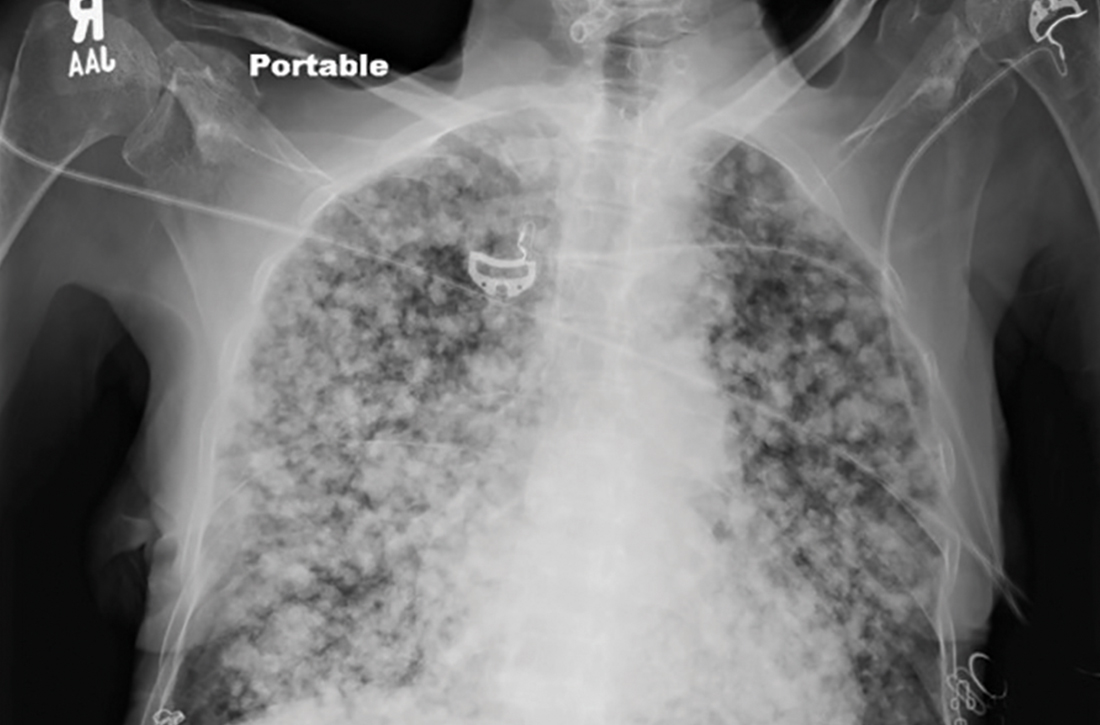
User login
15th Report on Carcinogens Adds to Its List
From environmental tobacco smoke to ultraviolet (UV) radiation, diesel exhaust particulates, lead, and now, chronic infection with Helicobacter pylori (H pylori)—the Report on Carcinogens has regularly updated the list of substances known or “reasonably anticipated” to cause cancer.
The 15th report, which is prepared by the National Toxicology Program (NTP) for the Department of Health and Human Services, has 8 new entries, bringing the number of human carcinogens (eg, metals, pesticides, and drugs) on the list to 256. (The first report, released in 1980, listed 26.) In addition to H pylori infection, this edition adds the flame-retardant chemical antimony trioxide, and 6 haloacetic acids found as water disinfection byproducts.
In 1971, then President Nixon declared “war on cancer” (the second leading cause of death in the US) and signed the National Cancer Act. In 1978, Congress ordered the Report on Carcinogens, to educate the public and health professionals on potential environmental carcinogenic hazards.
Perhaps disheartening to know that even with 256 entries, the list probably understates the number of carcinogens humans and other creatures are exposed to. But things can change with time. Each list goes through a rigorous round of reviews. Sometimes substances are “delisted” after, for instance, litigation or new research. Saccharin, for example, was removed from the ninth edition. It was listed as “reasonably anticipated” in 1981, based on “sufficient evidence of carcinogenicity in experimental animals.” It was removed, however, after extensive review of decades of saccharin use determined that the data were not sufficient to meet current criteria. Further research had revealed, also, that the observed bladder tumors in rats arose from a mechanism not relevant to humans.
Other entries, such as the controversial listing of the cancer drug tamoxifen, walk a fine line between risk and benefit. Tamoxifen, first listed in the ninth report (and still in the 15th report), was included because studies revealed that it could increase the risk of uterine cancer in women. But there also was conclusive evidence that it may prevent or delay breast cancer in women who are at high risk.
Ultimately, the report’s authors make it clear that it is for informative value and guidance, not necessarily a dictate. As one report put it: “Personal decisions concerning voluntary exposures to carcinogenic agents need to be based on additional information that is beyond the scope” of the report.
“As the identification of carcinogens is a key step in cancer prevention,” said Rick Woychik, PhD, director of the National Institute of Environmental Health Sciences and NTP, “publication of the report represents an important government activity towards improving public health.”
From environmental tobacco smoke to ultraviolet (UV) radiation, diesel exhaust particulates, lead, and now, chronic infection with Helicobacter pylori (H pylori)—the Report on Carcinogens has regularly updated the list of substances known or “reasonably anticipated” to cause cancer.
The 15th report, which is prepared by the National Toxicology Program (NTP) for the Department of Health and Human Services, has 8 new entries, bringing the number of human carcinogens (eg, metals, pesticides, and drugs) on the list to 256. (The first report, released in 1980, listed 26.) In addition to H pylori infection, this edition adds the flame-retardant chemical antimony trioxide, and 6 haloacetic acids found as water disinfection byproducts.
In 1971, then President Nixon declared “war on cancer” (the second leading cause of death in the US) and signed the National Cancer Act. In 1978, Congress ordered the Report on Carcinogens, to educate the public and health professionals on potential environmental carcinogenic hazards.
Perhaps disheartening to know that even with 256 entries, the list probably understates the number of carcinogens humans and other creatures are exposed to. But things can change with time. Each list goes through a rigorous round of reviews. Sometimes substances are “delisted” after, for instance, litigation or new research. Saccharin, for example, was removed from the ninth edition. It was listed as “reasonably anticipated” in 1981, based on “sufficient evidence of carcinogenicity in experimental animals.” It was removed, however, after extensive review of decades of saccharin use determined that the data were not sufficient to meet current criteria. Further research had revealed, also, that the observed bladder tumors in rats arose from a mechanism not relevant to humans.
Other entries, such as the controversial listing of the cancer drug tamoxifen, walk a fine line between risk and benefit. Tamoxifen, first listed in the ninth report (and still in the 15th report), was included because studies revealed that it could increase the risk of uterine cancer in women. But there also was conclusive evidence that it may prevent or delay breast cancer in women who are at high risk.
Ultimately, the report’s authors make it clear that it is for informative value and guidance, not necessarily a dictate. As one report put it: “Personal decisions concerning voluntary exposures to carcinogenic agents need to be based on additional information that is beyond the scope” of the report.
“As the identification of carcinogens is a key step in cancer prevention,” said Rick Woychik, PhD, director of the National Institute of Environmental Health Sciences and NTP, “publication of the report represents an important government activity towards improving public health.”
From environmental tobacco smoke to ultraviolet (UV) radiation, diesel exhaust particulates, lead, and now, chronic infection with Helicobacter pylori (H pylori)—the Report on Carcinogens has regularly updated the list of substances known or “reasonably anticipated” to cause cancer.
The 15th report, which is prepared by the National Toxicology Program (NTP) for the Department of Health and Human Services, has 8 new entries, bringing the number of human carcinogens (eg, metals, pesticides, and drugs) on the list to 256. (The first report, released in 1980, listed 26.) In addition to H pylori infection, this edition adds the flame-retardant chemical antimony trioxide, and 6 haloacetic acids found as water disinfection byproducts.
In 1971, then President Nixon declared “war on cancer” (the second leading cause of death in the US) and signed the National Cancer Act. In 1978, Congress ordered the Report on Carcinogens, to educate the public and health professionals on potential environmental carcinogenic hazards.
Perhaps disheartening to know that even with 256 entries, the list probably understates the number of carcinogens humans and other creatures are exposed to. But things can change with time. Each list goes through a rigorous round of reviews. Sometimes substances are “delisted” after, for instance, litigation or new research. Saccharin, for example, was removed from the ninth edition. It was listed as “reasonably anticipated” in 1981, based on “sufficient evidence of carcinogenicity in experimental animals.” It was removed, however, after extensive review of decades of saccharin use determined that the data were not sufficient to meet current criteria. Further research had revealed, also, that the observed bladder tumors in rats arose from a mechanism not relevant to humans.
Other entries, such as the controversial listing of the cancer drug tamoxifen, walk a fine line between risk and benefit. Tamoxifen, first listed in the ninth report (and still in the 15th report), was included because studies revealed that it could increase the risk of uterine cancer in women. But there also was conclusive evidence that it may prevent or delay breast cancer in women who are at high risk.
Ultimately, the report’s authors make it clear that it is for informative value and guidance, not necessarily a dictate. As one report put it: “Personal decisions concerning voluntary exposures to carcinogenic agents need to be based on additional information that is beyond the scope” of the report.
“As the identification of carcinogens is a key step in cancer prevention,” said Rick Woychik, PhD, director of the National Institute of Environmental Health Sciences and NTP, “publication of the report represents an important government activity towards improving public health.”
Fewer than half with severe aortic stenosis get new valves
The chance that patients with severe aortic stenosis (AS) will receive aortic valve replacement (AVR) is worse than the flip of a coin, even a decade after the gamechanging transcatheter option became available, a new study suggests.
Of the study’s 6,150 patients with an indication or potential indication for AVR, 48% received the procedure at Massachusetts General Hospital and its partner institution Brigham and Women’s Hospital, both in Boston – both of which have active, high-volume transcatheter and surgical AVR (TAVR/SAVR) programs.
“Essentially, this is a best-case scenario. So, unfortunately, I think on the national level we are likely to see rates that are far worse than what we observed here,” senior author Sammy Elmariah, MD, PhD, Massachusetts General Hospital, told this news organization.
The volume of AVR increased more than 10-fold over the 18-year study period (2000 to 2017), driven by the exponential growth of TAVR, he noted. However, the graying of America led to an even greater increase in the number of patients with severe AS and an indication for AVR.
The study, led by Shawn X. Li, MD, MBA, of Mass General, was published in the March 8 issue of the Journal of the American College of Cardiology.
Previous research has provided equally compelling data on the undertreatment of AS, including a 2021 study using natural language processing (NLP) that found AVR use was just 35.6% within 1 year of diagnosis and varied wildly among managing cardiologists.
The present study used NLP tools to identify symptoms consistent with severe AS in the medical record coupled with echocardiographic data from 10,795 patients with severe AS (valve area <1 cm2). Patients were divided into four AS subtypes and then classified as having a class 1 indication (high-gradient AS with symptoms or reduced ejection fraction [EF]) or a potential class 2a indication (low-gradient AS with symptoms) for AVR.
Among patients with high-gradient AS and class 1 indication for AVR, 1 in 3 did not receive AVR over the study period, including 30% with a normal EF and 47% with a low EF.
In those with low-gradient AS, 67% with a normal EF and 62% with a low EF did not receive AVR. The low-gradient groups were significantly less likely to receive AVR both in the entire study period and in the more contemporary period from 2014 to 2017, despite the valvular heart disease guideline 2014 update indicating AVR was “reasonable” in patients with low-gradient AS – a 2a recommendation upgraded to class 1 in the most recent 2020 update.
Better survival
In patients with a class 1 or potential class 2a indication, AVR was associated with a significantly lower risk of mortality in all four AS subgroups:
- High gradient/normal EF: 3% vs. 15%; adjusted hazard ratio, 0.42
- High-gradient/low EF: 16% vs. 72%; aHR, 0.28
- Low-gradient/normal EF: 5% vs. 14%; aHR, 0.73
- Low-gradient/low EF: 11% vs. 34%; aHR, 0.48; P < .001 for all
“I think what we need to do is change the paradigm, such that patients with a valve area that is less than or equal to 1 [cm2] is severe aortic stenosis until proven otherwise, and that essentially establishes a premise by which we default to treat these patients unless we can prove that it is in fact moderate,” Dr. Elmariah said.
Unfortunately, the opposite is currently true today, he said, and the default is not to treat and put patients through surgery or an invasive TAVR procedure unless physicians can definitively prove that it is severe AS. But they’re not always correct and don’t always have the ability to truly differentiate moderate from severe disease.
“The question, therefore, is ‘What do we do with those patients?’” Dr. Elmariah asked. “I think if a patient has symptoms, then we are obligated to intervene, given the stark difference in mortality that one sees when these patients go undertreated.”
Sounding the alarm
Robert Bonow, MD, a professor of cardiology at Northwestern University in Chicago and a writing committee member for the 2014 guideline update, said the study is a “big wake-up call” and “the take-home message is that we are missing some patients who have treatable aortic stenosis.”
The sheer magnitude of the problem, however, can be difficult to fully ascertain from administrative data like this, he said. Notably, patients who did not receive AVR were significantly older, with 37% aged 81-90 years and 12% over age 90, and had a lower hematocrit and lower estimated glomerular filtration rate. But it’s not clear how many had cancer, end-stage renal disease, or severe lung disease, which could have factored into the decision to undergo AVR.
“What’s also an issue is that over 50% of patients had low gradient disease, which is very problematic and takes careful assessment in an individual patient,” said Dr. Bonow, who is also editor-in-chief of JAMA Cardiology. “That’s all being generated by a low valve area of less than 1 cm2 from echo reports, so that’s not necessarily a careful prospective echo assessment ... so some of the patients with low-gradient disease may not have true severe aortic stenosis.”
Dr. Elmariah agreed that echocardiogram reports are not always clear cut and pointed out that referral to a valve specialist was highly predictive of whether or not a patient underwent AVR, supporting the class 1 guideline recommendation.
He also noted that Mass General is launching the DETECT-AS trial to determine whether electronic physician notifications highlighting clinical practice guideline recommendations will improve AVR utilization over standard care in 940 patients with severe AS on echocardiogram, defined by a valve area less than 1 cm2.
Reached for comment, Catherine Otto, MD, director of the Heart Valve Clinic at the University of Washington, Seattle, and a fellow member of the 2014 guideline writing committee, said “this adds to the data [that] we’re undertreating severe aortic stenosis, and it continues to be surprising given the availability of transcatheter options.”
The biggest challenge is trying to find out why it persists, which is difficult to determine from these data, she said. Whether that’s because the diagnosis is being missed or whether there are barriers to access because cardiologists aren’t understanding the indications or patients aren’t understanding what’s being offered, isn’t clear.
“The other [issue], of course, is are there inappropriate inequities in care? Is it fewer women, age-related, ethnic/racial-related; is it financial? Do people have coverage to get the treatment they need in our country?” Dr. Otto said. “All of those issues are areas that need to be addressed, and I think that is a concern we all have.”
An accompanying editorial points out that the “key lever” in combating undertreatment of AS is getting patients seen by a multidisciplinary heart team and details other possible solutions, such as adding process metrics regarding evaluation and treatment of AS to hospital performance.
“We track quality when AVR is performed (desirable), but how a hospital system performs in getting individuals treated who would benefit from AVR remains a complete blind spot,” write Brian Lindman, MD, MSc, and Angela Lowenstern, MD, MHS, both of Vanderbilt University Medical Center, Nashville, Tenn.
“Is it appropriate to consider the hospital ‘high performing’ when data from Li et al. show a 2-year absolute mortality difference from 9% to 56% based on treatment versus nontreatment with AVR for various AS patient subgroups?” they add.
Dr. Lindman and Dr. Lowenstern observe that having a 50% utilization rate for an effective therapy for a deadly cancer or stenting of ST-segment elevation myocardial infarction (STEMI) would generate negative headlines and a collective commitment to swift action by multiple stakeholders to address what would be “incontrovertibly unacceptable.”
