User login
Complex link between gut microbiome and immunotherapy response in advanced melanoma
A large-scale than previously thought.
Overall, researchers identified a panel of species, including Roseburia spp. and Akkermansia muciniphila, associated with responses to ICI therapy. However, no single species was a “fully consistent biomarker” across the studies, the authors explain.
This “machine learning analysis confirmed the link between the microbiome and overall response rates (ORRs) and progression-free survival (PFS) with ICIs but also revealed limited reproducibility of microbiome-based signatures across cohorts,” Karla A. Lee, PhD, a clinical research fellow at King’s College London, and colleagues report. The results suggest that “the microbiome is predictive of response in some, but not all, cohorts.”
The findings were published online Feb. 28 in Nature Medicine.
Despite recent advances in targeted therapies for melanoma, less than half of the those who receive a single-agent ICI respond, and those who receive combination ICI therapy often suffer from severe drug toxicity problems. That is why finding patients more likely to respond to a single-agent ICI has become a priority.
Previous studies have identified the gut microbiome as “a potential biomarker of response, as well as a therapeutic target” in melanoma and other malignancies, but “little consensus exists on which microbiome characteristics are associated with treatment responses in the human setting,” the authors explain.
To further clarify the microbiome–immunotherapy relationship, the researchers performed metagenomic sequencing of stool samples collected from 165 ICI-naive patients with unresectable stage III or IV cutaneous melanoma from 5 observational cohorts in the Netherlands, United Kingdom, and Spain. These data were integrated with 147 samples from publicly available datasets.
First, the authors highlighted the variability in findings across these observational studies. For instance, they analyzed stool samples from one UK-based observational study of patients with melanoma (PRIMM-UK) and found a small but statistically significant difference in the microbiome composition of immunotherapy responders versus nonresponders (P = .05) but did not find such an association in a parallel study in the Netherlands (PRIMM-NL, P = .61).
The investigators also explored biomarkers of response across different cohorts and found several standouts. In trials using ORR as an endpoint, two uncultivated Roseburia species (CAG:182 and CAG:471) were associated with responses to ICIs. For patients with available PFS data, Phascolarctobacterium succinatutens and Lactobacillus vaginalis were “enriched in responders” across 7 datasets and significant in 3 of the 8 meta-analysis approaches. A muciniphila and Dorea formicigenerans were also associated with ORR and PFS at 12 months in several meta-analyses.
However, “no single bacterium was a fully consistent biomarker of response across all datasets,” the authors wrote.
Still, the findings could have important implications for the more than 50% of patients with advanced melanoma who don’t respond to single-agent ICI therapy.
“Our study shows that studying the microbiome is important to improve and personalize immunotherapy treatments for melanoma,” study coauthor Nicola Segata, PhD, principal investigator in the Laboratory of Computational Metagenomics, University of Trento, Italy, said in a press release. “However, it also suggests that because of the person-to-person variability of the gut microbiome, even larger studies must be carried out to understand the specific gut microbial features that are more likely to lead to a positive response to immunotherapy.”
Coauthor Tim Spector, PhD, head of the Department of Twin Research & Genetic Epidemiology at King’s College London, added that “the ultimate goal is to identify which specific features of the microbiome are directly influencing the clinical benefits of immunotherapy to exploit these features in new personalized approaches to support cancer immunotherapy.”
In the meantime, he said, “this study highlights the potential impact of good diet and gut health on chances of survival in patients undergoing immunotherapy.”
This study was coordinated by King’s College London, CIBIO Department of the University of Trento and European Institute of Oncology in Italy, and the University of Groningen in the Netherlands, and was funded by the Seerave Foundation. Dr. Lee, Dr. Segata, and Dr. Spector have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large-scale than previously thought.
Overall, researchers identified a panel of species, including Roseburia spp. and Akkermansia muciniphila, associated with responses to ICI therapy. However, no single species was a “fully consistent biomarker” across the studies, the authors explain.
This “machine learning analysis confirmed the link between the microbiome and overall response rates (ORRs) and progression-free survival (PFS) with ICIs but also revealed limited reproducibility of microbiome-based signatures across cohorts,” Karla A. Lee, PhD, a clinical research fellow at King’s College London, and colleagues report. The results suggest that “the microbiome is predictive of response in some, but not all, cohorts.”
The findings were published online Feb. 28 in Nature Medicine.
Despite recent advances in targeted therapies for melanoma, less than half of the those who receive a single-agent ICI respond, and those who receive combination ICI therapy often suffer from severe drug toxicity problems. That is why finding patients more likely to respond to a single-agent ICI has become a priority.
Previous studies have identified the gut microbiome as “a potential biomarker of response, as well as a therapeutic target” in melanoma and other malignancies, but “little consensus exists on which microbiome characteristics are associated with treatment responses in the human setting,” the authors explain.
To further clarify the microbiome–immunotherapy relationship, the researchers performed metagenomic sequencing of stool samples collected from 165 ICI-naive patients with unresectable stage III or IV cutaneous melanoma from 5 observational cohorts in the Netherlands, United Kingdom, and Spain. These data were integrated with 147 samples from publicly available datasets.
First, the authors highlighted the variability in findings across these observational studies. For instance, they analyzed stool samples from one UK-based observational study of patients with melanoma (PRIMM-UK) and found a small but statistically significant difference in the microbiome composition of immunotherapy responders versus nonresponders (P = .05) but did not find such an association in a parallel study in the Netherlands (PRIMM-NL, P = .61).
The investigators also explored biomarkers of response across different cohorts and found several standouts. In trials using ORR as an endpoint, two uncultivated Roseburia species (CAG:182 and CAG:471) were associated with responses to ICIs. For patients with available PFS data, Phascolarctobacterium succinatutens and Lactobacillus vaginalis were “enriched in responders” across 7 datasets and significant in 3 of the 8 meta-analysis approaches. A muciniphila and Dorea formicigenerans were also associated with ORR and PFS at 12 months in several meta-analyses.
However, “no single bacterium was a fully consistent biomarker of response across all datasets,” the authors wrote.
Still, the findings could have important implications for the more than 50% of patients with advanced melanoma who don’t respond to single-agent ICI therapy.
“Our study shows that studying the microbiome is important to improve and personalize immunotherapy treatments for melanoma,” study coauthor Nicola Segata, PhD, principal investigator in the Laboratory of Computational Metagenomics, University of Trento, Italy, said in a press release. “However, it also suggests that because of the person-to-person variability of the gut microbiome, even larger studies must be carried out to understand the specific gut microbial features that are more likely to lead to a positive response to immunotherapy.”
Coauthor Tim Spector, PhD, head of the Department of Twin Research & Genetic Epidemiology at King’s College London, added that “the ultimate goal is to identify which specific features of the microbiome are directly influencing the clinical benefits of immunotherapy to exploit these features in new personalized approaches to support cancer immunotherapy.”
In the meantime, he said, “this study highlights the potential impact of good diet and gut health on chances of survival in patients undergoing immunotherapy.”
This study was coordinated by King’s College London, CIBIO Department of the University of Trento and European Institute of Oncology in Italy, and the University of Groningen in the Netherlands, and was funded by the Seerave Foundation. Dr. Lee, Dr. Segata, and Dr. Spector have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large-scale than previously thought.
Overall, researchers identified a panel of species, including Roseburia spp. and Akkermansia muciniphila, associated with responses to ICI therapy. However, no single species was a “fully consistent biomarker” across the studies, the authors explain.
This “machine learning analysis confirmed the link between the microbiome and overall response rates (ORRs) and progression-free survival (PFS) with ICIs but also revealed limited reproducibility of microbiome-based signatures across cohorts,” Karla A. Lee, PhD, a clinical research fellow at King’s College London, and colleagues report. The results suggest that “the microbiome is predictive of response in some, but not all, cohorts.”
The findings were published online Feb. 28 in Nature Medicine.
Despite recent advances in targeted therapies for melanoma, less than half of the those who receive a single-agent ICI respond, and those who receive combination ICI therapy often suffer from severe drug toxicity problems. That is why finding patients more likely to respond to a single-agent ICI has become a priority.
Previous studies have identified the gut microbiome as “a potential biomarker of response, as well as a therapeutic target” in melanoma and other malignancies, but “little consensus exists on which microbiome characteristics are associated with treatment responses in the human setting,” the authors explain.
To further clarify the microbiome–immunotherapy relationship, the researchers performed metagenomic sequencing of stool samples collected from 165 ICI-naive patients with unresectable stage III or IV cutaneous melanoma from 5 observational cohorts in the Netherlands, United Kingdom, and Spain. These data were integrated with 147 samples from publicly available datasets.
First, the authors highlighted the variability in findings across these observational studies. For instance, they analyzed stool samples from one UK-based observational study of patients with melanoma (PRIMM-UK) and found a small but statistically significant difference in the microbiome composition of immunotherapy responders versus nonresponders (P = .05) but did not find such an association in a parallel study in the Netherlands (PRIMM-NL, P = .61).
The investigators also explored biomarkers of response across different cohorts and found several standouts. In trials using ORR as an endpoint, two uncultivated Roseburia species (CAG:182 and CAG:471) were associated with responses to ICIs. For patients with available PFS data, Phascolarctobacterium succinatutens and Lactobacillus vaginalis were “enriched in responders” across 7 datasets and significant in 3 of the 8 meta-analysis approaches. A muciniphila and Dorea formicigenerans were also associated with ORR and PFS at 12 months in several meta-analyses.
However, “no single bacterium was a fully consistent biomarker of response across all datasets,” the authors wrote.
Still, the findings could have important implications for the more than 50% of patients with advanced melanoma who don’t respond to single-agent ICI therapy.
“Our study shows that studying the microbiome is important to improve and personalize immunotherapy treatments for melanoma,” study coauthor Nicola Segata, PhD, principal investigator in the Laboratory of Computational Metagenomics, University of Trento, Italy, said in a press release. “However, it also suggests that because of the person-to-person variability of the gut microbiome, even larger studies must be carried out to understand the specific gut microbial features that are more likely to lead to a positive response to immunotherapy.”
Coauthor Tim Spector, PhD, head of the Department of Twin Research & Genetic Epidemiology at King’s College London, added that “the ultimate goal is to identify which specific features of the microbiome are directly influencing the clinical benefits of immunotherapy to exploit these features in new personalized approaches to support cancer immunotherapy.”
In the meantime, he said, “this study highlights the potential impact of good diet and gut health on chances of survival in patients undergoing immunotherapy.”
This study was coordinated by King’s College London, CIBIO Department of the University of Trento and European Institute of Oncology in Italy, and the University of Groningen in the Netherlands, and was funded by the Seerave Foundation. Dr. Lee, Dr. Segata, and Dr. Spector have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Methotrexate plus leflunomide proves effective for PsA
A new study has found that methotrexate plus leflunomide outperforms methotrexate alone as a treatment option for patients with psoriatic arthritis (PsA).
“We believe that prescribing this combination in routine practice is viable when combined with shared decision-making and strict monitoring of side effects,” write Michelle L.M. Mulder, MD, of the department of rheumatology at Sint Maartenskliniek in Nijmegen, the Netherlands, and her coauthors. Their findings were published in The Lancet Rheumatology.
The latest treatment guidelines from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the European Alliance of Associations for Rheumatology recommend conventional synthetic disease-modifying antirheumatic drugs for patients with active PsA, but Dr. Mulder and her colleagues note a distinct lack of information on their effectiveness, especially this particular combination.
To assess the efficacy and safety of methotrexate plus leflunomide, they launched a single-center, double-blind, randomized trial that included 78 Dutch patients with PsA. The majority of the participants in this trial – dubbed COMPLETE-PsA – were men (64%), and the median age of the patients was 55 years. All had active disease at baseline; the median swollen joint count (SJC) and tender joint count were 4.0 in both groups.
Participants were assigned to receive either methotrexate plus leflunomide (n = 39) or methotrexate plus placebo (n = 39). After 16 weeks, mean Psoriatic Arthritis Disease Activity Score (PASDAS) had improved for patients in the combination therapy group in comparison with the monotherapy group (3.1; standard deviation, 1.4 vs. 3.7; SD, 1.3; treatment difference, –0.6; 90% confidence interval, –1.0 to –0.1; P = .025). The combination therapy group also achieved PASDAS low disease activity at a higher rate (59%) than did the monotherapy group (34%; P = .019).
Other notable differences after 16 weeks included improvements in SJC for 66 joints (–3.0 in the combination therapy group vs. –2.0 in the monotherapy group) and significantly better skin and nail measures – such as active psoriasis and change in body surface area – in the methotrexate plus leflunomide group.
When asked who should be prescribed the combination therapy and who should be prescribed methotrexate going forward, Dr. Mulder told this news organization, “At the moment, we have insufficient knowledge on who will benefit most or who will develop clinically relevant side effects. It seems warranted to discuss with every patient which approach they would prefer. This could be a step-down or -up approach.
“We hope to be able to better predict treatment response and side effects in the future via post hoc analysis of our study and via extensive flow-cytometric phenotyping of immune blood cells taken at baseline,” she added.
Three patients in the combination therapy group experienced serious adverse events, two of which were deemed unrelated to leflunomide. The most frequently occurring adverse events were nausea or vomiting, tiredness, and elevated alanine aminotransferase. Mild adverse events were more common in the methotrexate plus leflunomide group. No participants died, and all patients with adverse events recovered completely.
“It appears good practice to do blood draws for laboratory tests on liver enzymes at least monthly for the first 4 months and every 4 months after that once stable dosing is achieved, as well as have a telephone consultation after 6-8 weeks to talk about possible side effects a patient might experience and change or add therapy if necessary,” Dr. Mulder added.
Study turns perception of combination therapy into reality
It had already been perceived by rheumatologists that methotrexate plus leflunomide was an effective combo for PsA, and this study reinforces those beliefs, Clementina López-Medina, MD, PhD, and colleagues from the University of Cordoba (Spain), write in an accompanying editorial.
They highlight this study’s notable strengths, one of which was defining “active disease” as two or more swollen joints, which opened the study up to a larger patient population. The editorialists also underline the confirmation that leflunomide plus methotrexate reduces both joint symptoms and skin involvement in this subset of patients, which had also been found in a previous study.
“Leflunomide is usually considered as a second-line option after methotrexate is unsuccessful,” they note, “despite the fact that methotrexate did not show superiority over placebo in previous trials.”
The editorialists were not surprised that the combination therapy was more toxic than the monotherapy. Rheumatologists could use these data to individualize treatment accordingly, they write, while keeping an eye on “gastrointestinal disturbances.”
Overall, Dr. López-Medina and colleagues say that the study results should “be considered not only in daily clinical practice but also in the development of future recommendations.”
Leflunomide: Forgotten no longer, at least for PsA
“I think we probably underutilize leflunomide,” Arthur Kavanaugh, MD, professor of medicine and director of the Center for Innovative Therapy at the University of California, San Diego, told this news organization. “Sometimes medicines get ‘old,’ for lack of a better term, and fall a little bit of out of favor, sometimes unnecessarily. Leflunomide falls into that category. Because it’s older, it doesn’t get as much buzz as what’s new and shiny.
“I was not surprised by the results on the joints,” he said, “because we know from previous studies that leflunomide works in that regard. What did surprise me is that the skin got better, especially with the combination.”
Regarding the side effects for the combination therapy, he commended the authors for limiting potential uncertainty by using such a high dose of methotrexate.
“By going with a dose of 25 mg [per week], no one can say, ‘They pulled their punches and methotrexate monotherapy would’ve been just as good if it was given at a higher dose,’ “ he said. “And they also used leflunomide at a high dose. It makes you wonder: Could you use lower doses, and do lower doses mean fewer lab test abnormalities? This positive study does lend itself to some other permutations in terms of study design.
“Even though this was a small study,” he added, “it brings us right back to: We should really consider leflunomide in the treatment of PsA.”
The authors acknowledge their study’s limitations, including the fact that it was conducted in a single country and the absence of a nontreatment placebo group. They also note the higher percentage of women in the methotrexate plus leflunomide group, “which might have lowered the treatment response and increased the adverse event rate, resulting in bias.”
The study was funded by a Regional Junior Researcher Grant from Sint Maartenskliniek. The authors reported numerous potential conflicts of interest, including receiving payment, research grants, and consulting and speaker fees from various pharmaceutical companies.
A version of this article first appeared on Medscape.com.
A new study has found that methotrexate plus leflunomide outperforms methotrexate alone as a treatment option for patients with psoriatic arthritis (PsA).
“We believe that prescribing this combination in routine practice is viable when combined with shared decision-making and strict monitoring of side effects,” write Michelle L.M. Mulder, MD, of the department of rheumatology at Sint Maartenskliniek in Nijmegen, the Netherlands, and her coauthors. Their findings were published in The Lancet Rheumatology.
The latest treatment guidelines from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the European Alliance of Associations for Rheumatology recommend conventional synthetic disease-modifying antirheumatic drugs for patients with active PsA, but Dr. Mulder and her colleagues note a distinct lack of information on their effectiveness, especially this particular combination.
