User login
PCOS common in adolescent girls with type 2 diabetes
Polycystic ovary syndrome is common in girls with type 2 diabetes, findings of a new study suggest, and authors say screening for PCOS is critical in this group.
In a systematic review and meta-analysis involving 470 girls (average age 12.9-16.1 years) with type 2 diabetes in six studies, the prevalence of PCOS was nearly 1 in 5 (19.58%; 95% confidence interval, 12.02%-27.14%; P = .002), substantially higher than that of PCOS in the general adolescent population.
PCOS, a complex endocrine disorder, occurs in 1.14%-11.04% of adolescent girls globally, according to the paper published online in JAMA Network Open.
The secondary outcome studied links to prevalence of PCOS with race and obesity.
Insulin resistance and compensatory hyperinsulinemia are present in 44%-70% of women with PCOS, suggesting that they are more likely to develop type 2 diabetes, according to the researchers led by Milena Cioana, BHSc, with the department of pediatrics, McMaster University, Hamilton, Ont.
Kelly A. Curran, MD, an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center in Oklahoma City, where she practices adolescent medicine, said in an interview that it has been known that women with PCOS have higher rates of diabetes and many in the field have suspected the relationship is bidirectional.
“In my clinical practice, I’ve seen a high percentage of women with type 2 diabetes present with irregular menses, some of whom have gone on to be diagnosed with PCOS,” said Dr. Curran, who was not involved with the study.
However, she said, she was surprised the prevalence of PCOS reported in this paper – nearly one in five – was so high. Early diagnosis is important for PCOS to prevent complications such as hypertension, hyperglycemia, and dyslipidemia.
Psychiatric conditions are also prevalent in patients with PCOS, including anxiety (18%), depression (16%), and ADHD (9%).
Dr. Curran agreed there is a need to screen for PCOS and to evaluate for other causes of irregular periods in patients with type 2 diabetes.
“Menstrual irregularities are often overlooked in young women without further work-up, especially in patients who have chronic illnesses,” she noted.
Results come with a caveat
However, the authors said, results should be viewed with caution because “studies including the larger numbers of girls did not report the criteria used to diagnose PCOS, which is a challenge during adolescence.”
Diagnostic criteria for PCOS during adolescence include the combination of menstrual irregularities according to time since their first period and clinical or biochemical hyperandrogenism after excluding other potential causes.
Dr. Curran explained that PCOS symptoms include irregular periods and acne which can overlap with normal changes in puberty. In her experience, PCOS is often diagnosed without patients meeting full criteria. She agreed further research with standardized criteria is urgently needed.
The European Society of Human Reproduction and Embryology/American Society of Reproductive Medicine, the Pediatric Endocrine Society, and the International Consortium of Paediatric Endocrinology guidelines suggest that using ultrasound to check the size of ovaries could help diagnose PCOS, but other guidelines are more conservative, the authors noted.
They added that “there is a need for a consensus to establish the pediatric criteria for diagnosing PCOS in adolescents to ensure accurate diagnosis and lower the misclassification rates.”
Assessing links to obesity and race
Still unclear, the authors wrote, is whether and how obesity and race affect prevalence of PCOS among girls with type 2 diabetes.
The authors wrote: “Although earlier studies suggested that obesity-related insulin resistance and hyperinsulinemia can contribute to PCOS pathogenesis, insulin resistance in patients with PCOS may be present independently of [body mass index]. Obesity seems to increase the risk of PCOS only slightly and might represent a referral bias for PCOS.”
Few studies included in the meta-analysis had race-specific data, so the authors were limited in assessing associations between race and PCOS prevalence.
“However,” they wrote, “our data demonstrate that Indian girls had the highest prevalence, followed by White girls, and then Indigenous girls in Canada.”
Further studies are needed to help define at-risk subgroups and evaluate treatment strategies, the authors noted.
They reported having no relevant financial relationships. Dr. Curran had no conflicts of interest.
Polycystic ovary syndrome is common in girls with type 2 diabetes, findings of a new study suggest, and authors say screening for PCOS is critical in this group.
In a systematic review and meta-analysis involving 470 girls (average age 12.9-16.1 years) with type 2 diabetes in six studies, the prevalence of PCOS was nearly 1 in 5 (19.58%; 95% confidence interval, 12.02%-27.14%; P = .002), substantially higher than that of PCOS in the general adolescent population.
PCOS, a complex endocrine disorder, occurs in 1.14%-11.04% of adolescent girls globally, according to the paper published online in JAMA Network Open.
The secondary outcome studied links to prevalence of PCOS with race and obesity.
Insulin resistance and compensatory hyperinsulinemia are present in 44%-70% of women with PCOS, suggesting that they are more likely to develop type 2 diabetes, according to the researchers led by Milena Cioana, BHSc, with the department of pediatrics, McMaster University, Hamilton, Ont.
Kelly A. Curran, MD, an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center in Oklahoma City, where she practices adolescent medicine, said in an interview that it has been known that women with PCOS have higher rates of diabetes and many in the field have suspected the relationship is bidirectional.
“In my clinical practice, I’ve seen a high percentage of women with type 2 diabetes present with irregular menses, some of whom have gone on to be diagnosed with PCOS,” said Dr. Curran, who was not involved with the study.
However, she said, she was surprised the prevalence of PCOS reported in this paper – nearly one in five – was so high. Early diagnosis is important for PCOS to prevent complications such as hypertension, hyperglycemia, and dyslipidemia.
Psychiatric conditions are also prevalent in patients with PCOS, including anxiety (18%), depression (16%), and ADHD (9%).
Dr. Curran agreed there is a need to screen for PCOS and to evaluate for other causes of irregular periods in patients with type 2 diabetes.
“Menstrual irregularities are often overlooked in young women without further work-up, especially in patients who have chronic illnesses,” she noted.
Results come with a caveat
However, the authors said, results should be viewed with caution because “studies including the larger numbers of girls did not report the criteria used to diagnose PCOS, which is a challenge during adolescence.”
Diagnostic criteria for PCOS during adolescence include the combination of menstrual irregularities according to time since their first period and clinical or biochemical hyperandrogenism after excluding other potential causes.
Dr. Curran explained that PCOS symptoms include irregular periods and acne which can overlap with normal changes in puberty. In her experience, PCOS is often diagnosed without patients meeting full criteria. She agreed further research with standardized criteria is urgently needed.
The European Society of Human Reproduction and Embryology/American Society of Reproductive Medicine, the Pediatric Endocrine Society, and the International Consortium of Paediatric Endocrinology guidelines suggest that using ultrasound to check the size of ovaries could help diagnose PCOS, but other guidelines are more conservative, the authors noted.
They added that “there is a need for a consensus to establish the pediatric criteria for diagnosing PCOS in adolescents to ensure accurate diagnosis and lower the misclassification rates.”
Assessing links to obesity and race
Still unclear, the authors wrote, is whether and how obesity and race affect prevalence of PCOS among girls with type 2 diabetes.
The authors wrote: “Although earlier studies suggested that obesity-related insulin resistance and hyperinsulinemia can contribute to PCOS pathogenesis, insulin resistance in patients with PCOS may be present independently of [body mass index]. Obesity seems to increase the risk of PCOS only slightly and might represent a referral bias for PCOS.”
Few studies included in the meta-analysis had race-specific data, so the authors were limited in assessing associations between race and PCOS prevalence.
“However,” they wrote, “our data demonstrate that Indian girls had the highest prevalence, followed by White girls, and then Indigenous girls in Canada.”
Further studies are needed to help define at-risk subgroups and evaluate treatment strategies, the authors noted.
They reported having no relevant financial relationships. Dr. Curran had no conflicts of interest.
Polycystic ovary syndrome is common in girls with type 2 diabetes, findings of a new study suggest, and authors say screening for PCOS is critical in this group.
In a systematic review and meta-analysis involving 470 girls (average age 12.9-16.1 years) with type 2 diabetes in six studies, the prevalence of PCOS was nearly 1 in 5 (19.58%; 95% confidence interval, 12.02%-27.14%; P = .002), substantially higher than that of PCOS in the general adolescent population.
PCOS, a complex endocrine disorder, occurs in 1.14%-11.04% of adolescent girls globally, according to the paper published online in JAMA Network Open.
The secondary outcome studied links to prevalence of PCOS with race and obesity.
Insulin resistance and compensatory hyperinsulinemia are present in 44%-70% of women with PCOS, suggesting that they are more likely to develop type 2 diabetes, according to the researchers led by Milena Cioana, BHSc, with the department of pediatrics, McMaster University, Hamilton, Ont.
Kelly A. Curran, MD, an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center in Oklahoma City, where she practices adolescent medicine, said in an interview that it has been known that women with PCOS have higher rates of diabetes and many in the field have suspected the relationship is bidirectional.
“In my clinical practice, I’ve seen a high percentage of women with type 2 diabetes present with irregular menses, some of whom have gone on to be diagnosed with PCOS,” said Dr. Curran, who was not involved with the study.
However, she said, she was surprised the prevalence of PCOS reported in this paper – nearly one in five – was so high. Early diagnosis is important for PCOS to prevent complications such as hypertension, hyperglycemia, and dyslipidemia.
Psychiatric conditions are also prevalent in patients with PCOS, including anxiety (18%), depression (16%), and ADHD (9%).
Dr. Curran agreed there is a need to screen for PCOS and to evaluate for other causes of irregular periods in patients with type 2 diabetes.
“Menstrual irregularities are often overlooked in young women without further work-up, especially in patients who have chronic illnesses,” she noted.
Results come with a caveat
However, the authors said, results should be viewed with caution because “studies including the larger numbers of girls did not report the criteria used to diagnose PCOS, which is a challenge during adolescence.”
Diagnostic criteria for PCOS during adolescence include the combination of menstrual irregularities according to time since their first period and clinical or biochemical hyperandrogenism after excluding other potential causes.
Dr. Curran explained that PCOS symptoms include irregular periods and acne which can overlap with normal changes in puberty. In her experience, PCOS is often diagnosed without patients meeting full criteria. She agreed further research with standardized criteria is urgently needed.
The European Society of Human Reproduction and Embryology/American Society of Reproductive Medicine, the Pediatric Endocrine Society, and the International Consortium of Paediatric Endocrinology guidelines suggest that using ultrasound to check the size of ovaries could help diagnose PCOS, but other guidelines are more conservative, the authors noted.
They added that “there is a need for a consensus to establish the pediatric criteria for diagnosing PCOS in adolescents to ensure accurate diagnosis and lower the misclassification rates.”
Assessing links to obesity and race
Still unclear, the authors wrote, is whether and how obesity and race affect prevalence of PCOS among girls with type 2 diabetes.
The authors wrote: “Although earlier studies suggested that obesity-related insulin resistance and hyperinsulinemia can contribute to PCOS pathogenesis, insulin resistance in patients with PCOS may be present independently of [body mass index]. Obesity seems to increase the risk of PCOS only slightly and might represent a referral bias for PCOS.”
Few studies included in the meta-analysis had race-specific data, so the authors were limited in assessing associations between race and PCOS prevalence.
“However,” they wrote, “our data demonstrate that Indian girls had the highest prevalence, followed by White girls, and then Indigenous girls in Canada.”
Further studies are needed to help define at-risk subgroups and evaluate treatment strategies, the authors noted.
They reported having no relevant financial relationships. Dr. Curran had no conflicts of interest.
FROM JAMA NETWORK OPEN
Optimal NIV Medicare access promotion – a hopeful way forward for users of NIV
Use of positive airway pressure (PAP) devices for treatment of sleep apnea was first described in 1981. Subsequent use of PAP devices expanded to treat patients with respiratory failure. While the treatment in this population has rapidly gained widespread use and undoubtedly has reduced morbidity and mortality in these populations, policies governing these prescriptions have not really kept up with the burgeoning need.
In 2020, Drs. Peter Gay and Robert Owens brought together a technical expert panel (TEP) to systematically review the CMS policies with an eye to remove “regulatory barriers” to improve access for these patients with the mantra: “the right device gets to the right patient at the right time.”
The panel focused on “Optimal NIV Medicare Access Promotion (ONMAP),” and members with specific expertise were recruited for five patient groups: Thoracic Restrictive Disorders (TRD), COPD, Central Sleep Apnea (CSA), Hypoventilation Syndromes (HVS), and Obstructive Sleep Apnea (OSA). Each group reviewed the current coverage, outlined the deficiencies, and suggested revisions. Herein, I will briefly highlight each group’s most important points.
TRD: The goal for this group was to bring the US standards of care closer to the rest of the world. This group advocates that the start of noninvasive ventilation (NIV) should be substantially earlier, to provide the largest improvement in disease outcome and stability. Other prominent features submitted included arterial blood gases (ABG) to not be the only form of CO2 measurement allowed; paying for a second device if patients are using NIV continuously; qualification for a BiPAP to include if vital capacity is ≤ 80%; and, to obtain a home mechanical ventilator, a patient must either fail BiPAP or have extreme loss of function, high pressure requirements, or need mouthpiece ventilation.
CSA: The big challenges with this diagnosis related to qualifying coverage language in the current policies, which are confusing for many providers. Additionally, these policies often deny certain PAP devices and/or oxygen therapy. The group proposed: a single definition of CSA; eliminate discussion of hypoventilation; mirror qualifying symptoms, and, continuing coverage, to the same as that for OSA treatment; and remove need for a prior failure of BiPAP without a backup rate (BUR). The group also had specific recommendations for when oxygen therapy should be covered in patients with CSA.
COPD: This group also focused on the oxygen therapy and promoting use of devices with a BUR. Two problematic areas included the requirement that nocturnal oxygen saturation must drop to ≤ 88% for at least 5 cumulative minutes, and, that patients must begin with an S mode device (no BUR) for at least 2 months and can only then be prescribed a device with a BUR if CO2 fails to drop. The group advocates for the removal of both, the need for a nocturnal oximetry test, and, to “try” an S mode device. The panel advocated giving the prescribing physician discretion in making this determination. The panel also provided recommendations on when a home mechanical ventilator (HMV) should be considered instead of BiPAP therapy.
HVS: Hypoventilation syndromes are a heterogeneous group of disorders with hypercapnia, defined as a Paco2 ≥45 mm Hg. This panel noted that the current coverage criteria are outdated and fail to recognize the spectrum of disease severity and advances in technology, which often leads to circumvention by prescribing more costly home mechanical ventilators (HMV). Consistent with the TRD group, this panel recommended acceptance of surrogate noninvasive end tidal and transcutaneous Pco2 and venous blood gases in lieu of arterial blood gases. Additionally, they suggested no longer requiring CO2 measures while using prescribed oxygen; eliminating the need for a sleep study to avoid delays in care for patients being discharged from the hospital; removing spirometry as a requirement; and no longer a failure of BiPAP without a BUR.
OSA: The initial purpose of examining OSA in this process was to examine when BiPAP should be utilized for treatment; however, it necessitated examination of the entire policy for PAP. The areas that were identified as needing revision included: expansion of the symptom list for patients with OSA; revising the “4 hour rule,” suggesting that 2 hours has been proven to provide benefit; eliminating the need for another sleep study to re-qualify for PAP or supplemental oxygen; and embracing telehealth as a way to improve accessibility for follow-up visits.
For details, please review the papers published in the November 2021 issue of the journal CHEST® (2021; 160[5]:1579-1990, e377-e543).
We now await what CMS will do with our recommendations and work for “the right device to the right patient at the right time.”
Acknowledgment: Drs. Gerald Criner, Nicholas Hill, Babak Mohklesi, Timothy Morgenthaler, and Lisa Wolfe assisted with the content.
Use of positive airway pressure (PAP) devices for treatment of sleep apnea was first described in 1981. Subsequent use of PAP devices expanded to treat patients with respiratory failure. While the treatment in this population has rapidly gained widespread use and undoubtedly has reduced morbidity and mortality in these populations, policies governing these prescriptions have not really kept up with the burgeoning need.
In 2020, Drs. Peter Gay and Robert Owens brought together a technical expert panel (TEP) to systematically review the CMS policies with an eye to remove “regulatory barriers” to improve access for these patients with the mantra: “the right device gets to the right patient at the right time.”
The panel focused on “Optimal NIV Medicare Access Promotion (ONMAP),” and members with specific expertise were recruited for five patient groups: Thoracic Restrictive Disorders (TRD), COPD, Central Sleep Apnea (CSA), Hypoventilation Syndromes (HVS), and Obstructive Sleep Apnea (OSA). Each group reviewed the current coverage, outlined the deficiencies, and suggested revisions. Herein, I will briefly highlight each group’s most important points.
TRD: The goal for this group was to bring the US standards of care closer to the rest of the world. This group advocates that the start of noninvasive ventilation (NIV) should be substantially earlier, to provide the largest improvement in disease outcome and stability. Other prominent features submitted included arterial blood gases (ABG) to not be the only form of CO2 measurement allowed; paying for a second device if patients are using NIV continuously; qualification for a BiPAP to include if vital capacity is ≤ 80%; and, to obtain a home mechanical ventilator, a patient must either fail BiPAP or have extreme loss of function, high pressure requirements, or need mouthpiece ventilation.
