User login
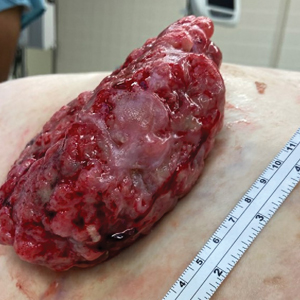
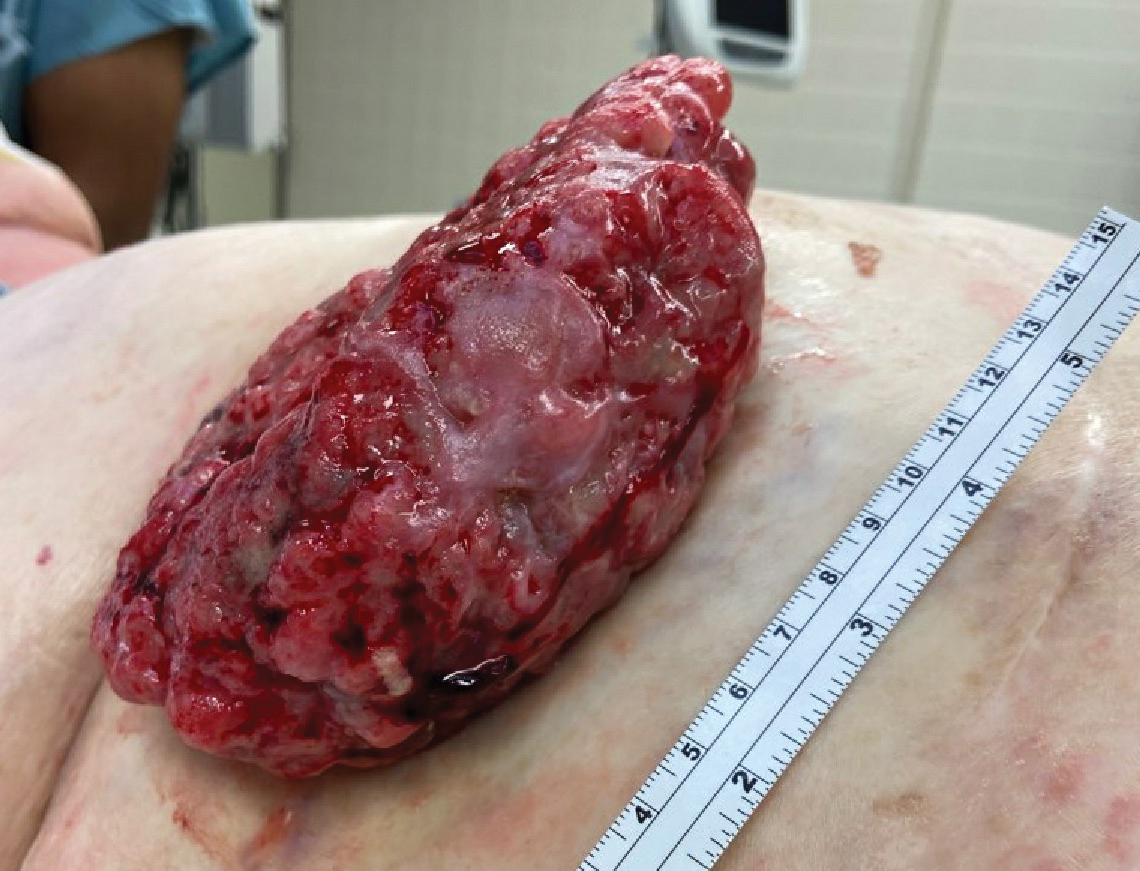
Fungating Mass on the Abdominal Wall
The Diagnosis: Basal Cell Carcinoma
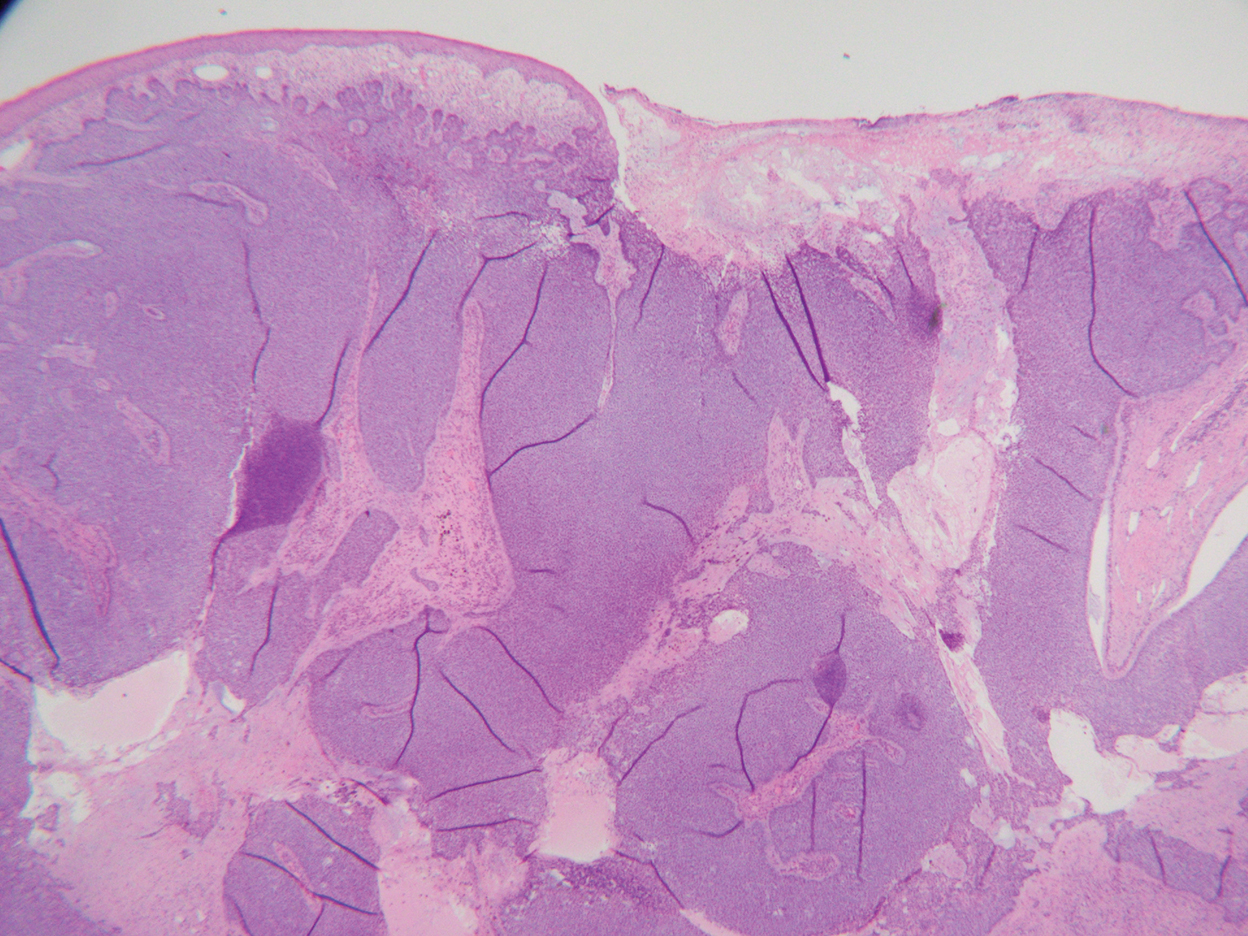
Histopathology was consistent with fungating basal cell carcinoma (BCC). The nodules were comprised of syncytial basaloid cells with high nuclear to cytoplasmic ratios, numerous mitotic figures, fibromyxoid stroma, and peripheral nuclear palisading (Figure). Fortunately, no perineural or lymphovascular invasion was identified, and the margins of the specimen were negative. Despite the high-risk nature of giant BCC, the mass was solitary without notable local invasion, leaving it amendable to surgery. On follow-up, the patient has remained recurrence free, and her hemoglobin level has since stabilized.
Skin cancer is the most common malignancy worldwide, and BCC accounts for more than 80% of nonmelanoma skin cancers in the United States. The incidence is on the rise due to the aging population and increasing cumulative skin exposure.1 Risk factors include both individual physical characteristics and environmental exposures. Individuals with lighter skin tones, red and blonde hair, and blue and green eyes are at an increased risk.2 UV radiation exposure is the most important cause of BCC.3 Chronic immunosuppression and exposure to arsenic, ionizing radiation, and psoralen plus UVA radiation also have been linked to the development of BCC.4-6 Basal cell carcinomas most commonly arise on sun-exposed areas such as the face, though more than 10% of cases appear on the trunk.7 Lesions characteristically remain localized, and growth rate is variable; however, when left untreated, BCCs have the potential to become locally destructive and difficult to treat.
Advanced BCCs are tumors that penetrate deeply into the skin. They often are not amenable to traditional therapy and/ or metastasize. Those that grow to a diameter greater than 5 cm, as in our patient, are known as giant BCCs. Only 0.5% to 1% of BCCs are giant BCCs8 ; they typically are more aggressive in nature with higher rates of local recurrence and metastasis. Individuals who develop giant BCCs either have had a delay in access to medical care or a history of BCC that was inadequately managed.9,10 During the COVID-19 pandemic, patient access to health care was substantially impacted during lockdowns. As in our patient, skin neoplasms and other medical conditions may present in later stages due to medical neglect.11,12 Metastasis is rare, even in advanced BCCs. A review of the literature from 1984 estimated that the incidence of metastasis of BCCs is 1 in 1000 to 35,000. Metastasis portends a poor prognosis with a median overall survival of 8 to 14 months.13 An updated review in 2013 found similar outcomes.14
The choice of management for BCCs depends on the risk for recurrence as well as individual patient factors. Characteristics such as tumor size, location, histology, whether it is a primary or recurrent lesion, and the presence of chronic skin disease determine the recurrence rate.15 The management of advanced BCCs often requires a multidisciplinary approach, as these neoplasms may not be amenable to local therapy without causing substantial morbidity. Mohs micrographic surgery is the treatment of choice for BCCs at high risk for recurrence.16 Standard surgical excision with postoperative margin assessment is acceptable when Mohs micrographic surgery is not available.17 Radiation therapy is an alternative for patients who are not candidates for surgery.18
Recently, improved understanding of the molecular pathogenesis of BCCs has led to the development of novel systemic therapies. The Hedgehog signaling pathway has been found to play a critical role in the development of most BCCs.19 Vismodegib and sonidegib are small-molecule inhibitors of the Hedgehog signaling pathway approved for the treatment of locally advanced and metastatic BCCs that are not amenable to surgery or radiation. Approximately 50% of advanced BCCs respond to these therapies; however, long-term treatment may be limited by intolerable side effects and the development of resistance.20 Basal cell carcinomas that spread to lymph nodes or distant sites are treated with traditional systemic therapy. Historically, conventional cytotoxic chemotherapies, such as platinum-containing regimens, were employed with limited benefit and notable morbidity.21
The differential diagnosis for our patient included several other cutaneous neoplasms. Squamous cell carcinoma is the second most common type of skin cancer. Similar to BCC, it can reach a substantial size if left untreated. Risk factors include chronic inflammation, exposure to radiation or chemical carcinogens, burns, human papillomavirus, and other chronic infections. Giant squamous cell carcinomas have high malignant potential and require imaging to assess the extent of invasion and for metastasis. Surgery typically is necessary for both staging and treatment. Adjuvant therapy also may be necessary.22,23
Internal malignant neoplasms rarely present as cutaneous metastases. Breast cancer, melanoma, and cancers of the upper respiratory tract most frequently metastasize to the skin. Although colorectal cancer (CRC) rarely metastasizes to the skin, it is an important cause of cutaneous metastasis due to its high incidence in the general population. When it does spread to the skin, CRC preferentially affects the abdominal wall. Lesions typically resemble the primary tumor but may appear anaplastic. The occurrence of cutaneous metastasis suggests latestage disease and carries a poor prognosis.24
Merkel cell carcinoma and melanoma are aggressive skin cancers with high mortality rates. The former is rarer but more lethal. Merkel cell carcinomas typically occur in elderly white men on sun-exposed areas of the skin. Tumors present as asymptomatic, rapidly expanding, blue-red, firm nodules. Immunosuppression and UV light exposure are notable risk factors.25 Of the 4 major subtypes of cutaneous melanoma, superficial spreading is the most common, followed by nodular, lentigo maligna, and acral lentiginous.26 Superficial spreading melanoma characteristically presents as an expanding asymmetric macule or thin plaque with irregular borders and variation in size and color (black, brown, or red). Nodular melanoma usually presents as symmetric in shape and color (amelanotic, black, or brown). Early recognition by both the patient and clinician is essential in preventing tumor growth and progression.27
Our patient’s presentation was highly concerning for cutaneous metastasis given her history of CRC. Furthermore, the finding of severe anemia was atypical for skin cancer and more characteristic of the prior malignancy. Imaging revealed a locally confined mass with no evidence of extension, lymph node involvement, or additional lesions. The diagnosis was clinched with histopathologic examination.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Lear JT, Tan BB, Smith AG, et al. Risk factors for basal cell carcinoma in the UK: case-control study in 806 patients. J R Soc Med. 1997; 90:371-374.
- Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer: I. basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
- Guo HR, Yu HS, Hu H, et al. Arsenic in drinking water and skin cancers: cell-type specificity (Taiwan, ROC). Cancer Causes Control. 2001;12:909-916.
- Lichter MD, Karagas MR, Mott LA, et al; The New Hampshire Skin Cancer Study Group. Therapeutic ionizing radiation and the incidence of basal cell carcinoma and squamous cell carcinoma. Arch Dermatol. 2000;136:1007-1011.
- Nijsten TEC, Stern RS. The increased risk of skin cancer is persistent after discontinuation of psoralen plus ultraviolet A: a cohort study. J Invest Dermatol. 2003;121:252-258.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
- Gualdi G, Monari P, Calzavara‐Pinton P, et al. When basal cell carcinomas became giant: an Italian multicenter study. Int J Dermatol. 2020;59:377-382.
- Randle HW, Roenigk RK, Brodland DG. Giant basal cell carcinoma (T3). who is at risk? Cancer. 1993;72:1624-1630.
- Archontaki M, Stavrianos SD, Korkolis DP, et al. Giant basal cell carcinoma: clinicopathological analysis of 51 cases and review of the literature. Anticancer Res. 2009;29:2655-2663.
- Shifat Ahmed SAK, Ajisola M, Azeem K, et al. Impact of the societal response to COVID-19 on access to healthcare for non-COVID-19 health issues in slum communities of Bangladesh, Kenya, Nigeria and Pakistan: results of pre-COVID and COVID-19 lockdown ssstakeholder engagements. BMJ Glob Health. 2020;5:E003042.
- Gomolin T, Cline A, Handler MZ. The danger of neglecting melanoma during the COVID-19 pandemic. J Dermatolog Treat. 2020;31:444-445.
- von Domarus H, Stevens PJ. Metastatic basal cell carcinoma. report of five cases and review of 170 cases in the literature. J Am Acad Dermatol. 1984;10:1043-1060.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616.
- Bøgelund FS, Philipsen PA, Gniadecki R. Factors affecting the recurrence rate of basal cell carcinoma. Acta Derm Venereol. 2007;87:330-334.
- Mosterd K, Krekels GAM, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9:1149-1156.
- Wetzig T, Woitek M, Eichhorn K, et al. Surgical excision of basal cell carcinoma with complete margin control: outcome at 5-year follow-up. Dermatology. 2010;220:363-369.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas. part 4: X-ray therapy. J Dermatol Surg Oncol. 1992;18:549-554.
- Tanese K, Emoto K, Kubota N, et al. Immunohistochemical visualization of the signature of activated Hedgehog signaling pathway in cutaneous epithelial tumors. J Dermatol. 2018;45:1181-1186.
- Basset-Séguin N, Hauschild A, Kunstfeld R, et al. Vismodegib in patients with advanced basal cell carcinoma: primary analysis of STEVIE, an international, open-label trial. Eur J Cancer. 2017;86:334-348.
- Carneiro BA, Watkin WG, Mehta UK, et al. Metastatic basal cell carcinoma: complete response to chemotherapy and associated pure red cell aplasia. Cancer Invest. 2006;24:396-400.
- Misiakos EP, Damaskou V, Koumarianou A, et al. A giant squamous cell carcinoma of the skin of the thoracic wall: a case report and review of the literature. J Med Case Rep. 2017;11:136.
- Wollina U, Bayyoud Y, Krönert C, et al. Giant epithelial malignancies (basal cell carcinoma, squamous cell carcinoma): a series of 20 tumors from a single center. J Cutan Aesthet Surg. 2012;5:12-19.
- Bittencourt MJS, Imbiriba AA, Oliveira OA, et al. Cutaneous metastasis of colorectal cancer. An Bras Dermatol. 2018;93:884-886.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
- Buettner PG, Leiter U, Eigentler TK, et al. Development of prognostic factors and survival in cutaneous melanoma over 25 years: an analysis of the Central Malignant Melanoma Registry of the German Dermatological Society. Cancer. 2005;103:616-624.
- Klebanov N, Gunasekera N, Lin WM, et al. The clinical spectrum of cutaneous melanoma morphology. J Am Acad Dermatol. 2019; 80:178-188.e3.
