User login
We’re Making Progress in the Fight Against GI Cancers
The House Appropriations Committee has included AGA-provided language on gastric and esophageal cancers in the FY25 Labor, Health, and Human Services report.
Gastric and esophageal cancers rates are rising and have a low 5-year survival rate and are highly fatal due to the lack of screening — despite both cancers typically being associated with reduced mortality. Delays in diagnosis lead to poor prognoses when the cancer is already at an advanced stage.
These cancers receive disproportionately low funding and have the lowest ratio of funding to lethality to any other cancer.
That’s why it’s crucial to close the gap and increase screening for GI cancers that are less commonly screened for.
AGA’s provided language encourages NIH to develop, test and implement screening strategies for gastric and esophageal cancers using non-endoscopic screening modalities, personalized clinical risk stratification for screenings and biomarker-based risk-stratification.
Why is this important?
This submission is the first time AGA language on gastric and esophageal cancer has been requested and included in the committee’s report. This illustrates the Committee’s recognition of the importance of GI cancer detection and the work being done by NIH.
What does this mean for GI?
This is an important first step to increasing access to cancer screenings! We look forward to working with our champions on Capitol Hill to increase patient access GI cancer screenings.
The House Appropriations Committee has included AGA-provided language on gastric and esophageal cancers in the FY25 Labor, Health, and Human Services report.
Gastric and esophageal cancers rates are rising and have a low 5-year survival rate and are highly fatal due to the lack of screening — despite both cancers typically being associated with reduced mortality. Delays in diagnosis lead to poor prognoses when the cancer is already at an advanced stage.
These cancers receive disproportionately low funding and have the lowest ratio of funding to lethality to any other cancer.
That’s why it’s crucial to close the gap and increase screening for GI cancers that are less commonly screened for.
AGA’s provided language encourages NIH to develop, test and implement screening strategies for gastric and esophageal cancers using non-endoscopic screening modalities, personalized clinical risk stratification for screenings and biomarker-based risk-stratification.
Why is this important?
This submission is the first time AGA language on gastric and esophageal cancer has been requested and included in the committee’s report. This illustrates the Committee’s recognition of the importance of GI cancer detection and the work being done by NIH.
What does this mean for GI?
This is an important first step to increasing access to cancer screenings! We look forward to working with our champions on Capitol Hill to increase patient access GI cancer screenings.
The House Appropriations Committee has included AGA-provided language on gastric and esophageal cancers in the FY25 Labor, Health, and Human Services report.
Gastric and esophageal cancers rates are rising and have a low 5-year survival rate and are highly fatal due to the lack of screening — despite both cancers typically being associated with reduced mortality. Delays in diagnosis lead to poor prognoses when the cancer is already at an advanced stage.
These cancers receive disproportionately low funding and have the lowest ratio of funding to lethality to any other cancer.
That’s why it’s crucial to close the gap and increase screening for GI cancers that are less commonly screened for.
AGA’s provided language encourages NIH to develop, test and implement screening strategies for gastric and esophageal cancers using non-endoscopic screening modalities, personalized clinical risk stratification for screenings and biomarker-based risk-stratification.
Why is this important?
This submission is the first time AGA language on gastric and esophageal cancer has been requested and included in the committee’s report. This illustrates the Committee’s recognition of the importance of GI cancer detection and the work being done by NIH.
What does this mean for GI?
This is an important first step to increasing access to cancer screenings! We look forward to working with our champions on Capitol Hill to increase patient access GI cancer screenings.
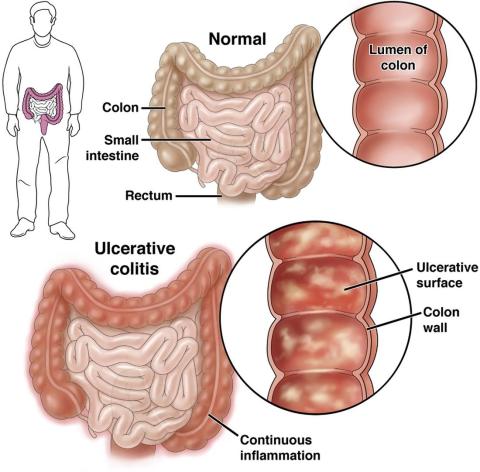
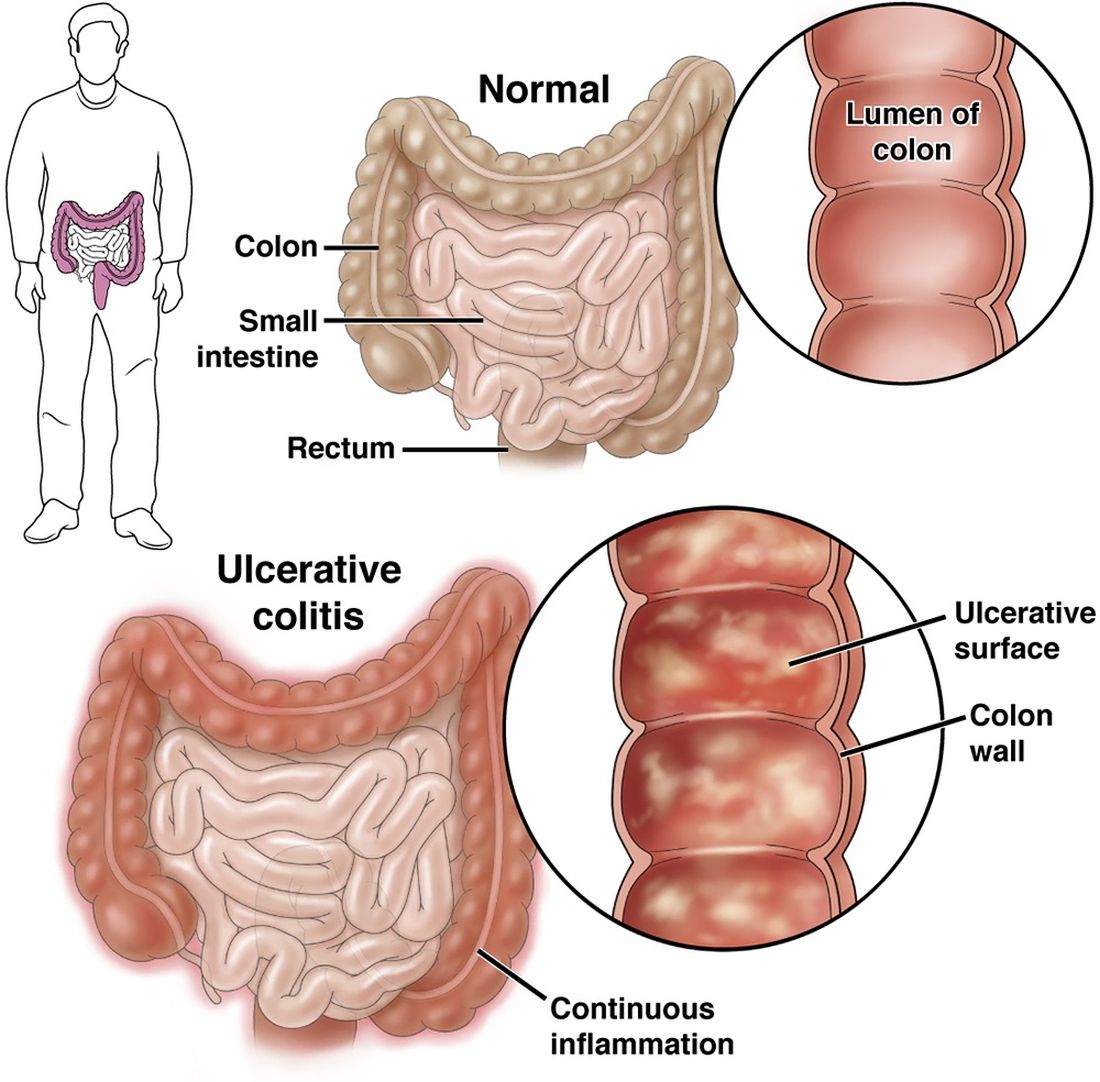
Check Out Our New Ulcerative Colitis Clinician Toolkit
Check out and bookmark AGA’s new ulcerative colitis toolkit, which compiles all our ulcerative colitis clinical guidance, continuing education resources, patient education, and FAQs into one convenient toolkit.
Curious about our other toolkits? Check out our toolkit on Crohn’s disease.
The new UC toolkit includes clinical guidance on:
- Role of biomarkers for the management of ulcerative colitis
- Medical management of moderate to severe ulcerative colitis
- Management of pouchitis and inflammatory pouch disorders
For more resources for ulcerative colitis patients, visit the Patient Center on the AGA website.
The AGA Patient Center has a variety of information that can be shared with your patients, including tips on diet, vaccine recommendations, and information on biosimilars.
Check out and bookmark AGA’s new ulcerative colitis toolkit, which compiles all our ulcerative colitis clinical guidance, continuing education resources, patient education, and FAQs into one convenient toolkit.
Curious about our other toolkits? Check out our toolkit on Crohn’s disease.
The new UC toolkit includes clinical guidance on:
- Role of biomarkers for the management of ulcerative colitis
- Medical management of moderate to severe ulcerative colitis
- Management of pouchitis and inflammatory pouch disorders
For more resources for ulcerative colitis patients, visit the Patient Center on the AGA website.
The AGA Patient Center has a variety of information that can be shared with your patients, including tips on diet, vaccine recommendations, and information on biosimilars.
Check out and bookmark AGA’s new ulcerative colitis toolkit, which compiles all our ulcerative colitis clinical guidance, continuing education resources, patient education, and FAQs into one convenient toolkit.
Curious about our other toolkits? Check out our toolkit on Crohn’s disease.
The new UC toolkit includes clinical guidance on:
- Role of biomarkers for the management of ulcerative colitis
- Medical management of moderate to severe ulcerative colitis
- Management of pouchitis and inflammatory pouch disorders
For more resources for ulcerative colitis patients, visit the Patient Center on the AGA website.
The AGA Patient Center has a variety of information that can be shared with your patients, including tips on diet, vaccine recommendations, and information on biosimilars.
Push, Fail, Push Harder: Olympic Athletes Who Became MDs
Your odds are 1 in 562,400.
Or, as Bill Mallon, the past president and cofounder of the International Society of Olympic Historians, has said, aspiring athletes have a 0.00000178% chance of making the Games.
Now imagine the odds of making the Olympics and then going on to become a physician. And maybe it’s not surprising that those who have done it credit the training they received as Olympic athletes as key to their success in medicine.
“Dealing with poor outcomes and having to get back up and try again,” said Olympian-turned-physician Ogonna Nnamani Silva, MD, “that reiterative process of trying to obtain perfection in your craft — that’s athletics 101.”
This connection isn’t just anecdotal. It has been discussed in medical journals and examined in surveys. The consensus is that, yes, there are specific characteristics elite athletes develop that physicians — regardless of their athletic background — can learn to apply to their work in medicine.
Maybe it’s something else, too: Certain mindsets don’t worry about long odds. They seek out crucibles again and again without concern for the heat involved. Because the outcome is worth it.
Here are four athletes who became high-performing physicians and how they did it.
The Gymnast/The Pediatric Surgeon
“Gymnastics helped me build a skill set for my career,” said Canadian Olympic gymnast-turned-pediatric orthopedic surgeon Lise Leveille, MD. “It led me to be successful as a medical student and ultimately obtain the job that I want in the area that I want working with the people that I want.”
The skills Dr. Leveille prizes include time management, teamwork, goal setting, and a strong work ethic, all of which propel an athlete to the crucial moment of “performance.”
“I miss performing,” said Dr. Leveille. “It defines who I was at that time. I miss being able to work toward something and then deliver when it counted” — like when she qualified for the 1998 Commonwealth games in Kuala Lumpur at 16.
The Canadian national team came third at that event, and Dr. Leveille built on that success at the Pan American Games, taking gold on the balance beam and as a team, and then qualifying for the Olympics at the 1999 World Championships. She competed in the team and five individual events at the 2000 Olympic Games in Sydney.
Though Dr. Leveille started gymnastics at age 3, her parents, both teachers, instilled in her the importance of education. Gymnastics opened academic doors for her, like being recruited to Stanford where she completed her undergraduate degree in biomedical engineering and human biology in 2004 before entering medical school at the University of British Columbia in Vancouver.
Now 41, Dr. Leveille accepts that she’ll never nail another gymnastics routine, but she channels that love of sticking the landing into the operating room at British Columbia Children’s Hospital, also in Vancouver.
“Some of the unknown variables within the operating room and how you deal with those unknown variables is exactly like showing up for a competition,” Dr. Leveille said. “When I have one of those cases where I have to perform under pressure and everything comes together, that’s exactly like nailing your routine when it counts most.”
The Pole Vaulter/The Emergency Medicine Physician
Tunisian American pole vaulter Leila Ben-Youssef, MD, had what could be considered a disappointing showing at the 2008 Olympic Games in Beijing. She collapsed from severe abdominal pain during the opening ceremony and had to be carried out. On the day of competition, she was still suffering. “I could barely run down the runway,” she recalled. “I cleared one bar. I was just happy to have been able to do that.”
When Dr. Ben-Youssef, who grew up in Montana, returned home, she underwent emergency surgery to remove the source of the pain: A large, benign tumor.
While some might be devastated by such bad luck, Dr. Ben-Youssef focuses on the success of her journey — the fact that she qualified and competed at the Olympics in the first place. The ability to accept setbacks is something she said comes with the territory.
“As an athlete, you’re always facing injury, and someone told me early in my career that the best athletes are the ones that know how to manage their expectations because it’s bound to happen,” she said. “So, there is disappointment. But recognizing that I did qualify for the Olympics despite being uncomfortable and having issues, I was still able to meet my goal.”
Prior to the games, Dr. Ben-Youssef had been accepted into medical school at the University of Washington School of Medicine at Montana State University in Bozeman, Montana. Thankfully, the school was supportive of Dr. Ben-Youssef’s Olympic dreams and allowed her to begin her studies a month behind her class. Upon her return from Beijing, she spent the rest of her medical school training with her head down, grinding.
“Medicine is hard,” said Dr. Ben-Youssef. “It’s grueling both physically and emotionally, and I think that’s similar to any elite sport. You’re going to deal with challenges and disappointment. I think having gone through that as an athlete really prepares you for the medical education system, for residency, and even for day-to-day work.”
Now a physician working in emergency medicine in Hawaii, Dr. Ben-Youssef feels the setbacks she experienced as an athlete help her connect with her patients as they deal with health challenges.
And as a volunteer pole vaulting coach for a local high school, Dr. Ben-Youssef has been able to surround herself with the positive, joyful energy of athletes. “Emergency medicine is often a sad place,” she said. “But in a sports environment, if people don’t succeed or are injured, there is still that energy there that strives for something, and it’s so fun to be around.”
The Rower/The Sports Medicine Specialist
Three-time US Olympic rower Genevra “Gevvie” Stone, MD, wanted to be a doctor even before she gave a thought to rowing. She was in eighth grade when she dislocated her knee for the third time. Her parents took her to a pediatric orthopedist, and Dr. Stone, according to her mom, declared: “That’s what I want to do when I grow up.”
“I’m a very stubborn person, and when I make a decision like that, I usually don’t veer from it,” Dr. Stone said.
That laser focus combined with a deep love of both sports and medicine has served Dr. Stone well. “Becoming a doctor and becoming an Olympian require you to dedicate not just your time and your energy but also your passion to that focus,” she said. “In both, you aren’t going to be successful if you don’t love what you’re doing. Finding the reward in it is what makes it achievable.”
Dr. Stone actually resisted rowing until she was 16 because both of her parents were Olympians in the sport and met on the US team. “It was their thing, and I didn’t want it to be my thing,” she recalled.