“In one of America’s leading health care systems, there was evidence of an overwhelming reduction in the risk of death with AVR in all AS subgroups examined, but <50% of patients with AS with an indication or potential indication for AVR were treated with an AVR. Let that set in; hear and internalize the alarm. The status quo is unacceptable. What will you do? What will we do?” they conclude.
The study was funded by Edwards Lifesciences. Dr. Elmariah has received research grants from the American Heart Association, National Institutes of Health, Edwards Lifesciences, Svelte Medical, Abbott Vascular, and Medtronic, and has received consulting fees from Edwards Lifesciences. Dr. Bonow and Dr. Otto have disclosed no relevant financial relationships. Dr. Lindman has received investigator-initiated research grants from Edwards. Dr. Lowenstern has received consulting fees from Edwards.
A version of this article first appeared on Medscape.com.
The chance that patients with severe aortic stenosis (AS) will receive aortic valve replacement (AVR) is worse than the flip of a coin, even a decade after the gamechanging transcatheter option became available, a new study suggests.
Of the study’s 6,150 patients with an indication or potential indication for AVR, 48% received the procedure at Massachusetts General Hospital and its partner institution Brigham and Women’s Hospital, both in Boston – both of which have active, high-volume transcatheter and surgical AVR (TAVR/SAVR) programs.
“Essentially, this is a best-case scenario. So, unfortunately, I think on the national level we are likely to see rates that are far worse than what we observed here,” senior author Sammy Elmariah, MD, PhD, Massachusetts General Hospital, told this news organization.
The volume of AVR increased more than 10-fold over the 18-year study period (2000 to 2017), driven by the exponential growth of TAVR, he noted. However, the graying of America led to an even greater increase in the number of patients with severe AS and an indication for AVR.
The study, led by Shawn X. Li, MD, MBA, of Mass General, was published in the March 8 issue of the Journal of the American College of Cardiology.
Previous research has provided equally compelling data on the undertreatment of AS, including a 2021 study using natural language processing (NLP) that found AVR use was just 35.6% within 1 year of diagnosis and varied wildly among managing cardiologists.
The present study used NLP tools to identify symptoms consistent with severe AS in the medical record coupled with echocardiographic data from 10,795 patients with severe AS (valve area <1 cm2). Patients were divided into four AS subtypes and then classified as having a class 1 indication (high-gradient AS with symptoms or reduced ejection fraction [EF]) or a potential class 2a indication (low-gradient AS with symptoms) for AVR.
Among patients with high-gradient AS and class 1 indication for AVR, 1 in 3 did not receive AVR over the study period, including 30% with a normal EF and 47% with a low EF.
In those with low-gradient AS, 67% with a normal EF and 62% with a low EF did not receive AVR. The low-gradient groups were significantly less likely to receive AVR both in the entire study period and in the more contemporary period from 2014 to 2017, despite the valvular heart disease guideline 2014 update indicating AVR was “reasonable” in patients with low-gradient AS – a 2a recommendation upgraded to class 1 in the most recent 2020 update.
Better survival
In patients with a class 1 or potential class 2a indication, AVR was associated with a significantly lower risk of mortality in all four AS subgroups:
- High gradient/normal EF: 3% vs. 15%; adjusted hazard ratio, 0.42
- High-gradient/low EF: 16% vs. 72%; aHR, 0.28
- Low-gradient/normal EF: 5% vs. 14%; aHR, 0.73
- Low-gradient/low EF: 11% vs. 34%; aHR, 0.48; P < .001 for all
“I think what we need to do is change the paradigm, such that patients with a valve area that is less than or equal to 1 [cm2] is severe aortic stenosis until proven otherwise, and that essentially establishes a premise by which we default to treat these patients unless we can prove that it is in fact moderate,” Dr. Elmariah said.
Unfortunately, the opposite is currently true today, he said, and the default is not to treat and put patients through surgery or an invasive TAVR procedure unless physicians can definitively prove that it is severe AS. But they’re not always correct and don’t always have the ability to truly differentiate moderate from severe disease.
“The question, therefore, is ‘What do we do with those patients?’” Dr. Elmariah asked. “I think if a patient has symptoms, then we are obligated to intervene, given the stark difference in mortality that one sees when these patients go undertreated.”
Sounding the alarm
Robert Bonow, MD, a professor of cardiology at Northwestern University in Chicago and a writing committee member for the 2014 guideline update, said the study is a “big wake-up call” and “the take-home message is that we are missing some patients who have treatable aortic stenosis.”
The sheer magnitude of the problem, however, can be difficult to fully ascertain from administrative data like this, he said. Notably, patients who did not receive AVR were significantly older, with 37% aged 81-90 years and 12% over age 90, and had a lower hematocrit and lower estimated glomerular filtration rate. But it’s not clear how many had cancer, end-stage renal disease, or severe lung disease, which could have factored into the decision to undergo AVR.
“What’s also an issue is that over 50% of patients had low gradient disease, which is very problematic and takes careful assessment in an individual patient,” said Dr. Bonow, who is also editor-in-chief of JAMA Cardiology. “That’s all being generated by a low valve area of less than 1 cm2 from echo reports, so that’s not necessarily a careful prospective echo assessment ... so some of the patients with low-gradient disease may not have true severe aortic stenosis.”
Dr. Elmariah agreed that echocardiogram reports are not always clear cut and pointed out that referral to a valve specialist was highly predictive of whether or not a patient underwent AVR, supporting the class 1 guideline recommendation.
He also noted that Mass General is launching the DETECT-AS trial to determine whether electronic physician notifications highlighting clinical practice guideline recommendations will improve AVR utilization over standard care in 940 patients with severe AS on echocardiogram, defined by a valve area less than 1 cm2.
Reached for comment, Catherine Otto, MD, director of the Heart Valve Clinic at the University of Washington, Seattle, and a fellow member of the 2014 guideline writing committee, said “this adds to the data [that] we’re undertreating severe aortic stenosis, and it continues to be surprising given the availability of transcatheter options.”
The biggest challenge is trying to find out why it persists, which is difficult to determine from these data, she said. Whether that’s because the diagnosis is being missed or whether there are barriers to access because cardiologists aren’t understanding the indications or patients aren’t understanding what’s being offered, isn’t clear.
“The other [issue], of course, is are there inappropriate inequities in care? Is it fewer women, age-related, ethnic/racial-related; is it financial? Do people have coverage to get the treatment they need in our country?” Dr. Otto said. “All of those issues are areas that need to be addressed, and I think that is a concern we all have.”
An accompanying editorial points out that the “key lever” in combating undertreatment of AS is getting patients seen by a multidisciplinary heart team and details other possible solutions, such as adding process metrics regarding evaluation and treatment of AS to hospital performance.
“We track quality when AVR is performed (desirable), but how a hospital system performs in getting individuals treated who would benefit from AVR remains a complete blind spot,” write Brian Lindman, MD, MSc, and Angela Lowenstern, MD, MHS, both of Vanderbilt University Medical Center, Nashville, Tenn.
“Is it appropriate to consider the hospital ‘high performing’ when data from Li et al. show a 2-year absolute mortality difference from 9% to 56% based on treatment versus nontreatment with AVR for various AS patient subgroups?” they add.
Dr. Lindman and Dr. Lowenstern observe that having a 50% utilization rate for an effective therapy for a deadly cancer or stenting of ST-segment elevation myocardial infarction (STEMI) would generate negative headlines and a collective commitment to swift action by multiple stakeholders to address what would be “incontrovertibly unacceptable.”
“In one of America’s leading health care systems, there was evidence of an overwhelming reduction in the risk of death with AVR in all AS subgroups examined, but <50% of patients with AS with an indication or potential indication for AVR were treated with an AVR. Let that set in; hear and internalize the alarm. The status quo is unacceptable. What will you do? What will we do?” they conclude.
The study was funded by Edwards Lifesciences. Dr. Elmariah has received research grants from the American Heart Association, National Institutes of Health, Edwards Lifesciences, Svelte Medical, Abbott Vascular, and Medtronic, and has received consulting fees from Edwards Lifesciences. Dr. Bonow and Dr. Otto have disclosed no relevant financial relationships. Dr. Lindman has received investigator-initiated research grants from Edwards. Dr. Lowenstern has received consulting fees from Edwards.
A version of this article first appeared on Medscape.com.
The chance that patients with severe aortic stenosis (AS) will receive aortic valve replacement (AVR) is worse than the flip of a coin, even a decade after the gamechanging transcatheter option became available, a new study suggests.
Of the study’s 6,150 patients with an indication or potential indication for AVR, 48% received the procedure at Massachusetts General Hospital and its partner institution Brigham and Women’s Hospital, both in Boston – both of which have active, high-volume transcatheter and surgical AVR (TAVR/SAVR) programs.
“Essentially, this is a best-case scenario. So, unfortunately, I think on the national level we are likely to see rates that are far worse than what we observed here,” senior author Sammy Elmariah, MD, PhD, Massachusetts General Hospital, told this news organization.
The volume of AVR increased more than 10-fold over the 18-year study period (2000 to 2017), driven by the exponential growth of TAVR, he noted. However, the graying of America led to an even greater increase in the number of patients with severe AS and an indication for AVR.
The study, led by Shawn X. Li, MD, MBA, of Mass General, was published in the March 8 issue of the Journal of the American College of Cardiology.
Previous research has provided equally compelling data on the undertreatment of AS, including a 2021 study using natural language processing (NLP) that found AVR use was just 35.6% within 1 year of diagnosis and varied wildly among managing cardiologists.
The present study used NLP tools to identify symptoms consistent with severe AS in the medical record coupled with echocardiographic data from 10,795 patients with severe AS (valve area <1 cm2). Patients were divided into four AS subtypes and then classified as having a class 1 indication (high-gradient AS with symptoms or reduced ejection fraction [EF]) or a potential class 2a indication (low-gradient AS with symptoms) for AVR.
Among patients with high-gradient AS and class 1 indication for AVR, 1 in 3 did not receive AVR over the study period, including 30% with a normal EF and 47% with a low EF.
In those with low-gradient AS, 67% with a normal EF and 62% with a low EF did not receive AVR. The low-gradient groups were significantly less likely to receive AVR both in the entire study period and in the more contemporary period from 2014 to 2017, despite the valvular heart disease guideline 2014 update indicating AVR was “reasonable” in patients with low-gradient AS – a 2a recommendation upgraded to class 1 in the most recent 2020 update.
Better survival
In patients with a class 1 or potential class 2a indication, AVR was associated with a significantly lower risk of mortality in all four AS subgroups:
- High gradient/normal EF: 3% vs. 15%; adjusted hazard ratio, 0.42
- High-gradient/low EF: 16% vs. 72%; aHR, 0.28
- Low-gradient/normal EF: 5% vs. 14%; aHR, 0.73
- Low-gradient/low EF: 11% vs. 34%; aHR, 0.48; P < .001 for all
“I think what we need to do is change the paradigm, such that patients with a valve area that is less than or equal to 1 [cm2] is severe aortic stenosis until proven otherwise, and that essentially establishes a premise by which we default to treat these patients unless we can prove that it is in fact moderate,” Dr. Elmariah said.
Unfortunately, the opposite is currently true today, he said, and the default is not to treat and put patients through surgery or an invasive TAVR procedure unless physicians can definitively prove that it is severe AS. But they’re not always correct and don’t always have the ability to truly differentiate moderate from severe disease.
“The question, therefore, is ‘What do we do with those patients?’” Dr. Elmariah asked. “I think if a patient has symptoms, then we are obligated to intervene, given the stark difference in mortality that one sees when these patients go undertreated.”
Sounding the alarm
Robert Bonow, MD, a professor of cardiology at Northwestern University in Chicago and a writing committee member for the 2014 guideline update, said the study is a “big wake-up call” and “the take-home message is that we are missing some patients who have treatable aortic stenosis.”
The sheer magnitude of the problem, however, can be difficult to fully ascertain from administrative data like this, he said. Notably, patients who did not receive AVR were significantly older, with 37% aged 81-90 years and 12% over age 90, and had a lower hematocrit and lower estimated glomerular filtration rate. But it’s not clear how many had cancer, end-stage renal disease, or severe lung disease, which could have factored into the decision to undergo AVR.
“What’s also an issue is that over 50% of patients had low gradient disease, which is very problematic and takes careful assessment in an individual patient,” said Dr. Bonow, who is also editor-in-chief of JAMA Cardiology. “That’s all being generated by a low valve area of less than 1 cm2 from echo reports, so that’s not necessarily a careful prospective echo assessment ... so some of the patients with low-gradient disease may not have true severe aortic stenosis.”
Dr. Elmariah agreed that echocardiogram reports are not always clear cut and pointed out that referral to a valve specialist was highly predictive of whether or not a patient underwent AVR, supporting the class 1 guideline recommendation.
He also noted that Mass General is launching the DETECT-AS trial to determine whether electronic physician notifications highlighting clinical practice guideline recommendations will improve AVR utilization over standard care in 940 patients with severe AS on echocardiogram, defined by a valve area less than 1 cm2.
Reached for comment, Catherine Otto, MD, director of the Heart Valve Clinic at the University of Washington, Seattle, and a fellow member of the 2014 guideline writing committee, said “this adds to the data [that] we’re undertreating severe aortic stenosis, and it continues to be surprising given the availability of transcatheter options.”
The biggest challenge is trying to find out why it persists, which is difficult to determine from these data, she said. Whether that’s because the diagnosis is being missed or whether there are barriers to access because cardiologists aren’t understanding the indications or patients aren’t understanding what’s being offered, isn’t clear.
“The other [issue], of course, is are there inappropriate inequities in care? Is it fewer women, age-related, ethnic/racial-related; is it financial? Do people have coverage to get the treatment they need in our country?” Dr. Otto said. “All of those issues are areas that need to be addressed, and I think that is a concern we all have.”
An accompanying editorial points out that the “key lever” in combating undertreatment of AS is getting patients seen by a multidisciplinary heart team and details other possible solutions, such as adding process metrics regarding evaluation and treatment of AS to hospital performance.
“We track quality when AVR is performed (desirable), but how a hospital system performs in getting individuals treated who would benefit from AVR remains a complete blind spot,” write Brian Lindman, MD, MSc, and Angela Lowenstern, MD, MHS, both of Vanderbilt University Medical Center, Nashville, Tenn.
“Is it appropriate to consider the hospital ‘high performing’ when data from Li et al. show a 2-year absolute mortality difference from 9% to 56% based on treatment versus nontreatment with AVR for various AS patient subgroups?” they add.
Dr. Lindman and Dr. Lowenstern observe that having a 50% utilization rate for an effective therapy for a deadly cancer or stenting of ST-segment elevation myocardial infarction (STEMI) would generate negative headlines and a collective commitment to swift action by multiple stakeholders to address what would be “incontrovertibly unacceptable.”
“In one of America’s leading health care systems, there was evidence of an overwhelming reduction in the risk of death with AVR in all AS subgroups examined, but <50% of patients with AS with an indication or potential indication for AVR were treated with an AVR. Let that set in; hear and internalize the alarm. The status quo is unacceptable. What will you do? What will we do?” they conclude.
The study was funded by Edwards Lifesciences. Dr. Elmariah has received research grants from the American Heart Association, National Institutes of Health, Edwards Lifesciences, Svelte Medical, Abbott Vascular, and Medtronic, and has received consulting fees from Edwards Lifesciences. Dr. Bonow and Dr. Otto have disclosed no relevant financial relationships. Dr. Lindman has received investigator-initiated research grants from Edwards. Dr. Lowenstern has received consulting fees from Edwards.
A version of this article first appeared on Medscape.com.
Clozapine interrupted: APA, others seek FDA forum on REMS
In a Feb. 14 letter, the groups asked the FDA to reconsider its new risk evaluation and mitigation strategy (REMS) for clozapine because of concerns it had the potential to cause abrupt discontinuation of the medication.
The groups cite an Institute for Safe Medication Practices (ISMP) report of a 40-year-old woman who was a long-time clozapine user, had a cardiac arrest, and died after she stopped taking the drug because her psychiatrist was unable to register for the updated version of the REMS.