The Diagnosis: Basal Cell Carcinoma
Histopathology was consistent with fungating basal cell carcinoma (BCC). The nodules were comprised of syncytial basaloid cells with high nuclear to cytoplasmic ratios, numerous mitotic figures, fibromyxoid stroma, and peripheral nuclear palisading (Figure). Fortunately, no perineural or lymphovascular invasion was identified, and the margins of the specimen were negative. Despite the high-risk nature of giant BCC, the mass was solitary without notable local invasion, leaving it amendable to surgery. On follow-up, the patient has remained recurrence free, and her hemoglobin level has since stabilized.

Skin cancer is the most common malignancy worldwide, and BCC accounts for more than 80% of nonmelanoma skin cancers in the United States. The incidence is on the rise due to the aging population and increasing cumulative skin exposure.1 Risk factors include both individual physical characteristics and environmental exposures. Individuals with lighter skin tones, red and blonde hair, and blue and green eyes are at an increased risk.2 UV radiation exposure is the most important cause of BCC.3 Chronic immunosuppression and exposure to arsenic, ionizing radiation, and psoralen plus UVA radiation also have been linked to the development of BCC.4-6 Basal cell carcinomas most commonly arise on sun-exposed areas such as the face, though more than 10% of cases appear on the trunk.7 Lesions characteristically remain localized, and growth rate is variable; however, when left untreated, BCCs have the potential to become locally destructive and difficult to treat.
Advanced BCCs are tumors that penetrate deeply into the skin. They often are not amenable to traditional therapy and/ or metastasize. Those that grow to a diameter greater than 5 cm, as in our patient, are known as giant BCCs. Only 0.5% to 1% of BCCs are giant BCCs8 ; they typically are more aggressive in nature with higher rates of local recurrence and metastasis. Individuals who develop giant BCCs either have had a delay in access to medical care or a history of BCC that was inadequately managed.9,10 During the COVID-19 pandemic, patient access to health care was substantially impacted during lockdowns. As in our patient, skin neoplasms and other medical conditions may present in later stages due to medical neglect.11,12 Metastasis is rare, even in advanced BCCs. A review of the literature from 1984 estimated that the incidence of metastasis of BCCs is 1 in 1000 to 35,000. Metastasis portends a poor prognosis with a median overall survival of 8 to 14 months.13 An updated review in 2013 found similar outcomes.14
The choice of management for BCCs depends on the risk for recurrence as well as individual patient factors. Characteristics such as tumor size, location, histology, whether it is a primary or recurrent lesion, and the presence of chronic skin disease determine the recurrence rate.15 The management of advanced BCCs often requires a multidisciplinary approach, as these neoplasms may not be amenable to local therapy without causing substantial morbidity. Mohs micrographic surgery is the treatment of choice for BCCs at high risk for recurrence.16 Standard surgical excision with postoperative margin assessment is acceptable when Mohs micrographic surgery is not available.17 Radiation therapy is an alternative for patients who are not candidates for surgery.18
Recently, improved understanding of the molecular pathogenesis of BCCs has led to the development of novel systemic therapies. The Hedgehog signaling pathway has been found to play a critical role in the development of most BCCs.19 Vismodegib and sonidegib are small-molecule inhibitors of the Hedgehog signaling pathway approved for the treatment of locally advanced and metastatic BCCs that are not amenable to surgery or radiation. Approximately 50% of advanced BCCs respond to these therapies; however, long-term treatment may be limited by intolerable side effects and the development of resistance.20 Basal cell carcinomas that spread to lymph nodes or distant sites are treated with traditional systemic therapy. Historically, conventional cytotoxic chemotherapies, such as platinum-containing regimens, were employed with limited benefit and notable morbidity.21
The differential diagnosis for our patient included several other cutaneous neoplasms. Squamous cell carcinoma is the second most common type of skin cancer. Similar to BCC, it can reach a substantial size if left untreated. Risk factors include chronic inflammation, exposure to radiation or chemical carcinogens, burns, human papillomavirus, and other chronic infections. Giant squamous cell carcinomas have high malignant potential and require imaging to assess the extent of invasion and for metastasis. Surgery typically is necessary for both staging and treatment. Adjuvant therapy also may be necessary.22,23
Internal malignant neoplasms rarely present as cutaneous metastases. Breast cancer, melanoma, and cancers of the upper respiratory tract most frequently metastasize to the skin. Although colorectal cancer (CRC) rarely metastasizes to the skin, it is an important cause of cutaneous metastasis due to its high incidence in the general population. When it does spread to the skin, CRC preferentially affects the abdominal wall. Lesions typically resemble the primary tumor but may appear anaplastic. The occurrence of cutaneous metastasis suggests latestage disease and carries a poor prognosis.24
Merkel cell carcinoma and melanoma are aggressive skin cancers with high mortality rates. The former is rarer but more lethal. Merkel cell carcinomas typically occur in elderly white men on sun-exposed areas of the skin. Tumors present as asymptomatic, rapidly expanding, blue-red, firm nodules. Immunosuppression and UV light exposure are notable risk factors.25 Of the 4 major subtypes of cutaneous melanoma, superficial spreading is the most common, followed by nodular, lentigo maligna, and acral lentiginous.26 Superficial spreading melanoma characteristically presents as an expanding asymmetric macule or thin plaque with irregular borders and variation in size and color (black, brown, or red). Nodular melanoma usually presents as symmetric in shape and color (amelanotic, black, or brown). Early recognition by both the patient and clinician is essential in preventing tumor growth and progression.27
Our patient’s presentation was highly concerning for cutaneous metastasis given her history of CRC. Furthermore, the finding of severe anemia was atypical for skin cancer and more characteristic of the prior malignancy. Imaging revealed a locally confined mass with no evidence of extension, lymph node involvement, or additional lesions. The diagnosis was clinched with histopathologic examination.
The Diagnosis: Basal Cell Carcinoma
Histopathology was consistent with fungating basal cell carcinoma (BCC). The nodules were comprised of syncytial basaloid cells with high nuclear to cytoplasmic ratios, numerous mitotic figures, fibromyxoid stroma, and peripheral nuclear palisading (Figure). Fortunately, no perineural or lymphovascular invasion was identified, and the margins of the specimen were negative. Despite the high-risk nature of giant BCC, the mass was solitary without notable local invasion, leaving it amendable to surgery. On follow-up, the patient has remained recurrence free, and her hemoglobin level has since stabilized.

Skin cancer is the most common malignancy worldwide, and BCC accounts for more than 80% of nonmelanoma skin cancers in the United States. The incidence is on the rise due to the aging population and increasing cumulative skin exposure.1 Risk factors include both individual physical characteristics and environmental exposures. Individuals with lighter skin tones, red and blonde hair, and blue and green eyes are at an increased risk.2 UV radiation exposure is the most important cause of BCC.3 Chronic immunosuppression and exposure to arsenic, ionizing radiation, and psoralen plus UVA radiation also have been linked to the development of BCC.4-6 Basal cell carcinomas most commonly arise on sun-exposed areas such as the face, though more than 10% of cases appear on the trunk.7 Lesions characteristically remain localized, and growth rate is variable; however, when left untreated, BCCs have the potential to become locally destructive and difficult to treat.
Advanced BCCs are tumors that penetrate deeply into the skin. They often are not amenable to traditional therapy and/ or metastasize. Those that grow to a diameter greater than 5 cm, as in our patient, are known as giant BCCs. Only 0.5% to 1% of BCCs are giant BCCs8 ; they typically are more aggressive in nature with higher rates of local recurrence and metastasis. Individuals who develop giant BCCs either have had a delay in access to medical care or a history of BCC that was inadequately managed.9,10 During the COVID-19 pandemic, patient access to health care was substantially impacted during lockdowns. As in our patient, skin neoplasms and other medical conditions may present in later stages due to medical neglect.11,12 Metastasis is rare, even in advanced BCCs. A review of the literature from 1984 estimated that the incidence of metastasis of BCCs is 1 in 1000 to 35,000. Metastasis portends a poor prognosis with a median overall survival of 8 to 14 months.13 An updated review in 2013 found similar outcomes.14
The choice of management for BCCs depends on the risk for recurrence as well as individual patient factors. Characteristics such as tumor size, location, histology, whether it is a primary or recurrent lesion, and the presence of chronic skin disease determine the recurrence rate.15 The management of advanced BCCs often requires a multidisciplinary approach, as these neoplasms may not be amenable to local therapy without causing substantial morbidity. Mohs micrographic surgery is the treatment of choice for BCCs at high risk for recurrence.16 Standard surgical excision with postoperative margin assessment is acceptable when Mohs micrographic surgery is not available.17 Radiation therapy is an alternative for patients who are not candidates for surgery.18
Recently, improved understanding of the molecular pathogenesis of BCCs has led to the development of novel systemic therapies. The Hedgehog signaling pathway has been found to play a critical role in the development of most BCCs.19 Vismodegib and sonidegib are small-molecule inhibitors of the Hedgehog signaling pathway approved for the treatment of locally advanced and metastatic BCCs that are not amenable to surgery or radiation. Approximately 50% of advanced BCCs respond to these therapies; however, long-term treatment may be limited by intolerable side effects and the development of resistance.20 Basal cell carcinomas that spread to lymph nodes or distant sites are treated with traditional systemic therapy. Historically, conventional cytotoxic chemotherapies, such as platinum-containing regimens, were employed with limited benefit and notable morbidity.21
The differential diagnosis for our patient included several other cutaneous neoplasms. Squamous cell carcinoma is the second most common type of skin cancer. Similar to BCC, it can reach a substantial size if left untreated. Risk factors include chronic inflammation, exposure to radiation or chemical carcinogens, burns, human papillomavirus, and other chronic infections. Giant squamous cell carcinomas have high malignant potential and require imaging to assess the extent of invasion and for metastasis. Surgery typically is necessary for both staging and treatment. Adjuvant therapy also may be necessary.22,23
Internal malignant neoplasms rarely present as cutaneous metastases. Breast cancer, melanoma, and cancers of the upper respiratory tract most frequently metastasize to the skin. Although colorectal cancer (CRC) rarely metastasizes to the skin, it is an important cause of cutaneous metastasis due to its high incidence in the general population. When it does spread to the skin, CRC preferentially affects the abdominal wall. Lesions typically resemble the primary tumor but may appear anaplastic. The occurrence of cutaneous metastasis suggests latestage disease and carries a poor prognosis.24
Merkel cell carcinoma and melanoma are aggressive skin cancers with high mortality rates. The former is rarer but more lethal. Merkel cell carcinomas typically occur in elderly white men on sun-exposed areas of the skin. Tumors present as asymptomatic, rapidly expanding, blue-red, firm nodules. Immunosuppression and UV light exposure are notable risk factors.25 Of the 4 major subtypes of cutaneous melanoma, superficial spreading is the most common, followed by nodular, lentigo maligna, and acral lentiginous.26 Superficial spreading melanoma characteristically presents as an expanding asymmetric macule or thin plaque with irregular borders and variation in size and color (black, brown, or red). Nodular melanoma usually presents as symmetric in shape and color (amelanotic, black, or brown). Early recognition by both the patient and clinician is essential in preventing tumor growth and progression.27
Our patient’s presentation was highly concerning for cutaneous metastasis given her history of CRC. Furthermore, the finding of severe anemia was atypical for skin cancer and more characteristic of the prior malignancy. Imaging revealed a locally confined mass with no evidence of extension, lymph node involvement, or additional lesions. The diagnosis was clinched with histopathologic examination.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Lear JT, Tan BB, Smith AG, et al. Risk factors for basal cell carcinoma in the UK: case-control study in 806 patients. J R Soc Med. 1997; 90:371-374.
- Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer: I. basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
- Guo HR, Yu HS, Hu H, et al. Arsenic in drinking water and skin cancers: cell-type specificity (Taiwan, ROC). Cancer Causes Control. 2001;12:909-916.
- Lichter MD, Karagas MR, Mott LA, et al; The New Hampshire Skin Cancer Study Group. Therapeutic ionizing radiation and the incidence of basal cell carcinoma and squamous cell carcinoma. Arch Dermatol. 2000;136:1007-1011.
- Nijsten TEC, Stern RS. The increased risk of skin cancer is persistent after discontinuation of psoralen plus ultraviolet A: a cohort study. J Invest Dermatol. 2003;121:252-258.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
- Gualdi G, Monari P, Calzavara‐Pinton P, et al. When basal cell carcinomas became giant: an Italian multicenter study. Int J Dermatol. 2020;59:377-382.
- Randle HW, Roenigk RK, Brodland DG. Giant basal cell carcinoma (T3). who is at risk? Cancer. 1993;72:1624-1630.
- Archontaki M, Stavrianos SD, Korkolis DP, et al. Giant basal cell carcinoma: clinicopathological analysis of 51 cases and review of the literature. Anticancer Res. 2009;29:2655-2663.
- Shifat Ahmed SAK, Ajisola M, Azeem K, et al. Impact of the societal response to COVID-19 on access to healthcare for non-COVID-19 health issues in slum communities of Bangladesh, Kenya, Nigeria and Pakistan: results of pre-COVID and COVID-19 lockdown ssstakeholder engagements. BMJ Glob Health. 2020;5:E003042.
- Gomolin T, Cline A, Handler MZ. The danger of neglecting melanoma during the COVID-19 pandemic. J Dermatolog Treat. 2020;31:444-445.
- von Domarus H, Stevens PJ. Metastatic basal cell carcinoma. report of five cases and review of 170 cases in the literature. J Am Acad Dermatol. 1984;10:1043-1060.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616.
- Bøgelund FS, Philipsen PA, Gniadecki R. Factors affecting the recurrence rate of basal cell carcinoma. Acta Derm Venereol. 2007;87:330-334.
- Mosterd K, Krekels GAM, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9:1149-1156.
- Wetzig T, Woitek M, Eichhorn K, et al. Surgical excision of basal cell carcinoma with complete margin control: outcome at 5-year follow-up. Dermatology. 2010;220:363-369.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas. part 4: X-ray therapy. J Dermatol Surg Oncol. 1992;18:549-554.
- Tanese K, Emoto K, Kubota N, et al. Immunohistochemical visualization of the signature of activated Hedgehog signaling pathway in cutaneous epithelial tumors. J Dermatol. 2018;45:1181-1186.
- Basset-Séguin N, Hauschild A, Kunstfeld R, et al. Vismodegib in patients with advanced basal cell carcinoma: primary analysis of STEVIE, an international, open-label trial. Eur J Cancer. 2017;86:334-348.
- Carneiro BA, Watkin WG, Mehta UK, et al. Metastatic basal cell carcinoma: complete response to chemotherapy and associated pure red cell aplasia. Cancer Invest. 2006;24:396-400.
- Misiakos EP, Damaskou V, Koumarianou A, et al. A giant squamous cell carcinoma of the skin of the thoracic wall: a case report and review of the literature. J Med Case Rep. 2017;11:136.
- Wollina U, Bayyoud Y, Krönert C, et al. Giant epithelial malignancies (basal cell carcinoma, squamous cell carcinoma): a series of 20 tumors from a single center. J Cutan Aesthet Surg. 2012;5:12-19.
- Bittencourt MJS, Imbiriba AA, Oliveira OA, et al. Cutaneous metastasis of colorectal cancer. An Bras Dermatol. 2018;93:884-886.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
- Buettner PG, Leiter U, Eigentler TK, et al. Development of prognostic factors and survival in cutaneous melanoma over 25 years: an analysis of the Central Malignant Melanoma Registry of the German Dermatological Society. Cancer. 2005;103:616-624.
- Klebanov N, Gunasekera N, Lin WM, et al. The clinical spectrum of cutaneous melanoma morphology. J Am Acad Dermatol. 2019; 80:178-188.e3.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Lear JT, Tan BB, Smith AG, et al. Risk factors for basal cell carcinoma in the UK: case-control study in 806 patients. J R Soc Med. 1997; 90:371-374.
- Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer: I. basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
- Guo HR, Yu HS, Hu H, et al. Arsenic in drinking water and skin cancers: cell-type specificity (Taiwan, ROC). Cancer Causes Control. 2001;12:909-916.
- Lichter MD, Karagas MR, Mott LA, et al; The New Hampshire Skin Cancer Study Group. Therapeutic ionizing radiation and the incidence of basal cell carcinoma and squamous cell carcinoma. Arch Dermatol. 2000;136:1007-1011.
- Nijsten TEC, Stern RS. The increased risk of skin cancer is persistent after discontinuation of psoralen plus ultraviolet A: a cohort study. J Invest Dermatol. 2003;121:252-258.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
- Gualdi G, Monari P, Calzavara‐Pinton P, et al. When basal cell carcinomas became giant: an Italian multicenter study. Int J Dermatol. 2020;59:377-382.
- Randle HW, Roenigk RK, Brodland DG. Giant basal cell carcinoma (T3). who is at risk? Cancer. 1993;72:1624-1630.
- Archontaki M, Stavrianos SD, Korkolis DP, et al. Giant basal cell carcinoma: clinicopathological analysis of 51 cases and review of the literature. Anticancer Res. 2009;29:2655-2663.
- Shifat Ahmed SAK, Ajisola M, Azeem K, et al. Impact of the societal response to COVID-19 on access to healthcare for non-COVID-19 health issues in slum communities of Bangladesh, Kenya, Nigeria and Pakistan: results of pre-COVID and COVID-19 lockdown ssstakeholder engagements. BMJ Glob Health. 2020;5:E003042.
- Gomolin T, Cline A, Handler MZ. The danger of neglecting melanoma during the COVID-19 pandemic. J Dermatolog Treat. 2020;31:444-445.
- von Domarus H, Stevens PJ. Metastatic basal cell carcinoma. report of five cases and review of 170 cases in the literature. J Am Acad Dermatol. 1984;10:1043-1060.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616.
