User login
The science of clean skin care and the clean beauty movement
. I see numerous social media posts, blogs, and magazine articles about toxic skin care ingredients, while more patients are asking their dermatologists about clean beauty products. So, I decided it was time to dissect the issues and figure out what “clean” really means to me.
The problem is that no one agrees on a clean ingredient standard for beauty products. Many companies, like Target, Walgreens/Boots, Sephora, Neiman Marcus, Whole Foods, and Ulta, have their own varying clean standards. Even Allure magazine has a “Clean Best of Beauty” seal. California has Proposition 65, otherwise known as the Safe Drinking Water and Toxic Enforcement Act of 1986, which contains a list of banned chemicals “known to the state to cause cancer or reproductive toxicity.” In January 2021, Hawai‘i law prohibited the sale of oxybenzone and octinoxate in sunscreens in response to scientific studies showing that these ingredients “are toxic to corals and other marine life.” The Environmental Working Group (EWG) rates the safety of ingredients based on carcinogenicity, developmental and reproductive toxicity, allergenicity, and immunotoxicity. The Cosmetic Ingredient Review (CIR), funded by the Personal Care Products Council, consists of a seven-member steering committee that has at least one dermatologist representing the American Academy of Dermatology and a toxicologist representing the Society of Toxicology. The CIR publishes detailed reviews of ingredients that can be easily found on PubMed and Google Scholar and closely reviews animal and human data and reports on safety and contact dermatitis risk.
Which clean beauty standard is best?
I reviewed most of the various standards, clean seals, laws, and safety reports and found significant discrepancies resulting from misunderstandings of the science, lack of depth in the scientific evaluations, lumping of ingredients into a larger category, or lack of data. The most salient cause of misinformation and confusion seems to be hyperbolic claims by the media and clean beauty advocates who do not understand the basic science.
When I conducted a survey of cosmetic chemists on my LinkedIn account, most of the chemists stated that “ ‘Clean Beauty’ is a marketing term, more than a scientific term.” None of the chemists could give an exact definition of clean beauty. However, I thought I needed a good answer for my patients and for doctors who want to use and recommend “clean skin care brands.”
A dermatologist’s approach to develop a clean beauty standard
Many of the standards combine all of the following into the “clean” designation: nontoxic to the environment (both the production process and the resulting ingredient), nontoxic to marine life and coral, cruelty-free (not tested on animals), hypoallergenic, lacking in known health risks (carcinogenicity, reproductive toxicity), vegan, and gluten free. As a dermatologist, I am a splitter more than a lumper, so I prefer that “clean” be split into categories to make it easier to understand. With that in mind, I will focus on clean beauty ingredients only as they pertain to health: carcinogenicity, endocrine effects, nephrotoxicity, hepatotoxicity, immunotoxicity, etc. This discussion will not consider environmental effects, reproductive toxicity (some ingredients may decrease fertility, which is beyond the scope of this article), ingredient sources, and sustainability, animal testing, or human rights violations during production. Those issues are important, of course, but for clarity and simplicity, we will focus on the health risks of skin care ingredients.
In this month’s column, I will focus on a few ingredients and will continue the discussion in subsequent columns. Please note that commercial standards such as Target standards are based on the product type (e.g., cleansers, sunscreens, or moisturizers). So, when I mention an ingredient not allowed by certain company standards, note that it can vary by product type. My comments pertain mostly to facial moisturizers and facial serums to try and simplify the information. The Good Face Project has a complete list of standards by product type, which I recommend as a resource if you want more detailed information.
Are ethanolamines safe or toxic in cosmetics?
Ethanolamines are common ingredients in surfactants, fragrances, and emulsifying agents and include cocamide diethanolamine (DEA), cocamide monoethanolamine (MEA), and triethanolamine (TEA). Cocamide DEA, lauramide DEA, linoleamide DEA, and oleamide DEA are fatty acid diethanolamides that may contain 4% to 33% diethanolamine.1 A Google search of toxic ingredients in beauty products consistently identifies ethanolamines among such offending product constituents. Table 1 reveals that ethanolamines are excluded from some standards and included in others (N = not allowed or restricted by amount used and Y = allowed with no restrictions). As you can see, the standards don’t correspond to the EWG rating of the ingredients, which ranges from 1 (low hazard) to 10 (high hazard).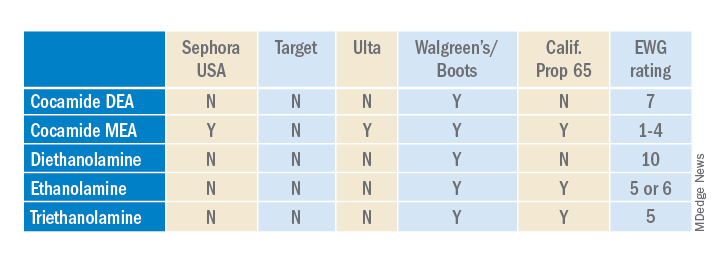
Why are ethanolamines sometimes considered safe and sometimes not?
Ethanolamines are reputed to be allergenic, but as we know as dermatologists, that does not mean that everyone will react to them. (In my opinion, allergenicity is a separate issue than the clean issue.) One study showed that TEA in 2.5% petrolatum had a 0.4% positive patch test rate in humans, which was thought to be related more to irritation than allergenicity.2 Cocamide DEA allergy is seen in those with hand dermatitis resulting from hand cleansers but is more commonly seen in metal workers.3 For this reason, these ethanolamines are usually found in rinse-off products to decrease exposure time. But there are many irritating ingredients not banned by Target, Sephora, and Ulta, so why does ethanolamine end up on toxic ingredient lists?
First, there is the issue of oral studies in animals. Oral forms of some ethanolamines have shown mild toxicity in rats, but topical forms have not been demonstrated to cause mutagenicity.1
For this reason, ethanolamines in their native form are considered safe.
The main issue with ethanolamines is that, when they are formulated with ingredients that break down into nitrogen, such as certain preservatives, the combination forms nitrosamines, such as N-nitrosodiethylamine (NDEA), which are carcinogenic.4 The European Commission prohibits DEA in cosmetics based on concerns about formation of these carcinogenic nitrosamines. Some standards limit ethanolamines to rinse-off products.5 The CIR panel concluded that diethanolamine and its 16 salts are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed and that TEA and TEA-related compounds are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed.6,7 The FDA states that there is no reason for consumers to be alarmed based on the use of DEA in cosmetics.8
The safety issues surrounding the use of ethanolamines in a skin care routine illustrate an important point: Every single product in the skin care routine should be compatible with the other products in the regimen. Using ethanolamines in a rinse-off product is one solution, as is ensuring that no other products in the skin care routine contain N-nitroso compounds that can combine with ethanolamines to form nitrosamines.
Are natural products safer?
Natural products are not necessarily any safer than synthetic products. Considering ethanolamines as the example here, note that cocamide DEA is an ethanolamine derived from coconut. It is often found in “green” or “natural” skin care products.9 It can still combine with N-nitroso compounds to form carcinogenic nitrosamines.
What is the bottom line? Are ethanolamines safe in cosmetics?
For now, if a patient asks if ethanolamine is safe in skin care, my answer would be yes, so long as the following is true:
- It is in a rinse-off product.
- The patient is not allergic to it.
- They do not have hand dermatitis.
- Their skin care routine does not include nitrogen-containing compounds like N-nitrosodiethanolamine (NDELA) or NDEA.
Conclusion
This column uses ethanolamines as an example to show the disparity in clean standards in the cosmetic industry. As you can see, there are multiple factors to consider. I will begin including clean information in my cosmeceutical critique columns to address some of these issues.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Cocamide DE. J Am Coll Toxicol. 1986;5(5).
2. Lessmann H et al. Contact Dermatitis. 2009 May;60(5):243-55.
3. Aalto-Korte K et al. 2014 Mar;70(3):169-74.
4. Kraeling ME et al. Food Chem Toxicol. 2004 Oct;42(10):1553-61.
5. Fiume MM et al. Int J Toxicol. 2015 Sep;34(2 Suppl):84S-98S.
6. Fiume MM.. Int J Toxicol. 2017 Sep/Oct;36(5_suppl2):89S-110S.
7. Fiume MM et al. Int J Toxicol. 2013 May-Jun;32(3 Suppl):59S-83S.
8. U.S. Food & Drug Administration. Diethanolamine. https://www.fda.gov/cosmetics/cosmetic-ingredients/diethanolamine. Accessed Feb. 12, 2022.
9. Aryanti N et al. IOP Conference Series: Materials Science and Engineering 2021 Feb 1 (Vol. 1053, No. 1, p. 012066). IOP Publishing.
. I see numerous social media posts, blogs, and magazine articles about toxic skin care ingredients, while more patients are asking their dermatologists about clean beauty products. So, I decided it was time to dissect the issues and figure out what “clean” really means to me.
The problem is that no one agrees on a clean ingredient standard for beauty products. Many companies, like Target, Walgreens/Boots, Sephora, Neiman Marcus, Whole Foods, and Ulta, have their own varying clean standards. Even Allure magazine has a “Clean Best of Beauty” seal. California has Proposition 65, otherwise known as the Safe Drinking Water and Toxic Enforcement Act of 1986, which contains a list of banned chemicals “known to the state to cause cancer or reproductive toxicity.” In January 2021, Hawai‘i law prohibited the sale of oxybenzone and octinoxate in sunscreens in response to scientific studies showing that these ingredients “are toxic to corals and other marine life.” The Environmental Working Group (EWG) rates the safety of ingredients based on carcinogenicity, developmental and reproductive toxicity, allergenicity, and immunotoxicity. The Cosmetic Ingredient Review (CIR), funded by the Personal Care Products Council, consists of a seven-member steering committee that has at least one dermatologist representing the American Academy of Dermatology and a toxicologist representing the Society of Toxicology. The CIR publishes detailed reviews of ingredients that can be easily found on PubMed and Google Scholar and closely reviews animal and human data and reports on safety and contact dermatitis risk.
Which clean beauty standard is best?
I reviewed most of the various standards, clean seals, laws, and safety reports and found significant discrepancies resulting from misunderstandings of the science, lack of depth in the scientific evaluations, lumping of ingredients into a larger category, or lack of data. The most salient cause of misinformation and confusion seems to be hyperbolic claims by the media and clean beauty advocates who do not understand the basic science.
When I conducted a survey of cosmetic chemists on my LinkedIn account, most of the chemists stated that “ ‘Clean Beauty’ is a marketing term, more than a scientific term.” None of the chemists could give an exact definition of clean beauty. However, I thought I needed a good answer for my patients and for doctors who want to use and recommend “clean skin care brands.”
A dermatologist’s approach to develop a clean beauty standard
Many of the standards combine all of the following into the “clean” designation: nontoxic to the environment (both the production process and the resulting ingredient), nontoxic to marine life and coral, cruelty-free (not tested on animals), hypoallergenic, lacking in known health risks (carcinogenicity, reproductive toxicity), vegan, and gluten free. As a dermatologist, I am a splitter more than a lumper, so I prefer that “clean” be split into categories to make it easier to understand. With that in mind, I will focus on clean beauty ingredients only as they pertain to health: carcinogenicity, endocrine effects, nephrotoxicity, hepatotoxicity, immunotoxicity, etc. This discussion will not consider environmental effects, reproductive toxicity (some ingredients may decrease fertility, which is beyond the scope of this article), ingredient sources, and sustainability, animal testing, or human rights violations during production. Those issues are important, of course, but for clarity and simplicity, we will focus on the health risks of skin care ingredients.
In this month’s column, I will focus on a few ingredients and will continue the discussion in subsequent columns. Please note that commercial standards such as Target standards are based on the product type (e.g., cleansers, sunscreens, or moisturizers). So, when I mention an ingredient not allowed by certain company standards, note that it can vary by product type. My comments pertain mostly to facial moisturizers and facial serums to try and simplify the information. The Good Face Project has a complete list of standards by product type, which I recommend as a resource if you want more detailed information.
Are ethanolamines safe or toxic in cosmetics?
Ethanolamines are common ingredients in surfactants, fragrances, and emulsifying agents and include cocamide diethanolamine (DEA), cocamide monoethanolamine (MEA), and triethanolamine (TEA). Cocamide DEA, lauramide DEA, linoleamide DEA, and oleamide DEA are fatty acid diethanolamides that may contain 4% to 33% diethanolamine.1 A Google search of toxic ingredients in beauty products consistently identifies ethanolamines among such offending product constituents. Table 1 reveals that ethanolamines are excluded from some standards and included in others (N = not allowed or restricted by amount used and Y = allowed with no restrictions). As you can see, the standards don’t correspond to the EWG rating of the ingredients, which ranges from 1 (low hazard) to 10 (high hazard).
Why are ethanolamines sometimes considered safe and sometimes not?
Ethanolamines are reputed to be allergenic, but as we know as dermatologists, that does not mean that everyone will react to them. (In my opinion, allergenicity is a separate issue than the clean issue.) One study showed that TEA in 2.5% petrolatum had a 0.4% positive patch test rate in humans, which was thought to be related more to irritation than allergenicity.2 Cocamide DEA allergy is seen in those with hand dermatitis resulting from hand cleansers but is more commonly seen in metal workers.3 For this reason, these ethanolamines are usually found in rinse-off products to decrease exposure time. But there are many irritating ingredients not banned by Target, Sephora, and Ulta, so why does ethanolamine end up on toxic ingredient lists?
First, there is the issue of oral studies in animals. Oral forms of some ethanolamines have shown mild toxicity in rats, but topical forms have not been demonstrated to cause mutagenicity.1
For this reason, ethanolamines in their native form are considered safe.
The main issue with ethanolamines is that, when they are formulated with ingredients that break down into nitrogen, such as certain preservatives, the combination forms nitrosamines, such as N-nitrosodiethylamine (NDEA), which are carcinogenic.4 The European Commission prohibits DEA in cosmetics based on concerns about formation of these carcinogenic nitrosamines. Some standards limit ethanolamines to rinse-off products.5 The CIR panel concluded that diethanolamine and its 16 salts are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed and that TEA and TEA-related compounds are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed.6,7 The FDA states that there is no reason for consumers to be alarmed based on the use of DEA in cosmetics.8
The safety issues surrounding the use of ethanolamines in a skin care routine illustrate an important point: Every single product in the skin care routine should be compatible with the other products in the regimen. Using ethanolamines in a rinse-off product is one solution, as is ensuring that no other products in the skin care routine contain N-nitroso compounds that can combine with ethanolamines to form nitrosamines.
Are natural products safer?
Natural products are not necessarily any safer than synthetic products. Considering ethanolamines as the example here, note that cocamide DEA is an ethanolamine derived from coconut. It is often found in “green” or “natural” skin care products.9 It can still combine with N-nitroso compounds to form carcinogenic nitrosamines.
What is the bottom line? Are ethanolamines safe in cosmetics?
For now, if a patient asks if ethanolamine is safe in skin care, my answer would be yes, so long as the following is true:
- It is in a rinse-off product.
- The patient is not allergic to it.
- They do not have hand dermatitis.
- Their skin care routine does not include nitrogen-containing compounds like N-nitrosodiethanolamine (NDELA) or NDEA.
Conclusion
This column uses ethanolamines as an example to show the disparity in clean standards in the cosmetic industry. As you can see, there are multiple factors to consider. I will begin including clean information in my cosmeceutical critique columns to address some of these issues.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Cocamide DE. J Am Coll Toxicol. 1986;5(5).
2. Lessmann H et al. Contact Dermatitis. 2009 May;60(5):243-55.
3. Aalto-Korte K et al. 2014 Mar;70(3):169-74.
4. Kraeling ME et al. Food Chem Toxicol. 2004 Oct;42(10):1553-61.
5. Fiume MM et al. Int J Toxicol. 2015 Sep;34(2 Suppl):84S-98S.
6. Fiume MM.. Int J Toxicol. 2017 Sep/Oct;36(5_suppl2):89S-110S.
7. Fiume MM et al. Int J Toxicol. 2013 May-Jun;32(3 Suppl):59S-83S.
8. U.S. Food & Drug Administration. Diethanolamine. https://www.fda.gov/cosmetics/cosmetic-ingredients/diethanolamine. Accessed Feb. 12, 2022.
9. Aryanti N et al. IOP Conference Series: Materials Science and Engineering 2021 Feb 1 (Vol. 1053, No. 1, p. 012066). IOP Publishing.
. I see numerous social media posts, blogs, and magazine articles about toxic skin care ingredients, while more patients are asking their dermatologists about clean beauty products. So, I decided it was time to dissect the issues and figure out what “clean” really means to me.
The problem is that no one agrees on a clean ingredient standard for beauty products. Many companies, like Target, Walgreens/Boots, Sephora, Neiman Marcus, Whole Foods, and Ulta, have their own varying clean standards. Even Allure magazine has a “Clean Best of Beauty” seal. California has Proposition 65, otherwise known as the Safe Drinking Water and Toxic Enforcement Act of 1986, which contains a list of banned chemicals “known to the state to cause cancer or reproductive toxicity.” In January 2021, Hawai‘i law prohibited the sale of oxybenzone and octinoxate in sunscreens in response to scientific studies showing that these ingredients “are toxic to corals and other marine life.” The Environmental Working Group (EWG) rates the safety of ingredients based on carcinogenicity, developmental and reproductive toxicity, allergenicity, and immunotoxicity. The Cosmetic Ingredient Review (CIR), funded by the Personal Care Products Council, consists of a seven-member steering committee that has at least one dermatologist representing the American Academy of Dermatology and a toxicologist representing the Society of Toxicology. The CIR publishes detailed reviews of ingredients that can be easily found on PubMed and Google Scholar and closely reviews animal and human data and reports on safety and contact dermatitis risk.
Which clean beauty standard is best?
I reviewed most of the various standards, clean seals, laws, and safety reports and found significant discrepancies resulting from misunderstandings of the science, lack of depth in the scientific evaluations, lumping of ingredients into a larger category, or lack of data. The most salient cause of misinformation and confusion seems to be hyperbolic claims by the media and clean beauty advocates who do not understand the basic science.
When I conducted a survey of cosmetic chemists on my LinkedIn account, most of the chemists stated that “ ‘Clean Beauty’ is a marketing term, more than a scientific term.” None of the chemists could give an exact definition of clean beauty. However, I thought I needed a good answer for my patients and for doctors who want to use and recommend “clean skin care brands.”
A dermatologist’s approach to develop a clean beauty standard
Many of the standards combine all of the following into the “clean” designation: nontoxic to the environment (both the production process and the resulting ingredient), nontoxic to marine life and coral, cruelty-free (not tested on animals), hypoallergenic, lacking in known health risks (carcinogenicity, reproductive toxicity), vegan, and gluten free. As a dermatologist, I am a splitter more than a lumper, so I prefer that “clean” be split into categories to make it easier to understand. With that in mind, I will focus on clean beauty ingredients only as they pertain to health: carcinogenicity, endocrine effects, nephrotoxicity, hepatotoxicity, immunotoxicity, etc. This discussion will not consider environmental effects, reproductive toxicity (some ingredients may decrease fertility, which is beyond the scope of this article), ingredient sources, and sustainability, animal testing, or human rights violations during production. Those issues are important, of course, but for clarity and simplicity, we will focus on the health risks of skin care ingredients.
In this month’s column, I will focus on a few ingredients and will continue the discussion in subsequent columns. Please note that commercial standards such as Target standards are based on the product type (e.g., cleansers, sunscreens, or moisturizers). So, when I mention an ingredient not allowed by certain company standards, note that it can vary by product type. My comments pertain mostly to facial moisturizers and facial serums to try and simplify the information. The Good Face Project has a complete list of standards by product type, which I recommend as a resource if you want more detailed information.
Are ethanolamines safe or toxic in cosmetics?
Ethanolamines are common ingredients in surfactants, fragrances, and emulsifying agents and include cocamide diethanolamine (DEA), cocamide monoethanolamine (MEA), and triethanolamine (TEA). Cocamide DEA, lauramide DEA, linoleamide DEA, and oleamide DEA are fatty acid diethanolamides that may contain 4% to 33% diethanolamine.1 A Google search of toxic ingredients in beauty products consistently identifies ethanolamines among such offending product constituents. Table 1 reveals that ethanolamines are excluded from some standards and included in others (N = not allowed or restricted by amount used and Y = allowed with no restrictions). As you can see, the standards don’t correspond to the EWG rating of the ingredients, which ranges from 1 (low hazard) to 10 (high hazard).
Why are ethanolamines sometimes considered safe and sometimes not?
Ethanolamines are reputed to be allergenic, but as we know as dermatologists, that does not mean that everyone will react to them. (In my opinion, allergenicity is a separate issue than the clean issue.) One study showed that TEA in 2.5% petrolatum had a 0.4% positive patch test rate in humans, which was thought to be related more to irritation than allergenicity.2 Cocamide DEA allergy is seen in those with hand dermatitis resulting from hand cleansers but is more commonly seen in metal workers.3 For this reason, these ethanolamines are usually found in rinse-off products to decrease exposure time. But there are many irritating ingredients not banned by Target, Sephora, and Ulta, so why does ethanolamine end up on toxic ingredient lists?
First, there is the issue of oral studies in animals. Oral forms of some ethanolamines have shown mild toxicity in rats, but topical forms have not been demonstrated to cause mutagenicity.1
For this reason, ethanolamines in their native form are considered safe.
The main issue with ethanolamines is that, when they are formulated with ingredients that break down into nitrogen, such as certain preservatives, the combination forms nitrosamines, such as N-nitrosodiethylamine (NDEA), which are carcinogenic.4 The European Commission prohibits DEA in cosmetics based on concerns about formation of these carcinogenic nitrosamines. Some standards limit ethanolamines to rinse-off products.5 The CIR panel concluded that diethanolamine and its 16 salts are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed and that TEA and TEA-related compounds are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed.6,7 The FDA states that there is no reason for consumers to be alarmed based on the use of DEA in cosmetics.8
The safety issues surrounding the use of ethanolamines in a skin care routine illustrate an important point: Every single product in the skin care routine should be compatible with the other products in the regimen. Using ethanolamines in a rinse-off product is one solution, as is ensuring that no other products in the skin care routine contain N-nitroso compounds that can combine with ethanolamines to form nitrosamines.
Are natural products safer?
Natural products are not necessarily any safer than synthetic products. Considering ethanolamines as the example here, note that cocamide DEA is an ethanolamine derived from coconut. It is often found in “green” or “natural” skin care products.9 It can still combine with N-nitroso compounds to form carcinogenic nitrosamines.
What is the bottom line? Are ethanolamines safe in cosmetics?
For now, if a patient asks if ethanolamine is safe in skin care, my answer would be yes, so long as the following is true:
- It is in a rinse-off product.
- The patient is not allergic to it.
- They do not have hand dermatitis.
- Their skin care routine does not include nitrogen-containing compounds like N-nitrosodiethanolamine (NDELA) or NDEA.
Conclusion
This column uses ethanolamines as an example to show the disparity in clean standards in the cosmetic industry. As you can see, there are multiple factors to consider. I will begin including clean information in my cosmeceutical critique columns to address some of these issues.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Cocamide DE. J Am Coll Toxicol. 1986;5(5).
2. Lessmann H et al. Contact Dermatitis. 2009 May;60(5):243-55.
3. Aalto-Korte K et al. 2014 Mar;70(3):169-74.
4. Kraeling ME et al. Food Chem Toxicol. 2004 Oct;42(10):1553-61.
5. Fiume MM et al. Int J Toxicol. 2015 Sep;34(2 Suppl):84S-98S.
6. Fiume MM.. Int J Toxicol. 2017 Sep/Oct;36(5_suppl2):89S-110S.
7. Fiume MM et al. Int J Toxicol. 2013 May-Jun;32(3 Suppl):59S-83S.
8. U.S. Food & Drug Administration. Diethanolamine. https://www.fda.gov/cosmetics/cosmetic-ingredients/diethanolamine. Accessed Feb. 12, 2022.
9. Aryanti N et al. IOP Conference Series: Materials Science and Engineering 2021 Feb 1 (Vol. 1053, No. 1, p. 012066). IOP Publishing.
Inside insulin (Part 2): Approaching a cure for type 1 diabetes?
Editor’s note: This is the second in a two-part series commemorating the 100-year anniversary of the first use of insulin in humans. Part 1 of this series examined the rivalry behind the discovery and use of insulin.
One hundred years ago, teenager Leonard Thompson was the first patient with type 1 diabetes to be successfully treated with insulin, granting him a reprieve from what was a certain death sentence at the time.
Since then, research has gathered pace. In the century since insulin’s discovery and first use, recombinant DNA technology has allowed for the engineering of the insulin molecule, providing numerous short- and long-acting analog versions. At the same time, technological leaps in automated insulin delivery and monitoring of blood glucose ensure more time with glucose in range and fewer life-threatening complications for those with type 1 diabetes fortunate enough to have access to the technology.
In spite of these advancements, there is still scope for further evolution of disease management, with the holy grail being the transplant of stem cell–derived islet cells capable of making insulin, ideally encased in some kind of protective device so that immunosuppression is not required.
Indeed, it is not unreasonable to “hope that type 1 diabetes will be a curable disease in the next 100 years,” said Elizabeth Stephens, MD, an endocrinologist who has type 1 diabetes and practices in Portland, Ore.
Type 1 diabetes: The past 100 years
The epidemiology of type 1 diabetes has shifted considerably since 1922. A century ago, given that average life expectancy in the United States was around 54 years, it was pretty much the only type of diabetes that doctors encountered. “There was some type 2 diabetes about in heavier people, but the focus was on type 1 diabetes,” noted Dr. Stephens.
Originally called juvenile diabetes because it was thought to only occur in children, “now 50% of people are diagnosed with type 1 diabetes ... over [the age of] 20,” explained Dr. Stephens.
In the United States, around 1.4 million adults 20 years and older, and 187,000 children younger than 20, have the disease, according to data from the National Diabetes Statistics Report 2020 by the Centers for Disease Control and Prevention. This total represents an increase of nearly 30% from 2017.
Over the years, theories as to the cause, or trigger, for type 1 diabetes “have included cow’s milk and [viral] infections,” said Dr. Stephens. “Most likely, there’s a genetic predisposition and some type of exposure, which creates the perfect storm to trigger disease.”
There are hints that COVID-19 might be precipitating type 1 diabetes in some people. Recently, the CDC found SARS-CoV-2 infection was associated with an increased risk for diabetes (all types) among youth, but not other acute respiratory infections. And two further studies from different parts of the world have recently identified an increase in the incidence of type 1 diabetes in children since the COVID-19 pandemic began, but the reasons remain unclear.
The global CoviDiab registry has also been established to collect data on patients with COVID-19–related diabetes.
The million-dollar question: Is COVID-19 itself is propagating type 1 diabetes or unmasking a predisposition to the disease sooner? The latter might be associated with a lower type 1 diabetes rate in the future, said Partha Kar, MBBS, OBE, national specialty advisor, diabetes, for National Health Service England.
“Right now, we don’t know the answer. Whichever way you look at it, it is likely there will be a rise in cases, and in countries where insulin is not freely available, healthcare systems need to have supply ready because insulin is lifesaving in type 1 diabetes,” Dr. Kar emphasized.
CGMs and automated insulin delivery: A ‘godsend’
A huge change has also been seen, most notably in the past 15 to 20 years, in the technological advancements that can help those with type 1 diabetes live an easier life.
Continuous glucose monitors (CGMs) and automated ways of delivering insulin, such as smart pens and insulin pumps, have made the daily life of a person with type 1 diabetes in the Western world considerably more comfortable.
CGMs provide a constant stream of data to an app, often wirelessly in sync with the insulin pump. However, on a global level, they are only available to a lucky few.
In England, pending National Institute for Health and Care Excellence) approval, any CGM should be available to all eligible patients with type 1 diabetes within the NHS from April 2022, Dr. Kar pointed out. In the United States, CGMs are often unaffordable and access is mostly dependent on a person’s health insurance.
Kersten Hall, PhD, a scientist and U.K.-based medical historian who recently wrote a book, “Insulin, the Crooked Timber” (Oxford, England: Oxford University Press, 2022) uncovering the lesser-known story behind the discovery of insulin, was diagnosed with adult-onset type 1 diabetes at the age of 41. Dr. Hall had always found the finger-prick blood glucose test to be a chore but now has a CGM.
“It’s a total game changer for me: a godsend. I can’t sing its praises enough,” he said. “All it involves is the swipe of the phone and this provides a reading which tells me if my glucose is too low, so I eat something, or too high, so I might [go for] a run.”
Brewing insulin at scale
As described by Dr. Hall in his book, the journey from treating Mr. Thompson in 1922 to treating the masses began when biochemist James Collip, MD, PhD, discovered a means of purifying the animal pancreas extracts used to treat the teenager.
But production at scale presented a further challenge. This was overcome in 1924 when Eli Lilly drew on a technique used in the beer brewing process – where pH guides bitterness – to purify and manufacture large amounts of insulin.
By 1936, a range of slower-acting cattle and pig-derived insulins, the first produced by Novo Nordisk Pharmaceuticals, were developed.
However, it took 8,000 lb (approximately 3,600 kg) of pancreas glands from 23,500 animals to make 1 lb (0.5 kg) of insulin, so a more efficient process was badly needed.
Dr. Hall, who is a molecular biologist as well as an author, explains that the use of recombinant DNA technology to produce human insulin, as done by Genentech in the late 70s, was a key development in the story of modern insulin products. Genentech then provided synthetic human insulin for Eli Lilly to conduct clinical trials.