CSA: The big challenges with this diagnosis related to qualifying coverage language in the current policies, which are confusing for many providers. Additionally, these policies often deny certain PAP devices and/or oxygen therapy. The group proposed: a single definition of CSA; eliminate discussion of hypoventilation; mirror qualifying symptoms, and, continuing coverage, to the same as that for OSA treatment; and remove need for a prior failure of BiPAP without a backup rate (BUR). The group also had specific recommendations for when oxygen therapy should be covered in patients with CSA.
COPD: This group also focused on the oxygen therapy and promoting use of devices with a BUR. Two problematic areas included the requirement that nocturnal oxygen saturation must drop to ≤ 88% for at least 5 cumulative minutes, and, that patients must begin with an S mode device (no BUR) for at least 2 months and can only then be prescribed a device with a BUR if CO2 fails to drop. The group advocates for the removal of both, the need for a nocturnal oximetry test, and, to “try” an S mode device. The panel advocated giving the prescribing physician discretion in making this determination. The panel also provided recommendations on when a home mechanical ventilator (HMV) should be considered instead of BiPAP therapy.
HVS: Hypoventilation syndromes are a heterogeneous group of disorders with hypercapnia, defined as a Paco2 ≥45 mm Hg. This panel noted that the current coverage criteria are outdated and fail to recognize the spectrum of disease severity and advances in technology, which often leads to circumvention by prescribing more costly home mechanical ventilators (HMV). Consistent with the TRD group, this panel recommended acceptance of surrogate noninvasive end tidal and transcutaneous Pco2 and venous blood gases in lieu of arterial blood gases. Additionally, they suggested no longer requiring CO2 measures while using prescribed oxygen; eliminating the need for a sleep study to avoid delays in care for patients being discharged from the hospital; removing spirometry as a requirement; and no longer a failure of BiPAP without a BUR.
OSA: The initial purpose of examining OSA in this process was to examine when BiPAP should be utilized for treatment; however, it necessitated examination of the entire policy for PAP. The areas that were identified as needing revision included: expansion of the symptom list for patients with OSA; revising the “4 hour rule,” suggesting that 2 hours has been proven to provide benefit; eliminating the need for another sleep study to re-qualify for PAP or supplemental oxygen; and embracing telehealth as a way to improve accessibility for follow-up visits.
For details, please review the papers published in the November 2021 issue of the journal CHEST® (2021; 160[5]:1579-1990, e377-e543).
We now await what CMS will do with our recommendations and work for “the right device to the right patient at the right time.”
Acknowledgment: Drs. Gerald Criner, Nicholas Hill, Babak Mohklesi, Timothy Morgenthaler, and Lisa Wolfe assisted with the content.
Use of positive airway pressure (PAP) devices for treatment of sleep apnea was first described in 1981. Subsequent use of PAP devices expanded to treat patients with respiratory failure. While the treatment in this population has rapidly gained widespread use and undoubtedly has reduced morbidity and mortality in these populations, policies governing these prescriptions have not really kept up with the burgeoning need.
In 2020, Drs. Peter Gay and Robert Owens brought together a technical expert panel (TEP) to systematically review the CMS policies with an eye to remove “regulatory barriers” to improve access for these patients with the mantra: “the right device gets to the right patient at the right time.”
The panel focused on “Optimal NIV Medicare Access Promotion (ONMAP),” and members with specific expertise were recruited for five patient groups: Thoracic Restrictive Disorders (TRD), COPD, Central Sleep Apnea (CSA), Hypoventilation Syndromes (HVS), and Obstructive Sleep Apnea (OSA). Each group reviewed the current coverage, outlined the deficiencies, and suggested revisions. Herein, I will briefly highlight each group’s most important points.
TRD: The goal for this group was to bring the US standards of care closer to the rest of the world. This group advocates that the start of noninvasive ventilation (NIV) should be substantially earlier, to provide the largest improvement in disease outcome and stability. Other prominent features submitted included arterial blood gases (ABG) to not be the only form of CO2 measurement allowed; paying for a second device if patients are using NIV continuously; qualification for a BiPAP to include if vital capacity is ≤ 80%; and, to obtain a home mechanical ventilator, a patient must either fail BiPAP or have extreme loss of function, high pressure requirements, or need mouthpiece ventilation.
CSA: The big challenges with this diagnosis related to qualifying coverage language in the current policies, which are confusing for many providers. Additionally, these policies often deny certain PAP devices and/or oxygen therapy. The group proposed: a single definition of CSA; eliminate discussion of hypoventilation; mirror qualifying symptoms, and, continuing coverage, to the same as that for OSA treatment; and remove need for a prior failure of BiPAP without a backup rate (BUR). The group also had specific recommendations for when oxygen therapy should be covered in patients with CSA.
COPD: This group also focused on the oxygen therapy and promoting use of devices with a BUR. Two problematic areas included the requirement that nocturnal oxygen saturation must drop to ≤ 88% for at least 5 cumulative minutes, and, that patients must begin with an S mode device (no BUR) for at least 2 months and can only then be prescribed a device with a BUR if CO2 fails to drop. The group advocates for the removal of both, the need for a nocturnal oximetry test, and, to “try” an S mode device. The panel advocated giving the prescribing physician discretion in making this determination. The panel also provided recommendations on when a home mechanical ventilator (HMV) should be considered instead of BiPAP therapy.
HVS: Hypoventilation syndromes are a heterogeneous group of disorders with hypercapnia, defined as a Paco2 ≥45 mm Hg. This panel noted that the current coverage criteria are outdated and fail to recognize the spectrum of disease severity and advances in technology, which often leads to circumvention by prescribing more costly home mechanical ventilators (HMV). Consistent with the TRD group, this panel recommended acceptance of surrogate noninvasive end tidal and transcutaneous Pco2 and venous blood gases in lieu of arterial blood gases. Additionally, they suggested no longer requiring CO2 measures while using prescribed oxygen; eliminating the need for a sleep study to avoid delays in care for patients being discharged from the hospital; removing spirometry as a requirement; and no longer a failure of BiPAP without a BUR.
OSA: The initial purpose of examining OSA in this process was to examine when BiPAP should be utilized for treatment; however, it necessitated examination of the entire policy for PAP. The areas that were identified as needing revision included: expansion of the symptom list for patients with OSA; revising the “4 hour rule,” suggesting that 2 hours has been proven to provide benefit; eliminating the need for another sleep study to re-qualify for PAP or supplemental oxygen; and embracing telehealth as a way to improve accessibility for follow-up visits.
For details, please review the papers published in the November 2021 issue of the journal CHEST® (2021; 160[5]:1579-1990, e377-e543).
We now await what CMS will do with our recommendations and work for “the right device to the right patient at the right time.”
Acknowledgment: Drs. Gerald Criner, Nicholas Hill, Babak Mohklesi, Timothy Morgenthaler, and Lisa Wolfe assisted with the content.
Inhaled corticosteroids for COVID-19
Since the onset of the pandemic, the role for corticosteroids (CS) as a therapy for COVID-19 has evolved. Initially, there was reluctance to use oral corticosteroids (OCS) outside of COVID-19-related sepsis or acute respiratory distress syndrome (ARDS). This was in keeping with community-acquired pneumonia (CAP) guidelines (Metlay JP, et al.Am J Respir Crit Care Med. 2019; 200:e45-e67) and reflected concerns that OCS might worsen outcomes in viral pneumonias. At my hospital, the reluctance to use OCS was extended to inhaled corticosteroids (ICS), with early protocols advising cessation in patients with COVID-19.
In fairness, the hesitation to use ICS was short-lived and reflected attempts to provide reasonable guidance during the early pandemic data vacuum. Over time, OCS therapy has gained acceptance as a treatment for moderate-to-severe COVID-19. On top of this, the relationship between COVID-19 and asthma has proved to be complicated. It seemed intuitive that asthmatics would fair worse in the face of a highly transmissible respiratory pathogen. Data on COVID-19 and asthma provide a mixed picture, though. It also appears that the interaction varies by phenotype (Zhu Z, et al. J Allergy Clin Immunol. 2020;146:327-329).
Improvements with OCS and the complicated interaction between COVID-19 and asthma led some to speculate that ICS, the primary treatment for asthma, may actually be protective. There is biologic plausibility to support this concept. Generally, we’ve seen a variety of immunomodulators show efficacy against moderate or severe disease. Specific to ICS, data have shown a down-regulation in COVID-19 gene expression and reduction in proteins required by the virus for cell entry. This includes a reduction in the evil, much maligned ACE-2 receptor (Peters M, et al. Am J Respir Crit Care Med. 2020;202:83-90).
Like much with COVID-19, the initial asthma phenotype and ICS data were observational and hypothesis- generating, at best. More recently, a series of randomized trials has tested the effects of ICS in patients with milder forms of COVID-19. The data are promising and are worth a thorough review by all physicians caring for COVID-19 outside of the hospital.
The STOIC trial (Ramakrishnan S, et al. Lancet Respir Med. 2021;9:763–772) randomized 146 patients to budesonide via dry powder inhaler (DPI), 800 ug twice per day (BID), versus usual care. The primary outcome was clinical deterioration, defined as presentation to acute or emergency care or need for hospitalization. There was a number of secondary outcomes designed to assess time-to-recovery, predominantly by self-report via questionnaires. The results were nothing short of spectacular. There was a significant difference in the primary outcome with a number-needed to treat (NNT) of only 8 to prevent one instance of COVID-19 deterioration. A number of the secondary outcomes reached significance, as well.
The PRINCIPLE trial, only available in preprint form (https://tinyurl.com/mr4cah7j), also randomized patients to budesonide via ICS vs usual care. PRINCIPLE is one of those cool, adaptive platform trials designed to evaluate multiple therapies simultaneously that have gained popularity in the pandemic era. These trials include predefined criteria for success and futility that allow treatments to be added and others to be dropped. The dosage of budesonide was identical to that in STOIC, and, again, it was delivered via DPI. By design, patients were older with co-morbidities, and there were two primary outcomes. The first was a composite of hospitalization and death, and the second was time to recovery.
The PRINCIPLE preprint is only an interim analysis. There were 751 and 1,028 patients who received budesonide and usual care, respectively. Time to recovery was significantly shorter in the budesonide group, but budesonide failed to meet their prespecified criteria for reducing hospitalization/death. The authors noted that the composite outcome of hospitalization or death did not occur at the rates originally anticipated, presumably due to high vaccination rates. This may have led to type II error.
In a third trial published online in November (Clemency BM, et al. JAMA Intern Med. 2021;10.1001/jamainternmed.2021.6759), patients were randomized to 640 micrograms per day of the ICS ciclesonide. Delivery was via metered-dose inhaler (MDI) for a total duration of 30 days. Unlike the STOIC and PRINCIPLE trials, this one wasn’t open label. It was blinded and placebo-controlled. The investigators found no difference in their primary outcome, time to resolution of symptoms. Ciclesonide did reduce the composite secondary outcome of ED visits or hospital admissions. The number needed to treat was 23.
Please indulge me while I overreact. It seems we’ve got a positive signal in all three. In the era of the Omnicron variant and limited health resources, a widely available therapy that curtails symptoms and prevents acute care visits and hospitalizations could have a tremendous impact. It doesn’t require administration in a clinic and, in theory, efficacy shouldn’t be affected by future mutations of the virus.
A more sober look mutes my enthusiasm. First, as the authors of the ciclesonide article note, open-label trials tracking subjective outcomes via self-assessment can be prone to bias. The ciclesonide trial was double-blinded and didn’t find a difference in time to symptom resolution, only the two open-label trials did. Second, the largest study (PRINCIPLE) didn’t show a difference in escalation of care.
Given, they defined “escalation” as hospitalization or death, and vaccines and patient selection (enrolled only outpatients with mild disease) made proving a statistical reduction difficult. However, in the text they state there wasn’t an improvement in “health care services use” either. In essence, the largest trial showed no change in escalation of care, and the trial with the best design did not show reduction in symptoms.
Although three randomized trials are enough for the inevitable meta-analysis that’ll be published soon; don’t expect it to shed much light. Combining data won’t be particularly helpful because the PRINCIPLE trial is larger than the other two combined, so its results will dominate any statistical analysis of combined data. Not to worry though – there are several more ICS COVID-19 trials underway (NCT04355637, NCT04331054, NCT04193878, NCT04330586, NCT04331054, NCT04331470, NCT04355637, NCT04356495, and NCT04381364). Providers will have to decide for themselves whether what we have so far is sufficient to change practice.
Dr. Holley is Program Director, Pulmonary and Critical Care Medicine Fellowship; and Associate Professor of Medicine USU, Walter Reed National Military Medical Center, Bethesda, Maryland. He also serves as Section Editor for Pulmonary Perspectives®.
Since the onset of the pandemic, the role for corticosteroids (CS) as a therapy for COVID-19 has evolved. Initially, there was reluctance to use oral corticosteroids (OCS) outside of COVID-19-related sepsis or acute respiratory distress syndrome (ARDS). This was in keeping with community-acquired pneumonia (CAP) guidelines (Metlay JP, et al.Am J Respir Crit Care Med. 2019; 200:e45-e67) and reflected concerns that OCS might worsen outcomes in viral pneumonias. At my hospital, the reluctance to use OCS was extended to inhaled corticosteroids (ICS), with early protocols advising cessation in patients with COVID-19.
In fairness, the hesitation to use ICS was short-lived and reflected attempts to provide reasonable guidance during the early pandemic data vacuum. Over time, OCS therapy has gained acceptance as a treatment for moderate-to-severe COVID-19. On top of this, the relationship between COVID-19 and asthma has proved to be complicated. It seemed intuitive that asthmatics would fair worse in the face of a highly transmissible respiratory pathogen. Data on COVID-19 and asthma provide a mixed picture, though. It also appears that the interaction varies by phenotype (Zhu Z, et al. J Allergy Clin Immunol. 2020;146:327-329).
Improvements with OCS and the complicated interaction between COVID-19 and asthma led some to speculate that ICS, the primary treatment for asthma, may actually be protective. There is biologic plausibility to support this concept. Generally, we’ve seen a variety of immunomodulators show efficacy against moderate or severe disease. Specific to ICS, data have shown a down-regulation in COVID-19 gene expression and reduction in proteins required by the virus for cell entry. This includes a reduction in the evil, much maligned ACE-2 receptor (Peters M, et al. Am J Respir Crit Care Med. 2020;202:83-90).
Like much with COVID-19, the initial asthma phenotype and ICS data were observational and hypothesis- generating, at best. More recently, a series of randomized trials has tested the effects of ICS in patients with milder forms of COVID-19. The data are promising and are worth a thorough review by all physicians caring for COVID-19 outside of the hospital.
The STOIC trial (Ramakrishnan S, et al. Lancet Respir Med. 2021;9:763–772) randomized 146 patients to budesonide via dry powder inhaler (DPI), 800 ug twice per day (BID), versus usual care. The primary outcome was clinical deterioration, defined as presentation to acute or emergency care or need for hospitalization. There was a number of secondary outcomes designed to assess time-to-recovery, predominantly by self-report via questionnaires. The results were nothing short of spectacular. There was a significant difference in the primary outcome with a number-needed to treat (NNT) of only 8 to prevent one instance of COVID-19 deterioration. A number of the secondary outcomes reached significance, as well.
The PRINCIPLE trial, only available in preprint form (https://tinyurl.com/mr4cah7j), also randomized patients to budesonide via ICS vs usual care. PRINCIPLE is one of those cool, adaptive platform trials designed to evaluate multiple therapies simultaneously that have gained popularity in the pandemic era. These trials include predefined criteria for success and futility that allow treatments to be added and others to be dropped. The dosage of budesonide was identical to that in STOIC, and, again, it was delivered via DPI. By design, patients were older with co-morbidities, and there were two primary outcomes. The first was a composite of hospitalization and death, and the second was time to recovery.
The PRINCIPLE preprint is only an interim analysis. There were 751 and 1,028 patients who received budesonide and usual care, respectively. Time to recovery was significantly shorter in the budesonide group, but budesonide failed to meet their prespecified criteria for reducing hospitalization/death. The authors noted that the composite outcome of hospitalization or death did not occur at the rates originally anticipated, presumably due to high vaccination rates. This may have led to type II error.
In a third trial published online in November (Clemency BM, et al. JAMA Intern Med. 2021;10.1001/jamainternmed.2021.6759), patients were randomized to 640 micrograms per day of the ICS ciclesonide. Delivery was via metered-dose inhaler (MDI) for a total duration of 30 days. Unlike the STOIC and PRINCIPLE trials, this one wasn’t open label. It was blinded and placebo-controlled. The investigators found no difference in their primary outcome, time to resolution of symptoms. Ciclesonide did reduce the composite secondary outcome of ED visits or hospital admissions. The number needed to treat was 23.
