User login
Reckoning with America’s alarming rise in anti-Asian hate
On March 16, the world was witness to a horrific act of violence when a gunman killed six Asian American women and two others at spas in the Atlanta, Georgia area. The attack prompted a national outcry and protests against the rising levels of hate and violence directed at Asian Americans and Pacific Islanders (AAPI), a community that has experienced a profound and disturbing legacy of racism in American history.
Despite this fact, my own understanding and awareness of the hate and racism experienced by the AAPI community, then and now, would be described as limited at best. Was I aware on some level? Perhaps. But if I’m being honest, I have not fully appreciated the unique experiences of AAPI colleagues, friends, and students.
That changed when I attended a White Coats Against Asian Hate & Racism rally, held by the George Washington University School of Medicine and Health Sciences 2 months after the Atlanta killings. Hearing my colleagues speak of their personal experiences, I quickly realized my lack of education on the subject of how systemic racism has long affected Asian Americans in this country.
Measuring the alarming rise in anti-Asian hate
The data supporting a rise in anti-Asian hate crimes have been staring us in the face for decades but have drawn increasing attention since the beginning of the COVID-19 pandemic, when these already distressingly high numbers experienced a steep rise.
Before looking at these figures, though, we must begin by defining what is considered a hate crime versus a hate incident. The National Asian Pacific American Bar Association and Asian & Pacific Islander American Health Forum have produced a beneficial summary document on precisely what separates these terms:
- A hate crime is a crime committed on the basis of the victim’s perceived or actual race, color, religion, national origin, sexual orientation, gender identity, or disability. It differs from “regular” crime in that its victims include the immediate crime target and others like them. Hate crimes affect families, communities, and, at times, an entire nation.
- A hate incident describes acts of prejudice that are not crimes and do not involve violence, threats, or property damage. The most common examples are isolated forms of speech, such as racial slurs.
Stop AAPI Hate (SAH) was founded in March 2020 as a coalition to track and analyze incidents of hate against this community. SAH’s 2020-2021 national report details 3,795 hate incidents that occurred from March 19, 2020, to Feb. 28, 2021. In a notable parallel to the Georgia killings, SAH found that Asian American women reported hate incidents 2.3 times more often than men and that businesses were the primary site of discrimination.
This rise in hate incidents has occurred in parallel with an increase in Asian American hate crimes. Recently, the Center for the Study of Hate and Extremism (CSUSB) released its Report to the Nation: Anti-Asian Prejudice & Hate Crime. I re-read that data point multiple times, thinking it must be in error. If you’re asking exactly why I was having difficulty accepting this data, you have to appreciate these two critical points:
- Per the CSUSB, anti-Asian hate crimes were already surging by 146% in 2020.
- This surge occurred while overall hate crimes dropped by 7%.
So, if 2020 was a surge, the first quarter of 2021 is a hurricane. What’s perhaps most concerning is that these data only capture reported cases and therefore are a fraction of the total.
Undoubtedly, we are living through an unprecedented rise in anti-Asian hate incidents and hate crimes since the start of the pandemic. This rise in hate-related events paralleled the many pandemic-related stressors (disease, isolation, economics, mental health, etc.). Should anyone have been surprised when this most recent deadly spike of anti-Asian hate occurred in the first quarter of 2021?
Hate’s toll on mental health
As a psychiatrist, I’ve spent my entire career working with dedicated teams to treat patients with mental health disorders. Currently, hate is not classified by the Diagnostic and Statistical Manual of Mental Disorders as a mental illness. However, I can’t think of another emotion that is a better candidate for further research and scientific instigation, if for no other reason than to better understand when prejudice and bias transform into hatred and crime.
Surprisingly, there has been relatively little research on the topic of hate in the fields of psychology and psychiatry. I’d be willing to wager that if you asked a typical graduating class of medical students to give you an actual working definition of the emotion of hate, most would be at a loss for words.
Dr. Fischer and Dr. Halperin published a helpful article that gives a functional perspective on hate. The authors cover a great deal of research on hate and offer the following four starting points valuable in considering it:
- “Hate is different from anger because an anger target is appraised as someone whose behavior can be influenced and changed.”
- “A hate target, on the contrary, implies appraisals of the other’s malevolent nature and malicious intent.”
- “Hate is characterized by appraisals that imply a stable perception of a person or group and thus the incapability to change the extremely negative characteristics attributed to the target of hate.”
- “Everyday observations also suggest that hate is so powerful that it does, not just temporarily but permanently, destroy relations between individuals or groups.”
When I view hate with these insights in mind, it completely changes how I choose to utilize the word or concept. Hate is an emotion whose goal/action tendency is to eliminate groups (not just people or obstacles) and destroy any current or future relationships. We can take this a step further in noting that hate spreads, not only to the intended targets but potentially my “own” group. Similar to secondhand smoke, there is no risk-free exposure to hate or racism.
In the past decade, a robust body of evidence has emerged that clearly illustrates the negative health impacts of racism. Dr. Paradies and colleagues performed a systematic meta-analysis explicitly focused on racism as a determinant of health, finding that it was associated with poorer mental health, including depression, anxiety, and psychological distress. Over the past two decades, researchers have increasingly looked at the effects of racial discrimination on the AAPI community. In their 2009 review article, Dr. Gee and colleagues identified 62 empirical articles assessing the relation between discrimination and health among Asian Americans. Most of the studies found that discrimination was associated with poorer health. Of the 40 studies focused on mental health, 37 reported that discrimination was associated with poorer outcomes.
SAH recently released its very illuminating Mental Health Report. Among several key findings, two in particular caught my attention. First, Asian Americans who have experienced racism are more stressed by anti-Asian hate than the pandemic itself. Second, one in five Asian Americans who have experienced racism display racial trauma, the psychological and emotional harm caused by racism. Given the rise in hate crimes, there must be concern regarding the level of trauma being inflicted upon the Asian American community.
A complete review of the health effects of racism is beyond this article’s scope. Still, the previously mentioned studies further support the need to treat racism in general, and specifically anti-Asian hate, as the urgent public health concern that it truly is. The U.S. government recently outlined an action plan to respond to anti-Asian violence, xenophobia, and bias. These are helpful first steps, but much more is required on a societal and individual level, given the mental health disparities faced by the AAPI community.
Determining the best ways to address this urgent public health concern can be overwhelming, exhausting, and outright demoralizing. The bottom line is that if we do nothing, communities and groups will continue to suffer the effects of racial hatred. These consequences are severe and transgenerational.
But we must start somewhere. For me, that begins by gaining a better understanding of the emotion of hate and my role in either facilitating or stopping it, and by listening, listening, and listening some more to AAPI colleagues, friends, and family about their lived experience with anti-Asian hate.
A version of this article first appeared on Medscape.com.
On March 16, the world was witness to a horrific act of violence when a gunman killed six Asian American women and two others at spas in the Atlanta, Georgia area. The attack prompted a national outcry and protests against the rising levels of hate and violence directed at Asian Americans and Pacific Islanders (AAPI), a community that has experienced a profound and disturbing legacy of racism in American history.
Despite this fact, my own understanding and awareness of the hate and racism experienced by the AAPI community, then and now, would be described as limited at best. Was I aware on some level? Perhaps. But if I’m being honest, I have not fully appreciated the unique experiences of AAPI colleagues, friends, and students.
That changed when I attended a White Coats Against Asian Hate & Racism rally, held by the George Washington University School of Medicine and Health Sciences 2 months after the Atlanta killings. Hearing my colleagues speak of their personal experiences, I quickly realized my lack of education on the subject of how systemic racism has long affected Asian Americans in this country.
Measuring the alarming rise in anti-Asian hate
The data supporting a rise in anti-Asian hate crimes have been staring us in the face for decades but have drawn increasing attention since the beginning of the COVID-19 pandemic, when these already distressingly high numbers experienced a steep rise.
Before looking at these figures, though, we must begin by defining what is considered a hate crime versus a hate incident. The National Asian Pacific American Bar Association and Asian & Pacific Islander American Health Forum have produced a beneficial summary document on precisely what separates these terms:
- A hate crime is a crime committed on the basis of the victim’s perceived or actual race, color, religion, national origin, sexual orientation, gender identity, or disability. It differs from “regular” crime in that its victims include the immediate crime target and others like them. Hate crimes affect families, communities, and, at times, an entire nation.
- A hate incident describes acts of prejudice that are not crimes and do not involve violence, threats, or property damage. The most common examples are isolated forms of speech, such as racial slurs.
Stop AAPI Hate (SAH) was founded in March 2020 as a coalition to track and analyze incidents of hate against this community. SAH’s 2020-2021 national report details 3,795 hate incidents that occurred from March 19, 2020, to Feb. 28, 2021. In a notable parallel to the Georgia killings, SAH found that Asian American women reported hate incidents 2.3 times more often than men and that businesses were the primary site of discrimination.
This rise in hate incidents has occurred in parallel with an increase in Asian American hate crimes. Recently, the Center for the Study of Hate and Extremism (CSUSB) released its Report to the Nation: Anti-Asian Prejudice & Hate Crime. I re-read that data point multiple times, thinking it must be in error. If you’re asking exactly why I was having difficulty accepting this data, you have to appreciate these two critical points:
- Per the CSUSB, anti-Asian hate crimes were already surging by 146% in 2020.
- This surge occurred while overall hate crimes dropped by 7%.
So, if 2020 was a surge, the first quarter of 2021 is a hurricane. What’s perhaps most concerning is that these data only capture reported cases and therefore are a fraction of the total.
Undoubtedly, we are living through an unprecedented rise in anti-Asian hate incidents and hate crimes since the start of the pandemic. This rise in hate-related events paralleled the many pandemic-related stressors (disease, isolation, economics, mental health, etc.). Should anyone have been surprised when this most recent deadly spike of anti-Asian hate occurred in the first quarter of 2021?
Hate’s toll on mental health
As a psychiatrist, I’ve spent my entire career working with dedicated teams to treat patients with mental health disorders. Currently, hate is not classified by the Diagnostic and Statistical Manual of Mental Disorders as a mental illness. However, I can’t think of another emotion that is a better candidate for further research and scientific instigation, if for no other reason than to better understand when prejudice and bias transform into hatred and crime.
Surprisingly, there has been relatively little research on the topic of hate in the fields of psychology and psychiatry. I’d be willing to wager that if you asked a typical graduating class of medical students to give you an actual working definition of the emotion of hate, most would be at a loss for words.
Dr. Fischer and Dr. Halperin published a helpful article that gives a functional perspective on hate. The authors cover a great deal of research on hate and offer the following four starting points valuable in considering it:
- “Hate is different from anger because an anger target is appraised as someone whose behavior can be influenced and changed.”
- “A hate target, on the contrary, implies appraisals of the other’s malevolent nature and malicious intent.”
- “Hate is characterized by appraisals that imply a stable perception of a person or group and thus the incapability to change the extremely negative characteristics attributed to the target of hate.”
- “Everyday observations also suggest that hate is so powerful that it does, not just temporarily but permanently, destroy relations between individuals or groups.”
When I view hate with these insights in mind, it completely changes how I choose to utilize the word or concept. Hate is an emotion whose goal/action tendency is to eliminate groups (not just people or obstacles) and destroy any current or future relationships. We can take this a step further in noting that hate spreads, not only to the intended targets but potentially my “own” group. Similar to secondhand smoke, there is no risk-free exposure to hate or racism.
In the past decade, a robust body of evidence has emerged that clearly illustrates the negative health impacts of racism. Dr. Paradies and colleagues performed a systematic meta-analysis explicitly focused on racism as a determinant of health, finding that it was associated with poorer mental health, including depression, anxiety, and psychological distress. Over the past two decades, researchers have increasingly looked at the effects of racial discrimination on the AAPI community. In their 2009 review article, Dr. Gee and colleagues identified 62 empirical articles assessing the relation between discrimination and health among Asian Americans. Most of the studies found that discrimination was associated with poorer health. Of the 40 studies focused on mental health, 37 reported that discrimination was associated with poorer outcomes.
SAH recently released its very illuminating Mental Health Report. Among several key findings, two in particular caught my attention. First, Asian Americans who have experienced racism are more stressed by anti-Asian hate than the pandemic itself. Second, one in five Asian Americans who have experienced racism display racial trauma, the psychological and emotional harm caused by racism. Given the rise in hate crimes, there must be concern regarding the level of trauma being inflicted upon the Asian American community.
A complete review of the health effects of racism is beyond this article’s scope. Still, the previously mentioned studies further support the need to treat racism in general, and specifically anti-Asian hate, as the urgent public health concern that it truly is. The U.S. government recently outlined an action plan to respond to anti-Asian violence, xenophobia, and bias. These are helpful first steps, but much more is required on a societal and individual level, given the mental health disparities faced by the AAPI community.
Determining the best ways to address this urgent public health concern can be overwhelming, exhausting, and outright demoralizing. The bottom line is that if we do nothing, communities and groups will continue to suffer the effects of racial hatred. These consequences are severe and transgenerational.
But we must start somewhere. For me, that begins by gaining a better understanding of the emotion of hate and my role in either facilitating or stopping it, and by listening, listening, and listening some more to AAPI colleagues, friends, and family about their lived experience with anti-Asian hate.
A version of this article first appeared on Medscape.com.
On March 16, the world was witness to a horrific act of violence when a gunman killed six Asian American women and two others at spas in the Atlanta, Georgia area. The attack prompted a national outcry and protests against the rising levels of hate and violence directed at Asian Americans and Pacific Islanders (AAPI), a community that has experienced a profound and disturbing legacy of racism in American history.
Despite this fact, my own understanding and awareness of the hate and racism experienced by the AAPI community, then and now, would be described as limited at best. Was I aware on some level? Perhaps. But if I’m being honest, I have not fully appreciated the unique experiences of AAPI colleagues, friends, and students.
That changed when I attended a White Coats Against Asian Hate & Racism rally, held by the George Washington University School of Medicine and Health Sciences 2 months after the Atlanta killings. Hearing my colleagues speak of their personal experiences, I quickly realized my lack of education on the subject of how systemic racism has long affected Asian Americans in this country.
Measuring the alarming rise in anti-Asian hate
The data supporting a rise in anti-Asian hate crimes have been staring us in the face for decades but have drawn increasing attention since the beginning of the COVID-19 pandemic, when these already distressingly high numbers experienced a steep rise.
Before looking at these figures, though, we must begin by defining what is considered a hate crime versus a hate incident. The National Asian Pacific American Bar Association and Asian & Pacific Islander American Health Forum have produced a beneficial summary document on precisely what separates these terms:
- A hate crime is a crime committed on the basis of the victim’s perceived or actual race, color, religion, national origin, sexual orientation, gender identity, or disability. It differs from “regular” crime in that its victims include the immediate crime target and others like them. Hate crimes affect families, communities, and, at times, an entire nation.
- A hate incident describes acts of prejudice that are not crimes and do not involve violence, threats, or property damage. The most common examples are isolated forms of speech, such as racial slurs.
Stop AAPI Hate (SAH) was founded in March 2020 as a coalition to track and analyze incidents of hate against this community. SAH’s 2020-2021 national report details 3,795 hate incidents that occurred from March 19, 2020, to Feb. 28, 2021. In a notable parallel to the Georgia killings, SAH found that Asian American women reported hate incidents 2.3 times more often than men and that businesses were the primary site of discrimination.
This rise in hate incidents has occurred in parallel with an increase in Asian American hate crimes. Recently, the Center for the Study of Hate and Extremism (CSUSB) released its Report to the Nation: Anti-Asian Prejudice & Hate Crime. I re-read that data point multiple times, thinking it must be in error. If you’re asking exactly why I was having difficulty accepting this data, you have to appreciate these two critical points:
- Per the CSUSB, anti-Asian hate crimes were already surging by 146% in 2020.
- This surge occurred while overall hate crimes dropped by 7%.
So, if 2020 was a surge, the first quarter of 2021 is a hurricane. What’s perhaps most concerning is that these data only capture reported cases and therefore are a fraction of the total.
Undoubtedly, we are living through an unprecedented rise in anti-Asian hate incidents and hate crimes since the start of the pandemic. This rise in hate-related events paralleled the many pandemic-related stressors (disease, isolation, economics, mental health, etc.). Should anyone have been surprised when this most recent deadly spike of anti-Asian hate occurred in the first quarter of 2021?
Hate’s toll on mental health
As a psychiatrist, I’ve spent my entire career working with dedicated teams to treat patients with mental health disorders. Currently, hate is not classified by the Diagnostic and Statistical Manual of Mental Disorders as a mental illness. However, I can’t think of another emotion that is a better candidate for further research and scientific instigation, if for no other reason than to better understand when prejudice and bias transform into hatred and crime.
Surprisingly, there has been relatively little research on the topic of hate in the fields of psychology and psychiatry. I’d be willing to wager that if you asked a typical graduating class of medical students to give you an actual working definition of the emotion of hate, most would be at a loss for words.
Dr. Fischer and Dr. Halperin published a helpful article that gives a functional perspective on hate. The authors cover a great deal of research on hate and offer the following four starting points valuable in considering it:
- “Hate is different from anger because an anger target is appraised as someone whose behavior can be influenced and changed.”
- “A hate target, on the contrary, implies appraisals of the other’s malevolent nature and malicious intent.”
- “Hate is characterized by appraisals that imply a stable perception of a person or group and thus the incapability to change the extremely negative characteristics attributed to the target of hate.”
- “Everyday observations also suggest that hate is so powerful that it does, not just temporarily but permanently, destroy relations between individuals or groups.”
When I view hate with these insights in mind, it completely changes how I choose to utilize the word or concept. Hate is an emotion whose goal/action tendency is to eliminate groups (not just people or obstacles) and destroy any current or future relationships. We can take this a step further in noting that hate spreads, not only to the intended targets but potentially my “own” group. Similar to secondhand smoke, there is no risk-free exposure to hate or racism.
In the past decade, a robust body of evidence has emerged that clearly illustrates the negative health impacts of racism. Dr. Paradies and colleagues performed a systematic meta-analysis explicitly focused on racism as a determinant of health, finding that it was associated with poorer mental health, including depression, anxiety, and psychological distress. Over the past two decades, researchers have increasingly looked at the effects of racial discrimination on the AAPI community. In their 2009 review article, Dr. Gee and colleagues identified 62 empirical articles assessing the relation between discrimination and health among Asian Americans. Most of the studies found that discrimination was associated with poorer health. Of the 40 studies focused on mental health, 37 reported that discrimination was associated with poorer outcomes.
SAH recently released its very illuminating Mental Health Report. Among several key findings, two in particular caught my attention. First, Asian Americans who have experienced racism are more stressed by anti-Asian hate than the pandemic itself. Second, one in five Asian Americans who have experienced racism display racial trauma, the psychological and emotional harm caused by racism. Given the rise in hate crimes, there must be concern regarding the level of trauma being inflicted upon the Asian American community.
A complete review of the health effects of racism is beyond this article’s scope. Still, the previously mentioned studies further support the need to treat racism in general, and specifically anti-Asian hate, as the urgent public health concern that it truly is. The U.S. government recently outlined an action plan to respond to anti-Asian violence, xenophobia, and bias. These are helpful first steps, but much more is required on a societal and individual level, given the mental health disparities faced by the AAPI community.
Determining the best ways to address this urgent public health concern can be overwhelming, exhausting, and outright demoralizing. The bottom line is that if we do nothing, communities and groups will continue to suffer the effects of racial hatred. These consequences are severe and transgenerational.
But we must start somewhere. For me, that begins by gaining a better understanding of the emotion of hate and my role in either facilitating or stopping it, and by listening, listening, and listening some more to AAPI colleagues, friends, and family about their lived experience with anti-Asian hate.
A version of this article first appeared on Medscape.com.
Certain gut bacteria tied to lower risk of diabetes
Having more diverse gut bacteria (greater microbiome richness) and specifically a greater abundance of 12 types of butyrate-producing bacteria were both associated with less insulin resistance and less type 2 diabetes, in a population-based observational study from the Netherlands.
Several studies have reported that there is less microbiome diversity in type 2 diabetes, Zhangling Chen, MD, PhD, of Erasmus Medical Center, Rotterdam, the Netherlands, and colleagues note.
Their study also identified a dozen types of bacteria that ferment dietary fiber (undigested carbohydrates) in the gut to produce butyrate, a short-chain fatty acid, which may play a role in protection against type 2 diabetes.
“The current study is the first, to our knowledge, to comprehensively investigate the associations between gut microbiome composition [and] type 2 diabetes in a large population-based sample … which we adjusted for a series of key confounders,” the researchers write.
“These findings suggest that higher gut microbial diversity, along with specifically more butyrate-producing bacteria, may play a role in the development of type 2 diabetes, which may help guide future prevention and treatment strategies,” they conclude in their study published online July 29 in JAMA Network Open.
Confirmation of previous work, plus some new findings
The study confirms what many smaller ones have repeatedly shown – that low gut microbiome diversity is associated with increased risks of obesity and type 2 diabetes, Nanette I. Steinle, MD, RDN, who was not involved in the research, said in an interview.
A diet rich in fiber and prebiotics promotes gut biome diversity, added Dr. Steinle, chief of the endocrinology and diabetes section at Maryland Veterans Affairs Medical Center in Baltimore.
The findings add to other research, she noted, such as a prospective trial in which a high-fiber diet induced changes in the gut microbe that were linked to better glycemic regulation (Science. 2018;359:1151-6) and a study of a promising probiotic formula to treat diabetes.
“An important next step,” according to Dr. Steinle, “is to provide interventions like healthy diet or specific fiber types to see what can be done to produce lasting shifts in the gut microbiome and if these shifts result in improved metabolic health.”
Natalia Shulzhenko, MD, PhD, said: “Some of associations of taxa [bacteria groupings] with type 2 diabetes reported by this study are new.”
Dr. Shulzhenko and colleagues recently published a review of the role of gut microbiota in type 2 diabetes pathophysiology that summarized evidence from 42 human studies as well as preclinical studies and clinical trials of probiotic treatments (EBioMedicine. 2020;51:102590).
“Besides adding new microbes to the list of potential pathobionts [organisms that can cause harm] and beneficial microbes for type 2 diabetes,” the findings by Dr. Chen and colleagues “support a notion that different members of the gut microbial community may have similar effects on type 2 diabetes in different individuals,” commonly known as “functional redundancy,” Dr. Shulzhenko, associate professor, Carlson College of Veterinary Medicine, Oregon State University, Corvallis, pointed out in an email.
Also “in line with previous studies,” the study shows that butyrate-producing bacteria are associated with type 2 diabetes.
She speculated that “these results will probably contribute to the body of knowledge that is needed to develop microbiota-based therapy and diagnostics.”
Which gut bacteria are linked with diabetes?
It is unclear which gut bacteria are associated with the development of type 2 diabetes, Dr. Chen and colleagues write.
To investigate this, they identified 1,418 participants from the Rotterdam Study and 748 participants from the LifeLines-DEEP study enrolled from January 2018 to December 2020. Of these participants, 193 had type 2 diabetes.
The participants provided stool samples that were used to measure gut microbiome composition using the 16S ribosomal RNA method. They also had blood tests to measure glucose and insulin, and researchers collected other demographic and medical data.
Participants in the Rotterdam study were older than in the LifeLines Deep study (mean age, 62 vs. 45 years). Both cohorts included slightly more men than women (58%).
Dr. Chen and colleagues identified 126 (bacteria) genera in the gut microbiome in the Rotterdam study and 184 genera in the LifeLines Deep study.
After correcting for age, sex, smoking, education, physical activity, alcohol intake, daily calories, body mass index, and use of lipid-lowering medication or proton pump inhibitors, higher microbiome diversity was associated with lower insulin resistance and a lower prevalence of type 2 diabetes.
A higher abundance of each of seven types of butyrate-producing bacteria – Christensenellaceae, Christensenellaceae R7 group, Marvinbryantia, Ruminococcaceae UCG-005, Ruminococcaceae UCG-008, Ruminococcaceae UCG-010, and Ruminococcaceae NK4A214 group – was associated with lower insulin resistance, after adjusting for confounders such as diet and medications (all P < .001).
And a higher abundance of each of five other types of butyrate-producing bacteria – Clostridiaceae 1, Peptostreptococcaceae, Clostridium sensu stricto 1, Intestinibacter, and Romboutsia – was associated with less type 2 diabetes (all P < .001).
Study limitations include that gut microbiome composition was determined from stool (fecal) samples, whereas the actual composition varies in different locations along the intestine, and the study also lacked information about butyrate concentrations in stool or blood, the researchers note.
They call for “future research [to] validate the hypothesis of butyrate-producing bacteria affecting glucose metabolism and diabetes risk via production of butyrate.”
The authors and Dr. Shulzhenko have reported no relevant financial relationships. Dr. Steinle has reported receiving funding from the National Institutes of Health and conducting a study funded by Kowa through the VA.
A version of this article first appeared on Medscape.com.
Having more diverse gut bacteria (greater microbiome richness) and specifically a greater abundance of 12 types of butyrate-producing bacteria were both associated with less insulin resistance and less type 2 diabetes, in a population-based observational study from the Netherlands.
Several studies have reported that there is less microbiome diversity in type 2 diabetes, Zhangling Chen, MD, PhD, of Erasmus Medical Center, Rotterdam, the Netherlands, and colleagues note.
Their study also identified a dozen types of bacteria that ferment dietary fiber (undigested carbohydrates) in the gut to produce butyrate, a short-chain fatty acid, which may play a role in protection against type 2 diabetes.
“The current study is the first, to our knowledge, to comprehensively investigate the associations between gut microbiome composition [and] type 2 diabetes in a large population-based sample … which we adjusted for a series of key confounders,” the researchers write.
“These findings suggest that higher gut microbial diversity, along with specifically more butyrate-producing bacteria, may play a role in the development of type 2 diabetes, which may help guide future prevention and treatment strategies,” they conclude in their study published online July 29 in JAMA Network Open.