To assess the efficacy and safety of methotrexate plus leflunomide, they launched a single-center, double-blind, randomized trial that included 78 Dutch patients with PsA. The majority of the participants in this trial – dubbed COMPLETE-PsA – were men (64%), and the median age of the patients was 55 years. All had active disease at baseline; the median swollen joint count (SJC) and tender joint count were 4.0 in both groups.
Participants were assigned to receive either methotrexate plus leflunomide (n = 39) or methotrexate plus placebo (n = 39). After 16 weeks, mean Psoriatic Arthritis Disease Activity Score (PASDAS) had improved for patients in the combination therapy group in comparison with the monotherapy group (3.1; standard deviation, 1.4 vs. 3.7; SD, 1.3; treatment difference, –0.6; 90% confidence interval, –1.0 to –0.1; P = .025). The combination therapy group also achieved PASDAS low disease activity at a higher rate (59%) than did the monotherapy group (34%; P = .019).
Other notable differences after 16 weeks included improvements in SJC for 66 joints (–3.0 in the combination therapy group vs. –2.0 in the monotherapy group) and significantly better skin and nail measures – such as active psoriasis and change in body surface area – in the methotrexate plus leflunomide group.
When asked who should be prescribed the combination therapy and who should be prescribed methotrexate going forward, Dr. Mulder told this news organization, “At the moment, we have insufficient knowledge on who will benefit most or who will develop clinically relevant side effects. It seems warranted to discuss with every patient which approach they would prefer. This could be a step-down or -up approach.
“We hope to be able to better predict treatment response and side effects in the future via post hoc analysis of our study and via extensive flow-cytometric phenotyping of immune blood cells taken at baseline,” she added.
Three patients in the combination therapy group experienced serious adverse events, two of which were deemed unrelated to leflunomide. The most frequently occurring adverse events were nausea or vomiting, tiredness, and elevated alanine aminotransferase. Mild adverse events were more common in the methotrexate plus leflunomide group. No participants died, and all patients with adverse events recovered completely.
“It appears good practice to do blood draws for laboratory tests on liver enzymes at least monthly for the first 4 months and every 4 months after that once stable dosing is achieved, as well as have a telephone consultation after 6-8 weeks to talk about possible side effects a patient might experience and change or add therapy if necessary,” Dr. Mulder added.
Study turns perception of combination therapy into reality
It had already been perceived by rheumatologists that methotrexate plus leflunomide was an effective combo for PsA, and this study reinforces those beliefs, Clementina López-Medina, MD, PhD, and colleagues from the University of Cordoba (Spain), write in an accompanying editorial.
They highlight this study’s notable strengths, one of which was defining “active disease” as two or more swollen joints, which opened the study up to a larger patient population. The editorialists also underline the confirmation that leflunomide plus methotrexate reduces both joint symptoms and skin involvement in this subset of patients, which had also been found in a previous study.
“Leflunomide is usually considered as a second-line option after methotrexate is unsuccessful,” they note, “despite the fact that methotrexate did not show superiority over placebo in previous trials.”
The editorialists were not surprised that the combination therapy was more toxic than the monotherapy. Rheumatologists could use these data to individualize treatment accordingly, they write, while keeping an eye on “gastrointestinal disturbances.”
Overall, Dr. López-Medina and colleagues say that the study results should “be considered not only in daily clinical practice but also in the development of future recommendations.”
Leflunomide: Forgotten no longer, at least for PsA
“I think we probably underutilize leflunomide,” Arthur Kavanaugh, MD, professor of medicine and director of the Center for Innovative Therapy at the University of California, San Diego, told this news organization. “Sometimes medicines get ‘old,’ for lack of a better term, and fall a little bit of out of favor, sometimes unnecessarily. Leflunomide falls into that category. Because it’s older, it doesn’t get as much buzz as what’s new and shiny.
“I was not surprised by the results on the joints,” he said, “because we know from previous studies that leflunomide works in that regard. What did surprise me is that the skin got better, especially with the combination.”
Regarding the side effects for the combination therapy, he commended the authors for limiting potential uncertainty by using such a high dose of methotrexate.
“By going with a dose of 25 mg [per week], no one can say, ‘They pulled their punches and methotrexate monotherapy would’ve been just as good if it was given at a higher dose,’ “ he said. “And they also used leflunomide at a high dose. It makes you wonder: Could you use lower doses, and do lower doses mean fewer lab test abnormalities? This positive study does lend itself to some other permutations in terms of study design.
“Even though this was a small study,” he added, “it brings us right back to: We should really consider leflunomide in the treatment of PsA.”
The authors acknowledge their study’s limitations, including the fact that it was conducted in a single country and the absence of a nontreatment placebo group. They also note the higher percentage of women in the methotrexate plus leflunomide group, “which might have lowered the treatment response and increased the adverse event rate, resulting in bias.”
The study was funded by a Regional Junior Researcher Grant from Sint Maartenskliniek. The authors reported numerous potential conflicts of interest, including receiving payment, research grants, and consulting and speaker fees from various pharmaceutical companies.
A version of this article first appeared on Medscape.com.
A new study has found that methotrexate plus leflunomide outperforms methotrexate alone as a treatment option for patients with psoriatic arthritis (PsA).
“We believe that prescribing this combination in routine practice is viable when combined with shared decision-making and strict monitoring of side effects,” write Michelle L.M. Mulder, MD, of the department of rheumatology at Sint Maartenskliniek in Nijmegen, the Netherlands, and her coauthors. Their findings were published in The Lancet Rheumatology.
The latest treatment guidelines from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the European Alliance of Associations for Rheumatology recommend conventional synthetic disease-modifying antirheumatic drugs for patients with active PsA, but Dr. Mulder and her colleagues note a distinct lack of information on their effectiveness, especially this particular combination.
To assess the efficacy and safety of methotrexate plus leflunomide, they launched a single-center, double-blind, randomized trial that included 78 Dutch patients with PsA. The majority of the participants in this trial – dubbed COMPLETE-PsA – were men (64%), and the median age of the patients was 55 years. All had active disease at baseline; the median swollen joint count (SJC) and tender joint count were 4.0 in both groups.
Participants were assigned to receive either methotrexate plus leflunomide (n = 39) or methotrexate plus placebo (n = 39). After 16 weeks, mean Psoriatic Arthritis Disease Activity Score (PASDAS) had improved for patients in the combination therapy group in comparison with the monotherapy group (3.1; standard deviation, 1.4 vs. 3.7; SD, 1.3; treatment difference, –0.6; 90% confidence interval, –1.0 to –0.1; P = .025). The combination therapy group also achieved PASDAS low disease activity at a higher rate (59%) than did the monotherapy group (34%; P = .019).
Other notable differences after 16 weeks included improvements in SJC for 66 joints (–3.0 in the combination therapy group vs. –2.0 in the monotherapy group) and significantly better skin and nail measures – such as active psoriasis and change in body surface area – in the methotrexate plus leflunomide group.
When asked who should be prescribed the combination therapy and who should be prescribed methotrexate going forward, Dr. Mulder told this news organization, “At the moment, we have insufficient knowledge on who will benefit most or who will develop clinically relevant side effects. It seems warranted to discuss with every patient which approach they would prefer. This could be a step-down or -up approach.
“We hope to be able to better predict treatment response and side effects in the future via post hoc analysis of our study and via extensive flow-cytometric phenotyping of immune blood cells taken at baseline,” she added.
Three patients in the combination therapy group experienced serious adverse events, two of which were deemed unrelated to leflunomide. The most frequently occurring adverse events were nausea or vomiting, tiredness, and elevated alanine aminotransferase. Mild adverse events were more common in the methotrexate plus leflunomide group. No participants died, and all patients with adverse events recovered completely.
“It appears good practice to do blood draws for laboratory tests on liver enzymes at least monthly for the first 4 months and every 4 months after that once stable dosing is achieved, as well as have a telephone consultation after 6-8 weeks to talk about possible side effects a patient might experience and change or add therapy if necessary,” Dr. Mulder added.
Study turns perception of combination therapy into reality
It had already been perceived by rheumatologists that methotrexate plus leflunomide was an effective combo for PsA, and this study reinforces those beliefs, Clementina López-Medina, MD, PhD, and colleagues from the University of Cordoba (Spain), write in an accompanying editorial.
They highlight this study’s notable strengths, one of which was defining “active disease” as two or more swollen joints, which opened the study up to a larger patient population. The editorialists also underline the confirmation that leflunomide plus methotrexate reduces both joint symptoms and skin involvement in this subset of patients, which had also been found in a previous study.
“Leflunomide is usually considered as a second-line option after methotrexate is unsuccessful,” they note, “despite the fact that methotrexate did not show superiority over placebo in previous trials.”
The editorialists were not surprised that the combination therapy was more toxic than the monotherapy. Rheumatologists could use these data to individualize treatment accordingly, they write, while keeping an eye on “gastrointestinal disturbances.”
Overall, Dr. López-Medina and colleagues say that the study results should “be considered not only in daily clinical practice but also in the development of future recommendations.”
Leflunomide: Forgotten no longer, at least for PsA
“I think we probably underutilize leflunomide,” Arthur Kavanaugh, MD, professor of medicine and director of the Center for Innovative Therapy at the University of California, San Diego, told this news organization. “Sometimes medicines get ‘old,’ for lack of a better term, and fall a little bit of out of favor, sometimes unnecessarily. Leflunomide falls into that category. Because it’s older, it doesn’t get as much buzz as what’s new and shiny.
“I was not surprised by the results on the joints,” he said, “because we know from previous studies that leflunomide works in that regard. What did surprise me is that the skin got better, especially with the combination.”
Regarding the side effects for the combination therapy, he commended the authors for limiting potential uncertainty by using such a high dose of methotrexate.
“By going with a dose of 25 mg [per week], no one can say, ‘They pulled their punches and methotrexate monotherapy would’ve been just as good if it was given at a higher dose,’ “ he said. “And they also used leflunomide at a high dose. It makes you wonder: Could you use lower doses, and do lower doses mean fewer lab test abnormalities? This positive study does lend itself to some other permutations in terms of study design.
“Even though this was a small study,” he added, “it brings us right back to: We should really consider leflunomide in the treatment of PsA.”
The authors acknowledge their study’s limitations, including the fact that it was conducted in a single country and the absence of a nontreatment placebo group. They also note the higher percentage of women in the methotrexate plus leflunomide group, “which might have lowered the treatment response and increased the adverse event rate, resulting in bias.”
The study was funded by a Regional Junior Researcher Grant from Sint Maartenskliniek. The authors reported numerous potential conflicts of interest, including receiving payment, research grants, and consulting and speaker fees from various pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM THE LANCET RHEUMATOLOGY
Active surveillance or maintenance after chemo induction in metastatic CRC?
Should patients with metastatic colorectal cancer (CRC) who respond to first-line treatment receive maintenance therapy, or are they better off on active surveillance?
In a recent trial designed to explore this question,
The researchers found that patients with metastatic CRC randomized to maintenance therapy with oral capecitabine (Xeloda) showed improved progression-free survival (PFS), compared with those being actively monitored – 3.88 versus 1.87 months.
But that benefit came at a cost. Patients on capecitabine experienced much worse toxicity and showed no overall survival benefit – 14.8 months in the capecitabine arm versus 15.2 in the surveillance arm.
The FOCUS4-N trial supports “the use of treatment breaks as safe management alternatives for patients” after induction, lead author Richard Adams, MD, an oncology professor at Cardiff University, Wales, and colleagues conclude in a study published in the Journal of Clinical Oncology.
Current treatment standards either require or recommend that patients with metastatic CRC receive maintenance therapy after induction chemotherapy, at least until they progress or experience excessive toxicity.
Although maintenance strategies have typically demonstrated a PFS benefit, that advantage may come at “the expense of ongoing ... toxicity.”
Dr. Adams and colleagues wanted to explore how patients would fare on an oral maintenance therapy with capecitabine, compared with active surveillance.
In the trial, researchers randomly assigned 254 patients who had responded to first-line therapy or had stable disease to either capecitabine (n = 127) or active monitoring (n = 127). Subjects were treated at 88 hospitals in the United Kingdom from 2014-2020. Most patients had widespread synchronous metastatic disease. About half had unresected primary tumors, and the majority had received doublet chemotherapy induction, which was irinotecan-based in 57%.
The authors found that patients receiving capecitabine maintenance therapy showed significant improvements in PFS (hazard ratio, 0.40; P < .0001), but also encountered considerably more toxicity than the surveillance group – including grade 2 or higher fatigue (25% vs. 12%), diarrhea (23% vs. 13%), and hand-foot skin reactions (26% vs. 3%).
Perhaps most notably, the treatment group did not experience an overall survival benefit (HR, 0.93; 95% confidence interval, 0.69-1.27; P = .66).
Overall, the authors noted, the main advantage of active maintenance was to delay the return of aggressive chemotherapy combinations for a few months.
With previous trials reporting pretty much the same findings, there is now “overwhelming level-one evidence” that surveillance is an “appropriate” option with “less time on therapy, lower toxicity, and in a number of studies, better quality of life,” which are all important factors for patients, according to Dr. Adams and colleagues in a follow-up letter.
A viable option
The current study pushes back on existing standards, which support maintenance therapy and can prevent patients from being offered active surveillance.
According to Dr. Adams and colleagues, “FOCUS4-N provides additional evidence to support the use of treatment breaks as safe management alternatives for patients who are stable or responding to first-line treatment for [metastatic] CRC.”
In addition, the FOCUS4-N trial suggests that oral capecitabine alone – instead of alongside intravenous bevacizumab (Avastin) every 3 weeks – is probably adequate for mCRC maintenance.
“It has been shown that single-agent bevacizumab is both ineffective and highly uneconomic” in the mCRC maintenance setting, “suggesting that it may be the capecitabine element of the combination that” provides the PFS benefit, the team noted.
An editorial accompanying the study also concluded that “treatment holidays are an equally viable and more cost-effective alternative” to maintenance and one of special interest to patients looking for a break after intensive induction.
“For patients, caregivers, and oncologists alike who are hesitant to ... stop therapy completely, it is important to note that” at least in trials, patients under surveillance “continue to be vigilantly monitored, including imaging every 8-12 weeks and symptom assessment every few weeks,” Pashtoon Murtaza Kasi, MD, medical oncologist at Weill Cornell Medicine/NewYork-Presbyterian, Manhattan, said in his editorial.
Interestingly, Dr. Kasi pointed out, the trial found no difference in quality-of-life scores between the study arms in the FOCUS4-N trial.
The two groups scored similarly on mobility, self-care, usual activities, anxiety, and depression, and patients in the capecitabine group reported slightly less pain and discomfort, which may have occurred because of delayed disease progression.
“Although it is somewhat reassuring that maintenance approaches do not adversely affect overall quality of life and functioning, it does not mean that these regimens are free from side effects,” Dr. Kasi writes, adding that it’s possible the surveys missed the impacts of increased toxicity with maintenance therapy.
Caveats to the study
A letter published in the Journal of Clinical Oncology raises questions about the generalizability of the FOCUS4-N results.
In the letter, Annika Kurreck, MD, and colleagues from Charity-University Medicine Berlin, Germany, highlighted that the trial only included patients without actionable biomarkers, which likely meant the study population had particularly aggressive disease. This possibility is supported by the “dramatically short” PFS reported in FOCUS4-N compared with prior maintenance versus surveillance investigations.
In addition, the letter writers caution that the study was underpowered to detect an overall survival benefit.
“Therefore, it might be hypothesized that FOCUS4-N comprised a cohort of patients with a rather aggressive tumor biology and/or high tumor load, leading to a quick failure of any de-escalation treatment strategy,” Dr. Kurreck and colleagues write.
In a response letter, Dr. Adams and his team countered that there’s no consistent evidence from past trials suggesting that patients with poorer prognostic features are unfit for surveillance. “We believe that it is a common misrepresentation of the evidence that all patients with worse prognostic features need to be maintained on active but toxic combination therapies for longer,” they said.
Instead of a blanket approach, maintenance versus surveillance should be “an assessment guided by the clinician listening to and guiding the patient rather than a molecular or biologically measurable parameter,” they write.
Dr. Adams and colleagues agreed that identifying subgroups of patients who are more likely to benefit from maintenance versus surveillance is required research, which “we plan to undertake.”
The work was funded by Cancer Research UK and the National Institute for Health Research. Many of the investigators had industry ties, including Dr. Adams, who reported various relationships with and payments from Merck, Amgen, and others. Dr. Kasi also had ties to several companies, including Bristol Myers Squibb, Lilly, and AstraZeneca. Dr. Kurreck and the other letter writers had numerous company ties as well, including relationships and payments from Roche, the maker of bevacizumab.
A version of this article first appeared on Medscape.com.
Should patients with metastatic colorectal cancer (CRC) who respond to first-line treatment receive maintenance therapy, or are they better off on active surveillance?
In a recent trial designed to explore this question,
The researchers found that patients with metastatic CRC randomized to maintenance therapy with oral capecitabine (Xeloda) showed improved progression-free survival (PFS), compared with those being actively monitored – 3.88 versus 1.87 months.
