User login
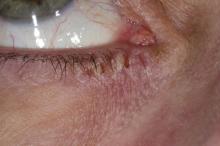
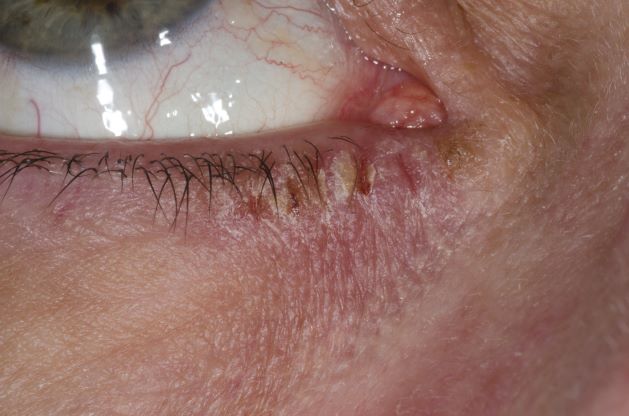
Woman with burning, itchy red eyes
This patient has the “atopic triad” of allergies, asthma, and atopic dermatitis. Atopic dermatitis around the eyes and on the eyelids often develops in teenage years and adulthood but may also occur in older persons. Occasionally, it can be the only manifestation of atopic dermatitis. The upper eyelids may appear scaly and fissured. The so-called "allergic shiners" (symmetric, dark circles beneath the lower eyelid) and Dennie-Morgan lines (extra skin folds under the lower eyelid) are often present.
The thin skin of the eyelids is particularly sensitive to irritants and allergens and is thus prone to develop dermatitis. Contact with the same trigger may not lead to a rash on other areas of skin. Upper, lower or both eyelids on one or both sides can be affected. The patient may report itching, stinging or burning, and the lids are red and scaly. They may swell. With persistence of the dermatitis, the eyelids become thickened with increased skin markings (lichenification). The eyelid margins may become involved (blepharitis). The appearance is similar, whatever the cause.
The basis of treatment for atopic dermatitis is to provide moisturization for dryness, allay pruritus, and manage inflammation of the eczematous lesions. Conservative initial management of eyelid dermatitis also includes gentle skin care and avoidance of fragrance and other known irritants in personal care, hair, and facial skin care products. Bland, fragrance-free emollients, such as petrolatum, may be applied directly to the eyelids.
Topical corticosteroids are one therapeutic option for eyelid dermatitis. However, only low-potency topical corticosteroids are safe, and only for short-term use, on the eyelids. Typically, they are used twice daily for 2-4 weeks. However, even with low-potency topical corticosteroids, the eyelids remain vulnerable to thinning, even atrophy. Because of these issues, topical calcineurin inhibitors are often the preferred treatment.
Patients with atopic dermatitis have an increased risk of comorbid eye diseases, including keratitis, conjunctivitis, and keratoconus. A careful clinical examination for associated erythema, crusting, and blepharitis many prompt a referral to an ophthalmologist.
Brian S. Kim, MD, Associate Professor, Department of Medicine, Division of Dermatology, Washington University School of Medicine, St. Louis, Missouri
Brian S. Kim, MD, has disclosed no relevant financial relationships.
This patient has the “atopic triad” of allergies, asthma, and atopic dermatitis. Atopic dermatitis around the eyes and on the eyelids often develops in teenage years and adulthood but may also occur in older persons. Occasionally, it can be the only manifestation of atopic dermatitis. The upper eyelids may appear scaly and fissured. The so-called "allergic shiners" (symmetric, dark circles beneath the lower eyelid) and Dennie-Morgan lines (extra skin folds under the lower eyelid) are often present.
The thin skin of the eyelids is particularly sensitive to irritants and allergens and is thus prone to develop dermatitis. Contact with the same trigger may not lead to a rash on other areas of skin. Upper, lower or both eyelids on one or both sides can be affected. The patient may report itching, stinging or burning, and the lids are red and scaly. They may swell. With persistence of the dermatitis, the eyelids become thickened with increased skin markings (lichenification). The eyelid margins may become involved (blepharitis). The appearance is similar, whatever the cause.
The basis of treatment for atopic dermatitis is to provide moisturization for dryness, allay pruritus, and manage inflammation of the eczematous lesions. Conservative initial management of eyelid dermatitis also includes gentle skin care and avoidance of fragrance and other known irritants in personal care, hair, and facial skin care products. Bland, fragrance-free emollients, such as petrolatum, may be applied directly to the eyelids.
Topical corticosteroids are one therapeutic option for eyelid dermatitis. However, only low-potency topical corticosteroids are safe, and only for short-term use, on the eyelids. Typically, they are used twice daily for 2-4 weeks. However, even with low-potency topical corticosteroids, the eyelids remain vulnerable to thinning, even atrophy. Because of these issues, topical calcineurin inhibitors are often the preferred treatment.
Patients with atopic dermatitis have an increased risk of comorbid eye diseases, including keratitis, conjunctivitis, and keratoconus. A careful clinical examination for associated erythema, crusting, and blepharitis many prompt a referral to an ophthalmologist.
Brian S. Kim, MD, Associate Professor, Department of Medicine, Division of Dermatology, Washington University School of Medicine, St. Louis, Missouri
Brian S. Kim, MD, has disclosed no relevant financial relationships.
This patient has the “atopic triad” of allergies, asthma, and atopic dermatitis. Atopic dermatitis around the eyes and on the eyelids often develops in teenage years and adulthood but may also occur in older persons. Occasionally, it can be the only manifestation of atopic dermatitis. The upper eyelids may appear scaly and fissured. The so-called "allergic shiners" (symmetric, dark circles beneath the lower eyelid) and Dennie-Morgan lines (extra skin folds under the lower eyelid) are often present.
The thin skin of the eyelids is particularly sensitive to irritants and allergens and is thus prone to develop dermatitis. Contact with the same trigger may not lead to a rash on other areas of skin. Upper, lower or both eyelids on one or both sides can be affected. The patient may report itching, stinging or burning, and the lids are red and scaly. They may swell. With persistence of the dermatitis, the eyelids become thickened with increased skin markings (lichenification). The eyelid margins may become involved (blepharitis). The appearance is similar, whatever the cause.
The basis of treatment for atopic dermatitis is to provide moisturization for dryness, allay pruritus, and manage inflammation of the eczematous lesions. Conservative initial management of eyelid dermatitis also includes gentle skin care and avoidance of fragrance and other known irritants in personal care, hair, and facial skin care products. Bland, fragrance-free emollients, such as petrolatum, may be applied directly to the eyelids.
Topical corticosteroids are one therapeutic option for eyelid dermatitis. However, only low-potency topical corticosteroids are safe, and only for short-term use, on the eyelids. Typically, they are used twice daily for 2-4 weeks. However, even with low-potency topical corticosteroids, the eyelids remain vulnerable to thinning, even atrophy. Because of these issues, topical calcineurin inhibitors are often the preferred treatment.
Patients with atopic dermatitis have an increased risk of comorbid eye diseases, including keratitis, conjunctivitis, and keratoconus. A careful clinical examination for associated erythema, crusting, and blepharitis many prompt a referral to an ophthalmologist.
Brian S. Kim, MD, Associate Professor, Department of Medicine, Division of Dermatology, Washington University School of Medicine, St. Louis, Missouri
Brian S. Kim, MD, has disclosed no relevant financial relationships.
A 21-year-old woman presents with burning, itchy red eyes that she rubs incessantly. On examination, she has an erythematic, scaly, pruritic rash on the upper and lower eyelids and below her eyes. She has no other outbreaks on the rest of her skin except for mild acne. A moisturizer has provided minimal relief for the itching but has not helped with the rash. She has a history of asthma, for which she uses an inhaler, and of hay fever, for which she takes an antihistamine. She also reports that she has had two episodes of conjunctivitis within the past year, which were treated with antibiotic eye drops.
D-dimer unreliable for ruling out pulmonary embolism in COVID-19
The plasma D-dimer assay has been used, along with clinical prediction scores, to rule out pulmonary embolism (PE) in critically ill patients for decades, but a new study suggests it may not be the right test to use in hospitalized COVID-19 patients.
The results showed that all hospitalized patients with COVID-19 and radiographic evidence of PE had plasma D-dimer levels of 0.05 mcg/mL or greater, the cutoff point for the diagnosis.
“If using D-dimer to exclude patients with PE, the increased values we found among 92.3% of patients suggest that this assay would be less useful than in the populations in which it was originally validated, among which a minority of patients had increased D-dimer values,” the authors write. “Setting higher D-dimer thresholds was associated with improved specificity at the cost of an increased false-negative rate that could be associated with an unacceptable patient safety risk.”
The inclusion of patients with D-dimer and computed tomography pulmonary angiography (CTPA) was necessary to estimate diagnostic performance, they note, but “this may have introduced selection bias by excluding patients unable to undergo CTPA.”
“Nonetheless, given the high pretest probability of PE and low specificity observed in this and other studies, these results suggest that use of D-dimer levels to exclude PE among patients hospitalized with COVID-19 may be inappropriate and have limited clinical utility,” they conclude.
Led by Constantine N. Logothetis, MD, from Morsani College of Medicine, University of South Florida, Tampa, the study was published online Oct. 8 as a Research Letter in JAMA Network Open.
Uncertain utility
The authors note that the availability of D-dimer samples routinely collected from hospitalized COVID-19 patients – as well as the heterogeneity of early, smaller studies – generated uncertainty about the utility of this assay.
This uncertainty prompted them to test the diagnostic accuracy of the D-dimer assay among a sample of 1,541 patients who were hospitalized with COVID-19 at their institution between January 2020 and February 2021 for a possible PE.
They compared plasma D-dimer concentrations with CTPA, the criterion standard for diagnosing PE, in 287 of those patients.
Overall, 118 patients (41.1%) required care in the ICU, and 27 patients (9.4%) died during hospitalization.
The investigators looked at the ability of plasma D-dimer levels collected on the same day as CTPA to diagnose PE.
Thirty-seven patients (12.9%) had radiographic evidence of PE, and 250 patients (87.1%) did not.
Overall, the vast majority of patients (92.3%; n = 265 patients) had plasma D-dimer levels of 0.05 mcg/mL or more, including all patients with PE and 225 of 250 patients without PE (91.2%).
The median D-dimer values were 1.0 mcg/mL for 250 patients without PE and 6.1 mcg/mL for 37 patients with PE.
D-dimer values ranged from 0.2 mcg/mL to 128 mcg/mL among patients without PE, and from 0.5 mcg/mL to more than 10,000 mcg/mL among patients with PE. Patients without PE had statistically significantly decreased mean D-dimer values (8.7 mcg/mL vs. 1.2 mcg/mL; P < .001).
A D-dimer concentration of 0.05 mcg/mL was associated with a sensitivity of 100%, specificity of 8.8%, negative predictive value (NPV) of 100%, positive predictive value (PPV) of 13.9%, and a negative likelihood ratio (NLR) of less than 0.1.
The age-adjusted threshold was associated with a sensitivity of 94.6%, specificity of 22.8%, NPV of 96.6%, PPV of 13.9%, and NLR of 0.24.
The authors note that all hospitalized patients with COVID-19 and radiographic evidence of PE had plasma D-dimer levels of 0.05 mcg/mL or greater.
D-dimer in VTE may not extrapolate to COVID-19
“The D-dimer test, which is a measure of circulating byproducts of blood clot dissolution, has long been incorporated into diagnostic algorithms for venous thromboembolic [VTE] disease, including deep vein thrombosis and pulmonary embolism. It is uncertain whether this diagnostic use of D-dimer testing can be extrapolated to the context of COVID-19 – an illness we now understand to be associated itself with intravascular thrombosis and fibrinolysis,” Matthew Tomey, MD, a cardiologist at Mount Sinai Morningside, New York, said in an interview.
“The authors of this study sought to evaluate the test characteristics of the D-dimer assay for diagnosis of pulmonary embolism in a consecutive series of 287 hospitalized patients with COVID-19 who underwent computed tomography pulmonary angiography (CTPA). This was a selected group of patients representing less than 20% of the 1,541 patients screened. Exclusion of data on the more than 80% of screened patients who did not undergo CTPA is a significant limitation of the study,” Dr. Tomey said.
“In the highly selected, small cohort studied, representing a group of patients at high pretest probability of pulmonary embolism, there was no patient with pulmonary embolism who had a D-dimer value less than 0.5 mcg/mL. Yet broad ranges of D-dimer values were observed in COVID-19 patients with (0.5 to >10,000 mcg/mL) and without (0.2 to 128 mcg/mL) pulmonary embolism,” he added.
Based on the presented data, it is likely true that very low levels of D-dimer decrease the likelihood of finding a pulmonary embolus on a CTPA, if it is performed, Dr. Tomey noted.
“Yet the data confirm that a wide range of D-dimer values can be observed in COVID-19 patients with or without pulmonary embolism. It is not clear at this time that D-dimer levels should be used as gatekeepers to diagnostic imaging studies such as CTPA when pretest suspicion of pulmonary embolism is high,” he said.
“This issue becomes relevant as we consider evolving data on use of anticoagulation in treatment of hospitalized patients with COVID-19. We learned this year that in critically ill patients hospitalized with COVID-19, routine therapeutic anticoagulation (with heparin) was not beneficial and potentially harmful when compared with usual thromboprophylaxis,” he concluded.
“As we strive to balance competing risks of bleeding and thrombosis, accurate diagnosis of pulmonary embolism is important to guide decision-making about therapeutic anticoagulation, including in COVID-19.”
