User login
When is catheter ablation a sound option for your patient with A-fib?
CASE
Jack Z, a 75-year-old man with well-controlled hypertension, diabetes controlled by diet, and atrial fibrillation (AF) presents to the family medicine clinic to establish care with you after moving to the community from out of town.
The patient describes a 1-year history of AF. He provides you with an echocardiography report from 6 months ago that shows no evidence of structural heart disease. He takes lisinopril, to control blood pressure; an anticoagulant; a beta-blocker; and amiodarone for rhythm control. Initially, he took flecainide, which was ineffective for rhythm control, before being switched to amiodarone. He had 2 cardioversion procedures, each time after episodes of symptoms. He does not smoke or drink alcohol.
Mr. Z describes worsening palpitations and shortness of breath over the past 9 months. Symptoms now include episodes of exertional fatigue, even when he is not having palpitations. Prior to the episodes of worsening symptoms, he tells you that he lived a “fairly active” life, golfing twice a week.
The patient’s previous primary care physician had encouraged him to talk to his cardiologist about “other options” for managing AF, because levels of his liver enzymes had started to rise (a known adverse effect of amiodarone1) when measured 3 months ago. He did not undertake that conversation, but asks you now about other treatments for AF.
Atrial fibrillation is the most common sustained cardiac arrhythmia, characterized by discordant electrical activation of the atria due to structural or electrophysiological abnormalities, or both. The disorder is associated with an increased rate of stroke and heart failure and is independently associated with a 1.5- to 2-fold risk of all-cause mortality.2
In this article, we review the pathophysiology of AF; management, including the role of, and indications for, catheter ablation; and patient- and disease-related factors associated with ablation (including odds of success, complications, risk of recurrence, and continuing need for thromboprophylaxis) that family physicians should consider when contemplating referral to a cardiologist or electrophysiologist for catheter ablation for AF.
What provokes AF?
AF is thought to occur as a result of an interaction among 3 phenomena:
- enhanced automaticity of abnormal atrial tissue
- triggered activity of ectopic foci within 1 or more pulmonary veins, lying within the left atrium
- re-entry, in which there is propagation of electrical impulses from an ectopic beat through another pathway.
Continue to: In patients who progress...
In patients who progress from paroxysmal to persistent AF (see “Subtypes,” below), 2 distinct pathways, facilitated by the presence of abnormal tissue, continuously activate one another, thus maintaining the arrhythmia. Myocardial tissue in the pulmonary veins is responsible for most ectopic electrical impulses in patients with drug-refractory AF (see “Rhythm control”).
Subtypes. For the purpose of planning treatment, AF is classified as:
- Paroxysmal. Terminates spontaneously or with intervention ≤ 7 days after onset.
- Persistent. Continuous and sustained for > 7 days.
- Longstanding persistent. Continuous for > 12 months.
- Permanent. The patient and physician accept that there will be no further attempt to restore or maintain sinus rhythm.
Goals of treatment
Primary management goals in patients with AF are 2-fold: control of symptoms and prevention of thromboembolism. A patient with new-onset AF who presents acutely with inadequate rate control and hemodynamic compromise requires urgent assessment to determine the cause of the arrhythmia and need for cardioversion.3 A symptomatic patient with AF who does not have high-risk features (eg, valvular heart disease, mechanical valves) might be a candidate for rhythm control in addition to rate control.3,4
Rate control. After evaluation in the hospital, a patient who has a rapid ventricular response but remains hemodynamically stable, without evidence of heart failure, should be initiated on a rate-controlling medication, such as a beta-blocker or nondihydropyridine calcium-channel blocker. A resting heart rate goal of < 80 beats per minute (bpm) is recommended for a symptomatic patient with AF. The heart rate goal can be relaxed, to < 110 bpm, in an asymptomatic patient with preserved left ventricular function.5,6
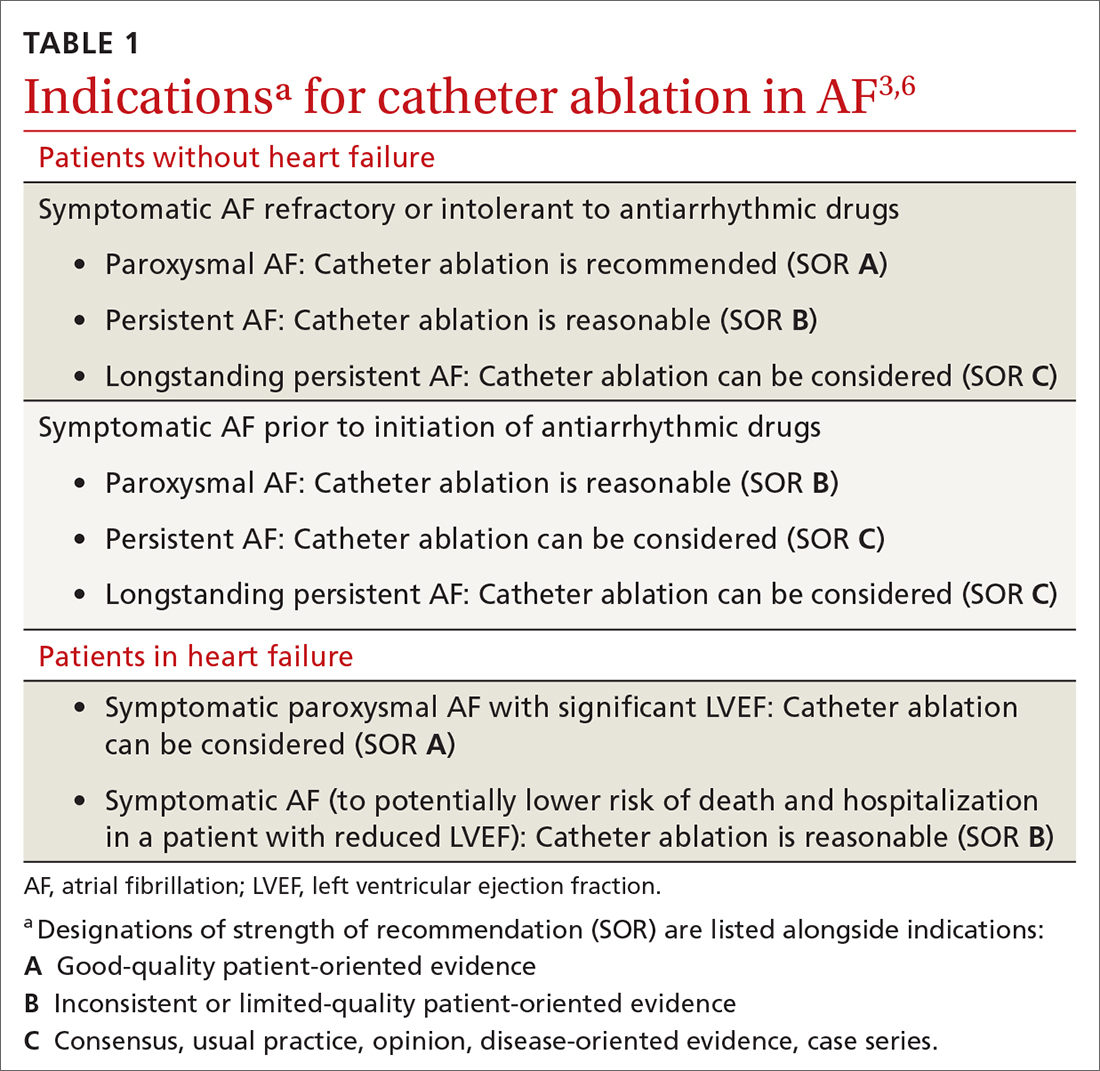
Rhythm control, indicated in patients who remain symptomatic on rate-controlling medication, can be achieved either with an antiarrhythmic drug (AAD) or by catheter ablation.4,5 In stable patients, rhythm control should be considered only after a thorough work-up for a reversible cause of AF, and can be achieved with an oral AAD or, in select patients, through catheter ablation (TABLE 13,6). Other indications for chronic rhythm control include treatment of patients with tachycardia-induced cardiomyopathy.5
A major study that documented the benefit of early rhythm control evaluated long-term outcomes in 2789 patients with AF who were undergoing catheter ablation.7 Patients were randomized to early rhythm control (catheter ablation or AAD) or “usual care”—ie, in this study, rhythm control limited to symptomatic patients. Primary outcomes were death from cardiovascular causes, stroke, and hospitalization with worsening heart failure or acute coronary syndrome. A first primary outcome event occurred in 249 patients (3.9/100 person-years) assigned to early rhythm control, compared to 316 (5.0 per 100 person-years) in the group assigned to usual care.
The study was terminated early (after 5.1 years) because of overwhelming evidence of efficacy (number need to treat = 7). Although early rhythm control was obtained through both catheter ablation and AAD (hazard ratio [HR] = 0.79; 96% CI, 0.66-0.94; P = .005), success was attributed to the use of catheter ablation for a rhythm-control strategy and its use among patients whose AF was present for < 1 year. Most patients in both treatment groups continued to receive anticoagulation, rate control, and optimization of cardiovascular risk.7
Continue to: Notably, direct studies...
Notably, direct studies comparing ablation and AAD have not confirmed the benefit of ablation over AAD in outcomes of all-cause mortality, bleeding, stroke, or cardiac arrest over a 5-year period.8
Adverse effects and mortality outcomes with AAD. Concern over using AAD for rhythm control is based mostly on adverse effects and long-term (1-year) mortality outcomes. Long-term AAD therapy has been shown to decrease the recurrence of AF—but without evidence to suggest other mortality benefits.
A meta-analysis of 59 randomized controlled trials reviewed 20,981 patients receiving AAD (including quinidine, disopyramide, propafenone, flecainide, metoprolol, amiodarone, dofetilide, dronedarone, and sotalol) for long-term effects on death, stroke, adverse reactions, and recurrence of AF.9 Findings at 10 months suggest that:
- Compared to placebo, amiodarone and sotalol increased the risk of all-cause mortality during the study period.
- There was minimal difference in mortality among patients taking dofetilide or dronedarone, compared to placebo.
- There were insufficient data to draw conclusions about the effect of disopyramide, flecainide, and propafenone on mortality.
Before starting a patient on AAD, the risk of arrhythmias and the potential for these agents to cause toxicity and adverse events should always be discussed.
CASE
You tell Mr. Z that you need to know the status of his comorbidities to make a recommendation about “other” management options, and proceed to take a detailed history.
Recent history. Mr. Z reveals that “today is a good day”: He has had “only 1” episode of palpitations, which resolved on its own. The previous episode, he explains, was 3 days ago, when palpitations were associated with lightheadedness and shortness of breath. He denies chest pains or swelling of the legs.
Physical exam. The patient appears spry, comfortable, and in no acute distress. Vital signs are within normal limits. A body mass index of 28.4 puts him in the “overweight” category. His blood pressure is 118/75 mm Hg.
Continue to: Cardiac examination...
Cardiac examination is significant for an irregular rhythm without murmurs, rubs, or gallops. His lungs are clear bilaterally; his abdomen is soft and nondistended. His extremities show no edema.
Testing. You obtain an electrocardiogram, which demonstrates a controlled ventricular rate of 88 bpm and AF. You order a complete blood count, comprehensive metabolic panel, tests of hemoglobin A1C and thyroid-stimulating hormone, lipid panel, echocardiogram, and a chest radiograph.
Results. The chest radiograph is negative for an acute cardiopulmonary process; cardiac size is normal. Aspartate aminotransferase and alanine aminotransferase levels are higher than twice the normal limit. The echocardiogram reveals an estimated left ventricular ejection fraction of 55% to 60%; no structural abnormalities are noted.
In which AF patients is catheter ablation indicated?
Ablation is recommended for select patients (TABLE 13,6) with symptomatic paroxysmal AF that is refractory to AAD or who are intolerant of AAD.3,6 It is a reasonable first-line therapy for high-performing athletes in whom AAD would affect athletic performance.3,10 It is also a reasonable option in select patients > 75 years and as an alternative to AAD therapy.3 Finally, catheter ablation should be considered in symptomatic patients with longstanding persistent AF and congestive heart failure, with or without reduced left ventricular ejection fraction.3
CASE
You inform Mr. Z that his symptoms are likely a result of symptomatic paroxysmal AF, which was refractory to flecainide and amiodarone, and that his abnormal liver function test results preclude continued use of amiodarone. You propose Holter monitoring to correlate timing of symptoms with the arrhythmia, but he reports this has been done, and the correlation confirmed, by his previous physician.
You explain that, because the diagnosis of symptomatic paroxysmal AF refractory to AADs has been confirmed, he is categorized as a patient who might benefit from catheter ablation, based on:
- the type of AF (ie, paroxysmal AF is associated with better ablation outcomes)
- persistent symptoms that are refractory to AADs
- his intolerance of AAD
- the length of time since onset of symptoms.
Mr. Z agrees to consider your recommendation.
Continue to: What are the benefits of catheter ablation?
What are the benefits of catheter ablation?
Ablation can be achieved through radiofrequency (RF) ablation, cryoablation, or newer, laser-based balloon ablation. Primary outcomes used to determine the success of any options for performing ablation include mortality, stroke, and hospitalization. Other endpoints include maintenance of sinus rhythm, freedom from AF, reduction in AF burden (estimated through patients’ report of symptoms, recurrence rate, need for a second ablation procedure, and serial long-term monitoring through an implantable cardiac monitoring device), quality of life, and prevention of AF progression.3
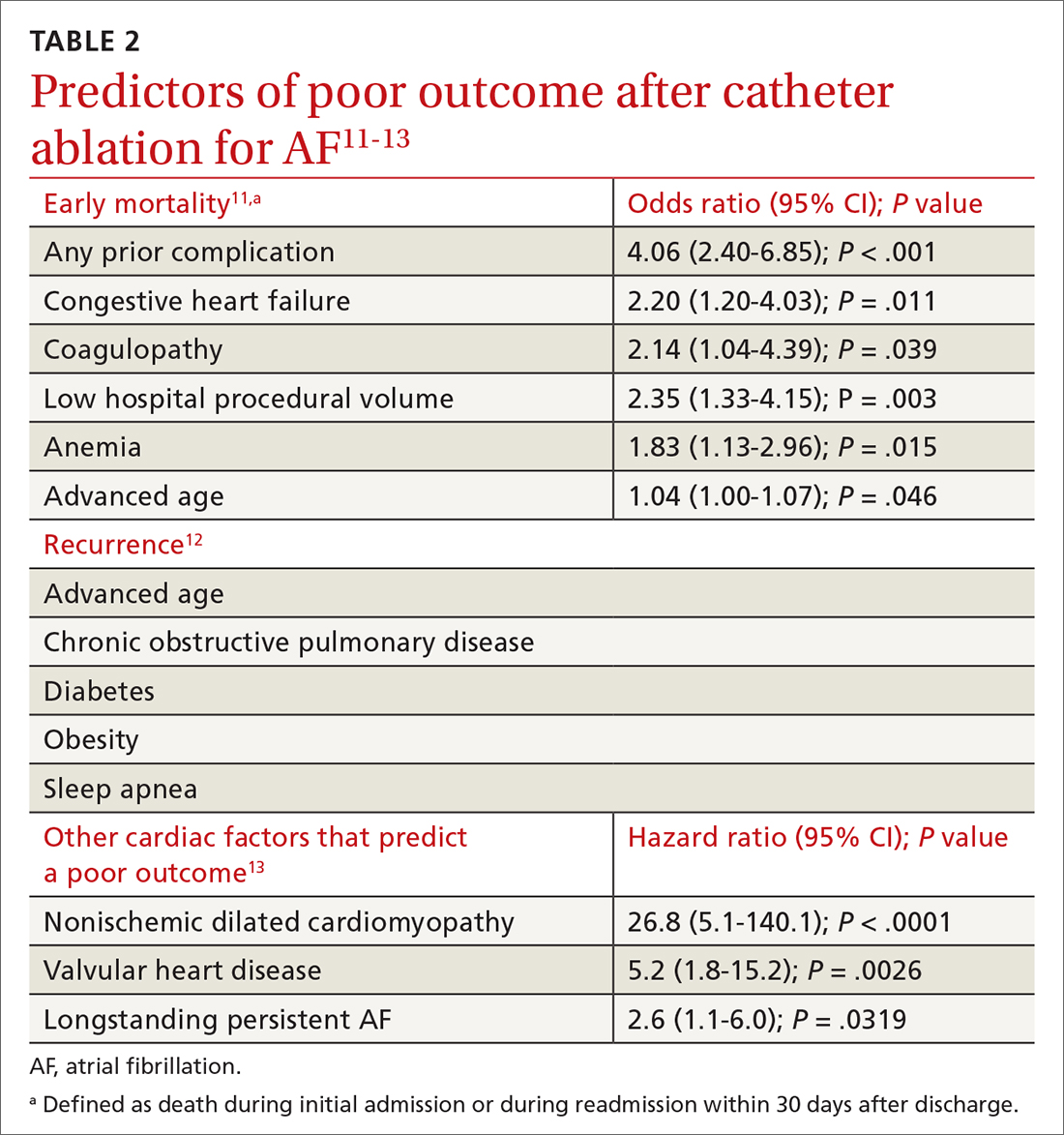
Patient and disease variables (TABLE 211-13). The success rate of catheter ablation, defined as freedom from either symptomatic or asymptomatic episodes of AF, is dependent on several factors,3,14 including:
- type of AF (paroxysmal or persistent)
- duration and degree of symptoms
- age
- sex
- comorbidities, including heart failure and structural heart or lung disease.
Overall, in patients with paroxysmal AF, an estimated 75% are symptom free 1 year after ablation.15 Patients with persistent and longstanding persistent AF experience a lower success rate.
RF catheter ablation has demonstrated superiority to AAD in reducing the need for cardioversion (relative risk [RR] = 0.62; 95% CI, 0.47-0.82) and cardiac-related hospitalization (RR = 0.27; 95% CI, 0.10-0.72;) at 12 months in patients with nonparoxysmal AF (persistent or longstanding persistent).16
Effect on mortality. Among patients with heart failure with reduced ejection fraction, long-term studies of cardiovascular outcomes 5 years post ablation concluded that ablation is associated with a decrease in all-cause mortality (RR = 0.69; 95% CI, 0.54-0.88; P = .003) and a reduction in hospitalization (RR = 0.62; 95% CI, 0.47-0.82; P = .0006); younger (< 65 years) and male patients derive greater benefit.6,17 Indications for ablation in patients with heart failure are similar to those in patients without heart failure; ablation can therefore be considered for select heart failure patients who remain symptomatic or for whom AAD has failed.3
Older patients. Ablation can be considered for patients > 75 years with symptomatic paroxysmal AF refractory to AAD or who are intolerant of AAD.3 A study that assessed the benefit of catheter ablation reviewed 587 older (> 75 years) patients with AF, of whom 324 were eligible for ablation. Endpoints were maintenance of sinus rhythm, stroke, death, and major bleeding. Return to normal sinus rhythm was an independent factor, associated with a decrease in the risk of mortality among all patient groups that underwent ablation (HR = 0.36; 95% CI, 0.2-0.63; P = .0005). Age > 75 years (HR = 1.09; 95% CI, 1.01-1.16; P > .02) and depressed ejection fraction < 40% (HR = 2.38; 95% CI, 1.28-4.4; P = .006) were determined to be unfavorable parameters for survival.18
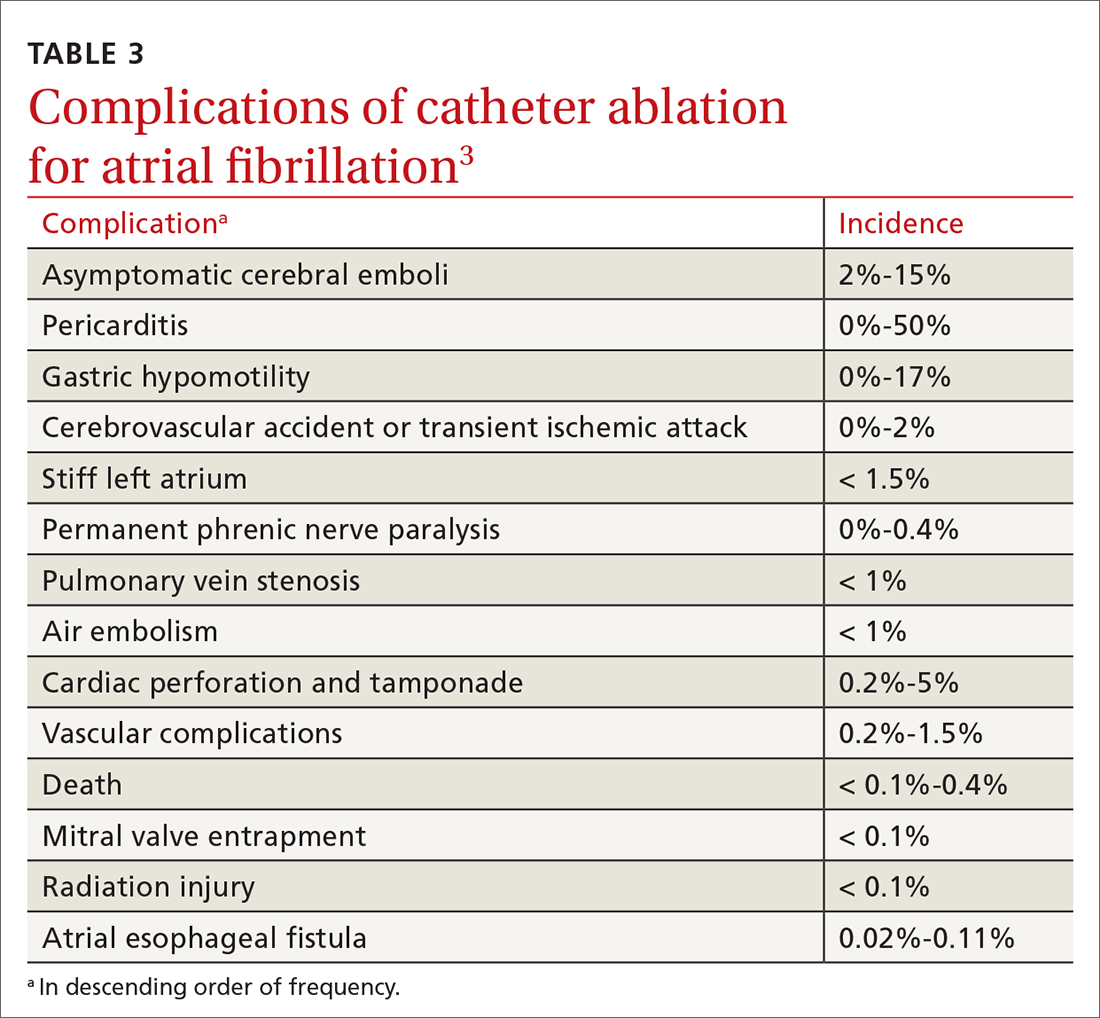
Complications and risks
Complications of catheter ablation for AF, although infrequent, can be severe (TABLE 33). Early mortality, defined as death during initial admission or 30-day readmission, occurs in approximately 0.5% of cases; half of deaths take place during readmission.11
Continue to: Complications vary...
Complications vary, based on the type and site of ablation.19,20 Cardiac tamponade or perforation, the most life-threatening complications, taken together occur in an estimated 1.9% of patients (odds ratio [OR] = 2.98; 95% CI, 1.36-6.56; P = .007).11 Other in-hospital complications independently predictive of death include any cardiac complications (OR = 12.8; 95% CI, 6.86 to 23.8; P < .001) and neurologic complications (cerebrovascular accident and transient ischemic attack) (OR = 8.72; 95% CI, 2.71-28.1; P < .001).
Other complications that do not cause death but might prolong the hospital stay include pericarditis without effusion, anesthesia-related complications, and vascular-access complications. Patients whose ablation is performed at an institution where the volume of ablations is low are also at higher risk of early mortality (OR = 2.35; 95% CI, 1.33-4.15; P = .003).16
Recurrence is common (TABLE 211-13). Risk of recurrence following ablation is significant; early (within 3 months after ablation) recurrence is seen in 50% of patients.21,22 However, this is a so-called "blanking period"—ie, a temporary period of inflammatory and proarrhythmic changes that are not predictors of later recurrence. The 5-year post-ablation recurrence rate is approximately 25.5%; longstanding persistent and persistent AF and the presence of comorbidities are major risk factors for recurrence.13,23
Recurrence is also associated with the type of procedure; pulmonary vein isolation, alone or in combination with another type of procedure, results in higher long-term success.21,23
Other variables affect outcome (TABLE 211-13). Following AF ablation, patients with nonparoxysmal AF at baseline, advanced age, sleep apnea and obesity, left atrial enlargement, and any structural heart disease tend to have a poorer long-term (5-year) outcome (ie, freedom from extended episodes of AF).3,13,23,24
Patients who undergo repeat procedures have higher arrhythmia-free survival; the highest ablation success rate is for patients with paroxysmal AF.13,23
Exposure to ionizing radiation. Fluoroscopy is required for multiple components of atrial mapping and ablation during RF ablation, including navigation, visualization, and monitoring of catheter placement. Patients undergoing this particular procedure therefore receive significant exposure to ionizing radiation. A reduction in, even complete elimination of, fluoroscopy has been achieved with:
- nonfluoroscopic 3-dimensional mapping systems25
- intracardiac echocardiography, which utilizes ultrasonographic imaging as the primary visual mode for tracking and manipulating the catheter
- robotic guided navigation.26-28
Continue to: CASE
CASE
At his return visit, Mr. Z says that he is concerned about, first, undergoing catheter ablation at his age and, second, the risks associated with the procedure. You explain that it is true that ablation is ideal in younger patients who have minimal comorbidities and that the risk of complications increases with age—but that there is no cutoff or absolute age contraindication to ablation.
You tell Mr. Z that you will work with him on risk-factor modification in anticipation of ablation. You also assure him that the decision whether to ablate must be a joint one—between him and a cardiologist experienced both in electrophysiology and in performing this highly technical procedure. And you explain that a highly practiced specialist can identify Mr. Z’s risk factors that might make ablation more difficult to perform and affect the long-term outcome.
With Mr. Z’s agreement, you screen for sleep apnea and start him on a lifestyle modification plan to achieve a more ideal weight, explaining that the risk of recurrence of AF after catheter ablation is increased by obesity and sleep apnea, in addition to age. You explain that, based on his CHA2DS2–VASc (congestive heart failure; hypertension; age, ≥ 75 years; diabetes; prior stroke, transient ischemic attack, or thromboembolism; vascular disease; age, 65 to 74 years; sex category) score of 3, he will remain on anticoagulation whether or not he has the ablation.
You refer the patient to the nearest high-volume cardiac ablation center.
Last, you caution Mr. Z that, based on his lipid levels, his 10-year risk of heart disease or stroke is elevated. You recommend treatment with a statin agent while he continues his other medications.
Delivering energy to myocardium
Myocardial tissue in pulmonary veins is responsible for most ectopic electrical impulses in patients with drug-refractory AF. The goal of catheter ablation in AF is destruction (scarring) of tissue that is the source of abnormal vein potentials.15
How RF ablation works. Ablation is most commonly performed using RF energy, a high-frequency form of electrical energy. Electrophysiology studies are carried out at the time of ablation by percutaneous, fluoroscopically guided insertion of 2 to 5 catheters, usually through the femoral or internal jugular vein, which are then positioned within several areas of the heart—usually, the right atrium, bundle of His, right ventricle, and coronary sinus.
Continue to: Electrical current...
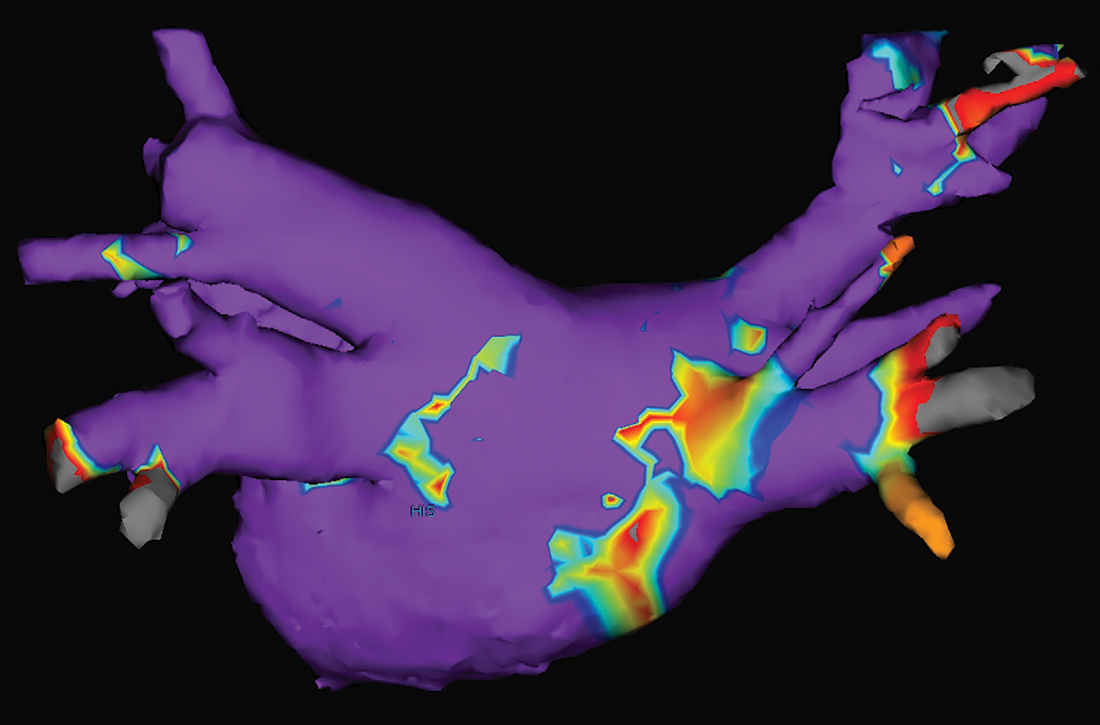
Electrical current is applied through the catheters from an external generator to stimulate the myocardium and thus determine its electrophysiologic properties. The anatomic and electrical activity of the left atrium and pulmonary veins is then identified, a technique known as electro-anatomical mapping (FIGURE). After arrhythmogenic myocardial tissue is mapped, ablation is carried out with RF energy through the catheter to the pathogenic myocardium from which arrhythmias are initiated or conducted. The result is thermal destruction of tissue and creation of small, shallow lesions that vary in size with the type of catheter and the force of contact pressure applied.3,29
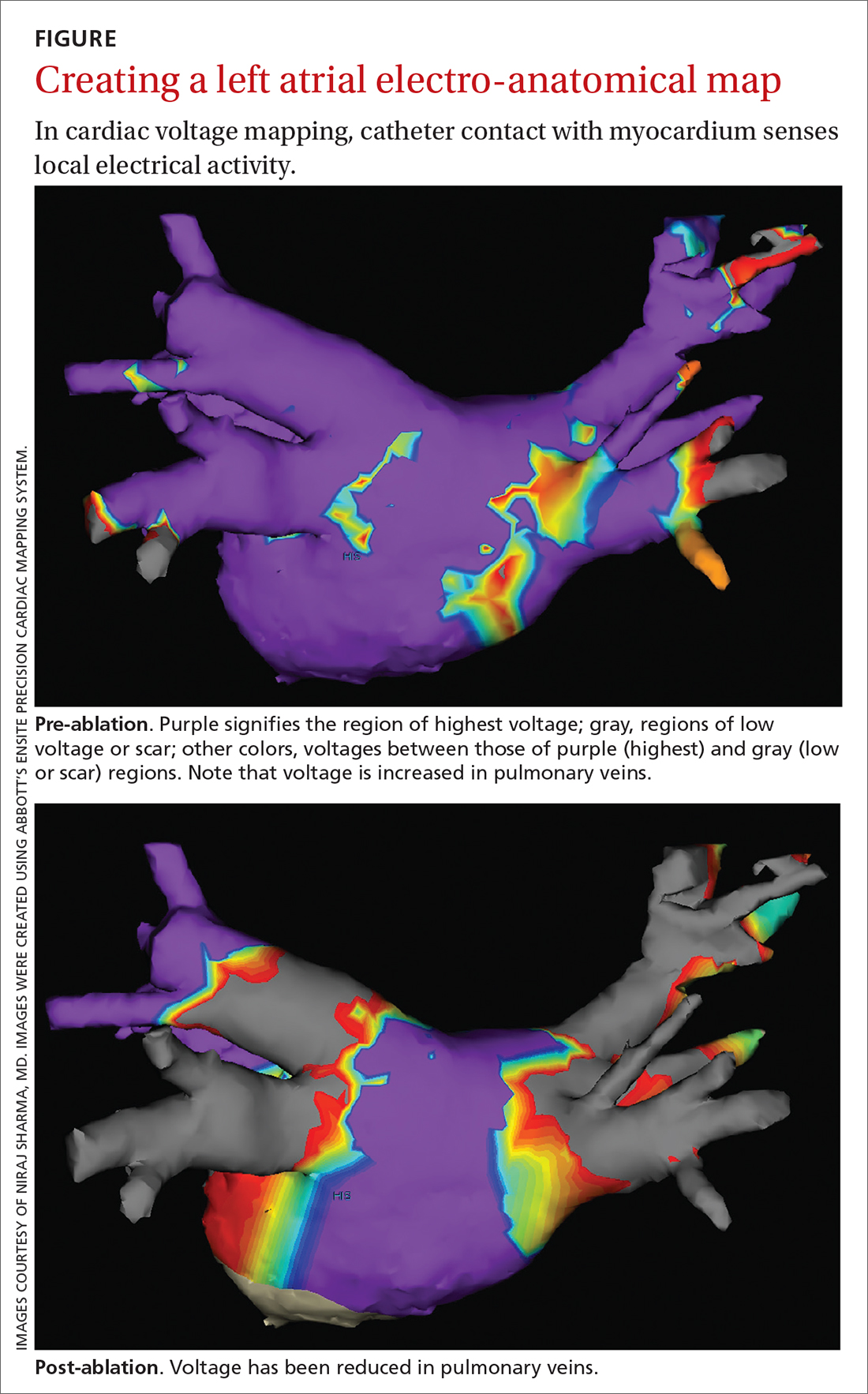
Other energy sources used in catheter ablation include cryothermal energy, which utilizes liquid nitrous oxide under pressure through a cryocatheter or cryoballoon catheter. Application of cryothermal energy freezes tissue and disrupts cell membranes and any electrical activity. Cryoballoon ablation has been shown to be similarly safe and efficacious as RF ablation in patients with paroxysmal AF.30,31
Newer laser-based balloon ablations are performed under ultrasonographic guidance and utilize arcs of laser energy delivered to the pathogenic myocardium.3
Thromboembolism prophylaxis
Oral anticoagulation to decrease the risk of stroke is initiated in all patients with AF, based on a thromboembolic risk profile determined by their CHA2DS2–VASc score, with anticoagulation recommended when the score is ≥ 2 in men and ≥ 3 in women. Options for anticoagulation include warfarin and one of the novel oral anticoagulants dabigatran, apixaban, rivaroxaban, and edoxaban.4 Recommendations are as follows3:
- For patients with a CHA2DS2–VASc score of ≥ 2 (men) or ≥ 3 (women), anticoagulation should be continued indefinitely, regardless of how successful the ablation procedure is.
- When patients choose to discontinue anticoagulation, they should be counseled in detail about the risk of doing so. The continued need for frequent arrhythmia monitoring should be emphasized.
The route from primary careto catheter ablation
Perform a thorough evaluation. Patients who present to you with palpitations should first undergo a routine workup for AF, followed by confirmation of the diagnosis. Exclude structural heart disease with echocardiography. Undertake monitoring, which is essential to determine whether symptoms are a reflection of the arrhythmia, using noncontinuous or continuous electrocardiographic (EKG) monitoring. Noncontinuous detection devices include:
- scheduled or symptom-initiated EKG
- a Holter monitor, worn for at least 24 hours and as long as 7 days
- trans-telephonic recordings and patient- or automatically activated devices
- an external loop recorder.32
Continuous EKG monitoring is more permanent (≥ 12 months). This is usually achieved through an implantable loop powered by a battery that lasts as long as 3 years.3
Ablation: Yes or no? Ablation is not recommended to avoid anticoagulation or when anticoagulation is contraindicated.5 With regard to specific patient criteria, the ideal patient:
- is symptomatic
- has failed AAD therapy
- does not have pulmonary disease
- has a normal or mildly dilated left atrium or normal or mildly reduced left ventricular ejection fraction.5
Continue to: There is no absolute age...
There is no absolute age or comorbidity contraindication to ablation. The patient should be referred to a cardiologist who has received appropriate training in electrophysiology, to identify comorbidities that (1) increase the technical difficulty of the procedure and baseline risk and (2) affect long-term outcome,12 and who performs the procedure in a center that has considerable experience with catheter ablation.33
Once the decision is made to perform ablation, you can provide strategies that optimize the outcome (freedom from AF episodes). Those tactics include weight loss and screening evaluation and, if indicated, treatment for sleep apnea.3
Protocol. Prior to the procedure, the patient fasts overnight; they might be asked to taper or discontinue cardiac medications that have electrophysiologic effects. Studies suggest a low risk of bleeding associated with catheter ablation; anticoagulation should therefore continue uninterrupted for patients undergoing catheter ablation for AF3,4,34,35; however, this practice varies with the cardiologist or electrophysiologist performing ablation.
Because of the length and complexity of the procedure, electro-anatomical mapping and ablation are conducted with the patient under general anesthesia.3 The patient is kept supine, and remains so for 2 to 4 hours afterward to allow for hemostasis at puncture sites.3
Patients might be monitored overnight, although same-day catheter ablation has been shown to be safe and cost-effective in select patients.36,37 Post ablation, patients follow up with the cardiologist and electrophysiologist. Long-term arrhythmia monitoring is required.3 Anticoagulation is continued for at least 2 months, and is discontinued based on the patient’s risk for stroke, utilizing their CHA2DS2–VASc score.3,4
CASE
At Mr. Z’s 6-month primary care follow-up, he confirms what has been reported to you as the referring physician: He had a successful catheter ablation and continues to have regular follow-up monitoring with the cardiologist. He is no longer taking amiodarone.
At this visit, he reports no recurrence of AF-associated symptoms or detectable AF on cardiac monitoring. He has lost 8 lbs. You counsel to him to continue to maintain a healthy lifestyle.
CORRESPONDENCE
Amimi S. Osayande MD, FAAFP, Northside-Gwinnett Family Medicine Residency Program, Strickland Family Medicine Center, 665 Duluth Highway, Suite 501, Lawrenceville, GA 30046; [email protected]
1. Amiodarone hydrochloride (marketed as Cordarone and Pacerone) information. Silver Spring, Md.: US Food & Drug Administration. Reviewed March 23, 2015. Accessed January 16, 2022. www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/amiodarone-hydrochloride-marketed-cordarone-and-pacerone-information
2. Gómez-Outes A, Suárez-Gea ML,García-Pinilla JM. Causes of death in atrial fibrillation: challenges and opportunities. Trends Cardiovasc Med. 2017;27:494-503. doi: 10.1016/j.tcm.2017.05.002
3. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/ APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Arrhythm. 2017;33:369-409. doi: 10.1016/j.joa.2017.08.001
4. Camm AJ, Lip GYH, De Caterina R, et al; ESC Committee for Practice Guidelines-CPG; Document Reviewers. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation—developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14:1385-1413. doi: 10.1093/europace/eus305
5. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071-2104. doi: 10.1161/CIR.0000000000000040
6. January CT, Wann LS, Calkins H, et al; Writing Group Members. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/ HRS guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019;16:e66-e93. doi: 10.1016/j.hrthm.2019.01.024
7. Kirchhof P, Camm AJ, Goette A, et al; EAST-AFNET 4 Trial Investigators. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305-1316. doi: 10.1056/ NEJMoa2019422
8. Packer DL, Mark DB, Robb RA, et al; CABANA Investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261-1274. doi: 10.1001/jama.2019.0693
9. Valembois L, Audureau E, Takeda A, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2019;9:CD005049. doi: 10.1002/14651858.CD005049
10. Koopman P, Nuyens D, Garweg C, et al. Efficacy of radiofrequency catheter ablation in athletes with atrial fibrillation. Europace. 2011;13:1386-1393. doi: 10.1093/europace/eur142
11. Hakalahti A, Biancari F, Nielsen JC, et al. Radiofrequency ablation vs. antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: systematic review and meta-analysis. Europace. 2015;17:370-378. doi: 10.1093/europace/euu376
12. Nyong J, Amit G, Adler AJ, et al. Efficacy and safety of ablation for people with non-paroxysmal atrial fibrillation. Cochrane Database Syst Rev. 2016;11:CD012088. doi: 10.1002/14651858. CD012088.pub2
13. Andrade JG, Champagne J, Dubuc M, et al; CIRCA-DOSE Study Investigators. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. 2019;140:1779-1788. doi: 10.1161/ CIRCULATIONAHA.119.042622
14. Asad ZUA, Yousif A, Khan MS, et al. Catheter ablation versus medical therapy for atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. Circ Arrhythm Electrophysiol. 2019;12:e007414. doi: 10.1161/ CIRCEP.119.007414
15. Nademanee K, Amnueypol M, Lee F, et al. Benefits and risks of catheter ablation in elderly patients with atrial fibrillation. Heart Rhythm. 2015;12:44-51. doi: 10.1016/j.hrthm.2014.09.049
16. Cheng EP, Liu CF, Yeo I, et al. Risk of mortality following catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2019;74: 2254-2264. doi: 10.1016/j.jacc.2019.08.1036
17. Brugada J, Katritsis DG, Arbelo E, et al; ESC Scientific Document Group. 2019 ESC Guidelines for the management of patients with supraventricular tachycardia. The Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Developed in collaboration with the Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2020;41:655-720. doi: 10.1093/eurheartj/ehz467
18. Hosseini SM, Rozen G, Saleh A, et al. Catheter ablation for cardiac arrhythmias: utilization and in-hospital complications, 2000 to 2013. JACC Clin Electrophysiol. 2017;3:1240-1248. doi: 10.1016/j.jacep.2017.05.005
19. Andrade JG, Macle L, Khairy P, et al. Incidence and significance of early recurrences associated with different ablation strategies for AF: a STAR-AF substudy. J Cardiovasc Electrophysiol. 2012;23:1295-1301. doi: 10.1111/j.1540-8167.2012.02399.x
20. Joshi S, Choi AD, Kamath GS, et al. Prevalence, predictors, and prognosis of atrial fibrillation early after pulmonary vein isolation: findings from 3 months of continuous automatic ECG loop recordings. J Cardiovasc Electrophysiol. 2009;20:1089-1094. doi: 10.1111/j.1540-8167.2009.01506.x
21. Weerasooriya R, Khairy P, Litalien J, et al. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160-166. doi: 10.1016/j.jacc.2010.05.061
22. Ouyang F, Tilz R, Chun J, et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation. 2010;122:2368-2377. doi: 10.1161/ CIRCULATIONAHA.110.946806
23. Tilz RR, Rillig A, Thum A-M, et al. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J Am Coll Cardiol. 2012;60: 1921-1929. doi: 10.1016/j.jacc.2012.04.060
24. Forkmann M, Schwab C, Busch S. [Catheter ablation of supraventricular tachycardia]. Herzschrittmacherther Elektrophysiol. 2019;30:336-342. doi: 10.1007/s00399-019-00654-x
25. Bulava A, Hanis J, Eisenberger M. Catheter ablation of atrial fibrillation using zero-fluoroscopy technique: a randomized trial. Pacing Clin Electrophysiol. 2015;38:797-806. doi: 10.1111/pace.12634
26. Haegeli LM, Stutz L, Mohsen M, et al. Feasibility of zero or near zero fluoroscopy during catheter ablation procedures. Cardiol J. 2019;26:226-232. doi: 10.5603/CJ.a2018.0029
27. Steven D, Servatius H, Rostock T, et al. Reduced fluoroscopy during atrial fibrillation ablation: benefits of robotic guided navigation. J Cardiovasc Electrophysiol. 2010;21:6-12. doi: 10.1111/j.1540-8167.2009.01592.x
28. General therapy for cardiac arrhythmias. In: Zipes DP, Libby P, Bonow RO, et al. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 11th ed. Elsevier; 2019.
29. Kuck K-H, Brugada J, Albenque J-P. Cryoballoon or radiofrequency ablation for atrial fibrillation. N Engl J Med. 2016;375: 1100-1101. doi: 10.1056/NEJMc1609160
30. Chen Y-H, Lu Z-Y, Xiang Y, et al. Cryoablation vs. radiofrequency ablation for treatment of paroxysmal atrial fibrillation: a systematic review and meta-analysis. Europace. 2017;19:784-794. doi: 10.1093/europace/euw330
31. Locati ET, Vecchi AM, Vargiu S, et al. Role of extended external loop recorders for the diagnosis of unexplained syncope, presyncope, and sustained palpitations. Europace. 2014;16:914-922. doi: 10.1093/europace/eut337
32. Calkins H, Kuck KH, Cappato R, et al; Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2012;9:632-696.e21. doi: 10.1016/j.hrthm.2011.12.016
33. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609-1678. doi: 10.1093/ europace/euw295
34. Nairooz R, Sardar P, Payne J, et al. Meta-analysis of major bleeding with uninterrupted warfarin compared to interrupted warfarin and heparin bridging in ablation of atrial fibrillation. Int J Cardiol. 2015;187:426-429. doi: 10.1016/j.ijcard.2015.03.376
35. Romero J, Cerrud-Rodriguez RC, Diaz JC, et al. Uninterrupted direct oral anticoagulants vs. uninterrupted vitamin K antagonists during catheter ablation of non-valvular atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. Europace. 2018;20:1612-1620. doi: 10.1093/europace/euy133
36. Deyell MW, Leather RA, Macle L, et al. Efficacy and safety of same-day discharge for atrial fibrillation ablation. JACC Clin Electrophysiol. 2020;6:609-619. doi: 10.1016/j.jacep.2020.02.009
37. Theodoreson MD, Chohan BC, McAloon CJ, et al. Same-day cardiac catheter ablation is safe and cost-effective: experience from a UK tertiary center. Heart Rhythm. 2015;12:1756-1761. doi: 10.1016/j.hrthm.2015.05.006
CASE
Jack Z, a 75-year-old man with well-controlled hypertension, diabetes controlled by diet, and atrial fibrillation (AF) presents to the family medicine clinic to establish care with you after moving to the community from out of town.
The patient describes a 1-year history of AF. He provides you with an echocardiography report from 6 months ago that shows no evidence of structural heart disease. He takes lisinopril, to control blood pressure; an anticoagulant; a beta-blocker; and amiodarone for rhythm control. Initially, he took flecainide, which was ineffective for rhythm control, before being switched to amiodarone. He had 2 cardioversion procedures, each time after episodes of symptoms. He does not smoke or drink alcohol.
Mr. Z describes worsening palpitations and shortness of breath over the past 9 months. Symptoms now include episodes of exertional fatigue, even when he is not having palpitations. Prior to the episodes of worsening symptoms, he tells you that he lived a “fairly active” life, golfing twice a week.
The patient’s previous primary care physician had encouraged him to talk to his cardiologist about “other options” for managing AF, because levels of his liver enzymes had started to rise (a known adverse effect of amiodarone1) when measured 3 months ago. He did not undertake that conversation, but asks you now about other treatments for AF.
Atrial fibrillation is the most common sustained cardiac arrhythmia, characterized by discordant electrical activation of the atria due to structural or electrophysiological abnormalities, or both. The disorder is associated with an increased rate of stroke and heart failure and is independently associated with a 1.5- to 2-fold risk of all-cause mortality.2
In this article, we review the pathophysiology of AF; management, including the role of, and indications for, catheter ablation; and patient- and disease-related factors associated with ablation (including odds of success, complications, risk of recurrence, and continuing need for thromboprophylaxis) that family physicians should consider when contemplating referral to a cardiologist or electrophysiologist for catheter ablation for AF.
What provokes AF?
AF is thought to occur as a result of an interaction among 3 phenomena:
- enhanced automaticity of abnormal atrial tissue
- triggered activity of ectopic foci within 1 or more pulmonary veins, lying within the left atrium
- re-entry, in which there is propagation of electrical impulses from an ectopic beat through another pathway.
Continue to: In patients who progress...
In patients who progress from paroxysmal to persistent AF (see “Subtypes,” below), 2 distinct pathways, facilitated by the presence of abnormal tissue, continuously activate one another, thus maintaining the arrhythmia. Myocardial tissue in the pulmonary veins is responsible for most ectopic electrical impulses in patients with drug-refractory AF (see “Rhythm control”).
Subtypes. For the purpose of planning treatment, AF is classified as:
- Paroxysmal. Terminates spontaneously or with intervention ≤ 7 days after onset.
- Persistent. Continuous and sustained for > 7 days.
- Longstanding persistent. Continuous for > 12 months.
- Permanent. The patient and physician accept that there will be no further attempt to restore or maintain sinus rhythm.
Goals of treatment
Primary management goals in patients with AF are 2-fold: control of symptoms and prevention of thromboembolism. A patient with new-onset AF who presents acutely with inadequate rate control and hemodynamic compromise requires urgent assessment to determine the cause of the arrhythmia and need for cardioversion.3 A symptomatic patient with AF who does not have high-risk features (eg, valvular heart disease, mechanical valves) might be a candidate for rhythm control in addition to rate control.3,4
Rate control. After evaluation in the hospital, a patient who has a rapid ventricular response but remains hemodynamically stable, without evidence of heart failure, should be initiated on a rate-controlling medication, such as a beta-blocker or nondihydropyridine calcium-channel blocker. A resting heart rate goal of < 80 beats per minute (bpm) is recommended for a symptomatic patient with AF. The heart rate goal can be relaxed, to < 110 bpm, in an asymptomatic patient with preserved left ventricular function.5,6
Rhythm control, indicated in patients who remain symptomatic on rate-controlling medication, can be achieved either with an antiarrhythmic drug (AAD) or by catheter ablation.4,5 In stable patients, rhythm control should be considered only after a thorough work-up for a reversible cause of AF, and can be achieved with an oral AAD or, in select patients, through catheter ablation (TABLE 13,6). Other indications for chronic rhythm control include treatment of patients with tachycardia-induced cardiomyopathy.5

