothesized to increase.1 The presence of tinea pseudoimbricata should alert dermatologists to the possible overuse of topical corticosteroids, and other forms of immunosuppression also should be considered.
- Lim SP, Smith AG. “Tinea pseudoimbricata”: tinea corporis in a renal transplant recipient mimicking the concentric rings of tinea imbricata. Clin Exp Dermatol. 2003;28:332-333.
- Batta K, Ramlogan D, Smith AG, et al. ‘Tinea indecisiva’ may mimic the concentric rings of tinea imbricata. Br J Dermatol. 2002;147:384.
- Mask-Bull L, Patel R, Tarbox MB. America’s first case of tinea pseudoimbricata. Am J Dermatol Venereol. 2015;4:15-17.
- Meena M, Mittal A. Tinea pseudo-imbricata. J Assoc Physicians India. 2018;66:79.
- Hay RJ, Reid S, Talwat E, et al. Immune responses of patients with tinea imbricata. Br J Dermatol. 1983;108:581-586.
- Dahl MV. Suppression of immunity and inflammation by products produced by dermatophytes. J Am Acad Dermatol. 1993;28(5 pt 1):S19-S23.
- Blutfield MS, Lohre JM, Pawich DA, et al. The immunologic response to Trichophyton rubrum in lower extremity fungal infections. J Fungi (Basel). 2015;1:130-137.
- De Hoog S, Monod M, Dawson T, et al. Skin fungi from colonization to infection [published online July 2017]. Microbiol Spectr. doi:10.1128/ microbiolspec.FUNK-0049-2016
- Lo Schiavo A, Ruocco E, Russo T, et al. Locus minoris resistentiae: an old but still valid way of thinking in medicine. Clin Dermatol. 2014;32:553-556.
- Ponsonby AL, Lucas RM, van der Mei IA. UVR, vitamin D and three autoimmune diseases—multiple sclerosis, type 1 diabetes, rheumatoid arthritis. Photochem Photobiol. 2005;81:1267-1275.
- Yazdani Abyaneh M, Griffith RD, Falto-Aizpurua L, et al. Narrowband ultraviolet B phototherapy in combination with other therapies for vitiligo: mechanisms and efficacies. J Eur Acad Dermatol Venereol. 2014;28:1610-1622.
The Diagnosis: Tinea Pseudoimbricata
Tinea pseudoimbricata and tinea indecisiva are synonyms describing cases of tinea corporis that manifest in scaly plaques in concentric rings evocative of those present in tinea imbricata. However, in contrast to tinea imbricata, cases of tinea pseudoimbricata are caused by dermatophytes other than Trichophyton concentricum. 1 Tinea pseudoimbricata usually presents in association with immunosuppression, either systemic or local, and can be produced by application of topical medications such as corticosteroids.2 Mask-Bull et al3 reported the case of a 21-year-old man in the United States with no history of immunosuppressive conditions who presented with scaly erythematous annular plaques on the lateral neck that resolved with 2 pulsed doses of terbinafine. Potassium hydroxide preparation and fungal culture were both consistent with Trichophyton tonsurans.3
Trichophyton concentricum is an anthropophilic species of dermatophyte endemic to areas within the South Pacific, Southeast Asia, and Central and South America. Infection with T concentricum produces tinea imbricata, which presents with concentric, scaly, annular rings. Cutaneous lesions of tinea imbricata caused by T concentricum have a more generalized distribution and more densely grouped, concentric circles than the cutaneous findings seen in patients with tinea pseudoimbricata.4 Affected patients typically demonstrate negative delayed-type hypersensitivity to T concentricum cytoplasmic antigen and T-lymphocyte hyporeactivity, which may contribute to the development of sequential waves of scaling observed in tinea imbricata.5
Trichophyton rubrum, the most common cause of tinea corporis, has been reported to cause some cases of tinea pseudoimbricata (indecisiva).1,2 It utilizes keratinases such as subtilisins (Sub3 and Sub4), leucine aminopeptidases (Lap1 and Lap2), and dipeptidyl peptidases (DppIV and DppV) to invade the skin. Once inside, mannans, glycoprotein constituents of the cell wall, are released and bind to the cell surface of mononuclear phagocytes, subsequently moving into the cell by phagocytosis, thereafter interfering with RNA synthesis that is necessary for presentation of antigens to appropriate T cells and allowing for initiation of chronic infection.6,7 The cytotoxic response to superficial dermatophyte infection is triggered by major histocompatibility complex class I molecule activation of CD8+ cells.6,8
Our case is of interest given the localization of the superficial dermatophyte infection to only vitiliginous skin. This distribution and appearance while undergoing narrowband UVB (NB-UVB) treatment is rare. We postulate that our patient likely represents a case of locus minoris resistentiae, a phenomenon in which an area of skin exhibits a compromised immune microenvironment that predisposes it to disease.9
In vitiligo, NB-UVB modulates the immune response by increasing IL-10, thereby promoting regulatory T-cell differentiation with suppression of autoreactive T cells and induction of direct T-lymphocyte apoptosis.10,11 Although the mechanism accounting for our patient’s presentation is unknown, we suspect NB-UVB–induced immunosuppression enabled persistence of the dermatophyte infection. The localization of the infection to the vitiliginous patches may result from the greater penetration of the UV light relative to the surrounding, normally pigmented skin. This relative difference in UV penetration would be expected to result in increased immunosuppression in the vitiliginous lesions and enhanced susceptibility to the fungal organisms.
Erythema annulare centrifugum is characterized by annular lesions with a trailing scale instead of the concentric rings seen in tinea pseudoimbricata. Erythema marginatum is seen in acute rheumatic fever and presents with a transient nonpruritic rash, usually on the trunk or extremities. Erythema migrans presents with fewer lesions that are less circinate in shape, and the patient often has a history of a tick bite. Tinea imbricata is caused by T concentricum, while tinea pseudoimbricata is caused by T tonsurans and other dermatophytes.
With the increasing use of immunosuppressant drugs, the prevalence of tinea pseudoimbricata is hypothesized to increase.1 The presence of tinea pseudoimbricata should alert dermatologists to the possible overuse of topical corticosteroids, and other forms of immunosuppression also should be considered.