Nonetheless, Dr. Stone easily fell into the sport in her late teens and was recruited by Princeton University. “I had grown up around Olympians and kind of took it for granted that if you worked hard enough and were decent at rowing, then you could be one of the best in the world, without really realizing how difficult it would be to achieve that,” she said.
Dr. Stone’s team won the NCAA Championship in 2006 and was invited to try out for the 2008 Olympic team at the US training center after she graduated from college. But she didn’t make it.
Instead, Dr. Stone entered medical school at Tufts University School of Medicine, Boston, thinking her competitive rowing career had come to end. But her love for the sport was still strong, and she realized she wasn’t finished.
After 2 years of medical school, Dr. Stone requested 2 years off so she might have another shot at making the Olympic team. The timing was right. She went to the London Olympics in 2012, graduated from medical school in 2014, and then took 2 more years off to train full time for the 2016 Olympics in Rio where she won silver.
At the 2020 Olympic Games in Tokyo, Dr. Stone took fifth place in the double sculls. While she continues to race the master’s circuit, she’s primarily dedicated to completing her sports medicine fellowship at University of Utah Health.
Fortunately, Dr. Stone’s parents, coaches, and teachers always supported her goals. “No one turned to me and told me I was crazy, just choose medicine or rowing,” she said. “Everyone said that if this is what you want to do, we’re here to support you, and I wouldn’t have been able to do it without that support.”
The Volleyball Player/The Plastic Surgeon
Dr. Nnamani Silva’s journey to the Olympics was also paved with an extensive list of supporters, beginning with her parents. And she has taken that sense of collaboration, coordination, and teamwork into her medical career.
The daughter of Nigerian immigrants who came to the United States to escape civil war, Dr. Nnamani Silva said her parents embraced the American dream. “To see what they were able to do with hard work, dedication, and sacrifice, I had no choice but to work hard because I saw their example. And that love for and belief in America was so strong in my house growing up,” she said.
Dreams of practicing medicine came first. A severe asthmatic growing up, Dr. Nnamani Silva recalled having wonderful doctors. “I had so many emergency room visits and hospitalizations,” she said. “But the doctors always gave me hope, and they literally transformed my life. I thought if I could pass that on to my future patients, that would be the greatest honor of my life.”
Volleyball gave Dr. Nnamani Silva the opportunity to attend Stanford, and she took time off during her junior year to train and compete in the 2004 Olympic Games in Athens. She also played for the United States at the 2008 Olympic Games in Beijing where the team took silver. Afterward, she continued to play overseas for several years.
At 33, and with a newborn daughter, Dr. Nnamani Silva returned to her original goal of becoming a doctor. She attended the University of California, San Francisco, and is currently a resident in the Harvard Plastic Surgery Program. She includes her husband, parents, and in-laws in this achievement, whom she said “saved” her. “There is no chance I would have finished medical school and survived residency without them.”
As a volleyball player, Dr. Nnamani Silva said she “believes in teams wholeheartedly,” valuing the exchange of energy and skill that she feels brings out the best in people. As a medical student, she initially didn’t realize how her previous life would apply to teamwork in the operating room. But it soon became clear.
“In surgery, when you harness the talents of everyone around you and you create that synergy, it’s an amazing feeling,” she said. And the stakes are often high. “It requires a lot of focus, discipline, determination, and resilience because you’re going to be humbled all the time.” Something athletes know a little bit about.
A version of this article first appeared on Medscape.com.
Your odds are 1 in 562,400.
Or, as Bill Mallon, the past president and cofounder of the International Society of Olympic Historians, has said, aspiring athletes have a 0.00000178% chance of making the Games.
Now imagine the odds of making the Olympics and then going on to become a physician. And maybe it’s not surprising that those who have done it credit the training they received as Olympic athletes as key to their success in medicine.
“Dealing with poor outcomes and having to get back up and try again,” said Olympian-turned-physician Ogonna Nnamani Silva, MD, “that reiterative process of trying to obtain perfection in your craft — that’s athletics 101.”
This connection isn’t just anecdotal. It has been discussed in medical journals and examined in surveys. The consensus is that, yes, there are specific characteristics elite athletes develop that physicians — regardless of their athletic background — can learn to apply to their work in medicine.
Maybe it’s something else, too: Certain mindsets don’t worry about long odds. They seek out crucibles again and again without concern for the heat involved. Because the outcome is worth it.
Here are four athletes who became high-performing physicians and how they did it.
The Gymnast/The Pediatric Surgeon
“Gymnastics helped me build a skill set for my career,” said Canadian Olympic gymnast-turned-pediatric orthopedic surgeon Lise Leveille, MD. “It led me to be successful as a medical student and ultimately obtain the job that I want in the area that I want working with the people that I want.”
The skills Dr. Leveille prizes include time management, teamwork, goal setting, and a strong work ethic, all of which propel an athlete to the crucial moment of “performance.”
“I miss performing,” said Dr. Leveille. “It defines who I was at that time. I miss being able to work toward something and then deliver when it counted” — like when she qualified for the 1998 Commonwealth games in Kuala Lumpur at 16.
The Canadian national team came third at that event, and Dr. Leveille built on that success at the Pan American Games, taking gold on the balance beam and as a team, and then qualifying for the Olympics at the 1999 World Championships. She competed in the team and five individual events at the 2000 Olympic Games in Sydney.
Though Dr. Leveille started gymnastics at age 3, her parents, both teachers, instilled in her the importance of education. Gymnastics opened academic doors for her, like being recruited to Stanford where she completed her undergraduate degree in biomedical engineering and human biology in 2004 before entering medical school at the University of British Columbia in Vancouver.
Now 41, Dr. Leveille accepts that she’ll never nail another gymnastics routine, but she channels that love of sticking the landing into the operating room at British Columbia Children’s Hospital, also in Vancouver.
“Some of the unknown variables within the operating room and how you deal with those unknown variables is exactly like showing up for a competition,” Dr. Leveille said. “When I have one of those cases where I have to perform under pressure and everything comes together, that’s exactly like nailing your routine when it counts most.”
The Pole Vaulter/The Emergency Medicine Physician
Tunisian American pole vaulter Leila Ben-Youssef, MD, had what could be considered a disappointing showing at the 2008 Olympic Games in Beijing. She collapsed from severe abdominal pain during the opening ceremony and had to be carried out. On the day of competition, she was still suffering. “I could barely run down the runway,” she recalled. “I cleared one bar. I was just happy to have been able to do that.”
When Dr. Ben-Youssef, who grew up in Montana, returned home, she underwent emergency surgery to remove the source of the pain: A large, benign tumor.
While some might be devastated by such bad luck, Dr. Ben-Youssef focuses on the success of her journey — the fact that she qualified and competed at the Olympics in the first place. The ability to accept setbacks is something she said comes with the territory.
“As an athlete, you’re always facing injury, and someone told me early in my career that the best athletes are the ones that know how to manage their expectations because it’s bound to happen,” she said. “So, there is disappointment. But recognizing that I did qualify for the Olympics despite being uncomfortable and having issues, I was still able to meet my goal.”
Prior to the games, Dr. Ben-Youssef had been accepted into medical school at the University of Washington School of Medicine at Montana State University in Bozeman, Montana. Thankfully, the school was supportive of Dr. Ben-Youssef’s Olympic dreams and allowed her to begin her studies a month behind her class. Upon her return from Beijing, she spent the rest of her medical school training with her head down, grinding.
“Medicine is hard,” said Dr. Ben-Youssef. “It’s grueling both physically and emotionally, and I think that’s similar to any elite sport. You’re going to deal with challenges and disappointment. I think having gone through that as an athlete really prepares you for the medical education system, for residency, and even for day-to-day work.”
Now a physician working in emergency medicine in Hawaii, Dr. Ben-Youssef feels the setbacks she experienced as an athlete help her connect with her patients as they deal with health challenges.
And as a volunteer pole vaulting coach for a local high school, Dr. Ben-Youssef has been able to surround herself with the positive, joyful energy of athletes. “Emergency medicine is often a sad place,” she said. “But in a sports environment, if people don’t succeed or are injured, there is still that energy there that strives for something, and it’s so fun to be around.”
The Rower/The Sports Medicine Specialist
Three-time US Olympic rower Genevra “Gevvie” Stone, MD, wanted to be a doctor even before she gave a thought to rowing. She was in eighth grade when she dislocated her knee for the third time. Her parents took her to a pediatric orthopedist, and Dr. Stone, according to her mom, declared: “That’s what I want to do when I grow up.”
“I’m a very stubborn person, and when I make a decision like that, I usually don’t veer from it,” Dr. Stone said.
That laser focus combined with a deep love of both sports and medicine has served Dr. Stone well. “Becoming a doctor and becoming an Olympian require you to dedicate not just your time and your energy but also your passion to that focus,” she said. “In both, you aren’t going to be successful if you don’t love what you’re doing. Finding the reward in it is what makes it achievable.”
Dr. Stone actually resisted rowing until she was 16 because both of her parents were Olympians in the sport and met on the US team. “It was their thing, and I didn’t want it to be my thing,” she recalled.
Nonetheless, Dr. Stone easily fell into the sport in her late teens and was recruited by Princeton University. “I had grown up around Olympians and kind of took it for granted that if you worked hard enough and were decent at rowing, then you could be one of the best in the world, without really realizing how difficult it would be to achieve that,” she said.
Dr. Stone’s team won the NCAA Championship in 2006 and was invited to try out for the 2008 Olympic team at the US training center after she graduated from college. But she didn’t make it.
Instead, Dr. Stone entered medical school at Tufts University School of Medicine, Boston, thinking her competitive rowing career had come to end. But her love for the sport was still strong, and she realized she wasn’t finished.
After 2 years of medical school, Dr. Stone requested 2 years off so she might have another shot at making the Olympic team. The timing was right. She went to the London Olympics in 2012, graduated from medical school in 2014, and then took 2 more years off to train full time for the 2016 Olympics in Rio where she won silver.
At the 2020 Olympic Games in Tokyo, Dr. Stone took fifth place in the double sculls. While she continues to race the master’s circuit, she’s primarily dedicated to completing her sports medicine fellowship at University of Utah Health.
Fortunately, Dr. Stone’s parents, coaches, and teachers always supported her goals. “No one turned to me and told me I was crazy, just choose medicine or rowing,” she said. “Everyone said that if this is what you want to do, we’re here to support you, and I wouldn’t have been able to do it without that support.”
The Volleyball Player/The Plastic Surgeon
Dr. Nnamani Silva’s journey to the Olympics was also paved with an extensive list of supporters, beginning with her parents. And she has taken that sense of collaboration, coordination, and teamwork into her medical career.
The daughter of Nigerian immigrants who came to the United States to escape civil war, Dr. Nnamani Silva said her parents embraced the American dream. “To see what they were able to do with hard work, dedication, and sacrifice, I had no choice but to work hard because I saw their example. And that love for and belief in America was so strong in my house growing up,” she said.
Dreams of practicing medicine came first. A severe asthmatic growing up, Dr. Nnamani Silva recalled having wonderful doctors. “I had so many emergency room visits and hospitalizations,” she said. “But the doctors always gave me hope, and they literally transformed my life. I thought if I could pass that on to my future patients, that would be the greatest honor of my life.”
Volleyball gave Dr. Nnamani Silva the opportunity to attend Stanford, and she took time off during her junior year to train and compete in the 2004 Olympic Games in Athens. She also played for the United States at the 2008 Olympic Games in Beijing where the team took silver. Afterward, she continued to play overseas for several years.
At 33, and with a newborn daughter, Dr. Nnamani Silva returned to her original goal of becoming a doctor. She attended the University of California, San Francisco, and is currently a resident in the Harvard Plastic Surgery Program. She includes her husband, parents, and in-laws in this achievement, whom she said “saved” her. “There is no chance I would have finished medical school and survived residency without them.”
As a volleyball player, Dr. Nnamani Silva said she “believes in teams wholeheartedly,” valuing the exchange of energy and skill that she feels brings out the best in people. As a medical student, she initially didn’t realize how her previous life would apply to teamwork in the operating room. But it soon became clear.
“In surgery, when you harness the talents of everyone around you and you create that synergy, it’s an amazing feeling,” she said. And the stakes are often high. “It requires a lot of focus, discipline, determination, and resilience because you’re going to be humbled all the time.” Something athletes know a little bit about.
A version of this article first appeared on Medscape.com.
Your odds are 1 in 562,400.
Or, as Bill Mallon, the past president and cofounder of the International Society of Olympic Historians, has said, aspiring athletes have a 0.00000178% chance of making the Games.
Now imagine the odds of making the Olympics and then going on to become a physician. And maybe it’s not surprising that those who have done it credit the training they received as Olympic athletes as key to their success in medicine.
“Dealing with poor outcomes and having to get back up and try again,” said Olympian-turned-physician Ogonna Nnamani Silva, MD, “that reiterative process of trying to obtain perfection in your craft — that’s athletics 101.”
This connection isn’t just anecdotal. It has been discussed in medical journals and examined in surveys. The consensus is that, yes, there are specific characteristics elite athletes develop that physicians — regardless of their athletic background — can learn to apply to their work in medicine.
Maybe it’s something else, too: Certain mindsets don’t worry about long odds. They seek out crucibles again and again without concern for the heat involved. Because the outcome is worth it.
Here are four athletes who became high-performing physicians and how they did it.
The Gymnast/The Pediatric Surgeon
“Gymnastics helped me build a skill set for my career,” said Canadian Olympic gymnast-turned-pediatric orthopedic surgeon Lise Leveille, MD. “It led me to be successful as a medical student and ultimately obtain the job that I want in the area that I want working with the people that I want.”
The skills Dr. Leveille prizes include time management, teamwork, goal setting, and a strong work ethic, all of which propel an athlete to the crucial moment of “performance.”
“I miss performing,” said Dr. Leveille. “It defines who I was at that time. I miss being able to work toward something and then deliver when it counted” — like when she qualified for the 1998 Commonwealth games in Kuala Lumpur at 16.
The Canadian national team came third at that event, and Dr. Leveille built on that success at the Pan American Games, taking gold on the balance beam and as a team, and then qualifying for the Olympics at the 1999 World Championships. She competed in the team and five individual events at the 2000 Olympic Games in Sydney.
Though Dr. Leveille started gymnastics at age 3, her parents, both teachers, instilled in her the importance of education. Gymnastics opened academic doors for her, like being recruited to Stanford where she completed her undergraduate degree in biomedical engineering and human biology in 2004 before entering medical school at the University of British Columbia in Vancouver.
Now 41, Dr. Leveille accepts that she’ll never nail another gymnastics routine, but she channels that love of sticking the landing into the operating room at British Columbia Children’s Hospital, also in Vancouver.
“Some of the unknown variables within the operating room and how you deal with those unknown variables is exactly like showing up for a competition,” Dr. Leveille said. “When I have one of those cases where I have to perform under pressure and everything comes together, that’s exactly like nailing your routine when it counts most.”
The Pole Vaulter/The Emergency Medicine Physician
Tunisian American pole vaulter Leila Ben-Youssef, MD, had what could be considered a disappointing showing at the 2008 Olympic Games in Beijing. She collapsed from severe abdominal pain during the opening ceremony and had to be carried out. On the day of competition, she was still suffering. “I could barely run down the runway,” she recalled. “I cleared one bar. I was just happy to have been able to do that.”
When Dr. Ben-Youssef, who grew up in Montana, returned home, she underwent emergency surgery to remove the source of the pain: A large, benign tumor.
While some might be devastated by such bad luck, Dr. Ben-Youssef focuses on the success of her journey — the fact that she qualified and competed at the Olympics in the first place. The ability to accept setbacks is something she said comes with the territory.