A major study that documented the benefit of early rhythm control evaluated long-term outcomes in 2789 patients with AF who were undergoing catheter ablation.7 Patients were randomized to early rhythm control (catheter ablation or AAD) or “usual care”—ie, in this study, rhythm control limited to symptomatic patients. Primary outcomes were death from cardiovascular causes, stroke, and hospitalization with worsening heart failure or acute coronary syndrome. A first primary outcome event occurred in 249 patients (3.9/100 person-years) assigned to early rhythm control, compared to 316 (5.0 per 100 person-years) in the group assigned to usual care.
The study was terminated early (after 5.1 years) because of overwhelming evidence of efficacy (number need to treat = 7). Although early rhythm control was obtained through both catheter ablation and AAD (hazard ratio [HR] = 0.79; 96% CI, 0.66-0.94; P = .005), success was attributed to the use of catheter ablation for a rhythm-control strategy and its use among patients whose AF was present for < 1 year. Most patients in both treatment groups continued to receive anticoagulation, rate control, and optimization of cardiovascular risk.7
Continue to: Notably, direct studies...
Notably, direct studies comparing ablation and AAD have not confirmed the benefit of ablation over AAD in outcomes of all-cause mortality, bleeding, stroke, or cardiac arrest over a 5-year period.8
Adverse effects and mortality outcomes with AAD. Concern over using AAD for rhythm control is based mostly on adverse effects and long-term (1-year) mortality outcomes. Long-term AAD therapy has been shown to decrease the recurrence of AF—but without evidence to suggest other mortality benefits.
A meta-analysis of 59 randomized controlled trials reviewed 20,981 patients receiving AAD (including quinidine, disopyramide, propafenone, flecainide, metoprolol, amiodarone, dofetilide, dronedarone, and sotalol) for long-term effects on death, stroke, adverse reactions, and recurrence of AF.9 Findings at 10 months suggest that:
- Compared to placebo, amiodarone and sotalol increased the risk of all-cause mortality during the study period.
- There was minimal difference in mortality among patients taking dofetilide or dronedarone, compared to placebo.
- There were insufficient data to draw conclusions about the effect of disopyramide, flecainide, and propafenone on mortality.
Before starting a patient on AAD, the risk of arrhythmias and the potential for these agents to cause toxicity and adverse events should always be discussed.
CASE
You tell Mr. Z that you need to know the status of his comorbidities to make a recommendation about “other” management options, and proceed to take a detailed history.
Recent history. Mr. Z reveals that “today is a good day”: He has had “only 1” episode of palpitations, which resolved on its own. The previous episode, he explains, was 3 days ago, when palpitations were associated with lightheadedness and shortness of breath. He denies chest pains or swelling of the legs.
Physical exam. The patient appears spry, comfortable, and in no acute distress. Vital signs are within normal limits. A body mass index of 28.4 puts him in the “overweight” category. His blood pressure is 118/75 mm Hg.
Continue to: Cardiac examination...
Cardiac examination is significant for an irregular rhythm without murmurs, rubs, or gallops. His lungs are clear bilaterally; his abdomen is soft and nondistended. His extremities show no edema.
Testing. You obtain an electrocardiogram, which demonstrates a controlled ventricular rate of 88 bpm and AF. You order a complete blood count, comprehensive metabolic panel, tests of hemoglobin A1C and thyroid-stimulating hormone, lipid panel, echocardiogram, and a chest radiograph.
Results. The chest radiograph is negative for an acute cardiopulmonary process; cardiac size is normal. Aspartate aminotransferase and alanine aminotransferase levels are higher than twice the normal limit. The echocardiogram reveals an estimated left ventricular ejection fraction of 55% to 60%; no structural abnormalities are noted.
In which AF patients is catheter ablation indicated?
Ablation is recommended for select patients (TABLE 13,6) with symptomatic paroxysmal AF that is refractory to AAD or who are intolerant of AAD.3,6 It is a reasonable first-line therapy for high-performing athletes in whom AAD would affect athletic performance.3,10 It is also a reasonable option in select patients > 75 years and as an alternative to AAD therapy.3 Finally, catheter ablation should be considered in symptomatic patients with longstanding persistent AF and congestive heart failure, with or without reduced left ventricular ejection fraction.3
CASE
You inform Mr. Z that his symptoms are likely a result of symptomatic paroxysmal AF, which was refractory to flecainide and amiodarone, and that his abnormal liver function test results preclude continued use of amiodarone. You propose Holter monitoring to correlate timing of symptoms with the arrhythmia, but he reports this has been done, and the correlation confirmed, by his previous physician.
You explain that, because the diagnosis of symptomatic paroxysmal AF refractory to AADs has been confirmed, he is categorized as a patient who might benefit from catheter ablation, based on:
- the type of AF (ie, paroxysmal AF is associated with better ablation outcomes)
- persistent symptoms that are refractory to AADs
- his intolerance of AAD
- the length of time since onset of symptoms.
Mr. Z agrees to consider your recommendation.
Continue to: What are the benefits of catheter ablation?
What are the benefits of catheter ablation?
Ablation can be achieved through radiofrequency (RF) ablation, cryoablation, or newer, laser-based balloon ablation. Primary outcomes used to determine the success of any options for performing ablation include mortality, stroke, and hospitalization. Other endpoints include maintenance of sinus rhythm, freedom from AF, reduction in AF burden (estimated through patients’ report of symptoms, recurrence rate, need for a second ablation procedure, and serial long-term monitoring through an implantable cardiac monitoring device), quality of life, and prevention of AF progression.3
Patient and disease variables (TABLE 211-13). The success rate of catheter ablation, defined as freedom from either symptomatic or asymptomatic episodes of AF, is dependent on several factors,3,14 including:
- type of AF (paroxysmal or persistent)
- duration and degree of symptoms
- age
- sex
- comorbidities, including heart failure and structural heart or lung disease.