The Diagnosis: Tinea Pseudoimbricata
Tinea pseudoimbricata and tinea indecisiva are synonyms describing cases of tinea corporis that manifest in scaly plaques in concentric rings evocative of those present in tinea imbricata. However, in contrast to tinea imbricata, cases of tinea pseudoimbricata are caused by dermatophytes other than Trichophyton concentricum. 1 Tinea pseudoimbricata usually presents in association with immunosuppression, either systemic or local, and can be produced by application of topical medications such as corticosteroids.2 Mask-Bull et al3 reported the case of a 21-year-old man in the United States with no history of immunosuppressive conditions who presented with scaly erythematous annular plaques on the lateral neck that resolved with 2 pulsed doses of terbinafine. Potassium hydroxide preparation and fungal culture were both consistent with Trichophyton tonsurans.3
Trichophyton concentricum is an anthropophilic species of dermatophyte endemic to areas within the South Pacific, Southeast Asia, and Central and South America. Infection with T concentricum produces tinea imbricata, which presents with concentric, scaly, annular rings. Cutaneous lesions of tinea imbricata caused by T concentricum have a more generalized distribution and more densely grouped, concentric circles than the cutaneous findings seen in patients with tinea pseudoimbricata.4 Affected patients typically demonstrate negative delayed-type hypersensitivity to T concentricum cytoplasmic antigen and T-lymphocyte hyporeactivity, which may contribute to the development of sequential waves of scaling observed in tinea imbricata.5
Trichophyton rubrum, the most common cause of tinea corporis, has been reported to cause some cases of tinea pseudoimbricata (indecisiva).1,2 It utilizes keratinases such as subtilisins (Sub3 and Sub4), leucine aminopeptidases (Lap1 and Lap2), and dipeptidyl peptidases (DppIV and DppV) to invade the skin. Once inside, mannans, glycoprotein constituents of the cell wall, are released and bind to the cell surface of mononuclear phagocytes, subsequently moving into the cell by phagocytosis, thereafter interfering with RNA synthesis that is necessary for presentation of antigens to appropriate T cells and allowing for initiation of chronic infection.6,7 The cytotoxic response to superficial dermatophyte infection is triggered by major histocompatibility complex class I molecule activation of CD8+ cells.6,8
Our case is of interest given the localization of the superficial dermatophyte infection to only vitiliginous skin. This distribution and appearance while undergoing narrowband UVB (NB-UVB) treatment is rare. We postulate that our patient likely represents a case of locus minoris resistentiae, a phenomenon in which an area of skin exhibits a compromised immune microenvironment that predisposes it to disease.9
In vitiligo, NB-UVB modulates the immune response by increasing IL-10, thereby promoting regulatory T-cell differentiation with suppression of autoreactive T cells and induction of direct T-lymphocyte apoptosis.10,11 Although the mechanism accounting for our patient’s presentation is unknown, we suspect NB-UVB–induced immunosuppression enabled persistence of the dermatophyte infection. The localization of the infection to the vitiliginous patches may result from the greater penetration of the UV light relative to the surrounding, normally pigmented skin. This relative difference in UV penetration would be expected to result in increased immunosuppression in the vitiliginous lesions and enhanced susceptibility to the fungal organisms.
Erythema annulare centrifugum is characterized by annular lesions with a trailing scale instead of the concentric rings seen in tinea pseudoimbricata. Erythema marginatum is seen in acute rheumatic fever and presents with a transient nonpruritic rash, usually on the trunk or extremities. Erythema migrans presents with fewer lesions that are less circinate in shape, and the patient often has a history of a tick bite. Tinea imbricata is caused by T concentricum, while tinea pseudoimbricata is caused by T tonsurans and other dermatophytes.
With the increasing use of immunosuppressant drugs, the prevalence of tinea pseudoimbricata is hypothesized to increase.1 The presence of tinea pseudoimbricata should alert dermatologists to the possible overuse of topical corticosteroids, and other forms of immunosuppression also should be considered.
- Lim SP, Smith AG. “Tinea pseudoimbricata”: tinea corporis in a renal transplant recipient mimicking the concentric rings of tinea imbricata. Clin Exp Dermatol. 2003;28:332-333.
- Batta K, Ramlogan D, Smith AG, et al. ‘Tinea indecisiva’ may mimic the concentric rings of tinea imbricata. Br J Dermatol. 2002;147:384.
- Mask-Bull L, Patel R, Tarbox MB. America’s first case of tinea pseudoimbricata. Am J Dermatol Venereol. 2015;4:15-17.
- Meena M, Mittal A. Tinea pseudo-imbricata. J Assoc Physicians India. 2018;66:79.
- Hay RJ, Reid S, Talwat E, et al. Immune responses of patients with tinea imbricata. Br J Dermatol. 1983;108:581-586.
- Dahl MV. Suppression of immunity and inflammation by products produced by dermatophytes. J Am Acad Dermatol. 1993;28(5 pt 1):S19-S23.
- Blutfield MS, Lohre JM, Pawich DA, et al. The immunologic response to Trichophyton rubrum in lower extremity fungal infections. J Fungi (Basel). 2015;1:130-137.
- De Hoog S, Monod M, Dawson T, et al. Skin fungi from colonization to infection [published online July 2017]. Microbiol Spectr. doi:10.1128/ microbiolspec.FUNK-0049-2016
- Lo Schiavo A, Ruocco E, Russo T, et al. Locus minoris resistentiae: an old but still valid way of thinking in medicine. Clin Dermatol. 2014;32:553-556.
- Ponsonby AL, Lucas RM, van der Mei IA. UVR, vitamin D and three autoimmune diseases—multiple sclerosis, type 1 diabetes, rheumatoid arthritis. Photochem Photobiol. 2005;81:1267-1275.
- Yazdani Abyaneh M, Griffith RD, Falto-Aizpurua L, et al. Narrowband ultraviolet B phototherapy in combination with other therapies for vitiligo: mechanisms and efficacies. J Eur Acad Dermatol Venereol. 2014;28:1610-1622.
- Lim SP, Smith AG. “Tinea pseudoimbricata”: tinea corporis in a renal transplant recipient mimicking the concentric rings of tinea imbricata. Clin Exp Dermatol. 2003;28:332-333.
- Batta K, Ramlogan D, Smith AG, et al. ‘Tinea indecisiva’ may mimic the concentric rings of tinea imbricata. Br J Dermatol. 2002;147:384.
- Mask-Bull L, Patel R, Tarbox MB. America’s first case of tinea pseudoimbricata. Am J Dermatol Venereol. 2015;4:15-17.
- Meena M, Mittal A. Tinea pseudo-imbricata. J Assoc Physicians India. 2018;66:79.
- Hay RJ, Reid S, Talwat E, et al. Immune responses of patients with tinea imbricata. Br J Dermatol. 1983;108:581-586.
- Dahl MV. Suppression of immunity and inflammation by products produced by dermatophytes. J Am Acad Dermatol. 1993;28(5 pt 1):S19-S23.
- Blutfield MS, Lohre JM, Pawich DA, et al. The immunologic response to Trichophyton rubrum in lower extremity fungal infections. J Fungi (Basel). 2015;1:130-137.
- De Hoog S, Monod M, Dawson T, et al. Skin fungi from colonization to infection [published online July 2017]. Microbiol Spectr. doi:10.1128/ microbiolspec.FUNK-0049-2016
- Lo Schiavo A, Ruocco E, Russo T, et al. Locus minoris resistentiae: an old but still valid way of thinking in medicine. Clin Dermatol. 2014;32:553-556.
- Ponsonby AL, Lucas RM, van der Mei IA. UVR, vitamin D and three autoimmune diseases—multiple sclerosis, type 1 diabetes, rheumatoid arthritis. Photochem Photobiol. 2005;81:1267-1275.
- Yazdani Abyaneh M, Griffith RD, Falto-Aizpurua L, et al. Narrowband ultraviolet B phototherapy in combination with other therapies for vitiligo: mechanisms and efficacies. J Eur Acad Dermatol Venereol. 2014;28:1610-1622.