But that benefit came at a cost. Patients on capecitabine experienced much worse toxicity and showed no overall survival benefit – 14.8 months in the capecitabine arm versus 15.2 in the surveillance arm.
The FOCUS4-N trial supports “the use of treatment breaks as safe management alternatives for patients” after induction, lead author Richard Adams, MD, an oncology professor at Cardiff University, Wales, and colleagues conclude in a study published in the Journal of Clinical Oncology.
Current treatment standards either require or recommend that patients with metastatic CRC receive maintenance therapy after induction chemotherapy, at least until they progress or experience excessive toxicity.
Although maintenance strategies have typically demonstrated a PFS benefit, that advantage may come at “the expense of ongoing ... toxicity.”
Dr. Adams and colleagues wanted to explore how patients would fare on an oral maintenance therapy with capecitabine, compared with active surveillance.
In the trial, researchers randomly assigned 254 patients who had responded to first-line therapy or had stable disease to either capecitabine (n = 127) or active monitoring (n = 127). Subjects were treated at 88 hospitals in the United Kingdom from 2014-2020. Most patients had widespread synchronous metastatic disease. About half had unresected primary tumors, and the majority had received doublet chemotherapy induction, which was irinotecan-based in 57%.
The authors found that patients receiving capecitabine maintenance therapy showed significant improvements in PFS (hazard ratio, 0.40; P < .0001), but also encountered considerably more toxicity than the surveillance group – including grade 2 or higher fatigue (25% vs. 12%), diarrhea (23% vs. 13%), and hand-foot skin reactions (26% vs. 3%).
Perhaps most notably, the treatment group did not experience an overall survival benefit (HR, 0.93; 95% confidence interval, 0.69-1.27; P = .66).
Overall, the authors noted, the main advantage of active maintenance was to delay the return of aggressive chemotherapy combinations for a few months.
With previous trials reporting pretty much the same findings, there is now “overwhelming level-one evidence” that surveillance is an “appropriate” option with “less time on therapy, lower toxicity, and in a number of studies, better quality of life,” which are all important factors for patients, according to Dr. Adams and colleagues in a follow-up letter.
A viable option
The current study pushes back on existing standards, which support maintenance therapy and can prevent patients from being offered active surveillance.
According to Dr. Adams and colleagues, “FOCUS4-N provides additional evidence to support the use of treatment breaks as safe management alternatives for patients who are stable or responding to first-line treatment for [metastatic] CRC.”
In addition, the FOCUS4-N trial suggests that oral capecitabine alone – instead of alongside intravenous bevacizumab (Avastin) every 3 weeks – is probably adequate for mCRC maintenance.
“It has been shown that single-agent bevacizumab is both ineffective and highly uneconomic” in the mCRC maintenance setting, “suggesting that it may be the capecitabine element of the combination that” provides the PFS benefit, the team noted.
An editorial accompanying the study also concluded that “treatment holidays are an equally viable and more cost-effective alternative” to maintenance and one of special interest to patients looking for a break after intensive induction.
“For patients, caregivers, and oncologists alike who are hesitant to ... stop therapy completely, it is important to note that” at least in trials, patients under surveillance “continue to be vigilantly monitored, including imaging every 8-12 weeks and symptom assessment every few weeks,” Pashtoon Murtaza Kasi, MD, medical oncologist at Weill Cornell Medicine/NewYork-Presbyterian, Manhattan, said in his editorial.
Interestingly, Dr. Kasi pointed out, the trial found no difference in quality-of-life scores between the study arms in the FOCUS4-N trial.
The two groups scored similarly on mobility, self-care, usual activities, anxiety, and depression, and patients in the capecitabine group reported slightly less pain and discomfort, which may have occurred because of delayed disease progression.
“Although it is somewhat reassuring that maintenance approaches do not adversely affect overall quality of life and functioning, it does not mean that these regimens are free from side effects,” Dr. Kasi writes, adding that it’s possible the surveys missed the impacts of increased toxicity with maintenance therapy.
Caveats to the study
A letter published in the Journal of Clinical Oncology raises questions about the generalizability of the FOCUS4-N results.
In the letter, Annika Kurreck, MD, and colleagues from Charity-University Medicine Berlin, Germany, highlighted that the trial only included patients without actionable biomarkers, which likely meant the study population had particularly aggressive disease. This possibility is supported by the “dramatically short” PFS reported in FOCUS4-N compared with prior maintenance versus surveillance investigations.
In addition, the letter writers caution that the study was underpowered to detect an overall survival benefit.
“Therefore, it might be hypothesized that FOCUS4-N comprised a cohort of patients with a rather aggressive tumor biology and/or high tumor load, leading to a quick failure of any de-escalation treatment strategy,” Dr. Kurreck and colleagues write.
In a response letter, Dr. Adams and his team countered that there’s no consistent evidence from past trials suggesting that patients with poorer prognostic features are unfit for surveillance. “We believe that it is a common misrepresentation of the evidence that all patients with worse prognostic features need to be maintained on active but toxic combination therapies for longer,” they said.
Instead of a blanket approach, maintenance versus surveillance should be “an assessment guided by the clinician listening to and guiding the patient rather than a molecular or biologically measurable parameter,” they write.
Dr. Adams and colleagues agreed that identifying subgroups of patients who are more likely to benefit from maintenance versus surveillance is required research, which “we plan to undertake.”
The work was funded by Cancer Research UK and the National Institute for Health Research. Many of the investigators had industry ties, including Dr. Adams, who reported various relationships with and payments from Merck, Amgen, and others. Dr. Kasi also had ties to several companies, including Bristol Myers Squibb, Lilly, and AstraZeneca. Dr. Kurreck and the other letter writers had numerous company ties as well, including relationships and payments from Roche, the maker of bevacizumab.
A version of this article first appeared on Medscape.com.
Should patients with metastatic colorectal cancer (CRC) who respond to first-line treatment receive maintenance therapy, or are they better off on active surveillance?
In a recent trial designed to explore this question,
The researchers found that patients with metastatic CRC randomized to maintenance therapy with oral capecitabine (Xeloda) showed improved progression-free survival (PFS), compared with those being actively monitored – 3.88 versus 1.87 months.
But that benefit came at a cost. Patients on capecitabine experienced much worse toxicity and showed no overall survival benefit – 14.8 months in the capecitabine arm versus 15.2 in the surveillance arm.
The FOCUS4-N trial supports “the use of treatment breaks as safe management alternatives for patients” after induction, lead author Richard Adams, MD, an oncology professor at Cardiff University, Wales, and colleagues conclude in a study published in the Journal of Clinical Oncology.
Current treatment standards either require or recommend that patients with metastatic CRC receive maintenance therapy after induction chemotherapy, at least until they progress or experience excessive toxicity.
Although maintenance strategies have typically demonstrated a PFS benefit, that advantage may come at “the expense of ongoing ... toxicity.”
Dr. Adams and colleagues wanted to explore how patients would fare on an oral maintenance therapy with capecitabine, compared with active surveillance.
In the trial, researchers randomly assigned 254 patients who had responded to first-line therapy or had stable disease to either capecitabine (n = 127) or active monitoring (n = 127). Subjects were treated at 88 hospitals in the United Kingdom from 2014-2020. Most patients had widespread synchronous metastatic disease. About half had unresected primary tumors, and the majority had received doublet chemotherapy induction, which was irinotecan-based in 57%.
The authors found that patients receiving capecitabine maintenance therapy showed significant improvements in PFS (hazard ratio, 0.40; P < .0001), but also encountered considerably more toxicity than the surveillance group – including grade 2 or higher fatigue (25% vs. 12%), diarrhea (23% vs. 13%), and hand-foot skin reactions (26% vs. 3%).
Perhaps most notably, the treatment group did not experience an overall survival benefit (HR, 0.93; 95% confidence interval, 0.69-1.27; P = .66).
Overall, the authors noted, the main advantage of active maintenance was to delay the return of aggressive chemotherapy combinations for a few months.
With previous trials reporting pretty much the same findings, there is now “overwhelming level-one evidence” that surveillance is an “appropriate” option with “less time on therapy, lower toxicity, and in a number of studies, better quality of life,” which are all important factors for patients, according to Dr. Adams and colleagues in a follow-up letter.
A viable option
The current study pushes back on existing standards, which support maintenance therapy and can prevent patients from being offered active surveillance.
According to Dr. Adams and colleagues, “FOCUS4-N provides additional evidence to support the use of treatment breaks as safe management alternatives for patients who are stable or responding to first-line treatment for [metastatic] CRC.”
In addition, the FOCUS4-N trial suggests that oral capecitabine alone – instead of alongside intravenous bevacizumab (Avastin) every 3 weeks – is probably adequate for mCRC maintenance.
“It has been shown that single-agent bevacizumab is both ineffective and highly uneconomic” in the mCRC maintenance setting, “suggesting that it may be the capecitabine element of the combination that” provides the PFS benefit, the team noted.
An editorial accompanying the study also concluded that “treatment holidays are an equally viable and more cost-effective alternative” to maintenance and one of special interest to patients looking for a break after intensive induction.
“For patients, caregivers, and oncologists alike who are hesitant to ... stop therapy completely, it is important to note that” at least in trials, patients under surveillance “continue to be vigilantly monitored, including imaging every 8-12 weeks and symptom assessment every few weeks,” Pashtoon Murtaza Kasi, MD, medical oncologist at Weill Cornell Medicine/NewYork-Presbyterian, Manhattan, said in his editorial.
Interestingly, Dr. Kasi pointed out, the trial found no difference in quality-of-life scores between the study arms in the FOCUS4-N trial.
The two groups scored similarly on mobility, self-care, usual activities, anxiety, and depression, and patients in the capecitabine group reported slightly less pain and discomfort, which may have occurred because of delayed disease progression.
“Although it is somewhat reassuring that maintenance approaches do not adversely affect overall quality of life and functioning, it does not mean that these regimens are free from side effects,” Dr. Kasi writes, adding that it’s possible the surveys missed the impacts of increased toxicity with maintenance therapy.
Caveats to the study
A letter published in the Journal of Clinical Oncology raises questions about the generalizability of the FOCUS4-N results.
In the letter, Annika Kurreck, MD, and colleagues from Charity-University Medicine Berlin, Germany, highlighted that the trial only included patients without actionable biomarkers, which likely meant the study population had particularly aggressive disease. This possibility is supported by the “dramatically short” PFS reported in FOCUS4-N compared with prior maintenance versus surveillance investigations.
In addition, the letter writers caution that the study was underpowered to detect an overall survival benefit.
“Therefore, it might be hypothesized that FOCUS4-N comprised a cohort of patients with a rather aggressive tumor biology and/or high tumor load, leading to a quick failure of any de-escalation treatment strategy,” Dr. Kurreck and colleagues write.
In a response letter, Dr. Adams and his team countered that there’s no consistent evidence from past trials suggesting that patients with poorer prognostic features are unfit for surveillance. “We believe that it is a common misrepresentation of the evidence that all patients with worse prognostic features need to be maintained on active but toxic combination therapies for longer,” they said.
Instead of a blanket approach, maintenance versus surveillance should be “an assessment guided by the clinician listening to and guiding the patient rather than a molecular or biologically measurable parameter,” they write.
Dr. Adams and colleagues agreed that identifying subgroups of patients who are more likely to benefit from maintenance versus surveillance is required research, which “we plan to undertake.”
The work was funded by Cancer Research UK and the National Institute for Health Research. Many of the investigators had industry ties, including Dr. Adams, who reported various relationships with and payments from Merck, Amgen, and others. Dr. Kasi also had ties to several companies, including Bristol Myers Squibb, Lilly, and AstraZeneca. Dr. Kurreck and the other letter writers had numerous company ties as well, including relationships and payments from Roche, the maker of bevacizumab.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF CLINICAL ONCOLOGY
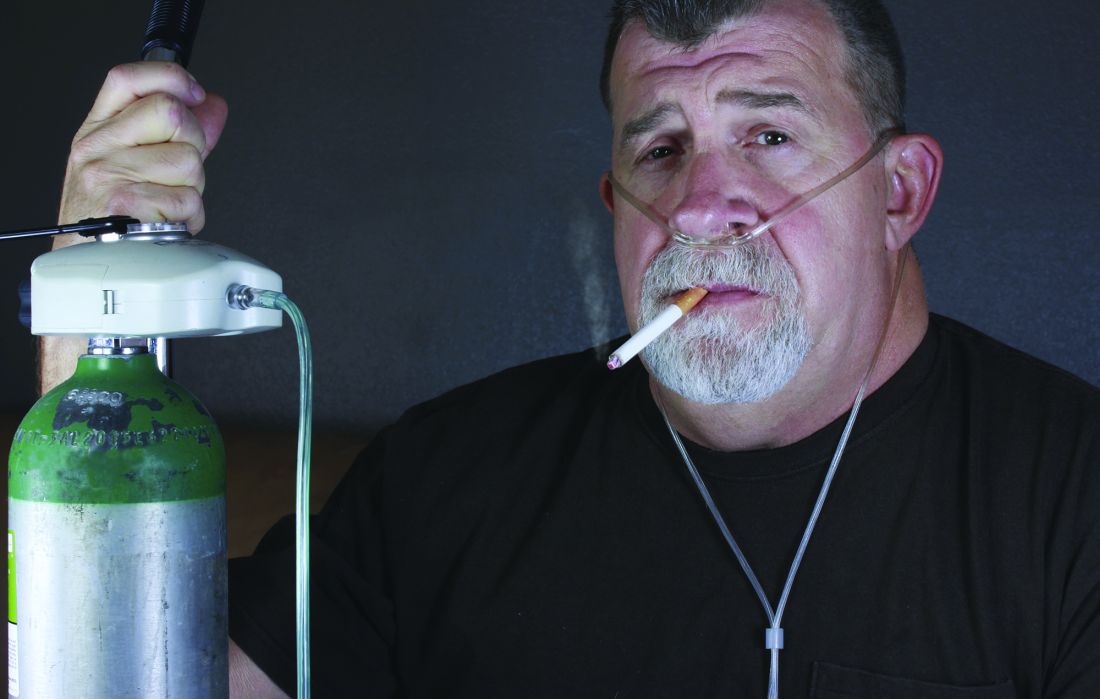
More smoking drives worse outcomes in interstitial lung disease
Heavier smoking significantly increased mortality in adults with progressive fibrosing interstitial lung disease (PF-ILD), based on data from 377 individuals.
The negative impact of smoking on pulmonary diseases is well documented, but the specific impact on patients with PF-ILD has not been well studied, Mark Platenburg, MD, of St. Antonious Hospital, Nieuwegein, the Netherlands, and colleagues wrote in Respiratory Medicine .
“Patients with PF-ILD or IPF [idiopathic pulmonary fibrosis] are prone to early mortality, indicating a need for prognostic [bio]marker studies for precision medicine,” they said.
The researchers identified adults older than 18 years with PF-ILD who were diagnosed at a single center. All study participants had at least 10% fibrosis, and showed either a decline of at least 10% in forced vital capacity, a 5.0%-9.9% relative FVC decline plus progressive respiratory symptoms and/or an increase in extent of fibrosis on subsequent high-resolution (HRCT progression), or progressive respiratory symptoms and HRCT progression over 24 months after ILD diagnosis.
Pack-years of smoking was a prognostic variable; the researchers also compared median transplant-free survival in heavy smokers and mild to moderate smokers. They also investigated the association between smoking quantity and emphysema in the study population.
Overall, the increased risk for mortality was 11%, 22%, and 44% in patients with 10, 20, and 40 pack-years of smoking, respectively.
Both the unadjusted and adjusted hazard ratio for pack-years were significant (1.014, P < .001 and 1.011, P = .022, respectively).
The median transplant-free survival of ever-smokers versus never-smokers with PF-ILD was 3.3 years versus 4.8 years; median transplant-free survival was 3.0 years for heavy smokers and 3.8 years for mild to moderate smokers. Similarly, median survival was 4.2 years in never-smokers versus 3.0 years in former smokers.
Emphysema was significantly more comment in heavy smokers, compared with never smokers and mild to moderate smokers (P < .001 for both).
“We observed a gradual decrease in survival starting from never to mild-moderate and subsequent heavy smokers supporting our finding that [pack-years] is an independent predictor for mortality in PF-ILD,” the researchers wrote. “This is an important message that clinicians could convey to their ILD patients, but also to patients at-risk for ILD.”
The study findings were limited by several factors, mainly the retrospective design, incomplete data for some patients, and lack of data on comorbidities, the researchers noted. However, the results strengthen the evidence for the detrimental effect of heavy smoking in PF-ILD, they said. Consequently, “efforts to reduce pack-years in those with, and at risk for, PF-ILD may translate into a survival benefit and should have high priority in clinical practice.”
The study was supported by grants from ZonMw TopZorg Care and TZO. The researchers had no financial conflicts to disclose.
Heavier smoking significantly increased mortality in adults with progressive fibrosing interstitial lung disease (PF-ILD), based on data from 377 individuals.
The negative impact of smoking on pulmonary diseases is well documented, but the specific impact on patients with PF-ILD has not been well studied, Mark Platenburg, MD, of St. Antonious Hospital, Nieuwegein, the Netherlands, and colleagues wrote in Respiratory Medicine .