“It is unacceptable for a REMS with unproven effectiveness at meeting its goal to carry risks of interruptions that can result in rehospitalization, acute exacerbation of psychosis, increased risk of suicide, and potentially fatal orthostatic hypotension/bradycardic syndromes associated with incorrect restarts,” the groups said in the letter.
“We feel certain that this case reported in the literature is not the only serious adverse outcome from the REMS and the transition,” they added.
The letter was signed by the American Psychiatric Association, the American Association for Community Psychiatry, the American Psychiatric Nurses Association, the College of Psychiatric and Neurologic Pharmacists, the National Alliance on Mental Illness, the National Association of State Mental Health Program Directors, and the National Council for Mental Wellbeing.
Clozapine can decrease the neutrophil count, which can lead to severe neutropenia, serious infection, and death. Consequently, the FDA put additional safety measures in place governing clozapine prescribing.
In 2015, a centralized clozapine REMS replaced separate prescribing registries that the drug manufacturers maintained. There were technical issues with the 2015 start-up of that website, including data migration problems and long call wait times, the FDA said.
Subsequently, the drug’s manufacturers then decided to change the REMS platform, which created new issues that led to high call volume and long wait times for clinicians and pharmacists who were trying to enroll.
Maintaining access
In November 2021, the FDA announced it would put some aspects of a planned switch on hold. A month later, the agency made further modifications to its plan.
The FDA said it would exercise “enforcement discretion” to try to maintain access to clozapine amid hitches with the REMS transition efforts. The agency also said at the time that it would not object if pharmacists dispensed clozapine without the usual authorization. In addition, wholesalers could ship the drug to pharmacies and health care settings without confirming REMS enrollment.
The FDA also held two December meetings to allow various stakeholders to air concerns.
In their letter, the APA and other groups asked if the FDA intends to continue with accommodations, such as allowing pharmacies to order clozapine from wholesalers without restriction.
“We do not feel the issues are resolved,” the groups said.
A version of this article first appeared on Medscape.com.
In a Feb. 14 letter, the groups asked the FDA to reconsider its new risk evaluation and mitigation strategy (REMS) for clozapine because of concerns it had the potential to cause abrupt discontinuation of the medication.
The groups cite an Institute for Safe Medication Practices (ISMP) report of a 40-year-old woman who was a long-time clozapine user, had a cardiac arrest, and died after she stopped taking the drug because her psychiatrist was unable to register for the updated version of the REMS.
“It is unacceptable for a REMS with unproven effectiveness at meeting its goal to carry risks of interruptions that can result in rehospitalization, acute exacerbation of psychosis, increased risk of suicide, and potentially fatal orthostatic hypotension/bradycardic syndromes associated with incorrect restarts,” the groups said in the letter.
“We feel certain that this case reported in the literature is not the only serious adverse outcome from the REMS and the transition,” they added.
The letter was signed by the American Psychiatric Association, the American Association for Community Psychiatry, the American Psychiatric Nurses Association, the College of Psychiatric and Neurologic Pharmacists, the National Alliance on Mental Illness, the National Association of State Mental Health Program Directors, and the National Council for Mental Wellbeing.
Clozapine can decrease the neutrophil count, which can lead to severe neutropenia, serious infection, and death. Consequently, the FDA put additional safety measures in place governing clozapine prescribing.
In 2015, a centralized clozapine REMS replaced separate prescribing registries that the drug manufacturers maintained. There were technical issues with the 2015 start-up of that website, including data migration problems and long call wait times, the FDA said.
Subsequently, the drug’s manufacturers then decided to change the REMS platform, which created new issues that led to high call volume and long wait times for clinicians and pharmacists who were trying to enroll.
Maintaining access
In November 2021, the FDA announced it would put some aspects of a planned switch on hold. A month later, the agency made further modifications to its plan.
The FDA said it would exercise “enforcement discretion” to try to maintain access to clozapine amid hitches with the REMS transition efforts. The agency also said at the time that it would not object if pharmacists dispensed clozapine without the usual authorization. In addition, wholesalers could ship the drug to pharmacies and health care settings without confirming REMS enrollment.
The FDA also held two December meetings to allow various stakeholders to air concerns.
In their letter, the APA and other groups asked if the FDA intends to continue with accommodations, such as allowing pharmacies to order clozapine from wholesalers without restriction.
“We do not feel the issues are resolved,” the groups said.
A version of this article first appeared on Medscape.com.
In a Feb. 14 letter, the groups asked the FDA to reconsider its new risk evaluation and mitigation strategy (REMS) for clozapine because of concerns it had the potential to cause abrupt discontinuation of the medication.
The groups cite an Institute for Safe Medication Practices (ISMP) report of a 40-year-old woman who was a long-time clozapine user, had a cardiac arrest, and died after she stopped taking the drug because her psychiatrist was unable to register for the updated version of the REMS.
“It is unacceptable for a REMS with unproven effectiveness at meeting its goal to carry risks of interruptions that can result in rehospitalization, acute exacerbation of psychosis, increased risk of suicide, and potentially fatal orthostatic hypotension/bradycardic syndromes associated with incorrect restarts,” the groups said in the letter.
“We feel certain that this case reported in the literature is not the only serious adverse outcome from the REMS and the transition,” they added.
The letter was signed by the American Psychiatric Association, the American Association for Community Psychiatry, the American Psychiatric Nurses Association, the College of Psychiatric and Neurologic Pharmacists, the National Alliance on Mental Illness, the National Association of State Mental Health Program Directors, and the National Council for Mental Wellbeing.
Clozapine can decrease the neutrophil count, which can lead to severe neutropenia, serious infection, and death. Consequently, the FDA put additional safety measures in place governing clozapine prescribing.
In 2015, a centralized clozapine REMS replaced separate prescribing registries that the drug manufacturers maintained. There were technical issues with the 2015 start-up of that website, including data migration problems and long call wait times, the FDA said.
Subsequently, the drug’s manufacturers then decided to change the REMS platform, which created new issues that led to high call volume and long wait times for clinicians and pharmacists who were trying to enroll.
Maintaining access
In November 2021, the FDA announced it would put some aspects of a planned switch on hold. A month later, the agency made further modifications to its plan.
The FDA said it would exercise “enforcement discretion” to try to maintain access to clozapine amid hitches with the REMS transition efforts. The agency also said at the time that it would not object if pharmacists dispensed clozapine without the usual authorization. In addition, wholesalers could ship the drug to pharmacies and health care settings without confirming REMS enrollment.
The FDA also held two December meetings to allow various stakeholders to air concerns.
In their letter, the APA and other groups asked if the FDA intends to continue with accommodations, such as allowing pharmacies to order clozapine from wholesalers without restriction.
“We do not feel the issues are resolved,” the groups said.
A version of this article first appeared on Medscape.com.
Managing overuse of food IgE panels: Multiple approaches needed
PHOENIX – For at least a decade, professional allergy and pediatrics societies have urged against using food IgE tests unless the patient has a history consistent with potential IgE-mediated food allergies. Yet virtually every health system offers these blood tests, and their inappropriate use – especially of panels that measure many allergens at once – remains a huge problem.
Beyond wasteful spending, excessive food IgE testing can lead patients to worry needlessly and to avoid foods they aren’t allergic to. For babies and toddlers, avoidance can drive up the risk of developing allergies to those foods later in life – a consequence that was convincingly proven by the LEAP study but has still not translated to a widespread change in practice.
“I think we all know that there’s just a lot of system-wide resistance to making these changes, and we don’t completely understand why,” Nicholas Hartog, MD, an allergist with Spectrum Health in Grand Rapids, Mich., told this news organization.
At the American Academy of Allergy, Asthma & Immunology annual meeting, one of Dr. Hartog’s residents, Courtney Cotter, DO, presented a poster detailing their team’s retrospective review of food panel ordering practices across Spectrum Health, a large, multispecialty physician group in west Michigan.
The team combed Epic health records to evaluate food IgE ordering from January 2016 to December 2021. They tracked monthly figures for the number of patients who underwent food IgE tests, the percentage of tested patients for whom food panels were available, and the number of food panels and total number of food IgE tests ordered. They compared average rates from the final 3 months with rates from the first 3 months, which predated the August 2016 establishment of an academic pediatric allergy/immunology department.
Initially, Dr. Hartog and his colleagues focused on educating doctors on appropriate use of food IgE tests through informal conversations and lectures, but, he said, “It’s really difficult to change physician behavior, so sometimes we have to go about it by making it hard to do the wrong thing.”
To that end, the team tried to eliminate the food panels. However, some lab staff feared the possibility of losing revenue if physicians decided to order these tests elsewhere. After more negotiations, the laboratory agreed in December 2019 to restrict and rework food IgE testing by dropping the number of panels from nine to two and by restricting the number of foods in those panels. For example, in the basic panel, “we limited it to just four allergens, so even if you order a panel, you’re not getting 20 results,” Dr. Hartog told this news organization. “I finally found a friendly pathologist who was very on board with this positive change.”
In December 2020, the team implemented yet another strategy: Epic alerts. Each time doctors request a food panel, they receive a pop-up message stating that panel tests are not recommended and asking if they wish to proceed.
The multipronged effort had a modest impact on the number of food panels ordered per month, which dipped from 112.7 to 84.7 for the first and last 3 months of the study. Monthly totals of individual food IgE tests showed a steeper drop, decreasing from 2,379 to 1,180 in the initial and final 3-month periods – a change Dr. Hartog attributes to the revamped food panels. They estimated the cost savings at around $40 per patient, “and we were getting on average about 200 patients a month, so it adds up,” he said.
But the Epic alerts seemed to have little effect. Over the duration of the study, the monthly number of IgE tests ordered per clinician did not change. Neither did the percentage of patients evaluated with a food panel. “The alerts pop up, but people are still ordering,” Dr. Hartog said.
On the whole, the analysis shows that, “despite major efforts to educate providers and the public about these things, it is rampantly disregarded and is a huge problem for our specialty and is likely causing harm to patients,” said allergist-immunologist Gerald Lee, MD, of Emory University in Atlanta.
Dr. Lee said that a common scenario for inappropriate food IgE testing is severe eczema. Many parents request blood tests because they assume their child’s skin condition is driven by food allergies. When the child turns up positive to various foods on panel tests, which have high false-positive rates, the physician may recommend eliminating those foods to improve the skin rash – which “actually delays introduction of the food and potentially increases the risk for food allergy,” Dr. Lee said. “That was a common practice when I was in fellowship (2011) and is widely prevalent today.”
Edwin Kim, MD, director of the UNC Food Allergy Initiative at the University of North Carolina at Chapel Hill, agrees that food IgE panels are wasteful and harmful. However, he thinks the solution is not to tell primary care physicians and pediatricians to stop using the tests. “We’re insinuating that they’re being used inappropriately, but the problem is that these are people that are patient facing, the patients are asking a question, and the appropriate tests aren’t there,” Dr. Kim said. “A big part of that problem is that the tests we have available to us are not good enough.”
The Spectrum Health analysis did not examine ICD codes associated with the food IgE tests or track which physicians ordered the tests. A 2016 retrospective review published in Pediatrics did evaluate ordering practices by specialty and found that primary care providers ordered “significantly more food allergen panels, tests for uncommon causes of food allergy, and generate higher cost per patient compared with allergists.”
Given the immense challenges with implementing system-wide changes, sometimes it can help to educate parents and families. “When you sit down and take 2 or 3 minutes to explain why this is a bad test and that I care about your kid but just don’t want inappropriate testing, they’re okay with it. They understand,” Dr. Hartog said. “When I teach residents, I make sure to emphasize that we have these conversations all the time.”
Dr. Hartog reports financial relationships with Binding Site (speaker), Regeneron (advisory board), Genentech (advisory board), Horizon Pharmaceuticals (advisory board, consulting, speaker), Takeda (speaker, advisory board) and Pharming Healthcare (advisory board, scientific steering committee, consulting), though none related to food allergy. Dr. Lee has disclosed no relevant financial relationships. Dr. Kim reports consultancy with Aimmune Therapeutics, Allako, AllerGenis, Belhaven Pharma, DBV Technologies, Duke Clinical Research Institute, and Nutricia; advisory board membership with ALK, DBV Technologies, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases and the Immune Tolerance Network; the National Center for Complementary and Integrative Health; Food Allergy Research and Education; and the Wallace Research Foundation.
A version of this article first appeared on Medscape.com.
PHOENIX – For at least a decade, professional allergy and pediatrics societies have urged against using food IgE tests unless the patient has a history consistent with potential IgE-mediated food allergies. Yet virtually every health system offers these blood tests, and their inappropriate use – especially of panels that measure many allergens at once – remains a huge problem.
Beyond wasteful spending, excessive food IgE testing can lead patients to worry needlessly and to avoid foods they aren’t allergic to. For babies and toddlers, avoidance can drive up the risk of developing allergies to those foods later in life – a consequence that was convincingly proven by the LEAP study but has still not translated to a widespread change in practice.
“I think we all know that there’s just a lot of system-wide resistance to making these changes, and we don’t completely understand why,” Nicholas Hartog, MD, an allergist with Spectrum Health in Grand Rapids, Mich., told this news organization.
At the American Academy of Allergy, Asthma & Immunology annual meeting, one of Dr. Hartog’s residents, Courtney Cotter, DO, presented a poster detailing their team’s retrospective review of food panel ordering practices across Spectrum Health, a large, multispecialty physician group in west Michigan.
The team combed Epic health records to evaluate food IgE ordering from January 2016 to December 2021. They tracked monthly figures for the number of patients who underwent food IgE tests, the percentage of tested patients for whom food panels were available, and the number of food panels and total number of food IgE tests ordered. They compared average rates from the final 3 months with rates from the first 3 months, which predated the August 2016 establishment of an academic pediatric allergy/immunology department.
Initially, Dr. Hartog and his colleagues focused on educating doctors on appropriate use of food IgE tests through informal conversations and lectures, but, he said, “It’s really difficult to change physician behavior, so sometimes we have to go about it by making it hard to do the wrong thing.”
To that end, the team tried to eliminate the food panels. However, some lab staff feared the possibility of losing revenue if physicians decided to order these tests elsewhere. After more negotiations, the laboratory agreed in December 2019 to restrict and rework food IgE testing by dropping the number of panels from nine to two and by restricting the number of foods in those panels. For example, in the basic panel, “we limited it to just four allergens, so even if you order a panel, you’re not getting 20 results,” Dr. Hartog told this news organization. “I finally found a friendly pathologist who was very on board with this positive change.”
In December 2020, the team implemented yet another strategy: Epic alerts. Each time doctors request a food panel, they receive a pop-up message stating that panel tests are not recommended and asking if they wish to proceed.
The multipronged effort had a modest impact on the number of food panels ordered per month, which dipped from 112.7 to 84.7 for the first and last 3 months of the study. Monthly totals of individual food IgE tests showed a steeper drop, decreasing from 2,379 to 1,180 in the initial and final 3-month periods – a change Dr. Hartog attributes to the revamped food panels. They estimated the cost savings at around $40 per patient, “and we were getting on average about 200 patients a month, so it adds up,” he said.
But the Epic alerts seemed to have little effect. Over the duration of the study, the monthly number of IgE tests ordered per clinician did not change. Neither did the percentage of patients evaluated with a food panel. “The alerts pop up, but people are still ordering,” Dr. Hartog said.
On the whole, the analysis shows that, “despite major efforts to educate providers and the public about these things, it is rampantly disregarded and is a huge problem for our specialty and is likely causing harm to patients,” said allergist-immunologist Gerald Lee, MD, of Emory University in Atlanta.
Dr. Lee said that a common scenario for inappropriate food IgE testing is severe eczema. Many parents request blood tests because they assume their child’s skin condition is driven by food allergies. When the child turns up positive to various foods on panel tests, which have high false-positive rates, the physician may recommend eliminating those foods to improve the skin rash – which “actually delays introduction of the food and potentially increases the risk for food allergy,” Dr. Lee said. “That was a common practice when I was in fellowship (2011) and is widely prevalent today.”