Please indulge me while I overreact. It seems we’ve got a positive signal in all three. In the era of the Omnicron variant and limited health resources, a widely available therapy that curtails symptoms and prevents acute care visits and hospitalizations could have a tremendous impact. It doesn’t require administration in a clinic and, in theory, efficacy shouldn’t be affected by future mutations of the virus.
A more sober look mutes my enthusiasm. First, as the authors of the ciclesonide article note, open-label trials tracking subjective outcomes via self-assessment can be prone to bias. The ciclesonide trial was double-blinded and didn’t find a difference in time to symptom resolution, only the two open-label trials did. Second, the largest study (PRINCIPLE) didn’t show a difference in escalation of care.
Given, they defined “escalation” as hospitalization or death, and vaccines and patient selection (enrolled only outpatients with mild disease) made proving a statistical reduction difficult. However, in the text they state there wasn’t an improvement in “health care services use” either. In essence, the largest trial showed no change in escalation of care, and the trial with the best design did not show reduction in symptoms.
Although three randomized trials are enough for the inevitable meta-analysis that’ll be published soon; don’t expect it to shed much light. Combining data won’t be particularly helpful because the PRINCIPLE trial is larger than the other two combined, so its results will dominate any statistical analysis of combined data. Not to worry though – there are several more ICS COVID-19 trials underway (NCT04355637, NCT04331054, NCT04193878, NCT04330586, NCT04331054, NCT04331470, NCT04355637, NCT04356495, and NCT04381364). Providers will have to decide for themselves whether what we have so far is sufficient to change practice.
Dr. Holley is Program Director, Pulmonary and Critical Care Medicine Fellowship; and Associate Professor of Medicine USU, Walter Reed National Military Medical Center, Bethesda, Maryland. He also serves as Section Editor for Pulmonary Perspectives®.
Since the onset of the pandemic, the role for corticosteroids (CS) as a therapy for COVID-19 has evolved. Initially, there was reluctance to use oral corticosteroids (OCS) outside of COVID-19-related sepsis or acute respiratory distress syndrome (ARDS). This was in keeping with community-acquired pneumonia (CAP) guidelines (Metlay JP, et al.Am J Respir Crit Care Med. 2019; 200:e45-e67) and reflected concerns that OCS might worsen outcomes in viral pneumonias. At my hospital, the reluctance to use OCS was extended to inhaled corticosteroids (ICS), with early protocols advising cessation in patients with COVID-19.
In fairness, the hesitation to use ICS was short-lived and reflected attempts to provide reasonable guidance during the early pandemic data vacuum. Over time, OCS therapy has gained acceptance as a treatment for moderate-to-severe COVID-19. On top of this, the relationship between COVID-19 and asthma has proved to be complicated. It seemed intuitive that asthmatics would fair worse in the face of a highly transmissible respiratory pathogen. Data on COVID-19 and asthma provide a mixed picture, though. It also appears that the interaction varies by phenotype (Zhu Z, et al. J Allergy Clin Immunol. 2020;146:327-329).
Improvements with OCS and the complicated interaction between COVID-19 and asthma led some to speculate that ICS, the primary treatment for asthma, may actually be protective. There is biologic plausibility to support this concept. Generally, we’ve seen a variety of immunomodulators show efficacy against moderate or severe disease. Specific to ICS, data have shown a down-regulation in COVID-19 gene expression and reduction in proteins required by the virus for cell entry. This includes a reduction in the evil, much maligned ACE-2 receptor (Peters M, et al. Am J Respir Crit Care Med. 2020;202:83-90).
Like much with COVID-19, the initial asthma phenotype and ICS data were observational and hypothesis- generating, at best. More recently, a series of randomized trials has tested the effects of ICS in patients with milder forms of COVID-19. The data are promising and are worth a thorough review by all physicians caring for COVID-19 outside of the hospital.
The STOIC trial (Ramakrishnan S, et al. Lancet Respir Med. 2021;9:763–772) randomized 146 patients to budesonide via dry powder inhaler (DPI), 800 ug twice per day (BID), versus usual care. The primary outcome was clinical deterioration, defined as presentation to acute or emergency care or need for hospitalization. There was a number of secondary outcomes designed to assess time-to-recovery, predominantly by self-report via questionnaires. The results were nothing short of spectacular. There was a significant difference in the primary outcome with a number-needed to treat (NNT) of only 8 to prevent one instance of COVID-19 deterioration. A number of the secondary outcomes reached significance, as well.
The PRINCIPLE trial, only available in preprint form (https://tinyurl.com/mr4cah7j), also randomized patients to budesonide via ICS vs usual care. PRINCIPLE is one of those cool, adaptive platform trials designed to evaluate multiple therapies simultaneously that have gained popularity in the pandemic era. These trials include predefined criteria for success and futility that allow treatments to be added and others to be dropped. The dosage of budesonide was identical to that in STOIC, and, again, it was delivered via DPI. By design, patients were older with co-morbidities, and there were two primary outcomes. The first was a composite of hospitalization and death, and the second was time to recovery.
The PRINCIPLE preprint is only an interim analysis. There were 751 and 1,028 patients who received budesonide and usual care, respectively. Time to recovery was significantly shorter in the budesonide group, but budesonide failed to meet their prespecified criteria for reducing hospitalization/death. The authors noted that the composite outcome of hospitalization or death did not occur at the rates originally anticipated, presumably due to high vaccination rates. This may have led to type II error.
In a third trial published online in November (Clemency BM, et al. JAMA Intern Med. 2021;10.1001/jamainternmed.2021.6759), patients were randomized to 640 micrograms per day of the ICS ciclesonide. Delivery was via metered-dose inhaler (MDI) for a total duration of 30 days. Unlike the STOIC and PRINCIPLE trials, this one wasn’t open label. It was blinded and placebo-controlled. The investigators found no difference in their primary outcome, time to resolution of symptoms. Ciclesonide did reduce the composite secondary outcome of ED visits or hospital admissions. The number needed to treat was 23.
Please indulge me while I overreact. It seems we’ve got a positive signal in all three. In the era of the Omnicron variant and limited health resources, a widely available therapy that curtails symptoms and prevents acute care visits and hospitalizations could have a tremendous impact. It doesn’t require administration in a clinic and, in theory, efficacy shouldn’t be affected by future mutations of the virus.
A more sober look mutes my enthusiasm. First, as the authors of the ciclesonide article note, open-label trials tracking subjective outcomes via self-assessment can be prone to bias. The ciclesonide trial was double-blinded and didn’t find a difference in time to symptom resolution, only the two open-label trials did. Second, the largest study (PRINCIPLE) didn’t show a difference in escalation of care.
Given, they defined “escalation” as hospitalization or death, and vaccines and patient selection (enrolled only outpatients with mild disease) made proving a statistical reduction difficult. However, in the text they state there wasn’t an improvement in “health care services use” either. In essence, the largest trial showed no change in escalation of care, and the trial with the best design did not show reduction in symptoms.
Although three randomized trials are enough for the inevitable meta-analysis that’ll be published soon; don’t expect it to shed much light. Combining data won’t be particularly helpful because the PRINCIPLE trial is larger than the other two combined, so its results will dominate any statistical analysis of combined data. Not to worry though – there are several more ICS COVID-19 trials underway (NCT04355637, NCT04331054, NCT04193878, NCT04330586, NCT04331054, NCT04331470, NCT04355637, NCT04356495, and NCT04381364). Providers will have to decide for themselves whether what we have so far is sufficient to change practice.
Dr. Holley is Program Director, Pulmonary and Critical Care Medicine Fellowship; and Associate Professor of Medicine USU, Walter Reed National Military Medical Center, Bethesda, Maryland. He also serves as Section Editor for Pulmonary Perspectives®.
Assisting Surgeons with Management: Initial Presentation of Abnormal Bleeding and Diagnosing of Fibroids
As an Advanced Practice Provider, when and why might a patient with uterine fibroids be scheduled to visit with you?
Ms. Haibach: Typically, with the flow of how our practice runs, a patient would schedule with me as an initial visit to explore their abnormal or heavy bleeding. Oftentimes, a patient is unsure with what they have going on medically and will view APPs as a safe place to start. Other times, I will see a patient for a general wellness exam who will mention heavier menses over the years or just a change in their bleeding pattern-- longer flow, things like that.
It may stem from something that seems out of the ordinary for them or a symptom impacting their life. For example, if a patient says, “I have to run home and change my clothes,” or “I'm bleeding through my bed sheets.” Those statements prompt further evaluation. At times, patients who have already been diagnosed with fibroids, will come to see me if they have chosen medical management over surgical management of their fibroids. They continue to follow up with me to reevaluate the success of their treatment plan periodically. So, whether I start them on a plan, or a physician does, they can follow up with me to revisit their medical plan and ensure it remains appropriate.
You touched on this a bit, but can you dive deeper into exactly what you are looking for as part of that visit?
Ms. Haibach: Definitely. With an initial consult to me, the number one question that I would ask my patients first is, what is your most bothersome symptom? With this question, I'm looking to determine: is it pain that brought you to me? Is it heavy bleeding? Do you feel bulk and bloaty? Are you having issues getting pregnant? Do you have bowel or bladder issues?
The information I get from that one initial question, helps guide the remainder of my visit. If bleeding is the main concern, we would focus on getting that under control. So, we need to suppress the menses with medication options. If bulk and bloating is the main concern, for instance the patient feels like they have a pregnant-looking abdomen, typically surgical options are warranted. If the main complaint is infertility, we do have fertility specialists in our practice who remove fibroids to aid patients in achieving pregnancy.
The most important purpose of this visit is to really listen to the patient to find out how these symptoms are impacting their daily lives. From there, I can use that information to guide my treatment plan.
So, once it is determined that the patient is a good surgical candidate, what would be the next steps?
Ms. Haibach: If at the end of my visit, I determine that a patient is potentially a suitable surgical candidate, the first thing I would do is order appropriate imaging. For example, if the patient is interested in uterine preservation for future fertility, she is likely going to opt for a laparoscopic myomectomy, where fibroids would be removed, and her uterus would be left in place. In that case, she would require an MRI for fibroid mapping. If a woman has completed childbearing, then oftentimes a pelvic ultrasound would suffice, at least to start, since she'd likely elect hysterectomy if she has reached her fertility goals.
I would also perform an endometrial biopsy to rule out malignant process before going into surgery. To optimize a patient for our MIGS surgeons, I gather a thorough medical history to ensure their comorbidities are appropriately managed. For example, diabetes is under control, sleep apnea is being treated, no active infections. If there is anything else going on that needs to be addressed, I'd refer them to the appropriate provider first.
Once I have acceptable imaging, a negative endometrial biopsy and an adequate medical history, I would then assist the patient in scheduling with one of the surgeons on my team for a consult and physical exam to determine surgery planning. Once they see our physician, a surgery date is booked. The patient would come back to see me within 30 days of surgery, and we would do a preoperative education appointment. I see them again 2 weeks after surgery for a post-op visit. We’d perform the post-of visit virtually in our practice. We would see the patient sooner if there are any other concerns that arise post-operatively.
What if the patient is not a surgical candidate? How do you as an APP assist in ongoing medical management?
Ms. Haibach: The presence of fibroids alone, without symptoms, often does not require surgical intervention. There are occasions where a patient is, for example, seen in the emergency room for abdominal pain, whereas they’ll get a CT scan of the abdomen pelvis, and a fibroid is incidentally found. At that point, they are instructed to see gynecology for follow-up. If the patient was unaware of the fibroid, has no symptoms and there's no concerning imaging features, then management with ongoing surveillance (repeat imaging and office follow up) and instructions on when to return is usually appropriate.
Depending on the symptoms, medical management typically includes hormonal suppression of menses in the form of a birth control pill or an IUD. If bleeding is the main concern, it is my goal to at least slow her bleeding, if not try to stop it. Not all women are good candidates for hormone therapy, so there is a medication option that is non-hormonal. In my role, I would start a medication plan for a patient and initiate a new medication such as hormonal suppression in the form of birth control, IUD, non-hormonal medications etc.
Typically, when I do that, I'll have the patient follow up with me in about two to three months to reassess the medication’s effectiveness. The goal of the reassessment is to determine if it is working for her life, to be sure there are no major side-effects, and just to make sure she is amenable to the plan. As part of the medical management, sometimes it is necessary to monitor blood counts for anemia to be certain that medical management is still appropriate for her.
From your experience in practicing, are you more likely to be visited by one age bracket or ethnicity over another?
Ms. Haibach: Actually, data tells us that most fibroids occur in women of reproductive age. They are also diagnosed in African American women two to three times more frequently than in white women. Fibroids are infrequently seen in premenstrual women. A relief of symptoms of the fibroids often occurs at the time of menopause, when the menstrual cyclicity seizes and steroid hormone levels decrease. My demographic is consistent with the above statistics. I tend to see women within the ages of 20’s-50’s and more often African Americans.
Was there anything else that you'd like to mention?
Ms. Haibach: Abnormal bleeding can be very stressful for women. APPs are a great place to start an abnormal bleeding or fibroid work-up. Patients should rest assure that although we cannot perform surgery, APPs can help get them in the right direction for the best care possible.
US Department of Health and Human Services, Office on Women’s Health. Uterine fibroids. (https://www.womenshealth.gov/a-z-topics/uterine-fibroids) Accessed 1/26/2022.
The American College of Obstetricians and Gynecologists. Uterine Fibroids. (https://www.acog.org/patient-resources/faqs/gynecologic-problems/uterine-fibroids) Accessed 1/26/2022.
As an Advanced Practice Provider, when and why might a patient with uterine fibroids be scheduled to visit with you?
Ms. Haibach: Typically, with the flow of how our practice runs, a patient would schedule with me as an initial visit to explore their abnormal or heavy bleeding. Oftentimes, a patient is unsure with what they have going on medically and will view APPs as a safe place to start. Other times, I will see a patient for a general wellness exam who will mention heavier menses over the years or just a change in their bleeding pattern-- longer flow, things like that.
It may stem from something that seems out of the ordinary for them or a symptom impacting their life. For example, if a patient says, “I have to run home and change my clothes,” or “I'm bleeding through my bed sheets.” Those statements prompt further evaluation. At times, patients who have already been diagnosed with fibroids, will come to see me if they have chosen medical management over surgical management of their fibroids. They continue to follow up with me to reevaluate the success of their treatment plan periodically. So, whether I start them on a plan, or a physician does, they can follow up with me to revisit their medical plan and ensure it remains appropriate.
You touched on this a bit, but can you dive deeper into exactly what you are looking for as part of that visit?
Ms. Haibach: Definitely. With an initial consult to me, the number one question that I would ask my patients first is, what is your most bothersome symptom? With this question, I'm looking to determine: is it pain that brought you to me? Is it heavy bleeding? Do you feel bulk and bloaty? Are you having issues getting pregnant? Do you have bowel or bladder issues?
The information I get from that one initial question, helps guide the remainder of my visit. If bleeding is the main concern, we would focus on getting that under control. So, we need to suppress the menses with medication options. If bulk and bloating is the main concern, for instance the patient feels like they have a pregnant-looking abdomen, typically surgical options are warranted. If the main complaint is infertility, we do have fertility specialists in our practice who remove fibroids to aid patients in achieving pregnancy.
The most important purpose of this visit is to really listen to the patient to find out how these symptoms are impacting their daily lives. From there, I can use that information to guide my treatment plan.
So, once it is determined that the patient is a good surgical candidate, what would be the next steps?
Ms. Haibach: If at the end of my visit, I determine that a patient is potentially a suitable surgical candidate, the first thing I would do is order appropriate imaging. For example, if the patient is interested in uterine preservation for future fertility, she is likely going to opt for a laparoscopic myomectomy, where fibroids would be removed, and her uterus would be left in place. In that case, she would require an MRI for fibroid mapping. If a woman has completed childbearing, then oftentimes a pelvic ultrasound would suffice, at least to start, since she'd likely elect hysterectomy if she has reached her fertility goals.
I would also perform an endometrial biopsy to rule out malignant process before going into surgery. To optimize a patient for our MIGS surgeons, I gather a thorough medical history to ensure their comorbidities are appropriately managed. For example, diabetes is under control, sleep apnea is being treated, no active infections. If there is anything else going on that needs to be addressed, I'd refer them to the appropriate provider first.
Once I have acceptable imaging, a negative endometrial biopsy and an adequate medical history, I would then assist the patient in scheduling with one of the surgeons on my team for a consult and physical exam to determine surgery planning. Once they see our physician, a surgery date is booked. The patient would come back to see me within 30 days of surgery, and we would do a preoperative education appointment. I see them again 2 weeks after surgery for a post-op visit. We’d perform the post-of visit virtually in our practice. We would see the patient sooner if there are any other concerns that arise post-operatively.
What if the patient is not a surgical candidate? How do you as an APP assist in ongoing medical management?