Confirmation of previous work, plus some new findings
The study confirms what many smaller ones have repeatedly shown – that low gut microbiome diversity is associated with increased risks of obesity and type 2 diabetes, Nanette I. Steinle, MD, RDN, who was not involved in the research, said in an interview.
A diet rich in fiber and prebiotics promotes gut biome diversity, added Dr. Steinle, chief of the endocrinology and diabetes section at Maryland Veterans Affairs Medical Center in Baltimore.
The findings add to other research, she noted, such as a prospective trial in which a high-fiber diet induced changes in the gut microbe that were linked to better glycemic regulation (Science. 2018;359:1151-6) and a study of a promising probiotic formula to treat diabetes.
“An important next step,” according to Dr. Steinle, “is to provide interventions like healthy diet or specific fiber types to see what can be done to produce lasting shifts in the gut microbiome and if these shifts result in improved metabolic health.”
Natalia Shulzhenko, MD, PhD, said: “Some of associations of taxa [bacteria groupings] with type 2 diabetes reported by this study are new.”
Dr. Shulzhenko and colleagues recently published a review of the role of gut microbiota in type 2 diabetes pathophysiology that summarized evidence from 42 human studies as well as preclinical studies and clinical trials of probiotic treatments (EBioMedicine. 2020;51:102590).
“Besides adding new microbes to the list of potential pathobionts [organisms that can cause harm] and beneficial microbes for type 2 diabetes,” the findings by Dr. Chen and colleagues “support a notion that different members of the gut microbial community may have similar effects on type 2 diabetes in different individuals,” commonly known as “functional redundancy,” Dr. Shulzhenko, associate professor, Carlson College of Veterinary Medicine, Oregon State University, Corvallis, pointed out in an email.
Also “in line with previous studies,” the study shows that butyrate-producing bacteria are associated with type 2 diabetes.
She speculated that “these results will probably contribute to the body of knowledge that is needed to develop microbiota-based therapy and diagnostics.”
Which gut bacteria are linked with diabetes?
It is unclear which gut bacteria are associated with the development of type 2 diabetes, Dr. Chen and colleagues write.
To investigate this, they identified 1,418 participants from the Rotterdam Study and 748 participants from the LifeLines-DEEP study enrolled from January 2018 to December 2020. Of these participants, 193 had type 2 diabetes.
The participants provided stool samples that were used to measure gut microbiome composition using the 16S ribosomal RNA method. They also had blood tests to measure glucose and insulin, and researchers collected other demographic and medical data.
Participants in the Rotterdam study were older than in the LifeLines Deep study (mean age, 62 vs. 45 years). Both cohorts included slightly more men than women (58%).
Dr. Chen and colleagues identified 126 (bacteria) genera in the gut microbiome in the Rotterdam study and 184 genera in the LifeLines Deep study.
After correcting for age, sex, smoking, education, physical activity, alcohol intake, daily calories, body mass index, and use of lipid-lowering medication or proton pump inhibitors, higher microbiome diversity was associated with lower insulin resistance and a lower prevalence of type 2 diabetes.
A higher abundance of each of seven types of butyrate-producing bacteria – Christensenellaceae, Christensenellaceae R7 group, Marvinbryantia, Ruminococcaceae UCG-005, Ruminococcaceae UCG-008, Ruminococcaceae UCG-010, and Ruminococcaceae NK4A214 group – was associated with lower insulin resistance, after adjusting for confounders such as diet and medications (all P < .001).
And a higher abundance of each of five other types of butyrate-producing bacteria – Clostridiaceae 1, Peptostreptococcaceae, Clostridium sensu stricto 1, Intestinibacter, and Romboutsia – was associated with less type 2 diabetes (all P < .001).
Study limitations include that gut microbiome composition was determined from stool (fecal) samples, whereas the actual composition varies in different locations along the intestine, and the study also lacked information about butyrate concentrations in stool or blood, the researchers note.
They call for “future research [to] validate the hypothesis of butyrate-producing bacteria affecting glucose metabolism and diabetes risk via production of butyrate.”
The authors and Dr. Shulzhenko have reported no relevant financial relationships. Dr. Steinle has reported receiving funding from the National Institutes of Health and conducting a study funded by Kowa through the VA.
A version of this article first appeared on Medscape.com.
Having more diverse gut bacteria (greater microbiome richness) and specifically a greater abundance of 12 types of butyrate-producing bacteria were both associated with less insulin resistance and less type 2 diabetes, in a population-based observational study from the Netherlands.
Several studies have reported that there is less microbiome diversity in type 2 diabetes, Zhangling Chen, MD, PhD, of Erasmus Medical Center, Rotterdam, the Netherlands, and colleagues note.
Their study also identified a dozen types of bacteria that ferment dietary fiber (undigested carbohydrates) in the gut to produce butyrate, a short-chain fatty acid, which may play a role in protection against type 2 diabetes.
“The current study is the first, to our knowledge, to comprehensively investigate the associations between gut microbiome composition [and] type 2 diabetes in a large population-based sample … which we adjusted for a series of key confounders,” the researchers write.
“These findings suggest that higher gut microbial diversity, along with specifically more butyrate-producing bacteria, may play a role in the development of type 2 diabetes, which may help guide future prevention and treatment strategies,” they conclude in their study published online July 29 in JAMA Network Open.
Confirmation of previous work, plus some new findings
The study confirms what many smaller ones have repeatedly shown – that low gut microbiome diversity is associated with increased risks of obesity and type 2 diabetes, Nanette I. Steinle, MD, RDN, who was not involved in the research, said in an interview.
A diet rich in fiber and prebiotics promotes gut biome diversity, added Dr. Steinle, chief of the endocrinology and diabetes section at Maryland Veterans Affairs Medical Center in Baltimore.
The findings add to other research, she noted, such as a prospective trial in which a high-fiber diet induced changes in the gut microbe that were linked to better glycemic regulation (Science. 2018;359:1151-6) and a study of a promising probiotic formula to treat diabetes.
“An important next step,” according to Dr. Steinle, “is to provide interventions like healthy diet or specific fiber types to see what can be done to produce lasting shifts in the gut microbiome and if these shifts result in improved metabolic health.”
Natalia Shulzhenko, MD, PhD, said: “Some of associations of taxa [bacteria groupings] with type 2 diabetes reported by this study are new.”
Dr. Shulzhenko and colleagues recently published a review of the role of gut microbiota in type 2 diabetes pathophysiology that summarized evidence from 42 human studies as well as preclinical studies and clinical trials of probiotic treatments (EBioMedicine. 2020;51:102590).
“Besides adding new microbes to the list of potential pathobionts [organisms that can cause harm] and beneficial microbes for type 2 diabetes,” the findings by Dr. Chen and colleagues “support a notion that different members of the gut microbial community may have similar effects on type 2 diabetes in different individuals,” commonly known as “functional redundancy,” Dr. Shulzhenko, associate professor, Carlson College of Veterinary Medicine, Oregon State University, Corvallis, pointed out in an email.
Also “in line with previous studies,” the study shows that butyrate-producing bacteria are associated with type 2 diabetes.
She speculated that “these results will probably contribute to the body of knowledge that is needed to develop microbiota-based therapy and diagnostics.”
Which gut bacteria are linked with diabetes?
It is unclear which gut bacteria are associated with the development of type 2 diabetes, Dr. Chen and colleagues write.
To investigate this, they identified 1,418 participants from the Rotterdam Study and 748 participants from the LifeLines-DEEP study enrolled from January 2018 to December 2020. Of these participants, 193 had type 2 diabetes.
The participants provided stool samples that were used to measure gut microbiome composition using the 16S ribosomal RNA method. They also had blood tests to measure glucose and insulin, and researchers collected other demographic and medical data.
Participants in the Rotterdam study were older than in the LifeLines Deep study (mean age, 62 vs. 45 years). Both cohorts included slightly more men than women (58%).
Dr. Chen and colleagues identified 126 (bacteria) genera in the gut microbiome in the Rotterdam study and 184 genera in the LifeLines Deep study.
After correcting for age, sex, smoking, education, physical activity, alcohol intake, daily calories, body mass index, and use of lipid-lowering medication or proton pump inhibitors, higher microbiome diversity was associated with lower insulin resistance and a lower prevalence of type 2 diabetes.
A higher abundance of each of seven types of butyrate-producing bacteria – Christensenellaceae, Christensenellaceae R7 group, Marvinbryantia, Ruminococcaceae UCG-005, Ruminococcaceae UCG-008, Ruminococcaceae UCG-010, and Ruminococcaceae NK4A214 group – was associated with lower insulin resistance, after adjusting for confounders such as diet and medications (all P < .001).
And a higher abundance of each of five other types of butyrate-producing bacteria – Clostridiaceae 1, Peptostreptococcaceae, Clostridium sensu stricto 1, Intestinibacter, and Romboutsia – was associated with less type 2 diabetes (all P < .001).
Study limitations include that gut microbiome composition was determined from stool (fecal) samples, whereas the actual composition varies in different locations along the intestine, and the study also lacked information about butyrate concentrations in stool or blood, the researchers note.
They call for “future research [to] validate the hypothesis of butyrate-producing bacteria affecting glucose metabolism and diabetes risk via production of butyrate.”
The authors and Dr. Shulzhenko have reported no relevant financial relationships. Dr. Steinle has reported receiving funding from the National Institutes of Health and conducting a study funded by Kowa through the VA.
A version of this article first appeared on Medscape.com.
Fulminant Hemorrhagic Bullae of the Upper Extremities Arising in the Setting of IV Placement During Severe COVID-19 Infection: Observations From a Major Consultative Practice
To the Editor:
A range of dermatologic manifestations of COVID-19 have been reported, including nonspecific maculopapular exanthems, urticaria, and varicellalike eruptions.1 Additionally, there have been sporadic accounts of cutaneous vasculopathic signs such as perniolike lesions, acro-ischemia, livedo reticularis, and retiform purpura.2 We describe exuberant hemorrhagic bullae occurring on the extremities of 2 critically ill patients with COVID-19. We hypothesized that the bullae were vasculopathic in nature and possibly exacerbated by peripheral intravenous (IV)–related injury.
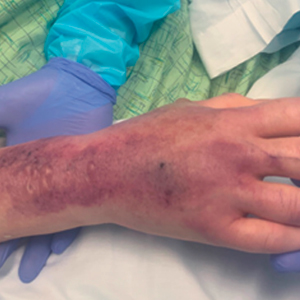
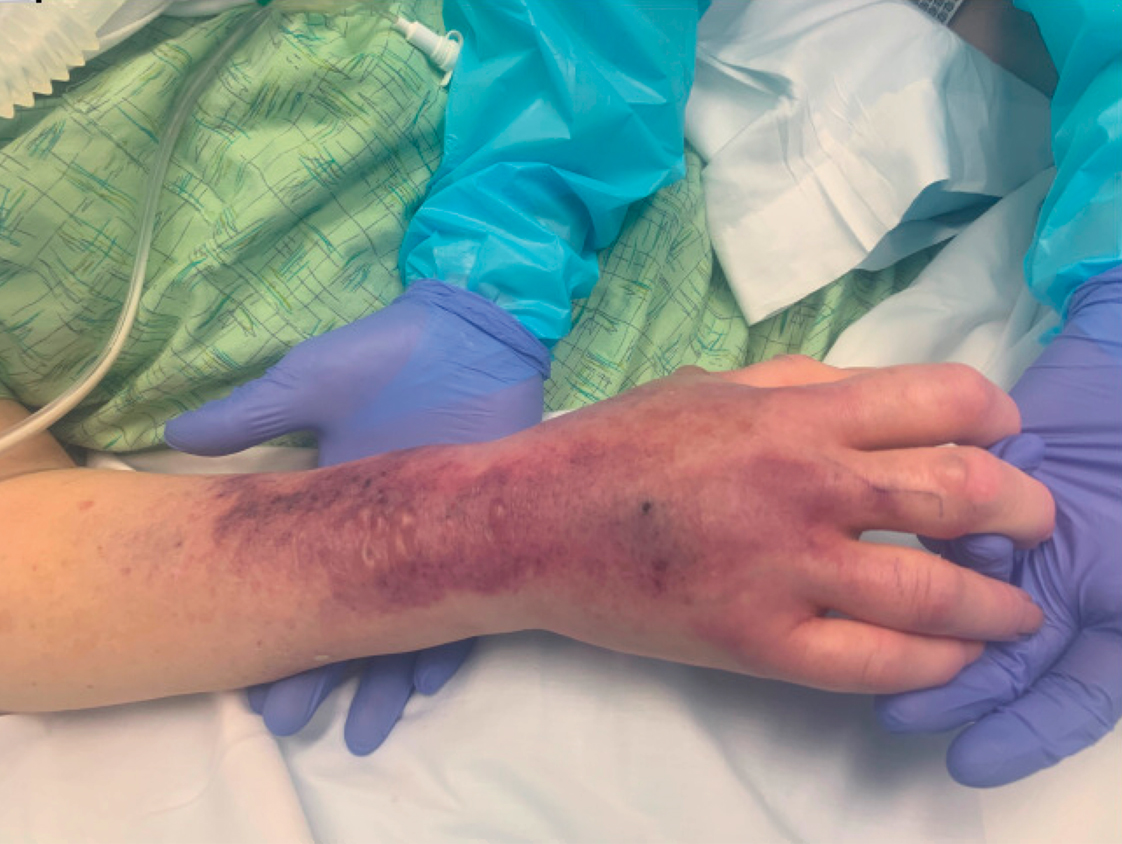
A 62-year-old woman with a history of diabetes mellitus and chronic obstructive pulmonary disease was admitted to the intensive care unit for acute hypoxemic respiratory failure secondary to COVID-19 infection. Dermatology was consulted for evaluation of blisters on the right arm. A new peripheral IV line was inserted into the patient’s right forearm for treatment of secondary methicillin-resistant Staphylococcus aureus pneumonia. The peripheral IV was inserted into the right proximal forearm for 2 days prior to development of ecchymosis and blisters. Intravenous medications included vancomycin, cefepime, methylprednisolone, and famotidine, as well as maintenance fluids (normal saline). Physical examination revealed extensive confluent ecchymoses with overlying tense bullae (Figure 1). Notable laboratory findings included an elevated D-dimer (peak of 8.67 μg/mL fibrinogen-equivalent units [FEUs], reference range <0.5 μg/mL FEU) and fibrinogen (789 mg/dL, reference range 200–400 mg/dL) levels. Three days later she developed worsening edema of the right arm, accompanied by more extensive bullae formation (Figure 2). Computed tomography of the right arm showed extensive subcutaneous stranding and subcutaneous edema. An orthopedic consultation determined that there was no compartment syndrome, and surgical intervention was not recommended. The patient’s course was complicated by multiorgan failure, and she died 18 days after admission.
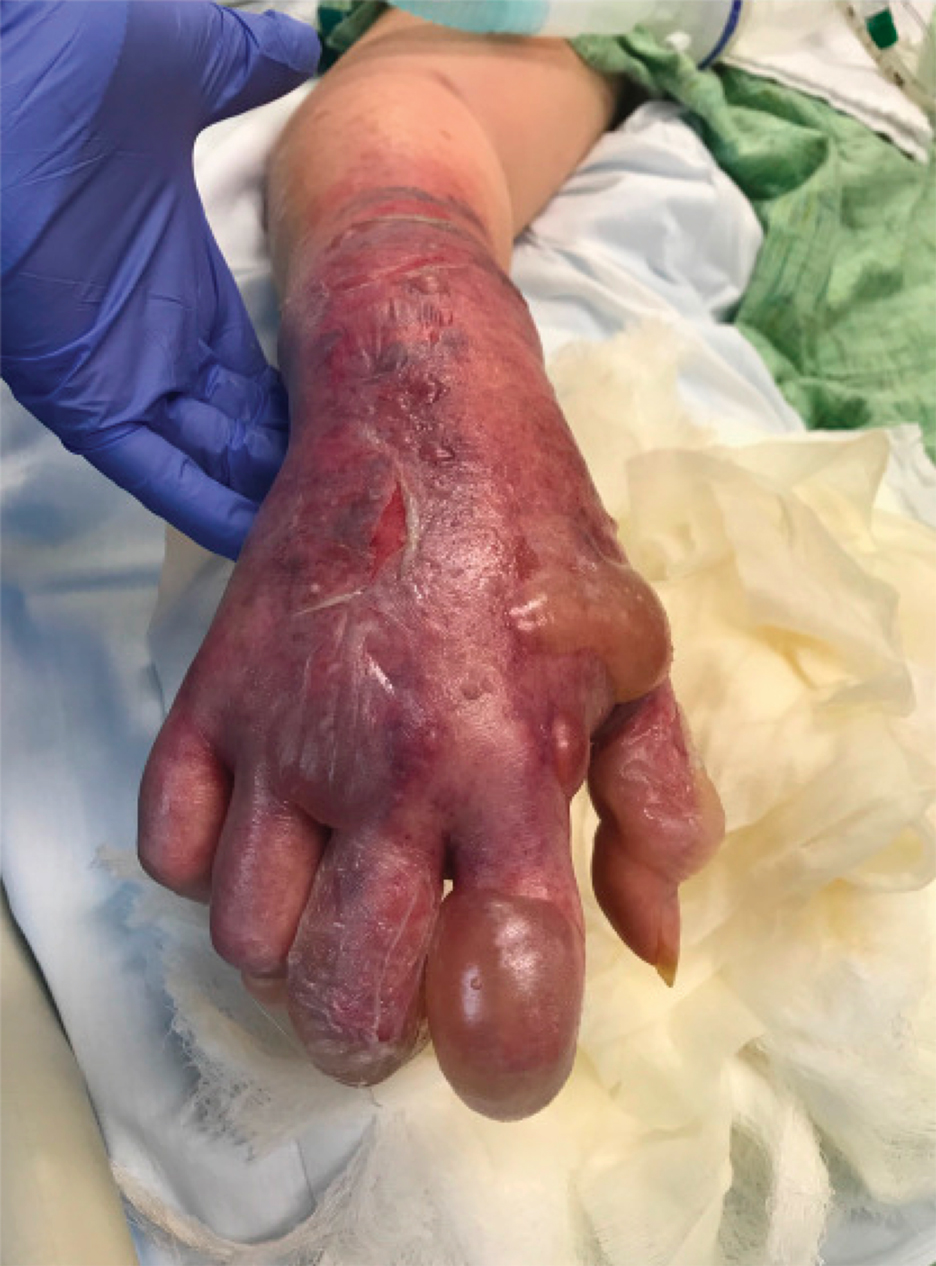
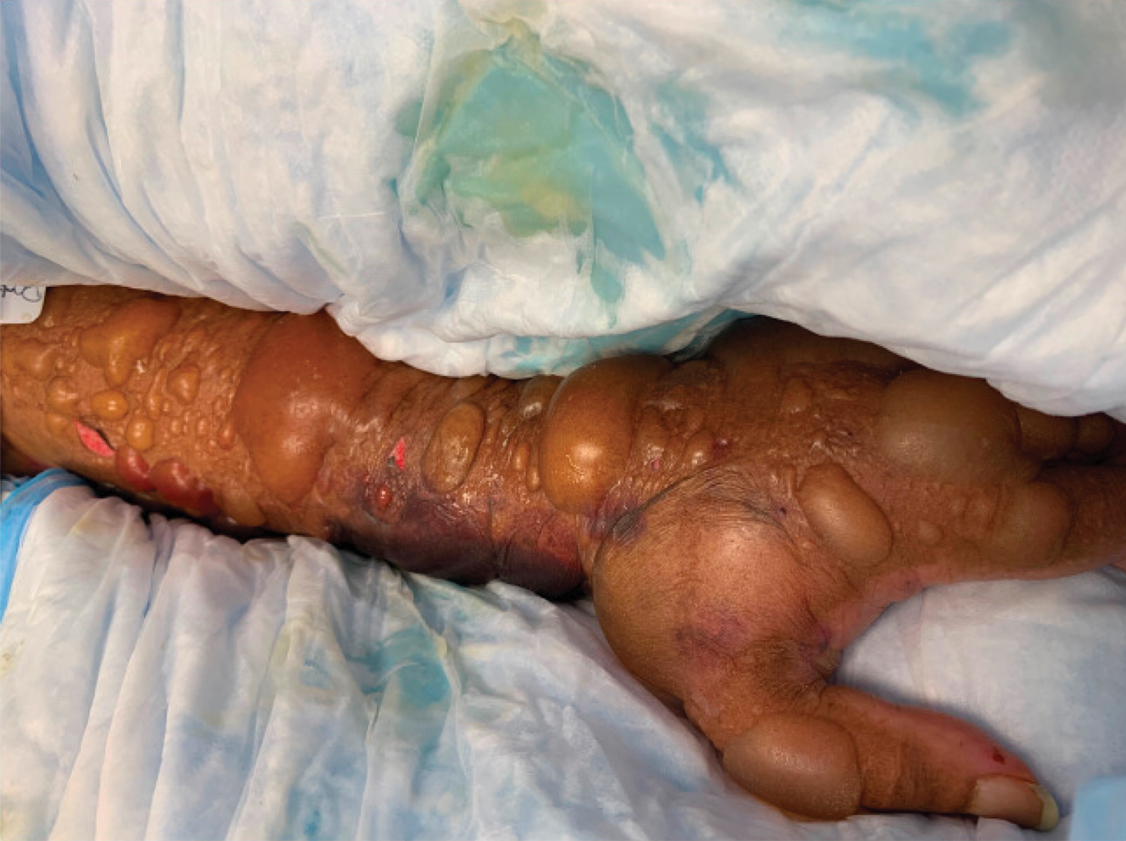
A 67-year-old man with coronary artery disease, diabetes mellitus, and hemiparesis secondary to stroke was admitted to the intensive care unit due to hypoxemia secondary to COVID-19 pneumonia. Dermatology was consulted for the evaluation of blisters on both arms. The right forearm peripheral IV line was used for 4 days prior to the development of cutaneous symptoms. Intravenous medications included cefepime, famotidine, and methylprednisolone. The left forearm peripheral IV line was in place for 1 day prior to the development of blisters and was used for the infusion of maintenance fluids (lactated Ringer’s solution). On the first day of the eruption, small bullae were noted at sites of prior peripheral IV lines (Figure 3). On day 3 of admission, the eruption progressed to larger and more confluent tense bullae with ecchymosis (Figure 4). Additionally, laboratory test results were notable for an elevated D-dimer (peak of >20.00 ug/mL FEU) and fibrinogen (748 mg/dL) levels. Computed tomography of the arms showed extensive subcutaneous stranding and fluid along the fascial planes of the arms, with no gas or abscess formation. Surgical intervention was not recommended following an orthopedic consultation. The patient’s course was complicated by acute kidney injury and rhabdomyolysis; he was later discharged to a skilled nursing facility in stable condition.

Reports from China indicate that approximately 50% of COVID-19 patients have elevated D-dimer levels and are at risk for thrombosis.3 We hypothesize that the exuberant hemorrhagic bullous eruptions in our 2 cases may be mediated in part by a hypercoagulable state secondary to COVID-19 infection combined with IV-related trauma or extravasation injury. However, a direct cytotoxic effect of the virus cannot be entirely excluded as a potential inciting factor. Other entities considered in the differential for localized bullae included trauma-induced bullous pemphigoid as well as bullous cellulitis. Both patients were treated with high-dose steroids as well as broad-spectrum antibiotics, which were expected to lead to improvement in symptoms of bullous pemphigoid and cellulitis, respectively; however, they did not lead to symptom improvement.
Extravasation injury results from unintentional administration of potentially vesicant substances into tissues surrounding the intended vascular channel.4 The mechanism of action of these injuries is postulated to arise from direct tissue injury from cytotoxic substances, elevated osmotic pressure, and reduced blood supply if vasoconstrictive substances are infused.5 In our patients, these injuries also may have promoted vascular occlusion leading to the brisk reaction observed. Although ecchymoses typically are associated with hypocoagulable states, both of our patients were noted to have normal platelet levels throughout hospitalization. Additionally, findings of elevated D-dimer and fibrinogen levels point to a hypercoagulable state. However, there is a possibility of platelet dysfunction leading to the observed cutaneous findings of ecchymoses. Thrombocytopenia is a common finding in patients with COVID-19 and is found to be associated with increased in-hospital mortality.6 Additional study of these reactions is needed given the propensity for multiorgan failure and death in patients with COVID-19 from suspected diffuse microvascular damage.3
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-19 pneumonia and acro-ischemia [in Chinese][published online March 28, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006.
- Mei H, Hu Y. Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19 [in Chinese][published online March 14, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E002.
- Sauerland C, Engelking C, Wickham R, et al. Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006;33:1134-1141.
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632.
- Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469‐1472.
To the Editor:
A range of dermatologic manifestations of COVID-19 have been reported, including nonspecific maculopapular exanthems, urticaria, and varicellalike eruptions.1 Additionally, there have been sporadic accounts of cutaneous vasculopathic signs such as perniolike lesions, acro-ischemia, livedo reticularis, and retiform purpura.2 We describe exuberant hemorrhagic bullae occurring on the extremities of 2 critically ill patients with COVID-19. We hypothesized that the bullae were vasculopathic in nature and possibly exacerbated by peripheral intravenous (IV)–related injury.
A 62-year-old woman with a history of diabetes mellitus and chronic obstructive pulmonary disease was admitted to the intensive care unit for acute hypoxemic respiratory failure secondary to COVID-19 infection. Dermatology was consulted for evaluation of blisters on the right arm. A new peripheral IV line was inserted into the patient’s right forearm for treatment of secondary methicillin-resistant Staphylococcus aureus pneumonia. The peripheral IV was inserted into the right proximal forearm for 2 days prior to development of ecchymosis and blisters. Intravenous medications included vancomycin, cefepime, methylprednisolone, and famotidine, as well as maintenance fluids (normal saline). Physical examination revealed extensive confluent ecchymoses with overlying tense bullae (Figure 1). Notable laboratory findings included an elevated D-dimer (peak of 8.67 μg/mL fibrinogen-equivalent units [FEUs], reference range <0.5 μg/mL FEU) and fibrinogen (789 mg/dL, reference range 200–400 mg/dL) levels. Three days later she developed worsening edema of the right arm, accompanied by more extensive bullae formation (Figure 2). Computed tomography of the right arm showed extensive subcutaneous stranding and subcutaneous edema. An orthopedic consultation determined that there was no compartment syndrome, and surgical intervention was not recommended. The patient’s course was complicated by multiorgan failure, and she died 18 days after admission.