Dr. Logothetis and Dr. Tomey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The plasma D-dimer assay has been used, along with clinical prediction scores, to rule out pulmonary embolism (PE) in critically ill patients for decades, but a new study suggests it may not be the right test to use in hospitalized COVID-19 patients.
The results showed that all hospitalized patients with COVID-19 and radiographic evidence of PE had plasma D-dimer levels of 0.05 mcg/mL or greater, the cutoff point for the diagnosis.
“If using D-dimer to exclude patients with PE, the increased values we found among 92.3% of patients suggest that this assay would be less useful than in the populations in which it was originally validated, among which a minority of patients had increased D-dimer values,” the authors write. “Setting higher D-dimer thresholds was associated with improved specificity at the cost of an increased false-negative rate that could be associated with an unacceptable patient safety risk.”
The inclusion of patients with D-dimer and computed tomography pulmonary angiography (CTPA) was necessary to estimate diagnostic performance, they note, but “this may have introduced selection bias by excluding patients unable to undergo CTPA.”
“Nonetheless, given the high pretest probability of PE and low specificity observed in this and other studies, these results suggest that use of D-dimer levels to exclude PE among patients hospitalized with COVID-19 may be inappropriate and have limited clinical utility,” they conclude.
Led by Constantine N. Logothetis, MD, from Morsani College of Medicine, University of South Florida, Tampa, the study was published online Oct. 8 as a Research Letter in JAMA Network Open.
Uncertain utility
The authors note that the availability of D-dimer samples routinely collected from hospitalized COVID-19 patients – as well as the heterogeneity of early, smaller studies – generated uncertainty about the utility of this assay.
This uncertainty prompted them to test the diagnostic accuracy of the D-dimer assay among a sample of 1,541 patients who were hospitalized with COVID-19 at their institution between January 2020 and February 2021 for a possible PE.
They compared plasma D-dimer concentrations with CTPA, the criterion standard for diagnosing PE, in 287 of those patients.
Overall, 118 patients (41.1%) required care in the ICU, and 27 patients (9.4%) died during hospitalization.
The investigators looked at the ability of plasma D-dimer levels collected on the same day as CTPA to diagnose PE.
Thirty-seven patients (12.9%) had radiographic evidence of PE, and 250 patients (87.1%) did not.
Overall, the vast majority of patients (92.3%; n = 265 patients) had plasma D-dimer levels of 0.05 mcg/mL or more, including all patients with PE and 225 of 250 patients without PE (91.2%).
The median D-dimer values were 1.0 mcg/mL for 250 patients without PE and 6.1 mcg/mL for 37 patients with PE.
D-dimer values ranged from 0.2 mcg/mL to 128 mcg/mL among patients without PE, and from 0.5 mcg/mL to more than 10,000 mcg/mL among patients with PE. Patients without PE had statistically significantly decreased mean D-dimer values (8.7 mcg/mL vs. 1.2 mcg/mL; P < .001).
A D-dimer concentration of 0.05 mcg/mL was associated with a sensitivity of 100%, specificity of 8.8%, negative predictive value (NPV) of 100%, positive predictive value (PPV) of 13.9%, and a negative likelihood ratio (NLR) of less than 0.1.
The age-adjusted threshold was associated with a sensitivity of 94.6%, specificity of 22.8%, NPV of 96.6%, PPV of 13.9%, and NLR of 0.24.
The authors note that all hospitalized patients with COVID-19 and radiographic evidence of PE had plasma D-dimer levels of 0.05 mcg/mL or greater.
D-dimer in VTE may not extrapolate to COVID-19
“The D-dimer test, which is a measure of circulating byproducts of blood clot dissolution, has long been incorporated into diagnostic algorithms for venous thromboembolic [VTE] disease, including deep vein thrombosis and pulmonary embolism. It is uncertain whether this diagnostic use of D-dimer testing can be extrapolated to the context of COVID-19 – an illness we now understand to be associated itself with intravascular thrombosis and fibrinolysis,” Matthew Tomey, MD, a cardiologist at Mount Sinai Morningside, New York, said in an interview.
“The authors of this study sought to evaluate the test characteristics of the D-dimer assay for diagnosis of pulmonary embolism in a consecutive series of 287 hospitalized patients with COVID-19 who underwent computed tomography pulmonary angiography (CTPA). This was a selected group of patients representing less than 20% of the 1,541 patients screened. Exclusion of data on the more than 80% of screened patients who did not undergo CTPA is a significant limitation of the study,” Dr. Tomey said.
“In the highly selected, small cohort studied, representing a group of patients at high pretest probability of pulmonary embolism, there was no patient with pulmonary embolism who had a D-dimer value less than 0.5 mcg/mL. Yet broad ranges of D-dimer values were observed in COVID-19 patients with (0.5 to >10,000 mcg/mL) and without (0.2 to 128 mcg/mL) pulmonary embolism,” he added.
Based on the presented data, it is likely true that very low levels of D-dimer decrease the likelihood of finding a pulmonary embolus on a CTPA, if it is performed, Dr. Tomey noted.
“Yet the data confirm that a wide range of D-dimer values can be observed in COVID-19 patients with or without pulmonary embolism. It is not clear at this time that D-dimer levels should be used as gatekeepers to diagnostic imaging studies such as CTPA when pretest suspicion of pulmonary embolism is high,” he said.
“This issue becomes relevant as we consider evolving data on use of anticoagulation in treatment of hospitalized patients with COVID-19. We learned this year that in critically ill patients hospitalized with COVID-19, routine therapeutic anticoagulation (with heparin) was not beneficial and potentially harmful when compared with usual thromboprophylaxis,” he concluded.
“As we strive to balance competing risks of bleeding and thrombosis, accurate diagnosis of pulmonary embolism is important to guide decision-making about therapeutic anticoagulation, including in COVID-19.”
Dr. Logothetis and Dr. Tomey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The plasma D-dimer assay has been used, along with clinical prediction scores, to rule out pulmonary embolism (PE) in critically ill patients for decades, but a new study suggests it may not be the right test to use in hospitalized COVID-19 patients.
The results showed that all hospitalized patients with COVID-19 and radiographic evidence of PE had plasma D-dimer levels of 0.05 mcg/mL or greater, the cutoff point for the diagnosis.
“If using D-dimer to exclude patients with PE, the increased values we found among 92.3% of patients suggest that this assay would be less useful than in the populations in which it was originally validated, among which a minority of patients had increased D-dimer values,” the authors write. “Setting higher D-dimer thresholds was associated with improved specificity at the cost of an increased false-negative rate that could be associated with an unacceptable patient safety risk.”
The inclusion of patients with D-dimer and computed tomography pulmonary angiography (CTPA) was necessary to estimate diagnostic performance, they note, but “this may have introduced selection bias by excluding patients unable to undergo CTPA.”
“Nonetheless, given the high pretest probability of PE and low specificity observed in this and other studies, these results suggest that use of D-dimer levels to exclude PE among patients hospitalized with COVID-19 may be inappropriate and have limited clinical utility,” they conclude.
Led by Constantine N. Logothetis, MD, from Morsani College of Medicine, University of South Florida, Tampa, the study was published online Oct. 8 as a Research Letter in JAMA Network Open.
Uncertain utility
The authors note that the availability of D-dimer samples routinely collected from hospitalized COVID-19 patients – as well as the heterogeneity of early, smaller studies – generated uncertainty about the utility of this assay.
This uncertainty prompted them to test the diagnostic accuracy of the D-dimer assay among a sample of 1,541 patients who were hospitalized with COVID-19 at their institution between January 2020 and February 2021 for a possible PE.
They compared plasma D-dimer concentrations with CTPA, the criterion standard for diagnosing PE, in 287 of those patients.
Overall, 118 patients (41.1%) required care in the ICU, and 27 patients (9.4%) died during hospitalization.
The investigators looked at the ability of plasma D-dimer levels collected on the same day as CTPA to diagnose PE.
Thirty-seven patients (12.9%) had radiographic evidence of PE, and 250 patients (87.1%) did not.
Overall, the vast majority of patients (92.3%; n = 265 patients) had plasma D-dimer levels of 0.05 mcg/mL or more, including all patients with PE and 225 of 250 patients without PE (91.2%).
The median D-dimer values were 1.0 mcg/mL for 250 patients without PE and 6.1 mcg/mL for 37 patients with PE.
D-dimer values ranged from 0.2 mcg/mL to 128 mcg/mL among patients without PE, and from 0.5 mcg/mL to more than 10,000 mcg/mL among patients with PE. Patients without PE had statistically significantly decreased mean D-dimer values (8.7 mcg/mL vs. 1.2 mcg/mL; P < .001).
A D-dimer concentration of 0.05 mcg/mL was associated with a sensitivity of 100%, specificity of 8.8%, negative predictive value (NPV) of 100%, positive predictive value (PPV) of 13.9%, and a negative likelihood ratio (NLR) of less than 0.1.
The age-adjusted threshold was associated with a sensitivity of 94.6%, specificity of 22.8%, NPV of 96.6%, PPV of 13.9%, and NLR of 0.24.
The authors note that all hospitalized patients with COVID-19 and radiographic evidence of PE had plasma D-dimer levels of 0.05 mcg/mL or greater.
D-dimer in VTE may not extrapolate to COVID-19
“The D-dimer test, which is a measure of circulating byproducts of blood clot dissolution, has long been incorporated into diagnostic algorithms for venous thromboembolic [VTE] disease, including deep vein thrombosis and pulmonary embolism. It is uncertain whether this diagnostic use of D-dimer testing can be extrapolated to the context of COVID-19 – an illness we now understand to be associated itself with intravascular thrombosis and fibrinolysis,” Matthew Tomey, MD, a cardiologist at Mount Sinai Morningside, New York, said in an interview.
“The authors of this study sought to evaluate the test characteristics of the D-dimer assay for diagnosis of pulmonary embolism in a consecutive series of 287 hospitalized patients with COVID-19 who underwent computed tomography pulmonary angiography (CTPA). This was a selected group of patients representing less than 20% of the 1,541 patients screened. Exclusion of data on the more than 80% of screened patients who did not undergo CTPA is a significant limitation of the study,” Dr. Tomey said.
“In the highly selected, small cohort studied, representing a group of patients at high pretest probability of pulmonary embolism, there was no patient with pulmonary embolism who had a D-dimer value less than 0.5 mcg/mL. Yet broad ranges of D-dimer values were observed in COVID-19 patients with (0.5 to >10,000 mcg/mL) and without (0.2 to 128 mcg/mL) pulmonary embolism,” he added.
Based on the presented data, it is likely true that very low levels of D-dimer decrease the likelihood of finding a pulmonary embolus on a CTPA, if it is performed, Dr. Tomey noted.
“Yet the data confirm that a wide range of D-dimer values can be observed in COVID-19 patients with or without pulmonary embolism. It is not clear at this time that D-dimer levels should be used as gatekeepers to diagnostic imaging studies such as CTPA when pretest suspicion of pulmonary embolism is high,” he said.
“This issue becomes relevant as we consider evolving data on use of anticoagulation in treatment of hospitalized patients with COVID-19. We learned this year that in critically ill patients hospitalized with COVID-19, routine therapeutic anticoagulation (with heparin) was not beneficial and potentially harmful when compared with usual thromboprophylaxis,” he concluded.
“As we strive to balance competing risks of bleeding and thrombosis, accurate diagnosis of pulmonary embolism is important to guide decision-making about therapeutic anticoagulation, including in COVID-19.”
Dr. Logothetis and Dr. Tomey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Medical comanagement did not improve hip fracture outcomes
Background: Medical comanagement of hip fracture patients is common. Prior evidence comes from mostly single-center studies, with most improvements being in process indicators such as length of stay and staff satisfaction.
Study design: Retrospective cohort study.
Setting: American College of Surgeons National Surgical Quality Improvement Program database.
Synopsis: With the NSQIP database targeted user file for hip fracture of 19,896 patients from 2016 to 2017, unadjusted analysis showed patients in the medical comanagement cohort were older with higher burden of comorbidities, higher morbidity (19.5% vs. 9.6%, odds ratio, 2.28; 95% CI, 1.98-2.63; P < .0001), and higher mortality rate (6.9% vs. 4.0%; OR, 1.79; 95% CI, 1.44-2.22; P < .0001). Both cohorts had similar proportion of patients participating in a standardized hip fracture program. After propensity score matching, patients in the comanagement cohort continued to show inferior morbidity (OR, 1.82; 95% CI, 1.52-2.20; P < .0001) and mortality (OR, 1.36; 95% CI, 1.02-1.81; P = .033).
This study failed to show superior outcomes in comanagement patients. The retrospective nature and propensity matching will lead to the question of unmeasured confounding in this large multinational database.
Bottom line: Medical comanagement of hip fractures was not associated with improved outcomes in the NSQIP database.
Citation: Maxwell BG, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: A propensity score–matched retrospective cohort analysis of the National Surgical Quality Improvement Project. J Hosp Med. 2020;15:468-74.
Dr. Lockwood is a hospitalist and chief of quality, performance, and patient safety at the Lexington (Ky.) VA Health Care System.
Background: Medical comanagement of hip fracture patients is common. Prior evidence comes from mostly single-center studies, with most improvements being in process indicators such as length of stay and staff satisfaction.
Study design: Retrospective cohort study.
Setting: American College of Surgeons National Surgical Quality Improvement Program database.