Ms. Haibach: The presence of fibroids alone, without symptoms, often does not require surgical intervention. There are occasions where a patient is, for example, seen in the emergency room for abdominal pain, whereas they’ll get a CT scan of the abdomen pelvis, and a fibroid is incidentally found. At that point, they are instructed to see gynecology for follow-up. If the patient was unaware of the fibroid, has no symptoms and there's no concerning imaging features, then management with ongoing surveillance (repeat imaging and office follow up) and instructions on when to return is usually appropriate.
Depending on the symptoms, medical management typically includes hormonal suppression of menses in the form of a birth control pill or an IUD. If bleeding is the main concern, it is my goal to at least slow her bleeding, if not try to stop it. Not all women are good candidates for hormone therapy, so there is a medication option that is non-hormonal. In my role, I would start a medication plan for a patient and initiate a new medication such as hormonal suppression in the form of birth control, IUD, non-hormonal medications etc.
Typically, when I do that, I'll have the patient follow up with me in about two to three months to reassess the medication’s effectiveness. The goal of the reassessment is to determine if it is working for her life, to be sure there are no major side-effects, and just to make sure she is amenable to the plan. As part of the medical management, sometimes it is necessary to monitor blood counts for anemia to be certain that medical management is still appropriate for her.
From your experience in practicing, are you more likely to be visited by one age bracket or ethnicity over another?
Ms. Haibach: Actually, data tells us that most fibroids occur in women of reproductive age. They are also diagnosed in African American women two to three times more frequently than in white women. Fibroids are infrequently seen in premenstrual women. A relief of symptoms of the fibroids often occurs at the time of menopause, when the menstrual cyclicity seizes and steroid hormone levels decrease. My demographic is consistent with the above statistics. I tend to see women within the ages of 20’s-50’s and more often African Americans.
Was there anything else that you'd like to mention?
Ms. Haibach: Abnormal bleeding can be very stressful for women. APPs are a great place to start an abnormal bleeding or fibroid work-up. Patients should rest assure that although we cannot perform surgery, APPs can help get them in the right direction for the best care possible.
As an Advanced Practice Provider, when and why might a patient with uterine fibroids be scheduled to visit with you?
Ms. Haibach: Typically, with the flow of how our practice runs, a patient would schedule with me as an initial visit to explore their abnormal or heavy bleeding. Oftentimes, a patient is unsure with what they have going on medically and will view APPs as a safe place to start. Other times, I will see a patient for a general wellness exam who will mention heavier menses over the years or just a change in their bleeding pattern-- longer flow, things like that.
It may stem from something that seems out of the ordinary for them or a symptom impacting their life. For example, if a patient says, “I have to run home and change my clothes,” or “I'm bleeding through my bed sheets.” Those statements prompt further evaluation. At times, patients who have already been diagnosed with fibroids, will come to see me if they have chosen medical management over surgical management of their fibroids. They continue to follow up with me to reevaluate the success of their treatment plan periodically. So, whether I start them on a plan, or a physician does, they can follow up with me to revisit their medical plan and ensure it remains appropriate.
You touched on this a bit, but can you dive deeper into exactly what you are looking for as part of that visit?
Ms. Haibach: Definitely. With an initial consult to me, the number one question that I would ask my patients first is, what is your most bothersome symptom? With this question, I'm looking to determine: is it pain that brought you to me? Is it heavy bleeding? Do you feel bulk and bloaty? Are you having issues getting pregnant? Do you have bowel or bladder issues?
The information I get from that one initial question, helps guide the remainder of my visit. If bleeding is the main concern, we would focus on getting that under control. So, we need to suppress the menses with medication options. If bulk and bloating is the main concern, for instance the patient feels like they have a pregnant-looking abdomen, typically surgical options are warranted. If the main complaint is infertility, we do have fertility specialists in our practice who remove fibroids to aid patients in achieving pregnancy.
The most important purpose of this visit is to really listen to the patient to find out how these symptoms are impacting their daily lives. From there, I can use that information to guide my treatment plan.
So, once it is determined that the patient is a good surgical candidate, what would be the next steps?
Ms. Haibach: If at the end of my visit, I determine that a patient is potentially a suitable surgical candidate, the first thing I would do is order appropriate imaging. For example, if the patient is interested in uterine preservation for future fertility, she is likely going to opt for a laparoscopic myomectomy, where fibroids would be removed, and her uterus would be left in place. In that case, she would require an MRI for fibroid mapping. If a woman has completed childbearing, then oftentimes a pelvic ultrasound would suffice, at least to start, since she'd likely elect hysterectomy if she has reached her fertility goals.
I would also perform an endometrial biopsy to rule out malignant process before going into surgery. To optimize a patient for our MIGS surgeons, I gather a thorough medical history to ensure their comorbidities are appropriately managed. For example, diabetes is under control, sleep apnea is being treated, no active infections. If there is anything else going on that needs to be addressed, I'd refer them to the appropriate provider first.
Once I have acceptable imaging, a negative endometrial biopsy and an adequate medical history, I would then assist the patient in scheduling with one of the surgeons on my team for a consult and physical exam to determine surgery planning. Once they see our physician, a surgery date is booked. The patient would come back to see me within 30 days of surgery, and we would do a preoperative education appointment. I see them again 2 weeks after surgery for a post-op visit. We’d perform the post-of visit virtually in our practice. We would see the patient sooner if there are any other concerns that arise post-operatively.
What if the patient is not a surgical candidate? How do you as an APP assist in ongoing medical management?
Ms. Haibach: The presence of fibroids alone, without symptoms, often does not require surgical intervention. There are occasions where a patient is, for example, seen in the emergency room for abdominal pain, whereas they’ll get a CT scan of the abdomen pelvis, and a fibroid is incidentally found. At that point, they are instructed to see gynecology for follow-up. If the patient was unaware of the fibroid, has no symptoms and there's no concerning imaging features, then management with ongoing surveillance (repeat imaging and office follow up) and instructions on when to return is usually appropriate.
Depending on the symptoms, medical management typically includes hormonal suppression of menses in the form of a birth control pill or an IUD. If bleeding is the main concern, it is my goal to at least slow her bleeding, if not try to stop it. Not all women are good candidates for hormone therapy, so there is a medication option that is non-hormonal. In my role, I would start a medication plan for a patient and initiate a new medication such as hormonal suppression in the form of birth control, IUD, non-hormonal medications etc.
Typically, when I do that, I'll have the patient follow up with me in about two to three months to reassess the medication’s effectiveness. The goal of the reassessment is to determine if it is working for her life, to be sure there are no major side-effects, and just to make sure she is amenable to the plan. As part of the medical management, sometimes it is necessary to monitor blood counts for anemia to be certain that medical management is still appropriate for her.
From your experience in practicing, are you more likely to be visited by one age bracket or ethnicity over another?
Ms. Haibach: Actually, data tells us that most fibroids occur in women of reproductive age. They are also diagnosed in African American women two to three times more frequently than in white women. Fibroids are infrequently seen in premenstrual women. A relief of symptoms of the fibroids often occurs at the time of menopause, when the menstrual cyclicity seizes and steroid hormone levels decrease. My demographic is consistent with the above statistics. I tend to see women within the ages of 20’s-50’s and more often African Americans.
Was there anything else that you'd like to mention?
Ms. Haibach: Abnormal bleeding can be very stressful for women. APPs are a great place to start an abnormal bleeding or fibroid work-up. Patients should rest assure that although we cannot perform surgery, APPs can help get them in the right direction for the best care possible.
US Department of Health and Human Services, Office on Women’s Health. Uterine fibroids. (https://www.womenshealth.gov/a-z-topics/uterine-fibroids) Accessed 1/26/2022.
The American College of Obstetricians and Gynecologists. Uterine Fibroids. (https://www.acog.org/patient-resources/faqs/gynecologic-problems/uterine-fibroids) Accessed 1/26/2022.
US Department of Health and Human Services, Office on Women’s Health. Uterine fibroids. (https://www.womenshealth.gov/a-z-topics/uterine-fibroids) Accessed 1/26/2022.
The American College of Obstetricians and Gynecologists. Uterine Fibroids. (https://www.acog.org/patient-resources/faqs/gynecologic-problems/uterine-fibroids) Accessed 1/26/2022.
Federal Practitioner 2022 Directory
- 4 Explanatory Notes and Abbreviation Key
- 9 Veterans Integrated Service Network (VISN) Guide
- 14 Department of Veterans Affairs Health Care Facilities
- 118 Centers of Excellence
- 135 TRICARE Region Guide
- 146 Department of Defense Health Care Facilities
- 4 Explanatory Notes and Abbreviation Key
- 9 Veterans Integrated Service Network (VISN) Guide
- 14 Department of Veterans Affairs Health Care Facilities
- 118 Centers of Excellence
- 135 TRICARE Region Guide
- 146 Department of Defense Health Care Facilities
- 4 Explanatory Notes and Abbreviation Key
- 9 Veterans Integrated Service Network (VISN) Guide
- 14 Department of Veterans Affairs Health Care Facilities
- 118 Centers of Excellence
- 135 TRICARE Region Guide
- 146 Department of Defense Health Care Facilities
Fewer diabetes complications with NOACs in patients with AFib
The new research, which was published in Annals of Internal Medicine, found that taking non–vitamin K oral anticoagulants was associated with reduced diabetes complications and lower mortality vs. taking warfarin in the group examined.
In their paper, the researchers present the outcomes of a retrospective cohort study involving 30,209 patients with atrial fibrillation and diabetes. Of these, 19,909 were treated with non–vitamin K oral anticoagulants (NOACs) – dabigatran, rivaroxaban, apixaban, or edoxaban – and 10,300 were treated with warfarin.
Dr. Huei-Kai Huang from the Hualien (Taiwan) Tzu Chi Hospital and coauthors wrote that, while diabetes mellitus is an important risk factor for stroke, there’s not yet a good understanding of the effect of different oral anticoagulants on the risk for diabetes-related complications in patients with atrial fibrillation and diabetes.
“Recent evidence has suggested that NOAC and warfarin may have different effects on glycemic control through the vitamin K–related mechanisms,” coauthor Yu-Kang Tu, PhD, from the College of Public Health at the National Taiwan University in Taipei said in an interview. “It was therefore natural to further evaluate whether NOAC could help decrease various diabetes-related complications, compared with warfarin.”
Hazards with NOACS vs. warfarin
The researchers found that patients treated with NOACs had a 16% lower hazard of macrovascular complications – a composite of coronary artery disease, stroke, and peripheral vascular disease (95% confidence interval, 0.78-0.91; P < .001) – and a 21% lower hazard of microvascular complications including dialysis and lower-extremity amputations (95% CI, 0.73-0.85; P < .001).
NOAC therapy was also associated a 22% lower hazard of death (95% CI, 0.75-0.82; P < .001) and a 9% lower hazard for glycemic emergency (95% CI, 0.83-0.99; P = .043), which the authors defined as a composite of diabetic ketoacidosis, hyperosmolar hyperglycemic state, and hypoglycemia.
In particular, patients treated with NOACs showed significantly lower hazards for coronary artery disease, stroke, dialysis, amputation of lower extremities, and death from cardiovascular and noncardiovascular causes, compared with warfarin users.
The study also found that patients on higher volumes of NOAC medication had greater reductions in mortality and diabetes complications.
“Although our main findings can be explained by the potential differences in underlying mechanisms of action between NOAC and warfarin, we were still surprised with the significantly lower risks of retinopathy, neuropathy, and hypoglycemia in patients taking NOAC with high medication possession ratio,” Dr. Tu said.
Study provides more diabetes-specific outcomes data
Commenting on the findings, Dr. Peter Rossing, head of complications research at the Steno Diabetes Center in Copenhagen said there has long been discussion about whether the newer and more expensive NOACs offer greater benefits to patient with diabetes – beyond stroke prevention – compared with the older and cheaper warfarin. As such, this study was important in providing more diabetes-specific outcomes data and in a large population.
“The effect size they find is certainly meaningful and relevant and should support decision-making,” Dr. Rossing noted in an interview. The finding of reduced risk of amputation and mortality “fits in line with theory that maybe if you block vitamin K, you get calcification, you get vascular damage that leads to failure of the kidney and leads to limb amputations, and that is potentially prevented or not developed when you give the NOACs.”
Dr. John Camm, professor of clinical cardiology at St George’s University of London, said the findings of the benefits of NOACs in this patient group ,were confirmation of earlier, smaller studies, and were important not just for patients with atrial fibrillation and diabetes, but also those prone to diabetes.
“We know from previous studies from the same database, and also from Korea, [for example], that patients who are treated with NOACs as opposed to warfarin develop less diabetes,” he explained.
Dr. Camm said many guidelines around the world now suggest NOACs, and, in some cases, even advise against using vitamin K antagonists as a first option, except in certain situations, such as when patients have rheumatic heart disease, mild to moderate mitral stenosis in rheumatic disease, or prosthetic heart valves.
The researchers applied two methods to account for covariates that may have influenced whether patients received one class of treatment or the other. These achieved ‘appropriate balance’ of baseline characteristics such as comorbidities and baseline medication use for diabetes and other conditions, Dr. Tu and colleagues wrote.
The benefits of NOACs were less evident in younger patients, and the reductions in mortality and diabetes complications associated with NOACs did not reach statistical significance in those aged under 65 years. Regarding this, Dr. Camm noted that there was a debate as to whether patients under 65 years with atrial fibrillation and diabetes should be put on an anticoagulant.
The study was funded by Hualien Tzu Chi Hospital. No conflicts of interest were declared.
The new research, which was published in Annals of Internal Medicine, found that taking non–vitamin K oral anticoagulants was associated with reduced diabetes complications and lower mortality vs. taking warfarin in the group examined.
In their paper, the researchers present the outcomes of a retrospective cohort study involving 30,209 patients with atrial fibrillation and diabetes. Of these, 19,909 were treated with non–vitamin K oral anticoagulants (NOACs) – dabigatran, rivaroxaban, apixaban, or edoxaban – and 10,300 were treated with warfarin.
Dr. Huei-Kai Huang from the Hualien (Taiwan) Tzu Chi Hospital and coauthors wrote that, while diabetes mellitus is an important risk factor for stroke, there’s not yet a good understanding of the effect of different oral anticoagulants on the risk for diabetes-related complications in patients with atrial fibrillation and diabetes.
“Recent evidence has suggested that NOAC and warfarin may have different effects on glycemic control through the vitamin K–related mechanisms,” coauthor Yu-Kang Tu, PhD, from the College of Public Health at the National Taiwan University in Taipei said in an interview. “It was therefore natural to further evaluate whether NOAC could help decrease various diabetes-related complications, compared with warfarin.”
Hazards with NOACS vs. warfarin
The researchers found that patients treated with NOACs had a 16% lower hazard of macrovascular complications – a composite of coronary artery disease, stroke, and peripheral vascular disease (95% confidence interval, 0.78-0.91; P < .001) – and a 21% lower hazard of microvascular complications including dialysis and lower-extremity amputations (95% CI, 0.73-0.85; P < .001).
NOAC therapy was also associated a 22% lower hazard of death (95% CI, 0.75-0.82; P < .001) and a 9% lower hazard for glycemic emergency (95% CI, 0.83-0.99; P = .043), which the authors defined as a composite of diabetic ketoacidosis, hyperosmolar hyperglycemic state, and hypoglycemia.
In particular, patients treated with NOACs showed significantly lower hazards for coronary artery disease, stroke, dialysis, amputation of lower extremities, and death from cardiovascular and noncardiovascular causes, compared with warfarin users.
The study also found that patients on higher volumes of NOAC medication had greater reductions in mortality and diabetes complications.
“Although our main findings can be explained by the potential differences in underlying mechanisms of action between NOAC and warfarin, we were still surprised with the significantly lower risks of retinopathy, neuropathy, and hypoglycemia in patients taking NOAC with high medication possession ratio,” Dr. Tu said.
Study provides more diabetes-specific outcomes data
Commenting on the findings, Dr. Peter Rossing, head of complications research at the Steno Diabetes Center in Copenhagen said there has long been discussion about whether the newer and more expensive NOACs offer greater benefits to patient with diabetes – beyond stroke prevention – compared with the older and cheaper warfarin. As such, this study was important in providing more diabetes-specific outcomes data and in a large population.
“The effect size they find is certainly meaningful and relevant and should support decision-making,” Dr. Rossing noted in an interview. The finding of reduced risk of amputation and mortality “fits in line with theory that maybe if you block vitamin K, you get calcification, you get vascular damage that leads to failure of the kidney and leads to limb amputations, and that is potentially prevented or not developed when you give the NOACs.”
Dr. John Camm, professor of clinical cardiology at St George’s University of London, said the findings of the benefits of NOACs in this patient group ,were confirmation of earlier, smaller studies, and were important not just for patients with atrial fibrillation and diabetes, but also those prone to diabetes.
“We know from previous studies from the same database, and also from Korea, [for example], that patients who are treated with NOACs as opposed to warfarin develop less diabetes,” he explained.