A 67-year-old man with coronary artery disease, diabetes mellitus, and hemiparesis secondary to stroke was admitted to the intensive care unit due to hypoxemia secondary to COVID-19 pneumonia. Dermatology was consulted for the evaluation of blisters on both arms. The right forearm peripheral IV line was used for 4 days prior to the development of cutaneous symptoms. Intravenous medications included cefepime, famotidine, and methylprednisolone. The left forearm peripheral IV line was in place for 1 day prior to the development of blisters and was used for the infusion of maintenance fluids (lactated Ringer’s solution). On the first day of the eruption, small bullae were noted at sites of prior peripheral IV lines (Figure 3). On day 3 of admission, the eruption progressed to larger and more confluent tense bullae with ecchymosis (Figure 4). Additionally, laboratory test results were notable for an elevated D-dimer (peak of >20.00 ug/mL FEU) and fibrinogen (748 mg/dL) levels. Computed tomography of the arms showed extensive subcutaneous stranding and fluid along the fascial planes of the arms, with no gas or abscess formation. Surgical intervention was not recommended following an orthopedic consultation. The patient’s course was complicated by acute kidney injury and rhabdomyolysis; he was later discharged to a skilled nursing facility in stable condition.


Reports from China indicate that approximately 50% of COVID-19 patients have elevated D-dimer levels and are at risk for thrombosis.3 We hypothesize that the exuberant hemorrhagic bullous eruptions in our 2 cases may be mediated in part by a hypercoagulable state secondary to COVID-19 infection combined with IV-related trauma or extravasation injury. However, a direct cytotoxic effect of the virus cannot be entirely excluded as a potential inciting factor. Other entities considered in the differential for localized bullae included trauma-induced bullous pemphigoid as well as bullous cellulitis. Both patients were treated with high-dose steroids as well as broad-spectrum antibiotics, which were expected to lead to improvement in symptoms of bullous pemphigoid and cellulitis, respectively; however, they did not lead to symptom improvement.
Extravasation injury results from unintentional administration of potentially vesicant substances into tissues surrounding the intended vascular channel.4 The mechanism of action of these injuries is postulated to arise from direct tissue injury from cytotoxic substances, elevated osmotic pressure, and reduced blood supply if vasoconstrictive substances are infused.5 In our patients, these injuries also may have promoted vascular occlusion leading to the brisk reaction observed. Although ecchymoses typically are associated with hypocoagulable states, both of our patients were noted to have normal platelet levels throughout hospitalization. Additionally, findings of elevated D-dimer and fibrinogen levels point to a hypercoagulable state. However, there is a possibility of platelet dysfunction leading to the observed cutaneous findings of ecchymoses. Thrombocytopenia is a common finding in patients with COVID-19 and is found to be associated with increased in-hospital mortality.6 Additional study of these reactions is needed given the propensity for multiorgan failure and death in patients with COVID-19 from suspected diffuse microvascular damage.3
To the Editor:
A range of dermatologic manifestations of COVID-19 have been reported, including nonspecific maculopapular exanthems, urticaria, and varicellalike eruptions.1 Additionally, there have been sporadic accounts of cutaneous vasculopathic signs such as perniolike lesions, acro-ischemia, livedo reticularis, and retiform purpura.2 We describe exuberant hemorrhagic bullae occurring on the extremities of 2 critically ill patients with COVID-19. We hypothesized that the bullae were vasculopathic in nature and possibly exacerbated by peripheral intravenous (IV)–related injury.
A 62-year-old woman with a history of diabetes mellitus and chronic obstructive pulmonary disease was admitted to the intensive care unit for acute hypoxemic respiratory failure secondary to COVID-19 infection. Dermatology was consulted for evaluation of blisters on the right arm. A new peripheral IV line was inserted into the patient’s right forearm for treatment of secondary methicillin-resistant Staphylococcus aureus pneumonia. The peripheral IV was inserted into the right proximal forearm for 2 days prior to development of ecchymosis and blisters. Intravenous medications included vancomycin, cefepime, methylprednisolone, and famotidine, as well as maintenance fluids (normal saline). Physical examination revealed extensive confluent ecchymoses with overlying tense bullae (Figure 1). Notable laboratory findings included an elevated D-dimer (peak of 8.67 μg/mL fibrinogen-equivalent units [FEUs], reference range <0.5 μg/mL FEU) and fibrinogen (789 mg/dL, reference range 200–400 mg/dL) levels. Three days later she developed worsening edema of the right arm, accompanied by more extensive bullae formation (Figure 2). Computed tomography of the right arm showed extensive subcutaneous stranding and subcutaneous edema. An orthopedic consultation determined that there was no compartment syndrome, and surgical intervention was not recommended. The patient’s course was complicated by multiorgan failure, and she died 18 days after admission.


A 67-year-old man with coronary artery disease, diabetes mellitus, and hemiparesis secondary to stroke was admitted to the intensive care unit due to hypoxemia secondary to COVID-19 pneumonia. Dermatology was consulted for the evaluation of blisters on both arms. The right forearm peripheral IV line was used for 4 days prior to the development of cutaneous symptoms. Intravenous medications included cefepime, famotidine, and methylprednisolone. The left forearm peripheral IV line was in place for 1 day prior to the development of blisters and was used for the infusion of maintenance fluids (lactated Ringer’s solution). On the first day of the eruption, small bullae were noted at sites of prior peripheral IV lines (Figure 3). On day 3 of admission, the eruption progressed to larger and more confluent tense bullae with ecchymosis (Figure 4). Additionally, laboratory test results were notable for an elevated D-dimer (peak of >20.00 ug/mL FEU) and fibrinogen (748 mg/dL) levels. Computed tomography of the arms showed extensive subcutaneous stranding and fluid along the fascial planes of the arms, with no gas or abscess formation. Surgical intervention was not recommended following an orthopedic consultation. The patient’s course was complicated by acute kidney injury and rhabdomyolysis; he was later discharged to a skilled nursing facility in stable condition.


Reports from China indicate that approximately 50% of COVID-19 patients have elevated D-dimer levels and are at risk for thrombosis.3 We hypothesize that the exuberant hemorrhagic bullous eruptions in our 2 cases may be mediated in part by a hypercoagulable state secondary to COVID-19 infection combined with IV-related trauma or extravasation injury. However, a direct cytotoxic effect of the virus cannot be entirely excluded as a potential inciting factor. Other entities considered in the differential for localized bullae included trauma-induced bullous pemphigoid as well as bullous cellulitis. Both patients were treated with high-dose steroids as well as broad-spectrum antibiotics, which were expected to lead to improvement in symptoms of bullous pemphigoid and cellulitis, respectively; however, they did not lead to symptom improvement.
Extravasation injury results from unintentional administration of potentially vesicant substances into tissues surrounding the intended vascular channel.4 The mechanism of action of these injuries is postulated to arise from direct tissue injury from cytotoxic substances, elevated osmotic pressure, and reduced blood supply if vasoconstrictive substances are infused.5 In our patients, these injuries also may have promoted vascular occlusion leading to the brisk reaction observed. Although ecchymoses typically are associated with hypocoagulable states, both of our patients were noted to have normal platelet levels throughout hospitalization. Additionally, findings of elevated D-dimer and fibrinogen levels point to a hypercoagulable state. However, there is a possibility of platelet dysfunction leading to the observed cutaneous findings of ecchymoses. Thrombocytopenia is a common finding in patients with COVID-19 and is found to be associated with increased in-hospital mortality.6 Additional study of these reactions is needed given the propensity for multiorgan failure and death in patients with COVID-19 from suspected diffuse microvascular damage.3
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-19 pneumonia and acro-ischemia [in Chinese][published online March 28, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006.
- Mei H, Hu Y. Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19 [in Chinese][published online March 14, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E002.
- Sauerland C, Engelking C, Wickham R, et al. Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006;33:1134-1141.
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632.
- Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469‐1472.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-19 pneumonia and acro-ischemia [in Chinese][published online March 28, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006.
- Mei H, Hu Y. Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19 [in Chinese][published online March 14, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E002.
- Sauerland C, Engelking C, Wickham R, et al. Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006;33:1134-1141.
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632.
- Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469‐1472.
Practice Points
- Hemorrhagic bullae are an uncommon cutaneous manifestation of COVID-19 infection in hospitalized individuals.
- Although there is no reported treatment for COVID-19–associated hemorrhagic bullae, we recommend supportive care and management of underlying etiology.
Shorter HCC screening intervals benefit high-risk patients
Ultrasonography screening intervals of less than 6-12 months were associated with early detection of hepatocellular carcinoma, as well as increased life expectancy and quality of life, according to data from a nationwide comparative effectiveness study of nearly 60,000 patients in Taiwan.
Many international societies, including the American Association for the Study of Liver Diseases, the Asian Pacific Association for the Study of the Liver, and the European Association for the Study of the Liver, recommend abdominal ultrasonography screening for hepatocellular carcinoma (HCC) with or without alpha-fetoprotein every 6 months for patients at increased risk for HCC, wrote Shih-Chiang Kuo, MD, of National Cheng Kung University, Tainan, Taiwan, and colleagues.
However, some studies do not support this recommendation, and data suggest that “adherence to regular screenings by high-risk patients has been inadequate, leading to reduced overall benefits of ultrasonography screening in real-world practice,” and the impact of screening schedules on quality of life has not been assessed, they said.
In a study published in JAMA Network Open, the researchers identified adults with newly diagnosed HCC from 2002 through 2015 using data from the Taiwan National Cancer Registry. Barcelona Clinic Liver Cancer (BCLC) staging information was available for 42,081 men and 17,113 women; the average age was 62 years for men and 69 years for women. The patients were divided into five cohorts based on the time between their last ultrasonography screening and an index date of 90 days before their HCC diagnosis. These groups were 6 months (0-6 months), 12 months (7-12 months), 24 months (13-24 months), 36 months (25-36 months), and longer than 36 months.
“For both sexes, the proportions of patients with HCC classified as being in earlier stages (stage 0 and A) were higher in subcohorts with shorter screening intervals since the most recent ultrasonography,” the researchers wrote.
The researchers also assessed quality of life measures using the European Quality of Life Five-Dimensions in 807 men (3,370 repeated assessments) and 252 women (1,044 repeated assessments). Among men, the loss of quality of life expectancy in terms of quality of life years (QALYs) was 10.0, 11.1, 12.1, 13.1, and 14.6 for screening intervals of 6 months, 12 months, 24 months, 36 months, and beyond 36 months, respectively. The corresponding QALYs for women at the same screening intervals were 9.0, 9.7, 10.3, 10.7, and 11.4, respectively.
In a subgroup analysis according to underlying liver disease, patients with underlying hepatitis B virus infection or cirrhosis showed the greatest benefits from shorter screening intervals. For those with hepatitis B virus infection, abdominal ultrasonography screening 6 months or less prior to diagnosis of HCC was associated with an additional 4.8 QALYs for men and 2.8 QALYs for women, compared with screening longer than 36 months prior to diagnosis. The corresponding savings in QALY for men and women with underlying cirrhosis was 4.8 QALYs and 2.4 QALYs. Patients with no underlying liver disease also benefited from shorter intervals, with potential savings of 3.2 QALYs for men and 1.6 QALYs for women in the 6-month screening groups, compared with the longer than 36 months groups.
However, less than half of the men overall underwent screening withing 6 months or 12 months before diagnosis (31.4% and 39.3%, respectively); for women, 42.2% received screening within 6 months of diagnosis and 51.9% received screening within 12 months.
The study findings were limited by several factors including the use of only the last screening before diagnosis, which allows the possibility that patients in the 6- or 12-month groups did not have regular screening, the researchers noted. In addition, the lack of data on quality of life for women with BCLC stage D might have caused an underestimation of quality of life loss, they said. However, the results were strengthened by the use of a national database and long follow-up period, they said.
The results support intervals of 6-12 months or less for regular ultrasonography screening as a way to improve early detection of HCC, “and may save lives and improve utility for patients with HCC from a lifetime perspective,” the researchers emphasized. “Because people with underlying risk factors (including hepatitis B virus or hepatitis C virus infection, cirrhosis, and alcoholic liver disease) showed only slightly more frequent ultrasonography screening than those without underlying risk factors, we recommend improving this clinical practice,” they concluded.
Impact of identifying risk
“This study is important because HCC remains the third leading cause of cancer deaths, and the 5-year survival rate is low,” said Atsushi Sakuraba, MD, of the University of Chicago, in an interview.
Dr. Sakuraba said that he was not surprised by any of the study findings. “Earlier diagnosis of cancer is often associated with improved outcome in many cancers,” he noted.
However, “Overutilization of resources may lead to increased health care costs, so correct identification of high-risk populations is needed,” Dr. Sakuraba said.
Additional research is warranted in several areas in order to make an impact on clinical practice, Dr. Sakuraba said, notably, “confirmation in other countries and ethnicities where the incidence of viral hepatitis varies.” Comparison to other tests, such as tumor markers, CT, and MRI, is needed as well, he concluded.
The study was supported by the Taiwan Ministry of Science and Technology. The researchers had no financial conflicts to disclose. Dr. Sakuraba had no financial conflicts to disclose.
Ultrasonography screening intervals of less than 6-12 months were associated with early detection of hepatocellular carcinoma, as well as increased life expectancy and quality of life, according to data from a nationwide comparative effectiveness study of nearly 60,000 patients in Taiwan.
Many international societies, including the American Association for the Study of Liver Diseases, the Asian Pacific Association for the Study of the Liver, and the European Association for the Study of the Liver, recommend abdominal ultrasonography screening for hepatocellular carcinoma (HCC) with or without alpha-fetoprotein every 6 months for patients at increased risk for HCC, wrote Shih-Chiang Kuo, MD, of National Cheng Kung University, Tainan, Taiwan, and colleagues.
However, some studies do not support this recommendation, and data suggest that “adherence to regular screenings by high-risk patients has been inadequate, leading to reduced overall benefits of ultrasonography screening in real-world practice,” and the impact of screening schedules on quality of life has not been assessed, they said.
In a study published in JAMA Network Open, the researchers identified adults with newly diagnosed HCC from 2002 through 2015 using data from the Taiwan National Cancer Registry. Barcelona Clinic Liver Cancer (BCLC) staging information was available for 42,081 men and 17,113 women; the average age was 62 years for men and 69 years for women. The patients were divided into five cohorts based on the time between their last ultrasonography screening and an index date of 90 days before their HCC diagnosis. These groups were 6 months (0-6 months), 12 months (7-12 months), 24 months (13-24 months), 36 months (25-36 months), and longer than 36 months.
“For both sexes, the proportions of patients with HCC classified as being in earlier stages (stage 0 and A) were higher in subcohorts with shorter screening intervals since the most recent ultrasonography,” the researchers wrote.
The researchers also assessed quality of life measures using the European Quality of Life Five-Dimensions in 807 men (3,370 repeated assessments) and 252 women (1,044 repeated assessments). Among men, the loss of quality of life expectancy in terms of quality of life years (QALYs) was 10.0, 11.1, 12.1, 13.1, and 14.6 for screening intervals of 6 months, 12 months, 24 months, 36 months, and beyond 36 months, respectively. The corresponding QALYs for women at the same screening intervals were 9.0, 9.7, 10.3, 10.7, and 11.4, respectively.
In a subgroup analysis according to underlying liver disease, patients with underlying hepatitis B virus infection or cirrhosis showed the greatest benefits from shorter screening intervals. For those with hepatitis B virus infection, abdominal ultrasonography screening 6 months or less prior to diagnosis of HCC was associated with an additional 4.8 QALYs for men and 2.8 QALYs for women, compared with screening longer than 36 months prior to diagnosis. The corresponding savings in QALY for men and women with underlying cirrhosis was 4.8 QALYs and 2.4 QALYs. Patients with no underlying liver disease also benefited from shorter intervals, with potential savings of 3.2 QALYs for men and 1.6 QALYs for women in the 6-month screening groups, compared with the longer than 36 months groups.
However, less than half of the men overall underwent screening withing 6 months or 12 months before diagnosis (31.4% and 39.3%, respectively); for women, 42.2% received screening within 6 months of diagnosis and 51.9% received screening within 12 months.
The study findings were limited by several factors including the use of only the last screening before diagnosis, which allows the possibility that patients in the 6- or 12-month groups did not have regular screening, the researchers noted. In addition, the lack of data on quality of life for women with BCLC stage D might have caused an underestimation of quality of life loss, they said. However, the results were strengthened by the use of a national database and long follow-up period, they said.
The results support intervals of 6-12 months or less for regular ultrasonography screening as a way to improve early detection of HCC, “and may save lives and improve utility for patients with HCC from a lifetime perspective,” the researchers emphasized. “Because people with underlying risk factors (including hepatitis B virus or hepatitis C virus infection, cirrhosis, and alcoholic liver disease) showed only slightly more frequent ultrasonography screening than those without underlying risk factors, we recommend improving this clinical practice,” they concluded.
Impact of identifying risk
“This study is important because HCC remains the third leading cause of cancer deaths, and the 5-year survival rate is low,” said Atsushi Sakuraba, MD, of the University of Chicago, in an interview.
Dr. Sakuraba said that he was not surprised by any of the study findings. “Earlier diagnosis of cancer is often associated with improved outcome in many cancers,” he noted.
However, “Overutilization of resources may lead to increased health care costs, so correct identification of high-risk populations is needed,” Dr. Sakuraba said.
Additional research is warranted in several areas in order to make an impact on clinical practice, Dr. Sakuraba said, notably, “confirmation in other countries and ethnicities where the incidence of viral hepatitis varies.” Comparison to other tests, such as tumor markers, CT, and MRI, is needed as well, he concluded.
The study was supported by the Taiwan Ministry of Science and Technology. The researchers had no financial conflicts to disclose. Dr. Sakuraba had no financial conflicts to disclose.
Ultrasonography screening intervals of less than 6-12 months were associated with early detection of hepatocellular carcinoma, as well as increased life expectancy and quality of life, according to data from a nationwide comparative effectiveness study of nearly 60,000 patients in Taiwan.
Many international societies, including the American Association for the Study of Liver Diseases, the Asian Pacific Association for the Study of the Liver, and the European Association for the Study of the Liver, recommend abdominal ultrasonography screening for hepatocellular carcinoma (HCC) with or without alpha-fetoprotein every 6 months for patients at increased risk for HCC, wrote Shih-Chiang Kuo, MD, of National Cheng Kung University, Tainan, Taiwan, and colleagues.
However, some studies do not support this recommendation, and data suggest that “adherence to regular screenings by high-risk patients has been inadequate, leading to reduced overall benefits of ultrasonography screening in real-world practice,” and the impact of screening schedules on quality of life has not been assessed, they said.
In a study published in JAMA Network Open, the researchers identified adults with newly diagnosed HCC from 2002 through 2015 using data from the Taiwan National Cancer Registry. Barcelona Clinic Liver Cancer (BCLC) staging information was available for 42,081 men and 17,113 women; the average age was 62 years for men and 69 years for women. The patients were divided into five cohorts based on the time between their last ultrasonography screening and an index date of 90 days before their HCC diagnosis. These groups were 6 months (0-6 months), 12 months (7-12 months), 24 months (13-24 months), 36 months (25-36 months), and longer than 36 months.
“For both sexes, the proportions of patients with HCC classified as being in earlier stages (stage 0 and A) were higher in subcohorts with shorter screening intervals since the most recent ultrasonography,” the researchers wrote.
The researchers also assessed quality of life measures using the European Quality of Life Five-Dimensions in 807 men (3,370 repeated assessments) and 252 women (1,044 repeated assessments). Among men, the loss of quality of life expectancy in terms of quality of life years (QALYs) was 10.0, 11.1, 12.1, 13.1, and 14.6 for screening intervals of 6 months, 12 months, 24 months, 36 months, and beyond 36 months, respectively. The corresponding QALYs for women at the same screening intervals were 9.0, 9.7, 10.3, 10.7, and 11.4, respectively.
In a subgroup analysis according to underlying liver disease, patients with underlying hepatitis B virus infection or cirrhosis showed the greatest benefits from shorter screening intervals. For those with hepatitis B virus infection, abdominal ultrasonography screening 6 months or less prior to diagnosis of HCC was associated with an additional 4.8 QALYs for men and 2.8 QALYs for women, compared with screening longer than 36 months prior to diagnosis. The corresponding savings in QALY for men and women with underlying cirrhosis was 4.8 QALYs and 2.4 QALYs. Patients with no underlying liver disease also benefited from shorter intervals, with potential savings of 3.2 QALYs for men and 1.6 QALYs for women in the 6-month screening groups, compared with the longer than 36 months groups.
However, less than half of the men overall underwent screening withing 6 months or 12 months before diagnosis (31.4% and 39.3%, respectively); for women, 42.2% received screening within 6 months of diagnosis and 51.9% received screening within 12 months.
The study findings were limited by several factors including the use of only the last screening before diagnosis, which allows the possibility that patients in the 6- or 12-month groups did not have regular screening, the researchers noted. In addition, the lack of data on quality of life for women with BCLC stage D might have caused an underestimation of quality of life loss, they said. However, the results were strengthened by the use of a national database and long follow-up period, they said.
The results support intervals of 6-12 months or less for regular ultrasonography screening as a way to improve early detection of HCC, “and may save lives and improve utility for patients with HCC from a lifetime perspective,” the researchers emphasized. “Because people with underlying risk factors (including hepatitis B virus or hepatitis C virus infection, cirrhosis, and alcoholic liver disease) showed only slightly more frequent ultrasonography screening than those without underlying risk factors, we recommend improving this clinical practice,” they concluded.
Impact of identifying risk
“This study is important because HCC remains the third leading cause of cancer deaths, and the 5-year survival rate is low,” said Atsushi Sakuraba, MD, of the University of Chicago, in an interview.
Dr. Sakuraba said that he was not surprised by any of the study findings. “Earlier diagnosis of cancer is often associated with improved outcome in many cancers,” he noted.
However, “Overutilization of resources may lead to increased health care costs, so correct identification of high-risk populations is needed,” Dr. Sakuraba said.
Additional research is warranted in several areas in order to make an impact on clinical practice, Dr. Sakuraba said, notably, “confirmation in other countries and ethnicities where the incidence of viral hepatitis varies.” Comparison to other tests, such as tumor markers, CT, and MRI, is needed as well, he concluded.
The study was supported by the Taiwan Ministry of Science and Technology. The researchers had no financial conflicts to disclose. Dr. Sakuraba had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
How heat kills: Deadly weather ‘cooking’ people from within
Millions of Americans have been languishing for weeks in the oppressive heat and humidity of a merciless summer. Deadly heat has already taken the lives of hundreds in the Pacific Northwest alone, with numbers likely to grow as the full impact of heat-related deaths eventually comes to light.
In the final week of July, the National Weather Service issued excessive heat warnings for 17 states, stretching from the West Coast, across the Midwest, down south into Louisiana and Georgia. Temperatures 10° to 15° F above average threaten the lives and livelihoods of people all across the country.
After a scorching heat wave in late June, residents of the Pacific Northwest are once again likely to see triple-digit temperatures in the coming days. With the heat, hospitals may face another surge of people with heat-related illnesses.
Erika Moseson, MD, a lung and intensive care specialist, witnessed firsthand the life-threatening impacts of soaring temperatures. She happened to be running her 10-bed intensive care unit in a suburban hospital in Gresham, Ore., about 15 miles east of Portland, the weekend of June 26. Within 12 hours, almost half her ICU beds were filled with people found unconscious on the street, in the bushes, or in their own beds, all because their body’s defenses had become overwhelmed by heat.
“It was unidentified person after unidentified person, coming in, same story, temperatures through the roof, comatose,” Dr. Moseson recalled. Young people in their 20s with muscle breakdown markers through the roof, a sign of rhabdomyolysis; people with no other medical problems that would have put them in a high-risk category.
As a lifelong Oregonian, she’d never seen anything like this before. “We’re all trained for it. I know what happens to you if you have heatstroke, I know how to treat it,” she trailed off, still finding it hard to believe. Still reeling from the number of cases in just a few hours. Still shocked that this happened on what’s supposed to be the cooler, rainforest side of Oregon.
Among those she treated and resuscitated, the memory of a patient that she lost continues to gnaw at her.
“I’ve gone back to it day after day since it happened,” she reflected.
Adults, in their 50s, living at home with their children. Just 1 hour prior, they’d all said goodnight. Then 1 hour later, when a child came to check in, both parents were unconscious.
Dr. Moseson shared how her team tried everything in their power for 18 hours to save the parent that was brought to her ICU. But like hundreds of others who went through the heat wave that weekend, her patient didn’t survive.
It was too late. From Dr. Moseson’s experience, it’s what happens “if you’re cooking a human.”
How heat kills
Regardless of where we live on the planet, humans maintain a consistent internal temperature around 98° F for our systems to function properly.
Our bodies have an entire temperature-regulating system to balance heat gain with heat loss so we don’t stray too far from our ideal range. The hypothalamus functions as the thermostat, communicating with heat sensors in our skin, muscles, and spinal cord. Based on signals about our core body temperature, our nervous system makes many decisions for us – opening up blood vessels in the peripheral parts of our body, pushing more blood toward the skin, and activating sweat glands to produce more sweat.
Sweat is one of the most powerful tools we have to maintain a safe internal temperature. Of course, there are some things under our control, such as removing clothing, drinking more water, and finding shade (or preferably air conditioning). But beyond that, it’s our ability to sweat that keeps us cool. When sweat evaporates into the air, heat from our skin goes with it, cooling us off.