Overall, in patients with paroxysmal AF, an estimated 75% are symptom free 1 year after ablation.15 Patients with persistent and longstanding persistent AF experience a lower success rate.
RF catheter ablation has demonstrated superiority to AAD in reducing the need for cardioversion (relative risk [RR] = 0.62; 95% CI, 0.47-0.82) and cardiac-related hospitalization (RR = 0.27; 95% CI, 0.10-0.72;) at 12 months in patients with nonparoxysmal AF (persistent or longstanding persistent).16
Effect on mortality. Among patients with heart failure with reduced ejection fraction, long-term studies of cardiovascular outcomes 5 years post ablation concluded that ablation is associated with a decrease in all-cause mortality (RR = 0.69; 95% CI, 0.54-0.88; P = .003) and a reduction in hospitalization (RR = 0.62; 95% CI, 0.47-0.82; P = .0006); younger (< 65 years) and male patients derive greater benefit.6,17 Indications for ablation in patients with heart failure are similar to those in patients without heart failure; ablation can therefore be considered for select heart failure patients who remain symptomatic or for whom AAD has failed.3
Older patients. Ablation can be considered for patients > 75 years with symptomatic paroxysmal AF refractory to AAD or who are intolerant of AAD.3 A study that assessed the benefit of catheter ablation reviewed 587 older (> 75 years) patients with AF, of whom 324 were eligible for ablation. Endpoints were maintenance of sinus rhythm, stroke, death, and major bleeding. Return to normal sinus rhythm was an independent factor, associated with a decrease in the risk of mortality among all patient groups that underwent ablation (HR = 0.36; 95% CI, 0.2-0.63; P = .0005). Age > 75 years (HR = 1.09; 95% CI, 1.01-1.16; P > .02) and depressed ejection fraction < 40% (HR = 2.38; 95% CI, 1.28-4.4; P = .006) were determined to be unfavorable parameters for survival.18
Complications and risks
Complications of catheter ablation for AF, although infrequent, can be severe (TABLE 33). Early mortality, defined as death during initial admission or 30-day readmission, occurs in approximately 0.5% of cases; half of deaths take place during readmission.11

Continue to: Complications vary...
Complications vary, based on the type and site of ablation.19,20 Cardiac tamponade or perforation, the most life-threatening complications, taken together occur in an estimated 1.9% of patients (odds ratio [OR] = 2.98; 95% CI, 1.36-6.56; P = .007).11 Other in-hospital complications independently predictive of death include any cardiac complications (OR = 12.8; 95% CI, 6.86 to 23.8; P < .001) and neurologic complications (cerebrovascular accident and transient ischemic attack) (OR = 8.72; 95% CI, 2.71-28.1; P < .001).
Other complications that do not cause death but might prolong the hospital stay include pericarditis without effusion, anesthesia-related complications, and vascular-access complications. Patients whose ablation is performed at an institution where the volume of ablations is low are also at higher risk of early mortality (OR = 2.35; 95% CI, 1.33-4.15; P = .003).16
Recurrence is common (TABLE 211-13). Risk of recurrence following ablation is significant; early (within 3 months after ablation) recurrence is seen in 50% of patients.21,22 However, this is a so-called "blanking period"—ie, a temporary period of inflammatory and proarrhythmic changes that are not predictors of later recurrence. The 5-year post-ablation recurrence rate is approximately 25.5%; longstanding persistent and persistent AF and the presence of comorbidities are major risk factors for recurrence.13,23
Recurrence is also associated with the type of procedure; pulmonary vein isolation, alone or in combination with another type of procedure, results in higher long-term success.21,23
Other variables affect outcome (TABLE 211-13). Following AF ablation, patients with nonparoxysmal AF at baseline, advanced age, sleep apnea and obesity, left atrial enlargement, and any structural heart disease tend to have a poorer long-term (5-year) outcome (ie, freedom from extended episodes of AF).3,13,23,24
Patients who undergo repeat procedures have higher arrhythmia-free survival; the highest ablation success rate is for patients with paroxysmal AF.13,23
Exposure to ionizing radiation. Fluoroscopy is required for multiple components of atrial mapping and ablation during RF ablation, including navigation, visualization, and monitoring of catheter placement. Patients undergoing this particular procedure therefore receive significant exposure to ionizing radiation. A reduction in, even complete elimination of, fluoroscopy has been achieved with:
- nonfluoroscopic 3-dimensional mapping systems25
- intracardiac echocardiography, which utilizes ultrasonographic imaging as the primary visual mode for tracking and manipulating the catheter
- robotic guided navigation.26-28
Continue to: CASE
CASE
At his return visit, Mr. Z says that he is concerned about, first, undergoing catheter ablation at his age and, second, the risks associated with the procedure. You explain that it is true that ablation is ideal in younger patients who have minimal comorbidities and that the risk of complications increases with age—but that there is no cutoff or absolute age contraindication to ablation.
You tell Mr. Z that you will work with him on risk-factor modification in anticipation of ablation. You also assure him that the decision whether to ablate must be a joint one—between him and a cardiologist experienced both in electrophysiology and in performing this highly technical procedure. And you explain that a highly practiced specialist can identify Mr. Z’s risk factors that might make ablation more difficult to perform and affect the long-term outcome.
With Mr. Z’s agreement, you screen for sleep apnea and start him on a lifestyle modification plan to achieve a more ideal weight, explaining that the risk of recurrence of AF after catheter ablation is increased by obesity and sleep apnea, in addition to age. You explain that, based on his CHA2DS2–VASc (congestive heart failure; hypertension; age, ≥ 75 years; diabetes; prior stroke, transient ischemic attack, or thromboembolism; vascular disease; age, 65 to 74 years; sex category) score of 3, he will remain on anticoagulation whether or not he has the ablation.
You refer the patient to the nearest high-volume cardiac ablation center.
Last, you caution Mr. Z that, based on his lipid levels, his 10-year risk of heart disease or stroke is elevated. You recommend treatment with a statin agent while he continues his other medications.
Delivering energy to myocardium
Myocardial tissue in pulmonary veins is responsible for most ectopic electrical impulses in patients with drug-refractory AF. The goal of catheter ablation in AF is destruction (scarring) of tissue that is the source of abnormal vein potentials.15
How RF ablation works. Ablation is most commonly performed using RF energy, a high-frequency form of electrical energy. Electrophysiology studies are carried out at the time of ablation by percutaneous, fluoroscopically guided insertion of 2 to 5 catheters, usually through the femoral or internal jugular vein, which are then positioned within several areas of the heart—usually, the right atrium, bundle of His, right ventricle, and coronary sinus.
Continue to: Electrical current...
Electrical current is applied through the catheters from an external generator to stimulate the myocardium and thus determine its electrophysiologic properties. The anatomic and electrical activity of the left atrium and pulmonary veins is then identified, a technique known as electro-anatomical mapping (FIGURE). After arrhythmogenic myocardial tissue is mapped, ablation is carried out with RF energy through the catheter to the pathogenic myocardium from which arrhythmias are initiated or conducted. The result is thermal destruction of tissue and creation of small, shallow lesions that vary in size with the type of catheter and the force of contact pressure applied.3,29

Other energy sources used in catheter ablation include cryothermal energy, which utilizes liquid nitrous oxide under pressure through a cryocatheter or cryoballoon catheter. Application of cryothermal energy freezes tissue and disrupts cell membranes and any electrical activity. Cryoballoon ablation has been shown to be similarly safe and efficacious as RF ablation in patients with paroxysmal AF.30,31
Newer laser-based balloon ablations are performed under ultrasonographic guidance and utilize arcs of laser energy delivered to the pathogenic myocardium.3
Thromboembolism prophylaxis
Oral anticoagulation to decrease the risk of stroke is initiated in all patients with AF, based on a thromboembolic risk profile determined by their CHA2DS2–VASc score, with anticoagulation recommended when the score is ≥ 2 in men and ≥ 3 in women. Options for anticoagulation include warfarin and one of the novel oral anticoagulants dabigatran, apixaban, rivaroxaban, and edoxaban.4 Recommendations are as follows3:
- For patients with a CHA2DS2–VASc score of ≥ 2 (men) or ≥ 3 (women), anticoagulation should be continued indefinitely, regardless of how successful the ablation procedure is.
- When patients choose to discontinue anticoagulation, they should be counseled in detail about the risk of doing so. The continued need for frequent arrhythmia monitoring should be emphasized.
The route from primary careto catheter ablation
Perform a thorough evaluation. Patients who present to you with palpitations should first undergo a routine workup for AF, followed by confirmation of the diagnosis. Exclude structural heart disease with echocardiography. Undertake monitoring, which is essential to determine whether symptoms are a reflection of the arrhythmia, using noncontinuous or continuous electrocardiographic (EKG) monitoring. Noncontinuous detection devices include:
- scheduled or symptom-initiated EKG
- a Holter monitor, worn for at least 24 hours and as long as 7 days
- trans-telephonic recordings and patient- or automatically activated devices
- an external loop recorder.32
Continuous EKG monitoring is more permanent (≥ 12 months). This is usually achieved through an implantable loop powered by a battery that lasts as long as 3 years.3
Ablation: Yes or no? Ablation is not recommended to avoid anticoagulation or when anticoagulation is contraindicated.5 With regard to specific patient criteria, the ideal patient:
- is symptomatic
- has failed AAD therapy
- does not have pulmonary disease
- has a normal or mildly dilated left atrium or normal or mildly reduced left ventricular ejection fraction.5
Continue to: There is no absolute age...
There is no absolute age or comorbidity contraindication to ablation. The patient should be referred to a cardiologist who has received appropriate training in electrophysiology, to identify comorbidities that (1) increase the technical difficulty of the procedure and baseline risk and (2) affect long-term outcome,12 and who performs the procedure in a center that has considerable experience with catheter ablation.33
Once the decision is made to perform ablation, you can provide strategies that optimize the outcome (freedom from AF episodes). Those tactics include weight loss and screening evaluation and, if indicated, treatment for sleep apnea.3
Protocol. Prior to the procedure, the patient fasts overnight; they might be asked to taper or discontinue cardiac medications that have electrophysiologic effects. Studies suggest a low risk of bleeding associated with catheter ablation; anticoagulation should therefore continue uninterrupted for patients undergoing catheter ablation for AF3,4,34,35; however, this practice varies with the cardiologist or electrophysiologist performing ablation.
Because of the length and complexity of the procedure, electro-anatomical mapping and ablation are conducted with the patient under general anesthesia.3 The patient is kept supine, and remains so for 2 to 4 hours afterward to allow for hemostasis at puncture sites.3
Patients might be monitored overnight, although same-day catheter ablation has been shown to be safe and cost-effective in select patients.36,37 Post ablation, patients follow up with the cardiologist and electrophysiologist. Long-term arrhythmia monitoring is required.3 Anticoagulation is continued for at least 2 months, and is discontinued based on the patient’s risk for stroke, utilizing their CHA2DS2–VASc score.3,4
CASE
At Mr. Z’s 6-month primary care follow-up, he confirms what has been reported to you as the referring physician: He had a successful catheter ablation and continues to have regular follow-up monitoring with the cardiologist. He is no longer taking amiodarone.
At this visit, he reports no recurrence of AF-associated symptoms or detectable AF on cardiac monitoring. He has lost 8 lbs. You counsel to him to continue to maintain a healthy lifestyle.
CORRESPONDENCE
Amimi S. Osayande MD, FAAFP, Northside-Gwinnett Family Medicine Residency Program, Strickland Family Medicine Center, 665 Duluth Highway, Suite 501, Lawrenceville, GA 30046; [email protected]
CASE
Jack Z, a 75-year-old man with well-controlled hypertension, diabetes controlled by diet, and atrial fibrillation (AF) presents to the family medicine clinic to establish care with you after moving to the community from out of town.
The patient describes a 1-year history of AF. He provides you with an echocardiography report from 6 months ago that shows no evidence of structural heart disease. He takes lisinopril, to control blood pressure; an anticoagulant; a beta-blocker; and amiodarone for rhythm control. Initially, he took flecainide, which was ineffective for rhythm control, before being switched to amiodarone. He had 2 cardioversion procedures, each time after episodes of symptoms. He does not smoke or drink alcohol.
Mr. Z describes worsening palpitations and shortness of breath over the past 9 months. Symptoms now include episodes of exertional fatigue, even when he is not having palpitations. Prior to the episodes of worsening symptoms, he tells you that he lived a “fairly active” life, golfing twice a week.
The patient’s previous primary care physician had encouraged him to talk to his cardiologist about “other options” for managing AF, because levels of his liver enzymes had started to rise (a known adverse effect of amiodarone1) when measured 3 months ago. He did not undertake that conversation, but asks you now about other treatments for AF.
Atrial fibrillation is the most common sustained cardiac arrhythmia, characterized by discordant electrical activation of the atria due to structural or electrophysiological abnormalities, or both. The disorder is associated with an increased rate of stroke and heart failure and is independently associated with a 1.5- to 2-fold risk of all-cause mortality.2
In this article, we review the pathophysiology of AF; management, including the role of, and indications for, catheter ablation; and patient- and disease-related factors associated with ablation (including odds of success, complications, risk of recurrence, and continuing need for thromboprophylaxis) that family physicians should consider when contemplating referral to a cardiologist or electrophysiologist for catheter ablation for AF.
What provokes AF?
AF is thought to occur as a result of an interaction among 3 phenomena:
- enhanced automaticity of abnormal atrial tissue
- triggered activity of ectopic foci within 1 or more pulmonary veins, lying within the left atrium
- re-entry, in which there is propagation of electrical impulses from an ectopic beat through another pathway.
Continue to: In patients who progress...
In patients who progress from paroxysmal to persistent AF (see “Subtypes,” below), 2 distinct pathways, facilitated by the presence of abnormal tissue, continuously activate one another, thus maintaining the arrhythmia. Myocardial tissue in the pulmonary veins is responsible for most ectopic electrical impulses in patients with drug-refractory AF (see “Rhythm control”).
Subtypes. For the purpose of planning treatment, AF is classified as:
- Paroxysmal. Terminates spontaneously or with intervention ≤ 7 days after onset.
- Persistent. Continuous and sustained for > 7 days.
- Longstanding persistent. Continuous for > 12 months.
- Permanent. The patient and physician accept that there will be no further attempt to restore or maintain sinus rhythm.
Goals of treatment
Primary management goals in patients with AF are 2-fold: control of symptoms and prevention of thromboembolism. A patient with new-onset AF who presents acutely with inadequate rate control and hemodynamic compromise requires urgent assessment to determine the cause of the arrhythmia and need for cardioversion.3 A symptomatic patient with AF who does not have high-risk features (eg, valvular heart disease, mechanical valves) might be a candidate for rhythm control in addition to rate control.3,4
Rate control. After evaluation in the hospital, a patient who has a rapid ventricular response but remains hemodynamically stable, without evidence of heart failure, should be initiated on a rate-controlling medication, such as a beta-blocker or nondihydropyridine calcium-channel blocker. A resting heart rate goal of < 80 beats per minute (bpm) is recommended for a symptomatic patient with AF. The heart rate goal can be relaxed, to < 110 bpm, in an asymptomatic patient with preserved left ventricular function.5,6
Rhythm control, indicated in patients who remain symptomatic on rate-controlling medication, can be achieved either with an antiarrhythmic drug (AAD) or by catheter ablation.4,5 In stable patients, rhythm control should be considered only after a thorough work-up for a reversible cause of AF, and can be achieved with an oral AAD or, in select patients, through catheter ablation (TABLE 13,6). Other indications for chronic rhythm control include treatment of patients with tachycardia-induced cardiomyopathy.5

A major study that documented the benefit of early rhythm control evaluated long-term outcomes in 2789 patients with AF who were undergoing catheter ablation.7 Patients were randomized to early rhythm control (catheter ablation or AAD) or “usual care”—ie, in this study, rhythm control limited to symptomatic patients. Primary outcomes were death from cardiovascular causes, stroke, and hospitalization with worsening heart failure or acute coronary syndrome. A first primary outcome event occurred in 249 patients (3.9/100 person-years) assigned to early rhythm control, compared to 316 (5.0 per 100 person-years) in the group assigned to usual care.
The study was terminated early (after 5.1 years) because of overwhelming evidence of efficacy (number need to treat = 7). Although early rhythm control was obtained through both catheter ablation and AAD (hazard ratio [HR] = 0.79; 96% CI, 0.66-0.94; P = .005), success was attributed to the use of catheter ablation for a rhythm-control strategy and its use among patients whose AF was present for < 1 year. Most patients in both treatment groups continued to receive anticoagulation, rate control, and optimization of cardiovascular risk.7
Continue to: Notably, direct studies...
Notably, direct studies comparing ablation and AAD have not confirmed the benefit of ablation over AAD in outcomes of all-cause mortality, bleeding, stroke, or cardiac arrest over a 5-year period.8
Adverse effects and mortality outcomes with AAD. Concern over using AAD for rhythm control is based mostly on adverse effects and long-term (1-year) mortality outcomes. Long-term AAD therapy has been shown to decrease the recurrence of AF—but without evidence to suggest other mortality benefits.
A meta-analysis of 59 randomized controlled trials reviewed 20,981 patients receiving AAD (including quinidine, disopyramide, propafenone, flecainide, metoprolol, amiodarone, dofetilide, dronedarone, and sotalol) for long-term effects on death, stroke, adverse reactions, and recurrence of AF.9 Findings at 10 months suggest that:
- Compared to placebo, amiodarone and sotalol increased the risk of all-cause mortality during the study period.
- There was minimal difference in mortality among patients taking dofetilide or dronedarone, compared to placebo.
- There were insufficient data to draw conclusions about the effect of disopyramide, flecainide, and propafenone on mortality.
Before starting a patient on AAD, the risk of arrhythmias and the potential for these agents to cause toxicity and adverse events should always be discussed.
CASE
You tell Mr. Z that you need to know the status of his comorbidities to make a recommendation about “other” management options, and proceed to take a detailed history.
Recent history. Mr. Z reveals that “today is a good day”: He has had “only 1” episode of palpitations, which resolved on its own. The previous episode, he explains, was 3 days ago, when palpitations were associated with lightheadedness and shortness of breath. He denies chest pains or swelling of the legs.
Physical exam. The patient appears spry, comfortable, and in no acute distress. Vital signs are within normal limits. A body mass index of 28.4 puts him in the “overweight” category. His blood pressure is 118/75 mm Hg.
Continue to: Cardiac examination...
Cardiac examination is significant for an irregular rhythm without murmurs, rubs, or gallops. His lungs are clear bilaterally; his abdomen is soft and nondistended. His extremities show no edema.
Testing. You obtain an electrocardiogram, which demonstrates a controlled ventricular rate of 88 bpm and AF. You order a complete blood count, comprehensive metabolic panel, tests of hemoglobin A1C and thyroid-stimulating hormone, lipid panel, echocardiogram, and a chest radiograph.
Results. The chest radiograph is negative for an acute cardiopulmonary process; cardiac size is normal. Aspartate aminotransferase and alanine aminotransferase levels are higher than twice the normal limit. The echocardiogram reveals an estimated left ventricular ejection fraction of 55% to 60%; no structural abnormalities are noted.
In which AF patients is catheter ablation indicated?
Ablation is recommended for select patients (TABLE 13,6) with symptomatic paroxysmal AF that is refractory to AAD or who are intolerant of AAD.3,6 It is a reasonable first-line therapy for high-performing athletes in whom AAD would affect athletic performance.3,10 It is also a reasonable option in select patients > 75 years and as an alternative to AAD therapy.3 Finally, catheter ablation should be considered in symptomatic patients with longstanding persistent AF and congestive heart failure, with or without reduced left ventricular ejection fraction.3
CASE
You inform Mr. Z that his symptoms are likely a result of symptomatic paroxysmal AF, which was refractory to flecainide and amiodarone, and that his abnormal liver function test results preclude continued use of amiodarone. You propose Holter monitoring to correlate timing of symptoms with the arrhythmia, but he reports this has been done, and the correlation confirmed, by his previous physician.
You explain that, because the diagnosis of symptomatic paroxysmal AF refractory to AADs has been confirmed, he is categorized as a patient who might benefit from catheter ablation, based on:
- the type of AF (ie, paroxysmal AF is associated with better ablation outcomes)
- persistent symptoms that are refractory to AADs
- his intolerance of AAD
- the length of time since onset of symptoms.
Mr. Z agrees to consider your recommendation.
Continue to: What are the benefits of catheter ablation?
What are the benefits of catheter ablation?
Ablation can be achieved through radiofrequency (RF) ablation, cryoablation, or newer, laser-based balloon ablation. Primary outcomes used to determine the success of any options for performing ablation include mortality, stroke, and hospitalization. Other endpoints include maintenance of sinus rhythm, freedom from AF, reduction in AF burden (estimated through patients’ report of symptoms, recurrence rate, need for a second ablation procedure, and serial long-term monitoring through an implantable cardiac monitoring device), quality of life, and prevention of AF progression.3
Patient and disease variables (TABLE 211-13). The success rate of catheter ablation, defined as freedom from either symptomatic or asymptomatic episodes of AF, is dependent on several factors,3,14 including:
- type of AF (paroxysmal or persistent)
- duration and degree of symptoms
- age
- sex
- comorbidities, including heart failure and structural heart or lung disease.