Synopsis: With the NSQIP database targeted user file for hip fracture of 19,896 patients from 2016 to 2017, unadjusted analysis showed patients in the medical comanagement cohort were older with higher burden of comorbidities, higher morbidity (19.5% vs. 9.6%, odds ratio, 2.28; 95% CI, 1.98-2.63; P < .0001), and higher mortality rate (6.9% vs. 4.0%; OR, 1.79; 95% CI, 1.44-2.22; P < .0001). Both cohorts had similar proportion of patients participating in a standardized hip fracture program. After propensity score matching, patients in the comanagement cohort continued to show inferior morbidity (OR, 1.82; 95% CI, 1.52-2.20; P < .0001) and mortality (OR, 1.36; 95% CI, 1.02-1.81; P = .033).
This study failed to show superior outcomes in comanagement patients. The retrospective nature and propensity matching will lead to the question of unmeasured confounding in this large multinational database.
Bottom line: Medical comanagement of hip fractures was not associated with improved outcomes in the NSQIP database.
Citation: Maxwell BG, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: A propensity score–matched retrospective cohort analysis of the National Surgical Quality Improvement Project. J Hosp Med. 2020;15:468-74.
Dr. Lockwood is a hospitalist and chief of quality, performance, and patient safety at the Lexington (Ky.) VA Health Care System.
Background: Medical comanagement of hip fracture patients is common. Prior evidence comes from mostly single-center studies, with most improvements being in process indicators such as length of stay and staff satisfaction.
Study design: Retrospective cohort study.
Setting: American College of Surgeons National Surgical Quality Improvement Program database.
Synopsis: With the NSQIP database targeted user file for hip fracture of 19,896 patients from 2016 to 2017, unadjusted analysis showed patients in the medical comanagement cohort were older with higher burden of comorbidities, higher morbidity (19.5% vs. 9.6%, odds ratio, 2.28; 95% CI, 1.98-2.63; P < .0001), and higher mortality rate (6.9% vs. 4.0%; OR, 1.79; 95% CI, 1.44-2.22; P < .0001). Both cohorts had similar proportion of patients participating in a standardized hip fracture program. After propensity score matching, patients in the comanagement cohort continued to show inferior morbidity (OR, 1.82; 95% CI, 1.52-2.20; P < .0001) and mortality (OR, 1.36; 95% CI, 1.02-1.81; P = .033).
This study failed to show superior outcomes in comanagement patients. The retrospective nature and propensity matching will lead to the question of unmeasured confounding in this large multinational database.
Bottom line: Medical comanagement of hip fractures was not associated with improved outcomes in the NSQIP database.
Citation: Maxwell BG, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: A propensity score–matched retrospective cohort analysis of the National Surgical Quality Improvement Project. J Hosp Med. 2020;15:468-74.
Dr. Lockwood is a hospitalist and chief of quality, performance, and patient safety at the Lexington (Ky.) VA Health Care System.
COVID-19 vaccination in MS: Lower response on certain medications
The results also show a reduced response to COVID vaccination in some patients on fingolimod.
The data come from a new series of vaccinated patients with MS from Madrid, which was presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
Presenting the data, Celia Oreja-Guevara, MD, Hospital Clínico San Carlos, Madrid, concluded that “currently approved COVID-19 vaccines appear safe in MS patients and are effective in most patients. However, vaccine strategy in patients treated with anti-CD20 and S1P inhibitors [such as fingolimod] need further study.”
“We showed that patients on ocrelizumab or rituximab had a very low or no antibody response to COVID vaccination,” she added. “However, some previous studies have shown some T-cell response to vaccination in these patients, and we are looking at that now.”
Assessing postvaccination antibody response
For the current study, the researchers analyzed the antibody response to COVID-19 vaccination at week 3, week 6, and month 3 after the first dose in 165 patients with MS and 200 healthy controls.
Of the patients with MS, 120 received both doses of mRNA vaccine and 42 received the AstraZeneca vaccine. The mean age of the MS patients was 45 years and 46 years in the healthy controls.
Adverse events were similar in the two groups, and no increase in relapse activity was seen in the patients with MS.
Mean antibody titers were slightly lower in the patients with MS versus the healthy controls. At 3 weeks, mean titers were 7,910 AU/mL in the patients with MS and 9,397 in the healthy controls. At 6 weeks, mean levels were 16,347 AU/mL in the patients with MS and 18,120 in the healthy controls.
Patients with MS treated with interferon-beta, glatiramer acetate, teriflunomide, dimethyl fumarate, cladribine, and natalizumab who received mRNA vaccines developed a similar postvaccination humoral response as the healthy controls at each of 3, 6, and 12 weeks after the first dose.
Patients with MS receiving the AstraZeneca vaccine mounted a lower humoral response than those receiving the mRNA vaccine, but this same effect was also seen in the healthy controls.
However, patients on the anti-CD20 drugs ocrelizumab or rituximab showed a lower humoral response to COVID vaccination. Only 3 of 20 patients who had been treated with ocrelizumab developed antibodies, but these patients had longer washout periods (at least 6 months) between receiving ocrelizumab and the COVID vaccine. All six patients treated with rituximab had no antibody response to the COVID vaccination.
Dr. Oreja-Guevara suggested that ocrelizumab-treated patients may have a worse outcome after COVID-19 infection. “In the first wave of infection in Madrid, we recorded five patients on ocrelizumab with COVID-19, four of whom were hospitalized,” she noted.
“In patients on ocrelizumab we need to try and have a long interval between giving this drug and giving the COVID vaccine. The longer the washout period, the more antibodies are seen,” she said.
She noted that two patients in the study received the COVID vaccine 1 year after ocrelizumab administration and had a normal humoral response, similar to the healthy controls.
The new anti-CD20 drug, ofatumumab, did not seem to affect the COVID vaccine antibody response as much as ocrelizumab or rituximab. In the current study, four of five patients treated with ofatumumab had an antibody response.
Dr. Oreja-Guevara suggested that this was probably because the depletion of B cells is not so strong with ofatumumab. “This drug is dosed every 4 weeks and it doesn’t deplete all the B cells and they are replaced quite quickly.”
Fingolimod is another MS drug that seems to affect the antibody response to COVID-19 vaccination.
Dr. Oreja-Guevara described the response to COVID vaccination in patients on fingolimod as “very variable.” Of 16 patients treated with fingolimod, 4 failed to develop a humoral response, 7 had a low antibody response, and 5 had a similar response to that seen in the healthy controls (three of these patients had also had a previous COVID-19 infection). The response to vaccination in fingolimod-treated patients did not appear to be related to lymphopenia.
Cellular response also impaired with fingolimod
These data are consistent with those from another cohort from Israel reported previously.
In that study, which was published earlier in 2021, a team led by Anat Achiron, MD, Sheba Medical Center, Tel Aviv, analyzed humoral immunity in 125 patients with MS 1 month after the second dose of the Pfizer COVID vaccine. A group of healthy people similarly vaccinated served as control.
Results showed that protective humoral immunity occurred in 97.9% of the control group after vaccination, compared with 100% in untreated patients and 100% in patients treated with cladribine but in just 22.7% of those treated with ocrelizumab and only 3.8% of those taking fingolimod.
For ocrelizumab-treated patients, the failure to mount appropriate IgG immune response was regardless of the absolute lymphocyte counts that were in the normal range or to the time interval from the last ocrelizumab treatment dose that ranged from 3.1 to 8.9 months, “suggesting the need to postpone the next dosing to enable an effective postvaccination humoral response,” the authors said.
They noted that the majority of the fingolimod-treated patients in the study had a low lymphocyte count (<1,000 cells/mm3), which may be the cause for failing to mount an immune response. But even in the small group of fingolimod-treated MS patients with an absolute lymphocyte count above 1,000 cells/mm3, no humoral response was detected.
At the ECTRIMS meeting, Dr. Achiron presented further results from this study on memory B-cell and T-cell responses to the COVID vaccine in these patients.
The results showed that COVID-specific B- and T-cell responses were only present in about half of healthy subjects, untreated patients with MS, and those treated with cladribine.
While the B-cell response was almost completely impaired in the ocrelizumab-treated patients, the T-cell response was present to the same extent as in the control group. But fingolimod patients showed no B- or T-cell responses.
Dr. Achiron concluded that patients on ocrelizumab should wait at least 9 months following the last dose before receiving COVID vaccination, and that patients taking fingolimod should consider a switch to a different medication.
But she pointed out that, despite the lack of humoral cellular responses in the fingolimod group, in this study there does not seem to have been an increase in COVID infection in patients taking fingolimod in a large registry study.
“This leads us to the idea that maybe lymphopenia is not the only story, and maybe innate immunity is playing a role. We still don’t really know the answer for that.”
Dr. Achiron said she was also surprised to see that even untreated and healthy subjects did not develop complete B-cell and T-cell responses after double COVID vaccination. And similar results have been seen in patients who have recovered from natural COVID infection, where the B-cell response is “not 100%,” she added.
“This points to the suggestion that everyone might need a third vaccination, MS patients or not,” she concluded.
A version of this article first appeared on Medscape.com.
The results also show a reduced response to COVID vaccination in some patients on fingolimod.
The data come from a new series of vaccinated patients with MS from Madrid, which was presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
Presenting the data, Celia Oreja-Guevara, MD, Hospital Clínico San Carlos, Madrid, concluded that “currently approved COVID-19 vaccines appear safe in MS patients and are effective in most patients. However, vaccine strategy in patients treated with anti-CD20 and S1P inhibitors [such as fingolimod] need further study.”
“We showed that patients on ocrelizumab or rituximab had a very low or no antibody response to COVID vaccination,” she added. “However, some previous studies have shown some T-cell response to vaccination in these patients, and we are looking at that now.”
Assessing postvaccination antibody response
For the current study, the researchers analyzed the antibody response to COVID-19 vaccination at week 3, week 6, and month 3 after the first dose in 165 patients with MS and 200 healthy controls.
Of the patients with MS, 120 received both doses of mRNA vaccine and 42 received the AstraZeneca vaccine. The mean age of the MS patients was 45 years and 46 years in the healthy controls.
Adverse events were similar in the two groups, and no increase in relapse activity was seen in the patients with MS.
Mean antibody titers were slightly lower in the patients with MS versus the healthy controls. At 3 weeks, mean titers were 7,910 AU/mL in the patients with MS and 9,397 in the healthy controls. At 6 weeks, mean levels were 16,347 AU/mL in the patients with MS and 18,120 in the healthy controls.
Patients with MS treated with interferon-beta, glatiramer acetate, teriflunomide, dimethyl fumarate, cladribine, and natalizumab who received mRNA vaccines developed a similar postvaccination humoral response as the healthy controls at each of 3, 6, and 12 weeks after the first dose.
Patients with MS receiving the AstraZeneca vaccine mounted a lower humoral response than those receiving the mRNA vaccine, but this same effect was also seen in the healthy controls.
However, patients on the anti-CD20 drugs ocrelizumab or rituximab showed a lower humoral response to COVID vaccination. Only 3 of 20 patients who had been treated with ocrelizumab developed antibodies, but these patients had longer washout periods (at least 6 months) between receiving ocrelizumab and the COVID vaccine. All six patients treated with rituximab had no antibody response to the COVID vaccination.
Dr. Oreja-Guevara suggested that ocrelizumab-treated patients may have a worse outcome after COVID-19 infection. “In the first wave of infection in Madrid, we recorded five patients on ocrelizumab with COVID-19, four of whom were hospitalized,” she noted.
“In patients on ocrelizumab we need to try and have a long interval between giving this drug and giving the COVID vaccine. The longer the washout period, the more antibodies are seen,” she said.
She noted that two patients in the study received the COVID vaccine 1 year after ocrelizumab administration and had a normal humoral response, similar to the healthy controls.
The new anti-CD20 drug, ofatumumab, did not seem to affect the COVID vaccine antibody response as much as ocrelizumab or rituximab. In the current study, four of five patients treated with ofatumumab had an antibody response.
Dr. Oreja-Guevara suggested that this was probably because the depletion of B cells is not so strong with ofatumumab. “This drug is dosed every 4 weeks and it doesn’t deplete all the B cells and they are replaced quite quickly.”
Fingolimod is another MS drug that seems to affect the antibody response to COVID-19 vaccination.
Dr. Oreja-Guevara described the response to COVID vaccination in patients on fingolimod as “very variable.” Of 16 patients treated with fingolimod, 4 failed to develop a humoral response, 7 had a low antibody response, and 5 had a similar response to that seen in the healthy controls (three of these patients had also had a previous COVID-19 infection). The response to vaccination in fingolimod-treated patients did not appear to be related to lymphopenia.
Cellular response also impaired with fingolimod
These data are consistent with those from another cohort from Israel reported previously.
In that study, which was published earlier in 2021, a team led by Anat Achiron, MD, Sheba Medical Center, Tel Aviv, analyzed humoral immunity in 125 patients with MS 1 month after the second dose of the Pfizer COVID vaccine. A group of healthy people similarly vaccinated served as control.
Results showed that protective humoral immunity occurred in 97.9% of the control group after vaccination, compared with 100% in untreated patients and 100% in patients treated with cladribine but in just 22.7% of those treated with ocrelizumab and only 3.8% of those taking fingolimod.
For ocrelizumab-treated patients, the failure to mount appropriate IgG immune response was regardless of the absolute lymphocyte counts that were in the normal range or to the time interval from the last ocrelizumab treatment dose that ranged from 3.1 to 8.9 months, “suggesting the need to postpone the next dosing to enable an effective postvaccination humoral response,” the authors said.