Over time, our sweat response can work better as we get used to warmer environments, a process that’s known as acclimatization. Over the period of a few days to weeks, the sweat glands of acclimated people can start making sweat at lower temperatures, produce more sweat, and absorb more salt back into our system, all to make us more efficient “sweaters.”
While someone who’s not used to the heat may only produce 1 liter of sweat per hour, people who have become acclimated can produce 2-3 liters every hour, allowing evaporation to eliminate more than two times the amount of heat.
Because the process of acclimatization can take some time, typically it’s the first throes of summer, or heat waves in places where people don’t typically see high temperatures, that are the most deadly. And of course, the right infrastructure, like access to air conditioning, also plays a large role in limiting heat-related death and hospitalization.
A 2019 study showed that heat-related hospitalizations peak at different temperatures in different places. For example, hospitalizations typically peak in Texas when the temperature hits 105° F. But they might be highest in the Pacific Northwest at just 81° F.
Even with acclimatization, there are limits to how much our bodies can adapt to heat. When the humidity goes up past 75%, there’s already so much moisture in the air that heat loss through evaporation no longer occurs.
It’s this connection between heat and humidity that can be deadly. This is why the heat index (a measure that takes into account temperature and relative humidity) and wet bulb globe temperature (a measure commonly used by the military and competitive athletes that takes into account temperature, humidity, wind speed, sun angle, and cloud cover) are both better at showing how dangerous the heat may be for our health, compared to temperature alone.
Kristie L. Ebi, PhD, a professor in the Center for Health and the Global Environment at the University of Washington, Seattle, has been studying the effects of heat and other climate-sensitive conditions on health for over 20 years. She stresses that it’s not just the recorded temperatures, but the prolonged exposure that kills.
If you never get a chance to bring down that core body temperature, if your internal temperatures stay above the range where your cells and your organs can work well for a long time, that’s when you can have the most dangerous effects of heat.
“It depends then on your age, your fitness, your individual physiology, underlying medical conditions, to how quickly that could affect the functioning of those organs. There’s lots of variability in there,” Dr. Ebi said.
Our hearts take on the brunt of the early response, working harder to pump blood toward the skin. Water and salt loss through our skin can start to cause electrolyte changes that can cause heat cramps and heat exhaustion. We feel tired, nauseated, dizzy. With enough water loss, we may become dehydrated, limiting the blood flow to our brains, causing us to pass out.
These early signs are like a car’s check engine light – systems are already being damaged, but resting, refueling, and, most importantly, turning off the heat are critical steps to prevent fatal injury.
If hazardous heat exposure continues and our internal temperatures continue to rise, nerves stop talking to each other, the proteins in our body unfold and lose their shape, and the cells of our organs disintegrate. This in turn sets off a fire alarm in our blood vessels, where a variety of chemical messengers, including “heat-shock proteins,” are released. The release of these inflammatory proteins, coupled with the loss of blood flow, eventually leads to the death of cells throughout the body, from the brain, to the heart, the muscles, and the kidneys.
This process is referred to as heatstroke. In essence, we melt from the inside.
At a certain point, this cascade can’t be reversed. Just like when you cool a melting block of ice, the parts that have melted will not go back to their original shape. It’s a similar process in our bodies, so delays in cooling and treatment can lead to death rates as high as 80%.
On the outside, we see people who look confused and disoriented, with hot skin and rapid breathing, and they may eventually become unconscious. Core body temperatures over 105° F clinch the diagnosis, but at the first sign of feeling unwell, cooling should be started.
There is no fancier or more effective treatment than that: Cool right away. In emergency rooms in Washington State, doctors used body bags filled with ice and water to cool victims of the heat wave in late June.
“It was all from heat ... that’s the thing, you feel so idiotic ... you’re like, ‘I’ve given you ice’ ... you bring their temperature down. But it’s already set off this cascade that you can’t stop,” Dr. Moseson said.
By the time Dr. Moseson’s patient made it to her, cooling with ice was just the beginning of the attempts to resuscitate and revive. The patient was already showing evidence of a process causing widespread bleeding and clotting, known as disseminated intravascular coagulation, along with damage to the heart and failing kidneys. Over 18 hours, her team cooled the patient, flooded the blood vessels with fluids and blood products, attempted to start dialysis, and inserted a breathing tube – all of the technology that is used to save people from serious cardiovascular collapse from other conditions. But nothing could reverse the melting that had already occurred.
Deaths from heat are 100% preventable. Until they’re not.
No respite
As Dr. Ebi says, the key to preventing heat-related death is to cool down enough to stabilize our internal cells and proteins before the irreversible cascade begins.
But for close to 80% of Americans who live in urban areas, temperatures can be even higher and more intolerable compared to surrounding areas because of the way we’ve designed our cities. In effect, we have unintentionally created hot zones called “urban heat islands.”
Jeremy Hoffman, PhD, chief scientist for the Science Museum of Virginia, explains that things like bricks, asphalt, and parking lots absorb more of the sun’s energy throughout the day and then emit that back into the air as heat throughout the afternoon and into the evening. This raises the air and surface temperatures in cities, relative to rural areas. When temperatures don’t cool enough at night, there’s no way to recover from the day’s heat. You start the next day still depleted, with less reserve to face the heat of a new day.
When you dig even deeper, it turns out that even within the same city, there are huge “thermal inequities,” as Dr. Hoffman calls them. In a 2019 study, he found that wealthier parts of cities had more natural spaces such as parks and tree-lined streets, compared to areas that had been intentionally “redlined,” or systematically deprived of investment. This pattern repeats itself in over 100 urban areas across the country and translates to huge temperature differences on the order of 10-20 degrees Fahrenheit within the same city, at the exact same time during a heat wave.
“In some ways, the way that we’ve decided to plan and build our cities physically turns up the thermostat by several tens of degrees during heat waves in particular neighborhoods,” Dr. Hoffman said.
Dr. Hoffman’s work showed that the city of Portland (where the death toll from the heat wave in late June was the highest) had some of the most intense differences between formerly redlined vs. tree-lined areas out of the more than 100 cities that he studied.
“Watching it play out, I was really concerned, not only as a climate scientist, but as a human. Understanding the urban heat island effect and the extreme nature of the inequity in our cities, thermally and otherwise, once you start to really recognize it, you can’t forget it.”
The most vulnerable
When it comes to identifying and protecting the people most vulnerable to heat stress and heat-related death, there is an ever-growing list of those most at risk. Unfortunately, very few recognize when they themselves are at risk, often until it’s too late.
According to Linda McCauley, PhD, dean of the Emory University School of Nursing in Atlanta, “the scope of who is vulnerable is quickly increasing.”
For example, we’re used to recognizing that pregnant women and young children are at risk. Public health campaigns have long advised us not to leave young children and pets in hot cars. We know that adolescents who play sports during hot summer months are at high risk for heat-related events and even death.
In Georgia, a 15-year-old boy collapsed and died after his first day back at football practice when the heat index was 105° F on July 26, even as it appears that all protocols for heat safety were being followed.
We recognize that outdoor workers face devastating consequences from prolonged exertion in the heat and must have safer working conditions.
The elderly and those with long-term medical and mental health conditions are also more vulnerable to heat. The elderly may not have the same warning signs and may not recognize that they are dehydrated until it is too late. In addition, their sweating mechanism weakens, and they may be taking medicines that interfere with their ability to regulate their temperature.
Poverty and inadequate housing are risk factors, especially for those in urban heat islands. For many people, their housing does not have enough cooling to protect them, and they can’t safely get themselves to cooling shelters.
These patterns for the most vulnerable fit for the majority of deaths in Oregon during the late June heat wave. Most victims were older, lived alone, and didn’t have air conditioning. But with climate change, the predictions are that temperatures will go higher and heat waves will last longer.
“There’s probably very few people today that are ‘immune’ to the effects of heat-related stress with climate change. All of us can be put in situations where we are susceptible,” Dr. McCauley said.
Dr. Moseson agreed. Many of her patients fit none of these risk categories – she treated people with no health problems in their 20s in her ICU, and the patient she lost would not traditionally have been thought of as high risk. That 50-something patient had no long-standing medical problems, and lived with family in a newly renovated suburban home that had air conditioning. The only problem was that the air conditioner had broken and there had been no rush to fix it based on past experience with Oregon summers.
Preventing heat deaths
Protecting ourselves and our families means monitoring the “simple things.” The first three rules are to make sure we’re drinking plenty of water – this means drinking whether we feel thirsty or not. If we’re not in an air-conditioned place, we’ve got to look for shade. And we need to take regular rest breaks.
Inside a home without air conditioning, placing ice in front of a fan to cool the air can work, but realistically, if you are in a place without air conditioning and the temperatures are approaching 90° F, it’s safest to find another place to stay, if possible.
For those playing sports, there are usually 1-week to 2-week protocols that allow for acclimatization when the season begins – this means starting slowly, without gear, and ramping up activity. Still, parents and coaches should watch advanced weather reports to make sure it’s safe to practice outside.
How we dress can also help us, so light clothing is key. And if we’re able to schedule activities for times when it is cooler, that can also protect us from overheating.
If anyone shows early signs of heat stress, removing clothing, cooling their bodies with cold water, and getting them out of the heat is critical. Any evidence of heatstroke is an emergency, and 911 should be called without delay. The faster the core temperature can be dropped, the better the chances for recovery.
On the level of communities, access to natural air conditioning in the form of healthy tree canopies, and trees at bus stops to provide shade can help a lot. According to Dr. Hoffman, these investments help almost right away. Reimagining our cities to remove the “hot zones” that we have created is another key to protecting ourselves as our climate changes.
Reaching our limits in a changing climate
Already, we are seeing more intense, more frequent, and longer-lasting heat waves throughout the country and across the globe.
Dr. Ebi, a coauthor of a recently released scientific analysis that found that the late June Pacific Northwest heat wave would have been virtually impossible without climate change, herself lived through the scorching temperatures in Seattle. Her work shows that the changing climate is killing us right now.
We are approaching a time where extreme temperatures and humidity will make it almost impossible for people to be outside in many parts of the world. Researchers have found that periods of extreme humid heat have more than doubled since 1979, and some places have already had wet-bulb temperatures at the limits of what scientists think humans can tolerate under ideal conditions, meaning for people in perfect health, completely unclothed, in gale-force winds, performing no activity. Obviously that’s less than ideal for most of us and helps explain why thousands of people die at temperatures much lower than our upper limit.
Dr. Ebi pointed out that the good news is that many local communities with a long history of managing high temperatures have a lot of knowledge to share with regions that are newly dealing with these conditions. This includes how local areas develop early warning and response systems with specific action plans.
But, she cautions, it’s going to take a lot of coordination and a lot of behavior change to stabilize the earth’s climate, understand our weak points, and protect our health.
For Dr. Moseson, this reality has hit home.
“I already spent the year being terrified that I as an ICU doctor was going to be the one who gave my mom COVID. Finally I’m vaccinated, she’s vaccinated. Now I’ve watched someone die because they don’t have AC. And my parents, they’re old-school Oregonians, they don’t have AC.”
A version of this article originally appeared on WebMD.com.
Millions of Americans have been languishing for weeks in the oppressive heat and humidity of a merciless summer. Deadly heat has already taken the lives of hundreds in the Pacific Northwest alone, with numbers likely to grow as the full impact of heat-related deaths eventually comes to light.
In the final week of July, the National Weather Service issued excessive heat warnings for 17 states, stretching from the West Coast, across the Midwest, down south into Louisiana and Georgia. Temperatures 10° to 15° F above average threaten the lives and livelihoods of people all across the country.
After a scorching heat wave in late June, residents of the Pacific Northwest are once again likely to see triple-digit temperatures in the coming days. With the heat, hospitals may face another surge of people with heat-related illnesses.
Erika Moseson, MD, a lung and intensive care specialist, witnessed firsthand the life-threatening impacts of soaring temperatures. She happened to be running her 10-bed intensive care unit in a suburban hospital in Gresham, Ore., about 15 miles east of Portland, the weekend of June 26. Within 12 hours, almost half her ICU beds were filled with people found unconscious on the street, in the bushes, or in their own beds, all because their body’s defenses had become overwhelmed by heat.
“It was unidentified person after unidentified person, coming in, same story, temperatures through the roof, comatose,” Dr. Moseson recalled. Young people in their 20s with muscle breakdown markers through the roof, a sign of rhabdomyolysis; people with no other medical problems that would have put them in a high-risk category.
As a lifelong Oregonian, she’d never seen anything like this before. “We’re all trained for it. I know what happens to you if you have heatstroke, I know how to treat it,” she trailed off, still finding it hard to believe. Still reeling from the number of cases in just a few hours. Still shocked that this happened on what’s supposed to be the cooler, rainforest side of Oregon.
Among those she treated and resuscitated, the memory of a patient that she lost continues to gnaw at her.
“I’ve gone back to it day after day since it happened,” she reflected.
Adults, in their 50s, living at home with their children. Just 1 hour prior, they’d all said goodnight. Then 1 hour later, when a child came to check in, both parents were unconscious.
Dr. Moseson shared how her team tried everything in their power for 18 hours to save the parent that was brought to her ICU. But like hundreds of others who went through the heat wave that weekend, her patient didn’t survive.
It was too late. From Dr. Moseson’s experience, it’s what happens “if you’re cooking a human.”
How heat kills
Regardless of where we live on the planet, humans maintain a consistent internal temperature around 98° F for our systems to function properly.
Our bodies have an entire temperature-regulating system to balance heat gain with heat loss so we don’t stray too far from our ideal range. The hypothalamus functions as the thermostat, communicating with heat sensors in our skin, muscles, and spinal cord. Based on signals about our core body temperature, our nervous system makes many decisions for us – opening up blood vessels in the peripheral parts of our body, pushing more blood toward the skin, and activating sweat glands to produce more sweat.
Sweat is one of the most powerful tools we have to maintain a safe internal temperature. Of course, there are some things under our control, such as removing clothing, drinking more water, and finding shade (or preferably air conditioning). But beyond that, it’s our ability to sweat that keeps us cool. When sweat evaporates into the air, heat from our skin goes with it, cooling us off.
Over time, our sweat response can work better as we get used to warmer environments, a process that’s known as acclimatization. Over the period of a few days to weeks, the sweat glands of acclimated people can start making sweat at lower temperatures, produce more sweat, and absorb more salt back into our system, all to make us more efficient “sweaters.”
While someone who’s not used to the heat may only produce 1 liter of sweat per hour, people who have become acclimated can produce 2-3 liters every hour, allowing evaporation to eliminate more than two times the amount of heat.
Because the process of acclimatization can take some time, typically it’s the first throes of summer, or heat waves in places where people don’t typically see high temperatures, that are the most deadly. And of course, the right infrastructure, like access to air conditioning, also plays a large role in limiting heat-related death and hospitalization.
A 2019 study showed that heat-related hospitalizations peak at different temperatures in different places. For example, hospitalizations typically peak in Texas when the temperature hits 105° F. But they might be highest in the Pacific Northwest at just 81° F.
Even with acclimatization, there are limits to how much our bodies can adapt to heat. When the humidity goes up past 75%, there’s already so much moisture in the air that heat loss through evaporation no longer occurs.
It’s this connection between heat and humidity that can be deadly. This is why the heat index (a measure that takes into account temperature and relative humidity) and wet bulb globe temperature (a measure commonly used by the military and competitive athletes that takes into account temperature, humidity, wind speed, sun angle, and cloud cover) are both better at showing how dangerous the heat may be for our health, compared to temperature alone.
Kristie L. Ebi, PhD, a professor in the Center for Health and the Global Environment at the University of Washington, Seattle, has been studying the effects of heat and other climate-sensitive conditions on health for over 20 years. She stresses that it’s not just the recorded temperatures, but the prolonged exposure that kills.
If you never get a chance to bring down that core body temperature, if your internal temperatures stay above the range where your cells and your organs can work well for a long time, that’s when you can have the most dangerous effects of heat.
“It depends then on your age, your fitness, your individual physiology, underlying medical conditions, to how quickly that could affect the functioning of those organs. There’s lots of variability in there,” Dr. Ebi said.
Our hearts take on the brunt of the early response, working harder to pump blood toward the skin. Water and salt loss through our skin can start to cause electrolyte changes that can cause heat cramps and heat exhaustion. We feel tired, nauseated, dizzy. With enough water loss, we may become dehydrated, limiting the blood flow to our brains, causing us to pass out.
These early signs are like a car’s check engine light – systems are already being damaged, but resting, refueling, and, most importantly, turning off the heat are critical steps to prevent fatal injury.
If hazardous heat exposure continues and our internal temperatures continue to rise, nerves stop talking to each other, the proteins in our body unfold and lose their shape, and the cells of our organs disintegrate. This in turn sets off a fire alarm in our blood vessels, where a variety of chemical messengers, including “heat-shock proteins,” are released. The release of these inflammatory proteins, coupled with the loss of blood flow, eventually leads to the death of cells throughout the body, from the brain, to the heart, the muscles, and the kidneys.
This process is referred to as heatstroke. In essence, we melt from the inside.
At a certain point, this cascade can’t be reversed. Just like when you cool a melting block of ice, the parts that have melted will not go back to their original shape. It’s a similar process in our bodies, so delays in cooling and treatment can lead to death rates as high as 80%.
On the outside, we see people who look confused and disoriented, with hot skin and rapid breathing, and they may eventually become unconscious. Core body temperatures over 105° F clinch the diagnosis, but at the first sign of feeling unwell, cooling should be started.
There is no fancier or more effective treatment than that: Cool right away. In emergency rooms in Washington State, doctors used body bags filled with ice and water to cool victims of the heat wave in late June.
“It was all from heat ... that’s the thing, you feel so idiotic ... you’re like, ‘I’ve given you ice’ ... you bring their temperature down. But it’s already set off this cascade that you can’t stop,” Dr. Moseson said.
By the time Dr. Moseson’s patient made it to her, cooling with ice was just the beginning of the attempts to resuscitate and revive. The patient was already showing evidence of a process causing widespread bleeding and clotting, known as disseminated intravascular coagulation, along with damage to the heart and failing kidneys. Over 18 hours, her team cooled the patient, flooded the blood vessels with fluids and blood products, attempted to start dialysis, and inserted a breathing tube – all of the technology that is used to save people from serious cardiovascular collapse from other conditions. But nothing could reverse the melting that had already occurred.
Deaths from heat are 100% preventable. Until they’re not.
No respite
As Dr. Ebi says, the key to preventing heat-related death is to cool down enough to stabilize our internal cells and proteins before the irreversible cascade begins.
But for close to 80% of Americans who live in urban areas, temperatures can be even higher and more intolerable compared to surrounding areas because of the way we’ve designed our cities. In effect, we have unintentionally created hot zones called “urban heat islands.”
Jeremy Hoffman, PhD, chief scientist for the Science Museum of Virginia, explains that things like bricks, asphalt, and parking lots absorb more of the sun’s energy throughout the day and then emit that back into the air as heat throughout the afternoon and into the evening. This raises the air and surface temperatures in cities, relative to rural areas. When temperatures don’t cool enough at night, there’s no way to recover from the day’s heat. You start the next day still depleted, with less reserve to face the heat of a new day.
When you dig even deeper, it turns out that even within the same city, there are huge “thermal inequities,” as Dr. Hoffman calls them. In a 2019 study, he found that wealthier parts of cities had more natural spaces such as parks and tree-lined streets, compared to areas that had been intentionally “redlined,” or systematically deprived of investment. This pattern repeats itself in over 100 urban areas across the country and translates to huge temperature differences on the order of 10-20 degrees Fahrenheit within the same city, at the exact same time during a heat wave.
“In some ways, the way that we’ve decided to plan and build our cities physically turns up the thermostat by several tens of degrees during heat waves in particular neighborhoods,” Dr. Hoffman said.
Dr. Hoffman’s work showed that the city of Portland (where the death toll from the heat wave in late June was the highest) had some of the most intense differences between formerly redlined vs. tree-lined areas out of the more than 100 cities that he studied.
“Watching it play out, I was really concerned, not only as a climate scientist, but as a human. Understanding the urban heat island effect and the extreme nature of the inequity in our cities, thermally and otherwise, once you start to really recognize it, you can’t forget it.”
The most vulnerable
When it comes to identifying and protecting the people most vulnerable to heat stress and heat-related death, there is an ever-growing list of those most at risk. Unfortunately, very few recognize when they themselves are at risk, often until it’s too late.
According to Linda McCauley, PhD, dean of the Emory University School of Nursing in Atlanta, “the scope of who is vulnerable is quickly increasing.”
For example, we’re used to recognizing that pregnant women and young children are at risk. Public health campaigns have long advised us not to leave young children and pets in hot cars. We know that adolescents who play sports during hot summer months are at high risk for heat-related events and even death.
In Georgia, a 15-year-old boy collapsed and died after his first day back at football practice when the heat index was 105° F on July 26, even as it appears that all protocols for heat safety were being followed.
We recognize that outdoor workers face devastating consequences from prolonged exertion in the heat and must have safer working conditions.
The elderly and those with long-term medical and mental health conditions are also more vulnerable to heat. The elderly may not have the same warning signs and may not recognize that they are dehydrated until it is too late. In addition, their sweating mechanism weakens, and they may be taking medicines that interfere with their ability to regulate their temperature.
Poverty and inadequate housing are risk factors, especially for those in urban heat islands. For many people, their housing does not have enough cooling to protect them, and they can’t safely get themselves to cooling shelters.
These patterns for the most vulnerable fit for the majority of deaths in Oregon during the late June heat wave. Most victims were older, lived alone, and didn’t have air conditioning. But with climate change, the predictions are that temperatures will go higher and heat waves will last longer.
“There’s probably very few people today that are ‘immune’ to the effects of heat-related stress with climate change. All of us can be put in situations where we are susceptible,” Dr. McCauley said.
Dr. Moseson agreed. Many of her patients fit none of these risk categories – she treated people with no health problems in their 20s in her ICU, and the patient she lost would not traditionally have been thought of as high risk. That 50-something patient had no long-standing medical problems, and lived with family in a newly renovated suburban home that had air conditioning. The only problem was that the air conditioner had broken and there had been no rush to fix it based on past experience with Oregon summers.
Preventing heat deaths
Protecting ourselves and our families means monitoring the “simple things.” The first three rules are to make sure we’re drinking plenty of water – this means drinking whether we feel thirsty or not. If we’re not in an air-conditioned place, we’ve got to look for shade. And we need to take regular rest breaks.
Inside a home without air conditioning, placing ice in front of a fan to cool the air can work, but realistically, if you are in a place without air conditioning and the temperatures are approaching 90° F, it’s safest to find another place to stay, if possible.
For those playing sports, there are usually 1-week to 2-week protocols that allow for acclimatization when the season begins – this means starting slowly, without gear, and ramping up activity. Still, parents and coaches should watch advanced weather reports to make sure it’s safe to practice outside.
How we dress can also help us, so light clothing is key. And if we’re able to schedule activities for times when it is cooler, that can also protect us from overheating.
If anyone shows early signs of heat stress, removing clothing, cooling their bodies with cold water, and getting them out of the heat is critical. Any evidence of heatstroke is an emergency, and 911 should be called without delay. The faster the core temperature can be dropped, the better the chances for recovery.
On the level of communities, access to natural air conditioning in the form of healthy tree canopies, and trees at bus stops to provide shade can help a lot. According to Dr. Hoffman, these investments help almost right away. Reimagining our cities to remove the “hot zones” that we have created is another key to protecting ourselves as our climate changes.
Reaching our limits in a changing climate
Already, we are seeing more intense, more frequent, and longer-lasting heat waves throughout the country and across the globe.
Dr. Ebi, a coauthor of a recently released scientific analysis that found that the late June Pacific Northwest heat wave would have been virtually impossible without climate change, herself lived through the scorching temperatures in Seattle. Her work shows that the changing climate is killing us right now.
We are approaching a time where extreme temperatures and humidity will make it almost impossible for people to be outside in many parts of the world. Researchers have found that periods of extreme humid heat have more than doubled since 1979, and some places have already had wet-bulb temperatures at the limits of what scientists think humans can tolerate under ideal conditions, meaning for people in perfect health, completely unclothed, in gale-force winds, performing no activity. Obviously that’s less than ideal for most of us and helps explain why thousands of people die at temperatures much lower than our upper limit.
Dr. Ebi pointed out that the good news is that many local communities with a long history of managing high temperatures have a lot of knowledge to share with regions that are newly dealing with these conditions. This includes how local areas develop early warning and response systems with specific action plans.
But, she cautions, it’s going to take a lot of coordination and a lot of behavior change to stabilize the earth’s climate, understand our weak points, and protect our health.
For Dr. Moseson, this reality has hit home.
“I already spent the year being terrified that I as an ICU doctor was going to be the one who gave my mom COVID. Finally I’m vaccinated, she’s vaccinated. Now I’ve watched someone die because they don’t have AC. And my parents, they’re old-school Oregonians, they don’t have AC.”
A version of this article originally appeared on WebMD.com.
Millions of Americans have been languishing for weeks in the oppressive heat and humidity of a merciless summer. Deadly heat has already taken the lives of hundreds in the Pacific Northwest alone, with numbers likely to grow as the full impact of heat-related deaths eventually comes to light.
In the final week of July, the National Weather Service issued excessive heat warnings for 17 states, stretching from the West Coast, across the Midwest, down south into Louisiana and Georgia. Temperatures 10° to 15° F above average threaten the lives and livelihoods of people all across the country.
After a scorching heat wave in late June, residents of the Pacific Northwest are once again likely to see triple-digit temperatures in the coming days. With the heat, hospitals may face another surge of people with heat-related illnesses.
