User login
HF prognosis differs according to iron deficiency definition
There’s overall agreement that iron deficiency is prevalent and portends a worse prognosis in patients with heart failure (HF), regardless of ejection fraction or anemia. What remains unclear, however, is which of the many definitions of iron deficiency most closely aligns with adverse outcomes.
Iron deficiency (ID) differs in chronic inflammatory conditions, such as chronic HF, and is defined in international guidelines as a ferritin less than 100 ng/mL or ferritin 100-299 ng/mL with a transferrin saturation (TSAT) less than 20%.
A new study examining four definitions of ID in more than 4,000 patients with HF revealed that TSAT and serum iron – but not guideline criteria – were independently associated with higher 5-year all-cause mortality, regardless of HF phenotype.
“The standard definition, the society guideline definition of iron deficiency, simply isn’t related to outcome at all. The lines for mortality are, more or less, superimposed,” senior author Andrew L. Clark, MD, Hull (England) University Teaching Hospital NHS Trust, told this news organization.
“So we do think, therefore, there’s a need for a rethink as to what constitutes a definition of iron definition in people with heart failure.”
The results were published in the Journal of the American College of Cardiology.
Previous studies have shown that guideline-defined ID is an independent predictor of mortality in chronic HF, but others have questioned its diagnostic and prognostic utility. A 2018 study using bone marrow iron staining as the gold standard showed that a TSAT of 19.8% or less or serum iron of 13 mcmol/L or less, but not ferritin, identified HF patients at the highest risk for death.
A 2016 report from the Hull LifeLab cohort also showed that the highest quintiles of ferritin had the worst all-cause and cardiovascular (CV) mortality.
Commenting on the new results, Maria Rosa Costanzo, MD, Midwest Cardiovascular Institute, Naperville, Ill., said “the first clinical implication is that we should not use these guidelines to define iron deficiency.
“The fundamental problem with the definition is that ferritin is not a good marker of iron deficiency because ferritin is an inflammatory marker,” she said. “So you could have high ferritin and still have iron deficiency because heart failure, like many other diseases, is an inflammatory state.”
In the present analysis of 4,422 patients referred to the Hull LifeLab clinic between 2001 and 2019, iron deficiency was defined using international guideline criteria, ferritin less than 100 ng/mL, TSAT less than 20%, and serum iron 13 mcmol/L or less.
In line with previous studies, the prevalence of ID was high, ranging from 44% to 68%, depending on the definition. ID was more common in women and in those with more severe symptoms, anemia, or preserved ejection fraction.
Overall, 5-year mortality was 34.5% (median follow-up, 49 months). Unadjusted mortality was lowest for patients with a serum ferritin less than 100 ng/mL and a TSAT greater than 20% and was highest for those with serum ferritin above 100 ng/mL with a TSAT less than 20%.
Serum iron levels and TSAT were highly correlated with each other (r = 0.92; P < .001). “Serum iron is almost entirely transferrin bound, and therefore a close association between serious iron and TSAT is not surprising,” noted the authors, led by Gabriele Masini, MD, University of Brescia (Italy).
After multivariate adjustment, TSAT less than 20% (hazard ratio, 1.27; P < .001) and serum iron of 13 mcmol/L or less (HR, 1.37; P < .001) were associated with greater all-cause mortality but not with CV mortality.
Serum ferritin less than 100 ng/mL tended to be associated with lower adjusted all-cause mortality (HR, 0.91; P = .09), whereas ferritin greater than 300 ng/mL was associated with lower all-cause (HR, 0.69, P < .001) and CV mortality (HR, 0.78; P = .048).
No association was found for guideline ID criteria and all-cause or CV mortality. Among patients fulfilling guideline ID criteria with a TSAT less than 20% and a ferritin 100 to 299 ng/mL, the adjusted hazard ratio for 5-year mortality was 1.82.
A ‘new iron age’
Although 3,011 (68%) patients met the guideline definition of ID, 32% of these patients had a TSAT of 20% or greater and serum iron above 13 mcmol/L, noted Dr. Costanzo.
“In other words, 30% of the patients do not have true iron deficiency,” she said. “If these patients are enrolled in trials of treatment for iron deficiency, they may spuriously reduce the efficacy of treatment.”
Intravenous iron has been shown to improve exercise capacity and quality of life in iron-deficient patients with HF in a series of trials, including FAIR-HF, CONFIRM-HF, and EFFECT-HF, and to reduce HF hospitalizations by 21% in the recent AFFIRM-AF trial.
Although from a single center, Dr. Clark said their findings are robust and hoped they spur a reanalysis of the data from older intravenous iron trials, as well as the IRONMAN trial expected later this year in patients with TSAT less than 20% or ferritin less than 100 ng/L.
“I would very much like to encourage industry to take our study and run with it a little bit and see if we can actually persuade them to rerun studies, maybe even very small-scale studies with a couple hundred patients, to see what the signal is using our definition of iron deficiency and seeing if we get a more striking immediate consequence from IV iron treatment as a result,” he said. “Because we think that we’ve now been able to define a group of patients whose iron deficiency is giving them a very poor prognosis and they, therefore, have much more to gain.”
In an accompanying editorial, Dr. Costanzo and coauthor James Januzzi, MD, of Massachusetts General Hospital and Harvard Medical School, both in Boston, also called for further research into better ID definitions and treatments.
“Diagnostically, soluble transferrin receptor levels may have the strongest correlation with the gold standard of bone marrow iron deficit, whereas new treatments, such as blockade of hepcidin, a key modulator of iron absorption and distribution, may emerge as an effective treatment for both absolute and functional ID,” they wrote.
“Ultimately, the study by Masini et al. places us squarely in a new iron age and underscores the great need for more investigation of the pathophysiology, clinical consequences, and treatment of iron deficiency in all patients with HF,” Dr. Costanzo and Dr. Januzzi concluded.
Dr. Masini reported having no relevant financial relationships. Dr. Januzzi is supported by the Hutter Family Professorship; is a trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, and Novartis; has received consulting income from Abbott Diagnostics, Boehringer Ingelheim, Janssen, Novartis, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda, and Vifor. Dr. Costanzo is a member of the board of directors for Nuwellis; is a consultant for Boehringer Ingelheim, V-Wave, and Nuwellis; and has received grant support from Novartis, Bayer, V-Wave, Nuwellis, and Abbott.
A version of this article first appeared on Medscape.com.
There’s overall agreement that iron deficiency is prevalent and portends a worse prognosis in patients with heart failure (HF), regardless of ejection fraction or anemia. What remains unclear, however, is which of the many definitions of iron deficiency most closely aligns with adverse outcomes.
Iron deficiency (ID) differs in chronic inflammatory conditions, such as chronic HF, and is defined in international guidelines as a ferritin less than 100 ng/mL or ferritin 100-299 ng/mL with a transferrin saturation (TSAT) less than 20%.
A new study examining four definitions of ID in more than 4,000 patients with HF revealed that TSAT and serum iron – but not guideline criteria – were independently associated with higher 5-year all-cause mortality, regardless of HF phenotype.
“The standard definition, the society guideline definition of iron deficiency, simply isn’t related to outcome at all. The lines for mortality are, more or less, superimposed,” senior author Andrew L. Clark, MD, Hull (England) University Teaching Hospital NHS Trust, told this news organization.
“So we do think, therefore, there’s a need for a rethink as to what constitutes a definition of iron definition in people with heart failure.”
The results were published in the Journal of the American College of Cardiology.
Previous studies have shown that guideline-defined ID is an independent predictor of mortality in chronic HF, but others have questioned its diagnostic and prognostic utility. A 2018 study using bone marrow iron staining as the gold standard showed that a TSAT of 19.8% or less or serum iron of 13 mcmol/L or less, but not ferritin, identified HF patients at the highest risk for death.
A 2016 report from the Hull LifeLab cohort also showed that the highest quintiles of ferritin had the worst all-cause and cardiovascular (CV) mortality.
Commenting on the new results, Maria Rosa Costanzo, MD, Midwest Cardiovascular Institute, Naperville, Ill., said “the first clinical implication is that we should not use these guidelines to define iron deficiency.
“The fundamental problem with the definition is that ferritin is not a good marker of iron deficiency because ferritin is an inflammatory marker,” she said. “So you could have high ferritin and still have iron deficiency because heart failure, like many other diseases, is an inflammatory state.”
In the present analysis of 4,422 patients referred to the Hull LifeLab clinic between 2001 and 2019, iron deficiency was defined using international guideline criteria, ferritin less than 100 ng/mL, TSAT less than 20%, and serum iron 13 mcmol/L or less.
In line with previous studies, the prevalence of ID was high, ranging from 44% to 68%, depending on the definition. ID was more common in women and in those with more severe symptoms, anemia, or preserved ejection fraction.
Overall, 5-year mortality was 34.5% (median follow-up, 49 months). Unadjusted mortality was lowest for patients with a serum ferritin less than 100 ng/mL and a TSAT greater than 20% and was highest for those with serum ferritin above 100 ng/mL with a TSAT less than 20%.
Serum iron levels and TSAT were highly correlated with each other (r = 0.92; P < .001). “Serum iron is almost entirely transferrin bound, and therefore a close association between serious iron and TSAT is not surprising,” noted the authors, led by Gabriele Masini, MD, University of Brescia (Italy).
After multivariate adjustment, TSAT less than 20% (hazard ratio, 1.27; P < .001) and serum iron of 13 mcmol/L or less (HR, 1.37; P < .001) were associated with greater all-cause mortality but not with CV mortality.
Serum ferritin less than 100 ng/mL tended to be associated with lower adjusted all-cause mortality (HR, 0.91; P = .09), whereas ferritin greater than 300 ng/mL was associated with lower all-cause (HR, 0.69, P < .001) and CV mortality (HR, 0.78; P = .048).
No association was found for guideline ID criteria and all-cause or CV mortality. Among patients fulfilling guideline ID criteria with a TSAT less than 20% and a ferritin 100 to 299 ng/mL, the adjusted hazard ratio for 5-year mortality was 1.82.
A ‘new iron age’
Although 3,011 (68%) patients met the guideline definition of ID, 32% of these patients had a TSAT of 20% or greater and serum iron above 13 mcmol/L, noted Dr. Costanzo.
“In other words, 30% of the patients do not have true iron deficiency,” she said. “If these patients are enrolled in trials of treatment for iron deficiency, they may spuriously reduce the efficacy of treatment.”
Intravenous iron has been shown to improve exercise capacity and quality of life in iron-deficient patients with HF in a series of trials, including FAIR-HF, CONFIRM-HF, and EFFECT-HF, and to reduce HF hospitalizations by 21% in the recent AFFIRM-AF trial.
Although from a single center, Dr. Clark said their findings are robust and hoped they spur a reanalysis of the data from older intravenous iron trials, as well as the IRONMAN trial expected later this year in patients with TSAT less than 20% or ferritin less than 100 ng/L.
“I would very much like to encourage industry to take our study and run with it a little bit and see if we can actually persuade them to rerun studies, maybe even very small-scale studies with a couple hundred patients, to see what the signal is using our definition of iron deficiency and seeing if we get a more striking immediate consequence from IV iron treatment as a result,” he said. “Because we think that we’ve now been able to define a group of patients whose iron deficiency is giving them a very poor prognosis and they, therefore, have much more to gain.”
In an accompanying editorial, Dr. Costanzo and coauthor James Januzzi, MD, of Massachusetts General Hospital and Harvard Medical School, both in Boston, also called for further research into better ID definitions and treatments.
“Diagnostically, soluble transferrin receptor levels may have the strongest correlation with the gold standard of bone marrow iron deficit, whereas new treatments, such as blockade of hepcidin, a key modulator of iron absorption and distribution, may emerge as an effective treatment for both absolute and functional ID,” they wrote.
“Ultimately, the study by Masini et al. places us squarely in a new iron age and underscores the great need for more investigation of the pathophysiology, clinical consequences, and treatment of iron deficiency in all patients with HF,” Dr. Costanzo and Dr. Januzzi concluded.
Dr. Masini reported having no relevant financial relationships. Dr. Januzzi is supported by the Hutter Family Professorship; is a trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, and Novartis; has received consulting income from Abbott Diagnostics, Boehringer Ingelheim, Janssen, Novartis, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda, and Vifor. Dr. Costanzo is a member of the board of directors for Nuwellis; is a consultant for Boehringer Ingelheim, V-Wave, and Nuwellis; and has received grant support from Novartis, Bayer, V-Wave, Nuwellis, and Abbott.
A version of this article first appeared on Medscape.com.
There’s overall agreement that iron deficiency is prevalent and portends a worse prognosis in patients with heart failure (HF), regardless of ejection fraction or anemia. What remains unclear, however, is which of the many definitions of iron deficiency most closely aligns with adverse outcomes.
Iron deficiency (ID) differs in chronic inflammatory conditions, such as chronic HF, and is defined in international guidelines as a ferritin less than 100 ng/mL or ferritin 100-299 ng/mL with a transferrin saturation (TSAT) less than 20%.
A new study examining four definitions of ID in more than 4,000 patients with HF revealed that TSAT and serum iron – but not guideline criteria – were independently associated with higher 5-year all-cause mortality, regardless of HF phenotype.
“The standard definition, the society guideline definition of iron deficiency, simply isn’t related to outcome at all. The lines for mortality are, more or less, superimposed,” senior author Andrew L. Clark, MD, Hull (England) University Teaching Hospital NHS Trust, told this news organization.
“So we do think, therefore, there’s a need for a rethink as to what constitutes a definition of iron definition in people with heart failure.”
The results were published in the Journal of the American College of Cardiology.
Previous studies have shown that guideline-defined ID is an independent predictor of mortality in chronic HF, but others have questioned its diagnostic and prognostic utility. A 2018 study using bone marrow iron staining as the gold standard showed that a TSAT of 19.8% or less or serum iron of 13 mcmol/L or less, but not ferritin, identified HF patients at the highest risk for death.
A 2016 report from the Hull LifeLab cohort also showed that the highest quintiles of ferritin had the worst all-cause and cardiovascular (CV) mortality.
Commenting on the new results, Maria Rosa Costanzo, MD, Midwest Cardiovascular Institute, Naperville, Ill., said “the first clinical implication is that we should not use these guidelines to define iron deficiency.
“The fundamental problem with the definition is that ferritin is not a good marker of iron deficiency because ferritin is an inflammatory marker,” she said. “So you could have high ferritin and still have iron deficiency because heart failure, like many other diseases, is an inflammatory state.”
In the present analysis of 4,422 patients referred to the Hull LifeLab clinic between 2001 and 2019, iron deficiency was defined using international guideline criteria, ferritin less than 100 ng/mL, TSAT less than 20%, and serum iron 13 mcmol/L or less.
In line with previous studies, the prevalence of ID was high, ranging from 44% to 68%, depending on the definition. ID was more common in women and in those with more severe symptoms, anemia, or preserved ejection fraction.
Overall, 5-year mortality was 34.5% (median follow-up, 49 months). Unadjusted mortality was lowest for patients with a serum ferritin less than 100 ng/mL and a TSAT greater than 20% and was highest for those with serum ferritin above 100 ng/mL with a TSAT less than 20%.
Serum iron levels and TSAT were highly correlated with each other (r = 0.92; P < .001). “Serum iron is almost entirely transferrin bound, and therefore a close association between serious iron and TSAT is not surprising,” noted the authors, led by Gabriele Masini, MD, University of Brescia (Italy).
After multivariate adjustment, TSAT less than 20% (hazard ratio, 1.27; P < .001) and serum iron of 13 mcmol/L or less (HR, 1.37; P < .001) were associated with greater all-cause mortality but not with CV mortality.