Human insulin most closely resembles porcine insulin structure and function, differing by only one amino acid, while human insulin differs from bovine insulin by three amino acid residues. This synthetic human insulin eliminated the allergies that the animal-derived products sometimes caused.
In the early 1980s, Eli Lilly produced Humulin, the first biosynthetic (made in Escherichia coli, hence the term, “bio”) human insulin.
This technology eventually “allowed for the alteration of specific amino acids in the sequence of the insulin protein to make insulin analogs [synthetic versions grown in E. coli and genetically altered for various properties] that act faster, or more slowly, than normal human insulin. By using the slow- and fast-acting insulins in combination, a patient can control their blood sugar levels with a much greater degree of finesse and precision,” Dr. Hall explained.
Today, a whole range of insulins are available, including ultra–rapid-acting, short-acting, intermediate-acting, long-acting, ultra–long-acting, and even inhaled insulin, although the latter is expensive, has been associated with side effects, and is less commonly used, according to Dr. Stephens.
Oral insulin formulations are even in the early stages of development, with candidate drugs by Generex and from the Oralis project.
“With insulin therapy, we try to reproduce the normal physiology of the healthy body and pancreas,” Dr. Stephens explained.
Insulin analogs are only made by three companies (Eli Lilly, Novo Nordisk, and Sanofi), and they are generally much more expensive than nonanalog human insulin. In the United Kingdom through the NHS, they cost twice as much.
In the United States today, one of the biggest barriers to proper care of type 1 diabetes is the cost of insulin, which can limit access. With the market controlled by these three large companies, the average cost of a unit of insulin in the United States, according to RAND research, was $98.17 in January 2021, compared with $7.52 in the United Kingdom and $12.00 in Canada.
Several U.S. states have enacted legislation capping insulin copayments to at, or under, $100 a month. But the federal Build Back Better Framework Act – which would cap copayments for insulin at $35 – currently hangs in the balance.
Alongside these moves, in 2020 the Food and Drug Administration approved the first interchangeable biosimilar insulin for type 1 diabetes (and insulin-dependent type 2 diabetes) in children and adults, called Semglee (Mylan Pharmaceuticals).
Biosimilars (essentially generic versions of branded insulins) are expected to be less expensive than branded analogs, but the indications so far are that they will only be around 20% cheaper.
“I totally fail to understand how the richest country in the world still has a debate about price caps, and we are looking at biosimilar markets to change the debate. This makes no sense to me at all,” stressed Dr. Kar. “For lifesaving drugs, they should be funded by the state.”
Insulin also remains unaffordable for many in numerous low- and middle-income countries, where most patients pay out-of-pocket for medicines. Globally, there are estimated to be around 30 million people who need insulin but cannot afford it.
How near to a cure in the coming decades?
Looking ahead to the coming years, if not the next 100, Dr. Stephens highlighted two important aspects of care.
First, the use of a CGM device in combination with an insulin pump (also known as a closed-loop system or artificial pancreas), where the CGM effectively tells the insulin pump how much insulin to automatically dispense, should revolutionize care.
A number of such closed-loop systems have recently been approved in both the United States, including systems from Medtronic and Omnipod, and Europe.
“I wear one of these and it’s been a life changer for me, but it doesn’t suit everyone because the technology can be cumbersome, but with time, hopefully things will become smaller and more accurate in insulin delivery,” Dr. Stephens added.
The second advance of interest is the development and transplantation of cells that produce insulin.
Dr. Stephens explained that someone living with type 1 diabetes has a lot to think about, not least, doing the math related to insulin requirement. “If we just had cells from a pancreas that could be transplanted and would do that for us, then it would be a total game changer.”
To date, Vertex Pharmaceuticals has successfully treated one patient – who had lived with type 1 diabetes for about 40 years and had recurrent episodes of severe hypoglycemia – with an infusion of stem cell–derived differentiated islet cells into his liver. The procedure resulted in near reversal of type 1 diabetes, with his insulin dose reduced from 34 to 3 units, and his hemoglobin A1c falling from 8.6% to 7.2%.
And although the patient, Brian Shelton, still needs to take immunosuppressive agents to prevent rejection of the stem cell–derived islets, “it’s a whole new life,” he recently told the New York Times.
Another company called ViaCyte is also working on a similar approach.
Whether this is a cure for type 1 diabetes is still debatable, said Anne Peters, MD, of the University of Southern California, Los Angeles. “Is it true? In a word, no. But we are part of the way there, which is much closer than we were 6 months ago.”
There are also ongoing clinical trials of therapeutic interventions to prevent or delay the trajectory from presymptomatic to clinical type 1 diabetes. The most advanced is the anti-CD3 monoclonal antibody teplizumab (Tzield, Provention Bio), which was rejected by the FDA in July 2021, but has since been refiled. The company expects to hear from the agency by the end of March 2022 as to whether the resubmission has been accepted.
Diabetes specialist nurses/educators keep it human
Dr. Hall said he concurs with the late eminent U.K. diabetes specialist Robert Tattersall’s observation on what he considers one of the most important advances in the management and treatment of type 1 diabetes: the human touch.
Referring to Dr. Tattersall’s book, “Diabetes: A Biography,” Dr. Hall quoted: “If asked what innovation had made the most difference to their lives in the 1980s, patients with type 1 diabetes in England would unhesitatingly have chosen not human insulin, but the spread of diabetes specialist nurses ... these people (mainly women) did more in the last two decades of the 20th century to improve the standard of diabetes care than any other innovation or drug.”
In the United States, diabetes specialist nurses were called diabetes educators until recently, when the name changed to certified diabetes care and education specialist.
“Above all, they have humanized the service and given the patient a say in the otherwise unequal relationship with all-powerful doctors,” concluded Dr. Hall, again quoting Dr. Tattersall.
A version of this article first appeared on Medscape.com.
Editor’s note: This is the second in a two-part series commemorating the 100-year anniversary of the first use of insulin in humans. Part 1 of this series examined the rivalry behind the discovery and use of insulin.
One hundred years ago, teenager Leonard Thompson was the first patient with type 1 diabetes to be successfully treated with insulin, granting him a reprieve from what was a certain death sentence at the time.
Since then, research has gathered pace. In the century since insulin’s discovery and first use, recombinant DNA technology has allowed for the engineering of the insulin molecule, providing numerous short- and long-acting analog versions. At the same time, technological leaps in automated insulin delivery and monitoring of blood glucose ensure more time with glucose in range and fewer life-threatening complications for those with type 1 diabetes fortunate enough to have access to the technology.
In spite of these advancements, there is still scope for further evolution of disease management, with the holy grail being the transplant of stem cell–derived islet cells capable of making insulin, ideally encased in some kind of protective device so that immunosuppression is not required.
Indeed, it is not unreasonable to “hope that type 1 diabetes will be a curable disease in the next 100 years,” said Elizabeth Stephens, MD, an endocrinologist who has type 1 diabetes and practices in Portland, Ore.
Type 1 diabetes: The past 100 years
The epidemiology of type 1 diabetes has shifted considerably since 1922. A century ago, given that average life expectancy in the United States was around 54 years, it was pretty much the only type of diabetes that doctors encountered. “There was some type 2 diabetes about in heavier people, but the focus was on type 1 diabetes,” noted Dr. Stephens.
Originally called juvenile diabetes because it was thought to only occur in children, “now 50% of people are diagnosed with type 1 diabetes ... over [the age of] 20,” explained Dr. Stephens.
In the United States, around 1.4 million adults 20 years and older, and 187,000 children younger than 20, have the disease, according to data from the National Diabetes Statistics Report 2020 by the Centers for Disease Control and Prevention. This total represents an increase of nearly 30% from 2017.
Over the years, theories as to the cause, or trigger, for type 1 diabetes “have included cow’s milk and [viral] infections,” said Dr. Stephens. “Most likely, there’s a genetic predisposition and some type of exposure, which creates the perfect storm to trigger disease.”
There are hints that COVID-19 might be precipitating type 1 diabetes in some people. Recently, the CDC found SARS-CoV-2 infection was associated with an increased risk for diabetes (all types) among youth, but not other acute respiratory infections. And two further studies from different parts of the world have recently identified an increase in the incidence of type 1 diabetes in children since the COVID-19 pandemic began, but the reasons remain unclear.
The global CoviDiab registry has also been established to collect data on patients with COVID-19–related diabetes.
The million-dollar question: Is COVID-19 itself is propagating type 1 diabetes or unmasking a predisposition to the disease sooner? The latter might be associated with a lower type 1 diabetes rate in the future, said Partha Kar, MBBS, OBE, national specialty advisor, diabetes, for National Health Service England.
“Right now, we don’t know the answer. Whichever way you look at it, it is likely there will be a rise in cases, and in countries where insulin is not freely available, healthcare systems need to have supply ready because insulin is lifesaving in type 1 diabetes,” Dr. Kar emphasized.
CGMs and automated insulin delivery: A ‘godsend’
A huge change has also been seen, most notably in the past 15 to 20 years, in the technological advancements that can help those with type 1 diabetes live an easier life.
Continuous glucose monitors (CGMs) and automated ways of delivering insulin, such as smart pens and insulin pumps, have made the daily life of a person with type 1 diabetes in the Western world considerably more comfortable.
CGMs provide a constant stream of data to an app, often wirelessly in sync with the insulin pump. However, on a global level, they are only available to a lucky few.
In England, pending National Institute for Health and Care Excellence) approval, any CGM should be available to all eligible patients with type 1 diabetes within the NHS from April 2022, Dr. Kar pointed out. In the United States, CGMs are often unaffordable and access is mostly dependent on a person’s health insurance.
Kersten Hall, PhD, a scientist and U.K.-based medical historian who recently wrote a book, “Insulin, the Crooked Timber” (Oxford, England: Oxford University Press, 2022) uncovering the lesser-known story behind the discovery of insulin, was diagnosed with adult-onset type 1 diabetes at the age of 41. Dr. Hall had always found the finger-prick blood glucose test to be a chore but now has a CGM.
“It’s a total game changer for me: a godsend. I can’t sing its praises enough,” he said. “All it involves is the swipe of the phone and this provides a reading which tells me if my glucose is too low, so I eat something, or too high, so I might [go for] a run.”
Brewing insulin at scale
As described by Dr. Hall in his book, the journey from treating Mr. Thompson in 1922 to treating the masses began when biochemist James Collip, MD, PhD, discovered a means of purifying the animal pancreas extracts used to treat the teenager.
But production at scale presented a further challenge. This was overcome in 1924 when Eli Lilly drew on a technique used in the beer brewing process – where pH guides bitterness – to purify and manufacture large amounts of insulin.
By 1936, a range of slower-acting cattle and pig-derived insulins, the first produced by Novo Nordisk Pharmaceuticals, were developed.
However, it took 8,000 lb (approximately 3,600 kg) of pancreas glands from 23,500 animals to make 1 lb (0.5 kg) of insulin, so a more efficient process was badly needed.
Dr. Hall, who is a molecular biologist as well as an author, explains that the use of recombinant DNA technology to produce human insulin, as done by Genentech in the late 70s, was a key development in the story of modern insulin products. Genentech then provided synthetic human insulin for Eli Lilly to conduct clinical trials.
Human insulin most closely resembles porcine insulin structure and function, differing by only one amino acid, while human insulin differs from bovine insulin by three amino acid residues. This synthetic human insulin eliminated the allergies that the animal-derived products sometimes caused.
In the early 1980s, Eli Lilly produced Humulin, the first biosynthetic (made in Escherichia coli, hence the term, “bio”) human insulin.
This technology eventually “allowed for the alteration of specific amino acids in the sequence of the insulin protein to make insulin analogs [synthetic versions grown in E. coli and genetically altered for various properties] that act faster, or more slowly, than normal human insulin. By using the slow- and fast-acting insulins in combination, a patient can control their blood sugar levels with a much greater degree of finesse and precision,” Dr. Hall explained.
Today, a whole range of insulins are available, including ultra–rapid-acting, short-acting, intermediate-acting, long-acting, ultra–long-acting, and even inhaled insulin, although the latter is expensive, has been associated with side effects, and is less commonly used, according to Dr. Stephens.
Oral insulin formulations are even in the early stages of development, with candidate drugs by Generex and from the Oralis project.
“With insulin therapy, we try to reproduce the normal physiology of the healthy body and pancreas,” Dr. Stephens explained.
Insulin analogs are only made by three companies (Eli Lilly, Novo Nordisk, and Sanofi), and they are generally much more expensive than nonanalog human insulin. In the United Kingdom through the NHS, they cost twice as much.
In the United States today, one of the biggest barriers to proper care of type 1 diabetes is the cost of insulin, which can limit access. With the market controlled by these three large companies, the average cost of a unit of insulin in the United States, according to RAND research, was $98.17 in January 2021, compared with $7.52 in the United Kingdom and $12.00 in Canada.
Several U.S. states have enacted legislation capping insulin copayments to at, or under, $100 a month. But the federal Build Back Better Framework Act – which would cap copayments for insulin at $35 – currently hangs in the balance.
Alongside these moves, in 2020 the Food and Drug Administration approved the first interchangeable biosimilar insulin for type 1 diabetes (and insulin-dependent type 2 diabetes) in children and adults, called Semglee (Mylan Pharmaceuticals).
Biosimilars (essentially generic versions of branded insulins) are expected to be less expensive than branded analogs, but the indications so far are that they will only be around 20% cheaper.
“I totally fail to understand how the richest country in the world still has a debate about price caps, and we are looking at biosimilar markets to change the debate. This makes no sense to me at all,” stressed Dr. Kar. “For lifesaving drugs, they should be funded by the state.”
Insulin also remains unaffordable for many in numerous low- and middle-income countries, where most patients pay out-of-pocket for medicines. Globally, there are estimated to be around 30 million people who need insulin but cannot afford it.
How near to a cure in the coming decades?
Looking ahead to the coming years, if not the next 100, Dr. Stephens highlighted two important aspects of care.
First, the use of a CGM device in combination with an insulin pump (also known as a closed-loop system or artificial pancreas), where the CGM effectively tells the insulin pump how much insulin to automatically dispense, should revolutionize care.
A number of such closed-loop systems have recently been approved in both the United States, including systems from Medtronic and Omnipod, and Europe.
“I wear one of these and it’s been a life changer for me, but it doesn’t suit everyone because the technology can be cumbersome, but with time, hopefully things will become smaller and more accurate in insulin delivery,” Dr. Stephens added.
The second advance of interest is the development and transplantation of cells that produce insulin.
Dr. Stephens explained that someone living with type 1 diabetes has a lot to think about, not least, doing the math related to insulin requirement. “If we just had cells from a pancreas that could be transplanted and would do that for us, then it would be a total game changer.”
To date, Vertex Pharmaceuticals has successfully treated one patient – who had lived with type 1 diabetes for about 40 years and had recurrent episodes of severe hypoglycemia – with an infusion of stem cell–derived differentiated islet cells into his liver. The procedure resulted in near reversal of type 1 diabetes, with his insulin dose reduced from 34 to 3 units, and his hemoglobin A1c falling from 8.6% to 7.2%.
And although the patient, Brian Shelton, still needs to take immunosuppressive agents to prevent rejection of the stem cell–derived islets, “it’s a whole new life,” he recently told the New York Times.
Another company called ViaCyte is also working on a similar approach.
Whether this is a cure for type 1 diabetes is still debatable, said Anne Peters, MD, of the University of Southern California, Los Angeles. “Is it true? In a word, no. But we are part of the way there, which is much closer than we were 6 months ago.”
There are also ongoing clinical trials of therapeutic interventions to prevent or delay the trajectory from presymptomatic to clinical type 1 diabetes. The most advanced is the anti-CD3 monoclonal antibody teplizumab (Tzield, Provention Bio), which was rejected by the FDA in July 2021, but has since been refiled. The company expects to hear from the agency by the end of March 2022 as to whether the resubmission has been accepted.
Diabetes specialist nurses/educators keep it human
Dr. Hall said he concurs with the late eminent U.K. diabetes specialist Robert Tattersall’s observation on what he considers one of the most important advances in the management and treatment of type 1 diabetes: the human touch.
Referring to Dr. Tattersall’s book, “Diabetes: A Biography,” Dr. Hall quoted: “If asked what innovation had made the most difference to their lives in the 1980s, patients with type 1 diabetes in England would unhesitatingly have chosen not human insulin, but the spread of diabetes specialist nurses ... these people (mainly women) did more in the last two decades of the 20th century to improve the standard of diabetes care than any other innovation or drug.”
In the United States, diabetes specialist nurses were called diabetes educators until recently, when the name changed to certified diabetes care and education specialist.
“Above all, they have humanized the service and given the patient a say in the otherwise unequal relationship with all-powerful doctors,” concluded Dr. Hall, again quoting Dr. Tattersall.
A version of this article first appeared on Medscape.com.
Editor’s note: This is the second in a two-part series commemorating the 100-year anniversary of the first use of insulin in humans. Part 1 of this series examined the rivalry behind the discovery and use of insulin.
One hundred years ago, teenager Leonard Thompson was the first patient with type 1 diabetes to be successfully treated with insulin, granting him a reprieve from what was a certain death sentence at the time.
Since then, research has gathered pace. In the century since insulin’s discovery and first use, recombinant DNA technology has allowed for the engineering of the insulin molecule, providing numerous short- and long-acting analog versions. At the same time, technological leaps in automated insulin delivery and monitoring of blood glucose ensure more time with glucose in range and fewer life-threatening complications for those with type 1 diabetes fortunate enough to have access to the technology.
In spite of these advancements, there is still scope for further evolution of disease management, with the holy grail being the transplant of stem cell–derived islet cells capable of making insulin, ideally encased in some kind of protective device so that immunosuppression is not required.
Indeed, it is not unreasonable to “hope that type 1 diabetes will be a curable disease in the next 100 years,” said Elizabeth Stephens, MD, an endocrinologist who has type 1 diabetes and practices in Portland, Ore.
Type 1 diabetes: The past 100 years
The epidemiology of type 1 diabetes has shifted considerably since 1922. A century ago, given that average life expectancy in the United States was around 54 years, it was pretty much the only type of diabetes that doctors encountered. “There was some type 2 diabetes about in heavier people, but the focus was on type 1 diabetes,” noted Dr. Stephens.
Originally called juvenile diabetes because it was thought to only occur in children, “now 50% of people are diagnosed with type 1 diabetes ... over [the age of] 20,” explained Dr. Stephens.
In the United States, around 1.4 million adults 20 years and older, and 187,000 children younger than 20, have the disease, according to data from the National Diabetes Statistics Report 2020 by the Centers for Disease Control and Prevention. This total represents an increase of nearly 30% from 2017.
Over the years, theories as to the cause, or trigger, for type 1 diabetes “have included cow’s milk and [viral] infections,” said Dr. Stephens. “Most likely, there’s a genetic predisposition and some type of exposure, which creates the perfect storm to trigger disease.”
There are hints that COVID-19 might be precipitating type 1 diabetes in some people. Recently, the CDC found SARS-CoV-2 infection was associated with an increased risk for diabetes (all types) among youth, but not other acute respiratory infections. And two further studies from different parts of the world have recently identified an increase in the incidence of type 1 diabetes in children since the COVID-19 pandemic began, but the reasons remain unclear.
The global CoviDiab registry has also been established to collect data on patients with COVID-19–related diabetes.
The million-dollar question: Is COVID-19 itself is propagating type 1 diabetes or unmasking a predisposition to the disease sooner? The latter might be associated with a lower type 1 diabetes rate in the future, said Partha Kar, MBBS, OBE, national specialty advisor, diabetes, for National Health Service England.
“Right now, we don’t know the answer. Whichever way you look at it, it is likely there will be a rise in cases, and in countries where insulin is not freely available, healthcare systems need to have supply ready because insulin is lifesaving in type 1 diabetes,” Dr. Kar emphasized.
CGMs and automated insulin delivery: A ‘godsend’
A huge change has also been seen, most notably in the past 15 to 20 years, in the technological advancements that can help those with type 1 diabetes live an easier life.
Continuous glucose monitors (CGMs) and automated ways of delivering insulin, such as smart pens and insulin pumps, have made the daily life of a person with type 1 diabetes in the Western world considerably more comfortable.
CGMs provide a constant stream of data to an app, often wirelessly in sync with the insulin pump. However, on a global level, they are only available to a lucky few.
In England, pending National Institute for Health and Care Excellence) approval, any CGM should be available to all eligible patients with type 1 diabetes within the NHS from April 2022, Dr. Kar pointed out. In the United States, CGMs are often unaffordable and access is mostly dependent on a person’s health insurance.
Kersten Hall, PhD, a scientist and U.K.-based medical historian who recently wrote a book, “Insulin, the Crooked Timber” (Oxford, England: Oxford University Press, 2022) uncovering the lesser-known story behind the discovery of insulin, was diagnosed with adult-onset type 1 diabetes at the age of 41. Dr. Hall had always found the finger-prick blood glucose test to be a chore but now has a CGM.
“It’s a total game changer for me: a godsend. I can’t sing its praises enough,” he said. “All it involves is the swipe of the phone and this provides a reading which tells me if my glucose is too low, so I eat something, or too high, so I might [go for] a run.”
Brewing insulin at scale
As described by Dr. Hall in his book, the journey from treating Mr. Thompson in 1922 to treating the masses began when biochemist James Collip, MD, PhD, discovered a means of purifying the animal pancreas extracts used to treat the teenager.
But production at scale presented a further challenge. This was overcome in 1924 when Eli Lilly drew on a technique used in the beer brewing process – where pH guides bitterness – to purify and manufacture large amounts of insulin.
By 1936, a range of slower-acting cattle and pig-derived insulins, the first produced by Novo Nordisk Pharmaceuticals, were developed.
However, it took 8,000 lb (approximately 3,600 kg) of pancreas glands from 23,500 animals to make 1 lb (0.5 kg) of insulin, so a more efficient process was badly needed.
Dr. Hall, who is a molecular biologist as well as an author, explains that the use of recombinant DNA technology to produce human insulin, as done by Genentech in the late 70s, was a key development in the story of modern insulin products. Genentech then provided synthetic human insulin for Eli Lilly to conduct clinical trials.
Human insulin most closely resembles porcine insulin structure and function, differing by only one amino acid, while human insulin differs from bovine insulin by three amino acid residues. This synthetic human insulin eliminated the allergies that the animal-derived products sometimes caused.
In the early 1980s, Eli Lilly produced Humulin, the first biosynthetic (made in Escherichia coli, hence the term, “bio”) human insulin.
This technology eventually “allowed for the alteration of specific amino acids in the sequence of the insulin protein to make insulin analogs [synthetic versions grown in E. coli and genetically altered for various properties] that act faster, or more slowly, than normal human insulin. By using the slow- and fast-acting insulins in combination, a patient can control their blood sugar levels with a much greater degree of finesse and precision,” Dr. Hall explained.
Today, a whole range of insulins are available, including ultra–rapid-acting, short-acting, intermediate-acting, long-acting, ultra–long-acting, and even inhaled insulin, although the latter is expensive, has been associated with side effects, and is less commonly used, according to Dr. Stephens.
Oral insulin formulations are even in the early stages of development, with candidate drugs by Generex and from the Oralis project.
“With insulin therapy, we try to reproduce the normal physiology of the healthy body and pancreas,” Dr. Stephens explained.
Insulin analogs are only made by three companies (Eli Lilly, Novo Nordisk, and Sanofi), and they are generally much more expensive than nonanalog human insulin. In the United Kingdom through the NHS, they cost twice as much.
In the United States today, one of the biggest barriers to proper care of type 1 diabetes is the cost of insulin, which can limit access. With the market controlled by these three large companies, the average cost of a unit of insulin in the United States, according to RAND research, was $98.17 in January 2021, compared with $7.52 in the United Kingdom and $12.00 in Canada.
Several U.S. states have enacted legislation capping insulin copayments to at, or under, $100 a month. But the federal Build Back Better Framework Act – which would cap copayments for insulin at $35 – currently hangs in the balance.
Alongside these moves, in 2020 the Food and Drug Administration approved the first interchangeable biosimilar insulin for type 1 diabetes (and insulin-dependent type 2 diabetes) in children and adults, called Semglee (Mylan Pharmaceuticals).
Biosimilars (essentially generic versions of branded insulins) are expected to be less expensive than branded analogs, but the indications so far are that they will only be around 20% cheaper.
“I totally fail to understand how the richest country in the world still has a debate about price caps, and we are looking at biosimilar markets to change the debate. This makes no sense to me at all,” stressed Dr. Kar. “For lifesaving drugs, they should be funded by the state.”
Insulin also remains unaffordable for many in numerous low- and middle-income countries, where most patients pay out-of-pocket for medicines. Globally, there are estimated to be around 30 million people who need insulin but cannot afford it.
How near to a cure in the coming decades?
Looking ahead to the coming years, if not the next 100, Dr. Stephens highlighted two important aspects of care.
First, the use of a CGM device in combination with an insulin pump (also known as a closed-loop system or artificial pancreas), where the CGM effectively tells the insulin pump how much insulin to automatically dispense, should revolutionize care.
A number of such closed-loop systems have recently been approved in both the United States, including systems from Medtronic and Omnipod, and Europe.
“I wear one of these and it’s been a life changer for me, but it doesn’t suit everyone because the technology can be cumbersome, but with time, hopefully things will become smaller and more accurate in insulin delivery,” Dr. Stephens added.
The second advance of interest is the development and transplantation of cells that produce insulin.
Dr. Stephens explained that someone living with type 1 diabetes has a lot to think about, not least, doing the math related to insulin requirement. “If we just had cells from a pancreas that could be transplanted and would do that for us, then it would be a total game changer.”
To date, Vertex Pharmaceuticals has successfully treated one patient – who had lived with type 1 diabetes for about 40 years and had recurrent episodes of severe hypoglycemia – with an infusion of stem cell–derived differentiated islet cells into his liver. The procedure resulted in near reversal of type 1 diabetes, with his insulin dose reduced from 34 to 3 units, and his hemoglobin A1c falling from 8.6% to 7.2%.
And although the patient, Brian Shelton, still needs to take immunosuppressive agents to prevent rejection of the stem cell–derived islets, “it’s a whole new life,” he recently told the New York Times.
Another company called ViaCyte is also working on a similar approach.
Whether this is a cure for type 1 diabetes is still debatable, said Anne Peters, MD, of the University of Southern California, Los Angeles. “Is it true? In a word, no. But we are part of the way there, which is much closer than we were 6 months ago.”
There are also ongoing clinical trials of therapeutic interventions to prevent or delay the trajectory from presymptomatic to clinical type 1 diabetes. The most advanced is the anti-CD3 monoclonal antibody teplizumab (Tzield, Provention Bio), which was rejected by the FDA in July 2021, but has since been refiled. The company expects to hear from the agency by the end of March 2022 as to whether the resubmission has been accepted.
Diabetes specialist nurses/educators keep it human
Dr. Hall said he concurs with the late eminent U.K. diabetes specialist Robert Tattersall’s observation on what he considers one of the most important advances in the management and treatment of type 1 diabetes: the human touch.
Referring to Dr. Tattersall’s book, “Diabetes: A Biography,” Dr. Hall quoted: “If asked what innovation had made the most difference to their lives in the 1980s, patients with type 1 diabetes in England would unhesitatingly have chosen not human insulin, but the spread of diabetes specialist nurses ... these people (mainly women) did more in the last two decades of the 20th century to improve the standard of diabetes care than any other innovation or drug.”
In the United States, diabetes specialist nurses were called diabetes educators until recently, when the name changed to certified diabetes care and education specialist.
“Above all, they have humanized the service and given the patient a say in the otherwise unequal relationship with all-powerful doctors,” concluded Dr. Hall, again quoting Dr. Tattersall.
A version of this article first appeared on Medscape.com.
Death of pig heart transplant patient is more a beginning than an end
The genetically altered pig’s heart “worked like a rock star, beautifully functioning,” the surgeon who performed the pioneering Jan. 7 xenotransplant procedure said in a press statement on the death of the patient, David Bennett Sr.
“He wasn’t able to overcome what turned out to be devastating – the debilitation from his previous period of heart failure, which was extreme,” said Bartley P. Griffith, MD, clinical director of the cardiac xenotransplantation program at the University of Maryland, Baltimore.
Representatives of the institution aren’t offering many details on the cause of Mr. Bennett’s death on March 8, 60 days after his operation, but said they will elaborate when their findings are formally published. But their comments seem to downplay the unique nature of the implanted heart itself as a culprit and instead implicate the patient’s diminished overall clinical condition and what grew into an ongoing battle with infections.
The 57-year-old Bennett, bedridden with end-stage heart failure, judged a poor candidate for a ventricular assist device, and on extracorporeal membrane oxygenation (ECMO), reportedly was offered the extraordinary surgery after being turned down for a conventional transplant at several major centers.
“Until day 45 or 50, he was doing very well,” Muhammad M. Mohiuddin, MD, the xenotransplantation program’s scientific director, observed in the statement. But infections soon took advantage of his hobbled immune system.
Given his “preexisting condition and how frail his body was,” Dr. Mohiuddin said, “we were having difficulty maintaining a balance between his immunosuppression and controlling his infection.” Mr. Bennett went into multiple organ failure and “I think that resulted in his passing away.”
Beyond wildest dreams
The surgeons confidently framed Mr. Bennett’s experience as a milestone for heart xenotransplantation. “The demonstration that it was possible, beyond the wildest dreams of most people in the field, even, at this point – that we were able to take a genetically engineered organ and watch it function flawlessly for 9 weeks – is pretty positive in terms of the potential of this therapy,” Dr. Griffith said.
But enough questions linger that others were more circumspect, even as they praised the accomplishment. “There’s no question that this is a historic event,” Mandeep R. Mehra, MD, of Harvard Medical School, and director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital, both in Boston, said in an interview.