“Patients with PF-ILD or IPF [idiopathic pulmonary fibrosis] are prone to early mortality, indicating a need for prognostic [bio]marker studies for precision medicine,” they said.
The researchers identified adults older than 18 years with PF-ILD who were diagnosed at a single center. All study participants had at least 10% fibrosis, and showed either a decline of at least 10% in forced vital capacity, a 5.0%-9.9% relative FVC decline plus progressive respiratory symptoms and/or an increase in extent of fibrosis on subsequent high-resolution (HRCT progression), or progressive respiratory symptoms and HRCT progression over 24 months after ILD diagnosis.
Pack-years of smoking was a prognostic variable; the researchers also compared median transplant-free survival in heavy smokers and mild to moderate smokers. They also investigated the association between smoking quantity and emphysema in the study population.
Overall, the increased risk for mortality was 11%, 22%, and 44% in patients with 10, 20, and 40 pack-years of smoking, respectively.
Both the unadjusted and adjusted hazard ratio for pack-years were significant (1.014, P < .001 and 1.011, P = .022, respectively).
The median transplant-free survival of ever-smokers versus never-smokers with PF-ILD was 3.3 years versus 4.8 years; median transplant-free survival was 3.0 years for heavy smokers and 3.8 years for mild to moderate smokers. Similarly, median survival was 4.2 years in never-smokers versus 3.0 years in former smokers.
Emphysema was significantly more comment in heavy smokers, compared with never smokers and mild to moderate smokers (P < .001 for both).
“We observed a gradual decrease in survival starting from never to mild-moderate and subsequent heavy smokers supporting our finding that [pack-years] is an independent predictor for mortality in PF-ILD,” the researchers wrote. “This is an important message that clinicians could convey to their ILD patients, but also to patients at-risk for ILD.”
The study findings were limited by several factors, mainly the retrospective design, incomplete data for some patients, and lack of data on comorbidities, the researchers noted. However, the results strengthen the evidence for the detrimental effect of heavy smoking in PF-ILD, they said. Consequently, “efforts to reduce pack-years in those with, and at risk for, PF-ILD may translate into a survival benefit and should have high priority in clinical practice.”
The study was supported by grants from ZonMw TopZorg Care and TZO. The researchers had no financial conflicts to disclose.
Heavier smoking significantly increased mortality in adults with progressive fibrosing interstitial lung disease (PF-ILD), based on data from 377 individuals.
The negative impact of smoking on pulmonary diseases is well documented, but the specific impact on patients with PF-ILD has not been well studied, Mark Platenburg, MD, of St. Antonious Hospital, Nieuwegein, the Netherlands, and colleagues wrote in Respiratory Medicine .
“Patients with PF-ILD or IPF [idiopathic pulmonary fibrosis] are prone to early mortality, indicating a need for prognostic [bio]marker studies for precision medicine,” they said.
The researchers identified adults older than 18 years with PF-ILD who were diagnosed at a single center. All study participants had at least 10% fibrosis, and showed either a decline of at least 10% in forced vital capacity, a 5.0%-9.9% relative FVC decline plus progressive respiratory symptoms and/or an increase in extent of fibrosis on subsequent high-resolution (HRCT progression), or progressive respiratory symptoms and HRCT progression over 24 months after ILD diagnosis.
Pack-years of smoking was a prognostic variable; the researchers also compared median transplant-free survival in heavy smokers and mild to moderate smokers. They also investigated the association between smoking quantity and emphysema in the study population.
Overall, the increased risk for mortality was 11%, 22%, and 44% in patients with 10, 20, and 40 pack-years of smoking, respectively.
Both the unadjusted and adjusted hazard ratio for pack-years were significant (1.014, P < .001 and 1.011, P = .022, respectively).
The median transplant-free survival of ever-smokers versus never-smokers with PF-ILD was 3.3 years versus 4.8 years; median transplant-free survival was 3.0 years for heavy smokers and 3.8 years for mild to moderate smokers. Similarly, median survival was 4.2 years in never-smokers versus 3.0 years in former smokers.
Emphysema was significantly more comment in heavy smokers, compared with never smokers and mild to moderate smokers (P < .001 for both).
“We observed a gradual decrease in survival starting from never to mild-moderate and subsequent heavy smokers supporting our finding that [pack-years] is an independent predictor for mortality in PF-ILD,” the researchers wrote. “This is an important message that clinicians could convey to their ILD patients, but also to patients at-risk for ILD.”
The study findings were limited by several factors, mainly the retrospective design, incomplete data for some patients, and lack of data on comorbidities, the researchers noted. However, the results strengthen the evidence for the detrimental effect of heavy smoking in PF-ILD, they said. Consequently, “efforts to reduce pack-years in those with, and at risk for, PF-ILD may translate into a survival benefit and should have high priority in clinical practice.”
The study was supported by grants from ZonMw TopZorg Care and TZO. The researchers had no financial conflicts to disclose.
FROM RESPIRATORY MEDICINE
FDA, DEA pushed to make gabapentin a controlled substance to stop ‘widespread misuse’
In a bid to stop abuse and diversion of the anticonvulsant gabapentin, a watchdog group is petitioning federal regulators to make the drug a controlled substance.
Gabapentin is a generic drug, best known under the brand name Neurontin. The petition also covers the related drug gabapentin enacarbil (Horizant).
Public Citizen requested that gabapentin come under the DEA’s Schedule V category, which already includes the similar drug pregabalin (Lyrica). Schedule V is the lowest rung on the DEA’s drug schedule, meaning it has lower potential for abuse then Schedule I through IV drugs. This tier also includes cough preparations with less than 200 milligrams of codeine.
Classifying gabapentin as a Schedule V drug would facilitate better tracking of the drug’s use and misuse and put in place educational and limitation requirements to mitigate the risk of addiction, overdose, and death, Michael Abrams, MPH, PhD, senior health researcher with Public Citizen’s Health Research Group, and colleagues write in the petition.
‘Widespread misuse’
There is “substantial evidence of widespread misuse” of gabapentin, plausibly helped by “extraordinary levels of off-label prescribing,” Public Citizen said in the petition.
Some estimates have pegged off-label use at more than 90%, with gabapentin prescribed for indications such as chronic cough, hiccups, postoperative pain, and postmenopausal hot flashes, the group said.
“Moreover, there are numerous reports indicating that gabapentin is widely used and diverted on the street to induce ‘highs’ or otherwise self-medicate,” Public Citizen said. “Both gabapentin and pregabalin have been empirically linked to the opioid overdose epidemic as drugs that potentiate the activity of these oftentimes deadly analgesics.”
This news organization tried several times to reach Azurity for comment but did not receive a response. Pfizer included gabapentin in the portfolio of drugs used to create the Viatris spin-off, which took place in 2020. Pfizer referred this news organization to Viatris for comment, but it also did not respond.
It is unclear how the FDA and DEA will respond to the petition. Public Citizen has received a reply from the FDA, in which the agency acknowledged receipt of the petition. However, the “acceptance of the petition for filing is a procedural matter and in no way reflects the agency’s decision on the substantive merits of the petition,” the FDA said in a letter.
As is common practice, the agency assigned a docket number for the petition, FDA-2022-P-0149. The docket’s website allows interested parties to track the issue.
‘Unnoticed’ abuse
There have been rising concerns about risks and abuse of gabapentin in recent years. In its petition, Public Citizen noted that the United Kingdom and several U.S. states have already sought tighter control of gabapentin prescriptions.
In 2019, the United Kingdom announced it would reclassify both pregabalin and gabapentin as class C controlled substances because of the rising numbers of deaths linked to the drugs.
As of November 2020, seven states – Alabama, Kentucky, Michigan, North Dakota, Tennessee, Virginia, and West Virginia – had classified gabapentin as a schedule V drug, while another 12 states required prescription monitoring of the drug, Public Citizen noted.
In 2018, researchers at the University of Louisville, Kentucky, a state that has been hit particularly hard by the opioid crisis, tried to draw more attention to the risks of gabapentin.
“Amid the opioid epidemic, abuse of a different prescription painkiller has widely gone unnoticed,” the University said in a press release at the time.
The release highlighted a study led by Rachel Vickers Smith, PhD, assistant professor in the University of Louisville School of Nursing that was published in Psychology of Addictive Behaviors.
It included 33 individuals who reported recent recreational use of gabapentin. Use of the drug was combined with buprenorphine, other opioids, cocaine, and caffeine to produce effects such as muscle relaxation, pain reduction, sleep induction, feeling drunk, and feeling “high.”
In the press release, Dr. Vickers Smith said individuals who abuse gabapentin often mix it with opioids, marijuana, cocaine, and opioid treatment medication, compounding side effects to the central nervous system that include euphoria and sedation.
In addition, some individuals who primarily abused opioid pain medication have turned to gabapentin after law-enforcement actions made it more difficult to obtain prescription opioids, she noted.
“People are looking for other drugs to substitute for opioids, and gabapentin has filled that place for some,” Dr. Vickers Smith said. “Some have said it gives them a high similar to opioids.”
FDA 2019 warning
In 2019, the FDA issued a warning about serious breathing difficulties associated with gabapentin and pregabalin in patients with respiratory risk factors.
These factors include opioid use and other drugs that depress the central nervous system, as well as conditions such as chronic obstructive pulmonary disease that reduce lung function. Older patients are also at higher risk, the FDA said.
The agency noted that gabapentinoids are often co-prescribed with opioids for for medical conditions and abused in combination with opioids. Data collected in 2016 from an office-based physician survey showed 14% of patient encounters involving gabapentin also involved opioids, the FDA said.
“Our evaluation shows that the use of these medicines, often referred to as gabapentinoids, has been growing for prescribed medical use, as well as misuse and abuse,” the agency said in its 2019 alert.
A version of this article first appeared on Medscape.com.
In a bid to stop abuse and diversion of the anticonvulsant gabapentin, a watchdog group is petitioning federal regulators to make the drug a controlled substance.
Gabapentin is a generic drug, best known under the brand name Neurontin. The petition also covers the related drug gabapentin enacarbil (Horizant).
Public Citizen requested that gabapentin come under the DEA’s Schedule V category, which already includes the similar drug pregabalin (Lyrica). Schedule V is the lowest rung on the DEA’s drug schedule, meaning it has lower potential for abuse then Schedule I through IV drugs. This tier also includes cough preparations with less than 200 milligrams of codeine.
Classifying gabapentin as a Schedule V drug would facilitate better tracking of the drug’s use and misuse and put in place educational and limitation requirements to mitigate the risk of addiction, overdose, and death, Michael Abrams, MPH, PhD, senior health researcher with Public Citizen’s Health Research Group, and colleagues write in the petition.
‘Widespread misuse’
There is “substantial evidence of widespread misuse” of gabapentin, plausibly helped by “extraordinary levels of off-label prescribing,” Public Citizen said in the petition.
Some estimates have pegged off-label use at more than 90%, with gabapentin prescribed for indications such as chronic cough, hiccups, postoperative pain, and postmenopausal hot flashes, the group said.
“Moreover, there are numerous reports indicating that gabapentin is widely used and diverted on the street to induce ‘highs’ or otherwise self-medicate,” Public Citizen said. “Both gabapentin and pregabalin have been empirically linked to the opioid overdose epidemic as drugs that potentiate the activity of these oftentimes deadly analgesics.”
This news organization tried several times to reach Azurity for comment but did not receive a response. Pfizer included gabapentin in the portfolio of drugs used to create the Viatris spin-off, which took place in 2020. Pfizer referred this news organization to Viatris for comment, but it also did not respond.
It is unclear how the FDA and DEA will respond to the petition. Public Citizen has received a reply from the FDA, in which the agency acknowledged receipt of the petition. However, the “acceptance of the petition for filing is a procedural matter and in no way reflects the agency’s decision on the substantive merits of the petition,” the FDA said in a letter.
As is common practice, the agency assigned a docket number for the petition, FDA-2022-P-0149. The docket’s website allows interested parties to track the issue.
‘Unnoticed’ abuse
There have been rising concerns about risks and abuse of gabapentin in recent years. In its petition, Public Citizen noted that the United Kingdom and several U.S. states have already sought tighter control of gabapentin prescriptions.
In 2019, the United Kingdom announced it would reclassify both pregabalin and gabapentin as class C controlled substances because of the rising numbers of deaths linked to the drugs.
As of November 2020, seven states – Alabama, Kentucky, Michigan, North Dakota, Tennessee, Virginia, and West Virginia – had classified gabapentin as a schedule V drug, while another 12 states required prescription monitoring of the drug, Public Citizen noted.
In 2018, researchers at the University of Louisville, Kentucky, a state that has been hit particularly hard by the opioid crisis, tried to draw more attention to the risks of gabapentin.
“Amid the opioid epidemic, abuse of a different prescription painkiller has widely gone unnoticed,” the University said in a press release at the time.
The release highlighted a study led by Rachel Vickers Smith, PhD, assistant professor in the University of Louisville School of Nursing that was published in Psychology of Addictive Behaviors.
It included 33 individuals who reported recent recreational use of gabapentin. Use of the drug was combined with buprenorphine, other opioids, cocaine, and caffeine to produce effects such as muscle relaxation, pain reduction, sleep induction, feeling drunk, and feeling “high.”
In the press release, Dr. Vickers Smith said individuals who abuse gabapentin often mix it with opioids, marijuana, cocaine, and opioid treatment medication, compounding side effects to the central nervous system that include euphoria and sedation.
In addition, some individuals who primarily abused opioid pain medication have turned to gabapentin after law-enforcement actions made it more difficult to obtain prescription opioids, she noted.
“People are looking for other drugs to substitute for opioids, and gabapentin has filled that place for some,” Dr. Vickers Smith said. “Some have said it gives them a high similar to opioids.”
FDA 2019 warning
In 2019, the FDA issued a warning about serious breathing difficulties associated with gabapentin and pregabalin in patients with respiratory risk factors.
These factors include opioid use and other drugs that depress the central nervous system, as well as conditions such as chronic obstructive pulmonary disease that reduce lung function. Older patients are also at higher risk, the FDA said.
The agency noted that gabapentinoids are often co-prescribed with opioids for for medical conditions and abused in combination with opioids. Data collected in 2016 from an office-based physician survey showed 14% of patient encounters involving gabapentin also involved opioids, the FDA said.
“Our evaluation shows that the use of these medicines, often referred to as gabapentinoids, has been growing for prescribed medical use, as well as misuse and abuse,” the agency said in its 2019 alert.
A version of this article first appeared on Medscape.com.
In a bid to stop abuse and diversion of the anticonvulsant gabapentin, a watchdog group is petitioning federal regulators to make the drug a controlled substance.
Gabapentin is a generic drug, best known under the brand name Neurontin. The petition also covers the related drug gabapentin enacarbil (Horizant).
Public Citizen requested that gabapentin come under the DEA’s Schedule V category, which already includes the similar drug pregabalin (Lyrica). Schedule V is the lowest rung on the DEA’s drug schedule, meaning it has lower potential for abuse then Schedule I through IV drugs. This tier also includes cough preparations with less than 200 milligrams of codeine.
Classifying gabapentin as a Schedule V drug would facilitate better tracking of the drug’s use and misuse and put in place educational and limitation requirements to mitigate the risk of addiction, overdose, and death, Michael Abrams, MPH, PhD, senior health researcher with Public Citizen’s Health Research Group, and colleagues write in the petition.
‘Widespread misuse’
There is “substantial evidence of widespread misuse” of gabapentin, plausibly helped by “extraordinary levels of off-label prescribing,” Public Citizen said in the petition.
Some estimates have pegged off-label use at more than 90%, with gabapentin prescribed for indications such as chronic cough, hiccups, postoperative pain, and postmenopausal hot flashes, the group said.
“Moreover, there are numerous reports indicating that gabapentin is widely used and diverted on the street to induce ‘highs’ or otherwise self-medicate,” Public Citizen said. “Both gabapentin and pregabalin have been empirically linked to the opioid overdose epidemic as drugs that potentiate the activity of these oftentimes deadly analgesics.”
This news organization tried several times to reach Azurity for comment but did not receive a response. Pfizer included gabapentin in the portfolio of drugs used to create the Viatris spin-off, which took place in 2020. Pfizer referred this news organization to Viatris for comment, but it also did not respond.
It is unclear how the FDA and DEA will respond to the petition. Public Citizen has received a reply from the FDA, in which the agency acknowledged receipt of the petition. However, the “acceptance of the petition for filing is a procedural matter and in no way reflects the agency’s decision on the substantive merits of the petition,” the FDA said in a letter.
As is common practice, the agency assigned a docket number for the petition, FDA-2022-P-0149. The docket’s website allows interested parties to track the issue.
‘Unnoticed’ abuse
There have been rising concerns about risks and abuse of gabapentin in recent years. In its petition, Public Citizen noted that the United Kingdom and several U.S. states have already sought tighter control of gabapentin prescriptions.
In 2019, the United Kingdom announced it would reclassify both pregabalin and gabapentin as class C controlled substances because of the rising numbers of deaths linked to the drugs.
