User login
Study links air pollution to psoriasis flares
Exposure to air pollution – even short term – may play a role in triggering psoriasis flares, according to new research from Italy, which found a significant association between exposure to higher levels of air pollution prior to patients presenting for psoriasis flares at medical visits, compared with visits unrelated to flares.
“We found that higher concentration of different air pollutants was associated with psoriasis flares in patients living in an industrialized city of the Po Valley” in Verona, Italy, report the authors of the study, published in JAMA Dermatology.
The findings underscore the need for clinicians to “consider environmental/external triggers in patients with chronic inflammatory diseases experiencing flares,” first author Francesco Bellinato, MD, of the Section of Dermatology and Venereology, University of Verona, Italy, told this news organization.
He and his coauthors conducted a case-crossover and cross-sectional longitudinal study that involved a retrospective analysis of data in 957 patients in Verona with chronic plaque psoriasis, who were evaluated every 3-4 months at an outpatient dermatology clinic for a median of 2.7 years.
Over the study period, disease flares, defined as an increase in the Psoriasis Area and Severity Index (PASI) of 5 or more points from the previous visit, occurred in 369 patients (38.6%), consistent with known flare rates in psoriasis. Participants in the study (mean age, 61) had median PASI scores of 12 during visits for psoriatic flares compared with PASI scores of 1 during control (no flare) visits (P < .001).
Evaluations of mean concentrations of several air pollutants within 10 miles of the patients over 4,398 visits showed that concentrations were significantly higher in the 60 days prior to the psoriasis flare, compared with control visits that were not related to flares (P < .05), after adjusting for factors including seasonality (by trimester, to adjust for weather conditions and UV/sunlight exposure) and the type of systemic psoriasis treatments patients were receiving (conventional or biological).
Increases in air pollutant levels prior to flares were observed among the 35.8% of patients who had a flare of at least a 50% increase in the PASI score, as well among the 47.2% of patients who had at least a 100% increase in PASI, compared with control visits not involving flares. In addition, mean and area-under-the-curve concentrations of air pollutants were higher in the 60 days before the visits among those with PASI 5 or greater, compared with those with PASI scores below 5, the authors add.
Dr. Bellinato noted that the associations were not limited to any particular subgroup. “The associations with air pollution and flares were observed in the entire population,” he said in an interview.
Vehicle, industry emissions
The pollutants that were measured were those mainly associated with fossil fuel combustion from vehicle and industry emissions, including carbon monoxide, nitrogen dioxide, other nitrogen oxides, benzene, coarse particulate matter (2.5-10.0 μm in diameter) and fine particulate matter (less than 2.5 μm in diameter).
They note that the risk of having a PASI score of 5 or greater was elevated even at thresholds of exposure that are largely considered safe. “Indeed, the risk for having a PASI score of 5 or greater was 40% to 50% higher at exposures as low as 20 μg/m3” of coarse particulate matter and 15 μg/m3 of fine particulate matter in the 60-day period prior to the visits, they write.
The authors referred to evidence linking air pollution with a worsening of a variety of inflammatory cutaneous diseases, including atopic dermatitis and acne, as well as photoaging. Psoriasis flares are known to be triggered by a variety of environmental factors, including infections or certain drugs; however, evidence of a role of air pollution has been lacking. Potential mechanisms linking the exposures to flares include the possibility that exhaust particles can activate skin resident T-cells, “resulting in abnormal production of proinflammatory cytokines including tumor necrosis factor α (TNF-α) and interleukins (ILs), including IL-1α, IL-1β, IL-6, and IL-8.8,” the authors write.
Their results, though inferring a causal relationship, fall short of showing a clear dose–response relationship between higher pollutant levels and an increased risk of psoriasis flares, possibly the result of a smaller sample size of subjects exposed to higher levels of pollution, they add.
Limitations of the study included the definition of flare, which used a clinical score that could be affected by other measurements, they point out, while strengths of the study included the large cohort of patients followed for over 7 years and the availability of daily measurements of air pollutants.
While the study suggests that environmental air pollutant fluctuations may affect psoriasis course,” the authors concluded, “further study is needed to examine whether these findings generalize to other populations and to better understand the mechanisms by which air pollution may affect psoriasis disease activity.”
Dr. Bellinato and four coauthors had no disclosures; the remaining authors had disclosures that included receiving personal fees from pharmaceutical companies that were outside of the submitted work.
A version of this article first appeared on Medscape.com.
Exposure to air pollution – even short term – may play a role in triggering psoriasis flares, according to new research from Italy, which found a significant association between exposure to higher levels of air pollution prior to patients presenting for psoriasis flares at medical visits, compared with visits unrelated to flares.
“We found that higher concentration of different air pollutants was associated with psoriasis flares in patients living in an industrialized city of the Po Valley” in Verona, Italy, report the authors of the study, published in JAMA Dermatology.
The findings underscore the need for clinicians to “consider environmental/external triggers in patients with chronic inflammatory diseases experiencing flares,” first author Francesco Bellinato, MD, of the Section of Dermatology and Venereology, University of Verona, Italy, told this news organization.
He and his coauthors conducted a case-crossover and cross-sectional longitudinal study that involved a retrospective analysis of data in 957 patients in Verona with chronic plaque psoriasis, who were evaluated every 3-4 months at an outpatient dermatology clinic for a median of 2.7 years.
Over the study period, disease flares, defined as an increase in the Psoriasis Area and Severity Index (PASI) of 5 or more points from the previous visit, occurred in 369 patients (38.6%), consistent with known flare rates in psoriasis. Participants in the study (mean age, 61) had median PASI scores of 12 during visits for psoriatic flares compared with PASI scores of 1 during control (no flare) visits (P < .001).
Evaluations of mean concentrations of several air pollutants within 10 miles of the patients over 4,398 visits showed that concentrations were significantly higher in the 60 days prior to the psoriasis flare, compared with control visits that were not related to flares (P < .05), after adjusting for factors including seasonality (by trimester, to adjust for weather conditions and UV/sunlight exposure) and the type of systemic psoriasis treatments patients were receiving (conventional or biological).
Increases in air pollutant levels prior to flares were observed among the 35.8% of patients who had a flare of at least a 50% increase in the PASI score, as well among the 47.2% of patients who had at least a 100% increase in PASI, compared with control visits not involving flares. In addition, mean and area-under-the-curve concentrations of air pollutants were higher in the 60 days before the visits among those with PASI 5 or greater, compared with those with PASI scores below 5, the authors add.
Dr. Bellinato noted that the associations were not limited to any particular subgroup. “The associations with air pollution and flares were observed in the entire population,” he said in an interview.
Vehicle, industry emissions
The pollutants that were measured were those mainly associated with fossil fuel combustion from vehicle and industry emissions, including carbon monoxide, nitrogen dioxide, other nitrogen oxides, benzene, coarse particulate matter (2.5-10.0 μm in diameter) and fine particulate matter (less than 2.5 μm in diameter).
They note that the risk of having a PASI score of 5 or greater was elevated even at thresholds of exposure that are largely considered safe. “Indeed, the risk for having a PASI score of 5 or greater was 40% to 50% higher at exposures as low as 20 μg/m3” of coarse particulate matter and 15 μg/m3 of fine particulate matter in the 60-day period prior to the visits, they write.
The authors referred to evidence linking air pollution with a worsening of a variety of inflammatory cutaneous diseases, including atopic dermatitis and acne, as well as photoaging. Psoriasis flares are known to be triggered by a variety of environmental factors, including infections or certain drugs; however, evidence of a role of air pollution has been lacking. Potential mechanisms linking the exposures to flares include the possibility that exhaust particles can activate skin resident T-cells, “resulting in abnormal production of proinflammatory cytokines including tumor necrosis factor α (TNF-α) and interleukins (ILs), including IL-1α, IL-1β, IL-6, and IL-8.8,” the authors write.
Their results, though inferring a causal relationship, fall short of showing a clear dose–response relationship between higher pollutant levels and an increased risk of psoriasis flares, possibly the result of a smaller sample size of subjects exposed to higher levels of pollution, they add.
Limitations of the study included the definition of flare, which used a clinical score that could be affected by other measurements, they point out, while strengths of the study included the large cohort of patients followed for over 7 years and the availability of daily measurements of air pollutants.
While the study suggests that environmental air pollutant fluctuations may affect psoriasis course,” the authors concluded, “further study is needed to examine whether these findings generalize to other populations and to better understand the mechanisms by which air pollution may affect psoriasis disease activity.”
Dr. Bellinato and four coauthors had no disclosures; the remaining authors had disclosures that included receiving personal fees from pharmaceutical companies that were outside of the submitted work.
A version of this article first appeared on Medscape.com.
Exposure to air pollution – even short term – may play a role in triggering psoriasis flares, according to new research from Italy, which found a significant association between exposure to higher levels of air pollution prior to patients presenting for psoriasis flares at medical visits, compared with visits unrelated to flares.
“We found that higher concentration of different air pollutants was associated with psoriasis flares in patients living in an industrialized city of the Po Valley” in Verona, Italy, report the authors of the study, published in JAMA Dermatology.
The findings underscore the need for clinicians to “consider environmental/external triggers in patients with chronic inflammatory diseases experiencing flares,” first author Francesco Bellinato, MD, of the Section of Dermatology and Venereology, University of Verona, Italy, told this news organization.
He and his coauthors conducted a case-crossover and cross-sectional longitudinal study that involved a retrospective analysis of data in 957 patients in Verona with chronic plaque psoriasis, who were evaluated every 3-4 months at an outpatient dermatology clinic for a median of 2.7 years.
Over the study period, disease flares, defined as an increase in the Psoriasis Area and Severity Index (PASI) of 5 or more points from the previous visit, occurred in 369 patients (38.6%), consistent with known flare rates in psoriasis. Participants in the study (mean age, 61) had median PASI scores of 12 during visits for psoriatic flares compared with PASI scores of 1 during control (no flare) visits (P < .001).
Evaluations of mean concentrations of several air pollutants within 10 miles of the patients over 4,398 visits showed that concentrations were significantly higher in the 60 days prior to the psoriasis flare, compared with control visits that were not related to flares (P < .05), after adjusting for factors including seasonality (by trimester, to adjust for weather conditions and UV/sunlight exposure) and the type of systemic psoriasis treatments patients were receiving (conventional or biological).
Increases in air pollutant levels prior to flares were observed among the 35.8% of patients who had a flare of at least a 50% increase in the PASI score, as well among the 47.2% of patients who had at least a 100% increase in PASI, compared with control visits not involving flares. In addition, mean and area-under-the-curve concentrations of air pollutants were higher in the 60 days before the visits among those with PASI 5 or greater, compared with those with PASI scores below 5, the authors add.
Dr. Bellinato noted that the associations were not limited to any particular subgroup. “The associations with air pollution and flares were observed in the entire population,” he said in an interview.
Vehicle, industry emissions
The pollutants that were measured were those mainly associated with fossil fuel combustion from vehicle and industry emissions, including carbon monoxide, nitrogen dioxide, other nitrogen oxides, benzene, coarse particulate matter (2.5-10.0 μm in diameter) and fine particulate matter (less than 2.5 μm in diameter).