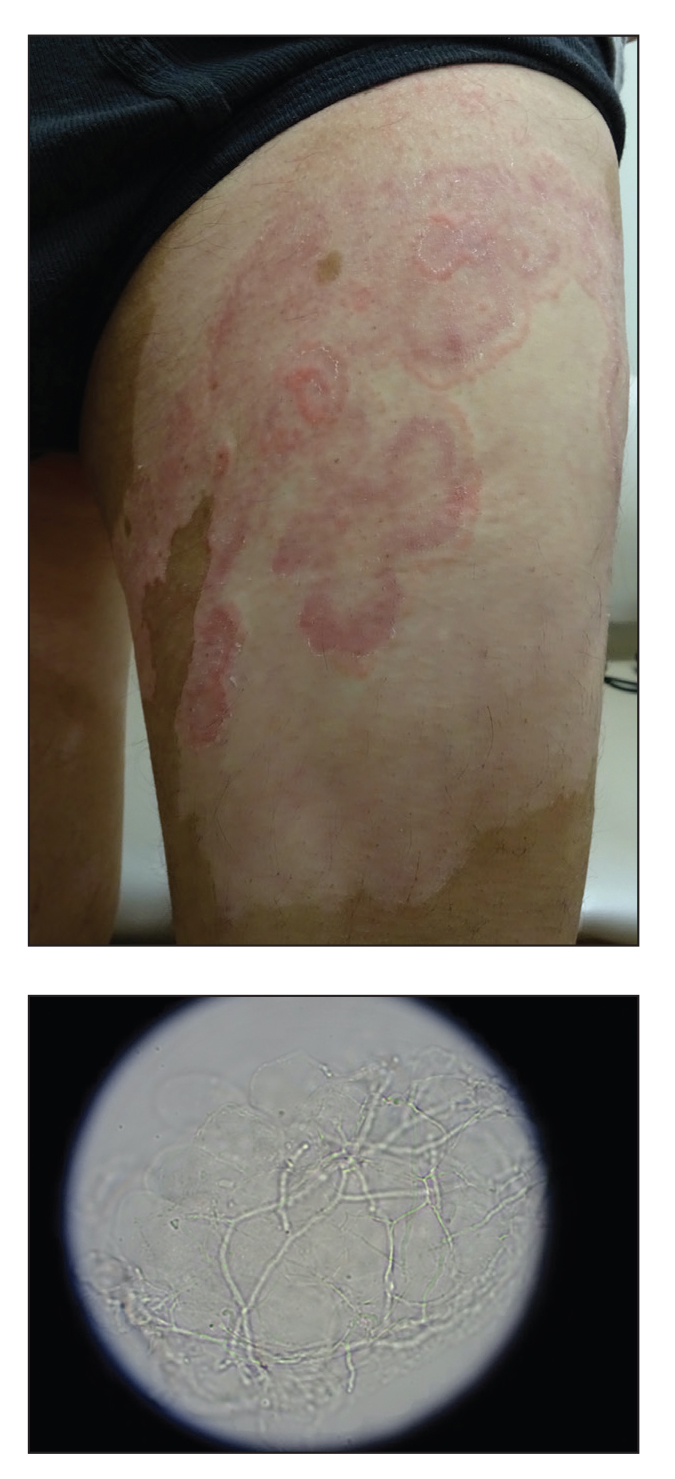
A 64-year-old man presented with generalized vitiligo. In addition to extensive depigmented macules, physical examination revealed the presence of onychomycosis and tinea corporis confirmed by microscopic examination of potassium hydroxide–treated superficial skin scrapings. Vitiligo treatment was postponed, and a 3-month course of oral terbinafine and naftifine cream was undertaken for the dermatophyte infections. Subsequent examination revealed that the patient’s tinea corporis had improved, though there were localized areas of persistence. Given the patient’s eagerness to treat his vitiligo, narrowband UVB phototherapy was started along with tolnaftate cream 1% for treatment of the residual tinea corporis. After 2 months of narrowband UVB, partial repigmentation of the vitiligo was observed; however, he had developed extensive pink, scaly, annular plaques in concentric rings within residual vitiliginous patches on the lower extremities (top). Repeat examination of potassium hydroxide–treated skin scrapings revealed numerous hyphae (bottom). A fungal culture identified Trichophyton rubrum.
FDA approves neoadjuvant pembro for triple-negative breast cancer
This approval is based on findings from the randomized, phase 3 KEYNOTE-522 trial, which showed significantly prolonged event-free survival with the pembrolizumab regimen versus neoadjuvant chemotherapy alone for previously untreated stage II-III TNBC.
This is the 30th indication for pembrolizumab in the United States.
The immunotherapy received accelerated approval in November 2020 for adjuvant use in locally recurrent unresectable or metastatic TNBC for patients whose tumors express programmed death–ligand-1, as determined by an FDA-approved test. That accelerated approval was based on results from the phase 3 KEYNOTE-355 trial. The approval has now been converted to a full approval on the basis of confirmatory data from the KEYNOTE-522, notes a statement from the manufacturer, Merck.
“Triple-negative is a difficult-to-treat type of breast cancer that unfortunately is more common in the U.S. in younger women and in Black women,” commented Vicki Goodman, MD, vice president of clinical research, Merck Research Laboratories. “We are proud to offer a new treatment option for patients faced with this challenging cancer. This neoadjuvant and adjuvant combination with pembrolizumab is the first immunotherapy regimen to be approved in high-risk early-stage TNBC, marking a meaningful milestone for the breast cancer community.”
In KEYNOTE-522, participants were randomly assigned to receive either placebo or pembrolizumab plus chemotherapy with carboplatin and paclitaxel, followed by doxorubicin or epirubicin and cyclophosphamide before surgery, as well as placebo or pembrolizumab as single-agent therapy after surgery.
The results from this trial, first reported in 2019 at the annual meeting of the European Society of Medical Oncology, showed that, for patients in the pembrolizumab arm of the trial, the pathological complete response rate was nearly 65% versus 51% among the patients who received placebo. The benefit was seen both in those whose tumors were positive and those whose tumors were negative for PD-L1 expression.
Among patients in the pembrolizumab arm, there was a 37% reduction in the risk for disease progression that precluded definitive surgery, a local/distant recurrence, a second primary cancer, or death from any cause (hazard ratio, 0.63).
Pembrolizumab can be associated with immune-mediated adverse reactions that may be severe or fatal, Merck noted.
These events “can occur in any organ system or tissue and can affect more than one body system simultaneously. Immune-mediated adverse reactions can occur at any time during or after treatment,” Merck warned. The company states: “Early identification and management of immune-mediated adverse reactions are essential.”
Treatment may need to be withheld or permanently discontinued, and corticosteroids may be needed, depending on the severity of the adverse reaction, according to the statement.
Infusion-related reactions can also occur. Because of its mechanism of action, pembrolizumab can cause fetal harm when administered to women during pregnancy.
A version of this article first appeared on Medscape.com.
This approval is based on findings from the randomized, phase 3 KEYNOTE-522 trial, which showed significantly prolonged event-free survival with the pembrolizumab regimen versus neoadjuvant chemotherapy alone for previously untreated stage II-III TNBC.
This is the 30th indication for pembrolizumab in the United States.
The immunotherapy received accelerated approval in November 2020 for adjuvant use in locally recurrent unresectable or metastatic TNBC for patients whose tumors express programmed death–ligand-1, as determined by an FDA-approved test. That accelerated approval was based on results from the phase 3 KEYNOTE-355 trial. The approval has now been converted to a full approval on the basis of confirmatory data from the KEYNOTE-522, notes a statement from the manufacturer, Merck.
“Triple-negative is a difficult-to-treat type of breast cancer that unfortunately is more common in the U.S. in younger women and in Black women,” commented Vicki Goodman, MD, vice president of clinical research, Merck Research Laboratories. “We are proud to offer a new treatment option for patients faced with this challenging cancer. This neoadjuvant and adjuvant combination with pembrolizumab is the first immunotherapy regimen to be approved in high-risk early-stage TNBC, marking a meaningful milestone for the breast cancer community.”