Serum ferritin less than 100 ng/mL tended to be associated with lower adjusted all-cause mortality (HR, 0.91; P = .09), whereas ferritin greater than 300 ng/mL was associated with lower all-cause (HR, 0.69, P < .001) and CV mortality (HR, 0.78; P = .048).
No association was found for guideline ID criteria and all-cause or CV mortality. Among patients fulfilling guideline ID criteria with a TSAT less than 20% and a ferritin 100 to 299 ng/mL, the adjusted hazard ratio for 5-year mortality was 1.82.
A ‘new iron age’
Although 3,011 (68%) patients met the guideline definition of ID, 32% of these patients had a TSAT of 20% or greater and serum iron above 13 mcmol/L, noted Dr. Costanzo.
“In other words, 30% of the patients do not have true iron deficiency,” she said. “If these patients are enrolled in trials of treatment for iron deficiency, they may spuriously reduce the efficacy of treatment.”
Intravenous iron has been shown to improve exercise capacity and quality of life in iron-deficient patients with HF in a series of trials, including FAIR-HF, CONFIRM-HF, and EFFECT-HF, and to reduce HF hospitalizations by 21% in the recent AFFIRM-AF trial.
Although from a single center, Dr. Clark said their findings are robust and hoped they spur a reanalysis of the data from older intravenous iron trials, as well as the IRONMAN trial expected later this year in patients with TSAT less than 20% or ferritin less than 100 ng/L.
“I would very much like to encourage industry to take our study and run with it a little bit and see if we can actually persuade them to rerun studies, maybe even very small-scale studies with a couple hundred patients, to see what the signal is using our definition of iron deficiency and seeing if we get a more striking immediate consequence from IV iron treatment as a result,” he said. “Because we think that we’ve now been able to define a group of patients whose iron deficiency is giving them a very poor prognosis and they, therefore, have much more to gain.”
In an accompanying editorial, Dr. Costanzo and coauthor James Januzzi, MD, of Massachusetts General Hospital and Harvard Medical School, both in Boston, also called for further research into better ID definitions and treatments.
“Diagnostically, soluble transferrin receptor levels may have the strongest correlation with the gold standard of bone marrow iron deficit, whereas new treatments, such as blockade of hepcidin, a key modulator of iron absorption and distribution, may emerge as an effective treatment for both absolute and functional ID,” they wrote.
“Ultimately, the study by Masini et al. places us squarely in a new iron age and underscores the great need for more investigation of the pathophysiology, clinical consequences, and treatment of iron deficiency in all patients with HF,” Dr. Costanzo and Dr. Januzzi concluded.
Dr. Masini reported having no relevant financial relationships. Dr. Januzzi is supported by the Hutter Family Professorship; is a trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, and Novartis; has received consulting income from Abbott Diagnostics, Boehringer Ingelheim, Janssen, Novartis, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda, and Vifor. Dr. Costanzo is a member of the board of directors for Nuwellis; is a consultant for Boehringer Ingelheim, V-Wave, and Nuwellis; and has received grant support from Novartis, Bayer, V-Wave, Nuwellis, and Abbott.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Sacral blisters
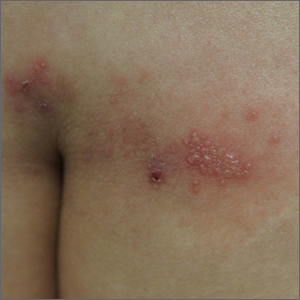
Grouped vesicles on an erythematous base should prompt concern for herpes viruses including varicella zoster (VZV) and herpes simplex (HSV). Polymerase chain reaction (PCR) testing for both VZV and HSV revealed this to be sacral HSV.
VZV classically presents in a dermatomal distribution, whereas HSV more commonly manifests along a single peripheral sensory nerve. Zosteriform presentations of HSV, however, have been reported.
Nongenital and nonoral HSV aren’t uncommon and can be associated with genital herpes, whether from self-inoculation or viremia.1 These outbreaks usually occur in the distribution of the pudendal nerve, which arises from the S2-S4 spinal nerves. There is an association of genital viral shedding even in the absence of lesions when sacral flaring manifests, and patients should be cautioned about sexual transmission or vertically transmitted perinatal infection in pregnant patients near term.
Treatment for an initial episode of genital infection with HSV is valacyclovir 1 g bid for 10 days. The regimen is ideally started within 48 to 72 hours of symptom onset.
This patient was empirically started on VZV dosing, then switched to HSV dosing when the PCR testing confirmed HSV. Knowledge of the exact pathogen is helpful in counseling the patient about the potential for spread and the risk of recurrence. With HSV, the patient may be prescribed a suppressive dose of valacyclovir 500 mg bid for 3 days, started at the onset of symptoms.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Vassantachart JM, Menter A. Recurrent lumbosacral herpes simplex virus infection. Proc (Bayl Univ Med Cent). 2016;29:48-49. doi:10.1080/08998280.2016.11929356

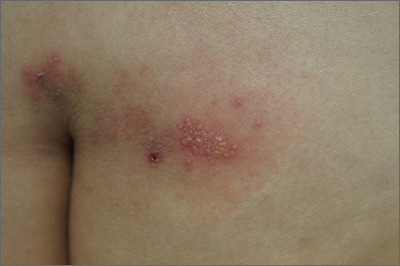
Grouped vesicles on an erythematous base should prompt concern for herpes viruses including varicella zoster (VZV) and herpes simplex (HSV). Polymerase chain reaction (PCR) testing for both VZV and HSV revealed this to be sacral HSV.
VZV classically presents in a dermatomal distribution, whereas HSV more commonly manifests along a single peripheral sensory nerve. Zosteriform presentations of HSV, however, have been reported.
Nongenital and nonoral HSV aren’t uncommon and can be associated with genital herpes, whether from self-inoculation or viremia.1 These outbreaks usually occur in the distribution of the pudendal nerve, which arises from the S2-S4 spinal nerves. There is an association of genital viral shedding even in the absence of lesions when sacral flaring manifests, and patients should be cautioned about sexual transmission or vertically transmitted perinatal infection in pregnant patients near term.
Treatment for an initial episode of genital infection with HSV is valacyclovir 1 g bid for 10 days. The regimen is ideally started within 48 to 72 hours of symptom onset.
This patient was empirically started on VZV dosing, then switched to HSV dosing when the PCR testing confirmed HSV. Knowledge of the exact pathogen is helpful in counseling the patient about the potential for spread and the risk of recurrence. With HSV, the patient may be prescribed a suppressive dose of valacyclovir 500 mg bid for 3 days, started at the onset of symptoms.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

Grouped vesicles on an erythematous base should prompt concern for herpes viruses including varicella zoster (VZV) and herpes simplex (HSV). Polymerase chain reaction (PCR) testing for both VZV and HSV revealed this to be sacral HSV.
VZV classically presents in a dermatomal distribution, whereas HSV more commonly manifests along a single peripheral sensory nerve. Zosteriform presentations of HSV, however, have been reported.
Nongenital and nonoral HSV aren’t uncommon and can be associated with genital herpes, whether from self-inoculation or viremia.1 These outbreaks usually occur in the distribution of the pudendal nerve, which arises from the S2-S4 spinal nerves. There is an association of genital viral shedding even in the absence of lesions when sacral flaring manifests, and patients should be cautioned about sexual transmission or vertically transmitted perinatal infection in pregnant patients near term.
Treatment for an initial episode of genital infection with HSV is valacyclovir 1 g bid for 10 days. The regimen is ideally started within 48 to 72 hours of symptom onset.
This patient was empirically started on VZV dosing, then switched to HSV dosing when the PCR testing confirmed HSV. Knowledge of the exact pathogen is helpful in counseling the patient about the potential for spread and the risk of recurrence. With HSV, the patient may be prescribed a suppressive dose of valacyclovir 500 mg bid for 3 days, started at the onset of symptoms.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Vassantachart JM, Menter A. Recurrent lumbosacral herpes simplex virus infection. Proc (Bayl Univ Med Cent). 2016;29:48-49. doi:10.1080/08998280.2016.11929356
1. Vassantachart JM, Menter A. Recurrent lumbosacral herpes simplex virus infection. Proc (Bayl Univ Med Cent). 2016;29:48-49. doi:10.1080/08998280.2016.11929356
Clinical Edge Journal Scan Commentary: RA February 2022
Several recent RA studies have addressed aspects of systemic illness other than joint pain and inflammation, including sleep, fatigue, psychosocial burden, and well-being. A cohort study by Lyne et al1 evaluated sleep duration and quality in 3,265 patients in the Swedish EIRA registry from 1-12 years after RA diagnosis. About 40% had problems in at least one sleep domain and the frequency of sleep problems increased somewhat with disease duration, but the strongest correlations with poor sleep were pain and functional impairment, suggesting that the overall activity of the RA was most important. Further research on improving sleep quality with improved control of disease activity would be helpful in supporting this hypothesis.
A systematic review by Shamail et al2 examined mental health outcomes in patients with RA taking Janus kinase (JAK) inhibitors, limiting the review to studies reporting SF-36 mental health outcomes. The resulting 19 studies encompassed over 14,000 patients and did demonstrate clinically meaningful changes in SF-36 scores compared to baseline in patients treated with JAK inhibitors. When compared to changes with placebo or disease-modifying antirheumatic drug (DMARD) treatment, JAK inhibitors appeared to have a benefit, though few studies showed a clinically meaningful difference. Given that other studies have shown improvement in mental health outcomes with other classes of RA treatments, it is not clear that this is an effect of the JAK inhibitor class rather than related to overall improvement in quality of life.
Fatigue is a prevalent concern among patients with RA and may significantly impact quality of life; its origins in RA are not well-understood but thought to be related to inflammation. A UK study of an inception cohort by Ifeseman et al3 examines fatigue in early RA; about 75% of participants reported a decreased vitality score compared to the mean in the UK general population. Of the approximately 729 study participants in the longitudinal analysis, trajectory modeling was used to identify two groups of people: one with an “average” vitality score and another with a score that was significantly reduced compared to average. This group had worse disease activity scores, Health Assessment Questionnaire (HAQ) scores, and pain, though as with the other studies mentioned above, it is not clear if fatigue is a feature of worse control of RA or related to ongoing central sensitization or “non-inflammatory” mechanisms.
Doumen et al4 analyzed interaction between psychosocial variables and disease activity in an early RA cohort and found that better baseline short form-36 (SF-36) scores as well as other measures of psychosocial burden and coping were associated with sustained Disease Activity Score 28 for Rheumatoid Arthritis with C-Reactive Protein (DAS-28-CRP) remission, while negative illness perception was associated with lower probability of sustained remission. Of the 287 patients who achieved DAS-28-CRP remission at week 16, the 231 patients who had a low psychosocial burden were more likely to remain in remission. Causality and direction are not established in this small study, so while evaluating psychosocial needs is relevant, as with the other studies mentioned above, caution must be used in attributing lack of improvement in disease activity to psychosocial burden or mood disorders.
References
- Lyne L et al. Sleep problems in rheumatoid arthritis over 12 years from diagnosis: results from the Swedish EIRA study. RMD Open. 2022;8:e001800 (Jan 5).
- Shamail GMH et al. Association between janus kinase inhibitors therapy and mental health outcome in rheumatoid arthritis: A systematic review and meta-analysis. Rheumatol Ther. 2021 (Dec 13).
- Ifesemen OS et al. Fatigue in early rheumatoid arthritis: data from the Early Rheumatoid Arthritis Network. Rheumatology (Oxford). 2021;keab861 (Dec 27).
- Doumen M et al. Psychosocial burden predicts sustained remission in early rheumatoid arthritis: unraveling the complex interplay of wellbeing and disease activity. Arthritis Care Res (Hoboken). 2021 (Dec 20).
Several recent RA studies have addressed aspects of systemic illness other than joint pain and inflammation, including sleep, fatigue, psychosocial burden, and well-being. A cohort study by Lyne et al1 evaluated sleep duration and quality in 3,265 patients in the Swedish EIRA registry from 1-12 years after RA diagnosis. About 40% had problems in at least one sleep domain and the frequency of sleep problems increased somewhat with disease duration, but the strongest correlations with poor sleep were pain and functional impairment, suggesting that the overall activity of the RA was most important. Further research on improving sleep quality with improved control of disease activity would be helpful in supporting this hypothesis.
A systematic review by Shamail et al2 examined mental health outcomes in patients with RA taking Janus kinase (JAK) inhibitors, limiting the review to studies reporting SF-36 mental health outcomes. The resulting 19 studies encompassed over 14,000 patients and did demonstrate clinically meaningful changes in SF-36 scores compared to baseline in patients treated with JAK inhibitors. When compared to changes with placebo or disease-modifying antirheumatic drug (DMARD) treatment, JAK inhibitors appeared to have a benefit, though few studies showed a clinically meaningful difference. Given that other studies have shown improvement in mental health outcomes with other classes of RA treatments, it is not clear that this is an effect of the JAK inhibitor class rather than related to overall improvement in quality of life.
Fatigue is a prevalent concern among patients with RA and may significantly impact quality of life; its origins in RA are not well-understood but thought to be related to inflammation. A UK study of an inception cohort by Ifeseman et al3 examines fatigue in early RA; about 75% of participants reported a decreased vitality score compared to the mean in the UK general population. Of the approximately 729 study participants in the longitudinal analysis, trajectory modeling was used to identify two groups of people: one with an “average” vitality score and another with a score that was significantly reduced compared to average. This group had worse disease activity scores, Health Assessment Questionnaire (HAQ) scores, and pain, though as with the other studies mentioned above, it is not clear if fatigue is a feature of worse control of RA or related to ongoing central sensitization or “non-inflammatory” mechanisms.
Doumen et al4 analyzed interaction between psychosocial variables and disease activity in an early RA cohort and found that better baseline short form-36 (SF-36) scores as well as other measures of psychosocial burden and coping were associated with sustained Disease Activity Score 28 for Rheumatoid Arthritis with C-Reactive Protein (DAS-28-CRP) remission, while negative illness perception was associated with lower probability of sustained remission. Of the 287 patients who achieved DAS-28-CRP remission at week 16, the 231 patients who had a low psychosocial burden were more likely to remain in remission. Causality and direction are not established in this small study, so while evaluating psychosocial needs is relevant, as with the other studies mentioned above, caution must be used in attributing lack of improvement in disease activity to psychosocial burden or mood disorders.
References
- Lyne L et al. Sleep problems in rheumatoid arthritis over 12 years from diagnosis: results from the Swedish EIRA study. RMD Open. 2022;8:e001800 (Jan 5).
- Shamail GMH et al. Association between janus kinase inhibitors therapy and mental health outcome in rheumatoid arthritis: A systematic review and meta-analysis. Rheumatol Ther. 2021 (Dec 13).
- Ifesemen OS et al. Fatigue in early rheumatoid arthritis: data from the Early Rheumatoid Arthritis Network. Rheumatology (Oxford). 2021;keab861 (Dec 27).
- Doumen M et al. Psychosocial burden predicts sustained remission in early rheumatoid arthritis: unraveling the complex interplay of wellbeing and disease activity. Arthritis Care Res (Hoboken). 2021 (Dec 20).