They note that the risk of having a PASI score of 5 or greater was elevated even at thresholds of exposure that are largely considered safe. “Indeed, the risk for having a PASI score of 5 or greater was 40% to 50% higher at exposures as low as 20 μg/m3” of coarse particulate matter and 15 μg/m3 of fine particulate matter in the 60-day period prior to the visits, they write.
The authors referred to evidence linking air pollution with a worsening of a variety of inflammatory cutaneous diseases, including atopic dermatitis and acne, as well as photoaging. Psoriasis flares are known to be triggered by a variety of environmental factors, including infections or certain drugs; however, evidence of a role of air pollution has been lacking. Potential mechanisms linking the exposures to flares include the possibility that exhaust particles can activate skin resident T-cells, “resulting in abnormal production of proinflammatory cytokines including tumor necrosis factor α (TNF-α) and interleukins (ILs), including IL-1α, IL-1β, IL-6, and IL-8.8,” the authors write.
Their results, though inferring a causal relationship, fall short of showing a clear dose–response relationship between higher pollutant levels and an increased risk of psoriasis flares, possibly the result of a smaller sample size of subjects exposed to higher levels of pollution, they add.
Limitations of the study included the definition of flare, which used a clinical score that could be affected by other measurements, they point out, while strengths of the study included the large cohort of patients followed for over 7 years and the availability of daily measurements of air pollutants.
While the study suggests that environmental air pollutant fluctuations may affect psoriasis course,” the authors concluded, “further study is needed to examine whether these findings generalize to other populations and to better understand the mechanisms by which air pollution may affect psoriasis disease activity.”
Dr. Bellinato and four coauthors had no disclosures; the remaining authors had disclosures that included receiving personal fees from pharmaceutical companies that were outside of the submitted work.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Q&A With JAAD Editor Dirk M. Elston, MD
who has authored more than 600 peer-reviewed publications and 92 textbook chapters.
After earning his undergraduate degree from Pennsylvania State University and his medical degree from Jefferson Medical College in Philadelphia, Dr. Elston completed an internship and a dermatology residency at Walter Reed Army Medical Center in Washington, as well as a dermatopathology fellowship at the Cleveland Clinic. He currently is professor and chair of the department of dermatology and dermatologic surgery at the Medical University of South Carolina in Charleston.
Dr. Elston is one of five authors of “Andrews’ Diseases of the Skin),” coauthor with Tammie Ferringer, MD, of the “Dermatopathology” textbook, and editor in chief of the Requisites in Dermatology series of textbooks. In 2018, he succeeded Bruce H. Thiers, MD, as editor of the Journal of the American Academy of Dermatology and in 2021, received the AAD’s Gold Medal Award, which is the academy’s highest honor.
In an interview, Dr. Elston reflected on his mentors, shared how he manages his many responsibilities as a clinician, teacher, and editor, and talked about the promising future of dermatology.
Who inspired you most to pursue a career in medicine? My grandmother, Annie Elston, was a physician and dedicated her life to helping others. She was a front-line medic during World War I, helped to run a neonatal syphilis ward after the war, and practiced pediatrics in New York City until her death. She was a great role model.
Did you enter medical school knowing that you wanted to become a dermatologist? If not, what was the turning point for you? I didn’t really know much about dermatology when I entered medical school. I fell in love with the specialty during a rotation.
What was the most memorable experience from your dermatology residency at Walter Reed Army Medical Center? There were so many interesting patients, including many tropical diseases.
Why did you choose to pursue a fellowship in dermatopathology? What was it about this subspeciality that piqued your interest? Great teachers, including Tim Berger, MD, George Lupton, MD, and Dean Pearson, MD. They inspired me to seek a dermpath fellowship and I was lucky enough to train with Wilma Bergfeld, MD.
In your opinion, what’s been the most important advance in dermatopathology to date?
Immunohistochemistry changed the specialty. Now molecular diagnostics is a second wave of major advancement.
How do you stay passionate about both dermatology and dermatopathology? The patients, residents, and fellows keep it interesting. It’s a two-way street. I learn as much as I teach.
You’ve had a remarkable run at the Journal of the American Academy of Dermatology, starting as deputy editor in 2008 before becoming editor in 2018. What’s been most rewarding about this role for you? It is a labor of love and such a privilege to see everyone’s best work.
During the peak of the COVID-19 pandemic, what were your most significant challenges from both a clinical and a personal standpoint? Fear of the unknown is always a challenge with a new epidemic and worse with a pandemic. The patients still needed to be seen but it was a challenge with some buildings closed and some personnel afraid to come to work.
Is there anything you would tell your younger self in terms of career advice? Enjoy every step of the journey.
Considering your various work responsibilities as a clinician, teacher, and editor, what’s your strategy for achieving a work-life balance? A good friend of mine is fond of saying that balance is an illusion. There is only resilience. I believe the truth lies somewhere in between. Make time for family, and decide what has to get done today and what can wait until tomorrow.
What development in dermatology are you most excited about in the next 5 years? We are in a golden age of therapeutic innovations that are life changing and lifesaving for our patients. I never would have believed I would see complete cures of patients with widely metastatic melanoma. From psoriasis to eczema to malignancy, our therapeutic armamentarium is dramatically better each year. It makes the practice of medicine exciting.
who has authored more than 600 peer-reviewed publications and 92 textbook chapters.
After earning his undergraduate degree from Pennsylvania State University and his medical degree from Jefferson Medical College in Philadelphia, Dr. Elston completed an internship and a dermatology residency at Walter Reed Army Medical Center in Washington, as well as a dermatopathology fellowship at the Cleveland Clinic. He currently is professor and chair of the department of dermatology and dermatologic surgery at the Medical University of South Carolina in Charleston.
Dr. Elston is one of five authors of “Andrews’ Diseases of the Skin),” coauthor with Tammie Ferringer, MD, of the “Dermatopathology” textbook, and editor in chief of the Requisites in Dermatology series of textbooks. In 2018, he succeeded Bruce H. Thiers, MD, as editor of the Journal of the American Academy of Dermatology and in 2021, received the AAD’s Gold Medal Award, which is the academy’s highest honor.
In an interview, Dr. Elston reflected on his mentors, shared how he manages his many responsibilities as a clinician, teacher, and editor, and talked about the promising future of dermatology.
Who inspired you most to pursue a career in medicine? My grandmother, Annie Elston, was a physician and dedicated her life to helping others. She was a front-line medic during World War I, helped to run a neonatal syphilis ward after the war, and practiced pediatrics in New York City until her death. She was a great role model.
Did you enter medical school knowing that you wanted to become a dermatologist? If not, what was the turning point for you? I didn’t really know much about dermatology when I entered medical school. I fell in love with the specialty during a rotation.
What was the most memorable experience from your dermatology residency at Walter Reed Army Medical Center? There were so many interesting patients, including many tropical diseases.
Why did you choose to pursue a fellowship in dermatopathology? What was it about this subspeciality that piqued your interest? Great teachers, including Tim Berger, MD, George Lupton, MD, and Dean Pearson, MD. They inspired me to seek a dermpath fellowship and I was lucky enough to train with Wilma Bergfeld, MD.
In your opinion, what’s been the most important advance in dermatopathology to date?
Immunohistochemistry changed the specialty. Now molecular diagnostics is a second wave of major advancement.
How do you stay passionate about both dermatology and dermatopathology? The patients, residents, and fellows keep it interesting. It’s a two-way street. I learn as much as I teach.
You’ve had a remarkable run at the Journal of the American Academy of Dermatology, starting as deputy editor in 2008 before becoming editor in 2018. What’s been most rewarding about this role for you? It is a labor of love and such a privilege to see everyone’s best work.
During the peak of the COVID-19 pandemic, what were your most significant challenges from both a clinical and a personal standpoint? Fear of the unknown is always a challenge with a new epidemic and worse with a pandemic. The patients still needed to be seen but it was a challenge with some buildings closed and some personnel afraid to come to work.
Is there anything you would tell your younger self in terms of career advice? Enjoy every step of the journey.
Considering your various work responsibilities as a clinician, teacher, and editor, what’s your strategy for achieving a work-life balance? A good friend of mine is fond of saying that balance is an illusion. There is only resilience. I believe the truth lies somewhere in between. Make time for family, and decide what has to get done today and what can wait until tomorrow.
What development in dermatology are you most excited about in the next 5 years? We are in a golden age of therapeutic innovations that are life changing and lifesaving for our patients. I never would have believed I would see complete cures of patients with widely metastatic melanoma. From psoriasis to eczema to malignancy, our therapeutic armamentarium is dramatically better each year. It makes the practice of medicine exciting.
who has authored more than 600 peer-reviewed publications and 92 textbook chapters.
After earning his undergraduate degree from Pennsylvania State University and his medical degree from Jefferson Medical College in Philadelphia, Dr. Elston completed an internship and a dermatology residency at Walter Reed Army Medical Center in Washington, as well as a dermatopathology fellowship at the Cleveland Clinic. He currently is professor and chair of the department of dermatology and dermatologic surgery at the Medical University of South Carolina in Charleston.
Dr. Elston is one of five authors of “Andrews’ Diseases of the Skin),” coauthor with Tammie Ferringer, MD, of the “Dermatopathology” textbook, and editor in chief of the Requisites in Dermatology series of textbooks. In 2018, he succeeded Bruce H. Thiers, MD, as editor of the Journal of the American Academy of Dermatology and in 2021, received the AAD’s Gold Medal Award, which is the academy’s highest honor.
In an interview, Dr. Elston reflected on his mentors, shared how he manages his many responsibilities as a clinician, teacher, and editor, and talked about the promising future of dermatology.
Who inspired you most to pursue a career in medicine? My grandmother, Annie Elston, was a physician and dedicated her life to helping others. She was a front-line medic during World War I, helped to run a neonatal syphilis ward after the war, and practiced pediatrics in New York City until her death. She was a great role model.
Did you enter medical school knowing that you wanted to become a dermatologist? If not, what was the turning point for you? I didn’t really know much about dermatology when I entered medical school. I fell in love with the specialty during a rotation.
What was the most memorable experience from your dermatology residency at Walter Reed Army Medical Center? There were so many interesting patients, including many tropical diseases.
Why did you choose to pursue a fellowship in dermatopathology? What was it about this subspeciality that piqued your interest? Great teachers, including Tim Berger, MD, George Lupton, MD, and Dean Pearson, MD. They inspired me to seek a dermpath fellowship and I was lucky enough to train with Wilma Bergfeld, MD.
In your opinion, what’s been the most important advance in dermatopathology to date?
Immunohistochemistry changed the specialty. Now molecular diagnostics is a second wave of major advancement.
How do you stay passionate about both dermatology and dermatopathology? The patients, residents, and fellows keep it interesting. It’s a two-way street. I learn as much as I teach.
You’ve had a remarkable run at the Journal of the American Academy of Dermatology, starting as deputy editor in 2008 before becoming editor in 2018. What’s been most rewarding about this role for you? It is a labor of love and such a privilege to see everyone’s best work.