Overall, in patients with paroxysmal AF, an estimated 75% are symptom free 1 year after ablation.15 Patients with persistent and longstanding persistent AF experience a lower success rate.
RF catheter ablation has demonstrated superiority to AAD in reducing the need for cardioversion (relative risk [RR] = 0.62; 95% CI, 0.47-0.82) and cardiac-related hospitalization (RR = 0.27; 95% CI, 0.10-0.72;) at 12 months in patients with nonparoxysmal AF (persistent or longstanding persistent).16
Effect on mortality. Among patients with heart failure with reduced ejection fraction, long-term studies of cardiovascular outcomes 5 years post ablation concluded that ablation is associated with a decrease in all-cause mortality (RR = 0.69; 95% CI, 0.54-0.88; P = .003) and a reduction in hospitalization (RR = 0.62; 95% CI, 0.47-0.82; P = .0006); younger (< 65 years) and male patients derive greater benefit.6,17 Indications for ablation in patients with heart failure are similar to those in patients without heart failure; ablation can therefore be considered for select heart failure patients who remain symptomatic or for whom AAD has failed.3
Older patients. Ablation can be considered for patients > 75 years with symptomatic paroxysmal AF refractory to AAD or who are intolerant of AAD.3 A study that assessed the benefit of catheter ablation reviewed 587 older (> 75 years) patients with AF, of whom 324 were eligible for ablation. Endpoints were maintenance of sinus rhythm, stroke, death, and major bleeding. Return to normal sinus rhythm was an independent factor, associated with a decrease in the risk of mortality among all patient groups that underwent ablation (HR = 0.36; 95% CI, 0.2-0.63; P = .0005). Age > 75 years (HR = 1.09; 95% CI, 1.01-1.16; P > .02) and depressed ejection fraction < 40% (HR = 2.38; 95% CI, 1.28-4.4; P = .006) were determined to be unfavorable parameters for survival.18
Complications and risks
Complications of catheter ablation for AF, although infrequent, can be severe (TABLE 33). Early mortality, defined as death during initial admission or 30-day readmission, occurs in approximately 0.5% of cases; half of deaths take place during readmission.11

Continue to: Complications vary...
Complications vary, based on the type and site of ablation.19,20 Cardiac tamponade or perforation, the most life-threatening complications, taken together occur in an estimated 1.9% of patients (odds ratio [OR] = 2.98; 95% CI, 1.36-6.56; P = .007).11 Other in-hospital complications independently predictive of death include any cardiac complications (OR = 12.8; 95% CI, 6.86 to 23.8; P < .001) and neurologic complications (cerebrovascular accident and transient ischemic attack) (OR = 8.72; 95% CI, 2.71-28.1; P < .001).
Other complications that do not cause death but might prolong the hospital stay include pericarditis without effusion, anesthesia-related complications, and vascular-access complications. Patients whose ablation is performed at an institution where the volume of ablations is low are also at higher risk of early mortality (OR = 2.35; 95% CI, 1.33-4.15; P = .003).16
Recurrence is common (TABLE 211-13). Risk of recurrence following ablation is significant; early (within 3 months after ablation) recurrence is seen in 50% of patients.21,22 However, this is a so-called "blanking period"—ie, a temporary period of inflammatory and proarrhythmic changes that are not predictors of later recurrence. The 5-year post-ablation recurrence rate is approximately 25.5%; longstanding persistent and persistent AF and the presence of comorbidities are major risk factors for recurrence.13,23
Recurrence is also associated with the type of procedure; pulmonary vein isolation, alone or in combination with another type of procedure, results in higher long-term success.21,23
Other variables affect outcome (TABLE 211-13). Following AF ablation, patients with nonparoxysmal AF at baseline, advanced age, sleep apnea and obesity, left atrial enlargement, and any structural heart disease tend to have a poorer long-term (5-year) outcome (ie, freedom from extended episodes of AF).3,13,23,24
Patients who undergo repeat procedures have higher arrhythmia-free survival; the highest ablation success rate is for patients with paroxysmal AF.13,23
Exposure to ionizing radiation. Fluoroscopy is required for multiple components of atrial mapping and ablation during RF ablation, including navigation, visualization, and monitoring of catheter placement. Patients undergoing this particular procedure therefore receive significant exposure to ionizing radiation. A reduction in, even complete elimination of, fluoroscopy has been achieved with:
- nonfluoroscopic 3-dimensional mapping systems25
- intracardiac echocardiography, which utilizes ultrasonographic imaging as the primary visual mode for tracking and manipulating the catheter
- robotic guided navigation.26-28
Continue to: CASE
CASE
At his return visit, Mr. Z says that he is concerned about, first, undergoing catheter ablation at his age and, second, the risks associated with the procedure. You explain that it is true that ablation is ideal in younger patients who have minimal comorbidities and that the risk of complications increases with age—but that there is no cutoff or absolute age contraindication to ablation.
You tell Mr. Z that you will work with him on risk-factor modification in anticipation of ablation. You also assure him that the decision whether to ablate must be a joint one—between him and a cardiologist experienced both in electrophysiology and in performing this highly technical procedure. And you explain that a highly practiced specialist can identify Mr. Z’s risk factors that might make ablation more difficult to perform and affect the long-term outcome.
With Mr. Z’s agreement, you screen for sleep apnea and start him on a lifestyle modification plan to achieve a more ideal weight, explaining that the risk of recurrence of AF after catheter ablation is increased by obesity and sleep apnea, in addition to age. You explain that, based on his CHA2DS2–VASc (congestive heart failure; hypertension; age, ≥ 75 years; diabetes; prior stroke, transient ischemic attack, or thromboembolism; vascular disease; age, 65 to 74 years; sex category) score of 3, he will remain on anticoagulation whether or not he has the ablation.
You refer the patient to the nearest high-volume cardiac ablation center.
Last, you caution Mr. Z that, based on his lipid levels, his 10-year risk of heart disease or stroke is elevated. You recommend treatment with a statin agent while he continues his other medications.
Delivering energy to myocardium
Myocardial tissue in pulmonary veins is responsible for most ectopic electrical impulses in patients with drug-refractory AF. The goal of catheter ablation in AF is destruction (scarring) of tissue that is the source of abnormal vein potentials.15
How RF ablation works. Ablation is most commonly performed using RF energy, a high-frequency form of electrical energy. Electrophysiology studies are carried out at the time of ablation by percutaneous, fluoroscopically guided insertion of 2 to 5 catheters, usually through the femoral or internal jugular vein, which are then positioned within several areas of the heart—usually, the right atrium, bundle of His, right ventricle, and coronary sinus.
Continue to: Electrical current...
Electrical current is applied through the catheters from an external generator to stimulate the myocardium and thus determine its electrophysiologic properties. The anatomic and electrical activity of the left atrium and pulmonary veins is then identified, a technique known as electro-anatomical mapping (FIGURE). After arrhythmogenic myocardial tissue is mapped, ablation is carried out with RF energy through the catheter to the pathogenic myocardium from which arrhythmias are initiated or conducted. The result is thermal destruction of tissue and creation of small, shallow lesions that vary in size with the type of catheter and the force of contact pressure applied.3,29