Several recent RA studies have addressed aspects of systemic illness other than joint pain and inflammation, including sleep, fatigue, psychosocial burden, and well-being. A cohort study by Lyne et al1 evaluated sleep duration and quality in 3,265 patients in the Swedish EIRA registry from 1-12 years after RA diagnosis. About 40% had problems in at least one sleep domain and the frequency of sleep problems increased somewhat with disease duration, but the strongest correlations with poor sleep were pain and functional impairment, suggesting that the overall activity of the RA was most important. Further research on improving sleep quality with improved control of disease activity would be helpful in supporting this hypothesis.
A systematic review by Shamail et al2 examined mental health outcomes in patients with RA taking Janus kinase (JAK) inhibitors, limiting the review to studies reporting SF-36 mental health outcomes. The resulting 19 studies encompassed over 14,000 patients and did demonstrate clinically meaningful changes in SF-36 scores compared to baseline in patients treated with JAK inhibitors. When compared to changes with placebo or disease-modifying antirheumatic drug (DMARD) treatment, JAK inhibitors appeared to have a benefit, though few studies showed a clinically meaningful difference. Given that other studies have shown improvement in mental health outcomes with other classes of RA treatments, it is not clear that this is an effect of the JAK inhibitor class rather than related to overall improvement in quality of life.
Fatigue is a prevalent concern among patients with RA and may significantly impact quality of life; its origins in RA are not well-understood but thought to be related to inflammation. A UK study of an inception cohort by Ifeseman et al3 examines fatigue in early RA; about 75% of participants reported a decreased vitality score compared to the mean in the UK general population. Of the approximately 729 study participants in the longitudinal analysis, trajectory modeling was used to identify two groups of people: one with an “average” vitality score and another with a score that was significantly reduced compared to average. This group had worse disease activity scores, Health Assessment Questionnaire (HAQ) scores, and pain, though as with the other studies mentioned above, it is not clear if fatigue is a feature of worse control of RA or related to ongoing central sensitization or “non-inflammatory” mechanisms.
Doumen et al4 analyzed interaction between psychosocial variables and disease activity in an early RA cohort and found that better baseline short form-36 (SF-36) scores as well as other measures of psychosocial burden and coping were associated with sustained Disease Activity Score 28 for Rheumatoid Arthritis with C-Reactive Protein (DAS-28-CRP) remission, while negative illness perception was associated with lower probability of sustained remission. Of the 287 patients who achieved DAS-28-CRP remission at week 16, the 231 patients who had a low psychosocial burden were more likely to remain in remission. Causality and direction are not established in this small study, so while evaluating psychosocial needs is relevant, as with the other studies mentioned above, caution must be used in attributing lack of improvement in disease activity to psychosocial burden or mood disorders.
References
- Lyne L et al. Sleep problems in rheumatoid arthritis over 12 years from diagnosis: results from the Swedish EIRA study. RMD Open. 2022;8:e001800 (Jan 5).
- Shamail GMH et al. Association between janus kinase inhibitors therapy and mental health outcome in rheumatoid arthritis: A systematic review and meta-analysis. Rheumatol Ther. 2021 (Dec 13).
- Ifesemen OS et al. Fatigue in early rheumatoid arthritis: data from the Early Rheumatoid Arthritis Network. Rheumatology (Oxford). 2021;keab861 (Dec 27).
- Doumen M et al. Psychosocial burden predicts sustained remission in early rheumatoid arthritis: unraveling the complex interplay of wellbeing and disease activity. Arthritis Care Res (Hoboken). 2021 (Dec 20).
Clinical Edge Journal Scan Commentary: PsA February 2022
Identifying risk factors associated with transition from cutaneous psoriasis to arthritic psoriasis remains a hot area of research. In a retrospective nested case-control study using the resources of the Rochester Epidemiology Project, Karmacharya et al1 identified 164 patients with incident PsA between 2000 and 2017. Among the 158 total patients satisfying study criteria, 64 (41%) had concurrent psoriasis and PsA and 94 (59%) had onset of psoriasis before PsA. The median time from psoriasis diagnosis to the incidence of PsA was 35.5 months with age at psoriasis onset (odds ratio [OR] per 10-year decrease 1.63; 95% CI 1.26-2.11) and its severity (OR for severe vs. mild 3.65; 95% CI 1.18-11.32) being associated with having a psoriasis diagnosis >1 year prior to incident PsA. Early onset as well as severe psoriasis is associated with the HLA- C*06 allele as is longer psoriasis-PsA latency. Although not evaluated in this study, this genetic factor, or other factors such as detection bias, may underly these observations.
Once diagnosed, stratification of PsA severity is important for planning treatment. Towards this goal, Dubash et al2 demonstrated that the presence of dactylitis indicates a more severe PsA phenotype. In a study of 177 disease-modifying antirheumatic drug (DMARD)-naive patients with early PsA, they found that those with dactylitis (46%) had significantly higher tender and swollen joint counts and C-reactive protein than those with non-dactylitic PsA. Ultrasound synovitis and erosions were also significantly more prevalent in dactylitic PsA. Thus, the presence of dactylitis indicates a more severe phenotype, and patients with dactylitis should be treated aggressively to improve long-term outcomes.
Novel therapies are being frequently evaluated in PsA and a recent target is interleukin (IL)-23, a key cytokine in the T-helper 17 (Th17) pathway and in the pathogenesis of psoriatic disease. Risankizumab is a novel monoclonal antibody targeting IL-23. In the double-blind phase 3 KEEPsAKE 1 study including 964 patients with active PsA and inadequate response to one or more conventional synthetic (cs) DMARDs. They were randomly assigned to receive 150 mg risankizumab or placebo, Kristensen et al3 demonstrated that, at week 24, at least a 20% improvement in the American College of Rheumatology score (ACR20) was achieved by a significantly higher proportion of patients receiving risankizumab vs. placebo (57.3% vs. 33.5%; P < .001). Treatment-emergent adverse events were mild-to-moderate and reported at similar frequencies in the risankizumab (40.4%) and placebo (38.7%) groups. Thus, risankizumab was efficacious in reducing clinical manifestations of PsA in patients with inadequate response to csDMARDs with no new adverse events. An important question when treating patients with PsA with targeted therapies is the need for concomitant therapy with csDMARDs. In a pooled analysis of 2 phase 3 trials, SELECT-PsA 1 and SELECT-PsA 2, 1,916 patients with active PsA with an inadequate response to ≥1 non-biologic (nb) DMARDs or biologic DMARDs were randomly assigned to placebo, 15 mg upadacitinib, or 30 mg upadacitinib as monotherapy or in combination with ≤2 nbDMARDs for 24 weeks, Nash et al4 demonstrated that at week 12, ACR20 response was achieved by a similar proportion of patients receiving 15 mg upadacitinib or 30 mg upadacitinib as monotherapy (15 mg: 33.7%; 95% CI 24.4%-43.1%; 30 mg: 45.7%; 95% CI 36.9%-54.5%) or combination therapy (15 mg: 34.0%; 95% CI 27.9%-40.1%; 30 mg: 39.6%; 95% CI 33.7%-45.5%). Adverse events were generally similar between monotherapy and combination therapy. Although, we don’t have information regarding the sustainability of the response, these data indicate that upadacitinib may be used without concomitant csDMARDs in PsA.
References
- Karmacharya P et al. Time to transition from psoriasis to psoriatic arthritis: A population-based study. Semin Arthritis Rheum. 2021(Dec 31):S0049-0172(21)00230-4.
- Dubash S et al. Dactylitis is an indicator of a more severe phenotype independently associated with greater SJC, CRP, ultrasound synovitis and erosive damage in DMARD-naive early psoriatic arthritis. Ann Rheum Dis. 2021(Dec 10):annrheumdis-2021-220964.
- Kristensen LE et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 1 trial. Ann Rheum Dis. 2022(Feb);81(2):225-231.
- Nash P et al. Upadacitinib as monotherapy and in combination with non-biologic disease-modifying antirheumatic drugs for psoriatic arthritis. Rheumatology (Oxford). 2021(Dec 3):keab905.
Identifying risk factors associated with transition from cutaneous psoriasis to arthritic psoriasis remains a hot area of research. In a retrospective nested case-control study using the resources of the Rochester Epidemiology Project, Karmacharya et al1 identified 164 patients with incident PsA between 2000 and 2017. Among the 158 total patients satisfying study criteria, 64 (41%) had concurrent psoriasis and PsA and 94 (59%) had onset of psoriasis before PsA. The median time from psoriasis diagnosis to the incidence of PsA was 35.5 months with age at psoriasis onset (odds ratio [OR] per 10-year decrease 1.63; 95% CI 1.26-2.11) and its severity (OR for severe vs. mild 3.65; 95% CI 1.18-11.32) being associated with having a psoriasis diagnosis >1 year prior to incident PsA. Early onset as well as severe psoriasis is associated with the HLA- C*06 allele as is longer psoriasis-PsA latency. Although not evaluated in this study, this genetic factor, or other factors such as detection bias, may underly these observations.
Once diagnosed, stratification of PsA severity is important for planning treatment. Towards this goal, Dubash et al2 demonstrated that the presence of dactylitis indicates a more severe PsA phenotype. In a study of 177 disease-modifying antirheumatic drug (DMARD)-naive patients with early PsA, they found that those with dactylitis (46%) had significantly higher tender and swollen joint counts and C-reactive protein than those with non-dactylitic PsA. Ultrasound synovitis and erosions were also significantly more prevalent in dactylitic PsA. Thus, the presence of dactylitis indicates a more severe phenotype, and patients with dactylitis should be treated aggressively to improve long-term outcomes.
Novel therapies are being frequently evaluated in PsA and a recent target is interleukin (IL)-23, a key cytokine in the T-helper 17 (Th17) pathway and in the pathogenesis of psoriatic disease. Risankizumab is a novel monoclonal antibody targeting IL-23. In the double-blind phase 3 KEEPsAKE 1 study including 964 patients with active PsA and inadequate response to one or more conventional synthetic (cs) DMARDs. They were randomly assigned to receive 150 mg risankizumab or placebo, Kristensen et al3 demonstrated that, at week 24, at least a 20% improvement in the American College of Rheumatology score (ACR20) was achieved by a significantly higher proportion of patients receiving risankizumab vs. placebo (57.3% vs. 33.5%; P < .001). Treatment-emergent adverse events were mild-to-moderate and reported at similar frequencies in the risankizumab (40.4%) and placebo (38.7%) groups. Thus, risankizumab was efficacious in reducing clinical manifestations of PsA in patients with inadequate response to csDMARDs with no new adverse events. An important question when treating patients with PsA with targeted therapies is the need for concomitant therapy with csDMARDs. In a pooled analysis of 2 phase 3 trials, SELECT-PsA 1 and SELECT-PsA 2, 1,916 patients with active PsA with an inadequate response to ≥1 non-biologic (nb) DMARDs or biologic DMARDs were randomly assigned to placebo, 15 mg upadacitinib, or 30 mg upadacitinib as monotherapy or in combination with ≤2 nbDMARDs for 24 weeks, Nash et al4 demonstrated that at week 12, ACR20 response was achieved by a similar proportion of patients receiving 15 mg upadacitinib or 30 mg upadacitinib as monotherapy (15 mg: 33.7%; 95% CI 24.4%-43.1%; 30 mg: 45.7%; 95% CI 36.9%-54.5%) or combination therapy (15 mg: 34.0%; 95% CI 27.9%-40.1%; 30 mg: 39.6%; 95% CI 33.7%-45.5%). Adverse events were generally similar between monotherapy and combination therapy. Although, we don’t have information regarding the sustainability of the response, these data indicate that upadacitinib may be used without concomitant csDMARDs in PsA.
References
- Karmacharya P et al. Time to transition from psoriasis to psoriatic arthritis: A population-based study. Semin Arthritis Rheum. 2021(Dec 31):S0049-0172(21)00230-4.
- Dubash S et al. Dactylitis is an indicator of a more severe phenotype independently associated with greater SJC, CRP, ultrasound synovitis and erosive damage in DMARD-naive early psoriatic arthritis. Ann Rheum Dis. 2021(Dec 10):annrheumdis-2021-220964.
- Kristensen LE et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 1 trial. Ann Rheum Dis. 2022(Feb);81(2):225-231.
- Nash P et al. Upadacitinib as monotherapy and in combination with non-biologic disease-modifying antirheumatic drugs for psoriatic arthritis. Rheumatology (Oxford). 2021(Dec 3):keab905.
Identifying risk factors associated with transition from cutaneous psoriasis to arthritic psoriasis remains a hot area of research. In a retrospective nested case-control study using the resources of the Rochester Epidemiology Project, Karmacharya et al1 identified 164 patients with incident PsA between 2000 and 2017. Among the 158 total patients satisfying study criteria, 64 (41%) had concurrent psoriasis and PsA and 94 (59%) had onset of psoriasis before PsA. The median time from psoriasis diagnosis to the incidence of PsA was 35.5 months with age at psoriasis onset (odds ratio [OR] per 10-year decrease 1.63; 95% CI 1.26-2.11) and its severity (OR for severe vs. mild 3.65; 95% CI 1.18-11.32) being associated with having a psoriasis diagnosis >1 year prior to incident PsA. Early onset as well as severe psoriasis is associated with the HLA- C*06 allele as is longer psoriasis-PsA latency. Although not evaluated in this study, this genetic factor, or other factors such as detection bias, may underly these observations.
Once diagnosed, stratification of PsA severity is important for planning treatment. Towards this goal, Dubash et al2 demonstrated that the presence of dactylitis indicates a more severe PsA phenotype. In a study of 177 disease-modifying antirheumatic drug (DMARD)-naive patients with early PsA, they found that those with dactylitis (46%) had significantly higher tender and swollen joint counts and C-reactive protein than those with non-dactylitic PsA. Ultrasound synovitis and erosions were also significantly more prevalent in dactylitic PsA. Thus, the presence of dactylitis indicates a more severe phenotype, and patients with dactylitis should be treated aggressively to improve long-term outcomes.
Novel therapies are being frequently evaluated in PsA and a recent target is interleukin (IL)-23, a key cytokine in the T-helper 17 (Th17) pathway and in the pathogenesis of psoriatic disease. Risankizumab is a novel monoclonal antibody targeting IL-23. In the double-blind phase 3 KEEPsAKE 1 study including 964 patients with active PsA and inadequate response to one or more conventional synthetic (cs) DMARDs. They were randomly assigned to receive 150 mg risankizumab or placebo, Kristensen et al3 demonstrated that, at week 24, at least a 20% improvement in the American College of Rheumatology score (ACR20) was achieved by a significantly higher proportion of patients receiving risankizumab vs. placebo (57.3% vs. 33.5%; P < .001). Treatment-emergent adverse events were mild-to-moderate and reported at similar frequencies in the risankizumab (40.4%) and placebo (38.7%) groups. Thus, risankizumab was efficacious in reducing clinical manifestations of PsA in patients with inadequate response to csDMARDs with no new adverse events. An important question when treating patients with PsA with targeted therapies is the need for concomitant therapy with csDMARDs. In a pooled analysis of 2 phase 3 trials, SELECT-PsA 1 and SELECT-PsA 2, 1,916 patients with active PsA with an inadequate response to ≥1 non-biologic (nb) DMARDs or biologic DMARDs were randomly assigned to placebo, 15 mg upadacitinib, or 30 mg upadacitinib as monotherapy or in combination with ≤2 nbDMARDs for 24 weeks, Nash et al4 demonstrated that at week 12, ACR20 response was achieved by a similar proportion of patients receiving 15 mg upadacitinib or 30 mg upadacitinib as monotherapy (15 mg: 33.7%; 95% CI 24.4%-43.1%; 30 mg: 45.7%; 95% CI 36.9%-54.5%) or combination therapy (15 mg: 34.0%; 95% CI 27.9%-40.1%; 30 mg: 39.6%; 95% CI 33.7%-45.5%). Adverse events were generally similar between monotherapy and combination therapy. Although, we don’t have information regarding the sustainability of the response, these data indicate that upadacitinib may be used without concomitant csDMARDs in PsA.