During the peak of the COVID-19 pandemic, what were your most significant challenges from both a clinical and a personal standpoint? Fear of the unknown is always a challenge with a new epidemic and worse with a pandemic. The patients still needed to be seen but it was a challenge with some buildings closed and some personnel afraid to come to work.
Is there anything you would tell your younger self in terms of career advice? Enjoy every step of the journey.
Considering your various work responsibilities as a clinician, teacher, and editor, what’s your strategy for achieving a work-life balance? A good friend of mine is fond of saying that balance is an illusion. There is only resilience. I believe the truth lies somewhere in between. Make time for family, and decide what has to get done today and what can wait until tomorrow.
What development in dermatology are you most excited about in the next 5 years? We are in a golden age of therapeutic innovations that are life changing and lifesaving for our patients. I never would have believed I would see complete cures of patients with widely metastatic melanoma. From psoriasis to eczema to malignancy, our therapeutic armamentarium is dramatically better each year. It makes the practice of medicine exciting.
Pharma should stop doing business in Russia, says ethicist
Should pharmaceutical companies continue to do business in Russia, running ongoing clinical trials, starting new ones, or continuing to sell their products there?
Some argue that medicine and science must not get enmeshed in politics, staying above the fray to protect their independence and credibility. Other defenders of business-as-usual say the pharmaceutical industry deals in health and aids the vulnerable. Humanitarianism requires continued interaction with Russia.
I think both arguments fail.
We are fighting a war with Russia. It is a war of economic strangulation, social isolation, and pushing Russia as hard as we can to become a pariah state so that internal pressure on Putin will cause him to rethink his cruel, unjustified invasion or the Russian people to replace him. This pressure must be harsh and it must happen quickly. Why?
Having failed to rapidly defeat the Ukrainian army in the war’s first weeks, Russian commanders are now resorting to the horrible barbarism they used in previous wars in Chechnya and Syria: flattening cities, attacking civilians, killing children with massive and indiscriminate firepower.
To mention one recent horror among many, Russian shelling destroyed a maternity hospital in Mariupol. Ukraine’s president, Volodymyr Zelensky, in bemoaning the Russians for their continuing series of war crimes called on the world to act.
“Mariupol. Direct Strike of Russian troops at the maternity hospital,” he wrote in a Twitter post. “People, children are under the wreckage. Atrocity! How much longer will the world be an accomplice ignoring terror?”
The Russian government’s response: “It is not the first time we have seen pathetic outcries concerning the so-called atrocities,” said Minister of Foreign Affairs Sergei Lavrov, claiming the hospital was being used as a base by an “ultra-radical” Ukrainian battalion.
Health and its preservation are key parts of the aim of medicine and science. There is no way that medicine and science can ignore what war does to health, what attacks on hospitals do to the sick and those who serve them there, the psychological toll that intentional terrorism takes on civilians and their defenders, and what the destruction of infrastructure means for the long-term well-being of Ukrainians.
There can be no collusion with war criminals. There can be no denial of the inextricable link between medicine, science, and politics. Medicine and science are controlled by political forces; their use for good or evil is driven by political considerations, and each doctor, scientist, and scientific society must take a stand when politics corrodes the underlying aims of research and healing.
How far does noncooperation with Russia go? Very, very far. All research, both ongoing and new, must cease immediately. Whatever can be done to minimize harm to existing subjects in a short period of time ought to be done, but that is it.
Similarly, no sale of medicines or therapies ought to be occurring, be they life-saving or consumer products. Putin will see to it that such shipments go to the military or are sold on the black market for revenue, and there is nothing pharma companies can do to stop that.
The Russian people need to be pinched not only by the loss of cheeseburgers and boutique coffee but by products they use to maintain their well-being. War is cruel that way, but if you tolerate a government that is bombing and shelling a peaceful neighbor to oblivion, then pharma must ensure that efforts to make Putin and his kleptocratic goons feel the wrath of their fellow citizens.
Given the realities of nuclear Armageddon, the civilized world must fight obvious barbarity as best it can with sanctions, financial assaults, property seizures, and forgoing commerce, including important raw materials and health products. War, even in a fiscal form, is not without terrible costs; but achieving a rapid, just resolution against tyranny permits no exceptions for pharma or any other business if it is a war that must be fought.
Dr. Caplan is director of the division of medical ethics at New York University. He has consulted with Johnson & Johnson’s Panel for Compassionate Drug Use.
A version of this article first appeared on Medscape.com.
Should pharmaceutical companies continue to do business in Russia, running ongoing clinical trials, starting new ones, or continuing to sell their products there?
Some argue that medicine and science must not get enmeshed in politics, staying above the fray to protect their independence and credibility. Other defenders of business-as-usual say the pharmaceutical industry deals in health and aids the vulnerable. Humanitarianism requires continued interaction with Russia.
I think both arguments fail.
We are fighting a war with Russia. It is a war of economic strangulation, social isolation, and pushing Russia as hard as we can to become a pariah state so that internal pressure on Putin will cause him to rethink his cruel, unjustified invasion or the Russian people to replace him. This pressure must be harsh and it must happen quickly. Why?
Having failed to rapidly defeat the Ukrainian army in the war’s first weeks, Russian commanders are now resorting to the horrible barbarism they used in previous wars in Chechnya and Syria: flattening cities, attacking civilians, killing children with massive and indiscriminate firepower.
To mention one recent horror among many, Russian shelling destroyed a maternity hospital in Mariupol. Ukraine’s president, Volodymyr Zelensky, in bemoaning the Russians for their continuing series of war crimes called on the world to act.
“Mariupol. Direct Strike of Russian troops at the maternity hospital,” he wrote in a Twitter post. “People, children are under the wreckage. Atrocity! How much longer will the world be an accomplice ignoring terror?”
The Russian government’s response: “It is not the first time we have seen pathetic outcries concerning the so-called atrocities,” said Minister of Foreign Affairs Sergei Lavrov, claiming the hospital was being used as a base by an “ultra-radical” Ukrainian battalion.
Health and its preservation are key parts of the aim of medicine and science. There is no way that medicine and science can ignore what war does to health, what attacks on hospitals do to the sick and those who serve them there, the psychological toll that intentional terrorism takes on civilians and their defenders, and what the destruction of infrastructure means for the long-term well-being of Ukrainians.
There can be no collusion with war criminals. There can be no denial of the inextricable link between medicine, science, and politics. Medicine and science are controlled by political forces; their use for good or evil is driven by political considerations, and each doctor, scientist, and scientific society must take a stand when politics corrodes the underlying aims of research and healing.
How far does noncooperation with Russia go? Very, very far. All research, both ongoing and new, must cease immediately. Whatever can be done to minimize harm to existing subjects in a short period of time ought to be done, but that is it.
Similarly, no sale of medicines or therapies ought to be occurring, be they life-saving or consumer products. Putin will see to it that such shipments go to the military or are sold on the black market for revenue, and there is nothing pharma companies can do to stop that.
The Russian people need to be pinched not only by the loss of cheeseburgers and boutique coffee but by products they use to maintain their well-being. War is cruel that way, but if you tolerate a government that is bombing and shelling a peaceful neighbor to oblivion, then pharma must ensure that efforts to make Putin and his kleptocratic goons feel the wrath of their fellow citizens.
Given the realities of nuclear Armageddon, the civilized world must fight obvious barbarity as best it can with sanctions, financial assaults, property seizures, and forgoing commerce, including important raw materials and health products. War, even in a fiscal form, is not without terrible costs; but achieving a rapid, just resolution against tyranny permits no exceptions for pharma or any other business if it is a war that must be fought.
Dr. Caplan is director of the division of medical ethics at New York University. He has consulted with Johnson & Johnson’s Panel for Compassionate Drug Use.
A version of this article first appeared on Medscape.com.
Should pharmaceutical companies continue to do business in Russia, running ongoing clinical trials, starting new ones, or continuing to sell their products there?
Some argue that medicine and science must not get enmeshed in politics, staying above the fray to protect their independence and credibility. Other defenders of business-as-usual say the pharmaceutical industry deals in health and aids the vulnerable. Humanitarianism requires continued interaction with Russia.
I think both arguments fail.
We are fighting a war with Russia. It is a war of economic strangulation, social isolation, and pushing Russia as hard as we can to become a pariah state so that internal pressure on Putin will cause him to rethink his cruel, unjustified invasion or the Russian people to replace him. This pressure must be harsh and it must happen quickly. Why?
Having failed to rapidly defeat the Ukrainian army in the war’s first weeks, Russian commanders are now resorting to the horrible barbarism they used in previous wars in Chechnya and Syria: flattening cities, attacking civilians, killing children with massive and indiscriminate firepower.
To mention one recent horror among many, Russian shelling destroyed a maternity hospital in Mariupol. Ukraine’s president, Volodymyr Zelensky, in bemoaning the Russians for their continuing series of war crimes called on the world to act.
“Mariupol. Direct Strike of Russian troops at the maternity hospital,” he wrote in a Twitter post. “People, children are under the wreckage. Atrocity! How much longer will the world be an accomplice ignoring terror?”
The Russian government’s response: “It is not the first time we have seen pathetic outcries concerning the so-called atrocities,” said Minister of Foreign Affairs Sergei Lavrov, claiming the hospital was being used as a base by an “ultra-radical” Ukrainian battalion.
Health and its preservation are key parts of the aim of medicine and science. There is no way that medicine and science can ignore what war does to health, what attacks on hospitals do to the sick and those who serve them there, the psychological toll that intentional terrorism takes on civilians and their defenders, and what the destruction of infrastructure means for the long-term well-being of Ukrainians.
There can be no collusion with war criminals. There can be no denial of the inextricable link between medicine, science, and politics. Medicine and science are controlled by political forces; their use for good or evil is driven by political considerations, and each doctor, scientist, and scientific society must take a stand when politics corrodes the underlying aims of research and healing.
How far does noncooperation with Russia go? Very, very far. All research, both ongoing and new, must cease immediately. Whatever can be done to minimize harm to existing subjects in a short period of time ought to be done, but that is it.
Similarly, no sale of medicines or therapies ought to be occurring, be they life-saving or consumer products. Putin will see to it that such shipments go to the military or are sold on the black market for revenue, and there is nothing pharma companies can do to stop that.
The Russian people need to be pinched not only by the loss of cheeseburgers and boutique coffee but by products they use to maintain their well-being. War is cruel that way, but if you tolerate a government that is bombing and shelling a peaceful neighbor to oblivion, then pharma must ensure that efforts to make Putin and his kleptocratic goons feel the wrath of their fellow citizens.
Given the realities of nuclear Armageddon, the civilized world must fight obvious barbarity as best it can with sanctions, financial assaults, property seizures, and forgoing commerce, including important raw materials and health products. War, even in a fiscal form, is not without terrible costs; but achieving a rapid, just resolution against tyranny permits no exceptions for pharma or any other business if it is a war that must be fought.
Dr. Caplan is director of the division of medical ethics at New York University. He has consulted with Johnson & Johnson’s Panel for Compassionate Drug Use.