- Bøgelund FS, Philipsen PA, Gniadecki R. Factors affecting the recurrence rate of basal cell carcinoma. Acta Derm Venereol. 2007;87:330-334.
- Mosterd K, Krekels GAM, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9:1149-1156.
- Wetzig T, Woitek M, Eichhorn K, et al. Surgical excision of basal cell carcinoma with complete margin control: outcome at 5-year follow-up. Dermatology. 2010;220:363-369.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas. part 4: X-ray therapy. J Dermatol Surg Oncol. 1992;18:549-554.
- Tanese K, Emoto K, Kubota N, et al. Immunohistochemical visualization of the signature of activated Hedgehog signaling pathway in cutaneous epithelial tumors. J Dermatol. 2018;45:1181-1186.
- Basset-Séguin N, Hauschild A, Kunstfeld R, et al. Vismodegib in patients with advanced basal cell carcinoma: primary analysis of STEVIE, an international, open-label trial. Eur J Cancer. 2017;86:334-348.
- Carneiro BA, Watkin WG, Mehta UK, et al. Metastatic basal cell carcinoma: complete response to chemotherapy and associated pure red cell aplasia. Cancer Invest. 2006;24:396-400.
- Misiakos EP, Damaskou V, Koumarianou A, et al. A giant squamous cell carcinoma of the skin of the thoracic wall: a case report and review of the literature. J Med Case Rep. 2017;11:136.
- Wollina U, Bayyoud Y, Krönert C, et al. Giant epithelial malignancies (basal cell carcinoma, squamous cell carcinoma): a series of 20 tumors from a single center. J Cutan Aesthet Surg. 2012;5:12-19.
- Bittencourt MJS, Imbiriba AA, Oliveira OA, et al. Cutaneous metastasis of colorectal cancer. An Bras Dermatol. 2018;93:884-886.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
- Buettner PG, Leiter U, Eigentler TK, et al. Development of prognostic factors and survival in cutaneous melanoma over 25 years: an analysis of the Central Malignant Melanoma Registry of the German Dermatological Society. Cancer. 2005;103:616-624.
- Klebanov N, Gunasekera N, Lin WM, et al. The clinical spectrum of cutaneous melanoma morphology. J Am Acad Dermatol. 2019; 80:178-188.e3.
A 77-year-old woman was admitted to the hospital with anemia (hemoglobin, 5.2 g/dL [reference range, 12.0–15.5 g/dL]) and a rapidly growing abdominal wall mass. She had a history of stage IIA colon cancer (T3N0M0) that was treated 5 years prior with a partial colon resection and adjuvant chemotherapy. She initially noticed a red scaly lesion developing around a scar from a prior surgery that had been stable for years. Over the last 2 months, the lesion rapidly expanded and would intermittently bleed. Physical examination revealed a 13×10×4.5-cm, pink-red, nodular, firm mass over the patient’s right upper quadrant. Computed tomography revealed a mass limited to the skin and superficial tissue. General surgery was consulted for excision of the mass.
ILAE offers first guide to treating depression in epilepsy
The new guidance highlights the high prevalence of depression among patients with epilepsy while offering the first systematic approach to treatment, reported lead author Marco Mula, MD, PhD, of Atkinson Morley Regional Neuroscience Centre at St George’s University Hospital, London, and colleagues.
“Despite evidence that depression represents a frequently encountered comorbidity [among patients with epilepsy], data on the treatment of depression in epilepsy [are] still limited and recommendations rely mostly on individual clinical experience and expertise,” the investigators wrote in Epilepsia.
Recommendations cover first-line treatment of unipolar depression in epilepsy without other psychiatric disorders.
For patients with mild depression, the guidance supports psychological intervention without pharmacologic therapy; however, if the patient wishes to use medication, has had a positive response to medication in the past, or nonpharmacologic treatments have previously failed or are unavailable, then SSRIs should be considered first-choice therapy. For moderate to severe depression, SSRIs are the first choice, according to Dr. Mula and colleagues.
“It has to be acknowledged that there is considerable debate in the psychiatric literature about the treatment of mild depression in adults,” the investigators noted. “A patient-level meta-analysis pointed out that the magnitude of benefit of antidepressant medications compared with placebo increases with severity of depression symptoms and it may be minimal or nonexistent, on average, in patients with mild or moderate symptoms.”
If a patient does not respond to first-line therapy, then venlafaxine should be considered, according to the guidance. When a patient does respond to therapy, treatment should be continued for at least 6 months, and when residual symptoms persist, treatment should be continued until resolution.
“In people with depression it is established that around two-thirds of patients do not achieve full remission with first-line treatment,” Dr. Mula and colleagues wrote. “In people with epilepsy, current data show that up to 50% of patients do not achieve full remission from depression. For this reason, augmentation strategies are often needed. They should be adopted by psychiatrists, neuropsychiatrists, or mental health professionals familiar with such therapeutic strategies.”
Beyond these key recommendations, the guidance covers a range of additional topics, including other pharmacologic options, medication discontinuation strategies, electroconvulsive therapy, light therapy, exercise training, vagus nerve stimulation, and repetitive transcranial magnetic stimulation.
Useful advice that counters common misconceptions
According to Jacqueline A. French, MD, a professor at NYU Langone Medical Center, Dr. Mula and colleagues are “top notch,” and their recommendations “hit every nail on the head.”
Dr. French, chief medical officer of The Epilepsy Foundation, emphasized the importance of the publication, which addresses two common misconceptions within the medical community: First, that standard antidepressants are insufficient to treat depression in patients with epilepsy, and second, that antidepressants may trigger seizures.
“The first purpose [of the publication] is to say, yes, these antidepressants do work,” Dr. French said, “and no, they don’t worsen seizures, and you can use them safely, and they are appropriate to use.”
Dr. French explained that managing depression remains a practice gap among epileptologists and neurologists because it is a diagnosis that doesn’t traditionally fall into their purview, yet many patients with epilepsy forgo visiting their primary care providers, who more frequently diagnose and manage depression. Dr. French agreed with the guidance that epilepsy specialists should fill this gap.
“We need to at least be able to take people through their first antidepressant, even though we were not trained to be psychiatrists,” Dr. French said. “That’s part of the best care of our patients.”
Imad Najm, MD, director of the Charles Shor Epilepsy Center, Cleveland Clinic, said the recommendations are a step forward in the field, as they are supported by clinical data, instead of just clinical experience and expertise.
Still, Dr. Najm noted that more work is needed to stratify risk of depression in epilepsy and evaluate a possible causal relationship between epilepsy therapies and depression.
He went on to emphasizes the scale of issue at hand, and the stakes involved.
“Depression, anxiety, and psychosis affect a large number of patients with epilepsy,” Dr. Najm said. “Clinical screening and recognition of these comorbidities leads to the institution of treatment options and significant improvement in quality of life. Mental health professionals should be an integral part of any comprehensive epilepsy center.”
The investigators disclosed relationships with Esai, UCB, Elsevier, and others. Dr. French is indirectly involved with multiple pharmaceutical companies developing epilepsy drugs through her role as director of The Epilepsy Study Consortium, a nonprofit organization. Dr. Najm reported no conflicts of interest.
The new guidance highlights the high prevalence of depression among patients with epilepsy while offering the first systematic approach to treatment, reported lead author Marco Mula, MD, PhD, of Atkinson Morley Regional Neuroscience Centre at St George’s University Hospital, London, and colleagues.
“Despite evidence that depression represents a frequently encountered comorbidity [among patients with epilepsy], data on the treatment of depression in epilepsy [are] still limited and recommendations rely mostly on individual clinical experience and expertise,” the investigators wrote in Epilepsia.
Recommendations cover first-line treatment of unipolar depression in epilepsy without other psychiatric disorders.
For patients with mild depression, the guidance supports psychological intervention without pharmacologic therapy; however, if the patient wishes to use medication, has had a positive response to medication in the past, or nonpharmacologic treatments have previously failed or are unavailable, then SSRIs should be considered first-choice therapy. For moderate to severe depression, SSRIs are the first choice, according to Dr. Mula and colleagues.
“It has to be acknowledged that there is considerable debate in the psychiatric literature about the treatment of mild depression in adults,” the investigators noted. “A patient-level meta-analysis pointed out that the magnitude of benefit of antidepressant medications compared with placebo increases with severity of depression symptoms and it may be minimal or nonexistent, on average, in patients with mild or moderate symptoms.”
If a patient does not respond to first-line therapy, then venlafaxine should be considered, according to the guidance. When a patient does respond to therapy, treatment should be continued for at least 6 months, and when residual symptoms persist, treatment should be continued until resolution.
“In people with depression it is established that around two-thirds of patients do not achieve full remission with first-line treatment,” Dr. Mula and colleagues wrote. “In people with epilepsy, current data show that up to 50% of patients do not achieve full remission from depression. For this reason, augmentation strategies are often needed. They should be adopted by psychiatrists, neuropsychiatrists, or mental health professionals familiar with such therapeutic strategies.”
Beyond these key recommendations, the guidance covers a range of additional topics, including other pharmacologic options, medication discontinuation strategies, electroconvulsive therapy, light therapy, exercise training, vagus nerve stimulation, and repetitive transcranial magnetic stimulation.
Useful advice that counters common misconceptions
According to Jacqueline A. French, MD, a professor at NYU Langone Medical Center, Dr. Mula and colleagues are “top notch,” and their recommendations “hit every nail on the head.”
Dr. French, chief medical officer of The Epilepsy Foundation, emphasized the importance of the publication, which addresses two common misconceptions within the medical community: First, that standard antidepressants are insufficient to treat depression in patients with epilepsy, and second, that antidepressants may trigger seizures.
“The first purpose [of the publication] is to say, yes, these antidepressants do work,” Dr. French said, “and no, they don’t worsen seizures, and you can use them safely, and they are appropriate to use.”
Dr. French explained that managing depression remains a practice gap among epileptologists and neurologists because it is a diagnosis that doesn’t traditionally fall into their purview, yet many patients with epilepsy forgo visiting their primary care providers, who more frequently diagnose and manage depression. Dr. French agreed with the guidance that epilepsy specialists should fill this gap.
“We need to at least be able to take people through their first antidepressant, even though we were not trained to be psychiatrists,” Dr. French said. “That’s part of the best care of our patients.”
Imad Najm, MD, director of the Charles Shor Epilepsy Center, Cleveland Clinic, said the recommendations are a step forward in the field, as they are supported by clinical data, instead of just clinical experience and expertise.
Still, Dr. Najm noted that more work is needed to stratify risk of depression in epilepsy and evaluate a possible causal relationship between epilepsy therapies and depression.
He went on to emphasizes the scale of issue at hand, and the stakes involved.
“Depression, anxiety, and psychosis affect a large number of patients with epilepsy,” Dr. Najm said. “Clinical screening and recognition of these comorbidities leads to the institution of treatment options and significant improvement in quality of life. Mental health professionals should be an integral part of any comprehensive epilepsy center.”
The investigators disclosed relationships with Esai, UCB, Elsevier, and others. Dr. French is indirectly involved with multiple pharmaceutical companies developing epilepsy drugs through her role as director of The Epilepsy Study Consortium, a nonprofit organization. Dr. Najm reported no conflicts of interest.
The new guidance highlights the high prevalence of depression among patients with epilepsy while offering the first systematic approach to treatment, reported lead author Marco Mula, MD, PhD, of Atkinson Morley Regional Neuroscience Centre at St George’s University Hospital, London, and colleagues.
“Despite evidence that depression represents a frequently encountered comorbidity [among patients with epilepsy], data on the treatment of depression in epilepsy [are] still limited and recommendations rely mostly on individual clinical experience and expertise,” the investigators wrote in Epilepsia.
Recommendations cover first-line treatment of unipolar depression in epilepsy without other psychiatric disorders.
For patients with mild depression, the guidance supports psychological intervention without pharmacologic therapy; however, if the patient wishes to use medication, has had a positive response to medication in the past, or nonpharmacologic treatments have previously failed or are unavailable, then SSRIs should be considered first-choice therapy. For moderate to severe depression, SSRIs are the first choice, according to Dr. Mula and colleagues.
“It has to be acknowledged that there is considerable debate in the psychiatric literature about the treatment of mild depression in adults,” the investigators noted. “A patient-level meta-analysis pointed out that the magnitude of benefit of antidepressant medications compared with placebo increases with severity of depression symptoms and it may be minimal or nonexistent, on average, in patients with mild or moderate symptoms.”
If a patient does not respond to first-line therapy, then venlafaxine should be considered, according to the guidance. When a patient does respond to therapy, treatment should be continued for at least 6 months, and when residual symptoms persist, treatment should be continued until resolution.
“In people with depression it is established that around two-thirds of patients do not achieve full remission with first-line treatment,” Dr. Mula and colleagues wrote. “In people with epilepsy, current data show that up to 50% of patients do not achieve full remission from depression. For this reason, augmentation strategies are often needed. They should be adopted by psychiatrists, neuropsychiatrists, or mental health professionals familiar with such therapeutic strategies.”
Beyond these key recommendations, the guidance covers a range of additional topics, including other pharmacologic options, medication discontinuation strategies, electroconvulsive therapy, light therapy, exercise training, vagus nerve stimulation, and repetitive transcranial magnetic stimulation.
Useful advice that counters common misconceptions
According to Jacqueline A. French, MD, a professor at NYU Langone Medical Center, Dr. Mula and colleagues are “top notch,” and their recommendations “hit every nail on the head.”
Dr. French, chief medical officer of The Epilepsy Foundation, emphasized the importance of the publication, which addresses two common misconceptions within the medical community: First, that standard antidepressants are insufficient to treat depression in patients with epilepsy, and second, that antidepressants may trigger seizures.
“The first purpose [of the publication] is to say, yes, these antidepressants do work,” Dr. French said, “and no, they don’t worsen seizures, and you can use them safely, and they are appropriate to use.”
Dr. French explained that managing depression remains a practice gap among epileptologists and neurologists because it is a diagnosis that doesn’t traditionally fall into their purview, yet many patients with epilepsy forgo visiting their primary care providers, who more frequently diagnose and manage depression. Dr. French agreed with the guidance that epilepsy specialists should fill this gap.
“We need to at least be able to take people through their first antidepressant, even though we were not trained to be psychiatrists,” Dr. French said. “That’s part of the best care of our patients.”
Imad Najm, MD, director of the Charles Shor Epilepsy Center, Cleveland Clinic, said the recommendations are a step forward in the field, as they are supported by clinical data, instead of just clinical experience and expertise.
Still, Dr. Najm noted that more work is needed to stratify risk of depression in epilepsy and evaluate a possible causal relationship between epilepsy therapies and depression.
He went on to emphasizes the scale of issue at hand, and the stakes involved.
“Depression, anxiety, and psychosis affect a large number of patients with epilepsy,” Dr. Najm said. “Clinical screening and recognition of these comorbidities leads to the institution of treatment options and significant improvement in quality of life. Mental health professionals should be an integral part of any comprehensive epilepsy center.”
The investigators disclosed relationships with Esai, UCB, Elsevier, and others. Dr. French is indirectly involved with multiple pharmaceutical companies developing epilepsy drugs through her role as director of The Epilepsy Study Consortium, a nonprofit organization. Dr. Najm reported no conflicts of interest.
FROM EPILEPSIA
Malnutrition common in patients with IBD
Malnutrition is common among patients with inflammatory bowel disease (IBD) and is associated with worse outcomes that can prolong hospitalizations and increase patients’ risk for death.
As many as 85% of inpatients with IBD may be malnourished, with the severity of malnutrition affected by disease activity, extent, and duration, said Kelly Issokson, MS, RD, CNSC, clinical nutrition coordinator in the IBD program in the division of gastroenterology at Cedars-Sinai Medical Center, Los Angeles.
“Malnutrition is a severe complication of IBD, and it should not be overlooked,” she said during an oral presentation at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
In patients with IBD, malabsorption, enteric losses, inadequate intake, and side effects of medical therapy can all lead to malnutrition, which in turn is an independent risk factor for venous thromboembolic events, nonelective surgery, longer hospital stays, and increased mortality.
In addition, malnutrition in IBD increases risk for infection and sepsis, and for perioperative complications, and can more than double the cost of care, compared with adequately nourished IBD patients, she said.
Ms. Issokson cited a definition of malnutrition from the American Society of Parenteral and Enteral Nutrition as “an acute or chronic state of overnutrition or undernutrition with or without inflammatory activity that has led to a change in body composition and diminished function.”
Lab findings of low albumin, low prealbumin, or isolated metrics such as weight loss or change in body mass index do not constitute malnutrition and should not be used to diagnosis it, Ms. Issokson cautioned.
Patients at low risk for malnutrition have no unintentional weight loss, are eating well, have minimal or no dietary restrictions, and no wasting. In contrast, high-risk patients have unintentional weight loss, decreased appetite and/or food intake, restrict multiple foods, or show signs of wasting.
Screening
“Nutrition screening is the first step in diagnosing a patient with malnutrition. This is a process of identifying individuals who may be at nutrition risk and benefit from assessment from a registered dietitian,” Ms. Issokson said.
The Malnutrition Screening Tool is quick, easy to administer, and requires minimal training. It can be used to screen adults for malnutrition regardless of age, medical history, or setting, she said.
The two-item instrument asks, “Have you recently lost weight without trying?” with a “no” scored as 0 and a “yes” scored as 2. The second question is, “Have you been eating poorly because of decreased appetite, with a “no” equal to 0 and a “yes” equal to 1. Patients with a score of 0 or 1 are not at risk, whereas patients with scores of 2 or 3 are deemed to be at risk for malnutrition and require further assessment by a dietitian.