They noted that the majority of the fingolimod-treated patients in the study had a low lymphocyte count (<1,000 cells/mm3), which may be the cause for failing to mount an immune response. But even in the small group of fingolimod-treated MS patients with an absolute lymphocyte count above 1,000 cells/mm3, no humoral response was detected.
At the ECTRIMS meeting, Dr. Achiron presented further results from this study on memory B-cell and T-cell responses to the COVID vaccine in these patients.
The results showed that COVID-specific B- and T-cell responses were only present in about half of healthy subjects, untreated patients with MS, and those treated with cladribine.
While the B-cell response was almost completely impaired in the ocrelizumab-treated patients, the T-cell response was present to the same extent as in the control group. But fingolimod patients showed no B- or T-cell responses.
Dr. Achiron concluded that patients on ocrelizumab should wait at least 9 months following the last dose before receiving COVID vaccination, and that patients taking fingolimod should consider a switch to a different medication.
But she pointed out that, despite the lack of humoral cellular responses in the fingolimod group, in this study there does not seem to have been an increase in COVID infection in patients taking fingolimod in a large registry study.
“This leads us to the idea that maybe lymphopenia is not the only story, and maybe innate immunity is playing a role. We still don’t really know the answer for that.”
Dr. Achiron said she was also surprised to see that even untreated and healthy subjects did not develop complete B-cell and T-cell responses after double COVID vaccination. And similar results have been seen in patients who have recovered from natural COVID infection, where the B-cell response is “not 100%,” she added.
“This points to the suggestion that everyone might need a third vaccination, MS patients or not,” she concluded.
A version of this article first appeared on Medscape.com.
The results also show a reduced response to COVID vaccination in some patients on fingolimod.
The data come from a new series of vaccinated patients with MS from Madrid, which was presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
Presenting the data, Celia Oreja-Guevara, MD, Hospital Clínico San Carlos, Madrid, concluded that “currently approved COVID-19 vaccines appear safe in MS patients and are effective in most patients. However, vaccine strategy in patients treated with anti-CD20 and S1P inhibitors [such as fingolimod] need further study.”
“We showed that patients on ocrelizumab or rituximab had a very low or no antibody response to COVID vaccination,” she added. “However, some previous studies have shown some T-cell response to vaccination in these patients, and we are looking at that now.”
Assessing postvaccination antibody response
For the current study, the researchers analyzed the antibody response to COVID-19 vaccination at week 3, week 6, and month 3 after the first dose in 165 patients with MS and 200 healthy controls.
Of the patients with MS, 120 received both doses of mRNA vaccine and 42 received the AstraZeneca vaccine. The mean age of the MS patients was 45 years and 46 years in the healthy controls.
Adverse events were similar in the two groups, and no increase in relapse activity was seen in the patients with MS.
Mean antibody titers were slightly lower in the patients with MS versus the healthy controls. At 3 weeks, mean titers were 7,910 AU/mL in the patients with MS and 9,397 in the healthy controls. At 6 weeks, mean levels were 16,347 AU/mL in the patients with MS and 18,120 in the healthy controls.
Patients with MS treated with interferon-beta, glatiramer acetate, teriflunomide, dimethyl fumarate, cladribine, and natalizumab who received mRNA vaccines developed a similar postvaccination humoral response as the healthy controls at each of 3, 6, and 12 weeks after the first dose.
Patients with MS receiving the AstraZeneca vaccine mounted a lower humoral response than those receiving the mRNA vaccine, but this same effect was also seen in the healthy controls.
However, patients on the anti-CD20 drugs ocrelizumab or rituximab showed a lower humoral response to COVID vaccination. Only 3 of 20 patients who had been treated with ocrelizumab developed antibodies, but these patients had longer washout periods (at least 6 months) between receiving ocrelizumab and the COVID vaccine. All six patients treated with rituximab had no antibody response to the COVID vaccination.
Dr. Oreja-Guevara suggested that ocrelizumab-treated patients may have a worse outcome after COVID-19 infection. “In the first wave of infection in Madrid, we recorded five patients on ocrelizumab with COVID-19, four of whom were hospitalized,” she noted.
“In patients on ocrelizumab we need to try and have a long interval between giving this drug and giving the COVID vaccine. The longer the washout period, the more antibodies are seen,” she said.
She noted that two patients in the study received the COVID vaccine 1 year after ocrelizumab administration and had a normal humoral response, similar to the healthy controls.
The new anti-CD20 drug, ofatumumab, did not seem to affect the COVID vaccine antibody response as much as ocrelizumab or rituximab. In the current study, four of five patients treated with ofatumumab had an antibody response.
Dr. Oreja-Guevara suggested that this was probably because the depletion of B cells is not so strong with ofatumumab. “This drug is dosed every 4 weeks and it doesn’t deplete all the B cells and they are replaced quite quickly.”
Fingolimod is another MS drug that seems to affect the antibody response to COVID-19 vaccination.
Dr. Oreja-Guevara described the response to COVID vaccination in patients on fingolimod as “very variable.” Of 16 patients treated with fingolimod, 4 failed to develop a humoral response, 7 had a low antibody response, and 5 had a similar response to that seen in the healthy controls (three of these patients had also had a previous COVID-19 infection). The response to vaccination in fingolimod-treated patients did not appear to be related to lymphopenia.
Cellular response also impaired with fingolimod
These data are consistent with those from another cohort from Israel reported previously.
In that study, which was published earlier in 2021, a team led by Anat Achiron, MD, Sheba Medical Center, Tel Aviv, analyzed humoral immunity in 125 patients with MS 1 month after the second dose of the Pfizer COVID vaccine. A group of healthy people similarly vaccinated served as control.
Results showed that protective humoral immunity occurred in 97.9% of the control group after vaccination, compared with 100% in untreated patients and 100% in patients treated with cladribine but in just 22.7% of those treated with ocrelizumab and only 3.8% of those taking fingolimod.
For ocrelizumab-treated patients, the failure to mount appropriate IgG immune response was regardless of the absolute lymphocyte counts that were in the normal range or to the time interval from the last ocrelizumab treatment dose that ranged from 3.1 to 8.9 months, “suggesting the need to postpone the next dosing to enable an effective postvaccination humoral response,” the authors said.
They noted that the majority of the fingolimod-treated patients in the study had a low lymphocyte count (<1,000 cells/mm3), which may be the cause for failing to mount an immune response. But even in the small group of fingolimod-treated MS patients with an absolute lymphocyte count above 1,000 cells/mm3, no humoral response was detected.
At the ECTRIMS meeting, Dr. Achiron presented further results from this study on memory B-cell and T-cell responses to the COVID vaccine in these patients.
The results showed that COVID-specific B- and T-cell responses were only present in about half of healthy subjects, untreated patients with MS, and those treated with cladribine.
While the B-cell response was almost completely impaired in the ocrelizumab-treated patients, the T-cell response was present to the same extent as in the control group. But fingolimod patients showed no B- or T-cell responses.
Dr. Achiron concluded that patients on ocrelizumab should wait at least 9 months following the last dose before receiving COVID vaccination, and that patients taking fingolimod should consider a switch to a different medication.
But she pointed out that, despite the lack of humoral cellular responses in the fingolimod group, in this study there does not seem to have been an increase in COVID infection in patients taking fingolimod in a large registry study.
“This leads us to the idea that maybe lymphopenia is not the only story, and maybe innate immunity is playing a role. We still don’t really know the answer for that.”
Dr. Achiron said she was also surprised to see that even untreated and healthy subjects did not develop complete B-cell and T-cell responses after double COVID vaccination. And similar results have been seen in patients who have recovered from natural COVID infection, where the B-cell response is “not 100%,” she added.
“This points to the suggestion that everyone might need a third vaccination, MS patients or not,” she concluded.
A version of this article first appeared on Medscape.com.
FROM ECTRIMS 2021
Many scientists face serious threats for speaking about COVID: Survey
, according to a survey published in Nature.
The survey of 321 scientists, largely from the United States, the United Kingdom, and Germany, found that 22% were threatened with physical or sexual violence and that 15% received death threats.
More than one quarter of scientists surveyed said they “always” or “usually” received comments from trolls or were personally attacked after speaking out about COVID-19. More than 40% suffered emotional or psychological distress as a result.
Some scientists said the experience of being trolled online or receiving personal attacks had a chilling effect on their willingness to speak to the media in the future.
Even scientists who had a high profile before the COVID-19 pandemic said in the Nature article that the abuse was a “new and unwelcome phenomenon tied to the pandemic.”
Some scientists reported anonymously that they were hesitant to speak about some topics after witnessing the abuse received by others.
“Shocking” results require action
An editorial in Nature calls the results of the survey “shocking” and says institutions at all levels must do more to “protect and defend scientists, and to condemn intimidation.
“Intimidation is unacceptable on any scale, and the findings should be of concern to all those who care about scientists’ well-being. Such behavior also risks discouraging researchers from contributing to public discussion — which would be a huge loss, given their expertise, during the pandemic,” the editorial states.
“Scientists and health officials should expect their research to be questioned and challenged, and should welcome critical feedback that is given in good faith. But threats of violence and extreme online abuse do nothing to encourage debate — and risk undermining science communication at a time when it has never mattered more,” the editorial concludes.
A number of scientists weighed in on the survey in a statement from the U.K. nonprofit organization, Science Media Center.
“Undoubtedly there is a danger that scientists who have themselves been, or had colleagues who have been attacked in ways that disturb one’s equilibrium, may decide to disengage from the media. This will be sad and result in overall harm,” warned Stephen Evans, MSc, with the London School of Hygiene and Tropical Medicine.
Simon Clarke, PhD, with the University of Reading, who responded to the Nature survey, said he is “glad to see so many fellow scientists took the time to reflect on their experiences.”
Dr. Clarke said he is “shocked and saddened to hear that so many fellow scientists have experienced death threats or threats of physical or sexual violence, simply for doing their job trying to communicate the scientific facts that are so important for society in understanding and responding to this global health emergency.”
Dr. Clarke said he too has had some “bad experiences after appearing in the media, particularly after calling out conspiracy theorists and some politicians, who seem to dislike having their pet theories debunked. I have on occasion been threatened with various forms of death, violence and lifelong imprisonment. I am fortunate to have felt able to ignore the threats I’ve received, but I know that some colleagues have had far worse experiences.”
Michael Head, PhD, with the University of Southampton, said there’s been “a huge amount of abuse aimed at everyone contributing to the pandemic response. This has included NHS frontline staff, and also scientists and academics providing thoughts and explanatory comments to the public.
“I myself have received plenty of abuse throughout the pandemic. For those of us who have been pulling apart anti-vaccine misinformation from pre-pandemic times, the presence of these attempts at intimidation is very wearying, but not surprising,” said Dr. Head.
“As a white, male academic, I would imagine I’m far less likely to receive abuse than a scientist making similar points but from a different demographic,” he said.
Susan Michie, FMedSci, with the University College London, said the findings of harassment and abuse of scientists during the pandemic align closely with what she and many U.K. women colleagues who have been prominent in speaking to the media have endured.
“The online abuse occurs most intensively after media engagements and especially after those that address restrictions to social mixing, the wearing of face masks or vaccination,” Dr. Michie said.
“This abuse has not put off many women colleagues I know from speaking to the media,” she said. “I think this is because they are well established in their careers and/or brave and very committed to communicating scientific understanding.
“They have also set up a variety of networks to support each other. However, I am concerned that it discourages early career scientists, especially young women and young women from minoritized ethnic backgrounds, from engaging with the media,” she said.
A version of this article first appeared on Medscape.com.
, according to a survey published in Nature.
The survey of 321 scientists, largely from the United States, the United Kingdom, and Germany, found that 22% were threatened with physical or sexual violence and that 15% received death threats.
More than one quarter of scientists surveyed said they “always” or “usually” received comments from trolls or were personally attacked after speaking out about COVID-19. More than 40% suffered emotional or psychological distress as a result.
Some scientists said the experience of being trolled online or receiving personal attacks had a chilling effect on their willingness to speak to the media in the future.
Even scientists who had a high profile before the COVID-19 pandemic said in the Nature article that the abuse was a “new and unwelcome phenomenon tied to the pandemic.”
Some scientists reported anonymously that they were hesitant to speak about some topics after witnessing the abuse received by others.
“Shocking” results require action
An editorial in Nature calls the results of the survey “shocking” and says institutions at all levels must do more to “protect and defend scientists, and to condemn intimidation.
“Intimidation is unacceptable on any scale, and the findings should be of concern to all those who care about scientists’ well-being. Such behavior also risks discouraging researchers from contributing to public discussion — which would be a huge loss, given their expertise, during the pandemic,” the editorial states.
“Scientists and health officials should expect their research to be questioned and challenged, and should welcome critical feedback that is given in good faith. But threats of violence and extreme online abuse do nothing to encourage debate — and risk undermining science communication at a time when it has never mattered more,” the editorial concludes.
A number of scientists weighed in on the survey in a statement from the U.K. nonprofit organization, Science Media Center.
“Undoubtedly there is a danger that scientists who have themselves been, or had colleagues who have been attacked in ways that disturb one’s equilibrium, may decide to disengage from the media. This will be sad and result in overall harm,” warned Stephen Evans, MSc, with the London School of Hygiene and Tropical Medicine.
Simon Clarke, PhD, with the University of Reading, who responded to the Nature survey, said he is “glad to see so many fellow scientists took the time to reflect on their experiences.”