In KEYNOTE-522, participants were randomly assigned to receive either placebo or pembrolizumab plus chemotherapy with carboplatin and paclitaxel, followed by doxorubicin or epirubicin and cyclophosphamide before surgery, as well as placebo or pembrolizumab as single-agent therapy after surgery.
The results from this trial, first reported in 2019 at the annual meeting of the European Society of Medical Oncology, showed that, for patients in the pembrolizumab arm of the trial, the pathological complete response rate was nearly 65% versus 51% among the patients who received placebo. The benefit was seen both in those whose tumors were positive and those whose tumors were negative for PD-L1 expression.
Among patients in the pembrolizumab arm, there was a 37% reduction in the risk for disease progression that precluded definitive surgery, a local/distant recurrence, a second primary cancer, or death from any cause (hazard ratio, 0.63).
Pembrolizumab can be associated with immune-mediated adverse reactions that may be severe or fatal, Merck noted.
These events “can occur in any organ system or tissue and can affect more than one body system simultaneously. Immune-mediated adverse reactions can occur at any time during or after treatment,” Merck warned. The company states: “Early identification and management of immune-mediated adverse reactions are essential.”
Treatment may need to be withheld or permanently discontinued, and corticosteroids may be needed, depending on the severity of the adverse reaction, according to the statement.
Infusion-related reactions can also occur. Because of its mechanism of action, pembrolizumab can cause fetal harm when administered to women during pregnancy.
A version of this article first appeared on Medscape.com.
This approval is based on findings from the randomized, phase 3 KEYNOTE-522 trial, which showed significantly prolonged event-free survival with the pembrolizumab regimen versus neoadjuvant chemotherapy alone for previously untreated stage II-III TNBC.
This is the 30th indication for pembrolizumab in the United States.
The immunotherapy received accelerated approval in November 2020 for adjuvant use in locally recurrent unresectable or metastatic TNBC for patients whose tumors express programmed death–ligand-1, as determined by an FDA-approved test. That accelerated approval was based on results from the phase 3 KEYNOTE-355 trial. The approval has now been converted to a full approval on the basis of confirmatory data from the KEYNOTE-522, notes a statement from the manufacturer, Merck.
“Triple-negative is a difficult-to-treat type of breast cancer that unfortunately is more common in the U.S. in younger women and in Black women,” commented Vicki Goodman, MD, vice president of clinical research, Merck Research Laboratories. “We are proud to offer a new treatment option for patients faced with this challenging cancer. This neoadjuvant and adjuvant combination with pembrolizumab is the first immunotherapy regimen to be approved in high-risk early-stage TNBC, marking a meaningful milestone for the breast cancer community.”
In KEYNOTE-522, participants were randomly assigned to receive either placebo or pembrolizumab plus chemotherapy with carboplatin and paclitaxel, followed by doxorubicin or epirubicin and cyclophosphamide before surgery, as well as placebo or pembrolizumab as single-agent therapy after surgery.
The results from this trial, first reported in 2019 at the annual meeting of the European Society of Medical Oncology, showed that, for patients in the pembrolizumab arm of the trial, the pathological complete response rate was nearly 65% versus 51% among the patients who received placebo. The benefit was seen both in those whose tumors were positive and those whose tumors were negative for PD-L1 expression.
Among patients in the pembrolizumab arm, there was a 37% reduction in the risk for disease progression that precluded definitive surgery, a local/distant recurrence, a second primary cancer, or death from any cause (hazard ratio, 0.63).
Pembrolizumab can be associated with immune-mediated adverse reactions that may be severe or fatal, Merck noted.
These events “can occur in any organ system or tissue and can affect more than one body system simultaneously. Immune-mediated adverse reactions can occur at any time during or after treatment,” Merck warned. The company states: “Early identification and management of immune-mediated adverse reactions are essential.”
Treatment may need to be withheld or permanently discontinued, and corticosteroids may be needed, depending on the severity of the adverse reaction, according to the statement.
Infusion-related reactions can also occur. Because of its mechanism of action, pembrolizumab can cause fetal harm when administered to women during pregnancy.
A version of this article first appeared on Medscape.com.
Untreatable, drug-resistant fungus found in Texas and Washington, D.C.
The CDC has reported two clusters of Candida auris infections resistant to all antifungal medications in long-term care facilities in 2021. Because these panresistant infections occurred without any exposure to antifungal drugs, the cases are even more worrisome. These clusters are the first time such nosocomial transmission has been detected.
In the District of Columbia, three panresistant isolates were discovered through screening for skin colonization with resistant organisms at a long-term acute care facility (LTAC) that cares for patients who are seriously ill, often on mechanical ventilation.
In Texas, the resistant organisms were found both by screening and in specimens from ill patients at an LTAC and a short-term acute care hospital that share patients. Two were panresistant, and five others were resistant to fluconazole and echinocandins.
These clusters occurred simultaneously and independently of each other; there were no links between the two institutions.
Colonization of skin with C. auris can lead to invasive infections in 5%-10% of affected patients. Routine skin surveillance cultures are not commonly done for Candida, although perirectal cultures for vancomycin-resistant enterococci and nasal swabs for MRSA have been done for years. Some areas, like Los Angeles, have recommended screening for C. auris in high-risk patients – defined as those who were on a ventilator or had a tracheostomy admitted from an LTAC or skilled nursing facility in Los Angeles County, New York, New Jersey, or Illinois.
In the past, about 85% of C. auris isolates in the United States have been resistant to azoles (for example, fluconazole), 33% to amphotericin B, and 1% to echinocandins. Because of generally strong susceptibility, an echinocandin such as micafungin or caspofungin has been the drug of choice for an invasive Candida infection.
C. auris is particularly difficult to deal with for several reasons. First, it can continue to live in the environment, on both dry or moist surfaces, for up to 2 weeks. Outbreaks have occurred both from hand (person-to-person) transmission or via inanimate surfaces that have become contaminated. Equally troublesome is that people become colonized with the yeast indefinitely.
Meghan Lyman, MD, of the fungal diseases branch of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, said in an interview that facilities might be slow in recognizing the problem and in identifying the organism. “We encounter problems in noninvasive specimens, especially urine,” Dr. Lyman added.
“Sometimes ... they consider Candida [to represent] colonization so they will often not speciate it.” She emphasized the need for facilities that care for ventilated patients to consider screening. “Higher priority ... are places in areas where there’s a lot of C. auris transmission or in nearby areas that are likely to get introductions.” Even those that do speciate may have difficulty identifying C. auris.
Further, Dr. Lyman stressed “the importance of antifungal susceptibility testing and testing for resistance. Because that’s also something that’s not widely available at all hospitals and clinical labs ... you can send it to the [CDC’s] antimicrobial resistance lab network” for testing.
COVID-19 has brought particular challenges. Rodney E. Rohde, PhD, MS, professor and chair, clinical lab science program, Texas State University, San Marcos, said in an interview that he is worried about all the steroids and broad-spectrum antibiotics patients receive.
They’re “being given medical interventions, whether it’s ventilators or [extracorporeal membrane oxygenation] or IVs or central lines or catheters for UTIs and you’re creating highways, right for something that may be right there,” said Dr. Rohde, who was not involved in the CDC study. “It’s a perfect storm, not just for C. auris, but I worry about bacterial resistance agents, too, like MRSA and so forth, having kind of a spike in those types of infections with COVID. So, it’s kind of a doubly dangerous time, I think.”
Multiresistant bacteria are a major health problem, causing illnesses in 2.8 million people annually in the United States, and causing about 35,000 deaths.