Assessment
Assessment for malnutrition involves a variety of factors, including anthropometric factors such as weight and BMI changes; biochemical markers such as fat-soluble vitamins, water-soluble vitamins, minerals, and urinary sodium; symptoms such as decreased appetite, abdominal pain, cramping or bloating, diarrhea, or urgency or obstructive symptoms; and body composition measures such as handgrip strength, biochemical impedance analysis, skinfold thickness, bone mineral density, and muscle mass.
Other nutritional assessment tools may include 24-hour recall of nutrition intake, diet history, and questions about eating behaviors, food allergies or intolerances, and cultural or religious food preferences.
Assessing food security is also important, especially during the current pandemic, Ms. Issokson emphasized.
“Is your patient running out of food? Do they have money to purchase food? Are they able to go to the grocery store to buy food? This is essential to know when you’re developing a nutrition plan,” she said.
A nutrition-focused physical exam should include assessment of skin manifestation, secondary to malnutrition or malabsorption, such as dry skin, delayed wound healing, stomatitis, scurvy, seborrheic dermatitis, bleeding, and periorificial and acral dermatitis or alopecia.
Diagnosis
Currently available malnutrition criteria have not been validated for use in patients with IBD, and further studies are needed to affirm their applicability to this population, Ms. Issokson said.
The Academy of Nutrition and Dietetics–American Society for Parenteral and Enteral Nutrition (AND-ASPEN) malnutrition criteria require measures of weight loss, energy intake, subcutaneous fat loss, subcutaneous muscle loss, general or local fluid accumulation, and handgrip strength to determine whether a patient is moderately or severely malnourished.
Ms. Issokson said that she finds the European Society for Clinical Nutrition and Metabolism Global Leadership Initiative on Malnutrition (ESPEN GLIM) criteria somewhat easier to use for diagnosis, as they consist of phenotypic and etiologic criteria, with patients who meet at least one of each being considered malnourished.
“When identified, document malnutrition, and of course intervene appropriately by referring to a dietitian providing education and supporting the patient to help them optimize their nutrition and improve their outcomes,” she concluded.
In a discussion following the session, panelist Neha Shah, MPH, RD, CNSC, a dietitian and health education specialist at the University of California, San Francisco, commented on the importance of malnutrition assessment in patients with IBD being considered for surgery.
Patients should be screened for malnutrition, and if they have a positive screen, “should be automatically referred to a registered dietitian specializing in IBD for a nutrition assessment,” she said.
“Certainly, a nutritional assessment, as Kelly has highlighted really well, will encompass an evaluation of various areas of health – patient history, food and nutrition history, changing anthropometrics, alterations in labs – and certainly going into further nutrition history with net food intolerance, intake from each food group, portions, access, support, culture, eating environment, skills in the kitchen, relationship with diet.”
Ms. Issokson is a board member of the Crohn’s & Colitis Foundation and a digital advisory board member of Avant Healthcare. Ms. Shah had no disclosures.
Malnutrition is common among patients with inflammatory bowel disease (IBD) and is associated with worse outcomes that can prolong hospitalizations and increase patients’ risk for death.
As many as 85% of inpatients with IBD may be malnourished, with the severity of malnutrition affected by disease activity, extent, and duration, said Kelly Issokson, MS, RD, CNSC, clinical nutrition coordinator in the IBD program in the division of gastroenterology at Cedars-Sinai Medical Center, Los Angeles.
“Malnutrition is a severe complication of IBD, and it should not be overlooked,” she said during an oral presentation at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
In patients with IBD, malabsorption, enteric losses, inadequate intake, and side effects of medical therapy can all lead to malnutrition, which in turn is an independent risk factor for venous thromboembolic events, nonelective surgery, longer hospital stays, and increased mortality.
In addition, malnutrition in IBD increases risk for infection and sepsis, and for perioperative complications, and can more than double the cost of care, compared with adequately nourished IBD patients, she said.
Ms. Issokson cited a definition of malnutrition from the American Society of Parenteral and Enteral Nutrition as “an acute or chronic state of overnutrition or undernutrition with or without inflammatory activity that has led to a change in body composition and diminished function.”
Lab findings of low albumin, low prealbumin, or isolated metrics such as weight loss or change in body mass index do not constitute malnutrition and should not be used to diagnosis it, Ms. Issokson cautioned.
Patients at low risk for malnutrition have no unintentional weight loss, are eating well, have minimal or no dietary restrictions, and no wasting. In contrast, high-risk patients have unintentional weight loss, decreased appetite and/or food intake, restrict multiple foods, or show signs of wasting.
Screening
“Nutrition screening is the first step in diagnosing a patient with malnutrition. This is a process of identifying individuals who may be at nutrition risk and benefit from assessment from a registered dietitian,” Ms. Issokson said.
The Malnutrition Screening Tool is quick, easy to administer, and requires minimal training. It can be used to screen adults for malnutrition regardless of age, medical history, or setting, she said.
The two-item instrument asks, “Have you recently lost weight without trying?” with a “no” scored as 0 and a “yes” scored as 2. The second question is, “Have you been eating poorly because of decreased appetite, with a “no” equal to 0 and a “yes” equal to 1. Patients with a score of 0 or 1 are not at risk, whereas patients with scores of 2 or 3 are deemed to be at risk for malnutrition and require further assessment by a dietitian.
Assessment
Assessment for malnutrition involves a variety of factors, including anthropometric factors such as weight and BMI changes; biochemical markers such as fat-soluble vitamins, water-soluble vitamins, minerals, and urinary sodium; symptoms such as decreased appetite, abdominal pain, cramping or bloating, diarrhea, or urgency or obstructive symptoms; and body composition measures such as handgrip strength, biochemical impedance analysis, skinfold thickness, bone mineral density, and muscle mass.
Other nutritional assessment tools may include 24-hour recall of nutrition intake, diet history, and questions about eating behaviors, food allergies or intolerances, and cultural or religious food preferences.
Assessing food security is also important, especially during the current pandemic, Ms. Issokson emphasized.
“Is your patient running out of food? Do they have money to purchase food? Are they able to go to the grocery store to buy food? This is essential to know when you’re developing a nutrition plan,” she said.
A nutrition-focused physical exam should include assessment of skin manifestation, secondary to malnutrition or malabsorption, such as dry skin, delayed wound healing, stomatitis, scurvy, seborrheic dermatitis, bleeding, and periorificial and acral dermatitis or alopecia.
Diagnosis
Currently available malnutrition criteria have not been validated for use in patients with IBD, and further studies are needed to affirm their applicability to this population, Ms. Issokson said.
The Academy of Nutrition and Dietetics–American Society for Parenteral and Enteral Nutrition (AND-ASPEN) malnutrition criteria require measures of weight loss, energy intake, subcutaneous fat loss, subcutaneous muscle loss, general or local fluid accumulation, and handgrip strength to determine whether a patient is moderately or severely malnourished.
Ms. Issokson said that she finds the European Society for Clinical Nutrition and Metabolism Global Leadership Initiative on Malnutrition (ESPEN GLIM) criteria somewhat easier to use for diagnosis, as they consist of phenotypic and etiologic criteria, with patients who meet at least one of each being considered malnourished.
“When identified, document malnutrition, and of course intervene appropriately by referring to a dietitian providing education and supporting the patient to help them optimize their nutrition and improve their outcomes,” she concluded.
In a discussion following the session, panelist Neha Shah, MPH, RD, CNSC, a dietitian and health education specialist at the University of California, San Francisco, commented on the importance of malnutrition assessment in patients with IBD being considered for surgery.
Patients should be screened for malnutrition, and if they have a positive screen, “should be automatically referred to a registered dietitian specializing in IBD for a nutrition assessment,” she said.
“Certainly, a nutritional assessment, as Kelly has highlighted really well, will encompass an evaluation of various areas of health – patient history, food and nutrition history, changing anthropometrics, alterations in labs – and certainly going into further nutrition history with net food intolerance, intake from each food group, portions, access, support, culture, eating environment, skills in the kitchen, relationship with diet.”
Ms. Issokson is a board member of the Crohn’s & Colitis Foundation and a digital advisory board member of Avant Healthcare. Ms. Shah had no disclosures.
Malnutrition is common among patients with inflammatory bowel disease (IBD) and is associated with worse outcomes that can prolong hospitalizations and increase patients’ risk for death.
As many as 85% of inpatients with IBD may be malnourished, with the severity of malnutrition affected by disease activity, extent, and duration, said Kelly Issokson, MS, RD, CNSC, clinical nutrition coordinator in the IBD program in the division of gastroenterology at Cedars-Sinai Medical Center, Los Angeles.
“Malnutrition is a severe complication of IBD, and it should not be overlooked,” she said during an oral presentation at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
In patients with IBD, malabsorption, enteric losses, inadequate intake, and side effects of medical therapy can all lead to malnutrition, which in turn is an independent risk factor for venous thromboembolic events, nonelective surgery, longer hospital stays, and increased mortality.
In addition, malnutrition in IBD increases risk for infection and sepsis, and for perioperative complications, and can more than double the cost of care, compared with adequately nourished IBD patients, she said.
Ms. Issokson cited a definition of malnutrition from the American Society of Parenteral and Enteral Nutrition as “an acute or chronic state of overnutrition or undernutrition with or without inflammatory activity that has led to a change in body composition and diminished function.”
Lab findings of low albumin, low prealbumin, or isolated metrics such as weight loss or change in body mass index do not constitute malnutrition and should not be used to diagnosis it, Ms. Issokson cautioned.
Patients at low risk for malnutrition have no unintentional weight loss, are eating well, have minimal or no dietary restrictions, and no wasting. In contrast, high-risk patients have unintentional weight loss, decreased appetite and/or food intake, restrict multiple foods, or show signs of wasting.
Screening
“Nutrition screening is the first step in diagnosing a patient with malnutrition. This is a process of identifying individuals who may be at nutrition risk and benefit from assessment from a registered dietitian,” Ms. Issokson said.
The Malnutrition Screening Tool is quick, easy to administer, and requires minimal training. It can be used to screen adults for malnutrition regardless of age, medical history, or setting, she said.
The two-item instrument asks, “Have you recently lost weight without trying?” with a “no” scored as 0 and a “yes” scored as 2. The second question is, “Have you been eating poorly because of decreased appetite, with a “no” equal to 0 and a “yes” equal to 1. Patients with a score of 0 or 1 are not at risk, whereas patients with scores of 2 or 3 are deemed to be at risk for malnutrition and require further assessment by a dietitian.
Assessment
Assessment for malnutrition involves a variety of factors, including anthropometric factors such as weight and BMI changes; biochemical markers such as fat-soluble vitamins, water-soluble vitamins, minerals, and urinary sodium; symptoms such as decreased appetite, abdominal pain, cramping or bloating, diarrhea, or urgency or obstructive symptoms; and body composition measures such as handgrip strength, biochemical impedance analysis, skinfold thickness, bone mineral density, and muscle mass.
Other nutritional assessment tools may include 24-hour recall of nutrition intake, diet history, and questions about eating behaviors, food allergies or intolerances, and cultural or religious food preferences.
Assessing food security is also important, especially during the current pandemic, Ms. Issokson emphasized.
“Is your patient running out of food? Do they have money to purchase food? Are they able to go to the grocery store to buy food? This is essential to know when you’re developing a nutrition plan,” she said.
A nutrition-focused physical exam should include assessment of skin manifestation, secondary to malnutrition or malabsorption, such as dry skin, delayed wound healing, stomatitis, scurvy, seborrheic dermatitis, bleeding, and periorificial and acral dermatitis or alopecia.
Diagnosis
Currently available malnutrition criteria have not been validated for use in patients with IBD, and further studies are needed to affirm their applicability to this population, Ms. Issokson said.
The Academy of Nutrition and Dietetics–American Society for Parenteral and Enteral Nutrition (AND-ASPEN) malnutrition criteria require measures of weight loss, energy intake, subcutaneous fat loss, subcutaneous muscle loss, general or local fluid accumulation, and handgrip strength to determine whether a patient is moderately or severely malnourished.
Ms. Issokson said that she finds the European Society for Clinical Nutrition and Metabolism Global Leadership Initiative on Malnutrition (ESPEN GLIM) criteria somewhat easier to use for diagnosis, as they consist of phenotypic and etiologic criteria, with patients who meet at least one of each being considered malnourished.
“When identified, document malnutrition, and of course intervene appropriately by referring to a dietitian providing education and supporting the patient to help them optimize their nutrition and improve their outcomes,” she concluded.
In a discussion following the session, panelist Neha Shah, MPH, RD, CNSC, a dietitian and health education specialist at the University of California, San Francisco, commented on the importance of malnutrition assessment in patients with IBD being considered for surgery.
Patients should be screened for malnutrition, and if they have a positive screen, “should be automatically referred to a registered dietitian specializing in IBD for a nutrition assessment,” she said.
“Certainly, a nutritional assessment, as Kelly has highlighted really well, will encompass an evaluation of various areas of health – patient history, food and nutrition history, changing anthropometrics, alterations in labs – and certainly going into further nutrition history with net food intolerance, intake from each food group, portions, access, support, culture, eating environment, skills in the kitchen, relationship with diet.”
Ms. Issokson is a board member of the Crohn’s & Colitis Foundation and a digital advisory board member of Avant Healthcare. Ms. Shah had no disclosures.
FROM THE CROHN’S & COLITIS CONGRESS
CDC, AAP issues new guidelines to better define developmental milestones
The Centers for Disease Control and Prevention and the American Academy of Pediatrics recently issued revised milestone guidelines for their developmental surveillance campaign, Learn the Signs, Act Early (LTSAE).
The new guidelines, published in Pediatrics, were drafted in “easy-to-understand” language and identify the behaviors that 75% or more of children should exhibit at certain ages based on developmental resources, existing data, and clinician experience. The previous milestone checklists, developed in 2004, used 50th percentile or average-age milestones.
The CDC, in collaboration with the AAP, convened a group of eight subject matter experts in various fields of child development, including a developmental pediatrician and researcher from Kennedy Krieger Institute, to develop new and clearer guidelines.
“The goals of the group were to identify evidence-informed milestones to include in CDC checklists, clarify when most children can be expected to reach a milestone (to discourage a wait-and-see approach), and support clinical judgment regarding screening between recommended ages,” wrote lead author Jennifer M. Zubler, MD, of the National Center on Birth Defects and Developmental Disabilities in Atlanta, and colleagues.
Key changes
The experts established 11 criteria for CDC surveillance milestones and tools, including milestones most children (75% or more) would be expected to reach by defined health supervision visit ages and those that are easily recognized in natural settings.
Criteria for developmental milestones and surveillance tools:
- Milestones are included at the age most (≥75%) children would be expected to demonstrate the milestone.
- Eliminate “warning signs.”
- Are easy for families of different social, cultural, and ethnic backgrounds to observe and use.
- Are able to be answered with yes, not yet, or not sure.
- Use plain language, avoiding vague terms like may, can, and begins.
- Are organized in developmental domains.
- Show progression of skills with age, when possible.
- Milestones are not repeated across checklists.
- Include open-ended questions.
- Include information for developmental promotion.
- Include information on how to act early if there are concerns.
The previous guidelines were critiqued by some clinicians as being “not helpful to individual families who had concerns about their child’s development,” and in some cases, led to delays in diagnoses as decision-makers opted for a “wait-and-see approach.”
“The earlier a child is identified with a developmental delay the better, as treatment as well as learning interventions can begin,” Paul Lipkin, MD, an associate professor of pediatrics at the Johns Hopkins University, Baltimore, said in an accompanying press release. “Revising the guidelines with expertise and data from clinicians in the field accomplishes these goals.”
Additional changes included new checklists for children between the ages of 15 and 30 months, additional social and emotional milestones, as well as the removal of complex language and duplicate milestones. The experts also developed new, open-ended questions to aid discussions with families.
“Review of a child’s development with these milestones opens up a continuous dialogue between a parent and the health care provider about their child’s present and future development,” said Dr. Lipkin.
Originally pioneered in 2005, the LTSAE awareness campaign provides free resources to clinicians and families to support early detection of children with developmental delays and disabilities. After the new guidelines were drafted, they were presented to parents of various racial groups, income levels, and educational backgrounds to confirm ease of use and understandability.
“These criteria and revised checklists can be used to support developmental surveillance, clinical judgment regarding additional developmental screening, and research in developmental surveillance processes,” wrote Dr. Zubler.
Expert perspective
“These new guidelines will allow us to catch more children with developmental delays as they raise the threshold to 75% of children achieving those milestones at that particular age,” Karalyn Kinsella, MD, a pediatrician in Cheshire, Conn., said in an interview.
Dr. Kinsella added that the new guidelines simplify the milestones and reduce redundancy across different developmental domains. “Most importantly, it gave me the opportunity to see just how great the CDC milestone tracker app is – I think parents would really like it.”
This project was supported by the CDC and Prevention of the Department of Health & Human Services. One author is a developer of the Ages & Stages Questionnaires and receives royalties from Brookes Publishing, the company that publishes this tool; the other authors have indicated they have no relevant conflicts of interest to disclose.
The Centers for Disease Control and Prevention and the American Academy of Pediatrics recently issued revised milestone guidelines for their developmental surveillance campaign, Learn the Signs, Act Early (LTSAE).
The new guidelines, published in Pediatrics, were drafted in “easy-to-understand” language and identify the behaviors that 75% or more of children should exhibit at certain ages based on developmental resources, existing data, and clinician experience. The previous milestone checklists, developed in 2004, used 50th percentile or average-age milestones.
The CDC, in collaboration with the AAP, convened a group of eight subject matter experts in various fields of child development, including a developmental pediatrician and researcher from Kennedy Krieger Institute, to develop new and clearer guidelines.