Erika Moseson, MD, a lung and intensive care specialist, witnessed firsthand the life-threatening impacts of soaring temperatures. She happened to be running her 10-bed intensive care unit in a suburban hospital in Gresham, Ore., about 15 miles east of Portland, the weekend of June 26. Within 12 hours, almost half her ICU beds were filled with people found unconscious on the street, in the bushes, or in their own beds, all because their body’s defenses had become overwhelmed by heat.
“It was unidentified person after unidentified person, coming in, same story, temperatures through the roof, comatose,” Dr. Moseson recalled. Young people in their 20s with muscle breakdown markers through the roof, a sign of rhabdomyolysis; people with no other medical problems that would have put them in a high-risk category.
As a lifelong Oregonian, she’d never seen anything like this before. “We’re all trained for it. I know what happens to you if you have heatstroke, I know how to treat it,” she trailed off, still finding it hard to believe. Still reeling from the number of cases in just a few hours. Still shocked that this happened on what’s supposed to be the cooler, rainforest side of Oregon.
Among those she treated and resuscitated, the memory of a patient that she lost continues to gnaw at her.
“I’ve gone back to it day after day since it happened,” she reflected.
Adults, in their 50s, living at home with their children. Just 1 hour prior, they’d all said goodnight. Then 1 hour later, when a child came to check in, both parents were unconscious.
Dr. Moseson shared how her team tried everything in their power for 18 hours to save the parent that was brought to her ICU. But like hundreds of others who went through the heat wave that weekend, her patient didn’t survive.
It was too late. From Dr. Moseson’s experience, it’s what happens “if you’re cooking a human.”
How heat kills
Regardless of where we live on the planet, humans maintain a consistent internal temperature around 98° F for our systems to function properly.
Our bodies have an entire temperature-regulating system to balance heat gain with heat loss so we don’t stray too far from our ideal range. The hypothalamus functions as the thermostat, communicating with heat sensors in our skin, muscles, and spinal cord. Based on signals about our core body temperature, our nervous system makes many decisions for us – opening up blood vessels in the peripheral parts of our body, pushing more blood toward the skin, and activating sweat glands to produce more sweat.
Sweat is one of the most powerful tools we have to maintain a safe internal temperature. Of course, there are some things under our control, such as removing clothing, drinking more water, and finding shade (or preferably air conditioning). But beyond that, it’s our ability to sweat that keeps us cool. When sweat evaporates into the air, heat from our skin goes with it, cooling us off.
Over time, our sweat response can work better as we get used to warmer environments, a process that’s known as acclimatization. Over the period of a few days to weeks, the sweat glands of acclimated people can start making sweat at lower temperatures, produce more sweat, and absorb more salt back into our system, all to make us more efficient “sweaters.”
While someone who’s not used to the heat may only produce 1 liter of sweat per hour, people who have become acclimated can produce 2-3 liters every hour, allowing evaporation to eliminate more than two times the amount of heat.
Because the process of acclimatization can take some time, typically it’s the first throes of summer, or heat waves in places where people don’t typically see high temperatures, that are the most deadly. And of course, the right infrastructure, like access to air conditioning, also plays a large role in limiting heat-related death and hospitalization.
A 2019 study showed that heat-related hospitalizations peak at different temperatures in different places. For example, hospitalizations typically peak in Texas when the temperature hits 105° F. But they might be highest in the Pacific Northwest at just 81° F.
Even with acclimatization, there are limits to how much our bodies can adapt to heat. When the humidity goes up past 75%, there’s already so much moisture in the air that heat loss through evaporation no longer occurs.
It’s this connection between heat and humidity that can be deadly. This is why the heat index (a measure that takes into account temperature and relative humidity) and wet bulb globe temperature (a measure commonly used by the military and competitive athletes that takes into account temperature, humidity, wind speed, sun angle, and cloud cover) are both better at showing how dangerous the heat may be for our health, compared to temperature alone.
Kristie L. Ebi, PhD, a professor in the Center for Health and the Global Environment at the University of Washington, Seattle, has been studying the effects of heat and other climate-sensitive conditions on health for over 20 years. She stresses that it’s not just the recorded temperatures, but the prolonged exposure that kills.
If you never get a chance to bring down that core body temperature, if your internal temperatures stay above the range where your cells and your organs can work well for a long time, that’s when you can have the most dangerous effects of heat.
“It depends then on your age, your fitness, your individual physiology, underlying medical conditions, to how quickly that could affect the functioning of those organs. There’s lots of variability in there,” Dr. Ebi said.
Our hearts take on the brunt of the early response, working harder to pump blood toward the skin. Water and salt loss through our skin can start to cause electrolyte changes that can cause heat cramps and heat exhaustion. We feel tired, nauseated, dizzy. With enough water loss, we may become dehydrated, limiting the blood flow to our brains, causing us to pass out.
These early signs are like a car’s check engine light – systems are already being damaged, but resting, refueling, and, most importantly, turning off the heat are critical steps to prevent fatal injury.
If hazardous heat exposure continues and our internal temperatures continue to rise, nerves stop talking to each other, the proteins in our body unfold and lose their shape, and the cells of our organs disintegrate. This in turn sets off a fire alarm in our blood vessels, where a variety of chemical messengers, including “heat-shock proteins,” are released. The release of these inflammatory proteins, coupled with the loss of blood flow, eventually leads to the death of cells throughout the body, from the brain, to the heart, the muscles, and the kidneys.
This process is referred to as heatstroke. In essence, we melt from the inside.
At a certain point, this cascade can’t be reversed. Just like when you cool a melting block of ice, the parts that have melted will not go back to their original shape. It’s a similar process in our bodies, so delays in cooling and treatment can lead to death rates as high as 80%.
On the outside, we see people who look confused and disoriented, with hot skin and rapid breathing, and they may eventually become unconscious. Core body temperatures over 105° F clinch the diagnosis, but at the first sign of feeling unwell, cooling should be started.
There is no fancier or more effective treatment than that: Cool right away. In emergency rooms in Washington State, doctors used body bags filled with ice and water to cool victims of the heat wave in late June.
“It was all from heat ... that’s the thing, you feel so idiotic ... you’re like, ‘I’ve given you ice’ ... you bring their temperature down. But it’s already set off this cascade that you can’t stop,” Dr. Moseson said.
By the time Dr. Moseson’s patient made it to her, cooling with ice was just the beginning of the attempts to resuscitate and revive. The patient was already showing evidence of a process causing widespread bleeding and clotting, known as disseminated intravascular coagulation, along with damage to the heart and failing kidneys. Over 18 hours, her team cooled the patient, flooded the blood vessels with fluids and blood products, attempted to start dialysis, and inserted a breathing tube – all of the technology that is used to save people from serious cardiovascular collapse from other conditions. But nothing could reverse the melting that had already occurred.
Deaths from heat are 100% preventable. Until they’re not.
No respite
As Dr. Ebi says, the key to preventing heat-related death is to cool down enough to stabilize our internal cells and proteins before the irreversible cascade begins.
But for close to 80% of Americans who live in urban areas, temperatures can be even higher and more intolerable compared to surrounding areas because of the way we’ve designed our cities. In effect, we have unintentionally created hot zones called “urban heat islands.”
Jeremy Hoffman, PhD, chief scientist for the Science Museum of Virginia, explains that things like bricks, asphalt, and parking lots absorb more of the sun’s energy throughout the day and then emit that back into the air as heat throughout the afternoon and into the evening. This raises the air and surface temperatures in cities, relative to rural areas. When temperatures don’t cool enough at night, there’s no way to recover from the day’s heat. You start the next day still depleted, with less reserve to face the heat of a new day.
When you dig even deeper, it turns out that even within the same city, there are huge “thermal inequities,” as Dr. Hoffman calls them. In a 2019 study, he found that wealthier parts of cities had more natural spaces such as parks and tree-lined streets, compared to areas that had been intentionally “redlined,” or systematically deprived of investment. This pattern repeats itself in over 100 urban areas across the country and translates to huge temperature differences on the order of 10-20 degrees Fahrenheit within the same city, at the exact same time during a heat wave.
“In some ways, the way that we’ve decided to plan and build our cities physically turns up the thermostat by several tens of degrees during heat waves in particular neighborhoods,” Dr. Hoffman said.
Dr. Hoffman’s work showed that the city of Portland (where the death toll from the heat wave in late June was the highest) had some of the most intense differences between formerly redlined vs. tree-lined areas out of the more than 100 cities that he studied.
“Watching it play out, I was really concerned, not only as a climate scientist, but as a human. Understanding the urban heat island effect and the extreme nature of the inequity in our cities, thermally and otherwise, once you start to really recognize it, you can’t forget it.”
The most vulnerable
When it comes to identifying and protecting the people most vulnerable to heat stress and heat-related death, there is an ever-growing list of those most at risk. Unfortunately, very few recognize when they themselves are at risk, often until it’s too late.
According to Linda McCauley, PhD, dean of the Emory University School of Nursing in Atlanta, “the scope of who is vulnerable is quickly increasing.”
For example, we’re used to recognizing that pregnant women and young children are at risk. Public health campaigns have long advised us not to leave young children and pets in hot cars. We know that adolescents who play sports during hot summer months are at high risk for heat-related events and even death.
In Georgia, a 15-year-old boy collapsed and died after his first day back at football practice when the heat index was 105° F on July 26, even as it appears that all protocols for heat safety were being followed.
We recognize that outdoor workers face devastating consequences from prolonged exertion in the heat and must have safer working conditions.
The elderly and those with long-term medical and mental health conditions are also more vulnerable to heat. The elderly may not have the same warning signs and may not recognize that they are dehydrated until it is too late. In addition, their sweating mechanism weakens, and they may be taking medicines that interfere with their ability to regulate their temperature.
Poverty and inadequate housing are risk factors, especially for those in urban heat islands. For many people, their housing does not have enough cooling to protect them, and they can’t safely get themselves to cooling shelters.
These patterns for the most vulnerable fit for the majority of deaths in Oregon during the late June heat wave. Most victims were older, lived alone, and didn’t have air conditioning. But with climate change, the predictions are that temperatures will go higher and heat waves will last longer.
“There’s probably very few people today that are ‘immune’ to the effects of heat-related stress with climate change. All of us can be put in situations where we are susceptible,” Dr. McCauley said.
Dr. Moseson agreed. Many of her patients fit none of these risk categories – she treated people with no health problems in their 20s in her ICU, and the patient she lost would not traditionally have been thought of as high risk. That 50-something patient had no long-standing medical problems, and lived with family in a newly renovated suburban home that had air conditioning. The only problem was that the air conditioner had broken and there had been no rush to fix it based on past experience with Oregon summers.
Preventing heat deaths
Protecting ourselves and our families means monitoring the “simple things.” The first three rules are to make sure we’re drinking plenty of water – this means drinking whether we feel thirsty or not. If we’re not in an air-conditioned place, we’ve got to look for shade. And we need to take regular rest breaks.
Inside a home without air conditioning, placing ice in front of a fan to cool the air can work, but realistically, if you are in a place without air conditioning and the temperatures are approaching 90° F, it’s safest to find another place to stay, if possible.
For those playing sports, there are usually 1-week to 2-week protocols that allow for acclimatization when the season begins – this means starting slowly, without gear, and ramping up activity. Still, parents and coaches should watch advanced weather reports to make sure it’s safe to practice outside.
How we dress can also help us, so light clothing is key. And if we’re able to schedule activities for times when it is cooler, that can also protect us from overheating.
If anyone shows early signs of heat stress, removing clothing, cooling their bodies with cold water, and getting them out of the heat is critical. Any evidence of heatstroke is an emergency, and 911 should be called without delay. The faster the core temperature can be dropped, the better the chances for recovery.
On the level of communities, access to natural air conditioning in the form of healthy tree canopies, and trees at bus stops to provide shade can help a lot. According to Dr. Hoffman, these investments help almost right away. Reimagining our cities to remove the “hot zones” that we have created is another key to protecting ourselves as our climate changes.
Reaching our limits in a changing climate
Already, we are seeing more intense, more frequent, and longer-lasting heat waves throughout the country and across the globe.
Dr. Ebi, a coauthor of a recently released scientific analysis that found that the late June Pacific Northwest heat wave would have been virtually impossible without climate change, herself lived through the scorching temperatures in Seattle. Her work shows that the changing climate is killing us right now.
We are approaching a time where extreme temperatures and humidity will make it almost impossible for people to be outside in many parts of the world. Researchers have found that periods of extreme humid heat have more than doubled since 1979, and some places have already had wet-bulb temperatures at the limits of what scientists think humans can tolerate under ideal conditions, meaning for people in perfect health, completely unclothed, in gale-force winds, performing no activity. Obviously that’s less than ideal for most of us and helps explain why thousands of people die at temperatures much lower than our upper limit.
Dr. Ebi pointed out that the good news is that many local communities with a long history of managing high temperatures have a lot of knowledge to share with regions that are newly dealing with these conditions. This includes how local areas develop early warning and response systems with specific action plans.
But, she cautions, it’s going to take a lot of coordination and a lot of behavior change to stabilize the earth’s climate, understand our weak points, and protect our health.
For Dr. Moseson, this reality has hit home.
“I already spent the year being terrified that I as an ICU doctor was going to be the one who gave my mom COVID. Finally I’m vaccinated, she’s vaccinated. Now I’ve watched someone die because they don’t have AC. And my parents, they’re old-school Oregonians, they don’t have AC.”
A version of this article originally appeared on WebMD.com.
Delta variant could drive herd immunity threshold over 80%
Because the Delta variant of SARS-CoV-2 spreads more easily than the original virus, the proportion of the population that needs to be vaccinated to reach herd immunity could be upward of 80% or more, experts say.
Also, it could be time to consider wearing an N95 mask in public indoor spaces regardless of vaccination status, according to a media briefing on Aug. 3 sponsored by the Infectious Diseases Society of America.
Furthermore, giving booster shots to the fully vaccinated is not the top public health priority now. Instead, third vaccinations should be reserved for more vulnerable populations – and efforts should focus on getting first vaccinations to unvaccinated people in the United States and around the world.
“The problem here is that the Delta variant is ... more transmissible than the original virus. That pushes the overall population herd immunity threshold much higher,” Ricardo Franco, MD, assistant professor of medicine at the University of Alabama at Birmingham, said during the briefing.
“For Delta, those threshold estimates go well over 80% and may be approaching 90%,” he said.
To put that figure in context, the original SARS-CoV-2 virus required an estimated 67% of the population to be vaccinated to achieve herd immunity. Also, measles has one of the highest herd immunity thresholds at 95%, Dr. Franco added.
Herd immunity is the point at which enough people are immunized that the entire population gains protection. And it’s already happening. “Unvaccinated people are actually benefiting from greater herd immunity protection in high-vaccination counties compared to low-vaccination ones,” he said.
Maximize mask protection
Unlike early in the COVID-19 pandemic with widespread shortages of personal protective equipment, face masks are now readily available. This includes N95 masks, which offer enhanced protection against SARS-CoV-2, Ezekiel J. Emanuel, MD, PhD, said during the briefing.
Following the July 27 CDC recommendation that most Americans wear masks indoors when in public places, “I do think we need to upgrade our masks,” said Dr. Emanuel, who is Diane v.S. Levy & Robert M. Levy professor at the University of Pennsylvania, Philadelphia.
“It’s not just any mask,” he added. “Good masks make a big difference and are very important.”
Mask protection is about blocking 0.3-mcm particles, “and I think we need to make sure that people have masks that can filter that out,” he said. Although surgical masks are very good, he added, “they’re not quite as good as N95s.” As their name implies, N95s filter out 95% of these particles.
Dr. Emanuel acknowledged that people are tired of COVID-19 and complying with public health measures but urged perseverance. “We’ve sacrificed a lot. We should not throw it away in just a few months because we are tired. We’re all tired, but we do have to do the little bit extra getting vaccinated, wearing masks indoors, and protecting ourselves, our families, and our communities.”
Dealing with a disconnect
In response to a reporter’s question about the possibility that the large crowd at the Lollapalooza music festival in Chicago could become a superspreader event, Dr. Emanuel said, “it is worrisome.”
“I would say that, if you’re going to go to a gathering like that, wearing an N95 mask is wise, and not spending too long at any one place is also wise,” he said.
On the plus side, the event was held outdoors with lots of air circulation, Dr. Emanuel said.
However, “this is the kind of thing where we’ve got a sort of disconnect between people’s desire to get back to normal ... and the fact that we’re in the middle of this upsurge.”
Another potential problem is the event brought people together from many different locations, so when they travel home, they could be “potentially seeding lots of other communities.”
Boosters for some, for now
Even though not officially recommended, some fully vaccinated Americans are seeking a third or booster vaccination on their own.
Asked for his opinion, Dr. Emanuel said: “We’re probably going to have to be giving boosters to immunocompromised people and people who are susceptible. That’s where we are going to start.”
More research is needed regarding booster shots, he said. “There are very small studies – and the ‘very small’ should be emphasized – given that we’ve given shots to over 160 million people.”
“But it does appear that the boosters increase the antibodies and protection,” he said.
Instead of boosters, it is more important for people who haven’t been vaccinated to get fully vaccinated.
“We need to put our priorities in the right places,” he said.
Emanuel noted that, except for people in rural areas that might have to travel long distances, access to vaccines is no longer an issue. “It’s very hard not to find a vaccine if you want it.”
A remaining hurdle is “battling a major disinformation initiative. I don’t think this is misinformation. I think there’s very clear evidence that it is disinformation – false facts about the vaccines being spread,” Dr. Emanuel said.
The breakthrough infection dilemma
Breakthrough cases “remain the vast minority of infections at this time ... that is reassuring,” Dr. Franco said.
Also, tracking symptomatic breakthrough infections remains easier than studying fully vaccinated people who become infected with SARS-CoV-2 but remain symptom free.
“We really don’t have a good handle on the frequency of asymptomatic cases,” Dr. Emanuel said. “If you’re missing breakthrough infections, a lot of them, you may be missing some [virus] evolution that would be very important for us to follow.” This missing information could include the emergence of new variants.
The asymptomatic breakthrough cases are the most worrisome group,” Dr. Emanuel said. “You get infected, you’re feeling fine. Maybe you’ve got a little sneeze or cough, but nothing unusual. And then you’re still able to transmit the Delta variant.”
The big picture
The upsurge in cases, hospitalizations, and deaths is a major challenge, Dr. Emanuel said. “We need to address that by getting many more people vaccinated right now with what are very good vaccines.”
“But it also means that we have to stop being U.S. focused alone.” He pointed out that Delta and other variants originated overseas, “so getting the world vaccinated ... has to be a top priority.”
“We are obviously all facing a challenge as we move into the fall,” Dr. Emanuel said. “With schools opening and employers bringing their employees back together, even if these groups are vaccinated, there are going to be major challenges for all of us.”
A version of this article first appeared on Medscape.com.
Because the Delta variant of SARS-CoV-2 spreads more easily than the original virus, the proportion of the population that needs to be vaccinated to reach herd immunity could be upward of 80% or more, experts say.
Also, it could be time to consider wearing an N95 mask in public indoor spaces regardless of vaccination status, according to a media briefing on Aug. 3 sponsored by the Infectious Diseases Society of America.
Furthermore, giving booster shots to the fully vaccinated is not the top public health priority now. Instead, third vaccinations should be reserved for more vulnerable populations – and efforts should focus on getting first vaccinations to unvaccinated people in the United States and around the world.
“The problem here is that the Delta variant is ... more transmissible than the original virus. That pushes the overall population herd immunity threshold much higher,” Ricardo Franco, MD, assistant professor of medicine at the University of Alabama at Birmingham, said during the briefing.
“For Delta, those threshold estimates go well over 80% and may be approaching 90%,” he said.
To put that figure in context, the original SARS-CoV-2 virus required an estimated 67% of the population to be vaccinated to achieve herd immunity. Also, measles has one of the highest herd immunity thresholds at 95%, Dr. Franco added.
Herd immunity is the point at which enough people are immunized that the entire population gains protection. And it’s already happening. “Unvaccinated people are actually benefiting from greater herd immunity protection in high-vaccination counties compared to low-vaccination ones,” he said.
Maximize mask protection
Unlike early in the COVID-19 pandemic with widespread shortages of personal protective equipment, face masks are now readily available. This includes N95 masks, which offer enhanced protection against SARS-CoV-2, Ezekiel J. Emanuel, MD, PhD, said during the briefing.
Following the July 27 CDC recommendation that most Americans wear masks indoors when in public places, “I do think we need to upgrade our masks,” said Dr. Emanuel, who is Diane v.S. Levy & Robert M. Levy professor at the University of Pennsylvania, Philadelphia.
“It’s not just any mask,” he added. “Good masks make a big difference and are very important.”
Mask protection is about blocking 0.3-mcm particles, “and I think we need to make sure that people have masks that can filter that out,” he said. Although surgical masks are very good, he added, “they’re not quite as good as N95s.” As their name implies, N95s filter out 95% of these particles.
Dr. Emanuel acknowledged that people are tired of COVID-19 and complying with public health measures but urged perseverance. “We’ve sacrificed a lot. We should not throw it away in just a few months because we are tired. We’re all tired, but we do have to do the little bit extra getting vaccinated, wearing masks indoors, and protecting ourselves, our families, and our communities.”
Dealing with a disconnect
In response to a reporter’s question about the possibility that the large crowd at the Lollapalooza music festival in Chicago could become a superspreader event, Dr. Emanuel said, “it is worrisome.”
“I would say that, if you’re going to go to a gathering like that, wearing an N95 mask is wise, and not spending too long at any one place is also wise,” he said.
On the plus side, the event was held outdoors with lots of air circulation, Dr. Emanuel said.
However, “this is the kind of thing where we’ve got a sort of disconnect between people’s desire to get back to normal ... and the fact that we’re in the middle of this upsurge.”
Another potential problem is the event brought people together from many different locations, so when they travel home, they could be “potentially seeding lots of other communities.”
Boosters for some, for now
Even though not officially recommended, some fully vaccinated Americans are seeking a third or booster vaccination on their own.
Asked for his opinion, Dr. Emanuel said: “We’re probably going to have to be giving boosters to immunocompromised people and people who are susceptible. That’s where we are going to start.”
More research is needed regarding booster shots, he said. “There are very small studies – and the ‘very small’ should be emphasized – given that we’ve given shots to over 160 million people.”
“But it does appear that the boosters increase the antibodies and protection,” he said.
Instead of boosters, it is more important for people who haven’t been vaccinated to get fully vaccinated.
“We need to put our priorities in the right places,” he said.
Emanuel noted that, except for people in rural areas that might have to travel long distances, access to vaccines is no longer an issue. “It’s very hard not to find a vaccine if you want it.”
A remaining hurdle is “battling a major disinformation initiative. I don’t think this is misinformation. I think there’s very clear evidence that it is disinformation – false facts about the vaccines being spread,” Dr. Emanuel said.
The breakthrough infection dilemma
Breakthrough cases “remain the vast minority of infections at this time ... that is reassuring,” Dr. Franco said.
Also, tracking symptomatic breakthrough infections remains easier than studying fully vaccinated people who become infected with SARS-CoV-2 but remain symptom free.
“We really don’t have a good handle on the frequency of asymptomatic cases,” Dr. Emanuel said. “If you’re missing breakthrough infections, a lot of them, you may be missing some [virus] evolution that would be very important for us to follow.” This missing information could include the emergence of new variants.
The asymptomatic breakthrough cases are the most worrisome group,” Dr. Emanuel said. “You get infected, you’re feeling fine. Maybe you’ve got a little sneeze or cough, but nothing unusual. And then you’re still able to transmit the Delta variant.”
The big picture
The upsurge in cases, hospitalizations, and deaths is a major challenge, Dr. Emanuel said. “We need to address that by getting many more people vaccinated right now with what are very good vaccines.”
“But it also means that we have to stop being U.S. focused alone.” He pointed out that Delta and other variants originated overseas, “so getting the world vaccinated ... has to be a top priority.”
“We are obviously all facing a challenge as we move into the fall,” Dr. Emanuel said. “With schools opening and employers bringing their employees back together, even if these groups are vaccinated, there are going to be major challenges for all of us.”
A version of this article first appeared on Medscape.com.
Because the Delta variant of SARS-CoV-2 spreads more easily than the original virus, the proportion of the population that needs to be vaccinated to reach herd immunity could be upward of 80% or more, experts say.
Also, it could be time to consider wearing an N95 mask in public indoor spaces regardless of vaccination status, according to a media briefing on Aug. 3 sponsored by the Infectious Diseases Society of America.
Furthermore, giving booster shots to the fully vaccinated is not the top public health priority now. Instead, third vaccinations should be reserved for more vulnerable populations – and efforts should focus on getting first vaccinations to unvaccinated people in the United States and around the world.
“The problem here is that the Delta variant is ... more transmissible than the original virus. That pushes the overall population herd immunity threshold much higher,” Ricardo Franco, MD, assistant professor of medicine at the University of Alabama at Birmingham, said during the briefing.
“For Delta, those threshold estimates go well over 80% and may be approaching 90%,” he said.
To put that figure in context, the original SARS-CoV-2 virus required an estimated 67% of the population to be vaccinated to achieve herd immunity. Also, measles has one of the highest herd immunity thresholds at 95%, Dr. Franco added.
Herd immunity is the point at which enough people are immunized that the entire population gains protection. And it’s already happening. “Unvaccinated people are actually benefiting from greater herd immunity protection in high-vaccination counties compared to low-vaccination ones,” he said.
Maximize mask protection
Unlike early in the COVID-19 pandemic with widespread shortages of personal protective equipment, face masks are now readily available. This includes N95 masks, which offer enhanced protection against SARS-CoV-2, Ezekiel J. Emanuel, MD, PhD, said during the briefing.
Following the July 27 CDC recommendation that most Americans wear masks indoors when in public places, “I do think we need to upgrade our masks,” said Dr. Emanuel, who is Diane v.S. Levy & Robert M. Levy professor at the University of Pennsylvania, Philadelphia.
“It’s not just any mask,” he added. “Good masks make a big difference and are very important.”