“As an athlete, you’re always facing injury, and someone told me early in my career that the best athletes are the ones that know how to manage their expectations because it’s bound to happen,” she said. “So, there is disappointment. But recognizing that I did qualify for the Olympics despite being uncomfortable and having issues, I was still able to meet my goal.”
Prior to the games, Dr. Ben-Youssef had been accepted into medical school at the University of Washington School of Medicine at Montana State University in Bozeman, Montana. Thankfully, the school was supportive of Dr. Ben-Youssef’s Olympic dreams and allowed her to begin her studies a month behind her class. Upon her return from Beijing, she spent the rest of her medical school training with her head down, grinding.
“Medicine is hard,” said Dr. Ben-Youssef. “It’s grueling both physically and emotionally, and I think that’s similar to any elite sport. You’re going to deal with challenges and disappointment. I think having gone through that as an athlete really prepares you for the medical education system, for residency, and even for day-to-day work.”
Now a physician working in emergency medicine in Hawaii, Dr. Ben-Youssef feels the setbacks she experienced as an athlete help her connect with her patients as they deal with health challenges.
And as a volunteer pole vaulting coach for a local high school, Dr. Ben-Youssef has been able to surround herself with the positive, joyful energy of athletes. “Emergency medicine is often a sad place,” she said. “But in a sports environment, if people don’t succeed or are injured, there is still that energy there that strives for something, and it’s so fun to be around.”
The Rower/The Sports Medicine Specialist
Three-time US Olympic rower Genevra “Gevvie” Stone, MD, wanted to be a doctor even before she gave a thought to rowing. She was in eighth grade when she dislocated her knee for the third time. Her parents took her to a pediatric orthopedist, and Dr. Stone, according to her mom, declared: “That’s what I want to do when I grow up.”
“I’m a very stubborn person, and when I make a decision like that, I usually don’t veer from it,” Dr. Stone said.
That laser focus combined with a deep love of both sports and medicine has served Dr. Stone well. “Becoming a doctor and becoming an Olympian require you to dedicate not just your time and your energy but also your passion to that focus,” she said. “In both, you aren’t going to be successful if you don’t love what you’re doing. Finding the reward in it is what makes it achievable.”
Dr. Stone actually resisted rowing until she was 16 because both of her parents were Olympians in the sport and met on the US team. “It was their thing, and I didn’t want it to be my thing,” she recalled.
Nonetheless, Dr. Stone easily fell into the sport in her late teens and was recruited by Princeton University. “I had grown up around Olympians and kind of took it for granted that if you worked hard enough and were decent at rowing, then you could be one of the best in the world, without really realizing how difficult it would be to achieve that,” she said.
Dr. Stone’s team won the NCAA Championship in 2006 and was invited to try out for the 2008 Olympic team at the US training center after she graduated from college. But she didn’t make it.
Instead, Dr. Stone entered medical school at Tufts University School of Medicine, Boston, thinking her competitive rowing career had come to end. But her love for the sport was still strong, and she realized she wasn’t finished.
After 2 years of medical school, Dr. Stone requested 2 years off so she might have another shot at making the Olympic team. The timing was right. She went to the London Olympics in 2012, graduated from medical school in 2014, and then took 2 more years off to train full time for the 2016 Olympics in Rio where she won silver.
At the 2020 Olympic Games in Tokyo, Dr. Stone took fifth place in the double sculls. While she continues to race the master’s circuit, she’s primarily dedicated to completing her sports medicine fellowship at University of Utah Health.
Fortunately, Dr. Stone’s parents, coaches, and teachers always supported her goals. “No one turned to me and told me I was crazy, just choose medicine or rowing,” she said. “Everyone said that if this is what you want to do, we’re here to support you, and I wouldn’t have been able to do it without that support.”
The Volleyball Player/The Plastic Surgeon
Dr. Nnamani Silva’s journey to the Olympics was also paved with an extensive list of supporters, beginning with her parents. And she has taken that sense of collaboration, coordination, and teamwork into her medical career.
The daughter of Nigerian immigrants who came to the United States to escape civil war, Dr. Nnamani Silva said her parents embraced the American dream. “To see what they were able to do with hard work, dedication, and sacrifice, I had no choice but to work hard because I saw their example. And that love for and belief in America was so strong in my house growing up,” she said.
Dreams of practicing medicine came first. A severe asthmatic growing up, Dr. Nnamani Silva recalled having wonderful doctors. “I had so many emergency room visits and hospitalizations,” she said. “But the doctors always gave me hope, and they literally transformed my life. I thought if I could pass that on to my future patients, that would be the greatest honor of my life.”
Volleyball gave Dr. Nnamani Silva the opportunity to attend Stanford, and she took time off during her junior year to train and compete in the 2004 Olympic Games in Athens. She also played for the United States at the 2008 Olympic Games in Beijing where the team took silver. Afterward, she continued to play overseas for several years.
At 33, and with a newborn daughter, Dr. Nnamani Silva returned to her original goal of becoming a doctor. She attended the University of California, San Francisco, and is currently a resident in the Harvard Plastic Surgery Program. She includes her husband, parents, and in-laws in this achievement, whom she said “saved” her. “There is no chance I would have finished medical school and survived residency without them.”
As a volleyball player, Dr. Nnamani Silva said she “believes in teams wholeheartedly,” valuing the exchange of energy and skill that she feels brings out the best in people. As a medical student, she initially didn’t realize how her previous life would apply to teamwork in the operating room. But it soon became clear.
“In surgery, when you harness the talents of everyone around you and you create that synergy, it’s an amazing feeling,” she said. And the stakes are often high. “It requires a lot of focus, discipline, determination, and resilience because you’re going to be humbled all the time.” Something athletes know a little bit about.
A version of this article first appeared on Medscape.com.
FDA OKs Voquezna for Heartburn Relief in Nonerosive Gastroesophageal Reflux Disease
It represents the third indication for the potassium-competitive acid blocker, which is already approved to treat all severities of erosive esophagitis and to eradicate Helicobacter pylori infection in combination with antibiotics.
The approval in nonerosive GERD was supported by results of the PHALCON-nonerosive GERD-301 study, a phase 3 randomized, placebo-controlled, double-blind, multicenter study evaluating the safety and efficacy of once-daily Voquezna in more than 700 adults with nonerosive GERD experiencing at least 4 days of heartburn per week.
“Vonoprazan was efficacious in reducing heartburn symptoms in patients with [nonerosive GERD], with the benefit appearing to begin as early as the first day of therapy. This treatment effect persisted after the initial 4-week placebo-controlled period throughout the 20-week extension period,” the study team wrote in a paper published online in Clinical Gastroenterology and Hepatology , and reported on by this news organization.
Voquezna “provides physicians with a novel, first-in-class treatment that can quickly and significantly reduce heartburn for many adult patients” with nonerosive GERD, Colin W. Howden, MD, AGAF, professor emeritus, University of Tennessee College of Medicine in Memphis, said in a news release.
The most common adverse events reported in patients treated with Voquezna during the 4-week placebo-controlled period were abdominal pain, constipation, diarrhea, nausea, and urinary tract infection.
Upper respiratory tract infection and sinusitis were also reported in patients who taking Voquezna in the 20-week extension phase of the trial.
Full prescribing information is available online.
A version of this article appeared on Medscape.com.
It represents the third indication for the potassium-competitive acid blocker, which is already approved to treat all severities of erosive esophagitis and to eradicate Helicobacter pylori infection in combination with antibiotics.
The approval in nonerosive GERD was supported by results of the PHALCON-nonerosive GERD-301 study, a phase 3 randomized, placebo-controlled, double-blind, multicenter study evaluating the safety and efficacy of once-daily Voquezna in more than 700 adults with nonerosive GERD experiencing at least 4 days of heartburn per week.
“Vonoprazan was efficacious in reducing heartburn symptoms in patients with [nonerosive GERD], with the benefit appearing to begin as early as the first day of therapy. This treatment effect persisted after the initial 4-week placebo-controlled period throughout the 20-week extension period,” the study team wrote in a paper published online in Clinical Gastroenterology and Hepatology , and reported on by this news organization.
Voquezna “provides physicians with a novel, first-in-class treatment that can quickly and significantly reduce heartburn for many adult patients” with nonerosive GERD, Colin W. Howden, MD, AGAF, professor emeritus, University of Tennessee College of Medicine in Memphis, said in a news release.
The most common adverse events reported in patients treated with Voquezna during the 4-week placebo-controlled period were abdominal pain, constipation, diarrhea, nausea, and urinary tract infection.
Upper respiratory tract infection and sinusitis were also reported in patients who taking Voquezna in the 20-week extension phase of the trial.
Full prescribing information is available online.
A version of this article appeared on Medscape.com.
It represents the third indication for the potassium-competitive acid blocker, which is already approved to treat all severities of erosive esophagitis and to eradicate Helicobacter pylori infection in combination with antibiotics.
The approval in nonerosive GERD was supported by results of the PHALCON-nonerosive GERD-301 study, a phase 3 randomized, placebo-controlled, double-blind, multicenter study evaluating the safety and efficacy of once-daily Voquezna in more than 700 adults with nonerosive GERD experiencing at least 4 days of heartburn per week.
“Vonoprazan was efficacious in reducing heartburn symptoms in patients with [nonerosive GERD], with the benefit appearing to begin as early as the first day of therapy. This treatment effect persisted after the initial 4-week placebo-controlled period throughout the 20-week extension period,” the study team wrote in a paper published online in Clinical Gastroenterology and Hepatology , and reported on by this news organization.
Voquezna “provides physicians with a novel, first-in-class treatment that can quickly and significantly reduce heartburn for many adult patients” with nonerosive GERD, Colin W. Howden, MD, AGAF, professor emeritus, University of Tennessee College of Medicine in Memphis, said in a news release.
The most common adverse events reported in patients treated with Voquezna during the 4-week placebo-controlled period were abdominal pain, constipation, diarrhea, nausea, and urinary tract infection.
Upper respiratory tract infection and sinusitis were also reported in patients who taking Voquezna in the 20-week extension phase of the trial.
Full prescribing information is available online.
A version of this article appeared on Medscape.com.
Healthcare Workers Face Gender-Based Violence
Across the world, healthcare workers experience workplace violence, which can differ by gender, seniority, and the type of workplace, according to a recent study.
An analysis found that men were more likely to report physical violence, while women were more likely to face nonphysical violence, such as verbal abuse, sexual harassment, and bullying.
“Our study was sparked by the increasing research on workplace violence in healthcare settings. Yet, there’s less empirical data about workplace violence based on gender, its effects on individuals and the collective workforce, and its subsequent impact on patient care and healthcare organizations,” study author Basnama Ayaz, a PhD candidate in nursing at the University of Toronto, told this news organization.
“Workplace violence in healthcare settings is a critical issue that requires attention and action from all stakeholders, including individual providers, healthcare and other institutions, policymakers, and the community,” she said. “By recognizing the problem and implementing evidence-based solutions, we can create safer work environments that protect healthcare workers and improve quality care for patients and organizational effectiveness.”
The study was published online in PLOS Global Public Health.
Widespread and Severe
Although women represent most of the healthcare workforce worldwide, hierarchical structures tend to reflect traditional gender norms, where men hold leadership positions and women serve in front-line care roles, said Ms. Ayaz. Women are often marginalized, and their concerns dismissed, which can exacerbate their vulnerability to gender-based workplace violence, she added.
To better understand these imbalances on a global scale, the investigators conducted a scoping review of the prevalence of and risk factors for gender-based workplace violence in healthcare settings. Participants included physicians, nurses, and midwives, between 2010 and 2024. Although the authors acknowledged that gender-based workplace violence affects the full gender spectrum, only a handful of studies included information about nonbinary personnel, so the review focused on men and women.
Among 226 studies, half focused on physicians, 22% focused on nurses, and 28% included physicians, nurses, midwives, and other medical workers. About 64% of studies reported a higher prevalence of all forms of workplace violence for women, including sexual violence, verbal abuse, discrimination, bullying, and physical violence, while 17% reported a higher prevalence for men.
Overall, across most countries, men experienced more physical violence than did women, and women experienced more verbal abuse, sexual harassment, and bullying. Female nurses were particularly likely to experience violence.
Healthcare workers were also more likely to experience violence if they were younger, less experienced, had a lower professional status, or were part of a minority group based on ethnicity, nationality, culture, or language. These factors were sensitive to gender, “reflecting women’s structural disadvantages in the workplace,” wrote the authors.
As a result of workplace violence, women were more likely to report changes in mental health and social behaviors, as well as dissatisfaction, burnout, and changes in their career goals.
The research team identified various factors linked to violent episodes. In clinical settings where most perpetrators were patients and their relatives, abuse and violence could be related to overcrowding, waiting time, and heavy workloads for healthcare providers. When supervisors or colleagues were the perpetrators, workplace violence appeared to be more likely with long hours, night shifts, and certain clinical settings, such as emergency departments, psychiatric settings, operating rooms, and maternity wards, said Ms. Ayaz. Sexual or gender harassment toward women was more prevalent in male-dominated surgical specialties.
“We were surprised by the extent and severity of workplace violence that healthcare workers face around the globe based on gender,” she said. “One aspect that stood out was the significant role that organizational culture and support systems play either in mitigating or exacerbating these incidents, particularly the power structures between and within professions.”
For instance, trainees in lower hierarchical positions often face a higher risk for violence, especially gender-based harassment, she said. Many times, they feel they can’t report these incidents to trainers or managers, who may also be the perpetrators, she added.
Addressing Systemic Issues
In 2002, the World Health Organization, International Council of Nurses, and other major medical and labor groups worldwide launched a program focused on ways to eliminate workplace violence in healthcare settings. Since 2020, the call for a solution has grown louder as clinicians, nurses, and other health professionals faced more physical and verbal violence during the COVID-19 pandemic, often leading to burnout.
“Workplace violence is very important because it is more prevalent in healthcare workers than in many other settings and is on the rise,” said Karen Abrams, MD, assistant professor of psychiatry at the University of Toronto. Dr. Abrams, who wasn’t involved with this study, has researched physicians’ experiences of stalking by patients.
Workplace violence “can affect physical and mental health and lead to burnout, depression, anxiety, and symptoms of PTSD,” said Dr. Abrams. “It can affect one’s sleep and concentration and, therefore, ability to perform one’s job.”
Dr. Ayaz and colleagues suggested recommendations to improve gender-based workplace violence, noting the complex and multifaceted aspects of enhancing current policies, fortifying institutional capacities to respond, and implementing tailored interventions. Changes are needed at various levels, including at the healthcare system and provincial, territorial, and national levels, she said.
In Canada, for instance, lawmakers passed a bill in 2021 that amended the national criminal code to make intimidation or bullying a healthcare worker punishable by as many as 10 years in prison. The changes also required courts to consider more serious penalties for offenders who target healthcare workers aggressively.
But more needs to be done, medical professional groups say. The Canadian Nurses Association and Canadian Federation of Nurses Unions, as well as provincial groups, have called for a pan-Canadian violence-prevention framework, targeted funding for violence prevention infrastructure, and an update to the nation’s health human resources strategy to address severe staffing shortages across the country.
“Canada needs a bold vision for the future of our healthcare. Amid an ongoing staffing crisis, the cracks in our public healthcare systems have only grown deeper and wider, with too many going without the care they need when they need it,” Linda Silas, president of the Canadian Federation of Nurses Unions, told this news organization.
“Access to care relies on safe staffing. Years of unsafe working conditions and insufficient staffing are pushing nurses out of our public healthcare system,” she said. “Working collaboratively, we can make healthcare jobs the best jobs in our communities.”