Dr. Clarke said he is “shocked and saddened to hear that so many fellow scientists have experienced death threats or threats of physical or sexual violence, simply for doing their job trying to communicate the scientific facts that are so important for society in understanding and responding to this global health emergency.”
Dr. Clarke said he too has had some “bad experiences after appearing in the media, particularly after calling out conspiracy theorists and some politicians, who seem to dislike having their pet theories debunked. I have on occasion been threatened with various forms of death, violence and lifelong imprisonment. I am fortunate to have felt able to ignore the threats I’ve received, but I know that some colleagues have had far worse experiences.”
Michael Head, PhD, with the University of Southampton, said there’s been “a huge amount of abuse aimed at everyone contributing to the pandemic response. This has included NHS frontline staff, and also scientists and academics providing thoughts and explanatory comments to the public.
“I myself have received plenty of abuse throughout the pandemic. For those of us who have been pulling apart anti-vaccine misinformation from pre-pandemic times, the presence of these attempts at intimidation is very wearying, but not surprising,” said Dr. Head.
“As a white, male academic, I would imagine I’m far less likely to receive abuse than a scientist making similar points but from a different demographic,” he said.
Susan Michie, FMedSci, with the University College London, said the findings of harassment and abuse of scientists during the pandemic align closely with what she and many U.K. women colleagues who have been prominent in speaking to the media have endured.
“The online abuse occurs most intensively after media engagements and especially after those that address restrictions to social mixing, the wearing of face masks or vaccination,” Dr. Michie said.
“This abuse has not put off many women colleagues I know from speaking to the media,” she said. “I think this is because they are well established in their careers and/or brave and very committed to communicating scientific understanding.
“They have also set up a variety of networks to support each other. However, I am concerned that it discourages early career scientists, especially young women and young women from minoritized ethnic backgrounds, from engaging with the media,” she said.
A version of this article first appeared on Medscape.com.
, according to a survey published in Nature.
The survey of 321 scientists, largely from the United States, the United Kingdom, and Germany, found that 22% were threatened with physical or sexual violence and that 15% received death threats.
More than one quarter of scientists surveyed said they “always” or “usually” received comments from trolls or were personally attacked after speaking out about COVID-19. More than 40% suffered emotional or psychological distress as a result.
Some scientists said the experience of being trolled online or receiving personal attacks had a chilling effect on their willingness to speak to the media in the future.
Even scientists who had a high profile before the COVID-19 pandemic said in the Nature article that the abuse was a “new and unwelcome phenomenon tied to the pandemic.”
Some scientists reported anonymously that they were hesitant to speak about some topics after witnessing the abuse received by others.
“Shocking” results require action
An editorial in Nature calls the results of the survey “shocking” and says institutions at all levels must do more to “protect and defend scientists, and to condemn intimidation.
“Intimidation is unacceptable on any scale, and the findings should be of concern to all those who care about scientists’ well-being. Such behavior also risks discouraging researchers from contributing to public discussion — which would be a huge loss, given their expertise, during the pandemic,” the editorial states.
“Scientists and health officials should expect their research to be questioned and challenged, and should welcome critical feedback that is given in good faith. But threats of violence and extreme online abuse do nothing to encourage debate — and risk undermining science communication at a time when it has never mattered more,” the editorial concludes.
A number of scientists weighed in on the survey in a statement from the U.K. nonprofit organization, Science Media Center.
“Undoubtedly there is a danger that scientists who have themselves been, or had colleagues who have been attacked in ways that disturb one’s equilibrium, may decide to disengage from the media. This will be sad and result in overall harm,” warned Stephen Evans, MSc, with the London School of Hygiene and Tropical Medicine.
Simon Clarke, PhD, with the University of Reading, who responded to the Nature survey, said he is “glad to see so many fellow scientists took the time to reflect on their experiences.”
Dr. Clarke said he is “shocked and saddened to hear that so many fellow scientists have experienced death threats or threats of physical or sexual violence, simply for doing their job trying to communicate the scientific facts that are so important for society in understanding and responding to this global health emergency.”
Dr. Clarke said he too has had some “bad experiences after appearing in the media, particularly after calling out conspiracy theorists and some politicians, who seem to dislike having their pet theories debunked. I have on occasion been threatened with various forms of death, violence and lifelong imprisonment. I am fortunate to have felt able to ignore the threats I’ve received, but I know that some colleagues have had far worse experiences.”
Michael Head, PhD, with the University of Southampton, said there’s been “a huge amount of abuse aimed at everyone contributing to the pandemic response. This has included NHS frontline staff, and also scientists and academics providing thoughts and explanatory comments to the public.
“I myself have received plenty of abuse throughout the pandemic. For those of us who have been pulling apart anti-vaccine misinformation from pre-pandemic times, the presence of these attempts at intimidation is very wearying, but not surprising,” said Dr. Head.
“As a white, male academic, I would imagine I’m far less likely to receive abuse than a scientist making similar points but from a different demographic,” he said.
Susan Michie, FMedSci, with the University College London, said the findings of harassment and abuse of scientists during the pandemic align closely with what she and many U.K. women colleagues who have been prominent in speaking to the media have endured.
“The online abuse occurs most intensively after media engagements and especially after those that address restrictions to social mixing, the wearing of face masks or vaccination,” Dr. Michie said.
“This abuse has not put off many women colleagues I know from speaking to the media,” she said. “I think this is because they are well established in their careers and/or brave and very committed to communicating scientific understanding.
“They have also set up a variety of networks to support each other. However, I am concerned that it discourages early career scientists, especially young women and young women from minoritized ethnic backgrounds, from engaging with the media,” she said.
A version of this article first appeared on Medscape.com.
New safety data regarding COVID vaccines
from the French National Agency for the Safety of Medicines and Health Products (ANSM).
The rare condition — more common in men than in women — is characterized by the sudden onset of severe pain in the shoulder, followed by arm paralysis. Its etiopathogenesis is not well understood, but vaccines, in particular the flu vaccine, have been implicated in some cases, the report states.
Six serious cases of the syndrome related to the Comirnaty (Pfizer) vaccine were reported by healthcare professionals and vaccinated individuals or their family and friends since the start of the monitoring program. Four of these cases occurred from September 3 to 16.
All six cases involved patients 19 to 69 years of age — two women and four men — who developed symptoms in the 50 days after vaccination. Half were reported after the first dose and half after the second dose. Four of the patients are currently recovering; the outcomes of the other two are unknown.
In the case of the Spikevax vaccine (Moderna), two cases of Parsonage-Turner syndrome were reported after vaccination (plus one that occurred after 50 days, which is currently being managed). The onset of symptoms in these two men — one in his early 30s and one in his early 60s — occurred less than 18 days after vaccination. One occurred after the first dose and one after the second dose. This timing indicates a possible link between the syndrome and the vaccine. Both men are currently in recovery.
This signal of mRNA vaccines is now “officially recognized,” according to the Pfizer and Moderna reports.
It is also considered a “potential signal” in the Vaxzevria (AstraZeneca) pharmacovigilance report, released October 8, which describes eight cases of Parsonage-Turner syndrome after vaccination.
Safety profile of mRNA COVID vaccines in youth
Between June 15, when children 12 years and older became eligible for vaccination, and August 26, there were 591 reports of potential adverse events — out of 6 million Pfizer doses administered — in 12- to 18-year-old children.
Of the 591 cases, 35.2% were deemed serious. The majority of these were cases of reactogenicity, malaise, or postvaccine discomfort (25%), followed by instances of myocarditis and pericarditis (15.9% and 7.2%, respectively). In eight of 10 cases, one of the first symptom reported was chest pain.
Myocarditis occurred in 39.4% of people after the first injection (mean time to onset, 13 days) and 54.5% after the second (mean time to onset, 4 days). Recorded progress was favorable in nearly nine of 10 cases.
Pericarditis occurred in 53.3% of people after the first injection (mean time to onset, 13 days), and 40.0% after the second (mean time to onset, 4 days).
Three cases of multisystem inflammatory syndrome in children (MISC) were reported after monitoring ended.
For this age group, “all reported events will continue to be monitored, especially serious events and multisystem inflammatory syndrome in children,” report authors conclude.
Data for adverse events after the Moderna vaccine remain limited, but the report stipulates that “the adverse events reported in 12- to 18-year-olds who received an injection do not display any particular pattern, compared with those reported in older subjects, with the exception of a roughly 100-fold lower incidence of reported adverse effects in the 12- to 17-year age group.”
No safety warnings for pregnant women
The pharmacovigilance report — which covered the period from December 27, 2020 to September 9, 2021 — “raises no safety warnings for pregnant or nursing women with any of the COVID-19 vaccines.” In addition, two recent studies — one published in JAMA and one in the New England Journal of Medicine — have shown no link between spontaneous miscarriage and mRNA vaccines.
“Moreover, it should be stressed that current data from the international literature consistently show that maternal SARS COV-2 infection increases the risk for fetal, maternal, and neonatal complications, and that this risk may increase with the arrival of the Alpha and Delta variants,” they write. “It is therefore important to reiterate the current recommendations to vaccinate all pregnant women, regardless of the stage of pregnancy.”
Some adverse effects, such as thromboembolic effects, in utero death, HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome, and uterine contractions, will continue to be monitored.
Questions regarding menstrual disorders
As for gynecological disorders reported after vaccination, questions still remain. “In most of the reported cases, it is difficult to accurately determine whether the vaccine played a role in the occurrence of menstrual/genital bleeding,” the authors of the pharmacovigilance monitoring report state.
“Nonetheless, these cases warrant attention,” they add, and further discussions with the French National Association of Obstetricians and Gynecologists and the French Society of Endocrinology are needed in regard to these potential safety signals.
A version of this article first appeared on Medscape.com.
from the French National Agency for the Safety of Medicines and Health Products (ANSM).
The rare condition — more common in men than in women — is characterized by the sudden onset of severe pain in the shoulder, followed by arm paralysis. Its etiopathogenesis is not well understood, but vaccines, in particular the flu vaccine, have been implicated in some cases, the report states.
Six serious cases of the syndrome related to the Comirnaty (Pfizer) vaccine were reported by healthcare professionals and vaccinated individuals or their family and friends since the start of the monitoring program. Four of these cases occurred from September 3 to 16.
All six cases involved patients 19 to 69 years of age — two women and four men — who developed symptoms in the 50 days after vaccination. Half were reported after the first dose and half after the second dose. Four of the patients are currently recovering; the outcomes of the other two are unknown.
In the case of the Spikevax vaccine (Moderna), two cases of Parsonage-Turner syndrome were reported after vaccination (plus one that occurred after 50 days, which is currently being managed). The onset of symptoms in these two men — one in his early 30s and one in his early 60s — occurred less than 18 days after vaccination. One occurred after the first dose and one after the second dose. This timing indicates a possible link between the syndrome and the vaccine. Both men are currently in recovery.
This signal of mRNA vaccines is now “officially recognized,” according to the Pfizer and Moderna reports.
It is also considered a “potential signal” in the Vaxzevria (AstraZeneca) pharmacovigilance report, released October 8, which describes eight cases of Parsonage-Turner syndrome after vaccination.
Safety profile of mRNA COVID vaccines in youth
Between June 15, when children 12 years and older became eligible for vaccination, and August 26, there were 591 reports of potential adverse events — out of 6 million Pfizer doses administered — in 12- to 18-year-old children.
Of the 591 cases, 35.2% were deemed serious. The majority of these were cases of reactogenicity, malaise, or postvaccine discomfort (25%), followed by instances of myocarditis and pericarditis (15.9% and 7.2%, respectively). In eight of 10 cases, one of the first symptom reported was chest pain.
Myocarditis occurred in 39.4% of people after the first injection (mean time to onset, 13 days) and 54.5% after the second (mean time to onset, 4 days). Recorded progress was favorable in nearly nine of 10 cases.
Pericarditis occurred in 53.3% of people after the first injection (mean time to onset, 13 days), and 40.0% after the second (mean time to onset, 4 days).
Three cases of multisystem inflammatory syndrome in children (MISC) were reported after monitoring ended.
For this age group, “all reported events will continue to be monitored, especially serious events and multisystem inflammatory syndrome in children,” report authors conclude.
Data for adverse events after the Moderna vaccine remain limited, but the report stipulates that “the adverse events reported in 12- to 18-year-olds who received an injection do not display any particular pattern, compared with those reported in older subjects, with the exception of a roughly 100-fold lower incidence of reported adverse effects in the 12- to 17-year age group.”
No safety warnings for pregnant women
The pharmacovigilance report — which covered the period from December 27, 2020 to September 9, 2021 — “raises no safety warnings for pregnant or nursing women with any of the COVID-19 vaccines.” In addition, two recent studies — one published in JAMA and one in the New England Journal of Medicine — have shown no link between spontaneous miscarriage and mRNA vaccines.
“Moreover, it should be stressed that current data from the international literature consistently show that maternal SARS COV-2 infection increases the risk for fetal, maternal, and neonatal complications, and that this risk may increase with the arrival of the Alpha and Delta variants,” they write. “It is therefore important to reiterate the current recommendations to vaccinate all pregnant women, regardless of the stage of pregnancy.”
Some adverse effects, such as thromboembolic effects, in utero death, HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome, and uterine contractions, will continue to be monitored.
Questions regarding menstrual disorders
As for gynecological disorders reported after vaccination, questions still remain. “In most of the reported cases, it is difficult to accurately determine whether the vaccine played a role in the occurrence of menstrual/genital bleeding,” the authors of the pharmacovigilance monitoring report state.