References
- Karmacharya P et al. Time to transition from psoriasis to psoriatic arthritis: A population-based study. Semin Arthritis Rheum. 2021(Dec 31):S0049-0172(21)00230-4.
- Dubash S et al. Dactylitis is an indicator of a more severe phenotype independently associated with greater SJC, CRP, ultrasound synovitis and erosive damage in DMARD-naive early psoriatic arthritis. Ann Rheum Dis. 2021(Dec 10):annrheumdis-2021-220964.
- Kristensen LE et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 1 trial. Ann Rheum Dis. 2022(Feb);81(2):225-231.
- Nash P et al. Upadacitinib as monotherapy and in combination with non-biologic disease-modifying antirheumatic drugs for psoriatic arthritis. Rheumatology (Oxford). 2021(Dec 3):keab905.
Omicron survives longer on plastic, skin than other COVID variants
, one possible explanation for why Omicron has spread so rapidly around the world.
In a lab experiment, samples of different variants were applied to pieces of plastic and human skin collected from autopsies, researchers from Kyoto Prefectural University of Medicine wrote in bioRxiv. A variant “survived” until it could no longer be detected on the surface.
“This study showed that the Omicron variant also has the highest environmental stability among VOCs (variants of concern), which suggests that this high stability might also be one of the factors that have allowed the Omicron variant to replace the Delta variant and spread rapidly,” the researchers wrote.
On plastic, the Omicron variant samples survived an average of 193.5 hours, a little more than 8 days. By comparison, the other survival times on plastic were 56 hours for the original COVID strain, 191.3 hours for Alpha, 156.6 hours for Beta, 59.3 hours for Gamma, and 114 hours for Delta.
On skin samples, the Omicron samples survived an average of 21.1 hours. The other variants had these average survival times on skin: 8.6 hours for the original version, 19.6 hours for Alpha, 19.1 hours for Beta, 11 hours for Gamma, and 16.8 hours for Delta.
The study found that the variants had more resistance to ethanol than the original strain of COVID. That said, all COVID samples were inactivated after being exposed to alcohol-based hand sanitizers for 15 seconds.
“Therefore, it is highly recommended that current infection control (hand hygiene) practices use disinfectants ... as proposed by the World Health Organization,” the researchers said.
The study has not been peer-reviewed.
A version of this article first appeared on WebMD.com.
, one possible explanation for why Omicron has spread so rapidly around the world.
In a lab experiment, samples of different variants were applied to pieces of plastic and human skin collected from autopsies, researchers from Kyoto Prefectural University of Medicine wrote in bioRxiv. A variant “survived” until it could no longer be detected on the surface.
“This study showed that the Omicron variant also has the highest environmental stability among VOCs (variants of concern), which suggests that this high stability might also be one of the factors that have allowed the Omicron variant to replace the Delta variant and spread rapidly,” the researchers wrote.
On plastic, the Omicron variant samples survived an average of 193.5 hours, a little more than 8 days. By comparison, the other survival times on plastic were 56 hours for the original COVID strain, 191.3 hours for Alpha, 156.6 hours for Beta, 59.3 hours for Gamma, and 114 hours for Delta.
On skin samples, the Omicron samples survived an average of 21.1 hours. The other variants had these average survival times on skin: 8.6 hours for the original version, 19.6 hours for Alpha, 19.1 hours for Beta, 11 hours for Gamma, and 16.8 hours for Delta.
The study found that the variants had more resistance to ethanol than the original strain of COVID. That said, all COVID samples were inactivated after being exposed to alcohol-based hand sanitizers for 15 seconds.
“Therefore, it is highly recommended that current infection control (hand hygiene) practices use disinfectants ... as proposed by the World Health Organization,” the researchers said.
The study has not been peer-reviewed.
A version of this article first appeared on WebMD.com.
, one possible explanation for why Omicron has spread so rapidly around the world.
In a lab experiment, samples of different variants were applied to pieces of plastic and human skin collected from autopsies, researchers from Kyoto Prefectural University of Medicine wrote in bioRxiv. A variant “survived” until it could no longer be detected on the surface.
“This study showed that the Omicron variant also has the highest environmental stability among VOCs (variants of concern), which suggests that this high stability might also be one of the factors that have allowed the Omicron variant to replace the Delta variant and spread rapidly,” the researchers wrote.
On plastic, the Omicron variant samples survived an average of 193.5 hours, a little more than 8 days. By comparison, the other survival times on plastic were 56 hours for the original COVID strain, 191.3 hours for Alpha, 156.6 hours for Beta, 59.3 hours for Gamma, and 114 hours for Delta.
On skin samples, the Omicron samples survived an average of 21.1 hours. The other variants had these average survival times on skin: 8.6 hours for the original version, 19.6 hours for Alpha, 19.1 hours for Beta, 11 hours for Gamma, and 16.8 hours for Delta.
The study found that the variants had more resistance to ethanol than the original strain of COVID. That said, all COVID samples were inactivated after being exposed to alcohol-based hand sanitizers for 15 seconds.
“Therefore, it is highly recommended that current infection control (hand hygiene) practices use disinfectants ... as proposed by the World Health Organization,” the researchers said.
The study has not been peer-reviewed.
A version of this article first appeared on WebMD.com.
Potential new standard of care for biliary tract cancer
, according to interim results from the TOPAZ-1 trial.
The risk of death for those taking durvalumab plus chemotherapy was 20% lower than for patients on chemotherapy alone. At 18 months, overall survival was 35.1% in the durvalumab group versus 25.6% for chemotherapy alone. By 2 years, overall survival was 24.9% versus 10.4%.
“TOPAZ-1 is the first phase 3 trial to show that adding immunotherapy to standard chemotherapy can increase survival in biliary tract cancer, and importantly, does so without inducing any new serious side effects,” said lead author Do-Youn Oh, MD, PhD, professor in the Division of Medical Oncology at Seoul National University Hospital and Seoul National University College of Medicine, Korea.
“The study met its primary endpoint at a prespecified interim analysis, and durvalumab plus gemcitabine and cisplatin demonstrated statistically significant and clinically meaningful prolonged overall survival compared with placebo plus chemotherapy,” she said.
“This is an effective first-line therapy and could become a new standard of care for patients with advanced biliary tract cancer,” she added.
Dr. Oh presented the results at the Gastrointestinal Cancers Symposium (GICS) 2022.
In a discussion of the paper, Nilofer Saba Azad, MD, from the department of oncology, Johns Hopkins Sidney Kimmel Cancer Center, Baltimore, noted that overall, “we are seeing enticing benefit in survival and response rate.”
“There is moderately strong preliminary clinical data and biological rationale that immune checkpoint may have some activity in biliary tract cancer,” she said. “The trial was adequately powered and accounted for important known clinical subsets, and [it] was placebo controlled. The results suggest a meaningful benefit for patients.”
However, she pointed out that there are still open questions, mostly having to do with the subgroup analysis.
Biliary tract cancer: Incidence is rising
Biliary tract cancers are a relatively rare and heterogeneous group of cancers, and global incidence is rising. “Advanced unresectable biliary tract cancer is an area of high unmet need due to its aggressive nature, limited treatment options, and poor prognosis,” explained Dr. Oh. “The first-line standard of care for advanced biliary tract cancers, gemcitabine and cisplatin, has remained unchanged for over a decade.”
Previous research has suggested that checkpoint inhibition may result in antitumor immune responses, she commented. A previous phase 2 trial showed that durvalumab combined with gemcitabine and cisplatin showed promising antitumor activity in advanced biliary tract cancer. This latest study is a larger phase 3 trial to investigate this effect further.
The study involved 365 patients with unresectable locally advanced, recurrent, or metastatic biliary tract cancers. Patients had one of three types of biliary tract cancer: 55% had intrahepatic cancers; 19% had extrahepatic cancers; and 25% had gallbladder cancer.
The trial was conducted in the U.S. and 17 countries in Europe, South America, and Asia. Nearly 55% of the cohort was from Asia, including South Korea, Thailand, Japan, and China.
All patients received chemotherapy with gemcitabine (1,000 mg/m2) and cisplatin (25 mg/m2 on days 1 and 8 every 3 weeks) for up to eight cycles.
Patients were randomized to receive either durvalumab (1,500 mg every 3 weeks) or placebo before chemotherapy and also to receive durvalumab (1,500 mg every 4 weeks) or placebo after chemotherapy until disease progression or unacceptable toxicity.
At approximately 1 year, the authors found that adding durvalumab significantly improved overall survival (hazard ratio, 0.80; P = .021).
Progression-free survival was also significantly better with durvalumab compared to placebo: 7.2 months versus 5.7 months (HR, 0.75; P = .001).
The overall response rate (ORR) was 26.7% with durvalumab and 18.7% with placebo.
The most common adverse events were anemia (experienced by 48.2% of patients), neutropenia (31.7%), and nausea (40.2%). Grade 3/4 adverse events occurred in 75.7% of patients receiving durvalumab versus 77.8% for placebo, indicating that the majority of side effects in both arms were from chemotherapy, Dr. Oh commented. Discontinuation of any study medication because of toxicity occurred in 8.9% and 11.4% of patients, respectively.
Enticing benefit, but questions remain
In her discussion of the paper, Dr. Azad pointed out that Asian patients comprised more than half of the cohort and appeared to derive more benefit from the investigational treatment compared to other groups. “So the question is if that is driving the benefit or just an increased benefit,” she said. “That is going to be an open question for our research community.”
Dr. Azad also noted that patients with nonmetastatic disease at enrollment did a little better, so more data are needed on how that affected the outcomes.
“PDL-1 just missed statistical significance, but that is something that will be further explored,” she said. “And we still have open questions about viral hepatitis, liver fluke infection, and cirrhosis, and I do hope that these will be included in the final analysis of the study.”
The GICS meeting is organized by the American Society of Clinical Oncology (ASCO), and the society highlighted these data in a press release. Cathy Eng, MD, FACP, ASCO expert in gastrointestinal cancers, commented in the statement: “TOPAZ-1 is the first phase 3 trial to demonstrate the benefit of immunotherapy for improved overall survival, in combination with chemotherapy, creating a new standard of care.”
The study received funding from AstraZeneca, marker of durvalumab. Dr. Oh and Dr. Azad reported relationships with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
, according to interim results from the TOPAZ-1 trial.
The risk of death for those taking durvalumab plus chemotherapy was 20% lower than for patients on chemotherapy alone. At 18 months, overall survival was 35.1% in the durvalumab group versus 25.6% for chemotherapy alone. By 2 years, overall survival was 24.9% versus 10.4%.
“TOPAZ-1 is the first phase 3 trial to show that adding immunotherapy to standard chemotherapy can increase survival in biliary tract cancer, and importantly, does so without inducing any new serious side effects,” said lead author Do-Youn Oh, MD, PhD, professor in the Division of Medical Oncology at Seoul National University Hospital and Seoul National University College of Medicine, Korea.
“The study met its primary endpoint at a prespecified interim analysis, and durvalumab plus gemcitabine and cisplatin demonstrated statistically significant and clinically meaningful prolonged overall survival compared with placebo plus chemotherapy,” she said.
“This is an effective first-line therapy and could become a new standard of care for patients with advanced biliary tract cancer,” she added.
Dr. Oh presented the results at the Gastrointestinal Cancers Symposium (GICS) 2022.
In a discussion of the paper, Nilofer Saba Azad, MD, from the department of oncology, Johns Hopkins Sidney Kimmel Cancer Center, Baltimore, noted that overall, “we are seeing enticing benefit in survival and response rate.”
“There is moderately strong preliminary clinical data and biological rationale that immune checkpoint may have some activity in biliary tract cancer,” she said. “The trial was adequately powered and accounted for important known clinical subsets, and [it] was placebo controlled. The results suggest a meaningful benefit for patients.”
However, she pointed out that there are still open questions, mostly having to do with the subgroup analysis.
Biliary tract cancer: Incidence is rising
Biliary tract cancers are a relatively rare and heterogeneous group of cancers, and global incidence is rising. “Advanced unresectable biliary tract cancer is an area of high unmet need due to its aggressive nature, limited treatment options, and poor prognosis,” explained Dr. Oh. “The first-line standard of care for advanced biliary tract cancers, gemcitabine and cisplatin, has remained unchanged for over a decade.”
Previous research has suggested that checkpoint inhibition may result in antitumor immune responses, she commented. A previous phase 2 trial showed that durvalumab combined with gemcitabine and cisplatin showed promising antitumor activity in advanced biliary tract cancer. This latest study is a larger phase 3 trial to investigate this effect further.
The study involved 365 patients with unresectable locally advanced, recurrent, or metastatic biliary tract cancers. Patients had one of three types of biliary tract cancer: 55% had intrahepatic cancers; 19% had extrahepatic cancers; and 25% had gallbladder cancer.
The trial was conducted in the U.S. and 17 countries in Europe, South America, and Asia. Nearly 55% of the cohort was from Asia, including South Korea, Thailand, Japan, and China.
All patients received chemotherapy with gemcitabine (1,000 mg/m2) and cisplatin (25 mg/m2 on days 1 and 8 every 3 weeks) for up to eight cycles.
Patients were randomized to receive either durvalumab (1,500 mg every 3 weeks) or placebo before chemotherapy and also to receive durvalumab (1,500 mg every 4 weeks) or placebo after chemotherapy until disease progression or unacceptable toxicity.
At approximately 1 year, the authors found that adding durvalumab significantly improved overall survival (hazard ratio, 0.80; P = .021).
Progression-free survival was also significantly better with durvalumab compared to placebo: 7.2 months versus 5.7 months (HR, 0.75; P = .001).
The overall response rate (ORR) was 26.7% with durvalumab and 18.7% with placebo.
The most common adverse events were anemia (experienced by 48.2% of patients), neutropenia (31.7%), and nausea (40.2%). Grade 3/4 adverse events occurred in 75.7% of patients receiving durvalumab versus 77.8% for placebo, indicating that the majority of side effects in both arms were from chemotherapy, Dr. Oh commented. Discontinuation of any study medication because of toxicity occurred in 8.9% and 11.4% of patients, respectively.
Enticing benefit, but questions remain
In her discussion of the paper, Dr. Azad pointed out that Asian patients comprised more than half of the cohort and appeared to derive more benefit from the investigational treatment compared to other groups. “So the question is if that is driving the benefit or just an increased benefit,” she said. “That is going to be an open question for our research community.”
Dr. Azad also noted that patients with nonmetastatic disease at enrollment did a little better, so more data are needed on how that affected the outcomes.
“PDL-1 just missed statistical significance, but that is something that will be further explored,” she said. “And we still have open questions about viral hepatitis, liver fluke infection, and cirrhosis, and I do hope that these will be included in the final analysis of the study.”
The GICS meeting is organized by the American Society of Clinical Oncology (ASCO), and the society highlighted these data in a press release. Cathy Eng, MD, FACP, ASCO expert in gastrointestinal cancers, commented in the statement: “TOPAZ-1 is the first phase 3 trial to demonstrate the benefit of immunotherapy for improved overall survival, in combination with chemotherapy, creating a new standard of care.”
The study received funding from AstraZeneca, marker of durvalumab. Dr. Oh and Dr. Azad reported relationships with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
, according to interim results from the TOPAZ-1 trial.