Other energy sources used in catheter ablation include cryothermal energy, which utilizes liquid nitrous oxide under pressure through a cryocatheter or cryoballoon catheter. Application of cryothermal energy freezes tissue and disrupts cell membranes and any electrical activity. Cryoballoon ablation has been shown to be similarly safe and efficacious as RF ablation in patients with paroxysmal AF.30,31
Newer laser-based balloon ablations are performed under ultrasonographic guidance and utilize arcs of laser energy delivered to the pathogenic myocardium.3
Thromboembolism prophylaxis
Oral anticoagulation to decrease the risk of stroke is initiated in all patients with AF, based on a thromboembolic risk profile determined by their CHA2DS2–VASc score, with anticoagulation recommended when the score is ≥ 2 in men and ≥ 3 in women. Options for anticoagulation include warfarin and one of the novel oral anticoagulants dabigatran, apixaban, rivaroxaban, and edoxaban.4 Recommendations are as follows3:
- For patients with a CHA2DS2–VASc score of ≥ 2 (men) or ≥ 3 (women), anticoagulation should be continued indefinitely, regardless of how successful the ablation procedure is.
- When patients choose to discontinue anticoagulation, they should be counseled in detail about the risk of doing so. The continued need for frequent arrhythmia monitoring should be emphasized.
The route from primary careto catheter ablation
Perform a thorough evaluation. Patients who present to you with palpitations should first undergo a routine workup for AF, followed by confirmation of the diagnosis. Exclude structural heart disease with echocardiography. Undertake monitoring, which is essential to determine whether symptoms are a reflection of the arrhythmia, using noncontinuous or continuous electrocardiographic (EKG) monitoring. Noncontinuous detection devices include:
- scheduled or symptom-initiated EKG
- a Holter monitor, worn for at least 24 hours and as long as 7 days
- trans-telephonic recordings and patient- or automatically activated devices
- an external loop recorder.32
Continuous EKG monitoring is more permanent (≥ 12 months). This is usually achieved through an implantable loop powered by a battery that lasts as long as 3 years.3
Ablation: Yes or no? Ablation is not recommended to avoid anticoagulation or when anticoagulation is contraindicated.5 With regard to specific patient criteria, the ideal patient:
- is symptomatic
- has failed AAD therapy
- does not have pulmonary disease
- has a normal or mildly dilated left atrium or normal or mildly reduced left ventricular ejection fraction.5
Continue to: There is no absolute age...
There is no absolute age or comorbidity contraindication to ablation. The patient should be referred to a cardiologist who has received appropriate training in electrophysiology, to identify comorbidities that (1) increase the technical difficulty of the procedure and baseline risk and (2) affect long-term outcome,12 and who performs the procedure in a center that has considerable experience with catheter ablation.33
Once the decision is made to perform ablation, you can provide strategies that optimize the outcome (freedom from AF episodes). Those tactics include weight loss and screening evaluation and, if indicated, treatment for sleep apnea.3
Protocol. Prior to the procedure, the patient fasts overnight; they might be asked to taper or discontinue cardiac medications that have electrophysiologic effects. Studies suggest a low risk of bleeding associated with catheter ablation; anticoagulation should therefore continue uninterrupted for patients undergoing catheter ablation for AF3,4,34,35; however, this practice varies with the cardiologist or electrophysiologist performing ablation.
Because of the length and complexity of the procedure, electro-anatomical mapping and ablation are conducted with the patient under general anesthesia.3 The patient is kept supine, and remains so for 2 to 4 hours afterward to allow for hemostasis at puncture sites.3
Patients might be monitored overnight, although same-day catheter ablation has been shown to be safe and cost-effective in select patients.36,37 Post ablation, patients follow up with the cardiologist and electrophysiologist. Long-term arrhythmia monitoring is required.3 Anticoagulation is continued for at least 2 months, and is discontinued based on the patient’s risk for stroke, utilizing their CHA2DS2–VASc score.3,4
CASE
At Mr. Z’s 6-month primary care follow-up, he confirms what has been reported to you as the referring physician: He had a successful catheter ablation and continues to have regular follow-up monitoring with the cardiologist. He is no longer taking amiodarone.
At this visit, he reports no recurrence of AF-associated symptoms or detectable AF on cardiac monitoring. He has lost 8 lbs. You counsel to him to continue to maintain a healthy lifestyle.
CORRESPONDENCE
Amimi S. Osayande MD, FAAFP, Northside-Gwinnett Family Medicine Residency Program, Strickland Family Medicine Center, 665 Duluth Highway, Suite 501, Lawrenceville, GA 30046; [email protected]
1. Amiodarone hydrochloride (marketed as Cordarone and Pacerone) information. Silver Spring, Md.: US Food & Drug Administration. Reviewed March 23, 2015. Accessed January 16, 2022. www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/amiodarone-hydrochloride-marketed-cordarone-and-pacerone-information
2. Gómez-Outes A, Suárez-Gea ML,García-Pinilla JM. Causes of death in atrial fibrillation: challenges and opportunities. Trends Cardiovasc Med. 2017;27:494-503. doi: 10.1016/j.tcm.2017.05.002
3. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/ APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Arrhythm. 2017;33:369-409. doi: 10.1016/j.joa.2017.08.001
4. Camm AJ, Lip GYH, De Caterina R, et al; ESC Committee for Practice Guidelines-CPG; Document Reviewers. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation—developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14:1385-1413. doi: 10.1093/europace/eus305
5. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071-2104. doi: 10.1161/CIR.0000000000000040
6. January CT, Wann LS, Calkins H, et al; Writing Group Members. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/ HRS guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019;16:e66-e93. doi: 10.1016/j.hrthm.2019.01.024
7. Kirchhof P, Camm AJ, Goette A, et al; EAST-AFNET 4 Trial Investigators. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305-1316. doi: 10.1056/ NEJMoa2019422
8. Packer DL, Mark DB, Robb RA, et al; CABANA Investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261-1274. doi: 10.1001/jama.2019.0693
9. Valembois L, Audureau E, Takeda A, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2019;9:CD005049. doi: 10.1002/14651858.CD005049
10. Koopman P, Nuyens D, Garweg C, et al. Efficacy of radiofrequency catheter ablation in athletes with atrial fibrillation. Europace. 2011;13:1386-1393. doi: 10.1093/europace/eur142
11. Hakalahti A, Biancari F, Nielsen JC, et al. Radiofrequency ablation vs. antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: systematic review and meta-analysis. Europace. 2015;17:370-378. doi: 10.1093/europace/euu376
12. Nyong J, Amit G, Adler AJ, et al. Efficacy and safety of ablation for people with non-paroxysmal atrial fibrillation. Cochrane Database Syst Rev. 2016;11:CD012088. doi: 10.1002/14651858. CD012088.pub2
13. Andrade JG, Champagne J, Dubuc M, et al; CIRCA-DOSE Study Investigators. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. 2019;140:1779-1788. doi: 10.1161/ CIRCULATIONAHA.119.042622
14. Asad ZUA, Yousif A, Khan MS, et al. Catheter ablation versus medical therapy for atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. Circ Arrhythm Electrophysiol. 2019;12:e007414. doi: 10.1161/ CIRCEP.119.007414
15. Nademanee K, Amnueypol M, Lee F, et al. Benefits and risks of catheter ablation in elderly patients with atrial fibrillation. Heart Rhythm. 2015;12:44-51. doi: 10.1016/j.hrthm.2014.09.049
16. Cheng EP, Liu CF, Yeo I, et al. Risk of mortality following catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2019;74: 2254-2264. doi: 10.1016/j.jacc.2019.08.1036
17. Brugada J, Katritsis DG, Arbelo E, et al; ESC Scientific Document Group. 2019 ESC Guidelines for the management of patients with supraventricular tachycardia. The Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Developed in collaboration with the Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2020;41:655-720. doi: 10.1093/eurheartj/ehz467
18. Hosseini SM, Rozen G, Saleh A, et al. Catheter ablation for cardiac arrhythmias: utilization and in-hospital complications, 2000 to 2013. JACC Clin Electrophysiol. 2017;3:1240-1248. doi: 10.1016/j.jacep.2017.05.005
19. Andrade JG, Macle L, Khairy P, et al. Incidence and significance of early recurrences associated with different ablation strategies for AF: a STAR-AF substudy. J Cardiovasc Electrophysiol. 2012;23:1295-1301. doi: 10.1111/j.1540-8167.2012.02399.x
20. Joshi S, Choi AD, Kamath GS, et al. Prevalence, predictors, and prognosis of atrial fibrillation early after pulmonary vein isolation: findings from 3 months of continuous automatic ECG loop recordings. J Cardiovasc Electrophysiol. 2009;20:1089-1094. doi: 10.1111/j.1540-8167.2009.01506.x
21. Weerasooriya R, Khairy P, Litalien J, et al. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160-166. doi: 10.1016/j.jacc.2010.05.061
22. Ouyang F, Tilz R, Chun J, et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation. 2010;122:2368-2377. doi: 10.1161/ CIRCULATIONAHA.110.946806
23. Tilz RR, Rillig A, Thum A-M, et al. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J Am Coll Cardiol. 2012;60: 1921-1929. doi: 10.1016/j.jacc.2012.04.060
24. Forkmann M, Schwab C, Busch S. [Catheter ablation of supraventricular tachycardia]. Herzschrittmacherther Elektrophysiol. 2019;30:336-342. doi: 10.1007/s00399-019-00654-x
25. Bulava A, Hanis J, Eisenberger M. Catheter ablation of atrial fibrillation using zero-fluoroscopy technique: a randomized trial. Pacing Clin Electrophysiol. 2015;38:797-806. doi: 10.1111/pace.12634
26. Haegeli LM, Stutz L, Mohsen M, et al. Feasibility of zero or near zero fluoroscopy during catheter ablation procedures. Cardiol J. 2019;26:226-232. doi: 10.5603/CJ.a2018.0029
27. Steven D, Servatius H, Rostock T, et al. Reduced fluoroscopy during atrial fibrillation ablation: benefits of robotic guided navigation. J Cardiovasc Electrophysiol. 2010;21:6-12. doi: 10.1111/j.1540-8167.2009.01592.x
28. General therapy for cardiac arrhythmias. In: Zipes DP, Libby P, Bonow RO, et al. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 11th ed. Elsevier; 2019.
29. Kuck K-H, Brugada J, Albenque J-P. Cryoballoon or radiofrequency ablation for atrial fibrillation. N Engl J Med. 2016;375: 1100-1101. doi: 10.1056/NEJMc1609160
30. Chen Y-H, Lu Z-Y, Xiang Y, et al. Cryoablation vs. radiofrequency ablation for treatment of paroxysmal atrial fibrillation: a systematic review and meta-analysis. Europace. 2017;19:784-794. doi: 10.1093/europace/euw330
31. Locati ET, Vecchi AM, Vargiu S, et al. Role of extended external loop recorders for the diagnosis of unexplained syncope, presyncope, and sustained palpitations. Europace. 2014;16:914-922. doi: 10.1093/europace/eut337
32. Calkins H, Kuck KH, Cappato R, et al; Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2012;9:632-696.e21. doi: 10.1016/j.hrthm.2011.12.016
33. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609-1678. doi: 10.1093/ europace/euw295
34. Nairooz R, Sardar P, Payne J, et al. Meta-analysis of major bleeding with uninterrupted warfarin compared to interrupted warfarin and heparin bridging in ablation of atrial fibrillation. Int J Cardiol. 2015;187:426-429. doi: 10.1016/j.ijcard.2015.03.376
35. Romero J, Cerrud-Rodriguez RC, Diaz JC, et al. Uninterrupted direct oral anticoagulants vs. uninterrupted vitamin K antagonists during catheter ablation of non-valvular atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. Europace. 2018;20:1612-1620. doi: 10.1093/europace/euy133
36. Deyell MW, Leather RA, Macle L, et al. Efficacy and safety of same-day discharge for atrial fibrillation ablation. JACC Clin Electrophysiol. 2020;6:609-619. doi: 10.1016/j.jacep.2020.02.009
37. Theodoreson MD, Chohan BC, McAloon CJ, et al. Same-day cardiac catheter ablation is safe and cost-effective: experience from a UK tertiary center. Heart Rhythm. 2015;12:1756-1761. doi: 10.1016/j.hrthm.2015.05.006
1. Amiodarone hydrochloride (marketed as Cordarone and Pacerone) information. Silver Spring, Md.: US Food & Drug Administration. Reviewed March 23, 2015. Accessed January 16, 2022. www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/amiodarone-hydrochloride-marketed-cordarone-and-pacerone-information
2. Gómez-Outes A, Suárez-Gea ML,García-Pinilla JM. Causes of death in atrial fibrillation: challenges and opportunities. Trends Cardiovasc Med. 2017;27:494-503. doi: 10.1016/j.tcm.2017.05.002
3. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/ APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Arrhythm. 2017;33:369-409. doi: 10.1016/j.joa.2017.08.001
4. Camm AJ, Lip GYH, De Caterina R, et al; ESC Committee for Practice Guidelines-CPG; Document Reviewers. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation—developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14:1385-1413. doi: 10.1093/europace/eus305
5. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071-2104. doi: 10.1161/CIR.0000000000000040
6. January CT, Wann LS, Calkins H, et al; Writing Group Members. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/ HRS guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019;16:e66-e93. doi: 10.1016/j.hrthm.2019.01.024
7. Kirchhof P, Camm AJ, Goette A, et al; EAST-AFNET 4 Trial Investigators. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305-1316. doi: 10.1056/ NEJMoa2019422
8. Packer DL, Mark DB, Robb RA, et al; CABANA Investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261-1274. doi: 10.1001/jama.2019.0693
9. Valembois L, Audureau E, Takeda A, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2019;9:CD005049. doi: 10.1002/14651858.CD005049
10. Koopman P, Nuyens D, Garweg C, et al. Efficacy of radiofrequency catheter ablation in athletes with atrial fibrillation. Europace. 2011;13:1386-1393. doi: 10.1093/europace/eur142
11. Hakalahti A, Biancari F, Nielsen JC, et al. Radiofrequency ablation vs. antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: systematic review and meta-analysis. Europace. 2015;17:370-378. doi: 10.1093/europace/euu376
12. Nyong J, Amit G, Adler AJ, et al. Efficacy and safety of ablation for people with non-paroxysmal atrial fibrillation. Cochrane Database Syst Rev. 2016;11:CD012088. doi: 10.1002/14651858. CD012088.pub2
13. Andrade JG, Champagne J, Dubuc M, et al; CIRCA-DOSE Study Investigators. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. 2019;140:1779-1788. doi: 10.1161/ CIRCULATIONAHA.119.042622
14. Asad ZUA, Yousif A, Khan MS, et al. Catheter ablation versus medical therapy for atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. Circ Arrhythm Electrophysiol. 2019;12:e007414. doi: 10.1161/ CIRCEP.119.007414
15. Nademanee K, Amnueypol M, Lee F, et al. Benefits and risks of catheter ablation in elderly patients with atrial fibrillation. Heart Rhythm. 2015;12:44-51. doi: 10.1016/j.hrthm.2014.09.049
16. Cheng EP, Liu CF, Yeo I, et al. Risk of mortality following catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2019;74: 2254-2264. doi: 10.1016/j.jacc.2019.08.1036
17. Brugada J, Katritsis DG, Arbelo E, et al; ESC Scientific Document Group. 2019 ESC Guidelines for the management of patients with supraventricular tachycardia. The Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Developed in collaboration with the Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2020;41:655-720. doi: 10.1093/eurheartj/ehz467
18. Hosseini SM, Rozen G, Saleh A, et al. Catheter ablation for cardiac arrhythmias: utilization and in-hospital complications, 2000 to 2013. JACC Clin Electrophysiol. 2017;3:1240-1248. doi: 10.1016/j.jacep.2017.05.005
19. Andrade JG, Macle L, Khairy P, et al. Incidence and significance of early recurrences associated with different ablation strategies for AF: a STAR-AF substudy. J Cardiovasc Electrophysiol. 2012;23:1295-1301. doi: 10.1111/j.1540-8167.2012.02399.x
20. Joshi S, Choi AD, Kamath GS, et al. Prevalence, predictors, and prognosis of atrial fibrillation early after pulmonary vein isolation: findings from 3 months of continuous automatic ECG loop recordings. J Cardiovasc Electrophysiol. 2009;20:1089-1094. doi: 10.1111/j.1540-8167.2009.01506.x
21. Weerasooriya R, Khairy P, Litalien J, et al. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160-166. doi: 10.1016/j.jacc.2010.05.061
22. Ouyang F, Tilz R, Chun J, et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation. 2010;122:2368-2377. doi: 10.1161/ CIRCULATIONAHA.110.946806
23. Tilz RR, Rillig A, Thum A-M, et al. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J Am Coll Cardiol. 2012;60: 1921-1929. doi: 10.1016/j.jacc.2012.04.060
24. Forkmann M, Schwab C, Busch S. [Catheter ablation of supraventricular tachycardia]. Herzschrittmacherther Elektrophysiol. 2019;30:336-342. doi: 10.1007/s00399-019-00654-x
25. Bulava A, Hanis J, Eisenberger M. Catheter ablation of atrial fibrillation using zero-fluoroscopy technique: a randomized trial. Pacing Clin Electrophysiol. 2015;38:797-806. doi: 10.1111/pace.12634
26. Haegeli LM, Stutz L, Mohsen M, et al. Feasibility of zero or near zero fluoroscopy during catheter ablation procedures. Cardiol J. 2019;26:226-232. doi: 10.5603/CJ.a2018.0029
27. Steven D, Servatius H, Rostock T, et al. Reduced fluoroscopy during atrial fibrillation ablation: benefits of robotic guided navigation. J Cardiovasc Electrophysiol. 2010;21:6-12. doi: 10.1111/j.1540-8167.2009.01592.x
28. General therapy for cardiac arrhythmias. In: Zipes DP, Libby P, Bonow RO, et al. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 11th ed. Elsevier; 2019.
29. Kuck K-H, Brugada J, Albenque J-P. Cryoballoon or radiofrequency ablation for atrial fibrillation. N Engl J Med. 2016;375: 1100-1101. doi: 10.1056/NEJMc1609160
30. Chen Y-H, Lu Z-Y, Xiang Y, et al. Cryoablation vs. radiofrequency ablation for treatment of paroxysmal atrial fibrillation: a systematic review and meta-analysis. Europace. 2017;19:784-794. doi: 10.1093/europace/euw330
31. Locati ET, Vecchi AM, Vargiu S, et al. Role of extended external loop recorders for the diagnosis of unexplained syncope, presyncope, and sustained palpitations. Europace. 2014;16:914-922. doi: 10.1093/europace/eut337
32. Calkins H, Kuck KH, Cappato R, et al; Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2012;9:632-696.e21. doi: 10.1016/j.hrthm.2011.12.016
33. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609-1678. doi: 10.1093/ europace/euw295
34. Nairooz R, Sardar P, Payne J, et al. Meta-analysis of major bleeding with uninterrupted warfarin compared to interrupted warfarin and heparin bridging in ablation of atrial fibrillation. Int J Cardiol. 2015;187:426-429. doi: 10.1016/j.ijcard.2015.03.376
35. Romero J, Cerrud-Rodriguez RC, Diaz JC, et al. Uninterrupted direct oral anticoagulants vs. uninterrupted vitamin K antagonists during catheter ablation of non-valvular atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. Europace. 2018;20:1612-1620. doi: 10.1093/europace/euy133
36. Deyell MW, Leather RA, Macle L, et al. Efficacy and safety of same-day discharge for atrial fibrillation ablation. JACC Clin Electrophysiol. 2020;6:609-619. doi: 10.1016/j.jacep.2020.02.009
37. Theodoreson MD, Chohan BC, McAloon CJ, et al. Same-day cardiac catheter ablation is safe and cost-effective: experience from a UK tertiary center. Heart Rhythm. 2015;12:1756-1761. doi: 10.1016/j.hrthm.2015.05.006
PRACTICE RECOMMENDATIONS
› Refer patients with atrial fibrillation (AF) to Cardiology for consideration of catheter ablation, a recommended treatment in select cases of (1) symptomatic paroxysmal AF in the setting of intolerance of antiarrhythmic drug therapy and (2) persistence of symptoms despite antiarrhythmic drug therapy. A
› Continue long-term oral anticoagulation therapy post ablation in patients with paroxysmal AF who have undergone catheter ablation if their CHA2DS2–VASc score is ≥ 2 (men) or ≥ 3 (women). C
› Regard catheter ablation as a reasonable alternative to antiarrhythmic drug therapy in select older patients with AF, and refer to a cardiologist as appropriate. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Side effects of COVID mRNA vaccines are mild and short, large study confirms
Data from the first 6 months after the rollout of mRNA COVID-19 vaccines in the United States released today show that adverse effects from shots are typically mild and short-lived.
Findings of the large study, compiled after nearly 300 million doses were administered, were published online March 7 in The Lancet Infectious Diseases.
Researchers, led by Hannah G. Rosenblum, MD, with the Centers for Disease Control and Prevention COVID Response Team, used passive U.S. surveillance data collected through the Vaccine Adverse Event Reporting System (VAERS), and the active system, v-safe, starting in December 2020 through the first 6 months of the U.S. COVID-19 vaccination program. V-safe is a voluntary, smartphone-based system set up in 2020 specifically for monitoring reactions to COVID-19 and health effects after vaccination. The health effects information from v-safe is presented in this study for the first time.
Of the 298.7 million doses of mRNA vaccines administered in the U.S. during the study period, VAERS processed 340,522 reports. Of those, 313,499 (92.1%) were nonserious; 22,527 (6.6%) were serious (nondeath); and 4,496 (1.3%) were deaths.
From v-safe reporting, researchers learned that about 71% of the 7.9 million participants reported local or systemic reactions, more frequently after dose 2 than after dose 1. Of those reporting reactions after dose 1, about two-thirds (68.6%) reported a local reaction and 52.7% reported a systemic reaction.
Among other findings:
- Injection-site pain occurred after dose 1 in 66.2% of participants and 68.6% after dose 2.
- One-third of participants (33.9%) reported fatigue after dose 1 and 55.7% after dose 2.
- Headache was reported among 27% of participants after dose 1 and 46.2% after dose 2.
- When injection site pain, fatigue, or headaches were reported, the reports were usually in the first week after vaccination.
- Reports of being unable to work or do normal daily activities, or instances of seeking medical care, occurred more commonly after dose 2 (32.1%) than after dose 1 (11.9%). Fewer than 1% of participants reported seeking medical care after dose 1 or 2 of the vaccine.
- Reactions and health effects were reported more often in female than in male recipients, and in people younger than 65 years, compared with older people.
- Serious adverse events, including myocarditis, have been identified following mRNA vaccinations, but the events are rare.
The authors wrote that these results are consistent with preauthorization clinical trials and early postauthorization reports.
“On the basis of our findings, mild to moderate transient reactogenicity should be anticipated,” they said, “particularly among younger and female vaccine recipients.”
‘Robust and reassuring data’
“The safety monitoring of the mRNA COVID-19 vaccines stands out as the most comprehensive of any vaccine in U.S. history. The use of these complementary monitoring systems has provided robust and reassuring data,” Matthew S. Krantz, MD, with the division of allergy, pulmonary, and critical care medicine at Vanderbilt University, Nashville, Tenn., and Elizabeth J. Phillips, MD, with the department of pathology, microbiology, and immunology at Vanderbilt, wrote in a related commentary in The Lancet Infectious Diseases.
They point out that the v-safe reports of reactions are consistent with those reported from clinical trials and a large population study in the United Kingdom.
Dr. Phillips said in a press release, “[A]lthough approximately one in 1,000 individuals vaccinated may have an adverse effect, most of these are nonserious. No unusual patterns emerged in the cause of death or serious adverse effects among VAERS reports. For adverse events of special interest, it is reassuring that there were no unexpected signals other than myopericarditis and anaphylaxis, already known to be associated with mRNA vaccines.”
The study authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Data from the first 6 months after the rollout of mRNA COVID-19 vaccines in the United States released today show that adverse effects from shots are typically mild and short-lived.
Findings of the large study, compiled after nearly 300 million doses were administered, were published online March 7 in The Lancet Infectious Diseases.
Researchers, led by Hannah G. Rosenblum, MD, with the Centers for Disease Control and Prevention COVID Response Team, used passive U.S. surveillance data collected through the Vaccine Adverse Event Reporting System (VAERS), and the active system, v-safe, starting in December 2020 through the first 6 months of the U.S. COVID-19 vaccination program. V-safe is a voluntary, smartphone-based system set up in 2020 specifically for monitoring reactions to COVID-19 and health effects after vaccination. The health effects information from v-safe is presented in this study for the first time.
Of the 298.7 million doses of mRNA vaccines administered in the U.S. during the study period, VAERS processed 340,522 reports. Of those, 313,499 (92.1%) were nonserious; 22,527 (6.6%) were serious (nondeath); and 4,496 (1.3%) were deaths.
From v-safe reporting, researchers learned that about 71% of the 7.9 million participants reported local or systemic reactions, more frequently after dose 2 than after dose 1. Of those reporting reactions after dose 1, about two-thirds (68.6%) reported a local reaction and 52.7% reported a systemic reaction.
Among other findings:
- Injection-site pain occurred after dose 1 in 66.2% of participants and 68.6% after dose 2.
- One-third of participants (33.9%) reported fatigue after dose 1 and 55.7% after dose 2.
- Headache was reported among 27% of participants after dose 1 and 46.2% after dose 2.
- When injection site pain, fatigue, or headaches were reported, the reports were usually in the first week after vaccination.
- Reports of being unable to work or do normal daily activities, or instances of seeking medical care, occurred more commonly after dose 2 (32.1%) than after dose 1 (11.9%). Fewer than 1% of participants reported seeking medical care after dose 1 or 2 of the vaccine.
- Reactions and health effects were reported more often in female than in male recipients, and in people younger than 65 years, compared with older people.
- Serious adverse events, including myocarditis, have been identified following mRNA vaccinations, but the events are rare.
The authors wrote that these results are consistent with preauthorization clinical trials and early postauthorization reports.
“On the basis of our findings, mild to moderate transient reactogenicity should be anticipated,” they said, “particularly among younger and female vaccine recipients.”
‘Robust and reassuring data’
“The safety monitoring of the mRNA COVID-19 vaccines stands out as the most comprehensive of any vaccine in U.S. history. The use of these complementary monitoring systems has provided robust and reassuring data,” Matthew S. Krantz, MD, with the division of allergy, pulmonary, and critical care medicine at Vanderbilt University, Nashville, Tenn., and Elizabeth J. Phillips, MD, with the department of pathology, microbiology, and immunology at Vanderbilt, wrote in a related commentary in The Lancet Infectious Diseases.
They point out that the v-safe reports of reactions are consistent with those reported from clinical trials and a large population study in the United Kingdom.
Dr. Phillips said in a press release, “[A]lthough approximately one in 1,000 individuals vaccinated may have an adverse effect, most of these are nonserious. No unusual patterns emerged in the cause of death or serious adverse effects among VAERS reports. For adverse events of special interest, it is reassuring that there were no unexpected signals other than myopericarditis and anaphylaxis, already known to be associated with mRNA vaccines.”
The study authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Data from the first 6 months after the rollout of mRNA COVID-19 vaccines in the United States released today show that adverse effects from shots are typically mild and short-lived.
Findings of the large study, compiled after nearly 300 million doses were administered, were published online March 7 in The Lancet Infectious Diseases.
Researchers, led by Hannah G. Rosenblum, MD, with the Centers for Disease Control and Prevention COVID Response Team, used passive U.S. surveillance data collected through the Vaccine Adverse Event Reporting System (VAERS), and the active system, v-safe, starting in December 2020 through the first 6 months of the U.S. COVID-19 vaccination program. V-safe is a voluntary, smartphone-based system set up in 2020 specifically for monitoring reactions to COVID-19 and health effects after vaccination. The health effects information from v-safe is presented in this study for the first time.
Of the 298.7 million doses of mRNA vaccines administered in the U.S. during the study period, VAERS processed 340,522 reports. Of those, 313,499 (92.1%) were nonserious; 22,527 (6.6%) were serious (nondeath); and 4,496 (1.3%) were deaths.
From v-safe reporting, researchers learned that about 71% of the 7.9 million participants reported local or systemic reactions, more frequently after dose 2 than after dose 1. Of those reporting reactions after dose 1, about two-thirds (68.6%) reported a local reaction and 52.7% reported a systemic reaction.
Among other findings:
- Injection-site pain occurred after dose 1 in 66.2% of participants and 68.6% after dose 2.
- One-third of participants (33.9%) reported fatigue after dose 1 and 55.7% after dose 2.
- Headache was reported among 27% of participants after dose 1 and 46.2% after dose 2.
- When injection site pain, fatigue, or headaches were reported, the reports were usually in the first week after vaccination.
- Reports of being unable to work or do normal daily activities, or instances of seeking medical care, occurred more commonly after dose 2 (32.1%) than after dose 1 (11.9%). Fewer than 1% of participants reported seeking medical care after dose 1 or 2 of the vaccine.
- Reactions and health effects were reported more often in female than in male recipients, and in people younger than 65 years, compared with older people.
- Serious adverse events, including myocarditis, have been identified following mRNA vaccinations, but the events are rare.
The authors wrote that these results are consistent with preauthorization clinical trials and early postauthorization reports.
“On the basis of our findings, mild to moderate transient reactogenicity should be anticipated,” they said, “particularly among younger and female vaccine recipients.”
‘Robust and reassuring data’
“The safety monitoring of the mRNA COVID-19 vaccines stands out as the most comprehensive of any vaccine in U.S. history. The use of these complementary monitoring systems has provided robust and reassuring data,” Matthew S. Krantz, MD, with the division of allergy, pulmonary, and critical care medicine at Vanderbilt University, Nashville, Tenn., and Elizabeth J. Phillips, MD, with the department of pathology, microbiology, and immunology at Vanderbilt, wrote in a related commentary in The Lancet Infectious Diseases.
They point out that the v-safe reports of reactions are consistent with those reported from clinical trials and a large population study in the United Kingdom.
Dr. Phillips said in a press release, “[A]lthough approximately one in 1,000 individuals vaccinated may have an adverse effect, most of these are nonserious. No unusual patterns emerged in the cause of death or serious adverse effects among VAERS reports. For adverse events of special interest, it is reassuring that there were no unexpected signals other than myopericarditis and anaphylaxis, already known to be associated with mRNA vaccines.”
The study authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psoriatic Arthritis: Presentation and Diagnosis
Psoriatic Arthritis: The Basics
No excess mortality seen in contemporary undifferentiated arthritis
Patients with undifferentiated arthritis (UA) that is defined according to contemporary criteria don’t appear to have the same excess mortality that is associated with rheumatoid arthritis, despite links between the two conditions.
UA has long been considered an earlier phase of RA, so similar management strategies are often used based on the assumption that outcomes and elevated mortality risk were similar between the two, but new findings reported in a research letter published in Annals of the Rheumatic Diseases challenge that assumption.
The change in the definition of UA that accompanied the introduction of new RA criteria in 2010 meant that some of the patients who previously met the criteria for UA now were classified as having RA, and “the remaining contemporary UA population (not fulfilling the 1987/2010 RA criteria) is largely autoantibody negative, presents with monoarthritis or oligoarthritis, and progresses less frequently to RA,” PhD candidate Marloes Verstappen of Leiden (Netherlands) University Medical Center, and coauthors wrote.
As the first large study on excess mortality in patients meeting contemporary criteria for UA, the authors said it suggests that the change in criteria for UA has served to increase the differences in mortality between it and RA.
“Further research and discussions are needed as to whether the management of contemporary UA should be similar to or different from that of RA,” they wrote.
The researchers conducted a longitudinal cohort study of 860 patients who met the conventional criteria for UA – they did not meet the 1987 RA criteria or other diagnosis – at baseline and 561 who met contemporary criteria for UA based on the fact that they did not meet the 1987 or 2010 RA criteria. There were also 762 patients who were diagnosed with RA according to the 1987 criteria, and 828 diagnosed according to the 2010 criteria. All of these patients were diagnosed between 1993 and 2008 and their median follow-up times ranged from 16.0 to 17.3 years, with a minimum of 10 years of follow-up.
The study found that, while there was a trend toward excess mortality in the conventional UA group (standardized mortality ratio, 1.11; 95% confidence interval, 0.96-1.27), there was no significant excess mortality in the contemporary UA patients (SMR, 1.05; 95% CI, 0.87-1.26).
In comparison, patients in both the 1987 RA criteria group and the 2010 criteria group showed significantly higher mortality. Among patients with anti–citrullinated protein antibody–positive disease, even early treatment with disease-modifying antirheumatic drugs and treat-to-target strategies didn’t reduce the excess mortality.
The study did find some suggestion of excess mortality among patients with contemporary UA and who were anti–citrullinated protein antibody positive, but the number of patients was small.
“Only a few percent of patients presenting with contemporary UA are autoantibody positive; these patients may be considered at increased risk to progress to RA,” the authors wrote.
The data also suggested that disease-modifying antirheumatic drugs didn’t alter excess mortality among patients with contemporary UA.
The study was supported by the Dutch Arthritis Foundation and the European Research Council. No conflicts of interest were declared.
Patients with undifferentiated arthritis (UA) that is defined according to contemporary criteria don’t appear to have the same excess mortality that is associated with rheumatoid arthritis, despite links between the two conditions.
UA has long been considered an earlier phase of RA, so similar management strategies are often used based on the assumption that outcomes and elevated mortality risk were similar between the two, but new findings reported in a research letter published in Annals of the Rheumatic Diseases challenge that assumption.
The change in the definition of UA that accompanied the introduction of new RA criteria in 2010 meant that some of the patients who previously met the criteria for UA now were classified as having RA, and “the remaining contemporary UA population (not fulfilling the 1987/2010 RA criteria) is largely autoantibody negative, presents with monoarthritis or oligoarthritis, and progresses less frequently to RA,” PhD candidate Marloes Verstappen of Leiden (Netherlands) University Medical Center, and coauthors wrote.
As the first large study on excess mortality in patients meeting contemporary criteria for UA, the authors said it suggests that the change in criteria for UA has served to increase the differences in mortality between it and RA.
“Further research and discussions are needed as to whether the management of contemporary UA should be similar to or different from that of RA,” they wrote.
The researchers conducted a longitudinal cohort study of 860 patients who met the conventional criteria for UA – they did not meet the 1987 RA criteria or other diagnosis – at baseline and 561 who met contemporary criteria for UA based on the fact that they did not meet the 1987 or 2010 RA criteria. There were also 762 patients who were diagnosed with RA according to the 1987 criteria, and 828 diagnosed according to the 2010 criteria. All of these patients were diagnosed between 1993 and 2008 and their median follow-up times ranged from 16.0 to 17.3 years, with a minimum of 10 years of follow-up.
The study found that, while there was a trend toward excess mortality in the conventional UA group (standardized mortality ratio, 1.11; 95% confidence interval, 0.96-1.27), there was no significant excess mortality in the contemporary UA patients (SMR, 1.05; 95% CI, 0.87-1.26).
In comparison, patients in both the 1987 RA criteria group and the 2010 criteria group showed significantly higher mortality. Among patients with anti–citrullinated protein antibody–positive disease, even early treatment with disease-modifying antirheumatic drugs and treat-to-target strategies didn’t reduce the excess mortality.
The study did find some suggestion of excess mortality among patients with contemporary UA and who were anti–citrullinated protein antibody positive, but the number of patients was small.
“Only a few percent of patients presenting with contemporary UA are autoantibody positive; these patients may be considered at increased risk to progress to RA,” the authors wrote.
The data also suggested that disease-modifying antirheumatic drugs didn’t alter excess mortality among patients with contemporary UA.
The study was supported by the Dutch Arthritis Foundation and the European Research Council. No conflicts of interest were declared.
Patients with undifferentiated arthritis (UA) that is defined according to contemporary criteria don’t appear to have the same excess mortality that is associated with rheumatoid arthritis, despite links between the two conditions.
UA has long been considered an earlier phase of RA, so similar management strategies are often used based on the assumption that outcomes and elevated mortality risk were similar between the two, but new findings reported in a research letter published in Annals of the Rheumatic Diseases challenge that assumption.
The change in the definition of UA that accompanied the introduction of new RA criteria in 2010 meant that some of the patients who previously met the criteria for UA now were classified as having RA, and “the remaining contemporary UA population (not fulfilling the 1987/2010 RA criteria) is largely autoantibody negative, presents with monoarthritis or oligoarthritis, and progresses less frequently to RA,” PhD candidate Marloes Verstappen of Leiden (Netherlands) University Medical Center, and coauthors wrote.
As the first large study on excess mortality in patients meeting contemporary criteria for UA, the authors said it suggests that the change in criteria for UA has served to increase the differences in mortality between it and RA.
“Further research and discussions are needed as to whether the management of contemporary UA should be similar to or different from that of RA,” they wrote.
The researchers conducted a longitudinal cohort study of 860 patients who met the conventional criteria for UA – they did not meet the 1987 RA criteria or other diagnosis – at baseline and 561 who met contemporary criteria for UA based on the fact that they did not meet the 1987 or 2010 RA criteria. There were also 762 patients who were diagnosed with RA according to the 1987 criteria, and 828 diagnosed according to the 2010 criteria. All of these patients were diagnosed between 1993 and 2008 and their median follow-up times ranged from 16.0 to 17.3 years, with a minimum of 10 years of follow-up.
The study found that, while there was a trend toward excess mortality in the conventional UA group (standardized mortality ratio, 1.11; 95% confidence interval, 0.96-1.27), there was no significant excess mortality in the contemporary UA patients (SMR, 1.05; 95% CI, 0.87-1.26).
In comparison, patients in both the 1987 RA criteria group and the 2010 criteria group showed significantly higher mortality. Among patients with anti–citrullinated protein antibody–positive disease, even early treatment with disease-modifying antirheumatic drugs and treat-to-target strategies didn’t reduce the excess mortality.
The study did find some suggestion of excess mortality among patients with contemporary UA and who were anti–citrullinated protein antibody positive, but the number of patients was small.
“Only a few percent of patients presenting with contemporary UA are autoantibody positive; these patients may be considered at increased risk to progress to RA,” the authors wrote.
The data also suggested that disease-modifying antirheumatic drugs didn’t alter excess mortality among patients with contemporary UA.
The study was supported by the Dutch Arthritis Foundation and the European Research Council. No conflicts of interest were declared.
FROM ANNALS OF the RHEUMATIC DISEASES
Screening with a tablet-based app elicits sensitive information in primary care
“Anyone who has been to a doctor’s office recently realizes that everyone there is very busy,” said David P. Miller Jr., MD, lead author of the paper published in JAMA Network Open, in an interview. “For our study, we programmed routine screening questions that nursing staff were asking at every visit into an app [called mPATH] that patients used on check-in.”
In particular, screening for depression, injurious falls, or intimate partner violence in a primary care setting is hampered not only by time constraints, but also staff discomfort and patients’ reluctance to disclose sensitive information, Dr. Miller of Wake Forest University, Winston-Salem, N.C., and colleagues explained in their paper.
Study methods and results
The researchers tested the app in three family practices and three internal medicine practices. They compared whether more patients were identified with depression, intimate partner violence, or fall risk in the 60 days of using the tablet-based app, compared with the 60-day period before introduction of the app, when nursing staff asked screening questions verbally. Patients were given the tablet and app to use at check-in, and results went into an electronic health record.
The study population included 23,026 individuals, aged 18 years and older who were seen between June 2019 and February 2020.
The post-app period was shortened to 30 days for the last two enrolled practices to avoid confounding from COVID-19, the researchers noted.
The primary outcome of the study was the proportion of patients who screened positive for a composite of depression, fall risk, or intimate partner violence.
“We found that [the app] significantly outperformed nursing staff in terms of detecting patients with depression or safety concerns,” Dr. Miller said in an interview. “By saving nurses time, we hope they can use the saved time to address patients’ identified concerns.”
Overall, the proportion of patients who screened positive for the composite outcome of depression, fall risk, or intimate partner violence increased from 8.7% to 19.5%. Increases were noted across all six participating clinics.
When broken out separately, the proportion of patients who screened positive for depression, based on Patient Health Questionnaire-2 scores of 2 or higher, increased from 1.5% to 4.2% from before to after the introduction of the tablet-based app. The proportion of patients screening positive for fall risk increased from 7.4% to 15.7%, and the proportion who screened positive for intimate partner violence increased from 0.1% to 2.9%.
Patient demographics were similar for the two time periods. Overall, 57.9% of patients were female, 80.5% were non-Hispanic White, and 13.5% were Black or African American. Patients ranged in age from 18-102 years, with a mean age of 59.7 years.
The association of app use on the primary outcome remained the same (adjusted odds ratio, 2.6) after accounting for patient characteristics.
Real-world setting supports clinical value
“One of the strengths of our study is that the mPATH app was delivered as usual care in the primary care clinics,” Dr. Miller said in an interview. “In other words, we relied entirely on clinical staff to hand the app to patients and transmit the screening results to the electronic health record. This allowed us to see how self-administered screening performs in the real world rather than in a research setting,” he said. “Another strength is our large sample size. We included more than 23,000 patients who were seen at one of six community-based primary care practices.”
“A few other studies have compared electronic self-administered screening with verbal screening, mainly in the areas of intimate partner violence or sexual health,” Dr. Miller noted. “However, these studies were administered by research staff and only included patients agreeing to be in a research study, which leaves many people out. What makes our study unique is that the primary care practices were using the self-screening app as part of their routine care,” he said.
“By analyzing deidentified data, we could see how self-administered screening compares to verbal screening among all patients in a real-world setting,” he added.
“We found that self-administered screening significantly outperforms verbal screening by clinical staff. Over twice as many patients with depression, fall risk, or intimate partner violence were identified by the app, compared to verbal screening,” said Dr. Miller. “We hope that clinics will look for ways to incorporate electronic self-screening in their usual processes. Self-administered screening not only saves staff time, but it does a much better job identifying patients with needs,” he said.
“The next step will be identifying the best way to incorporate digital health apps like mPATH into usual workflows,” Dr. Miller said. “We are currently conducting an implementation science trial of the mPATH app to learn this.”
App allows patients privacy in responses
“The study is important for assessing the physical and mental well-being of patients at all health care practices in general and in primary care practices in particular, said Noel Deep, MD, a general internist in group practice in Antigo, Wisc., in an interview. “This study provides the data that can be leveraged to provide this type of virtual or electronic options for patients to answer these sensitive questions,” he said.
“It provides them the opportunity to answer the questions truthfully and without fear of being judged by the staff who traditionally ask these questions,” he emphasized.
Dr. Deep was not surprised by the study outcomes.
“Almost all primary care practices administer these questionnaires to their patients, whether at their annual wellness exams or the Medicare wellness exams,” he said. “Many times, the staff asking these questions might introduce some of their personal bias or not ask the questions in a nonjudgmental manner, which may not elicit the right answers from the patients.”
The clinical value of the study is that it prompts physicians and health care organizations to consider adopting other modalities to collect screening information “that is comfortable to the patients, reproducible, patient-friendly, easily accessed by the patients and reviewable by their physicians, and, more importantly is private and maintains patient confidentiality,” said Dr. Deep.
Study needs to be replicated in rural, small communities
“I would like to see the study done among more diverse ethnic, age, socioeconomic, education, geographic, and physician practice–size populations,” which would reinforce the value of the tablet-based app if such studies yielded similar results, Dr. Deep said.
Privacy is especially important for practices in smaller communities/rural communities, such as the one where Dr. Deep practices, as everyone knows everyone in these kinds of places, he said.
“I understand that we are all sworn to maintaining patient confidentiality, but that may not be what the patients perceive. That is why I would like to see what the study finds in rural or small communities,” Dr. Deep explained.
The study was supported by the National Cancer Institute. Dr. Miller and coauthor Dr. Ajay Dharod are the coinventors of the mPATH app, and they and Wake Forest University Health Sciences have an ownership interest should the app be commercialized. Dr. Deep had no financial conflicts to disclose.
“Anyone who has been to a doctor’s office recently realizes that everyone there is very busy,” said David P. Miller Jr., MD, lead author of the paper published in JAMA Network Open, in an interview. “For our study, we programmed routine screening questions that nursing staff were asking at every visit into an app [called mPATH] that patients used on check-in.”
In particular, screening for depression, injurious falls, or intimate partner violence in a primary care setting is hampered not only by time constraints, but also staff discomfort and patients’ reluctance to disclose sensitive information, Dr. Miller of Wake Forest University, Winston-Salem, N.C., and colleagues explained in their paper.
Study methods and results
The researchers tested the app in three family practices and three internal medicine practices. They compared whether more patients were identified with depression, intimate partner violence, or fall risk in the 60 days of using the tablet-based app, compared with the 60-day period before introduction of the app, when nursing staff asked screening questions verbally. Patients were given the tablet and app to use at check-in, and results went into an electronic health record.
The study population included 23,026 individuals, aged 18 years and older who were seen between June 2019 and February 2020.
The post-app period was shortened to 30 days for the last two enrolled practices to avoid confounding from COVID-19, the researchers noted.
The primary outcome of the study was the proportion of patients who screened positive for a composite of depression, fall risk, or intimate partner violence.
“We found that [the app] significantly outperformed nursing staff in terms of detecting patients with depression or safety concerns,” Dr. Miller said in an interview. “By saving nurses time, we hope they can use the saved time to address patients’ identified concerns.”
Overall, the proportion of patients who screened positive for the composite outcome of depression, fall risk, or intimate partner violence increased from 8.7% to 19.5%. Increases were noted across all six participating clinics.
When broken out separately, the proportion of patients who screened positive for depression, based on Patient Health Questionnaire-2 scores of 2 or higher, increased from 1.5% to 4.2% from before to after the introduction of the tablet-based app. The proportion of patients screening positive for fall risk increased from 7.4% to 15.7%, and the proportion who screened positive for intimate partner violence increased from 0.1% to 2.9%.
Patient demographics were similar for the two time periods. Overall, 57.9% of patients were female, 80.5% were non-Hispanic White, and 13.5% were Black or African American. Patients ranged in age from 18-102 years, with a mean age of 59.7 years.
The association of app use on the primary outcome remained the same (adjusted odds ratio, 2.6) after accounting for patient characteristics.
Real-world setting supports clinical value
“One of the strengths of our study is that the mPATH app was delivered as usual care in the primary care clinics,” Dr. Miller said in an interview. “In other words, we relied entirely on clinical staff to hand the app to patients and transmit the screening results to the electronic health record. This allowed us to see how self-administered screening performs in the real world rather than in a research setting,” he said. “Another strength is our large sample size. We included more than 23,000 patients who were seen at one of six community-based primary care practices.”
“A few other studies have compared electronic self-administered screening with verbal screening, mainly in the areas of intimate partner violence or sexual health,” Dr. Miller noted. “However, these studies were administered by research staff and only included patients agreeing to be in a research study, which leaves many people out. What makes our study unique is that the primary care practices were using the self-screening app as part of their routine care,” he said.
“By analyzing deidentified data, we could see how self-administered screening compares to verbal screening among all patients in a real-world setting,” he added.
“We found that self-administered screening significantly outperforms verbal screening by clinical staff. Over twice as many patients with depression, fall risk, or intimate partner violence were identified by the app, compared to verbal screening,” said Dr. Miller. “We hope that clinics will look for ways to incorporate electronic self-screening in their usual processes. Self-administered screening not only saves staff time, but it does a much better job identifying patients with needs,” he said.
“The next step will be identifying the best way to incorporate digital health apps like mPATH into usual workflows,” Dr. Miller said. “We are currently conducting an implementation science trial of the mPATH app to learn this.”
App allows patients privacy in responses
“The study is important for assessing the physical and mental well-being of patients at all health care practices in general and in primary care practices in particular, said Noel Deep, MD, a general internist in group practice in Antigo, Wisc., in an interview. “This study provides the data that can be leveraged to provide this type of virtual or electronic options for patients to answer these sensitive questions,” he said.
“It provides them the opportunity to answer the questions truthfully and without fear of being judged by the staff who traditionally ask these questions,” he emphasized.
Dr. Deep was not surprised by the study outcomes.
“Almost all primary care practices administer these questionnaires to their patients, whether at their annual wellness exams or the Medicare wellness exams,” he said. “Many times, the staff asking these questions might introduce some of their personal bias or not ask the questions in a nonjudgmental manner, which may not elicit the right answers from the patients.”
The clinical value of the study is that it prompts physicians and health care organizations to consider adopting other modalities to collect screening information “that is comfortable to the patients, reproducible, patient-friendly, easily accessed by the patients and reviewable by their physicians, and, more importantly is private and maintains patient confidentiality,” said Dr. Deep.
Study needs to be replicated in rural, small communities
“I would like to see the study done among more diverse ethnic, age, socioeconomic, education, geographic, and physician practice–size populations,” which would reinforce the value of the tablet-based app if such studies yielded similar results, Dr. Deep said.
Privacy is especially important for practices in smaller communities/rural communities, such as the one where Dr. Deep practices, as everyone knows everyone in these kinds of places, he said.