As of November 2020, seven states – Alabama, Kentucky, Michigan, North Dakota, Tennessee, Virginia, and West Virginia – had classified gabapentin as a schedule V drug, while another 12 states required prescription monitoring of the drug, Public Citizen noted.
In 2018, researchers at the University of Louisville, Kentucky, a state that has been hit particularly hard by the opioid crisis, tried to draw more attention to the risks of gabapentin.
“Amid the opioid epidemic, abuse of a different prescription painkiller has widely gone unnoticed,” the University said in a press release at the time.
The release highlighted a study led by Rachel Vickers Smith, PhD, assistant professor in the University of Louisville School of Nursing that was published in Psychology of Addictive Behaviors.
It included 33 individuals who reported recent recreational use of gabapentin. Use of the drug was combined with buprenorphine, other opioids, cocaine, and caffeine to produce effects such as muscle relaxation, pain reduction, sleep induction, feeling drunk, and feeling “high.”
In the press release, Dr. Vickers Smith said individuals who abuse gabapentin often mix it with opioids, marijuana, cocaine, and opioid treatment medication, compounding side effects to the central nervous system that include euphoria and sedation.
In addition, some individuals who primarily abused opioid pain medication have turned to gabapentin after law-enforcement actions made it more difficult to obtain prescription opioids, she noted.
“People are looking for other drugs to substitute for opioids, and gabapentin has filled that place for some,” Dr. Vickers Smith said. “Some have said it gives them a high similar to opioids.”
FDA 2019 warning
In 2019, the FDA issued a warning about serious breathing difficulties associated with gabapentin and pregabalin in patients with respiratory risk factors.
These factors include opioid use and other drugs that depress the central nervous system, as well as conditions such as chronic obstructive pulmonary disease that reduce lung function. Older patients are also at higher risk, the FDA said.
The agency noted that gabapentinoids are often co-prescribed with opioids for for medical conditions and abused in combination with opioids. Data collected in 2016 from an office-based physician survey showed 14% of patient encounters involving gabapentin also involved opioids, the FDA said.
“Our evaluation shows that the use of these medicines, often referred to as gabapentinoids, has been growing for prescribed medical use, as well as misuse and abuse,” the agency said in its 2019 alert.
A version of this article first appeared on Medscape.com.
Oncology groups support Ukraine, one cuts ties with Russian docs
As many in the world react with sanctions imposed on Russia after its invasion of Ukraine, the oncology community has now stepped into the fray.
All the large cancer organizations have put out statements in support of Ukraine, but one group has gone further and cut its ties with Russia.
“The international cancer specialist network, OncoAlert, severed all cooperation with doctors in Russia as part of the Western sanctions,” the group announced on its Twitter page, which is decorated with a blue and yellow ribbon and declares that it “stands with Ukraine.”
“The OncoAlert Network is nonpolitical but we cannot stand idle and not take a stand against this aggression toward our Ukrainian friends & colleagues,” the group said. “The network will be pulling out of ALL collaborations & congresses in Russia.”
Not surprisingly, the post was inundated with a barrage of inflammatory and politically laced tweets from Russian and Chinese users. Many of them repeated the same phrase about “violating the Hippocratic oath and the Geneva convention,” used foul language, and slammed the United States for past military actions in other parts of the world.
A prominent Russian oncologist also responded, posting a video in which he discussed the situation more coherently and without mudslinging or scripted phrases. Andrey Kaprin, MD, PhD, is chief oncologist of the Russian Federation as well as director general of the Federal State Budgetary Institution, NMRCC, of the Ministry of Health of the Russian Federation. He says they continue to maintain relations with the largest and best known oncologic organizations. “We haven’t felt any deterioration in our relationship yet, and of course, we hope that this won’t happen.”
Dr. Kaprin said he believes OncoAlert will return to cooperation with Russia, and that “reason will prevail.”
“No one is protected from cancer, not even doctors, and that is why there should be no politics here,” he said.
Dr. Kaprin was speaking from Russia state-affiliated media, so it was not an independent commentary. Several of the Twitter responses to his video, primarily from non-Russians, were less than complimentary.
One user replied: “Cancer is rife in the Kremlin.”
Another post pointed out the hypocrisy of Russians being upset that OncoAlert was cutting ties with them. “What about sick Ukrainian kids, having to shelter in hospital basement, not having lifesaving surgeries because Russia decided to invade a democratic country?”
And another post was not buying the story that “reason will prevail,” in that the doctor’s talk seemed to contradict the reality of the situation. “I guess for every child #Russia murders they get cut off a little more from the civilized world?”
Cancer patients vulnerable
The war in Ukraine is an “unfolding humanitarian emergency,” said the World Health Organization, and it has called on top-level officials involved in the Russian invasion to ensure access for delivery of essential medical, surgical, and trauma supplies to help the Ukrainian people and refugees in neighboring countries. A shortage of oxygen, insulin, cancer therapies, and other essential supplies will continue to grow more dire in the weeks and months ahead, WHO officials predict.
One of the more heartbreaking reports described how pediatric cancer patients have been moved to hospital basements that are serving as temporary bomb shelters. Hospital staff continue to try to provide limited treatment when possible, even though essential supplies are dwindling.
“These children suffer more because they need to stay alive to fight with the cancer – and this fight cannot wait,” Lesia Lysytsia, MD, a doctor at Okhmatdyt, the country’s largest children’s hospital in Kyiv, said in an NBC news report.
For some children, the only treatment available is a basic form of chemotherapy, and at the Kyiv Regional Oncology Center, the situation became so dire for children in need of blood transfusions that physicians began to transfuse blood from parent to child.
“Our patients, they will die,” Dr. Lysytsia said. “We will calculate how many people or soldiers have died in attacks, but we will never calculate how many patients weren’t diagnosed of disease in time, how many patients died because they didn’t receive treatment. It’s an epic amount of people.”
Response from oncology community
Many of the large American oncology groups have issued strong statements expressing their support for Ukraine and offering assistance.
The American Cancer Society has partnered with the American Society of Clinical Oncology and the Sidney Kimmel Cancer Center–Jefferson Health to support all Ukrainian cancer patients and their families. The groups are engaging a network of oncologists and oncology nurses to provide support through the ACS Clinician Volunteer Corps.
The ACS and ASCO are making free cancer resources available in English, Ukrainian, Polish, and Russian through their patient information websites (available here and here), with additional patient education resources planned.
The ACS noted that there are more than 179,000 newly diagnosed patients with cancer among the Ukrainian people “suffering from Russia’s unprovoked aggression.”
“Disruptions to cancer treatment pose a grave risk to the survival of Ukrainian patients with cancer,” commented Karen Knudsen, PhD, CEO at the ACS.
ASCO also issued its own statement, declaring that it stands with “our Ukrainian members, the worldwide oncology community, and health care providers around the globe in condemning Russia’s unprovoked war on Ukraine.”
The society notes that it represents oncology professionals in Ukraine and neighboring countries including Poland, Romania, Moldova, Slovakia, and Hungary, which are now receiving thousands of refugees from the Russian invasion.
“We are hearing daily reports of cancer treatment interrupted by acts of war, including damage to medical facilities and shortages of critical supplies. Countless patients now need to find cancer care in new and unfamiliar surroundings with limited medical records and minimal resources,” the society commented.
The American Association for Cancer Research also issued a statement by President David A. Tuveson, MD, PhD, and CEO Margaret Foti, PhD, MD (hc). The organization has more than 50,000 members around the world, and they “stand in solidarity with the citizens of Ukraine during the Russian attack on their country.”
“This abhorrent war, which has been instigated by Russia’s leaders, is isolating and interrupting the lifesaving work of scientists and clinicians in Ukraine and Russia, threatening years of effective research collaborations and community building,” the AACR comments. “Limiting the exchange of innovative ideas, practices, and data across borders will significantly retard cancer research and have an adverse effect on public health.”
Perhaps the most subdued statement came from the European Society of Medical Oncology, in a brief release entitled: “Against Any War.” The society expressed profound sadness about the unfolding tragedy in Ukraine and the suffering of people. “We would like to confirm our solidarity and unconditioned support to all oncology professionals and cancer patients, with no geographical boundaries.”
ESMO also said that they were reviewing possibilities “to be of concrete help for our members and their patients, in collaboration with national and transnational oncology societies, as well as the International Cancer Foundation.”
A version of this article first appeared on Medscape.com.
As many in the world react with sanctions imposed on Russia after its invasion of Ukraine, the oncology community has now stepped into the fray.
All the large cancer organizations have put out statements in support of Ukraine, but one group has gone further and cut its ties with Russia.
“The international cancer specialist network, OncoAlert, severed all cooperation with doctors in Russia as part of the Western sanctions,” the group announced on its Twitter page, which is decorated with a blue and yellow ribbon and declares that it “stands with Ukraine.”
“The OncoAlert Network is nonpolitical but we cannot stand idle and not take a stand against this aggression toward our Ukrainian friends & colleagues,” the group said. “The network will be pulling out of ALL collaborations & congresses in Russia.”
Not surprisingly, the post was inundated with a barrage of inflammatory and politically laced tweets from Russian and Chinese users. Many of them repeated the same phrase about “violating the Hippocratic oath and the Geneva convention,” used foul language, and slammed the United States for past military actions in other parts of the world.
A prominent Russian oncologist also responded, posting a video in which he discussed the situation more coherently and without mudslinging or scripted phrases. Andrey Kaprin, MD, PhD, is chief oncologist of the Russian Federation as well as director general of the Federal State Budgetary Institution, NMRCC, of the Ministry of Health of the Russian Federation. He says they continue to maintain relations with the largest and best known oncologic organizations. “We haven’t felt any deterioration in our relationship yet, and of course, we hope that this won’t happen.”
Dr. Kaprin said he believes OncoAlert will return to cooperation with Russia, and that “reason will prevail.”
“No one is protected from cancer, not even doctors, and that is why there should be no politics here,” he said.
Dr. Kaprin was speaking from Russia state-affiliated media, so it was not an independent commentary. Several of the Twitter responses to his video, primarily from non-Russians, were less than complimentary.
One user replied: “Cancer is rife in the Kremlin.”
Another post pointed out the hypocrisy of Russians being upset that OncoAlert was cutting ties with them. “What about sick Ukrainian kids, having to shelter in hospital basement, not having lifesaving surgeries because Russia decided to invade a democratic country?”
And another post was not buying the story that “reason will prevail,” in that the doctor’s talk seemed to contradict the reality of the situation. “I guess for every child #Russia murders they get cut off a little more from the civilized world?”
Cancer patients vulnerable
The war in Ukraine is an “unfolding humanitarian emergency,” said the World Health Organization, and it has called on top-level officials involved in the Russian invasion to ensure access for delivery of essential medical, surgical, and trauma supplies to help the Ukrainian people and refugees in neighboring countries. A shortage of oxygen, insulin, cancer therapies, and other essential supplies will continue to grow more dire in the weeks and months ahead, WHO officials predict.
One of the more heartbreaking reports described how pediatric cancer patients have been moved to hospital basements that are serving as temporary bomb shelters. Hospital staff continue to try to provide limited treatment when possible, even though essential supplies are dwindling.
“These children suffer more because they need to stay alive to fight with the cancer – and this fight cannot wait,” Lesia Lysytsia, MD, a doctor at Okhmatdyt, the country’s largest children’s hospital in Kyiv, said in an NBC news report.
For some children, the only treatment available is a basic form of chemotherapy, and at the Kyiv Regional Oncology Center, the situation became so dire for children in need of blood transfusions that physicians began to transfuse blood from parent to child.
“Our patients, they will die,” Dr. Lysytsia said. “We will calculate how many people or soldiers have died in attacks, but we will never calculate how many patients weren’t diagnosed of disease in time, how many patients died because they didn’t receive treatment. It’s an epic amount of people.”
Response from oncology community
Many of the large American oncology groups have issued strong statements expressing their support for Ukraine and offering assistance.
The American Cancer Society has partnered with the American Society of Clinical Oncology and the Sidney Kimmel Cancer Center–Jefferson Health to support all Ukrainian cancer patients and their families. The groups are engaging a network of oncologists and oncology nurses to provide support through the ACS Clinician Volunteer Corps.
The ACS and ASCO are making free cancer resources available in English, Ukrainian, Polish, and Russian through their patient information websites (available here and here), with additional patient education resources planned.
The ACS noted that there are more than 179,000 newly diagnosed patients with cancer among the Ukrainian people “suffering from Russia’s unprovoked aggression.”
“Disruptions to cancer treatment pose a grave risk to the survival of Ukrainian patients with cancer,” commented Karen Knudsen, PhD, CEO at the ACS.
ASCO also issued its own statement, declaring that it stands with “our Ukrainian members, the worldwide oncology community, and health care providers around the globe in condemning Russia’s unprovoked war on Ukraine.”
The society notes that it represents oncology professionals in Ukraine and neighboring countries including Poland, Romania, Moldova, Slovakia, and Hungary, which are now receiving thousands of refugees from the Russian invasion.
“We are hearing daily reports of cancer treatment interrupted by acts of war, including damage to medical facilities and shortages of critical supplies. Countless patients now need to find cancer care in new and unfamiliar surroundings with limited medical records and minimal resources,” the society commented.
The American Association for Cancer Research also issued a statement by President David A. Tuveson, MD, PhD, and CEO Margaret Foti, PhD, MD (hc). The organization has more than 50,000 members around the world, and they “stand in solidarity with the citizens of Ukraine during the Russian attack on their country.”
“This abhorrent war, which has been instigated by Russia’s leaders, is isolating and interrupting the lifesaving work of scientists and clinicians in Ukraine and Russia, threatening years of effective research collaborations and community building,” the AACR comments. “Limiting the exchange of innovative ideas, practices, and data across borders will significantly retard cancer research and have an adverse effect on public health.”
Perhaps the most subdued statement came from the European Society of Medical Oncology, in a brief release entitled: “Against Any War.” The society expressed profound sadness about the unfolding tragedy in Ukraine and the suffering of people. “We would like to confirm our solidarity and unconditioned support to all oncology professionals and cancer patients, with no geographical boundaries.”
ESMO also said that they were reviewing possibilities “to be of concrete help for our members and their patients, in collaboration with national and transnational oncology societies, as well as the International Cancer Foundation.”
A version of this article first appeared on Medscape.com.
As many in the world react with sanctions imposed on Russia after its invasion of Ukraine, the oncology community has now stepped into the fray.
All the large cancer organizations have put out statements in support of Ukraine, but one group has gone further and cut its ties with Russia.
“The international cancer specialist network, OncoAlert, severed all cooperation with doctors in Russia as part of the Western sanctions,” the group announced on its Twitter page, which is decorated with a blue and yellow ribbon and declares that it “stands with Ukraine.”
“The OncoAlert Network is nonpolitical but we cannot stand idle and not take a stand against this aggression toward our Ukrainian friends & colleagues,” the group said. “The network will be pulling out of ALL collaborations & congresses in Russia.”
Not surprisingly, the post was inundated with a barrage of inflammatory and politically laced tweets from Russian and Chinese users. Many of them repeated the same phrase about “violating the Hippocratic oath and the Geneva convention,” used foul language, and slammed the United States for past military actions in other parts of the world.
A prominent Russian oncologist also responded, posting a video in which he discussed the situation more coherently and without mudslinging or scripted phrases. Andrey Kaprin, MD, PhD, is chief oncologist of the Russian Federation as well as director general of the Federal State Budgetary Institution, NMRCC, of the Ministry of Health of the Russian Federation. He says they continue to maintain relations with the largest and best known oncologic organizations. “We haven’t felt any deterioration in our relationship yet, and of course, we hope that this won’t happen.”
Dr. Kaprin said he believes OncoAlert will return to cooperation with Russia, and that “reason will prevail.”
“No one is protected from cancer, not even doctors, and that is why there should be no politics here,” he said.
Dr. Kaprin was speaking from Russia state-affiliated media, so it was not an independent commentary. Several of the Twitter responses to his video, primarily from non-Russians, were less than complimentary.
One user replied: “Cancer is rife in the Kremlin.”
Another post pointed out the hypocrisy of Russians being upset that OncoAlert was cutting ties with them. “What about sick Ukrainian kids, having to shelter in hospital basement, not having lifesaving surgeries because Russia decided to invade a democratic country?”
And another post was not buying the story that “reason will prevail,” in that the doctor’s talk seemed to contradict the reality of the situation. “I guess for every child #Russia murders they get cut off a little more from the civilized world?”
Cancer patients vulnerable
The war in Ukraine is an “unfolding humanitarian emergency,” said the World Health Organization, and it has called on top-level officials involved in the Russian invasion to ensure access for delivery of essential medical, surgical, and trauma supplies to help the Ukrainian people and refugees in neighboring countries. A shortage of oxygen, insulin, cancer therapies, and other essential supplies will continue to grow more dire in the weeks and months ahead, WHO officials predict.
One of the more heartbreaking reports described how pediatric cancer patients have been moved to hospital basements that are serving as temporary bomb shelters. Hospital staff continue to try to provide limited treatment when possible, even though essential supplies are dwindling.