Still, “I don’t think we should just conclude that it was the patient’s frailty or death from infection,” Dr. Mehra said. With so few details available, “I would be very careful in prematurely concluding that the problem did not reside with the heart but with the patient. We cannot be sure.”
For example, he noted, “6 to 8 weeks is right around the time when some cardiac complications, like accelerated forms of vasculopathy, could become evident.” Immune-mediated cardiac allograft vasculopathy is a common cause of heart transplant failure.
Or, “it could as easily have been the fact that immunosuppression was modified at 6 to 7 weeks in response to potential infection, which could have led to a cardiac compromise,” Dr. Mehra said. “We just don’t know.”
“It’s really important that this be reported in a scientifically accurate way, because we will all learn from this,” Lori J. West, MD, DPhil, said in an interview.
Little seems to be known for sure about the actual cause of death, “but the fact there was not hyperacute rejection is itself a big step forward. And we know, at least from the limited information we have, that it did not occur,” observed Dr. West, who directs the Alberta Transplant Institute, Edmonton, and the Canadian Donation and Transplantation Research Program. She is a professor of pediatrics with adjunct positions in the departments of surgery and microbiology/immunology.
Dr. West also sees Mr. Bennett’s struggle with infections and adjustments to his unique immunosuppressive regimen, at least as characterized by his care team, as in line with the experience of many heart transplant recipients facing the same threat.
“We already walk this tightrope with every transplant patient,” she said. Typically, they’re put on a somewhat standardized immunosuppressant regimen, “and then we modify it a bit, either increasing or decreasing it, depending on the posttransplant course.” The regimen can become especially intense in response to new signs of rejection, “and you know that that’s going to have an impact on susceptibility to all kinds of infections.”
Full circle
The porcine heart was protected along two fronts against assault from Mr. Bennett’s immune system and other inhospitable aspects of his physiology, either of which could also have been obstacles to success: Genetic modification (Revivicor) of the pig that provided the heart, and a singularly aggressive antirejection drug regimen for the patient.
The knockout of three genes targeting specific porcine cell-surface carbohydrates that provoke a strong human antibody response reportedly averted a hyperacute rejection response that would have caused the graft to fail almost immediately.
Other genetic manipulations, some using CRISPR technology, silenced genes encoded for porcine endogenous retroviruses. Others were aimed at controlling myocardial growth and stemming graft microangiopathy.
Mr. Bennett himself was treated with powerful immunosuppressants, including an investigational anti-CD40 monoclonal antibody (KPL-404, Kiniksa Pharmaceuticals) that, according to UMSOM, inhibits a well-recognized pathway critical to B-cell proliferation, T-cell activation, and antibody production.
“I suspect the patient may not have had rejection, but unfortunately, that intense immunosuppression really set him up – even if he had been half that age – for a very difficult time,” David A. Baran, MD, a cardiologist from Sentara Advanced Heart Failure Center, Norfolk, Va., who studies transplant immunology, said in an interview.
“This is in some ways like the original heart transplant in 1967, when the ability to do the surgery evolved before understanding of the immunosuppression needed. Four or 5 years later, heart transplantation almost died out, before the development of better immunosuppressants like cyclosporine and later tacrolimus,” Dr. Baran said.
“The current age, when we use less immunosuppression than ever, is based on 30 years of progressive success,” he noted. This landmark xenotransplantation “basically turns back the clock to a time when the intensity of immunosuppression by definition had to be extremely high, because we really didn’t know what to expect.”
Emerging role of xeno-organs
Xenotransplantation has been touted as potential strategy for expanding the pool of organs available for transplantation. Mr. Bennett’s “breakthrough surgery” takes the world “one step closer to solving the organ shortage crisis,” his surgeon, Dr. Griffith, announced soon after the procedure. “There are simply not enough donor human hearts available to meet the long list of potential recipients.”
But it’s not the only proposed approach. Measures could be taken, for example, to make more efficient use of the human organs that become available, partly by opening the field to additional less-than-ideal hearts and loosening regulatory mandates for projected graft survival.
“Every year, more than two-thirds of donor organs in the United States are discarded. So it’s not actually that we don’t have enough organs, it’s that we don’t have enough organs that people are willing to take,” Dr. Baran said. Still, it’s important to pursue all promising avenues, and “the genetic manipulation pathway is remarkable.”
But “honestly, organs such as kidneys probably make the most sense” for early study of xenotransplantation from pigs, he said. “The waiting list for kidneys is also very long, but if the kidney graft were to fail, the patient wouldn’t die. It would allow us to work out the immunosuppression without putting patients’ lives at risk.”
Often overlooked in assessments of organ demand, Dr. West said, is that “a lot of patients who could benefit from a transplant will never even be listed for a transplant.” It’s not clear why; perhaps they have multiple comorbidities, live too far from a transplant center, “or they’re too big or too small. Even if there were unlimited organs, you could never meet the needs of people who could benefit from transplantation.”
So even if more available donor organs were used, she said, there would still be a gap that xenotransplantation could help fill. “I’m very much in favor of research that allows us to continue to try to find a pathway to xenotransplantation. I think it’s critically important.”
Unquestionably, “we now need to have a dialogue to entertain how a technology like this, using modern medicine with gene editing, is really going to be utilized,” Dr. Mehra said. The Bennett case “does open up the field, but it also raises caution.” There should be broad participation to move the field forward, “coordinated through either societies or nationally allocated advisory committees that oversee the movement of this technology, to the next step.”
Ideally, that next step “would be to do a safety clinical trial in the right patient,” he said. “And the right patient, by definition, would be one who does not have a life-prolonging option, either mechanical circulatory support or allograft transplantation. That would be the goal.”
Dr. Mehra has reported receiving payments to his institution from Abbott for consulting; consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and serving on a scientific advisory board NuPulseCV, Leviticus, and FineHeart. Dr. Baran disclosed consulting for Getinge and LivaNova; speaking for Pfizer; and serving on trial steering committees for CareDx and Procyrion, all unrelated to xenotransplantation. Dr. West has declared no relevant conflicts.
A version of this article first appeared on Medscape.com.
The genetically altered pig’s heart “worked like a rock star, beautifully functioning,” the surgeon who performed the pioneering Jan. 7 xenotransplant procedure said in a press statement on the death of the patient, David Bennett Sr.
“He wasn’t able to overcome what turned out to be devastating – the debilitation from his previous period of heart failure, which was extreme,” said Bartley P. Griffith, MD, clinical director of the cardiac xenotransplantation program at the University of Maryland, Baltimore.
Representatives of the institution aren’t offering many details on the cause of Mr. Bennett’s death on March 8, 60 days after his operation, but said they will elaborate when their findings are formally published. But their comments seem to downplay the unique nature of the implanted heart itself as a culprit and instead implicate the patient’s diminished overall clinical condition and what grew into an ongoing battle with infections.
The 57-year-old Bennett, bedridden with end-stage heart failure, judged a poor candidate for a ventricular assist device, and on extracorporeal membrane oxygenation (ECMO), reportedly was offered the extraordinary surgery after being turned down for a conventional transplant at several major centers.
“Until day 45 or 50, he was doing very well,” Muhammad M. Mohiuddin, MD, the xenotransplantation program’s scientific director, observed in the statement. But infections soon took advantage of his hobbled immune system.
Given his “preexisting condition and how frail his body was,” Dr. Mohiuddin said, “we were having difficulty maintaining a balance between his immunosuppression and controlling his infection.” Mr. Bennett went into multiple organ failure and “I think that resulted in his passing away.”
Beyond wildest dreams
The surgeons confidently framed Mr. Bennett’s experience as a milestone for heart xenotransplantation. “The demonstration that it was possible, beyond the wildest dreams of most people in the field, even, at this point – that we were able to take a genetically engineered organ and watch it function flawlessly for 9 weeks – is pretty positive in terms of the potential of this therapy,” Dr. Griffith said.
But enough questions linger that others were more circumspect, even as they praised the accomplishment. “There’s no question that this is a historic event,” Mandeep R. Mehra, MD, of Harvard Medical School, and director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital, both in Boston, said in an interview.
Still, “I don’t think we should just conclude that it was the patient’s frailty or death from infection,” Dr. Mehra said. With so few details available, “I would be very careful in prematurely concluding that the problem did not reside with the heart but with the patient. We cannot be sure.”
For example, he noted, “6 to 8 weeks is right around the time when some cardiac complications, like accelerated forms of vasculopathy, could become evident.” Immune-mediated cardiac allograft vasculopathy is a common cause of heart transplant failure.
Or, “it could as easily have been the fact that immunosuppression was modified at 6 to 7 weeks in response to potential infection, which could have led to a cardiac compromise,” Dr. Mehra said. “We just don’t know.”
“It’s really important that this be reported in a scientifically accurate way, because we will all learn from this,” Lori J. West, MD, DPhil, said in an interview.
Little seems to be known for sure about the actual cause of death, “but the fact there was not hyperacute rejection is itself a big step forward. And we know, at least from the limited information we have, that it did not occur,” observed Dr. West, who directs the Alberta Transplant Institute, Edmonton, and the Canadian Donation and Transplantation Research Program. She is a professor of pediatrics with adjunct positions in the departments of surgery and microbiology/immunology.
Dr. West also sees Mr. Bennett’s struggle with infections and adjustments to his unique immunosuppressive regimen, at least as characterized by his care team, as in line with the experience of many heart transplant recipients facing the same threat.
“We already walk this tightrope with every transplant patient,” she said. Typically, they’re put on a somewhat standardized immunosuppressant regimen, “and then we modify it a bit, either increasing or decreasing it, depending on the posttransplant course.” The regimen can become especially intense in response to new signs of rejection, “and you know that that’s going to have an impact on susceptibility to all kinds of infections.”
Full circle
The porcine heart was protected along two fronts against assault from Mr. Bennett’s immune system and other inhospitable aspects of his physiology, either of which could also have been obstacles to success: Genetic modification (Revivicor) of the pig that provided the heart, and a singularly aggressive antirejection drug regimen for the patient.
The knockout of three genes targeting specific porcine cell-surface carbohydrates that provoke a strong human antibody response reportedly averted a hyperacute rejection response that would have caused the graft to fail almost immediately.
Other genetic manipulations, some using CRISPR technology, silenced genes encoded for porcine endogenous retroviruses. Others were aimed at controlling myocardial growth and stemming graft microangiopathy.
Mr. Bennett himself was treated with powerful immunosuppressants, including an investigational anti-CD40 monoclonal antibody (KPL-404, Kiniksa Pharmaceuticals) that, according to UMSOM, inhibits a well-recognized pathway critical to B-cell proliferation, T-cell activation, and antibody production.
“I suspect the patient may not have had rejection, but unfortunately, that intense immunosuppression really set him up – even if he had been half that age – for a very difficult time,” David A. Baran, MD, a cardiologist from Sentara Advanced Heart Failure Center, Norfolk, Va., who studies transplant immunology, said in an interview.
“This is in some ways like the original heart transplant in 1967, when the ability to do the surgery evolved before understanding of the immunosuppression needed. Four or 5 years later, heart transplantation almost died out, before the development of better immunosuppressants like cyclosporine and later tacrolimus,” Dr. Baran said.
“The current age, when we use less immunosuppression than ever, is based on 30 years of progressive success,” he noted. This landmark xenotransplantation “basically turns back the clock to a time when the intensity of immunosuppression by definition had to be extremely high, because we really didn’t know what to expect.”
Emerging role of xeno-organs
Xenotransplantation has been touted as potential strategy for expanding the pool of organs available for transplantation. Mr. Bennett’s “breakthrough surgery” takes the world “one step closer to solving the organ shortage crisis,” his surgeon, Dr. Griffith, announced soon after the procedure. “There are simply not enough donor human hearts available to meet the long list of potential recipients.”
But it’s not the only proposed approach. Measures could be taken, for example, to make more efficient use of the human organs that become available, partly by opening the field to additional less-than-ideal hearts and loosening regulatory mandates for projected graft survival.
“Every year, more than two-thirds of donor organs in the United States are discarded. So it’s not actually that we don’t have enough organs, it’s that we don’t have enough organs that people are willing to take,” Dr. Baran said. Still, it’s important to pursue all promising avenues, and “the genetic manipulation pathway is remarkable.”
But “honestly, organs such as kidneys probably make the most sense” for early study of xenotransplantation from pigs, he said. “The waiting list for kidneys is also very long, but if the kidney graft were to fail, the patient wouldn’t die. It would allow us to work out the immunosuppression without putting patients’ lives at risk.”
Often overlooked in assessments of organ demand, Dr. West said, is that “a lot of patients who could benefit from a transplant will never even be listed for a transplant.” It’s not clear why; perhaps they have multiple comorbidities, live too far from a transplant center, “or they’re too big or too small. Even if there were unlimited organs, you could never meet the needs of people who could benefit from transplantation.”
So even if more available donor organs were used, she said, there would still be a gap that xenotransplantation could help fill. “I’m very much in favor of research that allows us to continue to try to find a pathway to xenotransplantation. I think it’s critically important.”
Unquestionably, “we now need to have a dialogue to entertain how a technology like this, using modern medicine with gene editing, is really going to be utilized,” Dr. Mehra said. The Bennett case “does open up the field, but it also raises caution.” There should be broad participation to move the field forward, “coordinated through either societies or nationally allocated advisory committees that oversee the movement of this technology, to the next step.”
Ideally, that next step “would be to do a safety clinical trial in the right patient,” he said. “And the right patient, by definition, would be one who does not have a life-prolonging option, either mechanical circulatory support or allograft transplantation. That would be the goal.”
Dr. Mehra has reported receiving payments to his institution from Abbott for consulting; consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and serving on a scientific advisory board NuPulseCV, Leviticus, and FineHeart. Dr. Baran disclosed consulting for Getinge and LivaNova; speaking for Pfizer; and serving on trial steering committees for CareDx and Procyrion, all unrelated to xenotransplantation. Dr. West has declared no relevant conflicts.
A version of this article first appeared on Medscape.com.
The genetically altered pig’s heart “worked like a rock star, beautifully functioning,” the surgeon who performed the pioneering Jan. 7 xenotransplant procedure said in a press statement on the death of the patient, David Bennett Sr.
“He wasn’t able to overcome what turned out to be devastating – the debilitation from his previous period of heart failure, which was extreme,” said Bartley P. Griffith, MD, clinical director of the cardiac xenotransplantation program at the University of Maryland, Baltimore.
Representatives of the institution aren’t offering many details on the cause of Mr. Bennett’s death on March 8, 60 days after his operation, but said they will elaborate when their findings are formally published. But their comments seem to downplay the unique nature of the implanted heart itself as a culprit and instead implicate the patient’s diminished overall clinical condition and what grew into an ongoing battle with infections.
The 57-year-old Bennett, bedridden with end-stage heart failure, judged a poor candidate for a ventricular assist device, and on extracorporeal membrane oxygenation (ECMO), reportedly was offered the extraordinary surgery after being turned down for a conventional transplant at several major centers.
“Until day 45 or 50, he was doing very well,” Muhammad M. Mohiuddin, MD, the xenotransplantation program’s scientific director, observed in the statement. But infections soon took advantage of his hobbled immune system.
Given his “preexisting condition and how frail his body was,” Dr. Mohiuddin said, “we were having difficulty maintaining a balance between his immunosuppression and controlling his infection.” Mr. Bennett went into multiple organ failure and “I think that resulted in his passing away.”
Beyond wildest dreams
The surgeons confidently framed Mr. Bennett’s experience as a milestone for heart xenotransplantation. “The demonstration that it was possible, beyond the wildest dreams of most people in the field, even, at this point – that we were able to take a genetically engineered organ and watch it function flawlessly for 9 weeks – is pretty positive in terms of the potential of this therapy,” Dr. Griffith said.
But enough questions linger that others were more circumspect, even as they praised the accomplishment. “There’s no question that this is a historic event,” Mandeep R. Mehra, MD, of Harvard Medical School, and director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital, both in Boston, said in an interview.
Still, “I don’t think we should just conclude that it was the patient’s frailty or death from infection,” Dr. Mehra said. With so few details available, “I would be very careful in prematurely concluding that the problem did not reside with the heart but with the patient. We cannot be sure.”
For example, he noted, “6 to 8 weeks is right around the time when some cardiac complications, like accelerated forms of vasculopathy, could become evident.” Immune-mediated cardiac allograft vasculopathy is a common cause of heart transplant failure.
Or, “it could as easily have been the fact that immunosuppression was modified at 6 to 7 weeks in response to potential infection, which could have led to a cardiac compromise,” Dr. Mehra said. “We just don’t know.”
“It’s really important that this be reported in a scientifically accurate way, because we will all learn from this,” Lori J. West, MD, DPhil, said in an interview.
Little seems to be known for sure about the actual cause of death, “but the fact there was not hyperacute rejection is itself a big step forward. And we know, at least from the limited information we have, that it did not occur,” observed Dr. West, who directs the Alberta Transplant Institute, Edmonton, and the Canadian Donation and Transplantation Research Program. She is a professor of pediatrics with adjunct positions in the departments of surgery and microbiology/immunology.
Dr. West also sees Mr. Bennett’s struggle with infections and adjustments to his unique immunosuppressive regimen, at least as characterized by his care team, as in line with the experience of many heart transplant recipients facing the same threat.
“We already walk this tightrope with every transplant patient,” she said. Typically, they’re put on a somewhat standardized immunosuppressant regimen, “and then we modify it a bit, either increasing or decreasing it, depending on the posttransplant course.” The regimen can become especially intense in response to new signs of rejection, “and you know that that’s going to have an impact on susceptibility to all kinds of infections.”
Full circle
The porcine heart was protected along two fronts against assault from Mr. Bennett’s immune system and other inhospitable aspects of his physiology, either of which could also have been obstacles to success: Genetic modification (Revivicor) of the pig that provided the heart, and a singularly aggressive antirejection drug regimen for the patient.
The knockout of three genes targeting specific porcine cell-surface carbohydrates that provoke a strong human antibody response reportedly averted a hyperacute rejection response that would have caused the graft to fail almost immediately.
Other genetic manipulations, some using CRISPR technology, silenced genes encoded for porcine endogenous retroviruses. Others were aimed at controlling myocardial growth and stemming graft microangiopathy.
Mr. Bennett himself was treated with powerful immunosuppressants, including an investigational anti-CD40 monoclonal antibody (KPL-404, Kiniksa Pharmaceuticals) that, according to UMSOM, inhibits a well-recognized pathway critical to B-cell proliferation, T-cell activation, and antibody production.
“I suspect the patient may not have had rejection, but unfortunately, that intense immunosuppression really set him up – even if he had been half that age – for a very difficult time,” David A. Baran, MD, a cardiologist from Sentara Advanced Heart Failure Center, Norfolk, Va., who studies transplant immunology, said in an interview.
“This is in some ways like the original heart transplant in 1967, when the ability to do the surgery evolved before understanding of the immunosuppression needed. Four or 5 years later, heart transplantation almost died out, before the development of better immunosuppressants like cyclosporine and later tacrolimus,” Dr. Baran said.
“The current age, when we use less immunosuppression than ever, is based on 30 years of progressive success,” he noted. This landmark xenotransplantation “basically turns back the clock to a time when the intensity of immunosuppression by definition had to be extremely high, because we really didn’t know what to expect.”
Emerging role of xeno-organs
Xenotransplantation has been touted as potential strategy for expanding the pool of organs available for transplantation. Mr. Bennett’s “breakthrough surgery” takes the world “one step closer to solving the organ shortage crisis,” his surgeon, Dr. Griffith, announced soon after the procedure. “There are simply not enough donor human hearts available to meet the long list of potential recipients.”
But it’s not the only proposed approach. Measures could be taken, for example, to make more efficient use of the human organs that become available, partly by opening the field to additional less-than-ideal hearts and loosening regulatory mandates for projected graft survival.
“Every year, more than two-thirds of donor organs in the United States are discarded. So it’s not actually that we don’t have enough organs, it’s that we don’t have enough organs that people are willing to take,” Dr. Baran said. Still, it’s important to pursue all promising avenues, and “the genetic manipulation pathway is remarkable.”
But “honestly, organs such as kidneys probably make the most sense” for early study of xenotransplantation from pigs, he said. “The waiting list for kidneys is also very long, but if the kidney graft were to fail, the patient wouldn’t die. It would allow us to work out the immunosuppression without putting patients’ lives at risk.”
Often overlooked in assessments of organ demand, Dr. West said, is that “a lot of patients who could benefit from a transplant will never even be listed for a transplant.” It’s not clear why; perhaps they have multiple comorbidities, live too far from a transplant center, “or they’re too big or too small. Even if there were unlimited organs, you could never meet the needs of people who could benefit from transplantation.”
So even if more available donor organs were used, she said, there would still be a gap that xenotransplantation could help fill. “I’m very much in favor of research that allows us to continue to try to find a pathway to xenotransplantation. I think it’s critically important.”
Unquestionably, “we now need to have a dialogue to entertain how a technology like this, using modern medicine with gene editing, is really going to be utilized,” Dr. Mehra said. The Bennett case “does open up the field, but it also raises caution.” There should be broad participation to move the field forward, “coordinated through either societies or nationally allocated advisory committees that oversee the movement of this technology, to the next step.”
Ideally, that next step “would be to do a safety clinical trial in the right patient,” he said. “And the right patient, by definition, would be one who does not have a life-prolonging option, either mechanical circulatory support or allograft transplantation. That would be the goal.”
Dr. Mehra has reported receiving payments to his institution from Abbott for consulting; consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and serving on a scientific advisory board NuPulseCV, Leviticus, and FineHeart. Dr. Baran disclosed consulting for Getinge and LivaNova; speaking for Pfizer; and serving on trial steering committees for CareDx and Procyrion, all unrelated to xenotransplantation. Dr. West has declared no relevant conflicts.
A version of this article first appeared on Medscape.com.
Morphology of Mycosis Fungoides and Sézary Syndrome in Skin of Color
Mycosis fungoides (MF) and Sézary syndrome (SS) are non-Hodgkin T-cell lymphomas that make up the majority of cutaneous T-cell lymphomas. These conditions commonly affect Black patients, with an incidence rate of 12.6 cases of cutaneous T-cell lymphomas per million individuals vs 9.8 per million individuals in non–skin of color (SoC) patients.1 However, educational resources tend to focus on the clinical manifestations of MF/SS in lighter skin types, describing MF as erythematous patches, plaques, or tumors presenting in non–sun-exposed areas of the skin and SS as generalized erythroderma.2 Skin of color, comprised of Fitzpatrick skin types (FSTs) IV to VI,3 is poorly represented across dermatology textbooks,4,5 medical student resources,6 and peer-reviewed publications,7 raising awareness for the need to address this disparity.
Skin of color patients with MF/SS display variable morphologies, including features such as hyperpigmentation and hypopigmentation,8 the latter being exceedingly rare in non-SoC patients.9 Familiarity with these differences among providers is essential to allow for equitable diagnosis and treatment across all skin types, especially in light of data predicting that by 2044 more than 50% of the US population will be people of color.10 Patients with SoC are of many ethnic and racial backgrounds, including Black, Hispanic, American Indian, Pacific Islander, and Asian.11
Along with morphologic differences, there also are several racial disparities in the prognosis and survival of patients with MF/SS. Black patients diagnosed with MF present with greater body surface area affected, and Black women with MF have reduced survival rates compared to their White counterparts.12 Given these racial disparities in survival and representation in educational resources, we aimed to quantify the frequency of various morphologic characteristics of MF/SS in patients with SoC vs non-SoC patients to facilitate better recognition of early MF/SS in SoC patients by medical providers.
Methods
We performed a retrospective chart review following approval from the institutional review board at Northwestern University (Chicago, Illinois). We identified all patients with FSTs IV to VI and biopsy-proven MF/SS who had been clinically photographed in our clinic from January 1998 to December 2019. Only photographs that were high quality enough to review morphologic features were included in our review. Fitzpatrick skin type was determined based on electronic medical record documentation. If photographs were available from multiple visits for the same patient, only those showing posttreatment nonactive lesions were included. Additionally, 36 patients with FSTs I to III (non-SoC) and biopsy-proven MF/SS were included in our review as a comparison with the SoC cohort. The primary outcomes for this study included the presence of scale, erythema, hyperpigmentation, hypopigmentation, violaceous color, lichenification, silver hue, dyschromia, alopecia, poikiloderma, atrophy, and ulceration in active lesions. Dyschromia was defined by the presence of both hypopigmentation and hyperpigmentation. Poikiloderma was defined by hypopigmentation and hyperpigmentation, telangiectasia, and atrophy. Secondary outcomes included evaluation of those same characteristics in posttreatment nonactive lesions. All photographs were independently assessed by 3 authors (M.L.E., C.J.W., J.M.M.), and discrepancies were resolved by further review of the photograph in question and discussion.
Statistical Analysis—Summary statistics were applied to describe demographic and clinical characteristics. The χ2 test was used for categorical variables. Results achieving P<.05 were considered statistically significant.
Results
We reviewed photographs of 111 patients across all skin types (8, FST I; 12, FST II; 16, FST III; 17, FST IV; 44, FST V; 14, FST VI). The cohort was 47% female, and the mean age was 49.7 years (range, 15–86 years). The majority of the cohort had early-stage MF (stage IA or IB). There were more cases of SS in the SoC cohort than the non-SoC cohort (Table). Only 5 photographs had discrepancies and required discussion among the reviewers to achieve consensus.
![Frequency of morphologic features found in skin of color (SoC [Fitzpatrick skin types IV–VI]) vs non-SoC (Fitzpatrick skin types I–III) patients with mycosis fungoides/Sézary syndrome Frequency of morphologic features found in skin of color (SoC [Fitzpatrick skin types IV–VI]) vs non-SoC (Fitzpatrick skin types I–III) patients with mycosis fungoides/Sézary syndrome](https://cdn.mdedge.com/files/s3fs-public/Espinosa_1.JPG)
Regarding morphologic characteristics in active lesions (Figure 1), scale was present in almost all patients (99% in SoC, 94% in non-SoC). Erythema was present in nearly all non-SoC patients (94%) but only in 69% of SoC patients (P=.003). Poikiloderma also was found to be present at higher frequencies in non-SoC patients compared with SoC patients (19% and 4%, respectively [P=.008]). However, hyperpigmentation (80% vs 39%), lichenification (43% vs 17%), and silver hue (25% vs 3%) were more common in SoC patients than non-SoC patients (P<.05). There were no significant differences in the remaining features, including hypopigmentation (39% vs 25%), dyschromia (24% vs 19%), violaceous color (44% vs 25%), atrophy (11% vs 22%), alopecia (23% vs 31%), and ulceration (16% vs 8%) between SoC and non-SoC patients (P>.05). Photographs of MF in patients with SoC can be seen in Figure 2.
![Representative photographs of mycosis fungoides (MF) in skin of color (Fitzpatrick skin types [FSTs] IV–VI) Representative photographs of mycosis fungoides (MF) in skin of color (Fitzpatrick skin types [FSTs] IV–VI)](https://cdn.mdedge.com/files/s3fs-public/CT109003003_e_Fig2_ABCDE.JPG)
Posttreatment (nonactive) photographs were available for 26 patients (6 non-SoC, 20 SoC). We found that across all FST groups, hyperpigmentation was more common than hypopigmentation in areas of previously active disease. Statistical analysis was not completed given that few non-SoC photographs were available for comparison.
Comment
This qualitative review demonstrates the heterogeneity of MF/SS in SoC patients and that these conditions do not present in this population with the classic erythematous patches and plaques found in non-SoC patients. We found that hyperpigmentation, lichenification, and silver hue were present at higher rates in patients with FSTs IV to VI compared to those with FSTs I to III, which had higher rates of erythema and poikiloderma. Familiarity with these morphologic features along with increased exposure to clinical photographs of MF/SS in SoC patients will aid in the visual recognition required for this diagnosis, since erythema is harder to identify in darker skin types. Recognizing the unique findings of MF in patients with SoC as well as in patients with lighter skin types will enable earlier diagnosis and treatment of MF/SS across all skin types. If MF is diagnosed and treated early, life expectancy is similar to that of patients without MF.13 However, the 5-year survival rate for advanced-stage MF/SS is 52% across all skin types, and studies have found that Black patients with advanced-stage disease have worse outcomes despite accounting for demographic factors and tumor stage.14,15 Given the worse outcomes in SoC patients with advanced-stage MF/SS, earlier diagnosis could help address this disparity.8,13,14 Similar morphologic features could be used in diagnosing other inflammatory conditions; studies have shown that the lack of recognition of erythema in Black children has led to delayed diagnosis of atopic dermatitis and subsequent inadequate treatment.16,17
The morphologic presentation of MF/SS in SoC patients also can influence an optimal treatment plan for this population. Hypopigmented MF responds better to phototherapy than hyperpigmented MF, as phototherapy has been shown to have decreased efficacy with increasing FST.18 Therefore, for patients with FSTs IV to VI, topical agents such as nitrogen mustard or bexarotene may be more suitable treatment options, as the efficacy of these treatments is independent of skin color.8 However, nitrogen mustard commonly leads to postinflammatory hyperpigmentation, and topical bexarotene may lead to erythema or irritation; therefore, providers must counsel patients on these possible side effects. For refractory disease, adjunct systemic treatments such as oral bexarotene, subcutaneous interferon, methotrexate, or radiation therapy may be considered.8
In addition to aiding in the prompt diagnosis and treatment of MF/SS in SoC patients, our findings may be used to better assess the extent of disease and distinguish between active MF/SS lesions vs xerosis cutis or residual dyschromia from previously treated lesions. It is important to note that these morphologic features must be taken into account with a complete history and work-up. The differential diagnosis of MF/SS includes conditions such as atopic dermatitis, psoriasis, tinea corporis, and drug reactions, which may have similar morphology in SoC.19
Limitations of our study include the single-center design and the use of photographs instead of in-person examination; however, our cutaneous lymphoma clinic serves a diverse patient population, and our 3 reviewers rated the photographs independently. Discussion amongst the reviewers to address discrepancies was only required for 5 photographs, indicating the high inter-reviewer reliability. Additionally, the original purpose of FST was to assess for the propensity of the skin to burn when undergoing phototherapy, not to serve as a marker for skin color. We recommend trainees and clinicians be mindful about the purpose of FST and to use inclusive language (eg, using the terms skin irritation, skin tenderness, or skin becoming darker from the sun instead of tanning) when determining FST in darker-skinned individuals.20 Future directions include examining if certain treatments are associated with prolonged dyschromia.