Edwin Kim, MD, director of the UNC Food Allergy Initiative at the University of North Carolina at Chapel Hill, agrees that food IgE panels are wasteful and harmful. However, he thinks the solution is not to tell primary care physicians and pediatricians to stop using the tests. “We’re insinuating that they’re being used inappropriately, but the problem is that these are people that are patient facing, the patients are asking a question, and the appropriate tests aren’t there,” Dr. Kim said. “A big part of that problem is that the tests we have available to us are not good enough.”
The Spectrum Health analysis did not examine ICD codes associated with the food IgE tests or track which physicians ordered the tests. A 2016 retrospective review published in Pediatrics did evaluate ordering practices by specialty and found that primary care providers ordered “significantly more food allergen panels, tests for uncommon causes of food allergy, and generate higher cost per patient compared with allergists.”
Given the immense challenges with implementing system-wide changes, sometimes it can help to educate parents and families. “When you sit down and take 2 or 3 minutes to explain why this is a bad test and that I care about your kid but just don’t want inappropriate testing, they’re okay with it. They understand,” Dr. Hartog said. “When I teach residents, I make sure to emphasize that we have these conversations all the time.”
Dr. Hartog reports financial relationships with Binding Site (speaker), Regeneron (advisory board), Genentech (advisory board), Horizon Pharmaceuticals (advisory board, consulting, speaker), Takeda (speaker, advisory board) and Pharming Healthcare (advisory board, scientific steering committee, consulting), though none related to food allergy. Dr. Lee has disclosed no relevant financial relationships. Dr. Kim reports consultancy with Aimmune Therapeutics, Allako, AllerGenis, Belhaven Pharma, DBV Technologies, Duke Clinical Research Institute, and Nutricia; advisory board membership with ALK, DBV Technologies, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases and the Immune Tolerance Network; the National Center for Complementary and Integrative Health; Food Allergy Research and Education; and the Wallace Research Foundation.
A version of this article first appeared on Medscape.com.
PHOENIX – For at least a decade, professional allergy and pediatrics societies have urged against using food IgE tests unless the patient has a history consistent with potential IgE-mediated food allergies. Yet virtually every health system offers these blood tests, and their inappropriate use – especially of panels that measure many allergens at once – remains a huge problem.
Beyond wasteful spending, excessive food IgE testing can lead patients to worry needlessly and to avoid foods they aren’t allergic to. For babies and toddlers, avoidance can drive up the risk of developing allergies to those foods later in life – a consequence that was convincingly proven by the LEAP study but has still not translated to a widespread change in practice.
“I think we all know that there’s just a lot of system-wide resistance to making these changes, and we don’t completely understand why,” Nicholas Hartog, MD, an allergist with Spectrum Health in Grand Rapids, Mich., told this news organization.
At the American Academy of Allergy, Asthma & Immunology annual meeting, one of Dr. Hartog’s residents, Courtney Cotter, DO, presented a poster detailing their team’s retrospective review of food panel ordering practices across Spectrum Health, a large, multispecialty physician group in west Michigan.
The team combed Epic health records to evaluate food IgE ordering from January 2016 to December 2021. They tracked monthly figures for the number of patients who underwent food IgE tests, the percentage of tested patients for whom food panels were available, and the number of food panels and total number of food IgE tests ordered. They compared average rates from the final 3 months with rates from the first 3 months, which predated the August 2016 establishment of an academic pediatric allergy/immunology department.
Initially, Dr. Hartog and his colleagues focused on educating doctors on appropriate use of food IgE tests through informal conversations and lectures, but, he said, “It’s really difficult to change physician behavior, so sometimes we have to go about it by making it hard to do the wrong thing.”
To that end, the team tried to eliminate the food panels. However, some lab staff feared the possibility of losing revenue if physicians decided to order these tests elsewhere. After more negotiations, the laboratory agreed in December 2019 to restrict and rework food IgE testing by dropping the number of panels from nine to two and by restricting the number of foods in those panels. For example, in the basic panel, “we limited it to just four allergens, so even if you order a panel, you’re not getting 20 results,” Dr. Hartog told this news organization. “I finally found a friendly pathologist who was very on board with this positive change.”
In December 2020, the team implemented yet another strategy: Epic alerts. Each time doctors request a food panel, they receive a pop-up message stating that panel tests are not recommended and asking if they wish to proceed.
The multipronged effort had a modest impact on the number of food panels ordered per month, which dipped from 112.7 to 84.7 for the first and last 3 months of the study. Monthly totals of individual food IgE tests showed a steeper drop, decreasing from 2,379 to 1,180 in the initial and final 3-month periods – a change Dr. Hartog attributes to the revamped food panels. They estimated the cost savings at around $40 per patient, “and we were getting on average about 200 patients a month, so it adds up,” he said.
But the Epic alerts seemed to have little effect. Over the duration of the study, the monthly number of IgE tests ordered per clinician did not change. Neither did the percentage of patients evaluated with a food panel. “The alerts pop up, but people are still ordering,” Dr. Hartog said.
On the whole, the analysis shows that, “despite major efforts to educate providers and the public about these things, it is rampantly disregarded and is a huge problem for our specialty and is likely causing harm to patients,” said allergist-immunologist Gerald Lee, MD, of Emory University in Atlanta.
Dr. Lee said that a common scenario for inappropriate food IgE testing is severe eczema. Many parents request blood tests because they assume their child’s skin condition is driven by food allergies. When the child turns up positive to various foods on panel tests, which have high false-positive rates, the physician may recommend eliminating those foods to improve the skin rash – which “actually delays introduction of the food and potentially increases the risk for food allergy,” Dr. Lee said. “That was a common practice when I was in fellowship (2011) and is widely prevalent today.”
Edwin Kim, MD, director of the UNC Food Allergy Initiative at the University of North Carolina at Chapel Hill, agrees that food IgE panels are wasteful and harmful. However, he thinks the solution is not to tell primary care physicians and pediatricians to stop using the tests. “We’re insinuating that they’re being used inappropriately, but the problem is that these are people that are patient facing, the patients are asking a question, and the appropriate tests aren’t there,” Dr. Kim said. “A big part of that problem is that the tests we have available to us are not good enough.”
The Spectrum Health analysis did not examine ICD codes associated with the food IgE tests or track which physicians ordered the tests. A 2016 retrospective review published in Pediatrics did evaluate ordering practices by specialty and found that primary care providers ordered “significantly more food allergen panels, tests for uncommon causes of food allergy, and generate higher cost per patient compared with allergists.”
Given the immense challenges with implementing system-wide changes, sometimes it can help to educate parents and families. “When you sit down and take 2 or 3 minutes to explain why this is a bad test and that I care about your kid but just don’t want inappropriate testing, they’re okay with it. They understand,” Dr. Hartog said. “When I teach residents, I make sure to emphasize that we have these conversations all the time.”
Dr. Hartog reports financial relationships with Binding Site (speaker), Regeneron (advisory board), Genentech (advisory board), Horizon Pharmaceuticals (advisory board, consulting, speaker), Takeda (speaker, advisory board) and Pharming Healthcare (advisory board, scientific steering committee, consulting), though none related to food allergy. Dr. Lee has disclosed no relevant financial relationships. Dr. Kim reports consultancy with Aimmune Therapeutics, Allako, AllerGenis, Belhaven Pharma, DBV Technologies, Duke Clinical Research Institute, and Nutricia; advisory board membership with ALK, DBV Technologies, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases and the Immune Tolerance Network; the National Center for Complementary and Integrative Health; Food Allergy Research and Education; and the Wallace Research Foundation.
A version of this article first appeared on Medscape.com.
Boosting daily exercise after age 70 tied to lower CVD risk
Increasingly active patterns of physical activity were linked with reduced rates of overall mortality and cardiovascular disease (CVD), but early rather than later in late life, in a 20-year follow-up cohort study.
In this population of people older than 65 years, researchers found that physical activity overall was associated with lower rates of incident CVD, particularly among men, and the association was strongest in people 70 to 75 years of age, rather than in older age groups.
They also looked at “trajectories,” or changes in activity over time, and found that a stable-high trajectory of activity was associated with a significantly lower risk for cardiovascular outcomes in men than in those with a stable-low trajectory. For women, more physical activity was consistently associated with lower CVD outcomes, although not statistically significantly so, except for overall mortality, which did reach significance.
Notably, the greatest reduction in cardiovascular risk was reported in people who did more than 20 minutes of physical exercise each day, and it was more pronounced in those 70 years of age.
Physical activity was also associated with a lower incidence of heart failure and coronary heart disease in older people, again especially early on in late life, reported Claudio Barbiellini Amidei, MD, University of Padua, Italy, and colleagues.
The data suggest that physical activity is more effective in preventing CVD onset when implemented early rather than later in life, noted Dr. Amidei in an email.
“The findings of our study are suggestive of a protective effect of physical activity in late-life on cardiovascular health. WHO recommendations for adults and older adults are to practice at least 20 minutes of moderate to vigorous physical activity per day. I believe this is a realistic target, and policy makers should raise awareness on the importance of achieving this goal at all ages, including in late-life,” Dr. Amidei said.
The study was published online Feb. 14 in Heart.
Previous research has demonstrated that the most benefit of high physical activity, compared with low, begins at about 60 years of age, and that is because younger people are at much lower risk, noted Carl “Chip” Lavie MD, FACC, medical director of cardiac rehabilitation and prevention, Ochsner Clinical School–The University of Queensland School of Medicine, New Orleans, who was not involved in the study.
“At quite old ages, for example over age 80, resistance exercise or weight training and balance training may be even more important than aerobic training,” he added.
Activity ‘trajectories’
The benefits of physical activity on cardiovascular risk are well established, the researchers note. Less clear is the role that trajectories of activity over time play, although research to date suggests a reduction in risk with increasing activity from mid-life to early old age, they write.
For the current analysis, the researchers assessed 3,099 Italian participants. Mean age was about 75 years, and baseline data were collected from 1995 to 1997.
Follow-up visits were conducted after 4 years and again after 7 years. Using hospital medical records and mortality data, the researchers were able to collect surveillance data through 2018. Hospital records, surveys, and clinical assessments helped them identify incident and prevalent cardiovascular diseases, such as stroke, coronary heart disease, and heart failure.
Participants’ physical activity patterns were classified as stable-high, low-increasing, high-decreasing, and stable-low. Exposure was evaluated at 70, 75, 80, and 85 years of age.
“In our analyses, we focused on moderate to vigorous physical activity, and these include a broad range of exercises, such as walking very briskly, playing tennis, [and] jogging, but comprise also other activities, such as gardening or doing household chores,” said Dr. Amidei.
Patterns of stable-low physical activity were linked to a significantly greater risk for cardiovascular outcomes in men than patterns of stable-high physical activity (hazard ratio, 0.48; 95% confidence interval, 0.27-0.86; P for trend = .002).
No significant relation was found between physical activity and stroke, the researchers note.
“The benefits of physical activity seem to lessen above the age of 75 years and seem more important in men,” noted Dr. Lavie. “This may be partly due to the higher risk of CVD in men. Women typically lag 13 to 15 years behind men for CVD but start catching up in older years.”
Limitations of the study include lack of information regarding physical activity during mid-life, the limited number of stroke events, the relatively few participants older than 85 years, and potential recall bias, the researchers note.
Another limitation was that the physical activity data were based on patient surveys collected 3 years apart and did not involve the use of an accelerometer, the researchers add.
“Future observational studies are required to confirm our findings and pathophysiological studies are warranted to examine the underlying biological mechanisms. Physical activity is likely to be beneficial at any age, but to summarize our findings, we could say that when it comes to being physically active, the sooner the better,” concluded Dr. Amidei.
Dr. Amidei reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Increasingly active patterns of physical activity were linked with reduced rates of overall mortality and cardiovascular disease (CVD), but early rather than later in late life, in a 20-year follow-up cohort study.
In this population of people older than 65 years, researchers found that physical activity overall was associated with lower rates of incident CVD, particularly among men, and the association was strongest in people 70 to 75 years of age, rather than in older age groups.
They also looked at “trajectories,” or changes in activity over time, and found that a stable-high trajectory of activity was associated with a significantly lower risk for cardiovascular outcomes in men than in those with a stable-low trajectory. For women, more physical activity was consistently associated with lower CVD outcomes, although not statistically significantly so, except for overall mortality, which did reach significance.
Notably, the greatest reduction in cardiovascular risk was reported in people who did more than 20 minutes of physical exercise each day, and it was more pronounced in those 70 years of age.
Physical activity was also associated with a lower incidence of heart failure and coronary heart disease in older people, again especially early on in late life, reported Claudio Barbiellini Amidei, MD, University of Padua, Italy, and colleagues.
The data suggest that physical activity is more effective in preventing CVD onset when implemented early rather than later in life, noted Dr. Amidei in an email.
“The findings of our study are suggestive of a protective effect of physical activity in late-life on cardiovascular health. WHO recommendations for adults and older adults are to practice at least 20 minutes of moderate to vigorous physical activity per day. I believe this is a realistic target, and policy makers should raise awareness on the importance of achieving this goal at all ages, including in late-life,” Dr. Amidei said.
The study was published online Feb. 14 in Heart.
Previous research has demonstrated that the most benefit of high physical activity, compared with low, begins at about 60 years of age, and that is because younger people are at much lower risk, noted Carl “Chip” Lavie MD, FACC, medical director of cardiac rehabilitation and prevention, Ochsner Clinical School–The University of Queensland School of Medicine, New Orleans, who was not involved in the study.
“At quite old ages, for example over age 80, resistance exercise or weight training and balance training may be even more important than aerobic training,” he added.
Activity ‘trajectories’
The benefits of physical activity on cardiovascular risk are well established, the researchers note. Less clear is the role that trajectories of activity over time play, although research to date suggests a reduction in risk with increasing activity from mid-life to early old age, they write.
For the current analysis, the researchers assessed 3,099 Italian participants. Mean age was about 75 years, and baseline data were collected from 1995 to 1997.
Follow-up visits were conducted after 4 years and again after 7 years. Using hospital medical records and mortality data, the researchers were able to collect surveillance data through 2018. Hospital records, surveys, and clinical assessments helped them identify incident and prevalent cardiovascular diseases, such as stroke, coronary heart disease, and heart failure.
Participants’ physical activity patterns were classified as stable-high, low-increasing, high-decreasing, and stable-low. Exposure was evaluated at 70, 75, 80, and 85 years of age.
“In our analyses, we focused on moderate to vigorous physical activity, and these include a broad range of exercises, such as walking very briskly, playing tennis, [and] jogging, but comprise also other activities, such as gardening or doing household chores,” said Dr. Amidei.
Patterns of stable-low physical activity were linked to a significantly greater risk for cardiovascular outcomes in men than patterns of stable-high physical activity (hazard ratio, 0.48; 95% confidence interval, 0.27-0.86; P for trend = .002).
No significant relation was found between physical activity and stroke, the researchers note.
“The benefits of physical activity seem to lessen above the age of 75 years and seem more important in men,” noted Dr. Lavie. “This may be partly due to the higher risk of CVD in men. Women typically lag 13 to 15 years behind men for CVD but start catching up in older years.”
Limitations of the study include lack of information regarding physical activity during mid-life, the limited number of stroke events, the relatively few participants older than 85 years, and potential recall bias, the researchers note.
Another limitation was that the physical activity data were based on patient surveys collected 3 years apart and did not involve the use of an accelerometer, the researchers add.
“Future observational studies are required to confirm our findings and pathophysiological studies are warranted to examine the underlying biological mechanisms. Physical activity is likely to be beneficial at any age, but to summarize our findings, we could say that when it comes to being physically active, the sooner the better,” concluded Dr. Amidei.
Dr. Amidei reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Increasingly active patterns of physical activity were linked with reduced rates of overall mortality and cardiovascular disease (CVD), but early rather than later in late life, in a 20-year follow-up cohort study.
In this population of people older than 65 years, researchers found that physical activity overall was associated with lower rates of incident CVD, particularly among men, and the association was strongest in people 70 to 75 years of age, rather than in older age groups.