The risk of death for those taking durvalumab plus chemotherapy was 20% lower than for patients on chemotherapy alone. At 18 months, overall survival was 35.1% in the durvalumab group versus 25.6% for chemotherapy alone. By 2 years, overall survival was 24.9% versus 10.4%.
“TOPAZ-1 is the first phase 3 trial to show that adding immunotherapy to standard chemotherapy can increase survival in biliary tract cancer, and importantly, does so without inducing any new serious side effects,” said lead author Do-Youn Oh, MD, PhD, professor in the Division of Medical Oncology at Seoul National University Hospital and Seoul National University College of Medicine, Korea.
“The study met its primary endpoint at a prespecified interim analysis, and durvalumab plus gemcitabine and cisplatin demonstrated statistically significant and clinically meaningful prolonged overall survival compared with placebo plus chemotherapy,” she said.
“This is an effective first-line therapy and could become a new standard of care for patients with advanced biliary tract cancer,” she added.
Dr. Oh presented the results at the Gastrointestinal Cancers Symposium (GICS) 2022.
In a discussion of the paper, Nilofer Saba Azad, MD, from the department of oncology, Johns Hopkins Sidney Kimmel Cancer Center, Baltimore, noted that overall, “we are seeing enticing benefit in survival and response rate.”
“There is moderately strong preliminary clinical data and biological rationale that immune checkpoint may have some activity in biliary tract cancer,” she said. “The trial was adequately powered and accounted for important known clinical subsets, and [it] was placebo controlled. The results suggest a meaningful benefit for patients.”
However, she pointed out that there are still open questions, mostly having to do with the subgroup analysis.
Biliary tract cancer: Incidence is rising
Biliary tract cancers are a relatively rare and heterogeneous group of cancers, and global incidence is rising. “Advanced unresectable biliary tract cancer is an area of high unmet need due to its aggressive nature, limited treatment options, and poor prognosis,” explained Dr. Oh. “The first-line standard of care for advanced biliary tract cancers, gemcitabine and cisplatin, has remained unchanged for over a decade.”
Previous research has suggested that checkpoint inhibition may result in antitumor immune responses, she commented. A previous phase 2 trial showed that durvalumab combined with gemcitabine and cisplatin showed promising antitumor activity in advanced biliary tract cancer. This latest study is a larger phase 3 trial to investigate this effect further.
The study involved 365 patients with unresectable locally advanced, recurrent, or metastatic biliary tract cancers. Patients had one of three types of biliary tract cancer: 55% had intrahepatic cancers; 19% had extrahepatic cancers; and 25% had gallbladder cancer.
The trial was conducted in the U.S. and 17 countries in Europe, South America, and Asia. Nearly 55% of the cohort was from Asia, including South Korea, Thailand, Japan, and China.
All patients received chemotherapy with gemcitabine (1,000 mg/m2) and cisplatin (25 mg/m2 on days 1 and 8 every 3 weeks) for up to eight cycles.
Patients were randomized to receive either durvalumab (1,500 mg every 3 weeks) or placebo before chemotherapy and also to receive durvalumab (1,500 mg every 4 weeks) or placebo after chemotherapy until disease progression or unacceptable toxicity.
At approximately 1 year, the authors found that adding durvalumab significantly improved overall survival (hazard ratio, 0.80; P = .021).
Progression-free survival was also significantly better with durvalumab compared to placebo: 7.2 months versus 5.7 months (HR, 0.75; P = .001).
The overall response rate (ORR) was 26.7% with durvalumab and 18.7% with placebo.
The most common adverse events were anemia (experienced by 48.2% of patients), neutropenia (31.7%), and nausea (40.2%). Grade 3/4 adverse events occurred in 75.7% of patients receiving durvalumab versus 77.8% for placebo, indicating that the majority of side effects in both arms were from chemotherapy, Dr. Oh commented. Discontinuation of any study medication because of toxicity occurred in 8.9% and 11.4% of patients, respectively.
Enticing benefit, but questions remain
In her discussion of the paper, Dr. Azad pointed out that Asian patients comprised more than half of the cohort and appeared to derive more benefit from the investigational treatment compared to other groups. “So the question is if that is driving the benefit or just an increased benefit,” she said. “That is going to be an open question for our research community.”
Dr. Azad also noted that patients with nonmetastatic disease at enrollment did a little better, so more data are needed on how that affected the outcomes.
“PDL-1 just missed statistical significance, but that is something that will be further explored,” she said. “And we still have open questions about viral hepatitis, liver fluke infection, and cirrhosis, and I do hope that these will be included in the final analysis of the study.”
The GICS meeting is organized by the American Society of Clinical Oncology (ASCO), and the society highlighted these data in a press release. Cathy Eng, MD, FACP, ASCO expert in gastrointestinal cancers, commented in the statement: “TOPAZ-1 is the first phase 3 trial to demonstrate the benefit of immunotherapy for improved overall survival, in combination with chemotherapy, creating a new standard of care.”
The study received funding from AstraZeneca, marker of durvalumab. Dr. Oh and Dr. Azad reported relationships with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM GICS 2022
Pruritus in elderly patients: Not a diagnosis
that has appeared seemingly out of the blue.
“They ask: ‘What happened? Why did I get this? Everything was going so well and all of a sudden, I get this itchy rash that keeps me up every night,’ ” Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland, said during the Revolutionizing Atopic Dermatitis symposium. “Is this elderly atopic dermatitis? Is that a real thing?”
But such patients often lack flexural involvement, which is a telltale sign of atopic dermatitis, “so I really struggle with making the diagnosis of new onset AD in the elderly,” he said, adding that existing medical literature on the topic is variable, with the use of terms that include chronic eczematous eruption of the elderly, chronic “eczematiform” eruption in the elderly, chronic eczematous eruption of the aged, eczematous dermatitis not otherwise specified, dermal hypersensitivity reaction, urticarial dermatitis, and eczematous drug eruptions.
“Pruritus of the elderly is not a diagnosis,” Dr. Simpson said. “That’s just a symptom with a million etiologies. Never put that as your assessment. You could put pruritic eruption or pruritus, but try to look for the cause.”
More than 50% of older patients have xerosis, according to a 2013 clinical review on pruritus in the elderly, by Timothy G. Berger, MD, and colleagues at the University of California, San Francisco, which includes advice on the evaluation and management of pruritus in this group of patients based on whether they have a rash or not. For a patient with no rash, Dr. Simpson said, the workup “includes ruling out xerosis, scabies, and effects of medications that could cause rash such as narcotics and Adderall; as well as a generalized pruritus workup including renal and hepatic function, blood count, and thyroid levels.”
In a separate analysis of pruritic elderly patients by the same authors, five rash-related diagnoses accounted for 75% of cases: eczematous dermatitis, lichen simplex/prurigo nodularis, subacute prurigo, transient acantholytic dermatosis, and neuropathic disorder. “Morphology of pruritus with rash is also important,” Dr. Simpson added. “Is it eczematous? Papular? Prurigo nodularis? This helps lead you in the right direction.”
Some case-control studies have shown that calcium channel blockers could be related to eczema in older patients.
“But there aren’t a lot of studies out there that show that when you stop your calcium channel blocker, your eczema gets better,” Dr. Simpson said. “I’m reluctant to stop medications to try to help their eczema. I haven’t had many good results doing that.”
In an abstract presented during the 2021 annual meeting of the Society of Investigative Dermatology, he and his colleagues prospectively reviewed 89 patients over age 65 who had been referred with new-onset eczema. Of these, 34 underwent drug cessation trials for 1-3 months. “Not one patient improved when they stopped medications,” Dr. Simpson said, but “multiple patients were hospitalized for discontinuing their cardiac and antihypertensive medications.” While this was a biased sample of patients coming to him with chronic eczema, “in my experience, if you have chronic eczema in an older patient, stopping medications is likely not going to help.”
Other diagnostic tips he offered included asking patients what skin products they’re using, considering patch testing, and considering biopsy to rule out cutaneous T-cell lymphoma or bullous pemphigoid. “If you’re not sure there’s a rash, you might need to do a pruritus workup,” he said. If an eczematous rash is present and no other cause is found, try treating it like AD, he added.
Dr. Simpson reported serving as an investigator for and consultant to numerous pharmaceutical companies.
that has appeared seemingly out of the blue.
“They ask: ‘What happened? Why did I get this? Everything was going so well and all of a sudden, I get this itchy rash that keeps me up every night,’ ” Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland, said during the Revolutionizing Atopic Dermatitis symposium. “Is this elderly atopic dermatitis? Is that a real thing?”
But such patients often lack flexural involvement, which is a telltale sign of atopic dermatitis, “so I really struggle with making the diagnosis of new onset AD in the elderly,” he said, adding that existing medical literature on the topic is variable, with the use of terms that include chronic eczematous eruption of the elderly, chronic “eczematiform” eruption in the elderly, chronic eczematous eruption of the aged, eczematous dermatitis not otherwise specified, dermal hypersensitivity reaction, urticarial dermatitis, and eczematous drug eruptions.
“Pruritus of the elderly is not a diagnosis,” Dr. Simpson said. “That’s just a symptom with a million etiologies. Never put that as your assessment. You could put pruritic eruption or pruritus, but try to look for the cause.”
More than 50% of older patients have xerosis, according to a 2013 clinical review on pruritus in the elderly, by Timothy G. Berger, MD, and colleagues at the University of California, San Francisco, which includes advice on the evaluation and management of pruritus in this group of patients based on whether they have a rash or not. For a patient with no rash, Dr. Simpson said, the workup “includes ruling out xerosis, scabies, and effects of medications that could cause rash such as narcotics and Adderall; as well as a generalized pruritus workup including renal and hepatic function, blood count, and thyroid levels.”
In a separate analysis of pruritic elderly patients by the same authors, five rash-related diagnoses accounted for 75% of cases: eczematous dermatitis, lichen simplex/prurigo nodularis, subacute prurigo, transient acantholytic dermatosis, and neuropathic disorder. “Morphology of pruritus with rash is also important,” Dr. Simpson added. “Is it eczematous? Papular? Prurigo nodularis? This helps lead you in the right direction.”
Some case-control studies have shown that calcium channel blockers could be related to eczema in older patients.
“But there aren’t a lot of studies out there that show that when you stop your calcium channel blocker, your eczema gets better,” Dr. Simpson said. “I’m reluctant to stop medications to try to help their eczema. I haven’t had many good results doing that.”
In an abstract presented during the 2021 annual meeting of the Society of Investigative Dermatology, he and his colleagues prospectively reviewed 89 patients over age 65 who had been referred with new-onset eczema. Of these, 34 underwent drug cessation trials for 1-3 months. “Not one patient improved when they stopped medications,” Dr. Simpson said, but “multiple patients were hospitalized for discontinuing their cardiac and antihypertensive medications.” While this was a biased sample of patients coming to him with chronic eczema, “in my experience, if you have chronic eczema in an older patient, stopping medications is likely not going to help.”
Other diagnostic tips he offered included asking patients what skin products they’re using, considering patch testing, and considering biopsy to rule out cutaneous T-cell lymphoma or bullous pemphigoid. “If you’re not sure there’s a rash, you might need to do a pruritus workup,” he said. If an eczematous rash is present and no other cause is found, try treating it like AD, he added.
Dr. Simpson reported serving as an investigator for and consultant to numerous pharmaceutical companies.
that has appeared seemingly out of the blue.
“They ask: ‘What happened? Why did I get this? Everything was going so well and all of a sudden, I get this itchy rash that keeps me up every night,’ ” Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland, said during the Revolutionizing Atopic Dermatitis symposium. “Is this elderly atopic dermatitis? Is that a real thing?”
But such patients often lack flexural involvement, which is a telltale sign of atopic dermatitis, “so I really struggle with making the diagnosis of new onset AD in the elderly,” he said, adding that existing medical literature on the topic is variable, with the use of terms that include chronic eczematous eruption of the elderly, chronic “eczematiform” eruption in the elderly, chronic eczematous eruption of the aged, eczematous dermatitis not otherwise specified, dermal hypersensitivity reaction, urticarial dermatitis, and eczematous drug eruptions.
“Pruritus of the elderly is not a diagnosis,” Dr. Simpson said. “That’s just a symptom with a million etiologies. Never put that as your assessment. You could put pruritic eruption or pruritus, but try to look for the cause.”
More than 50% of older patients have xerosis, according to a 2013 clinical review on pruritus in the elderly, by Timothy G. Berger, MD, and colleagues at the University of California, San Francisco, which includes advice on the evaluation and management of pruritus in this group of patients based on whether they have a rash or not. For a patient with no rash, Dr. Simpson said, the workup “includes ruling out xerosis, scabies, and effects of medications that could cause rash such as narcotics and Adderall; as well as a generalized pruritus workup including renal and hepatic function, blood count, and thyroid levels.”
In a separate analysis of pruritic elderly patients by the same authors, five rash-related diagnoses accounted for 75% of cases: eczematous dermatitis, lichen simplex/prurigo nodularis, subacute prurigo, transient acantholytic dermatosis, and neuropathic disorder. “Morphology of pruritus with rash is also important,” Dr. Simpson added. “Is it eczematous? Papular? Prurigo nodularis? This helps lead you in the right direction.”
Some case-control studies have shown that calcium channel blockers could be related to eczema in older patients.
“But there aren’t a lot of studies out there that show that when you stop your calcium channel blocker, your eczema gets better,” Dr. Simpson said. “I’m reluctant to stop medications to try to help their eczema. I haven’t had many good results doing that.”
In an abstract presented during the 2021 annual meeting of the Society of Investigative Dermatology, he and his colleagues prospectively reviewed 89 patients over age 65 who had been referred with new-onset eczema. Of these, 34 underwent drug cessation trials for 1-3 months. “Not one patient improved when they stopped medications,” Dr. Simpson said, but “multiple patients were hospitalized for discontinuing their cardiac and antihypertensive medications.” While this was a biased sample of patients coming to him with chronic eczema, “in my experience, if you have chronic eczema in an older patient, stopping medications is likely not going to help.”
Other diagnostic tips he offered included asking patients what skin products they’re using, considering patch testing, and considering biopsy to rule out cutaneous T-cell lymphoma or bullous pemphigoid. “If you’re not sure there’s a rash, you might need to do a pruritus workup,” he said. If an eczematous rash is present and no other cause is found, try treating it like AD, he added.
Dr. Simpson reported serving as an investigator for and consultant to numerous pharmaceutical companies.
FROM REVOLUTIONIZING AD 2021
Uptake uncertain for potent new LDL-lowerer inclisiran
As inclisiran, a first-in-class LDL-cholesterol lowering drug, enters the U.S. market following Food and Drug Administration approval in December 2021, several issues muddy how popular inclisiran will be in actual practice. That’s despite stellar phase 3 trial evidence for safety, tolerability, and a potent lipid-lowering effect.
The active ingredient of inclisiran (Leqvio) is a small interfering RNA (siRNA) molecule that shuts down production of the PCSK9 (proprotein convertase subtilisin/kexin type 9) protein, an enzyme that’s made and functions primarily in the liver and degrades cellular receptors for LDL cholesterol. Inhibiting PCSK9 production means LDL-cholesterol receptors accumulate and boost the ability of liver cells to pull more LDL cholesterol out of blood.
PCSK9 inhibition is the most potent LDL-cholesterol lowering method now available, and it works well in patients who have maxed out LDL reduction by diet and statin treatment. The siRNA of inclisiran is tweaked to target the molecule to the surface of liver cells following subcutaneous injection. Other modifications of the siRNA give it stability that allows twice-a-year dosing, although patients receive a third injection during their first year to hasten a maximum treatment effect.