The authors received no specific funding for the study. Ms. Ayaz, Dr. Abrams, and Ms. Silas reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Across the world, healthcare workers experience workplace violence, which can differ by gender, seniority, and the type of workplace, according to a recent study.
An analysis found that men were more likely to report physical violence, while women were more likely to face nonphysical violence, such as verbal abuse, sexual harassment, and bullying.
“Our study was sparked by the increasing research on workplace violence in healthcare settings. Yet, there’s less empirical data about workplace violence based on gender, its effects on individuals and the collective workforce, and its subsequent impact on patient care and healthcare organizations,” study author Basnama Ayaz, a PhD candidate in nursing at the University of Toronto, told this news organization.
“Workplace violence in healthcare settings is a critical issue that requires attention and action from all stakeholders, including individual providers, healthcare and other institutions, policymakers, and the community,” she said. “By recognizing the problem and implementing evidence-based solutions, we can create safer work environments that protect healthcare workers and improve quality care for patients and organizational effectiveness.”
The study was published online in PLOS Global Public Health.
Widespread and Severe
Although women represent most of the healthcare workforce worldwide, hierarchical structures tend to reflect traditional gender norms, where men hold leadership positions and women serve in front-line care roles, said Ms. Ayaz. Women are often marginalized, and their concerns dismissed, which can exacerbate their vulnerability to gender-based workplace violence, she added.
To better understand these imbalances on a global scale, the investigators conducted a scoping review of the prevalence of and risk factors for gender-based workplace violence in healthcare settings. Participants included physicians, nurses, and midwives, between 2010 and 2024. Although the authors acknowledged that gender-based workplace violence affects the full gender spectrum, only a handful of studies included information about nonbinary personnel, so the review focused on men and women.
Among 226 studies, half focused on physicians, 22% focused on nurses, and 28% included physicians, nurses, midwives, and other medical workers. About 64% of studies reported a higher prevalence of all forms of workplace violence for women, including sexual violence, verbal abuse, discrimination, bullying, and physical violence, while 17% reported a higher prevalence for men.
Overall, across most countries, men experienced more physical violence than did women, and women experienced more verbal abuse, sexual harassment, and bullying. Female nurses were particularly likely to experience violence.
Healthcare workers were also more likely to experience violence if they were younger, less experienced, had a lower professional status, or were part of a minority group based on ethnicity, nationality, culture, or language. These factors were sensitive to gender, “reflecting women’s structural disadvantages in the workplace,” wrote the authors.
As a result of workplace violence, women were more likely to report changes in mental health and social behaviors, as well as dissatisfaction, burnout, and changes in their career goals.
The research team identified various factors linked to violent episodes. In clinical settings where most perpetrators were patients and their relatives, abuse and violence could be related to overcrowding, waiting time, and heavy workloads for healthcare providers. When supervisors or colleagues were the perpetrators, workplace violence appeared to be more likely with long hours, night shifts, and certain clinical settings, such as emergency departments, psychiatric settings, operating rooms, and maternity wards, said Ms. Ayaz. Sexual or gender harassment toward women was more prevalent in male-dominated surgical specialties.
“We were surprised by the extent and severity of workplace violence that healthcare workers face around the globe based on gender,” she said. “One aspect that stood out was the significant role that organizational culture and support systems play either in mitigating or exacerbating these incidents, particularly the power structures between and within professions.”
For instance, trainees in lower hierarchical positions often face a higher risk for violence, especially gender-based harassment, she said. Many times, they feel they can’t report these incidents to trainers or managers, who may also be the perpetrators, she added.
Addressing Systemic Issues
In 2002, the World Health Organization, International Council of Nurses, and other major medical and labor groups worldwide launched a program focused on ways to eliminate workplace violence in healthcare settings. Since 2020, the call for a solution has grown louder as clinicians, nurses, and other health professionals faced more physical and verbal violence during the COVID-19 pandemic, often leading to burnout.
“Workplace violence is very important because it is more prevalent in healthcare workers than in many other settings and is on the rise,” said Karen Abrams, MD, assistant professor of psychiatry at the University of Toronto. Dr. Abrams, who wasn’t involved with this study, has researched physicians’ experiences of stalking by patients.
Workplace violence “can affect physical and mental health and lead to burnout, depression, anxiety, and symptoms of PTSD,” said Dr. Abrams. “It can affect one’s sleep and concentration and, therefore, ability to perform one’s job.”
Dr. Ayaz and colleagues suggested recommendations to improve gender-based workplace violence, noting the complex and multifaceted aspects of enhancing current policies, fortifying institutional capacities to respond, and implementing tailored interventions. Changes are needed at various levels, including at the healthcare system and provincial, territorial, and national levels, she said.
In Canada, for instance, lawmakers passed a bill in 2021 that amended the national criminal code to make intimidation or bullying a healthcare worker punishable by as many as 10 years in prison. The changes also required courts to consider more serious penalties for offenders who target healthcare workers aggressively.
But more needs to be done, medical professional groups say. The Canadian Nurses Association and Canadian Federation of Nurses Unions, as well as provincial groups, have called for a pan-Canadian violence-prevention framework, targeted funding for violence prevention infrastructure, and an update to the nation’s health human resources strategy to address severe staffing shortages across the country.
“Canada needs a bold vision for the future of our healthcare. Amid an ongoing staffing crisis, the cracks in our public healthcare systems have only grown deeper and wider, with too many going without the care they need when they need it,” Linda Silas, president of the Canadian Federation of Nurses Unions, told this news organization.
“Access to care relies on safe staffing. Years of unsafe working conditions and insufficient staffing are pushing nurses out of our public healthcare system,” she said. “Working collaboratively, we can make healthcare jobs the best jobs in our communities.”
The authors received no specific funding for the study. Ms. Ayaz, Dr. Abrams, and Ms. Silas reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Across the world, healthcare workers experience workplace violence, which can differ by gender, seniority, and the type of workplace, according to a recent study.
An analysis found that men were more likely to report physical violence, while women were more likely to face nonphysical violence, such as verbal abuse, sexual harassment, and bullying.
“Our study was sparked by the increasing research on workplace violence in healthcare settings. Yet, there’s less empirical data about workplace violence based on gender, its effects on individuals and the collective workforce, and its subsequent impact on patient care and healthcare organizations,” study author Basnama Ayaz, a PhD candidate in nursing at the University of Toronto, told this news organization.
“Workplace violence in healthcare settings is a critical issue that requires attention and action from all stakeholders, including individual providers, healthcare and other institutions, policymakers, and the community,” she said. “By recognizing the problem and implementing evidence-based solutions, we can create safer work environments that protect healthcare workers and improve quality care for patients and organizational effectiveness.”
The study was published online in PLOS Global Public Health.
Widespread and Severe
Although women represent most of the healthcare workforce worldwide, hierarchical structures tend to reflect traditional gender norms, where men hold leadership positions and women serve in front-line care roles, said Ms. Ayaz. Women are often marginalized, and their concerns dismissed, which can exacerbate their vulnerability to gender-based workplace violence, she added.
To better understand these imbalances on a global scale, the investigators conducted a scoping review of the prevalence of and risk factors for gender-based workplace violence in healthcare settings. Participants included physicians, nurses, and midwives, between 2010 and 2024. Although the authors acknowledged that gender-based workplace violence affects the full gender spectrum, only a handful of studies included information about nonbinary personnel, so the review focused on men and women.
Among 226 studies, half focused on physicians, 22% focused on nurses, and 28% included physicians, nurses, midwives, and other medical workers. About 64% of studies reported a higher prevalence of all forms of workplace violence for women, including sexual violence, verbal abuse, discrimination, bullying, and physical violence, while 17% reported a higher prevalence for men.
Overall, across most countries, men experienced more physical violence than did women, and women experienced more verbal abuse, sexual harassment, and bullying. Female nurses were particularly likely to experience violence.
Healthcare workers were also more likely to experience violence if they were younger, less experienced, had a lower professional status, or were part of a minority group based on ethnicity, nationality, culture, or language. These factors were sensitive to gender, “reflecting women’s structural disadvantages in the workplace,” wrote the authors.
As a result of workplace violence, women were more likely to report changes in mental health and social behaviors, as well as dissatisfaction, burnout, and changes in their career goals.
The research team identified various factors linked to violent episodes. In clinical settings where most perpetrators were patients and their relatives, abuse and violence could be related to overcrowding, waiting time, and heavy workloads for healthcare providers. When supervisors or colleagues were the perpetrators, workplace violence appeared to be more likely with long hours, night shifts, and certain clinical settings, such as emergency departments, psychiatric settings, operating rooms, and maternity wards, said Ms. Ayaz. Sexual or gender harassment toward women was more prevalent in male-dominated surgical specialties.
“We were surprised by the extent and severity of workplace violence that healthcare workers face around the globe based on gender,” she said. “One aspect that stood out was the significant role that organizational culture and support systems play either in mitigating or exacerbating these incidents, particularly the power structures between and within professions.”
For instance, trainees in lower hierarchical positions often face a higher risk for violence, especially gender-based harassment, she said. Many times, they feel they can’t report these incidents to trainers or managers, who may also be the perpetrators, she added.
Addressing Systemic Issues
In 2002, the World Health Organization, International Council of Nurses, and other major medical and labor groups worldwide launched a program focused on ways to eliminate workplace violence in healthcare settings. Since 2020, the call for a solution has grown louder as clinicians, nurses, and other health professionals faced more physical and verbal violence during the COVID-19 pandemic, often leading to burnout.
“Workplace violence is very important because it is more prevalent in healthcare workers than in many other settings and is on the rise,” said Karen Abrams, MD, assistant professor of psychiatry at the University of Toronto. Dr. Abrams, who wasn’t involved with this study, has researched physicians’ experiences of stalking by patients.
Workplace violence “can affect physical and mental health and lead to burnout, depression, anxiety, and symptoms of PTSD,” said Dr. Abrams. “It can affect one’s sleep and concentration and, therefore, ability to perform one’s job.”
Dr. Ayaz and colleagues suggested recommendations to improve gender-based workplace violence, noting the complex and multifaceted aspects of enhancing current policies, fortifying institutional capacities to respond, and implementing tailored interventions. Changes are needed at various levels, including at the healthcare system and provincial, territorial, and national levels, she said.
In Canada, for instance, lawmakers passed a bill in 2021 that amended the national criminal code to make intimidation or bullying a healthcare worker punishable by as many as 10 years in prison. The changes also required courts to consider more serious penalties for offenders who target healthcare workers aggressively.
But more needs to be done, medical professional groups say. The Canadian Nurses Association and Canadian Federation of Nurses Unions, as well as provincial groups, have called for a pan-Canadian violence-prevention framework, targeted funding for violence prevention infrastructure, and an update to the nation’s health human resources strategy to address severe staffing shortages across the country.
“Canada needs a bold vision for the future of our healthcare. Amid an ongoing staffing crisis, the cracks in our public healthcare systems have only grown deeper and wider, with too many going without the care they need when they need it,” Linda Silas, president of the Canadian Federation of Nurses Unions, told this news organization.
“Access to care relies on safe staffing. Years of unsafe working conditions and insufficient staffing are pushing nurses out of our public healthcare system,” she said. “Working collaboratively, we can make healthcare jobs the best jobs in our communities.”
The authors received no specific funding for the study. Ms. Ayaz, Dr. Abrams, and Ms. Silas reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Is Immunotherapy Best for Unresectable HCC with Moderate Liver Dysfunction?
In the last 10 years, clinical outcomes have improved for patients with unresectable hepatocellular carcinoma (uHCC). The cancer generally comes with chronic liver inflammation, and liver cirrhosis is present in up to 80% of cases.
Clinical trials that have tested systemic immunotherapies have excluded patients who don’t fall into the Child-Pugh class A criteria (CP-A) for liver disease, which is the least severe of the Child-Pugh classes A-C. Therefore, there has been much debate about whether patients who have more liver disease (moderate liver dysfunction) and fit under CP-B criteria, instead of CP-A, should be treated with immune checkpoint inhibitor (ICI) therapy or best supportive care (BSC).
A new study, led by Claudia Angela Maria Fulgenzi, MD, with the Department of Surgery and Cancer at the Imperial College London, England, published in JAMA Oncology on July 18, uses an alternative way to compare outcomes following two different paths of care for uHCC patients with moderate liver dysfunction.
How was the study done and what did the investigators find?
Researchers performed a retrospective, multicenter, international clinical case series of patients treated in routine practice in tertiary care centers across Europe, the United States, and Asia. They compared data from uHCC patients with CP-B who were receiving first-line ICI-based treatment regimens (n = 187) with a cohort of matched patients with CP-B receiving BSC (n = 156). The first-line immunotherapies were the monotherapy nivolumab or the combination (atezolizumab plus bevacizumab).
Immunotherapy was linked with significantly lower risk of death, compared with best supportive care.
ICI exposure was associated with a reduction of about 50% in the risk of death (hazard ratio, 0.55; 95% CI, 0.35-0.86; P < .001).
Is immunotherapy or best supportive care the superior treatment?
The authors wrote that the results point to “improved survival in association with ICI treatment, compared with BSC in patients with uHCC with CP-B liver dysfunction.”
According to the study’s senior author David Pinato, MD, PhD, “this is the first study to suggest that there might be an advantage [of treatment with immunotherapy] in a proportion of people with Child-Pugh B liver dysfunction and particularly so in those patients with more limited disease and portal vein tumor thrombosis.”
Will the findings of this study make treatment allocation for patients with uHCC and moderate liver dysfunction (CP-B) less controversial?
Because it is a retrospective study, Dr. Pinato said in an interview, that the findings are not definitive, but can be used to inform future randomized controlled trials.
Dr. Pinato, who is also with the Imperial College London, added that the findings may also introduce a new question.
Although the study was not powered to look at survival differences across the two immunotherapy options given to the patients, there did not seem to be a striking difference between using one immunotherapy (nivolumab) or a combination (atezolizumab plus bevacizumab), he said.
“This is quite important because we know that combinations are significantly superior to monotherapy in patients with normal liver function but based on our study we might say that this provides preliminary evidence that [superiority of combination therapy] might not be true if the liver function is worse.”
What do these findings add to the literature about how best to treat patients with uHCC and suboptimal liver function?
Without evidence of efficacy and safety for the group in previous studies, the widespread recommendation for those with moderate dysfunction has been BSC.
These findings “pave the way to select potential patient subgroups in clinical practice,” Dr. Pinato said. It also suggests that the safety level of immunotherapy treatments is acceptable in this patient population, so they are not necessarily disadvantaged compared to patients with more preserved liver function.
“This is the best level of evidence currently available to guide treatment decisions in patients with Child-Pugh B who have been universally excluded by prospective clinical trials and for whom there is no randomized comparison,” Dr. Pinato said.
Dr. Pinato reported personal fees from Roche, AstraZeneca, Eisai, Mina Therapeutics, Starpharma, Lift Biosciences, Boston Scientific, and Avammune, and grants from GSK, MSD, and BMS outside the submitted work. Dr. Fulgenzi has no disclosures. Other authors of the new research have multiple ties with pharmaceutical companies. Complete disclosures are available with the full text of the journal article.
In the last 10 years, clinical outcomes have improved for patients with unresectable hepatocellular carcinoma (uHCC). The cancer generally comes with chronic liver inflammation, and liver cirrhosis is present in up to 80% of cases.
Clinical trials that have tested systemic immunotherapies have excluded patients who don’t fall into the Child-Pugh class A criteria (CP-A) for liver disease, which is the least severe of the Child-Pugh classes A-C. Therefore, there has been much debate about whether patients who have more liver disease (moderate liver dysfunction) and fit under CP-B criteria, instead of CP-A, should be treated with immune checkpoint inhibitor (ICI) therapy or best supportive care (BSC).
A new study, led by Claudia Angela Maria Fulgenzi, MD, with the Department of Surgery and Cancer at the Imperial College London, England, published in JAMA Oncology on July 18, uses an alternative way to compare outcomes following two different paths of care for uHCC patients with moderate liver dysfunction.
How was the study done and what did the investigators find?
Researchers performed a retrospective, multicenter, international clinical case series of patients treated in routine practice in tertiary care centers across Europe, the United States, and Asia. They compared data from uHCC patients with CP-B who were receiving first-line ICI-based treatment regimens (n = 187) with a cohort of matched patients with CP-B receiving BSC (n = 156). The first-line immunotherapies were the monotherapy nivolumab or the combination (atezolizumab plus bevacizumab).
Immunotherapy was linked with significantly lower risk of death, compared with best supportive care.
ICI exposure was associated with a reduction of about 50% in the risk of death (hazard ratio, 0.55; 95% CI, 0.35-0.86; P < .001).
Is immunotherapy or best supportive care the superior treatment?
The authors wrote that the results point to “improved survival in association with ICI treatment, compared with BSC in patients with uHCC with CP-B liver dysfunction.”
According to the study’s senior author David Pinato, MD, PhD, “this is the first study to suggest that there might be an advantage [of treatment with immunotherapy] in a proportion of people with Child-Pugh B liver dysfunction and particularly so in those patients with more limited disease and portal vein tumor thrombosis.”
Will the findings of this study make treatment allocation for patients with uHCC and moderate liver dysfunction (CP-B) less controversial?
Because it is a retrospective study, Dr. Pinato said in an interview, that the findings are not definitive, but can be used to inform future randomized controlled trials.
Dr. Pinato, who is also with the Imperial College London, added that the findings may also introduce a new question.
Although the study was not powered to look at survival differences across the two immunotherapy options given to the patients, there did not seem to be a striking difference between using one immunotherapy (nivolumab) or a combination (atezolizumab plus bevacizumab), he said.
“This is quite important because we know that combinations are significantly superior to monotherapy in patients with normal liver function but based on our study we might say that this provides preliminary evidence that [superiority of combination therapy] might not be true if the liver function is worse.”
What do these findings add to the literature about how best to treat patients with uHCC and suboptimal liver function?
Without evidence of efficacy and safety for the group in previous studies, the widespread recommendation for those with moderate dysfunction has been BSC.
These findings “pave the way to select potential patient subgroups in clinical practice,” Dr. Pinato said. It also suggests that the safety level of immunotherapy treatments is acceptable in this patient population, so they are not necessarily disadvantaged compared to patients with more preserved liver function.
“This is the best level of evidence currently available to guide treatment decisions in patients with Child-Pugh B who have been universally excluded by prospective clinical trials and for whom there is no randomized comparison,” Dr. Pinato said.
Dr. Pinato reported personal fees from Roche, AstraZeneca, Eisai, Mina Therapeutics, Starpharma, Lift Biosciences, Boston Scientific, and Avammune, and grants from GSK, MSD, and BMS outside the submitted work. Dr. Fulgenzi has no disclosures. Other authors of the new research have multiple ties with pharmaceutical companies. Complete disclosures are available with the full text of the journal article.
In the last 10 years, clinical outcomes have improved for patients with unresectable hepatocellular carcinoma (uHCC). The cancer generally comes with chronic liver inflammation, and liver cirrhosis is present in up to 80% of cases.
Clinical trials that have tested systemic immunotherapies have excluded patients who don’t fall into the Child-Pugh class A criteria (CP-A) for liver disease, which is the least severe of the Child-Pugh classes A-C. Therefore, there has been much debate about whether patients who have more liver disease (moderate liver dysfunction) and fit under CP-B criteria, instead of CP-A, should be treated with immune checkpoint inhibitor (ICI) therapy or best supportive care (BSC).
A new study, led by Claudia Angela Maria Fulgenzi, MD, with the Department of Surgery and Cancer at the Imperial College London, England, published in JAMA Oncology on July 18, uses an alternative way to compare outcomes following two different paths of care for uHCC patients with moderate liver dysfunction.
How was the study done and what did the investigators find?
Researchers performed a retrospective, multicenter, international clinical case series of patients treated in routine practice in tertiary care centers across Europe, the United States, and Asia. They compared data from uHCC patients with CP-B who were receiving first-line ICI-based treatment regimens (n = 187) with a cohort of matched patients with CP-B receiving BSC (n = 156). The first-line immunotherapies were the monotherapy nivolumab or the combination (atezolizumab plus bevacizumab).
Immunotherapy was linked with significantly lower risk of death, compared with best supportive care.
ICI exposure was associated with a reduction of about 50% in the risk of death (hazard ratio, 0.55; 95% CI, 0.35-0.86; P < .001).
Is immunotherapy or best supportive care the superior treatment?
The authors wrote that the results point to “improved survival in association with ICI treatment, compared with BSC in patients with uHCC with CP-B liver dysfunction.”
According to the study’s senior author David Pinato, MD, PhD, “this is the first study to suggest that there might be an advantage [of treatment with immunotherapy] in a proportion of people with Child-Pugh B liver dysfunction and particularly so in those patients with more limited disease and portal vein tumor thrombosis.”
Will the findings of this study make treatment allocation for patients with uHCC and moderate liver dysfunction (CP-B) less controversial?
Because it is a retrospective study, Dr. Pinato said in an interview, that the findings are not definitive, but can be used to inform future randomized controlled trials.
Dr. Pinato, who is also with the Imperial College London, added that the findings may also introduce a new question.
Although the study was not powered to look at survival differences across the two immunotherapy options given to the patients, there did not seem to be a striking difference between using one immunotherapy (nivolumab) or a combination (atezolizumab plus bevacizumab), he said.
“This is quite important because we know that combinations are significantly superior to monotherapy in patients with normal liver function but based on our study we might say that this provides preliminary evidence that [superiority of combination therapy] might not be true if the liver function is worse.”
What do these findings add to the literature about how best to treat patients with uHCC and suboptimal liver function?
Without evidence of efficacy and safety for the group in previous studies, the widespread recommendation for those with moderate dysfunction has been BSC.
These findings “pave the way to select potential patient subgroups in clinical practice,” Dr. Pinato said. It also suggests that the safety level of immunotherapy treatments is acceptable in this patient population, so they are not necessarily disadvantaged compared to patients with more preserved liver function.
“This is the best level of evidence currently available to guide treatment decisions in patients with Child-Pugh B who have been universally excluded by prospective clinical trials and for whom there is no randomized comparison,” Dr. Pinato said.
Dr. Pinato reported personal fees from Roche, AstraZeneca, Eisai, Mina Therapeutics, Starpharma, Lift Biosciences, Boston Scientific, and Avammune, and grants from GSK, MSD, and BMS outside the submitted work. Dr. Fulgenzi has no disclosures. Other authors of the new research have multiple ties with pharmaceutical companies. Complete disclosures are available with the full text of the journal article.
FROM JAMA ONCOLOGY
Cost of Drugs Can Be Breathtaking for COPD Patients
For patients with chronic obstructive pulmonary disease (COPD), the Global Initiative on Obstructive Lung Disease recommends long-term term pharmacologic and nonpharmacologic therapies based on each patient’s symptoms and disease severity.
Yet even the most effective drugs work only when patients take them as directed, and according to the World Health Organization, fewer than half of all patients worldwide are fully compliant with long-term COPD drug regimens.
And as a recent cross-sectional study showed, nearly one in six patients in the United States reported missing a COPD drug dose, lowering the dose, or delaying filling a prescription for financial reasons.
“I care for patients with COPD as their pulmonologist, and this is a very common problem that we see in clinical practice,” said Meredith McCormack, MD, a pulmonary and critical care medicine physician and associate director of the Pulmonary & Critical Care Medicine Division at Johns Hopkins University in Baltimore.
Dr. McCormack, a national spokesperson for The American Lung Association, said that she shows new patients the photos of all available inhalers and asks which ones they have and how they take them.
“I would say that a majority of the time people are taking their medicines slightly differently than prescribed, and often, this is due to cost,” she said.
Serious Consequences
Cost-related medication nonadherence (CRN), as investigators term it, can have major health effects and can be significantly more costly in the long run due to increased hospitalization rates, higher morbidity, and greater risk for COPD-related death associated with suboptimal care.
“For some patients even a month or two of being off medications increases the risk of having exacerbations, having more symptoms, [and] having a decline in their lung function,” said Douglas M. Beach, MD, a pulmonologist at Beth Israel Deaconess Medical Center in Boston.
In the aforementioned cross-sectional study, published in the open access journal BMC Public Health, Xin Wen, MD, from the Jiamusi (China) University School of Public Health, and colleagues looked at data on a representative sample of US adults who participated in the US National Health Interview Survey from 2013 through 2020.
The sample included 15,928 persons aged 18 years or older with a self-reported history of COPD who completed a CRN survey including the following questions:
During the past 12 months, have you
- Skipped medication doses to save money?
- Taken less medicine than prescribed to save money?
- Delayed filling a prescription to save money?
The investigators found that a weighted 18.56% of participants representing 2.39 million persons with COPD answered “yes” to one of the questions.
Translated into representative population numbers, that works out to an estimated 1.61 million persons with COPD missing doses, 1.72 million taking lower doses than those prescribed, and 2.03 million delaying filling prescriptions to save money.
A multivariable logistic regression analysis showed that those who were most likely to be nonadherent for financial reasons were patients younger than 65 years, women, persons with low family income, those who lacked health insurance, and patients with multiple comorbidities, the authors found.
Financial Barriers
One of the biggest barriers to COPD medication adherence is, somewhat paradoxically, insurance status, particularly Medicare, said Corinne Young, MSN, FNP-C, FCCP, from Colorado Springs Pulmonary Consultants.
“What’s so unfair about Medicare is that patients have to buy a drug plan, so they have to already pay for an extra plan to have access to drugs, and the plans vary because there are so many choices,” she said in an interview.
Elderly patients may be confused about the available options and may choose the Medicare Advantage plan with the lowest monthly premiums, which have the highest annual deductibles, usually in the $5000-and-up range, she said.
In addition, the Medicare Part D prescription coverage gap, commonly known as the “donut hole,” requires patients to pay a percentage of drug costs above a certain limit ($5030 in 2024) until a yearly out-of-pocket limit (currently $8000) is reached, after which the plan will again pick up most of the costs.
Although makers of inhalers have voluntarily agreed to limit monthly co-pays to $35 for uninsured patients, Medicare plans require insured patients to shell out considerably more, with 30 days of Trelegy Ellipta (fluticasone furoate, umeclidinium, and vilanterol) setting patients back nearly $350 per month, according to a recent search of costs for a United Healthcare Medicare Advantage plan.
Chasing Lower-Cost Options
“I have a lot of patients who use Canadian pharmacies to try to get around it, and I have a lot of patients who make a trip to Mexico every year and load up. I have patients who don’t take their inhalers as they are supposed to in order to make them last longer, and I have patients who take the inhalers of other family members,” Ms. Young said.
Humayun Anjum, MD, FCCP, from Dallas Pulmonary and Critical Care in Dallas, Texas, said in an interview that when patients claim that a prescribed drug isn’t working as expected for them, financial pressures may be partly to blame.
“When you investigate a little bit more, that’s where things become a bit more clear, and the patient may say ‘yeah, I stopped using this inhaler because it was costing me 200 bucks a month and I’m already on other medications,’ ” he said.
He noted that, when possible, he will steer patients toward discount prescription services such as GoodRx, which offers discounts at local pharmacies, or Mark Cuban Cost Plus Drugs, an online pharmacy that offers generic versions of Advair Diskus (fluticasone propionate/salmeterol) at a 100-50 mcg dose for $94.70, a savings of $307.30 over retail pharmacies, according to the company’s website.
Dr. Beach noted that Beth Israel Deaconess has a pharmacist embedded in the pulmonary clinic who can help eligible patients get financial assistance to pay for their medications.
“The influencing factors of CRN are multifaceted and necessitating more rigorous research. Health policy interventions focusing on reducing drug costs, delaying disease progression, preventing exacerbations, and reducing the risk of comorbidities may improve the economic burden of COPD and its outcomes,” Dr. Wen and colleagues wrote.
The study by Dr. Wen and colleagues was funded by grants from Chinese national and academic sources. Dr. McCormack has served as a consultant to Aridis, Boehringer Ingelheim, GlaxoSmithKline, MCG Diagnostics, ndd Medical Technologies, and UpToDate. Ms. Young, Dr. Anjum, and Dr. Beach reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
For patients with chronic obstructive pulmonary disease (COPD), the Global Initiative on Obstructive Lung Disease recommends long-term term pharmacologic and nonpharmacologic therapies based on each patient’s symptoms and disease severity.
Yet even the most effective drugs work only when patients take them as directed, and according to the World Health Organization, fewer than half of all patients worldwide are fully compliant with long-term COPD drug regimens.
And as a recent cross-sectional study showed, nearly one in six patients in the United States reported missing a COPD drug dose, lowering the dose, or delaying filling a prescription for financial reasons.
“I care for patients with COPD as their pulmonologist, and this is a very common problem that we see in clinical practice,” said Meredith McCormack, MD, a pulmonary and critical care medicine physician and associate director of the Pulmonary & Critical Care Medicine Division at Johns Hopkins University in Baltimore.
Dr. McCormack, a national spokesperson for The American Lung Association, said that she shows new patients the photos of all available inhalers and asks which ones they have and how they take them.
“I would say that a majority of the time people are taking their medicines slightly differently than prescribed, and often, this is due to cost,” she said.
Serious Consequences
Cost-related medication nonadherence (CRN), as investigators term it, can have major health effects and can be significantly more costly in the long run due to increased hospitalization rates, higher morbidity, and greater risk for COPD-related death associated with suboptimal care.
“For some patients even a month or two of being off medications increases the risk of having exacerbations, having more symptoms, [and] having a decline in their lung function,” said Douglas M. Beach, MD, a pulmonologist at Beth Israel Deaconess Medical Center in Boston.
In the aforementioned cross-sectional study, published in the open access journal BMC Public Health, Xin Wen, MD, from the Jiamusi (China) University School of Public Health, and colleagues looked at data on a representative sample of US adults who participated in the US National Health Interview Survey from 2013 through 2020.
The sample included 15,928 persons aged 18 years or older with a self-reported history of COPD who completed a CRN survey including the following questions:
During the past 12 months, have you
- Skipped medication doses to save money?
- Taken less medicine than prescribed to save money?
- Delayed filling a prescription to save money?
The investigators found that a weighted 18.56% of participants representing 2.39 million persons with COPD answered “yes” to one of the questions.
Translated into representative population numbers, that works out to an estimated 1.61 million persons with COPD missing doses, 1.72 million taking lower doses than those prescribed, and 2.03 million delaying filling prescriptions to save money.
A multivariable logistic regression analysis showed that those who were most likely to be nonadherent for financial reasons were patients younger than 65 years, women, persons with low family income, those who lacked health insurance, and patients with multiple comorbidities, the authors found.
Financial Barriers
One of the biggest barriers to COPD medication adherence is, somewhat paradoxically, insurance status, particularly Medicare, said Corinne Young, MSN, FNP-C, FCCP, from Colorado Springs Pulmonary Consultants.
“What’s so unfair about Medicare is that patients have to buy a drug plan, so they have to already pay for an extra plan to have access to drugs, and the plans vary because there are so many choices,” she said in an interview.
Elderly patients may be confused about the available options and may choose the Medicare Advantage plan with the lowest monthly premiums, which have the highest annual deductibles, usually in the $5000-and-up range, she said.
In addition, the Medicare Part D prescription coverage gap, commonly known as the “donut hole,” requires patients to pay a percentage of drug costs above a certain limit ($5030 in 2024) until a yearly out-of-pocket limit (currently $8000) is reached, after which the plan will again pick up most of the costs.
Although makers of inhalers have voluntarily agreed to limit monthly co-pays to $35 for uninsured patients, Medicare plans require insured patients to shell out considerably more, with 30 days of Trelegy Ellipta (fluticasone furoate, umeclidinium, and vilanterol) setting patients back nearly $350 per month, according to a recent search of costs for a United Healthcare Medicare Advantage plan.
Chasing Lower-Cost Options
“I have a lot of patients who use Canadian pharmacies to try to get around it, and I have a lot of patients who make a trip to Mexico every year and load up. I have patients who don’t take their inhalers as they are supposed to in order to make them last longer, and I have patients who take the inhalers of other family members,” Ms. Young said.
Humayun Anjum, MD, FCCP, from Dallas Pulmonary and Critical Care in Dallas, Texas, said in an interview that when patients claim that a prescribed drug isn’t working as expected for them, financial pressures may be partly to blame.
“When you investigate a little bit more, that’s where things become a bit more clear, and the patient may say ‘yeah, I stopped using this inhaler because it was costing me 200 bucks a month and I’m already on other medications,’ ” he said.
He noted that, when possible, he will steer patients toward discount prescription services such as GoodRx, which offers discounts at local pharmacies, or Mark Cuban Cost Plus Drugs, an online pharmacy that offers generic versions of Advair Diskus (fluticasone propionate/salmeterol) at a 100-50 mcg dose for $94.70, a savings of $307.30 over retail pharmacies, according to the company’s website.
Dr. Beach noted that Beth Israel Deaconess has a pharmacist embedded in the pulmonary clinic who can help eligible patients get financial assistance to pay for their medications.
“The influencing factors of CRN are multifaceted and necessitating more rigorous research. Health policy interventions focusing on reducing drug costs, delaying disease progression, preventing exacerbations, and reducing the risk of comorbidities may improve the economic burden of COPD and its outcomes,” Dr. Wen and colleagues wrote.
The study by Dr. Wen and colleagues was funded by grants from Chinese national and academic sources. Dr. McCormack has served as a consultant to Aridis, Boehringer Ingelheim, GlaxoSmithKline, MCG Diagnostics, ndd Medical Technologies, and UpToDate. Ms. Young, Dr. Anjum, and Dr. Beach reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
For patients with chronic obstructive pulmonary disease (COPD), the Global Initiative on Obstructive Lung Disease recommends long-term term pharmacologic and nonpharmacologic therapies based on each patient’s symptoms and disease severity.
Yet even the most effective drugs work only when patients take them as directed, and according to the World Health Organization, fewer than half of all patients worldwide are fully compliant with long-term COPD drug regimens.
And as a recent cross-sectional study showed, nearly one in six patients in the United States reported missing a COPD drug dose, lowering the dose, or delaying filling a prescription for financial reasons.
“I care for patients with COPD as their pulmonologist, and this is a very common problem that we see in clinical practice,” said Meredith McCormack, MD, a pulmonary and critical care medicine physician and associate director of the Pulmonary & Critical Care Medicine Division at Johns Hopkins University in Baltimore.
Dr. McCormack, a national spokesperson for The American Lung Association, said that she shows new patients the photos of all available inhalers and asks which ones they have and how they take them.
“I would say that a majority of the time people are taking their medicines slightly differently than prescribed, and often, this is due to cost,” she said.
Serious Consequences
Cost-related medication nonadherence (CRN), as investigators term it, can have major health effects and can be significantly more costly in the long run due to increased hospitalization rates, higher morbidity, and greater risk for COPD-related death associated with suboptimal care.
“For some patients even a month or two of being off medications increases the risk of having exacerbations, having more symptoms, [and] having a decline in their lung function,” said Douglas M. Beach, MD, a pulmonologist at Beth Israel Deaconess Medical Center in Boston.
In the aforementioned cross-sectional study, published in the open access journal BMC Public Health, Xin Wen, MD, from the Jiamusi (China) University School of Public Health, and colleagues looked at data on a representative sample of US adults who participated in the US National Health Interview Survey from 2013 through 2020.
The sample included 15,928 persons aged 18 years or older with a self-reported history of COPD who completed a CRN survey including the following questions:
During the past 12 months, have you
- Skipped medication doses to save money?
- Taken less medicine than prescribed to save money?
- Delayed filling a prescription to save money?
The investigators found that a weighted 18.56% of participants representing 2.39 million persons with COPD answered “yes” to one of the questions.
Translated into representative population numbers, that works out to an estimated 1.61 million persons with COPD missing doses, 1.72 million taking lower doses than those prescribed, and 2.03 million delaying filling prescriptions to save money.
A multivariable logistic regression analysis showed that those who were most likely to be nonadherent for financial reasons were patients younger than 65 years, women, persons with low family income, those who lacked health insurance, and patients with multiple comorbidities, the authors found.
Financial Barriers
One of the biggest barriers to COPD medication adherence is, somewhat paradoxically, insurance status, particularly Medicare, said Corinne Young, MSN, FNP-C, FCCP, from Colorado Springs Pulmonary Consultants.
“What’s so unfair about Medicare is that patients have to buy a drug plan, so they have to already pay for an extra plan to have access to drugs, and the plans vary because there are so many choices,” she said in an interview.
Elderly patients may be confused about the available options and may choose the Medicare Advantage plan with the lowest monthly premiums, which have the highest annual deductibles, usually in the $5000-and-up range, she said.
In addition, the Medicare Part D prescription coverage gap, commonly known as the “donut hole,” requires patients to pay a percentage of drug costs above a certain limit ($5030 in 2024) until a yearly out-of-pocket limit (currently $8000) is reached, after which the plan will again pick up most of the costs.
Although makers of inhalers have voluntarily agreed to limit monthly co-pays to $35 for uninsured patients, Medicare plans require insured patients to shell out considerably more, with 30 days of Trelegy Ellipta (fluticasone furoate, umeclidinium, and vilanterol) setting patients back nearly $350 per month, according to a recent search of costs for a United Healthcare Medicare Advantage plan.
Chasing Lower-Cost Options
“I have a lot of patients who use Canadian pharmacies to try to get around it, and I have a lot of patients who make a trip to Mexico every year and load up. I have patients who don’t take their inhalers as they are supposed to in order to make them last longer, and I have patients who take the inhalers of other family members,” Ms. Young said.
Humayun Anjum, MD, FCCP, from Dallas Pulmonary and Critical Care in Dallas, Texas, said in an interview that when patients claim that a prescribed drug isn’t working as expected for them, financial pressures may be partly to blame.
“When you investigate a little bit more, that’s where things become a bit more clear, and the patient may say ‘yeah, I stopped using this inhaler because it was costing me 200 bucks a month and I’m already on other medications,’ ” he said.
He noted that, when possible, he will steer patients toward discount prescription services such as GoodRx, which offers discounts at local pharmacies, or Mark Cuban Cost Plus Drugs, an online pharmacy that offers generic versions of Advair Diskus (fluticasone propionate/salmeterol) at a 100-50 mcg dose for $94.70, a savings of $307.30 over retail pharmacies, according to the company’s website.
Dr. Beach noted that Beth Israel Deaconess has a pharmacist embedded in the pulmonary clinic who can help eligible patients get financial assistance to pay for their medications.
“The influencing factors of CRN are multifaceted and necessitating more rigorous research. Health policy interventions focusing on reducing drug costs, delaying disease progression, preventing exacerbations, and reducing the risk of comorbidities may improve the economic burden of COPD and its outcomes,” Dr. Wen and colleagues wrote.
The study by Dr. Wen and colleagues was funded by grants from Chinese national and academic sources. Dr. McCormack has served as a consultant to Aridis, Boehringer Ingelheim, GlaxoSmithKline, MCG Diagnostics, ndd Medical Technologies, and UpToDate. Ms. Young, Dr. Anjum, and Dr. Beach reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Could total mesometrial resection become a new standard treatment for cervical cancer?
These findings suggest that TMMR may be considered a primary treatment option for both early-stage and locally advanced cervical cancer confined to the Müllerian compartment, reported lead author Henrik Falconer, MD, PhD, of Karolinska Institutet, Stockholm, Sweden, and colleagues.
What is the rationale behind TMMR?
“Current international guidelines [for cervical cancer] are primarily based on retrospective case series and a small number of outdated randomized controlled trials,” the investigators wrote in EClinicalMedicine, part of The Lancet publication platform. “The stage-dependent treatment recommendations, with surgery advised for early-stage and radiation therapy for locally advanced disease, may be considered too simplistic, suggesting that early stages of cervical cancer cannot be controlled with surgical resection alone or that locally advanced cervical cancer is inoperable.”
This mindset, they noted, overlooks the complexities of cancer spread. In contrast, TMMR and similar surgical approaches based on the cancer field model are mapped along routes of locoregional dissemination, leading to “excellent local control” in more than 600 cases at the University Hospital of Leipzig, Leipzig, Germany.
To date, however, TMMR’s adoption has been limited, and it has not been compared directly with current guideline treatments, prompting the present study.
What methods were used to compare TMMR with standard treatment?
The study compared TMMR plus therapeutic lymph node dissection (tLND) without adjuvant radiation versus standard treatment (ST) for early-stage (FIGO 2009 IB1, IIA1) and locally advanced (FIGO 2009 IB2, IIA2, IIB) cervical cancer. Standard treatment for patients with early-stage disease involved radical hysterectomy and pelvic lymphadenectomy, with adjuvant chemoradiation dependent upon final pathology. Those with locally advanced disease received definitive chemoradiation.
Data for the standard treatment group were drawn from population-based registries in Sweden, while those for the TMMR group came from the Leipzig Mesometrial Resection Study Database. The final dataset included 1,007 women treated between 2011 and 2020, with 733 undergoing standard treatment and 274 receiving TMMR.
Outcomes included RFS and OS, adjusted for clinical and tumor-related variables.
How did TMMR compare with standard treatment?
TMMR was associated with superior oncologic outcomes compared with standard treatment for early-stage cervical cancer.
Specifically, 5-year RFS was 91.2% for TMMR versus 81.8% for standard therapy (P = .002). In the adjusted analysis, TMMR was associated with a significantly lower hazard of recurrence (hazard ratio [HR], 0.39; 95% CI, 0.22–0.69) and death (HR, 0.42; 95% CI, 0.21-0.86). Also favoring TMMR, absolute difference in the risk of recurrence at 5 years was 9.4% (95% CI 3.2–15.7). In addition, 5-year OS was better in the TMMR group, at 93.3%, compared with 90.3% for standard treatment (P = .034).
Among patients with locally advanced disease, no significant differences in RFS or OS were observed.
Are these data strong enough to make TMMR the new standard treatment?
Dr. Falconer and colleagues concluded that TMMR with tLND “may replace the standard treatment approach in early-stage cervical cancer and furthermore be evaluated as an option in locally advanced cervical cancer confined to the Müllerian compartment.”
While the investigators anticipated demands for randomized controlled trials, they questioned the value of such studies, suggesting that any control arm would be “based on inconsistent or flawed concepts.”
Susan C. Modesitt, MD, director of the gynecologic oncology division of Winship Cancer Institute of Emory University, Atlanta, offered a different perspective.
“They do show encouraging data in the early stage,” Dr. Modesitt said in an interview, “but I would still want to see a randomized controlled trial, because we’ve been burned before.”
She cited the LACC trial, which dispelled strong convictions about the alleged superiority of minimally invasive radical hysterectomy.
“We thought minimally invasive was so good, and we should be doing that to everybody, but we did a trial, and we found worse outcomes,” Dr. Modesitt said. “More of those early-stage women died.”
Dr. Modesitt also pointed out the lack of safety data in the present publication.
“TMMR is a bigger procedure, so I would expect more complications,” she said, noting that rates of urinary injury, nerve injury, and readmission need to be considered alongside efficacy outcomes.
How does TMMR fit into the current treatment landscape for cervical cancer?
“This is a very niche surgery that most places don’t do,” Dr. Modesitt said.
She pointed out that “multiple variations” on the standard radical hysterectomy have been proposed in the past, such as the laterally extended endopelvic resection.
“[TMMR] is not a new concept,” she said. “It’s just a question of how radical it is.”
Instead of developing new types of radical surgery, she said, the trend in the United States is toward de-escalation of surgical treatments altogether, with greater reliance upon medical options, such as immunotherapy.
“[This study] is thought provoking, and I applaud them for doing it,” Dr. Modesitt said. “But I’m not going to go out and do that on my next patient.”
This study was supported by grants from Centre for Clinical Research Sörmland (Sweden) and Region Stockholm (Sweden). Dr. Falconer is a board member of Surgical Science.
These findings suggest that TMMR may be considered a primary treatment option for both early-stage and locally advanced cervical cancer confined to the Müllerian compartment, reported lead author Henrik Falconer, MD, PhD, of Karolinska Institutet, Stockholm, Sweden, and colleagues.
What is the rationale behind TMMR?
“Current international guidelines [for cervical cancer] are primarily based on retrospective case series and a small number of outdated randomized controlled trials,” the investigators wrote in EClinicalMedicine, part of The Lancet publication platform. “The stage-dependent treatment recommendations, with surgery advised for early-stage and radiation therapy for locally advanced disease, may be considered too simplistic, suggesting that early stages of cervical cancer cannot be controlled with surgical resection alone or that locally advanced cervical cancer is inoperable.”
This mindset, they noted, overlooks the complexities of cancer spread. In contrast, TMMR and similar surgical approaches based on the cancer field model are mapped along routes of locoregional dissemination, leading to “excellent local control” in more than 600 cases at the University Hospital of Leipzig, Leipzig, Germany.
To date, however, TMMR’s adoption has been limited, and it has not been compared directly with current guideline treatments, prompting the present study.
What methods were used to compare TMMR with standard treatment?
The study compared TMMR plus therapeutic lymph node dissection (tLND) without adjuvant radiation versus standard treatment (ST) for early-stage (FIGO 2009 IB1, IIA1) and locally advanced (FIGO 2009 IB2, IIA2, IIB) cervical cancer. Standard treatment for patients with early-stage disease involved radical hysterectomy and pelvic lymphadenectomy, with adjuvant chemoradiation dependent upon final pathology. Those with locally advanced disease received definitive chemoradiation.
Data for the standard treatment group were drawn from population-based registries in Sweden, while those for the TMMR group came from the Leipzig Mesometrial Resection Study Database. The final dataset included 1,007 women treated between 2011 and 2020, with 733 undergoing standard treatment and 274 receiving TMMR.
Outcomes included RFS and OS, adjusted for clinical and tumor-related variables.
How did TMMR compare with standard treatment?
TMMR was associated with superior oncologic outcomes compared with standard treatment for early-stage cervical cancer.
Specifically, 5-year RFS was 91.2% for TMMR versus 81.8% for standard therapy (P = .002). In the adjusted analysis, TMMR was associated with a significantly lower hazard of recurrence (hazard ratio [HR], 0.39; 95% CI, 0.22–0.69) and death (HR, 0.42; 95% CI, 0.21-0.86). Also favoring TMMR, absolute difference in the risk of recurrence at 5 years was 9.4% (95% CI 3.2–15.7). In addition, 5-year OS was better in the TMMR group, at 93.3%, compared with 90.3% for standard treatment (P = .034).
Among patients with locally advanced disease, no significant differences in RFS or OS were observed.
Are these data strong enough to make TMMR the new standard treatment?
Dr. Falconer and colleagues concluded that TMMR with tLND “may replace the standard treatment approach in early-stage cervical cancer and furthermore be evaluated as an option in locally advanced cervical cancer confined to the Müllerian compartment.”
While the investigators anticipated demands for randomized controlled trials, they questioned the value of such studies, suggesting that any control arm would be “based on inconsistent or flawed concepts.”
Susan C. Modesitt, MD, director of the gynecologic oncology division of Winship Cancer Institute of Emory University, Atlanta, offered a different perspective.
“They do show encouraging data in the early stage,” Dr. Modesitt said in an interview, “but I would still want to see a randomized controlled trial, because we’ve been burned before.”
She cited the LACC trial, which dispelled strong convictions about the alleged superiority of minimally invasive radical hysterectomy.
“We thought minimally invasive was so good, and we should be doing that to everybody, but we did a trial, and we found worse outcomes,” Dr. Modesitt said. “More of those early-stage women died.”
Dr. Modesitt also pointed out the lack of safety data in the present publication.
“TMMR is a bigger procedure, so I would expect more complications,” she said, noting that rates of urinary injury, nerve injury, and readmission need to be considered alongside efficacy outcomes.
How does TMMR fit into the current treatment landscape for cervical cancer?
“This is a very niche surgery that most places don’t do,” Dr. Modesitt said.
She pointed out that “multiple variations” on the standard radical hysterectomy have been proposed in the past, such as the laterally extended endopelvic resection.
“[TMMR] is not a new concept,” she said. “It’s just a question of how radical it is.”
Instead of developing new types of radical surgery, she said, the trend in the United States is toward de-escalation of surgical treatments altogether, with greater reliance upon medical options, such as immunotherapy.
“[This study] is thought provoking, and I applaud them for doing it,” Dr. Modesitt said. “But I’m not going to go out and do that on my next patient.”
This study was supported by grants from Centre for Clinical Research Sörmland (Sweden) and Region Stockholm (Sweden). Dr. Falconer is a board member of Surgical Science.
These findings suggest that TMMR may be considered a primary treatment option for both early-stage and locally advanced cervical cancer confined to the Müllerian compartment, reported lead author Henrik Falconer, MD, PhD, of Karolinska Institutet, Stockholm, Sweden, and colleagues.
What is the rationale behind TMMR?
“Current international guidelines [for cervical cancer] are primarily based on retrospective case series and a small number of outdated randomized controlled trials,” the investigators wrote in EClinicalMedicine, part of The Lancet publication platform. “The stage-dependent treatment recommendations, with surgery advised for early-stage and radiation therapy for locally advanced disease, may be considered too simplistic, suggesting that early stages of cervical cancer cannot be controlled with surgical resection alone or that locally advanced cervical cancer is inoperable.”
This mindset, they noted, overlooks the complexities of cancer spread. In contrast, TMMR and similar surgical approaches based on the cancer field model are mapped along routes of locoregional dissemination, leading to “excellent local control” in more than 600 cases at the University Hospital of Leipzig, Leipzig, Germany.
To date, however, TMMR’s adoption has been limited, and it has not been compared directly with current guideline treatments, prompting the present study.
What methods were used to compare TMMR with standard treatment?
The study compared TMMR plus therapeutic lymph node dissection (tLND) without adjuvant radiation versus standard treatment (ST) for early-stage (FIGO 2009 IB1, IIA1) and locally advanced (FIGO 2009 IB2, IIA2, IIB) cervical cancer. Standard treatment for patients with early-stage disease involved radical hysterectomy and pelvic lymphadenectomy, with adjuvant chemoradiation dependent upon final pathology. Those with locally advanced disease received definitive chemoradiation.
Data for the standard treatment group were drawn from population-based registries in Sweden, while those for the TMMR group came from the Leipzig Mesometrial Resection Study Database. The final dataset included 1,007 women treated between 2011 and 2020, with 733 undergoing standard treatment and 274 receiving TMMR.
Outcomes included RFS and OS, adjusted for clinical and tumor-related variables.
How did TMMR compare with standard treatment?
TMMR was associated with superior oncologic outcomes compared with standard treatment for early-stage cervical cancer.
Specifically, 5-year RFS was 91.2% for TMMR versus 81.8% for standard therapy (P = .002). In the adjusted analysis, TMMR was associated with a significantly lower hazard of recurrence (hazard ratio [HR], 0.39; 95% CI, 0.22–0.69) and death (HR, 0.42; 95% CI, 0.21-0.86). Also favoring TMMR, absolute difference in the risk of recurrence at 5 years was 9.4% (95% CI 3.2–15.7). In addition, 5-year OS was better in the TMMR group, at 93.3%, compared with 90.3% for standard treatment (P = .034).
Among patients with locally advanced disease, no significant differences in RFS or OS were observed.
Are these data strong enough to make TMMR the new standard treatment?
Dr. Falconer and colleagues concluded that TMMR with tLND “may replace the standard treatment approach in early-stage cervical cancer and furthermore be evaluated as an option in locally advanced cervical cancer confined to the Müllerian compartment.”
While the investigators anticipated demands for randomized controlled trials, they questioned the value of such studies, suggesting that any control arm would be “based on inconsistent or flawed concepts.”
Susan C. Modesitt, MD, director of the gynecologic oncology division of Winship Cancer Institute of Emory University, Atlanta, offered a different perspective.
“They do show encouraging data in the early stage,” Dr. Modesitt said in an interview, “but I would still want to see a randomized controlled trial, because we’ve been burned before.”
She cited the LACC trial, which dispelled strong convictions about the alleged superiority of minimally invasive radical hysterectomy.
“We thought minimally invasive was so good, and we should be doing that to everybody, but we did a trial, and we found worse outcomes,” Dr. Modesitt said. “More of those early-stage women died.”
Dr. Modesitt also pointed out the lack of safety data in the present publication.
“TMMR is a bigger procedure, so I would expect more complications,” she said, noting that rates of urinary injury, nerve injury, and readmission need to be considered alongside efficacy outcomes.
How does TMMR fit into the current treatment landscape for cervical cancer?
“This is a very niche surgery that most places don’t do,” Dr. Modesitt said.
She pointed out that “multiple variations” on the standard radical hysterectomy have been proposed in the past, such as the laterally extended endopelvic resection.
“[TMMR] is not a new concept,” she said. “It’s just a question of how radical it is.”
Instead of developing new types of radical surgery, she said, the trend in the United States is toward de-escalation of surgical treatments altogether, with greater reliance upon medical options, such as immunotherapy.
“[This study] is thought provoking, and I applaud them for doing it,” Dr. Modesitt said. “But I’m not going to go out and do that on my next patient.”
This study was supported by grants from Centre for Clinical Research Sörmland (Sweden) and Region Stockholm (Sweden). Dr. Falconer is a board member of Surgical Science.
FROM THE LANCET
Study Detects Bacteria in Tattoo, Permanent Makeup Inks
When US researchers tested 75 unopened and sealed tattoo and permanent makeup inks from 14 different manufacturers, they discovered that about 35% of the products were contaminated with bacteria.
They detected both aerobic bacteria and anaerobic bacteria, which thrive in low-oxygen environments like the dermal layer of the skin.
“This suggests that contaminated tattoo inks could be a source of infection from both types of bacteria,” Seong-Jae Peter Kim, PhD, a microbiologist with the Division of Microbiology, National Center for Toxicological Research, US Food and Drug Administration, who worked on the study, said in a news release.
The findings “are concerning,” said Waleed Javaid, MD, professor of medicine and director of infection prevention and control for the Mount Sinai Health System in New York City. “This contamination poses a significant health risk, as these inks are injected into the dermal layer of the skin, creating an environment conducive to bacterial infections,” said Dr. Javaid, who wasn’t involved in the study, which was published online in Applied and Environmental Microbiology.
New Body Art Culture
Tattoos are more popular than ever, and it is estimated that at least 32% of people in the United States have at least one tattoo. And the rise in popularity has coincided with an increase in ink-related infections.
This new research joins previous studies that have demonstrated that commercial tattoo and permanent makeup inks are often contaminated with pathogenic microorganisms.
Of the 75 ink samples that Dr. Kim and colleagues tested, 26 were contaminated with 34 bacterial isolates classified into 14 genera and 22 species. Among the 34 bacterial isolates, 19 were identified as possibly pathogenic bacterial strains.
Two species — Cutibacterium acnes (four strains) and Staphylococcus epidermidis (two strains) — were isolated under anaerobic conditions.
Two possibly pathogenic bacterial strains — Staphylococcus saprophyticus and C acnes — were isolated from the same two ink samples, indicating that tattoo and permanent makeup inks can harbor both aerobic (S saprophyticus) and anaerobic (C acnes) bacteria.
There was no significant association between sterility claims on the ink label and the absence of bacterial contamination.
“The presence of bacteria like Cutibacterium acnes and Staphylococcus epidermidis, which can cause skin infections and other complications, underscores the potential danger to individuals receiving tattoos or permanent makeup,” Dr. Javaid explained.
The results “emphasize the importance of monitoring these products for both aerobic and anaerobic bacteria, including possibly pathogenic microorganisms,” Dr. Kim said in the news release.
The next steps, according to the researchers, include developing more efficient and accurate microbial detection methods for tattoo inks to streamline the monitoring process and examining the occurrence, co-occurrence, and diversity of microbial contaminants in tattoo inks to prevent future contamination.
Counseling Patients
Healthcare professionals play a “crucial role in counseling patients about the risks associated with tattoos. They should inform patients about the potential for infections, allergic reactions, and other complications related to tattooing and permanent ink,” said Dr. Javaid.
Specific advice can include ensuring that the tattoo parlor adheres to strict hygiene practices and verifying that tattoo inks are from reputable sources and, if possible, have undergone sterilization.
Clinicians should discuss the importance of proper aftercare to minimize the risk for infection, recommend patients with compromised immune systems or skin conditions to reconsider getting a tattoo, and encourage patients to be aware of the signs of infection and to seek medical attention promptly if any symptoms arise.
“Enhanced regulatory measures would help reduce the risk of infections and ensure safer tattooing practices for consumers,” Dr. Javaid said. The findings of Dr. Kim and colleagues “indicate that current manufacturing and sterilization processes are inadequate.”
Regulations could include stricter manufacturing standards to ensure sterility, the mandatory testing of inks for microbial contamination before they reach the market, clear labeling requirements that accurately reflect the sterility and safety of products, and regular inspections and audits of tattoo ink manufacturers, he said, which could encourage the development of more effective sterilization techniques to eliminate bacterial contamination.
The FDA has created a document — Think Before You Ink: Tattoo Safety — for consumers who are considering getting a tattoo.
A version of this article first appeared on Medscape.com.
When US researchers tested 75 unopened and sealed tattoo and permanent makeup inks from 14 different manufacturers, they discovered that about 35% of the products were contaminated with bacteria.
They detected both aerobic bacteria and anaerobic bacteria, which thrive in low-oxygen environments like the dermal layer of the skin.
“This suggests that contaminated tattoo inks could be a source of infection from both types of bacteria,” Seong-Jae Peter Kim, PhD, a microbiologist with the Division of Microbiology, National Center for Toxicological Research, US Food and Drug Administration, who worked on the study, said in a news release.
The findings “are concerning,” said Waleed Javaid, MD, professor of medicine and director of infection prevention and control for the Mount Sinai Health System in New York City. “This contamination poses a significant health risk, as these inks are injected into the dermal layer of the skin, creating an environment conducive to bacterial infections,” said Dr. Javaid, who wasn’t involved in the study, which was published online in Applied and Environmental Microbiology.
New Body Art Culture
Tattoos are more popular than ever, and it is estimated that at least 32% of people in the United States have at least one tattoo. And the rise in popularity has coincided with an increase in ink-related infections.
This new research joins previous studies that have demonstrated that commercial tattoo and permanent makeup inks are often contaminated with pathogenic microorganisms.
Of the 75 ink samples that Dr. Kim and colleagues tested, 26 were contaminated with 34 bacterial isolates classified into 14 genera and 22 species. Among the 34 bacterial isolates, 19 were identified as possibly pathogenic bacterial strains.
Two species — Cutibacterium acnes (four strains) and Staphylococcus epidermidis (two strains) — were isolated under anaerobic conditions.
Two possibly pathogenic bacterial strains — Staphylococcus saprophyticus and C acnes — were isolated from the same two ink samples, indicating that tattoo and permanent makeup inks can harbor both aerobic (S saprophyticus) and anaerobic (C acnes) bacteria.
There was no significant association between sterility claims on the ink label and the absence of bacterial contamination.
“The presence of bacteria like Cutibacterium acnes and Staphylococcus epidermidis, which can cause skin infections and other complications, underscores the potential danger to individuals receiving tattoos or permanent makeup,” Dr. Javaid explained.
The results “emphasize the importance of monitoring these products for both aerobic and anaerobic bacteria, including possibly pathogenic microorganisms,” Dr. Kim said in the news release.
The next steps, according to the researchers, include developing more efficient and accurate microbial detection methods for tattoo inks to streamline the monitoring process and examining the occurrence, co-occurrence, and diversity of microbial contaminants in tattoo inks to prevent future contamination.
Counseling Patients
Healthcare professionals play a “crucial role in counseling patients about the risks associated with tattoos. They should inform patients about the potential for infections, allergic reactions, and other complications related to tattooing and permanent ink,” said Dr. Javaid.
Specific advice can include ensuring that the tattoo parlor adheres to strict hygiene practices and verifying that tattoo inks are from reputable sources and, if possible, have undergone sterilization.
Clinicians should discuss the importance of proper aftercare to minimize the risk for infection, recommend patients with compromised immune systems or skin conditions to reconsider getting a tattoo, and encourage patients to be aware of the signs of infection and to seek medical attention promptly if any symptoms arise.
“Enhanced regulatory measures would help reduce the risk of infections and ensure safer tattooing practices for consumers,” Dr. Javaid said. The findings of Dr. Kim and colleagues “indicate that current manufacturing and sterilization processes are inadequate.”
Regulations could include stricter manufacturing standards to ensure sterility, the mandatory testing of inks for microbial contamination before they reach the market, clear labeling requirements that accurately reflect the sterility and safety of products, and regular inspections and audits of tattoo ink manufacturers, he said, which could encourage the development of more effective sterilization techniques to eliminate bacterial contamination.
The FDA has created a document — Think Before You Ink: Tattoo Safety — for consumers who are considering getting a tattoo.
A version of this article first appeared on Medscape.com.
When US researchers tested 75 unopened and sealed tattoo and permanent makeup inks from 14 different manufacturers, they discovered that about 35% of the products were contaminated with bacteria.
They detected both aerobic bacteria and anaerobic bacteria, which thrive in low-oxygen environments like the dermal layer of the skin.
“This suggests that contaminated tattoo inks could be a source of infection from both types of bacteria,” Seong-Jae Peter Kim, PhD, a microbiologist with the Division of Microbiology, National Center for Toxicological Research, US Food and Drug Administration, who worked on the study, said in a news release.
The findings “are concerning,” said Waleed Javaid, MD, professor of medicine and director of infection prevention and control for the Mount Sinai Health System in New York City. “This contamination poses a significant health risk, as these inks are injected into the dermal layer of the skin, creating an environment conducive to bacterial infections,” said Dr. Javaid, who wasn’t involved in the study, which was published online in Applied and Environmental Microbiology.
New Body Art Culture
Tattoos are more popular than ever, and it is estimated that at least 32% of people in the United States have at least one tattoo. And the rise in popularity has coincided with an increase in ink-related infections.
This new research joins previous studies that have demonstrated that commercial tattoo and permanent makeup inks are often contaminated with pathogenic microorganisms.
Of the 75 ink samples that Dr. Kim and colleagues tested, 26 were contaminated with 34 bacterial isolates classified into 14 genera and 22 species. Among the 34 bacterial isolates, 19 were identified as possibly pathogenic bacterial strains.