A version of this article first appeared on Medscape.com.
AGA Clinical Practice Update: Commentary on noninvasive CRC screening
A new expert commentary from the American Gastroenterological Association focuses on noninvasive screening options for colorectal cancer (CRC), as well as approaches to ensure quality in noninvasive screening programs. The commentary was published in Gastroenterology.
The American Cancer Society reported in its Cancer Facts & Figures 2021 report that lifetime risk of CRC in the United States is 4%, and those with above average risk are recommended to undergo CRC screening at an earlier age, with colonoscopy as a screening modality. Between 75% and 80% of the U.S. population is considered at average risk, and this is the group covered by the expert commentary. In this group, CRC rates jump from 35.1 to 61.2 cases per 100,000 people between the ages of 45-49 years and 50-54 years. Early-onset (before 50) CRC accounts for 12% of all cases and 7% of CRC-related deaths.
The authors noted that the U.S. Preventive Services Task Force made a grade B recommendation for individuals to begin screening at age 45, regardless of screening method, and their modeling suggests that screening initialization at 45 rather than 50 years increases life-years gained by 6.2% at the cost of a 17% increase in colonoscopies.
According to the commentary authors, a hybrid approach combining annual fecal immunochemical testing (FIT) at age 45-49, followed by colonoscopy between ages 50 and 70, could result in substantial gains in life-years while prioritizing colonoscopies for advancing age, which is associated with increased risk of advanced adenomas (AA) and CRC.
Exploring options
For stool-based CRC screening, FIT has generally replaced guaiac fecal occult blood testing because of better patient adherence and fewer restrictions on medicine and diet. FIT can produce a quantitative result measured in micrograms of hemoglobin per gram, or qualitatively positive above a threshold of 20 mcg per gram. The MTsDNA (Cologuard) test combines FIT with two DNA methylation markers, KRAS mutation screening, and a measurement of total human DNA, with use of an algorithm of combined results to determine positivity. It is approved only for average-risk individuals aged 45-85.
In cases where MTsDNA tests positive, but colonoscopy reveals no findings, an aerodigestive cancer could be present. However, this is considered rare based on a study that revealed that 2.4% of patients with discordant results developed an aerodigestive cancer during a median 5.4 years of follow-up, compared with 1.1% of cases with negative MTsDNA and negative colonoscopy. The difference was not statistically significant. The commentary authors suggest that no further testing is required after a negative high-quality colonoscopy and that patients can resume screening at normal intervals with any of the recommended tests.
The Septin 9 blood test (Epi proColon) is another screening option, and is FDA approved for average-risk individuals older than 50 years. It detects methylation of the promoter region of the Septin 9 gene. It has a 48% sensitivity and 91.5% specificity for CRC, as well as a sensitivity of 11.2% for AA. One model found that Septin 9 screening every 1 or 2 years could lead to more quality-adjusted life-years gained and prevention of more deaths than annual FIT, but with more colonoscopies. CRC screening guidelines do not endorse Septin 9, but screening studies are in progress to assess its performance.
Ensuring quality
“The linchpin for effective noninvasive screening programs is adherence, and several measures of adherence are required,” the authors wrote. To ensure high quality of noninvasive screening programs, it is important to create metrics and employ continuous monitoring of compliance, and to initiate changes when adherence and outcomes lag. Important metrics include patient compliance, rapid reporting of test results, timely implementation of follow-up colonoscopies, and systems put in place to restore patients to appropriate CRC screening intervals.
The authors suggested several specific metrics and attainable performance goals. The ratio of tests completed within 1 year to tests ordered should reach 90% or more. Outreach should be conducted to patients who do not complete testing within 1 month of the order. All patients should be contacted with 2 weeks of test results, and those who test negative should be made aware of the appropriate interval for future screening, along with the method of contact.
At least 80% of patients who receive a positive test should be offered a colonoscopy date within 3 months, and all within 6 months, because delay past that time is associated with greater risk of AA, CRC, and advanced-stage CRC. Within 6 months of a positive noninvasive test, at least 95% of patients should have undergone a colonoscopy, unless they are too ill, have moved, or cannot be reached. “Quality metrics for noninvasive screening programs should be set and program performance should be measured and ideally reported publicly,” the authors summarized. “Poor adherence at any level should trigger review of established protocols and facilitate change to ensure high-quality screening.”
Two authors disclosed relationships with Freenome and/or Check-Cap, but the third disclosed no conflicts.
A new expert commentary from the American Gastroenterological Association focuses on noninvasive screening options for colorectal cancer (CRC), as well as approaches to ensure quality in noninvasive screening programs. The commentary was published in Gastroenterology.
The American Cancer Society reported in its Cancer Facts & Figures 2021 report that lifetime risk of CRC in the United States is 4%, and those with above average risk are recommended to undergo CRC screening at an earlier age, with colonoscopy as a screening modality. Between 75% and 80% of the U.S. population is considered at average risk, and this is the group covered by the expert commentary. In this group, CRC rates jump from 35.1 to 61.2 cases per 100,000 people between the ages of 45-49 years and 50-54 years. Early-onset (before 50) CRC accounts for 12% of all cases and 7% of CRC-related deaths.
The authors noted that the U.S. Preventive Services Task Force made a grade B recommendation for individuals to begin screening at age 45, regardless of screening method, and their modeling suggests that screening initialization at 45 rather than 50 years increases life-years gained by 6.2% at the cost of a 17% increase in colonoscopies.
According to the commentary authors, a hybrid approach combining annual fecal immunochemical testing (FIT) at age 45-49, followed by colonoscopy between ages 50 and 70, could result in substantial gains in life-years while prioritizing colonoscopies for advancing age, which is associated with increased risk of advanced adenomas (AA) and CRC.
Exploring options
For stool-based CRC screening, FIT has generally replaced guaiac fecal occult blood testing because of better patient adherence and fewer restrictions on medicine and diet. FIT can produce a quantitative result measured in micrograms of hemoglobin per gram, or qualitatively positive above a threshold of 20 mcg per gram. The MTsDNA (Cologuard) test combines FIT with two DNA methylation markers, KRAS mutation screening, and a measurement of total human DNA, with use of an algorithm of combined results to determine positivity. It is approved only for average-risk individuals aged 45-85.
In cases where MTsDNA tests positive, but colonoscopy reveals no findings, an aerodigestive cancer could be present. However, this is considered rare based on a study that revealed that 2.4% of patients with discordant results developed an aerodigestive cancer during a median 5.4 years of follow-up, compared with 1.1% of cases with negative MTsDNA and negative colonoscopy. The difference was not statistically significant. The commentary authors suggest that no further testing is required after a negative high-quality colonoscopy and that patients can resume screening at normal intervals with any of the recommended tests.
The Septin 9 blood test (Epi proColon) is another screening option, and is FDA approved for average-risk individuals older than 50 years. It detects methylation of the promoter region of the Septin 9 gene. It has a 48% sensitivity and 91.5% specificity for CRC, as well as a sensitivity of 11.2% for AA. One model found that Septin 9 screening every 1 or 2 years could lead to more quality-adjusted life-years gained and prevention of more deaths than annual FIT, but with more colonoscopies. CRC screening guidelines do not endorse Septin 9, but screening studies are in progress to assess its performance.
Ensuring quality
“The linchpin for effective noninvasive screening programs is adherence, and several measures of adherence are required,” the authors wrote. To ensure high quality of noninvasive screening programs, it is important to create metrics and employ continuous monitoring of compliance, and to initiate changes when adherence and outcomes lag. Important metrics include patient compliance, rapid reporting of test results, timely implementation of follow-up colonoscopies, and systems put in place to restore patients to appropriate CRC screening intervals.
The authors suggested several specific metrics and attainable performance goals. The ratio of tests completed within 1 year to tests ordered should reach 90% or more. Outreach should be conducted to patients who do not complete testing within 1 month of the order. All patients should be contacted with 2 weeks of test results, and those who test negative should be made aware of the appropriate interval for future screening, along with the method of contact.
At least 80% of patients who receive a positive test should be offered a colonoscopy date within 3 months, and all within 6 months, because delay past that time is associated with greater risk of AA, CRC, and advanced-stage CRC. Within 6 months of a positive noninvasive test, at least 95% of patients should have undergone a colonoscopy, unless they are too ill, have moved, or cannot be reached. “Quality metrics for noninvasive screening programs should be set and program performance should be measured and ideally reported publicly,” the authors summarized. “Poor adherence at any level should trigger review of established protocols and facilitate change to ensure high-quality screening.”
Two authors disclosed relationships with Freenome and/or Check-Cap, but the third disclosed no conflicts.
A new expert commentary from the American Gastroenterological Association focuses on noninvasive screening options for colorectal cancer (CRC), as well as approaches to ensure quality in noninvasive screening programs. The commentary was published in Gastroenterology.
The American Cancer Society reported in its Cancer Facts & Figures 2021 report that lifetime risk of CRC in the United States is 4%, and those with above average risk are recommended to undergo CRC screening at an earlier age, with colonoscopy as a screening modality. Between 75% and 80% of the U.S. population is considered at average risk, and this is the group covered by the expert commentary. In this group, CRC rates jump from 35.1 to 61.2 cases per 100,000 people between the ages of 45-49 years and 50-54 years. Early-onset (before 50) CRC accounts for 12% of all cases and 7% of CRC-related deaths.
The authors noted that the U.S. Preventive Services Task Force made a grade B recommendation for individuals to begin screening at age 45, regardless of screening method, and their modeling suggests that screening initialization at 45 rather than 50 years increases life-years gained by 6.2% at the cost of a 17% increase in colonoscopies.
According to the commentary authors, a hybrid approach combining annual fecal immunochemical testing (FIT) at age 45-49, followed by colonoscopy between ages 50 and 70, could result in substantial gains in life-years while prioritizing colonoscopies for advancing age, which is associated with increased risk of advanced adenomas (AA) and CRC.
Exploring options
For stool-based CRC screening, FIT has generally replaced guaiac fecal occult blood testing because of better patient adherence and fewer restrictions on medicine and diet. FIT can produce a quantitative result measured in micrograms of hemoglobin per gram, or qualitatively positive above a threshold of 20 mcg per gram. The MTsDNA (Cologuard) test combines FIT with two DNA methylation markers, KRAS mutation screening, and a measurement of total human DNA, with use of an algorithm of combined results to determine positivity. It is approved only for average-risk individuals aged 45-85.
In cases where MTsDNA tests positive, but colonoscopy reveals no findings, an aerodigestive cancer could be present. However, this is considered rare based on a study that revealed that 2.4% of patients with discordant results developed an aerodigestive cancer during a median 5.4 years of follow-up, compared with 1.1% of cases with negative MTsDNA and negative colonoscopy. The difference was not statistically significant. The commentary authors suggest that no further testing is required after a negative high-quality colonoscopy and that patients can resume screening at normal intervals with any of the recommended tests.