“These children suffer more because they need to stay alive to fight with the cancer – and this fight cannot wait,” Lesia Lysytsia, MD, a doctor at Okhmatdyt, the country’s largest children’s hospital in Kyiv, said in an NBC news report.
For some children, the only treatment available is a basic form of chemotherapy, and at the Kyiv Regional Oncology Center, the situation became so dire for children in need of blood transfusions that physicians began to transfuse blood from parent to child.
“Our patients, they will die,” Dr. Lysytsia said. “We will calculate how many people or soldiers have died in attacks, but we will never calculate how many patients weren’t diagnosed of disease in time, how many patients died because they didn’t receive treatment. It’s an epic amount of people.”
Response from oncology community
Many of the large American oncology groups have issued strong statements expressing their support for Ukraine and offering assistance.
The American Cancer Society has partnered with the American Society of Clinical Oncology and the Sidney Kimmel Cancer Center–Jefferson Health to support all Ukrainian cancer patients and their families. The groups are engaging a network of oncologists and oncology nurses to provide support through the ACS Clinician Volunteer Corps.
The ACS and ASCO are making free cancer resources available in English, Ukrainian, Polish, and Russian through their patient information websites (available here and here), with additional patient education resources planned.
The ACS noted that there are more than 179,000 newly diagnosed patients with cancer among the Ukrainian people “suffering from Russia’s unprovoked aggression.”
“Disruptions to cancer treatment pose a grave risk to the survival of Ukrainian patients with cancer,” commented Karen Knudsen, PhD, CEO at the ACS.
ASCO also issued its own statement, declaring that it stands with “our Ukrainian members, the worldwide oncology community, and health care providers around the globe in condemning Russia’s unprovoked war on Ukraine.”
The society notes that it represents oncology professionals in Ukraine and neighboring countries including Poland, Romania, Moldova, Slovakia, and Hungary, which are now receiving thousands of refugees from the Russian invasion.
“We are hearing daily reports of cancer treatment interrupted by acts of war, including damage to medical facilities and shortages of critical supplies. Countless patients now need to find cancer care in new and unfamiliar surroundings with limited medical records and minimal resources,” the society commented.
The American Association for Cancer Research also issued a statement by President David A. Tuveson, MD, PhD, and CEO Margaret Foti, PhD, MD (hc). The organization has more than 50,000 members around the world, and they “stand in solidarity with the citizens of Ukraine during the Russian attack on their country.”
“This abhorrent war, which has been instigated by Russia’s leaders, is isolating and interrupting the lifesaving work of scientists and clinicians in Ukraine and Russia, threatening years of effective research collaborations and community building,” the AACR comments. “Limiting the exchange of innovative ideas, practices, and data across borders will significantly retard cancer research and have an adverse effect on public health.”
Perhaps the most subdued statement came from the European Society of Medical Oncology, in a brief release entitled: “Against Any War.” The society expressed profound sadness about the unfolding tragedy in Ukraine and the suffering of people. “We would like to confirm our solidarity and unconditioned support to all oncology professionals and cancer patients, with no geographical boundaries.”
ESMO also said that they were reviewing possibilities “to be of concrete help for our members and their patients, in collaboration with national and transnational oncology societies, as well as the International Cancer Foundation.”
A version of this article first appeared on Medscape.com.
Physicians beware: Feds start tracking information-blocking claims
The federal government’s efforts to thwart information blocking are underway. As such,
Recently, the Office of the National Coordinator revealed that the Department of Health & Humans Services has received 299 reports of information blocking since inviting anyone who suspected that health care providers, IT developers, or health information networks/exchanges might have interfered with access, exchange, or use of EHI through the Report Information Blocking Portal on April 5, 2021.
The vast majority of these claims – 211 – were filed against providers, while 46 alleged incidents of information blocking were by health IT developers, and two claims point to health information networks/ exchanges. The other 25 claims did not appear to present a claim of information blocking.
Of the 274 possible claims of information blocking recently released by ONC, 176 were made by patients.
The ONC has sent all possible claims to the HHS’s Office of the Inspector General. The claims have not yet been investigated and substantiated.
Do the stats tell the story?
The numbers in the recent ONC report do not shed much light on how much impact the regulations are having on information sharing. Health care providers, including physicians, might not yet be complying with the rules because monetary penalties are not in place.
Indeed, HHS has yet to spell out exactly what the disincentives on providers will be, though the 21st Century Cures Act stipulates that regulators could fine up to $1 million per information-blocking incident.
“Some providers might be saying, ‘I’m not going to be penalized at this point … so I can take a little bit longer to think about how I come into compliance.’ That could be just one factor of a host of many that are affecting compliance. We also are still in the middle of a public health emergency. So it’s hard to say at this point” exactly how the regulations will affect information blocking, Lauren Riplinger, vice president of policy and public affairs at the American Health Information Management Association, Chicago, said in an interview.
A long time coming
The government first zeroed in on ensuring that patients have access to their information in 2016 when President Obama signed the Cures Act into law. The legislation directed ONC to implement a standardized process for the public to report claims of possible information blocking.
The initiative appears to be picking up steam. The ONC is expected to release monthly reports on the cumulative number of information-blocking claims. The announcement of associated penalties is expected sometime in the future.
Industry leaders are advising health care providers to brush up on compliance. Physicians can look to professional groups such as the American Medical Association, the Medical Group Management Association, and other specialty associations for guidance. In addition, the ONC is educating providers on the rule.
“The ONC has provided a lot of great content for the past couple months, not only in terms of putting out FAQs to help clarify some of the gray areas in the rule, but they also have produced a series of provider-specific webinars where they walk through a potential scenario and address the extent to the rules apply,” Ms. Riplinger said.
With education, more is better
These efforts, however, could be expanded, according to MGMA.
“There is a general awareness of the rules, but we encourage ONC to continue educating the provider community: More FAQs and educational webinars would be helpful,” Claire Ernst, director of government affairs for MGMA, said in an interview. “A June 2021 MGMA poll found that 51% of medical groups said they needed more government guidance on complying with the new information-blocking rules.”
Although ONC already has provided some “scenario-based” education, more of this type of guidance could prove valuable.
“This rule is that it is very circumstance based. … and so it’s those more nuanced cases that I think are more challenging for providers to know whether or not they are engaging in information blocking,” Ms. Riplinger noted.
For example, a physician might choose to not upload lab test results to a patient portal and prefer to wait to discuss the results directly with the patient, which could potentially be construed as information blocking under the regulations.
The MGMA is requesting that ONC take a second look at these situations – and possibly adjust the regulations.
“MGMA has heard concerns about the impact of providing immediate results to patients before medical groups have the time to thoroughly review test results and discuss them compassionately with their patients,” Ms. Ernst said. “To address this, ONC could expand the current definition of harm to account for other unintended consequences, such as emotional distress, or provide more flexibility in terms of the time frame.”
A version of this article first appeared on Medscape.com.
The federal government’s efforts to thwart information blocking are underway. As such,
Recently, the Office of the National Coordinator revealed that the Department of Health & Humans Services has received 299 reports of information blocking since inviting anyone who suspected that health care providers, IT developers, or health information networks/exchanges might have interfered with access, exchange, or use of EHI through the Report Information Blocking Portal on April 5, 2021.
The vast majority of these claims – 211 – were filed against providers, while 46 alleged incidents of information blocking were by health IT developers, and two claims point to health information networks/ exchanges. The other 25 claims did not appear to present a claim of information blocking.
Of the 274 possible claims of information blocking recently released by ONC, 176 were made by patients.
The ONC has sent all possible claims to the HHS’s Office of the Inspector General. The claims have not yet been investigated and substantiated.
Do the stats tell the story?
The numbers in the recent ONC report do not shed much light on how much impact the regulations are having on information sharing. Health care providers, including physicians, might not yet be complying with the rules because monetary penalties are not in place.
Indeed, HHS has yet to spell out exactly what the disincentives on providers will be, though the 21st Century Cures Act stipulates that regulators could fine up to $1 million per information-blocking incident.
“Some providers might be saying, ‘I’m not going to be penalized at this point … so I can take a little bit longer to think about how I come into compliance.’ That could be just one factor of a host of many that are affecting compliance. We also are still in the middle of a public health emergency. So it’s hard to say at this point” exactly how the regulations will affect information blocking, Lauren Riplinger, vice president of policy and public affairs at the American Health Information Management Association, Chicago, said in an interview.
A long time coming
The government first zeroed in on ensuring that patients have access to their information in 2016 when President Obama signed the Cures Act into law. The legislation directed ONC to implement a standardized process for the public to report claims of possible information blocking.
The initiative appears to be picking up steam. The ONC is expected to release monthly reports on the cumulative number of information-blocking claims. The announcement of associated penalties is expected sometime in the future.
Industry leaders are advising health care providers to brush up on compliance. Physicians can look to professional groups such as the American Medical Association, the Medical Group Management Association, and other specialty associations for guidance. In addition, the ONC is educating providers on the rule.
“The ONC has provided a lot of great content for the past couple months, not only in terms of putting out FAQs to help clarify some of the gray areas in the rule, but they also have produced a series of provider-specific webinars where they walk through a potential scenario and address the extent to the rules apply,” Ms. Riplinger said.
With education, more is better
These efforts, however, could be expanded, according to MGMA.
“There is a general awareness of the rules, but we encourage ONC to continue educating the provider community: More FAQs and educational webinars would be helpful,” Claire Ernst, director of government affairs for MGMA, said in an interview. “A June 2021 MGMA poll found that 51% of medical groups said they needed more government guidance on complying with the new information-blocking rules.”
Although ONC already has provided some “scenario-based” education, more of this type of guidance could prove valuable.
“This rule is that it is very circumstance based. … and so it’s those more nuanced cases that I think are more challenging for providers to know whether or not they are engaging in information blocking,” Ms. Riplinger noted.
For example, a physician might choose to not upload lab test results to a patient portal and prefer to wait to discuss the results directly with the patient, which could potentially be construed as information blocking under the regulations.
The MGMA is requesting that ONC take a second look at these situations – and possibly adjust the regulations.
“MGMA has heard concerns about the impact of providing immediate results to patients before medical groups have the time to thoroughly review test results and discuss them compassionately with their patients,” Ms. Ernst said. “To address this, ONC could expand the current definition of harm to account for other unintended consequences, such as emotional distress, or provide more flexibility in terms of the time frame.”
A version of this article first appeared on Medscape.com.
The federal government’s efforts to thwart information blocking are underway. As such,
Recently, the Office of the National Coordinator revealed that the Department of Health & Humans Services has received 299 reports of information blocking since inviting anyone who suspected that health care providers, IT developers, or health information networks/exchanges might have interfered with access, exchange, or use of EHI through the Report Information Blocking Portal on April 5, 2021.
The vast majority of these claims – 211 – were filed against providers, while 46 alleged incidents of information blocking were by health IT developers, and two claims point to health information networks/ exchanges. The other 25 claims did not appear to present a claim of information blocking.
Of the 274 possible claims of information blocking recently released by ONC, 176 were made by patients.
The ONC has sent all possible claims to the HHS’s Office of the Inspector General. The claims have not yet been investigated and substantiated.
Do the stats tell the story?
The numbers in the recent ONC report do not shed much light on how much impact the regulations are having on information sharing. Health care providers, including physicians, might not yet be complying with the rules because monetary penalties are not in place.
Indeed, HHS has yet to spell out exactly what the disincentives on providers will be, though the 21st Century Cures Act stipulates that regulators could fine up to $1 million per information-blocking incident.
“Some providers might be saying, ‘I’m not going to be penalized at this point … so I can take a little bit longer to think about how I come into compliance.’ That could be just one factor of a host of many that are affecting compliance. We also are still in the middle of a public health emergency. So it’s hard to say at this point” exactly how the regulations will affect information blocking, Lauren Riplinger, vice president of policy and public affairs at the American Health Information Management Association, Chicago, said in an interview.
A long time coming
The government first zeroed in on ensuring that patients have access to their information in 2016 when President Obama signed the Cures Act into law. The legislation directed ONC to implement a standardized process for the public to report claims of possible information blocking.
The initiative appears to be picking up steam. The ONC is expected to release monthly reports on the cumulative number of information-blocking claims. The announcement of associated penalties is expected sometime in the future.
Industry leaders are advising health care providers to brush up on compliance. Physicians can look to professional groups such as the American Medical Association, the Medical Group Management Association, and other specialty associations for guidance. In addition, the ONC is educating providers on the rule.
“The ONC has provided a lot of great content for the past couple months, not only in terms of putting out FAQs to help clarify some of the gray areas in the rule, but they also have produced a series of provider-specific webinars where they walk through a potential scenario and address the extent to the rules apply,” Ms. Riplinger said.
With education, more is better
These efforts, however, could be expanded, according to MGMA.
“There is a general awareness of the rules, but we encourage ONC to continue educating the provider community: More FAQs and educational webinars would be helpful,” Claire Ernst, director of government affairs for MGMA, said in an interview. “A June 2021 MGMA poll found that 51% of medical groups said they needed more government guidance on complying with the new information-blocking rules.”
Although ONC already has provided some “scenario-based” education, more of this type of guidance could prove valuable.
“This rule is that it is very circumstance based. … and so it’s those more nuanced cases that I think are more challenging for providers to know whether or not they are engaging in information blocking,” Ms. Riplinger noted.
For example, a physician might choose to not upload lab test results to a patient portal and prefer to wait to discuss the results directly with the patient, which could potentially be construed as information blocking under the regulations.
The MGMA is requesting that ONC take a second look at these situations – and possibly adjust the regulations.
“MGMA has heard concerns about the impact of providing immediate results to patients before medical groups have the time to thoroughly review test results and discuss them compassionately with their patients,” Ms. Ernst said. “To address this, ONC could expand the current definition of harm to account for other unintended consequences, such as emotional distress, or provide more flexibility in terms of the time frame.”
A version of this article first appeared on Medscape.com.
Dietary fiber tied to lower dementia risk
, new research shows.
Investigators administered a dietary survey to 3,700 healthy adults at midlife and then followed them for up to 20 years. They found that participants who consumed the most fiber had approximately a 25% lower risk of developing dementia in later life.
“This study showed that people with a high intake of dietary fiber, especially soluble fiber, have a lower risk of dementia,” study investigator Kazumasa Yamagishi, MD, PhD, professor, department of public health medicine, faculty of medicine and health, Services Research and Development Center, University of Tsukuba, Japan, said in an interview.
“There are still many unknowns about the causes of dementia, and it is not appropriate to determine causality based on the results of a single cohort study. However, the results of this study can be said to be one of the findings that will lead to the prevention of dementia,” Dr. Yamagishi said.
The study was published online Feb. 6 in Nutritional Neuroscience.
Brain-gut interaction
Brain-gut interaction has recently received attention for its potential involvement in the development of dementia. “The concept of brain-gut interaction emerged from the idea that the central nervous system communicates bidirectionally with the gastrointestinal tract, suggesting that the gut microbiome may influence brain plasticity and cognitive function,” the authors wrote.
A diet high in soluble fiber attenuates neuroinflammation in mouse models. Other animal studies have suggested that insoluble fiber might also have a beneficial effect on the microbiome.
The researchers wanted to see whether dietary fiber intake – especially soluble fiber – is associated with a reduced risk of dementia. They also investigated whether there was any difference between dementia in patients with vs. without a history of stroke.
In a previous study, these same researchers reported an inverse association between eating beans, which are high in fiber, and risk of disabling dementia. In the current study, the researchers extended the analyses to dietary fiber intake of total, soluble, and insoluble fibers, as well as other fiber-containing foods, such potatoes, vegetables, and fruits. However, they distinguished potatoes from other vegetables because the composition of starch in potatoes differs.
“Dietary fiber is a nutrient found in grains, potatoes, vegetables, and fruits and is known to affect intestinal bacteria,” Dr. Yamagishi said. “Recently, some experimental studies have shown that intestinal bacteria may be involved in cognitive functions as well as diseases of the digestive tract. However, there have been no studies that have actually examined the relationship between dietary fiber intake and the subsequent risk of dementia in large numbers of general people.”
The researchers turned to participants in the Circulatory Risk in Communities Study (CIRCS), an ongoing dynamic community cohort study involving five communities in Japan. The current study focused on communities where disabling dementia surveillance is conducted.
Participants (n = 3,739) ranged in age from 40 to 64 years (mean age, 51 years) at the time they completed the 24-hour dietary recall survey, and they participated in annual health checkups from 1985 to 1999. Potential risk factors for disabling dementia were measured at the time the dietary surveys were conducted. Participants were then followed for a median of 19.7 years (1999-2020) to confirm incident, disabling dementia.
“Disabling dementia” was defined as dementia that required care under the National Long-Term Care Insurance System and was further categorized on the basis of having a history or not having a history of stroke.
The researchers divided participants into quartiles, based on the amount of total, soluble, and insoluble intake reported in their surveys. They found that men tended to consume less total fiber compared to women.
Unclear mechanism
During follow-up, 670 participants developed disabling dementia.