“The goals of the group were to identify evidence-informed milestones to include in CDC checklists, clarify when most children can be expected to reach a milestone (to discourage a wait-and-see approach), and support clinical judgment regarding screening between recommended ages,” wrote lead author Jennifer M. Zubler, MD, of the National Center on Birth Defects and Developmental Disabilities in Atlanta, and colleagues.
Key changes
The experts established 11 criteria for CDC surveillance milestones and tools, including milestones most children (75% or more) would be expected to reach by defined health supervision visit ages and those that are easily recognized in natural settings.
Criteria for developmental milestones and surveillance tools:
- Milestones are included at the age most (≥75%) children would be expected to demonstrate the milestone.
- Eliminate “warning signs.”
- Are easy for families of different social, cultural, and ethnic backgrounds to observe and use.
- Are able to be answered with yes, not yet, or not sure.
- Use plain language, avoiding vague terms like may, can, and begins.
- Are organized in developmental domains.
- Show progression of skills with age, when possible.
- Milestones are not repeated across checklists.
- Include open-ended questions.
- Include information for developmental promotion.
- Include information on how to act early if there are concerns.
The previous guidelines were critiqued by some clinicians as being “not helpful to individual families who had concerns about their child’s development,” and in some cases, led to delays in diagnoses as decision-makers opted for a “wait-and-see approach.”
“The earlier a child is identified with a developmental delay the better, as treatment as well as learning interventions can begin,” Paul Lipkin, MD, an associate professor of pediatrics at the Johns Hopkins University, Baltimore, said in an accompanying press release. “Revising the guidelines with expertise and data from clinicians in the field accomplishes these goals.”
Additional changes included new checklists for children between the ages of 15 and 30 months, additional social and emotional milestones, as well as the removal of complex language and duplicate milestones. The experts also developed new, open-ended questions to aid discussions with families.
“Review of a child’s development with these milestones opens up a continuous dialogue between a parent and the health care provider about their child’s present and future development,” said Dr. Lipkin.
Originally pioneered in 2005, the LTSAE awareness campaign provides free resources to clinicians and families to support early detection of children with developmental delays and disabilities. After the new guidelines were drafted, they were presented to parents of various racial groups, income levels, and educational backgrounds to confirm ease of use and understandability.
“These criteria and revised checklists can be used to support developmental surveillance, clinical judgment regarding additional developmental screening, and research in developmental surveillance processes,” wrote Dr. Zubler.
Expert perspective
“These new guidelines will allow us to catch more children with developmental delays as they raise the threshold to 75% of children achieving those milestones at that particular age,” Karalyn Kinsella, MD, a pediatrician in Cheshire, Conn., said in an interview.
Dr. Kinsella added that the new guidelines simplify the milestones and reduce redundancy across different developmental domains. “Most importantly, it gave me the opportunity to see just how great the CDC milestone tracker app is – I think parents would really like it.”
This project was supported by the CDC and Prevention of the Department of Health & Human Services. One author is a developer of the Ages & Stages Questionnaires and receives royalties from Brookes Publishing, the company that publishes this tool; the other authors have indicated they have no relevant conflicts of interest to disclose.
The Centers for Disease Control and Prevention and the American Academy of Pediatrics recently issued revised milestone guidelines for their developmental surveillance campaign, Learn the Signs, Act Early (LTSAE).
The new guidelines, published in Pediatrics, were drafted in “easy-to-understand” language and identify the behaviors that 75% or more of children should exhibit at certain ages based on developmental resources, existing data, and clinician experience. The previous milestone checklists, developed in 2004, used 50th percentile or average-age milestones.
The CDC, in collaboration with the AAP, convened a group of eight subject matter experts in various fields of child development, including a developmental pediatrician and researcher from Kennedy Krieger Institute, to develop new and clearer guidelines.
“The goals of the group were to identify evidence-informed milestones to include in CDC checklists, clarify when most children can be expected to reach a milestone (to discourage a wait-and-see approach), and support clinical judgment regarding screening between recommended ages,” wrote lead author Jennifer M. Zubler, MD, of the National Center on Birth Defects and Developmental Disabilities in Atlanta, and colleagues.
Key changes
The experts established 11 criteria for CDC surveillance milestones and tools, including milestones most children (75% or more) would be expected to reach by defined health supervision visit ages and those that are easily recognized in natural settings.
Criteria for developmental milestones and surveillance tools:
- Milestones are included at the age most (≥75%) children would be expected to demonstrate the milestone.
- Eliminate “warning signs.”
- Are easy for families of different social, cultural, and ethnic backgrounds to observe and use.
- Are able to be answered with yes, not yet, or not sure.
- Use plain language, avoiding vague terms like may, can, and begins.
- Are organized in developmental domains.
- Show progression of skills with age, when possible.
- Milestones are not repeated across checklists.
- Include open-ended questions.
- Include information for developmental promotion.
- Include information on how to act early if there are concerns.
The previous guidelines were critiqued by some clinicians as being “not helpful to individual families who had concerns about their child’s development,” and in some cases, led to delays in diagnoses as decision-makers opted for a “wait-and-see approach.”
“The earlier a child is identified with a developmental delay the better, as treatment as well as learning interventions can begin,” Paul Lipkin, MD, an associate professor of pediatrics at the Johns Hopkins University, Baltimore, said in an accompanying press release. “Revising the guidelines with expertise and data from clinicians in the field accomplishes these goals.”
Additional changes included new checklists for children between the ages of 15 and 30 months, additional social and emotional milestones, as well as the removal of complex language and duplicate milestones. The experts also developed new, open-ended questions to aid discussions with families.
“Review of a child’s development with these milestones opens up a continuous dialogue between a parent and the health care provider about their child’s present and future development,” said Dr. Lipkin.
Originally pioneered in 2005, the LTSAE awareness campaign provides free resources to clinicians and families to support early detection of children with developmental delays and disabilities. After the new guidelines were drafted, they were presented to parents of various racial groups, income levels, and educational backgrounds to confirm ease of use and understandability.
“These criteria and revised checklists can be used to support developmental surveillance, clinical judgment regarding additional developmental screening, and research in developmental surveillance processes,” wrote Dr. Zubler.
Expert perspective
“These new guidelines will allow us to catch more children with developmental delays as they raise the threshold to 75% of children achieving those milestones at that particular age,” Karalyn Kinsella, MD, a pediatrician in Cheshire, Conn., said in an interview.
Dr. Kinsella added that the new guidelines simplify the milestones and reduce redundancy across different developmental domains. “Most importantly, it gave me the opportunity to see just how great the CDC milestone tracker app is – I think parents would really like it.”
This project was supported by the CDC and Prevention of the Department of Health & Human Services. One author is a developer of the Ages & Stages Questionnaires and receives royalties from Brookes Publishing, the company that publishes this tool; the other authors have indicated they have no relevant conflicts of interest to disclose.
FROM PEDIATRICS
Sports experts on T2D: Boost activity, cut sedentary time
The American College of Sports Medicine (ACSM) has issued new recommendations for exercise/physical activity in people with type 2 diabetes, which update a 2010 joint ACSM/American Diabetes Association position statement.
The guidance has been published in the February issue of Medicine & Science in Sports & Exercise.
“This consensus statement provides a brief summary of the current evidence and extends and updates the prior recommendations,” the authors explain.
In the past decade, there has been a “considerable amount” of research about exercise in people with type 2 diabetes, they add, while the prevalence of diabetes has steadily increased.
The updated recommendations have been “expanded to include physical activity – a broader, more comprehensive definition of human movement than planned exercise – and reducing sedentary time,” the authors note.
“The latest guidelines are applicable to most individuals with diabetes, including youth, with a few exceptions and modifications,” lead author Jill A. Kanaley, PhD, said in a press release from the ACSM.
The key takeaway is that “all individuals [with type 2 diabetes] should engage in regular physical activity, reduce sedentary time, and break up sitting time with frequent activity breaks,” said Dr. Kanaley, a professor in the department of nutrition and exercise physiology, University of Missouri, Columbia.
“Exercise can play an important role in managing type 2 diabetes, and workouts can be modified to fit the abilities of most people,” she stressed.
And those with type 2 diabetes who want to lose weight “should consider workouts of moderately high volume for 4 to 5 days per week,” she added.
Six key tips for physical activity in adults with type 2 diabetes
The consensus statement gives six key tips for physical activity in adults with type 2 diabetes, as follows.
- Regular aerobic exercise improves glycemic management; meta-analyses have reported fewer daily hyperglycemic episodes and reductions in A1c of 0.5%-0.7%.
- High-intensity resistance exercise, when performed safely, is better than low-to-moderate intensity resistance exercise for glucose management and attenuation of insulin levels. Resistance exercise typically results in improvements of 10% to 15% in strength, bone mineral density, blood pressure, lipid profile, skeletal muscle mass, and insulin sensitivity.
- Exercise after meals, such as taking a walk after dinner at one’s own pace, takes advantage of the blood glucose-stabilizing effects of exercise.
- Reduce sedentary time by taking regular breaks for small “doses” of physical activity, which can modestly attenuate postprandial glucose and insulin levels, particularly in individuals with insulin resistance and a higher body mass index.
- To prevent hypoglycemia during or after exercise, people taking insulin or insulin secretagogues should increase carbohydrate intake, or if possible, reduce insulin.
- People who are taking beta blockers should not rely on a heart monitor to measure workout intensity. They could ask a certified exercise professional about using ratings of perceived exertion to track how a workout feels.
Other recommendations
The consensus statement also summarizes precautions that people with complications of type 2 diabetes (such as neuropathy, retinopathy, kidney disease, and hypertension) should take.
Low impact exercises for flexibility can help introduce sedentary people to physical activity, the consensus group writes. Balance exercises can be helpful for older adults.
Weight loss greater than 5% can benefit A1c, blood lipid, and blood pressure levels. Moderate exercise 4 to 5 days a week can reduce visceral fat.
In studies of youth with type 2 diabetes, intensive lifestyle interventions plus metformin were not superior to metformin alone for managing glycemia. Physical activity goals are the same for youth with or without diabetes.
Pregnant women with diabetes should participate in at least 20 to 30 minutes of moderate-intensity exercise most days of the week.
Participating in an exercise program before and after bariatric surgery may enhance surgical outcomes.
Dr. Kanaley has reported receiving a grant from the National Institutes of Health. Disclosures for the other authors are listed in the article.
A version of this article first appeared on Medscape.com.
The American College of Sports Medicine (ACSM) has issued new recommendations for exercise/physical activity in people with type 2 diabetes, which update a 2010 joint ACSM/American Diabetes Association position statement.
The guidance has been published in the February issue of Medicine & Science in Sports & Exercise.
“This consensus statement provides a brief summary of the current evidence and extends and updates the prior recommendations,” the authors explain.
In the past decade, there has been a “considerable amount” of research about exercise in people with type 2 diabetes, they add, while the prevalence of diabetes has steadily increased.
The updated recommendations have been “expanded to include physical activity – a broader, more comprehensive definition of human movement than planned exercise – and reducing sedentary time,” the authors note.
“The latest guidelines are applicable to most individuals with diabetes, including youth, with a few exceptions and modifications,” lead author Jill A. Kanaley, PhD, said in a press release from the ACSM.
The key takeaway is that “all individuals [with type 2 diabetes] should engage in regular physical activity, reduce sedentary time, and break up sitting time with frequent activity breaks,” said Dr. Kanaley, a professor in the department of nutrition and exercise physiology, University of Missouri, Columbia.
“Exercise can play an important role in managing type 2 diabetes, and workouts can be modified to fit the abilities of most people,” she stressed.
And those with type 2 diabetes who want to lose weight “should consider workouts of moderately high volume for 4 to 5 days per week,” she added.
Six key tips for physical activity in adults with type 2 diabetes
The consensus statement gives six key tips for physical activity in adults with type 2 diabetes, as follows.
- Regular aerobic exercise improves glycemic management; meta-analyses have reported fewer daily hyperglycemic episodes and reductions in A1c of 0.5%-0.7%.
- High-intensity resistance exercise, when performed safely, is better than low-to-moderate intensity resistance exercise for glucose management and attenuation of insulin levels. Resistance exercise typically results in improvements of 10% to 15% in strength, bone mineral density, blood pressure, lipid profile, skeletal muscle mass, and insulin sensitivity.
- Exercise after meals, such as taking a walk after dinner at one’s own pace, takes advantage of the blood glucose-stabilizing effects of exercise.
- Reduce sedentary time by taking regular breaks for small “doses” of physical activity, which can modestly attenuate postprandial glucose and insulin levels, particularly in individuals with insulin resistance and a higher body mass index.
- To prevent hypoglycemia during or after exercise, people taking insulin or insulin secretagogues should increase carbohydrate intake, or if possible, reduce insulin.
- People who are taking beta blockers should not rely on a heart monitor to measure workout intensity. They could ask a certified exercise professional about using ratings of perceived exertion to track how a workout feels.
Other recommendations
The consensus statement also summarizes precautions that people with complications of type 2 diabetes (such as neuropathy, retinopathy, kidney disease, and hypertension) should take.
Low impact exercises for flexibility can help introduce sedentary people to physical activity, the consensus group writes. Balance exercises can be helpful for older adults.
Weight loss greater than 5% can benefit A1c, blood lipid, and blood pressure levels. Moderate exercise 4 to 5 days a week can reduce visceral fat.
In studies of youth with type 2 diabetes, intensive lifestyle interventions plus metformin were not superior to metformin alone for managing glycemia. Physical activity goals are the same for youth with or without diabetes.
Pregnant women with diabetes should participate in at least 20 to 30 minutes of moderate-intensity exercise most days of the week.
Participating in an exercise program before and after bariatric surgery may enhance surgical outcomes.
Dr. Kanaley has reported receiving a grant from the National Institutes of Health. Disclosures for the other authors are listed in the article.
A version of this article first appeared on Medscape.com.
The American College of Sports Medicine (ACSM) has issued new recommendations for exercise/physical activity in people with type 2 diabetes, which update a 2010 joint ACSM/American Diabetes Association position statement.
The guidance has been published in the February issue of Medicine & Science in Sports & Exercise.
“This consensus statement provides a brief summary of the current evidence and extends and updates the prior recommendations,” the authors explain.
In the past decade, there has been a “considerable amount” of research about exercise in people with type 2 diabetes, they add, while the prevalence of diabetes has steadily increased.
The updated recommendations have been “expanded to include physical activity – a broader, more comprehensive definition of human movement than planned exercise – and reducing sedentary time,” the authors note.
“The latest guidelines are applicable to most individuals with diabetes, including youth, with a few exceptions and modifications,” lead author Jill A. Kanaley, PhD, said in a press release from the ACSM.
The key takeaway is that “all individuals [with type 2 diabetes] should engage in regular physical activity, reduce sedentary time, and break up sitting time with frequent activity breaks,” said Dr. Kanaley, a professor in the department of nutrition and exercise physiology, University of Missouri, Columbia.
“Exercise can play an important role in managing type 2 diabetes, and workouts can be modified to fit the abilities of most people,” she stressed.
And those with type 2 diabetes who want to lose weight “should consider workouts of moderately high volume for 4 to 5 days per week,” she added.
Six key tips for physical activity in adults with type 2 diabetes
The consensus statement gives six key tips for physical activity in adults with type 2 diabetes, as follows.
- Regular aerobic exercise improves glycemic management; meta-analyses have reported fewer daily hyperglycemic episodes and reductions in A1c of 0.5%-0.7%.
- High-intensity resistance exercise, when performed safely, is better than low-to-moderate intensity resistance exercise for glucose management and attenuation of insulin levels. Resistance exercise typically results in improvements of 10% to 15% in strength, bone mineral density, blood pressure, lipid profile, skeletal muscle mass, and insulin sensitivity.
- Exercise after meals, such as taking a walk after dinner at one’s own pace, takes advantage of the blood glucose-stabilizing effects of exercise.
- Reduce sedentary time by taking regular breaks for small “doses” of physical activity, which can modestly attenuate postprandial glucose and insulin levels, particularly in individuals with insulin resistance and a higher body mass index.
- To prevent hypoglycemia during or after exercise, people taking insulin or insulin secretagogues should increase carbohydrate intake, or if possible, reduce insulin.
- People who are taking beta blockers should not rely on a heart monitor to measure workout intensity. They could ask a certified exercise professional about using ratings of perceived exertion to track how a workout feels.
Other recommendations
The consensus statement also summarizes precautions that people with complications of type 2 diabetes (such as neuropathy, retinopathy, kidney disease, and hypertension) should take.
Low impact exercises for flexibility can help introduce sedentary people to physical activity, the consensus group writes. Balance exercises can be helpful for older adults.
Weight loss greater than 5% can benefit A1c, blood lipid, and blood pressure levels. Moderate exercise 4 to 5 days a week can reduce visceral fat.
In studies of youth with type 2 diabetes, intensive lifestyle interventions plus metformin were not superior to metformin alone for managing glycemia. Physical activity goals are the same for youth with or without diabetes.
Pregnant women with diabetes should participate in at least 20 to 30 minutes of moderate-intensity exercise most days of the week.
Participating in an exercise program before and after bariatric surgery may enhance surgical outcomes.
Dr. Kanaley has reported receiving a grant from the National Institutes of Health. Disclosures for the other authors are listed in the article.
A version of this article first appeared on Medscape.com.
Derms in survey say climate change is impacting their patients
in which the majority of participants said their patients are already being impacted.
Almost 80% of the 148 participants who responded to an electronic survey reported this belief.