“Nonetheless, these cases warrant attention,” they add, and further discussions with the French National Association of Obstetricians and Gynecologists and the French Society of Endocrinology are needed in regard to these potential safety signals.
A version of this article first appeared on Medscape.com.
from the French National Agency for the Safety of Medicines and Health Products (ANSM).
The rare condition — more common in men than in women — is characterized by the sudden onset of severe pain in the shoulder, followed by arm paralysis. Its etiopathogenesis is not well understood, but vaccines, in particular the flu vaccine, have been implicated in some cases, the report states.
Six serious cases of the syndrome related to the Comirnaty (Pfizer) vaccine were reported by healthcare professionals and vaccinated individuals or their family and friends since the start of the monitoring program. Four of these cases occurred from September 3 to 16.
All six cases involved patients 19 to 69 years of age — two women and four men — who developed symptoms in the 50 days after vaccination. Half were reported after the first dose and half after the second dose. Four of the patients are currently recovering; the outcomes of the other two are unknown.
In the case of the Spikevax vaccine (Moderna), two cases of Parsonage-Turner syndrome were reported after vaccination (plus one that occurred after 50 days, which is currently being managed). The onset of symptoms in these two men — one in his early 30s and one in his early 60s — occurred less than 18 days after vaccination. One occurred after the first dose and one after the second dose. This timing indicates a possible link between the syndrome and the vaccine. Both men are currently in recovery.
This signal of mRNA vaccines is now “officially recognized,” according to the Pfizer and Moderna reports.
It is also considered a “potential signal” in the Vaxzevria (AstraZeneca) pharmacovigilance report, released October 8, which describes eight cases of Parsonage-Turner syndrome after vaccination.
Safety profile of mRNA COVID vaccines in youth
Between June 15, when children 12 years and older became eligible for vaccination, and August 26, there were 591 reports of potential adverse events — out of 6 million Pfizer doses administered — in 12- to 18-year-old children.
Of the 591 cases, 35.2% were deemed serious. The majority of these were cases of reactogenicity, malaise, or postvaccine discomfort (25%), followed by instances of myocarditis and pericarditis (15.9% and 7.2%, respectively). In eight of 10 cases, one of the first symptom reported was chest pain.
Myocarditis occurred in 39.4% of people after the first injection (mean time to onset, 13 days) and 54.5% after the second (mean time to onset, 4 days). Recorded progress was favorable in nearly nine of 10 cases.
Pericarditis occurred in 53.3% of people after the first injection (mean time to onset, 13 days), and 40.0% after the second (mean time to onset, 4 days).
Three cases of multisystem inflammatory syndrome in children (MISC) were reported after monitoring ended.
For this age group, “all reported events will continue to be monitored, especially serious events and multisystem inflammatory syndrome in children,” report authors conclude.
Data for adverse events after the Moderna vaccine remain limited, but the report stipulates that “the adverse events reported in 12- to 18-year-olds who received an injection do not display any particular pattern, compared with those reported in older subjects, with the exception of a roughly 100-fold lower incidence of reported adverse effects in the 12- to 17-year age group.”
No safety warnings for pregnant women
The pharmacovigilance report — which covered the period from December 27, 2020 to September 9, 2021 — “raises no safety warnings for pregnant or nursing women with any of the COVID-19 vaccines.” In addition, two recent studies — one published in JAMA and one in the New England Journal of Medicine — have shown no link between spontaneous miscarriage and mRNA vaccines.
“Moreover, it should be stressed that current data from the international literature consistently show that maternal SARS COV-2 infection increases the risk for fetal, maternal, and neonatal complications, and that this risk may increase with the arrival of the Alpha and Delta variants,” they write. “It is therefore important to reiterate the current recommendations to vaccinate all pregnant women, regardless of the stage of pregnancy.”
Some adverse effects, such as thromboembolic effects, in utero death, HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome, and uterine contractions, will continue to be monitored.
Questions regarding menstrual disorders
As for gynecological disorders reported after vaccination, questions still remain. “In most of the reported cases, it is difficult to accurately determine whether the vaccine played a role in the occurrence of menstrual/genital bleeding,” the authors of the pharmacovigilance monitoring report state.
“Nonetheless, these cases warrant attention,” they add, and further discussions with the French National Association of Obstetricians and Gynecologists and the French Society of Endocrinology are needed in regard to these potential safety signals.
A version of this article first appeared on Medscape.com.
Failure to communicate ‘doc-to-doc’ blamed for patient’s death
alleging that his death would have been avoided had there been better communication between the surgical oncologist and the treatment team.
The patient was a 49-year-old man who was experiencing chronic pain in his right ear. He saw a local ear, nose, and throat specialist, who could find no apparent cause after conducting a physical exam.
A CT scan revealed a 1.4-cm mass in the right pharyngeal space. A 1.6-cm lymph node in the right retropharyngeal/parapharyngeal carotid space was affected.
The following week, the patient underwent a positron-emission tomography scan and was subsequently referred to a head and neck surgical oncologist.
The surgeon performed a right radical tonsillectomy and pharyngectomy. During the surgery, the patient experienced significant bleeding complications. The surgeon was able to remove the tonsillar mass but could not resect the affected lymph node, owing to its proximity to the carotid artery. The affected lymph node was not removed, and the patient was informed that the problem would be addressed at another time.
Pathology revealed stage III squamous cell carcinoma (T3N0M0) that was HPV/p16 positive.
According to the lawsuit, which was reported in Expert Witness Newsletter, a critical error occurred.
The surgical oncologist apparently did not clearly communicate the situation to the rest of the clinicians involved in the patient’s care. The patient was treated as if the entire cancer had been surgically resected. He never underwent follow-up surgery to address the enlarged lymph node.
Because the care team believed that the patient had undergone a complete surgical resection, follow-up treatment consisted of radiotherapy without concurrent chemotherapy.
The patient underwent radiotherapy to a dose of 60 Gy over 30 treatment days.
About 5 months later, the patient once again presented with ear pain on the right side and difficulty speaking. Imaging showed that there was recurrence of a mass in his right parapharyngeal carotid space. Biopsy results indicated recurrent/progressive squamous cell carcinoma. The patient underwent a second round of radiotherapy. This time, he received concurrent chemotherapy.
Four months later, the patient presented to the emergency department complaining of episodes of syncope. Imaging revealed that the mass in his right parapharyngeal carotid space had increased in size, causing carotid stenosis. The patient was hospitalized for 4 days and was treated with steroids. The day after his discharge, he died at home.
Carotid blowout syndrome due to negligence
An autopsy was performed, and the cause of death was determined to be an acute massive bleed secondary to perforation of the right artery, which was “encased by a partially necrotic poorly differentiated squamous cell carcinoma.” This is known as carotid blowout syndrome.
After his death, the patient’s family contacted an attorney, who hired several expert witnesses to review the case. The alleged negligence by the head and neck oncologist was described as follows:
- There was a failure to appropriately assess the patient’s neck anatomy, and the entire tumor was not surgically removed.
- Frank disease tissue was left behind, and the disease subsequently progressed.
- The surgery was never completed; the cancer progressed and ultimately took the patient’s life.
- There was a failure to communicate the fact that the cancer had not been completely resected.
The alleged negligence by the radiation oncologist was described as follows:
- There was a failure to realize that the tumor had not been completely resected.
- The patient was given a suboptimal radiation dose of 60 Gy, which would have been appropriate only had the tumor been completely resected.
- There was a failure to give a radiation dose of 70 Gy (ie, the appropriate dose for remaining tumor).
The medical oncologist was alleged to have been negligent because chemotherapy was not given when indicated.
Very high stakes
None of the treating physicians were named in the lawsuit. Only the medical center where the treatment was given was named. The center is affiliated with an Ivy League university.
The patient was an extremely wealthy man who had worked as an insurance executive and investor. His premature death resulted in the loss of a massive amount of earnings, and the plaintiffs asked for a sum of $34 million as compensation. Because doctors do not carry insurance sufficient to cover that amount and generally do not have personal assets of that amount, the plaintiff targeted the hospital.
“The plaintiff knows that the physicians will never be able to pay an 8-figure settlement, so instead they go after the hospital itself,” says the newsletter. “The physicians simply become pawns in a protracted legal game.”
The lawsuit was settled out of court in 2021 for an undisclosed amount.
A version of this article first appeared on Medscape.com.
alleging that his death would have been avoided had there been better communication between the surgical oncologist and the treatment team.
The patient was a 49-year-old man who was experiencing chronic pain in his right ear. He saw a local ear, nose, and throat specialist, who could find no apparent cause after conducting a physical exam.
A CT scan revealed a 1.4-cm mass in the right pharyngeal space. A 1.6-cm lymph node in the right retropharyngeal/parapharyngeal carotid space was affected.
The following week, the patient underwent a positron-emission tomography scan and was subsequently referred to a head and neck surgical oncologist.
The surgeon performed a right radical tonsillectomy and pharyngectomy. During the surgery, the patient experienced significant bleeding complications. The surgeon was able to remove the tonsillar mass but could not resect the affected lymph node, owing to its proximity to the carotid artery. The affected lymph node was not removed, and the patient was informed that the problem would be addressed at another time.
Pathology revealed stage III squamous cell carcinoma (T3N0M0) that was HPV/p16 positive.
According to the lawsuit, which was reported in Expert Witness Newsletter, a critical error occurred.
The surgical oncologist apparently did not clearly communicate the situation to the rest of the clinicians involved in the patient’s care. The patient was treated as if the entire cancer had been surgically resected. He never underwent follow-up surgery to address the enlarged lymph node.
Because the care team believed that the patient had undergone a complete surgical resection, follow-up treatment consisted of radiotherapy without concurrent chemotherapy.
The patient underwent radiotherapy to a dose of 60 Gy over 30 treatment days.
About 5 months later, the patient once again presented with ear pain on the right side and difficulty speaking. Imaging showed that there was recurrence of a mass in his right parapharyngeal carotid space. Biopsy results indicated recurrent/progressive squamous cell carcinoma. The patient underwent a second round of radiotherapy. This time, he received concurrent chemotherapy.
Four months later, the patient presented to the emergency department complaining of episodes of syncope. Imaging revealed that the mass in his right parapharyngeal carotid space had increased in size, causing carotid stenosis. The patient was hospitalized for 4 days and was treated with steroids. The day after his discharge, he died at home.
Carotid blowout syndrome due to negligence
An autopsy was performed, and the cause of death was determined to be an acute massive bleed secondary to perforation of the right artery, which was “encased by a partially necrotic poorly differentiated squamous cell carcinoma.” This is known as carotid blowout syndrome.
After his death, the patient’s family contacted an attorney, who hired several expert witnesses to review the case. The alleged negligence by the head and neck oncologist was described as follows:
- There was a failure to appropriately assess the patient’s neck anatomy, and the entire tumor was not surgically removed.
- Frank disease tissue was left behind, and the disease subsequently progressed.
- The surgery was never completed; the cancer progressed and ultimately took the patient’s life.
- There was a failure to communicate the fact that the cancer had not been completely resected.
The alleged negligence by the radiation oncologist was described as follows:
- There was a failure to realize that the tumor had not been completely resected.
- The patient was given a suboptimal radiation dose of 60 Gy, which would have been appropriate only had the tumor been completely resected.
- There was a failure to give a radiation dose of 70 Gy (ie, the appropriate dose for remaining tumor).
The medical oncologist was alleged to have been negligent because chemotherapy was not given when indicated.
Very high stakes
None of the treating physicians were named in the lawsuit. Only the medical center where the treatment was given was named. The center is affiliated with an Ivy League university.
The patient was an extremely wealthy man who had worked as an insurance executive and investor. His premature death resulted in the loss of a massive amount of earnings, and the plaintiffs asked for a sum of $34 million as compensation. Because doctors do not carry insurance sufficient to cover that amount and generally do not have personal assets of that amount, the plaintiff targeted the hospital.
“The plaintiff knows that the physicians will never be able to pay an 8-figure settlement, so instead they go after the hospital itself,” says the newsletter. “The physicians simply become pawns in a protracted legal game.”
The lawsuit was settled out of court in 2021 for an undisclosed amount.
A version of this article first appeared on Medscape.com.
alleging that his death would have been avoided had there been better communication between the surgical oncologist and the treatment team.
The patient was a 49-year-old man who was experiencing chronic pain in his right ear. He saw a local ear, nose, and throat specialist, who could find no apparent cause after conducting a physical exam.
A CT scan revealed a 1.4-cm mass in the right pharyngeal space. A 1.6-cm lymph node in the right retropharyngeal/parapharyngeal carotid space was affected.
The following week, the patient underwent a positron-emission tomography scan and was subsequently referred to a head and neck surgical oncologist.
The surgeon performed a right radical tonsillectomy and pharyngectomy. During the surgery, the patient experienced significant bleeding complications. The surgeon was able to remove the tonsillar mass but could not resect the affected lymph node, owing to its proximity to the carotid artery. The affected lymph node was not removed, and the patient was informed that the problem would be addressed at another time.
Pathology revealed stage III squamous cell carcinoma (T3N0M0) that was HPV/p16 positive.
According to the lawsuit, which was reported in Expert Witness Newsletter, a critical error occurred.
The surgical oncologist apparently did not clearly communicate the situation to the rest of the clinicians involved in the patient’s care. The patient was treated as if the entire cancer had been surgically resected. He never underwent follow-up surgery to address the enlarged lymph node.