Total fiber intake was “inversely and linearly” associated with risk of incident dementia, the authors reported, with each successive quartile associated with a lower risk compared to the lowest quartile (P for trend = .03).
The association remained after adjustment for potential factors that might affect dementia onset, such as body mass index, systolic blood pressure, antihypertensive medication use, serum total cholesterol, cholesterol-lowering medication, and diabetes (P for trend = .05).
“The inverse association was more evident for soluble fiber intake and was confined to dementia without a history of stroke,” the authors reported. Moreover, potatoes, not vegetables or fruits, showed a similar association.
“The mechanisms are currently unknown but might involve the interactions that take place between the gut and the brain,” Dr. Yamagishi said in a release.
“One possibility is that soluble fiber regulates the composition of gut bacteria. This composition may affect neuroinflammation, which plays a role in the onset of dementia,” he suggested. “It’s also possible that dietary fiber may reduce other risk factors for dementia, such as body weight, blood pressure, lipids, and glucose levels.”
The authors noted several limitations. For example, they did not distinguish between Alzheimer’s and non-Alzheimer’s dementia. Moreover, they classified dietary habits on the basis of a single survey, and participants’ dietary patterns might have changed over the study period.
In addition, Dr. Yamagishi noted, it is “important to confirm the association in other populations.”
Balance is key
In an interview, Uma Naidoo, MD, director of nutritional and lifestyle psychiatry, Massachusetts General Hospital, and nutrition educator at Harvard Medical School, both in Boston, said the study “adds to the growing pool of evidence suggesting that a diet rich in colorful, plant-based foods can benefit our neurological and psychiatric health, especially as we age.”
Dr. Naidoo, a chef and the author of “This Is Your Brain on Food,” who was not involved in the study, continued, “In nutritional psychiatry, balance is key and therefore consuming a well-rounded diet including ample amounts of fiber – particularly from sources like steel-cut oats, beans, lentils, and numerous other fruits and vegetables – can be part of a healthy lifestyle and prevention against cognitive decline in later years.
“While the study authors admit to limitations within the study, in my opinion, eating healthier has so many mental and physical health benefits that it’s a nutritional psychiatry no-brainer,” she added.
The study was partly supported by Health and Labour Science Research Grants for Dementia from the Ministry of Health, Labour and Welfare of Japan; JSPS Kakenhi; FULLHAP; and the Osaka University International Joint Research Promotion Programme with University College London. The authors and Dr. Naidoo report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
Investigators administered a dietary survey to 3,700 healthy adults at midlife and then followed them for up to 20 years. They found that participants who consumed the most fiber had approximately a 25% lower risk of developing dementia in later life.
“This study showed that people with a high intake of dietary fiber, especially soluble fiber, have a lower risk of dementia,” study investigator Kazumasa Yamagishi, MD, PhD, professor, department of public health medicine, faculty of medicine and health, Services Research and Development Center, University of Tsukuba, Japan, said in an interview.
“There are still many unknowns about the causes of dementia, and it is not appropriate to determine causality based on the results of a single cohort study. However, the results of this study can be said to be one of the findings that will lead to the prevention of dementia,” Dr. Yamagishi said.
The study was published online Feb. 6 in Nutritional Neuroscience.
Brain-gut interaction
Brain-gut interaction has recently received attention for its potential involvement in the development of dementia. “The concept of brain-gut interaction emerged from the idea that the central nervous system communicates bidirectionally with the gastrointestinal tract, suggesting that the gut microbiome may influence brain plasticity and cognitive function,” the authors wrote.
A diet high in soluble fiber attenuates neuroinflammation in mouse models. Other animal studies have suggested that insoluble fiber might also have a beneficial effect on the microbiome.
The researchers wanted to see whether dietary fiber intake – especially soluble fiber – is associated with a reduced risk of dementia. They also investigated whether there was any difference between dementia in patients with vs. without a history of stroke.
In a previous study, these same researchers reported an inverse association between eating beans, which are high in fiber, and risk of disabling dementia. In the current study, the researchers extended the analyses to dietary fiber intake of total, soluble, and insoluble fibers, as well as other fiber-containing foods, such potatoes, vegetables, and fruits. However, they distinguished potatoes from other vegetables because the composition of starch in potatoes differs.
“Dietary fiber is a nutrient found in grains, potatoes, vegetables, and fruits and is known to affect intestinal bacteria,” Dr. Yamagishi said. “Recently, some experimental studies have shown that intestinal bacteria may be involved in cognitive functions as well as diseases of the digestive tract. However, there have been no studies that have actually examined the relationship between dietary fiber intake and the subsequent risk of dementia in large numbers of general people.”
The researchers turned to participants in the Circulatory Risk in Communities Study (CIRCS), an ongoing dynamic community cohort study involving five communities in Japan. The current study focused on communities where disabling dementia surveillance is conducted.
Participants (n = 3,739) ranged in age from 40 to 64 years (mean age, 51 years) at the time they completed the 24-hour dietary recall survey, and they participated in annual health checkups from 1985 to 1999. Potential risk factors for disabling dementia were measured at the time the dietary surveys were conducted. Participants were then followed for a median of 19.7 years (1999-2020) to confirm incident, disabling dementia.
“Disabling dementia” was defined as dementia that required care under the National Long-Term Care Insurance System and was further categorized on the basis of having a history or not having a history of stroke.
The researchers divided participants into quartiles, based on the amount of total, soluble, and insoluble intake reported in their surveys. They found that men tended to consume less total fiber compared to women.
Unclear mechanism
During follow-up, 670 participants developed disabling dementia.
Total fiber intake was “inversely and linearly” associated with risk of incident dementia, the authors reported, with each successive quartile associated with a lower risk compared to the lowest quartile (P for trend = .03).
The association remained after adjustment for potential factors that might affect dementia onset, such as body mass index, systolic blood pressure, antihypertensive medication use, serum total cholesterol, cholesterol-lowering medication, and diabetes (P for trend = .05).
“The inverse association was more evident for soluble fiber intake and was confined to dementia without a history of stroke,” the authors reported. Moreover, potatoes, not vegetables or fruits, showed a similar association.
“The mechanisms are currently unknown but might involve the interactions that take place between the gut and the brain,” Dr. Yamagishi said in a release.
“One possibility is that soluble fiber regulates the composition of gut bacteria. This composition may affect neuroinflammation, which plays a role in the onset of dementia,” he suggested. “It’s also possible that dietary fiber may reduce other risk factors for dementia, such as body weight, blood pressure, lipids, and glucose levels.”
The authors noted several limitations. For example, they did not distinguish between Alzheimer’s and non-Alzheimer’s dementia. Moreover, they classified dietary habits on the basis of a single survey, and participants’ dietary patterns might have changed over the study period.
In addition, Dr. Yamagishi noted, it is “important to confirm the association in other populations.”
Balance is key
In an interview, Uma Naidoo, MD, director of nutritional and lifestyle psychiatry, Massachusetts General Hospital, and nutrition educator at Harvard Medical School, both in Boston, said the study “adds to the growing pool of evidence suggesting that a diet rich in colorful, plant-based foods can benefit our neurological and psychiatric health, especially as we age.”
Dr. Naidoo, a chef and the author of “This Is Your Brain on Food,” who was not involved in the study, continued, “In nutritional psychiatry, balance is key and therefore consuming a well-rounded diet including ample amounts of fiber – particularly from sources like steel-cut oats, beans, lentils, and numerous other fruits and vegetables – can be part of a healthy lifestyle and prevention against cognitive decline in later years.
“While the study authors admit to limitations within the study, in my opinion, eating healthier has so many mental and physical health benefits that it’s a nutritional psychiatry no-brainer,” she added.
The study was partly supported by Health and Labour Science Research Grants for Dementia from the Ministry of Health, Labour and Welfare of Japan; JSPS Kakenhi; FULLHAP; and the Osaka University International Joint Research Promotion Programme with University College London. The authors and Dr. Naidoo report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
Investigators administered a dietary survey to 3,700 healthy adults at midlife and then followed them for up to 20 years. They found that participants who consumed the most fiber had approximately a 25% lower risk of developing dementia in later life.
“This study showed that people with a high intake of dietary fiber, especially soluble fiber, have a lower risk of dementia,” study investigator Kazumasa Yamagishi, MD, PhD, professor, department of public health medicine, faculty of medicine and health, Services Research and Development Center, University of Tsukuba, Japan, said in an interview.
“There are still many unknowns about the causes of dementia, and it is not appropriate to determine causality based on the results of a single cohort study. However, the results of this study can be said to be one of the findings that will lead to the prevention of dementia,” Dr. Yamagishi said.
The study was published online Feb. 6 in Nutritional Neuroscience.
Brain-gut interaction
Brain-gut interaction has recently received attention for its potential involvement in the development of dementia. “The concept of brain-gut interaction emerged from the idea that the central nervous system communicates bidirectionally with the gastrointestinal tract, suggesting that the gut microbiome may influence brain plasticity and cognitive function,” the authors wrote.
A diet high in soluble fiber attenuates neuroinflammation in mouse models. Other animal studies have suggested that insoluble fiber might also have a beneficial effect on the microbiome.
The researchers wanted to see whether dietary fiber intake – especially soluble fiber – is associated with a reduced risk of dementia. They also investigated whether there was any difference between dementia in patients with vs. without a history of stroke.
In a previous study, these same researchers reported an inverse association between eating beans, which are high in fiber, and risk of disabling dementia. In the current study, the researchers extended the analyses to dietary fiber intake of total, soluble, and insoluble fibers, as well as other fiber-containing foods, such potatoes, vegetables, and fruits. However, they distinguished potatoes from other vegetables because the composition of starch in potatoes differs.
“Dietary fiber is a nutrient found in grains, potatoes, vegetables, and fruits and is known to affect intestinal bacteria,” Dr. Yamagishi said. “Recently, some experimental studies have shown that intestinal bacteria may be involved in cognitive functions as well as diseases of the digestive tract. However, there have been no studies that have actually examined the relationship between dietary fiber intake and the subsequent risk of dementia in large numbers of general people.”
The researchers turned to participants in the Circulatory Risk in Communities Study (CIRCS), an ongoing dynamic community cohort study involving five communities in Japan. The current study focused on communities where disabling dementia surveillance is conducted.
Participants (n = 3,739) ranged in age from 40 to 64 years (mean age, 51 years) at the time they completed the 24-hour dietary recall survey, and they participated in annual health checkups from 1985 to 1999. Potential risk factors for disabling dementia were measured at the time the dietary surveys were conducted. Participants were then followed for a median of 19.7 years (1999-2020) to confirm incident, disabling dementia.
“Disabling dementia” was defined as dementia that required care under the National Long-Term Care Insurance System and was further categorized on the basis of having a history or not having a history of stroke.
The researchers divided participants into quartiles, based on the amount of total, soluble, and insoluble intake reported in their surveys. They found that men tended to consume less total fiber compared to women.
Unclear mechanism
During follow-up, 670 participants developed disabling dementia.
Total fiber intake was “inversely and linearly” associated with risk of incident dementia, the authors reported, with each successive quartile associated with a lower risk compared to the lowest quartile (P for trend = .03).
The association remained after adjustment for potential factors that might affect dementia onset, such as body mass index, systolic blood pressure, antihypertensive medication use, serum total cholesterol, cholesterol-lowering medication, and diabetes (P for trend = .05).
“The inverse association was more evident for soluble fiber intake and was confined to dementia without a history of stroke,” the authors reported. Moreover, potatoes, not vegetables or fruits, showed a similar association.
“The mechanisms are currently unknown but might involve the interactions that take place between the gut and the brain,” Dr. Yamagishi said in a release.
“One possibility is that soluble fiber regulates the composition of gut bacteria. This composition may affect neuroinflammation, which plays a role in the onset of dementia,” he suggested. “It’s also possible that dietary fiber may reduce other risk factors for dementia, such as body weight, blood pressure, lipids, and glucose levels.”
The authors noted several limitations. For example, they did not distinguish between Alzheimer’s and non-Alzheimer’s dementia. Moreover, they classified dietary habits on the basis of a single survey, and participants’ dietary patterns might have changed over the study period.
In addition, Dr. Yamagishi noted, it is “important to confirm the association in other populations.”
Balance is key
In an interview, Uma Naidoo, MD, director of nutritional and lifestyle psychiatry, Massachusetts General Hospital, and nutrition educator at Harvard Medical School, both in Boston, said the study “adds to the growing pool of evidence suggesting that a diet rich in colorful, plant-based foods can benefit our neurological and psychiatric health, especially as we age.”
Dr. Naidoo, a chef and the author of “This Is Your Brain on Food,” who was not involved in the study, continued, “In nutritional psychiatry, balance is key and therefore consuming a well-rounded diet including ample amounts of fiber – particularly from sources like steel-cut oats, beans, lentils, and numerous other fruits and vegetables – can be part of a healthy lifestyle and prevention against cognitive decline in later years.
“While the study authors admit to limitations within the study, in my opinion, eating healthier has so many mental and physical health benefits that it’s a nutritional psychiatry no-brainer,” she added.
The study was partly supported by Health and Labour Science Research Grants for Dementia from the Ministry of Health, Labour and Welfare of Japan; JSPS Kakenhi; FULLHAP; and the Osaka University International Joint Research Promotion Programme with University College London. The authors and Dr. Naidoo report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NUTRITIONAL NEUROSCIENCE
Treatment Advances in Moderate to Severe Active Ulcerative Colitis
Treatment for moderate to severe active ulcerative colitis (UC) has evolved, and with more effective treatment comes higher standards for disease control.
The initial goal is clinical response, followed by clinical remission, endoscopic remission, and — the ultimate goal — histologic remission.
The majority of UC medications have been studied for clinical and endoscopic remission. Recent clinical trials, however, have evaluated the emerging targeted therapies ustekinumab and ozanimod for histologic remission and found positive results.
Dr Bincy Abraham, director of the Fondren Inflammatory Bowel Disease Program at Houston Methodist in Houston, Texas, reports on UC treatment milestones and how emerging targeted therapies can help achieve these goals.
She also discusses patient monitoring to ensure response to therapy as well as medication adjustments should response prove inadequate.
--
Professor of Clinical Medicine, Department of Internal Medicine and Gastroenterology; Director, Fondren Inflammatory Bowel Disease Program, Underwood Center for Digestive Disorders, Houston Methodist, Houston, Texas
Bincy Abraham, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AbbVie; BMS; Janssen; Pfizer; Takeda; Medtronic
Serve(d) as a speaker or a member of a speakers bureau for: AbbVie; BMS; Janssen; Pfizer; Takeda
Received research grant from: Takeda; BMS; Genentech
Treatment for moderate to severe active ulcerative colitis (UC) has evolved, and with more effective treatment comes higher standards for disease control.
The initial goal is clinical response, followed by clinical remission, endoscopic remission, and — the ultimate goal — histologic remission.
The majority of UC medications have been studied for clinical and endoscopic remission. Recent clinical trials, however, have evaluated the emerging targeted therapies ustekinumab and ozanimod for histologic remission and found positive results.
Dr Bincy Abraham, director of the Fondren Inflammatory Bowel Disease Program at Houston Methodist in Houston, Texas, reports on UC treatment milestones and how emerging targeted therapies can help achieve these goals.
She also discusses patient monitoring to ensure response to therapy as well as medication adjustments should response prove inadequate.
--
Professor of Clinical Medicine, Department of Internal Medicine and Gastroenterology; Director, Fondren Inflammatory Bowel Disease Program, Underwood Center for Digestive Disorders, Houston Methodist, Houston, Texas
Bincy Abraham, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AbbVie; BMS; Janssen; Pfizer; Takeda; Medtronic
Serve(d) as a speaker or a member of a speakers bureau for: AbbVie; BMS; Janssen; Pfizer; Takeda
Received research grant from: Takeda; BMS; Genentech
Treatment for moderate to severe active ulcerative colitis (UC) has evolved, and with more effective treatment comes higher standards for disease control.
The initial goal is clinical response, followed by clinical remission, endoscopic remission, and — the ultimate goal — histologic remission.
The majority of UC medications have been studied for clinical and endoscopic remission. Recent clinical trials, however, have evaluated the emerging targeted therapies ustekinumab and ozanimod for histologic remission and found positive results.
Dr Bincy Abraham, director of the Fondren Inflammatory Bowel Disease Program at Houston Methodist in Houston, Texas, reports on UC treatment milestones and how emerging targeted therapies can help achieve these goals.
She also discusses patient monitoring to ensure response to therapy as well as medication adjustments should response prove inadequate.
--
Professor of Clinical Medicine, Department of Internal Medicine and Gastroenterology; Director, Fondren Inflammatory Bowel Disease Program, Underwood Center for Digestive Disorders, Houston Methodist, Houston, Texas
Bincy Abraham, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AbbVie; BMS; Janssen; Pfizer; Takeda; Medtronic
Serve(d) as a speaker or a member of a speakers bureau for: AbbVie; BMS; Janssen; Pfizer; Takeda
Received research grant from: Takeda; BMS; Genentech

Borderline personality disorder: Is there an optimal therapy?