Dr. Camm said many guidelines around the world now suggest NOACs, and, in some cases, even advise against using vitamin K antagonists as a first option, except in certain situations, such as when patients have rheumatic heart disease, mild to moderate mitral stenosis in rheumatic disease, or prosthetic heart valves.
The researchers applied two methods to account for covariates that may have influenced whether patients received one class of treatment or the other. These achieved ‘appropriate balance’ of baseline characteristics such as comorbidities and baseline medication use for diabetes and other conditions, Dr. Tu and colleagues wrote.
The benefits of NOACs were less evident in younger patients, and the reductions in mortality and diabetes complications associated with NOACs did not reach statistical significance in those aged under 65 years. Regarding this, Dr. Camm noted that there was a debate as to whether patients under 65 years with atrial fibrillation and diabetes should be put on an anticoagulant.
The study was funded by Hualien Tzu Chi Hospital. No conflicts of interest were declared.
The new research, which was published in Annals of Internal Medicine, found that taking non–vitamin K oral anticoagulants was associated with reduced diabetes complications and lower mortality vs. taking warfarin in the group examined.
In their paper, the researchers present the outcomes of a retrospective cohort study involving 30,209 patients with atrial fibrillation and diabetes. Of these, 19,909 were treated with non–vitamin K oral anticoagulants (NOACs) – dabigatran, rivaroxaban, apixaban, or edoxaban – and 10,300 were treated with warfarin.
Dr. Huei-Kai Huang from the Hualien (Taiwan) Tzu Chi Hospital and coauthors wrote that, while diabetes mellitus is an important risk factor for stroke, there’s not yet a good understanding of the effect of different oral anticoagulants on the risk for diabetes-related complications in patients with atrial fibrillation and diabetes.
“Recent evidence has suggested that NOAC and warfarin may have different effects on glycemic control through the vitamin K–related mechanisms,” coauthor Yu-Kang Tu, PhD, from the College of Public Health at the National Taiwan University in Taipei said in an interview. “It was therefore natural to further evaluate whether NOAC could help decrease various diabetes-related complications, compared with warfarin.”
Hazards with NOACS vs. warfarin
The researchers found that patients treated with NOACs had a 16% lower hazard of macrovascular complications – a composite of coronary artery disease, stroke, and peripheral vascular disease (95% confidence interval, 0.78-0.91; P < .001) – and a 21% lower hazard of microvascular complications including dialysis and lower-extremity amputations (95% CI, 0.73-0.85; P < .001).
NOAC therapy was also associated a 22% lower hazard of death (95% CI, 0.75-0.82; P < .001) and a 9% lower hazard for glycemic emergency (95% CI, 0.83-0.99; P = .043), which the authors defined as a composite of diabetic ketoacidosis, hyperosmolar hyperglycemic state, and hypoglycemia.
In particular, patients treated with NOACs showed significantly lower hazards for coronary artery disease, stroke, dialysis, amputation of lower extremities, and death from cardiovascular and noncardiovascular causes, compared with warfarin users.
The study also found that patients on higher volumes of NOAC medication had greater reductions in mortality and diabetes complications.
“Although our main findings can be explained by the potential differences in underlying mechanisms of action between NOAC and warfarin, we were still surprised with the significantly lower risks of retinopathy, neuropathy, and hypoglycemia in patients taking NOAC with high medication possession ratio,” Dr. Tu said.
Study provides more diabetes-specific outcomes data
Commenting on the findings, Dr. Peter Rossing, head of complications research at the Steno Diabetes Center in Copenhagen said there has long been discussion about whether the newer and more expensive NOACs offer greater benefits to patient with diabetes – beyond stroke prevention – compared with the older and cheaper warfarin. As such, this study was important in providing more diabetes-specific outcomes data and in a large population.
“The effect size they find is certainly meaningful and relevant and should support decision-making,” Dr. Rossing noted in an interview. The finding of reduced risk of amputation and mortality “fits in line with theory that maybe if you block vitamin K, you get calcification, you get vascular damage that leads to failure of the kidney and leads to limb amputations, and that is potentially prevented or not developed when you give the NOACs.”
Dr. John Camm, professor of clinical cardiology at St George’s University of London, said the findings of the benefits of NOACs in this patient group ,were confirmation of earlier, smaller studies, and were important not just for patients with atrial fibrillation and diabetes, but also those prone to diabetes.
“We know from previous studies from the same database, and also from Korea, [for example], that patients who are treated with NOACs as opposed to warfarin develop less diabetes,” he explained.
Dr. Camm said many guidelines around the world now suggest NOACs, and, in some cases, even advise against using vitamin K antagonists as a first option, except in certain situations, such as when patients have rheumatic heart disease, mild to moderate mitral stenosis in rheumatic disease, or prosthetic heart valves.
The researchers applied two methods to account for covariates that may have influenced whether patients received one class of treatment or the other. These achieved ‘appropriate balance’ of baseline characteristics such as comorbidities and baseline medication use for diabetes and other conditions, Dr. Tu and colleagues wrote.
The benefits of NOACs were less evident in younger patients, and the reductions in mortality and diabetes complications associated with NOACs did not reach statistical significance in those aged under 65 years. Regarding this, Dr. Camm noted that there was a debate as to whether patients under 65 years with atrial fibrillation and diabetes should be put on an anticoagulant.
The study was funded by Hualien Tzu Chi Hospital. No conflicts of interest were declared.
FROM ANNALS OF INTERNAL MEDICINE
Early-onset severe COPD: Similar physical symptoms, but higher depression rates
Younger and older patients with severe chronic obstructive pulmonary disease have similar pulmonary and physical health limitations, based on data from 1,058 adults.
Although chronic obstructive pulmonary disease (COPD) generally appears in older patients, the prevalence among adults aged 45-55 years was 6.5% in 2014-2015, wrote Rosanne J.H.C.G. Beijers, PhD, of Maastricht (the Netherlands) University Medical Center, and colleagues. However, data on the early-onset COPD phenotype are limited. In particular, the extent to which younger patients with early-onset severe COPD experienced the same physical and mental health problems as older patients with similar degree of airflow limitation has not been examined, they said.
In a study published in Clinical Nutrition, the researchers analyzed data from adults with COPD who were referred for pulmonary rehabilitation at a single center between July 2013 and August 2018. Severe disease was defined as FEV1< 50%, and early onset was defined as younger than 55 years. The mean age difference between older and younger patient groups was 15.8 years.
The study population included 79 individuals with early-onset severe disease, 54 with early-onset mild to moderate disease, 158 older adults with severe disease, and 103 older adults with mild to moderate disease. The researchers compared disease markers including body composition, physical performance, and mental health between the groups. A significantly greater proportion of the early-onset group were women, compared to the older group (64% vs. 44%).
In comparing early-onset and older patients with severe COPD, the researchers found that clinical characteristics were similar for body composition, skeletal muscle index, fat percentage, and bone mineral content, and for physical performance factors including the percent predicted maximal work capacity (Wmax), 6-minute walk test, and isokinetic strength. However, a higher prevalence of depression appeared in the early-onset severe-disease patients, compared with the older severe-disease patients (51.9% vs. 32.7%; P = .029).
Although the prevalence of depression was not based on a clinical diagnosis, this finding should prompt health care professionals to pay more attention to psychosocial and emotional well-being in early-onset severe COPD patients, the researchers noted.
In comparing early-onset severe-disease patients and early-onset patients with mild to moderate disease, patients with early-onset severe COPD had significantly lower exercise performance, based on a 6-minute walk test and percent predicted Wmax. However, body composition and isokinetic muscle strength were not significantly different between both early-onset groups.
The findings were limited by several factors including the relatively small number of early-onset patients and the lack of data on whether older patients were diagnosed with severe COPD at a younger age, and more research using age and lung function at the time of diagnosis is needed, the researchers noted. However, the results highlight the importance of early identification of patients at risk for early-onset severe COPD, they said. “Within these individuals at risk, special attention should also be paid to the development of extrapulmonary disease manifestations such as exercise limitations, impaired body composition, and psychological and emotional problems,” the researchers said. “Subsequently, intervention strategies need to be applied that not only focus on the regular advice of quitting smoking but also include decreasing the exposure to air pollutants and promoting a healthy lifestyle including physical activity and a healthy diet,” they added.
The study received no outside funding. Lead author Dr. Beijers had no financial conflicts to disclose.
Younger and older patients with severe chronic obstructive pulmonary disease have similar pulmonary and physical health limitations, based on data from 1,058 adults.
Although chronic obstructive pulmonary disease (COPD) generally appears in older patients, the prevalence among adults aged 45-55 years was 6.5% in 2014-2015, wrote Rosanne J.H.C.G. Beijers, PhD, of Maastricht (the Netherlands) University Medical Center, and colleagues. However, data on the early-onset COPD phenotype are limited. In particular, the extent to which younger patients with early-onset severe COPD experienced the same physical and mental health problems as older patients with similar degree of airflow limitation has not been examined, they said.
In a study published in Clinical Nutrition, the researchers analyzed data from adults with COPD who were referred for pulmonary rehabilitation at a single center between July 2013 and August 2018. Severe disease was defined as FEV1< 50%, and early onset was defined as younger than 55 years. The mean age difference between older and younger patient groups was 15.8 years.
The study population included 79 individuals with early-onset severe disease, 54 with early-onset mild to moderate disease, 158 older adults with severe disease, and 103 older adults with mild to moderate disease. The researchers compared disease markers including body composition, physical performance, and mental health between the groups. A significantly greater proportion of the early-onset group were women, compared to the older group (64% vs. 44%).
In comparing early-onset and older patients with severe COPD, the researchers found that clinical characteristics were similar for body composition, skeletal muscle index, fat percentage, and bone mineral content, and for physical performance factors including the percent predicted maximal work capacity (Wmax), 6-minute walk test, and isokinetic strength. However, a higher prevalence of depression appeared in the early-onset severe-disease patients, compared with the older severe-disease patients (51.9% vs. 32.7%; P = .029).
Although the prevalence of depression was not based on a clinical diagnosis, this finding should prompt health care professionals to pay more attention to psychosocial and emotional well-being in early-onset severe COPD patients, the researchers noted.
In comparing early-onset severe-disease patients and early-onset patients with mild to moderate disease, patients with early-onset severe COPD had significantly lower exercise performance, based on a 6-minute walk test and percent predicted Wmax. However, body composition and isokinetic muscle strength were not significantly different between both early-onset groups.
The findings were limited by several factors including the relatively small number of early-onset patients and the lack of data on whether older patients were diagnosed with severe COPD at a younger age, and more research using age and lung function at the time of diagnosis is needed, the researchers noted. However, the results highlight the importance of early identification of patients at risk for early-onset severe COPD, they said. “Within these individuals at risk, special attention should also be paid to the development of extrapulmonary disease manifestations such as exercise limitations, impaired body composition, and psychological and emotional problems,” the researchers said. “Subsequently, intervention strategies need to be applied that not only focus on the regular advice of quitting smoking but also include decreasing the exposure to air pollutants and promoting a healthy lifestyle including physical activity and a healthy diet,” they added.
The study received no outside funding. Lead author Dr. Beijers had no financial conflicts to disclose.
Younger and older patients with severe chronic obstructive pulmonary disease have similar pulmonary and physical health limitations, based on data from 1,058 adults.
Although chronic obstructive pulmonary disease (COPD) generally appears in older patients, the prevalence among adults aged 45-55 years was 6.5% in 2014-2015, wrote Rosanne J.H.C.G. Beijers, PhD, of Maastricht (the Netherlands) University Medical Center, and colleagues. However, data on the early-onset COPD phenotype are limited. In particular, the extent to which younger patients with early-onset severe COPD experienced the same physical and mental health problems as older patients with similar degree of airflow limitation has not been examined, they said.
In a study published in Clinical Nutrition, the researchers analyzed data from adults with COPD who were referred for pulmonary rehabilitation at a single center between July 2013 and August 2018. Severe disease was defined as FEV1< 50%, and early onset was defined as younger than 55 years. The mean age difference between older and younger patient groups was 15.8 years.
The study population included 79 individuals with early-onset severe disease, 54 with early-onset mild to moderate disease, 158 older adults with severe disease, and 103 older adults with mild to moderate disease. The researchers compared disease markers including body composition, physical performance, and mental health between the groups. A significantly greater proportion of the early-onset group were women, compared to the older group (64% vs. 44%).
In comparing early-onset and older patients with severe COPD, the researchers found that clinical characteristics were similar for body composition, skeletal muscle index, fat percentage, and bone mineral content, and for physical performance factors including the percent predicted maximal work capacity (Wmax), 6-minute walk test, and isokinetic strength. However, a higher prevalence of depression appeared in the early-onset severe-disease patients, compared with the older severe-disease patients (51.9% vs. 32.7%; P = .029).
Although the prevalence of depression was not based on a clinical diagnosis, this finding should prompt health care professionals to pay more attention to psychosocial and emotional well-being in early-onset severe COPD patients, the researchers noted.
In comparing early-onset severe-disease patients and early-onset patients with mild to moderate disease, patients with early-onset severe COPD had significantly lower exercise performance, based on a 6-minute walk test and percent predicted Wmax. However, body composition and isokinetic muscle strength were not significantly different between both early-onset groups.
The findings were limited by several factors including the relatively small number of early-onset patients and the lack of data on whether older patients were diagnosed with severe COPD at a younger age, and more research using age and lung function at the time of diagnosis is needed, the researchers noted. However, the results highlight the importance of early identification of patients at risk for early-onset severe COPD, they said. “Within these individuals at risk, special attention should also be paid to the development of extrapulmonary disease manifestations such as exercise limitations, impaired body composition, and psychological and emotional problems,” the researchers said. “Subsequently, intervention strategies need to be applied that not only focus on the regular advice of quitting smoking but also include decreasing the exposure to air pollutants and promoting a healthy lifestyle including physical activity and a healthy diet,” they added.
The study received no outside funding. Lead author Dr. Beijers had no financial conflicts to disclose.
FROM CLINICAL NUTRITION
Medical students help dispel kids’ fears with teddy bear clinics
In December 2021, 26 medical students at Florida State University (FSU) waltzed into the FSU Child Care and Early Learning Center loaded with armfuls of plushy, cute teddy bears. For the first time in several years, the Pediatric Interest Group opened the doors to their teddy bear clinic – an annual event that gives students an opportunity to practice their leadership skills while also helping to demystify trips to the doctor for the young participants.
At the clinic, children aged 2-4 emulate basic medical practices on their fuzzy patients under the guidance of the students.
Teddy bear clinics were started by FSU’s College of Medicine Family Medicine Interest Group in 2018, but it slowed to a halt until second-year medical student Taylor Posey approached the Pediatric Interest Group during her tenure as the group’s president about reinstating a similar program. At FSU, interest groups allow students who are not quite sure which field of medicine they’d like to pursue to gain experience in any they have interest in.
“Pediatrics is the reason I wanted to go to medical school,” Ms. Posey told this news organization. “So it was great that working on this project really solidified the thought that I did the right thing. It’s great to watch the volunteers and children interact together.”
The clinic divides the children into three groups: 2-year-old toddlers, 3-year-old “tweens,” and 4-year-old pre-K children.
The toddlers paint white handprints on black construction paper to “create” x-rays and learn about them. The tweens are given medical equipment such as paper stethoscopes, thermometers, Band Aids, cotton balls, and Q-Tips to put into their very own doctor bags, which are really just folders with the emblematic red plus sign sticker attached to the front. The Pre-K kids are tasked with giving their teddy bears medical exams under the watchful eye of the medical students. Together, they examine the teddy bear’s eyes, heart, and lungs.
“There’s growing research out there that says medical play – which can be defined as children playing as if they were the parents of the teddy bear, learning about a diagnosis, and treating it – decreases the anxiety in children when they go to visit a doctor. Having real medical equipment that the children can manipulate as opposed to plastic toys really makes a big difference,” Ms. Posey said.
One of Ms. Posey’s peers worked with her to create developmentally appropriate activities for the children. Ms. Posey said that some of the ideas for the clinic came from Pinterest boards.
“The planning of it worked really well. I was expecting things to fall through, but they didn’t,” Ms. Posey said. “It can be tough working with young children and trying to do activities with them so that you’re not doing too much but also not having too low of expectations.”
“It was really a massive success on all fronts,” said Mary P. Norton, MD, an assistant professor of pediatrics and faculty Pediatric Interest Group advisor for the clinic. “The ability to be in the community and get hands-on experience has been really cut down by the pandemic, and this allowed for our students to be able to go out in person and apply what they learned in the classroom with the age group they want to work with, which is fantastic.”
Perhaps the most impactful aspect of the clinic is its ability to help ease children’s fears about visits to the doctor. “We want to allow children to have a voice and give them a space to be a part of their treatment plan,” Dr. Norton said. “We want to say, ‘Your voice matters, you’re not a passive being,’ so that they’re a part of that relationship and show them that their experience is important. We hope these clinics aid in forming a partnership between parents, children, and doctors.”