They also looked at “trajectories,” or changes in activity over time, and found that a stable-high trajectory of activity was associated with a significantly lower risk for cardiovascular outcomes in men than in those with a stable-low trajectory. For women, more physical activity was consistently associated with lower CVD outcomes, although not statistically significantly so, except for overall mortality, which did reach significance.
Notably, the greatest reduction in cardiovascular risk was reported in people who did more than 20 minutes of physical exercise each day, and it was more pronounced in those 70 years of age.
Physical activity was also associated with a lower incidence of heart failure and coronary heart disease in older people, again especially early on in late life, reported Claudio Barbiellini Amidei, MD, University of Padua, Italy, and colleagues.
The data suggest that physical activity is more effective in preventing CVD onset when implemented early rather than later in life, noted Dr. Amidei in an email.
“The findings of our study are suggestive of a protective effect of physical activity in late-life on cardiovascular health. WHO recommendations for adults and older adults are to practice at least 20 minutes of moderate to vigorous physical activity per day. I believe this is a realistic target, and policy makers should raise awareness on the importance of achieving this goal at all ages, including in late-life,” Dr. Amidei said.
The study was published online Feb. 14 in Heart.
Previous research has demonstrated that the most benefit of high physical activity, compared with low, begins at about 60 years of age, and that is because younger people are at much lower risk, noted Carl “Chip” Lavie MD, FACC, medical director of cardiac rehabilitation and prevention, Ochsner Clinical School–The University of Queensland School of Medicine, New Orleans, who was not involved in the study.
“At quite old ages, for example over age 80, resistance exercise or weight training and balance training may be even more important than aerobic training,” he added.
Activity ‘trajectories’
The benefits of physical activity on cardiovascular risk are well established, the researchers note. Less clear is the role that trajectories of activity over time play, although research to date suggests a reduction in risk with increasing activity from mid-life to early old age, they write.
For the current analysis, the researchers assessed 3,099 Italian participants. Mean age was about 75 years, and baseline data were collected from 1995 to 1997.
Follow-up visits were conducted after 4 years and again after 7 years. Using hospital medical records and mortality data, the researchers were able to collect surveillance data through 2018. Hospital records, surveys, and clinical assessments helped them identify incident and prevalent cardiovascular diseases, such as stroke, coronary heart disease, and heart failure.
Participants’ physical activity patterns were classified as stable-high, low-increasing, high-decreasing, and stable-low. Exposure was evaluated at 70, 75, 80, and 85 years of age.
“In our analyses, we focused on moderate to vigorous physical activity, and these include a broad range of exercises, such as walking very briskly, playing tennis, [and] jogging, but comprise also other activities, such as gardening or doing household chores,” said Dr. Amidei.
Patterns of stable-low physical activity were linked to a significantly greater risk for cardiovascular outcomes in men than patterns of stable-high physical activity (hazard ratio, 0.48; 95% confidence interval, 0.27-0.86; P for trend = .002).
No significant relation was found between physical activity and stroke, the researchers note.
“The benefits of physical activity seem to lessen above the age of 75 years and seem more important in men,” noted Dr. Lavie. “This may be partly due to the higher risk of CVD in men. Women typically lag 13 to 15 years behind men for CVD but start catching up in older years.”
Limitations of the study include lack of information regarding physical activity during mid-life, the limited number of stroke events, the relatively few participants older than 85 years, and potential recall bias, the researchers note.
Another limitation was that the physical activity data were based on patient surveys collected 3 years apart and did not involve the use of an accelerometer, the researchers add.
“Future observational studies are required to confirm our findings and pathophysiological studies are warranted to examine the underlying biological mechanisms. Physical activity is likely to be beneficial at any age, but to summarize our findings, we could say that when it comes to being physically active, the sooner the better,” concluded Dr. Amidei.
Dr. Amidei reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Upadacitinib reduces extraintestinal manifestations of ulcerative colitis: Study
The selective Janus kinase (JAK) inhibitor upadacitinib resolved more extraintestinal manifestations (EIMs) of ulcerative colitis than placebo, according to an analysis of phase 3 study findings.
The 45-mg induction dose of upadacitinib, for example, resolved more anemia, peripheral arthropathy, and axial arthropathy than placebo. The 15-mg and 30-mg maintenance doses were also associated with greater resolution of EIMs, with the higher dose producing a significantly greater reduction in comparison with placebo.
“Upadacitinib was highly effective in decreasing ulcerative colitis activity, which is triggering the extraintestinal manifestations,” lead author Jean-Frederick Colombel, MD, told this news organization.
Dr. Colombel, a gastroenterologist and professor of medicine at Mount Sinai Icahn School of Medicine, New York, said he was not surprised to see this, given that the oral JAK inhibitor “has also demonstrated efficacy in rheumatoid arthritis, psoriatic arthritis, atopic dermatitis, and ankylosing spondylitis.”
He presented the study findings during an oral presentation Feb. 19 at the 17th congress of the European Crohn’s and Colitis Organisation.
In the United States, AbbVie is seeking approval from the U.S. Food and Drug Administration for upadacitinib (RINVOQ) to treat active, moderate to severe ulcerative colitis. The company submitted regulatory applications in September 2021 for this indication.
About 1 in 4 report EIMs
The researchers evaluated the U-ACHIEVE and U-ACCOMPLISH 8-week induction studies, in which 660 participants were randomly assigned to receive upadacitinib 45 mg and 328 patients were assigned to receive placebo. At baseline, 25% of treated patients and 27% of the placebo group had experienced at least one EIM.
The researchers also assessed outcomes of the 52-week U-ACHIEVE maintenance trial, in which 154 people with ulcerative colitis were randomly assigned to receive 30-mg upadacitinib, 148 to receive 15-mg upadacitinib, and 149 to receive placebo. Between 24% and 27% of participants in these groups reported at least one EIM at baseline.
Key findings
In the pooled induction studies, a higher proportion of participants in the upadacitinib group achieved resolution of any EIM at 8 weeks, compared with the placebo group (40% vs. 33%).
Regarding specific EIMs, a higher proportion of those in the upadacitinib group had achieved resolution of peripheral or axial arthropathies at 8 weeks, compared with the placebo group (55% vs. 42%), and more participants experienced resolution of anemia (38% vs. 33%).
Similar effects were observed in the maintenance study. Resolution of any EIM at 52 weeks was experienced by 66% of those in the 30-mg upadacitinib group, compared with 42% in the 15-mg upadacitinib group and 24% in the placebo group. The 30-mg results were significantly different than placebo (P < .001).
Regarding specific EIMs, a higher proportion of the 30-mg upadacitinib group experienced resolution of peripheral or axial arthropathies at week 52, compared with the 15-mg and placebo groups (67% vs. 39% vs. 22%). The difference was statistically significant between the 30-mg and placebo groups (P = .010) but not between the 15-mg and placebo groups.
More participants in the 30-mg group also experienced resolution of anemia, compared with the 15-mg group and the placebo group (71% vs. 50% vs. 36%). Once again, the difference between 30-mg group and placebo was significant (P = .019).
When asked what physicians should be most concerned about when prescribing upadacitinib, Dr. Colombel pointed to the risks of serious infections, including herpes zoster. He added that there are potential risks of cardiovascular events, deep vein thrombosis, and cancers, “but it is too early to tell.”
Dr. Colombel advised physicians to follow the prescribing information for upadacitinib following FDA approval.
An ‘excellent presentation’
“Upadacitinib is a promising new option for patients with ulcerative colitis,” ECCO 2022 session cochair Annemarie de Vries, MD, PhD, told this news organization.
“The excellent presentation by Prof. Colombel further supports the high expectations by providing evidence on the effect of upadacitinib on extraintestinal manifestations,” said Dr. De Vries, a gastroenterologist at Erasmus Medical Center, Rotterdam, the Netherlands.
“For further conclusions on drug positioning, we have to await real-world data and head-to-head trials versus anti-TNF agents and vedolizumab,” she added.
The study was sponsored by AbbVie. Dr. Colombel has received research support from and is a speaker and consultant for AbbVie. Dr. De Vries reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The selective Janus kinase (JAK) inhibitor upadacitinib resolved more extraintestinal manifestations (EIMs) of ulcerative colitis than placebo, according to an analysis of phase 3 study findings.
The 45-mg induction dose of upadacitinib, for example, resolved more anemia, peripheral arthropathy, and axial arthropathy than placebo. The 15-mg and 30-mg maintenance doses were also associated with greater resolution of EIMs, with the higher dose producing a significantly greater reduction in comparison with placebo.
“Upadacitinib was highly effective in decreasing ulcerative colitis activity, which is triggering the extraintestinal manifestations,” lead author Jean-Frederick Colombel, MD, told this news organization.
Dr. Colombel, a gastroenterologist and professor of medicine at Mount Sinai Icahn School of Medicine, New York, said he was not surprised to see this, given that the oral JAK inhibitor “has also demonstrated efficacy in rheumatoid arthritis, psoriatic arthritis, atopic dermatitis, and ankylosing spondylitis.”
He presented the study findings during an oral presentation Feb. 19 at the 17th congress of the European Crohn’s and Colitis Organisation.
In the United States, AbbVie is seeking approval from the U.S. Food and Drug Administration for upadacitinib (RINVOQ) to treat active, moderate to severe ulcerative colitis. The company submitted regulatory applications in September 2021 for this indication.
About 1 in 4 report EIMs
The researchers evaluated the U-ACHIEVE and U-ACCOMPLISH 8-week induction studies, in which 660 participants were randomly assigned to receive upadacitinib 45 mg and 328 patients were assigned to receive placebo. At baseline, 25% of treated patients and 27% of the placebo group had experienced at least one EIM.
The researchers also assessed outcomes of the 52-week U-ACHIEVE maintenance trial, in which 154 people with ulcerative colitis were randomly assigned to receive 30-mg upadacitinib, 148 to receive 15-mg upadacitinib, and 149 to receive placebo. Between 24% and 27% of participants in these groups reported at least one EIM at baseline.
Key findings
In the pooled induction studies, a higher proportion of participants in the upadacitinib group achieved resolution of any EIM at 8 weeks, compared with the placebo group (40% vs. 33%).
Regarding specific EIMs, a higher proportion of those in the upadacitinib group had achieved resolution of peripheral or axial arthropathies at 8 weeks, compared with the placebo group (55% vs. 42%), and more participants experienced resolution of anemia (38% vs. 33%).
Similar effects were observed in the maintenance study. Resolution of any EIM at 52 weeks was experienced by 66% of those in the 30-mg upadacitinib group, compared with 42% in the 15-mg upadacitinib group and 24% in the placebo group. The 30-mg results were significantly different than placebo (P < .001).
Regarding specific EIMs, a higher proportion of the 30-mg upadacitinib group experienced resolution of peripheral or axial arthropathies at week 52, compared with the 15-mg and placebo groups (67% vs. 39% vs. 22%). The difference was statistically significant between the 30-mg and placebo groups (P = .010) but not between the 15-mg and placebo groups.
More participants in the 30-mg group also experienced resolution of anemia, compared with the 15-mg group and the placebo group (71% vs. 50% vs. 36%). Once again, the difference between 30-mg group and placebo was significant (P = .019).
When asked what physicians should be most concerned about when prescribing upadacitinib, Dr. Colombel pointed to the risks of serious infections, including herpes zoster. He added that there are potential risks of cardiovascular events, deep vein thrombosis, and cancers, “but it is too early to tell.”
Dr. Colombel advised physicians to follow the prescribing information for upadacitinib following FDA approval.
An ‘excellent presentation’
“Upadacitinib is a promising new option for patients with ulcerative colitis,” ECCO 2022 session cochair Annemarie de Vries, MD, PhD, told this news organization.
“The excellent presentation by Prof. Colombel further supports the high expectations by providing evidence on the effect of upadacitinib on extraintestinal manifestations,” said Dr. De Vries, a gastroenterologist at Erasmus Medical Center, Rotterdam, the Netherlands.
“For further conclusions on drug positioning, we have to await real-world data and head-to-head trials versus anti-TNF agents and vedolizumab,” she added.
The study was sponsored by AbbVie. Dr. Colombel has received research support from and is a speaker and consultant for AbbVie. Dr. De Vries reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The selective Janus kinase (JAK) inhibitor upadacitinib resolved more extraintestinal manifestations (EIMs) of ulcerative colitis than placebo, according to an analysis of phase 3 study findings.
The 45-mg induction dose of upadacitinib, for example, resolved more anemia, peripheral arthropathy, and axial arthropathy than placebo. The 15-mg and 30-mg maintenance doses were also associated with greater resolution of EIMs, with the higher dose producing a significantly greater reduction in comparison with placebo.
“Upadacitinib was highly effective in decreasing ulcerative colitis activity, which is triggering the extraintestinal manifestations,” lead author Jean-Frederick Colombel, MD, told this news organization.
Dr. Colombel, a gastroenterologist and professor of medicine at Mount Sinai Icahn School of Medicine, New York, said he was not surprised to see this, given that the oral JAK inhibitor “has also demonstrated efficacy in rheumatoid arthritis, psoriatic arthritis, atopic dermatitis, and ankylosing spondylitis.”
He presented the study findings during an oral presentation Feb. 19 at the 17th congress of the European Crohn’s and Colitis Organisation.
In the United States, AbbVie is seeking approval from the U.S. Food and Drug Administration for upadacitinib (RINVOQ) to treat active, moderate to severe ulcerative colitis. The company submitted regulatory applications in September 2021 for this indication.
About 1 in 4 report EIMs
The researchers evaluated the U-ACHIEVE and U-ACCOMPLISH 8-week induction studies, in which 660 participants were randomly assigned to receive upadacitinib 45 mg and 328 patients were assigned to receive placebo. At baseline, 25% of treated patients and 27% of the placebo group had experienced at least one EIM.
The researchers also assessed outcomes of the 52-week U-ACHIEVE maintenance trial, in which 154 people with ulcerative colitis were randomly assigned to receive 30-mg upadacitinib, 148 to receive 15-mg upadacitinib, and 149 to receive placebo. Between 24% and 27% of participants in these groups reported at least one EIM at baseline.
Key findings
In the pooled induction studies, a higher proportion of participants in the upadacitinib group achieved resolution of any EIM at 8 weeks, compared with the placebo group (40% vs. 33%).
Regarding specific EIMs, a higher proportion of those in the upadacitinib group had achieved resolution of peripheral or axial arthropathies at 8 weeks, compared with the placebo group (55% vs. 42%), and more participants experienced resolution of anemia (38% vs. 33%).
Similar effects were observed in the maintenance study. Resolution of any EIM at 52 weeks was experienced by 66% of those in the 30-mg upadacitinib group, compared with 42% in the 15-mg upadacitinib group and 24% in the placebo group. The 30-mg results were significantly different than placebo (P < .001).
Regarding specific EIMs, a higher proportion of the 30-mg upadacitinib group experienced resolution of peripheral or axial arthropathies at week 52, compared with the 15-mg and placebo groups (67% vs. 39% vs. 22%). The difference was statistically significant between the 30-mg and placebo groups (P = .010) but not between the 15-mg and placebo groups.
More participants in the 30-mg group also experienced resolution of anemia, compared with the 15-mg group and the placebo group (71% vs. 50% vs. 36%). Once again, the difference between 30-mg group and placebo was significant (P = .019).
When asked what physicians should be most concerned about when prescribing upadacitinib, Dr. Colombel pointed to the risks of serious infections, including herpes zoster. He added that there are potential risks of cardiovascular events, deep vein thrombosis, and cancers, “but it is too early to tell.”
Dr. Colombel advised physicians to follow the prescribing information for upadacitinib following FDA approval.
An ‘excellent presentation’
“Upadacitinib is a promising new option for patients with ulcerative colitis,” ECCO 2022 session cochair Annemarie de Vries, MD, PhD, told this news organization.
“The excellent presentation by Prof. Colombel further supports the high expectations by providing evidence on the effect of upadacitinib on extraintestinal manifestations,” said Dr. De Vries, a gastroenterologist at Erasmus Medical Center, Rotterdam, the Netherlands.