The survey was designed and distributed to the membership of various dermatological organizations by Misha Rosenbach, MD, and coauthors. The results were published in the British Journal of Dermatology.
Asked also about specific types of climate-driven phenomena with a current – or future – impact on their patients, 80.1% reported that they believed that increased exposure to ultraviolet radiation (UVR) is impactful, or will be. Changes in temporal or geographic patterns of vector-borne illnesses were affirmed by 78.7%, and an increase in social displacement caused by extreme weather or other events was affirmed by 67.1% as having an impact on their patients currently or in the future.
Other phenomena affirmed by respondents as already having an impact or impacting patients in the future were an increased incidence of heat exposure or heat-related illness (58.2%); an increase in rates of inflammatory skin disease flares (43.2%); increased incidence of waterborne infections (42.5%); and increased rates of allergic contact dermatitis (29.5%).
The survey was sent to the membership of the American Society of Dermatologic Surgery, the Society for Pediatric Dermatology, the Society for Investigative Dermatology, and the American Academy of Dermatology’s Climate Change Expert Resource Group (ERG), among other organizations.
The study design and membership overlap made it impossible to calculate a response rate, the authors said, but they estimated it to be about 10%.
Almost all respondents were from the United States, and most (86.3%) practiced in an academic setting. The findings are similar to those of an online survey of members of the International Society of Dermatology (ISD), published in 2020, which found that 89% of 158 respondents believed climate change will impact the incidence of skin diseases in their area.
“Physicians, including dermatologists, are starting to understand the impact of the climate crisis on both their patients and themselves ... both through lived experiences and [issues raised] more in the scientific literature and in meetings,” Dr. Rosenbach, associate professor of dermatology at the University of Pennsylvania, Philadelphia, said in an interview.
A majority of participants in the U.S. survey agreed they have a responsibility to bring awareness of the health effects of climate change to patients (77.2%) and to policymakers (88.6%). (In the ISD survey, 88% said they believed that dermatologists should play an advocacy role in climate change-related issues).
Only a minority of respondents in the U.S. survey said that they would feel comfortable discussing climate change with their patients (37.2%). Almost one-third of the respondents said they would like to be better informed about climate change before doing so. And 81.8% said they would like to read more about the dermatological effects of climate change in scientific journals.
“There continues to be unfilled interest in education and advocacy regarding climate change, suggesting a ‘practice gap’ even among dermatologists,” Dr. Rosenbach and his colleagues wrote, noting opportunities for professional organizations and journals to provide more resources and “actionable items” regarding climate change.
Some dermatologists have been taking action, in the meantime, to reduce the carbon footprint of their practices and institutions. Reductions in facility energy consumption, and reductions in medical waste/optimization of recycling, were each reported by more than one-third of survey respondents.
And almost half indicated that their practice or institution had increased capacity for telemedicine or telecommuting in response to climate change. Only 8% said their practice or institution had divested from fossil fuel stocks and/or bonds.
“There are a lot of sustainability-in-medicine solutions that are actually cost-neutral or cost-saving for practices,” said Dr. Rosenbach, who is a founder and co-chair of the AAD’s ERG on Climate Change and Environmental Issues.
Research in dermatology is starting to quantify the environmental impact of some of these changes. In a research letter also published in the British Journal of Dermatology, researchers from Cardiff University and the department of dermatology at University Hospital of Wales, described how they determined that reusable surgical packs used for skin surgery are more sustainable than single-use packs because of their reduced cost and reduced greenhouse gas emissions.
Such single-site reports are “early feeders” into what will become a stream of larger studies quantifying the impact of measures taken in dermatology, Dr. Rosenbach said.
Across medicine, there is evidence that health care professionals are now seeing climate change as a threat to their patients. In a multinational survey published last year in The Lancet Planetary Health, 77% of 3,977 participants said that climate change will cause a moderate or great deal of harm for their patients.
Climate change will be discussed at the AAD’s annual meeting in late March in a session devoted to the topic, and as part of a broader session on controversies in dermatology.
Dr. Rosenbach and two of the five authors of the dermatology research letter are members of the AAD’s ERG on climate change, but in the publication they noted that they were not writing on behalf of the AAD. None of the authors reported any disclosures, and there was no funding source for the survey.
in which the majority of participants said their patients are already being impacted.
Almost 80% of the 148 participants who responded to an electronic survey reported this belief.
The survey was designed and distributed to the membership of various dermatological organizations by Misha Rosenbach, MD, and coauthors. The results were published in the British Journal of Dermatology.
Asked also about specific types of climate-driven phenomena with a current – or future – impact on their patients, 80.1% reported that they believed that increased exposure to ultraviolet radiation (UVR) is impactful, or will be. Changes in temporal or geographic patterns of vector-borne illnesses were affirmed by 78.7%, and an increase in social displacement caused by extreme weather or other events was affirmed by 67.1% as having an impact on their patients currently or in the future.
Other phenomena affirmed by respondents as already having an impact or impacting patients in the future were an increased incidence of heat exposure or heat-related illness (58.2%); an increase in rates of inflammatory skin disease flares (43.2%); increased incidence of waterborne infections (42.5%); and increased rates of allergic contact dermatitis (29.5%).
The survey was sent to the membership of the American Society of Dermatologic Surgery, the Society for Pediatric Dermatology, the Society for Investigative Dermatology, and the American Academy of Dermatology’s Climate Change Expert Resource Group (ERG), among other organizations.
The study design and membership overlap made it impossible to calculate a response rate, the authors said, but they estimated it to be about 10%.
Almost all respondents were from the United States, and most (86.3%) practiced in an academic setting. The findings are similar to those of an online survey of members of the International Society of Dermatology (ISD), published in 2020, which found that 89% of 158 respondents believed climate change will impact the incidence of skin diseases in their area.
“Physicians, including dermatologists, are starting to understand the impact of the climate crisis on both their patients and themselves ... both through lived experiences and [issues raised] more in the scientific literature and in meetings,” Dr. Rosenbach, associate professor of dermatology at the University of Pennsylvania, Philadelphia, said in an interview.
A majority of participants in the U.S. survey agreed they have a responsibility to bring awareness of the health effects of climate change to patients (77.2%) and to policymakers (88.6%). (In the ISD survey, 88% said they believed that dermatologists should play an advocacy role in climate change-related issues).
Only a minority of respondents in the U.S. survey said that they would feel comfortable discussing climate change with their patients (37.2%). Almost one-third of the respondents said they would like to be better informed about climate change before doing so. And 81.8% said they would like to read more about the dermatological effects of climate change in scientific journals.
“There continues to be unfilled interest in education and advocacy regarding climate change, suggesting a ‘practice gap’ even among dermatologists,” Dr. Rosenbach and his colleagues wrote, noting opportunities for professional organizations and journals to provide more resources and “actionable items” regarding climate change.
Some dermatologists have been taking action, in the meantime, to reduce the carbon footprint of their practices and institutions. Reductions in facility energy consumption, and reductions in medical waste/optimization of recycling, were each reported by more than one-third of survey respondents.
And almost half indicated that their practice or institution had increased capacity for telemedicine or telecommuting in response to climate change. Only 8% said their practice or institution had divested from fossil fuel stocks and/or bonds.
“There are a lot of sustainability-in-medicine solutions that are actually cost-neutral or cost-saving for practices,” said Dr. Rosenbach, who is a founder and co-chair of the AAD’s ERG on Climate Change and Environmental Issues.
Research in dermatology is starting to quantify the environmental impact of some of these changes. In a research letter also published in the British Journal of Dermatology, researchers from Cardiff University and the department of dermatology at University Hospital of Wales, described how they determined that reusable surgical packs used for skin surgery are more sustainable than single-use packs because of their reduced cost and reduced greenhouse gas emissions.
Such single-site reports are “early feeders” into what will become a stream of larger studies quantifying the impact of measures taken in dermatology, Dr. Rosenbach said.
Across medicine, there is evidence that health care professionals are now seeing climate change as a threat to their patients. In a multinational survey published last year in The Lancet Planetary Health, 77% of 3,977 participants said that climate change will cause a moderate or great deal of harm for their patients.
Climate change will be discussed at the AAD’s annual meeting in late March in a session devoted to the topic, and as part of a broader session on controversies in dermatology.
Dr. Rosenbach and two of the five authors of the dermatology research letter are members of the AAD’s ERG on climate change, but in the publication they noted that they were not writing on behalf of the AAD. None of the authors reported any disclosures, and there was no funding source for the survey.
in which the majority of participants said their patients are already being impacted.
Almost 80% of the 148 participants who responded to an electronic survey reported this belief.
The survey was designed and distributed to the membership of various dermatological organizations by Misha Rosenbach, MD, and coauthors. The results were published in the British Journal of Dermatology.
Asked also about specific types of climate-driven phenomena with a current – or future – impact on their patients, 80.1% reported that they believed that increased exposure to ultraviolet radiation (UVR) is impactful, or will be. Changes in temporal or geographic patterns of vector-borne illnesses were affirmed by 78.7%, and an increase in social displacement caused by extreme weather or other events was affirmed by 67.1% as having an impact on their patients currently or in the future.
Other phenomena affirmed by respondents as already having an impact or impacting patients in the future were an increased incidence of heat exposure or heat-related illness (58.2%); an increase in rates of inflammatory skin disease flares (43.2%); increased incidence of waterborne infections (42.5%); and increased rates of allergic contact dermatitis (29.5%).
The survey was sent to the membership of the American Society of Dermatologic Surgery, the Society for Pediatric Dermatology, the Society for Investigative Dermatology, and the American Academy of Dermatology’s Climate Change Expert Resource Group (ERG), among other organizations.
The study design and membership overlap made it impossible to calculate a response rate, the authors said, but they estimated it to be about 10%.
Almost all respondents were from the United States, and most (86.3%) practiced in an academic setting. The findings are similar to those of an online survey of members of the International Society of Dermatology (ISD), published in 2020, which found that 89% of 158 respondents believed climate change will impact the incidence of skin diseases in their area.
“Physicians, including dermatologists, are starting to understand the impact of the climate crisis on both their patients and themselves ... both through lived experiences and [issues raised] more in the scientific literature and in meetings,” Dr. Rosenbach, associate professor of dermatology at the University of Pennsylvania, Philadelphia, said in an interview.
A majority of participants in the U.S. survey agreed they have a responsibility to bring awareness of the health effects of climate change to patients (77.2%) and to policymakers (88.6%). (In the ISD survey, 88% said they believed that dermatologists should play an advocacy role in climate change-related issues).
Only a minority of respondents in the U.S. survey said that they would feel comfortable discussing climate change with their patients (37.2%). Almost one-third of the respondents said they would like to be better informed about climate change before doing so. And 81.8% said they would like to read more about the dermatological effects of climate change in scientific journals.
“There continues to be unfilled interest in education and advocacy regarding climate change, suggesting a ‘practice gap’ even among dermatologists,” Dr. Rosenbach and his colleagues wrote, noting opportunities for professional organizations and journals to provide more resources and “actionable items” regarding climate change.
Some dermatologists have been taking action, in the meantime, to reduce the carbon footprint of their practices and institutions. Reductions in facility energy consumption, and reductions in medical waste/optimization of recycling, were each reported by more than one-third of survey respondents.
And almost half indicated that their practice or institution had increased capacity for telemedicine or telecommuting in response to climate change. Only 8% said their practice or institution had divested from fossil fuel stocks and/or bonds.
“There are a lot of sustainability-in-medicine solutions that are actually cost-neutral or cost-saving for practices,” said Dr. Rosenbach, who is a founder and co-chair of the AAD’s ERG on Climate Change and Environmental Issues.
Research in dermatology is starting to quantify the environmental impact of some of these changes. In a research letter also published in the British Journal of Dermatology, researchers from Cardiff University and the department of dermatology at University Hospital of Wales, described how they determined that reusable surgical packs used for skin surgery are more sustainable than single-use packs because of their reduced cost and reduced greenhouse gas emissions.
Such single-site reports are “early feeders” into what will become a stream of larger studies quantifying the impact of measures taken in dermatology, Dr. Rosenbach said.
Across medicine, there is evidence that health care professionals are now seeing climate change as a threat to their patients. In a multinational survey published last year in The Lancet Planetary Health, 77% of 3,977 participants said that climate change will cause a moderate or great deal of harm for their patients.
Climate change will be discussed at the AAD’s annual meeting in late March in a session devoted to the topic, and as part of a broader session on controversies in dermatology.
Dr. Rosenbach and two of the five authors of the dermatology research letter are members of the AAD’s ERG on climate change, but in the publication they noted that they were not writing on behalf of the AAD. None of the authors reported any disclosures, and there was no funding source for the survey.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Small group of higher-volume antibiotic prescribers identified
“Higher-volume prescribers prescribed antibiotics to a larger share of their patient panel and their prescribing rate was 60% higher than that of lower-volume prescribers, indicating that their prescribing practices might be independent of the number of beneficiaries under their care,” Katryna A. Gouin, MPH, and associates wrote in the Morbidity and Mortality Weekly Report.
In 2019, 41% of all Part D antibiotics – that’s 24.4 million prescriptions – were prescribed by 69,835 higher-volume prescribers. The other 59% of all antibiotics were prescribed by the 627,000 lower-volume health care providers included in the analysis (those who prescribed fewer than 11 antibiotics were excluded), Ms. Gouin of Chenega in Anchorage, Alaska, and associates noted.
The analysis involved the Medicare Part D Prescribers by Provider data set and defined the highest-volume prescribers “as those in the highest 10th percentile of prescriber-level antibiotic volume (number of antibiotic prescriptions filled) across all Medicare providers nationwide,” the investigators explained.
The antibiotic-prescribing rate for the higher-volume prescribers was 680 prescriptions per 1,000 beneficiaries, which was 60% higher than the 426 prescriptions per 1,000 among the lower 90% of prescribers. Another way to look at it: The top 10% of health care providers “wrote a median of 284 antibiotic prescriptions, compared with a median of 41 among lower-volume prescribers,” the investigators said.
Physicians in internal medicine and family practice, the two largest medical specialties, were the most likely to be 10-percenters, accounting for 24.6% and 27.5%, respectively, of the higher-volume group. They were followed by nurse practitioners (14.1%) and physician assistants (7.4%), who were classified as specialists for the purposes of the study, Ms. Gouin and associates said.
The only other group of physicians among the top six specialties were urologists, who represented 6.8% of high-volume prescribers but only 1% of all prescribers, they noted.
The highest antibiotic prescription rate in the six largest groups of providers occurred among dentists, whose highest-prescribing practitioners wrote 1,271 prescriptions per 1,000 beneficiaries. Even the lower-prescribing 90% of dentists prescribed more antibiotics (1,068 per 1,000) than did the higher-prescribing family physicians (611 per 1,000) and internists (590 per 1,000), the researchers said.
The prescribing rates for all the other specialties that were not included separately also were higher than the family physicians’ and internists’. These rates were 850 per 1,000 beneficiaries for the higher-prescribers and 360 per 1,000 for the lower-prescribers, the researchers wrote.
The considerable differences in prescribing practices between specialties and even among those of the same specialty present “opportunities for improved prescribing through antibiotic stewardship activities focusing on these higher-volume prescribers, independent of specialty,” Ms. Gouin and associates wrote.
“Higher-volume prescribers prescribed antibiotics to a larger share of their patient panel and their prescribing rate was 60% higher than that of lower-volume prescribers, indicating that their prescribing practices might be independent of the number of beneficiaries under their care,” Katryna A. Gouin, MPH, and associates wrote in the Morbidity and Mortality Weekly Report.
In 2019, 41% of all Part D antibiotics – that’s 24.4 million prescriptions – were prescribed by 69,835 higher-volume prescribers. The other 59% of all antibiotics were prescribed by the 627,000 lower-volume health care providers included in the analysis (those who prescribed fewer than 11 antibiotics were excluded), Ms. Gouin of Chenega in Anchorage, Alaska, and associates noted.
The analysis involved the Medicare Part D Prescribers by Provider data set and defined the highest-volume prescribers “as those in the highest 10th percentile of prescriber-level antibiotic volume (number of antibiotic prescriptions filled) across all Medicare providers nationwide,” the investigators explained.
The antibiotic-prescribing rate for the higher-volume prescribers was 680 prescriptions per 1,000 beneficiaries, which was 60% higher than the 426 prescriptions per 1,000 among the lower 90% of prescribers. Another way to look at it: The top 10% of health care providers “wrote a median of 284 antibiotic prescriptions, compared with a median of 41 among lower-volume prescribers,” the investigators said.
Physicians in internal medicine and family practice, the two largest medical specialties, were the most likely to be 10-percenters, accounting for 24.6% and 27.5%, respectively, of the higher-volume group. They were followed by nurse practitioners (14.1%) and physician assistants (7.4%), who were classified as specialists for the purposes of the study, Ms. Gouin and associates said.
The only other group of physicians among the top six specialties were urologists, who represented 6.8% of high-volume prescribers but only 1% of all prescribers, they noted.
The highest antibiotic prescription rate in the six largest groups of providers occurred among dentists, whose highest-prescribing practitioners wrote 1,271 prescriptions per 1,000 beneficiaries. Even the lower-prescribing 90% of dentists prescribed more antibiotics (1,068 per 1,000) than did the higher-prescribing family physicians (611 per 1,000) and internists (590 per 1,000), the researchers said.
The prescribing rates for all the other specialties that were not included separately also were higher than the family physicians’ and internists’. These rates were 850 per 1,000 beneficiaries for the higher-prescribers and 360 per 1,000 for the lower-prescribers, the researchers wrote.