The Septin 9 blood test (Epi proColon) is another screening option, and is FDA approved for average-risk individuals older than 50 years. It detects methylation of the promoter region of the Septin 9 gene. It has a 48% sensitivity and 91.5% specificity for CRC, as well as a sensitivity of 11.2% for AA. One model found that Septin 9 screening every 1 or 2 years could lead to more quality-adjusted life-years gained and prevention of more deaths than annual FIT, but with more colonoscopies. CRC screening guidelines do not endorse Septin 9, but screening studies are in progress to assess its performance.
Ensuring quality
“The linchpin for effective noninvasive screening programs is adherence, and several measures of adherence are required,” the authors wrote. To ensure high quality of noninvasive screening programs, it is important to create metrics and employ continuous monitoring of compliance, and to initiate changes when adherence and outcomes lag. Important metrics include patient compliance, rapid reporting of test results, timely implementation of follow-up colonoscopies, and systems put in place to restore patients to appropriate CRC screening intervals.
The authors suggested several specific metrics and attainable performance goals. The ratio of tests completed within 1 year to tests ordered should reach 90% or more. Outreach should be conducted to patients who do not complete testing within 1 month of the order. All patients should be contacted with 2 weeks of test results, and those who test negative should be made aware of the appropriate interval for future screening, along with the method of contact.
At least 80% of patients who receive a positive test should be offered a colonoscopy date within 3 months, and all within 6 months, because delay past that time is associated with greater risk of AA, CRC, and advanced-stage CRC. Within 6 months of a positive noninvasive test, at least 95% of patients should have undergone a colonoscopy, unless they are too ill, have moved, or cannot be reached. “Quality metrics for noninvasive screening programs should be set and program performance should be measured and ideally reported publicly,” the authors summarized. “Poor adherence at any level should trigger review of established protocols and facilitate change to ensure high-quality screening.”
Two authors disclosed relationships with Freenome and/or Check-Cap, but the third disclosed no conflicts.
FROM GASTROENTEROLOGY
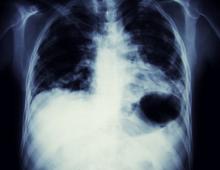
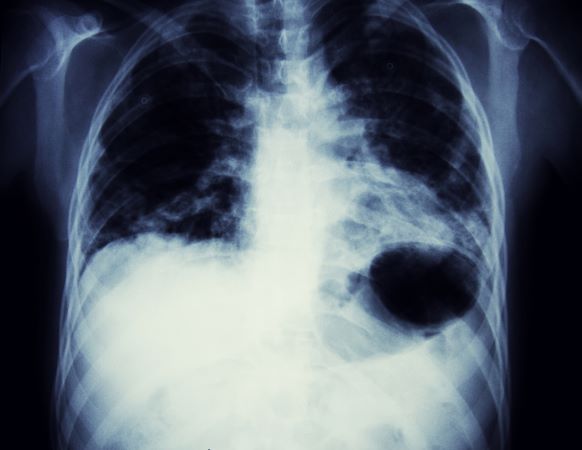
Cough and moderate hoarseness
Based on the patient's presentation, history, and imaging results, the likely diagnosis is non–small cell lung cancer (NSCLC) of an adenocarcinoma subtype. NSCLC accounts for about 80% of all lung cancer cases. Adenocarcinoma, in particular, is the most common type of lung cancer in the United States, accounting for about 40% of cases. This subtype is also the most common histology among nonsmokers. Still, individuals aged 55 to 77 years with a smoking history of 30 pack-years or more are considered to be the highest-risk group for lung cancer; those who quit less than 15 years ago — like the patient in the present case — are still considered to be in this risk group. Most cases of lung cancer are diagnosed at a late stage when symptoms have already begun to manifest. However, it should be noted that women are more likely to develop adenocarcinoma, are generally younger when they present with symptoms, and are more likely to present with localized disease. It remains to be proven whether the use of HRT affects the risk for lung cancer in women. Deaths from lung cancer, and in particular NSCLC, were shown to be higher among patients undergoing HRT, though no increase in lung cancer death was reported in women receiving estrogen alone.
In addition to the imaging described in this case, workup for NSCLC should include immunohistochemical (IHC) analyses to identify tumor type and lineage (adenocarcinoma, squamous cell carcinoma, metastatic malignancy, or primary pleural mesothelioma). Separate IHC analyses are then used to guide treatment decisions, identifying whether anaplastic lymphoma kinase inhibitor therapy or programmed death-ligand 1 inhibitor therapy would be appropriate.
Tissue should also be conserved for molecular testing. Management of NSCLC is primarily informed by the presence of targetable mutations. Among adenocarcinoma cases, the most common mutations are in the EGFR and KRAS genes. KRAS mutations, unlike EGFR mutations, are associated with a history of smoking and are considered prognostic biomarkers. Because overlapping targetable alterations are uncommon, patients who are confirmed to be harboring KRAS mutations will likely not benefit from additional molecular testing. Presence of the KRAS mutation suggests a poor response to EGFR tyrosine kinase inhibitors, though it does not appear to impact chemotherapeutic efficacy. Although no targeted therapies are yet available for this population, immune checkpoint inhibitors appear to be beneficial. National Comprehensive Cancer Network guidelines advise that all patients with adenocarcinoma be tested for EGFR mutations and that DNA mutational analysis is the preferred method.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Based on the patient's presentation, history, and imaging results, the likely diagnosis is non–small cell lung cancer (NSCLC) of an adenocarcinoma subtype. NSCLC accounts for about 80% of all lung cancer cases. Adenocarcinoma, in particular, is the most common type of lung cancer in the United States, accounting for about 40% of cases. This subtype is also the most common histology among nonsmokers. Still, individuals aged 55 to 77 years with a smoking history of 30 pack-years or more are considered to be the highest-risk group for lung cancer; those who quit less than 15 years ago — like the patient in the present case — are still considered to be in this risk group. Most cases of lung cancer are diagnosed at a late stage when symptoms have already begun to manifest. However, it should be noted that women are more likely to develop adenocarcinoma, are generally younger when they present with symptoms, and are more likely to present with localized disease. It remains to be proven whether the use of HRT affects the risk for lung cancer in women. Deaths from lung cancer, and in particular NSCLC, were shown to be higher among patients undergoing HRT, though no increase in lung cancer death was reported in women receiving estrogen alone.
In addition to the imaging described in this case, workup for NSCLC should include immunohistochemical (IHC) analyses to identify tumor type and lineage (adenocarcinoma, squamous cell carcinoma, metastatic malignancy, or primary pleural mesothelioma). Separate IHC analyses are then used to guide treatment decisions, identifying whether anaplastic lymphoma kinase inhibitor therapy or programmed death-ligand 1 inhibitor therapy would be appropriate.
Tissue should also be conserved for molecular testing. Management of NSCLC is primarily informed by the presence of targetable mutations. Among adenocarcinoma cases, the most common mutations are in the EGFR and KRAS genes. KRAS mutations, unlike EGFR mutations, are associated with a history of smoking and are considered prognostic biomarkers. Because overlapping targetable alterations are uncommon, patients who are confirmed to be harboring KRAS mutations will likely not benefit from additional molecular testing. Presence of the KRAS mutation suggests a poor response to EGFR tyrosine kinase inhibitors, though it does not appear to impact chemotherapeutic efficacy. Although no targeted therapies are yet available for this population, immune checkpoint inhibitors appear to be beneficial. National Comprehensive Cancer Network guidelines advise that all patients with adenocarcinoma be tested for EGFR mutations and that DNA mutational analysis is the preferred method.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Based on the patient's presentation, history, and imaging results, the likely diagnosis is non–small cell lung cancer (NSCLC) of an adenocarcinoma subtype. NSCLC accounts for about 80% of all lung cancer cases. Adenocarcinoma, in particular, is the most common type of lung cancer in the United States, accounting for about 40% of cases. This subtype is also the most common histology among nonsmokers. Still, individuals aged 55 to 77 years with a smoking history of 30 pack-years or more are considered to be the highest-risk group for lung cancer; those who quit less than 15 years ago — like the patient in the present case — are still considered to be in this risk group. Most cases of lung cancer are diagnosed at a late stage when symptoms have already begun to manifest. However, it should be noted that women are more likely to develop adenocarcinoma, are generally younger when they present with symptoms, and are more likely to present with localized disease. It remains to be proven whether the use of HRT affects the risk for lung cancer in women. Deaths from lung cancer, and in particular NSCLC, were shown to be higher among patients undergoing HRT, though no increase in lung cancer death was reported in women receiving estrogen alone.
In addition to the imaging described in this case, workup for NSCLC should include immunohistochemical (IHC) analyses to identify tumor type and lineage (adenocarcinoma, squamous cell carcinoma, metastatic malignancy, or primary pleural mesothelioma). Separate IHC analyses are then used to guide treatment decisions, identifying whether anaplastic lymphoma kinase inhibitor therapy or programmed death-ligand 1 inhibitor therapy would be appropriate.
Tissue should also be conserved for molecular testing. Management of NSCLC is primarily informed by the presence of targetable mutations. Among adenocarcinoma cases, the most common mutations are in the EGFR and KRAS genes. KRAS mutations, unlike EGFR mutations, are associated with a history of smoking and are considered prognostic biomarkers. Because overlapping targetable alterations are uncommon, patients who are confirmed to be harboring KRAS mutations will likely not benefit from additional molecular testing. Presence of the KRAS mutation suggests a poor response to EGFR tyrosine kinase inhibitors, though it does not appear to impact chemotherapeutic efficacy. Although no targeted therapies are yet available for this population, immune checkpoint inhibitors appear to be beneficial. National Comprehensive Cancer Network guidelines advise that all patients with adenocarcinoma be tested for EGFR mutations and that DNA mutational analysis is the preferred method.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
A 56-year-old woman presents with dyspnea, a persistent cough, and moderate hoarseness. She has no significant medical history other than thyroiditis. Her current medications include hormone replacement therapy (HRT). Although the patient reports a 20–pack-year history of smoking tobacco, she notes that she quit 11 years ago and has not been previously screened for lung cancer. A chest radiograph is ordered, which demonstrates a mass in the upper lobe of the right lung.
CV risk biomarkers tentatively identified in psoriatic disease
The risk of cardiovascular (CV) events in patients with psoriatic disease rises with higher levels of two cardiac biomarkers in a manner independent of risk calculated by the Framingham Risk Score (FRS), a longitudinal cohort study has shown. But researchers who conducted the study note that neither of the two biomarkers identified in the study – cardiac troponin I (cTnI) and N-terminal pro-brain-type natriuretic peptide (NT-proBNP) – led to an improvement in predictive performance when combined with the FRS, despite their association with carotid plaque burden.
Psoriasis and psoriatic arthritis are both associated with greater risk of CV morbidity and mortality, partly because of systemic inflammation that leads to atherogenesis. Measures of CV risk such as the FRS rely on traditional measures of CV risk and thus are likely to underestimate the CV event risk of people with psoriatic disease, according to the authors of the new study, published online in Arthritis & Rheumatology. The effort was led by Keith Colaço, MSc; Lihi Eder, MD, PhD; and other researchers affiliated with the University of Toronto.