Inclisiran’s FDA approval relied on results from three pivotal trials that together enrolled 3,660 patients with either atherosclerotic cardiovascular disease (ASCVD), ASCVD risk equivalents, or heterozygous familial hypercholesterolemia (HeFH), and LDL-cholesterol levels of at least 70 mg/dL in those with established ASCVD, or at least 100 mg/dL in other patients. (HeFH and ASCVD are the drug’s approved indications.) Pooled data from the three trials showed that inclisiran was safe and well tolerated during 18 months and produced an average LDL-cholesterol reduction after 510 days (1.4 years) of about 51% compared to baseline after correction for placebo effects (J Am Coll Cardiol. 2021 Mar 9;77 [9]:1182-93).
These data showed inclisiran was about as safe and effective for reducing LDL-cholesterol as agents from another class of PCSK9 inhibitors that rely on injected antibodies to inactivate PCSK9. Two agents from this class, alirocumab (Praluent) and evolocumab (Repatha), both came on the U.S. market in 2015. Although their performance in routine practice during the ensuing 6-plus years has been as safe and effective as what they showed in their respective registration trials, they have faced a rocky uptake road that’s been primarily hindered by the hefty price tag that both drugs carry.
Prior-authorization blues
When they first came out, evolocumab and alirocumab were burdened by annual drug costs of roughly $14,000, a fact that led to widespread prior-authorization and copay barriers set up by U.S. insurers. Although these barriers gradually lessened over time, in part aided by a substantial price cut for both drugs that led to annual drug costs more in the range of $6,000/year, they remain relatively pricey and are still not easy to start in patients because of prior-authorization requirements, said clinicians.
Recent penetration of the older PCSK9 inhibitors into eligible U.S. patients “is only about 1%-2%, based on the latest data,” said Michael H. Davidson, MD, a lipid specialist and director of Preventive Cardiology at the University of Chicago.
“We have these great, effective drugs, but they haven’t really made an impact over the past 5 years,” because of very limited uptake, a situation Dr. Davidson called “very disappointing,” during an interview.
Given this recent history, inclisiran, another expensive PCSK9 inhibitor, may face similar coverage pushback as it hits the U.S. market with a retail price, announced by its manufacturer Novartis, of $3,250/dose. This means that patients who start the drug and receive their initial dose, a second dose after 3 months, and then additional doses every 6 months, rack up a drug cost of close to $10,000 the first year on the drug and $6,500 each subsequent year.
This treatment schedule highlights the major logistical difference that distinguishes inclisiran from the antibody-based PCSK9 inhibitors, which are given by repeated subcutaneous injection every 2 or 4 weeks, usually with patients self-injecting the drugs at home. The less-frequent dosing schedule for inclisiran prompted the drug’s developers to schedule injections by a clinician in an office setting in the pivotal trials, which led to labeling for inclisiran that specifies administration only by a health care professional.
The ‘buy-and-bill’ coverage model
This difference in drug administration between inclisiran and the antibody-based PCSK9 inhibitors set up Novartis to promote insurance reimbursement for inclisiran using a “buy-and-bill” paradigm that was first developed for oncology drugs and which may provide a loophole around the prior-authorization roadblocks that hindered early uptake of the antibody-based PCSK9 inhibitors.
It’s also an approach that has made U.S. clinicians unsure how it will play out in practice. Infrequent inclisiran dosing may also boost patient compliance.
“Adherence is the greatest challenge in preventive cardiology, and thus inclisiran has the potential to be a game changer,” commented Christie M. Ballantyne, MD, professor and chief of cardiology at Baylor College of Medicine, Houston.
“Will it be easier for physicians to write a prescription and for patients to get the medication without a demanding and frustrating prior-authorization process?” he wondered during an interview. “I’m waiting to see how this unfolds, especially in systems where pharmacy is not fully integrated with the outpatient setting. In some ways, this is as big of an experiment as was development of the drug,” Dr. Ballantyne said.
Although the prior-authorization hoops for evolocumab and alirocumab have become easier to jump through, “most physicians don’t have the resources to handle it and don’t bother,” noted Dr. Davidson, and he’s concerned that infrastructure challenges will also hamper the buy-and-bill strategy for inclisiran.
He also expressed skepticism that the prior-authorization barrier will disappear. “Payers don’t want to open a large population to a very expensive drug without some gatekeeping,” he said, while acknowledging that in late January 2022 he did not yet have personal experience administering inclisiran or navigating its insurance reimbursement.
Boosting patient compliance
Dr. Davidson agreed that the prospect for enhanced patient compliance with inclisiran was intriguing and had already drawn the interest of some of his patients.
“There is a lot of appeal” to a treatment that’s only given once every 6 months, he said. “Compliance is a major issue, and this is less work for patients.”
“The biggest possible attraction of inclisiran is that it is given twice a year, but whether this plays out as anticipated in the real world need to be seen,” cautioned Vijay Nambi, MD, a cardiologist at the Michael E. DeBakey VA Hospital, Houston, and at Baylor College of Medicine who has written about inclisiran. He noted that while two doses a year is “on paper very attractive,” this scheme opens the door to missed or delayed appointments because of vacations, other patient travel, or events like a pandemic.
“The biggest pro for inclisiran is the dosing schedule,” said Chandni Bardolia, PharmD, a drug information specialist at Tabula Rasa Healthcare, Moorestown, N.J., who has analyzed and written about inclisiran and other lipid-lowering medications. “Twice yearly dosing following initiation will be a huge benefit to improve adherence and reduce the number of injections.”
However, inclisiran’s attractive dosing schedule as well as its safety and potent efficacy do not tell the whole story, she highlighted in an interview.
Inclisiran’s clinical evidence still cooking
“I see inclisiran as a last-line drug, mainly because the current alternatives have more safety and efficacy data,” Dr. Bardolia said.
Inclisiran’s “cost and the fact that there are other agents with clinical outcome data already available [alirocumab and evolocumab] means inclisiran is not a first-line agent after statins,” agreed Dr. Nambi.
The FDA based its inclisiran approval entirely on the drug’s demonstrated safety and LDL-lowering efficacy. The cardiovascular outcomes trial for inclisiran, ORION-4, with about 15,000 enrolled patients, started in 2018 and remains in progress with full results expected in 2026.
The lack of clinical outcomes data for inclisiran is a major limitation, said Neil J. Stone, MD, a cardiologist and professor at Northwestern University, Chicago, and vice chair of the panel that wrote the most recent cholesterol guideline for the American College of Cardiology and American Heart Association.
“My greatest concern is the lack of outcome trial data. That’s very important,” Dr. Stone said in an interview.
But others minimize this limitation given the overwhelming evidence that links lower levels of LDL-cholesterol to reduced clinical events.
Most clinicians “support lower LDL as a surrogate” for reduced clinical events, “just like blood pressure and hemoglobin A1c,” noted Dr. Davidson, although he conceded that a “substantial minority wants to wait to see inclisiran’s outcome benefits.”
It’s all about price
While opinions are mixed on the need for clinical outcomes data, experts are more uniform in seeing drug prices that run to several thousands per year as the main uptake issue.
“We need to look at the cost-efficacy with inclisiran, and we need benefit data to determine this,” said Dr. Stone.
“Outcomes data are central to characterizing value. I imagine that costs will impact adoption and dissemination” of inclisiran, commented Paul L. Hess, MD, a cardiologist at the Rocky Mountain Regional VA Medical Center, Denver.
Patient interest in less frequent dosing will be important for driving use, but “ultimately cost will be the most important driving factor,” for inclisiran uptake, commented Robert H. Eckel, MD, an endocrinologist affiliated with the University of Colorado School of Medicine, Aurora.
Dr. Davidson has ties to New Amsterdam Pharma and Amgen, which markets evolocumab (Repatha). Dr. Ballantyne is a consultant to numerous companies, including Amgen and Regeneron, which market alirocumab (Praluent). Dr. Nambi has been a site investigator for studies sponsored by Amgen, and by Merck, which markets the LDL-cholesterol drug ezetimibe (Zetia) and is developing an oral PCSK9 inhibitor (he said that the views he expressed are his own and don’t represent that of the department of Veterans Affairs or Baylor.) Dr. Bardolia had no disclosures beyond her employment at Tabula Rasa Healthcare. Dr. Stone, Dr. Hess, and Dr. Eckel had no relevant disclosures.
As inclisiran, a first-in-class LDL-cholesterol lowering drug, enters the U.S. market following Food and Drug Administration approval in December 2021, several issues muddy how popular inclisiran will be in actual practice. That’s despite stellar phase 3 trial evidence for safety, tolerability, and a potent lipid-lowering effect.
The active ingredient of inclisiran (Leqvio) is a small interfering RNA (siRNA) molecule that shuts down production of the PCSK9 (proprotein convertase subtilisin/kexin type 9) protein, an enzyme that’s made and functions primarily in the liver and degrades cellular receptors for LDL cholesterol. Inhibiting PCSK9 production means LDL-cholesterol receptors accumulate and boost the ability of liver cells to pull more LDL cholesterol out of blood.
PCSK9 inhibition is the most potent LDL-cholesterol lowering method now available, and it works well in patients who have maxed out LDL reduction by diet and statin treatment. The siRNA of inclisiran is tweaked to target the molecule to the surface of liver cells following subcutaneous injection. Other modifications of the siRNA give it stability that allows twice-a-year dosing, although patients receive a third injection during their first year to hasten a maximum treatment effect.
Inclisiran’s FDA approval relied on results from three pivotal trials that together enrolled 3,660 patients with either atherosclerotic cardiovascular disease (ASCVD), ASCVD risk equivalents, or heterozygous familial hypercholesterolemia (HeFH), and LDL-cholesterol levels of at least 70 mg/dL in those with established ASCVD, or at least 100 mg/dL in other patients. (HeFH and ASCVD are the drug’s approved indications.) Pooled data from the three trials showed that inclisiran was safe and well tolerated during 18 months and produced an average LDL-cholesterol reduction after 510 days (1.4 years) of about 51% compared to baseline after correction for placebo effects (J Am Coll Cardiol. 2021 Mar 9;77 [9]:1182-93).
These data showed inclisiran was about as safe and effective for reducing LDL-cholesterol as agents from another class of PCSK9 inhibitors that rely on injected antibodies to inactivate PCSK9. Two agents from this class, alirocumab (Praluent) and evolocumab (Repatha), both came on the U.S. market in 2015. Although their performance in routine practice during the ensuing 6-plus years has been as safe and effective as what they showed in their respective registration trials, they have faced a rocky uptake road that’s been primarily hindered by the hefty price tag that both drugs carry.
Prior-authorization blues
When they first came out, evolocumab and alirocumab were burdened by annual drug costs of roughly $14,000, a fact that led to widespread prior-authorization and copay barriers set up by U.S. insurers. Although these barriers gradually lessened over time, in part aided by a substantial price cut for both drugs that led to annual drug costs more in the range of $6,000/year, they remain relatively pricey and are still not easy to start in patients because of prior-authorization requirements, said clinicians.
Recent penetration of the older PCSK9 inhibitors into eligible U.S. patients “is only about 1%-2%, based on the latest data,” said Michael H. Davidson, MD, a lipid specialist and director of Preventive Cardiology at the University of Chicago.
“We have these great, effective drugs, but they haven’t really made an impact over the past 5 years,” because of very limited uptake, a situation Dr. Davidson called “very disappointing,” during an interview.
Given this recent history, inclisiran, another expensive PCSK9 inhibitor, may face similar coverage pushback as it hits the U.S. market with a retail price, announced by its manufacturer Novartis, of $3,250/dose. This means that patients who start the drug and receive their initial dose, a second dose after 3 months, and then additional doses every 6 months, rack up a drug cost of close to $10,000 the first year on the drug and $6,500 each subsequent year.
This treatment schedule highlights the major logistical difference that distinguishes inclisiran from the antibody-based PCSK9 inhibitors, which are given by repeated subcutaneous injection every 2 or 4 weeks, usually with patients self-injecting the drugs at home. The less-frequent dosing schedule for inclisiran prompted the drug’s developers to schedule injections by a clinician in an office setting in the pivotal trials, which led to labeling for inclisiran that specifies administration only by a health care professional.
The ‘buy-and-bill’ coverage model
This difference in drug administration between inclisiran and the antibody-based PCSK9 inhibitors set up Novartis to promote insurance reimbursement for inclisiran using a “buy-and-bill” paradigm that was first developed for oncology drugs and which may provide a loophole around the prior-authorization roadblocks that hindered early uptake of the antibody-based PCSK9 inhibitors.
It’s also an approach that has made U.S. clinicians unsure how it will play out in practice. Infrequent inclisiran dosing may also boost patient compliance.
“Adherence is the greatest challenge in preventive cardiology, and thus inclisiran has the potential to be a game changer,” commented Christie M. Ballantyne, MD, professor and chief of cardiology at Baylor College of Medicine, Houston.
“Will it be easier for physicians to write a prescription and for patients to get the medication without a demanding and frustrating prior-authorization process?” he wondered during an interview. “I’m waiting to see how this unfolds, especially in systems where pharmacy is not fully integrated with the outpatient setting. In some ways, this is as big of an experiment as was development of the drug,” Dr. Ballantyne said.
Although the prior-authorization hoops for evolocumab and alirocumab have become easier to jump through, “most physicians don’t have the resources to handle it and don’t bother,” noted Dr. Davidson, and he’s concerned that infrastructure challenges will also hamper the buy-and-bill strategy for inclisiran.
He also expressed skepticism that the prior-authorization barrier will disappear. “Payers don’t want to open a large population to a very expensive drug without some gatekeeping,” he said, while acknowledging that in late January 2022 he did not yet have personal experience administering inclisiran or navigating its insurance reimbursement.
Boosting patient compliance
Dr. Davidson agreed that the prospect for enhanced patient compliance with inclisiran was intriguing and had already drawn the interest of some of his patients.
“There is a lot of appeal” to a treatment that’s only given once every 6 months, he said. “Compliance is a major issue, and this is less work for patients.”
“The biggest possible attraction of inclisiran is that it is given twice a year, but whether this plays out as anticipated in the real world need to be seen,” cautioned Vijay Nambi, MD, a cardiologist at the Michael E. DeBakey VA Hospital, Houston, and at Baylor College of Medicine who has written about inclisiran. He noted that while two doses a year is “on paper very attractive,” this scheme opens the door to missed or delayed appointments because of vacations, other patient travel, or events like a pandemic.
“The biggest pro for inclisiran is the dosing schedule,” said Chandni Bardolia, PharmD, a drug information specialist at Tabula Rasa Healthcare, Moorestown, N.J., who has analyzed and written about inclisiran and other lipid-lowering medications. “Twice yearly dosing following initiation will be a huge benefit to improve adherence and reduce the number of injections.”
However, inclisiran’s attractive dosing schedule as well as its safety and potent efficacy do not tell the whole story, she highlighted in an interview.
Inclisiran’s clinical evidence still cooking
“I see inclisiran as a last-line drug, mainly because the current alternatives have more safety and efficacy data,” Dr. Bardolia said.
Inclisiran’s “cost and the fact that there are other agents with clinical outcome data already available [alirocumab and evolocumab] means inclisiran is not a first-line agent after statins,” agreed Dr. Nambi.
The FDA based its inclisiran approval entirely on the drug’s demonstrated safety and LDL-lowering efficacy. The cardiovascular outcomes trial for inclisiran, ORION-4, with about 15,000 enrolled patients, started in 2018 and remains in progress with full results expected in 2026.