Dr. Rohde raised another, rarely mentioned concern. “We’re in crisis mode. People are leaving our field more than they ever had before. The medical laboratory is being decimated because people have burned out after these past 14 months. And so I worry just about competent medical laboratory professionals that are on board to deal with these types of other crises that are popping up within hospitals and long-term care facilities. It kind of keeps me awake.”
Dr. Rohde and Dr. Lyman shared their concern that COVID caused a decrease in screening for other infections and drug-resistant organisms. Bare-bones staffing and shortages of personal protective equipment have likely fueled the spread of these infections as well.
In an outbreak of C. auris in a Florida hospital’s COVID unit in 2020, 35 of 67 patients became colonized, and 6 became ill. The epidemiologists investigating thought that contaminated gowns or gloves, computers, and other equipment were likely sources of transmission.
Low pay, especially in nursing homes, is another problem Dr. Rohde mentioned. It’s an additional problem in both acute and long-term care that “some of the lowest-paid people are the environmental services people, and so the turnover is crazy.” Yet, we rely on them to keep everyone safe. He added that, in addition to pay, he “tries to give them the appreciation and the recognition that they really deserve.”
There are a few specific measures that can be taken to protect patients. Dr. Lyman concluded. “The best way is identifying cases and really ensuring good infection control to prevent the spread.” It’s back to basics – limiting broad-spectrum antibiotics and invasive medical devices, and especially good handwashing and thorough cleaning.
Dr. Lyman and Dr. Rohde have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The CDC has reported two clusters of Candida auris infections resistant to all antifungal medications in long-term care facilities in 2021. Because these panresistant infections occurred without any exposure to antifungal drugs, the cases are even more worrisome. These clusters are the first time such nosocomial transmission has been detected.
In the District of Columbia, three panresistant isolates were discovered through screening for skin colonization with resistant organisms at a long-term acute care facility (LTAC) that cares for patients who are seriously ill, often on mechanical ventilation.
In Texas, the resistant organisms were found both by screening and in specimens from ill patients at an LTAC and a short-term acute care hospital that share patients. Two were panresistant, and five others were resistant to fluconazole and echinocandins.
These clusters occurred simultaneously and independently of each other; there were no links between the two institutions.
Colonization of skin with C. auris can lead to invasive infections in 5%-10% of affected patients. Routine skin surveillance cultures are not commonly done for Candida, although perirectal cultures for vancomycin-resistant enterococci and nasal swabs for MRSA have been done for years. Some areas, like Los Angeles, have recommended screening for C. auris in high-risk patients – defined as those who were on a ventilator or had a tracheostomy admitted from an LTAC or skilled nursing facility in Los Angeles County, New York, New Jersey, or Illinois.
In the past, about 85% of C. auris isolates in the United States have been resistant to azoles (for example, fluconazole), 33% to amphotericin B, and 1% to echinocandins. Because of generally strong susceptibility, an echinocandin such as micafungin or caspofungin has been the drug of choice for an invasive Candida infection.
C. auris is particularly difficult to deal with for several reasons. First, it can continue to live in the environment, on both dry or moist surfaces, for up to 2 weeks. Outbreaks have occurred both from hand (person-to-person) transmission or via inanimate surfaces that have become contaminated. Equally troublesome is that people become colonized with the yeast indefinitely.
Meghan Lyman, MD, of the fungal diseases branch of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, said in an interview that facilities might be slow in recognizing the problem and in identifying the organism. “We encounter problems in noninvasive specimens, especially urine,” Dr. Lyman added.
“Sometimes ... they consider Candida [to represent] colonization so they will often not speciate it.” She emphasized the need for facilities that care for ventilated patients to consider screening. “Higher priority ... are places in areas where there’s a lot of C. auris transmission or in nearby areas that are likely to get introductions.” Even those that do speciate may have difficulty identifying C. auris.
Further, Dr. Lyman stressed “the importance of antifungal susceptibility testing and testing for resistance. Because that’s also something that’s not widely available at all hospitals and clinical labs ... you can send it to the [CDC’s] antimicrobial resistance lab network” for testing.
COVID-19 has brought particular challenges. Rodney E. Rohde, PhD, MS, professor and chair, clinical lab science program, Texas State University, San Marcos, said in an interview that he is worried about all the steroids and broad-spectrum antibiotics patients receive.
They’re “being given medical interventions, whether it’s ventilators or [extracorporeal membrane oxygenation] or IVs or central lines or catheters for UTIs and you’re creating highways, right for something that may be right there,” said Dr. Rohde, who was not involved in the CDC study. “It’s a perfect storm, not just for C. auris, but I worry about bacterial resistance agents, too, like MRSA and so forth, having kind of a spike in those types of infections with COVID. So, it’s kind of a doubly dangerous time, I think.”
Multiresistant bacteria are a major health problem, causing illnesses in 2.8 million people annually in the United States, and causing about 35,000 deaths.
Dr. Rohde raised another, rarely mentioned concern. “We’re in crisis mode. People are leaving our field more than they ever had before. The medical laboratory is being decimated because people have burned out after these past 14 months. And so I worry just about competent medical laboratory professionals that are on board to deal with these types of other crises that are popping up within hospitals and long-term care facilities. It kind of keeps me awake.”
Dr. Rohde and Dr. Lyman shared their concern that COVID caused a decrease in screening for other infections and drug-resistant organisms. Bare-bones staffing and shortages of personal protective equipment have likely fueled the spread of these infections as well.
In an outbreak of C. auris in a Florida hospital’s COVID unit in 2020, 35 of 67 patients became colonized, and 6 became ill. The epidemiologists investigating thought that contaminated gowns or gloves, computers, and other equipment were likely sources of transmission.
Low pay, especially in nursing homes, is another problem Dr. Rohde mentioned. It’s an additional problem in both acute and long-term care that “some of the lowest-paid people are the environmental services people, and so the turnover is crazy.” Yet, we rely on them to keep everyone safe. He added that, in addition to pay, he “tries to give them the appreciation and the recognition that they really deserve.”
There are a few specific measures that can be taken to protect patients. Dr. Lyman concluded. “The best way is identifying cases and really ensuring good infection control to prevent the spread.” It’s back to basics – limiting broad-spectrum antibiotics and invasive medical devices, and especially good handwashing and thorough cleaning.
Dr. Lyman and Dr. Rohde have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The CDC has reported two clusters of Candida auris infections resistant to all antifungal medications in long-term care facilities in 2021. Because these panresistant infections occurred without any exposure to antifungal drugs, the cases are even more worrisome. These clusters are the first time such nosocomial transmission has been detected.
In the District of Columbia, three panresistant isolates were discovered through screening for skin colonization with resistant organisms at a long-term acute care facility (LTAC) that cares for patients who are seriously ill, often on mechanical ventilation.
In Texas, the resistant organisms were found both by screening and in specimens from ill patients at an LTAC and a short-term acute care hospital that share patients. Two were panresistant, and five others were resistant to fluconazole and echinocandins.
These clusters occurred simultaneously and independently of each other; there were no links between the two institutions.
Colonization of skin with C. auris can lead to invasive infections in 5%-10% of affected patients. Routine skin surveillance cultures are not commonly done for Candida, although perirectal cultures for vancomycin-resistant enterococci and nasal swabs for MRSA have been done for years. Some areas, like Los Angeles, have recommended screening for C. auris in high-risk patients – defined as those who were on a ventilator or had a tracheostomy admitted from an LTAC or skilled nursing facility in Los Angeles County, New York, New Jersey, or Illinois.