Conclusion
In our single-institution retrospective study, we found differences in the morphologic presentation of MF/SS in SoC patients vs non-SoC patients. While erythema is a common feature in non-SoC patients, clinical features of hyperpigmentation, lichenification, and silver hue should be carefully evaluated in the diagnosis of MF/SS in SoC patients. Knowledge of the heterogenous presentation of MF/SS in patients with SoC allows for expedited diagnosis and treatment, leading to better clinical outcomes. Valuable resources, including Taylor and Kelly’s Dermatology for Skin of Color, the Skin of Color Society, and VisualDx educate providers on how dermatologic conditions present in darker skin types. However, there is still work to be done to enhance diversity in educational resources in order to provide equitable care to patients of all skin types.
- Korgavkar K, Xiong M, Weinstock M. Changing incidence trends of cutaneous T-cell lymphoma. JAMA Dermatol. 2013;149:1295-1299. doi:10.1001/jamadermatol.2013.5526
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.E1-E16; quiz 221-222. doi:10.1016/j.jaad.2013.07.049
- Tull RZ, Kerby E, Subash JJ, et al. Ethnic skin centers in the United States: where are we in 2020?. J Am Acad Dermatol. 2020;83:1757-1759. doi:10.1016/j.jaad.2020.03.054
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196. doi:10.1016/j.jaad.2020.04.084
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Jones VA, Clark KA, Shobajo MT, et al. Skin of color representation in medical education: an analysis of popular preparatory materials used for United States medical licensing examinations. J Am Acad Dermatol. 2021;85:773-775. doi:10.1016/j.jaad.2020.07.112
- Montgomery SN, Elbuluk N. A quantitative analysis of research publications focused on the top chief complaints in skin of color patients. J Am Acad Dermatol. 2021;85:241-242. doi:10.1016/j.jaad.2020.08.031
- Hinds GA, Heald P. Cutaneous T-cell lymphoma in skin of color. J Am Acad Dermatol. 2009;60:359-375; quiz 376-378. doi:10.1016/j.jaad.2008.10.031
- Ardigó M, Borroni G, Muscardin L, et al. Hypopigmented mycosis fungoides in Caucasian patients: a clinicopathologic study of 7 cases. J Am Acad Dermatol. 2003;49:264-270. doi:10.1067/s0190-9622(03)00907-1
- Colby SL, Ortman JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. Updated October 8, 2021. Accessed February 28, 2022. https://www.census.gov/library/publications/2015/demo/p25-1143.html
- Taylor SC, Kyei A. Defining skin of color. In: Kelly AP, Taylor SC, Lim HW, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Huang AH, Kwatra SG, Khanna R, et al. Racial disparities in the clinical presentation and prognosis of patients with mycosis fungoides. J Natl Med Assoc. 2019;111:633-639. doi:10.1016/j.jnma.2019.08.006
- Kim YH, Jensen RA, Watanabe GL, et al. Clinical stage IA (limited patch and plaque) mycosis fungoides. a long-term outcome analysis. Arch Dermatol. 1996;132:1309-1313.
- Scarisbrick JJ, Prince HM, Vermeer MH, et al. Cutaneous lymphoma international consortium study of outcome in advanced stages of mycosis fungoides and Sézary syndrome: effect of specific prognostic markers on survival and development of a prognostic model. J Clin Oncol. 2015;33:3766-3773. doi:10.1200/JCO.2015.61.7142
- Nath SK, Yu JB, Wilson LD. Poorer prognosis of African-American patients with mycosis fungoides: an analysis of the SEER dataset, 1988 to 2008. Clin Lymphoma Myeloma Leuk. 2014;14:419-423. doi:10.1016/j.clml.2013.12.018
- Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol. 2002;147:920-925. doi:10.1046/j.1365-2133.2002.04965.x
- Poladian K, De Souza B, McMichael AJ. Atopic dermatitis in adolescents with skin of color. Cutis. 2019;104:164-168.
- Yones SS, Palmer RA, Garibaldinos TT, et al. Randomized double-blind trial of the treatment of chronic plaque psoriasis: efficacy of psoralen-UV-A therapy vs narrowband UV-B therapy. Arch Dermatol. 2006;142:836-842. doi:10.1001/archderm.142.7.836
- Currimbhoy S, Pandya AG. Cutaneous T-cell lymphoma. In: Kelly AP, Taylor SC, Lim HW, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
Mycosis fungoides (MF) and Sézary syndrome (SS) are non-Hodgkin T-cell lymphomas that make up the majority of cutaneous T-cell lymphomas. These conditions commonly affect Black patients, with an incidence rate of 12.6 cases of cutaneous T-cell lymphomas per million individuals vs 9.8 per million individuals in non–skin of color (SoC) patients.1 However, educational resources tend to focus on the clinical manifestations of MF/SS in lighter skin types, describing MF as erythematous patches, plaques, or tumors presenting in non–sun-exposed areas of the skin and SS as generalized erythroderma.2 Skin of color, comprised of Fitzpatrick skin types (FSTs) IV to VI,3 is poorly represented across dermatology textbooks,4,5 medical student resources,6 and peer-reviewed publications,7 raising awareness for the need to address this disparity.
Skin of color patients with MF/SS display variable morphologies, including features such as hyperpigmentation and hypopigmentation,8 the latter being exceedingly rare in non-SoC patients.9 Familiarity with these differences among providers is essential to allow for equitable diagnosis and treatment across all skin types, especially in light of data predicting that by 2044 more than 50% of the US population will be people of color.10 Patients with SoC are of many ethnic and racial backgrounds, including Black, Hispanic, American Indian, Pacific Islander, and Asian.11
Along with morphologic differences, there also are several racial disparities in the prognosis and survival of patients with MF/SS. Black patients diagnosed with MF present with greater body surface area affected, and Black women with MF have reduced survival rates compared to their White counterparts.12 Given these racial disparities in survival and representation in educational resources, we aimed to quantify the frequency of various morphologic characteristics of MF/SS in patients with SoC vs non-SoC patients to facilitate better recognition of early MF/SS in SoC patients by medical providers.
Methods
We performed a retrospective chart review following approval from the institutional review board at Northwestern University (Chicago, Illinois). We identified all patients with FSTs IV to VI and biopsy-proven MF/SS who had been clinically photographed in our clinic from January 1998 to December 2019. Only photographs that were high quality enough to review morphologic features were included in our review. Fitzpatrick skin type was determined based on electronic medical record documentation. If photographs were available from multiple visits for the same patient, only those showing posttreatment nonactive lesions were included. Additionally, 36 patients with FSTs I to III (non-SoC) and biopsy-proven MF/SS were included in our review as a comparison with the SoC cohort. The primary outcomes for this study included the presence of scale, erythema, hyperpigmentation, hypopigmentation, violaceous color, lichenification, silver hue, dyschromia, alopecia, poikiloderma, atrophy, and ulceration in active lesions. Dyschromia was defined by the presence of both hypopigmentation and hyperpigmentation. Poikiloderma was defined by hypopigmentation and hyperpigmentation, telangiectasia, and atrophy. Secondary outcomes included evaluation of those same characteristics in posttreatment nonactive lesions. All photographs were independently assessed by 3 authors (M.L.E., C.J.W., J.M.M.), and discrepancies were resolved by further review of the photograph in question and discussion.
Statistical Analysis—Summary statistics were applied to describe demographic and clinical characteristics. The χ2 test was used for categorical variables. Results achieving P<.05 were considered statistically significant.

Results
We reviewed photographs of 111 patients across all skin types (8, FST I; 12, FST II; 16, FST III; 17, FST IV; 44, FST V; 14, FST VI). The cohort was 47% female, and the mean age was 49.7 years (range, 15–86 years). The majority of the cohort had early-stage MF (stage IA or IB). There were more cases of SS in the SoC cohort than the non-SoC cohort (Table). Only 5 photographs had discrepancies and required discussion among the reviewers to achieve consensus.
![Frequency of morphologic features found in skin of color (SoC [Fitzpatrick skin types IV–VI]) vs non-SoC (Fitzpatrick skin types I–III) patients with mycosis fungoides/Sézary syndrome Frequency of morphologic features found in skin of color (SoC [Fitzpatrick skin types IV–VI]) vs non-SoC (Fitzpatrick skin types I–III) patients with mycosis fungoides/Sézary syndrome](https://cdn.mdedge.com/files/s3fs-public/Espinosa_1.JPG)
Regarding morphologic characteristics in active lesions (Figure 1), scale was present in almost all patients (99% in SoC, 94% in non-SoC). Erythema was present in nearly all non-SoC patients (94%) but only in 69% of SoC patients (P=.003). Poikiloderma also was found to be present at higher frequencies in non-SoC patients compared with SoC patients (19% and 4%, respectively [P=.008]). However, hyperpigmentation (80% vs 39%), lichenification (43% vs 17%), and silver hue (25% vs 3%) were more common in SoC patients than non-SoC patients (P<.05). There were no significant differences in the remaining features, including hypopigmentation (39% vs 25%), dyschromia (24% vs 19%), violaceous color (44% vs 25%), atrophy (11% vs 22%), alopecia (23% vs 31%), and ulceration (16% vs 8%) between SoC and non-SoC patients (P>.05). Photographs of MF in patients with SoC can be seen in Figure 2.
![Representative photographs of mycosis fungoides (MF) in skin of color (Fitzpatrick skin types [FSTs] IV–VI) Representative photographs of mycosis fungoides (MF) in skin of color (Fitzpatrick skin types [FSTs] IV–VI)](https://cdn.mdedge.com/files/s3fs-public/CT109003003_e_Fig2_ABCDE.JPG)
Posttreatment (nonactive) photographs were available for 26 patients (6 non-SoC, 20 SoC). We found that across all FST groups, hyperpigmentation was more common than hypopigmentation in areas of previously active disease. Statistical analysis was not completed given that few non-SoC photographs were available for comparison.
Comment
This qualitative review demonstrates the heterogeneity of MF/SS in SoC patients and that these conditions do not present in this population with the classic erythematous patches and plaques found in non-SoC patients. We found that hyperpigmentation, lichenification, and silver hue were present at higher rates in patients with FSTs IV to VI compared to those with FSTs I to III, which had higher rates of erythema and poikiloderma. Familiarity with these morphologic features along with increased exposure to clinical photographs of MF/SS in SoC patients will aid in the visual recognition required for this diagnosis, since erythema is harder to identify in darker skin types. Recognizing the unique findings of MF in patients with SoC as well as in patients with lighter skin types will enable earlier diagnosis and treatment of MF/SS across all skin types. If MF is diagnosed and treated early, life expectancy is similar to that of patients without MF.13 However, the 5-year survival rate for advanced-stage MF/SS is 52% across all skin types, and studies have found that Black patients with advanced-stage disease have worse outcomes despite accounting for demographic factors and tumor stage.14,15 Given the worse outcomes in SoC patients with advanced-stage MF/SS, earlier diagnosis could help address this disparity.8,13,14 Similar morphologic features could be used in diagnosing other inflammatory conditions; studies have shown that the lack of recognition of erythema in Black children has led to delayed diagnosis of atopic dermatitis and subsequent inadequate treatment.16,17
The morphologic presentation of MF/SS in SoC patients also can influence an optimal treatment plan for this population. Hypopigmented MF responds better to phototherapy than hyperpigmented MF, as phototherapy has been shown to have decreased efficacy with increasing FST.18 Therefore, for patients with FSTs IV to VI, topical agents such as nitrogen mustard or bexarotene may be more suitable treatment options, as the efficacy of these treatments is independent of skin color.8 However, nitrogen mustard commonly leads to postinflammatory hyperpigmentation, and topical bexarotene may lead to erythema or irritation; therefore, providers must counsel patients on these possible side effects. For refractory disease, adjunct systemic treatments such as oral bexarotene, subcutaneous interferon, methotrexate, or radiation therapy may be considered.8
In addition to aiding in the prompt diagnosis and treatment of MF/SS in SoC patients, our findings may be used to better assess the extent of disease and distinguish between active MF/SS lesions vs xerosis cutis or residual dyschromia from previously treated lesions. It is important to note that these morphologic features must be taken into account with a complete history and work-up. The differential diagnosis of MF/SS includes conditions such as atopic dermatitis, psoriasis, tinea corporis, and drug reactions, which may have similar morphology in SoC.19
Limitations of our study include the single-center design and the use of photographs instead of in-person examination; however, our cutaneous lymphoma clinic serves a diverse patient population, and our 3 reviewers rated the photographs independently. Discussion amongst the reviewers to address discrepancies was only required for 5 photographs, indicating the high inter-reviewer reliability. Additionally, the original purpose of FST was to assess for the propensity of the skin to burn when undergoing phototherapy, not to serve as a marker for skin color. We recommend trainees and clinicians be mindful about the purpose of FST and to use inclusive language (eg, using the terms skin irritation, skin tenderness, or skin becoming darker from the sun instead of tanning) when determining FST in darker-skinned individuals.20 Future directions include examining if certain treatments are associated with prolonged dyschromia.
Conclusion
In our single-institution retrospective study, we found differences in the morphologic presentation of MF/SS in SoC patients vs non-SoC patients. While erythema is a common feature in non-SoC patients, clinical features of hyperpigmentation, lichenification, and silver hue should be carefully evaluated in the diagnosis of MF/SS in SoC patients. Knowledge of the heterogenous presentation of MF/SS in patients with SoC allows for expedited diagnosis and treatment, leading to better clinical outcomes. Valuable resources, including Taylor and Kelly’s Dermatology for Skin of Color, the Skin of Color Society, and VisualDx educate providers on how dermatologic conditions present in darker skin types. However, there is still work to be done to enhance diversity in educational resources in order to provide equitable care to patients of all skin types.
Mycosis fungoides (MF) and Sézary syndrome (SS) are non-Hodgkin T-cell lymphomas that make up the majority of cutaneous T-cell lymphomas. These conditions commonly affect Black patients, with an incidence rate of 12.6 cases of cutaneous T-cell lymphomas per million individuals vs 9.8 per million individuals in non–skin of color (SoC) patients.1 However, educational resources tend to focus on the clinical manifestations of MF/SS in lighter skin types, describing MF as erythematous patches, plaques, or tumors presenting in non–sun-exposed areas of the skin and SS as generalized erythroderma.2 Skin of color, comprised of Fitzpatrick skin types (FSTs) IV to VI,3 is poorly represented across dermatology textbooks,4,5 medical student resources,6 and peer-reviewed publications,7 raising awareness for the need to address this disparity.
Skin of color patients with MF/SS display variable morphologies, including features such as hyperpigmentation and hypopigmentation,8 the latter being exceedingly rare in non-SoC patients.9 Familiarity with these differences among providers is essential to allow for equitable diagnosis and treatment across all skin types, especially in light of data predicting that by 2044 more than 50% of the US population will be people of color.10 Patients with SoC are of many ethnic and racial backgrounds, including Black, Hispanic, American Indian, Pacific Islander, and Asian.11
Along with morphologic differences, there also are several racial disparities in the prognosis and survival of patients with MF/SS. Black patients diagnosed with MF present with greater body surface area affected, and Black women with MF have reduced survival rates compared to their White counterparts.12 Given these racial disparities in survival and representation in educational resources, we aimed to quantify the frequency of various morphologic characteristics of MF/SS in patients with SoC vs non-SoC patients to facilitate better recognition of early MF/SS in SoC patients by medical providers.
Methods
We performed a retrospective chart review following approval from the institutional review board at Northwestern University (Chicago, Illinois). We identified all patients with FSTs IV to VI and biopsy-proven MF/SS who had been clinically photographed in our clinic from January 1998 to December 2019. Only photographs that were high quality enough to review morphologic features were included in our review. Fitzpatrick skin type was determined based on electronic medical record documentation. If photographs were available from multiple visits for the same patient, only those showing posttreatment nonactive lesions were included. Additionally, 36 patients with FSTs I to III (non-SoC) and biopsy-proven MF/SS were included in our review as a comparison with the SoC cohort. The primary outcomes for this study included the presence of scale, erythema, hyperpigmentation, hypopigmentation, violaceous color, lichenification, silver hue, dyschromia, alopecia, poikiloderma, atrophy, and ulceration in active lesions. Dyschromia was defined by the presence of both hypopigmentation and hyperpigmentation. Poikiloderma was defined by hypopigmentation and hyperpigmentation, telangiectasia, and atrophy. Secondary outcomes included evaluation of those same characteristics in posttreatment nonactive lesions. All photographs were independently assessed by 3 authors (M.L.E., C.J.W., J.M.M.), and discrepancies were resolved by further review of the photograph in question and discussion.
Statistical Analysis—Summary statistics were applied to describe demographic and clinical characteristics. The χ2 test was used for categorical variables. Results achieving P<.05 were considered statistically significant.

Results
We reviewed photographs of 111 patients across all skin types (8, FST I; 12, FST II; 16, FST III; 17, FST IV; 44, FST V; 14, FST VI). The cohort was 47% female, and the mean age was 49.7 years (range, 15–86 years). The majority of the cohort had early-stage MF (stage IA or IB). There were more cases of SS in the SoC cohort than the non-SoC cohort (Table). Only 5 photographs had discrepancies and required discussion among the reviewers to achieve consensus.
![Frequency of morphologic features found in skin of color (SoC [Fitzpatrick skin types IV–VI]) vs non-SoC (Fitzpatrick skin types I–III) patients with mycosis fungoides/Sézary syndrome Frequency of morphologic features found in skin of color (SoC [Fitzpatrick skin types IV–VI]) vs non-SoC (Fitzpatrick skin types I–III) patients with mycosis fungoides/Sézary syndrome](https://cdn.mdedge.com/files/s3fs-public/Espinosa_1.JPG)
Regarding morphologic characteristics in active lesions (Figure 1), scale was present in almost all patients (99% in SoC, 94% in non-SoC). Erythema was present in nearly all non-SoC patients (94%) but only in 69% of SoC patients (P=.003). Poikiloderma also was found to be present at higher frequencies in non-SoC patients compared with SoC patients (19% and 4%, respectively [P=.008]). However, hyperpigmentation (80% vs 39%), lichenification (43% vs 17%), and silver hue (25% vs 3%) were more common in SoC patients than non-SoC patients (P<.05). There were no significant differences in the remaining features, including hypopigmentation (39% vs 25%), dyschromia (24% vs 19%), violaceous color (44% vs 25%), atrophy (11% vs 22%), alopecia (23% vs 31%), and ulceration (16% vs 8%) between SoC and non-SoC patients (P>.05). Photographs of MF in patients with SoC can be seen in Figure 2.
![Representative photographs of mycosis fungoides (MF) in skin of color (Fitzpatrick skin types [FSTs] IV–VI) Representative photographs of mycosis fungoides (MF) in skin of color (Fitzpatrick skin types [FSTs] IV–VI)](https://cdn.mdedge.com/files/s3fs-public/CT109003003_e_Fig2_ABCDE.JPG)
Posttreatment (nonactive) photographs were available for 26 patients (6 non-SoC, 20 SoC). We found that across all FST groups, hyperpigmentation was more common than hypopigmentation in areas of previously active disease. Statistical analysis was not completed given that few non-SoC photographs were available for comparison.
Comment
This qualitative review demonstrates the heterogeneity of MF/SS in SoC patients and that these conditions do not present in this population with the classic erythematous patches and plaques found in non-SoC patients. We found that hyperpigmentation, lichenification, and silver hue were present at higher rates in patients with FSTs IV to VI compared to those with FSTs I to III, which had higher rates of erythema and poikiloderma. Familiarity with these morphologic features along with increased exposure to clinical photographs of MF/SS in SoC patients will aid in the visual recognition required for this diagnosis, since erythema is harder to identify in darker skin types. Recognizing the unique findings of MF in patients with SoC as well as in patients with lighter skin types will enable earlier diagnosis and treatment of MF/SS across all skin types. If MF is diagnosed and treated early, life expectancy is similar to that of patients without MF.13 However, the 5-year survival rate for advanced-stage MF/SS is 52% across all skin types, and studies have found that Black patients with advanced-stage disease have worse outcomes despite accounting for demographic factors and tumor stage.14,15 Given the worse outcomes in SoC patients with advanced-stage MF/SS, earlier diagnosis could help address this disparity.8,13,14 Similar morphologic features could be used in diagnosing other inflammatory conditions; studies have shown that the lack of recognition of erythema in Black children has led to delayed diagnosis of atopic dermatitis and subsequent inadequate treatment.16,17
The morphologic presentation of MF/SS in SoC patients also can influence an optimal treatment plan for this population. Hypopigmented MF responds better to phototherapy than hyperpigmented MF, as phototherapy has been shown to have decreased efficacy with increasing FST.18 Therefore, for patients with FSTs IV to VI, topical agents such as nitrogen mustard or bexarotene may be more suitable treatment options, as the efficacy of these treatments is independent of skin color.8 However, nitrogen mustard commonly leads to postinflammatory hyperpigmentation, and topical bexarotene may lead to erythema or irritation; therefore, providers must counsel patients on these possible side effects. For refractory disease, adjunct systemic treatments such as oral bexarotene, subcutaneous interferon, methotrexate, or radiation therapy may be considered.8
In addition to aiding in the prompt diagnosis and treatment of MF/SS in SoC patients, our findings may be used to better assess the extent of disease and distinguish between active MF/SS lesions vs xerosis cutis or residual dyschromia from previously treated lesions. It is important to note that these morphologic features must be taken into account with a complete history and work-up. The differential diagnosis of MF/SS includes conditions such as atopic dermatitis, psoriasis, tinea corporis, and drug reactions, which may have similar morphology in SoC.19
Limitations of our study include the single-center design and the use of photographs instead of in-person examination; however, our cutaneous lymphoma clinic serves a diverse patient population, and our 3 reviewers rated the photographs independently. Discussion amongst the reviewers to address discrepancies was only required for 5 photographs, indicating the high inter-reviewer reliability. Additionally, the original purpose of FST was to assess for the propensity of the skin to burn when undergoing phototherapy, not to serve as a marker for skin color. We recommend trainees and clinicians be mindful about the purpose of FST and to use inclusive language (eg, using the terms skin irritation, skin tenderness, or skin becoming darker from the sun instead of tanning) when determining FST in darker-skinned individuals.20 Future directions include examining if certain treatments are associated with prolonged dyschromia.
Conclusion
In our single-institution retrospective study, we found differences in the morphologic presentation of MF/SS in SoC patients vs non-SoC patients. While erythema is a common feature in non-SoC patients, clinical features of hyperpigmentation, lichenification, and silver hue should be carefully evaluated in the diagnosis of MF/SS in SoC patients. Knowledge of the heterogenous presentation of MF/SS in patients with SoC allows for expedited diagnosis and treatment, leading to better clinical outcomes. Valuable resources, including Taylor and Kelly’s Dermatology for Skin of Color, the Skin of Color Society, and VisualDx educate providers on how dermatologic conditions present in darker skin types. However, there is still work to be done to enhance diversity in educational resources in order to provide equitable care to patients of all skin types.
- Korgavkar K, Xiong M, Weinstock M. Changing incidence trends of cutaneous T-cell lymphoma. JAMA Dermatol. 2013;149:1295-1299. doi:10.1001/jamadermatol.2013.5526
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.E1-E16; quiz 221-222. doi:10.1016/j.jaad.2013.07.049
- Tull RZ, Kerby E, Subash JJ, et al. Ethnic skin centers in the United States: where are we in 2020?. J Am Acad Dermatol. 2020;83:1757-1759. doi:10.1016/j.jaad.2020.03.054
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196. doi:10.1016/j.jaad.2020.04.084
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Jones VA, Clark KA, Shobajo MT, et al. Skin of color representation in medical education: an analysis of popular preparatory materials used for United States medical licensing examinations. J Am Acad Dermatol. 2021;85:773-775. doi:10.1016/j.jaad.2020.07.112
- Montgomery SN, Elbuluk N. A quantitative analysis of research publications focused on the top chief complaints in skin of color patients. J Am Acad Dermatol. 2021;85:241-242. doi:10.1016/j.jaad.2020.08.031
- Hinds GA, Heald P. Cutaneous T-cell lymphoma in skin of color. J Am Acad Dermatol. 2009;60:359-375; quiz 376-378. doi:10.1016/j.jaad.2008.10.031
- Ardigó M, Borroni G, Muscardin L, et al. Hypopigmented mycosis fungoides in Caucasian patients: a clinicopathologic study of 7 cases. J Am Acad Dermatol. 2003;49:264-270. doi:10.1067/s0190-9622(03)00907-1
- Colby SL, Ortman JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. Updated October 8, 2021. Accessed February 28, 2022. https://www.census.gov/library/publications/2015/demo/p25-1143.html
- Taylor SC, Kyei A. Defining skin of color. In: Kelly AP, Taylor SC, Lim HW, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Huang AH, Kwatra SG, Khanna R, et al. Racial disparities in the clinical presentation and prognosis of patients with mycosis fungoides. J Natl Med Assoc. 2019;111:633-639. doi:10.1016/j.jnma.2019.08.006
- Kim YH, Jensen RA, Watanabe GL, et al. Clinical stage IA (limited patch and plaque) mycosis fungoides. a long-term outcome analysis. Arch Dermatol. 1996;132:1309-1313.
- Scarisbrick JJ, Prince HM, Vermeer MH, et al. Cutaneous lymphoma international consortium study of outcome in advanced stages of mycosis fungoides and Sézary syndrome: effect of specific prognostic markers on survival and development of a prognostic model. J Clin Oncol. 2015;33:3766-3773. doi:10.1200/JCO.2015.61.7142
- Nath SK, Yu JB, Wilson LD. Poorer prognosis of African-American patients with mycosis fungoides: an analysis of the SEER dataset, 1988 to 2008. Clin Lymphoma Myeloma Leuk. 2014;14:419-423. doi:10.1016/j.clml.2013.12.018
- Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol. 2002;147:920-925. doi:10.1046/j.1365-2133.2002.04965.x
- Poladian K, De Souza B, McMichael AJ. Atopic dermatitis in adolescents with skin of color. Cutis. 2019;104:164-168.
- Yones SS, Palmer RA, Garibaldinos TT, et al. Randomized double-blind trial of the treatment of chronic plaque psoriasis: efficacy of psoralen-UV-A therapy vs narrowband UV-B therapy. Arch Dermatol. 2006;142:836-842. doi:10.1001/archderm.142.7.836
- Currimbhoy S, Pandya AG. Cutaneous T-cell lymphoma. In: Kelly AP, Taylor SC, Lim HW, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
- Korgavkar K, Xiong M, Weinstock M. Changing incidence trends of cutaneous T-cell lymphoma. JAMA Dermatol. 2013;149:1295-1299. doi:10.1001/jamadermatol.2013.5526
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.E1-E16; quiz 221-222. doi:10.1016/j.jaad.2013.07.049
- Tull RZ, Kerby E, Subash JJ, et al. Ethnic skin centers in the United States: where are we in 2020?. J Am Acad Dermatol. 2020;83:1757-1759. doi:10.1016/j.jaad.2020.03.054
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196. doi:10.1016/j.jaad.2020.04.084
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Jones VA, Clark KA, Shobajo MT, et al. Skin of color representation in medical education: an analysis of popular preparatory materials used for United States medical licensing examinations. J Am Acad Dermatol. 2021;85:773-775. doi:10.1016/j.jaad.2020.07.112
- Montgomery SN, Elbuluk N. A quantitative analysis of research publications focused on the top chief complaints in skin of color patients. J Am Acad Dermatol. 2021;85:241-242. doi:10.1016/j.jaad.2020.08.031
- Hinds GA, Heald P. Cutaneous T-cell lymphoma in skin of color. J Am Acad Dermatol. 2009;60:359-375; quiz 376-378. doi:10.1016/j.jaad.2008.10.031
- Ardigó M, Borroni G, Muscardin L, et al. Hypopigmented mycosis fungoides in Caucasian patients: a clinicopathologic study of 7 cases. J Am Acad Dermatol. 2003;49:264-270. doi:10.1067/s0190-9622(03)00907-1
- Colby SL, Ortman JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. Updated October 8, 2021. Accessed February 28, 2022. https://www.census.gov/library/publications/2015/demo/p25-1143.html
- Taylor SC, Kyei A. Defining skin of color. In: Kelly AP, Taylor SC, Lim HW, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Huang AH, Kwatra SG, Khanna R, et al. Racial disparities in the clinical presentation and prognosis of patients with mycosis fungoides. J Natl Med Assoc. 2019;111:633-639. doi:10.1016/j.jnma.2019.08.006
- Kim YH, Jensen RA, Watanabe GL, et al. Clinical stage IA (limited patch and plaque) mycosis fungoides. a long-term outcome analysis. Arch Dermatol. 1996;132:1309-1313.