The lack of clinical outcomes data for inclisiran is a major limitation, said Neil J. Stone, MD, a cardiologist and professor at Northwestern University, Chicago, and vice chair of the panel that wrote the most recent cholesterol guideline for the American College of Cardiology and American Heart Association.
“My greatest concern is the lack of outcome trial data. That’s very important,” Dr. Stone said in an interview.
But others minimize this limitation given the overwhelming evidence that links lower levels of LDL-cholesterol to reduced clinical events.
Most clinicians “support lower LDL as a surrogate” for reduced clinical events, “just like blood pressure and hemoglobin A1c,” noted Dr. Davidson, although he conceded that a “substantial minority wants to wait to see inclisiran’s outcome benefits.”
It’s all about price
While opinions are mixed on the need for clinical outcomes data, experts are more uniform in seeing drug prices that run to several thousands per year as the main uptake issue.
“We need to look at the cost-efficacy with inclisiran, and we need benefit data to determine this,” said Dr. Stone.
“Outcomes data are central to characterizing value. I imagine that costs will impact adoption and dissemination” of inclisiran, commented Paul L. Hess, MD, a cardiologist at the Rocky Mountain Regional VA Medical Center, Denver.
Patient interest in less frequent dosing will be important for driving use, but “ultimately cost will be the most important driving factor,” for inclisiran uptake, commented Robert H. Eckel, MD, an endocrinologist affiliated with the University of Colorado School of Medicine, Aurora.
Dr. Davidson has ties to New Amsterdam Pharma and Amgen, which markets evolocumab (Repatha). Dr. Ballantyne is a consultant to numerous companies, including Amgen and Regeneron, which market alirocumab (Praluent). Dr. Nambi has been a site investigator for studies sponsored by Amgen, and by Merck, which markets the LDL-cholesterol drug ezetimibe (Zetia) and is developing an oral PCSK9 inhibitor (he said that the views he expressed are his own and don’t represent that of the department of Veterans Affairs or Baylor.) Dr. Bardolia had no disclosures beyond her employment at Tabula Rasa Healthcare. Dr. Stone, Dr. Hess, and Dr. Eckel had no relevant disclosures.
As inclisiran, a first-in-class LDL-cholesterol lowering drug, enters the U.S. market following Food and Drug Administration approval in December 2021, several issues muddy how popular inclisiran will be in actual practice. That’s despite stellar phase 3 trial evidence for safety, tolerability, and a potent lipid-lowering effect.
The active ingredient of inclisiran (Leqvio) is a small interfering RNA (siRNA) molecule that shuts down production of the PCSK9 (proprotein convertase subtilisin/kexin type 9) protein, an enzyme that’s made and functions primarily in the liver and degrades cellular receptors for LDL cholesterol. Inhibiting PCSK9 production means LDL-cholesterol receptors accumulate and boost the ability of liver cells to pull more LDL cholesterol out of blood.
PCSK9 inhibition is the most potent LDL-cholesterol lowering method now available, and it works well in patients who have maxed out LDL reduction by diet and statin treatment. The siRNA of inclisiran is tweaked to target the molecule to the surface of liver cells following subcutaneous injection. Other modifications of the siRNA give it stability that allows twice-a-year dosing, although patients receive a third injection during their first year to hasten a maximum treatment effect.
Inclisiran’s FDA approval relied on results from three pivotal trials that together enrolled 3,660 patients with either atherosclerotic cardiovascular disease (ASCVD), ASCVD risk equivalents, or heterozygous familial hypercholesterolemia (HeFH), and LDL-cholesterol levels of at least 70 mg/dL in those with established ASCVD, or at least 100 mg/dL in other patients. (HeFH and ASCVD are the drug’s approved indications.) Pooled data from the three trials showed that inclisiran was safe and well tolerated during 18 months and produced an average LDL-cholesterol reduction after 510 days (1.4 years) of about 51% compared to baseline after correction for placebo effects (J Am Coll Cardiol. 2021 Mar 9;77 [9]:1182-93).
These data showed inclisiran was about as safe and effective for reducing LDL-cholesterol as agents from another class of PCSK9 inhibitors that rely on injected antibodies to inactivate PCSK9. Two agents from this class, alirocumab (Praluent) and evolocumab (Repatha), both came on the U.S. market in 2015. Although their performance in routine practice during the ensuing 6-plus years has been as safe and effective as what they showed in their respective registration trials, they have faced a rocky uptake road that’s been primarily hindered by the hefty price tag that both drugs carry.
Prior-authorization blues
When they first came out, evolocumab and alirocumab were burdened by annual drug costs of roughly $14,000, a fact that led to widespread prior-authorization and copay barriers set up by U.S. insurers. Although these barriers gradually lessened over time, in part aided by a substantial price cut for both drugs that led to annual drug costs more in the range of $6,000/year, they remain relatively pricey and are still not easy to start in patients because of prior-authorization requirements, said clinicians.
Recent penetration of the older PCSK9 inhibitors into eligible U.S. patients “is only about 1%-2%, based on the latest data,” said Michael H. Davidson, MD, a lipid specialist and director of Preventive Cardiology at the University of Chicago.
“We have these great, effective drugs, but they haven’t really made an impact over the past 5 years,” because of very limited uptake, a situation Dr. Davidson called “very disappointing,” during an interview.
Given this recent history, inclisiran, another expensive PCSK9 inhibitor, may face similar coverage pushback as it hits the U.S. market with a retail price, announced by its manufacturer Novartis, of $3,250/dose. This means that patients who start the drug and receive their initial dose, a second dose after 3 months, and then additional doses every 6 months, rack up a drug cost of close to $10,000 the first year on the drug and $6,500 each subsequent year.
This treatment schedule highlights the major logistical difference that distinguishes inclisiran from the antibody-based PCSK9 inhibitors, which are given by repeated subcutaneous injection every 2 or 4 weeks, usually with patients self-injecting the drugs at home. The less-frequent dosing schedule for inclisiran prompted the drug’s developers to schedule injections by a clinician in an office setting in the pivotal trials, which led to labeling for inclisiran that specifies administration only by a health care professional.
The ‘buy-and-bill’ coverage model
This difference in drug administration between inclisiran and the antibody-based PCSK9 inhibitors set up Novartis to promote insurance reimbursement for inclisiran using a “buy-and-bill” paradigm that was first developed for oncology drugs and which may provide a loophole around the prior-authorization roadblocks that hindered early uptake of the antibody-based PCSK9 inhibitors.
It’s also an approach that has made U.S. clinicians unsure how it will play out in practice. Infrequent inclisiran dosing may also boost patient compliance.
“Adherence is the greatest challenge in preventive cardiology, and thus inclisiran has the potential to be a game changer,” commented Christie M. Ballantyne, MD, professor and chief of cardiology at Baylor College of Medicine, Houston.
“Will it be easier for physicians to write a prescription and for patients to get the medication without a demanding and frustrating prior-authorization process?” he wondered during an interview. “I’m waiting to see how this unfolds, especially in systems where pharmacy is not fully integrated with the outpatient setting. In some ways, this is as big of an experiment as was development of the drug,” Dr. Ballantyne said.
Although the prior-authorization hoops for evolocumab and alirocumab have become easier to jump through, “most physicians don’t have the resources to handle it and don’t bother,” noted Dr. Davidson, and he’s concerned that infrastructure challenges will also hamper the buy-and-bill strategy for inclisiran.
He also expressed skepticism that the prior-authorization barrier will disappear. “Payers don’t want to open a large population to a very expensive drug without some gatekeeping,” he said, while acknowledging that in late January 2022 he did not yet have personal experience administering inclisiran or navigating its insurance reimbursement.
Boosting patient compliance
Dr. Davidson agreed that the prospect for enhanced patient compliance with inclisiran was intriguing and had already drawn the interest of some of his patients.
“There is a lot of appeal” to a treatment that’s only given once every 6 months, he said. “Compliance is a major issue, and this is less work for patients.”
“The biggest possible attraction of inclisiran is that it is given twice a year, but whether this plays out as anticipated in the real world need to be seen,” cautioned Vijay Nambi, MD, a cardiologist at the Michael E. DeBakey VA Hospital, Houston, and at Baylor College of Medicine who has written about inclisiran. He noted that while two doses a year is “on paper very attractive,” this scheme opens the door to missed or delayed appointments because of vacations, other patient travel, or events like a pandemic.
“The biggest pro for inclisiran is the dosing schedule,” said Chandni Bardolia, PharmD, a drug information specialist at Tabula Rasa Healthcare, Moorestown, N.J., who has analyzed and written about inclisiran and other lipid-lowering medications. “Twice yearly dosing following initiation will be a huge benefit to improve adherence and reduce the number of injections.”
However, inclisiran’s attractive dosing schedule as well as its safety and potent efficacy do not tell the whole story, she highlighted in an interview.
Inclisiran’s clinical evidence still cooking
“I see inclisiran as a last-line drug, mainly because the current alternatives have more safety and efficacy data,” Dr. Bardolia said.
Inclisiran’s “cost and the fact that there are other agents with clinical outcome data already available [alirocumab and evolocumab] means inclisiran is not a first-line agent after statins,” agreed Dr. Nambi.
The FDA based its inclisiran approval entirely on the drug’s demonstrated safety and LDL-lowering efficacy. The cardiovascular outcomes trial for inclisiran, ORION-4, with about 15,000 enrolled patients, started in 2018 and remains in progress with full results expected in 2026.
The lack of clinical outcomes data for inclisiran is a major limitation, said Neil J. Stone, MD, a cardiologist and professor at Northwestern University, Chicago, and vice chair of the panel that wrote the most recent cholesterol guideline for the American College of Cardiology and American Heart Association.
“My greatest concern is the lack of outcome trial data. That’s very important,” Dr. Stone said in an interview.
But others minimize this limitation given the overwhelming evidence that links lower levels of LDL-cholesterol to reduced clinical events.
Most clinicians “support lower LDL as a surrogate” for reduced clinical events, “just like blood pressure and hemoglobin A1c,” noted Dr. Davidson, although he conceded that a “substantial minority wants to wait to see inclisiran’s outcome benefits.”
It’s all about price
While opinions are mixed on the need for clinical outcomes data, experts are more uniform in seeing drug prices that run to several thousands per year as the main uptake issue.
“We need to look at the cost-efficacy with inclisiran, and we need benefit data to determine this,” said Dr. Stone.
“Outcomes data are central to characterizing value. I imagine that costs will impact adoption and dissemination” of inclisiran, commented Paul L. Hess, MD, a cardiologist at the Rocky Mountain Regional VA Medical Center, Denver.
Patient interest in less frequent dosing will be important for driving use, but “ultimately cost will be the most important driving factor,” for inclisiran uptake, commented Robert H. Eckel, MD, an endocrinologist affiliated with the University of Colorado School of Medicine, Aurora.
Dr. Davidson has ties to New Amsterdam Pharma and Amgen, which markets evolocumab (Repatha). Dr. Ballantyne is a consultant to numerous companies, including Amgen and Regeneron, which market alirocumab (Praluent). Dr. Nambi has been a site investigator for studies sponsored by Amgen, and by Merck, which markets the LDL-cholesterol drug ezetimibe (Zetia) and is developing an oral PCSK9 inhibitor (he said that the views he expressed are his own and don’t represent that of the department of Veterans Affairs or Baylor.) Dr. Bardolia had no disclosures beyond her employment at Tabula Rasa Healthcare. Dr. Stone, Dr. Hess, and Dr. Eckel had no relevant disclosures.
Presence of autoantibodies most predictive of long COVID in study
Other significant early predictors of prolonged COVID symptoms – which the researchers called postacute sequelae – were having type 2 diabetes, SARS-CoV-2 RNAemia, and Epstein-Barr virus (EBV) viremia, Yapeng Su, PhD, of the Institute for Systems Biology (ISB) in Seattle, and colleagues wrote in Cell.
Having EBV viremia suggested that latent EBV has been reactivated, the authors noted.
“The most important postacute sequelae [that is conditions that are consequences of a disease] of COVID is the presence of autoantibodies,” James R. Heath, PhD, president of ISB and a bioengineering professor at the University of Washington, Seattle, said in an interview. “It’s about two times more important than the others.”
Dr. Heath and coauthors said early detection of this and other variables could prompt earlier aggressive treatment in patients susceptible to long COVID and ward off lingering symptoms.
“These predictive measures of long COVID can also help to better inform patients of their possible disease course,” study coauthor Daniel G. Chen, an undergraduate researcher at ISB, said in an interview. “We were also able to partially resolve the immunological underpinnings of some postacute sequelae of COVID in a way that suggested potential therapies, and the timing of those therapies.”
For example, he continued, the use of antivirals very early in the infectious course may mitigate the later development of long COVID. “This will, of course, have to be explored in an appropriately designed clinical trial.
“We also identified biomarkers of certain types of long COVID, such as neurological sequelae. Those biomarkers can help define the condition, which is a first step towards developing treatments.”
Study findings
With COVID patients monitored for 2 or 3 months, the study findings of the international “multiomic profiling” analysis include:
- Subclinical patient autoantibodies that reduce anti–SARS-CoV-2 antibodies suggest there is immune dysregulation during COVID-19 infection.
- Reactivation of latent other viruses during initial infection may be contributing to long COVID.
- Gastrointestinal postacute sequelae of COVID presents with a unique postacute expansion of cytotoxic T cells.
- SARS-CoV-2–specific and cytomegalovirus-specific CD8+ T cells displayed unique dynamics during recovery from infection.
According to the authors, as many as 69% of COVID-19 patients suffer from long COVID – a range of new, recurrent, or ongoing problems 4 or more weeks following initial SARS-CoV-2 infection. These may include memory loss, gastrointestinal distress, fatigue, anosmia, and shortness of breath.
Long COVID has been associated with acute disease severity, and is suspected to be related to autoimmune factors and unresolved viral fragments, according to the paper.
Research methods
The international study did a deep and detailed dive into multiple molecular markers of long COVID. It enrolled 209 COVID-19 patients with varying degrees of disease severity and matched them to 457 healthy controls. The researchers’ goal was to identify discrete and quantifiable long COVID factors and guide possible preemptive treatment.
Patients were assessed at three time points: at initial diagnosis, during the acute disease phase about a week later, and again 2 to 3 months post onset of symptoms after recovery from the acute phase of COVID. At the third assessment, some patients had lingering symptoms such as fatigue (52% ), cough (25%), and loss of taste or sense of smell (18%).
Blood draws were analyzed for autoantibodies and SARS-CoV-2–specific antibodies, global plasma proteomic and metabolomic profiles, and single-cell multiomic characterizations of peripheral blood mononuclear cells.
Each blood draw was paired with nasal-swab and plasma measurements of SARS-CoV-2 viral load and the data sets were integrated with electronic health records and self-reported patient symptoms to guide the interpretation of the molecular signatures of long COVID.
Author conclusions
The authors found an association between T2 hyperinflammation and long COVID–anticipating autoantibodies. This association further implies that hyperinflammation-controlling therapies in the acute stage of COVID may influence whether a patient experiences long COVID. “However, the detailed timing and context of these therapies matter, and, thus, future well-controlled studies will be needed to test these and other therapeutic implications,” Dr. Su and colleagues wrote.
Moreover, the negative correlations between anti–SARS-CoV-2 IgG and certain autoantibodies may suggest that patients with elevated autoantibody levels are more susceptible to breakthrough infections, the authors said.