Mask protection is about blocking 0.3-mcm particles, “and I think we need to make sure that people have masks that can filter that out,” he said. Although surgical masks are very good, he added, “they’re not quite as good as N95s.” As their name implies, N95s filter out 95% of these particles.
Dr. Emanuel acknowledged that people are tired of COVID-19 and complying with public health measures but urged perseverance. “We’ve sacrificed a lot. We should not throw it away in just a few months because we are tired. We’re all tired, but we do have to do the little bit extra getting vaccinated, wearing masks indoors, and protecting ourselves, our families, and our communities.”
Dealing with a disconnect
In response to a reporter’s question about the possibility that the large crowd at the Lollapalooza music festival in Chicago could become a superspreader event, Dr. Emanuel said, “it is worrisome.”
“I would say that, if you’re going to go to a gathering like that, wearing an N95 mask is wise, and not spending too long at any one place is also wise,” he said.
On the plus side, the event was held outdoors with lots of air circulation, Dr. Emanuel said.
However, “this is the kind of thing where we’ve got a sort of disconnect between people’s desire to get back to normal ... and the fact that we’re in the middle of this upsurge.”
Another potential problem is the event brought people together from many different locations, so when they travel home, they could be “potentially seeding lots of other communities.”
Boosters for some, for now
Even though not officially recommended, some fully vaccinated Americans are seeking a third or booster vaccination on their own.
Asked for his opinion, Dr. Emanuel said: “We’re probably going to have to be giving boosters to immunocompromised people and people who are susceptible. That’s where we are going to start.”
More research is needed regarding booster shots, he said. “There are very small studies – and the ‘very small’ should be emphasized – given that we’ve given shots to over 160 million people.”
“But it does appear that the boosters increase the antibodies and protection,” he said.
Instead of boosters, it is more important for people who haven’t been vaccinated to get fully vaccinated.
“We need to put our priorities in the right places,” he said.
Emanuel noted that, except for people in rural areas that might have to travel long distances, access to vaccines is no longer an issue. “It’s very hard not to find a vaccine if you want it.”
A remaining hurdle is “battling a major disinformation initiative. I don’t think this is misinformation. I think there’s very clear evidence that it is disinformation – false facts about the vaccines being spread,” Dr. Emanuel said.
The breakthrough infection dilemma
Breakthrough cases “remain the vast minority of infections at this time ... that is reassuring,” Dr. Franco said.
Also, tracking symptomatic breakthrough infections remains easier than studying fully vaccinated people who become infected with SARS-CoV-2 but remain symptom free.
“We really don’t have a good handle on the frequency of asymptomatic cases,” Dr. Emanuel said. “If you’re missing breakthrough infections, a lot of them, you may be missing some [virus] evolution that would be very important for us to follow.” This missing information could include the emergence of new variants.
The asymptomatic breakthrough cases are the most worrisome group,” Dr. Emanuel said. “You get infected, you’re feeling fine. Maybe you’ve got a little sneeze or cough, but nothing unusual. And then you’re still able to transmit the Delta variant.”
The big picture
The upsurge in cases, hospitalizations, and deaths is a major challenge, Dr. Emanuel said. “We need to address that by getting many more people vaccinated right now with what are very good vaccines.”
“But it also means that we have to stop being U.S. focused alone.” He pointed out that Delta and other variants originated overseas, “so getting the world vaccinated ... has to be a top priority.”
“We are obviously all facing a challenge as we move into the fall,” Dr. Emanuel said. “With schools opening and employers bringing their employees back together, even if these groups are vaccinated, there are going to be major challenges for all of us.”
A version of this article first appeared on Medscape.com.
New moms latched on to remote breastfeeding help. Will demand wane as pandemic fades?
Madison Cano knew she wanted to breastfeed her son, Theo. But breastfeeding was painful for her. The skin on her breasts was chafed and blistered last July when she returned home from the hospital. And Theo sometimes screamed during feedings.
Ms. Cano, 30, realized she needed help to get the short- and long-term health benefits of breastfeeding for moms and babies. New studies (Am J Obstet Gynecol. 2021 Mar 26. doi: 10.1016/j.ajog.2021.03.023) also have shown that COVID-vaccinated mothers pass protective antibodies on to their newborns. However, Ms. Cano lives in Montrose in western Colorado, 60 miles away from her lactation counselor, Ali Reynolds, in Grand Junction – and it was during the thick of the pandemic.
She messaged Ms. Reynolds on Facebook and took photos and recorded videos of herself breastfeeding so Reynolds could offer advice and encouragement from afar. It worked. She no longer had pain. Ms. Cano is still breastfeeding Theo, who just turned 1.
“I don’t think I would have understood what was happening and been able to work through it without that resource,” said Ms. Cano.
Support for breastfeeding was upended last year, when it no longer seemed safe to take a baby class at the hospital or invite a nurse into one’s home. Hospitals, lactation counselors, and support groups turned to virtual platforms like Zoom or phone calls. That made lactation support accessible to struggling families during the pandemic, said Danielle Harmon, executive director of the United States Lactation Consultant Association.
Today, although lactation specialists have more options to safely meet in person with families after their COVID-19 vaccinations, many are choosing to continue virtual classes, keeping alive the online communities they created and relying on the technology that worked for many families. Virtual options especially help those in remote areas or those with limited transportation access, breastfeeding experts say.
Right before the pandemic, for example, Sandrine Druon typically had one or two moms attend in-person meetings she held for La Leche League of Longmont at the First Evangelical Lutheran Church or at a Ziggi’s Coffee shop. But because they could no longer meet in person, last June she launched two monthly virtual meetings. Now, an online meeting will typically include 9 or 10 moms. She started an online Spanish-speaking meeting in May and parents joined from their homes in several states and even from other countries. She hopes eventually to have a mix of online and in-person meetings.
The virtual switch hasn’t worked for everyone. Ms. Harmon said the logistics of video support remain difficult, along with privacy concerns on platforms that could be hacked. Other lactation experts noted Black and Hispanic mothers are sometimes still left behind. So lactation specialists are trying to learn from the pandemic on what worked – and what didn’t – to reach all kinds of new parents.
Before the pandemic, 84% of U.S. mothers breastfed at least initially, according to 2019 data from the Centers for Disease Control and Prevention, while Colorado had a 93% rate.
The pandemic hasn’t seemed to change the picture, said Stacy Miller, Colorado’s breastfeeding coordinator for the Special Supplemental Nutrition Program for Women, Infants, and Children, shorthanded as WIC. Citing state birth certificate data, Ms. Miller said preliminary breastfeeding rates among families discharged from Colorado hospitals remained similar in the first quarter of 2021 to rates from 2020 or 2019.
Throughout the pandemic, lactation specialists have tried to offer convenient options for parents. St. Joseph Hospital in Denver launched virtual breastfeeding support groups that still occur today, in addition to breastfeeding help during families’ hospital stays, said Katie Halverstadt, the hospital’s clinical nurse manager of lactation and family education.
Last year in North Carolina, experts adapted an in-person prenatal breastfeeding program to an interactive video platform in English and Spanish. A separate effort on New York’s Long Island successfully converted in-person breastfeeding support to phone and video calls in 2020.
To help support parents in Grand Junction, Colo., Ms. Reynolds expanded her private practice, Valley Lactation, by offering virtual appointments while continuing to see some clients in their homes. That hybrid model continues today, although Ms. Reynolds said the demand for virtual or phone appointments has decreased lately as the country reopens.
Paying out-of-pocket for appointments is a hurdle her clients face, said Ms. Reynolds, but she encourages them to submit claims for telehealth or in-person visits to their health insurance companies for reimbursement. Early in the pandemic, telehealth rules were relaxed to encourage more telephone and virtual appointments – many of which have been covered by insurance.
But insurance coverage for lactation support will likely continue to be an issue independent of whether pandemic telehealth rules expire, Ms. Harmon said. While the Affordable Care Act mandates that insurance companies cover lactation support and supplies, such as breast pumps, Ms. Harmon said reimbursement is often spotty. Mirroring Medicaid, insurance providers often cover services only from licensed providers, she said, but just four states – Georgia, New Mexico, Oregon, and Rhode Island – license lactation consultants.
Experts such as Jennifer Schindler-Ruwisch, an assistant professor at Fairfield (Conn.) University, found the pandemic may have exacerbated breastfeeding barriers for those without access to online technology or translation services, among other things. She published one of the first studies in the United States to examine COVID’s effect on lactation services by collecting experiences from lactation support providers in Connecticut, including many working in WIC programs. For income-eligible WIC families, all breastfeeding classes, peer groups and one-on-one consultations are free.
Birdie Johnson, a doula who provides breastfeeding and other postpartum support to Black families as part of Sacred Seeds Black Doula Collective of Colorado, said virtual support groups during the pandemic also did not meet her clients’ needs for connection and interaction. Social media built communities online, particularly by normalizing breastfeeding struggles among Black parents, she said, but obstacles remained.
“COVID brought our community together and at the same time destroyed it,” Ms. Johnson said.
Black parents in the United States already had lower rates of breastfeeding than Asian or White parents, according to 2017 CDC data, and both Black and Hispanic parents have had lower rates of exclusively breastfeeding their babies at 6 months, which is what the American Academy of Pediatrics recommends. Socioeconomics and lack of workplace support have been found to contribute to the gap. Research also has found Black mothers are more likely than White moms to be introduced to infant formula at hospitals.
A scarcity of Black health care providers in lactation, women’s health and pediatrics is a continuing concern, Ms. Johnson said. In Colorado last year, the Colorado Breastfeeding Coalition, the Center for African American Health, Elephant Circle and Families Forward Resource Center held three training sessions for people of color to become lactation specialists, said Ms. Halverstadt, who chairs the coalition.
Jefferson County, which encompasses much of Denver’s western suburbs, is now training at least a dozen Spanish-speaking community members for lactation certification. In addition to classes, the trainees log clinical hours in breastfeeding support, sometimes during virtual meetings of a Spanish-speaking support group called Cuenta Conmigo Lactancia.
“You are more confident and more at ease with someone who knows your language, your culture and who is part of the community,” said Brenda Rodriguez, a dietitian and certified lactation consultant for Jefferson County Public Health, which reaches roughly 400 breastfeeding families each month through its WIC programs.
Angelica Pereda, a maternal and child health registered nurse, is part of that training program. Ms. Pereda, who is Hispanic and bilingual, gave birth to son Ahmias in April 2020 and struggled with breastfeeding because he could not latch on to her breasts. A lactation consultant could not come into her home during the pandemic, and she was skeptical of virtual consultations because of privacy concerns. So she pumped her breast milk and bottle-fed it to her son.
Her experience gave her newfound empathy for families, and she wants to help other Spanish-speaking parents find solutions – whether in person or virtually.
“There is just not enough breastfeeding support in general, but especially when that support is in a different language,” said Ms. Pereda.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Madison Cano knew she wanted to breastfeed her son, Theo. But breastfeeding was painful for her. The skin on her breasts was chafed and blistered last July when she returned home from the hospital. And Theo sometimes screamed during feedings.
Ms. Cano, 30, realized she needed help to get the short- and long-term health benefits of breastfeeding for moms and babies. New studies (Am J Obstet Gynecol. 2021 Mar 26. doi: 10.1016/j.ajog.2021.03.023) also have shown that COVID-vaccinated mothers pass protective antibodies on to their newborns. However, Ms. Cano lives in Montrose in western Colorado, 60 miles away from her lactation counselor, Ali Reynolds, in Grand Junction – and it was during the thick of the pandemic.
She messaged Ms. Reynolds on Facebook and took photos and recorded videos of herself breastfeeding so Reynolds could offer advice and encouragement from afar. It worked. She no longer had pain. Ms. Cano is still breastfeeding Theo, who just turned 1.
“I don’t think I would have understood what was happening and been able to work through it without that resource,” said Ms. Cano.
Support for breastfeeding was upended last year, when it no longer seemed safe to take a baby class at the hospital or invite a nurse into one’s home. Hospitals, lactation counselors, and support groups turned to virtual platforms like Zoom or phone calls. That made lactation support accessible to struggling families during the pandemic, said Danielle Harmon, executive director of the United States Lactation Consultant Association.
Today, although lactation specialists have more options to safely meet in person with families after their COVID-19 vaccinations, many are choosing to continue virtual classes, keeping alive the online communities they created and relying on the technology that worked for many families. Virtual options especially help those in remote areas or those with limited transportation access, breastfeeding experts say.
Right before the pandemic, for example, Sandrine Druon typically had one or two moms attend in-person meetings she held for La Leche League of Longmont at the First Evangelical Lutheran Church or at a Ziggi’s Coffee shop. But because they could no longer meet in person, last June she launched two monthly virtual meetings. Now, an online meeting will typically include 9 or 10 moms. She started an online Spanish-speaking meeting in May and parents joined from their homes in several states and even from other countries. She hopes eventually to have a mix of online and in-person meetings.
The virtual switch hasn’t worked for everyone. Ms. Harmon said the logistics of video support remain difficult, along with privacy concerns on platforms that could be hacked. Other lactation experts noted Black and Hispanic mothers are sometimes still left behind. So lactation specialists are trying to learn from the pandemic on what worked – and what didn’t – to reach all kinds of new parents.
Before the pandemic, 84% of U.S. mothers breastfed at least initially, according to 2019 data from the Centers for Disease Control and Prevention, while Colorado had a 93% rate.
The pandemic hasn’t seemed to change the picture, said Stacy Miller, Colorado’s breastfeeding coordinator for the Special Supplemental Nutrition Program for Women, Infants, and Children, shorthanded as WIC. Citing state birth certificate data, Ms. Miller said preliminary breastfeeding rates among families discharged from Colorado hospitals remained similar in the first quarter of 2021 to rates from 2020 or 2019.
Throughout the pandemic, lactation specialists have tried to offer convenient options for parents. St. Joseph Hospital in Denver launched virtual breastfeeding support groups that still occur today, in addition to breastfeeding help during families’ hospital stays, said Katie Halverstadt, the hospital’s clinical nurse manager of lactation and family education.
Last year in North Carolina, experts adapted an in-person prenatal breastfeeding program to an interactive video platform in English and Spanish. A separate effort on New York’s Long Island successfully converted in-person breastfeeding support to phone and video calls in 2020.
To help support parents in Grand Junction, Colo., Ms. Reynolds expanded her private practice, Valley Lactation, by offering virtual appointments while continuing to see some clients in their homes. That hybrid model continues today, although Ms. Reynolds said the demand for virtual or phone appointments has decreased lately as the country reopens.
Paying out-of-pocket for appointments is a hurdle her clients face, said Ms. Reynolds, but she encourages them to submit claims for telehealth or in-person visits to their health insurance companies for reimbursement. Early in the pandemic, telehealth rules were relaxed to encourage more telephone and virtual appointments – many of which have been covered by insurance.
But insurance coverage for lactation support will likely continue to be an issue independent of whether pandemic telehealth rules expire, Ms. Harmon said. While the Affordable Care Act mandates that insurance companies cover lactation support and supplies, such as breast pumps, Ms. Harmon said reimbursement is often spotty. Mirroring Medicaid, insurance providers often cover services only from licensed providers, she said, but just four states – Georgia, New Mexico, Oregon, and Rhode Island – license lactation consultants.
Experts such as Jennifer Schindler-Ruwisch, an assistant professor at Fairfield (Conn.) University, found the pandemic may have exacerbated breastfeeding barriers for those without access to online technology or translation services, among other things. She published one of the first studies in the United States to examine COVID’s effect on lactation services by collecting experiences from lactation support providers in Connecticut, including many working in WIC programs. For income-eligible WIC families, all breastfeeding classes, peer groups and one-on-one consultations are free.
Birdie Johnson, a doula who provides breastfeeding and other postpartum support to Black families as part of Sacred Seeds Black Doula Collective of Colorado, said virtual support groups during the pandemic also did not meet her clients’ needs for connection and interaction. Social media built communities online, particularly by normalizing breastfeeding struggles among Black parents, she said, but obstacles remained.
“COVID brought our community together and at the same time destroyed it,” Ms. Johnson said.
Black parents in the United States already had lower rates of breastfeeding than Asian or White parents, according to 2017 CDC data, and both Black and Hispanic parents have had lower rates of exclusively breastfeeding their babies at 6 months, which is what the American Academy of Pediatrics recommends. Socioeconomics and lack of workplace support have been found to contribute to the gap. Research also has found Black mothers are more likely than White moms to be introduced to infant formula at hospitals.
A scarcity of Black health care providers in lactation, women’s health and pediatrics is a continuing concern, Ms. Johnson said. In Colorado last year, the Colorado Breastfeeding Coalition, the Center for African American Health, Elephant Circle and Families Forward Resource Center held three training sessions for people of color to become lactation specialists, said Ms. Halverstadt, who chairs the coalition.
Jefferson County, which encompasses much of Denver’s western suburbs, is now training at least a dozen Spanish-speaking community members for lactation certification. In addition to classes, the trainees log clinical hours in breastfeeding support, sometimes during virtual meetings of a Spanish-speaking support group called Cuenta Conmigo Lactancia.
“You are more confident and more at ease with someone who knows your language, your culture and who is part of the community,” said Brenda Rodriguez, a dietitian and certified lactation consultant for Jefferson County Public Health, which reaches roughly 400 breastfeeding families each month through its WIC programs.
Angelica Pereda, a maternal and child health registered nurse, is part of that training program. Ms. Pereda, who is Hispanic and bilingual, gave birth to son Ahmias in April 2020 and struggled with breastfeeding because he could not latch on to her breasts. A lactation consultant could not come into her home during the pandemic, and she was skeptical of virtual consultations because of privacy concerns. So she pumped her breast milk and bottle-fed it to her son.
Her experience gave her newfound empathy for families, and she wants to help other Spanish-speaking parents find solutions – whether in person or virtually.
“There is just not enough breastfeeding support in general, but especially when that support is in a different language,” said Ms. Pereda.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Madison Cano knew she wanted to breastfeed her son, Theo. But breastfeeding was painful for her. The skin on her breasts was chafed and blistered last July when she returned home from the hospital. And Theo sometimes screamed during feedings.
Ms. Cano, 30, realized she needed help to get the short- and long-term health benefits of breastfeeding for moms and babies. New studies (Am J Obstet Gynecol. 2021 Mar 26. doi: 10.1016/j.ajog.2021.03.023) also have shown that COVID-vaccinated mothers pass protective antibodies on to their newborns. However, Ms. Cano lives in Montrose in western Colorado, 60 miles away from her lactation counselor, Ali Reynolds, in Grand Junction – and it was during the thick of the pandemic.
She messaged Ms. Reynolds on Facebook and took photos and recorded videos of herself breastfeeding so Reynolds could offer advice and encouragement from afar. It worked. She no longer had pain. Ms. Cano is still breastfeeding Theo, who just turned 1.
“I don’t think I would have understood what was happening and been able to work through it without that resource,” said Ms. Cano.
Support for breastfeeding was upended last year, when it no longer seemed safe to take a baby class at the hospital or invite a nurse into one’s home. Hospitals, lactation counselors, and support groups turned to virtual platforms like Zoom or phone calls. That made lactation support accessible to struggling families during the pandemic, said Danielle Harmon, executive director of the United States Lactation Consultant Association.
Today, although lactation specialists have more options to safely meet in person with families after their COVID-19 vaccinations, many are choosing to continue virtual classes, keeping alive the online communities they created and relying on the technology that worked for many families. Virtual options especially help those in remote areas or those with limited transportation access, breastfeeding experts say.
Right before the pandemic, for example, Sandrine Druon typically had one or two moms attend in-person meetings she held for La Leche League of Longmont at the First Evangelical Lutheran Church or at a Ziggi’s Coffee shop. But because they could no longer meet in person, last June she launched two monthly virtual meetings. Now, an online meeting will typically include 9 or 10 moms. She started an online Spanish-speaking meeting in May and parents joined from their homes in several states and even from other countries. She hopes eventually to have a mix of online and in-person meetings.
The virtual switch hasn’t worked for everyone. Ms. Harmon said the logistics of video support remain difficult, along with privacy concerns on platforms that could be hacked. Other lactation experts noted Black and Hispanic mothers are sometimes still left behind. So lactation specialists are trying to learn from the pandemic on what worked – and what didn’t – to reach all kinds of new parents.
Before the pandemic, 84% of U.S. mothers breastfed at least initially, according to 2019 data from the Centers for Disease Control and Prevention, while Colorado had a 93% rate.
The pandemic hasn’t seemed to change the picture, said Stacy Miller, Colorado’s breastfeeding coordinator for the Special Supplemental Nutrition Program for Women, Infants, and Children, shorthanded as WIC. Citing state birth certificate data, Ms. Miller said preliminary breastfeeding rates among families discharged from Colorado hospitals remained similar in the first quarter of 2021 to rates from 2020 or 2019.
Throughout the pandemic, lactation specialists have tried to offer convenient options for parents. St. Joseph Hospital in Denver launched virtual breastfeeding support groups that still occur today, in addition to breastfeeding help during families’ hospital stays, said Katie Halverstadt, the hospital’s clinical nurse manager of lactation and family education.
Last year in North Carolina, experts adapted an in-person prenatal breastfeeding program to an interactive video platform in English and Spanish. A separate effort on New York’s Long Island successfully converted in-person breastfeeding support to phone and video calls in 2020.
To help support parents in Grand Junction, Colo., Ms. Reynolds expanded her private practice, Valley Lactation, by offering virtual appointments while continuing to see some clients in their homes. That hybrid model continues today, although Ms. Reynolds said the demand for virtual or phone appointments has decreased lately as the country reopens.
Paying out-of-pocket for appointments is a hurdle her clients face, said Ms. Reynolds, but she encourages them to submit claims for telehealth or in-person visits to their health insurance companies for reimbursement. Early in the pandemic, telehealth rules were relaxed to encourage more telephone and virtual appointments – many of which have been covered by insurance.
But insurance coverage for lactation support will likely continue to be an issue independent of whether pandemic telehealth rules expire, Ms. Harmon said. While the Affordable Care Act mandates that insurance companies cover lactation support and supplies, such as breast pumps, Ms. Harmon said reimbursement is often spotty. Mirroring Medicaid, insurance providers often cover services only from licensed providers, she said, but just four states – Georgia, New Mexico, Oregon, and Rhode Island – license lactation consultants.
Experts such as Jennifer Schindler-Ruwisch, an assistant professor at Fairfield (Conn.) University, found the pandemic may have exacerbated breastfeeding barriers for those without access to online technology or translation services, among other things. She published one of the first studies in the United States to examine COVID’s effect on lactation services by collecting experiences from lactation support providers in Connecticut, including many working in WIC programs. For income-eligible WIC families, all breastfeeding classes, peer groups and one-on-one consultations are free.
Birdie Johnson, a doula who provides breastfeeding and other postpartum support to Black families as part of Sacred Seeds Black Doula Collective of Colorado, said virtual support groups during the pandemic also did not meet her clients’ needs for connection and interaction. Social media built communities online, particularly by normalizing breastfeeding struggles among Black parents, she said, but obstacles remained.
“COVID brought our community together and at the same time destroyed it,” Ms. Johnson said.
Black parents in the United States already had lower rates of breastfeeding than Asian or White parents, according to 2017 CDC data, and both Black and Hispanic parents have had lower rates of exclusively breastfeeding their babies at 6 months, which is what the American Academy of Pediatrics recommends. Socioeconomics and lack of workplace support have been found to contribute to the gap. Research also has found Black mothers are more likely than White moms to be introduced to infant formula at hospitals.
A scarcity of Black health care providers in lactation, women’s health and pediatrics is a continuing concern, Ms. Johnson said. In Colorado last year, the Colorado Breastfeeding Coalition, the Center for African American Health, Elephant Circle and Families Forward Resource Center held three training sessions for people of color to become lactation specialists, said Ms. Halverstadt, who chairs the coalition.
Jefferson County, which encompasses much of Denver’s western suburbs, is now training at least a dozen Spanish-speaking community members for lactation certification. In addition to classes, the trainees log clinical hours in breastfeeding support, sometimes during virtual meetings of a Spanish-speaking support group called Cuenta Conmigo Lactancia.
“You are more confident and more at ease with someone who knows your language, your culture and who is part of the community,” said Brenda Rodriguez, a dietitian and certified lactation consultant for Jefferson County Public Health, which reaches roughly 400 breastfeeding families each month through its WIC programs.
Angelica Pereda, a maternal and child health registered nurse, is part of that training program. Ms. Pereda, who is Hispanic and bilingual, gave birth to son Ahmias in April 2020 and struggled with breastfeeding because he could not latch on to her breasts. A lactation consultant could not come into her home during the pandemic, and she was skeptical of virtual consultations because of privacy concerns. So she pumped her breast milk and bottle-fed it to her son.
Her experience gave her newfound empathy for families, and she wants to help other Spanish-speaking parents find solutions – whether in person or virtually.
“There is just not enough breastfeeding support in general, but especially when that support is in a different language,” said Ms. Pereda.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
No higher risk of rheumatic and musculoskeletal disease flares seen after COVID-19 vaccination
Double-dose vaccination against SARS-CoV-2 in patients with rheumatic and musculoskeletal diseases (RMDs) doesn’t appear to boost the risk of flares, a new study shows. The risk of adverse effects after vaccination is high, just as it is in the general population, but no patients experienced allergic reactions.
“Our findings suggest that COVID-19 vaccines are safe in patients with rheumatic and musculoskeletal diseases. Overall, rates of flare are low and mild, while local and systemic reactions should be anticipated,” lead author Caoilfhionn Connolly, MD, MSc, a rheumatology fellow at Johns Hopkins University, Baltimore, said in an interview. “Many of our patients are at increased risk of severe infection and or complications from COVID-19. It has been shown that patients with RMDs are more willing to reconsider vaccination if it is recommended by a physician, and these data should help inform these critical discussions.”
The study appeared Aug. 4 in Arthritis & Rheumatology.