- Scarisbrick JJ, Prince HM, Vermeer MH, et al. Cutaneous lymphoma international consortium study of outcome in advanced stages of mycosis fungoides and Sézary syndrome: effect of specific prognostic markers on survival and development of a prognostic model. J Clin Oncol. 2015;33:3766-3773. doi:10.1200/JCO.2015.61.7142
- Nath SK, Yu JB, Wilson LD. Poorer prognosis of African-American patients with mycosis fungoides: an analysis of the SEER dataset, 1988 to 2008. Clin Lymphoma Myeloma Leuk. 2014;14:419-423. doi:10.1016/j.clml.2013.12.018
- Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol. 2002;147:920-925. doi:10.1046/j.1365-2133.2002.04965.x
- Poladian K, De Souza B, McMichael AJ. Atopic dermatitis in adolescents with skin of color. Cutis. 2019;104:164-168.
- Yones SS, Palmer RA, Garibaldinos TT, et al. Randomized double-blind trial of the treatment of chronic plaque psoriasis: efficacy of psoralen-UV-A therapy vs narrowband UV-B therapy. Arch Dermatol. 2006;142:836-842. doi:10.1001/archderm.142.7.836
- Currimbhoy S, Pandya AG. Cutaneous T-cell lymphoma. In: Kelly AP, Taylor SC, Lim HW, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
Practice Points
- Dermatologists should be familiar with the variable morphology of mycosis fungoides (MF)/Sézary syndrome (SS) exhibited by patients of all skin types to ensure prompt diagnosis and treatment.
- Patients with skin of color (SoC)(Fitzpatrick skin types IV–VI) with MF/SS are more likely than non-SoC patients (Fitzpatrick skin types I–III) to present with hyperpigmentation, a silver hue, and lichenification, whereas non-SoC patients commonly present with erythema and poikiloderma.
Many rheumatologists in Ukraine become refugees amid chaos
On the morning of Feb. 24, rheumatologist Olena Garmish woke at 5:50 a.m. from the blasts of rocket fire in Kiev, Ukraine, and saw the explosions through her window
She described that next week to this news organization: air sirens 20 hours a day, fearing death 24 hours a day, and growing food shortages.
Dr. Garmish, executive director of the Association of Rheumatologists of Ukraine, said she continued working at a Kiev hospital until March 4, but then had to leave the country with her children and has traveled to two other countries since. Now she is looking for employment abroad after 22 years as a clinical researcher and practitioner.
“We lost our jobs and rheumatology practice,” she said. Now, she says, she provides online consultations to patients as much as she can.
As air strikes continued Tuesday in Ukraine’s capital city and elsewhere throughout the country, rheumatologists are among citizens forced to upend their personal and professional lives and make the best decisions they can to keep themselves and their families safe.
Roman Yatsyshyn, MD, professor at Ivano-Frankivsk National Medical University in Ivano-Frankivsk, Ukraine, and vice president of the Association of Rheumatologists of Ukraine, told this news organization that many rheumatologists, like Dr. Garmish, have been forced to close their practices and flee the country. The hope is that the moves are temporary, he said.
He said rheumatologists there are having very different experiences depending on their proximity to the shelling.
Dmytro Rekalov, MD, PhD, who has been a practicing rheumatologist for 20 years, said he has had to relocate – he hopes temporarily – to western Ukraine.
He told this news organization that the battles are about 40 km (25 miles) from him.
“I have a small private rheumatology clinic in Zaporizhzhia [in southeastern Ukraine], so if they invade our city, I’ll have to close my clinic and find another place to live and to practice in.” Zaporizhzhia is home to the largest nuclear plant in Europe, a facility that came under attack earlier this month.
Doctors from areas under siege have been forced to move to quieter locations and consult with patients remotely, Dr. Yatsyshyn said.
“Moreover, all doctors are actively volunteering, helping refugees, and supporting our military at the front,” he said, adding that medications are in short supply.
“We express our sincere gratitude to the world and European medical communities for their help for Ukraine at this time. Medicines and medical devices come to Ukraine from many countries around the world every day,” he said.
Dr. Yatsyshyn said the Ministry of Health of Ukraine is coordinating delivery of medications.
“However, there is still a need for an uninterrupted supply of basic antirheumatic drugs, cytostatics, glucocorticosteroids, analgesics, and nonsteroidal anti-inflammatory drugs. We will be grateful if such help will continue to come from our colleagues,” Dr. Yatsyshyn said.
In most cases, he says, rheumatologists stay in touch with their patients via social media and apps, Skype, and Zoom.
“We have also created professional and patient groups in chat rooms,” he said. “There, we can respond quickly to current issues in different regions. If necessary, we send medicines in case of their absence or danger in certain regions of the country. Rheumatologists have set up a joint group for online counseling and exchange.”
Some rheumatologists have been retrained as emergency physicians, he said. In areas with less military activity, rheumatologists continue to treat patients at their practices. In places where it is relatively calm, rheumatologists consult not only local patients but also migrants from other regions affected by the war, Dr. Yatsyshyn explained.
The Association of Rheumatologists of Ukraine continues its activities, he said.
“We monitor the problems of our colleagues, their relocations, security, and the opportunity to work. In close cooperation with the Ministry of Health, we monitor the provision of necessary medicines to our patients. We are very grateful for the help of our colleagues from European associations, the United States, pharmaceutical companies, medical centers, universities, and volunteer organizations.”
“We have two other big requests to the entire medical and scientific community,” Dr. Yatsyshyn said. “To suspend the membership of all Russian medical communities in European and world associations (including EULAR, EUSTAR, Lupus Academy, ACR, British Society of Rheumatology, and others) with a ban on attending international forums just as almost all sports and art organizations in Europe and the civilized world have done.”
The second request, he said, is “to close the sky over Ukraine to stop killing children, civilians, destroying Ukrainian memories, and to destroy Ukrainians as a nation. We pray for this to all the conscious world.”
EULAR, the European Alliance of Associations for Rheumatology, said in a statement, “EULAR has stood for peace in Europe and globally, and for improving the lives of people with rheumatic and musculoskeletal diseases, for 75 years. We are committed to the tradition of humanity and peace and are deeply concerned about the general situation of the people in Ukraine. We will do our utmost to contribute to alleviate the suffering. To this end we are urgently exploring options together with other biomedical partners. Please also help to support the people in Ukraine, for example by donating to UNHCR (the UN refugee agency) or ICRC (International Committee of the Red Cross).
A version of this article first appeared on Medscape.com.
On the morning of Feb. 24, rheumatologist Olena Garmish woke at 5:50 a.m. from the blasts of rocket fire in Kiev, Ukraine, and saw the explosions through her window
She described that next week to this news organization: air sirens 20 hours a day, fearing death 24 hours a day, and growing food shortages.
Dr. Garmish, executive director of the Association of Rheumatologists of Ukraine, said she continued working at a Kiev hospital until March 4, but then had to leave the country with her children and has traveled to two other countries since. Now she is looking for employment abroad after 22 years as a clinical researcher and practitioner.
“We lost our jobs and rheumatology practice,” she said. Now, she says, she provides online consultations to patients as much as she can.
As air strikes continued Tuesday in Ukraine’s capital city and elsewhere throughout the country, rheumatologists are among citizens forced to upend their personal and professional lives and make the best decisions they can to keep themselves and their families safe.
Roman Yatsyshyn, MD, professor at Ivano-Frankivsk National Medical University in Ivano-Frankivsk, Ukraine, and vice president of the Association of Rheumatologists of Ukraine, told this news organization that many rheumatologists, like Dr. Garmish, have been forced to close their practices and flee the country. The hope is that the moves are temporary, he said.
He said rheumatologists there are having very different experiences depending on their proximity to the shelling.
Dmytro Rekalov, MD, PhD, who has been a practicing rheumatologist for 20 years, said he has had to relocate – he hopes temporarily – to western Ukraine.
He told this news organization that the battles are about 40 km (25 miles) from him.
“I have a small private rheumatology clinic in Zaporizhzhia [in southeastern Ukraine], so if they invade our city, I’ll have to close my clinic and find another place to live and to practice in.” Zaporizhzhia is home to the largest nuclear plant in Europe, a facility that came under attack earlier this month.
Doctors from areas under siege have been forced to move to quieter locations and consult with patients remotely, Dr. Yatsyshyn said.
“Moreover, all doctors are actively volunteering, helping refugees, and supporting our military at the front,” he said, adding that medications are in short supply.
“We express our sincere gratitude to the world and European medical communities for their help for Ukraine at this time. Medicines and medical devices come to Ukraine from many countries around the world every day,” he said.
Dr. Yatsyshyn said the Ministry of Health of Ukraine is coordinating delivery of medications.
“However, there is still a need for an uninterrupted supply of basic antirheumatic drugs, cytostatics, glucocorticosteroids, analgesics, and nonsteroidal anti-inflammatory drugs. We will be grateful if such help will continue to come from our colleagues,” Dr. Yatsyshyn said.
In most cases, he says, rheumatologists stay in touch with their patients via social media and apps, Skype, and Zoom.
“We have also created professional and patient groups in chat rooms,” he said. “There, we can respond quickly to current issues in different regions. If necessary, we send medicines in case of their absence or danger in certain regions of the country. Rheumatologists have set up a joint group for online counseling and exchange.”
Some rheumatologists have been retrained as emergency physicians, he said. In areas with less military activity, rheumatologists continue to treat patients at their practices. In places where it is relatively calm, rheumatologists consult not only local patients but also migrants from other regions affected by the war, Dr. Yatsyshyn explained.
The Association of Rheumatologists of Ukraine continues its activities, he said.
“We monitor the problems of our colleagues, their relocations, security, and the opportunity to work. In close cooperation with the Ministry of Health, we monitor the provision of necessary medicines to our patients. We are very grateful for the help of our colleagues from European associations, the United States, pharmaceutical companies, medical centers, universities, and volunteer organizations.”
“We have two other big requests to the entire medical and scientific community,” Dr. Yatsyshyn said. “To suspend the membership of all Russian medical communities in European and world associations (including EULAR, EUSTAR, Lupus Academy, ACR, British Society of Rheumatology, and others) with a ban on attending international forums just as almost all sports and art organizations in Europe and the civilized world have done.”
The second request, he said, is “to close the sky over Ukraine to stop killing children, civilians, destroying Ukrainian memories, and to destroy Ukrainians as a nation. We pray for this to all the conscious world.”
EULAR, the European Alliance of Associations for Rheumatology, said in a statement, “EULAR has stood for peace in Europe and globally, and for improving the lives of people with rheumatic and musculoskeletal diseases, for 75 years. We are committed to the tradition of humanity and peace and are deeply concerned about the general situation of the people in Ukraine. We will do our utmost to contribute to alleviate the suffering. To this end we are urgently exploring options together with other biomedical partners. Please also help to support the people in Ukraine, for example by donating to UNHCR (the UN refugee agency) or ICRC (International Committee of the Red Cross).
A version of this article first appeared on Medscape.com.
On the morning of Feb. 24, rheumatologist Olena Garmish woke at 5:50 a.m. from the blasts of rocket fire in Kiev, Ukraine, and saw the explosions through her window
She described that next week to this news organization: air sirens 20 hours a day, fearing death 24 hours a day, and growing food shortages.
Dr. Garmish, executive director of the Association of Rheumatologists of Ukraine, said she continued working at a Kiev hospital until March 4, but then had to leave the country with her children and has traveled to two other countries since. Now she is looking for employment abroad after 22 years as a clinical researcher and practitioner.
“We lost our jobs and rheumatology practice,” she said. Now, she says, she provides online consultations to patients as much as she can.
As air strikes continued Tuesday in Ukraine’s capital city and elsewhere throughout the country, rheumatologists are among citizens forced to upend their personal and professional lives and make the best decisions they can to keep themselves and their families safe.
Roman Yatsyshyn, MD, professor at Ivano-Frankivsk National Medical University in Ivano-Frankivsk, Ukraine, and vice president of the Association of Rheumatologists of Ukraine, told this news organization that many rheumatologists, like Dr. Garmish, have been forced to close their practices and flee the country. The hope is that the moves are temporary, he said.
He said rheumatologists there are having very different experiences depending on their proximity to the shelling.
Dmytro Rekalov, MD, PhD, who has been a practicing rheumatologist for 20 years, said he has had to relocate – he hopes temporarily – to western Ukraine.
He told this news organization that the battles are about 40 km (25 miles) from him.
“I have a small private rheumatology clinic in Zaporizhzhia [in southeastern Ukraine], so if they invade our city, I’ll have to close my clinic and find another place to live and to practice in.” Zaporizhzhia is home to the largest nuclear plant in Europe, a facility that came under attack earlier this month.
Doctors from areas under siege have been forced to move to quieter locations and consult with patients remotely, Dr. Yatsyshyn said.
“Moreover, all doctors are actively volunteering, helping refugees, and supporting our military at the front,” he said, adding that medications are in short supply.
“We express our sincere gratitude to the world and European medical communities for their help for Ukraine at this time. Medicines and medical devices come to Ukraine from many countries around the world every day,” he said.
Dr. Yatsyshyn said the Ministry of Health of Ukraine is coordinating delivery of medications.
“However, there is still a need for an uninterrupted supply of basic antirheumatic drugs, cytostatics, glucocorticosteroids, analgesics, and nonsteroidal anti-inflammatory drugs. We will be grateful if such help will continue to come from our colleagues,” Dr. Yatsyshyn said.
In most cases, he says, rheumatologists stay in touch with their patients via social media and apps, Skype, and Zoom.
“We have also created professional and patient groups in chat rooms,” he said. “There, we can respond quickly to current issues in different regions. If necessary, we send medicines in case of their absence or danger in certain regions of the country. Rheumatologists have set up a joint group for online counseling and exchange.”
Some rheumatologists have been retrained as emergency physicians, he said. In areas with less military activity, rheumatologists continue to treat patients at their practices. In places where it is relatively calm, rheumatologists consult not only local patients but also migrants from other regions affected by the war, Dr. Yatsyshyn explained.
The Association of Rheumatologists of Ukraine continues its activities, he said.
“We monitor the problems of our colleagues, their relocations, security, and the opportunity to work. In close cooperation with the Ministry of Health, we monitor the provision of necessary medicines to our patients. We are very grateful for the help of our colleagues from European associations, the United States, pharmaceutical companies, medical centers, universities, and volunteer organizations.”
“We have two other big requests to the entire medical and scientific community,” Dr. Yatsyshyn said. “To suspend the membership of all Russian medical communities in European and world associations (including EULAR, EUSTAR, Lupus Academy, ACR, British Society of Rheumatology, and others) with a ban on attending international forums just as almost all sports and art organizations in Europe and the civilized world have done.”
The second request, he said, is “to close the sky over Ukraine to stop killing children, civilians, destroying Ukrainian memories, and to destroy Ukrainians as a nation. We pray for this to all the conscious world.”
EULAR, the European Alliance of Associations for Rheumatology, said in a statement, “EULAR has stood for peace in Europe and globally, and for improving the lives of people with rheumatic and musculoskeletal diseases, for 75 years. We are committed to the tradition of humanity and peace and are deeply concerned about the general situation of the people in Ukraine. We will do our utmost to contribute to alleviate the suffering. To this end we are urgently exploring options together with other biomedical partners. Please also help to support the people in Ukraine, for example by donating to UNHCR (the UN refugee agency) or ICRC (International Committee of the Red Cross).
A version of this article first appeared on Medscape.com.
Lung cancer with ILD patients fare poorly after thoracic radiotherapy
Most lung cancer patients with interstitial lung disease will not benefit from thoracic radiotherapy, based on data from a systematic review of 24 studies.
Thoracic radiotherapy remains a key part of lung cancer treatment for early and metastatic disease. However, patients with both small cell lung cancer (SCLC) and non–small cell lung cancer (NSCLC) associated with interstitial lung disease (ILD) fare worse than do those without ILD, often because of acute exacerbation of ILD and severe or fatal pneumonitis, wrote Animesh Saha, MD, of Apollo Multi-Specialty Hospitals, Kolkata, India, and colleagues. Consequently, clinicians may hesitate to offer radiotherapy to these patients.
In a review published in Clinical Oncology, the researchers identified 24 studies, including phase II and phase III randomized or nonrandomized trials, as well as prospective, observational studies and retrospective real-world studies. The goal of the review was to report the incidence and predictors of radiation pneumonitis associated with different types of thoracic radiotherapy for lung cancer patients with ILD, the researchers said. Treatment types included curative-intent fractionated radiotherapy or chemoradiotherapy or moderately hypofractionated (nonstereotactic ablative radiotherapy [SABR]) and hyperfractionated radiotherapy as well as particle beam therapies.
The studies included patients with SCLC or NSCLC and any form of ILD, including subclinical, radiologically diagnosed, or symptomatic, the researchers said.
Overall, the median incidence of grade 3 or higher radiation pneumonitis was 19.7%; the median incidence in patients treated with conventional radical radiotherapy, SABR, and particle beam therapy was 31.8%, 11.9%, and 20.25%, respectively.
Eighteen studies reported grade 5 radiation pneumonitis; the overall median incidence was 6%, but as high as 60% in some studies. When separated by treatment type, the median incidence was 2.7%, 6.25%, and 6.25%, respectively, in patients treated with radical radiotherapy (non-SABR), SABR, and particle beam therapy.
Independent predictors of severe radiation pneumonitis (grade 2 or higher and grade 3 or higher) included subclinical or radiological ILD, the researchers said. Among ILD subtypes, studies have shown increased risk for severe radiation pneumonitis among those with non-IPF or non-UIP pattern fibrosis.
In addition, patient-related factors of low forced vital capacity (FVC) and low forced expiratory volume in 1 second (FEV1), have been associated with severe radiation pneumonitis, the researchers said. They also found increased risk for patients with lower lobe tumor location compared to other lobes.
As for treatment-related factors, a history of gemcitabine chemotherapy was associated with an increased risk of grade 3 or higher radiation pneumonitis.
“There is always concern about using thoracic radiotherapy in lung cancer patients with coexisting ILD in view of the risks involved,” the researchers wrote in their discussion of the findings. “Although thoracic radiotherapy is expected to produce similar local control, overall survival is worse in lung cancer patients with ILD than without, probably due to the poor prognosis associated with ILD and associated treatment-related mortality,” they said.
The findings were limited by several factors including the heterogeneity of the studies and study population and the retrospective design of most of the studies, the researchers noted.
However, the results highlight the increased risk of severe and fatal radiation pneumonitis in lung cancer patients with ILD and the need for careful patient selection and counseling if thoracic radiotherapy is to be considered, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Most lung cancer patients with interstitial lung disease will not benefit from thoracic radiotherapy, based on data from a systematic review of 24 studies.
Thoracic radiotherapy remains a key part of lung cancer treatment for early and metastatic disease. However, patients with both small cell lung cancer (SCLC) and non–small cell lung cancer (NSCLC) associated with interstitial lung disease (ILD) fare worse than do those without ILD, often because of acute exacerbation of ILD and severe or fatal pneumonitis, wrote Animesh Saha, MD, of Apollo Multi-Specialty Hospitals, Kolkata, India, and colleagues. Consequently, clinicians may hesitate to offer radiotherapy to these patients.
In a review published in Clinical Oncology, the researchers identified 24 studies, including phase II and phase III randomized or nonrandomized trials, as well as prospective, observational studies and retrospective real-world studies. The goal of the review was to report the incidence and predictors of radiation pneumonitis associated with different types of thoracic radiotherapy for lung cancer patients with ILD, the researchers said. Treatment types included curative-intent fractionated radiotherapy or chemoradiotherapy or moderately hypofractionated (nonstereotactic ablative radiotherapy [SABR]) and hyperfractionated radiotherapy as well as particle beam therapies.
The studies included patients with SCLC or NSCLC and any form of ILD, including subclinical, radiologically diagnosed, or symptomatic, the researchers said.
Overall, the median incidence of grade 3 or higher radiation pneumonitis was 19.7%; the median incidence in patients treated with conventional radical radiotherapy, SABR, and particle beam therapy was 31.8%, 11.9%, and 20.25%, respectively.
Eighteen studies reported grade 5 radiation pneumonitis; the overall median incidence was 6%, but as high as 60% in some studies. When separated by treatment type, the median incidence was 2.7%, 6.25%, and 6.25%, respectively, in patients treated with radical radiotherapy (non-SABR), SABR, and particle beam therapy.
Independent predictors of severe radiation pneumonitis (grade 2 or higher and grade 3 or higher) included subclinical or radiological ILD, the researchers said. Among ILD subtypes, studies have shown increased risk for severe radiation pneumonitis among those with non-IPF or non-UIP pattern fibrosis.
In addition, patient-related factors of low forced vital capacity (FVC) and low forced expiratory volume in 1 second (FEV1), have been associated with severe radiation pneumonitis, the researchers said. They also found increased risk for patients with lower lobe tumor location compared to other lobes.
As for treatment-related factors, a history of gemcitabine chemotherapy was associated with an increased risk of grade 3 or higher radiation pneumonitis.
“There is always concern about using thoracic radiotherapy in lung cancer patients with coexisting ILD in view of the risks involved,” the researchers wrote in their discussion of the findings. “Although thoracic radiotherapy is expected to produce similar local control, overall survival is worse in lung cancer patients with ILD than without, probably due to the poor prognosis associated with ILD and associated treatment-related mortality,” they said.
The findings were limited by several factors including the heterogeneity of the studies and study population and the retrospective design of most of the studies, the researchers noted.
However, the results highlight the increased risk of severe and fatal radiation pneumonitis in lung cancer patients with ILD and the need for careful patient selection and counseling if thoracic radiotherapy is to be considered, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Most lung cancer patients with interstitial lung disease will not benefit from thoracic radiotherapy, based on data from a systematic review of 24 studies.
Thoracic radiotherapy remains a key part of lung cancer treatment for early and metastatic disease. However, patients with both small cell lung cancer (SCLC) and non–small cell lung cancer (NSCLC) associated with interstitial lung disease (ILD) fare worse than do those without ILD, often because of acute exacerbation of ILD and severe or fatal pneumonitis, wrote Animesh Saha, MD, of Apollo Multi-Specialty Hospitals, Kolkata, India, and colleagues. Consequently, clinicians may hesitate to offer radiotherapy to these patients.
In a review published in Clinical Oncology, the researchers identified 24 studies, including phase II and phase III randomized or nonrandomized trials, as well as prospective, observational studies and retrospective real-world studies. The goal of the review was to report the incidence and predictors of radiation pneumonitis associated with different types of thoracic radiotherapy for lung cancer patients with ILD, the researchers said. Treatment types included curative-intent fractionated radiotherapy or chemoradiotherapy or moderately hypofractionated (nonstereotactic ablative radiotherapy [SABR]) and hyperfractionated radiotherapy as well as particle beam therapies.
The studies included patients with SCLC or NSCLC and any form of ILD, including subclinical, radiologically diagnosed, or symptomatic, the researchers said.
Overall, the median incidence of grade 3 or higher radiation pneumonitis was 19.7%; the median incidence in patients treated with conventional radical radiotherapy, SABR, and particle beam therapy was 31.8%, 11.9%, and 20.25%, respectively.
Eighteen studies reported grade 5 radiation pneumonitis; the overall median incidence was 6%, but as high as 60% in some studies. When separated by treatment type, the median incidence was 2.7%, 6.25%, and 6.25%, respectively, in patients treated with radical radiotherapy (non-SABR), SABR, and particle beam therapy.
Independent predictors of severe radiation pneumonitis (grade 2 or higher and grade 3 or higher) included subclinical or radiological ILD, the researchers said. Among ILD subtypes, studies have shown increased risk for severe radiation pneumonitis among those with non-IPF or non-UIP pattern fibrosis.
In addition, patient-related factors of low forced vital capacity (FVC) and low forced expiratory volume in 1 second (FEV1), have been associated with severe radiation pneumonitis, the researchers said. They also found increased risk for patients with lower lobe tumor location compared to other lobes.
As for treatment-related factors, a history of gemcitabine chemotherapy was associated with an increased risk of grade 3 or higher radiation pneumonitis.
“There is always concern about using thoracic radiotherapy in lung cancer patients with coexisting ILD in view of the risks involved,” the researchers wrote in their discussion of the findings. “Although thoracic radiotherapy is expected to produce similar local control, overall survival is worse in lung cancer patients with ILD than without, probably due to the poor prognosis associated with ILD and associated treatment-related mortality,” they said.
The findings were limited by several factors including the heterogeneity of the studies and study population and the retrospective design of most of the studies, the researchers noted.
However, the results highlight the increased risk of severe and fatal radiation pneumonitis in lung cancer patients with ILD and the need for careful patient selection and counseling if thoracic radiotherapy is to be considered, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM CLINICAL ONCOLOGY
Early MS biomarkers may improve prediction of long-term outcomes
WEST PALM BEACH, FL – , new research suggests.
The research shows that once standard clinical models can be incorporated into practice, the early measurement of these biomarkers will provide useful information in predicting who may be at risk of poorer outcomes, researcher Gauruv Bose, MD, Brigham Multiple Sclerosis Center, Ann Romney Center for Neurologic Diseases, Brigham and Women’s Hospital, Harvard Medical School, Boston, told this news organization.
The findings were presented at annual meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS).
Better together?
Although higher baseline sNfL levels in MS have previously been linked to greater brain atrophy and other long-term outcomes, and sGFAP changes are also associated with inflammation and damage through the disease course, less is known about longer-term effects of the two biomarker measures combined, Dr. Bose said.
“The value of using both sNfL and sGFAP in predictive models is of interest, since one correlates with neuroaxonal damage, while the other has correlated with astrocytic glial damage/cell turnover – potentially, though differently, reflecting inflammatory damage and neurodegeneration,” he added.
To investigate the relationship, the researchers evaluated patients with MS enrolled at the Brigham Multiple Sclerosis Center. All underwent neurologic examinations every 6 months, and MRI scans and blood samples were collected every year. Some had more than 20 years of follow-up.
The first study involved 144 patients (mean age, 37.4 years) from whom two samples of sNfL and sGFAP were collected within 3 years of MS onset.
The median baseline sNfL level was 10.7 pg/mL, and 50 patients (34.7%) already showed increases in sNfL at the 1-year follow-up. Their median sGFAP level at onset was 96 pg/mL, and 59 patients (41%) showed increases in sGFAP at the 1-year follow-up.
Results showed that higher baseline sNfL levels were significantly associated with increased risk for MS relapse at 10 years (hazard ratio, 1.34; P = .04), as well as with the development of new MRI lesions (HR, 1.35; P = .022).
Of the study group, 25 (17.4%) developed secondary progressive MS (SPMS) by the 10-year follow-up. For those prognostic assessments, the investigators compared utilization of a model using well-established clinical predictors of SPMS with and without the inclusion of sNfL and sGFAP.
The clinical model included key factors such as age, sex, body mass index, Extended Disability Status Scale (EDSS), timed 25-foot walk, and other measures.
The researchers found the clinical model alone predicted 10-year outcomes with an area under the receiver operating characteristic curve (AUC) of 0.75. However, with the addition of baseline sNfL and sGFAP measures, the AUC was improved to 0.79 (P = .0008).
Furthermore, the inclusion of additional follow-up sNfL and sGFAP measurements taken after baseline further improved the model’s AUC (0.82; P = .046).
The addition of the sNfL and sGFAP measures to the clinical models also improved the prediction of disability in MS at 10 years on EDSS (P = .068), as well as prediction of 10-year brain T2 lesion volume (P = .009) and brain parenchymal fraction (P = .04).
Relapse predictor?
In the second study, Dr. Bose and colleagues evaluated the role of the two serum measures in predicting relapse after disease-modifying therapy (DMT) discontinuation. That study included 42 patients who discontinued DMT treatment after having been disease-activity free for 2 years while on the drugs. They were compared with 36 patients who had similar characteristics and had continued DMT treatment.
All patients (mean age, 44.5 years) had a mean of 7.4 years since prior disease activity.
Increases in sNfL following DMT discontinuation, but not before, were associated with a significantly greater risk for clinical disease worsening at a mean follow-up of 7.5 years (HR, 9.4; P = .007). Change in sGFAP was associated with new MRI lesions (HR, 8.3; P = .039), compared with no changes.
“The crux of this study” was that patients with increased biomarker levels after stopping DMTs “were at a significantly higher risk for disease activity in the future compared to those whose biomarker levels remained stable,” Dr. Bose noted.
“We think this finding, if replicated in another cohort, has the potential to be included in guidelines regarding stopping DMT in patients with MS,” he added.
Clinically useful?
Jeffrey Cohen, MD, current president of ACTRIMS, said the first study supports mounting evidence on how sNfL and sGFAP at onset can predict future disease and have the potential to improve current predictive models.
“Combining clinical, MRI, and serum biomarkers into a single model works better than any of the three factors individually,” said Dr. Cohen, who is director of the Mellen Center for MS Treatment and Research and professor of neurology at the Cleveland Clinic.
“For the clinician, this information may help with treatment selection,” he added.
Dr. Cohen noted that the suggestion that the biomarkers could also be helpful in predicting relapse after discontinuation is of importance.
“Increasingly, we are considering this issue in the clinical setting,” he said. However, he also noted some caveats.
“Interpretation of the results of the study is not straightforward, illustrating the complexity of the issue,” Dr. Cohen said. “One issue is that the patients in the study were relatively young, with an average age of 45, which is not a group in which we typically would consider stopping therapy.”
Dr. Bose has received a postdoctoral fellowship grant from the Multiple Sclerosis Society of Canada. Dr. Cohen reports having received personal compensation for consulting for Biogen, Bristol-Myers Squibb, Convelo, Genentech, Janssen, NervGen, Novartis, and PSI; speaking for H3 Communications; and serving as an editor of the Multiple Sclerosis Journal.