The considerable differences in prescribing practices between specialties and even among those of the same specialty present “opportunities for improved prescribing through antibiotic stewardship activities focusing on these higher-volume prescribers, independent of specialty,” Ms. Gouin and associates wrote.
“Higher-volume prescribers prescribed antibiotics to a larger share of their patient panel and their prescribing rate was 60% higher than that of lower-volume prescribers, indicating that their prescribing practices might be independent of the number of beneficiaries under their care,” Katryna A. Gouin, MPH, and associates wrote in the Morbidity and Mortality Weekly Report.
In 2019, 41% of all Part D antibiotics – that’s 24.4 million prescriptions – were prescribed by 69,835 higher-volume prescribers. The other 59% of all antibiotics were prescribed by the 627,000 lower-volume health care providers included in the analysis (those who prescribed fewer than 11 antibiotics were excluded), Ms. Gouin of Chenega in Anchorage, Alaska, and associates noted.
The analysis involved the Medicare Part D Prescribers by Provider data set and defined the highest-volume prescribers “as those in the highest 10th percentile of prescriber-level antibiotic volume (number of antibiotic prescriptions filled) across all Medicare providers nationwide,” the investigators explained.
The antibiotic-prescribing rate for the higher-volume prescribers was 680 prescriptions per 1,000 beneficiaries, which was 60% higher than the 426 prescriptions per 1,000 among the lower 90% of prescribers. Another way to look at it: The top 10% of health care providers “wrote a median of 284 antibiotic prescriptions, compared with a median of 41 among lower-volume prescribers,” the investigators said.
Physicians in internal medicine and family practice, the two largest medical specialties, were the most likely to be 10-percenters, accounting for 24.6% and 27.5%, respectively, of the higher-volume group. They were followed by nurse practitioners (14.1%) and physician assistants (7.4%), who were classified as specialists for the purposes of the study, Ms. Gouin and associates said.
The only other group of physicians among the top six specialties were urologists, who represented 6.8% of high-volume prescribers but only 1% of all prescribers, they noted.
The highest antibiotic prescription rate in the six largest groups of providers occurred among dentists, whose highest-prescribing practitioners wrote 1,271 prescriptions per 1,000 beneficiaries. Even the lower-prescribing 90% of dentists prescribed more antibiotics (1,068 per 1,000) than did the higher-prescribing family physicians (611 per 1,000) and internists (590 per 1,000), the researchers said.
The prescribing rates for all the other specialties that were not included separately also were higher than the family physicians’ and internists’. These rates were 850 per 1,000 beneficiaries for the higher-prescribers and 360 per 1,000 for the lower-prescribers, the researchers wrote.
The considerable differences in prescribing practices between specialties and even among those of the same specialty present “opportunities for improved prescribing through antibiotic stewardship activities focusing on these higher-volume prescribers, independent of specialty,” Ms. Gouin and associates wrote.
FROM THE MMWR
PACAP38- and VIP-induced cluster headache attacks do not appear to alter CGRP levels
such as tryptase and histamine, a new study has found.
“Whether cluster headache attacks provoked by CGRP and PACAP38/VIP are mediated by distinct signaling pathways will be worth investigating in forthcoming studies,” wrote Lanfranco Pellesi, MD, of the Danish Headache Center at the University of Copenhagen, and his coauthors. The study was published in Cephalalgia.
To assess how these biochemical variables might contribute to cluster headache attacks, the researchers launched a randomized, double-blind trial of data from 44 Danish participants with cluster headache. The average age of the patients was 38 years; 14 had active episodic cluster headache, 15 had episodic cluster headache in remission, and 15 had chronic cluster headache.
All patients received a continuous infusion of either PACAP38 (10 pmol/kg per minute) or VIP (8 pmol/kg per minute) over a 20-minute period, using a time- and volume-controlled infusion pump. Blood was collected for analysis at fixed time points, including at baseline, at the end of the infusion, 10 minutes after the infusion, and 70 minutes after the infusion. Technical problems led to missing values in 285 out of 1,144 planned plasma samples.
PACAP38 infusion resulted in a cluster headache attack in 13 of the 44 participants and VIP induced an attack in 12 of the 44. No differences in plasma CGRP (P = .7074), tryptase (P = .6673), and histamine (P = .4792) levels were found between patients who developed attacks and those who did not, and the plasma concentrations did not differ among the various blood-drawing time points.
There was also no difference in plasma CGRP levels between patients with active episodic cluster headache, those with episodic cluster headache in remission, and those with chronic cluster headache. After post hoc analysis, plasma tryptase and plasma histamine levels were similar among the three cluster headache patient groups.
The final link to the cluster headache puzzle has not yet been found
“We know a lot about cluster headache: how it presents, how we can stop it acutely, and how we can stop it preventively. But we don’t know everything about all the neurotransmitters involved, the triggers that start an attack, or the causes of pain,” Alan Rapoport, MD, professor of neurology at the University of California, Los Angeles (UCLA), and past president of the International Headache Society, said in an interview. “This study was performed to find the answer to a small piece of the puzzle. Is CGRP the missing link for patients who begin a cluster attack, or should we be looking elsewhere?
“I would be cautious and say it appears that it doesn’t seem to be related, but further studies may show something different,” he added. “The reason for my qualification: There is a monoclonal antibody [galcanezumab], which grabs CGRP and prevents it from docking on its receptor, that has been approved for preventive treatment of episodic cluster headache. When you have episodic cluster, go into a cluster period, and take galcanezumab, it could and should decrease the number of attacks that you would ordinarily have had. That means it is related somewhat. But it certainly doesn’t work for everyone, so more investigations like this are needed.”
“What’s important about this study is that it opens up the possibility that there is another way into the cluster attack that could be operationalized for therapeutic purposes,” Peter Goadsby, MD, PhD, professor of neurology at UCLA and president of the American Headache Society, said in an interview.
When asked about the authors’ stated interest in investigating “if monoclonal antibodies targeting the CGRP pathway prevent PACAP38- or VIP-induced cluster headache attacks” as a follow-up, Dr. Goadsby strongly backed the idea. “If I sound excited about actually exploring whether that was a useful treatment or not, it’s because cluster headache is a dreadful condition. And the sooner you could work out whether it was useful or put the money into something else, well, that’s where I’d go.
“I think the principle here of doing experimental medicine, getting into human work with targets like this at the earliest possible time, is something that is not done as often as would be appropriate,” he added. “There is not enough investment, in my view, in early phase experimental work, which really just gets to that next step. Broadly speaking, the encouragement and support of experimental medicine is crucial to developing new therapies.”
The authors recognized their study’s potential limitations, including it’s being an exploratory study with results that should be interpreted cautiously. They acknowledged discrepancies with previous studies of plasma CGRP during cluster headache attacks, offering “different methodologies, including intra-assay differences and the location of blood sampling” as a possible reason. They also explained that some of the data are missing “completely at random” due to their policy of discarding all observations with incomplete laboratory measurements, adding that the impact on their sample size was “only modest.”
“In spite of these limitations,” Dr. Rapoport said, “this is an excellent study that shows us that PACAP38- and VIP-induced cluster headache attacks are not associated with alterations in plasma CGRP or in histamine and tryptase.”
Regarding potential conflicts of interest, one author reported being employed at the testing lab where the histamine measurements were conducted, as did another author who serves as the lab’s scientific adviser. A third author reported receiving personal fees from various pharmaceutical companies.
such as tryptase and histamine, a new study has found.
“Whether cluster headache attacks provoked by CGRP and PACAP38/VIP are mediated by distinct signaling pathways will be worth investigating in forthcoming studies,” wrote Lanfranco Pellesi, MD, of the Danish Headache Center at the University of Copenhagen, and his coauthors. The study was published in Cephalalgia.
To assess how these biochemical variables might contribute to cluster headache attacks, the researchers launched a randomized, double-blind trial of data from 44 Danish participants with cluster headache. The average age of the patients was 38 years; 14 had active episodic cluster headache, 15 had episodic cluster headache in remission, and 15 had chronic cluster headache.
All patients received a continuous infusion of either PACAP38 (10 pmol/kg per minute) or VIP (8 pmol/kg per minute) over a 20-minute period, using a time- and volume-controlled infusion pump. Blood was collected for analysis at fixed time points, including at baseline, at the end of the infusion, 10 minutes after the infusion, and 70 minutes after the infusion. Technical problems led to missing values in 285 out of 1,144 planned plasma samples.
PACAP38 infusion resulted in a cluster headache attack in 13 of the 44 participants and VIP induced an attack in 12 of the 44. No differences in plasma CGRP (P = .7074), tryptase (P = .6673), and histamine (P = .4792) levels were found between patients who developed attacks and those who did not, and the plasma concentrations did not differ among the various blood-drawing time points.
There was also no difference in plasma CGRP levels between patients with active episodic cluster headache, those with episodic cluster headache in remission, and those with chronic cluster headache. After post hoc analysis, plasma tryptase and plasma histamine levels were similar among the three cluster headache patient groups.
The final link to the cluster headache puzzle has not yet been found
“We know a lot about cluster headache: how it presents, how we can stop it acutely, and how we can stop it preventively. But we don’t know everything about all the neurotransmitters involved, the triggers that start an attack, or the causes of pain,” Alan Rapoport, MD, professor of neurology at the University of California, Los Angeles (UCLA), and past president of the International Headache Society, said in an interview. “This study was performed to find the answer to a small piece of the puzzle. Is CGRP the missing link for patients who begin a cluster attack, or should we be looking elsewhere?
“I would be cautious and say it appears that it doesn’t seem to be related, but further studies may show something different,” he added. “The reason for my qualification: There is a monoclonal antibody [galcanezumab], which grabs CGRP and prevents it from docking on its receptor, that has been approved for preventive treatment of episodic cluster headache. When you have episodic cluster, go into a cluster period, and take galcanezumab, it could and should decrease the number of attacks that you would ordinarily have had. That means it is related somewhat. But it certainly doesn’t work for everyone, so more investigations like this are needed.”
“What’s important about this study is that it opens up the possibility that there is another way into the cluster attack that could be operationalized for therapeutic purposes,” Peter Goadsby, MD, PhD, professor of neurology at UCLA and president of the American Headache Society, said in an interview.
When asked about the authors’ stated interest in investigating “if monoclonal antibodies targeting the CGRP pathway prevent PACAP38- or VIP-induced cluster headache attacks” as a follow-up, Dr. Goadsby strongly backed the idea. “If I sound excited about actually exploring whether that was a useful treatment or not, it’s because cluster headache is a dreadful condition. And the sooner you could work out whether it was useful or put the money into something else, well, that’s where I’d go.
“I think the principle here of doing experimental medicine, getting into human work with targets like this at the earliest possible time, is something that is not done as often as would be appropriate,” he added. “There is not enough investment, in my view, in early phase experimental work, which really just gets to that next step. Broadly speaking, the encouragement and support of experimental medicine is crucial to developing new therapies.”
The authors recognized their study’s potential limitations, including it’s being an exploratory study with results that should be interpreted cautiously. They acknowledged discrepancies with previous studies of plasma CGRP during cluster headache attacks, offering “different methodologies, including intra-assay differences and the location of blood sampling” as a possible reason. They also explained that some of the data are missing “completely at random” due to their policy of discarding all observations with incomplete laboratory measurements, adding that the impact on their sample size was “only modest.”
“In spite of these limitations,” Dr. Rapoport said, “this is an excellent study that shows us that PACAP38- and VIP-induced cluster headache attacks are not associated with alterations in plasma CGRP or in histamine and tryptase.”
Regarding potential conflicts of interest, one author reported being employed at the testing lab where the histamine measurements were conducted, as did another author who serves as the lab’s scientific adviser. A third author reported receiving personal fees from various pharmaceutical companies.
such as tryptase and histamine, a new study has found.
“Whether cluster headache attacks provoked by CGRP and PACAP38/VIP are mediated by distinct signaling pathways will be worth investigating in forthcoming studies,” wrote Lanfranco Pellesi, MD, of the Danish Headache Center at the University of Copenhagen, and his coauthors. The study was published in Cephalalgia.
To assess how these biochemical variables might contribute to cluster headache attacks, the researchers launched a randomized, double-blind trial of data from 44 Danish participants with cluster headache. The average age of the patients was 38 years; 14 had active episodic cluster headache, 15 had episodic cluster headache in remission, and 15 had chronic cluster headache.
All patients received a continuous infusion of either PACAP38 (10 pmol/kg per minute) or VIP (8 pmol/kg per minute) over a 20-minute period, using a time- and volume-controlled infusion pump. Blood was collected for analysis at fixed time points, including at baseline, at the end of the infusion, 10 minutes after the infusion, and 70 minutes after the infusion. Technical problems led to missing values in 285 out of 1,144 planned plasma samples.
PACAP38 infusion resulted in a cluster headache attack in 13 of the 44 participants and VIP induced an attack in 12 of the 44. No differences in plasma CGRP (P = .7074), tryptase (P = .6673), and histamine (P = .4792) levels were found between patients who developed attacks and those who did not, and the plasma concentrations did not differ among the various blood-drawing time points.
There was also no difference in plasma CGRP levels between patients with active episodic cluster headache, those with episodic cluster headache in remission, and those with chronic cluster headache. After post hoc analysis, plasma tryptase and plasma histamine levels were similar among the three cluster headache patient groups.
The final link to the cluster headache puzzle has not yet been found
“We know a lot about cluster headache: how it presents, how we can stop it acutely, and how we can stop it preventively. But we don’t know everything about all the neurotransmitters involved, the triggers that start an attack, or the causes of pain,” Alan Rapoport, MD, professor of neurology at the University of California, Los Angeles (UCLA), and past president of the International Headache Society, said in an interview. “This study was performed to find the answer to a small piece of the puzzle. Is CGRP the missing link for patients who begin a cluster attack, or should we be looking elsewhere?
“I would be cautious and say it appears that it doesn’t seem to be related, but further studies may show something different,” he added. “The reason for my qualification: There is a monoclonal antibody [galcanezumab], which grabs CGRP and prevents it from docking on its receptor, that has been approved for preventive treatment of episodic cluster headache. When you have episodic cluster, go into a cluster period, and take galcanezumab, it could and should decrease the number of attacks that you would ordinarily have had. That means it is related somewhat. But it certainly doesn’t work for everyone, so more investigations like this are needed.”
“What’s important about this study is that it opens up the possibility that there is another way into the cluster attack that could be operationalized for therapeutic purposes,” Peter Goadsby, MD, PhD, professor of neurology at UCLA and president of the American Headache Society, said in an interview.
When asked about the authors’ stated interest in investigating “if monoclonal antibodies targeting the CGRP pathway prevent PACAP38- or VIP-induced cluster headache attacks” as a follow-up, Dr. Goadsby strongly backed the idea. “If I sound excited about actually exploring whether that was a useful treatment or not, it’s because cluster headache is a dreadful condition. And the sooner you could work out whether it was useful or put the money into something else, well, that’s where I’d go.
“I think the principle here of doing experimental medicine, getting into human work with targets like this at the earliest possible time, is something that is not done as often as would be appropriate,” he added. “There is not enough investment, in my view, in early phase experimental work, which really just gets to that next step. Broadly speaking, the encouragement and support of experimental medicine is crucial to developing new therapies.”
The authors recognized their study’s potential limitations, including it’s being an exploratory study with results that should be interpreted cautiously. They acknowledged discrepancies with previous studies of plasma CGRP during cluster headache attacks, offering “different methodologies, including intra-assay differences and the location of blood sampling” as a possible reason. They also explained that some of the data are missing “completely at random” due to their policy of discarding all observations with incomplete laboratory measurements, adding that the impact on their sample size was “only modest.”
“In spite of these limitations,” Dr. Rapoport said, “this is an excellent study that shows us that PACAP38- and VIP-induced cluster headache attacks are not associated with alterations in plasma CGRP or in histamine and tryptase.”
Regarding potential conflicts of interest, one author reported being employed at the testing lab where the histamine measurements were conducted, as did another author who serves as the lab’s scientific adviser. A third author reported receiving personal fees from various pharmaceutical companies.
FROM CEPHALALGIA
FDA okays 6-month implanted Eversense CGM for diabetes
The U.S. Food and Drug Administration has approved a new second-generation version of the implanted continuous glucose monitoring (CGM) system Eversense (Senseonics) that lasts for 6 months.
The Eversense E3 CGM system doubles the wear time from 3 months with the previous Eversense device approved in the United States in 2018. As before, the new system is approved for adults with diabetes aged 18 years and older.
This means that it will be the longest lasting CGM system available in the United States, with essentially two sensor insertion and removal procedures per year, the company said.
Data from the pivotal PROMISE trial of the 6-month version were presented at the American Diabetes Association Scientific Sessions in 2021, as reported by this news organization.
An older 6-month wear time version (Eversense XL) has been available in Europe since 2017. The new second-generation 6-month system is currently under regulatory review there.
The PROMISE trial included 181 participants with diabetes, about two-thirds with type 1 and one-third with type 2 diabetes, at eight clinical research sites.
“We repeatedly hear from our patients with diabetes that what they desire is a long-lasting sensor that is also highly accurate ... The next generation Eversense E3 System delivers on both,” said PROMISE study principal investigator Satish Garg, MD, professor of medicine and director of the adult diabetes program at the Barbara Davis Center, University of Colorado, Aurora, in a company press release.
The Eversense E3 consists of a fluorescence-based sensor, a transmitter, and a smartphone app that displays glucose values, trends, and alerts. The sensor is inserted subcutaneously into the upper arm by a certified health care professional in a brief office procedure. The transmitter is placed on the skin on top of the sensor. Glucose data are sent to the app automatically every 5 minutes.