According to Dr. Connolly, the researchers launched their study to better understand the effect of two-dose SARS-CoV-2 messenger RNA vaccinations authorized for emergency use by the Food and Drug Administration (Pfizer and Moderna vaccines) on immunosuppressed patients. As she noted, patients with RMDs were largely excluded from vaccine trials, and “studies have shown that some patients with RMDs are hesitant about vaccination due to the lack of safety data.”
Some data about this patient population have started to appear. A study published July 21 in Lancet Rheumatology found that patients with systemic lupus erythematosus (SLE) reported few flares within a median of 3 days after receiving one or two doses of various COVID-19 vaccines. Side effects were common but were mainly mild or moderate.
For the new study, researchers surveyed 1,377 patients with RMDs who’d received two vaccination doses between December 2020 and April 2021. The patients had a median age of 47, 92% were female, and 10% were non-White. The patients had a variety of RMDs, including inflammatory arthritis (47%), SLE (20%), overlap connective tissue disease (20%), and Sjögren’s syndrome and myositis (each 5%).
A total of 11% said they’d experienced a flare that required treatment, but none were severe. In comparison, 56% of patients said they had experienced a flare of their RMD in the 6 months prior to their first vaccine dose. Several groups had a greater likelihood of flares, including those who’d had COVID-19 previously (adjusted incidence rate ratio [IRR], 2.09; P = .02). “COVID-19 can cause both acute and delayed inflammatory syndromes through activation of the immune system,” Dr. Connolly said. “Vaccination possibly triggered further activation of the immune system resulting in disease flare. This is an area that warrants further research.”
Patients who took combination immunomodulatory therapy were also more likely to flare after vaccination (IRR, 1.95; P < .001). And patients were more likely to report flares after vaccination if they experienced a flare in the 6 months preceding vaccination (IRR, 2.36; P < .001). “This may suggest more active disease at baseline,” Dr. Connolly said. “It is difficult to differentiate whether these patients would have experienced flare even without the vaccine.”
A number of factors didn’t appear to affect the likelihood of flares: gender, age, ethnicity, type of RMD, and type of vaccine.
Local and systemic side effects were frequently reported, including injection site pain (87% and 86% after first and second doses, respectively) and fatigue (60% and 80%, respectively). As is common among people receiving COVID-19 vaccines, side effects were more frequent after the second dose.
As for future research, “we are evaluating the long-term safety and efficacy of the COVID-19 vaccines in patients with RMDs,” study coauthor Julie J. Paik, MD, assistant professor of medicine at Johns Hopkins, said in an interview. “We are also evaluating the impact of changes in immunosuppression around vaccination.”
The study was funded by the Ben-Dov family and grants from several institutes of the National Institutes of Health. Dr. Connolly and Dr. Paik reported no relevant disclosures. Other study authors reported various financial relationships with pharmaceutical companies.
Double-dose vaccination against SARS-CoV-2 in patients with rheumatic and musculoskeletal diseases (RMDs) doesn’t appear to boost the risk of flares, a new study shows. The risk of adverse effects after vaccination is high, just as it is in the general population, but no patients experienced allergic reactions.
“Our findings suggest that COVID-19 vaccines are safe in patients with rheumatic and musculoskeletal diseases. Overall, rates of flare are low and mild, while local and systemic reactions should be anticipated,” lead author Caoilfhionn Connolly, MD, MSc, a rheumatology fellow at Johns Hopkins University, Baltimore, said in an interview. “Many of our patients are at increased risk of severe infection and or complications from COVID-19. It has been shown that patients with RMDs are more willing to reconsider vaccination if it is recommended by a physician, and these data should help inform these critical discussions.”
The study appeared Aug. 4 in Arthritis & Rheumatology.
According to Dr. Connolly, the researchers launched their study to better understand the effect of two-dose SARS-CoV-2 messenger RNA vaccinations authorized for emergency use by the Food and Drug Administration (Pfizer and Moderna vaccines) on immunosuppressed patients. As she noted, patients with RMDs were largely excluded from vaccine trials, and “studies have shown that some patients with RMDs are hesitant about vaccination due to the lack of safety data.”
Some data about this patient population have started to appear. A study published July 21 in Lancet Rheumatology found that patients with systemic lupus erythematosus (SLE) reported few flares within a median of 3 days after receiving one or two doses of various COVID-19 vaccines. Side effects were common but were mainly mild or moderate.
For the new study, researchers surveyed 1,377 patients with RMDs who’d received two vaccination doses between December 2020 and April 2021. The patients had a median age of 47, 92% were female, and 10% were non-White. The patients had a variety of RMDs, including inflammatory arthritis (47%), SLE (20%), overlap connective tissue disease (20%), and Sjögren’s syndrome and myositis (each 5%).
A total of 11% said they’d experienced a flare that required treatment, but none were severe. In comparison, 56% of patients said they had experienced a flare of their RMD in the 6 months prior to their first vaccine dose. Several groups had a greater likelihood of flares, including those who’d had COVID-19 previously (adjusted incidence rate ratio [IRR], 2.09; P = .02). “COVID-19 can cause both acute and delayed inflammatory syndromes through activation of the immune system,” Dr. Connolly said. “Vaccination possibly triggered further activation of the immune system resulting in disease flare. This is an area that warrants further research.”
Patients who took combination immunomodulatory therapy were also more likely to flare after vaccination (IRR, 1.95; P < .001). And patients were more likely to report flares after vaccination if they experienced a flare in the 6 months preceding vaccination (IRR, 2.36; P < .001). “This may suggest more active disease at baseline,” Dr. Connolly said. “It is difficult to differentiate whether these patients would have experienced flare even without the vaccine.”
A number of factors didn’t appear to affect the likelihood of flares: gender, age, ethnicity, type of RMD, and type of vaccine.
Local and systemic side effects were frequently reported, including injection site pain (87% and 86% after first and second doses, respectively) and fatigue (60% and 80%, respectively). As is common among people receiving COVID-19 vaccines, side effects were more frequent after the second dose.
As for future research, “we are evaluating the long-term safety and efficacy of the COVID-19 vaccines in patients with RMDs,” study coauthor Julie J. Paik, MD, assistant professor of medicine at Johns Hopkins, said in an interview. “We are also evaluating the impact of changes in immunosuppression around vaccination.”
The study was funded by the Ben-Dov family and grants from several institutes of the National Institutes of Health. Dr. Connolly and Dr. Paik reported no relevant disclosures. Other study authors reported various financial relationships with pharmaceutical companies.
Double-dose vaccination against SARS-CoV-2 in patients with rheumatic and musculoskeletal diseases (RMDs) doesn’t appear to boost the risk of flares, a new study shows. The risk of adverse effects after vaccination is high, just as it is in the general population, but no patients experienced allergic reactions.
“Our findings suggest that COVID-19 vaccines are safe in patients with rheumatic and musculoskeletal diseases. Overall, rates of flare are low and mild, while local and systemic reactions should be anticipated,” lead author Caoilfhionn Connolly, MD, MSc, a rheumatology fellow at Johns Hopkins University, Baltimore, said in an interview. “Many of our patients are at increased risk of severe infection and or complications from COVID-19. It has been shown that patients with RMDs are more willing to reconsider vaccination if it is recommended by a physician, and these data should help inform these critical discussions.”
The study appeared Aug. 4 in Arthritis & Rheumatology.
According to Dr. Connolly, the researchers launched their study to better understand the effect of two-dose SARS-CoV-2 messenger RNA vaccinations authorized for emergency use by the Food and Drug Administration (Pfizer and Moderna vaccines) on immunosuppressed patients. As she noted, patients with RMDs were largely excluded from vaccine trials, and “studies have shown that some patients with RMDs are hesitant about vaccination due to the lack of safety data.”
Some data about this patient population have started to appear. A study published July 21 in Lancet Rheumatology found that patients with systemic lupus erythematosus (SLE) reported few flares within a median of 3 days after receiving one or two doses of various COVID-19 vaccines. Side effects were common but were mainly mild or moderate.
For the new study, researchers surveyed 1,377 patients with RMDs who’d received two vaccination doses between December 2020 and April 2021. The patients had a median age of 47, 92% were female, and 10% were non-White. The patients had a variety of RMDs, including inflammatory arthritis (47%), SLE (20%), overlap connective tissue disease (20%), and Sjögren’s syndrome and myositis (each 5%).
A total of 11% said they’d experienced a flare that required treatment, but none were severe. In comparison, 56% of patients said they had experienced a flare of their RMD in the 6 months prior to their first vaccine dose. Several groups had a greater likelihood of flares, including those who’d had COVID-19 previously (adjusted incidence rate ratio [IRR], 2.09; P = .02). “COVID-19 can cause both acute and delayed inflammatory syndromes through activation of the immune system,” Dr. Connolly said. “Vaccination possibly triggered further activation of the immune system resulting in disease flare. This is an area that warrants further research.”
Patients who took combination immunomodulatory therapy were also more likely to flare after vaccination (IRR, 1.95; P < .001). And patients were more likely to report flares after vaccination if they experienced a flare in the 6 months preceding vaccination (IRR, 2.36; P < .001). “This may suggest more active disease at baseline,” Dr. Connolly said. “It is difficult to differentiate whether these patients would have experienced flare even without the vaccine.”
A number of factors didn’t appear to affect the likelihood of flares: gender, age, ethnicity, type of RMD, and type of vaccine.
Local and systemic side effects were frequently reported, including injection site pain (87% and 86% after first and second doses, respectively) and fatigue (60% and 80%, respectively). As is common among people receiving COVID-19 vaccines, side effects were more frequent after the second dose.
As for future research, “we are evaluating the long-term safety and efficacy of the COVID-19 vaccines in patients with RMDs,” study coauthor Julie J. Paik, MD, assistant professor of medicine at Johns Hopkins, said in an interview. “We are also evaluating the impact of changes in immunosuppression around vaccination.”
The study was funded by the Ben-Dov family and grants from several institutes of the National Institutes of Health. Dr. Connolly and Dr. Paik reported no relevant disclosures. Other study authors reported various financial relationships with pharmaceutical companies.
FROM ARTHRITIS & RHEUMATOLOGY
The Surfside tragedy: A call for healing the healers
The mental health toll from the Surfside, Fla., Champlain Tower collapse will be felt by our patients for years to come. As mental health professionals in Miami-Dade County, it has been difficult to deal with the catastrophe layered on the escalating COVID-19 crisis.
With each passing day after the June 24 incident, we all learned who the 98 victims were. In session after session, the enormous impact of this unfathomable tragedy unfolded. Some mental health care professionals were directly affected with the loss of family members; some lost patients, and a large number of our patients lost someone or knew someone who lost someone. It was reminiscent of our work during the COVID-19 crisis when we found that we were dealing with the same stressors as those of our patients. As it was said then, we were all in the same storm – just in very different boats.
It was heartening to see how many colleagues rushed to the site of the building where family waiting areas were established. So many professionals wanted to assist that some had to be turned away.
The days right after the collapse were agonizing for all as we waited and hoped for survivors to be found. Search teams from across the United States and from Mexico and Israel – specifically, Israeli Defense Forces personnel with experience conducting operations in the wake of earthquakes in both Haiti and Nepal, took on the dangerous work. When no one was recovered after the first day, hope faded, and after 10 days, the search and rescue efforts turned to search and recovery. We were indeed a county and community in mourning.
According to Lina Haji, PsyD, GIA Miami, in addition to the direct impact of loss, clinicians who engaged in crisis response and bereavement counseling with those affected by the Surfside tragedy were subjected to vicarious trauma. Mental health care providers in the Miami area not only experienced the direct effect of this tragedy but have been hearing details and harrowing stories about the unimaginable experiences their patients endured over those critical weeks. Vicarious trauma can result in our own symptoms, compassion fatigue, or burnout as clinicians. This resulted in a call for mental health providers to come to the aid of their fellow colleagues.
So, on the 1-month anniversary of the initial collapse, at the urging of Patricia Stauber, RN, LCSW, a clinician with more than 30 years’ experience in providing grief counseling in hospital and private practice settings; Antonello Bonci, MD, the founder of GIA Miami; Charlotte Tomic, director of public relations for the Institute for the Study of Global Antisemitism; and I cohosted what we hope will be several Mental Health Appreciation retreats. Our goal was to create a space to focus on healing the healers. We had hoped to hold an in-person event, but at the last moment we opted for a Zoom-based event because COVID-19 cases were rising rapidly again.
Working on the front lines
Cassie Feldman, PsyD, a licensed clinical psychologist with extensive experience working with grief, loss, end of life, and responding to trauma-related consults, reflected on her experience responding to the collapse in the earliest days – first independently at the request of community religious leaders and then as part of CADENA Foundation, a nonprofit organization dedicated to rescue, humanitarian aid, and disaster response and prevention worldwide.
Dr. Feldman worked alongside other mental health professionals, local Miami-Dade police and fire officials, and the domestic and international rescue teams (CADENA’s Go Team from Mexico and the Israeli Defense Force’s Search and Rescue Delegation), providing Psychological First Aid, crisis intervention, and disaster response to the victims’ families and survivors.
This initially was a 24-hour coverage effort, requiring Dr. Feldman and her colleagues to clear their schedules, and at times to work 18-hour shifts in the early days of the crisis to address the need for consistency and continuity. Their commitment was to show up for the victims’ families and survivors, fully embracing the chaos and the demands of the situation. She noted that the disaster brought out the best of her and her colleagues.
They divided and conquered the work, alongside clinicians from Jewish Community Services and Project Chai intervening acutely where possible, and coordinating long-term care plans for those survivors and members of the victims’ support networks in need of consistent care.
Dr. Feldman reflected on the notion that we have all been processing losses prior to this – loss of normalcy because of the pandemic, loss of people we loved as a result, other personal losses – and that this community tragedy is yet another loss to disentangle. It didn’t feel good or natural for her to passively absorb the news knowing she had both the skill set and capacity to take on an active supportive role. The first days at the community center were disorganized; it was hard to know who was who and what was what. She described parents crying out for their children and children longing for their parents. Individuals were so overcome with emotion that they grew faint. Friends and families flooded in but were unaware of how to be fully supportive. The level of trauma was so high that the only interventions that were absorbed were those that were nonverbal or that fully addressed practical needs. People were frightened and in a state of shock.
Day by day, more order ensued and the efforts became more coordinated, but it became apparent to her that the “family reunification center” was devoid of reunification. She and her colleagues’ primary role became aiding the police department in making death notifications to the families and being supportive of the victims’ families and their loved ones during and in between the formal briefings, where so many concerned family members and friends gathered and waited.
“As the days went on, things became more structured and predictable,” Dr. Feldman noted. “We continued to connect with the victims’ families and survivors, [listened to] their stories, shared meals with them, spent downtime with them, began to intimately know their loved ones, and all the barriers they were now facing. We became invested in them, their unique intricacies, and to care deeply for them like our own families and loved ones. Small talk and conversation morphed into silent embraces where spoken words weren’t necessary.”
Dr. Feldman said some of her earliest memories were visiting ICU patients alongside her father, a critical care and ICU physician. Her father taught her that nonverbal communication and connection can be offered to patients in the most poignant moments of suffering.
Her “nascent experiences in the ICU,” she said, taught her that “the most useful of interventions was just being with people in their pain and bearing witness at times when there were just no words.”
Dr. Feldman said that when many of her colleagues learned about the switch from rescue to recovery, the pull was to jump in their cars and drive to the hotel where the families were based to offer support.
The unity she witnessed – from the disparate clinicians who were virtual strangers before the incident but a team afterward, from the families and the community, and from the first responders and rescue teams – was inspiring, Dr. Feldman said.
“We were all forced to think beyond ourselves, push ourselves past our limits, and unify in a way that remedied this period in history of deep fragmentation,” she said.
Understanding the role of psychoneuroimmunology
In another presentation during the Zoom event, Ms. Stauber offered her insights about the importance of support among mental health clinicians.
She cited research on women with HIV showing that those who are part of a support group had a stronger immune response than those who were not.
Ms. Stauber said the impact of COVID-19 and its ramifications – including fear, grief over losing loved ones, isolation from friends and family, and interference/cessation of normal routines – has put an enormous strain on clinicians and clients. One of her clients had to take her mother to the emergency room – never to see her again. She continues to ask: “If I’d been there, could I have saved her?”
Another client whose husband died of COVID-19–related illness agonizes over not being able to be at her husband’s side, not being able to hold his hand, not being able to say goodbye.
She said other cultures are more accepting of suffering as a condition of life and the acknowledgment that our time on earth is limited.
The “quick fix for everything” society carries over to people’s grief, said Ms. Stauber. As a result, many find it difficult to appreciate how much time it takes to heal.
Normal uncomplicated grief can take approximately 2-3 years, she said. By then, the shock has been wearing off, the emotional roller coaster of loss is calming down, coping skills are strengthened, and life can once again be more fulfilling or meaningful. Complicated grief or grief with trauma takes much longer, said Ms. Stauber, who is a consultant with a national crisis and debriefing company providing trauma and bereavement support to Fortune 500 companies.
Trauma adds another complexity to loss. To begin to appreciate the rough road ahead, Ms. Stauber said, it is important to understand the basic challenges facing grieving people.
“This is where our profession may be needed; we are providing support for those suffering the immense pain of loss in a world that often has difficulty being present or patient with loss,” she said. “We are indeed providing an emotional life raft.”
Ultimately, self-care is critical, Ms. Stauber said. “Consider self-care a job requirement” to be successful. She also offered the following tips for self-care:
1. Share your own loss experience with a caring and nonjudgmental person.
2. Consider ongoing supervision and consultation with colleagues who understand the nature of your work.
3. Be willing to ask for help.
4. Be aware of risks and countertransference in our work.
5. Attend workshops.
6. Remember that you do not have to and cannot do it all by yourself – we absolutely need more grief and trauma trained therapists.
7. Involve yourself in activities outside of work that feed your soul and nourish your spirit.
8. Schedule play.
9. Develop a healthy self-care regimen to remain present doing this work.
10. Consider the benefits of exercise.
11. Enjoy the beauty and wonder of nature.
12. Consider yoga, meditation, spa retreats – such as Kripalu, Miraval, and Canyon Ranch.
13. Spend time with loving family and friends.
14. Adopt a pet.
15. Eat healthy foods; get plenty of rest.
16. Walk in the rain.
17. Listen to music.
18. Enjoy a relaxing bubble bath.
19. Sing, dance, and enjoy the blessings of this life.
20. Love yourself; you truly can be your own best friend.
To advocate on behalf of mental health for patients, we must do the same for mental health professionals. The retreat was well received, and we learned a lot from our speakers. After the program, we offered a 45-minute yoga class and then 30-minute sound bowl meditation. We plan to repeat the event in September to help our community deal with the ongoing stress of such overwhelming loss.
While our community will never be the same, we hope that, by coming together, we can all find a way to support one another and strive to help ourselves and others manage as we navigate yet another unprecedented crisis.
Dr. Ritvo, who has more than 30 years’ experience in psychiatry, practices telemedicine. She is author of “BeKindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018). Dr. Ritvo has no disclosures.
The mental health toll from the Surfside, Fla., Champlain Tower collapse will be felt by our patients for years to come. As mental health professionals in Miami-Dade County, it has been difficult to deal with the catastrophe layered on the escalating COVID-19 crisis.
With each passing day after the June 24 incident, we all learned who the 98 victims were. In session after session, the enormous impact of this unfathomable tragedy unfolded. Some mental health care professionals were directly affected with the loss of family members; some lost patients, and a large number of our patients lost someone or knew someone who lost someone. It was reminiscent of our work during the COVID-19 crisis when we found that we were dealing with the same stressors as those of our patients. As it was said then, we were all in the same storm – just in very different boats.
It was heartening to see how many colleagues rushed to the site of the building where family waiting areas were established. So many professionals wanted to assist that some had to be turned away.
The days right after the collapse were agonizing for all as we waited and hoped for survivors to be found. Search teams from across the United States and from Mexico and Israel – specifically, Israeli Defense Forces personnel with experience conducting operations in the wake of earthquakes in both Haiti and Nepal, took on the dangerous work. When no one was recovered after the first day, hope faded, and after 10 days, the search and rescue efforts turned to search and recovery. We were indeed a county and community in mourning.
According to Lina Haji, PsyD, GIA Miami, in addition to the direct impact of loss, clinicians who engaged in crisis response and bereavement counseling with those affected by the Surfside tragedy were subjected to vicarious trauma. Mental health care providers in the Miami area not only experienced the direct effect of this tragedy but have been hearing details and harrowing stories about the unimaginable experiences their patients endured over those critical weeks. Vicarious trauma can result in our own symptoms, compassion fatigue, or burnout as clinicians. This resulted in a call for mental health providers to come to the aid of their fellow colleagues.
So, on the 1-month anniversary of the initial collapse, at the urging of Patricia Stauber, RN, LCSW, a clinician with more than 30 years’ experience in providing grief counseling in hospital and private practice settings; Antonello Bonci, MD, the founder of GIA Miami; Charlotte Tomic, director of public relations for the Institute for the Study of Global Antisemitism; and I cohosted what we hope will be several Mental Health Appreciation retreats. Our goal was to create a space to focus on healing the healers. We had hoped to hold an in-person event, but at the last moment we opted for a Zoom-based event because COVID-19 cases were rising rapidly again.
Working on the front lines
Cassie Feldman, PsyD, a licensed clinical psychologist with extensive experience working with grief, loss, end of life, and responding to trauma-related consults, reflected on her experience responding to the collapse in the earliest days – first independently at the request of community religious leaders and then as part of CADENA Foundation, a nonprofit organization dedicated to rescue, humanitarian aid, and disaster response and prevention worldwide.
Dr. Feldman worked alongside other mental health professionals, local Miami-Dade police and fire officials, and the domestic and international rescue teams (CADENA’s Go Team from Mexico and the Israeli Defense Force’s Search and Rescue Delegation), providing Psychological First Aid, crisis intervention, and disaster response to the victims’ families and survivors.
This initially was a 24-hour coverage effort, requiring Dr. Feldman and her colleagues to clear their schedules, and at times to work 18-hour shifts in the early days of the crisis to address the need for consistency and continuity. Their commitment was to show up for the victims’ families and survivors, fully embracing the chaos and the demands of the situation. She noted that the disaster brought out the best of her and her colleagues.
They divided and conquered the work, alongside clinicians from Jewish Community Services and Project Chai intervening acutely where possible, and coordinating long-term care plans for those survivors and members of the victims’ support networks in need of consistent care.
Dr. Feldman reflected on the notion that we have all been processing losses prior to this – loss of normalcy because of the pandemic, loss of people we loved as a result, other personal losses – and that this community tragedy is yet another loss to disentangle. It didn’t feel good or natural for her to passively absorb the news knowing she had both the skill set and capacity to take on an active supportive role. The first days at the community center were disorganized; it was hard to know who was who and what was what. She described parents crying out for their children and children longing for their parents. Individuals were so overcome with emotion that they grew faint. Friends and families flooded in but were unaware of how to be fully supportive. The level of trauma was so high that the only interventions that were absorbed were those that were nonverbal or that fully addressed practical needs. People were frightened and in a state of shock.
Day by day, more order ensued and the efforts became more coordinated, but it became apparent to her that the “family reunification center” was devoid of reunification. She and her colleagues’ primary role became aiding the police department in making death notifications to the families and being supportive of the victims’ families and their loved ones during and in between the formal briefings, where so many concerned family members and friends gathered and waited.
“As the days went on, things became more structured and predictable,” Dr. Feldman noted. “We continued to connect with the victims’ families and survivors, [listened to] their stories, shared meals with them, spent downtime with them, began to intimately know their loved ones, and all the barriers they were now facing. We became invested in them, their unique intricacies, and to care deeply for them like our own families and loved ones. Small talk and conversation morphed into silent embraces where spoken words weren’t necessary.”
Dr. Feldman said some of her earliest memories were visiting ICU patients alongside her father, a critical care and ICU physician. Her father taught her that nonverbal communication and connection can be offered to patients in the most poignant moments of suffering.
Her “nascent experiences in the ICU,” she said, taught her that “the most useful of interventions was just being with people in their pain and bearing witness at times when there were just no words.”
Dr. Feldman said that when many of her colleagues learned about the switch from rescue to recovery, the pull was to jump in their cars and drive to the hotel where the families were based to offer support.
The unity she witnessed – from the disparate clinicians who were virtual strangers before the incident but a team afterward, from the families and the community, and from the first responders and rescue teams – was inspiring, Dr. Feldman said.
“We were all forced to think beyond ourselves, push ourselves past our limits, and unify in a way that remedied this period in history of deep fragmentation,” she said.
Understanding the role of psychoneuroimmunology
In another presentation during the Zoom event, Ms. Stauber offered her insights about the importance of support among mental health clinicians.
She cited research on women with HIV showing that those who are part of a support group had a stronger immune response than those who were not.
Ms. Stauber said the impact of COVID-19 and its ramifications – including fear, grief over losing loved ones, isolation from friends and family, and interference/cessation of normal routines – has put an enormous strain on clinicians and clients. One of her clients had to take her mother to the emergency room – never to see her again. She continues to ask: “If I’d been there, could I have saved her?”
Another client whose husband died of COVID-19–related illness agonizes over not being able to be at her husband’s side, not being able to hold his hand, not being able to say goodbye.
She said other cultures are more accepting of suffering as a condition of life and the acknowledgment that our time on earth is limited.
The “quick fix for everything” society carries over to people’s grief, said Ms. Stauber. As a result, many find it difficult to appreciate how much time it takes to heal.
Normal uncomplicated grief can take approximately 2-3 years, she said. By then, the shock has been wearing off, the emotional roller coaster of loss is calming down, coping skills are strengthened, and life can once again be more fulfilling or meaningful. Complicated grief or grief with trauma takes much longer, said Ms. Stauber, who is a consultant with a national crisis and debriefing company providing trauma and bereavement support to Fortune 500 companies.
Trauma adds another complexity to loss. To begin to appreciate the rough road ahead, Ms. Stauber said, it is important to understand the basic challenges facing grieving people.