Schema is a form of psychotherapy that focuses on experiential approaches rather than on behavior change.
The findings from an international randomized controlled trial underscore the importance of offering both individual and group approaches to patients with BPD, study investigator Arnoud Arntz, PhD, professor in the department of clinical psychology at the University of Amsterdam, told this news organization.
“In the Netherlands, there’s a big push from mental health institutes to deliver treatments in group therapy [only] because people think it’s more cost-effective; but these findings question that idea,” Dr. Arntz said.
The findings were published online March 2 in JAMA Psychiatry.
Early childhood experiences
Patients with BPD exhibit extreme sensitivity to interpersonal slights, intense and volatile emotions, and impulsive behaviors. Many abuse drugs, self-harm, or attempt suicide.
Evidence-based guidelines recommend psychotherapy as the primary treatment for BPD.
Schema therapy uses techniques from traditional psychotherapy but focuses on an experiential strategy. It also delves into early childhood experiences, which is relevant because patients with BPD often experienced abuse or neglect early in life.
As well, with this approach, therapists take on a sort of parenting role with patients to try to meet needs “that were frustrated in childhood,” said Dr. Arntz.
Previous research has suggested both individual and group schema therapy help reduce BPD symptoms, but the effectiveness of combining these two approaches has been unclear.
The current study included 495 adult patients (mean age, 33.6 years; 86.2% women) enrolled at 15 sites in five countries: the Netherlands, England, Greece, Germany, and Australia. All participants had a Borderline Personality Disorder Severity Index IV (BPDSI-IV) score of more than 20.
The BPDSI-IV score ranges from 0 to 90, with a score of 15 being the cutoff for a BPD diagnosis.
Investigators randomly assigned participants to one of three arms: predominantly group schema therapy, combined individual and group schema therapy, and treatment as usual – which was the optimal psychological treatment available at the site.
The two schema therapy arms, whether group or individual, involved a similar number of sessions each week. However, the frequency was gradually reduced over the course of the study.
Improved severity
The primary outcome was change in BPD severity as assessed at baseline, 6 months, 12 months, 18 months, 24 months, and 36 months with the BPDSI-IV total score.
Researchers first compared both the group therapy and the combination therapy with treatment as usual and found that together, the two schema arms were superior for reducing total BPDSI-IV score, with a medium to large effect size (Cohen d, 0.73; 95% confidence interval, .29-1.18; P = .001).
The difference was significant at 1.5 years (mean difference, 2.38; 95% CI, .27-4.49; P = .03).
When the treatment arms were compared separately, the combination therapy was superior to both the group therapy (Cohen d, 0.84; 95% CI, .09-1.59; P = .03) and to treatment as usual (Cohen d, 1.14; 95% CI, .57-1.71; P < .001).
The effectiveness of the predominantly group therapy did not differ significantly from that of treatment as usual.
The difference in effectiveness of combined therapy compared with treatment as usual became significant at 1 year. It became significant at 2.5 years compared with predominantly group therapy.
Treatment retention
In both schema arms, session frequency was tapered to only once a month; and in year 3, no further treatment was offered. However, symptom improvement continued during years 2 and 3.
Dr. Arntz explained this could be because patients realized they could apply what they learned after therapy was discontinued, which boosted their self-confidence.
Treatment retention was greater with combined therapy compared to the other options.
There was also improvement in several secondary outcomes, including happiness and quality of life, in most patients. However, patterns of outcomes for societal and work functioning improved more for those in either arm that received schema therapy.
“Group therapy seems to offer something that is important for learning to cooperate with other people. At work, you often have to collaborate with people who are not necessarily your friends,” Dr. Arntz noted.
The number of suicide attempts declined over time, with the combination arm being significantly superior to treatment as usual. During the study period, three patients died from suicide: one from each treatment arm. Another death had an unknown cause.
Overall, the results suggest that group and individual sessions address different needs of patients, the investigators noted.
While patients may learn to get along with others in a group setting, they may be more comfortable discussing severe trauma or suicidal thoughts in one-on-one sessions with a therapist, they added.
Strengths, weaknesses
Commenting for this news organization, John M. Oldham, MD, Distinguished Emeritus Professor, Menninger Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, said the study had a number of strengths, including its size and “good, solid” methodology.
“This is another big study that demonstrates a well-established form of psychotherapy leads to effective improvement in patients with borderline,” said Dr. Oldham, who was not involved in the research.
However, he noted a number of study limitations. First, training for therapists to deliver schema therapy is not always readily available. In addition, schema therapists in the study “were pretty junior,” with some appearing to be “trained on the job,” he said.
Dr. Oldham noted that cost may be another deterrent to implementing this therapeutic approach. Only those with substantial financial resources could afford once-a-week group therapy and once-a-week individual therapy for 2 years, at least in the United States, he said.
Because patients had to be willing to undergo therapy for 2 years to be enrolled in the study, the results may not be generalizable to the entire BPD population, Dr. Oldham added. “Many borderline patients would turn around and walk out the door if asked to commit to that,” he said.
So the study population may be “better attuned and receptive to therapy” and less impaired compared to many patients with this condition, Dr. Oldham said.
He also said the study did not compare individual schema therapy alone with group schema alone.
Study sites were supported by the Netherlands Organization for Health Research and Development and the Netherlands Foundation for Mental Health Study; Else Kröner-Fresenius-Stiftung; Australian Rotary Health; Greek Society of Schema Therapy, First Department of Psychiatry of the Medical School of the University of Athens, and Institut für Verhaltenstherapie Ausbildung Hamburg; South London and Maudsley NHS Foundation Trust and the Research Center Experimental Psychopathology, Maastricht University and Bradford District Care NHS Foundation Trust. Dr. Arntz has received grants from the Netherlands Organization for Health Research and Development and the Netherlands Foundation for Mental Health. Dr. Oldham reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Schema is a form of psychotherapy that focuses on experiential approaches rather than on behavior change.
The findings from an international randomized controlled trial underscore the importance of offering both individual and group approaches to patients with BPD, study investigator Arnoud Arntz, PhD, professor in the department of clinical psychology at the University of Amsterdam, told this news organization.
“In the Netherlands, there’s a big push from mental health institutes to deliver treatments in group therapy [only] because people think it’s more cost-effective; but these findings question that idea,” Dr. Arntz said.
The findings were published online March 2 in JAMA Psychiatry.
Early childhood experiences
Patients with BPD exhibit extreme sensitivity to interpersonal slights, intense and volatile emotions, and impulsive behaviors. Many abuse drugs, self-harm, or attempt suicide.
Evidence-based guidelines recommend psychotherapy as the primary treatment for BPD.
Schema therapy uses techniques from traditional psychotherapy but focuses on an experiential strategy. It also delves into early childhood experiences, which is relevant because patients with BPD often experienced abuse or neglect early in life.
As well, with this approach, therapists take on a sort of parenting role with patients to try to meet needs “that were frustrated in childhood,” said Dr. Arntz.
Previous research has suggested both individual and group schema therapy help reduce BPD symptoms, but the effectiveness of combining these two approaches has been unclear.
The current study included 495 adult patients (mean age, 33.6 years; 86.2% women) enrolled at 15 sites in five countries: the Netherlands, England, Greece, Germany, and Australia. All participants had a Borderline Personality Disorder Severity Index IV (BPDSI-IV) score of more than 20.
The BPDSI-IV score ranges from 0 to 90, with a score of 15 being the cutoff for a BPD diagnosis.
Investigators randomly assigned participants to one of three arms: predominantly group schema therapy, combined individual and group schema therapy, and treatment as usual – which was the optimal psychological treatment available at the site.
The two schema therapy arms, whether group or individual, involved a similar number of sessions each week. However, the frequency was gradually reduced over the course of the study.
Improved severity
The primary outcome was change in BPD severity as assessed at baseline, 6 months, 12 months, 18 months, 24 months, and 36 months with the BPDSI-IV total score.
Researchers first compared both the group therapy and the combination therapy with treatment as usual and found that together, the two schema arms were superior for reducing total BPDSI-IV score, with a medium to large effect size (Cohen d, 0.73; 95% confidence interval, .29-1.18; P = .001).
The difference was significant at 1.5 years (mean difference, 2.38; 95% CI, .27-4.49; P = .03).
When the treatment arms were compared separately, the combination therapy was superior to both the group therapy (Cohen d, 0.84; 95% CI, .09-1.59; P = .03) and to treatment as usual (Cohen d, 1.14; 95% CI, .57-1.71; P < .001).
The effectiveness of the predominantly group therapy did not differ significantly from that of treatment as usual.
The difference in effectiveness of combined therapy compared with treatment as usual became significant at 1 year. It became significant at 2.5 years compared with predominantly group therapy.
Treatment retention
In both schema arms, session frequency was tapered to only once a month; and in year 3, no further treatment was offered. However, symptom improvement continued during years 2 and 3.
Dr. Arntz explained this could be because patients realized they could apply what they learned after therapy was discontinued, which boosted their self-confidence.
Treatment retention was greater with combined therapy compared to the other options.
There was also improvement in several secondary outcomes, including happiness and quality of life, in most patients. However, patterns of outcomes for societal and work functioning improved more for those in either arm that received schema therapy.
“Group therapy seems to offer something that is important for learning to cooperate with other people. At work, you often have to collaborate with people who are not necessarily your friends,” Dr. Arntz noted.
The number of suicide attempts declined over time, with the combination arm being significantly superior to treatment as usual. During the study period, three patients died from suicide: one from each treatment arm. Another death had an unknown cause.
Overall, the results suggest that group and individual sessions address different needs of patients, the investigators noted.
While patients may learn to get along with others in a group setting, they may be more comfortable discussing severe trauma or suicidal thoughts in one-on-one sessions with a therapist, they added.
Strengths, weaknesses
Commenting for this news organization, John M. Oldham, MD, Distinguished Emeritus Professor, Menninger Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, said the study had a number of strengths, including its size and “good, solid” methodology.
“This is another big study that demonstrates a well-established form of psychotherapy leads to effective improvement in patients with borderline,” said Dr. Oldham, who was not involved in the research.
However, he noted a number of study limitations. First, training for therapists to deliver schema therapy is not always readily available. In addition, schema therapists in the study “were pretty junior,” with some appearing to be “trained on the job,” he said.
Dr. Oldham noted that cost may be another deterrent to implementing this therapeutic approach. Only those with substantial financial resources could afford once-a-week group therapy and once-a-week individual therapy for 2 years, at least in the United States, he said.
Because patients had to be willing to undergo therapy for 2 years to be enrolled in the study, the results may not be generalizable to the entire BPD population, Dr. Oldham added. “Many borderline patients would turn around and walk out the door if asked to commit to that,” he said.
So the study population may be “better attuned and receptive to therapy” and less impaired compared to many patients with this condition, Dr. Oldham said.
He also said the study did not compare individual schema therapy alone with group schema alone.
Study sites were supported by the Netherlands Organization for Health Research and Development and the Netherlands Foundation for Mental Health Study; Else Kröner-Fresenius-Stiftung; Australian Rotary Health; Greek Society of Schema Therapy, First Department of Psychiatry of the Medical School of the University of Athens, and Institut für Verhaltenstherapie Ausbildung Hamburg; South London and Maudsley NHS Foundation Trust and the Research Center Experimental Psychopathology, Maastricht University and Bradford District Care NHS Foundation Trust. Dr. Arntz has received grants from the Netherlands Organization for Health Research and Development and the Netherlands Foundation for Mental Health. Dr. Oldham reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Schema is a form of psychotherapy that focuses on experiential approaches rather than on behavior change.
The findings from an international randomized controlled trial underscore the importance of offering both individual and group approaches to patients with BPD, study investigator Arnoud Arntz, PhD, professor in the department of clinical psychology at the University of Amsterdam, told this news organization.
“In the Netherlands, there’s a big push from mental health institutes to deliver treatments in group therapy [only] because people think it’s more cost-effective; but these findings question that idea,” Dr. Arntz said.
The findings were published online March 2 in JAMA Psychiatry.
Early childhood experiences
Patients with BPD exhibit extreme sensitivity to interpersonal slights, intense and volatile emotions, and impulsive behaviors. Many abuse drugs, self-harm, or attempt suicide.
Evidence-based guidelines recommend psychotherapy as the primary treatment for BPD.
Schema therapy uses techniques from traditional psychotherapy but focuses on an experiential strategy. It also delves into early childhood experiences, which is relevant because patients with BPD often experienced abuse or neglect early in life.
As well, with this approach, therapists take on a sort of parenting role with patients to try to meet needs “that were frustrated in childhood,” said Dr. Arntz.
Previous research has suggested both individual and group schema therapy help reduce BPD symptoms, but the effectiveness of combining these two approaches has been unclear.
The current study included 495 adult patients (mean age, 33.6 years; 86.2% women) enrolled at 15 sites in five countries: the Netherlands, England, Greece, Germany, and Australia. All participants had a Borderline Personality Disorder Severity Index IV (BPDSI-IV) score of more than 20.
The BPDSI-IV score ranges from 0 to 90, with a score of 15 being the cutoff for a BPD diagnosis.
Investigators randomly assigned participants to one of three arms: predominantly group schema therapy, combined individual and group schema therapy, and treatment as usual – which was the optimal psychological treatment available at the site.
The two schema therapy arms, whether group or individual, involved a similar number of sessions each week. However, the frequency was gradually reduced over the course of the study.
Improved severity
The primary outcome was change in BPD severity as assessed at baseline, 6 months, 12 months, 18 months, 24 months, and 36 months with the BPDSI-IV total score.
Researchers first compared both the group therapy and the combination therapy with treatment as usual and found that together, the two schema arms were superior for reducing total BPDSI-IV score, with a medium to large effect size (Cohen d, 0.73; 95% confidence interval, .29-1.18; P = .001).
The difference was significant at 1.5 years (mean difference, 2.38; 95% CI, .27-4.49; P = .03).
When the treatment arms were compared separately, the combination therapy was superior to both the group therapy (Cohen d, 0.84; 95% CI, .09-1.59; P = .03) and to treatment as usual (Cohen d, 1.14; 95% CI, .57-1.71; P < .001).
The effectiveness of the predominantly group therapy did not differ significantly from that of treatment as usual.
The difference in effectiveness of combined therapy compared with treatment as usual became significant at 1 year. It became significant at 2.5 years compared with predominantly group therapy.
Treatment retention
In both schema arms, session frequency was tapered to only once a month; and in year 3, no further treatment was offered. However, symptom improvement continued during years 2 and 3.
Dr. Arntz explained this could be because patients realized they could apply what they learned after therapy was discontinued, which boosted their self-confidence.
Treatment retention was greater with combined therapy compared to the other options.
There was also improvement in several secondary outcomes, including happiness and quality of life, in most patients. However, patterns of outcomes for societal and work functioning improved more for those in either arm that received schema therapy.
“Group therapy seems to offer something that is important for learning to cooperate with other people. At work, you often have to collaborate with people who are not necessarily your friends,” Dr. Arntz noted.
The number of suicide attempts declined over time, with the combination arm being significantly superior to treatment as usual. During the study period, three patients died from suicide: one from each treatment arm. Another death had an unknown cause.
Overall, the results suggest that group and individual sessions address different needs of patients, the investigators noted.
While patients may learn to get along with others in a group setting, they may be more comfortable discussing severe trauma or suicidal thoughts in one-on-one sessions with a therapist, they added.
Strengths, weaknesses
Commenting for this news organization, John M. Oldham, MD, Distinguished Emeritus Professor, Menninger Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, said the study had a number of strengths, including its size and “good, solid” methodology.
“This is another big study that demonstrates a well-established form of psychotherapy leads to effective improvement in patients with borderline,” said Dr. Oldham, who was not involved in the research.
However, he noted a number of study limitations. First, training for therapists to deliver schema therapy is not always readily available. In addition, schema therapists in the study “were pretty junior,” with some appearing to be “trained on the job,” he said.
Dr. Oldham noted that cost may be another deterrent to implementing this therapeutic approach. Only those with substantial financial resources could afford once-a-week group therapy and once-a-week individual therapy for 2 years, at least in the United States, he said.
Because patients had to be willing to undergo therapy for 2 years to be enrolled in the study, the results may not be generalizable to the entire BPD population, Dr. Oldham added. “Many borderline patients would turn around and walk out the door if asked to commit to that,” he said.
So the study population may be “better attuned and receptive to therapy” and less impaired compared to many patients with this condition, Dr. Oldham said.
He also said the study did not compare individual schema therapy alone with group schema alone.
Study sites were supported by the Netherlands Organization for Health Research and Development and the Netherlands Foundation for Mental Health Study; Else Kröner-Fresenius-Stiftung; Australian Rotary Health; Greek Society of Schema Therapy, First Department of Psychiatry of the Medical School of the University of Athens, and Institut für Verhaltenstherapie Ausbildung Hamburg; South London and Maudsley NHS Foundation Trust and the Research Center Experimental Psychopathology, Maastricht University and Bradford District Care NHS Foundation Trust. Dr. Arntz has received grants from the Netherlands Organization for Health Research and Development and the Netherlands Foundation for Mental Health. Dr. Oldham reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.