A version of this article first appeared on Medscape.com.
WEST PALM BEACH, FL – , new research suggests.
The research shows that once standard clinical models can be incorporated into practice, the early measurement of these biomarkers will provide useful information in predicting who may be at risk of poorer outcomes, researcher Gauruv Bose, MD, Brigham Multiple Sclerosis Center, Ann Romney Center for Neurologic Diseases, Brigham and Women’s Hospital, Harvard Medical School, Boston, told this news organization.
The findings were presented at annual meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS).
Better together?
Although higher baseline sNfL levels in MS have previously been linked to greater brain atrophy and other long-term outcomes, and sGFAP changes are also associated with inflammation and damage through the disease course, less is known about longer-term effects of the two biomarker measures combined, Dr. Bose said.
“The value of using both sNfL and sGFAP in predictive models is of interest, since one correlates with neuroaxonal damage, while the other has correlated with astrocytic glial damage/cell turnover – potentially, though differently, reflecting inflammatory damage and neurodegeneration,” he added.
To investigate the relationship, the researchers evaluated patients with MS enrolled at the Brigham Multiple Sclerosis Center. All underwent neurologic examinations every 6 months, and MRI scans and blood samples were collected every year. Some had more than 20 years of follow-up.
The first study involved 144 patients (mean age, 37.4 years) from whom two samples of sNfL and sGFAP were collected within 3 years of MS onset.
The median baseline sNfL level was 10.7 pg/mL, and 50 patients (34.7%) already showed increases in sNfL at the 1-year follow-up. Their median sGFAP level at onset was 96 pg/mL, and 59 patients (41%) showed increases in sGFAP at the 1-year follow-up.
Results showed that higher baseline sNfL levels were significantly associated with increased risk for MS relapse at 10 years (hazard ratio, 1.34; P = .04), as well as with the development of new MRI lesions (HR, 1.35; P = .022).
Of the study group, 25 (17.4%) developed secondary progressive MS (SPMS) by the 10-year follow-up. For those prognostic assessments, the investigators compared utilization of a model using well-established clinical predictors of SPMS with and without the inclusion of sNfL and sGFAP.
The clinical model included key factors such as age, sex, body mass index, Extended Disability Status Scale (EDSS), timed 25-foot walk, and other measures.
The researchers found the clinical model alone predicted 10-year outcomes with an area under the receiver operating characteristic curve (AUC) of 0.75. However, with the addition of baseline sNfL and sGFAP measures, the AUC was improved to 0.79 (P = .0008).
Furthermore, the inclusion of additional follow-up sNfL and sGFAP measurements taken after baseline further improved the model’s AUC (0.82; P = .046).
The addition of the sNfL and sGFAP measures to the clinical models also improved the prediction of disability in MS at 10 years on EDSS (P = .068), as well as prediction of 10-year brain T2 lesion volume (P = .009) and brain parenchymal fraction (P = .04).
Relapse predictor?
In the second study, Dr. Bose and colleagues evaluated the role of the two serum measures in predicting relapse after disease-modifying therapy (DMT) discontinuation. That study included 42 patients who discontinued DMT treatment after having been disease-activity free for 2 years while on the drugs. They were compared with 36 patients who had similar characteristics and had continued DMT treatment.
All patients (mean age, 44.5 years) had a mean of 7.4 years since prior disease activity.
Increases in sNfL following DMT discontinuation, but not before, were associated with a significantly greater risk for clinical disease worsening at a mean follow-up of 7.5 years (HR, 9.4; P = .007). Change in sGFAP was associated with new MRI lesions (HR, 8.3; P = .039), compared with no changes.
“The crux of this study” was that patients with increased biomarker levels after stopping DMTs “were at a significantly higher risk for disease activity in the future compared to those whose biomarker levels remained stable,” Dr. Bose noted.
“We think this finding, if replicated in another cohort, has the potential to be included in guidelines regarding stopping DMT in patients with MS,” he added.
Clinically useful?
Jeffrey Cohen, MD, current president of ACTRIMS, said the first study supports mounting evidence on how sNfL and sGFAP at onset can predict future disease and have the potential to improve current predictive models.
“Combining clinical, MRI, and serum biomarkers into a single model works better than any of the three factors individually,” said Dr. Cohen, who is director of the Mellen Center for MS Treatment and Research and professor of neurology at the Cleveland Clinic.
“For the clinician, this information may help with treatment selection,” he added.
Dr. Cohen noted that the suggestion that the biomarkers could also be helpful in predicting relapse after discontinuation is of importance.
“Increasingly, we are considering this issue in the clinical setting,” he said. However, he also noted some caveats.
“Interpretation of the results of the study is not straightforward, illustrating the complexity of the issue,” Dr. Cohen said. “One issue is that the patients in the study were relatively young, with an average age of 45, which is not a group in which we typically would consider stopping therapy.”
Dr. Bose has received a postdoctoral fellowship grant from the Multiple Sclerosis Society of Canada. Dr. Cohen reports having received personal compensation for consulting for Biogen, Bristol-Myers Squibb, Convelo, Genentech, Janssen, NervGen, Novartis, and PSI; speaking for H3 Communications; and serving as an editor of the Multiple Sclerosis Journal.
A version of this article first appeared on Medscape.com.
WEST PALM BEACH, FL – , new research suggests.
The research shows that once standard clinical models can be incorporated into practice, the early measurement of these biomarkers will provide useful information in predicting who may be at risk of poorer outcomes, researcher Gauruv Bose, MD, Brigham Multiple Sclerosis Center, Ann Romney Center for Neurologic Diseases, Brigham and Women’s Hospital, Harvard Medical School, Boston, told this news organization.
The findings were presented at annual meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS).
Better together?
Although higher baseline sNfL levels in MS have previously been linked to greater brain atrophy and other long-term outcomes, and sGFAP changes are also associated with inflammation and damage through the disease course, less is known about longer-term effects of the two biomarker measures combined, Dr. Bose said.
“The value of using both sNfL and sGFAP in predictive models is of interest, since one correlates with neuroaxonal damage, while the other has correlated with astrocytic glial damage/cell turnover – potentially, though differently, reflecting inflammatory damage and neurodegeneration,” he added.
To investigate the relationship, the researchers evaluated patients with MS enrolled at the Brigham Multiple Sclerosis Center. All underwent neurologic examinations every 6 months, and MRI scans and blood samples were collected every year. Some had more than 20 years of follow-up.
The first study involved 144 patients (mean age, 37.4 years) from whom two samples of sNfL and sGFAP were collected within 3 years of MS onset.
The median baseline sNfL level was 10.7 pg/mL, and 50 patients (34.7%) already showed increases in sNfL at the 1-year follow-up. Their median sGFAP level at onset was 96 pg/mL, and 59 patients (41%) showed increases in sGFAP at the 1-year follow-up.
Results showed that higher baseline sNfL levels were significantly associated with increased risk for MS relapse at 10 years (hazard ratio, 1.34; P = .04), as well as with the development of new MRI lesions (HR, 1.35; P = .022).
Of the study group, 25 (17.4%) developed secondary progressive MS (SPMS) by the 10-year follow-up. For those prognostic assessments, the investigators compared utilization of a model using well-established clinical predictors of SPMS with and without the inclusion of sNfL and sGFAP.
The clinical model included key factors such as age, sex, body mass index, Extended Disability Status Scale (EDSS), timed 25-foot walk, and other measures.
The researchers found the clinical model alone predicted 10-year outcomes with an area under the receiver operating characteristic curve (AUC) of 0.75. However, with the addition of baseline sNfL and sGFAP measures, the AUC was improved to 0.79 (P = .0008).
Furthermore, the inclusion of additional follow-up sNfL and sGFAP measurements taken after baseline further improved the model’s AUC (0.82; P = .046).
The addition of the sNfL and sGFAP measures to the clinical models also improved the prediction of disability in MS at 10 years on EDSS (P = .068), as well as prediction of 10-year brain T2 lesion volume (P = .009) and brain parenchymal fraction (P = .04).
Relapse predictor?
In the second study, Dr. Bose and colleagues evaluated the role of the two serum measures in predicting relapse after disease-modifying therapy (DMT) discontinuation. That study included 42 patients who discontinued DMT treatment after having been disease-activity free for 2 years while on the drugs. They were compared with 36 patients who had similar characteristics and had continued DMT treatment.
All patients (mean age, 44.5 years) had a mean of 7.4 years since prior disease activity.
Increases in sNfL following DMT discontinuation, but not before, were associated with a significantly greater risk for clinical disease worsening at a mean follow-up of 7.5 years (HR, 9.4; P = .007). Change in sGFAP was associated with new MRI lesions (HR, 8.3; P = .039), compared with no changes.
“The crux of this study” was that patients with increased biomarker levels after stopping DMTs “were at a significantly higher risk for disease activity in the future compared to those whose biomarker levels remained stable,” Dr. Bose noted.
“We think this finding, if replicated in another cohort, has the potential to be included in guidelines regarding stopping DMT in patients with MS,” he added.
Clinically useful?
Jeffrey Cohen, MD, current president of ACTRIMS, said the first study supports mounting evidence on how sNfL and sGFAP at onset can predict future disease and have the potential to improve current predictive models.
“Combining clinical, MRI, and serum biomarkers into a single model works better than any of the three factors individually,” said Dr. Cohen, who is director of the Mellen Center for MS Treatment and Research and professor of neurology at the Cleveland Clinic.
“For the clinician, this information may help with treatment selection,” he added.
Dr. Cohen noted that the suggestion that the biomarkers could also be helpful in predicting relapse after discontinuation is of importance.
“Increasingly, we are considering this issue in the clinical setting,” he said. However, he also noted some caveats.
“Interpretation of the results of the study is not straightforward, illustrating the complexity of the issue,” Dr. Cohen said. “One issue is that the patients in the study were relatively young, with an average age of 45, which is not a group in which we typically would consider stopping therapy.”
Dr. Bose has received a postdoctoral fellowship grant from the Multiple Sclerosis Society of Canada. Dr. Cohen reports having received personal compensation for consulting for Biogen, Bristol-Myers Squibb, Convelo, Genentech, Janssen, NervGen, Novartis, and PSI; speaking for H3 Communications; and serving as an editor of the Multiple Sclerosis Journal.
A version of this article first appeared on Medscape.com.
Reporting from ACTRIMS Forumn 2022
Air trapping common in patients with long COVID
, according to a prospective study that compared 100 COVID-19 survivors who had persistent symptoms and 106 healthy control persons.
“Something is going on in the distal airways related to either inflammation or fibrosis that is giving us a signal of air trapping,” noted senior author Alejandro P. Comellas, MD, in a press release. The study was stimulated by reports from University of Iowa clinicians noting that many patients with initial SARS-CoV-2 infection who were either hospitalized or were treated in the ambulatory setting later reported shortness of breath and other respiratory symptoms indicative of chronic lung disease.
Study results
Investigators classified patients (mean age, 48 years; 66 women) with post-acute sequelae of COVID-19 according to whether they were ambulatory (67%), hospitalized (17%), or required treatment in the intensive care unit (16%). They then compared CT findings of patients who had COVID-19 and persistent symptoms with those of a healthy control group.
COVID-19 severity did not affect the percentage of cases of lung with air trapping among these patients. Air trapping occurred at rates of 25.4% among ambulatory patients, 34.6% in hospitalized patients, and in 27.3% of those requiring intensive care (P = .10). The percentage of lungs affected by air trapping in ambulatory participants was sharply and significantly higher than in healthy controls (25.4% vs. 7.2%; P < .001). Also, air trapping persisted; it was still present in 8 of 9 participants who underwent imaging more than 200 days post diagnosis.
Qualitative analysis of chest CT images showed that the most common imaging abnormality was air trapping (58%); ground glass opacities (GGOs) were found in 51% (46/91), note Dr. Comellas and coauthors. This suggests ongoing lung inflammation, edema, or fibrosis. These symptoms are often observed during acute COVID-19, frequently in an organizing pneumonia pattern, and have been shown to persist for months after infection in survivors of severe disease. The mean percentage of total lung classified as having regional GGOs on chest CT scans was 13.2% and 28.7%, respectively, in the hospitalized and ICU groups, both very much higher than in the ambulatory group, at 3.7% (P < .001 for both). Among healthy controls, the GGO rate on chest CT was only 0.06% (P < .001).
In addition, air trapping correlated with the ratio of residual volume to total lung capacity (r = 0.6; P < .001) but not with spirometry results. In fact, the investigators did not observe airflow obstruction by spirometry in any group, suggesting that air trapping in these patients involves only small rather than large airways and that these small airways contribute little to total airway resistance. Only when a large percentage, perhaps 75% or more, of all small airways are obstructed will spirometry pick up small airways disease, the authors observe.
Continuing disease
The findings taken together suggest that functional small airways disease and air trapping are a consequence of SARS-CoV-2 infection, according to Dr. Comellas. “If a portion of patients continues to have small airways disease, then we need to think about the mechanisms behind it,” he said. “It could be something related to inflammation that’s reversible, or it may be something related to a scar that is irreversible, and then we need to look at ways to prevent further progression of the disease.” Furthermore, “studies aimed at determining the natural history of functional small airways disease in patients with post-acute sequelae of COVID-19 and the biological mechanisms that underlie these findings are urgently needed to identify therapeutic and preventative interventions,” Dr. Comellas, professor of internal medicine at Carver College of Medicine, University of Iowa, Iowa City, concluded.
The study limitations, the authors state, include the fact that theirs was a single-center study that enrolled participants infected early during the COVID-19 pandemic and did not include patients with Delta or Omicron variants, thus limiting the generalizability of the findings.
The study was published in Radiology.
The reported findings “indicate a long-term impact on bronchiolar obstruction,” states Brett M. Elicker, MD, professor of clinical radiology, University of California, San Francisco, in an accompanying editorial . Because collagen may be absorbed for months after an acute insult, it is not entirely clear whether the abnormalities seen in the current study will be permanent. He said further, “the presence of ground glass opacity and/or fibrosis on CT were most common in the patients admitted to the ICU and likely correspond to post-organizing pneumonia and/or post-diffuse alveolar damage fibrosis.”
Dr. Elicker also pointed out that organizing pneumonia is especially common among patients with COVID-19 and is usually highly steroid-responsive. The opacities improve or resolve with treatment, but sometimes residual fibrosis occurs. “Longer-term studies assessing the clinical and imaging manifestations 1-2 years after the initial infection are needed to fully ascertain the permanent manifestations of post-COVID fibrosis.”
The study was supported by grants from the National Institutes of Health. The authors and Dr. Elicker have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a prospective study that compared 100 COVID-19 survivors who had persistent symptoms and 106 healthy control persons.
“Something is going on in the distal airways related to either inflammation or fibrosis that is giving us a signal of air trapping,” noted senior author Alejandro P. Comellas, MD, in a press release. The study was stimulated by reports from University of Iowa clinicians noting that many patients with initial SARS-CoV-2 infection who were either hospitalized or were treated in the ambulatory setting later reported shortness of breath and other respiratory symptoms indicative of chronic lung disease.
Study results
Investigators classified patients (mean age, 48 years; 66 women) with post-acute sequelae of COVID-19 according to whether they were ambulatory (67%), hospitalized (17%), or required treatment in the intensive care unit (16%). They then compared CT findings of patients who had COVID-19 and persistent symptoms with those of a healthy control group.
COVID-19 severity did not affect the percentage of cases of lung with air trapping among these patients. Air trapping occurred at rates of 25.4% among ambulatory patients, 34.6% in hospitalized patients, and in 27.3% of those requiring intensive care (P = .10). The percentage of lungs affected by air trapping in ambulatory participants was sharply and significantly higher than in healthy controls (25.4% vs. 7.2%; P < .001). Also, air trapping persisted; it was still present in 8 of 9 participants who underwent imaging more than 200 days post diagnosis.
Qualitative analysis of chest CT images showed that the most common imaging abnormality was air trapping (58%); ground glass opacities (GGOs) were found in 51% (46/91), note Dr. Comellas and coauthors. This suggests ongoing lung inflammation, edema, or fibrosis. These symptoms are often observed during acute COVID-19, frequently in an organizing pneumonia pattern, and have been shown to persist for months after infection in survivors of severe disease. The mean percentage of total lung classified as having regional GGOs on chest CT scans was 13.2% and 28.7%, respectively, in the hospitalized and ICU groups, both very much higher than in the ambulatory group, at 3.7% (P < .001 for both). Among healthy controls, the GGO rate on chest CT was only 0.06% (P < .001).
In addition, air trapping correlated with the ratio of residual volume to total lung capacity (r = 0.6; P < .001) but not with spirometry results. In fact, the investigators did not observe airflow obstruction by spirometry in any group, suggesting that air trapping in these patients involves only small rather than large airways and that these small airways contribute little to total airway resistance. Only when a large percentage, perhaps 75% or more, of all small airways are obstructed will spirometry pick up small airways disease, the authors observe.
Continuing disease
The findings taken together suggest that functional small airways disease and air trapping are a consequence of SARS-CoV-2 infection, according to Dr. Comellas. “If a portion of patients continues to have small airways disease, then we need to think about the mechanisms behind it,” he said. “It could be something related to inflammation that’s reversible, or it may be something related to a scar that is irreversible, and then we need to look at ways to prevent further progression of the disease.” Furthermore, “studies aimed at determining the natural history of functional small airways disease in patients with post-acute sequelae of COVID-19 and the biological mechanisms that underlie these findings are urgently needed to identify therapeutic and preventative interventions,” Dr. Comellas, professor of internal medicine at Carver College of Medicine, University of Iowa, Iowa City, concluded.
The study limitations, the authors state, include the fact that theirs was a single-center study that enrolled participants infected early during the COVID-19 pandemic and did not include patients with Delta or Omicron variants, thus limiting the generalizability of the findings.
The study was published in Radiology.
The reported findings “indicate a long-term impact on bronchiolar obstruction,” states Brett M. Elicker, MD, professor of clinical radiology, University of California, San Francisco, in an accompanying editorial . Because collagen may be absorbed for months after an acute insult, it is not entirely clear whether the abnormalities seen in the current study will be permanent. He said further, “the presence of ground glass opacity and/or fibrosis on CT were most common in the patients admitted to the ICU and likely correspond to post-organizing pneumonia and/or post-diffuse alveolar damage fibrosis.”
Dr. Elicker also pointed out that organizing pneumonia is especially common among patients with COVID-19 and is usually highly steroid-responsive. The opacities improve or resolve with treatment, but sometimes residual fibrosis occurs. “Longer-term studies assessing the clinical and imaging manifestations 1-2 years after the initial infection are needed to fully ascertain the permanent manifestations of post-COVID fibrosis.”
The study was supported by grants from the National Institutes of Health. The authors and Dr. Elicker have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a prospective study that compared 100 COVID-19 survivors who had persistent symptoms and 106 healthy control persons.
“Something is going on in the distal airways related to either inflammation or fibrosis that is giving us a signal of air trapping,” noted senior author Alejandro P. Comellas, MD, in a press release. The study was stimulated by reports from University of Iowa clinicians noting that many patients with initial SARS-CoV-2 infection who were either hospitalized or were treated in the ambulatory setting later reported shortness of breath and other respiratory symptoms indicative of chronic lung disease.
Study results
Investigators classified patients (mean age, 48 years; 66 women) with post-acute sequelae of COVID-19 according to whether they were ambulatory (67%), hospitalized (17%), or required treatment in the intensive care unit (16%). They then compared CT findings of patients who had COVID-19 and persistent symptoms with those of a healthy control group.
COVID-19 severity did not affect the percentage of cases of lung with air trapping among these patients. Air trapping occurred at rates of 25.4% among ambulatory patients, 34.6% in hospitalized patients, and in 27.3% of those requiring intensive care (P = .10). The percentage of lungs affected by air trapping in ambulatory participants was sharply and significantly higher than in healthy controls (25.4% vs. 7.2%; P < .001). Also, air trapping persisted; it was still present in 8 of 9 participants who underwent imaging more than 200 days post diagnosis.
Qualitative analysis of chest CT images showed that the most common imaging abnormality was air trapping (58%); ground glass opacities (GGOs) were found in 51% (46/91), note Dr. Comellas and coauthors. This suggests ongoing lung inflammation, edema, or fibrosis. These symptoms are often observed during acute COVID-19, frequently in an organizing pneumonia pattern, and have been shown to persist for months after infection in survivors of severe disease. The mean percentage of total lung classified as having regional GGOs on chest CT scans was 13.2% and 28.7%, respectively, in the hospitalized and ICU groups, both very much higher than in the ambulatory group, at 3.7% (P < .001 for both). Among healthy controls, the GGO rate on chest CT was only 0.06% (P < .001).
In addition, air trapping correlated with the ratio of residual volume to total lung capacity (r = 0.6; P < .001) but not with spirometry results. In fact, the investigators did not observe airflow obstruction by spirometry in any group, suggesting that air trapping in these patients involves only small rather than large airways and that these small airways contribute little to total airway resistance. Only when a large percentage, perhaps 75% or more, of all small airways are obstructed will spirometry pick up small airways disease, the authors observe.
Continuing disease
The findings taken together suggest that functional small airways disease and air trapping are a consequence of SARS-CoV-2 infection, according to Dr. Comellas. “If a portion of patients continues to have small airways disease, then we need to think about the mechanisms behind it,” he said. “It could be something related to inflammation that’s reversible, or it may be something related to a scar that is irreversible, and then we need to look at ways to prevent further progression of the disease.” Furthermore, “studies aimed at determining the natural history of functional small airways disease in patients with post-acute sequelae of COVID-19 and the biological mechanisms that underlie these findings are urgently needed to identify therapeutic and preventative interventions,” Dr. Comellas, professor of internal medicine at Carver College of Medicine, University of Iowa, Iowa City, concluded.
The study limitations, the authors state, include the fact that theirs was a single-center study that enrolled participants infected early during the COVID-19 pandemic and did not include patients with Delta or Omicron variants, thus limiting the generalizability of the findings.
The study was published in Radiology.
The reported findings “indicate a long-term impact on bronchiolar obstruction,” states Brett M. Elicker, MD, professor of clinical radiology, University of California, San Francisco, in an accompanying editorial . Because collagen may be absorbed for months after an acute insult, it is not entirely clear whether the abnormalities seen in the current study will be permanent. He said further, “the presence of ground glass opacity and/or fibrosis on CT were most common in the patients admitted to the ICU and likely correspond to post-organizing pneumonia and/or post-diffuse alveolar damage fibrosis.”
Dr. Elicker also pointed out that organizing pneumonia is especially common among patients with COVID-19 and is usually highly steroid-responsive. The opacities improve or resolve with treatment, but sometimes residual fibrosis occurs. “Longer-term studies assessing the clinical and imaging manifestations 1-2 years after the initial infection are needed to fully ascertain the permanent manifestations of post-COVID fibrosis.”
The study was supported by grants from the National Institutes of Health. The authors and Dr. Elicker have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM RADIOLOGY
2022 Update on gynecologic cancer
Despite the challenges of an ongoing COVID-19 pandemic, researchers in 2021 delivered practice-changing studies in gynecologic oncology. In this cancer Update, we highlight 4 studies that shed light on the surgical and systemic therapies that may improve outcomes for patients with cancers of the ovary, endometrium, and cervix. We review DESKTOP III, a trial that investigated the role of cytoreductive surgery in patients with recurrent ovarian cancer, and SENTOR, a study that evaluated the performance of sentinel lymph node biopsy in patients with high-grade endometrial cancers. Additionally, we examine 2 studies of systemic therapy that reveal the growing role of targeted therapies and immuno-oncology in the treatment of gynecologic malignancies.
A new era for patients with BRCA mutation–associated ovarian cancer
Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:1721-1731.
Ovarian cancer remains the most lethal gynecologic malignancy due to the frequency of advanced-stage diagnosis and frequent relapse after primary therapy. But for ovarian cancer patients with inherited mutations of the BRCA1 or BRCA2 genes, poly(ADP-ribose) polymerase (PARP) inhibitors, a class of oral anticancer medicines that target DNA repair, have ushered in a new era in which the possibility of long-term remission, and even cure, is more likely than at any other time.
Olaparib trial details
The SOLO1 study was a double-blind, placebo-controlled, phase 3 trial that investigated the role of PARP inhibitor maintenance therapy with olaparib in patients with pathologic BRCA1 or BRCA2 mutations who responded to platinum-based chemotherapy administered for a newly diagnosed, advanced-stage ovarian cancer.1 The study enrolled 391 patients, with 260 randomly assigned to receive olaparib for 24 months and 131 patients randomly assigned to receive placebo tablets. Most patients in the study had a mutation in the BRCA1 gene (72%), 27% had a BRCA2 mutation, and 1% had mutations in both genes.
The primary analysis of SOLO1 was published in 2018 and was based on a median follow-up of 3.4 years.1 That study showed that olaparib maintenance therapy resulted in a large progression-free survival benefit and led to its approval by the US Food and Drug Administration (FDA) as a maintenance therapy for patients with BRCA-mutated advanced ovarian cancer who responded to first-line platinum-based chemotherapy.
In 2021, Banerjee and colleagues updated the progression-free survival results for the SOLO1 trial after 5 years of follow-up.2 In this study, the patients randomly assigned to olaparib maintenance therapy had a persistent and statistically significant progression-free survival benefit, with the median progression-free survival reaching 56 months among the olaparib group compared with 13.8 months in the placebo group (hazard ratio [HR], 0.33; 95% confidence interval [CI], 0.25–0.43).2 Olaparib maintenance therapy resulted in more clinically significant adverse events, including anemia and neutropenia. Serious adverse events occurred in 55 (21%) of the olaparib-treated patients and 17 (13%) of the placebo-treated patients, but no treatment-related adverse events were fatal.
The updated progression-free survival data from the SOLO1 study provides important and promising evidence that frontline PARP inhibitor maintenance therapy may affect long-term remission in an unprecedented proportion of patients with BRCA-related ovarian cancer. Significant, sustained benefit was seen well beyond the end of treatment, and median progression-free survival was an astonishing 3.5 years longer in the olaparib treatment group than among patients who received placebo therapy.
Continue to: Cytoreductive surgery for recurrent ovarian cancer improves survival in well-selected patients...
Cytoreductive surgery for recurrent ovarian cancer improves survival in well-selected patients
Harter P, Sehouli J, Vergote I, et al; DESKTOP III Investigators. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med. 2021;385:2123- 2131.
In the DESKTOP III trial, Harter and colleagues contribute results to the ongoing discourse surrounding treatment options for patients with recurrent, platinum-sensitive ovarian cancer.3 Systemic therapies continue to be the mainstay of treatment in this setting; however, several groups have attempted to evaluate the role of secondary cytoreductive surgery in this setting.4,5
Specific inclusion criteria employed
The DESKTOP III investigators randomly assigned 407 patients with platinum-sensitive recurrent ovarian cancer to secondary cytoreductive surgery followed by platinum-based chemotherapy (n = 206) or platinum-based chemotherapy alone (n = 201).3 An essential aspect of the study’s design was the use of specific inclusion criteria known to identify patients with a high likelihood of complete resection at the time of secondary cytoreduction.6,7 Patients were eligible only if they had at least a 6-month remission following platinum-based chemotherapy, had a complete resection at their previous surgery, had no restriction on physical activity, and had ascites of no more than 500 mL.
Surgery group had superior overall and progression-free survival
After a median follow-up of approximately 70 months, patients randomly assigned to surgery had superior overall survival (53.7 months) compared with those assigned to chemotherapy alone (46.0 months; HR, 0.75; 95% CI, 0.59–0.96).3 Progression-free survival also was improved among patients who underwent surgery (median 18.4 vs 12.7 months; HR, 0.66; 95% CI, 0.54–0.82). Subgroup analyses did not identify any subset of patients who did not benefit from surgery. Whether a complete resection was achieved at secondary cytoreduction was highly prognostic: Patients who had a complete resection had a median overall survival of 61.9 months compared with 27.7 months in patients with residual disease. There were no deaths within 90 days of surgery.
The DESKTOP III trial provides compelling evidence that secondary cytoreductive surgery improves overall and progression-free survival among well-selected patients with recurrent, platinum-sensitive ovarian cancer. These results differ from those of a recently reported Gynecologic Oncology Group (GOG) trial that failed to detect a survival benefit for secondary cytoreductive surgery among patients with platinum-sensitive recurrent ovarian cancer.5 Key differences, which might explain the studies’ seemingly contradictory results, were that the GOG study had fewer specific eligibility criteria than the DESKTOP III trial, and that bevacizumab was administered much more frequently in the GOG study. It is therefore reasonable to discuss the possible benefits of secondary cytoreductive surgery with patients who meet DESKTOP III eligibility criteria, with a focus toward shared decision making and a candid discussion of the potential risks and benefits of secondary cytoreduction.
Continue to: Immunotherapy enters first-line treatment regimen for advanced cervical cancer...
Immunotherapy enters first-line treatment regimen for advanced cervical cancer
Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Persistent, recurrent, and metastatic cervical cancer carries a very poor prognosis: Most patients progress less than a year after starting treatment, and fewer than half survive for 2 years. First-line treatment in this setting has been platinum-based chemotherapy, often given with bevacizumab, a humanized monoclonal antibody that inhibits tumor growth by blocking angiogenesis.8 Pembrolizumab, an immune checkpoint inhibitor, targets cancer cells by blocking their ability to evade the immune system, and it is FDA approved and widely administered to patients with advanced cervical cancer who progress after first-line treatment.9
Addition of pembrolizumab extended survival
In the KEYNOTE-826 trial, Colombo and colleagues investigated the efficacy of incorporating an immune checkpoint inhibitor into the first-line treatment regimen for patients with persistent, recurrent, and metastatic cervical cancer.10 Researchers in this double-blinded, phase 3, randomized controlled trial assigned 617 patients to receive pembrolizumab or placebo concurrently with the investigator’s choice platinum-based chemotherapy. Bevacizumab was administered at the discretion of the treating oncologist.