“This is where our profession may be needed; we are providing support for those suffering the immense pain of loss in a world that often has difficulty being present or patient with loss,” she said. “We are indeed providing an emotional life raft.”
Ultimately, self-care is critical, Ms. Stauber said. “Consider self-care a job requirement” to be successful. She also offered the following tips for self-care:
1. Share your own loss experience with a caring and nonjudgmental person.
2. Consider ongoing supervision and consultation with colleagues who understand the nature of your work.
3. Be willing to ask for help.
4. Be aware of risks and countertransference in our work.
5. Attend workshops.
6. Remember that you do not have to and cannot do it all by yourself – we absolutely need more grief and trauma trained therapists.
7. Involve yourself in activities outside of work that feed your soul and nourish your spirit.
8. Schedule play.
9. Develop a healthy self-care regimen to remain present doing this work.
10. Consider the benefits of exercise.
11. Enjoy the beauty and wonder of nature.
12. Consider yoga, meditation, spa retreats – such as Kripalu, Miraval, and Canyon Ranch.
13. Spend time with loving family and friends.
14. Adopt a pet.
15. Eat healthy foods; get plenty of rest.
16. Walk in the rain.
17. Listen to music.
18. Enjoy a relaxing bubble bath.
19. Sing, dance, and enjoy the blessings of this life.
20. Love yourself; you truly can be your own best friend.
To advocate on behalf of mental health for patients, we must do the same for mental health professionals. The retreat was well received, and we learned a lot from our speakers. After the program, we offered a 45-minute yoga class and then 30-minute sound bowl meditation. We plan to repeat the event in September to help our community deal with the ongoing stress of such overwhelming loss.
While our community will never be the same, we hope that, by coming together, we can all find a way to support one another and strive to help ourselves and others manage as we navigate yet another unprecedented crisis.
Dr. Ritvo, who has more than 30 years’ experience in psychiatry, practices telemedicine. She is author of “BeKindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018). Dr. Ritvo has no disclosures.
The mental health toll from the Surfside, Fla., Champlain Tower collapse will be felt by our patients for years to come. As mental health professionals in Miami-Dade County, it has been difficult to deal with the catastrophe layered on the escalating COVID-19 crisis.
With each passing day after the June 24 incident, we all learned who the 98 victims were. In session after session, the enormous impact of this unfathomable tragedy unfolded. Some mental health care professionals were directly affected with the loss of family members; some lost patients, and a large number of our patients lost someone or knew someone who lost someone. It was reminiscent of our work during the COVID-19 crisis when we found that we were dealing with the same stressors as those of our patients. As it was said then, we were all in the same storm – just in very different boats.
It was heartening to see how many colleagues rushed to the site of the building where family waiting areas were established. So many professionals wanted to assist that some had to be turned away.
The days right after the collapse were agonizing for all as we waited and hoped for survivors to be found. Search teams from across the United States and from Mexico and Israel – specifically, Israeli Defense Forces personnel with experience conducting operations in the wake of earthquakes in both Haiti and Nepal, took on the dangerous work. When no one was recovered after the first day, hope faded, and after 10 days, the search and rescue efforts turned to search and recovery. We were indeed a county and community in mourning.
According to Lina Haji, PsyD, GIA Miami, in addition to the direct impact of loss, clinicians who engaged in crisis response and bereavement counseling with those affected by the Surfside tragedy were subjected to vicarious trauma. Mental health care providers in the Miami area not only experienced the direct effect of this tragedy but have been hearing details and harrowing stories about the unimaginable experiences their patients endured over those critical weeks. Vicarious trauma can result in our own symptoms, compassion fatigue, or burnout as clinicians. This resulted in a call for mental health providers to come to the aid of their fellow colleagues.
So, on the 1-month anniversary of the initial collapse, at the urging of Patricia Stauber, RN, LCSW, a clinician with more than 30 years’ experience in providing grief counseling in hospital and private practice settings; Antonello Bonci, MD, the founder of GIA Miami; Charlotte Tomic, director of public relations for the Institute for the Study of Global Antisemitism; and I cohosted what we hope will be several Mental Health Appreciation retreats. Our goal was to create a space to focus on healing the healers. We had hoped to hold an in-person event, but at the last moment we opted for a Zoom-based event because COVID-19 cases were rising rapidly again.
Working on the front lines
Cassie Feldman, PsyD, a licensed clinical psychologist with extensive experience working with grief, loss, end of life, and responding to trauma-related consults, reflected on her experience responding to the collapse in the earliest days – first independently at the request of community religious leaders and then as part of CADENA Foundation, a nonprofit organization dedicated to rescue, humanitarian aid, and disaster response and prevention worldwide.
Dr. Feldman worked alongside other mental health professionals, local Miami-Dade police and fire officials, and the domestic and international rescue teams (CADENA’s Go Team from Mexico and the Israeli Defense Force’s Search and Rescue Delegation), providing Psychological First Aid, crisis intervention, and disaster response to the victims’ families and survivors.
This initially was a 24-hour coverage effort, requiring Dr. Feldman and her colleagues to clear their schedules, and at times to work 18-hour shifts in the early days of the crisis to address the need for consistency and continuity. Their commitment was to show up for the victims’ families and survivors, fully embracing the chaos and the demands of the situation. She noted that the disaster brought out the best of her and her colleagues.
They divided and conquered the work, alongside clinicians from Jewish Community Services and Project Chai intervening acutely where possible, and coordinating long-term care plans for those survivors and members of the victims’ support networks in need of consistent care.
Dr. Feldman reflected on the notion that we have all been processing losses prior to this – loss of normalcy because of the pandemic, loss of people we loved as a result, other personal losses – and that this community tragedy is yet another loss to disentangle. It didn’t feel good or natural for her to passively absorb the news knowing she had both the skill set and capacity to take on an active supportive role. The first days at the community center were disorganized; it was hard to know who was who and what was what. She described parents crying out for their children and children longing for their parents. Individuals were so overcome with emotion that they grew faint. Friends and families flooded in but were unaware of how to be fully supportive. The level of trauma was so high that the only interventions that were absorbed were those that were nonverbal or that fully addressed practical needs. People were frightened and in a state of shock.
Day by day, more order ensued and the efforts became more coordinated, but it became apparent to her that the “family reunification center” was devoid of reunification. She and her colleagues’ primary role became aiding the police department in making death notifications to the families and being supportive of the victims’ families and their loved ones during and in between the formal briefings, where so many concerned family members and friends gathered and waited.
“As the days went on, things became more structured and predictable,” Dr. Feldman noted. “We continued to connect with the victims’ families and survivors, [listened to] their stories, shared meals with them, spent downtime with them, began to intimately know their loved ones, and all the barriers they were now facing. We became invested in them, their unique intricacies, and to care deeply for them like our own families and loved ones. Small talk and conversation morphed into silent embraces where spoken words weren’t necessary.”
Dr. Feldman said some of her earliest memories were visiting ICU patients alongside her father, a critical care and ICU physician. Her father taught her that nonverbal communication and connection can be offered to patients in the most poignant moments of suffering.
Her “nascent experiences in the ICU,” she said, taught her that “the most useful of interventions was just being with people in their pain and bearing witness at times when there were just no words.”
Dr. Feldman said that when many of her colleagues learned about the switch from rescue to recovery, the pull was to jump in their cars and drive to the hotel where the families were based to offer support.
The unity she witnessed – from the disparate clinicians who were virtual strangers before the incident but a team afterward, from the families and the community, and from the first responders and rescue teams – was inspiring, Dr. Feldman said.
“We were all forced to think beyond ourselves, push ourselves past our limits, and unify in a way that remedied this period in history of deep fragmentation,” she said.
Understanding the role of psychoneuroimmunology
In another presentation during the Zoom event, Ms. Stauber offered her insights about the importance of support among mental health clinicians.
She cited research on women with HIV showing that those who are part of a support group had a stronger immune response than those who were not.
Ms. Stauber said the impact of COVID-19 and its ramifications – including fear, grief over losing loved ones, isolation from friends and family, and interference/cessation of normal routines – has put an enormous strain on clinicians and clients. One of her clients had to take her mother to the emergency room – never to see her again. She continues to ask: “If I’d been there, could I have saved her?”
Another client whose husband died of COVID-19–related illness agonizes over not being able to be at her husband’s side, not being able to hold his hand, not being able to say goodbye.
She said other cultures are more accepting of suffering as a condition of life and the acknowledgment that our time on earth is limited.
The “quick fix for everything” society carries over to people’s grief, said Ms. Stauber. As a result, many find it difficult to appreciate how much time it takes to heal.
Normal uncomplicated grief can take approximately 2-3 years, she said. By then, the shock has been wearing off, the emotional roller coaster of loss is calming down, coping skills are strengthened, and life can once again be more fulfilling or meaningful. Complicated grief or grief with trauma takes much longer, said Ms. Stauber, who is a consultant with a national crisis and debriefing company providing trauma and bereavement support to Fortune 500 companies.
Trauma adds another complexity to loss. To begin to appreciate the rough road ahead, Ms. Stauber said, it is important to understand the basic challenges facing grieving people.
“This is where our profession may be needed; we are providing support for those suffering the immense pain of loss in a world that often has difficulty being present or patient with loss,” she said. “We are indeed providing an emotional life raft.”
Ultimately, self-care is critical, Ms. Stauber said. “Consider self-care a job requirement” to be successful. She also offered the following tips for self-care:
1. Share your own loss experience with a caring and nonjudgmental person.
2. Consider ongoing supervision and consultation with colleagues who understand the nature of your work.
3. Be willing to ask for help.
4. Be aware of risks and countertransference in our work.
5. Attend workshops.
6. Remember that you do not have to and cannot do it all by yourself – we absolutely need more grief and trauma trained therapists.
7. Involve yourself in activities outside of work that feed your soul and nourish your spirit.
8. Schedule play.
9. Develop a healthy self-care regimen to remain present doing this work.
10. Consider the benefits of exercise.
11. Enjoy the beauty and wonder of nature.
12. Consider yoga, meditation, spa retreats – such as Kripalu, Miraval, and Canyon Ranch.
13. Spend time with loving family and friends.
14. Adopt a pet.
15. Eat healthy foods; get plenty of rest.
16. Walk in the rain.
17. Listen to music.
18. Enjoy a relaxing bubble bath.
19. Sing, dance, and enjoy the blessings of this life.
20. Love yourself; you truly can be your own best friend.
To advocate on behalf of mental health for patients, we must do the same for mental health professionals. The retreat was well received, and we learned a lot from our speakers. After the program, we offered a 45-minute yoga class and then 30-minute sound bowl meditation. We plan to repeat the event in September to help our community deal with the ongoing stress of such overwhelming loss.
While our community will never be the same, we hope that, by coming together, we can all find a way to support one another and strive to help ourselves and others manage as we navigate yet another unprecedented crisis.
Dr. Ritvo, who has more than 30 years’ experience in psychiatry, practices telemedicine. She is author of “BeKindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018). Dr. Ritvo has no disclosures.
How many years of aromatase inhibitor therapy in breast cancer?
That’s a question that has been vexing oncologists for more than a decade, but final results from a large study now suggest that, after an initial 5 years of adjuvant endocrine therapy with tamoxifen and/or an AI, an additional 5 years of AI therapy offer no clinical benefits, compared with an additional 2 years of therapy.
What’s more, 5 years of additional treatment were associated with a significantly increased risk for fractures, compared with 2 years of therapy, Michael Gnant, MD, from the Medical University of Vienna, and colleagues reported in the phase 3 Austrian Breast and Colorectal Cancer Study Group Trial 16 (ABCSG-16).
“Thus, in postmenopausal women with hormone receptor–positive breast cancer who had received 5 years of adjuvant endocrine therapy, the extension of aromatase inhibitor therapy for 2 years rather than 5 years was sufficient to maximize the benefits of such therapy in most patients without extending the exposure to toxic effects,” they concluded.
The study was published online on July 28, 2021, in the New England Journal of Medicine.
The new results “in patients with hormone-receptor–positive breast cancer who are at low or average risk underscore the importance of avoiding iatrogenic complications when making these decisions,” Pamela J. Goodwin, MD, from the Lunenfeld–Tanenbaum Research Institute at the University of Toronto, wrote in an accompanying editorial.
She noted that these latest results echo those of two earlier studies: the DATA trial and the optimistically named IDEAL trial, both of which showed no benefit of short-term versus longer-term adjuvant endocrine therapy with regard to either disease-free survival (DFS) or overall survival (OS).
“These results provide strong evidence against the routine use of more than 2 years of extended aromatase inhibitor therapy in women who are at low or average risk, similar to those who were included in this trial,” Dr. Goodwin commented.
ABCSG-16 details
Dr. Gnant and colleagues enrolled 3,484 women aged 80 years or younger with HR-positive stage I, II, or III breast cancer for whom there was no evidence of recurrence at baseline. The patients had all received adjuvant tamoxifen or an AI for 5 years; for some patients, the two drugs were administered in sequence until 12 months before randomization.
The patients were randomly assigned to receive oral anastrozole 1 mg daily for either 2 or 5 additional years.
Among all patients, 3,208 had experienced no recurrence 2 years after randomization. These patients were included in the primary DFS analysis. Of this group, 1,635 (51%) had received tamoxifen alone for 5 years, 235 (7.3%) had received an AI alone, and 41.7% had received sequential AIs and tamoxifen.
At a median follow-up of 118 months after randomization, disease progression or death occurred in 670 women – 335 in each trial arm.
The rate of DFS 10 years after randomization was 73.6% in the 2-year additional-therapy group and 73.9% in the 5-year group. The hazard ratio for disease recurrence or death was 0.99 and was not significant. After adjustment for potential confounding factors, the HR remained essentially unchanged (HR, 1.0).
Overall survival at 8 years, a secondary endpoint, was virtually identical between the groups, at 87.5% in the 2-year group and 87.3% in the 5-year group. The HR for death from any cause was 1.02 and was not significant.
The HR for contralateral breast cancer was 1.15, and the HR for a second primary cancer was 1.06; neither was statistically significant.
Although the use of bone-targeted medication was similar between the groups, among patients in the 2-year group, the incidence of clinical bone fractures 5 years after randomization was lower, at 4.7% versus 6.3%, which translates to an HR for fracture with 5 additional years of AI therapy of 1.35 (95% confidence interval, 1.00-1.84).
Adverse events with anastrozole were consistent with its known toxicity profile. At least one serious adverse event occurred in 26.5% of patients in the 2-year group and in 40.2% in the 5-year group. Investigator-assessed serious adverse events that were judged to be related to anastrozole occurred in 2.3% and 4.0% of patients, respectively.
Osteoarthritis, the most frequently reported adverse event, was documented in 1.7% of patients in the 2-year group and in 4.3% in the 5-year group.
AI duration still unclear
“We did not investigate the value of extending adjuvant endocrine therapy per se, since the benefit of extending aromatase inhibitors after 5 years of adjuvant tamoxifen has been well established. In contrast, the most effective duration of adjuvant aromatase inhibitor therapy remains unclear in randomized trials,” Dr. Gnant and colleagues commented.
In her editorial, Dr. Goodwin summarized potential approaches for improving late outcomes for patients with hormone receptor–positive breast cancers, including the use of biomarkers to detect minimal residual disease and therapies to treat it.
She noted that the detection of circulating tumor cells in patients with HR-positive breast cancer 5 years after diagnosis is associated with a 13-fold increase in risk for recurrence and a median time to clinical recurrence of 2.8 years.
“This interval may be sufficiently long that therapeutic intervention can prevent the development of incurable clinical metastases. Continued improvement in these assays and the development of new therapies that target the unique biologic features of dormant cells will no doubt be required for a major reduction in late recurrence risk,” she wrote.
The study was supported by AstraZeneca and the Austrian Breast and Colorectal Cancer Study Group. Dr. Gnant has received lecture fees from AstraZeneca and others. Dr. Goodwin has received institutional research funding from the Breast Cancer Research Foundation and EPIC Sciences.
A version of this article first appeared on Medscape.com.
That’s a question that has been vexing oncologists for more than a decade, but final results from a large study now suggest that, after an initial 5 years of adjuvant endocrine therapy with tamoxifen and/or an AI, an additional 5 years of AI therapy offer no clinical benefits, compared with an additional 2 years of therapy.
What’s more, 5 years of additional treatment were associated with a significantly increased risk for fractures, compared with 2 years of therapy, Michael Gnant, MD, from the Medical University of Vienna, and colleagues reported in the phase 3 Austrian Breast and Colorectal Cancer Study Group Trial 16 (ABCSG-16).
“Thus, in postmenopausal women with hormone receptor–positive breast cancer who had received 5 years of adjuvant endocrine therapy, the extension of aromatase inhibitor therapy for 2 years rather than 5 years was sufficient to maximize the benefits of such therapy in most patients without extending the exposure to toxic effects,” they concluded.
The study was published online on July 28, 2021, in the New England Journal of Medicine.
The new results “in patients with hormone-receptor–positive breast cancer who are at low or average risk underscore the importance of avoiding iatrogenic complications when making these decisions,” Pamela J. Goodwin, MD, from the Lunenfeld–Tanenbaum Research Institute at the University of Toronto, wrote in an accompanying editorial.
She noted that these latest results echo those of two earlier studies: the DATA trial and the optimistically named IDEAL trial, both of which showed no benefit of short-term versus longer-term adjuvant endocrine therapy with regard to either disease-free survival (DFS) or overall survival (OS).
“These results provide strong evidence against the routine use of more than 2 years of extended aromatase inhibitor therapy in women who are at low or average risk, similar to those who were included in this trial,” Dr. Goodwin commented.
ABCSG-16 details
Dr. Gnant and colleagues enrolled 3,484 women aged 80 years or younger with HR-positive stage I, II, or III breast cancer for whom there was no evidence of recurrence at baseline. The patients had all received adjuvant tamoxifen or an AI for 5 years; for some patients, the two drugs were administered in sequence until 12 months before randomization.
The patients were randomly assigned to receive oral anastrozole 1 mg daily for either 2 or 5 additional years.
Among all patients, 3,208 had experienced no recurrence 2 years after randomization. These patients were included in the primary DFS analysis. Of this group, 1,635 (51%) had received tamoxifen alone for 5 years, 235 (7.3%) had received an AI alone, and 41.7% had received sequential AIs and tamoxifen.
At a median follow-up of 118 months after randomization, disease progression or death occurred in 670 women – 335 in each trial arm.
The rate of DFS 10 years after randomization was 73.6% in the 2-year additional-therapy group and 73.9% in the 5-year group. The hazard ratio for disease recurrence or death was 0.99 and was not significant. After adjustment for potential confounding factors, the HR remained essentially unchanged (HR, 1.0).
Overall survival at 8 years, a secondary endpoint, was virtually identical between the groups, at 87.5% in the 2-year group and 87.3% in the 5-year group. The HR for death from any cause was 1.02 and was not significant.
The HR for contralateral breast cancer was 1.15, and the HR for a second primary cancer was 1.06; neither was statistically significant.
Although the use of bone-targeted medication was similar between the groups, among patients in the 2-year group, the incidence of clinical bone fractures 5 years after randomization was lower, at 4.7% versus 6.3%, which translates to an HR for fracture with 5 additional years of AI therapy of 1.35 (95% confidence interval, 1.00-1.84).
Adverse events with anastrozole were consistent with its known toxicity profile. At least one serious adverse event occurred in 26.5% of patients in the 2-year group and in 40.2% in the 5-year group. Investigator-assessed serious adverse events that were judged to be related to anastrozole occurred in 2.3% and 4.0% of patients, respectively.
Osteoarthritis, the most frequently reported adverse event, was documented in 1.7% of patients in the 2-year group and in 4.3% in the 5-year group.
AI duration still unclear
“We did not investigate the value of extending adjuvant endocrine therapy per se, since the benefit of extending aromatase inhibitors after 5 years of adjuvant tamoxifen has been well established. In contrast, the most effective duration of adjuvant aromatase inhibitor therapy remains unclear in randomized trials,” Dr. Gnant and colleagues commented.
In her editorial, Dr. Goodwin summarized potential approaches for improving late outcomes for patients with hormone receptor–positive breast cancers, including the use of biomarkers to detect minimal residual disease and therapies to treat it.
She noted that the detection of circulating tumor cells in patients with HR-positive breast cancer 5 years after diagnosis is associated with a 13-fold increase in risk for recurrence and a median time to clinical recurrence of 2.8 years.
“This interval may be sufficiently long that therapeutic intervention can prevent the development of incurable clinical metastases. Continued improvement in these assays and the development of new therapies that target the unique biologic features of dormant cells will no doubt be required for a major reduction in late recurrence risk,” she wrote.
The study was supported by AstraZeneca and the Austrian Breast and Colorectal Cancer Study Group. Dr. Gnant has received lecture fees from AstraZeneca and others. Dr. Goodwin has received institutional research funding from the Breast Cancer Research Foundation and EPIC Sciences.
A version of this article first appeared on Medscape.com.
That’s a question that has been vexing oncologists for more than a decade, but final results from a large study now suggest that, after an initial 5 years of adjuvant endocrine therapy with tamoxifen and/or an AI, an additional 5 years of AI therapy offer no clinical benefits, compared with an additional 2 years of therapy.
What’s more, 5 years of additional treatment were associated with a significantly increased risk for fractures, compared with 2 years of therapy, Michael Gnant, MD, from the Medical University of Vienna, and colleagues reported in the phase 3 Austrian Breast and Colorectal Cancer Study Group Trial 16 (ABCSG-16).
“Thus, in postmenopausal women with hormone receptor–positive breast cancer who had received 5 years of adjuvant endocrine therapy, the extension of aromatase inhibitor therapy for 2 years rather than 5 years was sufficient to maximize the benefits of such therapy in most patients without extending the exposure to toxic effects,” they concluded.
The study was published online on July 28, 2021, in the New England Journal of Medicine.
The new results “in patients with hormone-receptor–positive breast cancer who are at low or average risk underscore the importance of avoiding iatrogenic complications when making these decisions,” Pamela J. Goodwin, MD, from the Lunenfeld–Tanenbaum Research Institute at the University of Toronto, wrote in an accompanying editorial.
She noted that these latest results echo those of two earlier studies: the DATA trial and the optimistically named IDEAL trial, both of which showed no benefit of short-term versus longer-term adjuvant endocrine therapy with regard to either disease-free survival (DFS) or overall survival (OS).
“These results provide strong evidence against the routine use of more than 2 years of extended aromatase inhibitor therapy in women who are at low or average risk, similar to those who were included in this trial,” Dr. Goodwin commented.
ABCSG-16 details
Dr. Gnant and colleagues enrolled 3,484 women aged 80 years or younger with HR-positive stage I, II, or III breast cancer for whom there was no evidence of recurrence at baseline. The patients had all received adjuvant tamoxifen or an AI for 5 years; for some patients, the two drugs were administered in sequence until 12 months before randomization.
The patients were randomly assigned to receive oral anastrozole 1 mg daily for either 2 or 5 additional years.
Among all patients, 3,208 had experienced no recurrence 2 years after randomization. These patients were included in the primary DFS analysis. Of this group, 1,635 (51%) had received tamoxifen alone for 5 years, 235 (7.3%) had received an AI alone, and 41.7% had received sequential AIs and tamoxifen.
At a median follow-up of 118 months after randomization, disease progression or death occurred in 670 women – 335 in each trial arm.
The rate of DFS 10 years after randomization was 73.6% in the 2-year additional-therapy group and 73.9% in the 5-year group. The hazard ratio for disease recurrence or death was 0.99 and was not significant. After adjustment for potential confounding factors, the HR remained essentially unchanged (HR, 1.0).
Overall survival at 8 years, a secondary endpoint, was virtually identical between the groups, at 87.5% in the 2-year group and 87.3% in the 5-year group. The HR for death from any cause was 1.02 and was not significant.
The HR for contralateral breast cancer was 1.15, and the HR for a second primary cancer was 1.06; neither was statistically significant.
Although the use of bone-targeted medication was similar between the groups, among patients in the 2-year group, the incidence of clinical bone fractures 5 years after randomization was lower, at 4.7% versus 6.3%, which translates to an HR for fracture with 5 additional years of AI therapy of 1.35 (95% confidence interval, 1.00-1.84).
Adverse events with anastrozole were consistent with its known toxicity profile. At least one serious adverse event occurred in 26.5% of patients in the 2-year group and in 40.2% in the 5-year group. Investigator-assessed serious adverse events that were judged to be related to anastrozole occurred in 2.3% and 4.0% of patients, respectively.
Osteoarthritis, the most frequently reported adverse event, was documented in 1.7% of patients in the 2-year group and in 4.3% in the 5-year group.
AI duration still unclear
“We did not investigate the value of extending adjuvant endocrine therapy per se, since the benefit of extending aromatase inhibitors after 5 years of adjuvant tamoxifen has been well established. In contrast, the most effective duration of adjuvant aromatase inhibitor therapy remains unclear in randomized trials,” Dr. Gnant and colleagues commented.
In her editorial, Dr. Goodwin summarized potential approaches for improving late outcomes for patients with hormone receptor–positive breast cancers, including the use of biomarkers to detect minimal residual disease and therapies to treat it.
She noted that the detection of circulating tumor cells in patients with HR-positive breast cancer 5 years after diagnosis is associated with a 13-fold increase in risk for recurrence and a median time to clinical recurrence of 2.8 years.
“This interval may be sufficiently long that therapeutic intervention can prevent the development of incurable clinical metastases. Continued improvement in these assays and the development of new therapies that target the unique biologic features of dormant cells will no doubt be required for a major reduction in late recurrence risk,” she wrote.
The study was supported by AstraZeneca and the Austrian Breast and Colorectal Cancer Study Group. Dr. Gnant has received lecture fees from AstraZeneca and others. Dr. Goodwin has received institutional research funding from the Breast Cancer Research Foundation and EPIC Sciences.
A version of this article first appeared on Medscape.com.