“We are desperately in need of biomarker science advancement in psoriatic arthritis for a variety of places of guidance: How to choose a medication more accurately for the patient in front of us – that is, getting to be more like oncologists who use biomarkers to pick the best treatment or combination. That’s an important need. A second important need is how to guide clinicians regarding risk prediction for things like persistent, severe disease activity, progressive structural damage from disease, and, in this case, predicting a very common comorbidity that occurs in [psoriasis and] psoriatic arthritis patients,” Philip J. Mease, MD, told this news organization when asked to comment on the study.
Such biomarkers could assist with patient counseling, according to Dr. Mease, who is director of rheumatology research at Swedish Medical Center/Providence St. Joseph Health and is a clinical professor at the University of Washington, both in Seattle. Some patients may struggle with advice to lose weight or adopt lifestyle measures to limit CV risk, and more accurate predictions of risk may serve as further motivation. “It could well be that if you have a biomarker that accurately predicts a coming cataclysm, that it will lead you to redouble your efforts to do whatever it takes to reduce cardiovascular risk,” he said.
Both cTnI and NT-proBNP have been linked to increased CV risk in the general population, but little work has been done in the context of rheumatologic diseases.
The researchers analyzed data from 358 patients seen at the University of Toronto. The mean follow-up was 3.69 years. After adjustment for CV risk factors, lipid-lowering therapy, and creatinine levels, there was an association between cTnI levels and total carotid plaque area (adjusted beta coefficient, 0.21; 95% confidence interval, 0-0.41), but not for levels of NT-proBNP.
Atherosclerosis progressed in 89 participants overall, but multivariate adjustment revealed no significant relationship between progression and cTnI or NT-proBNP levels.
Separately, the researchers analyzed 1,000 individuals with psoriatic arthritis (n = 648) or with psoriasis and no arthritis (n = 352) whom they followed for a mean of 7.1 years after the patients underwent evaluation during 2002-2019. After adjustment for FRS, there was an association between the risk of a CV event and each 1–standard deviation increase in both cTnI (hazard ratio, 3.02; 95% CI, 1.12-8.16) and NT-proBNP (HR, 2.02; 95% CI, 1.28-3.18).
The combination of both biomarkers with the FRS predicted higher CV risk (HR, 1.91; 95% CI, 1.23-2.97). Neither biomarker made a statistically significant difference in changing CV risk prediction when added individually to FRS, although cTnI trended toward significance (HR, 2.60; 95% CI, 0.98-6.87).
Instead of the carotid plaque burden, Dr. Mease would have liked to have seen the authors evaluate calcium scores in coronary arteries as measured by CT. “I would have loved to have seen the researchers using that in addition to the carotid plaque assessment, to see what that would show us about these patients,” he said.
Only a small number of patients experienced CV events during the study period, which will likely make it necessary to conduct larger studies to identify a clear relationship. “You need a registry-type study with probably many hundreds if not thousands of patients in order to identify whether or not adding troponin could be useful to what we typically measure with patients when we’re trying to assess their risk,” Dr. Mease said.
The study was supported in part by the National Psoriasis Foundation and the Arthritis Society. Individual researchers have received support from a range of sources, including the Enid Walker Estate, the Women’s College Research Institute, the Arthritis Society, the National Psoriasis Foundation, the Edward Dunlop Foundation, the Ontario Ministry of Science and Innovation, and a Pfizer Chair Research Award. Some of the researchers have financial relationships with pharmaceutical companies that market drugs for psoriasis and psoriatic arthritis.
A version of this article first appeared on Medscape.com.
The risk of cardiovascular (CV) events in patients with psoriatic disease rises with higher levels of two cardiac biomarkers in a manner independent of risk calculated by the Framingham Risk Score (FRS), a longitudinal cohort study has shown. But researchers who conducted the study note that neither of the two biomarkers identified in the study – cardiac troponin I (cTnI) and N-terminal pro-brain-type natriuretic peptide (NT-proBNP) – led to an improvement in predictive performance when combined with the FRS, despite their association with carotid plaque burden.
Psoriasis and psoriatic arthritis are both associated with greater risk of CV morbidity and mortality, partly because of systemic inflammation that leads to atherogenesis. Measures of CV risk such as the FRS rely on traditional measures of CV risk and thus are likely to underestimate the CV event risk of people with psoriatic disease, according to the authors of the new study, published online in Arthritis & Rheumatology. The effort was led by Keith Colaço, MSc; Lihi Eder, MD, PhD; and other researchers affiliated with the University of Toronto.
“We are desperately in need of biomarker science advancement in psoriatic arthritis for a variety of places of guidance: How to choose a medication more accurately for the patient in front of us – that is, getting to be more like oncologists who use biomarkers to pick the best treatment or combination. That’s an important need. A second important need is how to guide clinicians regarding risk prediction for things like persistent, severe disease activity, progressive structural damage from disease, and, in this case, predicting a very common comorbidity that occurs in [psoriasis and] psoriatic arthritis patients,” Philip J. Mease, MD, told this news organization when asked to comment on the study.
Such biomarkers could assist with patient counseling, according to Dr. Mease, who is director of rheumatology research at Swedish Medical Center/Providence St. Joseph Health and is a clinical professor at the University of Washington, both in Seattle. Some patients may struggle with advice to lose weight or adopt lifestyle measures to limit CV risk, and more accurate predictions of risk may serve as further motivation. “It could well be that if you have a biomarker that accurately predicts a coming cataclysm, that it will lead you to redouble your efforts to do whatever it takes to reduce cardiovascular risk,” he said.
Both cTnI and NT-proBNP have been linked to increased CV risk in the general population, but little work has been done in the context of rheumatologic diseases.
The researchers analyzed data from 358 patients seen at the University of Toronto. The mean follow-up was 3.69 years. After adjustment for CV risk factors, lipid-lowering therapy, and creatinine levels, there was an association between cTnI levels and total carotid plaque area (adjusted beta coefficient, 0.21; 95% confidence interval, 0-0.41), but not for levels of NT-proBNP.
Atherosclerosis progressed in 89 participants overall, but multivariate adjustment revealed no significant relationship between progression and cTnI or NT-proBNP levels.
Separately, the researchers analyzed 1,000 individuals with psoriatic arthritis (n = 648) or with psoriasis and no arthritis (n = 352) whom they followed for a mean of 7.1 years after the patients underwent evaluation during 2002-2019. After adjustment for FRS, there was an association between the risk of a CV event and each 1–standard deviation increase in both cTnI (hazard ratio, 3.02; 95% CI, 1.12-8.16) and NT-proBNP (HR, 2.02; 95% CI, 1.28-3.18).
The combination of both biomarkers with the FRS predicted higher CV risk (HR, 1.91; 95% CI, 1.23-2.97). Neither biomarker made a statistically significant difference in changing CV risk prediction when added individually to FRS, although cTnI trended toward significance (HR, 2.60; 95% CI, 0.98-6.87).
Instead of the carotid plaque burden, Dr. Mease would have liked to have seen the authors evaluate calcium scores in coronary arteries as measured by CT. “I would have loved to have seen the researchers using that in addition to the carotid plaque assessment, to see what that would show us about these patients,” he said.
Only a small number of patients experienced CV events during the study period, which will likely make it necessary to conduct larger studies to identify a clear relationship. “You need a registry-type study with probably many hundreds if not thousands of patients in order to identify whether or not adding troponin could be useful to what we typically measure with patients when we’re trying to assess their risk,” Dr. Mease said.
The study was supported in part by the National Psoriasis Foundation and the Arthritis Society. Individual researchers have received support from a range of sources, including the Enid Walker Estate, the Women’s College Research Institute, the Arthritis Society, the National Psoriasis Foundation, the Edward Dunlop Foundation, the Ontario Ministry of Science and Innovation, and a Pfizer Chair Research Award. Some of the researchers have financial relationships with pharmaceutical companies that market drugs for psoriasis and psoriatic arthritis.
A version of this article first appeared on Medscape.com.
The risk of cardiovascular (CV) events in patients with psoriatic disease rises with higher levels of two cardiac biomarkers in a manner independent of risk calculated by the Framingham Risk Score (FRS), a longitudinal cohort study has shown. But researchers who conducted the study note that neither of the two biomarkers identified in the study – cardiac troponin I (cTnI) and N-terminal pro-brain-type natriuretic peptide (NT-proBNP) – led to an improvement in predictive performance when combined with the FRS, despite their association with carotid plaque burden.
Psoriasis and psoriatic arthritis are both associated with greater risk of CV morbidity and mortality, partly because of systemic inflammation that leads to atherogenesis. Measures of CV risk such as the FRS rely on traditional measures of CV risk and thus are likely to underestimate the CV event risk of people with psoriatic disease, according to the authors of the new study, published online in Arthritis & Rheumatology. The effort was led by Keith Colaço, MSc; Lihi Eder, MD, PhD; and other researchers affiliated with the University of Toronto.
“We are desperately in need of biomarker science advancement in psoriatic arthritis for a variety of places of guidance: How to choose a medication more accurately for the patient in front of us – that is, getting to be more like oncologists who use biomarkers to pick the best treatment or combination. That’s an important need. A second important need is how to guide clinicians regarding risk prediction for things like persistent, severe disease activity, progressive structural damage from disease, and, in this case, predicting a very common comorbidity that occurs in [psoriasis and] psoriatic arthritis patients,” Philip J. Mease, MD, told this news organization when asked to comment on the study.
Such biomarkers could assist with patient counseling, according to Dr. Mease, who is director of rheumatology research at Swedish Medical Center/Providence St. Joseph Health and is a clinical professor at the University of Washington, both in Seattle. Some patients may struggle with advice to lose weight or adopt lifestyle measures to limit CV risk, and more accurate predictions of risk may serve as further motivation. “It could well be that if you have a biomarker that accurately predicts a coming cataclysm, that it will lead you to redouble your efforts to do whatever it takes to reduce cardiovascular risk,” he said.
Both cTnI and NT-proBNP have been linked to increased CV risk in the general population, but little work has been done in the context of rheumatologic diseases.
The researchers analyzed data from 358 patients seen at the University of Toronto. The mean follow-up was 3.69 years. After adjustment for CV risk factors, lipid-lowering therapy, and creatinine levels, there was an association between cTnI levels and total carotid plaque area (adjusted beta coefficient, 0.21; 95% confidence interval, 0-0.41), but not for levels of NT-proBNP.
Atherosclerosis progressed in 89 participants overall, but multivariate adjustment revealed no significant relationship between progression and cTnI or NT-proBNP levels.
Separately, the researchers analyzed 1,000 individuals with psoriatic arthritis (n = 648) or with psoriasis and no arthritis (n = 352) whom they followed for a mean of 7.1 years after the patients underwent evaluation during 2002-2019. After adjustment for FRS, there was an association between the risk of a CV event and each 1–standard deviation increase in both cTnI (hazard ratio, 3.02; 95% CI, 1.12-8.16) and NT-proBNP (HR, 2.02; 95% CI, 1.28-3.18).
The combination of both biomarkers with the FRS predicted higher CV risk (HR, 1.91; 95% CI, 1.23-2.97). Neither biomarker made a statistically significant difference in changing CV risk prediction when added individually to FRS, although cTnI trended toward significance (HR, 2.60; 95% CI, 0.98-6.87).
Instead of the carotid plaque burden, Dr. Mease would have liked to have seen the authors evaluate calcium scores in coronary arteries as measured by CT. “I would have loved to have seen the researchers using that in addition to the carotid plaque assessment, to see what that would show us about these patients,” he said.
Only a small number of patients experienced CV events during the study period, which will likely make it necessary to conduct larger studies to identify a clear relationship. “You need a registry-type study with probably many hundreds if not thousands of patients in order to identify whether or not adding troponin could be useful to what we typically measure with patients when we’re trying to assess their risk,” Dr. Mease said.
The study was supported in part by the National Psoriasis Foundation and the Arthritis Society. Individual researchers have received support from a range of sources, including the Enid Walker Estate, the Women’s College Research Institute, the Arthritis Society, the National Psoriasis Foundation, the Edward Dunlop Foundation, the Ontario Ministry of Science and Innovation, and a Pfizer Chair Research Award. Some of the researchers have financial relationships with pharmaceutical companies that market drugs for psoriasis and psoriatic arthritis.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS & RHEUMATOLOGY
Type 2 Diabetes Comorbidities
Migraine Pathophysiology
An 11-year-old female presented with skin discoloration on her back
Becker’s nevus
The history and physical exam are most consistent with Becker’s nevus, also known as Becker’s melanosis. This is a benign cutaneous hamartoma, usually found in males, characterized by a large, irregularly shaped brown patch, often with hypertrichosis.1 Becker’s nevus can be congenital but is more commonly noticed in late childhood or early adolescence, with thickening, increased pigmentation, and hair growth. Becker’s nevus is considered an overgrowth of epidermal pigment cells and hair follicles and is thought to be attributable to postzygotic mutations (with ACTB mutations most reported).1 It is often located unilaterally on the upper trunk but is occasionally present elsewhere on the body. Acne may occasionally develop within the nevus, which is believed to be triggered by puberty-associated androgens.1 The lesion tends to persist indefinitely but has no propensity for malignant transformation.
Becker’s nevus is generally an isolated skin lesion without other anomalies. However, in rare instances, it may be associated with ipsilateral breast hypoplasia or hypoplastic defects of the muscle, skin, or skeleton, which is known as Becker’s nevus syndrome.2 Treatment is not medically warranted for an isolated Becker’s nevus but may be pursued for cosmetic reasons. Although treatment is generally discouraged because of variable success, laser hair removal and laser therapy may be pursued to address the hypertrichosis and hyperpigmentation, respectively.
What is on the differential?
A café-au-lait macule (CALM) is a light- to dark-brown, oval lesion that commonly presents at birth or in early childhood. CALMs vary widely in size from less than 1.5 cm to more than 20 cm in diameter. They are asymptomatic and grow in proportion to the individual over time.3 Becker’s nevus can be distinguished from CALMs by the development of hypertrichosis, typical location and course, and other skin changes within the nevus.
Postinflammatory hyperpigmentation (PIH) is characterized by asymptomatic, darkened macules or patches that are brown to blue-gray in color. It is one of the most common causes of hyperpigmentation, particularly in skin of color, and can take months to years to resolve. PIH is caused by increased melanin production in response to a cutaneous inflammatory process, such as a drug reaction, allergy, mechanical or thermal injury, infection, phototoxicity, or an underlying skin condition.3 Our patient’s history with the lack of an inciting inflammatory process is more consistent with Becker’s nevus.
Erythema ab igne is a cutaneous reaction to heat that presents as a hyperpigmented patch with a reticular or mottled configuration and superficial venular telangiectasia. The lesion is initially erythematous and progresses to a pale pink to purplish dark-brown color.4 Causes include long-term use of a heating pad, laptop, electric blanket, or a hot water bottle. The absence of prolonged heat exposure in our patient’s history does not favor erythema ab igne.
Pigmentary mosaicism is characterized by a distinctive pattern of hyperpigmentation that follows the lines of ectodermal embryologic development, known as the lines of Blaschko.5 This condition is also known as linear and whorled nevoid hypermelanosis because of its streaky or swirl-like pattern. Pigmentary mosaicism can be present at birth or appear within the first few weeks of life. It is caused by genetic heterogeneity in neuroectodermal cells, which results in skin with areas of varying colors. Pigmentary mosaicism is unlikely in this case as our patient’s lesion does not follow the lines of Blaschko.
Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is the vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. Ms. Laborada and Dr. Eichenfield have no relevant financial disclosures.
References
1. Atzmony L et al. J Cutan Pathol. 2020;47(8):681-5.
2. Danarti R et al. J Am Acad Dermatol. 2004;51(6):965-9.
3. Paller A and Mancini AJ. “Hurwitz Clinical Pediatric Dermatology: A textbook of skin disorders of childhood and adolescence” 4th ed. Philadelphia: Elsevier Saunders, 2011.
4. Patel DP. JAMA Dermatol. 2017;153(7):685.
5. Kromann AB et al. Orphanet J Rare Dis. 2018;13(1):39.
Becker’s nevus
The history and physical exam are most consistent with Becker’s nevus, also known as Becker’s melanosis. This is a benign cutaneous hamartoma, usually found in males, characterized by a large, irregularly shaped brown patch, often with hypertrichosis.1 Becker’s nevus can be congenital but is more commonly noticed in late childhood or early adolescence, with thickening, increased pigmentation, and hair growth. Becker’s nevus is considered an overgrowth of epidermal pigment cells and hair follicles and is thought to be attributable to postzygotic mutations (with ACTB mutations most reported).1 It is often located unilaterally on the upper trunk but is occasionally present elsewhere on the body. Acne may occasionally develop within the nevus, which is believed to be triggered by puberty-associated androgens.1 The lesion tends to persist indefinitely but has no propensity for malignant transformation.
Becker’s nevus is generally an isolated skin lesion without other anomalies. However, in rare instances, it may be associated with ipsilateral breast hypoplasia or hypoplastic defects of the muscle, skin, or skeleton, which is known as Becker’s nevus syndrome.2 Treatment is not medically warranted for an isolated Becker’s nevus but may be pursued for cosmetic reasons. Although treatment is generally discouraged because of variable success, laser hair removal and laser therapy may be pursued to address the hypertrichosis and hyperpigmentation, respectively.
What is on the differential?
A café-au-lait macule (CALM) is a light- to dark-brown, oval lesion that commonly presents at birth or in early childhood. CALMs vary widely in size from less than 1.5 cm to more than 20 cm in diameter. They are asymptomatic and grow in proportion to the individual over time.3 Becker’s nevus can be distinguished from CALMs by the development of hypertrichosis, typical location and course, and other skin changes within the nevus.
Postinflammatory hyperpigmentation (PIH) is characterized by asymptomatic, darkened macules or patches that are brown to blue-gray in color. It is one of the most common causes of hyperpigmentation, particularly in skin of color, and can take months to years to resolve. PIH is caused by increased melanin production in response to a cutaneous inflammatory process, such as a drug reaction, allergy, mechanical or thermal injury, infection, phototoxicity, or an underlying skin condition.3 Our patient’s history with the lack of an inciting inflammatory process is more consistent with Becker’s nevus.
Erythema ab igne is a cutaneous reaction to heat that presents as a hyperpigmented patch with a reticular or mottled configuration and superficial venular telangiectasia. The lesion is initially erythematous and progresses to a pale pink to purplish dark-brown color.4 Causes include long-term use of a heating pad, laptop, electric blanket, or a hot water bottle. The absence of prolonged heat exposure in our patient’s history does not favor erythema ab igne.
Pigmentary mosaicism is characterized by a distinctive pattern of hyperpigmentation that follows the lines of ectodermal embryologic development, known as the lines of Blaschko.5 This condition is also known as linear and whorled nevoid hypermelanosis because of its streaky or swirl-like pattern. Pigmentary mosaicism can be present at birth or appear within the first few weeks of life. It is caused by genetic heterogeneity in neuroectodermal cells, which results in skin with areas of varying colors. Pigmentary mosaicism is unlikely in this case as our patient’s lesion does not follow the lines of Blaschko.
Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is the vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. Ms. Laborada and Dr. Eichenfield have no relevant financial disclosures.
References
1. Atzmony L et al. J Cutan Pathol. 2020;47(8):681-5.
2. Danarti R et al. J Am Acad Dermatol. 2004;51(6):965-9.
3. Paller A and Mancini AJ. “Hurwitz Clinical Pediatric Dermatology: A textbook of skin disorders of childhood and adolescence” 4th ed. Philadelphia: Elsevier Saunders, 2011.
4. Patel DP. JAMA Dermatol. 2017;153(7):685.
5. Kromann AB et al. Orphanet J Rare Dis. 2018;13(1):39.
Becker’s nevus
The history and physical exam are most consistent with Becker’s nevus, also known as Becker’s melanosis. This is a benign cutaneous hamartoma, usually found in males, characterized by a large, irregularly shaped brown patch, often with hypertrichosis.1 Becker’s nevus can be congenital but is more commonly noticed in late childhood or early adolescence, with thickening, increased pigmentation, and hair growth. Becker’s nevus is considered an overgrowth of epidermal pigment cells and hair follicles and is thought to be attributable to postzygotic mutations (with ACTB mutations most reported).1 It is often located unilaterally on the upper trunk but is occasionally present elsewhere on the body. Acne may occasionally develop within the nevus, which is believed to be triggered by puberty-associated androgens.1 The lesion tends to persist indefinitely but has no propensity for malignant transformation.
Becker’s nevus is generally an isolated skin lesion without other anomalies. However, in rare instances, it may be associated with ipsilateral breast hypoplasia or hypoplastic defects of the muscle, skin, or skeleton, which is known as Becker’s nevus syndrome.2 Treatment is not medically warranted for an isolated Becker’s nevus but may be pursued for cosmetic reasons. Although treatment is generally discouraged because of variable success, laser hair removal and laser therapy may be pursued to address the hypertrichosis and hyperpigmentation, respectively.
What is on the differential?
A café-au-lait macule (CALM) is a light- to dark-brown, oval lesion that commonly presents at birth or in early childhood. CALMs vary widely in size from less than 1.5 cm to more than 20 cm in diameter. They are asymptomatic and grow in proportion to the individual over time.3 Becker’s nevus can be distinguished from CALMs by the development of hypertrichosis, typical location and course, and other skin changes within the nevus.
Postinflammatory hyperpigmentation (PIH) is characterized by asymptomatic, darkened macules or patches that are brown to blue-gray in color. It is one of the most common causes of hyperpigmentation, particularly in skin of color, and can take months to years to resolve. PIH is caused by increased melanin production in response to a cutaneous inflammatory process, such as a drug reaction, allergy, mechanical or thermal injury, infection, phototoxicity, or an underlying skin condition.3 Our patient’s history with the lack of an inciting inflammatory process is more consistent with Becker’s nevus.
Erythema ab igne is a cutaneous reaction to heat that presents as a hyperpigmented patch with a reticular or mottled configuration and superficial venular telangiectasia. The lesion is initially erythematous and progresses to a pale pink to purplish dark-brown color.4 Causes include long-term use of a heating pad, laptop, electric blanket, or a hot water bottle. The absence of prolonged heat exposure in our patient’s history does not favor erythema ab igne.
Pigmentary mosaicism is characterized by a distinctive pattern of hyperpigmentation that follows the lines of ectodermal embryologic development, known as the lines of Blaschko.5 This condition is also known as linear and whorled nevoid hypermelanosis because of its streaky or swirl-like pattern. Pigmentary mosaicism can be present at birth or appear within the first few weeks of life. It is caused by genetic heterogeneity in neuroectodermal cells, which results in skin with areas of varying colors. Pigmentary mosaicism is unlikely in this case as our patient’s lesion does not follow the lines of Blaschko.
Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is the vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. Ms. Laborada and Dr. Eichenfield have no relevant financial disclosures.
References
1. Atzmony L et al. J Cutan Pathol. 2020;47(8):681-5.
2. Danarti R et al. J Am Acad Dermatol. 2004;51(6):965-9.
3. Paller A and Mancini AJ. “Hurwitz Clinical Pediatric Dermatology: A textbook of skin disorders of childhood and adolescence” 4th ed. Philadelphia: Elsevier Saunders, 2011.
4. Patel DP. JAMA Dermatol. 2017;153(7):685.
5. Kromann AB et al. Orphanet J Rare Dis. 2018;13(1):39.
Drug survival study looks at what lasts longest in RA, axSpA, PsA, and psoriasis
Survival rates of biologics and other novel immunomodulatory drugs vary substantially across chronic inflammatory diseases, and rates are highest for rituximab in rheumatoid arthritis (RA) and golimumab in axial spondyloarthritis (axSpA), but with similar rates seen for most drugs used in the treatment of psoriasis and psoriatic arthritis (PsA), according to findings from a study of two Danish registries.
Drug survival refers to “the probability that patients will remain on a given drug, and is a proxy for efficacy as well as safety in daily clinical practice,” wrote Alexander Egeberg, MD, PhD, of the department of dermatology at Copenhagen University Hospital–Bispebjerg, and colleagues. Although the use of biologics has expanded for inflammatory diseases, real-world data on drug survival in newer agents such as interleukin (IL)-17, IL-23, and Janus kinase inhibitors are lacking, they said.
In a study published in Seminars in Arthritis and Rheumatism, the researchers reviewed data from the DANBIO and DERMBIO registries of patients in Denmark with inflammatory diseases including rheumatoid arthritis (RA), axial spondyloarthritis (AxSpA), psoriatic arthritis (PsA), and psoriasis.
The study population included 12,089 adults: 5,104 with RA, 2,157 with AxSpA, 2,251 with PsA, and 2,577 with psoriasis. Patients’ mean age at the time of first treatment for these conditions was 57.8 years, 42.3 years, 49 years, and 45 years, respectively. Participants were treated with biologics or novel small molecule therapies for RA, AxSpA, PsA, or psoriasis between January 2015 and May 2021 (from the DANBIO database) and November 2009 to November 2019 (DERMBIO database).
In adjusted models, drug survival in RA was highest for rituximab followed by baricitinib, etanercept, and tocilizumab. Drug survival in AxSpA was highest for golimumab, compared with all other drugs, followed by secukinumab and etanercept. Survival was lowest for infliximab. In PsA, drug survival was roughly equal for most drugs, including golimumab, secukinumab, and ixekizumab, with the lowest survival observed for tofacitinib and infliximab, compared with all other drugs. Drug survival in psoriasis was highest with guselkumab, followed by ustekinumab and IL-17 inhibitors.
However, the number of treatment series “was low for some drugs, and not all differences were statistically significant, which could influence the overall interpretability of these findings,” the researchers noted in their discussion.
Notably, the high treatment persistence for rituximab in RA patients needs further confirmation, the researchers said. “In Denmark, rituximab is often the biologic drug of choice in RA patients with a history of cancer while there is a reluctancy to use TNF [tumor necrosis factor] inhibitors in such patients; this may have prolonged the drug survival for rituximab treated patients due to limited treatment alternatives,” they said.
The findings were limited by several factors, including the observational study design and changes in guidelines over the course of the study, the researchers noted. Other limitations included the inability to adjust for certain variables, such as antibody status, body weight, and smoking, because of missing data, and a lack of data on the underlying reasons for drug discontinuation, they said.
However, the results were strengthened by the large number of patients and completeness of the registries, the researchers emphasized. The range in responses to different drug types across diseases supports the need for individualized treatments with attention to underlying disease, patient profile, and treatment history, they concluded.
The study received no outside funding. Eight coauthors reported financial ties to a number of pharmaceutical companies.
Survival rates of biologics and other novel immunomodulatory drugs vary substantially across chronic inflammatory diseases, and rates are highest for rituximab in rheumatoid arthritis (RA) and golimumab in axial spondyloarthritis (axSpA), but with similar rates seen for most drugs used in the treatment of psoriasis and psoriatic arthritis (PsA), according to findings from a study of two Danish registries.
Drug survival refers to “the probability that patients will remain on a given drug, and is a proxy for efficacy as well as safety in daily clinical practice,” wrote Alexander Egeberg, MD, PhD, of the department of dermatology at Copenhagen University Hospital–Bispebjerg, and colleagues. Although the use of biologics has expanded for inflammatory diseases, real-world data on drug survival in newer agents such as interleukin (IL)-17, IL-23, and Janus kinase inhibitors are lacking, they said.
In a study published in Seminars in Arthritis and Rheumatism, the researchers reviewed data from the DANBIO and DERMBIO registries of patients in Denmark with inflammatory diseases including rheumatoid arthritis (RA), axial spondyloarthritis (AxSpA), psoriatic arthritis (PsA), and psoriasis.
The study population included 12,089 adults: 5,104 with RA, 2,157 with AxSpA, 2,251 with PsA, and 2,577 with psoriasis. Patients’ mean age at the time of first treatment for these conditions was 57.8 years, 42.3 years, 49 years, and 45 years, respectively. Participants were treated with biologics or novel small molecule therapies for RA, AxSpA, PsA, or psoriasis between January 2015 and May 2021 (from the DANBIO database) and November 2009 to November 2019 (DERMBIO database).
In adjusted models, drug survival in RA was highest for rituximab followed by baricitinib, etanercept, and tocilizumab. Drug survival in AxSpA was highest for golimumab, compared with all other drugs, followed by secukinumab and etanercept. Survival was lowest for infliximab. In PsA, drug survival was roughly equal for most drugs, including golimumab, secukinumab, and ixekizumab, with the lowest survival observed for tofacitinib and infliximab, compared with all other drugs. Drug survival in psoriasis was highest with guselkumab, followed by ustekinumab and IL-17 inhibitors.
However, the number of treatment series “was low for some drugs, and not all differences were statistically significant, which could influence the overall interpretability of these findings,” the researchers noted in their discussion.
Notably, the high treatment persistence for rituximab in RA patients needs further confirmation, the researchers said. “In Denmark, rituximab is often the biologic drug of choice in RA patients with a history of cancer while there is a reluctancy to use TNF [tumor necrosis factor] inhibitors in such patients; this may have prolonged the drug survival for rituximab treated patients due to limited treatment alternatives,” they said.
The findings were limited by several factors, including the observational study design and changes in guidelines over the course of the study, the researchers noted. Other limitations included the inability to adjust for certain variables, such as antibody status, body weight, and smoking, because of missing data, and a lack of data on the underlying reasons for drug discontinuation, they said.
However, the results were strengthened by the large number of patients and completeness of the registries, the researchers emphasized. The range in responses to different drug types across diseases supports the need for individualized treatments with attention to underlying disease, patient profile, and treatment history, they concluded.
The study received no outside funding. Eight coauthors reported financial ties to a number of pharmaceutical companies.
Survival rates of biologics and other novel immunomodulatory drugs vary substantially across chronic inflammatory diseases, and rates are highest for rituximab in rheumatoid arthritis (RA) and golimumab in axial spondyloarthritis (axSpA), but with similar rates seen for most drugs used in the treatment of psoriasis and psoriatic arthritis (PsA), according to findings from a study of two Danish registries.
Drug survival refers to “the probability that patients will remain on a given drug, and is a proxy for efficacy as well as safety in daily clinical practice,” wrote Alexander Egeberg, MD, PhD, of the department of dermatology at Copenhagen University Hospital–Bispebjerg, and colleagues. Although the use of biologics has expanded for inflammatory diseases, real-world data on drug survival in newer agents such as interleukin (IL)-17, IL-23, and Janus kinase inhibitors are lacking, they said.
In a study published in Seminars in Arthritis and Rheumatism, the researchers reviewed data from the DANBIO and DERMBIO registries of patients in Denmark with inflammatory diseases including rheumatoid arthritis (RA), axial spondyloarthritis (AxSpA), psoriatic arthritis (PsA), and psoriasis.
The study population included 12,089 adults: 5,104 with RA, 2,157 with AxSpA, 2,251 with PsA, and 2,577 with psoriasis. Patients’ mean age at the time of first treatment for these conditions was 57.8 years, 42.3 years, 49 years, and 45 years, respectively. Participants were treated with biologics or novel small molecule therapies for RA, AxSpA, PsA, or psoriasis between January 2015 and May 2021 (from the DANBIO database) and November 2009 to November 2019 (DERMBIO database).
In adjusted models, drug survival in RA was highest for rituximab followed by baricitinib, etanercept, and tocilizumab. Drug survival in AxSpA was highest for golimumab, compared with all other drugs, followed by secukinumab and etanercept. Survival was lowest for infliximab. In PsA, drug survival was roughly equal for most drugs, including golimumab, secukinumab, and ixekizumab, with the lowest survival observed for tofacitinib and infliximab, compared with all other drugs. Drug survival in psoriasis was highest with guselkumab, followed by ustekinumab and IL-17 inhibitors.
However, the number of treatment series “was low for some drugs, and not all differences were statistically significant, which could influence the overall interpretability of these findings,” the researchers noted in their discussion.
Notably, the high treatment persistence for rituximab in RA patients needs further confirmation, the researchers said. “In Denmark, rituximab is often the biologic drug of choice in RA patients with a history of cancer while there is a reluctancy to use TNF [tumor necrosis factor] inhibitors in such patients; this may have prolonged the drug survival for rituximab treated patients due to limited treatment alternatives,” they said.
The findings were limited by several factors, including the observational study design and changes in guidelines over the course of the study, the researchers noted. Other limitations included the inability to adjust for certain variables, such as antibody status, body weight, and smoking, because of missing data, and a lack of data on the underlying reasons for drug discontinuation, they said.
However, the results were strengthened by the large number of patients and completeness of the registries, the researchers emphasized. The range in responses to different drug types across diseases supports the need for individualized treatments with attention to underlying disease, patient profile, and treatment history, they concluded.
The study received no outside funding. Eight coauthors reported financial ties to a number of pharmaceutical companies.
FROM SEMINARS IN ARTHRITIS AND RHEUMATISM