Because the care team believed that the patient had undergone a complete surgical resection, follow-up treatment consisted of radiotherapy without concurrent chemotherapy.
The patient underwent radiotherapy to a dose of 60 Gy over 30 treatment days.
About 5 months later, the patient once again presented with ear pain on the right side and difficulty speaking. Imaging showed that there was recurrence of a mass in his right parapharyngeal carotid space. Biopsy results indicated recurrent/progressive squamous cell carcinoma. The patient underwent a second round of radiotherapy. This time, he received concurrent chemotherapy.
Four months later, the patient presented to the emergency department complaining of episodes of syncope. Imaging revealed that the mass in his right parapharyngeal carotid space had increased in size, causing carotid stenosis. The patient was hospitalized for 4 days and was treated with steroids. The day after his discharge, he died at home.
Carotid blowout syndrome due to negligence
An autopsy was performed, and the cause of death was determined to be an acute massive bleed secondary to perforation of the right artery, which was “encased by a partially necrotic poorly differentiated squamous cell carcinoma.” This is known as carotid blowout syndrome.
After his death, the patient’s family contacted an attorney, who hired several expert witnesses to review the case. The alleged negligence by the head and neck oncologist was described as follows:
- There was a failure to appropriately assess the patient’s neck anatomy, and the entire tumor was not surgically removed.
- Frank disease tissue was left behind, and the disease subsequently progressed.
- The surgery was never completed; the cancer progressed and ultimately took the patient’s life.
- There was a failure to communicate the fact that the cancer had not been completely resected.
The alleged negligence by the radiation oncologist was described as follows:
- There was a failure to realize that the tumor had not been completely resected.
- The patient was given a suboptimal radiation dose of 60 Gy, which would have been appropriate only had the tumor been completely resected.
- There was a failure to give a radiation dose of 70 Gy (ie, the appropriate dose for remaining tumor).
The medical oncologist was alleged to have been negligent because chemotherapy was not given when indicated.
Very high stakes
None of the treating physicians were named in the lawsuit. Only the medical center where the treatment was given was named. The center is affiliated with an Ivy League university.
The patient was an extremely wealthy man who had worked as an insurance executive and investor. His premature death resulted in the loss of a massive amount of earnings, and the plaintiffs asked for a sum of $34 million as compensation. Because doctors do not carry insurance sufficient to cover that amount and generally do not have personal assets of that amount, the plaintiff targeted the hospital.
“The plaintiff knows that the physicians will never be able to pay an 8-figure settlement, so instead they go after the hospital itself,” says the newsletter. “The physicians simply become pawns in a protracted legal game.”
The lawsuit was settled out of court in 2021 for an undisclosed amount.
A version of this article first appeared on Medscape.com.
‘Fascinating’ link between Alzheimer’s and COVID-19
The findings could lead to new treatment targets to slow progression and severity of both diseases.
Investigators found that a single genetic variant in the oligoadenylate synthetase 1 (OAS1) gene increases the risk for AD and that related variants in the same gene increase the likelihood of severe COVID-19 outcomes.
“These findings may allow us to identify new drug targets to slow progression of both diseases and reduce their severity,” Dervis Salih, PhD, senior research associate, UK Dementia Research Institute, University College London, said in an interview.
“Our work also suggests new approaches to treat both diseases with the same drugs,” Dr. Salih added.
The study was published online Oct. 7 in Brain.
Shared genetic network
The OAS1 gene is expressed in microglia, a type of immune cell that makes up around 10% of all cells in the brain.
In earlier work, investigators found evidence suggesting a link between the OAS1 gene and AD, but the function of the gene in microglia was unknown.
To further investigate the gene’s link to AD, they sequenced genetic data from 2,547 people – half with AD, and half without.
The genotyping analysis confirmed that the single-nucleotide polymorphism (SNP) rs1131454 within OAS1 is significantly associated with AD.
Given that the same OAS1 locus has recently been linked with severe COVID-19 outcomes, the researchers investigated four variants on the OAS1 gene.
Results indicate that SNPs within OAS1 associated with AD also show linkage to SNP variants associated with critical illness in COVID-19.
The rs1131454 (risk allele A) and rs4766676 (risk allele T) are associated with AD, and rs10735079 (risk allele A) and rs6489867 (risk allele T) are associated with critical illness with COVID-19, the investigators reported. All of these risk alleles dampen expression of OAS1.
“This study also provides strong new evidence that interferon signaling by the innate immune system plays a substantial role in the progression of Alzheimer’s,” said Dr. Salih.
“Identifying this shared genetic network in innate immune cells will allow us with future work to identify new biomarkers to track disease progression and also predict disease risk better for both disorders,” he added.
‘Fascinating’ link
In a statement from the UK nonprofit organization, Science Media Center, Kenneth Baillie, MBChB, with the University of Edinburgh, said this study builds on a discovery he and his colleagues made last year that OAS1 variants are associated with severe COVID-19.
“In the ISARIC4C study, we recently found that this is probably due to a change in the way cell membranes detect viruses, but this mechanism doesn’t explain the fascinating association with Alzheimer’s disease reported in this new work,” Dr. Baillie said.
“It is often the case that the same gene can have different roles in different parts of the body. Importantly, it doesn’t mean that having COVID-19 has any effect on your risk of Alzheimer’s,” he added.
Also weighing in on the new study, Jonathan Schott, MD, professor of neurology, University College London, noted that dementia is the “main preexisting health condition associated with COVID-19 mortality, accounting for about one in four deaths from COVID-19 between March and June 2020.
“While some of this excessive mortality may relate to people with dementia being overrepresented in care homes, which were particularly hard hit by the pandemic, or due to general increased vulnerability to infections, there have been questions as to whether there are common factors that might increase susceptibility both to developing dementia and to dying from COVID-19,” Dr. Schott explained.
This “elegant paper” provides evidence for the latter, “suggesting a common genetic mechanism both for Alzheimer’s disease and for severe COVID-19 infection,” Dr. Schott said.
“The identification of a genetic risk factor and elucidation of inflammatory pathways through which it may increase risk has important implications for our understanding of both diseases, with potential implications for novel treatments,” he added.
The study was funded by the UK Dementia Research Institute. The authors have disclosed no relevant financial relationships. Dr. Schott serves as chief medical officer for Alzheimer’s Research UK and is clinical adviser to the UK Dementia Research Institute. Dr. Baillie has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The findings could lead to new treatment targets to slow progression and severity of both diseases.
Investigators found that a single genetic variant in the oligoadenylate synthetase 1 (OAS1) gene increases the risk for AD and that related variants in the same gene increase the likelihood of severe COVID-19 outcomes.
“These findings may allow us to identify new drug targets to slow progression of both diseases and reduce their severity,” Dervis Salih, PhD, senior research associate, UK Dementia Research Institute, University College London, said in an interview.
“Our work also suggests new approaches to treat both diseases with the same drugs,” Dr. Salih added.
The study was published online Oct. 7 in Brain.
Shared genetic network
The OAS1 gene is expressed in microglia, a type of immune cell that makes up around 10% of all cells in the brain.
In earlier work, investigators found evidence suggesting a link between the OAS1 gene and AD, but the function of the gene in microglia was unknown.
To further investigate the gene’s link to AD, they sequenced genetic data from 2,547 people – half with AD, and half without.
The genotyping analysis confirmed that the single-nucleotide polymorphism (SNP) rs1131454 within OAS1 is significantly associated with AD.
Given that the same OAS1 locus has recently been linked with severe COVID-19 outcomes, the researchers investigated four variants on the OAS1 gene.
Results indicate that SNPs within OAS1 associated with AD also show linkage to SNP variants associated with critical illness in COVID-19.
The rs1131454 (risk allele A) and rs4766676 (risk allele T) are associated with AD, and rs10735079 (risk allele A) and rs6489867 (risk allele T) are associated with critical illness with COVID-19, the investigators reported. All of these risk alleles dampen expression of OAS1.
“This study also provides strong new evidence that interferon signaling by the innate immune system plays a substantial role in the progression of Alzheimer’s,” said Dr. Salih.
“Identifying this shared genetic network in innate immune cells will allow us with future work to identify new biomarkers to track disease progression and also predict disease risk better for both disorders,” he added.
‘Fascinating’ link
In a statement from the UK nonprofit organization, Science Media Center, Kenneth Baillie, MBChB, with the University of Edinburgh, said this study builds on a discovery he and his colleagues made last year that OAS1 variants are associated with severe COVID-19.
“In the ISARIC4C study, we recently found that this is probably due to a change in the way cell membranes detect viruses, but this mechanism doesn’t explain the fascinating association with Alzheimer’s disease reported in this new work,” Dr. Baillie said.
“It is often the case that the same gene can have different roles in different parts of the body. Importantly, it doesn’t mean that having COVID-19 has any effect on your risk of Alzheimer’s,” he added.
Also weighing in on the new study, Jonathan Schott, MD, professor of neurology, University College London, noted that dementia is the “main preexisting health condition associated with COVID-19 mortality, accounting for about one in four deaths from COVID-19 between March and June 2020.
“While some of this excessive mortality may relate to people with dementia being overrepresented in care homes, which were particularly hard hit by the pandemic, or due to general increased vulnerability to infections, there have been questions as to whether there are common factors that might increase susceptibility both to developing dementia and to dying from COVID-19,” Dr. Schott explained.
This “elegant paper” provides evidence for the latter, “suggesting a common genetic mechanism both for Alzheimer’s disease and for severe COVID-19 infection,” Dr. Schott said.
“The identification of a genetic risk factor and elucidation of inflammatory pathways through which it may increase risk has important implications for our understanding of both diseases, with potential implications for novel treatments,” he added.
The study was funded by the UK Dementia Research Institute. The authors have disclosed no relevant financial relationships. Dr. Schott serves as chief medical officer for Alzheimer’s Research UK and is clinical adviser to the UK Dementia Research Institute. Dr. Baillie has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The findings could lead to new treatment targets to slow progression and severity of both diseases.
Investigators found that a single genetic variant in the oligoadenylate synthetase 1 (OAS1) gene increases the risk for AD and that related variants in the same gene increase the likelihood of severe COVID-19 outcomes.
“These findings may allow us to identify new drug targets to slow progression of both diseases and reduce their severity,” Dervis Salih, PhD, senior research associate, UK Dementia Research Institute, University College London, said in an interview.
“Our work also suggests new approaches to treat both diseases with the same drugs,” Dr. Salih added.
The study was published online Oct. 7 in Brain.
Shared genetic network
The OAS1 gene is expressed in microglia, a type of immune cell that makes up around 10% of all cells in the brain.
In earlier work, investigators found evidence suggesting a link between the OAS1 gene and AD, but the function of the gene in microglia was unknown.
To further investigate the gene’s link to AD, they sequenced genetic data from 2,547 people – half with AD, and half without.
The genotyping analysis confirmed that the single-nucleotide polymorphism (SNP) rs1131454 within OAS1 is significantly associated with AD.
Given that the same OAS1 locus has recently been linked with severe COVID-19 outcomes, the researchers investigated four variants on the OAS1 gene.
Results indicate that SNPs within OAS1 associated with AD also show linkage to SNP variants associated with critical illness in COVID-19.
The rs1131454 (risk allele A) and rs4766676 (risk allele T) are associated with AD, and rs10735079 (risk allele A) and rs6489867 (risk allele T) are associated with critical illness with COVID-19, the investigators reported. All of these risk alleles dampen expression of OAS1.
“This study also provides strong new evidence that interferon signaling by the innate immune system plays a substantial role in the progression of Alzheimer’s,” said Dr. Salih.
“Identifying this shared genetic network in innate immune cells will allow us with future work to identify new biomarkers to track disease progression and also predict disease risk better for both disorders,” he added.
‘Fascinating’ link
In a statement from the UK nonprofit organization, Science Media Center, Kenneth Baillie, MBChB, with the University of Edinburgh, said this study builds on a discovery he and his colleagues made last year that OAS1 variants are associated with severe COVID-19.
“In the ISARIC4C study, we recently found that this is probably due to a change in the way cell membranes detect viruses, but this mechanism doesn’t explain the fascinating association with Alzheimer’s disease reported in this new work,” Dr. Baillie said.
“It is often the case that the same gene can have different roles in different parts of the body. Importantly, it doesn’t mean that having COVID-19 has any effect on your risk of Alzheimer’s,” he added.
Also weighing in on the new study, Jonathan Schott, MD, professor of neurology, University College London, noted that dementia is the “main preexisting health condition associated with COVID-19 mortality, accounting for about one in four deaths from COVID-19 between March and June 2020.
“While some of this excessive mortality may relate to people with dementia being overrepresented in care homes, which were particularly hard hit by the pandemic, or due to general increased vulnerability to infections, there have been questions as to whether there are common factors that might increase susceptibility both to developing dementia and to dying from COVID-19,” Dr. Schott explained.
This “elegant paper” provides evidence for the latter, “suggesting a common genetic mechanism both for Alzheimer’s disease and for severe COVID-19 infection,” Dr. Schott said.
“The identification of a genetic risk factor and elucidation of inflammatory pathways through which it may increase risk has important implications for our understanding of both diseases, with potential implications for novel treatments,” he added.
The study was funded by the UK Dementia Research Institute. The authors have disclosed no relevant financial relationships. Dr. Schott serves as chief medical officer for Alzheimer’s Research UK and is clinical adviser to the UK Dementia Research Institute. Dr. Baillie has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Even one vaccinated member can cut family’s COVID risk
The chances are reduced even further with each additional vaccinated or otherwise immune family member, according to new data.