In the past, about 85% of C. auris isolates in the United States have been resistant to azoles (for example, fluconazole), 33% to amphotericin B, and 1% to echinocandins. Because of generally strong susceptibility, an echinocandin such as micafungin or caspofungin has been the drug of choice for an invasive Candida infection.
C. auris is particularly difficult to deal with for several reasons. First, it can continue to live in the environment, on both dry or moist surfaces, for up to 2 weeks. Outbreaks have occurred both from hand (person-to-person) transmission or via inanimate surfaces that have become contaminated. Equally troublesome is that people become colonized with the yeast indefinitely.
Meghan Lyman, MD, of the fungal diseases branch of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, said in an interview that facilities might be slow in recognizing the problem and in identifying the organism. “We encounter problems in noninvasive specimens, especially urine,” Dr. Lyman added.
“Sometimes ... they consider Candida [to represent] colonization so they will often not speciate it.” She emphasized the need for facilities that care for ventilated patients to consider screening. “Higher priority ... are places in areas where there’s a lot of C. auris transmission or in nearby areas that are likely to get introductions.” Even those that do speciate may have difficulty identifying C. auris.
Further, Dr. Lyman stressed “the importance of antifungal susceptibility testing and testing for resistance. Because that’s also something that’s not widely available at all hospitals and clinical labs ... you can send it to the [CDC’s] antimicrobial resistance lab network” for testing.
COVID-19 has brought particular challenges. Rodney E. Rohde, PhD, MS, professor and chair, clinical lab science program, Texas State University, San Marcos, said in an interview that he is worried about all the steroids and broad-spectrum antibiotics patients receive.
They’re “being given medical interventions, whether it’s ventilators or [extracorporeal membrane oxygenation] or IVs or central lines or catheters for UTIs and you’re creating highways, right for something that may be right there,” said Dr. Rohde, who was not involved in the CDC study. “It’s a perfect storm, not just for C. auris, but I worry about bacterial resistance agents, too, like MRSA and so forth, having kind of a spike in those types of infections with COVID. So, it’s kind of a doubly dangerous time, I think.”
Multiresistant bacteria are a major health problem, causing illnesses in 2.8 million people annually in the United States, and causing about 35,000 deaths.
Dr. Rohde raised another, rarely mentioned concern. “We’re in crisis mode. People are leaving our field more than they ever had before. The medical laboratory is being decimated because people have burned out after these past 14 months. And so I worry just about competent medical laboratory professionals that are on board to deal with these types of other crises that are popping up within hospitals and long-term care facilities. It kind of keeps me awake.”
Dr. Rohde and Dr. Lyman shared their concern that COVID caused a decrease in screening for other infections and drug-resistant organisms. Bare-bones staffing and shortages of personal protective equipment have likely fueled the spread of these infections as well.
In an outbreak of C. auris in a Florida hospital’s COVID unit in 2020, 35 of 67 patients became colonized, and 6 became ill. The epidemiologists investigating thought that contaminated gowns or gloves, computers, and other equipment were likely sources of transmission.
Low pay, especially in nursing homes, is another problem Dr. Rohde mentioned. It’s an additional problem in both acute and long-term care that “some of the lowest-paid people are the environmental services people, and so the turnover is crazy.” Yet, we rely on them to keep everyone safe. He added that, in addition to pay, he “tries to give them the appreciation and the recognition that they really deserve.”
There are a few specific measures that can be taken to protect patients. Dr. Lyman concluded. “The best way is identifying cases and really ensuring good infection control to prevent the spread.” It’s back to basics – limiting broad-spectrum antibiotics and invasive medical devices, and especially good handwashing and thorough cleaning.
Dr. Lyman and Dr. Rohde have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Midodrine reduces fainting in young patients
.
Vasovagal syncope is the most common cause of fainting and is often triggered by dehydration and upright posture, according to Johns Hopkins Medicine. But it can also be caused by stimuli like the sight of blood or sudden emotional distress. The stimulus causes the heart rate and blood pressure to drop rapidly, according to the Mayo Clinic.
The randomized, placebo-controlled, double-blind trial included 133 patients with recurrent vasovagal syncope and was published in Annals of Internal Medicine. Those that received midodrine were less likely to have one syncope episode (28 of 66 [42%]), compared with those that took the placebo (41 of 67 [61%]). The absolute risk reduction for vasovagal syncope was 19 percentage points (95% confidence interval, 2-36 percentage points).
The study included patients from 25 university hospitals in Canada, the United States, Mexico, and the United Kingdom, who were followed for 12 months. The trial participants were highly symptomatic for vasovagal syncope, having experienced a median of 23 episodes in their lifetime and 5 syncope episodes in the last year, and they had no comorbid conditions.
“We don’t have many arrows in our quiver,” said Robert Sheldon, MD, PhD, a cardiologist at University of Calgary (Alta.) and lead author of the study, referring to the lack of evidence-based treatments for syncope.
For 20 years Dr. Sheldon’s lab has been testing drugs that showed some potential. While a previous study of fludrocortisone (Florinef ) showed some benefit, “[midodrine] was the first to be unequivocally, slam-dunk positive,” he said in an interview.
Earlier trials of midodrine
Other studies have shown midodrine to prevent syncope on tilt tests. There have been two randomized trials where midodrine significantly reduced vasovagal syncope, but one of these was short and in children, and the other one was open label with no placebo control.
“Risk reduction was very high in previous studies,” Dr. Sheldon said in an interview. But, because they were open label, there was a huge placebo effect, he noted.
“There were no adequately done, adequately powered [studies] that have been positive,” Dr. Sheldon added.
New study methods and outcomes
The study published in Annals of Internal Medicine this week included patients over 18 years of age with a Calgary Syncope Symptom Score of at least 2. All were educated on lifestyle measures that can prevent syncopes before beginning to take 5 mg of study drug or placebo three times daily, 4 hours apart.
In these cases, the study authors wrote, “taking medication three times a day seems worth the effort.” But in patients with a lower frequency of episodes, midodrine might not have an adequate payoff.
These results are “impressive,” said Roopinder K. Sandhu, MD, MPH, clinical electrophysiologist at Cedar-Sinai in Los Angeles. “This study demonstrated that midodrine is the first medical therapy, in addition to education and lifestyle measures, to unequivocally pass the scrutiny of an international, placebo-controlled, RCT to show a significant reduction in syncope recurrence in a younger population with frequent syncope events.”
“[Taking midodrine] doesn’t carry the long-term consequence of pacemakers,” she added.
Study limitations
Limitations of the new study include its small size and short observation period, the authors wrote. Additionally, a large proportion of patients enrolled were also from a single center in Calgary that specializes in syncope care. Twenty-seven patients in the trial stopped taking their assigned medication during the year observation period, but the authors concluded these participants “likely would bias the results against midodrine.”
For doctors considering midodrine for their patients, it’s critical to confirm the diagnosis and to try patient education first, Dr. Sheldon advised.
Lifestyle factors like hydration, adequate sodium intake, and squatting or lying down when the syncope is coming on can sufficiently suppress syncopes in two-thirds of patients, he noted.
This is a treatment for young people, Dr. Sandhu said. The median age in the trial was 35, so patients taking midodrine should be younger than 50. Midodrine is also not effective in patients with high blood pressure or heart failure, she said.
“[Midodrine] is easy to use but kind of a pain at first,” Dr. Sheldon noted. Every patient should start out taking 5 mg doses, three times a day – during waking hours. But then you have to adjust the dosage, “and it’s tricky,” he said.