“For further conclusions on drug positioning, we have to await real-world data and head-to-head trials versus anti-TNF agents and vedolizumab,” she added.
The study was sponsored by AbbVie. Dr. Colombel has received research support from and is a speaker and consultant for AbbVie. Dr. De Vries reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ECCO 2022
Let’s be more careful about the data—and commentary—we publish
In a recent letter to the editor, “25-hydroxyvitamin D concentration is key to analyzing vitamin D’s effects” (J Fam Pract. 2021;70:472), Dr. Grant links vitamin D supplementation with important health outcomes. He concludes that the positivity rate of SARS-CoV-2 was only 5.9% in people with higher concentrations of 25(OH)D vs 12.5% in those with lower concentrations. This is a flawed conclusion on the face of it, because the great confabulatory factor is behavior. Is it possible that those more likely to take supplemental vitamin D do so as a result of overall healthier lifestyles and choices (eg, vaccinations)? As health care representatives, we must be very careful about the data we publish and the commentary we attach to it, lest we advertise inadvertent follies. I see so much of that in our “peer-reviewed literature.”
I came to medicine as a chemist, and the rigors of peer review impressed upon the hard (fundamental) sciences are markedly different from those we “claim” adherence to in medicine. I find that some of the medical literature and study designs fall short of what would pass muster in the fundamental science industry. That is a shame! Such statements, as discussed here, have to be served for public consumption, and even to our colleagues, with a generous helping of skepticism and qualification.
RA Segal, MD, MPH
Gainesville, FL
In a recent letter to the editor, “25-hydroxyvitamin D concentration is key to analyzing vitamin D’s effects” (J Fam Pract. 2021;70:472), Dr. Grant links vitamin D supplementation with important health outcomes. He concludes that the positivity rate of SARS-CoV-2 was only 5.9% in people with higher concentrations of 25(OH)D vs 12.5% in those with lower concentrations. This is a flawed conclusion on the face of it, because the great confabulatory factor is behavior. Is it possible that those more likely to take supplemental vitamin D do so as a result of overall healthier lifestyles and choices (eg, vaccinations)? As health care representatives, we must be very careful about the data we publish and the commentary we attach to it, lest we advertise inadvertent follies. I see so much of that in our “peer-reviewed literature.”
I came to medicine as a chemist, and the rigors of peer review impressed upon the hard (fundamental) sciences are markedly different from those we “claim” adherence to in medicine. I find that some of the medical literature and study designs fall short of what would pass muster in the fundamental science industry. That is a shame! Such statements, as discussed here, have to be served for public consumption, and even to our colleagues, with a generous helping of skepticism and qualification.
RA Segal, MD, MPH
Gainesville, FL
In a recent letter to the editor, “25-hydroxyvitamin D concentration is key to analyzing vitamin D’s effects” (J Fam Pract. 2021;70:472), Dr. Grant links vitamin D supplementation with important health outcomes. He concludes that the positivity rate of SARS-CoV-2 was only 5.9% in people with higher concentrations of 25(OH)D vs 12.5% in those with lower concentrations. This is a flawed conclusion on the face of it, because the great confabulatory factor is behavior. Is it possible that those more likely to take supplemental vitamin D do so as a result of overall healthier lifestyles and choices (eg, vaccinations)? As health care representatives, we must be very careful about the data we publish and the commentary we attach to it, lest we advertise inadvertent follies. I see so much of that in our “peer-reviewed literature.”
I came to medicine as a chemist, and the rigors of peer review impressed upon the hard (fundamental) sciences are markedly different from those we “claim” adherence to in medicine. I find that some of the medical literature and study designs fall short of what would pass muster in the fundamental science industry. That is a shame! Such statements, as discussed here, have to be served for public consumption, and even to our colleagues, with a generous helping of skepticism and qualification.
RA Segal, MD, MPH
Gainesville, FL
Innumerable pulmonary nodules
A 56-year-old woman with a history of a thyroid goiter following a partial thyroidectomy presented to the emergency department with shortness of breath, progressive weakness, and fatigue. She also reported a poor appetite and unintentional weight loss of approximately 40 lbs over the past 2 months but had not sought medical care. She denied having a cough, chest pain, hemoptysis, hematemesis, fevers, chills, or night sweats.
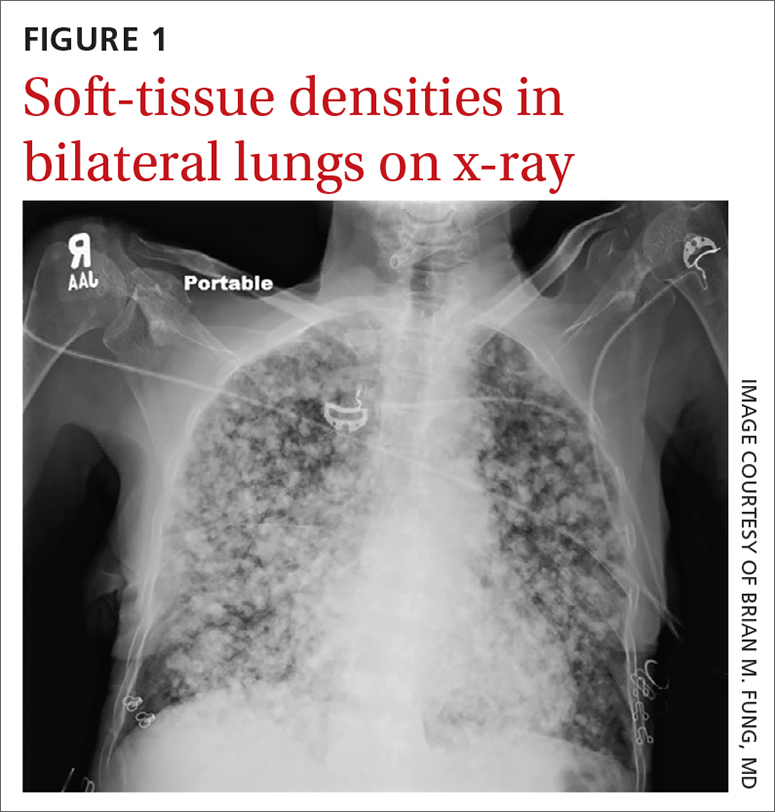
Physical examination revealed a cachectic woman with tachycardia and tachypnea, along with diffuse coarse rales throughout both lungs. The patient’s initial heart rate was 101 beats/min; respiratory rate, 25 breaths/min; and oxygen saturation, 74% on room air. The results of a complete blood count and comprehensive metabolic panel were within normal limits. A chest radiograph was performed, showing innumerable subcentimeter- and pericentimeter-sized soft-tissue densities in both lungs (FIGURE 1). Computed tomography (CT) of the chest was subsequently obtained to better characterize the nodules (FIGURES 2A and 2B). The CT revealed innumerable noncalcified pulmonary nodules as well as multiple hilar masses suggestive of malignancy; however, infectious etiologies could not be ruled out.
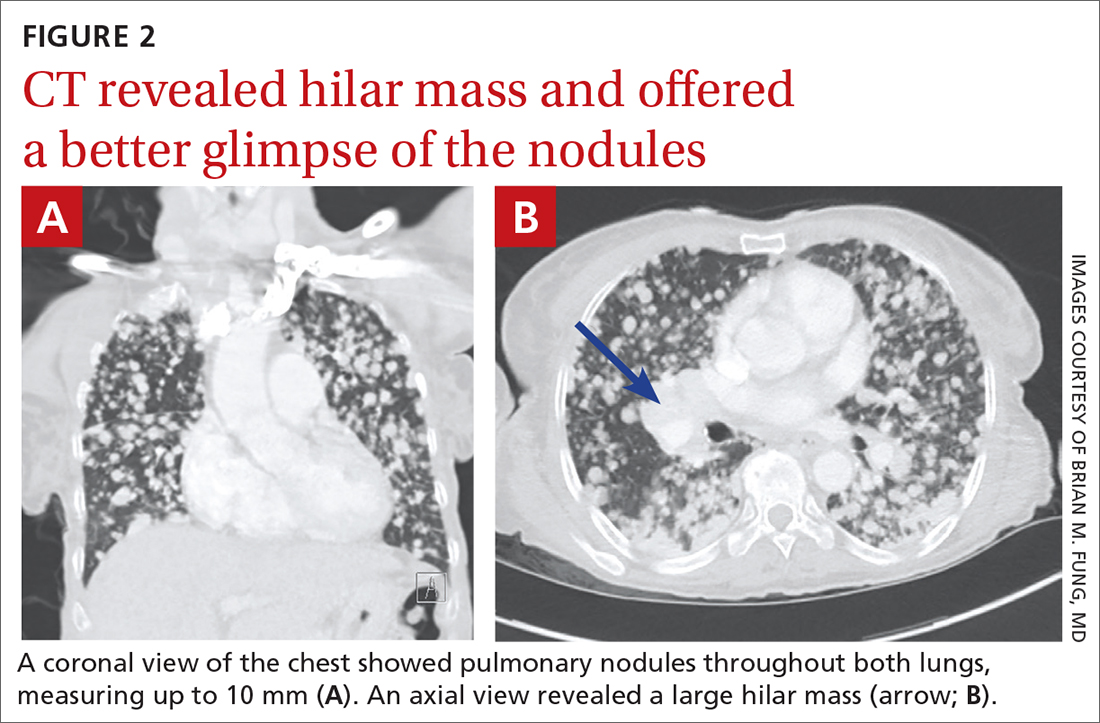
As the patient was visiting from a tuberculosis-endemic area, she was empirically started on rifampin, isoniazid, pyrazinamide, and ethambutol (RIPE) therapy for treatment of Mycobacterium tuberculosis infection. Subsequently, polymerase chain reaction (PCR) testing and acid-fast bacillus sputum smear came back negative for M tuberculosis and RIPE therapy was stopped. Bacterial culture and fungal screens for Pneumocystis jirovecii, Coccidioides, Cryptococcus, and Histoplasma capsulatum were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Metastatic follicular thyroid carcinoma
A biopsy of one of the lung nodules was performed and showed strong positive staining for thyroglobulin and thyroid transcription factor-1, establishing a diagnosis of metastatic follicular thyroid carcinoma. Further imaging revealed additional metastases to the brain, liver, adrenal glands, and spine.
Follicular thyroid carcinoma is the second most common histologic type of thyroid cancer, comprising 10% to 15% of thyroid cancer cases.1 Distant metastases are not uncommon in this histologic type; about 11% of patients have distant metastases at presentation.2 Compared with papillary thyroid cancer, which spreads via the lymphatics, the follicular type can metastasize via vascular invasion, thus leading to its “aggressiveness” and frequent occurrence of metastasis outside the neck (most commonly to the lung).1 For this reason, an early diagnosis is important.3 In this case, the patient revealed that she hadn’t sought medical care at the onset of her symptoms due to financial limitations and limited access to medical providers; this likely contributed to the patient’s diagnosis at a late stage of disease.
Infectious and septic diseases are part of the differential Dx
The differential diagnosis for diffuse pulmonary nodules includes various infections, silicosis, coal worker’s pneumoconiosis, septic pulmonary emboli, and pulmonary sarcoidosis. All of these diagnoses can cause constitutional, as well as pulmonary, symptoms.
Infections, such as pulmonary tuberculosis, pulmonary coccidioidomycosis, or other cavitating infections, can be ruled out with specific testing for the organism of concern and/or culture.
Silicosis and coal worker’s pneumoconiosis generally cause lesions in the upper lobes and require a significant occupational exposure to silica or coal, respectively.
Continue to: Septic pulmonary emboli
Septic pulmonary emboli are usually seen in the context of sepsis, and there are positive blood cultures at the time of imaging.
Pulmonary sarcoidosis typically manifests with hilar and mediastinal lymphadenopathy and is often accompanied by other organ involvement.
There are options for treatment if diagnosis is made early
Thyroidectomy, radioiodine treatment, and thyroid-stimulating hormone suppressive therapy are the most commonly used therapies for treatment of follicular thyroid carcinoma.4 If follicular thyroid carcinoma is caught early (with only local involvement), 5-year relative survival rates are near 100%.5 However, outcomes are generally poor in patients who are of older age and those who have macronodular lung metastases or multiple bone metastases.6
Our patient was started on levothyroxine. Radioiodine and targeted drug therapy with a tyrosine kinase inhibitor were attempted, but the patient was unable to tolerate them due to her poor functional status. She was thus transitioned to hospice care.
1. Aschebrook-Kilfoy B, Grogan RH, Ward MH, et al. Follicular thyroid cancer incidence patterns in the United States, 1980-2009. Thyroid. 2013;23:1015-1021. doi: 10.1089/thy.2012.0356
2. Shaha AR, Shah JP, Loree TR. Differentiated thyroid cancer presenting initially with distant metastasis. Am J Surg. 1997;174:474-476. doi: 10.1016/s0002-9610(97)00158-x
3. Lim IIP, Hochman T, Blumberg SN, et al. Disparities in the initial presentation of differentiated thyroid cancer in a large public hospital and adjoining university teaching hospital. Thyroid. 2012;22:269-274. doi: 10.1089/thy.2010.0385
4. Grani G, Lamartina L, Durante C, et al. Follicular thyroid cancer and Hürthle cell carcinoma: challenges in diagnosis, treatment, and clinical management. Lancet Diabetes Endocrinol. 2018;6:500-514. doi: 10.1016/S2213-8587(17)30325-X
5. Thyroid cancer survival rates, by type and stage. American Cancer Society. Updated January 25, 2021. Accessed February 1, 2022. www.cancer.org/cancer/thyroid-cancer/detection-diagnosis-staging/survival-rates.html
6. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892-2899. doi: 10.1210/jc.2005-2838
A 56-year-old woman with a history of a thyroid goiter following a partial thyroidectomy presented to the emergency department with shortness of breath, progressive weakness, and fatigue. She also reported a poor appetite and unintentional weight loss of approximately 40 lbs over the past 2 months but had not sought medical care. She denied having a cough, chest pain, hemoptysis, hematemesis, fevers, chills, or night sweats.

Physical examination revealed a cachectic woman with tachycardia and tachypnea, along with diffuse coarse rales throughout both lungs. The patient’s initial heart rate was 101 beats/min; respiratory rate, 25 breaths/min; and oxygen saturation, 74% on room air. The results of a complete blood count and comprehensive metabolic panel were within normal limits. A chest radiograph was performed, showing innumerable subcentimeter- and pericentimeter-sized soft-tissue densities in both lungs (FIGURE 1). Computed tomography (CT) of the chest was subsequently obtained to better characterize the nodules (FIGURES 2A and 2B). The CT revealed innumerable noncalcified pulmonary nodules as well as multiple hilar masses suggestive of malignancy; however, infectious etiologies could not be ruled out.

As the patient was visiting from a tuberculosis-endemic area, she was empirically started on rifampin, isoniazid, pyrazinamide, and ethambutol (RIPE) therapy for treatment of Mycobacterium tuberculosis infection. Subsequently, polymerase chain reaction (PCR) testing and acid-fast bacillus sputum smear came back negative for M tuberculosis and RIPE therapy was stopped. Bacterial culture and fungal screens for Pneumocystis jirovecii, Coccidioides, Cryptococcus, and Histoplasma capsulatum were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Metastatic follicular thyroid carcinoma
A biopsy of one of the lung nodules was performed and showed strong positive staining for thyroglobulin and thyroid transcription factor-1, establishing a diagnosis of metastatic follicular thyroid carcinoma. Further imaging revealed additional metastases to the brain, liver, adrenal glands, and spine.
Follicular thyroid carcinoma is the second most common histologic type of thyroid cancer, comprising 10% to 15% of thyroid cancer cases.1 Distant metastases are not uncommon in this histologic type; about 11% of patients have distant metastases at presentation.2 Compared with papillary thyroid cancer, which spreads via the lymphatics, the follicular type can metastasize via vascular invasion, thus leading to its “aggressiveness” and frequent occurrence of metastasis outside the neck (most commonly to the lung).1 For this reason, an early diagnosis is important.3 In this case, the patient revealed that she hadn’t sought medical care at the onset of her symptoms due to financial limitations and limited access to medical providers; this likely contributed to the patient’s diagnosis at a late stage of disease.
Infectious and septic diseases are part of the differential Dx
The differential diagnosis for diffuse pulmonary nodules includes various infections, silicosis, coal worker’s pneumoconiosis, septic pulmonary emboli, and pulmonary sarcoidosis. All of these diagnoses can cause constitutional, as well as pulmonary, symptoms.
Infections, such as pulmonary tuberculosis, pulmonary coccidioidomycosis, or other cavitating infections, can be ruled out with specific testing for the organism of concern and/or culture.
Silicosis and coal worker’s pneumoconiosis generally cause lesions in the upper lobes and require a significant occupational exposure to silica or coal, respectively.
Continue to: Septic pulmonary emboli
Septic pulmonary emboli are usually seen in the context of sepsis, and there are positive blood cultures at the time of imaging.
Pulmonary sarcoidosis typically manifests with hilar and mediastinal lymphadenopathy and is often accompanied by other organ involvement.
There are options for treatment if diagnosis is made early
Thyroidectomy, radioiodine treatment, and thyroid-stimulating hormone suppressive therapy are the most commonly used therapies for treatment of follicular thyroid carcinoma.4 If follicular thyroid carcinoma is caught early (with only local involvement), 5-year relative survival rates are near 100%.5 However, outcomes are generally poor in patients who are of older age and those who have macronodular lung metastases or multiple bone metastases.6
Our patient was started on levothyroxine. Radioiodine and targeted drug therapy with a tyrosine kinase inhibitor were attempted, but the patient was unable to tolerate them due to her poor functional status. She was thus transitioned to hospice care.
A 56-year-old woman with a history of a thyroid goiter following a partial thyroidectomy presented to the emergency department with shortness of breath, progressive weakness, and fatigue. She also reported a poor appetite and unintentional weight loss of approximately 40 lbs over the past 2 months but had not sought medical care. She denied having a cough, chest pain, hemoptysis, hematemesis, fevers, chills, or night sweats.

Physical examination revealed a cachectic woman with tachycardia and tachypnea, along with diffuse coarse rales throughout both lungs. The patient’s initial heart rate was 101 beats/min; respiratory rate, 25 breaths/min; and oxygen saturation, 74% on room air. The results of a complete blood count and comprehensive metabolic panel were within normal limits. A chest radiograph was performed, showing innumerable subcentimeter- and pericentimeter-sized soft-tissue densities in both lungs (FIGURE 1). Computed tomography (CT) of the chest was subsequently obtained to better characterize the nodules (FIGURES 2A and 2B). The CT revealed innumerable noncalcified pulmonary nodules as well as multiple hilar masses suggestive of malignancy; however, infectious etiologies could not be ruled out.

As the patient was visiting from a tuberculosis-endemic area, she was empirically started on rifampin, isoniazid, pyrazinamide, and ethambutol (RIPE) therapy for treatment of Mycobacterium tuberculosis infection. Subsequently, polymerase chain reaction (PCR) testing and acid-fast bacillus sputum smear came back negative for M tuberculosis and RIPE therapy was stopped. Bacterial culture and fungal screens for Pneumocystis jirovecii, Coccidioides, Cryptococcus, and Histoplasma capsulatum were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Metastatic follicular thyroid carcinoma
A biopsy of one of the lung nodules was performed and showed strong positive staining for thyroglobulin and thyroid transcription factor-1, establishing a diagnosis of metastatic follicular thyroid carcinoma. Further imaging revealed additional metastases to the brain, liver, adrenal glands, and spine.
Follicular thyroid carcinoma is the second most common histologic type of thyroid cancer, comprising 10% to 15% of thyroid cancer cases.1 Distant metastases are not uncommon in this histologic type; about 11% of patients have distant metastases at presentation.2 Compared with papillary thyroid cancer, which spreads via the lymphatics, the follicular type can metastasize via vascular invasion, thus leading to its “aggressiveness” and frequent occurrence of metastasis outside the neck (most commonly to the lung).1 For this reason, an early diagnosis is important.3 In this case, the patient revealed that she hadn’t sought medical care at the onset of her symptoms due to financial limitations and limited access to medical providers; this likely contributed to the patient’s diagnosis at a late stage of disease.
Infectious and septic diseases are part of the differential Dx
The differential diagnosis for diffuse pulmonary nodules includes various infections, silicosis, coal worker’s pneumoconiosis, septic pulmonary emboli, and pulmonary sarcoidosis. All of these diagnoses can cause constitutional, as well as pulmonary, symptoms.
Infections, such as pulmonary tuberculosis, pulmonary coccidioidomycosis, or other cavitating infections, can be ruled out with specific testing for the organism of concern and/or culture.
Silicosis and coal worker’s pneumoconiosis generally cause lesions in the upper lobes and require a significant occupational exposure to silica or coal, respectively.
Continue to: Septic pulmonary emboli
Septic pulmonary emboli are usually seen in the context of sepsis, and there are positive blood cultures at the time of imaging.
Pulmonary sarcoidosis typically manifests with hilar and mediastinal lymphadenopathy and is often accompanied by other organ involvement.
There are options for treatment if diagnosis is made early
Thyroidectomy, radioiodine treatment, and thyroid-stimulating hormone suppressive therapy are the most commonly used therapies for treatment of follicular thyroid carcinoma.4 If follicular thyroid carcinoma is caught early (with only local involvement), 5-year relative survival rates are near 100%.5 However, outcomes are generally poor in patients who are of older age and those who have macronodular lung metastases or multiple bone metastases.6
Our patient was started on levothyroxine. Radioiodine and targeted drug therapy with a tyrosine kinase inhibitor were attempted, but the patient was unable to tolerate them due to her poor functional status. She was thus transitioned to hospice care.
1. Aschebrook-Kilfoy B, Grogan RH, Ward MH, et al. Follicular thyroid cancer incidence patterns in the United States, 1980-2009. Thyroid. 2013;23:1015-1021. doi: 10.1089/thy.2012.0356
2. Shaha AR, Shah JP, Loree TR. Differentiated thyroid cancer presenting initially with distant metastasis. Am J Surg. 1997;174:474-476. doi: 10.1016/s0002-9610(97)00158-x
3. Lim IIP, Hochman T, Blumberg SN, et al. Disparities in the initial presentation of differentiated thyroid cancer in a large public hospital and adjoining university teaching hospital. Thyroid. 2012;22:269-274. doi: 10.1089/thy.2010.0385
4. Grani G, Lamartina L, Durante C, et al. Follicular thyroid cancer and Hürthle cell carcinoma: challenges in diagnosis, treatment, and clinical management. Lancet Diabetes Endocrinol. 2018;6:500-514. doi: 10.1016/S2213-8587(17)30325-X
5. Thyroid cancer survival rates, by type and stage. American Cancer Society. Updated January 25, 2021. Accessed February 1, 2022. www.cancer.org/cancer/thyroid-cancer/detection-diagnosis-staging/survival-rates.html
6. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892-2899. doi: 10.1210/jc.2005-2838
1. Aschebrook-Kilfoy B, Grogan RH, Ward MH, et al. Follicular thyroid cancer incidence patterns in the United States, 1980-2009. Thyroid. 2013;23:1015-1021. doi: 10.1089/thy.2012.0356
2. Shaha AR, Shah JP, Loree TR. Differentiated thyroid cancer presenting initially with distant metastasis. Am J Surg. 1997;174:474-476. doi: 10.1016/s0002-9610(97)00158-x
3. Lim IIP, Hochman T, Blumberg SN, et al. Disparities in the initial presentation of differentiated thyroid cancer in a large public hospital and adjoining university teaching hospital. Thyroid. 2012;22:269-274. doi: 10.1089/thy.2010.0385
4. Grani G, Lamartina L, Durante C, et al. Follicular thyroid cancer and Hürthle cell carcinoma: challenges in diagnosis, treatment, and clinical management. Lancet Diabetes Endocrinol. 2018;6:500-514. doi: 10.1016/S2213-8587(17)30325-X
5. Thyroid cancer survival rates, by type and stage. American Cancer Society. Updated January 25, 2021. Accessed February 1, 2022. www.cancer.org/cancer/thyroid-cancer/detection-diagnosis-staging/survival-rates.html
6. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892-2899. doi: 10.1210/jc.2005-2838
Early menopause, early dementia risk, study suggests
Earlier menopause appears to be associated with a higher risk of dementia, and earlier onset of dementia, compared with menopause at normal age or later, according to a large study.
“Being aware of this increased risk can help women practice strategies to prevent dementia and to work with their physicians to closely monitor their cognitive status as they age,” study investigator Wenting Hao, MD, with Shandong University, Jinan, China, says in a news release.
The findings were presented in an e-poster March 1 at the Epidemiology, Prevention, Lifestyle & Cardiometabolic Health (EPI|Lifestyle) 2022 conference sponsored by the American Heart Association.
UK Biobank data
Dr. Hao and colleagues examined health data for 153,291 women who were 60 years old on average when they became participants in the UK Biobank.
Age at menopause was categorized as premature (younger than age 40), early (40 to 44 years), reference (45 to 51), 52 to 55 years, and 55+ years.
Compared with women who entered menopause around age 50 years (reference), women who experienced premature menopause were 35% more likely to develop some type of dementia later in life (hazard ratio, 1.35; 95% confidence interval, 1.22 to 1.91).
Women with early menopause were also more likely to develop early-onset dementia, that is, before age 65 (HR, 1.31; 95% confidence interval, 1.07 to 1.72).
Women who entered menopause later (at age 52+) had dementia risk similar to women who entered menopause at the average age of 50 to 51 years.
The results were adjusted for relevant cofactors, including age at last exam, race, educational level, cigarette and alcohol use, body mass index, cardiovascular disease, diabetes, income, and leisure and physical activities.
Blame it on estrogen?
Reduced estrogen levels may be a factor in the possible connection between early menopause and dementia, Dr. Hao and her colleagues say.
Estradiol plays a key role in a range of neurological functions, so the reduction of endogenous estrogen at menopause may aggravate brain changes related to neurodegenerative disease and speed up progression of dementia, they explain.
“We know that the lack of estrogen over the long term enhances oxidative stress, which may increase brain aging and lead to cognitive impairment,” Dr. Hao adds.
Limitations of the study include reliance on self-reported information about age at menopause onset.
Also, the researchers did not evaluate dementia rates in women who had a naturally occurring early menopause separate from the women with surgery-induced menopause, which may affect the results.
Finally, the data used for this study included mostly White women living in the U.K. and may not generalize to other populations.
Supportive evidence, critical area of research
The U.K. study supports results of a previously reported Kaiser Permanente study, which showed women who entered menopause at age 45 or younger were at 28% greater dementia risk, compared with women who experienced menopause after age 45.
Reached for comment, Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, noted that nearly two-thirds of Americans with Alzheimer’s are women.
“We know Alzheimer’s and other dementias impact a greater number of women than men, but we don’t know why,” she told this news organization.
“Lifelong differences in women may affect their risk or affect what is contributing to their underlying biology of the disease, and we need more research to better understand what may be these contributing factors,” said Dr. Snyder.
“Reproductive history is one critical area being studied. The physical and hormonal changes that occur during menopause – as well as other hormonal changes throughout life – are considerable, and it’s important to understand what impact, if any, these changes may have on the brain,” Dr. Snyder added.
“The potential link between reproduction history and brain health is intriguing, but much more research in this area is needed to understand these links,” she said.
The study was funded by the Start-up Foundation for Scientific Research at Shandong University. Dr. Hao and Dr. Snyder have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Earlier menopause appears to be associated with a higher risk of dementia, and earlier onset of dementia, compared with menopause at normal age or later, according to a large study.
“Being aware of this increased risk can help women practice strategies to prevent dementia and to work with their physicians to closely monitor their cognitive status as they age,” study investigator Wenting Hao, MD, with Shandong University, Jinan, China, says in a news release.
The findings were presented in an e-poster March 1 at the Epidemiology, Prevention, Lifestyle & Cardiometabolic Health (EPI|Lifestyle) 2022 conference sponsored by the American Heart Association.
UK Biobank data
Dr. Hao and colleagues examined health data for 153,291 women who were 60 years old on average when they became participants in the UK Biobank.
Age at menopause was categorized as premature (younger than age 40), early (40 to 44 years), reference (45 to 51), 52 to 55 years, and 55+ years.
Compared with women who entered menopause around age 50 years (reference), women who experienced premature menopause were 35% more likely to develop some type of dementia later in life (hazard ratio, 1.35; 95% confidence interval, 1.22 to 1.91).
Women with early menopause were also more likely to develop early-onset dementia, that is, before age 65 (HR, 1.31; 95% confidence interval, 1.07 to 1.72).
Women who entered menopause later (at age 52+) had dementia risk similar to women who entered menopause at the average age of 50 to 51 years.
The results were adjusted for relevant cofactors, including age at last exam, race, educational level, cigarette and alcohol use, body mass index, cardiovascular disease, diabetes, income, and leisure and physical activities.
Blame it on estrogen?
Reduced estrogen levels may be a factor in the possible connection between early menopause and dementia, Dr. Hao and her colleagues say.
Estradiol plays a key role in a range of neurological functions, so the reduction of endogenous estrogen at menopause may aggravate brain changes related to neurodegenerative disease and speed up progression of dementia, they explain.
“We know that the lack of estrogen over the long term enhances oxidative stress, which may increase brain aging and lead to cognitive impairment,” Dr. Hao adds.
Limitations of the study include reliance on self-reported information about age at menopause onset.
Also, the researchers did not evaluate dementia rates in women who had a naturally occurring early menopause separate from the women with surgery-induced menopause, which may affect the results.
Finally, the data used for this study included mostly White women living in the U.K. and may not generalize to other populations.
Supportive evidence, critical area of research
The U.K. study supports results of a previously reported Kaiser Permanente study, which showed women who entered menopause at age 45 or younger were at 28% greater dementia risk, compared with women who experienced menopause after age 45.
Reached for comment, Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, noted that nearly two-thirds of Americans with Alzheimer’s are women.
“We know Alzheimer’s and other dementias impact a greater number of women than men, but we don’t know why,” she told this news organization.
“Lifelong differences in women may affect their risk or affect what is contributing to their underlying biology of the disease, and we need more research to better understand what may be these contributing factors,” said Dr. Snyder.
“Reproductive history is one critical area being studied. The physical and hormonal changes that occur during menopause – as well as other hormonal changes throughout life – are considerable, and it’s important to understand what impact, if any, these changes may have on the brain,” Dr. Snyder added.
“The potential link between reproduction history and brain health is intriguing, but much more research in this area is needed to understand these links,” she said.
The study was funded by the Start-up Foundation for Scientific Research at Shandong University. Dr. Hao and Dr. Snyder have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Earlier menopause appears to be associated with a higher risk of dementia, and earlier onset of dementia, compared with menopause at normal age or later, according to a large study.
“Being aware of this increased risk can help women practice strategies to prevent dementia and to work with their physicians to closely monitor their cognitive status as they age,” study investigator Wenting Hao, MD, with Shandong University, Jinan, China, says in a news release.
The findings were presented in an e-poster March 1 at the Epidemiology, Prevention, Lifestyle & Cardiometabolic Health (EPI|Lifestyle) 2022 conference sponsored by the American Heart Association.
UK Biobank data
Dr. Hao and colleagues examined health data for 153,291 women who were 60 years old on average when they became participants in the UK Biobank.
Age at menopause was categorized as premature (younger than age 40), early (40 to 44 years), reference (45 to 51), 52 to 55 years, and 55+ years.
Compared with women who entered menopause around age 50 years (reference), women who experienced premature menopause were 35% more likely to develop some type of dementia later in life (hazard ratio, 1.35; 95% confidence interval, 1.22 to 1.91).
Women with early menopause were also more likely to develop early-onset dementia, that is, before age 65 (HR, 1.31; 95% confidence interval, 1.07 to 1.72).
Women who entered menopause later (at age 52+) had dementia risk similar to women who entered menopause at the average age of 50 to 51 years.
The results were adjusted for relevant cofactors, including age at last exam, race, educational level, cigarette and alcohol use, body mass index, cardiovascular disease, diabetes, income, and leisure and physical activities.
Blame it on estrogen?
Reduced estrogen levels may be a factor in the possible connection between early menopause and dementia, Dr. Hao and her colleagues say.
Estradiol plays a key role in a range of neurological functions, so the reduction of endogenous estrogen at menopause may aggravate brain changes related to neurodegenerative disease and speed up progression of dementia, they explain.
“We know that the lack of estrogen over the long term enhances oxidative stress, which may increase brain aging and lead to cognitive impairment,” Dr. Hao adds.
Limitations of the study include reliance on self-reported information about age at menopause onset.
Also, the researchers did not evaluate dementia rates in women who had a naturally occurring early menopause separate from the women with surgery-induced menopause, which may affect the results.
Finally, the data used for this study included mostly White women living in the U.K. and may not generalize to other populations.
Supportive evidence, critical area of research
The U.K. study supports results of a previously reported Kaiser Permanente study, which showed women who entered menopause at age 45 or younger were at 28% greater dementia risk, compared with women who experienced menopause after age 45.
Reached for comment, Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, noted that nearly two-thirds of Americans with Alzheimer’s are women.
“We know Alzheimer’s and other dementias impact a greater number of women than men, but we don’t know why,” she told this news organization.
“Lifelong differences in women may affect their risk or affect what is contributing to their underlying biology of the disease, and we need more research to better understand what may be these contributing factors,” said Dr. Snyder.
“Reproductive history is one critical area being studied. The physical and hormonal changes that occur during menopause – as well as other hormonal changes throughout life – are considerable, and it’s important to understand what impact, if any, these changes may have on the brain,” Dr. Snyder added.
“The potential link between reproduction history and brain health is intriguing, but much more research in this area is needed to understand these links,” she said.
The study was funded by the Start-up Foundation for Scientific Research at Shandong University. Dr. Hao and Dr. Snyder have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Depression, suicidal ideation continue to plague physicians: Survey
Now, as they bear the weight of a multiyear pandemic alongside the perpetual struggle to maintain some semblance of work-life balance, their resiliency has been stretched to the brink.
In 2022, the Medscape Physician Suicide Report surveyed more than 13,000 physicians in 29 specialties who were candid about their experiences with suicidal thoughts, how they support their besieged colleagues, and their go-to coping strategies.
Overall, 21% of physicians reported having feelings of depression. Of those, 24% had clinical depression and 64% had colloquial depression. Physicians who felt sad or blue decreased slightly, compared with the 2021 report, but the number of physicians experiencing severe depression rose 4%.
One in 10 physicians said they have thought about or attempted suicide. However, the number of physicians with suicidal thoughts dropped to 9%, down substantially from the 22% who reported similar feelings in 2020.
Still, there was a slight uptick in women physicians contemplating suicide, likely linked to their larger share of childcare and family responsibilities.
“They have needed to pull double duty even more than usual, and that may have increased their sense of burnout and vulnerability to suicidal thoughts,” said Andrea Giedinghagen, MD, assistant professor in the department of psychiatry at Washington University in St. Louis, and coauthor of “Physician Suicide: A Call to Action
Fighting the stigma of seeking mental health help
Although the number of physicians attempting, but not completing suicide, has remained steady at 1% for several years, the recent passage of the Dr. Lorna Breen Health Care Provider Protection Act by Congress aims to drive that figure even lower. Dr. Breen, an ED physician at New York–Presbyterian Hospital, died by suicide in April 2020. Overwhelmed by the onslaught of COVID patients, Dr. Breen was reluctant to seek mental health services for fear of being ostracized.
“Many physicians don’t seek mental health care due to fear of negative consequences in the workplace, including retribution, exclusion, loss of license, or even their job,” Gary Price, MD, president of The Physicians Foundation, told this news organization. “This was the experience of Dr. Lorna Breen. She was convinced that if she talked to a professional, she would lose her medical license. Perhaps if Dr. Breen was equipped with the accurate information – there is no mental health reporting requirement in her state’s medical license application – it might have saved her life.”
This same stigma was reflected in the survey, with one physician saying: “I’m afraid that if I spoke to a therapist, I’d have to report receiving psychiatric treatment to credentialing or licensing boards.” Roughly 40% of survey respondents, regardless of age, chose not to disclose their suicidal thoughts to anyone, not even a family member or suicide hotline. And just a tiny portion of physicians (10% of men and 13% of women) said that a colleague had discussed their suicidal thoughts with them.
“There is a longstanding culture of silence around physician mental health in the medical community,” said Dr. Price. “The strategies within the Act are critical to fixing this culture and making it acceptable and normalized for physicians to seek mental health care,” and for it to “become a fundamental and ongoing element of being a practicing physician.”
As part of the legislation, the Department of Health & Human Services must award grants to hospitals, medical associations, and other entities to facilitate mental health programs for providers. They must also establish policy recommendations and conduct campaigns to improve providers’ mental and behavioral health, encourage providers to seek mental health support and assistance, remove barriers to such treatment, and identify best practices to prevent suicide and promote resiliency
Addressing barriers to mental health
The new bill is a step in the right direction, but Dr. Price said health organizations must do more to address the six key structural barriers that are “discouraging physicians from seeking [mental health] help,” such as the inclusion of “intrusive mental health questions on medical board, hospital credentialing, and malpractice insurance applications.”
In addition, employers should allow physicians to seek out-of-network mental health services, if necessary, and not cause further humiliation by requiring them to be treated by colleagues within their hospital system. A similar proposal has recently been introduced and is gaining traction in Utah, following the suicide of ED physician Scott Jolley, MD, in 2021 after he was admitted for psychiatric care where he worked.
Diminishing the stigma surrounding physicians’ mental health encourages a more open dialogue, so if a colleague reaches out – listen. “Start by thanking the colleague for sharing the information: ‘I’m sure that wasn’t easy but I appreciate that you respect me enough to share this. Let’s talk more,’ ” said Michael F. Myers, MD, professor of clinical psychiatry at State University of New York, Brooklyn . “Then ask what you can do to help, which cuts down on the sense of isolation that colleague may feel.”
According to the survey, many physicians have developed strategies to support their happiness and mental health. Although fewer than 10% said reducing work hours or transitioning to a part-time schedule was most effective, the majority of physicians relied on spending time with family and friends (68%) – a choice that has considerable benefits.
“Close and intimate relationships are the single most protective factor for our mental health,” said Peter Yellowlees, MBBS, MD, chief wellness officer for UC Davis Health and professor of psychiatry at the University of California, Davis. “Isolation and loneliness are very important stressors, and we know that about 25% of the population reports being lonely.”
A version of this article first appeared on Medscape.com.
Now, as they bear the weight of a multiyear pandemic alongside the perpetual struggle to maintain some semblance of work-life balance, their resiliency has been stretched to the brink.
In 2022, the Medscape Physician Suicide Report surveyed more than 13,000 physicians in 29 specialties who were candid about their experiences with suicidal thoughts, how they support their besieged colleagues, and their go-to coping strategies.
Overall, 21% of physicians reported having feelings of depression. Of those, 24% had clinical depression and 64% had colloquial depression. Physicians who felt sad or blue decreased slightly, compared with the 2021 report, but the number of physicians experiencing severe depression rose 4%.
One in 10 physicians said they have thought about or attempted suicide. However, the number of physicians with suicidal thoughts dropped to 9%, down substantially from the 22% who reported similar feelings in 2020.
Still, there was a slight uptick in women physicians contemplating suicide, likely linked to their larger share of childcare and family responsibilities.
“They have needed to pull double duty even more than usual, and that may have increased their sense of burnout and vulnerability to suicidal thoughts,” said Andrea Giedinghagen, MD, assistant professor in the department of psychiatry at Washington University in St. Louis, and coauthor of “Physician Suicide: A Call to Action
Fighting the stigma of seeking mental health help
Although the number of physicians attempting, but not completing suicide, has remained steady at 1% for several years, the recent passage of the Dr. Lorna Breen Health Care Provider Protection Act by Congress aims to drive that figure even lower. Dr. Breen, an ED physician at New York–Presbyterian Hospital, died by suicide in April 2020. Overwhelmed by the onslaught of COVID patients, Dr. Breen was reluctant to seek mental health services for fear of being ostracized.
“Many physicians don’t seek mental health care due to fear of negative consequences in the workplace, including retribution, exclusion, loss of license, or even their job,” Gary Price, MD, president of The Physicians Foundation, told this news organization. “This was the experience of Dr. Lorna Breen. She was convinced that if she talked to a professional, she would lose her medical license. Perhaps if Dr. Breen was equipped with the accurate information – there is no mental health reporting requirement in her state’s medical license application – it might have saved her life.”
This same stigma was reflected in the survey, with one physician saying: “I’m afraid that if I spoke to a therapist, I’d have to report receiving psychiatric treatment to credentialing or licensing boards.” Roughly 40% of survey respondents, regardless of age, chose not to disclose their suicidal thoughts to anyone, not even a family member or suicide hotline. And just a tiny portion of physicians (10% of men and 13% of women) said that a colleague had discussed their suicidal thoughts with them.
“There is a longstanding culture of silence around physician mental health in the medical community,” said Dr. Price. “The strategies within the Act are critical to fixing this culture and making it acceptable and normalized for physicians to seek mental health care,” and for it to “become a fundamental and ongoing element of being a practicing physician.”
As part of the legislation, the Department of Health & Human Services must award grants to hospitals, medical associations, and other entities to facilitate mental health programs for providers. They must also establish policy recommendations and conduct campaigns to improve providers’ mental and behavioral health, encourage providers to seek mental health support and assistance, remove barriers to such treatment, and identify best practices to prevent suicide and promote resiliency
Addressing barriers to mental health
The new bill is a step in the right direction, but Dr. Price said health organizations must do more to address the six key structural barriers that are “discouraging physicians from seeking [mental health] help,” such as the inclusion of “intrusive mental health questions on medical board, hospital credentialing, and malpractice insurance applications.”
In addition, employers should allow physicians to seek out-of-network mental health services, if necessary, and not cause further humiliation by requiring them to be treated by colleagues within their hospital system. A similar proposal has recently been introduced and is gaining traction in Utah, following the suicide of ED physician Scott Jolley, MD, in 2021 after he was admitted for psychiatric care where he worked.
Diminishing the stigma surrounding physicians’ mental health encourages a more open dialogue, so if a colleague reaches out – listen. “Start by thanking the colleague for sharing the information: ‘I’m sure that wasn’t easy but I appreciate that you respect me enough to share this. Let’s talk more,’ ” said Michael F. Myers, MD, professor of clinical psychiatry at State University of New York, Brooklyn . “Then ask what you can do to help, which cuts down on the sense of isolation that colleague may feel.”
According to the survey, many physicians have developed strategies to support their happiness and mental health. Although fewer than 10% said reducing work hours or transitioning to a part-time schedule was most effective, the majority of physicians relied on spending time with family and friends (68%) – a choice that has considerable benefits.
“Close and intimate relationships are the single most protective factor for our mental health,” said Peter Yellowlees, MBBS, MD, chief wellness officer for UC Davis Health and professor of psychiatry at the University of California, Davis. “Isolation and loneliness are very important stressors, and we know that about 25% of the population reports being lonely.”
A version of this article first appeared on Medscape.com.
Now, as they bear the weight of a multiyear pandemic alongside the perpetual struggle to maintain some semblance of work-life balance, their resiliency has been stretched to the brink.
In 2022, the Medscape Physician Suicide Report surveyed more than 13,000 physicians in 29 specialties who were candid about their experiences with suicidal thoughts, how they support their besieged colleagues, and their go-to coping strategies.
Overall, 21% of physicians reported having feelings of depression. Of those, 24% had clinical depression and 64% had colloquial depression. Physicians who felt sad or blue decreased slightly, compared with the 2021 report, but the number of physicians experiencing severe depression rose 4%.
One in 10 physicians said they have thought about or attempted suicide. However, the number of physicians with suicidal thoughts dropped to 9%, down substantially from the 22% who reported similar feelings in 2020.
Still, there was a slight uptick in women physicians contemplating suicide, likely linked to their larger share of childcare and family responsibilities.
“They have needed to pull double duty even more than usual, and that may have increased their sense of burnout and vulnerability to suicidal thoughts,” said Andrea Giedinghagen, MD, assistant professor in the department of psychiatry at Washington University in St. Louis, and coauthor of “Physician Suicide: A Call to Action
Fighting the stigma of seeking mental health help
Although the number of physicians attempting, but not completing suicide, has remained steady at 1% for several years, the recent passage of the Dr. Lorna Breen Health Care Provider Protection Act by Congress aims to drive that figure even lower. Dr. Breen, an ED physician at New York–Presbyterian Hospital, died by suicide in April 2020. Overwhelmed by the onslaught of COVID patients, Dr. Breen was reluctant to seek mental health services for fear of being ostracized.
“Many physicians don’t seek mental health care due to fear of negative consequences in the workplace, including retribution, exclusion, loss of license, or even their job,” Gary Price, MD, president of The Physicians Foundation, told this news organization. “This was the experience of Dr. Lorna Breen. She was convinced that if she talked to a professional, she would lose her medical license. Perhaps if Dr. Breen was equipped with the accurate information – there is no mental health reporting requirement in her state’s medical license application – it might have saved her life.”
This same stigma was reflected in the survey, with one physician saying: “I’m afraid that if I spoke to a therapist, I’d have to report receiving psychiatric treatment to credentialing or licensing boards.” Roughly 40% of survey respondents, regardless of age, chose not to disclose their suicidal thoughts to anyone, not even a family member or suicide hotline. And just a tiny portion of physicians (10% of men and 13% of women) said that a colleague had discussed their suicidal thoughts with them.
“There is a longstanding culture of silence around physician mental health in the medical community,” said Dr. Price. “The strategies within the Act are critical to fixing this culture and making it acceptable and normalized for physicians to seek mental health care,” and for it to “become a fundamental and ongoing element of being a practicing physician.”
As part of the legislation, the Department of Health & Human Services must award grants to hospitals, medical associations, and other entities to facilitate mental health programs for providers. They must also establish policy recommendations and conduct campaigns to improve providers’ mental and behavioral health, encourage providers to seek mental health support and assistance, remove barriers to such treatment, and identify best practices to prevent suicide and promote resiliency
Addressing barriers to mental health
The new bill is a step in the right direction, but Dr. Price said health organizations must do more to address the six key structural barriers that are “discouraging physicians from seeking [mental health] help,” such as the inclusion of “intrusive mental health questions on medical board, hospital credentialing, and malpractice insurance applications.”
In addition, employers should allow physicians to seek out-of-network mental health services, if necessary, and not cause further humiliation by requiring them to be treated by colleagues within their hospital system. A similar proposal has recently been introduced and is gaining traction in Utah, following the suicide of ED physician Scott Jolley, MD, in 2021 after he was admitted for psychiatric care where he worked.
Diminishing the stigma surrounding physicians’ mental health encourages a more open dialogue, so if a colleague reaches out – listen. “Start by thanking the colleague for sharing the information: ‘I’m sure that wasn’t easy but I appreciate that you respect me enough to share this. Let’s talk more,’ ” said Michael F. Myers, MD, professor of clinical psychiatry at State University of New York, Brooklyn . “Then ask what you can do to help, which cuts down on the sense of isolation that colleague may feel.”
According to the survey, many physicians have developed strategies to support their happiness and mental health. Although fewer than 10% said reducing work hours or transitioning to a part-time schedule was most effective, the majority of physicians relied on spending time with family and friends (68%) – a choice that has considerable benefits.
“Close and intimate relationships are the single most protective factor for our mental health,” said Peter Yellowlees, MBBS, MD, chief wellness officer for UC Davis Health and professor of psychiatry at the University of California, Davis. “Isolation and loneliness are very important stressors, and we know that about 25% of the population reports being lonely.”
A version of this article first appeared on Medscape.com.