Currently, the Pediatric Interest Group is hoping to have an annual teddy bear clinic. In the future, they hope to increase it to one a semester.
“These registered student organizations are 100% student run – student ideas, student volunteers, connections, and partnerships,” Dr. Norton said. “This clinic was all Taylor and all of the students. I can’t say how proud she is [to be] taking the time out of her busy medical student schedule to organize this for herself, her peers, and for these children.”
A version of this article first appeared on Medscape.com.
In December 2021, 26 medical students at Florida State University (FSU) waltzed into the FSU Child Care and Early Learning Center loaded with armfuls of plushy, cute teddy bears. For the first time in several years, the Pediatric Interest Group opened the doors to their teddy bear clinic – an annual event that gives students an opportunity to practice their leadership skills while also helping to demystify trips to the doctor for the young participants.
At the clinic, children aged 2-4 emulate basic medical practices on their fuzzy patients under the guidance of the students.
Teddy bear clinics were started by FSU’s College of Medicine Family Medicine Interest Group in 2018, but it slowed to a halt until second-year medical student Taylor Posey approached the Pediatric Interest Group during her tenure as the group’s president about reinstating a similar program. At FSU, interest groups allow students who are not quite sure which field of medicine they’d like to pursue to gain experience in any they have interest in.
“Pediatrics is the reason I wanted to go to medical school,” Ms. Posey told this news organization. “So it was great that working on this project really solidified the thought that I did the right thing. It’s great to watch the volunteers and children interact together.”
The clinic divides the children into three groups: 2-year-old toddlers, 3-year-old “tweens,” and 4-year-old pre-K children.
The toddlers paint white handprints on black construction paper to “create” x-rays and learn about them. The tweens are given medical equipment such as paper stethoscopes, thermometers, Band Aids, cotton balls, and Q-Tips to put into their very own doctor bags, which are really just folders with the emblematic red plus sign sticker attached to the front. The Pre-K kids are tasked with giving their teddy bears medical exams under the watchful eye of the medical students. Together, they examine the teddy bear’s eyes, heart, and lungs.
“There’s growing research out there that says medical play – which can be defined as children playing as if they were the parents of the teddy bear, learning about a diagnosis, and treating it – decreases the anxiety in children when they go to visit a doctor. Having real medical equipment that the children can manipulate as opposed to plastic toys really makes a big difference,” Ms. Posey said.
One of Ms. Posey’s peers worked with her to create developmentally appropriate activities for the children. Ms. Posey said that some of the ideas for the clinic came from Pinterest boards.
“The planning of it worked really well. I was expecting things to fall through, but they didn’t,” Ms. Posey said. “It can be tough working with young children and trying to do activities with them so that you’re not doing too much but also not having too low of expectations.”
“It was really a massive success on all fronts,” said Mary P. Norton, MD, an assistant professor of pediatrics and faculty Pediatric Interest Group advisor for the clinic. “The ability to be in the community and get hands-on experience has been really cut down by the pandemic, and this allowed for our students to be able to go out in person and apply what they learned in the classroom with the age group they want to work with, which is fantastic.”
Perhaps the most impactful aspect of the clinic is its ability to help ease children’s fears about visits to the doctor. “We want to allow children to have a voice and give them a space to be a part of their treatment plan,” Dr. Norton said. “We want to say, ‘Your voice matters, you’re not a passive being,’ so that they’re a part of that relationship and show them that their experience is important. We hope these clinics aid in forming a partnership between parents, children, and doctors.”
Currently, the Pediatric Interest Group is hoping to have an annual teddy bear clinic. In the future, they hope to increase it to one a semester.
“These registered student organizations are 100% student run – student ideas, student volunteers, connections, and partnerships,” Dr. Norton said. “This clinic was all Taylor and all of the students. I can’t say how proud she is [to be] taking the time out of her busy medical student schedule to organize this for herself, her peers, and for these children.”
A version of this article first appeared on Medscape.com.
In December 2021, 26 medical students at Florida State University (FSU) waltzed into the FSU Child Care and Early Learning Center loaded with armfuls of plushy, cute teddy bears. For the first time in several years, the Pediatric Interest Group opened the doors to their teddy bear clinic – an annual event that gives students an opportunity to practice their leadership skills while also helping to demystify trips to the doctor for the young participants.
At the clinic, children aged 2-4 emulate basic medical practices on their fuzzy patients under the guidance of the students.
Teddy bear clinics were started by FSU’s College of Medicine Family Medicine Interest Group in 2018, but it slowed to a halt until second-year medical student Taylor Posey approached the Pediatric Interest Group during her tenure as the group’s president about reinstating a similar program. At FSU, interest groups allow students who are not quite sure which field of medicine they’d like to pursue to gain experience in any they have interest in.
“Pediatrics is the reason I wanted to go to medical school,” Ms. Posey told this news organization. “So it was great that working on this project really solidified the thought that I did the right thing. It’s great to watch the volunteers and children interact together.”
The clinic divides the children into three groups: 2-year-old toddlers, 3-year-old “tweens,” and 4-year-old pre-K children.
The toddlers paint white handprints on black construction paper to “create” x-rays and learn about them. The tweens are given medical equipment such as paper stethoscopes, thermometers, Band Aids, cotton balls, and Q-Tips to put into their very own doctor bags, which are really just folders with the emblematic red plus sign sticker attached to the front. The Pre-K kids are tasked with giving their teddy bears medical exams under the watchful eye of the medical students. Together, they examine the teddy bear’s eyes, heart, and lungs.
“There’s growing research out there that says medical play – which can be defined as children playing as if they were the parents of the teddy bear, learning about a diagnosis, and treating it – decreases the anxiety in children when they go to visit a doctor. Having real medical equipment that the children can manipulate as opposed to plastic toys really makes a big difference,” Ms. Posey said.
One of Ms. Posey’s peers worked with her to create developmentally appropriate activities for the children. Ms. Posey said that some of the ideas for the clinic came from Pinterest boards.
“The planning of it worked really well. I was expecting things to fall through, but they didn’t,” Ms. Posey said. “It can be tough working with young children and trying to do activities with them so that you’re not doing too much but also not having too low of expectations.”
“It was really a massive success on all fronts,” said Mary P. Norton, MD, an assistant professor of pediatrics and faculty Pediatric Interest Group advisor for the clinic. “The ability to be in the community and get hands-on experience has been really cut down by the pandemic, and this allowed for our students to be able to go out in person and apply what they learned in the classroom with the age group they want to work with, which is fantastic.”
Perhaps the most impactful aspect of the clinic is its ability to help ease children’s fears about visits to the doctor. “We want to allow children to have a voice and give them a space to be a part of their treatment plan,” Dr. Norton said. “We want to say, ‘Your voice matters, you’re not a passive being,’ so that they’re a part of that relationship and show them that their experience is important. We hope these clinics aid in forming a partnership between parents, children, and doctors.”
Currently, the Pediatric Interest Group is hoping to have an annual teddy bear clinic. In the future, they hope to increase it to one a semester.
“These registered student organizations are 100% student run – student ideas, student volunteers, connections, and partnerships,” Dr. Norton said. “This clinic was all Taylor and all of the students. I can’t say how proud she is [to be] taking the time out of her busy medical student schedule to organize this for herself, her peers, and for these children.”
A version of this article first appeared on Medscape.com.
Treatment duration for acute otitis media – so many choices
Twenty years ago, the dilemma in treating acute otitis media (AOM) was which among 10-plus antibiotics to prescribe. A recent column discussed the evolving pathogen distribution in AOM and its effects on antibiotic choices.1 But here we consider treatment duration. Until the past decade, AOM treatment (except azithromycin) involved 10-day courses. But lately, 10-day antibiotic regimens for uncomplicated infections are disappearing. Shorter-course recommendations are the new norm because of the evolving clinical data showing that an appropriately chosen antibiotic (in partnership with host defenses and source control) resolves infection faster than was previously thought. Shorter courses make sense because of fewer adverse effects, less distortion of normal flora, and less likely induction of pathogen resistance. Table 4.12 in the newest 2021-2024 SOID Redbook lists three antibiotic durations for AOM, and actually there are more than that.
Why so many duration options? Clinical data show that not all AOM is alike and short courses work for subsets of AOM because, besides antibiotics, key elements in AOM resolution are host anatomy and immunity. Bacterial AOM results from a combination of refluxed pathogens in the middle ear being trapped when the eustachian tube malfunctions (infection occurs when middle ear plumbing gets stopped up). If the eustachian tube spontaneously drains and the host immune response slows/stops pathogen growth, no antibiotics are needed. Indeed, a sizable proportion of mild/moderate AOM episodes spontaneously resolve, particularly in children over 2 years old. So a high likelihood of spontaneous remission allows an initial 0-days duration option (watchful waiting) or delayed antibiotics (rescue prescriptions) for older children.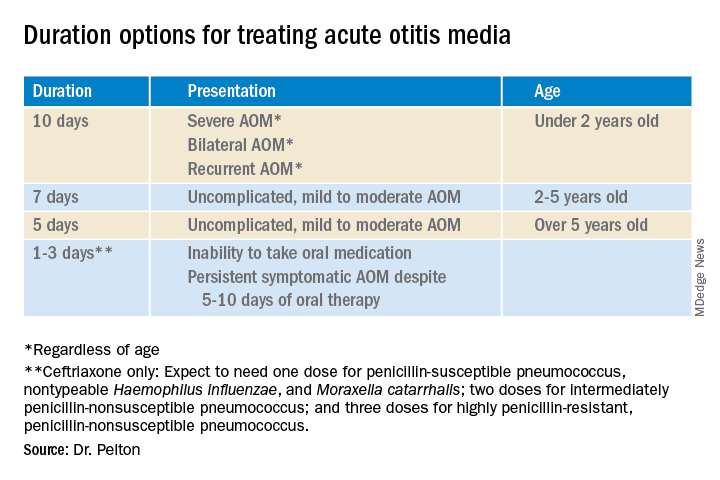
That said, when one chooses to initially prescribe antibiotics for AOM, different durations are recommended. Table 1 has my suggestions.
Data that gave me better microbiological understanding of why oral AOM trials less than 10 days were successful involved purulent AOM drainage from children who had pressure-equalizing (PE) tubes.2 The authors randomized children to either standard-dose amoxicillin-clavulanate or placebo. Of note, 95% of pathogens were susceptible to the antibiotic; 5% were pneumococcus intermediately resistant to penicillin. The authors sampled ear drainage daily for 7 days. Figure 1 shows that cultures remained positive in only around 5% of children by day 3-5 of antibiotics, but viable bacteria persisted through 7 days in over half of placebo recipients. Remember, both groups benefited from a form of source control (drainage of the middle ear via PE tubes). So, if antibiotics can do the job in 3-5 days, why continue antibiotics beyond 5 days?
Anatomy and severity. In children over 5 years old (reasonably mature eustachian tube anatomy) with nonrecurrent (no AOM in past month), nonsevere (no otalgia or high fever) AOM, 5 days is enough. But 2- to 5-year-olds (less mature anatomy) need 7 days and those <2 years old (least mature plumbing) need 10 days. Likewise, severe AOM usually warrants 10 days. Some experts recommend 10 days for bilateral AOM as well.
These age/severity differences make sense because failures are more frequent with:
1. Younger age.3 While not proven, my hypothesis is that “natural” source control (spontaneous internal draining the middle ear into the nasopharynx [NP]) is less frequent in younger children because they have less mature eustachian tube systems. Further, reflux of persisting NP organisms could restart a new AOM episode even if the original pathogen was eliminated by a short 5-day course.
2. Severe AOM. A rationale for longer courses in severe AOM (ear pain, high fever) is that high middle-ear pressures (indicated by degree of tympanic membrane bulging and ear pain) could impede antibiotic penetration, or that high initial bacterial loads (perhaps indicated by systemic fever) require more antibiotic. And finally, return to baseline eustachian tube function may take longer if severe AOM caused enhanced inflammation.
3. Recurrent AOM. (AOM within 1 prior month) – With recurrent AOM, the second “hit” to the eustachian tube may lead to more dysfunction, so a longer antibiotic course may be required to allow more complete source control and more time for more complete functional recovery after a repeated inflammatory injury.
4. Bilateral AOM. Two independent but infected sites mean twice the chance for failure. So, a longer course could allow more time for both sites to undergo “natural” source control.4
More bacteria – more antibiotic? So, is more antibiotic really needed for a higher bacterial load? In vitro this is known as the “inoculum effect,” particularly for beta-lactam drugs, for example, amoxicillin and cephalosporins. Laboratory susceptibility testing is performed with a specifically defined quantity of bacteria (105 bacteria/mL) and the minimum inhibitory concentration (MIC) is the lowest antibiotic concentration that stops bacterial growth. We know that drugs will likely fail if the MIC exceeds the achievable antibiotic concentration at the infection site. But is it as simple as just exceeding the MIC at the infection site? No, pharmacodynamics tell us that overall antibiotic exposure is also important. For example, to be successful, beta-lactam concentrations need to be above the MIC for 40%-50% of the day.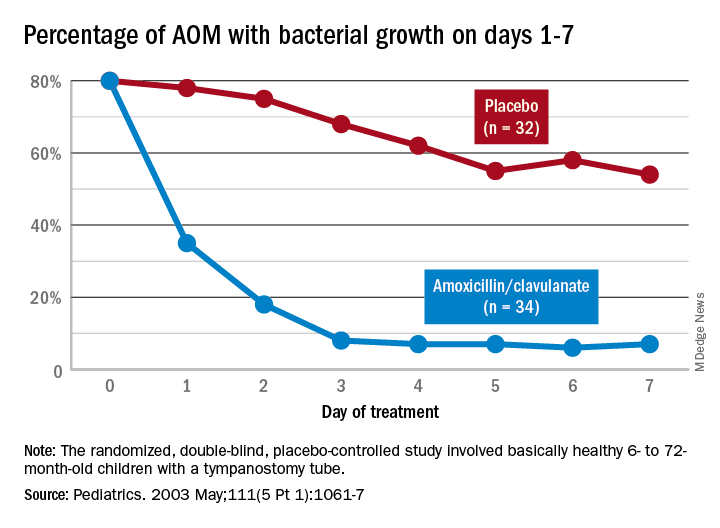
Higher MIC with higher bacterial load. Particularly for beta-lactams, testing with a quantity of bacteria >105/mL produces a higher MIC in vitro. This suggests that clinical failure could occur, even when our in vivo dosing leads to 40%-50% above the “standard” MIC that was obtained from testing the lab standard of 105/mL bacteria, when the infected site’s (middle ear) bacterial load is >105/mL (such higher bacterial loads occur in up to 30% of AOM).5 One way to negate inoculum effect is source control (drain the abscess or debridement), which reduces the bacterial load as well as allowing better antibiotic penetration– both favoring infection resolution. But with suboptimal source control, for example, the middle ear is not drained externally or internally, longer courses (more antibiotic exposure) could aid resolution. Whether the exposure can be administered as higher doses in fewer days or standard doses for more days is debatable but consider that a single parenteral dose of ceftriaxone successfully resolves AOM not attributable to penicillin-nonsusceptible pneumococcus.6Bottom line: Even though the number of potential antibiotics has contracted in the past 20 years, the need to individualize AOM treatment remains important and duration choices are more complex. Indeed, AOM comes in different flavors with patient age, clinical presentation, and episode frequency dictating the choice of duration.
Dr. Christopher J. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. Email him at [email protected].
References
1. Pichichero ME. MDedge. 2022 Jan 11.
2. Ruohola A et al. Pediatrics. 2003;111(5):1061-7.
3. Hoberman A et al. N Engl J Med. 2016;375(25):2446-56.
4. Pichichero ME et al. Otolaryngol Head Neck Surg. 2001;124(4):381-7.
5. Harrison CJ et al. Pediatr Infect Dis. 1985;4(6):641-6.
6. Leibovitz E et al. Pediatr Infect Dis. 2000;19(11):1040-5.
Twenty years ago, the dilemma in treating acute otitis media (AOM) was which among 10-plus antibiotics to prescribe. A recent column discussed the evolving pathogen distribution in AOM and its effects on antibiotic choices.1 But here we consider treatment duration. Until the past decade, AOM treatment (except azithromycin) involved 10-day courses. But lately, 10-day antibiotic regimens for uncomplicated infections are disappearing. Shorter-course recommendations are the new norm because of the evolving clinical data showing that an appropriately chosen antibiotic (in partnership with host defenses and source control) resolves infection faster than was previously thought. Shorter courses make sense because of fewer adverse effects, less distortion of normal flora, and less likely induction of pathogen resistance. Table 4.12 in the newest 2021-2024 SOID Redbook lists three antibiotic durations for AOM, and actually there are more than that.