Lead author Peter Nordström, MD, PhD, with the unit of geriatric medicine, Umeå (Sweden) University, said in an interview the message is important for public health: “When you vaccinate, you do not just protect yourself but also your relatives.”
The findings were published online on Oct. 11, 2021, in JAMA Internal Medicine.
Researchers analyzed data from 1,789,728 individuals from 814,806 families from nationwide registries in Sweden. All individuals had acquired immunity either from previously being infected with SARS-CoV-2 or by being fully vaccinated (that is, having received two doses of the Moderna, Pfizer, or Oxford/AstraZeneca vaccines). Persons were considered for inclusion until May 26, 2021.
Each person with immunity was matched in a 1:1 ratio to a person without immunity from a cohort of individuals with families that had from two to five members. Families with more than five members were excluded because of small sample sizes.
Primarily nonimmune families in which there was one immune family member had a 45%-61% lower risk of contracting COVID-19 (hazard ratio, 0.39-0.55; 95% confidence interval, 0.37-0.61; P < .001).
The risk reduction increased to 75%-86% when two family members were immune (HR, 0.14-0.25; 95% CI, 0.11-0.27; P < .001).
It increased to 91%-94% when three family members were immune (HR, 0.06-0.09; 95% CI, 0.04-0.10; P < .001) and to 97% with four immune family members (HR, 0.03; 95% CI, 0.02-0.05; P < .001).
“The results were similar for the outcome of COVID-19 infection that was severe enough to warrant a hospital stay,” the authors wrote. They listed as an example that, in three-member families in which two members were immune, the remaining nonimmune family member had an 80% lower risk for hospitalization (HR, 0.20; 95% CI, 0.10-0.43; P < .001).
Global implications
Dr. Nordström said the team used the family setting because it was more easily identifiable as a cohort with the national registries and because COVID-19 is spread among people in close contact with each other. The findings have implications for other groups that spend large amounts of time together and for herd immunity, he added.
The findings may be particularly welcome in regions of the world where vaccination rates are very low. The authors noted that most of the global population has not yet been vaccinated and that “it is anticipated that most of the population in low-income countries will be unable to receive a vaccine in 2021, with current vaccination rates suggesting that completely inoculating 70%-85% of the global population may take up to 5 years.”
Jill Foster, MD, a pediatric infectious disease specialist at the University of Minnesota, Minneapolis, said in an interview she agrees that the news could encourage countries that have very low vaccination rates.
This study may help motivate areas with few resources to start small, she said: “Even one is better than zero.”
She added that this news could also help ease the minds of families that have immunocompromised members or in which there are children who are too young to be vaccinated.
With these data, she said, people can see there’s something they can do to help protect a family member.
Dr. Foster said that although it’s intuitive to think that the more vaccinated people there are in a family, the safer people are, “it’s really nice to see the data coming out of such a large dataset.”
The authors acknowledged that a limitation of the study is that, at the time the study was conducted, the Delta variant was uncommon in Sweden. It is therefore unclear whether the findings regarding immunity are still relevant in Sweden and elsewhere now that the Delta strain is dominant.
The authors reported no relevant financial relationships. Dr. Foster has received grant support from Moderna.
A version of this article first appeared on Medscape.com.
The chances are reduced even further with each additional vaccinated or otherwise immune family member, according to new data.
Lead author Peter Nordström, MD, PhD, with the unit of geriatric medicine, Umeå (Sweden) University, said in an interview the message is important for public health: “When you vaccinate, you do not just protect yourself but also your relatives.”
The findings were published online on Oct. 11, 2021, in JAMA Internal Medicine.
Researchers analyzed data from 1,789,728 individuals from 814,806 families from nationwide registries in Sweden. All individuals had acquired immunity either from previously being infected with SARS-CoV-2 or by being fully vaccinated (that is, having received two doses of the Moderna, Pfizer, or Oxford/AstraZeneca vaccines). Persons were considered for inclusion until May 26, 2021.
Each person with immunity was matched in a 1:1 ratio to a person without immunity from a cohort of individuals with families that had from two to five members. Families with more than five members were excluded because of small sample sizes.
Primarily nonimmune families in which there was one immune family member had a 45%-61% lower risk of contracting COVID-19 (hazard ratio, 0.39-0.55; 95% confidence interval, 0.37-0.61; P < .001).
The risk reduction increased to 75%-86% when two family members were immune (HR, 0.14-0.25; 95% CI, 0.11-0.27; P < .001).
It increased to 91%-94% when three family members were immune (HR, 0.06-0.09; 95% CI, 0.04-0.10; P < .001) and to 97% with four immune family members (HR, 0.03; 95% CI, 0.02-0.05; P < .001).
“The results were similar for the outcome of COVID-19 infection that was severe enough to warrant a hospital stay,” the authors wrote. They listed as an example that, in three-member families in which two members were immune, the remaining nonimmune family member had an 80% lower risk for hospitalization (HR, 0.20; 95% CI, 0.10-0.43; P < .001).
Global implications
Dr. Nordström said the team used the family setting because it was more easily identifiable as a cohort with the national registries and because COVID-19 is spread among people in close contact with each other. The findings have implications for other groups that spend large amounts of time together and for herd immunity, he added.
The findings may be particularly welcome in regions of the world where vaccination rates are very low. The authors noted that most of the global population has not yet been vaccinated and that “it is anticipated that most of the population in low-income countries will be unable to receive a vaccine in 2021, with current vaccination rates suggesting that completely inoculating 70%-85% of the global population may take up to 5 years.”
Jill Foster, MD, a pediatric infectious disease specialist at the University of Minnesota, Minneapolis, said in an interview she agrees that the news could encourage countries that have very low vaccination rates.
This study may help motivate areas with few resources to start small, she said: “Even one is better than zero.”
She added that this news could also help ease the minds of families that have immunocompromised members or in which there are children who are too young to be vaccinated.
With these data, she said, people can see there’s something they can do to help protect a family member.
Dr. Foster said that although it’s intuitive to think that the more vaccinated people there are in a family, the safer people are, “it’s really nice to see the data coming out of such a large dataset.”
The authors acknowledged that a limitation of the study is that, at the time the study was conducted, the Delta variant was uncommon in Sweden. It is therefore unclear whether the findings regarding immunity are still relevant in Sweden and elsewhere now that the Delta strain is dominant.
The authors reported no relevant financial relationships. Dr. Foster has received grant support from Moderna.
A version of this article first appeared on Medscape.com.
The chances are reduced even further with each additional vaccinated or otherwise immune family member, according to new data.
Lead author Peter Nordström, MD, PhD, with the unit of geriatric medicine, Umeå (Sweden) University, said in an interview the message is important for public health: “When you vaccinate, you do not just protect yourself but also your relatives.”
The findings were published online on Oct. 11, 2021, in JAMA Internal Medicine.
Researchers analyzed data from 1,789,728 individuals from 814,806 families from nationwide registries in Sweden. All individuals had acquired immunity either from previously being infected with SARS-CoV-2 or by being fully vaccinated (that is, having received two doses of the Moderna, Pfizer, or Oxford/AstraZeneca vaccines). Persons were considered for inclusion until May 26, 2021.
Each person with immunity was matched in a 1:1 ratio to a person without immunity from a cohort of individuals with families that had from two to five members. Families with more than five members were excluded because of small sample sizes.
Primarily nonimmune families in which there was one immune family member had a 45%-61% lower risk of contracting COVID-19 (hazard ratio, 0.39-0.55; 95% confidence interval, 0.37-0.61; P < .001).
The risk reduction increased to 75%-86% when two family members were immune (HR, 0.14-0.25; 95% CI, 0.11-0.27; P < .001).
It increased to 91%-94% when three family members were immune (HR, 0.06-0.09; 95% CI, 0.04-0.10; P < .001) and to 97% with four immune family members (HR, 0.03; 95% CI, 0.02-0.05; P < .001).
“The results were similar for the outcome of COVID-19 infection that was severe enough to warrant a hospital stay,” the authors wrote. They listed as an example that, in three-member families in which two members were immune, the remaining nonimmune family member had an 80% lower risk for hospitalization (HR, 0.20; 95% CI, 0.10-0.43; P < .001).
Global implications
Dr. Nordström said the team used the family setting because it was more easily identifiable as a cohort with the national registries and because COVID-19 is spread among people in close contact with each other. The findings have implications for other groups that spend large amounts of time together and for herd immunity, he added.
The findings may be particularly welcome in regions of the world where vaccination rates are very low. The authors noted that most of the global population has not yet been vaccinated and that “it is anticipated that most of the population in low-income countries will be unable to receive a vaccine in 2021, with current vaccination rates suggesting that completely inoculating 70%-85% of the global population may take up to 5 years.”
Jill Foster, MD, a pediatric infectious disease specialist at the University of Minnesota, Minneapolis, said in an interview she agrees that the news could encourage countries that have very low vaccination rates.
This study may help motivate areas with few resources to start small, she said: “Even one is better than zero.”
She added that this news could also help ease the minds of families that have immunocompromised members or in which there are children who are too young to be vaccinated.
With these data, she said, people can see there’s something they can do to help protect a family member.
Dr. Foster said that although it’s intuitive to think that the more vaccinated people there are in a family, the safer people are, “it’s really nice to see the data coming out of such a large dataset.”
The authors acknowledged that a limitation of the study is that, at the time the study was conducted, the Delta variant was uncommon in Sweden. It is therefore unclear whether the findings regarding immunity are still relevant in Sweden and elsewhere now that the Delta strain is dominant.
The authors reported no relevant financial relationships. Dr. Foster has received grant support from Moderna.
A version of this article first appeared on Medscape.com.
CDC: Children just as vulnerable to COVID as adults
The study, which focused on 1,000 schools in Arizona’s Maricopa and Pima counties, found that there were 113 COVID-19 outbreaks in schools without mask requirements in the first month of in-person learning. There were 16 outbreaks in schools with mask requirements.
“Masks in schools work to protect our children, to keep them and their school communities safe, and to keep them in school for in-person learning,” CDC Director Rochelle Walensky, MD, said at an Oct. 13 White House briefing.
But, she said, more than 95% of schools across the country had remained open through the end of September, despite 1,800 school closures affecting nearly 1 million students.
Protection for children in school is just one piece of the puzzle, Dr. Walensky said – there must also be COVID-safe practices at home to limit transmission. A CDC study published in October found that children had similar infection rates, compared with adults, confirming there is risk to people of all ages.
“For those children not yet eligible for vaccination, the best protection we can provide them is to make sure everyone around them in the household is vaccinated and to make sure they’re wearing a mask in school and during indoor extracurricular activities,” Dr. Walensky said.
Meanwhile, Pfizer’s vaccine for children ages 5-11 may be approved by early November. The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee will meet Oct. 26 to discuss available data, and the CDC’s Advisory Committee on Immunization Practices will meet Nov. 2. A decision is expected soon after.
A version of this article first appeared on WebMD.com.
The study, which focused on 1,000 schools in Arizona’s Maricopa and Pima counties, found that there were 113 COVID-19 outbreaks in schools without mask requirements in the first month of in-person learning. There were 16 outbreaks in schools with mask requirements.
“Masks in schools work to protect our children, to keep them and their school communities safe, and to keep them in school for in-person learning,” CDC Director Rochelle Walensky, MD, said at an Oct. 13 White House briefing.
But, she said, more than 95% of schools across the country had remained open through the end of September, despite 1,800 school closures affecting nearly 1 million students.
Protection for children in school is just one piece of the puzzle, Dr. Walensky said – there must also be COVID-safe practices at home to limit transmission. A CDC study published in October found that children had similar infection rates, compared with adults, confirming there is risk to people of all ages.
“For those children not yet eligible for vaccination, the best protection we can provide them is to make sure everyone around them in the household is vaccinated and to make sure they’re wearing a mask in school and during indoor extracurricular activities,” Dr. Walensky said.
Meanwhile, Pfizer’s vaccine for children ages 5-11 may be approved by early November. The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee will meet Oct. 26 to discuss available data, and the CDC’s Advisory Committee on Immunization Practices will meet Nov. 2. A decision is expected soon after.
A version of this article first appeared on WebMD.com.
The study, which focused on 1,000 schools in Arizona’s Maricopa and Pima counties, found that there were 113 COVID-19 outbreaks in schools without mask requirements in the first month of in-person learning. There were 16 outbreaks in schools with mask requirements.
“Masks in schools work to protect our children, to keep them and their school communities safe, and to keep them in school for in-person learning,” CDC Director Rochelle Walensky, MD, said at an Oct. 13 White House briefing.
But, she said, more than 95% of schools across the country had remained open through the end of September, despite 1,800 school closures affecting nearly 1 million students.
Protection for children in school is just one piece of the puzzle, Dr. Walensky said – there must also be COVID-safe practices at home to limit transmission. A CDC study published in October found that children had similar infection rates, compared with adults, confirming there is risk to people of all ages.
“For those children not yet eligible for vaccination, the best protection we can provide them is to make sure everyone around them in the household is vaccinated and to make sure they’re wearing a mask in school and during indoor extracurricular activities,” Dr. Walensky said.
Meanwhile, Pfizer’s vaccine for children ages 5-11 may be approved by early November. The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee will meet Oct. 26 to discuss available data, and the CDC’s Advisory Committee on Immunization Practices will meet Nov. 2. A decision is expected soon after.
A version of this article first appeared on WebMD.com.