“Many patients with high autoantibodies simultaneously have low protective antibodies that neutralize SARS-CoV-2, and that’s going to make them more susceptible to breakthrough infections,” Mr. Chen explained.*
“Detectability of most [long COVID-19 factors] at COVID diagnosis emphasizes the importance of early disease measurements for understanding emergent chronic conditions and suggests [long COVID] treatment strategies,” they wrote.
According to Mr. Chen, there are clear similarities in underlying immunobiology between patients with COVID autoantibodies and patients with systemic lupus erythematosus.
“These findings are also helping us frame our thinking around other chronic autoimmune conditions, such as postacute Lyme syndrome, for example,” said Dr. Heath.
The bottom line, said Mr. Chen, is that measuring early long COVID indicators may result in preventive treatments. “An example is the cortisol deficiency we see in certain long COVID patients. There are known treatments such as cortisol replacement therapy that should be explored for this group.”
Outside expert’s take on findings
Commenting on the study, Sherry Hsiang-Yi Chou, MD, who was not involved in the research, called the study a very important first step in understanding the path of this complex phenomenon and perhaps other conditions with long-term side effects.
“The researchers have done huge amount of innovative scientific work. They’ve shown the DNA signature of how our bodies respond to this disease,” said Dr. Chou, who is chief of the division of neurocritical care at Northwestern Medicine in Chicago.
“This type of research will help us scientifically understand and differentiate the various syndromes within long COVID. It will help identify who’s at risk for different aspects of this syndrome and lead to following them for longer periods in clinical trials,” she added.
The authors acknowledged that lengthier studies in larger cohorts were needed to see which patients will develop long-term chronic postacute sequelae of COVID.
This research was supported by the Wilke Family Foundation, the Parker Institute for Cancer Immunotherapy, Merck, and the Biomedical Advanced Research and Development Authority. Other support came from the National Institutes of Health, the Bill and Melinda Gates Foundation, Saint John’s Cancer Center, Fred Hutchinson Cancer Research Center, and the European Union’s Horizon 2020 research and innovation program. Dr. Heath is a cofounder of Pact Pharma. He and several coauthors disclosed various ties to multiple private-sector companies. Mr. Chen and Dr. Chou had no competing interests.
*Correction, 1/28: An earlier version of this story misidentified Daniel G. Chen, an undergraduate researcher at ISB.
Other significant early predictors of prolonged COVID symptoms – which the researchers called postacute sequelae – were having type 2 diabetes, SARS-CoV-2 RNAemia, and Epstein-Barr virus (EBV) viremia, Yapeng Su, PhD, of the Institute for Systems Biology (ISB) in Seattle, and colleagues wrote in Cell.
Having EBV viremia suggested that latent EBV has been reactivated, the authors noted.
“The most important postacute sequelae [that is conditions that are consequences of a disease] of COVID is the presence of autoantibodies,” James R. Heath, PhD, president of ISB and a bioengineering professor at the University of Washington, Seattle, said in an interview. “It’s about two times more important than the others.”
Dr. Heath and coauthors said early detection of this and other variables could prompt earlier aggressive treatment in patients susceptible to long COVID and ward off lingering symptoms.
“These predictive measures of long COVID can also help to better inform patients of their possible disease course,” study coauthor Daniel G. Chen, an undergraduate researcher at ISB, said in an interview. “We were also able to partially resolve the immunological underpinnings of some postacute sequelae of COVID in a way that suggested potential therapies, and the timing of those therapies.”
For example, he continued, the use of antivirals very early in the infectious course may mitigate the later development of long COVID. “This will, of course, have to be explored in an appropriately designed clinical trial.
“We also identified biomarkers of certain types of long COVID, such as neurological sequelae. Those biomarkers can help define the condition, which is a first step towards developing treatments.”
Study findings
With COVID patients monitored for 2 or 3 months, the study findings of the international “multiomic profiling” analysis include:
- Subclinical patient autoantibodies that reduce anti–SARS-CoV-2 antibodies suggest there is immune dysregulation during COVID-19 infection.
- Reactivation of latent other viruses during initial infection may be contributing to long COVID.
- Gastrointestinal postacute sequelae of COVID presents with a unique postacute expansion of cytotoxic T cells.
- SARS-CoV-2–specific and cytomegalovirus-specific CD8+ T cells displayed unique dynamics during recovery from infection.
According to the authors, as many as 69% of COVID-19 patients suffer from long COVID – a range of new, recurrent, or ongoing problems 4 or more weeks following initial SARS-CoV-2 infection. These may include memory loss, gastrointestinal distress, fatigue, anosmia, and shortness of breath.
Long COVID has been associated with acute disease severity, and is suspected to be related to autoimmune factors and unresolved viral fragments, according to the paper.
Research methods
The international study did a deep and detailed dive into multiple molecular markers of long COVID. It enrolled 209 COVID-19 patients with varying degrees of disease severity and matched them to 457 healthy controls. The researchers’ goal was to identify discrete and quantifiable long COVID factors and guide possible preemptive treatment.
Patients were assessed at three time points: at initial diagnosis, during the acute disease phase about a week later, and again 2 to 3 months post onset of symptoms after recovery from the acute phase of COVID. At the third assessment, some patients had lingering symptoms such as fatigue (52% ), cough (25%), and loss of taste or sense of smell (18%).
Blood draws were analyzed for autoantibodies and SARS-CoV-2–specific antibodies, global plasma proteomic and metabolomic profiles, and single-cell multiomic characterizations of peripheral blood mononuclear cells.
Each blood draw was paired with nasal-swab and plasma measurements of SARS-CoV-2 viral load and the data sets were integrated with electronic health records and self-reported patient symptoms to guide the interpretation of the molecular signatures of long COVID.
Author conclusions
The authors found an association between T2 hyperinflammation and long COVID–anticipating autoantibodies. This association further implies that hyperinflammation-controlling therapies in the acute stage of COVID may influence whether a patient experiences long COVID. “However, the detailed timing and context of these therapies matter, and, thus, future well-controlled studies will be needed to test these and other therapeutic implications,” Dr. Su and colleagues wrote.
Moreover, the negative correlations between anti–SARS-CoV-2 IgG and certain autoantibodies may suggest that patients with elevated autoantibody levels are more susceptible to breakthrough infections, the authors said.
“Many patients with high autoantibodies simultaneously have low protective antibodies that neutralize SARS-CoV-2, and that’s going to make them more susceptible to breakthrough infections,” Mr. Chen explained.*
“Detectability of most [long COVID-19 factors] at COVID diagnosis emphasizes the importance of early disease measurements for understanding emergent chronic conditions and suggests [long COVID] treatment strategies,” they wrote.
According to Mr. Chen, there are clear similarities in underlying immunobiology between patients with COVID autoantibodies and patients with systemic lupus erythematosus.
“These findings are also helping us frame our thinking around other chronic autoimmune conditions, such as postacute Lyme syndrome, for example,” said Dr. Heath.
The bottom line, said Mr. Chen, is that measuring early long COVID indicators may result in preventive treatments. “An example is the cortisol deficiency we see in certain long COVID patients. There are known treatments such as cortisol replacement therapy that should be explored for this group.”
Outside expert’s take on findings
Commenting on the study, Sherry Hsiang-Yi Chou, MD, who was not involved in the research, called the study a very important first step in understanding the path of this complex phenomenon and perhaps other conditions with long-term side effects.
“The researchers have done huge amount of innovative scientific work. They’ve shown the DNA signature of how our bodies respond to this disease,” said Dr. Chou, who is chief of the division of neurocritical care at Northwestern Medicine in Chicago.
“This type of research will help us scientifically understand and differentiate the various syndromes within long COVID. It will help identify who’s at risk for different aspects of this syndrome and lead to following them for longer periods in clinical trials,” she added.
The authors acknowledged that lengthier studies in larger cohorts were needed to see which patients will develop long-term chronic postacute sequelae of COVID.
This research was supported by the Wilke Family Foundation, the Parker Institute for Cancer Immunotherapy, Merck, and the Biomedical Advanced Research and Development Authority. Other support came from the National Institutes of Health, the Bill and Melinda Gates Foundation, Saint John’s Cancer Center, Fred Hutchinson Cancer Research Center, and the European Union’s Horizon 2020 research and innovation program. Dr. Heath is a cofounder of Pact Pharma. He and several coauthors disclosed various ties to multiple private-sector companies. Mr. Chen and Dr. Chou had no competing interests.
*Correction, 1/28: An earlier version of this story misidentified Daniel G. Chen, an undergraduate researcher at ISB.
Other significant early predictors of prolonged COVID symptoms – which the researchers called postacute sequelae – were having type 2 diabetes, SARS-CoV-2 RNAemia, and Epstein-Barr virus (EBV) viremia, Yapeng Su, PhD, of the Institute for Systems Biology (ISB) in Seattle, and colleagues wrote in Cell.
Having EBV viremia suggested that latent EBV has been reactivated, the authors noted.
“The most important postacute sequelae [that is conditions that are consequences of a disease] of COVID is the presence of autoantibodies,” James R. Heath, PhD, president of ISB and a bioengineering professor at the University of Washington, Seattle, said in an interview. “It’s about two times more important than the others.”
Dr. Heath and coauthors said early detection of this and other variables could prompt earlier aggressive treatment in patients susceptible to long COVID and ward off lingering symptoms.
“These predictive measures of long COVID can also help to better inform patients of their possible disease course,” study coauthor Daniel G. Chen, an undergraduate researcher at ISB, said in an interview. “We were also able to partially resolve the immunological underpinnings of some postacute sequelae of COVID in a way that suggested potential therapies, and the timing of those therapies.”
For example, he continued, the use of antivirals very early in the infectious course may mitigate the later development of long COVID. “This will, of course, have to be explored in an appropriately designed clinical trial.
“We also identified biomarkers of certain types of long COVID, such as neurological sequelae. Those biomarkers can help define the condition, which is a first step towards developing treatments.”
Study findings
With COVID patients monitored for 2 or 3 months, the study findings of the international “multiomic profiling” analysis include:
- Subclinical patient autoantibodies that reduce anti–SARS-CoV-2 antibodies suggest there is immune dysregulation during COVID-19 infection.
- Reactivation of latent other viruses during initial infection may be contributing to long COVID.
- Gastrointestinal postacute sequelae of COVID presents with a unique postacute expansion of cytotoxic T cells.
- SARS-CoV-2–specific and cytomegalovirus-specific CD8+ T cells displayed unique dynamics during recovery from infection.
According to the authors, as many as 69% of COVID-19 patients suffer from long COVID – a range of new, recurrent, or ongoing problems 4 or more weeks following initial SARS-CoV-2 infection. These may include memory loss, gastrointestinal distress, fatigue, anosmia, and shortness of breath.
Long COVID has been associated with acute disease severity, and is suspected to be related to autoimmune factors and unresolved viral fragments, according to the paper.
Research methods
The international study did a deep and detailed dive into multiple molecular markers of long COVID. It enrolled 209 COVID-19 patients with varying degrees of disease severity and matched them to 457 healthy controls. The researchers’ goal was to identify discrete and quantifiable long COVID factors and guide possible preemptive treatment.
Patients were assessed at three time points: at initial diagnosis, during the acute disease phase about a week later, and again 2 to 3 months post onset of symptoms after recovery from the acute phase of COVID. At the third assessment, some patients had lingering symptoms such as fatigue (52% ), cough (25%), and loss of taste or sense of smell (18%).
Blood draws were analyzed for autoantibodies and SARS-CoV-2–specific antibodies, global plasma proteomic and metabolomic profiles, and single-cell multiomic characterizations of peripheral blood mononuclear cells.
Each blood draw was paired with nasal-swab and plasma measurements of SARS-CoV-2 viral load and the data sets were integrated with electronic health records and self-reported patient symptoms to guide the interpretation of the molecular signatures of long COVID.
Author conclusions
The authors found an association between T2 hyperinflammation and long COVID–anticipating autoantibodies. This association further implies that hyperinflammation-controlling therapies in the acute stage of COVID may influence whether a patient experiences long COVID. “However, the detailed timing and context of these therapies matter, and, thus, future well-controlled studies will be needed to test these and other therapeutic implications,” Dr. Su and colleagues wrote.
Moreover, the negative correlations between anti–SARS-CoV-2 IgG and certain autoantibodies may suggest that patients with elevated autoantibody levels are more susceptible to breakthrough infections, the authors said.
“Many patients with high autoantibodies simultaneously have low protective antibodies that neutralize SARS-CoV-2, and that’s going to make them more susceptible to breakthrough infections,” Mr. Chen explained.*
“Detectability of most [long COVID-19 factors] at COVID diagnosis emphasizes the importance of early disease measurements for understanding emergent chronic conditions and suggests [long COVID] treatment strategies,” they wrote.
According to Mr. Chen, there are clear similarities in underlying immunobiology between patients with COVID autoantibodies and patients with systemic lupus erythematosus.
“These findings are also helping us frame our thinking around other chronic autoimmune conditions, such as postacute Lyme syndrome, for example,” said Dr. Heath.
The bottom line, said Mr. Chen, is that measuring early long COVID indicators may result in preventive treatments. “An example is the cortisol deficiency we see in certain long COVID patients. There are known treatments such as cortisol replacement therapy that should be explored for this group.”
Outside expert’s take on findings
Commenting on the study, Sherry Hsiang-Yi Chou, MD, who was not involved in the research, called the study a very important first step in understanding the path of this complex phenomenon and perhaps other conditions with long-term side effects.
“The researchers have done huge amount of innovative scientific work. They’ve shown the DNA signature of how our bodies respond to this disease,” said Dr. Chou, who is chief of the division of neurocritical care at Northwestern Medicine in Chicago.
“This type of research will help us scientifically understand and differentiate the various syndromes within long COVID. It will help identify who’s at risk for different aspects of this syndrome and lead to following them for longer periods in clinical trials,” she added.
The authors acknowledged that lengthier studies in larger cohorts were needed to see which patients will develop long-term chronic postacute sequelae of COVID.
This research was supported by the Wilke Family Foundation, the Parker Institute for Cancer Immunotherapy, Merck, and the Biomedical Advanced Research and Development Authority. Other support came from the National Institutes of Health, the Bill and Melinda Gates Foundation, Saint John’s Cancer Center, Fred Hutchinson Cancer Research Center, and the European Union’s Horizon 2020 research and innovation program. Dr. Heath is a cofounder of Pact Pharma. He and several coauthors disclosed various ties to multiple private-sector companies. Mr. Chen and Dr. Chou had no competing interests.
*Correction, 1/28: An earlier version of this story misidentified Daniel G. Chen, an undergraduate researcher at ISB.
FROM CELL
Management of Opioid Use Disorder in Primary Care Settings With a Focus on Long-Acting Medication Formulations
After participating in the activity, PCPs should be able to:
- Assess a patient with possible signs and symptoms of opioid use disorder
- Identify criteria necessary to make a diagnosis of opioid use disorder
- Recognize factors that should be considered to tailor treatments for patients with opioid use disorder
- Select the best treatment option for patients with opioid use disorder
Click here to access this content now
After participating in the activity, PCPs should be able to:
- Assess a patient with possible signs and symptoms of opioid use disorder
- Identify criteria necessary to make a diagnosis of opioid use disorder
- Recognize factors that should be considered to tailor treatments for patients with opioid use disorder
- Select the best treatment option for patients with opioid use disorder
Click here to access this content now
After participating in the activity, PCPs should be able to:
- Assess a patient with possible signs and symptoms of opioid use disorder
- Identify criteria necessary to make a diagnosis of opioid use disorder
- Recognize factors that should be considered to tailor treatments for patients with opioid use disorder
- Select the best treatment option for patients with opioid use disorder
Click here to access this content now