If a patient experiences goosebumps or the sensation of worms crawling in the hair, the dose might be too much, Dr. Sheldon noted.
If the patient is still fainting, first consider when they are fainting, he said. If it’s around the time they should take another dose, it might be trough effect.
Dr. Sandhu was not involved in the study, but Cedar Sinai was a participating center, and she considers Dr. Sheldon to be a mentor. Dr. Sandhu also noted that she has published papers with Dr. Sheldon, who reported no conflicts.
.
Vasovagal syncope is the most common cause of fainting and is often triggered by dehydration and upright posture, according to Johns Hopkins Medicine. But it can also be caused by stimuli like the sight of blood or sudden emotional distress. The stimulus causes the heart rate and blood pressure to drop rapidly, according to the Mayo Clinic.
The randomized, placebo-controlled, double-blind trial included 133 patients with recurrent vasovagal syncope and was published in Annals of Internal Medicine. Those that received midodrine were less likely to have one syncope episode (28 of 66 [42%]), compared with those that took the placebo (41 of 67 [61%]). The absolute risk reduction for vasovagal syncope was 19 percentage points (95% confidence interval, 2-36 percentage points).
The study included patients from 25 university hospitals in Canada, the United States, Mexico, and the United Kingdom, who were followed for 12 months. The trial participants were highly symptomatic for vasovagal syncope, having experienced a median of 23 episodes in their lifetime and 5 syncope episodes in the last year, and they had no comorbid conditions.
“We don’t have many arrows in our quiver,” said Robert Sheldon, MD, PhD, a cardiologist at University of Calgary (Alta.) and lead author of the study, referring to the lack of evidence-based treatments for syncope.
For 20 years Dr. Sheldon’s lab has been testing drugs that showed some potential. While a previous study of fludrocortisone (Florinef ) showed some benefit, “[midodrine] was the first to be unequivocally, slam-dunk positive,” he said in an interview.
Earlier trials of midodrine
Other studies have shown midodrine to prevent syncope on tilt tests. There have been two randomized trials where midodrine significantly reduced vasovagal syncope, but one of these was short and in children, and the other one was open label with no placebo control.
“Risk reduction was very high in previous studies,” Dr. Sheldon said in an interview. But, because they were open label, there was a huge placebo effect, he noted.
“There were no adequately done, adequately powered [studies] that have been positive,” Dr. Sheldon added.
New study methods and outcomes
The study published in Annals of Internal Medicine this week included patients over 18 years of age with a Calgary Syncope Symptom Score of at least 2. All were educated on lifestyle measures that can prevent syncopes before beginning to take 5 mg of study drug or placebo three times daily, 4 hours apart.
In these cases, the study authors wrote, “taking medication three times a day seems worth the effort.” But in patients with a lower frequency of episodes, midodrine might not have an adequate payoff.
These results are “impressive,” said Roopinder K. Sandhu, MD, MPH, clinical electrophysiologist at Cedar-Sinai in Los Angeles. “This study demonstrated that midodrine is the first medical therapy, in addition to education and lifestyle measures, to unequivocally pass the scrutiny of an international, placebo-controlled, RCT to show a significant reduction in syncope recurrence in a younger population with frequent syncope events.”
“[Taking midodrine] doesn’t carry the long-term consequence of pacemakers,” she added.
Study limitations
Limitations of the new study include its small size and short observation period, the authors wrote. Additionally, a large proportion of patients enrolled were also from a single center in Calgary that specializes in syncope care. Twenty-seven patients in the trial stopped taking their assigned medication during the year observation period, but the authors concluded these participants “likely would bias the results against midodrine.”
For doctors considering midodrine for their patients, it’s critical to confirm the diagnosis and to try patient education first, Dr. Sheldon advised.
Lifestyle factors like hydration, adequate sodium intake, and squatting or lying down when the syncope is coming on can sufficiently suppress syncopes in two-thirds of patients, he noted.
This is a treatment for young people, Dr. Sandhu said. The median age in the trial was 35, so patients taking midodrine should be younger than 50. Midodrine is also not effective in patients with high blood pressure or heart failure, she said.
“[Midodrine] is easy to use but kind of a pain at first,” Dr. Sheldon noted. Every patient should start out taking 5 mg doses, three times a day – during waking hours. But then you have to adjust the dosage, “and it’s tricky,” he said.
If a patient experiences goosebumps or the sensation of worms crawling in the hair, the dose might be too much, Dr. Sheldon noted.
If the patient is still fainting, first consider when they are fainting, he said. If it’s around the time they should take another dose, it might be trough effect.
Dr. Sandhu was not involved in the study, but Cedar Sinai was a participating center, and she considers Dr. Sheldon to be a mentor. Dr. Sandhu also noted that she has published papers with Dr. Sheldon, who reported no conflicts.
.
Vasovagal syncope is the most common cause of fainting and is often triggered by dehydration and upright posture, according to Johns Hopkins Medicine. But it can also be caused by stimuli like the sight of blood or sudden emotional distress. The stimulus causes the heart rate and blood pressure to drop rapidly, according to the Mayo Clinic.
The randomized, placebo-controlled, double-blind trial included 133 patients with recurrent vasovagal syncope and was published in Annals of Internal Medicine. Those that received midodrine were less likely to have one syncope episode (28 of 66 [42%]), compared with those that took the placebo (41 of 67 [61%]). The absolute risk reduction for vasovagal syncope was 19 percentage points (95% confidence interval, 2-36 percentage points).
The study included patients from 25 university hospitals in Canada, the United States, Mexico, and the United Kingdom, who were followed for 12 months. The trial participants were highly symptomatic for vasovagal syncope, having experienced a median of 23 episodes in their lifetime and 5 syncope episodes in the last year, and they had no comorbid conditions.
“We don’t have many arrows in our quiver,” said Robert Sheldon, MD, PhD, a cardiologist at University of Calgary (Alta.) and lead author of the study, referring to the lack of evidence-based treatments for syncope.
For 20 years Dr. Sheldon’s lab has been testing drugs that showed some potential. While a previous study of fludrocortisone (Florinef ) showed some benefit, “[midodrine] was the first to be unequivocally, slam-dunk positive,” he said in an interview.
Earlier trials of midodrine
Other studies have shown midodrine to prevent syncope on tilt tests. There have been two randomized trials where midodrine significantly reduced vasovagal syncope, but one of these was short and in children, and the other one was open label with no placebo control.
“Risk reduction was very high in previous studies,” Dr. Sheldon said in an interview. But, because they were open label, there was a huge placebo effect, he noted.
“There were no adequately done, adequately powered [studies] that have been positive,” Dr. Sheldon added.
New study methods and outcomes
The study published in Annals of Internal Medicine this week included patients over 18 years of age with a Calgary Syncope Symptom Score of at least 2. All were educated on lifestyle measures that can prevent syncopes before beginning to take 5 mg of study drug or placebo three times daily, 4 hours apart.
In these cases, the study authors wrote, “taking medication three times a day seems worth the effort.” But in patients with a lower frequency of episodes, midodrine might not have an adequate payoff.
These results are “impressive,” said Roopinder K. Sandhu, MD, MPH, clinical electrophysiologist at Cedar-Sinai in Los Angeles. “This study demonstrated that midodrine is the first medical therapy, in addition to education and lifestyle measures, to unequivocally pass the scrutiny of an international, placebo-controlled, RCT to show a significant reduction in syncope recurrence in a younger population with frequent syncope events.”
“[Taking midodrine] doesn’t carry the long-term consequence of pacemakers,” she added.