“I understand that we are all sworn to maintaining patient confidentiality, but that may not be what the patients perceive. That is why I would like to see what the study finds in rural or small communities,” Dr. Deep explained.
The study was supported by the National Cancer Institute. Dr. Miller and coauthor Dr. Ajay Dharod are the coinventors of the mPATH app, and they and Wake Forest University Health Sciences have an ownership interest should the app be commercialized. Dr. Deep had no financial conflicts to disclose.
“Anyone who has been to a doctor’s office recently realizes that everyone there is very busy,” said David P. Miller Jr., MD, lead author of the paper published in JAMA Network Open, in an interview. “For our study, we programmed routine screening questions that nursing staff were asking at every visit into an app [called mPATH] that patients used on check-in.”
In particular, screening for depression, injurious falls, or intimate partner violence in a primary care setting is hampered not only by time constraints, but also staff discomfort and patients’ reluctance to disclose sensitive information, Dr. Miller of Wake Forest University, Winston-Salem, N.C., and colleagues explained in their paper.
Study methods and results
The researchers tested the app in three family practices and three internal medicine practices. They compared whether more patients were identified with depression, intimate partner violence, or fall risk in the 60 days of using the tablet-based app, compared with the 60-day period before introduction of the app, when nursing staff asked screening questions verbally. Patients were given the tablet and app to use at check-in, and results went into an electronic health record.
The study population included 23,026 individuals, aged 18 years and older who were seen between June 2019 and February 2020.
The post-app period was shortened to 30 days for the last two enrolled practices to avoid confounding from COVID-19, the researchers noted.
The primary outcome of the study was the proportion of patients who screened positive for a composite of depression, fall risk, or intimate partner violence.
“We found that [the app] significantly outperformed nursing staff in terms of detecting patients with depression or safety concerns,” Dr. Miller said in an interview. “By saving nurses time, we hope they can use the saved time to address patients’ identified concerns.”
Overall, the proportion of patients who screened positive for the composite outcome of depression, fall risk, or intimate partner violence increased from 8.7% to 19.5%. Increases were noted across all six participating clinics.
When broken out separately, the proportion of patients who screened positive for depression, based on Patient Health Questionnaire-2 scores of 2 or higher, increased from 1.5% to 4.2% from before to after the introduction of the tablet-based app. The proportion of patients screening positive for fall risk increased from 7.4% to 15.7%, and the proportion who screened positive for intimate partner violence increased from 0.1% to 2.9%.
Patient demographics were similar for the two time periods. Overall, 57.9% of patients were female, 80.5% were non-Hispanic White, and 13.5% were Black or African American. Patients ranged in age from 18-102 years, with a mean age of 59.7 years.
The association of app use on the primary outcome remained the same (adjusted odds ratio, 2.6) after accounting for patient characteristics.
Real-world setting supports clinical value
“One of the strengths of our study is that the mPATH app was delivered as usual care in the primary care clinics,” Dr. Miller said in an interview. “In other words, we relied entirely on clinical staff to hand the app to patients and transmit the screening results to the electronic health record. This allowed us to see how self-administered screening performs in the real world rather than in a research setting,” he said. “Another strength is our large sample size. We included more than 23,000 patients who were seen at one of six community-based primary care practices.”
“A few other studies have compared electronic self-administered screening with verbal screening, mainly in the areas of intimate partner violence or sexual health,” Dr. Miller noted. “However, these studies were administered by research staff and only included patients agreeing to be in a research study, which leaves many people out. What makes our study unique is that the primary care practices were using the self-screening app as part of their routine care,” he said.
“By analyzing deidentified data, we could see how self-administered screening compares to verbal screening among all patients in a real-world setting,” he added.
“We found that self-administered screening significantly outperforms verbal screening by clinical staff. Over twice as many patients with depression, fall risk, or intimate partner violence were identified by the app, compared to verbal screening,” said Dr. Miller. “We hope that clinics will look for ways to incorporate electronic self-screening in their usual processes. Self-administered screening not only saves staff time, but it does a much better job identifying patients with needs,” he said.
“The next step will be identifying the best way to incorporate digital health apps like mPATH into usual workflows,” Dr. Miller said. “We are currently conducting an implementation science trial of the mPATH app to learn this.”
App allows patients privacy in responses
“The study is important for assessing the physical and mental well-being of patients at all health care practices in general and in primary care practices in particular, said Noel Deep, MD, a general internist in group practice in Antigo, Wisc., in an interview. “This study provides the data that can be leveraged to provide this type of virtual or electronic options for patients to answer these sensitive questions,” he said.
“It provides them the opportunity to answer the questions truthfully and without fear of being judged by the staff who traditionally ask these questions,” he emphasized.
Dr. Deep was not surprised by the study outcomes.
“Almost all primary care practices administer these questionnaires to their patients, whether at their annual wellness exams or the Medicare wellness exams,” he said. “Many times, the staff asking these questions might introduce some of their personal bias or not ask the questions in a nonjudgmental manner, which may not elicit the right answers from the patients.”
The clinical value of the study is that it prompts physicians and health care organizations to consider adopting other modalities to collect screening information “that is comfortable to the patients, reproducible, patient-friendly, easily accessed by the patients and reviewable by their physicians, and, more importantly is private and maintains patient confidentiality,” said Dr. Deep.
Study needs to be replicated in rural, small communities
“I would like to see the study done among more diverse ethnic, age, socioeconomic, education, geographic, and physician practice–size populations,” which would reinforce the value of the tablet-based app if such studies yielded similar results, Dr. Deep said.
Privacy is especially important for practices in smaller communities/rural communities, such as the one where Dr. Deep practices, as everyone knows everyone in these kinds of places, he said.
“I understand that we are all sworn to maintaining patient confidentiality, but that may not be what the patients perceive. That is why I would like to see what the study finds in rural or small communities,” Dr. Deep explained.
The study was supported by the National Cancer Institute. Dr. Miller and coauthor Dr. Ajay Dharod are the coinventors of the mPATH app, and they and Wake Forest University Health Sciences have an ownership interest should the app be commercialized. Dr. Deep had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Painful Ulcerating Lesions on the Breast
The Diagnosis: Cystic Neutrophilic Granulomatous Mastitis
The histopathologic findings in our patient were characteristic of cystic neutrophilic granulomatous mastitis (CNGM), a rare granulomatous mastitis associated with Corynebacterium and suppurative lipogranulomas. Although not seen in our patient, the lipid vacuoles may contain gram-positive bacilli.1 The surrounding mixed inflammatory infiltrate contains Langerhans giant cells, lymphocytes, and neutrophils. Cystic neutrophilic granulomatous mastitis is seen in parous women of reproductive age. Physical examination demonstrates a palpable painful mass on the breast. Wound cultures frequently are negative, likely due to difficulty culturing Corynebacterium and prophylactic antibiotic treatment. Given the association with Corynebacterium species, early diagnosis of CNGM is essential in offering patients the most appropriate treatment. Prolonged antibiotic therapy specifically directed to corynebacteria is required, sometimes even beyond resolution of clinical symptoms. The diagnosis of CNGM often is missed or delayed due to its rarity and many potential mimickers. Clinically, CNGM may be virtually impossible to discern from invasive carcinoma.1
Our patient was treated with vancomycin and cefepime with incision and drainage as an inpatient. Upon discharge, she was started on prednisone 1 mg/kg daily tapered by 10 mg every 5 days over 1 month and doxycycline 100 mg twice daily. She was then transitioned to topical hydrocortisone and bacitracin; she reported decreased swelling and pain. No new lesions formed after the initiation of therapy; however, most lesions remained open. Cystic neutrophilic granulomatous mastitis remains a challenging entity to treat, with a variable response rate reported in the literature for antibiotics such as doxycycline and systemic and topical steroids as well as immunosuppressants including methotrexate.2,3
Cystic neutrophilic granulomatous mastitis can be distinguished from hidradenitis suppurativa clinically because ulcerating lesions can involve the superior portions of the breast in CNGM, whereas hidradenitis suppurativa typically is restricted to the lower intertriginous parts of the breast. Other mimics of CNGM can be distinguished with biopsy. Histology of pyoderma gangrenosum lacks prominent granuloma formation. Although sarcoidosis and mycobacterial infection show prominent granulomas, neither show the characteristic lipogranulomas seen in CNGM. Additionally, the granulomas of sarcoidosis are much larger and deeper than CNGM. Mycobacterial granulomas also typically reveal bacilli with acid-fast bacilli staining or via wound culture.
- Wu JM, Turashvili G. Cystic neutrophilic granulomatous mastitis: an update. J Clin Pathol. 2020;73:445-453. doi:10.1136/jclinpath-2019-206180
- Steuer AB, Stern MJ, Cobos G, et al. Clinical characteristics and medical management of idiopathic granulomatous mastitis. JAMA Dermatol. 2020;156:460-464. doi:10.1001/jamadermatol.2019.4516
- Dobinson HC, Anderson TP, Chambers ST, et al. Antimicrobial treatment options for granulomatous mastitis caused by Corynebacterium species [published online July 1, 2015]. J Clin Microbiol. 2015;53:2895-2899. doi:10.1128/JCM.00760-15
The Diagnosis: Cystic Neutrophilic Granulomatous Mastitis
The histopathologic findings in our patient were characteristic of cystic neutrophilic granulomatous mastitis (CNGM), a rare granulomatous mastitis associated with Corynebacterium and suppurative lipogranulomas. Although not seen in our patient, the lipid vacuoles may contain gram-positive bacilli.1 The surrounding mixed inflammatory infiltrate contains Langerhans giant cells, lymphocytes, and neutrophils. Cystic neutrophilic granulomatous mastitis is seen in parous women of reproductive age. Physical examination demonstrates a palpable painful mass on the breast. Wound cultures frequently are negative, likely due to difficulty culturing Corynebacterium and prophylactic antibiotic treatment. Given the association with Corynebacterium species, early diagnosis of CNGM is essential in offering patients the most appropriate treatment. Prolonged antibiotic therapy specifically directed to corynebacteria is required, sometimes even beyond resolution of clinical symptoms. The diagnosis of CNGM often is missed or delayed due to its rarity and many potential mimickers. Clinically, CNGM may be virtually impossible to discern from invasive carcinoma.1
Our patient was treated with vancomycin and cefepime with incision and drainage as an inpatient. Upon discharge, she was started on prednisone 1 mg/kg daily tapered by 10 mg every 5 days over 1 month and doxycycline 100 mg twice daily. She was then transitioned to topical hydrocortisone and bacitracin; she reported decreased swelling and pain. No new lesions formed after the initiation of therapy; however, most lesions remained open. Cystic neutrophilic granulomatous mastitis remains a challenging entity to treat, with a variable response rate reported in the literature for antibiotics such as doxycycline and systemic and topical steroids as well as immunosuppressants including methotrexate.2,3
Cystic neutrophilic granulomatous mastitis can be distinguished from hidradenitis suppurativa clinically because ulcerating lesions can involve the superior portions of the breast in CNGM, whereas hidradenitis suppurativa typically is restricted to the lower intertriginous parts of the breast. Other mimics of CNGM can be distinguished with biopsy. Histology of pyoderma gangrenosum lacks prominent granuloma formation. Although sarcoidosis and mycobacterial infection show prominent granulomas, neither show the characteristic lipogranulomas seen in CNGM. Additionally, the granulomas of sarcoidosis are much larger and deeper than CNGM. Mycobacterial granulomas also typically reveal bacilli with acid-fast bacilli staining or via wound culture.
The Diagnosis: Cystic Neutrophilic Granulomatous Mastitis
The histopathologic findings in our patient were characteristic of cystic neutrophilic granulomatous mastitis (CNGM), a rare granulomatous mastitis associated with Corynebacterium and suppurative lipogranulomas. Although not seen in our patient, the lipid vacuoles may contain gram-positive bacilli.1 The surrounding mixed inflammatory infiltrate contains Langerhans giant cells, lymphocytes, and neutrophils. Cystic neutrophilic granulomatous mastitis is seen in parous women of reproductive age. Physical examination demonstrates a palpable painful mass on the breast. Wound cultures frequently are negative, likely due to difficulty culturing Corynebacterium and prophylactic antibiotic treatment. Given the association with Corynebacterium species, early diagnosis of CNGM is essential in offering patients the most appropriate treatment. Prolonged antibiotic therapy specifically directed to corynebacteria is required, sometimes even beyond resolution of clinical symptoms. The diagnosis of CNGM often is missed or delayed due to its rarity and many potential mimickers. Clinically, CNGM may be virtually impossible to discern from invasive carcinoma.1
Our patient was treated with vancomycin and cefepime with incision and drainage as an inpatient. Upon discharge, she was started on prednisone 1 mg/kg daily tapered by 10 mg every 5 days over 1 month and doxycycline 100 mg twice daily. She was then transitioned to topical hydrocortisone and bacitracin; she reported decreased swelling and pain. No new lesions formed after the initiation of therapy; however, most lesions remained open. Cystic neutrophilic granulomatous mastitis remains a challenging entity to treat, with a variable response rate reported in the literature for antibiotics such as doxycycline and systemic and topical steroids as well as immunosuppressants including methotrexate.2,3
Cystic neutrophilic granulomatous mastitis can be distinguished from hidradenitis suppurativa clinically because ulcerating lesions can involve the superior portions of the breast in CNGM, whereas hidradenitis suppurativa typically is restricted to the lower intertriginous parts of the breast. Other mimics of CNGM can be distinguished with biopsy. Histology of pyoderma gangrenosum lacks prominent granuloma formation. Although sarcoidosis and mycobacterial infection show prominent granulomas, neither show the characteristic lipogranulomas seen in CNGM. Additionally, the granulomas of sarcoidosis are much larger and deeper than CNGM. Mycobacterial granulomas also typically reveal bacilli with acid-fast bacilli staining or via wound culture.
- Wu JM, Turashvili G. Cystic neutrophilic granulomatous mastitis: an update. J Clin Pathol. 2020;73:445-453. doi:10.1136/jclinpath-2019-206180
- Steuer AB, Stern MJ, Cobos G, et al. Clinical characteristics and medical management of idiopathic granulomatous mastitis. JAMA Dermatol. 2020;156:460-464. doi:10.1001/jamadermatol.2019.4516
- Dobinson HC, Anderson TP, Chambers ST, et al. Antimicrobial treatment options for granulomatous mastitis caused by Corynebacterium species [published online July 1, 2015]. J Clin Microbiol. 2015;53:2895-2899. doi:10.1128/JCM.00760-15
- Wu JM, Turashvili G. Cystic neutrophilic granulomatous mastitis: an update. J Clin Pathol. 2020;73:445-453. doi:10.1136/jclinpath-2019-206180
- Steuer AB, Stern MJ, Cobos G, et al. Clinical characteristics and medical management of idiopathic granulomatous mastitis. JAMA Dermatol. 2020;156:460-464. doi:10.1001/jamadermatol.2019.4516
- Dobinson HC, Anderson TP, Chambers ST, et al. Antimicrobial treatment options for granulomatous mastitis caused by Corynebacterium species [published online July 1, 2015]. J Clin Microbiol. 2015;53:2895-2899. doi:10.1128/JCM.00760-15
A 36-year-old puerperal woman presented with painful, unilateral, ulcerating breast lesions (top) of 3 months’ duration that developed during pregnancy and drained pus with blood. No improvement was seen with antibiotics or incision and drainage. Biopsy of a lesion showed stellate granulomas with cystic spaces and suppurative lipogranulomas where central lipid vacuoles were rimmed by neutrophils and an outer cuff of epithelioid histiocytes (bottom). Acid-fast bacilli, Grocott-Gomori methenamine-silver, Gram, and Steiner staining did not reveal any microorganisms. Additionally, wound cultures were negative.
Hair loss affects more than half of postmenopausal women
Female-pattern hair loss (FPHL) was identified in 52% of postmenopausal women, and 4% of these cases involved extensive baldness, based on data from 178 individuals.
FPHL can develop at any time from teenage years through and beyond menopause, wrote Sukanya Chaikittisilpa, MD, of Chulalongkorn University, Bangkok, and colleagues.
The cause of FPHL remains uncertain, but the presence of estrogen receptors in hair follicles suggests that the hormone changes of menopause may affect hair growth, the researchers said.
In a study published in Menopause, the researchers evaluated 178 postmenopausal women aged 50-65 years for FPHL. FPLH was determined based on photographs and on measures of hormone levels, hair density, and hair diameter.
The overall prevalence of FPHL was 52.2%. The hair loss was divided into three categories indicating mild, moderate, and severe (Ludwig grades I, II, and III) with prevalence of 73.2%, 22.6%, and 4.3%, respectively. The prevalence of FPHL also increased with age and time since menopause. In a simple logistic regression analysis, age 56 years and older and more than 6 years since menopause were significantly associated with FPHL (odds ratios, 3.41 and 1.98, respectively).
However, after adjustment for multiple variables, only a body mass index of 25 kg/m2 or higher also was associated with increased prevalence of FPHL (adjusted OR, 2.65).
A total of 60% of the study participants met criteria for low self-esteem, including all the women in the severe hair loss category.
“The postmenopausal women with FPHL in our cohort had lower total hair density, terminal hair density, hair thickness, hair unit density, and average hair per unit than those with normal hair patterns,” although vellus hair density was higher in women with FPHL, the researchers wrote in their discussion of the findings. This distinction may be caused in part by the shortened hair cycle and reduced anagen phase of velluslike follicles, they said.
The study findings were limited by several factors, including the cross-sectional design and the inclusion of only women from a single menopause clinic, which may not reflect FPHL in the general population, as well as the reliance on patients’ recall, the researchers noted. Another limitation was the inability to assess postmenopausal hormone levels, they added.
However, “This study may be the first FPHL study conducted in a menopause clinic that targeted only healthy postmenopausal women,” they wrote. More research is needed to determine the potential role of estrogen and testosterone on FPHL in postmenopausal women, and whether a history of polycystic ovarian syndrome has an effect, they said. Meanwhile, current study results may help clinicians and patients determine the most appropriate menopausal hormone therapies for postmenopausal women with FPHL, they concluded.
Consider lifestyle and self-esteem issues
The current study is important at this time because a larger proportion of women are either reaching menopause or are menopausal, said Constance Bohon, MD, a gynecologist in private practice in Washington, in an interview.
“Whatever we in the medical community can do to help women transition into the menopausal years with the least anxiety is important,” including helping women feel comfortable about their appearance, she said.
“For women in the peri- and postmenopausal years, hair loss is a relatively common concern,” Dr. Bohon said. However, in the current study, “I was surprised that it was associated with low self-esteem and obesity,” she noted. “For these women, it would be interesting to know whether they also had concerns about the appearance of their bodies, or just their hair loss,” she said. The question is whether the hair loss in and of itself caused low self-esteem in the study population, or whether it exacerbated their already poor self-assessment, Dr. Bohon said. “Another consideration is that perhaps these women were already feeling the effects of aging and were trying to change their appearance by using hair dyes, and now they find themselves losing hair as well,” she noted.
The takeaway message for clinicians is that discussions with perimenopausal and postmenopausal women should include the topic of hair loss along with hot flashes and night sweats, said Dr. Bohon.
Women who are experiencing hair loss or concerned about the possibility of hair loss should ask their doctors about possible interventions that may mitigate or prevent further hair loss, she said.
As for additional research, “the most important issue is to determine the factors that are associated with hair loss in the perimenopausal and postmenopausal years,” Dr. Bohon said. Research questions should include impact of dyeing or straightening hair on the likelihood of hair loss, and whether women with more severe hot flashes/night sweats and/or sleeplessness have more hair loss than women who do not experience any of the symptoms as they go through menopause, she emphasized.
Other considerations are whether certain diets or foods are more common among women who have more hair loss, and whether weight loss into a normal range or weight gain into a body mass index greater than 25 kg/m2 affects hair loss, said Dr. Bohon. Also, don’t discount the impact of stress, and whether women who have lost hair identify certain stressful times that preceded their hair loss, as well as what medications could be associated with hair loss, and whether hormone therapy might prevent hair loss, she said.
The study was supported by the Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University. The researchers had no financial conflicts to disclose. Dr. Bohon had no financial conflicts to disclose and serves on the Editorial Advisory Board of Ob.Gyn. News.
Female-pattern hair loss (FPHL) was identified in 52% of postmenopausal women, and 4% of these cases involved extensive baldness, based on data from 178 individuals.
FPHL can develop at any time from teenage years through and beyond menopause, wrote Sukanya Chaikittisilpa, MD, of Chulalongkorn University, Bangkok, and colleagues.
The cause of FPHL remains uncertain, but the presence of estrogen receptors in hair follicles suggests that the hormone changes of menopause may affect hair growth, the researchers said.
In a study published in Menopause, the researchers evaluated 178 postmenopausal women aged 50-65 years for FPHL. FPLH was determined based on photographs and on measures of hormone levels, hair density, and hair diameter.
The overall prevalence of FPHL was 52.2%. The hair loss was divided into three categories indicating mild, moderate, and severe (Ludwig grades I, II, and III) with prevalence of 73.2%, 22.6%, and 4.3%, respectively. The prevalence of FPHL also increased with age and time since menopause. In a simple logistic regression analysis, age 56 years and older and more than 6 years since menopause were significantly associated with FPHL (odds ratios, 3.41 and 1.98, respectively).
However, after adjustment for multiple variables, only a body mass index of 25 kg/m2 or higher also was associated with increased prevalence of FPHL (adjusted OR, 2.65).
A total of 60% of the study participants met criteria for low self-esteem, including all the women in the severe hair loss category.
“The postmenopausal women with FPHL in our cohort had lower total hair density, terminal hair density, hair thickness, hair unit density, and average hair per unit than those with normal hair patterns,” although vellus hair density was higher in women with FPHL, the researchers wrote in their discussion of the findings. This distinction may be caused in part by the shortened hair cycle and reduced anagen phase of velluslike follicles, they said.
The study findings were limited by several factors, including the cross-sectional design and the inclusion of only women from a single menopause clinic, which may not reflect FPHL in the general population, as well as the reliance on patients’ recall, the researchers noted. Another limitation was the inability to assess postmenopausal hormone levels, they added.
However, “This study may be the first FPHL study conducted in a menopause clinic that targeted only healthy postmenopausal women,” they wrote. More research is needed to determine the potential role of estrogen and testosterone on FPHL in postmenopausal women, and whether a history of polycystic ovarian syndrome has an effect, they said. Meanwhile, current study results may help clinicians and patients determine the most appropriate menopausal hormone therapies for postmenopausal women with FPHL, they concluded.
Consider lifestyle and self-esteem issues
The current study is important at this time because a larger proportion of women are either reaching menopause or are menopausal, said Constance Bohon, MD, a gynecologist in private practice in Washington, in an interview.
“Whatever we in the medical community can do to help women transition into the menopausal years with the least anxiety is important,” including helping women feel comfortable about their appearance, she said.
“For women in the peri- and postmenopausal years, hair loss is a relatively common concern,” Dr. Bohon said. However, in the current study, “I was surprised that it was associated with low self-esteem and obesity,” she noted. “For these women, it would be interesting to know whether they also had concerns about the appearance of their bodies, or just their hair loss,” she said. The question is whether the hair loss in and of itself caused low self-esteem in the study population, or whether it exacerbated their already poor self-assessment, Dr. Bohon said. “Another consideration is that perhaps these women were already feeling the effects of aging and were trying to change their appearance by using hair dyes, and now they find themselves losing hair as well,” she noted.
The takeaway message for clinicians is that discussions with perimenopausal and postmenopausal women should include the topic of hair loss along with hot flashes and night sweats, said Dr. Bohon.
Women who are experiencing hair loss or concerned about the possibility of hair loss should ask their doctors about possible interventions that may mitigate or prevent further hair loss, she said.
As for additional research, “the most important issue is to determine the factors that are associated with hair loss in the perimenopausal and postmenopausal years,” Dr. Bohon said. Research questions should include impact of dyeing or straightening hair on the likelihood of hair loss, and whether women with more severe hot flashes/night sweats and/or sleeplessness have more hair loss than women who do not experience any of the symptoms as they go through menopause, she emphasized.
Other considerations are whether certain diets or foods are more common among women who have more hair loss, and whether weight loss into a normal range or weight gain into a body mass index greater than 25 kg/m2 affects hair loss, said Dr. Bohon. Also, don’t discount the impact of stress, and whether women who have lost hair identify certain stressful times that preceded their hair loss, as well as what medications could be associated with hair loss, and whether hormone therapy might prevent hair loss, she said.
The study was supported by the Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University. The researchers had no financial conflicts to disclose. Dr. Bohon had no financial conflicts to disclose and serves on the Editorial Advisory Board of Ob.Gyn. News.
Female-pattern hair loss (FPHL) was identified in 52% of postmenopausal women, and 4% of these cases involved extensive baldness, based on data from 178 individuals.
FPHL can develop at any time from teenage years through and beyond menopause, wrote Sukanya Chaikittisilpa, MD, of Chulalongkorn University, Bangkok, and colleagues.
The cause of FPHL remains uncertain, but the presence of estrogen receptors in hair follicles suggests that the hormone changes of menopause may affect hair growth, the researchers said.
In a study published in Menopause, the researchers evaluated 178 postmenopausal women aged 50-65 years for FPHL. FPLH was determined based on photographs and on measures of hormone levels, hair density, and hair diameter.
The overall prevalence of FPHL was 52.2%. The hair loss was divided into three categories indicating mild, moderate, and severe (Ludwig grades I, II, and III) with prevalence of 73.2%, 22.6%, and 4.3%, respectively. The prevalence of FPHL also increased with age and time since menopause. In a simple logistic regression analysis, age 56 years and older and more than 6 years since menopause were significantly associated with FPHL (odds ratios, 3.41 and 1.98, respectively).
However, after adjustment for multiple variables, only a body mass index of 25 kg/m2 or higher also was associated with increased prevalence of FPHL (adjusted OR, 2.65).
A total of 60% of the study participants met criteria for low self-esteem, including all the women in the severe hair loss category.
“The postmenopausal women with FPHL in our cohort had lower total hair density, terminal hair density, hair thickness, hair unit density, and average hair per unit than those with normal hair patterns,” although vellus hair density was higher in women with FPHL, the researchers wrote in their discussion of the findings. This distinction may be caused in part by the shortened hair cycle and reduced anagen phase of velluslike follicles, they said.
The study findings were limited by several factors, including the cross-sectional design and the inclusion of only women from a single menopause clinic, which may not reflect FPHL in the general population, as well as the reliance on patients’ recall, the researchers noted. Another limitation was the inability to assess postmenopausal hormone levels, they added.
However, “This study may be the first FPHL study conducted in a menopause clinic that targeted only healthy postmenopausal women,” they wrote. More research is needed to determine the potential role of estrogen and testosterone on FPHL in postmenopausal women, and whether a history of polycystic ovarian syndrome has an effect, they said. Meanwhile, current study results may help clinicians and patients determine the most appropriate menopausal hormone therapies for postmenopausal women with FPHL, they concluded.
Consider lifestyle and self-esteem issues
The current study is important at this time because a larger proportion of women are either reaching menopause or are menopausal, said Constance Bohon, MD, a gynecologist in private practice in Washington, in an interview.
“Whatever we in the medical community can do to help women transition into the menopausal years with the least anxiety is important,” including helping women feel comfortable about their appearance, she said.
“For women in the peri- and postmenopausal years, hair loss is a relatively common concern,” Dr. Bohon said. However, in the current study, “I was surprised that it was associated with low self-esteem and obesity,” she noted. “For these women, it would be interesting to know whether they also had concerns about the appearance of their bodies, or just their hair loss,” she said. The question is whether the hair loss in and of itself caused low self-esteem in the study population, or whether it exacerbated their already poor self-assessment, Dr. Bohon said. “Another consideration is that perhaps these women were already feeling the effects of aging and were trying to change their appearance by using hair dyes, and now they find themselves losing hair as well,” she noted.
The takeaway message for clinicians is that discussions with perimenopausal and postmenopausal women should include the topic of hair loss along with hot flashes and night sweats, said Dr. Bohon.
Women who are experiencing hair loss or concerned about the possibility of hair loss should ask their doctors about possible interventions that may mitigate or prevent further hair loss, she said.
As for additional research, “the most important issue is to determine the factors that are associated with hair loss in the perimenopausal and postmenopausal years,” Dr. Bohon said. Research questions should include impact of dyeing or straightening hair on the likelihood of hair loss, and whether women with more severe hot flashes/night sweats and/or sleeplessness have more hair loss than women who do not experience any of the symptoms as they go through menopause, she emphasized.
Other considerations are whether certain diets or foods are more common among women who have more hair loss, and whether weight loss into a normal range or weight gain into a body mass index greater than 25 kg/m2 affects hair loss, said Dr. Bohon. Also, don’t discount the impact of stress, and whether women who have lost hair identify certain stressful times that preceded their hair loss, as well as what medications could be associated with hair loss, and whether hormone therapy might prevent hair loss, she said.
The study was supported by the Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University. The researchers had no financial conflicts to disclose. Dr. Bohon had no financial conflicts to disclose and serves on the Editorial Advisory Board of Ob.Gyn. News.
FROM MENOPAUSE
FDA approves neoadjuvant nivolumab/chemo for early-stage NSCLC
in combination with platinum-doublet chemotherapy, regardless of PDL-1 status.
Nivolumab is the first immune checkpoint inhibitor to be approved for resectable NSCLC; its three prior NSCLC indications are for metastatic disease, the agency said in its announcement.
Approval was based on the CheckMate 816 trial, which randomized 358 patients evenly to either nivolumab plus platinum doublets or to platinum doublets alone every 3 weeks for up to 3 cycles.
Trial participants had histologically confirmed stage IB, II, or IIIA disease, which was measurable by RECIST criteria. They were enrolled regardless of tumor PD-L1 status.
At surgery, the pathologic complete response rate was 24% in the nivolumab arm versus 2.2% in the chemotherapy-alone group.
Median event-free survival was 31.6 months with nivolumab but 20.8 months without it, which translated to a 37% reduction in the risk for progression, recurrence, or death following surgery. A trend toward better overall survival was not statistically significant, Bristol Myers Squibb said in its own announcement.
Nivolumab’s new neoadjuvant indication is for adult patients with resectable NSCLC (tumors greater than or equal to 4 cm or node positive). The recommended dosage is 360 mg in combination with platinum-doublet chemotherapy on the same day every 3 weeks for three cycles.
In a press release from Bristol Myers Squibb, CheckMate 816 investigator and Dana-Farber Cancer Institute thoracic oncologist Mark Awad, MD, PhD, called the approval “a turning point in how we treat resectable NSCLC.”
Patients with known EGFR mutations or ALK translocations, grade 2 or higher peripheral neuropathy, active autoimmune disease, or medical conditions requiring systemic immunosuppression were excluded.
There were no fatal adverse events in the nivolumab arm, but 30% of participants had serious adverse events, most commonly pneumonia and vomiting.
The most common side effects across all grades were nausea (38%), constipation (34%), fatigue (26%), decreased appetite (20%), and rash (20%). Surgical complications and hospital lengths were similar between the two study groups.
Rival checkpoint inhibitor pembrolizumab is also being investigated for neoadjuvant NSCLC.
A version of this article first appeared on Medscape.com.
in combination with platinum-doublet chemotherapy, regardless of PDL-1 status.
Nivolumab is the first immune checkpoint inhibitor to be approved for resectable NSCLC; its three prior NSCLC indications are for metastatic disease, the agency said in its announcement.
Approval was based on the CheckMate 816 trial, which randomized 358 patients evenly to either nivolumab plus platinum doublets or to platinum doublets alone every 3 weeks for up to 3 cycles.
Trial participants had histologically confirmed stage IB, II, or IIIA disease, which was measurable by RECIST criteria. They were enrolled regardless of tumor PD-L1 status.
At surgery, the pathologic complete response rate was 24% in the nivolumab arm versus 2.2% in the chemotherapy-alone group.
Median event-free survival was 31.6 months with nivolumab but 20.8 months without it, which translated to a 37% reduction in the risk for progression, recurrence, or death following surgery. A trend toward better overall survival was not statistically significant, Bristol Myers Squibb said in its own announcement.
Nivolumab’s new neoadjuvant indication is for adult patients with resectable NSCLC (tumors greater than or equal to 4 cm or node positive). The recommended dosage is 360 mg in combination with platinum-doublet chemotherapy on the same day every 3 weeks for three cycles.
In a press release from Bristol Myers Squibb, CheckMate 816 investigator and Dana-Farber Cancer Institute thoracic oncologist Mark Awad, MD, PhD, called the approval “a turning point in how we treat resectable NSCLC.”
Patients with known EGFR mutations or ALK translocations, grade 2 or higher peripheral neuropathy, active autoimmune disease, or medical conditions requiring systemic immunosuppression were excluded.
There were no fatal adverse events in the nivolumab arm, but 30% of participants had serious adverse events, most commonly pneumonia and vomiting.
The most common side effects across all grades were nausea (38%), constipation (34%), fatigue (26%), decreased appetite (20%), and rash (20%). Surgical complications and hospital lengths were similar between the two study groups.
Rival checkpoint inhibitor pembrolizumab is also being investigated for neoadjuvant NSCLC.
A version of this article first appeared on Medscape.com.
in combination with platinum-doublet chemotherapy, regardless of PDL-1 status.
Nivolumab is the first immune checkpoint inhibitor to be approved for resectable NSCLC; its three prior NSCLC indications are for metastatic disease, the agency said in its announcement.
Approval was based on the CheckMate 816 trial, which randomized 358 patients evenly to either nivolumab plus platinum doublets or to platinum doublets alone every 3 weeks for up to 3 cycles.
Trial participants had histologically confirmed stage IB, II, or IIIA disease, which was measurable by RECIST criteria. They were enrolled regardless of tumor PD-L1 status.
At surgery, the pathologic complete response rate was 24% in the nivolumab arm versus 2.2% in the chemotherapy-alone group.
Median event-free survival was 31.6 months with nivolumab but 20.8 months without it, which translated to a 37% reduction in the risk for progression, recurrence, or death following surgery. A trend toward better overall survival was not statistically significant, Bristol Myers Squibb said in its own announcement.
Nivolumab’s new neoadjuvant indication is for adult patients with resectable NSCLC (tumors greater than or equal to 4 cm or node positive). The recommended dosage is 360 mg in combination with platinum-doublet chemotherapy on the same day every 3 weeks for three cycles.
In a press release from Bristol Myers Squibb, CheckMate 816 investigator and Dana-Farber Cancer Institute thoracic oncologist Mark Awad, MD, PhD, called the approval “a turning point in how we treat resectable NSCLC.”
Patients with known EGFR mutations or ALK translocations, grade 2 or higher peripheral neuropathy, active autoimmune disease, or medical conditions requiring systemic immunosuppression were excluded.
There were no fatal adverse events in the nivolumab arm, but 30% of participants had serious adverse events, most commonly pneumonia and vomiting.
The most common side effects across all grades were nausea (38%), constipation (34%), fatigue (26%), decreased appetite (20%), and rash (20%). Surgical complications and hospital lengths were similar between the two study groups.
Rival checkpoint inhibitor pembrolizumab is also being investigated for neoadjuvant NSCLC.
A version of this article first appeared on Medscape.com.
Isolated Nodule and Generalized Lymphadenopathy
The Diagnosis: Blastic Plasmacytoid Dendritic Cell Neoplasm
A diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN) was rendered. Subsequent needle core biopsy of a left axillary lymph node as well as bone marrow aspiration and biopsy revealed a similar diffuse blastoid infiltrate with an identical immunophenotype to that in the skin biopsy from the pretibial mass and peripheral blood.
Previously known as blastic natural killer cell leukemia/lymphoma or agranular CD4+/CD56+ hematodermic neoplasm/tumor, BPDCN is a rare, clinically aggressive hematologic malignancy derived from the precursors of plasmacytoid dendritic cells. It often is diagnostically challenging, particularly when presenting at noncutaneous sites and in unusual (young) patient populations.1 It was included with other myeloid neoplasms in the 2008 World Health Organization classification; however, in the 2017 classification it was categorized as a separate entity. Blastic plasmacytoid dendritic cell neoplasm typically presents in the skin of elderly patients (age range at diagnosis, 61–67 years) with or without bone marrow involvement and systemic dissemination.1,2 The skin is the most common clinical site of disease in typical cases of BPDCN and often precedes bone marrow involvement. Thus, skin biopsy often is the key to making the diagnosis. Diagnosis of BPDCN may be delayed because of diagnostic pitfalls. Patients usually present with asymptomatic solitary or multiple lesions.3-5 Blastic plasmacytoid dendritic cell neoplasm can present as an isolated purplish nodule or bruiselike papule or more commonly as disseminated purplish nodules, papules, and macules. Isolated nodules are found on the head and lower limbs and can be more than 10 cm in diameter. Peripheral blood and bone marrow may be minimally involved at presentation but invariably become involved with the progression of disease. Cytopenia can occur at diagnosis and in a minority of severe cases indicates bone marrow failure.2-6
Skin involvement of BPDCN is thought to be secondary to the expression of skin migration molecules, such as cutaneous lymphocyte-associated antigen, one of the E-selectin ligands, which binds to E-selectin on high endothelial venules. In addition, the local dermal microenvironment of chemokines binding CXCR3, CXCR4, CCR6, or CCR7 present on neoplastic cells possibly leads to skin involvement. The full mechanism underlying the cutaneous tropism is still to be elucidated.4-7 Infiltration of the oral mucosa is seen in some patients, but it may be underreported. Mucosal disease typically appears similarly to cutaneous disease.
The cutaneous differential diagnosis for BPDCN depends on the clinical presentation, extent of disease spread, and thickness of infiltration. It includes common nonneoplastic diseases such as traumatic ecchymoses; purpuric disorders; extramedullary hematopoiesis; and soft-tissue neoplasms such as angiosarcoma, Kaposi sarcoma, neuroblastoma, and vascular metastases, as well as skin involvement by other hematologic neoplasms. An adequate incisional biopsy rather than a punch or shave biopsy is recommended for diagnosis. Dermatologists should alert the pathologist that BPDCN is in the clinical differential diagnosis when possible so that judicious use of appropriate immunophenotypic markers such as CD123, CD4, CD56, and T-cell leukemia/lymphoma protein 1 will avoid misdiagnosis of this aggressive condition, in addition to excluding acute myeloid leukemia, which also may express 3 of the above markers. However, most cases of acute myeloid leukemia lack terminal deoxynucleotidyl transferase (TdT) and express monocytic and other myeloid markers. Terminal deoxynucleotidyl transferase is positive in approximately one-third of cases of BPDCN, with expression in 10% to 80% of cells.1
It is important to include BPDCN in the differential diagnosis of immunophenotypically aberrant hematologic tumors. Diffuse large B-cell lymphoma, leg type, accounts for 4% of all primary cutaneous B-cell lymphomas.1 Compared with BPDCN, diffuse large B-cell lymphoma usually occurs in an older age group and is of B-cell lineage. Morphologically, these neoplasms are composed of a monotonous, diffuse, nonepidermotropic infiltrate of confluent sheets of centroblasts and immunoblasts (Figure 1). They may share immunohistochemical markers of CD79a, multiple myeloma 1, Bcl-2, and Bcl-6; however, they lack plasmacytoid dendritic cell (PDC)– associated antigens such as CD4, CD56, CD123, and T-cell leukemia/lymphoma protein 1.1
Adult T-cell leukemia/lymphoma is a neoplasm histologically composed of highly pleomorphic medium- to large-sized T cells with an irregular multilobated nuclear contour, so-called flower cells, in the peripheral blood. The nuclear chromatin is coarse and clumped with prominent nucleoli. Blastlike cells with dispersed chromatin are present in variable proportions. Most patients present with widespread lymph node and peripheral blood involvement. Skin is involved in more than half of patients with an epidermal as well as dermal pattern of infiltration (mainly perivascular)(Figure 2). Adult T-cell leukemia/lymphoma is endemic in several regions of the world, and the distribution is closely linked to the prevalence of human T-cell lymphotropic virus type 1 in the population. This neoplasm is of T-cell lineage and may share CD4 but not PDC-associated antigens with BPDCN.1
Cutaneous involvement by T-cell lymphoblastic leukemia/lymphoma (T-LBL) is a rare occurrence with a frequency of approximately 4.3%.8 T-cell lymphoblastic leukemia/lymphoma usually presents as multiple skin lesions throughout the body. Almost all cutaneous T-LBL cases are seen in association with bone marrow and/or mediastinal, lymph node, or extranodal involvement. Cutaneous T-LBLs present as a diffuse monomorphous infiltrate located in the entire dermis and subcutis without epidermotropism, composed of medium to large blasts with finely dispersed chromatin and relatively prominent nucleoli (Figure 3). Immunophenotyping studies show an immature T-cell immunophenotype, with expression of TdT (usually uniform), CD7, and cytoplasmic CD3 and an absence of PDC-associated antigens.8
Primary cutaneous γδ T-cell lymphoma (PCGDTL) is a neoplasm primarily involving the skin. Often rapidly fatal, PCGDTL has a broad clinical spectrum that may include indolent variants—subcutaneous, epidermotropic, and dermal. Patients typically present with nodular lesions that progress to ulceration and necrosis. Early lesions can be confused with erythema nodosum, mycosis fungoides, or infection. Histologically, they show variable epidermotropism as well as dermal and subcutaneous involvement by medium to large cells with coarse clumped chromatin (Figure 4). Large blastic cells with vesicular nuclei and prominent nucleoli are infrequent. In contrast to BPCDN, the neoplastic lymphocytes in dermal and subcutaneous PCGDTL typically are positive for T-cell intracellular antigen-1 and granzyme B with loss of CD4.9
At the time of presentation, 27% to 87% of BPDCN patients will have bone marrow involvement, 22% to 28% will have blood involvement, and 6% to 41% will have lymph node involvement.1-4,6,7,10,11 The clinical course is aggressive, with a median survival of 10.0 to 19.8 months, irrespective of the initial pattern of disease.1 Most cases have shown initial response to multiagent chemotherapy, but relapses with subsequent resistance to drugs regularly have been observed. Age has an adverse impact of prognosis. Low TdT expression has been associated with shorter survival.1 Approximately 10% to 20% of cases of BPDCN are associated with or develop into chronic myelogenous leukemia, myelodysplastic syndrome, or acute myeloid leukemia.1,4 Pediatric patients have a greater 5-year overall survival rate than older patients, and overall survival worsens with increasing age. The extent of cutaneous involvement and presence of systemic involvement at initial presentation do not seem to be strong predictors of survival.1,2,5-7,10-12 In a retrospective analysis of 90 patients, Julia et al12 found that the type of skin disease did not predict survival. Specifically, the presence of nodular lesions and disseminated skin involvement were not adverse prognostic factors compared with macular lesions limited to 1 or 2 body areas.12
- Facchetti F, Petrella T, Pileri SA. Blastic plasmacytoid dendritic cells neoplasm. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. World Health Organization; 2017:174-177.
- Jegalian AG, Facchetti F, Jaffe ES. Plasmacytoid dendritic cells: physiologic roles and pathologic states. Adv Anat Pathol. 2009;16:392-404.
- Shi Y, Wang E. Blastic plasmacytoid dendritic cell neoplasm: a clinicopathologic review. Arch Pathol Lab Med. 2014;138:564-569.
- Khoury JD, Medeiros LJ, Manning JT, et al. CD56(+) TdT(+) blastic natural killer cell tumor of the skin: a primitive systemic malignancy related to myelomonocytic leukemia. Cancer. 2002;94:2401-2408.
- Kolerova A, Sergeeva I, Krinitsyna J, et al. Blastic plasmacytoid dendritic cell neoplasm: case report and literature overview. Indian J Dermatol. 2020;65:217-221.
- Hirner JP, O’Malley JT, LeBoeuf NR. Blastic plasmacytoid dendritic cell neoplasm: the dermatologist’s perspective. Hematol Oncol Clin North Am. 2020;34:501-509.
- Guiducii C, Tripodo C, Gong M, et al. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J Exp Med. 2010;207:2931-2942.
- Khurana S, Beltran M, Jiang L, et al. Primary cutaneous T-cell lymphoblastic lymphoma: case report and literature review. Case Rep Hematol. 2019;2019:3540487. doi:10.1155/2019/3540487
- Gladys TE, Helm MF, Anderson BE, et al. Rapid onset of widespread nodules and lymphadenopathy. Cutis. 2020;106:132, 153-155.
- Gregorio J, Meller S, Conrad C, et al. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207:2921-2930.
- Guru Murthy GS, Pemmaraju N, Attallah E. Epidemiology and survival of blastic plasmacytoid dendritic cell neoplasm. Leuk Res. 2018;73:21-23.
- Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2012;169:579-586.
The Diagnosis: Blastic Plasmacytoid Dendritic Cell Neoplasm
A diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN) was rendered. Subsequent needle core biopsy of a left axillary lymph node as well as bone marrow aspiration and biopsy revealed a similar diffuse blastoid infiltrate with an identical immunophenotype to that in the skin biopsy from the pretibial mass and peripheral blood.
Previously known as blastic natural killer cell leukemia/lymphoma or agranular CD4+/CD56+ hematodermic neoplasm/tumor, BPDCN is a rare, clinically aggressive hematologic malignancy derived from the precursors of plasmacytoid dendritic cells. It often is diagnostically challenging, particularly when presenting at noncutaneous sites and in unusual (young) patient populations.1 It was included with other myeloid neoplasms in the 2008 World Health Organization classification; however, in the 2017 classification it was categorized as a separate entity. Blastic plasmacytoid dendritic cell neoplasm typically presents in the skin of elderly patients (age range at diagnosis, 61–67 years) with or without bone marrow involvement and systemic dissemination.1,2 The skin is the most common clinical site of disease in typical cases of BPDCN and often precedes bone marrow involvement. Thus, skin biopsy often is the key to making the diagnosis. Diagnosis of BPDCN may be delayed because of diagnostic pitfalls. Patients usually present with asymptomatic solitary or multiple lesions.3-5 Blastic plasmacytoid dendritic cell neoplasm can present as an isolated purplish nodule or bruiselike papule or more commonly as disseminated purplish nodules, papules, and macules. Isolated nodules are found on the head and lower limbs and can be more than 10 cm in diameter. Peripheral blood and bone marrow may be minimally involved at presentation but invariably become involved with the progression of disease. Cytopenia can occur at diagnosis and in a minority of severe cases indicates bone marrow failure.2-6
Skin involvement of BPDCN is thought to be secondary to the expression of skin migration molecules, such as cutaneous lymphocyte-associated antigen, one of the E-selectin ligands, which binds to E-selectin on high endothelial venules. In addition, the local dermal microenvironment of chemokines binding CXCR3, CXCR4, CCR6, or CCR7 present on neoplastic cells possibly leads to skin involvement. The full mechanism underlying the cutaneous tropism is still to be elucidated.4-7 Infiltration of the oral mucosa is seen in some patients, but it may be underreported. Mucosal disease typically appears similarly to cutaneous disease.
The cutaneous differential diagnosis for BPDCN depends on the clinical presentation, extent of disease spread, and thickness of infiltration. It includes common nonneoplastic diseases such as traumatic ecchymoses; purpuric disorders; extramedullary hematopoiesis; and soft-tissue neoplasms such as angiosarcoma, Kaposi sarcoma, neuroblastoma, and vascular metastases, as well as skin involvement by other hematologic neoplasms. An adequate incisional biopsy rather than a punch or shave biopsy is recommended for diagnosis. Dermatologists should alert the pathologist that BPDCN is in the clinical differential diagnosis when possible so that judicious use of appropriate immunophenotypic markers such as CD123, CD4, CD56, and T-cell leukemia/lymphoma protein 1 will avoid misdiagnosis of this aggressive condition, in addition to excluding acute myeloid leukemia, which also may express 3 of the above markers. However, most cases of acute myeloid leukemia lack terminal deoxynucleotidyl transferase (TdT) and express monocytic and other myeloid markers. Terminal deoxynucleotidyl transferase is positive in approximately one-third of cases of BPDCN, with expression in 10% to 80% of cells.1
It is important to include BPDCN in the differential diagnosis of immunophenotypically aberrant hematologic tumors. Diffuse large B-cell lymphoma, leg type, accounts for 4% of all primary cutaneous B-cell lymphomas.1 Compared with BPDCN, diffuse large B-cell lymphoma usually occurs in an older age group and is of B-cell lineage. Morphologically, these neoplasms are composed of a monotonous, diffuse, nonepidermotropic infiltrate of confluent sheets of centroblasts and immunoblasts (Figure 1). They may share immunohistochemical markers of CD79a, multiple myeloma 1, Bcl-2, and Bcl-6; however, they lack plasmacytoid dendritic cell (PDC)– associated antigens such as CD4, CD56, CD123, and T-cell leukemia/lymphoma protein 1.1