The system includes an on-body vibratory alert as well as alerts on the app for high and low blood glucose values. Eversense readings may be used for treatment decisions, but users still must perform fingerstick glucose checks for calibration.
The regulatory review for the Eversense E3 was delayed for a year due to the COVID-19 pandemic. It will be distributed in the United States through a partnership with Ascensia Diabetes Care beginning in the second quarter of 2022, according to a Senseonics statement.
In addition, “the company expects the majority of its expenses for 2022 to be for research and development for ongoing feasibility and pivotal clinical trials for additional products in its product pipeline, including the start of its 365-day pivotal trial.”
Health care providers who want to offer the Eversense CGM System to their patients can sign up here or call 844-SENSE4U (844-736-7348).
Patients interested in getting started on Eversense can sign up here and will be among the first to know when Eversense E3 is commercially available.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved a new second-generation version of the implanted continuous glucose monitoring (CGM) system Eversense (Senseonics) that lasts for 6 months.
The Eversense E3 CGM system doubles the wear time from 3 months with the previous Eversense device approved in the United States in 2018. As before, the new system is approved for adults with diabetes aged 18 years and older.
This means that it will be the longest lasting CGM system available in the United States, with essentially two sensor insertion and removal procedures per year, the company said.
Data from the pivotal PROMISE trial of the 6-month version were presented at the American Diabetes Association Scientific Sessions in 2021, as reported by this news organization.
An older 6-month wear time version (Eversense XL) has been available in Europe since 2017. The new second-generation 6-month system is currently under regulatory review there.
The PROMISE trial included 181 participants with diabetes, about two-thirds with type 1 and one-third with type 2 diabetes, at eight clinical research sites.
“We repeatedly hear from our patients with diabetes that what they desire is a long-lasting sensor that is also highly accurate ... The next generation Eversense E3 System delivers on both,” said PROMISE study principal investigator Satish Garg, MD, professor of medicine and director of the adult diabetes program at the Barbara Davis Center, University of Colorado, Aurora, in a company press release.
The Eversense E3 consists of a fluorescence-based sensor, a transmitter, and a smartphone app that displays glucose values, trends, and alerts. The sensor is inserted subcutaneously into the upper arm by a certified health care professional in a brief office procedure. The transmitter is placed on the skin on top of the sensor. Glucose data are sent to the app automatically every 5 minutes.
The system includes an on-body vibratory alert as well as alerts on the app for high and low blood glucose values. Eversense readings may be used for treatment decisions, but users still must perform fingerstick glucose checks for calibration.
The regulatory review for the Eversense E3 was delayed for a year due to the COVID-19 pandemic. It will be distributed in the United States through a partnership with Ascensia Diabetes Care beginning in the second quarter of 2022, according to a Senseonics statement.
In addition, “the company expects the majority of its expenses for 2022 to be for research and development for ongoing feasibility and pivotal clinical trials for additional products in its product pipeline, including the start of its 365-day pivotal trial.”
Health care providers who want to offer the Eversense CGM System to their patients can sign up here or call 844-SENSE4U (844-736-7348).
Patients interested in getting started on Eversense can sign up here and will be among the first to know when Eversense E3 is commercially available.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved a new second-generation version of the implanted continuous glucose monitoring (CGM) system Eversense (Senseonics) that lasts for 6 months.
The Eversense E3 CGM system doubles the wear time from 3 months with the previous Eversense device approved in the United States in 2018. As before, the new system is approved for adults with diabetes aged 18 years and older.
This means that it will be the longest lasting CGM system available in the United States, with essentially two sensor insertion and removal procedures per year, the company said.
Data from the pivotal PROMISE trial of the 6-month version were presented at the American Diabetes Association Scientific Sessions in 2021, as reported by this news organization.
An older 6-month wear time version (Eversense XL) has been available in Europe since 2017. The new second-generation 6-month system is currently under regulatory review there.
The PROMISE trial included 181 participants with diabetes, about two-thirds with type 1 and one-third with type 2 diabetes, at eight clinical research sites.
“We repeatedly hear from our patients with diabetes that what they desire is a long-lasting sensor that is also highly accurate ... The next generation Eversense E3 System delivers on both,” said PROMISE study principal investigator Satish Garg, MD, professor of medicine and director of the adult diabetes program at the Barbara Davis Center, University of Colorado, Aurora, in a company press release.
The Eversense E3 consists of a fluorescence-based sensor, a transmitter, and a smartphone app that displays glucose values, trends, and alerts. The sensor is inserted subcutaneously into the upper arm by a certified health care professional in a brief office procedure. The transmitter is placed on the skin on top of the sensor. Glucose data are sent to the app automatically every 5 minutes.
The system includes an on-body vibratory alert as well as alerts on the app for high and low blood glucose values. Eversense readings may be used for treatment decisions, but users still must perform fingerstick glucose checks for calibration.
The regulatory review for the Eversense E3 was delayed for a year due to the COVID-19 pandemic. It will be distributed in the United States through a partnership with Ascensia Diabetes Care beginning in the second quarter of 2022, according to a Senseonics statement.
In addition, “the company expects the majority of its expenses for 2022 to be for research and development for ongoing feasibility and pivotal clinical trials for additional products in its product pipeline, including the start of its 365-day pivotal trial.”
Health care providers who want to offer the Eversense CGM System to their patients can sign up here or call 844-SENSE4U (844-736-7348).
Patients interested in getting started on Eversense can sign up here and will be among the first to know when Eversense E3 is commercially available.
A version of this article first appeared on Medscape.com.
Study questions need for repeat Lp(a) testing
Repeat testing of lipoprotein(a) to assess a patient’s cardiovascular risk doesn’t seem to yield any additional helpful information, and a one-time baseline measure of Lp(a) molar concentration could be sufficient to help define lifetime risk, suggests a large analysis of a national database in the United Kingdom.
The study examined the correlation between baseline and first follow-up measures of Lp(a) molar concentration and incident coronary artery disease among 16,017 individuals in a cohort of the UK Biobank, a prospective observational study of about 500,000 middle-aged people recruited between 2006 and 2010 with ongoing follow-up.
Results showed found little change in Lp(a) molar concentration measures from baseline to an average of 4.4 years afterward, but did find an association between statin usage and significant increases in Lp(a) in people with high baseline levels. The study was published online on Feb. 14 in the Journal of the American College of Cardiology.
The baseline and follow-up Lp(a) molar concentration measures “are highly correlated with 85% of the repeat values being within 25 nmol/L of each other,” senior author Pradeep Natarajan, MD, MMSc, of Massachusetts General Hospital, Boston, said in an interview. “When predicting events, the follow-up Lp(a) concentration did not yield additional information beyond the baseline Lp(a).”
Additionally, the study found that statin therapy didn’t lead to meaningful changes in Lp(a) molar concentration levels. Patients on statins who had baseline Lp(a) above 70 nmol/L “had modest follow-up concentrations, but this did not appreciably change atherosclerotic cardiovascular disease risks,” Dr. Natarajan said. “For patients without clinical cardiovascular disease who are not on medicines that markedly change Lp(a), additional Lp(a) assessments are unlikely to provide additional prognostic information beyond the baseline Lp(a) measurement.”
Added lead author Mark Trinder, MSc: “These findings suggest that, in the absence of therapies substantially altering Lp(a), a single accurate measurement of Lp(a) molar concentration is an efficient method to inform atherosclerotic cardiovascular disease risk.” Mr. Trinder is an MD/PhD candidate at the Centre for Heart Lung Innovation at the University of British Columbia, Vancouver, and a visiting scholar in medical and population genetics and the Cardiovascular Disease Initiative at the Broad Institute of MIT and Harvard in Cambridge, Mass.
This study claims to be unique for two reasons: It reported on repeat Lp(a) measurements among the general population rather than a clinical trial, and it assessed the influence of statins on Lp(a) molar concentration rather than Lp(a) mass.
“Lp(a) molar concentration aims to mitigate challenges with mass assays, which are influenced by assay size,” Dr. Natarajan said. However, he noted that major clinical trials of investigative drugs for lowering Lp(a), specifically the ongoing HORIZON trial (NCT04023552), are using Lp(a) mass rather than molar concentration.
“There is an imperfect correlation between the two,” Dr. Natarajan said. “Depending on the results of this trial and others, and evaluation of both mass and molar concentration assays, we will then be able to better understand the path forward. These issues and the multiple assays have been challenging for both the clinical and scientific community.”
Santica Marcovina, ScD, PhD, coauthor of the invited commentary (J Am Coll Cardiol. 2022 Feb 14. doi: 10.1016/j.jacc.2021.11.053), said in an interview that the study’s major contribution to the literature is the finding that the molar concentration of Lp(a) appears to be stable regardless of statin use. “This important finding provides evidence that no longitudinal measurements of Lp(a) are needed in the primary prevention of atherosclerotic CVD and that once-in-a-lifetime measurement may reliably allow clinicians to assess whether or not Lp(a)-related risk is present in their patients,” she said. Dr. Marcovina is senior director of clinical laboratory sciences at Medpace Reference Laboratories, Cincinnati.
She noted that this study provides an actionable strategy for cardiologists. “Considering the clinical benefits, the relative low cost for measuring Lp(a), the fact that measurements need to be performed only once in the vast majority of individuals, all point to the implementation of Lp(a) general screening as soon as possible.”
Dr. Natarajan has financial relationships with Amgen, Apple, AstraZeneca, Boston Scientific, Blackstone Life Sciences, Genentech and Novartis. Dr. Marcovina has provided consulting for Roche, Denka, and Novartis, and has received research support from Amgen through Medpace.
Repeat testing of lipoprotein(a) to assess a patient’s cardiovascular risk doesn’t seem to yield any additional helpful information, and a one-time baseline measure of Lp(a) molar concentration could be sufficient to help define lifetime risk, suggests a large analysis of a national database in the United Kingdom.
The study examined the correlation between baseline and first follow-up measures of Lp(a) molar concentration and incident coronary artery disease among 16,017 individuals in a cohort of the UK Biobank, a prospective observational study of about 500,000 middle-aged people recruited between 2006 and 2010 with ongoing follow-up.
Results showed found little change in Lp(a) molar concentration measures from baseline to an average of 4.4 years afterward, but did find an association between statin usage and significant increases in Lp(a) in people with high baseline levels. The study was published online on Feb. 14 in the Journal of the American College of Cardiology.
The baseline and follow-up Lp(a) molar concentration measures “are highly correlated with 85% of the repeat values being within 25 nmol/L of each other,” senior author Pradeep Natarajan, MD, MMSc, of Massachusetts General Hospital, Boston, said in an interview. “When predicting events, the follow-up Lp(a) concentration did not yield additional information beyond the baseline Lp(a).”
Additionally, the study found that statin therapy didn’t lead to meaningful changes in Lp(a) molar concentration levels. Patients on statins who had baseline Lp(a) above 70 nmol/L “had modest follow-up concentrations, but this did not appreciably change atherosclerotic cardiovascular disease risks,” Dr. Natarajan said. “For patients without clinical cardiovascular disease who are not on medicines that markedly change Lp(a), additional Lp(a) assessments are unlikely to provide additional prognostic information beyond the baseline Lp(a) measurement.”
Added lead author Mark Trinder, MSc: “These findings suggest that, in the absence of therapies substantially altering Lp(a), a single accurate measurement of Lp(a) molar concentration is an efficient method to inform atherosclerotic cardiovascular disease risk.” Mr. Trinder is an MD/PhD candidate at the Centre for Heart Lung Innovation at the University of British Columbia, Vancouver, and a visiting scholar in medical and population genetics and the Cardiovascular Disease Initiative at the Broad Institute of MIT and Harvard in Cambridge, Mass.
This study claims to be unique for two reasons: It reported on repeat Lp(a) measurements among the general population rather than a clinical trial, and it assessed the influence of statins on Lp(a) molar concentration rather than Lp(a) mass.
“Lp(a) molar concentration aims to mitigate challenges with mass assays, which are influenced by assay size,” Dr. Natarajan said. However, he noted that major clinical trials of investigative drugs for lowering Lp(a), specifically the ongoing HORIZON trial (NCT04023552), are using Lp(a) mass rather than molar concentration.
“There is an imperfect correlation between the two,” Dr. Natarajan said. “Depending on the results of this trial and others, and evaluation of both mass and molar concentration assays, we will then be able to better understand the path forward. These issues and the multiple assays have been challenging for both the clinical and scientific community.”
Santica Marcovina, ScD, PhD, coauthor of the invited commentary (J Am Coll Cardiol. 2022 Feb 14. doi: 10.1016/j.jacc.2021.11.053), said in an interview that the study’s major contribution to the literature is the finding that the molar concentration of Lp(a) appears to be stable regardless of statin use. “This important finding provides evidence that no longitudinal measurements of Lp(a) are needed in the primary prevention of atherosclerotic CVD and that once-in-a-lifetime measurement may reliably allow clinicians to assess whether or not Lp(a)-related risk is present in their patients,” she said. Dr. Marcovina is senior director of clinical laboratory sciences at Medpace Reference Laboratories, Cincinnati.
She noted that this study provides an actionable strategy for cardiologists. “Considering the clinical benefits, the relative low cost for measuring Lp(a), the fact that measurements need to be performed only once in the vast majority of individuals, all point to the implementation of Lp(a) general screening as soon as possible.”
Dr. Natarajan has financial relationships with Amgen, Apple, AstraZeneca, Boston Scientific, Blackstone Life Sciences, Genentech and Novartis. Dr. Marcovina has provided consulting for Roche, Denka, and Novartis, and has received research support from Amgen through Medpace.
Repeat testing of lipoprotein(a) to assess a patient’s cardiovascular risk doesn’t seem to yield any additional helpful information, and a one-time baseline measure of Lp(a) molar concentration could be sufficient to help define lifetime risk, suggests a large analysis of a national database in the United Kingdom.
The study examined the correlation between baseline and first follow-up measures of Lp(a) molar concentration and incident coronary artery disease among 16,017 individuals in a cohort of the UK Biobank, a prospective observational study of about 500,000 middle-aged people recruited between 2006 and 2010 with ongoing follow-up.
Results showed found little change in Lp(a) molar concentration measures from baseline to an average of 4.4 years afterward, but did find an association between statin usage and significant increases in Lp(a) in people with high baseline levels. The study was published online on Feb. 14 in the Journal of the American College of Cardiology.
The baseline and follow-up Lp(a) molar concentration measures “are highly correlated with 85% of the repeat values being within 25 nmol/L of each other,” senior author Pradeep Natarajan, MD, MMSc, of Massachusetts General Hospital, Boston, said in an interview. “When predicting events, the follow-up Lp(a) concentration did not yield additional information beyond the baseline Lp(a).”
Additionally, the study found that statin therapy didn’t lead to meaningful changes in Lp(a) molar concentration levels. Patients on statins who had baseline Lp(a) above 70 nmol/L “had modest follow-up concentrations, but this did not appreciably change atherosclerotic cardiovascular disease risks,” Dr. Natarajan said. “For patients without clinical cardiovascular disease who are not on medicines that markedly change Lp(a), additional Lp(a) assessments are unlikely to provide additional prognostic information beyond the baseline Lp(a) measurement.”
Added lead author Mark Trinder, MSc: “These findings suggest that, in the absence of therapies substantially altering Lp(a), a single accurate measurement of Lp(a) molar concentration is an efficient method to inform atherosclerotic cardiovascular disease risk.” Mr. Trinder is an MD/PhD candidate at the Centre for Heart Lung Innovation at the University of British Columbia, Vancouver, and a visiting scholar in medical and population genetics and the Cardiovascular Disease Initiative at the Broad Institute of MIT and Harvard in Cambridge, Mass.
This study claims to be unique for two reasons: It reported on repeat Lp(a) measurements among the general population rather than a clinical trial, and it assessed the influence of statins on Lp(a) molar concentration rather than Lp(a) mass.
“Lp(a) molar concentration aims to mitigate challenges with mass assays, which are influenced by assay size,” Dr. Natarajan said. However, he noted that major clinical trials of investigative drugs for lowering Lp(a), specifically the ongoing HORIZON trial (NCT04023552), are using Lp(a) mass rather than molar concentration.
“There is an imperfect correlation between the two,” Dr. Natarajan said. “Depending on the results of this trial and others, and evaluation of both mass and molar concentration assays, we will then be able to better understand the path forward. These issues and the multiple assays have been challenging for both the clinical and scientific community.”
Santica Marcovina, ScD, PhD, coauthor of the invited commentary (J Am Coll Cardiol. 2022 Feb 14. doi: 10.1016/j.jacc.2021.11.053), said in an interview that the study’s major contribution to the literature is the finding that the molar concentration of Lp(a) appears to be stable regardless of statin use. “This important finding provides evidence that no longitudinal measurements of Lp(a) are needed in the primary prevention of atherosclerotic CVD and that once-in-a-lifetime measurement may reliably allow clinicians to assess whether or not Lp(a)-related risk is present in their patients,” she said. Dr. Marcovina is senior director of clinical laboratory sciences at Medpace Reference Laboratories, Cincinnati.
She noted that this study provides an actionable strategy for cardiologists. “Considering the clinical benefits, the relative low cost for measuring Lp(a), the fact that measurements need to be performed only once in the vast majority of individuals, all point to the implementation of Lp(a) general screening as soon as possible.”
Dr. Natarajan has financial relationships with Amgen, Apple, AstraZeneca, Boston Scientific, Blackstone Life Sciences, Genentech and Novartis. Dr. Marcovina has provided consulting for Roche, Denka, and Novartis, and has received research support from Amgen through Medpace.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY