User login
Gut microbiome species predict type 2 diabetes
according to results from a 15-year follow-up study of more than 5,000 people in Finland.
“We are not aware of previous long-term prospective studies of the associations between type 2 diabetes and the gut microbiome similar to the current study,” stated the authors of the study, published online Jan. 31, 2022, in Diabetes Care.
Though requiring further validation, the results “build on and extend previous mainly cross-sectional evidence and further support links between dietary habits, metabolic diseases, and type 2 diabetes that are modulated by the gut microbiome,” the authors wrote.
The findings are from a prospective study of data on fecal samples from 5,572 people in Finland in 2002 in the FINRISK 2002 population cohort. In 2017, the samples were sent for sequencing as follow-up.
Of note, the study excluded people with prevalent diabetes at baseline, including those being treated with antidiabetic drugs such as metformin.
Four species, two clusters associated with type 2 diabetes development
Over a median follow-up of 15.8 years, 432 (7.8%) participants went on to have a diagnosis of type 2 diabetes, and the presence of four species and two clusters at baseline were significantly associated with the development of type 2 diabetes.
The four species include Clostridium citroniae (hazard ratio, 1.21; unadjusted P = .02), C. bolteae (HR, 1.20; unadjusted P = .01), Tyzzerella nexilis (HR, 1.17; unadjusted P = .03), and Ruminococcus gnavus (HR, 1.17; P = .04).
And the two positively associated clusters mostly consisted of the same species (both HR, 1.18).
Importantly, the associations were nearly the same among participants in eastern and western Finland, which are known for having unique genetic as well as lifestyle differences that impact morbidity and mortality.
“Three of these taxa could be clustered together by proportional abundance in both geographic areas, and combined abundance of the four taxa was also predictive of incident type 2 diabetes,” the authors wrote.
They noted that the identified species have been previously associated with type 2 diabetes and appear to be linked in some ways to the quality of diet and with other metabolic diseases, such as fatty liver disease.
C. citroniae, for instance, has been associated with trimethylamine N-oxide (TMAO), a compound likely linked to the intake of red meat, and the authors noted that a direct association between red meat intake and type 2 diabetes risk has been known for more than 15 years.
TMAO has also been associated with adipose tissue inflammation and impeded hepatic insulin signaling, which are all involved in increased insulin resistance, high blood glucose levels, and type 2 diabetes, the authors explained.
R. gnavus has been previously associated with obesity in humans and animals. And the bacterial species is also “potentially related to glucose metabolism regulation and linked to increases in inflammatory cytokines, both of which are related to type 2 diabetes pathophysiology,” the authors reported.
Stepping stone toward improved prediction
Coauthor Teemu J. Niiranen, MD, PhD, of the division of medicine, Turku (Finland) University Hospital, noted that, while prior studies have linked type 2 diabetes with distinctive characteristics of gut microbiome composition, most studies have not included prospective data, and long-term studies have been lacking.
Furthermore, many of the studies could have been confounded by the use of antidiabetic drugs that could influence gut microbiome composition, including metformin, which was excluded in the current study.
“We avoid several of the biases related to cross-sectional studies, such as the confounding effects of diabetes medications,” Dr. Niiranen said in an interview.
“We also know the temporal sequence of the exposure and the outcome, and that the changes in the gut microbiome preceded the development of diabetes,” he said. “All in all, a cohort study like this provides a much greater level of evidence than cross-sectional studies.”
Dr. Niiranen noted, however, that “although we demonstrate that certain gut microbiome changes are associated with greater risk of future diabetes, we are still quite far from clinical use.”
In addition to needing to replicate the results in other ethnic groups and locations, “we would need to find optimal clinical cutoffs for clinical decision-making and demonstrate the amount increase in predictive ability, compared with conventional diabetes risk factors,” he said.
The study nevertheless “serves as a stepping stone toward the goal of improved prediction and the development of effective treatments for type 2 diabetes through modification of the gut microbiome,” the authors wrote.
Other research has shed light on gut bacteria that appear to be linked to the prevention rather than the development of diabetes, identifying species that help produce butyrate, a short-chain fatty acid that may in fact provide protection against type 2 diabetes.
And additional research does suggest potential clinical implications. Efforts to improve insulin sensitivity via the gut through fecal microbial transplantation are also making headway, with an oral capsule formulation showing benefit among patients with severe obesity.
The research was funded in part by grants from the Finnish Cultural Foundation, the Finnish Foundation for Cardiovascular Research, the Emil Aaltonen Foundation, the Finnish Medical Foundation, the Sigrid Jusélius Foundation, and the Academy of Finland.
A version of this article first appeared on Medscape.com.
according to results from a 15-year follow-up study of more than 5,000 people in Finland.
“We are not aware of previous long-term prospective studies of the associations between type 2 diabetes and the gut microbiome similar to the current study,” stated the authors of the study, published online Jan. 31, 2022, in Diabetes Care.
Though requiring further validation, the results “build on and extend previous mainly cross-sectional evidence and further support links between dietary habits, metabolic diseases, and type 2 diabetes that are modulated by the gut microbiome,” the authors wrote.
The findings are from a prospective study of data on fecal samples from 5,572 people in Finland in 2002 in the FINRISK 2002 population cohort. In 2017, the samples were sent for sequencing as follow-up.
Of note, the study excluded people with prevalent diabetes at baseline, including those being treated with antidiabetic drugs such as metformin.
Four species, two clusters associated with type 2 diabetes development
Over a median follow-up of 15.8 years, 432 (7.8%) participants went on to have a diagnosis of type 2 diabetes, and the presence of four species and two clusters at baseline were significantly associated with the development of type 2 diabetes.
The four species include Clostridium citroniae (hazard ratio, 1.21; unadjusted P = .02), C. bolteae (HR, 1.20; unadjusted P = .01), Tyzzerella nexilis (HR, 1.17; unadjusted P = .03), and Ruminococcus gnavus (HR, 1.17; P = .04).
And the two positively associated clusters mostly consisted of the same species (both HR, 1.18).
Importantly, the associations were nearly the same among participants in eastern and western Finland, which are known for having unique genetic as well as lifestyle differences that impact morbidity and mortality.
“Three of these taxa could be clustered together by proportional abundance in both geographic areas, and combined abundance of the four taxa was also predictive of incident type 2 diabetes,” the authors wrote.
They noted that the identified species have been previously associated with type 2 diabetes and appear to be linked in some ways to the quality of diet and with other metabolic diseases, such as fatty liver disease.
C. citroniae, for instance, has been associated with trimethylamine N-oxide (TMAO), a compound likely linked to the intake of red meat, and the authors noted that a direct association between red meat intake and type 2 diabetes risk has been known for more than 15 years.
TMAO has also been associated with adipose tissue inflammation and impeded hepatic insulin signaling, which are all involved in increased insulin resistance, high blood glucose levels, and type 2 diabetes, the authors explained.
R. gnavus has been previously associated with obesity in humans and animals. And the bacterial species is also “potentially related to glucose metabolism regulation and linked to increases in inflammatory cytokines, both of which are related to type 2 diabetes pathophysiology,” the authors reported.
Stepping stone toward improved prediction
Coauthor Teemu J. Niiranen, MD, PhD, of the division of medicine, Turku (Finland) University Hospital, noted that, while prior studies have linked type 2 diabetes with distinctive characteristics of gut microbiome composition, most studies have not included prospective data, and long-term studies have been lacking.
Furthermore, many of the studies could have been confounded by the use of antidiabetic drugs that could influence gut microbiome composition, including metformin, which was excluded in the current study.
“We avoid several of the biases related to cross-sectional studies, such as the confounding effects of diabetes medications,” Dr. Niiranen said in an interview.
“We also know the temporal sequence of the exposure and the outcome, and that the changes in the gut microbiome preceded the development of diabetes,” he said. “All in all, a cohort study like this provides a much greater level of evidence than cross-sectional studies.”
Dr. Niiranen noted, however, that “although we demonstrate that certain gut microbiome changes are associated with greater risk of future diabetes, we are still quite far from clinical use.”
In addition to needing to replicate the results in other ethnic groups and locations, “we would need to find optimal clinical cutoffs for clinical decision-making and demonstrate the amount increase in predictive ability, compared with conventional diabetes risk factors,” he said.
The study nevertheless “serves as a stepping stone toward the goal of improved prediction and the development of effective treatments for type 2 diabetes through modification of the gut microbiome,” the authors wrote.
Other research has shed light on gut bacteria that appear to be linked to the prevention rather than the development of diabetes, identifying species that help produce butyrate, a short-chain fatty acid that may in fact provide protection against type 2 diabetes.
And additional research does suggest potential clinical implications. Efforts to improve insulin sensitivity via the gut through fecal microbial transplantation are also making headway, with an oral capsule formulation showing benefit among patients with severe obesity.
The research was funded in part by grants from the Finnish Cultural Foundation, the Finnish Foundation for Cardiovascular Research, the Emil Aaltonen Foundation, the Finnish Medical Foundation, the Sigrid Jusélius Foundation, and the Academy of Finland.
A version of this article first appeared on Medscape.com.
according to results from a 15-year follow-up study of more than 5,000 people in Finland.
“We are not aware of previous long-term prospective studies of the associations between type 2 diabetes and the gut microbiome similar to the current study,” stated the authors of the study, published online Jan. 31, 2022, in Diabetes Care.
Though requiring further validation, the results “build on and extend previous mainly cross-sectional evidence and further support links between dietary habits, metabolic diseases, and type 2 diabetes that are modulated by the gut microbiome,” the authors wrote.
The findings are from a prospective study of data on fecal samples from 5,572 people in Finland in 2002 in the FINRISK 2002 population cohort. In 2017, the samples were sent for sequencing as follow-up.
Of note, the study excluded people with prevalent diabetes at baseline, including those being treated with antidiabetic drugs such as metformin.
Four species, two clusters associated with type 2 diabetes development
Over a median follow-up of 15.8 years, 432 (7.8%) participants went on to have a diagnosis of type 2 diabetes, and the presence of four species and two clusters at baseline were significantly associated with the development of type 2 diabetes.
The four species include Clostridium citroniae (hazard ratio, 1.21; unadjusted P = .02), C. bolteae (HR, 1.20; unadjusted P = .01), Tyzzerella nexilis (HR, 1.17; unadjusted P = .03), and Ruminococcus gnavus (HR, 1.17; P = .04).
And the two positively associated clusters mostly consisted of the same species (both HR, 1.18).
Importantly, the associations were nearly the same among participants in eastern and western Finland, which are known for having unique genetic as well as lifestyle differences that impact morbidity and mortality.
“Three of these taxa could be clustered together by proportional abundance in both geographic areas, and combined abundance of the four taxa was also predictive of incident type 2 diabetes,” the authors wrote.
They noted that the identified species have been previously associated with type 2 diabetes and appear to be linked in some ways to the quality of diet and with other metabolic diseases, such as fatty liver disease.
C. citroniae, for instance, has been associated with trimethylamine N-oxide (TMAO), a compound likely linked to the intake of red meat, and the authors noted that a direct association between red meat intake and type 2 diabetes risk has been known for more than 15 years.
TMAO has also been associated with adipose tissue inflammation and impeded hepatic insulin signaling, which are all involved in increased insulin resistance, high blood glucose levels, and type 2 diabetes, the authors explained.
R. gnavus has been previously associated with obesity in humans and animals. And the bacterial species is also “potentially related to glucose metabolism regulation and linked to increases in inflammatory cytokines, both of which are related to type 2 diabetes pathophysiology,” the authors reported.
Stepping stone toward improved prediction
Coauthor Teemu J. Niiranen, MD, PhD, of the division of medicine, Turku (Finland) University Hospital, noted that, while prior studies have linked type 2 diabetes with distinctive characteristics of gut microbiome composition, most studies have not included prospective data, and long-term studies have been lacking.
Furthermore, many of the studies could have been confounded by the use of antidiabetic drugs that could influence gut microbiome composition, including metformin, which was excluded in the current study.
“We avoid several of the biases related to cross-sectional studies, such as the confounding effects of diabetes medications,” Dr. Niiranen said in an interview.
“We also know the temporal sequence of the exposure and the outcome, and that the changes in the gut microbiome preceded the development of diabetes,” he said. “All in all, a cohort study like this provides a much greater level of evidence than cross-sectional studies.”
Dr. Niiranen noted, however, that “although we demonstrate that certain gut microbiome changes are associated with greater risk of future diabetes, we are still quite far from clinical use.”
In addition to needing to replicate the results in other ethnic groups and locations, “we would need to find optimal clinical cutoffs for clinical decision-making and demonstrate the amount increase in predictive ability, compared with conventional diabetes risk factors,” he said.
The study nevertheless “serves as a stepping stone toward the goal of improved prediction and the development of effective treatments for type 2 diabetes through modification of the gut microbiome,” the authors wrote.
Other research has shed light on gut bacteria that appear to be linked to the prevention rather than the development of diabetes, identifying species that help produce butyrate, a short-chain fatty acid that may in fact provide protection against type 2 diabetes.
And additional research does suggest potential clinical implications. Efforts to improve insulin sensitivity via the gut through fecal microbial transplantation are also making headway, with an oral capsule formulation showing benefit among patients with severe obesity.
The research was funded in part by grants from the Finnish Cultural Foundation, the Finnish Foundation for Cardiovascular Research, the Emil Aaltonen Foundation, the Finnish Medical Foundation, the Sigrid Jusélius Foundation, and the Academy of Finland.
A version of this article first appeared on Medscape.com.
FROM DIABETES CARE
Double-dose COVID-19 vaccines showed limited effectiveness against Omicron
, as determined on the basis of data from more than 800,000 Omicron-infected individuals.
Early laboratory data suggested a substantially lower neutralizing antibody response to the Omicron variant, compared with both the original COVID-19 strain and the Delta variant, write Nick Andrews, PhD, of the United Kingdom Health Security Agency, London, and colleagues.
Vaccines have shown high levels of effectiveness against symptomatic disease and severe disease and death resulting from the original COVID-19 virus and the Alpha variant and modest effectiveness against the Beta and Delta variants, they say.
“Neutralizing antibodies correlate with protection against reinfection and vaccine effectiveness against infection; therefore, reduced vaccine effectiveness against the omicron variant is anticipated on the basis of these early laboratory findings,” they explain.
In a study published in the New England Journal of Medicine, the researchers identified 886,774 adults aged 18 years and older who had been infected with the Omicron variant, 204,154 who had been infected with the Delta variant, and 1,572,621 symptomatic control patients who tested negative for COVID-19 between Nov. 27, 2021, and Jan. 12, 2022. The participants had been vaccinated with two doses of BNT162b2 (Pfizer–BioNTech), ChAdOx1 nCoV-19 (AstraZeneca), or mRNA-1273 (Moderna) vaccine, plus a booster given at least 175 days after a second dose, after Sept. 13, 2021.
Vaccine effectiveness was calculated after primary immunization at weeks 2-4, 5-9, 10-14, 15-19, 20-24, and 25 or longer after the second dose, and at 2-4, 5-9, and 10 or more weeks after boosters.
Omicron infections that occurred starting 14 or more days after a booster occurred a median of 39 days after the booster.
“Vaccine effectiveness was lower for the Omicron variant than for the Delta variant at all intervals after vaccination and for all combinations of primary courses and booster doses investigated,” the researchers write.
Individuals who received two doses of ChAdOx1 nCoV-19 had almost no protection against symptomatic disease caused by Omicron from 20-24 weeks after the second dose. For individuals who received two doses of BNT162b2, effectiveness was 65.5% 2-4 weeks after the second dose, but effectiveness declined to 15.4% after 15-19 weeks and to 8.8% after 25 or more weeks. For individuals who received two doses of mRNA-1273, vaccine effectiveness was 75.1% after 2-4 weeks, but effectiveness declined to 14.9% after 25 or more weeks.
Boosters created a short-term improvement in vaccine effectiveness against the Omicron variant, but this effect also declined over time.
Among individuals who received primary doses of ChAdOx1 nCoV-19, vaccine effectiveness increased to 62.4% 2-4 weeks after a BNT162b2 booster, then declined to 39.6% after 10 or more weeks. After an mRNA-1273 booster, vaccine effectiveness increased to 70.1% at 2-4 weeks and decreased to 60.9% at 5-9 weeks.
Among individuals who received primary doses of BNT162b2, vaccine effectiveness increased to 67.2% 2-4 weeks after a BNT162b2 booster, then declined to 45.7% at 10 or more weeks. After an mRNA-1273 booster, vaccine effectiveness increased to 73.9% at 2-4 weeks, then declined to 64.4% at 5-9 weeks.
Among individuals who received primary doses of mRNA-1273, vaccine effectiveness increased to 64.9% 2-4 weeks after a BNT162b2 booster and 66.3% 2-4 weeks after an mRNA-1273 booster.
The study findings were limited by potential confounding from study participants who had traveled and may have had different levels of vaccine coverage and by the inability to break down estimates on the basis of age and clinical risk that might affect vaccine effectiveness, the researchers note. Other limitations include a lack of data on vaccine effectiveness for a longer period after boosters, they say.
However, the results are consistent with neutralization data for the Omicron variant in studies from the United Kingdom, South Africa, and Germany, they write. “Our findings support maximizing coverage with third doses of vaccine in highly vaccinated populations such as in the United Kingdom. Further follow-up will be needed to assess protection against severe disease and the duration of protection after booster vaccination,” they conclude.
Focus on severe disease prevention
Paul Offit, MD, of the University of Pennsylvania, Philadelphia, addressed the topic of vaccine effectiveness in an op-ed published on March 4 in The Philadelphia Inquirer. The following is adapted from the op-ed, with his permission.
“The goal of the COVID vaccine – as is true for all vaccines – is to prevent serious illness,” Dr. Offit wrote.
“For most people with normal immune systems, two doses of mRNA vaccines appear to do exactly that. But not everyone,” wrote Dr. Offit, who serves as director of the Vaccine Education Center at the Children’s Hospital of Philadelphia and also serves on the Food and Drug Administration’s Vaccine Advisory Committee. “Three doses are required to induce high levels of protection against serious illness for people over 65 years of age or for people with other conditions that make them vulnerable, which can be anything from being overweight to having cancer. For people who are immune compromised, four doses might be required,” he noted.
Frequent vaccine boosting, although it may help prevent milder cases of COVID-19, such as those seen with the Omicron variant, is impractical, Dr. Offit emphasized. Instead, a newer, variant-specific vaccine might be needed if a variant emerges that overrides the protection against severe disease currently afforded by the available vaccines, he said. “But we’re not there yet. For now, we are going to have to realize that it is virtually impossible to prevent mild COVID without frequent boosting. So, let’s learn to accept that the goal of COVID vaccines is to prevent severe and not mild illness and stop talking about frequent boosting. Otherwise, we will never be able to live our lives as before,” he wrote.
The study was supported by the U.K. Health Security Agency. The researchers and Dr. Offit have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, as determined on the basis of data from more than 800,000 Omicron-infected individuals.
Early laboratory data suggested a substantially lower neutralizing antibody response to the Omicron variant, compared with both the original COVID-19 strain and the Delta variant, write Nick Andrews, PhD, of the United Kingdom Health Security Agency, London, and colleagues.
Vaccines have shown high levels of effectiveness against symptomatic disease and severe disease and death resulting from the original COVID-19 virus and the Alpha variant and modest effectiveness against the Beta and Delta variants, they say.
“Neutralizing antibodies correlate with protection against reinfection and vaccine effectiveness against infection; therefore, reduced vaccine effectiveness against the omicron variant is anticipated on the basis of these early laboratory findings,” they explain.
In a study published in the New England Journal of Medicine, the researchers identified 886,774 adults aged 18 years and older who had been infected with the Omicron variant, 204,154 who had been infected with the Delta variant, and 1,572,621 symptomatic control patients who tested negative for COVID-19 between Nov. 27, 2021, and Jan. 12, 2022. The participants had been vaccinated with two doses of BNT162b2 (Pfizer–BioNTech), ChAdOx1 nCoV-19 (AstraZeneca), or mRNA-1273 (Moderna) vaccine, plus a booster given at least 175 days after a second dose, after Sept. 13, 2021.
Vaccine effectiveness was calculated after primary immunization at weeks 2-4, 5-9, 10-14, 15-19, 20-24, and 25 or longer after the second dose, and at 2-4, 5-9, and 10 or more weeks after boosters.
Omicron infections that occurred starting 14 or more days after a booster occurred a median of 39 days after the booster.
“Vaccine effectiveness was lower for the Omicron variant than for the Delta variant at all intervals after vaccination and for all combinations of primary courses and booster doses investigated,” the researchers write.
Individuals who received two doses of ChAdOx1 nCoV-19 had almost no protection against symptomatic disease caused by Omicron from 20-24 weeks after the second dose. For individuals who received two doses of BNT162b2, effectiveness was 65.5% 2-4 weeks after the second dose, but effectiveness declined to 15.4% after 15-19 weeks and to 8.8% after 25 or more weeks. For individuals who received two doses of mRNA-1273, vaccine effectiveness was 75.1% after 2-4 weeks, but effectiveness declined to 14.9% after 25 or more weeks.
Boosters created a short-term improvement in vaccine effectiveness against the Omicron variant, but this effect also declined over time.
Among individuals who received primary doses of ChAdOx1 nCoV-19, vaccine effectiveness increased to 62.4% 2-4 weeks after a BNT162b2 booster, then declined to 39.6% after 10 or more weeks. After an mRNA-1273 booster, vaccine effectiveness increased to 70.1% at 2-4 weeks and decreased to 60.9% at 5-9 weeks.
Among individuals who received primary doses of BNT162b2, vaccine effectiveness increased to 67.2% 2-4 weeks after a BNT162b2 booster, then declined to 45.7% at 10 or more weeks. After an mRNA-1273 booster, vaccine effectiveness increased to 73.9% at 2-4 weeks, then declined to 64.4% at 5-9 weeks.
Among individuals who received primary doses of mRNA-1273, vaccine effectiveness increased to 64.9% 2-4 weeks after a BNT162b2 booster and 66.3% 2-4 weeks after an mRNA-1273 booster.
The study findings were limited by potential confounding from study participants who had traveled and may have had different levels of vaccine coverage and by the inability to break down estimates on the basis of age and clinical risk that might affect vaccine effectiveness, the researchers note. Other limitations include a lack of data on vaccine effectiveness for a longer period after boosters, they say.
However, the results are consistent with neutralization data for the Omicron variant in studies from the United Kingdom, South Africa, and Germany, they write. “Our findings support maximizing coverage with third doses of vaccine in highly vaccinated populations such as in the United Kingdom. Further follow-up will be needed to assess protection against severe disease and the duration of protection after booster vaccination,” they conclude.
Focus on severe disease prevention
Paul Offit, MD, of the University of Pennsylvania, Philadelphia, addressed the topic of vaccine effectiveness in an op-ed published on March 4 in The Philadelphia Inquirer. The following is adapted from the op-ed, with his permission.
“The goal of the COVID vaccine – as is true for all vaccines – is to prevent serious illness,” Dr. Offit wrote.
“For most people with normal immune systems, two doses of mRNA vaccines appear to do exactly that. But not everyone,” wrote Dr. Offit, who serves as director of the Vaccine Education Center at the Children’s Hospital of Philadelphia and also serves on the Food and Drug Administration’s Vaccine Advisory Committee. “Three doses are required to induce high levels of protection against serious illness for people over 65 years of age or for people with other conditions that make them vulnerable, which can be anything from being overweight to having cancer. For people who are immune compromised, four doses might be required,” he noted.
Frequent vaccine boosting, although it may help prevent milder cases of COVID-19, such as those seen with the Omicron variant, is impractical, Dr. Offit emphasized. Instead, a newer, variant-specific vaccine might be needed if a variant emerges that overrides the protection against severe disease currently afforded by the available vaccines, he said. “But we’re not there yet. For now, we are going to have to realize that it is virtually impossible to prevent mild COVID without frequent boosting. So, let’s learn to accept that the goal of COVID vaccines is to prevent severe and not mild illness and stop talking about frequent boosting. Otherwise, we will never be able to live our lives as before,” he wrote.
The study was supported by the U.K. Health Security Agency. The researchers and Dr. Offit have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, as determined on the basis of data from more than 800,000 Omicron-infected individuals.
Early laboratory data suggested a substantially lower neutralizing antibody response to the Omicron variant, compared with both the original COVID-19 strain and the Delta variant, write Nick Andrews, PhD, of the United Kingdom Health Security Agency, London, and colleagues.
Vaccines have shown high levels of effectiveness against symptomatic disease and severe disease and death resulting from the original COVID-19 virus and the Alpha variant and modest effectiveness against the Beta and Delta variants, they say.
“Neutralizing antibodies correlate with protection against reinfection and vaccine effectiveness against infection; therefore, reduced vaccine effectiveness against the omicron variant is anticipated on the basis of these early laboratory findings,” they explain.
In a study published in the New England Journal of Medicine, the researchers identified 886,774 adults aged 18 years and older who had been infected with the Omicron variant, 204,154 who had been infected with the Delta variant, and 1,572,621 symptomatic control patients who tested negative for COVID-19 between Nov. 27, 2021, and Jan. 12, 2022. The participants had been vaccinated with two doses of BNT162b2 (Pfizer–BioNTech), ChAdOx1 nCoV-19 (AstraZeneca), or mRNA-1273 (Moderna) vaccine, plus a booster given at least 175 days after a second dose, after Sept. 13, 2021.
Vaccine effectiveness was calculated after primary immunization at weeks 2-4, 5-9, 10-14, 15-19, 20-24, and 25 or longer after the second dose, and at 2-4, 5-9, and 10 or more weeks after boosters.
Omicron infections that occurred starting 14 or more days after a booster occurred a median of 39 days after the booster.
“Vaccine effectiveness was lower for the Omicron variant than for the Delta variant at all intervals after vaccination and for all combinations of primary courses and booster doses investigated,” the researchers write.
Individuals who received two doses of ChAdOx1 nCoV-19 had almost no protection against symptomatic disease caused by Omicron from 20-24 weeks after the second dose. For individuals who received two doses of BNT162b2, effectiveness was 65.5% 2-4 weeks after the second dose, but effectiveness declined to 15.4% after 15-19 weeks and to 8.8% after 25 or more weeks. For individuals who received two doses of mRNA-1273, vaccine effectiveness was 75.1% after 2-4 weeks, but effectiveness declined to 14.9% after 25 or more weeks.
Boosters created a short-term improvement in vaccine effectiveness against the Omicron variant, but this effect also declined over time.
Among individuals who received primary doses of ChAdOx1 nCoV-19, vaccine effectiveness increased to 62.4% 2-4 weeks after a BNT162b2 booster, then declined to 39.6% after 10 or more weeks. After an mRNA-1273 booster, vaccine effectiveness increased to 70.1% at 2-4 weeks and decreased to 60.9% at 5-9 weeks.
Among individuals who received primary doses of BNT162b2, vaccine effectiveness increased to 67.2% 2-4 weeks after a BNT162b2 booster, then declined to 45.7% at 10 or more weeks. After an mRNA-1273 booster, vaccine effectiveness increased to 73.9% at 2-4 weeks, then declined to 64.4% at 5-9 weeks.
Among individuals who received primary doses of mRNA-1273, vaccine effectiveness increased to 64.9% 2-4 weeks after a BNT162b2 booster and 66.3% 2-4 weeks after an mRNA-1273 booster.
The study findings were limited by potential confounding from study participants who had traveled and may have had different levels of vaccine coverage and by the inability to break down estimates on the basis of age and clinical risk that might affect vaccine effectiveness, the researchers note. Other limitations include a lack of data on vaccine effectiveness for a longer period after boosters, they say.
However, the results are consistent with neutralization data for the Omicron variant in studies from the United Kingdom, South Africa, and Germany, they write. “Our findings support maximizing coverage with third doses of vaccine in highly vaccinated populations such as in the United Kingdom. Further follow-up will be needed to assess protection against severe disease and the duration of protection after booster vaccination,” they conclude.
Focus on severe disease prevention
Paul Offit, MD, of the University of Pennsylvania, Philadelphia, addressed the topic of vaccine effectiveness in an op-ed published on March 4 in The Philadelphia Inquirer. The following is adapted from the op-ed, with his permission.
“The goal of the COVID vaccine – as is true for all vaccines – is to prevent serious illness,” Dr. Offit wrote.
“For most people with normal immune systems, two doses of mRNA vaccines appear to do exactly that. But not everyone,” wrote Dr. Offit, who serves as director of the Vaccine Education Center at the Children’s Hospital of Philadelphia and also serves on the Food and Drug Administration’s Vaccine Advisory Committee. “Three doses are required to induce high levels of protection against serious illness for people over 65 years of age or for people with other conditions that make them vulnerable, which can be anything from being overweight to having cancer. For people who are immune compromised, four doses might be required,” he noted.
Frequent vaccine boosting, although it may help prevent milder cases of COVID-19, such as those seen with the Omicron variant, is impractical, Dr. Offit emphasized. Instead, a newer, variant-specific vaccine might be needed if a variant emerges that overrides the protection against severe disease currently afforded by the available vaccines, he said. “But we’re not there yet. For now, we are going to have to realize that it is virtually impossible to prevent mild COVID without frequent boosting. So, let’s learn to accept that the goal of COVID vaccines is to prevent severe and not mild illness and stop talking about frequent boosting. Otherwise, we will never be able to live our lives as before,” he wrote.
The study was supported by the U.K. Health Security Agency. The researchers and Dr. Offit have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Does adjunctive oxytocin infusion during balloon cervical ripening improve labor induction?
Evidence summary
Time to delivery is shortened with combined therapy
Two recent high-quality meta-analyses investigated the effect of adding oxytocin to transcervical Foley balloon placement for cervical dilation. A network meta-analysis, including 30 RCTs (with 6465 pregnant patients), examined the efficacy of multiple combinations of cervical ripening methods.1 A subset of 7 trials (n = 1313) compared oxytocin infusion with transcervical Foley (inflated to 30-60 mL) to Foley alone. Patients were at > 24 weeks’ gestation with a live fetus and undergoing elective or medical induction of labor; exclusion criteria were standard contraindications to vaginal delivery.
Compared to Foley alone, Foley plus oxytocin reduced both the time to the primary outcome of vaginal delivery (mean duration [MD] = –4.2 h; 95% CI, –1.9 to –6.5) and the time to overall (vaginal and cesarean) delivery (MD = –3.1 h; 95% CI, –1.5 to –4.6). There were no differences in rates of cesarean section, chorioamnionitis, epidural use, or neonatal intensive care unit admission. This analysis did not stratify by parity.1
In a standard meta-analysis, researchers identified 6 RCTs (N = 1133) comparing transcervical Foley balloon and oxytocin to Foley balloon alone for cervical ripening in pregnant patients at > 23 weeks’ gestation (1 trial was limited to patients at > 37 weeks’ gestation).2 Foley balloons were inflated with 30 to 60 mL saline, and oxytocin infusions started at 1 to 2 mU/min and were titrated up to 10 to 40 mU/min. Balloon time was usually 12 hours, but not always stated.
The authors found no statistically significant difference in cesarean rates (the primary outcome) between Foley plus oxytocin vs Foley alone (relative risk [RR] = 0.91; 95% CI, 0.76-1.1). Overall delivery within 12 hours was more likely with combined therapy (RR of remaining pregnant = 0.46; 95% CI, 0.34-0.63), but delivery at 24 hours was not (RR = 0.94; 95% CI, 0.92-1.05). However, in a sub-analysis by parity, nulliparous women who received combined therapy had higher overall delivery rates in 24 hours than did multiparous women (RR = 0.77; 95% CI, 0.62-0.97).2
Adding oxytocin may allow shorter transcervical balloon times
One recent RCT (N = 177) compared labor induction with oxytocin and a single trans-cervical balloon (Cook catheter with only the intrauterine balloon inflated) removed at either 6 or 12 hours.3 Patients were pregnant women (mean age, 31 years) with a term singleton vertex pregnancy, a Bishop score ≤ 6, and no contraindications to vaginal delivery. All patients received a balloon inflated to 60 mL with an oxytocin infusion (2-30 mU/min). The intervention group had the balloon removed at 6 hours, while the control group had it removed at 12 hours.
The mean Bishop score changed by 6 points in each group. Time to overall delivery (the primary outcome) was significantly shorter with 6 hours of balloon time than with 12 hours (19.2 vs 24.3 h; P < .04). Overall delivery within 24 hours was also significantly more likely in the 6-hour group (67.4% vs 47.4%; P < .01), although vaginal delivery in 24 hours did not change (74% vs 59%; P = .07). No differences were seen in cesarean delivery rates or maternal or neonatal morbidity rates.
A look at fixed-dose vs titrated oxytocin
Another RCT (N = 116) examined the effectiveness of cervical ripening using a Foley balloon plus either fixed-dose or titrated low-dose oxytocin.4 Patients (mean age, 26 years) had singleton pregnancies at ≥ 37 weeks’ gestation with a Bishop score < 6 and presented for induction of labor. Foley balloons were inflated to 30 mL, and patients received either a fixed oxytocin infusion of 2 mU/min or a titrated infusion starting at 1 mU/min, increasing by 2 mU/min every 30 minutes to a maximum of 20 mU/min.
Continue to: Thre was no statistically...
There was no statistically significant difference in median time from Foley placement to overall delivery (the primary outcome) between the fixed low-dose and incremental low-dose groups in either nulliparous women (24 vs 19 h; P = .18) or multiparous women (16 vs 12 h; P = .68). The authors acknowledged the study may have been underpowered to detect a true difference.
Recommendations from others
A 2009 Practice Bulletin from the American College of Obstetricians and Gynecologists (ACOG) recommended the Foley catheter as a reasonable and effective alternative to prostaglandins for cervical ripening and the induction of labor (based on good-quality evidence).5 The guideline stated that Foley catheter placement before oxytocin induction reduced both the duration of labor and risk of cesarean delivery, but that the use of oxytocin along with a Foley catheter did not appear to shorten the time to delivery.
Editor’s takeaway
High-quality evidence shows us that the addition of oxytocin to balloon cervical ripening shortens the time to delivery. This newer evidence may prompt an update to the 2009 ACOG statement.
1. Orr L, Reisinger-Kindle K, Roy A, et al. Combination of Foley and prostaglandins versus Foley and oxytocin for cervical ripening: a network meta-analysis. Am J Obstet Gynecol. 2020;223:743.e1-743.e17. doi: 10.1016/j.ajog.2020.05.007
2. Gallagher LT, Gardner B, Rahman M, et al. Cervical ripening using Foley balloon with or without oxytocin: a systematic review and meta-analysis. Am J Perinatol. 2019;36:406-421. doi: 10.1055/s-0038-1668577
3. Lassey SC, Haber HR, Kanbergs A, et al. Six vs twelve hours of single balloon catheter placement with oxytocin administration for labor induction: a randomized controlled trial. Am J Obstet Gynecol. 2021:S0002-9378(21)00185-X. doi: 10.1016/j.ajog.2021.03.021
4. Fitzpatrick CB, Grotegut CA, Bishop TS, et al. Cervical ripening with Foley balloon plus fixed versus incremental low-dose oxytocin: a randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25:1006-1010. doi: 10.3109/14767058.2011.607522
5. ACOG Practice Bulletin No. 107: Induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386-397. doi: 10.1097/AOG.0b013e3181b48ef5
Evidence summary
Time to delivery is shortened with combined therapy
Two recent high-quality meta-analyses investigated the effect of adding oxytocin to transcervical Foley balloon placement for cervical dilation. A network meta-analysis, including 30 RCTs (with 6465 pregnant patients), examined the efficacy of multiple combinations of cervical ripening methods.1 A subset of 7 trials (n = 1313) compared oxytocin infusion with transcervical Foley (inflated to 30-60 mL) to Foley alone. Patients were at > 24 weeks’ gestation with a live fetus and undergoing elective or medical induction of labor; exclusion criteria were standard contraindications to vaginal delivery.
Compared to Foley alone, Foley plus oxytocin reduced both the time to the primary outcome of vaginal delivery (mean duration [MD] = –4.2 h; 95% CI, –1.9 to –6.5) and the time to overall (vaginal and cesarean) delivery (MD = –3.1 h; 95% CI, –1.5 to –4.6). There were no differences in rates of cesarean section, chorioamnionitis, epidural use, or neonatal intensive care unit admission. This analysis did not stratify by parity.1
In a standard meta-analysis, researchers identified 6 RCTs (N = 1133) comparing transcervical Foley balloon and oxytocin to Foley balloon alone for cervical ripening in pregnant patients at > 23 weeks’ gestation (1 trial was limited to patients at > 37 weeks’ gestation).2 Foley balloons were inflated with 30 to 60 mL saline, and oxytocin infusions started at 1 to 2 mU/min and were titrated up to 10 to 40 mU/min. Balloon time was usually 12 hours, but not always stated.
The authors found no statistically significant difference in cesarean rates (the primary outcome) between Foley plus oxytocin vs Foley alone (relative risk [RR] = 0.91; 95% CI, 0.76-1.1). Overall delivery within 12 hours was more likely with combined therapy (RR of remaining pregnant = 0.46; 95% CI, 0.34-0.63), but delivery at 24 hours was not (RR = 0.94; 95% CI, 0.92-1.05). However, in a sub-analysis by parity, nulliparous women who received combined therapy had higher overall delivery rates in 24 hours than did multiparous women (RR = 0.77; 95% CI, 0.62-0.97).2
Adding oxytocin may allow shorter transcervical balloon times
One recent RCT (N = 177) compared labor induction with oxytocin and a single trans-cervical balloon (Cook catheter with only the intrauterine balloon inflated) removed at either 6 or 12 hours.3 Patients were pregnant women (mean age, 31 years) with a term singleton vertex pregnancy, a Bishop score ≤ 6, and no contraindications to vaginal delivery. All patients received a balloon inflated to 60 mL with an oxytocin infusion (2-30 mU/min). The intervention group had the balloon removed at 6 hours, while the control group had it removed at 12 hours.
The mean Bishop score changed by 6 points in each group. Time to overall delivery (the primary outcome) was significantly shorter with 6 hours of balloon time than with 12 hours (19.2 vs 24.3 h; P < .04). Overall delivery within 24 hours was also significantly more likely in the 6-hour group (67.4% vs 47.4%; P < .01), although vaginal delivery in 24 hours did not change (74% vs 59%; P = .07). No differences were seen in cesarean delivery rates or maternal or neonatal morbidity rates.
A look at fixed-dose vs titrated oxytocin
Another RCT (N = 116) examined the effectiveness of cervical ripening using a Foley balloon plus either fixed-dose or titrated low-dose oxytocin.4 Patients (mean age, 26 years) had singleton pregnancies at ≥ 37 weeks’ gestation with a Bishop score < 6 and presented for induction of labor. Foley balloons were inflated to 30 mL, and patients received either a fixed oxytocin infusion of 2 mU/min or a titrated infusion starting at 1 mU/min, increasing by 2 mU/min every 30 minutes to a maximum of 20 mU/min.
Continue to: Thre was no statistically...
There was no statistically significant difference in median time from Foley placement to overall delivery (the primary outcome) between the fixed low-dose and incremental low-dose groups in either nulliparous women (24 vs 19 h; P = .18) or multiparous women (16 vs 12 h; P = .68). The authors acknowledged the study may have been underpowered to detect a true difference.
Recommendations from others
A 2009 Practice Bulletin from the American College of Obstetricians and Gynecologists (ACOG) recommended the Foley catheter as a reasonable and effective alternative to prostaglandins for cervical ripening and the induction of labor (based on good-quality evidence).5 The guideline stated that Foley catheter placement before oxytocin induction reduced both the duration of labor and risk of cesarean delivery, but that the use of oxytocin along with a Foley catheter did not appear to shorten the time to delivery.
Editor’s takeaway
High-quality evidence shows us that the addition of oxytocin to balloon cervical ripening shortens the time to delivery. This newer evidence may prompt an update to the 2009 ACOG statement.
Evidence summary
Time to delivery is shortened with combined therapy
Two recent high-quality meta-analyses investigated the effect of adding oxytocin to transcervical Foley balloon placement for cervical dilation. A network meta-analysis, including 30 RCTs (with 6465 pregnant patients), examined the efficacy of multiple combinations of cervical ripening methods.1 A subset of 7 trials (n = 1313) compared oxytocin infusion with transcervical Foley (inflated to 30-60 mL) to Foley alone. Patients were at > 24 weeks’ gestation with a live fetus and undergoing elective or medical induction of labor; exclusion criteria were standard contraindications to vaginal delivery.
Compared to Foley alone, Foley plus oxytocin reduced both the time to the primary outcome of vaginal delivery (mean duration [MD] = –4.2 h; 95% CI, –1.9 to –6.5) and the time to overall (vaginal and cesarean) delivery (MD = –3.1 h; 95% CI, –1.5 to –4.6). There were no differences in rates of cesarean section, chorioamnionitis, epidural use, or neonatal intensive care unit admission. This analysis did not stratify by parity.1
In a standard meta-analysis, researchers identified 6 RCTs (N = 1133) comparing transcervical Foley balloon and oxytocin to Foley balloon alone for cervical ripening in pregnant patients at > 23 weeks’ gestation (1 trial was limited to patients at > 37 weeks’ gestation).2 Foley balloons were inflated with 30 to 60 mL saline, and oxytocin infusions started at 1 to 2 mU/min and were titrated up to 10 to 40 mU/min. Balloon time was usually 12 hours, but not always stated.
The authors found no statistically significant difference in cesarean rates (the primary outcome) between Foley plus oxytocin vs Foley alone (relative risk [RR] = 0.91; 95% CI, 0.76-1.1). Overall delivery within 12 hours was more likely with combined therapy (RR of remaining pregnant = 0.46; 95% CI, 0.34-0.63), but delivery at 24 hours was not (RR = 0.94; 95% CI, 0.92-1.05). However, in a sub-analysis by parity, nulliparous women who received combined therapy had higher overall delivery rates in 24 hours than did multiparous women (RR = 0.77; 95% CI, 0.62-0.97).2
Adding oxytocin may allow shorter transcervical balloon times
One recent RCT (N = 177) compared labor induction with oxytocin and a single trans-cervical balloon (Cook catheter with only the intrauterine balloon inflated) removed at either 6 or 12 hours.3 Patients were pregnant women (mean age, 31 years) with a term singleton vertex pregnancy, a Bishop score ≤ 6, and no contraindications to vaginal delivery. All patients received a balloon inflated to 60 mL with an oxytocin infusion (2-30 mU/min). The intervention group had the balloon removed at 6 hours, while the control group had it removed at 12 hours.
The mean Bishop score changed by 6 points in each group. Time to overall delivery (the primary outcome) was significantly shorter with 6 hours of balloon time than with 12 hours (19.2 vs 24.3 h; P < .04). Overall delivery within 24 hours was also significantly more likely in the 6-hour group (67.4% vs 47.4%; P < .01), although vaginal delivery in 24 hours did not change (74% vs 59%; P = .07). No differences were seen in cesarean delivery rates or maternal or neonatal morbidity rates.
A look at fixed-dose vs titrated oxytocin
Another RCT (N = 116) examined the effectiveness of cervical ripening using a Foley balloon plus either fixed-dose or titrated low-dose oxytocin.4 Patients (mean age, 26 years) had singleton pregnancies at ≥ 37 weeks’ gestation with a Bishop score < 6 and presented for induction of labor. Foley balloons were inflated to 30 mL, and patients received either a fixed oxytocin infusion of 2 mU/min or a titrated infusion starting at 1 mU/min, increasing by 2 mU/min every 30 minutes to a maximum of 20 mU/min.
Continue to: Thre was no statistically...
There was no statistically significant difference in median time from Foley placement to overall delivery (the primary outcome) between the fixed low-dose and incremental low-dose groups in either nulliparous women (24 vs 19 h; P = .18) or multiparous women (16 vs 12 h; P = .68). The authors acknowledged the study may have been underpowered to detect a true difference.
Recommendations from others
A 2009 Practice Bulletin from the American College of Obstetricians and Gynecologists (ACOG) recommended the Foley catheter as a reasonable and effective alternative to prostaglandins for cervical ripening and the induction of labor (based on good-quality evidence).5 The guideline stated that Foley catheter placement before oxytocin induction reduced both the duration of labor and risk of cesarean delivery, but that the use of oxytocin along with a Foley catheter did not appear to shorten the time to delivery.
Editor’s takeaway
High-quality evidence shows us that the addition of oxytocin to balloon cervical ripening shortens the time to delivery. This newer evidence may prompt an update to the 2009 ACOG statement.
1. Orr L, Reisinger-Kindle K, Roy A, et al. Combination of Foley and prostaglandins versus Foley and oxytocin for cervical ripening: a network meta-analysis. Am J Obstet Gynecol. 2020;223:743.e1-743.e17. doi: 10.1016/j.ajog.2020.05.007
2. Gallagher LT, Gardner B, Rahman M, et al. Cervical ripening using Foley balloon with or without oxytocin: a systematic review and meta-analysis. Am J Perinatol. 2019;36:406-421. doi: 10.1055/s-0038-1668577
3. Lassey SC, Haber HR, Kanbergs A, et al. Six vs twelve hours of single balloon catheter placement with oxytocin administration for labor induction: a randomized controlled trial. Am J Obstet Gynecol. 2021:S0002-9378(21)00185-X. doi: 10.1016/j.ajog.2021.03.021
4. Fitzpatrick CB, Grotegut CA, Bishop TS, et al. Cervical ripening with Foley balloon plus fixed versus incremental low-dose oxytocin: a randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25:1006-1010. doi: 10.3109/14767058.2011.607522
5. ACOG Practice Bulletin No. 107: Induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386-397. doi: 10.1097/AOG.0b013e3181b48ef5
1. Orr L, Reisinger-Kindle K, Roy A, et al. Combination of Foley and prostaglandins versus Foley and oxytocin for cervical ripening: a network meta-analysis. Am J Obstet Gynecol. 2020;223:743.e1-743.e17. doi: 10.1016/j.ajog.2020.05.007
2. Gallagher LT, Gardner B, Rahman M, et al. Cervical ripening using Foley balloon with or without oxytocin: a systematic review and meta-analysis. Am J Perinatol. 2019;36:406-421. doi: 10.1055/s-0038-1668577
3. Lassey SC, Haber HR, Kanbergs A, et al. Six vs twelve hours of single balloon catheter placement with oxytocin administration for labor induction: a randomized controlled trial. Am J Obstet Gynecol. 2021:S0002-9378(21)00185-X. doi: 10.1016/j.ajog.2021.03.021
4. Fitzpatrick CB, Grotegut CA, Bishop TS, et al. Cervical ripening with Foley balloon plus fixed versus incremental low-dose oxytocin: a randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25:1006-1010. doi: 10.3109/14767058.2011.607522
5. ACOG Practice Bulletin No. 107: Induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386-397. doi: 10.1097/AOG.0b013e3181b48ef5
EVIDENCE-BASED ANSWER:
YES. Compared to the use of a transcervical balloon alone, combined cervical ripening with a balloon catheter and oxytocin shortens the time to overall delivery by 3 hours and the time to vaginal delivery by 4 hours, without altering the rate of cesarean section (strength of recommendation [SOR]: A, network meta-analysis). The effect is more pronounced in nulliparous patients (SOR: A, meta-analysis).
When combined therapy is used, 6 hours of balloon time may result in faster delivery than 12 hours (SOR: B, single randomized controlled trial [RCT]). Fixed-dose oxytocin and titrated oxytocin appear to have similar effect when combined with a cervical ripening balloon (SOR: C, underpowered RCT).
FDA committee recommends 2022-2023 influenza vaccine strains
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee has chosen the influenza vaccine strains for the 2022-2023 season in the northern hemisphere, which begins in the fall of 2022.
On March 3, the committee unanimously voted to endorse the World Health Organization’s recommendations as to which influenza strains to include for coverage by vaccines for the upcoming flu season. Two of the four recommended strains are different from last season.
The committee also heard updates on flu activity this season. So far, data from the U.S. Flu Vaccine Effectiveness (VE) network, which consists of seven study sites, have not shown that the vaccine is protective against influenza A. “We can say that it is not highly effective,” Brendan Flannery, PhD, who leads the U.S. Flu VE network for the Centers for Disease Control and Prevention, said in an interview. He was not involved with the advisory committee meeting. Flu activity this season has been low, he explained, so there are fewer cases his team can use to estimate vaccine efficacy. “If there’s some benefit, it’s hard for us to show that now,” he said.
Vaccine strains
The panel voted to include a A/Darwin/9/2021-like strain for the H3N2 component of the vaccine; this is changed from A/Cambodia/e0826360/2020. For the influenza B Victoria lineage component, the committee voted to include a B/Austria/1359417/2021-like virus, a swap from this year’s B/Washington/02/2019-like virus. These changes apply to the egg-based, cell-culture, and recombinant vaccines. Both new strains were included in WHO’s 2022 influenza vaccine strain recommendations for the southern hemisphere.
For the influenza A H1N1 component, the group also agreed to include a A/Victoria/2570/2019 (H1N1) pdm09-like virus for the egg-based vaccine and the A/Wisconsin/588/2019 (H1N1) pdm09-like virus for cell culture or recombinant vaccines. These strains were included for the 2021-2022 season. The panel also voted for the inclusion of a B/Phuket/3073/2013-like virus (B/Yamagata lineage) as the second influenza B strain for the quadrivalent egg-based, cell culture, or recombinant vaccines, which is unchanged from this flu season.
‘Sporadic’ flu activity
While there was an uptick in influenza activity this year compared to the 2020-2021 season, hospitalization rates are lower than in the four seasons preceding the pandemic (from 2016-2017 to 2019-2020). As of Feb. 26, the cumulative hospitalization rate for this flu season was 5.2 hospitalizations per 100,000 individuals. There have been eight pediatric deaths due to influenza so far this season, compared to one pediatric death reported to the CDC during the 2020-2021 flu season.
About 4.1% of specimens tested at clinical laboratories were positive for flu. Since Oct. 30, 2.7% of specimens have been positive for influenza this season. Nearly all viruses detected (97.7%) have been influenza A.
Lisa Grohskopf, MD, MPH, a medical officer in the influenza division at the CDC who presented the data at the meeting, described flu activity this season as “sporadic” and noted that activity is increasing in some areas of the country. According to CDC’s weekly influenza surveillance report, most states had minimal influenza-like illness (ILI) activity, although Arkansas, Idaho, Iowa, Kansas, Minnesota, and Utah had slightly higher ILI activity as of Feb. 26. Champaign-Urbana, Illinois; St. Cloud, Minnesota; and Brownwood, Texas, had the highest levels of flu activity in the country.
Low vaccine effectiveness
As of Jan. 22, results from the U.S. Flu VE network do not show statistically significant evidence that the flu vaccine is effective. Currently, the vaccine is estimated to be 8% effective against preventing influenza A infection (95% confidence interval, –31% to 36%) and 14% effective against preventing A/H3N2 infection (95% CI, –28% to 43%) for people aged 6 months and older.
The network did not have enough data to provide age-specific VE estimates or estimates of effectiveness against influenza B. This could be due to low flu activity relative to prepandemic years, Dr. Flannery said. Of the 2,758 individuals enrolled in the VE flu network this season, just 147 (5%) tested positive for the flu this season. This is the lowest positivity rate observed in the Flu VE network participants with respiratory illness over the past 10 flu seasons, Dr. Grohskopf noted. In comparison, estimates from the 2019 to 2020 season included 4,112 individuals, and 1,060 tested positive for flu.
“We are really at the bare minimum of what we can use for a flu vaccine effectiveness estimate,” Dr. Flannery said about the more recent data. The network was not able to produce any estimates about flu vaccine effectiveness for the 2020-2021 season because of historically low flu activity.
The Department of Defense also presented vaccine efficacy estimates for the 2021–2022 season. The vaccine has been 36% effective (95% CI, 28%-44%) against all strains of the virus, 33% effective against influenza A (95% CI, 24%-41%), 32% effective against A/H3N2 (95% CI, 3%-53%), and 59% effective against influenza B (95% CI, 42%-71%). These results are from a young, healthy adult population, Lieutenant Commander Courtney Gustin, DrPH, MSN, told the panel, and they may not be reflective of efficacy rates across all age groups.
Though these findings suggest there is low to no measurable benefit against influenza A, Dr. Flannery said the CDC still recommends getting the flu vaccine, as it can be protective against other circulating flu strains. “We have been able to demonstrate protection against other H3 [viruses], B viruses, and H1 viruses in the past,” he said. And as these results only show protection against mild disease, “there is still possibility that there’s benefit against more severe disease,” he added. Studies measuring effectiveness against more severe outcomes are not yet available.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee has chosen the influenza vaccine strains for the 2022-2023 season in the northern hemisphere, which begins in the fall of 2022.
On March 3, the committee unanimously voted to endorse the World Health Organization’s recommendations as to which influenza strains to include for coverage by vaccines for the upcoming flu season. Two of the four recommended strains are different from last season.
The committee also heard updates on flu activity this season. So far, data from the U.S. Flu Vaccine Effectiveness (VE) network, which consists of seven study sites, have not shown that the vaccine is protective against influenza A. “We can say that it is not highly effective,” Brendan Flannery, PhD, who leads the U.S. Flu VE network for the Centers for Disease Control and Prevention, said in an interview. He was not involved with the advisory committee meeting. Flu activity this season has been low, he explained, so there are fewer cases his team can use to estimate vaccine efficacy. “If there’s some benefit, it’s hard for us to show that now,” he said.
Vaccine strains
The panel voted to include a A/Darwin/9/2021-like strain for the H3N2 component of the vaccine; this is changed from A/Cambodia/e0826360/2020. For the influenza B Victoria lineage component, the committee voted to include a B/Austria/1359417/2021-like virus, a swap from this year’s B/Washington/02/2019-like virus. These changes apply to the egg-based, cell-culture, and recombinant vaccines. Both new strains were included in WHO’s 2022 influenza vaccine strain recommendations for the southern hemisphere.
For the influenza A H1N1 component, the group also agreed to include a A/Victoria/2570/2019 (H1N1) pdm09-like virus for the egg-based vaccine and the A/Wisconsin/588/2019 (H1N1) pdm09-like virus for cell culture or recombinant vaccines. These strains were included for the 2021-2022 season. The panel also voted for the inclusion of a B/Phuket/3073/2013-like virus (B/Yamagata lineage) as the second influenza B strain for the quadrivalent egg-based, cell culture, or recombinant vaccines, which is unchanged from this flu season.
‘Sporadic’ flu activity
While there was an uptick in influenza activity this year compared to the 2020-2021 season, hospitalization rates are lower than in the four seasons preceding the pandemic (from 2016-2017 to 2019-2020). As of Feb. 26, the cumulative hospitalization rate for this flu season was 5.2 hospitalizations per 100,000 individuals. There have been eight pediatric deaths due to influenza so far this season, compared to one pediatric death reported to the CDC during the 2020-2021 flu season.
About 4.1% of specimens tested at clinical laboratories were positive for flu. Since Oct. 30, 2.7% of specimens have been positive for influenza this season. Nearly all viruses detected (97.7%) have been influenza A.
Lisa Grohskopf, MD, MPH, a medical officer in the influenza division at the CDC who presented the data at the meeting, described flu activity this season as “sporadic” and noted that activity is increasing in some areas of the country. According to CDC’s weekly influenza surveillance report, most states had minimal influenza-like illness (ILI) activity, although Arkansas, Idaho, Iowa, Kansas, Minnesota, and Utah had slightly higher ILI activity as of Feb. 26. Champaign-Urbana, Illinois; St. Cloud, Minnesota; and Brownwood, Texas, had the highest levels of flu activity in the country.
Low vaccine effectiveness
As of Jan. 22, results from the U.S. Flu VE network do not show statistically significant evidence that the flu vaccine is effective. Currently, the vaccine is estimated to be 8% effective against preventing influenza A infection (95% confidence interval, –31% to 36%) and 14% effective against preventing A/H3N2 infection (95% CI, –28% to 43%) for people aged 6 months and older.
The network did not have enough data to provide age-specific VE estimates or estimates of effectiveness against influenza B. This could be due to low flu activity relative to prepandemic years, Dr. Flannery said. Of the 2,758 individuals enrolled in the VE flu network this season, just 147 (5%) tested positive for the flu this season. This is the lowest positivity rate observed in the Flu VE network participants with respiratory illness over the past 10 flu seasons, Dr. Grohskopf noted. In comparison, estimates from the 2019 to 2020 season included 4,112 individuals, and 1,060 tested positive for flu.
“We are really at the bare minimum of what we can use for a flu vaccine effectiveness estimate,” Dr. Flannery said about the more recent data. The network was not able to produce any estimates about flu vaccine effectiveness for the 2020-2021 season because of historically low flu activity.
The Department of Defense also presented vaccine efficacy estimates for the 2021–2022 season. The vaccine has been 36% effective (95% CI, 28%-44%) against all strains of the virus, 33% effective against influenza A (95% CI, 24%-41%), 32% effective against A/H3N2 (95% CI, 3%-53%), and 59% effective against influenza B (95% CI, 42%-71%). These results are from a young, healthy adult population, Lieutenant Commander Courtney Gustin, DrPH, MSN, told the panel, and they may not be reflective of efficacy rates across all age groups.
Though these findings suggest there is low to no measurable benefit against influenza A, Dr. Flannery said the CDC still recommends getting the flu vaccine, as it can be protective against other circulating flu strains. “We have been able to demonstrate protection against other H3 [viruses], B viruses, and H1 viruses in the past,” he said. And as these results only show protection against mild disease, “there is still possibility that there’s benefit against more severe disease,” he added. Studies measuring effectiveness against more severe outcomes are not yet available.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee has chosen the influenza vaccine strains for the 2022-2023 season in the northern hemisphere, which begins in the fall of 2022.
On March 3, the committee unanimously voted to endorse the World Health Organization’s recommendations as to which influenza strains to include for coverage by vaccines for the upcoming flu season. Two of the four recommended strains are different from last season.
The committee also heard updates on flu activity this season. So far, data from the U.S. Flu Vaccine Effectiveness (VE) network, which consists of seven study sites, have not shown that the vaccine is protective against influenza A. “We can say that it is not highly effective,” Brendan Flannery, PhD, who leads the U.S. Flu VE network for the Centers for Disease Control and Prevention, said in an interview. He was not involved with the advisory committee meeting. Flu activity this season has been low, he explained, so there are fewer cases his team can use to estimate vaccine efficacy. “If there’s some benefit, it’s hard for us to show that now,” he said.
Vaccine strains
The panel voted to include a A/Darwin/9/2021-like strain for the H3N2 component of the vaccine; this is changed from A/Cambodia/e0826360/2020. For the influenza B Victoria lineage component, the committee voted to include a B/Austria/1359417/2021-like virus, a swap from this year’s B/Washington/02/2019-like virus. These changes apply to the egg-based, cell-culture, and recombinant vaccines. Both new strains were included in WHO’s 2022 influenza vaccine strain recommendations for the southern hemisphere.
For the influenza A H1N1 component, the group also agreed to include a A/Victoria/2570/2019 (H1N1) pdm09-like virus for the egg-based vaccine and the A/Wisconsin/588/2019 (H1N1) pdm09-like virus for cell culture or recombinant vaccines. These strains were included for the 2021-2022 season. The panel also voted for the inclusion of a B/Phuket/3073/2013-like virus (B/Yamagata lineage) as the second influenza B strain for the quadrivalent egg-based, cell culture, or recombinant vaccines, which is unchanged from this flu season.
‘Sporadic’ flu activity
While there was an uptick in influenza activity this year compared to the 2020-2021 season, hospitalization rates are lower than in the four seasons preceding the pandemic (from 2016-2017 to 2019-2020). As of Feb. 26, the cumulative hospitalization rate for this flu season was 5.2 hospitalizations per 100,000 individuals. There have been eight pediatric deaths due to influenza so far this season, compared to one pediatric death reported to the CDC during the 2020-2021 flu season.
About 4.1% of specimens tested at clinical laboratories were positive for flu. Since Oct. 30, 2.7% of specimens have been positive for influenza this season. Nearly all viruses detected (97.7%) have been influenza A.
Lisa Grohskopf, MD, MPH, a medical officer in the influenza division at the CDC who presented the data at the meeting, described flu activity this season as “sporadic” and noted that activity is increasing in some areas of the country. According to CDC’s weekly influenza surveillance report, most states had minimal influenza-like illness (ILI) activity, although Arkansas, Idaho, Iowa, Kansas, Minnesota, and Utah had slightly higher ILI activity as of Feb. 26. Champaign-Urbana, Illinois; St. Cloud, Minnesota; and Brownwood, Texas, had the highest levels of flu activity in the country.
Low vaccine effectiveness
As of Jan. 22, results from the U.S. Flu VE network do not show statistically significant evidence that the flu vaccine is effective. Currently, the vaccine is estimated to be 8% effective against preventing influenza A infection (95% confidence interval, –31% to 36%) and 14% effective against preventing A/H3N2 infection (95% CI, –28% to 43%) for people aged 6 months and older.
The network did not have enough data to provide age-specific VE estimates or estimates of effectiveness against influenza B. This could be due to low flu activity relative to prepandemic years, Dr. Flannery said. Of the 2,758 individuals enrolled in the VE flu network this season, just 147 (5%) tested positive for the flu this season. This is the lowest positivity rate observed in the Flu VE network participants with respiratory illness over the past 10 flu seasons, Dr. Grohskopf noted. In comparison, estimates from the 2019 to 2020 season included 4,112 individuals, and 1,060 tested positive for flu.
“We are really at the bare minimum of what we can use for a flu vaccine effectiveness estimate,” Dr. Flannery said about the more recent data. The network was not able to produce any estimates about flu vaccine effectiveness for the 2020-2021 season because of historically low flu activity.
The Department of Defense also presented vaccine efficacy estimates for the 2021–2022 season. The vaccine has been 36% effective (95% CI, 28%-44%) against all strains of the virus, 33% effective against influenza A (95% CI, 24%-41%), 32% effective against A/H3N2 (95% CI, 3%-53%), and 59% effective against influenza B (95% CI, 42%-71%). These results are from a young, healthy adult population, Lieutenant Commander Courtney Gustin, DrPH, MSN, told the panel, and they may not be reflective of efficacy rates across all age groups.
Though these findings suggest there is low to no measurable benefit against influenza A, Dr. Flannery said the CDC still recommends getting the flu vaccine, as it can be protective against other circulating flu strains. “We have been able to demonstrate protection against other H3 [viruses], B viruses, and H1 viruses in the past,” he said. And as these results only show protection against mild disease, “there is still possibility that there’s benefit against more severe disease,” he added. Studies measuring effectiveness against more severe outcomes are not yet available.
A version of this article first appeared on Medscape.com.
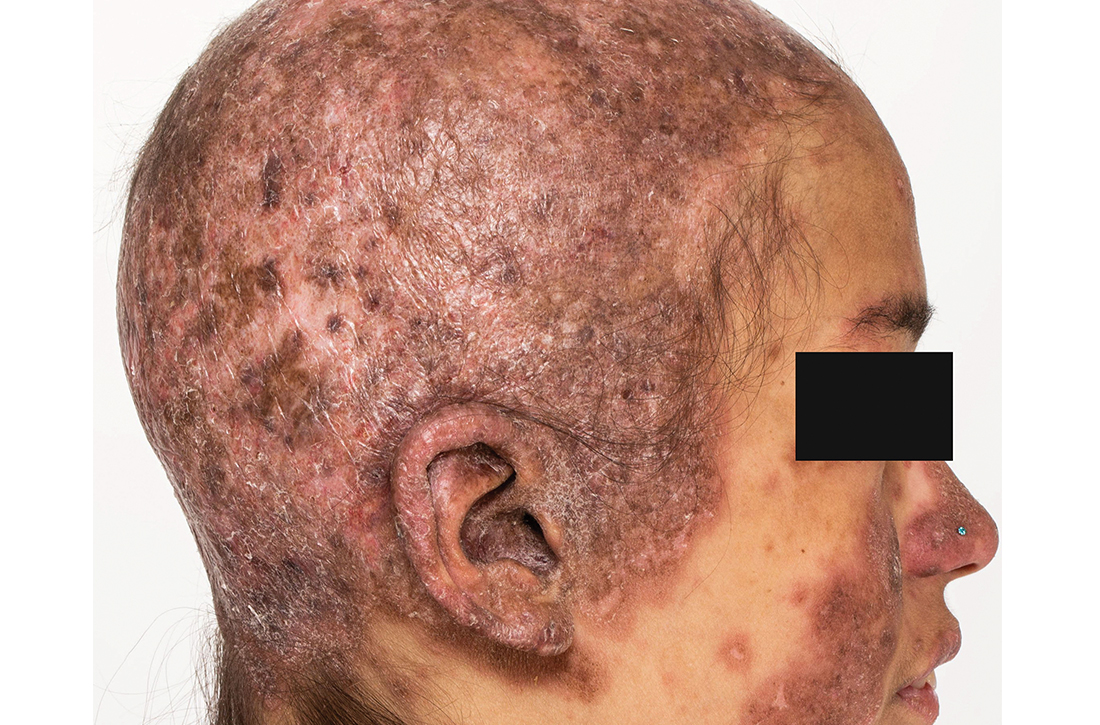
Extensive scarring alopecia and widespread rash
A 23-year-old woman with systemic lupus erythematosus (SLE) and a history of poor adherence to recommended treatment presented with a widespread pruritic rash and diffuse hair loss. The rash had rapidly progressed following sun exposure during the summer. The patient cited her mental health status (anxiety, depression), socioeconomic factors, and challenges with prescription insurance coverage as reasons for nonadherence to treatment.
Clinical examination revealed diffuse scarring alopecia and abnormal pigmentation of the scalp (FIGURE 1A), as well as large, red-brown, scaly, atrophic plaques on the face, ears, extremities, back, and buttocks (FIGURES 1B and 1C).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Generalized chronic cutaneouslupus erythematosus
The clinical features of our patient were most consistent with generalized chronic cutaneous lupus erythematosus (CCLE), which is 1 of 3 subtypes of cutaneous lupus erythematosus (CLE). The other 2 are acute and subacute cutaneous lupus erythematosus (ACLE and SCLE, respectively). CCLE is further divided into 3 distinct entities: discoid lupus erythematosus (DLE), chilblain lupus erythematosus, and lupus erythematosus panniculitis.
Distinguishing between the different forms of cutaneous lupus can be challenging; diagnosis is based on differences in clinical features and duration of skin changes, as well as biopsy and lab results.1 The clinical features of our patient were most consistent with DLE, based on the scarring alopecia with scaly atrophic plaques, dyspigmentation, and exacerbation following sun exposure.
DLE is the most common form of CCLE and frequently manifests in a localized, photosensitive distribution involving the scalp, ears, and/or face.2 Less commonly, it can demonstrate a more generalized distribution involving the trunk and/or extremities (reported incidence of 1.04 per 100,000 people).3 Longstanding DLE lesions commonly exhibit scarring and dyspigmentation. DLE occurs in approximately 15% to 30% of SLE patients,4 whereas about 10% of patients with DLE will progress to SLE.3
Positive antinuclear antibodies (ANA) are found in 54% of patients with CCLE, compared to 74% and 81% of patients with SCLE and ACLE, respectively.5 Thus, a negative ANA should not rule out the possibility of CLE.
Comprehensive lab work and biopsy could expose a systemic origin
While our patient already had a diagnosis of SLE, many patients will present with no prior history of autoimmune connective tissue disease, and, in that case, the objective should be to confirm the diagnosis and evaluate for systemic involvement. This includes a thorough review of systems; skin biopsy; complete blood count; liver function tests; urinalysis; and measurement of creatinine, inflammatory markers, ANA, extractable nuclear antigens, double-stranded DNA, complement levels (C3, C4, total), and antiphospholipid antibodies.6
Continue to: Biopsy
Biopsy features of DLE include vacuolar interface dermatitis, basement membrane zone thickening, follicular plugging, superficial and deep perivascular and periadnexal lymphohistiocytic inflammation with plasma cells, and increased mucin deposition. Direct immunofluorescence biopsy may show a continuous granular immunoglobulin (Ig) G/IgA/IgM and C3 band at the basement membrane zone.
Abnormal serologic tests may support the diagnosis of SLE based on American College of Rheumatology criteria and could suggest additional organ involvement or associated conditions, such as lupus nephritis or antiphospholipid syndrome (respectively). Currently, no clear consensus exists on monitoring patients with cutaneous lupus for systemic disease.
A gamut of skin-changing conditions should be considered
The differential diagnosis in this case includes SCLE, dermatitis, tinea corporis, cutaneous drug eruptions, and graft-versus-host disease (GVHD).
SCLE classically manifests with annular or psoriasiform lesions on the sun-exposed areas of the upper trunk (eg, the chest, neck, and upper extremities), while the central face and scalp are typically spared. Differentiating between generalized DLE and SCLE may be the most difficult, given similarities in the associated skin changes.
Dermatitis (atopic or contact) manifests as pruritic erythematous eczematous plaques, most commonly involving the flexural areas in atopic dermatitis and an exposure-dependent distribution pattern in contact dermatitis. The patient may have a history of atopy.
Continue to: Tinea corporis
Tinea corporis will manifest with annular scaly patches or plaques and may demonstrate erythematous papules around hair follicles in Majocchi granuloma. A positive potassium hydroxide exam demonstrating fungal hyphae confirms the diagnosis.
Cutaneous drug eruptions can have various morphologies and timing of onset. Certain photosensitive drug reactions can be triggered or exacerbated with sun exposure. Therefore, it is necessary to obtain a thorough medication history, including any new medications that were started within the past 4 to 6 weeks, although onset can be delayed beyond this timeframe.
GVHD is a complication that more commonly follows allogeneic hematopoietic stem cell transplants, although it may be seen following solid-organ transplantation or transfusion of nonirradiated blood. Chronic GVHD has an onset ≥ 100 days after transplant and is divided into nonsclerotic (lichenoid, atopic dermatitis-like, psoriasiform, poikilodermatous) and sclerotic morphologies.
Successful Tx requires adherence but may not prevent flare-ups
First-line treatment options for severe and widespread skin manifestations of CLE include photoprotection, smoking cessation, topical corticosteroids, hydroxychloroquine, and systemic corticosteroids. Second-line treatments include chloroquine, methotrexate, or mycophenolate mofetil; thalidomide or lenalidomide may be considered for patients with refractory disease.7,8
With successful treatment and photoprotection, patients may achieve significant skin clearing. Occasional flares, especially during warmer months, may occur if they are not diligent about photoprotection. Systemic treatments will also improve the patient’s systemic symptoms if the patient has concomitant SLE.
Our patient was advised to use topical steroids and to restart hydroxychloroquine 300 mg/d and mycophenolate mofetil 1000 mg/d (a regimen with which she had previously been nonadherent). The patient followed up with her family physician for assessment of her other medical issues. No new interventions for her mental health were initiated during this visit, as the severity of her depression was considered mild. She was referred to a case manager to navigate multiple medical appointments and prescription insurance coverage issues. The patient’s dose of mycophenolate mofetil was increased gradually to 3 g/d, and the patient experienced improvement in both her cutaneous lesions and systemic symptoms.
1. Petty AJ, Floyd L, Henderson C, et al. Cutaneous lupus erythematosus: progress and challenges. Curr Allergy Asthma Rep. 2020;20:12. doi: 10.1007/s11882-020-00906-8
2. Kuhn A, Landmann A. The classification and diagnosis of cutaneous lupus erythematosus. J Autoimmun. 2014;48-49:14-19. doi: 10.1016/j.jaut.2014.01.021
3. Durosaro O, Davis MDP, Reed KB, et al. Incidence of cutaneous lupus erythematosus, 1965-2005: a population-based study. Arch Dermatol. 2009;145:249-253. doi: 10.1001/archdermatol.2009.21
4. Merola JF. Overview of cutaneous lupus erythematosus. UpToDate. Updated September 19, 2021. Accessed February 17, 2022. www.uptodate.com/contents/overview-of-cutaneous-lupus-erythematosus
5. Biazar C, Sigges J, Patsinakidis N, et al. Cutaneous lupus erythematosus: first multicenter database analysis of 1002 patients from the European Society of Cutaneous Lupus Erythematosus (EUSCLE). Autoimmun Rev. 2013;12:444-454. doi: 10.1016/j.autrev.2012.08.019
6. O’Brien JC, Chong BF. not just skin deep: systemic disease involvement in patients with cutaneous lupus. J Investig Dermatol Symp Proc. 2017;18:S69-S74. doi: 10.1016/j.jisp.2016.09.001
7. Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: update of therapeutic options part I. J Am Acad Dermatol. 2011;65:e179-e193. doi: 10.1016/j.jaad.2010.06.018
8. Kindle SA, Wetter DA, Davis MDP, et al. Lenalidomide treatment of cutaneous lupus erythematosus: the Mayo Clinic experience. Int J Dermatol. 2016;55:e431-e439. doi: 10.1111/ijd.13226
A 23-year-old woman with systemic lupus erythematosus (SLE) and a history of poor adherence to recommended treatment presented with a widespread pruritic rash and diffuse hair loss. The rash had rapidly progressed following sun exposure during the summer. The patient cited her mental health status (anxiety, depression), socioeconomic factors, and challenges with prescription insurance coverage as reasons for nonadherence to treatment.
Clinical examination revealed diffuse scarring alopecia and abnormal pigmentation of the scalp (FIGURE 1A), as well as large, red-brown, scaly, atrophic plaques on the face, ears, extremities, back, and buttocks (FIGURES 1B and 1C).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Generalized chronic cutaneouslupus erythematosus
The clinical features of our patient were most consistent with generalized chronic cutaneous lupus erythematosus (CCLE), which is 1 of 3 subtypes of cutaneous lupus erythematosus (CLE). The other 2 are acute and subacute cutaneous lupus erythematosus (ACLE and SCLE, respectively). CCLE is further divided into 3 distinct entities: discoid lupus erythematosus (DLE), chilblain lupus erythematosus, and lupus erythematosus panniculitis.
Distinguishing between the different forms of cutaneous lupus can be challenging; diagnosis is based on differences in clinical features and duration of skin changes, as well as biopsy and lab results.1 The clinical features of our patient were most consistent with DLE, based on the scarring alopecia with scaly atrophic plaques, dyspigmentation, and exacerbation following sun exposure.
DLE is the most common form of CCLE and frequently manifests in a localized, photosensitive distribution involving the scalp, ears, and/or face.2 Less commonly, it can demonstrate a more generalized distribution involving the trunk and/or extremities (reported incidence of 1.04 per 100,000 people).3 Longstanding DLE lesions commonly exhibit scarring and dyspigmentation. DLE occurs in approximately 15% to 30% of SLE patients,4 whereas about 10% of patients with DLE will progress to SLE.3
Positive antinuclear antibodies (ANA) are found in 54% of patients with CCLE, compared to 74% and 81% of patients with SCLE and ACLE, respectively.5 Thus, a negative ANA should not rule out the possibility of CLE.
Comprehensive lab work and biopsy could expose a systemic origin
While our patient already had a diagnosis of SLE, many patients will present with no prior history of autoimmune connective tissue disease, and, in that case, the objective should be to confirm the diagnosis and evaluate for systemic involvement. This includes a thorough review of systems; skin biopsy; complete blood count; liver function tests; urinalysis; and measurement of creatinine, inflammatory markers, ANA, extractable nuclear antigens, double-stranded DNA, complement levels (C3, C4, total), and antiphospholipid antibodies.6
Continue to: Biopsy
Biopsy features of DLE include vacuolar interface dermatitis, basement membrane zone thickening, follicular plugging, superficial and deep perivascular and periadnexal lymphohistiocytic inflammation with plasma cells, and increased mucin deposition. Direct immunofluorescence biopsy may show a continuous granular immunoglobulin (Ig) G/IgA/IgM and C3 band at the basement membrane zone.
Abnormal serologic tests may support the diagnosis of SLE based on American College of Rheumatology criteria and could suggest additional organ involvement or associated conditions, such as lupus nephritis or antiphospholipid syndrome (respectively). Currently, no clear consensus exists on monitoring patients with cutaneous lupus for systemic disease.
A gamut of skin-changing conditions should be considered
The differential diagnosis in this case includes SCLE, dermatitis, tinea corporis, cutaneous drug eruptions, and graft-versus-host disease (GVHD).
SCLE classically manifests with annular or psoriasiform lesions on the sun-exposed areas of the upper trunk (eg, the chest, neck, and upper extremities), while the central face and scalp are typically spared. Differentiating between generalized DLE and SCLE may be the most difficult, given similarities in the associated skin changes.
Dermatitis (atopic or contact) manifests as pruritic erythematous eczematous plaques, most commonly involving the flexural areas in atopic dermatitis and an exposure-dependent distribution pattern in contact dermatitis. The patient may have a history of atopy.
Continue to: Tinea corporis
Tinea corporis will manifest with annular scaly patches or plaques and may demonstrate erythematous papules around hair follicles in Majocchi granuloma. A positive potassium hydroxide exam demonstrating fungal hyphae confirms the diagnosis.
Cutaneous drug eruptions can have various morphologies and timing of onset. Certain photosensitive drug reactions can be triggered or exacerbated with sun exposure. Therefore, it is necessary to obtain a thorough medication history, including any new medications that were started within the past 4 to 6 weeks, although onset can be delayed beyond this timeframe.
GVHD is a complication that more commonly follows allogeneic hematopoietic stem cell transplants, although it may be seen following solid-organ transplantation or transfusion of nonirradiated blood. Chronic GVHD has an onset ≥ 100 days after transplant and is divided into nonsclerotic (lichenoid, atopic dermatitis-like, psoriasiform, poikilodermatous) and sclerotic morphologies.
Successful Tx requires adherence but may not prevent flare-ups
First-line treatment options for severe and widespread skin manifestations of CLE include photoprotection, smoking cessation, topical corticosteroids, hydroxychloroquine, and systemic corticosteroids. Second-line treatments include chloroquine, methotrexate, or mycophenolate mofetil; thalidomide or lenalidomide may be considered for patients with refractory disease.7,8
With successful treatment and photoprotection, patients may achieve significant skin clearing. Occasional flares, especially during warmer months, may occur if they are not diligent about photoprotection. Systemic treatments will also improve the patient’s systemic symptoms if the patient has concomitant SLE.
Our patient was advised to use topical steroids and to restart hydroxychloroquine 300 mg/d and mycophenolate mofetil 1000 mg/d (a regimen with which she had previously been nonadherent). The patient followed up with her family physician for assessment of her other medical issues. No new interventions for her mental health were initiated during this visit, as the severity of her depression was considered mild. She was referred to a case manager to navigate multiple medical appointments and prescription insurance coverage issues. The patient’s dose of mycophenolate mofetil was increased gradually to 3 g/d, and the patient experienced improvement in both her cutaneous lesions and systemic symptoms.
A 23-year-old woman with systemic lupus erythematosus (SLE) and a history of poor adherence to recommended treatment presented with a widespread pruritic rash and diffuse hair loss. The rash had rapidly progressed following sun exposure during the summer. The patient cited her mental health status (anxiety, depression), socioeconomic factors, and challenges with prescription insurance coverage as reasons for nonadherence to treatment.
Clinical examination revealed diffuse scarring alopecia and abnormal pigmentation of the scalp (FIGURE 1A), as well as large, red-brown, scaly, atrophic plaques on the face, ears, extremities, back, and buttocks (FIGURES 1B and 1C).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Generalized chronic cutaneouslupus erythematosus
The clinical features of our patient were most consistent with generalized chronic cutaneous lupus erythematosus (CCLE), which is 1 of 3 subtypes of cutaneous lupus erythematosus (CLE). The other 2 are acute and subacute cutaneous lupus erythematosus (ACLE and SCLE, respectively). CCLE is further divided into 3 distinct entities: discoid lupus erythematosus (DLE), chilblain lupus erythematosus, and lupus erythematosus panniculitis.
Distinguishing between the different forms of cutaneous lupus can be challenging; diagnosis is based on differences in clinical features and duration of skin changes, as well as biopsy and lab results.1 The clinical features of our patient were most consistent with DLE, based on the scarring alopecia with scaly atrophic plaques, dyspigmentation, and exacerbation following sun exposure.
DLE is the most common form of CCLE and frequently manifests in a localized, photosensitive distribution involving the scalp, ears, and/or face.2 Less commonly, it can demonstrate a more generalized distribution involving the trunk and/or extremities (reported incidence of 1.04 per 100,000 people).3 Longstanding DLE lesions commonly exhibit scarring and dyspigmentation. DLE occurs in approximately 15% to 30% of SLE patients,4 whereas about 10% of patients with DLE will progress to SLE.3
Positive antinuclear antibodies (ANA) are found in 54% of patients with CCLE, compared to 74% and 81% of patients with SCLE and ACLE, respectively.5 Thus, a negative ANA should not rule out the possibility of CLE.
Comprehensive lab work and biopsy could expose a systemic origin
While our patient already had a diagnosis of SLE, many patients will present with no prior history of autoimmune connective tissue disease, and, in that case, the objective should be to confirm the diagnosis and evaluate for systemic involvement. This includes a thorough review of systems; skin biopsy; complete blood count; liver function tests; urinalysis; and measurement of creatinine, inflammatory markers, ANA, extractable nuclear antigens, double-stranded DNA, complement levels (C3, C4, total), and antiphospholipid antibodies.6
Continue to: Biopsy
Biopsy features of DLE include vacuolar interface dermatitis, basement membrane zone thickening, follicular plugging, superficial and deep perivascular and periadnexal lymphohistiocytic inflammation with plasma cells, and increased mucin deposition. Direct immunofluorescence biopsy may show a continuous granular immunoglobulin (Ig) G/IgA/IgM and C3 band at the basement membrane zone.
Abnormal serologic tests may support the diagnosis of SLE based on American College of Rheumatology criteria and could suggest additional organ involvement or associated conditions, such as lupus nephritis or antiphospholipid syndrome (respectively). Currently, no clear consensus exists on monitoring patients with cutaneous lupus for systemic disease.
A gamut of skin-changing conditions should be considered
The differential diagnosis in this case includes SCLE, dermatitis, tinea corporis, cutaneous drug eruptions, and graft-versus-host disease (GVHD).
SCLE classically manifests with annular or psoriasiform lesions on the sun-exposed areas of the upper trunk (eg, the chest, neck, and upper extremities), while the central face and scalp are typically spared. Differentiating between generalized DLE and SCLE may be the most difficult, given similarities in the associated skin changes.
Dermatitis (atopic or contact) manifests as pruritic erythematous eczematous plaques, most commonly involving the flexural areas in atopic dermatitis and an exposure-dependent distribution pattern in contact dermatitis. The patient may have a history of atopy.
Continue to: Tinea corporis
Tinea corporis will manifest with annular scaly patches or plaques and may demonstrate erythematous papules around hair follicles in Majocchi granuloma. A positive potassium hydroxide exam demonstrating fungal hyphae confirms the diagnosis.
Cutaneous drug eruptions can have various morphologies and timing of onset. Certain photosensitive drug reactions can be triggered or exacerbated with sun exposure. Therefore, it is necessary to obtain a thorough medication history, including any new medications that were started within the past 4 to 6 weeks, although onset can be delayed beyond this timeframe.
GVHD is a complication that more commonly follows allogeneic hematopoietic stem cell transplants, although it may be seen following solid-organ transplantation or transfusion of nonirradiated blood. Chronic GVHD has an onset ≥ 100 days after transplant and is divided into nonsclerotic (lichenoid, atopic dermatitis-like, psoriasiform, poikilodermatous) and sclerotic morphologies.
Successful Tx requires adherence but may not prevent flare-ups
First-line treatment options for severe and widespread skin manifestations of CLE include photoprotection, smoking cessation, topical corticosteroids, hydroxychloroquine, and systemic corticosteroids. Second-line treatments include chloroquine, methotrexate, or mycophenolate mofetil; thalidomide or lenalidomide may be considered for patients with refractory disease.7,8
With successful treatment and photoprotection, patients may achieve significant skin clearing. Occasional flares, especially during warmer months, may occur if they are not diligent about photoprotection. Systemic treatments will also improve the patient’s systemic symptoms if the patient has concomitant SLE.
Our patient was advised to use topical steroids and to restart hydroxychloroquine 300 mg/d and mycophenolate mofetil 1000 mg/d (a regimen with which she had previously been nonadherent). The patient followed up with her family physician for assessment of her other medical issues. No new interventions for her mental health were initiated during this visit, as the severity of her depression was considered mild. She was referred to a case manager to navigate multiple medical appointments and prescription insurance coverage issues. The patient’s dose of mycophenolate mofetil was increased gradually to 3 g/d, and the patient experienced improvement in both her cutaneous lesions and systemic symptoms.
1. Petty AJ, Floyd L, Henderson C, et al. Cutaneous lupus erythematosus: progress and challenges. Curr Allergy Asthma Rep. 2020;20:12. doi: 10.1007/s11882-020-00906-8
2. Kuhn A, Landmann A. The classification and diagnosis of cutaneous lupus erythematosus. J Autoimmun. 2014;48-49:14-19. doi: 10.1016/j.jaut.2014.01.021
3. Durosaro O, Davis MDP, Reed KB, et al. Incidence of cutaneous lupus erythematosus, 1965-2005: a population-based study. Arch Dermatol. 2009;145:249-253. doi: 10.1001/archdermatol.2009.21
4. Merola JF. Overview of cutaneous lupus erythematosus. UpToDate. Updated September 19, 2021. Accessed February 17, 2022. www.uptodate.com/contents/overview-of-cutaneous-lupus-erythematosus
5. Biazar C, Sigges J, Patsinakidis N, et al. Cutaneous lupus erythematosus: first multicenter database analysis of 1002 patients from the European Society of Cutaneous Lupus Erythematosus (EUSCLE). Autoimmun Rev. 2013;12:444-454. doi: 10.1016/j.autrev.2012.08.019
6. O’Brien JC, Chong BF. not just skin deep: systemic disease involvement in patients with cutaneous lupus. J Investig Dermatol Symp Proc. 2017;18:S69-S74. doi: 10.1016/j.jisp.2016.09.001
7. Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: update of therapeutic options part I. J Am Acad Dermatol. 2011;65:e179-e193. doi: 10.1016/j.jaad.2010.06.018
8. Kindle SA, Wetter DA, Davis MDP, et al. Lenalidomide treatment of cutaneous lupus erythematosus: the Mayo Clinic experience. Int J Dermatol. 2016;55:e431-e439. doi: 10.1111/ijd.13226
1. Petty AJ, Floyd L, Henderson C, et al. Cutaneous lupus erythematosus: progress and challenges. Curr Allergy Asthma Rep. 2020;20:12. doi: 10.1007/s11882-020-00906-8
2. Kuhn A, Landmann A. The classification and diagnosis of cutaneous lupus erythematosus. J Autoimmun. 2014;48-49:14-19. doi: 10.1016/j.jaut.2014.01.021
3. Durosaro O, Davis MDP, Reed KB, et al. Incidence of cutaneous lupus erythematosus, 1965-2005: a population-based study. Arch Dermatol. 2009;145:249-253. doi: 10.1001/archdermatol.2009.21
4. Merola JF. Overview of cutaneous lupus erythematosus. UpToDate. Updated September 19, 2021. Accessed February 17, 2022. www.uptodate.com/contents/overview-of-cutaneous-lupus-erythematosus
5. Biazar C, Sigges J, Patsinakidis N, et al. Cutaneous lupus erythematosus: first multicenter database analysis of 1002 patients from the European Society of Cutaneous Lupus Erythematosus (EUSCLE). Autoimmun Rev. 2013;12:444-454. doi: 10.1016/j.autrev.2012.08.019
6. O’Brien JC, Chong BF. not just skin deep: systemic disease involvement in patients with cutaneous lupus. J Investig Dermatol Symp Proc. 2017;18:S69-S74. doi: 10.1016/j.jisp.2016.09.001
7. Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: update of therapeutic options part I. J Am Acad Dermatol. 2011;65:e179-e193. doi: 10.1016/j.jaad.2010.06.018
8. Kindle SA, Wetter DA, Davis MDP, et al. Lenalidomide treatment of cutaneous lupus erythematosus: the Mayo Clinic experience. Int J Dermatol. 2016;55:e431-e439. doi: 10.1111/ijd.13226
Dyspareunia: Keys to biopsychosocial evaluation and treatment planning
Dyspareunia is persistent or recurrent pain before, during, or after sexual contact and is not limited to cisgender individuals or vaginal intercourse.1-3 With a prevalence as high as 45% in the United States,2-5 it is one of the most common complaints in gynecologic practices.5,6
Causes and contributing factors
There are many possible causes of dyspareunia.2,4,6 While some patients have a single cause, most cases are complex, with multiple overlapping causes and maintaining factors.4,6 Identifying each contributing factor can help you appropriately address all components.
Physical conditions. The range of physical contributors to dyspareunia includes inflammatory processes, structural abnormalities, musculoskeletal dysfunctions, pelvic organ disorders, injuries, iatrogenic effects, infections, allergic reactions, sensitization, hormonal changes, medication effects, adhesions, autoimmune disorders, and other pain syndromes (TABLE 12-4,6-11).

Inadequate arousal. One of the primary causes of pain during vaginal penetration is inadequate arousal and lubrication.1,2,9-11 Arousal is the phase of the sexual response cycle that leads to genital tumescence and prepares the genitals for sexual contact through penile/clitoral erection, vaginal engorgement, and lubrication, which prevents pain and enhances pleasurable sensation.9-11
While some physical conditions can lead to an inability to lubricate, the most common causes of inadequate lubrication are psychosocial-behavioral, wherein patients have the same physical ability to lubricate as patients without genital pain but do not progress through the arousal phase.9-11 Behavioral factors such as inadequate or ineffective foreplay can fail to produce engorgement and lubrication, while psychosocial factors such as low attraction to partner, relationship stressors, anxiety, or low self-esteem can have an inhibitory effect on sexual arousal.1,2,9-11 Psychosocial and behavioral factors may also be maintaining factors or consequences of dyspareunia, and need to be assessed and treated.1,2,9-11
Psychological trauma. Exposure to psychological traumas and the development of posttraumatic stress disorder (PTSD) have been linked with the development of pain disorders in general and dyspareunia specifically. Most patients seeking treatment for chronic pain disorders have a history of physical or sexual abuse.12 Changes in physiologic processes (eg, neurochemical, endocrine) that occur with PTSD interfere with the sexual response cycle, and sexual traumas specifically have been linked with pelvic floor dysfunction.13,14 Additionally, when PTSD is caused by a sexual trauma, even consensual sexual encounters can trigger flashbacks, intrusive memories, hyperarousal, and muscle tension that interfere with the sexual response cycle and contribute to genital pain.13
Vaginismus is both a physiologic and psychological contributor to dyspareunia.1,2,4 Patients experiencing pain can develop anxiety about repeated pain and involuntarily contract their pelvic muscles, thereby creating more pain, increasing anxiety, decreasing lubrication, and causing pelvic floor dysfunction.1-4,6 Consequently, all patients with dyspareunia should be assessed and continually monitored for symptoms of vaginismus.
Continue to: Anxiety
Anxiety. As with other pain disorders, anxiety develops around pain triggers.10,15 When expecting sexual activity, patients can experience extreme worry and panic attacks.10,15,16 The distress of sexual encounters can interfere with physiologic arousal and sexual desire, impacting all phases of the sexual response cycle.1,2
Relationship issues. Difficulty engaging in or avoidance of sexual activity can interfere with romantic relationships.2,10,16 Severe pain or vaginismus contractions can prevent penetration, leading to unconsummated marriages and an inability to conceive through intercourse.10 The distress surrounding sexual encounters can precipitate erectile dysfunction in male partners, or partners may continue to demand sexual encounters despite the patient’s pain, further impacting the relationship and heightening sexual distress.10 These stressors have led to relationships ending, patients reluctantly agreeing to nonmonogamy to appease their partners, and patients avoiding relationships altogether.10,16
Devalued self-image. Difficulties with sexuality and relationships impact the self-image of patients with dyspareunia. Diminished self-image may include feeling “inadequate” as a woman and as a sexual partner, or feeling like a “failure.”16 Women with dyspareunia often have more distress related to their body image, physical appearance, and genital self-image than do women without genital pain.17 Feeling resentment toward their body, or feeling “ugly,” embarrassed, shamed, “broken,” and “useless” also contribute to increased depressive symptoms found in patients with dyspareunia.16,18
Making the diagnosis
Most patients do not report symptoms unless directly asked2,7; therefore, it is recommended that all patients be screened as a part of an initial intake and before any genital exam (TABLE 22-4,6,7,9,11,19,20).4,7,21 If this screen is positive, a separate appointment may be needed for a thorough evaluation and before any attempt is made at a genital exam.4,7
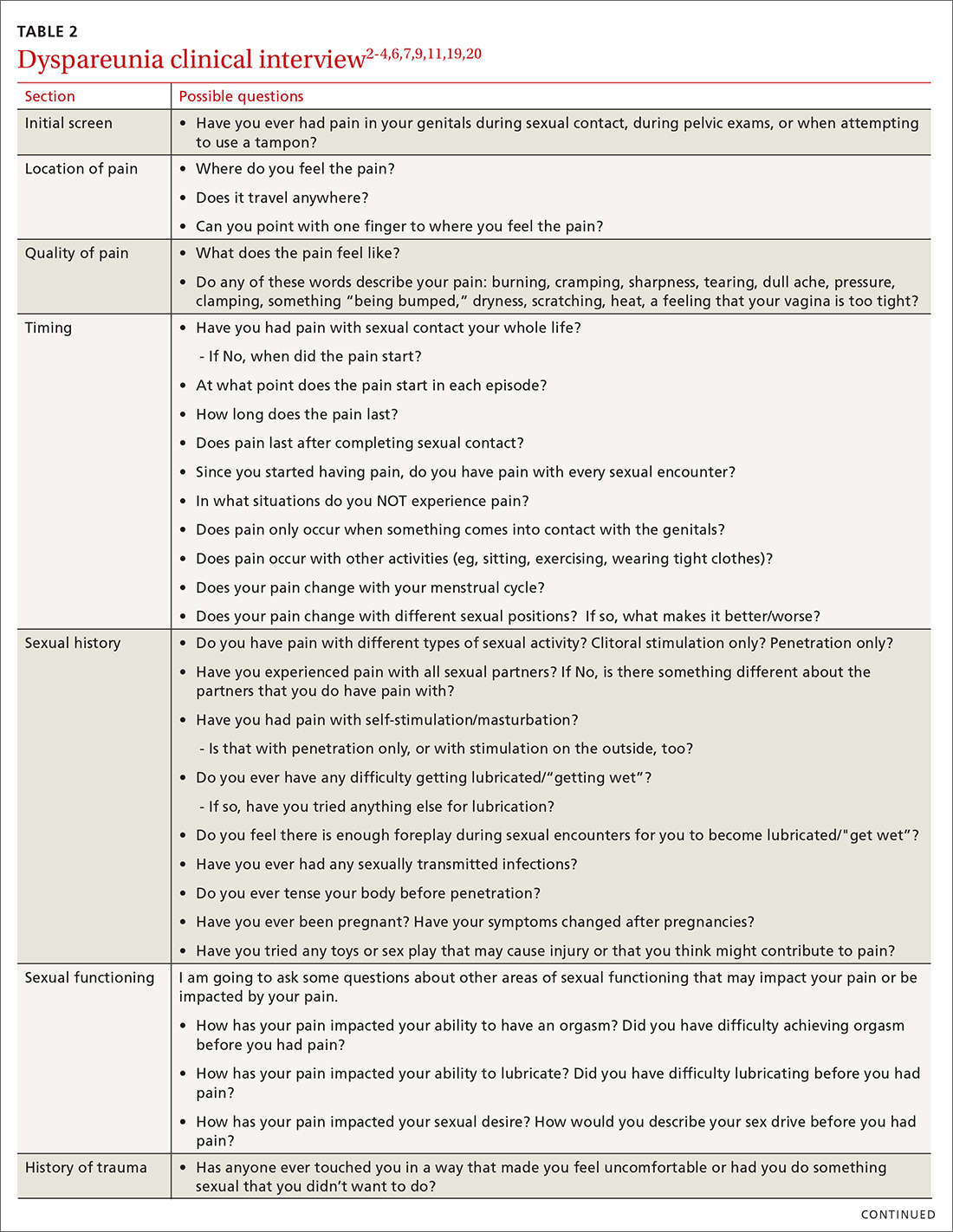
Items to include in the clinical interview
Given the range of possible causes of dyspareunia and its contributing factors and symptoms, a thorough clinical interview is essential. Begin with a review of the patient’s complete medical and surgical history to identify possible known contributors to genital pain.4 Pregnancy history is of particular importance as the prevalence of postpartum dyspareunia is 35%, with risk being greater for patients who experienced dyspareunia symptoms before pregnancy.22

Knowing the location and quality of pain is important for differentiating between possible diagnoses, as is specifying dyspareunia as lifelong or acquired, superficial or deep, and primary or secondary.1-4,6 Confirm the specific location(s) of pain—eg, at the introitus, in the vestibule, on the labia, in the perineum, or near the clitoris.2,4,6 A diagram or model may be needed to help patients to localize pain.4
To help narrow the differential, include the following elements in your assessment: pain quality, timing (eg, initial onset, episode onset, episode duration, situational triggers), alleviating factors, symptoms in surrounding structures (eg, bladder, bowel, muscles, bones), sexual history, other areas of sexual functioning, history of psychological trauma, relationship effects, and mental health (TABLE 22-4,6,7,9,11,19,20 and Table 323-28). Screening for a history of sexual trauma is particularly important, as a recent systematic review and meta-analysis found that women with a history of sexual assault had a 42% higher risk of gynecologic problems overall, a 74% higher risk of dyspareunia, and a 71% higher risk of vaginismus than women without a history of sexual assault.29 Using measures such as the Female Sexual Function Index or the McGill Pain Questionnaire can help patients more thoroughly describe their symptoms (TABLE 323-28).3
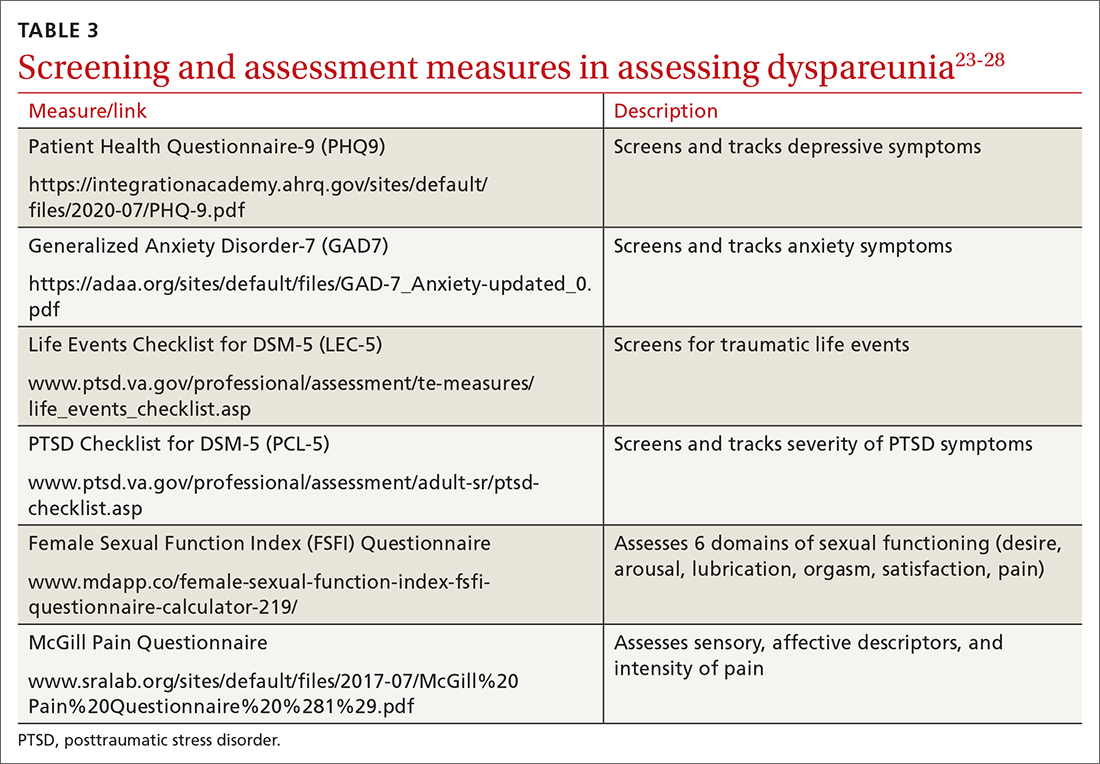
Continue to: Guidelines for the physical exam
Guidelines for the physical exam
Before the exam, ensure the patient has not used any topical genital treatment in the past 2 weeks that may interfere with sensitivity to the exam.4 To decrease patients’ anxiety about the exam, remind them that they can stop the exam at any time.7 Also consider offering the use of a mirror to better pinpoint the location of pain, and to possibly help the patient learn more about her anatomy.2,7
Begin the exam by palpating surrounding areas that may be involved in pain, including the abdomen and musculoskeletal features.3,6,19 Next visually inspect the external genitalia for lesions, abrasions, discoloration, erythema, or other abnormal findings.2,3,6 Ask the patient for permission before contacting the genitals. Because the labia may be a site of pain, apply gentle pressure in retracting it to fully examine the vestibule.6,7 Contraction of the pelvic floor muscles during approach or initial palpation could signal possible vaginismus.4
After visual inspection of external genitalia, use a cotton swab to map the vulva and vestibule in a clockwise fashion to precisely identify any painful locations.2-4,6 If the patient’s history of pain has been intermittent, it’s possible that the cotton swab will not elicit pain on the day of the initial exam, but it may on other days.4
Begin the internal exam by inserting a single finger into the first inch of the vagina and have the patient squeeze and release to assess tenderness, muscle tightness, and control.2,6 Advance the finger further into the vagina and palpate clockwise, examining the levator muscles, obturator muscles, rectum, urethra, and bladder for abnormal tightness or reproduction of pain.2,4,6 Complete a bimanual exam to evaluate the pelvic organs and adnexa.2,4 If indicated, a more thorough evaluation of pelvic floor musculature can be performed by a physical therapist or gynecologist who specializes in pelvic pain.2-4
If the patient consents to further evaluation, consider using a small speculum, advanced slowly, for further internal examination, noting any lesions, abrasions, discharge, ectropion, or tenderness.2-4,7 A rectal exam may also be needed in cases of deep dyspareunia.6 Initial work-up may include a potassium hydroxide wet prep, sexually transmitted infection testing, and pelvic ultrasound.2,4 In some cases, laparoscopy or biopsy may be needed.2,4
Treatments for common causes
Treatment often begins with education about anatomy, to help patients communicate about symptoms and engage more fully in their care.3 Additional education may be needed on genital functioning and the necessity of adequate stimulation and lubrication prior to penetration.1,2,9-11 A discussion of treatments for the wide range of possible causes of dyspareunia is outside the scope of this article. However, some basic behavioral changes may help patients address some of the more common contributing factors.
For example, if vaginal infection is suspected, advise patients to discontinue the use of harsh soaps, known vaginal irritants (eg, perfumed products, bath additives), and douches.3 Recommend using only preservative- and alcohol-free lubricants for sexual contact, and avoiding lubricants with added functions (eg, warming).3 It’s worth noting that avoidance of tight clothing and thong underwear due to possible risk for infections may not be necessary. A recent study found that women who frequently wore thong underwear (more than half of the time) were no more likely to develop urinary tract infections, yeast vaginitis, or bacterial vaginosis than those who avoid such items.30 However, noncotton underwear fabric, rather than tightness, was associated with yeast vaginitis30; therefore, patients may want to consider using only breathable underwear.3
Continue to: Medication
Medication. Medication may be used to treat the underlying contributing conditions or the symptom of pain directly. Some common options are particularly important for patients whose dyspareunia does not have an identifiable cause. These medications include anti-inflammatory agents, topical anesthetics, tricyclic antidepressants, and hormonal treatments.2-4 Since effectiveness varies based on subtypes of pain, select a medication according to the location, timing, and hypothesized mechanism of pain.3,31,32
Medication for deep pain. A meta-analysis and systematic review found that patients with some types of chronic pelvic pain with pain deep in the vagina or pelvis experienced greater than 50% reduction in pain using medroxyprogesterone acetate compared with placebo.33 Other treatments for deep pain depend on physical exam findings.
Medication for superficial pain. Many remedies have been tried, with at least 26 different treatments for vulvodynia pain alone.16 Only some of these treatments have supporting evidence. For patients with vulvar pain, an intent-to-treat RCT found that patients using a topical steroid experienced a 23% reduction in pain from pre-treatment to 6-month follow-up.32
Surgery is also effective for vulvar pain.34,35 For provoked vestibulodynia (in which pain is localized to the vestibule and triggered by contact with the vulva), or vulvar vestibulitis, RCTs have found that vestibulectomy has stronger effects on pain than other treatments,31,35 with a 53% reduction in pain during intercourse and a 70% reduction in vestibular pain overall.35 However, while vestibulectomy is effective for provoked vestibulodynia, it is not recommended for generalized vulvodynia, in which pain is diffuse across the vulva and occurs without vulvar contact.34
Unsupported treatments. A number of other treatments have not yet been found effective. Although lidocaine for vulvar pain is often used, RCTs have not found any significant reduction in symptoms, and a double-blind RCT found that lidocaine ointment actually performed worse than placebo.31,34 Similarly, oral tricyclics have not been found to decrease vulvar pain more than placebo in double-blind studies.31,34 Furthermore, a meta-analysis of RCTs comparing treatments with placebo for vestibular pain found no significant decrease in dyspareunia for topical conjugated estrogen, topical lidocaine, oral desipramine, oral desipramine with topical lidocaine, laser therapy, or transcranial direct current.32
Tx risks to consider. Risks and benefits of dyspareunia treatment options should be thoroughly weighed and discussed with the patient.2-4 Vestibulectomy, despite reducing pain for many patients, has led to increased pain for 9% of patients who underwent the procedure.35 Topical treatments may lead to allergic reactions, inflammation, and worsening of symptoms,4 and hormonal treatments have been found to increase the risk of weight gain and bloating and are not appropriate for patients trying to conceive.33
Coordinate care with other providers
While medications and surgery can reduce pain, they have not been shown to improve other aspects of sexual functioning such as sexual satisfaction, frequency of sexual intercourse, or overall sense of sexual functioning.35 Additionally, pain reduction does not address muscle tension, anxiety, self-esteem, and relationship problems. As a result, a multidisciplinary approach is generally needed.3,4,32,33
Continue to: Physical therapists
Physical therapists. Pelvic floor physical therapists are often members of the dyspareunia treatment team and can provide a thorough evaluation and treatment of pelvic floor disorders.2-4 An RCT with intent-to-treat analysis found that pain was reduced by 71% following pelvic floor physical therapy.36 Another RCT found that 90% of patients reported a clinically meaningful decrease in pain with pelvic floor physical therapy.37 In addition to addressing pain, pelvic floor physical therapy has also been found to improve sexual functioning, sexual satisfaction, distress, and patient perception of improvement.34,36,37
Behavioral health specialists. Psychotherapists, especially those trained in sex therapy, couples therapy, or cognitive behavioral therapy (CBT), are also typically on the treatment team. Multiple RCTs have found evidence of CBT’s effectiveness in the direct treatment of dyspareunia pain. Bergeron et al35 found a 37.5% reduction in vulvar vestibulitis pain intensity during intercourse after patients completed group CBT. Another intent-to-treat RCT found that patients receiving CBT experienced more pain reduction (~ 30%) than patients who were treated with a topical steroid.38
In addition to having a direct impact on pain, CBT has also been found to have a clinically and statistically significant positive impact on other aspects of sexual experience, such as overall sexuality, self-efficacy, overall sexual functioning, frequency of intercourse, and catastrophizing.34,38 A recent meta-analysis of RCTs found that about 80% of vaginismus patients were able to achieve penetrative intercourse after treatment with behavioral sex therapy or CBT.39 This success rate was not exceeded by physical or surgical treatments.39
When PTSD is thought to be a contributing factor, trauma therapy will likely be needed in addition to treatments for dyspareunia. First-line treatments for PTSD include cognitive processing therapy, prolonged exposure, trauma-focused CBT, and cognitive therapy.40
Psychotherapists can also help patients reduce anxiety, reintroduce sexual contact without triggering pain or anxiety, address emotional and self-esteem effects of dyspareunia, address relationship issues, and refocus sexual encounters on pleasure rather than pain avoidance.2-4 Despite patient reports of high treatment satisfaction following therapy,38 many patients may initially lack confidence in psychotherapy as a treatment for pain35 and may need to be educated on its effectiveness and multidimensional benefits.
Gynecologists. Often a gynecologist with specialization in pelvic pain is an essential member of the team for diagnostic clarification, recommendation of treatment options, and performance of more advanced treatments.2,3 If pain has become chronic, the patient may also benefit from a pain management team and support groups.2,3
Follow-up steps
Patients who screen negative for dyspareunia should be re-screened periodically. Continue to assess patients diagnosed with dyspareunia for vaginismus symptoms (if they are not initially present) to ensure that the treatment plan is appropriately adjusted. Once treatment has begun, ask about adverse effects and confidence in the treatment plan to minimize negative impacts on treatment adherence and to anticipate a need for a change in the treatment approach.31,35 In addition to tracking treatment effects on pain, continue to assess for patient-centered outcomes such as emotional functioning, self-esteem, and sexual and relationship satisfaction.34 The Female Sexual Function Index can be a useful tool to track symptoms.27,34
Finally, patients who do not experience sufficient improvement in symptoms and functioning with initial treatment may need continued support and encouragement. Given the broad range of contributing factors and the high number of potential treatments, patients may find hope in learning that multiple other treatment options may be available.
CORRESPONDENCE
Adrienne A. Williams, PhD, Department of Family and Community Medicine, University of Illinois at Chicago College of Medicine, 1919 W Taylor Street, MC 663, Chicago, IL 60612; [email protected]
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Ed. American Psychiatric Publishing; 2013.
2. Seehusen DA, Baird DC, Bode DV. Dyspareunia in women. Am Fam Phys. 2014;90:465-470.
3. Sorensen J, Bautista KE, Lamvu G, et al. Evaluation and treatment of female sexual pain: a clinical review. Cureus. 2018;10:e2379.
4. MacNeill C. Dyspareunia. Obstet Gynecol Clin North Am. 2006;33:565-77.
5. Latthe P, Latthe M, Say L, et al. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health. 2006;6:177.
6. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009;113:1124-1136.
7. Williams AA, Williams M. A guide to performing pelvic speculum exams: a patient-centered approach to reducing iatrogenic effects. Teach Learn Med. 2013;25:383-391.
8. Ünlü Z, Yentur A, Çakil N. Pudendal nerve neuropathy: An unknown-rare cause of pelvic pain. Arch Rheumatol. 2016;31:102-103.
9. Dewitte M, Borg C, Lowenstein L. A psychosocial approach to female genital pain. Nat Rev Urol. 2018;15:25-41.
10. Masters WH, Johnson VE. Human Sexual Inadequacy. 1st ed. Little, Brown; 1970.
11. Rathus SA, Nevid JS, Fichner-Rathus L. Human Sexuality in a World of Diversity. 5th ed. Allyn and Bacon; 2002.
12. Bailey BE, Freedenfeld RN, Kiser RS, et al. Lifetime physical and sexual abuse in chronic pain patients: psychosocial correlates and treatment outcomes. Disabil Rehabil. 2003;25:331-342.
13. Yehuda R, Lehrner A, Rosenbaum TY. PTSD and sexual dysfunction in men and women. J Sex Med. 2015;12:1107-1119.
14. Postma R, Bicanic I, van der Vaart H, et al. Pelvic floor muscle problems mediate sexual problems in young adult rape victims. J Sex Med. 2013;10:1978-1987.
15. Binik YM, Bergeron S, Khalifé S. Dyspareunia and vaginismus: so-called sexual pain. In: Leiblum SR, ed. 4th ed. Principles and Practice of Sex Therapy. The Guilford Press; 2007:124-156.
16. Ayling K, Ussher JM. “If sex hurts, am I still a woman?” The subjective experience of vulvodynia in hetero-sexual women. Arch Sex Behav. 2008;37:294-304.
17. Pazmany E, Bergeron S, Van Oudenhove L, et al. Body image and genital self-image in pre-menopausal women with dyspareunia. Arch Sex Behav. 2013;42:999-1010.
18. Maillé DL, Bergeron S, Lambert B. Body image in women with primary and secondary provoked vestibulodynia: a controlled study. J Sex Med. 2015;12:505-515.
19. Ryan L, Hawton K. Female dyspareunia. BMJ. 2004;328:1357.
20. Waldura JF, Arora I, Randall AM, et al. Fifty shades of stigma: exploring the health care experiences of kink-oriented patients. J Sex Med. 2016;13:1918-1929.
21. Hinchliff S, Gott M. Seeking medical help for sexual concerns in mid- and later life: a review of the literature. J Sex Res. 2011;48:106-117.
22. Banaei M, Kariman N, Ozgoli G, et al. Prevalence of postpartum dyspareunia: a systematic review and meta-analysis. Int J Gynaecol Obstet. 2021;153:14-24.
23. Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509-515.
24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
25. U.S. Department of Veterans Affairs. PTSD: National Center for PTSD. Life events checklist for DSM-5 (LEC-5). Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp
26. Weathers FW, Litz BT, Keane TM, et al. The PTSD checklist for DSM-5 (PCL-5). 2013. Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp
27. Rosen R, Brown C, Heiman J, et al. The female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191-208.
28. Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191-197.
29. Hassam T, Kelso E, Chowdary P, et al. Sexual assault as a risk factor for gynaecological morbidity: an exploratory systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;255:222-230.
30. Hamlin AA, Sheeder J, Muffly TM. Brief versus thong hygiene in obstetrics and gynecology (B-THONG): a survey study. J Obstet Gynaecol Res. 2019;45:1190-1196.
31. Foster DC, Kotok MB, Huang LS, et al. Oral desipramine and topical lidocaine for vulvodynia: a randomized controlled trial. Obstet Gynecol. 2010;116:583-593.
32. Pérez-López FR, Bueno-Notivol J, Hernandez AV, et al. Systematic review and meta-analysis of the effects of treatment modalities for vestibulodynia in women. Eur J Contracept Reprod Health Care. 2019;24:337-346.
33. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;(3):CD008797.
34. Goldstein AT, Pukall CF, Brown C, et al. Vulvodynia: assessment and treatment. J Sex Med. 2016;13:572-590.
35. Bergeron S, Binik YM, Khalifé S, et al. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91:297-306.
36. Schvartzman R, Schvartzman L, Ferreira CF, et al. Physical therapy intervention for women with dyspareunia: a randomized clinical trial. J Sex Marital Ther. 2019;45:378-394.
37. Morin M, Dumoulin C, Bergeron S, et al. Multimodal physical therapy versus topical lidocaine for provoked vestibulodynia: a multicenter, randomized trial. Am J Obstet Gynecol. 2021;224:189.e1-189.e12.
38. Bergeron S, Khalifé S, Dupuis M-J, et al. A randomized clinical trial comparing group cognitive-behavioral therapy and a topical steroid for women with dyspareunia. J Consult Clin Psychol. 2016;84:259-268.
39. Maseroli E, Scavello I, Rastrelli G, et al. Outcome of medical and psychosexual interventions for vaginismus: a systematic review and meta-analysis. J Sex Med. 2018;15:1752-1764.
40. American Psychological Association. Clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. 2017. Accessed February 3, 2022. www.apa.org/ptsd-guideline/ptsd.pdf
Dyspareunia is persistent or recurrent pain before, during, or after sexual contact and is not limited to cisgender individuals or vaginal intercourse.1-3 With a prevalence as high as 45% in the United States,2-5 it is one of the most common complaints in gynecologic practices.5,6
Causes and contributing factors
There are many possible causes of dyspareunia.2,4,6 While some patients have a single cause, most cases are complex, with multiple overlapping causes and maintaining factors.4,6 Identifying each contributing factor can help you appropriately address all components.
Physical conditions. The range of physical contributors to dyspareunia includes inflammatory processes, structural abnormalities, musculoskeletal dysfunctions, pelvic organ disorders, injuries, iatrogenic effects, infections, allergic reactions, sensitization, hormonal changes, medication effects, adhesions, autoimmune disorders, and other pain syndromes (TABLE 12-4,6-11).

Inadequate arousal. One of the primary causes of pain during vaginal penetration is inadequate arousal and lubrication.1,2,9-11 Arousal is the phase of the sexual response cycle that leads to genital tumescence and prepares the genitals for sexual contact through penile/clitoral erection, vaginal engorgement, and lubrication, which prevents pain and enhances pleasurable sensation.9-11
While some physical conditions can lead to an inability to lubricate, the most common causes of inadequate lubrication are psychosocial-behavioral, wherein patients have the same physical ability to lubricate as patients without genital pain but do not progress through the arousal phase.9-11 Behavioral factors such as inadequate or ineffective foreplay can fail to produce engorgement and lubrication, while psychosocial factors such as low attraction to partner, relationship stressors, anxiety, or low self-esteem can have an inhibitory effect on sexual arousal.1,2,9-11 Psychosocial and behavioral factors may also be maintaining factors or consequences of dyspareunia, and need to be assessed and treated.1,2,9-11
Psychological trauma. Exposure to psychological traumas and the development of posttraumatic stress disorder (PTSD) have been linked with the development of pain disorders in general and dyspareunia specifically. Most patients seeking treatment for chronic pain disorders have a history of physical or sexual abuse.12 Changes in physiologic processes (eg, neurochemical, endocrine) that occur with PTSD interfere with the sexual response cycle, and sexual traumas specifically have been linked with pelvic floor dysfunction.13,14 Additionally, when PTSD is caused by a sexual trauma, even consensual sexual encounters can trigger flashbacks, intrusive memories, hyperarousal, and muscle tension that interfere with the sexual response cycle and contribute to genital pain.13
Vaginismus is both a physiologic and psychological contributor to dyspareunia.1,2,4 Patients experiencing pain can develop anxiety about repeated pain and involuntarily contract their pelvic muscles, thereby creating more pain, increasing anxiety, decreasing lubrication, and causing pelvic floor dysfunction.1-4,6 Consequently, all patients with dyspareunia should be assessed and continually monitored for symptoms of vaginismus.
Continue to: Anxiety
Anxiety. As with other pain disorders, anxiety develops around pain triggers.10,15 When expecting sexual activity, patients can experience extreme worry and panic attacks.10,15,16 The distress of sexual encounters can interfere with physiologic arousal and sexual desire, impacting all phases of the sexual response cycle.1,2
Relationship issues. Difficulty engaging in or avoidance of sexual activity can interfere with romantic relationships.2,10,16 Severe pain or vaginismus contractions can prevent penetration, leading to unconsummated marriages and an inability to conceive through intercourse.10 The distress surrounding sexual encounters can precipitate erectile dysfunction in male partners, or partners may continue to demand sexual encounters despite the patient’s pain, further impacting the relationship and heightening sexual distress.10 These stressors have led to relationships ending, patients reluctantly agreeing to nonmonogamy to appease their partners, and patients avoiding relationships altogether.10,16
Devalued self-image. Difficulties with sexuality and relationships impact the self-image of patients with dyspareunia. Diminished self-image may include feeling “inadequate” as a woman and as a sexual partner, or feeling like a “failure.”16 Women with dyspareunia often have more distress related to their body image, physical appearance, and genital self-image than do women without genital pain.17 Feeling resentment toward their body, or feeling “ugly,” embarrassed, shamed, “broken,” and “useless” also contribute to increased depressive symptoms found in patients with dyspareunia.16,18
Making the diagnosis
Most patients do not report symptoms unless directly asked2,7; therefore, it is recommended that all patients be screened as a part of an initial intake and before any genital exam (TABLE 22-4,6,7,9,11,19,20).4,7,21 If this screen is positive, a separate appointment may be needed for a thorough evaluation and before any attempt is made at a genital exam.4,7

Items to include in the clinical interview
Given the range of possible causes of dyspareunia and its contributing factors and symptoms, a thorough clinical interview is essential. Begin with a review of the patient’s complete medical and surgical history to identify possible known contributors to genital pain.4 Pregnancy history is of particular importance as the prevalence of postpartum dyspareunia is 35%, with risk being greater for patients who experienced dyspareunia symptoms before pregnancy.22

Knowing the location and quality of pain is important for differentiating between possible diagnoses, as is specifying dyspareunia as lifelong or acquired, superficial or deep, and primary or secondary.1-4,6 Confirm the specific location(s) of pain—eg, at the introitus, in the vestibule, on the labia, in the perineum, or near the clitoris.2,4,6 A diagram or model may be needed to help patients to localize pain.4
To help narrow the differential, include the following elements in your assessment: pain quality, timing (eg, initial onset, episode onset, episode duration, situational triggers), alleviating factors, symptoms in surrounding structures (eg, bladder, bowel, muscles, bones), sexual history, other areas of sexual functioning, history of psychological trauma, relationship effects, and mental health (TABLE 22-4,6,7,9,11,19,20 and Table 323-28). Screening for a history of sexual trauma is particularly important, as a recent systematic review and meta-analysis found that women with a history of sexual assault had a 42% higher risk of gynecologic problems overall, a 74% higher risk of dyspareunia, and a 71% higher risk of vaginismus than women without a history of sexual assault.29 Using measures such as the Female Sexual Function Index or the McGill Pain Questionnaire can help patients more thoroughly describe their symptoms (TABLE 323-28).3

Continue to: Guidelines for the physical exam
Guidelines for the physical exam
Before the exam, ensure the patient has not used any topical genital treatment in the past 2 weeks that may interfere with sensitivity to the exam.4 To decrease patients’ anxiety about the exam, remind them that they can stop the exam at any time.7 Also consider offering the use of a mirror to better pinpoint the location of pain, and to possibly help the patient learn more about her anatomy.2,7
Begin the exam by palpating surrounding areas that may be involved in pain, including the abdomen and musculoskeletal features.3,6,19 Next visually inspect the external genitalia for lesions, abrasions, discoloration, erythema, or other abnormal findings.2,3,6 Ask the patient for permission before contacting the genitals. Because the labia may be a site of pain, apply gentle pressure in retracting it to fully examine the vestibule.6,7 Contraction of the pelvic floor muscles during approach or initial palpation could signal possible vaginismus.4
After visual inspection of external genitalia, use a cotton swab to map the vulva and vestibule in a clockwise fashion to precisely identify any painful locations.2-4,6 If the patient’s history of pain has been intermittent, it’s possible that the cotton swab will not elicit pain on the day of the initial exam, but it may on other days.4
Begin the internal exam by inserting a single finger into the first inch of the vagina and have the patient squeeze and release to assess tenderness, muscle tightness, and control.2,6 Advance the finger further into the vagina and palpate clockwise, examining the levator muscles, obturator muscles, rectum, urethra, and bladder for abnormal tightness or reproduction of pain.2,4,6 Complete a bimanual exam to evaluate the pelvic organs and adnexa.2,4 If indicated, a more thorough evaluation of pelvic floor musculature can be performed by a physical therapist or gynecologist who specializes in pelvic pain.2-4
If the patient consents to further evaluation, consider using a small speculum, advanced slowly, for further internal examination, noting any lesions, abrasions, discharge, ectropion, or tenderness.2-4,7 A rectal exam may also be needed in cases of deep dyspareunia.6 Initial work-up may include a potassium hydroxide wet prep, sexually transmitted infection testing, and pelvic ultrasound.2,4 In some cases, laparoscopy or biopsy may be needed.2,4
Treatments for common causes
Treatment often begins with education about anatomy, to help patients communicate about symptoms and engage more fully in their care.3 Additional education may be needed on genital functioning and the necessity of adequate stimulation and lubrication prior to penetration.1,2,9-11 A discussion of treatments for the wide range of possible causes of dyspareunia is outside the scope of this article. However, some basic behavioral changes may help patients address some of the more common contributing factors.
For example, if vaginal infection is suspected, advise patients to discontinue the use of harsh soaps, known vaginal irritants (eg, perfumed products, bath additives), and douches.3 Recommend using only preservative- and alcohol-free lubricants for sexual contact, and avoiding lubricants with added functions (eg, warming).3 It’s worth noting that avoidance of tight clothing and thong underwear due to possible risk for infections may not be necessary. A recent study found that women who frequently wore thong underwear (more than half of the time) were no more likely to develop urinary tract infections, yeast vaginitis, or bacterial vaginosis than those who avoid such items.30 However, noncotton underwear fabric, rather than tightness, was associated with yeast vaginitis30; therefore, patients may want to consider using only breathable underwear.3
Continue to: Medication
Medication. Medication may be used to treat the underlying contributing conditions or the symptom of pain directly. Some common options are particularly important for patients whose dyspareunia does not have an identifiable cause. These medications include anti-inflammatory agents, topical anesthetics, tricyclic antidepressants, and hormonal treatments.2-4 Since effectiveness varies based on subtypes of pain, select a medication according to the location, timing, and hypothesized mechanism of pain.3,31,32
Medication for deep pain. A meta-analysis and systematic review found that patients with some types of chronic pelvic pain with pain deep in the vagina or pelvis experienced greater than 50% reduction in pain using medroxyprogesterone acetate compared with placebo.33 Other treatments for deep pain depend on physical exam findings.
Medication for superficial pain. Many remedies have been tried, with at least 26 different treatments for vulvodynia pain alone.16 Only some of these treatments have supporting evidence. For patients with vulvar pain, an intent-to-treat RCT found that patients using a topical steroid experienced a 23% reduction in pain from pre-treatment to 6-month follow-up.32
Surgery is also effective for vulvar pain.34,35 For provoked vestibulodynia (in which pain is localized to the vestibule and triggered by contact with the vulva), or vulvar vestibulitis, RCTs have found that vestibulectomy has stronger effects on pain than other treatments,31,35 with a 53% reduction in pain during intercourse and a 70% reduction in vestibular pain overall.35 However, while vestibulectomy is effective for provoked vestibulodynia, it is not recommended for generalized vulvodynia, in which pain is diffuse across the vulva and occurs without vulvar contact.34
Unsupported treatments. A number of other treatments have not yet been found effective. Although lidocaine for vulvar pain is often used, RCTs have not found any significant reduction in symptoms, and a double-blind RCT found that lidocaine ointment actually performed worse than placebo.31,34 Similarly, oral tricyclics have not been found to decrease vulvar pain more than placebo in double-blind studies.31,34 Furthermore, a meta-analysis of RCTs comparing treatments with placebo for vestibular pain found no significant decrease in dyspareunia for topical conjugated estrogen, topical lidocaine, oral desipramine, oral desipramine with topical lidocaine, laser therapy, or transcranial direct current.32
Tx risks to consider. Risks and benefits of dyspareunia treatment options should be thoroughly weighed and discussed with the patient.2-4 Vestibulectomy, despite reducing pain for many patients, has led to increased pain for 9% of patients who underwent the procedure.35 Topical treatments may lead to allergic reactions, inflammation, and worsening of symptoms,4 and hormonal treatments have been found to increase the risk of weight gain and bloating and are not appropriate for patients trying to conceive.33
Coordinate care with other providers
While medications and surgery can reduce pain, they have not been shown to improve other aspects of sexual functioning such as sexual satisfaction, frequency of sexual intercourse, or overall sense of sexual functioning.35 Additionally, pain reduction does not address muscle tension, anxiety, self-esteem, and relationship problems. As a result, a multidisciplinary approach is generally needed.3,4,32,33
Continue to: Physical therapists
Physical therapists. Pelvic floor physical therapists are often members of the dyspareunia treatment team and can provide a thorough evaluation and treatment of pelvic floor disorders.2-4 An RCT with intent-to-treat analysis found that pain was reduced by 71% following pelvic floor physical therapy.36 Another RCT found that 90% of patients reported a clinically meaningful decrease in pain with pelvic floor physical therapy.37 In addition to addressing pain, pelvic floor physical therapy has also been found to improve sexual functioning, sexual satisfaction, distress, and patient perception of improvement.34,36,37
Behavioral health specialists. Psychotherapists, especially those trained in sex therapy, couples therapy, or cognitive behavioral therapy (CBT), are also typically on the treatment team. Multiple RCTs have found evidence of CBT’s effectiveness in the direct treatment of dyspareunia pain. Bergeron et al35 found a 37.5% reduction in vulvar vestibulitis pain intensity during intercourse after patients completed group CBT. Another intent-to-treat RCT found that patients receiving CBT experienced more pain reduction (~ 30%) than patients who were treated with a topical steroid.38
In addition to having a direct impact on pain, CBT has also been found to have a clinically and statistically significant positive impact on other aspects of sexual experience, such as overall sexuality, self-efficacy, overall sexual functioning, frequency of intercourse, and catastrophizing.34,38 A recent meta-analysis of RCTs found that about 80% of vaginismus patients were able to achieve penetrative intercourse after treatment with behavioral sex therapy or CBT.39 This success rate was not exceeded by physical or surgical treatments.39
When PTSD is thought to be a contributing factor, trauma therapy will likely be needed in addition to treatments for dyspareunia. First-line treatments for PTSD include cognitive processing therapy, prolonged exposure, trauma-focused CBT, and cognitive therapy.40
Psychotherapists can also help patients reduce anxiety, reintroduce sexual contact without triggering pain or anxiety, address emotional and self-esteem effects of dyspareunia, address relationship issues, and refocus sexual encounters on pleasure rather than pain avoidance.2-4 Despite patient reports of high treatment satisfaction following therapy,38 many patients may initially lack confidence in psychotherapy as a treatment for pain35 and may need to be educated on its effectiveness and multidimensional benefits.
Gynecologists. Often a gynecologist with specialization in pelvic pain is an essential member of the team for diagnostic clarification, recommendation of treatment options, and performance of more advanced treatments.2,3 If pain has become chronic, the patient may also benefit from a pain management team and support groups.2,3
Follow-up steps
Patients who screen negative for dyspareunia should be re-screened periodically. Continue to assess patients diagnosed with dyspareunia for vaginismus symptoms (if they are not initially present) to ensure that the treatment plan is appropriately adjusted. Once treatment has begun, ask about adverse effects and confidence in the treatment plan to minimize negative impacts on treatment adherence and to anticipate a need for a change in the treatment approach.31,35 In addition to tracking treatment effects on pain, continue to assess for patient-centered outcomes such as emotional functioning, self-esteem, and sexual and relationship satisfaction.34 The Female Sexual Function Index can be a useful tool to track symptoms.27,34
Finally, patients who do not experience sufficient improvement in symptoms and functioning with initial treatment may need continued support and encouragement. Given the broad range of contributing factors and the high number of potential treatments, patients may find hope in learning that multiple other treatment options may be available.
CORRESPONDENCE
Adrienne A. Williams, PhD, Department of Family and Community Medicine, University of Illinois at Chicago College of Medicine, 1919 W Taylor Street, MC 663, Chicago, IL 60612; [email protected]
Dyspareunia is persistent or recurrent pain before, during, or after sexual contact and is not limited to cisgender individuals or vaginal intercourse.1-3 With a prevalence as high as 45% in the United States,2-5 it is one of the most common complaints in gynecologic practices.5,6
Causes and contributing factors
There are many possible causes of dyspareunia.2,4,6 While some patients have a single cause, most cases are complex, with multiple overlapping causes and maintaining factors.4,6 Identifying each contributing factor can help you appropriately address all components.
Physical conditions. The range of physical contributors to dyspareunia includes inflammatory processes, structural abnormalities, musculoskeletal dysfunctions, pelvic organ disorders, injuries, iatrogenic effects, infections, allergic reactions, sensitization, hormonal changes, medication effects, adhesions, autoimmune disorders, and other pain syndromes (TABLE 12-4,6-11).

Inadequate arousal. One of the primary causes of pain during vaginal penetration is inadequate arousal and lubrication.1,2,9-11 Arousal is the phase of the sexual response cycle that leads to genital tumescence and prepares the genitals for sexual contact through penile/clitoral erection, vaginal engorgement, and lubrication, which prevents pain and enhances pleasurable sensation.9-11
While some physical conditions can lead to an inability to lubricate, the most common causes of inadequate lubrication are psychosocial-behavioral, wherein patients have the same physical ability to lubricate as patients without genital pain but do not progress through the arousal phase.9-11 Behavioral factors such as inadequate or ineffective foreplay can fail to produce engorgement and lubrication, while psychosocial factors such as low attraction to partner, relationship stressors, anxiety, or low self-esteem can have an inhibitory effect on sexual arousal.1,2,9-11 Psychosocial and behavioral factors may also be maintaining factors or consequences of dyspareunia, and need to be assessed and treated.1,2,9-11
Psychological trauma. Exposure to psychological traumas and the development of posttraumatic stress disorder (PTSD) have been linked with the development of pain disorders in general and dyspareunia specifically. Most patients seeking treatment for chronic pain disorders have a history of physical or sexual abuse.12 Changes in physiologic processes (eg, neurochemical, endocrine) that occur with PTSD interfere with the sexual response cycle, and sexual traumas specifically have been linked with pelvic floor dysfunction.13,14 Additionally, when PTSD is caused by a sexual trauma, even consensual sexual encounters can trigger flashbacks, intrusive memories, hyperarousal, and muscle tension that interfere with the sexual response cycle and contribute to genital pain.13
Vaginismus is both a physiologic and psychological contributor to dyspareunia.1,2,4 Patients experiencing pain can develop anxiety about repeated pain and involuntarily contract their pelvic muscles, thereby creating more pain, increasing anxiety, decreasing lubrication, and causing pelvic floor dysfunction.1-4,6 Consequently, all patients with dyspareunia should be assessed and continually monitored for symptoms of vaginismus.
Continue to: Anxiety
Anxiety. As with other pain disorders, anxiety develops around pain triggers.10,15 When expecting sexual activity, patients can experience extreme worry and panic attacks.10,15,16 The distress of sexual encounters can interfere with physiologic arousal and sexual desire, impacting all phases of the sexual response cycle.1,2
Relationship issues. Difficulty engaging in or avoidance of sexual activity can interfere with romantic relationships.2,10,16 Severe pain or vaginismus contractions can prevent penetration, leading to unconsummated marriages and an inability to conceive through intercourse.10 The distress surrounding sexual encounters can precipitate erectile dysfunction in male partners, or partners may continue to demand sexual encounters despite the patient’s pain, further impacting the relationship and heightening sexual distress.10 These stressors have led to relationships ending, patients reluctantly agreeing to nonmonogamy to appease their partners, and patients avoiding relationships altogether.10,16
Devalued self-image. Difficulties with sexuality and relationships impact the self-image of patients with dyspareunia. Diminished self-image may include feeling “inadequate” as a woman and as a sexual partner, or feeling like a “failure.”16 Women with dyspareunia often have more distress related to their body image, physical appearance, and genital self-image than do women without genital pain.17 Feeling resentment toward their body, or feeling “ugly,” embarrassed, shamed, “broken,” and “useless” also contribute to increased depressive symptoms found in patients with dyspareunia.16,18
Making the diagnosis
Most patients do not report symptoms unless directly asked2,7; therefore, it is recommended that all patients be screened as a part of an initial intake and before any genital exam (TABLE 22-4,6,7,9,11,19,20).4,7,21 If this screen is positive, a separate appointment may be needed for a thorough evaluation and before any attempt is made at a genital exam.4,7

Items to include in the clinical interview
Given the range of possible causes of dyspareunia and its contributing factors and symptoms, a thorough clinical interview is essential. Begin with a review of the patient’s complete medical and surgical history to identify possible known contributors to genital pain.4 Pregnancy history is of particular importance as the prevalence of postpartum dyspareunia is 35%, with risk being greater for patients who experienced dyspareunia symptoms before pregnancy.22

Knowing the location and quality of pain is important for differentiating between possible diagnoses, as is specifying dyspareunia as lifelong or acquired, superficial or deep, and primary or secondary.1-4,6 Confirm the specific location(s) of pain—eg, at the introitus, in the vestibule, on the labia, in the perineum, or near the clitoris.2,4,6 A diagram or model may be needed to help patients to localize pain.4
To help narrow the differential, include the following elements in your assessment: pain quality, timing (eg, initial onset, episode onset, episode duration, situational triggers), alleviating factors, symptoms in surrounding structures (eg, bladder, bowel, muscles, bones), sexual history, other areas of sexual functioning, history of psychological trauma, relationship effects, and mental health (TABLE 22-4,6,7,9,11,19,20 and Table 323-28). Screening for a history of sexual trauma is particularly important, as a recent systematic review and meta-analysis found that women with a history of sexual assault had a 42% higher risk of gynecologic problems overall, a 74% higher risk of dyspareunia, and a 71% higher risk of vaginismus than women without a history of sexual assault.29 Using measures such as the Female Sexual Function Index or the McGill Pain Questionnaire can help patients more thoroughly describe their symptoms (TABLE 323-28).3

Continue to: Guidelines for the physical exam
Guidelines for the physical exam
Before the exam, ensure the patient has not used any topical genital treatment in the past 2 weeks that may interfere with sensitivity to the exam.4 To decrease patients’ anxiety about the exam, remind them that they can stop the exam at any time.7 Also consider offering the use of a mirror to better pinpoint the location of pain, and to possibly help the patient learn more about her anatomy.2,7
Begin the exam by palpating surrounding areas that may be involved in pain, including the abdomen and musculoskeletal features.3,6,19 Next visually inspect the external genitalia for lesions, abrasions, discoloration, erythema, or other abnormal findings.2,3,6 Ask the patient for permission before contacting the genitals. Because the labia may be a site of pain, apply gentle pressure in retracting it to fully examine the vestibule.6,7 Contraction of the pelvic floor muscles during approach or initial palpation could signal possible vaginismus.4
After visual inspection of external genitalia, use a cotton swab to map the vulva and vestibule in a clockwise fashion to precisely identify any painful locations.2-4,6 If the patient’s history of pain has been intermittent, it’s possible that the cotton swab will not elicit pain on the day of the initial exam, but it may on other days.4
Begin the internal exam by inserting a single finger into the first inch of the vagina and have the patient squeeze and release to assess tenderness, muscle tightness, and control.2,6 Advance the finger further into the vagina and palpate clockwise, examining the levator muscles, obturator muscles, rectum, urethra, and bladder for abnormal tightness or reproduction of pain.2,4,6 Complete a bimanual exam to evaluate the pelvic organs and adnexa.2,4 If indicated, a more thorough evaluation of pelvic floor musculature can be performed by a physical therapist or gynecologist who specializes in pelvic pain.2-4
If the patient consents to further evaluation, consider using a small speculum, advanced slowly, for further internal examination, noting any lesions, abrasions, discharge, ectropion, or tenderness.2-4,7 A rectal exam may also be needed in cases of deep dyspareunia.6 Initial work-up may include a potassium hydroxide wet prep, sexually transmitted infection testing, and pelvic ultrasound.2,4 In some cases, laparoscopy or biopsy may be needed.2,4
Treatments for common causes
Treatment often begins with education about anatomy, to help patients communicate about symptoms and engage more fully in their care.3 Additional education may be needed on genital functioning and the necessity of adequate stimulation and lubrication prior to penetration.1,2,9-11 A discussion of treatments for the wide range of possible causes of dyspareunia is outside the scope of this article. However, some basic behavioral changes may help patients address some of the more common contributing factors.
For example, if vaginal infection is suspected, advise patients to discontinue the use of harsh soaps, known vaginal irritants (eg, perfumed products, bath additives), and douches.3 Recommend using only preservative- and alcohol-free lubricants for sexual contact, and avoiding lubricants with added functions (eg, warming).3 It’s worth noting that avoidance of tight clothing and thong underwear due to possible risk for infections may not be necessary. A recent study found that women who frequently wore thong underwear (more than half of the time) were no more likely to develop urinary tract infections, yeast vaginitis, or bacterial vaginosis than those who avoid such items.30 However, noncotton underwear fabric, rather than tightness, was associated with yeast vaginitis30; therefore, patients may want to consider using only breathable underwear.3
Continue to: Medication
Medication. Medication may be used to treat the underlying contributing conditions or the symptom of pain directly. Some common options are particularly important for patients whose dyspareunia does not have an identifiable cause. These medications include anti-inflammatory agents, topical anesthetics, tricyclic antidepressants, and hormonal treatments.2-4 Since effectiveness varies based on subtypes of pain, select a medication according to the location, timing, and hypothesized mechanism of pain.3,31,32
Medication for deep pain. A meta-analysis and systematic review found that patients with some types of chronic pelvic pain with pain deep in the vagina or pelvis experienced greater than 50% reduction in pain using medroxyprogesterone acetate compared with placebo.33 Other treatments for deep pain depend on physical exam findings.
Medication for superficial pain. Many remedies have been tried, with at least 26 different treatments for vulvodynia pain alone.16 Only some of these treatments have supporting evidence. For patients with vulvar pain, an intent-to-treat RCT found that patients using a topical steroid experienced a 23% reduction in pain from pre-treatment to 6-month follow-up.32
Surgery is also effective for vulvar pain.34,35 For provoked vestibulodynia (in which pain is localized to the vestibule and triggered by contact with the vulva), or vulvar vestibulitis, RCTs have found that vestibulectomy has stronger effects on pain than other treatments,31,35 with a 53% reduction in pain during intercourse and a 70% reduction in vestibular pain overall.35 However, while vestibulectomy is effective for provoked vestibulodynia, it is not recommended for generalized vulvodynia, in which pain is diffuse across the vulva and occurs without vulvar contact.34
Unsupported treatments. A number of other treatments have not yet been found effective. Although lidocaine for vulvar pain is often used, RCTs have not found any significant reduction in symptoms, and a double-blind RCT found that lidocaine ointment actually performed worse than placebo.31,34 Similarly, oral tricyclics have not been found to decrease vulvar pain more than placebo in double-blind studies.31,34 Furthermore, a meta-analysis of RCTs comparing treatments with placebo for vestibular pain found no significant decrease in dyspareunia for topical conjugated estrogen, topical lidocaine, oral desipramine, oral desipramine with topical lidocaine, laser therapy, or transcranial direct current.32
Tx risks to consider. Risks and benefits of dyspareunia treatment options should be thoroughly weighed and discussed with the patient.2-4 Vestibulectomy, despite reducing pain for many patients, has led to increased pain for 9% of patients who underwent the procedure.35 Topical treatments may lead to allergic reactions, inflammation, and worsening of symptoms,4 and hormonal treatments have been found to increase the risk of weight gain and bloating and are not appropriate for patients trying to conceive.33
Coordinate care with other providers
While medications and surgery can reduce pain, they have not been shown to improve other aspects of sexual functioning such as sexual satisfaction, frequency of sexual intercourse, or overall sense of sexual functioning.35 Additionally, pain reduction does not address muscle tension, anxiety, self-esteem, and relationship problems. As a result, a multidisciplinary approach is generally needed.3,4,32,33
Continue to: Physical therapists
Physical therapists. Pelvic floor physical therapists are often members of the dyspareunia treatment team and can provide a thorough evaluation and treatment of pelvic floor disorders.2-4 An RCT with intent-to-treat analysis found that pain was reduced by 71% following pelvic floor physical therapy.36 Another RCT found that 90% of patients reported a clinically meaningful decrease in pain with pelvic floor physical therapy.37 In addition to addressing pain, pelvic floor physical therapy has also been found to improve sexual functioning, sexual satisfaction, distress, and patient perception of improvement.34,36,37
Behavioral health specialists. Psychotherapists, especially those trained in sex therapy, couples therapy, or cognitive behavioral therapy (CBT), are also typically on the treatment team. Multiple RCTs have found evidence of CBT’s effectiveness in the direct treatment of dyspareunia pain. Bergeron et al35 found a 37.5% reduction in vulvar vestibulitis pain intensity during intercourse after patients completed group CBT. Another intent-to-treat RCT found that patients receiving CBT experienced more pain reduction (~ 30%) than patients who were treated with a topical steroid.38
In addition to having a direct impact on pain, CBT has also been found to have a clinically and statistically significant positive impact on other aspects of sexual experience, such as overall sexuality, self-efficacy, overall sexual functioning, frequency of intercourse, and catastrophizing.34,38 A recent meta-analysis of RCTs found that about 80% of vaginismus patients were able to achieve penetrative intercourse after treatment with behavioral sex therapy or CBT.39 This success rate was not exceeded by physical or surgical treatments.39
When PTSD is thought to be a contributing factor, trauma therapy will likely be needed in addition to treatments for dyspareunia. First-line treatments for PTSD include cognitive processing therapy, prolonged exposure, trauma-focused CBT, and cognitive therapy.40
Psychotherapists can also help patients reduce anxiety, reintroduce sexual contact without triggering pain or anxiety, address emotional and self-esteem effects of dyspareunia, address relationship issues, and refocus sexual encounters on pleasure rather than pain avoidance.2-4 Despite patient reports of high treatment satisfaction following therapy,38 many patients may initially lack confidence in psychotherapy as a treatment for pain35 and may need to be educated on its effectiveness and multidimensional benefits.
Gynecologists. Often a gynecologist with specialization in pelvic pain is an essential member of the team for diagnostic clarification, recommendation of treatment options, and performance of more advanced treatments.2,3 If pain has become chronic, the patient may also benefit from a pain management team and support groups.2,3
Follow-up steps
Patients who screen negative for dyspareunia should be re-screened periodically. Continue to assess patients diagnosed with dyspareunia for vaginismus symptoms (if they are not initially present) to ensure that the treatment plan is appropriately adjusted. Once treatment has begun, ask about adverse effects and confidence in the treatment plan to minimize negative impacts on treatment adherence and to anticipate a need for a change in the treatment approach.31,35 In addition to tracking treatment effects on pain, continue to assess for patient-centered outcomes such as emotional functioning, self-esteem, and sexual and relationship satisfaction.34 The Female Sexual Function Index can be a useful tool to track symptoms.27,34
Finally, patients who do not experience sufficient improvement in symptoms and functioning with initial treatment may need continued support and encouragement. Given the broad range of contributing factors and the high number of potential treatments, patients may find hope in learning that multiple other treatment options may be available.
CORRESPONDENCE
Adrienne A. Williams, PhD, Department of Family and Community Medicine, University of Illinois at Chicago College of Medicine, 1919 W Taylor Street, MC 663, Chicago, IL 60612; [email protected]
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Ed. American Psychiatric Publishing; 2013.
2. Seehusen DA, Baird DC, Bode DV. Dyspareunia in women. Am Fam Phys. 2014;90:465-470.
3. Sorensen J, Bautista KE, Lamvu G, et al. Evaluation and treatment of female sexual pain: a clinical review. Cureus. 2018;10:e2379.
4. MacNeill C. Dyspareunia. Obstet Gynecol Clin North Am. 2006;33:565-77.
5. Latthe P, Latthe M, Say L, et al. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health. 2006;6:177.
6. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009;113:1124-1136.
7. Williams AA, Williams M. A guide to performing pelvic speculum exams: a patient-centered approach to reducing iatrogenic effects. Teach Learn Med. 2013;25:383-391.
8. Ünlü Z, Yentur A, Çakil N. Pudendal nerve neuropathy: An unknown-rare cause of pelvic pain. Arch Rheumatol. 2016;31:102-103.
9. Dewitte M, Borg C, Lowenstein L. A psychosocial approach to female genital pain. Nat Rev Urol. 2018;15:25-41.
10. Masters WH, Johnson VE. Human Sexual Inadequacy. 1st ed. Little, Brown; 1970.
11. Rathus SA, Nevid JS, Fichner-Rathus L. Human Sexuality in a World of Diversity. 5th ed. Allyn and Bacon; 2002.
12. Bailey BE, Freedenfeld RN, Kiser RS, et al. Lifetime physical and sexual abuse in chronic pain patients: psychosocial correlates and treatment outcomes. Disabil Rehabil. 2003;25:331-342.
13. Yehuda R, Lehrner A, Rosenbaum TY. PTSD and sexual dysfunction in men and women. J Sex Med. 2015;12:1107-1119.
14. Postma R, Bicanic I, van der Vaart H, et al. Pelvic floor muscle problems mediate sexual problems in young adult rape victims. J Sex Med. 2013;10:1978-1987.
15. Binik YM, Bergeron S, Khalifé S. Dyspareunia and vaginismus: so-called sexual pain. In: Leiblum SR, ed. 4th ed. Principles and Practice of Sex Therapy. The Guilford Press; 2007:124-156.
16. Ayling K, Ussher JM. “If sex hurts, am I still a woman?” The subjective experience of vulvodynia in hetero-sexual women. Arch Sex Behav. 2008;37:294-304.
17. Pazmany E, Bergeron S, Van Oudenhove L, et al. Body image and genital self-image in pre-menopausal women with dyspareunia. Arch Sex Behav. 2013;42:999-1010.
18. Maillé DL, Bergeron S, Lambert B. Body image in women with primary and secondary provoked vestibulodynia: a controlled study. J Sex Med. 2015;12:505-515.
19. Ryan L, Hawton K. Female dyspareunia. BMJ. 2004;328:1357.
20. Waldura JF, Arora I, Randall AM, et al. Fifty shades of stigma: exploring the health care experiences of kink-oriented patients. J Sex Med. 2016;13:1918-1929.
21. Hinchliff S, Gott M. Seeking medical help for sexual concerns in mid- and later life: a review of the literature. J Sex Res. 2011;48:106-117.
22. Banaei M, Kariman N, Ozgoli G, et al. Prevalence of postpartum dyspareunia: a systematic review and meta-analysis. Int J Gynaecol Obstet. 2021;153:14-24.
23. Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509-515.
24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
25. U.S. Department of Veterans Affairs. PTSD: National Center for PTSD. Life events checklist for DSM-5 (LEC-5). Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp
26. Weathers FW, Litz BT, Keane TM, et al. The PTSD checklist for DSM-5 (PCL-5). 2013. Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp
27. Rosen R, Brown C, Heiman J, et al. The female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191-208.
28. Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191-197.
29. Hassam T, Kelso E, Chowdary P, et al. Sexual assault as a risk factor for gynaecological morbidity: an exploratory systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;255:222-230.
30. Hamlin AA, Sheeder J, Muffly TM. Brief versus thong hygiene in obstetrics and gynecology (B-THONG): a survey study. J Obstet Gynaecol Res. 2019;45:1190-1196.
31. Foster DC, Kotok MB, Huang LS, et al. Oral desipramine and topical lidocaine for vulvodynia: a randomized controlled trial. Obstet Gynecol. 2010;116:583-593.
32. Pérez-López FR, Bueno-Notivol J, Hernandez AV, et al. Systematic review and meta-analysis of the effects of treatment modalities for vestibulodynia in women. Eur J Contracept Reprod Health Care. 2019;24:337-346.
33. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;(3):CD008797.
34. Goldstein AT, Pukall CF, Brown C, et al. Vulvodynia: assessment and treatment. J Sex Med. 2016;13:572-590.
35. Bergeron S, Binik YM, Khalifé S, et al. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91:297-306.
36. Schvartzman R, Schvartzman L, Ferreira CF, et al. Physical therapy intervention for women with dyspareunia: a randomized clinical trial. J Sex Marital Ther. 2019;45:378-394.
37. Morin M, Dumoulin C, Bergeron S, et al. Multimodal physical therapy versus topical lidocaine for provoked vestibulodynia: a multicenter, randomized trial. Am J Obstet Gynecol. 2021;224:189.e1-189.e12.
38. Bergeron S, Khalifé S, Dupuis M-J, et al. A randomized clinical trial comparing group cognitive-behavioral therapy and a topical steroid for women with dyspareunia. J Consult Clin Psychol. 2016;84:259-268.
39. Maseroli E, Scavello I, Rastrelli G, et al. Outcome of medical and psychosexual interventions for vaginismus: a systematic review and meta-analysis. J Sex Med. 2018;15:1752-1764.
40. American Psychological Association. Clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. 2017. Accessed February 3, 2022. www.apa.org/ptsd-guideline/ptsd.pdf
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Ed. American Psychiatric Publishing; 2013.
2. Seehusen DA, Baird DC, Bode DV. Dyspareunia in women. Am Fam Phys. 2014;90:465-470.
3. Sorensen J, Bautista KE, Lamvu G, et al. Evaluation and treatment of female sexual pain: a clinical review. Cureus. 2018;10:e2379.
4. MacNeill C. Dyspareunia. Obstet Gynecol Clin North Am. 2006;33:565-77.
5. Latthe P, Latthe M, Say L, et al. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health. 2006;6:177.
6. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009;113:1124-1136.
7. Williams AA, Williams M. A guide to performing pelvic speculum exams: a patient-centered approach to reducing iatrogenic effects. Teach Learn Med. 2013;25:383-391.
8. Ünlü Z, Yentur A, Çakil N. Pudendal nerve neuropathy: An unknown-rare cause of pelvic pain. Arch Rheumatol. 2016;31:102-103.
9. Dewitte M, Borg C, Lowenstein L. A psychosocial approach to female genital pain. Nat Rev Urol. 2018;15:25-41.
10. Masters WH, Johnson VE. Human Sexual Inadequacy. 1st ed. Little, Brown; 1970.
11. Rathus SA, Nevid JS, Fichner-Rathus L. Human Sexuality in a World of Diversity. 5th ed. Allyn and Bacon; 2002.
12. Bailey BE, Freedenfeld RN, Kiser RS, et al. Lifetime physical and sexual abuse in chronic pain patients: psychosocial correlates and treatment outcomes. Disabil Rehabil. 2003;25:331-342.
13. Yehuda R, Lehrner A, Rosenbaum TY. PTSD and sexual dysfunction in men and women. J Sex Med. 2015;12:1107-1119.
14. Postma R, Bicanic I, van der Vaart H, et al. Pelvic floor muscle problems mediate sexual problems in young adult rape victims. J Sex Med. 2013;10:1978-1987.
15. Binik YM, Bergeron S, Khalifé S. Dyspareunia and vaginismus: so-called sexual pain. In: Leiblum SR, ed. 4th ed. Principles and Practice of Sex Therapy. The Guilford Press; 2007:124-156.
16. Ayling K, Ussher JM. “If sex hurts, am I still a woman?” The subjective experience of vulvodynia in hetero-sexual women. Arch Sex Behav. 2008;37:294-304.
17. Pazmany E, Bergeron S, Van Oudenhove L, et al. Body image and genital self-image in pre-menopausal women with dyspareunia. Arch Sex Behav. 2013;42:999-1010.
18. Maillé DL, Bergeron S, Lambert B. Body image in women with primary and secondary provoked vestibulodynia: a controlled study. J Sex Med. 2015;12:505-515.
19. Ryan L, Hawton K. Female dyspareunia. BMJ. 2004;328:1357.
20. Waldura JF, Arora I, Randall AM, et al. Fifty shades of stigma: exploring the health care experiences of kink-oriented patients. J Sex Med. 2016;13:1918-1929.
21. Hinchliff S, Gott M. Seeking medical help for sexual concerns in mid- and later life: a review of the literature. J Sex Res. 2011;48:106-117.
22. Banaei M, Kariman N, Ozgoli G, et al. Prevalence of postpartum dyspareunia: a systematic review and meta-analysis. Int J Gynaecol Obstet. 2021;153:14-24.
23. Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509-515.
24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
25. U.S. Department of Veterans Affairs. PTSD: National Center for PTSD. Life events checklist for DSM-5 (LEC-5). Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp
26. Weathers FW, Litz BT, Keane TM, et al. The PTSD checklist for DSM-5 (PCL-5). 2013. Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp
27. Rosen R, Brown C, Heiman J, et al. The female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191-208.
28. Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191-197.
29. Hassam T, Kelso E, Chowdary P, et al. Sexual assault as a risk factor for gynaecological morbidity: an exploratory systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;255:222-230.
30. Hamlin AA, Sheeder J, Muffly TM. Brief versus thong hygiene in obstetrics and gynecology (B-THONG): a survey study. J Obstet Gynaecol Res. 2019;45:1190-1196.
31. Foster DC, Kotok MB, Huang LS, et al. Oral desipramine and topical lidocaine for vulvodynia: a randomized controlled trial. Obstet Gynecol. 2010;116:583-593.
32. Pérez-López FR, Bueno-Notivol J, Hernandez AV, et al. Systematic review and meta-analysis of the effects of treatment modalities for vestibulodynia in women. Eur J Contracept Reprod Health Care. 2019;24:337-346.
33. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;(3):CD008797.
34. Goldstein AT, Pukall CF, Brown C, et al. Vulvodynia: assessment and treatment. J Sex Med. 2016;13:572-590.
35. Bergeron S, Binik YM, Khalifé S, et al. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91:297-306.
36. Schvartzman R, Schvartzman L, Ferreira CF, et al. Physical therapy intervention for women with dyspareunia: a randomized clinical trial. J Sex Marital Ther. 2019;45:378-394.
37. Morin M, Dumoulin C, Bergeron S, et al. Multimodal physical therapy versus topical lidocaine for provoked vestibulodynia: a multicenter, randomized trial. Am J Obstet Gynecol. 2021;224:189.e1-189.e12.
38. Bergeron S, Khalifé S, Dupuis M-J, et al. A randomized clinical trial comparing group cognitive-behavioral therapy and a topical steroid for women with dyspareunia. J Consult Clin Psychol. 2016;84:259-268.
39. Maseroli E, Scavello I, Rastrelli G, et al. Outcome of medical and psychosexual interventions for vaginismus: a systematic review and meta-analysis. J Sex Med. 2018;15:1752-1764.
40. American Psychological Association. Clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. 2017. Accessed February 3, 2022. www.apa.org/ptsd-guideline/ptsd.pdf
PRACTICE RECOMMENDATIONS
› Screen all patients for sexual dysfunctions, as patients often do not report symptoms on their own. B
› Refer patients with dyspareunia for psychotherapy to address both pain and psychosocial causes and sequela of dyspareunia. A
› Refer patients with dyspareunia for pelvic floor physical therapy to address pain and sexual functioning. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
COVID-19 found in 29 types of animals, scientists say
according to researchers’ latest tally.
In most cases, humans infect animals, and animals don’t transmit the virus back to humans. But scientists have expressed concerns about recent research that shows some animals – such as mink and deer – appear to be able to spread the virus to humans.
In addition, the virus will likely continue to circulate in wild animals, which could lead to new mutations, some of which may make the virus less susceptible to people’s immunity from current vaccines. Researchers are calling for better surveillance of animals, especially in the wild, to track any new variants.
“It could be evolving in hosts we are not aware of,” Eman Anis, PhD, an assistant professor of microbiology at the University of Pennsylvania, Philadelphia, told the Philadelphia Inquirer.
Scientists have identified the virus in a growing list of animals, according to the Centers for Disease Control and Prevention, including cats, dogs, ferrets, gorillas, hamsters, hippos, hyenas, mice, otters, pigs, rabbits, and tigers. In many cases, humans spread the coronavirus to pets at home or to wildlife in zoos and sanctuaries.
In the study, published in bioRxiv, researchers identified a person who tested positive after close contact with infected white-tailed deer. The coronavirus had evolved dozens of mutations not found in other strains.
Even with the changes, the virus they found doesn’t appear different enough to evade current vaccines, the researchers reported. The vaccines target the spike protein on the outside of coronavirus cells, and the mutations that happened in deer occurred elsewhere in the virus.
At the same time, scientists have noted that this points to the need to step up monitoring in wild animals before mutations become a problem.
“This is no need to panic, but this is not something we can ignore,” Suresh Kuchipudi, PhD, a professor of veterinary and biomedical sciences at Pennsylvania State University in University Park, told the Inquirer.
Dr. Kuchipudi, who wasn’t involved with the Canadian study, has done other studies that found COVID-19 in deer. As the coronavirus continues to circulate in deer, more mutations will arise, he noted.
“It’s hard to predict what evolution’s going to come up with,” Frederic Bushman, a microbiology professor at the University of Pennsylvania, told the Inquirer.
“The virus will probably change different ways in different animals. Some of them probably won’t infect humans as well,” he said. “But the fear is that maybe some new one will come along that does infect humans well.”
A version of this article first appeared on WebMD.com.
according to researchers’ latest tally.
In most cases, humans infect animals, and animals don’t transmit the virus back to humans. But scientists have expressed concerns about recent research that shows some animals – such as mink and deer – appear to be able to spread the virus to humans.
In addition, the virus will likely continue to circulate in wild animals, which could lead to new mutations, some of which may make the virus less susceptible to people’s immunity from current vaccines. Researchers are calling for better surveillance of animals, especially in the wild, to track any new variants.
“It could be evolving in hosts we are not aware of,” Eman Anis, PhD, an assistant professor of microbiology at the University of Pennsylvania, Philadelphia, told the Philadelphia Inquirer.
Scientists have identified the virus in a growing list of animals, according to the Centers for Disease Control and Prevention, including cats, dogs, ferrets, gorillas, hamsters, hippos, hyenas, mice, otters, pigs, rabbits, and tigers. In many cases, humans spread the coronavirus to pets at home or to wildlife in zoos and sanctuaries.
In the study, published in bioRxiv, researchers identified a person who tested positive after close contact with infected white-tailed deer. The coronavirus had evolved dozens of mutations not found in other strains.
Even with the changes, the virus they found doesn’t appear different enough to evade current vaccines, the researchers reported. The vaccines target the spike protein on the outside of coronavirus cells, and the mutations that happened in deer occurred elsewhere in the virus.
At the same time, scientists have noted that this points to the need to step up monitoring in wild animals before mutations become a problem.
“This is no need to panic, but this is not something we can ignore,” Suresh Kuchipudi, PhD, a professor of veterinary and biomedical sciences at Pennsylvania State University in University Park, told the Inquirer.
Dr. Kuchipudi, who wasn’t involved with the Canadian study, has done other studies that found COVID-19 in deer. As the coronavirus continues to circulate in deer, more mutations will arise, he noted.
“It’s hard to predict what evolution’s going to come up with,” Frederic Bushman, a microbiology professor at the University of Pennsylvania, told the Inquirer.
“The virus will probably change different ways in different animals. Some of them probably won’t infect humans as well,” he said. “But the fear is that maybe some new one will come along that does infect humans well.”
A version of this article first appeared on WebMD.com.
according to researchers’ latest tally.
In most cases, humans infect animals, and animals don’t transmit the virus back to humans. But scientists have expressed concerns about recent research that shows some animals – such as mink and deer – appear to be able to spread the virus to humans.
In addition, the virus will likely continue to circulate in wild animals, which could lead to new mutations, some of which may make the virus less susceptible to people’s immunity from current vaccines. Researchers are calling for better surveillance of animals, especially in the wild, to track any new variants.
“It could be evolving in hosts we are not aware of,” Eman Anis, PhD, an assistant professor of microbiology at the University of Pennsylvania, Philadelphia, told the Philadelphia Inquirer.
Scientists have identified the virus in a growing list of animals, according to the Centers for Disease Control and Prevention, including cats, dogs, ferrets, gorillas, hamsters, hippos, hyenas, mice, otters, pigs, rabbits, and tigers. In many cases, humans spread the coronavirus to pets at home or to wildlife in zoos and sanctuaries.
In the study, published in bioRxiv, researchers identified a person who tested positive after close contact with infected white-tailed deer. The coronavirus had evolved dozens of mutations not found in other strains.
Even with the changes, the virus they found doesn’t appear different enough to evade current vaccines, the researchers reported. The vaccines target the spike protein on the outside of coronavirus cells, and the mutations that happened in deer occurred elsewhere in the virus.
At the same time, scientists have noted that this points to the need to step up monitoring in wild animals before mutations become a problem.
“This is no need to panic, but this is not something we can ignore,” Suresh Kuchipudi, PhD, a professor of veterinary and biomedical sciences at Pennsylvania State University in University Park, told the Inquirer.
Dr. Kuchipudi, who wasn’t involved with the Canadian study, has done other studies that found COVID-19 in deer. As the coronavirus continues to circulate in deer, more mutations will arise, he noted.
“It’s hard to predict what evolution’s going to come up with,” Frederic Bushman, a microbiology professor at the University of Pennsylvania, told the Inquirer.
“The virus will probably change different ways in different animals. Some of them probably won’t infect humans as well,” he said. “But the fear is that maybe some new one will come along that does infect humans well.”
A version of this article first appeared on WebMD.com.
A-fib prevention, treatment, and screening: Where does the evidence lead us?
Atrial fibrillation (AF) is a common problem confronting family physicians. In this issue of JFP, we offer 2 articles about AF: one on prevention and one on treatment. Both provide evidence-based guidance to help you refine your care. But gaps remain. I’ll get to that in a bit.
Prevention. This month’s PURL1 discusses a randomized controlled trial (RCT) that enrolled moderate alcohol drinkers with AF.2 Compared to those who continued to drink moderately, those who reduced their alcohol consumption to 2 drinks per week had a significant reduction in recurrent AF (73% vs 53%), fewer hospitalizations (20% vs 9%), and less moderate or severe symptoms (32% vs 10%). Although previous studies of moderate alcohol consumption have shown positive effects on heart disease, this study and other more recent studies cast serious doubt on this assertion.3
Treatment. In their applied evidence article, Osayande and Sharma4 pose the question: When is catheter ablation a sound option for your patient with AF? They give us an excellent, evidence-based answer and remind us that we must focus on the treatment goals: to prevent stroke and to control symptoms. They recommend a stepwise approach, starting with rate control, progressing to rhythm control, and saving catheter ablation for resistant cases. In nearly all cases, anticoagulation to prevent stroke must be a part of treatment, with the exception of those with very low risk (so-called “lone atrial fibrillation”).
Screening. And what about screening for asymptomatic AF? The US Preventive Services Task Force recently reaffirmed its conclusion that there is insufficient evidence for screening for asymptomatic AF (a topic discussed in an online Practice Alert Brief5).6 Since wearable exercise-monitoring devices can detect heart arrhythmias (and are advertised for this purpose), a patient may present after receiving a notification about asymptomatic AF. What shall we do in these cases? The dilemma is that your patient will know she has a potentially dangerous condition, but there is no evidence that treating it will result in more benefit than harm.
A recently published study suggests that we should be very cautious in recommending treatment. In an RCT of patients ages 70 to 90 years, 1501 patients received an implantable loop recorder, while 4503 received routine health care; median follow-up was 64.5 months.7 Although more cases of AF were detected (32% in the monitored group vs 12% in the usual care group), and oral anticoagulation treatment was started more frequently (30% vs 13%, respectively), there was no significant difference in the proportion of patients who had a stroke or systemic arterial embolism (4.5% vs 5.6%).7 Until we have more data, reassurance seems to be the best recommendation for asymptomatic AF.
1. Thiel DJ, Marshall RC, Rogers TS. Alcohol abstinence reduces A-fib burden in drinkers. J Fam Pract. 2022;71:85-87.
2. Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382:20-28. doi: 10.1056/NEJMoa1817591
3. Hoek AG, van Oort S, Mukamal KJ, et al. Alcohol consumption and cardiovascular disease risk: placing new data in context [published online ahead of print, 2022 Feb 7]. Curr Atheroscler Rep. doi: 10.1007/s11883-022-00992-1
4. Osayande AS, Sharma N. When is catheter ablation a sound option for your patient with A-fib? J Fam Pract. 2022;71:54-62.
5. Campos-Outcalt D. USPSTF releases updated guidance on asymptomatic A-fb. J Fam Pract. 2022;3. Accessed February 18, 2022. www.mdedge.com/familymedicine/article/251911/cardiology/uspstf-releases-updated-guidance-asymptomatic-fib
6. USPSTF. Screening for atrial fibrillation: US Preventive Services Task Force recommendation statement. JAMA. 2022;327:360-367. doi: 10.1001/jama.2021.23732
7. Svendsen JH, Diederichsen SZ, Hojberg S, et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): a randomised controlled trial. Lancet. 2021;398:1507-1516.
Atrial fibrillation (AF) is a common problem confronting family physicians. In this issue of JFP, we offer 2 articles about AF: one on prevention and one on treatment. Both provide evidence-based guidance to help you refine your care. But gaps remain. I’ll get to that in a bit.
Prevention. This month’s PURL1 discusses a randomized controlled trial (RCT) that enrolled moderate alcohol drinkers with AF.2 Compared to those who continued to drink moderately, those who reduced their alcohol consumption to 2 drinks per week had a significant reduction in recurrent AF (73% vs 53%), fewer hospitalizations (20% vs 9%), and less moderate or severe symptoms (32% vs 10%). Although previous studies of moderate alcohol consumption have shown positive effects on heart disease, this study and other more recent studies cast serious doubt on this assertion.3
Treatment. In their applied evidence article, Osayande and Sharma4 pose the question: When is catheter ablation a sound option for your patient with AF? They give us an excellent, evidence-based answer and remind us that we must focus on the treatment goals: to prevent stroke and to control symptoms. They recommend a stepwise approach, starting with rate control, progressing to rhythm control, and saving catheter ablation for resistant cases. In nearly all cases, anticoagulation to prevent stroke must be a part of treatment, with the exception of those with very low risk (so-called “lone atrial fibrillation”).
Screening. And what about screening for asymptomatic AF? The US Preventive Services Task Force recently reaffirmed its conclusion that there is insufficient evidence for screening for asymptomatic AF (a topic discussed in an online Practice Alert Brief5).6 Since wearable exercise-monitoring devices can detect heart arrhythmias (and are advertised for this purpose), a patient may present after receiving a notification about asymptomatic AF. What shall we do in these cases? The dilemma is that your patient will know she has a potentially dangerous condition, but there is no evidence that treating it will result in more benefit than harm.
A recently published study suggests that we should be very cautious in recommending treatment. In an RCT of patients ages 70 to 90 years, 1501 patients received an implantable loop recorder, while 4503 received routine health care; median follow-up was 64.5 months.7 Although more cases of AF were detected (32% in the monitored group vs 12% in the usual care group), and oral anticoagulation treatment was started more frequently (30% vs 13%, respectively), there was no significant difference in the proportion of patients who had a stroke or systemic arterial embolism (4.5% vs 5.6%).7 Until we have more data, reassurance seems to be the best recommendation for asymptomatic AF.
Atrial fibrillation (AF) is a common problem confronting family physicians. In this issue of JFP, we offer 2 articles about AF: one on prevention and one on treatment. Both provide evidence-based guidance to help you refine your care. But gaps remain. I’ll get to that in a bit.
Prevention. This month’s PURL1 discusses a randomized controlled trial (RCT) that enrolled moderate alcohol drinkers with AF.2 Compared to those who continued to drink moderately, those who reduced their alcohol consumption to 2 drinks per week had a significant reduction in recurrent AF (73% vs 53%), fewer hospitalizations (20% vs 9%), and less moderate or severe symptoms (32% vs 10%). Although previous studies of moderate alcohol consumption have shown positive effects on heart disease, this study and other more recent studies cast serious doubt on this assertion.3
Treatment. In their applied evidence article, Osayande and Sharma4 pose the question: When is catheter ablation a sound option for your patient with AF? They give us an excellent, evidence-based answer and remind us that we must focus on the treatment goals: to prevent stroke and to control symptoms. They recommend a stepwise approach, starting with rate control, progressing to rhythm control, and saving catheter ablation for resistant cases. In nearly all cases, anticoagulation to prevent stroke must be a part of treatment, with the exception of those with very low risk (so-called “lone atrial fibrillation”).
Screening. And what about screening for asymptomatic AF? The US Preventive Services Task Force recently reaffirmed its conclusion that there is insufficient evidence for screening for asymptomatic AF (a topic discussed in an online Practice Alert Brief5).6 Since wearable exercise-monitoring devices can detect heart arrhythmias (and are advertised for this purpose), a patient may present after receiving a notification about asymptomatic AF. What shall we do in these cases? The dilemma is that your patient will know she has a potentially dangerous condition, but there is no evidence that treating it will result in more benefit than harm.
A recently published study suggests that we should be very cautious in recommending treatment. In an RCT of patients ages 70 to 90 years, 1501 patients received an implantable loop recorder, while 4503 received routine health care; median follow-up was 64.5 months.7 Although more cases of AF were detected (32% in the monitored group vs 12% in the usual care group), and oral anticoagulation treatment was started more frequently (30% vs 13%, respectively), there was no significant difference in the proportion of patients who had a stroke or systemic arterial embolism (4.5% vs 5.6%).7 Until we have more data, reassurance seems to be the best recommendation for asymptomatic AF.
1. Thiel DJ, Marshall RC, Rogers TS. Alcohol abstinence reduces A-fib burden in drinkers. J Fam Pract. 2022;71:85-87.
2. Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382:20-28. doi: 10.1056/NEJMoa1817591
3. Hoek AG, van Oort S, Mukamal KJ, et al. Alcohol consumption and cardiovascular disease risk: placing new data in context [published online ahead of print, 2022 Feb 7]. Curr Atheroscler Rep. doi: 10.1007/s11883-022-00992-1
4. Osayande AS, Sharma N. When is catheter ablation a sound option for your patient with A-fib? J Fam Pract. 2022;71:54-62.
5. Campos-Outcalt D. USPSTF releases updated guidance on asymptomatic A-fb. J Fam Pract. 2022;3. Accessed February 18, 2022. www.mdedge.com/familymedicine/article/251911/cardiology/uspstf-releases-updated-guidance-asymptomatic-fib
6. USPSTF. Screening for atrial fibrillation: US Preventive Services Task Force recommendation statement. JAMA. 2022;327:360-367. doi: 10.1001/jama.2021.23732
7. Svendsen JH, Diederichsen SZ, Hojberg S, et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): a randomised controlled trial. Lancet. 2021;398:1507-1516.
1. Thiel DJ, Marshall RC, Rogers TS. Alcohol abstinence reduces A-fib burden in drinkers. J Fam Pract. 2022;71:85-87.
2. Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382:20-28. doi: 10.1056/NEJMoa1817591
3. Hoek AG, van Oort S, Mukamal KJ, et al. Alcohol consumption and cardiovascular disease risk: placing new data in context [published online ahead of print, 2022 Feb 7]. Curr Atheroscler Rep. doi: 10.1007/s11883-022-00992-1
4. Osayande AS, Sharma N. When is catheter ablation a sound option for your patient with A-fib? J Fam Pract. 2022;71:54-62.
5. Campos-Outcalt D. USPSTF releases updated guidance on asymptomatic A-fb. J Fam Pract. 2022;3. Accessed February 18, 2022. www.mdedge.com/familymedicine/article/251911/cardiology/uspstf-releases-updated-guidance-asymptomatic-fib
6. USPSTF. Screening for atrial fibrillation: US Preventive Services Task Force recommendation statement. JAMA. 2022;327:360-367. doi: 10.1001/jama.2021.23732
7. Svendsen JH, Diederichsen SZ, Hojberg S, et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): a randomised controlled trial. Lancet. 2021;398:1507-1516.
Pan-coronavirus vaccines may be key to fighting future pandemics
As the COVID-19 pandemic winds down – for the time being at least – efforts are ramping up to develop next-generation vaccines that can protect against future novel coronaviruses and variants. Several projects are presenting clever combinations of viral parts to the immune system that evoke a robust and hopefully lasting response.
The coming generation of “pan” vaccines aims to tamp down SARS-CoV-2, its closest relatives, and whatever may come into tamer respiratory viruses like the common cold. Whatever the eventual components of this new generation of vaccines, experts agree on the goal: preventing severe disease and death. And a broader approach is critical.
“All the vaccines have been amazing. But we’re playing a whack-a-mole game with the variants. We need to take a step back and ask if a pan-variant vaccine is possible. That’s important because Omicron isn’t the last variant,” said Jacob Lemieux, MD, PhD, instructor in medicine and infectious disease specialist at Massachusetts General Hospital, Boston.
A broad spectrum vaccine
The drive to create a vaccine that would deter multiple coronaviruses arose early, among many researchers. An article published in Nature in May 2020 by National Institute of Allergy and Infectious Diseases researcher Luca T. Giurgea, MD, and colleagues said it all in the title: “Universal coronavirus vaccines: the time to start is now.”
Their concerns? The diversity of bat coronaviruses poised to jump into humans; the high mutability of the spike gene that the immune response recognizes; and the persistence of mutations in an RNA virus, which can’t repair errors.
Work on broader vaccines began in several labs as SARS-CoV-2 spawned variant after variant.
On Sept. 28, NIAID announced funding for developing ‘pan-coronavirus’ vaccines – the quotation marks theirs to indicate that a magic bullet against any new coronavirus is unrealistic. “These new awards are designed to look ahead and prepare for the next generation of coronaviruses with pandemic potential,” said NIAID director Anthony S. Fauci, MD. An initial three awards went to groups at the University of Wisconsin, Brigham and Women’s Hospital, and Duke University.
President Biden mentioned the NIAID funding in his State of the Union Address. He also talked about how the Biomedical Advanced Research and Development Authority, founded in 2006 to prepare for public health emergencies, is spearheading development of new vaccine platforms and vaccines that target a broader swath of pathogen parts.
Meanwhile, individual researchers from eclectic fields are finding new ways to prevent future pandemics.
Artem Babaian, PhD, a computational biologist at the University of Cambridge (England), had the idea to probe National Institutes of Health genome databases, going back more than a decade, for overlooked novel coronaviruses. He started the project while he was between jobs as the pandemic was unfurling, using a telltale enzyme unique to the RNA viruses to fish out COVID cousins. The work is published in Nature and the data freely available at serratus.io.
Among the nearly 132,000 novel RNA viruses Dr. Babaian’s team found, 9 were from previously unrecognized coronaviruses. The novel nine came from “ecologically diverse sources”: a seahorse, an axolotl, an eel, and several fishes. Deciphering the topographies of these coronaviruses may provide clues to developing vaccines that stay ahead of future pandemics.
But optics are important in keeping expectations reasonable. “‘Universal vaccine’ is a misnomer. I think about it as ‘broad spectrum vaccines.’ It’s critical to be up front that these vaccines can never guarantee immunity against all coronaviruses. There are no absolutes in biology, but they hopefully will work against the dangers that we do know exist. A vaccine that mimics exposure to many coronaviruses could protect against a currently unknown coronavirus, especially if slower-evolving antigens are included,” Dr. Babaian said in an interview.
Nikolai Petrovsky, MD, PhD, of Flinders University, Adelaide, and the biotechnology company Vaccine Pty, agrees, calling a literal pan-coronavirus vaccine a “pipe dream. What I do think is achievable is a broadly protective, pan–CoV-19 vaccine – I can say that because we have already developed and tested it, combining antigens rather than trying just one that can do everything.”
Immunity lures
The broader vaccines in development display viral antigens, such as spike proteins, to the immune system on diverse frameworks. Here are a few approaches.
Ferritin nanoparticles: A candidate vaccine from the emerging infectious diseases branch of Water Reed National Military Medical Center began phase 1 human trials in April 2021. Called SpFN, the vaccine consists of arrays of ferritin nanoparticles linked to spike proteins from various variants and species. Ferritin is a protein that binds and stores iron in the body.
“The repetitive and ordered display of the coronavirus spike protein on a multifaced nanoparticle may stimulate immunity in such a way as to translate into significantly broader protection,” said Walter Reed’s branch director and vaccine coinventor Kayvon Modjarrad, MD, PhD.
A second vaccine targets only the “bullseye” part of the spike that the virus uses to attach and gain access to human cells, called the receptor-binding domain (RBD), of SARS-CoV-2 variants and of the virus behind the original SARS. The preclinical data appeared in Science Translational Medicine.
Barton Haynes, MD and colleagues at the Duke Human Vaccine Institute are also using ferritin to design and develop a “pan-betacoronavirus vaccine,” referring to the genus to which SARS-CoV-2 belongs. They say their results in macaques, published in Nature, “demonstrate that current mRNA-based vaccines may provide some protection from future outbreaks of zoonotic betacoronaviruses.”
Mosaic nanoparticles: Graduate student Alexander Cohen is leading an effort at CalTech, in the lab of Pamela Bjorkman, PhD, that uses nanoparticles consisting of proteins from a bacterium (Strep pyogenes) to which RBDs from spike proteins of four or eight different betacoronaviruses are attached. The strategy demonstrates that the whole is greater than the sum of the parts.
“Alex’s results show that it is possible to raise diverse neutralizing antibody responses, even against coronavirus strains that were not represented on the injected nanoparticle. We are hopeful that this technology could be used to protect against future animal coronaviruses that cross into humans,” said Dr. Björkman. The work appeared in Science.
Candidate vaccines from Inovio Pharmaceuticals also use a mosaic spike strategy, but with DNA rings (plasmids) rather than nanoparticles. One version works against pre-Omicron variants and is being tested against Omicron, and another with “pan–COVID-19” coverage has tested well in animal models. Inovio’s vaccines are delivered into the skin using a special device that applies an electric pulse that increases the cells’ permeability.
Chimeric spikes: Yet another approach is to fashion vaccines from various parts of the betacoronaviruses that are most closely related to SARS-CoV-2 – the pathogens behind Middle East respiratory syndrome and severe acute respiratory syndrome as well as several bat viruses and a few pangolin ones. The abundance and ubiquity of these viruses provide a toolbox of sorts, with instructions written in the language of RNA, from which to select, dissect, recombine, and customize vaccines.
“SARS-like viruses can recombine and exhibit great genetic diversity in several parts of the genome. We designed chimeric spikes to improve coverage of a multiplexed vaccine,” said David Martinez, PhD.
His team at the University of North Carolina at Chapel Hill has developed mRNA vaccines that deliver “scrambled coronavirus spikes” representing various parts, not just the RBD, as described in Science.
In mice, the chimeric vaccines elicit robust T- and B-cell immune responses, which stimulate antibody production and control other facets of building immunity.
Beyond the spike bullseye
The challenge of developing pan-coronavirus vaccines is dual. “The very best vaccines are highly specific to each strain, and the universal vaccines have to sacrifice effectiveness to get broad coverage. Life is a trade-off.” Dr. Petrovsky told this news organization.
Efforts to broaden vaccine efficacy venture beyond targeting the RBD bullseyes of the spike triplets that festoon the virus. Some projects are focusing on less changeable spike parts that are more alike among less closely related coronaviruses than is the mutation-prone RBD. For example, the peptides that twist into the “stem-helix” portion of the part of the spike that adheres to host cells are the basis of some candidate vaccines now in preclinical studies.
Still other vaccines aren’t spike based at all. French company Osivax, for example, is working on a vaccine that targets the nucleocapsid protein that shields the viral RNA. The hope is that presenting various faces of the pathogen may spark immunity beyond an initial antibody rush and evoke more diverse and lasting T-cell responses.
With the myriad efforts to back up the first generation of COVID-19 vaccines with new ones offering broader protection, it appears that science may have finally learned from history.
“After the SARS outbreak, we lost interest and failed to complete development of a vaccine for use in case of a recurrent outbreak. We must not make the same mistake again,” Dr. Giurgea and colleagues wrote in their Nature article about universal coronavirus vaccines.
A version of this article first appeared on Medscape.com.
As the COVID-19 pandemic winds down – for the time being at least – efforts are ramping up to develop next-generation vaccines that can protect against future novel coronaviruses and variants. Several projects are presenting clever combinations of viral parts to the immune system that evoke a robust and hopefully lasting response.
The coming generation of “pan” vaccines aims to tamp down SARS-CoV-2, its closest relatives, and whatever may come into tamer respiratory viruses like the common cold. Whatever the eventual components of this new generation of vaccines, experts agree on the goal: preventing severe disease and death. And a broader approach is critical.
“All the vaccines have been amazing. But we’re playing a whack-a-mole game with the variants. We need to take a step back and ask if a pan-variant vaccine is possible. That’s important because Omicron isn’t the last variant,” said Jacob Lemieux, MD, PhD, instructor in medicine and infectious disease specialist at Massachusetts General Hospital, Boston.
A broad spectrum vaccine
The drive to create a vaccine that would deter multiple coronaviruses arose early, among many researchers. An article published in Nature in May 2020 by National Institute of Allergy and Infectious Diseases researcher Luca T. Giurgea, MD, and colleagues said it all in the title: “Universal coronavirus vaccines: the time to start is now.”
Their concerns? The diversity of bat coronaviruses poised to jump into humans; the high mutability of the spike gene that the immune response recognizes; and the persistence of mutations in an RNA virus, which can’t repair errors.
Work on broader vaccines began in several labs as SARS-CoV-2 spawned variant after variant.
On Sept. 28, NIAID announced funding for developing ‘pan-coronavirus’ vaccines – the quotation marks theirs to indicate that a magic bullet against any new coronavirus is unrealistic. “These new awards are designed to look ahead and prepare for the next generation of coronaviruses with pandemic potential,” said NIAID director Anthony S. Fauci, MD. An initial three awards went to groups at the University of Wisconsin, Brigham and Women’s Hospital, and Duke University.
President Biden mentioned the NIAID funding in his State of the Union Address. He also talked about how the Biomedical Advanced Research and Development Authority, founded in 2006 to prepare for public health emergencies, is spearheading development of new vaccine platforms and vaccines that target a broader swath of pathogen parts.
Meanwhile, individual researchers from eclectic fields are finding new ways to prevent future pandemics.
Artem Babaian, PhD, a computational biologist at the University of Cambridge (England), had the idea to probe National Institutes of Health genome databases, going back more than a decade, for overlooked novel coronaviruses. He started the project while he was between jobs as the pandemic was unfurling, using a telltale enzyme unique to the RNA viruses to fish out COVID cousins. The work is published in Nature and the data freely available at serratus.io.
Among the nearly 132,000 novel RNA viruses Dr. Babaian’s team found, 9 were from previously unrecognized coronaviruses. The novel nine came from “ecologically diverse sources”: a seahorse, an axolotl, an eel, and several fishes. Deciphering the topographies of these coronaviruses may provide clues to developing vaccines that stay ahead of future pandemics.
But optics are important in keeping expectations reasonable. “‘Universal vaccine’ is a misnomer. I think about it as ‘broad spectrum vaccines.’ It’s critical to be up front that these vaccines can never guarantee immunity against all coronaviruses. There are no absolutes in biology, but they hopefully will work against the dangers that we do know exist. A vaccine that mimics exposure to many coronaviruses could protect against a currently unknown coronavirus, especially if slower-evolving antigens are included,” Dr. Babaian said in an interview.
Nikolai Petrovsky, MD, PhD, of Flinders University, Adelaide, and the biotechnology company Vaccine Pty, agrees, calling a literal pan-coronavirus vaccine a “pipe dream. What I do think is achievable is a broadly protective, pan–CoV-19 vaccine – I can say that because we have already developed and tested it, combining antigens rather than trying just one that can do everything.”
Immunity lures
The broader vaccines in development display viral antigens, such as spike proteins, to the immune system on diverse frameworks. Here are a few approaches.
Ferritin nanoparticles: A candidate vaccine from the emerging infectious diseases branch of Water Reed National Military Medical Center began phase 1 human trials in April 2021. Called SpFN, the vaccine consists of arrays of ferritin nanoparticles linked to spike proteins from various variants and species. Ferritin is a protein that binds and stores iron in the body.
“The repetitive and ordered display of the coronavirus spike protein on a multifaced nanoparticle may stimulate immunity in such a way as to translate into significantly broader protection,” said Walter Reed’s branch director and vaccine coinventor Kayvon Modjarrad, MD, PhD.
A second vaccine targets only the “bullseye” part of the spike that the virus uses to attach and gain access to human cells, called the receptor-binding domain (RBD), of SARS-CoV-2 variants and of the virus behind the original SARS. The preclinical data appeared in Science Translational Medicine.
Barton Haynes, MD and colleagues at the Duke Human Vaccine Institute are also using ferritin to design and develop a “pan-betacoronavirus vaccine,” referring to the genus to which SARS-CoV-2 belongs. They say their results in macaques, published in Nature, “demonstrate that current mRNA-based vaccines may provide some protection from future outbreaks of zoonotic betacoronaviruses.”
Mosaic nanoparticles: Graduate student Alexander Cohen is leading an effort at CalTech, in the lab of Pamela Bjorkman, PhD, that uses nanoparticles consisting of proteins from a bacterium (Strep pyogenes) to which RBDs from spike proteins of four or eight different betacoronaviruses are attached. The strategy demonstrates that the whole is greater than the sum of the parts.
“Alex’s results show that it is possible to raise diverse neutralizing antibody responses, even against coronavirus strains that were not represented on the injected nanoparticle. We are hopeful that this technology could be used to protect against future animal coronaviruses that cross into humans,” said Dr. Björkman. The work appeared in Science.
Candidate vaccines from Inovio Pharmaceuticals also use a mosaic spike strategy, but with DNA rings (plasmids) rather than nanoparticles. One version works against pre-Omicron variants and is being tested against Omicron, and another with “pan–COVID-19” coverage has tested well in animal models. Inovio’s vaccines are delivered into the skin using a special device that applies an electric pulse that increases the cells’ permeability.
Chimeric spikes: Yet another approach is to fashion vaccines from various parts of the betacoronaviruses that are most closely related to SARS-CoV-2 – the pathogens behind Middle East respiratory syndrome and severe acute respiratory syndrome as well as several bat viruses and a few pangolin ones. The abundance and ubiquity of these viruses provide a toolbox of sorts, with instructions written in the language of RNA, from which to select, dissect, recombine, and customize vaccines.
“SARS-like viruses can recombine and exhibit great genetic diversity in several parts of the genome. We designed chimeric spikes to improve coverage of a multiplexed vaccine,” said David Martinez, PhD.
His team at the University of North Carolina at Chapel Hill has developed mRNA vaccines that deliver “scrambled coronavirus spikes” representing various parts, not just the RBD, as described in Science.
In mice, the chimeric vaccines elicit robust T- and B-cell immune responses, which stimulate antibody production and control other facets of building immunity.
Beyond the spike bullseye
The challenge of developing pan-coronavirus vaccines is dual. “The very best vaccines are highly specific to each strain, and the universal vaccines have to sacrifice effectiveness to get broad coverage. Life is a trade-off.” Dr. Petrovsky told this news organization.
Efforts to broaden vaccine efficacy venture beyond targeting the RBD bullseyes of the spike triplets that festoon the virus. Some projects are focusing on less changeable spike parts that are more alike among less closely related coronaviruses than is the mutation-prone RBD. For example, the peptides that twist into the “stem-helix” portion of the part of the spike that adheres to host cells are the basis of some candidate vaccines now in preclinical studies.
Still other vaccines aren’t spike based at all. French company Osivax, for example, is working on a vaccine that targets the nucleocapsid protein that shields the viral RNA. The hope is that presenting various faces of the pathogen may spark immunity beyond an initial antibody rush and evoke more diverse and lasting T-cell responses.
With the myriad efforts to back up the first generation of COVID-19 vaccines with new ones offering broader protection, it appears that science may have finally learned from history.
“After the SARS outbreak, we lost interest and failed to complete development of a vaccine for use in case of a recurrent outbreak. We must not make the same mistake again,” Dr. Giurgea and colleagues wrote in their Nature article about universal coronavirus vaccines.
A version of this article first appeared on Medscape.com.
As the COVID-19 pandemic winds down – for the time being at least – efforts are ramping up to develop next-generation vaccines that can protect against future novel coronaviruses and variants. Several projects are presenting clever combinations of viral parts to the immune system that evoke a robust and hopefully lasting response.
The coming generation of “pan” vaccines aims to tamp down SARS-CoV-2, its closest relatives, and whatever may come into tamer respiratory viruses like the common cold. Whatever the eventual components of this new generation of vaccines, experts agree on the goal: preventing severe disease and death. And a broader approach is critical.
“All the vaccines have been amazing. But we’re playing a whack-a-mole game with the variants. We need to take a step back and ask if a pan-variant vaccine is possible. That’s important because Omicron isn’t the last variant,” said Jacob Lemieux, MD, PhD, instructor in medicine and infectious disease specialist at Massachusetts General Hospital, Boston.
A broad spectrum vaccine
The drive to create a vaccine that would deter multiple coronaviruses arose early, among many researchers. An article published in Nature in May 2020 by National Institute of Allergy and Infectious Diseases researcher Luca T. Giurgea, MD, and colleagues said it all in the title: “Universal coronavirus vaccines: the time to start is now.”
Their concerns? The diversity of bat coronaviruses poised to jump into humans; the high mutability of the spike gene that the immune response recognizes; and the persistence of mutations in an RNA virus, which can’t repair errors.
Work on broader vaccines began in several labs as SARS-CoV-2 spawned variant after variant.
On Sept. 28, NIAID announced funding for developing ‘pan-coronavirus’ vaccines – the quotation marks theirs to indicate that a magic bullet against any new coronavirus is unrealistic. “These new awards are designed to look ahead and prepare for the next generation of coronaviruses with pandemic potential,” said NIAID director Anthony S. Fauci, MD. An initial three awards went to groups at the University of Wisconsin, Brigham and Women’s Hospital, and Duke University.
President Biden mentioned the NIAID funding in his State of the Union Address. He also talked about how the Biomedical Advanced Research and Development Authority, founded in 2006 to prepare for public health emergencies, is spearheading development of new vaccine platforms and vaccines that target a broader swath of pathogen parts.
Meanwhile, individual researchers from eclectic fields are finding new ways to prevent future pandemics.
Artem Babaian, PhD, a computational biologist at the University of Cambridge (England), had the idea to probe National Institutes of Health genome databases, going back more than a decade, for overlooked novel coronaviruses. He started the project while he was between jobs as the pandemic was unfurling, using a telltale enzyme unique to the RNA viruses to fish out COVID cousins. The work is published in Nature and the data freely available at serratus.io.
Among the nearly 132,000 novel RNA viruses Dr. Babaian’s team found, 9 were from previously unrecognized coronaviruses. The novel nine came from “ecologically diverse sources”: a seahorse, an axolotl, an eel, and several fishes. Deciphering the topographies of these coronaviruses may provide clues to developing vaccines that stay ahead of future pandemics.
But optics are important in keeping expectations reasonable. “‘Universal vaccine’ is a misnomer. I think about it as ‘broad spectrum vaccines.’ It’s critical to be up front that these vaccines can never guarantee immunity against all coronaviruses. There are no absolutes in biology, but they hopefully will work against the dangers that we do know exist. A vaccine that mimics exposure to many coronaviruses could protect against a currently unknown coronavirus, especially if slower-evolving antigens are included,” Dr. Babaian said in an interview.
Nikolai Petrovsky, MD, PhD, of Flinders University, Adelaide, and the biotechnology company Vaccine Pty, agrees, calling a literal pan-coronavirus vaccine a “pipe dream. What I do think is achievable is a broadly protective, pan–CoV-19 vaccine – I can say that because we have already developed and tested it, combining antigens rather than trying just one that can do everything.”
Immunity lures
The broader vaccines in development display viral antigens, such as spike proteins, to the immune system on diverse frameworks. Here are a few approaches.
Ferritin nanoparticles: A candidate vaccine from the emerging infectious diseases branch of Water Reed National Military Medical Center began phase 1 human trials in April 2021. Called SpFN, the vaccine consists of arrays of ferritin nanoparticles linked to spike proteins from various variants and species. Ferritin is a protein that binds and stores iron in the body.
“The repetitive and ordered display of the coronavirus spike protein on a multifaced nanoparticle may stimulate immunity in such a way as to translate into significantly broader protection,” said Walter Reed’s branch director and vaccine coinventor Kayvon Modjarrad, MD, PhD.
A second vaccine targets only the “bullseye” part of the spike that the virus uses to attach and gain access to human cells, called the receptor-binding domain (RBD), of SARS-CoV-2 variants and of the virus behind the original SARS. The preclinical data appeared in Science Translational Medicine.
Barton Haynes, MD and colleagues at the Duke Human Vaccine Institute are also using ferritin to design and develop a “pan-betacoronavirus vaccine,” referring to the genus to which SARS-CoV-2 belongs. They say their results in macaques, published in Nature, “demonstrate that current mRNA-based vaccines may provide some protection from future outbreaks of zoonotic betacoronaviruses.”
Mosaic nanoparticles: Graduate student Alexander Cohen is leading an effort at CalTech, in the lab of Pamela Bjorkman, PhD, that uses nanoparticles consisting of proteins from a bacterium (Strep pyogenes) to which RBDs from spike proteins of four or eight different betacoronaviruses are attached. The strategy demonstrates that the whole is greater than the sum of the parts.
“Alex’s results show that it is possible to raise diverse neutralizing antibody responses, even against coronavirus strains that were not represented on the injected nanoparticle. We are hopeful that this technology could be used to protect against future animal coronaviruses that cross into humans,” said Dr. Björkman. The work appeared in Science.
Candidate vaccines from Inovio Pharmaceuticals also use a mosaic spike strategy, but with DNA rings (plasmids) rather than nanoparticles. One version works against pre-Omicron variants and is being tested against Omicron, and another with “pan–COVID-19” coverage has tested well in animal models. Inovio’s vaccines are delivered into the skin using a special device that applies an electric pulse that increases the cells’ permeability.
Chimeric spikes: Yet another approach is to fashion vaccines from various parts of the betacoronaviruses that are most closely related to SARS-CoV-2 – the pathogens behind Middle East respiratory syndrome and severe acute respiratory syndrome as well as several bat viruses and a few pangolin ones. The abundance and ubiquity of these viruses provide a toolbox of sorts, with instructions written in the language of RNA, from which to select, dissect, recombine, and customize vaccines.
“SARS-like viruses can recombine and exhibit great genetic diversity in several parts of the genome. We designed chimeric spikes to improve coverage of a multiplexed vaccine,” said David Martinez, PhD.
His team at the University of North Carolina at Chapel Hill has developed mRNA vaccines that deliver “scrambled coronavirus spikes” representing various parts, not just the RBD, as described in Science.
In mice, the chimeric vaccines elicit robust T- and B-cell immune responses, which stimulate antibody production and control other facets of building immunity.
Beyond the spike bullseye
The challenge of developing pan-coronavirus vaccines is dual. “The very best vaccines are highly specific to each strain, and the universal vaccines have to sacrifice effectiveness to get broad coverage. Life is a trade-off.” Dr. Petrovsky told this news organization.
Efforts to broaden vaccine efficacy venture beyond targeting the RBD bullseyes of the spike triplets that festoon the virus. Some projects are focusing on less changeable spike parts that are more alike among less closely related coronaviruses than is the mutation-prone RBD. For example, the peptides that twist into the “stem-helix” portion of the part of the spike that adheres to host cells are the basis of some candidate vaccines now in preclinical studies.
Still other vaccines aren’t spike based at all. French company Osivax, for example, is working on a vaccine that targets the nucleocapsid protein that shields the viral RNA. The hope is that presenting various faces of the pathogen may spark immunity beyond an initial antibody rush and evoke more diverse and lasting T-cell responses.
With the myriad efforts to back up the first generation of COVID-19 vaccines with new ones offering broader protection, it appears that science may have finally learned from history.
“After the SARS outbreak, we lost interest and failed to complete development of a vaccine for use in case of a recurrent outbreak. We must not make the same mistake again,” Dr. Giurgea and colleagues wrote in their Nature article about universal coronavirus vaccines.
A version of this article first appeared on Medscape.com.
Vaccine update: The latest recommendations from ACIP
In a typical year, the Advisory Committee on Immunization Practices (ACIP) has three 1.5- to 2-day meetings to make recommendations for the use of new and existing vaccines in the US population. However, 2021 was not a typical year. Last year, ACIP held 17 meetings for a total of 127 hours. Most of these were related to vaccines to prevent COVID-19. There are now 3 COVID-19 vaccines authorized for use in the United States: the 2-dose mRNA-based Pfizer-BioNTech/Comirnaty and Moderna COVID-19 vaccines and the single-dose adenovirus, vector-based Janssen (Johnson & Johnson) COVID-19 vaccine.
TABLE 11 includes the actions taken by the ACIP from late 2020 through 2021 related to COVID-19 vaccines. All of these recommendations except 1 occurred after the US Food and Drug Administration (FDA) approved the product using an emergency use authorization (EUA). The exception is the recommendation for use of the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) for those ages 16 years and older, which was approved under the normal process 8 months after widespread use under an EUA.
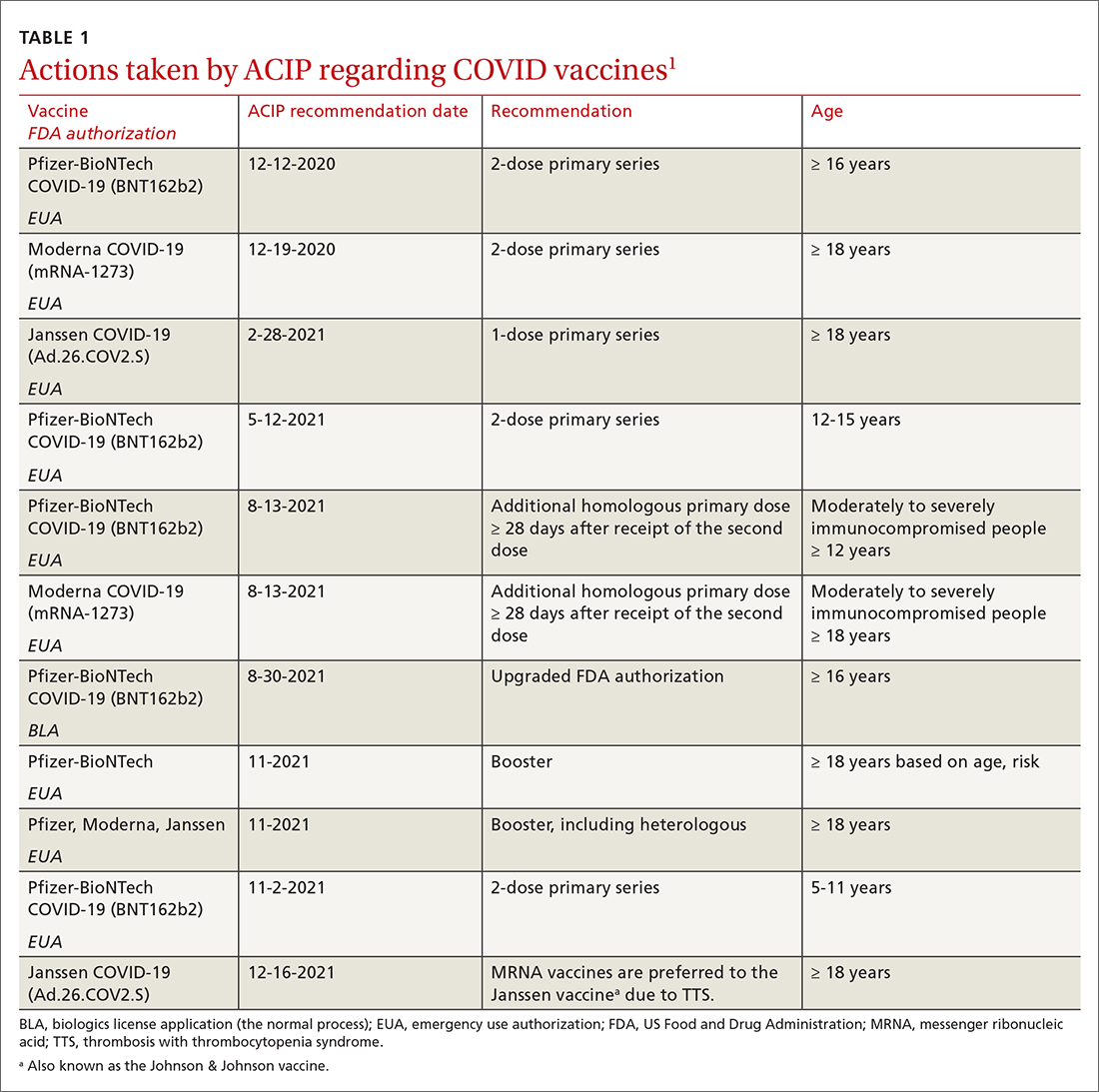
Hepatitis B vaccine now for all nonimmune adults up through 59 years
Since the introduction of hepatitis B (HepB) vaccines in 1980, the incidence of hepatitis B virus (HBV) infections in the United States has been reduced dramatically; there were an estimated 287,000 cases in 19852 and 19,200 in 2014.3 However, the incidence among adults has not declined in recent years and among someage groups has actually increased. Among those ages 40 to 49 years, the rate went from 1.9 per 100,000 in 20114 to 2.7 per 100,000 population in 2019.5 In those ages 50 to 59, there was an increase from 1.1 to 1.6 per 100,000 population over the same period of time.4,5
Recommendations for using HepB vaccine in adults have been based on risk that involves individual behavior, occupation, and medical conditions (TABLE 26). The presence of these risk factors is often unknown to medical professionals, who rarely ask about or document them. And patients can be reluctant to disclose them for fear of being stigmatized. The consequence has been a low rate of vaccination in at-risk adults.

At its November 2021 meeting, ACIP accepted the advice of the Hepatitis Work Group to move to a universal adult recommendation through age 59.7 ACIP believed that the incidence of acute infection in those ages 60 and older was too low to merit a universal recommendation. The new recommendation states that
Multiple HepB vaccine products are available for adults. Two are recombinant-based and require 3 doses: Engerix-B (GlaxoSmithKline) and Recombivax HB (Merck). One is recombinant based and requires only 2 doses: Heplisav-B (Dynavax Technologies). A new product recently approved by the FDA, PREHEVBRIO (VBI Vaccines), is another recombinant 3-dose option that the ACIP will consider early in 2022. HepB and HepA vaccines can also be co-administered with Twinrix (GlaxoSmithKline).
Pneumococcal vaccines: New PCV vaccines alter prescribing choices
The ACIP recommendations for pneumococcal vaccines in adults have been very confusing, involving 2 vaccines: PCV13 (Prevnar13, Pfizer) and PPSV23 (Pneumovax23, Merck). Both PCV13 and PPSV23 given in series were recommended for immunocompromised patients, but only PPSV23 was recommended for those with chronic medical conditions. For those 65 and older, PPSV23 was recommended for all individuals (including those with no chronic or immunocompromising condition), and PCV13 was recommended for those with immunocompromising conditions. Other adults in this older age group could receive PCV13 based on individual risk and shared clinical decision making.8
Continue to: This past year...
This past year, 2 new PCV vaccines were approved by the FDA: PCV15 (Vaxneuvance, Merck) and PCV20 (Prevnar20, Pfizer). While considering these new vaccines, the ACIP re-assessed its entire approval of pneumococcal vaccines. First, they retained the cutoff for universal pneumococcal vaccination at 65 years. For those younger than 65, they combined chronic medical conditions and immunocompromising conditions into a single at-risk group (TABLE 39). They then issued the same recommendation for older adults and those younger than 65 with risks: to receive a PCV vaccine, either PCV15 or PCV20. If they receive PCV15, it should be followed by PPSV23. PPSV23 is not recommended for those who receive PCV20. Therefore,

Recombinant zoster vaccine (RZV) has been licensed and recommended in the United States since 2017 in a 2-dose schedule for adults ages 50 years and older. In the summer of 2021, the FDA expanded the indication for use of RZV to include individuals 18 to 49 years of age who are or will be immunodeficient or immunosuppressed due to known disease or therapy. In October, the ACIP agreed and recommended 2 RZV doses for those 19 years and older in these risk groups (TABLE 410).

This recommendation was based on the elevated risk of herpes zoster documented in those with immune-suppressing conditions and therapies. In the conditions studied, the incidence in these younger adults exceeded that for older adults, for whom the vaccine is recommended.10 There are many immune conditions and immune-suppressing medications. The ACIP Zoster Work Group did not have efficacy and safety information on the use of RZV in each one of them, even though their recommendation includes them all. Many of these patients are under the care of specialists whose specialty societies had been recommending zoster vaccine for their patients, off label, prior to the FDA authorization.
Rabies vaccine is now available in 2-dose schedule
People who should receive rabies pre-exposure prophylaxis (PrEP) with rabies vaccine include laboratory personnel who work with rabies virus, biologists who work with bats, animal care professionals, wildlife biologists, veterinarians, and travelers who may be at risk of encountering rabid dogs. The recommendation has been for 3 doses of rabies vaccine at 0, 7, and 21-28 days. The ACIP voted at its June 2021 meeting to adopt a 2-dose PrEP schedule of 0 and 7 days.11 This will be especially helpful to travelers who want to complete the recommended doses prior to departure. Those who have sustained risk over time can elect to have a third dose after 21 days and before 3 years, or elect to have titers checked. More detailed clinical advice will be published in the CDC’s Morbidity and Mortality Weekly Report in 2022.
Dengue vaccine: New rec for those 9-16 years
In 2019, the FDA approved the first dengue vaccine for use in the United States for children 9 to 16 years old who had laboratory-confirmed previous dengue virus infection and who were living in an area where dengue is endemic. The CYD-TDV dengue vaccine (Dengvaxia) is a live-attenuated tetravalent vaccine built on a yellow fever vaccine backbone. Its effectiveness is 82% for prevention of symptomatic dengue, 79% for prevention of dengue-associated hospitalizations, and 84% against severe dengue.12
Continue to: Dengue viruses...
Dengue viruses (DENV) are transmitted by Aedes mosquitoes. There are 4 serotypes of dengue, and all 4 appear to be circulating in most endemic countries. Clinical disease varies from a mild febrile illness to severe disease. The most common clinical presentation includes sudden onset of fever, headache, retro-orbital pain, myalgia and arthralgia, abdominal pain, and nausea.
Severe disease includes plasma leakage, shock, respiratory distress, severe bleeding, and organ failure. While severe dengue can occur with a primary infection, a second infection with a different DENV increases the risk of severe dengue. A small increased risk of severe dengue occurs when dengue infection occurs after vaccination in those with no evidence of previous dengue infection. It is felt that the vaccine serves as a primary infection that increases the risk of severe dengue with subsequent infections. This is the reason that the vaccine is recommended only for those with a documented previous dengue infection.
At its June 2021 meeting, the ACIP recommended 3-doses of Dengvaxia, administered at 0, 6, and 12 months, for individuals 9 to 16 years of age who have laboratory confirmation of previous dengue infection and live in endemic areas.12 These areas include the territories and affiliated states of Puerto Rico, American Samoa, US Virgin Islands, Federated States of Micronesia, Republic of Marshall Islands, and the Republic of Palau. Puerto Rico accounts for 85% of the population of these areas and 95% of reported dengue cases.12The reason for the delay between FDA approval and the ACIP recommendation was the need to wait for a readily available, accurate laboratory test to confirm previous dengue infection, which is now available. There are other dengue vaccines in development including 2 live-attenuated, tetravalent vaccine candidates in Phase 3 trials.
1. ACIP. COVID-19 vaccine recommendations. Accessed February 8, 2022. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
2. CDC. Division of viral hepatitis. Disease burden from viral hepatitis A, B, and C in the United States. Accessed February 8 2022. www.cdc.gov/hepatitis/PDFs/disease_burden.pdf
3. CDC. Surveillance for viral hepatitis – United States, 2014. Hepatitis B. Accessed February 8, 2022. https://www.cdc.gov/hepatitis/statistics/2014surveillance/commentary.htm#:~:text=HEPATITIS%20B-,Acute%20Hepatitis%20B,B%20cases%20occurred%20in%202014
4. CDC. Viral hepatitis surveillance: United States, 2011. Hepatitis B. Accessed February 8, 2022. www.cdc.gov/hepatitis/statistics/2011surveillance/pdfs/2011HepSurveillanceRpt.pdf
5. CDC. Viral hepatitis surveillance report, 2019. Hepatitis B. Accessed February 8, 2022. www.cdc.gov/hepatitis/statistics/2019surveillance/HepB.htm
6. Schillie S, Harris A, Link-Gelles R, et al. Recommendations of the Advisory Committee on Immunization Practices for use of a hepatitis B vaccine with a novel adjuvant. MMWR Morb Mortal Wkly Rep. 2018;67:455-458.
7. CDC. Advisory Committee on Immunization Practices. Meeting recommendations, November 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/index.html
8. Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:1069-1075.
9. Kobayashi M. Considerations for use of PCV15 and PCV20 in U.S. adults. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-02/24-25/05-Pneumococcal-Kobayashi.pdf
10. Anderson TC, Masters NB, Guo A, et al. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:80-84.
11. CDC. ACIP recommendations. June 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/recommendations.html
12. Paz-Bailey G. Dengue vaccine. Evidence to recommendation framework. Presented to the ACIP June 24, 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-Dengue-Paz-Bailey-508.pdf
In a typical year, the Advisory Committee on Immunization Practices (ACIP) has three 1.5- to 2-day meetings to make recommendations for the use of new and existing vaccines in the US population. However, 2021 was not a typical year. Last year, ACIP held 17 meetings for a total of 127 hours. Most of these were related to vaccines to prevent COVID-19. There are now 3 COVID-19 vaccines authorized for use in the United States: the 2-dose mRNA-based Pfizer-BioNTech/Comirnaty and Moderna COVID-19 vaccines and the single-dose adenovirus, vector-based Janssen (Johnson & Johnson) COVID-19 vaccine.
TABLE 11 includes the actions taken by the ACIP from late 2020 through 2021 related to COVID-19 vaccines. All of these recommendations except 1 occurred after the US Food and Drug Administration (FDA) approved the product using an emergency use authorization (EUA). The exception is the recommendation for use of the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) for those ages 16 years and older, which was approved under the normal process 8 months after widespread use under an EUA.

Hepatitis B vaccine now for all nonimmune adults up through 59 years
Since the introduction of hepatitis B (HepB) vaccines in 1980, the incidence of hepatitis B virus (HBV) infections in the United States has been reduced dramatically; there were an estimated 287,000 cases in 19852 and 19,200 in 2014.3 However, the incidence among adults has not declined in recent years and among someage groups has actually increased. Among those ages 40 to 49 years, the rate went from 1.9 per 100,000 in 20114 to 2.7 per 100,000 population in 2019.5 In those ages 50 to 59, there was an increase from 1.1 to 1.6 per 100,000 population over the same period of time.4,5
Recommendations for using HepB vaccine in adults have been based on risk that involves individual behavior, occupation, and medical conditions (TABLE 26). The presence of these risk factors is often unknown to medical professionals, who rarely ask about or document them. And patients can be reluctant to disclose them for fear of being stigmatized. The consequence has been a low rate of vaccination in at-risk adults.

At its November 2021 meeting, ACIP accepted the advice of the Hepatitis Work Group to move to a universal adult recommendation through age 59.7 ACIP believed that the incidence of acute infection in those ages 60 and older was too low to merit a universal recommendation. The new recommendation states that
Multiple HepB vaccine products are available for adults. Two are recombinant-based and require 3 doses: Engerix-B (GlaxoSmithKline) and Recombivax HB (Merck). One is recombinant based and requires only 2 doses: Heplisav-B (Dynavax Technologies). A new product recently approved by the FDA, PREHEVBRIO (VBI Vaccines), is another recombinant 3-dose option that the ACIP will consider early in 2022. HepB and HepA vaccines can also be co-administered with Twinrix (GlaxoSmithKline).
Pneumococcal vaccines: New PCV vaccines alter prescribing choices
The ACIP recommendations for pneumococcal vaccines in adults have been very confusing, involving 2 vaccines: PCV13 (Prevnar13, Pfizer) and PPSV23 (Pneumovax23, Merck). Both PCV13 and PPSV23 given in series were recommended for immunocompromised patients, but only PPSV23 was recommended for those with chronic medical conditions. For those 65 and older, PPSV23 was recommended for all individuals (including those with no chronic or immunocompromising condition), and PCV13 was recommended for those with immunocompromising conditions. Other adults in this older age group could receive PCV13 based on individual risk and shared clinical decision making.8
Continue to: This past year...
This past year, 2 new PCV vaccines were approved by the FDA: PCV15 (Vaxneuvance, Merck) and PCV20 (Prevnar20, Pfizer). While considering these new vaccines, the ACIP re-assessed its entire approval of pneumococcal vaccines. First, they retained the cutoff for universal pneumococcal vaccination at 65 years. For those younger than 65, they combined chronic medical conditions and immunocompromising conditions into a single at-risk group (TABLE 39). They then issued the same recommendation for older adults and those younger than 65 with risks: to receive a PCV vaccine, either PCV15 or PCV20. If they receive PCV15, it should be followed by PPSV23. PPSV23 is not recommended for those who receive PCV20. Therefore,

Recombinant zoster vaccine (RZV) has been licensed and recommended in the United States since 2017 in a 2-dose schedule for adults ages 50 years and older. In the summer of 2021, the FDA expanded the indication for use of RZV to include individuals 18 to 49 years of age who are or will be immunodeficient or immunosuppressed due to known disease or therapy. In October, the ACIP agreed and recommended 2 RZV doses for those 19 years and older in these risk groups (TABLE 410).

This recommendation was based on the elevated risk of herpes zoster documented in those with immune-suppressing conditions and therapies. In the conditions studied, the incidence in these younger adults exceeded that for older adults, for whom the vaccine is recommended.10 There are many immune conditions and immune-suppressing medications. The ACIP Zoster Work Group did not have efficacy and safety information on the use of RZV in each one of them, even though their recommendation includes them all. Many of these patients are under the care of specialists whose specialty societies had been recommending zoster vaccine for their patients, off label, prior to the FDA authorization.
Rabies vaccine is now available in 2-dose schedule
People who should receive rabies pre-exposure prophylaxis (PrEP) with rabies vaccine include laboratory personnel who work with rabies virus, biologists who work with bats, animal care professionals, wildlife biologists, veterinarians, and travelers who may be at risk of encountering rabid dogs. The recommendation has been for 3 doses of rabies vaccine at 0, 7, and 21-28 days. The ACIP voted at its June 2021 meeting to adopt a 2-dose PrEP schedule of 0 and 7 days.11 This will be especially helpful to travelers who want to complete the recommended doses prior to departure. Those who have sustained risk over time can elect to have a third dose after 21 days and before 3 years, or elect to have titers checked. More detailed clinical advice will be published in the CDC’s Morbidity and Mortality Weekly Report in 2022.
Dengue vaccine: New rec for those 9-16 years
In 2019, the FDA approved the first dengue vaccine for use in the United States for children 9 to 16 years old who had laboratory-confirmed previous dengue virus infection and who were living in an area where dengue is endemic. The CYD-TDV dengue vaccine (Dengvaxia) is a live-attenuated tetravalent vaccine built on a yellow fever vaccine backbone. Its effectiveness is 82% for prevention of symptomatic dengue, 79% for prevention of dengue-associated hospitalizations, and 84% against severe dengue.12
Continue to: Dengue viruses...
Dengue viruses (DENV) are transmitted by Aedes mosquitoes. There are 4 serotypes of dengue, and all 4 appear to be circulating in most endemic countries. Clinical disease varies from a mild febrile illness to severe disease. The most common clinical presentation includes sudden onset of fever, headache, retro-orbital pain, myalgia and arthralgia, abdominal pain, and nausea.
Severe disease includes plasma leakage, shock, respiratory distress, severe bleeding, and organ failure. While severe dengue can occur with a primary infection, a second infection with a different DENV increases the risk of severe dengue. A small increased risk of severe dengue occurs when dengue infection occurs after vaccination in those with no evidence of previous dengue infection. It is felt that the vaccine serves as a primary infection that increases the risk of severe dengue with subsequent infections. This is the reason that the vaccine is recommended only for those with a documented previous dengue infection.
At its June 2021 meeting, the ACIP recommended 3-doses of Dengvaxia, administered at 0, 6, and 12 months, for individuals 9 to 16 years of age who have laboratory confirmation of previous dengue infection and live in endemic areas.12 These areas include the territories and affiliated states of Puerto Rico, American Samoa, US Virgin Islands, Federated States of Micronesia, Republic of Marshall Islands, and the Republic of Palau. Puerto Rico accounts for 85% of the population of these areas and 95% of reported dengue cases.12The reason for the delay between FDA approval and the ACIP recommendation was the need to wait for a readily available, accurate laboratory test to confirm previous dengue infection, which is now available. There are other dengue vaccines in development including 2 live-attenuated, tetravalent vaccine candidates in Phase 3 trials.
In a typical year, the Advisory Committee on Immunization Practices (ACIP) has three 1.5- to 2-day meetings to make recommendations for the use of new and existing vaccines in the US population. However, 2021 was not a typical year. Last year, ACIP held 17 meetings for a total of 127 hours. Most of these were related to vaccines to prevent COVID-19. There are now 3 COVID-19 vaccines authorized for use in the United States: the 2-dose mRNA-based Pfizer-BioNTech/Comirnaty and Moderna COVID-19 vaccines and the single-dose adenovirus, vector-based Janssen (Johnson & Johnson) COVID-19 vaccine.
TABLE 11 includes the actions taken by the ACIP from late 2020 through 2021 related to COVID-19 vaccines. All of these recommendations except 1 occurred after the US Food and Drug Administration (FDA) approved the product using an emergency use authorization (EUA). The exception is the recommendation for use of the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) for those ages 16 years and older, which was approved under the normal process 8 months after widespread use under an EUA.

Hepatitis B vaccine now for all nonimmune adults up through 59 years
Since the introduction of hepatitis B (HepB) vaccines in 1980, the incidence of hepatitis B virus (HBV) infections in the United States has been reduced dramatically; there were an estimated 287,000 cases in 19852 and 19,200 in 2014.3 However, the incidence among adults has not declined in recent years and among someage groups has actually increased. Among those ages 40 to 49 years, the rate went from 1.9 per 100,000 in 20114 to 2.7 per 100,000 population in 2019.5 In those ages 50 to 59, there was an increase from 1.1 to 1.6 per 100,000 population over the same period of time.4,5
Recommendations for using HepB vaccine in adults have been based on risk that involves individual behavior, occupation, and medical conditions (TABLE 26). The presence of these risk factors is often unknown to medical professionals, who rarely ask about or document them. And patients can be reluctant to disclose them for fear of being stigmatized. The consequence has been a low rate of vaccination in at-risk adults.

At its November 2021 meeting, ACIP accepted the advice of the Hepatitis Work Group to move to a universal adult recommendation through age 59.7 ACIP believed that the incidence of acute infection in those ages 60 and older was too low to merit a universal recommendation. The new recommendation states that
Multiple HepB vaccine products are available for adults. Two are recombinant-based and require 3 doses: Engerix-B (GlaxoSmithKline) and Recombivax HB (Merck). One is recombinant based and requires only 2 doses: Heplisav-B (Dynavax Technologies). A new product recently approved by the FDA, PREHEVBRIO (VBI Vaccines), is another recombinant 3-dose option that the ACIP will consider early in 2022. HepB and HepA vaccines can also be co-administered with Twinrix (GlaxoSmithKline).
Pneumococcal vaccines: New PCV vaccines alter prescribing choices
The ACIP recommendations for pneumococcal vaccines in adults have been very confusing, involving 2 vaccines: PCV13 (Prevnar13, Pfizer) and PPSV23 (Pneumovax23, Merck). Both PCV13 and PPSV23 given in series were recommended for immunocompromised patients, but only PPSV23 was recommended for those with chronic medical conditions. For those 65 and older, PPSV23 was recommended for all individuals (including those with no chronic or immunocompromising condition), and PCV13 was recommended for those with immunocompromising conditions. Other adults in this older age group could receive PCV13 based on individual risk and shared clinical decision making.8
Continue to: This past year...
This past year, 2 new PCV vaccines were approved by the FDA: PCV15 (Vaxneuvance, Merck) and PCV20 (Prevnar20, Pfizer). While considering these new vaccines, the ACIP re-assessed its entire approval of pneumococcal vaccines. First, they retained the cutoff for universal pneumococcal vaccination at 65 years. For those younger than 65, they combined chronic medical conditions and immunocompromising conditions into a single at-risk group (TABLE 39). They then issued the same recommendation for older adults and those younger than 65 with risks: to receive a PCV vaccine, either PCV15 or PCV20. If they receive PCV15, it should be followed by PPSV23. PPSV23 is not recommended for those who receive PCV20. Therefore,

Recombinant zoster vaccine (RZV) has been licensed and recommended in the United States since 2017 in a 2-dose schedule for adults ages 50 years and older. In the summer of 2021, the FDA expanded the indication for use of RZV to include individuals 18 to 49 years of age who are or will be immunodeficient or immunosuppressed due to known disease or therapy. In October, the ACIP agreed and recommended 2 RZV doses for those 19 years and older in these risk groups (TABLE 410).

This recommendation was based on the elevated risk of herpes zoster documented in those with immune-suppressing conditions and therapies. In the conditions studied, the incidence in these younger adults exceeded that for older adults, for whom the vaccine is recommended.10 There are many immune conditions and immune-suppressing medications. The ACIP Zoster Work Group did not have efficacy and safety information on the use of RZV in each one of them, even though their recommendation includes them all. Many of these patients are under the care of specialists whose specialty societies had been recommending zoster vaccine for their patients, off label, prior to the FDA authorization.
Rabies vaccine is now available in 2-dose schedule
People who should receive rabies pre-exposure prophylaxis (PrEP) with rabies vaccine include laboratory personnel who work with rabies virus, biologists who work with bats, animal care professionals, wildlife biologists, veterinarians, and travelers who may be at risk of encountering rabid dogs. The recommendation has been for 3 doses of rabies vaccine at 0, 7, and 21-28 days. The ACIP voted at its June 2021 meeting to adopt a 2-dose PrEP schedule of 0 and 7 days.11 This will be especially helpful to travelers who want to complete the recommended doses prior to departure. Those who have sustained risk over time can elect to have a third dose after 21 days and before 3 years, or elect to have titers checked. More detailed clinical advice will be published in the CDC’s Morbidity and Mortality Weekly Report in 2022.
Dengue vaccine: New rec for those 9-16 years
In 2019, the FDA approved the first dengue vaccine for use in the United States for children 9 to 16 years old who had laboratory-confirmed previous dengue virus infection and who were living in an area where dengue is endemic. The CYD-TDV dengue vaccine (Dengvaxia) is a live-attenuated tetravalent vaccine built on a yellow fever vaccine backbone. Its effectiveness is 82% for prevention of symptomatic dengue, 79% for prevention of dengue-associated hospitalizations, and 84% against severe dengue.12
Continue to: Dengue viruses...
Dengue viruses (DENV) are transmitted by Aedes mosquitoes. There are 4 serotypes of dengue, and all 4 appear to be circulating in most endemic countries. Clinical disease varies from a mild febrile illness to severe disease. The most common clinical presentation includes sudden onset of fever, headache, retro-orbital pain, myalgia and arthralgia, abdominal pain, and nausea.
Severe disease includes plasma leakage, shock, respiratory distress, severe bleeding, and organ failure. While severe dengue can occur with a primary infection, a second infection with a different DENV increases the risk of severe dengue. A small increased risk of severe dengue occurs when dengue infection occurs after vaccination in those with no evidence of previous dengue infection. It is felt that the vaccine serves as a primary infection that increases the risk of severe dengue with subsequent infections. This is the reason that the vaccine is recommended only for those with a documented previous dengue infection.
At its June 2021 meeting, the ACIP recommended 3-doses of Dengvaxia, administered at 0, 6, and 12 months, for individuals 9 to 16 years of age who have laboratory confirmation of previous dengue infection and live in endemic areas.12 These areas include the territories and affiliated states of Puerto Rico, American Samoa, US Virgin Islands, Federated States of Micronesia, Republic of Marshall Islands, and the Republic of Palau. Puerto Rico accounts for 85% of the population of these areas and 95% of reported dengue cases.12The reason for the delay between FDA approval and the ACIP recommendation was the need to wait for a readily available, accurate laboratory test to confirm previous dengue infection, which is now available. There are other dengue vaccines in development including 2 live-attenuated, tetravalent vaccine candidates in Phase 3 trials.
1. ACIP. COVID-19 vaccine recommendations. Accessed February 8, 2022. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
2. CDC. Division of viral hepatitis. Disease burden from viral hepatitis A, B, and C in the United States. Accessed February 8 2022. www.cdc.gov/hepatitis/PDFs/disease_burden.pdf
3. CDC. Surveillance for viral hepatitis – United States, 2014. Hepatitis B. Accessed February 8, 2022. https://www.cdc.gov/hepatitis/statistics/2014surveillance/commentary.htm#:~:text=HEPATITIS%20B-,Acute%20Hepatitis%20B,B%20cases%20occurred%20in%202014
4. CDC. Viral hepatitis surveillance: United States, 2011. Hepatitis B. Accessed February 8, 2022. www.cdc.gov/hepatitis/statistics/2011surveillance/pdfs/2011HepSurveillanceRpt.pdf
5. CDC. Viral hepatitis surveillance report, 2019. Hepatitis B. Accessed February 8, 2022. www.cdc.gov/hepatitis/statistics/2019surveillance/HepB.htm
6. Schillie S, Harris A, Link-Gelles R, et al. Recommendations of the Advisory Committee on Immunization Practices for use of a hepatitis B vaccine with a novel adjuvant. MMWR Morb Mortal Wkly Rep. 2018;67:455-458.
7. CDC. Advisory Committee on Immunization Practices. Meeting recommendations, November 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/index.html
8. Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:1069-1075.
9. Kobayashi M. Considerations for use of PCV15 and PCV20 in U.S. adults. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-02/24-25/05-Pneumococcal-Kobayashi.pdf
10. Anderson TC, Masters NB, Guo A, et al. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:80-84.
11. CDC. ACIP recommendations. June 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/recommendations.html
12. Paz-Bailey G. Dengue vaccine. Evidence to recommendation framework. Presented to the ACIP June 24, 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-Dengue-Paz-Bailey-508.pdf
1. ACIP. COVID-19 vaccine recommendations. Accessed February 8, 2022. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
2. CDC. Division of viral hepatitis. Disease burden from viral hepatitis A, B, and C in the United States. Accessed February 8 2022. www.cdc.gov/hepatitis/PDFs/disease_burden.pdf
3. CDC. Surveillance for viral hepatitis – United States, 2014. Hepatitis B. Accessed February 8, 2022. https://www.cdc.gov/hepatitis/statistics/2014surveillance/commentary.htm#:~:text=HEPATITIS%20B-,Acute%20Hepatitis%20B,B%20cases%20occurred%20in%202014
4. CDC. Viral hepatitis surveillance: United States, 2011. Hepatitis B. Accessed February 8, 2022. www.cdc.gov/hepatitis/statistics/2011surveillance/pdfs/2011HepSurveillanceRpt.pdf
5. CDC. Viral hepatitis surveillance report, 2019. Hepatitis B. Accessed February 8, 2022. www.cdc.gov/hepatitis/statistics/2019surveillance/HepB.htm
6. Schillie S, Harris A, Link-Gelles R, et al. Recommendations of the Advisory Committee on Immunization Practices for use of a hepatitis B vaccine with a novel adjuvant. MMWR Morb Mortal Wkly Rep. 2018;67:455-458.
7. CDC. Advisory Committee on Immunization Practices. Meeting recommendations, November 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/index.html
8. Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:1069-1075.
9. Kobayashi M. Considerations for use of PCV15 and PCV20 in U.S. adults. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-02/24-25/05-Pneumococcal-Kobayashi.pdf
10. Anderson TC, Masters NB, Guo A, et al. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:80-84.
11. CDC. ACIP recommendations. June 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/recommendations.html
12. Paz-Bailey G. Dengue vaccine. Evidence to recommendation framework. Presented to the ACIP June 24, 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-Dengue-Paz-Bailey-508.pdf