Adult T-cell leukemia/lymphoma is a neoplasm histologically composed of highly pleomorphic medium- to large-sized T cells with an irregular multilobated nuclear contour, so-called flower cells, in the peripheral blood. The nuclear chromatin is coarse and clumped with prominent nucleoli. Blastlike cells with dispersed chromatin are present in variable proportions. Most patients present with widespread lymph node and peripheral blood involvement. Skin is involved in more than half of patients with an epidermal as well as dermal pattern of infiltration (mainly perivascular)(Figure 2). Adult T-cell leukemia/lymphoma is endemic in several regions of the world, and the distribution is closely linked to the prevalence of human T-cell lymphotropic virus type 1 in the population. This neoplasm is of T-cell lineage and may share CD4 but not PDC-associated antigens with BPDCN.1

Cutaneous involvement by T-cell lymphoblastic leukemia/lymphoma (T-LBL) is a rare occurrence with a frequency of approximately 4.3%.8 T-cell lymphoblastic leukemia/lymphoma usually presents as multiple skin lesions throughout the body. Almost all cutaneous T-LBL cases are seen in association with bone marrow and/or mediastinal, lymph node, or extranodal involvement. Cutaneous T-LBLs present as a diffuse monomorphous infiltrate located in the entire dermis and subcutis without epidermotropism, composed of medium to large blasts with finely dispersed chromatin and relatively prominent nucleoli (Figure 3). Immunophenotyping studies show an immature T-cell immunophenotype, with expression of TdT (usually uniform), CD7, and cytoplasmic CD3 and an absence of PDC-associated antigens.8