Two species — Cutibacterium acnes (four strains) and Staphylococcus epidermidis (two strains) — were isolated under anaerobic conditions.
Two possibly pathogenic bacterial strains — Staphylococcus saprophyticus and C acnes — were isolated from the same two ink samples, indicating that tattoo and permanent makeup inks can harbor both aerobic (S saprophyticus) and anaerobic (C acnes) bacteria.
There was no significant association between sterility claims on the ink label and the absence of bacterial contamination.
“The presence of bacteria like Cutibacterium acnes and Staphylococcus epidermidis, which can cause skin infections and other complications, underscores the potential danger to individuals receiving tattoos or permanent makeup,” Dr. Javaid explained.
The results “emphasize the importance of monitoring these products for both aerobic and anaerobic bacteria, including possibly pathogenic microorganisms,” Dr. Kim said in the news release.
The next steps, according to the researchers, include developing more efficient and accurate microbial detection methods for tattoo inks to streamline the monitoring process and examining the occurrence, co-occurrence, and diversity of microbial contaminants in tattoo inks to prevent future contamination.
Counseling Patients
Healthcare professionals play a “crucial role in counseling patients about the risks associated with tattoos. They should inform patients about the potential for infections, allergic reactions, and other complications related to tattooing and permanent ink,” said Dr. Javaid.
Specific advice can include ensuring that the tattoo parlor adheres to strict hygiene practices and verifying that tattoo inks are from reputable sources and, if possible, have undergone sterilization.
Clinicians should discuss the importance of proper aftercare to minimize the risk for infection, recommend patients with compromised immune systems or skin conditions to reconsider getting a tattoo, and encourage patients to be aware of the signs of infection and to seek medical attention promptly if any symptoms arise.
“Enhanced regulatory measures would help reduce the risk of infections and ensure safer tattooing practices for consumers,” Dr. Javaid said. The findings of Dr. Kim and colleagues “indicate that current manufacturing and sterilization processes are inadequate.”
Regulations could include stricter manufacturing standards to ensure sterility, the mandatory testing of inks for microbial contamination before they reach the market, clear labeling requirements that accurately reflect the sterility and safety of products, and regular inspections and audits of tattoo ink manufacturers, he said, which could encourage the development of more effective sterilization techniques to eliminate bacterial contamination.
The FDA has created a document — Think Before You Ink: Tattoo Safety — for consumers who are considering getting a tattoo.
A version of this article first appeared on Medscape.com.
FROM APPLIED AND ENVIRONMENTAL MICROBIOLOGY
‘Chemoresistance Can Be Reversed’: Toughest Cancers Targeted
In the war against cancer, doctors and patients have long reached for three main weapons to target diseased cells: chemotherapy, radiation, and surgery.
But new research published this month in the journal Nature Materials suggests that manipulating the tissue around those cells — a strategy known as
“Our study shows the importance of the tumor microenvironment and its properties in dictating how cancer progresses and responds to drug treatment,” said first author Bauer LeSavage, PhD, who conducted the study as a postdoctoral researcher in the Bioengineering Department at Stanford University, Stanford, California. “It also demonstrates that chemoresistance can be reversed.”
Each year, about 66,000 people are diagnosed with pancreatic cancer, and 52,000 die from it. It is a particularly lethal type of cancer, with 5-year survival rates hovering around 7% — a rate that has not improved much since 1996 when the first-line chemotherapy drug gemcitabine was approved.
It looks different from many cancers, said Lynn Matrisian, PhD, chief science officer for the nonprofit Pancreatic Cancer Action Network. Instead of a tumorous mass, it is made of islands of cancer cells surrounded by unusually dense fibrous tissue known as the extracellular matrix, which can collapse blood vessels and prevent drugs from reaching the tumor.
For the study, Dr. LeSavage and his team engineered synthetic but lifelike three-dimensional pancreas tissue with varying degrees of stiffness and different biochemical properties. Then they inserted bits of real tumors from patients with pancreatic cancer, watched them grow, and tried to kill them with drugs.
They found that cells growing in a stiff matrix were more resistant to chemotherapy than those growing in a softer matrix. But the story didn’t end there.
They also found that high amounts of the tissue-strengthening protein hyaluronic acid in stiff tissue seemed to signal the cancer cells to develop tiny pumps on their surface which shuttled out the drugs before they could take effect.
When the researchers moved the cancer cells into either a softer matrix or a stiff matrix in which the hyaluronic acid receptor, called CD44, was blocked, the chemotherapy drugs started working again.
“This suggests that if we can disrupt the stiffness signaling that’s happening through the CD44 receptor, we could make patients’ pancreatic cancer treatable again by normal chemotherapy,” said senior study author Sarah Heilshorn, PhD, a professor of materials science and engineering at Stanford. “These results suggest an exciting new direction for new drug development.”
Targeting Nearby Tissue: A Novel Approach to Fighting Chemoresistance
The study is not the first to suggest that chemically targeting the microenvironment surrounding a tumor can influence how patients respond to treatment.
In one recent clinical trial, patients with metastatic pancreatic cancer were given an experimental drug to inhibit a protein called connective tissue growth factor, reduce fibrous tissue, and make pancreatic tumors easier to surgically remove. Results have not been published yet.
Other research suggests that the generic blood pressure drug losartan, when given in combination with chemotherapy and radiation, can boost survival in patients with advanced pancreatic cancer by, in part, improving the health of blood vessels that carry drugs to the tumor.
But other studies of such mechanotherapeutics have yielded inconclusive results, said Dr. Matrisian.
“This paper points to another reason why we should not give up on this approach,” she said.
Ning Wang, PhD, director of the new Institute for Mechanobiology at Northeastern University College of Engineering, Boston, said there is no question that the composition of a tumor’s environment can influence how cancer progresses or responds to drugs. The new paper, he said, adds an important new chapter to the evolving story.
“But it’s very complicated. It’s not as simple as saying make it softer or stiffer and you can change the outcome for the patient,” Dr. Wang said.
In fact, some research has shown that tissue becomes stiffer when cancer arises so it can contain it from spreading.
In one animal study of pancreatic cancer that had spread to the liver, administering drugs to soften the surrounding tissue, or stroma, actually had the opposite effect — accelerating tumor growth and reducing survival rates.
Dr. Wang also noted that any drug designed to influence the extracellular matrix would need to be extremely localized, to prevent damage to other tissues, like bone or heart muscle.
Dr. LeSavage said he sees the paper as a case study in how important the extracellular matrix is and an example of how artificially grown organs or tissues can play a key role in testing how drugs work or don’t work.
He imagines a day when doctors could personalize treatments by taking a bit of a patient’s tumor, growing it in artificial tissue, and seeing how different tissue-altering drugs affect different therapies.
“This isn’t something that is just unique to pancreatic cancer,” he said, noting that the extracellular matrix throughout the body interacts with different cancers. “If we could take someone who has a chemoresistant tumor and convert it into something that is sensitive to existing therapies again, we could give them a second chance.”
A version of this article appeared on Medscape.com.
In the war against cancer, doctors and patients have long reached for three main weapons to target diseased cells: chemotherapy, radiation, and surgery.
But new research published this month in the journal Nature Materials suggests that manipulating the tissue around those cells — a strategy known as
“Our study shows the importance of the tumor microenvironment and its properties in dictating how cancer progresses and responds to drug treatment,” said first author Bauer LeSavage, PhD, who conducted the study as a postdoctoral researcher in the Bioengineering Department at Stanford University, Stanford, California. “It also demonstrates that chemoresistance can be reversed.”
Each year, about 66,000 people are diagnosed with pancreatic cancer, and 52,000 die from it. It is a particularly lethal type of cancer, with 5-year survival rates hovering around 7% — a rate that has not improved much since 1996 when the first-line chemotherapy drug gemcitabine was approved.
It looks different from many cancers, said Lynn Matrisian, PhD, chief science officer for the nonprofit Pancreatic Cancer Action Network. Instead of a tumorous mass, it is made of islands of cancer cells surrounded by unusually dense fibrous tissue known as the extracellular matrix, which can collapse blood vessels and prevent drugs from reaching the tumor.
For the study, Dr. LeSavage and his team engineered synthetic but lifelike three-dimensional pancreas tissue with varying degrees of stiffness and different biochemical properties. Then they inserted bits of real tumors from patients with pancreatic cancer, watched them grow, and tried to kill them with drugs.
They found that cells growing in a stiff matrix were more resistant to chemotherapy than those growing in a softer matrix. But the story didn’t end there.
They also found that high amounts of the tissue-strengthening protein hyaluronic acid in stiff tissue seemed to signal the cancer cells to develop tiny pumps on their surface which shuttled out the drugs before they could take effect.
When the researchers moved the cancer cells into either a softer matrix or a stiff matrix in which the hyaluronic acid receptor, called CD44, was blocked, the chemotherapy drugs started working again.
“This suggests that if we can disrupt the stiffness signaling that’s happening through the CD44 receptor, we could make patients’ pancreatic cancer treatable again by normal chemotherapy,” said senior study author Sarah Heilshorn, PhD, a professor of materials science and engineering at Stanford. “These results suggest an exciting new direction for new drug development.”
Targeting Nearby Tissue: A Novel Approach to Fighting Chemoresistance
The study is not the first to suggest that chemically targeting the microenvironment surrounding a tumor can influence how patients respond to treatment.
In one recent clinical trial, patients with metastatic pancreatic cancer were given an experimental drug to inhibit a protein called connective tissue growth factor, reduce fibrous tissue, and make pancreatic tumors easier to surgically remove. Results have not been published yet.
Other research suggests that the generic blood pressure drug losartan, when given in combination with chemotherapy and radiation, can boost survival in patients with advanced pancreatic cancer by, in part, improving the health of blood vessels that carry drugs to the tumor.
But other studies of such mechanotherapeutics have yielded inconclusive results, said Dr. Matrisian.
“This paper points to another reason why we should not give up on this approach,” she said.
Ning Wang, PhD, director of the new Institute for Mechanobiology at Northeastern University College of Engineering, Boston, said there is no question that the composition of a tumor’s environment can influence how cancer progresses or responds to drugs. The new paper, he said, adds an important new chapter to the evolving story.
“But it’s very complicated. It’s not as simple as saying make it softer or stiffer and you can change the outcome for the patient,” Dr. Wang said.
In fact, some research has shown that tissue becomes stiffer when cancer arises so it can contain it from spreading.
In one animal study of pancreatic cancer that had spread to the liver, administering drugs to soften the surrounding tissue, or stroma, actually had the opposite effect — accelerating tumor growth and reducing survival rates.
Dr. Wang also noted that any drug designed to influence the extracellular matrix would need to be extremely localized, to prevent damage to other tissues, like bone or heart muscle.
Dr. LeSavage said he sees the paper as a case study in how important the extracellular matrix is and an example of how artificially grown organs or tissues can play a key role in testing how drugs work or don’t work.
He imagines a day when doctors could personalize treatments by taking a bit of a patient’s tumor, growing it in artificial tissue, and seeing how different tissue-altering drugs affect different therapies.
“This isn’t something that is just unique to pancreatic cancer,” he said, noting that the extracellular matrix throughout the body interacts with different cancers. “If we could take someone who has a chemoresistant tumor and convert it into something that is sensitive to existing therapies again, we could give them a second chance.”
A version of this article appeared on Medscape.com.
In the war against cancer, doctors and patients have long reached for three main weapons to target diseased cells: chemotherapy, radiation, and surgery.
But new research published this month in the journal Nature Materials suggests that manipulating the tissue around those cells — a strategy known as
“Our study shows the importance of the tumor microenvironment and its properties in dictating how cancer progresses and responds to drug treatment,” said first author Bauer LeSavage, PhD, who conducted the study as a postdoctoral researcher in the Bioengineering Department at Stanford University, Stanford, California. “It also demonstrates that chemoresistance can be reversed.”
Each year, about 66,000 people are diagnosed with pancreatic cancer, and 52,000 die from it. It is a particularly lethal type of cancer, with 5-year survival rates hovering around 7% — a rate that has not improved much since 1996 when the first-line chemotherapy drug gemcitabine was approved.
It looks different from many cancers, said Lynn Matrisian, PhD, chief science officer for the nonprofit Pancreatic Cancer Action Network. Instead of a tumorous mass, it is made of islands of cancer cells surrounded by unusually dense fibrous tissue known as the extracellular matrix, which can collapse blood vessels and prevent drugs from reaching the tumor.
For the study, Dr. LeSavage and his team engineered synthetic but lifelike three-dimensional pancreas tissue with varying degrees of stiffness and different biochemical properties. Then they inserted bits of real tumors from patients with pancreatic cancer, watched them grow, and tried to kill them with drugs.
They found that cells growing in a stiff matrix were more resistant to chemotherapy than those growing in a softer matrix. But the story didn’t end there.
They also found that high amounts of the tissue-strengthening protein hyaluronic acid in stiff tissue seemed to signal the cancer cells to develop tiny pumps on their surface which shuttled out the drugs before they could take effect.
When the researchers moved the cancer cells into either a softer matrix or a stiff matrix in which the hyaluronic acid receptor, called CD44, was blocked, the chemotherapy drugs started working again.
“This suggests that if we can disrupt the stiffness signaling that’s happening through the CD44 receptor, we could make patients’ pancreatic cancer treatable again by normal chemotherapy,” said senior study author Sarah Heilshorn, PhD, a professor of materials science and engineering at Stanford. “These results suggest an exciting new direction for new drug development.”
Targeting Nearby Tissue: A Novel Approach to Fighting Chemoresistance
The study is not the first to suggest that chemically targeting the microenvironment surrounding a tumor can influence how patients respond to treatment.
In one recent clinical trial, patients with metastatic pancreatic cancer were given an experimental drug to inhibit a protein called connective tissue growth factor, reduce fibrous tissue, and make pancreatic tumors easier to surgically remove. Results have not been published yet.
Other research suggests that the generic blood pressure drug losartan, when given in combination with chemotherapy and radiation, can boost survival in patients with advanced pancreatic cancer by, in part, improving the health of blood vessels that carry drugs to the tumor.
But other studies of such mechanotherapeutics have yielded inconclusive results, said Dr. Matrisian.
“This paper points to another reason why we should not give up on this approach,” she said.
Ning Wang, PhD, director of the new Institute for Mechanobiology at Northeastern University College of Engineering, Boston, said there is no question that the composition of a tumor’s environment can influence how cancer progresses or responds to drugs. The new paper, he said, adds an important new chapter to the evolving story.
“But it’s very complicated. It’s not as simple as saying make it softer or stiffer and you can change the outcome for the patient,” Dr. Wang said.
In fact, some research has shown that tissue becomes stiffer when cancer arises so it can contain it from spreading.
In one animal study of pancreatic cancer that had spread to the liver, administering drugs to soften the surrounding tissue, or stroma, actually had the opposite effect — accelerating tumor growth and reducing survival rates.
Dr. Wang also noted that any drug designed to influence the extracellular matrix would need to be extremely localized, to prevent damage to other tissues, like bone or heart muscle.
Dr. LeSavage said he sees the paper as a case study in how important the extracellular matrix is and an example of how artificially grown organs or tissues can play a key role in testing how drugs work or don’t work.
He imagines a day when doctors could personalize treatments by taking a bit of a patient’s tumor, growing it in artificial tissue, and seeing how different tissue-altering drugs affect different therapies.
“This isn’t something that is just unique to pancreatic cancer,” he said, noting that the extracellular matrix throughout the body interacts with different cancers. “If we could take someone who has a chemoresistant tumor and convert it into something that is sensitive to existing therapies again, we could give them a second chance.”
A version of this article appeared on Medscape.com.