The proportion of patients who survived at least 2 years following randomization was significantly higher among those assigned to pembrolizumab compared with placebo (53% vs 42%; HR, 0.67, 95% CI, 0.54–0.84).10 Similarly, median progression-free survival was superior among patients who received pembrolizumab compared with those who received placebo (10.4 months vs 8.2 months; HR, 0.65; 95% CI, 0.53–0.79). The role of bevacizumab in conjunction with pembrolizumab and platinum-based chemotherapy was not elucidated in this study because bevacizumab administration was not randomly assigned.
Anemia and neutropenia were the most common adverse events and were more frequent in the pembrolizumab group, but there were no new safety concerns related to concurrent use of pembrolizumab with cytotoxic chemotherapy and bevacizumab. Importantly, subgroup analysis results suggested that pembrolizumab was effective only in patients whose tumors expressed PD-L1 (programmed death ligand 1), a biomarker of pembrolizumab sensitivity in cervical cancer.
In light of the significant improvements in overall and progression-free survival demonstrated in the KEYNOTE-826 trial, in October 2021, the FDA approved the use of frontline pembrolizumab alongside platinum-based chemotherapy, with or without bevacizumab, for treatment of patients with persistent, recurrent, or metastatic cervical cancers that express PD-L1.
Continue to: Endometrial cancer surgical staging...
Endometrial cancer surgical staging: Is sentinel lymph node biopsy a viable option for high-risk histologies?
Cusimano MC, Vicus D, Pulman K, et al. Assessment of sentinel lymph node biopsy vs lymphadenectomy for intermediate- and high-grade endometrial cancer staging. JAMA Surg. 2021;156:157-164.
The use of intraoperative sentinel lymph node mapping and biopsy to identify lymph node metastases among patients undergoing surgical staging for endometrial cancer has become increasingly common. Lymph node status is an important prognostic factor, and it guides adjuvant treatment decisions in endometrial cancer. However, traditional pelvic and para-aortic lymphadenectomy is associated with increased risk of lower-extremity lymphedema, postoperative complications, and intraoperative injury.
Sentinel lymph node biopsy seeks to identify lymph node metastases while minimizing surgical morbidity by identifying and excising only lymph nodes that directly receive lymphatic drainage from the uterus. The combination of a fluorescent dye (indocyanine green) and near infrared cameras have led to the broad adoption of sentinel lymph node biopsy in endometrial cancer staging surgery. This practice is supported by prospective studies that demonstrate the high diagnostic accuracy of this approach.11,12 However, because most patients included in prior studies had low-grade endometrial cancer, the utility of sentinel lymph node biopsy in cases of high-grade histology has been less clear.
Sentinel lymph node biopsy vs lymphadenectomy for staging
In the SENTOR trial, Cusimano and colleagues examined the diagnostic accuracy of sentinel lymph node mapping and biopsy, using indocyanine green, in patients with intermediate- or high-grade early-stage endometrial cancer.13
All eligible patients (N = 156) underwent traditional or robot-assisted laparoscopic hysterectomy with sentinel lymph node biopsy. Subsequently, patients with grade 2 endometrioid carcinoma underwent bilateral pelvic lymphadenectomy, and those with high-grade histology (grade 3 endometrioid, serous, carcinosarcoma, clear cell, undifferentiated or dedifferentiated, and mixed high grade) underwent bilateral pelvic and para-aortic lymphadenectomy. The investigators evaluated the diagnostic characteristics of sentinel lymph node biopsy, treating complete lymphadenectomy as the gold standard.
Of the 156 patients enrolled, the median age was 65.5 and median body mass index was 27.5; 126 patients (81%) had high-grade histology. The sentinel lymph node biopsy had a sensitivity of 96% (95% CI, 81%–100%), identifying 26 of the 27 patients with nodal metastases. The false-negative rate was 4% (95% CI, 0%–9%) and the negative predictive value was 99% (95% CI, 96%–100%). Intraoperative adverse events occurred in 5 patients (3%), but none occurred during the sentinel lymph node biopsy. ●
The high sensitivity and negative predictive value of sentinel lymph node biopsy in the intermediate- and high-grade cohort included in the SENTOR trial are concordant with prior studies that predominantly included patients with low-grade endometrial cancer. These findings suggest that sentinel lymph node mapping and biopsy is a reasonable option for surgical staging, not only for patients with low-grade endometrial cancer but also for those with intermediate- and high-grade disease.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:1721-1731.
- Harter P, Sehouli J, Vergote I, et al; DESKTOP III Investigators. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med. 2021;385:2123-2131.
- Shi T, Zhu J, Feng Y, et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:439-449.
- Coleman RL, Spiritos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Harter P, du Bois A, Hahmann M, et al; Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Committee; AGO Ovarian Cancer Study Group. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol. 2006;13:1702-1710.
- Harter P, Sehouli J, Reuss A, et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: the Multicenter Intergroup Study DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int J Gynecol Cancer. 2011;21: 289-295.
- Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390:1654-1663.
- Frenel JS, Le Tourneau C, O’Neil B, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. J Clin Oncol. 2017;35:4035-4041.
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
- Rossi EC, Kowalski L, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18:384-392.
- Ballester M, Dubernard G, Lecuru F, et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicentre study (SENTIENDO). Lancet Oncol. 2011;12: 469-476.
- Cusimano MC, Vicus D, Pulman K, et al. Assessment of sentinel lymph node biopsy vs lymphadenectomy for intermediate- and high-grade endometrial cancer staging. JAMA Surg. 2021;156:157-164.
Despite the challenges of an ongoing COVID-19 pandemic, researchers in 2021 delivered practice-changing studies in gynecologic oncology. In this cancer Update, we highlight 4 studies that shed light on the surgical and systemic therapies that may improve outcomes for patients with cancers of the ovary, endometrium, and cervix. We review DESKTOP III, a trial that investigated the role of cytoreductive surgery in patients with recurrent ovarian cancer, and SENTOR, a study that evaluated the performance of sentinel lymph node biopsy in patients with high-grade endometrial cancers. Additionally, we examine 2 studies of systemic therapy that reveal the growing role of targeted therapies and immuno-oncology in the treatment of gynecologic malignancies.
A new era for patients with BRCA mutation–associated ovarian cancer
Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:1721-1731.
Ovarian cancer remains the most lethal gynecologic malignancy due to the frequency of advanced-stage diagnosis and frequent relapse after primary therapy. But for ovarian cancer patients with inherited mutations of the BRCA1 or BRCA2 genes, poly(ADP-ribose) polymerase (PARP) inhibitors, a class of oral anticancer medicines that target DNA repair, have ushered in a new era in which the possibility of long-term remission, and even cure, is more likely than at any other time.
Olaparib trial details
The SOLO1 study was a double-blind, placebo-controlled, phase 3 trial that investigated the role of PARP inhibitor maintenance therapy with olaparib in patients with pathologic BRCA1 or BRCA2 mutations who responded to platinum-based chemotherapy administered for a newly diagnosed, advanced-stage ovarian cancer.1 The study enrolled 391 patients, with 260 randomly assigned to receive olaparib for 24 months and 131 patients randomly assigned to receive placebo tablets. Most patients in the study had a mutation in the BRCA1 gene (72%), 27% had a BRCA2 mutation, and 1% had mutations in both genes.
The primary analysis of SOLO1 was published in 2018 and was based on a median follow-up of 3.4 years.1 That study showed that olaparib maintenance therapy resulted in a large progression-free survival benefit and led to its approval by the US Food and Drug Administration (FDA) as a maintenance therapy for patients with BRCA-mutated advanced ovarian cancer who responded to first-line platinum-based chemotherapy.
In 2021, Banerjee and colleagues updated the progression-free survival results for the SOLO1 trial after 5 years of follow-up.2 In this study, the patients randomly assigned to olaparib maintenance therapy had a persistent and statistically significant progression-free survival benefit, with the median progression-free survival reaching 56 months among the olaparib group compared with 13.8 months in the placebo group (hazard ratio [HR], 0.33; 95% confidence interval [CI], 0.25–0.43).2 Olaparib maintenance therapy resulted in more clinically significant adverse events, including anemia and neutropenia. Serious adverse events occurred in 55 (21%) of the olaparib-treated patients and 17 (13%) of the placebo-treated patients, but no treatment-related adverse events were fatal.
The updated progression-free survival data from the SOLO1 study provides important and promising evidence that frontline PARP inhibitor maintenance therapy may affect long-term remission in an unprecedented proportion of patients with BRCA-related ovarian cancer. Significant, sustained benefit was seen well beyond the end of treatment, and median progression-free survival was an astonishing 3.5 years longer in the olaparib treatment group than among patients who received placebo therapy.
Continue to: Cytoreductive surgery for recurrent ovarian cancer improves survival in well-selected patients...
Cytoreductive surgery for recurrent ovarian cancer improves survival in well-selected patients
Harter P, Sehouli J, Vergote I, et al; DESKTOP III Investigators. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med. 2021;385:2123- 2131.
In the DESKTOP III trial, Harter and colleagues contribute results to the ongoing discourse surrounding treatment options for patients with recurrent, platinum-sensitive ovarian cancer.3 Systemic therapies continue to be the mainstay of treatment in this setting; however, several groups have attempted to evaluate the role of secondary cytoreductive surgery in this setting.4,5
Specific inclusion criteria employed
The DESKTOP III investigators randomly assigned 407 patients with platinum-sensitive recurrent ovarian cancer to secondary cytoreductive surgery followed by platinum-based chemotherapy (n = 206) or platinum-based chemotherapy alone (n = 201).3 An essential aspect of the study’s design was the use of specific inclusion criteria known to identify patients with a high likelihood of complete resection at the time of secondary cytoreduction.6,7 Patients were eligible only if they had at least a 6-month remission following platinum-based chemotherapy, had a complete resection at their previous surgery, had no restriction on physical activity, and had ascites of no more than 500 mL.
Surgery group had superior overall and progression-free survival
After a median follow-up of approximately 70 months, patients randomly assigned to surgery had superior overall survival (53.7 months) compared with those assigned to chemotherapy alone (46.0 months; HR, 0.75; 95% CI, 0.59–0.96).3 Progression-free survival also was improved among patients who underwent surgery (median 18.4 vs 12.7 months; HR, 0.66; 95% CI, 0.54–0.82). Subgroup analyses did not identify any subset of patients who did not benefit from surgery. Whether a complete resection was achieved at secondary cytoreduction was highly prognostic: Patients who had a complete resection had a median overall survival of 61.9 months compared with 27.7 months in patients with residual disease. There were no deaths within 90 days of surgery.
The DESKTOP III trial provides compelling evidence that secondary cytoreductive surgery improves overall and progression-free survival among well-selected patients with recurrent, platinum-sensitive ovarian cancer. These results differ from those of a recently reported Gynecologic Oncology Group (GOG) trial that failed to detect a survival benefit for secondary cytoreductive surgery among patients with platinum-sensitive recurrent ovarian cancer.5 Key differences, which might explain the studies’ seemingly contradictory results, were that the GOG study had fewer specific eligibility criteria than the DESKTOP III trial, and that bevacizumab was administered much more frequently in the GOG study. It is therefore reasonable to discuss the possible benefits of secondary cytoreductive surgery with patients who meet DESKTOP III eligibility criteria, with a focus toward shared decision making and a candid discussion of the potential risks and benefits of secondary cytoreduction.
Continue to: Immunotherapy enters first-line treatment regimen for advanced cervical cancer...
Immunotherapy enters first-line treatment regimen for advanced cervical cancer
Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Persistent, recurrent, and metastatic cervical cancer carries a very poor prognosis: Most patients progress less than a year after starting treatment, and fewer than half survive for 2 years. First-line treatment in this setting has been platinum-based chemotherapy, often given with bevacizumab, a humanized monoclonal antibody that inhibits tumor growth by blocking angiogenesis.8 Pembrolizumab, an immune checkpoint inhibitor, targets cancer cells by blocking their ability to evade the immune system, and it is FDA approved and widely administered to patients with advanced cervical cancer who progress after first-line treatment.9
Addition of pembrolizumab extended survival
In the KEYNOTE-826 trial, Colombo and colleagues investigated the efficacy of incorporating an immune checkpoint inhibitor into the first-line treatment regimen for patients with persistent, recurrent, and metastatic cervical cancer.10 Researchers in this double-blinded, phase 3, randomized controlled trial assigned 617 patients to receive pembrolizumab or placebo concurrently with the investigator’s choice platinum-based chemotherapy. Bevacizumab was administered at the discretion of the treating oncologist.
The proportion of patients who survived at least 2 years following randomization was significantly higher among those assigned to pembrolizumab compared with placebo (53% vs 42%; HR, 0.67, 95% CI, 0.54–0.84).10 Similarly, median progression-free survival was superior among patients who received pembrolizumab compared with those who received placebo (10.4 months vs 8.2 months; HR, 0.65; 95% CI, 0.53–0.79). The role of bevacizumab in conjunction with pembrolizumab and platinum-based chemotherapy was not elucidated in this study because bevacizumab administration was not randomly assigned.
Anemia and neutropenia were the most common adverse events and were more frequent in the pembrolizumab group, but there were no new safety concerns related to concurrent use of pembrolizumab with cytotoxic chemotherapy and bevacizumab. Importantly, subgroup analysis results suggested that pembrolizumab was effective only in patients whose tumors expressed PD-L1 (programmed death ligand 1), a biomarker of pembrolizumab sensitivity in cervical cancer.
In light of the significant improvements in overall and progression-free survival demonstrated in the KEYNOTE-826 trial, in October 2021, the FDA approved the use of frontline pembrolizumab alongside platinum-based chemotherapy, with or without bevacizumab, for treatment of patients with persistent, recurrent, or metastatic cervical cancers that express PD-L1.
Continue to: Endometrial cancer surgical staging...
Endometrial cancer surgical staging: Is sentinel lymph node biopsy a viable option for high-risk histologies?
Cusimano MC, Vicus D, Pulman K, et al. Assessment of sentinel lymph node biopsy vs lymphadenectomy for intermediate- and high-grade endometrial cancer staging. JAMA Surg. 2021;156:157-164.
The use of intraoperative sentinel lymph node mapping and biopsy to identify lymph node metastases among patients undergoing surgical staging for endometrial cancer has become increasingly common. Lymph node status is an important prognostic factor, and it guides adjuvant treatment decisions in endometrial cancer. However, traditional pelvic and para-aortic lymphadenectomy is associated with increased risk of lower-extremity lymphedema, postoperative complications, and intraoperative injury.
Sentinel lymph node biopsy seeks to identify lymph node metastases while minimizing surgical morbidity by identifying and excising only lymph nodes that directly receive lymphatic drainage from the uterus. The combination of a fluorescent dye (indocyanine green) and near infrared cameras have led to the broad adoption of sentinel lymph node biopsy in endometrial cancer staging surgery. This practice is supported by prospective studies that demonstrate the high diagnostic accuracy of this approach.11,12 However, because most patients included in prior studies had low-grade endometrial cancer, the utility of sentinel lymph node biopsy in cases of high-grade histology has been less clear.
Sentinel lymph node biopsy vs lymphadenectomy for staging
In the SENTOR trial, Cusimano and colleagues examined the diagnostic accuracy of sentinel lymph node mapping and biopsy, using indocyanine green, in patients with intermediate- or high-grade early-stage endometrial cancer.13
All eligible patients (N = 156) underwent traditional or robot-assisted laparoscopic hysterectomy with sentinel lymph node biopsy. Subsequently, patients with grade 2 endometrioid carcinoma underwent bilateral pelvic lymphadenectomy, and those with high-grade histology (grade 3 endometrioid, serous, carcinosarcoma, clear cell, undifferentiated or dedifferentiated, and mixed high grade) underwent bilateral pelvic and para-aortic lymphadenectomy. The investigators evaluated the diagnostic characteristics of sentinel lymph node biopsy, treating complete lymphadenectomy as the gold standard.
Of the 156 patients enrolled, the median age was 65.5 and median body mass index was 27.5; 126 patients (81%) had high-grade histology. The sentinel lymph node biopsy had a sensitivity of 96% (95% CI, 81%–100%), identifying 26 of the 27 patients with nodal metastases. The false-negative rate was 4% (95% CI, 0%–9%) and the negative predictive value was 99% (95% CI, 96%–100%). Intraoperative adverse events occurred in 5 patients (3%), but none occurred during the sentinel lymph node biopsy. ●
The high sensitivity and negative predictive value of sentinel lymph node biopsy in the intermediate- and high-grade cohort included in the SENTOR trial are concordant with prior studies that predominantly included patients with low-grade endometrial cancer. These findings suggest that sentinel lymph node mapping and biopsy is a reasonable option for surgical staging, not only for patients with low-grade endometrial cancer but also for those with intermediate- and high-grade disease.
Despite the challenges of an ongoing COVID-19 pandemic, researchers in 2021 delivered practice-changing studies in gynecologic oncology. In this cancer Update, we highlight 4 studies that shed light on the surgical and systemic therapies that may improve outcomes for patients with cancers of the ovary, endometrium, and cervix. We review DESKTOP III, a trial that investigated the role of cytoreductive surgery in patients with recurrent ovarian cancer, and SENTOR, a study that evaluated the performance of sentinel lymph node biopsy in patients with high-grade endometrial cancers. Additionally, we examine 2 studies of systemic therapy that reveal the growing role of targeted therapies and immuno-oncology in the treatment of gynecologic malignancies.
A new era for patients with BRCA mutation–associated ovarian cancer
Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:1721-1731.
Ovarian cancer remains the most lethal gynecologic malignancy due to the frequency of advanced-stage diagnosis and frequent relapse after primary therapy. But for ovarian cancer patients with inherited mutations of the BRCA1 or BRCA2 genes, poly(ADP-ribose) polymerase (PARP) inhibitors, a class of oral anticancer medicines that target DNA repair, have ushered in a new era in which the possibility of long-term remission, and even cure, is more likely than at any other time.
Olaparib trial details
The SOLO1 study was a double-blind, placebo-controlled, phase 3 trial that investigated the role of PARP inhibitor maintenance therapy with olaparib in patients with pathologic BRCA1 or BRCA2 mutations who responded to platinum-based chemotherapy administered for a newly diagnosed, advanced-stage ovarian cancer.1 The study enrolled 391 patients, with 260 randomly assigned to receive olaparib for 24 months and 131 patients randomly assigned to receive placebo tablets. Most patients in the study had a mutation in the BRCA1 gene (72%), 27% had a BRCA2 mutation, and 1% had mutations in both genes.
The primary analysis of SOLO1 was published in 2018 and was based on a median follow-up of 3.4 years.1 That study showed that olaparib maintenance therapy resulted in a large progression-free survival benefit and led to its approval by the US Food and Drug Administration (FDA) as a maintenance therapy for patients with BRCA-mutated advanced ovarian cancer who responded to first-line platinum-based chemotherapy.
In 2021, Banerjee and colleagues updated the progression-free survival results for the SOLO1 trial after 5 years of follow-up.2 In this study, the patients randomly assigned to olaparib maintenance therapy had a persistent and statistically significant progression-free survival benefit, with the median progression-free survival reaching 56 months among the olaparib group compared with 13.8 months in the placebo group (hazard ratio [HR], 0.33; 95% confidence interval [CI], 0.25–0.43).2 Olaparib maintenance therapy resulted in more clinically significant adverse events, including anemia and neutropenia. Serious adverse events occurred in 55 (21%) of the olaparib-treated patients and 17 (13%) of the placebo-treated patients, but no treatment-related adverse events were fatal.
The updated progression-free survival data from the SOLO1 study provides important and promising evidence that frontline PARP inhibitor maintenance therapy may affect long-term remission in an unprecedented proportion of patients with BRCA-related ovarian cancer. Significant, sustained benefit was seen well beyond the end of treatment, and median progression-free survival was an astonishing 3.5 years longer in the olaparib treatment group than among patients who received placebo therapy.
Continue to: Cytoreductive surgery for recurrent ovarian cancer improves survival in well-selected patients...
Cytoreductive surgery for recurrent ovarian cancer improves survival in well-selected patients
Harter P, Sehouli J, Vergote I, et al; DESKTOP III Investigators. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med. 2021;385:2123- 2131.
In the DESKTOP III trial, Harter and colleagues contribute results to the ongoing discourse surrounding treatment options for patients with recurrent, platinum-sensitive ovarian cancer.3 Systemic therapies continue to be the mainstay of treatment in this setting; however, several groups have attempted to evaluate the role of secondary cytoreductive surgery in this setting.4,5
Specific inclusion criteria employed
The DESKTOP III investigators randomly assigned 407 patients with platinum-sensitive recurrent ovarian cancer to secondary cytoreductive surgery followed by platinum-based chemotherapy (n = 206) or platinum-based chemotherapy alone (n = 201).3 An essential aspect of the study’s design was the use of specific inclusion criteria known to identify patients with a high likelihood of complete resection at the time of secondary cytoreduction.6,7 Patients were eligible only if they had at least a 6-month remission following platinum-based chemotherapy, had a complete resection at their previous surgery, had no restriction on physical activity, and had ascites of no more than 500 mL.
Surgery group had superior overall and progression-free survival
After a median follow-up of approximately 70 months, patients randomly assigned to surgery had superior overall survival (53.7 months) compared with those assigned to chemotherapy alone (46.0 months; HR, 0.75; 95% CI, 0.59–0.96).3 Progression-free survival also was improved among patients who underwent surgery (median 18.4 vs 12.7 months; HR, 0.66; 95% CI, 0.54–0.82). Subgroup analyses did not identify any subset of patients who did not benefit from surgery. Whether a complete resection was achieved at secondary cytoreduction was highly prognostic: Patients who had a complete resection had a median overall survival of 61.9 months compared with 27.7 months in patients with residual disease. There were no deaths within 90 days of surgery.
The DESKTOP III trial provides compelling evidence that secondary cytoreductive surgery improves overall and progression-free survival among well-selected patients with recurrent, platinum-sensitive ovarian cancer. These results differ from those of a recently reported Gynecologic Oncology Group (GOG) trial that failed to detect a survival benefit for secondary cytoreductive surgery among patients with platinum-sensitive recurrent ovarian cancer.5 Key differences, which might explain the studies’ seemingly contradictory results, were that the GOG study had fewer specific eligibility criteria than the DESKTOP III trial, and that bevacizumab was administered much more frequently in the GOG study. It is therefore reasonable to discuss the possible benefits of secondary cytoreductive surgery with patients who meet DESKTOP III eligibility criteria, with a focus toward shared decision making and a candid discussion of the potential risks and benefits of secondary cytoreduction.
Continue to: Immunotherapy enters first-line treatment regimen for advanced cervical cancer...
Immunotherapy enters first-line treatment regimen for advanced cervical cancer
Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Persistent, recurrent, and metastatic cervical cancer carries a very poor prognosis: Most patients progress less than a year after starting treatment, and fewer than half survive for 2 years. First-line treatment in this setting has been platinum-based chemotherapy, often given with bevacizumab, a humanized monoclonal antibody that inhibits tumor growth by blocking angiogenesis.8 Pembrolizumab, an immune checkpoint inhibitor, targets cancer cells by blocking their ability to evade the immune system, and it is FDA approved and widely administered to patients with advanced cervical cancer who progress after first-line treatment.9
Addition of pembrolizumab extended survival
In the KEYNOTE-826 trial, Colombo and colleagues investigated the efficacy of incorporating an immune checkpoint inhibitor into the first-line treatment regimen for patients with persistent, recurrent, and metastatic cervical cancer.10 Researchers in this double-blinded, phase 3, randomized controlled trial assigned 617 patients to receive pembrolizumab or placebo concurrently with the investigator’s choice platinum-based chemotherapy. Bevacizumab was administered at the discretion of the treating oncologist.
The proportion of patients who survived at least 2 years following randomization was significantly higher among those assigned to pembrolizumab compared with placebo (53% vs 42%; HR, 0.67, 95% CI, 0.54–0.84).10 Similarly, median progression-free survival was superior among patients who received pembrolizumab compared with those who received placebo (10.4 months vs 8.2 months; HR, 0.65; 95% CI, 0.53–0.79). The role of bevacizumab in conjunction with pembrolizumab and platinum-based chemotherapy was not elucidated in this study because bevacizumab administration was not randomly assigned.
Anemia and neutropenia were the most common adverse events and were more frequent in the pembrolizumab group, but there were no new safety concerns related to concurrent use of pembrolizumab with cytotoxic chemotherapy and bevacizumab. Importantly, subgroup analysis results suggested that pembrolizumab was effective only in patients whose tumors expressed PD-L1 (programmed death ligand 1), a biomarker of pembrolizumab sensitivity in cervical cancer.
In light of the significant improvements in overall and progression-free survival demonstrated in the KEYNOTE-826 trial, in October 2021, the FDA approved the use of frontline pembrolizumab alongside platinum-based chemotherapy, with or without bevacizumab, for treatment of patients with persistent, recurrent, or metastatic cervical cancers that express PD-L1.
Continue to: Endometrial cancer surgical staging...
Endometrial cancer surgical staging: Is sentinel lymph node biopsy a viable option for high-risk histologies?
Cusimano MC, Vicus D, Pulman K, et al. Assessment of sentinel lymph node biopsy vs lymphadenectomy for intermediate- and high-grade endometrial cancer staging. JAMA Surg. 2021;156:157-164.
The use of intraoperative sentinel lymph node mapping and biopsy to identify lymph node metastases among patients undergoing surgical staging for endometrial cancer has become increasingly common. Lymph node status is an important prognostic factor, and it guides adjuvant treatment decisions in endometrial cancer. However, traditional pelvic and para-aortic lymphadenectomy is associated with increased risk of lower-extremity lymphedema, postoperative complications, and intraoperative injury.
Sentinel lymph node biopsy seeks to identify lymph node metastases while minimizing surgical morbidity by identifying and excising only lymph nodes that directly receive lymphatic drainage from the uterus. The combination of a fluorescent dye (indocyanine green) and near infrared cameras have led to the broad adoption of sentinel lymph node biopsy in endometrial cancer staging surgery. This practice is supported by prospective studies that demonstrate the high diagnostic accuracy of this approach.11,12 However, because most patients included in prior studies had low-grade endometrial cancer, the utility of sentinel lymph node biopsy in cases of high-grade histology has been less clear.
Sentinel lymph node biopsy vs lymphadenectomy for staging
In the SENTOR trial, Cusimano and colleagues examined the diagnostic accuracy of sentinel lymph node mapping and biopsy, using indocyanine green, in patients with intermediate- or high-grade early-stage endometrial cancer.13
All eligible patients (N = 156) underwent traditional or robot-assisted laparoscopic hysterectomy with sentinel lymph node biopsy. Subsequently, patients with grade 2 endometrioid carcinoma underwent bilateral pelvic lymphadenectomy, and those with high-grade histology (grade 3 endometrioid, serous, carcinosarcoma, clear cell, undifferentiated or dedifferentiated, and mixed high grade) underwent bilateral pelvic and para-aortic lymphadenectomy. The investigators evaluated the diagnostic characteristics of sentinel lymph node biopsy, treating complete lymphadenectomy as the gold standard.
Of the 156 patients enrolled, the median age was 65.5 and median body mass index was 27.5; 126 patients (81%) had high-grade histology. The sentinel lymph node biopsy had a sensitivity of 96% (95% CI, 81%–100%), identifying 26 of the 27 patients with nodal metastases. The false-negative rate was 4% (95% CI, 0%–9%) and the negative predictive value was 99% (95% CI, 96%–100%). Intraoperative adverse events occurred in 5 patients (3%), but none occurred during the sentinel lymph node biopsy. ●
The high sensitivity and negative predictive value of sentinel lymph node biopsy in the intermediate- and high-grade cohort included in the SENTOR trial are concordant with prior studies that predominantly included patients with low-grade endometrial cancer. These findings suggest that sentinel lymph node mapping and biopsy is a reasonable option for surgical staging, not only for patients with low-grade endometrial cancer but also for those with intermediate- and high-grade disease.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:1721-1731.
- Harter P, Sehouli J, Vergote I, et al; DESKTOP III Investigators. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med. 2021;385:2123-2131.
- Shi T, Zhu J, Feng Y, et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:439-449.
- Coleman RL, Spiritos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Harter P, du Bois A, Hahmann M, et al; Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Committee; AGO Ovarian Cancer Study Group. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol. 2006;13:1702-1710.
- Harter P, Sehouli J, Reuss A, et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: the Multicenter Intergroup Study DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int J Gynecol Cancer. 2011;21: 289-295.
- Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390:1654-1663.
- Frenel JS, Le Tourneau C, O’Neil B, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. J Clin Oncol. 2017;35:4035-4041.
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
- Rossi EC, Kowalski L, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18:384-392.
- Ballester M, Dubernard G, Lecuru F, et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicentre study (SENTIENDO). Lancet Oncol. 2011;12: 469-476.
- Cusimano MC, Vicus D, Pulman K, et al. Assessment of sentinel lymph node biopsy vs lymphadenectomy for intermediate- and high-grade endometrial cancer staging. JAMA Surg. 2021;156:157-164.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:1721-1731.
- Harter P, Sehouli J, Vergote I, et al; DESKTOP III Investigators. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med. 2021;385:2123-2131.
- Shi T, Zhu J, Feng Y, et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:439-449.
- Coleman RL, Spiritos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Harter P, du Bois A, Hahmann M, et al; Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Committee; AGO Ovarian Cancer Study Group. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol. 2006;13:1702-1710.
- Harter P, Sehouli J, Reuss A, et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: the Multicenter Intergroup Study DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int J Gynecol Cancer. 2011;21: 289-295.
- Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390:1654-1663.
- Frenel JS, Le Tourneau C, O’Neil B, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. J Clin Oncol. 2017;35:4035-4041.
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
- Rossi EC, Kowalski L, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18:384-392.
- Ballester M, Dubernard G, Lecuru F, et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicentre study (SENTIENDO). Lancet Oncol. 2011;12: 469-476.
- Cusimano MC, Vicus D, Pulman K, et al. Assessment of sentinel lymph node biopsy vs lymphadenectomy for intermediate- and high-grade endometrial cancer staging. JAMA Surg. 2021;156:157-164.
Nonstress test and maximal vertical pocket vs the biophysical profile: Equivocal or equivalent?
CASE 1 Pregnant patient endures extensive wait and travel times to have antenatal testing
Pregnant at age 35 without comorbidities, Ms. H was instructed to schedule weekly biophysical profiles (BPP) after 36 weeks’ gestation for advanced maternal age. She receives care at a community office 25 miles from the hospital where she will deliver. Ms. H must complete her antenatal testing at the hospital where the sonographer performs BPPs. She sees her physician at the nearby clinic and then takes public transit to the hospital. She waits 2 hours to be seen then makes her way back home. Her prenatal care visit, which usually takes 30 minutes, turns into a 5-hour ordeal. Ms. H delivered a healthy baby at 39 weeks. Unfortunately, she was fired from her job for missing too many workdays.
Antenatal testing has become routine, and it is costly
For the prescriber, antenatal testing is simple: Order a weekly ultrasound exam to reduce the risk of stillbirth, decrease litigation, generate income, and maximize patient satisfaction (with the assumption that everyone likes to peek at their baby). Recommending antenatal testing has—with the best intentions—become a habit and therefore is difficult to break. However, the American College of Obstetricians and Gynecologists (ACOG) recognizes that “there is a paucity of evidenced-based recommendations on the timing and frequency of antenatal fetal surveillance because of the challenges of conducting prospective trials in pregnancies complicated by stillbirths and the varying conditions that place pregnancies at high risk for stillbirth. As a result, evidence for the efficacy of antenatal fetal surveillance, when available, is largely circumstantial.”1
Antenatal testing without an evidence-based indication can be costly for the health care system, insurance companies, and patients. Many clinics, especially those in rural communities, do not have the equipment or personnel to complete antenatal testing on site. Asking a pregnant patient to travel repeatedly to another location for antenatal testing can increase her time off from work, complicate childcare, pose a financial burden, and lead to nonadherence. As clinicians, it is imperative that we work with our patients to create an individualized care plan to minimize these burdens and increase adherence.
Antenatal fetal surveillance can be considered for conditions in which stillbirth is reported more frequently than 0.8 per 1,000.
Advanced maternal age and stillbirth risk
One of the most common reasons for antenatal testing is advanced maternal age, that is, age older than 35. According to the Centers for Disease Control and Prevention and the National Vital Statistics System, from 2000 to 2012, 46 states and the District of Columbia (DC) reported an increase in first birth rates for women aged 35 to 39. Thirty-one states and DC saw a rise among women aged 40 to 44 in the same period (FIGURE).2
Advanced maternal age is an independent risk factor for stillbirth, with women aged 35 to 39 at 1.9-fold increased risk and women older than age 40 with a 2.4-fold higher risk compared with women younger than age 30.3 In a review of 44 studies including nearly 45,000,000 births, case-control studies, versus cohort studies, demonstrated a higher odds for stillbirth among women aged 35 and older (odds ratio [OR], 2.39; 95% confidence interval [CI], 1.57-3.66 vs OR, 1.73; 95% CI, 1.6-1.87).4 Now, many women older than age 35 may have a concomitant risk factor, such as diabetes or hypertension, that requires antenatal testing. However, for those without other risk factors, nearly 863 antenatal tests and 71 inductions would need to be completed to reduce the number of stillbirths by 1. Antenatal testing for women older than age 35 without other risk factors should be individualized through shared decision making.5 See the ACOG committee opinion for a table that outlines factors associated with an increased risk of stillbirth and suggested strategies for antenatal surveillance after viability.1
Continue to: CASE 2 Patient with high BPP score and altered...
CASE 2 Patient with high BPP score and altered fetal movements delivered for nonreassuring fetal heart rate
Ms. Q was undergoing weekly BPPs for diet-controlled gestational diabetes and a prepregnancy body mass index (BMI) of 52. At 37 weeks’ gestation, she had a BPP score of 8/8. However, it took almost 30 minutes to see 2 discrete body or limb movements. Ms. Q mentioned to the nurse taking her vitals after the BPP that the baby’s movements had changed over the previous few days, especially after contractions. Ms. Q then completed a nonstress test (NST); she had 2 contractions and 2 fetal heart rate decelerations, each lasting approximately 60 seconds. Ms. Q was sent to labor and delivery for prolonged monitoring, and she was delivered that day for a nonreassuring fetal heart rate tracing. Meconium-stained amniotic fluid and a tight triple nuchal cord were noted at delivery.
BPP considerations
While considered an in-depth look at the fetal status, BPPs may not predict overall fetal well-being during acute changes, such as umbilical cord compression or placental abruption. BPPs take longer to complete, require a trained sonographer, and include components like fetal breathing that may be influenced by such factors as nicotine,6-8 labor,9 rupture of membranes,10 magnesium sulfate,11 and infection.12
If medically indicated, which antenatal surveillance technique is right for your patient?
Frequently used antepartum fetal surveillance techniques include maternal perception of fetal movement or “kick counting,” NST, BPP, modified BPP, contraction stress test (CST), and umbilical artery Doppler velocimetry.
Worldwide, the most common form of antenatal surveillance is fetal kick counting. It is noninvasive, can be completed frequently, may decrease maternal anxiety, may improve maternal-fetal bonding, and is free.13 According to the results of a 2020 meta-analysis of 468,601 fetuses, however, there was no difference in perinatal death among patients who assessed fetal movements (0.54%) and those who did not (0.59%).14 There was a statistically significant increase in induction of labor, cesarean delivery, and preterm delivery among patients who counted fetal movements. Women who perceive a decrease in fetal movement should seek medical attention from a health care provider.
An evaluation for decreased fetal movement typically includes taking a history that focuses on risk factors that may increase stillbirth, including hypertension, growth restriction, fetal anomalies, diabetes, and substance use, and auscultation with a fetal Doppler. In the absence of risk factors and the presence of a normal fetal heartbeat, pregnant women should be reassured of fetal well-being. In a pregnancy at greater than 28 weeks, a 20-minute NST can be completed as well; this has become part of the standard workup of decreased fetal movement in developed countries. A reactive NST indicates normal fetal autonomic function in real time and a low incidence of stillbirth (1.9/1,000) within 1 week.15
Additionally, by measuring the amniotic fluid volume using the largest maximal vertical pocket (MVP), clinicians can gain insight into overall uteroplacental function. The combination of the NST and the MVP—otherwise known as a modified BPP—provides both short-term acid-base status and long-term uteroplacental function. The incidence of stillbirth in the 1 week after a modified BPP has been reported to be 0.8/1,000, which is equivalent to stillbirth incidence using a full BPP (0.8/1,000).16 The negative predictive value for both the modified BPP and the BPP is 99.9%—equivalent.
The case for modified BPP use
The modified BPP requires less time, is less costly (cost savings of approximately 50%), does not require a specialized sonographer, and can be performed in local community clinics.
Perhaps the initial antepartum surveillance test of choice should be the modified BPP, with the BPP used in cases in which the results of a modified BPP are abnormal. ●
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for MaternalFetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion no. 828. Obstet Gynecol. 2021;137:e177-197.
- Mathews TJ, Hamilton BE. First births to older women continue to rise. NCHS Data Brief, No. 152. Hyattsville, MD: National Center for Health Statistics; 2014.
- Fretts RC, Schmittdiel J, McLean FH, et al. Increased maternal age and the risk of fetal death. N Engl J Med. 1995;333: 953-957.
- Lean SC, Derricott H, Jones RL, et al. Advanced maternal age and adverse pregnancy outcomes: a systematic review and meta-analysis. PLoS One. 2017;12:e0186287.
- Fretts RC, Elkins EB, Myers ER, et al. Should older women have antepartum testing to prevent unexplained stillbirth? Obstet Gynecol. 2004;104:56-64.
- Manning F, Wyn Pugh E, Boddy K. Effect of cigarette smoking on fetal breathing movements in normal pregnancies. Br Med J. 1975;1:552-553.
- Manning FA, Feyerabend C. Cigarette smoking and fetal breathing movements. Br J Obstet Gynecol. 1976;83:262-270.
- Gennser G, Marsal K, Brantmark B. Maternal smoking and fetal breathing movements. Am J Obstet Gynecol. 1975;123:861-867.
- Boylan P, O’Donovan P, Owens OJ. Fetal breathing movements and the diagnosis of labor: a prospective analysis of 100 cases. Obstet Gynecol. 1985;66:517-520.
- Kivikoski AI, Amon E, Vaalamo PO, et al. Effect of thirdtrimester premature rupture of membranes on fetal breathing movements: a prospective case-control study. Am J Obstet Gynecol. 1988;159:1474-1477.
- Peaceman AM, Meyer BA, Thorp JA, et al. The effect of magnesium sulfate tocolysis on the fetal biophysical profile. Am J Obstet Gynecol. 1989;161:771-774.
- Vintzileos AM, Campbell WA, Nochimson DJ, et al. The fetal biophysical profile in patients with premature rupture of the membranes—an early predictor of fetal infection. Am J Obstet Gynecol. 1985;152:501-516.
- Liston RM, Bloom K, Zimmer P. The psychological effects of counting fetal movements. Birth. 1994;21:135-140.
- Bellussi F, Po’ G, Livi A, et al. Fetal movement counting and perinatal mortality: a systematic review and meta-analysis. Obstet Gynecol. 2020;135:453-462.
- Freeman RK, Anderson G, Dorchester W. A prospective multiinstitutional study of antepartum fetal heart rate monitoring. I. Risk of perinatal mortality and morbidity according to antepartum fetal heart rate test results. Am J Obstet Gynecol. 1982;143:771-777.
- Miller DA , Rabello YA, Paul RH. The modified biophysical profile: antepartum testing in the 1990s. Am J Obstet Gynecol. 1996;174:812-817.
CASE 1 Pregnant patient endures extensive wait and travel times to have antenatal testing
Pregnant at age 35 without comorbidities, Ms. H was instructed to schedule weekly biophysical profiles (BPP) after 36 weeks’ gestation for advanced maternal age. She receives care at a community office 25 miles from the hospital where she will deliver. Ms. H must complete her antenatal testing at the hospital where the sonographer performs BPPs. She sees her physician at the nearby clinic and then takes public transit to the hospital. She waits 2 hours to be seen then makes her way back home. Her prenatal care visit, which usually takes 30 minutes, turns into a 5-hour ordeal. Ms. H delivered a healthy baby at 39 weeks. Unfortunately, she was fired from her job for missing too many workdays.
Antenatal testing has become routine, and it is costly
For the prescriber, antenatal testing is simple: Order a weekly ultrasound exam to reduce the risk of stillbirth, decrease litigation, generate income, and maximize patient satisfaction (with the assumption that everyone likes to peek at their baby). Recommending antenatal testing has—with the best intentions—become a habit and therefore is difficult to break. However, the American College of Obstetricians and Gynecologists (ACOG) recognizes that “there is a paucity of evidenced-based recommendations on the timing and frequency of antenatal fetal surveillance because of the challenges of conducting prospective trials in pregnancies complicated by stillbirths and the varying conditions that place pregnancies at high risk for stillbirth. As a result, evidence for the efficacy of antenatal fetal surveillance, when available, is largely circumstantial.”1
Antenatal testing without an evidence-based indication can be costly for the health care system, insurance companies, and patients. Many clinics, especially those in rural communities, do not have the equipment or personnel to complete antenatal testing on site. Asking a pregnant patient to travel repeatedly to another location for antenatal testing can increase her time off from work, complicate childcare, pose a financial burden, and lead to nonadherence. As clinicians, it is imperative that we work with our patients to create an individualized care plan to minimize these burdens and increase adherence.
Antenatal fetal surveillance can be considered for conditions in which stillbirth is reported more frequently than 0.8 per 1,000.
Advanced maternal age and stillbirth risk
One of the most common reasons for antenatal testing is advanced maternal age, that is, age older than 35. According to the Centers for Disease Control and Prevention and the National Vital Statistics System, from 2000 to 2012, 46 states and the District of Columbia (DC) reported an increase in first birth rates for women aged 35 to 39. Thirty-one states and DC saw a rise among women aged 40 to 44 in the same period (FIGURE).2

Advanced maternal age is an independent risk factor for stillbirth, with women aged 35 to 39 at 1.9-fold increased risk and women older than age 40 with a 2.4-fold higher risk compared with women younger than age 30.3 In a review of 44 studies including nearly 45,000,000 births, case-control studies, versus cohort studies, demonstrated a higher odds for stillbirth among women aged 35 and older (odds ratio [OR], 2.39; 95% confidence interval [CI], 1.57-3.66 vs OR, 1.73; 95% CI, 1.6-1.87).4 Now, many women older than age 35 may have a concomitant risk factor, such as diabetes or hypertension, that requires antenatal testing. However, for those without other risk factors, nearly 863 antenatal tests and 71 inductions would need to be completed to reduce the number of stillbirths by 1. Antenatal testing for women older than age 35 without other risk factors should be individualized through shared decision making.5 See the ACOG committee opinion for a table that outlines factors associated with an increased risk of stillbirth and suggested strategies for antenatal surveillance after viability.1
Continue to: CASE 2 Patient with high BPP score and altered...
CASE 2 Patient with high BPP score and altered fetal movements delivered for nonreassuring fetal heart rate
Ms. Q was undergoing weekly BPPs for diet-controlled gestational diabetes and a prepregnancy body mass index (BMI) of 52. At 37 weeks’ gestation, she had a BPP score of 8/8. However, it took almost 30 minutes to see 2 discrete body or limb movements. Ms. Q mentioned to the nurse taking her vitals after the BPP that the baby’s movements had changed over the previous few days, especially after contractions. Ms. Q then completed a nonstress test (NST); she had 2 contractions and 2 fetal heart rate decelerations, each lasting approximately 60 seconds. Ms. Q was sent to labor and delivery for prolonged monitoring, and she was delivered that day for a nonreassuring fetal heart rate tracing. Meconium-stained amniotic fluid and a tight triple nuchal cord were noted at delivery.
BPP considerations
While considered an in-depth look at the fetal status, BPPs may not predict overall fetal well-being during acute changes, such as umbilical cord compression or placental abruption. BPPs take longer to complete, require a trained sonographer, and include components like fetal breathing that may be influenced by such factors as nicotine,6-8 labor,9 rupture of membranes,10 magnesium sulfate,11 and infection.12
If medically indicated, which antenatal surveillance technique is right for your patient?
Frequently used antepartum fetal surveillance techniques include maternal perception of fetal movement or “kick counting,” NST, BPP, modified BPP, contraction stress test (CST), and umbilical artery Doppler velocimetry.
Worldwide, the most common form of antenatal surveillance is fetal kick counting. It is noninvasive, can be completed frequently, may decrease maternal anxiety, may improve maternal-fetal bonding, and is free.13 According to the results of a 2020 meta-analysis of 468,601 fetuses, however, there was no difference in perinatal death among patients who assessed fetal movements (0.54%) and those who did not (0.59%).14 There was a statistically significant increase in induction of labor, cesarean delivery, and preterm delivery among patients who counted fetal movements. Women who perceive a decrease in fetal movement should seek medical attention from a health care provider.
An evaluation for decreased fetal movement typically includes taking a history that focuses on risk factors that may increase stillbirth, including hypertension, growth restriction, fetal anomalies, diabetes, and substance use, and auscultation with a fetal Doppler. In the absence of risk factors and the presence of a normal fetal heartbeat, pregnant women should be reassured of fetal well-being. In a pregnancy at greater than 28 weeks, a 20-minute NST can be completed as well; this has become part of the standard workup of decreased fetal movement in developed countries. A reactive NST indicates normal fetal autonomic function in real time and a low incidence of stillbirth (1.9/1,000) within 1 week.15
Additionally, by measuring the amniotic fluid volume using the largest maximal vertical pocket (MVP), clinicians can gain insight into overall uteroplacental function. The combination of the NST and the MVP—otherwise known as a modified BPP—provides both short-term acid-base status and long-term uteroplacental function. The incidence of stillbirth in the 1 week after a modified BPP has been reported to be 0.8/1,000, which is equivalent to stillbirth incidence using a full BPP (0.8/1,000).16 The negative predictive value for both the modified BPP and the BPP is 99.9%—equivalent.
The case for modified BPP use
The modified BPP requires less time, is less costly (cost savings of approximately 50%), does not require a specialized sonographer, and can be performed in local community clinics.
Perhaps the initial antepartum surveillance test of choice should be the modified BPP, with the BPP used in cases in which the results of a modified BPP are abnormal. ●
CASE 1 Pregnant patient endures extensive wait and travel times to have antenatal testing
Pregnant at age 35 without comorbidities, Ms. H was instructed to schedule weekly biophysical profiles (BPP) after 36 weeks’ gestation for advanced maternal age. She receives care at a community office 25 miles from the hospital where she will deliver. Ms. H must complete her antenatal testing at the hospital where the sonographer performs BPPs. She sees her physician at the nearby clinic and then takes public transit to the hospital. She waits 2 hours to be seen then makes her way back home. Her prenatal care visit, which usually takes 30 minutes, turns into a 5-hour ordeal. Ms. H delivered a healthy baby at 39 weeks. Unfortunately, she was fired from her job for missing too many workdays.
Antenatal testing has become routine, and it is costly
For the prescriber, antenatal testing is simple: Order a weekly ultrasound exam to reduce the risk of stillbirth, decrease litigation, generate income, and maximize patient satisfaction (with the assumption that everyone likes to peek at their baby). Recommending antenatal testing has—with the best intentions—become a habit and therefore is difficult to break. However, the American College of Obstetricians and Gynecologists (ACOG) recognizes that “there is a paucity of evidenced-based recommendations on the timing and frequency of antenatal fetal surveillance because of the challenges of conducting prospective trials in pregnancies complicated by stillbirths and the varying conditions that place pregnancies at high risk for stillbirth. As a result, evidence for the efficacy of antenatal fetal surveillance, when available, is largely circumstantial.”1
Antenatal testing without an evidence-based indication can be costly for the health care system, insurance companies, and patients. Many clinics, especially those in rural communities, do not have the equipment or personnel to complete antenatal testing on site. Asking a pregnant patient to travel repeatedly to another location for antenatal testing can increase her time off from work, complicate childcare, pose a financial burden, and lead to nonadherence. As clinicians, it is imperative that we work with our patients to create an individualized care plan to minimize these burdens and increase adherence.
Antenatal fetal surveillance can be considered for conditions in which stillbirth is reported more frequently than 0.8 per 1,000.
Advanced maternal age and stillbirth risk
One of the most common reasons for antenatal testing is advanced maternal age, that is, age older than 35. According to the Centers for Disease Control and Prevention and the National Vital Statistics System, from 2000 to 2012, 46 states and the District of Columbia (DC) reported an increase in first birth rates for women aged 35 to 39. Thirty-one states and DC saw a rise among women aged 40 to 44 in the same period (FIGURE).2

Advanced maternal age is an independent risk factor for stillbirth, with women aged 35 to 39 at 1.9-fold increased risk and women older than age 40 with a 2.4-fold higher risk compared with women younger than age 30.3 In a review of 44 studies including nearly 45,000,000 births, case-control studies, versus cohort studies, demonstrated a higher odds for stillbirth among women aged 35 and older (odds ratio [OR], 2.39; 95% confidence interval [CI], 1.57-3.66 vs OR, 1.73; 95% CI, 1.6-1.87).4 Now, many women older than age 35 may have a concomitant risk factor, such as diabetes or hypertension, that requires antenatal testing. However, for those without other risk factors, nearly 863 antenatal tests and 71 inductions would need to be completed to reduce the number of stillbirths by 1. Antenatal testing for women older than age 35 without other risk factors should be individualized through shared decision making.5 See the ACOG committee opinion for a table that outlines factors associated with an increased risk of stillbirth and suggested strategies for antenatal surveillance after viability.1
Continue to: CASE 2 Patient with high BPP score and altered...
CASE 2 Patient with high BPP score and altered fetal movements delivered for nonreassuring fetal heart rate
Ms. Q was undergoing weekly BPPs for diet-controlled gestational diabetes and a prepregnancy body mass index (BMI) of 52. At 37 weeks’ gestation, she had a BPP score of 8/8. However, it took almost 30 minutes to see 2 discrete body or limb movements. Ms. Q mentioned to the nurse taking her vitals after the BPP that the baby’s movements had changed over the previous few days, especially after contractions. Ms. Q then completed a nonstress test (NST); she had 2 contractions and 2 fetal heart rate decelerations, each lasting approximately 60 seconds. Ms. Q was sent to labor and delivery for prolonged monitoring, and she was delivered that day for a nonreassuring fetal heart rate tracing. Meconium-stained amniotic fluid and a tight triple nuchal cord were noted at delivery.
BPP considerations
While considered an in-depth look at the fetal status, BPPs may not predict overall fetal well-being during acute changes, such as umbilical cord compression or placental abruption. BPPs take longer to complete, require a trained sonographer, and include components like fetal breathing that may be influenced by such factors as nicotine,6-8 labor,9 rupture of membranes,10 magnesium sulfate,11 and infection.12
If medically indicated, which antenatal surveillance technique is right for your patient?
Frequently used antepartum fetal surveillance techniques include maternal perception of fetal movement or “kick counting,” NST, BPP, modified BPP, contraction stress test (CST), and umbilical artery Doppler velocimetry.
Worldwide, the most common form of antenatal surveillance is fetal kick counting. It is noninvasive, can be completed frequently, may decrease maternal anxiety, may improve maternal-fetal bonding, and is free.13 According to the results of a 2020 meta-analysis of 468,601 fetuses, however, there was no difference in perinatal death among patients who assessed fetal movements (0.54%) and those who did not (0.59%).14 There was a statistically significant increase in induction of labor, cesarean delivery, and preterm delivery among patients who counted fetal movements. Women who perceive a decrease in fetal movement should seek medical attention from a health care provider.
An evaluation for decreased fetal movement typically includes taking a history that focuses on risk factors that may increase stillbirth, including hypertension, growth restriction, fetal anomalies, diabetes, and substance use, and auscultation with a fetal Doppler. In the absence of risk factors and the presence of a normal fetal heartbeat, pregnant women should be reassured of fetal well-being. In a pregnancy at greater than 28 weeks, a 20-minute NST can be completed as well; this has become part of the standard workup of decreased fetal movement in developed countries. A reactive NST indicates normal fetal autonomic function in real time and a low incidence of stillbirth (1.9/1,000) within 1 week.15
Additionally, by measuring the amniotic fluid volume using the largest maximal vertical pocket (MVP), clinicians can gain insight into overall uteroplacental function. The combination of the NST and the MVP—otherwise known as a modified BPP—provides both short-term acid-base status and long-term uteroplacental function. The incidence of stillbirth in the 1 week after a modified BPP has been reported to be 0.8/1,000, which is equivalent to stillbirth incidence using a full BPP (0.8/1,000).16 The negative predictive value for both the modified BPP and the BPP is 99.9%—equivalent.
The case for modified BPP use
The modified BPP requires less time, is less costly (cost savings of approximately 50%), does not require a specialized sonographer, and can be performed in local community clinics.
Perhaps the initial antepartum surveillance test of choice should be the modified BPP, with the BPP used in cases in which the results of a modified BPP are abnormal. ●
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for MaternalFetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion no. 828. Obstet Gynecol. 2021;137:e177-197.
- Mathews TJ, Hamilton BE. First births to older women continue to rise. NCHS Data Brief, No. 152. Hyattsville, MD: National Center for Health Statistics; 2014.
- Fretts RC, Schmittdiel J, McLean FH, et al. Increased maternal age and the risk of fetal death. N Engl J Med. 1995;333: 953-957.
- Lean SC, Derricott H, Jones RL, et al. Advanced maternal age and adverse pregnancy outcomes: a systematic review and meta-analysis. PLoS One. 2017;12:e0186287.
- Fretts RC, Elkins EB, Myers ER, et al. Should older women have antepartum testing to prevent unexplained stillbirth? Obstet Gynecol. 2004;104:56-64.
- Manning F, Wyn Pugh E, Boddy K. Effect of cigarette smoking on fetal breathing movements in normal pregnancies. Br Med J. 1975;1:552-553.
- Manning FA, Feyerabend C. Cigarette smoking and fetal breathing movements. Br J Obstet Gynecol. 1976;83:262-270.
- Gennser G, Marsal K, Brantmark B. Maternal smoking and fetal breathing movements. Am J Obstet Gynecol. 1975;123:861-867.
- Boylan P, O’Donovan P, Owens OJ. Fetal breathing movements and the diagnosis of labor: a prospective analysis of 100 cases. Obstet Gynecol. 1985;66:517-520.
- Kivikoski AI, Amon E, Vaalamo PO, et al. Effect of thirdtrimester premature rupture of membranes on fetal breathing movements: a prospective case-control study. Am J Obstet Gynecol. 1988;159:1474-1477.
- Peaceman AM, Meyer BA, Thorp JA, et al. The effect of magnesium sulfate tocolysis on the fetal biophysical profile. Am J Obstet Gynecol. 1989;161:771-774.
- Vintzileos AM, Campbell WA, Nochimson DJ, et al. The fetal biophysical profile in patients with premature rupture of the membranes—an early predictor of fetal infection. Am J Obstet Gynecol. 1985;152:501-516.
- Liston RM, Bloom K, Zimmer P. The psychological effects of counting fetal movements. Birth. 1994;21:135-140.
- Bellussi F, Po’ G, Livi A, et al. Fetal movement counting and perinatal mortality: a systematic review and meta-analysis. Obstet Gynecol. 2020;135:453-462.
- Freeman RK, Anderson G, Dorchester W. A prospective multiinstitutional study of antepartum fetal heart rate monitoring. I. Risk of perinatal mortality and morbidity according to antepartum fetal heart rate test results. Am J Obstet Gynecol. 1982;143:771-777.
- Miller DA , Rabello YA, Paul RH. The modified biophysical profile: antepartum testing in the 1990s. Am J Obstet Gynecol. 1996;174:812-817.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for MaternalFetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion no. 828. Obstet Gynecol. 2021;137:e177-197.
- Mathews TJ, Hamilton BE. First births to older women continue to rise. NCHS Data Brief, No. 152. Hyattsville, MD: National Center for Health Statistics; 2014.
- Fretts RC, Schmittdiel J, McLean FH, et al. Increased maternal age and the risk of fetal death. N Engl J Med. 1995;333: 953-957.
- Lean SC, Derricott H, Jones RL, et al. Advanced maternal age and adverse pregnancy outcomes: a systematic review and meta-analysis. PLoS One. 2017;12:e0186287.
- Fretts RC, Elkins EB, Myers ER, et al. Should older women have antepartum testing to prevent unexplained stillbirth? Obstet Gynecol. 2004;104:56-64.
- Manning F, Wyn Pugh E, Boddy K. Effect of cigarette smoking on fetal breathing movements in normal pregnancies. Br Med J. 1975;1:552-553.
- Manning FA, Feyerabend C. Cigarette smoking and fetal breathing movements. Br J Obstet Gynecol. 1976;83:262-270.
- Gennser G, Marsal K, Brantmark B. Maternal smoking and fetal breathing movements. Am J Obstet Gynecol. 1975;123:861-867.
- Boylan P, O’Donovan P, Owens OJ. Fetal breathing movements and the diagnosis of labor: a prospective analysis of 100 cases. Obstet Gynecol. 1985;66:517-520.
- Kivikoski AI, Amon E, Vaalamo PO, et al. Effect of thirdtrimester premature rupture of membranes on fetal breathing movements: a prospective case-control study. Am J Obstet Gynecol. 1988;159:1474-1477.
- Peaceman AM, Meyer BA, Thorp JA, et al. The effect of magnesium sulfate tocolysis on the fetal biophysical profile. Am J Obstet Gynecol. 1989;161:771-774.
- Vintzileos AM, Campbell WA, Nochimson DJ, et al. The fetal biophysical profile in patients with premature rupture of the membranes—an early predictor of fetal infection. Am J Obstet Gynecol. 1985;152:501-516.
- Liston RM, Bloom K, Zimmer P. The psychological effects of counting fetal movements. Birth. 1994;21:135-140.
- Bellussi F, Po’ G, Livi A, et al. Fetal movement counting and perinatal mortality: a systematic review and meta-analysis. Obstet Gynecol. 2020;135:453-462.
- Freeman RK, Anderson G, Dorchester W. A prospective multiinstitutional study of antepartum fetal heart rate monitoring. I. Risk of perinatal mortality and morbidity according to antepartum fetal heart rate test results. Am J Obstet Gynecol. 1982;143:771-777.
- Miller DA , Rabello YA, Paul RH. The modified biophysical profile: antepartum testing in the 1990s. Am J Obstet Gynecol. 1996;174:812-817.