Primary cutaneous γδ T-cell lymphoma (PCGDTL) is a neoplasm primarily involving the skin. Often rapidly fatal, PCGDTL has a broad clinical spectrum that may include indolent variants—subcutaneous, epidermotropic, and dermal. Patients typically present with nodular lesions that progress to ulceration and necrosis. Early lesions can be confused with erythema nodosum, mycosis fungoides, or infection. Histologically, they show variable epidermotropism as well as dermal and subcutaneous involvement by medium to large cells with coarse clumped chromatin (Figure 4). Large blastic cells with vesicular nuclei and prominent nucleoli are infrequent. In contrast to BPCDN, the neoplastic lymphocytes in dermal and subcutaneous PCGDTL typically are positive for T-cell intracellular antigen-1 and granzyme B with loss of CD4.9

At the time of presentation, 27% to 87% of BPDCN patients will have bone marrow involvement, 22% to 28% will have blood involvement, and 6% to 41% will have lymph node involvement.1-4,6,7,10,11 The clinical course is aggressive, with a median survival of 10.0 to 19.8 months, irrespective of the initial pattern of disease.1 Most cases have shown initial response to multiagent chemotherapy, but relapses with subsequent resistance to drugs regularly have been observed. Age has an adverse impact of prognosis. Low TdT expression has been associated with shorter survival.1 Approximately 10% to 20% of cases of BPDCN are associated with or develop into chronic myelogenous leukemia, myelodysplastic syndrome, or acute myeloid leukemia.1,4 Pediatric patients have a greater 5-year overall survival rate than older patients, and overall survival worsens with increasing age. The extent of cutaneous involvement and presence of systemic involvement at initial presentation do not seem to be strong predictors of survival.1,2,5-7,10-12 In a retrospective analysis of 90 patients, Julia et al12 found that the type of skin disease did not predict survival. Specifically, the presence of nodular lesions and disseminated skin involvement were not adverse prognostic factors compared with macular lesions limited to 1 or 2 body areas.12
The Diagnosis: Blastic Plasmacytoid Dendritic Cell Neoplasm
A diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN) was rendered. Subsequent needle core biopsy of a left axillary lymph node as well as bone marrow aspiration and biopsy revealed a similar diffuse blastoid infiltrate with an identical immunophenotype to that in the skin biopsy from the pretibial mass and peripheral blood.
Previously known as blastic natural killer cell leukemia/lymphoma or agranular CD4+/CD56+ hematodermic neoplasm/tumor, BPDCN is a rare, clinically aggressive hematologic malignancy derived from the precursors of plasmacytoid dendritic cells. It often is diagnostically challenging, particularly when presenting at noncutaneous sites and in unusual (young) patient populations.1 It was included with other myeloid neoplasms in the 2008 World Health Organization classification; however, in the 2017 classification it was categorized as a separate entity. Blastic plasmacytoid dendritic cell neoplasm typically presents in the skin of elderly patients (age range at diagnosis, 61–67 years) with or without bone marrow involvement and systemic dissemination.1,2 The skin is the most common clinical site of disease in typical cases of BPDCN and often precedes bone marrow involvement. Thus, skin biopsy often is the key to making the diagnosis. Diagnosis of BPDCN may be delayed because of diagnostic pitfalls. Patients usually present with asymptomatic solitary or multiple lesions.3-5 Blastic plasmacytoid dendritic cell neoplasm can present as an isolated purplish nodule or bruiselike papule or more commonly as disseminated purplish nodules, papules, and macules. Isolated nodules are found on the head and lower limbs and can be more than 10 cm in diameter. Peripheral blood and bone marrow may be minimally involved at presentation but invariably become involved with the progression of disease. Cytopenia can occur at diagnosis and in a minority of severe cases indicates bone marrow failure.2-6
Skin involvement of BPDCN is thought to be secondary to the expression of skin migration molecules, such as cutaneous lymphocyte-associated antigen, one of the E-selectin ligands, which binds to E-selectin on high endothelial venules. In addition, the local dermal microenvironment of chemokines binding CXCR3, CXCR4, CCR6, or CCR7 present on neoplastic cells possibly leads to skin involvement. The full mechanism underlying the cutaneous tropism is still to be elucidated.4-7 Infiltration of the oral mucosa is seen in some patients, but it may be underreported. Mucosal disease typically appears similarly to cutaneous disease.
The cutaneous differential diagnosis for BPDCN depends on the clinical presentation, extent of disease spread, and thickness of infiltration. It includes common nonneoplastic diseases such as traumatic ecchymoses; purpuric disorders; extramedullary hematopoiesis; and soft-tissue neoplasms such as angiosarcoma, Kaposi sarcoma, neuroblastoma, and vascular metastases, as well as skin involvement by other hematologic neoplasms. An adequate incisional biopsy rather than a punch or shave biopsy is recommended for diagnosis. Dermatologists should alert the pathologist that BPDCN is in the clinical differential diagnosis when possible so that judicious use of appropriate immunophenotypic markers such as CD123, CD4, CD56, and T-cell leukemia/lymphoma protein 1 will avoid misdiagnosis of this aggressive condition, in addition to excluding acute myeloid leukemia, which also may express 3 of the above markers. However, most cases of acute myeloid leukemia lack terminal deoxynucleotidyl transferase (TdT) and express monocytic and other myeloid markers. Terminal deoxynucleotidyl transferase is positive in approximately one-third of cases of BPDCN, with expression in 10% to 80% of cells.1
It is important to include BPDCN in the differential diagnosis of immunophenotypically aberrant hematologic tumors. Diffuse large B-cell lymphoma, leg type, accounts for 4% of all primary cutaneous B-cell lymphomas.1 Compared with BPDCN, diffuse large B-cell lymphoma usually occurs in an older age group and is of B-cell lineage. Morphologically, these neoplasms are composed of a monotonous, diffuse, nonepidermotropic infiltrate of confluent sheets of centroblasts and immunoblasts (Figure 1). They may share immunohistochemical markers of CD79a, multiple myeloma 1, Bcl-2, and Bcl-6; however, they lack plasmacytoid dendritic cell (PDC)– associated antigens such as CD4, CD56, CD123, and T-cell leukemia/lymphoma protein 1.1

Adult T-cell leukemia/lymphoma is a neoplasm histologically composed of highly pleomorphic medium- to large-sized T cells with an irregular multilobated nuclear contour, so-called flower cells, in the peripheral blood. The nuclear chromatin is coarse and clumped with prominent nucleoli. Blastlike cells with dispersed chromatin are present in variable proportions. Most patients present with widespread lymph node and peripheral blood involvement. Skin is involved in more than half of patients with an epidermal as well as dermal pattern of infiltration (mainly perivascular)(Figure 2). Adult T-cell leukemia/lymphoma is endemic in several regions of the world, and the distribution is closely linked to the prevalence of human T-cell lymphotropic virus type 1 in the population. This neoplasm is of T-cell lineage and may share CD4 but not PDC-associated antigens with BPDCN.1

Cutaneous involvement by T-cell lymphoblastic leukemia/lymphoma (T-LBL) is a rare occurrence with a frequency of approximately 4.3%.8 T-cell lymphoblastic leukemia/lymphoma usually presents as multiple skin lesions throughout the body. Almost all cutaneous T-LBL cases are seen in association with bone marrow and/or mediastinal, lymph node, or extranodal involvement. Cutaneous T-LBLs present as a diffuse monomorphous infiltrate located in the entire dermis and subcutis without epidermotropism, composed of medium to large blasts with finely dispersed chromatin and relatively prominent nucleoli (Figure 3). Immunophenotyping studies show an immature T-cell immunophenotype, with expression of TdT (usually uniform), CD7, and cytoplasmic CD3 and an absence of PDC-associated antigens.8

Primary cutaneous γδ T-cell lymphoma (PCGDTL) is a neoplasm primarily involving the skin. Often rapidly fatal, PCGDTL has a broad clinical spectrum that may include indolent variants—subcutaneous, epidermotropic, and dermal. Patients typically present with nodular lesions that progress to ulceration and necrosis. Early lesions can be confused with erythema nodosum, mycosis fungoides, or infection. Histologically, they show variable epidermotropism as well as dermal and subcutaneous involvement by medium to large cells with coarse clumped chromatin (Figure 4). Large blastic cells with vesicular nuclei and prominent nucleoli are infrequent. In contrast to BPCDN, the neoplastic lymphocytes in dermal and subcutaneous PCGDTL typically are positive for T-cell intracellular antigen-1 and granzyme B with loss of CD4.9

At the time of presentation, 27% to 87% of BPDCN patients will have bone marrow involvement, 22% to 28% will have blood involvement, and 6% to 41% will have lymph node involvement.1-4,6,7,10,11 The clinical course is aggressive, with a median survival of 10.0 to 19.8 months, irrespective of the initial pattern of disease.1 Most cases have shown initial response to multiagent chemotherapy, but relapses with subsequent resistance to drugs regularly have been observed. Age has an adverse impact of prognosis. Low TdT expression has been associated with shorter survival.1 Approximately 10% to 20% of cases of BPDCN are associated with or develop into chronic myelogenous leukemia, myelodysplastic syndrome, or acute myeloid leukemia.1,4 Pediatric patients have a greater 5-year overall survival rate than older patients, and overall survival worsens with increasing age. The extent of cutaneous involvement and presence of systemic involvement at initial presentation do not seem to be strong predictors of survival.1,2,5-7,10-12 In a retrospective analysis of 90 patients, Julia et al12 found that the type of skin disease did not predict survival. Specifically, the presence of nodular lesions and disseminated skin involvement were not adverse prognostic factors compared with macular lesions limited to 1 or 2 body areas.12
- Facchetti F, Petrella T, Pileri SA. Blastic plasmacytoid dendritic cells neoplasm. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. World Health Organization; 2017:174-177.
- Jegalian AG, Facchetti F, Jaffe ES. Plasmacytoid dendritic cells: physiologic roles and pathologic states. Adv Anat Pathol. 2009;16:392-404.
- Shi Y, Wang E. Blastic plasmacytoid dendritic cell neoplasm: a clinicopathologic review. Arch Pathol Lab Med. 2014;138:564-569.
- Khoury JD, Medeiros LJ, Manning JT, et al. CD56(+) TdT(+) blastic natural killer cell tumor of the skin: a primitive systemic malignancy related to myelomonocytic leukemia. Cancer. 2002;94:2401-2408.
- Kolerova A, Sergeeva I, Krinitsyna J, et al. Blastic plasmacytoid dendritic cell neoplasm: case report and literature overview. Indian J Dermatol. 2020;65:217-221.
- Hirner JP, O’Malley JT, LeBoeuf NR. Blastic plasmacytoid dendritic cell neoplasm: the dermatologist’s perspective. Hematol Oncol Clin North Am. 2020;34:501-509.
- Guiducii C, Tripodo C, Gong M, et al. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J Exp Med. 2010;207:2931-2942.
- Khurana S, Beltran M, Jiang L, et al. Primary cutaneous T-cell lymphoblastic lymphoma: case report and literature review. Case Rep Hematol. 2019;2019:3540487. doi:10.1155/2019/3540487
- Gladys TE, Helm MF, Anderson BE, et al. Rapid onset of widespread nodules and lymphadenopathy. Cutis. 2020;106:132, 153-155.
- Gregorio J, Meller S, Conrad C, et al. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207:2921-2930.
- Guru Murthy GS, Pemmaraju N, Attallah E. Epidemiology and survival of blastic plasmacytoid dendritic cell neoplasm. Leuk Res. 2018;73:21-23.
- Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2012;169:579-586.
- Facchetti F, Petrella T, Pileri SA. Blastic plasmacytoid dendritic cells neoplasm. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. World Health Organization; 2017:174-177.
- Jegalian AG, Facchetti F, Jaffe ES. Plasmacytoid dendritic cells: physiologic roles and pathologic states. Adv Anat Pathol. 2009;16:392-404.
- Shi Y, Wang E. Blastic plasmacytoid dendritic cell neoplasm: a clinicopathologic review. Arch Pathol Lab Med. 2014;138:564-569.
- Khoury JD, Medeiros LJ, Manning JT, et al. CD56(+) TdT(+) blastic natural killer cell tumor of the skin: a primitive systemic malignancy related to myelomonocytic leukemia. Cancer. 2002;94:2401-2408.
- Kolerova A, Sergeeva I, Krinitsyna J, et al. Blastic plasmacytoid dendritic cell neoplasm: case report and literature overview. Indian J Dermatol. 2020;65:217-221.
- Hirner JP, O’Malley JT, LeBoeuf NR. Blastic plasmacytoid dendritic cell neoplasm: the dermatologist’s perspective. Hematol Oncol Clin North Am. 2020;34:501-509.
- Guiducii C, Tripodo C, Gong M, et al. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J Exp Med. 2010;207:2931-2942.
- Khurana S, Beltran M, Jiang L, et al. Primary cutaneous T-cell lymphoblastic lymphoma: case report and literature review. Case Rep Hematol. 2019;2019:3540487. doi:10.1155/2019/3540487
- Gladys TE, Helm MF, Anderson BE, et al. Rapid onset of widespread nodules and lymphadenopathy. Cutis. 2020;106:132, 153-155.
- Gregorio J, Meller S, Conrad C, et al. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207:2921-2930.
- Guru Murthy GS, Pemmaraju N, Attallah E. Epidemiology and survival of blastic plasmacytoid dendritic cell neoplasm. Leuk Res. 2018;73:21-23.
- Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2012;169:579-586.
A 23-year-old man presented with skin that bruised easily, pancytopenia, recent fatigue, fever, and loss of appetite, along with a nontender, brown-purple, left anterior pretibial mass of 2 years’ duration (top). Computed tomography showed diffuse lymphadenopathy involving the inguinal, mesenteric, retroperitoneal, mediastinal, and axillary regions. A biopsy of the mass showed a dense monomorphous infiltrate of medium-sized blastoid cells with small or inconspicuous nucleoli (bottom). The lesion diffusely involved the dermis and extended into the subcutaneous tissue but spared the epidermis. Flow cytometry immunophenotyping of peripheral blood neoplastic cells (bottom [inset]) showed high-level expression of CD123 together with expression of CD4, CD56, CD45RA, and CD43 but a lack of expression of any other myelomonocytic or lymphoid lineage–associated markers.