Why so many duration options? Clinical data show that not all AOM is alike and short courses work for subsets of AOM because, besides antibiotics, key elements in AOM resolution are host anatomy and immunity. Bacterial AOM results from a combination of refluxed pathogens in the middle ear being trapped when the eustachian tube malfunctions (infection occurs when middle ear plumbing gets stopped up). If the eustachian tube spontaneously drains and the host immune response slows/stops pathogen growth, no antibiotics are needed. Indeed, a sizable proportion of mild/moderate AOM episodes spontaneously resolve, particularly in children over 2 years old. So a high likelihood of spontaneous remission allows an initial 0-days duration option (watchful waiting) or delayed antibiotics (rescue prescriptions) for older children.
That said, when one chooses to initially prescribe antibiotics for AOM, different durations are recommended. Table 1 has my suggestions.
Data that gave me better microbiological understanding of why oral AOM trials less than 10 days were successful involved purulent AOM drainage from children who had pressure-equalizing (PE) tubes.2 The authors randomized children to either standard-dose amoxicillin-clavulanate or placebo. Of note, 95% of pathogens were susceptible to the antibiotic; 5% were pneumococcus intermediately resistant to penicillin. The authors sampled ear drainage daily for 7 days. Figure 1 shows that cultures remained positive in only around 5% of children by day 3-5 of antibiotics, but viable bacteria persisted through 7 days in over half of placebo recipients. Remember, both groups benefited from a form of source control (drainage of the middle ear via PE tubes). So, if antibiotics can do the job in 3-5 days, why continue antibiotics beyond 5 days?
Anatomy and severity. In children over 5 years old (reasonably mature eustachian tube anatomy) with nonrecurrent (no AOM in past month), nonsevere (no otalgia or high fever) AOM, 5 days is enough. But 2- to 5-year-olds (less mature anatomy) need 7 days and those <2 years old (least mature plumbing) need 10 days. Likewise, severe AOM usually warrants 10 days. Some experts recommend 10 days for bilateral AOM as well.
These age/severity differences make sense because failures are more frequent with:
1. Younger age.3 While not proven, my hypothesis is that “natural” source control (spontaneous internal draining the middle ear into the nasopharynx [NP]) is less frequent in younger children because they have less mature eustachian tube systems. Further, reflux of persisting NP organisms could restart a new AOM episode even if the original pathogen was eliminated by a short 5-day course.
2. Severe AOM. A rationale for longer courses in severe AOM (ear pain, high fever) is that high middle-ear pressures (indicated by degree of tympanic membrane bulging and ear pain) could impede antibiotic penetration, or that high initial bacterial loads (perhaps indicated by systemic fever) require more antibiotic. And finally, return to baseline eustachian tube function may take longer if severe AOM caused enhanced inflammation.
3. Recurrent AOM. (AOM within 1 prior month) – With recurrent AOM, the second “hit” to the eustachian tube may lead to more dysfunction, so a longer antibiotic course may be required to allow more complete source control and more time for more complete functional recovery after a repeated inflammatory injury.
4. Bilateral AOM. Two independent but infected sites mean twice the chance for failure. So, a longer course could allow more time for both sites to undergo “natural” source control.4
More bacteria – more antibiotic? So, is more antibiotic really needed for a higher bacterial load? In vitro this is known as the “inoculum effect,” particularly for beta-lactam drugs, for example, amoxicillin and cephalosporins. Laboratory susceptibility testing is performed with a specifically defined quantity of bacteria (105 bacteria/mL) and the minimum inhibitory concentration (MIC) is the lowest antibiotic concentration that stops bacterial growth. We know that drugs will likely fail if the MIC exceeds the achievable antibiotic concentration at the infection site. But is it as simple as just exceeding the MIC at the infection site? No, pharmacodynamics tell us that overall antibiotic exposure is also important. For example, to be successful, beta-lactam concentrations need to be above the MIC for 40%-50% of the day.
Higher MIC with higher bacterial load. Particularly for beta-lactams, testing with a quantity of bacteria >105/mL produces a higher MIC in vitro. This suggests that clinical failure could occur, even when our in vivo dosing leads to 40%-50% above the “standard” MIC that was obtained from testing the lab standard of 105/mL bacteria, when the infected site’s (middle ear) bacterial load is >105/mL (such higher bacterial loads occur in up to 30% of AOM).5 One way to negate inoculum effect is source control (drain the abscess or debridement), which reduces the bacterial load as well as allowing better antibiotic penetration– both favoring infection resolution. But with suboptimal source control, for example, the middle ear is not drained externally or internally, longer courses (more antibiotic exposure) could aid resolution. Whether the exposure can be administered as higher doses in fewer days or standard doses for more days is debatable but consider that a single parenteral dose of ceftriaxone successfully resolves AOM not attributable to penicillin-nonsusceptible pneumococcus.6Bottom line: Even though the number of potential antibiotics has contracted in the past 20 years, the need to individualize AOM treatment remains important and duration choices are more complex. Indeed, AOM comes in different flavors with patient age, clinical presentation, and episode frequency dictating the choice of duration.
Dr. Christopher J. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. Email him at [email protected].
References
1. Pichichero ME. MDedge. 2022 Jan 11.
2. Ruohola A et al. Pediatrics. 2003;111(5):1061-7.
3. Hoberman A et al. N Engl J Med. 2016;375(25):2446-56.
4. Pichichero ME et al. Otolaryngol Head Neck Surg. 2001;124(4):381-7.
5. Harrison CJ et al. Pediatr Infect Dis. 1985;4(6):641-6.
6. Leibovitz E et al. Pediatr Infect Dis. 2000;19(11):1040-5.
Twenty years ago, the dilemma in treating acute otitis media (AOM) was which among 10-plus antibiotics to prescribe. A recent column discussed the evolving pathogen distribution in AOM and its effects on antibiotic choices.1 But here we consider treatment duration. Until the past decade, AOM treatment (except azithromycin) involved 10-day courses. But lately, 10-day antibiotic regimens for uncomplicated infections are disappearing. Shorter-course recommendations are the new norm because of the evolving clinical data showing that an appropriately chosen antibiotic (in partnership with host defenses and source control) resolves infection faster than was previously thought. Shorter courses make sense because of fewer adverse effects, less distortion of normal flora, and less likely induction of pathogen resistance. Table 4.12 in the newest 2021-2024 SOID Redbook lists three antibiotic durations for AOM, and actually there are more than that.
Why so many duration options? Clinical data show that not all AOM is alike and short courses work for subsets of AOM because, besides antibiotics, key elements in AOM resolution are host anatomy and immunity. Bacterial AOM results from a combination of refluxed pathogens in the middle ear being trapped when the eustachian tube malfunctions (infection occurs when middle ear plumbing gets stopped up). If the eustachian tube spontaneously drains and the host immune response slows/stops pathogen growth, no antibiotics are needed. Indeed, a sizable proportion of mild/moderate AOM episodes spontaneously resolve, particularly in children over 2 years old. So a high likelihood of spontaneous remission allows an initial 0-days duration option (watchful waiting) or delayed antibiotics (rescue prescriptions) for older children.
That said, when one chooses to initially prescribe antibiotics for AOM, different durations are recommended. Table 1 has my suggestions.
Data that gave me better microbiological understanding of why oral AOM trials less than 10 days were successful involved purulent AOM drainage from children who had pressure-equalizing (PE) tubes.2 The authors randomized children to either standard-dose amoxicillin-clavulanate or placebo. Of note, 95% of pathogens were susceptible to the antibiotic; 5% were pneumococcus intermediately resistant to penicillin. The authors sampled ear drainage daily for 7 days. Figure 1 shows that cultures remained positive in only around 5% of children by day 3-5 of antibiotics, but viable bacteria persisted through 7 days in over half of placebo recipients. Remember, both groups benefited from a form of source control (drainage of the middle ear via PE tubes). So, if antibiotics can do the job in 3-5 days, why continue antibiotics beyond 5 days?
Anatomy and severity. In children over 5 years old (reasonably mature eustachian tube anatomy) with nonrecurrent (no AOM in past month), nonsevere (no otalgia or high fever) AOM, 5 days is enough. But 2- to 5-year-olds (less mature anatomy) need 7 days and those <2 years old (least mature plumbing) need 10 days. Likewise, severe AOM usually warrants 10 days. Some experts recommend 10 days for bilateral AOM as well.
These age/severity differences make sense because failures are more frequent with:
1. Younger age.3 While not proven, my hypothesis is that “natural” source control (spontaneous internal draining the middle ear into the nasopharynx [NP]) is less frequent in younger children because they have less mature eustachian tube systems. Further, reflux of persisting NP organisms could restart a new AOM episode even if the original pathogen was eliminated by a short 5-day course.
2. Severe AOM. A rationale for longer courses in severe AOM (ear pain, high fever) is that high middle-ear pressures (indicated by degree of tympanic membrane bulging and ear pain) could impede antibiotic penetration, or that high initial bacterial loads (perhaps indicated by systemic fever) require more antibiotic. And finally, return to baseline eustachian tube function may take longer if severe AOM caused enhanced inflammation.
3. Recurrent AOM. (AOM within 1 prior month) – With recurrent AOM, the second “hit” to the eustachian tube may lead to more dysfunction, so a longer antibiotic course may be required to allow more complete source control and more time for more complete functional recovery after a repeated inflammatory injury.
4. Bilateral AOM. Two independent but infected sites mean twice the chance for failure. So, a longer course could allow more time for both sites to undergo “natural” source control.4
More bacteria – more antibiotic? So, is more antibiotic really needed for a higher bacterial load? In vitro this is known as the “inoculum effect,” particularly for beta-lactam drugs, for example, amoxicillin and cephalosporins. Laboratory susceptibility testing is performed with a specifically defined quantity of bacteria (105 bacteria/mL) and the minimum inhibitory concentration (MIC) is the lowest antibiotic concentration that stops bacterial growth. We know that drugs will likely fail if the MIC exceeds the achievable antibiotic concentration at the infection site. But is it as simple as just exceeding the MIC at the infection site? No, pharmacodynamics tell us that overall antibiotic exposure is also important. For example, to be successful, beta-lactam concentrations need to be above the MIC for 40%-50% of the day.
Higher MIC with higher bacterial load. Particularly for beta-lactams, testing with a quantity of bacteria >105/mL produces a higher MIC in vitro. This suggests that clinical failure could occur, even when our in vivo dosing leads to 40%-50% above the “standard” MIC that was obtained from testing the lab standard of 105/mL bacteria, when the infected site’s (middle ear) bacterial load is >105/mL (such higher bacterial loads occur in up to 30% of AOM).5 One way to negate inoculum effect is source control (drain the abscess or debridement), which reduces the bacterial load as well as allowing better antibiotic penetration– both favoring infection resolution. But with suboptimal source control, for example, the middle ear is not drained externally or internally, longer courses (more antibiotic exposure) could aid resolution. Whether the exposure can be administered as higher doses in fewer days or standard doses for more days is debatable but consider that a single parenteral dose of ceftriaxone successfully resolves AOM not attributable to penicillin-nonsusceptible pneumococcus.6Bottom line: Even though the number of potential antibiotics has contracted in the past 20 years, the need to individualize AOM treatment remains important and duration choices are more complex. Indeed, AOM comes in different flavors with patient age, clinical presentation, and episode frequency dictating the choice of duration.
Dr. Christopher J. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. Email him at [email protected].
References
1. Pichichero ME. MDedge. 2022 Jan 11.
2. Ruohola A et al. Pediatrics. 2003;111(5):1061-7.
3. Hoberman A et al. N Engl J Med. 2016;375(25):2446-56.
4. Pichichero ME et al. Otolaryngol Head Neck Surg. 2001;124(4):381-7.
5. Harrison CJ et al. Pediatr Infect Dis. 1985;4(6):641-6.
6. Leibovitz E et al. Pediatr Infect Dis. 2000;19(11):1040-5.
Organ transplantation: Unvaccinated need not apply
I agree with most advice given by the affable TV character Ted Lasso. “Every choice is a chance,” he said. Pandemic-era physicians must now consider whether a politically motivated choice to decline COVID-19 vaccination should negatively affect the chance to receive an organ donation.
And in confronting these choices, we have a chance to educate the public on the complexities of the organ allocation process.
A well-informed patient’s personal choice should be honored, even if clinicians disagree, if it does not affect the well-being of others. For example, I once had a patient in acute leukemic crisis who declined blood products because she was a Jehovah’s Witness. She died. Her choice affected her longevity only.
Compare that decision with awarding an organ to an individual who has declined readily available protection of that organ. Weigh that choice against the fact that said protection is against an infectious disease that has killed over 5.5 million worldwide.
Some institutions stand strong, others hedge their bets
Admirably, Loyola University Health System understands that difference. They published a firm stand on transplant candidacy and COVID-19 vaccination status in the Journal of Heart and Lung Transplant. Daniel Dilling, MD, medical director of the lung transplantation program , and Mark Kuczewski, PhD, a professor of medical ethics at Loyola University Chicago, Maywood, Ill., wrote that: “We believe that requiring vaccination against COVID-19 should not be controversial when we focus strictly on established frameworks and practices surrounding eligibility for wait-listing to receive a solid organ transplant.”
The Cleveland Clinic apparently agrees. In October 2021, they denied a liver transplant to Michelle Vitullo of Ohio, whose daughter had been deemed “a perfect match.” Her daughter, also unvaccinated, stated: “Being denied for a nonmedical reason for someone’s beliefs that are different to yours, I mean that’s not how that should be.”
But vaccination status is a medical reason, given well-established data regarding increased mortality among the immunosuppressed. Ms. Vitullo then said: “We are trying to get to UPMC [University of Pittsburgh Medical Center] as they don’t require a vaccination.”
The public information page on transplant candidacy from UPMC reads (my italics): It is recommended that all transplant candidates, transplant recipients, and their household members receive COVID-19 vaccination when the vaccine is available to them. It is preferred that transplant candidates are vaccinated more than 2 weeks before transplantation.
I reached out to UPMC for clarification and was told by email that “we do not have a policy regarding COVID-19 vaccination requirement for current transplant candidates.” Houston Methodist shares the same agnostic stance.
Compare these opinions with Brigham and Women’s Hospital, where the requirements are resolute: “Like most other transplant programs across the country, the COVID-19 vaccine is one of several vaccines and lifestyle behaviors that are required for patients awaiting solid organ transplant.”
They add that “transplant candidates must also receive the seasonal influenza and hepatitis B vaccines, follow other healthy behaviors, and demonstrate they can commit to taking the required medications following transplant.”
In January 2022, Brigham and Women’s Hospital declared 31-year-old D.J. Ferguson ineligible for a heart transplant because he declined to be vaccinated against COVID-19. According to the New York Post and ABC News, his physicians resorted to left ventricular assist device support. His mother, Tracy Ferguson, is quoted as saying: “He’s not an antivaxxer. He has all of his vaccines.” I’ll just leave that right there.
Unfortunately, Michelle Vitullo’s obituary was published in December 2021. Regardless of whether she received her liver transplant, the outcome is tragic, and whatever you think of this family’s battle playing out in the glare of the national spotlight, their loss is no less devastating.
The directed-donation aspect of this case poses an interesting question. A news anchor asked the mother and daughter: “If you both accept the risks, why doesn’t the hospital just let you try?” The answers are obvious to us clinicians. Performing a transplantation in an unvaccinated patient could lead to their early death if they became infected because of their immunocompromised state, would open the door for transplantation of any patient who is unvaccinated for anything, including influenza and hepatitis B, which could result in the preventable waste of organs, and puts other vulnerable hospitalized patients at risk during the initial transplant stay and follow-up.
That’s not to mention the potential legal suit. Never has a consent form dissuaded any party from lodging an accusation of wrongful death or medical malpractice. In the face of strong data on higher mortality in unvaccinated, immunocompromised patients, a good lawyer could charge that the institution and transplant surgeons should have known better, regardless of the donor and recipient’s willingness to accept the risks.
The Vitullo and Ferguson cases are among many similar dilemmas surrounding transplant candidacy across the United States.
University of Virginia Health in Charlottesville denied 42-year-old Shamgar Connors a kidney transplant because he is unvaccinated, despite a previous COVID-19 infection. In October 2021, Leilani Lutali of Colorado was denied a kidney by UCHealth because she declined vaccination.
As Ted Lasso says: “There’s a bunch of crazy stuff on Twitter.”
Predictably, social media is full of public outcry. “Some cold-hearted people on here” tweeted one. “What if it was one of your loved ones who needed a transplant?” Another tweeted the Hippocratic oath with the comment that “They all swore under this noble ‘oat’, but I guess it’s been forgotten.” (This was followed with a photo of a box of Quaker Oats in a failed attempt at humor.) These discussions among the Twitterati highlight the depths of misunderstanding on organ transplantation.
To be fair, unless you have been personally involved in the decision-making process for transplant candidacy, there is little opportunity to be educated. I explain to my anxious patients and their families that a donor organ is like a fumbled football. There may be well over 100 patients at all levels of transplant status in many geographic locations diving for that same organ.