Study limitations
Limitations of the new study include its small size and short observation period, the authors wrote. Additionally, a large proportion of patients enrolled were also from a single center in Calgary that specializes in syncope care. Twenty-seven patients in the trial stopped taking their assigned medication during the year observation period, but the authors concluded these participants “likely would bias the results against midodrine.”
For doctors considering midodrine for their patients, it’s critical to confirm the diagnosis and to try patient education first, Dr. Sheldon advised.
Lifestyle factors like hydration, adequate sodium intake, and squatting or lying down when the syncope is coming on can sufficiently suppress syncopes in two-thirds of patients, he noted.
This is a treatment for young people, Dr. Sandhu said. The median age in the trial was 35, so patients taking midodrine should be younger than 50. Midodrine is also not effective in patients with high blood pressure or heart failure, she said.
“[Midodrine] is easy to use but kind of a pain at first,” Dr. Sheldon noted. Every patient should start out taking 5 mg doses, three times a day – during waking hours. But then you have to adjust the dosage, “and it’s tricky,” he said.
If a patient experiences goosebumps or the sensation of worms crawling in the hair, the dose might be too much, Dr. Sheldon noted.
If the patient is still fainting, first consider when they are fainting, he said. If it’s around the time they should take another dose, it might be trough effect.
Dr. Sandhu was not involved in the study, but Cedar Sinai was a participating center, and she considers Dr. Sheldon to be a mentor. Dr. Sandhu also noted that she has published papers with Dr. Sheldon, who reported no conflicts.
FROM ANNALS OF INTERNAL MEDICINE
Increases in new COVID cases among children far outpace vaccinations
New COVID-19 cases in children soared by almost 86% over the course of just 1 week, while the number of 12- to 17-year-old children who have received at least one dose of vaccine rose by 5.4%, according to two separate sources.
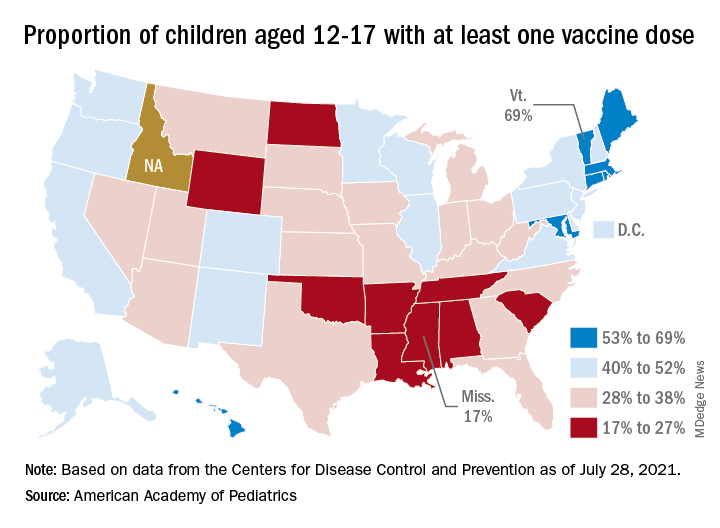
Meanwhile, the increase over the past 2 weeks – from 23,551 new cases for July 16-22 to almost 72,000 – works out to almost 205%, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
Children represented 19.0% of the cases reported during the week of July 23-29, and they have made up 14.3% of all cases since the pandemic began, with the total number of cases in children now approaching 4.2 million, the AAP and CHA said in their weekly COVID report. About 22% of the U.S. population is under the age of 18 years.
As of Aug. 2, just over 9.8 million children aged 12-17 years had received at least one dose of the COVID vaccine, which was up by about 500,000, or 5.4%, from a week earlier, based on data from the Centers for Disease Control and Prevention.
Children aged 16-17 have reached a notable milestone on the journey that started with vaccine approval in December: 50.2% have gotten at least one dose and 40.3% are fully vaccinated. Among children aged 12-15 years, the proportion with at least one dose of vaccine is up to 39.5%, compared with 37.1% the previous week, while 29.0% are fully vaccinated (27.8% the week before), the CDC said on its COVID Data Tracker.
The national rates for child vaccination, however, tend to hide the disparities between states. There is a gap between Mississippi (lowest), where just 17% of children aged 12-17 years have gotten at least one dose, and Vermont (highest), which is up to 69%. Vermont also has the highest rate of vaccine completion (60%), while Alabama and Mississippi have the lowest (10%), according to a solo report from the AAP.
New COVID-19 cases in children soared by almost 86% over the course of just 1 week, while the number of 12- to 17-year-old children who have received at least one dose of vaccine rose by 5.4%, according to two separate sources.

Meanwhile, the increase over the past 2 weeks – from 23,551 new cases for July 16-22 to almost 72,000 – works out to almost 205%, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
Children represented 19.0% of the cases reported during the week of July 23-29, and they have made up 14.3% of all cases since the pandemic began, with the total number of cases in children now approaching 4.2 million, the AAP and CHA said in their weekly COVID report. About 22% of the U.S. population is under the age of 18 years.
As of Aug. 2, just over 9.8 million children aged 12-17 years had received at least one dose of the COVID vaccine, which was up by about 500,000, or 5.4%, from a week earlier, based on data from the Centers for Disease Control and Prevention.
Children aged 16-17 have reached a notable milestone on the journey that started with vaccine approval in December: 50.2% have gotten at least one dose and 40.3% are fully vaccinated. Among children aged 12-15 years, the proportion with at least one dose of vaccine is up to 39.5%, compared with 37.1% the previous week, while 29.0% are fully vaccinated (27.8% the week before), the CDC said on its COVID Data Tracker.
The national rates for child vaccination, however, tend to hide the disparities between states. There is a gap between Mississippi (lowest), where just 17% of children aged 12-17 years have gotten at least one dose, and Vermont (highest), which is up to 69%. Vermont also has the highest rate of vaccine completion (60%), while Alabama and Mississippi have the lowest (10%), according to a solo report from the AAP.
New COVID-19 cases in children soared by almost 86% over the course of just 1 week, while the number of 12- to 17-year-old children who have received at least one dose of vaccine rose by 5.4%, according to two separate sources.

Meanwhile, the increase over the past 2 weeks – from 23,551 new cases for July 16-22 to almost 72,000 – works out to almost 205%, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
Children represented 19.0% of the cases reported during the week of July 23-29, and they have made up 14.3% of all cases since the pandemic began, with the total number of cases in children now approaching 4.2 million, the AAP and CHA said in their weekly COVID report. About 22% of the U.S. population is under the age of 18 years.
As of Aug. 2, just over 9.8 million children aged 12-17 years had received at least one dose of the COVID vaccine, which was up by about 500,000, or 5.4%, from a week earlier, based on data from the Centers for Disease Control and Prevention.
Children aged 16-17 have reached a notable milestone on the journey that started with vaccine approval in December: 50.2% have gotten at least one dose and 40.3% are fully vaccinated. Among children aged 12-15 years, the proportion with at least one dose of vaccine is up to 39.5%, compared with 37.1% the previous week, while 29.0% are fully vaccinated (27.8% the week before), the CDC said on its COVID Data Tracker.
The national rates for child vaccination, however, tend to hide the disparities between states. There is a gap between Mississippi (lowest), where just 17% of children aged 12-17 years have gotten at least one dose, and Vermont (highest), which is up to 69%. Vermont also has the highest rate of vaccine completion (60%), while Alabama and Mississippi have the lowest (10%), according to a solo report from the AAP.