The transplant team is tasked with finding the best match, determining who is the sickest, assessing time for transport of that organ, and, above all, who will be the best steward of that organ.
Take heart transplantation, for instance. Approximately 3,500 patients in the United States are awaiting one each year. Instead of facing an almost certain death within 5 years, a transplant recipient has a chance at a median survival of 12-13 years. The cost of a heart transplant is approximately $1.38 million, according to Milliman, a consulting firm. This is “an incredibly resource intensive procedure,” including expenditures for transportation, antirejection medication, office visits, physician fees, ICU stays, rejection surveillance, and acute rejection therapies.
Transplant denial is nothing new
People get turned down for organ transplants all the time. My patient with end-stage dilated cardiomyopathy was denied a heart transplant when it was discovered that he had scores of outstanding parking tickets. This was seen as a surrogate for an inability to afford his antirejection medication.
Another patient swore that her positive cotinine levels were caused by endless hours at the bingo hall where second-hand smoke swirled. She was also denied. Many potential candidates who are in acute decline hold precariously to newfound sobriety. They are denied. A patient’s boyfriend told the transplant team that he couldn’t be relied upon to drive her to her appointments. She was denied.
Many people who engage in antisocial behaviors have no idea that these actions may result in the denial of an organ transplant should their future selves need one. These are hard lines, but everyone should agree that the odds of survival are heavily in favor of the consistently adherent.
We should take this opportunity to educate the public on how complicated obtaining an organ transplant can be. More than 6,000 people die each year waiting for an organ because of the supply-and-demand disparities in the transplantation arena. I’m willing to bet that many of the loudest protesters in favor of unvaccinated transplant recipients have not signed the organ donor box on the back of their driver’s license. This conversation is an opportunity to change that and remind people that organ donation may be their only opportunity to save a fellow human’s life.
Again, to quote Ted Lasso: “If you care about someone and you got a little love in your heart, there ain’t nothing you can’t get through together.” That philosophy should apply to the tasks of selecting the best organ donors as well as the best organ recipients.
And every organ should go to the one who will honor their donor and their donor’s family by taking the best care of that ultimate gift of life, including being vaccinated against COVID-19.
Dr. Walton-Shirley is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. She disclosed no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
I agree with most advice given by the affable TV character Ted Lasso. “Every choice is a chance,” he said. Pandemic-era physicians must now consider whether a politically motivated choice to decline COVID-19 vaccination should negatively affect the chance to receive an organ donation.
And in confronting these choices, we have a chance to educate the public on the complexities of the organ allocation process.
A well-informed patient’s personal choice should be honored, even if clinicians disagree, if it does not affect the well-being of others. For example, I once had a patient in acute leukemic crisis who declined blood products because she was a Jehovah’s Witness. She died. Her choice affected her longevity only.
Compare that decision with awarding an organ to an individual who has declined readily available protection of that organ. Weigh that choice against the fact that said protection is against an infectious disease that has killed over 5.5 million worldwide.
Some institutions stand strong, others hedge their bets
Admirably, Loyola University Health System understands that difference. They published a firm stand on transplant candidacy and COVID-19 vaccination status in the Journal of Heart and Lung Transplant. Daniel Dilling, MD, medical director of the lung transplantation program , and Mark Kuczewski, PhD, a professor of medical ethics at Loyola University Chicago, Maywood, Ill., wrote that: “We believe that requiring vaccination against COVID-19 should not be controversial when we focus strictly on established frameworks and practices surrounding eligibility for wait-listing to receive a solid organ transplant.”
The Cleveland Clinic apparently agrees. In October 2021, they denied a liver transplant to Michelle Vitullo of Ohio, whose daughter had been deemed “a perfect match.” Her daughter, also unvaccinated, stated: “Being denied for a nonmedical reason for someone’s beliefs that are different to yours, I mean that’s not how that should be.”
But vaccination status is a medical reason, given well-established data regarding increased mortality among the immunosuppressed. Ms. Vitullo then said: “We are trying to get to UPMC [University of Pittsburgh Medical Center] as they don’t require a vaccination.”
The public information page on transplant candidacy from UPMC reads (my italics): It is recommended that all transplant candidates, transplant recipients, and their household members receive COVID-19 vaccination when the vaccine is available to them. It is preferred that transplant candidates are vaccinated more than 2 weeks before transplantation.
I reached out to UPMC for clarification and was told by email that “we do not have a policy regarding COVID-19 vaccination requirement for current transplant candidates.” Houston Methodist shares the same agnostic stance.
Compare these opinions with Brigham and Women’s Hospital, where the requirements are resolute: “Like most other transplant programs across the country, the COVID-19 vaccine is one of several vaccines and lifestyle behaviors that are required for patients awaiting solid organ transplant.”
They add that “transplant candidates must also receive the seasonal influenza and hepatitis B vaccines, follow other healthy behaviors, and demonstrate they can commit to taking the required medications following transplant.”
In January 2022, Brigham and Women’s Hospital declared 31-year-old D.J. Ferguson ineligible for a heart transplant because he declined to be vaccinated against COVID-19. According to the New York Post and ABC News, his physicians resorted to left ventricular assist device support. His mother, Tracy Ferguson, is quoted as saying: “He’s not an antivaxxer. He has all of his vaccines.” I’ll just leave that right there.
Unfortunately, Michelle Vitullo’s obituary was published in December 2021. Regardless of whether she received her liver transplant, the outcome is tragic, and whatever you think of this family’s battle playing out in the glare of the national spotlight, their loss is no less devastating.
The directed-donation aspect of this case poses an interesting question. A news anchor asked the mother and daughter: “If you both accept the risks, why doesn’t the hospital just let you try?” The answers are obvious to us clinicians. Performing a transplantation in an unvaccinated patient could lead to their early death if they became infected because of their immunocompromised state, would open the door for transplantation of any patient who is unvaccinated for anything, including influenza and hepatitis B, which could result in the preventable waste of organs, and puts other vulnerable hospitalized patients at risk during the initial transplant stay and follow-up.
That’s not to mention the potential legal suit. Never has a consent form dissuaded any party from lodging an accusation of wrongful death or medical malpractice. In the face of strong data on higher mortality in unvaccinated, immunocompromised patients, a good lawyer could charge that the institution and transplant surgeons should have known better, regardless of the donor and recipient’s willingness to accept the risks.
The Vitullo and Ferguson cases are among many similar dilemmas surrounding transplant candidacy across the United States.
University of Virginia Health in Charlottesville denied 42-year-old Shamgar Connors a kidney transplant because he is unvaccinated, despite a previous COVID-19 infection. In October 2021, Leilani Lutali of Colorado was denied a kidney by UCHealth because she declined vaccination.
As Ted Lasso says: “There’s a bunch of crazy stuff on Twitter.”
Predictably, social media is full of public outcry. “Some cold-hearted people on here” tweeted one. “What if it was one of your loved ones who needed a transplant?” Another tweeted the Hippocratic oath with the comment that “They all swore under this noble ‘oat’, but I guess it’s been forgotten.” (This was followed with a photo of a box of Quaker Oats in a failed attempt at humor.) These discussions among the Twitterati highlight the depths of misunderstanding on organ transplantation.
To be fair, unless you have been personally involved in the decision-making process for transplant candidacy, there is little opportunity to be educated. I explain to my anxious patients and their families that a donor organ is like a fumbled football. There may be well over 100 patients at all levels of transplant status in many geographic locations diving for that same organ.
The transplant team is tasked with finding the best match, determining who is the sickest, assessing time for transport of that organ, and, above all, who will be the best steward of that organ.
Take heart transplantation, for instance. Approximately 3,500 patients in the United States are awaiting one each year. Instead of facing an almost certain death within 5 years, a transplant recipient has a chance at a median survival of 12-13 years. The cost of a heart transplant is approximately $1.38 million, according to Milliman, a consulting firm. This is “an incredibly resource intensive procedure,” including expenditures for transportation, antirejection medication, office visits, physician fees, ICU stays, rejection surveillance, and acute rejection therapies.
Transplant denial is nothing new
People get turned down for organ transplants all the time. My patient with end-stage dilated cardiomyopathy was denied a heart transplant when it was discovered that he had scores of outstanding parking tickets. This was seen as a surrogate for an inability to afford his antirejection medication.
Another patient swore that her positive cotinine levels were caused by endless hours at the bingo hall where second-hand smoke swirled. She was also denied. Many potential candidates who are in acute decline hold precariously to newfound sobriety. They are denied. A patient’s boyfriend told the transplant team that he couldn’t be relied upon to drive her to her appointments. She was denied.
Many people who engage in antisocial behaviors have no idea that these actions may result in the denial of an organ transplant should their future selves need one. These are hard lines, but everyone should agree that the odds of survival are heavily in favor of the consistently adherent.
We should take this opportunity to educate the public on how complicated obtaining an organ transplant can be. More than 6,000 people die each year waiting for an organ because of the supply-and-demand disparities in the transplantation arena. I’m willing to bet that many of the loudest protesters in favor of unvaccinated transplant recipients have not signed the organ donor box on the back of their driver’s license. This conversation is an opportunity to change that and remind people that organ donation may be their only opportunity to save a fellow human’s life.
Again, to quote Ted Lasso: “If you care about someone and you got a little love in your heart, there ain’t nothing you can’t get through together.” That philosophy should apply to the tasks of selecting the best organ donors as well as the best organ recipients.
And every organ should go to the one who will honor their donor and their donor’s family by taking the best care of that ultimate gift of life, including being vaccinated against COVID-19.
Dr. Walton-Shirley is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. She disclosed no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
I agree with most advice given by the affable TV character Ted Lasso. “Every choice is a chance,” he said. Pandemic-era physicians must now consider whether a politically motivated choice to decline COVID-19 vaccination should negatively affect the chance to receive an organ donation.
And in confronting these choices, we have a chance to educate the public on the complexities of the organ allocation process.
A well-informed patient’s personal choice should be honored, even if clinicians disagree, if it does not affect the well-being of others. For example, I once had a patient in acute leukemic crisis who declined blood products because she was a Jehovah’s Witness. She died. Her choice affected her longevity only.
Compare that decision with awarding an organ to an individual who has declined readily available protection of that organ. Weigh that choice against the fact that said protection is against an infectious disease that has killed over 5.5 million worldwide.
Some institutions stand strong, others hedge their bets
Admirably, Loyola University Health System understands that difference. They published a firm stand on transplant candidacy and COVID-19 vaccination status in the Journal of Heart and Lung Transplant. Daniel Dilling, MD, medical director of the lung transplantation program , and Mark Kuczewski, PhD, a professor of medical ethics at Loyola University Chicago, Maywood, Ill., wrote that: “We believe that requiring vaccination against COVID-19 should not be controversial when we focus strictly on established frameworks and practices surrounding eligibility for wait-listing to receive a solid organ transplant.”
The Cleveland Clinic apparently agrees. In October 2021, they denied a liver transplant to Michelle Vitullo of Ohio, whose daughter had been deemed “a perfect match.” Her daughter, also unvaccinated, stated: “Being denied for a nonmedical reason for someone’s beliefs that are different to yours, I mean that’s not how that should be.”
But vaccination status is a medical reason, given well-established data regarding increased mortality among the immunosuppressed. Ms. Vitullo then said: “We are trying to get to UPMC [University of Pittsburgh Medical Center] as they don’t require a vaccination.”
The public information page on transplant candidacy from UPMC reads (my italics): It is recommended that all transplant candidates, transplant recipients, and their household members receive COVID-19 vaccination when the vaccine is available to them. It is preferred that transplant candidates are vaccinated more than 2 weeks before transplantation.
I reached out to UPMC for clarification and was told by email that “we do not have a policy regarding COVID-19 vaccination requirement for current transplant candidates.” Houston Methodist shares the same agnostic stance.
Compare these opinions with Brigham and Women’s Hospital, where the requirements are resolute: “Like most other transplant programs across the country, the COVID-19 vaccine is one of several vaccines and lifestyle behaviors that are required for patients awaiting solid organ transplant.”
They add that “transplant candidates must also receive the seasonal influenza and hepatitis B vaccines, follow other healthy behaviors, and demonstrate they can commit to taking the required medications following transplant.”
In January 2022, Brigham and Women’s Hospital declared 31-year-old D.J. Ferguson ineligible for a heart transplant because he declined to be vaccinated against COVID-19. According to the New York Post and ABC News, his physicians resorted to left ventricular assist device support. His mother, Tracy Ferguson, is quoted as saying: “He’s not an antivaxxer. He has all of his vaccines.” I’ll just leave that right there.
Unfortunately, Michelle Vitullo’s obituary was published in December 2021. Regardless of whether she received her liver transplant, the outcome is tragic, and whatever you think of this family’s battle playing out in the glare of the national spotlight, their loss is no less devastating.
The directed-donation aspect of this case poses an interesting question. A news anchor asked the mother and daughter: “If you both accept the risks, why doesn’t the hospital just let you try?” The answers are obvious to us clinicians. Performing a transplantation in an unvaccinated patient could lead to their early death if they became infected because of their immunocompromised state, would open the door for transplantation of any patient who is unvaccinated for anything, including influenza and hepatitis B, which could result in the preventable waste of organs, and puts other vulnerable hospitalized patients at risk during the initial transplant stay and follow-up.
That’s not to mention the potential legal suit. Never has a consent form dissuaded any party from lodging an accusation of wrongful death or medical malpractice. In the face of strong data on higher mortality in unvaccinated, immunocompromised patients, a good lawyer could charge that the institution and transplant surgeons should have known better, regardless of the donor and recipient’s willingness to accept the risks.
The Vitullo and Ferguson cases are among many similar dilemmas surrounding transplant candidacy across the United States.
University of Virginia Health in Charlottesville denied 42-year-old Shamgar Connors a kidney transplant because he is unvaccinated, despite a previous COVID-19 infection. In October 2021, Leilani Lutali of Colorado was denied a kidney by UCHealth because she declined vaccination.
As Ted Lasso says: “There’s a bunch of crazy stuff on Twitter.”
Predictably, social media is full of public outcry. “Some cold-hearted people on here” tweeted one. “What if it was one of your loved ones who needed a transplant?” Another tweeted the Hippocratic oath with the comment that “They all swore under this noble ‘oat’, but I guess it’s been forgotten.” (This was followed with a photo of a box of Quaker Oats in a failed attempt at humor.) These discussions among the Twitterati highlight the depths of misunderstanding on organ transplantation.
To be fair, unless you have been personally involved in the decision-making process for transplant candidacy, there is little opportunity to be educated. I explain to my anxious patients and their families that a donor organ is like a fumbled football. There may be well over 100 patients at all levels of transplant status in many geographic locations diving for that same organ.
The transplant team is tasked with finding the best match, determining who is the sickest, assessing time for transport of that organ, and, above all, who will be the best steward of that organ.
Take heart transplantation, for instance. Approximately 3,500 patients in the United States are awaiting one each year. Instead of facing an almost certain death within 5 years, a transplant recipient has a chance at a median survival of 12-13 years. The cost of a heart transplant is approximately $1.38 million, according to Milliman, a consulting firm. This is “an incredibly resource intensive procedure,” including expenditures for transportation, antirejection medication, office visits, physician fees, ICU stays, rejection surveillance, and acute rejection therapies.
Transplant denial is nothing new
People get turned down for organ transplants all the time. My patient with end-stage dilated cardiomyopathy was denied a heart transplant when it was discovered that he had scores of outstanding parking tickets. This was seen as a surrogate for an inability to afford his antirejection medication.
Another patient swore that her positive cotinine levels were caused by endless hours at the bingo hall where second-hand smoke swirled. She was also denied. Many potential candidates who are in acute decline hold precariously to newfound sobriety. They are denied. A patient’s boyfriend told the transplant team that he couldn’t be relied upon to drive her to her appointments. She was denied.
Many people who engage in antisocial behaviors have no idea that these actions may result in the denial of an organ transplant should their future selves need one. These are hard lines, but everyone should agree that the odds of survival are heavily in favor of the consistently adherent.
We should take this opportunity to educate the public on how complicated obtaining an organ transplant can be. More than 6,000 people die each year waiting for an organ because of the supply-and-demand disparities in the transplantation arena. I’m willing to bet that many of the loudest protesters in favor of unvaccinated transplant recipients have not signed the organ donor box on the back of their driver’s license. This conversation is an opportunity to change that and remind people that organ donation may be their only opportunity to save a fellow human’s life.
Again, to quote Ted Lasso: “If you care about someone and you got a little love in your heart, there ain’t nothing you can’t get through together.” That philosophy should apply to the tasks of selecting the best organ donors as well as the best organ recipients.
And every organ should go to the one who will honor their donor and their donor’s family by taking the best care of that ultimate gift of life, including being vaccinated against COVID-19.
Dr. Walton-Shirley is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. She disclosed no relevant conflicts of interest. A version of this article first appeared on Medscape.com.






